Ireland’s Dedicated Hospital Professional Publication

IN THIS ISSUE:
NEWS: Revised Pay for Hospital Pharmacists
Page 6
REPORT:
Medicines Shortages reaching ‘Critical’ Point Page 9
CONFERENCE: 58th IACR Annual Conference
Page 14
Med. 2020;382(6):514-524.
4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2022. © National Comprehensive Cancer Network, Inc. 2021. All rights reserved. Published June 21, 2022. Accessed July 29, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
ABBREVIATED PRESCRIBING INFORMATION
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Kisqali (ribociclib) 200 mg film-coated tablets
Presentation: Film coated tablets (FCT) containing 200 mg of ribociclib and 0.344 mg soya lecithin.
Indications: Kisqali is indicated for the treatment of women with hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy
In pre or perimenopausal women, the endocrine therapy should be combined with a luteinising hormone releasing hormone (LHRH) agonist.
Dosage and administration:
Adults: The recommended dose is 600 mg (3 x 200 mg FCT) taken orally, once daily for 21 consecutive days followed by 7 days off treatment, resulting in a complete cycle of 28 days.
Kisqali should be used together with 2.5 mg letrozole or another aromatase inhibitor or with 500 mg fulvestrant.
When Kisqali is used in combination with an aromatase inhibitor, the aromatase inhibitor should be taken orally once daily continuously throughout the 28 day cycle. Please refer to the Summary of Product Characteristics (SmPC) of the aromatase inhibitor for additional details.
When Kisqali is used in combination with fulvestrant, fulvestrant is administered intramuscularly on days 1, 15 and 29, and once monthly thereafter. Please refer to the SmPC of fulvestrant for additional details.
Treatment of pre and perimenopausal women with the approved Kisqali combinations should also include an LHRH agonist in accordance with local clinical practice.
Management of severe or intolerable adverse reactions (ARs) may require temporary dose interruption, reduction or discontinuation of Kisqali. Please see section 4.2 of SmPC for recommended dose modification guidelines.
renal impairment. Caution should be used in patients with severe renal impairment with close monitoring for signs of toxicity. ♦Hepatic impairment: Mild: No dose adjustment is necessary. Moderate or severe: Dose adjustment is required, and the starting dose of 400 mg once daily is recommended. ♦Elderly (>65 years): No dose adjustment is required. ♦Pediatrics(<18 years): Safety and efficacy have not been established. Contraindications: Hypersensitivity to the active substance or to peanut, soya or any of the excipients.
Warnings/Precautions: ♦Neutropenia was most frequently reported AR. A complete blood count (CBC) should be performed before initiating treatment. CBC should be monitored every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. Febrile neutropenia was reported in 1.4% of patients exposed to Kisqali in the phase III clinical studies. Patients should be instructed to report any fever promptly.Based on the severity of the neutropenia, Kisqali may require dose interruption, reduction, or discontinuation.
♦Hepatobiliary toxicity - increases in
transaminases have been reported. Liver function tests (LFTs) should be performed before initiating treatment. LFTs should be monitored every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. If grade ≥ 2 abnormalities are noted, more frequent monitoring is recommended. Recommendations for patients who have elevated AST/ALT grade ≥ 3 at baseline have not been established. Based on the severity of transaminase elevations, Kisqali may require dose interruption, reduction, or discontinuation. ♦QT interval prolongation has been reported with Kisqali. The use of Kisqali should be avoided in patients who have already or who are at significant risk of developing QTc prolongation. The ECG should be assessed prior to initiation of treatment. Treatment with Kisqali should be initiated only in patients with QTcF values <450 msec. The ECG should be repeated at approximately Day 14 of the first cycle and at the beginning of the second cycle, then as clinically indicated. In case of QTcF prolongation during treatment, more frequent ECG monitoring is recommended Appropriate monitoring of serum electrolytes (including potassium, calcium, phosphorous, and magnesium) should be performed prior to initiation of treatment, at the beginning of the first 6 cycles, and then as clinically indicated. Any abnormality should be corrected before the start of Kisqali treatment. Based on the observed QT prolongation during treatment, Kisqali may require dose interruption, reduction, or discontinuation. Based on the E2301 study QTcF interval data, Kisqali is not recommended for use in combination with tamoxifen. ♦Critical visceral disease. The efficacy and safety of ribociclib have not been studied in patients with critical visceral disease. ♦Severe cutaneous reactions Toxic epidermal necrolysis (TEN) has been reported with Kisqali treatment. If signs and symptoms suggestive of severe cutaneous reactions (e.g. progressive widespread skin rash often with blisters or mucosal lesions) appear, Kisqali should be discontinued immediately. ♦Interstitial lung disease/pneumonitis ILD/pneumonitis has been reported with CDK4/6 inhibitors including Kisqali. Based on the severity of the ILD/pneumonitis, which may be fatal, Kisqali may require dose interruption, reduction or discontinuation as described in SmPC. Patients should be monitored for pulmonary symptoms indicative of ILD/pneumonitis which may include hypoxia, cough and dyspnoea and dose modifications should be managed in accordance with Table 5 (see section 4.2)
♦Blood creatinine increase ribociclib may cause blood creatinine increase – if this occurs it is recommended that further assessment of the renal function be performed to exclude renal impairment.
♦CYP3A4 substrates. ribociclib may interact with medicinal products which are metabolised via CYP3A4, which may lead to increased serum concentrations of CYP3A4 substrates (see section 4.5). Caution is recommended in case of concomitant use with sensitive CYP3A4 substrates with a narrow therapeutic index and the SmPC of the other product should be consulted for the recommendations regarding co administration with CYP3A4 inhibitors.
Pregnancy, Fertility and Lacation
♦Pregnancy: Pregnancy status should be verified prior to starting treatment as Kisqali can cause foetal harm when administered to a pregnant woman.
♦Women of childbearing potential who are receiving Kisqali should use effective contraception (e.g. double-barrier contraception) during therapy and for at least 21 days after stopping treatment with Kisqali. ♦Breast feeding: Patients receiving Kisqali should not breast feed for at least 21 days after the last dose. ♦Fertility: There are no clinical data available regarding effects of ribociclib on fertility. Based on animal studies, ribociclib may impair fertility in males of reproductive potential.
♦Effects on ability to drive and use machines Patients should be advised to be cautious when driving or using machines in case they experience fatigue, dizziness or vertigo during treatment with Kisqali.
Interactions: ♦Concomitant use of strong CYP3A4 inhibitors should be avoided, including, but not limited to, clarithromycin, indinavir, itraconazole, ketoconazole, lopinavir, ritonavir, nefazodone, nelfinavir, posaconazole, saquinavir, telaprevir, telithromycin, verapamil, and voriconazole. Alternative concomitant medicinal products with less potential to inhibit CYP3A4 should be considered. Patients should be monitored for ARs. If concomitant use of a strong CYP3A4 inhibitor cannot be avoided, the dose of Kisqali should be reduced (see section 4.2 of SmPC). ♦Grapefruit or grapefruit juice should be avoided. ♦Concomitant use of strong CYP3A4 inducers should be avoided, including, but not limited to, phenytoin, rifampicin, carbamazepine and St John’s Wort (Hypericum perforatum). An alternative medicinal product with no or minimal potential to induce CYP3A4 should be considered. ♦Caution is recommended when Kisqali is administered with sensitive CYP3A4 substrates with narrow therapeutic index (including, but not limited to, alfentanil, ciclosporin, everolimus, fentanyl, sirolimus, and tacrolimus), and their dose may need to be reduced. ♦Concomitant administration of Kisqali at the 600 mg dose with the following CYP3A4 substrates should be avoided: alfuzosin, amiodarone, cisapride, pimozide, quinidine, ergotamine, dihydroergotamine, quetiapine, lovastatin, simvastatin, sildenafil, midazolam, triazolam. ♦Caution and monitoring for toxicity are advised during concomitant treatment with sensitive substrates of drug transporters P-gp, BCRP, OATP1B1/1B3, OCT1, OCT2, MATE1 and BSEP which exhibit a narrow therapeutic index, including but not limited to digoxin, pitavastatin, pravastatin, rosuvastatin and metformin. ♦Co-administration of Kisqali with medicinal products with known potential to prolong the QT interval should be avoided such as anti-arrhythmic medicinal products (including, but not limited to, amiodarone, disopyramide, procainamide, quinidine and sotalol) and other medicinal products known to prolong the QT interval including, but not limited to, chloroquine, halofantrine, clarithromycin, ciprofloxacin, levofloxacin, azithromycin, haloperidol, methadone, moxifloxacin, bepridil, pimozide and intravenous ondansetron. Kisqali is not recommended for use in combination with tamoxifen.
Adverse reactions: ♦Very common: Infections, neutropenia, leukopenia, anaemia lymphopenia, decreased appetite, headache, dizziness, dyspnoea, cough, nausea, diarrhoea, vomiting, constipation, stomatitis, abdominal pain, dyspepsia alopecia, rash, pruritus, back pain, fatigue, peripheral oedema, asthenia, pyrexia, abnormal liver function tests. ♦Common:, thrombocytopenia, febrile neutropenia, hypocalcaemia, hypokalaemia, hypophosphataemia, vertigo, lacrimation increased, dry eye, syncope, dysgeusia, , hepatotoxicity, erythema, dry skin, vitiligo, dry mouth, oropharyngeal pain, blood creatinine increased, electrocardiogram
FEATURE: Potential New Treatment for Asthma
Page 18
CPD: Post-Biologic Treatment for Psoriasis
Page 31
ONCOLOGY FOCUS: Radionuclide Therapy in Advanced Prostate Cancer
Page 36
ONCOLOGY FOCUS: Cancer Associated Venous Thromboembolism
Page 48
HOSPITAL PROFESSIONAL NEWS
IRELAND
HPN February 2023 Issue 105 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only
Special populations: ♦Renal impairment: Mild or moderate: No dose adjustment is necessary. Severe: A starting dose of 200 mg is recommended in patients with severe renal impairment. Kisqali has not been studied in breast cancer patients with severe
Kisqali can be taken with or without food (see section 4.5).The tablets should be swallowed whole and should not be chewed, crushed or split prior to swallowing.
for a
of adverse reactions. Legal Category: POM Pack sizes: Unit packs containing 21, 42 or 63 FCTs. Not all pack sizes may be marketed. Marketing Authorisation Holder: Novartis Europharm Limited Vista Building, Elm Park, Merrion Road, Dublin 4 Ireland Marketing Authorisation Numbers: EU/1/17/1221/003 & 005. Full prescribing information is available on request from Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Dublin 4. Tel: 01 2601255 or at www.medicines.ie Prescribing information last revised: April 2022 November 2022 | IE253121 © 2022 Novartis Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Merrion Road, Dublin 4, D04 A9N6 REFERENCES: 1. Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival results from the phase III MONALEESA-2 trial of postmenopausal patients with HR+/HER2− advanced breast cancer treated with endocrine therapy ± ribociclib. Presented at: European Society of Medical Oncology; September 16-21, 2021. 2. Im S-A, Lu Y-S, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307-316. 3. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J
QT prolonged. ♦Please refer to SmPC
full list
KISQALI is not indicated for concomitant use with tamoxifen4 1L, first line; 2L, second line; ET, endocrine therapy; LHRH, luteinizing hormonereleasing hormone, aBC, advanced breast cancer. ESMO - European society of medical oncology SABC - San Antonio Breast Cancer Conference ASCO - American Society of Clinical Oncology Reporting suspected adverse reactions of the medicinal product is important to Novartis and the HPRA. It allows continued monitoring of the benefit/risk profile of the medicinal product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by calling 01 2080 612. National Comprehensive Cancer Network® (NCCN®) now recognizes ribociclib (KISQALI®) + ET, a Category 1 preferred treatment option, for showing an OS BENEFIT IN 1L PATIENTS with HR+/HER2- mBC4 NCCN RECOMMENDED KISQALI—the only CDK4/6 inhibitor with statistically significant overall survival across all 3 phase III trials1–3
TO LIVE WELL WITH HIV


DEVELOPED WITH THE HIV COMMUNITY, FOR THE HIV COMMUNITY









For information and resources to support those living with HIV to live well visit www.hivfindyourfour.ie



Find Your Four is a campaign developed and funded by Gilead Sciences, in collaboration with the HIV community.

UK-UNB-3430 | January 2023

Government must answer ‘999’ call P4
Capacity in Irish Hospitals reaches breaking point P5
Hospital Pharmacists finally receive commitment to pay revision P6

Medicines Shortage Index provides worrying insights P9
New Platform launched to tackle Obesity P12
Robotic First at Galway University Hospital P17


REGULARS
Feature: Precision Medicine for COPD P26
CPD: Psoriasis P31
Oncology Focus: Triple Negative Breast Cancer P40
Oncology Focus: Lung Cancer P52
Feature: Ulcerative Colitis P77
P80
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system or transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562
GROUP DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com
EDITOR
Kelly Jo Eastwood
DIGITAL MARKETING & EDITORIAL EXECUTIVE
Emilia Bayliss
emilia@hospitalprofessionalnews.ie
EDITORIAL editorial@hospitalprofessionalnews.ie
ACCOUNTS
Rachel Wilson cs.ipn@btconnect.com
SALES EXECUTIVE
Aoife Tremere aoife.t@hospitalprofessionalnews.ie

CONTRIBUTORS
N Tangney | DM Murphy
Tomás P. Carroll | Ronan C. Heeney
Geraldine Kelly | Gerry McElvaney
Dr Jonathan Briody | Mr Richard Walsh

Professor Kilian Walsh | Amina Babar
Mary N. O’Reilly | Michaela J. Higgins

Lucy Norris | Theresa Lowry Lehnen
Síle Griffin | Fiona Griffin
Tatiana Larmak | Naoise Irwin
Professor Joe M. O’Sullivan
Yasmina E. Hernandez Santana
Patrick T. Walsh
DESIGN DIRECTOR

Ian Stoddart Design
Editor

As 2023 and a New Year gets underway, unfortunately some lingering issues from the past remain. Notably medicine shortages and the pressures being faced by Ireland’s hospitals.
On page 4, we detail how the Irish Hospital Consultants Association (IHCA) has urged the Government to treat the growing crisis in the health service as an ‘emergency 999 call’, given that almost 900,000 people are on some form of NTPF waiting list, a record 931 admitted patients were treated on trolleys last week, and 918 permanent Consultant posts nationally are either vacant or filled on a temporary or agency basis.
IHCA President Professor Robert Landers said: “This is a lifethreatening situation for patients seeking care in our hospitals and requires a ‘999- like’ emergency response from Government.”
The Medicines Shortage Index, prepared by Azure Pharmaceuticals, has found that there are 224 medicines currently unavailable, an increase of 12 medicines in seven days (at 11 January). Among the additional medicines to go out-of-stock in the past weeks are Phenytoin which is used to treat epilepsy.
Analysis by the group found that 40% of the medicines currently out of stock are provided to the Irish market by a single supply. Furthermore, the survey has shown that medicine manufacturers, including companies producing medicines domestically, are getting paid up to four times as much for their products abroad than in Ireland.
The EU is preparing to stockpile drugs and oblige manufacturers to guarantee supplies in efforts to tackle the on-going medicines shortages.
The European Commission will also try to reduce reliance on China and increase domestic production capacity, the commission told the Financial Times — which reported the news. In a written response to the Greek government, EU health commissioner Stella Kyriakides outlined the plan. The commission will intervene to ensure “strategic autonomy” in basic medicines through a “systemic industrial policy”.
You can read more about this in our Report starting on page 9 of this issue.
Our Special Focus this month is on Oncology, tying in with World Cancer Day on Saturday, 4th February. We have some excellent clinically contributed content, including Triple Negative Breast Cancer: A Background and Cost in Ireland; Common Urological Side effects of Radiotherapy, and Biomarkers for Cancer
Associated Venous Thromboembolism.
I hope you enjoy the issue.
3 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023 February Issue Issue 105 4
Contents Foreword
Clinical R&D:
12 6 17 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews
Government must answer ‘999’ Call
The Irish Hospital Consultants Association (IHCA) has urged the Government to treat the growing crisis in the health service as an ‘emergency 999 call’, given that almost 900,000 people are on some form of NTPF waiting list, a record 931 admitted patients were treated on trolleys last week, and 918 permanent Consultant posts nationally are either vacant or filled on a temporary or agency basis.
The appeal from Consultants for an emergency response from Government comes as the latest figures from the National Treatment Purchase Fund (NTPF) released today reveal that 870,000 people were on some form of NTFP waiting list at the end of December – a reduction of just 9,000 compared with the start of 2022.
Minister for Health Stephen Donnelly launched the Government’s ¤350 million Waiting List Action Plan for 2022 last February2 which committed to reducing active waiting lists for acute scheduled care by 18% (more than 132,00) by the end of 2022.
Before leaving office, former HSE CEO Paul Reid said that the HSE could not forecast whether a reduction in the waiting lists of 18%, 15% or even 10% could be achieved by the end of 2022.3 In fact, today’s NTPF figures confirm the three main waiting lists

covered by the plan decreased by just 29,800 or 4%. This decrease takes account of 71,000 people who were removed from the waiting lists without any treatment in the first nine months of 2022 through an NTPF ‘validation programme’.
Growth in a number of pre-admit, planned and suspension lists not widely publicised means that overall NTPF waiting lists reduced by just 9,180 in 2022, and have risen by a staggering 286,500 (49%) since the publication of Sláintecare in 2017. Including the additional 243,000 people on waiting lists for CTs, MRIs or ultrasounds nationally5 sees the total number of people awaiting hospital care now amount to over 1.1 million.
New analysis from the IHCA shows that the HSE is 102,500 outpatient appointments and procedures away from meeting the 18% reduction target for the end of 2022, including almost 97,000 outpatient appointments and around 5,500 inpatient/day case procedures and GI scopes.
Last month, the number of patients awaiting inpatient/day case treatment increased for the fourth month in a row, and at 81,568 are at the highest level since the peak of the pandemic in April 2020. Consultants have raised their concerns that the combination
of the unprecedented spike in hospital overcrowding, the longstanding deficits in hospital capacity and the record vacant Consultant posts could see the number of people on hospital waiting lists reach new record highs in the months ahead, due to the continued cancellation of essential scheduled care across the country.
Commenting on today’s waiting lists, IHCA President Professor Robert Landers said:
“This is a life-threatening situation for patients seeking care in our hospitals and requires a ‘999like’ emergency response from Government. With almost 900,000 people on waiting lists, over 900 vacant Consultant posts and 900 admitted patients treated on a chair or trolley on a single day last week, the numbers paint a stark picture of the crisis facing the health service.
“But we cannot forget that the vast waiting lists represent individual adults or children who could be experiencing pain, suffering or the psychological distress at not knowing when they will be able to receive the hospital treatment they need. This is a wholly unacceptable situation.
“Regrettably, in the short-term, waiting lists are likely to deteriorate further due to the cancellation of many outpatient appointments,
inpatient admissions, and day case procedures, including chemotherapy and dialysis.
“The Government needs to urgently put in place a credible plan to provide care to the 1.1 million people awaiting essential hospital assessment and treatment. This can only be achieved by addressing the twin deficits of a shortage of Consultants and a lack of sufficient public hospital capacity.
“The fact that 918 permanent Consultant posts, 23% of the total number approved, cannot be filled as needed is a shocking signal to Government and health service management that the current conditions in our public hospitals are not up to the standard required by skilled medical and surgical specialists to practise effectively. This in turn is driving our highly trained specialists abroad to pursue their careers in countries where they work in collaborative and supportive health services.”
4 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Professor Robert Landers, President, IHCA
“The Government needs to urgently put in place a credible plan to provide care to the 1.1 million people awaiting essential hospital assessment and treatment”
Hospital Capacity hits new Heights

The Irish Hospital Consultants Association (IHCA) has expressed its concern over the continued pressures being faced by Ireland’s acute hospital services as extreme levels of overcrowding in our emergency departments and un-safe capacity limits hit record new highs.
Commenting, a spokesperson for the IHCA said, “The Irish health service is once again the focus of public, political and media attention. The level of coverage of individual and collective experiences from within our hospitals over the past number of weeks is only comparable to that of the start of the Covid-19 pandemic. It is not inconceivable that we could see 1,000 admitted patients being
treated on trolleys on a single day in the weeks ahead.
“Public hospital staff are working tirelessly attempting to provide appropriate levels of care to patients. Consultants are on call 24/7, often practising over and above recommended levels, but the reality is there simply aren’t enough of us to meet increased demand. We are still working with 40% less Consultant staffing in Ireland, compared to the EU average.
“What compounds this further is the failure of Government to put in place bed and staffing commitments dating back years. When Consultants do eventually get to see patients, we face restricted care flows
due to inadequate bed and theatre capacity, and required staffing levels.
“The Winter Plan 2022/23 committed to appointing 51 additional Emergency Medicine Consultants, yet as we hit the peak of winter illness, none of these have been appointed as yet on a permanent basis, with just approximately 20 locum Consultants in place. This is not surprising given that it took on average 9 months to fill a vacant Consultant post in 2022.
“In addition, the Department of Health has no credible plan to urgently put in place the 1,400 additional public hospital beds that need to be opened more speedily
than anticipated in what is an outof-date 2018 Capacity Review.
“As a result, those of us on the ground in hospitals and delivering care in the community are consistently left to firefight for ourselves with the limited and overstretched resources we have.
“The HSE’s National Service Plan 2023 is now due and must be published as soon as possible, taking all this into consideration. To move away from this constant wheel of crises, the Government must put in place the capacity expansion that is needed and the HSE must empower hospitals and community services to make decisions and take the actions needed to provide timely and safe care to their patients.”
Nominations open for Medical Council Election
The Medical Council has announced the procedures for the election of the six directly elected members of the Medical Council.
The Medical Council, which is the regulatory body for doctors, has a statutory role in protecting the public by promoting the highest professional standards amongst doctors practising in Ireland, and is made up of 25 members, with a majority of 13 non-medical members and 12 medical members. Members are appointed through a mixture of elections, nominations by a
number of bodies and through the public appointments system.
In order to be eligible for nomination for election to the Medical Council, a medical professional must be on the register of medical practitioners, and must be practising medicine in the State (but excluding any visiting EEA practitioner) on 15th February 2023, the day before nominations close. They must be nominated for election by 10 registered medical practitioners practising medicine in the State.
An independent Returning Officer has been appointed and, in accordance with the provisions of section 17(1) and (8) of the Medical Practitioners Act 2007, will conduct the election process. The Medical Council is encouraging all interested and eligible doctors to consider running for election.
The closing date for receipt of nomination papers is Thursday, 16th February 2023 at 1pm. The independent Returning Officer will attend at the offices of the
Medical Council to receive nomination papers, which can be submitted via post or in person on 16th February, 2023 between the hours of 10am – 1pm.
Nomination papers and other information, including information on the roles and responsibilities of Council members, which is included in the Medical Council’s Corporate Governance Framework regarding the election, are available on the Medical Council website www.medicalcouncil.ie/elections
5 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
News
Revised Pay and New Posts for Hospital Pharmacists
Forsa National Secretary Linda Kelly

The circular activates the recommendations contained in the 2017 McLoughlin Report and subsequent negotiations. It confirms improvements to the basic grade pharmacist salary scale, while increment dates remain unchanged.
The Department of Health and HSE have issued circulars confirming the implementation of a revised pay scale and revised management posts for hospital pharmacists, agreed with Fórsa and the HPAI following the Building Momentum review last year.
The new pay scale sees the removal of the first three points of the existing scale and will result in an adjustment to salary for most, while the old salary scale will be maintained in the Department of Health consolidated salary scales for pension purposes.
The introduction of the new pharmacy executive services manager post is also confirmed by the circular and takes effect from the same date. Details of how existing postholders will be designated to those posts are also outlined in the circular.
National secretary Linda Kelly said the circular’s confirmation of new scales and agreed management posts marked the conclusion of a lengthy union campaign to ensure the recommendations of the McLoughlin report were activated: “Despite a long drawn-out negotiation, our hospital pharmacist members were determined to achieve what has now been confirmed in this circular.
“A clause in the review of Building Momentum last year provided the
New Study on Parkinson’s Disease
The activity of multiple genes in the brain varies according to sex among people with Parkinson’s disease, a new study reports.
These differences may contribute to discrepancies in how Parkinson’s tends to affect men and women. “While males have a higher age-adjusted disease incidence and are more frequently affected by muscle rigidity, females present more often with disabling tremors,” its researchers noted.
The reasons for these sex-based differences are poorly understood. To learn more, a trio of researchers in Luxembourg conducted a metaanalysis, a type of study where scientists pool and collectively analyze data from multiple studies. Specifically, this work focused on datasets of gene expression in samples of brain tissue collected after death from people with Parkinson’s and matched adults without the disease as controls.
Gene expression refers to how much individual genes are “turned on or off” in terms of their activity. They assessed nearly 12,000 genes, looking for those whose activity levels were significantly altered in patients versus controls according to sex. They identified 1,146 differentially expressed genes (DEGs) in men with Parkinson’s and 118 DEGs in female patients.
Partly, the higher number of DEGs in men could be due to the larger number of male samples included in the analysis, allowing greater statistical power to identify significantly altered gene activity, the researchers noted.
However, analyses involving a subset of the men also generally yielded higher numbers of genes with altered activity. As such, “the lower number of significant DEGs in females may at least partly reflect a different disease manifestation and progression,” the team wrote.
The scientists divided the DEGs into three categories: 36 were female specific, meaning
significant activity changes were detected in women only, while 539 were male specific. Another 37 DEGs were sex dimorphic, meaning they showed opposite trends between sexes (higher levels in women but lower levels in men, or vice versa).Many of the genes identified in these analyses “are involved in cellular processes and organelles previously described to display pathological [disease-driving] alterations in PD [Parkinson’s disease],” the researchers wrote.
For example, several of these genes are involved in the production of dopamine or the function of mitochondria, the cell’s powerhouses, and lysosomes, the cell’s recycling centers. Dopamine is the major brain chemical messenger that’s progressively lost in Parkinson’s, while mitochondrial damage and lysosomal impairment have been implicated in the disease.
Further analyses were conducted to assess the biochemical pathways most affected by these gene activity changes. Results indicated that malespecific and sex-dimorphic DEGs were commonly involved in mitochondrial function and cellular energy metabolism, while femalespecific DEGs more commonly associated with inflammation.
necessary negotiating opportunity to ensure these measures were introduced, and we were able to confirm the outcome to pharmacist members last November.
“The new HSE circular confirms that all the necessary arrangements are now in place, and I want to acknowledge the great work of our hospital pharmacist representatives throughout this process. It demonstrates, yet again, the real power of trade union membership and collective bargaining.
“Implementation discussions on the remaining recommendations related to the deputy pharmacy executive services manager posts and the creation of advanced specialists posts will now commence,” she said.
“No significant overlap was observed between the pathways with female DEGs and male DEGs, suggesting that instead of affecting different genes in the same pathways, sex-specific changes tend to affect diverse pathways,” the team wrote.
While many of the DEGs have known functions in Parkinson’srelated pathways, most have not been specifically linked to Parkinson’s before.
Notably, one exception was the NR4A2 gene, whose activity was generally lower in Parkinson’s patients relative to controls, but the effect was much more pronounced in men.
This gene has a “key regulatory role in the maintenance of dopamine metabolism and inflammatory gene expression,” the researchers wrote, and its reduced activity has been linked to brain aging and increased production of the alpha-synuclein protein, a hallmark of Parkinson’s.
Preclinical work has implicated NR4A2 activity changes in Parkinson’s and suggested that the protein it encodes may be a useful treatment target.
Two other sex-dependent DEGs of interest with Parkinson’sassociated regulatory functions were CA2 and EFNA1
6 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
“No significant overlap was observed between the pathways with female DEGs and male DEGs, suggesting that instead of affecting different genes in the same pathways, sex-specific changes tend to affect diverse pathways”
Standard LCIG*
... a reduced levodopa dose can be given
*Levodopa-Carbidopa Intestinal Gel 1. Lecigon Summary of Product Characteristics, 2021.
The same effective and stable plasma levodopa levels are achieved
ABBREVIATED PRESCRIBING INFORMATION. Lecigon 20 mg/ml + 5 mg/ml + 20 mg/ml intestinal gel. 1 ml contains 20 mg levodopa, 5 mg carbidopa monohydrate and 20 mg entacapone. Presentation: Yellow or yellowish-red opaque viscous gel. Indications: Treatment of advanced Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available oral combinations of Parkinson medicinal products have not given satisfactory results. Dosage and administration: Adults/Elderly: Administration by a portable infusion pump directly into the duodenum or upper jejunum via a percutaneous endoscopic gastrostomy(PEG) tube or radiological gastrojejunostony tube. Please consult Summary of Product Characteristics (SmPC) for further information. Only pump Crono LECIG (CE 0476) may be used for the administration of Lecigon. The dose should be titrated to achieve the optimal clinical response in the individual patient, which involves maximising the functional ON-time during the day by minimising the number and duration of OFF episodes (bradykinesia) and minimising ON-time with disabling dyskinesia. Total dose/day of Lecigon is composed of three individually adjusted doses: the morning bolus dose, the continuous maintenance dose, and extra bolus doses. Treatment is usually limited to the patient’s awake period. If medically justified, Lecigon can be administered up to 24 hours/day. The maximum recommended daily dose is 100 ml (2000 mg levodopa, 500 mg carbidopa monohydrate and 2000 mg entacapone. Please consult SmPC for further information. Total morning dose is usually 5–10 ml (100–200 mg levodopa) but not exceeding 15 ml (300 mg levodopa). Continuous maintenance dose is usually 0.7–5.0 ml/hour (15–100 mg levodopa/hour). Extra bolus doses are given if the patient becomes hypokinetic and are normally less than 3ml. An increase in the continuous maintenance dose should be considered if the need for extra doses exceeds 5 doses per day. Please consult SmPC for further information. After initial titration, the morning dose and maintenance dose are fine-tuned over the course of a few weeks. Lecigon is initially given as monotherapy. If needed, other anti-Parkinsonian medicinal products can be taken concurrently. If treatment with other anti-Parkinsonian medicinal products is discontinued or changed, it may be necessary to adjust the doses of Lecigon. Sudden deterioration in response with recurring motor fluctuations may indicate that the tube has dislocated to the stomach. This needs confirmation by X-ray and may require repositioning. Please consult SmPC for further information. The cartridge is for single use only and should not be used for more than 24 hours. Children: There is no relevant indication for use in children and adolescents. Contraindications: Hypersensitivity to the active substances or any of the excipients, narrow-angle glaucoma, severe heart failure, severe cardiac arrhythmia, acute stroke, severe hepatic impairment. Non-selective MAO-inhibitors and selective MAO type A inhibitors are contraindicated and should be discontinued at least two weeks prior to initiating therapy with Lecigon. Conditions in which adrenergics are contraindicated. Previous neuroleptic malignant syndrome (NMS) and/or non-traumatic rhabdomyolysis. Suspected undiagnosed skin lesions or a history of melanoma. Please consult SmPC for further information. Warnings and precautions: Not recommended for the treatment of drug-induced extrapyramidal reactions. Caution in ischaemic heart disease, severe cardiovascular or pulmonary disease, bronchial asthma, renal, hepatic or endocrine disease, or history of peptic ulcer disease or of convulsions, past or current psychosis, chronic wide-angle glaucoma, concomitant administration of antipsychotics with dopamine receptor blocking properties or with medicines which may cause orthostatic hypotension. In patients with a history of myocardial infarction who have residual atrial nodal or ventricular arrhythmias, cardiac function should be monitored with particular care during the period of initial dosage adjustments. Monitor all patients for mental changes, depression with suicidal tendencies, and other serious mental changes. Neuroleptic malignant like syndrome with secondary rhabdomyolysis may occur on abrupt dose reduction/discontinuation of Lecigon. Patients should be monitored for the development of impulse control disorders and review of treatment is recommended if such symptoms develop. Patients and caregivers are advised to monitor for melanomas on a regular basis when using Lecigon. Dose may need to be adjusted downwards to avoid levodopa-induced dyskinesia. Periodically evaluate hepatic, haematopoietic, cardiovascular and renal function during extended therapy. Lecigon contains hydrazine, a degradation product of carbidopa that can be genotoxic and possibly carcinogenic. Reported complications for levodopa/carbidopa in clinical studies include bezoar, ileus, implant site erosion/ulcer, intestinal haemorrhage, intestinal ischaemia, intestinal obstruction, intestinal perforation, intussusception, pancreatitis, peritonitis, pneumoperitoneum and post-operative wound infection. Sudden or gradual worsening of bradykinesia may indicate an obstruction in the tubing system and should be investigated. Weight loss has been associated with the active substances contained in Lecigon, and caregivers should therefore be aware of weight loss. Monitoring of weight is recommended to avoid severe weight loss. Prolonged or persistent diarrhoea that appears during use of entacapone could be a sign of colitis. In case of prolonged or persistent diarrhoea, treatment with the medicinal product should be discontinued and other appropriate medical treatment and investigation considered. Replacement of Lecigon with either levodopa and a DDC inhibitor without entacapone or other dopaminergic therapy may be necessary and should be done slowly. For patients who experience progressive anorexia, asthenia and weight loss within a relatively short period of time, a general medical evaluation including liver function assessment should be considered. Please consult SmPC for further information. Interactions: Antihypertensives, antidepressants, anticholinergics, dopamine receptor antagonists, benzodiazepines, isoniazide, phenytoin, papaverine, sympathicomimetrics , iron, protein-rich diet, amantadine and dopamine agonists (e.g. piribedil) may increase levodopa-related adverse events. Lecigon dose adjustment may be needed when used with these medicines. Lecigon can be taken with MAO type B inhibitors (e.g. selegiline) although serious orthostatic hypotension may occur and the dose of levodopa may need to be reduced. Lecigon may affect metabolism of medicinal products such as S-warfarin and patients should be monitored during initiation with Lecigon therapy when used with this medicine. Please consult SmPC for further information. Pregnancy and lactation: Lecigon is not recommended during pregnancy or in women of childbearing potential not using contraception unless the benefits for the mother outweigh the possible risks to the foetus. It is unknown whether carbidopa and entacapone or their metabolites are excreted in human milk. Breastfeeding should be avoided during treatment with Lecigon. Driving and operation of machinery: Caution; Lecigon can have a major influence on the ability to drive and use machines. Refrain if somnolence and/or sudden sleep episodes occur. Undesirable effects: Weight loss, anxiety, depression, insomnia, dyskinesia, Parkinson’s disease /exacerbation of parkinsonism (e.g. bradykinesia), orthostatic hypotension, nausea, constipation, diarrhoea, pain in muscles and tissues, musculoskeletal pain, chromaturia, fall. Complications of the device and surgery: Postoperative wound infection, abdominal pain, excessive granulation tissue, complication of device insertion, incision site erythema, post-procedural discharge, procedural pain, procedural site reaction. Refer to SmPC for other undesirable effects. Adverse events should be reported via HPRA Pharmacovigilance, website: www.hpra.ie. Special precautions for storage: Store in a refrigerator 2°C - 8°C. Do not freeze. Store in the original package in order to protect from light. For storage instructions after first opening of the medicinal product, refer to the summary of product characteristics. Pack size: 7 x 47 ml cartridges. Marketing authorisation holder: LobSor Pharmaceuticals AB, Kålsängsgränd 10 D, SE-753 19 Uppsala, Sweden. Distributed by Clonmel Healthcare Ltd, Clonmel, Co. Tipperary. Marketing authorisation number: PA23144/001/001. Medicinal product subject to medical prescription. A copy of the summary of product characteristics is available upon request or alternatively please go to: www.clonmelhealthcare.ie Last revision date: March 2022. Date of Preparation: October 2022. 2022/ADV/LEC/268H

A combination of three effective Parkinson’s therapies in one convenient gel formulation for intestinal infusion1
Levodopa (20 mg/ml) Carbidopa monohydrate (5 mg/ml) Entacapone (20 mg/ml) Increased levodopa bioavailability with LECIGON®
Highest Recorded Figure of Newly Notified Cases of HIV
HIV Ireland has called for increased investment in nationwide sexual health services, including personnel and resources, to ensure Ireland can meet its global commitment to end new HIV transmissions. Statistics published weekly by the Health Protection Surveillance Centre indicate that more 750 cases of HIV have been notified in 2022, more than double the number for the same period in 2021. However, despite the increase in notifications, falling rates of new transmissions occurring within Ireland give cause for optimism.
The HPSC records newly notified case as those who have recently acquired the virus together with people already living with the virus and transferring their care to Ireland.
“Under diagnosis of HIV remains a concern. The European Centre
for Disease Prevention and Control has said that an estimated 1 in 8 people living with HIV in the EU/ EEA area remain undiagnosed,” said Stephen O’Hare Executive, Director of HIV Ireland.
“In order to decrease the number of undiagnosed cases,” continued Mr O’Hare, “timely access to early testing and subsequent linkage to care is vital.”
“While treatment for HIV is available free at the point of use in the public health system in Ireland, barriers to accessing testing persists,” he added.
This year, UNAIDS has called on the world to unite to end the inequalities that underpin and perpetuate HIV transmission, including increasing investment in new strategies and user-friendly approaches to more widely available HIV testing, treatment
and prevention to improve early diagnosis, end new HIV transmissions, and end AIDSrelated deaths.
The stigma and exclusion faced by people living with HIV and marginalised populations remains a significant barrier to accessing testing, treatment uptake, adherence to medication and seeking support.
HIV-related stigma still persists including among health care professionals. Research conducted this year by Dr Elena Vaughan of NUI Galway and supported by HIV Ireland found that among healthcare workers who were not HIV specialists, 83% claimed knowledge of ‘Undetectable = Untransmittable’ (U=U) and treatment as prevention. However, 40% said they would still be nervous about drawing blood from
Body Clock effect on Respiratory Conditions
a person living with HIV leading to unnecessary ‘extra’ precautions, e.g., excessive use of PPE.
This year, HIV Ireland is once again promoting its Glow Red for World AIDS Day campaign to shine a light on the impact of HIV-related stigma. Over 40 buildings and landmarks around Ireland will light up in red to show their support and to mark 40 years since the first reports of HIV and AIDS on our shores.
HIV stigma is real and ending HIV stigma is a real possibility. As internationally renowned Irish HIV activist and ambassador of this year’s Glow Red campaign, Rebecca Tallon de Havilland, has said, “We can end stigma by strengthening the voices of people living with HIV. Using my voice has empowered me to overcome selfstigma and live my best life.”
usual function to recruit other immune cells to the lungs. More cells than are needed are brought to the lungs, resulting in damaging inflammation.
This could have implications for individuals such as shift workers or people with erratic sleeping/eating patterns in that they are more likely to experience more severe respiratory infections.
This study pinpoints lung fibroblasts as a potential target in the treatment of lung diseases that display this kind of inflammation, a change in approach from most current therapeutic options which focus on easing symptoms rather than treating the cause.
Researchers at RCSI University of Medicine and Health Sciences have discovered how disruption of the circadian clock can lead to increased inflammation in the lungs, which may have negative effects on chronic lung diseases and respiratory infections.
The paper, published in The FASEB Journal, looked at the molecular clock in fibroblasts, a cell type which is abundant in the lung. When the regular rhythm of the fibroblast clock was lost, it resulted in an increased inflammatory response and therefore, worse symptoms.

Lead author Shannon Cox, School of Pharmacy and Biomolecular Sciences at RCSI, said, “This study links together previous knowledge to confirm the importance of lung fibroblasts in inflammation and highlights the negative consequences of disruption to our circadian clock, such as poor night-time sleep, in lung disease. We can apply this understanding when developing therapies and interventions for patients.”

Disturbing the circadian clock in lung fibroblasts impacts their
The majority of this study was supported through funding provided to Professor Annie Curtis by the Science Foundation Ireland Career Development Award (CDA) programme and by the Irish Research Council through a Laureate Award.
Further support was provided by National Heart, Lung, and Blood Institute grant, a Boehringer Ingelheim Fonds travel grant and an RCSI School of Postgraduate Studies International Secondment Award.
The PMI’s Annual Pharma Summit will be held on March 30th, 2023 at Croke Park
Our theme for the day is “Partnering to Improve Human Health” and we will be exploring this theme throughout the day from four vantage points: Technology, Government, Cross Company and Intra Company.
The day will be highly interactive with a mix of keynote speakers and panel discussions with plenty of opportunities to catch up with industry colleagues and make new connections.
Further details regarding speakers will be revealed over the coming weeks.
8 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Professor Annie Curtis
Medicine Shortages Reaching EU Level and ‘Critical’ Point
Medicines shortages are not a new phenomenon. Certainly since the advent of Covid-19, Brexit and the Northern Ireland protocol, the issue is becoming more critical.
The mainstream media has been increasingly reporting on the situation during the start of this new year, as a severe shortage of OTC cough syrups for adults and children has alarmed parents and caregivers. Sprays for sore throats, dissolvable paracetamol powder and soluble aspirin are also widely unavailable.
The Medicines Shortage Index, prepared by Azure Pharmaceuticals, has found that there are 224 medicines currently unavailable, an increase of 12 medicines in seven days (at 11 January). Among the additional medicines to go out-of-stock in the past week are Phenytoin which is used to treat epilepsy.
There is a major shortage of over-the-counter cough syrups for adults and children. Sprays for sore throats, dissolvable paracetamol powder and soluble aspirin are also widely unavailable.
Antibiotics used to treat bacterial and respiratory infections, including Amoxicillin, Penicillin and Cefalexin, are also in scarce supply.
Analysis by the group found that 40% of the medicines currently out of stock are provided to the Irish market by a single supply.
Furthermore, the survey has shown that medicine manufacturers, including companies producing medicines domestically, are getting paid up to four times as much for their products abroad than in Ireland.

The authors of the Medicine Shortages Index, also identified that medicine shortages have further worsened.
Azure has analysed the average prices paid for 10 essential mainstream medicines by Irish, UK and European governments.
Its analysis shows that, on average, the UK and EU member governments are paying twice as much to manufacturers than the comparative prices that the Irish government/industry agreement allows. Some countries are paying up to four times more than Ireland, giving rise to serious shortages for patients
here as manufacturers choose to maximise returns through supplying higher price markets.
Azure is currently not supplying any of the above medicines to the Irish market.
The UK government and the majority of the 27 EU member state governments have taken specific measures in response to the escalating medicine shortage issue. This includes, changes to medicine pricing rules, stockpiling of key products, the introduction of an export ban of key drugs, and provision of additional powers to pharmacists. To date, the Department of Health is yet to meaningfully respond to this deepening challenge.
Commenting, Sandra Gannon, General Manager, Azure Pharmaceuticals said:
“In less than a decade, we have gone full circle on what we pay for mainstream medicines with Ireland now paying substantially less than neighbouring countries for a range of medicines. As a result, manufacturers are choosing
to supply their medicines to those countries who will pay better prices. This in turn gives rise to growing medicines shortages and discontinuations here with patients unable to source the medicines they need. We are paying the price for not paying the price.
“The government appears to be at best misinformed and at worst, in denial about the root cause of this worsening problem. Changing legislation to give extra powers to pharmacists should form a key part of a package of solutions, but that alone will not resolve matters. The price we pay, and a medicines pricing agreement that is no longer fit-for-purpose, is at the heart of this issue.
“The HPRA has a co-ordinating role to manage shortages but is can only respond with regulatory measures, as it acknowledged itself in a statement this week. This issue requires a mix of actions by the Department of Health. To date, that has been lacking.”
40% of the medicines out of stock this month in Ireland have
just a single supplier, leaving pharmacists without licensed alternatives for patients. It also leaves Ireland out of sync with the rest of Europe, with a recently published European Commission report showing the EU wide singled-sourced average standing at 25%.
Heed Warnings
Medicines for Ireland (MFI) are also urging Government to heed recent warnings from GPs and pharmacists nationwide on the growing risk of medicines shortages as inflation, energy and transport costs continue to rise, and global supply chain disruptions persist.
Medicines for Ireland members are the suppliers of the majority of medicine in Ireland to the HSE and patients directly and played a pivotal role in a new Framework Agreement on the supply and pricing of non-originator, generic, biosimilar, and hybrid medicines, announced by Government last year.
9 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Report
Medicine Strength Pack Size IE € UK € EU Average € Treatment For Paracetamol 500 mg tablet 100 pack 1.73 3.05 7.35 Pain Amoxicillin 500mg capsule 100 pack 16.15 31.75 24.46 Infection Lorazepam 1g tablet 100 pack 3.77 7.63 11.43 Anixety Prednisolone 5mg tablet 98 pack 3.06 4.2 7.49 Inflammation/Asthma Nitrofurantoin 100mg Caps 30 pack 6.73 13.03 11.91 Urinary tract infection Amisulpride 200mg 60 pack 31.68 39.08 60.99 Schizophrenia Clonazepam 0.5mg 100 pack 4.33 35.46 13.59 Epilepsy Co-Amoxiclav 125/31mg/5ml 100 pack 1.38 6.25 4.12 Infection Tamoxifen 20mg tabs 30 pack 4.58 3.16 9.11 Breast Cancer Ipratropium Nebules 250mg/ml x 2ml 20 pack 4.5 7.46 7.95 COPD
Sandra Gannon, General Manager, Azure Pharmaceuticals
Commenting on increasing medicine shortages, Medicines for Ireland Chairperson, Padraic O’Brien has said “In Ireland and throughout Europe, soaring energy costs, inflation and supply chain disturbances have contributed to thousands of generic medicines disappearing from the European and Irish market.”
“MFI members are willing to work directly with Government to help tackle this serious issue and prevent potential medicines shortages. Our aim is to deliver industry insights and extend our expertise to help improve the development of medicinal pricing
and procurement policies in Ireland and safeguard the supply of medicines to Ireland.”
According to the Health Products Regulatory Authority (HPRA) website there are currently 187 medicines in short supply in Ireland. Without intervention this situation has the potential to significantly worsen.

Mr O’Brien added, “As a small market Ireland is more likely to be badly impacted by inflationary pressure and as costs continue to rise, market conditions will become increasingly unviable for companies supplying generic medicines to Irish hospitals and
pharmacies. Additionally, in some cases, our reimbursement prices for certain medicines are too low compared to other EU countries”.
“Price adjustments in Ireland are historically downward only, where other European countries employ flexible pricing mechanisms that allows reimbursement prices to rise for medicines that are in short supply. Ireland does not have such a mechanism and is therefore further disadvantaged.”
A recent MFI members survey found that 91% of MFI members experienced increased costs associated with import and/or manufacturing of pharmaceutical
and medical products for the Irish market in 2022. While all MFI member companies envisage increases in transportation costs over the next 12 to 24 months.
“Our main focus is to help Government ensure market conditions in Ireland remain sustainable in order to retain and secure access to reliable and affordable treatment for Irish patients. We believe it is time for us to revisit our work with Government and the HSE on the Framework Agreement on Supply and Pricing and develop improvements to mitigate against supply risks.” concluded Mr O’Brien.
10 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Report
HPRA Statement
The Healthcare Products Regulatory Authority said in a statement, “Due to a combination of factors, including the level of respiratory illnesses in the community, a significant increase in demand for medicines used to treat seasonal conditions such as colds and flus has been observed over recent weeks. In some cases, this demand has been 2-3 times the normal level seen during the same period in previous years. From discussions with suppliers and regulators in other countries, the Health Products Regulatory Authority (HPRA) understands that similar trends have been observed
in other European countries who have experienced significant increases in demand.
“The HPRA has been engaging with all stakeholders, including suppliers, with a view to ensuring a coordinated response to this increased demand. The key focus at all times has been to ensure that suitable medicines remain available to treat all patients.
“In the case of medicines used most often in Ireland, there are typically multiple forms, strengths, brands, and generic medicines available from various sources. Where some individual medicines are in short supply, alternative options such as alternative
strengths, brands, and generic medicines remain available to ensure continuity of treatment. In some cases where the medicine initially prescribed for the patient is unavailable, patients may be switched to a suitable therapeutic alternative following appropriate consultation with a healthcare professional. This approach is also consistent with national antimicrobial prescribing guidelines. “Suitable medicines continue to be available to treat respiratory illnesses and their symptoms in both adults and children. Taking into account the wide range of available medicines to treat respiratory illnesses, there is no need for healthcare professionals to order extra quantities of medicines, or for doctors to issue additional prescriptions. Similarly, patients and the general public are asked not to seek supplies of medicines over and above their normal requirements. Doing so will
disrupt existing stock levels and hamper the supply of medicines for others.
“Further to the HPRA’s regular and ongoing engagement with industry, we have been informed that in a number of instances suppliers have increased production and sourced additional stock to respond to this recent increase in demand. Although the HPRA has no role in procuring medicines, we continue to engage with the suppliers to obtain updates and remains open to expediting regulatory procedures to enable supply of additional stock, where possible.
“The HPRA fulfils a coordinating role in Ireland’s response to managing medicine shortages when they occur. In each case, the HPRA works with relevant stakeholders as necessary, to coordinate an effective approach to the management of a confirmed product shortage.”
The EU is preparing to stockpile drugs and oblige manufacturers to guarantee supplies in efforts to tackle the on-going medicines shortages.
The European Commission will also try to reduce reliance on China and increase domestic production capacity, the commission told the Financial Times — which reported the news. In a written response to the Greek government, EU health commissioner Stella Kyriakides outlined the plan.
The commission will intervene to ensure “strategic autonomy” in basic medicines through a “systemic industrial policy”.
It plans to propose legislation to “secure access to medicines for all patients in need and to avoid any market disruption of medicines”.
This would require “stronger obligations for supply, earlier notification of shortages and withdrawals and enhanced transparency of stocks”, the commission said.
This follows moves by the Greek health minister last week to bring in changes to address shortages there of inhalers and antibiotics. Greece is putting an export ban in place, but the Financial Times reports this is not being considered by the EU for now.
Greece is also listing alternatives to drugs which are in short supply, as is done in Britain.
This is something the Irish Pharmacy Union has repeatedly called for under the title of a “Serious Shortage Protocol” including calls made in early December when shortages became apparent.
Ms Kyriakides said in a written statement the commission was suspending some regulations and working with EU companies to increase capacity.
“Discussions with industry have already taken place and they are aware that they must rapidly step up production of these medicines,” she said.
Responding to the crisis, the International Generic and Biosimilar Medicines Association (IGBA), a Brussels-based industry group, told the FT that doctors are prescribing antibiotics more often.

It called on governments to share more information about their disease forecasts and loosen trade restrictions to allow drugs and raw materials to move more freely to countries with shortages.
They should also force wholesalers, pharmacies and hospitals to stop stockpiling antibiotics, according to the IGBA.
11
HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Warning signs of sudden cardiac arrest provide opportunity
Primary care visits rise sharply in the weeks immediately preceding a sudden cardiac arrest, according to results from the ESCAPE-NET project. The project is backed by the European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC) and the European Resuscitation Council (ERC).
“Contrary to the general assumption, sudden cardiac arrest does not strike entirely unheralded, as ESCAPE-NET data have shown that patients attend primary care more often in the run up to an arrest compared to usual,” said Dr. Hanno Tan, ESCAPE-NET project leader and cardiologist, Amsterdam University Medical Centre AMC, the Netherlands.
“This insight may provide a lead for efforts to identify individuals at imminent risk of sudden cardiac arrest so that it can be prevented.”
Sudden cardiac arrest causes one in five deaths in industrialised countries. Most sudden cardiac arrests occur in the community in people not known to be at risk. A cardiac arrhythmia, called
ventricular fibrillation, causes the heart to stop pumping and blood flow ceases. If blood flow is not restored quickly, the individual passes out and dies within 10 to 20 minutes.
ESCAPE-NET was set up to improve both prevention and treatment. During the five-year EU Horizon 2020-funded scientific project, which concludes on 1 January 2023, scientists have investigated the causes of ventricular fibrillation so that it can be prevented and have examined resuscitation strategies in an effort to improve survival rates.
Developing effective prevention and treatment approaches required information on genetic and environmental risk factors from large study cohorts of sudden cardiac arrest patients – which were previously unavailable. The 16 ESCAPE-NET scientific partners joined forces to create a shared harmonised database of more than 100,000 sudden cardiac arrest victims. Dr. Tan said: “This resource can be used by scientists across
the world, including researchers outside of the ESCAPE-NET consortium, to conduct studies on sudden cardiac arrest. This should accelerate knowledge gathering on this condition and ultimately reduce the societal burden of sudden cardiac arrest.”
A biobank with DNA samples from 10,000 well-phenotyped sudden cardiac arrest victims has also been created. “This biobank will serve to increase our understanding of the genetic causes of sudden cardiac arrest,” said Dr. Tan.
More than 100 ESCAPE-NET research papers have been published in peer reviewed journals. Among them are European Heart Journal, Circulation, Journal of the American College of Cardiology, Journal of the American Heart Association, Nature Genetics, The Lancet Public Health, Resuscitation, and EP Europace. Scientific discoveries include the finding that citizen-rescuers
provide less rapid resuscitation care to women than to men, and that women consequently have lower survival chances than men. Dr. Tan said: “This eye-opener must lead to public awareness campaigns aimed at narrowing the gender gap in sudden cardiac arrest management.”
Novel data have been collected on the sudden cardiac arrest risk associated with the use of various commonly used, noncardiac drugs in different European countries.4 Dr. Tan said: “This information may lead to the safer use of these drugs.”
He concluded: “Sudden cardiac arrest is a pressing public health problem that has so far been extremely hard to solve, largely because of the lack of difficult to obtain detailed clinical data and biological samples. ESCAPE-NET has made important steps by establishing a database, biobank and knowledge base that may be used in future studies to solve this problem.”
New Medically Led Platform to Tackle Obesity
and product designer Peter Lumley with support from senior team members including world-leading obesity scientists and clinicians, Professor Carel Le Roux and Dr Werd Al-Najim, UCD School of Medicine and UCD Conway Institute and Professor Alex Miras, Ulster University.
Beyondbmi, a University College
Dublin (UCD) HealthTech spin-out company supported by NovaUCD, has announced closing a ¤525,000 pre-seed funding round, advised by CKS Finance.

Beyondbmi has developed a 12-month weight management programme, designed by the leading medical weight management experts, to enable clients achieve long-term weight loss and health gain in a sustainable way. Beyondbmi uses the latest scientific research and therapies to create individually
tailored treatment plans which includes detailed assessments, medication, dietary plan, and behavioural health adjustments.
The funding will be used by the company to launch its proprietary Beyondbmi platform and demonstrate medical outcomes by giving people living with excess body fat access to doctorprescribed weight loss medication, nutritional therapy and one-to-one accountability coaching.
A UCD School of Medicine spinout Beyondbmi was founded by medical doctor Dr Harriet Treacy
Dr Harriet Treacy, co-founder, Beyondbmi said, “As a practising doctor, I have treated 100’s of patients who are experiencing health conditions they are not aware are a direct result of excess body fat. Conditions such as high blood pressure, type 2 diabetes, anxiety and depression and increasingly, female health challenges like PCOS and infertility. Until now, I have had few medically-proven solutions I can point them towards to manage weight issues. This led me to spend time with global obesity experts to understand how we could design a solution which was based on the science of obesity.”
She added, “According to the World Health Organization it is now estimated that over 60% of the population are living with overweight or obesity with a staggering 220 health complications directly linked to these. Our mission at Beyondbmi is to provide a scientificallybased alternative to the outdated approach of ‘eat less, move more’ which, research has shown, is an ineffective long-term approach to weight management. For too long, the symptom of hunger associated with excess fat accumulation has been treated as an issue of willpower which causes untold harm to people who often internalise the failure of the treatment as a personal or moral failure.”
“Thankfully, we now have more effective solutions in the form of new medical drug treatments, combined with nutritional therapy and behavioural support which targets the underlying biology responsible for the feeling of hunger. This results in, on average, 15% weight loss over little more than a year as demonstrated in the STEP trials, and we are excited that Beyondbmi will provide a solution that aims to deliver these results in a real world setting.”
12 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Professor Alex Miras, Dr Harriet Treacy, Professor Carel Le Roux and Dr Werd Al-Najim.
News
Credit: Maura Hickey
SUSPECT ATTR-CM

(TRANSTHYRETIN AMYLOID CARDIOMYOPATHY)

A LIFE-THREATENING DISEASE THAT CAN GO UNDETECTED
Life-threatening, underrecognized, and underdiagnosed, ATTR-CM is a rare condition found in mostly older patients in which misfolded transthyretin proteins deposit in the heart.1-7 It is vital to recognize the diagnostic clues so you can identify this disease.
CONSIDER THE FOLLOWING CLINICAL CLUES, ESPECIALLY IN COMBINATION, TO RAISE SUSPICION FOR ATTR-CM AND THE NEED FOR FURTHER TESTING
heart failure with preserved ejection fraction in patients typically over 60 years old5-7
to standard heart failure therapies (ACEi, ARBs, and beta blockers)8-10
between QRS voltage and left ventricular (LV) wall thickness11-13
of carpal tunnel syndrome or lumbar spinal stenosis3,8,14-20
showing increased LV wall thickness6,13,16,21,22
H FpEF I NTOLERANCE DISCORDANCE DIAGNOSIS E CHO N ERVOUS SYSTEM
LEARN HOW TO RECOGNIZE THE CLUES OF ATTR-CM AT:


—autonomic nervous system dysfunction-including gastrointestinal complaints or unexplained weight loss6,16,23,24
3. Connors LH, Sam F, Skinner M, et al. Heart failure due to age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282-290. 4. Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098. 5. Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2(2):113-122. 6. Maurer MS, Hanna M, Grogan M, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68(2):161-172.
7. González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585-2594. 8. Narotsky DL, Castano A, Weinsaft JW, Bokhari S, Maurer MS. Wild-type transthyretin cardiac amyloidosis: novel insights from advanced imaging. Can J Cardiol. 2016;32(9):1166.e1-1166.e10. 9. Brunjes DL, Castano A, Clemons A, Rubin J, Maurer MS. Transthyretin cardiac amyloidosis in older Americans. J Card Fail. 2016;22(12):996-1003. 10. Castaño A, Drach BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163-178.
11. Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49:9-13. 12. Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114(7):1089-1093. 13. Quarta CC, Solomon D, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840-1849. 14. Connors LH, Prokaeva T, Lim A, et al. Cardiac amyloidosis in African Americans: Comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158(4):607-614. 15. Nakagawa M, Sekijima Y, Yazaki M, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23(1):58-63. 16. Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203-1212. 17. Sperry BW, Reyes BA, Ikram A, et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018;72(17): 2040-2050. 18. Westermark P, Westermark GT, Suhr OB, Berg S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. 2014;119(3):223-228. 19. Yanagisawa A, Ueda M, Sueyoshi T, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28(2):201-207. 20. Sueyoshi T, Ueda M, Jono H, et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Hum Pathol. 2011;42(9):1259-1264. 21. Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442-1448. 22. Ternacle J, Bodez D, Guellich A, et al. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging 2016;9(2):126-138. 23. Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63-76. 24. Swiecicki PL, Zhen DB, Mauermann ML, et al. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid. 2015;22(2):123-131.
The health information contained in this ad is provided for educational purposes only. Date of Preparation: February 2021 GCMA code: PP-RDP-IRL-0105
References 1. Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23(4):209-213.
2. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357-1377.
S U S P E C T A N D D E T E C T . I E
Precision Medicine and Novel Therapeutic Strategies in Detection and Treatment of Cancer: Highlights from the 58th IACR Annual Conference
Written by: Sean P. Kennedy1,*,Oliver Treacy2, Emma H. Allott3,4, Alex J. Eustace5, Niamh Lynam-Lennon6, Niamh Buckley7 and Tracy Robson8
1School of Biological, Health and Sports Sciences, Technological University Dublin, D07 ADY7 Dublin, Ireland
2Discipline of Pharmacology and Therapeutics, College of Medicine, Nursing and Health Sciences, University of Galway, H91 TK33 Galway, Ireland
3Patrick G. Johnston Centre for Cancer Research, Queen’s University Belfast, Belfast BT9 7AE, UK
4Department of Histopathology and Morbid Anatomy, Trinity Translational Medicine Institute, Trinity College Dublin, D08 HD53 Dublin, Ireland
5National Institute for Cellular Biotechnology, Dublin City University, D09 NR58 Dublin, Ireland
6Department of Surgery, Trinity St James’s Cancer Institute, Trinity Translational Medicine Institute, St James’s Hospital, Trinity College Dublin, D02 PN40 Dublin, Ireland
7School of Pharmacy, Queen’s University Belfast, 97 Lisburn Road, Belfast BT9 7AE, UK
8School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons Ireland, D02 YN77 Dublin, Ireland
The Irish Association for Cancer Research (IACR) held its 58th annual conference from the 30th of March to the 1st of April 2022, in Cork, Ireland. The following article is a report of knowledge conveyed at the conference. There was a focus on cancer prevention in both “Early Detection” and “Cancer Vaccines” sessions, and cancer treatment in “Novel Therapeutics” and “Breakthrough Research” sessions. The research presented at this conference highlighted the interplay of both prevention and treatment, as many speakers focused on the same cancer but at different times in the treatment process. There was a push for more non-invasive early detection techniques, the current impact of cancer vaccines and new ways of further stratifying selection criteria for easier identification of high-risk individuals. This was coupled with novel treatment strategies and identification of new therapeutic targets.
Cancer is a disease that has a considerable impact on society and on the individual. Currently, 1 in 2 men and 1 in 3 women will be diagnosed with cancer within their lifetime. In Ireland, the incidence of cancer is over 45,000 cases per annum (www.cancer.ie (accessed on 1 July 2022) and is expected to double again by 2050. Not only does this disease have a huge impact on the individual, but it has the most devastating economic impact of any cause of death in the world (www.cancer.org (accessed 1 July 2022)). The global economic impact of cancer was $895 billion dollars in 2008. This is 19% higher than heart disease, which has the second largest impact at $753 billion (www.cancer.org (accessed 1 July 2022)).
The ability to both accurately target and treat cancer has been

the benchmark for success in the cancer research community, since the introduction of targeted therapeutics by Sidney Farber in 1948.1 With Sidney Farber’s seminal discovery of anti-cancer agents, it was said that “only through the cooperation of both prevention and treatment, will it be possible to produce a significant reduction in cancer mortality”. In the past 30 years, there has been a 32% drop in the cancer mortality rate, which has been attributed to, among other things, improvements in prevention and treatment (www.cancer.org (accessed 1 July 2022)). At the 58th Irish Association of Cancer Research (IACR) conference, the sessions were reflective of this mantra, in that we had speakers looking towards early detection of cancer, preventative “cancer vaccines” and treatment through both mechanistic understanding of pathways as well as targeting them through novel therapeutics.
Early Detection and Diagnosis
Like two sides of the same coin, prevention and treatment go hand in hand. The ultimate goal of a health care professional is to prevent a disease from taking hold and requiring treatment. In Prof. Sam Janes group at University College London (UCL) they pose the question “Could there be a future that we prescribe a cancer preventative, when people quit smoking?” When adjusted for both age and sex, global lung cancer mortality (18%) is almost double the 2nd highest mortality cancer, colorectal (9.4%),2 however when it comes to treatment costs, in the case of non-small cell lung cancer (NSCLC), the vast majority of money is spent on treatment of high grade tumours, carrying lower survival rates, and not on the detection and prevention of the disease.3
Prof. Janes echoes remarks made by Peter B Bach, MD of Memorial Sloan Kettering Cancer Centre (MSK), that cost of therapy in established, high grade cancers are soaring on a logarithmic scale and unsustainable.4 To combat increasing costs in later stages, Prof. Janes’ lab look to eliminate those costs through detection at earlier stages. A way of determining whether preinvasive lung carcinoma-in-situ (CIS) lesions progress to invasive cancer or regress to low grade dysplasia5 is highlighted by Prof. Janes. The difference is observed through upregulation of genomic instability CIN genes (ACTL6A, ELAVL1, MAD2L1, NEK2, OIP5) in their ability to predict progression through pre invasive CIS, however CIS lesions are similar to cancer on a molecular level.5
This led Prof. Janes to his next question, “What are the immune characteristics of these pre-
cancerous lesions?”. Evidence of different evolutionary trajectories in gene expression for early precancerous legions in tumour evasion of the immune system,6 as well as both progressive and regressive CIS lesions with both immune “hot and cold” phenotypes, threaten to muddy the waters.7 Prof. Janes then went on to show that regressive and progressive legions can be successfully stratified via chromosomal instability which predicts progressive legions, while immune competence predicts regressive legions.7 In a more translational approach, Prof. Janes then goes on to describe the EARL study (NCT03870152) which is the first randomised trial of lung cancer prevention, testing electrocautery versus surveillance of histologically confirmed high-grade pre-invasive airway lesions. This collaborative trial between Cancer Research UK and the lung cancer centre of excellence at UCL is still recruiting and it aims to reduce cancer mortality in the same way other trials have successfully done through low dose computed topography (LDCT).8
Finally, Prof. Janes talked about the SUMMIT study (NCT03934866) in which the objectives were twofold; to assess the feasibility of LDCT screening of a large metropolitan population and to assess the accuracy of a blood biomarker for multiple cancers. Despite road blocks to success like COVID-19 and historically poor uptake and retention of patients in lung screening trials in general,9 the SUMMIT study has recently reported high patient satisfaction with the study.10
Systems Biology Approach
When trying to tease apart complex biological systems and
14 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE Conference Report
Professor Sam Janes, UCD
illuminate non-canonical pathways involved in cancer progression, systems biology is a tool which can deliver significant insight into otherwise opaque systems. In the Irish Cancer Society lecture and selected talks session, Prof. Chris Sweeney highlighted a systems biology approach towards uncovering an unknown regulator of Nuclear Factor-Kappa B (NF-κB) and its involvement in “lethal” prostate cancer. Both canonical and non-canonical NF-κB and its involvement in cancer have been described and interrogated at length over the last 2 decades.11,12
Prof. Sweeney began the talk highlighting early ventures into understanding the mechanism behind NF-κB being present in early prostate cancer but expression having no clear association with “lethal” prostate cancer.13 While it is clear that NFκB is activated and overexpressed in multiple types of prostate cancer,13 it was shown that there is an increased nuclear localisation in cancer cells14 and correlation of NF-κB immunoreactivity with disease recurrence,15 highlighting that if increased NF-κB is nearly always present in early prostate cancer, then what determines whether NF-κB drives “lethal” prostate cancer? To address this question, Prof. Sweeney utilised a systems biology approach in defining a key regulator of NF-κB activation in “lethal” prostate cancer, namely Tristetraprolin (TTP) (also called ZFP36).
NF-κB activation is controlled by both positive and negative feedback mechanisms which leads, inevitably, to multiple layers of complexity.16 Through a collaboration with Prof. Travis Gerke’s lab and a bioinformatical approach to the question posed earlier, it was elucidated that low TTP expression is associated
with “lethal” prostate cancer.17 This conclusion was reached by reconstructing specific NF-κB activation pathways in the context of “lethal” prostate cancer through 3 steps. Firstly, the data was analysed heterogeneously (that is that the NF-κB pathway was defined in a large and diverse dataset), the data was then configured via a Bayesian, biologyspecific integration and then finally, differential gene profiles were queried with a clinical context. This led to the finding that TPP is a putative prostate cancer tumour suppressor gene which regulates NF-κB via increased mRNA degradation of the NF-κB activator (TNF-α) and prevention of the nuclear translocation of the p65 subunit of NF-κB.17
Prof. Sweeney showed currently unpublished work using both the cancer genome atlas (TCGA) RNAseq and Dana Farber Cancer Institute (DFCI) IHC data that TPP loss decreases disease-free survival in primary human prostate cancer and compounds impact of PTEN loss in localised prostate cancer by ~40%. To observe the effects of TPP loss on prostate cancer progression alone and in combination with PTEN loss, a genetically modified mouse model was developed recapitulating that phenotype. The result was acceleration of prostate cancer progression, driven by PTEN loss which was also reflected in human clinical correlation data. TPP loss facilitated increased tumour cell proliferation, invasion and dissemination, as well as quicker progression to castration-resistant prostate cancer.
In the final part of Prof. Sweeney’s presentation, he discussed the use of Dimeththylaminoparthenolide (DMAPT) as a way of targeting NF-κB, highlighting its excellent toxicity profile, bioavailability and notably a protectant quality against radiation and cisplatin in normal tissue, while conversely a sensitizer in tumours.18 Despite NF-κB having a range of pathway “on and off ramps”, it is a viable and druggable target in prostate cancer. DMAPT is a unique anticancer agent in that it displays a preferential effect on cancer cells over non-cancer cells, due to cancer cells having an aberrant glutathione balance19,20 and a greater reliance for survival on constitutive NF-κB activation.11,12,16 Prof. Sweeney went further and showed how DMAPT limits radiation-induced apoptosis in non-cancer but increases in cancer tissue and prevents radiation-induced mitochondrial damage in prostate and bladder cancer.18 Prof. Sweeney concluded his presentation with a whole host of unpublished, in vivo
model data (lung, urothelial, bladder, kidney and prostate) in which he demonstrated in vivo radiation efficacy against cancer cells and protection for normal cells. Preliminary human pharmacokinetic data showed a modest suppression of NF-κB in patients with acute myeloid leukemia using a limited dose of DMAPT. This promising result paves the way for work on an encapsulated formulation and clinical trials thereafter.
Cancer Vaccines
Cancer Vaccines are constructed with the goal of inducing an immune response against tumour antigens.21 Despite great success with both Human papilloma virus (HPV)22 and Hepatitis B virus (HBV),23 there have been constraints which dictate the success of the vaccine approach. Heterogeneity of a tumour has long been a limiting factor when it comes to treatment, as it allows for multiple different avenues of therapeutic resistance and as cancer vaccination can be raised to a single epitope and can’t account for various neoantigens simultaneously, this problem persists. To combat this heterogeneity, some groups have looked at targeting heterogeneity with individual, personalised vaccines, however, that brings with it a whole host of logistical and regulatory problems.24
Prof. Benoit Van den Eynde of the Ludwig Institute for Cancer Research (LCR) has been looking at tackling heterogeneity through targeting MAGE-type antigens in lung cancer.


Prof. Van den Eynde showed that there have been many attempts so far to develop MAGE-targeting cancer vaccines. Some very large clinical trials involving
thousands of patients have been carried out, aimed at evaluating a recombinant MAGE-A3 protein vaccine and adjuvant formulations in NSCLC and melanoma patients bearing MAGE-A3 expressing tumours. However, both have unfortunately failed (NCT00480025) (NCT00796445). With advances in oncology, it is hypothesized that the reasons for these failures arise from suboptimal induction of CD8+ T cells and the vaccine not utilized in combination with checkpoint inhibitors. To rectify this, Prof. Van den Eynde proposed the use of a heterologous primeboost (a Chimpanzee Adenovirus Oxford (ChAdOx) recombinant, in combination with a Modified Vaccinia Ankara (MVA)) viral vector, which has been shown to induce a strong CD8+ T cell response in humans.25
The reason MAGE-A3 is such an attractive vaccination target is because MAGE-A3 is the most frequently expressed MAGE-type antigen in human cancers, as well as the established oncogenic role of many MAGE-type antigens.26,27
It is also necessary to target NYESO-1 in order to induce a strong CD8+ response which was missing from the unsuccessful trials. Taking on lessons from the past, Prof. Van Den Eynde then spoke of a clinical trial project in which he can use a vaccine to “heat the cold tumours” and sensitise them to anti-PD-1 (pro-T cell). This approach in which they combine the ChAdOx1/MVA/ MAGE-A3/NY-ESO-1 vaccine with the new standard of care, namely chemotherapy (Carboplatin and Paclitaxel) and anti-PD1 (pembrolizumab), in MAGE-A3/ NY-ESO-1 tumours was performed in DBA/2 mice with 15V4T3 (NSCLC) cell-derived tumours. After optimisation of treatment schedules and exposure to the vaccine, a significant benefit of the vaccine/anti-PD-1/chemo combination was observed, when compared to any other combination of each component.28 This has led to the development of a collaborative (VOLT) Phase 1/2a clinical trial on NSCLC in which inclusion criteria are MAGE-A3 +/ NY-ESO-1 expression and the two arms are separated into vaccine/ standard of care (SoC) against SoC alone. The implications of a functional, interchangeable cancer vaccine which promotes CD8+ T cell infiltration are huge in terms of personalised medicine.
Cancer Epigenetics
Formerly seen as a genetic disease, cancer has been shown to be a disease of many hats, including epigenetic abnormalities.29 In recent years, many avenues of epigenetics have
15 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Professor Chris Sweeney
Professor Benoit Van den Eynde
been investigated, including noncoding mRNA expression, DNA methylation and in the case of Prof. Adrian Bracken’s lab at Trinity College Dublin (TCD), histone modifications. Prof. Bracken began the talk showing recent rounds of NGS sequencing and the key oncogenes and tumour suppressors highlighted30 and a reminder that chromatin-modifying proteins are one of the main contributors to that list.
Two different groups applied genome-scale sequencing to pediatric diffuse intrinsic pontine gliomas (DIPG) and found that this deadly brain cancer was caused by a frequent (80%) somatic mutation in Histone H3 and Lysine 27 (more commonly written H3K27me#).31,32 Prof. Bracken then shows how Polycomb Repressive Complexes 2 (PRC2) mediated H3K27me3 recruits PRC1 to compact chromatin. EZH2 (a component of PRC2) is recurrently mutated in lymphomas with increased H3K27me3 and decreased H3K27me2.33 It was shown previously that core PRC2 is also deleted in various cancers such as T cell leukemia, myeloid malignancies, and malignant peripheral nerve sheath tumours.34
To address these destructive epigenetic mutations in EZH2, Prof. Bracken’s lab has aided in the development of highly selective EZH2 inhibitors. Utilizing a high throughput screening process for inhibitors of the PRC2 complex, followed by pharmacodynamic and pharmacokinetic optimization, low nanomolar potency small molecule EZH2 inhibitors were generated.35 These small molecules displayed remarkable selectivity for EZH2 and showed similar efficacy against wild-type and mutant forms of EZH2. One of these therapeutics, Tazemetostat, produced clinically
meaningful and durable responses, has a favourable safety profile in heavily pretreated patients with follicular lymphoma and has FDAgranted, accelerated approval for adult patients with relapsed or refractory follicular lymphoma whose tumors are positive for an EZH2 mutation.36
To understand how H3K27M2/3 disrupts the epigenome and trial these new small molecule inhibitors, Prof. Bracken’s lab created a clinically-relevant model of DIPG.37 Inhibition of EZH2 enzymatic activity through Tazemetostat was shown to selectively reverse aberrant repression of neurodevelopmental genes associated with DIPG.37 Prof. Bracken then surmised the talk with a thought on both understanding and effectively targeting the right H3K27 aberrant phenotype, either through EZH2 or Histone deacetylase inhibitors (HDAC) in aberrant repression associated with elevated H3K27me3 expression or reduced gene expression associated with elevated H3K27ac expression.38
Novel Therapeutic Strategies
This year’s winner of the European Association of Cancer Research (EACR) senior investigator prize was Dr. Daniela Ottaviani of the Royal College of Surgeons Ireland (RCSI) and their work on CDK12 as a novel therapeutic target in endocrine therapy-resistant breast cancer was presented. Despite being one of the most studied forms of cancer, breast cancer still eludes researchers with resistance channels to therapeutics. Out of 2.3 million women diagnosed with breast cancer in 2020, 73% of cases will be estrogen receptor positive (ER+) and human epidermal growth factor receptor 2 negative (HER2-) and of this 73% treated, a staggering 40% of ER+/HER2patients will develop resistance to endocrine therapy.39 Endocrine resistance represents a major clinical problem and often leads to secondary metastatic disease so to combat this, strategies to overcome such resistance and identify new actionable targets are needed.40 Because of its prevalence in advanced metastatic breast cancer41 and association with worse survival outcome in ER+ breast cancer,42 Dr. Ottaviani elected to investigate the functional and mechanistic role of cyclin-dependent kinase 12 (CDK12) in endocrine resistant metastatic breast cancer, and evaluate its use as a new therapeutic target.
Through whole exome sequencing, Dr. Ottaviani showed CDK12 amplification is a frequent and

common alteration found in advanced breast cancer (n = 78). When utilising RNA sequencing, CDK12 gene expression was also shown to be increased in metastasis vs. primary breast tumours (n = 45). This was further confirmed through liquid chromatography—mass spectrometry (LC-MS) of ER+ primary breast tumours with good and poor outcomes and high CDK12 protein expression were shown in ER+ primary tumours with poor outcome (n = 10). Pathway analysis revealed CDK12-driven transcriptome in endocrine resistance drives ER+ signalling, contributing to cancer cell growth, cell cycle progression and proliferation. Upon looking at transcription factors and chromatin regulators potentially cooperating with CDK12, MED1 stood out as a potential target for its known involvement with CDK12 in tumourigenesis.43 MED1 was seen as a potential transcriptional partner of CDK12 as ligand-bound ER recruits the MED1/Mediator complex to initiate targeted gene transcription.44 Dr. Ottaviani went on to show how MED1 and ER are depleted from the chromatin upon siCDK12, indicating CDK12 knockdown disrupts MED1 and ER recruitment to the chromatin. Following CDK12 knockdown, apoptosis was significantly increased as evidenced by both RNA sequencing and PARP cleavage western blots. CT7116 (a CDK12 inhibitor) (300 nM) was then shown to significantly inhibit viability and clonogenic growth of ER+ cell and organoid models of advanced breast cancer.
Dr. Ottaviani showed CDK12 facilitates endocrine resistance in ER+ breast tumours by promoting estrogen-dependent growth signalling through modulation of MED1 and ER chromatin accessibility and then displayed pharmacological targeting of CDK12 against viability of cell and organoid models of ER+ advanced breast cancer. These preliminary results support the potential use of
CDK12 inhibition in the treatment of endocrine therapy-resistant breast cancer.
Conclusions
Prevention and treatment are akin to diplomacy and war. When diplomacy fails, war becomes inevitable. In that same way, both prevention and treatment are important avenues of attack and are highlighted at the 58th IACR annual conference. This conference couldn’t have been better concluded than with the final speaker and recipient of the IACR Award for Outstanding Contribution to Cancer Medicine and Research, Prof. John Reynolds. The academic head of clinical surgery at St. James Hospital and Trinity College Dublin (TCD), Prof. Reynolds is also the national lead in Ireland for oesophageal and gastric cancer. He is the president of the Irish Society of Clinical Nutrition and European Society of Diseases of the Oesophagus, so it was poignant when he spoke about one of the more deceptively preventable diseases, malnutrition.
Prof. Reynolds highlighted the famous Lancet article linking body-mass index and incidence of cancer,45 which identified Oesophageal adenocarcinoma as the cancer with the highest incidence risk per 5 kg/m2 bodyweight. Throughout the talk, Prof. Reynolds championed taking the initiative with terms such as “prehabilitation” and “preoperative” when talking about nutrition-related carcinomas and talked about a gap in the Irish dietetic services which leaves little opportunity for early intervention or effective ongoing support. To finish on a positive note, Prof. Reynolds highlighted the ICBP SURVMARK-2 study, displaying Ireland’s 5-year net survival of both Oesophageal and Stomach cancer and how survival has increased by 11% in both cancers over the last 20 years46 due in no small part to preventative and early intervention.
References available on request
16 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Conference Report
Professor Adrian Bracken
New Accreditation for Beaumont Hospital CR Department
The Cardiac Rehabilitation (CR) Department of Beaumont Hospital was recently accredited by the European Association of Preventive Cardiology (EAPC) in the category of secondary prevention and cardiac rehabilitation.
Beaumont Hospital provides CR to the largest annual patient volume in Ireland, and is modelled on the Mayo Clinic’s ‘highperformance’ CR programme. Our comprehensive programme of multidisciplinary care provides supervised exercise training, education, risk factor modification, stress management and psychological support for patients and their families.
To earn EAPC centre accreditation, Beaumont Hospital participated in a rigorous application process requiring extensive documentation of the CR programme’s

practices, and its performance was benchmarked against key performance indictors (KPIs) associated with international standards of cardiac rehabilitation and secondary prevention.
“We are very proud of this accreditation as it confirms our commitment to providing patients with guideline-driven, stateof-the-art and person-centred cardiovascular care”, said Helen Newton, CR Programme Manager.
“With this accreditation programme, the European Association of Preventive Cardiology (EAPC) aims to set the standards for preventive cardiology practice, thus improving quality of care and cardiovascular health,” says Prof Michael Papadakis, EAPC President. “We congratulate Beaumont Hospital on this achievement.”
Robotic Guided Coronary Intervention First
Galway University Hospitals has carried out the first Robotic Guided Coronary Intervention in Ireland and the UK.
The innovative procedure combines the benefits of coronary intervention with the precision of robotics, offering a range of benefits to patients.
The new technology is used in stent procedures to relieve blockages in the arteries of the heart. It allows for greater precision in positioning stents, allowing the Interventional Cardiologists to move the stent a millimetre at a time.
It also allows the medical team to have an enhanced, close up view of the angiographic images and information during the entire procedure.
The new technology allows Interventional Cardiologists to use the robot as an extension of their own hand, allowing for robotic precision and details visualization when positioning of guide catheters, guidewires and balloon/ stent catheters.
Professor Faisal Sharif, Consultant Cardiologist, who carried out the first procedure in UHG welcomed the addition of the CorPath Robotic Angioplasy as a game changer.

“Robotic innovation has come a long way in the last decade. And we in Galway are delighted to have performed the first Robotic Guided Coronary Intervention in Ireland and the UK.”
“The main advantage of robotics is that it is safe and very precise in stent placement. It allows the accurate placement for up to 1mm at a time,” he said.
The use of robotics in the procedure will also benefit staff, reducing their exposure to radiation.
“Traditionally, the coronary stent placement procedure is performed in the Cardiac Cath Lab resulting in staff exposure to radiation. The second main advantage of using Robotics is the reduction in radiation exposure for the staff.”
“We recently successfully completed the first case and going forward we will be performing these procedures regularly. I would like to thank Science Foundation Ireland, University of Galway and University Hospital Galway for their support towards this innovation,” added Prof Sharif.
Chris Kane, General Manager of Galway University Hospitals welcomed the introduction of the new technology adding that it will further benefit patients.
“Innovations such as this are transforming medicine and will have a significant impact on the future care for patients. This stateof-the-art robotics will enhance patient care for our patients across the West and Northwest of Ireland,” she added.
17 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
News
Dr Simone Fezzi, Professor Faisal Sharif and Dr Max Wagener
Beaumont Hospital’s CR Department staff with the Mayo Clinic Faculty (Cardiac Rehabilitation) and the President of the Irish Association of Cardiac Rehabilitation (IACR)
Asthma
Potential new treatments for asthma
 Written by N Tangney1, DM Murphy1
Written by N Tangney1, DM Murphy1
exposure to certain triggers, e.g. viruses, allergens, aspirin.1
Asthma overview
Asthma is a heterogenous condition characterised by airway obstruction and hyper-responsiveness with chronic inflammation and subsequent bronchial remodelling. Genetic make-up may predispose people to developing asthma after
There are two typical phenotypes – Th2-high (‘type 2’) and Th2low. This refers to whether or not the disease process is driven by T-helper type 2 cells (Th2). Th2 cells promote eosinophilic airway inflammation by the generation of interleukin (IL)-4, IL-5, and IL-13 which manifests as a high eosinophils, high fractional exhaled nitric oxide (FeNO), high immunoglobulin E (IgE), or allergic phenotype (Figure 1). These individuals make up approximately 50% of asthma patients.2 This is clinically relevant as certain therapeutics in severe asthma are aimed at these targets in this group.
Current treatments
Asthma therapy has evolved over centuries.3 The Ebers Papyrus from 1550 BC mentions inhalations from heated henbane (Hyoscyamus
niger, a plant from which hyoscine is extracted) used as treatment for a condition likely to be asthma. In China circa 1000 BC Ma Huang, a plant from which ephedrine was eventually extracted, was used. Moses Maimonides, a Spanish physician of the 12th century, advised dry air, an even temper, and chicken soup for asthma. Atropa belladonna (deadly nightshade) was used in 1869 by Henry Salter which eventually led to the isolation of ipratropium, an ingredient in some modern inhalers (Figure 2). Medicines such as cortisone and antihistamines are relatively recent products of the 20th century.
Modern asthma guidelines focus on a process of shared decision-making depending on the patient’s own views, goals, and capabilities. The Global Initiative for Asthma (GINA) guidelines involve a step-wise approach depending
on symptom frequency.4 Over the last number of years the importance of early introduction of inhaled corticosteroids (ICS) in the asthma treatment paradigm has been increasingly emphasised. Treatment with inhaled corticosteroids is now recommended at all stages of treatment on either a regular or as required basis. The treatment paradigm is based on achieving control with escalating levels of therapy to attempt to achieve this goal.
Severe asthma is defined as “uncontrolled despite adherence with maximal optimized high dose ICS-long-acting beta agonist treatment and management of contributory factors”. These individuals should be referred for specialist assessment where phenotype and appropriate treatment options are assessed. Patients with Th2-high phenotypes may be considered for targeted biologic therapy while advanced options for Th2-low asthmatics are limited, as fewer biological targets have been identified to date.

Though only a minority of patients has severe asthma (5-10%), this group make up the majority of the associated healthcare costs and so there is a pressing need to improve therapeutics for this group, particularly for the Th2-low patients.
Alongside pharmacological methods, it is crucial to address other elements which may be corrected such as inhaler technique, adherence, triggers (beta-blockers, non-steroidal anti-inflammatories) and weight.5 One should always consider comorbidities and optimise these issues as they can often contribute to symptoms under the guise of asthma, e.g. rhinosinusitis, gastrooesophageal reflux, anxiety.

Smart inhalers
The prevalence of poor inhaler technique ranges from 14 – 90% depending on the device and context.6 Smart inhalers are a new technology capable of assessing inhaler technique and tracking compliance. These can be linked to phones and send reminders when medications are due. Some can detect high pollution or pollen in the area. Given compliance can be a major issue in asthma treatment, these may provide a
18 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
N Tangney
1The Department of Respiratory Medicine, Cork University Hospital, Cork, Ireland
Figure 1: Asthma pathophysiology. Credit: Medscape
This is a promotional advertisement from LEO Pharma for IE healthcare professionals.

For the treatment of moderate to severe atopic dermatitis in adult and adolescent patients 12 years and older who are candidates for systemic therapy1

TIME TO PRESS PLAY
Prescribing Information for Adtralza® (tralokinumab) 150 mg solution for injection in pre-filled syringe


Please refer to the full Summary of Product Characteristics (SmPC) (www.medicines.ie) before prescribing.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Indications: Treatment of moderate-tosevere atopic dermatitis in adult and adolescent patients 12 years and older who are candidates for systemic therapy. Active ingredients: Each pre-filled syringe contains 150 mg of tralokinumab in 1 mL solution (150 mg/mL). Dosage and administration: Posology: The recommended dose of tralokinumab for adult and adolescent patients 12 years and older is an initial dose of 600 mg (four 150 mg injections) followed by 300 mg (two 150 mg injections) administered every other week as subcutaneous injection. Every fourth week dosing may be considered for patients who achieve clear or almost clear skin after 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve further with continued treatment every other week beyond 16 weeks. Tralokinumab can be used with or without topical corticosteroids. The use of topical corticosteroids, when appropriate, may provide an additional effect to the overall efficacy of tralokinumab. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas. If a dose is missed, the dose should be administered as soon as possible and then dosing should be resumed at the regular scheduled time. No dose adjustment is recommended for elderly patients, patients with renal impairment or patients with hepatic impairment. For patients with high body weight (>100 kg), who achieve clear or almost clear skin after 16 weeks of treatment, reducing the dosage to every fourth week might not be appropriate. The safety and efficacy of tralokinumab in children below the age of 12 years have not yet been established. Method of administration: Subcutaneous use. The pre-filled syringe should not be shaken. After removing the pre-filled syringes from the refrigerator, they should be allowed to reach room temperature by waiting for 30 minutes before injecting. Tralokinumab is administered by subcutaneous injection into the thigh or abdomen, except the 5 cm around the navel. If somebody else administers the injection, the upper arm can also be used. For the initial 600 mg dose, four 150 mg tralokinumab injections should be administered consecutively in different injection
IL, interleukin.
sites within the same body area. It is recommended to rotate the injection site with each dose. Tralokinumab should not be injected into skin that is tender, damaged or has bruises or scars. A patient may self-inject tralokinumab or the patient’s caregiver may administer tralokinumab if their healthcare professional determines that this is appropriate. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: If a systemic hypersensitivity reaction (immediate or delayed) occurs, administration of tralokinumab should be discontinued and appropriate therapy initiated. Patients treated with tralokinumab who develop conjunctivitis that does not resolve following standard treatment should undergo ophthalmological examination. Patients with pre-existing helminth infections should be treated before initiating treatment with tralokinumab. If patients become infected while receiving tralokinumab and do not respond to antihelminth treatment, treatment with tralokinumab should be discontinued until infection resolves. Live and live attenuated vaccines should not be given concurrently with tralokinumab. Fertility, pregnancy and lactation: There is limited data from the use of tralokinumab in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure, it is preferable to avoid the use of tralokinumab during pregnancy. It is unknown whether tralokinumab is excreted in human milk or absorbed systemically after ingestion. Animal studies did not show any effects on male and female reproductive organs and on sperm count, motility and morphology.
Side effects: Very common (≥1/10): Upper respiratory tract infections. Common (≥1/100 to <1/10): conjunctivitis, conjunctivitis allergic, eosinophilia, injection site reaction. Uncommon (≥1/1,000 to <1/100): keratitis. Precautions for storage: Store in a refrigerator (2°C-8°C). Do not freeze. Store in the original package in order to protect from light. Legal category: POM. Marketing authorisation number and holder: EU/1/21/1554/002. LEO Pharma A/S, Ballerup, Denmark. Last revised: October 2022
Reference number: REF-22407
Reporting of Suspected Adverse Reactions
Adverse events should be reported.
Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie
Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com
References: 1. Adtralza® SPC. 2. Duggan S. Drugs 2021;81(14):1657–1663. 3. Bieber T. Allergy 2020;75:54–62.
Date of preparation: December 2022
more at www.adtralza.ie Adtralza®
Learn
The first licensed
biologic that
inhibits
IL-13
alone, 1,2 a key driver of atopic dermatitis signs and symptoms.3
IE/MAT-62263 Further information can be found in the Summary of Product Characteristics or from: LEO Pharma, Cashel Road, Dublin 12, Ireland. E-mail: medical-info.ie@leo-pharma.com
® Registered trademark
Not an actual patient. For illustrative purposes only. Individual results may vary.
temper, and chicken soup for asthma. Atropa belladonna (deadly nightshade) was used in Salter which eventually led to the isolation of ipratropium, an ingredient in some modern Medicines such as cortisone and antihistamines are relatively recent products of the 20th century.

Asthma
solution for some users which could have a positive effect on overall symptom management.
Anti-IgE
Credit: Colin Underhill/Alamy Stock Photo
The NAVIGATOR study consisted of 1,061 patients with severe, uncontrolled asthma (including those with low eosinophils).14 Compared to placebo, treatment with tezepulumab resulted in an overall rate ratio of 0.44 for asthma exacerbation rates and also had a greater improvement in prebronchodilation FEV1 and scores on ACQ-6, AQLQ, and asthma symptom diary (ASD).
illustrated some potential benefit in patients with severe, refractory asthma but these early results have yet to be replicated in large clinical trials.23
Anti-IL13/Anti-IL33
There have been numerous trials investigating therapies targeting IL-13 and IL-33. These are two cytokines thought to play a complex role in signalling cascades in asthma, particularly type 2 inflammation. Unfortunately, to date, there have not been any significantly positive results from trials of anti-IL13 or anti-IL33 therapies.24, 25, 26, 27
Th2-low options
Omalizumab (Xolair) is a monoclonal anti-IgE antibody which is currently indicated in severe allergic asthma as a 2 – 4 weekly subcutaneous injection. It is indicated in patients >6 years old with proven IgEmediated, severe asthma. Higher blood eosinophils and FeNO and childhood-onset asthma may predict a better response to treatment. It can also be considered for use in pregnancy thanks to its favourable safety profile. It has been shown to reduce severe exacerbations by 44% and significantly improve quality of life.7
the 2022 GINA guidelines though for Th2-high asthmatics only. It is a two-weekly subcutaneous injection. Randomised control trials (RCT) have shown it reduces the rate of severe asthma exacerbations to a greater extent than those receiving placebo.
The SOURCE trial evaluated its OCS-sparing effect, however there was no significant reduction in OCS dose compared with placebo.15 WAYFINDER is an ongoing study which aims to build on these results16 and DESTINATION is an extension from NAVIGATOR and SOURCE examining long term safety and asthma exacerbations.17 PASSAGE is a phase 4 trial underway that will examine tezepulumab’s effectiveness in a real world population.18
OTHER TARGETS:
sthma guidelines focus on a process of shared decision-making depending on the patient’s own and capabilities. The Global Initiative for Asthma (GINA) guidelines involve a step-wise depending on symptom frequency (4). Over the last number of years the importance of early of inhaled corticosteroids (ICS) in the asthma treatment paradigm has been increasingly Treatment with inhaled corticosteroids is now recommended at all stages of treatment on regular or as required basis. The treatment paradigm is based on achieving control with escalating therapy to attempt to achieve this goal.
In a study of 1,902 patients, the annual rate of severe exacerbations was 0.46 in the dupilumab group compared to 0.87 in the placebo group.10 There was a 0.32 L increase in forced expiratory volume (FEV1) compared to 0.14 L in the placebo group and ACQ-5 and asthma quality of life questionnaire (AQLQ) scores were lower in the treatment arm. A greater effect was seen with higher baseline eosinophils. A subsequent post hoc analysis showed similar outcomes in both allergic and non-allergic phenotypes (based on IgE).11
GATA-3 inhibitor
GATA3 is a transcription factor that controls the production of certain Th2 cytokines such as IL-4, 5 and 13 whose overexpression has been observed in severe asthma. A GATA3-specifc DNA enzyme, SB010, has been developed as an oral inhaler that cleaves GATA3 mRNA thus reducing cytokine production.19
is defined as “uncontrolled despite adherence with maximal optimized high dose ICS-longagonist treatment and management of contributory factors”. These individuals should be specialist assessment where phenotype and appropriate treatment options are assessed. Th2-high phenotypes may be considered for targeted biologic therapy while advanced h2-low asthmatics are limited, as fewer biological targets have been identified to date minority of patients has severe asthma (5-10%), this group make up the majority of the
Anti-IL5
Three anti-IL5 biologics are currently available for use in severe asthma – benralizumab (Fasenra), mepolizumab (Nucala), and reslizumab (Cinqaero). They are indicated in patients with high blood eosinophils and frequent exacerbations. Higher blood eosinophils, adult-onset asthma, and nasal polyposis may predict a better response. Benralizumab and mepolizumab are given as subcutaneous injections while reslizumab is administered as an intravenous infusion. Anti-IL5 use has been shown to significantly reduce both hospital and nonhospital exacerbations, GP visits, and oral corticosteroid (OCS) use.8 Switching to IL5 inhibitors in patients who are not fully controlled on omalizumab has been associated with a significant reduction in hospital and community exacerbation rates with a non-significant reduction in asthma control questionnaire (ACQ) score.9
Anti-IL4
Dupilumab (Dupixent), an anti-IL4 biologic, is another new addition to
A study by Rabe et al showed that dupilumab had a significant reductive effect on OCS dose with 48% no longer requiring OCS vs 25% with placebo, regardless of eosinophil levels.12
Its use is further supported by a phase 2 trial which showed increased lung function and reduced severe exacerbation rate (53.7 - 70.5% depending on dose) in 611 patients treated with dupilumab irrespective of baseline eosinophil count.13
Anti-TSLP
Tezepulumab (Tezspire), a four-weekly subcutaneous injection, inhibits thymic stromal lymphopoeitin (TSLP) which acts further upstream in the inflammatory cascade than most biologics and as a result affects both Th2-low and Th2-high asthmatics. It is the first such biologic to be approved in the EU and is included in the 2022 GINA guidelines. Evidence is based largely on two RCTs – NAVIGATOR and SOURCE.
A phase 2 RCT investigating the efficacy of SB010 in patients with mild, allergic type asthma (as per GINA guidelines) showed clinical potential and the GIANT-1 (GATA3 Inhibition in Asthmatics Not controlled on standard medication with Type-2) trial is a follow on study for patients with moderate to severe type-2 asthma that is currently underway.
Tyrosine kinase inhibitors
Mast cells are activated by stem cell factor (SCF) binding to the c-kit receptor (a tyrosine kinase receptor (TKR)). It has been shown that inhibition of the SCF/c-kit pathway leads to a reduction in histamine and mast cells, as well as eosinophil infiltration, IL-4 and airway hyper-responsiveness in vivo20, revealing a potential therapeutic pathway. Another TKR which may be of value is that of platelet-derived growth factor (PDGF) receptor which has been shown to play a role in chronic tissue remodelling in asthma. Oral masitinib has demonstrated clinical efficacy in early studies with a phase 3 trial underway for masitinib treatment in patients with severe uncontrolled, eosinophilic asthma which will hopefully yield positive results.21, 22 Imatinib
There is a paucity of treatment options available in severe, Th2-low asthma as fewer feasible therapeutic targets have been identified to date. One option to consider in this group is the addition of maintenance azithromycin, a macrolide antibiotic that can also act as an immunomodulator in respiratory conditions such as asthma, bronchiectasis, or COPD. This is given either three times per week or once daily and should be trialled for at least six months. The AMAZES trial has shown a significant reduction in exacerbations from 64% to 49% along with a significant improvement in quality of life with the addition of azithromycin.28 Another possible option for the refractory Th2-low group is bronchial thermoplasty. This is a bronchoscopic technique which uses heat to reduce smooth muscle in the airway in order to decrease its bronchoconstrictive capabilities. In patients with severe asthma this procedure has been shown to improve AQLQ score and reduce severe exacerbations in the following year29 while also maintaining efficacy at 10 years post-procedure.30
Conclusion
Treatment advances in asthma have come a long way since the days of henbane inhalation. The illness still however places a major burden on healthcare systems and treatment in particular groups, such as the Th2-low asthmatics, has not kept pace. With the introduction of targeted monoclonal therapies, treatment for eligible patients has been revolutionised. The optimal therapies and switches between these agents are still being elucidated. Thankfully, continued research across the globe resulting in new treatment avenues casts a promising light for asthma therapy in the future.
References available on request
20 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 1: Atropa belladonna
Figure 2: Atropa belladonna Credit: Colin Underhill/Alamy Stock Photo
ABBREVIATED PRESCRIBING INFORMATION – IRELAND
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
Apexxnar®▼ suspension for injection in pre-filled syringe
Pneumococcal polysaccharide conjugate vaccine (20-valent, adsorbed)

Presentation: Each 0.5 mL dose of Apexxnar contains 2.2 micrograms of each of the following polysaccharide serotypes: 1, 3, 4, 5, 6A, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F and 4.4 micrograms of polysaccharide serotype 6B. Each polysaccharide is conjugated to the CRM197 carrier protein and adsorbed on aluminium phosphate. 1 dose (0.5 mL) contains approximately 51 µg CRM197 carrier protein and 0.125 mg aluminium.
Indications: Active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older. Apexxnar should be used in accordance with o cial recommendations taking into consideration the risk of invasive disease and pneumonia in di erent age groups, underlying comorbidities as well as the variability of serotype epidemiology in di erent geographical areas.
Dosage and Administration: For intramuscular injection. Individuals ≥ 18 years of age and older: One single dose. The need for revaccination with a subsequent dose of Apexxnar has not been established. Based on the clinical experience with Prevenar 13, if the use of 23-valent pneumococcal polysaccharide vaccine is considered appropriate, Apexxnar should be given first. Special populations: There are no data with Apexxnar in special populations. Limited experience from clinical studies with Prevenar 13 are available in adults at higher risk of pneumococcal infection either immunocompromised individuals or following bone marrow transplantation. Based on these data the following posology was recommended for Prevenar 13: Individuals at higher risk of pneumococcal infection (e.g., individuals with sickle cell disease or HIV infection), including those previously vaccinated with 1 or more doses of PPSV23, were recommended to receive at least 1 dose of Prevenar 13. In individuals with a hematopoietic stem cell transplant (HSCT), the recommended immunisation series with Prevenar 13 consisted of 4 doses of 0.5 mL each. The primary series consisted of 3 doses, with the first dose given 3 to 6 months after HSCT and with an interval of at least 1 month between doses. A booster dose was recommended 6 months after the third dose.
Contra-indications: Hypersensitivity to the active substances, to any of the excipients, or to diphtheria toxoid.
Warnings and Precautions: Do not administer intravascularly. Appropriate medical treatment and supervision must be available in case of anaphylaxis. Vaccination should be postponed in individuals su ering from acute severe febrile illness. However, the presence of a minor infection, such as a cold, should not result in the deferral of vaccination. It should not be given to individuals with thrombocytopenia or a bleeding disorder that would contraindicate intramuscular injection. The risk of bleeding in patients with coagulation disorders needs to be carefully evaluated before intramuscular administration of any vaccine, and subcutaneous administration should be considered if the potential benefit clearly outweighs the risks. Apexxnar will only protect against Streptococcus pneumoniae serotypes included in the vaccine and will not protect against other microorganisms that cause invasive disease and pneumonia. As with any vaccine, Apexxnar may not protect all individuals receiving the vaccine from pneumococcal disease. Safety and immunogenicity data on Apexxnar are not available for individuals in immunocompromised groups. Vaccination should be considered on an individual basis. Individuals with impaired immune responsiveness, whether due to the use of immuno-suppressive therapy, a genetic
References
APEXXNAR. Summary of Product Characteristics.
defect, human immunodeficiency virus (HIV) infection, or other causes, may have reduced antibody response to active immunisation. The clinical relevance of this is unknown. In adults across all studied age groups, formal non-inferiority criteria were met although numerically lower geometric mean titres were observed with Apexxnar for most of the serotypes compared to Prevenar 13, however the clinical relevance of this observation for immunocompromised individuals is unknown.
Drug Interactions: Apexxnar may be administered concomitantly with seasonal influenza vaccine (QIV; surface antigen, inactivated, adjuvanted). In subjects with underlying conditions associated with a high risk of developing life-threatening pneumococcal disease, consideration may be given to separating administrations of QIV and Apexxnar (e.g., by approximately 4 weeks). Apexxnar can be administered concomitantly with COVID-19 mRNA vaccine (nucleoside modified).
Fertility, Pregnancy & Lactation: There are no data from the use of Apexxnar in pregnant women. Animal studies do not indicate direct or indirect harmful e ects with respect to reproductive toxicity. Administration of Apexxnar in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and foetus. It is unknown whether Apexxnar is excreted in human milk. Side E ects: As Apexxnar contains the same 13 serotype-specific capsular polysaccharide conjugates and the same vaccine excipients as Prevenar 13, the adverse reactions already identified for Prevenar 13 have been adopted for Apexxnar. In clinical trials, the safety profile of Apexxnar was similar to that of Prevenar 13. No new adverse reactions were identified as compared to Prevenar 13. When Apexxnar was administered to adults aged ≥ 65 years together with the third (booster) dose of a COVID-19 mRNA vaccine (nucleoside modified), the tolerability profile generally resembled that of the COVID-19 mRNA vaccine (nucleoside modified) administered alone. Very common (≥ 1/10): Headache, joint pain, muscle pain, vaccination-site pain/tenderness, fatigue. Common (≥ 1/100 to < 1/10): Vaccination-site induration/ swelling, vaccination-site erythema, pyrexia. Uncommon (≥ 1/1,000 to < 1/100): Hypersensitivity reaction including face oedema, dyspnoea, bronchospasm, diarrhoea, nausea, vomiting, rash, angioedema, vaccination-site pruritus, lymphadenopathy, vaccination-site urticaria, chills. The following adverse reactions have been spontaneously reported during the postmarketing use of Prevenar 13, which may also occur with Apexxnar. These events were reported voluntarily from a population of uncertain size, so it is not possible to reliably estimate their frequency or to establish, for all events, a causal relationship to vaccine exposure: Anaphylactic/anaphylactoid reaction, including shock, erythema multiforme, vaccination-site dermatitis. Additional information in special populations in studies with Prevenar 13: Adults with HIV infection have similar frequencies of adverse reactions, except that pyrexia and vomiting were very common and nausea common. Adults with an HSCT have similar frequencies of adverse reactions, except that pyrexia, vomiting, and diarrhoea were very common.
For full prescribing information see the Summary of Product Characteristics.
Legal Category: S1A. Package Quantities: Pack of 1 single-dose pre-filled syringe (with separate needle).

Marketing Authorisation Numbers: EU/1/21/1612/002. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium.
For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500.
Last revised: 12/2022.
Ref: PE 3_0.
Froes F, Roche N, Blasi F. Pneumococcal vaccination and chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2017;12:3457-3468.


PP-PNR-IRL-0023 | Date of Preparation: January 2023
APEXXNAR® is indicated for active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.1
▼
older.
1.
2.
Designed to deliver long-lasting immunity.1,2
Helps protect against the 20 Streptococcus pneumoniae serotypes included in the vaccine.1
Respiratory
The Top 5 Respiratory Conditions placing the biggest burden on Irish Health Care



Chronic Obstructive Pulmonary Disease (COPD):
Chronic obstructive pulmonary disease (COPD) is a common, preventable, treatable disease that is characterised by persistent respiratory symptoms of breathlessness and wheeze, and airflow obstruction that is not fully reversible. The natural timeline of the disease features symptomatic exacerbations and progressive decline in lung function with resultant significant impact on quality of life.1 It is estimated that up to 500,000
Respiratory disease carries a very significant healthcare burden worldwide. Compared with other European Countries Ireland is affected with higher rates of respiratory disease.
In 2003 the Irish Thoracic Society (ITS) published a report entitled ‘Ireland Needs Healthier Airways and Lungs - the Evidence’ which was the first report to highlight the scale and impact of respiratory disease in this country. More recently, in 2018, the ITS published ‘Respiratory Health of the Nation’, once again bringing focus to the burden of respiratory disease on the Irish Health Care system. However, much of this respiratory disease burden could be prevented, or potentially detected earlier, with improved public health awareness and lifestyle interventions.

people in Ireland have COPD with only approximately half of these formally diagnosed.2 Spirometry is key to diagnosing non-reversible airflow obstruction in COPD. Smoking is the most important risk factor for developing COPD with approximately 40-50% of lifelong smokers developing the disease.3 Prevalence increases with age and is also higher in areas with high social deprivation.2
COPD is Ireland’s fourth leading cause of death after cardiovascular disease, stroke and lung cancer.4 While the vast majority of COPD
22 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Written by C. Hayes & Professor Eddie Moloney, Respiratory Consultant, Tallaght University Hospital
C. Hayes
Professor Eddie Moloney
cases are managed in the primary care setting, COPD further places a notable burden on tertiary care services.
Ireland had the highest hospitalisation rate for COPD among the 37 OECD member countries in 2020 and COPD accounts for 3.4% of all hospital bed days.
Of particular relevance to the Irish population, the American Thoracic Society and European Respiratory Society both recommend testing for Alpha-1 Antitrypsin Deficiency (AATD) in all patients with COPD, regardless of age or smoking history. After Cystic Fibrosis, AATD is the most common genetic condition in Ireland, with an estimated 250,000 people with mild or moderate deficiency and 3,000 with severe deficiency. Smoking cessation is of particular importance in this cohort as even those with mild deficiency are at significantly higher risk of developing COPD with cigarette exposure.5
Many effective treatments are available for COPD. However, prevention is key and all patients should be encouraged and offered assistance with smoking cessation.1 Inhaled bronchodilators comprising of long-acting muscarinic antagonist (LAMA) and/ or long-acting beta agonist (LABA) agents are typically the first line for pharmacological management.1
Patient education on the importance of inhaler technique and adherence is essential. The benefit of inhaled corticosteroids (ICS) in COPD is topical. The blood eosinophil count (BEC)
has been shown to be predictive of response to ICS therapy in those COPD patients who need a step up in therapy from LAMA/ LABA therapy.6 Current guidelines suggest considering their use in those with a BEC of >300 per μL and to consider avoiding in those with BEC <100 per μL.1
Influenza and pneumococcal vaccination are recommended and can decrease the rate of acute exacerbations. Prophylactic macrolides, particularly azithromycin, can be considered for those non-smoker COPD patients with recurrent infective exacerbations despite maximal inhaled therapy.1
Non-pharmacological management and risk factor modification are also key components of treatment. Those with chronic hypoxaemia may warrant long-term oxygen therapy, especially in the presence of coexisting pulmonary hypertension. Pulmonary rehabilitation is a cornerstone of management and has been shown to be beneficial in improving both symptoms, exercise tolerance and decreased health care utilisation.7
More recently the use of surgical or bronchoscopic interventions should be considered in those with advanced disease. Lung volume reduction surgery and endobronchial valves can be an option in appropriately selected patients with advanced emphysema and evidence of significant air trapping/lung hyperinflation causing marked symptoms.8, 9 In carefully selected patients with severe
and progressive COPD, referral for lung transplantation may be appropriate. COPD (AATD and non-AATD) is the most common indication for lung transplantation globally accounting for 40% of all lung transplants performed.10 Benefits have been shown in both survival and functional capacity. However, the decision to proceed with transplant and the timing of referral remains complex.10
Asthma:
Asthma is a chronic, eosinophilic inflammatory condition of the airways characterised by the presence of episodic and reversible airflow obstruction. Common symptoms include cough, wheeze, dyspnoea and chest tightness, often with diurnal variation. It is estimated that approximately 450,000 people in Ireland currently have a diagnosis of asthma comprising 1 in 10 children.11 While globally the mortality and number of hospital admissions from asthma has declined significantly over the past 30 years, Ireland continues to have one of the highest prevalences of asthma in Europe today.3
While the vast majority of asthma cases are mild, 5-10% of patients will have severe asthma which is defined by the Global Initiative for Asthma (GINA) as ‘asthma that is uncontrolled despite high dose ICS-LABA, or that requires high dose ICS-LABA to remain controlled’.12 Although a minority of cases, these severe asthma patients comprise a large proportion of the Irish healthcare and resource burden associated with asthma.
There are approximately 8,000 asthma related admissions to Irish hospitals every year and the total cost of asthma care to the state is approximately ¤472 million per annum.11
Asthma management has evolved significantly over the last 20 years particularly with the emergence of biological agents. GINA guidelines recommend a stepwise approach to asthma management with continual re-assessment of asthma control and treatment side effects and subsequent escalation or de-escalation of treatment as appropriate. Education on the adequacy of inhaler technique and adherence is a key component of management. Prior studies have shown at least 50% of patients do not take controller medications as prescribed and up to 80% cannot use their inhaler correctly.12 Smoking cessation and trigger avoidance also play a key role in management.
The preferred initial treatment for patients with mild asthma is now as needed low dose ICS-formoterol. Escalation of treatment typically includes regular ICS-LABA therapy and then intensification of ICS dose. Additional options then include adding a LAMA or a leukotriene receptor antagonist.
Biologic agents against several cytokines involved in the asthma inflammatory pathway have shown great success in recent years. These include targeted therapies against IL- 5/IL-5R, IL-4R and more recently thymic stromal lymphopoietin. These agents have shown to be highly

23 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Respiratory
effective in reducing exacerbation rates, improving symptom control and decreasing the need for maintenance corticosteroid in appropriate patients,; predominantly those who exhibit signs of type 2/eosinophilic airway inflammation.12
Lung Cancer:
Lung cancer is the leading cause of cancer-related deaths in Ireland in both males and females. There were 3271 new lung cancer cases diagnosed in Ireland in 2020, with the majority of cases being advanced, and non-operable, at diagnosis.134 Approximately 85-90% are related to smoking. The incidence of lung cancer is higher in lower socioeconomic groups. This is likely due to higher rates of smoking but may also reflect greater occupational exposure to harmful dust, fibres and fumes.2 Advice and encouragement of smoking cessation at every healthcare contact is essential to try and decrease the significant public health burden associated with lung cancer. All smokers should be offered either nicotine replacement therapy or other smoking cessation medication, and counselling, which has been shown to increase the chances of successfully quitting.14
There have been many recent advances in lung cancer treatment which have improved outcomes and offered hope for further breakthroughs in the future. The management of metastatic non-small cell lung cancer (NSCLC) has been revolutionised by the development of targeted therapies based on the presence of certain driver mutations. Many of these mutations exist with several available therapeutic options.15
Immune checkpoint inhibitors have drastically changed the management of many lung malignancies. These may be used alone or in combination with chemotherapy and have significantly improved survival in appropriate patients.16
Pneumonia:
Acute lower respiratory tract infections (LRTI) are a significant cause of morbidity and mortality in both children and adults.
Pneumonia is the 5th most common cause of death in Ireland.2 In 2016 pneumonia and LRTI accounted for 4.5% of all inpatient hospitalisations and 6.3% of all inpatient bed days.2 However, a significant majority of patients are treated in the community with hospitalisations representing only a small proportion of the overall burden placed on the healthcare service. Over 90% of deaths from pneumonia are in those aged 70 years and over.2

Lack of new antibiotics and expanding resistance to established agents are increasingly important threats to health and pneumonia survival, with approximately 10% of Streptococcus pneumoniae isolates now resistant to commonly used penicillin and/or macrolide antibiotics.17
Risk factors for pneumonia include older age, smoking, pre-existing lung disease, immunosuppression or neurological dysfunction which can increase risk of aspiration. Preventive measures including smoking cessation and vaccination are key to try and reduce disease impact on healthcare services. This includes promotion of the yearly influenza vaccine for the general public and pneumococcal polysaccharide (PPV23) vaccine for those over 65 or for those under 65 at higher risk of pneumococcal disease, including patients with Diabetes or chronic lung, heart, liver, or kidney disease.18
Pneumococcal polysaccharide (PPV23) vaccination has been shown to be an effective preventive measure, significantly decreasing the risk of both invasive and non-invasive pneumococcal pneumonia.19 Seasonal influenza vaccination is an important public health measure which can help decrease the significant burden placed on the health service every winter. Efficacy varies depending on a number of factors including age, baseline health, immune function and the degree of match between vaccine antigens and circulating virus strains.20 However, even for those with breakthrough influenza infection, vaccination
is associated with reduced mortality and disease severity. In addition, in patients with underlying cardiovascular disease, influenza vaccination is associated with a significant reduction in cardiovascular mortality and major adverse cardiovascular events.21
Pneumonia due to Covid-19 infection is beyond the scope of this article and has been excluded.
Obstructive Sleep Apnoea: Sleep disordered breathing encompasses a broad spectrum of sleep-related breathing disorders. The most common form of sleep disordered breathing is obstructive sleep apnoea (OSA). OSA is a disorder that is characterized by progressive snoring, obstructive apneas due to repetitive collapsibility of the upper airway during sleep, usually noticed by a bed partner, arousals and awakenings during sleep, and waking unrefreshed . Patients may experience symptoms including daytime sleepiness, depressed mood, irritability, cognitive dysfunction, and work and road traffic related accidents.22
Obesity is the most common predisposing factor and risk correlates with BMI and neck circumference.23 OSA prevalence is increasing mainly due to rising rates of obesity worldwide. It is estimated that one billion people on the planet have OSA.24 The excessive daytime somnolence can lead to significant decline in quality of life and cognitive performance as well as an increase in road traffic and occupational accidents.25 Moreover, the major health burden of patients with OSA relates to the cardiovascular system. Epidemiological studies
have demonstrated an independent relationship between OSA and the development of hypertension, atrial fibrillation and other cardiac arrhythmias, coronary artery disease, congestive cardiac failure and stroke.26
Access to diagnostics has improved in recent years with the availability of home sleep apnoea testing or home polysomnography. In appropriate patients with a high pretest probability of moderate to severe, uncomplicated OSA, this is a viable alternative to overnight hospital polysomnography and carries potential benefits including patient convenience and reduced time to diagnosis and commencement of treatment.27
Early recognition of OSA is important as effective treatments are available. Weight loss and exercise should be recommended to all patients with OSA who are overweight or obese. This can further lead to reductions in blood pressure, metabolic parameters and markers of disease severity.268 Continuous positive airway pressure (CPAP) is the mainstay of treatment for OSA. There is extensive evidence showing its benefits in improving symptoms and quality of life as well as reducing cardiovascular mortality and morbidity.289 Monitoring and encouragement of adherence is important as this can improve the potential benefits of CPAP therapy.
In patients with mild to moderate OSA who decline CPAP or fail to respond to it, oral appliances such as mandibular advancement devices are an alternative therapy that have been shown to improve signs and symptoms of OSA.29
References available on request
24 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
* Patients with moderate-to-severe COPD not adequately treated by a combination of ICS/LABA, or LAMA/LABA.1

** A worsening of symptoms or a history of exacerbations treated with antibiotics or oral corticosteroids in the past 12 months.
ICS, inhaled corticosteroid; LABA, long-acting ß2-agonist; LAMA, long-acting muscarinic antagonist
TRELEGY Ellipta is generally well tolerated. Common adverse reactions include: pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, constipation, arthralgia, back pain1
References: 1. TRELEGY Ellipta SmPC, 2022. Available at www.medicines.ie. Accessed October 2022. 2. Lipson DA et al. Am J Respir Crit Care Med 2017; 196:438–446. 3. Halpin DMG;ERJ Open Research;2021;7;1-11.
Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) Prescribing information. Please consult the full Summary of Product Characteristics (SmPC) before prescribing Trelegy Ellipta (fluticasone furoate/umeclidinium/vilanterol [as trifenatate]) inhalation powder. Each single inhalation of fluticasone furoate (FF) 100 micrograms (mcg), umeclidinium bromide (UMEC) 62.5 micrograms and vilanterol as trifenatate (VI) 25 mcg provides a delivered dose of 92 mcg FF, 55 mcg UMEC and 22 mcg VI. Indications: Maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an inhaled corticosteroid (ICS) and a long-acting ß2-agonist (LABA) or a combination of a LABA and a long acting muscarinic antagonist. Dosage and administration: One inhalation once daily at the same time each day. Contraindications: Hypersensitivity to the active substances or to any of the excipients (lactose monohydrate & magnesium stearate). Precautions: Paradoxical bronchospasm, unstable or life-threatening cardiovascular disease or heart rhythm abnormalities, convulsive disorders or thyrotoxicosis, pulmonary tuberculosis or patients with chronic or untreated infections, narrow-angle glaucoma, urinary retention, hypokalaemia, patients predisposed to low levels of serum potassium, diabetes mellitus. In patients with moderate to severe hepatic impairment patients should be monitored for systemic corticosteroid-related adverse reactions. Eye symptoms such as blurred vision may be due to underlying serious conditions such as cataract, glaucoma or central serous chorioretinopathy (CSCR); consider referral to ophthalmologist. Increased incidence of pneumonia has been observed in patients with COPD receiving inhaled corticosteroids. Risk factors for pneumonia include: current smokers, old age, patients with a history of prior pneumonia, patients with a low body mass index and severe COPD. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take Trelegy. Acute symptoms: Not for acute symptoms, use short-acting inhaled bronchodilator. Warn patients to seek medical advice if short-acting inhaled bronchodilator use increases. Therapy should not be abruptly stopped without physician supervision due to risk of symptom recurrence. Systemic effects: Systemic effects of ICSs may occur, particularly at high doses for long periods, but much less likely than with oral corticosteroids. Interactions with other medicinal products: Caution should be exercised with concurrent use of ß-blockers. Caution is advised when co-administering with strong CYP3A4 inhibitors (e.g. ketoconazole, ritonavir, cobicistat-containing products), hypokalaemic treatments or non-potassium-sparing diuretics. Co-administration with other long-acting muscarinic antagonists or long acting ß2-adrenergic agonists is not recommended. Pregnancy and breast-feeding: Experience limited. Balance risks against benefits. Side effects: Common (≥1/100 to <1/10): pneumonia, upper respiratory tract infection, bronchitis, pharyngitis, rhinitis, sinusitis, influenza, nasopharyngitis, candidiasis of mouth and throat, urinary tract infection, headache, cough, oropharyngeal pain, arthralgia, back pain. Uncommon (≥1/1,000 to <1/100): viral respiratory tract infection, dysgeusia, vision blurred, glaucoma, eye pain, supraventricular tachyarrhythmia, tachycardia, atrial fibrillation, dysphonia, dry mouth, fractures. Rare (≥1/10,000 to <1/1,000): Hypersensitivity reactions, including anaphylaxis, angioedema, urticaria, and rash, intraocular pressure increased. Marketing Authorisation (MA) Holder: GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. MA No. [EU/1/17/1236/002]. Legal category: POM B. Last date of revision: July 2022. Code: PI-6725. Further information available on request from GlaxoSmithKline, 12 Riverwalk, Citywest Business Campus, Dublin 24. Tel: 01-4955000.



CAN TOO*1,2
IT KEEPS GOING SO THEY
is a 31% significant increase in the odds of patients on TRELEGY Ellipta improving their health status/HRQoL compared with those on non-Ellipta Multiple Inhaler Triple therapy (MITT)3 Striving for a life uninterrupted For COPD patients on treatment with ICS/ LABA and at risk of exacerbation**1
There
Find out more here: www.trelegy.ie or request a visit from a GSK representative ©2022 GSK Group of Companies or its licensor Trademarks are owned by or licensed to the GSK Group of Companies PM-IE-FVU-ADVT-220007 | December 2022 TRELEGY Ellipta was developed in collaboration with Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to
GlaxoSmithKline on 1800 244 255.
Precision Medicine
Alpha-1 Antitrypsin Deficiency (AATD) – Precision Medicine for COPD?
AAT protein production is controlled by the SERPINA1 gene. Every individual copies (also called alleles) of the SERPINA1 gene. Each allele is equally responsible dominant) for producing its share of AAT protein. The normal SERPINA1 M and produces fully functional M AAT protein. If a person inherits 2 normal alleles, they produce enough AAT protein to protect their lungs and are having the “MM” phenotype.
Written by Tomás P. Carroll1,2, Ronan C. Heeney1,2, Geraldine Kelly2, Gerry McElvaney1
Author Affiliations: 1Irish Centre for Genetic Lung Disease, RCSI Education and Research Centre, Beaumont Hospital, Dublin 9. 2Alpha-1 Foundation Ireland - Correspondence: tcarroll@rcsi.ie
More than 200 alleles of the SERPINA1 gene exist resulting in a large variety variants of AAT. These mutations can impair the quantity or quality produced, or sometimes both. Common mutations include “I”, “S” and “Z”. have many different allele combinations resulting in various phenotypes such MZ, SZ, IZ, and ZZ. This gives rise to a spectrum of deficiency from moderate SZ) to severe (ZZ)(Figure 2)
Tomás P. Carroll
Alpha-1 Antitrypsin Deficiency (AATD) – Precision Medicine for COPD?
 Written by Tomás P. Carroll1,2, Ronan C. Heeney1,2, Geraldine Kelly McElvaney1
Written by Tomás P. Carroll1,2, Ronan C. Heeney1,2, Geraldine Kelly McElvaney1
Author Affiliations: 1Irish Centre for Genetic Lung Disease, RCSI Research Centre, Beaumont Hospital, Dublin 9. 2Alpha-1 Foundation Ireland
Correspondence: tcarroll@rcsi.ie

What is Alpha-1 Antitrypsin Deficiency?
What is Alpha-1 Antitrypsin Deficiency?
Alpha-1 antitrypsin deficiency (AATD) is a genetic condition that causes low serum levels of AAT. Deficiency allows proteases such as NE to cause irreversible damage to fragile lung tissue, a process dramatically accelerated by cigarette smoke. The lung tissue destruction manifests as emphysema or chronic obstructive pulmonary disease (COPD)(Figure 1).
Alpha-1 antitrypsin (AAT) is a protein produced primarily by the liver that is responsible for inhibiting neutrophil elastase (NE) as well as several other proteases AAT provides a protective screen for the lungs against proteases released by cigarette smoke and other inhaled irritants. These proteases can damage lung tissue if left uncontrolled AAT is an acute-phase protein and rises during infection and inflammation, a clue to its additional immuno-modulatory properties.
Alpha-1 antitrypsin (AAT) is a protein produced primarily by the liver that is responsible for inhibiting neutrophil elastase (NE) as well as several other proteases. AAT provides a protective screen for the lungs against proteases released from white blood cells activated by cigarette smoke and other inhaled irritants. These proteases can damage lung tissue if left uncontrolled. AAT is an acute-phase protein and rises during infection and inflammation, a clue to its additional immunomodulatory properties.
AAT protein production is controlled by the SERPINA1 gene. Every individual has 2 copies (also called alleles) of the SERPINA1 gene. Each allele is equally responsible (co-dominant) for producing its share of AAT protein. The normal SERPINA1


Figure 2 Average serum AAT concentrations (g/L) between the different phenotypes (data from the Irish National Targeted Detection Programme Normal range for AAT is 1.0 – 2.0 g/L (red dotted lines on graph).
AAT phenotypes are confirmed in the laboratory by the technique of isoelectric where the AAT variants are separated on agarose gel by an electrical current. protein stops on the gel and the resulting banding pattern it produces reveals protein “phenotype” unique to each combination of alleles (Figure 3)
Alpha-1 antitrypsin deficiency (AATD) is a genetic condition that causes low serum levels of AAT. Deficiency allows proteases such as NE to cause irreversible damage to fragile lung tissue, a process dramatically accelerated by cigarette smoke The lung tissue destruction manifests as emphysema or chronic obstructive pulmonary disease (COPD)(Figure 1).
allele is called M and produces fully functional M AAT protein. If a person inherits 2 normal SERPINA1 alleles, they produce enough AAT protein to protect their lungs and are referred to as having the “MM” phenotype. More than 200 alleles of the SERPINA1 gene exist resulting in a large variety of abnormal variants of AAT. These mutations can impair the quantity or quality of the protein produced, or sometimes both. Common mutations include “I”, “S” and “Z”. A person can have many different allele combinations resulting in various phenotypes such as MM, MS, MZ, SZ, IZ, and ZZ. This gives rise to a spectrum of deficiency from moderate (MZ and SZ) to severe (ZZ)(Figure 2).
AAT phenotypes are confirmed in the laboratory by the technique of isoelectric focusing where the AAT variants are separated on agarose gel by an electrical current. Where the protein stops on the gel and
the resulting banding pattern it produces reveals a specific protein “phenotype” unique to each combination of alleles (Figure 3). Further genetic analysis is sometimes required to determine the precise mutation causing a deficiency (known as the "genotype"), but this usually applies to rare or novel mutations. The further genetic testing takes the form of allele-specific genotyping for known mutations,
most severe by 80-85%)
26 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 1. The role of alpha-1 antitrypsin (AAT) in normal and AAT deficient individuals.
Figure 1. The role of alpha-1 antitrypsin (AAT) in normal and AAT deficient individuals
Figure 2. Average serum AAT concentrations (g/L) between the different AAT phenotypes (data from the Irish National Targeted Detection Programme for AATD). Normal range for AAT is 1.0 – 2.0 g/L
Figure 3. Isoelectric focusing technique used phenotypes are shown with ZZ the
Figure 3. Isoelectric focusing technique used to diagnose AATD. The 6 major AAT phenotypes are shown with ZZ the most severe AATD phenotype (serum AAT reduced by 80-85%)
or mild shortness of breath even in the absence of airflow obstruction and some may be minimally affected or asymptomatic. With regard smokers who have yet to develop significant respiratory disease, we would encourage testing for AATD given the high prevalence in the Irish population and the potential for significant risk factor modification in the form of rapid smoking cessation in affected individuals.
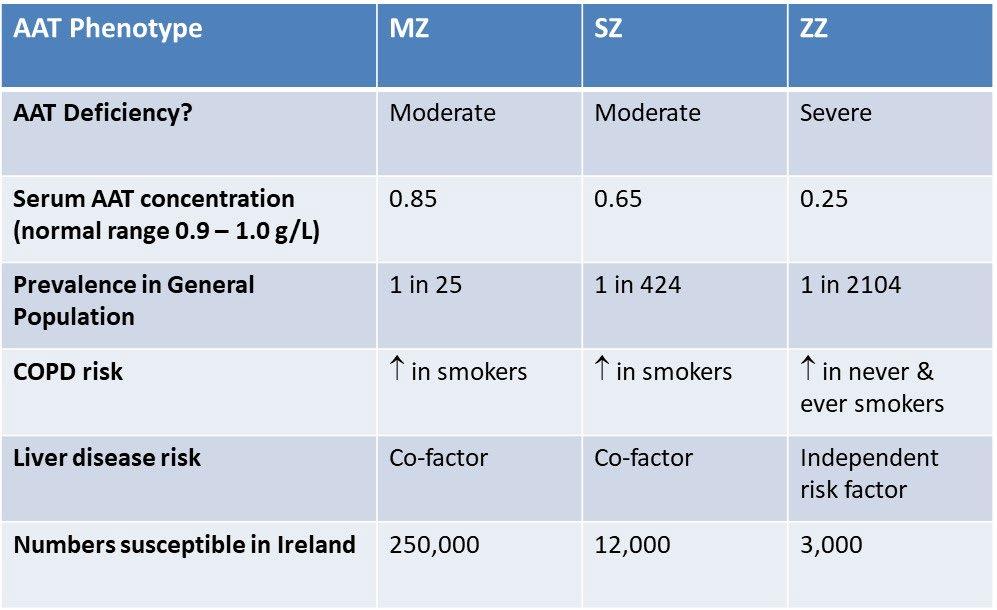
While AATD causes lung disease through causes lung disease through the absence of AAT, it causes liver disease through the gain of a toxic function. The Z mutation causes the AAT protein to misfold and adopt an abnormal shape, which results in hepatocyte accumulation. This can result in the development of hepatic steatosis, cirrhosis and hepatocellular carcinoma. This may ultimately progress to liver transplantation or death. The progression to cirrhosis is also accelerated by lifestyle factors such as alcohol consumption and is another reason for testing in order to guide lifestyle modification.
Clinical Manifestations of AATD
These are called “Q0” or “null” mutations. These mutations are named after the place of birth of the index or first case, examples include the novel Q0dublin and Q0cork mutations.
Clinical Manifestations of AATD
or gene sequencing for novel mutations. The full range of diagnostic techniques used to investigate AATD at the National Centre of Expertise for AATD at Beaumont Hospital are presented in Figure 4. Through the national targeted detection programme for AATD, several ultra-rare mutations have been found to exist in the Irish population that produce no detectable AAT in blood.
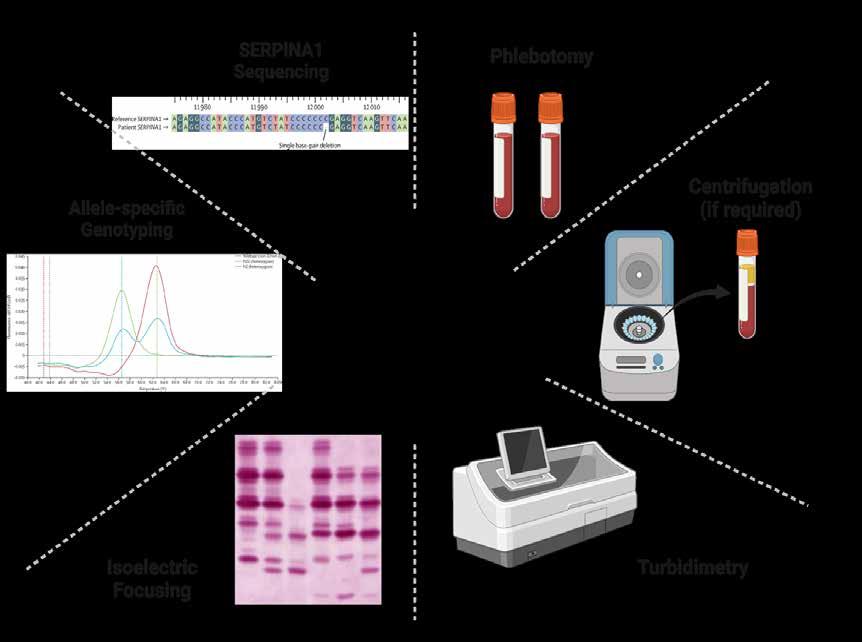
The risk of disease in AATD is influenced by the type of AATD (severe or moderate) in combination with lifestyle and occupational exposures (e.g. smoking)(Figure 5). Nevertheless, the organs most commonly affected by AATD are the lungs and the liver. The progression to
significant airflow obstruction in the form of COPD and emphysema is greatly accelerated in smokers. Patients with AATD also have bronchial hyper-responsiveness, which can mimic asthma. Patients can also present with bronchiectasis, which may manifest itself as recurrent respiratory tract infections. Given the wide variety of different phenotypes that exist; it should be noted that clinical expression is highly variable, even within individuals with the same phenotype. Lung symptoms can be as simple as a persistent cough
Rarer manifestations of AATD include a skin condition called panniculitis, which presents as inflammation of the subcutaneous fat. Erythema nodosum is a form of panniculitis and as such AATD should be screened for in patients presenting with erythema nodosum, especially necrotising erythema nodosum as it can be effectively treated with intravenous purified AAT. In fact, the contribution of AATD to panniculitis continues to be underrecognised. Panniculitis can be fatal and often occurs suddenly in otherwise healthy individuals. Importantly, AATD-related panniculitis can be effectively treated with intravenous infusions of plasma-purified AAT protein, and in some cases represents a treatment that is life-saving (Franciosi et al, 2015) when firstline treatment with dapsone fails. AATD is also linked to ANCAassociated vasculitis, likely due to the imbalance between the anti-protease effects of AAT and neutrophil-derived proteases. Patients presenting with ANCAassociated vasculitis should be screened for AATD.
to cirrhosis is also accelerated by lifestyle factors such as alcohol consumption and is another reason for testing in order to guide lifestyle modification
The Prevalence of AATD in Ireland
It is known that 1 in 25 individuals carry the Z mutation and that ~265,000 people have a clinically relevant phenotype of some description. Currently, over 22,000
The risk of disease in AATD is influenced by the type of AATD (severe or moderate) in combination with lifestyle and occupational exposures (e.g. smoking)(Figure 5). Nevertheless, the organs most commonly affected by AATD are the lungs and the liver. The progression to significant airflow obstruction in the form of COPD and emphysema is greatly accelerated in smokers Patients with AATD also have bronchial hyperresponsiveness, which can mimic asthma. Patients can also present with bronchiectasis, which may manifest itself as recurrent respiratory tract infections. Given the wide variety clinical expression is highly Lung symptoms can be as or mild shortness of breath even in the absence of airflow asymptomatic. With regard smokers , we would encourage testing for the potential for significant in the form of rapid smoking cessation in affected individuals. its absence (loss of function); it causes liver causes the AAT protein to in hepatocyte accumulation. This can result in the development of hepatic steatosis, cirrhosis and hepatocellular carcinoma. This may ultimately progress to liver transplantation or death The progression
27 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Figure 4. The various diagnostic approaches used on the path to a diagnosis of AATD.
Figure 4. The various diagnostic approaches used on the path to a diagnosis of AATD
Figure 5. Key facts about the three most common forms of AATD found in Ireland (MZ, SZ, and ZZ)
Figure 5. Key facts about the three most common forms of AATD found in Ireland (MZ, SZ, and ZZ)
Precision Medicine

o Adults with panniculitis.
o Siblings of those with AATD.
o Individuals with unexplained liver disease, including neonates and the elderly.
o Individuals with ANCAassociated vasculitis.
National Centre of Expertise for AATD
Are There Positives to a Diagnosis?
More information for healthcare professionals can be found on the Alpha-1 Foundation Ireland website (www.alpha1.ie). Getting tested for AATD is very simple and requires a single standard 3ml serum/plasma blood bottle. The sample is sent to the Alpha-1 Foundation Ireland laboratory at the RCSI Education and Research Centre at Beaumont Hospital. Testing is free as part of the HSEfunded national targeted detection programme. Of note, severe AATD can also be picked up by a common laboratory test called serum protein electrophoresis (or SPE). For a person with severe AATD, the alpha-1 globulin fraction will be visibly reduced and marked as abnormal.
If the patient is agreeable, they can be referred to the National Centre of Expertise for AATD at Beaumont Hospital. Here, patient investigations are tailored to look for diseases associated with AATD and patients are provided with education about their condition and their particular phenotype. Investigations include spirometry, oscillometry, and CT thorax to evaluate lung disease, in addition to abdominal ultrasound and transient elastography to evaluate liver disease. Patients may also be invited to take part in clinical trials investigating new treatments for both lung and liver disease.
patient referrals from anywhere in the island of Ireland with many patients travelling from northern Ireland. More details can be found on www.alpha1.ie.
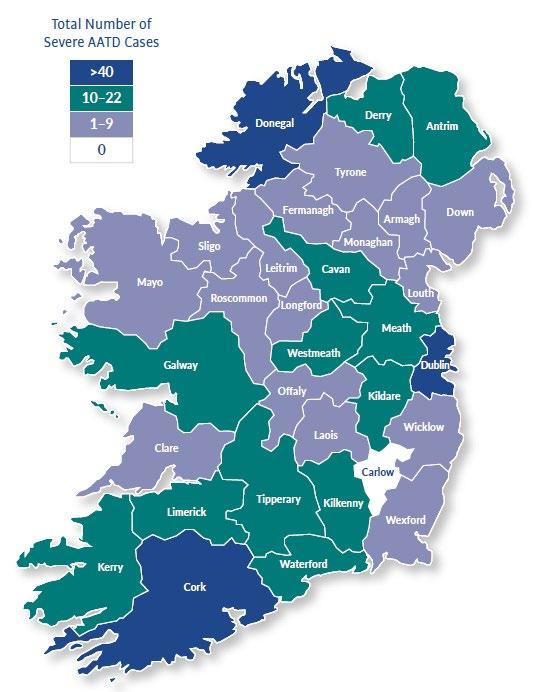
There are notable positive aspects to a diagnosis of AATD. These cannot be overstated, and most notably, individuals can avail of expert specialist care at the national AATD centre at Beaumont Hospital Knowing the degree of AATD can be a useful motivator for smoking cessation or avoidance, for consideration of workplace gases, fumes and dusts, and for other health promotion measures such as flu vaccination.
o Adults with asthma and airflow obstruction that is incompletely reversed by treatment.
So Who Should be Tested and How?
o Asymptomatic individuals with airflow obstruction on lung function testing.
Due to the genetic nature of the condition patients often enquire about what it means to have AATD, and the implications for family planning/children and firstdegree relatives. At the national centre, we can provide a more tailored discussion around these topics and explain the inheritance patterns of the condition. The centre is headed by Professor Gerry McElvaney, an international leader in the field of AATD. It should be noted that we accept patient referrals from anywhere in the island of Ireland with many patients travelling from northern Ireland. More details can be found on www.alpha1.ie.
We have recently shown that people with AATD are highly motivated to quit smoking. Our study of participants on the national AATD registry found that parental smoking was significantly associated with smoking and having a higher pack year history than AATD individuals without a parental smoking history. Therefore, individuals with AATD whose parents smoke are primed for a “double hit” increasing their risk of COPD due to inheritance of both genetic and social factors (increased likelihood of smoking)(Figure 7 This highlights the importance of targeted smoking cessation efforts in people with AATD not only to reduce lung disease risk in the individual, but to also reduce the likelihood of their children ever smoking and thereby mitigating the future burden of COPD While numerous AATD registries report a low percentage of ZZ active smokers, this was the first study of its kind to specifically evaluate smoking cessation in an AATD cohort and further highlights the behaviour modification benefits of intervening in this cohort.
people have been screened for AATD on the island of Ireland in a national targeted detection programme and approximately 5,000 have been found to carry an abnormal phenotype. Our screening efforts have identified over 450 out of the estimated 3,000 ZZ (most severely affected) AATD individuals who live with the condition on the island of Ireland (Figure 6). This is the tip of the iceberg, as there are an estimated 85% of individuals with ZZ AATD yet to be diagnosed. We rely on each individual physician’s clinical acumen to consider AATD part of the differential for diseases such as COPD, refractory asthma, liver fibrosis and liver cirrhosis, erythema nodosa and the vasculitides.
o Adults with emphysema or status).
If a patient is found to have low levels of AAT on screening (or by SPE); then their sample is automatically referred for confirmatory AAT phenotyping. Once the test is completed, a comprehensive report is sent back to the healthcare provider. The report explains the patient’s AAT level and AAT phenotype and details what disease that individual is likely to suffer in the presence/absence of risk factors (smoke/alcohol). Typically, the turnaround time for phenotyping is approximately 14 to 20 days and can be done sooner if there is a clinical emergency.
Are There Positives to a Diagnosis?
The criteria for screening in Ireland follow WHO and ATS/ERS recommendations2 which suggest the following groups be screened:
So Who Should be Tested and How?
The criteria for screening in Ireland follow WHO and ATS/ERS recommendations which suggest the following groups be screened:
o Adults with emphysema or COPD (regardless of age/ smoking status).
There are notable positive aspects to a diagnosis of AATD. Individuals can avail of expert specialist care at the national AATD centre at Beaumont Hospital. Knowing the
28 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
(Figure 6). This is the tip of the iceberg, as there are an estimated 85% of individuals with ZZ
AATD yet to be diagnosed We rely on each individual physician’s clinical acumen to consider AATD part of the differential for diseases such as COPD, refractory asthma, liver fibrosis and liver cirrhosis, erythema nodosa and the vasculitides.
Figure 6. The geographical distribution of severe AATD in Ireland to date.
Figure 6. The geographical distribution of severe AATD in Ireland to date
Figure 7. The concept of a “double-hit” in AATD families related to genetic and social risks
Equally important is the knowledge of AATD acquired by the wider family when a member diagnosed. Family screening is strongly recommended as it can identify additional at relatives who may also benefit from expert care and rapid smoking cessation (see Figure 8).
learning coping strategies and receiving peer to peer support. The conference also covers exciting new research and new treatment prospects in the field of AATD. Giving patients who live with AATD a better understanding of their condition and the supports available empowers them to make informed decisions about their health.
The National AATD Registry
by Alpha-1 Foundation Ireland team members, Ms. Geraldine Kelly, Ronan Heeney, and Dr. Tomás Carroll. The site contains a wealth of knowledge about AATD in general as well as upcoming events of interest to the AATD community. Of note, the global Alpha-1 conference will take place in April 2023 in Dublin. This is a great opportunity for patients and healthcare providers to learn more about AATD from leading researchers in the field.
The Future
Alpha-1 Foundation Ireland Resources
degree of AATD can be a useful motivator for smoking cessation or avoidance, for consideration of workplace gases, fumes and dusts, and for other health promotion measures such as flu vaccination.
The National AATD Registry
Equally important is the knowledge of AATD acquired by the wider family when a member is diagnosed. Family screening is strongly recommended as it can identify additional at risk relatives who may also benefit from expert care and rapid smoking cessation (see Figure 8).
When patients are seen in our national centre for expertise in Beaumont Hospital, they are given the opportunity to take part in the national AATD registry (www. alpha1.ie/who-we-are/nationalalpha-1-registry). This confidential registry seeks to improve the care of patients living with AATD and allows doctors involved in the care of patients with AATD an easy and rapid method of determining if a patient might benefit from taking part in new clinical trials testing new treatments. The registry allows the consolidation of relevant key results and parameters for patients with AATD in order to effectively track a patient’s clinical status. The registry has proved its value generating several important publications which have deepened our knowledge around AATD.
results and parameters for patients with AATD in order to effectively clinical status. The registry has proved its value generating several which have deepened our knowledge around AATD4,5.
national Alpha-1 patient conference is usually held each year that provides patients an invaluable insight into their condition through meeting other people affected by the condition. It gives patients an overview of what can be expected from living with the condition as well as learning coping strategies and receiving peer to peer support. The conference also covers exciting new research and new treatment prospects in the field of AATD. Giving patients who live with AATD a better understanding of their condition and supports available empowers them to make informed decisions about their health.
We have recently shown that people with AATD are highly motivated to quit smoking. Our study of participants on the national AATD registry found that parental smoking was significantly associated with smoking and having a higher pack year history than AATD individuals without a parental smoking history. Therefore, individuals with AATD whose parents smoke are primed for a “double hit” increasing their risk of COPD due to inheritance of both genetic and social factors (increased likelihood of smoking) (Figure 7). This highlights the importance of targeted smoking cessation efforts in people with AATD, not only to reduce lung disease risk in the individual, but to also reduce the likelihood of their children ever smoking and thereby mitigating the future burden of COPD. While numerous AATD registries report a low percentage of ZZ active smokers, this was the first study of its kind to specifically evaluate smoking cessation in an AATD cohort and further highlights the behaviour modification benefits of intervening in this cohort.
A national Alpha-1 patient conference is usually held each year that provides patients an invaluable insight into their condition through meeting other people affected by the condition. It gives patients an overview of what can be expected from living with the condition as well as
Alpha-1 Foundation Ireland Resources
The Alpha-1 Foundation Ireland website (www.alpha1.ie) has been a source of information for patients seeking guidance on their condition and was particularly valuable during the COVID-19 pandemic with a huge increase in site visitors. The website is hosted

When patients are seen in our national centre for expertise in Beaumont Hospital, they given the opportunity to take part in the national AATD registry (www.alpha1.ie/whoare/national-alpha-1-registry). This confidential registry seeks to improve the care of patients living with AATD and allows doctors involved in the care of patients with AATD easy and rapid method of determining if a patient might benefit from taking part in new clinical trials testing new treatments. The registry allows the consolidation of relevant key
In 2023, the only specific treatment for severe AATDrelated emphysema continues to be intravenous purified AAT. Unfortunately, this therapy is not widely available in Ireland and is not reimbursed by the government. Historically, the absence of a treatment has been an obstacle to more widespread testing for AATD. However, there are ongoing clinical trials taking place at the National Centre of Expertise for AATD. The trials are testing new medications that attempt to correct the misfolded AAT protein and allow it to be secreted from the liver to reach and protect the lungs. Patients with severe AATD could be eligible for trials of new medications that may one day potentially cure their condition. A recent groundbreaking study published in the New England Journal of Medicine described the first effective treatment for liver disease caused by severe AATD (Strnad et al, 2022). It is an exciting time with many other potential treatments for AATD in development.
The Alpha-1 Foundation Ireland website (www.alpha1.ie) has information for patients seeking guidance on their condition and was during the COVID-19 pandemic with a huge increase in site visitors by Alpha-1 Foundation Ireland team members, Ms. Geraldine Kelly Dr. Tomás Carroll. The site contains a wealth of knowledge about well as upcoming events of interest to the AATD community. Of note, conference will take place in April 2023 in Dublin. This is a great opportunity and healthcare providers to learn more about AATD from leading researchers
In the future it is envisaged that AATD screening could potentially be included in the new-born screening programme, which would equip affected individuals with a much earlier diagnosis, better knowledge, and more control over their own condition through risk factor avoidance. We are also working with the new respiratory integrated care hubs to raise awareness of AATD. Ultimately, it is only through increasing public and professional awareness that we can improve the lives of those living with AATD. People found to have AATD have a wealth of resources and available to them, but this only applies to the lucky minority who are correctly diagnosed. More work is needed to increase the detection of this common, yet rarely diagnosed condition with a modifiable outcome in terms of COPD risk.
References available on request
29 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Figure 8. Typical AATD family tree. ZZ (severe AATD), MZ (moderate AATD), and MM (unaffected) phenotypes are shown.
Figure 8. Typical AATD family tree. ZZ (severe AATD), MZ (moderate AATD), and MM (unaffected) phenotypes are shown
Figure 9. Alpha-1 Foundation Ireland core activities and services.
Figure 9. Alpha-1 Foundation Ireland core activities and services
for the right patient, at the right time
• Long-term safety and efficacy profile spanning 5 years in psoriasis (PsO) and psoriatic arthritis (PsA)1,2
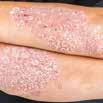
• Improved quality of life sustained up to 5 years1,2
• No laboratory prescreening or ongoing drug-specific monitoring1
• No label warning against use with live vaccines1
• 9-hour half-life, rapid clearance1
Is now the right time to move your patients on to OTEZLA?
OTEZLA® (apremilast) 10mg, 20mg and 30mg film coated-tablets
Brief Prescribing Information
Refer to the Summary of Product Characteristics (SPC) before prescribing
Further information is available upon request
Presentation: 10mg, 20mg and 30mg film coated-tablets.
Indications: Psoriatic arthritis: OTEZLA, alone or in combination with Disease Modifying Antirheumatic Drugs (DMARDs), is indicated for the treatment of active psoriatic arthritis (PsA) in adult patients who have had an inadequate response or who have been intolerant to a prior DMARD therapy. Psoriasis: OTEZLA is indicated for the treatment of moderate to severe chronic plaque psoriasis in adult patients who failed to respond to or who have a contraindication to, or are intolerant to other systemic therapy including ciclosporine, methotrexate or psoralen and ultraviolet-A light (PUVA).
Dosage and administration: Treatment with OTEZLA should be initiated by specialists experienced in the diagnosis and treatment of psoriasis or psoriatic arthritis. The recommended dose of OTEZLA is 30mg twice daily taken orally in the AM and PM, approximately 12 hours apart, with no food restrictions. The film-coated tablets should be swallowed whole. An initial dose titration is required per the following schedule: Day 1: 10mg in the AM; Day 2: 10mg in the AM and 10 mg in the PM; Day 3: 10mg in the AM and 20mg in the PM; Day 4: 20mg in the AM and 20mg in the PM; Day 5: 20mg in the AM and 30mg in the PM; Day 6 and thereafter: 30mg twice daily in the AM and PM. No re-titration is required after initial titration. If patients miss a dose, the next dose should be taken as soon as possible. If it is close to the time for their next dose, the missed dose should not be taken and the next dose should be taken at the regular time.
Patients with severe renal impairment: The dose of OTEZLA should be reduced to 30mg once daily in patients with severe renal impairment (creatinine clearance of less than 30mL per minute estimated by the Cockcroft-Gault equation). For initial dose titration in this group, it is recommended that OTEZLA is titrated using only the AM doses and the PM doses be skipped. Paediatric population: The safety and efficacy of OTEZLA in children aged 0 to 17 years have not been established. No data is available.
Contraindications: Hypersensitivity to the active substance(s) or to any of the excipients. OTEZLA is contraindicated in pregnancy. Pregnancy should be excluded before treatment can be initiated. Special warnings and precautions: Diarrhoea, nausea and vomiting: Severe diarrhoea, nausea, and vomiting associated with the use of OTEZLA have been reported. Most events occurred within the first few weeks of treatment. In some cases, patients were hospitalized. Patients 65 years of age or older may be at a higher risk of complications. Discontinuation of treatment may be necessary. Psychiatric disorders: OTEZLA is associated with
an increased risk of psychiatric disorders such as insomnia and depression. Instances of suicidal ideation and behaviour, including suicide, have been observed in patients with or without history of depression. The risks and benefits of starting or continuing treatment with OTEZLA should be carefully assessed if patients report previous or existing psychiatric symptoms or if concomitant treatment with other medicinal products likely to cause psychiatric events is intended. Patients and caregivers should be instructed to notify the prescriber of any changes in behaviour or mood and of any suicidal ideation. If patients suffered from new or worsening psychiatric symptoms, or suicidal ideation or suicidal attempt is identified, it is recommended to discontinue treatment with OTEZLA. Severe renal impairment: See dosage and administration section. Underweight patients: OTEZLA may cause weight loss. Patients who are underweight at the start of treatment should have their body weight monitored regularly. In the event of unexplained and clinically significant weight loss, these patients should be evaluated by a medical practitioner and discontinuation of treatment should be considered. Lactose content: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
Interactions: Co-administration of strong cytochrome P450 3A4 (CYP3A4) enzyme inducer, rifampicin, resulted in a reduction of systemic exposure of OTEZLA, which may result in a loss of efficacy of OTEZLA. Therefore, the use of strong CYP3A4 enzyme inducers (e.g. rifampicin, phenobarbital, carbamazepine, phenytoin and St. John’s Wort) with OTEZLA is not recommended.
In clinical studies, OTEZLA has been administered concomitantly with topical therapy (including corticosteroids, coal tar shampoo and salicylic acid scalp preparations) and UVB phototherapy.
OTEZLA can be co-administered with a potent CYP3A4 inhibitor such as ketoconazole, as well as with methotrexate in psoriatic arthritis patients and with oral contraceptives.


Pregnancy, lactation and fertility: Women of childbearing potential should use an effective method of contraception to prevent pregnancy during treatment. OTEZLA should not be used during breast-feeding. No fertility data is available in humans.
Undesirable effects: Psychiatric disorders: In clinical studies and post-marketing experience, uncommon cases of suicidal ideation and behaviour, were reported, while completed suicide was reported post-marketing.
The most commonly reported adverse reactions with OTEZLA in these indications are gastrointestinal (GI) disorders including diarrhoea (15.7%) and nausea (13.9%). These GI adverse reactions generally occurred within the first 2 weeks of treatment and usually resolved within 4 weeks.

Adverse reactions reported in the psoriatic arthritis and/or psoriasis clinical trial programme and post marketing experience
include: very common (≥1/10) diarrhoea*, nausea*; common (≥1/100 to <1/10) bronchitis, upper respiratory tract infection, nasopharyngitis*, decreased appetite*, insomnia, depression, migraine*, tension headache*, headache*, cough, vomiting*, dyspepsia, frequent bowel movements, upper abdominal pain*, gastroesophageal reflux disease, back pain*, fatigue; uncommon (≥1/1,000 to <1/100) hypersensitivity, suicidal ideation and behaviour, gastrointestinal haemorrhage, rash, urticaria, weight loss; not known (cannot be estimated from the available data) angioedema. *At least one of these adverse reactions was reported as serious. Please consult the SPC for a full description of undesirable events.
Pharmaceutical Precautions: Do not store above 30ºC. Legal category: POM. Presentation and Marketing Authorisation
Numbers: Initiation pack containing 27 film coated tablets (4 x 10mg, 4 x 20mg, 19 x 30mg) - EU/1/14/981/001; 30mg film coated tablets in a pack size of 56 tablets - EU/1/14/981/002. Marketing Authorisation Holder: Amgen Europe B.V. Minervum 7061, 4817 ZK Breda, The Netherlands. Further information is available from Amgen Ireland Limited, 21 Northwood Court, Santry, Dublin D09 TX31. OTEZLA is a trademark owned or licensed by Amgen Inc., its subsidiaries, or affiliates.
Date of preparation: April 2020 (Ref: IE-OTZ-2000019).
Adverse reactions/events should be reported to the Health Products Regulatory Authority (HPRA) using the available methods via www.hpra.ie. Adverse events should also be reported to Amgen Limited on +44 (0)1223 436441.
Abbreviations: PDE4, phosphodiesterase-4; PsA, psoriatic arthritis; PsO, psoriasis.
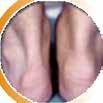
References: 1. OTEZLA (apremilast). Summary of Product Characteristics; 2. Kavanaugh A, et al. Arthritis Res Ther. 2019;21:118;
3. Augustin M, et al. J Eur Assoc Dermatol Venereol. 2021;35:123–134;




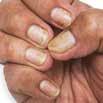

4. Wollenhaupt J, et al. Presented at EULAR 2020; 3–6 June 2020; Virtual: Poster FRI0365; 5. Crowley JA, et al. Presented at the 73rd Annual Meeting of the American Academy of Dermatology; 20–24 March 2015; San Francisco, CA: P894; 6. Rich P, et al. J Am Acad Dermatol. 2016;74(1):134–142; 7. Reich K, et al. Dermatol Ther. 2022;12:203–221.
© 2022 Amgen Inc. All rights reserved. Amgen Ireland Ltd., 21 Northwood Court, Santry, Dublin 9 IE-OTZ-0622-00004
Date of preparation:
August 2022
OTEZLA is an intracellular PDE4 inhibitor with demonstrated efficacy in high-impact areas, which can improve your patient’s quality of life1–7
OTEZLA is the simple oral choice for your adult patients with moderate to severe psoriasis or active psoriatic arthritis1
Palms Scalp Nail Itch Genital
PsO Plaque psoriasis
Enthesitis Dactylitis Nail involvement Skin psoriasis
Limited joint involvement
PsA Psoriatic arthritis
Images depict fictional patients.
CPD
60 Second Summary
In the real-world APPRECIATE study (NCT02740218), most patients with psoriasis demonstrated notable improvements on disease severity measures and reported clinically meaningful treatment benefits with apremilast.


We aim to further describe patientrelevant needs and benefits and patient satisfaction with apremilast, including subgroup analyses based on patient characteristics.
APPRECIATE, a multinational, retrospective, cross-sectional study, enrolled patients with chronic plaque psoriasis who started apremilast according to the European label. Patient Benefit Index (PBI; range 0 (no patient-relevant benefit) to 4 (maximum patient-relevant benefit), global PBI score ≥ 1 indicating minimum patient-relevant benefit and ≥ 3 indicating high benefit) and nine-item Treatment Satisfaction Questionnaire for Medication (TSQM-9; range 0–100) were assessed 6 (± 1) months after apremilast initiation and summarized descriptively. Relationships between global PBI and TSQM-9 assessments were analyzed by Pearson correlations. Of 480 enrolled patients, 347 (72.3%) had remained on apremilast at 6 (± 1) months; 90.9% (300/330) achieved global PBI score ≥ 1. Mean (standard deviation) global PBI score was 2.8 (1.2). Higher achievement of global PBI score ≥ 3 was observed in patients with no prior treatments (61.1% (22/36)) or prior phototherapy (64.6% (42/65)) versus prior conventional systemic (54.4% (100/184)) or biologic (38.6% (17/44)) treatment. Strong correlations were observed between the global PBI score and the TSQM-9 global satisfaction and effectiveness subscale scores. Patients continuing apremilast for 6 (± 1) months in APPRECIATE reported patient-relevant treatment benefits. Findings suggest that receiving apremilast earlier versus later in treatment management is consistent with greater improvements in patient-relevant treatment outcomes.
1Institute for Health Services Research in Dermatology and Nursing (IVDP), University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany - 2The Dermatology Centre, NIHR Manchester Biomedical Research Centre, Salford Royal Hospital, The University of Manchester, Manchester, UK - 3Department of Dermatology, Lausanne University Hospital CHUV and University of Lausanne, Lausanne, Switzerland - 4Department of Dermatology, Clinic Hietzing, Vienna, Austria - 5Unit of Dermatology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden - 6Department of Dermatology and Allergy, Technical University of Munich, Munich, Germany - 7Dermatologikum Hamburg, Hamburg, Germany - 8College of Biomedical and Health Sciences, Cardiff University, Cardiff, UK - 9Amgen Europe GmbH, Rotkreuz, Switzerland - 10The Dermatology Centre, NIHR Manchester Biomedical Research Centre, Salford Royal Hospital, The University of Manchester, Manchester, UK
1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN.
Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
Amgen® has no editorial oversight of the CPD programmes included in these modules

Real-World Experience of Patient-Relevant Benefits and Treatment Satisfaction with Apremilast in Patients with Psoriasis: An Analysis of the APPRECIATE Study
Plaque psoriasis is a long-term immune-mediated inflammatory condition that requires ongoing routine clinical care and treatment to address high disease burden, impaired daily functioning, and reduced health-related quality of life (HRQoL).1,2,3,4 In this complex disease, interactions between genetic and environmental factors contribute to inflammation and clinical symptoms.5, 6 Pruritus due to psoriasis can be intense and is a major determinant of quality of life impairment.7, 8 Psoriasis is also accompanied by other distressful symptoms, including skin lesions in highly visible special areas such as the scalp, nails, and palmoplantar areas, as well as other medical and psychological comorbidities.2, 3, 9,10,11 Clinical trials assess a treatment’s efficacy and safety, but patients who meet stringent inclusion and exclusion criteria in clinical trials may not reflect those in real-world clinical practice. Real-world studies can provide valuable insights into the use of psoriasis treatments in diverse types of patients encountered in clinical practice.12 The use of patient-reported outcomes has gained prominence among healthcare providers as they allow physicians to evaluate treatment effectiveness with regard to the patient’s functional status and HRQoL.13, 14 Understanding the patient’s perspective
can also provide insights into reasons for treatment adherence or nonadherence.15
Treatment effectiveness has been shown to strongly influence treatment satisfaction and may affect patients’ treatment preferences. However, studies of patients undergoing treatment for a range of conditions, including psoriasis, show that some patients report high treatment satisfaction despite a lack of treatment effectiveness based on physician- and patient-rated assessments.16,17,18,19 Other aspects of treatment such as safety, route of administration, and compatibility with lifestyle can also affect overall treatment satisfaction.18,19,20 Investigating the relationship between patient-relevant benefits and treatment satisfaction can help provide a more holistic approach to assessing the effectiveness of psoriasis treatments.20 Considering that 52.3% of patients with psoriasis reported being dissatisfied with their current treatment in the US National Psoriasis Foundations surveys,12 a better understanding of patient-relevant treatment benefits may support efforts to improve treatment satisfaction and effectiveness.
The APPRECIATE study (NCT02740218) was a
multinational, retrospective, cross-sectional study of patients with psoriasis who started treatment with apremilast, an oral phosphodiesterase 4 inhibitor, in real-world clinical practice.21 Apremilast is approved in Europe for the treatment of adult patients with moderate to severe chronic plaque psoriasis who failed to respond to, have a contraindication to, or are intolerant of other systemic therapy, including cyclosporine, methotrexate, or psoralen and ultraviolet A light (PUVA). In APPRECIATE, patients demonstrated notable mean improvements on assessments of disease severity (i.e., Psoriasis Area and Severity Index (PASI), psoriasis-involved body surface area (BSA) and HRQoL (i.e., Dermatology Life Quality Index (DLQI)) at 6 (± 1) months after apremilast initiation.21 The majority of patients in APPRECIATE also achieved the minimum patientrelevant benefit on the Patient Benefit Index (PBI; global PBI score ≥ 1) with ongoing apremilast treatment for 6 (± 1) months.21
The PBI is a validated patientreported outcomes measure for skin diseases that evaluates the benefits of treatment with respect to treatment-related patient needs.22, 23 The PBI was developed to enable assessment of patientrelevant benefits, taking into
31
AUTHORS: Written by Toni Maria Klein1, Christine Blome1, C. Elise Kleyn2, Curdin Conrad3, Paul G. Sator4, Mona Ståhle5, Kilian Eyerich6, Marc Alexander Radtke7, Christine Bundy8, Myriam Cordey9, Christopher E. M. Griffiths10 & Matthias Augustin1
CPD 96: PSORIASIS
Continuing Professional Development
CPD
Continuous Professional Development is supported by Amgen®
1. Data shown are based on patients who continued apremilast at 6 (± 1) months’ follow-up and answered the PBI (n = 330). Possible scores range from 0 (no benefit) to 4 (maximal benefit)
1. Data shown are based on patients who continued apremilast at 6 (± 1) months’ follow-up and answered the PBI (n 330). Possible scores range from 0 (no benefit) to 4 (maximal benefit)
2. PBI Patient Benefit Index, SD standard deviation
2. PBI Patient Benefit Index, SD standard deviation
3. *Each patient was allocated to only one prior treatment category (by hierarchical order as displayed) even if more than one prior psoriasis therapy had been documented. One patient received other treatment
4. †Patients may have received other conventional systemics (e.g., retinoids)
account each patient’s individual treatment preferences and goals.22 Here, we further describe patientrelevant needs and benefits in all patients and in subgroups based on clinically relevant patient characteristics. In addition, we analyze the association between patient-relevant benefits and patients’ treatment satisfaction.
METHODS
Study Design and Data Collection

APPRECIATE was a multinational, retrospective, observational, crosssectional study of patients with psoriasis treated with apremilast in real-world clinical settings in Europe (Fig. 1). This analysis was conducted using data from 87 study sites in six countries in Europe (Austria, Germany, Ireland, Sweden, Switzerland, and the UK). Data collection took place between May 2016 and July 2018. The study was approved by the relevant ethics committees and was conducted in accordance with the principles of the Declaration of Helsinki.21 Written informed consent was obtained from patients before study participation.
At the time of study inclusion (i.e., 6 (± 1) months after initiating apremilast treatment), patient demographics, medical history, comorbid conditions, psoriasis disease characteristics, and
adverse events were obtained retrospectively from medical records. All assessments were performed during routine clinical visits. Physicians and patients completed study-specific questionnaires designed to assess patient needs and treatment goals. Patients completed the PBI for skin diseases (PBI-S or PBI) and the nine-item Treatment Satisfaction Questionnaire for Medication (TSQM-9).
The PBI weighs treatment benefits in various domains based on their relative importance, as defined by the individual patients’ needs.22, 23 This measure consists of a patient needs questionnaire (PNQ) and patient benefit questionnaire (PBQ), both of which include the same 25 items (Supplementary Table 1).21, 23 The PNQ evaluates the relevance of the 25 items as treatment goals for the individual patient.22 The PBQ measures the extent to which the current therapy contributed to achieving each treatment goal.22 The range of possible scores on the PNQ and PBQ is 0 (no importance/benefit) to 4 (maximum importance/ benefit).22 The PNQ and PBQ are used to calculate the global PBI score, which is calculated as the mean of all PBQ items weighted by the relative importance of corresponding PNQ items for each patient.22 A global PBI score ≥ 1
is considered the minimum patient-relevant benefit.23 High benefit was defined as a global PBI score ≥ 3.21 Although the PNQ and PBQ are typically completed before and after treatment,22 both the PNQ and PBQ were completed by the patient at study inclusion (i.e., 6 (± 1) months after apremilast initiation) owing to the retrospective study design of APPRECIATE.
The TSQM-9 is a validated nine-item instrument that includes an effectiveness scale, a convenience scale, and a global satisfaction scale. Scores on each scale range from 0 to 100, with higher scores indicating greater treatment satisfaction.24
Statistical Analyses
All analyses were descriptive for all endpoints and used pooled data from the six European countries participating in APPRECIATE. Data are reported as observed without imputations for missing values (i.e., patients with missing data were excluded). Subgroup analyses of global PBI scores by age group (< 35 years, 35–65 years, > 65 years), sex (male, female), psoriasis in special areas (nail, scalp, palmoplantar, or none), and number of prior systemic treatments or phototherapies (0, 1, 2, or > 2) were conducted. Additional subgroup analyses
evaluated global PBI scores by type of prior treatment (i.e., patients were allocated to one category in the following hierarchical ranking order: no prior treatment, phototherapy (but no other treatment), conventional systemic (may have had phototherapy but no other systemics), biologic (may have had phototherapy and/or conventional systemics)); by previous conventional systemic treatment (i.e., patients who received prior methotrexate but not fumarates, patients who received prior fumarates but not methotrexate, or patients who previously received both methotrexate and fumarates); by presence or absence of symptoms of psoriasis or psoriasis in special areas (i.e., pruritus or scalp, nail, or palmoplantar involvement); by age at psoriasis diagnosis (≤ 40 years, > 40 years); and by presence or absence of psoriatic arthritis (PsA). The top needs (responses of ‘quite’ and ‘very’ on the PNQ) and responses indicating at least a moderate benefit for the top needs after apremilast treatment (responses of ‘moderately’, ‘quite’, or ‘very’ on the PBQ) were evaluated in patients continuing apremilast treatment at 6 (± 1) months and in a post hoc subgroup analysis of patients with and without PsA. Preplanned correlation analyses using Pearson’s correlation
32 CPD 96: PSORIASIS
1
Table
Global PBI score 330 2.8 (1.2) Global PBI
0 36 3.0 (1.1) 1 143 2.8 (1.1) 2 84 2.8 (1.2) > 2 67 2.5 (1.3) Global PBI score by type of prior treatment* None 36 3.0 (1.1) Phototherapy 65 3.0 (1.0) Conventional systemics 184 2.8 (1.2) Biologics 44 2.4 (1.3) Global PBI score by prior treatment Methotrexate without fumarates† 93 2.7 (1.2) Fumarates without methotrexate 38 2.8 (1.2) Both methotrexate and fumarates† 16 2.6 (1.2)
score by number of prior systemic treatments or phototherapies
Continuous Professional Development is supported by Amgen®
coefficients were performed to evaluate the association between the global PBI score and TSQM-9 global satisfaction score, TSQM-9 effectiveness score, and TSQM-9 convenience score.
RESULTS
Patients
Of the 605 patients who were contacted about participating, 484 agreed to participate and enrolled in the study;21 four patients were later determined to be ineligible and were excluded from analyses (Fig. 2). A total of 124/480 (25.8%) patients in the full analysis population had PsA, 64 (51.6%) of whom had a rheumatologistconfirmed diagnosis. A total of 347/480 (72.3%) patients were continuing treatment with apremilast at the 6-month assessment (Fig. 2). Among patients continuing apremilast with a PBI score available for analysis, 36/330 (10.9%) patients had no prior treatment, 65/330 (19.7%) had prior phototherapy, 184/330 (55.8%) had prior conventional systemic treatment, and 44/330 (13.3%) had prior biologic treatment, as assigned to a single category by hierarchical order (i.e., in the order presented above; Fig.
2). One patient (0.2%) received other prior treatment.
Demographics and disease characteristics for the 480 patients in the study population have been previously reported.21 Briefly, mean age was 51.3 (standard deviation (SD) = 15.2) years, and 53.8% (n = 258) of patients were male. The mean time since psoriasis diagnosis was 18.6 (SD = 14.3) years. At apremilast initiation, mean psoriasis-involved BSA was 25.4% (SD = 23.5%, n = 141), mean PASI score was 12.5 (SD = 8.4, n = 350), and mean DLQI score was 13.4 (SD = 7.5, n = 205).
PBI Analyses
There were 451/480 patients (94.0%) with a PBI score available for analysis. The mean global PBI score was 2.4 (SD = 1.4) among all patients and 2.8 (SD = 1.2) among patients continuing apremilast at 6 (± 1) months (Table 1).21 In patients continuing apremilast treatment, 52.4% (182/347) reported a high treatment benefit (global PBI score ≥ 3). Among patients continuing apremilast treatment with a PBI score available for analysis (n = 330), types of prior treatments included: biologics (n = 44), including adalimumab
(22/44, 50.0%), etanercept (15/44, 34.1%), ustekinumab (9/44, 20.5%), secukinumab (9/44, 20.5%), infliximab (6/44, 13.6%), efalizumab (3/44, 6.8%), ixekizumab (2/44, 4.5%), tildrakizumab (1/44, 2.3%), and certolizumab (1/44, 2.3%); conventional systemics (n = 184), including methotrexate (109/184, 59.2%), fumaric acid (54/184, 29.3%), acitretin (34/184, 18.5%), cyclosporine (23/184, 12.5%), retinoids (14/184, 7.6%), glucocorticoids (9/184, 4.9%), leflunomide (2/184, 1.1%), and steroids (1/184, 0.5%); and phototherapy (n = 65), including narrow band UVB (30/65, 46.2%), UVA/UVB (n = 22/65, 33.8%), PUVA (13/65, 20.0%), and one (1.5%) unknown.

The top ten patient treatment needs, as identified in the PNQ, are presented in Fig. 3 for patients with non-missing data. More than 90% of patients responded that ‘regain control of the disease’, ‘have confidence in the therapy’, ‘get better skin quickly’, and ‘be healed of all skin defects’ were quite or very important needs. More than 48% of patients reported a benefit (answers of quite or very) on the PBQ for all of the top ten treatment needs; seven
of the top ten benefits overall corresponded to the top ten needs identified in the PNQ.
Similar mean global PBI scores were observed in patients without (2.3 (SD = 1.5)) versus those with (2.4 (SD = 1.4)) ≥ 1 symptom of psoriasis or psoriasis in special areas (i.e., pruritus or scalp, nail, or palmoplantar involvement).
In a subgroup analysis of global PBI scores based on number of prior systemic treatments, mean global PBI scores generally decreased as number of prior systemic treatments for psoriasis increased, and the lowest mean PBI scores were observed for patients who had prior biologic treatment (Table 1). In patients with prior conventional systemic treatment, mean PBI scores were generally similar among patients whose prior treatment included methotrexate without fumarates, fumarates without methotrexate, or both methotrexate and fumarates (Table 1). The proportion of patients reporting a high benefit (global PBI score ≥ 3) was greater in patients who had received fewer prior treatments versus those with more prior treatments; in patients who had received prior treatment with phototherapy versus conventional systemics and biologics; and in patients who had prior treatment with fumarates but not methotrexate versus prior methotrexate but not fumarates or prior treatment with both fumarates and methotrexate.
Mean global PBI scores at followup were similar in patients with versus without comorbid PsA (2.8 (SD = 1.0) versus 2.8 (SD = 1.2), respectively). Patients with and without comorbid PsA shared eight of ten top treatment needs based on the PNQ. The two treatment goals of ‘to have fewer side effects’ and ‘to be free of pain’ were identified as high needs only in patients with comorbid PsA (83.9% (99/118) and 82.2% (97/118), respectively), and the two treatment goals of ‘need less time for daily treatment’ and ‘be able to engage in normal leisure activities’ were identified as high needs only in patients without comorbid PsA (81.2% (233/287) and 77.9% (225/289), respectively. Most patients with and without PsA reported a high benefit on all of their ten top treatment needs.
33
Continuous Professional Development is supported by Amgen®
Figure 2
Similar mean global PBI scores were observed in subgroups of patients based on age (< 35 years: 2.3 (SD = 1.4); 35–65 years: 2.4 (SD = 1.4); > 65 years: 2.5 (SD = 1.3)), sex (male: 2.3 (SD = 1.4); female: 2.4 (SD = 1.4)), and age at psoriasis diagnosis (≤ 40 years: 2.3 (SD = 1.4); > 40 years: 2.5 (SD = 1.3)). The proportions of patients reporting a high benefit of apremilast treatment (global PBI score ≥ 3) were greater in older patients (> 65 years: 47.1% (49/104)) versus younger patients (< 35 years: 40.5% (32/79); 35–65 years: 40.7% (121/297)) and similar in subgroups based on sex (male: 42.2% (109/258); female: 41.9% (93/222)) and age at psoriasis diagnosis (≤ 40 years: 41.5% (136/328); > 40 years: 43.5% (64/147)).
Association Between Global PBI Scores and Ratings of Apremilast Treatment Satisfaction and Effectiveness
Mean TSQM subscale scores were 59.8 (SD = 28.9; n = 455) for the effectiveness subscale, 82.4 (SD = 18.0; n = 462) for the convenience subscale, and 58.2 (SD = 31.2; n = 462) for the global satisfaction subscale. Strong positive associations were observed between the global PBI score and the global satisfaction and effectiveness TSQM-9 subscale scores, respectively (Fig. 6). A weak positive correlation was found between global PBI score and the convenience subscale of the TSQM-9.

Discussion
Patient-reported outcome measures can provide valuable assessments of treatment benefits that are important for patients’ lives.13, 22 Because some factors that contribute to overall disease burden, such as pruritus and psoriasis in special areas, are not fully captured by traditional clinical measures of disease severity, patientreported outcome measures can help physicians to obtain a broader evaluation of treatment effectiveness.13, 14, 25, 26 The PBI is an assessment of patient-relevant treatment
benefits validated for use with psoriasis patients.22, 23 In the APPRECIATE study, apremilast demonstrated improvements in disease severity measures at 6 (± 1) months after apremilast initiation.21 Results from the current analysis of patient-reported outcomes from APPRECIATE suggest that patients continuing apremilast treatment at 6 (± 1) months experienced treatment benefits, particularly patients with no prior treatments and those without prior conventional systemic or biologic treatment. The proportion of patients who reported a high benefit (defined as global PBI score ≥ 3) with 6 (± 1) months of apremilast treatment was highest among patients who had been previously treated with phototherapy or who had not been previously treated with any other systemic psoriasis treatment. Our findings suggest that receiving apremilast treatment earlier rather than later in treatment management is consistent with greater improvements in patient-relevant treatment outcomes. This might be due to increased benefit of apremilast in this patient group. Based on patients’ responses to the PBI, the treatment needs of psoriasis patients in APPRECIATE extended beyond skin improvement and encompassed other physical, psychological, and other patientrelevant benefits associated with apremilast treatment. Many of the top ten needs identified in the APPRECIATE study were consistent with the top needs identified by psoriasis patients in a validation study of the PBI,22 as well as analyses of PBI data from large psoriasis registries in Europe (the German PsoBest registry and the national psoriasis registry of Switzerland).27, 28 Our findings on the PNQ and PBQ items in the APPRECIATE study revealed that most patients continuing apremilast treatment for 6 (± 1) months (72.3% of enrolled patients) reported treatment benefits on the top patient-reported key treatment needs. APPRECIATE patients with comorbid PsA shared most of the same treatment needs and had similar benefit responses as patients
without PsA and the overall study population; however, being free of pain and having fewer side effects were top treatment needs in patients with PsA but not in patients without PsA. In the APPRECIATE study, patient ratings of global satisfaction and treatment effectiveness of apremilast were strongly correlated with the global PBI score, thus confirming that the PBI measure can provide insights into psoriasis treatment effectiveness that are relevant to patients and physicians. In addition, a small group of patients had a high global PBI score but low satisfaction with treatment effectiveness, suggesting that patients also consider factors other than treatment effectiveness when assessing their overall satisfaction with apremilast treatment. In support of this concept, a systematic review that evaluated treatment preferences and satisfaction among psoriasis patients found that some patients rated attributes associated with the treatment process (such as access or mode of delivery) as more important than efficacy outcomes.20 In APPRECIATE, patient ratings of the convenience of apremilast treatment only weakly correlated with patient assessments of apremilast benefits, and this weak correlation may reflect that ratings of treatment benefit may not be based on treatment convenience.
Some limitations of this study include those inherent to retrospective analyses such as recall bias and data that are missing or not recorded based on chart review. Analyses including patients who continued treatment may not be representative of all patients who started or stopped treatment; the benefit of apremilast may differ in the overall population of study patients who started apremilast treatment, and data have shown that patients in APPRECIATE who discontinued apremilast treatment had lower global PBI scores and TSQM-9 subscale scores than those who continued treatment.21 Also, confounding was possible in the prior treatment subgroups because patients who had greater numbers of prior treatments may have been more likely to have had prior biologic treatment. As the prior treatment subgroups were not randomized, some characteristics may have been unbalanced between the subgroups. The number of prior treatments may have been related to disease severity or may have influenced the patient’s ability to assess treatment benefits, and these considerations may limit the interpretation of study findings. Also, findings should be interpreted with caution owing to the small sample sizes in some of the subgroups. In addition, because there were no predefined criteria to screen and enroll patients with comorbid PsA, patients with comorbid PsA in this study may not be representative of the general population of patients with psoriasis and comorbid PsA. References available on request

34 CPD 96: PSORIASIS
3 Continuous Professional Development is supported by Amgen®
Figure
Cancer Report launched at St Vincent’s
St Vincent’s University Hospital has published its Cancer Report 2020/2021 on behalf of the St Vincent’s Hospital Group (SVHG). SVHG encompasses St. Vincent’s Private Hospital (SVPH), St. Vincent’s University Hospital (SVUH) and St Michael’s Hospital (SMH) supported in teaching and research by University College Dublin (UCD). The report details statistics with regard to cancer patient numbers during 2020/2021 and outlines key updates relating to the diagnosis, treatment, care and research of cancer at the Hospital Group.
St. Vincent’s University Hospital (SVUH) is a centre of excellence for cancer care, one of eight nationally designated adult cancer centres and is the nationally designated centre for neuroendocrine tumours (NET), pancreatic cancer and sarcoma. The hospital is also one of the largest providers of care for patients with breast, prostate, colorectal, lung and gynae cancer and is one of three adult stem cell transplant centres in the country.
SVUH has a long tradition of cancer research. The recent Cancer Clinical Trial Research Infrastructure Funding award to the UCD Clinical Research Centre, by the Health Research Board, will enhance and expand cancer research at the hospital.
In 2021, in order to support the delivery of the SVUH Strategic Cancer Plan and OECI accreditation, the management of cancer services was re-organised into a Cancer Directorate. The Cancer Centre Directorate is responsible for the delivery of the Cancer Strategic Plan, the Strategic Cancer Research Plan and progressing the OECI Cancer Centre Accreditation programme. From an operational perspective, this streamlines the management of cancer services and enables the hospital to better manage the increasing levels of activity, patient complexity and acuity that is forecast, primarily driven by Ireland’s growing and aging population.
Speaking at the launch, Dr. David Fennelly, Clinical Director for Cancer Care said, “I am delighted to launch the annual Cancer Report on behalf of The SVHG Cancer Directorate. It is the first step on the path to Cancer Centre Accreditation for the St. Vincent Hospital Group.”
A summary of the main findings in the St. Vincent’s Healthcare Group (SVHG) 2020/2021 Cancer Report are as follows:
Despite COVID-19 restrictions, the hospital diagnosed 4,754 patients in 2020 and 5,623 in 2021 (compared with 4,779 patients in 2019).
The number of cancer cases in 2020 was marginally lower than 2019. Driven primarily by the March/April 2020 lockdown, which saw fewer patients attending diagnostic services at the hospital, particularly the Rapid Access Clinics for breast, prostate and lung cancers. Attendances and diagnosis picked up significantly in
2021, with diagnosed cancers up over 18% on 2019 levels and the number of patients discussed at multidisciplinary meetings up over 9% on 2020.
Skin Cancer accounted for approximately half of all cancers diagnosed at the hospital in 2021, with breast, colorectal, lung and urological cancers accounting for another 25%.

5,669 patient referrals were received in the symptomatic breast service in 2021, 44% of which are triaged as urgent and 56% as non-urgent.
Similar patterns were observed in the rapid access prostate clinic. Prostate RAC attendances in 2020 were 72% of 2019 levels. The Lung RAC attendances were up 8% to 436 in 2020 compared to 401 attendances in 2019. In 2021, 116 prostate cancers and 203 lung cancers were diagnosed through St. Vincent’s University Hospitals Rapid Access Clinics.
Professor of Medical Oncology Appointment
RCSI University of Medicine and Health Sciences has announced the appointment of Professor Jarushka Naidoo as Academic Professor of Medical Oncology in the RCSI Department of Medicine.

Professor Naidoo will lead RCSI’s innovative medical cancer research team to further its growth and development, and continue its work to secure funding for vital international cancer research.
In her career to date, Professor Naidoo has developed and led a portfolio of clinical trials and translational studies in lung cancer, immunotherapy and immune-related toxicity. She is an established leader in these fields with over one hundred articles published in publications such as New England Journal of Medicine, Journal of Clinical Oncology and JAMA Oncology.
Key to Professor Naidoo’s role will be to grow the clinical and translational research focus of lung cancer and immunotherapy at RCSI. She will do this by building clinical and research teams to
advance cancer clinical trials, clinical research, and governance.
Overall, she will enhance the number of high-quality cancer clinical trials at these centres, and translate research findings into new treatments and trials that will improve patient care and outcomes.
New treatments
Professor Naidoo is a Consultant Medical Oncologist at Beaumont Hospital and was previously an honorary clinical professor at RCSI. She is Chair of the Lung DiseaseSpecific Subgroup of Cancer Trials Ireland, a role she took up in 2020 after returning to Ireland from John Hopkins University in the United States where she worked as Assistant Professor of Oncology. She also founded the national research group, the Irish Lung Cancer Alliance.
Professor Naidoo commented on her appointment: "Advancing clinical trials for new targeted cancer therapies is critical to improving quality of life and outcomes for people with cancer. Collaboration amongst experts in cancer research is vital to ensure that every resource possible is used to make discoveries and introduce new treatments that
will have lifelong impacts on population health.
“In my new role as Academic Professor of Medical Oncology at RCSI, I look forward to building collaborations between clinicians and scientists that will impact on research and ultimately increase access to clinical trials for patients worldwide.”
HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023 35 ONCOLOGY: NEWS
Professor Jarushka Naidoo, Academic Professor of Medical Oncology in the RCSI Department of Medicine
Targeted Radionuclide therapy in advanced prostate cancer
Written by: Professor Joe M. O’Sullivan MD FRCPI FFRRCSI FRCR Chair of Radiation Oncology, Queen’s University Belfast Consultant Clinical Oncologist, Northern Ireland Cancer Centre, Belfast

prostate cancer (mCRPC).2

The ambitious dose escalation scheme was facilitated by autologous peripheral blood stem cell transfusion. In an initial phase 1 dose escalation trial, we established a safe and tolerable administered activity of 5000MBq. Stem cells were mobilized using gCSF. We demonstrated excellent tolerability of the therapy and encouraging evidence of efficacy however the overall cost and resource usage of the procedure were ultimately prohibitive to further development.
Introduction
Ionising radiation has been used as an effective cancer therapy for over 100 years. With the primary target of cancer cell DNA, most radiation therapy is delivered by linear accelerators in the form of external beam radiotherapy. An alternative way of administering therapeutic radiation to patients is to intravenously inject certain radioactive chemicals, known as radionuclides which can, by various means, gain close access to cancer cells and deliver ionising radiation at a molecular level as part of the radioactive decay process. Radionuclide therapy has been important in the treatment of metastatic prostate cancer for over 30 years, and a recently approved therapy, Lutetium-177- PSMA617 (PLUVITCO ™, Novartis) has heralded a new and exciting era for the field.
Early Days of Radionuclide Therapy in prostate cancer
I first encountered the use of radionuclide therapy in advanced prostate cancer as a trainee radiation oncologist in St. Luke’s Hospital in Dublin back in the mid-1990’s. Patients with metastatic prostate cancer, no longer responding to castration therapy (the only life-prolonging systemic therapy available at the time), with severe uncontrolled bone pain, were referred for treatment with Strontium-89. It was my job as one of the trainee oncologists to assess the patient and then to inject the therapy as an intravenous bolus injection
using a lead covered syringe. It is tough to think back on those men with advanced prostate cancer in severe pain, and without access to the multiple life-prolonging therapies now available to use in current practice.
Strontium-89 was the 1st radionuclide licensed for use in advanced prostate cancer. Strontium has very similar properties to calcium, and when injected intravenously as Strontium-89 Chloride, it will (like calcium) preferentially accumulate in mineral bone. This is useful in prostate cancer where bone is the dominant metastatic site, and the phenotype of the majority of bone metastases is ‘bone-forming’ or osteoblastic. These over-active areas of mineral bone adjacent to the metastatic deposits in the bone marrow ‘suck in’ the injected Strontium-89 which becomes incorporated into the hydroxyapatite of the mineral bone and allowing the delivery of ionising radiation.
In the case of Strontium-89, the radiation is in the form of betaparticles. These tiny ionising particles are similar to electrons and can travel over a radius of approximately 1-2 mm causing the formation of free radicals which in turn interact with cellular DNA. Around the same time as the emergence of Strontium-89 as a therapy in advanced prostate cancer, another beta-emitting radionuclide, Samarium-153 EDTMP was licensed. This radioisotope was not inherently bone-targeting but was made
so by conjugation with a bisphosphonate (EDTMP).
Both Strontium-89 and Samarium-153 EDTMP were tested in multiple clinical trials in metastatic prostate cancer during the 1990’s. Most of these trials included relatively small numbers of patients with pain control/pain response as the primary endpoint. Prospective studies demonstrated a pain response rate in the order of 60-70%. It uncertain whether these therapies resulted in any meaningful alteration in the course of disease in these men. In particular, there was never any evidence of an overall survival benefit resulting from the use of these therapies, although none of the studies were properly powered to make that assessment.1
Increasing the efficacy of Radionuclide therapy
During the early 2000’s there was considerable interest in enhancing the efficacy of radionuclide therapy in prostate cancer. Trials of Strontium-89 or Samarium-153 EDTMP combined with chemotherapy were conducted without much improvement in outcomes. The major challenge with dose intensification of radionuclide therapy is bone marrow toxicity. While working at Royal Marsden Hospital/Institute of Cancer Research I led a clinical research programme exploring the potential of dose escalation of Rhenium-186 HEDP (a beta-emitting boneseeking radionuclide) in men with metastatic castration resistant
In 2004, I was appointed to Queen’s University Belfast and continued my radionuclide research at the Northern Ireland Cancer Centre, Belfast. I developed a phase 1 trial explore the potential for combining repeated doses of Rhenium-186 HEDP with Docetaxel chemotherapy (The TAXIUM Trial) The study established a safe schedule of repeated Rhenium-186 HEDP combined with Docetaxel. I then developed a randomised phase 2 trial (The TAXIUM 2 Trial) which compared standard therapy with Cabazitaxel Chemotherapy and repeated Rhenium-188 HEDP. While well tolerated, the combination was not superior to Cabazitaxel alone.3
Radium-223
The real game changer in the field of radionuclide therapy in advanced prostate cancer came in the form of Radium-223, developed in Oslo by Algeta and subsequently acquired by Bayer. Radium-223 is like Strontium-89, a calcium mimetic. The big difference is that Radium-223 decays by emitting Alpha particles (2 protons + 2 neutrons). These relatively massive (at an atomic level) entities cause massive DNA damage over a very short range (~100 microns) in tissue. The advantage of this short range of range of ionising radiation is the relative protection of the bone marrow and the potential to deliver the therapy in cycles akin to chemotherapy. The phase 3 ALSYMPCA trial demonstrated an overall survival advantage in metastatic castration resistant prostate cancer (mCRPC) over standard of care for 6 cycles of Radium-223 (XOFIGO ™)
36 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: PROSTATE CANCER
and the radionuclide became available for clinical use in 2013.4 Radium-223 has been shown to be well tolerated in men with mCRPC and continues to be an important treatment option for men with castration resistant prostate cancer metastatic to bone.
Following the success of Radium-223 in mCRPC, I developed a phase 1/2 clinical trial which explored the role of Radium-223 in castration sensitive prostate cancer in combination with Androgen deprivation therapy, Docetaxel and external beam radiotherapy to the prostate and pelvic nodes. The ADRRAD trial finished recruitment in 2019 and published in 2021 establishing the safety and feasibility of this schedule. Several lines of parallel, translational science investigations from this study are now bearing fruit.6
Targeting PSMA

One of the major challenges with Radium-223 is that it only targets
bone. While bone is the dominant site of spread in advanced prostate cancer, metastases are also commonly found in liver, lung and brain as well as lymph nodes. A new target was needed and a protein named Prostate Surface Membrane Antigen (PSMA), highly expressed in advanced prostate cancer, has proven to be an excellent candidate. The position of the PSMA molecule (Figure 1)5 on prostate cells depends on the state of malignant transformation, being localized to the cytoplasmic and apical side of the prostate epithelium in benign cells and as malignancy evolves, PSMA moves from the cytoplasm to the luminal surface of the prostatic ducts, allowing the presentation of a large extracellular domain which is amenable to ligand binding. The normal role of PSMA is as yet uncertain however it appears likely to have a transport function across the cell membrane as PSMA ligands are internalized through endocytosis. One very important
feature of the PSMA molecule is that expression generally increases with Gleason score, and as the patient progresses to metastatic castration-resistant prostate cancer (mCRPC). PSMA expression is not confined to prostate cells and can also in salivary glands, duodenal mucosa, proximal renal tubular cells, and neuroendocrine cells in the colon.
The PSMA molecule was first targeted by PET tracers enabling its use as a imaging tool with significantly improved sensitivity and specificity over conventional imaging such as isotope bone scan, CT, and MRI. Combining a therapeutic radionuclide (for example Lutetium-177) with a PSMA avid ligand (e.g. PSMA617, a small molecule inhibitor of PSMA) opened the possibility of delivering ionising radiation directly to prostate cancer cells. This type of therapeutic approach is known
as a radioligand therapy (RLT).
Recent data on demonstrating overall survival benefit in mCRPC means that this agent will the 1st PSMA targeted radioligand available in the clinic.
177Lu-PSMA-617 (PLUVITCO ™)
Results of large international, industry sponsored randomised trial testing the benefit of 177LuPSMA-617, (The VISION Trial), have recently been published.7 In this trial, men with mCRPC who had prior therapy with at least one androgen-receptor–pathway inhibitor (e.g. Abiraterone, Enzalutamide) and (unless they were ineligible) at least one taxane (e.g. Docetaxel, Cabazitaxel) and with PSMA-positive metastases visible on a PSMA PET-CT scan (gallium-68 (68Ga)–labeled PSMA11) were randomly assigned
37 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023 PSMA molecule [5]
Figure 1, PSMA molecule5
in a 2:1 ratio to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks for four to six cycles) plus standard care (e.g. hormone therapy, local radiotherapy), or standard care alone. Standard care could not include cytotoxic chemotherapy, immunotherapy or other radionuclides including Radium-223.
In total, 831 patients were randomised. With a median follow-up of 20.9 months, patients randomised to receive 177Lu-PSMA-617 plus standard care were shown to have significantly prolonged radiographic progression free survival compared to the control arm (median, 8.7 vs. 3.4 months; hazard ratio for progression or death, 0.40, P<0.001) and overall survival (median, 15.3 vs. 11.3 months; hazard ratio for death, 0.62; P<0.001) (Figure 2). All the key secondary end points were significantly in favour of 177Lu-PSMA-617. From the toxicity perspective, there were significantly more grade 3 or above adverse events (largely haematological) seen with 177Lu-

PSMA-617 than in the control arm (52.7% vs. 38.0%), however quality of life did not appear to be adversely affected.
This study recruited patients in the 3rd line setting of mCRPC and a median survival of the patients in the control arm of just over 11 months is a good indicator of the advanced nature of the disease in this cohort of men.
177Lu-PSMA-617 has achieved regulatory approval in US and UK with EMA approval expected in the coming months and will join the armamentarium of available lifeprolonging therapies in this space.
Implementing 177Lu-PSMA-617 will not be without its challenges including radioprotection, access to PSMA PET, and availability of a trained workforce in particular physicists and nuclear medicine technologists. Experience of using Radium-223 over the past 8 years will likely help many departments manage the new therapy.
Future Directions
As well as being used in earlier prostate cancer disease states, future directions for PSMAtargeted radionuclide therapy include the use of dosimetry to
personalise the dose received by the patient, alternate radioisotopes in particular the alpha-emitters, Actinium-225 and Thorium-227, and combination with radiosensitisers. I believe it is a very exciting time for radionuclide therapy in cancer which will hopefully result in better outcomes for our patients.
References
1. Brady D, Parker CC, O'Sullivan JM. Bone-targeting radiopharmaceuticals including radium-223. Cancer J. 2013 JanFeb;19(1):71-8. doi: 10.1097/ PPO.0b013e318282479b. PMID: 23337760.
2. O'Sullivan JM, McCready VR, Flux G, Norman AR, Buffa FM, Chittenden S, et al. High activity Rhenium-186 HEDP with autologous peripheral blood stem cell rescue: a phase I study in progressive hormone refractory prostate cancer metastatic to bone. Br J Cancer. 2002;86(11):1715-20.
3. van Dodewaard-de Jong JM, de Klerk JM, Bloemendal HJ, van Bezooijen BP, de Haas MJ, Wilson RH, and O’Sullivan JM. A phase I study of combined docetaxel and repeated high activity 186Re-HEDP in
castration-resistant prostate cancer (CRPC) metastatic to bone (the TAXIUM trial). Eur J Nucl Med Mol Imaging. 2011;38(11):1990-8.
4. Parker C, Nilsson S, Heinrich D, O’Sullivan JM et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23.
5. O’Driscoll et al, British J Pharmacol 2016, 173, 3041
6. Turner PG, Jain S, Cole A, Grey A, Mitchell D, Prise KM, and O’Sullivan JM. Toxicity and Efficacy of Concurrent Androgen Deprivation Therapy, Pelvic Radiotherapy, and Radium-223 in Patients with De Novo Metastatic Hormone-Sensitive Prostate Cancer. Clin Cancer Res. 2021;27(16):4549-56.
7. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N, El-Haddad G, Park CH, Beer TM, Armour A, Pérez-Contreras WJ, DeSilvio M, Kpamegan E, Gericke G, Messmann RA, Morris MJ, Krause BJ; VISION Investigators. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Jun 23. doi: 10.1056/ NEJMoa2107322.

RESPIRATORY: PNEUMONIA FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Figure 2, Overall Survival from Vision Trial7
38 ONCOLOGY: PROSTATE CANCER
Figure 2, Overall Survival from Vision Trial [7]
Pemetrexed 25 mg/mL
Pemetrexed 25 mg/mL
Launching
Launching
A room temperature stable ready-to-dilute concentrate
A room temperature stable ready-to-dilute concentrate
Pemetrexed 25 mg/mL
Pemetrexed 25 mg/mL
A multi-targeted anti-cancer antifolate agent In three presentations
A multi-targeted anti-cancer antifolate agent In three presentations
PRESCRIBING INFORMATION: Consult the Summary of Product Characteristics for further information. Additional information is available upon request Pemetrexed Fresenius Kabi 25mg/ml concentrate for solution for infusion. Active ingredients: Pemetrexed diacid. One vial of 4ml concentrate contains 100mg pemetrexed. One vial of 20ml concentrate contains 500mg pemetrexed. One vial of 40ml concentrate contains 1,000mg pemetrexed. Excipients with known effect – 4ml vial - 964mg hydroxypropylbetadex, 20ml vial – 4820mg hydroxypropylbetadex, 40ml vial – 9640mg hydroxypropylbetadex. Indications: In combination with cisplatin - treatment of chemotherapy naïve patients with unresectable malignant pleural mesothelioma, first line treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology. Monotherapy – Maintenance treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology in patients whose disease has not progressed immediately following platinum-based chemotherapy, second line treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology. Posology and method of administration: Only administer under the supervision of a physician qualified in the use of anti-cancer chemotherapy. Administer as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. See SmPC for further information on handling, administering and dilution. In combination with cisplatin – Recommended dose is 500mg/m2 of body surface area (BSA) administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. Recommended dose of cisplatin is 75mg/m2 BSA infused over two hours approximately 30 minutes after completion of the pemetrexed infusion on the first day of each 21-day cycle. Patients must receive adequate anti-emetic treatment and appropriate hydration prior to and/or after receiving cisplatin (see also cisplatin SmPC). As single agent – In the treatment of non-small cell lung cancer after prior chemotherapy, recommended dose is 500mg/m2 BSA administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. Pre-medication regimen: To reduce the incidence and severity of skin reactions, a corticosteroid should be given the day prior to, on the day of, and the day after pemetrexed administration. Corticosteroid should be equivalent to 4mg of dexamethasone orally twice daily. Patients must also receive vitamin supplementation; oral folic acid or a multivitamin containing folic acid (350 to 1000 micrograms) on a daily basis. At least five doses of folic acid must be taken during the seven days preceding the first dose of pemetrexed and dosing must continue during the full course of therapy and for 21 days after the last dose of pemetrexed. Patients must also receive an intramuscular injection of vitamin B12 (1000 micrograms) in the week preceding the first dose of pemetrexed and once every three cycles thereafter. Subsequent vitamin B12 injections may be given on the same day as pemetrexed. Monitoring: Before each dose complete blood count including differential white cell count (WCC) and platelet count. Prior to each chemotherapy administration blood chemistry tests should be collected to evaluate renal and hepatic function. Before the start of any cycle of chemotherapy, patients are required to have absolute neutrophil count (ANC) ≥ 1500 cells/mm3 and platelets ≥ 100,000 cells/mm3. Creatinine clearance should be ≥ 45 ml/min.
Total bilirubin should be ≤ 1.5 times upper limit of normal. Alkaline phosphatase (AP), aspartate aminotransferase (AST or SGOT) and alanine aminotransferase (ALT or SGPT) should be ≤ 3 times upper limit of normal. AP, AST and ALT ≤ 5 times upper limit of normal is acceptable if liver has tumour involvement. Dose adjustments: At the start of a subsequent cycle should be based on nadir haematologic counts or maximum non-haematologic toxicity from the preceding cycle of therapy. Treatment may be delayed to allow sufficient time for recovery. Upon recovery patients should be retreated using the guidelines as follows, which are applicable for pemetrexed Fresenius Kabi used as single agent or in combination with cisplatin. Haematologic toxicities (dose modify both pemetrexed and cisplatin) – Nadir ANC < 500/mm3 and nadir platelets ≥ 50,000/mm3 or nadir platelets < 50,000/mm3 regardless of nadir ANC: 75% of previous dose. Nadir platelets < 50,000/mm3 with bleeding regardless of nadir ANC: 50% of previous dose. Non-haematologic toxicities ≥ Grade 3 (excluding neurotoxicity) - pemetrexed Fresenius Kabi should be withheld until resolution to less than or equal to the patient’s pre-therapy value. Treatment should be resumed accordingly – Any grade 3 or 4 toxicities except mucositis, or any diarrhoea requiring hospitalisation or grade 3 or 4 diarrhoea: 75% of previous dose (mg/m2) of pemetrexed and cisplatin. Grade 3 or 4 mucositis: 50% of previous pemetrexed dose (mg/m2) and 100% of previous cisplatin dose (mg/m2). Neurotoxicity – Patients should discontinue therapy if Grade 3 or 4 neurotoxicity is observed. CTC Grade 0-1: 100% of previous pemetrexed and cisplatin dose (mg/m2). CTC Grade 2: 100% of previous pemetrexed dose (mg/m2) and 50% of previous cisplatin dose (mg/m2). Discontinue treatment with pemetrexed Fresenius Kabi if a patient experiences any haematologic or non-haematologic Grade 3 or 4 toxicity after 2 dose reductions or immediately if Grade 3 or 4 neurotoxicity is observed. Elderly – No dose reductions other than those recommended for all patients are necessary.
PRESCRIBING INFORMATION: Consult the Summary of Product Characteristics for further information. Additional information is available upon request Pemetrexed Fresenius Kabi 25mg/ml concentrate for solution for infusion. Active ingredients: Pemetrexed diacid. One vial of 4ml concentrate contains 100mg pemetrexed. One vial of 20ml concentrate contains 500mg pemetrexed. One vial of 40ml concentrate contains 1,000mg pemetrexed. Excipients with known effect – 4ml vial - 964mg hydroxypropylbetadex, 20ml vial – 4820mg hydroxypropylbetadex, 40ml vial – 9640mg hydroxypropylbetadex. Indications: In combination with cisplatin - treatment of chemotherapy naïve patients with unresectable malignant pleural mesothelioma, first line treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology. Monotherapy – Maintenance treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology in patients whose disease has not progressed immediately following platinum-based chemotherapy, second line treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology. Posology and method of administration: Only administer under the supervision of a physician qualified in the use of anti-cancer chemotherapy. Administer as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. See SmPC for further information on handling, administering and dilution. In combination with cisplatin – Recommended dose is 500mg/m2 of body surface area (BSA) administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. Recommended dose of cisplatin is 75mg/m2 BSA infused over two hours approximately 30 minutes after completion of the pemetrexed infusion on the first day of each 21-day cycle. Patients must receive adequate anti-emetic treatment and appropriate hydration prior to and/or after receiving cisplatin (see also cisplatin SmPC). As single agent – In the treatment of non-small cell lung cancer after prior chemotherapy, recommended dose is 500mg/m2 BSA administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. Pre-medication regimen: To reduce the incidence and severity of skin reactions, a corticosteroid should be given the day prior to, on the day of, and the day after pemetrexed administration. Corticosteroid should be equivalent to 4mg of dexamethasone orally twice daily. Patients must also receive vitamin supplementation; oral folic acid or a multivitamin containing folic acid (350 to 1000 micrograms) on a daily basis. At least five doses of folic acid must be taken during the seven days preceding the first dose of pemetrexed and dosing must continue during the full course of therapy and for 21 days after the last dose of pemetrexed. Patients must also receive an intramuscular injection of vitamin B12 (1000 micrograms) in the week preceding the first dose of pemetrexed and once every three cycles thereafter. Subsequent vitamin B12 injections may be given on the same day as pemetrexed. Monitoring: Before each dose complete blood count including differential white cell count (WCC) and platelet count. Prior to each chemotherapy administration blood chemistry tests should be collected to evaluate renal and hepatic function. Before the start of any cycle of chemotherapy, patients are required to have absolute neutrophil count (ANC) ≥ 1500 cells/mm3 and platelets ≥ 100,000 cells/mm3. Creatinine clearance should be ≥ 45 ml/min. Total bilirubin should be ≤ 1.5 times upper limit of normal. Alkaline phosphatase (AP), aspartate aminotransferase (AST or SGOT) and alanine aminotransferase (ALT or SGPT) should be ≤ 3 times upper limit of normal. AP, AST and ALT ≤ 5 times upper limit of normal is acceptable if liver has tumour involvement. Dose adjustments: At the start of a subsequent cycle should be based on nadir haematologic counts or maximum non-haematologic toxicity from the preceding cycle of therapy. Treatment may be delayed to allow sufficient time for recovery. Upon recovery patients should be retreated using the guidelines as follows, which are applicable for pemetrexed Fresenius Kabi used as single agent or in combination with cisplatin. Haematologic toxicities (dose modify both pemetrexed and cisplatin) – Nadir ANC < 500/mm3 and nadir platelets ≥ 50,000/mm3, or nadir platelets < 50,000/mm3 regardless of nadir ANC: 75% of previous dose. Nadir platelets < 50,000/mm3 with bleeding regardless of nadir ANC: 50% of previous dose. Non-haematologic toxicities ≥ Grade 3 (excluding neurotoxicity) - pemetrexed Fresenius Kabi should be withheld until resolution to less than or equal to the patient’s pre-therapy value. Treatment should be resumed accordingly – Any grade 3 or 4 toxicities except mucositis, or any diarrhoea requiring hospitalisation or grade 3 or 4 diarrhoea: 75% of previous dose (mg/m2) of pemetrexed and cisplatin. Grade 3 or 4 mucositis: 50% of previous pemetrexed dose (mg/m2) and 100% of previous cisplatin dose (mg/m2).


Neurotoxicity – Patients should discontinue therapy if Grade 3 or 4 neurotoxicity is observed. CTC Grade 0-1: 100% of previous pemetrexed and cisplatin dose (mg/m2). CTC Grade 2: 100% of previous pemetrexed dose (mg/m2) and 50% of previous cisplatin dose (mg/m2). Discontinue treatment with pemetrexed Fresenius Kabi if a patient experiences any haematologic or non-haematologic Grade 3 or 4 toxicity after 2 dose reductions or immediately if Grade 3 or 4 neurotoxicity is observed. Elderly – No dose reductions other than those recommended for all patients are necessary.
Fresenius Kabi Limited
Fresenius Kabi Ireland
Unit 3B Fingal Bay, Balbriggan, Co. Dublin, Ireland
Unit 3B Fingal Bay, Balbriggan,
Paediatric population – No relevant use in malignant pleural mesothelioma and non-small cell lung cancer. Renal impairment – (Standard Cockcroft and Gault formula or GFR measured Tc99m-DPTA serum clearance method). In clinical studies patients with creatinine clearance of ≥ 45ml/min required no dose adjustments other than those recommended for all patients. Not recommended in patients with creatinine clearance below 45ml/min. Hepatic impairment – Patients with hepatic impairment have not been specifically studied. Contraindications: Hypersensitivity to the active substance or to any of the excipients, breast-feeding, concomitant yellow fever vaccine. Special warnings and precautions for use: Monitor patients for myelosuppression. Pemetrexed should not be given until ANC returns to ≥ 1500 cells/mm3 and platelet count to ≥ 100,000 cells/mm3. Dose reductions for subsequent cycles based on nadir ANC, platelet count and maximum non-haematologic toxicity seen from previous cycle. All patients must be instructed to take folic acid and vitamin B12 as a prophylactic measure to reduce treatment-related toxicity. Pre-treatment with dexamethasone (or equivalent) can reduce the incidence and severity of skin reactions. Not recommended in patients with creatinine clearance of < 45ml/min. Patients with mild to moderate renal insufficiency (creatinine clearance 45-79ml/min) should avoid taking NSAIDs, or acetylsalicylic acid (> 1.3g daily) for 2 days before, on the day of, and 2 days following pemetrexed administration. In patients with mild to moderate renal insufficiency NSAIDs with long elimination half-lives should be interrupted for at least 5 days prior to, on the day of, and at least 2 days following pemetrexed administration. Patients should be regularly monitored for acute tubular necrosis, decreased renal function and signs and symptoms of nephrogenic diabetes insipidus. Drainage of third space fluid collection prior to pemetrexed treatment should be considered but may not be necessary. Patients should receive adequate antiemetic treatment and appropriate hydration prior to and/or after receiving treatment. Serious cardiovascular and cerebrovascular events have been uncommonly reported during clinical studies with pemetrexed, usually when given in combination with another cytotoxic agent. Concomitant use of live attenuated vaccines is not recommended. Sexually mature males are advised not to father a child during treatment and up to 6 months thereafter. Contraceptive measures or abstinence are recommended. Possibility of irreversible infertility; men advised to seek counselling on sperm storage before starting treatment. Women of childbearing potential must use effective contraception during treatment with pemetrexed. Cases of radiation pneumonitis reported in patients treated with radiation either prior, during or subsequent to pemetrexed therapy; particular attention should be paid to these patients and caution with other radiosensitising agents. Cases of radiation recall reported in patients who received radiotherapy weeks or years previously. Accumulation of cyclodextrins may occur in patients with moderate to severe renal dysfunction. May cause fatigue, patients should be cautioned against driving or operating machinery if this occurs. Undesirable effects: (Associated with pemetrexed monotherapy or in combination with cisplatin) Infection, pharyngitis, sepsis, dermo-hypodermitis, neutropenia, leukopenia, decreased haemoglobin, febrile neutropenia, decreased platelet count, pancytopenia, autoimmune haemolytic anaemia, hypersensitivity, anaphylactic shock, dehydration, taste disorder, peripheral motor neuropathy, peripheral sensory neuropathy, dizziness, cerebrovascular accident, ischemic stroke, intracranial haemorrhage, conjunctivitis, dry eye, increased lacrimation, keratoconjunctivitis sicca, eyelid oedema, ocular surface disease, cardiac failure, arrhythmia, angina, myocardial infarction, coronary artery disease, supraventricular arrhythmia, peripheral ischaemia, pulmonary embolism, interstitial pneumonitis, stomatitis, anorexia, vomiting, diarrhoea, nausea, dyspepsia, constipation, abdominal pain, rectal haemorrhage, gastrointestinal haemorrhage, intestinal perforation, oesophagitis, colitis, increased alanine aminotransferase, increased aspartate aminotransferase, hepatitis, rash, skin exfoliation, hyperpigmentation, pruritis, erythema multiforme, alopecia, urticaria, erythema, Stevens-Johnson syndrome, toxic epidermal necrolysis, pemphigoid, dermatitis bullous, acquired epidermolysis bullosa, erythematous oedema, pseudocellulitis, dermatitis, eczema, prurigo, decreased creatinine clearance, increased blood creatinine, renal failure, decreased GFR, nephrogenic diabetes insipidus, renal tubular necrosis, fatigue, pyrexia, pain, oedema, chest pain, mucosal inflammation, increased GGT, radiation oesophagitis, radiation pneumonitis, recall phenomenon. Legal Category: POM Marketing Authorisation Numbers: EU/1/16/1115/003, EU/1/16/1115/004, EU/1/16/1115/005. Marketing Authorisation Holder: Fresenius Kabi Deutschland GmbH, Else-Kroner-Straße 1, 61352 Bad Homburg v.d.Höhe, Germany. Further Information: See the SmPC for further details. Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra.ie Adverse events should also be reported to Fresenius Kabi Limited via email Pharmacovigilance GB@Fresenius-Kabi.com. Date of Preparation: May 2021 Job code: API/Pemetrexed/02
Paediatric population – No relevant use in malignant pleural mesothelioma and non-small cell lung cancer. Renal impairment – (Standard Cockcroft and Gault formula or GFR measured Tc99m-DPTA serum clearance method). In clinical studies patients with creatinine clearance of ≥ 45ml/min required no dose adjustments other than those recommended for all patients. Not recommended in patients with creatinine clearance below 45ml/min. Hepatic impairment – Patients with hepatic impairment have not been specifically studied. Contraindications: Hypersensitivity to the active substance or to any of the excipients, breast-feeding, concomitant yellow fever vaccine. Special warnings and precautions for use: Monitor patients for myelosuppression. Pemetrexed should not be given until ANC returns to ≥ 1500 cells/mm3 and platelet count to ≥ 100,000 cells/mm3. Dose reductions for subsequent cycles based on nadir ANC, platelet count and maximum non-haematologic toxicity seen from previous cycle. All patients must be instructed to take folic acid and vitamin B12 as a prophylactic measure to reduce treatment-related toxicity. Pre-treatment with dexamethasone (or equivalent) can reduce the incidence and severity of skin reactions. Not recommended in patients with creatinine clearance of < 45ml/min. Patients with mild to moderate renal insufficiency (creatinine clearance 45-79ml/min) should avoid taking NSAIDs, or acetylsalicylic acid (> 1.3g daily) for 2 days before, on the day of, and 2 days following pemetrexed administration. In patients with mild to moderate renal insufficiency NSAIDs with long elimination half-lives should be interrupted for at least 5 days prior to, on the day of, and at least 2 days following pemetrexed administration. Patients should be regularly monitored for acute tubular necrosis, decreased renal function and signs and symptoms of nephrogenic diabetes insipidus. Drainage of third space fluid collection prior to pemetrexed treatment should be considered but may not be necessary. Patients should receive adequate antiemetic treatment and appropriate hydration prior to and/or after receiving treatment. Serious cardiovascular and cerebrovascular events have been uncommonly reported during clinical studies with pemetrexed, usually when given in combination with another cytotoxic agent. Concomitant use of live attenuated vaccines is not recommended. Sexually mature males are advised not to father a child during treatment and up to 6 months thereafter. Contraceptive measures or abstinence are recommended. Possibility of irreversible infertility; men advised to seek counselling on sperm storage before starting treatment. Women of childbearing potential must use effective contraception during treatment with pemetrexed. Cases of radiation pneumonitis reported in patients treated with radiation either prior, during or subsequent to pemetrexed therapy; particular attention should be paid to these patients and caution with other radiosensitising agents. Cases of radiation recall reported in patients who received radiotherapy weeks or years previously. Accumulation of cyclodextrins may occur in patients with moderate to severe renal dysfunction. May cause fatigue, patients should be cautioned against driving or operating machinery if this occurs. Undesirable effects: (Associated with pemetrexed monotherapy or in combination with cisplatin) Infection, pharyngitis, sepsis, dermo-hypodermitis, neutropenia, leukopenia, decreased haemoglobin, febrile neutropenia, decreased platelet count, pancytopenia, autoimmune haemolytic anaemia, hypersensitivity, anaphylactic shock, dehydration, taste disorder, peripheral motor neuropathy, peripheral sensory neuropathy, dizziness, cerebrovascular accident, ischemic stroke, intracranial haemorrhage, conjunctivitis, dry eye, increased lacrimation, keratoconjunctivitis sicca, eyelid oedema, ocular surface disease, cardiac failure, arrhythmia, angina, myocardial infarction, coronary artery disease, supraventricular arrhythmia, peripheral ischaemia, pulmonary embolism, interstitial pneumonitis, stomatitis, anorexia, vomiting, diarrhoea, nausea, dyspepsia, constipation, abdominal pain, rectal haemorrhage, gastrointestinal haemorrhage, intestinal perforation, oesophagitis, colitis, increased alanine aminotransferase, increased aspartate aminotransferase, hepatitis, rash, skin exfoliation, hyperpigmentation, pruritis, erythema multiforme, alopecia, urticaria, erythema, Stevens-Johnson syndrome, toxic epidermal necrolysis, pemphigoid, dermatitis bullous, acquired epidermolysis bullosa, erythematous oedema, pseudocellulitis, dermatitis, eczema, prurigo, decreased creatinine clearance, increased blood creatinine, renal failure, decreased GFR, nephrogenic diabetes insipidus, renal tubular necrosis, fatigue, pyrexia, pain, oedema, chest pain, mucosal inflammation, increased GGT, radiation oesophagitis, radiation pneumonitis, recall phenomenon. Legal Category: POM Marketing Authorisation Numbers: EU/1/16/1115/003, EU/1/16/1115/004, EU/1/16/1115/005. Marketing Authorisation Holder: Fresenius Kabi Deutschland GmbH, Else-Kroner-Straße 1, 61352 Bad Homburg v.d.Höhe, Germany. Further Information: See the SmPC for further details. Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra.ie. Adverse events should also be reported to Fresenius Kabi Limited via email Pharmacovigilance GB@Fresenius-Kabi.com. Date of Preparation: May 2021 Job code: API/Pemetrexed/02
Website: www.fresenius-kabi.com/ie/
Email: FK-enquiries.ireland@fresenius-kabi.com
Phone: +353 (0)1 841 3030
Website: www.fresenius-kabi.com/ie/
Email: FK-enquiries.ireland@fresenius-kabi.com


Phone: +353 (0)1 841 3030
14:42
Date of Prep: May 2021
Ref: IV/ONCO/PEME/01/2021
19763_FK_Pemetrexed_A4_4pp_Brochure_v4_Option_A.indd 1 27/04/2021 14:42
19763_FK_Pemetrexed_A4_4pp_Brochure_v4_Option_A.indd 27/04/2021 14:42
Date of Prep: May 2021 Ref: IV/ONCO/PEME/01/2021
19763_FK_Pemetrexed_A4_4pp_Brochure_v4_Option_A.indd 1 27/04/2021 14:42
Fresenius Kabi Limited Fresenius Kabi Ireland
19763_FK_Pemetrexed_A4_4pp_Brochure_v4_Option_A.indd 27/04/2021
Triple Negative Breast Cancer: Background and Cost in Ireland
 Written by Dr Jonathan Briody & Amina Babar
Written by Dr Jonathan Briody & Amina Babar
Bio: Dr Briody is a health economist conducting an economic evaluation of intervention in, and prevention of, diabetes at RCSI University of Medicine and Health Sciences. He is supervising the MSc in Health Economics of co-author Amina Babar at the University of York


achieve a complete response to treatment and prolonged periods of remission when treating this disease.
treatment of TNBC. Nevertheless, some targeted therapies used to treat other types of cancer, such as PARP inhibitors and immune checkpoint inhibitors, are being studied in clinical trials to treat TNBC.
Breast cancer accounts for 30% of all female cancer cases and is the most common cancer in women worldwide, constituting just under 10% of annual cancer diagnoses in Ireland. On average, 88.3% of women diagnosed with breast cancer are expected to survive for at least five years. Triplenegative breast cancer (TNBC) is a subtype characterized by a lack of expression of estrogen receptors, progesterone receptors, and human epidermal growth factor receptor 2 (HER2).
One in every eight breast cancer cases diagnosed in Ireland is triple negative. TNBC is more common in younger women, with the highest rates occurring in women under 40. TNBC can be more resistant to chemotherapy, often the primary treatment option for this breast cancer subtype. Cancer cells similarly do not respond to hormone therapies, such as tamoxifen and aromatase inhibitors. These factors make it more difficult to
This disease is also more likely to spread. Breast cancer is described as being locally advanced when it is limited to the breast and nearby lymph nodes. When the cancer extends to an area of the body other than the original site, it is said to have metastasized. The risk ratio for developing metastasis in triple-negative disease is particularly high in the first five years following diagnosis compared to other breast tumours. Within ten years of diagnosis, triple-negative breast cancer patients are significantly more likely (23%) than other breast cancer patients to develop a visceral metastasis as the primary site of distant recurrence.
Five-year survival rates are 11% in TNBC that has metastasized to the bones, lungs, or liver.
Targeted immunotherapies, targeting specific proteins or pathways in cancer cells, are critical in treating many breast cancers and have dramatically altered the survival landscape once the disease metastasizes. However, there are currently no targeted therapies that are expressly approved for the
Much of the promise in improving treatment outcomes for TNBC comes from such clinical trials. In the IMpassion130 study, atezolizumab with nab-paclitaxel was more effective than nab-paclitaxel alone in extending progression-free survival for patients with metastatic triplenegative breast cancer. The phase III KEYNOTE-355 trial found that in patients with locally recurrent inoperable or metastatic PD-L1positive TNBC, the combination of pembrolizumab and chemotherapy significantly improved overall and progression-free survival. A phase III clinical trial (GeparQuinto) found that the combination of paclitaxel and carboplatin was more effective at improving PFS compared to docetaxel in patients with TNBC.
Establishing emerging therapy options is only one of many steps required for adoption in the clinic. The effectiveness and the efficiency of interventions in health are quickly becoming of equal importance in increasingly constrained healthcare systems. National policy requires a health economic analysis to assess the best use of limited resources and provides a definite structure in which investments in treatment strategies which maximize the overall health produced in the care system are prioritized. Subsequently, the highest degree of net health the system can provide can be realized.
Ireland's Health Service Executive (HSE) provides guidance on the
cost-effectiveness of treatment options for TNBC. According to HSE recommendations, treatments are most commonly considered cost-effective for treating TNBC in Ireland if an incremental cost-effectiveness ratio (ICER) of ¤45,000 per quality-adjusted life year (QALY) is met. That is, the cost difference between a suggested novel therapy and standard of care, divided by the difference in outcomes between these therapies, must be less than or equal to ¤45,000 per QALY gained. When the proposed intervention cost exceeds the cost-effectiveness threshold, the applicant company may enter into confidential negotiations with the HSE to reduce the initially proposed drug or technology price.
As the prevalence of TNBC increases with advances in screening and shifts in population dynamics, this suggests an increasing demand for novel therapies in a disease that is challenging to treat under standard of care. Such interventions are expected to present a higher cost burden. Where the benefits of novel strategies are not balanced with costs, the burden to the Irish healthcare system will be passed onto patients via financially diminished service provision and declines in access to other forms of care. Ongoing health economic analysis for the treatment of TNBC can help guide such treatment decisions to ensure that healthcare resources are utilized in such a way as to maximize the overall level of health produced by the health system, resulting in a net gain for all users.
40 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: BREAST CANCER
Amina Babar, MSc Health Economics, University of York. BSc in Economics and Finance
The effectiveness and the efficiency of interventions in health are quickly becoming of equal importance in increasingly constrained healthcare systems
Ireland and Northern Ireland Abbreviated Prescribing Information: XTANDITM (enzalutamide) 40 mg film-coated tablets. For full prescribing information, refer to the Summary of Product Characteristics (SPC). Presentation: 40 mg film-coated tablets each containing 40 mg of enzalutamide. Indications: The treatment of adult men with metastatic hormone-sensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy. The treatment of adult men with high-risk nonmetastatic castration-resistant prostate cancer (CRPC). The treatment of adult men with metastatic CRPC who are asymptomatic or mildly symptomatic after failure of androgen deprivation therapy in whom chemotherapy is not yet clinically indicated. The treatment of adult men with metastatic CRPC whose disease has progressed on or after docetaxel therapy. Posology and administration: Treatment with enzalutamide should be initiated and supervised by specialist physicians experienced in the medical treatment of prostate cancer. The recommended dose is 160 mg enzalutamide (four 40 mg film-coated tablets) as a single oral daily dose. The tablets should be swallowed whole with water, and can be taken with or without food. Medical castration with a luteinising hormone-releasing hormone (LHRH) analogue should be continued during treatment of patients not surgically castrated. If a patient experiences a ≥ Grade 3 toxicity or an intolerable adverse reaction, dosing should be withheld for one week or until symptoms improve to ≤ Grade 2, then resumed at the same or a reduced dose (120 mg or 80 mg) if warranted. Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SPC. Women who are or may become pregnant. Special warnings and precautions for use: Risk of seizure: Use of enzalutamide has been associated with seizure. The decision to continue treatment in patients who develop seizures should be taken case by case. Posterior reversible encephalopathy syndrome: There have been rare reports of posterior reversible encephalopathy syndrome (PRES) in patients receiving XTANDI. PRES is a rare, reversible, neurological disorder which can present with rapidly evolving symptoms including seizure, headache, confusion, blindness, and other visual and neurological disturbances, with or without associated hypertension. A diagnosis of PRES requires confirmation by brain imaging, preferably magnetic resonance imaging (MRI). Discontinuation of XTANDI in patients who develop PRES is recommended. Second Primary Malignancies: Cases of second primary malignancies have been reported in patients treated with enzalutamide in clinical studies. In phase 3 clinical studies, the most frequently reported events in enzalutamide treated patients, and greater than placebo, were bladder cancer (0.3%), adenocarcinoma of the colon (0.2%), transitional cell carcinoma (0.2%) and bladder transitional cell carcinoma (0.1%). Patients should be advised to promptly seek the attention of their physician if they notice signs of gastrointestinal bleeding, macroscopic haematuria, or other symptoms such as dysuria or urinary urgency develop during treatment with enzalutamide. Concomitant use with other medicinal products: Enzalutamide is a potent enzyme inducer and may lead to loss of efficacy of many commonly used medicinal products. A review of concomitant medicinal products should therefore be conducted when initiating enzalutamide treatment.
Concomitant use of enzalutamide with medicinal products that are sensitive substrates of many metabolising enzymes or transporters should generally be avoided if their therapeutic effect is of large importance to the patient, and if dose adjustments cannot easily be performed based on monitoring of efficacy or plasma concentrations. Co-administration with warfarin and coumarin-like anticoagulants should be avoided. If XTANDI is co-administered with an anticoagulant metabolised by CYP2C9 (such as warfarin or acenocoumarol), additional International Normalised Ratio (INR) monitoring should be conducted. Renal impairment: Caution is required in patients with severe renal impairment as enzalutamide has not been studied in this patient population. Severe hepatic impairment: An increased half-life of enzalutamide has been observed in patients with severe hepatic impairment, possibly related to increased tissue distribution. The clinical relevance of this observation remains unknown. A prolonged time to reach steady state concentrations is however anticipated, and the time to maximum pharmacological effect as well as time for onset and decline of enzyme induction may be increased. Recent cardiovascular disease: The phase 3 studies excluded patients with recent myocardial infarction (in the past 6 months) or unstable angina (in the past 3 months), New York Heart Association Class (NYHA) III or IV heart failure except if Left Ventricular Ejection Fraction (LVEF) ≥ 45%, bradycardia or uncontrolled hypertension. This should be taken into account if XTANDI is prescribed in these patients. Androgen deprivation therapy may prolong the QT interval: In patients with a history of or risk factors for QT prolongation and in patients receiving concomitant medicinal products that might prolong the QT interval physicians should assess the benefit risk ratio including the potential for Torsade de pointes prior to initiating XTANDI. Use with chemotherapy: The safety and efficacy of concomitant use of XTANDI with cytotoxic chemotherapy has not been established. Co-administration of enzalutamide has no clinically relevant effect on the pharmacokinetics of intravenous docetaxel; however, an increase in the occurrence of docetaxel-induced neutropenia cannot be excluded. Hypersensitivity reactions: Hypersensitivity reactions manifested by symptoms including, but not limited to, rash, or face, tongue, lip, or pharyngeal oedema have been observed with enzalutamide. Severe cutaneous adverse reactions (SCARs) have been reported with enzalutamide. At the time of prescription patients should be advised of the signs and symptoms and monitored closely for skin reactions.
Excipients: This medicine contains less than 1 mmol sodium (less than 23 mg) per
film-coated tablet, that is to say essentially ‘sodium-free’. Interactions: Potential for other medicinal products to affect enzalutamide exposures: CYP2C8 plays an important role in the elimination of enzalutamide and in the formation of its active metabolite. Strong inhibitors (e.g. gemfibrozil) of CYP2C8 are to be avoided or used with caution during enzalutamide treatment. If patients must be co-administered a strong CYP2C8 inhibitor, the dose of enzalutamide should be reduced to 80 mg once daily. No dose adjustment is necessary when XTANDI is co-administered with inducers of CYP2C8. CYP3A4 plays a minor role in the metabolism of enzalutamide. No dose adjustment is necessary when XTANDI is co-administered with inhibitors or inducers of CYP3A4. Potential for enzalutamide to affect exposures to other medicinal products: Enzalutamide is a potent enzyme inducer and increases the synthesis of many enzymes and transporters; therefore, interaction with many common medicinal products that are substrates of enzymes or transporters is expected. Enzymes that may be induced include CYP3A in the liver and gut, CYP2B6, CYP2C9, CYP2C19, and uridine 5’-diphospho-glucuronosyltransferase (UGTs - glucuronide conjugating enzymes). Some transporters may also be induced, e.g. multidrug resistanceassociated protein 2 (MRP2) and the organic anion transporting polypeptide 1B1 (OATP1B1). In vivo studies have shown that enzalutamide is a strong inducer of CYP3A4 and a moderate inducer of CYP2C9 and CYP2C19. The full induction potential of enzalutamide may not occur until approximately 1 month after the start of treatment, when steady-state plasma concentrations of enzalutamide are reached, although some induction effects may be apparent earlier. Patients taking medicinal products that are substrates of CYP2B6, CYP3A4, CYP2C9, CYP2C19 or UGT1A1 should be evaluated for possible loss of pharmacological effects (or increase in effects in cases where active metabolites are formed) during the first month of enzalutamide treatment, and dose adjustment should be considered as appropriate. In consideration of the long half-life of enzalutamide (5.8 days), effects on enzymes may persist for one month or longer after stopping enzalutamide. A gradual dose reduction of the concomitant medicinal product may be necessary when stopping enzalutamide treatment. In vitro data indicate that enzalutamide may be an inhibitor of the efflux transporter P-gp. A mild inhibitory effect of enzalutamide, at steadystate, on P-gp was observed in a study in patients with prostate cancer that received a single oral dose of the probe P-gp substrate digoxin before and concomitantly with enzalutamide (concomitant administration followed at least 55 days of once daily dosing of 160 mg enzalutamide). The AUC and Cmax of digoxin increased by 33% and 17%, respectively. Medicinal products with a narrow therapeutic range that are substrates for P-gp (e.g. colchicine, dabigatran etexilate, digoxin) should be used with caution when administered concomitantly with XTANDI and may require dose adjustment to maintain optimal plasma concentrations. At steady state, enzalutamide did not cause a clinically meaningful change in exposure to the probe breast cancer resistance protein (BCRP) substrate rosuvastatin in patients with prostate cancer that received a single oral dose of rosuvastatin before and concomitantly with enzalutamide (concomitant administration followed at least 55 days of once daily dosing of 160 mg enzalutamide). The AUC of rosuvastatin decreased by 14% while Cmax increased by 6%. No dose adjustment is necessary when a BCRP substrate is co administered with XTANDI. Based on in vitro data, inhibition of MRP2 (in the intestine), as well as organic anion transporter 3 (OAT3) and organic cation transporter 1 (OCT1) (systemically) cannot be excluded. Theoretically, induction of these transporters is also possible, and the net effect is presently unknown. Since androgen deprivation treatment may prolong the QT interval, the concomitant use of XTANDI with medicinal products known to prolong the QT interval or medicinal products able to induce Torsade de pointes should be carefully evaluated. Fertility, pregnancy and lactation: There are no human data on the use of XTANDI in pregnancy and this medicinal product is not for use in women of childbearing potential. This medicine may cause harm to the unborn child or potential loss of pregnancy if taken by women who are pregnant. It is not known whether enzalutamide or its metabolites are present in semen. A condom is required during and for 3 months after treatment with enzalutamide if the patient is engaged in sexual activity with a pregnant woman. If the patient engages in sexual intercourse with a woman of childbearing potential a condom and another form of birth control must be used during and for 3 months after treatment. Enzalutamide is not for use in women. Enzalutamide is contraindicated in women who are, or who may become, pregnant. It is not known if enzalutamide is present in human milk. Enzalutamide and/or its metabolites are secreted in rat milk. Animal studies showed that enzalutamide affected the reproductive system in male rats and dogs. Effects on ability to drive and use machines: XTANDI may have a moderate influence on the ability to drive and use machines as psychiatric and neurologic events including seizure have been reported. Patients should be advised of the potential risk of experiencing a psychiatric or neurological event while driving or operating machines. Undesirable effects: Summary of the safety profile: The most common adverse reactions are asthenia/ fatigue, hot flush, hypertension, fractures, and fall. Other important adverse reactions include ischemic heart disease and seizure. Seizure occurred in 0.5% of enzalutamidetreated patients, 0.2% of placebo-treated patients, and 0.3% in bicalutamide-treated patients. Rare cases of posterior reversible encephalopathy syndrome have been reported in enzalutamide-treated patients. Adverse reactions observed during clinical studies are listed below by frequency category. Frequency categories are defined as follows: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥
XTANDI™ is indicated for the treatment of adult men with metastatic castrationresistant prostate cancer who are asymptomatic or mildly symptomatic after failure of androgendeprivation therapy, in whom chemotherapy is not yet clinically indicated.1
XTANDI is also indicated for the treatment of adult men with metastatic castration – resistant prostate cancer whose disease has progressed on or after docetaxel.1






1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000); not known (cannot be estimated from the available data). Adverse reactions are presented according to Medical Dictionary for Regulatory Activities (MedDRA) system organ classification and within each frequency grouping they are presented in order of decreasing seriousness. Adverse reactions identified in controlled clinical trials and post-marketing: Blood and lymphatic system disorders: Uncommon: leucopenia, neutropenia; Not known*: thrombocytopenia. Immune system disorders: Not known*: face oedema, tongue oedema, lip oedema, pharyngeal oedema Psychiatric disorders: Common: anxiety; Uncommon: visual hallucination. Nervous system disorders: Common: headache, memory impairment, amnesia, disturbance in attention, dysgeusia, restless legs syndrome; Uncommon: cognitive disorder, seizure¥; Not known*: posterior reversible encephalopathy syndrome. Cardiac disorders: Common: ischemic heart disease†; Not known*: QT-prolongation. Vascular disorders: Very common: hot flush, hypertension. Gastrointestinal disorders: Not known*: nausea, vomiting, diarrhoea. Skin and subcutaneous tissue disorders: Common: dry skin, pruritus; Not known*: erythema multiforme, rash. Musculoskeletal and connective tissue disorders: Very common: fractures‡ Not known*: myalgia, muscle spasms, muscular weakness, back pain. Reproductive system and breast disorder: Common: gynaecomastia. General disorders and administration site conditions: Very common: asthenia, fatigue. Injury, poisoning and procedural complications: Very common: fall. * Spontaneous reports from post-marketing experience. ¥ As evaluated by narrow Standardised MedDRA Queries (SMQs) of ‘Convulsions’ including convulsion, grand mal convulsion, complex partial seizures, partial seizures and status epilepticus. This includes rare cases of seizure with complications leading to death. † As evaluated by narrow SMQs of ‘Myocardial Infarction’ and ‘Other Ischemic Heart Disease’ including the following preferred terms observed in at least two patients in randomized placebo-controlled phase 3 studies: angina pectoris, coronary artery disease, myocardial infarctions, acute myocardial infarction, acute coronary syndrome, angina unstable, myocardial ischaemia and arteriosclerosis coronary artery. ‡ Includes all preferred terms with the word ‘fracture’ in bones. Description of selected adverse reactions: Seizure: In controlled clinical studies, 24 patients (0.5%) experienced a seizure out of 4403 patients treated with a daily dose of 160 mg enzalutamide, whereas four patients (0.2%) receiving placebo and one patient (0.3%) receiving bicalutamide experienced a seizure. Dose appears to be an important predictor of the risk of seizure, as reflected by preclinical data, and data from a doseescalation study. In the controlled clinical studies, patients with prior seizure or risk factors for seizure were excluded. In the 9785 CL 0403 (UPWARD) single-arm trial to assess incidence of seizure in patients with predisposing factors for seizure (whereof 1.6% had a history of seizures), 8 of 366 (2.2%) patients treated with enzalutamide experienced a seizure. The median duration of treatment was 9.3 months. The mechanism by which enzalutamide may lower the seizure threshold is not known but could be related to data from in vitro studies showing that enzalutamide and its active metabolite bind to and can inhibit the activity of the GABA-gated chloride channel.
Ischemic Heart Disease: In randomized placebo-controlled clinical studies, ischemic heart disease occurred in 3.9% of patients treated with enzalutamide plus ADT compared to 1.5% patients treated with placebo plus ADT. Fifteen (0.4%) patients treated with enzalutamide and 2 (0.1%) patients treated with placebo had an ischemic heart disease event that led to death. Prescribers should consult the full SPC in relation to other adverse reactions. Overdose: There is no antidote for enzalutamide. In the event of an overdose, treatment with enzalutamide should be stopped and general supportive measures initiated taking into consideration the half-life of 5.8 days. Patients may be at increased risk of seizures following an overdose. Cost (excluding VAT): XTANDI 40 mg film-coated tablets x 112: Ireland: POA. Northern Ireland: £2,734.67. Legal classification: Ireland: POM/S1A. Northern Ireland: POM. Marketing Authorisation number: XTANDI 40 mg film-coated tablets: EU/1/13/846/002 Marketing Authorisation Holder: Astellas Pharma Europe B.V., Sylviusweg 62, 2333 BE Leiden, The Netherlands. Date of preparation of Prescribing Information: October 2022 Job number: MAT-UKE-XTD-2022-00004 Further information is available on request from: Ireland: Astellas Pharma Co. Ltd., Tel.: +353 1 467 1555. Northern Ireland: Astellas Pharma Ltd, Medical Information 0800 783 5018. For full prescribing information, refer to the SPCs, which may be found at www.medicines.ie (Ireland) and https://www.emcmedicines.com/en-GB/ northernireland/ (Northern Ireland).
Adverse events should be reported. For Ireland, Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance, Website: www.hpra.ie or Astellas Pharma Co. Ltd. Tel: +353 1 467 1555, E-mail: irishdrugsafety@astellas.com
Adverse events should be reported. For Northern Ireland, reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Astellas Pharma Ltd on 0800 783 5018.

MAT-IE-XTD-2022-00015 Date of preparation: November 2022
References: 1. XTANDI Summary of Product Characteristics.
Inhibition of IRE1α RNase activity sensitizes patient-derived acute myeloid leukaemia cells to proteasome inhibitors

Written by: Stuart Creedican1 , 2 , 3, Claire M. Robinson1, 2, Katarzyna Mnich1, 2, Md Nahidul Islam2, Eva Szegezdi1, 2 , Ruth Clifford4, 5, Janusz Krawczyk6, 7, John B. Patterson8, Stephen P. FitzGerald9, Mark Summers3, Ciaran Richardson3, Kenneth Martin3, Adrienne M. Gorman1, 2, and Afshin Samali1, 2

1Apoptosis Research Centre, University of Galway, Galway Ireland, 2School of Biological and Chemical Sciences, University of Galway, Galway Ireland, 3Randox Teoranta, Dungloe, Co. Donegal Ireland, 4Limerick Digital Cancer Research Centre, HRI, School of Medicine, University of Limerick, Limerick Ireland, 5Department of Haematology, University Hospital Limerick, Limerick Ireland, 6School of Medicine, University of Galway, Galway Ireland, 7Department of Haematology, Galway University Hospital, Galway Ireland, 8Orinove Inc., Newbury Park California, USA, 9Randox Laboratories Ltd, Crumlin, Co. Antrim UK
Despite improvements in prognostic stratification and optimization of therapeutic intervention in acute myeloid leukaemia (AML) patients, longterm survival is low. Clinical trials suggest proteasome inhibitors may be beneficial, but further interrogation of the molecular consequences of proteasome inhibition in AML is warranted to identify novel approaches that enhance their efficacy.1 In multiple myeloma (MM), resistance to proteasome inhibitors can occur upon activation of the unfolded protein response (UPR), a stress response pathway that can control cell fate.2 Inositol-requiring enzyme 1 alpha (IRE1α) is one of three stress sensors that mediates UPR signalling. IRE1α activity occurs via its RNase domain resulting in cleavage of a 26-nucleotide intron from X-Box Binding Protein 1 (XBP1) mRNA leading to formation of a transcription factor, XBP1s. XBP1s enhances cell survival by increasing transcription of genes associated with protein folding, endoplasmic reticulumassociated degradation (ERAD) and phospholipid synthesis. We demonstrate that an IRE1 RNase inhibitor (MKC8866), in combination with proteasome
inhibitors, significantly decreases XBP1s levels and increases cell death in AML cell lines and patient-derived AML cells. In addition, this combination treatment can successfully target the CD34+CD38- population and reduce clonogenic ability.

In some cancer types, targeting IRE1α alone may promote cell death.3 We investigated the impact of inhibiting basal IRE1 RNase activity with MKC8866 in three AML cell lines: U937, Molm 13 and KG1a. Expression of XBP1s protein was quantified using a previously described biochip array assay.4 In all cell lines, XBP1s was detected and was significantly decreased with MKC8866 treatment (Figure 1A, left panel). This had no impact on cell death (Figure 1A, right panel). Treatment with either bortezomib (BTZ) or carfilzomib (CFZ) increased XBP1s in KG1a and U937 cells. MKC8866 reversed this increase (Figure 1B,C, left panels). BTZ or CFZ caused cell death in KG1a and U937 cells and MKC8866 co-treatment significantly enhanced AML cell death (Figure 1B,C, right panels).
Bone marrow mesenchymal stromal cells (BMSCs) protect AML cells from chemotherapymediated killing, which can be modelled in vitro by co-culture with HS-5 cells, an immortalized BMSC feeder cell line. 5 We hypothesized that BMSC would protect AML cells treated with proteasome inhibitor(s), and that IRE1α inhibitors may overcome this protection. KG1a and U937 cells were co-cultured with HS-5 cells and treated with BTZ or CFZ and DMSO or MKC8866. In both AML cell lines, MKC8866 reduced XBP1s levels in proteasome inhibitor-treated cells (Figure 1D,E,
42 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: LEUKAEMIA
1
Professor Afshin Samali
Figure
left panels). Significantly increased AML cell death was observed in the combination treatments that included CFZ (p < 0.0001); increased cell death was observed in the combination treatments with BTZ but this was not significant (Figure 1D,E, right panels).
We next examined combination treatment in a more clinically relevant setting, using CFZ rather than BTZ as it displayed a superior cytotoxicity profile in combination experiments (Figure 1). Twelve primary bone marrow aspirates from patient-derived mononuclear cells (MNCs) were isolated from AML patients and grown in the HS-5 co-culture system. Patientderived cells were treated with DMSO or MKC8866. Similar to AML cell lines, treatment with MKC8866 did not affect primary AML cell viability (Figure 2A). However, there was increased cell death in AML patient-derived MNCs when they were treated with CFZ in combination with MKC8866 (Figure 2B). As the CD34+ CD38- cells are responsible for resistance and relapse, the impact of combination treatments on this population was
examined. MKC8866 enhanced CFZ-mediated cytotoxicity in the CD34+ CD38- population (Figure 2C) as well as increasing the anticlonogenic effects of CFZ (Figure 2D). Active IRE1α-XBP1s signalling can contribute to regulation of cytokines and chemokines, many of which can influence AML cell behaviour.6, 7 We investigated the impact of combination treatment on the expression of 12 diseaserelevant cytokines and chemokines using Evidence Evolution® Cytokine Array multiplexed assay. Levels of interleukin-6 (IL-6) were decreased in MKC8866-treated samples while levels of monocyte chemoattractant protein (MCP-1) were reduced in the AML patient co-cultures when they received MKC8866 and were further decreased in samples receiving combination treatments (Figure 2E,F).

XBP1s levels in MM patients positively correlate with patient outcome when treated with BTZ.8 No such correlative studies have been performed in AML. We compared basal XBP1s levels with viability of patient cells after combination treatment. XBP1s
levels significantly correlated with MKC8866-mediated enhancement of 20 nM and 100 nM CFZ treatment (Figure 2G,H). The cells that were most responsive to MKC8866 plus CFZ co-treatment when compared with CFZ treatment alone had the highest XBP1s and vice versa.
This study proposes IRE1α-XBP1s pathway inhibition as an adjunctive strategy for improving efficacy of proteasome inhibitors in AML. Proteasome inhibitors significantly increased XBP1s levels, and targeting the IRE1α-XBP1s axis with MKC8866 in the presence of CFZ significantly enhanced cell death in AML cell lines and patient samples. We demonstrated this in a clinically relevant co-culture model of AML, where resistance to therapy-induced cell death is often an issue.
Although the AML cells used in this study constitutively expressed XBP1s, it was only in conditions of proteasome inhibitor-induced upregulation of XBP1s that targeting this pathway provided therapeutic benefit. This suggests that while XBP1s may be basally active in AML cells, these cells are not reliant on XBP1s for survival. Despite this, basal levels of XBP1s in AML cells can prognosticate response to combination therapy and we demonstrate the utility
of an XBP1 biochip to predict patient response. Sun et al. previously reported that IRE1α inhibition enhances the cytotoxic effects of BTZ in AML cell lines. 3 In contrast to our findings, the same group demonstrated that targeting IRE1α alone decreased AML cell viability. 3 Perhaps this was due to higher basal XBP1s in their cells and, therefore, enhanced sensitivity to IRE1 RNase inhibition, as a wide range of XBP1s mRNA levels has previously been reported in bigger AML cohorts.9 Notably, different IRE1α RNase inhibitors were also used, and off-target toxicity could account for the differences observed.3
Acute myeloid leukaemia–stroma interactions have been implicated in promoting drug resistance and increasing the fraction of quiescent AML cells.10, 11 We demonstrate that CFZ and MKC8866 combination treatment can overcome HS-5 protective effects. This cotreatment also reduces proportional survival in the leukaemic stem cell (LSC)-containing CD34+CD38population, an effect that has previously been shown to be stronger with CFZ than BTZ treatment.11 Signalling via the IRE1α-XBP1s arm of the UPR preserves the self-renewal capacity of pre-LSCs and enables their clonal dominance over haematopoietic stem cells indicating an important role for XBP1s in LSC renewal.12 In this study, it is conceivable that LSC viability is decreased by the same mechanism and this contributes to the reduced clonogenic capacity observed with combination treatment. We also observe significant reductions in IL-6 and MCP-1 in combination treated cells, both of which impact haematopoietic stem cell phenotype.13, 14 These changes likely contribute to a more hostile environment for AML cells and contribute to increased AML cell death. Together, the results would suggest that combination treatment could improve patient outcomes and relapse rates.
In conclusion, we demonstrate the efficacy of a novel combination treatment using CFZ with MKC8866 in ex vivo AML models, to decrease the viability of AML cells. We propose that the combination treatment of CFZ and MKC8866 warrants further investigation as a treatment strategy for AML patients.
43 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Figure 2
Evolving Role of Liquid Biopsies in Management of Breast Cancer
 Written by Mary N. O’Reilly, Michaela J. Higgins, St. Vincent’s University Hospital
Written by Mary N. O’Reilly, Michaela J. Higgins, St. Vincent’s University Hospital


and exosomes. We will discuss how they are currently used and their potential future applications.
Methods of biopsy
Circulating tumour cells
Summary
Liquid biopsies have the potential to become a gold standard method of diagnosing and monitoring those with breast cancer in the near future. The wealth of information that liquid biopsies can provide has numerous applications including diagnostic, prognostic, therapeutic response prediction and detection of relapse in those who were treated with curative intent. This review will discuss the role of three potential biomarkers for breast cancer which can be obtained from liquid biopsies; circulating tumour cells, circulating tumour DNA and exosomes.
Introduction
Breast cancer is the most common cancer among females (after skin cancer) in the world. In recent years, large scale clinical trials of new interventions and treatments have led to significantly improved diagnosis, therapy and survival for breast cancer patients. We are moving away from traditional cytotoxic chemotherapy in favour of more targeted and therefore potentially less toxic therapies.
Precision oncology describes efforts made to determine and target the drivers of a cancer at a molecular level. To date, most analyses of tumours have been performed on histopathological tissue samples. In contrast, so called ‘liquid biopsies’ have the potential to facilitate diagnosis, prognosis, measurement of tumour burden and response to therapy via simple, non-invasive blood sampling. (Figure 1) Liquid biopsy has the potential to inform the treating physicians decisions in a number of ways including decision to omit or include adjuvant therapy after surgery by detecting minimal residual disease, detecting early recurrence and analysis of response to disease so the treatment can be altered if it is not effective. Here we review the most common types of ‘liquid biopsies’ available; namely, circulating tumour cells (CTCs), circulating tumour DNA (ctDNA),

Tissue biopsy is the traditional method of diagnosing cancer. While this is the best practice and continues to be standard of care for patients, it has considerable drawbacks. Tumours may present in challenging locations such as lung, liver or bone which cannot easily be accessed without interventional radiology expertise and space.1 For the patients, there are associated risks of pain, infection, bleeding and damage to local structures depending on the area which needs to be biopsied. Liquid biopsy by contrast, takes the form of a small volume (approx. 10 ml) venous blood sample which can be collected in a low cost, minimally invasive manner.2 After diagnosis and treatment commencement, it can be used in conjunction with tissue biopsy to assess response to treatment and disease relapse. Table 1 compares the advantages and disadvantages of these approaches.
CTCs are cells which have entered systemic circulation after dislodgement from the primary tumour.3 Some primary tumours shed millions of cells a day but only a tiny proportion of these will develop as a distant metastasis.4 Identification of the cell surface molecule epithelial cell adhesion molecule EpCAM3 is the most commonly used technique to identify CTCs. Tumour biomarker profiles, such as the HER2 receptor and oestrogen receptor status which are used to guide treatment, can change over the disease course thus sampling of a new metastasis is recommended.5 Obtaining a repeat biopsy is not always possible for a number of reasons such as deteriorating patient condition or a difficult to access tumour location. CTC examination has the potential to not only guide treatment decisions which will result in a better prognosis, but also to spare breast cancer patients the potential toxicities of treatments to which their cancer has become resistant. The DETECT III trial analysed patient who had HER2-
44 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: BREAST CANCER
Mary O’Reilly Michaela Higgins
Figure 1: Information which can be obtained from a liquid biopsy. Created using www.biorender.com
Figure 1: Information which can be obtained from a liquid biopsy. Created using www.biorender.com
Tissue Biopsy
Liquid Biopsy Advantages Disadvantages Advantages Disadvantages
Confers essential histological confirmation which remains the gold standard for cancer diagnosis

Cancers are heterogenous and a tissue biopsy only reflects one portion of the tumour
Potential to reflect the overall genomic landscape of the tumour
Can be difficult to access depending on site of tumour
Resource-intensive – expertise to perform the procedure, operating room and nursing staff
Easily collected peripheral blood sample
Less risk of patient morbidity related to an adverse event from the biopsy
Invasive/painful for the patient Can be serially collected to reflect tumour molecular changes over time eg to identify emergence of resistance to treatment
Long turnaround time – a number of weeks for next generation sequencing
Challenges with preparation and storage of samples may impact biomarker interpretation e.g. ER status of decalcified bone sample
Circulating tumour cells
Rapid turnaround time- 7-10 days for next generation sequencing results
Can be used as a complementary diagnostic tool for molecular analysis
Liquid biopsy technologies available predominantly in the research setting and require laboratory expertise which is not yet routinely available. The technologies themselves are evolving rapidly
Unable to detect histological tissue type from which the tumour arises
May not be sensitive enough to detect low volume disease eg detection of minimal residual disease or early recurrence
May be insufficient for next generation sequencing
positive CTCs but HER2-negative metastatic breast cancer (by prior tissue sample). Patients were randomised to receive either standard treatment or standard treatment with lapatinib6 which is a small molecule tyrosine kinase inhibitor targeting HER2. The study showed a survival advantage among patients who received lapatinib thus suggesting that CTC-driven change in therapy was useful and that the detection of HER2positive CTCs was predictive of benefit for patients. CTCs can also be useful to guide prognostication in breast cancer.
A pooled analysis of the phase II multicentre trials BEVERLY -1 and 2 showed that patients who had detectable CTCs at baseline had a shorter overall survival compared with those who did not .7 The SUCCESS A trial showed that in high risk early breast cancer the presence of CTCs two years after completion of chemotherapy was associated with decreased overall survival.8 The IMENEO study was a large international
meta-analysis which analysed the clinical validity of CTC count in early, non-metastatic breast cancer patients who received neoadjuvant therapy. Data from 2,156 patients was collected and again it was found that those who had CTC detected at baseline had worse overall survival outcomes.9 Gao et al10 analysed whether CTC could be used in breast cancer screening. They found that use of combined imaging, ultrasound or mammogram with CTC increased the accuracy of breast cancer diagnosis.
Although there has been great enthusiasm among patients and physicians to adopt routine usage of CTCs, there are a number of limitations. The studies above suggest that at present CTCs should be utilised as a complementary test performed to add information to other examinations. A significant limitation is the fact that CTC detection is based on identification of epithelial markers, which are
sometimes lost. Some CTCs undergo transformation from epithelial to mesenchymal cells. This transition increases the cells ability to establish peripheral micrometastases.3 These cells become undetectable by several commonly used detection methods.11 The laboratory techniques used to detect CTCs
and their sensitivities are very much in evolution and improving.12
Circulating tumour DNA
CTCs are cells which have entered systemic circulation after dislodgement from the primary tumour[3]. Some primary tumours shed millions of cells a day but only a tiny proportion of these will develop as a distant metastasis[4]. Identification of the cell surface molecule epithelial cell adhesion molecule EpCAM[3] is the most commonly used technique to identify CTCs. Tumour biomarker profiles, such as the HER2 receptor and oestrogen receptor status which are used to guide treatment, can change over the disease course thus sampling of a new metastasis is recommended[5]. Obtaining a repeat biopsy is not always possible for a number of reasons such as deteriorating patient condition or a difficult to access tumour location. CTC examination has the potential to not only guide treatment decisions which will result in a better prognosis, but also to spare breast cancer patients the potential toxicities of treatments to which their cancer has become resistant. The DETECT III trial analysed patient who had HER2-positive CTCs but HER2-negative metastatic breast cancer (by prior tissue sample). Patients were randomised to receive either standard treatment or standard treatment with lapatinib[6] which is a small molecule tyrosine kinase inhibitor targeting HER2. The study showed a survival advantage among patients who received lapatinib thus suggesting that CTC-driven change in therapy was useful and that the detection of HER2positive CTCs was predictive of benefit for patients. CTCs can also be useful to guide prognostication in breast cancer. A pooled analysis of the phase II multicentre trials BEVERLY -1 and 2 showed that patients who had detectable CTCs at
ctDNA is tumour- specific free DNA fragments released during apoptosis from the primary tumour or associated metastatic deposits which can serve as a biomarker for diagnosis, prognosis and
45 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Table 1: Comparison of liquid and tissue biopsies
The potential for the widespread clinical use of liquid biopsies in the detection and management of modern breast cancers is huge and attractive to patients and their treating physicians
Circulating Tumour DNA Circulating tumour cells Advantages Disadvantages Advantages Disadvantages
Allows for detection of presence of cancer even in cases of low tumour burden

Can be used to monitor response to therapy as well as development of resistance to therapy
Proteomic and metabolomic analysis not possible
Low signal to noise ratio in early stage disease
Proteomic and metabolomic analysis can be used to provide additional information
Allow structural evaluation of cancer phenotype
Heterogenicity of CTCs can make interpretation complicated
Not routinely available at present outside of research setting in Ireland. Work is needed to improve sensitivity and specificity of this test before they are incorporated into clinical practice
Not routinely available at present outside of research setting in Ireland. Work is needed to improve sensitivity and specificity of this test before they are incorporated into clinical practice
Exosomes
prediction.13 It is thought that ctDNA is released by all tumour sites; including metastases and therefore it may provide invaluable information with regards to tumour heterogeneity. Tumour-specific mutations allow the tumour DNA to be differentiated from other nonspecific free DNA which originates from normal cells. There are two ways in which ctDNA is currently used for therapeutics14; the first is using a targeted approach to detect specific mutations in genes which are actionable such as PIK3CA and ERBB2. The other method is an untargeted approach where whole genome sequencing is performed on the DNA. This yields a vast quantity of information only a small fraction of which may be actionable.
to two years earlier than imaging in patients with early- stage breast cancer.15 A number of studies have described concordance between primary tumour and ctDNA. Adalsteinsson et al analysed samples from 41 patients with either breast or prostate cancer, they found 88% concordance of clonal somatic mutations between ctDNA and matched tumour biopsies.16
Amenable to immunolabelling techniques
of ctDNA in their plasma to asses response to treatment. 19 of these patients (95%) had fluctuations in the levels of ctDNA detected which reflected response to therapy seen on imaging.
Exosomes
into protein identical to the original cancer cell. This method of communication confers phenotypic traits of the cancer to previously heathy cells. In this way some exosomes play a role in mediating cancer progression and metastasis.
A further type of liquid biopsy in development for breast cancer is based on exosomes. Exosomes are extracellular vesicles with a diameter ranging from 50 to 150nm. They are released from their cell of origin, enveloped in a lipid bilayer containing molecular constituents from their cell of origin such as DNA, RNA, proteins and metabolites [19]. Exosomes are stable and plentiful in bodily fluids. Patients with breast cancer have been shown to have substantially higher levels of exosomes in their blood than matched cancer free controls[20]. Given this distinction, there may be a role for the use of exosomes in the diagnosis detection of breast cancer. Exosomes from malignant cells have a different microRNA (miRNA) and protein profile compared to normal cells allowing analysis of the tumour status and potentially treatment response in vitro at a particular or serial timepoints[21]. Exosomes are taken up by receiver cells which can translate the miRNA into protein identical to the original cancer cell. This method of communication confers phenotypic traits of the cancer to previously heathy cells. In this way some exosomes play a role in mediating cancer progression and metastasis.
Conclusion
CtDNA can detect occult tumours and therefore has the potential to become gold standard for diagnosis to detect the presence of tumour DNA before it is identifiable on imaging. The first custom built next generation sequencing based ctDNA test, Signatera was developed in 2019. This technology set out to detect early disease recurrence after surgery as well as detection of post-operative minimal residual disease. Coombes et al demonstrated that this test could identify disease relapse up
The predictive value of ctDNA was evaluated in a pooled analysis of MONALEESA2,3 and 7 trials.17 The ctDNA from 1507 oestrogen receptor positive, HER2-negative metastatic breast cancer patients were analysed by next generation sequencing. Tumours harbouring mutations in FRS2, PRKCA, MDM2, ERBB2, AKT1 and/or BRCA1/2 identified by ctDNA were associated with improved progression free survival with the CDK4/6 inhibitor ribociclib in combination with endocrine therapy when compared to endocrine therapy alone. Dawson et al18 performed a proof of analysis study where they assessed the presence of ctDNA in patients with metastatic breast cancer and somatic genomic alterations. ctDNA was detected in 97% (29/30) of patients. 20 patients had serial assessment
A further type of liquid biopsy in development for breast cancer is based on exosomes. Exosomes are extracellular vesicles with a diameter ranging from 50 to 150nm. They are released from their cell of origin, enveloped in a lipid bilayer containing molecular constituents from their cell of origin such as DNA, RNA, proteins and metabolites.19 Exosomes are stable and plentiful in bodily fluids. Patients with breast cancer have been shown to have substantially higher levels of exosomes in their blood than matched cancer free controls.20 Given this distinction, there may be a role for the use of exosomes in the detection of breast cancer. Exosomes from malignant cells have a different microRNA (miRNA) and protein profile compared to normal cells allowing analysis of the tumour status and potentially treatment response in vitro at a particular or serial timepoints.21 Exosomes are taken up by receiver cells which can translate the miRNA
Conclusion
The potential for the widespread clinical use of liquid biopsies in the detection and management of modern breast cancers is huge and attractive to patients and their treating physicians. Liquid biopsies have various applications across many aspects of the breast cancer journey from molecular profiling at diagnosis which will guide therapeutic decisions; including identification of those who should receive adjuvant therapy to surveillance and early detection of occult metastases. Clinical studies suggest that several techniques measuring in particular circulating tumour cells or cell free tumour DNA, are sensitive, reliable and may rapidly inform treatment of patients with breast cancer. We anticipate and hope that these assays will become available to Irish patients outside the research setting in the next few years. References available on request
46 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: BREAST CANCER
detected in 97% (29/30) of patients. 20 patients had serial assessment of ctDNA in their
Table 2: Comparison of ctDNA and CTCs
plasma to asses response to treatment. 19 of these patients (95%) had fluctuations in the levels of ctDNA detected which reflected response to therapy seen on imaging.
Table 2: Comparison of ctDNA and CTCs

NOW
(upadacitinib) INDICATED
ULCERATIVE COLITIS1
is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1 AbbVie® is a registered trademark of AbbVie Inc. RINVOQ® and its design are registered trademarks of AbbVie Inc. REFERENCES: 1. RINVOQ® summary of product characteristics, available at www.medicines.ie. JAK: Janus kinase; UC: ulcerative colitis. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. Legal Classification: POM(S1A). IE-RNQG-220007 | August 2022 Full Summary of Product Characteristics is available at medicines.ie ▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie. Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
RINVOQ®
FOR ADULTS WITH MODERATE TO SEVERELY ACTIVE
*RINVOQ®
Biomarkers for Cancer Associated Venous Thromboembolism
 Written by Lucy Norris PhD. Coagulation research laboratory, Dept of Obstetrics and Gynaecology, Trinity St. James’s Cancer Institute, Trinity Centre for Health Sciences, St. James Hospital, Dublin 8
Written by Lucy Norris PhD. Coagulation research laboratory, Dept of Obstetrics and Gynaecology, Trinity St. James’s Cancer Institute, Trinity Centre for Health Sciences, St. James Hospital, Dublin 8

Cancer associated venous thromboembolism
Venous thromboembolism(VTE) is a potentially lethal complication of cancer and is the leading cause of death in cancer patients after the cancer itself. Cancer associated VTE increases morbidity and can impact patient’s quality of life, frequently resulting in delays or interruptions in cancer treatment. Despite advances in preventative treatment, the rate of cancer associated VTE has risen substantially in the last 20 years particularly in patients receiving chemotherapy. Up to 20% of patients will experience VTE during their cancer journey and the risk of VTE in cancer patients is 4-7-fold higher than in the general population. Patient, tumour, and treatment factors all contribute to the risk of VTE in cancer. Tumour site is a major determinant of VTE risk; pancreatic, brain, ovarian and lung cancer are associated with the highest VTE rates. The risk of VTE in cancer patients is increasing in tandem with the rising rate of cancer worldwide and the increased survival of cancer patients.
Cancer treatments are a significant contributor to VTE risk, in particular cancer surgery with resulting immobility and hospital stay. Chemotherapy is also a major contributor to VTE risk. The effects of chemotherapy and surgery can be additive. Ovarian cancer patients who cannot be optimally debulked are frequently treated with neoadjuvant platinum-based chemotherapy followed by interval
debulking surgery which further exacerbates VTE risk. Recent studies of patients with ovarian cancer receiving neoadjuvant chemotherapy have reported VTE rates as high as 27%.

Emerging evidence suggests that newer targeted cancer therapies are also prothrombotic. Immune checkpoint inhibitors, frequently used in lung cancer, are associated with an increased risk of both venous and arterial
thrombotic events, particularly in those tumours with specific molecular profiles eg ALK-/ROStranslocations in non small cell lung cancer. It is unclear however whether these events are linked to the biology of the underlying cancer or are due to a mechanistic effect of the therapy.
Pathogenesis of VTE in cancer
The pathogenesis of VTE was described by Virchow in the middle of the 19th century as a triad of venous stasis, hypercoagulability and endothelial damage which interact to promote the development of a thrombus (Figure 1). In a cancer patient, all three components of the Virchow’s triad are present. A bulky tumour can lead to venous compression and venous stasis, tumours express procoagulant material which, when released into the circulation, can trigger activation of the coagulation cascade. Damage to the vascular endothelium due to chemotherapy or surgery creates a procoagulant surface for the developing thrombus.

Cancer is now recognised as a pro-inflammatory condition. Reports suggest that tumours express pro-inflammatory cytokines which interact with the coagulation pathway (Figure 2).
Obesity, a risk factor for many cancers, has been associated with a state of low-grade systemic inflammation. This is characterized by an adipose tissue driven
48 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: VTE
Figure 1. Virchow’s Triad
acute-phase response resulting in secretion of cytokines.
VTE risk assessment in cancerthe importance of biomarkers
Although VTE is a significant cause of morbidity and mortality in the general population, effective anticoagulant treatments are available for treatment and prevention of this disease. However VTE in cancer patients represents a significant clinical problem, since these patients have higher rates of both bleeding and VTE recurrence compared with the non-cancer population. This highlights the need for a careful risk/benefit assessment of prophylaxis.

Patients are particularly at risk of VTE following cancer surgery. As a result, international guidelines recommend extended (28 day) prophylaxis with Low Molecular Weight Heparin(LMWH) for all patients having major pelvic/ abdominal cancer surgery.

However, VTE still occurs in a significant number of patients post-surgery despite extended LMWH prophylaxis which suggests that the dose of LMWH or duration of prophylaxis may
be inadequate in these patients. In contrast, recent advances in minimally invasive surgery (MIS) have led to questions regarding the benefits of extended prophylaxis in lower risk women undergoing MIS. VTE rates following MIS in cancer patients are reported to be low and many investigators feel that extended prophylaxis is neither warranted or beneficial in these patients. Current recommended risk assessment methods for surgical patients (Caprini score) have limited sensitivity in cancer patients and are seldom used in practice.
In patients undergoing chemotherapy, recent guidelines have suggested that primary VTE prophylaxis with direct oral anticoagulants (DOACs) should be considered for intermediate/high risk ambulatory cancer patients following risk assessment. However, in practice uptake is low, even in high risk patients, due largely to concerns over reported bleeding risks associated with DOACS.
The recommended tool for VTE risk assessment during
chemotherapy is the Khorana score, a risk model based on five easily available clinical parameters which has been extensively validated in mixed cancer studies. However, the Khorana score performs poorly in in some patient groups including those at high risk of thrombosis such as lung and ovarian cancer. The guidelines recommend a single assessment prior to treatment but the interplay between cancer, coagulation activation and chemotherapy is a dynamic process and is unlikely to be accurately represented by a single risk assessment at the start of therapy. For optimal VTE prophylaxis in the cancer patients, ongoing VTE risk assessment is required.
Biomarkers for pro-coagulant activity offer the opportunity for serial assessment of cancer patients This approach offers a more tailored approach to prophylaxis, minimising the risk of bleeding complications. Research by our group and others has identified several biomarkers which can be used alone or in combination with clinical variables for prediction of VTE in cancer patients.
BIOMARKERS FOR PREDICTION OF VTE IN CANCER
D-dimer
D-dimer is the fragment produced when fibrin is cleaved and thus represents fibrin turnover and coagulation activation. D-dimer is widely used as a diagnostic biomarker for VTE, although highly sensitive and easily measured in the hospital laboratory, the specificity of D-dimer is reduced in many conditions including infection, surgery, and cardiovascular disease. D-dimer has been identified as the strongest prognostic biomarker for VTE in patients with cancer. The Vienna CAT score is a nomogram combining cancer site with D-dimer levels for prediction of VTE in a mixed cancer population. In lung cancer and in pancreatic cancer D-dimer pre-treatment levels have been used to predict VTE in patients undergoing chemotherapy. However due to the low specificity of D-dimer, the positive predictability of this biomarker is low. In ovarian cancer, D-dimer is used to triage patients and high levels are an indicator of advanced disease rather than VTE risk. Several groups have attempted to dynamically assess D-dimer levels as a marker for VTE during chemotherapy with limited success due to the variability of D-dimer during treatment and the difficulty of selecting appropriate cut-offs to account for comorbidities.
Thrombin generation
Global markers of hypercoagulability are attractive candidates as predictors of VTE as they capture the composite effects of activators and inhibitors of the haemostatic system which drive thrombus formation. The thrombin generation assay quantifies the enzymatic thrombin activity that can be triggered in plasma. Measurement of thrombin generation has a positive predictive value independent of D-Dimer. Recent developments have transformed the thrombin generation assay into a high throughput assay suitable for clinical use however there are still issues with variability and thrombin generation has yet to gain widespread acceptance in the clinic.
We and others have shown that thrombin generation is increased in patients who subsequently
49 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Figure 2: Pathogenesis of VTE in cancer patients
develop cancer associated VTE and can be used as a predictive biomarker. Our group has developed the Thrombogyn score, a risk score developed from clinical risk factors for prediction of VTE in gynaecological cancer patients post surgery. We extended the Thrombogyn score with the addition of two biomarkers thrombin generation (ETP) and D-dimer, which were measured pre-operatively. Using this extended Thrombogyn score, a high risk group was identified of which, 42% of patients developed VTE. The extended score had a sensitivity of 95.7% compared with 72% for the score without biomarker data. The Khorana score has a sensitivity of 35-50% for high risk patients.
Factor VIIIc
Further work by our group has identified the key determinants of the increased thrombin generation in cancer patients who develop VTE. In both treatment naïve and neoadjuvant treated gynaecological cancer patients, factor VIIIc was increased in cancer patients who subsequently developed VTE compared with those who remained thrombosis free. Raised factor VIIIc levels were associated with 2.8 fold increase in VTE adjusted for age, stage of cancer and chemotherapy. This is in agreement with several groups who have shown that Factor VIIIc can predict VTE in a mixed group of cancer patients.
Thrombomodulin and the activated protein C pathway
The protein C pathway is a major regulator of thrombin
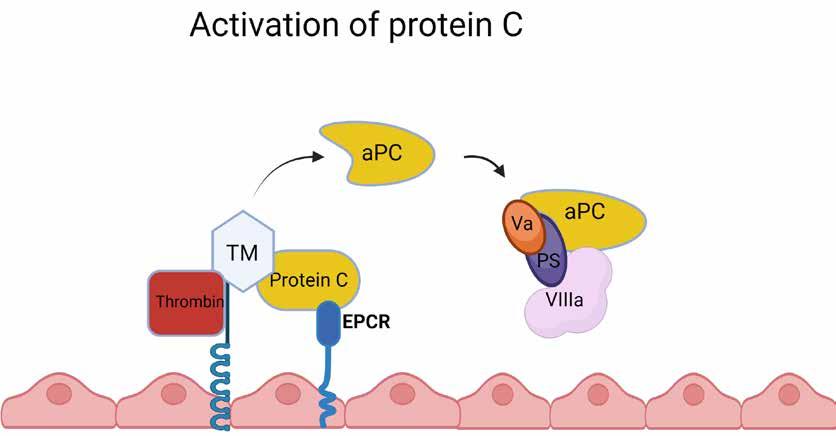
production. Binding of thrombin to thrombomodulin(TM) on the endothelial cell surface leads to the activation of protein C(aPC) which is accelerated by the endothelial protein C receptor(EPCR) (Figure 3).
Endothelial damage resulting in reduced expression of TM on the endothelial surface can cause a reduction in the activation of protein C. Failure of protein C activation or resistance to the inhibitory effects of aPC (both genetic and acquired) is a common cause of VTE in the non cancer population. Our recent data has shown that chemotherapy alters the aPC pathway. We observed reduced soluble TM in patients who developed VTE following neoadjuvant chemotherapy suggesting that reduced generation of aPC may be implicated in chemotherapy associated VTE. TM levels were inversely correlated with thrombin generation suggesting a direct relationship between TM and thrombus formation in cancer associated VTE. These changes do not occur in similar patients who are treatment naïve suggesting that this is a treatment effect.
P-selectin
In vivo and in vitro data published by our group has shown that platelets play a role in metastasis, forming a so called “platelet cloak” around tumour cells as they escape from the primary tumour into the circulation. This allows them to evade natural defence mechanisms in the body and is

linked to poor prognosis. The adhesion molecule P-selectin, is expressed on the platelet membrane following platelet activation. s-P- Selectin is cleaved and shed in the circulation and has been reported as a marker of VTE in cancer patients. This underlines the role that platelets have not only in metastasis, but also in cancer associated VTE. Recent studies have shown that the mechanisms by which P-selectin mediates cancer associated VTE are linked to cancer associated inflammation and metastasis. P-selectin has been shown to be an independent predictor of VTE and, along with factor VIII and D-dimer, was elevated longitudinally in a small cohort of cancer patients sampled serially prior to the development of VTE.
New approaches to biomarker discovery

Our research and those of many groups has focussed on investigating procoagulant biomarkers for VTE in circulating
blood. With the availability of multiplex assays and arrays, several hundred potential biomarkers can be investigated from a small sample. Using this approach, candidate biomarkers unrelated to coagulation such as stromal cell–derived factor-1 (SDF1), thyroid-stimulating hormone (TSH), and monocyte chemotactic protein 4 and growth hormone (GH) and interleukin-1 receptor type 1 (IL-1R1) have recently been identified. The mechanism by which these cytokines and hormones induce thrombus formation is not understood and prospective longitudinal analysis will determine their future role in VTE risk prediction. At a molecular level, tumour expressed microRNAs have been shown to be predictive of VTE in colorectal cancer. In glioblastoma, targeted next generation sequencing has been used to identify tumour mutations associated with VTE.
Conclusion
Cancer associated VTE is a significant medical problem which persists throughout the course of the disease. Effective and safe prevention of VTE is required particularly in the era of evolving targeted therapies with unknown thrombotic potential. Current risk models do not provide the sensitivity to accurately identify patients who will most benefit from prophylaxis. Serial measurement of biomarkers used alone, or in combination with clinical risk factors can increase the sensitivity of risk prediction and allow personalised treatment so that the right patient gets prophylaxis at the right time. Longitudinal studies are required to identify the most effective combinations for dynamic assessment of VTE risk.
References available on request
RESPIRATORY: PNEUMONIA FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
50 ONCOLOGY: VTE
Figure 3. Activation of protein C
Breakthrough in research for Metastatic Breast Cancer
New figures announced by Breast Cancer Ireland point to a substantial increase in survival rates beyond 5 years of those living with metastatic breast cancer, the most severe form of the disease, from 19% to 32%*. Ireland’s leading breast cancer charity has also revealed for the first time that there are approximately 950** females in Ireland living with distant metastatic disease or metastatic breast cancer, the most challenging area from a scientific research and treatment perspective. Although rates of recovery from metastatic breast cancer are lower than for other forms of cancer, the number of women living in Ireland with the disease is steadily growing, with continuing clinical trials development, creating new and improved treatments and therapies.
Hope can also be found in the results of ground-breaking new research conducted by the Breast Cancer Ireland funded research team at RCSI, University of Medicine and Health Sciences in Dublin, which has uncovered a potential new target to manage breast cancer that has spread to the brain.
 Prof Leonie Young, Scientific Director of the Beaumont
Prof Leonie Young, Scientific Director of the Beaumont
RCSI Cancer Centre and leader of the Endocrine Oncology Research Group at RCSI explains “When it comes to brain metastases, clinical management includes surgical resection or radiation therapy –currently there are no targeted therapeutic options. However, in looking at patient tumours from both the breast and the brain, our research team has identified RNA Methylation as an essential mechanism which enables the tumour to evolve and progress. Our research has shown that targeting this mechanism, with drugs currently in trial for other cancer types (FTO Inhibitors) could prove significantly beneficial for patients with breast cancer that has metastasized to the brain.
In addition to the announcement of these research findings and to mark World Cancer Day on 4th February, Breast Cancer Ireland has launched #MetastaticMatters a campaign focussing on those living with a metastatic diagnosis***. The campaign aims to highlight the symptoms of metastatic disease to be aware of, and also to increase understanding of how and why this occurs. The campaign will be supported by a heavyweight digital, PR and communications campaign ensuring that the
voices and concerns of those living with metastatic disease in Ireland are heard, understood and valued - making the breast cancer community feel like a place that ALL those affected belong.


Speaking at the launch of the campaign, Breast Cancer Ireland
CEO Aisling Hurley said, “The narrative around metastatic breast cancer needs to evolve… and it is beginning to do so. It is undeniably a very worrying diagnosis but from a research perspective, there is hope to be found and findings indicate that it is getting more hopeful by the day. We see many women and men in this country living with metastatic disease…and in fact living well. In this sense, it’s important to drive conversations around metastatic breast cancer and show what it can look like, and what it does look like for so many people. The idea of living with an incurable disease can be a hard one to get
our heads around, but in the last 5 years significant strides are being made to change the prognosis of stage four breast cancer, helping patients live longer, fuller lives”
Rachel McKenna, a Breast Cancer Ireland Patient Supporter, currently living with stage 4 breast and bone cancer added “The dichotomy of metastatic breast cancer often creates confusion - a stage four cancer diagnosis may become terminal and yet it's possible to live a full life with this incurable illness for a potentially long time. It’s hard to get your ahead around at times - we'll be on treatment for the rest of our life, but people live with this – and many live well. A diagnosis of metastatic cancer is serious and incredibly scary, but as treatments advance, and research is underway by some of the finest cancer experts across the globe, patients have more options than ever”
To mark World Cancer Day on 4th February, Breast Cancer Ireland has also announced its funding of The Orla Byrne PHD Scholarship, awarded to student Louise Watson at RCSI – a four-year study into understanding key regulatory networks in breast cancer brain metastases. Orla Byrne was a much-loved Patient Ambassador for Breast Cancer Ireland for many years, and an Outreach & Education Coordinator, who sadly passed away from metastatic breast cancer in November 2021 aged just 42.
51 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
ONCOLOGY: NEWS
Breast Cancer Ireland CEO, Aisling Huley
“The narrative around metastatic breast cancer needs to evolve… and it is beginning to do so. It is undeniably a very worrying diagnosis but from a research perspective, there is hope to be found and findings indicate that it is getting more hopeful by the day”
Why does cancer occur and is it possible to avoid its development?
Written by Tatiana Iarmak – http:// orcid.org/0000-0001-5371-2958. Lecturer of the Department of Advanced Training of Junior Medical Specialists at the Municipal Health Care Institution 'Kharkiv Regional Medical Vocational College', Kharkiv, Ukraine. An independent trainer, consultant to medical facilities and non-medical facilities to instruct medical staff on how to safely provide services to patients/clients.

Corresponding Author details: iarmak.tat@gmail.com
Ancient medicine left a huge legacy to the modern medicine, which has been using very actively the relevant today knowledge of ancient Greek, ancient Roman and other physicians, philosophers, and scientists. The tumors have been known for a very long time. Information about them was first obtained from the ancient Egyptian manuscripts “Papyrus of Edwin Smith” and “Papyrus of Ebers” (1600 BC). In ancient Egypt, not only bodies, but also individual organs of dead people were mummified - during mummification, the malignant formations are preserved much better than healthy tissues, thanks to that, modern scientists have information about the ancient Egyptians, who had cancer of various localizations more than 4000 years ago. With the help of modern technologies, such as computed tomography (CT) and others, the scientists can discover the traces of tumors in ancient mummies. The scientists all over the world have been studying tumors for many years. Michael Zimmerman, paleopathologist, professor at Villanova University (USA) and at the KNH Center for Biomedical Egyptology at the University of Manchester, for the first time histologically verified the
diagnosis of the colorectal cancer, while studying the mummy of an ancient Egyptian. The traces of a tumor – a fibrous dysplasia in the rib of a Neanderthal man, who lived about 120 thousand years ago, was discovered in Croatia more than 100 years ago, and only today this find has attracted the attention of the scientists from the University of Pennsylvania (USA) and is considered to be the oldest archaeological find. Dr. Kat Arney
– a British writer and scientist, who took part in the study of the remains of the Neanderthal, in her book "Rebel Cell: Cancer, evolution and the science of life" (2020) talks about the role of cancer in human life and how it can be stopped. Ann Rosalie David – a British egyptologist and emeritus professor at the University of Manchester with Michael R. Zimmerman in her book “Cancer: an old disease, a new disease or something in between?” (2010) talks about the role of the environmental carcinogens in the occurrence of tumors, the need for a deeper study of their etiology and pathogenesis, and the development of the effective prevention methods.

The “father of medicine”, Hippocrates, in his work “Carcinoma” for the first time called the tumor “karkinos” (cancer or crab), since it looks like these crustacean animals. Hippocrates gave a description to many types of cancer: skin, nasopharynx, breast, rectum, etc. He also introduced the term "onkos", oncology was named from - a science, which
studies the causes of tumors, the mechanisms of their development, the clinical course and develops the diagnostic methods, treatment and prevention. Aulus Cornelius Celsus - an ancient Roman physician, translated the Greek word καρκίνος into Latin cancer - this term is used in modern medicine today. Hippocrates and Celsus considered the main method of treatment to be the surgical removal of the external tumors, however, operations were not recommended for the internal tumors, because in ancient times, complex operations were more lethal than the tumor itself. In the medical treatises of ancient India, China, Babylon, there are also references to malignant neoplasms. Swiss physician, alchemist, philosopher of the Renaissance, one of the founders of iatrochemistry (the direction of alchemy of the 16th-17th centuries for the preparation of chemical drugs), Paracelsus, described the miners' disease "Schneeberg lung disease" or "Schneeberg miners' disease", which main cause was a constant inhalation of the ore dust rocks, containing the radioactive substances, in particular, radon

RESPIRATORY: PNEUMONIA FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 52 ONCOLOGY: CANCER DEVELOPMENT
“Not every cause which reaches the body has an effect on it and no disease can be caused without a corresponding susceptibility of the organism.” Avicenna
A tumour removed by surgery in 1689)
and its decay products in the stagnant air of mines. This lung disease was later identified as the lung cancer. An English surgeon Percivall Pott proved in 1775 that chemicals can be carcinogens, i.e. cause the development of malignant tumors. He described the scrotum cancer in chimney sweepers, caused by the soot from chimneys, and called it the "chimney sweep cancer". Chimney sweepers themselves called this disease the "soot wart." The world's first law to protect a person from carcinogens in the workplace was passed on July 5, 1788.
Until the 19th century, the oncological diseases were recorded quite rarely. An incredible growth of the oncopathology has arisen, particularly, in the last two decades, which is more associated with the various bad habits (smoking, drinking alcohol, unhealthy diet, etc.), polluted ecology, and an increase in the human life expectancy. Cancer is the second leading cause of death in the world after cardiovascular diseases, but the upward trend in oncological diseases may soon put it in a leading position. The variety of species, types, and forms of malignant tumors is causing an increasing concern among specialists. The cause, the mechanism of the occurrence, the origin of the malignant tumors are not fully understood. A number of outstanding scientists have created many theories of the emergence and the development of tumors. German scientist of the second half of the 19th century, Rudolf Virchow, is the founder of
a cellular pathology, a creator of the theory of irritation, in other words, a the theory of the onset of tumors, according to which, a frequent irritation or trauma of the tissues of the human body (stomach, rectum, cervix, and other organs) accelerates the processes of the cell division and can cause their malignant growth.
In 1911, Francis Peyton Rous, an American pathologist, Nobel laureate for his discovery of tumorinducing viruses (1966) proved that a malignant tumor of chickens (Rous sarcoma) can be transmitted from a sick bird to a healthy one by the acellular filtrates – viruses, which are the etiological factor in the origin of tumors. The viral theory of cancer had been making its way for many years. Rous received the prize only 55 years after his discovery. Many scientists over time have confirmed the viral and infectious nature of cancer, i.e. an ability to be transmitted from person to person. The theory has more than 110 years of history. 20% of all tumors in the world are caused by viruses.
Currently, the viruses which cause tumors in humans include:
a. Epstein-Barr virus (EBV) or human herpes virus type 4 (HHV4) - the first isolated virus, which causes Burkitt's lymphoma (1964), nasopharyngeal cancer (carcinoma), stomach cancer, and many diseases of infectious (infectious mononucleosis, etc.) and of non-infectious nature. EBV is one of the most common viruses among the world's population (latent carriage - 95%) - human papilloma virus. Today, more than 100 types of HPV are known.
b. Harald zur Hausen, a German scientist, isolated the first oncogenic human papillomavirus (HPV) type 16 in 1983 and in 1984 - HPV type 18. It is these two types of HPV that cause 70% of cases of anogenital cancer in men and women. In 2008, Hausen received the Nobel Prize for his discovery of the human papillomavirus as the cause of cervical cancer. HPV is most commonly transmitted sexually (80%); a possible infection of the fetus from the mother during childbirth, through microtrauma of the mucous membrane of different localization (larynx, pharynx, etc.) and skin. A latent carriage under the adverse conditions can turn into cancerous tumors within 15-20 years. The first HPV vaccine was introduced in 2006 (developed by Australian scientist Ian Frazer and Chinese scientist Zhou Jian), which protects against the development of the cervical cancer. In 2019, Ian Frazer and Chinese scientists at the Xiamen University started to develop a new vaccine, which will help stop the development of cancer in people who already have it.


c. hepatitis B virus (HBV) was discovered in 1963 by Baruch Blumberg, an American scientist, who received the Nobel Prize for this discovery in 1976. Hepatitis B is transmitted parenterallythrough medical instruments or materials infected with blood or biological fluids (during manipulations, operations, dental procedures); damaged skin and mucous membranes (piercing, tattoo, manicure, pedicure), intravenous drug injections; sexually, from mother
to fetus during childbirth, with breast milk; kisses (saliva), transplantation (transplantation of organs and tissues), by fomite transmission (personal hygiene items-toothbrush, shaving razor, scissors). In 5% of the patients with chronic hepatitis B, the primary liver cancer develops and is more often recorded in men, suffering from cirrhosis of the liver (20%). Maurice Hilleman – an American vaccinologist developed the first plasma vaccine in 1981. Subsequently, the vaccine has been improved many times.
Vaccination against hepatitis B is included in the vaccination calendar in all countries of Europe and America. Thanks to the vaccine, the incidence of hepatitis B has decreased by 80%-90%.
d. hepatitis D virus (HDV)discovered by an award-winning Italian virologist Mario Rizzetto in 1977 in patients with hepatitis B. Hepatitis D develops only in the presence of the hepatitis B virus in the human body and provokes the occurrence of the hepatocellular carcinoma (the primary cancer). Transmission routes are identical to hepatitis B. The hepatitis B vaccine protects against the hepatitis D infection.
e. hepatitis C virus (HCV) was discovered in 1989 by American scientists Harvey J. Alter, Michael Houghton and Charles M. Rice, who jointly became Nobel Prize winners for their discovery of the Hepatitis C virus in 2020. The hepatitis C is called an "affectionate killer”, because it can be latent in the human body for many years. Chronic hepatitis C can cause cirrhosis of the liver and a hepatocellular carcinoma (primary liver cancer). The routes of transmission are identical to the hepatitis B. The vaccine has not been developed yet.
f. human T-lymphotropic virus (HTLV-1) - an oncogenic retrovirus - the causative agent T-cell lymphoma mostly in adults and a HTLV-1-associated myelopathy (tropical spastic paraparesis). The routes of transmission are identical to the hepatitis B and C. Lifelong carriage.
g. herpes virus HHV-8, associated with the Kaposi's sarcoma was discovered in 1994 by an American virologist, professor of the American Cancer Society, Yuan Chang. She received numerous awards for her cancer researches. The transmission routes of HHV-8 are identical to those of the hepatitis B, C, human T-lymphotropic viruses. In
53 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
One of the most common cancers in Ireland is bowel cancer which kills about 1,000 people every year. Most patients are unaware of the disease, because in the early stages there are no manifestations. The incidence of cancer increases with age. However, in recent years, 400,000 children have been diagnosed with cancer every year, which has become one of the main causes of death among children and adolescents.
addition to blood, the saliva poses a serious danger. Crowded living, non-compliance with the sanitary and hygienic rules can lead to early infection in childhood.
h. Merkel cell polyomavirus provokes Merkel's sarcoma - an aggressive form of the skin cancer, more common in the elderly and immunocompromised people; was identified by Yuan Chang in 2008.

i. A virus, which causes severe complications in patients with glioblastoma (a brain tumor is one of the most lethal types of human cancer) is Cytomegalovirus (CMV); it belongs to the Herpesviridae type 5 family (HHV-5). CMV was first discovered by the German pathologist Hugo Ribbert in 1881 and isolated in 1956 by American scientists Thomas Huckle Weller, M. Smith and Wallace P. Rowe. CMV does not cause cancer, but it can accelerate the development, complicate the course of an existing disease and lead to death due to the reactivation (studies by Chang-Hyuk Kwon, Ohio State University Comprehensive Cancer Center). The infection occurs similarly to the hepatitis B, C, D, and also transplacentally (penetrates the blood-placental barrier) at any stage of pregnancy, provoking the occurrence of the intrauterine infections. The virus is contained in almost all human biological fluids: blood, semen, vaginal secretions, saliva, tears, etc. and persists for life in the human body (the latent carriage from 60% to 90% of the world population). All the viral infections which provoke the development of cancer are the infections, associated with the provision of medical care (hospital infections), and often occur in medical institutions when the anti-epidemic and sanitary and hygienic regimens are not observed.
They are also included in the list of absolute contradictions for donating blood.
Helicobacter pylori (H. Pylori) is a causative agent of the intestinal bacterial infection, discovered in 1979 by Australian scientists John Robin Warren and Barry James Marshall. For this discovery they received the Nobel Prize in 2005. H. pylori is registered in all countries of the world. In developed countries, the level of latent carriage of H. pylori is 15-30%, in developing countries - 50-70%. In 1994, the International Agency for Research on Cancer (IARC) recognized the H. pylori infection as the firstorder carcinogen, which means that H. pylori has an unconditional link with the occurrence of the stomach cancer. H. pylori lives in the stomach, provokes the development of gastritis, gastric and duodenal ulcers, leading to cancer. Non-observance of the sanitary and hygienic rules plays an negative role in the transmission of infection, so the main route of transmission is fomite transmission, in other words, by touching the contaminated household objects. The transmission factors are dirty hands, other people's dishes (cups, spoons, etc.), even kisses. It is also possible to become infected with H. pylori during the passage of an endoscopic examination – fibrogastroscopy, because of a poor-quality disinfection and sterilization of a fibrogastroscope. Also, in the formation of oncogenesis a serious threat is posed by the parasitic diseases (helminthiases) - a class of trematodes: a. Schistosoma haematobium causes squamous cell carcinoma of the bladder. Infection is possible through a contact with water, polluted with the helminth eggs (bathing,
fishing). b. Clonorchis sinensis provokes the gallbladder cancer (cholangiocarcinoma).
c. Opisthorchis Viverrini causes liver cancer, hepatocellular carcinoma and pancreatic cancer. Clonorchiasis and opisthorchiasis can be contracted by eating raw, lightly salted or undercooked freshwater fish. In case of opisthorchiasis, the infection occurs more often by the fish which is part of the carp family. These types of helminthiases are included in the IARC list of carcinogens.
In 1965, by the decision of the World Health Assembly (WHA), the International Agency for Research on Cancer (IARC) was established as an independent structure of WHO. IARC has coordinated the international researches of the causes of cancer in humans, has studied the mechanisms of carcinogenesis, and has developed the cancer prevention strategies, especially in low- and middle-income regions. The results of the IARC researches have served as the basis for the development of the cancer prevention and control programs. The IARC list includes 116 names of carcinogens (from the Latin “cancer” - cancer and ancient Greek "γεννάω" - I give birth), i.e. substances which contribute to the development of cancer. They are divided into: a. chemical: benzene is part of gasoline, plastics, dyes, rubber, some drugs; dioxins are formed during the burning of the household waste; tobacco products; food additives - E 121, E 123, etc., prohibited by law (WHO, UN organizations - Food and Agriculture Organization - FAO); ethanol in alcoholic beverages, etc.; b. physical: ionizing and ultraviolet radiation; c. biological: viruses, bacteria, helminthiases (84% - WHO data).
Chronic infections, malnutrition, stress upset the balance of the human body and lead to a decrease in immunity and can cause the development of cancer. According to the immunological theory, when the function of the immune system is impaired, the cancer cells “escape” without being recognized by the immune system, which leads to the appearance of neoplasms.
In 2018, James P. Allison, an American immunologist and a Japanese scientist Tasuku Honjo received the Nobel Prize for their discovery of the anticancer therapy by suppressing the negative immune regulation. Thanks to their discovery, the drugs for tumor immunotherapy have been created in recent years, which can slow down the process of the tumor development even at the fourth stage. Even Hippocrates
spoke about the psychological factors that affect health and provoke the development of tumors and recommended "to beware of the emotional unrest ...". An ancient Roman physician and philosopher Galen suggested, that the temperament affects the development of tumors: melancholic people get cancer more often than the sanguine people. The psychosomatic theory of cancer is directly related to stress. Stress (tension, pressure) is the reaction of the human body in response to various kinds of stimuli - stressors, as negative (distress) associated with the long-term experiences - trauma, loss of a loved one, conflicts in the family, at work; abrupt changes in lifestyle - retirement, frequent relocations, emigration, war, etc., and positive (eustress) naturemarriage, childbirth, admission to the University, etc. Most adults and elderly cancer patients do not want to talk about their problems, but carry them in themselves, thereby, aggravating the disease, while many children, adolescents, young people with cancer actively use the concept of “oncological coming out” (“disclosure”, "exit") - they share their experiences on social media; participate in forums, talk shows; seek help, emotional and financial support. On the contrary, adults and elderly people are mostly not ready to open up, share, and some do not own or do not have modern gadgets. A Canadian scientist, the founder of the doctrine of stress, Hans Selye, in 1936 published his first work “Syndrome caused by various damaging agents”, in which he used the term “general adaptation syndrome” (from Latin “adaptare” - to adapt, Greek “syndrome” - combination). Stress plays a huge role in everyone's life. It can be acute, which ends quickly and chronic or permanent, debilitating, leading to poor health, depression (often a person feels very lonely, unwanted) and the possible occurrence of cancer. Stress releases the stress hormones adrenaline, norepinephrine, and cortisol. The researches by scientists from the United States and Germany have shown that after a few years of recovery from cancer, the immune cells (neutrophils) and the increased levels of the hormones norepinephrine and cortisol can contribute to the recurrence of tumors, which leads to death, as well as to the development of oncology at an early age. Most types of cancer are genetically determined, but not all and not always are inherited. There are many more theories, however, in recent years, scientists have come to a conclusion that the development of cancer comes from the influence of many risk
RESPIRATORY: PNEUMONIA FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 54 ONCOLOGY: CANCER DEVELOPMENT
factors, the factors potentially dangerous to human health, contributing to the occurrence of diseases, in particular, the malignant tumors. These include: the use of tobacco, alcohol; chronic infections; abortions and childbirth of the first child after 35 years; living near power lines; lack of physical activity, malnutrition - fast food, high-calorie, fatty, spicy foods, lack or few vegetable and fruit in the diet; obesity or overweight; negative emotional state; stress and nervous strain; polluted ecology and more (WHO data). The polyetiological theory of cancer – when the carcinogenic factors (chemical physical, biological), infectious, parasitic, fungal diseases; the state of the immune, neurohumoral systems of the human body; genetic predisposition, risk factors (smoking, alcohol, etc.), polluted environment, contribute to the emergence and the development of cancer.
Cancer is a global problem. Cancer affects all the organs and tissues of a person. 19.3 million people worldwide developed cancer in 2020, out of which 10 million died. The number of cancer cases by 2040 will be 47% higher than in 2020. Every 5th inhabitant on Earth gets cancer, every 8th man and 11th woman die. 70% of all deaths occur in countries with low and middle living standards (WHO data). The most commonly diagnosed cancers are breast, lung, colon, rectum, prostate and stomach ones. One of the most common


cancers in Ireland is bowel cancer which kills about 1,000 people every year. Most patients are unaware of the disease, because in the early stages there are no manifestations. The incidence of cancer increases with age. However, in recent years, 400,000 children have been diagnosed with cancer every year, which has become one of the main causes of death among children and adolescents. In 2050, 27 million new cases of cancer and 17.5 million deaths are being forecast (International Union Against Cancer, UICC). On February 4, 2021, the WHO Regional Office for Europe (WHO/Europe) launched an initiative “United Action against Cancer” - from prevention and early detection to diagnosis, treatment and palliative care. In the framework of this initiative, the innovative approaches to the fight against cancer are being developed. The long-term goal is “the elimination of cancer as a life-threatening disease in the European Region”. Since February 4, 2005, the World Cancer Day has been celebrated annually under the auspices of the International Union Against Cancer, UICC in order to draw the attention of the world governments and public organizations to the problem of cancer. The World Cancer Day in 2022-2024 has been dedicated to the theme - "Everyone deserves access to cancer care". Every year the World Breast Cancer Day is celebrated in October and the World Pancreatic Cancer Day (initiated by WHO) in November. On November 17, 2021, the WHO
launched a historic movement, bringing together the global community to eliminate the cervical cancer for the first time and unveiled the Global Strategy to Accelerate the Elimination of the Cervical Cancer as a Public Health Issue. The resolution was adopted by 194 countries. In December 2022, WHO presented data on the resistance of the bacterial infections to the antimicrobial drugs (AMR) - more than 50% are resistant. Bacterial infections are common complications of cancer that must be treated with antibiotics. AMR will slow the progress in cancer prevention and treatment for many years. “Antimicrobial resistance undermines the modern medicine and endangers the lives of millions of people,” WHO Director-General Tedros Adhanom Ghebreyesus said in the report. The tumors of breast and cervix are women's health and every woman has the right to be healthy. According to WHO, the mortality from cervical cancer will increase by 22% by 2030. As a way to draw attention to the problem, many types of malignant tumors are distinguished by different colors of the symbolic ribbon. For example, a lavender ribbon means cancer of all types, pink - breast, turquoise - cervix, purple – pancreas.
The basis of the fight against oncological diseases is prevention, which prioritizes a healthy lifestyle, as it provides each person with individual health, well-being and longevity. For that, it is necessary a proper nutrition - more vegetables, fruits and berries in the diet, eating low-calorie foods - cereals (buckwheat, rice, etc.), beans; physical activity (running, exercise, etc.), walking in the fresh air; positive emotions; UV protection; giving up bad habits - smoking, alcohol, drugs; prevention of the infectious diseases (viral, bacterial, parasitic, fungal); vaccination against hepatitis B and human papillomavirus, since other vaccines against carcinogenic viruses have not yet been developed; strengthening of the immune system, in particular, by the intake of vitamins C, group B; A, E, etc.; minerals - magnesium, zinc, potassium, calcium, etc.; antioxidants - selenium, etc.; early diagnosis. Unfortunately, it is impossible to completely avoid the effect of carcinogens on the human body. They are everywhere: at home - household chemicals, microwave ovens, furniture and harmful wood materials (chipboard, fiberboard, MDF), plastic, replacing the harmless natural wood; at work - when professional activity is associated with the hazardous production,
such as chemical productionthe manufacture of medicinal, disinfectant preparations, varnishes, paints, etc., building materials, food, glass, and others; in the environment - radiation, vehicle exhaust gases, soil, water, etc. The scientists in many countries have been conducting thousands of studies to reduce the risk of the development of tumors, to prevent metastases, to create a cure, a cancer vaccine and enable humanity to live without cancer. Ukrainian scientists headed by V. Litvinov, the director of the Antarctic Center (NANC) at the polar station "Akademik Vernadsky" in Antarctica, have created a cure for skin cancer (melanoma - one of the most aggressive forms of malignant tumors in humans) based on the herbaceous plant meadow grass - it tolerates low temperatures (- 40°C), ultraviolet irradiation, has anti-melanoma, antiviral, anti-inflammatory, antibacterial action. The medicine made from the meadow grass was called "Melovit". Currently, the drug is being tested at the Educational and Scientific Center "Institute of Biology and Medicine" of the Kyiv National University. Taras Shevchenko. Professor of Cellular Dermatology and OncologyPenny Lovat and the scientists at Newcastle University created the "AMBLor" test to detect melanoma. The test is applied to a biopsy after the removal of the primary melanoma and reveals the individual level of a possible cancer recurrence (low, high) in each patient. The research is still ongoing. The scientists from the Australian Edith Cowan University (ECU) have developed a test for the early diagnosis of melanoma, which will detect the specific antibodies formed already in the very early stages of melanoma, which will reduce mortality and save the lives of millions of people. The test is at the stage of clinical trials.
Conclusions:
Cancer is a global problem in all countries of the world. Every year, millions of people fall ill with various types of cancer and about half of them die. Healthy lifestyle, preventive measures, early diagnosis, identification and elimination of the risk factors, new methods of diagnostics and treatment, programs of the WHO and other organizations involved in the problem of cancer, will make it possible to reduce the risks of the occurrence and the development of tumors, reduce the death rate from cancer and even prevent it.
55 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
What’s Driving the Rise of Colon Cancer in Young Adults?
 Written by
Written by
Colorectal cancer (CRC) is the third most common cancer worldwide and the second most common cause of cancer-related death. The incidence of early-onset CRC (EO-CRC) in those younger than 50 years is rising globally. Early-onset cancers now account for approximately 10% of all new diagnoses of CRC. It is estimated that 1 in 10 colon cancers and 1 in 4 rectal cancers will be diagnosed in adults younger than 50 years. This rise is striking, and there is an urgency to understand what is driving this epidemiologic shift.
There is strong evidence that the development of CRC is related to diet and lifestyle. Studies have shown evidence that red and processed meat and alcohol increase the risk of CRC. Higher sugar-sweetened beverage intake in adolescence and adulthood has been shown to be associated with a higher risk of EO-CRC among women. Dairy products and whole grains seem to have a protective role, as does increased physical activity, whereas sedentary behavior is associated with an increased risk of EO-CRC.
There are also emerging data linking the gut microbiome with the development of CRC. Gut microbes interact with the host immune system and can influence the antitumor immune response. Patients with CRC have reduced bacterial diversity, as compared with healthy persons, and studies indicate that Firmicutes, Bacteroidetes, enterotoxigenic Bacteroides fragilis, and the oral anaerobe Fusobacterium nucleatum are enriched in CRCs.
Nearly 30% of patients with
EO-CRC have a family history of this cancer in at least 1 firstdegree relative. Population-level data indicate a 3% to 5% overall prevalence of hereditary CRCs, of which Lynch syndrome is the most common. Despite being the most common inherited risk factor for CRC, Lynch syndrome is undiagnosed in most affected patients, who are unaware that they are at high risk for cancer. Only half of patients found to have a pathogenic germline variant report a family history of CRC in a first-degree relative. The syndrome is because of a germline mutation in 1 or more mismatch repair (MMR) genes (MLH1, MSH2, MSH6, PMS2, or EPCAM) that results in deficiency of the MMR protein critical in the repair of DNA base pair “mistakes,” producing a phenotype called microsatellite instability-high. Current guidelines recommend universal testing of all newly diagnosed CRCs for MMR and microsatellite instability status to detect Lynch syndrome.9 Although this remains an important patient population, most EO-CRCs are sporadic and not attributable to any germline alterations in cancer risk genes.
Given these findings and the limited sensitivity of clinical criteria to determine which patients are at high risk, the position statement of the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer recommends conducting genetic testing by using next-generation sequencing and the use of a germline multigene panel in all patients with CRC who are
under the age of 50. Similarly, the National Comprehensive Cancer Network recommends genetic risk counselling and evaluation for all persons with EO-CRC.
EO-CRCs are most often detected in the rectum, followed in prevalence by the distal colon; more than 70% of EO-CRCs are in the left colon at presentation. They more frequently display adverse histopathologic features— poor differentiation, perineural invasion, venous invasion, and mucinous and/or signet-cell morphologic conditions. Patients typically present with a more advanced disease. Patients with EO-CRCs are often unaware of this disease and its symptoms, which are frequently attributed to common, benign conditions such as hemorrhoids. Patients under the age of 50 years, especially men, are less likely than older patients to have a primary care provider, to seek primary care, and to be insured. Health care providers may also be more likely to defer or forgo testing in younger patients than they would be in older, symptomatic patients.
Screening for CRC: Recommended Ages, Test Options

Most CRCs develop through the adenoma–carcinoma sequence, presenting opportunities to prevent cancer by removing its precursor lesions, in addition to identifying CRC in its earliest, curable stages. Approximately 70% of sporadic CRCs develop from adenomatous polyps and 25% to 30% arise from sessile serrated lesions (SSLs) through the SSL-to-carcinoma pathway. CRC screening efforts are directed toward removal of adenomas and SSLs and detection of early-stage CRC.14 Screening for CRC has been one of the most powerful tools to have made an impact on cancer-specific mortality.
In 2018, the American Cancer Society lowered the recommended age to begin CRC screening from 50 to 45 years, on the basis of modeling data suggesting that beginning screening at the age of 45 was associated with a higher number of predicted life years gained and an improved balance between the screening burden and the benefit.15 It was only in the last year that the U.S. Preventive Services Task Force also adopted this screening recommendation. Patients with a family history of CRC, including people who have a first-degree relative in whom CRC

or an advanced adenoma has been diagnosed before the age of 60 years or people who have 2 firstdegree relatives who have received a diagnosis at any age are advised to begin undergoing screening colonoscopy at the age of 40 or at an age that is 10 years younger than the age of the family member with the earliest diagnosis.
Patients with a family history of CRC… are advised to begin undergoing screening colonoscopy at the age of 40 or at an age that is 10 years younger than the age of the family member with the earliest diagnosis. There are several effective screening tests available, including stool testing, such as fecal immunochemical testing and multitarget DNA testing and screening based on visualization such as colonoscopy or sigmoidoscopy and CT colonography. A major limitation of non–colonoscopy-based CRC screening tests is that a positive test result requires a follow-up colonoscopy. Fecal occult blood testing has been largely replaced by fecal immunochemical testing (FIT), which has higher sensitivity for CRC. The FIT sampling technique is easier, as many tests require a single fecal sample, the test has a higher adherence rate, and no dietary modifications or medication restrictions are required. New screening tools such as blood-based screening tests are also being developed. Although colonoscopy remains the gold standard, the mantra is that any screening is better than no screening.
There are unique clinical challenges for patients with EO-CRC. Diagnostic delays result from the low level of suspicion from primary care providers, financial toxicity for patients who are in the prime of their earning potential, sexual and fertility considerations, and long-term survivorship concerns. Survivors may experience chronic side effects after surgery, radiation, and chemotherapy that can last through the remainder of life. Regular follow-up with oncologists and primary providers is needed to look for late effects. Focused support for patients with EO-CRC during their therapy, attention to survivorship for survivors, and education for primary providers and oncologists on diagnosing and caring for these patients are needed.
Dr. Asha Karippot is a medical oncologist at Texas Oncology in Plano, Texas
RESPIRATORY: PNEUMONIA 56 ONCOLOGY: COLON CANCER FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Dr. Aparna Parikh is an expert in gastrointestinal cancers with a focus on young adults with colorectal cancer and also sees patients with pancreatic cancer at Massachusetts General Hospital in Boston
& colecalciferol (vitamin D3 ) DESUNIN ®

• Available in 800 IU tablets and 4000 IU tablets
• Daily flexible dosing of 800 IU - 4000 IU Vitamin D3
ABBREVIATED PRESCRIBING INFORMATION: Desunin (colecalciferol), 800 IU & 4000 IU Tablets.
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Indications, Dosage and Administration:
Desunin 800 IU: Desunin 800 IU is indicated for the prevention and treatment of vitamin D deficiency in adults and adolescents.
In addition to specific osteoporosis treatment of patients who are at risk of vitamin D deficiency, preferably in combination with calcium
Desunin 4000 IU: Desunin 4000 IU is indicated for the treatment of vitamin D deficiency in adults and adolescents.

Vitamin D deficiency is defined as serum levels of 25-hydroxycolecalciferol (25(OH)D) < 25 nmol/l.
Recommended dose: One tablet per day.
The dose should be adjusted dependent upon desirable serum levels of 25-hydroxycolecalciferol (25(OH)D), the severity of the disease and the patient´s response to treatment.
The daily dose should not exceed 4000 IU.
Pediatric population
The safety and efficacy of Desunin in children under 12 years have not been established.
Dosage in hepatic impairment
No dose adjustment is required.
Dosage in renal impairment
Desunin should not be used in patients with severe renal impairment (see section 4.3 of the SmPC).
Administration: The tablets can be swallowed whole or crushed. The tablets can be taken with food.
Presentation: Tablets
Contraindications:
• Diseases and/or conditions resulting in hypercalcaemia or hypercalciuria.
• Nephrolithiasis
• Nephrocalcinosis
• Hypervitaminosis D
• Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SmPC
Warnings and precautions:
Desunin should be prescribed with caution to patients suffering from sarcoidosis due to risk of increased metabolism of vitamin D into its active form. These patients should be monitored with regard to the calcium content in serum and urine.
During long-term treatment, serum calcium levels should be followed, and renal function should be monitored through measurements of serum creatinine. Monitoring is especially important in elderly patients on concomitant treatment with cardiac glycosides or diuretics (see section 4.5 of the SmPC) and in patients with a high tendency to calculus formation. In case of hypercalciuria (exceeding 300 mg (7.5 mmol)/24 hours) or signs of impaired renal function the dose should be reduced, or the treatment discontinued.
Desunin should be used with caution in patients with impairment of renal function and the effect on calcium and phosphate levels should be monitored. The risk of soft tissue calcification should be taken into account. In patients with severe renal insufficiency, vitamin D in the form of colecalciferol is not metabolised normally and other forms of vitamin D should be used.
The content of vitamin D (800 IU or 4000IU) in Desunin should be considered when prescribing other medicinal products containing vitamin D. Additional doses of vitamin D should be taken under close medical supervision. In such cases it is necessary to monitor serum calcium levels and urinary calcium excretion frequently. Excipients: Desunin contain sucrose, isomalt and sodium. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine.
This medicine contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
Interactions with other medicinal products and other forms of interactions:
Thiazide diuretics reduce the urinary excretion of calcium. Due to the increased risk of hypercalcaemia, serum calcium should be regularly monitored during concomitant use of thiazide diuretics.
Concomitant use of phenytoin or barbiturates may reduce the effect of vitamin D since the metabolism increases.

Excessive dosing of vitamin D can induce hypercalcaemia, which may increase the risk of digitalis toxicity and serious arrhythmias due to the additive inotropic effects. The electrocardiogram (ECG) and serum calcium levels of patients should be closely monitored.
Glucocorticoid steroids may increase vitamin D metabolism and elimination. During concomitant use, it may be necessary to increase the dose of Desunin tablets.
Simultaneous treatment with ion exchange resins such as cholestyramine or laxatives such as paraffin oil may reduce the gastrointestinal absorption of vitamin D.
Fertility, pregnancy and lactation:
Fertility - There are no data on the effect of Desunin on fertility. However, normal endogenous levels of vitamin D are not expected to have any adverse effects on fertility.
Pregnancy - Desunin should be used during pregnancy, only in the case of a vitamin D deficiency. Desunin is not recommended during pregnancy in patients without a vitamin D deficiency as the daily intake should not exceed 600 IU vitamin D.
Studies in animals have shown reproductive toxicity of high doses of vitamin D (see section 5.3 of the SmPC).
There are no indications that vitamin D at therapeutic doses is teratogenic in humans.
Breast-feeding - Vitamin-D can be used during breast-feeding. Vitamin D3 passes into breast milk. This should be considered when giving additional vitamin D to the child.
Undesirable effects:
Very common (≥1/10): None
Common (>1/100, <1/10): None
For details of uncommon, rare and very rarely reported adverse events and those of unknown frequency, see SmPC.
Reporting of adverse reactions:
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL – Dublin 2; Tel: +353 1 6764971; Fax: + 353 1 6762517. Website: www.hpra.ie. E-mail: medsafety@hpra.ie.
reactions/events should also be reported to the marketing authorisation holder at the email address: pv.ireland@viatris.com or phone 0044(0)8001218267.
Job code: DES-2021-0020 Date of preparation: April 2021 www.Viatris.com
Legal Category: Product subject to prescription which may be renewed (B) Marketing Authorisation Number: PA2010/058/001&004 Marketing Authorisation Holder: Mylan IRE Healthcare Limited, Unit 35/36, Grange Parade,
Industrial Estate,
13,
Full Prescribing Information available on request from: Viatris, Dublin 17. Phone
8322250. Date of revision of Abbreviated Prescribing Information: 15 March 2021 Reference Number: IE-AbPI-Desunin-v003 Viatris/Mylan IRE Healthcare Limited, Unit 35/36, Grange Parade, Baldoyle Industrial Estate, Dublin 13, Ireland.
Adverse
Baldoyle
Dublin
Ireland
01
Vitamin D: A complementary nutritional therapy for treatment of glioblastoma?
Written by Fiona Griffin, BSc
Author bios:
Síle Griffin, BSc, MSc, PhD, received her BSc in Neuroscience from the University College Cork, Ireland in 2009, MSc in Stem Cells and Regeneration from the University of Bristol, UK in 2010, and PhD in Neuroscience from Keele University, UK in 2015. Her doctoral research focused on stem cell therapies for neurodegenerative diseases, in particular the role of vitamins in brain development and repair. Following this, Síle worked as a Scientist on the Brain Health Platform at DuPont Nutrition & Biosciences, Finland conducting pre-clinical and clinical trials to establish nutritional therapies for cognitive health. In 2021, Síle was awarded a Marie Sk�odowska Curie Career-FIT PLUS Fellowship at the University of Limerick/ Marigot Ltd., Ireland to work on a nutritional strategy using a marine and plant-based dietary supplement to support bone health. ORCID: https:// orcid. org/0000-0002-6670-5084

Fiona Griffin, BSc, received her BSc in Radiation Therapy from Trinity College Dublin, Ireland in 2017. Her undergraduate thesis explored the therapeutic potential of melatonin for breast cancer radiation therapy patients (DOI: 10.1080/09553002.2018.1446227). Fiona is currently studying Graduate Entry Medicine at the University of Limerick, Ireland, alongside part-time employment as a Senior Radiation Therapist at the University Hospital Limerick, Ireland. Both authors contributed equally to this article.


Glioblastoma accounts for approximately 50% of all brain tumours in adults and is considered incurable due to its heterogeneity and complex pathogenesis.1 Despite advancement of modern therapies against glioblastoma, it remains a deadly disease with poor prognosis and significantly impacts on quality of life throughout the disease course.2
Median patient survival rates range between 14-16 months following diagnosis, and a five-year survival rate of 9.8% in patients,1 resulting in a critical public health issue. Indeed, treatment of glioblastoma
remains the most challenging task in clinical oncology. Current therapeutic management involves maximal surgical resection of the tumour along with radiation and concomitant adjuvant temozolomide (TMZ) therapy.3 However, glioblastoma has a poor response to current conventional chemotherapeutics due to varying side effects along with a relatively short half-life of TMZ-based chemotherapy (1.8 hours).1 1,25-Dihydroxyvitamin D3 has emerged as a target of interest to be co-administered with different brain cancer treatments due its anti-proliferative and prodifferentiation effects in the CNS,
including gliomas, and ability to cross the blood-brainbarrier.4-6
Indeed, early studies conducted on rat glioma demonstrated that such cells respond to 1,25-dihydroxyvitamin D3.7 Thus, there are long-standing academic and patientspecific interests in vitamin D supplementation as a possible concomitant therapy to counteract tumour growth or reduce cancer risk.
Vitamin D and Vitamin D Analogues: Regulators of cell proliferation
Multiple in vitro studies have shown that 1,25-dihydroxyvitamin D3 promotes a proliferation-todifferentiation switch in several cell types by promoting progression through the cell cycle and subsequently driving the cells to a more differentiated phenotype.5,8 This has been reported to occur via regulation of cell cycle protein and senescence markers.2 The mechanisms underpinning these anti-proliferative properties elicited by 1,25-dihydroxyvitamin D3 differ across different cell types and cell lines derived from the same type of cancer.2
Some of the known effects induced by 1,25-dihydroxyvitamin D3 are mediated through the nuclear vitamin D receptor (VDR), a transcription factor belonging to the superfamily of nuclear receptors for steroid hormones.9 VDR is almost ubiquitously expressed throughout the human body, including the CNS, and functions by regulating over 500 genes by the ligated VDR protein’s binding to vitamin D response elements, subsequently leading to gene activation and suppression.10
Notably, increased levels of VDR expression have been reported in different cancer types, particularly in glioblastoma versus lower-grade gliomas.11 More recent evidence also reports overexpression of a new oncogene, MED12, in glioblastoma patients which identifies as an important mediator of VDR signalling and an attractive target for future studies in the context of glioblastoma pathogenesis.12
It is important to note that preclinical data reveal that the levels of 1,25-dihydroxyvitamin D3 needed to significantly suppress cellular proliferation is greater than normal physio logical levels.13 For example, the most active metabolite of the 1,25-dihydroxyvitamin D3 hormone, calcitriol, exerts therapeutic effects at concentrations of 10 8 to 10 4 M,13 thus leading to serious side effects such as hypercalcaemia and possible complications during cancer treatment.2
This has therefore led to the production of safer alternative synthetic analogues of 1,25-dihydroxyvitamin D3, including tacalcitol, calcipotriol, ML-344, EM1, CB1093, EB1089, KH1060, MC903 and MC1288, all of which have proved to be able to induce anti-tumour activity in glioblastoma without giving rise to severe hypercalcemic side effects and bioavailability issues.1 Importantly, synthetic vitamin D analogues have been shown to interact with the VDR,14 and are also reported to suppress cellular proliferation and viability in different cancer cells.15-17 Evidence reported by Salomón et al. showed that glioblastoma associated with VDR expression is linked with a better long-term survival of patients, thus supporting a role for VDR in glioma progression.18
The group also investigated the role of VDR in cellular survival, migration and/or invasion (i.e., important processes in glioma progression) using human glioblastoma T98G cells, a cell line that does express VDR. They found that silencing VDR in the T98G cell line significantly increased cellular survival, whereas supplementation with calcitriol (the active 1,25-dihydroxyvitamin D3 hormone) subsequently increased VDR mRNA and protein levels and suppressed glioma cell survival.18 Similarly, the ability of 1,25-dihydroxyvitamin D3 to suppress migration and proliferation in the T98G human glioblastoma cells was reported by Emanuelsson et al.19 The group also demonstrated
58 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: GLIOBLASTOMA
Síle Griffin, BSc, MSc, PhD
significant suppression of proliferation and migration of T98G cells by both calcipotriol and tacalcitol, with stronger effects observed with tacalcitol.19
Vitamin D and Vitamin D
Analogues: Modulators of glioma risk and progression
Clinicians commonly use circulating vitamin D (25-hydroxyvitamin D3) to determine the index of vitamin D status in the body.20 As defined by the Endocrine Society’s Practice Guidelines of Vitamin D, circulating 25-hydroxyvitamin D3 serum level in humans lower than 20ng/ ml, from 20 to 30ng/ml, and higher than 30ng/ ml are indicative of a deficiency, a relative insufficiency, and a sufficiency of vitamin D, respectively.21

Interestingly, some epidemiological studies and clinical observations indicate a connection between vitamin D deficiency or low circulating 25-hydroxyvitamin D3 serum levels (due to limited sun exposure essential to convert cholecalciferol to Vitamin D and/ or poor dietary intake) to an increased risk of developing gliomas and put forth a potential application of this vitamin as a biomarker in glioblastoma prevention or earlier prognosis, reviewed in.16,22 Epidemiological data also suggest a higher risk of brain tumours in adults, to winter births16 and comparative studies on blood bank specimens correlate higher prediagnosis serum 25-hydroxyvitamin D3 levels to lower risk of glioblastoma in men over age 56 years.23
Glioblastoma patients with 25-hydroxyvitamin D3 serum levels greater than 30ng/mL prior to initiation of chemotherapy and radiation demonstrate longer overall survival,16 highlighting the strong potential of supplemental vitamin D to reduce mortality in patients compared to non-users. Patients supplementing vitamin D following diagnosis of glioblastoma have also been reported to have a survival advantage.24 Data from preclinical studies have highlighted the potential of combining the synergistic effects of 1,25-dihydroxyvitamin D3 effects with other therapeutic options for glioblastoma.
For example, one study showed that TMZ and vitamin D co-administration significantly inhibited tumour progression, concomitantly enhancing survival duration in rat glioblastoma orthotopic xenograft models, when compared to TMZ treatment alone.25 However, despite
extensive in vitro and animal studies in this field, limited small-scale clinical trials have been conducted to thoroughly evaluate the safety and efficacy of concomitant treatment with vitamin D or vitamin D on its own in the treatment of glioblastoma. A phase II clinical trial conducted by Trouillas et al., investigated adjunct alfacalcidiol (vitamin D analogue) administration in synergy with classical surgery–radiotherapy–chemotherapy treatments during treatment for malignant glioblastoma.
The study reported safety of the supplementation in addition to induction of a progressive and durable regression of the tumour in some patients.26 Ongoing phase I/ II clinical trials are currently underway to determine the combinatorial effects of calcitriol with other chemotherapeutic agents on glioma and other brain tumours. In a phase I/ II clinical trial, the efficacy and
toxicity of long-term highdose
1,25-dihydroxyvitamin D3 (daily dose of 4000 IU) with concurrent chemoradiotherapy containing TMZ followed by adjuvant chemotherapy containing TMZ is being investigated in newly diagnosed glioblastoma patients (ClinicalTrials.gov Identifier: NCT01181193).
In another phase 1 trial, the effectiveness, and maximum tolerated doses of subcutaneous and/or oral calcitriol combined with intravenous carboplatin is being investigated in the treatment of advanced solid brain tumours (ClinicalTrials.gov Identifier: NCT00008086).
Conclusion and future perspectives
Findings based on different mouse, rodent, and human glioma cell lines report that 1,25-dihydroxyvitamin D3 and vitamin D analogues may promote cell cycle arrest, apoptosis,
anti-migratory and anti-invasive effects in various types of brain cancer cells. These compounds also appear to function synergistically when combined with other cancer therapeutics for glioma. Although the preclinical and epidemiologic data are persuasive, the relevance of findings based on in vitro and preclinical studies must eventually be validated in well-designed human clinical trials to support the assertion of 1,25-dihydroxyvitamin D3 as a complementary nutritional therapy in the treatment of glioblastoma.
Furthermore, future research should also focus on the development of improved vitamin D analogues, which are efficient in low doses and safe for co-administration to glioblastoma patients whose tumours express a vitamin D-responsive receptor.
References available on request

59 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Refractory Metastatic Colorectal Cancer
Efficacy, safety and prognostic factors in patients with refractory metastatic colorectal cancer treated with trifluridine/tipiracil plus bevacizumab in a real-world setting
Colorectal cancer (CRC) is the second highest cause of cancerrelated death worldwide, with an estimated 1.8 million new cases and > 880,000 deaths in 2018.1 Evidence-based guidelines recommend cytotoxic chemotherapy (e.g. oxaliplatin, irinotecan or fluoropyrimidines) as first- and second-line treatment in patients with metastatic CRC (mCRC), with the addition of anti-epidermal growth factor receptor (EGFR) agents (i.e. cetuximab or panitumumab) in those with wild-type RAS tumours or the anti-vascular endothelial growth factor (VEGF) agent bevacizumab2. However, a high proportion of patients develop progressive disease (PD) after receiving standard chemotherapy, with > 40% receiving at least three lines of treatment.3 Treatment recommendations now include the multi-kinase inhibitor regorafenib and trifluridine/tipiracil (TAS-102) as third-line treatment options in these patients.2

The efficacy and safety of TAS-102, a combination of a thymidine-based nucleic acid analogue (trifluridine)
and a thymidine phosphorylase inhibitor (tipiracil), have been demonstrated in clinical studies of patients with previously treated mCRC, with significantly improved overall survival (OS) compared with placebo.4,5,6 An exploratory analysis of the RECOURSE clinical study4 identified patients with low tumour burden and less aggressive disease (i.e. ≥ 18 months since metastatic disease diagnosis) as having improved survival outcomes with TAS-102 monotherapy.7
The survival benefits with TAS-102 monotherapy are modest and there is a need for improved treatment options in patients with refractory mCRC. Several phase I/II and phase II studies have investigated the efficacy and safety of TAS-102 in combination with bevacizumab.8,9,10,11,12,13 These studies included the single-arm Japanese C-TASK FORCE study in 25 patients with refractory mCRC, which reported a centrallyassessed median progression-free survival (PFS) of 3.7 months and a median OS of 11.4 months with TAS-102 plus bevacizumab9. In a Danish randomised study in 93 patients with refractory mCRC, TAS-102 plus bevacizumab was associated with significantly
improved median PFS (4.6 vs 2.6 months, hazard ratio [HR] 0.45, p = 0.001) and median OS (9.4 vs 6.7 months, HR 0.55, p = 0.028) compared with TAS-102 monotherapy.10
Previous retrospective studies of Japanese patients with refractory mCRC have also indicated that the TAS-102 plus bevacizumab combination provides significant survival benefits compared with TAS-102 monotherapy in routine clinical practice;14,15,16 however, real-world data on the use of TAS-102 plus bevacizumab in non-Asian populations are limited. The aim of this real-world study was to evaluate the efficacy, safety and prognostic factors of TAS-102 plus bevacizumab in patients with refractory mCRC in routine clinical practice in Spain.
RESULTS
Population characteristics
Thirty-five patients were treated with TAS-102 plus bevacizumab between July 2019 and October 2021 and were included in this study. Patients had a median (range) age of 65 (41–82) years and 31.4% were aged ≥ 70 years. The majority of patients
(88.6%) had undergone primary tumour resection, 77.1% had an Eastern Cooperative Oncology performance status (ECOG PS) of 0–1, 80.0% had liver metastases, and 71.4% were diagnosed with metastatic disease ≥ 18 months before starting TAS-102 plus bevacizumab. Previous treatment included anti-VEGF therapy in 94.3% of patients (bevacizumab in 57.1% patients, aflibercept in 8.6%, or both bevacizumab and aflibercept in 28.6% patients); 68.6% were receiving TAS-102 plus bevacizumab as third-line treatment. None of the patients had previously received regorafenib.
TAS-102 was started at a reduced dose (30 mg/m2) in seven patients (20.0%) and no patients started bevacizumab at reduced doses. Prophylactic granulocyte colony-stimulating factor (G-CSF) treatment was administered to five patients (14.3%). Patients received a median of 4 cycles of TAS-102 plus bevacizumab (range 2–15 cycles).
Efficacy
In total, 31 of 35 patients (88.6%) were evaluable for response; two patients (5.7%) were not evaluable
60 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: COLORECTAL CANCER
Response, n (%) N = 31 Best overall response CR 0 PR 1 (3.2) SD 15 (48.4) PD 15 (48.4) ORR (CR + PR) 1 (3.2) DCR (CR + PR + SD) 16 (51.6)
Written by: Nieves Martínez-Lago, Teresa Calleja Chucla, Beatriz Alonso De Castro, Rafael Varela Ponte, Cristina Reboredo Rendo, Martin Igor Gomez-Randulfe Rodriguez, Sofia Silva Diaz, Begoña Graña Suarez, Juan de la Cámara Gomez, Fernando Busto Fernández, María Mateos Salvador & Margarita Reboredo Lopez
1. CR complete response, DCR disease control rate, ORR overall response rate, PD progressive disease, PR partial response, SD stable disease.
Table 1
MAKE TIME
for more moments that matter
Extend overall survival for patients in 3rd line mCRC1
trifluridine/tipiracil
Lonsurf® (Trifluridine/ tipiracil): Abbreviated Prescribing Information: Please refer to the Summary of Product Characteristics before prescribing COMPOSITION*: Lonsurf 15 mg/6.14 mg: film-coated tablet containing 15 mg trifluridine and 6.14 mg tipiracil (as hydrochloride). Lonsurf 20 mg/8.19 mg: film-coated tablet containing 20 mg trifluridine and 8.19 mg tipiracil (as hydrochloride). INDICATION*: As monotherapy for the treatment of adult patients with metastatic colorectal cancer who have been previously treated with, or are not considered candidates for, available therapies including fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapies, anti-VEGF agents, and anti-EGFR agents. As monotherapy for the treatment of adult patients with metastatic gastric cancer including adenocarcinoma of the gastroesophageal junction, who have been previously treated with at least two prior systemic treatment regimens for advanced disease. DOSAGE AND ADMINISTRATION*: Recommended starting dose: 35 mg/m2/dose taken orally twice daily on Days 1 to 5 and Days 8 to 12 of each 28-day cycle, within 1 hour after completion of the morning and evening meals (20mg/m2/dose for patients with severe renal impairment). Dosage calculated according to body surface area, not exceeding 80 mg/dose. Possible dosing adjustments based on individual safety and tolerability: permitted dose reductions to a minimum dose of 20 mg/m2 twice daily (15mg/m2/dose for patients with severe renal impairment), dose escalation not permitted after a dose reduction. CONTRAINDICATIONS*: Hypersensitivity to the active substances or to any of the excipients. WARNINGS *: Bone marrow suppression: Complete blood cell counts must be obtained prior to initiation of therapy, prior to each cycle and as needed. Treatment must not be started if absolute neutrophil count < 1.5 x 109/L, if platelet counts < 75 x 109/L, or if unresolved Grade 3 or 4 non-haematological clinically relevant toxicity. Patient should be monitored closely for infections, appropriate measures should be administered as clinically indicated. Gastrointestinal toxicity: anti-emetic, anti-diarrhoeal and other measures should be administered as clinically indicated, dose modifications should be applied as necessary. Renal impairment: not recommended if end-stage renal disease. Patients with renal impairment should be monitored closely; patients with moderate or severe renal impairment should be more frequently monitored for haematological toxicities. Hepatic impairment: not recommended if baseline moderate or severe hepatic impairment. Proteinuria: monitoring by dipstick urinalysis recommended prior to starting and during therapy. Excipients: contain lactose. INTERACTIONS*: Precautions: medicinal products that interact with nucleoside transporters CNT1, ENT1 and ENT2, inhibitors of OCT2 or MATE1, human thymidine kinase substrates (e.g. zidovudine), hormonal contraceptives. FERTIL-
ITY*. PREGNANCY AND BREASTFEEDING*: Not recommended. CONTRACEPTION*: For women and men, highly effective contraceptive measures must be used during treatment and for 6 months after stopping treatment. DRIVE & USE MACHINES*: Fatigue, dizziness or malaise may occur. UNDESIRABLE EFFECTS*: Very common: Neutropenia, leukopenia, anaemia, thrombocytopenia, decreased appetite, diarrhoea, nausea, vomiting, fatigue. Common: Lower respiratory tract infection, febrile neutropenia, lymphopenia, hypoalbuminaemia, dysgeusia, neuropathy peripheral, dyspnoea, abdominal pain, constipation, stomatitis, oral disorder, hyperbilirubinaemia, Palmar-plantarerythrodysaesthesia syndrome, rash, alopecia, pruritus, dry skin, proteinuria, pyrexia, oedema, mucosal inflammation, malaise, hepatic enzyme increased, blood alkaline phosphatase increased, weight decreased. Uncommon: Septic shock, enteritis infectious, lung infection, biliary tract infection, influenza, urinary tract infection, gingivitis, herpes zoster, tinea pedis, candida infection, bacterial infection, infection, neutropenic sepsis, upper respiratory tract infection, conjunctivitis, cancer pain, pancytopenia, granulocytopenia, monocytopenia, erythropenia, leukocytosis, monocytosis, dehydration, hyperglycaemia, hyperkalaemia, hypokalaemia, hypophosphataemia, hypernatraemia, hyponatraemia, hypocalcaemia, gout, anxiety, insomnia, neurotoxicity, dysaesthesia, hyperaesthesia, hypoaesthesia, syncope, paraesthesia, burning sensation, lethargy, dizziness, headache, visual acuity reduced, vision blurred, diplopia, cataract, dry eye, vertigo, ear discomfort, angina pectoris, arrhythmia, palpitations, embolism, hypertension, hypotension, flushing, pulmonary embolism, pleural effusion, rhinorrhoea, dysphonia, oropharyngeal pain, epistaxis, cough, enterocolitis haemorrhagic, gastrointestinal haemorrhage, pancreatitis acute, ascites, ileus, subileus, colitis, gastritis, reflux gastritis, oesophagitis, impaired gastric emptying, abdominal distension, anal inflammation, mouth ulceration, dyspepsia, gastrooesophageal reflux disease, proctalgia, buccal polyp, gingival bleeding, glossitis, periodontal disease, tooth disorder, retching, flatulence, breath odour, hepatotoxicity, biliary dilatation, skin exfoliation, urticaria, photosensitivity reaction, erythema, acne, hyperhidrosis, blister, nail disorder, joint swelling, arthralgia, bone pain, myalgia, musculoskeletal pain, muscular weakness, muscle spasms, pain in extremity, renal failure, cystitis noninfective, micturition disorder, haematuria, leukocyturia, menstrual disorder, general physical health deterioration, pain, feeling of body temperature change, xerosis, discomfort, blood creatinine increased, electrocardiogram QT prolonged, international normalised ratio increased, activated partial thromboplastin time prolonged, blood urea increased, blood lactate dehydrogenase increased, protein total decreased, C-reactive protein increased, haematocrit decreased. Post-marketing experience: interstitial lung disease. OVERDOSE* PROPERTIES*: Trifluridine is an antineoplastic thymidine-based nucleoside analogue and tipiracil hydrochloride is a thymidine phosphorylase (TPase) inhibitor. Following uptake into cancer cells, trifluridine, is phosphorylated by thymidine kinase, further metabolised in cells to a deoxyribonucleic acid DNA substrate, and incorporated directly into DNA, preventing cell proliferation. However, trifluridine is rapidly degraded by TPase and readily metabolised by a first-pass effect following oral administration, hence the inclusion of the TPase inhibitor, tipiracil hydrochloride.PRESENTATION* Pack of 20 or 60 film-coated tablets. Marketing
Authorisation Holder LES LABORATOIRES SERVIER, 50 rue Carnot, 92284 Suresnes cedex France. www.servier.com. Marketing Authorisation: EU/1/16/1096/001-006. Legal Classification for Supply: POM. Further information available from: Servier Laboratories (Ireland) Ltd., Second Floor, 19 Lr. George’s Street, Dun Laoghaire, Co. Dublin A96 ER84, Ireland, Tel (01) 6638110, www.servier.ie.
*For complete information, please refer to the Summary of Product Characteristics available on medicines.ie. Date of last revision of text: January 2021 (Date of last approved SmPC: December 2020)
Reference: 1. Lonsurf SmPC December 2020

Date of preparation of item September 2021. 2122c1LNPressAd A4

due to an early death, and two patients (5.7%) had response assessment pending at the time of the analysis. After a median followup of 11.6 months, 15 patients (48.4%) had PD, one (3.2%) had achieved partial response (PR) and no patients had complete response (CR) (Table 1). The overall response rate (ORR) and disease control rate (DCR) were 3.2% and 51.6%, respectively.

Based on Kaplan–Meier estimates, patients had a median PFS of 4.3 months [95% confidence interval (CI) 3.4–5.1 months] (Fig. 1a) and a median OS of 9.3 months (95% CI 6.6–12.1 months) (Fig. 1b).
In the univariate regression analysis, prognostic factors associated with significantly improved OS were the absence (vs presence) of peritoneal metastases and grade 1–2 (vs grade 3) tumour
histological grade. The absence of peritoneal metastases was also associated with significantly improved PFS.
Safety
The most common adverse events (AEs) of any grade were neutropenia (74.3%), asthenia (65.7%), anaemia (54.8%) and thrombocytopenia (34.3%) (Table 2). The most frequent grade 3–4
AEs were neutropenia (45.7%), asthenia (17.1%) and nausea/ vomiting (8.6%). There were no reports of febrile neutropenia and no treatment-related deaths. Neutropenia was managed by reducing the dose of TAS-102 in five patients (38.1%) and administration of G-CSF prophylaxis in five patients (33.3%), while in the other patients, treatment was delayed until recovery. In this study, 37.1% of patients required a dose reduction of TAS-102 (20% required one dose level reduction (–5 mg/m2), and 17.1% required two dose level reduction (–10 mg/ m2) from baseline dose level. No patients required dose reduction or discontinuation of bevacizumab.
Discussion
In this real-world study of TAS-102 plus bevacizumab treatment in patients with refractory mCRC, efficacy and safety data were generally consistent with those of previous clinical studies, including the Japanese C-TASK FORCE study9 and the Danish phase II study.10 The ORR in our study (3.2%) was slightly higher than that reported in C-TASK FORCE (0% by central assessment)9 and the Danish study (2%)10, whereas the DCR was slightly lower in our study (51.6%) than in earlier studies (64% and 67%, respectively).9,10 In our study, the median PFS (4.3 months) was similar to that reported in the earlier studies (3.7 and 4.6 months, respectively), while the median OS (9.3 months) was similar to that of the Danish study (9.4 months)10, but slightly lower than in C-TASK FORCE (11.4 months).9
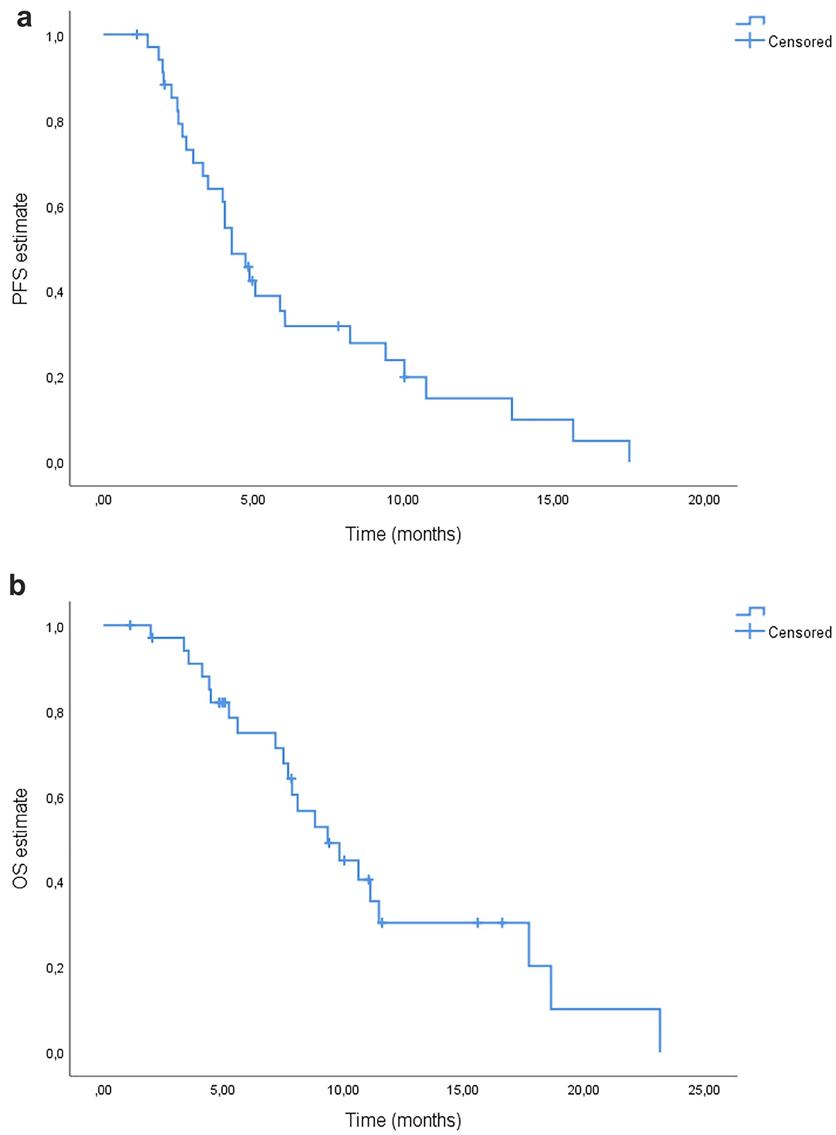
The efficacy of TAS-102 plus bevacizumab in our study was also generally comparable with that reported in previous real-world retrospective studies of Japanese patients with refractory mCRC, in which the median PFS with TAS-102 plus bevacizumab was 3.7 months16 or 4.4 months14, and the median OS ranged from 8.6 to 14.4 months.14,15,16
Patients with refractory mCRC often have poor prognosis.17 In our real-world study, 22.9% of patients had an ECOG PS of 2 and therefore may be more representative of patients with refractory mCRC in routine clinical practice than the previous C-TASK FORCE and Danish clinical studies, which excluded patients with ECOG PS of 2.9,10 In the previous Japanese realworld studies of TAS-102 plus bevacizumab, the proportion of patients with ECOG PS of 2 (or modified Glasgow prognostic
62 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: COLORECTAL CANCER
Figure 1a and 1b
Table 2 Summary of the most frequent all grade and grade 3/4 adverse events.
score of 2) was also much lower (1.4–4.8%)14,15,16 than in our study. Therefore, our study indicates that TAS-102 plus bevacizumab continues to be effective in patients with refractory mCRC and poor performance status scores.
In the univariate analysis of prognostic factors for survival, our study showed that OS and PFS were significantly improved in patients without peritoneal metastases, and those with low tumour histological grade had significantly improved OS. However, patients with low tumour burden (< 3 metastatic sites) had significantly worse PFS compared with those with ≥ 3 metastatic sites. Although this result seems counterintuitive, the low tumour burden may be an indicator of treatment intensity or
the finding may be a statistical artefact associated with the small population size of our study. Another study found no difference in survival outcomes between patients CRC with three versus four metastatic sites.18 Moreover, a large database analysis of the correlates of survival showed that the organ affected by metastasis was an important determinant of survival.19 Further research is needed to determine whether it is the number of metastatic sites or the organs affected by metastases that has the greatest impact on survival outcomes.
Previous studies have identified other baseline prognostic factors associated with improved clinical outcomes with TAS-102 (either as monotherapy or combined with bevacizumab), including modified

Glasgow prognostic score20, the Tabernero prognostic factors [i.e. low tumour burden, less aggressive disease (≥ 18 months since diagnosis of metastatic disease) and liver metastases],7 high lymphocyte-to-monocyte ratio (≥ 3.18),21 and the TASRECOSMO predictive model [i.e. general status, neutrophil-tolymphocyte ratio, KRAS, NRAS and BRAF mutation status, carcinoembryonic antigen (CEA) and alkaline phosphatase (ALP) levels, and time since metastatic disease diagnosis].17 However, our study did not identify liver metastases or the time since diagnosis of metastasis < 18 months (i.e. the Tabernero factors) as being prognostic of OS or PFS, and we did not examine mutational status, CEA or ALP levels, or lymphocyte-to-monocyte or neutrophil-to-lymphocyte ratios as potential prognostic factors.
In our study, TAS-102 plus bevacizumab was associated with manageable toxicities, with the most common grade 3–4 AEs being neutropenia, asthenia and nausea/vomiting. The incidence of grade 3–4 neutropenia (45.7%) was lower than that reported in the C-TASK FORCE study (72%)9 and the Danish study (67%)10, and was slightly lower than in previous Japanese real-world studies (48.2–52.4%).14,15,16
Furthermore, no patients developed febrile neutropenia in our study, while the incidence of this event was 16% and 6%, respectively, in C-TASK FORCE and the Danish study,9,10 and 0–3.3% in the Japanese real-world studies.14,15,16 The lower levels of
haematological toxicity observed in our study may have been due to the relatively high proportion of patients who received prophylactic G-CSF therapy (14.3%). Of note, several studies have reported that chemotherapy-induced neutropenia with TAS-102 (with or without bevacizumab) is associated with improved survival outcomes,22,23,24 which highlights the importance of G-CSF prophylaxis to prevent or manage neutropenia and allow for continued TAS-102 plus bevacizumab treatment without the need for dose reduction.
The limitations of our study include its retrospective, singlearm, single-centre design and its small population size (N = 35). An ongoing international phase III study (SUNLIGHT; NCT04737187) is currently investigating the efficacy and safety of TAS-102 plus bevacizumab versus TAS102 monotherapy as third-line treatment in patients with refractory mCRC, and has a target enrolment of 490 patients.25 This open-label, multicentre study aims to further confirm the clinical benefits of TAS-102 plus bevacizumab over TAS-102 monotherapy in a large population of patients with refractory mCRC; results are expected in 2023.
In conclusion, this real-world study confirms the efficacy and safety of TAS-102 plus bevacizumab in patients with refractory mCRC in routine clinical practice, with survival and tolerability outcomes that were generally consistent with previous clinical and real-world studies of patients in this setting.
AE, n (%) All grades Grade 3–4 Neutropenia 26 (74.3) 16 (45.7) Asthenia 23 (65.7) 6 (17.1) Anaemia 17 (54.8) 2 (5.7) Thrombocytopenia 12 (34.3) 2 (5.7) Diarrhoea 12 (34.3) 2 (5.7) Hepatic function abnormalities 8 (22.9) 0 Nausea/vomiting 6 (17.1) 3 (8.6) Bleeding 4 (11.4) 0 Hypertension 2 (5.7) 1 (2.9) Venous thromboembolism 0 0 Febrile neutropenia 0 0
63
1. AE adverse event.
HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Table 2 Summary of the most frequent all grade and grade 3/4 adverse events
Common Urological Side effects of Radiotherapy: A management Guide for Non Urologists to help improve patient management and outcomes
Written by
Introduction
Radiotherapy is a well recognised and necessary treatment modality when considering a multidisciplinary approach to the management of urological cancers as both primary treatment and also as an adjunct in the palliative setting.
The increased utilisation of active surveillance for low grade low volume prostate cancer and novel therapies for bladder cancer may have resulted in a decrease in the prevalence of radiotherapy primary treatment sessions however radiotherapy continue’s to be an essential aspect in the treatment for prostate and bladder cancer. And as an adjunct in testicular cancer.
Similar to any intervention for cancer, Radiotherapy treatment can have side effects. Due to the anatomy of the pelvis and lower abdomen the treatment of urinary tract malignancy sees radiotherapy related complications not only related to the urinary tract but also the gastro-intestinal tract. These complications may include radiation cystitis, ureteric stricture,, urethral stricture, urinary fistula and secondary malignancies. The prevalence of these side effects can often be underestimated as they may develop as a late complication
Professor Kilian Walsh FRCSIUrol, Consultant Urological Surgeon, University College Hospital Galway


and may not be observed in the follow-up period or present as an emergency. A difficulty for treatment strategies can be that patients are likely to be older and have increased comorbidities. These side effects can impact on the patient's quality of life and prove both difficult and costly to manage.

In this article we will discuss the management of these complications in an attempt to improve understanding of how they are optimally treated so as to improve the outcomes for patients whom are unfortunate enough to suffer a radiotherapy related complication.
Radiation Cystitis
This is the most recognised and prevalent urological side effect of radiation therapy occurring in 5-10% of those receiving pelvic irradiation, most commonly presenting in patients whom have undergone treatment for prostate and bladder cancer. One study found that it accounted for over 51.2% of radiotherapy side-effect related urological admissions.
Acute radiation cystitis is common and usually self limiting, occurring sometimes shortly after radiation exposure. Impairment of the urothelium leads to damage to deeper tissues due to contact with
urine and spread of inflammation. A self limiting course of acute radiation cystitis may last for up to 3 months and is characterised by severe lower urinary tract symptoms such as dysuria, frequency and urgency. In a case of chronic radiation cystitis visible haematuria is the major presenting symptom and may vary in severity from mild to life threateninghypovolemic shock. It will be associated with lower urinary tract symptoms such as dysuria, frequency, urgency and nocturia as well as formation of large blood clots which may increase the risk of urinary retention.
Due to the non-specific presentation of radiation cystitis in its presentation other causes of haematuria must be investigated and excluded when these patients present as an emergency. These include urinary tract infections, coagulopathies and secondary malignancy.
Management
Acute radiation cystitis is usually a self limiting condition which may be managed conservatively and the mainstay of treatment involves management of symptoms. Anticholinergic medications such as tolteridine and solifenacin can be utilised in these cases to help reduce frequency, urgency and other lower urinary tract symptoms. Patients should be reviewed regularly.
Stabilisation, Irrigation and clot removal
Management of chronic radiation cystitis with visible haematuria is more complicated. In the acute setting if severe visible haematuria leading to hypovolemic shock is present, intravenous fluid resuscitation and blood transfusion must be considered. Once the patient has been stabilised a 22 or 24 french transurethral Silicone urinary catheter should be inserted into the bladder to commence a bladder washout followed by irrigation with saline and regular bladder washouts with an irrigating syringe should be continued until the urine is clear. This is considered first line therapy. There are other therapies available to treat continued bleeding.
If the bleeding does not settle a bladder washout with an Elik Evacuator under general anaesthetic or spinal block may be required.
Intravesical therapies
Intravesical therapies using formalin and alum can be helpful once in contact with the damaged urothelium and may reduce ongoing bleeding.
Response rates with these agents typically range from 60-90% however there can be side effects with these medications which patients must be made aware of,. Alum has a more favourable side effect profile so may be used initially. Formalin is only recommended in cases of intractable hemorrhagic cystitis as it can cause severe bladder wall fibrosis leading possibly to the requirement of a urinary diversion if the fibrosis is very severe.
Systemic therapies
Systemic therapies are a more appealing option for treatment as they do not require hospitalisation however there is limited evidence to support their use. Therapies that have been used in treatment include WF10 which reduces inflammation, induces natural immunity and stimulates cellular defence mechanisms however it only rarely reverses the haematuria.
Hyperbaric Oxygen
Hyperbaric oxygen (HBOT) therapy involves a special pressurised treatment chamber which delivers 100% oxygen at a higher pressure than is present in the atmosphere. This stimulates neo-angiogenesis facilitating reepithelialization healing. Complete resolution of haematuria has been found in 3496% of patients following HBOT therapy. This usually involves 2040 sessions so is seen as costly and resources are limited. HBOT can be construed an alternative to surgery if other more conservative measures fail
Ablative therapies
Ablation of damaged submucosal vasculature using laser therapy or diathermy is associated with a complete response in 75%-
64 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: RADIOTHERAPY
Mr Richard Walsh, Final Med University College Dublin
TREAT EARLY.1 DELAY DISEASE PROGRESSION.1 EXTEND LIFE.1
Use ERLEADA® earlier and keep other
treatment options for later stages1
ERLEADA® (apalutamide) is indicated for the treatment of adult men with:2
• non-metastatic castration-resistant prostate cancer (nmCRPC) who are at high risk* of developing metastatic disease
• metastatic hormone-sensitive prostate cancer (mHSPC) in combination with ADT
Visit the Janssen Medical Cloud to learn more about ERLEADA®
ERLEADA®▼ 60 mg film-coated tablets PRESCRIBING INFORMATION. ACTIVE
INGREDIENT(S): Apalutamide. Please refer to Summary of Product Characteristics (SmPC) before prescribing. INDICATION(S): Treatment of adult men: with non metastatic castration resistant prostate cancer (nm CRPC), at high risk of developing metastatic disease; with metastatic hormone-sensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy (ADT). DOSAGE & ADMINISTRATION: Adults: 240 mg (four 60 mg tablets) single daily dose, swallowed whole with or without food. Continue medical castration with gonadotropin releasing hormone analogue (GnRHa) throughout in patients not surgically castrated. Elderly: No dose adjustment. Renal impairment: Mild to moderate - no dose adjustment. Severe - not studied; caution advised. If treating, monitor patients for side effects, reduce dose if required. Hepatic impairment: Baseline mild or moderate (Child Pugh Class A and B) - no dose adjustment. Severe - not recommended.
CONTRAINDICATIONS: Hypersensitivity to active substance or any excipient. Women who are or may become pregnant. SPECIAL WARNINGS & PRECAUTIONS: Seizure: History of seizures or other predisposing factors - not recommended. Discontinue permanently if seizure develops during treatment. Risk of seizure may be increased in patients receiving concomitant medicinal products that lower the seizure threshold. Ischaemic heart disease and ischaemic cerebrovascular disorders occurred, including events leading to death; monitor for signs and symptoms of ischaemic heart disease AND ischaemic cerebrovascular disorders, optimise management of risk factors: hypertension, diabetes, dyslipidaemia. Falls and fractures: Reported; evaluate patients for risk before starting Erleada; continue monitoring and manage fractures, consider use of bone-targeted agents. Concomitant use with other medicinal products: Potent enzyme inducer; may lead to loss of efficacy of concomitant medication. Review concomitant medication and refer to SmPC for further guidance. Avoid co-administration with warfarin and coumarin-like anticoagulants. If coadministered with an anticoagulant metabolised by CYP2C9 (e.g. warfarin or acenocoumarol), conduct additional International Normalised Ratio (INR) monitoring. Recent cardiovascular disease: Monitor patients with clinically significant cardiovascular disease for risk factors (e.g. hypercholesterolaemia, hypertriglyceridaemia, other cardio-metabolic disorders) as safety in patients with clinically significant cardiovascular disease in the past 6 months has not been established. Androgen deprivation therapy may prolong QT interval: Assess benefit-risk including potential for Torsade de pointes prior to initiating Erleada in patients with a history or risk factors for QT prolongation and those receiving concomitant medicinal products that might prolong QT interval. Severe Cutaneous Adverse Reactions (SCARs): Including drug reaction with eosinophilia and systemic symptoms (DRESS) and Stevens Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Advise patients of signs
and symptoms suggestive of DRESS or SJS/TEN If these symptoms are observed, withdraw Erleada immediately and patients to seek immediate medical consultation. Do not restart Erleada in patients who have experienced DRESS or SJS/TEN while taking Erleada at any time and consider an alternative treatment. Effects on ability to drive and use machines: Seizures reported; potential risk for driving or operating machines. SIDE EFFECTS: Refer to SmPC for other side effects. Very common: Hot flush, hypertension, diarrhoea, skin rash, fracture, arthralgia, fatigue, weight decreased, fall, decreased appetite. Common: Hypothyroidism, hypercholesterolaemia, hypertriglyceridaemia, dysgeusia, ischaemic heart disease, ischaemic cerebrovascular disorders, pruritus, alopecia, muscle spasm. Other side effects: Seizure, QT prolongation, DRESS, SJS/TEN. LEGAL CATEGORY: Prescription only medicines. PRESENTATIONS, PACK SIZES, MARKETING AUTHORISATION NUMBER(S): Blister pack, 112, EU/1/18/1342/001. MARKETING AUTHORISATION HOLDER: JANSSENCILAG INTERNATIONAL NV, Turnhoutseweg 30, B-2340 Beerse, Belgium. FURTHER INFORMATION IS AVAILABLE FROM: Janssen Sciences Ireland UC, Barnahely, Ringaskiddy, IRL – Co. Cork P43 FA46. Prescribing information last revised: December 2022.
Adverse events should be reported. ▼ This medicinal product is subject to additional monitoring and it is therefore important to report any suspected adverse events related to this medicinal product. Healthcare professionals are asked to report any suspected adverse events via: HPRA Pharmacovigilance, Website: www.hpra.ie. Adverse events should also be reported to, Janssen Sciences Ireland UC on 1 800 709 122 or at dsafety@its.jnj.com.
*High risk is defined as patients with a PSA doubling time of ≤10 months.1 ADT = androgen deprivation therapy
References: 1. Smith MR, et al. Apalutamide and Overall Survival in Prostate Cancer. Eur Urol. 2021;79(1):150–158. 2. ERLEADA® (apalutamide). Summary of Product Characteristics. Available from: www.medicines.ie
Date of preparation: January 2023 | CP-361339

97% of cases. This is performed in the operating Theatre via a cystoscope with either a rollerball diathermy or Holmium or Green Light Laser.
Interventional Therapies
In intractable cases Interventional radiology can perform embolisation of the iliac arteries, this has been shown to reduce haematuria with an efficacy of up to 100%. Gluteal pain is a recognised complication of this procedure. Bilateral Percutaneous nephrostomy for urinary drainage, cutaneous ureterostomy and ileal conduit formation are all considered if the Haematuria is persistent despite all the previous measures.. A transverse colon conduit is preferable over an ileal conduit if there is a concern that the small bowel has been irradiated, urinary diversion with or without cystectomy is considered a definitive treatment however it will carry a high morbidity and mortality and is a last resort
Urinary Fistulae
Fistulae may prove the most difficult to treat of the urological side effects of radiotherapy (Mundy AR). A fistula is defined as an abnormal connection between two body parts, such as an organ or blood vessel and another structure. The most common fistulae to form as a result of radiotherapy include vesicovaginal, rectovaginal and rectovesical. Fistulae which occur due to radiation tend to be large and multiple. The neck of the bladder receives the highest dose of radiation during external beam radiotherapy for prostate cancer and therefore is the most common site for a fistula should it occur. The pathophysiology of their formation is related to hypoxia and necrosis of the tissue with radiation. Subsequent weakness and fragility of tissues allows for abnormal connections to develop.
The presentation of a fistula is dependent on its location. A fistula between the urinary tract and GI tract will lead to symptoms such as recurrent urinary tract infections (and associated lower urinary tract symptoms), pneumaturia and faeces in the urine. A vesicovaginal fistula will lead to leakage of urine from the vagina.
Imaging of the fistula with CT scanning and contrast to determine size, location and surrounding tissue viability is a mainstay of diagnosis and planning for surgery. Cystoscopy
and direct visualisation is the preferred method for this. A cystogram using methylene blue dye can also be used.
Management
Management of a urinary fistula secondary to radiotherapy is complex. Surgery is considered the mainstay of curative treatment however due to previous irradiation of surrounding tissues healing can often be impaired after surgery. This means that ,unfortunately, failure of treatment and relapses are more common than in non-irradiated tissues. The most important aspect of surgical management is to provide diversion of urine away from the site of the fistula so that it will not recur.
The principles of fistula repair are to excise the fistulous tract, close the opening from each organ and lay a flap or graft between the two repaired edges so that they heal independently.

Rectourethral Fistula
Initial management for a rectourethral fistula involves intestinal and urinary diversion to reduce the risk of major infection and sepsis. Further management depends on factors such as the size of the fistula and the comorbidities of the patient. Transperineal access to the fistula is the most common technique with transanal and trans-sphincteric approaches being considered a flap utilising the gracilis muscle can be used to protect the repair
Vesicovaginal Fistula
In these cases either a transvaginal, transabdominal or combined approach can be used to repair the fistula. Omental, peritoneal or labial flaps are often used to cover the repair. Urinary drainage for 10 days via a suprapubic catheter is required and a cystogram will confirm the integrity of the repair.
Urinary Strictures:
Ureteric Strictures
Strictures of the ureters tend to occur as a late complication of radiation exposure and more commonly than urethral strictures. Symptoms for this depend on the degree of stenosis, they can present with hydronephrosis on imaging, symptomatic loin pain or recurrent upper urinary tract infections.
For diagnosis urea and creatinine may show impaired renal function, physical examination, x-ray imaging, ultrasound, CT scan, MRI scan and diagnostic ureteroscopy.
Management
Initial management is to ensure passage of urine this may be done through a percutaneous nephrostomy or ureteral stent.
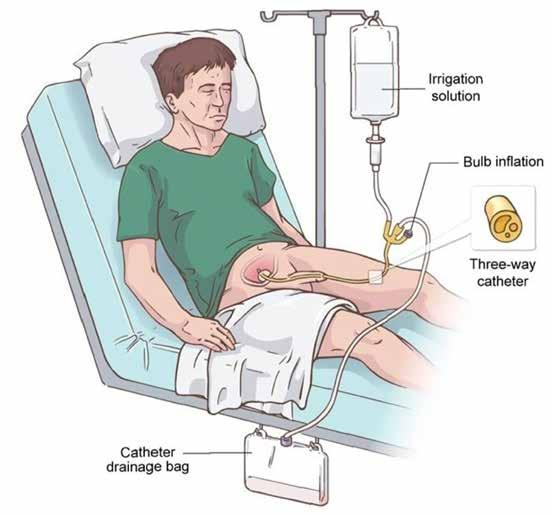
Minimally invasive techniques include balloon dilatation and stent placement are preferred as an alternative to open surgery. The surgical methods of choice involve reconstruction of the ureter with a buccal graft, Trans Uretero-ureterostomy, Boari flap for lower third ureteric strictures or if necessary a urinary diversion and stoma if the ureteric tissue is considered too unhealthy for reconstruction.
Urethral Strictures
A urethral stricture can lead to voiding dysfunction and subsequently upper urinary tract damage. High total radiation dose is the most common risk factor for development of a urethral stricture with necrosis and hypoxia. Presentation includes irritative and obstructive symptoms with reduced urinary flow and possibly urinary retention.
Management
Endoscopic treatment such as urethral dilatation and direct internal urethrotomy are minimally invasive but are associated with a significant risk of recurrence however for patients who are unsuitable for major reconstructive surgery this method is suitable.
Urethroplasty is the mainstay of surgical treatment. This involves removal of the stricture and primary anastomosis. In the case
of a longer stenosis a substitution urethroplasty using a graft (buccal most commonly). Urinary incontinence is a significant risk of this procedure.
Secondary Malignancy
Radiation induced secondary malignancies are a rare late side effect of radiation therapy Epidemiological studies show a higher rate of bladder cancer in patients receiving radiation for prostate cancer compared with patients who underwent surgery or watchful waiting. These bladder cancers are commonly high grade and muscle invasive when diagnosed. Therefore it is important to be vigilant of the risk of bladder cancer in patients who have received radiation for prostate cancer and if they have a life expectancy of over 20 years the possibility of secondary malignancy needs to be included in their management discussion and a cystoscopy is indicated in the presence of any haematuria.
Conclusion
Radiotherapy is an important and effective modality in the treatment of urological malignancies. The side effects associated with radiotherapy can be significant and often underestimated. The side effects may present many years after radiotherapy treatment is administered and can affect quality of life outcomes for cancer survivor patients. Management can be complex however with an understanding of the appropriate strategies and with adequate resources they can be treated effectively in high quality urology centres with appropriate support. References available on request
FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: RADIOTHERAPY
Continuous bladder irrigation (CBI)
Three-way catheter
Catheter drainage bag
66
Irrigation solution Bulb inflation


www.bbraun.ie
A Comprehensive Overview of Lung Cancer
An interview with Theresa Lowry Lehnen (PhD), Clinical Nurse Practitioner and Associate Lecturer South East Technological University

survival rate nearly 100%) after complete resection,” Theresa adds.
Lung cancer is divided into two broad histologic classes: smallcell lung carcinomas (SCLC) and non-small cell lung carcinomas (NSCLC), which grow and spread differently.
Theresa continues, “About 1 in 7 of all lung cancers is the small cell type. Smoking is the major risk factor for small cell lung cancer. SCLCs are the most dedifferentiated cancers and tend to be central mediastinal tumours. SCLCs comprise 10%–15% of all lung cancers. They are extremely aggressive, disseminating rapidly into submucosal lymphatic vessels and regional lymph nodes, and are almost always present without bronchial invasion.
Lung cancer is the 5th most common cancer in Ireland, with almost 2,700 people being diagnosed each year. It affects both women and men, usually over the age of 50.

Lung cancer can be treated with surgery, drug therapies and radiotherapy, depending on the type and where it is found.
Lung cancer is a highly invasive, rapidly metastasising and prevalent cancer accounting for approximately 12.4% of all cancers globally. It is one of the most frequently diagnosed cancers, and the leading cause of cancer-related deaths worldwide. There are an estimated 2 million new cases and 1·76 million related deaths annually. 70% of patients diagnosed with lung cancer present with advanced stage disease.
We spoke with Theresa Lowry Lehnen, PhD, Clinical Nurse Practitioner and Associate Lecturer South East Technological University to gain a better insight into the overview of this condition.
“The pathophysiology of lung cancer is complex and incompletely understood, she told us. “It is theorised that repeated exposure to carcinogens, such as cigarette smoke leads to dysplasia of lung epithelium. If the exposure continues, it leads to genetic mutations and affects protein synthesis. This, in turn, disrupts the cell cycle and promotes carcinogenesis.
“The most common genetic mutations responsible for lung cancer development are MYC, BCL2, and p53 for small cell lung cancer and EGFR, KRAS, and p16 for non-small cell lung cancer. Approximately 90% of lung cancer cases are caused by smoking and the use of tobacco products. Lung diseases such as idiopathic pulmonary fibrosis increase the risk of lung cancer independent of smoking.
“Other factors such as radon gas, asbestos, air pollution, and chronic infections can also contribute to lung carcinogenesis. Multiple inherited and acquired mechanisms of susceptibility to lung cancer have been proposed. Highly heterogeneous, lung cancer can occur in many different sites in the bronchial tree, presenting variable signs and symptoms depending on its anatomic location.”
Squamous cell lung cancers represent 25%–30% of all lung cancers and tend to arise in the main bronchi and advance to the carina. Adenocarcinomas account for approximately 40% of all lung cancers and consist of tumours arising in peripheral bronchi.
Bronchioloalveolar cancers (BAC), now reclassified into adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA), develop in the alveoli and spread through the interalveolar connections.
“Both AIS and MIA have good disease-free survival rates (5-year
“Small cell carcinoma is composed of round, oval, or angulated cells, with a small amount of cytoplasm, and are about the size of a resting lymphocyte. No distinct nucleoli are seen and they usually stain positive with chromogranin or synaptophysin. SCLCs are extensively necrotic. The WHO previously classified SCLC into three cell subtypes: oat cell, intermediate cell, and combined cell (SCLC with NSCLC component, squamous, or adenocarcinoma).
“However, studies have shown that such classification does not have much clinical significance or prognostic value. Regardless of stage, the current prognosis for patients with SCLC is poor despite improvements in diagnosis and therapy during the past three decades. Without treatment, SCLC has the most aggressive clinical course of any type of pulmonary tumour, with median survival from diagnosis of only 2 to 4 months. About 10% of the total population of SCLC patients remains free of disease during the 2 years from the start of therapy, which is the time period during which most relapses occur.”
Even these patients, however, are at risk of dying from lung cancer, she explains. “The overall survival rate at 5 years is 5% to 10%. An important prognostic factor for SCLC is the extent of disease. Patients with limited disease (LD) have a better prognosis than patients with extensive disease
(ED). For patients with LD, median survival of 16 to 24 months and 5-year survival rates of 14% with current forms of treatment have been reported.
“Patients diagnosed with LD who smoke should be encouraged to stop smoking before undergoing combined-modality therapy because continued smoking may compromise survival. Improved long-term survival in patients with LD has been shown with combined-modality therapy. Non-small cell lung carcinomas is any type of epithelial lung cancer other than small cell lung cancer. Most lung cancers are NSCLC and there are four main types; squamous cell carcinoma (25%), large cell carcinoma (10%), adenocarcinoma (40%), and not otherwise specified (NOS).”
Presentation
There are no specific signs and symptoms for lung cancer. Theresa adds, “Most patients already have advanced disease at the time of presentation. Lung cancer symptoms occur due to local effects of the tumour, such as cough due to bronchial compression by the tumour due to distant metastasis, strokelike symptoms secondary to brain metastasis, paraneoplastic syndrome, and kidney stones due to persistent hypercalcemia.” Symptoms can include, difficulty breathing; a cough that does not go away or a change in a long-term cough; repeated chest infections that won’t go away, even after antibiotics; feeling more tired than usual; hoarse voice; coughing up blood-stained phlegm; pain in the chest, especially when coughing or breathing in; loss of appetite / weight loss; swelling around the face and neck and difficulty in swallowing.
Staging
The tumour, node, metastasis (TNM) staging system for lung cancer is an internationally accepted system used to characterise the extent of disease. The TNM system combines features of the tumour into disease stage groups that correlate with survival and are linked to recommendations for treatment. T describes the size of the tumour and any spread of cancer into nearby tissue. N
68 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: LUNG CANCER


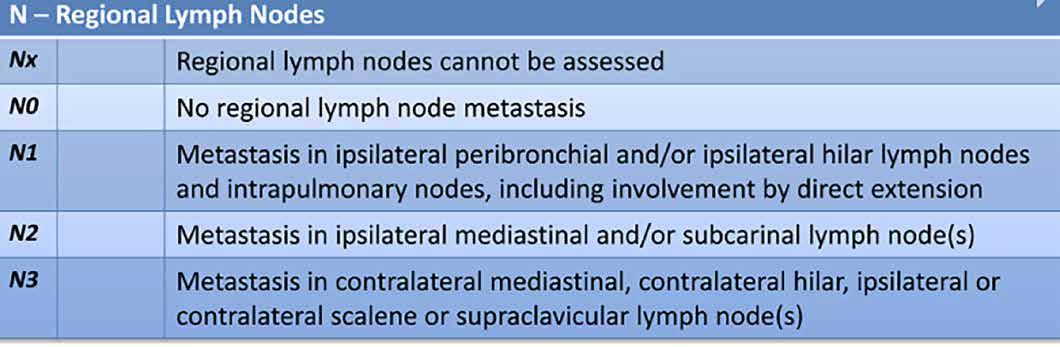
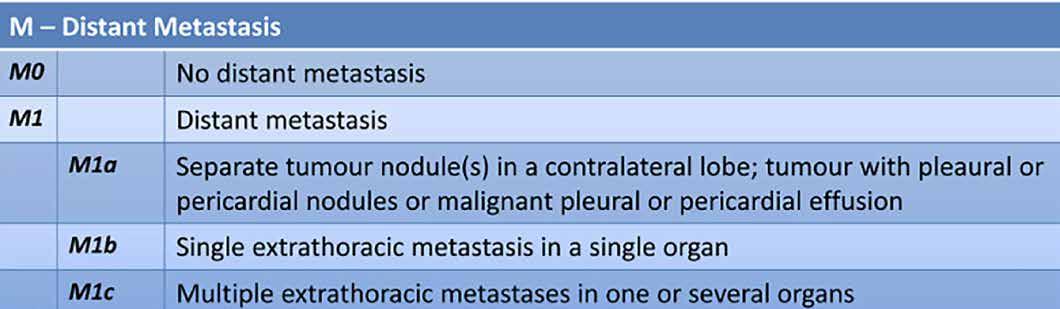
69 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023 TNM Staging System International Association for the study of lung cancer: https://www.iaslc.org/
describes the spread of cancer to nearby lymph nodes. M describes metastasis-the spread of cancer to other parts of the body. Doctors also sometimes use a simpler staging system for small cell lung cancer, describing it as limited disease (LD) or extensive disease (ED).
Lung cancer evaluation requires radiological and invasive staging:

Radiological staging
• Contrast-enhanced CT chest with extension to upper abdomen up to the level of the adrenal glands.
• Imaging with PET or PET-CT directed at sites of potential metastasis when symptoms or focal findings are present or when chest CT shows evidence of advanced disease.
Theresa continues, “Intravenous (IV) contrast enhancement is preferable as it may distinguish mediastinal invasion of the primary tumour or metastatic lymph nodes from vascular structures. The significant advantage of CT is that it provides an accurate anatomic definition of the tumour within the thorax, which helps clinicians decide the optimal biopsy site.
“CT can also identify the tumour-related atelectasis; post obstructive pneumonitis; intra- or extra-thoracic metastatic disease; and co-existing lung disease. The main objective of a CT scan is to identify the extent of the tumour, its anatomical location, and the lymph node involvement. TNM
staging relies heavily on lymph node involvement. PET scanning allows in vivo determination of metabolic and pathologic processes. It provides limited anatomic resolution but provides information on the primary tumour's metabolic activity, mediastinal involvement, and potential distant metastases.”
Invasive staging
After CT and PET scans, the next step is to obtain tissue or pathologic confirmation of malignancy, confirm the staging and histological differentiation of cancer.
“Procedures include: Bronchoscopic endobronchial ultrasound-transbronchial needle aspiration (TBNA); EndoscopicTBNA; Mediastinoscopy; Thoracoscopy or video-assisted thoracoscopy (VATS).
“Staging procedures for SCLC are important to distinguish patients with disease limited to their thorax from those with distant metastases. At the time of initial diagnosis, approximately twothirds of patients with SCLC have clinical evidence of metastases; most of the remaining patients have clinical evidence of extensive nodal involvement in the hilar, mediastinal, and sometimes supraclavicular regions.
“Determining the stage of cancer allows an assessment of prognosis and a determination of treatment, particularly when chest radiation therapy or surgical excision is added to chemotherapy for patients

with LD. If ED is confirmed, further evaluation should be individualised according to the signs and symptoms unique to the individual patient. Standard staging procedures include: A thorough physical examination; Routine blood counts and serum chemistries; Chest and upper abdominal computed tomography (CT) scanning; A radionuclide bone scan; A brain magnetic resonance imaging scan or CT scan; Bone marrow aspirate or biopsy in selected patients in which treatment would change based on the results.
“Staging procedures for NSCLC: In non-small cell lung cancer, the determination of stage has important therapeutic and prognostic implications. Careful initial diagnostic evaluation to define the location and to determine the extent of primary and metastatic tumour involvement is critical for the appropriate care of patients.
“In general, symptoms, physical signs, laboratory findings, and perceived risk of distant metastasis lead to an evaluation for distant metastatic disease. Additional tests such as bone scans and computed tomography (CT)/ magnetic resonance imaging (MRI) of the brain may be performed if initial assessments suggest metastases or if patients with stage III disease are under consideration for aggressive local and combined modality treatments.
“Procedures used to determine stage include: history; physical examination; routine laboratory
evaluations; Chest x-ray; Chest CT scan with infusion of contrast material; Fluorine F 18-fludeoxyglucose positron emission tomography scanning. Procedures used to obtain tissue samples include bronchoscopy, mediastinoscopy, or anterior mediastinotomy.”
TREATMENT
Treatment-Small cell lung cancer:
Theresa notes that chemotherapy and radiation therapy have been shown to improve survival for patients with small cell lung cancer. Chemotherapy improves the survival of patients with limitedstage disease (LD) or extensivestage disease (ED), but it is curative in only a minority of patients.
“Because patients with SCLC tend to develop distant metastases, localised forms of treatment, such as surgical resection or radiation therapy, rarely produce long-term survival. With incorporation of current chemotherapy regimens into the treatment program, however, survival is prolonged, with at least a fourfold to fivefold improvement in median survival compared with patients who are given no therapy,” she says.
“SCLC is highly radiosensitive and thoracic radiation therapy improves survival of patients with LD and ED tumors.
“The role of surgery in the management of patients with SCLC is unproven. Small case series and population studies
RESPIRATORY: PNEUMONIA FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
70 ONCOLOGY: LUNG CANCER
have reported favourable outcomes for the minority of LD patients with very limited disease, with small tumours pathologically confined to the lung of origin or the lung and ipsilateral hilar lymph nodes from surgical resection with adjuvant chemotherapy.
“Patients who have undergone surgery and then diagnosed with SCLC generally receive adjuvant chemotherapy with or without radiation therapy. In patients who receive chemotherapy with radiation therapy, there is no improvement in survival with the addition of surgery. Given the absence of data from randomised trials, the role of surgery in the management of individual patients with SCLC must be considered, both in terms of potential benefit and risk from the surgical procedure.
“Studies have evaluated the role of immune checkpoint inhibitors in frontline treatment of patients with extensive-stage SCLC. Two PD-L1 inhibitors, atezolizumab and durvalumab, demonstrated prolongation of overall survival when combined with platinum and etoposide, compared with the same combination chemotherapy regimen alone.
“Chemotherapy for patients with ED SCLC is commonly given as a two-drug combination of platinum and etoposide in doses associated with at least moderate toxic effects (as in limited-stage SCLC). Cisplatin is associated with significant toxic effects and requires hydration, which can be problematic in patients with cardiovascular disease. Carboplatin is active in SCLC, is dosed according to renal function, and is associated with less nonhaematological toxic effects.
Other regimens appear to produce similar survival outcomes but have been studied less extensively or are in less common use.
“Radiation therapy to sites of metastatic disease unlikely to be immediately palliated by chemotherapy, especially brain, epidural, and bone metastases, is a standard treatment option for patients with ED SCLC. Patients with ED treated with chemotherapy who have achieved a response can be considered for thoracic radiation therapy.”
Standard treatment options for patients with recurrent smallcell lung cancer (SCLC) include: chemotherapy, immune checkpoint modulation; palliative care.
Theresa adds, “Although second-line chemotherapy has been shown to produce tumour regression, responses are usually short lived. The median survival is
rarely more than 12 months and usually less than 6 months after second-line therapy. Response to first-line chemotherapy predicts the subsequent response to second-line therapy.”
Treatment-Non-small cell lung cancer:
As a class, NSCLC is usually less sensitive to chemotherapy and radiation therapy compared with SCLC.
“Patients with resectable disease may be cured by surgery or surgery followed by chemotherapy. Local control can be achieved with radiation therapy in a large number of patients with unresectable disease, but cure is seen only in a small number of patients.
“Patients with locally advanced unresectable disease may achieve long-term survival with radiation therapy combined with chemotherapy. Patients with advanced metastatic disease may achieve improved survival and palliation of symptoms with chemotherapy, targeted agents, and other supportive measures.
“In non-small cell lung cancer (NSCLC), results of standard treatment are poor except for the most localised cancers. Treatment decisions are based on knowledge of histologic type; tumour size and location; involvement of pleura; surgical margins; status and location of lymph nodes; tumour grade; and lymphovascular invasion. Surgery is potentially the most curative therapeutic option. Postoperative chemotherapy may provide an additional benefit to patients with resected NSCLC. Radiation therapy combined with chemotherapy can produce a cure in a small number of patients and can provide palliation in most patients.
“Prophylactic cranial irradiation may reduce the incidence of brain metastases, but there is no evidence of a survival benefit and the effect of prophylactic cranial irradiation on quality of life is unknown. In patients with advanced-stage disease, chemotherapy or epidermal growth factor receptor (EGFR) kinase inhibitors offer modest improvements in median survival, although overall survival is poor.”
Outlook
Treatment of patients with lung cancer requires multidisciplinary co-operation and is based on surgery, radiotherapy, systemic treatments including chemotherapy, targeted therapies,
and immune checkpoint inhibitors, and supportive care including end-of-life care. Treatment depends on tumour characteristics, tumour stage and patient-related factors.
Theresa notes, “Although chemotherapy remains a cornerstone of treatment, targeted therapies and immune checkpoint inhibitors improve the treatment of metastatic cancers, and are hoped to improve the outcome of adjuvant and induction therapies.”
She adds, “Scientists are trying to develop sputum and blood markers that could be used to detect lung cancer early. Two active areas of research involve: analysing blood samples to identify whether tumour cells or molecular markers in the blood will help diagnose lung cancer early; and examining sputum samples for the presence of abnormal cells or molecular markers that identify individuals who may need more follow-up.”
Machine learning is a method that allows computers to learn how to predict certain outcomes. In lung cancer, researchers are using computer algorithms to create computer-aided programs that are better able to identify cancer in CT scans than radiologists or pathologists. In one artificial intelligence study, researchers trained a computer program to diagnose two types of lung cancer with 97% accuracy, as well as detect cancer-related genetic mutations.
Immunotherapies work with the body's immune system to help fight cancer and are a major focus in lung cancer treatment research today. Clinical trials are ongoing to look at new combinations of immunotherapies with or without chemotherapy to treat lung cancer.

Theresa adds, “Successful immunotherapy of lung cancer requires a better understanding of immune escape and immunosuppression in lung cancer, and learning how to monitor immunity, edit immunity, and reactivate immunity in cancer. Learning to understand cancer immunosurveillance, immunoediting, and how to reactivate cancer immunity can embark on a future full of possibilities to use immunotherapy as a reliable lung cancer treatment.
“An immune checkpoint inhibitor is a drug that blocks proteins on immune system cells which then allows them to fight cancer. Several immune
checkpoint inhibitors have been approved for advanced lung cancer. These inhibitors, which increase the strength of anticancer immune responses, target the proteins PD-L1 and PD-1. Patients whose tumours test high for PD-L1 proteins may be more responsive to this type of treatment than others, but more studies are needed. A key issue with immunotherapies is deciding which patients are most likely to benefit. One marker for immunotherapy response is tumour mutational burden, or TMB, which is the total number of mutations in the DNA of the cancer cells.
“In lung cancer, positive responses to checkpoint inhibitors have been linked with a high TMB in some trials.
“Targeted treatments identify and attack certain types of cancer cells with less harm to normal cells. In recent years, many targeted therapies have become available for advanced lung cancer and more are in development.
“EGFR inhibitors block the activity of a protein called epidermal growth factor receptor (EGFR). EGFR may be found at higher levels than normal on cancer cells, causing them to grow and divide. Some drugs that target EGFR are approved for treating lung cancer.
“ROS1 Inhibitors: The ROS1 gene makes the protein ROS1, which is involved in cell signalling and cell growth. A small percentage of non-small cell lung cancer patients have altered forms of the ROS1 gene. Some drugs are approved as treatments for patients with these alterations.
“BRAF Inhibitors: The BRAF gene makes the protein, B-Raf, which is involved in sending signals in cells and cell growth. This gene may be altered in some patients with nonsmall cell lung cancer, which can increase the growth and spread of cancer cells. A combination of drugs which targets a specific mutation in the BRAF gene, and a drug which targets a protein called MEK, has been approved in some countries as treatment for certain patients with non-small cell lung cancer.
“Other Inhibitors: Some non-small cell lung cancers have mutations in the NRTK-1 and NRTK2 genes that can be treated with targeted therapy. Inhibitors of other targets that drive some lung cancers are currently being tested in clinical trials,” Theresa concludes.
71 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Has the National Fall in Smoking Rates in Ireland Been Replicated in Cancer Patients? A 5-Year Report
Tobacco smoking has been causally linked as an independent risk factor in the pathogenesis and development of a wide variety of cancers.1 However, the implications of continued smoking after a cancer diagnosis are equally deleterious and the benefits of smoking cessation after a cancer diagnosis are often undervalued.2 Tobacco smoking is associated with an increased risk of recurrence,3,4,5 the development of second primary cancers,3,6,7,8 poorer treatment outcomes (including treatment-related complications and toxicities),3,4,9 lower survival rates and decreased quality of life,3,10 and pulmonary, cardiovascular and wound complications leading to increased length of hospital stay.11,12 Mortality among lung cancer patients is higher with increased smoking and home/work second-hand smoke exposure.13 There is a growing body of evidence that smoking cessation improves outcomes following the diagnosis of several different cancers.8,14,15 Some of these smoking-related complications are seen to decrease by 19% with each week of cessation.11 Notwithstanding the adverse outcomes, a significant number of people who have been diagnosed with cancer continue to smoke.10,16 Some of the important issues to be addressed to promote smoking cessation among cancer patients include: (i) a lack of awareness of smoking harms, (ii) lack of tailored interventions to promote smoking cessation among cancer patients,2 (iii) limited opportunities for conversation around smoking cessation17 and (iv) suboptimal use of smoking cessation interventions by cancer patients.17
Even though approximately 69% of tobacco smokers express a desire to quit and 52% have attempted to quit in the previous year,18 only 4–7% are able to quit without the use of smoking cessation supports.19 Although patients with head and neck (H and N) and lung cancers (LC) have shown a higher motivation to quit with quit rates as high as 70%, they also have high relapse rates
of up to 50% within 6 months.19,20
Evidence-based smoking cessation supports with consideration to the special needs of cancer patients are therefore needed. As part of a feasibility study, with the objective of assessing the development of a hospital-based, high-intensity, peri-treatment behavioural smoking cessation programme as part of routine care for cancer patients throughout Ireland, a review of smoking rates among cancer patients in Ireland was undertaken.
Materials and Methods
Rates of current and past smoking during a five-year study period among cancer patients in Ireland were evaluated using Hospital Inpatient Enquiry (HIPE) data. HIPE is a database which collects administrative, demographic and clinical data on discharges from, and deaths in, Irish acute public hospitals.21 HIPE data were requested for the five most recent years available (2014–2018). Data were received as summary tables representing number of discharges (17 years and older) with a principal diagnosis of cancer, for all cancer types combined (overall) and individual cancers (including breast cancer (BC), lung cancer, cervical cancer (CC) and H and N cancer), sorted by age, gender and smoking status (current and past smoking). Cells with five or fewer discharges were suppressed and not disclosed for reasons of confidentiality. HIPE discharges are coded using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10AM); Australian Classification of Health Interventions (ACHI); and Australian Coding Standards (ACS), 8th Edition.21
Smoking is collected using ICD10-AM diagnosis codes which are coded as an additional diagnosis. In the HIPE data, ‘current smoking’ was documented using code Z72.0 ‘Tobacco use— current’, which indicated if the patient has smoked tobacco (any amount) within the last month or there was documentation of
‘hazardous use’ (a pattern of substance use that increases the risk of harmful consequences for the user) of tobacco. Current smoking in our dataset also included code F17.1 ‘harmful use’ of tobacco (the clinician has clearly documented a relationship between a particular condition(s) and smoking (even if the patient has ceased smoking)), and code F17.2 ‘tobacco dependence syndrome’. ‘Past smoking’ (code Z86.43) was documented if the patient had smoked tobacco (any amount) in the past but excluding the last month.22
Prevalence over five years (2014–2018) was calculated for overall, age-specific and genderspecific smoking rates for patients with all cancer types combined, BC (female), LC, CC and H and N cancer. The relative change in the rate of current smoking from 2014 to 2018 among males and females (overall cancers) was calculated (2014 rate 2018 rate/2014 rate) × (100). The smoking rates among cancer patients were compared to the general population in Ireland (using ‘Healthy Ireland’ survey reports, a national health and lifestyle survey in people aged 15 and over in Ireland)23 and in Europe (European Tobacco Use: Trends Report 2019)24 and with the global trends of smoking among cancer patients. The study hypothesis was that smoking rates in cancer patients in Ireland do not follow the same trend as seen in the general population.

Results
The total number of discharges with a primary diagnosis of malignancy for all cancer types combined and for individual cancers reported by HIPE during 2014–2018 are presented in Table 1. From 2014 to 2018, discharges with a BC diagnosis were highest (range 8.8–9.0%) compared to other cancers (LC (range 3.1–3.6%), CC (range 1.6–1.4%) and H and N (1%)).
There was an increase in current smoking from 10.5 to 11.7% among cancer patients (overall) from 2014 to 2017 (Table 2). There
was a similar rise in the rate of past smokers among cancer patients (overall) from 15.2 to 21% during the same period (Table 3). There was a minor decrease of 0.3% in both current and past smoking in 2018 (Table 2 and Table 3).
Rates of current and past smoking for all cancers combined and individual cancers overall, gender-specific and by age group are shown in Table 2 and Table 3. Current and past smoking rates from 2014 to 2018 were consistently higher among males than females among all types of cancers, with the exception of current and past smoking in H and N cancer in 2017 and 2015/2018, respectively. Current smoking rates among overall cancer patients were higher in males than females (2014–2018). However, the relative increase in the current smoking rate was higher among females (9.1%) than males (7.6%) during 2014–2018. Current smoking rates were consistently higher among the 50–59 years old age group (range 14–15.9%), whereas past smoking rates were consistently higher among the 70 years and older age group (range 17.5–24.5%) for all types of cancers (overall) during 2014–2018. Among all cancer types, LC patients had the highest rate of current (range 24.7–24%) and past smoking (range 30.3–38%), whereas BC patients had the lowest rates of current (range 5.7–6.6%) and past smoking (7.2–12.8%) during this period.
Discussion
The proportion of survivors has increased among cancer patients and, with increased survival, more attention is now directed to survivorship.25 The present analysis aimed to estimate the prevalence of smoking among cancer patients admitted to Irish public hospitals during 2014–2018. Current smoking rates among cancer patients overall increased during 2014–2017, followed by a small reduction of 0.3% in 2018. Current smoking was consistently highest among 50–59-year-old cancer patients and among
72 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE ONCOLOGY: SMOKING
Written by: Patricia Fitzpatrick1,2, Nancy Bhardwaj1,*, Ailsa Lyons2, Kirsten Doherty2, Kate Frazer3, Amanda McCann4,5,Vikram Niranjan1, Shiraz Syed2 and Patricia Fox3
1School of Public Health, Physiotherapy & Sports Science, University College Dublin, D04 V1W8 Dublin, Ireland 2Department of Preventive Medicine & Health Promotion, St. Vincent’s University Hospital, D04 T6F4 Dublin, Ireland 3School of Nursing, Midwifery and Health Systems, University College Dublin, D04 V1W8 Dublin, Ireland 4Conway Institute of Biomolecular and Biomedical Research, University College Dublin, D04 V1W8 Dublin, Ireland 5School of Medicine, University College Dublin, D04 V1W8 Dublin, Ireland *Author to whom correspondence should be addressed.
typescombinedandforindividualcancersreportedbyHIPEduring2014–2018arepresentedinTable 1.From2014to2018,dischargeswithaBCdiagnosiswerehighest(range 8.8–9.0%)comparedtoothercancers(LC(range3.1–3.6%),CC(range1.6–1.4%)andHand N(1%)).

Table 1. Total number of discharges with a diagnosis of cancer (all combined and individual cancer) reported by HIPE during 2014–2018
Table1. Totalnumberofdischargeswithadiagnosisofcancer(allcombinedandindividualcancer) reportedbyHIPEduring2014–2018.
interlinking primary care and hospital services.41
males during 2014–2018. Current smoking rates were highest among LC patients and lowest among BC patients. The rates found are relatively low compared to the general populations (overall and gender-specific) of Europe and Ireland. Almost 29% of the population smokes tobacco in Europe,24 with the prevalence of current smoking ranging from 13–46% across countries during 2016.24 A minor fall of 0.3% was observed among cancer patients in 2018; in contrast, smoking rates in the Irish general population fell by 6% (from 23 to 17%) between 2015 and 2019.26 In the present analysis, current smoking was higher in males than females in overall cancer patients during 2014–2018 and in most years in LC patients. The upward trend was seen similarly in both genders. Similar gender differences are seen in the overall European and the Irish general populations.23,24 In this study, current smoking was highest in the 50–59-year-old age group (range 14–16%), older than the Irish general population highest prevalence age group of 25–34 years.23 These results point to our study hypothesis that smoking trends in cancer patients in Ireland do not follow that seen in the general population.
While smoking among the general population is widely evaluated in developed countries, there are few studies evaluating smoking trends among cancer patients.
In the National Cancer Institute’s (NCI) Cancer Trends Progress Report 2015–2019, smoking rates were higher in cancer patients than in the general United States (US) population, but the trend was downwards with an annual percentage change of 1.56% (2015–2018) followed by a small rise in 2019.25 In our study, current smoking rates were lower among cancer patients (overall) than the concurrent rate in the general population (2015–2019) of Ireland, but the upward trend was in contrast to the national decline in smoking rates.23,26
Based on the 2017 US National Health Interview Survey data (n =
26,742), Gritz et al. (2020) reported that among cancer survivors the rates of current smoking was 13.1% versus 26.1% for past smoking (smoked at least 100 cigarettes in their life but were not smoking at the time of the survey)27 which is slightly higher than our findings of current and past smoking (11.76% and 21.02%, respectively, in 2017). Current and past smoking rates reported for individual cancers by Gritz et al. were 12.64% and 23.27% for BC and 32.24% and 30.76% for CC, respectively,27 which were much higher than the present analysis where current and past smoking was 6.11% and 10.83% for BC and 17% and 17.73% for CC, respectively. However, the rates in LC patients reported by Gritz and colleagues were much lower than in our study (10.9% vs. 27.26% and 34.43% vs. 39.09%, respectively).27
Therewasanincreaseincurrentsmokingfrom10.5to11.7%amongcancerpatients (overall)from2014to2017(Table 2).Therewasasimilarriseintherateofpastsmokers amongcancerpatients(overall)from15.2to21%duringthesameperiod(Table 3).There wasaminordecreaseof0.3%inbothcurrentandpastsmokingin2018(Tables 2 and 3).
Table2. Five-year(2014–2018)trendofcurrentsmokingamongallcancer(combined),breast,lung, cervicalandheadandneckcancerpatientsoverallandage-andgender-specificinIreland.
Continued smoking is associated with lower survival rates among cancer patients. The National Cancer Registry of Ireland reported that cancer patients who were current smokers had significantly increased mortality compared to never smokers (HR = 1.36, 95% CI: 1.21–1.52).28 Given the high smoking rates among cancer patients, it is critical that healthcare professionals advocate smoking cessation among all patients including those diagnosed with cancer. However, in practice it is recognised that patients do not receive sufficient encouragement from their health care providers to quit smoking.29,30 Some of the barriers to broach the subject of smoking cessation from oncology healthcare professionals include (i) concerns around the negative impact of the therapeutic relationship, (ii) perceptions that such discussions were not appropriate,31 (ii) likely to be ineffective,17,31 or (iii) stigmatising31 and (v) inadequate training in smoking cessation.17,31 Hospital smoking cessation services are imperative to smoking cessation among cancer patients, but underutilisation has also been observed. In the US, in 2012, the Joint Commission set a
quality standard for hospitals and recommended that all admitted patients identified as current smokers should receive smoking cessation advice. Notwithstanding the recommendations, many hospitals failed to comply.32,33,34,35 Motivation to stop smoking is often high immediately after diagnosis—a time that can be taken advantage of as a key ‘teachable moment’.11 Patientrelated barriers to smoking cessation include being in a younger age group, depression, high nicotine dependence, living with someone who smokes and socio-economic difficulties,11,36 stress (related to the cancer diagnosis), a wish to maintain personal control and a sense of ‘normal’ self and being unconvinced or not accepting the connection between smoking, cancer and health.17 KaramHage et al. also identified the lack of a tobacco treatment programme integrated with cancer treatment as a barrier.12 These can be addressed by considering communication style and the need for prolonged and intensified counselling and pharmacotherapy support.11
Early integration of general practitioners (GPs) with cancer patient care can potentiate and help to bridge the lack of onsite smoking cessation services in hospitals,37 and the advantage in smoking cessation through a GP is attributable to frequent visits by cancer patients.38 The advice from the GP can be delivered in various formats such as very brief, brief or intensive. In a UK study of 42 clinical trials between 1972 and 2012 on smoking cessation advice including 31,000 smokers, primary care was found to be the most common site for advice; brief intervention resulted in increased quit rates.39 Smoking cessation services in the UK encourages all GPs to identify patients who smoke and provide smoking cessation advice, following which 4-week behavioural support is also provided.40 Smoking cessation should be viewed as an interdisciplinary intervention,
The Irish National Cancer Strategy 2017–2026 stated that smoking has the biggest impact on cancer prevention. Tobacco Free Ireland, the National Tobacco Control strategy aims to make Ireland tobacco-free (prevalence of less than 5%) by 2025, and has been recognized as a crucial part of cancer prevention.42 Daily smoking has declined to 14% among those over the age of 15 in Ireland, with a further 3% occasional smokers in 2019.26 The National Tobacco Control Strategy also advises on providing appropriate smoking cessation supports as part of the treatment regime and care planning for all cancer survivors42 and recognises smoking cessation services as an important element of tobacco control.37 The National Brief Intervention Programme (Making Every Contact Count) embraces behaviour change support as a routine part of health care practice and delivery to facilitate prevention of smoking.42 There are a wide range of smoking cessation services provided by the Health Service Executive (HSE) in Ireland, including a QUITline phone service for cessation support, online and social media supports on Quit.ie and Facebook, primary care supports provided by GPs, pharmacists and dentists, HSE quit clinics and courses, and medication and nicotine replacement therapies.43
The Irish Cancer Society runs ‘Daffodil Centres’ in many large hospitals in Ireland which provide onsite information, support and advice on smoking cessation as part of cancer prevention.44 Both in-hospital smoking cessation programmes and those provided by the HSE in Ireland are not disease- or time-specific and the services available for cancer patients are the same. It is unknown how routinely or consistently smoking cessation interventions are being offered to patients with cancer. When the service is offered, rates of uptake and attendance are unclear, but anecdotally it is known that fewer cancer patients are referred.
To our knowledge, in the last ten years this is the first study from Ireland and Europe to evaluate the prevalence of smoking among cancer patients. This study has been conducted using data from the HIPE system, a computerbased information system that captures data on discharges from 62 acute public hospitals in Ireland. Among eight national health information systems, it has
CancerType NumberofDischargesReported 20142015201620172018 Allcancers 2700925606261672652727276 n (%) n (%) n (%) n (%) n (%) Breast 2369(8.8)2325(9.1)2250(8.6)2307(8.7)2469(9.0) Lung 835(3.1)871(3.4)1003(3.8)988(3.7)995(3.6) Cervical 426(1.6)402(1.6)320(1.2)389(1.5)387(1.4) HeadandNeck254(1.0)257(1.0)258(1.0)271(1.0)234(1.0)
CancerType Categories Year 20142015201620172018 Allcancer 10.5311.2411.2011.7611.44 Breastcancer(female) 5.906.626.446.115.71 Lungcancer 24.7426.5324.0227.2624.11 Cervicalcancer 11.0014.6712.8017.0019.80 HeadandNeckcancer 3.148.5612.7910.337.69 Allcancers Male 12.0812.4512.4313.0713.00 Female 8.819.929.8310.319.72 Breastcancer Female 5.906.626.446.115.71 Lungcancer Male 26.2527.0022.5627.0524.72 Female22.7825.9525.6827.5123.33 Cervicalcancer Female11.0014.6712.8017.0019.80 HeadandNeckcancer Male ~9.0016.558.10~ Female ~8.007.9613.00~
73 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Therewasanincreaseincurrentsmokingfrom10.5to11.7%amongcancerpatients (overall)from2014to2017(Table 2).Therewasasimilarriseintherateofpastsmokers amongcancerpatients(overall)from15.2to21%duringthesameperiod(Table 3).There wasaminordecreaseof0.3%inbothcurrentandpastsmokingin2018(Tables 2 and 3).
Table 2. Five-year (2014–2018) trend of current smoking among all cancer (combined), breast, lung, cervical and head and neck cancer patients overall and age- and gender-specific in Ireland
Five-year(2014–2018)trendofcurrentsmokingamongallcancer(combined),breast,lung, cervicalandheadandneckcancerpatientsoverallandage-andgender-specificinIreland.

~ denotes five or fewer cases reported to HIPE or cases that have been suppressed for reason of confidentiality and not disclosed.
the largest database, contributing to the strength of the study.45 This is an important but underresearched topic in Ireland. The data in this study will provide the basis for planning of smoking cessation services for cancer patients; the exact nature of such services will depend on the final collated results from our feasibility study.
~denotesfiveorfewercasesreportedtoHIPEorcasesthathavebeensuppressedforreasonofconfidentiality andnotdisclosed.
However, there are some limitations with the use of the HIPE system. The collection of smoking-related codes is very much dependent on the doctors and nursing staff recording this information in the notes, which may not be consistent across hospitals; therefore, it is recognised that smoking documentation may be an
underestimate.22 As there is no code for ‘never smoked’ in the WHO ICD classification used for HIPE, it is difficult to ascertain the degree of underestimation of tobacco use.22 This problem has been highlighted in other reviews also.46 It was observed in the NCI’s clinical cancer trials that only 21.9% of clinicians assessed current smoking and 2.6%
secondhand smoke exposure at enrolment and only 4.5% assessed tobacco use during follow up and 0.6% assessed secondhand smoke exposure during follow up.46 Another limitation is that HIPE data represents the number of discharges and there is no unique identification number for patients. As patients may be admitted more than once, there may be
Variables CancerType Categories Year 20142015201620172018 Overallcurrent smokingrates (%) Allcancer 10.5311.2411.2011.7611.44 Breastcancer(female) 5.906.626.446.115.71 Lungcancer 24.7426.5324.0227.2624.11 Cervicalcancer 11.0014.6712.8017.0019.80 HeadandNeckcancer 3.148.5612.7910.337.69 Gender-specific rates(%) Allcancers Male 12.0812.4512.4313.0713.00 Female 8.819.929.8310.319.72 Breastcancer Female 5.906.626.446.115.71 Lungcancer Male 26.2527.0022.5627.0524.72 Female22.7825.9525.6827.5123.33 Cervicalcancer Female11.0014.6712.8017.0019.80 HeadandNeckcancer Male ~9.0016.558.10~ Female ~8.007.9613.00~ Int.J.Environ.Res.PublicHealth 2022, 19,2348 4of10 Table2. Cont. Variables CancerType Categories Year 20142015201620172018 Age-specific rates(%) Allcancers Lessthan40years10.6011.0811.7710.379.97 40–49years11.9312.7412.0313.5113.60 50–59years14.0014.5215.2515.9115.53 60–69years12.3313.6213.3913.8313.20 70yearsandover7.367.587.538.298.08 Breastcancer Lessthan40years10.506.956.484.396.62 40–49years5.805.585.215.314.85 50–59years6.348.568.637.547.27 60–69years7.118.088.168.527.22 70yearsandover3.084.054.094.023.34 Lungcancer Lessthan40years~ ~20.68~ ~ 40–49years28.4432.0032.8938.0021.90 50–59years35.8235.4032.5034.9234.91 60–69years26.0231.4927.1028.5227.40 70yearsandover18.0818.2117.5322.2718.21 Cervicalcancer Lessthan40years7.8214.40~6.1823.57 40–59years12.1619.6614.5821.5425.97 60yearsandover12.296.60~18.917.27 HeadandNeckcancer Lessthan40years~16.3621.73~ ~ 40–59years~8.9011.53~10.00 60yearsandover~5.1810.4411.477.20
Variables CancerType Categories Year 20142015201620172018 Overall past-smoking rates(%) Allcancer 15.2217.4619.7021.0220.75 Breastcancer(female) 7.269.1111.0210.8312.83 Lungcancer 30.3434.3437.5939.0938.09 Cervicalcancer 11.268.9514.0617.7313.17 RESPIRATORY: PNEUMONIA FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 74 ONCOLOGY: SMOKING
Table3. Five-year(2014–2018)trendofpastsmokingamongallcancers(combined),breast,lung, cervicalandheadandneckcancerpatientsoverallandbyageandgenderinIreland.
SIMPONI delivers long-term disease control, maintaining efficacy and safety profile over 4 years.2
SIMPONI is indicated for treatment of moderately to severely active ulcerative colitis in adult patients who have had an inadequate response to conventional therapy including corticosteroids and 6-mercaptopurine (6-MP) or azathioprine (AZA), or who are intolerant to or have medical contraindications for such therapies.1

Simponi 50 mg, 100 mg Solution for Injection in pre-filled pen Simponi 50 mg Solution for Injection in pre-filled syringe (golimumab)
ABRIDGED PRODUCT INFORMATION Refer to Summary of Product Characteristics before prescribing PRESENTATION Simponi 50 mg solution for injection in pre-filled pen Simponi 50 mg solution for injection in pre-filled syringe Simponi 100 mg solution for injection in pre-filled pen INDICATIONS Rheumatoid Arthritis (RA): Simponi, in combination with methotrexate (MTX), is indicated for: the treatment of moderate to severe, active rheumatoid arthritis in adults when the response to disease-modifying anti-rheumatic drug (DMARD) therapy including MTX has been inadequate; the treatment of severe, active and progressive rheumatoid arthritis in adults not previously treated with MTX. Simponi, in combination with MTX, has been shown to reduce the rate of progression of joint damage as measured by X-ray and to improve physical function; Psoriatic Arthritis (PsA): Simponi, alone or in combination with MTX, is indicated for the treatment of active and progressive PsA in adults when the response to DMARD therapy has been inadequate. Simponi has been shown to reduce the rate of progression of peripheral joint damage as measured by X-ray in patients with polyarticular symmetrical subtypes of the disease and to improve physical function. Ankylosing Spondylitis (AS): Simponi is indicated for the treatment of severe, active AS in adults who have responded inadequately to conventional therapy. Non-radiographic axial spondyloarthritis (nr-Axial SpA): Simponi is indicated for the treatment of severe, active nr-Axial SpA who have had an inadequate response to or are intolerant to NSAIDs Ulcerative colitis (UC): Simponi is indicated for treatment of moderately to severely active UC in adult patients who have had an inadequate response to conventional therapy including corticosteroids and 6-mercaptopurine (6-MP) or azathioprine (AZA), or who are intolerant to or have medical contraindications for such therapies. Polyarticular juvenile idiopathic arthritis (pJIA): Simponi 50mg in combination with MTX is indicated for the treatment of polyarticular juvenile idiopathic arthritis in children with a body weight of at least 40 kg, who have responded inadequately to previous therapy with MTX. DOSAGE AND ADMINISTRATION Simponi should be injected subcutaneously. Treatment should be initiated and supervised by qualified physicians experienced in the diagnosis and treatment of RA, PsA, AS, nr-Axial SpA, UC or pJIA. After proper training in subcutaneous injection technique, patients may self-inject, if their physician deems it appropriate. RA: Simponi 50 mg given once a month, on the same date each month, concomitantly with MTX. PsA: Simponi 50 mg given once a month, on the same date each month, alone or in combination with MTX. AS and nr-Axial SpA: Simponi 50 mg given once a month, on the same date each month. Clinical response is usually achieved within 12-14 weeks of treatment (3 or 4 doses). Continued therapy should be reconsidered in patients who show no evidence of therapeutic benefit within this time period. In patients weighing more than 100 kg who do not achieve an adequate clinical response after 3 or 4 doses, increasing the dose of golimumab to 100 mg once a month may be considered, taking into account the increased risk of certain serious adverse reactions with the 100 mg dose compared with the 50 mg dose. UC: Patients weighing < 80 kg: Simponi given as an initial dose of 200 mg, followed by 100 mg at week 2. Patients who have an adequate response should receive 50 mg at week 6 and every 4 weeks thereafter. Patients who have an inadequate response may benefit from continuing with 100 mg at week 6 and every 4 weeks thereafter. Patients weighing ≥ 80 kg: Simponi given as an initial dose of 200 mg, followed by 100 mg at week 2, then 100 mg every 4 weeks. During maintenance treatment, corticosteroids may be tapered, following clinical practice guidelines. Clinical response is usually achieved within 12-14 weeks of treatment (after 4 doses). pJIA: Simponi 50 mg administered once a month, on the same date each month, for children with a body weight of at least 40 kg. Clinical response is usually achieved within 12 to 14 weeks of treatment (after 3-4 doses). Missed dose: If a patient forgets to inject Simponi on the planned date, the forgotten dose should be injected as soon as the patient remembers. The patient should be instructed not to inject a double dose. Older patients (≥ 65 years): no dose adjustment required. Paediatric patients (<18 years): For indications other than pJIA, Simponi is not recommended. Patients with renal and hepatic impairment: Simponi is not recommended. CONTRAINDICATIONS Patients with a hypersensitivity to golimumab or any of the excipients; Patients with active tuberculosis (TB) or other severe infection such as sepsis and opportunistic infections; patients with moderate or severe heart failure (NYHA class III/IV). PRECAUTIONS AND WARNINGS Infections: Patients must be monitored closely for infection before, during and for 5 months after cessation of treatment. Exercise caution when considering golimumab in patients with chronic infection or a history of recurrent infection including use of concomitant immunosuppressive therapy. Golimumab should not be given to patients with clinically important active infection. Patients should be advised of the potential risk factors. Bacterial infections (including sepsis and pneumonia), mycobacterial (including TB), invasive fungal and opportunistic infections, including fatalities, have been reported. The invasive fungal infection should be suspected if they develop a serious systemic illness. There was a greater incidence of serious infections, including opportunistic infections and TB, in patients receiving golimumab 100 mg compared with patients receiving golimumab 50 mg. Serious infections have occurred in patients on concomitant immunosuppressive therapy that, in addition to their underlying disease, could predispose them to infection. There have been reports of active TB in patients receiving golimumab, including patients previously treated for latent TB. Patients should be evaluated for active or latent TB before golimumab treatment. All such tests should be recorded on the Patient Reminder Card provided with the product. If active TB is diagnosed, treatment with golimumab should not be initiated. If latent TB is diagnosed, treatment with anti-TB therapy must be initiated before initiation of golimumab. Patients on golimumab should be monitored closely for signs and symptoms of active TB and advised to seek medical advice if signs and/or symptoms of TB appear. Hepatitis B (HBV) reactivation: Reactivation of HBV occurred in patients receiving golimumab who were chronic carriers. Some cases had a fatal outcome. Patients should be tested for HBV infection before initiating treatment with golimumab Malignancies and lymphoproliferative disorders: Caution is advised when considering golimumab treatment in patients with history of malignancy or continuing treatment in patients who develop a malignancy, additional caution should be exercised in patients with increased risk for malignancy due to heavy smoking. A risk for the development of malignancies in children and adolescents cannot be excluded. Rare cases, usually fatal, of hepatosplenic T-cell lymphoma (HSTCL) have been reported, the majority of cases occurred in adolescent and young males nearly all on concomitant treatment with azathioprine (AZA) or 6-mercaptopurine (6–MP). The potential risk with the combination of AZA or 6-MP and golimumab should be carefully considered. A risk for the development for HSTCL in patients treated with TNF-blockers cannot be excluded. Colon dysplasia/carcinoma - Screen for dysplasia in all patients with UC who are at increased risk or had a prior history for dysplasia or colon carcinoma. In newly diagnosed dysplasia patients the risks and benefits of continued golimumab use should be carefully assessed. Melanoma and Merkel cell carcinoma (all TNF-blocking agents including golimumab) have been reported, periodic skin examination is recommended, particularly for
patients with risk factors for skin cancer. Heart Failure: Golimumab should be used with caution in patients with mild heart failure (NYHA class I/II). Patients should be closely monitored and golimumab must be discontinued in patients who develop new or worsening symptoms of heart failure. Some cases had a fatal outcome. Neurological events: Use of anti-TNF therapy, including golimumab, has been associated with cases of new onset or exacerbation of clinical symptoms and/or radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis and peripheral demyelinating disorders. Discontinuation of golimumab should be considered if these disorders develop. Carefully consider the benefits and risks before initiation of therapy in patients with a history of demyelinating disorders. Surgery: Patients requiring surgery whilst on golimumab therapy should be closely monitored for infections. Autoimmune processes: If a patient develops symptoms suggestive of a lupus-like syndrome following treatment with golimumab and is positive for antibodies against double-stranded DNA, treatment should be discontinued. Haematological reactions: There have been post-marketing reports of pancytopenia, leukopenia, neutropenia, agranulocytosis, aplastic anaemia, and thrombocytopaenia in patients receiving TNF-blockers, including golimumab. Patients should be advised to seek medical attention if they develop signs and symptoms suggestive of blood dyscrasias. Discontinuation should be considered in patients with significant haematologic abnormalities. Vaccinations/therapeutic infectious agents: It is recommended that live vaccines or any therapeutic infectious agents should not be given concurrently. Allergic reactions: If an anaphylactic reaction or other serious allergic reaction occurs, administration of golimumab should be discontinued immediately, and suitable treatment initiated. The needle cover of the pre-filled pen contains latex and may cause allergic reactions in those sensitive to latex. Special populations: Older patients (≥ 65 years): Adverse events, serious adverse events and serious infections in patients aged ≥65 were comparable to those observed in younger patients. However, caution should be exercised when treating the elderly, particular attention should be paid to infections. There were no patients age 45 and over in the nr-Axial SpA study. Paediatric patients (<18 years): Vaccinations: it is recommended that prior to initiating golimumab therapy, paediatric patients be brought up to date with all immunisations in agreement with current immunisation guidelines. Excipients: Simponi contains sorbitol (E420). In patients with rare hereditary problems of fructose intolerance, the additive effect of concomitantly administered products containing sorbitol (or fructose) and dietary intake of sorbitol (or fructose) should be taken into account. INTERACTIONS Combination of golimumab and other biological therapeutics used to treat the same conditions as golimumab, including anakinra and abatacept is not recommended. PREGNANCY AND LACTATION Administration of golimumab is not recommended during pregnancy or breast-feeding. Women of childbearing potential should use adequate contraception and continue its use for at least 6 months after the last golimumab treatment. SIDE EFFECTS Refer to SmPC for complete information on side effects
Very Common (≥ 1/10): upper respiratory tract infection; Common (≥1/100): bacterial infections, lower respiratory tract infections, viral infections, bronchitis, sinusitis, superficial fungal infections, abscess, leukopenia (including neutropenia), anaemia, allergic reactions, autoantibody positive, depression, insomnia, dizziness, headache, paraesthesia, hypertension, asthma and related symptoms, dyspepsia, gastrointestinal and abdominal pain, nausea, gastrointestinal inflammatory disorders, stomatitis, alanine aminotransferase increased, aspartate aminotransferase increased, pruritus, rash, alopecia, dermatitis, pyrexia, asthenia, injection site reaction, chest discomfort, bone fractures were reported. Serious, including fatal adverse events have been reported including septic shock, lymphoma, leukaemia, melanoma, Merkel cell carcinoma, hepatosplenic T-cell lymphoma*, leukopenia, thrombocytopaenia, pancytopaenia, aplastic anaemia, agranulocytosis, serious systemic hypersensitivity reactions (including anaphylactic reaction), skin exfoliation, vasculitis (systemic), sarcoidosis, demyelinating disorders, congestive heart failure, arrhythmia, ischaemic coronary artery disease, thrombosis, interstitial lung disease and lupus-like syndrome. * Observed with other TNF-blocking agents. Paediatric population: pJIA: The safety of golimumab has been studied in a phase III study of 173 pJIA patients from 2 to 17 years of age. The average follow-up was approximately two years. In this study, the type and frequency of adverse events reported were generally similar to those seen in adult RA studies. PACKAGE QUANTITIES 1 x 50 mg pre-filled pen containing 50 mg of golimumab in 0.5 ml solution for injection 1 x 50 mg pre-filled syringe containing 50 mg of golimumab in 0.5 ml solution for injection 1 x 100 mg pre-filled pen containing 100 mg of golimumab in 1 ml solution for injection Legal Category: Prescription Only Medicine. Marketing Authorisation Number 50 mg Pre-filled Pen EU/1/09/546/001 50 mg
Pre-filled Syringe EU/1/09/546/003 100 mg Pre-filled
Pen EU/1/09/546/005 Marketing Authorisation Holder Janssen Biologics B.V., Einsteinweg 101, 2333 CB Leiden, The Netherlands Date of Revision of Text: February 2019 © Merck Sharp & Dohme Ireland (Human Health) Limited 2019. All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin 18 or from www.medicines.ie. X-083G-Feb2019. Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie Adverse events should also be reported to MSD (Tel: 01-299 8700) References: 1. Simponi (Golimumab) Summary of Product Characteristics (SmPC). Last updated November 2020. Available at www.medicines.ie 2. Reinisch W et al. Long-term Benefit of Golimumab for Patients with Moderately-to-Severely Active Ulcerative Colitis: Results from the PURSUIT-Maintenance Extension. J Crohns Colitis. 2018;12(9):1053-1066 IE-GOL-00203 Date of Preparation: August 2022 Choose a different path for your Ulcerative Colitis patients’ future.
Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7 Ireland
Image by permission of Sussex Wildlife Trust
RESPIRATORY: PNEUMONIA
Five-year(2014–2018)trendofpastsmokingamongallcancers(combined),breast,lung, cervicalandheadandneckcancerpatientsoverallandbyageandgenderinIreland.

~ denotes five or fewer cases reported to HIPE or cases that have been suppressed for reason of confidentiality and not disclosed.
some duplicate data.45 However, the HIPE data are recorded for each patient admitted to public hospitals in Ireland and coded using standard international classification systems. Despite the limitation of duplicate episodes of care, as the number of discharges in each cancer group were comparable during the 5-year study period (2014–2018), this
andnotdisclosed.
limitation should be stable over the study period and trend analysis was meaningful.
Conclusions
In conclusion, smoking trends in cancer patients show a worrying upward trend. Cancer patients being routinely asked about smoking by their
healthcare professionals and its documentation is crucial to smoking cessation. Robust documentation of smoking status will also support careful evaluation of smoking among cancer patients in future studies. Referral of cancer patients who smoke to hospital-based or community smoking cessation services is pivotal to increase their chances
Ratesofcurrentandpastsmokingforallcancerscombinedandindividualcancers overall,gender-specificandbyagegroupareshowninTables 2 and 3.Currentandpast smokingratesfrom2014to2018wereconsistentlyhigheramongmalesthanfemales amongalltypesofcancers,withtheexceptionofcurrentandpastsmokinginHandN cancerin2017and2015/2018,respectively.Currentsmokingratesamongoverallcancer patientswerehigherinmalesthanfemales(2014–2018).However,therelativeincrease inthecurrentsmokingratewashigheramongfemales(9.1%)thanmales(7.6%)during 2014–2018.Currentsmokingrateswereconsistentlyhigheramongthe50–59yearsoldage group(range14–15.9%),whereaspastsmokingrateswereconsistentlyhigheramongthe 70yearsandolderagegroup(range17.5–24.5%)foralltypesofcancers(overall)during 2014–2018.Amongallcancertypes,LCpatientshadthehighestrateofcurrent(range 24.7–24%)andpastsmoking(range30.3–38%),whereasBCpatientshadthelowestratesof
of quitting and reduce smoking relapses. It is important that there is an accurate way to capture smoking rates in cancer patients to facilitate a national review; routine documentation of smoking status in medical and nursing charts, and improved capture in the HIPE database is imperative.
References available on request
Int.J.Environ.Res.PublicHealth 2022, 19,2348 5of10
Variables CancerType Categories Year 20142015201620172018 Age-specific rates(%) Allcancers Lessthan40years7.607.569.8610.529.59 40–49years11.0410.9614.3914.3014.36 50–59years12.9716.0817.5617.2717.75 60–69years16.8719.1521.5023.6823.22 70yearsandover17.5120.5222.6624.5023.91 Breastcancer Lessthan40years4.905.348.104.945.42 40–49years8.006.0411.9611.2412.86 50–59years7.0110.1310.139.1210.53 60–69years8.8912.1311.4712.7916.26 70yearsandover6.178.7811.5812.3914.45 Lungcancer Lessthan40years~ ~20.68~ ~ 40–49years~ ~23.6828.0035.23 50–59years21.7629.1933.0022.6821.22 60–69years30.8732.9236.7038.2537.84 70yearsandover34.2839.0841.0643.6941.74 Cervicalcancer Lessthan40years13.045.9311.8121.6410.56 40–59years12.1611.2313.8813.2513.63 60yearsandover8.198.4918.1821.6215.45 HeadandNeckcancer Lessthan40years~ ~ ~20.00~ 40–59years~ ~17.9424.4620.00 60yearsandover9.0025.1824.6229.50~ ~denotesfiveorfewercasesreportedtoHIPEorcasesthathavebeensuppressedforreasonofconfidentiality
Table3. Cont.
40–59years~8.9011.53~10.00 60yearsandover~5.1810.4411.477.20 ~denotesfiveorfewercasesreportedtoHIPEorcasesthathavebeensuppressedforreasonofconfidentiality
Variables CancerType Categories Year 20142015201620172018 Overall past-smoking rates(%) Allcancer 15.2217.4619.7021.0220.75 Breastcancer(female) 7.269.1111.0210.8312.83 Lungcancer 30.3434.3437.5939.0938.09 Cervicalcancer 11.268.9514.0617.7313.17 HeadandNeckcancer 8.2615.9518.2125.8316.23 Gender-specific rates(%) Allcancers Male 18.9021.942425.924.79 Female11.1312.611515.616.27 Breastcancer Female 7.269.1111.0210.8312.83 Lungcancer Male 31.3336.0040.4043.5040.87 Female29.0432.2034.4033.7434.52 Cervicalcancer Female11.268.9514.0617.7313.17 HeadandNeckcancer Male ~14.5022.0628.3010.86 Female ~17.4613.2722.7623.95
Table 3. Five-year (2014–2018) trend of past smoking among all cancers (combined), breast, lung, cervical and head and neck cancer patients overall and by age and gender in Ireland
FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 76 ONCOLOGY: SMOKING
IL-36: a therapeutic target for ulcerative colitis?
Written by Yasmina E. Hernandez Santana, Naoise Irwin & Patrick T. Walsh
a) National Children’s Research Centre, Children’s Health Ireland Crumlin,
b)
Department of Clinical Medicine, School of Medicine, Trinity College Dublin
Introduction
Ulcerative colitis (UC) is a chronic gastrointestinal inflammatory condition characterized by inflammation of the intestinal mucosa, which can extend from the rectum to the entire length of the colon. Together with Crohn’s Disease, it represents the most frequent manifestation of Inflammatory Bowel Disease (IBD) and its most frequent symptoms include abdominal pain and diarrhea with presence of blood. Although the precise etiology of UC is poorly understood, many factors contribute to this condition including genetic background, microbiome dysbiosis, dietary and environmental factors, and dysregulated mucosal inflammation.1 The incidence of UC is increasing among both adult and pediatric patients in developed countries, and the prevalence of disease has been estimated to range from 2.42 to 2943 cases per 100,000 persons in Europe.4 Furthermore, the total direct costs for UC annually are estimated at between 12.5 and 29.1 billion euros in Europe with costs associated with hospitalizations and surgery increasing with disease severity.5 While historically, therapeutic approaches have been aimed at alleviating symptoms of disease, more recently the focus has shifted toward inhibiting specific pathways associated with dysregulated inflammation and promoting mucosal healing as a means of more effectively treating patients. Despite significant new and emerging advances in this regard, it is estimated that up to 30–40% of patients are unresponsive to currently available therapeutic options. This underlines an important need to improve and individualize treatment approaches.
Current therapeutic strategies for UC
Arguably the most significant advance in UC treatment in recent decades has coincided with the development of biotherapeutics aimed at targeting specific inflammatory cytokines. Prior to this, the use of agents such as sulfonamides,
aminosalicylates, corticosteroids, and antibiotics, although successful, were beset with issues including high rates of relapse and deleterious side effects.6–13
Toward the end of the last century, disease modifying immunosuppressant agents such as cyclosporine and tacrolimus emerged as new therapeutic approaches, underscoring the validation of targeting aberrant inflammation as an effective strategy for UC.14 This was quickly followed by the emergence of monoclonal antibodies (mAbs) aimed at neutralizing proinflammatory cytokines, initially focused on treating patients for whom traditional approaches had failed.15 The first monoclonal antibody licensed for treatment of IBD was infliximab, an antiTNF antibody.16 Subsequently, further anti-TNF agents such as adalimumab and golimumab have also entered the clinic.17,18 A second successful cytokinedirected approach has been to target the shared p40 subunit of IL-12 and IL-23 which are key instructive cytokines in driving proinflammatory T helper 1/17 responses. Ustekinumab, which directly targets the p40 subunit, has been approved for moderate to severe UC.19 Further mAbs, which specifically target the p19 subunit of IL-23 including brazikumab, risankizumab, guselkumab, and mirikizumab, are also currently being evaluated as potential therapeutics in IBD.20
In addition to anti-cytokine therapies, new biotherapeutics have also been developed with the aim of restricting pathogenic immune cell infiltration to the gut. These include vedolizumab, which binds the α4β7 integrin and is suggested to inhibit leukocyte migration toward the intestinal mucosa.21,22 Interestingly, Feagan et al. demonstrated that vedolizumab was efficacious as both an induction and maintenance therapy for UC.23 These findings have been supported by subsequent studies which have also reported a favorable safety profile.24,25
Another biotherapeutic that limits the migration of lymphocytes


to the gut is the sphingosine 1-phosphate (S1P) receptor modulator, ozanimod. The results of phase II and III clinical trials have shown that ozanimod enhances clinical remission in patients with moderate to severe UC and has a satisfactory longterm safety profile.26,27 Similar approaches, currently under clinical development include anti-adhesion molecules such as AJM347 (α4β7-integrin inhibitor), AJM300 (anti-α4integrin antibody), ontalizumab (anti-MadCAM1 antibody), and etrolizumab (anti-β7-integrin antibody).28 The efficacy and safety of etrolizumab as a treatment for UC has been evaluated in phase 3 clinical trials enrolling over 3,000 patients.25 Recently reported findings indicate that while treatment with etrolizumab can provide benefit to patients during the induction phase, such improvements were less evident in maintenance studies.26,27,29–31
Most recently, small molecule inhibitors of the Janus kinases (JAK) 1/3 signaling cascade have gained considerable attention as potential treatments for IBD.32 While tofacitinib (JAK1-3) Inhibitor is approved for UC, several other inhibitors currently under clinical evaluation include peficitinib (JAK3 inhibitor), TD-1473 (gut-selective pan-JAK inhibitor), deucravacitinib (TYK2 inhibitor), filgotinib (JAK1 inhibitor), and upadacitinib (JAK1 inhibitor). Filgotinib was shown to effectively induce and maintain clinical remission in patients with moderate to severe UC33 and
has recently been approved in Europe as a treatment for UC.
Furthermore, a recent metaanalysis of data from 28 clinical trials showed that upadacitinib resulted in improved induction of clinical remission of moderate to severe UC in comparison to a range of other small molecules and biologics but was also more likely to lead to adverse events.30 While the landscape of new therapeutic entities for UC continues to expand, there is an appreciation that not all patients will be responsive to specific treatment approaches. This is due to an incomplete understanding of the complex nature of disease etiology and pathogenesis coupled with the individualized nature of disease progression among patients. While combination therapeutic approaches have been proposed and are being evaluated in patients that do not respond to single modes of therapy,34,35 there is a pressing need to identify biomarker signatures of patient responsiveness to define which approaches are most likely to achieve improved patient outcomes and the appropriate timing of such interventions. An ever growing understanding of the pathologic pathways driving mechanisms of disease continues to reveal new targets which may be relevant in certain patients.
One such emerging target is the IL-36 subfamily of cytokines which appear to play a distinct role in regulating the balance between inflammation and homeostasis in the intestine.
77 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Ulcerative
Yasmina E. Hernandez Santana Patrick T. Walsh
Colitis
Ulcerative Colitis
Figure 1. Therapeutic interventions in IL-36-mediated inflammation. Neutrophil-derived proteases process IL-36 cytokines (IL-36a, IL-36B and IL-36y) into fully active forms via proteolytic cleavage. Activation of IL-36 agonists allows their interaction with the IL-36 receptor (IL-36R) and recruitment of the interleukin-1 receptor accessory protein (IL-1-RAcP) to form an IL-36R/IL-1RAcP heterodimer. The formation of this receptor complex initiates NFkB and MAPK signalling and a subsequent IL-36-mediated inflammatory response. The IL-36 receptor antagonist (IL-36Ra) exists naturally and inhibits the pro-inflammatory response elicited by IL-36 agonists by binding to the IL-36R and preventing the association of IL-36R and IL-1RAcP. Therapeutics are also capable of inhibiting this IL-36-mediated inflammatory cascade. Spesolimab, for example, is a monoclonal antibody that binds to the IL-36R and prevents its interaction with IL-36 agonists. Additional therapeutics include Anti-IL-1RAcP, which interacts with IL-1RAcP to inhibit the formation of the IL-36R/IL-1RAcP complex, and protease inhibitors, which prevent the processing of IL-36 agonists into active forms. A-552 is a more recently discovered small molecule that exclusively antagonizes IL-36y to attenuate IL-36y-specific responses.
Role of IL-36 cytokine family in UC
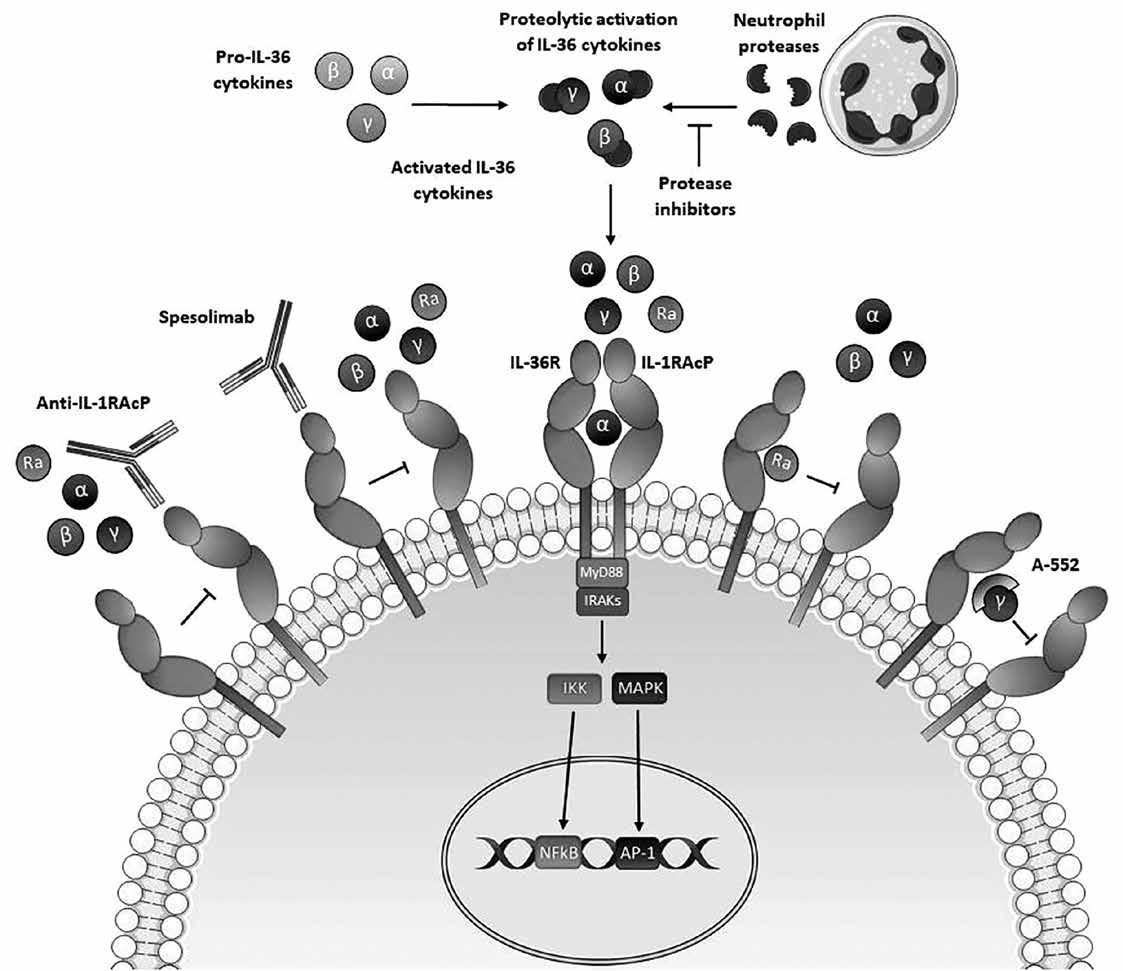
The IL-36 cytokines are members of the broader IL-1 cytokine family. The IL-36 family comprises three agonist ligands, IL-36 alpha (IL-1F6), IL-36 beta (IL-1F8), and IL-36 gamma (IL-1F9), and two antagonist ligands IL-36 Ra
(IL-1F5) and IL-38. All members bind to the IL-36 receptor, a heterodimer composed of the specific IL-36 R (IL-1Rrp2) and IL-1RAcP chains.36,37 IL36 cytokines are secreted by epithelial cells and mononuclear cells in the gut36 and are thought to be secreted in a non-active form that requires proteolytic
processing for full activation. It is noteworthy that neutrophilderived proteases, including proteinase-3, elastase, and cathepsin G, have been shown to activate IL-36 family members in vitro,38,39 and neutrophil infiltration is a characteristic of the inflamed gastrointestinal mucosa in UC.40 Indeed, the inhibition of
gastrointestinal mucosal protease activity has been proposed as a potential therapeutic option for IBD.41
IL-36 cytokines have been identified as key pathogenic drivers of a rare autoinflammatory disease condition known as Deficiency of Interleukin-36 Receptor Antagonist (DITRA), which is associated with lossof-function mutations in the IL36RN gene and manifests primarily as chronic generalized pustular psoriasis (GPP).42–45 These discoveries have prompted further investigations into the role of these cytokines, not only in more common dermal diseases, but also in chronic inflammatory bowel disease.
Elevated levels of IL-36α and IL-36γ expression have been
78 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
described in the inflamed colonic mucosa of UC patients as well as in several murine models of disease.46–49 Despite this, the precise role of IL-36 cytokines in this setting remains controversial. While some studies have described their role in promoting intestinal inflammation through the recruitment and activation of neutrophils, inflammatory monocytes, and pro-inflammatory CD4 + T cells,50,51 others have demonstrated that IL-36 promotes resolution of intestinal inflammation through enhanced mucosal healing.48,49,52 More recently, IL-36 has been implicated in driving fibrosis as a result of chronic intestinal inflammation by Scheibe and colleagues,53 who observed high levels of IL36 cytokine expression in fibrotic tissue in the colon of patients affected with IBD. In addition, several studies have indicated that IL-36 cytokines can influence microbiome dysbiosis which likely plays a significant role in intestinal homeostasis and inflammation.52,54 Although further studies are required, in order to fully elucidate the specific mechanisms through which IL-36 cytokines influence disease pathogenesis in UC, several ongoing clinical trials are evaluating whether inhibition of IL-36 R signaling can improve patient outcomes.
Potential therapies focused on IL-36 R inhibition
As evidence accumulates that current gold standard treatment approaches for UC remain ineffective among large numbers of patients,55 efforts to identify new and improved targets for this cohort have continued. With the emergence of IL-36 cytokines as instructive signals in gastrointestinal inflammation several strategies aimed at their inhibition have been developed (Figure 1). Spesolimab (BI655130) is a humanized monoclonal IgG1 antibody against IL-36 R that has shown therapeutic potential in a phase I proof-of-concept study in patients affected with Generalized Pustular Psoriasis (GPP).56 More recently, a follow on phase II clinical trial (NCT03782792) also reported enhanced disease remission in GPP patients treated with spesolimab. However, it was also noted that treatment was accompanied by the development of anti-drug anitbodies and infection in some patients,55 indicating that further, more expansive trials are warranted. Spesolimab is also currently under clinical investigation in
UC. Importantly, these trials will evaluate efficacy in non-responder UC patients (NCT03482635),56 who have demonstrated neither a clinical response during the induction period nor any improvement in symptomatology following previous treatment with TNF antagonists and/or vedolizumab. In addition, healing of the intestinal mucosa is being evaluated in patients with mild or moderate UC receiving spesolimab as a combination treatment with TNF-inhibitors (NCT03123120),57 while the safety and long-term efficacy of IL-36 R blockade with spesolimab in UC are also under evaluation (NCT03648541).58 Although at earlier stages in development, several other approaches aimed at targeting IL-36 activity may offer therapeutic potential for UC. For example, specific protease inhibitors of cathepsin G and elastase have been shown to inhibit IL-36 pathogenic activity in the context of psoriatic disease59 and may constitute a potential therapy for other inflammatory diseases including UC. While blockade of the IL36R with spesolimab will inhibit the signaling of all IL-36 ligands, specific targeting of individual IL-36 cytokines may represent a more directed approach. This may prove more beneficial given the reported dichotomous roles of IL-36 cytokines in inflammation versus resolution. In this regard, the approach of Todorovic and colleagues who developed a specific antagonist of IL-36γ, named A-552, with validation in preclinical models of psoriasis, is of interest.60 In contrast, in the inflamed intestine a broader inhibitory approach may be more appropriate given the reported influence of other IL-1 family members in mucosal inflammation. Recently, Højen and colleagues61 developed a monoclonal antibody targeting the shared IL-1RAcP which was found to be effective in blocking inflammation driven by IL-1 family members, including IL-36 cytokines, in several mouse models of disease.61
Expert opinion
Emerging data continue to implicate IL-36 cytokines as central instructive signals in the pathogenesis of UC. While this data has provided a rationale for the evaluation of monoclonal antibodies against IL-36 R in the clinic, much remains to be resolved concerning how IL-36 family members direct intestinal inflammation and homeostasis.
Outstanding issues include the relative importance of the specific IL-36 cytokine family members to the pathogenesis of UC, and whether they can elicit differential effects at different phases of the disease. While most reports are in agreement that the expression of both IL36α and IL-36γ are elevated in the inflamed colon,62 whether there is functional redundancy between both cytokines has not been fully investigated. This may be of particular relevance if their expression is evident at different temporal phases in the development of chronic intestinal inflammation as observed in UC. Indeed, we have recently demonstrated that IL-36α expression is most significantly elevated in newly diagnosed, treatment-naïve patients presenting with UC, indicating potential mechanistic significance during the earliest phases of disease pathogenesis.63 Uncovering the precise roles of individual IL-36 cytokines in orchestrating intestinal inflammation and/or resolution will also likely assist in resolving the controversy surrounding the apparent dichotomous roles of IL-36 in the intestine as described above.
Related to this is the question of identifying which specific cell subsets are responsible for mediating the effects of IL-36 R signaling in the intestine during the initiation and chronic phases of UC. Several reports have demonstrated that IL36 R expression is detected on diverse immune and parenchymal cell subsets and consequently can play diverse roles in colonic inflammation.48,53,63 Recent advances in single cell transcriptomic and proteomic analysis from the inflamed intestines of UC patients will likely be very informative in this regard.64
In addition, the development of cell-specific knock-out mice, as recently reported,65,66 will be helpful in uncovering mechanistic insights into the distinct roles of IL-36 at different stages of disease, as has previously been described for the related IL-1 family member, IL-18.67
The possible influence of IL36 cytokines on the intestinal microbiome also remains an important area for investigation which remains relatively unaddressed.52,54 As a deeper understanding is gained of how alterations in the intestinal microbiome influences disease pathogenesis in UC, the

potential role of IL-36 cytokines as mediators of dysbiosis, and how this might influence disease progression is an area which warrants further investigation.
While the results of early clinical trials of spesolimab among patients with UC will provide critically important information in advancing the validation of the IL-36 family as a viable target, elucidating answers to basic questions surrounding their mechanism of action, such as those highlighted above, will continue to be invaluable in efforts to maximize the clinical success of IL-36 as a viable target for UC. In addition, the recent establishment of proof-of-concept for spesolimab in generalized pustular psoriasis will also likely be informative.56 As further clinical data on the use of spesolimab is reported, the possible identification of specific molecular biomarker signatures to facilitate the potential of utilizing IL-36 inhibition in an individualized fashion will be important.68 Such advances will not only assist in determining which patients might benefit most from IL-36 directed treatment but will also help identify the circumstances in which patients will likely benefit more from a combination therapeutic approach.
References available on request
79 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Clinical R&D
ALZECURE SELECTS CD AND ENTERS NEXT DEVELOPMENT PHASE WITH ALZSTATIN ACD680 AGAINST ALZHEIMER'S
AlzeCure Pharma AB (publ) (FN STO: ALZCUR), a pharmaceutical company that develops a broad portfolio of small molecule drug candidates for diseases affecting the central nervous system, with projects in both Alzheimer’s disease and pain, has announced that the company has chosen a candidate drug (CD) and started the preclinical development phase with the company's preventive and disease-modifying candidate drug Alzstatin® ACD680.
ACD680 is being developed within AlzeCure's Alzstatin platform, with the aim of developing a preventive and disease-modifying drug for the early treatment of Alzheimer's disease. In the project, a CD has now been selected which will continue into the preclinical development program, which includes preclinical safety and tolerability studies, as well as formulation work and stability testing.
In Alzheimer's disease, a protein, amyloid beta (Aβ42), accumulates into larger aggregates, such as plaques, which have a harmful effect on nerve cells and their function. ACD680 is a so-called gamma-secretase modulator (GSM), which constitutes a promising class of small-molecule Aβ42-lowering anti-amyloidogenic substances for preventive and disease-modifying treatment of Alzheimer's disease. The GSM thereby affects the production of the very building block of the harmful amyloid aggregates and exhibits several key properties that distinguish it from antibody treatments, including that it can be taken in tablet form, easily crosses the blood-brain barrier and can be produced more cost-effectively.
”With Alzstatin, we want to offer a preventive and disease-modifying treatment against Alzheimer's in the form of an oral therapy, which is non-invasive for patients. In addition to affecting an important disease mechanism, ACD680 also derives from a new series of molecules that, among other things, are expected to provide benefits from a patent perspective, with a significantly longer patent period,” said Gunnar Nordvall, project leader and Director of Medicinal Chemistry at AlzeCure.
”We are very pleased to have begun preclinical development with ACD680. We hereby build further on the communicated strategy to strengthen the project portfolio with the development
of several candidates in parallel and also demonstrate AlzeCure's capacity in terms of development and delivery. With the increased interest in the Alzheimer field, we see exciting commercial opportunities for Alzstatin going forward,” said AlzeCure’s CEO Martin Jönsson.
NEW MUMS VIRTUAL WELLBEING HUB INTRODUCED AT UNIVERSITY HOSPITAL GALWAY
The Maternity Department at University Hospital Galway (UHG) has introduced a ‘New Mums Virtual Wellbeing Hub’ which provides online support, advice and signposting on postnatal services available to mothers and their families both in the hospital and the community.

The hub is a collaborative initiative between the Maternity Department, UHG and Community Healthcare West. The team includes a Midwife, Social Worker, Paediatrician, GP, Pharmacist, Public Health Nurse, Dietician, Physiotherapist, Lactation Consultant, Urogynaecology Specialist Midwife and a member of the Perinatal Mental Health Team.
Carmel Connolly, Clinical Nurse Manger 2 and Project Lead
said, “The lack of support and connection, change in identity and unrealistic expectations of parenthood due to the influence of social media has affected maternal wellbeing. Feeling overwhelmed, exhausted and lonely is not uncommon.
“Listening to our mothers concerns led to the design of this new service. Mothers need to connect with other mothers. There is a real fatigue and loneliness after giving birth for many women. They need consistent, evidenced-based information and signposting to services available to them in Galway.
“We launched the hub in November and had 19 couples in attendance. It is so reassuring to see the difference the hub has already created in the lives of mothers and their families. There has already been increased referrals to Physiotherapy, Lactation Consultant and Urogynaecology Specialists. Mothers have connected with these services as a direct result of attending the wellbeing hub.
“The virtual hub is live on the third Tuesday of every month at 11am and booking is available via www. ughmaternity.com.”
One mother who attended the hub said, “It was lovely to be able to connect with other mothers. I was having breastfeeding issues and I knew who to contact after the hub. It is reassuring to see the healthcare team from the hospital and the community working together. It is a fantastic service.”
Another mother said, “I wish this service was available after my first baby. I did not know what was normal. I was so down in myself and was embarrassed to look for help. I picked up lots of skills from the Clinical Psychologist. I am very grateful for the support of this hub.”
KINARUS THERAPEUTICS REPORTS PRECLINICAL DATA IN LUNG FIBROSIS AND DISCLOSES CLINICAL DEVELOPMENT PLAN
Kinarus Therapeutics AG (SIX:KNRS) “Kinarus”, a Swiss clinical-stage biopharmaceutical company has announced preclinical data supporting the potential effectiveness of its lead clinical candidate, KIN001, as an oral treatment for idiopathic pulmonary fibrosis (IPF). Kinarus has developed a protocol for a Phase 2 clinical trial in patients with IPF in consultation with key
From left, Ailish Killilea, Assistant Director of Public Health Nursing, Community Healthcare West; Helen Murphy, Director of Midwifery, University Hospital Galway; Carmel Connolly Project Lead and Clinical Midwife Manager 2, University Hospital Galway; June Barrett Dietitian, University Hospital Galway; Peter Kidd, Pharmacist, University Hospital Galway; Anne Marie Grealish Assistant Director of Midwifery, University Hospital Galway; Eithne Gilligan, Clinical Nurse Manager 2, Community Midwives, University Hospital Galway; Marie Conway, Masters Student, Social Work Department, University Hospital Galway and Mary Moran, Midwife, Parent Education Services, University Hospital Galway
80 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
experts, including PD Dr. Katrin Hostettler, Senior Physician in Pulmonology, at the University Hospital of Basel.
In a mouse model of lung injury, KIN001 significantly reduced lung weights and tissue fibrosis score vs. controls. KIN001 was more effective in direct comparison to pirfenidone, a marketed IPF therapy. The combination of KIN001 with pirfenidone demonstrated greater reduction in lung fibrosis, indicating the potential for additional benefit of KIN001 in combination with the current standard of care. In order to understand the mechanisms of action of KIN001, global gene expression changes in lung tissue were measured by the RNAseq method. KIN001 significantly reduced the upregulation of multiple key inflammatory cytokines and chemokines implicated in the pathology of lung fibrosis.
“These preclinical data strongly support the potential of KIN001 to be an effective treatment for IPF and fibrotic disorders of the lung and other organs, both in single or combination therapy.” said Thierry Fumeaux, Chief Medical Officer of Kinarus. “Many patients stop therapy with the currently available drugs, due to side effects and lack of efficacy. Our data demonstrate that KIN001 possesses broad anti-inflammatory and anti-fibrotic properties which increase the probability that a patient may respond to KIN001. Together with our superior safety profile, demonstrated in clinical testing to date, this indicates that KIN001 may be more effective and better tolerated than available drugs. These data also support the potential of KIN001 to reduce severity and long-term organ damage in COVID-19.”
The Phase 2 study in IPF will be a 52-week, double-blind, randomized, placebo-controlled trial evaluating the effect of oral KIN001 on Forced Vital Capacity (FVC) in 80 patients with IPF. This study will enroll patients on current standard of care including Ofev and Esbriet. Patients not currently treated with either drug will also be included since a substantial number of patients end treatment with these two drugs due to safety concerns.
Kinarus is currently conducting a Phase 2 trial of KIN001 to treat ambulatory COVID-19 patients (“KINFAST”) with a positive SARS-CoV-2 test, which is actively recruiting in Switzerland and Germany. The primary endpoint of the study is the reduction in the severity and duration of Covid-19 symptoms.
LONG COVID PATIENT EDUCATION PROGRAMME IN GALWAY UNIVERSITY HOSPITALS WINS NATIONAL HEALTHCARE AWARD
A group patient support programme developed by team members from the Long COVID and post COVID clinics in Galway University Hospitals has won the Irish Medical Times Irish Healthcare Award in the patient education category.

Long COVID is estimated to occur in 10-20% of people infected with the SARS-CoV-2 virus and people can experience a wide range of symptoms including fatigue; brain fog, non-restorative sleep, pain and breathlessness. These symptoms often impact on a person’s quality of life and daily functions with work, education, parenting and self-care all being affected. The patient education programme in GUH is in place since July 2021 and aims to enable patients to manage their personal symptom profile in a supportive and structured environment. The programme uses a Multi-Disciplinary approach to help patients learn more about the condition and manage their symptoms.
The programme covers a range of areas including fatigue management, brain fog, sleep restructuring, post-exertional malaise, pacing, breathing pattern changes and relaxation. 70% of the group participants identified work as a key area of concern,
with household tasks, parenting, self-care and leisure activities also being common problems among many. A problem solving approach is taken, enabling each person to apply the knowledge in a way that is unique to their symptom profile and personal circumstances.
This programme is delivered online, to make it accessible to patients across the West of Ireland, by specially trained occupational therapists and physiotherapists. The programme runs every week for one hour in duration for a total of four weeks. Referrals to the program are accepted via the long COVID clinic
Speaking of the achievement Ciara Breen, Occupational Therapy Manager said; “After an evaluation of the first groups was completed the results showcased significant improvements in the areas targeted in the programme. These included improved fatigue, cognition, function and mood. Patient feedback has been very positive to date, and the group approach also created a firm foundation for the therapeutic relationship to flourish for those with additional one-toone goals afterwards.
“Given the increasing evidence that recovery after COVID-19 infection is prolonged for a significant proportion of people, and has a complicated and overlapping symptom profile, this group programme highlights the value of supported self-management programs for individuals with long COVID and the feasibility of delivery of this education in an online group format.
“This programme would not be possible without the support of our Infectious Diseases and Respiratory consultants, and the
dedication of our MDT in GUH. It is our aim to continue to strive towards improving care and services for patients.”
Chris Kane, General Manager of Galway University Hospitals added, “This award is a recognition of the adaptability of our post COVID and long COVID clinical teams, being able to respond so innovatively to the varied needs of this patient group is a huge achievement. I want to congratulate the team and thank them for continuing to put patient care at the centre of our services.”
FDA ACCEPTS NIRSEVIMAB APPLICATION AS FIRST PROTECTIVE OPTION AGAINST RSV DISEASE FOR ALL INFANTS
The U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) has accepted the Biologics License Application (BLA) for nirsevimab for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in newborns and infants entering or during their first RSV season and for children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
Nirsevimab is being developed jointly by Sanofi and AstraZeneca and, if approved, would be the first protective option for the broad infant population, including those born healthy, at term or preterm, or with specific health conditions. The FDA has indicated they will work to expedite their review. The Prescription Drug User Fee Act date, the FDA target action date for their decision, is in the third quarter of 2023.
Thomas Triomphe, Executive Vice President, Vaccines, Sanofi said, “This is a landmark file acceptance in the US as it brings us one step closer to offering the first and only broadly protective option against RSV disease designed for all infants. Given the unprecedented number of otherwise healthy infants who have been hospitalized with RSV this year in the US and the recurrent pattern of RSV epidemics year after year, it is our intention to make nirsevimab available, if approved in time, for the 2023/2024 season to help alleviate the burden of RSV on families and the healthcare system.”
RSV is a very contagious virus that can lead to serious respiratory illness, according to the Centers for Disease Control and Prevention (CDC).10 In the US, RSV is the leading cause of hospitalisation for babies under one.11 Any infant can be
81 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Accepting the Irish Healthcare Award on behalf of the GUH Long COVID service patient education team: Catherine O Sullivan, Physiotherapy Manager; Gillian Collins, Clinical Specialist Occupational Therapist and Ciara Breen, Occupational Therapy Manager
Clinical R&D
hospitalized in their first RSV season: about 75% of infants hospitalized for RSV in the U.S. are born at term, with no underlying conditions.12-14 The current 2022/23 RSV season has placed a particularly high burden on infants and families in the United Stated with the American Academy of Pediatrics (AAP) requesting the White House declare an emergency to support the national response to the alarming surge of pediatric hospitalizations due to RSV and influenza.
Dr William Muller, Associate Professor, Pediatrics, Northwestern University Feinberg School of Medicine and Scientific Director, Clinical and Community Trials, Ann & Robert H. Lurie Children’s Hospital of Chicago, Illinois added, "A substantial burden of disease from RSV affects infants, families, and healthcare providers every year. Effective interventions to prevent RSV are a critical need. This year in the US, we’ve seen first-hand how frightening the impact of this respiratory disease is on our patients and how stressful it is on the healthcare system, highlighting the urgency of addressing this problem."
The submission was based on results from the Phase 3 MELODY, Phase 2/3 MEDLEY and Phase
2b trials.1-8 Results across the MELODY and Phase 2b trials showed that nirsevimab demonstrated consistent protection of approximately 80%, against medically attended RSV disease with a single dose.1-5
In these trials, nirsevimab helped protect an all-infant population (including healthy term, late preterm, and preterm infants, as well as infants with specific health conditions) against RSV disease requiring medical care, including physician office, urgent care, emergency room visits and hospitalizations, through the duration of the RSV season.1-8 The safety profile of nirsevimab was similar to placebo. Nirsevimab also demonstrated a comparable safety and tolerability profile to palivizumab in the Phase 2/3 MEDLEY trial.7-9
ZENAS BIOPHARMA
ANNOUNCES FIRST PATIENT
based therapies, has announced that the first patient has been dosed in the INDIGO Phase 3 registrational study of obexelimab. The INDIGO study will evaluate the clinical efficacy and safety of obexelimab treatment in the prevention of IgG4-related disease (IgG4-RD) flare. Obexelimab is a high-affinity bifunctional antibody that inhibits B-cell lineages by simultaneously binding to CD19 and FcƳRIIB, thereby downregulating B-cell activity in patients with autoimmune diseases associated with autoantibodies, such as IgG4-RD.
“IgG4-RD is a chronic and serious fibroinflammatory condition that can affect nearly any organ system and can have a profound impact on many patients, leading to severe organ damage or death,” said Hua Mu, M.D., Ph.D., Chief Executive Officer at Zenas. “There are no currently approved treatments for patients living with IgG4-RD. Based upon the promising data from a Phase 2 study of obexelimab in IgG4-RD patients, we are excited to continue to evaluate the potential of obexelimab in the INDIGO study.”
About the INDIGO Study
The INDIGO study is a global multicenter, randomized, doubleblind, placebo-controlled study enrolling up to 200 adults with active IgG4-RD signs/ symptoms (i.e., flare) that require steroid therapy. Patients will be randomized in a ratio of 1:1 to receive either obexelimab or placebo, administered as subcutaneous injections.
The primary endpoint of INDIGO is time to first IgG4-RD flare (defined as the reappearance of previous signs/symptoms or appearance of new signs/symptoms of IgG4-RD) that requires initiation of rescue therapy from randomization to Week 52. Safety will be evaluated throughout the study duration.
More information on the INDIGO study (NCT05662241) is available at clinicaltrials.gov.
OVERCROWDING CRISIS RISKS DELAYED CANCER DETECTION
for only urgent cases to attend Emergency Departments.
“Cancer detection is time-critical: any delays to a cancer diagnosis being picked up can negatively impact patients. Our concern is that people may be delaying seeking medical advice because of the crisis across the health service, and when this is added to wait-times for diagnostic tests for those who do present with symptoms, there may be delayed diagnoses of cancer.
“We already know that around 1 in 10 cancer cases expected to be picked up in 2020 were not, due to the effects of the pandemic, which has continued to be a factor in the last two years.
“People in treatment may also be worried about contacting or attending hospitals if they are feeling unwell, due to fears of being admitted through Emergency Departments. We are very concerned about the chill effect this could have on them getting in touch with their cancer teams.
“Government needs to do much more to ensure that timely and appropriate care is available to everyone who needs it. Patients still face unacceptably long delays, and our health service is woefully under-resourced to meet the pressures that are already emerging from the growing number of people being diagnosed with cancer.
“Lastly, we would urge anyone experiencing symptoms that are unexplained, unusual or persistent, or who is currently undergoing cancer treatment, to please still seek medical advice.
“If that isn’t possible, please contact the Irish Cancer Society to speak to a nurse who can give information, support and advice for free on 1800 200 700.
The RCSI funding was announced by Minister for Further and Higher Education, Research, Innovation and Science, Simon Harris TD, as part of an overall investment of ¤53.3 million for the advancement of high-quality and high-impact research activities in institutions across Ireland.
Professor David Henshall, Professor of Molecular Physiology and Neuroscience at RCSI and Director of the SFI FutureNeuro Research Centre, is leading the new project to establish a Core Medium Throughput Facility at the University. The infrastructure will enable researchers to rapidly screen compounds and gene therapies for effects on the electrical activity of neurons in models of brain disease, establishing advanced drug discovery capabilities.
Commenting on the significance of the facility, Professor Henshall said: “This new facility will provide streamlined screening and data analysis for our scientists, enabling faster research outputs across a number of projects that aim to develop urgently-needed treatments to improve quality of life and outcomes for people living with neurological diseases.
“The investment will help our team to advance scientific research at a faster pace and answer new research questions to identify novel drug targets in epilepsy, autism, multiple sclerosis, pain, neurodegenerative, psychiatric, and motor neuron diseases. It will enhance our attractiveness to industry collaborators and strengthen our ability to win competitive EU and international research funding awards.”
The project is co-funded by RCSI and FutureNeuro with each contributing ¤80,000 towards the facility with technical support to the value of a further ¤35,000 each.
DOSED IN PHASE 3 CLINICAL STUDY OF OBEXELIMAB FOR THE TREATMENT OF IMMUNOGLOBULIN G4RELATED DISEASE
Efforts to catch cancer cases early may be at risk due to concerns among patients and the public about overcrowding in the healthcare system, the Irish Cancer Society has warned.
(IGG4-RD)
Zenas BioPharma, a global biopharmaceutical company committed to becoming a leader in the development and commercialization of immune-
The Society’s Director of Advocacy Rachel Morrogh said: “We are concerned that people with cancer symptoms are putting off seeking medical care because they do not think it is serious enough amid the ongoing hospital overcrowding crisis, and the call
“Even amid the current difficulties accessing care, nobody should suffer alone at home and we don’t want anyone to be afraid, so please pick up the phone and make contact with us.”
SFI AWARDS ¤1.5M TO RCSI FOR NEW FACILITY TO ADVANCE NEUROLOGICAL RESEARCH
RCSI University of Medicine and Health Sciences has been awarded funding of ¤1.5m for a new facility to support neurological research through the Science Foundation Ireland (SFI) Research Infrastructure Fund.
Professor Fergal O’Brien, Deputy Vice-Chancellor for Research and Innovation at RCSI said: “I would like to congratulate Prof. Henshall and everyone involved for securing this important funding through the SFI Research Infrastructure Programme. This project will make more scientific discoveries possible for neurological researchers in RCSI and at our SFI FutureNeuro Research Centre by speeding up drug and biomarker discovery and screening programmes.
“This facility will also have a significant impact on the national research landscape, by providing Irish researchers access to cuttingedge research infrastructure that will enhance high quality research
82 FEBRUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
activities and innovation in areas of strategic importance. I am confident that this enhanced capability will promote and facilitate opportunities for partnerships that will lead to a variety of discoveries and ultimately benefit the health of patients.”
The SFI Research Infrastructure Programme supports the research community in building and sustaining cutting edge infrastructure in order to accomplish high-quality, impactful and innovative research. The programme facilitates broad usage across Ireland and to encourage partnerships and collaboration between different cohorts of researchers in Ireland; for example, between universities, technological universities, institutes of technology, other eligible research bodies, researchers in the Republic of Ireland and Northern Ireland, and between different cohorts of researchers in Ireland.
NEW CROSS-BORDER HEALTH PARTNERSHIP WILL SUPPORT PROCUREMENT OF IRISH MEDTECH SMES IN SECONDARY CARE
Supported by InterTradeIreland’s Synergy initiative, the All-Island MedTech SMEs (AIMS) is being delivered by Health Innovation Hub Ireland (HIHI) and the Health Innovation Research Alliance Northern Ireland (HIRANI). AIMs brings together a wide group including SMEs, academics and healthcare procurement, to capture procurement barriers encountered by MedTech SMEs in the Northern
Irish and Irish public healthcare systems (Health and Social Care (HSC) and Health Service Executive (HSE) respectively). By involving all stakeholders, AIMs will focus on developing a framework to support procurement and improve MedTech adoption in secondary care across both healthcare systems.
HIHI National Director Dr. Tanya Mulcahy said, “We have been working with MedTech SMEs for nearly ten years in HIHI. The size and liquidity of companies means that smaller entities are often precluded from the procurement process. AIMS will look at how we can address barriers such as these so that indigenous MedTech can scale and grow and our health systems can choose from a wider pool of innovation.”
Ultimately, the All-Island MedTech SMEs (AIMS) is bringing together Irish SMEs and procurement to develop and propose a model for innovative procurement for allisland MedTech adoption in Irish health services North and South.
AIMS is funded by InterTradeIreland’s Synergy initiative. A cross-border cluster initiative set up by InterTradeIreland, the crossborder trade and business development body. It aims to scale cross border collaboration among SMEs and other players such as universities, third sector organisations and government agencies using cluster and networking supports.
HIRANI Programme Manager Dr Siobhán McGrath said, “We are ambitious with AIMs because we know that there are solutions to ensure that Irish MedTech SMEs can thrive in the indigenous
market. Through creating a network of health stakeholders North and South, capturing common challenges, sharing knowledge in relation to all-island supply chains and procurement channels, we can deliver this.”
START-UP OF THE YEAR AWARD
An early-stage Medtech start-up focused on transforming obesity care has won the 2022 University College Dublin Start-Up of the Year Award.

MetHealth received the award, and its ¤30,000 prize fund, after being declared the overall winner of the 2022 UCD VentureLaunch Accelerator Programme – which aims to equip its participants with the knowledge and skills required to work as part of a team leading a new commercial venture.

The annual Programme, which is run by NovaUCD, aims to support the creation and launch of sustainable and profitable new start-ups emerging from University College Dublin.
As winners of ‘Start-Up of the Year’ MetHealth will receive a prize package worth ¤30,000, which includes a ¤10,000 cash prize sponsored by AMD, a professional service package sponsored by Deloitte and Bryan Maguire Financial Services and incubation space at NovaUCD.
The company is developing a biomarker-based risk-stratification platform and associated digital health solution that can identify patients with complications of obesity, including liver disease.
Members of the MetHealth included Dr Fiona McGillicuddy, who took part in the VentureLaunch programme, and Professor Stephen Pennington, UCD School of Medicine and
UCD Conway Institute; Associate Professor Catherine Mooney, UCD School of Computer Science and UCD Conway Institute, Dr Rachel Byrne, UCD School of Medicine, Dr Anna Antoniadi, UCD School of Computer Science and Aleksandra Dudzik, UCD School of Medicine.
“The global prevalence of obesity is increasing significantly. In the EU alone some 1.2 million people are dying annually due to obesity complications, including from liver disease,” said Dr Fiona McGillicuddy.
“Early identification of those patients with liver inflammation is key but this currently involves painful invasive tests. We are developing a novel biomarkerbased platform technology which can, using a blood sample, stratify patients with obesity who are at high risk of complications of obesity including liver disease.
“This non-invasive test eliminates pain for the patient, is significantly more cost effective than an invasive test, can lead to early interventions for high-risk patients and can aid clinical trial recruitment for new drugs targeting the liver.”
She added: “On behalf of the MetHealth team I am delighted to win the 2022 UCD Start-Up of the Year Award following the completion of the VentureLaunch Accelerator Programme at NovaUCD.
“We currently plan to spin-out of UCD at the end of 2023 when we will be seeking to raise ¤2 million in seed funding to set up the MetHealth laboratory and develop a market ready prototype.”
83 HOSPITALPROFESSIONALNEWS.IE | HPN • FEBRUARY 2023
Professor David Henshall, Professor of Molecular Physiology and Neuroscience at RCSI
Dr Fiona McGillicuddy, MetHealth
KEYTRUDA: helping to redefine overall survival expectations for more patients with mNSCLC1-4

KEYTRUDA® (pembrolizumab)


ABRIDGED PRODUCT INFORMATION Refer to Summary of Product Characteristics before prescribing. PRESENTATION KEYTRUDA 25 mg/mL: One vial of 4 mL of concentrate contains 100 mg of pembrolizumab. INDICATIONS KEYTRUDA as monotherapy is indicated for the treatment of adults and adolescents aged 12 years and older with advanced (unresectable or metastatic) melanoma. KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults and adolescents aged 12 years and older with Stage IIB, IIC or III melanoma and who have undergone complete resection. KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥50% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations. KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR or ALK positive mutations. KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR or ALK positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA. KEYTRUDA as monotherapy is indicated for the treatment of adult and paediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma (cHL) who have failed autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT is not a treatment option.
KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who have received prior platinum-containing chemotherapy. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who are not eligible for cisplatin-containing chemotherapy and whose tumours express PD L1 with a combined positive score (CPS) ≥ 10. KEYTRUDA as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the first-line treatment of metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC) in adults whose tumours express PD-L1 with a CPS ≥ 1. KEYTRUDA as monotherapy is indicated for the treatment of recurrent or metastatic HNSCC in adults whose tumours express PD-L1 with a ≥ 50% TPS and progressing on or after platinum-containing chemotherapy. KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of advanced renal cell carcinoma (RCC) in adults. KEYTRUDA, in combination with lenvatinib, is indicated for the first line treatment of advanced renal cell carcinoma in adults. KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with renal cell carcinoma at increased risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions. Microsatellite instability high (MSI-H) or mismatch repair deficient (dMMR) cancers Colorectal cancer (CRC) KEYTRUDA as monotherapy is indicated for adults with MSI-H or dMMR colorectal cancer in the following settings: - first line treatment of metastatic colorectal cancer - treatment of unresectable or metastatic colorectal cancer after previous fluoropyrimidine based combination therapy. Non-colorectal cancers KEYTRUDA as monotherapy is indicated for the treatment of the following MSI H or dMMR tumours in adults with (a) advanced or recurrent endometrial carcinoma, who have disease progression on or following prior treatment with a platinum containing therapy in any setting and who are not candidates for curative surgery or radiation, (b) unresectable or metastatic gastric, small intestine, or biliary cancer, who have disease progression on or following at least one prior therapy. KEYTRUDA, in combination with platinum and fluoropyrimidine based chemotherapy, is indicated for the first-line treatment of locally advanced unresectable or metastatic carcinoma of the oesophagus or HER-2 negative gastroesophageal junction adenocarcinoma in adults whose tumours express PD-L1 with a CPS ≥ 10. KEYTRUDA, in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, is indicated for the treatment of adults with locally advanced, or early stage triple negative breast cancer at high risk of recurrence. KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of locally recurrent unresectable or metastatic triple negative breast cancer in adults whose tumours express PD L1 with a CPS ≥ 10 and who have not received prior chemotherapy for metastatic disease. KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of advanced or recurrent endometrial carcinoma in adults who have disease progression on or following prior treatment with a platinum containing therapy in any setting and who are not candidates for curative surgery or radiation. KEYTRUDA, in combination with chemotherapy with or without bevacizumab, is indicated for the treatment of persistent, recurrent, or metastatic cervical cancer in adults whose tumours express PD L1 with a CPS ≥ 1. DOSAGE AND ADMINISTRATION See SmPC for full details. Therapy must be initiated and supervised by specialist physicians experienced in the treatment of cancer. The recommended dose of KEYTRUDA in adults is either 200 mg every 3 weeks or 400 mg every 6 weeks administered as an intravenous infusion over 30 minutes. The recommended dose of KEYTRUDA as monotherapy in paediatric patients aged 3 years and older with cHL or patients aged 12 years and older with melanoma is 2 mg/kg bodyweight (up to a maximum of 200 mg), every 3 weeks administered as an intravenous infusion over 30 minutes. For use in combination, see the Summary of Product Characteristics (SmPC) for the concomitant therapies. KEYTRUDA must not be administered as an intravenous push or bolus injection. When administering KEYTRUDA as part of a combination with intravenous chemotherapy, KEYTRUDA should be administered first. Treat patients until disease progression or unacceptable toxicity (and up to maximum duration of therapy if specified for an indication). For the adjuvant treatment of melanoma or RCC, KEYTRUDA should be administered until disease recurrence, unacceptable toxicity, or for a duration of up to one year. Refer to the SmPC for dosing in neoadjuvant and adjuvant treatment of locally advanced, or early stage triple-negative breast cancer at high risk of recurrence. KEYTRUDA, as monotherapy or as combination therapy, should be permanently discontinued (a) For Grade 4 toxicity except for: endocrinopathies that are controlled with replacement hormones; or haematological toxicity, only in patients with cHL in which KEYTRUDA should be withheld until adverse reactions recover to Grade 0-1; (b) If corticosteroid dosing cannot be reduced to ≤10 mg prednisone or equivalent per day within 12 weeks; (c) If a treatment-related toxicity does not resolve to Grade 0 1 within 12 weeks after last dose of KEYTRUDA; (d) If any event occurs a second time at Grade ≥ 3 severity. Patients must be given the Patient Alert Card and be informed about the risks of KEYTRUDA. Special populations Elderly: No dose adjustment necessary. Renal impairment: No dose adjustment needed for mild or moderate renal impairment. No studies in severe renal impairment. Hepatic impairment: No dose adjustment needed for mild or moderate hepatic impairment. No studies in severe hepatic impairment. Paediatric population: Safety and efficacy in children below 18 years of age not established except in paediatric patients with melanoma or cHL. CONTRAINDICATIONS Hypersensitivity to the active substance or to any excipients. PRECAUTIONS AND WARNINGS Assessment of PD-L1 status When assessing the PD-L1 status of
or false positive determinations.
administration of corticosteroids and/or supportive care. Immune related adverse reactions have also occurred after the last dose of pembrolizumab. Immune-related adverse reactions affecting more than one body system can occur simultaneously. Immune-related adverse reactions are immune-related pneumonitis, immune-related colitis, immune-related hepatitis, immune-related nephritis, immune-related
endocrinopathies (including adrenal insufficiency, hypophysitis, type 1 diabetes mellitus, diabetic ketoacidosis, hypothyroidism, and hyperthyroidism), Immune-related skin adverse reactions (also including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)), Refer to SmPC for more information and management of immune-related adverse reactions. Complications of allogeneic Haematopoietic Stem Cell Transplant (HSCT): Cases of graft-versus-host-disease (GVHD) and hepatic veno-occlusive disease (VOD) have been observed in patients with classical Hodgkin lymphoma undergoing allogeneic HSCT after previous exposure to pembrolizumab. Infusion-related reactions: Grades 1, 2, 3 or 4 infusion reactions including hypersensitivity and anaphylaxis, could be seen with pembrolizumab treatment. Refer to SmPC for more information and management of infusion-related reactions. Overdose: There is no information on overdose with pembrolizumab. In case of overdose, monitor closely for signs or symptoms of adverse reactions and treat appropriately. INTERACTIONS No formal pharmacokinetic drug interaction studies have been conducted with pembrolizumab. No metabolic drug drug interactions are expected. The use of systemic corticosteroids or immunosuppressants before starting pembrolizumab should be avoided because of their potential interference with the pharmacodynamic activity and efficacy of pembrolizumab. Corticosteroids can be used as premedication, when pembrolizumab is used in combination with chemotherapy, as antiemetic prophylaxis and/or to alleviate chemotherapy-related adverse reactions. FERTILITY, PREGNANCY AND LACTATION Women of childbearing potential Women of childbearing potential should use effective contraception during treatment with pembrolizumab and for at least 4 months after the last dose of pembrolizumab. Pregnancy No data on use in pregnant women. Do not use during pregnancy unless the clinical condition of the woman requires treatment with pembrolizumab. Breast-feeding It is unknown whether pembrolizumab is secreted in human milk. A risk to newborns/ infants cannot be excluded. Fertility No clinical data available. SIDE EFFECTS Refer to SmPC for complete information on side effects. Pembrolizumab is most commonly associated with immune-related adverse reactions. Most of these reactions resolved with appropriate medical treatment or withdrawal of pembrolizumab. The most serious adverse reactions were immune-and infusion-related adverse reactions. When pembrolizumab is administered in combination with axitinib or lenvatinib, refer to the SmPC for axitinib or lenvatinib prior to initiation of treatment. For additional lenvatinib safety information related to advanced RCC see the SmPC for Kisplyx and for advanced EC see the SmPC for Lenvima. Monotherapy: Very Common: anaemia, hypothyroidism, decreased appetite, headache, dyspnea, cough, abdominal pain, nausea, vomiting, constipation, musculoskeletal pain, arthralgia, asthenia, oedema, pyrexia, diarrhoea, pruritus, rash, fatigue. Common: pneumonia, thrombocytopenia, neutropenia, lymphopenia, hyponatraemia, hypokalaemia, hypocalcaemia, insomnia, neuropathy peripheral, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, hyperthyroidism, insomnia, dizziness, dysgeusia, pneumonitis, colitis, dry mouth, hepatitis, severe skin reactions, vitiligo, dry skin, eczema, alopecia, dermatitis acneiform, erythema, dermatitis, myositis, pain in extremity, arthritis, influenza like illness, chills, AST and ALT increases, increase in blood alkaline phosphatase, hypercalcaemia, blood bilirubin increased, blood creatinine increased, infusion related reaction. In combination with chemotherapy: Very Common: neutropenia, anaemia, thrombocytopenia, leukopenia, hypothyroidism, hypokalaemia, decreased appetite, insomnia, neuropathy peripheral, headache, dizziness, dysgeusia, dyspnoea, cough, nausea, diarrhoea, vomiting, abdominal pain, constipation, alopecia, rash, pruritus, arthralgia, musculoskeletal pain, myositis, pyrexia, fatigue, asthenia, oedema, ALT increase, AST increased. Common: pneumonia, febrile neutropenia, lymphopenia, infusion related reaction, adrenal insufficiency, thyroiditis, hyperthyroidism, hyponatraemia, hypocalcaemia, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, pneumonitis, colitis, gastritis, dry mouth, hepatitis, severe skin reactions, erythema, dermatitis acneiform, dermatitis, dry skin, eczema, pain in extremity, arthritis, acute kidney injury, influenza-like illness, chills, blood creatinine increased, blood alkaline phosphatase increased, hypercalcaemia, blood bilirubin increased. In combination with axitinib or lenvatinib: Very Common: urinary tract infection, anaemia, hypothyroidism, decreased appetite, headache, dysgeusia, hypertension, dyspnoea, cough, diarrhoea, abdominal pain, nausea, vomiting, constipation, rash, pruritus, arthralgia, musculoskeletal pain, myositis, pain in extremity, fatigue, asthenia, oedema, pyrexia, lipase increased, alanine aminotransferase increased, aspartate aminotransferase increased, blood creatinine increased. Common: pneumonia, neutropenia, thrombocytopenia, lymphopenia, leukopenia, infusion-related reaction, adrenal insufficiency, hyperthyroidism, thyroiditis, hyponatraemia, hypokalaemia, hypocalcaemia, insomnia, dizziness, neuropathy peripheral, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), pneumonitis, colitis, pancreatitis, gastritis, dry mouth, hepatitis, severe skin reactions, dermatitis, dry skin, erythema, dermatitis acneiform, alopecia, arthritis, nephritis, influenza like illness, chills, amylase increased, blood bilirubin increased, blood alkaline phosphatase increased, hypercalcaemia. PACKAGE QUANTITIES KEYTRUDA 25 mg/mL: 4 mL of concentrate in a 10 mL Type I clear glass vial.
Legal Category: POM. Marketing Authorisation numbers EU/1/15/1024/002 Marketing Authorisation holder Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands. Date of revision: October 2022. © 2022 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin, D18 X5K7 or from www.medicines.ie. Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-2998700) II126 Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-2998700)


Immune-related adverse reactions
adverse reactions, including severe
pembrolizumab. Most immune related adverse reactions occurring during treatment with
interruptions of pembrolizumab,
the tumour, it is important that a well-validated and robust methodology is chosen to minimise false negative
Immune-related
and fatal cases, have occurred in patients receiving
pembrolizumab were reversible and managed with
Reference 1. Keytruda Summary of Product Characteristics, October 2022, available at www.medicines.ie.
Gandhi L, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. 3. Paz-Ares L, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379:2040–2051. 4. Reck, M et al. Pembrolizumab versus chemotherapy for PDL1 positive-non-small cell lung cancer, N Eng J Med 2016, 375(19)1823-1833. ALK=anaplastic lymphoma kinase; EGFR=epidermal growth factor receptor; mNSCLC=metastatic non–small cell lung carcinoma; PDL1=programmed death ligand 1. Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7, Ireland. IE-KEY-00670 Date of Preparation: November 2022 Line Histology PD-L1 <1% or unknown PD-L1 1-49% PD-L1 >50% 1st line Combination* Therapy2 NONSQUAMOUS √ √ √ 1st line Combination** Therapy 3 SQUAMOUS √ √ √ 1st line*** Monotherapy4 NON-SQUAMOUS AND SQUAMOUS X X √ * KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR or ALK positive mutations. ** KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults. *** KEYTRUDA, as monotherapy, is indicated for the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a≥ 50% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations.
2.
(pembrolizumab) Infusion 25mg/ml











































 Written by N Tangney1, DM Murphy1
Written by N Tangney1, DM Murphy1




















 Written by Tomás P. Carroll1,2, Ronan C. Heeney1,2, Geraldine Kelly McElvaney1
Written by Tomás P. Carroll1,2, Ronan C. Heeney1,2, Geraldine Kelly McElvaney1














































 Written by Mary N. O’Reilly, Michaela J. Higgins, St. Vincent’s University Hospital
Written by Mary N. O’Reilly, Michaela J. Higgins, St. Vincent’s University Hospital



 Written by Lucy Norris PhD. Coagulation research laboratory, Dept of Obstetrics and Gynaecology, Trinity St. James’s Cancer Institute, Trinity Centre for Health Sciences, St. James Hospital, Dublin 8
Written by Lucy Norris PhD. Coagulation research laboratory, Dept of Obstetrics and Gynaecology, Trinity St. James’s Cancer Institute, Trinity Centre for Health Sciences, St. James Hospital, Dublin 8





 Prof Leonie Young, Scientific Director of the Beaumont
Prof Leonie Young, Scientific Director of the Beaumont









































