IN THIS ISSUE:
NEWS: Hospital Pharmacy Shortages Survey Page 4
MEDICINES: Mazars Report welcomed by pharmaceutical industry Page 7
CONFERENCE: History of Urology Page 10
FEATURE: Ovarian Cancer Page 17

CPD: Diabetes and Insulin Prescribing Page 31
GASTROENTEROLOGY FOCUS: Colon Capsule Endoscopy Page 36
ENDOCRINOLOGY FOCUS: Personalised approaches to IBS Page 43
HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HPN March 2023 Issue 106 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only NOW RINVOQ® (upadacitinib) INDICATED FOR ADULTS WITH MODERATE TO SEVERELY ACTIVE ULCERATIVE COLITIS1 *RINVOQ® is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1 AbbVie® is a registered trademark of AbbVie Inc. RINVOQ® and its design are registered trademarks of AbbVie Inc. REFERENCES: 1. RINVOQ® summary of product characteristics, available at www.medicines.ie. JAK: Janus kinase; UC: ulcerative colitis. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. Legal Classification: POM(S1A). IE-RNQG-220007 August 2022 Full Summary of Product Characteristics is available at medicines.ie ▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie. Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
Flucloxacillin Powder for solution for injection/infusion


PRESCRIBING INFORMATION: Consult the Summary of Product Characteristics for further information. Additional information is available upon request. Flucloxacillin 500mg, 1000mg and 2000mg powder for solution for injection/infusion. Active ingredients: As flucloxacillin sodium, each 10mL vial contains 500mg flucloxacillin (25.5mg sodium approximately), 20mL vial contains 1000mg flucloxacillin (51mg sodium approximately), 50mL vial contains 2000mg flucloxacillin (102mg sodium approximately). Indications: For the treatment of the following infections due to beta-lactamase producing staphylococci and other sensitive Gram-positive organisms such as streptococci – skin and soft tissue infections (like abscesses, cellulitis, infected burns, impetigo), upper respiratory tract infections (like pharyngitis, tonsillitis, sinusitis), lower respiratory tract infections (like pneumonia, bronchopneumonia, pulmonary abscess), bone and joint infections (like osteomyelitis and arthritis), endocarditis. Prophylaxis in cardiovascular surgery (valve prostheses, artery prostheses) and orthopedic surgery (arthroplasty, osteosynthesis and arthrotomy). Consideration should be given to official guidance on appropriate use of antibacterial agents. Posology and method of administration: Parenteral therapy is indicated if oral route impracticable or unsuitable as in the case of severe diarrhoea or vomiting and particularly for urgent treatment of severe infection. Routes of administration: 500mg vial - Intramuscular (im), intravenous (iv), intrapleural and intraarticular. 1000mg vial and 2000mg vial – intramuscular and intravenous routes only. Intravenous injection/ infusion should be administered slowly. Dosage depends on age, weight and renal function as well as the severity and nature of the infection. Adults and adolescents ≥ 12 years – Total daily dosage of 1g to 4g, administered in three to four divided doses, by iv or im injection. Severe infections: up to 8g per day administered in four infusions (over 20 to 30 min). No single bolus injection or infusion should exceed 2g. Maximum dose of 12g per day should not be exceeded. In surgical prophylaxis: 2g iv (bolus or infusion) upon induction of anesthesia, to be repeated every 6h for 24h in vascular and orthopedic surgery, and for 48h in cardiac or coronary surgery. Methicillin-susceptible Staphylococcus aureus. Endocarditis: 2g every 6h, increasing to 2g every 4h in patients weighing >85kg. Children under 12 years of age – In mild to moderate infections: 25 to 50mg/kg/24 hours administered in three to four equally divided doses by im or iv injection. Severe infections: Up to 100mg/kg/24 hours in three to four divided doses. No single bolus injection or infusion should exceed 33mg/kg. Methicillin-susceptible Staphylococcus aureus. Endocarditis: 200mg/kg/24 hours in three to four divided doses. Premature infants, neonates, sucklings and infants – Risk of kernicterus; only use in premature infants and neonates after rigorous risk-benefit assessment. Treatment is generally with 25mg to 50mg/kg/24 hours divided into three or four equal doses.




Maximum daily dose 100mg/kg/24 hours. Abnormal renal function – Renal excretion slowed in patients with renal insufficiency. In severe renal insufficiency (creatinine clearance <10ml/min) consider dose reduction or extension of dose interval. Maximum recommended dose in adults is 1g every 8 to 12 hours. In anuric patients maximum dosage is 1g every 12 h. Flucloxacillin not significantly removed by dialysis hence no supplementary dosages need be administered either during or at end of dialysis period. Hepatic impairment – Dose reduction not necessary. Intrapleural and intraarticular – Usual dose is 250mg to 500mg once daily. Contraindications: Hypersensitivity to the active substance or excipients, should not be given to patients with history of hypersensitivity to beta-lactam antibiotics, patients with previous history of flucloxacillin-associated jaundice/hepatic dysfunction. Not suitable for ocular or subconjunctival administration or intrathecal injection. Special warnings and precautions for use: Before initiating therapy with flucloxacillin check patient history concerning previous hypersensitivity reactions to beta-lactams. Cross sensitivity between penicillins and cephalosporins is well documented. Serious and
occasionally fatal hypersensitivity reactions have been reported in patients receiving beta-lactam antibiotics, occurrence more frequent with parenteral compared to oral therapy. Reactions more likely to occur in patients with history of beta-lactam hypersensitivity. If allergic reaction occurs discontinue flucloxacillin and institute appropriate therapy. Feverish generalized erythema associated with pustula occurring at treatment initiation may be a symptom of acute generalised exanthematous pustulosis (AGEP); in case of AGEP diagnosis discontinue flucloxacillin and subsequent administration is contraindicated. Use with caution in patients with evidence of hepatic dysfunction, patients ≥50 years of age and those with serious underlying disease; hepatic events may be severe and in extremely rare circumstances deaths have been reported. Flucloxacillin solutions reconstituted with local anesthetics (lidocaine) should not be given by iv administration. Adjust dose in renal impairment. Special caution essential in newborns due to risk of hyperbilirubinaemia and potential for high serum levels of flucloxacillin due to reduced rate of renal excretion. Regular monitoring of blood count, hepatic and renal function recommended during prolonged treatment. Pseudomembranous colitis can occur; if this develops discontinue flucloxacillin and initiate appropriate therapy. Prolonged use may occasionally result in overgrowth of nonsusceptible organisms. Caution when administered concomitantly with paracetamol due to increased risk of high anion gap metabolic acidosis (HAGMA). High risk of HAGMA particularly in patients with severe renal impairment, sepsis or malnutrition especially if maximum daily doses of paracetamol used. Following co-administration close monitoring recommended to detect the appearance of HAGMA (including testing for urinary 5-oxoproline). If flucloxacillin is continued after paracetamol cessation, it is advisable to ensure no HAGMA signals. Particular caution with drug-induced liver injury in patients with HLA-B*5701 haplotype; frequency of these disorders increasing in HIV-infected patients who may be at increased risk of exposure to flucloxacillin. Hypokalaemia (potentially life threatening) can occur especially at high doses and may be resistant to potassium supplementation. Regularly measure potassium levels during high dose flucloxacillin therapy. Attention warranted when combining flucloxacillin with hypokalaemia inducing diuretics or when other risk factors for development of hypokalaemia present. Contains sodium, consider in patients on a sodium controlled diet. Undesirable effects: Common (>1/100 to <1/10): Minor gastrointestinal disturbances. Uncommon (>1/1000 to <1/100): Rash, urticaria, purpura. Very rare (<1/10,000): Neutropenia (including agranulocytosis), thrombocytopenia, eosinophilia, haemolytic anaemia, anaphylactic shock, angioneurotic oedema, high anion gap metabolic acidosis, pseudomembranous colitis, neurological disorders with convulsions possible with high dose iv injection in patients with renal failure, hepatitis, cholestatic jaundice, changes in liver function laboratory test results, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, arthralgia, myalgia, interstitial nephritis, fever sometimes develops more than 48 hours after start of treatment. Frequency not known: Acute generalized exanthematous pustulosis, phlebitis. Legal Category: POM Marketing Authorisation Numbers: PA2059/069/001, PA2059/069/002, PA2059/069/003. Marketing Authorisation Holder: Fresenius Kabi Deutschland GmbH, Else-Kroener Strasse 1, Bad Homburg v.d.H 61352, Germany Further Information: See the SmPC for further details. Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra.ie. Adverse events should also be reported to Fresenius Kabi Limited via email Pharmacovigilance.GB@Fresenius-Kabi.com. Date of Preparation: August 2021 IE-IVF-2100004
of Prep: August 2021
500mg 1000mg 2000mg Fresenius Kabi Limited Fresenius Kabi Ireland Unit 3B Fingal Bay, Balbriggan, Co. Dublin, Ireland Website: www.fresenius-kabi.com/ie/ Email: FK-enquiries.ireland@fresenius-kabi.com Phone: +353 (0)1 841 3030
Date
Ref: IE-IVF-2100007
Pharmacy Departments
renew Memorandum of
Understanding P5
Medicine shortages index standing at 247 P6
Mazars Report welcomed by pharmaceutical industry P7

Advances in the field of HIV P20
Promoting equality and diversity in Gender orientation
sexual health P23
Women in Surgery Fellowship for Dr Murphy P29
REGULARS
CPD: Insulin Prescribing P31
Endocrinology Focus: Liver Disease and Pregnancy P39
Endocrinology Focus: Pancreatic Cancer P50
Endocrinology Focus: Diabetes P56
Feature: Lipid Guidelines for clinical practice P72
Clinical R&D: P80
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562

GROUP DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood
EDITORIAL
editorial@hospitalprofessionalnews.ie
ACCOUNTS
Rachel Wilson cs.ipn@btconnect.com

SALES EXECUTIVE
Aoife Tremere aoife.t@hospitalprofessionalnews.ie
CONTRIBUTORS
Bernadette Carter | Ann Piercy
Dr Ken Patterson | Niall Davis

Diana Hogan-Murphy | Eimear Gibbons
Dr OB Kelly | Barry Hall
Samantha Vega | Cassandra Herron
Omar Elsherif | Ms Rand Al-Najim
Professor Humphrey J O’Connor
Jemma Henry | Theresa Lowry-Lehnen
Tatiana Iamak | Dr Jonathan Briody
Dr Kate McCann | Dr Werd Al-Najim
Professor Alex Miras | Aideen Stack
Professor Fiona Lyon

Professor Mary Higgins
DESIGN DIRECTOR

Ian Stoddart Design
Editor
Healthcare workforce shortages are not a new phenomenon triggered by the COVID-19 pandemic. They were simply exacerbated by it. Shortages of nurses or physicians in hospitals, particularly in rural areas are frequently featured in news outlets across Europe.
However, not only these professions are affected but also the pharmacy workforce, including pharmacists, technicians and others. Especially those working in hospitals have to cope with staffing which is insufficient to meet patients' needs. To address the problem of pharmacy workforce shortages, the European Association of Hospital Pharmacists (EAHP) and its members started an in-depth analysis of the situation in Europe.
A workshop conducted with delegates from EAHP's member countries in June last year was followed by an Investigation of the Hospital Pharmacy Profession in Europe that closed in the first quarter of 2023. “High-quality education in all European countries is of utmost importance for addressing the workforce problems of pharmacists to ensure that the profession remains an integral part of healthcare,” says EAHP. You can read more about this on page 5 of this issue.
In other news, the decision by Minister for Health, Stephen Donnelly T.D. to publish the Mazars Report has been welcomed by the Irish Pharmaceutical Healthcare Association as a positive step towards providing Irish patients with faster access to lifechanging new medicines. Turn to page 7 for the full details.
In one of your leading clinical features this month, Professor Fiona Lyon discusses recent advances in the field of HIV. Professor Lyon is Medical Director/Clinical Lead in Sexual Health, HSE Sexual Health and Crisis Pregnancy Programme and Consultant in Genitourinary Medicine, GUIDE Clinic, St. James’s Hospital.
“Four decades since the first reports of HIV and AIDS in Ireland, massive advances have been made in the field of HIV medicine such that in 2023 individuals newly diagnosed with HIV have the potential for life expectancy similar to that of the general population and new HIV infections could be prevented,” says Professor Lyon.
“In 2023, there is a vast range of HIV treatment and prevention options available. It is critical that our health system and broader society is responsive and adaptable to ensure the best possible outcomes for people living with HIV (PLWH) and those at risk of acquiring HIV.” You can read the full article on page 20.
Meanwhile, our special focus for March is on Gastroenterology/ Endocrinology & Nutrition and we have some excellent contributed content, including personalised approaches to IBS, Pancreatic cancer and diabetes care in Ireland.
I hope you enjoy the issue.
3 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 March Issue Issue 106 7
Contents Foreword
23 9 29 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews
Health service has ‘eye off the ball’
A leading Professor at the University of Limerick has said that the continued trend towards imposing a corporate model in our public health system is compounding the service’s inability to deliver care to patients.
Speaking in a New Video as part of the Irish Hospital Consultants Association’s (IHCA) Care Can’t Wait campaign, Professor Orla Muldoon says too much emphasis is being placed on Key Performance Indicators (KPIs) and not enough on providing the capacity and resources required for treating patients.

The Professor of Psychology explains how this in turn has created a ‘management class’ in the public health service of which many decision-makers are not medical professionals.
She questions why the medical and surgical specialists and leaders in hospitals are not “trusted to manage the system” or the people they care for on a daily basis.
Commenting, she said, “We have this paradox where patients, once they get into the system, feel the quality of care is good. Yet, we don’t really believe the people providing that care can take
Support Award for Ashley
leadership roles in the delivery of the service.”
Recent studies show almost 8 in every 10 Consultants are screening positive for burnout as they struggle to cope in an overstretched public hospital system where demand has outstripped supply.
Professor Muldoon says that this is the case across the entire health system as “those who we have tasked with caring for our most vulnerable” increasingly describe the management structure and use of performance indicators as “frustrating”.
Professor Muldoon says this also shows that far too much focus is being placed on counting performance indicators rather than on providing the capacity and resources for the delivery of essential services to patients.
“This model of using KPIs to identify areas of improvement works in the private, corporate sector, because those entities are given all sorts of resources to help achieve their targets. But in the public health system you have more people counting performance indicators, but no extra resources allocated to the actual care.”
New Hospital Pharmacy Medicine Shortage Survey
Medicines availability in the new year started very much as it ended in 2022. News outlets across Europe reported the increase in respiratory infections, in particular in children, leading to shortages of vital treatment options, including antibiotics. At the same time, healthcare professionals, patient representatives and notified bodies were ringing the alarm bells due to increasing concerns about medical device shortages.
Congratulations to Ashley Bazin, Team Leader on the Oncology & Haematology Clinical Trials with Tallaght University Hospital (TUH) who has been awarded the Irish Cancer Society Support Staff of the Year Award for 2023, in recognition of her contribution to driving forward Clinical Trials in TUH.
The Support staff of the Year Award recognises an individual who on a daily basis goes above and beyond the call of duty to support the cancer research being carried out across the country.
The PMI’s Annual Pharma Summit will be held on March 30th, 2023 at Croke Park

Five years passed since the last comprehensive investigation by the European Association of Hospital Pharmacists (EAHP) into the problem of medicine shortages. Since new data is vital – especially ahead of the upcoming revision of the general pharmaceutical legislation – EAHP has decided to launch a new Shortage Survey, this time looking at both the shortage of medicines and medical devices in the hospital environment.
The 2023 Shortage Survey, focused on medicinal products and medical devices, seeks to investigate the
reasons and impacts of shortages on patients in European hospitals as well as possible solutions. Besides hospital pharmacists, EAHP’s Survey targets other healthcare professionals working in hospitals, like nurses and physicians, as well as patients and their carers that have experienced medicine shortages during their hospital stay.
EAHP has been advocating on the issue of medicines shortages for more than a decade. Five surveys were conducted by EAHP in 2013, 2014, 2018, 2019 and 2020, with the last one focusing also on the pandemic preparedness of pharmacists. The results of these surveys have provided an overview of the severity of the problem as well as its impact on overall patient care.
Healthcare professionals and patients impacted by a medicine shortage during their treatment in a hospital are invited to provide feedback until 30 April 2023.
The theme for the day is “Partnering to Improve Human Health” and the event will be exploring this theme throughout the day from four vantage points: Technology, Government, Cross Company and Intra Company. The day will be highly interactive with a mix of keynote speakers and panel discussions with plenty of opportunities to catch up with industry colleagues and make new connections. Visit www.thepmi.com for further information.
4 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Pharmacy Renews Memorandum of Understanding
Pictured are representatives from Beacon Hospital Pharmacy Department and the School of Pharmacy and Biomolecular Science, RCSI as they renewed a Memorandum of Understanding

Last month, representatives renewed the Memorandum of Understanding between Beacon Hospital’s Pharmacy Department and RCSI School of Pharmacy and Biomolecular Sciences. Through collaboration and cooperation, Beacon Hospital and RCSI are mutually committed to the on-going development, delivery and promotion of a person-centred model of care for Pharmacy Practice in accordance with international best practice. The continued success of this collaborative partnership is based on the engagement, co-operation, good-will and mutual respect demonstrated by both Beacon Hospital Pharmacy Department and RCSI School of Pharmacy and Biomolecular Sciences. Both have effectively collaborated together previously and are committed to continuing this arrangement into the future.
Renewed Memorandum of Understanding
This agreement will provide for RCSI undergraduate pharmacy students to receive placementbased pharmacy education at Beacon Hospital to facilitate the development of clinical pharmacy knowledge.
Additionally the RCSI School of Pharmacy and Biomolecular Sciences will facilitate the
development of educational content and training programmes which will support the education of Pharmacy Staff Members in Beacon Hospital.
Similarly, through this agreement there will be opportunities for collaborative research between RCSI and Beacon Hospital. In particular this research will be directed at improving patient outcomes and experience, through enhancing the
underpinning pharmacy services at Beacon Hospital.
Academic Appointments
Catherine Nugent reappointed as Honorary Clinical Associate Professor at RCSI.
Keira Hall reappointed as Honorary Senior Lecturer at RCSI. Seamus Dunne and Hilary Ward new appointments as Honorary Clinical Lecturers at RCSI.
Future-Proofing the hospital pharmacy workforce
Healthcare workforce shortages are not a new phenomenon triggered by the COVID-19 pandemic. They were simply exacerbated by it. Shortages of nurses or physicians in hospitals, particularly in rural areas are frequently featured in news outlets across Europe. However, not only these professions are affected but also the pharmacy workforce, including pharmacists, technicians and others. Especially those working in hospitals have to cope with staffing which is insufficient to meet patients' needs.
To address the problem of pharmacy workforce shortages, the European Association of Hospital Pharmacists (EAHP) and its members started an in-depth analysis of the situation in Europe. A workshop conducted with delegates from EAHP's member countries in June last year was followed by an Investigation of the Hospital Pharmacy Profession in Europe that closed in the first quarter of 2023. Touching
on the state-of-the-art of the profession and specifically on the European Statements of Hospital Pharmacy, the investigation collected information on their implementation, the size of the profession and other pharmacyspecific practice areas.
Scientific achievements for example in the field of advanced therapy medicinal products are leading to increasingly complex medication-related problems, specific handling and preparations and related issues. In addition, new competencies and tasks widened the scope of engagement of hospital pharmacists in multi-professional teams in the hospital setting and beyond. Medicines reconciliation, medication optimisation, bedside counselling or being part of the antimicrobial stewardship team are just a few of the clinical pharmacy services that should be provided to all patients across Europe by hospital pharmacists as part of the multidisciplinary care
team. To ensure the availability of these vital services a resilient workforce is required.
Other aspects requiring a futureproof pharmacy workforce are the increasing individualisation of care, growing medicine shortage problems requiring interventions by hospital pharmacists and supporting personnel, and rising healthcare costs. The latter can be addressed with the help of pharmacy expertise linked to the procurement of medicines and medical devices and health technology assessments (HTAs) but is also associated with a larger need for the workforce to handle often very specific needs of the patients. Another very important chapter for optimal patient outcomes and safety is the interface of care. Thus, the need for highly educated and specialised professionals in medication and medication-related processes that can ensure the seamless transfer of patients between healthcare settings is growing.
High-quality education in all European countries is of utmost importance for addressing the workforce problems of pharmacists to ensure that the profession remains an integral part of healthcare. Education needs to go hand in hand with hiring enough pharmacy personnel, including support staff, and finding the appropriate balance of training a sufficient number of students each year to robustly grow the pharmacy profession in each country.
For EAHP it is clearly time to act for patients in all countries around Europe. Concrete action that ensures a resilient and futureproof pharmacy profession by for example training an adequate number of students to fill the growing number of vacancies in clinical, community, hospital and industrial pharmacies is urgently needed. To address the workforce gaps, EAHP plans to put forward a new position paper on the hospital pharmacy workforce that proposes short and long-term measures.
5 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
News
Medicine Shortages Index at 247
“There’s an awareness in other European countries that market related factors need to be tackled. Medicines shortages are not just winter specific, and shortages are not only occurring as a result of exceptional circumstances. There are systemic factors that need resolution”
Medicine shortages in Ireland continue to persist with 247 different medicines used by Irish patients currently out-of-stock, as a new trend affecting supply develops, according to the latest Medicine Shortage Index.
The latest figures show the number of medicine shortages in Ireland up an additional 19 medicines in short supply since the end of last month, and a 38% increase since the Index began in October.
Of the 247 medicines currently unavailable, 13 are listed on the World Medical Organisation’s (WHO) ‘critical medicines’ list.
The latest shortages analysis indicates a new trend of medicines that are stored or delivered using plastic components now increasingly in short supply. These medicines include nasal sprays, inhalers for the treatment of asthma and 11 different eye drop products.

Other medicines still in short supply across multiple suppliers in the past week include those that treat epilepsy, and medicines used for the treatment of high blood pressure.
Many antibiotics like Amoxicillin and Penicillin and commonly used over-the-counter medicines like Benylin ™ and Dioralyte ™ are still difficult for patients to source.
The Medicine Shortage Index, prepared by industry experts, Azure Pharmaceuticals, analyses data made publicly available by the Health Products Regulatory Authority (HPRA).
Other European countries have already taken specific policy measures to date in response to the escalating medicine shortage issue. Portugal, the UK, Germany, and Switzerland have all taken a range of price related policy measures in response to the problem, including price increases for lower priced medicines.
While, Sweden, Denmark and Malta, which all use tenders to set reimbursement prices, have all experienced price increases
due to lack of supply of core medicines. To date, the Irish Department of Health is yet to meaningfully respond to this deepening challenge.
Medicine shortages will continue grow incrementally unless political will is shown in Ireland to take measures, like those carried out by other EU nations, to meaningfully tackle the issue, Sandra Gannon, Azure CEO, said:
“One of the means we have to protect our domestic supply of stock, to prevent these important medicines from running out, is through pricing. Other European countries have already recognised this fact and taken measures to mitigate against situations where their stocks run out. For example, Portugal recently raised its pricing by up to 5% for cheap medicines.
“Weaknesses in the supply chain alone highlight the imperative of revisiting the pricing framework for medicines to protect supply of stock and protect Irish patients.”
Doctors encouraged to train in Ireland
Irish postgraduate medical colleges were in Dubai recently to discuss the large range of medical training opportunities in Ireland for doctors from the United Arab Emirates and the wider Gulf region. A new website www. postgraduatemedicaltraining.ie was launched by Her Excellency Ambassador Alison Milton, Irish Ambassador to the UAE, detailing Ireland’s clinical postgraduate training opportunities for doctors from across the GCC. It also shares information on relocation support and benefits of training in Ireland.
The clinical residency and fellowship programmes provided by Irish medical colleges are internationally recognised and fully certified. They offer handson supervised training, as well as opportunities for clinical research. Doctors from the UAE will have the opportunity to move to Ireland and learn from leading experts to develop the medical, communication, management and interpersonal skills required to provide world-class care and enhance the clinical environments in which they operate.
“The Royal College of Physicians of Ireland has strong links with the United Arab Emirates, partnering with healthcare organisations in the UAE to provide postgraduate medical training opportunities since 2013. These partnerships are highly valued by the College,” Professor Mary Horgan, President of the Royal College of Physicians of Ireland said.
“Medical graduates from the United Arab Emirates make significant contributions to our medical teams. Graduates report
Commenting on the perception that medicine shortages are a result of exceptional circumstances and are a one-off situation, Ms Gannon pointed to the level of EU activity on the topic, as well as the focus of the European Medicines regulator (EMA) on medicine shortages as evidence that this problem is not going away without serious intervention and planning.
“There’s an awareness in other European countries that market related factors need to be tackled. Medicines shortages are not just winter specific, and shortages are not only occurring as a result of exceptional circumstances. There are systemic factors that need resolution.
“Each patient has different needs and reducing the problem down to exceptional circumstances alone diminishes the quality of life impact that each patient experiences with their illness.”
Medicine shortages experts from Europe and Ireland recently said in Dublin, at a panel discussion examining medicine shortages, that the situation will continue to worsen without the political will to implement measures that put the needs of patients first.
a very positive experience and, upon returning home to the UAE, they are in a strong position to advance their medical careers and contribute to the development of healthcare in the UAE. There are a growing number of fantastic opportunities for doctors from the UAE to come to Ireland for transformative postgraduate medical training across many specialties and this new website is a comprehensive resource for doctors to explore what’s available,” Prof Horgan said.
6 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Positive Step to Faster Medicines Access
The decision by Minister for Health, Stephen Donnelly T.D. to publish the Mazars Report has been welcomed by the Irish Pharmaceutical Healthcare Association as a positive step towards providing Irish patients with faster access to life-changing new medicines.

IPHA, which represents the major international biopharmaceutical companies in Ireland, commended the Minister’s decision to publish the report and to establish a Working Group to review and improve the reimbursement system for new medicines.
The organisation recognised that the initiative is the first of its kind since the passage of the Health (Pricing and Supply of Medical Goods) Act 2013, describing it as a valuable opportunity for key stakeholders, including industry and patient groups, to contribute to improved patient care and health outcomes.
IPHA said the Working Group’s role in reviewing and improving the reimbursement process was especially important considering
the many new advances in science and the potential to raise standards of patient care through faster access to newer and better medicines and treatments.
It applauded the Minister’s decision to act immediately to enhance transparency in the reimbursement system through the introduction of a tracker system and indicative timelines, stating that it would result in better planning and management of applications for medicine reimbursement.
IPHA said it welcomed the opportunity to engage in the Working Group, to present its own reform proposals and to hear the view of other stakeholders. It stated, “We will put forward ideas in good faith and will also listen to what is asked of us. Our members are very conscious of industry’s responsibilities to improve the process in a collaborative way.
We will:
• Bring forward proposals to support the further development of national medicines policy.
Child and Youth Mental Health Lead
• Present the case for an increase in personnel and IT resourcing for the HSE’s Corporate Pharmaceutical Unit, the HSE Drugs Group and the National CPE.
• Explore ways for earlier reimbursement for certain treatments, such as early access schemes, as is done in other countries. IPHA companies are ready with some rare disease, cancer and end-of-life treatments that can be pathfinders for this new approach in 2023 and 2024.
• Advocate for an increase in the HSE’s decision authority table thresholds and more generally for a well-structured commercial framework, as exists in other countries.”
IPHA said there were “many other practical steps that can be taken to improve the processes that
fully respect the HSE’s obligations under the 2013 Health Act. We will study the Mazars Report carefully and provide any observations arising from it to the Minister.”
Michael O’Connell, IPHA President said: “Over the past three budgets, the Government has allocated almost ¤100 million to new medicines. The announcement today is a further indication of the Minister’s continued commitment to ensure that patients have faster access to new, innovative medicines. I thank the Minister for driving this reform and bringing all stakeholders together in doing so”.
Minister for Mental Health and Older People Mary Butler welcomed the opening of the recruitment process for the post of Child and Youth Mental Health Lead in the HSE. This key new role will provide leadership, operational oversight, and delegated management of all service delivery across child and youth mental health services across the country. They will also be responsible for managing and coordinating service planning activities, partnership and capacity building, the development of service plans, and setting of service standards right across child and youth mental health services in Ireland.The post holder will report to the HSE National Director for Community Operations and will be supported by a dedicated team for which funding has been provided.
This role, and the wider Child and Youth Mental Health office, will result in improved links with the National Clinical Advisor Group Lead Mental Health, which in turn will support the development of current and future youth mental healthrelated National Clinical Programmes.
Minister Butler stated, “The progression of this role has been a key priority for me over the past year, and it will play a crucial part in ensuring that integrated mental health services for young people have a more centralised and evidence-based focus within the HSE. “We will see the completed reviews and audits arising from the Maskey Report, along with the Final Report of the Mental Health Commission on CAMHS later this year, which together will give us real time data never available before to support this new post. Importantly, there will be new support staff to underpin this welcome initiative. I will ensure that the HSE also progresses as quickly as possible the new post of National Clinical Lead for Youth Mental Health as announced in the Dáil over the last week.”
Pharmacy Role in Hygiene
Advice on hygiene given by pharmacies has widened from oral care to infection control measures, according to a report by the FIP Global Pharmaceutical Observatory (GPO) published last month.
A literature review found educating people on hygiene measures such as handwashing and disinfection to be a novel community pharmacy service during the pandemic. The FIP GPO also conducted a cross-country survey through five FIP member organisations (n=60).
More than half of the respondents reported that avoidance of respiratory, viral, communicable, and food and water borne infections were the most common “germ concerns” expressed to them by the public. The study also looked into current and future learning needs of pharmacy teams.
The findings from the literature review showed that the concept of providing hygiene care advice has been differently shaped preand post-COVID times. Before the COVID-19 pandemic, the
area of focus was limited to oral hygiene. All studies reviewed in the literature reinforced the vital role of community pharmacists as the public’s first choice of healthcare provider in mitigating the spread of the disease as well as their prominent contribution to overall emergency management.
There was also an unequivocal need to support, train and educate community pharmacists and their teams to improve their knowledge because the concept of increasing personal hygiene awareness
is a focus area of community pharmacists in developed and developing countries.
The report states, “As evidenced throughout this report, community pharmacists and their teams can play a leading role in providing reliable recommendations and educating patients on health hygiene, engaging and empowering patients in self-care, and minimising the spread of contagious diseases, ultimately contributing to improving our communities’ overall health.”
7 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
News
IPHA President Michael O’Connell
Blood Pressure Monitoring outside Hospital
The European Union has awarded a European consortium ¤4.4million for the SMARTSHAPE project to focus on developing an implantable medical device for continuous blood pressure monitoring.
Hypertension is the leading global contributor to premature death, accounting for more than 9 million deaths a year. Elevated blood pressure is a chronic lifetime risk factor that can lead to serious cardiovascular events if undiagnosed or poorly controlled. Many high-risk patients require long-term monitoring to tailor drug treatments and improve healthcare outcomes, but there is no clinically accepted method of continuous beat-to-beat blood pressure
monitoring that patients can use outside of the hospital setting.
The SMARTSHAPE consortium is led by Professor William Wijns, a Science Foundation Ireland (SFI) funded Research Professor in Interventional Cardiology at University of Galway’s College of Medicine, Nursing and Health Sciences.
According to Professor Wijns, “The best innovations start with a clinical need. Patients who require monitoring are better off in their own homes rather than in a hospital setting. There is a huge market opportunity for a medicalgrade, user-friendly, and minimally invasive solution for continuous blood pressure monitoring.”
Professor Wijns is also a Funded Investigator at CÚRAM, the SFI research centre for medical devices based at University of Galway which focuses on developing biomedical implants, therapeutic and diagnostic devices that address the needs of patients living with chronic illness.
Dr Atif Shahzad, joint director of the Smart Sensors Lab at the University of Galway and a research fellow at the Institute of Metabolism and Systems Research at the University of Birmingham, said: “Our SMARTSHAPE consortium has developed an IP-protected technologically disruptive sensor for continuous
pressure measurement. There are challenges related to biocompatibility, longevity, and delivery to the target tissue, and these need to be overcome to deliver the sensor to the market.”
Dr Shahzad added: “This project will address these challenges by formulating an innovative biomaterial: a novel temperaturedependent shape memory polymer (SMP). SMPs will enable the development of a microsensor that can be curled up, introduced into the body through a minimally invasive procedure, and ‘opened up’ when placed at body temperature to take a predefined shape.”
Experts Call for National Life Sciences Strategy to Improve Irish Patient Care
Michael O’Connell, Country Director, Biogen, Mairead McCaul, MSD, Michael O’Connell, IPHA, Brenda Dooley, AXIS Consulting, and Matt Moran, BioPharmaChem, shared key insight on ongoing trends in the pharmaceutical sector
Technology Assessments (HTA) to be made on new medicines; much longer than in other jurisdictions such as Scotland, which is between 6 and 12 months.
Panel of Guaranteed Irish pharmaceutical experts call on Government and key stakeholders to collaborate to achieve more timely access to medicines for Irish patients.

The Annual Guaranteed Irish Pharmaceutical Forum, hosted by Guaranteed Irish featured an industry leading line-up within the pharmaceutical sector. The panel discussion featured industry thought leaders Mairead McCaul Managing Director of MSD Ireland Human Health, Matt Moran Director of BioPharmaChem Ireland, Ibec, Brenda Dooley CEO AXIS Consulting and Michael O’Connell Country Director of Biogen and President of the Irish Pharmaceutical Healthcare Association (IPHA). The attendees included more than 100 registrations from pharmaceutical businesses across the country.
Industry Successes to Date
The panel outlined Ireland’s position as a major global player in pharmaceutical production, employing over 30,000 people in Ireland directly, and a further 30,000 indirectly, with Irish exports exceeding ¤100 billion. Ireland is now the largest net exporter of pharmaceuticals in the EU accounting for over 50% of all exports from the country and is one of the main hubs of manufacturing pharmaceuticals and biopharmaceuticals in the world, with 24 of the 25 global leaders in the industry based in Ireland.
Current Industry Challenges
While Ireland is a global leader in the pharmaceutical industry, timely access to medicines in Ireland is a concern. Periods of up to 2.5 years are the norm for full Health
Resourcing, and the availability of resources to evaluate medicines is a factor in the delayed reimbursement of medicines. As we move to an era of ever-more complex medicines, innovations, Gene & Cell Therapy (GCT) and potential cures; without regular and important conversations between Government, key stakeholders and industry leaders, Ireland is likely to be left behind on the global stage, as will patients. A robust system is needed to ensure the cost effectiveness of new innovations are affordable to both the state and the consumers. The panel of experts also highlighted the challenges facing the industry including talent retention and building a workforce for the future of pharma in Ireland, including tackling the ever-present problem of training educators to help them prepare the next generation for skillsets which may not exist yet.
Future Industry Opportunities
The panel also discussed the need for a life sciences strategy
for Government, stating that 18 of the 20 biggest life sciences manufacturers in the world operate here in Ireland, and that some of these companies in the reimbursement process are finding it difficult to get their innovations to patients in reasonable timeframes. Timely patient access to medicines should be a primary goal of any Government and Health Minister, regardless of political stance or party allegiance. As of January 2023, there is no firm life sciences strategy from Government which incorporates medicines and patient access to medicines.
Panel Call to Government
1. Timely patient access
2. Commitment to development of life sciences strategy
3. Providing additional resources to evaluate and reimburse medicines quickly
4. Attract R&D and clinical trials to Ireland as a boutique destination with experience in the pharmaceutical industry
Finally, Mairéad McCaul, Managing Director of MSD Ireland Human Health, praised Guaranteed Irish, acknowledging the role the organization plays in promoting Ireland and facilitating networking opportunities for likeminded industry experts and leaders to share ideas and create new key relationships, and position Ireland as an ideal location for further pharmaceutical investment.
8 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Sharing Visions in Stroke Care
The first in-person conference to focus on ‘The vision for a comprehensive stroke care pathway in Ireland’ was hosted by the iPASTAR (Improving Pathways for Acute Stroke and Rehabilitation) programme at the RCSI Education and Research Centre, Beaumont Hospital.
iPASTAR is a collaborative doctoral training award funded by the Health Research Board Collaborative Doctoral Awards Programme, and hosted by RCSI and UCD.
The conference brought together healthcare professionals, academics, Public and Patient Involvement (PPI) partners and key stakeholders to celebrate the European Stroke Organisation (ESO) accreditation of the Stroke Service at Beaumont Hospital as a comprehensive stroke centre and present iPASTAR team’s vision for comprehensive stroke care pathway in Ireland.
The new Beaumont stroke unit is the first in Ireland and the UK to be awarded the prestigious ESO accreditation.
Commenting on the success of the event, Professor Frances Horgan, iPASTAR lead and Professor at the School of Physiotherapy, RCSI, said, “The discussions at the symposium today have encompassed the collaborative, co-designed approach that iPASTAR aims to achieve. Bringing clinicians, academics and patients together to address the challenges and opportunities in stroke care will enable us to make positive, evidence-based changes, optimising the patient pathway.”
A ‘design sprint’ workshop preceded the conference where
participants heard from the PPI partners in the project, including the perspectives from a stroke survivor on their experience of life after stroke and being involved in iPASTAR and discussed an ‘ideal’ stroke pathway, from acute care,
Date for your Diary
transition to home to living well and healthy after stroke.
During the symposium, iPASTAR PhD research scholars Dr Deirdre McCartan, Geraldine O’Callaghan, Patricia Hall and Clare Fitzgerald
RCSI Centre for Professionalism in Medicine and Health Sciences, supported by the Bon Secours Health System (Lead Sponsor) and Medical Protection Society are delighted to announce the date for their annual conference:

Professionalism: The Cost of Caring
Join them on the 28th April 2023 for a day of exciting talks and presentations. This year they are delighted to host a hybrid (online and in person) event which gives you the opportunity to participate and engage with our conference, no matter where you are in the world.
presented an update on their PhD projects, which focus on delivery of integrated stroke care for patients, from the hospital, to rehabilitation in the community, and living well after stroke.
The event focuses on Medical Professionalism and promises to be a great day of exciting talks and presentations from an international panel of speakers from Canada, USA, Australia, UK, UAE and Ireland! Joined by colleagues such as Johanna Westbrook from University of Sydney, Colin West from the Mayo Clinic and Yvonne Steinert from McGill, Dr Henry Marsh of “Do No
Registration for the online event is FREE! Please note there is a nominal fee to attend in person 5 CPD points.
Registration Link - https://bit.ly/MedProf23Registration
Put the date in your diary and register now. Don’t forget to follow on Twitter and use #MedProf23

9 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
News
Ian Carter, CEO, RCSI Hospital’s Group; Prof. Fergal O’Brien, Deputy Vice Chancellor for Research and Innovation, RCSI; Prof. Frances Horgan, Profesith
Harm” fame, amongst many others.
Urology Congress
History Congress to offer ‘Inspiration and Perspective’
EAU23 will be held in Milan March 10th-13th, 2023


“For a fresh perspective, several non-urologists will be participating including the prolific Belgian author Kristien Hemmerechts. The role of women in urology and the EAU in particular is of course a very current debate. The number of female urologists is increasing, but their involvement in the EAU’s offices and boards is still lacking. We think it’s very important to focus on role of women in urology and by extension the EAU and we think Mrs. Hemmerechts can provide a feministic point of view on the challenges for women in medicine or professional organisations.
The EAU History Office is hosting another edition of the International Congress on the History of Urology to coincide with EAU23 and to mark the end of the EAU’s 50th Anniversary year. On 10 March 2023, the first day of EAU23 in Milan, there will be a full-day programme subtitled ‘Paradigm Shifts in Urology: 50 Years of Major Developments’. The History Congress will examine some highlights in urology that coincide with the EAU’s five decades of excellence. The selected speakers are experts in their respective fields, including medical pioneers and key figures from EAU history.
The two chairs of the Congress, History Office chairman Prof. Philip Van Kerrebroeck (Antwerp, BE) and his predecessor Prof. Dirk Schultheiss (Giessen, DE) give a preview of some of the themes and topics that will be covered.
Celebrating uniqueness of urology
The EAU conference organisers state, “It’s important to realise that urology is one of the few subspecialties within surgery that was able to retain diagnostics and the conservative part of treatment. Most other fields within surgery have an equivalent within internal medicine. Gastroenterological surgeons, have gastroenterologists, cardiac surgeons have the cardiologists, and so on. Within the field of urology, this has never been the case. This has historical reasons: the development of specific techniques like endoscopy that urologists always kept for themselves.
“If you talk to younger colleagues about why they want to become urologists, this is one reason they give: urology is not only dealing with surgical aspects but also the conservative and diagnostic aspects in terms of therapy. You see a patient from diagnostics to therapy and often beyond.
A half-century
“The general theme of the History Congress is the development of urology over the last 50 years, divided by subspecialty. First of all, this congress marks the end of the EAU’s anniversary celebrations that started at EAU22 and we wanted to highlight what happened in urology in the EAU’s lifetime. Secondly, we wanted to illustrate how much has changed in our field in only 50 years. It’s amazing: if you look how urologists worked, in case of diagnostics and therapies, it’s completely different. Some elements have persisted but there are so many more new modalities or older modalities that have been transformed.
“All medical specialties have had huge developments since the early 1970s, but in urology perhaps there were more dramatic changes. Even over the course of our careers, the amount of open surgery for kidney stones, or for instance cases of horseshoe kidney, have now almost completely been eradicated. The advent of laparoscopic and robotic techniques have even more reduced the need for open surgery and this trend was adopted particularly quickly in urology. Urodynamics
has become digitalized and computerised. Urology can also be seen as a pioneer in the development of prosthetics, for instance in sphincter and penile prostheses, and in the development of neuromodulation in functional problems.
“Reviewing 50 years is not only a review of what we achieved, but also of what was not achieved, or still problematic, or even new problems we created. Looking at sexual reassignment surgery, what was completely normal only 20 years ago is already considered outdated or ridiculous.
A chance to meet pioneers
“Because the programme is focused on the past 50 years, it also allows us to invite people who ‘wrote history’ in this period. Prof. Patrick Walsh is joining us from the United States to talk about his role in the discovery of nerve-sparing radical prostatectomy. His work in the 1970s and 1980s caused a shift in the thinking around radical prostatectomy. Another big name from the history of our field is Prof. Tony Mundy, leading expert in urethral strictures who will undoubtedly share a great historic overview of his expertise.
“The programme is organised around a variety of important subspecialties and feature some provocative and conversationstarting sessions. It will be chaired by members of the EAU History Office and also the EAU Board. The programme will be unique in that we will have four of the EAU’s (former) Secretary Generals taking part in some capacity. We will have speakers from all across the world, young and old.
Inspiration and perspective
“We always hope that participants take away a new consideration for their work. History can be humbling! We also think the programme and speakers will offer inspiration that will help our colleagues in their daily practice from the next day on. That’s what sets us apart from other professions: medicine requires an inspiration: empathy, individual contact, psychological aspects at every level, and the focus on the patient. That’s what a congress like ours can offer.”
The International Congress on the History of Urology is free to attend for all EAU23 delegates and requires no separate registration. It replaces the History Office’s usual “Special Session” but its Poster Session will be held as usual.
10 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
50mgs once daily
BETMIGA 25 mg prolonged-release tablets & BETMIGA 50 mg prolonged-release tablets.
Prescribing Information: BETMIGA™ (mirabegron)
For full prescribing information, refer to the Summary of Product Characteristics (SPC). Name: BETMIGA 25 mg prolonged-release tablets & BETMIGA 50 mg prolonged-release tablets. Presentation: Prolongedrelease tablets containing 25 mg or 50 mg mirabegron. Indication: Symptomatic treatment of urgency, increased micturition frequency and/or urgency incontinence as may occur in adult patients with overactive bladder (OAB) syndrome. Posology and administration: The recommended dose is 50 mg orally once daily in adults (including elderly patients). Mirabegron should not be used in paediatrics for OAB. A reduced dose of 25 mg once daily is recommended for special populations (please see the full SPC for information on special populations). The tablet should be taken with liquids, swallowed whole and is not to be chewed, divided, or crushed. The tablet may be taken with or without food. Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SPC. Severe uncontrolled hypertension defined as systolic blood pressure ≥ 180 mm Hg and/or diastolic blood pressure ≥ 110 mm Hg. Warnings and Precautions: Renal impairment: BETMIGA has not been studied in patients with end stage renal disease (eGFR < 15 ml/min/1.73 m2 or patients requiring haemodialysis) and, therefore, it is not recommended for use in this patient population. Data are limited in patients with severe renal impairment (eGFR 15 to 29 ml/min/1.73 m2); based on a pharmacokinetic study (see section 5.2 of the SPC) a dose of 25 mg once daily is recommended in this population. This medicinal product is not recommended for use in patients with severe renal impairment (eGFR 15 to 29 ml/min/1.73 m2) concomitantly receiving strong CYP3A inhibitors (see section 4.5 of the SPC).
Hepatic impairment: BETMIGA has not been studied in patients with severe hepatic impairment (ChildPugh Class C) and, therefore, it is not recommended for use in this patient population. This medicinal product is not recommended for use in patients with moderate hepatic impairment (Child-Pugh B) concomitantly receiving strong CYP3A inhibitors (see section 4.5 of the SPC). Hypertension: Mirabegron can increase blood pressure. Blood pressure should be measured at baseline and periodically during treatment with mirabegron, especially in hypertensive patients. Data are limited in patients with stage 2 hypertension (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 100 mm Hg). Patients with congenital or acquired QT prolongation: BETMIGA, at therapeutic doses, has not demonstrated clinically relevant QT prolongation in clinical studies (see section 5.1 of the SPC). However, since patients with a known history of QT prolongation or patients who are taking medicinal products known to prolong the QT interval were not included in these studies, the effects of mirabegron in these patients is unknown.
Caution should be exercised when administering mirabegron in these patients. Patients with bladder outlet obstruction and patients taking antimuscarinics medicinal products for OAB: Urinary retention in patients with bladder outlet obstruction (BOO) and in patients taking antimuscarinic medicinal products for the treatment of OAB has been reported in postmarketing experience in patients taking mirabegron. A
controlled clinical safety study in patients with BOO did not demonstrate increased urinary retention in patients treated with BETMIGA; however, BETMIGA should be administered with caution to patients with clinically significant BOO. BETMIGA should also be administered with caution to patients taking antimuscarinic medicinal products for the treatment of OAB. Interactions: Caution is advised if mirabegron is co-administered with medicinal products with a narrow therapeutic index and significantly metabolised by CYP2D6. Caution is also advised if mirabegron is co-administered with CYP2D6 substrates that are individually dose titrated. In patients with mild to moderate renal impairment or mild hepatic impairment, concomitantly receiving strong CYP3A inhibitors, the recommended dose is 25 mg once daily. For patients who are initiating a combination of mirabegron and digoxin (P-gp substrate), the lowest dose for digoxin should be prescribed initially (see the SPC for full prescribing information). The potential for inhibition of P-gp by mirabegron should be considered when BETMIGA is combined with sensitive P-gp substrates. Increases in mirabegron exposure due to drug-drug interactions may be associated with increases in pulse rate. Pregnancy and lactation: BETMIGA is not recommended in women of childbearing potential not using contraception. This medicinal product is not recommended during pregnancy. BETMIGA should not be administered during breast-feeding. Undesirable effects: Summary of the safety profile: The safety of BETMIGA was evaluated in 8433 adult patients with OAB, of which 5648 received at least one dose of mirabegron in the phase 2/3 clinical program, and 622 patients received BETMIGA for at least 1 year (365 days). In the three 12-week phase 3 double blind, placebo controlled studies, 88% of the patients completed treatment with this medicinal product, and 4% of the patients discontinued due to adverse events. Most adverse reactions were mild to moderate in severity. The most common adverse reactions reported for adult patients treated with BETMIGA 50 mg during the three 12-week phase 3 double blind, placebo controlled studies are tachycardia and urinary tract infections. The frequency of tachycardia was 1.2% in patients receiving BETMIGA 50 mg. Tachycardia led to discontinuation in 0.1% patients receiving BETMIGA 50 mg. The frequency of urinary tract infections was 2.9% in patients receiving BETMIGA 50 mg. Urinary tract infections led to discontinuation in none of the patients receiving BETMIGA 50 mg. Serious adverse reactions included atrial fibrillation (0.2%). Adverse reactions observed during the 1-year (long term) active controlled (muscarinic antagonist) study were similar in type and severity to those observed in the three 12-week phase 3 double blind, placebo controlled studies. Adverse reactions: The following list reflects the adverse reactions observed with mirabegron in adults with OAB in the three 12-week phase 3 double blind, placebo controlled studies. The frequency of adverse reactions is defined as follows: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000) and not known (cannot be established from the available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. The adverse events are grouped by MedDRA system organ class. Infections and infestations:
Common: Urinary tract infection, Uncommon: Vaginal infection, Cystitis. Psychiatric disorders: Not known (cannot be estimated from the available data): Insomnia*, Confusional state*. Nervous system disorders:
Common: Headache*, Dizziness*. Eye disorders: Rare: Eyelid oedema. Cardiac disorders: Common: Tachycardia, Uncommon: Palpitation, Atrial fibrillation. Vascular disorders: Very rare: Hypertensive crisis*. Gastrointestinal disorders: Common: Nausea*, Constipation*, Diarrhoea*, Uncommon: Dyspepsia, Gastritis, Rare: Lip oedema. Skin and subcutaneous tissue disorders: Uncommon: Urticaria, Rash, Rash macular, Rash papular, Pruritus, Rare: Leukocytoclastic vasculitis, Purpura, Angioedema*. Musculoskeletal and connective tissue disorders: Uncommon: Joint swelling. Renal and urinary disorders: Rare: Urinary retention*. Reproductive system and breast disorders: Uncommon: Vulvovaginal pruritus. Investigations: Uncommon: Blood pressure increased, GGT increased, AST increased, ALT increased. * signifies adverse reactions observed during post-marketing experience. Prescribers should consult the SPC in relation to other adverse reactions. Overdose: Treatment for overdose should be symptomatic and supportive. In the event of overdose, pulse rate, blood pressure, and ECG monitoring is recommended. Basic NHS Cost: Great Britain (GB)/Northern Ireland(NI): BETMIGA 50 mg x 30 = £29, BETMIGA 25 mg x 30 tablets = £29. Ireland (IE): POA. Legal classification: POM. Marketing Authorisation number(s): (GB): PLGB 00166/0415-0416. NI/IE: EU/1/12/809/001-006, EU/1/12/809/008-013, EU/1/12/809/015018. Marketing Authorisation Holder: GB: Astellas Pharma Ltd., 300 Dashwood Lang Road, Bourne Business Park, Addlestone, United Kingdom, KT15 2NX. NI/IE: Astellas Pharma Europe B.V. Sylviusweg 62, 2333 BE Leiden, The Netherlands. Date of Preparation of Prescribing information: January 2023. Job bag number: MAT-IE-BET-2023-00001. Further information available from: GB/NI: Astellas Pharma Ltd, Medical Information: 0800 783 5018. IE: Astellas Pharma Co. Ltd., Tel.: +353 1 467 1555. For full prescribing information, please see the Summary of Product Characteristics, which may be found at: GB: www.medicines.org.uk; NI: https://www.emcmedicines.com/en-GB/northernireland/; IE: www.medicines.ie.
United Kingdom (GB/NI)

Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Astellas Pharma Ltd. on 0800 783 5018. Ireland
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance, Website: www.hpra.ie or Astellas Pharma Co. Ltd. Tel: +353 1 467 1555, E-mail: irishdrugsafety@astellas.com.

Date of preparation: January 2023 References: 1. Freeman R, et al. Current Medical Research and Opinion 2017. https://doi.org/10.1080/03007995.2017.1419170. MAT-IE-BET-2023-00003
His 14th walk in the park since the day he started BETMIGA1
First ‘Incisionless’ surgery for Achalasia
Galway University Hospitals (GUH) has become the first hospital in Ireland to introduce an ‘incisionless’ minimally invasive surgery to help correct blockages of the oesophagus.
Achalasia is a rare condition which causes a blockage in the muscles of the lower oesophagus causing dysphagia or difficulty in swallowing, including in pronounced cases, liquids.
The procedure was carried out by Mr Paul Carroll, Consultant Oesophagogastric and General Surgeon, specialising in minimally invasive surgery and endoscopic
surgery for oesophago-gastric cancer and benign disease.
Traditionally, treatment of Achalasia involves procedures to repeatedly dilate/open the oesophagus or, alternatively, requires laparoscopic surgery. The traditional surgery divides the muscles of the lower oesophagus through several small incisions in the abdomen.
However, surgeons in GUH have now undertaken an incisionless procedure using Peroral Endoscopic Myotomy (POEM). POEM uses an endoscope, a narrow flexible tube with a
camera, to breach the lining of the oesophagus. The endoscope is inserted through the mouth and can be positioned into the space between the oesophageal lining and the muscles, allowing it to divide these muscles internally. The breach is then closed with special clips.
One of the significant benefits of this procedure is the ability to treat a subtype of achalasia with chest pain. This requires a longer division of the muscles along the length of the oesophagus, which is traditionally challenging to treat with standard surgery. POEM can also be used as a
Cross Border Network for Rare Diseases
University College Dublin (UCD), Queen’s University Belfast, and 33 partners launched the All-Ireland Rare Disease Interdisciplinary Research Network (RAiN).
Individual rare diseases may be rare but collectively impact more than 400,000 people across the island of Ireland. There are as many people living with rare diseases across Ireland as live with diabetes, yet rare diseases receive much less recognition and support.
Many people living with a rare disease experience chronic debilitating illness, with more than 30 percent of children with rare diseases dying before their fifth birthday. RAiN will help evaluate the quality of life and management of people living with
rare diseases on the island of Ireland and internationally.
Co-lead of RAiN for UCD, Associate Professor Suja Somanadhan said, “This all-Island interdisciplinary rare disease research network will serve as a hub to support collaboration and connection between members across the Island, which includes researchers, early career investigators, industrial partners and Public and Patient Involvement expert groups.”
Co-lead of RAiN for QUB, Professor Amy Jayne McKnight added, “I’m delighted that so many individuals in our local rare disease community have come together to establish this network and look forward to working in partnership with those working, often on a shoestring budget, to improve the lives of people living with rare diseases.”
Officially launching the network, Vice President for Equality, Diversity, and Inclusion at UCD Professor Colin Scott said: “People living with rare disorders face significant health and social inequalities. The All-Island interdisciplinary rare disease research network helps ‘level up’ the rare disease community across Ireland. I am delighted to launch this unprecedented collaborative approach led by UCD and QUB with government, local authority, charity, patient and caregiver partners across Ireland.”
RAiN is funded by the Department of the Taoiseach from the Shared Island strand of Irish Research Council’s ‘New Foundations’ awards. The network builds on established
rescue or second line intervention where other treatments have failed. Patients are discharged the following morning without pain and with resolution of symptoms. The introduction of this procedure has added to the expanding Minimally Invasive Upper GI programme in Galway and the Saolta University Health Care Group, which also provides endoscopic surgical interventions for early oesophageal and gastric cancers. GUH is one of only two centres in Ireland with this capability.
Mr Paul Carroll, Consultant Oesophagogastric and General Surgeon at Galway University Hospitals thanked his team for their support in bringing the procedure to Irish patients. “I am personally delighted that I have been able to introduce this procedure into Ireland for treatment of this disease process. It would not have been possible without the support and training I received whilst on fellowship in the University of Toronto and finally without the unwavering support of the late Marie Farragher, Clinical Nurse Manager in theatre and her team of nurses for pushing boundaries with me,” he added.
north-south research partnerships between UCD and Queen’s*. By fostering collaboration among researchers, practitioners, policymakers, patients and families working on rare diseases, RAiN will advance health service developments, leverage funding, and facilitate internationally excellent translational rare disease research.
Monthly seminars will discuss research that addresses the significant unmet health, social, psychological, and educational needs of children, young people affected by rare disease and their families. Nurtured research partnerships will inspire and empower early career researchers as emerging leaders for interdisciplinary rare disease research.
12 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
News
From Left, Máire Cooper, Theatre Staff Nurse; Noreen Keelan, Senior Anaesthetic Staff Nurse; Paul Carroll, Consultant Oesophagogastric and General Surgeon; Ashitosh Waidande, CNM 2, GI Theatre; and Deirdre Hoade, Senior Staff Nurse, Theatre
Liver Cancer
Primary liver cancers with a focus on Hepatocellular Carcinoma
 Written by
Written by
Introduction
Hepatocellular cancer (HCC) and cholangiocarcinoma represent the most common forms of liver cancer with the former accounting for over 80% of cases1 . Cholangiocarcinomas arise from the bile ducts and will not be discussed in this article however should be highlighted as a rising cause of liver cancer. Indeed the numbers of new cases and deaths from liver cancer are expected to increase by more than 55% by 20401. This highlights the urgent need for national strategies and international collaboration to deal with this impending disease burden. The management of HCC has seen remarkable changes in the past 5 years. Up until 2017 sorafenib was the only drug available to patients with advanced HCC. We now have two new standard immune checkpoint inhibitor (ICI) combinations in the first line setting, demonstrating superiority over sorafenib while lenvatinib represents a further oral therapy. A number of additional oral tyrosine kinase inhibitors have shown benefit in the second line setting. It is now inevitable that these agents will be integrated earlier in the management of HCC, with trials in the adjuvant and neoadjuvant setting ongoing. How countries will cope with the cost of these new therapies has not been addressed and highlights continued global disparities in cancer care.
Epidemiology
Hepatocellular carcinoma is the 3rd most common cause of cancer related mortality world-wide with an estimated 5 year survival rate of 18%. The majority of HCC arise on a background of chronic liver disease with 80-90% of patients having underlying liver cirrhosis. HCC most commonly occurs in the 6th decade and occurs more frequently in males. The spectrum of underlying liver disease varies globally with a predominance of hepatitis B and C in Asia while alcohol and non-alcoholic fatty liver disease (NAFLD) dominate in Western Societies. Less common causes of HCC include haemochromatosis, alpha-1antitrypsin deficiency and drug induced liver injury.
Notably there has been a rise in the incidence of HCC in Ireland and Europe due to obesity and the prevalence of fatty liver
disease while levels of alcohol consumption remain high 2. Europe has the highest levels of alcohol intake in the world3, while NAFLD and nonalcoholic steatohepatitis (NASH) are predicted to become the leading cause of end stage liver disease 4. Excessive alcohol use, obesity and diabetes are risk factors for HCC that require urgent public health strategies.
Prevention and screening

Globally, the advent of hepatitis B vaccination, has together with the development of antivirals agents for hepatitis C resulted in a decline in viral associated HCC highlighting effective prevention strategies. NAFLD and progression to NASH is inextricably linked to obesity and diabetes and is largely due to modern Western diets enriched in excess sugars and fats together with a more sedentary lifestyle. Smoking may contribute to HCC risk particularly in the presence of other risk factors while aspirin and coffee have been found to be protective5 6. The risks of alcohol and liver cirrhosis are well known , however tackling the issues of obesity and excessive alcohol consumption will require significant cultural changes and government intervention. The EASL-Lancet liver commission have provided recommendations on achieving healthier livers for all Europeans underscoring the need for multi-disciplinary input and national policies 7 The prevention of chronic liver disease will represent one of the most effective ways in reducing mortality from HCC.
Earlier detection of HCC is further critical, particularly as management strategies evolve. International guidelines and liver societies recommend surveillance of high risk populations using ultrasound with or without alpha fetoprotein (AFP)8. Its noteworthy that not everyone with liver disease or indeed cirrhosis will develop HCC. Nevertheless HCC when detected early is highly curable. Ultrasound screening in the presence of obesity can be challenging and the role of AFP in screening has been controversial. Novel strategies such as a liquid biopsy or a blood test to evaluate cell free circulating tumour DNA will likely become part of screening protocols in the future.9
Making a Diagnosis and staging HCC
HCC is unusual compared to other cancers in that a diagnosis can be made without a biopsy confirming histology. Liver imaging with a CT or MRI in the setting of chronic liver disease reveals characteristic features of tumour enhancement which is specific for HCC. However not all HCC will have such obvious characteristics on CT or MRI and as such the liver imaging reporting and data system (LI-RADS, LR) was developed for radiology reporting without need for a biopsy. This is an important point so that registries adequately capture the burden of HCC that exists if pathology has not been obtained. The role of biopsy of early lesions has been controversial because of concerns with regard to seeding. Biopsy however is increasingly being employed especially as systemic therapies are being used before surgery or transplant. Furthermore it has been demonstrated that imaging alone wrongly calls 9% of cases labelled as HCC in the advanced setting. This would mean inappropriate treatments in those patients10
The staging of HCC is integral to prognosis and management and the Barcelona Clinic Liver Criteria (BCLC) staging system is the most widely used. While the tumour burden and presence of portal vein invasion and or extrahepatic disease is critical to staging, equally important is the assessment of liver function and the consideration of patient
performance status11. BCLC classifies HCC into very early stage (BCLC 0), early stage (BCLC A), intermediate stage (BCLC B), advanced stage (BCLC C) and terminal stage (BCLC D).
Curative options BCLC 0, A and B
BCLC 0 and A HCC should be considered for curative options including surgical resection, transplant and ablation. Resection is ideal for single lesions where liver function is preserved. In patients where there is multifocal disease or where there is decompensated cirrhosis, liver transplant is a potential option. Surgical considerations in the presence of cirrhosis are complex and all eligible patients should be discussed at a multidisciplinary team meeting at a high volume centre. Liver transplantation in eligible patients has a 5 year survival rate of 75-80%12. Liver transplantation has the advantage of treating underlying liver cirrhosis while providing the widest possible surgical margin. Most commonly the Milan Criteria13 are used to select patients considered eligible for liver transplant. These criteria (one lesion ≥ 2 cm and ≤ 5 cm, or up to 3 lesions, each ≥ 1 cm and ≤ 3 cm, with no evidence of vascular invasion or extra-hepatic metastases) are today in many centres considered too restrictive and downstaging protocols will seek to employ locoregional or systemic therapies to render patients beyond Milan criteria eligible. This accepts that tumour biology is superior to size
13 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
Dr. Ruth Hutch, Oncology SHO, Trinity St. James's Cancer Institute
Prof. Grainne O'Kane, Medical Oncologist, Trinity St James's Cancer Institute
Liver Cancer
and number when considering patients for transplant and a number of extended criteria exist across the world14. In addition to surgical options, thermal ablative strategies are usually employed for tumours < 3cm15 or in larger lesions unsuitable to other curative options. To date adjuvant drug trials have been negative however a number of adjuvant studies evaluating immune checkpoint inhibitor combinations are due to report this year.
Intermediate stage disease (BCLC B) and local approaches
One of the biggest changes to the BCLC 2022 update was in intermediate stage disease. It is now recognized that some BCLC B HCC are amenable to curative therapies in extended transplant criteria and there are also some BCLC B HCC with a larger tumour volume who should be considered for a systemic first approach.
Historically this cohort of patients were predominantly treated with trans-arterial chemoembolization (TACE). HCC are hypervascular tumours deriving most blood supply from the hepatic artery. After accessing the bloody supply to the HCC, TACE should be completed in a selective manner. This can be performed conventionally (cTACE) where a drug carried by lipiodol is give in an arterial injection, followed by arterial embolization. The second option is using drug eluding beads (DEB-TACE). Both are equally effective in controlling disease however DEB-TACE is thought associated with less toxic side
effects including abdominal pain15. Although DEB TACE is more expensive, patients can be managed with oral analgesia and have a shorter inpatient stay16 Both options can be delivered on an outpatient based protocol, where resources are in place which can save substantially on costs17
TACE has been shown to improve survival over symptom control and in early studies 63% of patients where alive at 2 years when treated with TACE18. TACE can also be used as a bridge to transplant providing tumour control while patients are on a transplant waiting list. TACE has also been shown to effectively downstage patients to within transplant criteria. Other locoregional therapies that can be used in these settings include stereotactic body radiation (SBRT) together with transarterial radioembolization (TARE). The role of SBRT is gaining particular attention, especially in the setting of macrovascular invasion. Locoregional therapy decisions should be made at an MDT and all should be considered in the treatment paradigm.
In 2023, in the era of rapidly evolving drug strategies the sequencing of local therapies and systemic treatments or how these can be combined becomes an important question19. What is crucial is that patients receiving locoregional treatments are discussed regularly to ensure that patients move treatments at the time of refractory disease and that liver function is preserved in order to afford patients the opportunities
to receive systemic treatments including immunotherapy combinations. Systemic therapies are being increasingly utilized in intermediate stage disease as a first option, ensuring access to drug with the possibility of employing locoregional therapies at a later stage. Many trials are currently ongoing in this space.
Advanced Disease (BCLC B/C)
Immune checkpoints are molecules expressed on a number of cells including immune cells and tumour cells that prevent an overactive immune response in organs such as the liver thus maintaining self-tolerance. In HCC, immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1)/programmed deathligand 1 (PD-L1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) have demonstrated activity particularly when combinations strategies are used and have resulted in new standards of care.
There are now four options (received EMA approval) for patients in the first line advanced setting for HCC: atezolizumab/ bevacizumab (preferred choice); durvalumab/tremelimumab (STRIDE regimen), lenvatinib and sorafenib.
Sorafenib, a multi-tyrosine kinase inhibitor was the only systemic oral therapy available to patients with advanced disease and preserved liver function until 2017 based on the results of the SHARP trial. There has now been a rapid growth in the armamentarium of agents
exhibiting activity in HCC. In 2018 the REFLECT randomized phase III trial demonstrated the noninferiority of Lenvatinib ( an oral tyrosine kinase inhibitor with broader receptor target) compared with sorafenib. The median overall survival (OS) was 13.6 months for patients treated with Lenvatinib compared with 12.3 months for patients treated with sorafenib (HR: 0.92; 95% CI: 0.79 to 1.06)20. Notably the progression free survival was almost double in the Lenvatinib arm (7.4 months vs. 3.7 months).
The landmark IMbrave150 trial however has changed the landscape of drug treatment in HCC. In this randomized phase III trial patients received either sorafenib or atezolizumab/ bevacizumab. Atezolizumab is a PD-L1 inhibitor and bevacizumab a VEGF antibody. The trial was interrupted at first analysis with the combination of atezolizumab/ bevacizumab demonstrating a superior survival of 19.2 months versus 13.2 months in the sorafenib arm21. Importantly the combination also showed benefits in quality of life with a median time to deterioration of 11.2 months with atezolizumab/bevacizumab compared to 3.6 months with sorafenib22. Because of the risks of bleeding with bevacizumab, especially in the population of patients with advanced HCC, all patients entering the study were required to have had an OGD which is still a requirement before prescribing the drugs today. The durability of immune checkpoint
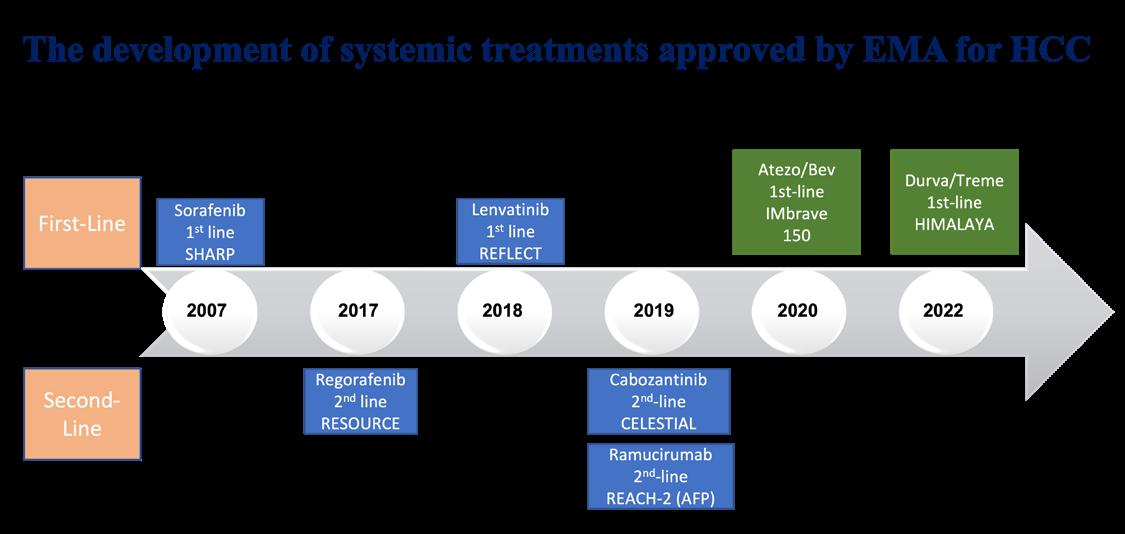
14 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
inhibitors is of particular interest in those achieving a response. In this regard the HIMALAYA trial demonstrated superiority over single agent tremelimumab (CTLA4 inhibitor)/durvalumab (PD-L1 inhibitor) – known as the STRIDE regimen versus sorafenib23 At three years with this regimen 30.7% of patients were alive compared to 20.7% with sorafenib. The STRIDE regimen provides an ICI combination option in patients with high risk varices not suitable for bevacizumab, while lenvatinib and sorafenib (less commonly) are alternatives. Studies in the second line setting were performed when sorafenib was the first choice of agent in first-line. Nevertheless the RESOURCE trial, CELESTIAL-2 and REACH-2 have demonstrated the efficacy of regorafenib, cabozantinib and ramucirumab post sorafenib in HCC expanding therapeutics available to patients.
Conclusions and future strategies
HCC is a rapidly rising cause of cancer related mortality in Ireland and Europe linked in particular to obesity and alcohol consumption. It is imperative that all patients with HCC and liver tumours are discussed at a multidisciplinary tumour board with radiologists, interventional radiologists, hepatologists, medical oncologists, HPB surgeons, radiation oncologists, nurse specialists and pathologists present. Palliative care physicians are also a crucial part of the team. This ensures patients access all possible treatment options,
particularly curative approaches in a field that’s rapidly evolving. It is now inevitable that immunotherapy combinations will be utilized earlier in HCC management including in the neo/adjuvant setting. Clinical trials access in Ireland needs to increase to allow for patients to avail of novel combinations given that many drugs available globally are not accessible to patients in Ireland as of yet. Biomarkers to select patients for the right treatment options are urgently needed and much research is ongoing in this area. Screening strategies are likely to evolve to include liquid biopsies, but novel approaches including new drugs and new diagnostics can be costly. It is therefore crucial that the government work with members of the multidisciplinary team, patients and key stakeholders to develop national strategies to tackle obesity, the metabolic syndrome and excess alcohol consumption while providing the infrastructure needed for continued research.
References
1. Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77(6):1598606. doi: 10.1016/j.jhep.2022.08.021 [published Online First: 2022/10/09]
2. Gaynor C, Iqbal M, Comber H, et al. Improving prognosis for patients with hepatocellular carcinoma in Ireland 1994-2008. Eur J Gastroenterol Hepatol 2017;29(2):221-24. doi: 10.1097/ MEG.0000000000000756 [published Online First: 2016/11/11]
3. WHO. Global status report on alcohol and health 2018. Sept 27 hwwipiiaF, 2023).
4. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018;69(4):896-904. doi: 10.1016/j. jhep.2018.05.036 [published Online First: 2018/06/11]
5. Lee YC, Cohet C, Yang YC, et al. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol 2009;38(6):1497-511. doi: 10.1093/ ije/dyp280 [published Online First: 2009/09/02]
6. Bravi F, Bosetti C, Tavani A, et al. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol 2013;11(11):1413-21 e1. doi: 10.1016/j.cgh.2013.04.039 [published Online First: 2013/05/11]
7. Karlsen TH, Sheron N, Zelber-Sagi S, et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022;399(10319):61-116. doi: 10.1016/S0140-6736(21)01701-3 [published Online First: 2021/12/06]
8. Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet 2022;400(10360):1345-62. doi: 10.1016/S0140-6736(22)01200-4 [published Online First: 2022/09/10]
9. Kisiel JB, Dukek BA, R VSRK, et al. Hepatocellular Carcinoma Detection by Plasma Methylated DNA: Discovery, Phase I Pilot, and Phase II Clinical Validation. Hepatology 2019;69(3):1180-92. doi: 10.1002/ hep.30244 [published Online First: 2018/09/01]
10. Childs A, Zakeri N, Ma YT, et al. Biopsy for advanced hepatocellular carcinoma: results of a multicentre UK audit. British journal of cancer 2021;125(10):1350-55. doi: 10.1038/ s41416-021-01535-2 [published Online First: 2021/09/17]
11. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76(3):681-93. doi: 10.1016/j.jhep.2021.11.018 [published Online First: 2021/11/22]
12. Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 2017;14(4):203-17. doi: 10.1038/ nrgastro.2016.193 [published Online First: 2017/01/06]
13. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334(11):693-9. doi: 10.1056/ NEJM199603143341104 [published Online First: 1996/03/14]
14. Mazzaferro V, Citterio D, Bhoori S, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol 2020;21(7):947-
56. doi: 10.1016/S14702045(20)30224-2 [published Online First: 2020/07/03]
15. Takayama T, Hasegawa K, Izumi N, et al. Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial). Liver Cancer 2022;11(3):20918. doi: 10.1159/000521665
[published Online First: 2022/08/12]
16. Kang YJ, Lee BC, Kim JK, et al. Conventional Versus Small Doxorubicin-eluting Bead Transcatheter Arterial Chemoembolization for Treating Barcelona Clinic Liver Cancer Stage 0/A Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2020;43(1):55-64. doi: 10.1007/ s00270-019-02349-9 [published Online First: 2019/10/28]
17. Fritsche MR, Watchmaker JM, Lipnik AJ, et al. Outpatient Transarterial Chemoembolization of Hepatocellular Carcinoma: Review of a Same-Day Discharge Strategy. J Vasc Interv Radiol 2018;29(4):55055. doi: 10.1016/j.jvir.2017.11.018
[published Online First: 2018/02/27]
18. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359(9319):17349. doi: 10.1016/S01406736(02)08649-X [published Online First: 2002/06/07]
19. Brar G, Kesselman A, Malhotra A, et al. Redefining IntermediateStage HCC Treatment in the Era of Immune Therapies. JCO Oncol Pract 2022;18(1):35-41. doi: 10.1200/OP.21.00227 [published Online First: 2021/07/14]
20. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391(10126):1163-73. doi: 10.1016/S0140-6736(18)30207-1
21. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382(20):1894-905. doi: 10.1056/NEJMoa1915745 [published Online First: 2020/05/14]
22. Galle PR, Finn RS, Qin S, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an openlabel, randomised, phase 3 trial. Lancet Oncol 2021;22(7):991-1001. doi: 10.1016/S1470-2045(21)001510 [published Online First: 2021/05/31]
23. Abou-Alfa GK LG, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De Toni EN, Rimassa L. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence. 2022 Jul 26;1(8):EVIDoa2100070.
15 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
ON THE RIGHT RIGHT AWAY1-3
STELARA® offers:
• Lasting steroid-free remission4

• Long term treatment persistence
• Reassuring safety and efficacy profile across Immunology (Dermatology, Rheumatology and Gastroenterology)
STELARA ® 45 mg and 90 mg solution for injection and 130 mg concentrate for solution for infusion PRESCRIBING INFORMATION. ACTIVE INGREDIENT(S): Ustekinumab. Please refer to Summary of Product Characteristics (SmPC) before prescribing. INDICATION(S): Plaque psoriasis adults: Treatment of moderate to severe plaque psoriasis in adults who failed to respond to, or who have a contraindication to, or are intolerant to other systemic therapies including ciclosporin, methotrexate or PUVA. Plaque psoriasis paediatrics: Moderate to severe plaque psoriasis in children and adolescent patients from 6 years of age, who are inadequately controlled by, or are intolerant to, other systemic therapies or phototherapies. Psoriatic arthritis: Alone or in combination with methotrexate for treatment of active psoriatic arthritis in adult patients when response to previous non-biological disease-modifying anti-rheumatic drug (DMARD) therapy has been inadequate. Crohn’s disease: Treatment of adult patients with moderately to severely active Crohn’s disease who had inadequate response with/ lost response to/were intolerant to either conventional therapy or TNFα antagonist or have contraindications to such therapies. Ulcerative colitis: Treatment of adult patients with moderately to severely active ulcerative colitis who had an inadequate response with/lost response to/were intolerant to either conventional therapy or a biologic or have contraindications to such therapies. DOSAGE & ADMINISTRATION: Adults: Under guidance and supervision of a physician experienced in diagnosis and treatment of psoriasis/psoriatic arthritis/Crohn’s disease/ulcerative colitis. Psoriasis or psoriatic arthritis. Subcutaneous (s.c.) injection. Avoid areas with psoriasis. Self-injecting patients or caregivers ensure appropriate training. Physicians are required to follow-up and monitor patients. Plaque psoriasis, adults & elderly: Patients up to and including 100kg, 45 mg at week 0 followed by a 45 mg dose at week 4, then every 12 weeks. Patients greater than 100 kg, 90 mg at week 0 followed by a 90 mg dose at week 4, then every 12 weeks (45 mg was less effective in these patients). Plaque psoriasis paediatrics (6 years and older): Patients under 60 kg, 0.75 mg/kg at week 0, followed by 0.75 mg/kg at week 4 then every 12 weeks thereafter. Patients 60 - 100kg, 45 mg at week 0 followed by 45 mg at week 4, then every 12 weeks. Patients greater than 100 kg, 90mg at week 0, followed by 90mg at week 4, then every 12 weeks. Psoriatic arthritis, adults & elderly: 45 mg at week 0 followed by a 45 mg dose at week 4, then every 12 weeks. Alternatively, 90 mg may be used in patients with a body weight greater than 100 kg. Consider discontinuation if no response after 28 weeks. Crohn’s disease and ulcerative colitis: Initial single intravenous infusion dose based on body weight (260 mg or 390 mg or 520 mg) diluted in sodium chloride solution and given over at least one hour.
At week 8 after intravenous dose, 90 mg s.c. dose is given; followed by every 12 weeks (or 8 weeks based on clinical judgement). Consider discontinuation if no response 16 weeks after the IV induction dose or 16 weeks after switching to the 8-weekly maintenance dose. Immunomodulators and/or corticosteroids may be continued but consider reducing/discontinuing corticosteroids if responding to Stelara. In Crohn’s disease, if therapy interrupted, resume s.c. every 8 weeks if safe/effective. Children: under 6 years - Not recommended for psoriasis. under 18 years - Not recommended for psoriatic arthritis, Crohn’s disease and ulcerative colitis. Renal & Hepatic impairment: Not studied. CONTRAINDICATIONS: Hypersensitivity to product; clinically important, active infection. SPECIAL
WARNINGS & PRECAUTIONS: Infections: Potential to increase risk of infections and reactivate latent infections. Opportunistic infections have been reported in patients treated with ustekinumab. Caution in patients with a chronic infection or history of recurrent infection, particularly TB. Patients should be evaluated for tuberculosis prior to initiation of STELARA. Consider anti-tuberculosis therapy prior to initiation of STELARA in patients with history of latent or active tuberculosis. Patients should seek medical advice if signs or symptoms suggestive of an infection occur. If a serious infection develops, closely monitor and STELARA should not be administered until infection resolves. Malignancies: Potential to increase risk of malignancy. No studies in patients with history of malignancy or in patients who develop malignancy while receiving STELARA. Monitor all patients, in particular those older than 60, patients with a medical history of prolonged immunosuppressant therapy or those with a history of PUVA treatment for nonmelanoma skin cancer. Concomitant immunosuppressive therapy: Caution, including when changing immunosuppressive biologic agents. Hypersensitivity reactions: Serious hypersensitivity reactions (anaphylaxis and angioedema) reported, in some cases several days after treatment. If these occur appropriate therapy should be instituted and STELARA discontinued. Latex sensitivity: Needle cover contains natural rubber (latex), may cause allergic reactions. Immunotherapy: Not known whether STELARA affects allergy immunotherapy. Serious skin conditions: Exfoliative dermatitis reported following treatment. Discontinue STELARA if drug reaction is suspected. Infusion-related anaphylactic reactions to the infusion have been reported. If a serious or life-threatening reaction is observed, appropriate therapy should be instituted and ustekinumab should be discontinued. Vaccines: Administration of live vaccines to infants is not recommended for six months following birth. Lupus-related conditions: Cases of lupus-related conditions have been reported in patients treated with ustekinumab. SIDE EFFECTS: Common: upper respiratory tract infection,
nasopharyngitis, sinusitis, dizziness, headache, oropharyngeal pain, diarrhoea, nausea, vomiting, pruritus, back pain, myalgia, arthralgia, fatigue, injection site erythema, injection site pain. Other side effects: cellulitis, serious hypersensitivity reactions (including anaphylaxis, angioedema), skin exfoliation, exfoliative dermatitis, lower respiratory tract infection. Very rare: bullous pemphigoid, cutaneous lupus erythematosus, lupus-like syndrome. Studies show adverse events reported in children 12 years and over with plaque psoriasis were similar to those seen in previous studies in adults with plaque psoriasis. Refer to SmPC for other side effects. LEGAL CATEGORY: Prescription Only Medicine (POM). PRESENTATIONS, PACK SIZES, MARKETING AUTHORISATION
NUMBER(S): 45 mg, 1 x vial, EU/1/08/494/001; 45 mg, 1 x 0.5 ml pre-filled syringe, EU/1/08/494/003; 90 mg, 1 x 1.0 ml pre-filled syringe, EU/1/08/494/004; 130 mg, 1 x vial, EU/1/08/494/005. MARKETING AUTHORISATION HOLDER: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium. FURTHER INFORMATION IS AVAILABLE FROM: Janssen Sciences Ireland UC, Barnahely, Ringaskiddy, IRL - Co. Cork, P43 FA46. Prescribing information last revised: December 2022.
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse events via: HPRA Pharmacovigilance Website: www.hpra.ie. Adverse events should also be reported to Janssen Sciences Ireland UC on 1800 709 122 or at dsafety@its.jnj.com.
© Janssen-Cilag International NV 2022
REFERENCES: 1. Agrawal M, et al. Gastroenterology. 2021;161(1):47-65. 2. Berg DR, et al. Inflamm Bowel Dis. 2019; 25(12): 1896-1905. 3. Loy L, et al. Expert Rev Gastroenterol Hepatol. 2019; 13(6): 547-555. 4. Sandborn WJ et al. UEGW Virtual 2020; 11–13 October 2020; Abstract OP110. 5. Koy Y, et al. Ail Phar and Ther 2021;54(3):292-301
6. Stelara® Summary of Product Characteristics, Available from www.medicinies.ie 7. Liefferinckx C, et al. J Crohns Colitis. 2019;13:1401-9.
8. Biemans VBC, et al. J Crohns Colitis. 2019;14:33-45. 9. af Björkesten CG, et al. Poster presented at ECCO 2020; P499. 10. Chaparro M, et al. Poster presented at ECCO 2020; P629.
CP-350301 l Date of preparation: January 2023


What is Ovarian Cancer?
Ovarian cancer is an umbrella term for a multitude of different types of cancer that affect the ovaries, fallopian tubes, and the primary peritoneal cavity. The peritoneal cavity, also known as the peritoneum, is a thin membrane that lines the abdominal cavity and covers many of the organs inside that cavity.
There are different types of ovarian cancer. The type of ovarian cancer you have depends on the type of cell it starts in.
• Epithelial ovarian cancer is cancer of the surface of the ovary (the epithelium), and is the most common ovarian cancer. Fallopian tube cancer and primary peritoneal cancer are also included in this type of ovarian cancer.
• Germ cell ovarian cancer derives from the reproductive cells of the ovaries. Germ cell ovarian cancer is rare.
• Stromal cells ovarian cancer derives from connective tissue cell. Stromal cells ovarian cancer is also very rare.
• Small cell carcinoma (SCCO) of the ovary is an extremely rare ovarian cancer and it is not certain whether the cells in SCCO are from ovarian epithelial cells, sex-cord stromal cells or germ cells.3
Ovarian cancer in Ireland:
Ireland has one of the highest rates of ovarian cancer in Europe. Ireland also has one of the highest death rates from ovarian cancer in Europe. On average 400 women are diagnosed with ovarian cancer each year in Ireland and 295 women die from ovarian cancer. It is the sixth most common cancer in Irish women after non-melanoma skin cancer and the fourth most common cause of death from cancer. It mainly affects women who have been through the menopause (usually over the age of 50) but it can also affect younger women.2
Lack of screening for ovarian cancer:
There is no reliable screening test for ovarian cancer and every person assigned female at birth is at risk. Delays in diagnoses is common due to this lack of screening and also because symptoms of ovarian cancer are often confused with other conditions. Most people are diagnosed once the cancer has
already spread, making it more difficult to treat. While every woman is at risk, ovarian cancer is often overlooked and underfunded.3
Risk factors for ovarian cancer:
• Getting older – most cases of ovarian cancer occur between the ages of 50 – 79 but younger people can also get ovarian cancer
• Family history – risk is higher for women with a family history of ovarian, breast, endometrial or colorectal cancer
• Faulty inherited genes – 5 to 15% are caused by an inherited faulty genes, such as BRCA1 & BRCA2 alterations
• Ethnicity - women of Ashkenazi Jewish descent are at a higher risk of carrying BRCA1 and BRCA2 alteration and hence have an increased risk of ovarian cancer

• Reproductive history – your risk is higher if you have not delivered children
• Smoking - an increase the risk of certain types of ovarian cancer such as mucinous ovarian cancer
• HRT – slight increased risk
• Medical condition –endometriosis or diabetes
• Being overweight or obesehaving excess body fat is linked to an increase in risk of ovarian cancer
Having one or more risk factors does not mean that you will definitely get ovarian cancer.3
Symptoms of ovarian cancer:
Let’s B.E.A.T. Ovarian Cancer:
• Bloating or an increase in the size of your abdomen
• Eating less and feeling full more quickly
• Abdominal and pelvic pain you feel most days
• Talk to your GP about your symptoms
Other possible symptoms may include:
• Needing to pass urine more often
• Tiredness that is unexplained
• Weight loss that is unexplained
• Changes in your bowel habit or symptoms of irritable bowel
Written by Bernadette Carter, Assistant Director of Nursing, The Marie Keating Foundation

syndrome, especially if this starts after the age of 50
• Abnormal bleeding – Any postmenopausal bleeding should always be checked by your primary health care provider or doctor3, 4
Diagnosis of ovarian cancer:
As mentioned, there is no routine screening test to diagnose ovarian cancer. A HPV cervical screening test (smear) will not detect ovarian cancer. While cervical screening is effective in early detection of cervical cancer, it is not a test for ovarian cancer.
It is very important to speak to your G.P. if you have any of the symptoms listed and if they persist for more than 3 weeks. Particularly if you have family history of ovarian or breast cancer or if you have a family history of the BRCA gene alteration (BRCA1 or BRCA2)
Your GP may do the following tests:
• Pelvic exam
• Transvaginal or pelvic ultrasound
• CA-125 blood test
Your GP may refer you to a specialist (Gynaecologist) who may request further tests to diagnose or rule out ovarian cancer. Tests that may be done include the following:
• CT scan - several x-rays are taken from different angles to create a detailed image of your ovaries
• Needle biopsy - a needle is passed through your tummy, under anaesthetic to retrieve a sample of ovary cells or fluid from around the ovaries, so it can be checked for cancer
• Laparoscopy - a small opening is made in your tummy under anaesthetic and a tube with a camera is inserted to look at your ovaries, a small tissue sample may also be removed for testing.
If ovarian cancer is diagnosed, these tests can help find check if the cancer has spread.5
Treatment for ovarian cancer: A multidisciplinary team (MDT) will discuss the best treatment option for each individual
Your treatment depends on several factors:
• Type of ovarian cancer
• Stage of the cancer (Size and Spread)
• Grade of the cancer – tells us how the cancer cells look in comparison to normal healthy cells
• Your general health
The main treatments for ovarian cancer are:
• Surgery
• Chemotherapy
• Radiotherapy
• Targeted Therapies (e.g. PARP inhibitors)
• Clinical trials
• A combination of treatments may be required5, 6
References
1. Cancer Research UK - https:// www.cancerresearchuk.org/ about-cancer/ovarian-cancer/ what-is-ovarian-cancer
2. NCRI report 2022 Ireland - NCRI_ AnnualStatisticalReport_2022.pdf
3. World Ovarian Cancer CoalitionWhat Is Ovarian Cancer? – World Ovarian Cancer Coalition
4. This is Gynaecological Oncology (GO) website – www.thisisGo.ie
5. HSE website - Ovarian cancerDiagnosis - HSE.ie
6. Cancer research UK - https:// www.cancerresearchuk.org/ about-cancer/ovarian-cancer/ treatment
17 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
Ovarian Cancer
CLINICALLY MEANINGFUL OVERALL SURVIVAL BENEFIT1*
In women with s/gBRCAm advanced ovarian cancer following response to first-line platinum-based chemotherapy
7-YEAR FOLLOW-UP OF THE SOLO-1 TRIAL EVALUATING LYNPARZA AS MAINTENANCE THERAPY IN NEWLY DIAGNOSED BRCAm ADVANCED OVARIAN CANCER SHOWED:

~2 out of 3 patients alive at 7 years
67% with LYNPARZA vs. 46.5% with placebo (HR=0.55; 95% CI: 0.40-0.76; P=0.0004)*1
~1-in-2 patients yet to receive a subsequent treatment at 7 years 45.3% of LYNPARZA vs. 20.6% with placebo (HR=0.37; 95% CI: 0.28-0.48)1
Consistent safety profile
With the primary analysis at the 7-year follow-up, with no new safety signals1,2
For expert opinion on what this data means for clinical practice please scan this QR code.
*7-years OS follow-up data are not statistically significant. P value of <0.0001
declare statistical significance.1
was required to
For additional information, refer to the Summary of Product Characteristics.
ABRIDGED PRESCRIBING INFORMATION
LYNPARZA® (olaparib) 150mg & 100mg FILM-COATED TABLETS
Consult Summary of Product Characteristics (SmPC) before prescribing.
Indication: Ovarian Cancer: As monotherapy for the maintenance treatment of adult patients with advanced (FIGO stages III and IV) BRCA1/2-mutated (germline and/or somatic) high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy. As monotherapy for the maintenance treatment of adult patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy. Lynparza in combination with bevacizumab: As maintenance treatment of adult patients with advanced (FIGO stages III and IV) high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) following completion of first-line platinum-based chemotherapy in combination with bevacizumab and whose cancer is associated with homologous recombination deficiency (HRD) positive status defined by either a BRCA1/2 mutation and/or genomic instability. Breast Cancer: As monotherapy or in combination with endocrine therapy for the adjuvant treatment of adult patients with germline BRCA1/2-mutations who have HER2-negative, high risk early breast cancer previously treated with neoadjuvant or adjuvant chemotherapy. As monotherapy for the treatment of adult patients with germline BRCA1/2-mutations, who have HER2 negative locally advanced or metastatic breast cancer. Patients should have previously been treated with an anthracycline and a taxane in the (neo)adjuvant or metastatic setting unless patients were not suitable for these treatments. Patients with hormone receptor (HR)-positive breast cancer should also have progressed on or after prior endocrine therapy, or be considered unsuitable for endocrine therapy. Adenocarcinoma of the pancreas: As monotherapy for the maintenance treatment of adult patients with germline BRCA1/2-mutations who have metastatic adenocarcinoma of the pancreas and have not progressed after a minimum of 16 weeks of platinum treatment within a first-line chemotherapy regimen. Prostate Cancer: As monotherapy for the treatment of adult patients with metastatic castration-resistant prostate cancer (mCRPC) and BRCA1/2-mutations (germline and/or somatic) who have progressed following prior therapy that included a new hormonal agent. In combination with abiraterone and prednisone or prednisolone for the treatment of adult patients with mCRPC in whom chemotherapy is not clinically indicated.
Presentation: 150mg and 100mg olaparib film-coated tablets.
Dosage and Administration: Treatment should be initiated and supervised by a physician experienced in the use of anticancer therapies. Patient selection: First-line maintenance treatment of BRCAmutated advanced ovarian cancer: Before Lynparza treatment is initiated for first-line maintenance treatment of high-grade epithelial ovarian cancer (EOC), fallopian tube cancer (FTC) or primary peritoneal cancer (PPC), patients must have confirmation of deleterious or suspected deleterious germline and/ or somatic mutations in the breast cancer susceptibility genes (BRCA) 1 or 2 using a validated test. Maintenance treatment of platinum-sensitive relapsed ovarian cancer: There is no requirement for BRCA1/2 testing prior to using Lynparza for the monotherapy maintenance treatment of relapsed EOC, FTC or PPC who are in a complete or partial response to platinum-based therapy. First-line maintenance treatment of HRD positive advanced ovarian cancer in combination with bevacizumab: Before Lynparza with bevacizumab treatment is initiated for the first-line maintenance treatment of EOC, FTC or PPC, patients must have confirmation of either deleterious or suspected deleterious BRCA1/2 mutation and/or genomic instability determined using a validated test. Adjuvant treatment of germline BRCAmutated high risk early breast cancer: Before Lynparza treatment is initiated for adjuvant treatment of HER2 negative high risk early breast cancer, patients must have confirmation of deleterious or suspected deleterious gBRCA1/2 mutation using a validated test. Monotherapy treatment of gBRCA1/2-mutated HER2-negative metastatic breast cancer: For germline breast cancer susceptibility genes (gBRCA1/2) mutated human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer, patients must have confirmation of deleterious or suspected deleterious gBRCA1/2 mutation before Lynparza treatment is initiated. gBRCA1/2 mutation status should be determined by an experienced laboratory using a validated test method. First-line maintenance treatment of gBRCA-mutated metastatic adenocarcinoma of the pancreas: For first-line maintenance treatment of germline BRCA1/2-mutated metastatic adenocarcinoma of the pancreas, patients must have confirmation of a deleterious or suspected deleterious gBRCA1/2 mutation before Lynparza treatment is initiated. gBRCA1/2 mutation status should be determined by an experienced laboratory using a validated test method. Monotherapy treatment of BRCA1/2-mutated metastatic castration-resistant prostate cancer: For BRCA1/2-mutated metastatic castration-resistant prostate cancer (mCRPC), patients must have confirmation of a deleterious or suspected deleterious BRCA1/2 mutation (using either tumour or blood sample) before Lynparza treatment is initiated. BRCA1/2 mutation status should be determined by an experienced laboratory using a validated test method. Treatment of mCRPC in combination with abiraterone and prednisone or prednisolone: No genomic testing is required prior to using Lynparza in combination with abiraterone and prednisone or prednisolone for the treatment of patients with mCRPC.
Genetic counselling for patients tested for mutations in BRCA1/2 genes should be performed. Posology: Recommended dose in monotherapy or in combination with bevacizumab for ovarian cancer or in combination with abiraterone and prednisone or prednisolone for prostate cancer or endocrine therapy is 300mg (two 150mg tablets) twice daily, equivalent to a total daily dose of 600mg. The 100mg tablet is available for dose reduction.
Tablets should be swallowed whole and not chewed, crushed, dissolved or divided and may be taken without regard to meals. Lynparza monotherapy: Patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy should start Lynparza treatment no later than 8 weeks after completion of their final dose of platinum-containing regimen. Lynparza in combination with bevacizumab: When Lynparza is used in combination with bevacizumab for the first-line maintenance treatment of high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer following completion of first-line platinumbased therapy with bevacizumab, the dose of bevacizumab is 15 mg/kg once every 3 weeks. Lynparza in combination with endocrine therapy: Please refer to the full product information of the endocrine therapy combination partner(s) (aromatase inhibitor/anti-oestrogen agent and/or LHRH) for the recommended posology. Lynparza in combination with abiraterone and prednisone or prednisolone: When Lynparza is used in combination with abiraterone for the treatment of patients with mCRPC, the dose of abiraterone is 1000 mg orally once daily. Abiraterone should be given with prednisone or prednisolone 5 mg orally twice daily. Please refer to the full product information for abiraterone. Duration of treatment: First-line maintenance treatment of BRCA-mutated advanced ovarian cancer: Patients can continue treatment until radiological disease progression, unacceptable toxicity or for up to 2 years if there is no radiological evidence of disease after 2 years of treatment. Patients with evidence of disease at 2 years, who in the opinion of the treating physician can derive further benefit from continuous treatment, can be treated beyond 2 years. Maintenance treatment of platinum-sensitive relapsed ovarian cancer: For patients with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer, it is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. First-line maintenance treatment of HRD positive advanced ovarian cancer in combination with bevacizumab: Patients can continue treatment with Lynparza until radiological disease progression, unacceptable toxicity or for up to 2 years if there is no radiological evidence of disease after 2 years of treatment. Patients with evidence of disease at 2 years, who in the opinion of the treating physician can derive further benefit from continuous Lynparza treatment, can be treated beyond 2 years. Adjuvant treatment of germline BRCA-mutated high risk early breast cancer: It is recommended that patients are treated for up to 1 year, or until disease recurrence, or unacceptable toxicity, whichever occurs first. Monotherapy treatment of gBRCA1/2-mutated HER2-negative metastatic breast cancer: It is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. There are no efficacy or safety data on maintenance retreatment with Lynparza following first or subsequent relapse in ovarian cancer patients or on retreatment of breast cancer patients. First-line maintenance treatment of gBRCAmutated metastatic adenocarcinoma of the pancreas: It is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. Monotherapy treatment of BRCA1/2mutated metastatic castration-resistant prostate cancer: It is recommended that treatment be continued until progression of the underlying disease or unacceptable toxicity. Medical castration with luteinising hormone releasing hormone (LHRH) analogue should be continued during treatment in patients not surgically castrated. Treatment of mCRPC in combination with abiraterone and prednisone or prednisolone: It is recommended that treatment be continued until progression of the underlying disease or unacceptable
toxicity when Lynparza is used in combination with abiraterone and prednisone or prednisolone. Treatment with a gonadotropin-releasing hormone (GnRH) analogue should be continued during treatment in all patients, or patients should have had prior bilateral orchiectomy. Please refer to the product information for abiraterone. There are no efficacy or safety data on retreatment with Lynparza in prostate cancer patients. If a patient misses a dose of Lynparza, they should take their next normal dose at its scheduled time. Dose adjustments: Treatment interruption to manage adverse reactions such as nausea, vomiting, diarrhoea, anaemia and dose reduction can be considered. Recommended dose reduction is to 250mg (one 150mg tablet and one 100mg tablet) twice daily, equivalent to a total daily dose of 500mg. If further dose reduction is required, then reduction to 200mg (two 100mg tablets) twice daily, equivalent to a total daily dose of 400mg is recommended. Concomitant use of strong or moderate CYP3A inhibitors is not recommended and alternative agents should be considered. If a strong CYP3A inhibitor must be co-administered, recommended dose reduction is to 100mg (one 100mg tablet) twice daily, equivalent to a total daily dose of 200mg. If a moderate CYP3A inhibitor must be co-administered, recommended dose reduction is to 150mg (one 150mg tablet) twice daily, equivalent to a total daily dose of 300mg. Elderly: No adjustment in starting dose is required. Renal impairment: For patients with moderate renal impairment (creatinine clearance 31 to 50 ml/min) the recommended dose is 200mg (two 100mg tablets) twice daily, equivalent to a total daily dose of 400mg. Lynparza can be administered in patients with mild renal impairment (creatinine clearance 51 to 80 ml/min) with no dose adjustment. No studies have been conducted in patients with severe renal impairment or end-stage renal disease (creatinine clearance ≤ 30 ml/min) and Lynparza is not recommended for use. It may only be used in patients with severe renal impairment if the benefit outweighs the potential risk with careful monitoring of renal function and adverse events. Hepatic impairment: Can be administered in patients with mild or moderate hepatic impairment (Child-Pugh A or B) with no dose adjustment. Not recommended in patients with severe hepatic impairment (Child-Pugh C). Contraindications: Hypersensitivity to the active substance or to any of the excipients. Breast-feeding during treatment and for 1 month after the last dose.
Warnings and Precautions: Haematological toxicity: Treatment should not be started in patients until they have recovered from haematological toxicity caused by previous anticancer therapy (haemoglobin, platelet and neutrophil levels should be ≤CTCAE grade 1). Baseline testing followed by monthly monitoring of complete blood counts is recommended for first 12 months of treatment and periodically thereafter. Treatment should be interrupted and appropriate haematological testing should be initiated if patient develops severe haematological toxicity or blood transfusion dependence. Myelodysplastic syndrome/ Acute Myeloid Leukaemia (MDS/AML): If MDS/AML is suspected, the patient should be referred to a haematologist for further investigations, including bone marrow analysis and blood sampling for cytogenetics. If, following investigation for prolonged haematological toxicity, MDS/AML is confirmed, Lynparza should be discontinued and the patient treated appropriately. Venous Thromboembolic Events: Monitor patients for clinical signs and symptoms of venous thrombosis and pulmonary embolism and treat as medically appropriate. Patients with a prior history of VTE may be more at risk of a further occurrence and should be monitored appropriately. Pneumonitis: Interrupt Lynparza treatment and promptly investigate as appropriate. Discontinue Lynparza if pneumonitis is confirmed and treat patient appropriately. Embryofoetal toxicity: Lynparza could cause foetal harm when administered to a pregnant woman. Pregnancy/contraception: Lynparza should not be used during pregnancy. Women of childbearing potential must use two forms of reliable contraception, before starting Lynparza, during therapy and 6 months after receiving the last dose. Two highly effective and complementary forms of contraception are recommended. Male patients and their female partners of childbearing potential should use reliable contraception during therapy and for 3 months after receiving the last dose. Sodium: This medicinal product contains less than 1 mmol sodium (23 mg) per 100 mg or 150 mg tablet, that is to say essentially “sodium-free”.
Drug Interactions: The recommended Lynparza monotherapy dose is not suitable for combination with myelosuppressive anticancer medicinal products. Caution and close monitoring if vaccines or immunosuppressant agents are co-administered. Effect of other drugs on Lynparza: Strong CYP3A inhibitors (e.g. itraconazole, telithromycin, clarithromycin, protease inhibitors boosted with ritonavir or cobicistat, boceprevir, telaprevir) or moderate CYP3A inhibitors (e.g. erythromycin, diltiazem, fluconazole, verapamil) are not recommended. If co-administered, the dose of Lynparza should be reduced. It is also not recommended to consume grapefruit juice. Strong CYP3A inducers (e.g. phenytoin, rifampicin, rifapentine, carbamazepine, nevirapine, phenobarbital, and St John’s Wort) are not recommended with Lynparza as the efficacy of Lynparza could be substantially reduced. The magnitude of the effect of moderate to strong inducers (e.g. efavirenz, rifabutin) on olaparib exposure is not established, therefore the co-administration of Lynparza with these medicinal products is also not recommended. Effect of Lynparza on other drugs: Caution and appropriate clinical monitoring is recommended when sensitive CYP3A substrates or substrates with a narrow therapeutic margin (e.g. simvastatin, cisapride, cyclosporine, ergot alkaloids, fentanyl, pimozide, sirolimus, tacrolimus and quetiapine) or P-gp substrates (e.g. simvastatin, pravastatin, dabigatran, digoxin and colchicine) are combined with Lynparza. Lynparza may reduce efficacy of hormonal contraceptives. Lynparza may increase the exposure to substrates of BCRP (e.g. methotrexate, rosuvastatin), OATP1B1 (e.g. bosentan, glibenclamide, repaglinide, statins and valsartan), OCT1, MATE1, MATE2K (e.g. metformin), OCT2 (e.g. serum creatinine), OAT3 (e.g. furosemide and methotrexate). Caution if co-administered with any statin.
Pregnancy and Lactation: Women of childbearing potential should not become pregnant while on Lynparza and not be pregnant at the beginning of treatment. A pregnancy test should be performed prior to treatment and considered regularly throughout treatment. The efficacy of some hormonal contraceptives may be reduced if co-administered with Lynparza. Therefore, an additional non-hormonal contraceptive method should be considered during treatment. For women with hormone dependent cancer, two non-hormonal contraceptives should be considered. Lynparza could cause foetal harm when administered to a pregnant woman Lynparza is contraindicated during breast-feeding and for 1 month after receiving last dose. Male patients must use a condom during therapy and for 3 months after receiving last dose when having sexual intercourse with a pregnant woman or with a woman of childbearing potential. Female partners of male patients must also use highly effective contraception. Male patients should not donate sperm during therapy and for 3 months after treatment.
Ability to Drive and Use Machines: Asthenia, fatigue and dizziness have been reported and patients who experience these symptoms should observe caution when driving or using machines.
Undesirable Events: Consult SmPC for full list of side effects. Very common: Anaemia, neutropenia, leukopenia, nausea, vomiting, diarrhoea, dyspepsia, dysgeusia, decreased appetite, fatigue (including asthenia), headache, dizziness, cough, dyspnoea. Common: Lymphopenia, thrombocytopenia, stomatitis, upper abdominal pain, rash, blood creatinine increased, venous thromboembolism. Uncommon: Myelodysplastic syndrome/Acute myeloid leukaemia, hypersensitivity, dermatitis, mean cell volume increased. Rare: Angioedema, erythema nodosum.
Legal Category: Product subject to prescription which may not be renewed (A)
Marketing Authorisation Number: EU/1/14/959/002-003 (100mg tablets); EU/1/14/959/004-005 (150mg tablets).
Marketing Authorisation Holder: AstraZeneca AB, SE151 85 Södertälje, Sweden. Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 7100.
LYNPARZA is a trade mark of the AstraZeneca group of companies.
Date of API preparation: 12/2022
Adverse events should be reported directly to: HPRA Pharmacovigilance, Website: www.hpra.ie Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
Veeva ID: IE-4543
References: 1. DiSilvestro P, et al. J Clin Oncol. 2022 @Epub ahead of print. doi: 10.1200/JCO.22.01549. 2. Moore K, et al. N Engl J Med. 2018;379(26):2495-2505. VEEVA ID: IE- 4503 | Date of Preparation February 2023
Advances in the Field of HIV
Written by Professor Fiona Lyons, Medical Director/Clinical Lead in Sexual Health, HSE Sexual Health and Crisis Pregnancy Programme Consultant in Genitourinary Medicine, GUIDE Clinic, St. James’s Hospital, Dublin

Clinical Professor in Genitourinary Medicine, School of Medicine, Trinity College Dublin
nationally, coordinated audit of PLWH attending all public HIV treatment services was conducted in 2018. This measured the number attending HIV services in 2017, the number on ART and the number who were virally suppressed. The audit found that 5,317 individuals attended a HIV treatment service in Ireland in 2017. Of these, 98.3% were on ART, of whom 95.4% were virally supressed to below the level of detection of the assay. A repeat audit is planned in 2023.
Availability and offer of HIV testing is essential to ensure that PLWH have the opportunity to benefit from early ART. HIV testing, and the role of Health Care Professionals in HIV testing is discussed later.
transmission is known). The second most commonly affected group, heterosexual females, account for 29% of diagnoses (where route of transmission is known) which is higher than in previous years.
• Among those with known region of origin, the majority of HIV cases (88%) are among migrants, defined as originating outside the country in which they were diagnosed. This proportion is higher than in previous years.
Global targets for eliminating HIV
Four decades since the first reports of HIV and AIDS in Ireland, massive advances have been made in the field of HIV medicine such that in 2023 individuals newly diagnosed with HIV have the potential for life expectancy similar to that of the general population and new HIV infections could be prevented.
HIV surveillance and epidemiology in Ireland
Surveillance of HIV is vital for understanding and responding to the latest trends and features of the HIV epidemic. HIV became a notifiable disease in 2011, the Health Protection Surveillance Centre produces weekly and annual reports.
The most recent annual report demonstrates an increase in the number of new HIV diagnoses in 2022 compared to 2020 and 2021, which may be explained by a number of factors including: easing of restrictions associated with the COVID19 pandemic;
resumption of normal testing services; increased migration to Ireland of people who are living with HIV and unmet need in HIV prevention services. The finalised report for 2022 is expected in quarter 2 2023.
Key features of the provisional surveillance data on 644 new diagnoses, corresponding to a rate of 13.5 per 100,000 population, from Q1-Q3 2022 include:
• The majority of HIV diagnoses (77%) are in people who were previously diagnosed positive outside Ireland (where history of previous diagnosis is known).
• The majority of HIV diagnoses are among males but a higher proportion of HIV diagnoses are in females, compared to recent years.
• The key population group affected by HIV continue to be gay, bisexual and men who have sex with men (gbMSM) accounting for 55% of HIV diagnoses (where route of
Outcomes for people living with HIV (PLWH)
Without antiretroviral therapy (ART) the majority of PLWH will develop profound HIV related immunosuppression, HIV associated opportunistic infections and ultimately succumb to an AIDS related illness.
Early initiation of ART before there has been a decline in CD4 count (the current marker of HIV related immunosuppression) affords PLWH net benefits (both in terms of AIDS and non-AIDS serious events). The UK HIV cohort study found that successfully treated PLWH have a normal life expectancy and that those who started ART with a low CD4 cell count significantly improve their life expectancy if they have a good CD4 cell count response and undetectable viral load.
Given the benefits of early initiation ART, international guidelines recommend that ART is offered to all PLWH regardless of CD4 count. In Ireland, ART is available and recommended for all PLWH attending services. The first
Goal 3.3 of the United Nations Sustainable Development Goals is to end the AIDS epidemic by 2030. The global COVID19 pandemic has significantly affected the ability to reach this goal. Recognising this, UNAIDS has reset the 2020 HIV global targets from 90-90-90 to 9595-95, the aim being that by 2025; 95% of PLWH will be diagnosed; 95% of people diagnosed will be receiving ART; and 95% of PLWH on ART will be virally suppressed and unable to pass on infection. The repeat national audit planned for 2023 will contribute to our understanding of how Ireland is doing in terms of reaching global HIV targets.
Combination HIV prevention strategies to eliminate new infections
It is well accepted that the best approach to HIV prevention is through a combination approach of behavioural interventions, structural interventions and biomedical interventions. These have been defined by UNAIDS as:
“Behavioural interventions for HIV prevention are applied to promote change in sexual behaviour, and to increase HIV service utilization and adherence to HIV services and behaviours.”
“Structural interventions are activities designed to address the underlying environmental vulnerabilities of HIV infection, including political, legal, economic, physical, social and cultural barriers such as inequitable gender norms or HIV-related stigma and discrimination.”
20 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
HIV
“Within structural interventions, it is important for us as Health Care Professionals to consider HIV-related stigma in healthcare settings where it remains a barrier to accessing screening, treatment and care for HIV”

HIV
Within structural interventions, it is important for us as Health Care Professionals to consider HIV-related stigma in healthcare settings where it remains a barrier to accessing testing, treatment and care for HIV. A study on the stigma experiences in healthcare settings of PLWH in Ireland found that experiences of enacted, anticipated and internalised stigma were common and that these experiences impacted participants' engagement with care and affected health-seeking behaviours and treatment adherence.
“Biomedical interventions use a mix of clinical and medical approaches and tools to reduce HIV transmission. These include condoms, needle and syringe distribution, opioid substitution therapy, voluntary medical male circumcision (VMMC), antiretroviral therapy for prevention, STI testing and treatment and HIV testing.” Within biomedical interventions needle and syringe distribution and opioid substitution therapy are well established in Ireland. A national condom distribution service was established in 2015 with a steady increase in the number of free condoms and lubricants supplied per year through a range of health care, community and social settings. VMMC is not practiced within the Irish setting as a HIV prevention intervention.
Antiretroviral therapy for HIV prevention
In addition to the significant individual benefits associated with ART for PLWH, ART used in a range of circumstances is central to HIV prevention,
specifically: Treatment as Prevention (TasP); PreExposure Prophylaxis (PrEP) and PostExposure Prophylaxis (PEP).
• Effective ART for people living with HIV has been shown in a large randomised controlled trial, conducted across a range of economic settings to avert HIV transmission. The real world protective effect of ART in preventing sexual transmission of HIV has been well established. These studies provide robust evidence in support of the statement “U=U”, undetectable equals untransmittable. Effective ART in pregnancy has similarly been shown to avert vertical transmission of HIV with vertical transmission rates of <0.4% since 2012 in England.
• ART can also be used following a potential HIV exposure, post exposure prophylaxis (PEP). PEP is a 28-day course of ART that must be started as soon as possible following a potential exposure and within 72 hours. PEP is available through most public STI clinics, some Emergency departments and sexual assault units. PEP is not available through community pharmacies. Irish PEP guidelines have been in place since 2012 with updates since then including 2022 updates which are currently out for consultation.
• Pre-exposure prophylaxis (PrEP) is the pre-emptive use of ART in HIV negative people to reduce the risk of HIV infection and has been
shown to be highly effective in preventing infection when used correctly. A health technology assessment concluded the successful implementation of a national PrEP programme would be safe, effective and cost-saving over the medium to long term in Ireland. A national PrEP programme commenced in late 2019. The HSE Sexual Health and Crisis Pregnancy Programme provides information resources for PrEP users and providers. (sexualwellbeing.ie)
HIV testing
HIV testing is an integral part of combination HIV prevention given the clear benefits of effective ART in preventing onward transmission in addition to the significant individual health benefits with early initiation of ART for PLWH. At present there are no HIV testing guidelines in Ireland. However, the key principles and testing recommendations of the British HIV association guidelines published in 2020 are readily applicable to Ireland.
For healthcare professionals working outside the area of HIV and related fields some principles of the BHIVA guidelines are important to highlight, specifically: all healthcare workers should be able to offer an HIV test in their setting; people should be made aware that they are being tested for HIV and that testing is voluntary; and in general, lengthy pre-test discussion is not required.
Whilst there are no specific HIV testing guidelines in Ireland, HIV testing is routinely offered in a

number of health care settings including opt-out antenatal screening which commenced in 1999; routine opt-out testing as part of STI screening and following a pilot study at St. James’s Hospital Dublin, routine opt-out HIV (hepatitis B and hepatitis C) testing for St. James’s Hospital Emergency Department attendees undergoing blood testing.
A number of medical conditions, referred to as HIV indicator conditions, are known to be associated with an undiagnosed HIV seroprevalence of at least 1 per 1000. These conditions include: malignant lymphoma; anal cancer/dysplasia; cervical dysplasia; community acquired pneumonia; seborrheic dermatitis; severe or atypical psoriasis; mononeuritis; candidemia; invasive pneumococcal disease; primary lung cancer and Guillain-Barre syndrome. Complete tables with the supporting references are available in the BHIVA guidelines. To assist non-HIV specialist healthcare professionals in providing HIV testing to people with HIV indicator conditions, OptTEST, has developed a training module, available at www.opttest.helmlms.com/login.
Summary
In 2023, there is a vast range of HIV treatment and prevention options available. It is critical that our health system and broader society is responsive and adaptable to ensure the best possible outcomes for people living with HIV (PLWH) and those at risk of acquiring HIV.
References available on request
22 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
GOSHH – Promoting Equality and Diversity
 Written by Ann Piercy, Personal Support Worker, KnowNow Community Worker
Written by Ann Piercy, Personal Support Worker, KnowNow Community Worker
GOSHH works in Gender Orientation Sexual Health and HIV.

GOSHH is a charity organisation which provides a safe, confidential, welcoming environment for everyone we work with. Our office is based in Limerick City and we cover Counties of Limerick, Clare, and North Tipperary. Our focus is on the promotion of equality and wellbeing of all with a positive and respectful ap-proach to sexual orientation and gender diversity. Our vision is to create an environment where the mental, emotional, physical and social well-being of everyone is promoted and sexual rights are respected, protected and fulfilled. We promote diversity and equity of all staff and service users, inclusive of a person’s gender (de-fined as male, female, trans or self-identified), marital or family status, sexual orientation, life-style, employment, age, disability (including mental health), religion, race, ethnic
origin and/or membership of the travelling community.
GOSHH was formed in 1989 as Limerick AIDS Alliance which operated until 1994 when the name changed to Red Ribbon Project reflecting the breadth of sexual health services provided throughout the Mid-West of Ireland. Gay Switchboard Limerick was founded in 1986 and be-came Rainbow Support Services in 2001 reflecting the wide variety of identities supported throughout the Mid-West of Ireland. Both projects joined together in 2013 and became GOSHH in 2014.
Sexually transmitted infections (STI) remain one of the most critical public health challenges facing our community, with approximately 500 new HIV cases occurring every year. The Health Protection Surveillance Centre (HPSC) collects data and generate reports on STIs in
Ireland on a weekly basis basing on demographic, gender and age. Health disparities in HIV are linked to a real complex of social determinants that directly affects the populations in the community that are mostly affected by this epidemic. Our community-based initiative approach to reduce HIV is funded by the HSE Sexual Health and Crisis Pregnancy Programme. In order to achieve health equity, every one has to have equal access to be health services. To prevent new HIV transmission, GOSHH provides quality health information and services through our helpline, social media platforms, face to face, outreach and mailing services.
Our services include free rapid HIV, syphilis and hepatitis C testing. As the first entry point of accessing testing services in the community, we provide both in-house and outreach service, saving time for both the clients and University Hospital Limerick (UHL) STI clinic in infection de-tection, making it easier and quicker for the clients to start their care. The rapid test takes about fifteen minutes from the time the clients walks in, filling forms and conducting the test. They have their results before leaving the space. If the test is reactive to either HIV, syphilis or hepatitis C, we facilitate the referral to the STI clinic for further assessment, support and treatment. Our outreach services visit 3rd level institutions, direct provision centres and internally displaced person centres, primary health care centres, homeless shelters and drug and alcohol rehabilitation centres. The programme also enhances and sustains partnerships and support communication strategies that promotes HIV prevention activities
in the community by promoting the self-testing programme sh24. ie which has been rolled out nationwide by the HSE. GOSHH have a good working relationship with the local STI clinic and other STI clinics in and around Ireland. We also offer peer support to people living with HIV, as well as those affected such as friends and family. There was an increase of people looking for support during COVID-19 in terms of information on how and where to access medical services, referrals to STI clinics and mental health. Because there was restriction in movement, those that were not registered with the Irish health system and instead opted to be going home for their medical check-ups found themselves stuck with no medication to continue their treatment. We made sure that every cli-ent was referred to an STI clinic near them.
Other support clients accessed during this period were mental health support which included telephone calls, text messages and zoom calls. There were challenges to providing mental health support for people living with HIV due to COVID restrictions as people were not allowed to leave their environment, for example with people living in direct provision centres, it was almost impossible to have a conversation with them without their privacy being intruded on as they live in shared accommodation.
GOSHH services include providing HIV training in transmission, prevention and treatment for people living with HIV, professionals working with people living with HIV or any groups at risk. We also provide free condom available in the foyer or can be ordered online via the GOSHH website.

23 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 News
WITH RAPID RESPONSES AND PROVEN SEE THE DIFFERENCE XELJANZ®


XELJANZ® (tofacitinib) Prescribing Information:



Please refer to the Summary of Product Characteristics (SmPC) before prescribing XELJANZ 5 mg or 10 mg film-coated tablets, XELJANZ 11 mg prolonged release tablets or XELJANZ 1 mg/mL oral solution. Presentation: Film-coated tablet containing tofacitinib citrate, equivalent to 5 mg or 10 mg tofacitinib. Prolonged-release tablets containing tofacitinib citrate, equivalent to 11 mg tofacitinib. Oral solution containing tofacitinib citrate, equivalent to 1 mg/mL tofacitinib. Indications: Please note not all presentations are licensed for all indications, please see dosage section for details: In combination with methotrexate (MTX) for the treatment of moderate to severe active rheumatoid arthritis (RA) in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying antirheumatic drugs. Can be given as monotherapy in case of intolerance to MTX or when treatment with MTX is inappropriate. In combination with MTX for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response or who have been intolerant to a prior disease modifying antirheumatic drug (DMARD) therapy. For the treatment of adult patients with active ankylosing spondylitis (AS) who have responded inadequately to conventional therapy. For the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent. For the treatment of active polyarticular juvenile idiopathic arthritis (JIA) (rheumatoid factor positive [RF+] or negative [RF-] polyarthritis, and extended oligoarthritis), and juvenile psoriatic arthritis in patients 2 years of age and older, who have responded inadequately to previous therapy with DMARDs. Can be given in combination with MTX or as monotherapy in case of intolerance to MTX or where continued treatment with MTX is inappropriate. Dosage: Treatment should be initiated and supervised by specialist physicians experienced in the diagnosis and treatment of the condition for which tofacitinib is indicated. Tofacitinib is given orally, with or without food. RA, PsA and AS: The recommended dose is 5 mg twice daily or 11 mg once daily which should not be exceeded. Treatment with tofacitinib 5 mg film coated tablets twice daily and tofacitinib 11 mg prolonged release tablet once daily may be switched between each other on the day following the last dose of either tablet. Available data suggest that clinical improvement in AS is observed within 16 weeks of initiation of treatment. Continued therapy should be carefully reconsidered in AS patients exhibiting no clinical improvement within this timeframe. UC: The recommended dose is 10 mg twice daily for induction for 8 weeks. For patients who do not achieve adequate therapeutic benefit by week 8, the induction dose of 10 mg twice daily can be extended for an additional 8 weeks (16 weeks total), followed by 5 mg twice daily for maintenance. Tofacitinib induction therapy should be discontinued in any patient who shows no evidence of therapeutic benefit by week 16. The recommended dose for maintenance treatment is tofacitinib 5 mg twice daily. Tofacitinib 10 mg twice daily for maintenance treatment is not recommended in patients with UC who have known venous thromboembolism (VTE) risk factors, unless there is no suitable alternative treatment available. For patients with UC who are not at increased risk for VTE, tofacitinib 10 mg twice daily may be considered if the patient experiences a decrease in response on tofacitinib 5 mg twice daily and failed to respond to alternative treatment options for UC such as tumour necrosis factor (TNF) inhibitor treatment. Tofacitinib 10 mg twice daily for maintenance treatment should be used for the shortest duration possible. The lowest effective dose needed to maintain response should be used. Polyarticular JIA and juvenile PsA: The recommended dose in patients 2 years of age and older: 10 kg - < 20 kg: 3.2 mg (3.2 mL oral solution) twice daily, 20 kg - < 40 kg: 4 mg (4 mL oral solution) twice daily, and ≥ 40 kg 5 mg (5 mL oral solution or 5 mg tablet) twice daily. Patients ≥ 40 kg treated with tofacitinib 5 mL oral solution twice daily may be switched to tofacitinib 5 mg tablets twice daily. Available data suggest that clinical improvement is observed in paediatric patients within 18 weeks of initiation. Continued therapy should be carefully reconsidered in a paediatric patient exhibiting no clinical improvement within this timeframe. Dose interruption and adjustment:
Tofacitinib treatment should be interrupted if a patient develops a serious infection until the infection is controlled. Interruption of dosing may be needed for management of dose-related laboratory abnormalities including lymphopenia, neutropenia, and anaemia. It is recommended not to initiate dosing in patients with an absolute lymphocyte count (ALC) less than 0.75×10 /L, an absolute neutrophil count (ANC) less than 1×10 /L or with haemoglobin less than 9 g/dL. It is recommended not to initiate dosing in paediatric patients with an absolute neutrophil count (ANC) less than 1.2×109/L or with haemoglobin less than 10 g/dL. Renal impairment: No dose adjustment is required in patients with mild or moderate renal impairment. Patients with severe renal impairment the dose should be reduced to 5 mg once daily when the indicated dose in the presence of normal renal function is 5 mg twice daily or 11 mg prolonged-release tablet once daily. Dose should be reduced to 5 mg twice daily when the indicated dose in the presence of normal renal function is 10 mg twice daily. Patients with severe renal impairment should remain on a reduced dose even after haemodialysis. Hepatic impairment: No dose adjustment is required in patients with mild hepatic impairment. Patients with moderate hepatic impairment dose should be reduced to 5 mg once daily when the indicated dose in the presence of normal hepatic function is 5 mg twice daily or 11 mg prolonged-release tablet once daily. Dose should be reduced to 5 mg twice daily when the indicated dose in the presence of normal hepatic function is 10 mg twice daily. Tofacitinib should not be used in patients with severe hepatic impairment. Elderly: No dose adjustment is required in patients aged 65 years and older. Use with caution as increased risk and severity of adverse events. See also Warnings & Precautions for use in patients over 65 years of age. Paediatric population: The safety and efficacy of tofacitinib in children less than 2 years of age with polyarticular JIA and juvenile PsA has not been established. The safety and efficacy of tofacitinib in children less than 18 years of age with other indications (e.g. ulcerative colitis) has not been established. The safety and efficacy of tofacitinib prolonged-release formulation in children aged less than 18 years have not been established. Interactions: Tofacitinib total daily dose should be reduced by half in patients receiving potent inhibitors of cytochrome P450 (CYP) 3A4 (e.g. ketoconazole) and in patients receiving 1 or more products that result in both moderate inhibition of CYP3A4 and potent inhibition of CYP2C19 (e.g. fluconazole). Coadministration with potent CYP inducers (e.g. rifampicin) may result in a loss of or reduced clinical response. Coadministration with potent inducers of CYP3A4 is not recommended. Contraindications: Hypersensitivity to any of the ingredients, active tuberculosis (TB), serious infections such as sepsis, or opportunistic infections, severe hepatic impairment, pregnancy and lactation. Warnings and Precautions: Patients treated with tofacitinib should be given a patient alert card. Use in patients over 65 years of age: Considering the increased risk of serious infections, myocardial infarction, and malignancies with tofacitinib in patients over 65 years of age, tofacitinib should only be used in patients over 65 years of age if no suitable treatment alternatives are available. Combination with other therapies: There was a higher incidence of adverse events for the combination of tofacitinib with MTX versus tofacitinib as monotherapy in RA clinical studies. Tofacitinib should be avoided in combination with biologics and potent immunosuppressants such as azathioprine, 6-mercaptopurine, ciclosporin and tacrolimus. Venous thromboembolism (VTE): Serious VTE events including pulmonary embolism (PE), some of which were fatal, and deep vein thrombosis (DVT), have been observed in patients taking tofacitinib. In a randomised post authorisation safety study in patients with rheumatoid arthritis who were 50 years of age or older with at least one additional
aFrom the start of treatment with XELJANZ in patients with moderately to severely active UC who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent. 3
XELJANZ 10 mg BID achieved higher rates of remission (primary endpoint) at week 8 vs placebo in OCTAVE Induction 1 (19% [88/476] vs 8% [10/122]; P=0.007) and OCTAVE Induction 2 (17% [71/429] vs 4% [4/112]; P<0.001)
XELJANZ 10 mg BID achieved higher rates of clinical response at week 8 vs placebo in OCTAVE Induction 1 (60% [285/476] vs 33% [40/122]; P<0.001) and OCTAVE Induction 2 (55% [236/429] vs 29% [32/112]; P<0.001) 1
In OCTAVE Sustain, for patients showing remission or clinical response following induction, XELJANZ 5 mg BID achieved higher rates of remission at week 52 (primary endpoint) vs placebo (34% [68/198] vs 11% [22/198]; P<0.001).1
Decreases in rectal bleeding and stool frequency Mayo subscores were observed as early as day 3 in patients treated with XELJANZ 10 mg BID 2

PP-XEL-IRL-0856 © 2023 Pfi zer Inc. All rights reserved. January 2023
PROVEN MAINTENANCE OF EFFICACY, CAN MAKE FROM THE START1,2,a


cardiovascular risk factor, a dose dependent increased risk for VTE was observed with tofacitinib compared to TNF inhibitors. In a post hoc exploratory analysis within this study, in patients with known VTE risk factors, occurrences of subsequent VTEs were observed more frequently in tofacitinib-treated patients that, at 12 months treatment, had D-dimer level greater than or equal to twice the upper limit of normal (2×ULN) versus those with D-dimer level <2×ULN. For patients with RA with known risk factors for VTE, consider testing D-dimer levels after approximately 12 months of treatment. If D-dimer test result is ≥ 2×ULN, confirm that clinical benefits outweigh risks prior to a decision on treatment continuation with tofacitinib. Tofacitinib should be used with caution in patients with known risk factors for VTE, regardless of indication and dose. Tofacitinib 10 mg twice daily for maintenance treatment is not recommended in patients with UC who have known VTE risk factors, unless there is no suitable alternative treatment available. Promptly evaluate patients with signs and symptoms of VTE and discontinue tofacitinib in patients with suspected VTE, regardless of dose or indication. Retinal venous thrombosis (RVT): Patientsexperiencing symptoms suggestiveofRVTshould be advised to promptly seek medical care. Infections: Seriousandsometimes fatal infections have been reported in patients administered tofacitinib. Rheumatoid arthritis patients taking corticosteroids may be predisposed to infection. Patients should be closely monitored for infections, with prompt diagnosis and treatment. Treatment should be interrupted if a serious infection develops. As there is a higher incidence of infections in the elderly and in the diabetic populations in general, caution should be used when treating the elderly and patients with diabetes. Tuberculosis: Patients should be evaluated for both active and latent TB prior to being treated with tofacitinib. Patients who test positive for latent TB should be treated with standard antimycobacterial therapy before administering tofacitinib. Viral Reactivation: Viral reactivation and cases of herpes zoster have been observed. Screening for viral hepatitis should be performed in accordance with clinical guidelines prior to starting therapy with tofacitinib. The impact on chronic viral hepatitis is not known. Major adverse cardiovascular events (MACE): MACE have been observed in patients taking tofacitinib. In a randomised post authorisation safety study in patients with RA who were 50 years of age or older with at least one additional cardiovascular risk factor, an increased incidence of myocardial infarctions was observed with tofacitinib compared to TNF inhibitors. In patients over 65 years of age, patients who are current or past smokers, and patients with other cardiovascular risk factors, tofacitinib should only be used if no suitable treatment alternatives are available. Diabetes: Dose adjustment of anti-diabetic medication may be necessary in the event that hypoglycaemia occurs following initiation of tofacitinib. Vaccinations: Prior to initiating tofacitinib it is recommended that all patients, particularly pJIA and jPsA patients, be brought up to date with all immunisations in agreement with current immunisation guidelines. Live vaccines should not be given concurrently with tofacitinib. Malignancy and lymphoproliferative disorder: Tofacitinib may affect host defences against malignancies. In a randomised post authorisation safety study in patients with RA who were 50 years of age or older with at least one additional cardiovascular risk factor, an increased incidence of malignancies excludingnon-melanomaskincancer(NMSC), particularlylungcancerandlymphoma,wasobservedwithtofacitinibcomparedtoTNF inhibitors. Lung cancers and lymphoma in patients treated with tofacitinib have also been observed in other clinical studies and in the post marketing setting. Other malignancies in patients treated with tofacitinib were observed in clinical studies and the post marketing setting, including, but not limited
References:
to, breast cancer, melanoma, prostate cancer, and pancreatic cancer. In patients over 65 years of age, patients who are current or past smokers, and patients with other malignancy risk factors tofacitinib should only be used if no suitable treatment alternatives are available. NMSCs have been reported in patients treated with tofacitinib; the risk of NMSC may be higher in patients treated with tofacitinib 10 mg twice daily than in patients treated with 5 mg twice daily. Periodic skin examination is recommended in patients at increased risk for skin cancer. Interstitial lung disease: Caution is recommended in patients with a history of chronic lung disease as they may be more prone to infection. Asian patients are known to be at higher risk of ILD, caution should be exercised with these patients. Gastrointestinal perforations: Tofacitinib should be used with caution in patients who may be at increased risk, e.g. history of diverticulitis or concomitant use of corticosteroids or NSAIDs. Hypersensitivity: Cases of hypersensitivity associated with tofacitinib administration have been reported. Allergic reactions included angioedema and urticaria; serious reactions have occurred. If any serious allergic or anaphylactic reaction occurs, tofacitinib should be discontinuedimmediately. Laboratory Parameters: Increasedincidenceoflymphopeniaandneutropeniahavebeenreported,anddecreasesinhaemoglobin,whichshouldbemonitored in accordance with the SmPC. Monitor ANC and haemoglobin at baseline, 4-8 weeks and 3 monthly, ALC at baseline and 3 monthly. Tofacitinib has been associated with increases in lipid parameters, maximal effects were observed within 6 weeks. Monitoring should be performed 8 weeks after initiation and managed according to hyperlipidaemia guidelines. Tofacitinib has been associated with liver enzyme elevations; use caution if initiating in patients with elevated liver enzymes particularly in combination with potentially hepatotoxic products. Routine monitoring of liver tests and prompt investigation of any observed liver enzyme elevations are recommended to identify potential cases of drug-induced liver injury. Gastrointestinal obstruction with a non-deformable prolonged-release formulation: Caution should be used when administering tofacitinib 11 mg prolonged release tablets to patients with pre-existing severe gastrointestinal narrowing (pathologic or iatrogenic). Pregnancy & Lactation: Use of tofacitinib during pregnancy and breast-feeding is contraindicated. Undesirable Effects: RA, PsA and A: The most common serious adverse reactions were serious infections; pneumonia, herpes zoster, UTIs, cellulitis, diverticulitis, appendicitis and opportunistic infections. The most commonly reported adverse reactions during the first 3 months in controlled clinical studies were headache, upper respiratory tract infections, diarrhoea, nausea and hypertension. UC: The most commonly reported adverse reactions in patients receiving tofacitinib 10 mg twice daily were headache, nasopharyngitis, nausea, and arthralgia. Commonly reported adverse reactions (>1/100 to <1/10) across all indications were pneumonia, influenza, herpes zoster, urinary tract infection, sinusitis, bronchitis, nasopharyngitis, pharyngitis, lymphopenia, anaemia, headache, hypertension, cough, abdominal pain, vomiting, diarrhoea, nausea, gastritis, dyspepsia, rash, arthralgia, peripheral oedema, increased creatine phosphokinase. Refer to section 4.8 of the SmPC for further information on side effects, including description of selected adverse reactions. Legal Category: S1A. Marketing Authorisation Number: EU/1/17/1178/003 – 5 mg (56 film-coated tablets); EU/1/17/1178/007 – 10 mg (56 filmcoated tablets); EU/1/17/1178/012 – 11 mg (28 prolonged-release tablets); EU/1/17/1178/015 1mg/mL oral solution. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium.
1. Sandborn WJ, Su C, Sands BE, et al; for the OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723-1736.

2. Hanauer S et al. Tofacitinib Induction Therapy Reduces Symptoms Within 3 Days for Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2019;17(1):139-147.
3. XELJANZ Summary of Product Characteristics.

further information on this medicine
For queries regarding product availability
contact:
6500. Last revised: 09/2022. Ref: XJ 18_0.
For
please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@ pfizer.com
please
Pfizer Healthcare Ireland, 9 Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24, + 353 1 467
Benign prostatic hyperplasia (BPH): Risk factors, aetiology, and management
Written by Dr Ken Patterson, Urology Registrar, Beaumont Hospital, Dublin; and Mr Niall Davis, Consultant Urologist and Senior Lecturer, Beaumont Hospital and the RCSI, Dublin
Benign prostatic hyperplasia (BPH) is a term used to describe the nonmalignant growth or hyperplasia of prostate tissue. The condition is characterised by an increase in epithelial and stromal cells in the peri-urethral area of the prostate. BPH is the most common cause of bladder outlet obstruction (BOO) and LUTS in men. It is extremely common and the prevalence of histological BPH at autopsy is as high as 90 per cent in men aged 81-90 years.1
The resultant effects of BPH on men are in the form of LUTS; the prevalence of which can range between 44 per cent in men between the ages of 40-59, and increasing to 70 per cent in males over 80 years of age.
LUTS can greatly affect the qualityof-life of these men, and due to the high prevalence of this condition in the community, can put pressure on both primary and secondary healthcare providers in both the investigation and management of this cohort of patients.
Case Study
CASE REPORT
Mr Jones, a 62-year-old architect, presents to his GP complaining of new onset of urinary symptoms over the past three months. His main complaints are urinary frequency, nocturia (two-three times a night), a slow stream and more recently, difficulty in initiating his stream. He denies any visible haematuria or dysuria, and his urine dipstick is normal. He has no history of urinary tract infections (UTIs). His GP calculates his International Prostate Symptom Score (IPSS) score to be 15, indicating moderate voiding symptoms. Mr Jones says his lower urinary tract symptoms (LUTS) are impacting on his daily schedule, as he feels tired in work from getting up at night and is urinating more frequently during the day in work. He drinks sixto-seven cups of tea during the day and has a cup at night before going to bed. The GP performs a digital rectal exam (DRE), which reveals an enlarged but smoothfeeling prostate gland. Bloods are sent, including a U/E and PSA. The GP provides a frequency/ volume
chart and advises Mr Jones to reduce his tea intake and switch to decaffeinated tea, and to limit his evening fluid intake.
At follow-up, his PSA is 0.75ng/ ml, and his creatinine is normal at 73μmol/L. He reports some improvement in his frequency and nocturia following the previous lifestyle advice provided by the GP, however he now has the sensation of incomplete emptying. His frequency volume chart outrules nocturnal polyuria. He is started on an alpha1-blocker, to which he has great relief of his symptoms.
Two years later he re-presents to his GP with symptoms suggestive of a UTI. He is treated in the community and he is referred to a urologist who performs a flexible cystoscopy, which confirms an enlarged, moderately occlusive prostate gland. Uroflowometry shows a reduced urinary stream with a reduced maximum flow rate (QMax), a prolonged and intermittent stream, and post-
void residual (PVR) of 185mls. His PSA is repeated and is now 1.6ng/ml and DRE again reveals a benign-feeling prostate gland. A 5-alpha reductase inhibitor (5-ARI) is added in combination with the alpha1-blocker. Repeat uroflowometry in six months shows an increased QMax and a PVR of just 50mls.
Primary care providers play an important role in the investigation and initial management of patients presenting with LUTS due to BPH. However, referral to a urologist is indicated in certain scenarios, including;
• Patients who present with acute urinary retention (AUR) or renal impairment due to BPH.
• Patients who fail to respond to or who are unable to tolerate the side-effects of medical therapy.
• Patients who present with visible or non-visible haematuria.
• Patients with signs or symptoms of high pressure chronic retention (tense, distended bladder, bedwetting, progressive renal failure).
Pathophysiology
Despite symptomatic BPH being first described from as early as 1649, the pathophysiology of this disease process remains relatively poorly understood. Interactions between the epithelium and the stroma are regulated by the androgen receptor and dihydrotestosterone (DHT). Both of these cause prostatic tissue enlargement with the development of characteristic enlarging benign nodules. About 90 per cent of serum testosterone is converted to the more potent DHT by the enzyme 5-alpha reductase-2 in prostatic tissue. This is not the only pathway that promotes prostatic growth, which is the basis as to why not all patients with BPH respond to 5-ARIs.
INVESTIGATIONS FOR LUTS
26 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Urology
TABLE 1: Recommended diagnostic investigations for LUTS
Always Medical history Clinical
Optional PSA Uroflowmetry
examination with DRE Validated symptom score questionnaire Frequency/volume chart Urinalysis
Post void residual Ultrasonography Cystourethroscopy Pressure-flow studies
Serum creatinine
Prescribing information: VESOMNI™ 6mg/0.4mg modified release tablets
For full prescribing information, refer to the Summary of Product Characteristics (SPC). Presentation: VESOMNI modified release tablets contain a layer of 6 mg solifenacin succinate, corresponding to 4.5 mg solifenacin free base and a layer of 0.4 mg tamsulosin hydrochloride, corresponding to 0.37 mg of tamsulosin free base. Indication: Treatment of moderate to severe storage symptoms (urgency, increased micturition frequency) and voiding symptoms associated with benign prostatic hyperplasia (BPH) in men who are not adequately responding to treatment with monotherapy. Posology and method of administration: Adult males,including older people: One VESOMNI tablet (6 mg/0.4 mg) once daily taken orally with or without food. The maximum daily dose is one VESOMNI tablet. The tablet must be swallowed whole, intact without biting or chewing. Do not crush the tablet. Specialpopulations(seealsocontraindicationsbelow): Renal impairment: Severerenalimpairment (creatinine clearance ≤ 30 mL/min): Treat with caution, maximum daily dose in these patients is one VESOMNI tablet. Hepatic impairment: Moderatehepaticimpairment(Child-Pugh score of 7-9): Treat with caution, maximum daily dose in these patients is one VESOMNI tablet. In patients with severe hepatic impairment (Child-Pugh score > 9), the use of VESOMNI is contraindicated. Concomitant treatment with moderate and strong inhibitors of CYP450 3A4: e.g. verapamil, ketoconazole, ritonavir, nelfinavir, itraconazole: Treat with caution, maximum daily dose should be limited to one VESOMNI tablet. Paediatricpopulation: There is no relevant indication for use of VESOMNI in children and adolescents. Contraindications: Patients with hypersensitivy to the active substance(s) or to any of the excipients (see SPC). Patients undergoing haemodialysis. Patients with severe hepatic impairment. Patients with severe renal impairment who are also treated with a strong cytochrome P450 (CYP)3A4 inhibitor e.g. ketoconazole. Patients with moderate hepatic impairment who are also treated with a strong CYP3A4 inhibitor e.g. ketoconazole. Patients with severe gastrointestinal conditions (including toxic megacolon), myasthenia gravis or narrow-angle glaucoma and patients at risk for these conditions. Patients with a history of orthostatic hypotension. Special Warnings and Precautions for Use: VESOMNI should be used with caution in patients with: severe renal impairment; risk of urinary retention; gastrointestinal obstructive disorders; risk of decreased gastrointestinal motility; hiatus hernia/gastroesophageal reflux and/or who are concurrently taking medicinal products (such as bisphosphonates) that can cause or exacerbate oesophagitis; autonomic neuropathy. The patient should be examined in order to exclude the presence of other conditions, which can cause similar symptoms to benign prostatic hyperplasia. Other causes of frequent urination (heart failure or renal disease) should be assessed before treatment with VESOMNI is initiated. If a urinary tract infection is present, appropriate antibacterial therapy should be started. QT prolongation and Torsade de Pointes have been observed in patients with risk factors, such as pre-existing long QT syndrome and hypokalaemia, who are treated with solifenacin succinate. Angioedema with airway obstruction has been reported in some patients on solifenacin succinate and tamsulosin. If angioedema occurs, VESOMNI should be discontinued and not restarted. Appropriate therapy and/or measures should be taken. Anaphylactic reaction has been reported in some patients treated with solifenacin succinate. In patients who develop anaphylactic reactions, VESOMNI should be discontinued and appropriate therapy and/or measures should be taken. As with other alpha1-adrenoceptor antagonists, a reduction in blood pressure can occur in individual cases during treatment with tamsulosin, as a result of which, rarely, syncope can occur. Patients starting treatment with VESOMNI should be cautioned to sit or lie down at the first signs of orthostatic hypotension (dizziness, weakness) until the symptoms have disappeared. The ‘Intraoperative Floppy Iris Syndrome’ (IFIS, a variant of small pupil syndrome) has been observed during cataract and glaucoma surgery in some patients on or previously treated with tamsulosin hydrochloride. IFIS may increase the risk of eye complications during and after the operation. Therefore, the initiation of therapy with VESOMNI in patients for whom cataract or glaucoma surgery is scheduled is not recommended. Discontinuing treatment with VESOMNI 1-2 weeks prior to cataract or glaucoma surgery is anecdotally considered helpful, but the benefit of treatment discontinuation has not been established. During pre-operative assessment, surgeons and ophthalmic teams should consider whether patients scheduled for cataract or glaucoma surgery are being or have been treated with VESOMNI in order to ensure that appropriate measures will be in place to manage IFIS during surgery. VESOMNI should be used with caution in combination with moderate and strong inhibitors of
A

combined force against LUTS and BPH
CYP3A4 and it should not be used in combination with strong inhibitors of CYP3A4, e.g., ketoconazole, in patients who are of the CYP2D6 poor metaboliser phenotype or who are using strong inhibitors of CYP2D6, e.g., paroxetine. Interactions: Pharmacological interactions: Concomitant medication with other anticholinergic medicinal products may result in more pronounced therapeutic and undesirable effects. Allow approximately one week after stopping treatment with VESOMNI before commencing any anticholinergic therapy. The therapeutic effect of solifenacin may be reduced by concomitant administration of cholinergic receptor agonists. Pharmacokinetic interactions: Pharmacokinetic interactions involving the potential for other medicinal products to affectVESOMNI exposures: Interactions with CYP3A4 and CYP2D6 inhibitors: See Contraindications, Posology and administration and Special warnings and precautions above. Concomitant administration may lead to increased exposure to both solifenacin (ketoconazole 400 mg/day resulted in a 1.5-fold increase in Cmax and a 2.8-fold increase in AUC). and tamsulosin (ketoconazole 400 mg/day resulted in a 2.2-fold increase in Cmax and a 2.8-fold increase in AUC). VESOMNI should be used with caution in combination with strong CYP3A4 inhibitors. VESOMNI should not be given together with strong CYP3A4 inhibitors in patients who are also CYP2D6 poor metabolizer phenotype or who are using strong CYP2D6 inhibitors. See SPC for details of the effects of other CYP3A4 and CYP2D6 inhibitors. Inducers: Inducers of CYP3A4 (e.g. rifampicin) may decrease the plasma concentrations of solifenacin and tamsulosin. Information available for the individual active substances: Solifenacin can reduce the effect of medicinal products that stimulate the motility of the gastrointestinal tract, such as metoclopramide and cisapride. Solifenacin did not affect the pharmacokinetics of digoxin, or the pharmacokinetics or effect on prothrombin time of R- or S-warfarin. Co-administration of tamsulosin and other alpha1-adrenoreceptor antagonists could lead to hypotensive effects. Diclofenac and warfarin may increase the elimination rate of tamsulosin. No interactions have been seen when tamsulosin was given concurrently with atenolol, enalapril or theophylline. Fertility, pregnancy and lactation: The effect of VESOMNI on fertility has not been established. Ejaculation disorders have been observed in short and long term clinical studies with tamsulosin. Events of ejaculation disorder, retrograde ejaculation and ejaculation failure have been reported in the post authorization phase. VESOMNI is not indicated for use in women. Driving and use of machines: No studies have been performed, however patients should be informed about the possible occurrence of dizziness, blurred vision, fatigue and uncommonly somnolence, which may negatively affect the ability to drive or use machines. Undesirable Effects: Summary of the safety profile: VESOMNI may cause anticholinergic undesirable effects of, in general, mild to moderate severity. The most frequently reported adverse reactions during the clinical studies performed for the development of VESOMNI were dry mouth (9.5%), followed by constipation (3.2%) and dyspepsia (including abdominal pain; 2.4%). Other common undesirable effects are dizziness (including vertigo; 1.4%), vision blurred (1.2%), fatigue (1.2%), and ejaculation disorder (including retrograde ejaculation; 1.5%). Acute urinary retention (0.3%, uncommon) is the most serious adverse drug reaction that has been observed during treatment with VESOMNI in clinical studies. List of adverse reactions: the ‘VESOMNI frequency’ below reflects adverse drug reactions that have been observed during the double-blind clinical studies performed for the development of VESOMNI (based on reports of treatment-related adverse events, which have been reported by at least two patients and occurred with a frequency higher than for placebo in the double-blind studies). The ‘solifenacin frequency’ and ‘tamsulosin frequency’ below reflect adverse drug reactions (ADRs) previously reported with one of the individual components (as presented in the Summary of Product Characteristics (SmPCs) of solifenacin 5 and 10 mg and tamsulosin 0.4 mg respectively that may also occur when receiving VESOMNI (some of these have not been observed during the clinical development program of VESOMNI). The frequency of adverse reactions is defined as follows: very
urinary tract infection, cystitis Immune system disorders: Not known: anaphylactic reaction* Metabolism and nutrition disorders: Not known: decreased appetite*, hyperkalemia* Psychiatric disorders: Very rare: hallucination*, confusional state* Not known: delirium* Nervous system disorders: Uncommon somnolence, dysgeusia Rare: dizziness*, headache* Eye disorders: Common: vision blurred Uncommon: dry eyes Not known: glaucoma* Cardiac disorders: Not known: palpitations*, Torsade de Pointes*, electrocardiogram QT prolongation*, atrial fibrillation*, tachycardia* Respiratory, thoracic and mediastinal disorders: Uncommon: nasal dryness Not known: dysphonia* Gastrointestinal disorders: Very common: dry mouth Common: dyspepsia, constipation, nausea, abdominal pain Uncommon: gastro-oesophageal reflux disease, dry throat Rare: vomiting*, colonic obstruction, faecal impaction, Not known: ileus*, abdominal discomfort* Hepatobiliary disorders: Not known: liver disorder*, liver function test abnormal* Skin and subcutaneous tissue disorders: Uncommon: dry skin Rare: pruritus*, rash* Very rare: urticaria*, angioedema*, erythema multiforme* Not known: exfoliative dermatitis* Musculoskeletal and connective tissue disorders: Not known: muscular weakness* Renal and urinary disorders: Uncommon: difficulty in micturition Rare: urinary retention***Not known: renal impairment* General disorders and administration site conditions:
Uncommon: fatigue, perhiperal oedema. Tamsulosin 0.4mg frequency#: Nervous system disorders:
Common: dizziness Uncommon: headache Rare: syncope Eye disorders: Not known: vision blurred*, Intraoperative Floppy Iris Syndrome (IFIS)**, visual impairment* Cardiac disorders: Uncommon: palpitations Not known: atrial fibrillation*, arrhythmia*, tachycardia* Vascular disorders: Uncommon: orthostatic hypotension Respiratory, thoracic and mediastinal disorders: Uncommon: rhinitis Not known: dyspnoea*, epistaxis* Gastrointestinal disorders: Uncommon: constipation, nausea, diarrhea, vomiting Skin and subcutaneous tissue disorders: Uncommon: pruritus, rash, urticaria Rare: angioedema Very Rare: Stevens-Johnson syndrome Not known: erythema multiforme*, exfoliative dermatitis* Reproductive system and breast disorders: Common: ejaculation disorders including retrograde ejaculation and ejaculation failure Very rare: priapism General disorders and administration site conditions: Uncommon: asthenia. #: The ADRs from solifenacin and tamsulosin included are the ADRs listed in the summary of product characteristics of both products. *: from post-marketing reporting. Because these spontaneously reported events are from the worldwide post-marketing experience, the frequency of events and the role of solifenacin or tamsulosin and their causation cannot be reliably determined. **: from post-marketing reporting, observed during cataract and glaucoma surgery. ***: see Special warnings and precautions for use. Long-termsafety of VESOMNI: The profile of undesirable effects seen with treatment up to 1 year was similar to that observed in the 12-week studies. The product is well-tolerated and no specific adverse reactions have been associated with long-term use. Description of selected adverse reactions: For urinary retention see Special warnings and precautions for use. Olderpeople: The therapeutic indication of VESOMNI, moderate to severe storage symptoms (urgency, increased micturition frequency) and voiding symptoms associated with BPH, is a disease affecting elderly men. The clinical development of VESOMNI has been performed in patients 45 to 91 years of age, with an average age of 65 years. Adverse reactions in the elderly population were similar to the younger population. Reporting of suspected adverse reactions: see below. Overdose: Overdosage with the combination of solifenacin and tamsulosin can potentially result in severe anticholinergic effects plus acute hypotension. Refer to SPC for details of treatment of overdose. Legal Category: POM (S1B). Nature
to
uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data). The adverse events are grouped by MedDRA system organ class preferred term (PT).
common
≥1/10);
1/100
VVESOMNI frequency: Nervous system disorders: Common: dizziness Eye disorders: Common: vision blurred Gastrointestinal disorders: Common: dry mouth, dyspepsia, constipation Skin and subcutaneous tissue disorders: Uncommon: pruritus Renal and urinary disorders: Uncommon: Urinary retention*** Reproductive system and breast disorders: Common: ejaculation disorders including retrograde ejaculation and ejaculation failure General disorders and administration site conditions: Common: fatigue Solifenacin 5mg & 10mg frequency#: Infections and infestations: Uncommon:
(
common (≥
<1/10);
and contents of container: Aluminium blister packs containing 30 tablets. Product Authorisation Number: PA1241/016/001. Marketing Authorisation holder: Astellas Pharma Co., Ltd. Further information is available from: Astellas Pharma Co., Ltd, Damastown Road, Damastown Industrial Park, Mulhuddart, Dublin 15. Phone: +3531 467 1555. Summary of Product Characteristics with full prescribing information available upon request. Job number: MAT-IE-VESOM-2023-00001 Date of preparation of API: January 2023 Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance, Website: www.hpra.ie or Astellas Pharma Co., Ltd. Tel: +353 1 467 1555, E-mail: irishdrugsafety@astellas.com.
49% of men with LUTS report bladder and prostate symptoms1 VESOMNI treats the symptoms of both the bladder and prostate 2 References: 1. Sexton CC, et al. BJU Int 2009; 103(Suppl 3): 12-23. 2. VESOMNI Summary of Product Characteristics. Date of preparation: January 23 Job code: MAT-IE-VESOM-2023-00002
DHT upregulates several growth factors in BPH such as fibroblast growth factor (FGF), epithelial growth factor (EGF), insulin-like growth factor (IGF), keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF).
Paracrine cellular alterations that include proliferation, quiescence, and apoptosis all result in prostatic tissue expansion.
The pathophysiological basis of BOO resulting from BPH has been extensively studied; it can be a result of both dynamic and static components of BPH:

1. Dynamic component: 1-adrenoreceptor mediated prostatic smooth muscle contraction. This effect is the rationale for α-adrenoreceptor blockade treatment.
2. Static component: This is a result of the volume effect of prostatic tissue hyperplasia.
Investigations
All patients presenting with bothersome LUTS thought to be as a result of BPH should have a thorough clinical history taken and clinical examination performed. Symptom severity should be assessed using a validated assessment tool, with the IPSS being the most commonly used assessment tool. DRE should always be performed to rule out the presence of a prostate malignancy and to assess prostate size and shape. Laboratory investigations that should be performed include urinalysis, serum creatinine measurement, and prostate specific antigen (PSA) measurement. Other useful investigations for BPH can include uroflowometry and PVR measurement, renal ultrasonography, frequency/volume charts, and pressure flow studies. Men should be counselled appropriately prior to PSA
measurement. A PSA level >1.4ng/ ml is a predictor of symptom progression in men with BPH. Patients with an elevated serum creatinine should be investigated with renal ultrasonography as this may be indicative of high pressure chronic urinary retention – there may be clues of this in the history if there is any history of bedwetting. The relationship between patients whom have an elevated serum creatinine and hydronephrosis on ultrasound is directly proportional: Creatinine <115mmol/l: 0.8 per cent, creatinine 115-130mmol/l: 9 percent, and creatinine >130mmol/l: 33 per cent.2
Management
Conservative management:
Despite a range of different treatment options being available to men with BPH-related LUTS, some men do not have symptoms significant enough to warrant treatment. Many men presenting to primary care reporting LUTS may simply be seeking reassurance that their symptoms do not raise suspicion of a significant prostate cancer. In many men reporting LUTS, simple lifestyle modification may be sufficient to alleviate their symptoms.
Avoiding bladder irritants in the diet such as caffeine and alcohol, restricting fluid intake in the evening time in men with nocturia, and encouraging men to double void in cases of incomplete emptying can have a significant impact on symptoms. Very few men who report mild LUTS progress to develop significant complications as a result.3 For every six men who present with LUTS; three-out-of-six will remain static, two will progress, and one will improve.
Medical therapy:
Medical treatment is an option for men with more severe LUTS, or in patients who have not
responded to, or have progressed on conservative management. Adrenoreceptors which are located within the stroma and smooth muscle of the prostate gland and bladder neck control contractility. Alpha-1-blockers target the dynamic component of symptomatic BPH, in which the smooth muscle contraction within the prostate inhibits emptying of the bladder. Alpha1-blockers that are currently available are doxazosin, terazosin, alfuzosin, tamsulosin, silodosin, and naftopidil.
In contrast, 5-ARIs target the static component of BPH, where the mass effect of the enlarged prostate gland prevents bladder emptying. The mechanism of action lies in the inhibition of the 5α-reductase enzyme, which leads to apoptosis of prostate epithelial cells, thus reducing prostate size and facilitating unobstructed bladder emptying.
The benefit of 5-ARIs in comparison with A1-blockers is that 5-ARIs reduce the risk of disease progression, with lower levels of AUR and a reduced need for surgical intervention seen in patients treated with 5-ARIs when compared with placebo.4 However, the time to clinical benefit in patients treated with 5-ARIs is only evident following at least six months of treatment, whereas symptomatic benefit is seen within days of starting treatment with A1-blockers.
Surgical treatment:
In recent years, novel therapies have been developed in the surgical treatment of BPH. The prostatic urethral lift or urolift is one such treatment which has gained popularity in recent years. This procedure involves the insertion of multiple suture-based implants into the prostate under cystoscopic guidance with the aim of relieving prostatic obstruction of the
urethra and bladder. The benefit of urolift is significantly lower sexual side-effects when compared with transurethral resection of the prostate (TURP), however, symptomatic improvement is reported as less pronounced.5
Holmium enucleation of the prostate (HoLEP) and photoselective vaporisation of the prostate (PVP) offer comparable symptomatic relief and are more haemostatic in nature so can offer reduced rates of complications.
6,7
TURP remains the primary standard treatment for surgical intervention of LUTS secondary to BPH, and has remained so for >80 years.8 TURP utilises electrocautery to unobstruct the urethral channel by internally coring out the prostate. It is recommended in patients with moderate-to-severe BPH-related LUTS and an enlarged prostate.9
TURP can be performed using monopolar or bipolar electrocautery systems. Each system has advantages and disadvantages, however, bipolar is becoming an increasingly popular choice in Ireland and appears to have lower rates of transurethral resection (TUR) syndrome – a potentially lifethreatening complication related to fluid overload and dilutional hyponatraemia. This is due to the fact that saline irrigation is used as opposed to the glycine irrigation used in monopolar TURP. Patients undergoing TURP should be counselled that it is performed under general or spinal anaesthetic. It usually requires an inpatient stay of between three and five days, and a urethral catheter is necessary postoperatively for the first one-to-two days. A more detailed explanation of the procedure, the risks and benefits, and post-operative care is available on the British Association of Urological Surgery (BAUS) website.
Conclusion
Due to our ever-ageing population, and the prevalence of BPH in the community, there is an increasing burden on our healthcare system at both primary and secondary care levels in the investigation and management of this patient cohort. Prostatic growth is a seemingly unavoidable consequence of ageing. The ultimate goal of treatment in these men should always be about improving their quality-of-life and arresting the progression of disease.
References available on request
28 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Urology
PROGRESS Women in Surgery Fellowship
Dr Evelyn Murphy has been announced by the Royal College of Surgeons in Ireland (RCSI) as the recipient of the 2023 PROGRESS Women in Surgery Fellowship.
The prestigious bursary, awarded by RCSI and supported by Olympus Medical, promotes female participation in surgical training at Fellowship level.
Dr Murphy will undertake a Fellowship in Minimally Invasive Surgery and Orthopaedic Foot and Ankle Surgery in Sydney. It will incorporate percutaneous surgery, sports surgery, trauma and total ankle replacement, covering basic and complex fusions and osteotomies and forefoot reconstructions.
Dr Murphy is undertaking Higher Specialist Training in Trauma and Orthopaedics at RCSI, due for completion in 2023, and is currently posted at Galway University Hospital. She has four postgraduate qualifications, has published over 55 papers and has been awarded and shortlisted for more than 15 prizes, bursaries and research grants for her work.

Following the Fellowship, Dr Murphy will be one of the pioneers in this field in Ireland.
Dr Murphy commented: “I am delighted to receive the 2023 PROGRESS Women in Surgery Fellowship. I am honoured to be
Dr Evelyn Murphy, recipient of the 2023 PROGRESS Women in Surgery Fellowship
the first Orthopaedic recipient of this award. This initiative, sponsored by Olympus and facilitated through RCSI, will support me during the fellowship. I intend to develop key skills in minimally invasive foot and ankle surgery. This fellowship will allow me to continue to innovate in the field of orthopaedics. This award highlights the importance of continued inclusion in the practice of surgery. It is very encouraging to see increased female participation and overall diversity in surgery as whole.”
Now in its fourth year, the PROGRESS Fellowship aims to nurture and develop the expertise and skill base of Irish female surgeons, enabling them to gain international exposure in their chosen fields, acquire additional surgical skills, access new technologies and contribute to the advancement of surgical science and practice on the island of Ireland.
Dr Murphy’s Fellowship was announced by RCSI Vice President Professor Deborah McNamara and presented by RCSI President Professor Laura Viana as part of RCSI’s Charter Meeting celebrations.
RCSI President Professor Laura Viani said: “I congratulate Dr Evelyn Murphy on receiving this very significant Fellowship. The future for women in surgery is brighter than ever before, and this award is testament to that. Indeed, this is the first time in RCSI’s history that the President and Vice President roles have both been held by women. I look forward to following Dr Murphy’s achievements during her time in Australia and beyond.”
Alison Harvey, Country Manager of Olympus Medical, said:
"Olympus is proud to continue our partnership with RCSI to award the distinguished PROGRESS Fellowship for talented female surgical trainees. It is a privilege to support Dr Murphy to develop her clinical experience and become an international expert in her field. We believe this Fellowship makes an important contribution to both enhancing the skill base of surgeons, and improving patient outcomes, here in Ireland."
For more information about the PROGRESS Women in Surgery Fellowship, visit www.rcsi.com
New ¤190 m Bon Secours Limerick Hospital
The Taoiseach, Leo Varadkar, has welcomed a new ¤190 million Bon Secours hospital in Ballysimon, Limerick, to be opened in 2025, which will create 250 new jobs and greatly expand services offered to all patients in the Mid-West.
Part of a wider ¤300 million national investment by Bon Secours Health System in line with its 2025 Strategic Plan, the Limerick hospital expects to be able to treat more than 50,000 patients annually through a wide range of services and technologically advanced care offered by the new facilities.
Bon Secours Health System Group Chief Executive, Bill Maher, said, “This new facility will transform day-care surgical and oncology
treatments available in Limerick, provide new services such as general medicine, a new cath lab and cardiology, and widen patientcentred services to a growing local population. It is the latest development in our ¤300m national investment strategy, with the MidWest now set to further benefit from the high-quality healthcare and technologically advanced services that are the Bon Secours hallmark. The Taoiseach’s support today is a real endorsement of our ongoing efforts to provide the highest quality in service provision, combining the latest technologies and approaches with compassion and personalised medical care. Our ambition is to continue to build greater partnership with the public system to increase national capacity, improve services and
jointly tackle waiting lists providing good help to those in need.”
Key features of the new facility will include:
75 Inpatient beds (all single ensuite rooms)
four bed Special Observation Unit (single en-suite rooms)
20 Endoscopy Recovery Bays and three procedure rooms
20-bay Day Surgical Unit
radiology Department
four operating theatres
10-bay Medical Assessment Unit
dedicated Ophthalmology Unit
pre-assessment Unit
minor op rooms
Last year Bon Secours Health System announced a ¤300 million national investment in its services as part of a new strategy, “Resilience, Reliability and Readiness – The 2025 Plan”, which includes new capital projects, state-of-the-art equipment, IT infrastructure, and the creation of 450 new jobs across the country. As well as its new hospital plans for Limerick, Bon Secours has recently opened new ¤10 million operating theatres in Cork. A ¤16m new Day Ward and Oncology Unit in Dublin is set to open in June, with Bon Secours Tralee also planning to open a new ¤10 million Medical Assessment Unit and surgical day ward in 2024.
29 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
News
• Help your patients find balance between HbA1c reduction and hypoglycaemic risk
Offers
1-7
† Toujeo® is available in easy-to-use pens,1,8,9 to be administered once daily at any time of the day, preferably at the same time every day.1 When needed, patients can administer Toujeo® up to 3 hours before or after their usual time of administration. Flexible dosing time was evaluated in two randomised, open-label clinical studies, in patients with T2DM.1

Prescribing Information: Toujeo (insulin glargine 300 units/ml) Please refer to Summary of Product Characteristics (SmPC) before prescribing. Presentation: Toujeo SoloStar and DoubleStar pre-filled pens. Each ml contains 300 units of insulin glargine. SoloStar pen contains 1.5ml (450 units) of solution for injection. DoubleStar pen contains 3ml (900 units) of solution for injection. Indication: Treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years. Dosage and Administration: Toujeo is administered subcutaneously, by injection into the abdominal wall, the deltoid or the thigh, once daily, at any time of the day, preferably at the same time every day. Injection sites must be rotated within a given injection area from one injection to the next in order to reduce the risk of lipodystrophy and cutaneous amyloidosis. The dose regimen (dose and timing) should be adjusted according to individual response. Do not administer intravenously. In type 1 diabetes mellitus, Toujeo must be combined with short-/rapidacting insulin to cover mealtime insulin requirements. In patients with type 2 diabetes mellitus, recommended daily starting dose is 0.2 units/kg followed by individual dose adjustments. Toujeo can also be given together with other anti-hyperglycaemic medicinal products. Switch between insulin glargine 100 units/ml and Toujeo: Insulin glargine 100 units/ml and Toujeo are not bioequivalent and are not directly interchangeable. When switching from insulin glargine 100 units/ml to Toujeo, this can be done on a unit-to-unit basis, but a higher Toujeo dose (approximately 10-18%) may be needed to achieve target ranges for plasma glucose levels. When switching from Toujeo to insulin glargine 100 units/ml, the dose should be reduced (approximately by 20%).
Switching from other basal insulins to Toujeo: A change of dose and/or timing of the basal insulin and concomitant anti-hyperglycaemic treatment may be required. Dose adjustments may also be required if the patient’s weight or lifestyle changes, the timing of insulin dose is changed, or other circumstances arise that increase susceptibility to hypo- or hyperglycaemia. Toujeo must not be mixed or diluted with any other insulin or other medicinal products. Close metabolic monitoring is recommended during a switch and in the initial weeks thereafter. SoloStar 1-80 units per single injection in steps of 1 unit and DoubleStar 2-160 units in steps of 2 units. When changing from Toujeo SoloStar to Toujeo DoubleStar, if the patient’s previous dose was an odd number, then the dose must be increased or decreased by 1 unit. Toujeo DoubleStar prefilled pen is recommended for patients requiring at least 20 units per day. Special Populations: Insulin requirements may be diminished in the elderly or patients with renal or hepatic impairment. Paediatric: When switching basal insulin to Toujeo, dose reduction of basal and bolus insulin needs to be considered on an individual basis, in order to minimise the risk of hypoglycaemia. The safety and efficacy of Toujeo in children and adolescents below 6 years of age have not been established. Contraindications: Hypersensitivity to insulin glargine or any excipients.

Precautions and Warnings: Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Toujeo is not the insulin of choice for treatment of diabetic ketoacidosis. Patients must be instructed to perform continuous rotation of the injection site to reduce the risk of developing lipodystrophy and cutaneous amyloidosis. There is a potential risk of delayed insulin absorption and worsened glycaemic control following insulin injections at sites with these reactions. A sudden change in the injection site to an unaffected area has been reported to result in hypoglycaemia. Blood
Intended for Healthcare Professionals in the Republic of Ireland only.

glucose monitoring is recommended after the change in the injection site, and dose adjustment of antidiabetic medications may be considered. Hypoglycaemia: In case of insufficient glucose control or a tendency to hyper/hypoglycaemic episodes, the patient’s adherence to the prescribed treatment regimen, injection sites and proper injection technique and all other relevant factors must be reviewed before dose adjustment is considered. Particular caution should be exercised, and intensified blood glucose monitoring is advisable for patients in whom hypoglycaemic episodes might be of clinical relevance and in those where dose adjustments may be required. Warning signs of hypoglycaemia may be changed, less pronounced or absent in certain risk groups, potentially resulting in severe hypoglycaemia and loss of consciousness. Risk groups include patients in whom glycaemic control is markedly improved, hypoglycaemia develops gradually, an autonomic neuropathy is present, or who are elderly. The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia. Intercurrent illness: Requires intensified metabolic monitoring and often it is necessary to adjust the insulin dose. Insulin antibodies: administration may cause insulin antibodies to form. Use with pioglitazone: Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs. Medication errors: Insulin labels must always be checked before each injection to avoid errors between Toujeo and other insulins. Patients must be instructed to never use a syringe to remove Toujeo from the SoloStar or DoubleStar prefilled pen, A new sterile needle must be attached before each injection. Needles must not be re-used. Pregnancy and lactation: There are no data from exposed pregnancies in controlled clinical trials. However, there is a large amount of data on use of insulin glargine 100 units/ml in pregnant women indicating no specific adverse effects on pregnancy and no specific malformative nor feto/neonatal toxicity. The use of Toujeo may be considered during pregnancy, if clinically needed. Careful monitoring of glucose control is essential. It is unknown if insulin glargine is excreted in breast milk. Interactions: Substances that affect glucose metabolism may require adjustment of insulin glargine. Adverse Reactions: Very common: Hypoglycaemia. Prolonged or severe hypoglycaemia may be life-threatening. Common: Lipohypertrophy, injection site reactions, including redness, pain, itching, hives, swelling, or inflammation. Prescribers should consult the SmPC in relation to other adverse reactions. Legal Category: POM. Marketing Authorisation Number: SoloStar 5 pen pack: EU/1/00/133/035; DoubleStar 5 pen pack: EU/1/00/133/038. Marketing Authorisation Holder: Sanofi Aventis Deutschland GmbH, D-65926 Frankfurt am Main, Germany. Further information is available from: Medical Information, Sanofi, 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com. Date of preparation: July 2022. MAT-IE-2200355 (V1.0)
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com
Abbreviations: HbA1c, Hemoglobin A1c; PD, Pharmacodynamics; PK, Pharmacokinetics; T1DM, Type 1 Diabetes Mellitus; T2DM, Type 2 Diabetes Mellitus.
References: 1. Toujeo® Summary of Product Characteristics July 2020. 2. Home PD, et al. Diabetes Care 2015;38(12):2217-2225. 3. Matsuhisa M, et al. Diabetes Obes Metab 2016;18(4):375-383. 4. Danne T, et al. Diabetes Care 2020; 43(7):1512–1519. 5. Riddle MC, et al. Diabetes Care 2014;37:2755-2762.
6. Yki-Jarvinen H, et al. Diabetes Care 2014;37:3235-3243. 7. Bolli GB, et al. Diabetes Obes Metab 2015;17:386-394. 8. Singh R, et al. Eur Endocrinol 2018;14:47-51. 9. Pohlmeier H, et al. J Diabetes Sci Technol 2017;11;263-269.
MAT-IE-2200486 (v1.0) | December 2022
•
flexibility † (+/- 3 hours) in dosing time1,8,9
FOR THE TREATMENT OF DIABETES MELLITUS IN ADULTS, ADOLESCENTS AND CHILDREN FROM THE AGE OF 6 YEARS1
From the start,1 there to help your patients on their basal insulin journey1 from 6 years
Toujeo® DoubleStar™ prefilled pen is recommended for patients requiring at least 20 units per day.1
CPD CPD
60 Second Summary
Diabetes mellitus (DM) is a heterogeneous complex metabolic condition characterised by hyperglycaemia with a degenerative potential resulting from changes in the production, secretion and/or inability of insulin to adequately exercise its effects.
DM is a leading cause of death globally and described as the most challenging health problem in the 21st century, driven primarily by rising levels of obesity and an ageing population.

This audit was conducted over one day in March 2022. The audit was approved by the local Clinical Audit Committee, piloted on two inpatients, and communicated to all data collectors prior to commencement. Generated data were anonymous and securely stored. Independent analysis was conducted by three researchers to confirm reliability of results.
This study identified 17% of inpatients had DM on audit day in GUH of which 9% were insulin dependent and 55% of all inpatients with DM were treated with insulin. This is similar to the most recently published National Health Service (NHS) National Diabetes Inpatient Audit (NaDIA) England 2019 report which found 18% of all inpatients in 188 NHS hospitals had documented DM.
Whilst the majority of inpatients were prescribed a meal time supplement, it was only documented clearly by nurses for approximately one in every three inpatients potentiating administration errors and significant patient harm.
Future quality improvement interventions for consideration to optimise patient care include implementing a dedicated insulin safety team that comprises a broad membership to facilitate sustainability and spread of interventions to improve insulin prescribing and administration practice and electronic prescribing as a part of the ongoing implementation of a new pharmacy system.
AUTHORS: D. Hogan-Murphy1, L. Reddington2, M.C. Dennedy2,3, L. Egan3,4, J. Okiro2, J. Given1, S. Donnellan1, M.R. Salehmohamed2
1. Department of Pharmacy, Galway University Hospitals, Galway, Ireland
2. Department of Endocrinology, Diabetes and Metabolism, Galway University Hospitals, Galway, Ireland
3. Department of Pharmacology and Therapeutics, School of Medicine, National University of Ireland, Galway, Ireland
4. Department of Gastroenterology, Galway University Hospitals, Galway, Ireland
1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
Insulin prescribing, administration, and glucose monitoring trends in a hospital setting
ABSTRACT
Aims: Insulin is a high-alert critical medication which can cause significant patient harm when used inappropriately. The aim of this study was to conduct a prospective audit on insulin prescribing, administration, and glucose monitoring trends in Galway University Hospitals.

Methods: This audit was conducted over one day in March 2022. The audit was approved by the local Clinical Audit Committee, piloted on two inpatients, and communicated to all data collectors prior to commencement. Generated data were anonymous and securely stored. Independent analysis was conducted by three researchers to confirm reliability of results.
Results: Four hundred and fifty-four inpatients were reviewed of which 17% [75] had diabetes and 9% [41] were prescribed insulin. The overall insulin error rate with one or more errors comprising prescribing and/or administration per inpatient drug record was 90% [37]. In total, 95% [235] insulin brand names and 89% [220] dose units were clearly prescribed, 84% [208] administration times were clearly specified by a prescriber, 87% [214] orders were signed, 58% [25] prescribers clearly documented their registration number/bleep/name at least once for contact purposes, 35% [30] meal time supplements were documented clearly by a nurse, 70% [202] administrations were double checked by a second person, 53% [142] administration times were documented by a nurse, and 26% [10] of inpatients were administered insulin by a nurse when not prescribed.
Conclusion: Results will assist in developing quality improvement initiatives to optimise patient care.
Introduction
Diabetes mellitus (DM) is a heterogeneous complex metabolic condition characterised by hyperglycaemia with a degenerative potential resulting from changes in the production, secretion and/or inability of insulin to adequately exercise its effects1 The most common classifications include Type 1 DM and Type 2 DM, the latter accounting for more than 90% of all cases2. Type 2 DM is characterised by insulin resistance and a relative deficiency of insulin secretion which progressively worsens over time3,4 Type 1 DM results in an absolute deficiency in beta-cell function with autoimmune destruction
of beta-cells a common origin5
Current estimates suggest more than half a billion adults live with DM worldwide, a rise of 16% since previous estimates in 20196. This is predicted to escalate to almost 800 million by 20456. In Ireland, in the absence of a national DM registry, the current approximate projection is 5.6%7
DM is a leading cause of death globally8 and described as the most challenging health problem in the 21st century9,10 driven primarily by rising levels of obesity and an ageing population9,11. A systematic review and meta-analysis on the epidemiology of DM and its complications amongst adults in Ireland found variables from
7–25% for retinopathy; 3–32% for neuropathy; and 3-5% for nephropathy12. The economic burden also plays heavily with Ireland ranked 7 th in the world for DM related health expenditure per person7, and as high as ¤1.4 billion annually with costs mostly associated with hospitalisations and treatment of complications6 Insulin is a high-alert medicine used in the treatment of DM which bears a heightened risk of causing significant patient harm. With limited Irish data availability and few local incidents reported, anecdotal evidence suggests insulin accounts for a substantial number of medication errors13,14. A review by the States
31 CPD 97: DIABETES
4 previous steps, log and record your findings. Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
Continuing Professional Development
Claims Agency of over 20,000 medication incidents reported by Irish acute hospitals in 2017 and 2018 found insulin was the fourth most commonly implicated therapeutic subgroup15, many comprising omissions leading to hyperglycaemia and inaccurate dosing leading to hyperglycaemia and hypoglycaemia16
Insulin has been identified as a significant medication safety concern in Galway University Hospitals (GUH). The aim of this study was to conduct a prospective audit on insulin prescribing, administration, and glucose monitoring trends in GUH in order to identify and develop agreed quality improvement initiatives to enhance patient care.
Methods
A prospective audit on insulin prescribing, administration, and glucose monitoring was conducted over one day in March 2022 on 24 wards in GUH. GUH comprises University Hospital Galway (UHG), a Model 4 public hospital, and Merlin Park University Hospital (MPUH), a Model 2 public hospital, and provides a comprehensive range of services to emergency and elective patients within the Saolta University Healthcare Group in the West of Ireland.
Inclusion criteria comprised inpatients prescribed/administered insulin in UHG and MPUH for the previous 72 hours until 9am on the morning of audit. Exclusion criteria comprised non-admitted patients, Day Wards, Emergency Department, Acute Medical Unit, Short Stay Unit, Emergency Surgical Unit, Critical Care including Post Anaesthetic Care Unit, Maternity Department, and Psychiatry Department. Content of the audit protocol and tool was informed by the research objective, local practices, and existing evidence-based international and national literature. The audit tool
Overall insulin error rate per inpatient drug record
The overall insulin error rate with one or more errors comprising prescribing and/or administration per inpatient drug record was 90% [n=41] as presented in Table 1.
Table 1: Overall insulin error rate per inpatient drug record
Insulin prescribing patterns
was piloted on a medical ward in UHG with two random inpatients prescribed and administered insulin. Minor amendments were made to its content and the pilot was excluded from data analysis.
Results
Nineteen inpatients [n=37; 51%] were prescribed the same insulin as pre-admission, four inpatients [n=37; 11%] were not prescribed the same insulin as pre-admission, and 14 inpatients [n=37; 38%] were not on insulin pre-admission and were either prescribed a meal time supplement [10 inpatients; n=37; 27%] or were newly prescribed insulin on admission [4 inpatients; n=37; 11%].
The audit was led by two lead researchers and conducted by 29 data collectors comprising endocrine consultant and nonconsultant hospital doctors, diabetes nurse specialists, and pharmacists. The audit tool was guided by the protocol which was communicated to all data collectors prior to the audit via video conferencing and faceto-face meetings and emails. This audit was conducted in accordance with the HSE Code of Governance (2021) and HSE Healthcare Audit Quality Assurance
and Verification Standards (2019) and was approved by the GUH Clinical Audit Committee prior to commencement. All audit forms were anonymous and securely stored in a locked cabinet and all generated data were securely stored on an encrypted password protected work computer. Any audit records will be destroyed after full dissemination of audit findings. Independent analysis was conducted by the two primary researchers and a specialist registrar in endocrinology to confirm reliability of results. This process involved independently inputting content of paper audit forms into excel, analysing data, and comparing results. No significant discrepancies were identified.
General participation and prevalence
A total of 247 insulin doses were prescribed of which 235 orders [95%] had the insulin name clearly documented Two hundred and twenty [89%] insulin dose units were clearly prescribed, 208 [84%] administration times were clearly specified by a prescriber, and 214 [87%] orders were signed by a prescriber (Figure 2).
In total, 454 inpatients were reviewed of which 41 [9%] were prescribed insulin and included in the audit. The number of inpatients using an Insulin and Glucose Monitoring Record was 117 [26%] of which 75 inpatients [17%] had a documented history of DM (Figure 1). This equates to 55% of all inpatients with DM were treated with insulin. Patient specialty comprised medical [27, 66%], surgical [12, 29%], and paediatric [2, 5%].
Overall insulin error rate per inpatient drug record
DM/Insulin Prevalence GUH
Number of inpatients documented with DM
Number of inpatients on Insulin
Number of inpatients using an Insulin and Glucose Monitoring Record
Number of inpatients excluded at time of data collection
Number of inpatients on ward
Overall insulin error rate per inpatient drug record
The overall insulin error rate with one or more errors comprising prescribing and/or inpatient drug record was 90% [n=41] as presented in Table 1.
32 CPD 97: DIABETES
Figure 1: DM/Insulin prevalence GUH
Error description Error type Insulin brand incorrect 0% Prescribing error 80% Insulin error 90% Insulin name not clearly prescribed 5% Insulin dose units not clearly prescribed 11% Insulin administration times not clearly specified by the prescriber 16% Insulin orders not signed by the prescriber 13% Prescriber MCRN/bleep/name unclear for contact purposes 42% Meal time supplement documented incorrectly 65% Insulin administration not double checked by a 2nd person 30% Administration error 89% Insulin administration times not documented 47% Insulin administered when not prescribed 26%
Table 1 Overall insulin error rate per inpatient drug record
Figure 1: DM/Insulin prevalence GUH
Figure 1: DM/Insulin prevalence GUH
508 54 117 41 75
Insulin Prescribing Patterns
What number of insulin orders have been signed by the prescriber?
What number of insulin administration times are clearly specified by the prescriber?
What number of insulin dose units are clearly prescribed?
What number of insulin orders have the name clearly prescribed?
What number of insulin doses have been prescribed?
The overall insulin error rate with one or more errors comprising prescribing and/or administration per inpatient drug record was 90% [n=41] as presented in Table 1.
Insulin prescribing patterns
person. Administration times were documented for 142 doses [n=270; 53%] as illustrated in Figure 3. Ten inpatients [n=39; 26%] were administered insulin by a nurse when not prescribed.
Glucose management
Twenty-five prescribers [n=43; 58%] clearly documented their medical council registration number (MCRN)/bleep/name on the insulin drug record at least once for contact purposes and 25 inpatients [61%] had the meal time supplement signed by a prescriber. The meal time supplement was documented clearly 30 times out of a total of 86 entries [35%].
Insulin administration patterns
Nineteen inpatients [n=37; 51%] were prescribed the same insulin as pre-admission, four inpatients [n=37; 11%] were not prescribed the same insulin as pre-admission, and 14 inpatients [n=37; 38%] were not on insulin pre-admission and were either prescribed a meal time supplement [10 inpatients; n=37; 27%] or were newly prescribed insulin on admission [4 inpatients; n=37; 11%].
Insulin Administration Patterns
A total of 247 insulin doses were prescribed of which 235 orders [95%] had the insulin name clearly documented. Two hundred and twenty [89%] insulin dose units were clearly prescribed, 208 [84%] administration times were clearly specified by a prescriber, and 214 [87%] orders were signed by a prescriber (Figure 2).
What number of doses with administration times are documented?
The GUH DM team reviewed/was contacted to review 21 inpatients insulin drug record [55%, n=38]. Twenty-five inpatients [61%; n=41] had changes to their insulin regimen made during their inpatient stay. This included changes to a brand name of insulin [4; n=37; 11%], the initiation of insulin and/or a meal time supplement [14; n=37; 38%], and/or a dose change [9; n=32; 28%; median 3; range 1-6]. Three inpatients had their insulin omitted after an episode of hypoglycaemia (blood glucose <4 mmol/L).
Discussion
What number of doses are double checked by an independent second person?
All inpatients not prescribed the same insulin as pre-admission were altered by the DM team during admission and were therefore appropriately changed. The remaining inpatients were not on insulin pre-admission and were either prescribed a meal time supplement or were newly prescribed insulin on admission. The overall insulin error rate comprising both prescribing [80%] and administration [89%] per inpatient drug record was 90%. This is significantly higher than the NaDIA 2019 report which found 18% of inpatient drug records had one or more insulin errors17 This figure is also higher than findings from an analysis of the National Reporting and Learning
Two hundred and eighty-seven doses were administered of which 202 [70%] were double checked by an independent second person. Administration times were documented for 142 doses [n=270; 53%] as illustrated in Figure 3 Ten inpatients [n=39; 26%] were administered insulin by a nurse when not prescribed.
System database of patient safety incidents concerning insulin reported from NHS providers in England and Wales over a six year period which found 61% of incidents occurred at the administration stage and 17% at the prescribing stage19 The most common medication error types were wrong dose, strength, or frequency followed by omitted or delayed insulin19 Most prescriptions in this audit had the insulin name, dose, and administration times clearly documented and signed by the prescriber which are positive findings. However, less than three out of five prescribers clearly documented their MCRN/ bleep/name on the insulin drug record at least once for contact purposes leading to possible time delays and patient care issues if communication is required. Whilst the majority of inpatients were prescribed a meal time supplement, it was only documented clearly by nurses for approximately one in every three inpatients potentiating administration errors and significant patient harm. Almost one in three insulin doses were also not double checked by an independent second person and almost half administration times were not documented. Insulin is a highalert medication which requires a two-person check of both dose
What number of doses have been administered?
Glucose management
Twenty-five prescribers [n=43; 58%] clearly documented their medical council registration number (MCRN)/bleep/name on the insulin drug record at least once for contact purposes and 25 inpatients [61%] had the meal time supplement signed by a prescriber. The meal time supplement was documented clearly 30 times out of a total of 86 entries [35%].
Insulin administration patterns
Two hundred and eighty-seven doses were administered of which 202 [70%] were double checked by an independent second
This study identified 17% of inpatients had DM on audit day in GUH of which 9% were insulin dependent and 55% of all inpatients with DM were treated with insulin. This is similar to the most recently published National Health Service (NHS) National Diabetes Inpatient Audit (NaDIA) England 2019 report which found 18% of all inpatients in 188 NHS hospitals had documented DM17 The NaDIA England and Wales 2015 report identified a lower prevalence of 6% of all inpatients in 206 NHS hospitals were insulin dependent and 36% of inpatients with DM were treated with insulin18 The majority of inpatients in this audit were medical and prescribed the same insulin as pre-admission.
The GUH DM team reviewed/was contacted to review 21 inpatient five inpatients [61%; n=41] had changes to their insulin regimen made during their included changes to a brand name of insulin [4; n=37; 11%], the initiation of insulin and/or a meal time supplement [14; n=37; 38%], and/or a dose change [9; n=32; 28%; their insulin omitted after an episode of hypoglycaemia (blood glucose <4 mmol/L).
33
Figure 2: Insulin prescribing patterns
Figure 3: Insulin administration patterns
247 235 220 208 214
202 142
Figure 2: Insulin prescribing patterns
preparation and administration at the bedside as well as precise administration timing to minimise hypoglycaemia, hyperglycaemia, wide glycaemic excursions, and diabetic ketoacidosis.
More than one in four inpatients were administered insulin by a nurse when not prescribed. A nurse may only administer a non-prescribed medication in a situation that requires immediate intervention in lifethreatening situations and there is no immediate access to an appropriate prescriber. All insulin doses should be prescribed prior to nurse administration.
The GUH DM team reviewed or was contacted to review more than half of the inpatients prescribed insulin. More than three out of five inpatients had changes to their insulin regimen made during their inpatient stay including brand of insulin, the initiation of insulin, and dose changes. Three inpatients had their insulin omitted after an episode of hypoglycaemia. This is lower than a study evaluating insulin information on discharge summaries in a United Kingdom (UK) hospital which found 33 out of 42 patients [79%] had changes made including the initiation and/or discontinuation of insulin therapy, insulin dose changes, and insulin preparation/brand20
Based on audit results as well as evidence from local insulin error reporting, local practices, and best practice, some of the current interventions implemented specific to insulin in GUH with support from hospital management and
Future quality improvement
Insulin Prescribing Patterns
interventions for consideration to optimise patient care include implementing a dedicated insulin safety team that comprises a broad membership to facilitate sustainability and spread of interventions to improve insulin prescribing and administration practice; electronic prescribing as a part of the ongoing implementation of a new pharmacy system; an insulin inpatient and discharge checklist; and promotion of an annual national audit on insulin prescribing, administration, and glucose monitoring trends similar to the UK. Once interventions are in situ it is anticipated that results of a reaudit in GUH will be favourable.
Declaration of Conflicts of Interest
The authors report no conflict of interest.
Corresponding Author:
Figure 2: Insulin prescribing patterns
Dr Diana Hogan-Murphy
Department of Pharmacy University Hospital Galway Galway
Insulin administration patterns
medication safety committees which are transferrable to other hospitals include an updated inpatient Insulin and Glucose Monitoring Drug Record; recruitment of a clinical pharmacist with a special interest in diabetes; implementation of mandatory e-learning for nurses and doctors on insulin prescribing and administration; updated/newly approved policies, procedures, protocols, and guidelines; piloting of a self-administration
policy; continuous education and training to patients as well as medical, nursing, and pharmacy undergraduate students and employees in GUH; expansion of a medication safety portal inclusive of educational videos; use of hospital screens and social media to disseminate prudent information; and further promotion of error reporting. No challenges were encountered as all key stakeholders were involved from the onset.
E-Mail: diana.hogan-murphy@hse.ie
Twenty-five prescribers [n=43; 58%] clearly documented their medical council (MCRN)/bleep/name on the insulin drug record at least once for contact purposes and had the meal time supplement signed by a prescriber. The meal time supplement was 30 times out of a total of 86 entries [35%].
References available on request Figure 3: Insulin administration patterns
Two hundred and eighty-seven doses were administered of which 202 [70%] were double independent second person. Administration times were documented for 142 doses illustrated in Figure 3 Ten inpatients [n=39; 26%] were administered insulin by a nurse
Insulin Administration Patterns
What number of doses with administration times are documented?
What number of doses are double checked by an independent second person?
What number of doses have been administered?
Figure 3: Insulin administration patterns
Glucose management
142
202
287
The GUH DM team reviewed/was contacted to review 21 inpatients insulin drug record five inpatients [61%; n=41] had changes to their insulin regimen made during their included changes to a brand name of insulin [4; n=37; 11%], the initiation of insulin
34 CPD 97: DIABETES
235
208 214
220
Cancer Inequalities and Deprivation
Cancer inequalities in Ireland 2004 -2018
The National Cancer Registry (NCRI) has published a new report entitled: Cancer inequalities in Ireland by deprivation, 2004-2018. This report measures differences in cancer incidence, five-year survival and stage at presentation between populations living in the most and least deprived areas in Ireland for the diagnosis period 2014-2018. Comparative information is also provided for earlier periods (20042008 and 2009-2013).
The report can be found at www.ncri.ie
Key findings include:
• Overall, there was a 7% higher age-standardised incidence for males and a 5% higher incidence for females living in most deprived areas compared to those living in the least deprived areas in 2014-2018
• Overall, there was lower fiveyear cancer survival rates in patients from the most deprived areas compared to those in the least deprived areas. Those in the most deprived areas had a 28% higher mortality risk due to cancer within five years of cancer diagnosis compared to those in the least deprived areas, having adjusted for age, gender, and cancer type.
• Differences in the types of cancers diagnosed in the most and least deprived areas, with a higher incidence of stomach, lung and cervical cancer in people living in most deprived areas, while those living in least deprived areas show a higher incidence of breast, prostate, and melanoma and nonmelanoma skin cancer.
• People living in the most deprived areas had a higher risk of late-stage presentation for breast and prostate cancers than those living in least deprived areas. No disparities in stage of presentation were found for lung or colorectal cancers when comparing the least and most deprived groups.
• Although cancer incidence rates have fallen and survival rates have improved over time across the majority of cancers and for all sectors of society, there is no evidence of any reduction in disparities between those living in the least and the most deprived areas.
Age-standardised cancer incidence: most vs. least deprived

Summary Figure 1.
Age-standardised cancer incidence, Ireland, 2014 -2018: comparison between the most and the least deprived quintiles.
Arrows indicate direction of difference and significance at the 95% confidence level
What was found?
Five-year survival
• The most deprived quintile of the population in 2014-2018 had significantly poorer five-year survival (mortality hazard 28% higher than the least deprived quintile ) for cancer as a whole, with similar findings for the two earlier periods.
• Five-year survival was poorer for the most deprived quintile of the population compared with the least deprived quintile for colorectal, lung, melanoma, breast and prostate cancers for the most recent period 2014-2018 and (with the exception of melanoma) for the two earlier periods.
• There was no significant narrowing or widening of survival disparities over time.
• This data is summarised in Figure 2.
A range of potential factors may contribute to such disparities, including differences in general health, exposure to particular risk factors, health-seeking behaviour (influencing early detection), access to healthcare, or other factors that may be linked to socioeconomic or geographic factors. Disentangling these factors and their relative importance is far from straightforward, and many challenges remain in tackling the root causes of such inequalities.
Chair of the NCRI Board Dr Jerome Coffey, welcomed the report and said, “These important data
underline the known links between socioeconomic deprivation and cancer incidence and survival, with no major reductions in disparities between groups over the time periods examined. Prevention, screening and early diagnosis are major elements of the National Cancer Strategy 2017-2026 and will have to remain as priorities in subsequent strategies.”
NCRI co-author Dr Niamh Bambury said, “There is an increased focus on cancer inequalities experienced across the cancer continuum as part of Europe’s Beating Cancer Plan. Monitoring cancer inequalities
8
is important to identify groups who might benefit from targeted risk reduction interventions and to assess the impact of cancer strategies. This report provides the most up-to-date, reliable information on the effect of deprivation on cancer incidence and five-year survival. The socioeconomic and individual factors that contribute to cancer disparities are manifold and require a whole system response. In the health field, advancement of policy initiatives, including Sláintecare and Healthy Ireland, are needed to help address the root causes of these disparities.”
35 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
Report
Colon Capsule Endoscopy- Establishing it’s Role in Clinical Practice
Over 20 years since its introduction, small bowel capsule endoscopy (SBCE) is now well established in many diagnostic algorithms, most notably obscure gastrointestinal bleeds.1 Colon capsule endoscopy (CCE) is a newer technique, with it’s second-generation system (PillCam COLON 2) only becoming commercially available in 2012.2-8 EGuidance from the European Society of Gastrointestinal Endoscopy (ESGE), updated in 2020, provided the first framework for healthcare providers with regards to indications, preparation, reporting and work up of findings for CCE.9 It advises that CCE is safe and appears accurate when used in average-risk individuals, and safe for use in those with incomplete colonoscopy and without stenosis. They recommend use of CCE if colonoscopy is not appropriate or not possible. They advise that there remains a paucity of studies based in the setting of screening, and in comparing CCE with radiological imaging or traditional endoscopic modalities.9
Polyp Detection and Colorectal Cancer Surveillance

National screening programmes face significant challenges in attempting to deliver timely and cost-effective screening. Bowel Screen is a two-stage population
based programme that currently offers free bowel screening to men and women aged 60-69 years in Ireland. National colonoscopy capacity has been a rate limiting step in expansion of this programme to those aged 55-74 years. Patient acceptance of colonoscopy as a screening tool is also low with uptake as low at 11% in certain subgroups of invitees.10 Effective, alternative screening pathways are required.
To date, CCE is playing a marginal role in screening but there is a growing body of evidence supporting its use. A recent systematic review of its use in a screening population by Vuik et al included 13 studies, comprising 2485 patients.11 Eight studies used CCE as a filter test after a positive FIT result and five studies used CCE for primary screening. The polyp detection rate of CCE was 24 % - 74 %. For polyps > 6 mm, sensitivity of CCE was 79 % - 96 % and specificity was 66 % - 97 %. For polyps ≥ 10 mm, sensitivity of CCE was 84 % - 97 %, which was superior to CTC. The CRC detection rate for completed CCEs was 93 %. They concluded that the accuracy of CCE was comparable to colonoscopy and superior to CTC, making it a good alternative for screening programmes. A more recent meta-analysis by
Kjolhede et al comparing CCE and colonoscopy with regards to polyp detection concluded that CCE did show high sensitivity and specificity for per-patients polyps compared to colonoscopy.12 CCE does perform well with regards to cancer detection.8, 13-16 In two studies, however, cancers were missed in the left colon due to the capsule battery dying and the capsule not viewing these areas.17, 18 There remains limited data regarding the ability of both CCE and CTC to detect laterally spreading tumours (LSTs) with good sensitivity and specificity.21,22 These non-polypoid lesions are also more difficult to pick up at traditional colonoscopy. CCE had a sensitivity of 0.86 for non-polypoid tumours in one review.21
Incomplete colonoscopies
Incomplete colonoscopies can occur in up 20% of patients and are associated with higher rates of missed lesions.23, 24 Reasons for incomplete colonoscopy include female gender, elder patients, long, redundant sigmoid colons, excessive looping, patient discomfort and a history of previous abdomino-pelvic surgeries.24, 25, 26 Four prospective studies have looked specifically at this group, with large number of polyps identified by CCE-2 in areas not reached by initial colonoscopy.27, 28, 29, 30 ESGE guidance now endorses the use of CCE in this situation.9
A prospective, single-centre, randomized trial, the VICOCA study, compared CCE and CTC in 290 individuals, using colonoscopy as the gold standard.31 It reported sensitivity, specificity and positive and negative predictive values of CCE for the detection of patients with any neoplastic lesion of 98.1%, 76.6%, 93.7% and 92.0%, respectively. CTC had sensitivity, specificity and positive and negative predictive values of 64.9%, 95.7%, 96.8% and 57.7%, respectively. In terms of detection of polyps > 6mm, the sensitivity of CCE and CTC was 96.1% and 79.3%. CCE was shown have
superior sensitivity for detecting serrated lesions (73.6% versus 32.9%; p < 0.001). Similarly the TOPAZ trial concluded that CCE should be considered comparable, if not superior, to CTC as a screening test.32
Excretion and Completion Rates


Preparation is of particular interest in CCE given there is no opportunity to flush the mucosa or suction as with colonoscopy, and adequate propulsion is required to ensure the capsule is excreted prior to the battery dying. The quality of the protocol is dependent on many factors including the laxative used, timing and volume of laxative, types of boosters used and timing of boosters and also patient tolerability. Great variation exists between studies with regards to excretion rates and bowel cleanliness. A recent meta-analysis found that excretion rates varied from 57%- 100% and adequate bowel prep ranged from 40%-100% and concluded that improvements are needed in both areas before widespread implementation of CCE into surveillance programmes.12 Low -volume PEG preparations, sodium phosphate free boosters, prokinetics and suppositories are now used with good success, reducing the volume of fluid the patients have to ingest without compromising polyp detection.33, 34 Prucalopride has been recently shown to improve CCE completion rates and polyp detection rates, as has castor oil.35, 36, 37, 38
Safety
CCE is a safe procedure with the main consideration being capsule retention. The majority of the studies to date report no preparation or capsule related adverse events.11, 12, 13, 39, 40, 41 Adverse events reported are generally due to the preparation and include nausea, vomiting or bloating.15, 16, 18, 20, 33 Baltes described one retained capsule in the ileum in a patient who subsequently had it removed
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 36 GASTROENTEROLOGY: ENDOSCOPY
Eimear Gibbons
Written by E. Gibbons, O.B. Kelly, B. Hall Department of Gastroenterology, Connolly Hospital, Blanchardstown
Barry Hall

HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication
ENDOCRINOLOGY: ENDOSCOPY
during resection for previously unknown Crohn’s disease.27
Capsule aspiration is a rare but documented complication which may occur in 0.1% of cases.42
Patient preference
CCE does appear to be more acceptable than colonoscopy.40, 43, 44, 45 A recent interim analysis asked over 14,000 participants in a screening programme (prior to their FIT result) whether they would prefer CCE or colonoscopy, with 50% choosing CCE versus 9% choosing colonoscopy.46 Disadvantages reported include longer wait time for results and unfamiliarity with the technology.43
Cost
An up-to-date analysis of costeffectiveness is needed. Recent ESGE guidance advised that CCE may be cost effective if it improves engagement in surveillance programmes.9 Hassan et al also
concluded that CCEs effect on cost of screening lies it in ability to improve compliance.47

Future for CCE
CCE has been demonstrated to be comparable to traditional colonoscopy and better than CTC for detection of colonic pathology.12 In comparative studies it has been shown to be useful in both symptomatic participants and after incomplete colonoscopy. It is not without drawbacks, however, and certain areas, including bowel preparation as previously described, require particular attention.
A large proportion of those who undergo CCE still require follow up with colonoscopy or sigmoidoscopy.44 Reasons for this include need for polypectomy, inadequate views of the mucosa or the battery dying before being excreted. Along with improving bowel preparation regimens and battery life, the use of FIT testing as a triage tool could help reduce
this burden. The optimum FIT level at which referral directly to CCE would be appropriate is yet to be determined.
The reading of colon capsules is time consuming and currently a rate limiting step in the expansion of capsule services in hospitals. Vuik et al reported a median time of 55 minutes for colon capsule reading.48 The combination of advances in artificial intelligence (AI) technologies, commercial interest and a growing body of supporting evidence suggests that the use of AI to augment day to day endoscopy will become a reality in the near future. AI software for colon capsules is currently in its infancy. It should help greatly with regards to polyp detection and also in speeding up reading.
Outside of polyp detection and screening, there is also a growing body of evidence for the use of CCE in inflammatory bowel disease (IBD) which is to be further explored. ESGE recent 2020 guidance advises there is currently insufficient data to support use of

CCE in diagnosis or surveillance of those with suspected or known IBD, however noted current preliminary data suggests it may of use in monitoring of disease activity in UC.9
Now the accuracy of CCE has been established, investment in training and artificial intelligence, along with continued robust data regarding efficacy are necessary to ensure CCE finds its place in routine clinical practice. Large scale initiatives, like the use of CCE in the National Health Service (NHS) UK urgent cancer pathway, will provide a wealth of further information in the coming months and years.44 Despite drawbacks, CCE is a viable diagnostic alternative to colonoscopy at an important time in service delivery. The increasing demand on colonoscopy waiting lists raises valid concerns regarding the ability of health services to deliver endoscopy in a timely fashion. It is clear that alternative diagnostic and surveillance modalities are necessary and that CCE has a central role to play.49
References available on request
St Luke's Radiation Oncology Network welcomes new Network Director
St Luke's Radiation Oncology Network is pleased to announce the appointment of Professor Charles Gillham as the new Network Director. Prof Gillham takes up the post having been a Consultant Radiation Oncologist in St Luke's Radiation Oncology Network for 15 years and the Clinical Lead at St Luke’s Centre at St James Hospital since 2013.
Professor Gillham sub-specialises in the radiotherapy treatment
of sarcomas, gynaecological, colorectal and haematological malignancies. He is the National Lead for Sarcoma, co-founder of the Irish Sarcoma Group, an active member of Cancer Trials Ireland and since 2015 has been the Chief Examiner in Radiation Oncology for the Faculty of Radiology, Royal College of Surgeons in Ireland.
Professor Gillham, Network Director, St Luke's Radiation Oncology Network stated: “It is
with great pleasure that I take up this new role as Network Director of the largest radiation oncology centre in Ireland at such an important time in healthcare. The Irish healthcare system is still recovering from the enormous impact of the pandemic and the cyberattack in 2021 with residual systemic challenges. SLRON launched its first ever strategy last year with a focus on ensuring the finest cancer workforce and
a commitment to research and technological innovation to provide the best possible care for patients. I look forward to implementing this ambitious plan for further development in optimised cancer care, underpinned by patient experience. My key priorities will be focused on patients, staff and education and to build on St Luke’s exemplary history of innovation, research, education and quality cancer service.”
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 38
News
Liver Disease and Pregnancy
 Written by:
Written by:
Cassandra Herron, UCD School of Medicine, University College Dublin


Mary Higgins, UCD Perinatal Research Centre, National Maternity Hospital
Omar Elsherif, Hepatology, St Vincents University Hospital Dublin

Introduction
Pregnancy is a normal physiological process and one of the most joyful and anticipated stages of life. However, some pregnancies can be more complicated with the development of medical problems such as gestational diabetes, pre-eclampsia or cholestasis. Additionally pregnancy may exacerbate pre-existing medical conditions. This article aims to briefly review issues in pregnancy relating to liver disease. We will start by reviewing the normal liver changes in pregnancy, pre-existing liver disease, and liver disease occurring only in pregnancy and finally highlight of the role of the multidisciplinary team and other relevant factors.
Normal liver changes in pregnancy
During pregnancy, the liver undergoes physiological and anatomical changes Physical examination findings include telangiectasias, spider angiomas and palmar erythema can be seen during the late half of the pregnancy because of the elevated levels of oestrogen. serum alkaline phosphatase (ALP) increases up
to four times in the third trimester as a result of the elevated levels of placental alkaline phosphatase. Other liver biochemical tests such as serum alanine transferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT) and total and free bilirubin typically remain within normal limits so any abnormal value should warrant further evaluation. As there is haemodilution, it is normal to have a mild decrease in serum albumin. Total cholesterol, triglyceride, fibrinogen and ceruloplasmin are augmented during pregnancy while prothrombin time (PT) and activated partial thromboplastin time (APTT) remain stable. Ultrasonography examination in pregnancy normally shows a normal biliary tree, increased fasting gallbladder volume and residual volume post contraction.
Pre-existing liver disease Hepatitis B
The prevalence of hepatitis B virus (HBV) is generally similar in the pregnant population as the general population and every pregnant woman should be screened for the HBV surface antigen (HBsAg). Women with chronic HBV (CHB) should have additional

tests such as HBV viral load, hepatitis B e antigen, and alanine aminotransferase level to assess for disease activity.
Pregnancy can affect the natural history of HBV, placing pregnant women with CHB at an increased risk for HBV flares (6-14%), particularly in the postpartum period (10-50%). The majority of these flares are self-limiting and hepatic decompensation is rare.
The majority of infant HBV infection is due to exposure to vaginal blood and secretions during delivery and in women with a viral load >200,000IU/ml, maternal to child transmission (MTCT) occurs in up to 1025% despite receiving HBV immunoglobulin and vaccination. For these women, the use of antiviral therapy in the third trimester is recommended. Among the antivirals considered safe in pregnancy tenofovir is preferred as it has the most robust safety data in pregnancy and a high barrier to resistance. Ideally treatment should be initiated early in the third trimester to allow sufficient time for the viral load to decrease to below 200,000IU/ml, and in women using antivirals for the sole purpose of decreasing
MTCT, treatment may be stopped shortly after delivery or continued if planning to breastfeed.
For the infant, an IM dose of 0.5mL Hepatitis B immunoglobulin and a HBV vaccine first dose be given within 12 hours of delivery is recommended, and has been shown to reduce rates of MTCT from 90% to 10%.
Hepatitis C
While most women with Hepatitis C infection have uneventful pregnancies, infection with the hepatitis C virus (HCV) has been associated with adverse pregnancy outcomes such as preterm birth, IUGR, congenital abnormalities, and increased risk for developing intrahepatic cholestasis of pregnancy (ICP). There is an approximate 5.8% risk of mother to child transmission (MTCT), which is the leading cause for HCV infection in children and can occur both in utero and in the peripartum period. This risk is elevated in women who inject drugs during pregnancy and nearly doubles in women that are coinfected with HIV.
There is currently no treatment for HCV that is approved for use during pregnancy, and as such the
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 39 ENDOCRINOLOGY: LIVER DISEASE & PREGNANCY
Samantha Vega
Samantha Vega Figueroa, Escuela de Medicina y Ciencias de la Salud Tesalud, Tecnológico de Monterrey Campus Ciudad de México
Cassandra Herron
Mary Higgins
Omar Elsherif
ENDOCRINOLOGY: LIVER DISEASE & PREGNANCY

indicator in women with pre-existing liver disease in planning a pregnancy. The score ranges from 6 to 40 and is used to assess how severe a patient’s liver disease is using serum creatinine (if patient has been dialyzed twice within last seven days then this value should be taken as 4.0), INR, and serum bilirubin.
Laboratory results
*(if dialized twice in last 7 days – take value as 4.0) mg/dl
MELD score = 3.8[Ln serum bilirubin] + 11.2[Ln INR] + 9.6[Ln serum creatinine] + 6.4
mainstay of care for these patient is to reduce the risk of MTCT as much as possible by avoiding prolonged rupture of membranes and invasive monitoring as much as is feasible and testing of infants born to infected mothers. Antiviral therapy has been used successfully in some cases with the aim of reducing the risk of MTCT, although more data is needed to support this approach across the board. Many people with HCV are asymptomatic, and screening in pregnant women is currently done on an at-risk basis, however there is evidence that routine screening for all pregnant women is cost effective.
Non-alcoholic fatty liver disease (NAFLD)
NAFLD is increasingly common in the Western world with a prevalence of approximately 20-30% and is characterised by hepatic steatosis in the absence of excessive alcohol intake. NAFLD is also associated with metabolic syndrome, which may increases the risk of adverse pregnancy outcomes such as preterm delivery, post-partum haemorrhage, and hypertensive complications and as such women with this disease should receive preconceptual counselling on the increased risks. Given the prevalence, it is likely that most people with NAFLD are unaware of their diagnosis.
The infants born to these mothers are at an increased risk of early obesity and childhood NAFLD, most likely due to multiple factors not just NAFLD. Currently the only treatment options for NAFLD are diet and exercise, and ideally patients would have their weight and blood sugar optimised prior to conception. Advice is given on pregnancy weight gain, which depends on the booking BMI. Close monitoring and treatment of associated risks such as hyperglycaemia and hypertension is also imperative during pregnancy. Moderate exercise during pregnancy has been associated with improved maternal
and fetal outcomes and could benefit these mothers and infants. Breastfeeding has also been shown to produce benefits in normalising glucose and triglycerides.
Autoimmune liver disease
Autoimmune hepatitis (AIH) is an immune-mediated liver disease characterised by chronic inflammation associated with increased gammaglobulins and the presence of specific serum autoantibodies. While the exact aetiological mechanism is still unknown, the disease is characterised by progressive destruction of the liver parenchyma which over time leads to liver cirrhosis.
The risk of complications generally corresponds to the stage of AIH at which the pregnancy occurs, with cirrhosis conferring a higher risk if associated with impaired liver function. Ideally a patient with AIH should be optimised before becoming pregnant. The model for end-stage liver disease (MELD) is often used as a prognostic indicator in women with preexisting liver disease in planning a pregnancy. The score ranges from 6 to 40 and is used to assess how severe a patient’s liver disease is using serum creatinine (if patient has been dialyzed twice within last seven days then this value should be taken as 4.0), INR, and serum bilirubin (see above).
Women with liver cirrhosis and a MELD score >10) are considered at higher risk for pregnancy, although patients with compensated cirrhosis can have successful pregnancies. Medical treatment of AIH during pregnancy is generally includes glucocorticoids such as prednisolonewith azathioprine. The most serious complications of AIH cirrhosis during pregnancy related to increased portal hypertension and variceal bleeding, particularly during the third trimester and ideally all patients should have an upper endoscopy to identify and prophylactically ligate any highrisk varices.
Up to 20% of women with AIH may “flare” during or shortly after pregnancy, which can be managed with higher doses of prednisolone. The incidence of flares increases with the post-partum return of normal immunity, and LFTS should be monitored during this period.
Pregnancy after liver transplantation
Liver transplantation is associated with a survival benefit and increased life expectancy in patients with decompensated cirrhosis. Advanced liver disease causing decompensated cirrhosis often causes infertility in women due to a lack of ovulation, however many of women that receive a liver transplant regain their fertility following transplant. For women wanting to conceive after liver transplant, planning can begin one year post transplant when the patient has achieved stable liver function and a period without acute cellular rejection.
The predominant risks in pregnancy after liver transplantation are acute cell rejection, loss of graft function, bacterial and viral infections, gestational diabetes (GDM), and pre-eclampsia/eclampsia.
Immunosuppressive drugs such as tacrolimus, and cyclosporin can be used during pregnancy, but do carry a small increased risk of hypertension, pre-eclampsia, and eclampsia, though this may be also as a result of the conditions themselves. Cyclosporin blood levels should be monitored due to the increased hepatic clearance of cyclosporin during pregnancy. Both of these drugs also carry an increased risk of transient hyperkalaemia in the newborn. Prednisolone can also be used during pregnancy, but carries the risk of growth restriction, GDM, preterm birth, and higher incidences of cleft palate and adrenal insufficiency in the newborn. Women with a liver transplant may benefit from GDM screening in the first trimester, and again at 24-28 weeks. In contrast to the above drugs, mycophenolate-
mofetil (MMF) is teratogenic and should be discontinued at least six-weeks prior to conception or ideally longer.
Women with liver cirrhosis and a MELD score >10) are considered at higher risk for pregnancy, although patients with compensated cirrhosis can have successful pregnancies. Medical treatment of AIH during pregnancy is generally includes glucocorticoids such as prednisolonewith azathioprine. The most serious
Liver disease in pregnancy Cholestasis
Intrahepatic cholestasis of pregnancy (IHCP) is the most common form of hepatic disease in pregnancy, it is a reversible cholestatic condition whose incidence is estimated at 0.51.8% in Europe. Risk factors include maternal age >35 years, multiparity, history of oral contraceptive use, fertility treatments and previous IHCP. Regarding pathogenesis, it is a multifactorial disease that arises in genetically predisposed women during the second or third trimester when their serum concentration of oestrogen reaches its peak and resolves spontaneously after birth when sex hormone levels fall. The diagnosis is suspected based on the symptom of pruritus that classically involve the palms and soles which worsens at night and is not associated with a rash, and abnormal elevated serum bile acids (>10μmol/L). IHCP is a diagnosis of exclusion once other causes of pruritis or abnormal LFTs have been ruled out.
Medical treatment includes oral ursodeoxycholic acid though other medications may be required if symptoms are refractory. Traditionally a diagnosis of IHCP, due to concerns regarding intrauterine death, has mandated early delivery (around 37 weeks’ gestation) though more recently guidelines have changed to provide more individualised care, depending on the clinical situation, wishes of the woman, and level of serum bile acid elevation.
Acute fatty liver
AFLP is a medical and obstetric emergency that affects 1 in 7,00020,000 and occurs in the late third trimester with microvesicular fatty infiltration of hepatocytes. Risk factors include multiple gestation
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 40
Value Bilirubin mg/dl Creatinine
INR %
mitochondrial dysfunction due to 3-hydroxyacyl-coenzyme A dehydrogenase (LCHAD) enzyme deficiency in the fetus which leads to accumulation of hepatotoxic fatty acid metabolites.
Diagnosis is made clinically with the aid of the Swansea criteria (Table 1, adapted from Mikolasevic, 2018), the presence of six or more features in the absence of other etiologies is suggestive of AFLP.Liver biopsy is considered the gold standard for diagnosing but is rarely done due to inherent risks in pregnancy.
Swansea Criteria
Nausea or vomiting
Abdominal pain (mainly epigastric)
Polydipsia/polyuria
Encephalopathy
Elevated bilirubin (>14 mmol/L)
Hypoglycemia (<4 mmol/L)
Elevated uric acid (>340 mmol/L)

Leukocytosis (>11x10^6/L)
Elevation in serum ammonia (>47 mmol/L)
Elevated transaminases (>42 IU/L)
Ascites or bright liver on ultrasound
Renal failure (Creatinine >150 mmol/L)
Coagulopathy (PT>14s or aPTT >34s)
Microvesicular steatosis on biopsy
The treatment is delivery of the fetus, which may be preterm and supportive care typically resulting in rapid recovery. However, management of complications may continue for days to weeks. This condition might recur in subsequent pregnancies. The international recommendation is that all women with AFLP and their children should have molecular testing for LCHAD.
and male fetus. The pathogenesis involves mitochondrial dysfunction due to 3-hydroxyacyl-coenzyme A dehydrogenase (LCHAD) enzyme deficiency in the fetus which leads to accumulation of hepatotoxic fatty acid metabolites.
Diagnosis is made clinically with the aid of the Swansea criteria (Table 1, adapted from Mikolasevic, 2018, above), the presence of six or more features in the absence of other etiologies is suggestive of AFLP.Liver biopsy is considered the gold standard for diagnosing but is rarely done due to inherent risks in pregnancy.
The treatment is delivery of the fetus, which may be preterm and supportive care typically resulting in rapid recovery. However, management of complications may continue for days to weeks. This condition might recur in subsequent pregnancies. The international recommendation is that all women with AFLP and their children should have molecular testing for LCHAD.
HELLP – Haemolysis, elevated liver enzymes and low platelets
HELLP syndrome is a rare and severe variant of preeclampsia which happens in 0.2%-0.9% of pregnancies. This condition occurs in the late second or third trimester or in the early postpartum period. Risk factors include family history of HELLP syndrome and antecedents of HELLP or preeclampsia in previous pregnancies.
The pathophysiology of this condition involves abnormal development of placental vasculature, maternal endothelial dysfunction that cause poor perfusion and platelet aggregation which results in clot formation, platelet consumption,
microangiopathic haemolytic anaemia and fibrin deposition in hepatic sinusoids leading to ischemic injury.
Signs and symptoms include hypertension, proteinuria, right upper quadrant abdominal pain, nausea and vomiting. Laboratory tests show elevated transaminases because of hepatocellular necrosis, lactate dehydrogenase, elevated unconjugated bilirubin and decreased haptoglobin due to haemolysis.
Complications of HELLP syndrome are disseminated intravascular coagulation (DIC), acute renal failure, pulmonary oedema, intracranial haemorrhage, placental abruption, hepatic infarction, haemorrhage or rupture; meanwhile fetal concerns include prematurity or intrauterine growth restriction, or tragically intrauterine death. While traditionally maternal mortality rate is reported as 5% with a fetal mortality rate of 40%, in the last thirty years increased recognition of the early symptoms and signs of HELLP has significantly reduced complications.
Treatment usually includes delivery and supportive care. Similar to pre-eclampsia, iatrogenic birth, supportive care, blood pressure management, seizure prophylaxis with magnesium sulphate are all part of management. If the gestational age is less than 34 weeks’ gestation maternal steroid (dexamethasone or betamethasone) can be administered to reduce neonatal complications including respiratory distress syndrome, intracranial haemorrhage or necrotising enterocolitis.
A crucially important consideration for both pre-eclampsia and HELLP is the misleading statement that
“delivery is curative” – there are multiple case reports of worsening disease in the postnatal period. For example, it is common for blood pressure to increase in the few days after birth as fluid sequestered in the interstitial space returns to the vascular tree. Liver function tests can worsen before normalising. There is a risk of venous thromboembolism (VTE), and careful consideration should be given to balancing the risks of bleeding to that of VTE. As a result, senior input from multiple specialities may be required to provide appropriate care. Occasionally intensive care admission is appropriate and recommended.
Role of the MDT
As with any other medical condition in pregnancy, pre-existing or pregnancy associated liver diseases require multidisciplinary care. At the centre of this is the concept of patient centred care, where the person themselves are the expert on their experience of a condition and the healthcare professionals experts on the medical condition. Individualisation of care to provide support is invaluable, while still providing care that is evidence based and appropriate.
For women or pregnant people with no pre-existing disease, antenatal care can be provided to normal risk women in a supported care pathway provided by midwives. Women or pregnant people with pre-existing disease can, depending on their condition, have care provided by an assisted or specialist care pathway with both midwifery and obstetricians involved. The role of a hepatologist is crucial for pre-existing disease, whereas conditions such as IHCP are within the practice of obstetricians and midwives unless
the disease is resistant to standard medications. Providing care using different “lenses” allows for more holistic care and it is incredibly useful to have joint clinics where patients can be seen by all relevant healthcare professionals with clear communication at all levels. Other healthcare professionals, including anaesthesiology, pharmacy, mental health support, neonatology, genetics, theatre staff, intensive care, medical scientists and pathology may also play a significant role in care of people with liver disease in pregnancy. Other factors to consider Pregnancy is obviously not a disease or a condition, and it would not be holistic to reduce it to the care of a disease. At the heart of this are two people – a mother and baby- and the wish for both to come through pregnancy, labour and birth safely. For some people pregnancy may be contraindicated and difficult decisions may be required for the family. Some people will choose to continue in the pregnancies having been informed and reflected on possible complications; others may choose not to continue. Other pregnancy care issues such as support, parent education, assessment of the growing fetus, nutrition, medication review, planning for birth and the postnatal period are all important. Not all women with liver disease, even transplantation, will require a caesarean birth, and vaginal birth may be appropriate for many.
Summary
Liver disease can affect pregnancies in many ways. For most, a diagnosis of cholestasis may be the most significant, with increased monitoring and perhaps earlier birth than planned. For a few, a complication such as AFLP, HELLP or liver haematoma due to PET may result in a long hospital stay and prolonged recovery. The role of the multidisciplinary team is crucial, and the person – women, pregnant person or transgender man with a uterus – remains in the centre of care.
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 41
Written by Aideen Stack, Health Psychologist
I am a Health Psychologist from Cork and I live with Ulcerative Colitis (UC). I was diagnosed with UC in 2019 while living in New Zealand. My symptoms were initially mild.

In January 2021 my UC symptoms returned, this time more serious. I was in the toilet 20 times a day, blood and mucous in my stools, significant weight loss and severe abdominal pain. I was admitted to hospital for the first time at the beginning of February, the week I was due to start my Health Psychology internship with the University of Auckland. Oral steroids helped the symptoms gradually subside and the gastroenterologist suggested I begin a biologic drug for longer term use. Over the next 8 months, I was admitted to hospital 6 times with varying degrees of success from biologics and steroids. I was physically and emotionally exhausted. On top of being unwell, I was continuing my degree with a high workload. Thankfully, the University of Auckland and my manager at my internship were supportive, I successfully complete my degree on time in 2022. I am grateful for the support of my husband, who was there with me every step of the way.
I was always curious about how stress could be impacting UC. I was at a significantly stressful point in my life in 2021 –completing my degree, saving for a wedding, living far from home and being in hospital during the COVID-19 pandemic. I asked my gastroenterologist at the time, who told me that there is “little evidence” to suggest that stress plays a part in symptom onset and severity in UC. I struggled to believe this given my background in Health Psychology and my interest in how the mind and body are constantly in sync.
Driven by personal experience and professional interest, I completed a literature review and gave a presentation on the Gut – Brain Axis. I believe that,
The Gut – Brain Axis and its role in Mental Health Presentations in IBD
given the link between the gut and mental stress, it is paramount that professionals working with individuals who have inflammatory conditions such as IBD understand the importance of the Gut – Brain Axis and its role in Mental Health. The communication between the central nervous system (CNS) and gut is referred to as the gut – brain axis. The autonomic nervous system, hypothalamic-pituitaryadrenal (HPA) axis, and nerves within the gastrointestinal (GI) tract link the gut and the brain. This system allows the brain to influence intestinal activities, and the gut to influence mood. Increasing evidence has associated an imbalance in the gut and CNS with symptomatology in gastrointestinal diseases such as Inflammatory Bowel Disease (IBD) while healthy gut function has been linked to regulated CNS function.
The brain and the gut are connected physically and biochemically. Physically, the gut and the brain are connected via the nervous system, in particular the Vagus nerve. Messages are continuously sent between the two. When the gut experiences disturbance, like in IBD, a message is sent to the brain that there is dysfunction. The brain interprets this as a stressor, eliciting the body’s stress response via the sympathetic nervous system. Part of the stress response is the secretion of the hormone cortisol. Cortisol has been found to increase intestinal permeability, influencing various GI disturbances seen in IBD (cramping, leaky gut, mucous). Following GI disturbance from cortisol, neural signals are then passed back to the brain, signalling disturbance, and this stress response is perpetuated.
The gut and the brain are also connected biochemically. Biochemical information from the gut is carried to the body and brain by cytokines (inflammatory proteins), neurotransmitters, and hormones. Inflammatory cytokines elevate inflammation in the body, as seen in IBD. Elevated inflammation can create the stress response, leading to increased levels of cortisol, thus increasing an individual’s experience with symptoms. This relationship between the gut and inflammation plays a significant role in the perpetuation of mental health stress, through the HPA response and the role of the gut – brain axis.
The gut microbiome plays an integral part in this system. The

composition of our microbiome influences intestinal permeability. A healthy microbiome forms a strong intestinal tissue, thus protecting the body from release of inflammatory. An unhealthy microbiome, disrupted by inflammation as well as by cortisol, leads to a more permeable intestinal wall allowing for the passing through of cytokines and pathogens into the blood vessels. These in turn activate the body’s stress response cycle, maintaining inflammation in the body and possible chronic stress.
To understand the role that the gut – brain axis plays in mental health, numerous studies have demonstrated that both acute and chronic stress interfere with intestinal barrier and induce adverse alterations in the gut microbiome. A disrupted barrier allows for pathogens and inflammatory cytokines to pass into the blood vessels, which cause systemic inflammation, resulting in the HPA stress response. If a person is under chronic stress from environmental factors, this cycle is constant. Presumably due to this cycle of systemic inflammation, the gut –brain axis has been implicated in several mood disorders. Anxiety and depression have well –established links to functional GI disruption. This link between GI disruptions and mood disorders is primarily due to the inflamed gut sending messages to the brain, resulting in the afore mentioned stress response. Interestingly, individuals with a diagnosis of depression have been found to have increased levels of inflammatory cytokines in the blood. When a person experiences a chronic stress response (as in mood disorders) they experience symptoms, such as fatigue, appetite disturbance, difficulty in concentrating and apathysymptoms also seen in IBD. As mentioned above, when the gut is disrupted by the stress response, the intestinal wall becomes more permeable, allowing the release of these cytokines into the blood. The body then reacts with a systemic inflammatory response. Research has demonstrated that inflammatory cytokine activates the HPA axis, hence contributing to the presentation of mental health issues in patients with gut related issues.
In conclusion, one cannot say for sure that environmental stress does not play a factor in symptoms of IBD due to the close link between inflammation in the
gut and the stress response. It is paramount for Health
Professionals working with IBD patients to understand this link and empower patients to manage their stress to soothe the Gut – Brain Axis, hence helping to reduce the perpetuation of the inflammatory system response.
References available on request
Crohn's and Colitis
Ireland is a support organisation in Ireland for people who are living with, or impacted by, Crohn's disease or ulcerative colitis (collectively known as Inflammatory Bowel Disease or IBD).
We are here to support people who are living with IBD, along with their parents, partners, family and friends. We work to improve the quality of life and well-being of the IBD community through sensitive support services, including advocacy, provision of information, training and events. We aim to encourage and educate healthcare professionals in the importance and value of peer support as an adjunct to their management of IBD.
Some of the negative impacts of living with IBD may include social exclusion, unemployment/ under-employment, disadvantage in education or the workplace, stigma, anxiety and depression - we aim to support people who experience some or all of these.
Services we provide:
• Provide membership/'no waiting' card
• Support helpline
• Peer Support (in person and online)
o Telephone
o One-to-one
o Group support
• Newsletters
• Gutcast: The first podcast for people living with IBD in Ireland
• Provide practical advice on a range of topics
• Support Research projects
• Advocacy and awareness campaigns
To refer a patient for support simple call Tel:+ 353 1 531 2983 or email :info@crohnscolitis.ie. Our website is www.crohnscolitis.ie

MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 42
GASTROENTEROLOGY
A personalised approach to Irritable Bowel Syndrome
Introduction
When you think about it, the gut is a marvellous machine! Simply eating food automatically sets the machine in motion and all being well we are blissfully unaware of digestion without pain, wind, or noise. In the lower gut, a feeling of rectal fullness disappears once defaecation is complete. The whole process of digestion critically depends on normal gut-brain contact with continuous crosstalk between the automatic nervous system within the gut, the Enteric Nervous System (ENS), and the Central Nervous System (CNS). If for whatever reason, at brain or gut level, that crosstalk between the ENS and CNS becomes disturbed or dysregulated the end result can be the development of a Disorder of Gut Brain Interaction (DGBI). Irritable Bowel Syndrome (IBS) is the commonist DGBI and perhaps the most troublesome. IBS is defined as a syndrome of
recurring abdominal pain at least one day per week in the last three months related to defaecation and associated with a change in bowel habit. Other symptoms of IBS can include excess wind, bloating and a feeling of incomplete rectal emptying.
IBS is very common affecting about 1 in 10 people, causes a significant negative impact on quality of life, social life and work productivity, and is associated with increased medical costs and healthcare utilisation. For unknown reasons, IBS is far commoner in women than men (7 of 10 patients) and women also bear the brunt of the condition with more severe symptoms, more comorbid somatic symptoms and increased need for second line medications. IBS is arbitrarily defined by the predominant disturbance in bowel habit. About 10 to 20% of patients have IBS Diarrhoea, 10 to 20% have IBS Constipation, and the
Professor Humphrey J. O’Connor MD FRCP AGAF, Trinity Academic Gastroenterology Group, Trinity Centre for Health Sciences, Tallaght University Hospital, Tallaght, Dublin 24 email: oconnor_hj@hotmail.com

majority of patients are IBS Mixed.
The IBS subtype has an important bearing on the choice of treatment options. Nearly 40% of primary care and gastroenterologist visits can be attributed to IBS. Longterm, about a third of patients
with IBS improve and symptoms disappear, symptoms remain unchanged in 30 to 50%, but can worsen in about 20%.

Diagnosis
It is important to remember that the majority of IBS patients are diagnosed and managed in primary care. The diagnosis of IBS is primarily based on patients having appropriate symptoms of abdominal pain and altered bowel habit and normal results in a limited number of tests. One of the dilemmas faced by IBS patients is their multiple symptoms, feeling so unwell, and yet all tests are normal. There is no specific test or biomarker for IBS but at the same time doctors and patients do not want to miss a serious or organic disease masquerading as IBS, particularly cancer. Basic tests can be summarised as the four C’s, Complete or full blood count, C reactive protein, Coeliac serology (tTg antibody) and faecal Calprotectin. Thyroid function tests are often added to this list. This set of tests will help exclude inflammatory bowel disease, microscopic colitis, coeliac disease and hyper or hypothyroidism. The need for invasive tests such as colonoscopy or scans is driven by the presence of “red flag symptoms” such as night waking, weight loss or non-hemorrhoidal rectal bleeding, and patient age. Current guidelines use an age cut-off of 45 or 50 years for colonoscopy but factors such as family history of colorectal cancer or patient worries and wishes should be respected. Another recent concern is the inexplicable and genuine rise in young-onset colorectal cancer, particularly in women, and this phenomenon
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 43 GASTROENTEROLOGY: IRRITABLE BOWEL SYNDROME
GASTROENTEROLOGY: IRRITABLE BOWEL SYNDROME

might also lower the age threshold for colonoscopy.
Long-term follow up of patients positively diagnosed with IBS is reassuring with only rare development of organic disease. Hence, although symptoms might persist and even change over the years, repeat testing should be avoided. Extensive and fruitless investigations only serve to undermine patients confidence in the diagnosis of IBS and in their healthcare provider. The fact that IBS does not increase the risk of colorectal cancer should also be emphasised.
Treatment
It is in the treatment of IBS that the word “Personalised” really becomes important. A good doctor-patient relationship is important in all aspects of clinical medicine but is paramount in the management of IBS. Dedicated time is needed in clinic to appreciate what are the patients’ predominant symptoms, concerns and preferences, previous treatments, and possible psychological comorbidities. Equally, time is needed to educate and explain to patients in plain language and diagrams the nature of IBS as a brain-gut disorder and the role of factors such as lifestyle, diet, stress, and gut bacteria etc. For many patients, education and the reassurance of normal test results as outlined above, coupled with lifestyle and dietary interventions are enough to ease symptoms and improve quality of
life. Increased exercise, healthy eating habits and reduced intake of alcohol, caffeine, fat and spicy or gas-producing foods have all been shown to have beneficial effects on IBS symptoms.

If medication is needed for mild to moderate IBS, the choice is largely driven by the predominant symptoms. Antispasmodics including mebeverine or alverine, the anticholinergic hyoscine, or peppermint oil have all been shown to have some impact on the pain of IBS and can be used as necessary. Water soluble fibre such as ispaghula or the osmotic laxative polyethylene glycol can help constipation. Loperamide is an effective antidiarrhoeal agent but does not improve overall IBS symptoms and again can be used as necessary.
Dietary Therapy
In a recently published study from the Netherlands on treatment preferences, IBS patients showed a strong preference for dietary intervention (48%) compared with pharmacological therapy (29%) or psychotherapy (23%). Since its introduction from Melbourne Australia over a decade ago, low FODMAP diet (LFD) has become the diet of choice worldwide in the management of IBS. LFD works on the premise that Fermentable Oligosaccharides, Disaccharides, Monosaccharides And Polyols are responsible for IBS symptoms by, among other things, creating an osmotic load in the small bowel and increasing colonic gas production. The upshot of at least ten clinical trials suggests
LFD can give effective symptom relief in about 50 - 70% of IBS patients and all IBS subtypes can show response with IBS Diarrhoea most favourable. In practice, patients firstly eliminate FODMAPs for an arbitrary 2-6 weeks to assess response. If symptom response is good foods are gradually reintroduced to ultimately personalise the diet to the minimum numbers of FODMAPs that need to be avoided. Personalised advice from a dietitian and providing written instructions have been shown to increase compliance and success. LFD comes with a number of caveats. We have very little longterm data on the effects of LFD and concerns have been raised about its nutritional adequacy and the reduction in fibre intake. We are also only beginning to assess combination treatments, for instance, LFD with prebiotics or probiotics, or LFD with psychological treatments.
Probiotics and Antibiotics
The human microbiome, the microorganisms that inhabit the gut, has received enormous attention in recent years with the realisation that a “good” microbiome is essential for health whereas a “disturbed” microbiome might contribute to ill health, including IBS. The fact that by-products of microbiome metabolism might actually interact with the ENS and play a role in brain-gut crosstalk has added further interest. Our colleagues at University College Cork have been to the forefront of this cutting edge research. By putting live microorganisms into the gut, probiotics are designed
to improve the microbiome. At least 50 clinical trials have tested the effects of probiotics in IBS. On balance, probiotics have been shown to improve global symptoms as well as bloating and excess wind. However, no solid recommendation can be given at this time on what particular strain or formulation of probiotic is best, or the subtype of IBS most likely to respond.
Ironically, antibiotics which might diminish the microbiome have also shown efficacy in IBS. Clinical trials of Rifaximin, an oral broadspectrum minimally absorbed antibiotic, have shown good effect particularly in non-constipated IBS. The course of Rifaximin lasts two weeks and can be repeated.
Moderate to Severe IBS
About 20% of IBS patients have really troublesome symptoms poorly responsive to first line treatments and dietary changes and will usually be attending a gastroenterology clinic. Added to their bothersome IBS symptoms many of these patients will have other gastrointestinal symptoms, extra intestinal symptoms apparently emanating from several organ systems, and psychological problems (Figure). In clinical practice, the most commonly reported extraintestinal problems include chronic fatigue, fibromyalgia, poor sleep, low back pain, chronic pelvic pain, dysuria, and dyspareunia (Table 1). A history of anxiety, depression or panic disorder is common enough in this group of patients but the extraintestinal symptoms can occur in the absence of mood disorder. On direct questioning some patients will give a history of unresolved childhood abuse. In
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 44
Table 1
this setting IBS is aptly described as a BioPsychoSocial disorder disorder and difficult to judge whether treatment should be directed to the brain or gut or both.

Gut
With greater understanding of the ENS over the past decade, the pharmaceutical industry has developed targeted oral treatments that act at gut level to help difficult IBS constipation or diarrhoea. Prucalopride (Resolor), linaclotide, plecanatide and lubiprostone are all prosecretory agents which have been shown to ease the symptoms of IBS Constipation. At present prucalopride is the only one of these medications available on prescription and perhaps the availability of the other agents is delayed for pharmacoeconomic reasons with linaclotide available on a named patient basis. Eluxadoline, a opioid receptor agonist, has proven efficacy in the management of IBS diarrhoea but again is not available on prescription in Ireland.
In difficult IBS Diarrhoea, it is worth remembering idiopathic bile acid diarrhoea (BAD) as a possible contributor to urgent watery stools and a real risk of faecal incontinence. In idiopathic BAD, increased bile synthesis seems to overpower absorptive capacity in the ileum, with consequent excess colonic bile acids which increase colonic motility, water secretion and mucosal permeability. Diagnostic tests are available for BAD but sometimes an easier
alternative is a treatment trial of a bile acid binder medication such as cholestyramine or colesevelam. A favourable response to these medications is tantamount to a positive diagnostic test.
Brain
Faced with difficult poorly responsive IBS, gastroenterologists have increasingly turned to treatment with low dose psychotropic agents, originally developed to treat psychological or psychiatric disorders. Before prescribing, open discussion is needed to reassure the patient that he or she is not being stigmatised with IBS as a psychiatric disorder and to explain potential medication benefits and risks. Sedation is the most common side effect but unwanted weight gain should also be mentioned. Low dose amitriptyline or nortriptyline are particularly useful in the management of IBS diarrhoea or IBS mixed. 10mg at bedtime also helps poor sleep and responders usually feel clinical benefit within a week or two. Nortriptyline may be less sedating than amitriptyline. Patients should be asked to promptly report side effects and a planned follow up contact at two weeks is very beneficial for a good trusted doctor-patient relationship in the long-term. If a patient has not responded to 10mg of amitriptyline or nortriptyline after two weeks and has not had any side effects, continue 10mg for
longer rather than increase the dose as cumulative benefit can occur over time. Increased dose potentially invites more side effects but on occasion the dose can escalate to 50mg daily. If a patient is doing well, treatment should continue for six to twelve months on the premise that a longer course might reduce the chance of symptom recurrence when the medication is slowly withdrawn. Tricyclic antidepressants tend to be avoided in patients with IBS constipation and a treatment trial of a low dose selective serotonin reuptake inhibitor, escitalopram at 5 to 10mg, is an alternative choice in that setting. Other related agents include mirtazapine 15mg at night for IBS Diarrhoea and functional dyspepsia and low dose duloxetine 30mg for abdominal pain and IBS Constipation.
Psychotherapy
A variety of different psychological treatments are effective in the management of IBS including cognitive behavioural therapy, gut-directed hypnotherapy, relaxation therapy, multicomponent psychological therapy and dynamic psychotherapy. Younger patients more often prefer psychotherapy than older patients and at least one study has shown gut-directed hypnotherapy worked best in young female IBS patients with associated anxiety. The practical difficulty in
Becoming a Standout Surgeon
Experts in the surgical field joined the RCSI Fellows and Members Office for its 'Becoming a Standout Surgeon' event last month as part of the RCSI Charter Meeting 2023. The aim of the event was to raise awareness amongst medical students, surgical trainees, interns, NCHDs and junior doctors of the opportunities available through the new RCSI affiliate membership programme and to provide guidance on pursuing a successful career in surgery.
Affiliate Membership of RCSI provides those considering a career in surgery and trainee surgeons with exclusive access to a range of ‘member-only’ services aimed at supporting and supplementing their personal, practical, academic and
professional development in the surgical field.
Professor David Healy, RCSI Council Member, was joined by an expert panel to discuss key surgical topics to guide the potential future surgical training programme applicants in the audience and provide them with an insight into the standards and the level of competition in the field. The panel of speakers, addressing an audience of more than 100, at the event included: Professor Kevin Barry, Director of National Surgical Training Programmes, RCSI; Mr Dara Kavanagh, Chair of the Core Surgical Training committee, RCSI; Ms Jessica Ryan, Irish Surgical Training Group (ISTG) President and Higher Surgical Trainee, RCSI;
psychological therapies is limited availability, high costs, and the difficulty of weekly appointments. Some of those difficulties may be overcome by mobile Apps such as Nerva which delivers a self-administered gut-directed hypnotherapy programme at home. In an ideal world, the clinic setting for difficult IBS would combine the services of a gastroenterologist, a specialist dietitian, and gastrointestinal psychotherapist.
Conclusions
IBS is a common, often troublesome DGBI that creates a multiplicity of symptoms both within and outside the gut. IBS disimproves quality of life and increases healthcare seeking. The burden of IBS falls very much on women more than men. Management of IBS calls for a personalised doctorpatient relationship that can deliver empathy, explanation, a measured diagnostic workup, and effective safe therapy mindful of the patients condition and preferences. In essence, optimum care of IBS patients requires an effective holistic approach that truly combines the science and art of medicine.
References on request
and
Ms Ciara Tallon, Career Designer, RCSI.
Development opportunities
As part of discussion on the Core Surgical Training (CST) selection criteria, Professor Kevin Barry and Mr Dara Kavanagh gave their expert advice on sitting the MRCS Part A and Part B examinations, the importance of timing and how to maximise Higher Surgical Training (HST) scores.
Professor Barry said: “I am very encouraged and pleased with the high standards demonstrated by the successful CST candidates. For example, 40% of the appointed candidates had already passed Part A of the MRCS exams and 15% have passed both parts of the MRCS exams which
is testament to the quality and dedication of the candidates.”
Ciara Tallon discussed career development opportunities in surgery and Jessica Ryan provided insight on how to successfully apply for CST and HST.
Future-focused
The ‘Becoming a Standout Surgeon’ event took place as part of Charter 2023, the annual RCSI symposium that reflects RCSI’s role and reach as a professional training body. This year’s meeting focussed on surgical care and planning for the future.
A recording of the full event, ‘Becoming a Standout Surgeon’ is available on-demand to RCSI Affiliate Members.
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 45
News
Girls with type 1 diabetes have worse outcomes compared with boys
Compared with boys, girls with type 1 diabetes have worse HbA1c, higher BMI and more diabetic ketoacidosis and require higher insulin doses, according to a systematic review published in Diabetologia.

“The observed disparities between sexes ... in our study populations can partially be attributed to puberty, yet this does not explain potential disparities in younger children,” Silvia A. G. de Vries, MD, PhD, from the department of vascular medicine at the Amsterdam University Medical Center, and colleagues wrote. “Factors that may play an important role at prepubertal ages are differences in the distribution of fat, insulin resistance, behavioural factors, growth hormone and the early influence of sex steroids. Treatment bias to the disadvantage of young girls may also influence daily clinical care, potentially affecting the treatment of risk factors.”
Researchers searched literature in MEDLINE up to June 2021 and identified 90 observational, cohort, cross-sectional and case-control studies with 643,217 people that included assessment of diabetes, sex characteristics,
sex distribution and children and/or adolescents with type 1 diabetes for review. All studies’ primary outcomes mentioning sex differences among children with type 1 diabetes were included. Most studies observed a higher HbA1c for girls at diagnosis and during treatment compared with boys. In addition, these studies demonstrated a steeper HbA1c increase over time among girls. Many studies also observed higher BMI, a higher prevalence of overweight or obesity and a higher prevalence of dyslipidemia among girls.
For boys, hypoglycemia and partial remission occurred more often whereas diabetic ketoacidosis and hospitalization occurred more often among girls. Most of the studies’ findings demonstrated that girls used pump therapy more frequently and needed higher insulin doses compared with boys.
Researchers also observed comorbidities, including thyroid disease and celiac disease, as more common among female participants compared with males. Every study reported lower quality of life among female participants.
According to the researchers, the large number of studies reporting comparable outcomes favoring one sex over the other within several clinical categories renders this evidence increasingly convincing.
“Sex disparities are observed in a variety of daily clinical variables and outcomes in the pediatric type 1 diabetes population, specifically among female adolescents. Most striking differences are seen regarding glycemic control, BMI, insulin dose, DKA and quality of life,” the researchers wrote. “These differences suggest a less favorable clinical profile for young female individuals, with potential consequences later in life.”
One in ten people around the world are living with diabetes, a lifelong condition listed by the World Health Organization in 2019 as one of the top ten leading causes of deaths globally.
The International Diabetes Federation (IDF) released new figures in December 2021 showing that more than half a billion adults are now living with diabetes worldwide — an alarming rise of 16% (74 million) since the previous IDF estimates in 2019.
Diabetes around the world in 2021:
• 537 million adults (20-79 years) are living with diabetes.
• 541 million adults have prediabetes, which places them at high risk of type 2 diabetes.
• The prevalence of diabetes worldwide is predicted to rise to 643 million by 2030 and 784 million by 2045.

Prevalence of Diabetes in Ireland
There is no National Diabetes Registry of people living with diabetes in Ireland, therefore all national estimates might not be fully accurate. However, as per a parliamentary question asked of the HSE in September 2021 regarding the prevalence of Type 1 diabetes in Ireland
the figures below used the data modelled from the Scottish Diabetes Register. In Scotland, the prevalence of diabetes was 5.6% of the total census population in 2020. The table below outlines the estimated prevalence of type 2 diabetes (87.9% of the total diabetes population) and type 1 diabetes (10.8% of the total diabetes population) based on the Scottish prevalence.
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 46 ENDOCRINOLOGY: DIABETES
Do You Know How Nutrition Can Impact Fertility?
Traditionally the main focus of nutrition and fertility has been on weight management, restoring ovulation function, and lifestyle factors such as quitting smoking and reducing alcohol intake. While the positive impact of these changes has been documented, there is emerging evidence to suggest that specific dietary strategies can optimise egg and sperm quality. Nutrition can also influence health conditions in females that affect fertility. As one in six heterosexual couples in Ireland may experience infertility,1 it is essential that health professionals are aware of how nutrition can impact fertility. It is also important that they understand the Fertility Dietitian’s role in assessing and managing nutrition and lifestyle factors that affect fertility.
Age, health conditions, mechanical issues e.g. blocked tubes, smoking, diet, obesity, and endocrine disruptors are all factors that can affect female fertility. By identifying the modifiable factors that can be influenced by nutrition and lifestyle changes, the impact of these factors can be modulated to optimise fertility.
The key objectives that a Fertility Dietitian will focus on to support

a woman’s fertility are optimising egg health, restoring ovulation, reducing inflammation, reducing oxidative stress, managing existing health conditions and improving the overall nutritional adequacy to support fertility and potential pregnancy. Knowing which objective is a priority and which dietary strategies to use are key considerations for the Fertility Dietitian. Optimising egg health is particularly important as age is a major factor affecting female fertility.2 As a woman ages, an increased percentage of eggs contain genetic abnormalities and older eggs are likely to accumulate more DNA errors.3 Oxidative stress — an imbalance between reactive oxygen species (ROS) and protective antioxidants — is associated with reduced oocyte and embryo quality.4,5
As males are found to be solely responsible for 20–30 percent of infertility cases and contribute to 50 percent of cases overall, it is vital that they are considered when advising nutrition and lifestyle changes.6 An accumulation of evidence shows that oxidative stress also plays a role in male infertility. The sperm membranes contain a large amount of long-chain polyunsaturated
Written by Jemma Henry, CORU Registered Dietitian, MINDI, Certified Fertility and Prenatal Dietitian, Post Graduate Diploma in Nutrition and Dietetics, BSC Biochemistry and Molecular Biology and Diploma in Food Science.


Email: jemmahenry@nourishmyfertility.ie Instagram: @nourishmyfertility

fatty acids, making them highly susceptible to oxidation.7 This can lead to an increased risk of DNA fragmentation and further impairments to sperm quality.8 Diet and lifestyle strategies to reduce oxidative stress are also important factors for the Fertility Dietitian to consider when aiming to optimise sperm health.
Nutritional Factors
Nutritional considerations when reducing oxidative stress include
increasing antioxidants and anti-inflammatory omega-3 fatty acids to counteract the adverse effects of oxidative stress. The question continues to arise whether there is an actual fertility diet. Currently, there is not enough evidence to determine an official ‘fertility diet’. Literature suggests that there are certain dietary patterns or strategies that can optimise fertility.9,10 These dietary patterns are associated with a Mediterranean-style diet which has been characterised by a high consumption of wholegrains, vegetables (including pulses), fruits, unrefined carbohydrates, olive oil, oily fish and with a low consumption of red meat.9 This diet pattern is in stark contrast to the western diet which is characterised by saturated fat, refined carbohydrates, processed meat and low levels of vitamins and minerals.9 The western diet is considered to be proinflammatory as it is associated with higher levels of inflammation which has been proposed to be related to poor fertility outcomes in both men and women.10 The dietary principles that are associated with the Mediterranean diet have also demonstrated associations with a lower risk of ovulatory fertility and a reduced risk of infertility.11 Studies have shown positive associations between the Mediterranean diet and improvements in Assisted Reproductive Technology (ART) outcomes such as higher embryo yield and the increased probability of achieving pregnancy.12,13 Other diets have been investigated for improvements in fertility in the
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 47
NUTRITION: FERTILITY
context of ART and unsurprisingly they share similar dietary patterns to the Mediterranean diet such as emphasising fruit, vegetables, wholegrains, fish and polyunsaturated fatty acids (PUFA) while limiting processed foods.14,15 Although the effect of dietary patterns on pregnancy outcomes in people using ART is still inconclusive, these studies show promise as potential strategies to improve ART-assisted outcomes.16 Healthy eating patterns have also demonstrated a beneficial effect on sperm parameters.17
The Fertility Dietitian will help individuals choose carbohydrates based on their food preferences while considering the glycaemic index (GI) and glycaemic load (GL) of the carbohydrates. The GI is the relative ranking of carbohydrates based on how a carbohydrate affects blood sugar. Carbohydrates that have a low GI rating will have less of an impact on blood sugar than those with a high GI rating. The Glycaemic load (GL) is a more accurate way to assess the effect of diet on blood glucose concentration because it accounts for carbohydrate quality through the GI and carbohydrate quantity through portion size.18 Studies have shown, that a higher dietary GL was associated with an increased risk of infertility due to anovulation and reduced fecundity (the ability to produce offspring) in women.18,19 The impact of high glycaemic foods has been also shown to impact sperm quality.20

The type of protein appears to be important regarding ovulatory infertility as Chavarro et al found that replacing animal protein with vegetable protein reduced the risk of infertility due to anovulation.21
Another nutritional consideration that the Fertility Dietitian will include in their nutritional assessment is ensuring that the client is receiving 400 μg of folic acid to prevent neural tube defects (NTDs).22 This dosage can be higher if they have been medically assessed to have increased requirements, for example, previous NTDs, malabsorption disorders, or obesity. Interestingly, there is growing evidence that folic acid could also improve other reproductive outcomes including promoting oocyte quality.23
Vitamin D is thought to play an important role in fertility as receptors are found in the ovaries, endometrium, placenta, pituitary gland, and sperm and testicles.24 Observational studies show that vitamin D deficiency is a risk marker for reduced fertility but is
still not clear whether vitamin D supplement improves fertility.25 In the case of vitamin D deficiency during infertility treatment, vitamin D supplementation may benefit women with disorders like polycystic ovary syndrome, insulin resistance, or low antimullerian hormone levels.26 Vitamin D deficiency and insufficiency are also associated with poorer ART outcomes in women.27 Currently, there is no specific recommendation for Vitamin D in Ireland for women trying to conceive. The EFSA has very recently updated its guidance to advise that 12-65-year-olds should supplement 15 μg (600 IU) of Vitamin D for the winter months or daily if individuals are of darkerskinned ethnicity or pregnant.67 Other authors have suggested higher levels of Vitamin D for those trying to conceive.25
Dietary strategies will also focus on increasing foods that contain nutrients such as Zinc, Iron, and B12. Zinc is involved in spermatogenesis, ovulation, and oocyte maturation while iron deficiency is associated with ovulatory infertility.28-30 Vitamin B12 deficiency individually and in combination with hyperhomocysteinemia is associated with early pregnancy loss.31
In practice, the Fertility Dietitian will encourage monounsaturated and polyunsaturated fats while reducing trans and saturated fat. The rationale for this is based on observational studies that have shown a higher amount of trans fats is associated with a higher risk of ovulatory infertility and reduced fecundability.32-33 Omega 3 fatty acids are essential fatty acids, that have a significant antiinflammatory effect.34 These have been associated with a higher probability of clinical pregnancy and live birth in women undergoing infertility treatment with ART.35
Most recently a systematic review summarised the emerging association between omega-3 intake through diet and favourable IVF/ICSI outcomes, possibly via its effect on egg quality.36
Caffeine and alcohol are also other important factors to consider as part of the nutritional assessment. The European Food Safety Authority guidelines advise women who are trying to conceive or are pregnant to keep caffeine intake below 200 mg to reduce negative pregnancy outcomes.37 While increased caffeine consumption is associated with spontaneous abortion and (>500mg per day) may affect time to pregnancy, there is a lack of evidence to show that there is an association between caffeine and fecundity or caffeine and IVF/ICSI outcomes.38-41 The HSE advises women who are trying to conceive to aim to avoid alcohol but to what extent alcohol consumption affects female fertility remains unclear.42 Female alcohol consumption is associated with reduced fecundability and another study also suggested that modest drinking levels may decrease fecundability if consumed during critical physiologic intervals of the menstrual cycle.43,44 Drinking more than 84g of alcohol per week (1 alcoholic beverage=12g of alcohol) was associated with reduced IVF/ICSI outcomes in both men and women.41 While there are studies showing how alcohol affects sperm quality in heavy drinkers there is little evidence to demonstrate alcohol’s effect on fecundability in the male.45,46
As well as looking at lifestyle factors such as smoking, reducing exposure to endocrine disruptors is also a significant part of the advice given by the Fertility Dietitian. Endocrine Disrupting Chemicals (EDCs) are ubiquitous exogenous substances that
can mimic or interfere with the endocrine system. Examples of EDCs include bisphenol A, phthalates, pesticides, and pollutants such as dioxin and polychlorinated biphenyls (endocrine society) The main way humans are exposed to them is through diet. EDCs can affect conception, in-utero development, neonatal life, and long-term child and maternal health by affecting both male and female reproductive processes. Complete avoidance is impossible, but dietary alterations such as avoiding plastic bottles and packaging have been shown to reduce exposure.47
Health conditions impacted by nutrition

In support of how important nutrition’s role is in managing female infertility, Kumar et al have proposed a self-administered screening tool to identify women that would benefit from nutritional intervention to improve fertility. This screening tool is awaiting validation. This publication also summarises health conditions such as Polycystic ovary syndrome (PCOS), Endometriosis and being over/underweight as health conditions that can be nutritionally optimised to improve female fertility.48
PCOS is the most common endocrine disorder in women of reproductive age. It is the leading cause of anovulatory infertility.49 The key role of dietary intervention with PCOS is to restore ovulation, restore menstrual cycle regularity, reduce insulin resistance and reduce weight if necessary. While lifestyle management is recommended as first-line treatment of PCOS, the optimal dietary composition is unclear.50 Focusing on low GI food is one of the approaches the Fertility Dietitian will use to reduce insulin resistance and restore menstrual regularity.51 As there is a high
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 48 NUTRITION: FERTILITY
prevalence of women in this PCOS population that are obese or overweight, research suggests that approximately 5 percent of weight reduction is adequate to improve insulin resistance, high levels of androgens, reproductive system dysfunctions, and fertility in overweight women with PCOS.50

Endometriosis is an oestrogendependent, chronic inflammatory disease affecting up to 10 percent of women of reproductive age.52,53 It is also a systemic condition and has been found on virtually all organs.54 Some women with Endometriosis are asymptomatic, while for others, it is a debilitating condition that can cause pain and infertility.55 The pathophysiology of infertility in mild cases of Endometriosis is not understood but it has been associated with anatomical disturbances in severe cases.54 It has also been linked to a negative impact on egg quality.53 As Endometriosis is an inflammatory condition, dietary approaches often include focusing on foods with anti-inflammatory properties such as omega 3 fatty acids and antioxidants from fruit and vegetables, herbs/spices that contain polyphenols/ flavonoids and reducing processed food.56 While the research suggests that increased consumption of omega-3 has a positive effect on Endometriosis the overall evidence for using an aninflammatory approach remains unclear.57 Higher amounts of red meat, alcohol and trans fats are associated with an increased
risk of Endometriosis.58-60 Part of the Fertility Dietitian’s role in this area of Endometriosis is symptom management. Visceral hypersensitivity is a key feature in both Irritable Bowel Syndrome (IBS) and Endometriosis and may explain why they share similar symptoms.61 A low FODMAP diet which has been investigated for its effectiveness for individuals with IBS may be an effective dietary strategy for women with gut symptoms and Endometriosis.62,61 A significant association between IBS, Eating Disorders (ED), and Endometriosis was demonstrated in the ENDONUT trial where the prevalence of IBS and ED in women with Endometriosis was higher than in the general population.63 This has further implications for the dietary strategy used by the Fertility Dietitian as they will have to also consider an individual’s relationship with food while achieving nutritional adequacy to support a potential pregnancy.
Weight management is also an important consideration for the Fertility Dietitian when trying to optimise fertility. Obesity is a known risk-factor for infertility. It has been shown to affect ovulatory function, time to conception and is associated with poor reproductive outcomes in assisted conception.64,65 However there is insufficient evidence to
suggest that short-term weight loss before ART improves outcomes.67 National Clinical Guidelines advise maintaining a BMI of 19-25 kg/m2 as part of recurrent miscarriage management advice.66 Low body weight can also affect fertility by impacting ovulation while increasing body weight may improve the chance of conception.64
Conclusion
Although questions still remain unanswered it is clear that nutrition and lifestyle play a critical role in impacting both male and female fertility. While we do not have an official fertility diet, there are dietary patterns/ strategies that can positively affect fertility. High-quality intervention studies are warranted to confirm these dietary patterns/ strategies with a view to developing dietary guidelines that go beyond the traditional advice of weight management, alcohol, and smoking.
As there is an abundance of misinformation on this topic on infertility forums, the internet, social media and from non-qualified practitioners, individuals struggling with infertility are trying diets and supplements which may further reduce their chances of conception and add to an already costly ART journey.
As nutrition and lifestyle factors are modifiable, it is vital that they are part of the infertility management treatment plan for both men and women. This nutrition and lifestyle advice should be given as a consultation to allow for a full nutritional assessment. The appropriate dietary patterns/ strategies and their rationale should be explained to the individual. Furthermore, the level of evidence underpinning each diet strategy discussed should also be explained in an easyto-understand manner. This will allow the individual to make an informed decision about their nutritional and lifestyle choices which may be impacting their fertility. This will undoubtedly reduce the use of alternative diets and non-evidenced-based supplements that are currently being widely used by men and women experiencing infertility. It is the unique skill set of the Fertility Dietitian that allows them to assess the available evidence and to determine the most appropriate nutritional plan in order to optimise fertility. The Fertility Dietitian is perfectly placed to be an integral part of the multidisciplinary team for infertility management.
References on request
New Study on clinically relevant treatments for heart attacks
CÚRAM SFI Research Centre for Medical Devices researchers have published in Nature Communications a key study establishing a new pre-clinical model to develop clinically relevant treatments for heart attacks.
Heart attacks (myocardial infarction (MI)) occur due to an acute complication of coronary artery disease and are a major cause of global mortality. The two main types of heart attack are ST-elevation (STEMI) and Non-ST elevation (NSTEMI). A non-ST-elevation is a type of heart attack that usually happens when your heart's oxygen needs are unmet. This condition gets its name because it doesn't have an easily identifiable electrical pattern like with an ST elevation that can be read from an electrocardiogram (ECG).
Patients who survive a heart attack have variable degrees of damage to their cardiac tissue, which can lead to heart failure in a significant proportion of these patients. In the last two decades, NSTEMIs have markedly risen in hospitalised patients. This subtype of heart attack results in a smaller amount of tissue damage compared to STEMIs. Still, importantly, recent clinical registry data shows that NSTEMIs are associated with higher long-term mortality than STEMIs.
Currently, preclinical models of heart attack mimic only fullthickness STEMI and hence cater only for an investigation into therapeutics and interventions directed at the NSTEMI subset of heart attack. In this new study, researchers have developed a preclinical model of NSTEMI by adopting a novel surgical
procedure that closely resembles the complexity of clinical cases in humans. Researchers validated the presented model by comparing it with an established method to achieve STEMIs. They performed a detailed analysis at the main acute and late time points after the induction of NSTEMI, at 7 and 28 days, respectively.
Professor Abhay Pandit, CÚRAM Scientific Director and senior author of the study, said, "There is a need in the field to adopt clinically relevant models to study NSTEMI pathophysiology and reveal its functional differences with STEMI induction. This new model will facilitate the translation of future research in the field, enabling the discovery of new clinically relevant treatments for patients."
Mr Mark Da Costa, Clinical Investigator at CÚRAM and
senior author of the study, added, "Currently, NSTEMI is the most common presentation of acute heart attack. The concern is that NSTEMI patients have lower in-patient (during their admission for the primary NSTEMI) and short-term mortality rates but significantly higher long-term mortality than those of STEMI patients. A Danish registry study of 8,889 patients showed that the 5-year mortality after NSTEMI was 16%, and another registry study highlighted a 10-year survival rate of only around 50%. To the best of our knowledge, there are currently no models that can reproduce both the functional and histological characteristics of NSTEMIs. This novel model may specifically serve as a preclinical foundation to study interventions that could combat the short and long-term effects of NSTEMI."
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 49
News
Pancreatic Cancer: A Public Health Burden
• Gender-more common in males than females
• Ethnicity-more common in African Americans than Caucasians
• Family history
• Blood group A patients have a 40% higher risk than those with other blood types
• Pancreatic microbiota
Presentation and Diagnosis
with recent-onset diabetes, and sometimes may present with recurrent deep vein thrombosis (DVT) due to hypercoagulability that prompts clinicians to suspect cancer and a full workup.
“Blood tests include, FBC, U+E, LFTs, coagulation-studies, glucose, and lipid-profile.”
Staging investigations for patients with confirmed pancreatic cancer include:
The global incidence and mortality of pancreatic cancer are increasing annually. Worldwide, the incidence is projected to rise to 18.6 per 100,000 by 2050, with the average annual growth of 1.1%, indicating that pancreatic cancer will pose a significant public health burden.
Theresa Lowry Lehnen, Clinical Nurse Practitioner and Associate Lecturer South East Technological University told us more about the 12th most common cancer worldwide.

Pancreatic cancer is the 11th most common cancer in women, and the 12th most common in men. Theresa notes, “Globally there were more than 495,000 new cases of pancreatic cancer in 2020, and it affects over 600 people in Ireland annually. Despite rapid advances in modern medicine and significant improvements in survival rates of many cancers, pancreatic cancer is still a highly fatal gastrointestinal cancer with difficulty in early detection, and a low 5-year survival rate.
“Pancreatic cancer 5-year survival rates range from 2% to 9%, with little difference between high and low-income, and middle-income countries. The 5-year survival rate varies globally in different regions and countries, but does not exceed 10%. It is predicted that patients with non-operative pancreatic cancer have a lower 5-year survival rate.
“The estimated global, 5-year survival rate for pancreatic cancer is about 5%. Approximately 90% of all cases are among people over 55 years of age, and the incidence
rates for both genders increases with age. Surgical resection is the only current option for a cure, however, only 20% of pancreatic cancer is surgically resectable at the time of diagnosis.”
Aetiology and Risk Factors

The aetiology of pancreatic cancer is poorly understood, Theresa says. 95% of tumours carry mutations in the K-RAS2 proto-oncogene, the activation of which leads to increased cell proliferation, loss of the normal response to apoptotic signals, dysplasia and ultimately cancer. In the pancreas, K-RAS mutations tend to cause the development of precancerous lesions called pancreatic intraepithelial neoplasia (PIN), which are present in up to a third of older people, but will only lead to cancer in about 1% of cases. It is estimated that it takes up to 20 years from the time of the first mutation to develop the fullblown disease.
Many non-modifiable and modifiable risk factors are associated with pancreatic cancer.
• Age older than 55 years
• Smoking
• Diabetes
• Obesity
• Chronic pancreatitis
• Cirrhosis of the liver
• Helicobacter pylori infection
• Work exposure to chemicals in the dry cleaning and metalworking industry
Theresa explains, “Symptoms of pancreatic cancer include; pain or discomfort in the abdomen, which may spread to the back; unexplained weight loss; indigestion; jaundice; loss of appetite; nausea; feeling full very quickly; a lasting change in bowel habits-steatorrhea; a new diagnosis of diabetes without weight gain; and tiredness.
“Sometimes there may be no signs or symptoms in the early stages of pancreatic cancer. Diagnostic tests include, history, examination, laboratory tests, transabdominal ultrasound, abdominal CT scan, PTC (percutaneous transhepatic cholangiography), MRCP scan (magnetic resonance cholangiopancreatography), Endoscopic Ultrasound (EUS), Laparoscopy and Biospy.
“General examination may reveal jaundice, pallor, skin excoriations or weight loss/cachexia. Abdominal examination may reveal epigastric tenderness; an upper abdominal mass; nodular hepatomegaly; ascites; and a palpable gallbladder. Courvoisier’s law states that the presence of a non-tender enlarged gallbladder means that jaundice is unlikely to be due to gallstones, as chronic cholecystitis causes the gallbladder to become shrunken and fibrotic. A palpable gallbladder is, therefore, a worrying sign. There may also be cervical lymphadenopathy.
“Patients with adenocarcinoma of pancreas typically present with painless jaundice (70%) usually due to obstruction of the common bile duct from the pancreatic head tumour. Weight loss occurs in about 90% of patients and abdominal pain in about 75%.
Weakness, pruritus from bile salts in the skin, anorexia, palpable, non-tender, distended gallbladder, acholic stools, and dark urine may be present. Patients may present
• Triple-phase “pancreas protocol” CT scan is the gold standard for assessment of pancreatic cancer. By taking images at different times after IV contrast administration, it provides detailed imaging of the tumour itself, its invasion into surrounding tissues, the degree of vascular infiltration and biliary tree dilatation, and presence of lymphatic or liver metastasis. It can be used to accurately predict surgical resectability in up to 90% of cases.
• Bone scan or PET scan can accurately detect distant metastases.
• Diagnostic laparoscopy is used to rule out intraperitoneal spread in high-risk patients.
• Biopsies for histopathology can be obtained percutaneously, endoscopically via EUS/ERCP, or laparoscopically and in some cases through an open procedure. Endoscopic biopsy is considered to be the safest and most appropriate approach. 8 Differential diagnosis includes acute pancreatitis, chronic pancreatitis, cholangitis, cholecystitis, choledochal cyst, peptic ulcer disease, cholangiocarcinoma, and gastric cancer.
Treatment and Management
Treatment for pancreatic cancer includes surgery, chemotherapy and radiotherapy. Theresa continues, “The treatment and management of pancreatic cancer is determined by whether the tumour is resectable or not, but other important factors include the patient’s overall health and fitness for major surgery and postoperative chemotherapy.
“There are surgical treatments available for both resectable cancers and palliate unresectable disease, and these are
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 50 ENDOCRINOLOGY: PANCREATIC CANCER
An interview with Theresa Lowry Lehnen (PhD), Clinical Nurse Practitioner and Associate Lecturer South East Technological University
The smallest of details make a world of difference to the way a violin sounds. Just as details can have a huge impact on patient outcomes.
For the treatment of Grade 1 and a subset of Grade 2 (Ki-67 index up to 10%) gastroenteropancreatic, neuroendocrine tumours of midgut, pancreatic or unknown origin (where hindgut have been excluded), in adults with unresectable locally advanced or metastatic disease.1
Abbreviated Prescribing Information
Somatuline® Autogel® (lanreotide acetate) solution for injection in a pre-filled syringe. See full Summary of Product Characteristics before prescribing. Available at: www.medicines.ie
Presentation: Pre-filled syringe containing a solution of lanreotide acetate 60, 90 or 120mg per syringe. Indications: [1] Long-term treatment of acromegaly when the circulating levels of Growth Hormone (GH) and/or Insulin-like Growth Factor-1 (IGF-1) remain abnormal after surgery and/or radiotherapy, or in patients who otherwise require medical treatment. [2] Relief of symptoms associated with acromegaly. [3] The treatment of Grade 1 and a subset of Grade 2 (Ki67 index up to 10%) gastroenteropancreatic neuroendocrine tumours (GEP-NETs) of midgut, pancreatic or unknown origin (where hindgut have been excluded), in adults with unresectable locally advanced or metastatic disease. [4] Treatment of symptoms associated with carcinoid tumours.
Dosage: Acromegaly: Starting dose 60 to 120mg administered via deep subcutaneous injection every 28 days. Previous treatment with other lanreotide acetate preparations affect suggested starting dose. Dose individualised according to patient’s response (judged by reduction in symptoms and/or reduction in GH and/or IGF1 levels). If complete control is obtained, the dose may be decreased or Somatuline Autogel 120mg given every 42-56 days. Neuroendocrine Tumours (NET) treatment: The recommended dose is one injection of Somatuline Autogel 120mg administered every 28 days. Continue treatment for as long as needed for tumour control. NET (carcinoid) symptoms: Starting dose 60 to 120mg administered via deep subcutaneous injection every 28 days. Dose adjusted according to degree of symptomatic relief obtained. Patients well controlled on a somatostatin analogue can be treated with Somatuline Autogel 120mg every 42-56 days. Elderly, renal and/or hepatic
impairment: No dose adjustment necessary due to the wide therapeutic window. Paediatrics: not recommended in children/adolescents due to lack of safety and efficacy data. Method of
Administration: Somatuline Autogel should be injected via deep subcutaneous route into the superior external quadrant of the buttock or in the upper outer thigh. For patients who receive a stable dose of Somatuline Autogel and after appropriate training, the injection may be given by the patient themselves or another trained person. In the case of self-injection, the injection should be given in the upper outer thigh. A healthcare professional should decide who should administer the injections. Regardless of the injection site, the skin should not be folded and the needle should be inserted rapidly and to its full length, perpendicularly to the skin. The injection site should alternate between the right and left side. Contraindications: Hypersensitivity to lanreotide, somatostatin or related peptides or to any of the excipients.
Warnings/Precautions: May reduce gallbladder motility and lead to gallstone formation. Patients may require periodic monitoring. There have been post-marketing reports of gallstones resulting in complications, including cholecystitis, cholangitis, and pancreatitis, requiring cholecystectomy in patients taking lanreotide. If complications of cholelithiasis are suspected, discontinue lanreotide and treat appropriately.
Patients treated with Somatuline Autogel may experience hypo- or hyperglycaemia. Blood glucose levels should be monitored at the start of the treatment or when the dose is altered; and any anti-diabetic medication should be adjusted accordingly. Slight decreases in thyroid function have been observed in patients with acromegaly. Thyroid function tests are recommended where clinically indicated. Somatuline Autogel may lead to a decrease of heart rate in patients without underlying cardiac problems. Sinus bradycardia may occur in patients with preexisting cardiac disorders. Care should be taken when initiating treatment in patients with
For access to the Somatuline® Autogel® website please visit: www.focus-on-living.ie and enter the following code: 454837
bradycardia. Interactions: The pharmacological gastrointestinal effects of lanreotide may result in a reduction of the intestinal absorption of co-administered drugs including ciclosporin. Concomitant administration of ciclosporin with lanreotide may decrease the relative bioavailability of ciclosporin and therefore may necessitate the adjustment of ciclosporin dose to maintain therapeutic levels. Concomitant administration of bromocriptine may increase the bioavailability of bromocriptine. Concomitant administration of bradycardia inducing drugs (e.g. beta blockers) may have an additive effect on the slight reduction of heart rate associated with lanreotide. Dose adjustments of such concomitant medications may be necessary. The limited published data available indicate that somatostatin analogues may decrease clearance of drugs metabolised via CYP450 enzymes. Drugs with a low therapeutic index mainly metabolised via CYP3A4 (e.g. quinidine, terfenadine) should be used with caution.
Pregnancy/Lactation: Pregnancy: There is a limited amount of data (less than 300 pregnancy outcomes) from the use of lanreotide in pregnant women. As a precautionary measure, it is preferable to avoid the use of Somatuline
Autogel during pregnancy. Lactation: Somatuline
Autogel should not be used during breastfeeding as it is not known whether it is excreted in human milk. Undesirable effects: Very common: Diarrhoea, loose stools, abdominal pain, cholelithiasis. Common: Nausea, vomiting, constipation, flatulence, abdominal distension, abdominal discomfort, dyspepsia, steatorrhoea, sinus bradycardia, hypoglycaemia, decreased appetite, hyperglycaemia, diabetes mellitus, alopecia, hypotrichosis, musculoskeletal pain, myalgia, dizziness, headache, lethargy, fatigue, asthenia, injection site reactions (pain, mass, induration, nodule, pruritus), biliary dilatation, ALAT increased, ASAT abnormal, ALAT abnormal, blood bilirubin increased, decreased weight, blood glucose increased, glycosylated haemoglobin increased, pancreatic enzymes
decreased. Post-marketing: Pancreatitis, allergic reactions (including angioedema, anaphylaxis, hypersensitivity), injection site abscess, cholecystitis and cholangitis. Prescribers should consult the Summary of Product Characteristics in relation to other side effects. Pharmaceutical
Particulars: Store in a refrigerator (2° C to 8°C) in the original package. Box of one 0.5ml prefilled syringe with automatic safety system and one needle. Legal category: POM. Marketing Authorisation Number(s): 60mg PA869/4/2, 90mg PA869/4/3, 120mg PA869/4/4. Marketing Authorisation Holder: Ipsen Pharmaceuticals Ltd, Blanchardstown Industrial Park, Blanchardstown, Dublin 15. Further information can be obtained from Ipsen Pharmaceuticals Ltd, Blanchardstown Industrial Park, Blanchardstown, Dublin 15, Ireland, Tel: (01)8098256. Somatuline® and Autogel® are registered trademarks. Date of Preparation of PI: September 2021. SOM-IE-000368
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie or email medsafety@hpra.ie. The HPRA can also be contacted on 016764971. Adverse events should also be reported to Ipsen via email at pharmacovigilance.uk-ie@ipsen.com or phone on +441753 627777, IE phone 018098256.
Reference:
1. Somatuline® Autogel® Summary of Product Characteristics. Available at www.medicines.ie
SOM-IE-000474. February 2023
Eb E
supported by the use of adjuvant medical therapies such as chemoradiotherapy and pain control measures. If the pancreatic adenocarcinoma is considered locally advanced it is unresectable, and neoadjuvant treatment with chemotherapy and/or radiation is typically preferred in this situation.
“There is a role for radiation therapy in combination with chemotherapy to treat locally advanced pancreatic cancer. Radiation therapy was originally used to alleviate the pain, but its use is now more widespread to shrink tumours and increase survival.
“Pylorus-preserving pancreaticoduodenectomy (Modified Whipple): Localised tumours in the head of the pancreas or periampullary region are suitable for pylorus-preserving pancreaticoduodenectomy, more commonly known as the modified Whipple procedure. It involves removal of the head of the pancreas, most of the duodenum, the common bile duct and the gallbladder, with or without extended dissection of additional lymph nodes.
“The traditional Whipple’s procedure also includes removal of the pylorus and antrum of the stomach, which is still occasionally necessary depending on the extent of the tumour. If the portal or superior mesenteric veins are involved, they can also sometimes be resected and reconstructed using vein grafts. Once the above organs have been
resected, the functional anatomy of the upper GI tract must be restored so the patient can still digest and absorb food.

“This is done by creating a Roux-en-Y loop from a segment of jejunum, and using end-toside anastamoses to make a pancreaticojejunostomy to drain pancreatic juice into the small bowel; a hepaticojejunostomy to drain bile into the small bowel, and a duodenojejunostomy to restore GI tract continuity. This is normally done via a large abdominal “rooftop” incision, but some centres do it laparoscopically. The Whipple is a high-risk procedure requiring careful pre-operative work-up and intensive post-operative monitoring and management.
“Complications of the Whipple procedure include bleeding, sepsis, bile and pancreatic leakage, anastamotic failure, delayed gastric emptying, and nutritional problems, and patients often remain in hospital for weeks or months while they recover. Patients require long-term pancreatic enzyme replacement in the form of Creon tablets taken with food. Five-year survival for patients with ductal carcinomas is 10-20%, and 40% for patients with other types of pancreatic cancer.
“Distal Pancreatectomy: Patients with localised tumours in the body or tail of the pancreas are suitable for a distal pancreatectomy. This involves removal of the body and tail of the pancreas as well as the spleen. Distal pancreatectomy is
a more straightforward procedure than the Whipple and can be done openly or laparoscopically.
“Complications are less frequent but include bleeding, sepsis, pancreatic leakage and pancreatic endocrine insufficiency resulting in diabetes. As the procedure involves a splenectomy, these patients are also at lifelong risk of potentially fatal overwhelming postsplenectomy infections (OPSI).
“Total pancreatectomy: Rarely, patients with localised tumours in or involving the neck of the pancreas, or diffuse cancers such as intraductal papillary mucinous neoplasm (IPMN) may be offered a total pancreatectomy. Complication rates are similar to a Whipple procedure, but with the added risk of post-splenectomy sepsis, and lifelong insulindependent diabetes, which can be difficult to manage.
“Palliative Surgical Treatments: Patients presenting with obstructive symptoms secondary to inoperable pancreatic cancer may be offered palliative surgical treatments to help control their symptoms. Biliary obstruction and jaundice is common in advanced disease. It leads to malaise, abdominal pain and severe pruritis, and can also put patients at risk of death from cholangitis, renal failure and severe coagulopathy.
“Management options include biliary stenting, endoscopically via an ERCP, or percutaneously using radiological
transhepatic techniques, and biliary bypass surgery with choledochojejunostomy, hepaticojejunostomy or sometimes a palliative ‘Whipple’ procedure. Duodenal obstruction is common and presents with symptoms of gastric outlet obstruction such as epigastric pain and profuse nonbilious vomiting. It can be treated endoscopically with duodenal stenting, or gastric bypass surgery using a gastrojejunostomy or Roux-en-Y bypass.
“Pancreatic duct obstruction can lead to pain and malabsorption. It can be treated with endoscopic pancreatic stenting or sometimes with pancreaticoduodenectomy.”
Theresa adds that pain is a significant problem in patients with pancreatic cancer and is generally managed with opiate analgesia, neuropathic adjuncts and the management of obstructive symptoms. “Medical therapies for exocrine cancers may be used as adjuncts to surgery, or as a primary palliative treatment in advanced disease. Patients with unresectable disease may be offered palliative chemotherapy. Although most pancreatic tumours are chemoresistant, gemcitabinebased regimes can delay disease progression and improve survival in patients with reasonable performance status.
“Radiotherapy can help with pain control, but does not improve survival. Pancreatic enzyme supplementation with Creon tablets can help with malabsorption and weight loss. Advanced metastatic cancer requires individualised palliative treatment with chemoradiotherapy and symptom control. Unresectable or metastatic functional endocrine tumours except for somatostatinomas can be managed medically with somatostatin analogues, which act to control symptoms by suppressing pancreatic hormone secretion.”
Theresa concludes that, “Despite major scientific and medical advances, mortality rates for pancreatic cancer have not improved much since the 1970s. Pancreatic cancer rates continue to rise, and will cause a major economic burden for all countries and related populations in the next two decades. A global prevention and control strategy for pancreatic cancer must include effective tobacco-control policy, recommendations for healthier lifestyles, increased screening, vaccination programmes, and education to improve public awareness and the need to take precautions.”
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 52 ENDOCRINOLOGY: PANCREATIC CANCER
Intestinal Healthcare-Associated Infections
"All diseases begin in the gut."
- Hippocrates
Key words: Gastroenterology, gastrointestinal tract, intestines, digestive system, intestinal infections, risk factors; healthcare-associated infections, hospital-acquired infections, nosocomial infections, outbreaks, viruses, enteric viruses, Clostridium difficile, C. difficile, rotaviruses, noroviruses, antibiotics, epidemiological surveillance, infection control, immunity, vaccines, prevention.
The aim of the study: Show the importance of the science of gastroenterology in the study of the gastrointestinal tract, digestive system; the urgency of the problem of the healthcare-associated infections (HAIs), in particular, of the intestinal bacterial infection C. difficile and intestinal rota- and norovirus hospital-acquired infections; the influence of the risk factors, antibiotics, seasonality, stays at a medical facility, the state of the immune system of the human body on the occurrence of the outbreaks and the spread of the intestinal HAIs; the importance of the infection prevention and control, the system of epidemiological surveillance, vaccination in preventing the occurrence and the development of HAIs.
Brief summary: This article examines the dependence of the occurrence of the intestinal HAIs on the type of the medical facility, age, seasonality, type of pathogen, mechanisms and ways of spreading of the causative agents of HAIs, immunity, compliance with the sanitary-hygienic and antiepidemic regimes in a medical facility.
Gastroenterology is a young science, which appeared only at the beginning of the 19th century and, as a medical specialty, developed at the end of the 19th century. Gastroenterology
(from Ancient Greek γαστήρ, γαστρός - stomach, Ancient Greek ἔντερον - intestines, Ancient Greek λόγος - teaching) is a science, which studies the gastrointestinal tract (GIT) of a person, its structure, functions and is engaged in prevention, diagnosis and treatment of the diseases of the digestive system. The founder of gastroenterology, a great French physiologist, Claude Bernard, whose studies showed an important role of the salivary glands, gastric and intestinal juices, pancreas in the process of digestion and assimilation of food, won two prestigious awards for his outstanding achievements in the field of physiology - Baly Medal (1875) - awarded once in two years (biennale) by the Royal College of Physicians of London and Copley Medal (1876) - the oldest scientific award of the Royal Society of London. William Beaumont – an American gastroenterologist proved the chemical nature of digestion. The William Beaumont Prize in Gastroenterology has been awarded annually since 2015 by the American Gastroenterology Association for the significant researches in the field of gastroenterology. Such outstanding German and Austrian
scientists, whose number of discoveries, inventions, awards and prizes can hardly be listed, as Theodor Billroth – a founder of modern abdominal surgery, Theodor Kocher - one of the founders of the European scientific surgery and Anton Eiselsberg - one of the best students of Billroth, made a huge contribution to the development of gastroenterology. Thanks to the following discoveries and inventions: a) gastroscopy (1868); b) the method of gastric intubation (1869, A. Kussmaul - German therapist); c) x-rays (1895, Wilhelm Röntgen - German physicist, Nobel Prize laureate in 1901); d) probes for aspiration of gastric juice - Einhorn duodenal tube (1910, Max Einhorn American gastroenterologist);
e) flexible fibrogastroscope (1958, B.I. Hirschowitz – American gastroenterologist with co-authors Curtiss and Peters); f) computed tomography (1979, Sir Godfrey Hounsfield - British electrical engineer and Allan CormackAmerican physicist Nobel Prize laureate); g) magnetic resonance imaging method (2003, Sir Peter Mansfield - British physicist and Paul Lauterbur - American chemist Nobel Prize laureate), there have been left practically no “blind spots” on the "map" of gastroenterology.
In 1958, there was created an international non-governmental professional medical federation,
Written by Tatiana Iarmak –http://orcid.org/0000-0001-53712958. Lecturer of the Department of Advanced Training of Junior Medical Specialists at the Municipal Health Care Institution 'Kharkiv Regional Medical Vocational College', Kharkiv, Ukraine. An independent trainer, consultant to medical facilities and non-medical facilities to instruct medical staff on how to safely provide services to patients/clients.

Corresponding Author details: iarmak.tat@gmail.com
the World Gastroenterology Organization (WGO), which united 110 national and regional associations of gastroenterologists, having more than 60,000 members. The goal of WGO was to increase the level of education in the field of gastroenterology worldwide, to develop effective methods for the prevention and treatment of the disorders and diseases of the digestive system. In 1992, the United European Gastroenterology (UEG) was founded - a nonprofit organization, which brought together 17 ordinary member societies and 49 national societies; has supported the digestive health research community, has developed the prevention; has improved the methods of treatment of the digestive diseases in Europe; and has provided the first-class education. May 29 marks the World Digestive Health Day, established at the initiative of the World Gastroenterology Organization with the aim of drawing attention of the society to the problems of the indigestion in humans and to find effective methods of prevention. Antimicrobial drug resistance (AMR) has been a worldwide problem for the past two decades.
In 2021, the Ireland’s Second One Health National Action Plan on Antimicrobial Resistance 20212025 was published, presenting 5 strategic goals:
1) Improve awareness and knowledge of the AMR;
2) Enhance surveillance of the antibiotic resistance and antibiotic use;
3) Reduce the spread of infection and disease;
4) Optimize the Use of Antibiotics in Human and Animal Health;
5) Promote research and sustainable investment in new medicines, diagnostic tools, vaccines and other interventions. The digestive system is one of the largest systems in human body, responsible for the digestion, assimilation of food and excretion of processed food. It performs the following functions:
1) hormonalproduces the hormones: secretin, serotonin, histamine, gastrin, etc. (Ernest Starling - an outstanding English physiologist introduced the term “hormone” into medicine and discovered the hormone secretin in 1905);
2) protective - about 80% of the cells of the immune system are in the intestine, therefore, a healthy intestine is a guarantee of immunity, which means a guarantee of a good health in general;
3) endocrine and other functions. The digestive organs include: oral cavity, esophagus, stomach, liver, pancreas, gallbladder, intestines: thin and thick, etc. Diseases of the digestive system are very common. More often, these are inflammatory acute and chronic processes of various parts of the digestive tract - the stomach (gastritis), liver (hepatitis), pancreas (pancreatitis), gallbladder and ducts (cholecystitis and cholangitis), small and large intestines (enteritis and colitis), etc.; which can lead to more serious diseases and processesstomach ulcers, ulcerative colitis and others. In addition, the disruption of the digestive tract can correlate with such complications as diarrhea, constipation, changes in the physiological rhythm of the bowel movements in patients with type 1 diabetes; can contribute to the development of chronic fatigue

HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 53 GASTROENTEROLOGY: INTESTINAL INFECTIONS
syndrome, skin diseases and more. All these processes occur for various reasons: malnutrition, stress, lack of physical activity, uncontrolled use of antibiotics, disruption of the immune, nervous, endocrine systems, etc. The intestine (called the “second brain”) controls the health of the human body as a whole and, also, affects the behavior, the mood and the quality of human life. However, the problems with the gastrointestinal tract can also be associated with the pathogens of infectious diseases - bacteria, viruses, fungi, parasites. In terms of morbidity and mortality, the acute intestinal infections are ranked 2nd in the world after the acute respiratory viral infections.

The greatest problem the acute intestinal infections create particularly in the context of medical facilities, contributing to the emergence and the development of the infections, associated with the provision of medical care (HAIs). HAI is “any clinically recognizable infectious disease, which occurs in patients as a result of their medical hospitalization, when seeking medical care, or in employees of a medical facility as a result of their work at such facility, regardless of the time of the onset of the symptoms of the disease” (WHO) . The share of the acute intestinal infections in the total number of the HAIs is 8-15%. HAIs affect the rates of morbidity, mortality, the quality of human life (moral damage), cause significant harm to the health of medical staff and, moreover, create economic problems in the healthcare systems of all countries of the world. Getting into a medical facility for treatment, surgery, childbirth; coming to an appointment or consultation with a family doctor; undergoing various endoscopic examinations and more, an individual must be protected from a possible infection with HAIs. Unfortunately, absolutely any person who got into a medical
facility risks of getting infected with HAIs; however, the highrisk groups, such as premature, newborn babies; children under age 3; elderly people; people with chronic diseases, who regularly undergo various medical examinations or have tests; patients with inflammatory bowel disease; pregnant women; immunocompromised people, are more susceptible to it. As a rule, the infection with HAIs occurs in patients, who have already been treated in hospitals of various profiles: surgical, neurological, psychiatric, maternity hospitals, etc. This increases the number of days spent at a medical facility, thus, the likelihood of more death cases. Many pathogens are already present in the body of a patient or a medical staff (carriage) and, therefore, the cross-infection from patient to patient or from patient to medical staff is also possible. Furthermore, the environment of a medical facility - air, surfaces, equipment, apparatus; underwear, patient care items, can serve as infection transmission factors. A large number of premises: wards, corridors, waiting rooms in medical facilities also contribute to the spread of infections. The causative agents of hospital strains, especially the intestinal ones, are resistant to antibiotics, disinfectants, therefore, very difficult to eliminate.
The causative agents of intestinal HAIs are numerousEnterococcus, Escherichia coli, Enterobacter spp., Salmonella and others, but the most common and recognized pathogens of the intestinal HAIs are Clostridioides (Clostridium) difficile, rotavirus and norovirus. C. difficile - a spore-forming, toxigenic bacterium, which lives in the large intestine of humans and animals and can remain in the environment in a form of spores for a long time (soil, water, etc.). In medical facilities, the endoscopic devices - fibrogastroscope, duodenoscope, etc.; surfaces -

equipment, furniture, etc., as well as hands of medical personnel can be contaminated with it. The pathogen was first described in 1935, but only in 1975 an American infectious disease scientist, John Gill Bartlett, discovered the Clostridioides difficile infection as the main cause of the nosocomial infections. In recent years, C. difficile accounts for 60% of all cases of diarrhea, associated with HAIs. C. difficile can cause outbreaks of nosocomial infections in intensive care units (ICU), hematology, oncology, surgery, maternity hospitals, etc. Infection is also possible in nursing homes, hospices, or on an outpatient basis (the risk of infection for the outpatients is less, than for the hospitalized). The main mechanism of transmission of C. difficile spores from person to person is fecal-oral - through the hands of personnel, contaminated food, drugs (especially in liquid dosage forms), etc. The main risk factors are a) uncontrolled, irrational use of antibiotics (they increase the risk of infection by 8 times, destroy a normal microflora of the large intestine, which ensures the colonization of the opportunistic and pathogenic microflora); b) hospitalization, c) surgical interventions on the gastrointestinal tract; d) various endoscopic studies; e) use of probes - nasogastric, gastric, duodenal, cleansing enemas. The toxins A and B of C. difficile cause diarrhea and inflammatory processes in the intestines in humans - pseudomembranous colitis (PMC), which is also called "nosocomial colitis", "antibiotic-associated colitis" or "C. difficile-associated colitis". PMC can be complicated by the toxic megacolon - a significant expansion of the lumen of the colon, leading to a sharp deterioration of the patient's condition, intestinal perforation, sepsis, possibly death. About 5% of C. difficile strains also have a binary toxin, which increases
the virulence and aggravates the course of the disease. For the past two decades, C. difficile has been the leading cause of the infectious hospital-acquired diarrhea and adult death in the United States, Canada, and Europe; for the most part, in developing countries. In Ireland, since January 2012, the new and recurrent infections have been reported as "C. difficile infection". According to the Q2 2022 National Report Enhanced Surveillance of Clostridioides (Clostridium) difficile Infection in Ireland, 207 cases were recognized as nosocomial cases, 187 of which were new. Prevention of C. difficile is aimed primarily at a proper hand hygiene - washing hands with soap and water is more preferable than using antiseptics, observing personal hygiene rules, observing sanitary-hygienic and anti-epidemic regimes in medical institution, a rational use of antibiotics, minimum length of stay at a medical facility.
Along with C. difficile, the enteric viruses, such as rotavirus and norovirus, are one of the main etiological causes of nosocomial infections. In 1973, Ruth Bishop, an outstanding Australian virologist, a winner of many prestigious awards, prizes and a holder of the Order of Australia (2019), professor, together with a group of researchers discovered rotavirus. Thanks to the structure similar to a wheel, the virus received a name "rota" - from Latin "wheel". The WHO approved the term "rotavirus" in 1979.
In the late 1990s, Ruth Bishop created the first oral rotavirus vaccine, which could be injected immediately after birth to prevent infection in the maternity hospital. The vaccine was included into the 2007 Australian vaccination calendar and then into the vaccination calendars of more than 110 countries around the world. Ruth Bishop’s rotavirus discovery and the creation of the vaccine helped save hundreds of thousands of children's lives. Currently, RotaTeq® and Rotarix® are two licensed oral vaccines in use worldwide. WHO recommends the inclusion of rotavirus vaccine for infants to all national immunization programs and strongly recommends vaccination in countries with a high mortality rate from diarrhea, which is the second leading cause of death from infections in children under 5 in the world. Rotavirus vaccines are already included in the vaccination schedules of 114 countries around the world, including Ireland. In Ukraine, the rotavirus
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 54 GASTROENTEROLOGY: INTESTINAL INFECTIONS
vaccine is not included into the vaccination calendar, it is advisory and can be obtained from private medical facilities. Also, WHO has developed the “Immunization Action Agenda (IA 2030) Programme - A Global Strategy to Leave No One Behind”, communicating the benefits and a need for vaccines.
Rotavirus belongs to the Reoviridae family and infects the mucosa of the small intestine. The disease proceeds, according to the type of gastroenteritis (diarrhea, vomiting), with a possible development of dehydration syndrome, which can lead to death. Rotavirus is one of the most common and contagious intestinal viruses in the world. The disease is more often recorded in premature infants, newborns and children under 3, as well as in children who are bottle-fed. In the structure of nosocomial viral intestinal infections, Rotavirus ranks first (70% and above). It is very stable in the environment, can remain for a long time on objects, equipment, apparatus in medical institutions; virus carriers are common among medical personnel (>20% - WHO data); dirty hands play a key role. Hospital rotavirus infections often occur in maternity hospitals, wards and departments for premature babies, departments of early childhood. In winter, the incidence of rotavirus gastroenteritis increases, which coincides with the rise in the incidence of the acute respiratory viral infections and may contribute to the emergence of nosocomial outbreaks of rotavirus infection due to the greater number of children in hospitals. The source of infection are patients with rotavirus gastroenteritis, virus carriers, medical staff. The mechanism of transmission is fecal-oral. Prevention is aimed at hand hygiene, compliance with the sanitary-hygienic and antiepidemic regime in the health care facilities - a thorough disinfection and sterilization of instruments and equipment, especially incubators (an apparatus for the temporary stay of premature or sick newborns to maintain their vitality).
Noroviruses are number two after rotaviruses, among the acute intestinal infections (about 1 billion cases in the world annually) and in countries where mass vaccination against rotavirus infection is carried out – number one. In 1968, American researchers discovered an unknown virus in schoolchildren in Norfolk (USA). Only 4 years later, in 1972, the virus was seen with an electron microscope and was
named Norwalk virus in honor of the city it was first discovered in. In 2002, the International Committee on Taxonomy of Viruses approved the name Norovirus. Noroviruses are highly contagious, especially when circulating indoors – in healthcare facilities, early childhood health centers, hospices, nursing homes, child care facilities, etc. The share of nosocomial noroviruses varies from 50% to 65%. Noroviruses are very contagious, remain in the environment for a long time (premises of medical facilities - wards, doctors' offices, etc.), and are resistant to disinfectants and antiseptics. They affect all age groups, especially children, elderly people and people with weakened immune systemsafter surgery, transplantology, with oncopathology and others. Hospital outbreaks of norovirus infection occur more often in the winter season (called "winter vomiting"), when the number of diseases and outbreaks increases among population. The bringing of norovirus is carried out exogenously - by patients, medical staff, visitors who are in the incubation period or by virus carriers. The infection is transmitted by the fecal-oral mechanism through contaminated food, water, surfaces, fomites (appliances, furniture, utensils, door handles, etc.) and then spreads within the medical facility. It proceeds in a form of gastroenteritis, accompanied by vomiting, diarrhea. The department or a hospital may be closed for quarantine due to the occurrence of a large-scale outbreak of norovirus infection for an indefinite period of time until a complete elimination of all cases of the disease. The admission of new patients and visitors during this period is prohibited. Infected catering staff and food distributors in medical facilities are suspended from work. Prevention is based on washing hands with soap and water for 30 seconds, as well as on the disinfection of rooms, objects, etc. in medical facilities. A norovirus vaccine has not yet been developed. A group of American researchers, led by Professor Nihal Altan-Bonnet of the US National Institute of Health, made a groundbreaking discovery that enteric viruses, such as norovirus and rotavirus, replicate in the salivary glands and can be transmitted through saliva (Ghosh et al., Nature 2022). Scientists suggest that the airborne route of transmission (the most contagious) of intestinal
viruses - by coughing, talking, kissing, sharing utensils or food, may contribute to a greater spread of these viruses. The results of the study will help develop more effective methods for the prevention, diagnosis and treatment of the diseases caused by the intestinal rotaand noroviruses.
In May 2022, WHO published the first-ever global report on infection prevention and control - a set of scientific and practical measures aimed at protecting the health of patients, medical workers and visitors to medical facilities from preventable infections, including those, resistant to antimicrobials during the provision of medical care. According to the report, every 7th patient out of 100 in high-income countries, every 15th in low- and middle-income countries contracts at least one HAI, and one in 10 dies. When infected with HAIs, which are resistant to antimicrobials, mortality increases two to three times. The correct organization of the infection prevention and control can reduce the number of HAIs by 70%, following the rules of the hand hygiene and other prevention methods. The key to safety of patients and staff is clean hands. By the decision of the UN General Assembly, together with the WHO, the Global Handwashing Day was established on May 5, 2008. Its motto: “Clean hands save lives!”. Hands are a major factor in the transmission of infectious agents, especially in the healthcare settings. Awareness of the population about the need to wash hands will help maintain health.
Over the past decades, several organizations have been created around the world to support patients, such as the International Alliance of Patients’ Organization (IAPO 1999), the World Patient Advocacy Alliance (October 27, 2004) and the World Patiens Alliance - a new International

Coalition (September 25, 2020), which all protect the interests of patients in all countries of the world. Patients’ safety is one of the main indicators of the quality of the healthcare services worldwide, therefore, the 72nd World Health Assembly has declared September 17 as the World Patient Safety Day since 2019. Ukraine supported this decision by the Decree of the President of Ukraine of September 4, 2019. The theme of the World Patient Safety Day in 2022 was “A safe use of drugs” and its slogan - “Drugs without harm”. In October 2022, the 1st World Patient Conference, "New Normal, New Challenges for Patients", took place in Rome.
Conclusions
The digestive system plays a huge role in human life. A healthy intestine protects the body from the occurrence of infectious diseases, namely, from the pathogens of intestinal infections, in particular, from HAIs; which are a serious problem in medical facilities of various profiles in all countries of the world. Optimization of the epidemiological surveillance system of HAIs caused by the intestinal pathogens: C. difficile, rotaviruses and noroviruses in medical facilities; proper organization of infection prevention and control: compliance with the sanitaryhygienic and anti-epidemic regimes – a competent use of disinfectants, taking into account the type of pathogen, especially in seasonal periods of the rising incidence; hand hygiene; rational use of antibiotics; reduction of hospital stays; isolation and quarantine measures will significantly reduce the risks of the occurrence and the spread of the intestinal HAIs, the incidence and mortality in healthcare facilities from HAIs.
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 55
Diabetes Care in Ireland
Estimating the population health impact and cost-effectiveness of implementing evidence-based diabetes care and prevention in Ireland
Written by Dr Jonathan Briody, Health Economist, Royal College of Surgeons in Ireland
double to almost half a million in the next 15 years unless population-wide prevention policies are instituted, according to HSE research. If unaddressed, the incremental national cost associated with this trend is anticipated to be greater than ¤100 million annually.
That is, to draw on advances in health impact modelling pioneered globally to develop a state-of-theart simulation model to prioritise interventions for Ireland that costeffectively stem the health burden of diabetes.
In this article, Dr Jonathan Briody, a health economist with the Royal College of Surgeons in Ireland, discusses a new project in which pioneering microsimulation models adapted from international programmes and validated for the first time in the Irish setting will be used to estimate the health economic consequences of incremental improvements in the quality of diabetic and pre-diabetic care in Ireland.

Diabetes mellitus is a metabolic disorder that occurs when the body cannot produce or properly use insulin, a hormone produced by the pancreas. Insulin is essential for regulating the amount of glucose (sugar) in the blood, as it helps the body to convert glucose into energy. When the body is unable to produce insulin, or when the insulin it produces is ineffective, glucose builds up in the blood, leading to high blood sugar levels or hyperglycemia, which damages the blood vessels and nerves in the body. Such uncontrolled diabetes has been associated with heart disease, stroke, blindness, kidney failure and amputation.
As a growing global public health threat, diabetes is placing an enormous burden on individuals, families, health systems, and national economies. Ireland is sharing this burden, as prevalence has doubled over the last two decades to 7%. There are an estimated 225,000 people living with the disease nationally.
An additional 17-19% of the population have pre-diabetes or undiagnosed diabetes, putting one-quarter of the Irish adult population at high risk for the morbidity stemming from chronic hyperglycemia.
Fortunately, randomised controlled trials have provided a diverse range of options to prevent diabetes and its complications. Multi-component lifestyle interventions for adults with pre-diabetes can reduce the incidence of diabetes by 50% or more. Where diabetes has not been prevented, achieving strict glycemic control reduces the risk of microvascular complications while lowering blood pressure and lipid levels significantly reduce myocardial infarction and stroke. These have been shown to be cost-effective, with blood pressure management, glycemic management, lipid management, and screening for complications all demonstrating favourable cost-effectiveness ratios. Likewise, structured lifestyle interventions have also been highly cost-effective, particularly when stratification approaches are applied to identify high-risk individuals.
Unfortunately, the direction of the diabetes epidemic in Ireland is less encouraging. Recent estimates indicate a significantly increased trend in the prevalence of type 1 diabetes with increasing age. Similarly, the number of type 2 diabetes patients is predicted to

Of late, the HSE, the Diabetes National Clinical Programme and the National Framework for the Integrated Prevention and Management of Chronic Disease have prioritised enhancing integrated care to achieve comprehensive risk factor management and avoid complications while also launching regional pilot programmes in diabetes prevention. However, such efforts are hampered by a lack of evidence to support optimal investment in the prevention and management of diabetes in Ireland.
This no longer needs to present a missed opportunity to reduce Ireland's disease burden. The Irish Research Council (IRC) has awarded researchers at the Royal College of Surgeons in Ireland University of Medicine and Health Sciences (RCSI) funds to collaborate with enterprise partner Diabetes Ireland on a research project of mutual interest via the Enterprise Partnership Scheme (EPS).
Professor Ed Gregg, Chair and Head of the School of Population Health at RCSI, and Professor Kathleen Bennett, Deputy Head of School – Research at the School of Population Health and Head of the Data Science Centre at RCSI, provide internationally established disease experience. Dr Jonathan Briody brings healtheconomic expertise at RCSI as the project awardee. Dr Kate Gajewska completes the team as Research and Advocacy Manager at Diabetes Ireland, the preeminent charity and advocacy organisation in Ireland dedicated to supporting people with diabetes.
Through strong collaboration with world-class experts in diabetes epidemiology, care, prevention, and economics nationally and internationally, this partnership will meet the objective of this project.
Particularly, a health economic model is being adapted to the Irish setting. This model will estimate the long-term health effects of diabetes in Ireland and determine the health consequences and cost-effectiveness of implementing new approaches to screen, manage, and prevent diabetes and its complications. The partnership will also provide Diabetes Ireland with critical estimates while adding significantly to the national infrastructure for evaluating healthcare policies for diabetes. This work consists of several primary aims, developing an innovative and sustainable Irish diabetes model, using this model to estimate the health impact and cost-effectiveness that could be achieved with optimal management in the Irish population with diabetes and evaluating early action to prevent diabetes. Central to all of this is the fundamental research question to be answered by this undertaking, the financial and population health consequences of applying advanced health economic modelling of diabetes to the Irish setting.
In sum, numerous evidencebased options exist to prevent diabetes and its complications. However, the potential health and economic consequences of appropriate implementation of these interventions have yet to be quantified for Ireland. This project is under development to produce some of the first evidence on the expected health benefit and cost-effectiveness of successfully integrated population-level care and prevention programmes in Ireland. The provision of this evidence in a manner that is actionable when planning prevention and treatment policies at a national level presents a critical opportunity to prioritise interventions that cost-effectively reduce the long-term health burdens of diabetes for all.
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 56 ENDOCRINOLOGY: DIABETES
• Help your patients find balance between HbA1c reduction and hypoglycaemic risk
Offers
1-7
† Toujeo® is available in easy-to-use pens,1,8,9 to be administered once daily at any time of the day, preferably at the same time every day.1 When needed, patients can administer Toujeo® up to 3 hours before or after their usual time of administration. Flexible dosing time was evaluated in two randomised, open-label clinical studies, in patients with T2DM.1

Prescribing Information: Toujeo (insulin glargine 300 units/ml) Please refer to Summary of Product Characteristics (SmPC) before prescribing. Presentation: Toujeo SoloStar and DoubleStar pre-filled pens. Each ml contains 300 units of insulin glargine. SoloStar pen contains 1.5ml (450 units) of solution for injection. DoubleStar pen contains 3ml (900 units) of solution for injection. Indication: Treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years. Dosage and Administration: Toujeo is administered subcutaneously, by injection into the abdominal wall, the deltoid or the thigh, once daily, at any time of the day, preferably at the same time every day. Injection sites must be rotated within a given injection area from one injection to the next in order to reduce the risk of lipodystrophy and cutaneous amyloidosis. The dose regimen (dose and timing) should be adjusted according to individual response. Do not administer intravenously. In type 1 diabetes mellitus, Toujeo must be combined with short-/rapidacting insulin to cover mealtime insulin requirements. In patients with type 2 diabetes mellitus, recommended daily starting dose is 0.2 units/kg followed by individual dose adjustments. Toujeo can also be given together with other anti-hyperglycaemic medicinal products. Switch between insulin glargine 100 units/ml and Toujeo: Insulin glargine 100 units/ml and Toujeo are not bioequivalent and are not directly interchangeable. When switching from insulin glargine 100 units/ml to Toujeo, this can be done on a unit-to-unit basis, but a higher Toujeo dose (approximately 10-18%) may be needed to achieve target ranges for plasma glucose levels. When switching from Toujeo to insulin glargine 100 units/ml, the dose should be reduced (approximately by 20%).
Switching from other basal insulins to Toujeo: A change of dose and/or timing of the basal insulin and concomitant anti-hyperglycaemic treatment may be required. Dose adjustments may also be required if the patient’s weight or lifestyle changes, the timing of insulin dose is changed, or other circumstances arise that increase susceptibility to hypo- or hyperglycaemia. Toujeo must not be mixed or diluted with any other insulin or other medicinal products. Close metabolic monitoring is recommended during a switch and in the initial weeks thereafter. SoloStar 1-80 units per single injection in steps of 1 unit and DoubleStar 2-160 units in steps of 2 units. When changing from Toujeo SoloStar to Toujeo DoubleStar, if the patient’s previous dose was an odd number, then the dose must be increased or decreased by 1 unit. Toujeo DoubleStar prefilled pen is recommended for patients requiring at least 20 units per day. Special Populations: Insulin requirements may be diminished in the elderly or patients with renal or hepatic impairment. Paediatric: When switching basal insulin to Toujeo, dose reduction of basal and bolus insulin needs to be considered on an individual basis, in order to minimise the risk of hypoglycaemia. The safety and efficacy of Toujeo in children and adolescents below 6 years of age have not been established. Contraindications: Hypersensitivity to insulin glargine or any excipients.

Precautions and Warnings: Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Toujeo is not the insulin of choice for treatment of diabetic ketoacidosis. Patients must be instructed to perform continuous rotation of the injection site to reduce the risk of developing lipodystrophy and cutaneous amyloidosis. There is a potential risk of delayed insulin absorption and worsened glycaemic control following insulin injections at sites with these reactions. A sudden change in the injection site to an unaffected area has been reported to result in hypoglycaemia. Blood
Intended for Healthcare Professionals in the Republic of Ireland only.

glucose monitoring is recommended after the change in the injection site, and dose adjustment of antidiabetic medications may be considered. Hypoglycaemia: In case of insufficient glucose control or a tendency to hyper/hypoglycaemic episodes, the patient’s adherence to the prescribed treatment regimen, injection sites and proper injection technique and all other relevant factors must be reviewed before dose adjustment is considered. Particular caution should be exercised, and intensified blood glucose monitoring is advisable for patients in whom hypoglycaemic episodes might be of clinical relevance and in those where dose adjustments may be required. Warning signs of hypoglycaemia may be changed, less pronounced or absent in certain risk groups, potentially resulting in severe hypoglycaemia and loss of consciousness. Risk groups include patients in whom glycaemic control is markedly improved, hypoglycaemia develops gradually, an autonomic neuropathy is present, or who are elderly. The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia. Intercurrent illness: Requires intensified metabolic monitoring and often it is necessary to adjust the insulin dose. Insulin antibodies: administration may cause insulin antibodies to form. Use with pioglitazone: Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs. Medication errors: Insulin labels must always be checked before each injection to avoid errors between Toujeo and other insulins. Patients must be instructed to never use a syringe to remove Toujeo from the SoloStar or DoubleStar prefilled pen, A new sterile needle must be attached before each injection. Needles must not be re-used. Pregnancy and lactation: There are no data from exposed pregnancies in controlled clinical trials. However, there is a large amount of data on use of insulin glargine 100 units/ml in pregnant women indicating no specific adverse effects on pregnancy and no specific malformative nor feto/neonatal toxicity. The use of Toujeo may be considered during pregnancy, if clinically needed. Careful monitoring of glucose control is essential. It is unknown if insulin glargine is excreted in breast milk. Interactions: Substances that affect glucose metabolism may require adjustment of insulin glargine. Adverse Reactions: Very common: Hypoglycaemia. Prolonged or severe hypoglycaemia may be life-threatening. Common: Lipohypertrophy, injection site reactions, including redness, pain, itching, hives, swelling, or inflammation. Prescribers should consult the SmPC in relation to other adverse reactions. Legal Category: POM. Marketing Authorisation Number: SoloStar 5 pen pack: EU/1/00/133/035; DoubleStar 5 pen pack: EU/1/00/133/038. Marketing Authorisation Holder: Sanofi Aventis Deutschland GmbH, D-65926 Frankfurt am Main, Germany. Further information is available from: Medical Information, Sanofi, 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com. Date of preparation: July 2022. MAT-IE-2200355 (V1.0)
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com
Abbreviations: HbA1c, Hemoglobin A1c; PD, Pharmacodynamics; PK, Pharmacokinetics; T1DM, Type 1 Diabetes Mellitus; T2DM, Type 2 Diabetes Mellitus.
References: 1. Toujeo® Summary of Product Characteristics July 2020. 2. Home PD, et al. Diabetes Care 2015;38(12):2217-2225. 3. Matsuhisa M, et al. Diabetes Obes Metab 2016;18(4):375-383. 4. Danne T, et al. Diabetes Care 2020; 43(7):1512–1519. 5. Riddle MC, et al. Diabetes Care 2014;37:2755-2762.
6. Yki-Jarvinen H, et al. Diabetes Care 2014;37:3235-3243. 7. Bolli GB, et al. Diabetes Obes Metab 2015;17:386-394. 8. Singh R, et al. Eur Endocrinol 2018;14:47-51. 9. Pohlmeier H, et al. J Diabetes Sci Technol 2017;11;263-269.
MAT-IE-2200486 (v1.0) | December 2022
•
flexibility † (+/- 3 hours) in dosing time1,8,9
FOR THE TREATMENT OF DIABETES MELLITUS IN ADULTS, ADOLESCENTS AND CHILDREN FROM THE AGE OF 6 YEARS1
From the start,1 there to help your patients on their basal insulin journey1 from 6 years
Toujeo® DoubleStar™ prefilled pen is recommended for patients requiring at least 20 units per day.1
Changing Perspectives – Let’s Talk About Obesity
Written By Dr Kate McCann, Emdoc Health Lifestyle Medicine

relationship between genetics and the other factors.
Biological: Underlying conditions such as hypothyroidism, polycystic ovarian syndrome, and Cushing’s syndrome are important to highlight here. Iatrogenic can be included here, including prescribed corticosteroids and many of the medications used to treat epilepsy as well as those use d in psychiatric care and pain management.
• Orthopaedic: Reduced mobility, osteoarthritis, plantar fasciitis, Achilles tendonitis, foot and ankle pain, low back pain, disc degeneration, spinal stenosis, increased rate of injury with movement including meniscal tears, rotator cuff tendonitis, knee dislocations, and an increase in incidence and severity of ankle fractures
• Fertility: subfertility in both men and women
4 March is world obesity day, and this year’s theme is “Changing Perspectives: Let’s Talk About Obesity.” And, more than ever, in the past year, we are talking about obesity - from reactions to the shocking the times editorial last october calling for an increase in fat-shaming to the polarised conversations sparked by the recent oscar-nominated film the whale - but are these the conversations we need to be having?
“DOCTORS DO NOT UNDERSTAND OBESITY.”
This quote is not from a patient but from Dr. Fatima Cody Stanford, MD, MPH, MDH Obesity Medicine Physician at Massachusetts General Hospital on primetime US television (60 minutes 1/1/23). She found that most medical schools don't teach that obesity is a disease -- even though obesity is now the second leading cause of preventable death in many countries. This leads to worse health outcomes for patients with obesity.
To provide the best care for patients with obesity, healthcare professionals must start with the fundamental understanding that:
Obesity is a chronic, multifactorial, relapsing, progressive disease with biological, genetic, psychological, environmental and societal causes.
This year marks 25 years since the National Institute of Health declared obesity a disease, and 10 years since the American Medical Association did likewise. It would surprise many doctors to learn that origins of obesity medicine
actually go back farther than that. The National Obesity Society was founded in the US in 1949 and the first International Congress on Obesity was held in 1973. Doctors then and since then were – and on occasion continue to be - actually criticised for medicalising excess weight, or insisting that weight management should be by experts. Later this spring, Dublin will host the European Obesity Meeting. And one of the focuses will be on how the rates of obesity have now reached epidemic proportions.
Over the past 20 years, the number of Irish children and adults who are above a healthy weight or have obesity have more than doubled to around 60% of the population. 25% of children and 20% of adolescent are above a healthy weight or have obesity. Because this potentially poses significant challenge to the delivery of Irish healthcare over the coming years, let’s talk about about obesity aetiology, complications, staging, and treatments.
Let’s Talk About Obesity and Aetiology
The causes of obesity are multifactorial disease including biological, genetic, psychological, environmental and societal. The relationship among these factors is complex and still not completely understood. Let’s just highlight a few of the many factors that have been identified:
Genetics: Emerging data is suggesting that genetics play much more of an role that has been previously appreciated, specifically the genes that affect behaviours such as appetite or metabolism, such as the body’s tendency to store adipose. Studies have found genes that may regulate hunger and satiety. There is much ongoing study to better understand the complex
Environmental: Among the most important environmental factors to highlight is prevalence of highly and ultra-processed foods in the Irish diet. In an outstanding recent piece in the Irish Times, “It delivers a taste bomb of pure pleasure, but ultraprocessed food is killing us” (30/1/2023) A panel of health and marketing experts lay out the stark reality that 60% of shopping trolleys in Ireland are now filled with hyperpalatable foods. These foods have a lower satiety index. One effect of food being cheaper, convenient. readily available and unnaturally tasty is over-consumption.
Societal: These factors include the increasing sedentary nature of jobs, urbanisation, changing agriculture and modalities of transportation, increasing screen time, and decreasing overall physical activity. Poverty, health literacy, education level, lack of sleep, shift work, and food distribution and marketings all been shown to be factors affecting obesity.
Psychological: Among the psychological factors contributing to obesity, one to highlight is Adverse Childhood Events (ACE). Multiple studies have demonstrated a strong relationship between recalled adverse child events and increased adult BMI.
Let’s talk about Obesity Complications
Obesity is a chronic and progressive disease; it is associated with significant complications. Both patients and healthcare professionals should be aware of the impact of obesity on healthrelated outcomes. Let’s highlight just some of the more significant:
• Liver: NAFLD and cirrhosis
• Cardiovascular: coronary artery disease, hypertension, dyslipidaemia, congestive heart failure, stroke
• Psychology: depression, eating disorder, body image problems, deliberate self-harm
• Respiratory: asthma, obstructive sleep apnoea, exertional dyspnoea
• Gastrointestinal: GORD, esophagitis, gallstones, diarrhoea, diverticular disease, polyps, and increased risk of cancers, including eosophageal, colorectal, gallbladder, pancreatic, and gastric cancers
• Endocrine: Type 2 diabetes and increased risk of thyroid cancers
• Renal and Urological: Chronic kidney disease, urinary incontinence, sexual dysfunction, urinary tract infections, nephrolithiasis, increased risk of renal and prostate cancers
• Neurological: Benign Intracranial Hypertension and increased risk of cognitive decline from Alzheimer’s disease and vascular dementias
• Oncological: Increased risk of additional cancers including ovarian, uterine, and thyroid cancers, myelomas, and meningiomas
While this is neither an exhaustive or in-depth exploration of the complications of obesity, it underlines that regardless of medical specialty, a better understanding of obesity as a disease is important for comprehensive patient care and better health outcomes.
Let’s Talk about Obesity Staging
Screening and identifying disease complications can also aid with stratifying risk. While BMI is commonly referred to when classifying obesity, it was never designed to be used in obesity management. It is more likely a question of when rather than

MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 58 ENDOCRINOLOGY: OBESITY
if BMI will cease to be used to classify obesity. At present, obesity is both classified by BMI (WHO criteria, Figure 1) and staged by disease process. Patient decision-making is more likely to be meaningfully guided by staging the disease. Currently, the most widely researched obesity staging tool is the Edmonton Obesity Staging System (EOSS, Figure 2). Patients at 0 have no complications identified. Patients at Stage 1 have mild symptoms or subclinical risk factors. Stage 2 is established co-morbidities or moderate functional limitations. Stage 3 is established end-organ damage or significant functional limitations. Stage 4 is severe end-stage organ damage, severe psychological co-morbidities, and severe functional limitations. For example, in a patient with BMI of 32 and pre-diabetes and prehypertension, their disease can be described as Class I, Stage 1 obesity. To use another example, in a patient with BMI of 46 with liver cirrhosis, congestive heart failure, and suicidal ideation, their disease would be termed Class III, Stage 4 obesity.
Let’s talk about Obesity Stigma
“Fat-shaming is the only way to beat the Obesity Crisis” was the horrifying headline printed in The Times on 28 October 2022. This spurred appropriately intense and swift reaction from both experts as well as patient advocacy groups. It is important to note that complications from obesity are not limited to those from the weight itself, but also directly from the weight stigma. Multiple studies of shown that patients who experience weight stigma also have higher rates of depression, negative body image, reduced self-esteem and increased cardiovascular and metabolic complications. The experience of weight stigma is also associated with increased risk of disordered eating, deliberate self harm, and all-cause mortality.
Most concerning to us, as physicians, should be that these studies have shown that patients with obesity are reluctant to seek healthcare when necessary due to their negative experiences with

STAGE 0
NO sign of obesity-related risk factors
NO physical symptoms
NO pyschological symptoms
NO functional limitations
STAGE 1
STAGE 2
SUBCLINICAL risk factors
-ORMILD physical or pyschological symptoms and/or impairment of well-being
ESTABLISHED obesity-related comorbidities requiring medical intervention
-ORMODERATE pyschological symptoms
-ORMODERATE functional limitations
weight bias in healthcare. Weight stigma itself – with or without the reluctance to seek healthcare when needed - can also lead to delay in diagnosis.
As health care professionals, the challenge we face is examining ourselves for unconscious weight bias or weight stigma. In this epidemic, both patients
STAGE 3

SIGNIFICANT obesity-related end-organ damage
-ORSIGNIFICANT pyschological symptoms
-ORSIGNIFICANT functional limitations
SIGNIFICANT impairment of well-being
STAGE 4
SEVERE (potential end stage) comorbidities
-ORSEVERELY disabling pyschological symptoms
-ORSEVERE functional limitations
and families need us to have the skills and compassion to the necessary supportive, non-judgemental conversations.
Let’s Talk about Obesity and Treatments
As I discuss with my own patients: weight management is not about weight loss for sake of weight loss, it is certainly not about aesthetics – the goal of care is health gains. It is about reducing the risk from obesity-related complications and all-cause mortality. Weigh loss is unfortunately targeted by both the cosmetic/aesthetics, social media influencers, and the wellness industry, many aspects of which are unregulated. Much of what is marketed is not evidence-based or is frankly predatory, including restrictive diets, unsafe practices, expensive supplements, or unrealistic weight loss goals.
Let’s Talk about Lifestyle Medicine: It’s worth first defining a term that is used so loosely, including by non-medics and influencers. In this context of obesity prevention and treatment, we are specifically using the term to refer to a well-defined and evidence-based medical specialty, currently recognised and standardised in US and UK. Lifestyle medicine plays a key role in the evidence-based management of many chronic diseases, and in this respect, its use in the treatment of obesity should not be considered differently. Lifestyle medicine is based on the pillars of good nutrition (including a healthy relationship with food), physical activity, good mental health, stress management, restorative sleep and social connections. The role of lifestyle medicine in the management of obesity is not an iteration of the outdated “eat less, move more.” Lifestyle medicine has an essential role in the multi-disciplinary approach to the prevention of obesity as
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 59
Figure 1. Edmonton Obesity Staging System (EOSS)
Figure 2. WHO Classification of Weight Status (BMI kg/m2)
REF: SHARMA AM & KUSHNER RF. INT J OBES 2009
well as the management of both psychological and physical comorbidities of obesity.
Let’s Talk about Pharmaceutical Management: The most effective pharmaceutical options available to patients in Ireland currently include GLP-1 analogues liraglutide and semaglutide. Current international guidelines recommend their use in individuals who are already making lifestyle changes and have a BMI of ≥ 30 kg/m2 or ≥ 27 kg/m2 with an obesity-related comorbidity. Semaglutide has been shown to be more effective in studies than liraglutide. However, liraglutide may be more affordable for select patients; since 1/1/2023, it is now reimbursed through DPS for patients who meet defined criteria.
GLP-1 analogues mimic the effects of glucagon-like peptide 1 and can help patients feel fuller faster as it slows gastric emptying. It also stimulates the production of extra insulin in response to rising blood sugar levels after eating. The advantages of GLP-1 analogues is that there is extensive data on pharmacokinetics and drug safety as it is has been used for years in the management of diabetes. The disadvantages can include cost as many patient are not currently eligible for reimbursement. The most common side effects include nausea, belching, bloating, and change in bowel habit though for many patients this can be managed with careful prescribing, patient-led increase of dosing, and attention to diet.
It is contraindicated in certain patients including those diabetic retinopathy or ketoacidosis, chronic kidney disease, history of thyroid cancer or multiple endocrine neoplasia syndrome, type 2. Furthermore, recent negative media coverage and social media influencers about the misuse of the drug have made some patients hesitant.
Let’s Talk about Surgical Management: Bariatric surgery - specifically gastric bypass or sleeve gastrectomy - is the most effective treatment for weight loss in patients with BMI over 40 or over 35 with associated comorbidities.
However, patients in Ireland currently face significant challenges accessing timely and safe bariatric surgery, including:
1. For patients with private health insurance, the reimbursement criteria may be different than international guidelines, and commonly requires patients to have a BMI of ≥45 or ≥ 40 with weight-related co-morbidities. Not all aspect of care may be covered or reimbursed.
2. For patients depending on public services, patients can currently expect to spend up to 5 years on waiting lists for an appointment with a comprehensive, multidisciplinary weight management service that includes bariatric surgery as a potential option.
3. Travel tourism, or traveling abroad to have bariatric surgery, has risks including
the recently well-publicised surgical complications. The low-risk option often presented to patients - especially those centres abroad marketing their intervention packages as cosmetic or all-incluseive - is the placement of a lap band. Globally, up to 40% of patients who have a lap band will seek to have it removed within 10 years due to side effects or complications. It is also has its limitations as a treatment for obesity as it has no metabolic effect. Treatment through these centres rarely includes access to necessary post-operative multidisciplinary care.
Obesity is a chronic disease and patients should be counselled from the outset that treatment will be lifelong, whether this involves lifestyle modifications, pharmaceuticals, or bariatric surgery. The biology is such that the patient’s body will, in short, take steps to defend its highest weight. Goals of care should include a plan for ongoing care and avoidance of weight cycling, which increases cardiometabolic risk.
Let’s Talk about Obesity and Current Challenges
The challenges facing patients with obesity are not limited just to weight-stigma in healthcare setting or difficulty accessing qualified medical care.
The Sars-CoV-2 pandemic has demonstrated that those with obesity are not only more likely to suffer from severe infections but also long term side effects. In multiple studies, women with obesity make up a disproportionate percentage
of patients in long covid clinics. While the aetiology of long covid is still not understood, emerging data does that obesity is an independent risk factor for continuing symptoms including brain fog, fatigue, pain, and shortness of breath more than 12 weeks after the infection.
The continued framing of obesity as an aesthetic or cosmetic problem poses a challenge in itself. Obesity is a disease; not a cosmetic or aesthetic issue. The continued perspective of obesity as cosmetic problem only serves to perpetuate weight stigma or obstruct reforms to insurance reimbursement guidelines, support for increased public funding, and/ or patient access to properly qualified experts.
We need to, as a profession, collectively shift our perspective to one that obesity is indeed a complex and chronic disease with real, stratifiable risks, validated disease staging, and effective evidence-based based management strategies. As healthcare professionals, we will need to advocate both with patients and on behalf of patients for public health measures. As a collective profession, we will need to stay up-to-date on emerging data and options for treatment.
Let’s Talk about Obesity – with understanding. Not just because of the Oscars or to mark how many years have passed or because of sensational headlines or a day of action on the calendar. It is time for this conversation because it is an epidemic with the potential for significant impact on patient mental and physical health outcomes and on the Irish healthcare system.
Let’s Talk about resources that can help Change Perspectives
Childhood Obesity. Obesity Reasearch and Care Group in RCSI and CHI@TempleStreet welcomes all healthcare professionals to take free online training.
https://childhoodobesity.ie/training/
SCOPE training and certification through World Obesity. Regisration, as well most of the online modules and courses, are completely free.

https://www.worldobesity.org/training-and-events-/scope/certification
Current Clinical Guidelines: https://asoi.info/ ASOI also has an image bank of nonstigmatising images for use in media and in presentations.
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 60 ENDOCRINOLOGY: OBESITY
Phramacotherapy, endoscopic devices, and bariatric surgery for the treatment of obesity
Dr Werd Al-Najim, Clinical Nutritionist, School of Medicine, University College Dublin; and ProHealth365 Physiotherapy and Nutrition
Prof Alex Miras, Clinical Professor of Medicine, Ulster University; and ProHealth365 ProHealth365 Physiotherapy and Nutrition

Ms Rand Al-Najim, Chartered Physiotherapist, ProHealth365 ProHealth365 Physiotherapy and Nutrition


Obesity is a chronic relapsing disease that acts as a gateway to many other non-communicable diseases, such as diabetes, cardiovascular disease and cancer. While the prevention of obesity is ideal for lowering the risk of developing these diseases, a large percentage of the population is already living with overweight and obesity. For example, in Ireland, just under 37% of people have a normal weight, six out of ten people are living with overweight or obesity.
The first weight management action most of the population takes is lifestyle intervention.

According to the Health Ireland Summary Report (2019), the most common actions were exercising more, eating fewer calories, and eating/drinking fewer sugarsweetened foods/drinks. These are all significant lifestyle interventions that will improve general health and result in some weight loss but may be challenging to maintain in the long term.
Several other obesity treatment options are available for those
who do not respond to selfdirected interventions; these include intensive lifestyle treatment combining nutritional and exercise therapy, pharmacotherapy, endoscopic devices, and bariatric surgery. Patients need to understand the pros and cons of each treatment, the amount of weight loss to be expected, and the lifetime commitment. All obesity treatments require life-long
follow-up. In a previous article published by Hospital Professional News, we covered the intensive lifestyle treatment combining nutritional and exercise therapy for obesity. This article will focus on pharmacotherapy, endoscopic devices, and bariatric surgery.
Available anti-obesity medications in Ireland
The use of anti-obesity
medications in conjunction with lifestyle interventions is indicated for people with obesity (BMI ≥30 kg/m2) or those who are overweight (BMI ≥27 kg/m2) with at least one weight-related health condition (eg, type 2 diabetes, cardiovascular disease, and osteoarthritis). Several anti-obesity medications are approved by regulatory authorities in Ireland for long-term weight management, summarised in table 1.
Orlistat
Orlistat is a gastric and pancreatic lipase inhibitor marketed under the names XENICAL® 120mg and Alli® 60 mg. This agent was first approved in 1998 to reduce dietary fat absorption by around 30%.
Orlistat is considered one of the safest anti-obesity medications currently available in the market as it is not absorbed from the gastrointestinal tract (GI), however as the fundamental action of the drug is to inhibit digestion and absorption of dietary fat, steatorrhoea can be unwelcomed and un-pleasant reality for patients when they overconsume fatty foods. Orlistat results in weight loss in a dose dependent manner but up to 4% in one year.
Naltrexone SR/Bupropion DR
Naltrexone
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 61 ENDOCRINOLOGY: OBESITY TREATMENT
SR/ Bupropion DR marketed under the name Contrave® in the United States
Dr Werd Al-Najim
Written by:
Ms Rand Al-Najim
Prof Alex Miras
and MysimbaTM in Europe. Naltrexone is an opioid receptor antagonist and Bupropion is a proopiomelanocortin (POMC) neuron stimulator. Taken together, the stimulation of POMC enhances the release of the satiety neurotransmitter α-melanocyte stimulating hormone (α-MSH) in the arcuate nucleus of the hypothalamus. This mechanism is modulated by mu-opioid receptors via an inhibitory feedback loop. Naltrexone blocks this inhibitory feedback thereby enhancing the release of α-MSH. Naltrexone also enhances the activity of the dopaminergic in the nucleus accumbens, ventral tegmental area and pre-frontal cortex, which are thought to be involved in some hedonic (reward) properties of food such as food cravings and improved inhibitory behaviour, resulting in around 5% weight loss.
Glucagon-like peptide-1 (GLP-1) analogue
GLP-1 is an incretin hormone released in response to nutrient ingestion. The class of GLP1 receptor agonists, of which liraglutide (Saxenda®) and semaglutide (Wegovy®) are currently approved for obesity management, inhibit food intake via action at GLP-1 receptors in several CNS areas (particularly the brainstem and hypothalamus)
and lead to 8-12% weight loss. Additionally, GLP-1 receptor agonists have peripheral actions, including slowing gastrointestinal transit, enhancing insulin secretion under conditions of hyperglycaemia, and decreasing blood pressure by increasing renal sodium excretion.
Hydrogels and endoscopic procedures
Endoscopic devices and oral hydrogels were developed to fill the gap in obesity management for people who are not candidates for, need to lose weight before, or prefer a less invasive alternative to bariatric surgery. All the endoscopic procedures have limited availability in Ireland.
Hydrogel
Oral hydrogel therapy obtained FDA approval in 2019 for adults with a BMI of 25·0 kg/m2 or more with or without comorbidities. This treatment involves ingestion of capsules containing a hydrogel matrix that absorbs water in the stomach to form gel pieces that occupy volume, thereby contributing to a feeling of satiety. The hydrogel is broken down in the large intestine and excreted in the faeces. Published data show that, at 24 weeks, mean weight loss was 6·4%. Common adverse effects associated with hydrogel include mild gastrointestinal symptoms such as abdominal discomfort, flatulence, and diarrhoea.
Few endoscopic treatments have shown durable clinical efficacy,
and some (eg, tube gastrostomy) have been withdrawn from the market. Endoscopic bariatric therapies in current clinical use include intragastric balloons and endoscopic gastroplasty.
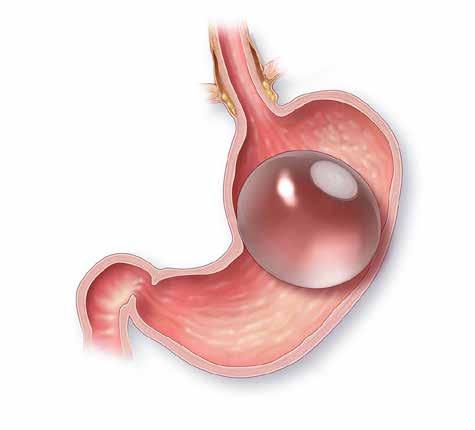
Intragastric balloons
Intragastric balloons reduces food intake by restricting gastric capacity and delaying gastric emptying, temporary, while they are in place. They are usually filled with fluid or air and removed after 6–12 months. Intragastric balloons lead to 15% weight loss. Complications with this procedure occur infrequently and include gastric outlet obstruction, gastric ulceration, and gastric perforation (0·04% to 1·00% of procedures). Intragastric balloons are only useful as a bridge to having another procedure done for people’s health (e.g. knee replacement) and they cannot be used long term for the treatment of obesity.
Endoscopic gastroplasty
Endoscopic gastroplasty reduces gastric capacity by infolding the greater curvature of the stomach with sutures (plication). Endoscopic gastroplasty lead to 13·6% weight loss after 1 year. About 1% of patients have complications, such as perigastric collections, major bleeding, and deep vein thrombosis.
Available bariatric surgery in Ireland
Bariatric surgery is an option for patients with a BMI of 35 kg/m2 or higher who have not responded to other interventions. Bariatric surgery procedures have evolved over the years starting from radical small bowel operations such as the jejunal-ileal bypass in the 1950s, to the gastric bypass in the late 1960s, gastric banding in the 1990s and the vertical sleeve gastrectomy.
Mechanism of action
Inactivation of gastric and pancreatic lipase
Central control of appetite; opioid receptor antagonist (naltrexone); and dopamine and noradrenaline reuptake inhibitor (bupropion)
Key indications
BMI ≥30 kg/m2 or BMI
≥27 kg/m2 with at least one weight-related health condition.
Efficacy
4% weight loss at 1 year
Adverse effects Steatorrhoea, oily faecal spotting, faecal urgency, and fat-soluble vitamin deficiency
BMI ≥30 kg/m2 or BMI
≥27 kg/m2 with at least one weight-related health condition
5% weight loss at 1 year
Dry mouth, nausea, vomiting, irritability, diarrhoea, constipation, headache, insomnia, dizziness, hypertension, seizures, and precipitation of mania
Central control of appetite (through GLP1 receptor agonism in hypothalamus and hindbrain) and peripheral actions eg, slowing gastrointestinal transit and reducing glucose levels
BMI ≥30 kg/m2 or BMI
≥27 kg/m2 with at least one weight-related health condition
8% weight loss at 1 year
Nausea, vomiting, diarrhoea, constipation, dyspepsia, abdominal pain, and headache
Central control of appetite (through GLP-1 receptor agonism in hypothalamus and hindbrain) and peripheral actions, slowing gastrointestinal transit, reducing glucose levels
BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with at least one weight-related health condition

12.5% weight loss at 68 weeks
Nausea, vomiting, diarrhoea, constipation, dyspepsia, abdominal pain, and headache
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 62 ENDOCRINOLOGY:
OBESITY TREATMENT
Table 1 Approved anti-obesity medications in Ireland
Orlistat Naltrexone-bupropion Liraglutide 3.0mg Semaglutide 2.4mg
The Gastric Balloon
The different bariatric procedures currently vary as regards their relative metabolic benefits, as well as surgical risks. The term metabolic surgery is now preferred to describe certain types of surgeries particularly gastric bypass and sleeve gastrectomy to more accurately capture their effects, not only on weight, but also diabetes and cardiometabolic risk. Moreover, this alternative terminology also points to the shift in emphasis it provides towards viewing the health benefits associated with weight loss rather than simply weight loss itself as being the key outcome.
Gastric Band
The Gastric Band (BAND) involves the insertion of an adjustable hallow plastic and silicone ring near the stomach’s upper end, immediately below the gastrooesophageal junction, creating a small pouch and a narrow passage to the remaining part of the stomach. This procedure causes a delayed emptying of food from the pouch and thus a feeling of fullness. The band is adjusted through injections of fluid in a subcutaneous port to change the size of the passage over time causing a reduction of 15% in total body weight.
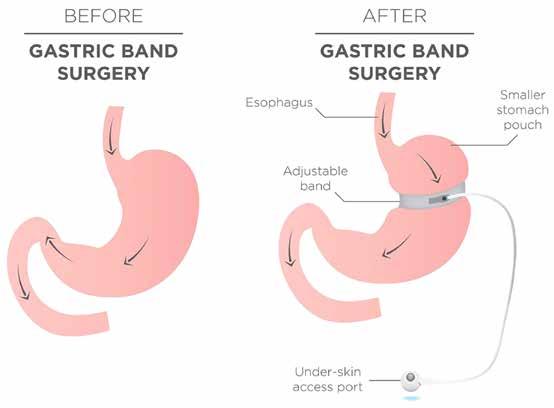
Vertical Sleeve Gastrectomy
Vertical Sleeve Gastrectomy (VSG) has become increasingly popular due to its relative simplicity and good clinical outcomes. VSG involves the excision of 70-80% of the stomach to make a small stomach that creates a high- pressure chamber which easily generates pressures sufficient to overcome pyloric sphincter tone and thus results in rapid gastric emptying.
Gastric Band
Roux-en-Y gastric bypass
The Roux-en-Y gastric bypass (RYGB) is the procedure many consider the gold standard due to its effectiveness in weight loss and weight loss maintenance. Nevertheless, RYGB is technically a more demanding procedure than VSG, but still very safe in good hands. In this procedure, the stomach is transected to generate a small gastric pouch 15 -30 ml in volume, and a lower larger gastric remnant excluded from contact with food. The gastric pouch is then anastomosed to the distal limb of a jejunotomy performed at the mid-jejunum thus forming the alimentary or Roux limb. In the absence of gastric reservoir function or sphincter control this rearrangement allows essentially instantaneous transfer of ingested food to the small intestinal lumen. Gastric, pancreatic and biliary secretions flow undiluted in the biliopancreatic limb proximal to the jejunal transection and are permitted to mix with chyme

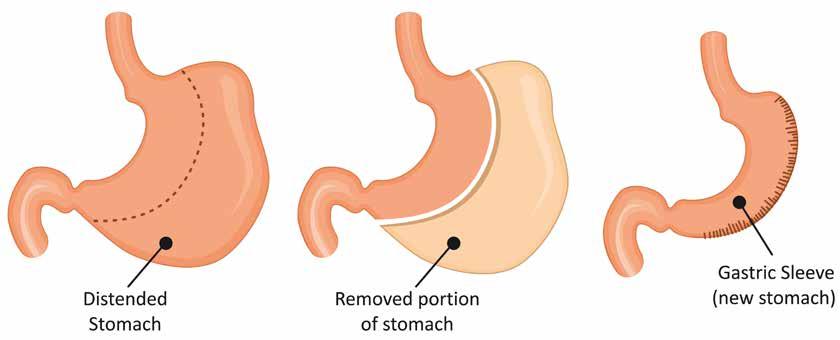
in a common channel formed by side-to-side anastomosis of the biliopancreatic limb 100150cm distal to the gastro-jejunal anastomosis.
In the short term, weight loss is similar between sleeve gastrectomy and Roux-en-Y gastric bypass (approximately 30% total bodyweight loss at 12 months). In contrast, long-term studies indicate greater weight loss with Roux-en-Y gastric bypass than sleeve gastrectomy (27% vs 23% total bodyweight loss) over 5–7 years’ follow-up. Bariatric surgery also leads to improvement or remission of other obesity-related comorbidities such as, hypertension, obstructive sleep apnoea, musculoskeletal pain, and overall quality of life.

Bariatric surgery has a very favourable risk to benefit profile, but this needs to be evaluated on a case-by-case basis before a decision to proceed is made. Potential complications can be classified as surgical, medical, nutritional, and psychological. These can be prevented or dealt with promptly by an experienced multidisciplinary team.
Take home message
Several options for obesity management are now available, including intensive lifestyle treatment combining nutritional and exercise therapy, pharmacotherapy, endoscopic devices, and bariatric surgery. The treatment option should take the patient’s medical status and wishes into consideration. There is no ‘one size fits all’. Usually, the patients want to be thin and happy. However, a more realistic outcome of all obesity treatments should be aimed at improving health and functionality. The amount of weight loss that needs to be achieved to improve overall health depends on the severity and present complications of obesity. For example, to improve the complication of obesity such as glycaemic control in type 2 diabetes as little as 5% weight loss makes a significant difference, while other complications such as sleep apnoea may require in excess of 10-15% weight loss to reduce the apnoea hypopnoea index below the threshold to require CPAP treatment. Therefore, a personalised decision should be made for each patient.
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 63
Vertical Sleeve Gastrectomy
Roux-en-Y Gastric Bypass (RNY)
Precision Medications for Irritable Bowel Syndrome with Predominant Constipation (IBS-C)
 Written by Robert Varley, GI SpR, Connolly Hospital
Written by Robert Varley, GI SpR, Connolly Hospital

How is IBS diagnosed?
IBS is a diagnosis of exclusion. All patients presenting with symptoms of IBS for the first time must be investigated with:
1. Full blood count (FBC)
2. C reactive protein (CRP) or erythrocyte sedimentation rate (ESR)
Irritable Bowel Syndrome (IBS) is one of the most common gastrointestinal disorders seen in clinical practice. In recent years, significant advances have been made in understanding its complex pathophysiology, with considerable new evidence published concerning its diagnosis, investigation and management. This led to the British Society of Gastroenterology (BSG) publishing updated IBS clinical practice guidelines in 2021.1 In this review, I will outline the approach to diagnosing and managing IBS with Predominant Constipation (IBS-C).
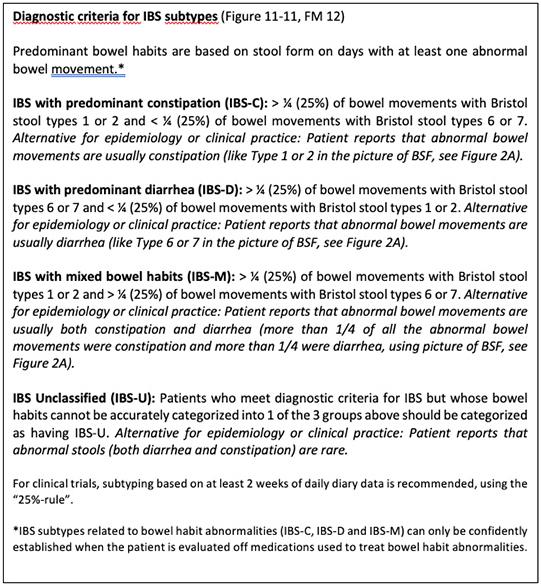
3. Coeliac serology: Anti-tissue transglutaminase (Anti-TTG) and immunoglobulins
4. Faecal calprotectin in under 45 year olds, to exclude inflammatory bowel disease
Local and national guidelines for colorectal and ovarian cancer screening should be followed. In Ireland, all patients aged 6070 should participate in Bowel Screen, the national colorectal cancer screening programme.
Colonoscopy is not indicated in IBS without alarm symptoms or
signs (summarised in Box 1).1
However, it should be considered in those patients with symptoms suggestive of IBS with diarrhoea who have atypical features and/or relevant risk factors for microscopic colitis (female sex, age ≥50 years, coexistent autoimmune disease, nocturnal or severe, watery, diarrhoea, duration of diarrhoea <12 months, weight loss or use of potential precipitating drugs including nonsteroidal anti-inflammatory drugs, proton pump inhibitors, etc)
A diagnosis of IBS should be made based on symptoms, the absence of alarm symptoms or signs, and no abnormalities on the above simple blood and stool tests. Referral to gastroenterology is warranted where there is diagnostic doubt, in patients with symptoms that are severe, or refractory to first-line treatments, or on individual patient request.
IBS is diagnosed using the Rome Criteria, most recently updated to Rome IV in 2016.2 Criteria must be fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis:
Recurrent abdominal pain on average at least 1 day/week in the last 3 months, associated with two or more of the following criteria:
1. Related to defaecation
2. Associated with a change in frequency of stool
3. Associated with a change in form (appearance) of stool
IBS is further subdivided by predominant symptom, summarised in Figures 1 + 2.2 As shown, IBS with Predominant Constipation (IBS-C) requires over 25% of abnormal bowel movements to be very solid (Bristol
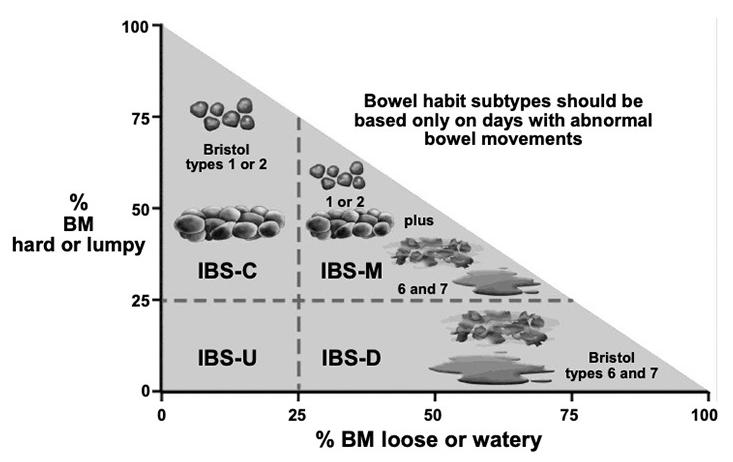
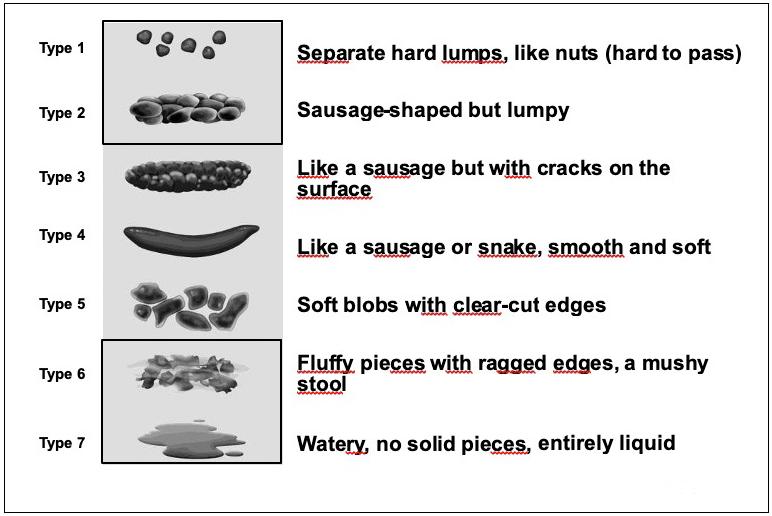 Figure 1: IBS Diagnostic Criteria
Figure2A: Bristol Stool Chart
Figure 1: IBS Diagnostic Criteria
Figure2A: Bristol Stool Chart
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 64 GASTROENTEROLOGY: IBS
Figure 2B: IBS Subtype Graph
stool type 1 and 2) with less than 25% being very loose (Bristol stool type 6 and 7).
How is IBS-C Managed?
The treatment algorithm for IBS is shown in Figure 3, with overall diet and lifestyle advice followed by treatments directed towards predominant symptoms1
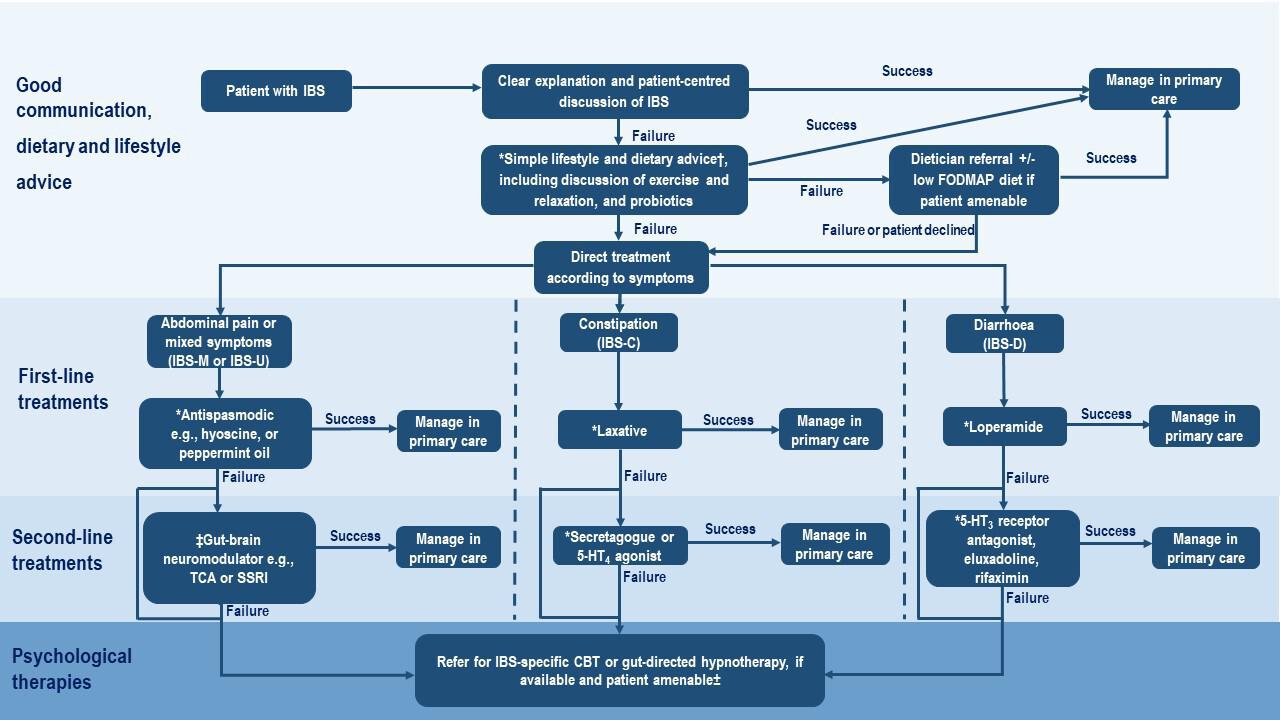
New patients should be given a clear explanation of the diagnosis, pathophysiology, natural history and the most common symptom triggers
IBS should be explained as a disorder of gut-brain interaction, with the impact of diet,
stress, cognitive, behavioural and emotional responses to symptoms, as well as postinfective changes
To manage expectations, there should be a realistic discussion regarding the limitations of treatments - while there is no cure, significant improvements in symptoms and quality of life are very achievable
In IBS-C, the treatment target is the normalisation of bowel movement pattern, defined as at least three completed spontaneous bowel movements per week
Treatment should commence with either dietary therapies or first-line drugs, as per patient
Second-line drugs are reserved for those not improving with these measures, with many second-line drugs only available in secondary care
The efficacy of a treatment should be evaluated after 3 months, and discontinued if no response with escalation to the next available treatment
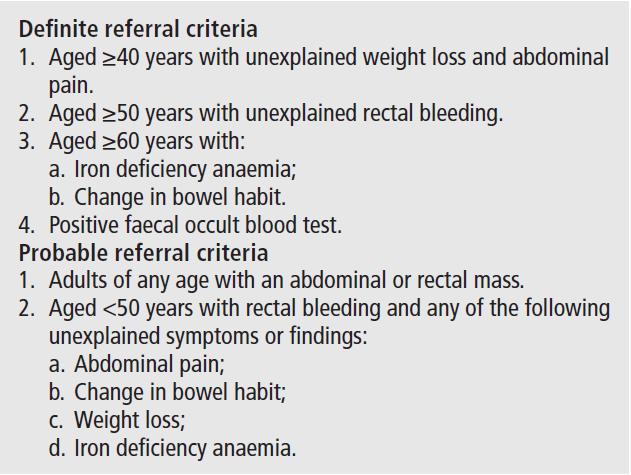
1. Fibre and Dietary Therapy:
First line dietary advice should be offered to all patients with IBS. This includes eating regular meals, maintaining adequate nutrition, limiting alcohol and caffeine intake, adjusting fibre intake, and reducing intake of spicy and fatty foods
For IBS-C, diet and lifestyle measures including drinking plenty of water, regular exercise and a high-fibre diet (up to 30g/ day) are recommended

Soluble fibre, such as isphagula (Fybogel), is a bulk-forming laxative and an effective treatment for IBS global symptoms and abdominal pain. This should be commenced at a low dose (3-4g/day) and built up gradually (to 20-30g/day) to avoid bloating
Insoluble fibre (e.g. wheat, bran) should be avoided as it may
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (i.e. the low FODMAPs diet) is an effective second-line dietary therapy for IBS global symptoms and abdominal pain. It is not effective for managing constipation. Its implementation should be supervised by a dietician, with foods reintroduced according to tolerance.
Food elimination diets based on IgG antibodies and a gluten-free diet are not recommended
2. Probiotics:
Probiotics significantly improve IBS global symptoms and abdominal pain
These is currently insufficient evidence to recommend a particular species or strain
Patients wishing to try probiotics are advised to take them for up to 12 weeks, and discontinue if no improvement in symptoms
Commonly prescribed probiotics include Alforex, Udos Super 8 and Symprove
3. Laxatives - First-line Drugs for IBS-C:
Laxative doses should be titrated according to symptoms
Both stimulant and osmotic laxatives are effective in the treatment of chronic idiopathic
Guidelines
Box 1: Lower Gastrointestinal alarm symptoms or signs that are referral criteria for suspected colorectal cancer
Figure 3: Treatment Algorithm for IBS
Gut: first published as 10.1136/gutjnl-2021-324598 on 26 April 2021. Downloaded HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 65
Figure 2 Treatment algorithm for IBS. *Review efficacy after 3 months of treatment and discontinue if no response. †As per the National Institute for Health and Care Excellence IBS dietary advice sheet, plus consider ispaghula. ‡TCAs should be first choice, starting at a dose of 10 mg at night, and titrating slowly (eg, by 10 mg/week) according to response and tolerability. Continue for at least 6 months if the patient reports
Examples include:
1. Stimulant laxatives: bisacodyl (Dulcolax), senna (Senokot), sodium picosulfate
2. Osmotic laxatives: lactulose (Duphalac), macrogol (Movicol), polyethylene glycol
Only osmotic laxatives have been formally evaluated for IBS-C, with limited efficacy seen - across two RCTs, polyethylene glycol significantly increased bowel movements in one trial with no significant effect on abdominal pain in either trial
4. Second-line Drugs for IBS-C: In patients with IBS-C and symptoms not improving with laxatives, escalation to secondline treatments should be considered. These include two medication classes:
1. Secretagogues: These activate ion channels on the intraluminal surface of
enterocytes, causing efflux of ions and water into the lumen, softening stool and accelerating transit. Examples include:
1) Linaclotide (Constella): A guanylate-cyclase c agonist that improves abdominal pain, bloating and frequency of bowel movements. The standard dose is 290μg OD. Across all trials, It is the most efficacious second-line IBS-C treatment but has the highest rate of side-effects, most commonly diarrhoea (over 10%)
2) Lubiprostone (Amitiza): A chloride channel activator that improves abdominal pain, bloating and frequency of bowel movements. The standard dose is 24μg BD. It is efficacious with lower rates of diarrhoea than linaclotide but nausea is common (30%)
3) Other secretagogues: Plecanatide and tenapanor
have similar efficacy and safety profiles to linaclotide and lubiprostone. They are licenced for IBS-C in the USA but not currently by the European Medical Agency (EMA)
2. 5HT4 Agonists: These are prokinetic agents, stimulating propulsive gut motility. Examples include:
1) Prucalopride (Resolor): Shown to improve abdominal pain, bloating and frequency of bowel movements in idiopathic chronic constipation. The standard dose is 2mg OD. To date, there have been no trials specifically for IBS-C
2) Tegaserod: Licenced in the USA for IBS-C in females under 65 years of age with no prior cardiovascular disease history. It is not currently licenced by the EMA
5. Psychological Therapies:
IBS-specific Cognitive Behavioural Therapy (CBT) and Gut-directed Hypnotherapy are
two areas of ongoing research that may be efficacious in global IBS symptoms
These should be considered when symptoms have not improved after 12 months of drug treatment, or earlier if locally available or patient preference
Their role specifically in IBS-C needs further evaluation
As you can see, there are many novel therapeutic options in IBS-C, offering patients many and varied treatments that can significantly improve symptoms as well as quality of life. Furthermore, this remains an area with considerable ongoing research, hopefully providing patients with even more treatment options in the coming years.
References:
1. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome, Vasant DH, et al. Gut 2021; 70: 1214 - 1240. doi: 10.1136/gutjnl-2021-324598
2. https://theromefoundation.org/ rome-iv/rome-iv-criteria/
A new study highlights how little we know about the vitamin D
status of ethnic minorities in Ireland
The study provides the most up to date findings on vitamin D intake and factors affecting vitamin D status of adults in Ireland, and has been published in the British Journal of Nutrition.
The study identifies groups most at risk of vitamin D deficiency and has highlighted gaps that exist in our understanding of why people are getting tested for vitamin D (a practice the researchers believe is often unnecessary and wasteful) along with how little we know about the vitamin D status of our ethnic minorities.
Currently 300,000 people in Ireland have osteoporosis (according to the Irish Osteoporosis Society), ensuring adequate vitamin D intake in early life can decrease the risk of osteoporosis/ fractures in later life.
The study investigated 383 participants, with a mean age of 56.0 years.
KEY FINDINGS
• The majority of adults (81%) not taking supplements did not

meet the recommended daily allowance (RDA) for vitamin D and this was the biggest risk factor for deficiency.
• Those with dark skin are nearly 4 times more likely to be vitamin D deficient.
• Participants with low vitamin D intakes, darker skin and who avoid the sun are most at risk of deficiency.
• Knowledge of recommended intakes was poor amongst participants yet there were high levels of inappropriate vitamin D testing. Half of those tested were unaware of the recommended daily allowance for vitamin D.
• Better awareness of vitamin D and need for adequate intakes should be promoted.
• Over a third (40%) of adults had their vitamin D levels tested for non-clinical reasons.
In a previous study, the research team have found that
approximately ¤60k was wasted per year in a Dublin hospital in unnecessary testing https://doi. org/10.1515/cclm-2021-0607. This places unnecessary burdens on laboratory and staff resources.
In the published study, it was found that over a third (40%) of adults had their vitamin D levels tested for no valid clinical reason with most being carried out as part a 'routine' clinic check. Only a minority (12%) were aware of the correct recommended daily amount and nearly half had no knowledge of any recommendations. While a minority (30%) felt they were familiar with vitamin D, the majority (86%) did still recognise it as important for bone health and 66% for immunity.
Researchers state that those ‘most at risk’ including adults with dark skin or with very poor intakes (those who don't eat fortified foods or supplements) and with little sun exposure may need more vitamin D to optimise their levels than recommended by the Food Safety
Authority of Ireland. Findings highlight the need for promotion of better awareness of vitamin D and of foods /fortified products that contain vitamin D and or supplements to improve status. They believe that fortification of staple food groups could also help address this issue and has been successful in other countries such as Finland.
Helena Scully, Mercers Glanbia Bone Research Fellow, MISA Institute, St James’s Hospital/ School of Medicine Trinity College and lead author of the study said, “We know that people living in Ireland are at-risk of vitamin D deficiency, particularly in the winter when we don’t get much sun. Our research indicates that those most at risk have inadequate vitamin D intake, dark skin types and avoid sun exposure. Choosing foods such as milk and cereal products with added vitamin D, and taking a supplement (15 micrograms or 600units per day), particularly in the winter can help prevent low vitamin D levels.”
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 66 GASTROENTEROLOGY: IBS
News
An Overview of Coeliac Disease
There are an estimated 50,000 people living with coeliac disease in Ireland, and a further 400,000 who are gluten intolerant. Furthermore, many cases of coeliac disease go undiagnosed Coeliac disease (CD) is an autoimmune disorder triggered by gluten ingestion in genetically predisposed individuals, resulting in mucosal inflammation and villous atrophy in the small intestine leading to malabsorption. It is a chronic disease and is not an allergy or food intolerance.
Hospital Professional News spoke to Theresa Lowry Lehnen, Clinical Nurse Practitioner and Associate Lecturer South East Technological University to gain further insights into this condition.

CD is one of the most common autoimmune conditions, with a prevalence of 0.5– 1% of the general population, with a female-to-male ratio ranging from 2:1 to 3:1.
Theresa told us that prevalence estimates based on serologic screens indicate the disorder may be present in 1/150 in Europe, and 1/250 in some parts of the US. She says, “Current prevalence estimates in some regions including Ireland are as high as 1/100. Onset of CD can occur at any age, although symptoms are most likely to develop in early childhood after weaning with gluten, and the introduction of cereals into the diet in the first year of life. Coeliac disease can also occur in later adulthood, and is often diagnosed in people aged 40-60 years.
“Coeliac disease is higher in firstdegree CD relatives (10–20%) and in other at-risk groups, particularly patients with Down syndrome, type 1 diabetes, or IgA deficiency. The condition is more common in people with Type 1 diabetes and autoimmune thyroid disease. Between 4 and 9% of people with Type 1 diabetes also have CD, compared with 1% in the general population. People with autoimmune thyroid disease are at a higher risk (1-4%) of having coeliac disease compared with 1% in the general population.”
Coeliac disease is a chronic condition, and currently, the only
treatment consists of permanent exclusion of gluten from the diet.
Aetiology and Pathophysiology
Coeliac disease is a hereditary disorder caused by sensitivity to the gliadin fraction of gluten, a protein found in wheat.
Theresa explains, “Similar proteins are present in rye and barley. In a genetically susceptible person, gluten-sensitive T cells are activated when glutenderived peptide epitopes are presented. The inflammatory response causes characteristic mucosal villous atrophy in the small intestine. This destruction, in turn, leads to the decreased functionality of the intestinal surface and malabsorption.
“The lack of nutrient absorption impacts directly on the digestive system but also indirectly on all the systems of the body. This results in generally poor health, and is why coeliac disease can have signs and symptoms arising from almost any body system, and not just the gastrointestinal system.”
Presentation
Coeliac disease is more commonly detected in infants and children younger than 3 years of age, and is characterised by diarrhoea, anorexia, pallor, abdominal distention, muscle wasting and failure to thrive. Stools are often soft, bulky, clay-coloured, and offensive. Older children may present with anaemia, weight loss or failure to grow normally. Adults may present with diarrhoea, bloating, constipation and abdominal pain. Lassitude, weakness, and anorexia are common. Mild and intermittent diarrhoea is sometimes the presenting symptom and steatorrhea ranging from mild to severe.
Theresa adds, “Some patients have weight loss, although rarely enough to become underweight. Anaemia, glossitis, angular stomatitis, and aphthous ulcers are often seen in these patients. Manifestations of vitamin D and calcium deficiencies such as osteomalacia, osteopenia and osteoporosis are common.
“Both males and females may have reduced fertility and women
may have amonorrhoea. About 10% of patients with coeliac disease develop Dermatitis herpetiformis (DH), a skin condition caused by gluten intolerance.
“Dermatitis herpetiformis is an intensely pruritic papulovesicular rash that is distributed symmetrically over the extensor areas of the elbows, knees, buttocks, shoulders, and scalp. It usually responds to the exclusion of gluten from the diet, but can take a long time for a gluten free diet to clear the rash. For Individuals with Dermatitis Herpetiformis, a skin biopsy is enough for diagnosis of both DH and coeliac disease. It is not necessary to perform an endoscopic biopsy, as the skin biopsy is definitive.
“Dapsone is a medication prescribed for DH, which is taken twice daily. Dapsone can cause side effects such as headache and depression and may need to be prescribed for up to 2 years. The lowest effective dose should be prescribed. Untreated coeliac disease leads to chronic ill health and complications. Complications which may or may not be present at diagnosis include osteoporosis, ulcerative jejunitis, malignancyintestinal lymphoma, functional hyposplenism, vitamin D deficiency and iron deficiency.
“Serological testing for coeliac disease should be offered to people with persistent unexplained abdominal or gastrointestinal symptoms; faltering growth; prolonged

fatigue; unexpected weight loss; severe or persistent mouth ulcers; unexplained iron, vitamin B12 or folate deficiency; type 1 diabetes at diagnosis; autoimmune thyroid disease at diagnosis; irritable bowel syndrome in adults; and first-degree relatives of people with coeliac disease.”
Diagnosis
A diagnosis of coeliac disease is suspected clinically, based on history and laboratory abnormalities suggestive of malabsorption. Family incidence is an indicator and coeliac disease should also be strongly considered in patients with iron deficiency without obvious gastrointestinal bleeding. A confirmed diagnosis depends on serologic markers and a smallbowel biopsy.
Anti-tissue transglutaminase antibody (tTG) and antiendomysial antibody (EMA) have sensitivity and specificity > 90%. These antibodies decrease in titre in patients on a gluten-free diet and are useful in monitoring dietary adherence.
Theresa adds that diagnostic serologic testing is done with patients following a glutencontaining diet and notes, “To obtain a reliable result the patient must have been consuming gluten for more than one meal a day for six weeks. It is important to advise the patient not to start a gluten-free diet until diagnosis is confirmed by a specialist, even if the results of a serological test are positive.
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 67 ENDOCRINOLOGY: COELIAC DISEASE
An interview with Theresa Lowry Lehnen (PhD), Clinical Nurse Practitioner and Associate Lecturer South East Technological University
“Coeliac disease is strongly associated with variations of the human leukocyte antigen (HLA) DQ gene. Research has shown that the HLA-DQ2 and HLA-DQ8 variants are almost always present in people with coeliac disease of white, Northern European background. Although less clear, these variants probably play a similar role in other ethnic groups.
“Testing for the HLA DQ2 and HLA DQ8 variants alone cannot be used to diagnose coeliac disease, as these genes are present in a large proportion of the population including people who do not have coeliac disease. NICE recommend to only consider using HLA DQ2 (DQ2.2and DQ2.5) DQ8 testing in diagnosis of coeliac disease in specialist settings, example children who are not having a biopsy or people who have already limited gluten from the diet and choose not to have a gluten challenge.
“Serological tests to investigate suspected coeliac disease in young people and adults should test for total immunoglobulin

A (IgA) and IgA tissue transglutaminase (tTG) as the first choice; IgA endomysial antibodies (EMA) if IgA tTG is weakly positive and consider using IgG EMA, IgG deamidated gliadin peptide (DGP) or IgG tTG if IgA is deficient. Serological tests to investigate suspected coeliac disease in children should test for total IgA and IgA tTG as the first choice, and consider using IgG EMA, IgG DGP or IgG tTG if IgA is deficient.”
Theresa adds, “If either serological test is negative, coeliac disease is unlikely. However, it is possible to have a negative test and still have coeliac disease for example patients already on a gluten free diet (GFD) and therefore the antibodies are negative. A minority of coeliac patients may have IgA deficiency and the screening test results can be misleading so, the total serum IgA should be checked to detect IgA deficiency.
“If either serological test is positive, the patient should have a diagnostic small-bowel biopsy. The gold standard for the diagnosis of coeliac disease is a duodenal mucosal biopsy which shows villous atrophy.4 Biopsy remains essential for the diagnosis of adult CD and cannot be replaced by serology alone. Exceptions are patients with coagulation disorders and pregnant women, in whom biopsy may not be feasible or should be postponed until postpartum.
“In coeliac disease, the damage to the lining of the intestine is classified according to the Marsh classification scale:
Marsh 0: lining of the intestine is normal and unlikely that the person has coeliac disease.
Marsh 1: increased number of lymphocytes seen, but there are normal villi.
Marsh 2: increased number of lymphocytes, the depressions in the lining of the intestine are deeper than normal, but normal villi length.
Marsh 3: the villi are becoming flattened.
Marsh 4: the villi are completely flattened.
“Originally the Marsh scale ranged from 0 to 4, with type 3 indicating coeliac disease. The scale has since been simplified to allow for more consistency and reproducibility between pathologists.”
There are situations when the diagnosis is not clear, she points out.
“Some patients experience symptoms despite no identified changes on the small bowel biopsy. There is also seronegative coeliac disease where despite typical symptoms and significant villous atrophy of the duodenal biopsy, there is no serological evidence of the disease.”
Treatment and Management
The main treatment of coeliac disease is a lifelong, strict, gluten free diet (GFD). These include wheat protein, gliadin, rye protein, secalin, and barley protein, hordein. The goal of treatment is to relieve symptoms, achieve mucosal healing, avoid complications of CD, and have a good quality of life with a nutritionally complete GFD.
“This is best achieved when patients are motivated, receive expert information and are involved in their own care and treatment. Follow-up and annual review are necessary to ensure adequate response to treatment, prevention of complications, and maintenance of motivation to remain gluten free.
“As a gluten-free diet is the primary treatment option for people with coeliac disease, it is important that a dietitian or HCP with a specialist interest in coeliac disease plays a significant role in the patients care and follow-up. Many of the common problems associated with the long-term management of coeliac disease happen because of nonadherence to a gluten-free diet.
“Patient education is important and a healthcare professional with a specialist knowledge of coeliac disease should explain to the patient, and their family members or carers the importance of a gluten-free diet, and provide detailed information to help the patient follow it. This should include: information on which types of food contain gluten and suitable alternatives, including gluten-free substitutes, explanations of food labelling information sources about gluten-free diets, recipe ideas and cookbooks, how to manage social situations, eating out and travelling away from home, including travel abroad, avoiding cross-contamination with gluten in the home, minimising the risk of accidental gluten intake when eating out, and the role of national and local coeliac support groups.
“Many everyday foods are gluten free including meat, vegetables, cheese, potatoes and rice. Foods containing gluten include, bread, pasta, cereals, biscuits, crackers, cakes, pastries, pies, gravies and sauces. However, gluten free varieties of these and other products exist. It is important to check the labels on food, as many products, particularly processed food contain gluten additives. Gluten may also be found in non-food products such as, some medications, postage stamps and lipstick.
MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 68 ENDOCRINOLOGY: COELIAC DISEASE
Modified
MARSH TYPE IEL / 100 ENTEROCYTES –JEJUNUM* IEL / 100 ENTEROCYTES –DUODENUM* CRYPT HYPERPLASIA VILLI 0 <40 <30 Normal Normal 1 >40 >30 Normal Normal 2 >40 >30 Increased Normal 3a >40 >30 Increased Mild atrophy 3b >40 >30 Increased Marked atrophy 3c >40 >30 Increased Complete atrophy
Marsh Classification of histologic findings in celiac disease (Oberhuber)
Modified Marsh Classification of histologic findings in celiac disease (Oberhuber)
“Oats contain a protein called avenin which is like gluten, but most people with coeliac disease can safely eat avenin. Before including oats in the diet, the CD patient should talk to a dietician or HCP, and check the oats are pure with no possibility of contamination from other grains. Oats should be avoided until the GFD is working well. They can be re-introduced into the diet, when the patient is symptom free, but should be stopped if symptoms re-occur.
“Symptoms usually improve over the course of several weeks once a gluten-free diet is initiated. Patients who do not respond, need further review, but also an assessment of compliance with the gluten free diet. Serology testing can assess compliance. Non-compliance can be unintentional, and an individual may be still ingesting gluten without realising it.
“Treatment and management involve assessing the impact of malabsorption on the body.
Nutritional deficiencies are common in CD and should be identified and treated. Full blood count, iron stores, folate, ferritin, vitamin B12, levels of vitamin D and other fat-soluble vitamins, and bone mineral density should

be monitored.3 A Dexa scan may be required for those with bone thinning or osteoporosis. A gluten-free diet itself can also be associated with lower levels of certain micronutrients. Fibre, iron, calcium, vitamin D, vitamin B6, vitamin B12 and folate deficiencies have all been noted in people following a gluten-free diet. Gluten-free foods themselves have been shown to be lower in thiamine, riboflavin, niacin, folate, iron and dietary fibre. This may primarily be because gluten-free foods tend not to be fortified, and be refined. Coeliac patients should be encouraged to consume foods high in iron and folate to combat these deficiencies, and supplements considered if recommended intakes cannot be achieved through diet alone.
“Calcium and vitamin D deficiencies may also be present and supplements necessary if intake is insufficient. Other nutritional deficiencies may include magnesium, zinc, niacin and riboflavin.”
Theresa notes that most nutritional deficiencies will resolve due to mucosal healing and increased absorption, once a gluten-free diet is established.
The key elements of managing coeliac disease through diet are to choose and eat foods that are gluten-free; consume a wellbalanced diet; including good sources of calcium, iron, vitamin D and B vitamins; eat foods that are rich in fibre; always check foods and fluids in the coeliac society list of gluten-free foods, and check food labels.

She adds, “The emphasis of dietary management in coeliac disease should focus on the nutritional quality of the diet, and not just simply ‘foods allowed or foods to avoid’.”
Prognosis, Outlook and Support
Prognosis for individuals diagnosed early and who remain compliant with a gluten-free diet is good, however, a lifelong diet completely free of gluten can be very costly and challenging, Theresa told us.
“Compliance with a gluten-free diet is difficult, and relapses are common. Many patients continue to experience symptoms, often due to imperfect adherence to a gluten-free diet. People with coeliac disease face a lifelong condition that can be emotionally and physically debilitating, and
which left untreated can lead to a significant reduction in quality of life. While a large selection of gluten free products is available in major supermarkets and food outlets and more supports available now than in the past, increased awareness of CD as a worldwide health problem is necessary, to help patients cope with the illness and its treatment.
“Patient support and information is an integral part of the management of CD. Clinician play a lead part by providing ongoing assessment, management, support and education. Key roles are to establish a therapeutic relationship with the patient, assess their understanding of the condition, establish goals and expectations for successful management of their condition, and evaluate their physical, emotional, and psychological well-being. Monitoring and evaluating symptoms, outcomes and responses to therapy, enables clinicians to play a pivotal role in managing coeliac disease, and improving the patient’s quality of life.
“Future drug therapies are currently in development with the hope of reducing the burden of living with coeliac disease, and improving long-term health outcomes. Clinical trials are in progress, but only a few have reached later clinical trial phases. Other scientific challenges include obtaining a better understanding of phenotypes and seronegative coeliac disease. There is ongoing work on developing possible non-dietary therapies that would enable people with coeliac disease to tolerate gluten. One of the main focuses of the research in this area is immune modulators. Ongoing research and therapeutic strategies aimed at developing a vaccine and desensitising those with CD to gliadin peptides, could provide a preventative and definitive cure for CD.
“Identification of future cure and/or alternative treatments to a gluten-free diet brings hope for CD patients, who are unavoidably burdened by dietary restrictions. The Coeliac Society of Ireland https://coeliac.ie/ is a registered charity that provides information and support to people diagnosed with coeliac disease throughout Ireland.”
HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023 69
Lipid Guidelines
Abbreviated lipid guidelines for clinical practice
Written by: Vincent Maher, ALMAR Centre, Tallaght University Hospital, Department of Cardiology, Trinity College Dublin; Joe Gallagher, Department of General Practice, University College Dublin; Ruth Agar, ALMAR Centre, Tallaght University Hospital, Department of Cardiology, Trinity College Dublin; Damian Griffin, Department of Chemical Pathology, University Hospital Galway; Niall Colwell, Tipperary University Hospital; Patricia O’Connor, Department of Clinical Pharmacology, St James Hospital, Dublin; Brendan McAdam, Beaumont Hospital Dublin; Gerald Tomkin, Department of Medicine, Trinity College Dublin; Daphne Owens, Department of Biochemistry, Royal College of Surgeons in Ireland; Mary Ryan, Department of Endocrinology, Bon Secours Hospital, Limerick; William Tormey, Department of Chemical Pathology, Beaumont Hospital & Maeve Durkan, Department of Endocrinology, Bon Secours Hospital, University College Cork
general practice was involved from the outset in this process to help guide the appropriate direction undertaken.9 Following preliminary meetings of a core group of interested parties, a working document was produced. A single paged document that could be viewed electronically and easily incorporated into practice computer desk tops was deemed the preferred option. Careful consideration was given to highlight key elements such as the need for abbreviated guidelines, who and how to test, risk groups and target lipid levels, practical steps and suggested lipid-lowering drugs and dosages required to achieve lipid targets. An explanatory page was also produced to help guide interpretation when needed. A number of hyperlink materials were also developed to help familiarise practitioners with information that would enhance their understanding of the basis for intervention and need for further screening as deemed necessary.10,11,12,13,14,15
Discussion
Clinical lipid guidelines based on the latest available information are developed by a panel of international experts.1, 2 They make recommendations according to the type and level of evidence supporting or not the best course of action in various circumstances. Despite an enormous effort to develop guidelines, their translation into clinical practice is often slow or challenging.3,4,5,6 Many with severe hypercholesterolaemia go undetected or inadequately treated.7 In the modern era of sound bites and information overload, the practicing physician and nurse have only a limited time to comprehend and utilise guidelines. Previous studies highlight that general practitioners expressed frustration concerning the length and accessibility of guidelines.8 Despite this, all practitioners hope to have the latest most appropriate information to deliver good clinical
practice. In an effort to bridge this gap, many have requested a brief summary, or clinical pathways to help them treat lipid disorders particularly within primary care. The distillation of the extensive knowledge base in guidelines into practical recommendations that are clearly visualised, understood and easy to use requires the collective effort of lipid specialists. Some members of the Irish Lipid Network which incorporates specialists engaged in advanced lipid management in the Republic of Ireland volunteered to undertake this task. They formulated an easy-to-use comprehensive short document with links to appropriate associated information in a manner that could be used electronically in clinical practice. This guide was reviewed and approved by the Quality and Safety in Practice Committee of the Irish College of General Practitioners prior to submission
to ensure ease of application in routine general practice.
Methods
A number of members of the Irish Lipid Network were involved in the production of a document that could facilitate interpretation and management of lipid disorders in Ireland. Those involved in specialist lipid clinics are already familiar with the various aspects of lipid guidelines. As a consequence, the focus was on how to produce a short document for the larger body of practitioners particularly those in primary care, to help them manage lipid disorders when time is limited and detailed study is not possible. The specialists who participated included cardiologists, chemical pathologists, endocrinologists, clinical pharmacologists, general practitioners, nurses and scientists. Given the need to widely disseminate lipid management information,
The extent of lipid problems in Ireland is significant, and lipid management4, 6 and lipid services are inadequate.15, 16 The significance of untreated lipid disorders on population health cannot be overestimated,17, 18 and early interventions could have far-reaching consequences on Irish lives and health care resource utilisation. Furthermore, evidence from our older population in Ireland also highlights inadequate treatment of cholesterol problems once diagnosed.19 Following detection, lowering of LDL cholesterol levels to very low levels yields a 22% cardiovascular risk reduction for each mmol/l LDLc reduction using statins. Additionally, lowering of non-HDLc levels in those with moderately elevated triglycerides further reduces cardiovascular risks.20 Treatment of those with severe hypertriglyceridaemia markedly reduces the risk of pancreatitis.21 It is unacceptable that any individual has undetected
70 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
COMBINING POWER AND CONFIDENCE
AGAINST LDL-C*
Rosuvastatin
10 mg
40 mg /10 mg
Rosuvastatin + Ezetimibe
20 mg /10 mg
High intensity statin single pill combination for LDL-C reduction1
Therapeutic Indications:
Primary Hypercholesterolaemia/Homozygous Familial Hypercholesterolaemia (HoFH) Suvezen is indicated for substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate products, as adjunct to diet for treatment of primary hypercholesterolaemia (heterozygous familial and non-familial) or homozygous familial hypercholesterolaemia.
Prevention of Cardiovascular Events Suvezen is indicated as substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently, at the same dose level as in the fixed dose combination, but as separate products to reduce the risk of cardiovascular events in patients with coronary heart disease (CHD) and a history of acute coronary syndrome (ACS).
Prescribing Information: Suvezen (rosuvastatin/ ezetimibe) film-coated tablets

Please refer to the Summary of Product Characteristics (SmPC) for full prescribing details.
Presentations: Suvezen 10mg/10mg, 20mg/10mg and 40mg/10mg: Each film-coated tablet contains 10mg; 20mg or 40mg of rosuvastatin (as rosuvastatin calcium) respectively, and 10mg ezetimibe. Indication: Suvezen is indicated for substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently at the same dose level as in the fixed combination, but as separate products, as adjunct to diet for treatment of primary hypercholesterolaemia (heterozygous familial and non-familial) or homozygous familial hypercholesterolaemia. Suvezen is indicated as a substitution therapy in adult patients who are adequately controlled with rosuvastatin and ezetimibe given concurrently, at the same dose level as in the fixed dose combination, but as separate products to reduce the risk of cardiovascular events in patients with coronary heart disease (CHD) and a history of acute coronary syndrome (ACS). Dosage and Administration: The patient should be on and continue, an appropriate lipid-lowering diet, during treatment with Suvezen. Suvezen is not suitable for initial therapy. Treatment initiation or dose adjustment, if necessary, should only be done with the monocomponents and after setting the appropriate doses the switch to the fixed dose combination of the appropriate strength is possible. Patient should use the strength corresponding to their previous treatment. The recommended dose is one Suvezen tablet daily. To be administered at any time of the day, with or without food. The tablet should be swallowed whole with a drink of water. If co-administered with bile acid sequestrant (BAS), administration of Suvezen should occur either ≥2 hours before or ≥4 hours after administration of a BAS. Special populations: Paediatric (<18 years): Safety and efficacy has not been established. Elderly (>70 years): Starting dose of 5 mg rosuvastatin is recommended. The combination is not suitable for initial therapy. Hepatic impairment: Mild: No dosage adjustment is required. Moderate/Severe: Treatment with Suvezen is not recommended. Renal impairment: Mild: No dose adjustment is necessary. Moderate (creatinine clearance <60 ml/ min): The recommended start dose is rosuvastatin 5mg. Race: The recommended start dose is rosuvastatin 5 mg for patients of Asian ancestry due to increased systemic exposure. The fixed dose combination is not suitable for initial therapy. Monocomponent preparations should be used to start the treatment or to modify the dose. Suvezen 40 mg/10 mg tablets are contraindicated in these patients. Genetic polymorphisms: In patients who are known to have specific types of genetic polymorphisms that can lead to increased rosuvastatin exposure, a lower daily dose of Suvezen is recommended. Dosage in patients with pre-disposing factors to myopathy: The recommended start dose is rosuvastatin 5mg in patients with pre-disposing factors to myopathy. Suvezen 40 mg/10 mg tablets are contraindicated in some of these patients. Concomitant therapy: The risk of myopathy (including rhabdomyolysis) is increased when Suvezen is administered concomitantly with certain medicinal products that may increase the plasma concentration of rosuvastatin (e.g. ciclosporin and certain protease inhibitors including combinations of ritonavir with atazanavir, lopinavir, and/or tipranavir). Whenever possible, alternative medications should be considered, and, if necessary, consider temporarily discontinuing Suvezen therapy. In situations where co-administration of these medicinal products with Suvezen is unavoidable, the benefit and the risk of concurrent treatment and rosuvastatin dosing adjustments should be carefully considered. Contraindications: Hypersensitivity to the active substances or excipients. Pregnancy, breast-feeding and in women of childbearing potential not using appropriate contraceptive measures. Active liver disease or any serum transaminase elevations which are unexplained, persistent or exceeding 3x the upper limit of normal (ULN). Severe renal impairment (creatinine clearance <30ml/min); myopathy or receiving concomitant ciclosporin. 40mg/10mg dose contraindicated in patients with predisposing factors for myopathy/rhabdomyolysis; such factors include: Moderate renal impairment (creatinine clearance <60ml/min), hypothyroidism, personal or family history of hereditary muscular disorders, previous history of muscular toxicity with another HMG-CoA reductase inhibitor or fibrate, alcohol abuse, situations where an increase in plasma levels of rosuvastatin may occur, Asian patients, concomitant use of fibrates.


Precautions and Warnings: Skeletal muscle effects: have been reported in rosuvastatin-treated patients with all doses and in particular with doses >20mg. As with other HMG-CoA reductase inhibitors, reporting rate for rhabdomyolysis is associated with use at doses >40mg. Post-marketing experience with ezetimibe, cases of myopathy and rhabdomyolysis have been reported. If myopathy is suspected based on muscle symptoms or is confirmed by a creatine phosphokinase (CPK) level, Suvezen and any of these other agents that the patient is taking concomitantly should be immediately discontinued. All patients starting therapy with Suvezen should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness, particularly if associated with malaise or fever. Creatine kinase (CK) measurement: CK should not be measured following strenuous exercise or in the presence of a plausible alternative cause of CK increase. If CK levels are significantly elevated at baseline (>5x ULN) a confirmatory test should be carried out within 5 – 7 days. If the repeat test confirms a baseline CK >5x ULN, treatment should not be started.
Patients with pre-disposing factors for myopathy/rhabdomyolysis: Caution should be exercised in these patients. Risk: benefit of treatment should be considered and clinical monitoring is recommended. CK levels should be measured in these patients. Therapy should be discontinued if CK levels are markedly elevated (>5x ULN) or if muscular symptoms are severe and cause daily discomfort. If symptoms resolve and CK levels return to normal, then consideration should be given to re-introducing treatment at the lowest dose. Immune-mediated necrotising myopathy (IMNM): Clinically characterised by proximal muscle weakness and elevated serum CK, has been reported very rarely during or after treatment with statins, including rosuvastatin, despite discontinuation of statin treatment. In clinical trials an increase in the incidence of myositis and myopathy has been seen in patients receiving other HMG-CoA reductase inhibitors together with fibric acid derivatives. Suvezen should not be used in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g. sepsis, hypotension, major surgery, trauma, severe metabolic, endocrine and electrolyte disorders; or uncontrolled seizures).
Liver effects: In controlled coadministration trials in patients receiving ezetimibe with statin, consecutive transaminase elevations ≥3x ULN have been observed. It is recommended that liver function tests be carried out prior to, and 3 months following, the initiation of treatment. Rosuvastatin should be discontinued or the dose reduced if the level of serum transaminases is >3x ULN. The reporting rate for serious events is higher at the 40mg dose. In patients with secondary hypercholesterolaemia caused by hypothyroidism or nephrotic syndrome, the underlying disease should be treated prior to initiating therapy with rosuvastatin. Liver disease and alcohol: As with other HMG-CoA reductase
Reference: 1. Suvezen Summary of Product Characteristics

* LDL-C: Low-density lipoprotein Cholesterol
inhibitors, rosuvastatin should be used with caution in patients who consume excessive quantities of alcohol and/ or have a history of liver disease. Renal effects: Proteinuria has been observed in patients treated with higher doses of rosuvastatin and was transient or intermittent in most cases. Proteinuria has not been shown to be predictive of acute or progressive renal disease. An assessment of renal function should be considered during routine follow-up of patients treated with a dose of 40mg. Diabetes mellitus: Some evidence suggests that statins raise blood glucose and in some patients, at high risk of future diabetes, may produce a level of hyperglycaemia where formal diabetes care is appropriate. This risk, however, is outweighed by the reduction in vascular risk with statins and therefore should not be a reason for stopping statin treatment. Patients at risk (fasting glucose 5.6 – 6.9mmol/l, BMI >30kg/m2, raised triglycerides, hypertension) should be monitored both clinically and biochemically according to national guidelines. Interstitial lung disease: Exceptional cases have been reported with some statins, especially with long term therapy. Presenting features can include dyspnoea, non-productive cough and deterioration in general health (fatigue, weight loss and fever). If it is suspected, statin therapy should be discontinued. Severe cutaneous adverse reactions: Stevens-Johnson syndrome (SJS) and drug reaction with eosinophilia and systemic symptoms (DRESS), which could be life-threatening or fatal, have been reported with rosuvastatin. If signs and symptoms suggestive of this reaction appears, Suvezen must be discontinued immediately and an alternative treatment should be considered. Treatment with Suvezen must not be restarted at any time. Protease inhibitors: Increased systemic exposure to rosuvastatin has been observed in subjects receiving rosuvastatin concomitantly with various protease inhibitors in combination with ritonavir. Consideration should be given both to the benefit of lipid lowering by use of Suvezen in HIV patients receiving protease inhibitors and the potential for increased rosuvastatin plasma concentrations when initiating and up titrating rosuvastatin doses in patients treated with protease inhibitors. The concomitant use with certain protease inhibitors is not recommended unless the dose of rosuvastatin is adjusted. Fibrates: The safety and efficacy of ezetimibe administered with fibrates have not been established. If cholelithiasis is suspected in a patient receiving Suvezen and fenofibrate, gallbladder investigations are indicated and therapy should be discontinued. Anticoagulants: If Suvezen is added to warfarin, another coumarin anticoagulant, or fluindione, the International Normalised Ratio (INR) should be appropriately monitored. Fusidic acid: Suvezen must not be co-administered with systemic formulations of fusidic acid or within 7 days of stopping fusidic acid treatment. In patients where the use of systemic fusidic acid is considered essential, statin treatment should be discontinued throughout the duration of fusidic acid treatment. There have been reports of rhabdomyolysis (including some fatalities) in patients receiving fusidic acid and statins in combination. The patient should be advised to seek medical advice immediately if they experience any symptoms of muscle weakness, pain or tenderness. Statin therapy may be reintroduced seven days after the last dose of fusidic acid. In exceptional circumstances, where prolonged systemic fusidic acid is needed, e.g., for the treatment of severe infections, the need for co-administration of Suvezen and fusidic acid should only be considered on a case by case basis and under close medical supervision. Suvezen contains lactose and sodium: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine. This medicine is essentially ‘sodium-free’. Interactions: Contraindicated combinations: Ciclosporin. Not recommended combinations: Fibrates and other lipid-lowering products, protease inhibitors, transporter protein inhibitors and fusidic acid. Other possible interactions: Cytochrome P450 enzymes, antacids, colestyramine, anticoagulants, Vitamin K antagonists, clopidogrel, ticagrelor, erythromycin, oral contraceptive/hormone replacement therapy. When coadministering rosuvastatin with other medicinal products known to increase exposure to rosuvastatin, doses should be adjusted (see SmPC for full details). The maximum daily dose should be adjusted so that the expected rosuvastatin exposure would not likely exceed that of a 40mg daily dose of rosuvastatin taken without interacting medicinal products. Fertility, Pregnancy and Breastfeeding: No clinical data are available on the use of ezetimibe during pregnancy. Potential risk from inhibition of HMG-CoA reductase outweighs the advantage of treatment during pregnancy. If a patient becomes pregnant during use of Suvezen, treatment should be discontinued immediately. Animal studies have shown excretion of medicinal product through breast milk. However, there are no data in humans. No clinical trial data on the effects of fertility in humans. Adverse Reactions: Adverse drug reactions previously reported with one of the individual components (ezetimibe or rosuvastatin) may be potential undesirable effects with Suvezen. Common: diabetes mellitus, headache, dizziness, constipation, nausea, abdominal pain, diarrhoea, flatulence, myalgia, ALT and/or AST increased, asthenia and fatigue. Uncommon: decreased appetite, paraesthesia, hot flush, hypertension, cough, dyspepsia, gastroesophageal reflux disease, nausea, dry mouth, gastritis, pruritus, rash, urticaria, arthralgia, muscle spasms, neck pain, back pain, muscular weakness, pain in extremity, ALT and/or AST increased, blood CPK increased, gamma-glutamyltransferase increased, liver function test abnormal, chest pain, pain, asthenia, oedema peripheral. Rare: thrombocytopenia, hypersensitivity reactions including angioedema, pancreatitis, increased hepatic transaminases, myopathy (including myositis), rhabdomyolysis, lupus-like syndrome and muscle rupture. Very rare: polyneuropathy, memory loss, jaundice, hepatitis, arthralgia, haematuria, gynaecomastia. Not known: thrombocytopenia, hypersensitivity (including rash, urticaria, anaphylaxis and angioedema), depression, peripheral neuropathy, sleep disturbances (including insomnia and nightmares), dizziness, paraesthesia, cough, dyspnoea, diarrhoea, pancreatitis, constipation, hepatitis, cholelithiasis, cholecystitis, Stevens Johnson syndrome, erythema multiforme, drug reaction with eosinophilia and systemic symptoms, immune-mediated necrotising myopathy, tendon disorders (sometimes complicated by rupture), myalgia, myopathy/rhabdomyolysis, oedema, asthenia. Prescribers should consult the SmPC in relation to other adverse reactions. Legal Category: POM. Marketing Authorisation Numbers: 10mg/10mg: PA0540/193/001; 20mg/10mg: PA0540/193/002; 40mg/10mg: PA0540/193/003. Marketing Authorisation Holder: Sanofi-Aventis Ireland Ltd. T/A SANOFI, Citywest Business Campus, Dublin 24, Ireland. Further information is available from: Sanofi, 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com Date of Preparation: December 2022 (MAT-IE-2200239 v1.0) Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com
MAT-IE-2101261 (v3.0) | January 2023
+ Ezetimibe
Rosuvastatin + Ezetimibe
Lipid Guidelines
serious lipid problems which are causing accelerated ASCVD or pancreatitis. As a consequence, when it comes to lipid disorders “What you don’t know may hurt you!”. Therefore, familiarisation of lipid management in primary care will facilitate more individuals being screened and more severe genetic disorders being identified earlier.22, 23 The objective of these abbreviated guidelines is to facilitate greater understanding by distilling the most pertinent, useable information from the ESC lipid management guidelines (2019) and the ESC Cardiovascular prevention in clinical practice guidelines (2021) related to lipid disorders. Although shortened versions are likely to eliminate a lot of explanatory information that enhances understanding and management, this approach allows interventions to be undertaken quickly in a reassuring manner
News
without the need for further detailed study. For those who wish to glean further information, appropriate links are provided to help in this regard. Since all of this information will be presented electronically on computer desktops, updates and additional information can easily be added. Auditing the use of this abbreviated lipid management tool will facilitate a better understanding of what really matters to practitioners.
As the format of limiting the information to a single page has been widely used in primary care for other conditions, it is likely that this approach to lipid management will also be useful. Endorsement by a group of specialists who are usually the main referral group for lipid management in Ireland may also help utilisation of these modified
guidelines. Like all new ventures, only time will tell if this approach is valuable. Certainly, presentation of preliminary versions of these guidelines was widely accepted by general practitioners and endorsed by the Irish College of General Practitioners.
Dissemination of this information and provision of appropriate software versions of these documents for incorporation on computer desk tops will require support. Ideally, monies needed for dissemination of this information should come from various sources to help maintain and expand the development of this tool. Independence from bias on any information provided needs to be ensured in order to engage the trust of practitioners on the validity of this approach long term. Finally, guidelines are only guidelines, and they will afford
Molecular Changes in Breast Cancer
practitioners with some of the information required to help their patients. It is likely that these abbreviated guidelines will require associated educational support in order to familiarise practitioners on how to use them and also to gain feedback on their utility. It is envisioned that educational meetings, videos and short practice guidelines will be provided. As a result of increased awareness and interventions, there will also be increasing numbers of patients that need specialist lipid management. In this regard, development of guidelines needs to be followed by the necessary provision of appropriately resourced specialist lipid centres if a seamless optimal lipid management service is to be provided nationally.
References availabe on request
Researchers at RCSI University of Medicine and Health Sciences and the Beaumont RCSI Cancer Centre have discovered key molecular changes that drive the spread of breast cancer to the brain.
The research findings, published in the journal Cancer Communications ahead of World Cancer Day (4 February), also identified potential new approaches to treating advanced breast cancer in the future.
With the support of Breast Cancer Ireland ambassadors, Professor Leonie Young and colleagues focused on breast cancer brain metastases, where an original
cancer in the breast establishes secondary tumours in the brain.
“Metastatic brain disease is a devastating illness that happens in up to a third of patients with advanced breast cancer,” explains Prof. Young, the study’s Principal Investigator and Scientific Director of the Opens in new windowBeaumont RCSI Cancer Centre.
“There are currently few treatment options when this happens, and they centre mainly on surgery and radiation treatment. The prognosis is not good for patients, and we urgently need new medicines to help them.”
Research for new ways to treat metastatic brain disease has tended to focus on changes in the DNA of cancerous cells, but Prof. Young took a different approach. She and her collaborators looked at changes in RNA, a type of molecule that helps to control how genes work.

“There had been some evidence that reversible changes in RNA were present in advanced brain metastatic disease,” says Prof. Young. “So we wanted to explore these changes, to understand them better, on the basis that they could potentially be used to indicate how the disease was progressing and possibly represent new targets for treatments to intervene.”
By examining cells in the lab and patient tumours from both the breast and the brain, the researchers mapped the patterns
of methylation in RNA, a natural process where the RNA is decorated with chemical structures called methyl groups that affect the way the RNA works. The scientists were able to show that RNA methylation was an essential mechanism that enables the tumour to evolve and progress.
“In this study we mapped the RNA methylation landscape in advanced breast cancer and determined its clinical relevance, and importantly patients and Breast Cancer Ireland ambassadors played a key role in this work,” says Prof. Young.
“Our research showed that targeting this mechanism with drugs called FTO inhibitors that are currently in trial for other cancer types could potentially be significantly beneficial for patients with breast cancer that has spread to the brain. Our hope is that these findings will translate into better outcomes for patients with advanced breast cancer.”
The study was a collaboration between the Endocrine Oncology Research Group at RCSI’s Department of Surgery and Ben May Department for Cancer Research at the University of Chicago, and was funded by Breast Cancer Ireland, Science Foundation Ireland, Breast Cancer NOW and the Irish Research Council.
72 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Professor Leonie Young, RCSI
Irish Women in Cardiology Survey
A report from the Irish women in cardiology survey, exploring Europe’s largest gender gap in cardiology
Written by
Bethany Wong, Alice Brennan, Department of Cardiology, Golden Jubilee Hospital, Glasgow; Stephanie James, Department of Cardiovascular Imaging, Beacon Hospital, Dublin; Lisa Brandon, Department of Cardiology, St James Hospital, Dublin; Deepti Ranganathan, Department of Cardiology, Mater Misercordiae Hospital, Dublin; Barbra Dalton, Irish Cardiac Society; Ken McDonald, School of Medicine, University College Dublin; Deirdre Ward, Department of Cardiology, Tallaght Hospital, Dublin
Lead author: Dr Bethany Wong is currently a Cardiology Specialist Registrar, a PhD candidate at University College Dublin and an Irish Clinical Academic Training (ICAT) fellow. She is a founding member of the Irish Women in Cardiology subgroup of the ICS. Through this, she has helped to set up a formalized mentorship programme for junior doctors, as well as fundraise for educational and networking events to improve gender equality in Irish Cardiology.

In the Republic of Ireland (ROI), 8% of public cardiology consultants are female; the lowest proportion in Europe, despite more females entering the medical workforce. The Medical Workforce Report 2020–211 stated that cardiology had the lowest female consultant ratio of any medical sub-speciality.1 Although, Northern Ireland (NI) is part of the UK, NI and ROI share an ‘all island’ professional society called the Irish Cardiac Society (ICS). Although there are currently no data as to the female consultant ratios in NI, it is likely similar to both the UK (13% female consultants2) and ROI.
Several publications examining the gender gap in cardiology globally2–5 have aimed to find solutions to improve gender discrepancy. Although Ireland ranks in the top 10 most genderequal countries in the world, with almost gender parity on educational attainment (99.8%) and Health and Survival (96.4%),6 this does not translate into cardiology. We sought to understand the perceptions of Irish trainees and consultants on aspects of working in cardiology to identify areas that can target this disparity.
Methods
University College Dublin research ethics committee approved this study. A questionnaire was created and adapted from previous studies assessing the reasons for gender discrepancies in cardiology.2–4,7
Irish Cardiac Society, distributed the survey to all cardiology trainees and consultants through its mailing list.
Descriptive and frequency analyses were used for demographic data. Comparisons between groups were conducted using independent samples t-tests or Mann–Whitney U tests. X2 tests were used to analyse gender response differences. Phi coefficient was used for assessing the effect size of X2 associations, with an effect value of 0.1 = small effect, 0.3 = moderate effect, 0.5 = large effect size. Two-sided P-values <0.05 were considered statistically significant. Themes for free text box answers were collated. Only one theme per participant response was allocated to ensure equal representation.
Results
There were 94 respondents with a response rate of 30%. Females made up 47 (50%) of respondents. A total of 54% were married, however, females were more likely to be single compared with their male counterparts (36% vs. 17%, P < 0.05). Females also reported higher levels of childcare responsibilities, with 19% providing >70% of the childcare duties, vs. 5% of males reporting to provide >70% of the childcare duties (P < 0.05, phi = 0.42).
Despite only one person (1%) reporting working less than full time (LTFT), the majority (53%) of the respondents said they would consider working LTFT, given the opportunity. The main reasons were more time with family, better work–life balance, and burnout. However, almost two-thirds (64%) of respondents felt their departments would not accommodate LTFT cardiologists.
A variety of sub-specialty fields within cardiology were represented including interventional cardiology (30%), imaging (20%), heart failure (13%), electrophysiology (12%), and general cardiology (16%). Of
note, males were twice as likely (28% vs. 14%, P-value <0.05, phi = 0.29) to choose specialities involving higher radiation exposure, such as intervention or electrophysiology.
Forty-eight per cent of respondents reported having experienced bullying, regardless of gender (females 53%, males 43%) or seniority. Consultants accounted for 60% of bullies. Only 1 in 2 respondents reported bullying to seniors (53%), and 46%, felt a lack of reporting system.
A total of 79% of females reported experiencing sexism, compared with 15% of males (P < 0.001, phi = 0.65). There was a significant difference (P = 0.001, phi = 0.40) in females (30%) compared with males (2%) reporting, missed opportunities for professional advancement based on their gender. Most females (85%) felt that training in cardiology was harder for female trainees, and this view was shared by 53% of male respondents. Themes included: sexism (19%), maternity difficulties (19%), childcare commitments (19%), cardiology being perceived as a male-dominated speciality (14%), and a lack of work flexibility (13%) as key reasons. Figure 1 demonstrates that females report
that their career prospects were significantly lower than males, whereas males reported that career prospects were the same. Overall, 70% of respondents, felt that cardiology would benefit from more female representation and having a mentor through their cardiology training (females = 91%, males = 54%).
Discussion
Irish female cardiology trainees and consultants report having experienced sexism (79%), bullying (53%), and a perceived lack of career advancement based on gender (30%). There are some similarities to the current study with the British Junior Cardiology Association (BJCA) report.2,8 This includes the significant gender difference in those pursuing procedural, high radiation, sub-specialities such as intervention or electrophysiology. In the UK, 9.4% of female trainees2 and 48% of female consultants7 experienced or witnessed sexism. This is lower than the 79% reported here, and additionally, this all-Ireland survey, asked respondents only if they themselves had experience sexism, and not if it had been witnessed, which would likely lead to a much higher reported rate.
73 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
Cardiology
Dr Wong
1 Comparison of perception of career prospects for female cardiologists between males and females. The figure ...
Cardiology
Figure 1 Comparison of perception of career prospects for female cardiologists between males and females. The figure demonstrates a scale from 1 to 10 of respondents answer to the question ‘In your opinion are career prospects for female cardiologists the same as those for male cardiologists in all cardiology sub-specialties?’ Where 1 is much lower and 10 is much higher. The lower the score, the lower the perceived career prospects for females compared with males. Females reported a median score of 3, which is a significantly lower than the median score of male respondents. Males reported (with a median score of 5), that career prospects for females are the same as males.
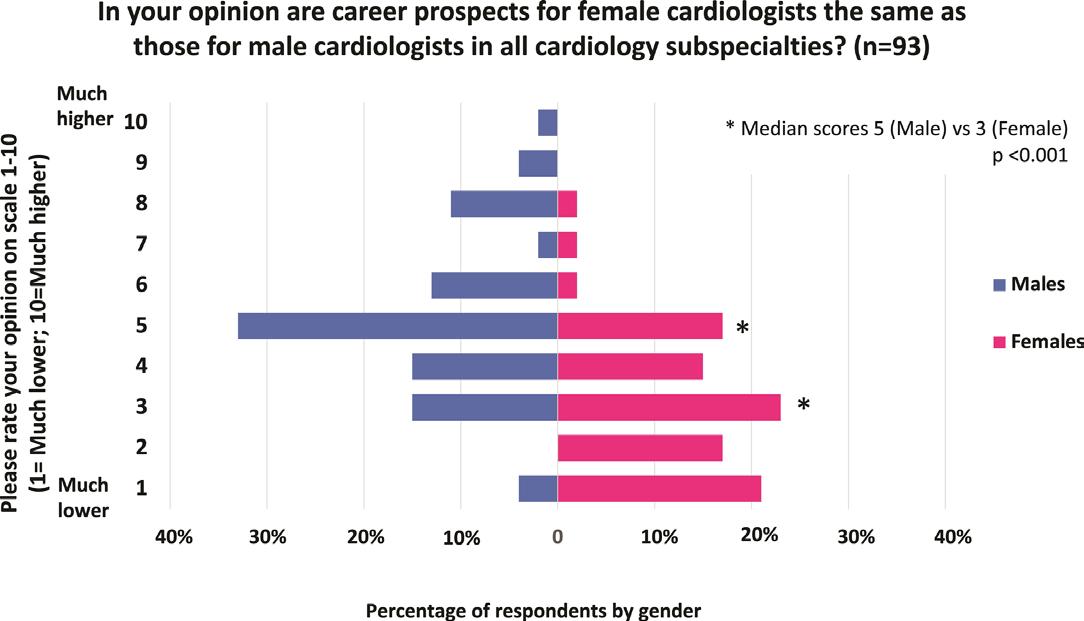
Despite rates of bullying in the UK being significantly lower (11% vs. 48%) consultants were the majority of perpetrators in both studies.8
In the UK, 9% of cardiology consultants and 6% of trainees work LTFT.9 In Ireland, to date there has been only one job share between two trainees since the inception of the cardiology training scheme in 1994 and the number of LTFT consultant cardiologists is <1%. This lack of flexibility in training creates an adverse environment for Irish Cardiologists, especially for women due to childcare responsibilities.
Despite a higher proportion of female cardiology trainees than ever before, a recent study demonstrated that gender parity in cardiology would not be reached in the next 50 years at this current rate.10 However, progress in Ireland could be faster given the national gender parity in other domains,6 which potentially allows restructuring of available resources and
policies from non-medical fields. Interestingly, recent evidence suggests that current national gender parity and its relationship with equality in cardiology is not straightforward, and indeed may have an inverse relationship. Recent work performed by the Pink International Young Academy of Cardiology group demonstrated that in Europe, countries with the most national gender parity had the worst representation of female leaders in cardiology.5 Conversely, Russia and Morocco who have the most female cardiology leaders, have the worst parity in gender nationally.
Volume 2, Issue 3, May 2022, oeac033, https://doi.org/10.1093/ehjopen/oeac033 slide may be subject to copyright: please see the slide notes for details.
males and consultants. Despite this, almost all female cardiology trainees and consultants in ROI/NI completed this survey, suggesting rates of sexism, bullying, and perception of lack of career advancement is a true reflection of female Irish cardiologists. Even though this survey had a good response rate of 30%, compared with other gender-based cardiology surveys (response rates 20–23%),4,7 it is unlikely to represent the experience of all cardiology trainees or consultants. Finally, another element that was not captured in this survey but has been well documented is salary discrepancies between sexes3,11 as well as sexual harassment.7
changing system-wide policies is needed. These policies should also prioritize systems and facilities to institute family friendly work environments which are already in place in non-medical fields in Ireland. Finally, improving women in leadership roles and mentorship of trainees is critical.
There are some limitations to this study. Firstly, there was participation bias with 50% female response rate, which is a higher representation than their proportion amongst Irish cardiologists. This bias is difficult to address, as the theme of this survey would appeal to women and those who have been affected by discrimination, making them more likely to partake. There was, however, under representation of
Programme directors in the USA have implemented strategies to promote gender diversity within their programmes.12 This includes implicit bias education, prioritizing diversity and equity in developing the match and interview process, and highlighting diversity initiatives in institutions. Some of these strategies could be adopted in Ireland. A fundamental gap highlighted in this survey is the lack of support structure to report discrimination, bullying, and harassment, without the fear of retaliation or stigmatization. This can be challenging, as each hospital human resources department, have different systems for reporting, none of which are anonymised and often do not result in any repercussions to the perpetrator. To target this,
Following on from this survey, Irish Women in Cardiology (WiC) have collaborated with BJCA WiC group and have set up a formalized mentorship programme. Currently, this has enrolled junior doctors, but we aim to extend recruitment to medical schools and subsequently secondary schools to target younger females who have yet to decide on a career. A step in improving equity in leadership positions has already started with a female consultant now sitting on the 10-person selection panel for ROI cardiology specialist training interviews and there is now an equal representation of female council members in ICS for the first time.
In conclusion, this study solidifies themes surrounding why women do not pursue a career in cardiology and presents real-life data on difficulties experienced in day-to-day clinical practice. This includes sexism, bullying, lack of flexible training, maternity, and childcare responsibilities as well as a ‘boys club’ environment that creates a glass ceiling.
References available on request
74 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
SUSPECT ATTR-CM

(TRANSTHYRETIN AMYLOID CARDIOMYOPATHY)

A LIFE-THREATENING DISEASE THAT CAN GO UNDETECTED
Life-threatening, underrecognized, and underdiagnosed, ATTR-CM is a rare condition found in mostly older patients in which misfolded transthyretin proteins deposit in the heart.1-7 It is vital to recognize the diagnostic clues so you can identify this disease.
CONSIDER THE FOLLOWING CLINICAL CLUES, ESPECIALLY IN COMBINATION, TO RAISE SUSPICION FOR ATTR-CM AND THE NEED FOR FURTHER TESTING
heart failure with preserved ejection fraction in patients typically over 60 years old5-7
to standard heart failure therapies (ACEi, ARBs, and beta blockers)8-10
between QRS voltage and left ventricular (LV) wall thickness11-13
of carpal tunnel syndrome or lumbar spinal stenosis3,8,14-20
showing increased LV wall thickness6,13,16,21,22
H FpEF I NTOLERANCE DISCORDANCE DIAGNOSIS E CHO N ERVOUS SYSTEM
LEARN HOW TO RECOGNIZE THE CLUES OF ATTR-CM AT:


—autonomic nervous system dysfunction-including gastrointestinal complaints or unexplained weight loss6,16,23,24
3. Connors LH, Sam F, Skinner M, et al. Heart failure due to age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282-290. 4. Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098. 5. Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2(2):113-122. 6. Maurer MS, Hanna M, Grogan M, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68(2):161-172.
7. González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585-2594. 8. Narotsky DL, Castano A, Weinsaft JW, Bokhari S, Maurer MS. Wild-type transthyretin cardiac amyloidosis: novel insights from advanced imaging. Can J Cardiol. 2016;32(9):1166.e1-1166.e10. 9. Brunjes DL, Castano A, Clemons A, Rubin J, Maurer MS. Transthyretin cardiac amyloidosis in older Americans. J Card Fail. 2016;22(12):996-1003. 10. Castaño A, Drach BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163-178.
11. Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49:9-13. 12. Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114(7):1089-1093. 13. Quarta CC, Solomon D, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840-1849. 14. Connors LH, Prokaeva T, Lim A, et al. Cardiac amyloidosis in African Americans: Comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158(4):607-614. 15. Nakagawa M, Sekijima Y, Yazaki M, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23(1):58-63. 16. Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203-1212. 17. Sperry BW, Reyes BA, Ikram A, et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018;72(17): 2040-2050. 18. Westermark P, Westermark GT, Suhr OB, Berg S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. 2014;119(3):223-228. 19. Yanagisawa A, Ueda M, Sueyoshi T, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28(2):201-207. 20. Sueyoshi T, Ueda M, Jono H, et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Hum Pathol. 2011;42(9):1259-1264. 21. Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442-1448. 22. Ternacle J, Bodez D, Guellich A, et al. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging 2016;9(2):126-138. 23. Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63-76. 24. Swiecicki PL, Zhen DB, Mauermann ML, et al. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid. 2015;22(2):123-131.
The health information contained in this ad is provided for educational purposes only. Date of Preparation: February 2021 GCMA code: PP-RDP-IRL-0105
References 1. Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23(4):209-213.
2. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357-1377.
S U S P E C T A N D D E T E C T . I E
Predicted cardiac and second cancer risks for patients undergoing VMAT for mediastinal Hodgkin lymphoma
Written by: Orla
A. Houlihan, Patrick G. Johnston Centre for Cancer Research, Queen’s University Belfast; St Luke’s Radiation Oncology Network, Dublin; Georgios Ntentas, Nuffield Department of Population Health, University of Oxford; Department of Medical Physics, Guy’s and St Thomas’ NHS Foundation Trust, London; School of Biomedical Engineering and Imaging Sciences, King’s College London; David J. Cutter, Nuffield Department of Population Health, University of Oxford; Oxford Cancer Centre, Oxford University Hospitals NHS Foundation Trust; Patricia Daly, St Luke’s Radiation Oncology Network, Dublin; Trinity St James’s Cancer Institute, St. James’s Hospital, Dublin; School of Medicine, Trinity College Dublin; Charles Gillham, St Luke’s Radiation Oncology Network, Dublin; Trinity St James’s Cancer Institute, St. James’s Hospital, Dublin; School of Medicine, Trinity College Dublin; Orla McArdle, St Luke’s Radiation Oncology Network, Dublin; and Frances K. Duane, St Luke’s Radiation Oncology Network, Dublin; Trinity St James’s Cancer Institute, St. James’s Hospital, Dublin, Ireland, School of Medicine, Trinity College Dublin
The majority of patients treated for lymphoma do not die from the disease1 and are at risk of longterm treatment-related morbidity and mortality.2 For early stage Hodgkin lymphoma (HL), 5 year overall survival rates are in excess of 90% with the use of combined modality therapy.1 Research has focused on identifying the minimum therapeutic intervention that will maintain outcomes and minimise survivor exposure to late toxicity of treatment.1, 3
Sequential clinical trials replaced historic extended radiation fields with smaller fields and reduced target volumes including involved field,3 involved site4 and involved node1 treatment approaches. Randomised data supported prescribed dose reductions in early favourable stage disease found to be adequately treated with 20 Gy instead of 30 Gy.5 Optimal photon radiotherapy (RT) planning techniques may further improve the therapeutic index for patients requiring treatment. Deep inspiration breath hold (DIBH) has been shown to reduce heart and lung doses.6, 7 Volumetric modulated arc therapy (VMAT) planning techniques used in combination with DIBH are associated with reduced heart, lung and breast doses.8,9,10 Proton beam therapy (PBT) may provide additional dosimetric advantages in the treatment of patients with mediastinal lymphoma and may reduce the risk of late effects.11
However, patients requiring RT to the mediastinum remain at an increased risk of late adverse effects. These include cardiovascular disease (CVD)12 and second malignancies such as breast, lung and oesophageal cancer,2 all of which cause morbidity and potentially excess mortality.
This study aims first to present the radiation doses received by individual patients who have undergone photon RT for mediastinal HL in a large multicentre radiation oncology network in Ireland, and second, to predict 30-year absolute mortality risks (AMR30) for CVD and second primary cancers. These data are intended to inform the need for ongoing treatment optimisation, to aid the consent process for future patients and to guide subsequent surveillance for lymphoma survivors.
MATERIALS AND METHODS Patient population
All patients who underwent RT for mediastinal lymphoma in a multicentre radiation oncology network between January 2016 and September 2019 inclusive were identified and their medical records were reviewed. There were no exclusion criteria regarding treatment of additional sites to the mediastinum, for example, axilla and neck. Patients who could tolerate DIBH were planned in both free breathing (FB) and DIBH and the optimal plan with greatest tumour coverage and lowest dose to organs at risk (OARs) was selected for treatment. Baseline characteristics were recorded including age, sex, smoking status, histological diagnosis, Ann Arbor stage, indication for treatment, RT dose fractionation schedule, use of DIBH and details of chemotherapy received. This study was approved by the St Luke’s Radiation Oncology Network ethics committee.
CT simulation and contouring
During RT CT simulation, patients were positioned with their arms by their sides and immobilised with the aid of a thermoplastic mask.
The clinical target volume (CTV) for each patient was delineated based on guidelines for involved site RT.13 During contouring, the patient’s pre-chemotherapy and interim or post-chemotherapy PET/CT was displayed on an adjacent monitor to aid delineation. The OARs of interest were contoured; whole heart, left ventricle, all four cardiac valves, lungs, female breasts, oesophagus and common carotid arteries. The left ventricle, cardiac valves and common carotid arteries were retrospectively contoured for the purposes of risk calculation for this study and were not included in the original planning process. Contour definitions and guides were derived from published atlases and information from anatomy and cardiac imaging textbooks and IMAIOS e-anatomy.14,15,16,17 CTV to planning target volume (PTV) margins of 5–15 mm were at the discretion of the treating clinician taking individual disease distribution into consideration.
Planning and dosimetric data collection
All patients were planned in the Eclipse planning system (Versions 13.6, 15.1 and 15.6), Varian Medical Systems, using VMAT. The standard approach for early stage favourable HL is to treat with 20 Gy in 10 fractions.5 Patients with early stage unfavourable HL were treated with 30 Gy in 15 fractions. All cases are discussed at a dedicated lymphoma MDT and our overall approach is to include radiotherapy in the treatment plan only when potential late effects are considered acceptable—for example, radiotherapy for early stage disease may be omitted in the case of a young woman for whom breast tissue will be included in the treatment field. Doses higher than 30 Gy were
used in the setting of residual PET/CT-positive disease. In these cases a total dose of 36–40 Gy is recommended.13
Individualised VMAT plans were generated for each patient. A generic starting point of two full arcs was further developed with the addition of partial arcs and modification of existing full arcs to achieve maximal coverage and conformality while minimising the dose to OARs.18 In our network, efforts are made to reduce the dose to all OARs to as low as reasonably achievable (ALARA). If possible, mean heart and lung doses were maintained < 5 Gy and 8 Gy respectively. In the setting of bulky mediastinal disease or residual PET/CT-positive disease in the mediastinum, higher lung and heart doses were accepted, with maximum accepted limits of mean lung dose (MLD) < 20 Gy, the volume of lung receiving ≥ 20 Gy (V20) less than 30% and mean heart dose (MHD) < 20 Gy. Mean breast dose was maintained < 4 Gy where possible. Each plan was optimised to achieve optimal conformity and PTV coverage of at least 98% of the PTV receiving at least 95% of the prescription dose. The mean doses to all contoured OARs were extracted, as well as PTV volume.
Late effects risk prediction
The treatment-related AMR30 for CVD and second primary cancers were predicted for all patients in this study. The risk prediction methods are described in detail in two previous studies.11, 19 In brief, for each patient, the background cumulative AMR30, in the absence of any lymphoma-related or treatment-related risks, was estimated for each disease of interest using mortality rates in the general Irish population
76 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Cardiology
and by taking into account the competing risk of death from other causes. These mortality rates were the most recent five year age- and sex-specific death rates from coronary heart disease (CHD), congestive heart failure (CHF), valvular heart disease (VHD), stroke and “other cardiac diseases” and from cancers of the lung, breast (females only) and oesophagus available from the World Health Organization (WHO) mortality database.20 The radiation-related increase in the mortality rates was calculated using individual radiation doses combined with published dose–response relationships. The dose responses allow the relative risk from modern radiation techniques to be estimated even though they are based on historical cohorts who received on average, higher radiation doses to normal tissues when treated. Mean heart dose was used to estimate risk of CHD,21 mean left ventricular dose (MLVD) for risk
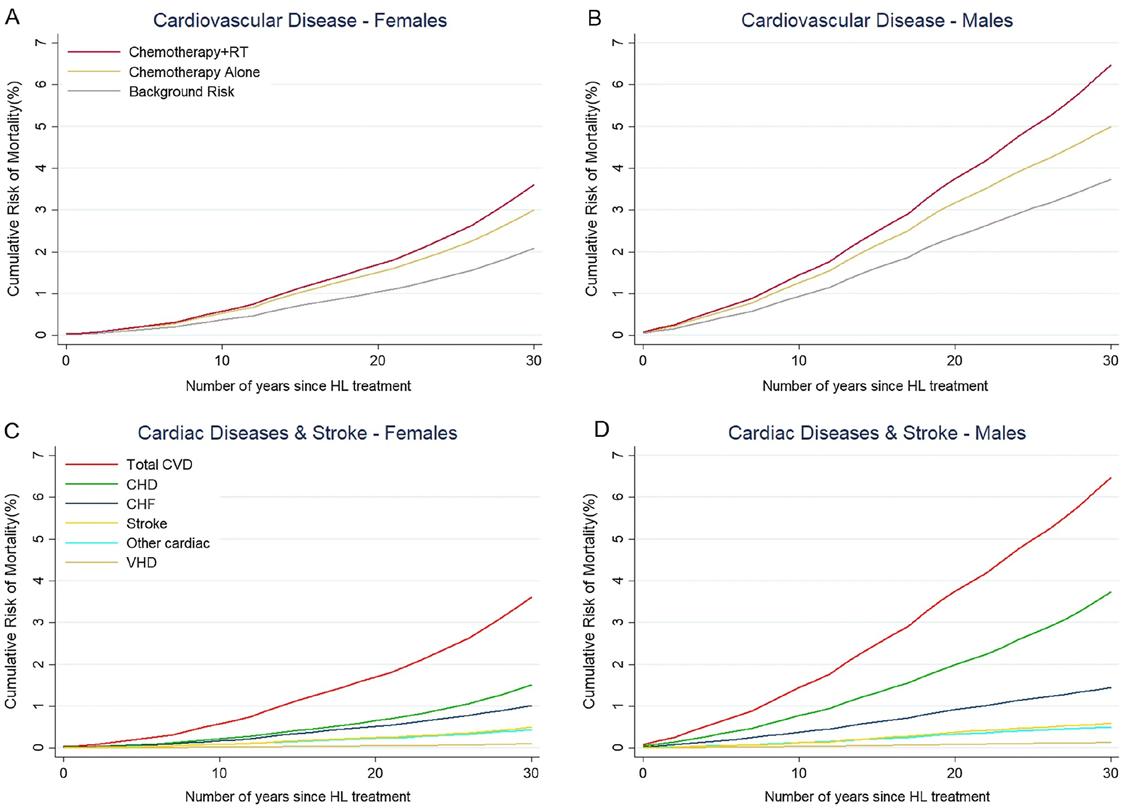
of CHF,22 a weighted average of the mean doses to the aortic valve (AVMean), the mitral valve (MVMean) and the tricuspid valve (TVMean) for risk of VHD23 and mean carotid artery doses (MCA) for risk of stroke.20, 24 For CHF, separate calculations were carried out including the effect of anthracycline chemotherapy without RT as well as for the effect of combined modality treatment.22 The sum of the mortality rates from CHD, CHF, VHD, stroke and “other cardiac diseases”, comprised the total CVD risk. Mean lung dose, mean breast dose (MBD) and mean oesophagus dose (MOD) were used to estimate risk from lung, breast and oesophageal cancers, respectively.24,25,26,27,28,29 Smoking status could not be taken into account due to unknown smoking status for approximately half of patients and lack of separate population rates for smokers and never smokers in Ireland.
Results
In total, 44 patients with mediastinal HL fulfilled the inclusion criteria for our study (21 male, 23 female). The median age at treatment was 28 years (range 17–72). The majority of patients (n = 27, 61%) were treated for early stage unfavourable disease. Two patients were treated for early stage favourable disease—both of these patients received 30 Gy in 15 fractions. In both cases they had a complete response documented on post-chemotherapy PET/CT but had persistent disease on interim PET/CT after 2 cycles of ABVD. All received chemotherapy, 98% (n = 43) received anthracycline regimens, 80% (n = 35) received 4–6 cycles ABVD. Median prescribed RT dose was 30 Gy (range 30–46 Gy). Forty-one percent (n = 18) were treated in DIBH.
Average PTV volume was 1007.7 cc. Mean heart dose was 9.8 Gy (range 0.2–23.8 Gy). The aortic and
pulmonary valves received higher doses of radiation than the mitral and tricuspid valves due to their more proximal location to the PTV.
The predicted background AMR30 from CVD was 2.88% and the excess AMR30 was 1.07% with chemotherapy and a further 1.11% with RT to a total of 5.06%. The chemotherapy-related excess AMR30 of CVD was 0.86% and 0.21% for CHF and other cardiac diseases, respectively. The radiation-related excess AMR30 of CVD was 0.79% for CHD, 0.04% for CHF, 0.01% for VHD, 0.13% for stroke, and 0.14% for other cardiac diseases. While the OARs of male patients did not receive greater radiation doses compared to the doses received by female patients, the predicted AMR30 for all CVD was greater in males due to their higher background risk, 3.74% versus 2.09% for females.
The predicted background AMR30 from second cancers was: lung
77 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
Figure 1
cancer 1.07%, breast cancer in females 1.00% and oesophageal cancer 0.20%. The excess mean AMR30 following RT was: lung cancer 2.20%, breast cancer in females 0.34%, and oesophageal cancer 0.28%, leading to a total AMR30 of 3.27, 1.34 and 0.48% from lung, breast and oesophageal cancer respectively. Among both male and female patients, the greatest additional risk was for lung cancer (1.89% for males, 2.48% for females). Since the background AMR30 from lung cancer was similar for male and female patients, the higher excess lung cancer risk in women was due to the higher lung doses. (Figure 1)
Discussion
This study provides a patientspecific prediction of treatmentrelated absolute mortality risk from mediastinal photon RT and chemotherapy in a cohort of HL survivors treated in a national oncology network in Ireland. Our findings show that risks are low but remain clinically significant. Efforts to reduce incidental radiation doses by introducing more advanced RT techniques and reducing target volumes by increasing use of DIBH should be continued. We also provide individualised risk estimates which can be used to inform future patients and increase their involvement in decision making regarding balancing risks and benefits from their treatment.
Lastly, we demonstrate that expanding long-term surveillance may provide a more holistic treatment approach to patients. For example, guidelines are in place for breast cancer screening but our data suggest the role of lung cancer screening may need to be considered.
For survivors treated with 30 Gy to the mediastinum, the radiation doses in our cohort are similar to those reported for modern photon RT in a recent review.30 Mean lung, heart and oesophagus doses were 10.6 Gy, 10.0 Gy and 15.8 Gy, respectively. Mean breast dose in female patients was 5.6 Gy.
Excess mean AMR30 of CVD following chemotherapy and RT was 2.18%, with 1.07% attributed to chemotherapy and 1.11% to RT. Our AMR30 were similar to those predicted in a recent study in a different population of early stage HL patients.19 RT was also associated with excess AMR30 for second cancers (lung cancer 2.20%, breast cancer in females 0.34%, and oesophageal cancer 0.28%). The total risk of lung cancer mortality more than tripled for women (from 1.02 to 3.50%) and more than doubled for men (from 1.11% to 3.00%). This is due to the higher mean lung doses recorded for women in this study as both men and women had on average similar background AMR30 in our study. The predicted risk of breast and oesophageal
cancer mortality was generally low and the RT-related increase of breast AMR30 in women was also low (0.34%). For oesophageal cancer it was tripled in women after RT (from 0.12 to 0.32%), with a smaller relative, but larger absolute, increase seen for men (from 0.30 to 0.66%). While the overall absolute mortality risks are low for patients with HL, these fatal effects remain clinically significant. These RT-related risks vary widely among patients, with a range of 0.12 to 3.46% for excess AMR30 of CVD and 0.60 to 8.79% for excess AMR30 of lung cancer mortality and 0.01 to 1.37% for oesophageal cancer mortality.
Approaches to reduce the risk of late cardiovascular and second cancer risks for patients with HL include the adoption of advanced radiotherapy techniques such as DIBH. While DIBH has the potential to reduce radiation dose to OARs in mediastinal lymphoma for some patients, it is not always superior to FB techniques and is not always tolerable by patients.18 In our network, despite dual planning for all patients who could tolerate DIBH, these plans were superior to FB plans for only 41% of the overall cohort. In our network, however, the same PTV margins are applied for both DIBH and FB plans. Enhanced image guidance techniques can facilitate the use of tighter PTV margins with DIBH, which likely would reduce the dose to OARs further
and provide added benefit.7, 10 Tighter margins combined with state of the art radiotherapy techniques such as butterfly VMAT9, 10 or full-arc butterfly VMAT31 could provide further OAR dose and risk reductions.
Alternatively, PBT may also offer an advantage compared to photon RT, however, it is not widely available yet for patients with HL due to higher cost and limited numbers of PBT centres, with no centre currently available in Ireland. Identifying patients who will derive greatest benefit from PBT would be beneficial but it remains a challenge in clinical practice. The International Lymphoma Radiation Oncology Group guidelines state that patients who could “greatly benefit” from PBT include those with mediastinal disease that extends below the origin of the left main coronary artery (LMCA), those for whom breast dose is a concern and heavily pre-treated patients at higher risk of radiationinduced toxicity.32 Another recent study showed that when CTV overlapped longitudinally with the heart by ≥ 40%, PBT reduced mean heart dose by 3.2 Gy (19% relative decrease), left ventricular dose by 5.6 Gy (50% relative decrease) and valvular doses by 5.1 Gy (24% relative decrease). These measures resulted in a reduction in total CVD AMR30 in the study among patients in Western Europe by 0.3% from 3.8% to 3.5%.11 For patients with axillary involvement,
78 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Cardiology
PBT reduced mean lung dose by 2.8 Gy (29% relative decrease) and lung cancer AMR30 among patients in Western Europe by 0.5% from 2.7 to 2.2%.11 Based on the above findings, we calculated the percentage of patients in our cohort falling within these subgroups. Forty-one percent (18/44) of patients had a CTV which overlapped longitudinally with the heart by ≥ 40%,11 70% (31/44) had mediastinal disease which extended below the origin of the LMCA32 and 52% (23/44) patients had disease which extended below the 7th thoracic vertebra.33 These patients could have potentially had reduced risk of radiation-related AMR30 from CVD if treated with PBT. In addition, the 16 patients (36%) who had axillary disease treated could have potentially had reduced risk of radiation-related AMR30 from second lung and breast cancer (females).11, 32 However, only dual planning with both photon RT and PBT within our cohort could provide a more individualised estimate as the range of absolute AMR30 benefit is wide amongst patients based on previously published results.22
A strength of our study is that we used an individualised risk prediction approach which takes into account individual OAR doses from radiotherapy CT-planning scans detailing anatomy and 3D dose distributions for each patient combined with the best available epidemiological evidence regarding the magnitude of the long-term risks of radiation and anthracycline chemotherapy in
HL survivors to predict AMR30, as well as up to date age and sex-specific background Irish risks as recently published by the WHO. We recognise that the dose–response relationships used as the basis for our methodology are currently based on data from patients treated with historical techniques but these relationships remain the best epidemiological evidence available that provide dose-response relationships between radiation dose and risk of cardiovascular disease and second cancers. The nature of late manifestation of such effects requires a long patient follow up and the results of such studies will always be behind the fast evolving RT technologies. These doseresponse relationships provide the relative risks (recognised from these historical treatments) of late effects versus radiation doses to the relevant OARs which can be used to estimate absolute risks for contemporary cohorts. The majority of cardiovascular risk dose–response relationships used in our study have been recently externally validated, giving us more confidence in their use.34 When newer studies are published that adequately define dose–response relationships within more modern cohorts, updates to the risk estimation models will be important. We also included all patients treated with mediastinal RT in a RT network delivering just over 40% of all RT courses nationally—this likely is a reasonable national representation of RT dosimetry for this cohort. Although only 44 patients
were identified this identifies a substantial ‘real world’ cohort who actually received these treatments rather than a more constrained planning study.
Our study has several limitations. We have included a population of varying clinical demographics, disease stages and RT doses, which, while reflective of daily clinical practice, makes it difficult to draw conclusions regarding more selected groups of patients. The retrospective nature of this study, specifically the retrospective contouring of cardiac substructures and carotid arteries, limits the interpretation of some of the risks that were predicted using these doses, as these doses and thus risks might have been slightly lower if these substructures were included in the optimiser. While our cohort received contemporary RT, there was scope to further optimise treatment e.g. reduce PTV margins for patients undergoing DIBH, add further OAR dose volume constraints. Despite these limitations, the doses presented and thus the estimated risks will likely be of interest to the wider lymphoma community and are reflective of a ‘real world’ cohort as many centres worldwide are currently introducing DIBH and advanced VMAT. This study includes patients treated 2016–2019 prior to updated recommendations of dose constraints published by the International Lymphoma Radiation Oncology Group in 2020.4 We have since revised our OAR dose constraints in line with this guideline and our results
National Rare Disease Plan in Pipeline
The Department of Health has marked Rare Disease Day by announcing it is to develop a new National Rare Disease Plan. Pledging support to people living with rare diseases the Minister Stephen Donnelly confirmed that work is due to commence on the new National Rare Disease Plan, in line with the commitment in the Programme for Government.
Improving and expanding the care and treatment of patients with rare diseases is a priority for the government, with work spearheaded by the HSE National Clinical Programme for Rare Diseases.
The government has also substantially increased funding for
new innovative medicines for rare diseases, investing ¤100 million over the last three Budgets.
During this time, more than 100 new medicines have been approved, including 34 orphan medicines to treat rare diseases.
In 2023, the government has committed ¤2.7 million to support the implementation of this Strategy.
Minister Donnelly said: “I understand that patients living with rare diseases and their families face huge challenges in managing and treating their conditions, and these challenges are heightened by the lack of information and research into these diseases.
“As a health service, we are working to change this.
Personalised treatments will help transform healthcare provision in Ireland, and this vision has already been laid out with the recent launch of the National Strategy for Accelerating Genetic and Genomic Medicine in Ireland.”
Close links have already been developed with EU partners. Last year the HSE was nominated as the National Competent Authority in an EU Joint Action of European Reference Networks for Rare Diseases, which enables greater coordination and sharing of best practices in key areas such as genetic testing.
Highlighting the importance of this EU-wide approach, the Chief Medical Officer Professor Breda Smyth said: “This collaboration
may influence other radiotherapy centres to also update their OAR dose constraints. Another limitation is that excess risks from smoking in the Irish population were not included in the risk prediction due to lack of separate population rates for smokers and never-smokers in Ireland. Smoking increases the risk of CVD and lung cancer and therefore we might have underestimated the risks for some patients. Smoking may increase the background AMR30 (and thus excess treatment-related risk) from CVD and lung cancer up to 14-fold compared to never smokers.11 Of note, incidence of CVD and second cancers were not calculated in this study. Incidence of CVD and breast cancer in particular would be higher than mortality risk, with additional effects on survivors’ quality of life and impact on health services research, therefore surveillance is even more crucial in these patients. Incidence rates in Ireland were not available for the cardiac endpoints reported.
Conclusions
In conclusion, for patients with mediastinal lymphoma predicted excess mortality risks from CVD and second cancers remain clinically significant despite contemporary chemotherapy and photon RT. Ongoing efforts to reduce the toxicity of combined modality treatment and implement techniques such as DIBH, reduced margins and advanced radiotherapy techniques including PBT are strongly encouraged. References available on request
has allowed us to enter into 18 European Reference Networks (ERNs) on Rare Diseases. These ERNs include representation from five academic hospitals and three universities and is coordinated by the National Rare Disease Office.
“This represents a significant achievement by the health service, to drive innovation, training and clinical research for highly specialised care.”
1 in 15 people live with a rare disease at some point in their life
Rare Disease Day aims to build awareness of rare diseases and the need for increased expertise and knowledge to help improve treatments.
79 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
News
Clinical R&D
CROSS BORDER NETWORK LAUNCHES TO BRING HOPE TO FAMILIES AFFECTED BY RARE
DISEASES
University College Dublin (UCD), Queen’s University Belfast, and 33 partners have launched the All-Ireland Rare Disease Interdisciplinary Research Network (RAiN).
Individual rare diseases may be rare but collectively impact more than 400,000 people across the island of Ireland. There are as many people living with rare diseases across Ireland as live with diabetes, yet rare diseases receive much less recognition and support.
Many people living with a rare disease experience chronic debilitating illness, with more than 30 percent of children with rare diseases dying before their fifth birthday. RAiN will help evaluate the quality of life and management of people living with rare diseases on the island of Ireland and internationally.
Commenting on the occasion of the launch, Taoiseach Leo Varadkar, TD said: “I am pleased to announce the launch of the All-Ireland Rare Diseases Interdisciplinary Research Network, which will strengthen cross border links between academic, clinical health, and social care researchers working to improve the lives of people affected by rare disorders. Through the RAiN network, researchers will work in partnership with patients and families to make real impacts in the day-to-day care, treatment and management for those living with conditions such as cystic fibrosis, PKU, chromosomal anomalies, and childhood cancers, and enhance cooperation in how we respond to these challenges on an all-island basis.”
Co-lead of RAiN for UCD, Associate Professor Suja Somanadhan said: “This all-Island interdisciplinary rare disease research network will serve as a hub to support collaboration and connection between members across the Island, which includes researchers, early career investigators, industrial partners and Public and Patient Involvement expert groups.”
Co-lead of RAiN for QUB, Professor Amy Jayne McKnight said: “I’m delighted that so many individuals in our local rare disease community have come together to establish this network and look forward to working in partnership with those working,
often on a shoestring budget, to improve the lives of people living with rare diseases.”
RAiN is funded by the Department of the Taoiseach from the Shared Island strand of Irish Research Council’s ‘New Foundations’ awards. The network builds on established north-south research partnerships between UCD and Queen’s*.
By fostering collaboration among researchers, practitioners, policymakers, patients and families working on rare diseases, RAiN will advance health service developments, leverage funding, and facilitate internationally excellent translational rare disease research.
Monthly seminars will discuss research that addresses the significant unmet health, social, psychological, and educational needs of children, young people affected by rare disease and their families. Nurtured research partnerships will inspire and empower early career researchers as emerging leaders for interdisciplinary rare disease research.
HOSPITAL SERVICES LIMITED (HSL) ANNOUNCES EXPANSION
Known for introducing innovative new health technologies to hospitals and healthcare facilities for the past 60 years across the island of Ireland, Hospital Services Limited (HSL) have expanded into

the UK with the launch of their new headquarters in the East Midlands, England, with the £1.7m investment aiming to grow the company’s footprint in the UK over the coming years.
A specialist distributor of medical and surgical equipment, consumable products, and healthcare IT solutions, HSL is one of the largest privately-owned distributors supplying the health sector in the UK and Ireland. In addition to supplying market leading technologies and solutions to its customers, HSL’s team provides on-going technical and clinical support to the end-user of our products.
HSL partners exclusively with many world-class manufacturers across several disciplines including, to name just a few, Richard Wolf and DTR Medical (general surgery), Hologic (mammography), Shimadzu (radiology), Fujifilm (endoscopy), and Haag-Streit and Ziemer (ophthalmology).
With financial backing from the Foresight Group, a leading independent infrastructure and private equity investment manager with over £6.5 billion of assets under management, HSL continues to grow its offering, both locally and across the UK, enabling its customers to focus on what is most important - the Patient.
Following continued growth and success in the UK where HSL
have established a reputation for supplying innovative, efficiencygrowing and high-quality radiology and healthtech, HSL have established a Britain-based team of 25 based across their new Draycott headquarters, an office in Bath, and positioned remotely across the regions of the UK including Wales.
The company also has ambitious plans to grow the team by at least 60% in the coming years through planned acquisitions and ongoing recruitment campaigns and has recently appointment Steve Leatherland as Regional Director of Operations in the UK. Commenting on his role in helping strategic growth for HSL Steve Leatherland said, “These are exciting times for HSL with a growing portfolio, an expanding team, and a working environment focused on creating a culture of customer centricity. With over 20 years’ experience in the healthcare service sector, I am looking forward to working with some of the best people and products in the marketplace.”
Graham Stewart, Commercial & Finance Director and Sam McMaster, Director of Telehealth photographed in HSL’s newly-opened Draycott headquarters
80 MARCH 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
DUPIXENT® (DUPILUMAB) APPROVED BY EUROPEAN COMMISSION AS THE FIRST AND ONLY TARGETED MEDICINE INDICATED FOR EOSINOPHILIC ESOPHAGITIS
The European Commission (EC) has expanded the marketing authorization for Dupixent® (dupilumab) in the European Union (EU) to treat eosinophilic esophagitis (EoE) in adults and adolescents 12 years and older, weighing at least 40 kg, who are inadequately controlled by, are intolerant to, or who are not candidates for conventional medicinal therapy. EoE is a chronic, progressive inflammatory disease that damages the esophagus and prevents it from working properly. With this approval, Dupixent is the first and only targeted medicine specifically indicated to treat EoE in Europe and the U.S.
Naimish Patel, M.D. Head of Global Development, Immunology and Inflammation at Sanofi said, “The impact of EoE on a patient’s daily life cannot be overstated – the narrowing and scarring of the esophagus can make something as simple as eating a painful and distressing experience, and may lead to choking and food impaction. With this latest approval for Dupixent, adults and adolescents in the EU suffering from the chronic and often debilitating symptoms of EoE now have the first and only targeted treatment option clinically proven to reduce both esophageal inflammation and damage, as well as improve swallowing ability, pain and health-related quality of life.”
George D. Yancopoulos, M.D., Ph.D. President and Chief Scientific Officer at Regeneron added, “This latest approval establishes Dupixent as the only targeted medicine specifically indicated for eosinophilic esophagitis in the European Union. Dupixent is also the only biologic shown in pivotal trials to help patients achieve histological remission, reduce difficulty swallowing and improve healthrelated quality of life – all of which are crucial to reducing the burden of this debilitating disease. Since its first approval, Dupixent has redefined the treatment of certain chronic diseases with underlying type 2 inflammation and is now indicated for five conditions in the European Union. We remain committed to investigating Dupixent’s potential in additional diseases in which IL-4 and IL-13 may play a key role.”
The EC decision is supported by 52-week data from a Phase
3 trial consisting of three parts (Part A, B and C). Part A and Part B investigated Dupixent 300 mg weekly (Part A n=42; Part B n=80) compared to placebo (Part A n=39; Part B n=79) for 24 weeks. Part C (n=188) observed patients who had continued on or switched to Dupixent from Parts A and B for an additional 28 weeks.
Dupixent patients in Parts A and B, respectively, experienced:
• An approximately 10 times higher rate of histological disease remission (60% and 59%), a co-primary endpoint, compared to placebo (5% and 6%).
• A 69% and 64% reduction in disease symptoms compared to 32% and 41% with placebo. Disease symptoms were measured using the Dysphagia Symptom Questionnaire (DSQ), on which Dupixent patients experienced a 21.9- and 23.8-point clinically meaningful improvement compared to a 9.6- and 13.9-point improvement for placebo, a coprimary endpoint. Swallowing improvement was observed as early as four weeks.
• A greater than seven-fold reduction in abnormal endoscopic findings from baseline (-3.2 and -4.5 points) compared to placebo (-0.3 and -0.6 points).
• Nominally significant improvements in swallowingrelated pain and health-related quality of life, as well as less frequent non-swallowing symptoms.
Histological disease remission, swallowing improvement and reduction in abnormal endoscopic findings were consistent with the overall population in patients who were uncontrolled, or not responsive to or not eligible for swallowed topical corticosteroids. Longer term efficacy in Part C was similar to results observed in Parts A and B.
The safety results of the trial were generally consistent with the known safety profile of Dupixent in its approved indications. The most common side effects across indications include injection site reactions, conjunctivitis, conjunctivitis allergic, arthralgia, oral herpes and eosinophilia. Adverse events more commonly observed in EoE patients treated with Dupixent (n=122) compared to placebo (n=117) included infections (32% vs. 25%). An additional adverse reaction of injection site bruising was reported
in the EoE trial. The safety profile through 52 weeks was generally consistent with the safety profile observed at 24 weeks.
PORTIUNCULA UNIVERSITY HOSPITAL LAUNCHES ‘LITTLE JOURNEY’ APP FOR CHILDREN
Portiuncula University Hospital has launched the ‘Little Journey’ virtual reality app to help children between the ages of 3 and 12, to prepare for surgery and allay their anxiety in advance of their hospital stay.
The ‘Little Journey’ app provides 360 degree views of all the areas the children will visit at the hospital as well as useful information about anaesthesiology tailored to the age of the child.
Dr Vinod Sudhir, Consultant Anaesthesiologist at Portiuncula University Hospital said, “Preoperative anxiety in children and parents is a very real phenomenon before undergoing general anaesthesia. As healthcare professionals we recognise the increasing impact of anxiety and the physiological negative impact it can have on the child’s and parent’s ability to cope with events surrounding the experience of anaesthesiology and surgery.
“Children may have anxiety around the multidisciplinary environment of theatre, their expectation of events, meeting unfamiliar people and even fear of pain. Parents/ guardians can accompany their young children to theatre and be present with them during induction of anaesthesia. Some of the parent’s anxiety comes from their fear of the unknown and the steps surrounding their role with their child at the time their child undergoes anaesthesia. By providing information through the app, which uses child-friendly animation, we can begin to prepare parents/guardians and children for surgery and ease their distress.
“The ‘Little Journey’ app was developed to help change the way children experience hospitals by empowering them and giving them the information they need to reduce their anxiety in a fun, interactive format. The app helps children to familiarise themselves with the hospital and the handy checklists included will help families to prepare in advance of the hospital stay.
“Last year the demand for general anaesthetic increased in children due to the increase of paediatric day case surgery at the hospital. We want to help alleviate any distress due to anxiety whether the child is presenting for surgery or investigative procedures.”
The ‘Little Journey’ app is free to download from Google Play Store or App Store. It can be used on a smartphone in 2D or using a 3D virtual reality headset. The app gives children the chance to meet the animated healthcare characters who will explain what they do and what happens on the day of surgery, see some of the equipment which will be used to care for them, find out more about what to expect on the day of their procedure and includes a virtual tour of the children’s day ward, the theatre room and the recovery room at Portiuncula University Hospital.
Portiuncula University Hospital launches ‘Little Journey’ virtual reality app to help children between the ages of 3 and 12, to prepare for surgery and allay their anxiety in advance of their hospital stay. From left, Elizabeth Fitzgerald, Theatre Staff Nurse; Breda Brady, Clinical Skills Facilitator, Paediatric Unit; 6 year old Diarmuid Fallon from Oran, County Roscommon and Dr Vinod Sudhir, Consultant Anaesthesiologist
81 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
Clinical R&D
JANSSEN PRESENTS UPDATED DATA DEMONSTRATING IMPROVED OUTCOMES FROM THE USE OF NIRAPARIB IN COMBINATION WITH ABIRATERONE ACETATE PLUS PREDNISONE AS A FIRST-LINE THERAPY IN PATIENTS WITH BRCA-POSITIVE METASTATIC CASTRATION-RESISTANT PROSTATE CANCER
The Janssen Pharmaceutical Companies of Johnson & Johnson has announced updated results from the Phase 3 MAGNITUDE study evaluating the investigational use of niraparib, a highly selective poly (ADP-ribose) polymerase (PARP) inhibitor, in combination with abiraterone acetate plus prednisone (AAP) in patients with metastatic castration-resistant prostate cancer (mCRPC) with or without specific homologous recombination repair (HRR) gene alterations, including BRCA mutations.1 Results will be featured in a Rapid Abstract Session (Abstract #170) at the American Society of Clinical Oncology’s Genitourinary (ASCO GU) Cancers Symposium 2023, taking place February 16-18.1
In the second interim analysis (IA2) of the MAGNITUDE study (NCT03748641), the treatment combination of niraparib and AAP, in comparison to placebo and AAP at 26.8 months of median followup, demonstrated a statistically significant prolongation in time to symptomatic progression (TSP) and continued consistent improvement of time-to-initiation of cytotoxic chemotherapy (TCC) in the HRR-positive population and a strong improvement in TSP for the BRCA subgroup of the HRR-positive population.1,2 Updated radiographic progression free survival (rPFS) results were consistent with the primary analysis which showed statistically significant benefit in both the HRRpositive population and BRCA subgroup.1,2,3 Additionally, a trend toward improvement in overall survival (OS) was observed in the BRCA subgroup.1,2 No new safety signals were identified.1,2 The most common adverse events for niraparib and AAP versus placebo and AAP, regardless of causality, were anemia (50.0 percent vs 22.7 percent, respectively), hypertension (33.0 percent vs 22.3 percent) and constipation (33.0 percent vs 15.6 percent).1,2 Patients without HRR gene alterations had no improvement in outcomes from the use of niraparib in combination with AAP.1,2
Notably, in the BRCA subgroup (8.1 months additional follow-up at IA2), rPFS by central review
demonstrated a consistent and clinically meaningful treatment effect favoring niraparib and AAP, with a median rPFS of 19.5 months at IA2 compared with 10.9 months for placebo and AAP (hazard ratio [HR], 0.55 (95 percent confidence interval [CI], 0.39-0.78).1,2 For patients with BRCA-positive mCRPC, preplanned sensitivity analysis evaluating rPFS by investigator review also showed benefit for niraparib and AAP (HR, 0.46 [95 percent CI, 0.32-0.67]).1,2 Further, results of the IA2 indicate that patients with BRCA mutations treated with niraparib and AAP experienced a trend towards delayed time to worst pain intensity (HR, 0.70 [95 percent CI, 0.44-1.12]) and pain interference (HR, 0.67 [95 percent CI, 0.40-1.12]) compared with placebo and AAP.1,2
“At Janssen, our goal is to provide treatment options that delay progression, prolong life, and support a better quality of life for those diagnosed with prostate cancer,” said Martin Vogel, EMEA Therapeutic Area Lead for Oncology, JanssenCilag GmbH. “The MAGNITUDE study highlights the importance of biomarker testing to identify those who will optimally benefit from the combination of niraparib and AAP. This is a crucial step in ensuring we can bring the right treatment to the right patients, based on their unique characteristics, and speaks to our wider commitment to exploring precision medicine to treat, intercept and prevent, and potentially one day cure, diseases like mCRPC.”
Prostate cancer is the most common cancer in men across Europe.4 In 2020, more than 473,000 patients were diagnosed in Europe and over 100,000 deaths were attributed to this challenging disease.5 Patients with mCRPC and HRR gene alterations, of which BRCA mutations are the most common, are more likely to have aggressive disease, poor outcomes and a shorter survival time.6,7,8,9 Up to 30% percent of mCRPC cancer cases have alterations in genes associated with HRR.3,10 Approximately 10 to 15 percent of patients with mCRPC have BRCA gene alterations.11,12
In April 2022, Janssen submitted a marketing authorisation application to the European Medicines Agency seeking approval for niraparib in combination with abiraterone acetate in the form of a dual action tablet (DAT), plus prednisone or prednisolone, based on data from the MAGNITUDE study.13 Marketing authorisation applications are under review across a number of countries globally.
EXAMINING RISE IN HEALTHRELATED CLAIMS
Minister for Health Stephen Donnelly has received government approval to establish an Interdepartmental Working Group to examine the rising cost of health-related claims and consider mechanisms to reduce costs. The Group will examine the rising cost of clinical negligence claims in the health system, with a particular focus on high value claims, and identify measures that could be put in place to reduce future costs.
The Group’s considerations will include developing a plan to implement risk management and other initiatives to reduce the occurrence of adverse incidents and to recommend measures to address patient concerns following the occurrence of adverse incidents, taking into account measures suggested by Dr Scally in his final implementation review report.
The Group will be chaired independently by an expert healthcare professional, Dr Rhona Mahony and will be comprised of membership from across key government departments and agencies.
Minister Donnelly stated:
“The most effective way to manage the cost of claims against the HSE is to minimise incidents of avoidable harm. While the department and the HSE work continuously to monitor and improve patient safety, reduce incidents of harm and to minimise risk, I am keen to take further action on this.
“The establishment of this Group, chaired by, Dr. Rhona Mahony and comprising membership from across key government departments and agencies, will be essential in identifying measures that could be put in place to improve outcomes for patients and reduce future costs. This is an important piece of work for patients and for the whole health service and I look forward to receiving the group’s findings and recommendations in due course.”
NEW PATIENT ADVICE AND LIAISON SERVICE COORDINATOR FOR LETTERKENNY UNIVERSITY HOSPITAL

Letterkenny University Hospital is delighted to announce the appointment of Angela Mc Closkey to the role of Patient Advice and Liaison Service (PALS) Coordinator. Angela is from Letterkenny and spent 19 years working as a Radiographer in Letterkenny
University Hospital prior to her appointment as PALS Coordinator.
The Patient Advice and Liaison Service Coordinator acts as a visible focal point for patients, families and carers and is often the main contact within the hospital. They help to resolve issues for patients and their families and work towards improving the patient experience at any available opportunity.
Speaking about her appointment
Angela said; “Within the hospital every contact we have with a patient can have a lasting impact and I try and make that contact as positive as possible. My role involves ensuring that the patient voice is heard either through the patient directly or through a nominated representative.
“If a patient wants to provide feedback or make a comment about the hospital and the care they received, I can assist them in doing so, or refer them to the appropriate person who will be able to assist them further.”
Sean Murphy, General Manager at Letterkenny University Hospital added; “This is the first appointment of a PALS Coordinator to Letterkenny University Hospital and I am delighted to welcome Angela to the role. PALS Coordinators have a very important function as they help to embed the voice of the patient in the workings of the hospital. Patients are at the centre of everything we do and through the PALS service we can learn more about the patient experience and put plans in place to make necessary improvements.”
ALLCARE PHARMACY ANNOUNCE NEW SPONSORSHIP
RTÉ Media Sales has announced Allcare Pharmacy as the new sponsor of Today with Claire
82 MARCH 2023 • HPN |
HOSPITALPROFESSIONALNEWS.IE
Angela Mc Closkey (PALS)
Byrne, RTÉ Radio 1, Monday –Friday, 10am-12pm.
Brokered by Javelin, the 11-month RTÉ Radio 1 sponsorship includes 6 x 10-second sponsor-credited stings per show, sponsorcredited promos in Rising Time, Liveline, Drivetime, Saturday with Colm O’Mongain and Brendan O’Connor as well as podcast and homepage sponsorship.
Today with Claire Byrne is currently joint seventh most listened-to radio programme in the country, with a daily audience of 344,000.
Caroline Burton, Head of Retail Marketing at Uniphar Group said “Allcare Pharmacy is delighted to partner with Claire Byrne and RTE Radio 1 in 2023. As Ireland’s largest community pharmacy network, it was important for us to find a partner which reflects the informative, entertaining and important topics of the day that matter to our customers. Today with Claire Byrne is a perfect fit for a trusted community pharmacy brand like Allcare Pharmacy.”

SAXENDA® (LIRAGLUTIDE 3MG) INJECTION APPROVED FOR REIMBURSEMENT IN IRELAND FOR ADULTS LIVING WITH OBESITY
Novo Nordisk have announced that Saxenda® (liraglutide 3mg) has been granted reimbursement in Ireland for the treatment of adults living with obesity.1 Liraglutide 3mg is the only obesity medicine reimbursed for people with an initial Body Mass Index (BMI) of ≥35kg/m2, pre-diabetes and at least one cardiovascular risk factor, in combination with a reduced calorie diet and increased physical activity.1,2
While people living with obesity may have been advised to ‘eat less
and move more’, clinical guidelines recommend moving beyond this simplistic approach to address the root drivers of obesity.6 Instead, people living with obesity should have access to evidence-informed interventions, including medical nutrition therapy, physical activity, psychological interventions, pharmacotherapy and surgery.6
Professor Donal O’Shea, HSE Clinical Lead for Obesity said: “Today’s announcement of liraglutide 3mg reimbursement for obesity, represents a landmark moment in Ireland’s changing response to how obesity is managed following the introduction of a new HSE Model of Care for the Management of Overweight and Obesity last year. Liraglutide 3mg has demonstrated, both in clinical studies and real-world use, clinically meaningful weight loss when combined with diet and exercise.”
7-12
Liraglutide 3mg, a human glucagon-like peptide-1 (GLP1) analogue, comes in pre-filled pens as a once daily injection. Reimbursement is through a managed access protocol, run by the HSE’s Medicines Management Programme, and those qualifying for reimbursement will need to satisfy the following eligibility criteria: Body Mass Index (BMI) of ≥35kg/m2, pre-diabetes and at least one cardiovascular risk factor. For full details on reimbursement criteria, please see: https://www. hse.ie/eng/about/who/cspd/ncps/ medicines-management/managedaccess-protocols/liraglutidesaxenda-/. For people living with obesity who do not meet these criteria, the medicine can still be prescribed and purchased at the individual’s expense.
Treatment with Liraglutide 3mg should be discontinued after 12 weeks on the 3mg/day dose if people have not lost at least 5% of their initial body weight.2 Further prescribing information can be found at: https://www.medicines. ie/medicines/saxenda-6-mg-mlsolution-for-injection-in-pre-filledpen-34785/spc
UCD RUGBY CLUB TO RAISE FUNDS FOR IRISH CANCER SOCIETY DAFFODIL DAY

UCD Rugby Club, sponsored by EY Ireland, has announced details of their fundraising drive to raise vital funds for the Irish Cancer Society’s annual Daffodil Day.
UCD Rugby Club made the announcement at the UCD Bowl.
Averil Power, CEO Of the Irish Cancer Society, said, “We are grateful to UCD Rugby Club for the incredible effort that they’ve put in
every year to run Daffodil Day on the UCD campus. We are also so grateful to our wonderful partners in EY for their support. The Irish Cancer Society typically gets three percent of our funding from the State. Therefore, we have to raise over twenty-five million euro every year to fund all of our free nursing, counselling and transport to cancer treatment, as well as our life-saving and life-changing cancer research and trials. We genuinely couldn’t provide all of the vital services that we do without the support of UCD Rugby Club and EY.”
Graham Reid, EY Ireland Partner and Head of Markets, added, “EY are very proud to support UCD RFC’s fundraising drive for The Irish Cancer Society, which is also EY Ireland’s Charity Partner this year. The Irish Cancer Society is an incredible community of patients, survivors, volunteers, supporters, health, social care professionals and researchers offering free care, advice and support for cancer patients and their loved ones. Their vision is that, by 2025, three out of every four cancer patients in Ireland will survive their diagnosis and, in future, no one in Ireland will die from the disease. We in EY want to play our part in helping them achieve that vision through fundraising and raising awareness of the signs, symptoms, and importance of early detection with all our people.”
Strength in numbers – Players and representatives from UCD Rugby Football Club are joined by representatives from The Irish Cancer Society and EY for the launch of their annual daffodil day fundraising drive in aid of The Irish Cancer Society
83 HOSPITALPROFESSIONALNEWS.IE | HPN • MARCH 2023
Pictured L-R are Caroline Burton, Head of Retail Marketing at Uniphar Group, Geraldine O’Leary, Commercial Director, RTÉ, and Aoife Hofler, Director, Javelin
BRING PROTECTION TO LIFE
BY REDUCING THE RISK OF THE COMPOSITE OF DECLINING KIDNEY FUNCTION (BY ≥ 50%), ESKD, AND RENAL OR CV DEATH IN CONJUNCTION WITH OTHER CKD THERAPIES WHEN COMPARED TO PLACEBO*1
FORXIGA IS INDICATED IN ADULT PATIENTS FOR THE TREATMENT OF CKD.2
treatment
GFR mL/min/1.73m2
FORXIGA 10 mg
Once daily
No titration required except in patients with severe hepatic impairment
*The DAPA-CKD study was an international, multicentre, randomised, double-blind, placebo-controlled study. In DAPA-CKD, the primary endpoint showed that FORXIGA in conjunction with other CKD therapies reduced the relative risk of the composite endpoint of ≥50% sustained decline in eGFR, reaching ESKD, and renal or CV death by 39% (5.3% ARR) vs placebo with other CKD therapies in 4304 adult patients with CKD with an eGFR of 75 to 25 mL/ min/1.73m2 and albuminuria (UACR ≥ 200 and ≤ 5000 mg/g) (median follow-up of 2.4 years; 9.2% vs 14.5%; HR 0.61; 95% CI (0.51 - 0.72; p<0.001).1 ESKD defined as sustained eGFR < 15 mL/min/1.73m2, chronic dialysis treatment or receiving a renal transplant. DAPA-CKD was stopped early due to efficacy benefit, because of the unplanned early stop, the secondary endpoints are considered nominally significant; Secondary endpoints showed that FORXIGA in conjunction with other CKD therapies reduced the relative risk of the composite of CV death or hHF by 29% (1.8% ARR: HR 0.71; 95% CI, 0.55 to 0.92; nominal p=0.009) and also reduced the relative risk of all-cause mortality by 31% (2.1% ARR; HR 0.69; 95% CI, 0.53 to 0.88; nominal p=0.004) vs placebo with other CKD therapies. There were comparable rates of the individual component of CV death, FORXIGA vs placebo (3.0% vs 3.7%; HR 0.81; 95% CI, 0.58, 1.12)1
The overall safety profile of dapagliflozin in patients with chronic kidney disease was consistent with the known safety profile of dapagliflozin.2
©AstraZeneca 2022. All Rights Reserved.

ABRIDGED PRESCRIBING INFORMATION
FORXIGA® (dapagliflozin) 5MG & 10MG FILMCOATED TABLETS.
Consult Summary of Product Characteristics (SmPC) before prescribing.
Indications: Adults and children aged 10 years and above: Type 2 diabetes mellitus: For the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise, as monotherapy when metformin is considered inappropriate due to intolerance, or in addition to other medicinal products for the treatment of type 2 diabetes. Adults: Heart Failure: Indicated in adults for the treatment of symptomatic chronic heart failure. Chronic kidney disease: Indicated in adults for the treatment of chronic kidney disease.
Presentation: Film-coated tablets. 5mg or 10mg of dapagliflozin (as propanediol monohydrate). Each 5mg tablet contains 25mg of lactose. Each 10mg tablet contains 50mg of lactose.
Dosage and Administration: Adults: Type 2 diabetes mellitus: The recommended dose is 10mg once daily. Consider a lower dose of insulin or insulin secretagogue such as a sulphonylurea when used in combination with dapagliflozin to reduce the risk of hypoglycaemia. Children and adolescents: No dose adjustment in children aged 10 years and above. No safety and efficacy data available for children below 10 years of age. Heart Failure: The recommended dose is 10mg once daily. Chronic kidney disease: The recommended dose is 10 mg once daily. Children and adolescents: <18 years: Safety and efficacy not yet established. Elderly: ≥65 years: No dose adjustment is recommended based on age. Renal impairment: No dose adjustment is required based on renal function. Due to limited experience, it is not recommended to initiate treatment with dapagliflozin in patients with GFR < 25 mL/min. If GFR falls below 45 mL/min, additional glucose lowering treatment should be considered in patients with diabetes mellitus if further glycaemic control is needed. Mild or moderate hepatic impairment: No dose adjustment. Severe hepatic impairment: Starting dose of 5mg is recommended, if well tolerated, dose may be increased to 10mg. Method of administration: Forxiga can be taken orally at any time of day with or without food. Tablets should be swallowed whole.
Contraindications: Hypersensitivity to dapagliflozin, or excipients. Warnings and Precautions: Dapagliflozin should not be used in patients with type 1 diabetes mellitus. Renal impairment: Due to limited experience, it is not recommended to initiate treatment with dapagliflozin in patients with GFR < 25 mL/min. The glucose lowering efficacy of dapagliflozin is dependent on renal function, and is reduced in patients with GFR < 45 mL/min and is likely absent in patients with severe renal impairment. Hepatic impairment: Exposure is increased in patients with severe hepatic impairment. Use in patients at risk of volume depletion and/or hypotension: Dapagliflozin increases diuresis which may lead to a modest decrease in blood pressure, it may be more pronounced in patients with very high blood glucose concentrations. Exercise caution in patients for whom a dapagliflozin induced drop in blood pressure could pose a risk, such as patients on anti hypertensive therapy with a history of hypotension or elderly patients. Careful monitoring of volume status and electrolytes is recommended in conditions leading to volume depletion, such as acute gastrointestinal illness. In volume depleted patients temporary interruption of dapagliflozin is recommended until volume depletion is corrected. Diabetic ketoacidosis (DKA): Rare cases of DKA, including life-threatening and fatal cases, have been reported in patients treated with SGLT2 inhibitors, including dapagliflozin. In a number of cases, the presentation of the condition was atypical with only moderately
increased blood glucose values, below 14mmol/L (250mg/dL). The risk of DKA must be considered in the event of non-specific symptoms such as nausea, vomiting, anorexia, abdominal pain, excessive thirst, difficulty breathing, confusion, unusual fatigue or sleepiness. Patients should be assessed for ketoacidosis immediately if these symptoms occur, regardless of blood glucose level. In patients where DKA is suspected or diagnosed, dapagliflozin treatment should be stopped immediately. Treatment should be interrupted in patients who are hospitalised for major surgical procedures or acute serious medical illnesses. Monitoring of ketones is recommended in these patients. Measurement of blood ketone levels is preferred to urine. Treatment with dapagliflozin may be restarted when the ketone values are normal and the patient’s condition has stabilised. Before initiating dapagliflozin, factors in patient history that may predispose to ketoacidosis should be considered. Patients who may be at higher risk of DKA include patients with a low beta cell function reserve (e.g. patients with low C peptide or latent autoimmune diabetes in adults (LADA) or patients with a history of pancreatitis), patients with conditions that lead to restricted food intake or severe dehydration, patients for whom insulin doses are reduced and patients with increased insulin requirements due to acute medical illness, surgery or alcohol abuse. SGLT2 inhibitors should be used with caution in these patients. Restarting SGLT2 inhibitor treatment in patients experiencing a DKA while on SGLT2 inhibitor treatment is not recommended, unless another clear precipitating factor is identified and resolved. Dapagliflozin should not be used for treatment of patients with type 1 diabetes mellitus. Necrotising fasciitis of the perineum (Fournier’s gangrene): Postmarketing cases have been reported in female and male patients taking SGLT2 inhibitors. Urgent surgical intervention and antibiotic treatment is required. Advise patients to seek medical attention if they experience a combination of pain, tenderness, erythema, or swelling in the genital or perineal area, with fever or malaise. Either uro-genital infection or perineal abscess may precede necrotising fasciitis. If suspected, discontinue Forxiga and institute prompt treatment (including antibiotics and surgical debridement). Urinary tract infections: Temporary interruption of dapagliflozin should be considered when treating pyelonephritis or urosepsis. Elderly (≥65 years): No dose adjustment is recommended based on age. Elderly patients are more likely to have impaired renal function, be treated with medicines such as anti-hypertensives or diuretics, and be at a greater risk of volume depletion. Cardiac failure: Experience with dapagliflozin in NYHA class IV is limited. Infiltrative cardiomyopathy: Patients with infiltrative cardiomyopathy have not been studied. Chronic kidney disease: There is no experience with dapagliflozin for the treatment of chronic kidney disease in patients without diabetes who do not have albuminuria. Patients with albuminuria may benefit more from treatment with dapagliflozin. Lower limb amputations: Counsel patients with diabetes on routine preventative foot care as an increase in cases of lower limb amputation (primarily of the toe) has been observed in long term, clinical studies in type 2 diabetes mellitus with SGLT2 inhibitors. Urine laboratory assessments: Patients will test positive for glucose in the urine due to mechanism of action. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take Forxiga.
Drug Interactions: Diuretics: Dapagliflozin may add to the diuretic effect of thiazide and loop diuretics and may increase the risk of dehydration and hypotension. Insulin and insulin secretagogues: Consider a lower dose of insulin or insulin secretagogue when used in combination with dapagliflozin to reduce the risk of hypoglycaemia. Interference with 1,5 AG assay: Monitoring glycaemic control with 1,5-AG assay is not recommended as measurements of 1,5 AG are unreliable in assessing glycaemic control in patients taking SGLT2 inhibitors. Alternative methods
should be used. Interference with lithium: Dapagliflozin may increase renal lithium excretion and the blood lithium levels may be decreased. Serum concentration of lithium should be monitored more frequently after dapagliflozin initiation and dose changes. Please refer the patient to the lithium prescribing doctor in order to monitor serum concentration of lithium.
Pregnancy and Lactation: Not recommended during the second and third trimesters of pregnancy. Treatment should be discontinued when pregnancy is detected. Do not use whilst breast-feeding.
Ability to Drive and Use Machines: Alert patients on the risk of hypoglycaemia when dapagliflozin is used in combination with a sulphonylurea or insulin.
Undesirable Events: Consult SmPC for full list of side effects. Very common (≥1/10): Hypoglycaemia (when used with SU or insulin). Common (≥1/100 to <1/10): Vulvovaginitis, balanitis and related genital infections, urinary tract infection, dizziness, rash, back pain, dysuria, polyuria, haematocrit increased, creatinine renal clearance decreased during initial treatment, dyslipidaemia. Uncommon (≥1/1,000 to < 1/100): Fungal infection, volume depletion, thirst, constipation, dry mouth, nocturia, vulvovaginal pruritus, pruritus genital, blood creatinine increased during initial treatment, blood urea increased, weight decreased. Rare (≥ 1/10,000 to < 1/1,000): Diabetic ketoacidosis (when used in type 2 diabetes mellitus). Very Rare (< 1/10,000): Angioedema, necrotising fasciitis of the perineum (Fournier’s gangrene), Tubulointerstitial nephritis. Legal Category: Product subject to prescription which may be renewed (B).
Marketing Authorisation Number: EU/1/12/795/002; EU/1/12/795/007.
Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden.
Further product information available on request from: AstraZeneca Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road North, Dublin 15. Tel: +353 1 609 71 00.
FORXIGA is a trademark of the AstraZeneca group of companies.
Date of API preparation: 02/2023 Veeva ID: IE-4664
References:
Adverse events should be reported directly to: HPRA Pharmacovigilance, Website: www.hpra.ie
Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899
1. Heerspink HJL et al. N Engl J Med. 2020;383(15):1436–1446.
2. FORXIGA 10 mg film-coated tablets. Summary of product characteristics.
ARR = absolute risk reduction; CKD = chronic kidney disease; CV = cardiovascular; DAPA-CKD = Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease; eGFR = estimated glomerular filtration rate; ESKD = end-stage kidney disease; hHF = hospitalisation for heart failure; HR = hazard ratio. HFrEF = heart failure with reduced ejection fraction; T2D = type 2 diabetes
Veeva ID: IE-4695 Date of Prep: February 2023


























 Written by
Written by










 Written by Ann Piercy, Personal Support Worker, KnowNow Community Worker
Written by Ann Piercy, Personal Support Worker, KnowNow Community Worker


























 Written by:
Written by:


































 Figure 1: IBS Diagnostic Criteria
Figure2A: Bristol Stool Chart
Figure 1: IBS Diagnostic Criteria
Figure2A: Bristol Stool Chart





















