IRELAND
Ireland’s Dedicated Hospital Professional Publication

ABBREVIATED PRESCRIBING INFORMATION
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Kisqali (ribociclib) 200 mg film-coated tablets Presentation: Film coated tablets (FCT) containing 200 mg of ribociclib and 0.344 mg soya lecithin.
Indications: Kisqali is indicated for the treatment of women with hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy In pre or perimenopausal women, the endocrine therapy should be combined with a luteinising hormone releasing hormone (LHRH) agonist.
Dosage and administration: Adults: The recommended dose is 600 mg (3 x 200 mg FCT) taken orally, once daily for 21 consecutive days followed by 7 days off treatment, resulting in a complete cycle of 28 days. Kisqali should be used together with 2.5 mg letrozole or another aromatase inhibitor or with 500 mg fulvestrant.
When Kisqali is used in combination with an aromatase inhibitor, the aromatase inhibitor should be taken orally once daily continuously throughout the 28 day cycle.
Please refer to the Summary of Product Characteristics (SmPC) of the aromatase inhibitor for additional details.
When Kisqali is used in combination with fulvestrant, fulvestrant is administered intramuscularly on days 1, 15 and 29, and once monthly thereafter. Please refer to the SmPC of fulvestrant for additional details.
Treatment of pre and perimenopausal women with the approved Kisqali combinations should also include an LHRH agonist in accordance with local clinical practice.
Management of severe or intolerable adverse reactions (ARs) may require temporary dose interruption, reduction or discontinuation of Kisqali. Please see section 4.2 of SmPC for recommended dose modification guidelines.
Kisqali can be taken with or without food (see section 4.5).The tablets should be swallowed whole and should not be chewed, crushed or split prior to swallowing.
Special populations: ♦Renal impairment: Mild or moderate: No dose adjustment is necessary. Severe: A starting dose of 200 mg is recommended in patients with severe renal impairment. Kisqali has not been studied in breast cancer patients with severe renal impairment. Caution should be used in patients with severe renal impairment with close monitoring for signs of toxicity. ♦Hepatic impairment: Mild: No dose adjustment is necessary. Moderate or severe: Dose adjustment is required, and the starting dose of 400 mg once daily is recommended. ♦Elderly (>65 years): No dose adjustment is required.
♦Pediatrics(<18 years): Safety and efficacy have not been established.
Contraindications: Hypersensitivity to the active substance or to peanut, soya or any of the excipients.
Warnings/Precautions: ♦Neutropenia was most frequently reported AR. A complete blood count (CBC) should be performed before initiating treatment. CBC should be monitored every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. Febrile neutropenia was reported in 1.4% of patients exposed to Kisqali in the phase III clinical studies. Patients should be instructed to report any fever promptly.Based on the severity of the neutropenia, Kisqali may require dose interruption, reduction, or discontinuation. ♦Hepatobiliary toxicity - increases in
Med. 2020;382(6):514-524.
transaminases have been reported. Liver function tests (LFTs) should be performed before initiating treatment. LFTs should be monitored every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. If grade ≥ 2 abnormalities are noted, more frequent monitoring is recommended.
Recommendations for patients who have elevated AST/ALT grade ≥ 3 at baseline have not been established. Based on the severity of transaminase elevations, Kisqali may require dose interruption, reduction, or discontinuation. ♦QT interval prolongation has been reported with Kisqali. The use of Kisqali should be avoided in patients who have already or who are at significant risk of developing QTc prolongation. The ECG should be assessed prior to initiation of treatment. Treatment with Kisqali should be initiated only in patients with QTcF values <450 msec. The ECG should be repeated at approximately Day 14 of the first cycle and at the beginning of the second cycle, then as clinically indicated. In case of QTcF prolongation during treatment, more frequent ECG monitoring is recommended Appropriate monitoring of serum electrolytes (including potassium, calcium, phosphorous, and magnesium) should be performed prior to initiation of treatment, at the beginning of the first 6 cycles, and then as clinically indicated. Any abnormality should be corrected before the start of Kisqali treatment. Based on the observed QT prolongation during treatment, Kisqali may require dose interruption, reduction, or discontinuation. Based on the E2301 study QTcF interval data, Kisqali is not recommended for use in combination with tamoxifen. ♦Critical visceral disease. The efficacy and safety of ribociclib have not been studied in patients with critical visceral disease. ♦Severe cutaneous reactions Toxic epidermal necrolysis (TEN) has been reported with Kisqali treatment. If signs and symptoms suggestive of severe cutaneous reactions (e.g. progressive widespread skin rash often with blisters or mucosal lesions) appear, Kisqali should be discontinued immediately. ♦Interstitial lung disease/pneumonitis ILD/pneumonitis has been reported with CDK4/6 inhibitors including Kisqali. Based on the severity of the ILD/pneumonitis, which may be fatal, Kisqali may require dose interruption, reduction or discontinuation as described in SmPC. Patients should be monitored for pulmonary symptoms indicative of ILD/pneumonitis which may include hypoxia, cough and dyspnoea and dose modifications should be managed in accordance with Table 5 (see section 4.2)
♦Blood creatinine increase ribociclib may cause blood creatinine increase – if this occurs it is recommended that further assessment of the renal function be performed to exclude renal impairment.
♦CYP3A4 substrates. ribociclib may interact with medicinal products which are metabolised via CYP3A4, which may lead to increased serum concentrations of CYP3A4 substrates (see section 4.5). Caution is recommended in case of concomitant use with sensitive CYP3A4 substrates with a narrow therapeutic index and the SmPC of the other product should be consulted for the recommendations regarding co administration with CYP3A4 inhibitors.
Pregnancy, Fertility and Lacation
♦Pregnancy: Pregnancy status should be verified prior to starting treatment as Kisqali can cause foetal harm when administered to a pregnant woman.
♦Women of childbearing potential who are receiving Kisqali should use effective contraception (e.g. double-barrier contraception) during therapy and for at least 21 days after stopping treatment with Kisqali. ♦Breast feeding: Patients receiving Kisqali should not breast feed for at least 21 days after the last dose. ♦Fertility: There are no clinical data available regarding effects of ribociclib on fertility. Based on animal studies, ribociclib may impair fertility in males of reproductive potential.
♦Effects on ability to drive and use machines Patients should be advised to be cautious when driving or using machines in case they experience fatigue, dizziness or vertigo during treatment with Kisqali.
Interactions: ♦Concomitant use of strong CYP3A4 inhibitors should be avoided, including, but not limited to, clarithromycin, indinavir, itraconazole, ketoconazole, lopinavir, ritonavir, nefazodone, nelfinavir, posaconazole, saquinavir, telaprevir, telithromycin, verapamil, and voriconazole. Alternative concomitant medicinal products with less potential to inhibit CYP3A4 should be considered. Patients should be monitored for ARs. If concomitant use of a strong CYP3A4 inhibitor cannot be avoided, the dose of Kisqali should be reduced (see section 4.2 of SmPC). ♦Grapefruit or grapefruit juice should be avoided. ♦Concomitant use of strong CYP3A4 inducers should be avoided, including, but not limited to, phenytoin, rifampicin, carbamazepine and St John’s Wort (Hypericum perforatum). An alternative medicinal product with no or minimal potential to induce CYP3A4 should be considered. ♦Caution is recommended when Kisqali is administered with sensitive CYP3A4 substrates with narrow therapeutic index (including, but not limited to, alfentanil, ciclosporin, everolimus, fentanyl, sirolimus, and tacrolimus), and their dose may need to be reduced. ♦Concomitant administration of Kisqali at the 600 mg dose with the following CYP3A4 substrates should be avoided: alfuzosin, amiodarone, cisapride, pimozide, quinidine, ergotamine, dihydroergotamine, quetiapine, lovastatin, simvastatin, sildenafil, midazolam, triazolam. ♦Caution and monitoring for toxicity are advised during concomitant treatment with sensitive substrates of drug transporters P-gp, BCRP, OATP1B1/1B3, OCT1, OCT2, MATE1 and BSEP which exhibit a narrow therapeutic index, including but not limited to digoxin, pitavastatin, pravastatin, rosuvastatin and metformin. ♦Co-administration of Kisqali with medicinal products with known potential to prolong the QT interval should be avoided such as anti-arrhythmic medicinal products (including, but not limited to, amiodarone, disopyramide, procainamide, quinidine and sotalol) and other medicinal products known to prolong the QT interval including, but not limited to, chloroquine, halofantrine, clarithromycin, ciprofloxacin, levofloxacin, azithromycin, haloperidol, methadone, moxifloxacin, bepridil, pimozide and intravenous ondansetron. Kisqali is not recommended for use in combination with tamoxifen.
Adverse reactions: ♦Very common: Infections, neutropenia, leukopenia, anaemia lymphopenia, decreased appetite, headache, dizziness, dyspnoea,
HOSPITAL
NEWS
PROFESSIONAL
HPN January 2023 Issue 104 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only
dry skin, vitiligo, dry mouth, oropharyngeal pain, blood creatinine increased, electrocardiogram QT prolonged. ♦Please refer to SmPC for a full list of adverse reactions. Legal Category: POM Pack sizes: Unit packs containing 21, 42 or 63 FCTs. Not all pack sizes may be marketed. Marketing Authorisation Holder: Novartis Europharm Limited Vista Building, Elm Park, Merrion Road, Dublin 4 Ireland Marketing Authorisation Numbers: EU/1/17/1221/003 & 005. Full prescribing information is available on request from Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Dublin 4. Tel: 01 2601255 or at www.medicines.ie Prescribing information last revised: April 2022 November 2022 | IE253121 © 2022 Novartis
D04 A9N6 REFERENCES: 1. Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival results from the phase III MONALEESA-2 trial of postmenopausal patients with
±
KISQALI is not indicated for concomitant use with tamoxifen4 1L, first line; 2L, second line; ET, endocrine therapy; LHRH, luteinizing hormonereleasing hormone, aBC, advanced breast cancer. ESMO - European society of medical oncology SABC - San Antonio Breast Cancer Conference ASCO - American Society of Clinical Oncology Reporting suspected adverse reactions of the medicinal product is important to Novartis and the HPRA. It allows continued monitoring of the benefit/risk profile of the medicinal product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by calling 01 2080 612. National Comprehensive Cancer Network® (NCCN®) now recognizes ribociclib (KISQALI®) + ET, a Category 1 preferred treatment option, for showing an OS BENEFIT IN 1L PATIENTS with HR+/HER2- mBC4 NCCN RECOMMENDED KISQALI—the only CDK4/6 inhibitor with statistically significant overall survival across all 3 phase III trials1–3 IN THIS ISSUE: NEWS: Consultant Contract proposals response Page 5 MEDICINES: IPHA publishes new Study on Medicine Competitiveness Page 6 REPORT: National Trends for Cancer in Ireland Page 10 BIOSIMILARS: Biosimilars and their use in Cancer Treatment Page 16 FEATURE: Osteoporosis Clinical Practice Guidelines Page 22 CPD: Overview of the Management of ATTR Page 31 RESPIRATORY FOCUS: Developments in Cystic Fibrosis Page 70
cough, nausea, diarrhoea, vomiting, constipation, stomatitis, abdominal pain, dyspepsia alopecia, rash, pruritus, back pain, fatigue, peripheral oedema, asthenia, pyrexia, abnormal liver function tests. ♦Common:, thrombocytopenia, febrile neutropenia, hypocalcaemia, hypokalaemia, hypophosphataemia, vertigo, lacrimation increased, dry eye, syncope, dysgeusia, , hepatotoxicity, erythema,
Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Merrion Road, Dublin 4,
HR+/HER2− advanced breast cancer treated with endocrine therapy
ribociclib. Presented at: European Society of Medical Oncology; September 16-21, 2021. 2. Im S-A, Lu Y-S, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307-316. 3. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J
4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2022. © National Comprehensive Cancer Network, Inc. 2021. All rights reserved. Published June 21, 2022. Accessed July 29, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
PAXLOVID is indicated for patients
at high risk of progression to severe COVID191
*
Recommendations in national guidelines may differ from the approved indications.
Key factors that put a patient at high risk for progression to severe COVID-19 include1:
60 years of age and older, regardless of comorbidities
Immunosuppressive disease or treatment
Diabetes
Overweight (BMI >25)
Cardiovascular disease Hypertension
Chronic lung disease (including asthma)
Chronic kidney disease**
Current smoker
Neurodevelopmental disorders Sickle cell disease
Active cancer Medically related technological dependence
PAXLOVID is taken orally, at home, twice daily for 5 days
1
Treatment should begin immediately following a positive COVID-19 test and within 5 days of symptom onset
For more information about PAXLOVID dosing adjustments, including the authorised SmPC, [Scan QR Code]
Learn more about potential drug interactions before treatment begins with the Interaction Finder on www.paxlovideducation.ie [Scan QR Code]
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions.
* Patients is defined as adults who do not require supplemental oxygen.1
** In patients with moderate renal impairment (eGFR ≥ 30 to < 60 mL/min), the dose of Paxlovid should be reduced to nirmatrelvir/ritonavir 150 mg/100 mg every 12 hours for 5 days to avoid over-exposure (this dose adjustment has not been clinically tested). Paxlovid should not be used in patients with severe renal impairment [eGFR < 30 mL/min, including patients with End Stage Renal Disease (ESRD) under haemodialysis].
Abbreviated Prescribing Information
Paxlovid® (nirmatrelvir/ritonavir) 150 mg + 100 mg film-coated tablets
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions. Please refer to the Summary of Product Characteristics (SmPC) before prescribing Paxlovid. Indications: Treatment of coronavirus disease 2019 (COVID-19) in adults who do not require supplemental oxygen and who are at increased risk for progression to severe COVID 19 (see section 5.1 of the SmPC). Presentation: Each pink film-coated tablet contains 150 mg of nirmatrelvir. Each white film-coated tablet contains 100 mg of ritonavir. Dosage: The recommended dosage is 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet) all taken together orally every 12 hours for 5 days. Paxlovid should be administered as soon as possible after a diagnosis of COVID-19 and within 5 days of symptom onset. Completion of the full 5-day treatment course is recommended even if the patient requires hospitalisation due to severe or critical COVID-19. In patients with moderate renal impairment, (eGFR ≥ 30 to < 60 mL/min) the dose of Paxlovid should be reduced to nirmatrelvir/ritonavir 150 mg/100 mg every 12 hours for 5 days. Paxlovid should not be used in patients with severe renal or severe hepatic impairment. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Medicinal products that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening reactions, as well as medicinal products that are potent CYP3A inducers where significantly reduced plasma nirmatrelvir/ritonavir concentrations may be associated with the potential for loss of virologic response and possible resistance. Medicinal products listed below are a guide and not considered a comprehensive list of all possible medicinal products that are contraindicated with Paxlovid: Alpha 1‑adrenoreceptor antagonist: alfuzosin; Analgesics: pethidine, propoxyphene; Antianginal: ranolazine; Anticancer drugs: neratinib, venetoclax; Antiarrhythmics: amiodarone, bepridil, dronedarone, encainide, flecainide, propafenone, quinidine; Antibiotics: fusidic acid, rifampicin; Anticonvulsants: carbamazepine, phenobarbital, phenytoin; Anti gout: colchicine; Antihistamines: astemizole, terfenadine; Antipsychotics/Neuroleptics: lurasidone, pimozide, clozapine, quetiapine; Ergot derivatives: dihydroergotamine, ergonovine, ergotamine, methylergonovine; GI motility agent: cisapride; Herbal products: St. John’s Wort (Hypericum perforatum); Lipid modifying agents: lovastatin, simvastatin, lomitapide; PDE5 inhibitors: avanafil, sildenafil, vardenafil; Sedative/ Hypnotics: clorazepate, diazepam, estazolam, flurazepam, oral midazolam, triazolam. Warnings and Precautions: Risk of serious adverse reactions due to interactions with other medicinal products: Due to effects on CYP3A metabolic pathways, potential for interactions should be considered with other medicinal products prior to and during Paxlovid therapy; concomitant medicinal products should be reviewed during Paxlovid therapy and the patient should be monitored for the adverse reactions associated with the concomitant medicinal products. The risk of interactions with concomitant medications during the 5-day treatment period for Paxlovid should be weighed against the risk of not receiving Paxlovid; please refer to Table 1 in SmPC section 4.5. Severe renal impairment: Paxlovid should not be used in patients with severe renal impairment (eGFR < 30 mL/min, including patients with ESRD under haemodialysis). Severe hepatic impairment: Paxlovid should not be used in patients with severe hepatic impairment. Hepatotoxicity: Caution should be exercised when administering Paxlovid to patients with pre-existing liver diseases, liver enzyme abnormalities or hepatitis. HIV resistance: As nirmatrelvir is coadministered with ritonavir, there may be a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection. Excipients: nirmatrelvir tablets contain lactose, patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine. Contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’. Drug Interactions: Nirmatrelvir and ritonavir are CYP3A substrates; therefore, medicinal products that induce CYP3A may decrease plasma concentrations and reduce therapeutic effect. Paxlovid (nirmatrelvir/ritonavir) is an inhibitor of CYP3A and may increase plasma concentrations of medicinal products that are primarily metabolised by CYP3A. Ritonavir has a high affinity for several cytochrome P450 (CYP) isoforms and may inhibit oxidation with the following ranked order: CYP3A4 > CYP2D6. Ritonavir also has a high affinity for P-glycoprotein (P-gp) and may inhibit this transporter. Ritonavir may induce glucuronidation and oxidation by CYP1A2, CYP2C8, CYP2C9 and CYP2C19 thereby increasing the biotransformation of some medicinal products metabolised by these pathways. As a conservative measure, the drug-drug interactions pertaining to ritonavir used in chronic HIV infection should apply for Paxlovid. Medicinal products listed here are a guide and not considered a comprehensive list of all possible medicinal products that may interact with nirmatrelvir/ritonavir: Amphetamine derivatives: amphetamine; Analgesics: buprenorphine, norbuprenorphine, piroxicam, fentanyl, methadone, morphine; Antiarrhythmics: digoxin; Antiasthmatic: theophylline; Anticancer: afatinib, abemaciclib, apalutamide, ceritinib, dasatinib, nilotinib, vincristine, vinblastine, encorafenib, fostamatinib, ibrutinib, venetoclax (contraindicated in some circumstances); Anticoagulants: rivaroxaban, vorapaxar, warfarin; Anticonvulsants: divalproex, lamotrigine, phenytoin; Antidepressants: amitriptyline, fluoxetine, imipramine, nortriptyline, paroxetine, sertraline, desipramine; Antihistamines: fexofenadine, loratadine; Anti infectives: rifabutin, voriconazole, ketoconazole, itraconazole, erythromycin, atovaquone, bedaquiline, delamanid, clarithromycin, sulfamethoxazole/trimethoprim; Anti HIV: efavirenz, maraviroc, raltegravir, zidovudine; Anti HCV: glecaprevir/pibrentasvir; Antipsychotics: haloperidol, risperidone, thioridazine; β2-agonist (long acting): salmeterol; Calcium channel antagonist: amlodipine, diltiazem, nifedipine; Endothelin antagonists: bosentan, riociguat; HMG Co A reductase: atorvastatin, fluvastatin, pravastatin, rosuvastatin; Hormonal contraceptive: ethinyl estradiol; Immunosuppressants: cyclosporine, tacrolimus, everolimus; PDE5 inhibitors: sildenafil (contraindicated in some circumstances), tadalafil; Sedatives/Hypnotics: parenteral midazolam, alprazolam, buspirone; Sleeping agent: zolpidem; Smoke cessation: buproprion; Steroids: inhaled, injectable or intranasal fluticasone propionate, budesonide, triamcinolone, dexamethasone, prednisolone; Thyroid hormone replacement therapy: levothyroxine. Please refer to Table 1 in SmPC section 4.5 for additional information on interaction with medicinal products / other forms of interaction. Fertility, pregnancy and lactation: Women of childbearing potential should avoid becoming pregnant during treatment with Paxlovid and as a precautionary measure for 7 days after completing Paxlovid. Patients using combined hormonal contraceptives should be advised to use an effective alternative contraceptive method or an additional barrier method of contraception during treatment and until after one complete menstrual cycle after stopping Paxlovid. Paxlovid is not recommended during pregnancy and in women of childbearing potential not using contraception unless the clinical condition requires treatment with Paxlovid. Breast feeding should be discontinued during treatment with Paxlovid and as a precautionary measure for 7 days after completing Paxlovid. Driving and operating machinery: Paxlovid is expected to have no influence on the ability to drive and use machines. Undesirable effects: Common (≥ 1/100 to < 1/10) adverse events reported were dysgeusia, headache, diarrhoea, vomiting and nausea. Uncommon (≥ 1/1,000 to < 1/100) adverse events reported were abdominal pain. Rare (≥ 1/10,000 to < 1/1,000) adverse events reported were malaise. See SmPC section 4.8 for full details.Legal Category: S1A. Package Quantities: 150 mg + 100 mg, 20 + 10 film-coated tablets. Marketing Authorisation Number: EU/1/22/1625/001. Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Brussels, Belgium. For further information on this medicine please contact: Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com For queries regarding product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 4676500. Last revised: 11/2022 Ref: PX 4_0
Reference: 1. PAXLOVID™ Summary of Product Characteristics. Pfizer Inc.; October 2022.
© 2022 Pfizer Inc. All rights reserved. PP-PAX-IRL-0096 | November 2022
Contents Foreword
Private Hospitals Association says new interim strategy needed P4
More collaboration needed in medicines competitiveness, study reports P6
NCHD Committee Ballot on new Agreement P8
Cancer Trends in Ireland latest report P10
World-renowned surgeon hosted at University Hospital Galway P12



Biosimilar medicines and their use in the treatment of cancer P16
All-Ireland approach to Psychosis P27
REGULARS
CPD: hATTR P31
Respiratory Focus: Pneumonia P50
Respiratory Focus: Smart Techniques P54
Respiratory Focus: Cystic Fibrosis P70
4
Editor
As Hospital Professional News was going to press with the first issue of 2023, the Irish Hospital Consultants Association (IHCA) was expressing dismay that the Government is on course to miss waiting list reduction targets for 2022 by a significant margin.
Minister for Health Stephen Donnelly launched the ¤350 million Waiting List Action Plan for 2022 in February which committed to reducing active waiting lists for acute scheduled care by 18% (more than 132,00) by the end of this year. However, figures show that by the end of November the numbers of those waiting for treatment will have only dropped by a modest 1.6% (fewer than 11,500).
IPN
Clifton
House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562 GROUP DIRECTOR
DIGITAL MARKETING
8 27 HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews


The latest NTPF figures confirm that the number of people on the three main waiting lists decreased from 720,056 at the start of 2022 to 708,590 at the end of November, nowhere near the planned 18% reduction.
Overall, a total of 887,500 people were on some form of hospital waiting list at the end of November, including almost 97,000 children.
When the additional 243,000 people awaiting CTs, MRIs or ultrasounds nationally are added, the total number awaiting hospital care is over 1.1 million – or more than one-fifth of the entire population.
Analysis from the IHCA shows that the HSE is now more than 120,800 outpatient appointments and procedures away from meeting the 18% reduction targets for the end of this year, including 115,100 outpatient appointments and around 5,700 inpatient or day case procedures and GI scopes.
In other news, the National Cancer Registry (NCRI) has published its 2022 Annual Statistical Report: Cancer in Ireland. 1994 – 2020. This report on the status of cancer in Ireland includes updated statistics on cancer incidence, mortality and survival for patients diagnosed in Ireland 1994 – 2020. You can read more about the report findings on page 10 whilst on page 16, Rebecca Parkin, AnneMarie DeFrein and Patricia Heckmann discuss biosimilar medicines and their use in the treatment of cancer.
The authors, from the National Cancer Control Programme state that, “As biologicals are high-cost components of cancer care, the availability of safe and effective biosimilars has huge potential to reduce cancer care costs and enable access to biologicals and other expensive cancer treatments for patients with cancer.”
Our Special Focus for January is in the field of Respiratory Care, with some excellent and current clinical updates contributed by leading specialists in this field.
I hope you enjoy the issue.
12 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication

3 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 January Issue Issue 104
Clinical R&D: P80
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system of transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors. PUBLISHER
Communications Ireland Ltd
EDITOR
EDITORIAL
Emilia Bayliss emilia@hospitalprofessionalnews.ie EDITORIAL editorial@hospitalprofessionalnews.ie ACCOUNTS Rachel Wilson cs.ipn@btconnect.com SALES EXECUTIVE Aoife Tremere aoife.t@hospitalprofessionalnews.ie CONTRIBUTORS Rebecca Parkin | AnneMarie DeFrein Patricia Heckmann | Rachel Flynn Catherine Armstrong | Fiona Heaney Barry O’Sullivan | Philip Watt Persijn Honkoop | Omar Usmani Matteo Bonini | Rebecca Weedle Ronan Ryan | Vincent Young Gerard Fitzmaurice | Dzufar Halim Sinead Walsh | Dr Sile Toland Dr Dan Ryan | Tatiana Larmak Edel Burton | Michelle O’Driscoll Professor John Carey Theresa Lowry-Lehnen Aoife Fleming DESIGN DIRECTOR Ian Stoddart Design
Natalie Maginnis n-maginnis@btconnect.com
Kelly Jo Eastwood
&
EXECUTIVE
Waiting for New Elective Hospitals Won’t Address Current Crisis
Jim Daly, CEO, Private Hospitals Association
even give the final green light to progress with any of these new elective facilities. These will then take a considerable time to build and commission thereafter. Realistically it would be 2028 or 2029 at the very earliest before we could hope to see the first elective hospital up and running.
elective hospitals and surgical hubs at existing hospitals, to address systemic problems associated with hospital waiting lists over the longer term, but says urgent action is needed right now to meet the needs of a record 897,0000 people currently awaiting either a hospital appointment or a hospital procedure in this country.
The Private Hospitals Association says a new interim strategy to urgently address the immediate and urgent problem of hospital waiting lists is needed immediately, as the government announced
more details on a planned new network of public elective hospitals and surgical hubs.
The PHA notes the update by the government on its strategic plan to progress with new
Advancement of Pharmacy
Private Hospitals Association CEO, Jim Daly, said, “The longterm strategy to clear elective lists is just that - we won’t know for at least 2 more years - end of 2025- if government will

“Patients can’t wait till 2029 for the hospital treatment that is urgently needed now. The State can act now to prioritise certain actions to help clear the unacceptable backlog of patients. We are disappointed at the slow pace of progress throughout 2022 with the Department, the HSE and the NTPF despite their recognition of private hospitals’ ability to provide quick, safe, high-quality healthcare to patients. The state needs to do more now for people languishing on public lists. This includes a strategic partnership and formal structures of cooperation to achieve waiting list reductions and improved mediumterm planning around same.”
Last month saw the launch of a toolkit to support pharmacy professional associations, universities and educators in advancing pharmaceutical education at all levels, from undergraduate to continuing professional development. The toolkit has been published by the International Pharmaceutical Federation (FIP).
Release of the FIP Education Transformation Toolkit follows the federation’s global call to action for advancing pharmaceutical education made in December 2021. The call outlined 18 actions needed to avert the predicted shortfall in health workers and to help the profession meet the United Nations Sustainable Development Goals. These actions included quality assurance of pharmacy education and training, and supporting the development of advanced specialist frameworks.
The toolkit brings together numerous globally relevant resources for each of the 18 recommended actions. “We have urged all stakeholders to take our recommended actions to advance pharmaceutical education. We are now providing them with added support to do so. This digital toolkit is a muchneeded compendium of FIP education resources that will serve to help all educators and trainers to provide quality education,” said Prof. Ralph Altiere, chair of FIP Education.
Hospital Pharmacy Congress 2023
There are a little bit more than 100 days left until hospital pharmacists from all across the world will come together for EAHP's annual Congress. Hosted this time in Lisbon, Portugal the profession will be coming together from the 22nd to the 24th of March 2023 for the 27th edition of EAHP's congress.

The Scientific Committee has put together an outstanding programme centring around the theme "From drug design to treatment success – What really matters to patients?".
Three keynotes will focus on the opportunities for personalised medicine, improvements in the communication of risks and benefits to patients and patient involvement in pharmacy practice research. Medication safety, compounding, communication, patient involvement, procurement, shortages and digitalisation are some of the topics covered by the different interactive sessions. Those registering for EAHP's Congress before the end of January will be able to benefit from a reduced registration rate.
4 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Consultants Respond to New Contract Proposals
Commenting on the agreement by Government of the proposed new Consultant contract today, the President of the Irish Hospital Consultants Association (IHCA), Professor Rob Landers, said, “We have communicated our views on Minister Donnelly’s proposal consistently to both the Minister himself and the Secretary General of the Department of Health.
“Currently, over 900,000 people are on a waiting list to see a hospital consultant. Over 900
permanent Consultant posts are unfilled. For a decade, we have been calling on Government to constructively engage so as to address these stark realities.
“Despite progress in discussions over the recent period, the Minister and Government have moved on and ended talks with representative bodies.
“There remain a number of critically important issues that need to be addressed to ensure
any proposed contract will reduce patient waiting times and address the Consultant recruitment and retention crisis.
“The Donnelly proposal must stand up against international standards in order to make Ireland’s health service a place that medical and surgical specialist want to work. This is the lens through which our members will evaluate this proposal.
“We will also be looking at this
from the perspective of the almost 1 million people currently waiting for essential care across the country, to assess whether the core objective of providing patients with timely essential care can be achieved through this proposed contract.
“We have a detailed consultation process to undertake with our membership now, including those currently working abroad, to determine Consultants’ final view on the Donnelly proposal.”
Irish Research Discovers new Brain Tumour Subtypes

Research lead by RCSI University of Medicine and Health Sciences has discovered three new subtypes of brain tumour which could help to identify new and effective therapies. The novel tumour subtypes are forms of glioblastoma, the most common and most aggressive form of adult primary brain cancer with no cure currently available.
The research, published in Annals of Oncology, has identified that glioblastoma tumours can be placed into three categories based on the different kind of non-cancer cells that can be found within the tumour. These so-called tumour microenvironment cells can include immune cells and blood vessel cells.
Currently, the majority of patients with glioblastoma are treated in the same way. Further investigation of these newly identified subtypes will mean that different patients could receive treatment specific to the cells in their own tumour. This type of targeted treatment is known as 'precision medicine'.
Senior Author and Lead Investigator, Professor Annette Byrne, Head of the RCSI Precision Cancer Medicine Group commented: "Glioblastoma patients currently have a poor prognosis due to limited treatment options so it is vital that new treatments be developed. Targeted treatment or 'precision medicine' has the potential to improve outcomes for these patients. We hope further analysis of the tumour
Advancing Hospital Pharmacy
Through
The
subtypes identified in this research, will provide the data needed to support future glioblastoma clinical trials in Ireland."
Precision medicine approaches could include the use of immune-targeting therapies (immunotherapies) in patients that have the tumour subtype defined by high levels of immune cells within their tumour microenvironment. An assessment
of glioblastoma clinical trial datasets by this research group provided support for this idea, showing that patients with this subtype of tumour may have an improved outcome when treated with immunotherapies compared to other subtypes.
The study’s first authors are Kieron White and Dr Kate Connor from the RCSI Precision Cancer Medicine Group, Department of Physiology.
9th
Sharing your feedback on the investigation should take approximately 45 minutes. The full set of questions is available in PDF format on EAHP's website. Visit www.eahp.eu for more details.
5 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
News
Professor Annette Byrne, Head of the RCSI Precision Cancer Medicine Group
its membership, the European Association of Hospital Pharmacists (EAHP) has reached out to chief pharmacists, directors of pharmacy and managers of the pharmacy in each hospital across Europe to obtain their feedback on the state of play of the profession. Until the
of January 2023, each European hospital pharmacy can contribute to the "Investigation of the hospital pharmacy profession in Europe – Assess and advance hospital pharmacy!".
investigation touches on the state-of-the art of the profession and specifically on the European Statements of Hospital Pharmacy. Chief pharmacists, directors of the pharmacy and managers of the pharmacy are encouraged to participate, but they can also delegate the provision of feedback to another pharmacist in their hospital pharmacy. Only one response per hospital pharmacy is needed.
IPHA Study
New Study on Medicines Competitiveness
funding, European research funding is more uniform across countries. The countries with the highest EU research spend per population are not the centres of innovation. This is a weak strategy. The European Commission should consider more strategic allocation of resources to foster the growth of world-leading research centres. It should sponsor a review of existing life science and industrial policies across Member States to identify success factors and opportunities for replication.
2. Rethink supply chain policies to attract ATMP investments in Europe
Europe could lose out to the US and Asia for new investments in biopharmaceutical research and production, according to a new study by consultants Charles River Associates (CRA) which urges more collaboration between industry and policy leaders to boost competitiveness.
The study, called ‘Factors Affecting the Location of Biopharmaceutical Investments and Implications for European Policy Priorities’, shows the industry’s declining competitiveness in Europe, with the global share of research and development investments, clinical trials and manufacturing output all waning. However, the study, for EFPIA, the representative organisation for Europe’s biopharmaceutical industry, finds that a key driver of most new investments is the location and performance of existing research and development or manufacturing footprint.
IPHA, a member of EFPIA and the representative organisation for Ireland’s biopharmaceutical industry, said recent investments in production sites across the country are based, in part, on an already-established performance culture built over several decades. But IPHA said Ireland should not be complacent about our attractiveness for future investments, with significant global competitive pressures making the outlook uncertain.
The study shows that:
• In 2002, the US spent $2 billion more than Europe on research and development. Today, that figure is $20 billion – a rise of 1000%. Of the total research and development investments made in the US, Europe, China and Japan, only 31% occurs in Europe. This has declined
steadily from 41% in 2001. China has grown its share from 1% to 8%.
• Clinical trial activity for advanced therapeutic medicinal products (ATMPs), which include cell and gene therapies, is twice as high in the US and almost three times as high in China than in Europe. The number of trials for these breakthrough treatments conducted in the US and Asia-Pacific region grew by 70% and 67%, respectively, between 2014 and 2021 while Europe remained stagnant.
• Europe accounted for a 19.3% share of global clinical trials activity in 2020, a drop of 6.3%, compared with a 25.6% average over the past 10 years.
IPHA, along with EFPIA, have urged policymakers in Europe to back innovation and supportive intellectual property rights in the current review of the pharmaceutical legislation by the European Commission. That means making Europe more competitive for jobs and investments in biopharmaceuticals, accelerating patients’ access to affordable medicines, investing in research for unmet medical needs and making regulatory pathways more agile and efficient.
The CRA study has several recommendations, including:
1. Incentive the development of world-class innovation hubs in the EU
Global research and development is expected to grow at a rate of 4.2% annually to $233 billion in 2026 – but it is moving out of the EU. While world-leading hubs like Boston, San Francisco and the UK’s ‘Golden Triangle’ get significant policy focus and strategic

ATMPs are the therapies of the future, with 804 next-generation biotherapeutics, including cell and gene therapies and mRNA technology, in a global pipeline of over 8,000 new medicines. The US has half the world’s ATMP manufacturing facilities. Asia is fast becoming the most competitive region for attracting ATMP clinical trials (255 in 2021), with Europe in decline (89 in 2021). For Europe to compete more effectively, it must recognise the complexity of these new technologies and build the interconnected ecosystem needed to develop them. IPHA has argued that Ireland should aspire to be a centre for supply chain and production and adopt a national policy for adopting ATMPs affordably and fast in the health system. The EU should take a more proactive role in fostering the growth of emerging ATMP clusters, providing support across the ecosystem of research and development, manufacturing and clinical trials. The old approach of siloed policymaking focused on innovation, manufacturing and healthcare sustainability does not work.
3. Support innovation by recognising the link between fast access and competitiveness for new investments
The traditional lifecycle of medicine development is changing. Medicines increasingly need to gain conditional approval to reach patients earlier to generate real-world evidence. Company research investment will increasingly be influenced by regulatory agility and flexibility and a supportive clinical trial
environment. The European Commission’s legislative proposals for the industry share some of these goals – supporting innovative trial designs and new methods for data generation and assessment. But policymakers do not take into account the link between fast access to new medicines and the attractiveness of Europe as a location for companies to locate research, clinical trials and manufacturing, particularly for new technologies. IPHA has argued for the closing of the gap between scale and speed – that we can have a major biopharmaceutical investment footprint and faster access to new medicines. By the end of this year, we will share with policymakers proposals aimed at improving the reimbursement process.
Oliver O’Connor, IPHA’s CEO and member of the Steering Group for the CRA study, said it was an important input into evidencebased policymaking.
“In a European context, Ireland has a very strong biopharmaceutical manufacturing footprint, with sites across the country making medicines for the world and generating corporation and payroll taxes which are helping the Government to deal with cost-ofliving living challenges. Although our heritage in innovation is helping to attract new investments in biopharmaceutical production, we can’t be complacent about the future. As the study shows, companies often site investments close to their headquarters and where the operating environment for innovation is optimal.
“Ireland has significant challenges to overcome, especially in giving more patients faster access to new medicines and attracting the next wave of investments in areas like ATMPs. We have ground to make up in data and digitalisation, and in plugging our clusters of higher education institutions into research networks in Europe and beyond.
“We share the European Commission’s goal of widening access to affordable medicines for all citizens while spurring innovation. But striking this delicate policy balance requires engaged dialogue so that the innovation ecosystem is not undermined and Europe can stay competitive, both in standards of care and in our capacity to keep the jobs we have and attract new ones,” said Mr O’Connor.
6 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Administration: Adults/Elderly: Administration by portable pump directly into the duodenum or upper jejeunum via a percutaneous endoscopic gastrostomy (PEG) or radiological gastrojejunostomy tube. Initially a nasoduodenal/nasojejunal tube should be considered to determine patient’s response before a PEG-J tube is placed. Where the physician considers this assessment is not necessary, the nasojejunal test phase may be waived and treatment initiated directly with placement of PEG-J. Duodopa is given initially as monotherapy and dose adjusted to optimal response for the individual patient. Total dose/day is composed of three individually adjusted doses: morning bolus, continuous maintenance and extra bolus doses administered over approximately 16 hours. Total morning dose usually 5-10ml (100-200mg levodopa) but not exceeding 15ml (300mg levodopa). Continuous maintenance dose should be between 1-10ml/hr (20200mg levodopa/hr) but usually 2-6ml/hr (40-120mg levodopa/hr). Maximum recommended daily dose is 200ml. Extra bolus doses (if patient becomes hypokinetic during the day) are normally 0.5-2.0ml. Increase maintenance dose if more than 5 extra bolus doses/day are needed. Fine adjustments to the morning bolus, maintenance and extra bolus doses should be made over a few weeks after the initial dose setting. Sudden deterioration in response with recurring motor fluctuations indicates tube may have moved from duodenum/ jejunum into stomach and needs repositioning. Medicine cassettes are for single use only and should not be used for longer than 24 hours, even if some medicinal product remains. Treatment is usually administered during the patient’s awake period. If medically justified, Duodopa may be administered for up to 24 hours. Opened cassettes should not be reused. Children: No relevant use in the paediatric population. Renal/hepatic impairment: No studies on the pharmacokinetics of carbidopa and levodopa in patients with hepatic or renal impairment. Dose titration should be conducted with caution in patients with severe renal and hepatic impairment. Contraindications, Warnings, Precautions etc: Contraindications: Hypersensitivity to ingredients, narrow-angle glaucoma, severe heart failure or cardiac arrhythmia, acute stroke. Conditions where adrenergics are contraindicated (e.g. pheochromocytoma, hyperthyroidism, Cushing’s syndrome). Nonselective MAO-inhibitors and selective MAO type A inhibitors are contraindicated and should be withdrawn at least two weeks before starting Duodopa. Duodopa should not be used in patients with suspicious undiagnosed skin lesions or a history of melanoma. Warnings/Precautions: Not recommended for drug-induced extrapyramidal reactions. Caution in severe pulmonary or cardiovascular disease, bronchial asthma, renal, hepatic or endocrine disease, or history of peptic ulcer disease or of convulsions, past or current psychosis, chronic wide-angle glaucoma, co-administration with antipsychotics with dopamine receptor blocking properties or with medicines which may cause orthostatic hypotension. In patients with a history of myocardial infarction who have residual nodal or ventricular arrhythmias, cardiac function should be monitored with care during initial dose adjustments. Monitor all patients for development of mental changes, depression with suicidal tendencies and other serious mental changes. Neuroleptic Malignant like Syndrome with secondary rhabdomyolysis has not been reported with Duodopa but may occur on abrupt dose reduction/withdrawal. Periodically evaluate hepatic, haematopoietic, cardiovascular and renal function during extended therapy. Increases in impulse control disorders have been reported and patients should be monitored and reviewed.
Patients and providers are advised to monitor for melanomas on a regular basis when using Duodopa. Ideally, periodic skin examinations should be performed by dermatologists. Dose may need to be adjusted downwards to avoid levodopa induced dyskinesia. Sudden or gradual worsening of bradykinesia may indicate an obstruction in the device and should be investigated. Duodopa contains hydrazine, a degradation product of carbidopa that can be genotoxic and possibly carcinogenic, clinical significance of exposure unknown. Reported complications in clinical studies include abscess, bezoar, ileus, implant site erosion/ulcer, intestinal haemorrhage, intestinal ischaemia, intestinal obstruction, intestinal perforation, intussusception, pancreatitis, peritonitis, pneumonia (including aspiration pneumonia), pneumoperitoneum, post-operative wound infection and sepsis. Before initiation of treatment, patients and caregivers should be warned of the potential risk of developing Dopamine Dysregulation Syndrome (DDS). Polyneuropathy has been reported in patients treated with levodopa/carbidopa intestinal gel. Before starting therapy evaluate patients for history or signs of polyneuropathy and known risk factors, and periodically thereafter. Drug Interactions: Antihypertensives, tricyclic antidepressants, anticholinergics, dopamine receptor antagonists, benzodiazepines, isoniazide, phenytoin, papaverine, sympathicomimetics, iron, protein-rich diet, COMT inhibitors (e.g. tolcapone, entacapone) amantadine. Duodopa dose adjustment may be needed when used with these drugs. Duodopa can be taken with MAO type B inhibitors (e.g. selegiline) although serious orthostatic hypotension may occur and the dose of levodopa may need to be reduced. Fertility, Pregnancy and Lactation: Limited data relating to the use of levodopa/carbidopa in pregnant women. Duodopa is not recommended during pregnancy. Breast-feeding should be discontinued during treatment with Duodopa. No adverse reactions on fertility have been observed in preclinical studies with carbidopa or levodopa alone. Ability to Drive and Operate Machinery: Caution; Duodopa can have a major influence on the ability to drive and use machines. Refrain from driving/operating machinery if somnolence and/or sudden sleep episodes occur. Side Effects: Very common: Weight decreased, Anxiety, Depression, Insomnia, Dyskinesia, Parkinson’s disease, Orthostatic hypotension, Nausea, Constipation, Fall, Common: Anaemia, Increased weight, Amino acid level increased (Metylmalonic acid increased), Blood homocysteine increased, Decreased appetite, Vitamin B6 & B12 deficiency, Abnormal dreams, Agitation, Confusional state, Hallucination, Impulsive behaviour, Psychotic disorder, Sleep attacks, Sleep disorder, Dizziness, Dystonia, Headache, Hypoaesthesia, On and off phenomenon, Paraesthesia, Polyneuropathy, Somnolence, Syncope, Tremor, Heart rate irregular, Hypertension, Hypotension, Dyspnoea, Oropharyngeal pain, Abdominal distension, Diarrhoea, Dry mouth, Dysgeusia, Dyspepsia, Dysphagia, Flatulence, Vomiting, Dermatitis contact, Hyperhidrosis, Oedema peripheral, Pruritus, Rash, Muscle spasms, Neck pain, Urinary incontinence, Urinary retention, Fatigue, Pain, Asthenia. Device and Procedure Related


Adverse Reactions: Very common: Postoperative wound infection, Abdominal pain, Excessive granulation tissue, Complications of device insertion, Incision site erythema, Post procedural discharge, Procedural pain, Procedural site reaction. Common: Incision site cellulitis, Post procedural infection, Abdominal discomfort, Abdominal pain upper, Peritonitis, Pneumoperitoneum, Pneumonia/Aspiration pneumonia, Device dislocation, Device occlusion, Gastrointestinal stoma complication, Incision site pain, Postoperative Ileus, Post procedural complication, Post procedural discomfort, Post procedural haemorrhage. Laboratory values: May change. Prescribers should consult the SmPC for the complete list of reported side effects. HCPs are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie. Marketing
Authorisation Number: PA 1824/2/1. Legal Category: POM (S1B). Further information is available from: AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24. Date of Revision: January 2021. PI/2/005
References: 1. Duodopa® Summary of Product Characteristics, available on www.medicines.ie 2. Nyholm D. The rationale for continuous dopaminergic stimulation in advanced Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(suppl):S13-S17. doi:10.1016/j.parkreldis.2007.06.005. IE-DUOD-210040. Date of Preparation: September 2021.
You can offer your patients continuous dopaminergic stimulation with Duodopa®1,2 Because ON TIME is their time1 In the treatment of advanced Parkinson’s disease Duodopa® is indicated for the treatment of advanced levodoparesponsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have not given satisfactory results1 People
patients. PRESCRIBING INFORMATION: Duodopa 20 mg/ml + 5 mg/ml intestinal gel (levodopa/carbidopa) Refer to Summary of Product Characteristics (SmPC) for full prescribing information. Presentation: Intestinal gel containing 20mg/ml levodopa and 5mg/ml carbidopa monohydrate. Indication: Advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available combinations of Parkinson medicinal products have
given satisfactory results.
in this piece are models, not actual
not
Dosage and
NCHD Committee Ballot on New Agreement
Dr John Cannon, Chairman, Irish Medical Organisation NCHD Committee
action up to and including industrial action in support of the #standingup4NCHD campaign which has run through the current year.
The NCHD Committee of the Irish Medical Organisation (IMO) is balloting NCHD members to seek support for an agreement with the HSE and the Department

of Health on eliminating unsafe and illegal hours. The agreement would see employers being penalised for noncompliance. The IMO has also secured
Oxycare Medical Facility Opens
agreement that an entirely new NCHD Contract will be negotiated starting in January.
The ballot commenced on the 12th December and concluded on 19th December. The NCHD Committee of the IMO is recommending a Yes vote in the ballot.
This breakthrough follows extended negotiations between the IMO NCHD Committee and the HSE and Department of Health. These negotiations were prompted by the overwhelming (97%) support of NCHDs for
Dr John Cannon, Chairman of the NCHD Committee of the IMO said, “This agreement has been hard-won, and we believe that while it represents progress in some key areas further reform on NCHD working conditions and training must be secured in the NCHD Contract negotiations which will commence in January 2023. NCHDs are doctors in training and their work/study and life balance must be protected to ensure safety for both doctors and patients. We view this very much as a first step in changing the culture in which NCHDs are treated within the system which is bad for doctors, bad for patients and leading to ever increasing levels of emigration.”
Minister for Health Stephen Donnelly TD has officially opened the Oxycare medical facility in Dublin, Ireland’s only internationally accredited medical hyperbaric oxygen treatment centre.
Oxycare provides medical Hyperbaric Oxygen treatment to patients referred by consultants around the country, with a mission to contribute to the health and wellbeing of patients by providing the highest standard of treatment to every patient. Conditions suitable for medical hyperbaric oxygen treatment include but are not limited to; enhancement of healing in oxygen deprived wounds, skin grafts and flaps post surgery, healing of heat burns, radiation injuries, crush-related injuries, idiopathic sudden sensorineural hearing loss.
Dr Gerry Molloy, co-founder of Oxycare said, “As Ireland’s first and only internationally-accredited hyperbaric oxygen treatment centre, Oxycare is positioned uniquely in the Irish medical landscape, with consultants and surgeons at several of the country’s top hospitals referring patients to our facility to undergo this treatment in order to enhance outcomes for those patients for whom standard care may not prove sufficient. The results of treatment – adhering to appropriate medical standards and protocols - are impressive, and are attested to in correspondence from various consultants who have referred their patients and who have now adopted this mode of treatment as an option to be considered for the most difficult cases.”
First National Genetics Strategy
The National Strategy for Accelerating Genetic and Genomic Medicine in Ireland was launched last month, the first ever strategy for Ireland.
The strategy outlines the planned development of an enhanced patient and family-centred genetic and genomic service that will be coordinated nationally.
The strategy was developed in collaboration with the Department of Health and healthcare professionals, international experts, academics, patient representatives and advocates. Provided for under this strategy is:
• the creation of a new national office for genetics and genomics
• the transition of genetics and genomics into routine care delivery
• targeted workforce planning and development
• ensuring Public and Patient Involvement (PPI) and partnership
• the strengthening of Ireland’s infrastructure to drive advances in this area
Speaking at the launch, Dr Colm Henry, HSE Chief Clinical Officer explained: “Advancing Ireland’s genetics and genomics service means improving healthcare for everyone in Ireland, because it will allow for increased disease prevention, better diagnostics, more targeted treatments, and better patient and family outcomes.
Through this new expert-informed strategy, we can work toward a future where genetic and genomic medicine will be part of routine
care delivery that can be accessed equitably across the country, from visits to the GP to extended care for rare disease or cancer.”
Dr Mark Bale, former Genomics Advisor to the UK Department of Health and Chair of the National Genetics and Genomics Strategy Steering Group, said: “In the development of this strategy, we’ve noted examples of excellence in genetics and genomics evident throughout the country. However, because of the collective, invaluable contributions of over 100 experts, healthcare professionals, advocates and patient representatives, Ireland now has a comprehensive strategy. This provides a way forward for progressing this exciting field of medicine to improve citizen health and wellbeing whilst advancing research, innovation,
and discovery. I have every confidence that Ireland will rise to this occasion, beginning with implementation in early 2023.”
Patient and public involvement was a key feature throughout the development of the strategy, and the HSE is committed to continuing to centre patient voices throughout its implementation.
Deirdre McNamara, Director of the HSE’s Strategic Programmes Office, said: “It was a pleasure and a privilege to have the input of so many patient representatives and advocates in this process, and their contributions certainly shaped the content of the strategy in many ways. It is essential that the needs of patients and their families continue to be at the heart of the design and development of any new genetic and genomic services or initiatives in Ireland.”
8 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Overprescribing among Older People: TILDA Study Overprescribing
Researchers from the Irish Longitudinal Study on Ageing (TILDA) have recently published a study which looked at overprescribing among older people near end of life in Ireland.

International evidence shows that people approaching end of life (EOL) have high prevalence of polypharmacy, including overprescribing. Overprescribing may have adverse side effects for mental and physical health and represents wasteful spending. Little is known about prescribing near EOL in Ireland.
The study authors comprised of Soraya Matthews, Dr Frank Moriarty, Dr Mark Ward, Dr Anne Nolan, Professor Charles Nomand, Professor Rose Anne Kenny and Peter May. They aimed to describe the prevalence of two undesirable outcomes, and to identify factors associated with these outcomes: potentially questionable prescribing, and potentially inadequate prescribing, in the last year of life (LYOL). They used The Irish Longitudinal Study on Ageing, a biennial nationally representative dataset on people aged 50+ in Ireland. Below we summarise their findings.
In a nationally representative sample of 525 older people with high mortality risk in Ireland, 401 (76%) had potentially inadequate and 294 (56%) potentially questionable medications. Prevalence of these prescribing patterns among those in LYOL was 62% and 38% respectively. One factor was significantly associated with potentially inadequate medications in LYOL: male (OR 4.40, p = .004) Three factors were associated with potentially questionable medications in LYOL: male (OR 3.37, p = .002); three or more activities of daily living (ADLs) (OR 3.97, p = .003); and outpatient hospital visits (OR 1.03, p = .02).
Comparatively lower prevalence of the specified prescribing patterns in LYOL is encouraging to the extent that they may indicate a level of deprescribing is occurring as people approach end of life. However, approximately 30,000 people die annually in Ireland (the majority of them over the age of 50), and our data suggest that a large proportion of these are at risk of inappropriate polypharmacy.
Tackling high prevalence of overprescribing near end of life requires ex ante identification of those at greatest risk. Multivariable regression results indicate that male sex is the strongest predictor of both undesirable outcomes. The odds of men receiving potentially inadequate medications were 4.4 times higher (than the odds of women receiving potentially inadequate medications), after controlling for other important predictors of prescribing. Similarly, the odds of men receiving potentially questionable medications were 3.4 times higher (than the odds of women receiving questionable medications), after controlling for the same predictors. Three or more ADL difficulties and outpatient engagements were also associated with potentially questionable medications, but neither cancer nor cardiovascular disease were associated with outcome.
Examining the prevalence of potentially inadequate and questionable medications among male and female participants, differences were noted across medications used in the prevention and treatment of cardiovascular disease (antiplatelet and anticoagulant drugs, lipid-lowering drugs), with higher prevalence among males, and to a lesser extent, higher prevalence of medication for osteoporosis among females. These patterns are reflective of the patterns of prescribing observed in middle-aged and
older adults more generally, with noted undertreatment of women compared to men given the same level of cardiovascular morbidity, while older men with similar risk of fracture to women are less likely to be treated for osteoporosis. This suggests that being of high mortality risk does little to reduce the disparity in prescribing between sexes. There were also high rates of medical or GP visit card eligibility (based on income) among those with questionable or inadequate medications, potentially reflecting an impact of higher burden and severity of co-morbidities, or greater access to care.
Much of the existing literature on inappropriate prescribing among people with limited life expectancy has focussed on use of preventive medications, where the likely benefits will not be realised within a patient’s life expectancy. Most studies have focussed on people with a specific life-limiting condition, most often cancer. The most often cited preventive medications prescribed inappropriately were lipid-lowering drugs and antihypertensives, similar to the findings of our study, as well as antidiabetic drugs, which had a relatively low prevalence in the current study. Often such medications are only stopped in the last month of life. This underlines the importance of reviewing and discussing the need and benefit of such long-term medications to align treatment with patients’ goals, in general but
particularly among people with life-limiting illness. It is also worth noting that while overprescribing is the focus of much research on optimal medication use near end of life, under prescribing is also an important issue, where use of symptom-relieving medications that enhance quality of life may be insufficient. Care for such patients should focus not just on reducing medications, but ensure therapy maximises quality and quantity of life and is aligned with patient priorities.
Ireland is ranked fourth worldwide for end-of-life care, meaning that there is relatively high per capita palliative care provision at the population level. Our paper highlights that even in a setting with well-established palliative care services, the risk of inappropriate prescribing among people with limited life expectancy in significant. However, these deficiencies in patient experience must be understood in the context of other challenges; previous research in TILDA demonstrated strong evidence for undermanagement of illness symptoms near end of life, whereas overprescribing is a form of overtreatment.
Thousands of older people die annually in Ireland with potentially inappropriate or questionable prescribing patterns. Gender differences for these outcomes are very large. Further work is needed to identify and reduce overprescribing near EOL in Ireland, particularly among men.
9 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
Professor Rose Anne Kenny, TILDA
Annual statistical report 2022
What are the most common cancers causing death?
Cancer in Ireland: Latest Report Published
In males, cancer of the prostate , colorectal (bowel), pancreas and oesophagus were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths, respectively. Colorectal (bowel) cancer was the 2nd most common cancer death in males during 2016-2018, but dropped to 3rd behind prostate cancer during 2018-2020.

Over 50% increase in numbers of cancer survivors compared with a decade ago
The National Cancer Registry (NCRI) has published its 2022 Annual Statistical Report: Cancer in Ireland. 1994 – 2020. This report on the status of cancer in Ireland includes updated statistics on cancer incidence, mortality and survival for patients diagnosed in Ireland 1994 – 2020.
The National Cancer Registry of Ireland is now in its 29th year of data collection, and in the 2022 annual statistical report, they summarise cancer data collected up to diagnosis year 2020. In additional to the more regular reporting of incidence, mortality and survival figures, this year they again provide additional focus on impacts of the COVID-19 pandemic on numbers of cancers diagnosed.
Key findings include:
• Over 50% increase in numbers of cancer survivors compared with a decade ago as, for the first time, the number of patients living after an invasive cancer diagnosis has exceeded the 200,000 mark, equivalent to 1 in 24 people in Ireland. This reflects the ongoing improvement in cancer survival.
• More complete data on the impact of the COVID-19 pandemic on cancer diagnoses indicates that the pandemic resulted in a 10% reduction in cancer diagnoses (based on all cancers) or 11% (based on microscopically verified cancers) in 2020, compared to what was expected that year.
• Median age at diagnosis for all cancer combined (excluding non-melanoma skin cancers) was 69 years in men and 67 years in women, with little change over time. The median age at death for all invasive cancers combined was 74 years in both men and women, an increase compared with the median of 72 years in both men and women during 1994-1998, consistent with improved cancer survival.
• Further evidence of improvements in colorectal cancer control in men, as this cancer drops from 2nd to 3rd most common cause of cancer deaths in men.
Top five most common causes of cancer death during 2018 -2020 Males Females
Annual statistical report 2022
What are the most common cancers causing death?
The report shows that lung cancer was the leading cause of cancer death in both sexes during 20182020. In males, cancer of the prostate, colorectal, pancreas and oesophagus were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths respectively. Colorectal cancer was the 2nd most common cancer death in males during 2016-2018 but dropped to 3rd behind prostate cancer during 2018-2020.

contained in the National Cancer Strategy 2017-2026.”
These figures exclude non -melanoma skin cancers, which are rarely fatal.

Top five most common causes of cancer death during 2018 -2020 Males Females
Number of cancer survivors
In females, cancer of the breast, colorectal, ovary and pancreas were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths respectively.
The report also shows, around 207,000 cancer patients or former cancer patients were alive in Ireland at the end of 2020 (about 4.2% or one in 24 of the Irish population).
Total=207,365 (100%)
Director of the National Cancer Registry, Professor Deirdre Murray states, “One notable milestone we report this year is that, by the end of 2020, for the first time, the number of people living after an invasive cancer diagnosis had exceeded the 200,000 mark to reach 207,000. This is equivalent to 4.2% of the population, or about 1 in 24 persons in Ireland, a >50% increase in numbers of cancer survivors compared with one decade ago. This reflects both an increase in the number of people being diagnosed with cancer every year and ongoing improvements in cancer survival, as also reported here.”
The six most common cancers among cancer survivors
The report authors state, “This year’s report is the first in which we have primarily reported age-standardised incidence and mortality rates based on the ‘newer’, 2013 European Standard Population (ESP), while still retaining equivalent figures using the older 1976 standard in the appendices for continuity. Age-standardisation is one of the key methods to control for different age distributions among populations or over time, to help ensure valid comparisons between countries, regions or periods.
How many previously diagnosed cancer patients are still alive?
The top six most common cancers among survivors were: breast cancer (23% of all cancer survivors), prostate cancer (21%), colorectal cancer (115) and skin melanoma (7%), non-Hodgkin lymphoma (4%) and lung cancer (4%) which together account for 70% of all cancer survivors.
Number of cancer survivors
Total=207,365 (100%)
Chair of the NCRI Board Dr Jerome Coffey, welcomed the report and said, “These data tell a clear story of improvements in cancer survival rates over time. Combined with increasing numbers of new cases the report describes the number of people alive and well following diagnosis and treatment. This further underlines the recommendations for service developments
6
6
The six most common cancers among cancer survivors

10 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
“This change is necessary to ensure such valid comparisons but one consequence of using a different standard is that the rates based on the 2013 standard are higher than those based on the 1976 standard. This does not imply any ‘real’ change in rates or risk and, in fact, overall rates of cancer incidence (allowing for population growth and ageing) have been stable or falling over the last decade.” National Cancer Registry Ireland
Lung cancer was the leading cause of cancer death in both sexes during 2018-2020
In females, cancer of the breast, colorectal (bowel), ovary and pancreas were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths, respectively.
How many previously diagnosed cancer patients are still alive?
About 207,000 cancer patients or former cancer patients were alive in Ireland at the end of 2020 (about 4.2% or 1 in 24 of the Irish population).
The top six most common cancers among survivors were: breast cancer (23% of all cancer survivors), prostate cancer (21%), colorectal (bowel) cancer (11%) and skin melanoma (7%), non-Hodgkin lymphoma (4%) and lung cancer (4%) which together account for 70% of all cancer survivors.
National Cancer Registry Ireland
Lung cancer was the leading cause of cancer death in both sexes during 2018-2020
In males, cancer of the prostate , colorectal (bowel), pancreas and oesophagus were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths, respectively. Colorectal (bowel) cancer was the 2nd most common cancer death in males during 2016-2018, but dropped to 3rd behind prostate cancer during 2018-2020.
In females, cancer of the breast, colorectal (bowel), ovary and pancreas were the 2nd, 3rd, 4th and 5th most common categories of cancer deaths, respectively.
About 207,000 cancer patients or former cancer patients were alive in Ireland at the end of 2020 (about 4.2% or 1 in 24 of the Irish population).
The top six most common cancers among survivors were: breast cancer (23% of all cancer survivors), prostate cancer (21%), colorectal (bowel) cancer (11%) and skin melanoma (7%), non-Hodgkin lymphoma (4%) and lung cancer (4%) which together account for 70% of all cancer survivors.
These figures exclude non -melanoma skin cancers, which are rarely fatal.
full
40 mg film-coated
of
to
40 mg film-coated
each containing 40 mg of enzalutamide. Indications: The treatment of adult men with metastatic hormone-sensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy. The treatment of adult men with high-risk nonmetastatic castration-resistant prostate cancer (CRPC). The treatment of adult men with metastatic CRPC who are asymptomatic or mildly symptomatic after failure of androgen deprivation therapy in whom chemotherapy is not yet clinically indicated. The treatment of adult men with metastatic CRPC whose disease has progressed on or after docetaxel therapy. Posology and administration: Treatment with enzalutamide should be initiated and supervised by specialist physicians experienced in the medical treatment of prostate cancer. The recommended dose is 160 mg enzalutamide (four 40 mg film-coated tablets) as a single oral daily dose. The tablets should be swallowed whole with water, and can be taken with or without food. Medical castration with a luteinising hormone-releasing hormone (LHRH) analogue should be continued during treatment of patients not surgically castrated. If a patient experiences a ≥ Grade 3 toxicity or an intolerable adverse reaction, dosing should be withheld for one week or until symptoms improve to ≤ Grade 2, then resumed at the same or a reduced dose (120 mg or 80 mg) if warranted. Contraindications: Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SPC. Women who are or may become pregnant. Special warnings and precautions for use: Risk of seizure: Use of enzalutamide has been associated with seizure. The decision to continue treatment in patients who develop seizures should be taken case by case. Posterior reversible encephalopathy syndrome: There have been rare reports of posterior reversible encephalopathy syndrome (PRES) in patients receiving XTANDI. PRES is a rare, reversible, neurological disorder which can present with rapidly evolving symptoms including seizure, headache, confusion, blindness, and other visual and neurological disturbances, with or without associated hypertension. A diagnosis of PRES requires confirmation by brain imaging, preferably magnetic resonance imaging (MRI). Discontinuation of XTANDI in patients who develop PRES is recommended. Second Primary Malignancies: Cases of second primary malignancies have been reported in patients treated with enzalutamide in clinical studies. In phase 3 clinical studies, the most frequently reported events in enzalutamide treated patients, and greater than placebo, were bladder cancer (0.3%), adenocarcinoma of the colon (0.2%), transitional cell carcinoma (0.2%) and bladder transitional cell carcinoma (0.1%). Patients should be advised to promptly seek the attention of their physician if they notice signs of gastrointestinal bleeding, macroscopic haematuria, or other symptoms such as dysuria or urinary urgency develop during treatment with enzalutamide. Concomitant use with other medicinal products: Enzalutamide is a potent enzyme inducer and may lead to loss of efficacy of many commonly used medicinal products. A review of concomitant medicinal products should therefore be conducted when initiating enzalutamide treatment. Concomitant use of enzalutamide with medicinal products that are sensitive substrates of many metabolising enzymes or transporters should generally be avoided if their therapeutic effect is of large importance to the patient, and if dose adjustments cannot easily be performed based on monitoring of efficacy or plasma concentrations. Co-administration with warfarin and coumarin-like anticoagulants should be avoided. If XTANDI is co-administered with an anticoagulant metabolised by CYP2C9 (such as warfarin or acenocoumarol), additional International Normalised Ratio (INR) monitoring should be conducted. Renal impairment: Caution is required in patients with severe renal impairment as enzalutamide has not been studied in this patient population. Severe hepatic impairment: An increased half-life of enzalutamide has been observed in patients with severe hepatic impairment, possibly related to increased tissue distribution. The clinical relevance of this observation remains unknown. A prolonged time to reach steady state concentrations is however anticipated, and the time to maximum pharmacological effect as well as time for onset and decline of enzyme induction may be increased. Recent cardiovascular disease: The phase 3 studies excluded patients with recent myocardial infarction (in the past 6 months) or unstable angina (in the past 3 months), New York Heart Association Class (NYHA) III or IV heart failure except if Left Ventricular Ejection Fraction (LVEF) ≥ 45%, bradycardia or uncontrolled hypertension. This should be taken into account if XTANDI is prescribed in these patients. Androgen deprivation therapy may prolong the QT interval: In patients with a history of or risk factors for QT prolongation and in patients receiving concomitant medicinal products that might prolong the QT interval physicians should assess the benefit risk ratio including the potential for Torsade de pointes prior to initiating XTANDI. Use with chemotherapy: The safety and efficacy of concomitant use of XTANDI with cytotoxic chemotherapy has not been established. Co-administration of enzalutamide has no clinically relevant effect on the pharmacokinetics of intravenous docetaxel; however, an increase in the occurrence of docetaxel-induced neutropenia cannot be excluded. Hypersensitivity reactions: Hypersensitivity reactions manifested by symptoms including, but not limited to, rash, or face, tongue, lip, or pharyngeal oedema have been observed with enzalutamide. Severe cutaneous adverse reactions (SCARs) have been reported with enzalutamide. At the time of prescription patients should be advised of the signs and symptoms and monitored closely for skin reactions. Excipients: This medicine contains less than 1 mmol sodium (less than 23 mg) per
film-coated tablet, that is to say essentially ‘sodium-free’. Interactions: Potential for other medicinal products to affect enzalutamide exposures: CYP2C8 plays an important role in the elimination of enzalutamide and in the formation of its active metabolite. Strong inhibitors (e.g. gemfibrozil) of CYP2C8 are to be avoided or used with caution during enzalutamide treatment. If patients must be co-administered a strong CYP2C8 inhibitor, the dose of enzalutamide should be reduced to 80 mg once daily. No dose adjustment is necessary when XTANDI is co-administered with inducers of CYP2C8. CYP3A4 plays a minor role in the metabolism of enzalutamide. No dose adjustment is necessary when XTANDI is co-administered with inhibitors or inducers of CYP3A4. Potential for enzalutamide to affect exposures to other medicinal products: Enzalutamide is a potent enzyme inducer and increases the synthesis of many enzymes and transporters; therefore, interaction with many common medicinal products that are substrates of enzymes or transporters is expected. Enzymes that may be induced include CYP3A in the liver and gut, CYP2B6, CYP2C9, CYP2C19, and uridine 5’-diphospho-glucuronosyltransferase (UGTs - glucuronide conjugating enzymes). Some transporters may also be induced, e.g. multidrug resistanceassociated protein 2 (MRP2) and the organic anion transporting polypeptide 1B1 (OATP1B1). In vivo studies have shown that enzalutamide is a strong inducer of CYP3A4 and a moderate inducer of CYP2C9 and CYP2C19. The full induction potential of enzalutamide may not occur until approximately 1 month after the start of treatment, when steady-state plasma concentrations of enzalutamide are reached, although some induction effects may be apparent earlier. Patients taking medicinal products that are substrates of CYP2B6, CYP3A4, CYP2C9, CYP2C19 or UGT1A1 should be evaluated for possible loss of pharmacological effects (or increase in effects in cases where active metabolites are formed) during the first month of enzalutamide treatment, and dose adjustment should be considered as appropriate. In consideration of the long half-life of enzalutamide (5.8 days), effects on enzymes may persist for one month or longer after stopping enzalutamide. A gradual dose reduction of the concomitant medicinal product may be necessary when stopping enzalutamide treatment. In vitro data indicate that enzalutamide may be an inhibitor of the efflux transporter P-gp. A mild inhibitory effect of enzalutamide, at steadystate, on P-gp was observed in a study in patients with prostate cancer that received a single oral dose of the probe P-gp substrate digoxin before and concomitantly with enzalutamide (concomitant administration followed at least 55 days of once daily dosing of 160 mg enzalutamide). The AUC and Cmax of digoxin increased by 33% and 17%, respectively. Medicinal products with a narrow therapeutic range that are substrates for P-gp (e.g. colchicine, dabigatran etexilate, digoxin) should be used with caution when administered concomitantly with XTANDI and may require dose adjustment to maintain optimal plasma concentrations. At steady state, enzalutamide did not cause a clinically meaningful change in exposure to the probe breast cancer resistance protein (BCRP) substrate rosuvastatin in patients with prostate cancer that received a single oral dose of rosuvastatin before and concomitantly with enzalutamide (concomitant administration followed at least 55 days of once daily dosing of 160 mg enzalutamide). The AUC of rosuvastatin decreased by 14% while Cmax increased by 6%. No dose adjustment is necessary when a BCRP substrate is co administered with XTANDI. Based on in vitro data, inhibition of MRP2 (in the intestine), as well as organic anion transporter 3 (OAT3) and organic cation transporter 1 (OCT1) (systemically) cannot be excluded. Theoretically, induction of these transporters is also possible, and the net effect is presently unknown. Since androgen deprivation treatment may prolong the QT interval, the concomitant use of XTANDI with medicinal products known to prolong the QT interval or medicinal products able to induce Torsade de pointes should be carefully evaluated. Fertility, pregnancy and lactation: There are no human data on the use of XTANDI in pregnancy and this medicinal product is not for use in women of childbearing potential. This medicine may cause harm to the unborn child or potential loss of pregnancy if taken by women who are pregnant. It is not known whether enzalutamide or its metabolites are present in semen. A condom is required during and for 3 months after treatment with enzalutamide if the patient is engaged in sexual activity with a pregnant woman. If the patient engages in sexual intercourse with a woman of childbearing potential a condom and another form of birth control must be used during and for 3 months after treatment. Enzalutamide is not for use in women. Enzalutamide is contraindicated in women who are, or who may become, pregnant. It is not known if enzalutamide is present in human milk. Enzalutamide and/or its metabolites are secreted in rat milk. Animal studies showed that enzalutamide affected the reproductive system in male rats and dogs. Effects on ability to drive and use machines: XTANDI may have a moderate influence on the ability to drive and use machines as psychiatric and neurologic events including seizure have been reported. Patients should be advised of the potential risk of experiencing a psychiatric or neurological event while driving or operating machines. Undesirable effects: Summary of the safety profile: The most common adverse reactions are asthenia/ fatigue, hot flush, hypertension, fractures, and fall. Other important adverse reactions include ischemic heart disease and seizure. Seizure occurred in 0.5% of enzalutamidetreated patients, 0.2% of placebo-treated patients, and 0.3% in bicalutamide-treated patients. Rare cases of posterior reversible encephalopathy syndrome have been reported in enzalutamide-treated patients. Adverse reactions observed during clinical studies are listed below by frequency category. Frequency categories are defined as follows: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥
XTANDI™ is indicated for the treatment of adult men with metastatic castrationresistant prostate cancer who are asymptomatic or mildly symptomatic after failure of androgendeprivation therapy, in whom chemotherapy is not yet clinically indicated.1
XTANDI is also indicated for the treatment of adult men with metastatic castration – resistant prostate cancer whose disease has progressed on or after docetaxel.1






1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000); not known (cannot be estimated from the available data). Adverse reactions are presented according to Medical Dictionary for Regulatory Activities (MedDRA) system organ classification and within each frequency grouping they are presented in order of decreasing seriousness. Adverse reactions identified in controlled clinical trials and post-marketing: Blood and lymphatic system disorders: Uncommon: leucopenia, neutropenia; Not known*: thrombocytopenia. Immune system disorders: Not known*: face oedema, tongue oedema, lip oedema, pharyngeal oedema Psychiatric disorders: Common: anxiety; Uncommon: visual hallucination. Nervous system disorders: Common: headache, memory impairment, amnesia, disturbance in attention, dysgeusia, restless legs syndrome; Uncommon: cognitive disorder, seizure¥; Not known*: posterior reversible encephalopathy syndrome. Cardiac disorders: Common: ischemic heart disease†; Not known*: QT-prolongation. Vascular disorders: Very common: hot flush, hypertension. Gastrointestinal disorders: Not known*: nausea, vomiting, diarrhoea. Skin and subcutaneous tissue disorders: Common: dry skin, pruritus; Not known*: erythema multiforme, rash. Musculoskeletal and connective tissue disorders: Very common: fractures‡ Not known*: myalgia, muscle spasms, muscular weakness, back pain. Reproductive system and breast disorder: Common: gynaecomastia. General disorders and administration site conditions: Very common: asthenia, fatigue. Injury, poisoning and procedural complications: Very common: fall. * Spontaneous reports from post-marketing experience. ¥ As evaluated by narrow Standardised MedDRA Queries (SMQs) of ‘Convulsions’ including convulsion, grand mal convulsion, complex partial seizures, partial seizures and status epilepticus. This includes rare cases of seizure with complications leading to death. † As evaluated by narrow SMQs of ‘Myocardial Infarction’ and ‘Other Ischemic Heart Disease’ including the following preferred terms observed in at least two patients in randomized placebo-controlled phase 3 studies: angina pectoris, coronary artery disease, myocardial infarctions, acute myocardial infarction, acute coronary syndrome, angina unstable, myocardial ischaemia and arteriosclerosis coronary artery. ‡ Includes all preferred terms with the word ‘fracture’ in bones. Description of selected adverse reactions: Seizure: In controlled clinical studies, 24 patients (0.5%) experienced a seizure out of 4403 patients treated with a daily dose of 160 mg enzalutamide, whereas four patients (0.2%) receiving placebo and one patient (0.3%) receiving bicalutamide experienced a seizure. Dose appears to be an important predictor of the risk of seizure, as reflected by preclinical data, and data from a doseescalation study. In the controlled clinical studies, patients with prior seizure or risk factors for seizure were excluded. In the 9785 CL 0403 (UPWARD) single-arm trial to assess incidence of seizure in patients with predisposing factors for seizure (whereof 1.6% had a history of seizures), 8 of 366 (2.2%) patients treated with enzalutamide experienced a seizure. The median duration of treatment was 9.3 months. The mechanism by which enzalutamide may lower the seizure threshold is not known but could be related to data from in vitro studies showing that enzalutamide and its active metabolite bind to and can inhibit the activity of the GABA-gated chloride channel. Ischemic Heart Disease: In randomized placebo-controlled clinical studies, ischemic heart disease occurred in 3.9% of patients treated with enzalutamide plus ADT compared to 1.5% patients treated with placebo plus ADT. Fifteen (0.4%) patients treated with enzalutamide and 2 (0.1%) patients treated with placebo had an ischemic heart disease event that led to death. Prescribers should consult the full SPC in relation to other adverse reactions. Overdose: There is no antidote for enzalutamide. In the event of an overdose, treatment with enzalutamide should be stopped and general supportive measures initiated taking into consideration the half-life of 5.8 days. Patients may be at increased risk of seizures following an overdose. Cost (excluding VAT): XTANDI 40 mg film-coated tablets x 112: Ireland: POA. Northern Ireland: £2,734.67. Legal classification: Ireland: POM/S1A. Northern Ireland: POM. Marketing Authorisation number: XTANDI 40 mg film-coated tablets: EU/1/13/846/002 Marketing Authorisation Holder: Astellas Pharma Europe B.V., Sylviusweg 62, 2333 BE Leiden, The Netherlands. Date of preparation of Prescribing Information: October 2022 Job number: MAT-UKE-XTD-2022-00004 Further information is available on request from: Ireland: Astellas Pharma Co. Ltd., Tel.: +353 1 467 1555. Northern Ireland: Astellas Pharma Ltd, Medical Information 0800 783 5018. For full prescribing information, refer to the SPCs, which may be found at www.medicines.ie (Ireland) and https://www.emcmedicines.com/en-GB/ northernireland/ (Northern Ireland).
Adverse events should be reported. For Ireland, Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance, Website: www.hpra.ie or Astellas Pharma Co. Ltd. Tel: +353 1 467 1555, E-mail: irishdrugsafety@astellas.com
Adverse events should be reported. For Northern Ireland, reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Astellas Pharma Ltd on 0800 783 5018.

MAT-IE-XTD-2022-00015 Date of preparation: November 2022
Ireland and Northern Ireland Abbreviated Prescribing Information: XTANDITM (enzalutamide)
tablets. For
prescribing information, refer
the Summary
Product Characteristics (SPC). Presentation:
tablets
References: 1. XTANDI Summary of Product Characteristics.
University Hospital Galway hosts world renowned surgeon

University Hospital Galway was delighted to welcome world pioneering surgeon, Professor Diego González Rivas to the hospital last week. During his visit Professor González Rivas, delivered a lecture to colleagues across the Department of Cardiothoracic Surgery entitled “The Journey of Uniportal Thoracic Surgery from VATS to Robotics”. He also performed two complex cases alongside the cardiothoracic team in UHG.
Prof González Rivas is a world renowned surgeon who has
pioneered multiple innovative techniques over the years. He was the first surgeon in the world to perform the single-port VATS technique. The single-port VATS surgical technique is innovative at world level, since it enables the removal of more complex tumours through a single incision. He also has over 15 years’ experience in performing major single-port lung surgery in patients without intubation and with spontaneous breathing.
Dr. González-Rivas was the pioneer surgeon in the world performing uniportal VATS lobectomies. Research has shown that patients who have undergone this surgery have demonstrated less postoperative pain, a speedier recovery and superior cosmetic results when compared to those where a multi-port approach was utilised.
He is the creator of the minimally invasive thoracic surgery unit in La Coruna, Spain and is the director of the uniportal VATS training programme at Shanghai Pulmonary Hospital which is the biggest thoracic centre in the world.
He is also a leader in the field of robotic surgery, performing singleport resections using an articulated robotic arm, without the need of an assistant and has developed single port robotic lung resection surgery (URATS) performing the first case in September 2021.

Patients Deserve Better Campaign
The Parkinsons Association of Ireland has been working with the Neurological Alliance of Ireland as part of their “Patients Deserve Better” campaign to call for action to tackle the shortage of one hundred specialist nurses in neurology services.
The Patients Deserve Better campaign is calling for 20
additional nurses each year over the next five years to tackle this shortfall.
A very successful pre budget campaign led to 23 additional nurses being announced in this years Budget. These additional nurses will cover conditions including multiple sclerosis, Parkinson’s disease, epilepsy and
rare neurological conditions and will be assigned by the National Clinical Programme in Neurology all across Ireland in response to local needs.
Mags Rogers, Executive Director of the Neurological Alliance of Ireland has highlighted the key role of collective patient advocacy in this campaign stating, “Over
Speaking about his visit to UHG, Prof González Rivas said, “I really enjoyed the hospitality of the Galway team. There is an excellent team here performing excellent surgery and I would like to be back for further collaboration.”
Welcoming the surgeon to the hospital, Mr Alan Soo, Consultant Cardiothoracic Surgeon and Clinical Lead for the Saolta University Health Care Group said, “We are delighted to host Prof González Rivas at UHG. Events such as these give us the opportunity to showcase the surgical capabilities of the unit. It also promotes collaboration and exchange of technique/ideas not limited to just surgery but anesthesia and nursing. These events promote shared learning among teams and inspire people to work together to develop a better health service.”
11,000 e mails have been sent to TD’s via the campaign website patientsdeservebetter.ie since the campaign was launched in October 2021. This is an example of how organisation’s representing people with neurological conditions can work together with these individuals and their families to achieve real change.”
12 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
News
Professor Diego González Rivas
Dr Alan Soo, Consultant Cardiothoracic Surgeon and Clinical Lead for the Saolta University Health Care Group with Professor Diego González Rivas at University Hospital Galway
Focusing on Medicines Access
David Delaney, Head of Policy and Market Access, Europe with Viatris and Medicines for Ireland recently met with the U.S. Embassy Ireland’s Anood Taqui in Dublin.

He said, “As Medicines for Europe and Medicines for Ireland are the representatives of the makers of the majority of medicines supplied daily in Europe, I was delighted to focus today on two common priority areas.”
“The first was the United State’s and Ireland’s trade relationship, one of the largest globally, with bilateral investment and trade totaling $1.6Trillion annually.
“The second area focused specifically on pharmaceuticals and stable patient access to medicines flowing globally from Ireland, discussing how to overcome challenges such as inflation spikes and medicines availability across the EU.
“Thanks again to U.S. Ambassador to Ireland Claire D Cronin and the U.S. Commercial Service team at the U.S. Embassy Dublin, including the indefatigable Padraig O'Connor , who help our member companies grow exports, jobs, investments and our links to U.S. companies supplying us in Ireland. And, to their colleagues, always
Next Steps for Elective Hospitals
David Delaney, Head of Policy and Market Access, Europe with Viatris and Medicines for Ireland with the U.S. Embassy Ireland’s Anood Taqui
ready to help us via 70+ other U.S. Commercial Service offices, 24/7 across the globe.
“With partners like this, the future looks bright for our sector in Ireland and patients globally who count on access to Ireland’s pharma exports ($67Billion in 2021).”
The Minister for Health Stephen Donnelly has received government approval of the next stage of the Enhanced Provision of Elective Care Programme and progression of the development of new Elective Hospitals in Cork and Galway.
The development of elective hospitals will change the way in which day case, scheduled procedures, surgeries, scans and outpatient services can be better arranged across the country, ensuring greater capacity in the future and help to address waiting lists.
The preferred sites, recommended to be brought forward into the planning phase, are at St Stephen's Hospital, Sarsfield Court in Cork, and Merlin Park in Galway.
The Cork centre with nine planned theatres, seven endoscopy suites and nine minor operation rooms is expected to provide 180,000 extra procedures, treatments and diagnostic appointments per year.
The Galway centre with a planned eight operating theatres, seven endoscopy suites and nine minor operation rooms is forecast to provide 175,000 additional procedures, treatments and diagnostic appointments per year.
The new national Elective Hospitals will be located in Cork, Galway and Dublin, and will provide services for the whole population, extending well beyond their traditional ‘catchment’ area. An announcement on the Dublin project will follow once its associated Business Case has been approved by Government.
New Videofluoroscopy Service for Portiuncula University Hospital

Portiuncula University Hospital (PUH) recently introduced a new Videofluoroscopy Service for patients in the hospital who need an x-ray assessment of their swallow.
The videofluoroscopy service is led out by the Speech and Language Therapy Department in partnership with the Radiology Department.
Videofluoroscopy is an x-ray examination of the swallow and is considered the ‘gold standard’ for the assessment of swallowing difficulties and can be used for assessment, diagnosis and management of dysphagia.
Dysphagia is the medical term used to describe swallowing difficulties which may be a symptom of a range of medical conditions such as; stroke, progressive neurological conditions for example Dementia and Parkinson’s disease, respiratory conditions, head and neck cancer, physical and
Fom left, Aisling Ní Cheannabháin, Speech and Language Therapist; Fiona Brennan, Senior Speech and Language Therapist; Anne-Marie Johnston, Senior Speech and Language Therapist; Sophie Linehan, Senior Speech and Language Therapist and Colette Ward, Senior Speech and Language Therapist
intellectual disabilities, palliative care needs or the normal ageing process.
Speaking about the new videofluoroscopy service at the hospital, Fiona Brennan, Senior Speech and Language Therapist said, “On behalf of myself and the team we are delighted to be able to offer this service to our in-patients at PUH as previously patients had to travel elsewhere for the procedure, which took time and was an inconvenience for patients. Having videofluoroscopy
will not only improve the range of services we can deliver but also provide a more positive experience for the patient.”
Caroline Hanrahan, Radiography Services Manager said, “We are delighted to work with our colleagues in the Speech and Language Therapy Department on this important diagnostic service.”
Keane,
“The new videofluoroscopy service will deliver the latest technology to patients and facilitate a more patient-centred approach to care. This new service will improve rehabilitation outcomes and support patients to return to their normal lives outside of hospital.”
13 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
News
James
Hospital Manager said,
Government on Course to Miss Targets
The Irish Hospital Consultants Association (IHCA) has today (Thursday 15th December 2022) expressed dismay that the Government is on course to miss waiting list reduction targets for 2022 by a significant margin.
Minister for Health Stephen Donnelly launched the ¤350 million Waiting List Action Plan for 2022 in February1 which committed to reducing active waiting lists for acute scheduled care by 18% (more than 132,00) by the end of this year. However, figures show that by the end of November the numbers of those waiting for treatment will have only dropped by a modest 1.6% (fewer than 11,500).
The latest NTPF figures confirm that the number of people on the
three main waiting lists decreased from 720,056 at the start of 2022 to 708,590 at the end of November, nowhere near the planned 18% reduction.2
Overall, a total of 887,500 people were on some form of hospital waiting list at the end of November, including almost 97,000 children.
When the additional 243,000 people awaiting CTs, MRIs or ultrasounds nationally are added, 3 the total number awaiting hospital care is over 1.1 million –or more than one-fifth of the entire population.
Analysis from the IHCA shows that the HSE is now more than 120,800 outpatient appointments and procedures away from meeting
the 18% reduction targets for the end of this year, including 115,100 outpatient appointments and around 5,700 inpatient or day case procedures and GI scopes.
IHCA President Professor Robert Landers said that filling the over 900 vacant Consultant posts is one target which simply cannot be missed.
“As Consultants, we need and want sustainable solutions to provide care to the 1.1 million people awaiting essential diagnostics or treatments in our hospitals. Increases in waiting times for planned procedures can mean patients’ conditions may significantly deteriorate, hampering their quality of life and potentially their future health outcomes.
Guiding Misinformation in Eczema
Cathal O’Connor
and misinformation around atopic eczema with the panel offering tips on what to look out for and also what to avoid.
Dermatologists in the country we have large waiting lists.”
“However, it is clear from these latest figures that the Government continues to fall short on actions and promises, leaving hospitals and Consultants unable to provide the timely access to care our patients need and deserve.
“With a record 918 permanent Consultant posts nationally either vacant or filled on a temporary or agency basis, an increase of 190 unfilled Consultant posts (+26%) in past two years, it is imperative that all vacant Consultant posts are filled and overall numbers are increased to at least the EU average if we are to effectively address the record hospital waiting lists.
“Failure to do this will mean it could take decades to tackle our waiting lists.”
The Irish Skin Foundation recently held a webinar on ‘Myth busting – Misinformation in Eczema’ which took place on World Eczema Day to help raise awareness of one of the world’s most prevalent skin conditions. The theme of the event focussed on some of the biggest myths
The panel included advocate and broadcaster, Paul Harriott, Professor Michelle Murphy, Consultant Dermatologist, SIVUH, Cork and Dr Cathal O’Connor, Clinical Research Fellow in Paediatric Dermatology, SIVUH, Cork.
Dr O’Connor said, “Right now is a very exciting time to be working within the field of eczema as we have so many excellent new treatments coming out. Unfortunately with only having too few Consultant


Dr O’Connor discussed his recent paper which was entitled ‘Scratching the surface: A review of online misinformation and conspiracy theories in atopic dermatitis’ and explained the thinking behind the research which was to help guide patients and families vulnerable to misinformation given the severe impact of atopic dermatitis on quality of life.
Eczema is non-contagious and affects approximately one in five children and one in 10 adults in Ireland.
The exact causes are unknown but genetics, defects in the skin
barrier, environmental allergens and abnormal function of the immune system all contribute.
Atopic eczema is the most common form and may run in families.
Symptoms include itchy, dry and scaly skin and/or a rash — all of which can be painful and become infected. The pigmentation of people with darker skin may also be affected.
Atopic eczema can be triggered by exposure to environmental irritants or allergens including cosmetics, detergents, toiletries, dust mites, pollen, animal dander and certain clothing materials.
It can also be triggered by emotional stress, and if the skin gets infected.
14 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Dr
This is a promotional advertisement from LEO Pharma for IE healthcare professionals.
For the treatment of moderate to severe atopic dermatitis in adult and adolescent patients 12 years and older who are candidates for systemic therapy1

TIME TO PRESS PLAY
Adtralza®

The first licensed biologic that inhibits IL-13 alone, 1,2 a key driver of atopic dermatitis signs and symptoms.3

Learn more at www.adtralza.ie
Prescribing Information for
pre-filled syringe
Adtralza® (tralokinumab) 150 mg
solution for injection in
Please refer to the full Summary of Product Characteristics (SmPC) (www.medicines.ie) before prescribing.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Indications: Treatment of moderate-tosevere atopic dermatitis in adult and adolescent patients 12 years and older who are candidates for systemic therapy. Active ingredients: Each pre-filled syringe contains 150 mg of tralokinumab in 1 mL solution (150 mg/mL). Dosage and administration: Posology: The recommended dose of tralokinumab for adult and adolescent patients 12 years and older is an initial dose of 600 mg (four 150 mg injections) followed by 300 mg (two 150 mg injections) administered every other week as subcutaneous injection. Every fourth week dosing may be considered for patients who achieve clear or almost clear skin after 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve further with continued treatment every other week beyond 16 weeks. Tralokinumab can be used with or without topical corticosteroids. The use of topical corticosteroids, when appropriate, may provide an additional effect to the overall efficacy of tralokinumab. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas. If a dose is missed, the dose should be administered as soon as possible and then dosing should be resumed at the regular scheduled time. No dose adjustment is recommended for elderly patients, patients with renal impairment or patients with hepatic impairment. For patients with high body weight (>100 kg), who achieve clear or almost clear skin after 16 weeks of treatment, reducing the dosage to every fourth week might not be appropriate. The safety and efficacy of tralokinumab in children below the age of 12 years have not yet been established. Method of administration: Subcutaneous use. The pre-filled syringe should not be shaken. After removing the pre-filled syringes from the refrigerator, they should be allowed to reach room temperature by waiting for 30 minutes before injecting. Tralokinumab is administered by subcutaneous injection into the thigh or abdomen, except the 5 cm around the navel. If somebody else administers the injection, the upper arm can also be used. For the initial 600 mg dose, four 150 mg tralokinumab injections should be administered consecutively in different injection
IL, interleukin.
sites within the same body area. It is recommended to rotate the injection site with each dose. Tralokinumab should not be injected into skin that is tender, damaged or has bruises or scars. A patient may self-inject tralokinumab or the patient’s caregiver may administer tralokinumab if their healthcare professional determines that this is appropriate. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: If a systemic hypersensitivity reaction (immediate or delayed) occurs, administration of tralokinumab should be discontinued and appropriate therapy initiated. Patients treated with tralokinumab who develop conjunctivitis that does not resolve following standard treatment should undergo ophthalmological examination. Patients with pre-existing helminth infections should be treated before initiating treatment with tralokinumab. If patients become infected while receiving tralokinumab and do not respond to antihelminth treatment, treatment with tralokinumab should be discontinued until infection resolves. Live and live attenuated vaccines should not be given concurrently with tralokinumab. Fertility, pregnancy and lactation: There is limited data from the use of tralokinumab in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure, it is preferable to avoid the use of tralokinumab during pregnancy. It is unknown whether tralokinumab is excreted in human milk or absorbed systemically after ingestion. Animal studies did not show any effects on male and female reproductive organs and on sperm count, motility and morphology.
Side effects: Very common (≥1/10): Upper respiratory tract infections. Common (≥1/100 to <1/10): conjunctivitis, conjunctivitis allergic, eosinophilia, injection site reaction. Uncommon (≥1/1,000 to <1/100): keratitis. Precautions for storage: Store in a refrigerator (2°C-8°C). Do not freeze. Store in the original package in order to protect from light. Legal category: POM. Marketing authorisation number and holder: EU/1/21/1554/002. LEO Pharma A/S, Ballerup, Denmark. Last revised: October 2022
Reference number: REF-22407
Reporting of Suspected Adverse Reactions
Adverse events should be reported.
Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie
Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com

References: 1. Adtralza® SPC. 2. Duggan S. Drugs 2021;81(14):1657–1663. 3. Bieber T. Allergy 2020;75:54–62.
Further information can be found in the Summary of Product Characteristics or from: LEO Pharma, Cashel Road, Dublin 12, Ireland.
E-mail: medical-info.ie@leo-pharma.com
® Registered trademark
Date of preparation: December 2022 IE/MAT-62263
Not an actual patient. For illustrative purposes only. Individual results may vary.
Biosimilars
Biological medicines: overview
Biosimilar medicines and their use in the treatment of cancer
Written by: Rebecca Parkin, AnneMarie DeFrein and Patricia Heckmann, National Cancer Control Programme
Introduction
Biological medicines (‘biologicals’) contain active substances from a biological source, such as living cells or organisms. Biological medicines are well established in clinical practice and in many cases they are indispensable for the treatment of serious and chronic conditions such as diabetes, autoimmune diseases and cancers.
cell systems and recombinant DNA technology. The EU legislation imposes strict requirements for the manufacture of all medicines:
(SACT ) and maximise funding for new medicines. The introduction of biosimilars for the treatment of cancer into the healthcare market in Ireland is welcomed by the National Cancer Control Programme (NCCP). In 2017, the NCCP convened a multidisciplinary working group, involving key stakeholders, to develop and agree a national guidance document on the use of biosimilars in the treatment of cancer.6
Key features of biological medicines
Most biological medicines in current clinical use contain active substances made of proteins. These can differ in size and structural complexity, from simple proteins like insulin or growth hormone to more complex ones such as coagulation factors or monoclonal antibodies (figure 1).
This article aims to outline the background to the development and approval of biosimilars, with a focus on their role in the treatment of cancer.
What are biological medicines and biosimilars?



Biomanufacturing strictly regulated
The manufacture of biological medicines tends to be more complex than for chemically-derived molecules. Most biological medicines are made by biotechnology, often using sophisticated
Cancer is recognised as a major health concern. The number of cancers diagnosed annually in Ireland is rising and the significant cost of new drug treatments for cancer, including biological medicines (or biologicals), has been cited as an ongoing challenge.1, 2 In recent years, in addition to their increased use in other diseases, biologicals have become a vital part of treatment for cancer, as both therapeutic and supportive care agents.3, 4 However, the high cost of these treatments places a significant burden on healthcare systems. The expiration of patents for biologicals has allowed the development of biosimilar medicines (biosimilars). A regulatory framework for the approval of biosimilars was established in Europe in 2003, with the USA implementing a framework in 2009.3 Since the introduction of the first biosimilar by the European Medicines Agency (EMA) in 2006, a number of biosimilars have been approved and safely used in the treatment of cancer.5 Their approval has led to increased market competition, resulting in a reduced cost burden and enabling accessibility of treatments to patients with cancer.5 As such, biosimilars represent one of the ways to obtain sustainability of systemic anti-cancer therapy
A biological medicine (or biological) is a medicine that contains an active substance made by a biological process or derived from a biological source.7 Biologicals include proteins such as hormones (erythropoietins, insulins and growth hormones) or enzymes that are naturally produced in the human body, and monoclonal antibodies. They may also be blood products, immunological medicinal products and advanced technology products such as gene and cell therapies.8 They vary in size and structural complexity, from simple proteins such as insulin or growth
hormone to more complex ones such as coagulation factors or monoclonal antibodies (see Figure 1).
Are biosimilars the same as generic medicines?
� EU manufacturers must hold a manufacturer’s license and are legally obliged to comply with Good Manufacturing Practice (GMP), the agreed standards to obtain a medicine with proven quality.
A biosimilar is a biological medicine that is highly similar to another biological (also known as a reference biological medicine) in terms of its quality, safety and efficacy.6, 7 A reference biological medicine is one which already has a marketing authorisation and has been approved for use in patients. Biosimilars contain a version of the active substance of an approved reference biological and should be used in the same way as the reference biological and in accordance with its Summary of Product Characteristics (SmPC).6, 7
� National regulatory authorities in the EU regularly inspect manufacturing sites for compliance with GMP requirements.

No, they are not the same. Generics are small-molecule drugs made from a fixed chemical structure that can be easily replicated, e.g. methotrexate, whereas biosimilars of advanced biologicals are much larger, complex molecules derived from living cells with inherent natural variability, e.g. monoclonal antibodies such as rituximab (see Figure 2). Because of their biological and variable nature, biosimilars cannot be regarded as generic medicines. As such, it should be remembered that “biosimilar” is a regulatory term, not a scientific one.6
How are biosimilars approved?
� If some manufacturing steps take place outside the EU, then non-EU manufacturers, importers and wholesale distributors are obliged to follow the same strict requirements and are also regularly inspected.
For biological medicines, some of the GMP requirements have been adapted to take into account their specific nature (e.g. use of appropriate aseptic techniques, refrigeration and other storage conditions, stability, transport etc.).
Biosimilars are reviewed by the EMA through a centralised procedure, following an application from the pharmaceutical company. The EMA provide a scientific opinion to the European Commission who approve the biosimilar medicine and grant it an EU-wide marketing authorisation. The legal framework and regulatory pathway agreed in the EU outlines that biosimilars
Figure 1. Examples of types of proteins in biological medicines approved in the EU
All biologicals have an inherent degree of natural variability due to their biological nature, i.e. no two batches of a product will be the exact same. This is also true for biosimilars, which will never be an exact copy of their biological counterparts because of the complex process needed for their production.5 EMA approval indicates that any differences between a biosimilar and its reference biological will have been shown not to affect safety or effectiveness,5 through a rigorous comparability exercise which is carried out at quality, non-clinical and clinical levels.
16 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Monoclonal antibody 150,000 daltons Growth hormone 22,000 daltons Insulin 5,808 daltons
Figure 1 Examples of different protein types in biologicals approved in the EU Adapted from5



















Figure 2 Molecular weight comparisons: small-molecule drugs versus larger biologicals. Not drawn to scale. Ave: Average; Da: Daltons; EPO: Erythropoietin; mAbs: Monoclonal antibodies Adapted from9
clinical studies are not required for all other approved indications.
This extrapolation of indications, versus a clinical trial, may have led to some initial hesitancy in the adoption of biosimilars. Research in the US has shown that uptake can be slow initially due to challenges such as policy change and lack of patient and provider education.12 An Irish study carried out in 2017 highlighted concerns held by medical specialists in relation to biosimilars and emphasised the need for educational initiatives.13 However, there is a growing body of evidence to support their effectiveness, with uptake in some countries near 100%.14 Increased uptake may be driven by financial incentives offered to encourage biosimilar prescribing.15
Interchangeability and substitution

In the context of biosimilars and reference biological medicines, it is important to be aware of the terminology for interchangeability and substitution practices in the EU.5

must be manufactured to the same quality standards as the reference biological medicine.5, 6 Since 2006, biosimilar versions of several reference biologicals have been approved in the EU and many are available in Ireland. In the Irish setting, after a biosimilar has gained EU approval, the company may submit a reimbursement application to the HSE, in line with the criteria outlined in the Framework Agreement on the Supply and Pricing of Generic, Biosimilar and Hybrid Medicines (2021).10
Animportantpointtobearinmindduringthedevelopmentofbiosimilarsistheunderstandingofthedifference betweencomparabilityandbiosimilarity.Comparabilityreferstoapostapprovalmanufacturingchangemadetoa biologicproductbytheproduct’smanufacturer.Suchchangestothemanufacturingprocessarerelativelycommon andmayincludeachangeinmanufacturingequipmentorchangesmadetoscale-upyieldortoreduceimpurities, andsoforth.Comparabilityoftheproductsbeforeandafterthemanufacturingchangeshavebeenmadeassuresthat theproducthasretaineditsattributes.Thus,thereisnoexpectationofadverseeffectonthesafetyorefficacyofthe productafterthechange [13].Suchmanufacturingchangesaresuccessfullymadebecausetheproductmanufacturer hasanexhaustiveunderstandingoftheproduct,includingitsdevelopmenthistory.Thisdoesnotimplythatover timethebiologicbecomesabiosimilarofitself.Biosimilarity,ontheotherhand,describestheconceptwhereby amanufacturerdevelopsamoleculetobesimilarinstructureandfunctiontoanexistingproduct,theRP.A biosimilarismanufacturedanewwithoutanypriorknowledgeofthedevelopmentalhistoryoftheRP;forthis reason,abiosimilarcanneverbeanexactreplicaofitsRP.Inthiscase,demonstrationofbiosimilarityisbasedona comprehensivecomparativecharacterizationoftheproposedbiosimilarthatisproducedusinganew linedevelopedbyanew/differentmanufacturer.






Extrapolation of indications
For the approval of biosimilars, a process called extrapolation of indications is used. This is where a biosimilar is approved for use in the same indication held by the reference biological medicine, even though it has not been directly studied in a comparative clinical trial.11 For a biosimilar to gain approval, it needs to demonstrate that there are no clinically meaningful differences relative to the reference medicine, in a sensitive patient population. Once comparability has been demonstrated in one indication (i.e. the most sensitive), confirmative
18 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Biosimilars
Biosimilars:whattheoncologistshouldknow Review Larger biologicals – More complex protein molecules – Produced in living cells – Have immunogenic potential Large biologicals – Complex protein molecules – Produced in living cells – Have immunogenic potential Small-molecule drugs – Simple, well-characterized chemical structure – Produced by chemical synthesis – Mostly non-immunogenic <100,000 Da <10,000 Da -100 Da mAbs -168,000 GCSF 18,800 Molecular mass HGH 22,000 Enoxaparin 4500 [mm] Insulin -3800 Acetylsalicylic acid 180 EPO 30,000 Figure1.Molecularweightcomparisons:small-moleculedrugsversuslargerbiologicals. Adaptedfrom [13] Ave:Average;Da:Daltons;EPO:Erythropoietin;mAbs:Monoclonalantibodies. RPcanimpactpharmacologicactivityandimmunogenicpotentialaswellasclinicalefficacyandsafetyofthe biosimilar,itisimportanttoidentifyandquantifythemearlyintheproductdevelopmentprocess
futuresciencegroup www.futuremedicine.com 1149
Interchangeability
The EMA defines interchangeability as ‘the possibility of exchanging one medicine for another medicine that is expected to have the same clinical effect. This could mean replacing a reference biological medicine with a biosimilar (or vice versa) or replacing one biosimilar with another’.5 This process must be carried out under the supervision of a physician. Hospital biosimilar policy should detail the process to be followed when changing a patient from one biological medicine to another.6, 7 This may include supplying the patient with dedicated patient information on the use of biosimilars and / or a discussion between the patient and prescribing physician.7
In September 2022, the EMA and the Heads of Medicines Agencies (HMA) issued a joint statement confirming that biosimilars approved in the EU are interchangeable (at prescriber level) with their reference medicine or with an equivalent biosimilar. While already practised in many Member States, this joint position on interchangeability harmonises the EU approach. Decisions regarding substitution at pharmacy-level, however, are managed by individual Member States.16
Substitution (at pharmacy level)
Unlike small-molecule drugs , biological medicines cannot be substituted at pharmacy level. The Health (Pricing and Supply of Medical Goods) Act 2013 specifically excludes biological medicines (including biosimilars) from being substituted at pharmacy level.17
Biosimilars in Ireland
In January 2016, the HSE Medicines Management Programme (HSE-MMP) highlighted the potential for biosimilars to significantly reduce drug expenditure and facilitate greater access to such treatments.18 On the introduction of a biosimilar to the Irish market, the 2021 Framework Agreement on the Supply and Pricing of Medicines provides for an automatic price reduction of 37.14% for patent-expired, non-exclusive biologicals in addition to the agreed rebates.10, 19 Since 2016, the HSE-MMP has supported the appropriate introduction of biosimilars into clinical use in Ireland, including implementing a prescribing initiative, which has resulted in a significant increase in biosimilar uptake in Ireland since 2019.18, 19
In 2018, the MMP commenced the best-value biological (BVB) medicine initiative to evaluate
different formulations has been a particular challenge to the uptake of some biosimilars used in the treatment of cancer. For example, rituximab and trastuzumab have both intravenous and subcutaneous formulations available for administration but a biosimilar is only available in intravenous form.
different formulations has been a particular challenge to the uptake of some biosimilars used in the treatment of cancer. For example, rituximab and trastuzumab have both intravenous and subcutaneous formulations available for administration but a biosimilar is only available in intravenous form.
Table 1 Biosimilars licensed in Ireland for supportive care agents used in the treatment of cancer Supportive care agent Cancer treatment indications
Table 1 Biosimilars licensed in Ireland for supportive care agents used in the treatment of cancer
Table 1 Biosimilars licensed in Ireland for supportive care agents used in the treatment of cancer Supportive care agent Cancer treatment indications
Filgrastim
Date of 1st approval*
Date of 1st approval*
• Management of neutropenia related to cancer treatment
Filgrastim • Management of neutropenia related to cancer treatment
• Mobilisation of peripheral blood progenitor cells 2008
• Mobilisation of peripheral blood progenitor cells 2008
Pegfilgrastim • Neutropenia related to cancer treatment 2018
Pegfilgrastim • Neutropenia related to cancer treatment 2018
Epoetin alpha • Anaemia related to cancer treatment 2009
Epoetin alpha • Anaemia related to cancer treatment 2009
Epoetin zeta • Anaemia related to cancer treatment 2007
Epoetin zeta • Anaemia related to cancer treatment 2007
Table 2 Biosimilars licensed in Ireland for monoclonal antibodies used in the treatment of cancer
Table 2 Biosimilars licensed in Ireland for monoclonal antibodies used in the treatment of cancer Monoclonal antibody Cancer treatment indications
Table 2 Biosimilars licensed in Ireland for monoclonal antibodies used in the treatment of cancer Monoclonal antibody Cancer treatment indications
Rituximab
Rituximab
Date of 1st approval*
Date of 1st approval*
• Non-Hodgkin's lymphoma and chronic lymphocytic leukaemia 2017
• Non-Hodgkin's lymphoma and chronic lymphocytic leukaemia 2017
Trastuzumab • HER2-positive metastatic breast cancer
Trastuzumab • HER2-positive metastatic breast cancer
2017
2017
• Early HER2-positive breast cancer in combination with chemotherapy
• Early HER2-positive breast cancer in combination with chemotherapy
• Metastatic gastric cancer
• Metastatic gastric cancer
Bevacizumab • Colorectal cancer
Bevacizumab • Colorectal cancer
• Unresectable, locally advanced, recurrent or metastatic non-squamous non-small cell lung cancer
• Unresectable, locally advanced, recurrent or metastatic non-squamous non-small cell lung cancer
• Advanced or metastatic renal cell cancer
• Advanced or metastatic renal cell cancer
• Recurrent ovarian, fallopian tube or primary peritoneal cancer
• Recurrent ovarian, fallopian tube or primary peritoneal cancer
• Persistent, recurrent or metastatic cancer of the uterine cervix
• Persistent, recurrent or metastatic cancer of the uterine cervix
• Recurrent glioblastoma
• Recurrent glioblastoma
2018
2018
*A number of biosimilar products are licensed in Ireland but availability of these products may vary
*A number of biosimilar products are licensed in Ireland but availability of these products may vary
*A number of biosimilar products are licensed in Ireland but availability of these products may vary
Conc lusion and future directions
Conc lusion and future directions
can be used for the treatment of chemotherapy-induced anaemia,3 see Table 1 above. Additionally, there are biosimilars of therapeutic agents including monoclonal antibodies (mAbs), see Table 2 above. The availability of different formulations has been a particular challenge to the uptake of some biosimilars used in the treatment of cancer. For example, rituximab and trastuzumab have both intravenous and subcutaneous formulations available for administration but a biosimilar is only available in intravenous form.
of biosimilars should follow a methodical, staged approach with engagement of all relevant stakeholders.6, 7, 23
therapeutic areas where there is potential for biosimilars to be introduced. The initiative aims to realise potential efficiencies from the availability of biosimilars, and to work with the HSE and clinicians to increase uptake of identified BVB medicines in Ireland. To date, the MMP has identified BVB medicines for TNF-α inhibitors (adalimumab and etanercept) under the High Tech Arrangement. They have recently initiated a BVB process for granulocyte stimulating factors (G-CSFs) which are used as supportive agents in the treatment of cancer.
Biosimilars for the treatment of cancer in Ireland
Biologicals and their biosimilar counterparts have been used in the treatment of cancer for many years and this use will grow as more patents expire. Biosimilars approved for the treatment of cancer are licensed by the EMA for all indications held by the reference product, based on extrapolation of efficacy and safety data.20 There are a number of biosimilars available in Ireland used in the treatment of cancer. These include the supportive care agents G-CSFs, used for the prevention and treatment of chemotherapy-induced neutropenia and epoetins, which
Conclusion and future directions
Cancer prevalence and the cost of cancer treatment continue to increase globally. The high costs, particularly of biologicals, may present a challenge in ensuring access for patients to optimal treatment. Cost and accessibility barriers can create disparities in treatment for cancer and resulting clinical outcomes (21) Biologicals and their biosimilar counterparts have an integral role to play in the treatment of cancer. As biologicals are high-cost components of cancer care, the availability of safe and effective biosimilars has huge potential to reduce cancer care costs and enable access to biologicals and other expensive cancer treatments for patients with cancer (22) The implementation of biosimilars should follow a methodical, staged approach with engagement of all relevant stakeholders (6, 7, 23).
Cancer prevalence and the cost of cancer treatment continue to increase globally. The high costs, particularly of biologicals, may present a challenge in ensuring access for patients to optimal treatment. Cost and accessibility barriers can create disparities in treatment for cancer and resulting clinical outcomes.21 Biologicals and their biosimilar counterparts have an integral role to play in the treatment of cancer. As biologicals are high-cost components of cancer care, the availability of safe and effective biosimilars has huge potential to reduce cancer care costs and enable access to biologicals and other expensive cancer treatments for patients with cancer.22 The implementation
Several additional biosimilar candidate drugs / medicines are being globally investigated and are at various stages of clinical development and regulatory approval.4 More than 10 years of research has shown that biosimilars are safe and effective.3 There has been a significant increase in the utilisation of biosimilars in Ireland since 2019,19 with a growing body of evidence and experience supporting broader uptake.
Cancer prevalence and the cost of cancer treatment continue to increase globally. The high costs, particularly of biologicals, may present a challenge in ensuring access for patients to optimal treatment. Cost and accessibility barriers can create disparities in treatment for cancer and resulting clinical outcomes (21). Biologicals and their biosimilar counterparts have an integral role to play in the treatment of cancer. As biologicals are high-cost components of cancer care, the availability of safe and effective biosimilars has huge potential to reduce cancer care costs and enable access to biologicals and other expensive cancer treatments for patients with cancer (22) The implementation of biosimilars should follow a methodical, staged approach with engagement of all relevant stakeholders (6, 7, 23)
The continued development and utilisation of biosimilars is welcomed by the NCCP, the broader HSE and a number of other Irish and European bodies. Optimising the use of biosimilars will facilitate access for patients and support health services to deal with the rising cost of new medicines.
For additional information, please refer to the NCCP Guidance on the use of Biosimilar Medicines in Cancer Treatment document (available on the NCCP website) or email oncologydrugs@cancercontrol.ie.
References available on request
19 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
Medicine Shortage Warning must be taken Seriously
Medicines for Ireland Chairperson, Padraic O’Brien

Medicines for Ireland members are the suppliers of the majority of medicine in Ireland to the HSE and patients directly and played a pivotal role in a new Framework Agreement on the supply and pricing of non-originator, generic, biosimilar, and hybrid medicines, announced by Government last year.
industry insights and extend our expertise to help improve the development of medicinal pricing and procurement policies in Ireland and safeguard the supply of medicines to Ireland.”
According to the Health Products Regulatory Authority (HPRA) website there are currently 187 medicines in short supply in Ireland. Without intervention this situation has the potential to significantly worsen.
flexible pricing mechanisms that allows reimbursement prices to rise for medicines that are in short supply. Ireland does not have such a mechanism and is therefore further disadvantaged.”
Medicines for Ireland (MFI) are urging Government to heed recent warnings from GPs and pharmacists nationwide on the growing risk of medicines shortages as inflation, energy and transport costs continue to rise, and global supply chain disruptions persist.
Commenting on increasing medicine shortages, Medicines for Ireland Chairperson, Padraic O’Brien has said “In Ireland and throughout Europe, soaring energy costs, inflation and supply chain disturbances have contributed to thousands of generic medicines disappearing from the European and Irish market.”
“MFI members are willing to work directly with Government to help tackle this serious issue and prevent potential medicines shortages. Our aim is to deliver
Mr O’Brien added, “As a small market Ireland is more likely to be badly impacted by inflationary pressure and as costs continue to rise, market conditions will become increasingly unviable for companies supplying generic medicines to Irish hospitals and pharmacies. Additionally, in some cases, our reimbursement prices for certain medicines are too low compared to other EU countries.”
“Price adjustments in Ireland are historically downward only, where other European countries employ
Strategic Plan for Hospital Pharmacy
A recent MFI members survey found that 91% of MFI members experienced increased costs associated with import and/or manufacturing of pharmaceutical and medical products for the Irish market in 2022. While all MFI member companies envisage increases in transportation costs over the next 12 to 24 months.
“Our main focus is to help Government ensure market conditions in Ireland remain sustainable in order to retain and secure access to reliable and affordable treatment for Irish patients. We believe it is time for us to revisit our work with Government and the HSE on the Framework Agreement on Supply and Pricing and develop improvements to mitigate against supply risks.” concluded Mr O’Brien.
A new strategic plan (2022-2027) and goals have been developed by FIP’s Hospital Pharmacy Section (HPS) following member consultation and deliberative, focused strategic planning sessions.
“Since 2008, the HPS Basel Statements on the Future of Hospital Pharmacy have been the guiding light for the work of our section and our members. The new HPS Strategic Plan 2022 creates a cross walk between the Basel Statements and the FIP Development Goals, with the intent of driving further alignment of the work of the HPS to these goals,” said HPS president Ryan Forrey.
Welcoming the new HPS strategy, FIP president Dominique Jordan said: “The FIP Development Goals were launched in 2020 as a framework for transforming the pharmacy profession over the next decade globally, regionally and nationally, in alignment with wider global imperatives, namely the United Nations Sustainable Development Goals. This new strategic plan for FIP’s HPS is an important part of implementing the transformation of pharmacy and we commend the section for its support in this mission, advancing our profession as One FIP.”
Sláintecare Consultant Contract
In a landmark move, the government has today approved a proposal from Minister for Health Stephen Donnelly for a new publiconly hospital consultant contract.
It follows the conclusion of negotiations on the new contract following extensive talks between the Department of Health (DoH), the Health Service Executive (HSE), the Irish Medical Organisation (IMO) and the Irish Hospital Consultants Association (IHCA).
Chair of the Process, Mr Tom Mallon, JC informed the Minister earlier this week that a second round of negotiations, which began last August, had come to a conclusion.
The new Sláintecare contract will include:
• basic pay of ¤209,915¤252,150 on a six-point scale
• a 37-hour week with an 8am to 10pm Monday to Friday and 8am to 6pm Saturday
• consultants will continue to receive additional remuneration for on-call duties and overtime as applicable
• a highly flexible contract that enables consultants opt for a variety of different work patterns including less than whole time; work sharing; compressed hours; flexible start and finish
times will be available to the greatest extent possible
• supporting consultants to participate in medical education training and research and enabling a greater focus on research and education in line with other jurisdictions
• consultants will be free, having met their commitment to their public contract, to engage in offsite private practice, in the same way as allowed in the NHS Minister Stephen Donnelly said, "The introduction of this new contract will be a landmark in delivering Universal Healthcare. I was pleased to be able to inform
my government colleagues that the negotiations between my department, the HSE, the IMO and the IHCA had come to a conclusion.
"This new contract will ensure that care will be provided when patients need it most. Consultant decision-making on-site results in reduced emergency admissions, shorter lengths of stay and more complete care plans for discharge. This new contract will not result in an increase in working hours for consultants but instead will focus on ensuring that these senior decision makers are present and delivering patient care when demand is highest.”
20 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE News
Recommendations on Electronic Sharing
In November 2022, the Health Information and Quality Authority (HIQA) published a set of recommendations to enable the electronic sharing of health information in older persons’ services. Read more to find out all you need to know.
Background
In common with many other countries, Ireland faces the challenge of an ageing population and it is expected that people aged 65 years or more will account for 50% of all healthcare activity by 2031. An older person typically receives care from a wide range of health and social care professionals across both acute and community settings. However, the health information of an older person is usually held across a number of IT systems or in paper records, making it difficult for health and social care professionals to easily access and share this information across all settings. This means that it is often difficult to get a full picture of the care needed for an older person. Additionally, the high number of different services an older person experiences, and the increasingly complex nature of older persons care, are challenges to the safe and effective sharing of health information in older persons’ services.
The need for change
These challenges in older persons’ services were intensified by the COVID-19 pandemic.
In August 2020, the COVID-19 Nursing Homes Expert Panel recommended the introduction of an integrated IT system to support the effective sharing of health information for older persons.
The recommendation from the Expert Panel looked for the safe and effective sharing of information along the care journey, which includes numerous settings, such as: residential care settings (including public and private nursing homes): day care settings; home support services (including public and private providers); GP practice management systems; and discharge planning in acute settings.
HIQA’s involvement
The Department of Health asked HIQA to explore how the
Written by Rachel Flynn, Director of Health Information and Standards, HIQA

capabilities recommended by the Expert Panel could be progressed and implemented. HIQA worked with stakeholders across health and social care, and ICT, to develop Recommendations on the ICT Enablement of Older Persons Services. The recommendations support the safe and effective electronic sharing of health information for older persons (and for other populations) across both public and private sectors, and are aligned to national eHealth goals under Sláintecare.
The recommendations also provide an analysis of the current situation nationally and international best practice. Informed by this evidence, they outline measures that would ensure that the capabilities requested by the Expert Panel are provided in line with national priorities.
Recommendations
The recommendations are:
Strategy and governance: A national health information strategy, with identified priorities and associated funding, is needed to provide strategic direction across the entire health information system. A clear overall strategy for ICT enablement of older persons services is also needed, to ensure that the capabilities
outlined by the Expert Panel are delivered. This should be developed by the appropriate governance structure(s).
Vision and roadmap: A shared vision must be developed, in collaboration with all stakeholders, with older persons being a critical stakeholder group. It is essential that stakeholders are fully engaged throughout the programme to ensure acceptance and adoption.
Standardised sharing of information: A suite of national standards are fundamental for the effective sharing of information and should include minimum datasets that are based on the actual needs of users. This will support the safe and effective sharing of appropriate information along the older person’s care journey, with those authorised to provide care having the information they need when they need it.
User engagement: All systems should meet basic user-centred principles and users should have the targeted training and broader digital education they need, such as through broad national initiatives. This will ensure that users have confidence in, and are ready to adopt, the systems that are
developed, which is a critical point of failure in some eHealth projects.
Next steps
To progress these recommendations, HIQA will develop a comprehensive suite of national standards to help facilitate the digital enablement of older persons services and the health information system in general for all populations.
Health information generates large volumes of data every day, and health and social care professionals spend a significant amount of their time handling and collecting it, and looking for and storing it. The current ICT infrastructure in Ireland’s health and social care services is highly fragmented with major gaps and silos of information, preventing the safe and effective transfer of health information. These recommendations aim to support the digital enablement of older persons services to ensure that high quality health information is easily available to all health and social care professionals providing care to older people at all stages along their patient journey. The Recommendations on the ICT Enablement of Older Persons Services can be read in full on www.hiqa.ie.
21 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
HIQA publishes recommendations to support electronic sharing of information in older persons’ services
HIQA Report
Osteoporosis
Osteoporosis Clinical Practice Guidelines: An Update
All at: Centre for Osteoporosis and Metabolic Bone Disease, Department of Rheumatology, Merlin Park Campus, Galway University Hospitals, Ireland.


School of Medicine and Health Sciences, University of Galway, Ireland.
Address correspondence to: john.j.carey@nuigalway.ie

fracture assessment or some combination of these.2,14,22-26
What are Clinical Practice Guidelines?
Clinical Practice guidelines (CPGs) form an essential part of modern medicine.1 In essence they provide a set of recommendations for health professionals derived by expert committees reflecting a summary of the evidence, opinions on the strength and relevance of the evidence, the benefits and harms, and consensus.1-3 CPGs can improve and standardise the assessment and management of important clinical disorders1,3 While the concept of evidencebased medicine is a good one, agreement between experts is not universal, particularly with respect to what constitutes good evidence, what to include, how much and for whom.1,4-12 An overly rigorous process compiling excessive output may prove redundant, and cause confusion.1,3,6 Practitioners confidence in, use of, and preference for CPGs varies.3,13,14
In osteoporosis huge gaps remain between best evidence and CPGs and what transpires in clinical practice.14-18 Key remaining challenges include clearly defining what constitutes quality, and harmonizing guidelines.13,14,16-20
What is Osteoporosis?
Osteoporosis is one of the commonest non-communicable diseases in the world today.2,13,14,16,19-24 This disease results in skeletal failure manifest as clinical events known as fragility fractures.13,14,16,19,20 Bone mineral density measurement is the test of choice for diagnosis of osteoporosis in older men and women without a prior fracture, which can also be incorporated into various algorithms to assess the risk of fracture and monitor the effectiveness of interventions over time.2,14,20,21,24 Osteoporosis and the associated fractures can be very detrimental to patients quality and quantity of life, and are associated with large healthcare costs.2,13,14,16,22-24 Although hundreds of CPGs have been formulated to address both primary and secondary prevention, considerable heterogeneity exists, but as outlined in table 1 the general principles are broadly similar.2,13,14,16,18-20,22-24 In this article we will focus on key principles captured in more recent American, Asian and European guidelines which we believe have relevance for managing our patients in Ireland.2,13,14,16,18-20,22-24
General Principles of Osteoporosis Clinical Practice Guidelines
The basic principles for the identification, evaluation and management of postmenopausal women and men aged 50 years and older are shared among osteoporosis experts, outlined in Table 1. However explicit terminology, level of detail and homogeneity of structure and content can vary.14,22-25 These contrast with recommendations from others whose expertise focuses on evidence rather than illness.2,26 Yet many of the core principles align with a universal goal – fracture preventionthough they differ in strength of recommendations, detail or philosophy of evidence.2,14,22-26
The first principle involves identification of those at risk for primary prevention, or with a prior fragility fracture for secondary prevention. A number of different strategies are suggested including case-finding, fracture liaison services, and screening those without prior fractures using either a multifactorial assessment tool, bone mineral density (BMD) measurement and vertebral
Measurement of BMD and fracture risk assessments are explored in great detail in some CPGs,22-25 while others are less specific or less enthusiastic,2,14,26 particularly when it comes to monitoring the effect of any intervention. There is general agreement screening (testing those without signs or symptoms of a disease) for postmenopausal women is clinically and cost effective, usually women aged 65 years and older. Although there is agreement screening older men is important, when, how and what to do requires further clarification and consensus.2,13,14,20-26 Importantly what is not explicitly stated is also important and needs more emphasis: not to screen healthy younger people who are not at risk. This is because all testing is imperfect, and overdiagnosis can occur, with important consequences for the patient including further unnecessary testing and concern, and unproven treatment. Unfortunately in Ireland multiple proposals for a national osteoporosis programme, and more recently an osteoporosis screening programme have been rejected. Contemporaneously a daft plan to provide free DXA scans for everyone who wants one is being funded by the tax payer which will do nothing to address quality, standards or appropriate osteoporosis care for our population. This will cost far more in the long run than one where scientific evidence and expert consensus is the foundation, and is already wreaking havoc with our patients.
Those identified at risk should undergo further evaluation to identify risk factors, in particular those which are amenable to modification such as smoking, excessive alcohol and glucocorticoid use. Assessment and management of fall risk is unanimous, though details on
22 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Written by: Professor John J. Carey, Consultant Physician in Rheumatology and Medicine, Clinical Lead in Osteoporosis, DXA and Fracture Liaison Services - Catherine Armstrong, Clinical Nurse Specialist in Osteoporosis and Fiona Heaney, Clinical Nurse Specialist in Osteoporosis
Professor John Carey
Catherine Armstrong
Fiona Heaney
MOVYMIA®: THE NEW TERIPARATIDE BIOSIMILAR FROM CLONMEL

Rebuild bone before it breaks again—with Movymia®1 RELIABLE: Movymia®’s quality, safety and efficacy is highly similar to its reference product1,2,* EFFECTIVE: Anabolic MoA effectively rebuilds bone through the stimulation of osteoblasts1,3 AFFORDABLE: Allows more eligible patients to benefit due to its cost advantage4,5 RE-USABLE: One high quality reuseable pen for the entire treatment period1
MOVYMIA 20 MICROGRAMS/80 MICROLITERS SOLUTION FOR INJECTION
Each dose of 80 microliters contains 20 micrograms of teriparatide. One cartridge of 2.4 ml of solution contains 600 micrograms of teriparatide (corresponding to 250 micrograms per ml). Presentation: Glass cartridge. Indications: Movymia is indicated in adults. Treatment of osteoporosis in postmenopausal women and men at increased risk of fracture. In postmenopausal women, a significant reduction in the incidence of vertebral and non-vertebral fractures but not hip fractures has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture. Dosage: The recommended dose is 20 micrograms administered once daily. Patients should receive calcium and vitamin D supplements if dietary intake is inadequate. The maximum total duration of treatment is 24 months. The 24 month course should not be repeated over a patient’s lifetime. Following cessation of teriparatide therapy, patients may be continued on other osteoporosis therapies. Teriparatide must not be used in severe renal impairment. Use with caution in moderate renal impairment and impaired hepatic function. Teriparatide should not be used in paediatric patients (less than 18 years), or young adults with open epiphyses. Method of administration: Movymia should be administered once daily by subcutaneous injection in the thigh or abdomen. It should be administered exclusively with the Movymia Pen reusable, multidose medicine delivery system and the injection needles which are listed as compatible in the instructions provided with the pen. The pen and injection needles are not included with Movymia. However, for treatment initiation a cartridge and pen pack should be used. Movymia must not be used with any other pen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or excipients. Pregnancy and Breast-feeding Pre-existing hypercalcaemia, severe renal impairment, metabolic bone diseases other than primary osteoporosis or glucocorticoid-induced osteoporosis, unexplained elevations of alkaline phosphatase, prior external beam or implant radiation therapy to the skeleton, patients with skeletal malignancies or bone metastases. Warnings and precautions: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection. Routine calcium monitoring during therapy is not required. Teriparatide may cause small increases in urinary calcium excretion, but the incidence of hypercalciuria did not differ from that in the placebo-treated patients in clinical trials. Teriparatide should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. When transient orthostatic hypotension occurred, it happened within the first several doses, was relieved by placing subjects in a reclining position, and did not preclude continued treatment. Caution should be exercised in patients with moderate renal impairment. Experience in the younger adult population, including premenopausal women, is limited. Treatment should only be initiated if the benefit clearly outweighs risks in this population. Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, teriparatide should be discontinued. The recommended treatment time of 24 months should not be exceeded. Contains sodium. Interactions: Digoxin, digitalis. Fertility, pregnancy and lactation: Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, Movymia should be discontinued. Movymia is contraindicated for use during pregnancy and breast-feeding. The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. Driving and operation of machinery: Teriparatide has no or negligible influence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Undesirable effects: Nausea, pain in limb, headache, dizziness. Refer to Summary of Product Characteristics for other adverse effects. Pack size: 1. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie. Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, German. Marketing authorisation number: EU/1/16/1161/001-003. Medicinal product subject to medical prescription. Date last revised: July 2019.

1. Movymia® SmPC. 2. Movymia® EPAR – public assessment report, available at: https://www.ema.europa.eu/documents/assessment-report/movymia-epar-public-assessment-report_en.pdf
3. Brixen KT et al. Basic Clin Pharmacol Toxicol. 200494(6):260–70. 4. Lyman GH et al. N Engl J Med. 2018378(21):2036–2044. 5. Janjigian YY et al. Future Oncol. 201814(23):2403–2414.* Forsteo® October 2019. 2019/ADV/TER/122H
DON’T WAIT UNTIL OSTEOPOROSIS STRIKES AGAIN
HEALTHCARE
Osteoporosis
Table 1. Comparison of Key Principles Included in Consensus Guidelines for Assessing and Managing Osteoporosis in postmenopausal women and men aged 50 years and older across North America, Europe and the Asia-Pacific Region.
Table 1. Comparison of Key Principles Included in Consensus Guidelines for Assessing and Managing Osteoporosis in postmenopausal women and men aged 50 years and older across North America, Europe and the Asia-Pacific Region
Principle North America14 USA24 Europe23! Asia-Pacific25 UK22
Identification of Patient
Evaluation of Risk Factors
Risk Assessment
Test
Vertebral Fracture Assessment
Risk
Activity
Osteoporosis Medication
Management
Local Bone Enhancement • •
Threshold
/ Quality •
Specialist Referral • • • • Special Populations • • Patient Education • •
Fracture Rehabilitation •
!: These guidelines are for postmenopausal women only.
!: These guidelines are for postmenopausal women only.
performance and management varies considerably. All agree addressing adequate nutrition, in particular an appropriate amount of intake of calcium and vitamin D. Exercise, physiotherapy and rehabilitation are widely acknowledged.14,22-25 The largest study ever performed which randomised postmenopausal women to calcium and vitamin D or placebo showed this practice does not reduce the risk of fracture, and actually more than doubles the risk in younger postmenopausal women.27
Treatment of low BMD with osteoporosis medication is the single most effective proven intervention for those with this disease or at high risk for fracture.2,13,14,16-20,22-26, and this principle has universal appeal to all CPGs . However this is where the greatest discordance exists in principles of who to treat, with what, for how long and how to monitor adherence, compliance and success.2,13,14,16,23-26 In general osteoporosis medications' are very safe and very effective once prescribed and taken correctly. While side-effects can occur, they are usually mild and benign.
Those identified at risk should undergo further evaluation to identify risk factors, in particular those which are amenable to modification such as smoking, excessive alcohol and glucocorticoid use. Assessment and management of fall risk is unanimous, though details on performance and management varies considerably. All agree addressing adequate nutrition, in particular an appropriate amount of intake of calcium and vitamin D. Exercise, physiotherapy and rehabilitation are widely acknowledged 14,22-25. The largest study ever performed which randomised
Unfortunately exaggerations of harm despite a lack of robust evidence to support this, in contrast to the huge evidence to support their use has limited their effectiveness in practice and undermined the whole principle of evidence-based medicine.2,13,16,17,19,22-26 Resolving this to ensure all women with postmenopausal osteoporosis and men aged 50 years and older with osteoporosis, in particular those with prior fragility fractures are prescribed them would have the greatest individual and population health benefit.15 Effective communication and discussion with patients to educate and empower them, and address their preferences and concerns is crucial for effective engagement.14,22
Some guidelines also address other areas critical to patient care, such as surgery, local bone enhancement procedures like kyphoplasty, pain management and rehabilitation of the patient post fracture.22-24 These important areas should not be overlooked as adequate pain control and a multidisciplinary assessment and management are essential
to hasten patient recovery and independence following such events. Ireland lacks such programmes, but services are gaining momentum nationally with the appointment for the first time of several advanced nurse practitioners in Ireland in 2022 to support fracture liaison services.
Monitoring of patients to address their ongoing needs, and treatment compliance and effectiveness is needed.2,13,14,20-26 This includes history and physical examinations, discussions around their lifestyle choices, other medical problems as well as their osteoporosis management and medications, and biochemical and DXA testing. Standards for monitoring BMD have been established for more than 20 years, which include sending patients back to the same centre where the least significant change is known (a combination of measurement error and random error with a 95% confidence interval) in order to know whether the BMD or other DXA features have actually changed or not. H.S.E. funded and insurance companies in Ireland are undermining this whole principle of appropriate care by sending
unsuspecting patients to different centres for repeat DXA scans which provides no clinically useful information. This is one more reason why a national programme is more essential than ever.
CPGs represent a roadmap to improve and standardise the care of patients. These are evidence-based consensus recommendations in so far as is possible. Today the principles for osteoporosis assessment and management are universally agreed across North America, Europe and the Asia-Pacific region.2,13,14,20-26 Experts agree a “one-size fits all approach” is not appropriate and tailored recommendations are needed for individual patients.1-6,10,13,14,20-26 However it is time a national programme for osteoporosis care in Ireland is put in place to provide basic standards of quality care for all patients with osteoporosis. Solutions to bring best practice into everyday use would be hugely rewarding, and far more clinically and cost effective for our patients and the taxpayer.15
References available on request
24 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
• • • • •
• • • • •
Fracture
• • • • •
DXA
• • • • •
• • • • Falls
• • • • •
Lifestyle • • • •
Nutrition • • • • •
Physical
• • • • •
• • • • • Pain
• • •
Intervention
• • • •
Monitoring • • • • • Audit




All-Ireland Approach to Psychosis
RCSI University of Medicine and Health Sciences is leading a network of world-leading psychosis experts on a new training and research programme to improve care for people who experience psychosis.
The Health Research Boardfunded PSI-STAR programme aims to address issues such as prediction and prevention of psychosis, reducing stigma related to the experience of psychosis, predicting physical health outcomes and life expectancy in people who experience psychosis and promoting their recovery.
The programme launch, which took place today at the National Gallery of Ireland, brought together a diverse team with organisational, arts, patient and public involvement (PPI), research, practitioner and academic expertise.

Professor David Cotter, Professor of Molecular Psychiatry, Department of Psychiatry, RCSI leads the PSI-STAR team which includes Co-Leads Professor Mary Cannon, Professor of Psychiatric Epidemiology and Youth Mental Health at RCSI and Professor Agnes Higgins, Professor in Mental Health, School of Nursing and Midwifery, Trinity College Dublin.
Professor Cotter commented: “I am delighted to be a part of this consortium which aims to
Professor David Cotter, Professor of Molecular Psychiatry, Department of Psychiatry, RCSI leads the PSI-STAR team which includes Co-Leads Professor Mary Cannon, Professor of Psychiatric Epidemiology and Youth Mental Health at RCSI and Professor Agnes Higgins, Professor in Mental Health, School of Nursing and Midwifery, Trinity College Dublin
bring about change in how we think about and respond to those experiencing psychosis. We will train future leaders in this field of research. The multidisciplinary approach within the PSI-STAR team, the meaningful inclusion of PPI and support of our collaborators will ensure we achieve this.”
Professor Agnes Higgins, Trinity College Dublin said: “The launch of PSI-STAR marks an important milestone for psychosis research in Ireland. Crucially, this programme will help amplify the voice of people with lived experience of psychosis throughout all stages of the research. A voice that is often unheard within the research agenda.”
Psychosis, which includes diagnoses including schizophrenia and bipolar disorder, occur in about three in a hundred people and usually start in adolescence or young adulthood. They can greatly
disrupt a young person's life in terms of their education, social relationships and career outcomes. It is important to understand the meaning of psychosis from multiple perspectives so that the best treatments and supports can be offered without delay.
The research team involves clinicians and academics from across Ireland in the fields of psychiatry, nursing, social work, sociology and psychology, including an academic with lived experience of psychosis. The programme is training five PhD students to be leaders in the field of psychosis and make a positive impact on people's lives.
Lack of Awareness on Vaccines
Almost half of people do not know what vaccines they should get as adults to prevent certain diseases, according to a survey carried out by Ipsos for the Irish Pharmaceutical Healthcare Association (IPHA).
The research, measuring public attitudes to vaccination in general and to Covid-19 vaccination, shows that 46% of people are not aware of the vaccines they should get as adults unless a healthcare professional tells them.
But 61% of people would get vaccinated for certain diseases if their doctor recommended the move.
Vaccines on the Health Service Executive’s adult vaccination schedule include flu, pneumococcal disease, whooping cough and travel vaccines, with
Hepatitis B, HPV, mumps and rubella available for some people.
While 96% of people know that vaccines are available for flu, vaccines awareness varies for some other diseases, including tuberculosis (71%), HPV (70%) and meningitis (65%).
Over three in five people, or 61%, believe that it is not necessary to get vaccinated for certain diseases because they have already been eradicated.
Half of people said lack of information about vaccines was the biggest barrier to vaccine uptake. Almost two in five people, or 39%, said a lack of knowledge about the risks of remaining unvaccinated could stop them getting protected. Smaller proportions of people cited inconvenience, distance and lack
of time as barriers, at 20%, 18% and 17%, respectively.
Over half of people, or 51%, have received two or more booster doses of Covid-19 vaccine while, overall, 93% of people aged 18 or over have been vaccinated for the disease at least once.
Over three in four people, or 77%, believe that children should study science at school, in part to learn more about vaccines.
The history of vaccination in Ireland spans almost two centuries, starting with smallpox in 1863. Now, smallpox is eradicated. Vaccines have eradicated diphtheria, a disease that suffocated children until the 1960s. Vaccines have eradicated or managed whopping cough, polio, measles, mumps and rubella, and meningitis. More recently, vaccines
Discussions at the launch event revolved around how collaborators including Mental Health Reform, GROW, Spunout, SHINE, Peer Advocacy, the theatre production company Brokentalkers, HSE and the Mental Health Commission could best work with the research programme and develop real-world applications of research to policy and practice. Sharing of lived experiences with psychosis and de-stigmatising is a main focus.
PSI-STAR is funded through a Health Research Board Collaborative Doctoral Award (CDA), a structured doctoral training program leading to the award of a PhD.
have been vital in tackling the HPV virus and Covid-19.
Bernard Mallee, Director of Communications and Advocacy at IPHA, said: “The impact of vaccines on human health has been transformative. Over 30 infectious diseases are vaccinepreventable. It is concerning, though, that there are wide knowledge gaps about some of the diseases vaccines can prevent. Encouragingly, many people heed doctors’ guidance about getting vaccinated. There is strong public support for teaching science in schools, indicating that, as a society, we value facts. Communities benefit from innovation, socially and economically, provided we make it part of our everyday lives.”
27 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
News
Hospital Pharmacy
Experiences of a Hospital Pharmacist Abroad
Written by Barry O’Sullivan, Manager, Outpatient Pharmacy, Imperial College London Diabetes Centre - Adjunct Clinical Lecturer, School of Pharmacy, University College Cork, Ireland
in Abu Dhabi and Al Ain across the full spectrum of diabetes and related complications under one roof. A one stop shop if you will.
The specialised focus, backed by customised software, allows patients to undergo all the necessary tests, obtain results, and receive tailored consultationbased diagnosis and prognosis during the same appointment.
During medical consultation the prescription is issued on the clinical software then the Pharmacy team will commence with the insurance approval, dispensing and clinical check.
If a patient wants to get the medication elsewhere, they are given a hard copy external prescription and get same dispensed in another Pharmacy in the Emirate.
Covid lockdowns gave us all time to think, to reflect and indeed plan for the brighter days ahead.
“What is next for me? Was the question that arose time and time again for me. The only possible solution to the question: change.
Pre and during Covid I undertook some studies to upskill. An MBA from University of Limerick and qualifying as a Chartered Management Accountant were the culmination of the endeavour.

For a number of years I had wanted to work in the Middle East, a totally different landscape but one with easy access to home. The idea of working there intrigued me. Western healthcare is held in high esteem, English the working language and healthcare is JCI accreditation which I had familiarity with. COVID lockdown gave me time to reflect on where I was and what was next. I decided to make the move happen.
I had applied for a few Pharmacist roles in the region, but the interest was one directional. There is a large supply of Pharmacists in the region already, not much need to import one more. For some healthcare workers, the route to the Middle East is navigated with the assistance of agencies, some of the large hospitals actively host recruitment drives for some
professions such as nurses. Less so for Pharmacists.
How my role came about was untraditional. For years I read the career paths of business people in Business section of the Sunday papers, a curiosity I apply to Linkedin also. On this platform I noted an Irish name in a prominent role in a large healthcare organisation based in the middle east. I sent a direct message to Teresa Quinn, Senior Director of Clinical Operations, Mubadala Health, along the lines of “your career trajectory is very impressive, what path did you take?”
A conversation ensued and after some initial discussion, my CV was requested. Over the course of a few weeks there was a few rounds of video calls culminating with an offer letter arriving into my inbox.
I was offered a position of Pharmacy Manager with Imperial College London Diabetes Centre, a comprehensive, state-of-theart outpatient facility specialising in diabetes treatment, research, training and public health awareness. The Centre opened in Abu Dhabi in 2006 as the first healthcare facility established by the state owned Mubadala Investment Company in partnership with Imperial College London.
Then started the administrative element to move to Abu Dhabi. It began in earnest in October, culminating with my Pharmacist licence for Abu Dhabi being issued late December.
My new employers assisted with all the various elements, such as the pre departure paperwork with Department of Health licence, verification of all previous role and qualifications and on arrival helping me get settled with the all important Emirates ID card, phone etc. On arrival new colleagues aided my settling in, especially Shukri Abu Ata.
Working as Pharmacy manager leading three clinics, two in Abu Dhabi and one in Al Ain poses a new challenge. It requires all elements of my clinical, financial and managerial skills.
Leading a team of over 70 across the three sites is a demanding job. Our team processes over 110,000 packs of medications every month
Our Home Delivery service ships over 4000 deliveries every month.
The day to day clinical processes are very similar to Ireland and the UK.
Imperial College London Diabetes Centre provides a comprehensive range of treatments at its branches
Healthcare in the UAE is a mix of private and public sectors, but expats will be using the private system. Different to Ireland in terms of the payer, the UAE national patient’s medications are typically covered by insurance. There can be a small co-pay but not much cost.
UAE nationals are covered under the Thiqa program. Citizens get a Thiqa card, which gives them full access to a large number of both private and public healthcare providers within the Daman network. To apply for this, you must be a UAE national (living in Abu Dhabi) and aged between 18 and 75.
For expats, employers and sponsors are responsible for providing health insurance for employees and their families. This includes coverage for one spouse and three children under 18.
When it comes to dependents, the employer is only required to pay 50% of the coverage, with the rest paid by either the sponsor or the employee. For additional dependents, the obligation falls to the employee to pay for their health insurance.
The day to day clinical work is pretty much identical to home. Evidence based care in line with best international practice is the standard. Governance structures are in line with internal best practice and we liaise with the Department of Health frequently.
28 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Any prescription collected face to face has a Pharmacist consultation, usually lasting around 7 minutes.
Indication, mechanism of action, side effects and so on are all detailed. For home delivery patients, this is done on the Phone if a new item or any changes.
Any clinical interventions on a prescription are discussed with the prescriber and noted on the patient file.
We use the MERP scale for near misses and medication errors, with a robust reporting structure and outcomes review for any required changes.
As a learning tool, medication errors and “interaction of the month” are shared with the clinical teams, an idea I copied from the excellent Medication Safety Minute run by St. James’ Hospital in Dublin.
Pharmacy supports the other clinical teams in the usual way with clinical input, supply and other considerations. We work closely with non clinical teams of procurement and given the insurance element, the Revenue Cycle Management team also. They act as the point of contact with the insurance companies.
Healthcare is an international village no matter where you are and the United Arab Emirates is no different. Indeed, some my colleagues have siblings working in healthcare in Ireland. Others have studied with Irish institutions such as the Royal College of Surgeons, both in Dublin and on their campuses in the Gulf region.
In the international village that is healthcare, the main currency is respect. The culture here is one of politeness and respect, especially amongst colleagues. We see, in Ireland and other countries, when healthcare staff aren’t paid respect
be it in terms of conditions or pay, they leave. The numbers of healthcare staff leaving the sector or the country highlights this.
Self doubt and uncertainty were inevitable for me with such a career move but focusing on what I did know, what I could do and what I could help improve allayed, for the most part, those negative feelings.
Having worked in a private hospital in Cork was a huge help as the JCI accreditation is applicable over here too.
Slowly but steadily I am getting used to the change in both personal and professional life.
Packing up and leaving Cork was not without an emotional cost. I am the youngest of seven, uncle of 14 and have a close relationship with my mother, Mary T. Leaving home at 4am on a January morning wasn’t an occasion full of smiles I’ll be honest.
From a work point of view, it feels like a huge opportunity to develop myself and in time, progress. In the short few months here, I have been involved wide array of projects using clinical and financial skills utilising both my Pharmacist and Chartered Management Accountant skillsets.
The learning curve is vertical at times, but often we know more than we realise and being recruited by such a prestigious organisation instils a sense of self belief that you can perform.
For me, at this moment in time, the United Arab Emirates is the best place for me to be. The standard of living and options of things to do is amazing. It’s safe, clean and friendly. In making the reasoned decision to move, there has been of course the element of doubt, is this the right thing to do? So far, for me it has been. For anyone else, there is only one way to find out.
Researcher of the Year Award for Professor Hardiman

Trinity’s Professor of Neurology Orla Hardiman was named 2022 Researcher of the Year at the prestigious Science Foundation Ireland Awards at the annual SFI Science Summit this week. Hardiman is a clinician scientist and a world authority on the causes, diagnosis, and treatment of Amyotrophic Lateral Sclerosis (ALS)/ Motor Neuron Disease (MND). She heads the Academic Unit of Neurology at Trinity College Dublin and leads the SFI Precision ALS Spoke.
The event also saw Trinity’s Dr Claire Gillan named SFI EarlyCareer Researcher of the Year, and Michael Morris, Director of the AMBER research centre, win the SFI award for Best International Engagement.
Dr Linda Doyle, Provost of Trinity College Dublin said, "Orla's worldleading research on the causes, diagnosis and treatment of Motor Neuron Disease (ALS) has had a huge impact on the lives of so many people. Her generosity and dedication are hugely inspiring. Orla's research has placed Ireland at the centre of international research collaboration on ALS. I am absolutely delighted that she has been announced as the SFI Researcher of the Year. It comes only two weeks after she received the 2022 Provost Innovation Award.
"I also want to congratulate Claire Gillan, Associate Professor in Trinity’s School of Psychology, on winning SFI's Early Career Researcher of the Year award.

"The success of Orla, Claire and Michael illustrates the sheer breadth of outstanding research taking place across Trinity."
Professor Orla Hardiman commented on the award, “I am greatly honoured to receive this prestigious award, which is a reflection of the hugely talented individuals with whom I have had the privilege of supervising, mentoring and collaborating over the years. I am also aware of the enormous benefits of being in a position to engage in international collaborations with like-minded clinician scientists. Understanding the processes that drive neurodegeneration is the “final frontier” in neuroscience. As clinician scientists, we seek to unravel the complexity of neurodegenerative disease in humans, and our work in Ireland has focussed on how best to enable the successful translation of laboratory discoveries to new drugs for those with different subtypes of disease. Our ultimate
collective objective is to ensure that we provide the right drug for the right patient at the right time. I am particularly conscious of my privileged position as a female leader in science, and of the importance of mentoring from experience other younger women as they juggle careers, family life and research. I am enormously grateful to both SFI and the HRB in enabling my scientific career over the years, and of course to my husband Gerry and my children for their ongoing love and support.”
29 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
News
Dr Claire Gillan
Professor Orla Hardiman

NOW RINVOQ® (upadacitinib) INDICATED FOR ADULTS WITH MODERATE TO SEVERELY ACTIVE ULCERATIVE COLITIS1 *RINVOQ® is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1 AbbVie® is a registered trademark of AbbVie Inc. RINVOQ® and its design are registered trademarks of AbbVie Inc. REFERENCES: 1. RINVOQ® summary of product characteristics, available at www.medicines.ie. JAK: Janus kinase; UC: ulcerative colitis. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. Legal Classification: POM(S1A). IE-RNQG-220007 | August 2022 Full Summary of Product Characteristics is available at medicines.ie ▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie. Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
Continuing Professional Development
CPD
60 Second Summary
Hereditary transthyretin-mediated amyloidosis (hATTR), also known as ATTRv (v for variant), is a rare, adult-onset, autosomal-dominant disorder, caused by heterozygous pathogenic variants in the transthyretin TTR gene. It is a multisystem disorder caused by the extracellular deposition of amyloid, derived from transthyretin, which progressively impairs the function of multiple tissues and organs culminating in increasing disability and death within 3–15 years of symptom onset.
An expert consensus statement on best practice for genetic diagnosis, clinical screening and management of individuals with, or at risk of, hATTR amyloidosis has been proposed to encourage a consistent approach to patient management in different regions of the UK and Ireland.
Recommendations were made for three different groups: (1) people with symptoms raising a possibility of hATTR amyloidosis; (2) people with biopsy-confirmed ATTR amyloidosis; and (3) people without symptoms who may have hATTR amyloidosis (i.e. relatives of people with identified TTR variants).
The recommendations outlined in this statement may help increase the identification of affected individuals, help them gain access to regular follow-up and, if needed, treatment to reduce disease progression once they become symptomatic.
In conclusion, this consensus statement provides best practice recommendations for the genetic testing and management of people with symptoms raising a possibility of hATTR amyloidosis, patients with biopsy-confirmed ATTR amyloidosis and pre-symptomatic TTR gene variant carriers. We believe these recommendations will help increase the identification of affected individuals, help them gain access to regular followup and, if needed, treatment to reduce disease progression once they become symptomatic.
1. REFLECT - Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY - If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN - If I have identified a
knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE - Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT - At this time you may like to record your learning for future use or assessment. Follow the
4 previous steps, log and record your findings.
Published by HPN.
Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.

Clinical and Genetic Evaluation of People with or at Risk of Hereditary ATTR Amyloidosis: An Expert Opinion and Consensus on Best Practice in Ireland and the UK
Hereditary transthyretin-mediated amyloidosis (hATTR), also known as ATTRv (v for variant), is a rare, adult-onset, autosomal-dominant disorder, caused by heterozygous pathogenic variants in the transthyretin TTR gene.1,2,3
It is a multisystem disorder caused by the extracellular deposition of amyloid, derived from transthyretin, which progressively impairs the function of multiple tissues and organs culminating in increasing disability and death within 3–15 years of symptom onset.1 2 3 4 5 6 7 8
The disease manifests with either predominantly axonal sensory, motor and autonomic neuropathies, or as an infiltrative cardiomyopathy.9 Many patients have disease affecting both the nerves (neuropathy) and heart (cardiomyopathy) and occasionally other organs, such as the kidneys and eyes.2 10 11 12 13 This heterogeneity, which differs according to TTR variant and its penetrance in each individual,3 makes the diagnosis of hATTR amyloidosis challenging, particularly in non-endemic regions, where patients often present with no known family history.9 10
TTR gene variants in the UK and Ireland differ to those commonly occurring in other geographic locations14,15,16,17 and may warrant
a unique approach with regards to screening and evaluation compared with other regions. The National Amyloidosis Centre (NAC) in London is a centrally funded National Health Service specialist unit available to patients from England, Scotland, Northern Ireland, Wales and the Republic of Ireland. The NAC provides both clinical and genetic diagnostic services. Despite this central facility, there is variance across the UK and Ireland with regards to the identification, diagnosis and monitoring of patients with, or at risk of, hATTR amyloidosis. Furthermore, with hATTR amyloidosis treatments typically being more effective if initiated at the early stages of disease,18, 19 there is a need to provide timely and equitable access to specialist healthcare services, genetic testing and counselling, and clinical investigation for families living in different regions of the UK and Ireland. Although patient support is available through the UK ATTR Amyloidosis Patients Association (http://ttramyloidosis. uk/), there are still challenges in accessing services to ensure that all individuals in the UK and Ireland with Hattr amyloidosis receive consistent, high-quality care.
Between 2020 and 2021, an expert panel from the UK and Ireland
convened to discuss proposals for the optimal clinical and genetic management specific to this region, for both patients and presymptomatic carriers. The aim of the discussions was to identify the current challenges and provide consensus recommendations. This article does not contain any new studies with human participants or animals performed by any of the authors.
Expert Panel and Consensus Process
The expert panel comprised 10 amyloidosis specialists, representing the multidisciplinary team, including internal medicine (PNH), renal medicine (JDG), neurology (MMR), clinical genetics (AJG, HC, RM), haematology (MREC) and cardiology (WEM, CJC, RC). Consensus and recommendations evolved during three virtual meetings, held between 29 July 2020 and 8 November 2021 and chaired by Professor Philip Hawkins of the NAC, UK.
Discussions focused on identification of pre-symptomatic TTR variant carriers, diagnostic and pre-symptomatic genetic testing and associated genetic counselling, clinical investigations (for cardiac and neurological disease) and patient access to
31
AUTHORS: Julian D. Gillmore, National Amyloidosis Centre, Division of Medicine, UCL, Royal Free Hospital, Rowland Hill Street, London, Mary M. Reilly, Centre for Neuromuscular Diseases, UCL Queen Square Institute of Neurology and National Hospital for Neurology and Neurosurgery, London, Caroline J. Coats, Queen Elizabeth University Hospital, Glasgow
Rob Cooper, Liverpool Heart and Chest Hospital, Helen Cox, Birmingham’s Women’s and Children’s Hospital, Mark R. E. Coyne, University College Dublin and Affiliated Hospitals, Dublin, Andrew J. Green, Department of Clinical Genetics, Children’s Health Ireland, Dublin, Ireland, School of Medicine, University College Dublin, Ruth McGowan, West of Scotland Centre for Genomic Medicine, Queen Elizabeth University Hospital, Glasgow; William E. Moody, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham & Philip N. Hawkins, National Amyloidosis Centre, Division of Medicine, UCL, Royal Free Hospital, Rowland Hill Street, London; Division of Medicine, Faculty of Medical Sciences, University College London
CPD 95: hATTR
CPD
Fig 1: Diagnostic testing algorithm for cardiac amyloidosis.18 99mTc technetium-99m, ATTR transthyretin amyloidosis, ATTRv hereditary transthyretin amyloidosis, ATTRwt wild-type transthyretin amyloidosis, AL light-chain amyloidosis, CMR cardiac magnetic resonance, DPD 3,3-diphosphono-1,2propanodicarboxylic acid, ECG electrocardiogram, HDMP hydroxymethylene diphosphonate, PYP pyrophosphate, SPECT single photon emission computed tomography, TTR transthyretin
specialist/multidisciplinary teams and support organizations. Experts shared their current practices, highlighting the process of care and services for the management of patients across the UK and Ireland. Following each meeting, the discussion was summarized in the form of a draft consensus statement which was shared and refined off-line by the expert panel. Following the third panel meeting, all members agreed and approved the final consensus statements.
Separate recommendations are provided for three distinct subgroups: (1) people with symptoms raising a possibility of hATTR amyloidosis; (2) people with biopsy-confirmed ATTR
amyloidosis; and (3) people without symptoms who may have hATTR amyloidosis (i.e. relatives of people with identified TTR variants).
Consensus Statement
People with Symptoms Raising a Possibility of hATTR Amyloidosis
Diagnostic Testing: Steps
The initial steps required for a confirmatory diagnosis of hATTR amyloidosis are determined by clinical signs/symptoms at presentation. The presence of ‘red flag’ neurological signs and symptoms, characteristic of hATTR amyloidosis, should prompt early genetic testing,9 20 while patients with cardiac signs/symptoms require further investigation using technetium-99m 3,3-diphosphono1,2-propanodicarboxylic acid (Tc-DPD) imaging (combined with haematological tests) to exclude light-chain (AL) amyloidosis and confirm ATTR amyloidosis (Fig. 1).18 21 Physicians should be encouraged to arrange diagnostic genetic testing for patients with a positive Tc-DPD imaging scan, to differentiate between hATTR amyloidosis or acquired ATTR wild-type amyloidosis. It is the responsibility of the referring clinician to obtain appropriate consent from the patient for genetic testing.22

Consensus points: Genetic testing should be considered in anyone suspected to have ATTR amyloidosis, as well as in anyone with a positive Tc-DPD scan. When TTR amyloid is suspected, there are two possibilities for testing via a simple blood test: single gene testing (sequencing of the TTR gene alone, particularly in cases where there is a strong suspicion of hATTR amyloidosis) or panel testing (any cardiomyopathy, neuropathy or amyloidosis panel test that includes TTR; the NAC has a hereditary amyloidosis panel that includes the TTR gene). The turnaround may be longer for panel testing, so single gene testing is recommended in cases where there is suspicion of hATTR amyloidosis.
Challenges: Access to genetic testing varies across the UK. In England, the National Genomic Testing Directory specifies the testing criteria for hereditary systemic amyloidosis (R204).23 In Scotland, Ireland, Northern Ireland and Wales, testing can be accessed by physicians in cases where hATTR amyloidosis is suspected.
Recommendations: Genetic testing should be considered early in the diagnostic work-up of patients suspected of having hATTR
amyloidosis. It is the responsibility of the referring clinician to obtain informed consent that is appropriate for the type of test performed on the patient. If a pathogenic variant, likely pathogenic variant or variant of uncertain significance is identified, follow-up should be arranged with Regional Clinical Genetics service to discuss the implications for the family and support cascade testing. Further specialist advice may be sought as required from an amyloidosis centre.
Assessments
For assessment of patients presenting with cardiomyopathy, we recommend the diagnostic algorithm recently proposed by Garcia-Pavia et al.18 (Fig. 1). For assessment of patients presenting with neuropathy, we propose a new diagnostic algorithm as shown in Fig. 2
Multidisciplinary Team and Specialist Support
Once a diagnosis is confirmed, it is important to define the level of support and treatment needed from the specialists and multidisciplinary teams for each patient (and their families).
Consensus points: All identified hATTR amyloidosis gene carriers
32 CPD 95: hATTR
should have access to an amyloidosis specialist or centre.
Recommendations: A baseline consultation with an amyloid disease specialist shortly after genetic diagnosis is recommended for all patients with confirmed hATTR amyloidosis. The purpose of this consultation is to provide context, alleviate patient anxiety, determine next steps for management and provide advice on treatment. A date for follow-up should be arranged following this initial baseline consultation.
People with Biopsy-Confirmed ATTR Amyloidosis
Diagnostic Testing: Steps
Consensus points: Genetic testing should be offered to all patients with biopsy-confirmed ATTR amyloidosis to determine whether the patient is affected by the hereditary form or the more common acquired (or wildtype) form of ATTR amyloidosis. Genetic testing should also be offered to all cases of neurological ATTR amyloidosis to identify the causative TTR variant.9 Testing provides information on the pathogenic TTR genetic variant if present, which can help inform

subsequent disease management choices.9 Genetic testing should generally be organized by the treating physician (often a neurologist or cardiologist, supported where appropriate by geneticists).
Challenges: Ensuring that all treating physicians are aware of the need for genetic testing of patients with ATTR amyloidosis will be a challenge.
Recommendations: Genetic counselling should be recommended to patients at the time of gene testing and followup genetic information should be provided for all patients with a confirmed pathogenic TTR gene variant.24 In addition, all first-degree relatives of the proband should be offered the option for referral to genetic services to discuss the options for pre-symptomatic cascade genetic testing.25, 26
Multidisciplinary Team and Specialist Support
Consensus points: All identified hATTR amyloidosis gene carriers should have access to an amyloidosis specialist or centre.
People Without Symptoms Who May Have hATTR Amyloidosis Variants (i.e. Relatives of People with Identified TTR Variants)
Cascade Genetic Testing and Genetic Counselling
Consensus points: As hATTR amyloidosis is inherited in an autosomal dominant pattern, pre-symptomatic first-degree relatives of a proband may be offered pre-symptomatic genetic testing to determine if they are at risk of developing hATTR amyloidosis.27 However, pre-symptomatic testing of pre-symptomatic children is not recommended until they are adults and can give informed consent to pre-symptomatic testing.26, 27 The exact penetrance of each variant is currently unknown and incomplete penetrance is frequent (i.e. not every gene carrier will go on to develop symptoms).27 28 Geneticists, and genetic counsellors in the UK and Ireland, have an important role in counselling each individual prior to pre-symptomatic genetic testing. All clinical geneticists should have prompt access to a cardiologist/neurologist or other physician with experience in treating patients with amyloidosis.
Fig 2: Diagnostic testing algorithm for neuropathy in patients with symptoms raising a possibility of hATTR amyloidosis *Advice regarding the best location for obtaining biopsy specimens can be obtained from an amyloidosis expert. ATTR transthyretin amyloidosis, DPD 3,3-diphosphono1,2-propanodicarboxylic acid imaging, TTR transthyretin
Challenges: If the proband’s relative is not symptomatic, genetic testing should be requested by genetics professionals, and these relatives should meet with a genetics professional prior to pre-symptomatic testing.
Recommendations: Genetic counselling should always be recommended before pre-symptomatic testing. Healthcare providers are advised to educate patients on the concept of penetrance as part of the counselling process.
Assessments and Management of Pre-symptomatic Carriers
Recommendations: Cardiac magnetic resonance imaging (MRI) using T1 mapping and extracellular
33
volume quantification, and Tc-DPD imaging, can detect pre-symptomatic cardiac disease.29

Neurological examination, nerve conduction studies and skin biopsies for epidermal nerve fibre density and amyloid deposits can detect pre-symptomatic neuropathy.20 27 Cardiac and neurological assessments should be offered within 10 years of the predicted age of onset relating to the phenotype associated with a particular TTR variant. Specific assessments may include cardiac blood biomarkers, electrocardiogram, echocardiography, Tc-DPD imaging, cardiac MRI, serial neurological clinical assessments, serial nerve conduction studies/electromyography, skin biopsies for epidermal nerve fibre density and amyloid deposits, and other assessments of autonomic/ ocular/gastrointestinal symptoms.20 Individual tests should be performed at a frequency deemed appropriate by the treating physician.
Challenges: the optimal frequency at which to repeat these investigations is unknown.
Multidisciplinary Team and Specialist Support
Consensus points: All identified TTR gene carriers should have access to an amyloidosis specialist and/or centre. Such a network of specialist amyloidosis centres is currently in development in the UK and Ireland.
Recommendations: Prompt consultation with an amyloidosis specialist is recommended for all patients who are found to have a pathogenic TTR gene variant. The primary aim of this consultation is to manage potential anxiety and to develop a plan for future monitoring at a frequency determined by the age of onset in the family, or in case of an index case, based on overall average age of onset. Moreover, patient information should be provided at an early stage and patients should be entered into a programme offering continued clinical surveillance. People with pre-symptomatic/potential disease are more likely to present earlier and engage with follow-up and supportive care if treatments are available.
Discussion
This publication is the outcome of an extensive consultation with experts from the UK and Ireland to outline the challenges and recommendations for genetic testing (and diagnosis) of hATTR amyloidosis. Our aim was to provide practical guidance to treating physicians, and emerging amyloid specialist centres, on genetic testing for patients with suspected symptoms of hATTR amyloidosis and biopsy-confirmed ATTR amyloidosis, as well as pre-symptomatic (and symptomatic)
family members affected by this disease. We believe that this statement is timely and relevant, as the number of confirmed cases of hATTR amyloidosis continues to grow (and may be much larger than previously recognized), owing to the increasing numbers of people coming forward for cascade genetic testing. The rapid and progressive nature of the disease (and the burden on patients and their families30) means that, with effective treatments now available, there is a clear need for timely screening, identification and follow-up of at-risk individuals to initiate treatment at the earliest onset of symptoms. Survival from symptom onset in patients with hATTR amyloidosis ranges from approximately 12 years in patients with the neurological phenotype (such as early-onset V50M) to less than 3 years in patients with cardiac symptoms associated with variants such as T80A and V142I.31 There is emerging evidence from the NAC, as well as other centres across Europe, that with the advent of Tc-DPD imaging, patients with hATTR amyloidosis are being diagnosed at an earlier disease stage—with associated improved survival time.32
In addition to the recommendations provided in this statement, we strongly advise treating physicians to encourage their patients with hATTR amyloidosis, as well as presymptomatic relatives, to participate in research and to enrol in independent, outcome-based registries run in collaboration with the European Rare Disease Network or the TRANSCEND study. The TRANSCEND study is a prospective, observational study aimed at improving our understanding of the natural history of hATTR amyloidosis and its response to treatment.
More than 130 amyloidogenic TTR variants have been identified worldwide;1, 2 Penetrance is an important consideration in hATTR amyloidosis, as it may be incomplete depending on the TTR variant. Thus, some variant carriers may remain asymptomatic for life while other TTR variants are associated with very rapid disease progression.33, 34 In Ireland, T80A (previously known as T60A) is the most common pathogenic variant with a founder population originating from the Donegal region of Ireland, typically giving rise to an hATTR amyloidosis-mixed phenotype.35 Meanwhile, in the UK, the predominant disease-causing TTR variants are V142I, T80A and V50M.14, 17
In conclusion, this consensus statement provides best practice recommendations for the genetic testing and management of people with symptoms raising a possibility of hATTR amyloidosis, patients with biopsyconfirmed ATTR amyloidosis and presymptomatic TTR gene variant carriers. We believe these recommendations will help increase the identification of affected individuals, help them gain access to regular follow-up and, if needed, treatment to reduce disease progression once they become symptomatic.
References available upon requst
34 CPD 95: hATTR
Bortezomib 2.5mg/ml Solution for Injection


Bortezomib is provided as a ready to use formulation for subcutaneous injection, and after dilution for intravenous injection.
Therapeutic Indications
Bortezomib as monotherapy or in combination with pegylated liposomal doxorubicin or dexamethasone is indicated for the treatment of adult patients with progressive multiple myeloma who have received at least 1 prior therapy and who have already undergone or are unsuitable for haematopoietic stem cell transplantation.
Bortezomib in combination with melphalan and prednisone is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for high-dose chemotherapy with haematopoietic stem cell transplantation.
Special Warning
Bortezomib should not be administered intrathecally.
Prescribing information
Bortezomib in combination with dexamethasone, or with dexamethasone and thalidomide, is indicated for the induction treatment of adult patients with previously untreated multiple myeloma who are eligible for high-dose chemotherapy with haematopoietic stem cell transplantation.
Bortezomib in combination with rituximab, cyclophosphamide, doxorubicin and prednisone is indicated for the treatment of adult patients with previously untreated mantle cell lymphoma who are unsuitable for haematopoietic stem cell transplantation.

Refer to the Full Summary of Product Characteristics (SmPC) before prescribing. Name and active ingredients: Bortezomib 2.5mg/ml solution for injection. 1ml solution for injection contains 2.5mg bortezomib (as a mannitol boronic ester). Pharmaceutical form: Solution for injection. Clear, colourless to light yellow solution with a pH of 4.0 – 5.5. Indications: As monotherapy or in combination for the treatment of adult patients with progressive multiple myeloma who have received at least 1 prior therapy and who have already undergone or are unsuitable for haematopoietic stem cell transplantation. In combination for the treatment of adult patients with previously untreated myeloma. For full details, refer to SmPC section 4.1. Posology and method of administration: The recommended dose is determined by the indication being treated. See SmPC section 4.2 for dosage, preparation and administration. Each vial contains an additional overfill of 0.2ml. Contraindications: Hypersensitivity to the active substance, to boron or to any of the excipients listed in section 6.1 of the SmPC. Acute diffuse infiltrative pulmonary and pericardial disease. When Bortezomib is given in combination with other medicinal products, refer to their Summaries of Product Characteristics for additional contraindications. Special warnings and precautions for use: Bortezomib is a cytotoxic agent. Therefore, caution should be used during handling and preparation of Bortezomib. Use of gloves and other protective clothing to prevent skin contact is recommended. Pregnant personnel should not handle this medicinal product. Aseptic technique must be strictly observed throughout the handling of Bortezomib, since it contains no preservative. There have been fatal cases of inadvertent intrathecal administration of bortezomib. Bortezomib is for subcutaneous or - after dilution - intravenous use. Bortezomib should not be administered intrathecally. For full details, refer to SmPC section 4.4. Interactions: The list of products to avoid concomitant use of or to closely monitor the use of can be found in SmPC section 4.5. Fertility, pregnancy and lactation: Female patients of childbearing potential and male patients must use effective contraceptive measures during and for 3 months following treatment. See SmPC section 4.6 for full details. Undesirable effects: Very common (≥1/10): thrombocytopenia, neutropenia, anaemia, decreased appetite, neuropathies, peripheral sensory neuropathy, dysaesthesia, neuralgia, nausea and vomiting symptoms, diarrhoea, constipation, musculoskeletal pain, pyrexia, fatigue and asthenia. For full information on adverse reactions, see SmPC section 4.8. Pack size: 1 x vial of 3.5mg/1.4ml. Legal Classification: POM. Marketing Authorisation Number: PA1774/008/001. Distributed by: Uniphar on behalf of Athlone Pharmaceuticals Ltd. For a copy of the SmPC or further medical information, please contact (00353) 86 8394447 or medical@kent-athlone.com Date of preparation: August 2022. Ref: IE22/001/SmPC February 2022. Additional information available on request.
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie) or by email medsafety@hpra.ie Adverse events should also be reported to Kent Athlone Pharma Group on (090) 666 1109 or medical@kent-athlone.com
Your partner of choice, when excellence matters.
For further information on this product, please contact Gavin Butler, Key Account Manager, Athlone Pharmaceuticals Ltd., Ballymurray, Co. Roscommon Tel: + 353 (0) 87 3354523 | Email: Gavin.Butler@kent-athlone.com
Date of preparation: November 2022 Ref: IE2022/015/00
Vial contains a 0.2ml overfill
Migraine
BoNT-A
efficacy in
high frequency migraine: an open label, single arm, exploratory study applying the PREEMPT paradigm
Written by Daniele Martinelli, Sebastiano Arceri and Cristina Tassorelli & Colleagues, Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy
Exclusion criteria
• Previous failure of >2 treatments with migraine preventive medications in order to avoid pharmacoresistance as a confounding variable;
◦ Migraine onset after age 50;
• Other primary/secondary headaches. Episodic tension-type headache was allowed if the patient could clearly distinguish the two types of attacks;
◦ Clinically relevant medical conditions;
◦ Hypersensitivity to BoNT-A or ‘Botox® ingredients.
This clinical trial was conducted in accordance with the principles laid down by the 18th World Medical Assembly (Helsinki, 1964), the ICH guidelines for Good Clinical Practice and it was approved by the local Ethics Committee. All subjects signed an informed consent before enrolment.
Primary endpoints
Migraine is a disabling condition that affects more than 14% of the population. The burden of migraine largely depends on the frequency of the attacks with which the disease manifests itself. Based on the number of monthly migraine days (MMD), migraine has been subdivided into low-to-moderate frequency (up to 7 MMD), high frequency (8–14 MMD) and chronic subtypes (≥15 monthly headache days),1 for both practical and research reasons.2 These numerical cut-offs have provided a rational framework for the allocation of resources and for the conduction of research studies and drug trials.
High frequency episodic migraine (HFEM) is associated to an increased risk of transformation into chronic migraine (CM).1 In this frame, focusing on preventive treatments that address specifically HFEM becomes extremely important both for lowering migraine
frequency and for preventing transition to chronic migraine.
In recent years, the headache field has witnessed a terrific development of new therapeutic strategies addressing the needs of migraine sufferers. Four monoclonal antibodies targeting calcitonin gene-related peptide (CGRP) are now available for the treatment of migraine with at least 4 MMD, but they are expensive and, in most countries, their subsidization is restricted to subjects who have previously failed at least 3 preventive treatments.3
Onabotulinum toxin-A (BoNT-A) proved effective in the prevention of chronic migraine in the large investigational program (Phase III REsearch Evaluating Migraine Prophylaxis Therapy, PREEMPT).4 Prior to the PREEMPT program, the efficacy of BoNT-A had also been evaluated in episodic migraine5–9 with conflicting results, probably due to methodological reasons. Indeed, the different
studies used lower doses and less injection points as compared to the PRREMPT paradigm. The aim of this exploratory study is to evaluate the efficacy and safety of BoNT-A delivered according to the PREEMPT paradigm in subjects with HFEM.
Methods
This is an open label, exploratory, single arm interventional trial conducted at a tertiary Headache Centre of the IRCCS Mondino Foundation Hospital in Pavia, Italy (March 2018- May 2020, reg. n° NCT 04578782 clinicaltrial.gov).
Inclusion criteria
• Migraine without or with aura,10 with a documented frequency of 8–14 migraine days/month (MMD) in the 3 months before screening and in the 28 days run-in period (documented via a prospective headache diary);

◦ 18–65 years of age.
• Reduction from baseline (run-in period) in the number of MMD in the 12-week period following the last BoNT-A treatment.
Secondary endpoints
Changes from baseline of the following variables during the last 12 weeks of the study:
• Monthly moderate-to-severe headache days;
• Monthly use of acute drugs;
• Migraine-related disability (MIDAS questionnaire);
• Quality of life (Migraine Specific Questionnaire, MSQ);
• Levels of anxiety and depression (Hospital Anxiety and Depression Scale, HADS).
Protocol design (Figure 1)
After the screening visit (V1), subjects entered the run-in period of 4 weeks. Enrolled subjects received BoNT-A according to the PREEMPT paradigm (155 U, 31
36 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Headache Science and Neurorehabilitation Center, IRCCS Mondino Foundation, Pavia, Italy

BOTOX® is indicated for symptom relief in adults fulfilling criteria for chronic migraine (headaches on ≥15 days per month of which at least 8 days with migraine) in patients who have responded inadequately or are intolerant of prophylactic migraine medications1 Legal category POM (S1A). Marketing Authorisation Numbers: PA 1824/017/1-3. Further information is available on request from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, D24 XN32 or at www.medicines.ie IE-BCM-220002 Date of preparation: April 2022 References: 1. BOTOX® Summary of Product Characteristics available on www.medicines.ie
Migraine
Figure 1
sites) (V2). Treatment was repeated 3 times every 12 weeks (V3-4-5). The final visit (V6) occurred at week 48. From the second BoNT-A cycle, the injection paradigm could be upgraded to the PREEMPT ‘followthe-pain’ version (195 UI, 39 sites), based on clinical judgement.

From V1 to V6 subjects completed daily a headache diary. MIDAS, MSQ and HADs were obtained at all visits. A monthly phone call was scheduled to check for adverse events.
Statistical analysis
For the primary outcome, assuming a minimum clinically meaningful mean difference of 2.0 MMD and an SD of 2.0, power of 80%, and α=.05, a sample size of at least 32 records (that is at least 16 participants completing the trial) was required (calculated with Open Source Epidemiologic Statistic for Public Health (www.openepi.com).11
Analysis was performed in the per-protocol population formed by participants who received the intervention and completed the 48-week follow-up. No imputation was done for the missing values. Variables were assessed for normality of distribution by evaluating skewness and kurtosis. MANOVA (multivariate analysis of variance) for repeated measures, with post-hoc Bonferroni’s correction, was used to analyse comparison over time of variables.
Results
Of the 50 screened subjects, 32 were enrolled in the study and 28 completed the trial. Reasons for dropping out were: adverse events (n. 1), consent withdrawal (n. 2), SARS-CoV-2 pandemic outbreak (n. 1).
At baseline, the population enrolled (81% women) had a mean age of 44.8 ± 11.9 years, 11.5 ± 2.2 headache days, of which 11.1 ± 2.3 were migraine days. The disease duration was 26.6 ± 13.5 years and neither major comorbidity nor pharmacoresistance were reported. Notably most of the patient presented none or just one previous failure of preventive treatment.
Ten patients were on stable concomitant preventive therapy (betablockers: 6, calcium channel antagonists: 1 and antiepileptic drugs: 3) throughout the entire trial period.
Migraine-associated disability was severe in all patients (MIDAS grade IV).
BoNT-A reduced the number of MMDs by 3.68 days ( 33.1%, p < 0.001) on average in the last 12-week observation period compared to baseline (Figure 2a). Thirty-nine percent of the patients had at least a 50% reduction in migraine days. BoNT-A also significantly reduced the number of moderate-to-severe headache days ( 33.9%, p < 0.001), the number of acute medications ( 22.9%, p = 0.029) and the days of their intake ( 24.6%, p = 0.021). All the endpoints were met already after the first administration and the benefits persisted throughout all the trial as shown in Figure 2b.
MIDAS and MSQ scores significantly improved since the first trimester ( 41.7%, p = 0.001 and 31.7%, p < 0.001 respectively in the last observation period).
Anxiety and depression levels declined but the variation did not reach a statistical significance.
Adverse events were transient and mild-to-moderate in severity. Only one patient discontinued the study drug due to a mild and selflimiting cutaneous allergic adverse reaction in the site of injection.
Discussion
To our knowledge, this is the first registered trial addressing the efficacy of BoNT-A delivered according to the PREEMPT paradigm specifically targeting subjects with HFEM. Our findings show that BoNT-A significantly reduced the frequency of migraine, the intake of acute medications and the burden of disease.
Previous studies investigating BoNT-A efficacy in episodic migraine yielded conflicting results. Some of these studies used markedly lower doses of drug, less or different injection sites, different volumes of injections and shorter treatment durations.5–7,9 Interestingly, using an approach similar to the present study over 3 treatment cycles, Aurora et al. failed to show efficacy in episodic migraine, but detected a signal of efficacy in the subgroup of migraine subjects with 12–15 headache days/month.8
The possible efficacy of BoNT-A in HFEM is also suggested by the findings reported in a realworld study where MMDs at 12 months decreased by 41.2% and analgesic use by 39.3%.12
BoNT-A disrupts neuropeptide secretion and receptor translocation related to trigeminal nociception, hence preventing pain sensitization through peripheral and possibly central mechanisms. Peripheral and central sensitization are deeply implicated in chronic migraine (13), and HFEM is clinically, and probably also
pathophysiologically, a transition state from low-moderate frequency episodic migraine to chronic migraine (1). Therefore, a drug that effectively counteracts trigeminal sensitization does hold promise in HFEM treatment, as already proven in CM.
In the evolving scenario of migraine prevention, the possibility to provide an additional therapeutic option to a subpopulation of migraine patients with a high burden of disease is extremely important to alleviate the impact of disease and, possibly, to prevent its transition to CM.
The limitations of the study are intrinsically linked to its design. It is however important to note that this is an investigator-initiated trial with very limited funding, but methodologically powered for the primary outcome measure. This latter was selected at a quite long follow-up to minimize the confounding effect of placebo. This said, caution is suggested with the present findings, until they are confirmed on larger-scale placebo-controlled studies.
Conclusion
In this study BoNT-A proved effective in the prevention of HFEM by significantly improving multiple outcome measures. The future possibility to use BONT-A in HFEM patients would be extremely important to improve their clinical condition and to minimize the risk of transformation into chronic migraine.
References available on request
38 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
New CEO for HSE
The HSE Board has approved the appointment of Mr Bernard Gloster as Chief Executive of the HSE, following an open, competitive selection process. Mr Gloster, who is currently Chief Executive of the state Child and Family Agency Tusla, is expected to take up the position in Spring 2023.
Before joining Tusla as CEO in September 2019, Mr Gloster spent more than 30 years working in the health services. He held several senior management
positions including Chief Officer of HSE Mid West Community Healthcare, and worked in or managed in both community and acute hospital operations.

He is a social care worker by profession, holds an MBA from Oxford Brookes University and an MSc in Management Practice from UCC.
Announcing the appointment the Chairman of the HSE Board Mr Ciarán Devane said: “I am delighted that someone of Bernard’s experience, both
within the HSE and externally within Tusla, is taking up this role. Bernard’s track record and commitment to public service will be invaluable as the HSE, like health services elsewhere, enters a period requiring great change and development.”
Welcoming the appointment, the Minister for Health Stephen Donnelly TD said: “Bernard Gloster is a public servant with a track record of innovation and reform across health and social care services over many years.
At a time of unprecedented investment in the HSE I know he is deeply committed to ensuring that the Irish health service becomes one of the best places in the world in which to be treated, and to work in. I am delighted that he is to take up this role. The challenge of leading our health services is among the most important roles in our public service and I look forward to working closely with Bernard as we work towards our goal of delivering universal health care for our people.”
39 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 Figure 2a & 2b News
Parkinson’s Disease

European Academy of Neurology/Movement Disorder Society
- European Section guideline on the treatment of Parkinson's disease: I. Invasive therapies - A Summary
lesioning of the thalamic ventralis intermedius (Vim) nucleus for tremor can be replaced by electrical stimulation.15 After the discovery of the pathophysiologic role of the subthalamic nucleus (STN) by Bergman and colleagues,16 DBS electrodes were implanted in this nucleus17 to improve the broader symptom spectrum of PD. Radiofrequency thermocoagulation pallidotomy18 was subsequently also largely replaced by electrical stimulation of this nucleus. A large number of case series19 and finally randomized controlled studies have established the concept of DBS of the STN or the globus pallidus internus (GPi) for the treatment of PD.20
only in a few movement disorder centers since then.25, 28 During the past decade, it has been used more widely, in parallel with the technological advances of infusion pump devices. Apomorphine can also be administered using intermittent subcutaneous pen injection for individual off periods, which is not discussed here.
Human fetal29, 30 or stem cell transplantation or gene therapy31-33 are experimental treatments of PD and have currently no relevance for clinical care.
Scope
The first European guideline on the treatment of Parkinson's disease (PD) was published in 2006 consisting of two parts, the early uncomplicated disease1 and late complicated disease,2 and were renewed in 2013 as recommendations of European Federation of Neurological Societies/Movement Disorder Society (MDS) for the diagnosis3 and management4 of PD. The European Academy of Neurology (EAN) in collaboration with the European section of the MDS (MDS-ES) has now begun to produce regular updates of the guidelines (GLs5) according to GRADE methodology. The GL task force has been set up from the two societies to realize this task. The new PD GLs will be separated into several chapters of which this review on invasive therapies is the first. We have included interventions requiring surgery or invasive medication delivery. Invasive treatments are usually considered for advanced PD and cover deep brain stimulation (DBS), pump therapies, and lesional therapies for the treatment of PD.
Radiofrequency thermocoagulation of the thalamus and pallidus internus has a long history of
treatment for PD. Initiated in the 1950s,6 it was practiced around the world in the following decades.7-10 These treatments were initially used because effective drugs to treat bradykinesia and tremor were not available yet. However, when levodopa (L-dopa) became available in the 1970s, lesional surgeries were largely abandoned with the exception of pallidotomy in some countries and thalamotomies for tremors unresponsive to oral treatments, both on a small scale. However, the occurrence of L-dopa–induced fluctuations and dyskinesia triggered the search for better interventions. As a result, lesional pallidotomies were rediscovered, and subsequently DBS started its steep rise in the 1990s. Because of the general impression that DBS results in fewer complications,11 which was confirmed by a randomized controlled trial in the year 2000,12 DBS has become the most frequently used intervention, and lesional procedures have usually been reserved for specific situations.
DBS developed out of the aforementioned radiofrequency thermocoagulation of the thalamus.13, 14 Benabid and colleagues discovered that
Radiosurgery of the Vim with proton beams has also been used since the late 1960s in few centers worldwide21, 22 initially only for this nucleus. Although these lesional procedures showed some benefits, few recent publications are available and are mostly case series, and detailed reports of adverse effects or longterm consequences are lacking. A very recent development is the introduction of magnetic resonance imaging–guided focused ultrasound surgery (MRgFUS). This is an incisionless therapy that has mainly been assessed for essential tremor, and trials for PD are only beginning. This technique is currently used within the thalamus but lesioning of the STN was also recently reported.23
Among pharmacological interventions, the concept of reducing dopaminergic hypersensitivity and maintaining constant plasma levels by continuous stimulation of dopaminergic terminals has emerged,24 resulting in the treatments of continuous subcutaneous infusion of apomorphine25 or intrajejunal application of L-dopa–carbidopa intestinal gel (LCIG) preparations.26 Apomorphine hydrochloride (apomorphine) has been proposed for the treatment of PD since the 1950s,27 but it had been used
Although there are individual GLs and evidence-based medicine reviews on DBS and radiofrequency thermocoagulation lesions, the whole spectrum of invasive interventions for PD has not been addressed in previous GLs. Furthermore, only the NICE-GL 2017,34 which had a more limited focus, used GRADE-methodology. The invasive interventions for PD share several features and merit a combined review: All are currently mostly used for advanced stage PD except STN-DBS and MRgFUS. They are all considered invasive procedures as apomorphine infusion requires continuous subcutaneous infusion, LCIG needs a percutaneous jejunostomy, MRgFUS is an incisionless intracerebral lesioning, and DBS and radiofrequency thermocoagulation require brain surgery through a burr hole. Furthermore, all are more expensive than standard drug treatments for PD. Health economics and cost-effectiveness of those interventions are beyond the scope of these GLs, but existing systematic reviews are available.35
Methods
The methodology for the development of these GLs has followed the framework provided by GRADE36 and the recommendations of the EAN on the development of a neurological management GL.5
40 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Written by Günther Deuschl, Angelo Antonini, Joao Costa, Katarzyna �mi�owska, Daniela Berg, Jean-Christophe Corvol, Giovanni Fabbrini, Joaquim Ferreira, Tom Foltynie, Pablo Mir, Annette Schrag, Klaus Seppi, Pille Taba, Evzen Ruzicka, Marianna Selikhova, Nicholas Henschke, Gemma Villanueva, Elena Moro
*Levodopa-Carbidopa Intestinal Gel 1. Lecigon Summary of Product Characteristics, 2021. ABBREVIATED PRESCRIBING INFORMATION. Lecigon 20 mg/ml + 5 mg/ml + 20 mg/ml intestinal gel. 1 ml contains 20 mg levodopa, 5 mg carbidopa monohydrate and 20 mg entacapone. Presentation: Yellow or yellowish-red opaque viscous gel. Indications: Treatment of advanced Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia when available oral combinations of Parkinson medicinal products have not given satisfactory results. Dosage and administration: Adults/Elderly: Administration by a portable infusion pump directly into the duodenum or upper jejunum via a percutaneous endoscopic gastrostomy(PEG) tube or radiological gastrojejunostony tube. Please consult Summary of Product Characteristics (SmPC) for further information. Only pump Crono LECIG (CE 0476) may be used for the administration of Lecigon. The dose should be titrated to achieve the optimal clinical response in the individual patient, which involves maximising the functional ON-time during the day by minimising the number and duration of OFF episodes (bradykinesia) and minimising ON-time with disabling dyskinesia. Total dose/day of Lecigon is composed of three individually adjusted doses: the morning bolus dose, the continuous maintenance dose, and extra bolus doses. Treatment is usually limited to the patient’s awake period. If medically justified, Lecigon can be administered up to 24 hours/day. The maximum recommended daily dose is 100 ml (2000 mg levodopa, 500 mg carbidopa monohydrate and 2000 mg entacapone. Please consult SmPC for further information. Total morning dose is usually 5–10 ml (100–200 mg levodopa) but not exceeding 15 ml (300 mg levodopa). Continuous maintenance dose is usually 0.7–5.0 ml/hour (15–100 mg levodopa/hour). Extra bolus doses are given if the patient becomes hypokinetic and are normally less than 3ml. An increase in the continuous maintenance dose should be considered if the need for extra doses exceeds 5 doses per day. Please consult SmPC for further information. After initial titration, the morning dose and maintenance dose are fine-tuned over the course of a few weeks. Lecigon is initially given as monotherapy. If needed, other anti-Parkinsonian medicinal products can be taken concurrently. If treatment with other anti-Parkinsonian medicinal products is discontinued or changed, it may be necessary to adjust the doses of Lecigon. Sudden deterioration in response with recurring motor fluctuations may indicate that the tube has dislocated to the stomach. This needs confirmation by X-ray and may require repositioning. Please consult SmPC for further information. The cartridge is for single use only and should not be used for more than 24 hours. Children: There is no relevant indication for use in children and adolescents. Contraindications: Hypersensitivity to the active substances or any of the excipients, narrow-angle glaucoma, severe heart failure, severe cardiac arrhythmia, acute stroke, severe hepatic impairment. Non-selective MAO-inhibitors and selective MAO type A inhibitors are contraindicated and should be discontinued at least two weeks prior to initiating therapy with Lecigon. Conditions in which adrenergics are contraindicated. Previous neuroleptic malignant syndrome (NMS) and/or non-traumatic rhabdomyolysis. Suspected undiagnosed skin lesions or a history of melanoma. Please consult SmPC for further information. Warnings and precautions: Not recommended for the treatment of drug-induced extrapyramidal reactions. Caution in ischaemic heart disease, severe cardiovascular or pulmonary disease, bronchial asthma, renal, hepatic or endocrine disease, or history of peptic ulcer disease or of convulsions, past or current psychosis, chronic wide-angle glaucoma, concomitant administration of antipsychotics with dopamine receptor blocking properties or with medicines which may cause orthostatic hypotension. In patients with a history of myocardial infarction who have residual atrial nodal or ventricular arrhythmias, cardiac function should be monitored with particular care during the period of initial dosage adjustments. Monitor all patients for mental changes, depression with suicidal tendencies, and other serious mental changes. Neuroleptic malignant like syndrome with secondary rhabdomyolysis may occur on abrupt dose reduction/discontinuation of Lecigon. Patients should be monitored for the development of impulse control disorders and review of treatment is recommended if such symptoms develop. Patients and caregivers are advised to monitor for melanomas on a regular basis when using Lecigon. Dose may need to be adjusted downwards to avoid levodopa-induced dyskinesia. Periodically evaluate hepatic, haematopoietic, cardiovascular and renal function during extended therapy. Lecigon contains hydrazine, a degradation product of carbidopa that can be genotoxic and possibly carcinogenic. Reported complications for levodopa/carbidopa in clinical studies include bezoar, ileus, implant site erosion/ulcer, intestinal haemorrhage, intestinal ischaemia, intestinal obstruction, intestinal perforation, intussusception, pancreatitis, peritonitis, pneumoperitoneum and post-operative wound infection. Sudden or gradual worsening of bradykinesia may indicate an obstruction in the tubing system and should be investigated. Weight loss has been associated with the active substances contained in Lecigon, and caregivers should therefore be aware of weight loss. Monitoring of weight is recommended to avoid severe weight loss. Prolonged or persistent diarrhoea that appears during use of entacapone could be a sign of colitis. In case of prolonged or persistent diarrhoea, treatment with the medicinal product should be discontinued and other appropriate medical treatment and investigation considered. Replacement of Lecigon with either levodopa and a DDC inhibitor without entacapone or other dopaminergic therapy may be necessary and should be done slowly. For patients who experience progressive anorexia, asthenia and weight loss within a relatively short period of time, a general medical evaluation including liver function assessment should be considered. Please consult SmPC for further information. Interactions: Antihypertensives, antidepressants, anticholinergics, dopamine receptor antagonists, benzodiazepines, isoniazide, phenytoin, papaverine, sympathicomimetrics , iron, protein-rich diet, amantadine and dopamine agonists (e.g. piribedil) may increase levodopa-related adverse events. Lecigon dose adjustment may be needed when used with these medicines. Lecigon can be taken with MAO type B inhibitors (e.g. selegiline) although serious orthostatic hypotension may occur and the dose of levodopa may need to be reduced. Lecigon may affect metabolism of medicinal products such as S-warfarin and patients should be monitored during initiation with Lecigon therapy when used with this medicine. Please consult SmPC for further information. Pregnancy and lactation: Lecigon is not recommended during pregnancy or in women of childbearing potential not using contraception unless the benefits for the mother outweigh the possible risks to the foetus. It is unknown whether carbidopa and entacapone or their metabolites are excreted in human milk. Breastfeeding should be avoided during treatment with Lecigon. Driving and operation of machinery: Caution; Lecigon can have a major influence on the ability to drive and use machines. Refrain if somnolence and/or sudden sleep episodes occur. Undesirable effects: Weight loss, anxiety, depression, insomnia, dyskinesia, Parkinson’s disease /exacerbation of parkinsonism (e.g. bradykinesia), orthostatic hypotension, nausea, constipation, diarrhoea, pain in muscles and tissues, musculoskeletal pain, chromaturia, fall. Complications of the device and surgery: Postoperative wound infection, abdominal pain, excessive granulation tissue, complication of device insertion, incision site erythema, post-procedural discharge, procedural pain, procedural site reaction. Refer to SmPC for other undesirable effects. Adverse events should be reported via HPRA Pharmacovigilance, website: www.hpra.ie. Special precautions for storage: Store in a refrigerator 2°C - 8°C. Do not freeze. Store in the original package in order to protect from light. For storage instructions after first opening of the medicinal product, refer to the summary of product characteristics. Pack size: 7 x 47 ml cartridges. Marketing authorisation holder: LobSor Pharmaceuticals AB, Kålsängsgränd 10 D, SE-753 19 Uppsala, Sweden. Distributed by Clonmel Healthcare Ltd, Clonmel, Co. Tipperary. Marketing authorisation number: PA23144/001/001. Medicinal
is available upon request or alternatively please go to: www.clonmelhealthcare.ie Last revision date: March 2022. Date of Preparation: October 2022.

A combination of three effective Parkinson’s therapies in one convenient gel formulation for intestinal infusion1
product characteristics
Standard LCIG* Levodopa (20 mg/ml) Carbidopa monohydrate (5 mg/ml) Entacapone (20 mg/ml) Increased levodopa bioavailability with LECIGON® ... a reduced levodopa dose can be given The same effective and stable plasma levodopa levels are achieved
product subject to medical prescription. A copy of the summary of
2022/ADV/LEC/268H
Parkinson’s Disease
Figure 1: Recommendations for invasive therapies tested in different patient groups with randomized controlled studies (for details of the recommendations, see the text). To facilitate the reader's overview, we present them along with this guideline's abbreviated recommendations for the various interventions. DBS, deep brain stimulation; Gpi, pallidum internum; L-dopa, levodopa; MRg, magnetic resonance imaging guided; PD, Parkinson's disease; STN, subthalamic nucleus; Vim, ventralis intermedius
Population/intervention/ comparison/outcome (PICO) questions were constructed according to GRADE standards and are shown in the Appendix S1 Methods section. References used in the current GLs were identified by performing a systematic search in the PubMed, Embase, Web of Science, and Cochrane Library databases as well as clinical trials registration via clinicaltrials.gov in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.37 The literature search for each PICO was conducted from the earliest possible date to December 31, 2020. Two people (from either EAN [K.S., G.D., E.M., A.A.] or Cochrane Response [G.V., N.H.]) independently screened all citations and abstracts identified by the search. Articles were selected based on the following eligibility criteria: (1) original research and/or (2) randomized clinical trial (RCT) and/or (3) metanalysis/systematic review and (4) performed in people with PD. Data were extracted in DistillerSR and summarized in GRADE evidence profiles. The writing group (G.D., A.A., J.C., K.S., E.M.) of the GLs shared the tasks of the systematic review and developed the first draft of the GLs. Every member of the GL group voted on each of the recommendations. Members with a possible conflict of interest for specific questions abstained from voting. Cochrane Response, a fee-for-service organization of Cochrane, was assisting the GL task force to produce these GLs.
For further details regarding search strategy, study selection, data extraction, assessment of risk of
bias, data synthesis, summarizing and interpreting results, see the full article Appendix section (details at end of article).
Outcomes
These GLs considered the following three main aspects important for clinical decision-making:
1. Objective outcomes are usually graded by the clinician with clinical scales and include measures of mobility as well as nonmotor outcomes if they are not patient reported (eg, instrumented measures of mobility, sleep). A typical example is the Unified Parkinson's Disease Rating Scale Part III (UPDRS-III).
2. Measures of function and/or well-being of the patient. The majority of reported outcomes are based on patient-reported outcome measures such as health-related quality of life (QoL; eg, 39-Item Parkinson's Disease Questionnaire [PDQ39]) or are completed by the clinician based on an interview with the patient (eg, Schwab and England scale, Unified Parkinson's Disease Rating Scale Part II [UPDRS-II]).

3. Adverse events (AEs) are to be balanced against the benefit. AEs are defined as any undesirable experience associated with the use of a medical (or a device) product in a patient and can cover all aspects of health. AEs are standardized into serious AEs (SAEs) and AEs. Death, life-treating conditions, hospitalization and prolonged hospitalization, disability,
and permanent damage are considered SAEs. For surgical studies, surgical SAEs are considered separately.
‘Critical’ and ‘important’ outcomes were defined in the PICOs. The critical outcomes were considered more relevant for decision making than the “important” outcomes. The data were analyzed as short-term effects (≤12 months) and medium-term effects (>12 months). For each critical and important outcome, the pooled estimates of effect are presented with 95% confidence intervals with the anchor-based minimal clinically important change (MCIC) for each outcome, the effect size as expressed by Cohen's d, the number of studies and participants, and the GRADE rating for the certainty of evidence. The MCIC was derived from previous studies and was the subject of a consensus within the GL group. Whenever anchorbased MCIC were not available, Cohens' d as a distribution-based statistical measures was used as the only benchmark.
Results and Recommendations
In total, the database search yielded 2600 articles on nonlesional therapies. Based on the aforementioned criteria, 13 studies were included. Specifically, eight studies on DBS versus best medical treatment (BMT), two studies on STN-DBS versus GPiDBS, one study on apomorphine, and two studies on LCIG.
For lesional therapies, 1297 articles were screened, and three studies were included (two on radiofrequency thermocoagulation of the pallidum and one on MRgFUS therapy).
Interventions, targeted brain structures, and patient profiles discussed in these GLs
The studies were to be separated by the following intervention types: DBS with implantation of an electrode that reversibly modulates brain circuits, radiofrequency thermocoagulation producing thermal localized lesions. Both procedures require brain surgery through a burr hole for each brain side. Radiosurgery is producing brain lesions with stereotactic radiation through the intact scalp. MRgFUS is producing lesions with high-energetic focused ultrasound. Both procedures are incisionless. Apomorphine pump treatment is applied through subcutaneous continuous infusion, and LCIG requiring a permanent percutaneous jejunostomy for infusion of L-dopa into the duodenum. The studies were also separated for the targeted nucleus in the brain (STN, GPi, and Vim). For early PD with early fluctuations and for early PD without fluctuations, studies have considered only the STN as the DBS target. For advanced PD, four of the six studies included only STN-DBS patients, whereas two studies had patients with mixed targets: one with 51/121 GPi, 60/121 STN, and 134 BMT40 and the other with 4/178 GPI, 174/178 STN, and 183 BMT.41
The benefits and risks of STN-DBS versus GPi-DBS are presented in a separate paragraph as there are two large RCTs comparing the effects between the two targets.42, 43 Mostly uncontrolled trials with radiofrequency thermocoagulation have been published for Vim,
42 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
GPi, and STN. MRgFUS and radiosurgery trials are available for Vim and STN. Finally, the different RCTs have recruited different patient groups. Most invasive interventions were tested for patients with advanced PD with fluctuations and dyskinesia that can no longer be satisfactorily treated with oral medication. This also includes treatment-resistant tremor.44 The group is labeled here as advanced PD. Patients with motor fluctuations since <3 years after onset can usually still be treated with medication but were specifically tested in RCTs and are labeled as early PD with early fluctuations.
An additional patient group are those who are not yet fluctuating and still have a favorable response to drugs even if extended medication regimens are needed. They are called early PD without fluctuations. Two additional specific patient groups are those with treatment-resistant tremor and those with predominant unilateral symptoms.
Figure 1 gives an overview of the different aspects of these GL recommendations.
Evidence, summary of findings, and recommendations
Presentation of the data is separated for nonlesional and lesional therapies. The effects of the interventions are discussed in the next sections and shown in an abbreviated form in Figure 2. They are also partly repeated in the next paragraphs. The wording of the results follows the suggestions of GRADE. All comparisons of
effects and adverse effects are against standard noninvasive treatments (medication).
Summary of findings
These RCTs show that STNDBS probably results in a large improvement of QoL, a large improvement of activities of daily living (ADLs), and a large improvement of motor impairment in these patients. There is likely to be a large effect on motor fluctuations and dyskinesia, the typical complications of long-term medical therapy, particularly with a moderate increase in daily ON time and a moderate reduction in daily OFF time. Hoehn and Yahr stage may be slightly improved. Cognition and depression as important outcomes are likely neither improved nor worsened. SAEs are more common for the surgically treated patient group than for those on BMT. A total of 152 SAEs are described in 604 patients (25%) in the DBS group versus 52 SAEs/469 patients (11%) in the BMT group. Most surgeryrelated AEs were reversible. Patients seeking DBS have more suicidal ideation and suicides than the general population48, 49 but rates in the treated group do not differ from the comparator group on BMT49 suggesting that this is not an effect of the treatment.
Considerations of the GL task force
Eligibility criteria are important for the selection of patients for this treatment. The most important preoperative predictor of outcome is response to L-dopa during
a formal L-dopa test.50 Most studies reported a minimum of 33% improvement as an inclusion criterion, and lower values have shown poorer treatment results.51 Tremor as a symptom is responding particularly well to STN-DBS, even if not well responding during the L-dopa test.52 Age may also be a predictor of response, but this has not yet been convincingly demonstrated because some studies have found no effect of age53 but others have, and most studies had an age limit at 70 years. There are also neurosurgical contraindications (eg, severe brain atrophy), uncontrolled depression, psychosis, or dementia. The expert group suggests consideration of overall health and biological age rather than the numerical age. Several uncontrolled retrospective and prospective studies report on the value of STN-DBS for the treatment of several nonmotor symptoms of the disease. This includes a reduction of mood fluctuations, hallucinations, and psychosis and improvements in urinary incontinence and sleep.52, 54-56 An open 3-year study in 67 STN-DBS patients matched with 84 medically treated patients showed significant differences on the nonmotor symptom scale, particularly for sleep, fatigue, and urinary symptoms.55
Long-term outcome of STN-DBS cannot be assessed in randomized controlled studies. However, a number of uncontrolled longterm studies of patients with advanced PD and STN stimulation are available and underwent
multiple meta-analyses.52, 57, 58 These reveal that STN-DBS is still effective beyond 15 years after the intervention, with significant improvement in motor complications and a stable reduction of dopaminergic drugs.59 Certainly, these cohorts are highly selected and most do not report the disease course of patients lost at follow-up. Maintenance of QoL above the preoperative level has been found for 5 years after surgery despite the natural progression of the disease.58, 59

The GL group concludes that STNDBS should be offered to eligible patients with PD with medically resistant fluctuations. Stringent inclusion and exclusion criteria need to be applied at specialized centers for each patient.
Recommendation 1: Offer STNDBS to people with advanced PD if fluctuations are not satisfactorily controlled with medication or if tremor cannot be controlled with medication (15 voters, 100%).
STN-DBS for Early PD with early fluctuations
Two studies were included in the review.60, 61 Overall, 271 patients with PD aged younger than 60 years and with fluctuations or dyskinesia for less than 3 years were randomly assigned to STN-DBS or BMT.
Summary of findings
STN-DBS in early PD with early fluctuation compared with BMT results in a large improvement of QoL, a large improvement of ADLs, and a large improvement of motor symptoms. The evidence
43 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
Figure 2
Parkinson’s Disease
is very uncertain about the effect on complications of therapy. Daily ON time without dyskinesia and OFF time may be improved. STN-DBS may result in no difference regarding cognition and apathy. STN-DBS may improve depression. The effect sizes are similar to advanced PD. DBS may improve impulsive–compulsive behaviors in the long term. STN-DBS probably improves gait62 and reduces the daily dosage of medication.61 STNDBS may increase the likelihood of experiencing SAEs. They occurred in 54.8% of the patients in the neurostimulation group and in 44.1% of those in the best medical treatment group. In the DBS group, 13.7% experienced gait impairment as an AE in contrast to 11.8% in BMT group. Long-term data are not yet published for this cohort.
Considerations of the GL task force
Inclusion criteria are important: Patients in this group were aged younger than 61 years at surgery, the improvement at the preoperative L-dopa test was 50% or higher, and there were no cognitive changes or uncontrolled psychiatric conditions. A secondary analysis showed that the effect on QoL depends on the baseline PDQ-39 score, with those having a worse PDQ-39 baseline score having better postoperative improvement.63 Thus, a subjectively relevant affection of QoL might be a further inclusion criterion.
The GL group concludes that STN-DBS can be offered to people with early PD and early fluctuations who fulfil the inclusion and exclusion criteria for DBS. Less is known about the long-term course of these patients than for patients with advanced disease.
Recommendation 2: Consider offering STN-DBS to people with early PD and early fluctuations (15 voters, 100%).
STN-DBS for people with early
PD without fluctuations
The study published by Charles and colleagues64, 65 is the only study on implantation of STN-DBS at a stage where fluctuations have not yet occurred. Inclusion criteria were the following: Hoehn and Yahr stage II in the off state, antiparkinsonian medications for more than 6 months but less than 4 years, and no current or prior
history of motor fluctuations. A total of 30 patients were randomly assigned, and outcomes were reported for 12 and 24 months of follow-up.
Summary of findings
The critical outcomes PDQ-39, ADLs (UPDRS-II), motor score while OFF (UPDRS-III), and disease complications (Unified Parkinson's Disease Rating Scale Part IV [UPDRS-IV]) were not different between the DBS and BMT groups. Similarly, medication change from baseline to 24 months was not significantly different between the DBS and BMT groups despite substantially increased dosages. Two serious AEs occurred in the DBS group.
Considerations of the GL task force
The study has limitations as the sample size was small (15 people with PD per arm). According to the inclusion criteria, patients eligible for the study did not experience motor fluctuations; however, the difference between the UPDRS-III in the OFF and the ON states was 43% at baseline, suggesting that the patients were already fluctuating. At 24 months of follow-up, the UPDRS-III score in the ON state did not differ from the baseline score, and there was worsening of the UPDRS-III score off medication compared with baseline. The authors stated that a neuroprotective effect can be seen in DBS group; however, no current evidence supports this view. Further studies are underway.
The GL group concludes that STN-DBS should not be offered to people with PD without fluctuations.
Recommendation 3: Do not offer DBS to people with early PD without fluctuations (16 voters, 100%).
STN-DBS versus GPi-DBS in PD
The majority of RCTs for DBS in PD have investigated STN-DBS. Nevertheless, in some countries, GPi-DBS is commonly used. There are only two RCT studies comparing GPi-DBS versus STN-DBS. The American Veterans Administration (VA) study43 randomly assigned 152 patients to GPi-DBS and 147 to STN-DBS. The Netherlands study randomly assigned 62 patients to GPi-DBS and 63 patients to STN-DBS.42 Duration of the studies was 24 months43 and 12 months,42 respectively.
Summary of findings
The analysis of both studies shows that most critical and important outcomes were not different. This applies for QoL (PDQ-39), ADLs (UPDRS-II) at 1 and 2 years, motor score (UPDRS-III in the off medication state) at 1 and 2 years, complications of therapy, ON time without troublesome dyskinesia, SAEs, Hoehn and Yahr staging, and cognition.53, 66 A minor improvement of depression in the GPi group was found in the US study.43 There was a greater reduction in L-dopa dosage in the STN groups of both studies. GPi-DBS was associated with a greater increase of daily dose of dopaminergic medication compared with STN-DBS both in the first year of follow-up and during a longer term follow-up.
Considerations of the GL task force
The 3-year, open-label, follow-up data of the VA study53 showed a stable result of the critical outcome parameters, whereas the 3-year follow-up of the Netherlands study67 concluded that motor symptoms and functioning during the off-drug phase were more improved after STN-DBS than after GPi-DBS, with no differences for cognition, mood, or behavior.
The studies show only minor differences between STN-DBS and GPi-DBS during the 3-year observation period. Although these differences may be important for particular patients, they do not allow prioritizing one treatment over the other. Therefore, both targets are similarly effective to treat symptoms of advanced PD and can both be recommended. The greater reduction of L-dopa for those treated with STN-DBS was considered an important clinical difference.
Recommendation 4: Both STNDBS and GPi-DBS are effective to treat symptoms of advanced PD with fluctuations, but dopaminergic medication can be more reduced with STN-DBS (16 voters, 100%).
The Summary of findings can be viewed via the full paper (details at the bottom of this article).
Future Developments
These GLs is the first to evaluate all currently available invasive treatments for PD and is part of a series that will cover all the other treatment options and essential diagnostic procedures for PD. There is only limited evidence for
several of the invasive treatments for PD. Using the rigorous GRADE methodology, the GLs only consider RCTs for evaluation but describe the spectrum of approved interventions. Economic evaluations of the treatment options are not yet included and will likely differ between regions with different health care systems and the large variation in the availability and costs of these treatments. However, the EAN is working on including this in the future. A number of careful studies are available that compare the costs for the different treatments for specific countries.35, 96-101
Several invasive interventions for PD have undergone a rapid development, with DBS of the STN and GPi being established treatments for the improvement of motor symptoms and healthrelated QoL.20 Further questions on the use of DBS for psychosocial impact and nonmotor symptoms in PD as well as the possible usefulness for axial abnormalities still need to be answered. Limited research activity is available for radiosurgery for PD.102 No randomized controlled studies on lesional procedures with radiofrequency thermocoagulation have been published, and this treatment may remain a last resort treatment for special cases in the hands of experienced specialized functional neurosurgeons. Research and use of MRgFUS is currently rapidly developing, but important questions are still open.23 One conceivable indication for this intervention is treatmentresistant parkinsonian tremor, but the first focused ultrasound thalamotomy trial failed the threshold of this GL because the study was underpowered. Infusion therapies are another active field of research, and other new forms of less-invasive interventions are currently being developed. New trials may also explore the treatment of other, particularly nonmotor, symptoms of PD.
The invasive treatments discussed should only be used for appropriately selected patients, but in those can profoundly change the lives of people with PD.
First published: 06 July 2022 https://doi.org/10.1111/ene.15386
This article is co-published by the European Journal of Neurology and Journal of the International Parkinson and Movement Disorder Society
44 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
LAMA + LABA
Abbreviated Prescribing Information
Eklira® Genuair® 322 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and a green dosage button. Each delivered dose contains 375 µg aclidinium bromide equivalent to 322 µg of aclidinium. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation use. Recommended dose is one inhalation of 322 micrograms aclidinium twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work di erently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents.


Contraindications: Hypersensitivity to aclidinium bromide or to any of the excipients. Warnings and Precautions: Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Use with caution in patients with symptomatic prostatic hyperplasia or bladder-neck obstruction or with narrow-angle glaucoma. Do not use in patients with rare hereditary problems of galactose intolerance, total lactose de ciency or glucose-galactose malabsorption.
Interactions: Do not administer with other anticholinergic-containing medicinal products. No other interactions expected. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Risk to newborns/infants cannot be excluded. Consider risk-bene t before using during lactation. Unlikely to a ect fertility at the recommended dose. Side-e ects: Common (1-10%): Sinusitis, nasopharyngitis, headache, cough, diarrhoea, nausea. Uncommon (0.1-1%): Dizziness, blurred vision, tachycardia, palpitations, dysphonia, dry mouth, stomatitis, rash, pruritus, urinary retention. Rare (0.01-0.1%): hypersensitivity. Not known: angioedema, anaphylactic reaction. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing Authorisation Number: EU/1/12/778/002 Marketing
Maintenance bronchodilator

dose is one inhalation of 340 µg/12






symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation
Patients
be







how
administer



correctly as the Genuair inhaler may work di erently from inhalers used previously. It is important to instruct the patients








read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Warnings and Precautions: Do not use in asthma. Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Discontinue if increases in pulse rate, blood pressure or changes in ECG occur. Use with caution in patients with a history of or known prolongation of the QTc interval or treated with products a ecting the QTc interval. Use with caution in patients with severe cardiovascular disorders, convulsive disorders, thyrotoxicosis and phaeochromocytoma. Hypokalaemia may occur, is usually transient and supplementation not needed. In patients with severe COPD, hypokalaemia may be potentiated by hypoxia and concomitant treatment. Use with caution in patients with symptomatic prostatic hyperplasia, urinary retention or with narrow-angle glaucoma. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Do not use in patients with rare hereditary problems of galactose intolerance, the Lapp lactase de ciency or glucose-galactose malabsorption. Interactions: Do not administer with other anticholinergic and/or long-acting β2-adrenergic agonist containing medicinal products. Caution in use with methylxanthine derivatives, steroids, non-potassium-sparing diuretics, β-adrenergic blockers or medicinal products known to prolong the QTc interval. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Consider risk-bene t before using during lactation. Unlikely to a ect fertility at the recommended dose. Sidee ects: Common (1-10%): Nasopharyngitis, urinary tract infection, sinusitis tooth abscess, insomnia, anxiety, headache, dizziness, tremor, cough, diarrhoea, nausea, dry mouth, myalgia, muscle spasms, peripheral oedema, increased blood creatine phosphokinase. Uncommon (0.1- 1%): Hypokalaemia, hyperglycaemia, agitation, dysgeusia, blurred vision, tachycardia, electrocardiogram QTc prolonged, palpitations, angina pectoris, dysphonia, throat irritation, stomatitis, rash, pruritus, urinary retention, increased blood pressure. Rare (0.01-0.1%): Hypersensitivity, bronchospasm, including paradoxical. Not known: anaphylactic reaction, angioedema. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing Authorisation Number: EU/1/14/963/001 Marketing Authorisation holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: October 2019

This medicinal product is subject to additional
will
identi cation of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA
Earlsfort
2; Tel: +353 1 6764971; Fax: +353 1 6762517.
Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744. References: 1. MIMS Ireland November 2020 2. Eklira® Genuair® Summary of Product Characteristics, last updated November 2019 3. Brimica® Genuair® Summary of Product Characteristics, last updated August 2019 4 Magnussen, H et al. COPD. 2019 Apr;16(2):196-205
monitoring. This
allow quick
Pharmacovigilance,
Terrace, IRL - Dublin
Website: www.hpra.ie; E-mail: medsafety@ hpra.ie.
Prescribing Information Brimica® Genuair® 340 micrograms/12 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and an orange dosage button. Each delivered dose contains 396 µg aclidinium bromide (equivalent to 340 µg of aclidinium) and 11.8 micrograms of formoterol fumarate dihydrate.
contains lactose.
LAMA Abbreviated
Also,
Use:
treatment to relieve
use. Recommended
µg twice daily.
should
instructed on
to
the product
to
Authorisation holder: AstraZeneca AB, SE151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: February 2020 Date of item: November 2020. IR-BRI-10-2020 This medicinal product is subject to additional monitoring. This will allow quick identi cation of new safety information. Healthcare professionals are asked to report any suspected adverse reactions to: HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.hpra.ie, e-mail: medsafety@ hpra.ie. Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744. Genuair®-has it ‘clicked’ yet? The ONLY pre lled inhaler with visual and audible feedback for confirmed dose delivery1-4 Genuair - a simple to use inhaler for patients with COPD4
Long-term management of patients with ATTR-CM
This consensus document from an international expert panel recommends a set of clinically feasible tools for the long-term monitoring of patients with transthyretin amyloid cardiomyopathy (ATTR-CM), including meaningful thresholds for defining disease progression and the frequency of measurements.
6MWT, 6-minute walk test; ATTR-CM, transthyretin amyloid cardiomyopathy; ECG, electrocardiogram; EQ-5D, EuroQol five dimensions; GLS, global longitudinal strain; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricular; LVEF, left ventricular ejection fraction; NAC, UK National Amyloidosis Centre; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; QoL, quality of life.
Garcia-Pavia P, Rapezzi C, Adler Y. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. European Heart Journal. 2021 Apr 7;42(16):1554–68.
Monitoring ATTR-CM in diagnosed patients

• There remains a need for practical evaluation tools that are feasible to conduct every 6 to 12 months Monitoring ATTR-CM in diagnosed patients
• Evaluation of parameters over time may be more meaningful than those with specific cut-off values; accurate baseline levels are crucial
• ATTR-CM phenotype and disease stage may impact the reliability of measurements used to monitor disease progression
Treatment effects and ATTR-CM progression
• Allow sufficient time to observe benefit of treatment effect, particularly in the first 6 to 12 months Treatment effects and ATTR-CM progression
• Clear endpoints indicating disease progression or stability are unknown
• Findings from Phase 3 trials, such as those from the Transthyretin Amyloidosis
Cardiomyopathyhy Clinical Trial (ATTR-ACT), and long term data may inform relevant endpoints for treatment goals
*The panel stressed that where ATTR-CM progression is indicated, measurements should not be interpreted as a recommendation to discontinue disease-modifying therapies
Summary
Once diagnosed, untreated patients have a median survival of ~2 to 3.5 years
As demonstrated by the post hoc analysis of ATTR-ACT and the LTE, early diagnosis and treatment is essential in the management of ATTR-CM2
Tafamidis significantly reduced all-cause mortality and CV-related hospitalizations vs placebo over 30 months (P=0.0006) in patients with ATTR-CM3
Data from the ATTR-ACT study and long-term extension study demonstrates long term efficacy and safety of tafamidis for the treatment of ATTR-CM
What is ATTR-CM
Transthyretin (trans-thy-re-tin) Amyloid Cardiomyopathy (ATTRCM) is an underdiagnosed and potentially fatal disease. It’s characterized by deposits of amyloid protein fibrils in the walls of the left ventricle, the main pumping chamber of the heart. In ATTR-CM, the amyloid protein is made of transthyretin. The amyloid protein deposits cause the heart walls to become stiff, resulting in the inability of the left ventricle to:
1. Properly relax and fill with blood
2. Adequately squeeze to pump blood out of the heart
What are the risk factors?
For Hereditary Transthyretin Amyloid Cardiomyopathy:
• A family member with ATTR-CM or heart failure
• Age 50+ (although symptoms begin anywhere from age 20 to age 80)
• Gender - primarily male
• Race - African American
For Wild-Type Transthyretin Amyloid Cardiomyopathy:
• Age 65+ • Gender
• Primarily male
What are the symptoms of ATTR-CM?
Symptoms are like those associated with heart failure:
• Shortness of breath is the most common, especially with minimal exertion and when lying down; the other symptoms usually occur after the shortness of breath is already there
• Coughing or wheezing, especially when lying down
• Swelling in the feet, ankles and legs
• Bloating in the abdomen
• Confusion or trouble thinking
• Increased heart rate

• Palpitations or abnormal heart rhythms
Additional symptoms:
• Numbness or tingling in the hands and feet
• Carpal Tunnel Syndrome
46 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
ATTR 14
Presented by Claudio Rapezzi, University of Ferrara, Ferrara, Italy; World Congress on Acute Heart Failure 2022
Criteria for disease progression in patients with ATTR-CM Extracted from: Garcia-Pavia P, Rapezzi C, Adler Y. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. European Heart Journal. 2021 Apr 7;42(16):1554–68
7.
Life-threatening, underrecognized, and underdiagnosed, ATTR-CM is a rare condition found in mostly older patients in which misfolded transthyretin proteins deposit in the heart.1-7 It is vital to recognize the diagnostic clues so you can identify this disease.
Wild-type
JW,
cardiac
heart failure with preserved ejection fraction in patients typically over 60 years old5-7
to standard heart failure therapies (ACEi, ARBs, and beta blockers)8-10
between QRS voltage and left ventricular (LV) wall thickness11-13
of carpal tunnel syndrome or lumbar spinal stenosis3,8,14-20
showing increased LV wall thickness6,13,16,21,22
—autonomic
system dysfunction-including
complaints or

weight loss6,16,23,24
A,
Eur Heart J. 2015;36(38):2585-2594. 8. Narotsky
Can J Cardiol. 2016;32(9):1166.e1-1166.e10. 9. Brunjes
Clemons A, Rubin J, Maurer MS. Transthyretin cardiac amyloidosis in older Americans. J Card Fail. 2016;22(12):996-1003. 10. Castaño A, Drach BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163-178. 11. Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49:9-13. 12. Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114(7):1089-1093. 13. Quarta CC, Solomon D, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840-1849. 14. Connors LH, Prokaeva T, Lim A, et al. Cardiac amyloidosis in African Americans: Comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158(4):607-614. 15. Nakagawa M, Sekijima Y, Yazaki M, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23(1):58-63. 16. Rapezzi C, Merlini G,
disease
Systemic
CC, et
and clinical courses of the 3 main types. Circulation. 2009;120(13):1203-1212. 17. Sperry BW,

amyloidosis in
A, et al. Tenosynovial and
BA,
release. J Am Coll Cardiol. 2018;72(17): 2040-2050. 18.
Sci. 2014;119(3):223-228. 19.
Ups J
Mod Pathol. 2015;28(2):201-207. 20.
the


T, Ueda M, Jono H, et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Hum Pathol. 2011;42(9):1259-1264. 21. Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442-1448. 22. Ternacle J, Bodez D, Guellich A, et al. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging 2016;9(2):126-138. 23. Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63-76. 24. Swiecicki PL, Zhen DB, Mauermann ML, et al. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid. 2015;22(2):123-131.
The health information contained in this ad is provided for educational purposes only.
Date of Preparation: February 2021 GCMA code: PP-RDP-IRL-0105
5.
nervous
gastrointestinal
unexplained
References 1. Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23(4):209-213. 2. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357-1377. 3. Connors LH, Sam F, Skinner M, et al. Heart failure due to age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282-290. 4. Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098.
Mohammed SF, Mirzoyev SA, Edwards WD,
et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2(2):113-122. 6. Maurer MS, Hanna M, Grogan M, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68(2):161-172.
LEARN HOW TO RECOGNIZE THE CLUES OF ATTR-CM AT: S U S P E C T A N D D E T E C T . I E CONSIDER THE FOLLOWING CLINICAL CLUES, ESPECIALLY IN COMBINATION, TO RAISE SUSPICION FOR ATTR-CM AND THE NEED FOR FURTHER TESTING SUSPECT ATTR-CM (TRANSTHYRETIN AMYLOID CARDIOMYOPATHY) A LIFE-THREATENING DISEASE THAT CAN GO UNDETECTED H FpEF I NTOLERANCE DISCORDANCE DIAGNOSIS E CHO N ERVOUS SYSTEM
González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction.
DL, Castano
Weinsaft
Bokhari S, Maurer MS.
transthyretin
amyloidosis: novel insights from advanced imaging.
DL, Castano A,
Quarta
al.
cardiac amyloidoses:
profiles
Reyes
Ikram
cardiac
patients undergoing carpal tunnel
Westermark P, Westermark GT, Suhr OB, Berg S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis.
Med
Yanagisawa A, Ueda M, Sueyoshi T, et al. Amyloid deposits derived from transthyretin in
ligamentum flavum as related to lumbar spinal canal stenosis.
Sueyoshi
How a Coronavirus Protease Inhibitor Works to Fight COVID-19
SARS-CoV-2, the virus that causes COVID-19, was sometimes called the “novel coronavirus” in the early days of the pandemic.
The term was fitting because SARS-CoV-2 was new; the world had not previously encountered this virus, though the scientific community was already quite familiar with other coronaviruses, including SARS-CoV-1, the virus that caused the 2003-2004 severe acute respiratory syndrome (SARS) outbreak, MERS-CoV that caused the Middle East respiratory syndrome (MERS) outbreak in 2012, as well as the common human coronaviruses responsible for billions of cases of the common cold.
There’s a reason we don’t yet have a cure for the common cold: viral infections are notoriously difficult to treat. That’s because viruses hide inside the cells of their hosts. Viruses hijack host cells and use the cells’ structures to crank out more copies of the virus, which then infect and hijack more hosts.
Destroying an invader that’s tucked into the cells of the body is a delicate task; you want to disable the invader without damaging the cell. Understanding the life cycle of viruses helps scientists develop medications that target the virus without affecting the host cells.
Breaking the SARS-CoV-2 life cycle
To summarize, for a virus to cause sickness in a human being, it must enter the body, then attach to and enter a host cell, then create new viral copies. Interrupting this process at any step may prevent infection, illness, or the spread of infection.
The virus that causes COVID-19, SARS-CoV-2, usually enters the body via the nose or mouth, but can also enter through the eyes by direct contact. The spike protein on the surface of the virus binds to cells that have a particular type of receptor on their surface. Like a key unlocking and opening a door, this connection allows the virus to enter the host cell.

Inside the cell, the virus releases its ribonucleic acid (RNA), a molecule that contains the genetic instructions that direct the cell to create proteins to make more viruses. The viral RNA is translated into a long protein chain called a polyprotein. Viral enzymes break the polyprotein into smaller protein units, which become the building blocks of new viral copies by replication of structural protein RNA. The new virus can then infect more cells.
The SARS-CoV-2 life cycle can potentially be stopped by interfering with any of these steps.
“You can inhibit the virus at the step of attachment,” says Devendra Rai, a senior scientist at Pfizer, “if you design a drug that binds to either the spike protein or the host receptor. Those are called entry inhibitors.”
Or “you can inhibit the virus at the step of making functional proteins by stopping the cleavage of the polyprotein,” Rai says. Medications aimed at stopping this step are protease inhibitors.
Another option is to stop the virus from making copies of its genetic blueprint. Medications that interrupt this step are called polymerase inhibitors.
The virus can also be inhibited at the assembly and release steps, which entail assembly of virus particle in the infected cell and its release from the infected cell, respectively. This is called viral release.
What is a protease inhibitor?

Medications that inhibit the cleavage of the polyprotein into functional proteins are called protease inhibitors. Protease is a protein-based enzyme that normally breaks the polyprotein into functional proteins, so blocking, or inhibiting, protease prevents this essential step of viral reproduction.
While the spike protein, the part of the virus that attaches to host cells, can be susceptible to mutations, the region of the polyprotein that produces protease is much less so, which means that it is far more likely to remain a stable target for a drug
“When you are designing an inhibitor, you want to design an inhibitor for a region that is not so readily mutated,” Rai says. The resulting protease inhibitor may thus retain efficacy versus variants of the same virus.
Pfizer's novel oral COVID-19 treatment is comprised of two protease inhibitors, nirmatrelvir and ritonavir that are administered together. Nirmatrelvir blocks SARS-CoV-2 main protease enzyme activity that would otherwise cleave the polyprotein into functional proteins.1 Ritonavir, another protease inhibitor, is not active against SARS-CoV-2; it is used to slow the metabolism of nirmatrelvir, allowing it to be active for longer at higher concentrations so it can most effectively interrupt viral replication.2
The Phase 2/3 EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High Risk Patients) was a randomized, placebo-controlled trial in nonhospitalized adult subjects with a laboratory confirmed diagnosis of SARS-CoV-2 infection. It
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
48 RESPIRATORY: COVID-19
found that, when treated within three days of symptom onset, Pfizer’s oral treatment reduced the risk of COVID-19 related hospitalization or death from any cause by 89% compared to placebo in non-hospitalized high-risk adults with COVID-19 .3
Consistent results were observed in the population treated within five days of symptom onset, with an 88% reduction of hospitalization or death. Treatment-emergent adverse events were comparable between the oral treatment (23%) and the placebo (24%), most of which were mild in intensity. Fewer serious adverse events (1.6% vs. 6.6%) and discontinuation of study drug due to adverse events (2.1% vs. 4.2%) were observed in patients dosed with the oral treatment, compared to the placebo, respectively.
On December 22, 2021, the U.S. Food and Drug Administration authorized the oral treatment for emergency use for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kg, or 88 lb) with positive results of direct severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death.4

The oral treatment should be initiated as soon as possible after a COVID-19 diagnosis has been made and within five days of symptom onset. The medication should be taken twice a day (morning and evening) for five days.5


“We believe this medication may be an important tool to help fight COVID-19, Rai says. “I'm so proud to have played a role in that.”
Pfizer's oral treatment has not been approved, but has been authorized for emergency use by the FDA to treat mild-to-moderate COVID-19 in patients 12 and older, weighing at least 40 kg, with positive results of SARS-CoV-2 viral testing, who are at high risk for progression to severe COVID-19, including hospitalization and death. Authorized only for the duration of the declaration that circumstances exist justifying the authorization unless the declaration is terminated or authorization revoked sooner. See EUA Fact Sheet: www.COVID19oralRx.com
References available on request
HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 49
Management of Pneumonia
An interview with Theresa Lowry Lehnen (PhD), Clinical Nurse Practitioner and Associate Lecturer South East Technological University

at least one other lower respiratory tract symptom); new focal chest signs on examination; and at least one systemic feature, either a symptom complex of sweating, fevers, shivers, aches and pains and/or temperature of 38°C or more. Any pneumonia acquired 48 hours after admission to an inpatient setting such as a hospital and not incubating at the time of admission is classed as HAP.
Pneumonia is a common acute respiratory infection that affects the lung parenchyma, i.e. the alveoli and distal airways in the lower respiratory tract. Rather than a single disease, pneumonia is an umbrella term for a group of syndromes caused by a variety of microorganisms resulting in varied manifestations and sequelae. It is a major health problem globally, associated with high morbidity and short and long-term mortality in all age groups.
We recently spoke to Clinical Nurse Practitioner and Associate Lecturer South East Technological University Theresa Lowry Lehnen to find out more about this common respiratory condition.
Pneumonia is broadly divided into two categories; communityacquired pneumonia (CAP) and hospital-acquired pneumonia (HAP). CAP can be defined as symptoms of an acute lower respiratory tract illness (cough and
Theresa explains, “Pneumonia occurs more commonly in susceptible individuals. Children < 5 years and adults > 70 years are the populations most affected. Lower respiratory tract infections (LRTIs) including pneumonia and bronchiolitis affected 489 million people globally in 2019, according to data from the 2019 Global Burden of Diseases (GBD) study.
“Aspiration pneumonia contributes 5–15% of all cases of community acquired pneumonia and is associated with worse outcomes, especially in older patients with multiple comorbidities,” she explains.

There is a lack of data about the incidence of aspiration pneumonia in patients with hospital acquired pneumonia. However Theresa adds, “In Europe, the annual incidence of CAP is estimated at 1.07–1.2 cases per 1,000 people, increasing to 14 cases per 1,000 amongst those aged 65 years and over, with a higher incidence in men. The 2019 GBD study showed that LRTI was responsible for over 2.49 million deaths, with mortality highest amongst patients over 70 years of age (1.23 million deaths).
“Pneumonia is the single largest infectious cause of death in children worldwide. 740, 180 children under the age of 5 died as a result of pneumonia in 2019, accounting for 14% of all deaths of children under five years of age and 22% of all deaths in children aged 1 to 5.
“Development of pneumonia largely depends on the host immune response, with pathogen characteristics having a less prominent role. A large variety of microorganisms including
bacteria, respiratory viruses and fungi can cause pneumonia, and there are geographical variations in their prevalence. The causative microorganisms for CAP and HAP differ significantly. The most common causative microorganisms involved in community acquired pneumonia are Streptococcus pneumoniae, Respiratory viruses, Haemophilus influenzae and other bacteria such as Mycoplasma pneumoniae and Legionella pneumophila.
“The most frequent causative microorganisms in hospital acquired pneumonia are Staphylococcus aureus (including MSSA and MRSA), Enterobacterales, non-fermenting gram-negative bacilli example, Pseudomonas aeruginosa, and Acinetobacter spp.
“Fungal infections are usually implicated in patients with certain predisposing immunocompromised states like HIV and organ transplant recipients, among others. However, often overlooked, some fungal species can cause pneumonia in immunocompetent individuals which results in a delay in diagnosis and leads to unfavourable outcomes.”
Pathophysiology
There is an intricate balance between the organisms residing in the lower respiratory tract and the local and systemic defence mechanisms (both innate and acquired), which when disturbed gives rise to inflammation of the lung parenchyma (pneumonia), Theresa told us.

Pic courtesy: Case courtesy of Dayu Gai, Radiopaedia.org, rID: 30865
“The resident macrophages serve to protect the lung from foreign pathogens. Ironically, the inflammatory reaction triggered by these macrophages is what is responsible for the histopathological and clinical findings seen in pneumonia. The macrophages engulf these pathogens and trigger signal molecules or cytokines like TNF-a, IL-8, and IL-1 that recruit inflammatory cells such as neutrophils to the site of infection. They also serve to present these antigens to the T cells that trigger both cellular and humoral defence mechanisms, activate complement and form antibodies against these organisms.
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
50 RESPIRATORY: PNEUMONIA
“This in turn, causes inflammation of the lung parenchyma, and makes the lining capillaries "leaky," which leads to exudative congestion and underlines the pathogenesis of pneumonia.”
Histopathology in pneumonia can be broadly divided into bronchopneumonia/lobular pneumonia or lobar pneumonia.
Lobar Pneumonia is diffuse consolidation involving the entire lobe of the lung and its evolvement can be broken down into 4 stages:
• Congestion: This stage is characterised by heavy and wet spongy lung tissue, diffuse congestion, vascular engorgement, and the accumulation of alveolar fluid rich in infective organisms. There are few red blood cells and neutrophils at this stage.
• Red hepatisation: This stage is characterised by marked infiltration of red blood cells, neutrophils, and fibrin into the alveolar fluid. The lungs appear red and firm, similar in appearance to a liver, hence the term hepatisation.
• Gray hepatisation: The red blood cells break down and are associated with fibrinopurulent exudates causing a red to gray colour transformation.
• Resolution: This stage is characterised by clearing of the exudates by resident macrophages, with or without residual scar tissue formation.
“Bronchopneumonia is characterised by suppurative inflammation localised in patches around bronchi which may or may not be localised to a single lung lobe. Very rarely, severe forms of pneumonia may result in the formation of a lung abscess, a complete breakdown of tissue and formation of pus-filled pockets in focal areas of the lung. The infection may spread to the pleural space forming a fibrinopurulent exudate filling this space, known as empyema,” she says.
Risk Factors
CAP: Prematurity, malnutrition, household air pollution, ambient particulates or suboptimal breastfeeding are main CAPrelated risk factors in children. In adults, COPD, diabetes mellitus, cardiovascular disease and chronic liver disease are the most frequent comorbidities that increase the risk of CAP. Men have a higher risk than women, which may be explained by differences in anatomy, behavioural, socioeconomic and lifestyle
factors. Immunocompromised patients have a higher risk of community acquired pneumonia than the general population. Several studies have demonstrated an association between lifestyle factors and the risk of CAP, including smoking, high alcohol consumption, being underweight due to poor-nutrition or underlying conditions that compromise the immune response, and poor living conditions.
HAP: Incidence of HAP is associated with various sociodemographic, clinical, and hospital environmental factors. Risk factors for hospital-acquired pneumonia include mechanical ventilation for > 48 h (VAP), residence in an ICU, duration of ICU or hospital stay, severity of underlying illness, and presence of comorbidities. Pseudomonas aeruginosa, Staphylococcus aureus, and Enterobacter are the most common causes of HAP.
Presentation and Diagnosis
Theresa continues, “The signs and symptoms of pneumonia are highly variable, and its clinical course can vary greatly between individuals. Symptoms may develop gradually over a number of days but may also appear much faster. Main presenting complaints include systemic signs such as fever with chills, malaise, loss of appetite, and myalgia. These findings are more common in viral compared to bacterial pneumonia.
“Pulmonary symptoms include cough with or without sputum production. Bacterial pneumonia is associated with purulent or rarely blood-tinged sputum. Viral pneumonia is associated with watery or occasionally

mucopurulent sputum production. There may be an associated pleuritic chest pain with the concomitant involvement of the pleura.
“Dyspnoea and a diffuse heaviness of the chest are also occasionally seen. Common findings on physical examination include tachypnoea, tachycardia, fever with or without chills, decreased or bronchial breath sounds, egophony and tactile fremitus, both suggestive of a consolidative process, crackles on auscultation of the affected regions of the lung and dullness on percussion.”
Treatment
“Antibiotics are the mainstay therapy for pneumonia, however, the agents used depend on a variety of host and pathogen factors. Ideally, therapy should be pathogen-directed, even though a pathogen is often not identified. As therapy must be started promptly, empirical therapy directed at the most likely aetiological pathogens is required. Empiric therapy is guided by resistance patterns prevalent in the region as well as patient risk factors for multi-drug resistant organisms.
“CAP management involves initial risk assessment of the patient and clinical findings to decide whether to manage the patient at home in the community, or in a hospital setting. Health professionals should take into account disease severity and how ill the individual is; clinical observations; social circumstances including the availability of support at home; comorbidities; age; and risk of non-adherence to medication.
“While often conceptualised as an acute, completely reversible disease, numerous studies have documented that CAP patients continue to have significantly greater mortality than expected over the following 2 to 5 years, with some studies suggesting even longer-term adverse impacts and reduced quality of life. A significant proportion of the excess mortality may be due to cardiovascular disease, either myocardial infarction or stroke, and heart failure.”
Theresa adds, “The mechanisms driving the excess of cardiovascular and cardiac disease has not been definitively determined, but accelerated atherosclerosis and direct cardiac damage during acute pneumonia are both strong hypotheses. Observational studies have also demonstrated a greater burden of long-term cognitive impairment, functional impairment, and depressive symptoms after pneumonia or sepsis. In community acquired pneumonia (CAP), antibiotics are started immediately. If symptoms are not improving as expected with antibiotics, therapy should be reviewed and escalated, or hospital referral considered. Severity is assessed using the CRB-65 score.
“The need for hospital referral should be assessed, and at review, re-assessed using the CRB65 criteria. At convalescence, it is important to ensure COVID-19, influenza and pneumococcal vaccinations are up to date.”
HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 51
*Alternative doxycycline dose: 100mg every 12 hours CRB65 is used in primary care to assess 30 day mortality risk in adults with pneumonia. The score is calculated by giving 1 point for each of the following prognostic features: Confusion (defined as a Mental Test Score of 8 or less, or new disorientation in person, place or time) Respiratory rate ≥30/minute Low blood presure systolic [<90 mmHg] or diastolic [≤60 mmHg] Age 65 or more Risk of death is stratified as follows, depending on CRB65 score: 0: low risk (less than 1% mortality risk). Consider hospital referral and assessment 1 or 2: intermediate risk (1% to 10% mortality risk). Consider hospital referral and assessment 3 or 4: high risk (more than 10% mortality risk). Immediate hospital referral
Community Acquired Pneumonia (Adults): Antibiotic Treatment Table
Assess using the CRB -65 score (each symptom or sign scores one point)
(Confusion, Respiratory rate ≥ 30/min, BP ≤ 90/60 mmHg, Age ≥ 65)
Drug Dose Duration Notes
CRB 65 Score Zero (0) Suitable for home treatment
Review if symptoms are not improving as expected with antibiotics and escalate therapy, or consider hospital referral.
Avoid in penicillin allergy.
Amoxicillin 500mg every 8 hours 5 days
If no response after 48 hours on Amoxicillin monotherapy, consider addition of Clarithromycin or change to Doxycycline
OR Doxycycline 200mg every 24 hours* 5 days Avoid in pregnancy.
Advise to take with a glass of water and sit upright for 30 minutes after taking.
Can take with food or milk if gastritis is an issue.
Absorption of doxycycline significantly impaired by antacids, iron/calcium/magnesium/zinc -containing products.
to advances in microbial detection and clinical epidemiology. Theresa concludes, “Previously thought to be a sterile space, the lung is now recognised as a complex ecosystem of microbes, with equally complex relationships to their host and each other. Widespread availability of rapid molecular diagnostic testing has provided a new insight into aetiologies of pneumonia that challenges paradigms set by earlier studies from microbiology cultures.
OR Clarithromycin (Second line in penicillin allergy)
500mg every 12 hours 5 days
Macrolides should be used with caution in pregnancy. Clarithromycin suitable only in 2nd and 3rd trimester in pregnancy. Alternative macrolide for all trimesters of pregnancy: Azithromycin 500mg stat then 250mg every 24 hours from Day 2 to Day 5.
CRB 65 Score 1-2 and assessed suitable for treatment in the community
Review if symptoms are not improving as expected with antibiotics and escalate therapy, or consider hospital referral.
Avoid amoxicillin in penicillin allergy.
Amoxicillin PLUS Clarithromycin
500mg - 1g every 8 hours 500mg every 12 hours
5 days
OR Doxycycline 200mg every 24 hours* 5 days
Macrolides should be used with caution in pregnancy. Clarithromycin suitable only in 2nd and 3rd trimester in pregnancy. Alternative macrolide for all trimesters of pregnancy: Azithromycin 500mg stat then 250mg eve ry 24 hours from Day 2 to Day 5.
Avoid in pregnancy.
Advise to take with a glass of water and sit upright for 30 minutes after taking.
Can take with food or milk if gastritis is an issue. Absorption of doxycycline significantly impaired by antacids, iron/calcium/magnesium/zinc -containing products.
CRB 65 Score 3 or more : urgent hospital admission
Administer Benzylpenicillin prior to transfer
Or
1.2g IV/IM N/A
Amoxicillin 1g PO N/A
*Alternative doxycycline dose: 100mg every 12 hours.
In non-severe infection, 200mg stat then 100mg every 24 hours can be considered.
• Pleuritic pain should be relieved using simple analgesia, and consider pulmonary embolism.
• Consider advising patients on hydration and smoking cessation where appropriate.
• Consider time off work for patients with CAP dependent on clinical assessment.
Avoid in penicillin allergy
Urgent hospital admission
Seek risk factors for Legionella and Staph.aureus infection
• Advise to consult pharmacist for symptom relief.
While patients should start to show improvement after 48 hours, complete recovery can be a long process and it is common for patients to experience symptoms for several weeks after treatment. Management of VAP and HAP is prolonged, complicated, and involves the use of broad-spectrum antibiotics. It involves early identification of signs of pneumonia and thorough evaluation before starting
empiric therapy. In treating HAP and VAP major concerns include the high prevalence of multi-drug resistance in the implicated organisms isolated from such patients. Major risk factors to take into consideration while estimating the risk for drug resistance include patient comorbidities, recent receipt of antibiotics, functional status, and severity of illness.
Outlook
Perspective on the pathogenesis of pneumonia is changing due
“Biomarkers, including C-reactive peptide, procalcitonin, and newer diagnostic technologies that combine microbial detection with inflammatory patterns are improving our understanding and refine the paradigm of lung infection, but to date have still to deliver meaningful clinical interventions.

“Ultrasound is an emerging technology that has expanded in its availability of point-ofcare testing with increasingly high quality. Several studies have suggested that lung ultrasound demonstrating airspace consolidation or focal distribution of B lines may have closer alignment with the clinician diagnosis of pneumonia or CT findings than chest radiographs. However, no studies currently exist that examine whether lung ultrasound improves diagnosis or outcomes. The perceived relative advantage over chest radiographs, degree of adoption, and consistency with this technique will influence the role of this technology in the future.
“Recent radiological and clinical research has questioned long standing concepts of pneumonia, especially community-acquired pneumonia, and challenged the radiological “gold standard” of a chest radiograph. Much work is being carried out to translate recent advances in understanding of lung infection, technological advances in diagnostic tools, and the leverage of clinical data into real improvements in management options and outcomes for patients with pneumonia. Substantial changes and improvements in diagnosis and treatment options can occur through challenging existing definitions, advancing technology, and adopting research designs that accommodate the complexity of patients and host–pathogen interactions.”
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE RESPIRATORY: PNEUMONIA 52
STUDY DESIGN
Double-blind, active comparator-controlled study in which pneumococcal vaccine-naïve participants 50 years of age and older were randomized to receive either VAXNEUVANCE (N=604) or PCV13 (N=601). aGMT ratio vs PCV13: 1.62 (95% Cl: 1.40-1.87).
Randomized controlled trials assessing the clinical efficacy of VAXNEUVANCE compared to PCV13 have not been conducted.
▼
VAXNEUVANCE®
Suspension for injection in pre-filled syringe. Pneumococcal polysaccharide conjugate vaccine (15-valent, adsorbed)
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SPC for how to report adverse reactions.
ABRIDGED PRODUCT INFORMATION Refer to Summary of Product Characteristics before prescribing. PRESENTATION 1 dose (0.5 mL) contains 2.0 mcg of each of the following pneumococcal polysaccharide serotypes: 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F and 4 mcg serotype 6B; 125 mcg aluminium (Al3+) and approximately 30 mcg CRM197 carrier protein. INDICATIONS For active immunisation to prevent invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older. Use in accordance with official recommendations. DOSAGE AND ADMINISTRATION Adults: Single dose of 0.5 ml given by intramuscular injection. Need for a booster dose not established. Children and adolescents aged under 18 years: Safety and efficacy not established. CONTRAINDICATIONS Hypersensitivity to any component of the vaccine or to any diphtheria toxoid containing vaccine. PRECAUTIONS AND WARNINGS Do not give intravascularly. Ensure appropriate facilities and medication are available in case of anaphylaxis. Delay vaccination in presence of acute, severe febrile illness or other significant infection. Use with caution in individuals on anticoagulants, or those with thrombocytopaenia or any coagulation disorder, as bleeding or bruising may occur. Vaccine may not provide complete protection in all recipients. Immunocompromised individuals may have reduced antibody response to Vaxneuvance. Availability of safety and immunogenicity data in presence of immunosuppression is limited to individuals with HIV infection. INTERACTIONS Can be administered concomitantly with seasonal quadrivalent influenza vaccine (split virion, inactivated); no data on concomitant administration of Vaxneuvance with other vaccines. Different injectable vaccines should always be administered at different injection sites. Immunosuppressive therapies may reduce the immune responses to vaccines. FERTILITY, PREGNANCY AND LACTATION Pregnancy and lactation: Limited experience in pregnant women. Only consider use in pregnancy when risk: benefit ratio is positive for mother and foetus. No
VAXNEUVANCE is indicated for active immunisation for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.1

VAXNEUVANCE contains 15 purified pneumococcal capsular polysaccharides from Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, 33F), each conjugated to a carrier protein (CRM197).1
VAXNEUVANCE elicits a T-cell dependent immune response to induce antibodies that enhance opsonisation, phagocytosis, and killing of pneumococci to protect against pneumococcal disease.1
VAXNEUVANCE is available to order privately from MSD msd@united-drug.com Freephone orderline 1800 200 845 Freefax orderline 1800 200 846
For more information on the VAXNEUVANCE visit MSDConnect.ie or scan the QR code with your phone.
1. Vaxneuvance Summary of Product Characteristics, September 2022, available at www.medicines.ie.
data available for use during breastfeeding; no human data on effect of Vaxneuvance on fertility. SIDE EFFECTS Refer to SmPC for complete information on side-effects: Very common: headache; myalgia; injection-site pain, swelling and erythema; fatigue. Common: arthralgia*; injection site pruritis. Common in adults 18 to 49 years of age: Dizziness; nausea; pyrexia; chills. *very common in adults 18 to 49 years of age PACKAGE QUANTITIES 1 single dose (0.5 mL) suspension in pre filled syringe with 2 separate needles. Legal Category: POM. Marketing Authorisation numbers EU/1/21/1591/005 Marketing Authorisation holder Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands. Date of revision: August 2022. © 2022 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin, D18 X5K7 or from www.medicines.ie. Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-2998700) Red Oak North, South County Business Park,
Ireland. IE-PVC-00002 Date of Preparation: October 2022 A NEW ADULT PNEUMOCOCCAL CONJUGATED VACCINE INCLUDING 15 SEROTYPES (PCV15) VAXNEUVANCE delivered a robust immune response to 15 S. pneumoniae serotypes, including:1
Leopardstown, Dublin D18 X5K7,
• Noninferior immune responses for the 13 serotypes shared with PCV13 • 62% higher immune responsea for serotype 3 • Superior immune responses for 2 unique serotypes, 22F and 33F
NEW
REFERENCE
The Current and Future Role of Technology in Respiratory Care
Written by: Persijn Honkoop, Omar Usmani & Matteo Bonini
Over the past few decades, technology and improvements in artificial intelligence (AI) have dramatically changed major sectors of our day-to-day lives, including the field of healthcare.
Thanks to the spread and easy availability of several innovative technological devices and systems, it is in fact at present possible to obtain, collect, and analyze vast amounts of data from individual patients, leading to improved and more targeted healthcare solutions. Such an approach of “Precision Medicine” has allowed public health policies and decision-making processes to adopt a delivery model that focuses on tailored traits, improving disease diagnosis and management, as well as helping to reduce the relevant burden of chronic respiratory diseases.
Electronic health (e-health) is a term coined in the late 1990s, to describe a broad range of digital technologies and interventions used by a variety of stakeholders, across different settings.1 E-health includes a wide range of subdomains, such as mobile health, telemedicine, medication trackers, wearables,
digital stethoscopes, and clinical decision-support systems (Fig. 1). E-health has been consistently shown to enhance the quality of care, improve adherence to therapy, and allow early detection of worsening in chronic pulmonary diseases.2 In respiratory care, e-health has also been reported to empower patients with tools

and skills to become optimal selfmanagers. Indeed, e-health allows proactive care by providing easy to use and personalized action plans. Furthermore, healthcare professionals may find e-health interventions valuable channels to actively interact with patients and to facilitate information exchange. In addition, the use of e-health is becoming widespread in the pharmaceutical industry and in research settings. Indeed, rather topically, with the public health emergency and the need to limit in-person appointments while maintaining access to care due to the COVID-19 pandemic, e-health services have significantly increased, giving the potential to provide patients with improved awareness of their pathological condition and foster their involvement in the care pathway. E-health programs are widely used in respiratory diseases,3, 4 particularly in patients affected by chronic conditions, such as chronic obstructive pulmonary diseases (COPD), asthma, and interstitial lung diseases.5 Remote patient monitoring involves continuous evaluation of patients’ clinical status, through realtime recordings or via analysis
of functional parameters and vital signs remotely collected. In this framework, tools, such as wearables, smart inhalers, and digital stethoscopes, are often connected continuously with mobile applications in order to deliver medical expertise depending on patients’ needs. The present review addresses the current and potential future role of major e-health tools and approaches in respiratory medicine, with the aim of providing readers with reliable and cutting-edge evidence to increase their awareness on the topic, and to allow them to optimally benefit from the latest innovation technology.
Role of Technology in Respiratory Medicine


Wearables
The concepts of “ubiquitous” and “pervasive” human wellbeing monitoring are becoming a reality as a consequence of the important advances in sensors, miniaturized processors, body area networks, and wireless
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
54 RESPIRATORY: SMART TECHNOLOGY
Figure
1
data transmission technologies, allowing the assessment of physical, physiological, and biochemical parameters in different environments without restriction of activity.6 “Wearable” means whatever a subject can wear, such as watches, patches, or devices, without limiting daily activities or restricting mobility. Presently, there are an overwhelming number of fashionable wearable devices, but not all of them are capable of reliably measuring the health status of the wearer.7 Conversely, there are plenty of sensors that measure physiological parameters, but are not in a wearable form. Wearable biomedical sensors are therefore a subset of devices that are able to both measure physiological parameters and be worn. Biomedical wearables are commonly worn on the wrist, earlobe, finger, or trouser belt. Several products have been tested for assessing and monitoring both individual (i.e., respiratory rate, oxygen saturation, heart rate, glycemia, physical activity) and environmental parameters (i.e., air temperature and humidity, allergen and pollutant air concentration).8
Pulse oximeters, for example, measure light absorption [which depends on the levels of oxygenated (oxyhemoglobin) and deoxygenated blood] through an electronic processor and a pair of small light-emitting diodes illuminating the skin.9 Pulse oximetry is commonly measured on the spot, but can also be continuously checked during daily activities, and at night to reveal potential effort-driven or nocturnal desaturations. Pulse oximetry has a crucial value in the assessment and management of acute dyspnoea, both in primary and secondary care. Currently, however, there is little evidence to support or refute the use of home pulse oximetry in the selfmanagement of asthma, although the oxygen saturation (SpO2) value is considered a valuable marker to identify patients with asthma exacerbations at risk of respiratory failure, and who need to be promptly hospitalized.10 Similarly, in COPD patients, low SpO2 has been shown to be a reliable marker of symptom worsening and the occurrence of an exacerbation episode.11, 12 Interestingly, more recent bio-wearables tested on COPD patients with SpO2 measured continuously have generated personalized alerts, based on intelligent algorithms, that distinguish physiological oxygen fluctuations from real signs of disease decline.13 Home telemonitoring systems, including pulse oximetry measurements, have also been tested with benefits in patients affected by interstitial
lung diseases, and in particular in idiopathic pulmonary fibrosis (IPF).14 Of current relevance, home pulse oximetry has become an essential monitoring tool during the COVID-19 pandemic, helping to assist self-isolated patients, and to detect early deterioration of the pulmonary function.15

In addition to blood oxygenation, pulmonary ventilation provides key information on abnormal respiratory patterns, especially in patients at risk of sudden deterioration in their clinical condition.16,17,18 Respiratory rate is considered one of the most reproducible indirect parameters of pulmonary ventilation, and can be measured by body surface wearable sensors placed on chest bands, shirts, bras, or trouser belts.19,20,21
Moreover, wearable devices have garnered significant attention as a safe and cost-effective healthcare device for tracking physical activity; this being highly correlated with health outcomes, such as disease morbidity, hospitalization and mortality.22 Given that most respiratory diseases are associated with cardiovascular comorbidities and muscular deficits, wearable devices have become a valuable tool to promote an active lifestyle. Exercise tolerance can be measured through several endpoints, such as distance walked/run, diaries and questionnaires, metabolic activity, heart rate, and body temperature. Remarkably, “fitness trackers” (i.e., Fitbit) can suggest personalized rehabilitation or training programs depending on the user’s goals and needs.23,24,25,26
Several wearable devices are being developed to monitor indoor and outdoor air quality, as well as other environmental data. Data can be transmitted via Bluetooth or wi-fi to mobile phones to create maps of air quality. This could be particularly helpful, for example, in atopic asthmatic patients, who should avoid irritants, allergens, and pollutants to minimize the risk of developing exacerbations.27 There are several barriers to the implementation of wearables in daily clinical practice. We have already mentioned the reliability of the results.7 There is also an issue with interoperability, how to get the data of these devices into the electronic medical record (EMR) that healthcare professionals (HCPs) use, and, furthermore, how to ensure the HCPs are not flooded with data. A well=designed implementation strategy is needed before introducing these to regular medical care.28, 29
Smart Inhalers
Inhaled medication is the keystone in the management of patients affected by chronic pulmonary diseases.30 Electronic monitoring of adherence to inhaler therapy has been feasible since about the 1980s, when the 'Nebuliser Chronolog' was first adopted in the academic setting.31 However, it is only in the past 10 years that the electronics have become sufficiently portable, reliable, and affordable to allow their extensive diffusion in the market. Such progress has been paralleled by significant improvements in wireless technologies and mobile computing, allowing information to be swiftly transferred from the inhaler to a personal device, following remote analysis, and shown to the user in a clear and attractive way.
It is well known that unsatisfactory adherence and poor inhalation technique to prescribed pharmacological treatment is one of the most relevant causes of lack of disease control among patients affected by chronic respiratory diseases. This has been repeatedly associated with negative clinical outcomes, impaired quality of life, and increased healthcare costs.32, 33
Electronic monitoring devices, also called “smart inhalers”, seem to have a crucial role in objectively monitoring adherence, and consequently providing precious real-time feedback to patients. Smart inhalers take advantage of a sensor attached to or integrated in the inhaler, and designed to connect, mostly via a Bluetooth system, to a mobile application. These devices can offer several functions, from simple tracking tools, only counting the usage of an inhaler, to more sophisticated devices that provide personalized alerts. Indeed, smart inhalers can remind patients to use their inhalers and provide personalized feedback depending on the time of use, inhalation technique, correlation with peak flow values, or questionnaire scores. Such information can be used, not only to detect and correct patient habits but also to guide physician decision processes in adjusting management. Several trials have been conducted testing smart inhaler use among COPD and asthma patients, showing adherence improvement and concomitant overall decrease in exacerbation rate and improved lung function.32, 34, 35
In 2007, Charles and co-authors36 published the results of a
randomized trial performed in more than 100 asthmatic patients, aiming to assess the impact of an audio-visual reminder feedback (AVRF). All the participants were given a smart inhaler device, although, in the control arm, this was exclusively adopted to record the compliance to treatment as the primary outcome. Study findings revealed a significantly improved adherence to the prescribed therapy in the intervention group over the observational period (88% vs. 66% of total intended doses).
The relevance of HCPs providing information concordant to those captured by smart inhalers was furthermore assessed in a randomized pilot trial, enrolling children referred to a respiratory outpatient clinic. Study results showed that adherence rate was significantly increased in children receiving feedback in comparison to those that did not, over a 4-month period (79% vs. 58%).37
In addition, the Study of Asthma Adherence Reminders, combining the potential of AVRF to that of personalized support offered by physicians to patients, strengthened the evidence of a marked difference in compliance between subjects assigned to the investigational arm and those allocated to the control group, although the study power was not determined in advance, this being just a secondary endpoint.38
Interestingly, Moran and co-workers, with the aim of evaluating the relationship between standard measures of adherence and more recently suggested scores/parameters, carried out a longitudinal observational study in a large sample of subjects diagnosed with COPD. Study results revealed that, despite 59 59% concordance between long-acting beta agonists used in combination with inhaled corticosteroid prescriptions and actual use, as measured by the number of delivered doses, a lower number of inhalations (47%) had actually been administered within the proper time-window.39
Advances in smart inhaler technology also permitted feedback on inhalation techniques. Such an innovative way of monitoring has importantly minimized the risk of critical errors when using both pressurized metered-dose inhalers and dry-powder inhalers, also allowing a more proper calculation of a reliable degree of adherence. In regard to this, smart inhaler
HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 55
feedback has been reported to be superior even to intensive education when referring to the share of doses taken appropriately and timely, thanks to a lower risk of over- or missed dosing, as well as of mistakes in the inhalation technique.40 Similar to wearables, smart inhalers also face barriers in implementation. For smart inhalers, specifically, the additional costs of the device can be an important barrier. Also, monitoring of adherence can be perceived as threatening by a patient, so it needs a proper introduction and an approach of positive feedback.
Portable Electronic Spirometers
Pulmonary function tests are useful references to optimal diagnosis and monitoring of patients with chronic respiratory diseases. Particularly for asthmatic and COPD patients, spirometry represents the gold standard test for disease diagnosis and classification, as well as a valuable aid for assessing disease severity, prognosis, and management.41 Despite its crucial role, spirometry is not performed as much as it should be, due to several reasons, including bulky devices, complex interpretation software, need for frequent calibration, maintenance, and supply costs, and special training for performing and interpreting tests. With the increasing demand for high-quality healthcare services, the advent of innovative and portable medical devices that can be used remotely and in resourceless conditions is improving delivery and quality of care. Multiple randomized controlled trials and systematic reviews have shown efficacy in improving asthma control and quality of life, while reducing the rate of severe asthma exacerbations.42, 43
Following an earlier report of selfmonitoring of pulmonary function using a home spirometer,44 a study investigated the reliability, feasibility, and impact of home-based measurement of forced vital capacity (FVC) and dyspnea in a US population of IPF patients.45 Another study conducted in a specialized center in the Netherlands confirmed the feasibility of a home monitoring program including real-time wireless home spirometry in IPF patients.46
Carpenter and coworkers recently conducted an elegant systematic review of commercially available portable electronic spirometers
designed for asthma patient use.47 The search strategy yielded 36 devices, 16 of which met inclusion criteria. Although all the devices were designed for use by patients with asthma, most were also suitable for other respiratory diseases. For instance, ten devices (62.5%) targeted COPD patients, six (37.5%) cystic fibrosis, and five (31.3%) bronchitis and emphysema. Three devices (18.8%) were also marketed to monitor lung function after lung transplant surgery. All the 16 portable spirometers provided testing for peak expiratory flow (PEF) and forced exhaled volume in the first second (FEV1), while 13 (81.3%) also allowed patients to measure FVC. Interestingly, half of the devices measured FEF25–75, which is an indirect endpoint of the extent to which the smaller airways are affected.48, 49 Ten devices provided graphical representations of lung function results. Seven gave patients immediate visual or audio feedback on whether they had performed the test correctly. Six devices had a traffic light system indicating whether patients’ pulmonary status was in the red (danger zone), yellow (caution), or green (safe) zone. Almost all the spirometers, either directly or via their associated apps, allowed patients to share lung function test results with their healthcare provider. Of note, however, only four devices had obtained approval from the FDA and the majority of them (65%) did not provide any information regarding how data security was addressed, which is a major barrier to implementation. It is also uncertain whether the other devices will eventually submit an application for FDA approval, since it is an arduous process. Moreover, it has to be repeated for every (major) change to the device. This is problematic for companies working according to a continuous improvement principle, whereby they frequently tweak the devices. Although it is understandable that very frequent resubmissions for FDA approval are bypassed from a company perspective, it limits their applicability, and these devices cannot be recommended by clinicians. Similar to wearables and smart inhalers, a proper integration of the data these devices generate into the EMR will be essential. For implementation to be a success, these data should aid a clinician in decision-making, rather than be overwhelming.50, 51
A commonly used device in clinical practice is the PIKO-1 (Pulmonary Data Services; Ferraris

Medical, Hertford, UK), which is able to measure both PEF and FEV1. The device has a built-in memory which stores the last measurements performed, and reports a comparison of each measurement to the patient’s reference value for that parameter. Data are also automatically transferred to a cloud platform which provides real-time feedback to the patient through a specifically designed app. It has been shown that, when regularly adopted, PIKO-1 and other similar devices can facilitate the patient's awareness of the disease, and encourage cooperation with the GP or respiratory physician.52
Digital Stethoscope
The stethoscope was invented in 1818 by a French physician, René Laennec.53 Lung sounds acquired by stethoscopes are extensively used in diagnosing and differentiating respiratory diseases. However, despite the huge effort made over recent decades to interpret these sounds and to identify diseases associated with certain patterns, effective stethoscope use is limited to the individual experience of practitioners. Electronic stethoscopes represent an innovative method to increase the accuracy of lung auscultation, by amplifying and recording lung sounds and transforming them into digital signals that can be later processed and shared. The digital stethoscope consists of three different modules, data acquisition, pre-processing, and signal processing, before the listener can appreciate the auscultated sound. The data acquisition module involves a microphone and a piezoelectric sensor. It is responsible for filtering, buffering, and amplification of the auscultated sounds, as well as the conversion of the acoustic sound to a digital signal. The pre-processing module filters the digital signal and removes any artefacts. These digital data are then forwarded to the signal-processing module, which packages the information in a higher-order classification, and clusters the data for a clinical diagnostic decision.
Gurung et al. performed a meta-analysis of studies aiming to understand the prognostic power of combining digital pulmonary auscultation with computerbased algorithms, showing that the specificity and sensitivity of identifying abnormal pulmonary sounds, using computer- based algorithms, were 85% and 80%, respectively.54
In an ongoing, multi-center clinical trial (clinicaltrials.gov identifier: NCT03503188), digital lung sounds and basic patient characteristics are being prospectively collected from patients with IPF and from symptom-matched control subjects. A well-established teaching device for lung sounds will be used as a reference.55
Further studies have tried to create a unique and standardized protocol for the correct use of digital stethoscopes, starting from the identification of specific chest auscultation points and the classification of sounds based on score grading scales. Nevertheless, there is the need of further and deeper analysis among a wider cohort of patients that could lead to record and classify a greater range of pathological sounds. This is an essential condition for the creation of a complete sound database that can enhance and improve the sensibility and specificity of electronic auscultation process.56, 57 Until that is created, the limited reliability is a major barrier to implementation. Also, users will have to get used to the sounds created in this way, because they will be (subtly) different to what doctors are used to. A lot of thought will also need to go into the proper implementation into the organization of care. Of course, these sort of devices might allow for long-distance consultations, which would provide an excellent solution for more rural areas. However, care still needs to be organized properly, and the doctor responsible will need to have sufficient data available to make an informed clinical decision.58
Artificial Intelligence (AI) Diagnosis
Recent years have seen an explosion of interest in the use of artificial intelligence and machine-learning (ML) techniques in medicine.59 AI may be defined as “the theory and development of computer systems able to perform tasks normally requiring human intelligence, such as visual perception, speech recognition, decision-making, and translation between languages”; ML is a subfield of AI in which statistical models are used to learn patterns from data to accomplish a specific task. Such a phenomenon has been driven by the development of deep neural networks (DNNs) that can process complex input data and output a classification, such as ’normal’ or ’abnormal’. DNNs are ’trained’ to use large banks of data that have been assigned the correct labels.
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE RESPIRATORY: SMART TECHNOLOGY 56
DNNs have shown the potential to equal or even surpass the accuracy of human experts in pattern recognition tasks, such as interpreting medical images or biosignals.60,61,62 Within respiratory medicine, the main applications of AI and machine learning thus far have been the interpretation of thoracic imaging, lung pathology slides,and physiological data, such as pulmonary function tests.63, 64
ML has been used for instance to create an algorithm for the classification of fibrotic lung diseases using high-resolution computed tomography (HRCT) of the lungs to distinguish between usual idiopathic pneumonia (UIP) and non-UIP patterns, with comparable accuracy to an expert radiologist. In particular, CALIPER (Computer-Aided Lung Informatics for Pathology Evaluation and Rating) analyzes and evaluates the severity of parenchymal pulmonary abnormalities (honeycombing, reticular abnormalities, groundglass opacities, emphysema) and progression over time in HRCT.65, 66
Furthermore, an integrated homemonitoring system (myAirCoach) has recently been tested with the aim of assessing its clinical effectiveness and acceptance, on top of usual care, in patients with asthma using inhalation medication.67 The myAirCoach
system consists of an inhaler adapter, an indoor air-quality monitor, a physical activity tracker, a portable spirometer, a fraction exhaled nitric oxide device, and an app (Fig. 2). The primary outcome has been asthma control, while secondary outcomes are exacerbations, quality of life, and patient acceptance. Subjects were recruited in 2 separate studies: in the first, 30 participants were randomized to either usual care or myAirCoach support for 3–6 months, while in the second study, 12 participants were provided with the myAirCoach system in a 3-month before–after study. Using the myAirCoach support system significantly improved asthma control and quality of life, with a reduction in severe asthma exacerbations. Participants reported positive attitudes toward the system.68 AI presents us with several new barriers. There are concerns regarding privacy and security of data, and questions regarding liability. AI can also impact negatively on patient autonomy.69

Conclusions
Given the scarcity of resources for managing chronic lung diseases, it is particularly important to consider the potential added

value of novel digitization concepts in respiratory medicine. However, before implementation, potential interventions need to be assessed for their efficacy and cost-effectiveness, and with more bonding between sectors and disciplines. Technology interventions have been proven to be acceptable to respiratory patients and physicians, and the relative ubiquity of mobile Internetand Bluetooth-connected devices makes real-time monitoring of pulmonary diseases extremely feasible in everyday life. Overall, the potential of technology tools in respiratory medicine relies on three fundamental aspects: the interaction between clinicians, the interaction between clinician and patient, and the interaction between patient and the health technology. Moreover, personalized technological approaches, as well as innovative care pathways to patients, could significantly reduce healthcare costs. However, an effective digital intervention must fulfil several important design criteria. Both inhaler devices and software should be intuitive and easy to use, and hardware should be unobtrusive, with accurate and objective measurement of
adherence and other parameters. Motivating patients to take an active role in managing their condition, and maintaining their interest in doing so, is of paramount importance in designing a successful future digital intervention. Lastly, the current lack of proper study power assessments and the challenges in establishing a minimal clinical difference particularly for the patient-reported outcomes makes it hard to compare and fully interpret study findings. There is, therefore, the need to establish widely agreed and adopted standards for conducting trials and reporting results on the role of technology in respiratory medicine. These should also take into consideration potential pitfalls related to e-health interventions, such as patient privacy protection, data fishing, and non-compliance with evidence-based medicine, guideline recommendations, and regulatory board statements.
HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 57
Figure 2
Robotic Assisted Thoracic Surgery – the Next Step

thoracotomy and sternotomy. At St James’s we offer a comprehensive minimally invasive thoracic surgical program with a minimally invasive approach undertaken in up to 70% of our lung cancer operations.
Minimally invasive approaches result in less pain, less blood loss, and a shorter length of stay in hospital as well as smaller incisions and improved cosmesis with similar long-term cancer survival. The improved recovery times can also lead to greater uptake of adjuvant treatment if indicated.
Robotic assisted thoracic surgery (RATS) represents the pinnacle of modern thoracic surgical practice. Pulmonary resections have seen many advances in both surgical technique and perioperative care since first performed in the 1800’s. Minimally invasive approaches have modified surgical practice, particularly in the past 20 years, and this evolution has demonstrated significant advantages for both patient and surgeon.
Robotic assisted thoracic surgery involves a surgeon using a console to control a number of robotic arms that enable more advanced minimally invasive surgery (Figure 1). We use three or four small incisions to perform operations using wristed robotic instruments under direct vision with a stereoscopic magnified high-definition camera. It offers advantages in many cases over traditional minimally
invasive approaches such as conventional video-assisted thoracic surgery (VATS) due to improved visualisation with the 3-dimensional camera and less pressure on port sites due to the pivot feature of the robotic ports. It enables the surgeon to operate in a more natural orientation as the instruments move in the same fluid manner as human wrist joints.
For the surgeon, there is more direct hand-eye co-ordination with advanced manoeuvrability of the wristed instruments giving improved ergonomics compared to conventional VATS surgery.

Minimally invasive approaches represent an evolution in surgical care from the traditional open approaches to the chest, such as

There remains some discussion regarding the advantages of robotic-assisted thoracic surgery compared with conventional video-assisted thoracic surgery, particularly given the significant financial costs involved. Despite that it is now generally accepted that robotic surgery results in smaller scars, less incidence of arrhythmia's and respiratory tract infections, lower blood loss, less pain, shorter length of hospitalisation, and faster overall recovery time with a resultant earlier return to normal activity.
The median length of stay for VATS lung resections is 4 days while it is three days for robotic surgery; with traditional open surgery it remains 6 days.
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
58 RESPIRATORY: ROBOTIC SURGERY
Figure 1: Intuitive da Vinci robotic assisted surgical platforms
Written by Ms Rebecca Weedle MCh FRCSI (CTh), Mr Ronan Ryan MD FRCSI (CTh), Mr Vincent Young MD FRCSI (CTh) & Mr Gerard J Fitzmaurice MSc FRCSI (CTh) Department of Cardiothoracic Surgery, St James’s Hospital, Dublin
Mr Vincent Young, Ms Rebecca Weedle and Mr Gerard J Fitzmaurice
For the surgeon, robotic surgery can be easier to learn than VATS particularly if already an experienced open surgeon or transitioning from the posterior VATS approach. There is a greater degree of precision with 10-times magnification allowing meticulous dissection and motion scaling eliminates tremor, there is minimal lung retraction / handling with the benefit of carbon dioxide (CO2) insufflation, and importantly the ability to undertake a systematic mediastinal lymph node dissection is comparable to an open approach. That said, there are some disadvantages including cost as it is significantly more expensive than VATS and one must accept that like a PC it will have a limited shelf-life and have to be upgraded or replaced about every 5-years.
the surgeon to control. The vision cart communicates between the other components and houses the energy technologies and screen for surgical assistants and scrub nurses to follow the operation.
Robotic-assisted thoracic surgery can be used for a wide variety of procedures in the chest, including on the lung, the diaphragm, and the mediastinum. RATS has particular advantages when dealing with mediastinal tumours enabling a higher degree of precision and facilitating a greater scope of patient access to minimally invasive surgical approaches. However the bulk of our thoracic surgical program are lung resections performed for the treatment of lung cancer.
and a much higher rate of airleak however in select patients it does offer an additional treatment avenue in the era of robotic surgery in patients with borderline respiratory reserve. Altorki and colleagues more recently presented similar results in the CALGB140503 Alliance study and publication of the full results is eagerly awaited. The robotic approach is particularly useful for segmentectomy due to the excellent anatomical visualisation and enhanced articulation of the instruments. It also enables the surgeon to undertake a comprehensive systematic mediastinal lymph node dissection which we know is so important amongst this patient population.
Robotic-assisted thoracic surgery can be used for a wide variety of procedures in the chest, including on the lung, the diaphragm, and the mediastinum. RATS has particular advantages when dealing with mediastinal tumours enabling a higher degree of precision and facilitating a greater scope of patient access to minimally invasive surgical approaches. However the bulk of our thoracic surgical program are lung resections performed for the treatment of lung cancer. At St James’s Hospital, we perform approximately 55% of all curative-intent lung cancer surgery in Ireland. Anatomical lung resections, predominately lobectomy, are the current gold standard for treating resectable non-small cell lung cancers however there has been recent interest in sub-lobar resections which are particularly amenable to a robotic approach.
So why did we consider a RATS program? There are three key tenets - it allows us to undertake more complex procedures via a minimally invasive approach building on our already significant minimally invasive program, there are smaller incisions with less pain for our patients, and we want to be able to offer state of the art treatment to our patient population.
At St James’s Hospital, we perform approximately 55% of all curative-intent lung cancer surgery in Ireland. Anatomical lung resections, predominately lobectomy, are the current gold standard for treating resectable non-small cell lung cancers however there has been recent interest in sub-lobar resections which are particularly amenable to a robotic approach.
Segmentectomy was primarily performed for patients who were not fit for lobectomy but may now be offered in select patients to treat small peripheral lung cancers with proven multi-level negative lymph nodes. There has been much interest in a recent study by the West Japan Oncology Group and Japan Clinical Oncology Group (JCOG0802) published in the Lancet in April 2022 that demonstrated a similar 5-year overall survival between patients with small (<2cm) peripheral tumours with pathologically confirmed negative lymph nodes who underwent segmentectomy or lobectomy. Caution lies in double the local relapse rate amongst the segmentectomy group at 10.5% and a much higher rate of airleak however in select patients it does offer an additional treatment avenue in the era of robotic surgery in patients with borderline respiratory reserve. Altorki and colleagues more recently presented similar results in the CALGB140503 Alliance study and publication of the full results is eagerly awaited. The robotic approach is particularly useful for segmentectomy due to the excellent anatomical visualisation and enhanced articulation of the instruments. It also enables the surgeon to undertake a comprehensive systematic mediastinal lymph node dissection which we know is so important amongst this patient population.
As the largest centre for lung cancer surgery in Ireland, we use the latest da Vinci Xi robotic platform. This consists of a surgeon’s console which connects to a patient cart and a vision cart. The surgeon uses the console to control the view of the target anatomy and the instruments in real time. There is no delay in the transmission of the movements of the surgeons hands to the tips of the instruments. A second training console is also available to enable another surgeon to assist or train with the operating surgeon. The patient cart, which holds the camera and instruments, is docked to the ports and specially designed instruments are placed through the ports for
Segmentectomy was primarily performed for patients who were not fit for lobectomy but may now be offered in select patients to treat small peripheral lung cancers with proven multi-level negative lymph nodes. There has been much interest in a recent study by the West Japan Oncology Group and Japan Clinical Oncology Group (JCOG0802) published in the Lancet in April 2022 that demonstrated a similar 5-year overall survival between patients with small (<2cm) peripheral tumours with pathologically confirmed negative lymph nodes who underwent segmentectomy or lobectomy. Caution lies in double the local relapse rate amongst the segmentectomy group at 10.5%
In Ireland lung cancer is the 3rd most common form of cancer but the leading cause of invasive cancer-related death in both men and women. Recently published outcomes from the National Cancer Registry show that lung cancer survival has improved more than 2.5-fold over the last 30 years, from 9% 5-year net survival in 1994-1998 to 24% in 2014-2018 (Figure 2). A significant part of this improvement is due to advanced surgical techniques and focused surgical care with improved technology as well as improved diagnostics and systemic therapies. Lung cancer screening has been shown to reduce lung cancer related mortality and large trials in the UK (UKLS), Europe (NELSON), and the US (NSLT) have all shown screening to be beneficial in targeted high risk populations. There is no public lung cancer CT screening available yet in Ireland, but this is likely to result in an increase in the detection of early stage lung cancers when introduced. Many of these cancers will be amenable to surgical treatment with a minimally invasive approach.


At St James’s Hospital, we launched our robotic thoracic surgical programme earlier this year and we have integrated it into our existing practice. One of our first patients was an 83-yearold gentleman with impaired lung function that placed him in a high risk surgical category. He had a large tumour measuring 55mm in his left lower lobe and he underwent a RATS left lower lobectomy and systematic mediastinal lymph node dissection, achieving complete resection on final histopathology. Once his chest drain was removed, he did not require any opiate analgesia. He mobilised independently and successfully completed his post-operative physiotherapy assessment on day 2. He went home well 3 days following surgery. Our patients have reported minimal pain after robotic-assisted thoracic surgery with many only requiring simple analgesia. We have found that elderly patients, in particular, with poor lung function are gaining a real advantage with this treatment modality.
As the largest centre for lung cancer surgery in Ireland, we aim to continually evolve and recognise that modern thoracic surgical oncology practice involves many individually small but combined significant steps that enhance the overall patient journey and contribute to improved overall survival.
As part of the evolution of our minimally invasive program at St James’s, we have developed a comprehensive enhanced recovery after thoracic surgery (ERATS) program. This involves a number of key components including prehabilitation, nutritional support, smoking cessation, avoidance of fasting and carbohydrate loading, regional anaesthesia, early mobilisation, and minimally invasive surgery. It is a 360-degree patient centred journey with input from a broad multi-disciplinary team with the sole aim to provide optimal patient outcomes in lung cancer care. Our robotic assisted thoracic surgical program is a welcome addition for our patients to assist them in this difficult journey.
Figure 2: Graphical representation demonstrating a significant improvement in overall 5-year net survival from lung cancer in Ireland over the past 25 years [National Cancer Registry Ireland]

HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 59
Figure 2: Graphical representation demonstrating a significant improvement in overall 5-year net survival from lung cancer in Ireland over the past 25 years [National Cancer Registry
Ireland]
New Frontiers of the Management of Chronic Obstructive Pulmonary Disease (COPD)
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory condition. It is among the leading causes of mortality worldwide. This is expected to rise, due to ongoing exposure to COPD risk factors in developing countries and an aging population in developed countries.1, 2 Because of its high prevalence and chronicity, COPD exerts a substantial burden on the healthcare system and resources, due to the need for ongoing chronic therapy, outpatient appointments, and frequent hospitalisations.3
COPD is a heterogeneous lung condition characterised by persistent respiratory symptoms (dyspnoea, cough, sputum production) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema), that cause persistent, often progressive, airflow obstruction.4 COPD management aims to improve the quality of life via symptom control and prevention of exacerbations.5 Therefore, the optimal management of COPD requires an integrated pathway of care involving patients, family, care-givers, and healthcare professionals as patients move between stability and acute exacerbations.6
Diagnosis of COPD is based on a triad of causative exposure (particularly smoking), symptoms, and airflow obstruction (FEV1/ FVC <70%) on spirometry. The HSE Integrated Model of Care for the Prevention and Management of Chronic Disease includes COPD.7 This model of care supports people to live well within the community, with ready
and equitable access to their GP, diagnostics, health and social care professional input and specialist opinion, as required. The focus is on keeping people well and on providing care as close to home as possible. The cornerstone of treatment for COPD is smoking cessation and must be stressed with those who continue to smoke at every encounter.7 The other key non pharmacological interventions are vaccinations and pulmonary rehabilitation. Approved pharmacological therapies include inhaled bronchodilators and corticosteroids, azithromycin, roflumilast and oxygen therapy.
There have been numerous research developments in the management of COPD patients who are still symptomatic, despite optimal treatment. These new frontiers include monoclonal antibodies (MAbs), hyaluronan, stem cells, new bronchoscopic interventions, and digital health.

Monoclonal antibodies (MAbs)
MAbs are immunoglobulins designed to specifically act on a target to produce desired therapeutic effects for patients.8 In respiratory medicine, omalizumab was the first MAb approved and used in severe asthma in the early 2000s. Since then, more targeted MAbs have been developed, such as mepolizumab and benralizumab, and offered to appropriate asthma populations. Numerous MAbs or biologic agents have been tried to target inflammatory pathways in COPD but most disappointingly failed to demonstrate significant findings. Interest in MAbs was re sparked from positive results in COPD patients with an eosinophilic
phenotype using mepolizumab (anti-IL5) in METREO, a phase 3 clinical trial.9 However, the small yet significant results using mepolizumab in moderate-tosevere COPD in METREO were not demonstrated in its parallel phase 3 clinical trial, METREX, nor in two other phase 3 clinical trials for benralizumab (anti-IL5 receptor) (GALATHEA, TERANOVA).10 A systematic review integrating all of these results showed that these MAbs are safe but require high doses to reduce moderateto-severe exacerbations in highly selected eosinophilic COPD.11 For now, MAbs used in asthma show positive but limited proof of efficacy in eosinophilic COPD. In terms of cost-effective balance, they are not yet approved by the FDA.11 Nevertheless, there are more ongoing studies to delineate these effects (RESOLUTE trial for benralizumab, NCT04053634) and with different agents (dupilumab in NOTUS, NCT04456673) (tezepelumab in COURSE, NCT04039113). MAbs still have a long way to go in COPD management, but they remain a huge potential to be utilised in the personalised treatment of COPD, especially in a very selected group.12
Matrix Biologics: Hyaluronan

Hyaluronan is a high molecular mass polysaccharide found as a major component in extracellular matrix, especially of soft connective tissue.13 It has a key role in wound repair with tissue regeneration, inflammation response and angiogenesis.14 In the lung, a high concentration of hyaluronan can be found and detected on the airway surface.15, 16 Hyaluronan molecules could be degraded to smaller fragments in acute inflammation and these smaller fragments could exert strong pro-inflammatory effects.17 New evidence suggests that the imbalance between hyaluronan and its smaller fragments may contribute to the pathogenesis of chronic airway disease.18 In animal models, aerosolized hyaluronan was found to reverse emphysema induced by neutrophil elastase.19
A phase 2 clinical trial on hyaluronan in the form of highmolecular-weight in COPD exacerbation demonstrated significant findings clinically in
terms of shortened duration of acute respiratory failure, need for non-invasive ventilation and length of stay in the hospital.20 This was only a pilot study with a small number of patients, but it has illustrated the safety and feasibility of further exploring hyaluronan as a potential agent in the management of COPD in the future.
Stem cells
Stem cells are a population of unspecialized cells with the potency to differentiate into different types of cells and with regenerative capability.21, 22 Stem cell therapy in regenerative medicine may have the potential to play a revolutionary role in the management of COPD or even in any other lung disease, moving away from treating symptoms and focusing more on regenerating damaged lung structures and restoring lung function.23
Based on preclinical research on COPD, stem cells may benefit patients in terms of improving airway inflammation, regenerating new lung tissues for ventilation, and angiogenesis of blood vessels to improve perfusion.24 Clinical trials of stem cells in COPD so far have not progressed from phase 2. A recent systematic review of stem cells in COPD patients demonstrated that whilst stem cells or its derivative product were safe, they only significantly improved the exercise capacity of COPD patients, with no improvement in lung function, pCO2 retention or hospitalisation from acute exacerbation.23 Thus, the success of stem cell therapy in animal models was not fully replicated and demonstrated in clinical trials, possibly due to the heterogeneity, small number of samples, and the severity of COPD patients enrolled.25
Larger, well-designed randomised controlled trials are required to quantify the beneficial effects of stem cells in COPD.
Bronchoscopic interventions
Lung hyperinflation occurs with advanced emphysema in COPD. It causes breathlessness as the diaphragm is unable to function properly. Surgical intervention may be indicated in a very carefully selected group

JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE RESPIRATORY: COPD 60
Dzufar Halim, Department of Respiratory Medicine, University Hospital Galway - University of Galway
Sinead Walsh, Department of Respiratory Medicine, University Hospital Galway - University of Galway
Written by
digital health.41 Examples are telemonitoring, telehealth, postdischarge care, hospital-at-home strategy, electronic records, big data analytics, environmentmonitoring applications and digital-technology-enabled homecare programmes.42
Digital health is an innovative solution to provide an integrated, patient-centred care model for the management of COPD and patients are perceptive to its role, especially with symptoms and anxiety management.43 In COPD management specifically, digital health technologies potentially can be used in a number of ways. These include pulmonary rehabilitation, inhaler technique training, adherence monitoring, recognition and early treatment of exacerbations, education about COPD and its management, remote monitoring and recognition of symptoms, feedback to patients and healthcare professionals, tools to avoid exacerbation triggers etc (Figure 1).40

Figure 1: Potential opportunities for digital innovations to facilitate effective COPD management (from Watson, A. and T.M.A. Wilkinson, Digital healthcare in COPD management: a narrative review on the advantages, pitfalls, and need for further research. Therapeutic Advances in Respiratory Disease, 2022. 16: p. 17534666221075493).
Figure 1: Potential opportunities for digital innovations to facilitate effective COPD management (from Watson, A. and T.M.A. Wilkinson, Digital healthcare in COPD management: a narrative review on the advantages, pitfalls, and need for further research. Therapeutic Advances in Respiratory Disease, 2022. 16: p. 17534666221075493).
of COPD patients if they are still symptomatic despite maximal medical therapy and pulmonary rehabilitation.26 The recognized surgical interventions for COPD are mainly giant bullectomy, lung volume reduction surgery (LVRS) and lung transplantation.27 LVRS, when performed on the right group, can improve both symptoms and mortality. However, open surgery is not without risk of severe complications, especially in the multimorbid COPD population. In the NETT trial, for example, LVRS is associated with high perioperative mortality and morbidity.28 This is one of the highest-risk procedures performed electively due to the fact that these patients generally have low lung function and increased age.29
Endoscopic Lung Volume Reduction (eLVR) is an alternative procedure which is less invasive and has better postoperative risk profiles compared to open surgery. Different techniques are available including an endobronchial oneway valve (EBV), self-activating coils, airway bypass stent, sealant, and thermal ablative techniques. The most widely studied and used technique in eLVR is EBV.30 Current literature shows a carefully selected group of patients who underwent EBV had significant improvement in exercise capacity and quality of life.31 These results are comparable to LVRS but with a lower rate of complications.32 An ongoing trial (CELEB) will further
clarify which one is a better option; LVRS or eLVR. (ISRCTN19684749)
There are new procedures targeting the chronic bronchitis group of COPD; nitrogen cryospray, bronchial rheoplasty and lung denervation technology. These procedures use a similar concept, that is to deliver a specific substance endobronchially via bronchoscopy to reduce airway secretions. The nitrogen cryospray uses liquid nitrogen to induce extreme cold to freeze and destroy abnormal cells.33 It has two clinical trials ongoing currently (NCT03892694, NCT03893370). A study published in 2020 showed that it was safe, feasible and significant symptom improvement.34 Bronchial rheoplasty is a technique applying non-thermal pulsed electrical fields to the abnormal airway epithelium. A small clinical trial in COPD patients with chronic bronchitis demonstrated that it is feasible, safe and associated with significant symptomatic relief.35 An ongoing trial, RheSolve (NCT04677465), will further elucidate the effects of this intervention.
Lung denervation technology uses radiofrequency ablation via bronchoscopy to disrupt parasympathetic innervation of the lung. By doing this, cholinergic hyperactivity and subsequently mucous secretions can be reduced. A phase 2 trial, AIRFLOW-2, showed that this

technology can reduce the risk of exacerbation and hospitalizations in patients with moderate to severe COPD with chronic bronchitis.36 An ongoing follow-up trial (AIRFLOW-3, NCT03639051) will further determine the effects of the technology on COPD exacerbation.
Digital health
Self-management is an essential part of chronic disease management as patients become more responsible for their healthcare and develop skills to manage their conditions more effectively.37 It is associated with an improvement in quality of life, reduction in hospitalisation, reduction in exacerbation duration, and lower healthcare costs.38 However, efforts to increase patients’ autonomy and active participation in their selfmanagement care can be difficult with barriers from both patients (motivation, ambivalence) and healthcare professionals (time, resources, skills).39
The recent rise of digital technologies and innovations during the COVID-19 pandemic has led to an emphasis on a different approach to address self-management and ultimately COPD care.40 The utilisation and medicalisation of digital technologies to make healthcare more enhanced, efficient, affordable, personalised and precise have been termed
Pulmonary rehabilitation (PR) is a vital component in managing breathless patients with COPD. Its use, especially postexacerbation, can provide better symptomatic relief and quality of life and improve mortality.44 However, barriers preventing full utilisation of this service include accessibility, inconvenience, lack of transportation and fewer PR centres.45 Using digital technologies, home PR could be an alternative to provide this key intervention. Current literature demonstrates that this is feasible and effective even in frail COPD populations.46, 47, 48 Digital health is projected to be the future of healthcare and therefore it will become the new frontier in how we manage patients with COPD.49
Summary
COPD is a heterogeneous condition that requires specific treatment for different types of phenotypes or endotypes. The pathogenesis needs further exploration to better understand this complex heterogeneity and the inflammatory pathway involved. There are numerous emerging therapies targeting different aspects of COPD, including monoclonal antibodies, matrix biologics (hyaluronan), stem cells, bronchoscopic interventions and digital health. Further research is still needed before they become mainstays of treatment, but the healthcare professionals and patients with COPD can look towards the future with optimism at the new frontiers of COPD management.
References available on request
HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 61
Lung Cancer in Non-Smokers
by Dr Sile Toland, Respiratory SpR, Beaumont Hospital
 Dr Dan Ryan, Consultant Respiratory Physician, Beaumont Hospital
Dr Dan Ryan, Consultant Respiratory Physician, Beaumont Hospital

likely due to the development of specific somatic mutations that drive malignancy. However, several studies have shown that increasing age is also a common predisposing factor. The increased risk with age is felt to be due to the longer duration of exposure to potential carcinogenic agents.14
Risk factors
The causative factors for lung cancer in never smokers are generally not well understood but several separate genetic and environmental factors are believed to contribute to lung cancer carcinogenesis which we discuss in the following sections

Second-hand smoke
Lung cancer is the leading cause of cancer death worldwide, causing about 1.8 million deaths per year.1 In Ireland, approximately 2,700 people are diagnosed with lung cancer every year.2 Exposure to tobacco smoke is the main aetiologic factor responsible for lung cancer, accounting for approximately two thirds of cases worldwide and its importance is demonstrated by the decline in lung cancer incidence and mortality that has accompanied the decline in smoking.3-5Despite the preponderance of tobacco smoking as its predominant aetiology, lung cancer is also a health problem in those with no history of smoking, accounting for between 10-20% of cases5,6 and despite the general reduction in lung cancer rates, the rate of lung cancer in non-smokers has been noted to be increasing.5

The term “never smoker” refers to individuals who have smoked less than 100 cigarettes in their lifetime. The majority of lung cancers in non-smokers occur in Non-Small Cell Lung Cancers (NSCLC) and particularly in lung adenocarcinoma.6 A much smaller subset of lung tumours (2%) called carcinoid tumours also show no major relationship to previous smoking history.6 With advances in the understanding of the molecular biology of cancer, substantial differences
have been found in the genetic and molecular characteristics of lung cancer in smokers versus non-smokers including clinically significant treatment differences and prognostic outcomes. Several studies have suggested that lung cancer in non-smokers is different enough from a biologic and epidemiologic perspective to be looked at as almost a completely different entity.7-10
Epidemiology
Worldwide, 10–20% of patients with lung cancer are non-smokers.11 There are major geographic and gender differences, particularly in Asia, where 60-80% of women with the disease are never smokers.12 Despite non-smoking women more frequently being diagnosed with lung cancer than nonsmoking men, they do have an overall decreased mortality when compared to men. In two American Cancer Society cancer prevention study cohorts, it showed agestandardised lung cancer mortality rates of 12 per 100,000 for never smoking men and 9.5 per 100,000 for never smoking women of European ancestry. In Asian populations, the rates were 26 and 16.1 per 100,000 for men and women, respectively.13
Lung cancer in non-smokers had classically been considered to occur at a younger age most
Second-hand smoke is an important risk factor for lung cancer amongst never smokers. Several studies suggest that approximately 15-35% of lung cancer among never smokers is
due to second-hand smoke and that risk may be increased in those with exposure prior to age 25.15,16 Analysis from a case-control study in Europe of 520,000 people estimated that the proportion of lung cancer related to secondhand smoke was 16 to 24 percent in non-smokers. In the European Prospective Investigation into Cancer and Nutrition study evaluating the risk of cancer in nearly 500,000 people, the risk of lung cancer in never smokers was significantly elevated in those with second-hand smoke exposure.17 A meta- analysis from 55 international studies showed specifically that spousal smoking increased the risk of lung cancer in a never smoking spouse.18
Radon
Radon is a gaseous decay product of uranium-238 and radium-226 and is capable of damaging respiratory epithelium through the emission of alpha particles.
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
62
RESPIRATORY: LUNG
Dr Sile Toland
CANCER
Written
and
Dr Dan Ryan
References:
3.
REPUBLIC OF IRELAND
Presentation: Brigatinib 180 mg, 90 mg and 30 mg film-coated tablets. Indications: As monotherapy for the treatment of adult patients with anaplastic lymphoma kinase (ALK)positive advanced non-small cell lung cancer (NSCLC) previously not treated with an ALK inhibitor. As monotherapy for the treatment of adult patients with ALK-positive advanced NSCLC previously treated with crizotinib. Dosage and administration: Recommended starting dose is 90 mg once daily for the first 7 days, then 180 mg once daily. If ALUNBRIG is interrupted for 14 days or longer for reasons other than adverse reactions, treatment should be resumed at 90 mg once daily for 7 days before increasing to the previously tolerated dose. If a dose is missed or vomiting occurs after taking a dose, an additional dose should not be administered and the next dose should be taken at the scheduled time. Treatment should continue as long as clinical benefit is observed. Dosing interruption and/or dose reduction may be required. Refer to SmPC for full dose adjustments. Paediatric population: No data are available. Elderly patients: Dose adjustment is not required. No available data on patients aged > 85 years. Hepatic impairment: A reduced starting dose of 60 mg once daily for the first 7 days, then 120 mg once daily is recommended for patients with severe hepatic impairment (ChildPugh class C). Renal impairment: A reduced starting dose of 60 mg once daily for the first 7 days, then 90 mg once daily is recommended for patients with severe renal impairment (eGFR <30 mL/min). Patients with severe renal impairment should be closely monitored for new or worsening respiratory symptoms (e.g., dyspnoea, cough, etc.) particularly in the first week. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and precautions: Refer to SmPC for recommended dose modifications. Pulmonary adverse reactions: Most occurred within the first 7 days of treatment, but they can occur later in treatment. Patients should be monitored for new or worsening respiratory symptoms (e.g., dyspnoea, cough, etc.), particularly in the first week of treatment. If interstitial lung disease/pneumonitis is suspected, the dose of ALUNBRIG should be withheld, and the patient evaluated for other causes of symptoms (e.g., pulmonary embolism, tumour progression, and infectious pneumonia). Dose modification may be required. Hypertension: Heart rate and blood pressure should be monitored regularly. Hypertension should be treated according to standard guidelines. Heart rate should be monitored more frequently in patients if concomitant use of a medicinal product known to cause bradycardia cannot be avoided. Withhold ALUNBRIG in patients with severe hypertension (≥ Grade 3) until hypertension has recovered to Grade 1 or to baseline. Modify dose accordingly. Bradycardia: Caution should be exercised when administering ALUNBRIG in combination with other agents known to cause bradycardia. If symptomatic bradycardia occurs, treatment with ALUNBRIG should be withheld and concomitant medicinal products known to cause bradycardia should be evaluated. Upon recovery, the dose should be modified according to SmPC. In case of life-threatening bradycardia, if no contributing concomitant medication is identified or in case of recurrence, treatment with ALUNBRIG should be discontinued. Visual disturbance: Advise
patients to report any visual symptoms. Consider ophthalmologic evaluation/dose reduction for new or worsening severe symptoms. Creatine phosphokinase (CPK) elevation: Patients should be advised to report any unexplained muscle pain, tenderness, or weakness. CPK levels should be monitored regularly during ALUNBRIG treatment. Based on the severity of the CPK elevation, and if associated with muscle pain or weakness, treatment with ALUNBRIG should be withheld, and the dose modified. Elevations of pancreatic enzymes: Lipase and amylase should be monitored regularly during treatment with ALUNBRIG. Based on the severity of the laboratory abnormalities, treatment with ALUNBRIG should be withheld, and the dose modified. Hepatotoxicity: Liver function, including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total bilirubin should be assessed prior to the initiation of ALUNBRIG and then every 2 weeks during the first 3 months of treatment. Thereafter, monitoring should be performed periodically. Based on the severity of the laboratory abnormalities, treatment should be withheld, and the dose modified. Hyperglycaemia: Fasting serum glucose should be assessed prior to initiation of ALUNBRIG and monitored periodically thereafter. Antihyperglycaemic medications should be initiated or optimised as needed. If adequate hyperglycaemic control cannot be achieved with optimal medical management, ALUNBRIG should be withheld until adequate hyperglycaemic control is achieved; upon recovery, dose reduce ALUNBRIG as per the SmPC or permanent discontinuation may be considered. Photosensitivity and photodermatosis: Photosensitivity to sunlight has occurred in patients treated with ALUNBRIG. Patients should be advised to avoid prolonged sun exposure while taking ALUNBRIG and for at least 5 days after discontinuation of treatment. When outdoors, advise patients to wear a hat and protective clothing and to use a broad-spectrum Ultraviolet A/B sunscreen and lip balm (SPF≥30). For severe photosensitivity reactions (≥Grade 3) withhold ALUNBRIG until recovery to baseline. The dose should be modified accordingly. Lactose: ALUNBRIG contains lactose monohydrate. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take ALUNBRIG. Sodium: ALUNBRIG is essentially ‘sodium-free’ containing less than 1 mmol sodium (23 mg) per tablet. Interactions: Avoid use with strong CYP3A inhibitors. Avoid use with strong and moderate CYP3A inducers. Please refer to SmPC for further information and guidance for situations where use cannot be avoided. Grapefruit or grapefruit juice should be avoided. Coadministration of ALUNBRIG with CYP3A substrates with a narrow therapeutic index should be avoided as ALUNBRIG may reduce their effectiveness. Coadministration of ALUNBRIG with substrates of P-glycoprotein, breast cancer resistance protein, organic cation transporter 1, multidrug and toxin extrusion protein (MATE) 1 and 2K may increase their plasma concentrations. Patients should be closely monitored when ALUNBRIG is co-administered with substrates of these transporters with a narrow therapeutic index. Fertility, pregnancy and lactation: Women of reproductive potential should be advised not to become pregnant and to use effective non-hormonal
contraception during treatment with ALUNBRIG and for at least 4 months following the final dose. Men should be advised not to father a child during ALUNBRIG treatment. Men with female partners of reproductive potential should be advised to use effective contraception during and for at least 3 months after the last ALUNBRIG treatment. No clinical data on the use of ALUNBRIG in pregnant women. ALUNBRIG should not be used during pregnancy unless the clinical condition of the mother requires treatment. Breastfeeding should be stopped during treatment with ALUNBRIG. No human data are available on the effect of ALUNBRIG on fertility. Undesirable effects: Very common (≥1/10): Pneumonia, upper respiratory tract infection, anaemia, lymphocyte count decreased, APTT increased, white blood cell count decreased, neutrophil count decreased, hyperglycaemia, hyperinsulinaemia, hypophosphataemia, hypomagnesaemia, hypercalcaemia, hyponatraemia, hypokalaemia, decreased appetite, headache, peripheral neuropathy, dizziness, visual disturbance, hypertension, cough, dyspnoea, lipase increased, diarrhoea, amylase increased, nausea, vomiting, abdominal pain, constipation, stomatitis, AST increased, ALT increased, alkaline phosphatase increased, rash, pruritus, blood CPK increased, myalgia, arthralgia, blood creatinine increased, fatigue, oedema, pyrexia. Common (≥1/100 to <1/10): Decreased platelet count, insomnia, memory impairment, dysgeusia, bradycardia, electrocardiogram QT prolonged, tachycardia, palpitations, pneumonitis, dry mouth, dyspepsia, flatulence, blood lactate dehydrogenase increased, hyperbilirubinaemia, dry skin, photosensitivity reaction, musculoskeletal chest pain, pain in extremity, musculoskeletal stiffness, non-cardiac chest pain, chest discomfort, pain, blood cholesterol increased, weight decreased. Other serious undesirable effects: Uncommon: (≥1/1,000 to <1/100): Pancreatitis. Refer to the SmPC for details on full side effect profile and interactions. Legal classification: POM. Marketing authorisation (MA) numbers: EU/1/18/1264/011, EU/1/18/1264/008, EU/1/18/1264/010, EU/1/18/1264/012.
Name and address of MA holder: Takeda Pharma A/S, Delta Park 45, 2665 Vallensbaek Strand, Denmark. Additional information is available on request at: medinfoemea@takeda.com. PI approval code: pi-01842. Date of Preparation: January 2022.

FIRST-LINE ESMO RECOMMENDATION2 A lunbr ig is indic ate d as monother apy for the treatment of adul t patient s wi th A L K+ aNS CLC previously: 3 - not treate d wi th an A L K inhibi tor, or, - previously treate d wi th cr izotinib NOW APPROVED FOR ONCE-DAILY FIRST-LINE TREATMENT OF ALK+ aNSCLC3 See ALK+ aNSCLC, Think Brain Metastases, Choose ALUNBRIG (brigatinib) 1
the Summary of Product Characteristics (SmPC)
prescribing
C-APROM/IE/ALUN/0051. Date
preparation: June 2022
ALUNBRIG®t(brigatinib) PRESCRIBING INFORMATION FOR
Refer to
before
Adverse Events should be reported to the Pharmacovigilance Unit at the Health Products Regulatory Authority. Reporting forms and information can be found at: www.hpra.ie. Adverse events should also be reported to Takeda at: AE.GBR-IRL@takeda.com
of
This medicinal product is subject to additional monitoring.
Takeda Oncology, and ALUNBRIG are registered trademarks of Takeda Pharmaceutical Company Limited. Other trademarks are the property of their respective owners. ©2022 Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. All rights reserved. t
ALK, anaplastic lymphoma kinase; aNSCLC, advanced non-small cell lung cancer; ESMO, European Society for Medical Oncology.
1. Camidge DR, et al. J Thorac Oncol 2021;16:2091-2108; 2. Planchard D, et al. Annals Oncol. 2018;29(S4): iv192–237 - Updated version published 15 September 2020. Available at: https://tinyurl.com/3853nnnu. Accessed June 2022.
Alunbrig (brigatinib) Summary of Product Characteristics. Available at: https://www.medicines.ie/medicines/alunbrig-34655/ Accessed June 2022.
Radon is present in soil, rock, and groundwater, and it can accumulate in homes.
Radon is classified as a Group 1 carcinogen and exposure to radon gas is thought to be the second most important cause of lung cancer worldwide after smoking.19 Internationally, the proportion of lung cancer cases estimated to be attributable to radon ranges from 3-14% depending on the average radon concentration in the country.20
This is of particular importance in Ireland, as Ireland has previously been estimated to have the eighth highest level of indoor radon concentrations amongst OECD countries and radon is estimated to cause approximately 350 cases of lung cancer in Ireland each year.21
A 2018 paper published in the Journal of Environmental Radioactivity showed that, in Ireland, those living in areas where 10%-20% of households are above the national reference level for radon exposure are about three times more likely to report a lung cancer diagnosis than those who live in areas with fewer than 1% of households above the national reference level. The most notable examination of radoninduced lung cancer within an Irish context resulted from two cases of lung cancer in non-smokers which prompted the discovery of a household with levels of indoor radon 250 times higher than the national reference level.22
Air Pollution
Outdoor air pollution is also associated with lung cancer risk. Using mean long-term ambient fine particulate matter (PM2.5) as a measure of air pollution, in the Cancer Prevention Study-II, there was a 15-27% increase in lung cancer mortality in never smokers for each 10 mcg/m3 increase in PM2.5 concentrations. In 2019, approximately 300,000 lung cancer deaths worldwide were attributed to PM2.5 exposure.23
Data from a breakthrough study in understanding how air pollution can lead to lung cancer, presented at the European Society of Medical Oncology congress in September 2022 conducted by researchers at the Francis Crick Institute and University College London showed a positive correlation with higher rates of EGFR-mutant
lung cancer and other types of cancer found in areas with higher concentrations of PM2.5. The researchers hypothesised that, when inhaled, PM2.5 particles trigger an alarm response in the lungs, causing the inflammation and activation of dormant cells carrying cancer-causing mutations. Mice with EGFR-mutant cells were exposed to air pollution concentrations normally found in cities, and found that the exposed animals were more likely to develop lung cancer than nonexposed mice. The discovery is of global impact because 99% of the world’s population currently live in areas that exceed WHOs annual limit for PM2.5.24
Other environmental factors
Other occupational exposures are known to increase lung cancer risk.
Commonly associated toxins include asbestos, chromium, and arsenic. In a French study of 1493 cases, some occupational exposure was identified in 9.4 percent of women and 48.6 percent of men who developed lung cancer as never smokers 25Arsenic has connected with an increased risk of lung cancer where it contaminates drinking water, such as in some areas of Taiwan and Chile.26 Asbestos exposure alone leads to a six-fold increase in relative risk of developing lung cancer, while asbestos exposure and smoking leads to a 59-fold increase.27Various dietary factors have been studied as possible causes of lung cancer. Higher intake of fruits and vegetables may be protective against lung cancer. One study from Italy showed a link between higher consumption of red meat and an increased risk for the development of lung cancer in never smokers28, and another report indicated that higher consumption of fish may protect against lung cancer in never smokers.29Indoor air pollutants such as fumes from cooking oil and the smoke from burning coal have been linked to lung cancer, particularly in Asia.30,31
Oestrogens
The role of oestrogens and other female hormones in lung cancer risk in women is uncertain, regardless of smoking status.33 Multiple studies have shown that the majority of NSCLCs express the oestrogen receptor beta and that the expression of this receptor is more common in never

smokers compared with smokers. Other studies have looked at the relationship of lung cancer incidence to early menopause use of hormone therapy, age at first birth, number of children, and use of tamoxifen. However, the results have been inconsistent in establishing a relationship. Data from the randomized WHI trial indicated that women assigned to postmenopausal oestrogen and progesterone had a higher risk of death from lung cancer without a higher incidence of the disease compared with women assigned to placebo . However, women randomised to oestrogen alone had no such increase in lung cancer mortality, nor in lung cancer incidence, compared with those on placebo.34 Further studies are required to establish the role, if any that oestrogen may play in lung cancer development and the effect that anti oestrogen therapies may have on treatment.
Lung disease
The risk of lung cancer among never smokers is increased in those with prior damage to the lungs from underlying pulmonary disease or exposure to radiation or chemotherapy. The extent of this risk is unclear because the vast majority of pulmonary disease is related to smoking. In idiopathic pulmonary fibrosis, an association does seem to be supported by the majority of studies, one study from Li et al in 2014 showed the prevalence rates of lung cancer in patients with IPF (4.8% to 48%) are much higher than patients without IPF (2.0% to 6.4%).35
A 2008 paper by Yang et al. showed that alpha-1-antitrypsin carriers are at a 70–100% increased risk of lung cancer, particularly adenocarcinoma and squamous cell subtypes, depending on smoking intensity, smokers were noted to have a 2–9-fold higher risk of lung cancer than never smokers.36With regards to non-cystic fibrosis bronchiectasis a population based cohort study published in September 2022 showed that the incidence of lung cancer in participants with bronchiectasis was significantly higher regardless of smoking status.37
Biological differences
1.Pathology
Adenocarcinoma is more common in never smokers as well as in light smokers and former smokers while squamous cell carcinoma and small cell lung cancer are seen with a higher frequency in heavy smokers.9,12 In a review of
published reports on the histology of lung cancer that included smoking data, adenocarcinoma was more common than squamous cell carcinoma among nonsmokers (62% versus 18%, based upon 5144 cases). By contrast, adenocarcinoma was less frequent among smokers (19% versus 53% in 21,853 cases).11,38,39
Genetic factors and Molecular biology
Multiple studies have shown an association between lung cancer in never smokers and a family history of lung cancer, suggesting a significant role for genetic factors.
In a case-control study of 257 cases of lung cancer among never smokers and an equivalent number of control never smokers, lung cancer was significantly more common among those with a positive family history.40
In another case-control study that included over 2400 relatives of 316 never smoker lung cancer cases, there was a 25 percent excess risk of any type of cancer among first-degree relatives of cases, this study also showed an increased risk of early-onset lung cancer (<50 years) among those relatives who smoked. While this study does not directly link a history of lung cancer in non-smokers to future generations developing lung cancer, it does show the importance of genetic factors leading to the development of cancer as well as the risk involved when genetically susceptible individuals partake in smoking.41
Contemporary advances in the understanding of the molecular biology of lung cancer have led to the identification of substantial genetic differences between lung cancer in smokers versus never smokers. These factors are arguably more important than any clinical differences. These genetic changes generally occur in genes that when mutated, fundamentally alter the activity of the cell to promote uncontrolled growth and division and are referred to as driver mutations. While these mutations also occur in smokers, the rate at which they occur in non-smokers is proportionally much higher and particularly in adenocarcinoma where prevalence of clinically actionable driver alterations in never smokers can be as high as 92% in certain populations.42,43
Epidermal Growth Factor receptor (EGFR) mutations
EGFR is a cell surface tyrosine kinase that regulates intracellular signalling pathways to control
RESPIRATORY: LUNG CANCER JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE 64
Table. Characteristics of Lung Cancer in Smokers vs Never Smokers
Non-Smokers
Smokers
Proportion of global lung cancer cases 10-20% 70-80%
Average age at diagnosis 70 70
Sex predominance Female Male
Stage IV at diagnosis 62% 49%
Histological makeup
• 50-60% adenocarcinoma
• 10-20% squamous cell carcinoma
• Small cell lung cancer is extremely rare

Prevalence of clinically actionable driver alterations 78-92% 50%
• Occurs across histological subtypes
• Strongest association with squamous cell carcinoma and small cell lung cancer
Dominant driver mutation EGFR KRAS
PDL-1 expression (Tumour proportion score >50%)
cellular proliferation. Specific mutations in genes encoding for EGFR (Del19/Exon 21 L858R) results in constitutive or sustained activation of these signalling pathways resulting in aggressive tumour phenotypes. EGFR mutations occur in about 15% of the general population with advanced lung Adenocarcinoma. However in non-smoking patients this rate increases to 30% or more. Furthermore the rates of EGFR positivity increase further in women and in patients from Asian populations where mutations can occur in 60% or more of cases in non-smokers.3,44 Importantly, significantly improved treatment response rates are seen in patients with EGFR mutations who are treated with tyrosine kinase inhibitors (TKIs). Higher response rates and better survival have been reported in never smokers with these drugs compared with current or former smokers.43
Anaplastic Lymphoma Kinase (ALK) gene mutations
The ALK gene also encodes a transmembrane tyrosine kinase
Lower (11.9%) Higher (28.3%)
that plays an important role in normal cell regulation activities in its normal state. In ALK-activated NSCLC, a rearrangement occurs with another gene ( EML4) to create an activated driver mutation that fuels unrestricted cell growth and division. ALK-rearranged NSCLC comprises 2% to 5% of all NSCLC cases but similarly to EGFR occurs in significantly higher numbers in light and never smokers which account for 70-80% of cases.45 Specific TKI inhibition in ALK positive patients also results in demonstrably better response rates compared to traditional platinum based therapies.45
V-Raf murine sarcoma viral oncogene homolog (BRAF) Mutation/Human epidermal growth factor receptor 2 (HER2)/c-ros oncogene (ROS 1)
There are a number of other cell surface Tyrosine Kinase mutations that can drive malignancy in NSCLC and specifically in nonsmokers. BRAF, HER 2 and ROS 1 are all surface tyrosine kinases that act as driver mutations in 1-2%
of lung adenocarcinoma cases and all occur significantly more often in patients who are nonsmokers.46-48 As such, testing for these specific mutations through immunohistochemistry analysis or Next Generation Sequencing (NGS) is increasingly mandated in advanced lung Adenocarcinoma in non-smokers.41-43 Furthermore, even broader testing for a wider spectrum of mutations should be considered through NGS in non-smoking patients if initial molecular profiling is negative as the proportion who will have a potentially targetable mutation is much higher than in smoking populations with advanced NSCLC and show good response rates.43
Summary
Non-smokers are defined as those who have smoked less than 100-lifetime cigarettes. Lung cancer in non-smokers almost exclusively occurs NSCLC or Carcinoid tumours and is more likely to occur in women with adenocarcinoma being the most common type. Although the aetiology for lung cancer among
Table. Characteristics of Lung Cancer in Smokers vs Never Smokers
never smokers is not always clear, risk factors include secondhand tobacco smoke, radon gas exposure, other environmental exposures and genetic factors. There are important differences at the molecular level between lung cancers arising in never smokers and those in smokers. The best understood of these are the abnormalities in Tyrosine Kinase pathways that occur much more frequently in patients who have never smoked. These include mutations in EGFR, ALK fusion oncogene, HER-2, BRAF, ROS-1 and other mutations for which targeted therapies offer potentially improved treatment response and survival rates. From a public health perspective It is important that patients are aware that lung cancer is a disease that does not exclusively affect current or former smokers and that any patient with persistent troubling chest symptoms should seek medical advice and further testing if required.
References available on request
HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 65
What to Know about the Acute Respiratory Viral Infections and the Flu?
Written by Tatiana Iarmak – http:// orcid.org/0000-0001-5371-2958.

Lecturer of the Department of Advanced Training of Junior Medical Specialists at the Municipal Health Care Institution 'Kharkiv Regional Medical Vocational College', Kharkiv, Ukraine. An independent trainer, consultant to medical facilities and non-medical facilities to instruct medical staff on how to safely provide services to patients/clients.
Corresponding Author details: iarmak.tat@gmail.com
The aim of the study: Show the relevance of the problem of the infectious diseases, in particular, of the acute respiratory and acute respiratory viral infections, influenza; the influence of the type of the pathogen, seasonality, age, the state of the body's immune system on the occurrence and the spread of the acute respiratory and the acute respiratory viral infections, influenza; the importance of prevention and immunoprophylaxis in preventing the occurrence and the spread of morbidity.
Brief summary: This article examines the dependence of the incidence of the acute respiratory and the acute respiratory viral infections, influenza, on the seasons of the year, the type of the pathogen, the ways of spreading of the infectious agents, immunity, lifestyle.

Background
Infectious diseases remain one of the main problem for all the countries and pose a serious threat to the whole world. They have been known to the mankind since ancient times. In ancient Greece, Hippocrates laid down the foundations of epidemiology - a science which studies the patterns of the occurrence and the spread of the infectious diseases among people, the measures of preventing and combatting them; also, introduced the concept of "epidemic" and outlined it in his collection of books "Epidemics".
In the 16th century, an outstanding Italian physician and astronomer, Girolamo Fracastoro, one of the founders of the modern epidemiology, the creator of the doctrine of infectious (contagious) diseases, singled out the concept of "infection" and three possible ways of infection: by contact with the patient, through the objects and through air (from the book "On contagion, contagious
diseases and treatment ", 1546). He also developed the preventive measures, such as the isolation of the patient, a separate clothing for those caring for the sick; marking the doors of the houses with a red cross when there were contagious patients; the closure of the places of trade, restricting the visits to churches, etc. Much of the above is still relevant today. Only in the second half of the 18th century did epidemiology become a science with its own laws and rules, which are still being elaborated. The discoveries of the two outstanding scientists in the 19th - 20th century, the founders of microbiology and immunology: Louis Pasteur (France) and Robert Koch (Germany), proved the bacterial nature of the infectious diseases and laid down the foundations of the artificial immunity with the help of the vaccines, as well as the following researches of many other scientists in the field of microbiology and virology allowed to divide diseases into infectious,
when the pathogen enters the human or animal body, and noninfectious. The 20th century was marked by a large number of discoveries of diseases of a viral nature. Viruses are widespread and very diverse. The ability of viruses to mutate – the variabilityleads to the emergence of the new strains and forms of diseases, the number of which increases every year. Currently, there are about 2 thousand infectious diseases. Infectious diseases (lat. "infectio") is a group of diseases resulting from an interaction of a pathogen, a microorganism (bacteria, viruses, fungi, etc.), with a macroorganism - a human or animal body. In this regard, infectious diseases are divided into anthroponoses, which are inherent (peculiar) only in humans and zoonoses or zooanthroponoses, inherent in animals and humans. As a rule, infectious diseases are contagious, in other words, transmitted from person-to-person, and can quickly spread among people.
The most contagious and common are the infections of the respiratory system – the respiratory infections. The respiratory system consists of the lungs and the respiratory tract: nose, pharynx, larynx, trachea, bronchi and bronchioles and performs a huge number of functions - respiratory, gas exchange, protective, thermoregulation, hematopoiesis, deposition, voice formation, olfactory, ventilation, suction, filtration, water, lipid metabolism functions and more. The respiratory tract infections are divided into upper respiratory tract infections and lower respiratory tract infections. The upper respiratory tract is the nasal cavity, paranasal sinuses, pharynx and part of the larynx above the vocal cords. The upper respiratory tract infections: rhinitis, sinusitis, pharyngitis, tonsillitis, epiglottitis, rhinosinusitis, etc. The lower respiratory tract is the larynx, trachea and bronchi. Infections of the lower respiratory tract: laryngitis, bronchitis, bronchiolitis, bronchopneumonia. The infections, affecting the lungs, are pneumonia. Infections of the lower respiratory tract, in terms of the frequency of
occurrence, are in the first place among the infectious diseases and in fourth place in the world in terms of mortality. Pneumonia is the leading cause of the early childhood death worldwide. In 2001, the pulmonologists from all countries organized a Forum of International Pulmonological Societies (FIRS). The societies included more than 70,000 professionals. The goal of the FIRS was to raise awareness of the population and the leaders of all the countries about the importance of the respiratory health, to develop the programs for a more effective prevention, improve the quality of an early detection of the respiratory diseases, and create a strategy to combat the respiratory system diseases, in particular, the respiratory infections. For a normal functioning, a person needs air (oxygen), water, sleep and food. Breathing is a vital necessity, therefore, the respiratory function is at the top of the list of human needs to stay alive. Without oxygen, an ordinary untrained person is able to hold out for 5-7 minutes, if longer, the irreversible changes occur in the cardiovascular system, in the brain - the oxygen starvation (hypoxia) of the vessels of the brain, and other organs, tissues, which leads to death. However, there is a category of people - freedivers, who use special techniques for training and can stay underwater for a long time (the record of 24 min. 33 sec. set in 2018 by a Croatian diver Budimir Šobat) at great depths without using professional equipment, with no oxygen, and without the detrimental consequences for their health.
About 90% of all infectious diseases are acute respiratory infections (lat. respiratiobreathing). This is the most common group of the diseases which causes the greatest economic damage to all the countries of the world compared to other infectious diseases. The acute respiratory infections or the acute respiratory diseases affect the organs of the respiratory system. They can be of various origin - bacterial, viral, fungal, etc. The respiratory infections are a specific type of an infectious
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
“A wise person would rather avoid diseases, than pick drugs” Thomas More
66
VIRAL
RESPIRATORY:
INFECTIONS

PP-BIO-IRL-0149 All rights
September 2021
Marketing
▼
reserved.
Legal Category: POM S1A.
Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information please contact: Pfizer Healthcare Ireland, 9 Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 or Medical Information on 1800 633 363 or at medical.information@pfizer.com Please see summary of product characteristics for more information. T This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Refer to section 4.8 of the SPC for how to report adverse reactions.
pathology. They have a long seasonality - autumn, winter, spring; all age groups can get sick but more often these are children, who attend preschool institutions or elementary school. Immunity after an infection is short-term type-specific - only inherent in a certain type of the causative agent of a disease. The cold season is the most fertile time for the spread of the viruses, especially the respiratory viruses. The acute respiratory infections can be caused by one type of the pathogen, for example, only by a virus or a bacteriummonoinfection; by a combination of different types of only viruses or only bacteria - coinfection; by simultaneously the different types of pathogens: viruses, bacteria, fungi and others - mixed infection. The mixed infection often occurs in a more severe form, for a longer time, with complications, since the interaction of the pathogens often changes their properties, which can lead to disability and death. With co- or mixed infection, the infection occurs simultaneously (in parallel) with the different types of pathogens. There is also a concept of superinfection, when another infection (secondary infection) joins an already existing infectious process. Infection occurs, as a rule, at the stage of recovery, when the human body is depleted, immune forces and resistance are reduced. Secondary infection prolongs the course of the disease, worsens the patient's condition, leads to complications, up to death. The most common bacterial pathogens are Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae Mycoplasma pneumoniae, Chlamydophila pneumoniae,
Legionella pneumophila, etc. Against the backdrop of a bacterial infection, the complications in a form of bronchitis or pneumonia may develop. The acute respiratory infection is caused by viruses: influenza, parainfluenza, rhinoviruses, reoviruses, adenoviruses, respiratory syncytial virus, coronavirus, etc. The respiratory syncytial virus is one of the main causative agents of the acute respiratory infections in children of the first 2 years of life (25%). Older children and adults are more likely to get influenza (40%-50%); and during an epidemic, the incidence rates rise up to 70%-90%. Currently, more than 300 viruses are known to cause the acute respiratory infection. More than 500 million people fall ill and more than 4 million die each year from the respiratory infections, especially in the countries with low living standards. About 40% of the respiratory infections in the cold season occur in a form of co-, mixed- and superinfections. Quite often, a viral infection, especially influenza, is complicated by a bacterial pneumonia; but in the last decade, the most common complication of the acute respiratory viral infection was the meningitis – an inflammation of the membranes of the brain and the spinal cord and the meningoencephalitis – an inflammation of the membranes of the brain and cerebral substance. Together with the acute respiratory infection, they significantly aggravate the patient's condition and can lead to death. About 300 thousand people die every year from meningitis and every fifth person who has been ill with it develops the irreversible


processes: hydrocephalus, mental retardation in children; epileptic seizures, psychosis, etc. WHO has classified meningococcal infection as a particularly dangerous infection. To prevent and control the meningitis, WHO has developed the first global roadmap, which sets out a concept and a strategy for the period up to 2030. When a viral infection is complicated by a bacterial infection, doctors often prescribe the antibiotic therapy. Unreasonable prescribing of antibiotics, as well as their sale without prescription in pharmacies leads to their uncontrolled use. The misuse of antibiotics is a major problem worldwide. About 50% of them are used not on purpose. About 1.3 million people die every year (WHO data) from infections, whose pathogens are resistant to antimicrobials. If the situation does not change, the annual number of deaths may increase up to 10 million (according to the WHO experts) by 2050, which will exceed the mortality from the cardiovascular and oncological diseases. On November 18, 2022 the World Antimicrobial Awareness Week took place under the auspices of the United Nations. Its organizers: the Food and Agriculture Organization of the United Nations (FAO), the United Nations Environment Program (UNEP), the World Health Organization (WHO) and the World Organization for Animal Health (OIE) presented the Multi-Stakeholder Partnership Platform to Combat Antimicrobial Resistance. WHO Director-General Tedros Adhanom Ghebreyesus said: "This platform will be critical in raising awareness of the issue... and gaining public support."
Of all the acute respiratory infections, the most dangerous infection is the flu. The history of influenza begins in the ancient times. The great Hippocrates and the ancient Roman historian Titus Livy for the first time in 412 BC described the symptoms of the flu. Hippocrates also noted the seasonality: “All diseases occur at any time of the year, but some of them often occur and worsen at certain times.” The name “flu” appeared only in the 17th century and came from the French word “grippe”, which means “grab”, another name for the flu is “influenza” (flu) from lat. “intrude”. In 1933, at the National Institute for Medical Research in England, the British virologists Christopher H. Andrewes., Wilson Smith and Patrick Laidlaw outlined the first human influenza virus type “A”; T. Francis and T. Medgill discovered the type “B” influenza virus in 1940; R. Taylor influenza discovered the type "C" virus in 1947. Influenza type “A” viruses pose the greatest danger, causing epidemics and pandemics; influenza type “B” - epidemic outbreaks and epidemics, influenza type “C”more often sporadic incidence. The process of the emergence and the spread of the infectious diseases among people is called an epidemic process. According to the degree of intensity of the spread, the epidemic process manifests itself in the form of a) sporadic morbidity - single cases of the disease which are not epidemically related to each other; b) epidemic outbreak - an epidemically linked incidence, which affects a small group, for instance, a kindergarten or a larger group, such as a college or a university prom; c) epidemicmass morbidity of people, covering a locality or country; d) pandemic -
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE RESPIRATORY: VIRAL INFECTIONS 68
mass morbidity of people, covering countries and continents.
The main route of transmission of influenza and the acute respiratory infection from a sick person to a healthy person is airborne. The acute respiratory infections are transmitted by the airborne droplets, which contribute to the rapid spread of the disease. When coughing, sneezing, talking, singing, and even breathing and yawning, the microparticles of saliva, mucus, sputum from the mucous membranes of the respiratory tract, enter the surrounding air and can be in it in a form of the fine aerosols, which poses the greatest danger within the radius of 2-3 meters in enclosed spaces. In a while, the aerosol settles down on the environmental objects (furniture, floor etc.), it remains there for a certain time (depending on the type of the pathogen); thus, a person gets infected by contacting the infected surfaces. Also, it can be transmitted by the household contact through the household items: dishes, dummies for babies, towels, bed linen. Moreover, influenza and the acute respiratory infections can be transmitted through the transplacental route (intrauterine - through the placenta), from a mother to the fetus during pregnancy and have a teratogenic (causes malformations) effect on the embryo and fetus, which can lead to various pathologies, congenital viral disease, death. In addition, a pregnant woman
may be at risk of miscarriage or premature birth. In order to prevent the severe consequences of the acute respiratory infection and influenza in pregnant women, it is recommended to inform women about possible complications and carry out preventive measures before the pregnancy occurs.
The scientists from Trinity College Dublin, Ireland, together with the scientists from the University of Melbourne in Australia, have identified a mechanism of the occurrence of the dangerous complications in women during pregnancy, when infected with the influenza virus. Professor John O'Leary, who holds the position of the Professor/Chair of Pathology in Trinity College Dublin described the influenza virus as a "vascular storm". The studies have shown that the virus provokes hyperactivity of the immune system, the long-term consequences of which can be manifested in a form of various diseases in the mother and child. "We will need more research to develop a specific antiviral therapy for pregnant women," said Prof John O'Leary.
To reduce the risk of the occurrence and the spread of the acute respiratory infections, it is necessary to carry out prevention. Prevention is divided into non-specific and specific. The non-specific prevention is aimed at strengthening of the immune system: daily routine, good sleep,
positive attitude, physical activity, a proper nutrition and drinking routine, walks in the fresh air etc. Also, the implementation of sanitary-hygienic and antiepidemic measures, especially in the cold season with an increase in the incidence of the respiratory infections: frequent washing of hands with soap or treating them with an antiseptic, since hands are the main factor in the transmission of an infection. Ventilation; wet cleaning of premises, including the door handles, switches, etc. with the use of the disinfecting solutions; treating phones and gadgets with an antiseptic; avoidance of the crowded places: public transport, theaters, concert halls, etc.; visiting medical institutions without necessity; going to a friend’s house when one is sick with the respiratory infection; staying home and not going to work or not taking children to school at the first signs of illness: runny nose, cough, fever will significantly reduce the possibility of infection and the spread of the acute respiratory infection. In public places, it is required to observe the "respiratory etiquette" - the rules of conduct aimed at limiting the spread of the respiratory diseases: - wearing a medical mask correctly - covering the nose and the mouth (reduces the risk of infection by 60% -80%).
The mask was invented by the surgeons Johann MikulichRadetsky and Paul Berger at the end of the 19th century. During the Spanish flu pandemic of 1918-1920, the masks were used en masse for the first time;
- when coughing and sneezing, to use a disposable paper handkerchief or napkin and discard immediately after use;


- sneezing and coughing into the elbow bend in the absence of a handkerchief, not into the palms or fists of the hands, since the pathogens are transmitted from the hands to the objects – the door handles, switches, handrails, ATM buttons, trolley handles in a store etc .;
- treating hands with an antiseptic or the antiseptic wipes; - not touching one’s face with dirty hands, especially the mouth, nose, eyes.
A specific prevention - the immunoprophylaxis or a vaccination, is aimed at protecting against possible infection and reducing the risk of complications. In 1952, the WHO established the Global Influenza Surveillance and Response System (GISRS). The WHO Global Influenza Surveillance Standards were updated in 2013. The strategy of the system for the manufacture of the influenza vaccines with adaptation to the virus mutations allows to update the composition of vaccines every year and save millions of lives.
Conclusions
The acute respiratory infections, the acute respiratory viral infections, influenza are the most common diseases in the general structure of the infectious diseases worldwide. An early detection and isolation of patients; compliance with the sanitary-hygienic and anti-epidemic regime, especially during the seasonal periods of an increase in the incidence, compliance with the rules of the "respiratory etiquette"; targeted use of the antibiotics; prevention of the post-respiratory infectious complications in women during pregnancy, after childbirth, in newborns; the organization of the non-specific and specific preventive measures - immunoprophylaxis, can reduce the risks of the occurrence and the spread of the acute respiratory infections among the population, as well as lower the level of morbidity and mortality, especially among children.
HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 69
Developments in Cystic Fibrosis Paediatric Care

Cystic Fibrosis Ireland, the national patient organisation has played a significant role in all these developments since our formation in 1963, as have at times individual patient advocates/spokespeople.
The new exciting CFTR
that was subsequently updated and replaced with the Model of Care for CF in 2019 published by the National Clinical Programme for CF, and its steering group, which includes CFI.
Of further crucial importance in the early years of CF paediatric care in Ireland was the emergence the first range of effective medications. These drug therapies include: Creon which is an enzyme that aids the digestion of food (1992); Pulmozyme, which helps thins CF related mucus (1994) and the nebulised Tobramycin that was the first effective antibiotic against Pseudomonas (1998).
Modulators address the underlying cause of cystic fibrosis, not just the symptoms. With some younger patients in particular, the impact of these drug therapies is so powerful that the sweat test used to diagnose CF at birth can sometimes return a negative result.
it is distressing that as a result of a dispute between Vertex and the HSE, 35 children with CF remain excluded from the drug therapy kaftrio that has helped revolutionise CF care in Ireland in recent years. This exclusion stems from a difference in interpretation of the innovative 2017 portfolio agreement for CFTR modulators and CFI continues to advocate resolution as soon as possible.

This is a time of hope and increased expectation for the health and future all people with cystic fibrosis in Ireland, especially young people and children. In my capacity as CEO of Cystic Fibrosis Ireland I have been meeting up with our many voluntary branches around the country in recent weeks as COVID-19 subsides, but still remains a concern. The general feedback of parents from these meetings is recognition of the significant progress in CF care in Ireland in recent years, with still more to do.
Much of the initial push for the concept of CF paediatric services in the 1960’s came from Professor Colman Saunders and his daughter Anne O’Dwyer, who was then a mother of twins with CF. Anne became the first chairperson of the CF Association of Ireland (CFAI) in 1963 – an early example of partnership in effective patient advocacy, a concept that remains important to this day.
Paediatric CF services commenced in Ireland in 1969 with the opening of a modest CF unit in the Children’s Hospital in Crumlin, with initial seed funding
provided by Cystic Fibrosis Ireland. As this centre proved effective, it gained support beyond the board of Crumlin hospital. Because of support from the Department of Health, the first CF paediatric consultant in Ireland, Dr. Edward Tempany, was appointed in 1971.
Because of the severity of CF and poor expectations of survival during this period, it was not until 1976 that the first CF adult consultant was appointed – Dr. Muiris Fitzgerald in St Vincent’s Hospital, Dublin. These two much respected clinicians are fondly remembered to this day. The challenges they faced in delivering CF services at this time were immense because of major gaps in facilities and medications and CF research was still at an early stage.
Major developments in CF paediatric care in more recent years includes: The addition of CF to the newborn screening Programme (2011) and the emergence of a network of specialised and shared care paediatric centres around the country that followed on from the recommendations of the CFI commissioned the Pollock Report
While these medications remain vital in CF care to this day, most focus and expectations are now on a range of drug therapies from Boston based company Vertex. These CFTR modulators commenced with Kalydeco (2013) and Orkambi (2017) and more recently Kaftrio (2020). Kaftrio in particular has demonstrated very significant increases in lung function and a reduction in hospitalisations that have been confirmed through real world data.
Thanks to a range of factors over the past decade, CF is now a much more manageable long term disease. The outlook for those with CF, particularly young people with CF, has never being so positive. This is reflected in the increasing positive data from the CF Registry of Ireland set up by CFI in 2002 and which became independent in 2005 and is based in UCD.
These improvements have come about through the partnership of clinicians; HSE funded CF specialised and shared care hospitals; the CF related health research undertaken and supported by many.
The next key infrastructure development in CF paediatric care will be the opening of the forthcoming Children’s Hospital Ireland in the St James campus.
CHI will host the merger of the 3 existing Dublin CF centres in Tallaght, Temple St and Crumlin and will herald in another new era if CF care for children and young people with CF.
Heath research remains a vital part of shaping clinical services. As part of the HRCI/HRB joint funding scheme, CFI is currently funding 2 major CF related research projects focussing on the transition form paediatric to adult services and CF related liver disease. CFI is also a partner in the CF Recover research project looking at real world outcomes from CFTR modulators, led by Professor Paul McNally (CHI Crumlin).
Finally, CFI wishes to thank the excellent CF clinical teams; the National Clinical Programme for CF; the HSE and most of all the thousands of people who support our work as an association as we look forward to celebrating 60 years of advocacy for better CF services and outcomes for people with CF in Ireland.
JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Written
by Philip Watt, CEO Cystic Fibrosis Ireland
RESPIRATORY: CYSTIC FIBROSIS 70
“Heath research remains a vital part of shaping clinical services. As part of the HRCI/HRB joint funding scheme, CFI is currently funding 2 major CF related research projects focussing on the transition form paediatric to adult services and CF related liver disease”


Early detection of recurrent lung cancer: Enhancing-nodule in post-radiation fibrosis
Introduction
Chemotherapy and radiotherapy remain the mainstay of non-small cell lung cancer treatment despite the advances in surgical management. It is important to differentiate expected post-treatment changes from lung cancer recurrence.1

Radiation-induced lung injury generally manifests with two distinct well-known clinical and pathologic phases: an early phase of transient radiation pneumonitis, which usually occurs within the first 6 months after completion of treatment and a later phase of chronic radiation fibrosis, which typically occurs at 6–12 months after completion of treatment.1
Case report
A 70-year-old male was referred for chest CT following an abnormal chest radiograph that showed a suspicious nodule. The subsequent contrast-enhanced chest CT scan revealed a 3.6 m lobulated enhancing mass in the superior segment of the left lower lobe. He underwent CT-guided trans-thoracic biopsy which revealed a stage T2a primary lung adenocarcinoma (Figure 1(a)).

Subsequent EBUS confirmed nodal metastases in a station 4L node. A PET-CT scan did not show any distant metastasis. He received chemotherapy with stereotactic body radiotherapy (SBRT) to the tumour (Figure 1(b)) and underwent 3–6 monthly surveillance CT scans for the next 2 years which showed no signs of recurrence. A chest CT performed 3 years out from his initial treatment was reported to show stable post-radiation fibrosis. The enhancing-nodule in postradiation fibrosis sign was not detected although in retrospect was present within the area of fibrosis (Figure1(c)). A repeat chest CT 6 months later for increasing
symptoms showed enlargement in the enhancing nodule which was detected and a biopsy proved recurrence and the patient was recommenced on chemotherapy (Figure1(d)).
Discussion
Libshitz et al.2 and Ikezoe et al.3 were the first authors to classify the CT appearances of radiation-induced lung injury postconventional radiation. Classifying such changes as early (<6 months) or late (6–12 months) phase with regard to the time interval after the end of treatment better corresponds to the clinical and pathologic aspects of radiationinduced lung abnormalities.
Our case report deals with recurrence of cancer in the late phase of radiation injury. Radiologic imaging manifestations of radiation fibrosis associated
with the relatively newer methods of radiation therapy have been classified according to one of three patterns described as modified conventional, masslike, or scar-like.4 It is difficult to differentiate mass-like radiation fibrosis from residual or recurrent lung cancer; however, loss of volume of the consolidation on follow-up favours fibrosis.
Imaging features that raise the suspicion of local tumour recurrence include alteration in the contour and dimensions of the fibrotic area, with the appearance of a homogeneous opacity without air bronchograms and with convex borders in the irradiated lung.5 Integrated PET/CT appears to provide higher accuracy than that available with CT alone for distinguishing residual or recurrent tumour from lung changes after radiation treatment in patients with non-small cell lung cancer.6
Figure 1. (a). Index scan demonstrating 3.6 cm lobulated enhancing mass in the superior segment of the left lower lobe. The mass was biopsy proven adenocarcinoma. (b). Follow-up scan post-chemo and radiotherapy after 3 years from index scan: Post-treatment fibrotic changes in the left hilum. No enhancing nodule within. (c). 6 months follow-up contrast-enhanced CT shows enhancing nodule measuring 1.8 cm within the post-treatment fibrotic changes, concerning for recurrence. This nodule was initially missed. (d). Interval increase in size of the enhancing nodule to 2.6 cm, in the 6 month-follow-up scan.
In our case report, we have identified an important sign to help detect recurrent lung cancer early on CT chest called the ‘enhancingnodule-in post-treatment fibrosissign’. It highlights the importance for chest radiologists to scrutinise areas of fibrosis for areas of enhancement within the fibrotic segment with great care. Our patient had fibrotic changes for several months post-treatment that did not show the sign; so, constant vigilance in interrogating areas of fibrosis for this sign is warranted. In conclusion, the enhancing-nodule sign is a useful sign to look for within areas of fibrosis for recurrent lung cancers and is best evaluated on soft tissue windows. The proposed sign can contribute to earlier detection of recurrent lung cancer. Of note, lung cancer can also be relatively hypoenhancing relative to adjacent fibrosis. While there have been studies discussing relative enhancement and iodine content as potential markers of recurrent lung cancer, such as status post-SBRT, this is an area that requires more research. This case report is therefore of interest in this developing field and useful to share.
References available on request
HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023 RESPIRATORY: FIBROSIS 73
Written by Dr Rituparna Saha, David Ryan, Department of Radiology, St Vincent’s University Hospital, Dublin, Dr Emer Hanrahan, Department of Oncology, St Vincent’s University Hospital Dublin and Professor Jonathan D Dodd, Department of Radiology, St Vincent’s University Hospital, Dublin, School of Medicine, University College Dublin
Antimicrobials
The protected antimicrobial process in a University Teaching Hospital: a qualitative interview study exploring the knowledge, attitudes, and experiences of healthcare professionals
 Written by E Burton, M O’Driscoll and A. Fleming, Pharmaceutical Care Research Group, School of Pharmacy, University College Cork
Written by E Burton, M O’Driscoll and A. Fleming, Pharmaceutical Care Research Group, School of Pharmacy, University College Cork


Enterobacteriaceae reducing from 10.87/1000 PD (number of MDR isolates per 1000 patient days) to 2.98/1000PD (p < 0.001).7 An antimicrobial cost reduction from ¤15,681 ± 1790 per month (total ¤78,409 in period 1) to¤713 ± 256 per month (total ¤4.989 in period 2), following the introduction of antimicrobial protection/restriction, was achieved in another study.8
Antimicrobial restriction or protection is an important and potential high impact Antimicrobial Stewardship (AMS) intervention in the hospital setting.1 All Irish hospitals have been advised to introduce a policy of antimicrobial protection/ restriction.1 The antimicrobials which are restricted vary between hospitals, but typically include meropenem, linezolid, caspofungin and amphotericin B and several others. Formulary restriction/protection curtails the overuse of broad spectrum, costly and new antimicrobials.1, 2 It establishes the requirement for local approval from an Infectious Disease physician, microbiologist, or other member of the AMS Team to sanction those antimicrobials.2 The latter strategy may involve approval numbers or codes displayed in patient records, or charts, which illustrate approval being granted.2 Ideally, a treatment plan is also included in the patient’s notes, outlining parameters such as duration of therapy.2 Notably, these mechanisms are in addition to standard procedures, including the performance of culture tests to confirm causative pathogens and determine antimicrobial sensitivity profiles.2
A systematic review of hospital AMS interventions found antimicrobial protection to be the most effective method to reduce the consumption of antimicrobials.3 Rates of success for antimicrobial protection were found to be 66–87%.3 Compared to persuasive practices (clinical intervention and feedback), restrictive practices (antimicrobial

protection/restriction) had at least a three-fold greater effect in achieving AMS goals.3 The benefits of antimicrobial restriction/ protection include reduced antimicrobial consumption, reduced antimicrobial resistance, and reduced expenditure.4 One study, involving 22 hospitals found that in those hospitals that restricted carbapenems, usage was consistently lower (approximately 20 days of therapy (DOT)/1000 patient days (PD)), depending on the year,
than the hospitals that did not restrict carbapenems.5 A recent meta-analysis found significant reductions in antimicrobial resistance after the introduction of antimicrobial protection, but only with non-fermenters (e.g., Pseudomonas species).6 Another study found that the incidence of all non-pseudomonal multidrug resistant (MDR) Gram-negative bacilli decreased significantly after carbapenem restriction, with that of extended spectrum beta lactamases (ESBL)-producing
There is a large body of published research investigating the impact of AMS interventions, however there is a lack of qualitative research investigating the antimicrobial restriction/protection process in the hospital setting. Existing qualitative studies focus on AMS interventions generally, with one study only considering the views of Infectious Disease Consultants, not other healthcare professionals.9 The importance of qualitative enquiry to investigate current AMS behaviours and practices in the clinical setting has been widely recommended. Expansion of qualitative research has been recommended in order to enhance the understanding of factors influencing antimicrobial prescribing decisions and AMS
74 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Edel Burton
Michelle O’Driscoll
Aoife Fleming
Launching
Launching
Flucloxacillin Powder for solution for injection/infusion
Flucloxacillin Powder for solution for injection/infusion
500mg 1000mg 2000mg
PRESCRIBING INFORMATION: Consult the Summary of Product Characteristics for further information. Additional information is available upon request. Flucloxacillin 500mg, 1000mg and 2000mg powder for solution for injection/infusion. Active ingredients: As flucloxacillin sodium, each 10mL vial contains 500mg flucloxacillin (25.5mg sodium approximately), 20mL vial contains 1000mg flucloxacillin (51mg sodium approximately), 50mL vial contains 2000mg flucloxacillin (102mg sodium approximately). Indications: For the treatment of the following infections due to beta-lactamase producing staphylococci and other sensitive Gram-positive organisms such as streptococci – skin and soft tissue infections (like abscesses, cellulitis, infected burns, impetigo), upper respiratory tract infections (like pharyngitis, tonsillitis, sinusitis), lower respiratory tract infections (like pneumonia, bronchopneumonia, pulmonary abscess), bone and joint infections (like osteomyelitis and arthritis), endocarditis. Prophylaxis in cardiovascular surgery (valve prostheses, artery prostheses) and orthopedic surgery (arthroplasty, osteosynthesis and arthrotomy). Consideration should be given to official guidance on appropriate use of antibacterial agents. Posology and method of administration: Parenteral therapy is indicated if oral route impracticable or unsuitable as in the case of severe diarrhoea or vomiting and particularly for urgent treatment of severe infection. Routes of administration: 500mg vial - Intramuscular (im), intravenous (iv), intrapleural and intraarticular. 1000mg vial and 2000mg vial – intramuscular and intravenous routes only. Intravenous injection/ infusion should be administered slowly. Dosage depends on age, weight and renal function as well as the severity and nature of the infection. Adults and adolescents ≥ 12 years – Total daily dosage of 1g to 4g, administered in three to four divided doses, by iv or im injection. Severe infections: up to 8g per day administered in four infusions (over 20 to 30 min). No single bolus injection or infusion should exceed 2g. Maximum dose of 12g per day should not be exceeded. In surgical prophylaxis: 2g iv (bolus or infusion) upon induction of anesthesia, to be repeated every 6h for 24h in vascular and orthopedic surgery, and for 48h in cardiac or coronary surgery. Methicillin-susceptible Staphylococcus aureus. Endocarditis: 2g every 6h, increasing to 2g every 4h in patients weighing >85kg. Children under 12 years of age – In mild to moderate infections: 25 to 50mg/kg/24 hours administered in three to four equally divided doses by im or iv injection. Severe infections: Up to 100mg/kg/24 hours in three to four divided doses. No single bolus injection or infusion should exceed 33mg/kg. Methicillin-susceptible Staphylococcus aureus. Endocarditis: 200mg/kg/24 hours in three to four divided doses. Premature infants, neonates, sucklings and infants – Risk of kernicterus; only use in premature infants and neonates after rigorous risk-benefit assessment. Treatment is generally with 25mg to 50mg/kg/24 hours divided into three or four equal doses. Maximum daily dose 100mg/kg/24 hours. Abnormal renal function – Renal excretion slowed in patients with renal insufficiency. In severe renal insufficiency (creatinine clearance <10ml/min) consider dose reduction or extension of dose interval. Maximum recommended dose in adults is 1g every 8 to 12 hours. In anuric patients maximum dosage is 1g every 12 h. Flucloxacillin not significantly removed by dialysis hence no supplementary dosages need be administered either during or at end of dialysis period. Hepatic impairment – Dose reduction not necessary. Intrapleural and intraarticular – Usual dose is 250mg to 500mg once daily. Contraindications: Hypersensitivity to the active substance or excipients, should not be given to patients with history of hypersensitivity to beta-lactam antibiotics, patients with previous history of flucloxacillin-associated jaundice/hepatic dysfunction. Not suitable for ocular or subconjunctival administration or intrathecal injection. Special warnings and precautions for use: Before initiating therapy with flucloxacillin check patient history concerning previous hypersensitivity reactions to beta-lactams. Cross sensitivity between penicillins and cephalosporins is well documented. Serious and
PRESCRIBING INFORMATION: Consult the Summary of Product Characteristics for further information. Additional information is available upon request. Flucloxacillin 500mg, 1000mg and 2000mg powder for solution for injection/infusion. Active ingredients: As flucloxacillin sodium, each 10mL vial contains 500mg flucloxacillin (25.5mg sodium approximately), 20mL vial contains 1000mg flucloxacillin (51mg sodium approximately), 50mL vial contains 2000mg flucloxacillin (102mg sodium approximately). Indications: For the treatment of the following infections due to beta-lactamase producing staphylococci and other sensitive Gram-positive organisms such as streptococci – skin and soft tissue infections (like abscesses, cellulitis, infected burns, impetigo), upper respiratory tract infections (like pharyngitis, tonsillitis, sinusitis), lower respiratory tract infections (like pneumonia, bronchopneumonia, pulmonary abscess), bone and joint infections (like osteomyelitis and arthritis), endocarditis. Prophylaxis in cardiovascular surgery (valve prostheses, artery prostheses) and orthopedic surgery (arthroplasty, osteosynthesis and arthrotomy). Consideration should be given to official guidance on appropriate use of antibacterial agents. Posology and method of administration: Parenteral therapy is indicated if oral route impracticable or unsuitable as in the case of severe diarrhoea or vomiting and particularly for urgent treatment of severe infection. Routes of administration: 500mg vial - Intramuscular (im), intravenous (iv), intrapleural and intraarticular. 1000mg vial and 2000mg vial – intramuscular and intravenous routes only. Intravenous injection/ infusion should be administered slowly. Dosage depends on age, weight and renal function as well as the severity and nature of the infection. Adults and adolescents ≥ 12 years – Total daily dosage of 1g to 4g, administered in three to four divided doses, by iv or im injection. Severe infections: up to 8g per day administered in four infusions (over 20 to 30 min). No single bolus injection or infusion should exceed 2g. Maximum dose of 12g per day should not be exceeded. In surgical prophylaxis: 2g iv (bolus or infusion) upon induction of anesthesia, to be repeated every 6h for 24h in vascular and orthopedic surgery, and for 48h in cardiac or coronary surgery. Methicillin-susceptible Staphylococcus aureus. Endocarditis 2g every 6h, increasing to 2g every 4h in patients weighing >85kg. Children under 12 years of age – In mild to moderate infections: 25 to 50mg/kg/24 hours administered in three to four equally divided doses by im or iv injection. Severe infections: Up to 100mg/kg/24 hours in three to four divided doses. No single bolus injection or infusion should exceed 33mg/kg. Methicillin-susceptible Staphylococcus aureus. Endocarditis: 200mg/kg/24 hours in three to four divided doses. Premature infants, neonates, sucklings and infants – Risk of kernicterus; only use in premature infants and neonates after rigorous risk-benefit assessment. Treatment is generally with 25mg to 50mg/kg/24 hours divided into three or four equal doses. Maximum daily dose 100mg/kg/24 hours. Abnormal renal function – Renal excretion slowed in patients with renal insufficiency. In severe renal insufficiency (creatinine clearance <10ml/min) consider dose reduction or extension of dose interval. Maximum recommended dose in adults is 1g every 8 to 12 hours. In anuric patients maximum dosage is 1g every 12 h. Flucloxacillin not significantly removed by dialysis hence no supplementary dosages need be administered either during or at end of dialysis period. Hepatic impairment – Dose reduction not necessary. Intrapleural and intraarticular – Usual dose is 250mg to 500mg once daily. Contraindications: Hypersensitivity to the active substance or excipients, should not be given to patients with history of hypersensitivity to beta-lactam antibiotics, patients with previous history of flucloxacillin-associated jaundice/hepatic dysfunction. Not suitable for ocular or subconjunctival administration or intrathecal injection. Special warnings and precautions for use: Before initiating therapy with flucloxacillin check patient history concerning previous hypersensitivity reactions to beta-lactams. Cross sensitivity between penicillins and cephalosporins is well documented. Serious and





Fresenius Kabi Limited
Fresenius Kabi Ireland Unit 3B Fingal Bay, Balbriggan, Co. Dublin, Ireland
Fresenius Kabi Limited Fresenius Kabi Ireland Unit 3B Fingal Bay, Balbriggan,
occasionally fatal hypersensitivity reactions have been reported in patients receiving beta-lactam antibiotics, occurrence more frequent with parenteral compared to oral therapy. Reactions more likely to occur in patients with history of beta-lactam hypersensitivity. If allergic reaction occurs discontinue flucloxacillin and institute appropriate therapy. Feverish generalized erythema associated with pustula occurring at treatment initiation may be a symptom of acute generalised exanthematous pustulosis (AGEP); in case of AGEP diagnosis discontinue flucloxacillin and subsequent administration is contraindicated. Use with caution in patients with evidence of hepatic dysfunction, patients ≥50 years of age and those with serious underlying disease; hepatic events may be severe and in extremely rare circumstances deaths have been reported. Flucloxacillin solutions reconstituted with local anesthetics (lidocaine) should not be given by iv administration. Adjust dose in renal impairment. Special caution essential in newborns due to risk of hyperbilirubinaemia and potential for high serum levels of flucloxacillin due to reduced rate of renal excretion. Regular monitoring of blood count, hepatic and renal function recommended during prolonged treatment. Pseudomembranous colitis can occur; if this develops discontinue flucloxacillin and initiate appropriate therapy. Prolonged use may occasionally result in overgrowth of nonsusceptible organisms. Caution when administered concomitantly with paracetamol due to increased risk of high anion gap metabolic acidosis (HAGMA). High risk of HAGMA particularly in patients with severe renal impairment, sepsis or malnutrition especially if maximum daily doses of paracetamol used. Following co-administration close monitoring recommended to detect the appearance of HAGMA (including testing for urinary 5-oxoproline). If flucloxacillin is continued after paracetamol cessation, it is advisable to ensure no HAGMA signals. Particular caution with drug-induced liver injury in patients with HLA-B*5701 haplotype; frequency of these disorders increasing in HIV-infected patients who may be at increased risk of exposure to flucloxacillin. Hypokalaemia (potentially life threatening) can occur especially at high doses and may be resistant to potassium supplementation. Regularly measure potassium levels during high dose flucloxacillin therapy. Attention warranted when combining flucloxacillin with hypokalaemia inducing diuretics or when other risk factors for development of hypokalaemia present. Contains sodium, consider in patients on a sodium controlled diet. Undesirable effects: Common (>1/100 to <1/10): Minor gastrointestinal disturbances. Uncommon (>1/1000 to <1/100): Rash, urticaria, purpura. Very rare (<1/10,000): Neutropenia (including agranulocytosis), thrombocytopenia, eosinophilia, haemolytic anaemia, anaphylactic shock, angioneurotic oedema, high anion gap metabolic acidosis, pseudomembranous colitis, neurological disorders with convulsions possible with high dose iv injection in patients with renal failure, hepatitis, cholestatic jaundice, changes in liver function laboratory test results, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, arthralgia, myalgia, interstitial nephritis, fever sometimes develops more than 48 hours after start of treatment. Frequency not known: Acute generalized exanthematous pustulosis, phlebitis. Legal Category: POM Marketing Authorisation Numbers: PA2059/069/001, PA2059/069/002, PA2059/069/003. Marketing Authorisation Holder: Fresenius Kabi Deutschland GmbH, Else-Kroener Strasse 1, Bad Homburg v.d.H 61352, Germany Further Information: See the SmPC for further details. Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra.ie. Adverse events should also be reported to Fresenius Kabi Limited via email Pharmacovigilance.GB@Fresenius-Kabi.com. Date of Preparation: August 2021 IE-IVF-2100004
occasionally fatal hypersensitivity reactions have been reported in patients receiving beta-lactam antibiotics, occurrence more frequent with parenteral compared to oral therapy. Reactions more likely to occur in patients with history of beta-lactam hypersensitivity. If allergic reaction occurs discontinue flucloxacillin and institute appropriate therapy. Feverish generalized erythema associated with pustula occurring at treatment initiation may be a symptom of acute generalised exanthematous pustulosis (AGEP); in case of AGEP diagnosis discontinue flucloxacillin and subsequent administration is contraindicated. Use with caution in patients with evidence of hepatic dysfunction, patients ≥50 years of age and those with serious underlying disease; hepatic events may be severe and in extremely rare circumstances deaths have been reported. Flucloxacillin solutions reconstituted with local anesthetics (lidocaine) should not be given by iv administration. Adjust dose in renal impairment. Special caution essential in newborns due to risk of hyperbilirubinaemia and potential for high serum levels of flucloxacillin due to reduced rate of renal excretion. Regular monitoring of blood count, hepatic and renal function recommended during prolonged treatment. Pseudomembranous colitis can occur; if this develops discontinue flucloxacillin and initiate appropriate therapy. Prolonged use may occasionally result in overgrowth of nonsusceptible organisms. Caution when administered concomitantly with paracetamol due to increased risk of high anion gap metabolic acidosis (HAGMA). High risk of HAGMA particularly in patients with severe renal impairment, sepsis or malnutrition especially if maximum daily doses of paracetamol used. Following co-administration close monitoring recommended to detect the appearance of HAGMA (including testing for urinary 5-oxoproline). If flucloxacillin is continued after paracetamol cessation, it is advisable to ensure no HAGMA signals. Particular caution with drug-induced liver injury in patients with HLA-B*5701 haplotype; frequency of these disorders increasing in HIV-infected patients who may be at increased risk of exposure to flucloxacillin. Hypokalaemia (potentially life threatening) can occur especially at high doses and may be resistant to potassium supplementation. Regularly measure potassium levels during high dose flucloxacillin therapy. Attention warranted when combining flucloxacillin with hypokalaemia inducing diuretics or when other risk factors for development of hypokalaemia present. Contains sodium, consider in patients on a sodium controlled diet. Undesirable effects: Common (>1/100 to <1/10): Minor gastrointestinal disturbances. Uncommon (>1/1000 to <1/100): Rash, urticaria, purpura. Very rare (<1/10,000): Neutropenia (including agranulocytosis), thrombocytopenia, eosinophilia, haemolytic anaemia, anaphylactic shock, angioneurotic oedema, high anion gap metabolic acidosis, pseudomembranous colitis, neurological disorders with convulsions possible with high dose iv injection in patients with renal failure, hepatitis, cholestatic jaundice, changes in liver function laboratory test results, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, arthralgia, myalgia, interstitial nephritis, fever sometimes develops more than 48 hours after start of treatment. Frequency not known: Acute generalized exanthematous pustulosis, phlebitis. Legal Category: POM Marketing Authorisation Numbers: PA2059/069/001, PA2059/069/002, PA2059/069/003. Marketing Authorisation Holder: Fresenius Kabi Deutschland GmbH, Else-Kroener Strasse 1, Bad Homburg v.d.H 61352, Germany Further Information: See the SmPC for further details. Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra.ie. Adverse events should also be reported to Fresenius Kabi Limited via email Pharmacovigilance.GB@Fresenius-Kabi.com. Date of Preparation: August 2021 IE-IVF-2100004
Website: www.fresenius-kabi.com/ie/ Email: FK-enquiries.ireland@fresenius-kabi.com Phone: +353 (0)1 841 3030
Website: www.fresenius-kabi.com/ie/ Email: FK-enquiries.ireland@fresenius-kabi.com Phone: +353 (0)1 841 3030

Date of Prep: August 2021 Ref: IE-IVF-2100007

Date of Prep: August 2021 Ref: IE-IVF-2100007
1000mg 2000mg
500mg
Antimicrobials
Area Demographic information
Knowledge of the process of antimicrobial protection
Education and Training
Interaction with the Antimicrobial Stewardship Team
Adherence to the protected antimicrobial process
Table 1
practices.10,11,12 In order to optimise the implementation of antimicrobial protection moving forward, it is necessary to explore the views and experiences of hospital healthcare professionals.
Study setting
The study hospital is a 350-bed voluntary general acute hospital, serving both public and private patients providing in-patient medical and surgical, day patient, outpatient, and emergency services. In the hospital, the process of antimicrobial protection is led by the AMS Team comprised of a Consultant Microbiologist, Infectious Disease Consultant and Antimicrobial Pharmacist. A hard copy of the restricted antimicrobial application form must be filled out by the prescribing doctor and the order approved by a member of the AMS Team, in order to use restricted antimicrobials.
Each ward has a Clinical Nurse Manager who is in charge of the ward, and a team of staff nurses. A Consultant is in charge of a team of junior doctors within their speciality (Registrar, Senior House Officer, Intern). Each ward is also assigned a Clinical Pharmacist.
Study design
Semi-structured interviews with key stakeholders in the antimicrobial protection process were conducted from 13 September to 10 October 2019 by the primary researcher (EB). This approach facilitated in-depth exploration of participants’ views and experiences.13, 14
Topic guide and interviewing
An interview topic guide (Table 1 above) was developed based on the study objectives, existing literature, and experience of the research team. Two pilot interviews, with a nurse and a
pharmacist were conducted; these were included in the final analysis. The topic guide was then revised to include more probing questions and further exploration of participants’ responses.
Sampling
Purposive and convenience sampling were used to recruit participants in the hospital. The sampling strategy recruited doctors, pharmacists and nurses involved in implementing the restricted antimicrobial process in the hospital. This included members of the AMS Team. No other inclusion or exclusion criteria were applied. Participants were invited to participate in the study by face-to-face invitation on the wards. Written informed consent was obtained.
Twelve interviews were conducted (including the two pilot interviews) involving all required HCPs for representation. A further three interviews were conducted to ensure that data saturation had been reached and that no new themes emerged, as per the Francis method.15
To support this, iterative data analysis begun in parallel with data collection. Interviews were audio-recorded and transcribed verbatim by the primary researcher. No repeat interviews were conducted. Field notes were handwritten after interviews.
Analysis
Framework analysis was used to analyse the transcripts.16
All transcripts were coded independently by one author (EB). QSR International’s NVivo 12 Qualitative Data Analysis Software was used to organise the coding. Initial, non-hierarchical codes were generated. These were reviewed in consultation with a second reviewer (MOD) with discussion
Issues discussed
Profession, grade, gender
Experience with the process
Awareness of the antimicrobials which are protected
Formal/informal training on the antimicrobial process
Formal/informal training on AMS
Antimicrobial guidelines
Approval/denial of protected antimicrobial requests
Efficiency of the process
Confidence executing the process
Professional practices with protected antimicrobials (prescribing, dispensing, administering)
of the final codes by all authors. Codes were then attributed to domains of the Theoretical Domains Framework (TDF).16
Results
Fifteen interviews were conducted. Interview participants included: 8 Doctors, 4 Pharmacists and 3 Nurses. Participant detail is displayed in Table 3. The interviews ranged from 8 to 15 min. Key themes are presented by identifying the relevant TDF domains.
Theoretical domains framework
The analysis identified key domains of the TDF16 that were found to be relevant, and they are described below. The other domains that were not identified (optimism, reinforcement, intentions, goals, and emotions) are not discussed as not enough references to the relevant constructs were made.
Knowledge
Participants’ knowledge of the antimicrobial protection process was inconsistent. Most participants had a general understanding of the steps involved in the process and the reasons behind it. All participating pharmacists and nurses, and most of the medical team participants could identify examples of restricted antimicrobials, with meropenem, linezolid and caspofungin being mentioned most frequently. In all professions, apart from pharmacy, participants relayed that level of experience and knowledge influenced the individual’s interaction with the process. Senior nurses were involved with the process more frequently than staff nurses, and junior doctors filled out the forms more than Consultants. Some reported that a selection of their medical colleagues did not realize
a form was required to order a restricted antimicrobial.
Most participants did not report engaging in any formal or informal AMS training, apart from their university education. Nursing staff stated that they receive “on-thejob learning” (Nurse 2) alone, in regard to this process.
Social influences
The correct communication channels and procedures were identified by interviewees as being important components of the process. Doctors and nurses discussed contacting pharmacy or the AMS Team for advice. Pharmacists contacted microbiology before proceeding if any queries arose. Informal wardbased discussions between these three disciplines were also seen to positively influence adherence to the process. Participants all expressed how positive teamwork encouraged them to engage with the process. Recognition of the reliance on fellow professionals, especially those in more senior or specialised roles, in terms of tasks, knowledge or procedure, offered participants opportunities to clarify and question items surrounding antimicrobial prescribing.
It’s kind of a two-way street you know, there has to be a lot of working together. (Doctor 5)
Nursing and pharmacy appeared to drive the restricted antimicrobial process, and adherence to it, at ward and dispensary level. Nurses discussed presenting the forms to fill out when the medical team wished to prescribe a restricted antimicrobial. At dispensary level, pharmacy was seen by all disciplines as the “gatekeepers” of the restricted antimicrobials. At ward level, pharmacists encouraged the medical team to complete the form, and follow the
76 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
correct procedure, or contacted the nurses to ask them to followup on the forms.
Pharmacy ensure that the forms are filled in completely and the supply is withheld until those forms are completed… (Pharmacist 1)
Those on the AMS Team spoke of the conflicts that can arise when medical opinions differ. Participants mentioned that at times the clinical opinion of a member of the AMS Team could contradict that of the Consultant ordering the restricted antimicrobial.

…people want to prescribe something and and I’ve been in opposition to it and that’s a major challenge at times (Doctor 3)
Other doctors spoke of factors which may lead to these discussions. The concept of the autonomy of the prescribing Consultant was discussed by junior doctors and members of the AMS Team. Acknowledgement that medical care and opinions can differ was evident.
At the end of the day like a consultant could do it whether the consultant microbiologist wants it or not you know what I mean?” (Doctor 4)
However, the majority of junior doctors acknowledged that the decision of the AMS Team is always taken on board and usually accepted. Restricted antimicrobials were used more frequently by medical teams, rather than surgical teams. However, members of the AMS Team mentioned that they would encourage the surgical teams to be more aware of AMS in the context of surgical prophylaxis. Surgical junior doctors mentioned that they would not have filled out the restricted form if they were not prompted to do so by a phone call from the Consultant Microbiologist.
I think one area where we really, it would be nice if the message was accepted more it would be em antimicrobial prophylaxis in the surgical groups as I think that grips on for too long. (Doctor 7)
Memory attention and decision processes
All participants were aware that the process for applying for a restricted antimicrobial existed. However, participants relayed the stages of the process with varying degrees of familiarity. Members of the medical team in particular were unfamiliar with the process beyond the point of filling in the form. They were unsure of who collected the
form, and its purpose when sent to pharmacy. Members of the nursing and pharmacy teams could relay all stages of the process.
Don’t know what happens once brought to pharmacy. But I think it’s to do with like them being able to order it in and stock and things like that. (Doctor 5)
The majority of participants believed that the process of antimicrobial restriction was superior in the study site than other hospitals. This was due to the interactive nature and strong ward presence of the AMS Team. However, others stated that they knew of other hospitals who were more stringent about withholding restricted antimicrobials. In most cases, pharmacy did not dispense the restricted antimicrobial unless the form was filled out. However, participants revealed that pharmacy was hesitant to leave a patient without a restricted antimicrobial, “as the very nature of an antibiotic is you have to use it quickly” (Pharmacist 2). Thus, at times a mutual understanding was reached. A form might be delivered the morning after receipt of the antimicrobial, due to clinical need, and availability of the AMS Team. So, we can’t say we’re not giving it to you, we give them one vial of
the antibiotic and we say to the nurses will ye go back and get the form for us before we can issue more. (Pharmacist 2)
Beliefs about consequences
Overall, participants agreed that the restricted antimicrobial process led to positive patient outcomes. They reported that it improves appropriateness of treatment, reduces exposure to antimicrobial side effects, and reduces length of hospital stay, as well as preventing resistance developing to these antimicrobials. They acknowledged that AMS policies need to be adhered to in order to protect these antimicrobials.
You’re giving them the right antibiotic em for their particular indication so it would definitely contribute positively to patient care. (Pharmacist 4)
Most participants spoke of the forms and AMS programmes in the hospital as having a positive impact on prescribing practices. The medical team were aware that restricted antimicrobials are usually last line, and that escalating from other agents should only be done under certain circumstances. The form is the final step resulting from that decision, with review by microbiology.
77 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
Antimicrobials
You know there’s certain flags on the form that em, would would spur the doctor on to contact micro and discuss it and maybe not prescribe the restricted antibiotic so quickly in the first place. (Pharmacist 2)
A minority of participants, mostly those in more senior roles, referred to the process resulting in cost savings as restricted antimicrobials are typically more expensive. It was claimed that the process aids in ‘addressing cost related issues” (PH4). The restrictive nature of the form acted like a ‘gatekeeper’ (Pharmacist 4). The interviewees made it evident that the benefit to the patient had to be weighed up against the economic effects.
I suppose we particularly want to monitor the use of those, some of them from a cost point of view. Say particularly like the antifungals; like Ambisome is very expensive…(Doctor 7)
Conversely, a minority of professionals considered the possibility of negative patient outcomes. Some participants believed that the process prevents or slows down access to potentially critical antimicrobials. Other participants discussed the consequences for patients if their restricted antimicrobial regime was not reviewed by microbiology. They were concerned that patients may be prescribed the incorrect antibiotic or regime without specialist review.
…there had been some medications that the nurses couldn’t administer because the pharmacy hadn’t yet received the antimicrobial form...(Doctor 1)
Environmental contexts and resources
Although some participants believed the form itself to be a barrier to adherence to the process, difficulty locating the hard-copy forms was the primary
logistical challenge reported by members of the medical teams.
Some stated that there was an absence of forms on certain wards, with differing filing systems or form versions between wards.
Huge difficulty finding the forms; they’re different places on the wards. (Doctor 2).
There were actually two different forms. I wasn’t sure which one was right to use. (Doctor 5).
Restricted antimicrobials are stored in the pharmacy. However, participants from pharmacy and nursing mentioned that restricted antimicrobials stored on the wards, for a particular patient, at times were used for other patients, in breach of the process.
Patient A and B on the ward, and A was prescribed meropenem and it was appropriate, and we got the form, and everything was perfect, and we filed it away, but patient B then was prescribed it but because there was there was a supply of meropenem out on the ward already... The ward didn’t need to come to pharmacy for it so they were using that supply so there could be a day a day and a half of patient B getting meropenem and it could be totally inappropriate. (Pharmacist 2)
The alteration of the process out of hours was discussed by the majority of participants and was perceived as a challenge due to reduced access to the AMS Team. Nursing staff reported that the procurement of the antimicrobials out of hours is their responsibility, regardless of whether the form is filled in and approved by the AMS Team or not.
We’d normally either try to get it from another ward or the Assistant Director of Nursing would just have to open pharmacy. It’s something prescribed. We kind of have to do as much as we can to get it. Even if we don’t have the form. (Nurse 2)
Acquiring the form at a later date is seen to be the responsibility of pharmacy. Junior members of the medical team who needed to escalate a patient to a restricted antimicrobial out of hours, document the details in the chart and follow up with the AMS Team the following day. Members of the AMS Team stated that any restricted antimicrobial that is commenced out of hours will usually be followed up on within twelve hours. Some questioned the utility of twenty-four-hour cover for the AMS Team.
A call to me at three in the morning to authorize meropenem…? I mean really what I think we have to maybe work more towards is the education of our Non-Consultant Hospital Doctors. (Doctor 7)
Social/professional role and identity
All participants were aware of their individual role in the process. Within professions, roles also differed depending on the grade of the healthcare professional, and their level of experience. All nurses interviewed stated that they assist in guiding the doctor through completing the form if they are unfamiliar with the process.
You know because I’d often be the one that would be liaising with pharmacy, so I am very I suppose I know what ones need the form now. (Nurse 2)
The nursing staff order the antimicrobials from pharmacy, administer them and review the duration of courses. Pharmacy is primarily involved in the process as the “gate keepers “(Pharmacist 4) at dispensary level, but also at ward level. Pharmacists check the form completeness and use their professional judgement to dispense the antimicrobial or not. The junior doctors liaise with the AMS Team on behalf of their team.
I feel my role is a link between
Appointment for Professor Naidoo

you know passing on the patient information and consulting with the consultant. (Doctor 5)
Statement of key findings
This qualitative study has found that the process of antimicrobial restriction in one Irish university teaching hospital is a multidisciplinary effort between pharmacy, medicine, and nursing. The findings contribute valuable insights to the adherence and implementation of a key AMS strategy, which could be optimised to improve the protection of important antimicrobials. A key finding of this study was that knowledge of the process differed by the level of experience of the healthcare professional, and their healthcare discipline. Clinical Nurse Managers interacted with the process on a regular basis, signposting the medical teams towards the form and liaising with pharmacy about the procurement of the restricted antimicrobials.
It was reported that staff nurses rarely engaged with the process. Within the medical teams, junior doctors were more familiar with filling in the forms than their consultant colleagues. However, this did differ depending on the speciality of the junior doctor.
The study found that the daily implementation of the process varies, depending on whether or not there are forms on the ward, whether the request is made during working hours or out of hours, and in some cases, patients were reported as being administered these antimicrobials without full authorisation.
Participants recommended that standardization of the process across all wards, an electronic version of the form, and formal education surrounding the process of antimicrobial protection and AMS were needed.
References available on request
Professor Naidoo will lead RCSI’s innovative medical cancer research team to further its growth and development, and continue its work to secure funding for vital international cancer research.
In her career to date, Professor Naidoo has developed and led a portfolio of clinical trials and translational studies in lung cancer, immunotherapy and immune-related toxicity. She is an established leader in these fields with over one hundred articles published in publications such as New England Journal of Medicine, Journal of Clinical Oncology and JAMA Oncology
Key to Professor Naidoo’s role will be to grow the clinical and translational research focus of lung cancer and immunotherapy at RCSI. She will do this by building clinical and research teams to advance cancer clinical trials, clinical research, and governance.
78 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
RCSI University of Medicine and Health Sciences has announced the appointment of Professor Jarushka Naidoo as Academic Professor of Medical Oncology in the RCSI Department of Medicine.
Professor Jarushka Naidoo
News
All-Ireland Guidelines Launched for Lymphoedema
The HSE, together with the Lymphoedema Network Northern Ireland and the Public Health Agency in Northern Ireland, have launched the first ‘All-Ireland Lymphoedema Guidelines for the Diagnosis, Assessment and Management of Lymphoedema’. More than 45,000 people in Ireland and Northern Ireland live with lymphoedema, an incurable and chronic illness that causes swelling in the body’s tissue. This evidencebased clinical practice guideline has been launched to support clinicians and ultimately improve the quality of life of people living with lymphoedema across Ireland. These guidelines support the implementation of good lymphoedema care in all settings and improve the experience and safety of people in our care. The guideline will provide
healthcare professionals with clear, evidence-based guidance on the diagnosis, assessment and management of patients with all types of lymphoedema.
Developed in collaboration with clinicians, service user representatives, and key stakeholders, including Higher Educations Institutions, across the Republic of Ireland and Northern Ireland, they are a guide for managers and commissioners to support the development and modernisation of pathways of lymphoedema care, education and research.
The ‘All Ireland Lymphoedema Guidelines for the Diagnosis, Assessment and Management of Lymphoedema’ provide:
• The most up-to-date, evidence based recommendations to
support best clinical practice, standardisation of care and improved patient outcomes.
• An in-depth evidence review and recognition of changes in pathways, healthcare practices and advances in technology that support the Sláintecare strategy.
• A progressive way of integrating specialist services and non-specialist services and promoting supported self management in the community.
• A focus on prevention and early detection in areas that are high risk for lymphoedema
• Education requirements for lymphoedema services and service users.
• An implementation and evaluation plan
Speaking about the guidelines, HSE Chief Clinical Officer, Dr Colm Henry said, “The availability of these guidelines will support the implementation of evidence based, standardised lymphoedema care in all health care settings and improve the experience and safety of people in our care. It will provide guidance to healthcare workers on prevention, early detection, effective treatments and supported self-care to ensure that every patient receives optimal care. The recommended treatment pathways are patient-centred and support the Sláintecare strategy of the right care, in the right place, at the right time.”
Visit hse.ie/lymphoedema to read the ‘All-Ireland Lymphoedema Guidelines for the Diagnosis, Assessment and Management of Lymphoedema’
First for Endoscopic Radial Artery Harvesting (ERAH)
University Hospital Galway (UHG) is the first hospital in the country to introduce a new minimally invasive surgery for coronary artery bypass grafting.

The endoscopic radial harvest programme reduces significantly the risk of complications and infection for the patient.
The procedure was carried out for the first time in Ireland by Dr Sadiq Siddiqui as part of the minimal access surgery programme in the Department of Cardiothoracic Surgery, University Hospital Galway. Dr Siddiqui is an integral member of the surgical team led by Consultant Cardiothoracic Surgeons; Mr Fabio Bartolozzi, Mr Ronan Kelly and, Mr Alan Soo.
Traditionally in coronary artery bypass grafting (CABG) operations, a number of arteries and veins are removed from their natural position and used as conduits to bring blood to the narrow or blocked coronary arteries on the surface of a patient’s heart.
The standard surgery leaves large scars on the patient’s legs and forearms that are often slow to heal and can be disfiguring. This new endoscopic approach leaves two tiny scars on the forearm in
The surgical team for the first endoscopic radial harvest surgery for coronary artery bypass grafting in Ireland at University Hospital Galway. From left: Mr Alan Soo, Consultant Cardiothoracic Surgeon; Dr Sadiq Siddiqui, Cardiothoracic Surgery Registrar and Mr Ronan Kelly, Consultant Cardiothoracic Surgeon. Missing from the photo Mr Fabio Bartolozzi, Consultant Cardiothoracic Surgeon
place of a foot long scar along the whole arm. These heal quicker and with less pain.
“The radial artery from the forearm has been shown to be a longer lasting and superior vessel to use in Coronary Artery Surgery operations particularly in younger patients,” said Dr Siddiqui.
After performing over 400 endoscopic saphenous vein harvests (EVH) in Galway as part of coronary artery bypass operations, Dr Siddiqui travelled to the Royal Brompton National Heart Hospital in London to further his training in endoscopic surgery. There he trained in this new technique to also harvest the radial artery from the left forearm.
“We are very grateful to the Royal Brompton Hospital for
the assistance and expertise,” he added.
Prof Pat Nash, Chief Clinical Director with the Saolta University Hospital Group added: “The Endoscopic Radial Artery (ERAH) programme will further advance the burgeoning minimally invasive cardiac surgery programme in Galway and improve the care of patient’s undergoing coronary artery surgery in Galway by reducing their risk of complications and infections.”
Chris Kane, hospital manager at GUH congratulated the team describing the innovating procedure as an important step in enhancing patient care.
“This healthcare innovation will greatly improve our patient’s care. Introducing new techniques and procedures is an important part of our role as a teaching hospital and I am delighted to see such progressive surgeries take place here,” she added.
79 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
News
¤3.8M FUNDING TO DEVELOP WEARABLE PATCH FOR TYPE 2 DIABETES
A project to develop a patch worn on the cheek that delivers peptide* treatments for Type 2 Diabetes has been awarded just under ¤3.8 million by the EU’s Horizon Europe RESILIENCE programme.
The ‘BUCCAL-PEP’ project will combine skills to develop a multifunctional biomaterial patch which allows, for the first time ever, delivery of peptide therapies across the cheek (buccal). Existing patches have already been designed for small molecules but these cannot effectively deliver peptide treatments by this route of administration. Type 2 Diabetes patients need the peptide insulin to treat the disease.
Type 2 Diabetes is the chronic disease of focus for the research because patients tend to prefer non-injected drug administration routes over injections, which improves patient compliance. BUCCAL-PEP has the potential to provide an alternative administration route for peptides other than by injections and oral routes, which could benefit the treatment of other conditions such as pain relief and certain cancers.
Prof David Brayden, Professor of Advanced Drug Delivery at University College Dublin, Fellow of UCD Conway Institute for Biomolecular and Biomedical Research, and Funded Investigator at CÚRAM SFI Research Centre for Medical Devices is the coordinator on the grant. He will lead a consortium of seven partners across Ireland, Denmark, Germany, France, the Netherlands, and the UK, including a large pharmaceutical company, Novo Nordisk (Denmark), several SMEs, and academic partners (listed below).
Professor Brayden said, “I am delighted to be the coordinator on this exciting new grant which combines academic and industry partners across the EU. Patients need alternative routes for large molecule delivery over injections as this has an impact over their willingness to adhere to therapy. Buccal administration has particular challenges and our project will attempt to address these using new patch designs.”
UCD Vice-President for Research, Innovation and Impact, Professor Orla Feely said, “We congratulate Prof David Brayden on bringing together this significant consortium project and securing equally significant EU funding. It is a key goal of
Horizon Europe programmes to connect academics, industry and varied stakeholders to produce research, new technologies and outcomes that empower people in ways that truly matter. In this case, the project will support the translation of research into clinical applications that have significant potential to really address the needs of patients living with chronic conditions.”
The patch design uses permeation enhancer, (a substance that boosts penetration), along with multiple biomaterials and a peptide cargo, enabling diffusion of the peptide across the mucosal surface of the cheek for effective delivery of the treatment.
Oral delivery of macromolecules including peptides – such as insulin – is one of the great challenges in pharmaceutical research: only five peptideanalogues are administered by tablets or capsules. Low bioavailability, dosage control, and restrictions in use (e.g. undesirable food interactions) remain key challenges. Buccal delivery has the added benefit of avoiding food effects on the absorption of peptides, a common inconvenient problem found with oral peptide administration.
BOOTS CUSTOMERS AND COLLEAGUES RAISE OVER ¤100,000 FOR THE IRISH CANCER SOCIETY NIGHT
NURSES THROUGH THE BOOTS NIGHT WALK
Boots customers and colleagues have raised over ¤100,000 for the
Irish Cancer Society Night Nurse service through the 2022 Boots Night Walk. The annual sponsored 5km walk saw participants and Boots colleagues raise ¤103,516 through fundraising and donations. The Irish Cancer Society Night Nurses provide in-home care through the night, for cancer patients requiring end-of-life care, whilst also providing rest and respite for the patient’s families. The service is provided free of charge, and is an invaluable support to families and their loved ones during what can be a particularly difficult time.
Boots has been a proud supporter of the Irish Cancer Society since 2012, and through the generosity of customers, patients and colleagues has raised over ¤2.6 million, equating to over 7,500 nights of care for the service.

Across Boots stores, 150 Boots Irish Cancer Society Information Pharmacists and over 50 Boots Cancer Beauty Advisors are specially trained and on hand to support cancer patients and their loved ones.
Stephen Watkins, Managing Director of Boots Ireland, says,”We are extremely proud to have raised ¤103,516 this year for the Irish Cancer Society Night Nurse service. Since 2012, our customers, patients and colleagues have supported this special service, providing care and comfort to cancer patients and their loved ones. This ongoing partnership helps further our commitment to care for our customers and patients in the
communities we serve at all stages of their life.”
Averil Power, CEO, Irish Cancer Society, says, “On behalf of everyone at the Irish Cancer Society, I would like to thank Boots Ireland colleagues and customers for their continued support and enthusiasm for the Boots Night Walk, an absolutely wonderful fundraising initiative for the Irish Cancer Society Night Nursing service.
The service allows end-of-life care for cancer patients in the comfort of their own homes, surrounded by their loved ones. With each year, demand for this valuable service grows. Approximately 97% of the Society’s funding comes from donations, so we simply could not provide this crucial service without your generosity. Thank you for enabling us to be there for cancer patients and their families across Ireland, when they need us most.”
FIRST ALL-IRELAND GUIDELINES LAUNCHED FOR PEOPLE LIVING WITH LYMPHOEDEMA
The HSE, together with the Lymphoedema Network Northern Ireland and the Public Health Agency in Northern Ireland, have launched the first ‘All-Ireland Lymphoedema Guidelines for the Diagnosis, Assessment and Management of Lymphoedema’.
Boots Night Walk for Night Nurses 2022, Martha Ryan, Stephen Watkins, Fionnuala O’Leary and Mary Twohill
80 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
Clinical R&D
More than 45,000 people in Ireland and Northern Ireland live with lymphoedema, an incurable and chronic illness that causes swelling in the body’s tissue. This evidencebased clinical practice guideline has been launched to support clinicians and ultimately improve the quality of life of people living with lymphoedema across Ireland.
These guidelines support the implementation of good lymphoedema care in all settings and improve the experience and safety of people in our care. The guideline will provide healthcare professionals with clear, evidence-based guidance on the diagnosis, assessment and management of patients with all types of lymphoedema.
Developed in collaboration with clinicians, service user representatives, and key stakeholders, including Higher Educations Institutions, across the Republic of Ireland and Northern Ireland, they are a guide for managers and commissioners to support the development and modernisation of pathways of lymphoedema care, education and research.
The ‘All Ireland Lymphoedema Guidelines for the Diagnosis, Assessment and Management of Lymphoedema’ provide:
• The most up-to-date, evidence based recommendations to support best clinical practice, standardisation of care and improved patient outcomes.
• An in-depth evidence review and recognition of changes in pathways, healthcare practices and advances in technology that support the Sláintecare strategy.
• A progressive way of integrating specialist services and non-specialist services and promoting supported self management in the community.
• A focus on prevention and early detection in areas that are high risk for lymphoedema
• Education requirements for lymphoedema services and service users.
• An implementation and evaluation plan
Speaking about the guidelines, HSE Chief Clinical Officer, Dr Colm Henry said: “The availability of these guidelines will support the implementation of evidence based, standardised lymphoedema care in all health care settings and improve the experience and safety of
people in our care. It will provide guidance to healthcare workers on prevention, early detection, effective treatments and supported self-care to ensure that every patient receives optimal care. The recommended treatment pathways are patient-centred and support the Sláintecare strategy of the right care, in the right place, at the right time.”
Visit hse.ie/lymphoedema to read the ‘All-Ireland Lymphoedema Guidelines for the Diagnosis, Assessment and Management of Lymphoedema’
PIRFENFAMOTIDOINE CLONMEL
Clonmel Healthcare is delighted to announce the launch of Pirfenidone Clonmel 267mg and 801mg film-coated tablets.

cobas MPXV targets two different regions of the MPXV genome, which are both less prone to mutations than other parts of the genome. This dual-target approach ensures that cobas MPXV will continue to detect the virus even if a mutation occurs in one of the target regions.
“When multiple clusters of monkeypox virus infection were initially reported in countries where the disease is not endemic, Roche was among the first companies to address virus concerns with test kits,” said Thomas Schinecker, CEO of Roche Diagnostics.
“In order to meet the testing needs and workflow demands of laboratories as well as expand access to safe and reliable diagnostic solutions, we developed the cobas MPXV on the fully automated and high-throughput cobas 6800/8800 system.”
The high-throughput solution can help individuals get the right results quickly. This is important so that patients are not subjected to unnecessary additional testing or isolation, and will have access to appropriate treatment as soon as possible.
Alongside Olivia Reilly of Clonmel Healthcare is Karl Henry, the newest face of Pharmaton, leading fitness and wellness expert, and Podcast host Karl Henry. Now moved to West Cork, Karl was at Hickey Pharmacy to promote Pharmaton’s latest immunity supplement, Immuno10; built to boost immunity and help you get back to living better faster.
Pharmaton is available over the counter from almost all pharmacies nationwide (RRP: ¤17.95). The recommended daily dose is one film-coated tablet per day in the morning with a glass of water, preferably at breakfast. Immuno10 is suitable for adults 18 years +. Food supplements should not replace a balanced diet or healthy lifestyle. Always read the label.
NEW PROGRAMME FOR HEART HEALTH
The Irish Heart Foundation’s High-Risk Prevention Project (HRPP) in General Practice works with patients from disadvantaged areas at high risk of heart disease and stroke.
Pirfenidone Clonmel is indicated in adults for the treatment of mild to moderate idiopathic pulmonary fibrosis (IPF).
Full prescribing information is available on request or alternatively please go to www. clonmel-health.ie. Medicinal product subject to medical prescription.
Please contact Clonmel Healthcare on 01-6204000 if you require any additional information.
PA 126/350/1-2. PA Holder: Clonmel Healthcare Ltd., Clonmel, Co. Tipperary. Date prepared: November 2022. 2022/ADV/ PIR/287H.
ROCHE RECEIVES U.S. FDA EMERGENCY USE AUTHORIZATION FOR ITS HIGH-THROUGHPUT TEST TO DETECT MONKEYPOX VIRUS
Roche has announced that the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for cobas® MPXV for use on the cobas® 6800/8800 Systems. The test is a real time PCR test for the qualitative detection of DNA from monkeypox virus (MPXV) in lesion swabs collected from individuals suspected of monkeypox infection by their healthcare provider.
Like many viruses, monkeypox cannot be conclusively diagnosed by symptoms alone. This is because many monkeypox symptoms closely resemble those of other rash-producing illnesses such as chickenpox, measles, bacterial skin infections, and even hives or allergies.
PHARMATON PHARMACY VISIT
Pharmaton Ambassador Karl Henry recently madea pharmacy visit to Hickey Pharmacy, Ballincollig, Cork (below).

A new heart health programme by the Irish Heart Foundation has shown to be beneficial to people at high risk of heart disease and stroke.
Launched in January 2021 and supported by the HSE, The Irish Heart Foundation’s High-Risk Prevention Project in General Practice works with patients from disadvantaged areas at high risk of heart disease and stroke and has helped patients make positive changes to their lifestyles and improve their health and wellbeing.
81 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
Clinical R&D
Despite taking place during the COVID-19 pandemic,the programme resulted in measurable positive change and feedback from the majority of participants.
Under the HSE Chronic disease management programme, those at high risk of cardiovascular disease are entitled to one annual GP visit and one practice nurse visit. Such high-risk patients require support to actively manage their risk factors.
This is challenging to achieve through an annual visit, particularly for vulnerable people and people in deprived areas.
The Irish Heart Foundation, therefore, identified that this group of people could benefit from the support of a tailored lifestyle behaviour change programme and developed the high-risk prevention project in general practice to help with this.
Six practices in disadvantaged areas in Dublin (4) and Wexford (2) were involved in the programme.
The HRPP consisted of six weekly one-to-one sessions, where patients were aided in introducing lifestyle changes that they would like to make such as increased physical activity. They were then supported to set goals and overcome barriers to making positive changes to their habits.
Across the six practices 304 people started the programme, and 260 successfully completed the six-week intervention showing a high patient retention rate (86%) despite the challenges of the pandemic., the average age of participants was 58.
In a preliminary review of the project, 72 patients who were followed up at 12 months demonstrated a significant reduction in BMI, waist circumference and overall body weight as well as improved dietary habits, increased physical activity and less sedentary behaviors.
Participants reported that the programme has improved their knowledge of how to better manage their health issues such as blood pressure, weight, and cholesterol. They also highlighted the benefit of the intervention being carried out at their local GP practice.
Practice Nurses who took part in the programme also reported a number of benefits from their experience with one stating, “I had a patient from 1st cohort come into me today for a check and he is doing brilliant, has lost a stone in 8 weeks. Blood pressure hugely improved and overall he feels a new
person. You know its such great motivation to see them embrace it and see the health benefits. He said that no one had ever taken any interest in his health before and found that alone motivational.”
NEW PARTNERSHIP TO INCREASE MENTAL HEALTH SUPPORTS
The HSE has announced a national partnership with digital mental health platform SilverCloud®. The partnership will help address the growing need for access to mental health support in Ireland.
According to research from Maynooth University and Trinity College earlier this year, 42% of Irish adults have a mental disorder. WHO research also indicates that mental health is one of the greatest social and economic challenges of our time, more pronounced now than before the pandemic.
Through this partnership, clinicians can refer to SilverCloud’s evidencebased digital CBT programmes. Active use of the digital platform in Ireland has doubled since the 12-month review of the pilot in April 2022. The service has seen over 10,000 referrals from Ireland based GPs, Primary Care Psychologists, Counselling in Primary Care and Jigsaw as they identify SilverCloud as a potential solution to support their patients.
Minister for Mental Health, Mary Butler TD said, “The announcement of this partnership with the Health Service Executive and digital mental health platform SilverCloud® for the provision of Guided
Digital Cognitive Behavioural Therapy (CBT) is hugely positive. I am passionate about improving access to and enhancing the reach of mental health services for people who require them. This announcement follows a very successful pilot which has seen the service receive over 10,000 referrals. This national rollout will be a key part of Ireland’s mental health strategy in the years ahead making it easier for people to secure the support they need.”
A new report published by SilverCloud, reviews the first 18-month application of the service in Ireland. 89 per cent of people provided access to SilverCloud felt that digital CBT would work for them and their needs prior to commencing a programme. The overall satisfaction rate of the programme was 94% post-use. Patient engagement with the programmes has seen a broad age range between 18-44 years (76%) and a representation across all 26 counties with Dublin, Cork and Galway leading in account volume.
Further results have shown the effectiveness of digital CBT:
• 49% of users with clinical levels of depression (excl. severe) transitioned to recovery.
• 53% of users with clinical levels of anxiety (excl. severe) transitioned to recovery.
• GPs are the source of the highest number of referrals, representing 89% of the total referrals.
• On average users spent 4.5 hours on the platform
“Barriers to access can be caused by finances, fear of stigma, or difficulties related to taking the first step to talk to someone. The SilverCloud digital CBT programmes can be accessed confidentially from one’s own home, therefore, waitlists for appointments and even location in remote areas of the country are no longer impediments.” - Dr Derek Richards, Chief Science Officer of SilverCloud, Amwell. “Additionally, a large body of research has now accumulated to demonstrate the effectiveness of guided digitally delivered CBT for depression and anxiety[4],[5],[6]. Commensurate with this research, the clinical outcomes and patient satisfaction ratings with the national digital CBT service are notable.”
HEALTH RESEARCH CHARITIES IRELAND RESEARCH IMPACT AWARD

Dr Mairéad O’Driscoll, CEO, Health Research Board, Professor Sally Ann Lynch, CHI at Temple Street and Crumlin, and UCD School of Medicine, and Dr Avril Kennan, CEO of Health Research Charities Ireland, pictured at the inaugural Health Research Charities Ireland Research Impact Award 2022, which was presented to Professor Lynch.
The award recognises the vital role of health research charities in funding research and the
Left to right Dr Mairéad O’Driscoll, Professor Sally Ann Lynch and Dr Avril Kennan
82 JANUARY 2023 • HPN | HOSPITALPROFESSIONALNEWS.IE
contributions of principal investigators, funded through the Health Research Charities Ireland/ Health Research Board Joint Funding Scheme.
Professor Lynch’s work, which was supported by the National Children’s Research Centre and the Children’s Health Foundation, Temple Street, was recognised for its real-world impact and for making a positive difference to patients’ lives. For more information, visit www.hrci.ie/joint-funding-scheme
GLOUP® GOES SUGAR FREE!
Gloup® Swallowing Gel has launched a new sugar free formulation called Gloup Zero® The raspberry flavoured gel can still be used from 2 years of age* to swallow solid medicines, including:

It works by moistening the mucous membranes in the mouth and throat cavity and allowing the tablets to pass smoothly via the oesophagus to the stomach.
To use put the tablets/ capsules on a tablespoon, add 5ml of Gloup Zero® and swallow. Multiple tablets / capsules can be taken at the same time.
Gloup Zero® is sugar free and is suitable for diabetics.
Please contact Clonmel Healthcare on 01-6204000 if you require any additional information.
*Can be used by anyone over the age of 2 years, who can initiate their own swallow.
Always read the label. Clonmel Healthcare Ltd., Clonmel, Co. Tipperary. Date prepared: November 2022. 2022/ ADV/GLO/183H.
UNIPHAR AND FRIENDS OF CROSS CHARITY INITIATIVE
A joint fundraising initiative between Uniphar plc and cancer charity Friends of Cross was marked at Trinity College recently with the handover of a cheque amounting to ¤110,000 to the Trinity St. James’s Cancer Institute. The Provost of Trinity College, Linda Doyle, accepted the cheque on behalf of the Institute, and said it was a wonderful act of

generosity that would greatly assist the important ground-breaking cancer research ongoing at the Trinity St. James’s campus.
Uniphar Chairman, Maurice Pratt and former Ireland rugby legend and Friends of Cross patron, Paul Wallace, were on hand to jointly carry out the official cheque handover to the Provost, which took place beneath the historic Trinity Campanile on the College’s front square.
The monies raised came from the Uniphar plc ‘Unity for Hope’ campaign which was supported by the company’s 2,900 staff members globally, with matched funding by Uniphar plc and from supporters of the Friends of Cross, all of whom ran novel fundraising campaigns throughout the 20-month pandemic lockdown period
The total amount raised came to ¤110,000 and will go towards continued support of life-saving cancer research at the Trinity Translational Medicine Institute, Trinity College Dublin and St. James’s Hospital.
Friends of CROSS has helped raise over ¤1 million for the cancer research charity over the past 12 years, which directly supports the education and training of students in their careers as young researchers. The funds also contribute to the purchase of
Professor John Reynolds, Professor of Surgery and Head of Department at Trinity College Dublin and St James’s Hospital Dublin; Paul Wallace, Patron Friends of Cross; Maurice Pratt, Chairman Uniphar PLC; and Dr Linda Doyle, Provost of Trinity College Dublin
state-of-the-art equipment, used by over 60 researchers in Ireland working across several cancer types including oesophageal cancer, breast cancer, colon cancer and lung cancer.
The Chairman of Uniphar, Maurice Pratt, said, “It is a great honour to present this significant donation to Trinity St James’s Cancer Institute after such a lengthy gap due to the Covid pandemic. Uniphar plc has over 2,900 staff globally, and each person participated in our ‘Unity for Hope’ cancer charity campaign by running micro fundraising initiatives from their homes and office spaces during the pandemic. The money they raised was then matched by Uniphar plc and a very welcome additional contribution was also made by the Friends of Cross. We are delighted to be finally handing the final cheque here to the wonderful team at the Trinity St. James’s Cancer Institute.”
83 HOSPITALPROFESSIONALNEWS.IE | HPN • JANUARY 2023
Tablets Capsules Vitamins Nutritional Supplements
Median recovery time (T4/T1 = 0.9) ≅ 3 minutes. • 2 mg/kg if recovery has occurred up to at least T2 following ROC- or VEC-induced block. Median recovery time (T4/T1 = 0.9) ≅ 2 minutes. Median recovery time (T4/T1= 0.9) is slightly faster with ROC- than VEC-induced block. Immediate reversal of ROC-induced block: 16 mg/kg. Median recovery time (T4/T1 = 0.9) ≅ 1.5 minutes when 16 mg/kg is given 3 minutes after a bolus dose of 1.2 mg/kg ROC. Not recommended for immediate reversal of VEC-induced block. Re administration of sugammadex: For post-operative recurrence of block after an initial dose of 2 mg/kg or 4 mg/kg, repeat dose of 4 mg/kg is recommended. Following a second dose of sugammadex, monitor the patient closely to ascertain sustained return of neuromuscular function. Re-administration of ROC or VEC after sugammadex: Up to 4 mg/kg Sugammadex a waiting time of 5 minutes for re-use of 1.2 mg/kg ROC; 4 hours waiting time for re-use of 0.6 mg/kg ROC or 0.1 mg/kg VEC. With ROC onset of NM block may be prolonged and duration of NM block may shortened. After immediate reversal, 16 mg/kg sugammadex, a waiting time of 24 hours is recommended. See SPC for patients with mild or moderate renal impairment. Special populations: Renal impairment: For mild and moderate renal impairment use adult dose. Not recommended in severe renal impairment (including patients requiring dialysis). Elderly: Use adult dose although recovery times are slower. Obese: In obese patients, including morbidly obese patients (body mass index ≥ 40 kg/m2), adult dose based on actual body weight. Hepatic impairment: Caution in patients with severe hepatic impairment, or impairment with coagulopathy. Children and adolescents (2-17 years): Bridion 100 mg/ml may be diluted to 10 mg/ml to increase the accuracy of dosing in the paediatric population. Routine reversal: A dose of 4 mg/kg sugammadex is recommended for reversal of rocuronium induced blockade if recovery has reached at least 1-2 PTC. A dose of 2 mg/kg is recommended for reversal of rocuronium induced blockade at reappearance of T2 Immediate reversal: Immediate reversal in children and adolescents has not been investigated. Term newborn infants and infants: Not recommended. CONTRA-INDICATIONS Hypersensitivity to sugammadex or to any excipients. PRECAU-


Sugammadex can be removed using haemodialysis with a high flux filter. INTERACTIONS Toremifene and fusidic acid may displace rocuronium or vecuronium from sugammadex and delay recovery (no clinically relevant capturing interactions are expected). Interaction of sugammadex with hormonal contraceptives may lead to a decrease in progesterone exposure equivalent to one missed daily dose of oral contraceptive (no displacement interactions are expected). In general sugammadex does not interfere with laboratory tests, with the possible exception of the serum progesterone assay where interference is observed at sugammadex plasma concentrations of 100 µg/ml plasma. In a study doses of 4 mg/kg and 16 mg/kg sugammadex resulted in maximum mean prolongations of the activated partial thromboplastin time by 17 and 22% respectively and prothrombin time by 11% and 22% respectively. These were of short duration (≤ 30 minutes). In in vitro experiments pharmacodynamic interaction was noted with vitamin K antagonists, unfractionated heparin, low molecular weight heparinoids, rivaroxaban and dabigatran. PREGNANCY AND LACTATION Pregnancy and Lactation: Caution in pregnant women. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from sugammadex therapy, taking into account the benefit of breast feeding for the child and the benefit of therapy for the woman. The effects on human fertility have not been investigated. SIDE EFFECTS Refer to SmPC for complete information on side effects Common (≥ 1/100 to < 1/10): Anaesthetic complications including movement of limbs or body or
TIONS AND WARNINGS Ventilatory support is mandatory for patients until adequate spontaneous respiration is restored following reversal of block. Should block reoccur following extubation, adequate ventilation should be provided. The use of lower than recommended doses may lead to an increased risk of neuromuscular blockade after an initial reversal and is not recommended. Caution should be exercised when considering the use of sugammadex in patients receiving anticoagulation for a pre-existing or co-morbid condition. An increased risk of bleeding cannot be excluded in patients with hereditary vitamin K dependent clotting factor deficiencies; with pre-existing coagulopathies; on coumarin derivatives and at an INR receive or VEC with mild or ROC should If a shorter waiting time is required, the ROC dose for a new NM blockade should be 1.2 mg/kg within 30 minutes after Sugammadex administration. immediate of hours is If block before recommended nonsteroidal blocking agent renal is If block is additional doses of anaesthetic and/or opioid should be given as clinically indicated. Marked bradycardia with cardiac arrest have been observed within minand agents such as has patients receiving or VEC in the ICU setting.
sugammadex in patients receiving anticoagulation for a pre-existing or co-morbid condition. An increased risk of bleeding cannot be excluded in patients with hereditary vitamin K dependent clotting factor deficiencies; with pre-existing coagulopathies; on coumarin derivatives and at an INR above 3.5; using coagulants who receive a dose of 16mg/kg sugammadex. If re administration of ROC or VEC is required in patients with mild or moderate renal impairment after routine sugammadex reversal a waiting time for re-use of 0.6 mg/kg ROC or 0.1 mg/kg VEC should be 24 hours. If a shorter waiting time is required, the ROC dose for a new NM blockade should be 1.2 mg/kg within 30 minutes after Sugammadex administration. For re-administration of ROC or VEC after immediate reversal a waiting time of 24 hours is recommended. If neuromuscular block is required before the recommended waiting time has passed, a nonsteroidal neuromuscular blocking agent should be used. The use in patients with severe renal impairment is not recommended including those requiring dialysis. If neuromuscular block is reversed, while anaesthesia is continued, additional doses of anaesthetic and/or opioid should be given as clinically indicated. Marked bradycardia with cardiac arrest have been observed within minutes after administration, closely monitor patients for haemodynamic changes during and after reversal. Treat with anti-cholinergic agents such as atropine if clinically significant bradycardia observed.Sugammadex has not been investigated in patients receiving ROC or VEC in the ICU setting.
Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7 Ireland
Date of preparation: March 2022. IE-XBR-00079


County Business Park, Leopardstown, Dublin 18 or from www.medicines.ie. EMEA/H/C/000885/II/0042 Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-299 8700) Date of preparation: March 2022. IE-XBR-00079 Red Oak North, South County Business Park, X5K7 ➜ Scan the QR code to register an account with MSDConnect.ie ➜ Registering only takes two minutes ➜ Discover videos and product information in our portal that will help to support you and keep you informed ➜ Keep up-to-date with news, announcements and events If you would like to hear more from MSD and keep up-to-date with the most useful clinical content across all our therapy areas then register now for MSDConnect.ie MSDCONNECT BRIDION® 100 MG/ML SOLUTION FOR INJECTION Sugammadex ABRIDGED PRODUCT INFORMATION Refer to Summary of Product Characteristics (SmPC) before prescribing PRESENTATION Vials of 200mg (2 ml) or 500mg (5 ml). INDICATIONS Reversal of rocuronium (ROC) or vecuronium (VEC) induced neuromuscular (NM) block in adults. For routine reversal of ROC-induced block in children and adolescents aged 2 to 17 years. DOSAGE AND ADMINISTRATION I.V. as a single bolus injection administered rapidly (within 10 seconds), into an existing I.V. line, by/under the supervision of an anaesthetist. Use appropriate technique to monitor recovery of NM block. Dose depends on the level of block to be reversed, not the anaesthetic regimen. Adults: Routine reversal following ROC- or VEC-induced block: • 4 mg/kg if recovery has reached at least 1 2 post-tetanic counts (PTC).
sugammadex to reverse block induced by nonsteroidal blockers such as succinylcholine or benzylisoquinolinium compounds, or steroidal blockers other than ROC or VEC. Conditions associated with prolonged circulation time such as cardiovascular disease, old age, or oedematous state may cause longer recovery times. Be prepared for possible drug hypersensitivity reactions. This medicinal product contains up to 9.7 mg sodium per ml, equivalent to 0.5% of the WHO recommended maximum daily intake of 2 g sodium for an adult. OVERDOSE No dose related adverse events nor serious adverse events were reported.
coughing during
grimacing, or suckling on the endotracheal tube, airway complication of anaesthesia, procedural hypertension and procedural
< 1/100): Hypersensitivity
reported side
the SmPC.
administered
dose labelled
blockade
on neuromuscular monitoring or clinical
of lower than recommended
increased risk of recurrence of neuromuscular blockade after initial reversal and is not recommended. HANDLING See
with infusion solutions. Physical incompatibility has been reported with verapamil, ondansetron and ranitidine. PACKAGE QUANTITIES 10 vials of 2 ml 10 vials of 5 ml LEGAL CATEGORY POM Marketing Authorisation Numbers EU/1/08/466/001-002 Marketing Authorisation Holder Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands Date of review of prescribing information: January 2022 © Merck Sharp and Dohme B.V., 2022. All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin 18 or from www.medicines.ie. EMEA/H/C/000885/II/0042 Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-299 8700)
Do not use
anaesthesia,
complication. Uncommon (≥ 1/1,000 to
reactions, including anaphylaxis, have occurred in some patients and volunteers. Other less common and rarely
effects are listed in
In clinical studies with subjects treated with rocuronium or vecuronium, where sugammadex was
using a
for the depth of neuromuscular blockade, an incidence of 0.2% was observed for recurrence of neuromuscular
as based
evidence. The use
doses may lead to an
SmPC for details of compatability

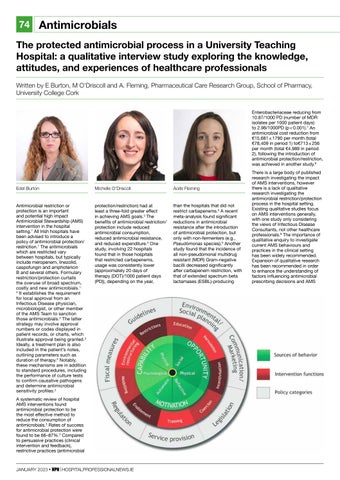





































































































































 Dr Dan Ryan, Consultant Respiratory Physician, Beaumont Hospital
Dr Dan Ryan, Consultant Respiratory Physician, Beaumont Hospital











 Written by E Burton, M O’Driscoll and A. Fleming, Pharmaceutical Care Research Group, School of Pharmacy, University College Cork
Written by E Burton, M O’Driscoll and A. Fleming, Pharmaceutical Care Research Group, School of Pharmacy, University College Cork


























