IN THIS ISSUE:
NEWS: New President
Association Page
REPORT: Medical Council publishes Workforce Intelligence Report Page
FEATURE: The Phases of Migraine Page



CPD: Lithium Therapy Page
WOMEN'S HEALTH FOCUS: Navigating Menopause in Thrombosis Page

WOMEN'S HEALTH FOCUS: Sepsis and Pregnancy Page
WOMEN'S HEALTH FOCUS: Metastatic Breast Cancer Page
HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HPN October 2022 Issue 101 HOSPITALPROFESSIONALNEWS.IE This Publication is for Healthcare Professionals Only
for Consultants
4
8
14
31
40
53
68 MARKETING AUTHORISATION HOLDER: AbbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061 Ludwigshafen, Germany. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie. LEGAL CLASSIFICATION: POM (S1A). Full Summary of Product Characteristics is available at www.medicines.ie Reference 1. RINVOQ Summary of Product Characteristics, available on www.medicines.ie ©2022 AbbVie Inc. All rights reserved. IE-RNQ_AD-220036 | March 2022 INTRODUCING FOR THE TREATMENT OF MODERATE TO SEVERE atopic dermatitis in adults and adolescents 12 years and older who are candidates for systemic therapy 1
TAFINLAR + MEKINIST
ABBREVIATED PRESCRIBING INFORMATION
Non-fixed dose combination of MEKINIST® and TAFINLAR®
Before prescribing Mekinist and Tafinlar in combination, please refer to the Summary of Product Characteristics (SmPC) of both products.
Presentation of each product: MEKINIST (trametinib) 0.5 mg and 2.0 mg film-coated tablets. Each film-coated tablet contains trametinib dimethyl sulfoxide equivalent to 0.5 mg and 2.0 mg of trametinib respectively. TAFINLAR (dabrafenib) 50 mg and 75 mg hard capsules. Each capsule contains dabrafenib mesilate, equivalent to 50 mg and 75 mg of dabrafenib respectively.
Indications: Combination of Mekinist and Tafinlar:


• for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.
• for the adjuvant treatment of adult patients with stage III melanoma with a BRAF V600 mutation following complete resection.
• for the treatment of adult patients with advanced non-small cell lung cancer (NSCLC) with a BRAF V600 mutation.
Dosage and Administration: Adults: recommended dose of Mekinist is 2mg once daily in combination with Tafinlar 150mg twice daily. Dose modification: Management of ADRs may require treatment interruption, dose reduction, or discontinuation. Elderly: No initial dose adjustment required in patients >65 years. Paediatrics: Safety and efficacy not established in patients <18 years. Renal impairment: No dose adjustment required in mild or moderate impairment. Caution advised in severe renal impairment. Hepatic impairment: No dose adjustment required in mild hepatic impairment. Caution advised in moderate and severe hepatic impairment. In the adjuvant melanoma setting, patients should be treated for a period of 12 months unless there is disease recurrence or unacceptable toxicity. Contraindications: Hypersensitivity to active substance or excipients. Warnings/Precautions for Mekinist (used as a monotherapy or in combination with Tafinlar): Left ventricular ejection fraction (LVEF) reduction/Left ventricular dysfunction: Cases of LVEF decrease have been reported. Should be used with caution when conditions could impair left ventricular fraction. All patients should be evaluated for LVEF prior to initiation of treatment with continued evaluation during treatment. Consider dose modification guidelines. In patients receiving Mekinist in combination with Tafinlar, there have been occasional reports of acute, severe LVEF due to myocarditis. Full recovery was observed when stopping treatment. Physicians should be alert to the possibility of myocarditis in patients who develop new or worsening cardiac signs or symptoms. Haemorrhage: Haemorrhagic events including major and fatal haemorrhagic events occurred in patients taking Mekinist as monotherapy and in combination with Tafinlar. Hypertension: Blood pressure should be measured at baseline and monitored during treatment with Mekinist, with control of hypertension by standard therapy as appropriate. Interstitial lung disease (ILD)/Pneumonitis: Mekinist should be withheld in patients with suspected ILD or pneumonitis, including patients presenting with new or progressive pulmonary symptoms and findings including cough, dyspnoea, hypoxia, pleural effusion, or infiltrates, pending clinical investigations. Mekinist should be permanently discontinued for patients diagnosed with treatment-related ILD or pneumonitis. If Mekinist is being used in combination with Tafinlar then therapy with Tafinlar may be continued at the same dose. Rhabdomyolysis: Signs or symptoms of rhabdomyolysis should warrant an appropriate clinical evaluation and treatment as indicated. Visual impairment: visual disturbances, including chorioretinopathy or retinal pigment epithelial detachment (RPED) and retinal vein occlusion (RVO) have been observed. Not recommended for patients with history of RVO. Ophthalmological evaluation should be performed at baseline and during treatment if clinically warranted. If retinal abnormality is observed, treatment should be interrupted immediately and referral to specialist should be considered. Permanently discontinue treatment if RVO is noticed. Rash: observed in 60% of patients in monotherapy and 24% of patients in combination with Tafinlar. Severe cutaneous adverse reactions (SCARs): SCARs, including Stevens-Johnson syndrome, and drug reaction with eosinophilia and systemic symptoms (DRESS), which can be life-threatening or fatal, have been reported with Mekinist in combination with Tafinlar. Before initiating treatment, patients should be advised of the signs and symptoms and monitored closely for skin reactions. If signs and symptoms
suggestive of SCARs appear, Mekinist and Tafinlar should be withdrawn. Deep vein thrombosis (DVT)/pulmonary embolism (PE): can occur when used as a monotherapy or in combination with Tafinlar. Seek immediate medical care if patients develop symptoms of DVT or PE. Pyrexia: Pyrexia including severe rigors, dehydration and hypotension (including acute renal insufficiency) reported. Incidence and severity increased when Mekinist used in combination with Tafinlar. Monitoring serum creatinine and other evidence of renal function impairment during and following severe pyrexia events. Serious non-infectious febrile events observed. For management of pyrexia, therapy should be interrupted if the patient’s temperature is ≥38°C (100.4°F). In case of recurrence, therapy can also be interrupted at the first symptom of pyrexia. Colitis and gastrointestinal perforation: Colitis and gastrointestinal perforation, including fatal outcome, have been reported. Treatment with Mekinist monotherapy or in combination with Tafinlar should be used with caution in patients with risk factors for gastrointestinal perforation, including a history of diverticulitis, metastases to the gastrointestinal tract and concomitant use of medications with a recognized risk of gastrointestinal perforation. If patients develop symptoms of colitis and gastrointestinal perforation they should immediately seek medical care. Sarcoidosis: Cases of sarcoidosis have been reported in patients treated with trametinib in combination with dabrafenib, mostly involving the skin, lung, eye and lymph nodes. In the majority of the cases, treatment with trametinib and dabrafenib was maintained. In case of a diagnosis of sarcoidosis, relevant treatment should be considered. It is important not to misinterpret sarcoidosis as disease progression Sodium: This medicine contains less than 1mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium free’. Warnings/Precautions for Tafinlar (used as a monotherapy or in combination with Mekinist): Pyrexia including severe rigors, dehydration and hypotension (including acute renal insufficiency) reported. Incidence and severity increased when used in combination with Mekinist. During and following severe pyrexia events, serum creatinine and other evidence of renal function should be monitored. Serious non-infections febrile events have been observed. For management of pyrexia therapy should be interrupted if the patient’s temperature is ≥38°C (100.4°F). In case of recurrence, therapy can also be interrupted at the first symptom of pyrexia. Cutaneous squamous cell carcinoma (cuSCC) and new primary melanoma: skin examination prior, during and for 6 months after discontinuation of treatment or until initiation of another anti-neoplastic therapy. Non-cutaneous secondary/recurrent malignancy: monitoring as clinically appropriate for up to 6 months after discontinuation of Tafinlar or until initiation of another anti-neoplastic therapy. Pancreatitis: unexplained abdominal pain should be promptly investigated to include measurement of serum amylase & lipase. Close monitoring when re-starting Tafinlar. Uveitis: monitoring patients for visual signs and symptoms during therapy. Colitis and gastrointestinal perforation: Colitis and gastrointestinal perforation, including fatal outcome, have been reported in patients taking dabrafenib in combination with trametinib. Sarcoidosis: Cases of sarcoidosis have been reported in patients treated with dabrafenib in combination with trametinib, mostly involving the skin, lung, eye and lymph nodes. In the majority of the cases, treatment with dabrafenib and trametinib was maintained. In case of a diagnosis of sarcoidosis, relevant treatment should be considered. It is important not to misinterpret sarcoidosis as disease progression. Interactions: Mekinist: Effect of trametinib on other medicinal products: Based on clinical data, no loss of efficacy of hormonal contraceptives is expected when co-administered with trametinib monotherapy Tafinlar: Effect of other medicinal products on dabrafenib: Caution with co-administration of strong inhibitors (e.g. ketoconazole, gemfibrozil, nefazodone, clarithromycin, ritonavir, saquinavir, telithromycin, itraconazole, voriconazole, posaconazole, atazanavir) of CYP2C8 or CYP3A4. Avoid co-administration with strong inducers (e.g. rifampicin, phenytoin, carbamazepine, phenobarbital, or St John’s wort (Hypericum perforatum) of CYP2C8 and CYP3A4. Avoid agents that increase gastric pH, when possible. Effect of dabrafenib on other medicinal products: Avoid concomitant use with medicinal products that are sensitive substrates of certain metabolising enzymes or transporters, if monitoring for efficacy and dose adjustment is not possible. Please refer to Section 4.5 of SPC for groups of medicinal products that can be affected. A drug utilisation review (DUR) is essential on initiating dabrafenib treatment. Exercise caution when co-administering with warfarin and consider additional INR (lnternational Normalised Ratio) monitoring. Exercise caution
REFERENCES:
Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Merrion Road, Dublin 4, D04 A9N6
TAFINLAR + MEKINIST improved distant metastasis-free survival
patients
distant metastasis

(n=432)
when co-administering with digoxin and additional monitoring is recommended. Effects of dabrafenib on substance transport systems: Tafinlar inhibits OATP1B1 and OATP1B3. Monitoring recommended of drugs that are sensitive substrates of OATP1B1 and OATP1B3 and known to have a narrow therapeutic index with regards to high peak concentrations (Cmax). Fertility, Pregnancy & Lactation: Women of child-bearing potential: When Mekinist and Tafinlar are use in combination, use effective methods of contraception during therapy and for at least 16 weeks after stopping treatment. Tafinlar may decrease the efficacy of oral or any systemic hormonal contraceptives; use an effective alternative method of contraception. Pregnancy: Caution should be exercised by considering the expected benefit to the mother against possible risk to the foetus. Breastfeeding: Caution should be exercised by considering the benefit of breastfeeding for the child and the benefit of therapy for the woman. Fertility: Mekinist may impair human fertility. Tafinlar represents a potential risk for impaired spermatogenesis, which may be irreversible. Ability to Drive and Use Machines: Trametinib and Dabrafenib have minor influence. Patients should be made aware of the potential for fatigue, dizziness and eye problems. Adverse reactions with Mekinist monotherapy in metastatic melanoma: Very common (≥ 1/10): hypertension, haemorrhage, cough; dyspnoea, diarrhoea, nausea, vomiting, constipation, abdominal pain, dry mouth, rash, dermatitis acneiform, dry skin, pruritus, alopecia, fatigue, oedema peripheral, pyrexia, asparate aminotransferase increased. common (≥ 1/100, < 1/10): folliculitis; paronychia; cellulitis; rash pustular; anaemia; hypersensitivity; dehydration; vision blurred; periorbital oedema; visual impairment; left ventricular dysfunction; ejection fraction decreased; bradycardia; lymphoedema; pneumonitis; stomatitis; erythema; palmar-plantar erythrodysaesthesia syndrome; skin fissures; skin chapped; face oedema; mucosal inflammation; asthenia; alanine aminotransferase increased; blood alkaline phosphatase increased; blood creatine phosphokinase increased. Adverse reactions with Tafinlar monotherapy: Very common (≥ 1/10): papilloma; decreased appetite; headache; cough; nausea, vomiting, diarrhoea; hyperkeratosis, alopecia, rash, PPE, arthralgia, myalgia, pain in extremity, pyrexia, fatigue, chills, asthenia. common (≥ 1/100, < 1/10): cuSCC; seborrhoeic keratosis; skin tags; basal cell carcinoma; hypophosphataemia; hyperglycaemia; constipation; dry skin; pruritus; actinic keratosis; skin lesion; erythema; photosensitivity reaction; influenza-like illness. Adverse reactions observed when Mekinist and Tafinlar are used in combination: Very common (≥ 1/10): nasopharyngitis, decreased appetite, headache, dizziness, hypertension, haemorrhage, cough; abdominal pain, constipation, diarrhoea, nausea, vomiting, alanine aminotransferase increased, aspartate aminotransferase increased, dry skin, pruritus, rash, erythema, arthralgia, myalgia, pain in extremity, muscle spasms, fatigue, oedema peripheral, pyrexia, chills, asthenia, influenza-like illness. common (≥ 1/100, < 1/10): urinary tract infection, cellulitis; folliculitis; paronychia; rash pustular; cuSCC; papilloma; seborrhoeic keratosis; neutropenia,; anaemia; thrombocytopenia; leukopenia; dehydration; hyponatraemia; hypophosphataemia; hyperglycaemia; vision blurred; visual impairment; uveitis; ejection fraction decreased; hypotension; lymphoedema; dyspnoea; dry mouth; stomatitis; dermatitis acneiform; actinic keratosis; night sweats; hyperkeratosis; alopecia; palmar-plantar erythrodysaesthesia syndrome; skin lesion; hyperhidrosis; panniculitis; skin fissures; photosensitivity; mucosal inflammation; face oedema; blood alkaline; phosphatase increased; gamma-glutamyltransferase increased; blood creatine phosphokinase increased. For more details on adverse reactions, please see SmPC. Pack Size: Mekinist is supplied in bottles of 30 tablets. Tafinlar is supplied in packs of 120 hard capsules. Legal category: POM. Marketing Authorisation Numbers: Mekinist: EU/1/14/931/002 (0.5mg), EU/1/14/931/006 (2.0mg). Tafinlar: EU/1/13/865/002 (50 mg), EU/1/13/865/004 (75 mg). Marketing Authorisation Holder: Novartis Europharm Ltd, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland.
Date of revision of text: Aug 2021.
Full prescribing information is available upon request from: Novartis Ireland Limited, Vista Building, Elm Park Business Park, Elm Park, Dublin 4. Tel: 01-2601255 or at www. medicines.ie. Detailed information on this product is also available on the website of the European Medicines Agency http://www.ema.europa.eu.
1. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 2017;377(19):1813-1823.
2. Kirkwood JM, Manola J, Ibrahim J, et al; for Eastern Cooperative Oncology Group. A pooled analysis of Eastern Cooperative Oncology Group and Intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10(5):1670-1677.
3. TAFINLAR Summary of Product Characteristics. Novartis Pharmaceuticals available at www.medicines.ie
4. Zelboraf Summary of Product characteristics. Roche available at www.medicines.ie 5. Cotellic Summary of Product Characteristics. Roche available at www.medicines.ie
6. Braftovi Summary of Product Characteristics. Pierre Fabre Medicament available at www.medicines.ie
7. Mektovi Summary of Product Characteristics.Pierre Fabre Medicament available at www.medicines.ie 8. Hauschild A, Dummer R, Schadendorf D, et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600–mutant stage III melanoma. J Clin Oncol. 2018;36(35):3441-3449. 9. Supplement to: Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813-1823.
10. Hauschild A, Dummer R, Santinami M, et al. Long-term benefit of adjuvant dabrafenib plus trametinib in patients with resected stage III BRAF V600-mutant melanoma: year analysis of COMBI-AD. Presented at: the American Society for Clinical Oncology Annual Meeting; May 29-31, 2020; Chicago, IL
OS, overall survival. * 3-year OS data from first interim analysis. a In 7% of patients, reasons for discontinuation before completion of 1 year of treatment included protocol deviation, lost to follow-up, investigator discretion, and decision by patient or proxy.2 PROTECT YOUR PATIENTS THROUGHOUT THEIR ADJUVANT JOURNEY ADJUVANT PATIENTS ALIVE AT 3 YEARS 86% 36 mo24 mo12 mo0 mo Interim analysis for OS had a median follow-up of 34 months1 Year 1 41% of patients taking placebo discontinued due to disease relapse (n=432) ONLY 5% of patients discontinued due to relapse 88% of patients were alive and relapse free after 12 months of treatment a COMBI-AD: 1 Year (on treatment, n=438) TAFINLAR + MEKINIST led to lower rates of discontinuation due to relapse8,9 Year 5 of patients were free of distant metastasis and alive in the TAFINLAR + MEKINIST arm (95% Cl, 61%-71%) 65% 54% of
(95% Cl, 49%-60%) were free of
in the placebo arm
COMBI-AD: 5 Years (4 years after treatment end, n=438)
10 April 2022 | IE200251 © 2022 Novartis
THE ONLY ORAL ADJUVANT THERAPY WITH 3-YEAR OS DATA1–7*
Contents Foreword
IPHA announces appointment of new President P5

Looking at new ways of investigating Osteoporosis Screening P6
Irish Medical Council highlights concerns in new Intelligence Report P8

Illegal Prescription Medicines still being sourced P10
New Network launched for Pharmacists P12
Focusing on Eye Health and Macular Odema P20
Fatigue in the Healthcare System P28
REGULARS
CPD: Lithium Therapy P31
Feature: Bladder Cancer P22
Women’s Health Focus: Menopause, Vaginal Atrophy & Urinary Incontinence P63
Women’s Health Focus: Importance of Sleep P66
Women’s Health Focus: Herpes Simplex Virus P76
Clinical R&D: P80
Hospital Professional News is a publication for Hospital Professionals and Professional educational bodies only. All rights reserved by Hospital Professional News. All material published in Hospital Professional News is copyright and no part of this magazine may be reproduced, stored in a retrieval system of transmitted in any form without written permission. IPN Communications Ltd have taken every care in compiling the magazine to ensure that it is correct at the time of going to press, however the publishers assume no responsibility for any effects from omissions or errors.
PUBLISHER
IPN Communications Ireland Ltd Clifton House, Lower Fitzwilliam Street, Dublin 2 (01) 669 0562

GROUP DIRECTOR
Natalie Maginnis n-maginnis@btconnect.com

EDITOR
Kelly Jo Eastwood
DIGITAL MARKETING & EDITORIAL EXECUTIVE
Danielle Norton danielle@hospitalprofessionalnews.ie
EDITORIAL
editorial@hospitalprofessionalnews.ie
ACCOUNTS

Rachel Wilson cs.ipn@btconnect.com
SALES EXECUTIVE
Aoife
Hazel
DESIGN
Stoddart
Editor
Two of our leading news stories this month pertain to the appointment of key individuals within the healthcare and medicines setting. Professor Robert Landers has been appointed as President of the Irish Hospital Consultants Association, taking over from Professor Alan Irvine. Professor Landers is eager to continue the work carried out to date by Professor Irvine stating, ““With around 900 permanent hospital Consultant posts currently vacant or not filled as needed, the onus is now on the Government to provide attractive working conditions in our health service, for our existing Consultants, our Consultants in training and the new medical talent we want to attract into permanent posts. A first step must be reversing the unilateral pay inequality imposed on Consultants appointed after 2012.”
Meanwhile, Michael O’Connor is the newly appointed President of the Irish Pharmaceutical Healthcare Authority, and has pledged to focus on achieving a better environment for access, competitiveness and innovation.

““In access, a better funding environment and a new Framework Agreement for the Supply and Pricing of Medicines is improving Ireland’s capacity to deliver the latest treatments to patients. Next year, IPHA member companies expect to launch 30 new medicines, potentially treating more than 7,000 patients,” he says. You can read more about this on page 5.
In other news, the Pharmacist Antimicrobial Stewardship Network (PANS-net) has recently been launched. The network is a fantastic opportunity for pharmacists across all settings to collaborate in education, promotion and implementation of best practice so we can achieve our shared goal of enabling responsible use of antimicrobials in all patients and limiting the emergence of antimicrobial resistance (AMR). Turn to page 12 for further details.
Saint John of God Hospital Pharmacy, the HSE National Medication Safety Programme, and the Irish Pharmacy Union have joined forces in a novel new initiative to launch a new patient Information Booklet on Lithium Therapy.
The joint initiative aims to promote safer lithium therapy and empower patients to engage more with their Healthcare Professional on all aspects of lithium therapy including monitoring and potential side-effects. Our Continuing Professional Development Module for October is written by Audrey Purcell from St John of God Hospital with further details of this initiative
Our Special Focus this month is in the field of Women’s Health. We have some excellent clinical articles on this subject including Dr Deirdre Lundy on navigating menopause in thrombosis patients, Dr Edward O’Sullivan who talks about migraine in women and Danielle Keane & colleagues on metastatic breast cancer.
I hope you enjoy the issue.
3 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022 October Issue Issue 101 5
10 8 28 HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication HOSPITALPROFESSIONALNEWS.IE @HospitalProNews HospitalProfessionalNews
Tremere aoife.t@hospitalprofessionalnews.ie CONTRIBUTORS
Breen | Ruth Morrow | Yair Daykan Dr Louise O’Toole | Helen Forristal Paddy McGeoghegan | Dale Whelehan Dr Naomi Alego | Audrey Purcell Dr Deirdre Lundy | Osas Edelbiri Fergal O’Shaughnessy | Fionnuala Ní Áinle Caitriona Cunningham | Dr Edward O’Sullivan Mary Higgins | Shideh Kiafer | Elaine Houlihan Maria Farren | Susan Knowles Darragh O’Reilly | Siaghal MacColgain Janis Morrissey | Dr Catherine Riordan Tatiana Lamak | Danielle Keane Grace Phillips | Nicola Mitchell | Barry O’Reilly Roisin Connolly | Josephine Hegarty Aoife Ni Eochaidh | Louise Delaney Theresa Lowry-Lehnen
DIRECTOR Ian
Design
New Consultants President issues Warning
Professor Robert Landers, The Irish Hospital Consultants Association President
a backlog of deferred care that could take up to 15 years to address even with the provision of significant additional resources.
Following the publication of the IHCA’s Pre-Budget 2023 submission, he again urged Government to deliver on its Budget promises by providing the necessary resources, capacity, and Consultants to assess and treat patients.
The Irish Hospital Consultants Association (IHCA) has announced that Professor Robert Landers, Consultant Histopathologist at University Hospital Waterford, has been appointed as the new President of the Association.

Professor Landers takes over the presidency from Professor Alan Irvine who has completed his twoyear term leading the Association,
which represents around 95% of hospital Consultants across the Irish health service.
Commenting upon his appointment Professor Robert Landers said, “With a record 910,000 people on waiting lists, never have the core values of this Association been more relevant.
I am eager to continue the work done by Prof Irvine in advocating for timely, quality care for patients
and the essential resources needed for the Consultants who treat them.’’
Professor Landers acknowledged that he is embarking on his two-year term at a time when the health service is confronted with severe challenges and deficits, including an extreme shortage of medical and surgical specialists which has resulted in unacceptable waiting lists and
Hospital Pharmacy Role in Clinical Trials
Clinical trials are essential for continuously improving patient outcomes and their quality of life.
Hospital pharmacists, as members of the multidisciplinary team, are needed to safely manage them.
With the entry into application of the Clinical Trial Regulation, the European Association of Hospital Pharmacists (EAHP) has decided to update its Position Paper on Clinical Trials in 2022.
One of the key contributions of the pharmacist is the promotion of patient safety by collaborating in the development of a research protocol, reviewing as a member of an advisory committee, establishing mechanisms that contribute to safety, and assuring compliance with local and national regulations and standards. Hospital pharmacists are at the forefront of patient
care and consequently also have a significant impact on patient management and thus should be further integrated into the work of ethics committees.
The position paper of EAHP touches on the role of hospital pharmacists in clinical trials, the involvement of different patient groups, the improvements in Europe's clinical trial landscape and the role of ethics committees in clinical trials. It contains several calls to action, namely
• EAHP calls on national governments to recognise the important roles that hospital pharmacists play in clinical trials by requiring their involvement to increase patient safety
• EAHP encourages regulators to further improve training
on clinical trials by anchoring it into both undergraduate and continuing education of pharmacists.
• EAHP recognises that not all patient groups are suitable candidates or fully represented in clinical trials. Where appropriate, efforts should be made – taking into account also all relevant constraints – to create clinical trials that also study the effects of new treatment options in diverse patient populations, so that also these groups could be provided with access to new medicines once approved.
• EAHP underlines the importance of utilising the full potential of the EU Clinical Trial Regulation by swiftly putting all necessary measures in place at the
“With around 900 permanent hospital Consultant posts currently vacant or not filled as needed, the onus is now on the Government to provide attractive working conditions in our health service, for our existing Consultants, our Consultants in training and the new medical talent we want to attract into permanent posts. A first step must be reversing the unilateral pay inequality imposed on Consultants appointed after 2012. This ongoing inequality is a major factor in Consultant workforce deficits, which in turn will continue to hamper our ability to deliver critical, timely care to patients.’’
Prof Landers will be joined in his role by two Vice Presidents: Prof Gabrielle Colleran, Consultant Radiologist, CHI at Temple Street/ NMH Holles Street (re-elected); and newly elected Prof Anne Doherty, Consultant Liaison Psychiatrist, Mater Hospital.
national level to successfully transition to this new regime.
• EAHP urges the Member States to ensure that the role of ethics committees under the new Clinical Trial Regulation remains strong in the interest of clinical trial participants.
EAHP Congress 2023
Meanwhile, the abstract submission for the 27th Congress of the European Association of Hospital Pharmacists (EAHP) is closing soon. Until the 1st of October, hospital pharmacists, other healthcare professionals and scientists interested in sharing their work at EAHP’s next Congress are encouraged to submit their research. Questions concerning the abstract submission process can be addressed to abstract@eahp.eu.
4 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
News
Biogen’s Michael O’Connell named as new IPHA President
Michael O’Connell, Country Director for Biogen, has been named President of the Irish Pharmaceutical Healthcare Association (IPHA), the representative body for the research-based biopharmaceutical industry.

Takeda’s Shane Ryan becomes Vice-President. Mr O’Connell takes over the role vacated by Pfizer’s Paul Reid after his two-year term. IPHA’s Board confirmed the appointments which take effect immediately.
The industry believes it can have a shaping role on the health and enterprise landscape in Ireland through the application of science in patients’ lives and the economic upside of jobs, investments and exports linked to innovation in medicines.
Mr O’Connell pledged to focus his presidency on achieving a better environment for access, competitiveness and innovation.
“This is an exciting time for the biopharmaceutical industry, in Ireland and globally. The pandemic has demonstrated the dividend of science, with protection through vaccines helping social and economic life to resume. In areas like access, competitiveness and innovation, we have new opportunities for progress. It is our role at IPHA to ensure that, by working with others, the environment is right to maximise the return on these opportunities for our society and the economy.
Biogen’s Michael O’Connell named as new IPHA President
“In access, a better funding environment and a new Framework Agreement for the Supply and Pricing of Medicines is improving Ireland’s capacity to deliver the latest treatments to patients. Next year, IPHA member companies expect to launch 30 new medicines, potentially treating more than 7,000 patients. The estimated cost of these medicines next year is ¤35 million. We will shortly bring forward proposals for a fitter and faster reimbursement process which, alongside sustained funding for new medicines, should narrow the gap between the completion of health technology assessments and the availability of new medicines for patients.
“Our industry, across commercial and manufacturing operations, is distributed regionally, generating employment, tax revenues and exports. Our ability to keep the production, research and commercial investments we have, and to attract new ones in biologics and next-generation therapies, will depend on how well we can compete in a volatile global trading environment. We must maintain diverse global supply chains and avoid blunt-instrument
policies like ‘near-shoring’ or ‘reshoring’ that would jeopardise supply chain resilience. We must ensure the entire medicines supply chain is insulated from energy rationing. We must keep working on the development of new training pathways for the skills needed in an ever-changing industry and take full advantage of developments like artificial intelligence, the internet of things, genomics and cell therapy.
“In Europe, as the European Commission works on a legislative proposal that will shape the operating environment for
Driving Forward Repurposing of Medicines
REMEDi4ALL launched last month with the aim of making a major leap forward in drug repurposing.
The Value Added Medicines Group, a sector group of Medicines for Europe, joins the initiative as a member of the REMEDI4ALL consortium.
This promising approach to drug development consisting in the identification, testing, and validation of new therapeutic indications for existing medications, is a developing field but faces numerous barriers and systemic inefficiencies. Still, its potential to significantly bring down times and costs of drug
development -it focuses on already approved, discontinued, shelved or investigational therapeutics- makes this novel strategy attractive for rare and neglected conditions, cancer, emerging public health threats such as COVID-19 or new drug combinations. It also translates into more sustainable health systems.
The REMEDI4ALL initiative will:
• develop an innovation platform supporting promising, high impact drug repurposing projects championed by patients in any phase of development and disease area;
• establish a global community that contributes to informing and shaping policy and advancing debate and knowledge exchange
EATRIS, the European infrastructure for translational medicine, will lead this multidisciplinary consortium involving 24 European organisations with the common goal of making cost-effective repurposed medicines more widely available.
To advance knowledge in the field of medicine repurposing and address substantial obstacles -fragmented and siloed
medicines innovation, it is vital that intellectual property rights be protected and strengthened. In parallel, we should take steps to improve equity of access to new medicines for all Europe’s citizens. Ireland should strongly champion innovation as the basis for new jobs, investments, vaccines, treatments and cures. We have a jobs-rich industry in Ireland and a strong pipeline of new medicines for better clinical care. All that shows that the policy environment has worked for innovation. Now is not the time to damage that environment. Let us resolve to bolster it,” said Mr O’Connell.
research; non-standardised datasets; heterogenous quality of computational tools; poor patient engagement or lack of incentives and policies to support and enhance drug repurposing- the European Union (EU) through the Horizon Europe (HE) programme will invest 23 million euros in REMEDi4ALL over the next 5 years. It is expected that, due to REMEDi4ALL, more (and better) repurposed therapeutics will be widely available thanks to more agile, cutting-edge development processes, ultimately contributing to increased sustainability of health systems.
5 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022 News
Osteoporosis Screening
University of Galway clinicians, computer scientists and engineers are using enhanced x-ray technology used to measure bone density in people across Galway, Leitrim and Sligo to develop new osteoporosis screening and testing strategies for early identification of the condition in patients.
Funded by the Health Research Board, the Dual-energy X-ray Absorptiometry Management Application Project (DXA MAP), uses state of the art machines to develop a personalised, patientcentred tool for osteoporosis screening and fracture prediction.
Professor of Medicine at University of Galway and Clinical Lead for DXA, Osteoporosis and Metabolic Bone Disorders, at Galway University Hospitals, John Carey said, “The cross disciplinary expertise enables the development of a smart screening methodology to reduce health costs, maximise healthcare efficiencies, reduce waiting times and improve patient care and quality of life.”
The DXA MAP tool will be underpinned by artificial intelligence, recommended diagnostic criteria, reference
standards and visualisation approaches to support osteoporosis and fracture risk prediction, clinical interpretation and clinical-patient communication. The DXA MAP project also aims to support clinician interpretation through more automated processes and could predict Covid-19 and multi-morbidity risk using DXA secondary-data.
The project will be carried out by the University’s College of Science and Engineering and the College of Medicine, Nursing and Health Science, and led by Dr
New Taskforce on Workforce Planning
Professor Anthony O’Regan, Dean of the Institute of Medicine at the Royal College of Physicians of Ireland (RCPI) will Chair a new National Taskforce on the NCHD Workforce announced by the Minister for Health Stephen Donnelly.

The purpose of the Taskforce is to put in place sustainable workforce planning strategies and policies to improve the NCHD experience
and to support present and future retention of NCHDs in Ireland.
On today’s announcement, Professor Anthony O’Regan, Chair of the National Taskforce on NCHD Workforce said:
"I have been deeply committed to training and education throughout my career. I welcome the opportunity to review and improve NCHD working and training resources across our clinical sites in Ireland. It is imperative that we develop a supportive environment
Commercial Activity of the Year Award
Attracta Brennan, Professor John Carey and Associate Professor Mary Dempsey.
It is estimated that up to 300,000 people in Ireland have osteoporosis. Although more common in females who have gone through the menopause, it can also affect men and children.
Osteoporosis is a condition that affects the inside of bones. It causes bones to become fragile, so they break easily. It is called the silent disease because there are no signs or symptoms prior to a person breaking (fracturing) bones.
commensurate to the talent of our trainee NCHD colleagues and future healthcare leaders."
The Taskforce will seek to improve the NCHD experience/work-life balance through the development and implementation of improved NCHD structures and supports in hospital sites. It will aim to further develop and foster a culture of education and training at clinical site level and plan for future configuration of the medical workforce to support delivery of healthcare in Ireland.
Congratulations to Dr Waleed Faisal and his team on winning the SSPC Commercial Activity of the Year Award, which was kindly sponsored by Varda Space Industries.

The award, presented at the SSPC 2022 Centre Symposium held on the 31st of September, recognized the team behind the patent platform microneedle technology, ArrayPatch, for their outstanding contribution to knowledge transfer and commercialization activities.
Enterprise Ireland (EI) recently awarded the team a second commercialisation fund to develop a medicated 'band-aid' to treat fungal infections of the fingernails and toenails (onychomycosis).
Ms Arefe Ariamanesh, Dr Caroline Blackshields, Dr Waleed Faisal and Dr Ziad Sartawi
Professor Anthony O'Regan
6 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE News
MICROGRAMS/80 MICROLITERS SOLUTION FOR INJECTION
MICROLITERS SOLUTION FOR INJECTION

SOLUTION FOR INJECTION
(corresponding
women and men at increased risk of fracture. In postmenopausal women, a significant reduction in

of
and non-vertebral fractures but not
has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk
per ml). Presentation: Glass cartridge. Indications: Movymia is indicated in adults. Treatment of osteoporosis in postmenopausal women and men at increased risk of fracture. In postmenopausal women, a significant reduction in the incidence of vertebral and non-vertebral fractures but not hip fractures has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture. Dosage: The recommended dose is 20 micrograms administered once daily. Patients should receive calcium and vitamin D supplements if dietary intake is inadequate. The maximum total duration of treatment is 24 months. The 24 month course should not be repeated over a patient’s lifetime. Following cessation of teriparatide therapy, patients may be continued on other osteoporosis therapies. Teriparatide must not be used in severe renal impairment. Use with caution in moderate renal impairment and impaired hepatic function. Teriparatide should not be used in paediatric patients (less than 18 years), or young adults with open epiphyses. Method of administration: Movymia should be administered once daily by subcutaneous injection in the thigh or abdomen. It should be administered exclusively with the Movymia Pen reusable, multidose medicine delivery system and the injection needles which are listed as compatible in the instructions provided with the pen. The pen and injection needles are not included with Movymia. However, for treatment initiation a cartridge and pen pack should be used. Movymia must not be used with any other pen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or excipients. Pregnancy and Breast-feeding Pre-existing hypercalcaemia, severe renal impairment, metabolic bone diseases other than primary osteoporosis or glucocorticoid-induced osteoporosis, unexplained elevations of alkaline phosphatase, prior external beam or implant radiation therapy to the skeleton, patients with skeletal malignancies or bone metastases. Warnings and precautions: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection. Routine calcium monitoring during therapy is not required. Teripa ratide may cause small increases in urinary calcium excretion, but the incidence of hypercalciuria did not differ from that in the placebo-treated patients in clinical trials. Teriparatide should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. When transient orthostatic hypotension occurred, it happened within the first several doses, was relieved by placing subjects in a reclining position, and did not preclude continued treatment. Caution should be exercised in patients with moderate renal impairment. Experience in the younger adult population, including premenopausal women, is limited. Treatment should only be initiated if the benefit clearly outweighs risks in this population. Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, teriparatide should be discontinued. The recommended treatment time of 24 months should not be exceeded. Contains sodium. Interactions: Digoxin, digitalis. Fertility, pregnancy and lactation: Women of child bearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, Movymia should be discontinued. Movymia is contraindicated for use during pregnancy and breast-feeding. The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. Driving and operation of machinery: Teriparatide has no or negligible influence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Undesirable effects: Nausea, pain in limb, headache, dizziness. Refer to Summary of Product Characteristics for other adverse effects. Pack size: 1. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie. Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, German. Marketing authorisation number: EU/1/16/1161/001-003. Medicinal product subject to medical prescription. Date last revised: July 2019.
Dosage: The recommended dose is 20 micrograms administered once daily. Patients should receive calcium and vitamin D supplements if dietary intake is inadequate. The maximum total duration of treatment is 24 months. The 24 month course should not be repeated over a patient’s lifetime. Following cessation of teriparatide therapy, patients may be continued on other osteoporosis therapies. Teriparatide must not be used in severe renal impairment. Use with caution in moderate renal impairment and impaired hepatic function. Teriparatide should not be used in paediatric patients (less than 18 years), or young adults with open epiphyses. Method of administration: Movymia should be administered once daily by subcutaneous injection in the thigh or abdomen. It should be administered exclusively with the Movymia Pen reusable, multidose medicine delivery system and the injection needles which are listed as compatible in the instructions provided with the pen. The pen and injection needles are not included with Movymia. However, for treatment initiation a cartridge and pen pack should be used. Movymia must not be used with any other pen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or excipients. Pregnancy and Breast-feeding Pre-existing hypercalcaemia, severe renal impairment, metabolic bone diseases other than primary osteoporosis or glucocorticoid-induced osteoporosis, unexplained elevations of alkaline phosphatase, prior external beam or implant radiation therapy to the skeleton, patients with skeletal malignancies or bone metastases. Warnings and precautions: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection. Routine calcium monitoring during therapy is not required. Teripa ratide may cause small increases in urinary calcium excretion, but the incidence of hypercalciuria did not differ from that in the placebo-treated patients in clinical trials. Teriparatide should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. When transient orthostatic hypotension occurred, it happened within the first several doses, was relieved by placing subjects in a reclining position, and did not preclude continued treatment. Caution should be exercised in patients with moderate renal impairment. Experience in the younger adult population, including premenopausal women, is limited. Treatment should only be initiated if the benefit clearly outweighs risks in this population. Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, teriparatide should be discontinued. The recommended treatment time of 24 months should not be exceeded. Contains sodium. Interactions: Digoxin, digitalis. Fertility, pregnancy and lactation: Women of child bearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, Movymia should be discontinued. Movymia is contraindicated for use during pregnancy and breast-feeding. The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. Driving and operation of machinery: Teriparatide has no or negligible influence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Undesirable effects: Nausea, pain in limb, headache, dizziness. Refer to Summary of Product Characteristics for other adverse effects. Pack size: 1. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie. Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, German. Marketing authorisation number: EU/1/16/1161/001-003. Medicinal product subject to medical prescription.
osteoporosis in postmenopausal women and men at increased risk of fracture. In postmenopausal women, a significant reduction in the incidence of vertebral and non-vertebral fractures but not hip fractures has been demonstrated. Treatment of osteoporosis associated with sustained systemic glucocorticoid therapy in women and men at increased risk for fracture. Dosage: The recommended dose is 20 micrograms administered once daily. Patients should receive calcium and vitamin D supplements if dietary intake is inadequate. The maximum total duration of treatment is 24 months. The 24 month course should not be repeated over a patient’s lifetime. Following cessation of teriparatide therapy, patients may be continued on other osteoporosis therapies. Teriparatide must not be used in severe renal impairment. Use with caution in moderate renal impairment and impaired hepatic function. Teriparatide should not be used in paediatric patients (less than 18 years), or young adults with open epiphyses. Method of administration: Movymia should be administered once daily by subcutaneous injection in the thigh or abdomen. It should be administered exclusively with the Movymia Pen reusable, multidose medicine delivery system and the injection needles which are listed as compatible in the instructions provided with the pen. The pen and injection needles are not included with Movymia. However, for treatment initiation a cartridge and pen pack should be used. Movymia must not be used with any other pen. Patients must be trained to use the proper injection techniques. Contraindications: Hypersensitivity to the active substance or excipients. Pregnancy and Breast-feeding Pre-existing hypercalcaemia, severe renal impairment, metabolic bone diseases other than primary osteoporosis or glucocorticoid-induced osteoporosis, unexplained elevations of alkaline phosphatase, prior external beam or implant radiation therapy to the skeleton, patients with skeletal malignancies or bone metastases. Warnings and precautions: In normocalcaemic patients, slight and transient elevations of serum calcium concentrations have been observed following teriparatide injection. Serum calcium concentrations reach a maximum between 4 and 6 hours and return to baseline by 16 to 24 hours after each dose of teriparatide. Therefore, if blood samples for serum calcium measurements are taken, this should be done at least 16 hours after the most recent teriparatide injection. Routine calcium monitoring during therapy is not required. Teripa ratide may cause small increases in urinary calcium excretion, but the incidence of hypercalciuria did not differ from that in the placebo-treated patients in clinical trials. Teriparatide should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition. In short-term clinical studies with teriparatide, isolated episodes of transient orthostatic hypotension were observed. Typically, an event began within 4 hours of dosing and spontaneously resolved within a few minutes to a few hours. When transient orthostatic hypotension occurred, it happened within the first several doses, was relieved by placing subjects in a reclining position, and did not preclude continued treatment. Caution should be exercised in patients with moderate renal impairment. Experience in the younger adult population, including premenopausal women, is limited. Treatment should only be initiated if the benefit clearly outweighs risks in this population. Women of childbearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, teriparatide should be discontinued. The recommended treatment time of 24 months should not be exceeded. Contains sodium. Interactions: Digoxin, digitalis. Fertility, pregnancy and lactation: Women of child bearing potential should use effective methods of contraception during use of teriparatide. If pregnancy occurs, Movymia should be discontinued. Movymia is contraindicated for use during pregnancy and breast-feeding. The effect of teriparatide on human foetal development has not been studied. The potential risk for humans is unknown. Driving and operation of machinery: Teriparatide has no or negligible influence on the ability to drive and use machines. Transient, orthostatic hypotension or dizziness was observed in some patients. These patients should refrain from driving or the use of machines until symptoms have subsided. Undesirable effects: Nausea, pain in limb, headache, dizziness. Refer to Summary of Product Characteristics for other adverse effects. Pack size: 1. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie. Marketing authorisation holder: STADA Arzneimittel AG, Stadastrasse 2-18, 61118 Bad Vilbel, German. Marketing authorisation number: EU/1/16/1161/001-003. Medicinal product subject to medical prescription. Date last revised: July 2019.
DON’T WAIT UNTIL OSTEOPOROSIS STRIKES AGAIN MOVYMIA®: THE NEW TERIPARATIDE BIOSIMILAR FROM CLONMEL HEALTHCARE Rebuild bone before it breaks again—with Movymia®1 RELIABLE: Movymia®’s quality, safety and efficacy is highly similar to its reference product1,2,* EFFECTIVE: Anabolic MoA effectively rebuilds bone through the stimulation of osteoblasts1,3 AFFORDABLE: Allows more eligible patients to benefit due to its cost advantage4,5 RE-USABLE: One high quality reuseable pen for the entire treatment period1 MOVYMIA 20 MICROGRAMS/80 MICROLITERS
Each dose of 80 microliters contains 20 micrograms of teriparatide. One cartridge of 2.4 ml of solution contains 600 micrograms of teriparatide
to 250 micrograms per ml). Presentation: Glass cartridge. Indications: Movymia is indicated in adults. Treatment of osteoporosis in postmenopausal
the incidence
vertebral
hip fractures
for fracture.
Date last revised: July 2019. 1. Movymia® SmPC. 2. Movymia® EPAR – public assessment report, available at: https://www.ema.europa.eu/documents/assessment-report/movymia-epar-public-assessment-report_en.pdf 3. Brixen KT et al. Basic Clin Pharmacol Toxicol. 200494(6):260–70. 4. Lyman GH et al. N Engl J Med. 2018378(21):2036–2044. 5. Janjigian YY et al. Future Oncol. 201814(23):2403–2414.* Forsteo® October 2019. 2019/ADV/TER/122H DON’T WAIT UNTIL OSTEOPOROSIS STRIKES AGAIN MOVYMIA®: THE NEW TERIPARATIDE BIOSIMILAR FROM CLONMEL HEALTHCARE Rebuild bone before it breaks again—with Movymia®1 RELIABLE: Movymia®’s quality, safety and efficacy is highly similar to its reference product1,2,* EFFECTIVE: Anabolic MoA effectively rebuilds bone through the stimulation of osteoblasts1,3 AFFORDABLE: Allows more eligible patients to benefit due to its cost advantage4,5 RE-USABLE: One high quality reuseable pen for the entire treatment period1 MOVYMIA 20
Each dose of 80 microliters contains 20 micrograms of teriparatide. One cartridge of 2.4 ml of solution contains 600 micrograms of teriparatide (corresponding to 250 micrograms
1. Movymia® SmPC. 2. Movymia® EPAR – public assessment report, available at: https://www.ema.europa.eu/documents/assessment-report/movymia-epar-public-assessment-report_en.pdf 3. Brixen KT et al. Basic Clin Pharmacol Toxicol. 200494(6):260–70. 4. Lyman GH et al. N Engl J Med. 2018378(21):2036–2044. 5. Janjigian YY et al. Future Oncol. 201814(23):2403–2414.* Forsteo® October 2019. 2019/ADV/TER/122H DON’T WAIT UNTIL OSTEOPOROSIS STRIKES AGAIN MOVYMIA®: THE NEW TERIPARATIDE BIOSIMILAR FROM CLONMEL HEALTHCARE Rebuild bone before it breaks again—with Movymia®1 RELIABLE: Movymia®’s quality, safety and efficacy is highly similar to its reference product1,2,* EFFECTIVE: Anabolic MoA effectively rebuilds bone through the stimulation of osteoblasts1,3 AFFORDABLE: Allows more eligible patients to benefit due to its cost advantage4,5 RE-USABLE: One high quality reuseable pen for the entire treatment period1 MOVYMIA 20 MICROGRAMS/80
Each dose of 80 microliters contains 20 micrograms of teriparatide. One cartridge of 2.4 ml of solution contains 600 micrograms of teriparatide (corresponding to 250 micrograms per ml). Presentation: Glass cartridge. Indications: Movymia is indicated in adults. Treatment of
1. Movymia® SmPC. 2. Movymia® EPAR – public assessment report, available at: https://www.ema.europa.eu/documents/assessment-report/movymia-epar-public-assessment-report_en.pdf 3. Brixen KT et al. Basic Clin Pharmacol Toxicol. 200494(6):260–70. 4. Lyman GH et al. N Engl J Med. 2018378(21):2036–2044. 5. Janjigian YY et al. Future Oncol. 201814(23):2403–2414.* Forsteo® October 2019. 2019/ADV/TER/122H
Further concerns highlighted in Medical Council’s Workforce Intelligence Report
For the first time in its Workforce Intelligence Report, the Medical Council is providing a summary of the key risks it has identified as part of the research project. Concern for patient safety is at the core of the five specific risks identified:
• General Division – 34.9% of clinically active doctors in Ireland are on the General Division. This represents a key risk to patient safety, as while there is an increase in the General Division, consultant and specialist posts are not being filled.
The Medical Council has launched the Medical Workforce Intelligence Report for 2021. The report provides a detailed analysis of the Medical Council’s registration data, focusing on demographics of those retaining and withdrawing from the medical register in Ireland. Much of the report highlights deficits in the system and the resulting risks attached to patient safety and care. The Medical Workforce Intelligence Report is a central document in informing workforce planning and improved patient safety in Ireland.
The report contains significant findings, which need to be addressed collaboratively amongst policymakers, educators, planners and employers.
Key Highlights:
• Overall, 21,680 doctors retained their place on the Medical Council’s register in 2021, with 18,424 or 85% of those being clinically active.
• Of the active registered doctors in Ireland, 53% were male and 47% female.
• Over one-third of all clinically active doctors in Ireland are on the General Division of the Register
• The number of new doctors who registered in 2021 was 2,605, which represented a 14% increase on the previous year.
• Of those, 1,717 were international graduates and 888 were Irish graduates.
• 49.8% of NCHDs occupied non-training posts
• 62% of doctors self-reported working more than 40 hours a week,
• Dublin has the largest number of working doctors with 7,426, which equates to 35% of the total.
• Non-Consultant Hospital Doctors (NCHDs) – patient safety is further highlighted as there is a considerable proportion of NCHDs required to perform the duties of hospital consultants
• Reliance on International Medical Graduates (IMGs) – the majority of NCHDs are trained overseas and do not have access to specialist training in Ireland. The health service is over-reliant on IMGs who report being overworked, undervalued, experiencing discrimination and unable to access specialist training. Aside from the individual impact on the doctors, the treatment of IMGs has serious implications for patient safety.
• Non-compliance with European Working Time Directive – in 2021, over one quarter of doctors reported working more than 48 hours a week, in contravention of the EWTD. This has further serious implications for patient safety.
Millions at Risk as HIV Progress Falters
New data from UNAIDS on the global HIV response reveals that during the last two years of COVID-19 and other global crises, progress against the HIV pandemic has faltered, resources have shrunk, and millions of lives are at risk as a result. The new report, In Danger, is being launched ahead of the International AIDS Conference in Montreal, Canada.
Globally the number of new infections dropped only 3.6% between 2020 and 2021, the
smallest annual decline in new HIV infections since 2016. Eastern Europe and central Asia, Middle East and North Africa, and Latin America have all seen increases in annual HIV infections over several years. In Asia and the Pacific – the world’s most populous region –UNAIDS data now shows new HIV infections are rising where they had been falling. Climbing infections in these regions are alarming. In eastern and southern Africa rapid progress from previous years significantly slowed in 2021. There is some
positive news, with notable declines in new HIV infections in western and central Africa and in the Caribbean, but even in these regions, the HIV response is threatened by a tightening resource crunch.
New infections occurred disproportionately among young women and adolescent girls, with a new infection every two minutes in this population in 2021. The gendered HIV impact, particularly for young African women and girls, occurred amidst
• Attrition – Acute doctor shortages within the Irish health system, especially at skilled and experienced consultant level, affect quality of care and can undermine patient safety. In 2021, doctors cited family and personal issues, lack of training opportunities, inadequate resourcing and work conditions as reasons for withdrawing.
The Medical Council highlights five recommendations for action:
• Commencing coordination and collaboration across all key stakeholders by setting up a Planning and Advisory Group to explore and plan workforce strategy
• Undertake a national consultation with individuals, patient groups and medical stakeholders to identify key priorities, issues, and challenges
• Identify priority workforce issues and contributing factors, determined by research and consultation
• Exploring the impact and feasibility or proposed approaches to ensure a fit-forpurpose approach
• The proposed strategy should not be developed in isolation, and health reforms and policies that are underway, including Sláintecare, Regional Health Areas, Healthy Ireland programmes and initiatives, national clinical programmes, and other developments should be considered.

disruption of key HIV treatment and prevention services, millions of girls out of school due to pandemics, and spikes in teenage pregnancies and gender-based violence. In sub-Saharan Africa, adolescent girls and young women are three times as likely to acquire HIV as adolescent boys and young men.
The report also shows that efforts to ensure that all people living with HIV are accessing life-saving antiretroviral treatment are faltering.
Medical Council CEO, Leo Kearns and Dr Suzanne Crowe, Medical Council President
8 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE News
DURABLE AND ROBUST 2,3
DOVATO

PART OF HEALTHY LIVING WITH HIV


THE FASTEST GROWING ORAL REGIMEN FOR PLHIV 1*
WITHOUT TDF, TAF AND ABC
DOVATO is indicated for the treatment of HIV-1 in adults and adolescents above 12 years weighing at least 40 kg, with no known or suspected resistance to the integrase inhibitor class, or lamivudine.
Abridged Prescribing Information Dovato (dolutegravir 50mg/lamivudine 300mg) tablets

See Summary of Product Characteristics (SmPC) before prescribing.
Presentation: Film-coated tablet containing dolutegravir sodium equivalent to 50 mg dolutegravir and 300 mg lamivudine debossed with “SV-137” on one face. Indication: HIV-1 in adults & adolescents above 12 years of age weighing >40kg, with no known or suspected resistance to the integrase inhibitor class, or lamivudine. Dosing: One tablet once daily with or without food. Use an additional 50mg tablet of dolutegravir approximately 12 hours after the dose of Dovato when co-administered with efavirenz, nevirapine, tipranavir/ritonavir, etravirine (without boosted PI), carbamazepine, oxcarbazepine, phenytoin, phenobarbital, St John’s Wort or rifampicin.
Elderly: Limited data in 65+ yrs. Renal impairment: Not recommended in patients with creatinine clearance < 30 mL/min. For patients with a sustained creatinine clearance between 30 and 49 mL/min see SmPC section 4.4. Hepatic impairment: Caution in severe hepatic impairment (Child-Pugh grade C). Contraindications: Hypersensitivity to any ingredient. Co-administration with substrates of OCT-2 with narrow therapeutic windows, such as fampridine. Special warnings/precautions: Risk of hypersensitivity reactions. Discontinue dolutegravir and other suspect agents immediately. Risks of osteonecrosis, immune reactivation syndrome. Monitor LFTs in Hepatitis B/C co-infection and ensure effective Hepatitis B therapy. Caution with metformin: monitor renal function and consider metformin dose adjustment. Use with etravirine requires boosted PI or increased dose of dolutegravir. Use with Mg/Al-containing antacids requires dosage separation. Use with calcium, multivitamins or iron also requires dosage separation if not taken at the same time with food. Use with cladribine or emtricitabine not recommended. When possible, avoid chronic co-administration of sorbitol or other osmotic acting alcohols (see SmPC
section 4.5). If unavoidable, consider more frequent viral load monitoring. Fertility, pregnancy and lactation: Human fertility - no data; animal fertility - studies indicate no effects. Women of childbearing potential (WOCBP) should be counselled about the potential risk of neural tube defects including consideration of effective contraceptive measures. If a woman plans pregnancy, the benefits and the risks of continuing treatment should be discussed with the patient. The safety and efficacy of a duel regime has not been studied in pregnancy. If a pregnancy is confirmed in the first trimester while on Dovato, the benefits and risks of continuing Dovato versus switching to another antiretroviral regimen should be discussed with the patient taking the gestational age and the critical time period of neural tube defect development into account (see SmPC section 4.6). There have been reports of mitochondrial dysfunction in HIV-negative infants exposed in utero and/or post-natally to nucleoside analogues. Do not breast-feed. Side effects: See SmPC for full details. Headache, GI disturbance, insomnia, abnormal dreams, depression, anxiety, dizziness, somnolence, rash, pruritus, alopecia, fatigue, arthralgia, myalgia, hypersensitivity, completed suicide, suicidal ideation or suicide attempt, panic attack, hepatitis, blood dyscrasias, acute hepatic failure, pancreatitis, angioedema, rhabdomyolysis, lactic acidosis, peripheral neuropathy. Elevations of bilirubin, ALT, AST and CPK. MA Nr: EU/1/19/1370/001. MA holder: ViiV Healthcare BV, Van Asch van Wijckstraat 55H, 3811 LP Amersfoort, Netherlands. Legal Category: POM A. Date of preparation of API: February 2022. Code: PI-6305. Further information available from GlaxoSmithKline, 12 Riverwalk, Citywest, Business Campus, Dublin 24. Tel: 01-4955000.
Adverse events should be reported directly to the Health Products Regulatory Authority (HPRA) on their website: www.hpra.ie. Adverse events should also be reported to GlaxoSmithKline on 1800 244 255.
*From November 2020 through October 2021, over 39 markets and territories with data sources available.
References: 1. Data on file. Regimen market share growth November 2020-October 2021. REF-146400. ViiV Healthcare group of companies. Research Triangle Park, NC. 2. Cahn P, Sierra Madero J, Arribas JR, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naïve adults with HIV-1 infection. AIDS. 2022;36(1): 39-48. doi:10.1097/QAD.0000000000003070 3. Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine (DTG/3TC) versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with HIV-1: results through week 144 from the phase 3, non-inferiority TANGO randomized trial. Clin Infect Dis. 2022;ciac036 and suppl 1-18. doi:10.1093/cid/ciac036


DOVATO is owned by or licensed to the ViiV Healthcare group of companies. ©2022 ViiV Healthcare group of companies or its licensor.
Date of preparation: March 2022 PM-IE-DLL-JRNA-220001

For your patients living with HIV, make DOVATO a part of their healthy future.
Virological suppression is the first step to achieving
Increased Awareness Calls on Prescription Medicines
• Analgesic medicines – 33,542 units detained (105,769 units detained Jan – June 2021).
• 43,707 units of Covid-19 medicines detained (23,172 units detained Jan – June 2021).
• 287 websites, e-commerce listings and/or social media pages amended or shutdown (167 Jan – June 2021).
According to Grainne Power, Director of Compliance with the HPRA, the reduction of detentions in the first half of 2022 cannot be linked to any one factor alone.

The Health Products Regulatory Authority (HPRA) has stated that whilst the volume of detained illegal medicines in the first half of 2022 has fallen significantly compared to the same period in 2021, a substantial amount of product is still being sourced online which poses risks to people’s health. The HPRA enforcement section detained
nearly half a million (486,088) dosage units of falsified and other illegal medicines between January and June 2022 (895,591 for the first half of 2021). Announcing its mid-year update of detention figures, the HPRA reminded the public of the health risks associated with prescription medicines purchased online from unauthorised sources.
In the first six months of 2022, the main categories of illegal products detained included sedatives (28%), anabolic steroids (12%), erectile dysfunction medicines (9%) and analgesics (7%). The breakdown is:
• Sedative medicines – 137,587 units detained (434,157 units detained Jan – June 2021).
• Anabolic steroids – 59,764 units detained (59,750 units detained Jan – June 2021).
• Erectile dysfunction - 41,635 units were detained (56,878 units detained Jan – June 2021).
¤3.2m for Degenerative Retinal Diseases
“Although the volume of detentions has decreased, we are still observing significant levels of potent, prescription medicines that are being illegally supplied into Ireland. These levels remain very concerning with so many people prepared to take a chance in ordering prescription medicines online. When you acquire medicines from unregulated sources, you simply have no idea what you are getting. This isn’t merely about people wasting money on falsified or counterfeit products, it is also about the very real dangers of significant side effects, of using a product without supervision where there is no guarantee of what it contains and of experiencing interactions with other medicines being taken. All of these risks have the potential to make your condition worse or cause serious harm to health,” Ms Power says.
The project (EYE-D) will target diseases that can result in severe loss of vision and are estimated to affect 224,000 people in Ireland, and 40 million people worldwide.
The funding for EYE-D was announced today by Simon Harris, Minister for Further and Higher Education, Research, Innovation and Science. Science Foundation Ireland will provide ¤1.6 million
funding to EYE-D, matched by project partners.
The proposed partnership involves separate collaborations with three companies: Roche, Disarm/Eli Lilly, private ophthalmology clinic, Progressive Vision Research, and the charity Fighting Blindness Ireland.
Cumulatively, these groups will fund ¤1.6 million to advance various research programmes focused on identifying the underlying causes of degenerative eye diseases.
Professor Matthew Campbell said, “We are excited about the potential developments that will emerge from this grant. Spearheading a project with a cumulative budget of ¤3.2 million will allow us to make a major impact on the international stage of vision research. In addition, our research endeavours put us in a perfect position to identify the cause of some of the most common forms of blindness.”
Co-PI, Professor Sarah Doyle, added, “This funding will allow us to build on the major successes our group has had in understanding degenerative eye diseases. Added to this, we can now recruit the most talented group of scientists internationally and place Ireland at the forefront of vision research.”

Professors Matthew Campbell and Sarah Doyle, Trinity College Dublin
Grainne Power, Director of Compliance, Health Products Regulatory Authority
“Although the volume of detentions has decreased, we are still observing significant levels of potent, prescription medicines that are being illegally supplied into Ireland”
10 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE News
relieve the discomfort

ABBREVIATED PRESCRIBING INFORMATION. Proctosedyl ointment. Each gram of ointment contains 5 mg of hydrocortisone, 5 mg of cinchocaine hydrochloride, 10 mg of aesculin and 10 mg of framycetin sulphate. Presentation: Yellowish white, translucent, homogeneous ointment. Indications: In the local management of pain, pruritus and inflammation associated with internal or external haemorrhoids, and such haemorrhoidal complications as fissures, proctitis, perianal eczema, and post-operative states. Dosage: Application to external surface or by means of the cannula into the rectum, twice daily and after each bowel movement. Treatment should last for a week. Method of administration: Topical, intrarectal and perianal. Contraindications: Use in the presence of untreated infections of viral, bacterial, tuberculous, parasitic or fungal origin. Use in patients hypersensitive to the active ingredient or any of the excipients. Warnings and precautions: Continuous treatment for longer than three weeks should be avoided in patients under the age of three years because of the possibility of adrenocortical suppression and growth retardation. Continuous application without interruption will result in local atrophy of the skin, striae, and superficial vascular dilation. Prolonged use of an anti-infective may result in the development of super-infection due to organisms, including fungi, resistant to that anti-infective. May cause local skin reactions (e.g. contact dermatitis). Before prescribing the product any potential malignancies should be excluded. Pheochromocytoma crisis, which can be fatal, has been reported after administration of corticosteroids. Corticosteroids should only be administered to patients with suspected or identified pheochromocytoma after an appropriate risk/benefit evaluation. Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids. Interactions: Co-treatment with CYP3A inhibitors, including cobicistat-containing products, is expected to increase the risk of systemic side-effects. The combination should be avoided unless the benefit outweighs the increased risk of systemic corticosteroid side effects, in which case patients should be monitored for systemic corticosteroid side effects. Pregnancy and lactation: This product should not be used in pregnancy or lactation unless considered essential by the physician. Driving and operation of machinery: Not applicable. Undesirable effects: Itching, pain or rash. Refer to Summary of Product Characteristics for other undesirable effects. Adverse events should be reported via HPRA Pharmacovigilance, website: www.hpra.ie. Pack size: 30 g. Marketing authorisation holder: Opella Healthcare France SAS T/A Sanofi, 82 Avenue Raspail, 94250 Gentilly, France. Marketing authorisation number: PA23180/002/001. Medicinal product subject to medical prescription. Last revision date: May 2022. Distributed in Ireland by Clonmel Healthcare Ltd, Waterford Road, Clonmel, Co. Tipperary, Ireland. 2022/ADV/PRO/120H


For the local management of pain, pruritus and inflammation associated with internal or external haemorrhoids, and such haemorrhoidal complications as fissures, proctitis, perianal eczema, and post-operative states.
New Network Launched for Pharmacists
We need to curb, and hopefully reverse, the growing trend of antimicrobial resistance in order to have effective antimicrobials to enable us to deliver an optimal level of healthcare. We need, as healthcare workers, to feel empowered to play our part by learning how to incorporate antimicrobial stewardship in our day to day practice. To assist all pharmacists in doing this a new network has been established the Pharmacist Antimicrobial Stewardship Network (PAMS-net)
Join the network
The PAMS-net webpage is hosted on the IIOP website. It provides a discussion forum and resources section. Members receive updates through the network and network events are organised regularly.
3 reasons to join the PAMS-net
The vision of the PAMS net is a more joined up approach to AMS across the pharmacy profession. Pharmacists across all settings have guided the development of this vision through participation in focus groups, feedback sessions and membership of the PAMS net working group.
knowledge and learning in the area of antimicrobial stewardship.
All pharmacists with an interest in responsible antimicrobial use are welcome to join this network. The network aims to bring together pharmacists from a diverse range of settings including community, hospital, education, researchers, other HSE and national bodies. The network welcomes pharmacists from all professional backgrounds, those who are considered experienced antimicrobial pharmacists and those who wish to further their knowledge.
antimicrobials are kept effective for future generations.
1. Networking & support through our online discussion forum and educational network events
As healthcare workers we all have a role to play as antimicrobial stewards, as detailed in the recently published HSE antimicrobial stewardship (AMS) guidance for all healthcare settings. We need to curb, and hopefully reverse, the growing trend of antimicrobial resistance in order to have effective antimicrobials to enable us to deliver an optimal level of healthcare. We need, as healthcare workers, to feel empowered to play our part by learning how to incorporate antimicrobial stewardship in our day-to-day practice. To assist all pharmacists in doing this a new network has been established the Pharmacist Antimicrobial Stewardship Network (PAMS-net)
Antimicrobial stewardship
PAMS-net vision
The functions of the network are:
2. CPD through our resources and educational network events
The PAMS-net launched on the 15th August 2022. It is a collaboration between the HSE Antimicrobial Resistance and Infection Control (AMRIC) team and the Irish Institute of Pharmacy (IIOP) and is open to all registered pharmacists.
• To share knowledge, information, learning and experience in AMS, both across and within sectors
• To provide a discussion forum for members
• To share AMS work and foster creativity and innovation in AMS
• To assist in the provision of CPD for pharmacists with an interest in AMS
Join the network
The vision of the PAMS-net is a more joined up approach to AMS across the pharmacy profession. Pharmacists across all settings have guided the development of this vision through participation in focus groups, feedback sessions and membership of the PAMS-net working group.
3. Protect antimicrobials for future generations! Optimising antimicrobial use in all patients reduces patient harms and it limits the development of antimicrobial resistance so that antimicrobials are kept effective for future generations.
The PAMS net webpage is hosted on the IIOP website. It provides a discussion forum and resources section. Members receive updates through the network and network events are organised regularly.
The functions of the network are:

• To share knowledge, information, learning and experience in AMS, both across and within sectors
3 reasons to join the PAMS net
1. Networking & support through our online discussion forum and educational network events
To become a member of the network go to the “Courses and Events” page on the IIOP website and click the “Forum” tab (see below).

The network is a fantastic opportunity for pharmacists across all settings to collaborate in education, promotion and implementation of best practice so we can achieve our shared goal of enabling responsible use of antimicrobials in all patients and limiting the emergence of antimicrobial resistance (AMR). Through the PAMS-net discussion forum and member events pharmacists can come together to provide support and advice to one another. Pharmacists across different settings can share knowledge and learning in the area of antimicrobial stewardship.
• To provide a discussion forum for members
2. CPD through our resources and educational network events
The PAMS-net launched on the 15th August 2022. It is a collaboration between the HSE Antimicrobial Resistance and Infection Control (AMRIC) team and the Irish Institute of Pharmacy (IIOP) and is open to all registered pharmacists.
The network is a fantastic opportunity for pharmacists across all settings to collaborate in education, promotion and implementation of best practice so we can achieve our shared goal of enabling responsible use of antimicrobials in all patients and limiting the emergence of antimicrobial resistance (AMR). Through the PAMS-net discussion forum and member events pharmacists can come together to provide support and advice to one another. Pharmacists across different settings can share
Antimicrobial stewardship (AMS) can encompass any action that promotes responsible use of antimicrobials. Some examples of AMS in pharmacy practice include: checking if antibiotic prescriptions align with antibiotic prescribing guidelines; recognising when an antibiotic agent, dose or duration is not in line with guidelines and highlighting this to the prescriber; providing advice when a patient presents with a self-limiting or viral infection.
Antimicrobial stewardship
Overuse and misuse of antimicrobials causes increasing AMR as well as other patient harms. Over the last 20 to 30 years we have seen many antimicrobials become less effective or even not effective at all for treatment of some infections due to AMR. AMS is vital in limiting and potentially reversing the development of AMR so that
• To share AMS work and foster creativity and innovation in AMS
3. Protect antimicrobials for future generations! Optimising antimicrobial use in all patients reduces patient harms and it limits the development of antimicrobial resistance so that antimicrobials are kept effective for future generations.
To learn more about antimicrobial stewardship and view national antibiotic prescribing guidelines and resources visit antibioticprescribing.ie.
• To assist in the provision of CPD for pharmacists with an interest in AMS
Keeping antibiotics effective for future generations -it’s everyone’s responsibility
All pharmacists with an interest in responsible antimicrobial use are welcome to join this network. The network aims to bring together pharmacists from a diverse range of settings including community, hospital, education, researchers, other HSE and national bodies. The network welcomes pharmacists from all professional backgrounds, those who are considered experienced antimicrobial pharmacists and those who wish to further their knowledge.

To become a member of the network go to the “Courses and Events” page on the IIOP website and click the “Forum” tab.
Antimicrobial stewardship (AMS) can encompass any action that promotes responsible use of antimicrobials. Some examples of AMS in pharmacy practice include: checking if antibiotic prescriptions align with antibiotic prescribing guidelines; recognising when an antibiotic agent, dose or duration is not in line with guidelines and highlighting this to the prescriber; providing advice when a patient presents with a self-limiting or viral infection.
Women in Leadership Event
The Pharmaceutical Manufacturers’ Institute (PMI) are hosted a Women in Leadership lunch which takes place on Thursday, October 13th in Kingswood House Hotel, Kingswood. Following on from the previous Women in Leadership event in May where Eimear Caslin was guest MC; for this event the team are joined by Melissa Fisher, General Manager with Viatris.
Overuse and misuse of antimicrobials causes increasing AMR as well as other patient harms. Over the last 20 to 30 years we have seen many antimicrobials become less effective or even not effective at all for treatment of some infections due to AMR. AMS is vital in limiting and potentially reversing the development of AMR so that antimicrobials are kept effective for future generations.
PAMS-net vision
Guest speaker is Lisa Clancy, Vice President of the Paralympics Olympics Ireland and Owner of Clansult – a consultancy agency. She has been to the fore in leadership across Irish sport for a number of years and in a number of different roles, and is currently creating a pathway for leaders of the future through the Women in Sport Leadership Programme. A passionate advocate for mentorship and driving women in leadership, Lisa works with companies, athletes & senior executives to help them reach their full potential and make a strategic impact.
A new network for pharmacists just launched - Pharmacist Antimicrobial Stewardship Network (PAMS-net)
12 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE News
recently published HSE antimicrobial stewardship (AMS) guidance for all healthcare settings
Pemetrexed 25 mg/mL
Launching
Launching
A room temperature stable ready-to-dilute concentrate
Pemetrexed 25 mg/mL
Pemetrexed 25 mg/mL
Consult the Summary of Product Characteristics for further information. Additional information is available upon request Pemetrexed Fresenius Kabi 25mg/ml concentrate for solution for infusion. Active ingredients: Pemetrexed diacid. One vial of 4ml concentrate contains 100mg pemetrexed. One vial of 20ml concentrate contains 500mg pemetrexed. One vial of 40ml concentrate contains 1,000mg pemetrexed. Excipients with known effect – 4ml vial - 964mg hydroxypropylbetadex, 20ml vial – 4820mg hydroxypropylbetadex, 40ml vial – 9640mg hydroxypropylbetadex. Indications: In combination with cisplatin - treatment of chemotherapy naïve patients with unresectable malignant pleural mesothelioma, first line treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology. Monotherapy – Maintenance treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology in patients whose disease has not progressed immediately following platinum-based chemotherapy, second line treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology. Posology and method of administration: Only administer under the supervision of a physician qualified in the use of anti-cancer chemotherapy. Administer as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. See SmPC for further information on handling, administering and dilution. In combination with cisplatin – Recommended dose is 500mg/m2 of body surface area (BSA) administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. Recommended dose of cisplatin is 75mg/m2 BSA infused over two hours approximately 30 minutes after completion of the pemetrexed infusion on the first day of each 21-day cycle. Patients must receive adequate anti-emetic treatment and appropriate hydration prior to and/or after receiving cisplatin (see also cisplatin SmPC). As single agent – In the treatment of non-small cell lung cancer after prior chemotherapy, recommended dose is 500mg/m2 BSA administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. Pre-medication regimen: To reduce the incidence and severity of skin reactions, a corticosteroid should be given the day prior to, on the day of, and the day after pemetrexed administration. Corticosteroid should be equivalent to 4mg of dexamethasone orally twice daily. Patients must also receive vitamin supplementation; oral folic acid or a multivitamin containing folic acid (350 to 1000 micrograms) on a daily basis. At least five doses of folic acid must be taken during the seven days preceding the first dose of pemetrexed and dosing must continue during the full course of therapy and for 21 days after the last dose of pemetrexed. Patients must also receive an intramuscular injection of vitamin B12 (1000 micrograms) in the week preceding the first dose of pemetrexed and once every three cycles thereafter. Subsequent vitamin B12 injections may be given on the same day as pemetrexed. Monitoring: Before each dose complete blood count including differential white cell count (WCC) and platelet count. Prior to each chemotherapy administration blood chemistry tests should be collected to evaluate renal and hepatic function. Before the start of any cycle of chemotherapy, patients are required to have absolute neutrophil count (ANC) ≥ 1500 cells/mm3 and platelets ≥ 100,000 cells/mm3. Creatinine clearance should be ≥ 45 ml/min. Total bilirubin should be ≤ 1.5 times upper limit of normal. Alkaline phosphatase (AP), aspartate aminotransferase (AST or SGOT) and alanine aminotransferase (ALT or SGPT) should be ≤ 3 times upper limit of normal. AP, AST and ALT ≤ 5 times upper limit of normal is acceptable if liver has tumour involvement. Dose adjustments: At the start of a subsequent cycle should be based on nadir haematologic counts or maximum non-haematologic toxicity from the preceding cycle of therapy. Treatment may be delayed to allow sufficient time for recovery. Upon recovery patients should be retreated using the guidelines as follows, which are applicable for pemetrexed Fresenius Kabi used as single agent or in combination with cisplatin. Haematologic toxicities (dose modify both pemetrexed and cisplatin) – Nadir ANC < 500/mm3 and nadir platelets ≥ 50,000/mm3, or nadir platelets < 50,000/mm3 regardless of nadir ANC: 75% of previous dose. Nadir platelets < 50,000/mm3 with bleeding regardless of nadir ANC: 50% of previous dose. Non-haematologic toxicities ≥ Grade 3 (excluding neurotoxicity) - pemetrexed Fresenius Kabi should be withheld until resolution to less than or equal to the patient’s pre-therapy value. Treatment should be resumed accordingly – Any grade 3 or 4 toxicities except mucositis, or any diarrhoea requiring hospitalisation or grade 3 or 4 diarrhoea: 75% of previous dose (mg/m2) of pemetrexed and cisplatin. Grade 3 or 4 mucositis: 50% of previous pemetrexed dose (mg/m2) and 100% of previous cisplatin dose (mg/m2). Neurotoxicity – Patients should discontinue therapy if Grade 3 or 4 neurotoxicity is observed. CTC Grade 0-1: 100% of previous pemetrexed and cisplatin dose (mg/m2). CTC Grade 2: 100% of previous pemetrexed dose (mg/m2) and 50% of previous cisplatin dose (mg/m2). Discontinue treatment with pemetrexed Fresenius Kabi if a patient experiences any haematologic or non-haematologic Grade 3 or 4 toxicity after 2 dose reductions or immediately if Grade 3 or 4 neurotoxicity is observed. Elderly – No dose reductions other than those recommended for all patients are necessary.

PRESCRIBING INFORMATION: Consult the Summary of Product Characteristics for further information. Additional information is available upon request Pemetrexed Fresenius Kabi 25mg/ml concentrate for solution for infusion. Active ingredients: Pemetrexed diacid. One vial of 4ml concentrate contains 100mg pemetrexed. One vial of 20ml concentrate contains 500mg pemetrexed. One vial of 40ml concentrate contains 1,000mg pemetrexed. Excipients with known effect – 4ml vial - 964mg hydroxypropylbetadex, 20ml vial – 4820mg hydroxypropylbetadex, 40ml vial – 9640mg hydroxypropylbetadex. Indications: In combination with cisplatin - treatment of chemotherapy naïve patients with unresectable malignant pleural mesothelioma, first line treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology. Monotherapy – Maintenance treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology in patients whose disease has not progressed immediately following platinum-based chemotherapy, second line treatment of locally advanced or metastatic non-small cell lung cancer other than predominantly squamous cell histology. Posology and method of administration: Only administer under the supervision of a physician qualified in the use of anti-cancer chemotherapy. Administer as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. See SmPC for further information on handling, administering and dilution. In combination with cisplatin – Recommended dose is 500mg/m2 of body surface area (BSA) administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. Recommended dose of cisplatin is 75mg/m2 BSA infused over two hours approximately 30 minutes after completion of the pemetrexed infusion on the first day of each 21-day cycle. Patients must receive adequate anti-emetic treatment and appropriate hydration prior to and/or after receiving cisplatin (see also cisplatin SmPC). As single agent – In the treatment of non-small cell lung cancer after prior chemotherapy, recommended dose is 500mg/m2 BSA administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. Pre-medication regimen: To reduce the incidence and severity of skin reactions, a corticosteroid should be given the day prior to, on the day of, and the day after pemetrexed administration. Corticosteroid should be equivalent to 4mg of dexamethasone orally twice daily. Patients must also receive vitamin supplementation; oral folic acid or a multivitamin containing folic acid (350 to 1000 micrograms) on a daily basis. At least five doses of folic acid must be taken during the seven days preceding the first dose of pemetrexed and dosing must continue during the full course of therapy and for 21 days after the last dose of pemetrexed. Patients must also receive an intramuscular injection of vitamin B12 (1000 micrograms) in the week preceding the first dose of pemetrexed and once every three cycles thereafter. Subsequent vitamin B12 injections may be given on the same day as pemetrexed. Monitoring: Before each dose complete blood count including differential white cell count (WCC) and platelet count. Prior to each chemotherapy administration blood chemistry tests should be collected to evaluate renal and hepatic function. Before the start of any cycle of chemotherapy, patients are required to have absolute neutrophil count (ANC) ≥ 1500 cells/mm3 and platelets ≥ 100,000 cells/mm3. Creatinine clearance should be ≥ 45 ml/min. Total bilirubin should be ≤ 1.5 times upper limit of normal. Alkaline phosphatase (AP), aspartate aminotransferase (AST or SGOT) and alanine aminotransferase (ALT or SGPT) should be ≤ 3 times upper limit of normal. AP, AST and ALT ≤ 5 times upper limit of normal is acceptable if liver has tumour involvement. Dose adjustments: At the start of a subsequent cycle should be based on nadir haematologic counts or maximum non-haematologic toxicity from the preceding cycle of therapy. Treatment may be delayed to allow sufficient time for recovery. Upon recovery patients should be retreated using the guidelines as follows, which are applicable for pemetrexed Fresenius Kabi used as single agent or in combination with cisplatin. Haematologic toxicities (dose modify both pemetrexed and cisplatin) – Nadir ANC < 500/mm3 and nadir platelets ≥ 50,000/mm3, or nadir platelets < 50,000/mm3 regardless of nadir ANC: 75% of previous dose. Nadir platelets < 50,000/mm3 with bleeding regardless of nadir ANC: 50% of previous dose. Non-haematologic toxicities ≥ Grade 3 (excluding neurotoxicity) - pemetrexed Fresenius Kabi should be withheld until resolution to less than or equal to the patient’s pre-therapy value. Treatment should be resumed accordingly – Any grade 3 or 4 toxicities except mucositis, or any diarrhoea requiring hospitalisation or grade 3 or 4 diarrhoea: 75% of previous dose (mg/m2) of pemetrexed and cisplatin. Grade 3 or 4 mucositis: 50% of previous pemetrexed dose (mg/m2) and 100% of previous cisplatin dose (mg/m2). Neurotoxicity – Patients should discontinue therapy if Grade 3 or 4 neurotoxicity is observed. CTC Grade 0-1: 100% of previous pemetrexed and cisplatin dose (mg/m2). CTC Grade 2: 100% of previous pemetrexed dose (mg/m2) and 50% of previous cisplatin dose (mg/m2). Discontinue treatment with pemetrexed Fresenius Kabi if a patient experiences any haematologic or non-haematologic Grade 3 or 4 toxicity after 2 dose reductions or immediately if Grade 3 or 4 neurotoxicity is observed. Elderly – No dose reductions other than those recommended for all patients are necessary.

Dublin, Ireland
Paediatric population – No relevant use in malignant pleural mesothelioma and non-small cell lung cancer. Renal impairment – (Standard Cockcroft and Gault formula or GFR measured Tc99m-DPTA serum clearance method). In clinical studies patients with creatinine clearance of ≥ 45ml/min required no dose adjustments other than those recommended for all patients. Not recommended in patients with creatinine clearance below 45ml/min. Hepatic impairment – Patients with hepatic impairment have not been specifically studied. Contraindications: Hypersensitivity to the active substance or to any of the excipients, breast-feeding, concomitant yellow fever vaccine. Special warnings and precautions for use: Monitor patients for myelosuppression. Pemetrexed should not be given until ANC returns to ≥ 1500 cells/mm3 and platelet count to ≥ 100,000 cells/mm3. Dose reductions for subsequent cycles based on nadir ANC, platelet count and maximum non-haematologic toxicity seen from previous cycle. All patients must be instructed to take folic acid and vitamin B12 as a prophylactic measure to reduce treatment-related toxicity. Pre-treatment with dexamethasone (or equivalent) can reduce the incidence and severity of skin reactions. Not recommended in patients with creatinine clearance of < 45ml/min. Patients with mild to moderate renal insufficiency (creatinine clearance 45-79ml/min) should avoid taking NSAIDs, or acetylsalicylic acid (> 1.3g daily) for 2 days before, on the day of, and 2 days following pemetrexed administration. In patients with mild to moderate renal insufficiency NSAIDs with long elimination half-lives should be interrupted for at least 5 days prior to, on the day of, and at least 2 days following pemetrexed administration. Patients should be regularly monitored for acute tubular necrosis, decreased renal function and signs and symptoms of nephrogenic diabetes insipidus. Drainage of third space fluid collection prior to pemetrexed treatment should be considered but may not be necessary. Patients should receive adequate antiemetic treatment and appropriate hydration prior to and/or after receiving treatment. Serious cardiovascular and cerebrovascular events have been uncommonly reported during clinical studies with pemetrexed, usually when given in combination with another cytotoxic agent. Concomitant use of live attenuated vaccines is not recommended. Sexually mature males are advised not to father a child during treatment and up to 6 months thereafter. Contraceptive measures or abstinence are recommended. Possibility of irreversible infertility; men advised to seek counselling on sperm storage before starting treatment. Women of childbearing potential must use effective contraception during treatment with pemetrexed. Cases of radiation pneumonitis reported in patients treated with radiation either prior, during or subsequent to pemetrexed therapy; particular attention should be paid to these patients and caution with other radiosensitising agents. Cases of radiation recall reported in patients who received radiotherapy weeks or years previously. Accumulation of cyclodextrins may occur in patients with moderate to severe renal dysfunction. May cause fatigue, patients should be cautioned against driving or operating machinery if this occurs. Undesirable effects: (Associated with pemetrexed monotherapy or in combination with cisplatin) Infection, pharyngitis, sepsis, dermo-hypodermitis, neutropenia, leukopenia, decreased haemoglobin, febrile neutropenia, decreased platelet count, pancytopenia, autoimmune haemolytic anaemia, hypersensitivity, anaphylactic shock, dehydration, taste disorder, peripheral motor neuropathy, peripheral sensory neuropathy, dizziness, cerebrovascular accident, ischemic stroke, intracranial haemorrhage, conjunctivitis, dry eye, increased lacrimation, keratoconjunctivitis sicca, eyelid oedema, ocular surface disease, cardiac failure, arrhythmia, angina, myocardial infarction, coronary artery disease, supraventricular arrhythmia, peripheral ischaemia, pulmonary embolism, interstitial pneumonitis, stomatitis, anorexia, vomiting, diarrhoea, nausea, dyspepsia, constipation, abdominal pain, rectal haemorrhage, gastrointestinal haemorrhage, intestinal perforation, oesophagitis, colitis, increased alanine aminotransferase, increased aspartate aminotransferase, hepatitis, rash, skin exfoliation, hyperpigmentation, pruritis, erythema multiforme, alopecia, urticaria, erythema, Stevens-Johnson syndrome, toxic epidermal necrolysis, pemphigoid, dermatitis bullous, acquired epidermolysis bullosa, erythematous oedema, pseudocellulitis, dermatitis, eczema, prurigo, decreased creatinine clearance, increased blood creatinine, renal failure, decreased GFR, nephrogenic diabetes insipidus, renal tubular necrosis, fatigue, pyrexia, pain, oedema, chest pain, mucosal inflammation, increased GGT, radiation oesophagitis, radiation pneumonitis, recall phenomenon. Legal Category: POM Marketing Authorisation Numbers: EU/1/16/1115/003, EU/1/16/1115/004, EU/1/16/1115/005. Marketing Authorisation Holder: Fresenius Kabi Deutschland GmbH, Else-Kroner-Straße 1, 61352 Bad Homburg v.d.Höhe, Germany. Further Information: See the SmPC for further details.
27/04/202114:42
27/04/2021 14:42
Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra.ie Adverse events should also be reported to Fresenius Kabi Limited via email Pharmacovigilance GB@Fresenius Kabi.com. Date of Preparation: May 2021 Job code: API/Pemetrexed/02
Paediatric population – No relevant use in malignant pleural mesothelioma and non-small cell lung cancer. Renal impairment – (Standard Cockcroft and Gault formula or GFR measured Tc99m-DPTA serum clearance method). In clinical studies patients with creatinine clearance of ≥ 45ml/min required no dose adjustments other than those recommended for all patients. Not recommended in patients with creatinine clearance below 45ml/min. Hepatic impairment – Patients with hepatic impairment have not been specifically studied. Contraindications: Hypersensitivity to the active substance or to any of the excipients, breast-feeding, concomitant yellow fever vaccine. Special warnings and precautions for use: Monitor patients for myelosuppression. Pemetrexed should not be given until ANC returns to ≥ 1500 cells/mm3 and platelet count to ≥ 100,000 cells/mm3. Dose reductions for subsequent cycles based on nadir ANC, platelet count and maximum non-haematologic toxicity seen from previous cycle. All patients must be instructed to take folic acid and vitamin B12 as a prophylactic measure to reduce treatment-related toxicity. Pre-treatment with dexamethasone (or equivalent) can reduce the incidence and severity of skin reactions. Not recommended in patients with creatinine clearance of < 45ml/min. Patients with mild to moderate renal insufficiency (creatinine clearance 45-79ml/min) should avoid taking NSAIDs, or acetylsalicylic acid (> 1.3g daily) for 2 days before, on the day of, and 2 days following pemetrexed administration. In patients with mild to moderate renal insufficiency NSAIDs with long elimination half-lives should be interrupted for at least 5 days prior to, on the day of, and at least 2 days following pemetrexed administration. Patients should be regularly monitored for acute tubular necrosis, decreased renal function and signs and symptoms of nephrogenic diabetes insipidus. Drainage of third space fluid collection prior to pemetrexed treatment should be considered but may not be necessary. Patients should receive adequate antiemetic treatment and appropriate hydration prior to and/or after receiving treatment. Serious cardiovascular and cerebrovascular events have been uncommonly reported during clinical studies with pemetrexed, usually when given in combination with another cytotoxic agent. Concomitant use of live attenuated vaccines is not recommended. Sexually mature males are advised not to father a child during treatment and up to 6 months thereafter. Contraceptive measures or abstinence are recommended. Possibility of irreversible infertility; men advised to seek counselling on sperm storage before starting treatment. Women of childbearing potential must use effective contraception during treatment with pemetrexed. Cases of radiation pneumonitis reported in patients treated with radiation either prior, during or subsequent to pemetrexed therapy; particular attention should be paid to these patients and caution with other radiosensitising agents. Cases of radiation recall reported in patients who received radiotherapy weeks or years previously. Accumulation of cyclodextrins may occur in patients with moderate to severe renal dysfunction. May cause fatigue, patients should be cautioned against driving or operating machinery if this occurs. Undesirable effects: (Associated with pemetrexed monotherapy or in combination with cisplatin) Infection, pharyngitis, sepsis, dermo-hypodermitis, neutropenia, leukopenia, decreased haemoglobin, febrile neutropenia, decreased platelet count, pancytopenia, autoimmune haemolytic anaemia, hypersensitivity, anaphylactic shock, dehydration, taste disorder, peripheral motor neuropathy, peripheral sensory neuropathy, dizziness, cerebrovascular accident, ischemic stroke, intracranial haemorrhage, conjunctivitis, dry eye, increased lacrimation, keratoconjunctivitis sicca, eyelid oedema, ocular surface disease, cardiac failure, arrhythmia, angina, myocardial infarction, coronary artery disease, supraventricular arrhythmia, peripheral ischaemia, pulmonary embolism, interstitial pneumonitis, stomatitis, anorexia, vomiting, diarrhoea, nausea, dyspepsia, constipation, abdominal pain, rectal haemorrhage, gastrointestinal haemorrhage, intestinal perforation, oesophagitis, colitis, increased alanine aminotransferase, increased aspartate aminotransferase, hepatitis, rash, skin exfoliation, hyperpigmentation, pruritis, erythema multiforme, alopecia, urticaria, erythema, Stevens-Johnson syndrome, toxic epidermal necrolysis, pemphigoid, dermatitis bullous, acquired epidermolysis bullosa, erythematous oedema, pseudocellulitis, dermatitis, eczema, prurigo, decreased creatinine clearance, increased blood creatinine, renal failure, decreased GFR, nephrogenic diabetes insipidus, renal tubular necrosis, fatigue, pyrexia, pain, oedema, chest pain, mucosal inflammation, increased GGT, radiation oesophagitis, radiation pneumonitis, recall phenomenon. Legal Category: POM Marketing Authorisation Numbers: EU/1/16/1115/003, EU/1/16/1115/004, EU/1/16/1115/005. Marketing Authorisation Holder: Fresenius Kabi Deutschland GmbH, Else-Kroner-Straße 1, 61352 Bad Homburg v.d.Höhe, Germany. Further Information: See the SmPC for further details. Adverse events should be reported. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra.ie. Adverse events should also be reported to Fresenius Kabi Limited via email Pharmacovigilance GB@Fresenius Kabi.com. Date of Preparation: May 2021 Job code: API/Pemetrexed/02
Website: www.fresenius-kabi.com/ie/ Email: FK-enquiries.ireland@fresenius-kabi.com Phone: +353 (0)1 841 3030

Website: www.fresenius-kabi.com/ie/
Email: FK-enquiries.ireland@fresenius-kabi.com

Phone: +353 (0)1 841 3030
Date of Prep: May 2021
Ref: IV/ONCO/PEME/01/2021
Date of Prep: May 2021
Ref: IV/ONCO/PEME/01/2021
A multi-targeted anti-cancer antifolate agent In three presentations Pemetrexed 25 mg/mL A room temperature stable ready-to-dilute concentrate 19763_FK_Pemetrexed_A4_4pp_Brochure_v4_Option_A.indd 1 27/04/2021 14:42
Fresenius Kabi Limited Fresenius Kabi Ireland Unit 3B Fingal Bay, Balbriggan, Co.
19763_FK_Pemetrexed_A4_4pp_Brochure_v4_Option_A.indd
A multi-targeted anti-cancer antifolate agent In three presentations
19763_FK_Pemetrexed_A4_4pp_Brochure_v4_Option_A.indd 1
Fresenius Kabi Limited Fresenius Kabi Ireland Unit 3B Fingal Bay, Balbriggan,
19763_FK_Pemetrexed_A4_4pp_Brochure_v4_Option_A.indd 27/04/202114:42
Migraine
Phases of Migraine - Before, During and After an Attack
Written by Hazel Breen Communications and Information Officer, Migraine Association of Ireland

In addition, the MAI have seen a significant spike in vestibular migraine diagnosis since the beginning of 2022, mostly in women.
• Slurring of speech
• Muscular weakness
• Loss of co-ordination
• Confusion
Migraine is a complex neurological condition that affects over 600,000 people in Ireland alone, that’s 1215% of our population! The Global Burden of Disease states that migraine is the 2nd most disabling disease in the world in their most recent figures (over an eighth of the global population). Migraine is often considered ‘just a headache’ which could not be further from the truth. “There are many types of migraine, many triggers, and many symptoms before, during and after a migraine attack that many people and health care professionals are unaware of.” – Hazel Breen, Communications Officer, Migraine Association of Ireland.
Migraine can be episodic or chronic. Episodic migraine lasts from 4-72 hours whereas chronic migraine can last 15 days or more per month! Before delving into the phases of migraine, let’s look at the different types of migraine and some of their symptoms first:
1. Migraine without aura - The majority of migraine sufferers have migraine without aura. The most common symptoms of migraine with aura are an intense throbbing headache, usually on one side of the head, worsened by movement and lasts anywhere from 4-72 hours.
2. Migraine with aura - refers to a range of neurological disturbances that occur before the headache begins, usually lasting about 20-60 minutes. Roughly 20% of people with migraine experience ‘aura’ in addition to some or all the symptoms.

3. Vestibular migraine - or migraine association vertigo (MAV) is a disorder which causes problems with the coordination of the sensory information sent to your brain from your eyes. Up to 40% of all migraine sufferers experience some vestibular symptoms during their lifetime, such as dizziness, sensitivity to light/ sound and stiffness in the neck. However, people who suffer with vestibular migraine specifically, experience a whole gamut of other symptoms, including but not limited to:
• Vertigo
• Other motion problems in the head, eyes, or body
• Diminished eye focus
• Muscle spasms in the upper spine
• And much more. Visit migraine. ie to see the full list of symptoms for each type of migraine condition
4. Hemiplegic migraine - is a rare type of migraine where the person experience many of usual migraine symptoms, but may also suffer from temporary numbness, weakness or even paralysis on one side of their body. The word hemiplegia comes from the Greek language where ‘hemi’ means half and ‘plegia’ from the words ‘plege’ or ‘plessein’, means stroke or meaning to strike. Unlike a stroke, the symptoms of hemiplegic migraine begin slowly and build up as the migraine progresses. Weakness and numbness usually go away within 24 hours but may last a few days. Hemiplegic migraine can be one of the most difficult types of migraine to diagnose as many of the symptoms can mimic stroke, seizures, or other conditions. MRIs, CT scans and other tests are done to rule out other more sinister causes of the symptoms.
5. Migraine aura without headache - affects about 1% of migraineurs where they experience migraine aura without ever having a headache. The most common symptoms of migraine aura are visual disturbances such as:
• Blind spots
• Flashing lights
• Zig-zag patterns
Other sensory symptoms include:
• Pins and needles on one side usually starting in the fingers/arm, sometimes spreading up into the face
Basilar migraine - (the new name for this is migraine with brain stem sura) is a rare form of migraine that includes symptoms such as loss of balance, double vision, blurred vision, difficulty in speaking and fainting. During the headache, some people lose consciousness. These are very frightening sensations for the migraine sufferer, and often people describe the feeling of terror and fear that they are about to suffer a stroke. Basilar migraine occurs when the circulation in the back of the brain or neck is affected. It usually affects young women but is sometimes seen in children too.
Ophthalmoplegic migraine - is a very rare type of migraine that occurs mainly in young people in which there is weakness of one or more of the muscles that move the eye. In addition to headache, symptoms of ophthalmoplegic migraine include dilation of the pupils, inability to move the eye upward, downward, or across, as well as a drooping of the upper eyelid.
The phases of migraine – Before, during and after the attack…
Being aware and informed on the different phases of a migraine attack can be very useful. You may get one, some, or all the following phases, which can vary from each attack in length or severity. This information and patterns can provide Healthcare Professionals with information that can help them make a diagnosis and provide the appropriate access to care and medication. Taking medication as soon as you
14 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
erenumab. Indications: Aimovig is indicated for prophylaxis of migraine in adults who have at least 4 migraine days per month. Dosage and administration: Adults: The recommended dose of Aimovig is 70 mg administered subcutaneously every 4 weeks. Some patients may benefit from a dosage of 140 mg once every 4 weeks. Each 140 mg dose is given either as one subcutaneous injection of 140 mg or as two subcutaneous injections of 70 mg. Aimovig is intended for patient self-administration in the abdomen, thigh, or, if someone else is giving the injection, also into the outer area of the upper arm. Administration should be performed by an individual who has been trained to administer the product. The needle cover of Aimovig prefilled pen contains dry natural rubber, which may cause allergic reactions in individuals sensitive to latex. Special populations: Paediatric patients: The safety and effectiveness of Aimovig has not been studied in paediatric patients. Elderly (aged 65 years and over): The safety and effectiveness of Aimovig has not been studied in elderly patients. No dose adjustment is necessary as the pharmacokinetics of erenumab are not affected by age. Renal impairment: No dose adjustment is necessary in patients with mild to moderate renal impairment. Hepatic impairment: No studies have been performed in patients with hepatic impairment. Hepatic clearance is not a major clearance pathway for erenumab. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Warnings and precautions: Hypersensitivity reactions: Serious hypersensitivity reactions, including rash, angioedema, and anaphylactic reactions, have been reported with erenumab in post-marketing experience. These reactions may occur within minutes, although some may occur more than one week after treatment. In that context, patients should be warned about the symptoms associated with hypersensitivity reactions. If a serious or severe hypersensitivity reaction occurs, initiate appropriate therapy and do not continue treatment with erenumab. Constipation: Constipation is a common undesirable effect of Aimovig and is usually mild or moderate in intensity. In a majority of the cases, the onset was reported after the first dose of Aimovig; however patients have also experienced constipation later on in the treatment. In most cases constipation resolved within three months. In the post marketing setting, constipation with serious complications has been reported with erenumab. In some of these cases hospitalisation was required, including cases where surgery was necessary. History of constipation or the concurrent use of medicinal products associated with decreased gastrointestinal motility may increase the risk for more severe constipation and the potential for constipation related
complications. Patients should be warned about the risk of constipation and advised to seek medical attention in case constipation does not resolve or worsens. Patients should seek medical attention immediately if they develop severe constipation. Constipation should be managed promptly as clinically appropriate. For severe constipation, discontinuation of treatment should be considered. Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Latex sensitive individuals: The removable cap of the Aimovig pre-filled pen contains dry natural rubber latex, which may cause Allergic reactions in individuals sensitive to latex Pregnancy, lactation, fertility: Pregnancy: There are a limited amount of data from the use of erenumab in pregnant women. As a precautionary measure it is preferable to avoid the use of Aimovig during pregnancy. Lactation: It is not known whether erenumab is present in human milk. The use of Aimovig could be considered during breastfeeding only if clinically needed. Fertility: There is no data available on the impact of Aimovig on male and female fertility. Animal studies showed no impact on female and male fertility. Adverse drug reactions: Common (≥1/100 to <1/10): Hypersensitivity reactions including anaphylaxis, angioedema, rash, swelling/ oedema and urticaria. Injection site reactions, constipation, muscle spasm, pruritus. Interactions: No effect on exposure of co-administered medicinal products is expected based on the metabolic pathway of monoclonal antibodies. No interactions with oral contraceptives (ethinyl estradiol and norgestimate) or sumatriptan were observed in studies with healthy volunteers. Legal Category: POM. Marketing Authorisation Holder: Novartis Europharm Ltd, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland. Marketing Authorisation Numbers: EU/1/18/1293/001 006. Date of last revision of Abbreviated Prescribing Information: September 2020. Full prescribing information is available upon request from: Novartis Ireland Limited, Vista Building, Elm Park Business Campus, Merrion Road, Dublin 4, Ireland, Tel: + 353 1 220 4100 or at www.medicines.ie. Detailed information on this product is also available on the website of the European Medicines Agency http://www.ema.europa.eu.
▼ This medicinal product is subject to additional monitoring. Reporting suspected adverse reactions of the medicinal product is important to Novartis and the HPRA.
It allows continued monitoring of the benefit/risk profile of the medicinal product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website: www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by calling 01 2080 612.

THIS IS PREVENTION1,2 THIS IS FEWER MIGRAINE DAYS.2,3 THIS IS LONG-TERM EVIDENCE.4 References: 1. Aimovig SmPC available at medicines.ie 2. Tepper S, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebocontrolled phase 2 trial. Lancet Neurol 2017; 16: 425-434. 3. Goadsby PJ, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017; 377: 2123-2132. 4. Ashina M, et al. Eur J Neurol. 2021 in Press Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial - PubMed (nih.gov) *Image used is not an actual Aimovig ® patient IE145414 | August 2021 ABBREVIATED PRESCRIBING INFORMATION ▼ Aimovig® (erenumab) 70 mg and 140 mg solution for injection in pre-filled pen Important note: Before prescribing, consult full prescribing information. Presentation: Aimovig 70 mg solution for injection in pre-filled pen. Each pre-filled pen contains 70 mg erenumab. Aimovig 140 mg solution for injection in pre-filled pen. Each pre-filled pen contains 140 mg
AIMOVIG® IS REIMBURSED FOR CHRONIC MIGRAINE PATIENTS WITH THREE OR MORE PREVIOUS PROPHYLACTIC TREATMENT FAILURES ▼®
Migraine
notice the pain may fully prevent an attack or shorten an attack. It’s important to note that migraine attacks in childhood are often much shorter than in adulthood which might make it easier to tell different headache stages in a child.
The phases of a migraine attack are as follows:
Premonitory phase
“When I feel a migraine attack coming on, I feel a fuzziness and pain building up in my head. I can feel very nauseous and sometimes I feel a bit dizzy. I would feel the headache building up to a more severe headache quite quickly.”
speech and hearing changes
Some people experience memory changes, feelings of fear and confusion, and more rarely, partial paralysis or fainting.
Aura is the result of a wave of nerve activity that spreads over the brain (known as cortical spreading depression). As this electrical wave spreads, the nerves fire in an abnormal way and this range of reversible neurological symptoms (aura) develop. This stage can last up to 60 minutes. In adults, they usually happen before the headache itself, but in children, they may happen at the same time as the headache. It is possible to have the aura symptoms without the headache, this is often referred to as ‘silent migraine’.
The Headache phase
often compared this to feeling of having a hangover but without the alcohol.”
- Susan Doyle, migraine patient
Recovery or Postdrome phase
This is the final phase of an attack, and it can take hours or days for a drained, fatigued or ‘hungover’ type feeling to fully disappear. Symptoms can be like those of the first stage (premonitory). Often, they mirror these symptoms. For example, if you lost your appetite at the beginning of the attack, you might be very hungry now. If you were tired, you might feel full of energy.
For further information:
Hazel Breen, Communications and Information Officer, Migraine Association of Ireland, Unit 14 Block 5 Port Tunnel Business Park Clonshaugh Dublin D17 WK24
Email: communications@migraine.ie
The Migraine Association of Ireland
- Susan Doyle, migraine patient
This is sometimes referred to as the onset of migraine or the warning stage in which certain physical and mental changes occur which can include:
feeling tired excessive yawning food cravings
changes in your mood such as feeling down or irritable (high or low)
feeling thirsty neck stiffness passing more urine (wee)
These feelings can last up to 24 hours.
Aura phase
Migraine without aura does not include this stage. The aura part of migraine includes a wide range of neurological symptoms usually before the headache stage. These symptoms include:
changes in sight (visual disturbances) such as dark spots, coloured spots, sparkles or ‘stars’, and zigzag lines
numbness or pins and needles weakness
dizziness or vertigo (sensation of spinning and poor balance)
“I become very ill with vomiting, and I must go to bed and block out all light and all noise and have a cold wet flannel over my head. These attacks could last a couple of hours, but in the past before I started Ajovy medication, I was getting them daily! If I wake up with a migraine, it will last most of the day even with extra medication and I would be completely incapacitated.”
- Susan Doyle, migraine patient
This phase can cause moderate to severe head pain. The headache is typically throbbing and is made worse by movement. It is usually on one side of the head, especially at the start of an attack. However, you can get pain on both sides, or all over the head. Nausea (sickness) and vomiting (being sick) can happen at this stage, and you may feel sensitive to light, sound, smell, and movement.
Painkillers work best when taken early in this stage. As you now know from the list of types of migraine and symptoms above, the symptoms for each person can vary as migraine is so individual and so can each attack be. Visit www.migraine.ie to read more detail on each type of migraine, their symptoms, and triggers too.
Resolution phase
Most attacks slowly fade away, but some stop suddenly after the person with migraine is sick or cries a lot. Sleep seems to help many people, even an hour or two can be enough to end an attack. Many children find that sleeping for just a few minutes can stop their attack.
“After each attack, I feel completely drained of any energy and completely ‘wiped out’! I have
Being aware of the different phases of a migraine attack can be helpful. It can help you prepare for an attack, get a diagnosis, and decide when to take acute or alternative treatment, such as painkillers or make migraine smart lifestyle changes. It is useful to have a rescue treatment plan for when attacks occur. This may include painkillers such as a triptan, a NSAID (e.g., ibuprofen) or paracetamol. It often also includes anti-sickness medication. Please note that too many painkillers can create a different type of headache called Medication Overuse Headache (MOH) so it is important for Healthcare professionals to advise to use only as prescribed or as directed on the package of medication.
For other people, being aware of the types, symptoms and phases of a migraine attack can help their understanding towards loved ones in your life with migraine. It may also help with the frustration and lack of understanding people often face around migraine, especially at work and in education. Therefore, migraine awareness and education are vital and must be taken seriously as it has a huge impact on just under one eighth of the Irish population which in turn impacts the workplace economy with sick leave equating to ¤252 million per year in Ireland. The better we as a nation get at managing migraine, the more beneficial it will be to everyone overall. People with migraine want to work, want to study, and want to be able to live life to the fullest but need awareness, education, the right access to care and medication and reasonable accommodations to do so. For more information visit www.migraine.ie
References:
Global Burden of Disease 2019
Migraine Trust, UK
Susan Doyle – migraine patient and sufferer

The Migraine Association of Ireland is Ireland’s only patient charity that provides information, support, education, and reassurance to the 600,000 people suffering with migraine and headache disorders in Ireland, whilst seeking further research, better treatments/access to care and increased public awareness of the condition. MAI services include providing free educational events/courses to help people manage their migraine, and certified HP courses for GPs and other HCPs. In addition, they lobby and advocate on behalf of migraineurs for access to new treatments and better access to care.
About migraine
Migraine is a complex neurological condition which is classified by the World Health Organisation as the 7th most disabling disease worldwide, the 4th for women. Migraine is the most common neurological condition in the world, affecting 12 – 15% of the population. It is 3 x times more common in women than it is in men and is usually inherited. On any given day in Ireland over 13,000 people suffer from migraine. It is a very individual condition. Some people experience only one or two attacks per year while others suffer on a weekly basis. An attack can last from 4 to 72 hours.
Individuals can experience migraine without aura, with aura, aura no headache, basilar, hemiplegic, ophthalmoplegic and vestibular. They can also experience chronic migraine, cluster headaches, medication overuse headache and new daily persistent headache.
Therefore, it is paramount that Migraine Ireland create wide scale awareness so migraineurs know they are ‘not alone’, learn to manage their condition and educate others about migraine.
Employers can reduce sick days per year if they were more aware of migraine as a condition and how vast it is. Sick days from migraine add up to over ¤240 million in losses for the Irish economy alone which could be avoided if employers were more educated about migraine.
16 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
Asthma
Management of Stable and Acute Asthma
Asthma is a heterogeneous disease, usually characterized by chronic airway inflammation which is defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, together with variable expiratory airflow limitation (GINA, 2022). This article seeks to address the management of asthma in adults both from a stable and an acute perspective.
The key to asthma management is education and empowering patients to manage their asthma as well as the use of appropriate treatment for the patient’s asthma phenotype, symptoms and lifestyle.
Management of Stable Asthma
The goals of asthma management are:
1. Symptom control: to achieve good control of symptoms and maintain normal activity levels
2. Risk reduction: to minimize future risk of exacerbations, fixed airflow limitation and medication side-effects (GINA 2022)
Assessment of asthma control involves assessing symptoms over the previous 4 weeks using the GINA Assessment of Asthma Control and the Asthma Control Test (ACT) and assessing risk factors for poor outcomes. Treatment issues should also be addressed at every visit and should include:
• Review of inhaler technique and adherence
• Asking about side-effects
• Reviewing the patient’s written asthma action plan?
• Exploring the patient’s attitudes and goals for their asthma?
The treatment and management of asthma should incorporate the following elements:
Education on the disease process
Management of trigger factors
Medication management –actions, inhaler technique, adverse events and adherence
Asthma Action Plan
Management of acute flare-ups of asthma
The goal of asthma management is for the patient to be optimally controlled on the minimum amount of medication. GINA (2022) provides health care professionals with a management approach based on control using a step wise approach. This assists health professionals with the titration of medications using as step down or step up approach in attempt to achieve this goal.
The cornerstone of asthma treatment is inhaled therapy as medications are directly targeted at the airways and therefore, are more effective. This also limits the amount of systemic absorption and reduces adverse events. Patients should be commenced
 Written by Ruth Morrow, Respiratory Nurse Specialist, Asthma Society of Ireland
Written by Ruth Morrow, Respiratory Nurse Specialist, Asthma Society of Ireland

on the appropriate step of the treatment guidelines which is dependent on the severity of their symptoms (GINA, 2022). Each patient is assigned to one of five treatment steps and patients may move up or down the steps depending on symptoms and the amount of reliever therapy being used. Inhaled glucocorticosteroids are the cornerstone of asthma treatment and are the most effective controller medications available. However, there are additional oral medications such as leukotriene receptor antagonists which can be added on and are very useful in patients who have an allergic component to their asthma, experience cold air bronchoconstriction and have exercise induced symptoms. These medications are also licensed for use in allergic rhinitis, a condition which 85% of people with asthma also have. Sublingual immunotherapy is also now recommended at all steps of
the guideline depending on the patient’s asthma phenotype.
In 2019, GINA updated their strategy which outlined significant changes to the way asthma is managed in adults and adolescents. The changes recognise a real sea change in the use of short acting bronchodilators (SABA) and the introduction of combination therapy of inhaled corticosteroids (ICS) and longacting bronchodilators (LABA) as “a needed therapy” at Step 1 and as a maintenance therapy at Step 2. Using a combination therapy as an “as-needed” therapy will require a significant change in the mindset of HCPs given that we haven't been using SABAs for the last 50 years to relieve asthma symptoms.
Why this change?
Inhaled SABA (Salbutamol, Terbutaline) have been firstline treatment for asthma for 50 years. Traditionally asthma was thought to be a disease of bronchoconstriction with SABA being the drug of choice. Added to this, rapid relief of symptoms, reliance on, patient satisfaction and their low cost have meant that SABAs were widely used, overused and over-relied upon. The perception by patients that their reliever “gives me control over my asthma”, so much so that they often don’t see the need for other treatment. However, research over the past number of years has shown that regular and frequent use of SABAs decrease bronchoprotection, increase rebound hyperresponsiveness, and decrease bronchodilator response (Hancox, 2000). Patients with apparently mild asthma are at risk of serious adverse events such as near fatal asthma, acute asthma and death from asthma. Regular or frequent use of SABAs are also associated with increased allergic response and increased eosinophilic airway inflammation
17 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
Asthma
(Aldridge, AJRCCM 2000). Patients who get 3 or more canisters of SABA per year (average 1.7 puffs/ day) are associated with higher risk of attendance to the emergency department (Stanford, AAAI 2012) and patients who receive 12 or more canisters per year are associated with higher risk of death (Suissa, AJRCCM 1994). A meta-analysis by Crossingham et al (Cochrane 2021) of four RCTs involving 9,565 patients demonstrated the benefits of LABA/ICS combination therapy showing a 55% reduction in severe exacerbations compared with SABA alone. ED visits or hospitalizations were 65% lower than with SABA alone and 37% lower than with daily ICS.
In their review of the literature, GINA found no evidence to support a Step 1 SABA-only approach. The lack of evidence for SABA-only treatment contrasted with the strong evidence for safety, efficacy and effectiveness of the treatments recommended in Steps 2-5 of the strategy i.e. ICS and ICS/LABA. For safety, GINA no longer recommends SABA-only treatment for Step 1. This decision was based on evidence that SABA-only treatment increases the risk of severe exacerbations, and that adding any ICS significantly reduces the risk. It is now recommended that all adults and adolescents with asthma should receive symptom-driven or regular low dose combination LABA/ICS-containing controller treatment, to reduce the risk of serious exacerbations (GINA, 2022). Patients who have symptoms more than twice a month should be prescribed ICS/ LABA twice daily (Step 2-5) and patients who have symptoms less than twice a month should use ICS/LABA on “an as-needed basis” (Step1). Daily ICS is no
longer listed as a Step 1 option as it has a high probability of poor adherence. It is now replaced by a more feasible as-needed controller option at Step 1.Patients should be offered self-management plans with instructions on how to adjust their medications in response to worsening symptoms and/or worsening PEFR. An example of a self-management plan is available on www.asthmasociety.ie.
Non-pharmacological management
The non-pharmacological management of asthma include management of trigger factors, smoking cessation, management of obesity and gastroesophageal reflux disease. Influenza vaccination is recommended for those with more severe asthma. Gastro-esophageal reflux can worsen asthma symptoms and treatment of reflux may improve asthma symptoms. Hormones can also play a significant role in asthma control. Some patients will experience worsening of their asthma symptoms pre-menstrually or during menstruation. During pregnancy, asthma control may improve, deteriorate or stay the same as pre-pregnancy. Asthma may also develop in women who are menopausal and very often require high doses of inhaled corticosteroids and have more difficult to control asthma..
Adherence with medication regimes
One of the biggest challenges in asthma management is adherence to medication as many patients may be asymptomatic and therefore “don’t feel the need to use their medication daily” Exploring the patient’s beliefs and attitudes can be useful in determining a rationale for non-adherence to medication regime. Saving medication until it is needed, fear of becoming addicted or the health professional didn’t listen are amongst reasons given by patients in the INCA study (Sulaiman et al, 2014). In the
current climate, cost a significant factor even for the person who has a medical card and should not be overlooked. Two proven ways to address non-adherence are shared decision-making between the health professional and the patient and motivation interviewing. Using motivational interviewing, the health professional can assess the individual’s likelihood to adhere to their medication or to nonpharmacological interventions.
Risk factors for poor outcomes
Patients who experience uncontrolled asthma symptoms, had one or more exacerbations in the previous year, the start of the patient’s usual ‘flare-up’ season (especially if autumn), has major psychological or socio-economic problems, poor adherence with controller medication and/or incorrect inhaler technique are at risk of an exacerbation in the coming months.
Assessment and Management of Acute asthma
Accurate and timely assessment of acute asthma exacerbations should be carried out to ensure a successful outcome. Table 1 (below) differentiates between a mild and severe acute exacerbation.
The management of acute asthma includes:
1. Oxygen therapy - 24% delivered by face mask (usually 1L/min) to maintain oxygen saturation 93-95%
2. Inhaled short-acting bronchodilator – 4-10 puffs of Salbutamol by spacer, or 5mg by nebulizer, every 20 min for first hour, then reassess severity. If symptoms persist, deteriorate or recur, give an additional 10 puffs per hour and admit to hospital
3. Oral corticosteroids – max 50mgs of oral steroids and continue for 5 -7 days
4. Additional treatments can include - For moderate/severe
exacerbations, Ipratropium bromide 80mcg (or 250mcg by nebulizer) every 20 minutes
Criteria for immediate transfer to secondary care include:
1. Features of severe exacerbation at initial or subsequent assessment
Patient is unable to speak or drink
Cyanosis
Subcostal retraction
Oxygen saturation <92% when breathing room air
Silent chest on auscultation
2. Lack of response to initial bronchodilator treatment
3. Persisting tachypnoea despite 3 administrations of inhaled SABA,
4. Unable to be managed at home Follow-up post exacerbation
All patients should be followed up regularly after an exacerbation, until symptoms and lung function return to normal. Patients are at increased risk during recovery from a further exacerbation. This provides an opportunity to review and update the patient’s asthma management, review inhaler technique and adherence and to ascertain if there was a cause ie new trigger factors, for this flare-up which might be helpful in preventing future flare-ups
At follow-up visit(s), the asthma review should include:
• The patient’s understanding of the cause of the flare-up
• Modifiable risk factors, e.g. smoking, weight loss if indicated, addressing new triggers
• Adherence with medications, and understanding of their purpose
• Reliever should be being used as-needed rather than routinely
• If controller medication was increased, the increased dose should be maintained for 3 weeks and possibly longer particularly, if during the winter or during Hayfever season
• Inhaler technique skills
• Written asthma action plan
Conclusion
This article has addressed stable and acute asthma management. The rationale for the introduction of LABA/ICS combination therapy has been explored following the changes to the GINA guidelines in 2019.
References available on request.
18 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
Symptoms Altered consciousness Oximetry on presentation (SaO2) Speech Pulse rate Peak Flow Central cyanosis Wheeze intensity Mild No >95% Sentences Pulse rate 100–120 bpm >50% predicted or best Absent Variable Severe Agitated, confused or drowsy <92% Words <50% of predicted or best Likely to be present Chest may be quiet Table 1: Assessment of acute exacerbation of asthma (GINA, 2022)


















Eye Health
Management of Macular Oedema
Macular oedema is the end common pathway for many prevalent ophthalmic conditions. It is one of the leading causes of central visual loss.
The condition is characterized by the accumulation of fluid in the outer plexiform layer and the inner nuclear layer of the retina; it occurs secondary to the breakdown of the blood-retinal barrier. This causes an abnormal passage of proteins into retinal tissues resulting in osmotic water retention and consequent swelling.
When macular oedema occurs, patients may be aware of a fall in their central vision, they may also experience distortion and reduced colour vision.
Macular oedema can be diagnosed at the slit-lamp using high magnification; however Ocular Coherence Tomography (OCT) imaging facilitates both detection and quantification of the oedema. In recent times, the availability of OCT imaging has increased, allowing patients to access this investigation through both optometric as well as ophthalmic practitioners. Depending on the aetiology of the macular oedema, it has a different nomenclature.
A common cause of macular oedema is retinal vein occlusion (RVO). This may be secondary to a central, branch or hemiretinal vein occlusion. The prevalence of RVO increases with age. The most common association with RVO is undiagnosed hypertension. It is to be recommended that when patients are diagnosed with macular oedema secondary to RVO that they undergo 24hour blood pressure monitoring.
Open-angle glaucoma, diabetes mellitus, and hyperlipidemia have all been implicated as other primary risk factors for RVO. A hypercoagulable state also predisposes to the development of RVO. Conditions such as polycythemia, multiple myeloma, cryoglobulinemia, and Waldenstrom macroglobulinemia place patients at a higher risk of developing RVO. In a young patient who presents with a RVO a full systemic workup is mandated to detect such potential underlying causes.
The primary treatment of macular oedema secondary to RVO is to address the underlying cause. Macular oedema secondary
OCT image of left macular oedema

to advanced hypertension can resolve following adequate hypertensive control. Otherwise, patients are treated with a course of intravitreal therapy. The agents used may either be steroids or more commonly an anti-Vascular endothelial growth factor (VEGF) agent. Such agents include ranibizumab (Lucentis), aflibercept (Eylea), and bevacizumab (Avastin). Intravitreal steroids have a longer duration of action but are associated with an increased risk of cataract formation and can cause raised intraocular pressure. When the macular oedema resolves, the patient’s visual acuity generally improves unless there is associated non perfusion of the macula. Patients may require ongoing treatment for several months to years to preserve their vision.
Patients with either Type 1 or Type 2 Diabetes Mellitus can develop diabetic macular oedema (DME). If the macular oedema does not involve the fovea, it can be treated by laser photocoagulation. However, laser scars can enlarge with time and encroach on the fovea leading to visual loss, so intravitreal therapy with either anti-VEGF agents or steroids is preferred by some for treating cases of DME with and without foveal involvement. Improving glycaemic control, regularising hypertension and treating hyperlipidaemia are known interventions to treat diabetic maculopathy. If DME has been present for some time, secondary structural changes can occur in the retina. These changes are termed disorganisation of retinal inner layers (DRIL). When these changes are seen, the visual prognosis for the patient is guarded.
Neovascular age related macular degeneration (nAMD) is associated with swelling of the central macula. Choroidal neovascularisation leads to both subretinal and intraretinal leakage of fluid as well as haemorrhage. The presence of chronic intraretinal fluid is associated with a poorer visual outcome. Treatment of nAMD is with sustained intravitreal anti-VEGF therapy to dry up the intraretinal fluid and prevent the development
of fibrosis. Patients are monitored with serial OCT imaging and their intravitreal treatment frequency adjusted accordingly.
Cataract surgery is a recognised cause of macular oedema. When this occurs, it is termed pseudophakic cystoid macular oedema (CMO). The cataract surgery may have been either a complicated or an uncomplicated procedure. The patient may initially have good vision post cataract surgery which then falls in the following month, or else the vision may fail to improve vision at all post operatively. Many patients have subclinical CMO that is only detectable on OCT imaging and is self-limiting in nature. CMO is believed to be secondary to intraocular inflammation. The majority of patients with CMO will undergo resolution of their macular oedema following a course of combined topical NSAIDs and steroids. Sometimes intensive hourly treatment is required ab initio. A small subset of patients will require either a periocular or an intravitreal injection of steroid to settle this inflammatory condition. If cataract surgery is planned for their fellow eye, prophylactic antiinflammatories are prescribed by many surgeons.
Macular oedema may also occur as a complication of other intraocular procedures. These procedures include panretinal photocoagulation and YAG laser. Cryotherapy is another procedure which is known to cause macular oedema.
Uveitis, no matter where it is located within the eye -anterior, intermediate, or posterior can result in macular oedema and consequently reduced vision.
The treatment of macular oedema secondary to uveitis is to treat the inflammatory cause with immunosuppressive agents. Chronic inflammatory changes at the macula can result in the development of choroidal neovascularisation.
Another cause of macular oedema is drug related. Fingolimid associated macular oedema (FAME) is well described.
Fingolimid is an oral diseasemodifying therapy indicated for the treatment of multiple sclerosis. The risk of FAME is dose dependent. A baseline OCT is required before commencing Fingolimid and the patients should undergo regular monitoring while on treatment.
Latanoprost is a topical therapy commonly used to treat primary open angle glaucoma and CMO is a recognised side effect. CMO has been reported following nicotinic acid and niacin supplementation with doses of greater than 1.5g/day.
Patients with retinitis pigmentosa have a reduced peripheral visual field. They may also develop CMO which will then further reduce their remaining central visual function. CMO in retinitis pigmentosa responds well to treatment with oral acetazolamide, topical dorzolamide or brinzolamide drops.
Macular oedema has a disparate range of causes and accordingly treatments. It can cause mild to profound visual loss, if left untreated as in the case of nAMD the visual loss can be irreversible.
OCT imaging facilitates the early detection of macular oedema and with early intervention, patients’ sight can be preserved.
Written by Dr Louise O’Toole, Consultant Medical Ophthalmologist, Bon Secours Hospital Glasnevin, Dublin - Mater Private Network, Dublin
20 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
Blackrock Health Summit
The inaugural Blackrock Health Summit took place last month at the Royal College of Physicians Ireland. The event brings together experts from across public and private healthcare, both nationally and internationally.



The Summit theme is Better Together – Partners in Innovative Healthcare, and it will cover an array of topics, such as how best to harness the power of medical innovation; how to empower strong and dynamic leadership across healthcare institutions, and how to create innovative models of healthcare, learning and sharing best practice.
The Summit comes at a moment of inflection for the Irish health system. Nine months after reopening the country, the impact of Covid within our hospitals remains significant. Nationwide treatment backlogs continue to build, and the Irish Hospital Consultants Association now believe they could take up to fifteen years to resolve.
Private hospitals are currently playing a significant role in Government waiting list plans and the Summit will offer an opportunity to discuss how the relationship between public and private health can be strengthened into the future.
Guest speakers from the Irish healthcare sector will include the Minister of State for Health Frank Feighan, Director of the National Cancer Registry Professor Deirdre Murray, as well as leaders from the Department of Health and HSE.
Following Blackrock Health’s signing of an EU-first healthcare partnership with the worldrenowned Cleveland Clinic in May, the Summit will feature as guest speakers Dr. Toby Cosgrove, former Cleveland Clinic Group CEO and Dr. Conor Delaney, CEO and President of the Cleveland Clinic Florida.
The Summit will also hear from members of the Blackrock Health executive leadership team including Group CEO Dr. Caroline Whelan and Chairman Bryan Harty, as well as medical experts from within the group, such as Blackrock Health Hermitage Clinic’s Clinical Director of Radiology Dr. John Sheehan.
Blackrock Health CEO Dr. Caroline Whelan said, “I and the rest of the Blackrock Health team are immensely excited to be hosting this Summit which brings together key figures from Irish and international healthcare to discuss some of the most important issues facing our sector.
“The aspiration of Blackrock Health is to be a leader among our peers in delivering world-class care to our patients, but we know
perfectly well that no organisation on its own is capable of bringing about the improvements we need to see.
“Covid has left a lasting imprint on our sector by creating backlogs which will take years to resolve. As we face another challenging winter against the backdrop of a cost-of-living crisis and economic difficulties which are keenly felt in our sector, the need to work together and to learn from one another has never been more urgent.”
Blackrock Health Chairman Bryan Harty added, “Today’s Summit is the keystone date on Blackrock Health’s calendar and we are extremely proud to be able to bring together so much expertise in healthcare: expertise from within the public and private sectors, from Ireland and from further afield.
“The challenges which exist in our sector are considerable, and every one of our speakers will have their own thoughts about these challenges and ideas about how best to tackle them. What unites us all is the shared commitment to excellence in the delivery of healthcare, and the common belief that we are indeed ‘Better together.’
“By working together more closely and learning how to maximise the value of our partnerships we can all ensure even better care and outcomes for our patients, who are at the centre of everything we do at Blackrock Health.”
Left to right Bryan Harty, Dr Caroline Whelan and Dr Eugene Murray
Minister of State at the Department of Health Frank Feighan, Dr Caroline Whelan and the Chief Executive of the HSE, Paul Reid
Minister of State at the Department of Health Frank Feighan and Dr Caroline Whelan
21 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022 News
Bladder Cancer
Positive Outcomes in Bladder Cancer
Risk factors, warning signs and the positive outcomes of early detection
Written by Helen Forristal, MSc (ANP) BSc (Professional Nursing) ENB 237 (Oncology Nursing), Director of Nursing Services at The Marie Keating Foundation

use certain organic chemicals also may have a higher risk of bladder cancer. It is always worth checking with your Health and Safety officer, about your safety when using certain chemicals. Thankfully there is far more regulation around the use and exposure to chemicals than there was many years ago.
Gender – Bladder cancer is much more common in men than in women.
Chronic bladder irritation and infections – Urinary tract infections, kidney and bladder stones, bladder catheters left in place a long time, and other causes of chronic bladder irritation have been linked with bladder cancer (especially squamous cell carcinoma of the bladder), but it’s not clear if they cause bladder cancer.

Sometimes the blood is there in such small amounts that you can’t see it.(called microscopic haematuria) But a urine test will still show if blood is present and if it is further tests will be carried out.
The bleeding is not usually painful but tell your doctor whether you had any pain when you peed (passed urine) with blood in it as it will help them to make their diagnosis.
It can also help your doctor if you tell them whether:
Each year in Ireland, 491 men and women are diagnosed with bladder cancer. Sadly, around 222 people die from the disease each year. But this does not have to be the case. When detected early, bladder cancer is very treatable.
Bladder cancer begins when cells in the bladder start to grow uncontrollably. As more cancer cells develop, they can form a tumour which can spread to other areas of the body. It usually takes a long time to develop, so it is most common in older people.
3 in 4 people (over 75%) who get bladder cancer in Ireland are 65 years or older. Although less common in people under 50, it is very worthwhile staying vigilant. More men than women get bladder cancer. This may be because more men than women have smoked or been exposed to chemicals/dyes at work in recent decades.
We don’t know exactly what causes most bladder cancers but there are some factors that may increase your risk
Smoking cigarettes increases your risk of bladder cancer. Over a third of all bladder cancers are caused by smoking. Your risk of getting bladder cancer if you smoke is up to 4 times that of someone who has never smoked.
People with the highest risk are those who:
• smoke heavily
• started smoking at an early age
• have smoked for a long time
• Cigar and pipe smoking can also increase your risk.
Certain industrial chemicals have been linked with bladder cancer. Workers in other industries that
If you have been diagnosed with bladder cancer, it is worth finding out if you have ever been exposed to a chemical mentioned here. If you have, talk to your urologist or cancer doctor.
• Arylamines
• Polycyclic aromatic hydrocarbons
• Chlorine and trihalomethanes
• Chlorine
There are also risk factors that are beyond your control
Race and ethnicity – Caucasians are about twice as likely to develop bladder cancer as Black and Hispanic people. Asian Americans and Native Americans have slightly lower rates of bladder cancer. The reasons for these differences are not well understood.
Age – Bladder cancer usually takes a long time to develop, so it is most common in older people. 3 in 4 people (over 75%) who get bladder cancer in Ireland are 65 years or older. It is rarer in people under 50.
Personal history of bladder or other urothelial cancers such as kidney
Genetics and family history –People who have family members with bladder cancer have a higher risk of getting it themselves.
Prior chemotherapy or radiation therapy
Signs and symptoms
These are the possible symptoms of bladder cancer. Your symptoms are unlikely to be cancer, but it’s important to get them checked by a doctor.
Blood in the urine
Blood in the urine is the most common symptom of bladder cancer. 4 out of 5 people with bladder cancer (80%) have some blood in their urine. Doctors call blood in the urine haematuria (pronounced heem-at-you-ree-ah). It is important to remember that blood in urine can sometimes be caused by stones in the urinary tract, a Urinary Tract Infection or even Prostatitis.
• There is blood only when you start to pee
• The blood is mixed with all the urine you pass
Problems with passing urine may be a symptom of other conditions but a bladder cancer should be ruled out.
You should see your doctor if you:
• Notice blood in your urine
• Need to pass urine very often
• Need to pass urine very suddenly
• Have pain when passing urine
Please go to your doctor if you have any concerns, it is better to have these signs and/or symptoms checked out early and remember that early detection saves lives. Many cancers can be treated and potentially cured if caught early.
For more information and support around bladder cancer or any of the most common cancers please go to www. mariekeating.ie or reach out to info@mariekeating.ie with any questions.
22 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
as monotherapy is indicated for the

in
• After at least 5 years of follow up KEYTRUDA monotherapy continued to show improved OS, ORR, and DOR compared with investigators choice of chemotherapy for patients with advanced platinum-resistant or platinum-refractory urothelial carcinoma.2

Results from KEYNOTE-045: A multicentre, open-label, randomised, phase 3 trial comparing KEYTRUDA with investigators choice of chemotherapy for patients with locally advanced or metastatic urothelial carcinoma with disease progression on or after platinum-containing chemotherapy.1,2
Superior Overall Survival 29% reduction in risk of death (HR, 0.71 [95%CI, 0.59–0.86]
Median OS: KEYTRUDA 10.1 months vs Chemotherapy 7.2 months
Longer Duration of Response KEYTRUDA 29.7 months vs Chemotherapy 4.4 months

5 Year Results1-3
Objective Response Rate KEYTRUDA 21.9% vs Chemotherapy 11.0%
There was no statistically significant difference between KEYTRUDA and Chemotherapy with respect to Progression Free Survival. Median PFS: KEYTRUDA 2.1 months vs Chemotherapy 3.3 months (HR, 0.95 [95%CI, 0.79-1.14]
After 5 years of follow up safety was consistent with that of previous reports.2 In the as-treated population, any grade treatment related adverse events of any cause occurred in 62% of participants in the pembrolizumab group and 90.6% of participants in the chemotherapy group. Grade 3 or worse adverse events of any cause occurred in 16.9% in the pembrolizumab group and 50.2% in the chemotherapy group.3
ABRIDGED PRODUCT INFORMATION Refer to Summary of Product Characteristics before prescribing. PRESENTATION KEYTRUDA 25 mg/mL: One vial of 4 mL of concentrate contains 100 mg of pembrolizumab. INDICATIONS KEYTRUDA as monotherapy is indicated for the treatment of adults and adolescents aged 12 years and older with advanced (unresectable or metastatic) melanoma. KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults and adolescents aged 12 years and older with Stage IIB, IIC or III melanoma and who have undergone complete resection. KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥50% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations. KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR or ALK positive mutations. KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or me tastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR or ALK positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA. KEYTRUDA as monotherapy is indicated for the treatment of adult and paediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma (cHL) who have failed autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT is not a treatment option. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who have received prior platinum-con taining chemotherapy. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who are not eligible for cisplatin-containing chemotherapy and whose tumours express PD L1 with a combined positive score (CPS) ≥ 10. KEYTRUDA as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the first-line treatment of metastatic or un resectable recurrent head and neck squamous cell carcinoma (HNSCC) in adults whose tumours express PD-L1 with a CPS ≥ 1. KEYTRUDA as monotherapy is indicated for the treatment of recurrent or metastatic HNSCC in adults whose tumours express PD-L1 with a ≥ 50% TPS and pro gressing on or after platinum-containing chemotherapy. KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of advanced renal cell carcinoma (RCC) in adults. KEYTRUDA, in
KEYTRUDA® (pembrolizumab)
Immune related adverse reactions have also occurred after the last dose of pembrolizumab. Immune-related adverse reactions affecting more than one body system can occur simultaneously. Immune-related adverse reactions are immune-related pneumonitis, immune-related colitis, immune-related hepatitis, immune-related nephritis, immune-related endocrinopathies (including adrenal insufficiency, hypophysitis, type 1 diabetes mellitus, diabetic ketoacidosis, hypothyroidism, and hyperthyroi dism), Immune-related skin adverse reactions (also including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)), Refer to SmPC for more information and management of immune-related adverse reactions. Complications of allogeneic Haematopoietic Stem Cell Trans plant (HSCT): Cases of graft-versus-host-disease (GVHD) and hepatic veno-occlusive disease (VOD) have been observed in patients with classical Hodgkin lymphoma undergoing allogeneic HSCT after previous exposure to pembrolizumab. Infusion-related reactions: Grades 1, 2, 3 or 4 infusion reactions including hypersensitivity and anaphylaxis, could be seen with pembrolizumab treatment. Refer to SmPC for more information and mana gement of infusion-related reactions. Overdose: There is no information on overdose with pembrolizumab. In case of overdose, monitor closely for signs or symptoms of adverse reactions and treat appropriately. INTERACTIONS No formal pharmacokinetic drug interaction studies have been conducted with pembrolizumab. No metabolic drug drug interactions are expected. The use of systemic corticosteroids or immunosuppressants before starting pembrolizumab should be avoided because of their potential interference with the pharmacodynamic activity and efficacy of pembro lizumab. Corticosteroids can be used as premedication, when pembrolizumab is used in combination with chemotherapy, as antiemetic prophylaxis and/or to alleviate chemotherapy-related adverse reactions. FERTILITY, PREGNANCY AND LACTATION Women of childbearing potential Women of childbearing potential should use effective contraception during treatment with pembrolizumab and for at least 4 months after the last dose of pembrolizumab. No data on use in pregnant women. Do not use during pregnancy unless the clinical condition of the woman requires treatment with pembrolizumab. Breast-feeding It is unknown whether pembrolizumab is secreted in human milk. A risk to newborns/ infants cannot be excluded. Fertility No clinical data available. SIDE EFFECTS Refer to SmPC for complete information on side effects. Pembrolizumab is most commonly asso ciated with immune-related adverse reactions. Most of these reactions resolved with appropriate medical treatment or withdrawal of pembrolizumab. The most serious adverse
have received prior platinum containing chemotherapy1
combination with lenvatinib, is indicated for the first line treatment of advanced renal cell carci noma in adults. KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with renal cell carcinoma at increased risk of recurren ce following nephrectomy, or following nephrectomy and resection of metastatic lesions. Microsatellite instability high (MSI-H) or mismatch repair deficient (dMMR) cancers Colorectal cancer (CRC) KEYTRUDA as monotherapy is indicated for adults with MSI-H or dMMR colorectal cancer in the following settings: - first line treatment of metastatic colorectal cancer - treatment of unresectable or metastatic colorectal cancer after previous fluoropyrimidine based combination therapy. Non-colorectal cancers KEYTRUDA as monotherapy is indicated for the treatment of the following MSI H or dMMR tumours in adults with (a) advanced or recurrent endometrial carcinoma, who have disease progression on or following prior treatment with a platinum containing therapy in any setting and who are not candidates for curative surgery or radiation, (b) unresectable or metastatic gastric, small intestine, or biliary cancer, who have disease progression on or following at least one prior therapy. KEYTRUDA, in combination with platinum and fluoropyrimidine based chemotherapy, is indicated for the first-line treatment of locally advanced unresectable or metastatic carcinoma of the oesophagus or HER-2 negative gastroesophageal junction adenocarcinoma in adults whose tumours express PD-L1 with a CPS ≥ 10. KEYTRUDA, in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery, is indicated for the treatment of adults with locally advanced, or early stage triple negative breast cancer at high risk of recurrence. KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of locally recurrent unresectable or metastatic triple negative breast cancer in adults whose tumours express PD L1 with a CPS ≥ 10 and who have not received prior chemotherapy for metastatic disease. KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of advanced or recurrent endometrial carcinoma in adults who have disease progression on or following prior treatment with a platinum containing therapy in any setting and who are not candidates for curative surgery or radiation. KEYTRUDA, in combination with chemotherapy with or without bevacizumab, is indicated for the treatment of persistent, recurrent, or metastatic cervical cancer in adults whose tu mours express PD L1 with a CPS ≥ 1. DOSAGE AND ADMINISTRATION See SmPC for full details. Therapy must be initiated and supervised by specialist physicians experienced in the treatment of cancer. The recommended dose of KEYTRUDA in adults is either 200 mg every 3 weeks or 400 mg every 6 weeks administered as an intravenous infusion over 30 minutes. The recommended dose of KEYTRUDA as monotherapy in paediatric patients aged 3 years and older with cHL or patients aged 12 years and older with melanoma is 2 mg/kg bodyweight (up to a maximum of 200 mg), every 3 weeks administered as an intravenous infusion over 30 minutes. For use in combination, see the Summary of Product Characteristics (SmPC) for the concomitant therapies. KEYTRUDA must not be administered as an intravenous push or bolus injection. When administering KEYTRUDA as part of a combination with intravenous chemotherapy, KEYTRUDA should be administered first. Treat patients until disease progres sion or unacceptable toxicity (and up to maximum duration of therapy if specified for an indication). For the adjuvant treatment of melanoma or RCC, KEYTRUDA should be administered until disease recurrence, unacceptable toxicity, or for a duration of up to one year. Refer to the SmPC for dosing in neoadjuvant and adjuvant treatment of locally advanced, or early stage triple-negative breast cancer at high risk of recurrence. KEYTRUDA, as monotherapy or as combination therapy, should be permanently discontinued (a) For Grade 4 toxicity except for: endocrinopathies that are controlled with replacement hormones; or haematological toxicity, only in patients with cHL in which KEYTRUDA should be withheld until adverse reactions recover to Grade 0-1; (b) If corticosteroid dosing cannot be reduced to ≤10 mg prednisone or equivalent per day within 12 weeks; (c) If a treatment-re lated toxicity does not resolve to Grade 0 1 within 12 weeks after last dose of KEYTRUDA; (d) If any event occurs a second time at Grade ≥ 3 seve rity. Patients must be given the Patient Alert Card and be informed about the risks of KEYTRUDA. Special populations Elderly: No dose adjustment necessary. Renal impairment: No dose adjustment needed for mild or moderate renal impairment. No studies in severe renal impairment. Hepatic impairment: No dose adjustment needed for mild hepatic impairment. No studies in moderate or severe hepatic impairment. Paediatric population: Safety and efficacy in children below 18 years of age not established except in paediatric patients with melanoma or cHL. CONTRAINDICATIONS Hypersensitivity to the active substance or to any excipients. PRECAUTIONS AND WARNINGS Assessment of PD-L1 status When assessing the PD-L1 status of the tumour, it is important that a well-validated and robust methodology is chosen to minimise false negative or false positive deter minations. Immune-related adverse reactions Immune-related adverse reactions, including severe and fatal cases, have occurred in patients recei ving pembrolizumab. Most immune related adverse reactions occurring during treatment with pembrolizumab were reversible and managed with interruptions of pembrolizumab, administration of corticosteroids and/or supportive care.
reactions were immune-and infusion-related adverse reactions. When pembrolizumab is administered in combination with axitinib or lenvatinib, refer to the SmPC for axitinib or lenvatinib prior to initiation of treatment. For additional lenvatinib safety information related to advanced RCC see the SmPC for Kisplyx and for advanced EC see the SmPC for Lenvima. Monotherapy: Very Common: anaemia, hypothyroidism, decreased appetite, headache, dyspnea, cough, abdominal pain, nausea, vomiting, constipation, musculoskeletal pain, arthralgia, asthenia, oede ma, pyrexia, diarrhoea, pruritus, rash, fatigue. Common: pneumonia, thrombocytopenia, neutropenia, lymphopenia, hyponatraemia, hypokalaemia, hypocalcaemia, insomnia, neuropathy peripheral, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, hyperthyroidism, insomnia, dizziness, dysgeusia, pneumonitis, colitis, dry mouth, hepatitis, severe skin reactions, vitiligo, dry skin, eczema, alopecia, dermatitis acnei form, erythema, dermatitis, myositis, pain in extremity, arthritis, influenza like illness, chills, AST and ALT increases, increase in blood alkaline phosphatase, hypercalcaemia, blood bilirubin increased, blood creatinine increased, infusion related reaction. In combination with chemotherapy: Very Common: neutropenia, anaemia, thrombocytopenia, leukopenia, hypothyroidism, hypokalaemia, decreased appetite, insomnia, neuropathy peripheral, headache, dizziness, dysgeusia, dyspnoea, cough, nausea, diarrhoea, vomiting, abdominal pain, constipation, alopecia, rash, pruritus, arthralgia, musculoskeletal pain, myositis, pyrexia, fatigue, asthenia, oedema, ALT increase, AST increased. Common: pneumonia, febrile neutrope nia, lymphopenia, infusion related reaction, adrenal insufficiency, thyroiditis, hyperthyroidism, hyponatraemia, hypocalcaemia, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, pneumonitis, colitis, gastritis, dry mouth, hepatitis, severe skin reactions, erythema, dermatitis acneiform, dermatitis, dry skin, eczema, pain in extremity, arthritis, acute kidney injury, influenza-like illness, chills, blood creatinine increa sed, blood alkaline phosphatase increased, hypercalcaemia, blood bilirubin increased. In combination with axitinib or lenvatinib: Very Common: urinary tract infection, anaemia, hypothyroidism, decreased appetite, headache, dysgeusia, hypertension, dyspnoea, cough, diarrhoea, abdominal pain, nausea, vomiting, constipation, rash, pruritus, arthralgia, musculoskeletal pain, myositis, pain in extremity, fatigue, asthenia, oedema, pyrexia, lipase increased, alanine aminotransferase increased, aspartate aminotransferase increased, blood creatinine increased. Common: pneumonia, neutropenia, thrombocytopenia, lymphopenia, leukopenia, infusion-related reaction, adrenal insufficiency, hyperthyroidism, thyroiditis, hyponatrae mia, hypokalaemia, hypocalcaemia, insomnia, dizziness, neuropathy peripheral, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), pneumonitis, colitis, pancreatitis, gastritis, dry mouth, hepatitis, severe skin reactions, dermatitis, dry skin, erythema, dermatitis acneiform, alopecia, arthritis, nephritis, influenza like illness, chills, amylase increased, blood bilirubin increased, blood alkaline phosphatase increased, hypercalcaemia. PACKAGE QUANTITIES KEYTRUDA 25 mg/mL: 4 mL of concentrate in a 10 mL Type I clear glass vial. Legal Category: POM. Marketing Autho risation numbers EU/1/15/1024/002 Marketing Authorisation holder Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Ne therlands. Date of revision: June 2022. © 2022 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin, D18 X5K7 or from www.medicines.ie. II111 Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-2998700) References 1. KEYTRUDA (pembrolizumab) SmPC. Available at: www.medicines.ie. Accessed July 2022 2. Bellmunt J et al. Five-year follow-up from phase III KEYNOTE-045 trial: Pembrolizumab versus investigator’s choice (paclitaxel, docetaxel, or vinflunine) in recurrent, advanced urothelial cancer. Poster (4532) presented ASCO 2021; virtual. 3. Necchi A et al. Three-year follow up from phase III KEYNOTE-045 trial: Pembrolizumab versus investigator’s choice (paclitaxel, docetaxel, or vinflunine) in recurrent, advanced urothelial cancer. Poster (919P) presented at ESMO 2019. Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7, Ireland. IE-KEY-00587 Date of Preparation: July 2022 (pembrolizumab) Infusion 25mg/ml KEYTRUDA
treatment of locally advanced or metastatic urothelial carcinoma
adults who
Epilepsy
A Focus on SUDEP
Written by Paddy McGeoghegan - Epilepsy Ireland Advocacy & Communications Manager
SUDEP is a word which can strike fear and worry amongst people with epilepsy and their families. In advance of SUDEP Action Day 2022 on October 19th, I was pleased to write about this often undiscussed, rare but devastating aspect of epilepsy.
Epilepsy-related deaths can be due to several factors – because of a prolonged seizure (status epilepticus) or seizure-related injury or drowning. Another major cause of epilepsyrelated deaths is SUDEP. Overall, it is estimated that there are approximately 130 epilepsyrelated deaths in Ireland each year. Of these, it estimated that 21-40 are due to SUDEP. An Epilepsy Ireland funded study being conducted by Dr. Yvonne Langan of St. James’s Hospital Dublin is currently seeking to establish a clearer picture of SUDEP and epilepsy-related death incidence in Ireland.
SUDEP stands for Sudden Unexpected Death in Epilepsy and is defined where there is death in a patient with epilepsy that is not due to trauma, drowning, status epilepticus, or other known causes but for which there is often evidence of an associated seizure.

Unfortunately, there is still a lot we don’t about SUDEP. Globally, epilepsy researchers are working to find out what the exact causes of SUDEP are. It is believed that respiratory abnormalities and/or cardiac arrhythmias play a central role. However, the exact mechanism behind why and how this happens remains unclear.
In the past, SUDEP was a subject that many people with epilepsy, their family members and their clinicians did not want to discuss. However, attitudes have changed. In 2020, an Epilepsy Ireland survey of 323 people with epilepsy revealed that 81% of people with epilepsy wanted to know more about SUDEP.
At Epilepsy Ireland, we are hopeful that research will eventually provide the answers to the questions about SUDEP we have today. However, in the absence of this breakthrough, it is best to focus on what we DO know, rather than what we don’t.
We do know that the risk of SUDEP varies from low to very low. We do know that it can affect any age group of those living with epilepsy but is more common in younger adults. We do know that the better a person’s epilepsy is controlled, the lesser the risk of SUDEP. We do know that certain types of seizures are not associated with SUDEP, such as absence or myoclonic seizures. However, the risk is higher if you have generalised tonic-clonic seizures. We do know that there are risk factors for SUDEP – some of which can be reduced by a person better understanding their epilepsy and using techniques to self-manage the condition.
Raising awareness of the risks to the epilepsy community and how they can be modified is the key aspect of SUDEP Action Day. There are actions that can be taken to help reduce the risk of SUDEP. Ensuring that people with epilepsy and their families are aware of these risks could help to avoid a potentially preventable death.
On SUDEP Action Day, we also share stories from families who have sadly lost a loved one due to SUDEP. These harrowing stories lay bare the impact of this rare but devastating aspect of epilepsy. Unfortunately, a common thread amongst these stories is how the person themselves or the wider family were unaware of SUDEP.
As Hospital Professionals in varying areas of our health service, the opportunity to discuss SUDEP may present to you. Our message is to have the conversation. Our most recent survey found how people with epilepsy want to know about SUDEP whether perceived at being at high risk or not; they want to know about it sooner rather than later; and they want to hear about it from their trusted medical teams.
Knowledge is power for our community – and simply knowing about SUDEP could make a substantial difference to how that person approaches and manages their condition.
I would invite readers of Hospital Professional News to join with us on October 19th to raise awareness of SUDEP; what can be done to reduce the risks; and remember those who are no longer with us. Please check out our website and all our social media channels in the lead up to the day and on the day itself.
Our team are here to assist your patients with any information they may require on SUDEP so please do not hesitate to get in touch with us if we can assist you in any way.
Visit www.epilepsy.ie to learn more about SUDEP and SUDEP Action Day.
Some of the key findings of the 2020 Epilepsy Ireland survey on SUDEP
24 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE


































Strength of Balance

Indicated for the treatment of moderate to severe active rheumatoid arthritis in adults who have responded inadequately to, or are intolerant to one or more disease modifying anti-rheumatic drugs.1 May be used as monotherapy or in combination with methotrexate.1
Refer to Summary of Product Characteristics (SmPC) before prescribing, and for full prescribing information.
JYSELECA® filgotinib 100 mg or 200 mg film-coated tablets. Indication: Jyseleca is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease modifying anti rheumatic drugs (DMARDs). Jyseleca may be used as monotherapy or in combination with methotrexate (MTX). Dosage: Adults: 200 mg once daily. Taken orally with/without food. It is recommended that tablets are swallowed whole. Laboratory Monitoring: Refer to the SmPC for information regarding laboratory monitoring and dose initiation or interruption. Elderly: A starting dose of 100 mg once daily is recommended for patients aged 75 years and older as clinical experience is limited. Renal impairment: No dose adjustment required in patients with estimated creatinine clearance (CrCl) ≥ 60 mL/min. A dose of 100 mg of filgotinib once daily is recommended for patients with moderate or severe renal impairment (CrCl 15 to < 60 mL/min). Not recommended in patients with CrCl < 15 mL/min. Hepatic impairment: Mild/moderate hepatic impairment: no dose adjustment required. Severe hepatic impairment: not recommended. Children (< 18years): Safety and efficacy not yet established. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Active tuberculosis (TB) or active serious infections. Pregnancy. Warnings/Precautions: See SmPC for full information. Immunosuppression: Combination use, with immunosuppressants e.g. ciclosporin, tacrolimus, biologics or other Janus kinase (JAK) inhibitors is not recommended as a risk of additive immunosuppression cannot be excluded. Infections: Infections, including serious infections such as pneumonia and opportunistic infections
e.g. tuberculosis (TB), oesophageal candidiasis, and cryptococcosis have been reported. Risk benefit should be assessed prior to initiating in patients with risk factors for infections (see SmPC). Patients should be closely monitored for the development of signs and symptoms of infections during and after filgotinib treatment. Treatment should be interrupted if the patient is not responding to antimicrobial therapy, until infection is controlled. There is a higher incidence of serious infections in the elderly aged 75 years and older, caution should be used when treating this population. Tuberculosis: Patients should be screened for TB before initiating filgotinib, and filgotinib should not be administered to patients with active TB. Viral reactivation: Cases of herpes virus reactivation (e.g., herpes zoster), were reported in clinical studies (see SmPC). In rheumatoid arthritis clinical studies, the risk of herpes zoster appeared to be higher in female patients, Asian patients, patients ≥ 50 years of age, patients with a medical history of herpes zoster, patients with a medical history of chronic lung disease and patients treated with filgotinib 200 mg once daily. If a patient develops herpes zoster, filgotinib treatment should be temporarily interrupted until the episode resolves. Screening for viral hepatitis and monitoring for reactivation should be performed. Malignancy: Immunomodulatory medicinal products may increase the risk of malignancies. Malignancies were observed in clinical studies (see SmPC). Fertility: In animal studies, decreased fertility, impaired spermatogenesis, and histopathological effects on male reproductive organs were observed (see SmPC). The potential effect of filgotinib on sperm production and male fertility in humans is currently unknown. Haematological abnormalities: Do not start therapy, or temporarily stop, if Absolute Neutrophil Count (ANC) <1 × 109 cells/L, ALC <0.5 × 109 cells/L or haemoglobin <8 g/dL. Temporarily stop therapy if
JYSELECA, GALAPAGOS and the JYSELECA and GALAPAGOS logos are registered trademarks of Galapagos NV. © 2022 Galapagos NV. All rights reserved.
these values are observed during routine patient management. Vaccinations: Use of live vaccines during, or immediately prior to, filgotinib treatment is not recommended. It is recommended that immunisations, including prophylactic zoster vaccinations, be updated in agreement with current immunisation guidelines prior to initiating filgotinib treatment. Lipids: Treatment with filgotinib was associated with dose dependent increases in lipid parameters, including total cholesterol, and high-density lipoprotein (HDL) levels, while low density lipoprotein (LDL) levels were slightly increased (see SmPC). Cardiovascular risk: Rheumatoid arthritis patients have an increased risk for cardiovascular disorders. Patients should have risk factors (e.g., hypertension, hyperlipidaemia) managed as part of usual standard of care. Venous thromboembolism: Events of deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving JAK inhibitors including filgotinib. Caution should be used in patients with risk factors for DVT/PE, such as older age, obesity, a medical history of DVT/PE, or patients undergoing surgery, and prolonged immobilisation. Lactose content: Contains lactose; patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take filgotinib. Pregnancy/Lactation: Filgotinib is contraindicated in pregnancy. Filgotinib should not be used during breastfeeding. Women of childbearing potential must use effective contraception during and for at least 1 week after cessation of treatment. Driving/Using machinery: No or negligible influence, however dizziness has been reported. Side effects: See SmPC for full information. Common (≥1/100 to <1/10): nausea, upper respiratory tract infection, urinary tract infection, dizziness and lymphopenia Uncommon (≥1/1000 to <1/100): herpes zoster, pneumonia, neutropenia,
hypercholesterolaemia and blood creatine phosphokinase increase. Serious side effects: See SmPC for full information Legal category: POM Pack: 30 filmcoated tablets/bottle Price: UK Basic NHS cost: £863.10; Ireland: POA Marketing authorisation number(s): Jyseleca 100mg film-coated tablets EU/1/20/1480/001 EU/1/20/1480/002 Jyseleca 200mg film-coated tablets EU/1/20/1480/003 EU/1/20/1480/004 Further information: Galapagos UK, Belmont House, 148 Belmont Road, Uxbridge UB8 1QS, United Kingdom 00800 7878 1345 medicalinfo@glpg.com Jyseleca® is a trademark. Date of Preparation: August 2022 IE-RA-FIL-202112-00003
Additional monitoring required
Adverse events should be reported. For Northern Ireland, reporting forms and information can be found at yellowcard.mhra.gov.uk or via the Yellow Card app (download from the Apple App Store or Google Play Store).
Adverse events should also be reported to Galapagos via email to DrugSafety.UK.Ireland@glpg.com or 00800 7878 1345
Adverse events should be reported. For Ireland, reporting forms and information can be found at www.hpra.ie and can be reported to HPRA on +353 1 6764971.
Adverse events should also be reported to Galapagos via email to DrugSafety.UK.Ireland@glpg.com or 00800 7878 1345

2022.
Combe B,
Rheum Dis 2021;doi:10.1136/annrheumdis-2020-219214.
2022 IE-RA-JY-202201-00001
September
References: 1. JYSELECA SPC. Available at: www.medicines.org.uk / www.medicines.ie. Last accessed: September
2.
et al. Ann
3. Genovese MC, et al. JAMA 2019;322 (4):315–325. 4. Westhovens R, et al. Ann Rheum Dis 2021;doi:10.1136/annrheumdis-2020-219213. 5. Combe B, et al. Poster presented virtually at ACR, November 3–10, 2021. 6. Buch MH, et al. Poster presented virtually at ACR, November 3–10, 2021. 7. Genovese MC, et al. Poster presented virtually at the European League Against Rheumatism (EULAR) 2020 E-Congress, June 3–6, 2020. JYSELECA shows more than 5x greater potency for JAK1 over JAK2/3 and TYK21* Balancing sustained efficacy2-6 with acceptable tolerability1,7 JYSELECA – a JAK1 preferential inhibitor for moderate to severe RA1
Visit strengthofbalance.co.uk to learn more *This is based on biochemical assays, the clinical consequence of this is unknown. Common adverse events (≥1/100 to <1/10) include: nausea, upper respiratory tract infection, urinary tract infection, dizziness, lymphopenia.1
Health Reform
Fatigue in Healthcare - Employee Blame or System Reclaim?
Written by
Dr Dale Whelehan

Introduction
In this article we provide a holistic understanding of fatigue in healthcare systems, including the interventions that are required to tackle long-standing issues of burnout in healthcare workers. Dale has previously described how fatigue occurs in individuals in our previous issue and is this time joined by fellow expert Naomi Algeo.
Dr Naomi Algeo is a Senior Occupational Therapist (Oncology/ Haematology), St. James’s Hospital. Her PhD research focused on the development of a workfocused programme to support women with breast cancer in returning to work. Naomi sits on the Development Group for Cancer Rehabilitation for the World Health Organisation and has presented widely internationally and nationally on the area of cancer survivorship and employment. She regularly presents on understanding and managing fatigue.
Context
The COVID-19 pandemic is the biggest challenge the healthcare system has faced in living memory and placed significant demands on individuals to change behaviours. At the forefront of these challenges was the expectation that healthcare workers (HCWs) defend through the front-facing role of virus management and mitigation. The ramifications for such personal sacrifices has been well documented by The World Health Organisation (WHO), who have previously identified HCWs as particularly vulnerable and susceptible to the effects of pandemics (Koh et al., 2003) for both physical and mental health. In
Dr Naomi Algeo

addition to the personal sacrifices, the pandemic placed severe strain on global healthcare services.
The health and well-being of HCWs, including minimising absenteeism is imperative to deliver, maintain and increase essential health and care services. However, the healthcare workforce have been exposed to higher risks of contracting COVID-19 (Nguyen et al., 2020), and therefore living with the long term consequences of COVID-19 (Ladds et al., 2020). Coupled with this, HCWs have a higher risk of experiencing high levels of occupational fatigue or “burnout” (Shanafelt et al., 2015). Fatigue is a common symptom experienced with both conditions (Ladds et al., 2020; van Dijk and Swaen, 2003), yet presents with different aetiologies and management strategies. While such issues are long standing in healthcare, the opportunity for change is more possible now than ever.
There is an imperative to provide innovative perspectives in changing culture towards fatigue in healthcare, by establishing systems which reduce fatigue risk, and enabling individuals to selfmanage their own fatigue levels. In this practical commentary we discuss the existing and emerging issues of fatigue in healthcare and the associated leadership considerations. We argue for the development of a systematic programme for fatigue identification, education, and mitigation to be designed by healthcare staff, implemented with good faith, and embedded through applying evidence-based behaviour change principles.
Worsening burnout since COVID-19
While ordinary levels of day-to-day fatigue are a normal emotional state resulting from time-ontask demands, chronic levels of occupational fatigue characterised in this instance as “burnout” is not. Defined by WHO in ICD-11 as a syndrome, this chronic fatigue is conceptualised as resulting from chronic workplace stress that is not successfully managed resulting in emotional exhaustion, mental distance from one’s job, and reduced professional efficacy (WHO, 2018). This is unsurprising given that this pandemic saw HCWs working increased hours, requiring additional PPE and most detrimentally greater disruption to sleep quality (Magnavita et al., 2020). Consistent levels of increased stress have also been associated with cognitive dysfunction such as impairment to the learning and memory regions of the brain (Kirschkaum et al., 1996) which can have impacts on work performance and ability. Neglecting occupational-based stressors may also lead to higher levels of clinical depression and anxiety (Teng et al., 2020). These psychological symptoms often go unnoticed within complex and busy systems, and particularly ones which are process and not human driven.
Emerging levels of Long-COVID Fatigue
The long-term consequences of COVID-19, also known as ‘LongCOVID’ (Perego et al., 2020), can onset after any severity of acute illness, with multi-dimensional clusters of often episodic symptoms. As per the WHO, Long-COVID “occurs in individuals with a history of probable or confirmed SARS CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.”. It is estimated that a minimum of 114,500 individuals who have had COVID-19 in Ireland are experiencing/will experience Long-COVID (Timoney, 2022).
Fatigue is one symptom which can be found to relapse and remit throughout the Long-COVID trajectory and has been cited as a common symptom across both acute and chronic phases (Tenforde et al., 2020). LongCOVID fatigue has not been
characterised, but is described similar to ME/CFS with persistent and disabling exhaustion, exercise intolerance, cognitive difficulty and musculoskeletal/joint pain. Fatigue has long-term implications for individuals and society. Townsend and colleagues (2020) reported persistent fatigue at ten-weeks for 52% of participants, culminating in a third unable to return to work. An important characteristic of Long-COVID fatigue is symptom exacerbated by exertion, similar to post-exertional malaise, where post-exertional exhaustion is a cardinal feature of the inability to produce sufficient energy on demand, among those with myalgic encephalomyelitis/ chronic fatigue syndrome (ME/ CFS) (Carruthers, 2011). Previous epidemics such as SARS, H1N1, and Ebola saw large proportions meet ME/CFS diagnostic criteria (Islam et al, 2020). Additionally, higher incidences of ME/CFS have been reported in HCWs (Jason et al., 1998) compared to the general population. Drawing from past experience, an opportunity exists to potentially mitigate against long-term consequences of COVID-19 for HCWs or where not possible, support recovery through meaningful intervention.
Changing the ways of working
If healthcare wants to make a meaningful difference in tackling fatigue levels in HCWs, interventions must recognise the interplay between rest and performance. Removing the structural barriers, particularly in settings where continuity of patient care is required, may optimise rest opportunities and reduce fatigue levels within the constraints of a system which requires 24-hour work . In the case of on-call work, efforts should be made in scheduling to minimise continuous hours of wakefulness before and during duty periods that are unscheduled – this means an end to 24 hour call rotas or more, and a greater level of consistency in scheduling which is biomathematically modelled, and not resource modelled. If HCWs feel fatigued in work, opportunities for rest and sleep could be provided through sleep pods or napping rooms, and having a protocol for returning to work following a rest period which controls for sleep inertia. In the same vein, optimising sleep conditions during rest periods is also important,
28 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
and procedures to minimise interruptions during non-work periods should be established and disseminated widely.
Modelling this shift from resource to individualised approaches requires good management and leadership in a context where there are ever increasing demands and limited resources. Emphasis on educating staff on the link between preventive measures and performance optimisation, providing goal-orientated activities to track changes in culture, and showing future opportunities for improved healthcare system provision is key to ensuring stakeholder buy-in.
Changing the culture
Employment legislation varies internationally, with differing implications for areas such as sick leave entitlements and reasonable accommodations. This financial pressure may expedite return to work, even if the individuals are not physically, mentally or cognitively ready to return, potentially amplifying Long-COVID related fatigue. Reasonable accommodations are widely recognised across Europe and are associated with positive health benefits (Neumark et al, 2015). They vary in nature and include a graded return to work, flexible hours, ergonomic modification, or change of role.
Reasonable accommodations should be tailored to HCWs and developed in collaboration with a professional specialised in the area e.g. occupational physician, occupational therapist, or occupational health nurse.
Establishing a graded return to work programme is important for those experiencing LongCOVID fatigue and draws on the energy conservation principle of pacing. Issues can arise where the ‘goalposts’ for a full return to work are set prematurely. Instead, it can be useful for HCWs to continually review their status with their employer. Two-way communication between employer-employee is key. Establishing a minimum time for rest should post-exertional malaise (PEM) exist, and allowing fatigue only to reach agreed levels upon graded return to work may offer the best means in working within the limitations of LongCOVID fatigue. This is facilitated by ensuring that surveillance of all exposed HCWs is consistent and that individuals are provided with additional psychological and workrelated support. Considerations should also be given regarding the type of work which HCWs are completing both in the context of day-to-day work and graded return to work plans. Tasks should be considered in respect
Poor knowledge of patient condition
Poor collegial support
Higher level of patient caseload & Emergency tasks
Variables on work-related cognative load
Poor communication with patients
Higher level of difficulty of task
Postprandial dip / Night-shift work
to how physically, cognitively and emotionally demanding they may be through self-reported outcome measurements. Ergonomic changes to reduce physical exertion, and ‘cognitive offloaders’ such as experience of having a team to support in decision-making may assist in managing and pacing in occupational settings.
Examples of known ‘cognitive loaders’ modelled from adapted to healthcare are described in Figure 1. These have applicability for individuals in self-management of performance, but also organisation systems such as planning oncall rotas in conjunction with a screening programme to detect ‘at risk’ personnel.
A number of safeguards are recommended for HCWs, including access to mental health services throughout their career trajectory may be useful for psychological needs, while ‘enveloping’ of work activity is important so as to prevent delayed onset of fatigue symptoms due to overload resulting
from increased physical demands. The Energy Envelope Theory stems from ME/CFS research and posits that those with fatigue should not expend more energy than they perceive themselves to have (Jason et al, 2013).
Finally, one area of managing stress is using positive psychological based interventions such as increasing self-awareness and emotional regulation, training in resiliency building and mindfulness based behaviour-change practices (Rogers, 2016). These challenge conventional norms of viewing stress as a harmful activity, instead mediating its activity in a way which protects professional performance. Meditation has been shown to reduce activity in stress-promoting regions of the brain (Zeller and Levin, 2013). The risks associated with psychological stressors such as anxiety were higher in those healthcare workers who reported decreased sleep quality during the COVID-19 pandemic (Magnavita et al., 2020), suggesting sleep also may also play a pivotal role for both viral management and occupational-stress managed fatigue.
Creating a system for fatigue tracking
In the absence of Fatigue Risk Management Systems (FRMS) in healthcare, drawing on best practice from parallel industries such as the International Civil Aviation Organisation (ICAO) FRMS task force (ICAO, 2016) could be an innovative approach in identifying occupational and non-occupational fatigue. Such systems mandate fatigue screening and identify at what point fatigue becomes decremental for personal wellbeing and professional performance. This allows timely intervention and mitigation. In the context of the COVID-19 pandemic, no screening outcome tools have been widely endorsed to identify fatigue in healthcare. Monitoring fatigue through fatigue logs which include aggravating and easing factors may be useful in helping to differentiate triggers of fatigue. Using tracking-devices, either automated or self-reported, to track known variables such as heart rate monitoring for PEM management, or which contribute to fatigue in people's personal lives such as sleep, physical
Figure 1: Workload considerations in healthcare setting in management of fatigue
29 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
Health Reform
activity and diet may highlight the influence of such lifestyle factors on their own fatigue. Feedback from tracking increases selfawareness within individuals and adapts behaviours at the point of ‘at risk’. It also informs institutional fatigue risk management responses by identifying high level data on fatigue levels within sections of the hospital. Clinical and organisational leadership would benefit from collaborative commitments to implement uniform fatigue screening programmes, throughout occupational health and clinical departments, to monitor performance within health systems.
Training Programme
Training staff of fatigue management
Ensuring effective training of all levels of stakeholders is important to maximise buy-in to fatigue reporting and mitigation. Training on fatigue and risks associated with fatigue can develop institutional capacity and resilience in adapting to the required behaviour changes. It can also encourage self-regulation of individuals' performance in identifying when ‘at risk’ of performance decrement. An example of a tailored fatigue education programme, modelled
and adapted off the ICAO programme (ICAO, 2016) and adapted to a healthcare setting is seen in Table 1 below. Learning and development departments could lead organisations through structured implementation of training programmes across a range of management levels.
Conclusion
There exists growing pressures on healthcare, particularly with the increasing ‘reactiveness’ of the system due to ongoing challenges beyond resource capability. This shouldn’t negate the need to recognise that fatigue in healthcare
An overview of fatigue risk management structure within the hospital, including the concepts of shared responsibility and encouraging effective reporting
Information about health consequences of fatigue
Information about social and environmental consequences of fatigue
Psychological training through evidence based approaches embedded in psychological resilience to work place stressors
The importance of self regulation of fatigue in performance in management
How to identify fatigue in themselves (self awareness) and in others
Mitigation strategies available to individuals for fatigue management (legislatively, institutionally, structurally, and individually)
Table 1: Example of a training curriculum on fatigue risk management for healthcare workers
personnel remains a significant concealed risk to sustainability of healthcare provision. The link between leadership and increased work engagement is important, as it is associated with acquiring additional job resources (Salanova and Schaufeli, 2008) which has positive implications developing systemic changes for fatigue management in healthcare systems. Similarly, through improving staff engagement by focusing on effective fatigue management strategies, there is evidence of strong association of recovery of the workforce (Schaufeli and Bakker, 2004) to sustain further phases of the impact of the pandemic and subsequent fallout. Recognising mitigation systems from successful industries such as aviation in identifying those at risk of fatigue gives healthcare an opportunity to contextualise relevant aspects of good practice and reflects the importance of challenging processes and offering leadership which transforms current practice to a model which is evidence based and effective. One of the leading healthcare innovators of our time, Atul Gawande, said “Better is possible. It does not take genius. It takes diligence. It takes moral clarity. It takes ingenuity. And above all, it takes a willingness to try”. With the increased focus on healthcare systems during the pandemic, one which praised healthcare workers as the front-of-the-frontline, this is an opportunity to show our staff we value them for their heroic efforts, by effectively challenging long-standing issues of poor workforce health and wellbeinglet us not waste it.
Irish Osteoporosis Society Annual Conference

Conclusion
The Irish Osteoporosis Society
Annual medical conference will be held virtually on Saturday 22nd October 2022.
The event, which is CPD applied for, features a wide ranging programme with the opening address being delivered by
President of the Society, Professor Moira O’Brien.
Other keynote speakers include; Dr Kevin McCarroll, Consultant Physician & Geriatrician at the Bone Health Unit in St James Hospital talking about ‘Hyperparathyroidism’
– Ms Aoife Ni Eochaidh, Chartered Physiotherapist, Clinical Specialist Physiotherapist at Bon Secour Consultant’s Clinic speaking about ‘Women’s Health’ and Dr Derek
There exists growing pressures on healthcare, particularly with the increasing ‘reactiveness’ of the system due to ongoing challenges beyond resource capability. This shouldn’t negate the need to recognise that fatigue in healthcare personnel remains a significant concealed risk to sustainability of healthcare provision. The link between leadership and increased work engagement is important, as it is associated with acquiring additional job resources (Salanova and Schaufeli, 2008) which has positive implications developing systemic changes for fatigue management in healthcare systems. Similarly, through improving staff engagement by focusing on effective fatigue management strategies, there is evidence of strong association of recovery of the workforce (Schaufeli and Bakker, 2004) to sustain further phases of the impact of the pandemic and subsequent fallout. Recognising mitigation systems from successful industries such as aviation in identifying those at risk of fatigue gives healthcare an opportunity to contextualise relevant aspects of good practice and reflects the importance of challenging processes and offering leadership which transforms current practice to a model which is evidence based and effective. One of the leading healthcare innovators
Bennett, President of the Irish American Orthopaedic Society and Member of the European Board of Orthopaedic & Trauma Surgeons who will be looking at ‘License to Kill – The Double O’s of Bone Health.’
Furthermore there will be oral abstracts and question and answer chaired sessions. You can find out more by visiting www.irishosteoporosis.ie
Table 1: Example of a training curriculum on fatigue risk management for healthcare workers
30 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
News
60 Second Summary
• Saint John of God Hospital Pharmacy, the HSE National Medication Safety Programme and the Irish Pharmacy Union have collaborated to launch a national patient information booklet on lithium therapy.
• This initiative is intended to promote and support safer lithium therapy, and empower patients to engage with their Healthcare Professional to discuss all aspects of lithium therapy, monitoring, and side-effects.
• The booklet has been produced by Audrey Purcell, Chief 2 Pharmacist, Saint John of God Hospital, and supported by Professor Dolores Keating, Chief 1 Pharmacist, and the Hospital Drug and Therapeutics Committee.

• It has been reviewed and endorsed by Ciara Kirke, HSE Clinical Lead, National Medication Safety Programme, and the national print supported by the HSE’s National Quality and Patient Safety Directorate.
• It has been edited and reviewed by the National Adult Literacy Agency and has successfully been awarded the plain English mark by NALA.
• It has been reviewed by the Irish Medication Safety Network, Irish Pharmacy Union and the College of Psychiatrists.
• The booklet contains an information section with important safety and clinical information on Lithium therapy, a programme of monitoring, and a record book to record essential information on lithium levels and blood test results.
• Lithium booklets will be distributed from the HSE directly to Hospital Pharmacies, and to Community Pharmacies via the IPU. GPs may sign-post patients to Community Pharmacists to avail of a booklet.
• The electronic template is available on the HSE website www. safermeds.ie and Saint John of God Hospital website https://www. stjohnofgodhospital.ie/images/ National_Lithium_Booklet_final_ version_September_2022.pdf
• Lithium booklets may be reordered from Saint John of God Hospital Pharmacy: hospital. pharmacy@sjog.ie
1. REFLECT Before reading this module, consider the following: Will this clinical area be relevant to my practice?
2. IDENTIFY If the answer is no, I may still be interested in the area but the article may not contribute towards my continuing professional development (CPD). If the answer is yes, I should identify any knowledge gaps in the clinical area.
3. PLAN If I have identified a knowledge gap - will this article satisfy those needs - or will more reading be required?
4. EVALUATE Did this article meet my learning needs - and how has my practise changed as a result?Have I identified further learning needs?
5. WHAT NEXT At this time you may like to record your learning for future use or assessment. Follow the 4 previous steps, log and record your findings.
Published by HPN. Copies can be downloaded from www.irishpharmacytraining.ie
Disclaimer: All material published is copyright, no part of this can be used in any other publication without permission of the publishers and author.
Lithium Therapy: a national patient safety and quality improvement initiative
1. BACKGROUND AND CLINICAL INFORMATION:
Lithium Indications:
• Bipolar Disorder: mania, hypomania and prophylaxis of Bipolar Disorder
• Recurrent Depressive Disorder: used to augment antidepressants
• Reduction of intentional self-harm and suicidality.
Lithium productsProduct: Formulation: Recommended dosing: Lithium level sampling time:
Priadel Tablets: 200mg+400mg (200mg tablet may be halved to facilitate 100mg dose )
Camcolit tablet EMP 400mg
EMP: Exempt Medicinal Product
Priadel Liquid EMP 520mg/5ml
Li-Liquid EMP 509mg/5ml
Li Liquid EMP 509mg/5ml

Lithium
Lithium Carbonate. 400mg tablet contains 10.8mmol/L Lithium.

Lithium Carbonate
Once daily: at night time (Prolonged Release)
Once daily: at night time (Prolonged Release)
Lithium Citrate
520mg/5ml : equivalent to Lithium Carbonate 204mg/5ml (calculate as 200mg/5ml)
equivalent to Lithium Carbonate 204mg/5ml (calculate as 200mg/5ml)
Lithium Citrate
Lithium Citrate
509mg/ml: equivalent to Lithium Carbonate 200mg/5ml (calculate as 200mg/5ml)
509mg/ml: equivalent to Lithium Carbonate 200mg/5ml (calculate as 200mg/5ml)
Once daily: at night time (Prolonged Release)
12 hours post dose (acceptable 10 14 hours)
time:
12 hours post dose (acceptable 10 14 hours)
12 hours post dose (acceptable 10 14 hours)
(Prolonged Release) 12 hours post dose (acceptable 10 14 hours)
Once daily:
Twice daily: morning and night (Immediate Release)
Twice daily: morning and night (Immediate Release)
Pre morning dose
Pre morning dose
Twice daily: morning and night (Immediate Release)
Twice daily: morning and night (Immediate Release)
Pre morning dose
Pre morning dose
AUTHORS: Audrey Purcell B(Sc) Pharm. MPSI. MSc. Psych Pharm.
Chief 2 Pharmacist, Saint John of God Hospital, Stillorgan, Co Dublin.
Honorary Senior Clinical Lecturer, Royal College of Surgeons in Ireland.
31
CPD 92: LITHIUM MANAGEMENT
CPD Continuing Professional Development CPD Product: Formulation: Recommended dosing: Lithium level sampling
Priadel Tablets: 200mg+400mg (200mg tablet may be halved to facilitate 100mg dose )
Carbonate. 400mg tablet contains 10.8mmol/L Lithium.
Camcolit tablet EMP 400mg EMP: Exempt Medicinal Product Lithium Carbonate
at night time
Priadel Liquid EMP 520mg/5ml Lithium Citrate 520mg/5ml :
before initiation
Hypothyroidism: Lithium has varied effects on Thyroid Hormone production and regulation, including inhibition of Iodine uptake in the Thyroid, inhibition of Thyroid Hormone synthesis and release, and hepatic conversion of free Thyroxine. Patient may present with typical hypothyroidism symptoms including lethargy, impaired cognition, weight gain, dry skin, and cold intolerance. Risk factors include females, older adults, family history of hypothyroidism, and presence of anti-thyroid antibodies.

Switching lithium products:
Prescribing:
Plasma levels:
Priadel tablets are recommended for routine use. The tablets have score- lines therefore they can be divided accurately to provide smaller dosage requirements. If a patient is unable to swallow tablets a liquid may be prescribed. It is essential that a switch from tablets to liquid is prescribed by their Doctor, and calculation confirmed by Pharmacist.
impairment or those below 50kg in weight, often require lower starting dose (eg 200mg), and maintenance doses.
• Significant change in patient’s sodium or fluid intake
• Last serum lithium level >0.8mmol/L
Starting dose may usually range from 400mg 800mg OD (nocte) in adults, depending on indication. Elderly patients, those with renal impairment or those below 50kg in weight, often require lower starting dose (eg 200mg), and maintenance doses.
Plasma levels:
Lithium plasma level should be checked 5-7 days after starting, after every dose change, and after addition/discontinuation of medication that can affect level.
Target levels:
• Interacting medicines. Key interacting medicines include: ACEI, ARBs, NSAIDs and Thiazide diuretics. (See summary below and BNF/ Stockley’s for exhaustive list).
Discontinuation:
Lithium plasma level should be checked 5-7 days after starting, after every dose change, and after addition/discontinuation of medication that can affect level.
Target levels:
Example: Switching patient from Priadel tablet (Carbonate) 800mg nocte to Priadel liquid (Citrate) = 400mg (10ml) BD morning and night. Lithium level to be checked 5-7 days post- switch.
Hyperparathyroidism and hypercalcaemia: Hypercalcaemia has been reported with Lithium therapy, which may or may not be related to drug-induced hyperparathyroidism. While lithium has been observed to affect Parathyroid Hormone levels after a single dose, long -term exposure is likely required to observe clinically relevant alterations in Calcium homeostasis.
Baseline work-up:
• ECG : if cardiac history, risk factors for QTc prolongation, concomitant medicines that prolong QTc
The minimum effective plasma level for prophylaxis in adults is 0.4mmol/L; optimal range is 0.60.8 mmol/L. A level of 0.4mmol/L may be effective in unipolar depression; 0.6 -1 mmol/L in Bipolar Disorder, and levels at the higher end of the range in mania (0.8-1mmol/L).
If a decision is made to discontinue lithium, the risk of relapse may be reduced by reducing the dose gradually. It is recommended to reduce the dose slowly over at least 4 weeks or longer, and preferably up to 3 months in Bipolar Disorder; except in medical emergency or overdose.
Polydipsia and polyuria: Common adverse effects associated with lithium. Patients may notice increased urinary frequency (> 3 L in 24 hours) due to poor urine concentration; and increased thirst, which is independent of dry mouth effects of lithium.
The minimum effective plasma level for prophylaxis in adults is 0.4mmol/L; optimal range is 0.6 0.8 mmol/L. A level of 0.4mmol/L may be effective in unipolar depression; 0.6 1 mmol/L in Bipolar Disorder, and levels at the higher end of the range in mania (0.8 1mmol/L).
• Weight and height
• Urea and Electrolytes
• Serum Creatinine+/-estimated Glomerular Filtration Rate (eGFR)
• Adjusted Calcium
• Thyroid Function Tests (TFTs): include Free T4 and Thyroid Stimulating Hormone (TSH). Patient should be euthyroid before initiation
• Full Blood Count
• Pregnancy test and review of contraception (in women of childbearing age).
Prescribing:
Starting dose may usually range from 400mg-800mg OD (nocte) in adults, depending on indication. Elderly patients, those with renal
Monitoring frequency:
TFTs, renal function, Calcium level, and weight check, recommended every 6 months; or every 3 months in at-risk patients.
Once stable, serum lithium levels recommended every 3 months for the first year, then every 6 months; or every 3 months in at-risk patients.
At risk patients include:
• Elderly (> 65 years)
• Have received less than 12 months treatment
• Renal impairment (eGFR<60ml/min)
• Impaired Thyroid function at last test
• Raised Calcium level (adjusted) at last test
• Poor symptom control or suspected poor adherence
ALARM BELL INTERACTIONS: THINK “ANTS” (SEE TABLE PAGE 33)
ADVERSE EFFECTS : ( NOT AN EXHAUSTIVE LIST)
Cardiac: Lithium may cause cardiac arrhythmia, including bradycardia, sinoatrial dysfunction (SA block), abnormal T waves on ECG (T-wave inversion), and STsegment depression.
Dermatological: Lithium may cause acne vulgaris and/or psoriasis (including exacerbation of both) in patients with and without either condition at baseline.
GI: Lithium may cause dyspepsia, diarrhoea, nausea, vomiting, dysgeusia (metallic or salty taste), gastritis and abdominal pain. Some effects (e.g. nausea) may occur early in treatment. Other effects may take longer to develop. Supratherapeutic lithium levels should be suspected with severe nausea, vomiting and diarrhoea.
Renal effects: Up to one-third of patients may develop some degree of decreased kidney function during the course of lithium therapy, with approximately 5% developing significant kidney impairment/failure.
Sexual dysfunction: Studies report rates of the various effects of 5-40 %. Effects can include decreased libido, impaired sexual arousal, and erectile dysfunction. Sexual dysfunction can negatively impact a patient’s quality of life.
Tremor: Lithium can cause tremor in up to 25% of patients, making it one of the most common adverse effects. This is commonly a bilateral, symmetrical hand tremor, which may spontaneously decrease over time as compensatory mechanisms develop within the patient. Course tremor and muscle twitching may be observed in lithium toxicity.
Tremor commonly begins early in treatment, but can develop later in treatment, with or without a dose increase. Risk factors include: higher doses/serum levels, medicines that can increase
Pregnancy test and review of contraception (in women of childbearing age)
32 CPD 92: LITHIUM MANAGEMENT
euthyroid
• Full Blood Count •
Name: Magnitude: Timescale: Additional Information:
A: ACE Inhibitors (ACEI) Angiotensin Receptor Blockers (ARBs)
Unpredictable
Up to four fold increase in lithium level
Develops over several weeks
Most likely to cause lithium toxicity within a month of starting
Sevenfold increased risk of hospitalisation for lithium toxicity in the elderly
Consider alternative anti hypertensive
If combination necessary, increased monitoring of lithium level and renal function required.
Clinic/Hospital for urgent lithium level and U+Es, and seek specialist advice.
Referral to secondary care may be required depending on the severity of symptoms.
LITHIUM THERAPY : A BLUE BOOK FOR SAFETY
N: Non Steroidal Anti inflammatories (NSAIDs)
T: Thiazide Diuretics
T: Thiazide Diuretics
Unpredictable
From 10% to more than four fold increase in lithium level
than four fold increase in lithium level
Unpredictable
Unpredictable
Up to four fold increase in lithium level
Up to four fold increase in lithium level
Variable: a few days to several months.
Variable: a few days to several months
Diclofenac: up to 23% increase in lithium level
S: Sodium
S: Sodium
Excess Sodium can reduce lithium levels
Excess Sodium can reduce lithium levels
Sodium restriction can lead to lithium toxicity.
Sodium restriction can lead to lithium toxicity.
Thiazides reduce renal clearance of lithium and levels can rise within a few days
Thiazides reduce renal clearance of lithium and levels can rise within a few days
Usually apparent in the first 10 days
Usually apparent in the first 10 days
Any effect will be apparent in the first month.
Any effect will be apparent in the first month.
lithium level, medicines known to induce tremor (e.g. antipsychotic, antidepressants), caffeine and older adults.
Weight gain: Increases of 4 to 7 Kg within the first year have been reported in the literature.
Effects on central mechanisms related to weight gain, satiety and metabolism are possible.
Increased consumption of highcalorie, sugary beverages from increased thirst with lithium could contribute
Diclofenac: up to 23% increase in lithium level
Ibuprofen: up to 25% increase in lithium level
Ibuprofen: up to 25% increase in lithium level
Patients should be advised to avoid OTC NSAIDs and use Paracetamol if OTC analgesic required
Patients should be advised to avoid OTC NSAIDs and use Paracetamol if OTC analgesic required
Thiazide diuretics should only be used where unavoidable and with strict monitoring
Thiazide diuretics should only be used where unavoidable and with strict monitoring
Loop diuretics may be safer Furosemide is probably the safest diuretic to use with lithium but frequent monitoring required.
Loop diuretics may be safer Furosemide is probably the safest diuretic to use with lithium but frequent monitoring required.
Consider high Sodium content OTC preparations and recommend suitable alternatives
Consider high Sodium content OTC preparations and recommend suitable alternatives
Care with Sodium content in effervescent formulation
Care with Sodium content in effervescent formulation
Refer to SPS Pharmacy NHS “Considering Sodium content of medicines”; particular care with products > 17mmol Sodium in maximum daily dose https://www.sps.nhs.uk/articles/consi dering sodium content of medicines/
Refer to SPS Pharmacy NHS “Considering Sodium content of medicines”; particular care with products > 17mmol Sodium in maximum daily dose https://www.sps.nhs.uk/articles/consi dering sodium content of medicines/
Example: Sodium Bicarbonate in antacids (Gaviscon); recommend Maalox instead.
Example: Sodium Bicarbonate in antacids (Gaviscon); recommend Maalox instead.
• The national patient information booklet has been produced by Audrey Purcell, Chief 2 Pharmacist, Saint John of God Hospital, and supported by Professor Dolores Keating, Chief Pharmacist, and Saint John of God Drug and Therapeutics Committee
• This initiative is intended to provide and promote safer lithium therapy, and empower patients to engage with their Healthcare Professional to discuss all aspects of lithium therapy, monitoring, and sideeffects. Appropriate information and monitoring is imperative to ensure best outcomes for patients on lithium therapy and reduce likelihood of harm.
• The booklet has been reviewed and endorsed by Ciara Kirke, HSE Clinical Lead, National Medication Safety Programme, and the national print supported by the HSE’s National Quality and Patient Safety Directorate.
• It has been edited and reviewed by the National Adult Literacy Agency and has successfully been awarded the plain English mark by NALA.
• It has been reviewed and endorsed by Saint John of God Hospital Drug and Therapeutics Committee, Irish Medication Safety Network, Irish Pharmacy Union, and the College of Psychiatrists.
The Lithium booklet contains:
• Essential clinical and safety information for patients
• Information on side-effects and signs of toxicity
Symptoms of toxicity:
For patients with symptoms of toxicity (eg diarrhoea, vomiting, coarse tremor, mental state changes or falls):
Withhold lithium, refer to GP/
Advise on appropriate OTC medicines and contra-
programme of monitoring to ensure safe and appropriate
33
•
indications • A
monitoring Name: Magnitude: Timescale: Additional Information: A: ACE Inhibitors (ACEI) Angiotensin Receptor Blockers (ARBs) Unpredictable Up to four fold increase in lithium level Develops over several weeks Most likely to cause lithium toxicity within a month of starting Sevenfold increased risk of hospitalisation for lithium toxicity in the elderly Consider alternative anti hypertensive If combination necessary, increased monitoring of lithium level and renal function required. N: Non Steroidal Anti inflammatories (NSAIDs) Unpredictable From 10% to more
What happens
If you have too much lithium
blood,
• A record book to record lithium levels and essential blood test results.
Healthcare Professionals provide essential support and are recommended to:

• Ensure patients have a lithium booklet
• Reinforce essential information verbally
• Refer patient to the booklet to be aware of potential side-effects and signs of toxicity
• Ensure the patient understands their own programme of monitoring

• Support patients to engage in appropriate blood test monitoring: keep their record book up to date, and have available at consultations with GP, Consultant, Pharmacist, Nurse.
National launch of the Lithium booklet:
• Saint John of God Hospital, the HSE National Medication Safety Programme and the Irish Pharmacy Union have collaborated to implement a national launch of the booklet.
• Lithium booklets will be distributed from the HSE directly to Hospital Pharmacies, and to Community Pharmacies via the IPU Review. GPs may sign-post patients to Community Pharmacists to avail of a booklet.



• Booklets can be ordered from Saint John of God Hospital: hospital.pharmacy@sjog.ie


Acknowledgements:
Many thanks to Aoife Carolan, Senior Pharmacist, Saint John of God Hospital, and Ciara Kirke, HSE Clinical Lead, for article peer-review.
References:
1. Goodwin GM, Haddad PM, Ferrier IN, Aronson JK, Barness TRH, Cipriani A, et al. Evidence-based guidelines for treating Bipolar Disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016: 30(6); 495-553. Available online https://www.bap.org.uk/ pdfs/BAP_Guidelines-Bipolar.pdf (accessed 15/07/2022)
2. The National Institute for Health and Care Excellence (NICE) Guideline Bipolar Disorder: Assessment and Management (NICE 2014). Available online https://www. nice.org.uk/guidance/cg185 (accessed 15/07/2022)
3. Up To Date. Lithium: Drug Information. Available online: www.uptodate.com
4. Taylor D, Barnes T, Young A. The Maudsley Prescribing Guidelines in Psychiatry. 14th ed. West Sussex (England): Wiley Blackwell; 2021.
ill.
Read the following list very carefully. If you get one or more of these problems
5. Bazire S. Psychotropic Drug Directory 2020/21. London: Lloyd-Reinhold Publications Limited; 2020.
6. Specialist Pharmacy Service NHS. July 2021. Lithium Monitoring. Available online https://www.sps.nhs.uk/monitorings/ lithium-monitoring/ (accessed 15/07/2022)
7. Specialist Pharmacy Service NHS. October 2021. Considering sodium content of medicines. Available online https://www. sps.nhs.uk/articles/considering-sodiumcontent-of-medicines/#:~:text=This%20 medicine%20contains%20less%20 than,say%20essentially%20 'sodium%2Dfree (accessed 15/07/2022)
8. Choice and Medication. Lithium Patient Information Leaflet. Available online https://www.choiceandmedication. org/stjohnofgodhospital/medication/ lithium-carbonate-and-citrate/ (accessed 15/07/2022)
Saint John of God Hospital
34 CPD 92: LITHIUM MANAGEMENT
There are two parts to this booklet: • an information section, and a record book. The Lithium Record book is on page 19. Page number 1. What is lithium and what is it used for? 3 2. What checks are needed before starting lithium? 4 3. How do I take lithium? 5 4. What blood tests do I have when I take lithium? 8 5. What side effects can lithium cause? 10 6. What happens if lithium level in my blood is too high? 13 7. What can make lithium blood levels too high? 14 8. Pregnancy and lithium: what do I need to know? 16 9. Alcohol and lithium: what do I need to know? 17 About this booklet Information section 2 13 6.
if lithium level in my blood is too high?
in your
this is called lithium toxicity (or lithium poisoning). This can make you very
at any time, talk to your doctor straight away. A small number of people may not have any immediate symptoms of toxicity when the lithium in their blood is too high. This is why it is important to have regular checks. Regular checks can prevent long-term problems. Symptoms if there is too much lithium in your system • Severe hand shake (tremor). Vomiting or severe nausea and persistent diarrhoea. • Muscle weakness. • Being unsteady on your feet. • Muscle twitches. • Slurring of words so that it is difficult for others to understand what you are saying. • Blurred vision. • Confusion. Feeling unusually sleepy. Date Daily Dose (mg): Lithium blood level (mmol/L) Kidney Checks (eGFR/ Creatinine) + Ref Range Thyroid checks TSH (mlU/L): + Ref Range Thyroid checks Free T4 (pmol/L): + Ref Range Adjusted Calcium (mmol/L): + Ref Range Weight/ BMI: Date of next blood test: Example 1: 2/3/22 Example 2: 2/3/22 600mg 600mg 0.8 0.8 eGFR 85 ✓ 2.59 (0.27-4.2) ✓ 13 (12-22) ✓ 2.25 (2.1-2.6) ✓ 67kg 67kg 6/6/22 6/6/22 1 2 3 4 5 6 7 8 19 Lithium therapy record book – your healthcare Produced by Audrey Purcell, Chief 2 Pharmacist, Saint John of God Hospital, Stillorgan, Co Dublin. Reviewed and endorsed by: all above Adapted with permission from the National Patient Safety Agency, UK. Version 2: February 2022. Safety Medication Irish Network Produced by Audrey Purcell Senior Pharmacist, and Colleagues, Saint John of God Hospital, Stillorgan, Co Dublin. Adapted with permission from the National Patient Safety Agency, UK. October 2013. To re-order please contact KPW Ballinasloe Tel: 090 9642297 Produced by Audrey Purcell, Chief 2 Pharmacist, Saint John of God Hospital, Stillorgan, Co Dublin. Reviewed and endorsed by: all above Adapted with permission from the National Patient Safety Agency, UK. Version 2: February 2022. Safety Medication Irish Network Produced by Audrey Purcell Senior Pharmacist, and Colleagues, Saint John of God Hospital, Stillorgan, Co Dublin. Adapted with permission from the National Patient Safety Agency, UK. October 2013. To re-order please contact KPW Ballinasloe Tel: 090 9642297
OSTEOPOROSIS
Efficacy of the Combination of Teriparatide and Denosumab in the Treatment of Postmenopausal Osteoporosis: A Meta-Analysis
 Written by Yang Sun1, Yue Li2, Jiangbi Li1, Xiaoping Xie1, Feng Gu1, Zhenjiang Sui1, Ke Zhang1, and Tiecheng Yu1,*
Written by Yang Sun1, Yue Li2, Jiangbi Li1, Xiaoping Xie1, Feng Gu1, Zhenjiang Sui1, Ke Zhang1, and Tiecheng Yu1,*
1Department of Orthopedics, The First Hospital of Jilin University, Jilin Changchun, China
2Department of Social Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
The most prevalent bone disease among aged women is postmenopausal osteoporosis (Kahwati et al., 2018). It is a major cause of fracture, which results in substantial morbidity, mortality, and financial burdens (Liu Y. et al., 2018; Nayak and Greenspan, 2018). Skeletal fragility and microarchitectural degeneration are hallmarks of the disease (Black and Rosen, 2016a). The most of patients with postmenopausal osteoporosis are probably attributable to genetically determined low bone mass combined with bone loss associated with oestrogen deficiency (Andreopoulou and Bockman, 2015). In the United States, osteoporosis causes millions of fractures each year, the vast majority of which occur in postmenopausal women (Black and Rosen, 2016a). Moreover, nearly 9 million osteoporosisrelated fractures occur each year (Liu GF. et al., 2018) The global cost of osteoporosis-related fractures is supposed to surpass $25.3 billion per year by 2025 (Burge et al., 2007).

Until now, a variety of pharmacotherapies have been available for postmenopausal osteoporosis (Khosla and Hofbauer, 2017). Postmenopausal osteoporosis medications could generally be divided into two categories. Antiresorptive medications like nitrogencontaining bisphosphonates and denosumab, a receptor activator of nuclear factor B ligand inhibitor, are the most commonly used drugs. The other category is the anabolic agents teriparatide [PTH-(1–34)] and PTH [PTH-(1–84)], which are mostly used for patients with serious and established osteoporosis. In addition to stimulating osteoblastic bone formation, these peptides also stimulate bone resorption (Dempster et al., 2012). Despite
the fact that therapeutic options for postmenopausal osteoporosis have increased during the past few years (Khosla and Hofbauer, 2017), no currently approved therapy seems to restore normal bone integrity in the most of patients with established osteoporosis and choices for severe patients are still constrained. Approved osteoporosis therapies are generally constrained to the prescription of a single drug with a set dosage and dosing
frequency. Combination treatment with anabolic and antiresorptive agents has been suggested as a method to improve the treatment efficacy. However, attempts to combine teriparatide or PTH with bisphosphonates have failed since no combination has ever been proven to be consistently better than monotherapy (Black et al., 2003; Finkelstein et al., 2003; Finkelstein et al., 2010; Cosman et al., 2011). With the publication of clinical trials
and the accumulation of clinical experience, we have found that the combination of teriparatide and denosumab probably has better efficacy in postmenopausal osteoporosis (Tsai et al., 2013; Leder et al., 2015; Idolazzi et al., 2016; Nakamura et al., 2017; Suzuki et al., 2019). To further corroborate the clinical value of the combination treatment, we have made this meta-analysis to provide the basis for this new treatment modality.
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022 35
WOMEN’S HEALTH
Figure 1
Methods
The Preferred Reporting Items for Systematic Reviews and MetaAnalysis (PRISMA) statement is followed in this study (Moher et al., 2009). A formal protocol was developed and registered on the INPLASY international platform of registered systematic review and meta-analysis protocols (INPLASY Protocol 202210092).
Results
Literature Search
The process of literature screening, study selection, and reasons for exclusion are depicted in the PRISMA statement flowchart (Figure 1). We found 336 records in our initial search. 10 articles were considered potentially eligible for inclusion after duplicates were removed and titles and abstracts were screened. 5 RCTs (Tsai et al., 2013; Leder et al., 2015; Idolazzi et al., 2016; Nakamura et al., 2017; Suzuki et al., 2019) were finally included in the metaanalysis after a thorough review of the full text.
Characteristics of the Included Studies
This meta-analysis included 6 studies published between 2013 and 2018. They included 297 patients with postmenopausal


osteoporosis accepting the combination treatment or monotherapy from Northern America (2 studies), Asia (2 studies), and Europe (1 study). The mean age of the included participants ranges from 67 to 75 years old. Population sizes ranged from 30 to 94. Among the selected studies, 3 of 5 trials compared combination with teriparatide, 5 of 5 compared combination with denosumab.
Quality Assessment
In 5 trials, an sufficient randomized sequence was generated, and 2 trials reported proper allocation concealment. In 5 trials, participant and personnel blinding was unclear or rarely reported. The blinding of outcome assessment was unclear in 2 trials and the other 3 trials were sorted as being at low risk. None of the 5 trials had incomplete outcome data, selective reporting, or other bias (Supplementary Figures S1, S2).
Primary Outcome
Mean Percent Change of BMD in Lumbar Spine
5 trials (Tsai et al., 2013; Leder et al., 2015; Idolazzi et al., 2016; Nakamura et al., 2017; Suzuki et al., 2019) with 297 patients provided the BMD data and were included in the analysis. Before conducting the meta-analysis, it is important to
WOMEN’S HEALTH OSTEOPOROSIS
ensure that there is no difference between the baseline values of the combination therapy and control therapy. The positive values indicates that the combination group is higher. The results are as follows : standard mean difference (SMD) =0.05, 95%CI: 0.16∼0.26; I2 = 23.4%, p = 0.243; Z = 0.47, p = 0.637 (Supplementary Figure S3). There was no significant difference in baseline values between groups. Compared with the monotherapy, combination treatment can significantly improve the BMD in the lumbar spine. The results are as follows : WMD = 2.91, 95%CI: 1.98 3.83; I2 = 38.7%, p = 0.121; Z = 6.16, p = 0.00 (Figure 2).
Mean Percent Change of BMD in Hip
5 trials (Tsai et al., 2013; Leder et al., 2015; Idolazzi et al., 2016; Nakamura et al., 2017; Suzuki et al., 2019) with 297 patients provided the BMD data and were included in the analysis. Before conducting the meta-analysis, it is important to ensure that there is no difference between the baseline values of the combination therapy and control therapy. The positive values indicates that the combination group is higher. The results are as follows : SMD = 0.10, 95%CI: 0.31∼0.10; I2 = 0.0%, p = 0.993; Z = 0.99, p = 0.322 (Supplementary Figure S4). There was no significant difference in baseline
values between groups. Compared with the monotherapy, combination treatment can significantly improve the BMD in hip. The results are as follows : WMD = 3.19, 95%CI: 2.25 4.13; I2 = 59.9%, p = 0.015; Z = 6.68, p = 0.00 (Figure 3).
Second Outcome
Mean Percent Change of CTX in Serum
3 trials (Tsai et al., 2013; Leder et al., 2015; Idolazzi et al., 2016) with 230 patients provided the CTX data and were included in the analysis. Before conducting the meta-analysis, it is important to ensure that there is no difference between the baseline values of the combination therapy and control therapy. The positive values indicates that the combination group is higher. The results are as follows : WMD = 0.03, 95%CI: 0.01∼0.08; I2 = 37.7%, p = 0.155; Z = 1.70, p = 0.09 (Supplementary Figure S5). There was no significant difference in baseline values between groups. We integrated the data and summarized them on a single graph (Supplementary Figure S6). The results are as follows. Compared with monotherapy, combination can decrease CTX levels significantly: WMD = 23.68, 95%CI: 33.82 13.54; I2 = 95.5%, p = 0.000; Z = 4.58, p = 0.361.
Mean Percent Change of 25(OH)D in Serum
2 trials (Nakamura et al., 2017; Suzuki et al., 2019) with 67 patients provided the 25(OH) D data and were included in the analysis. Before conducting the meta-analysis, it is important to ensure that there is no difference between the baseline values of the combination therapy and control therapy. The positive values indicates that the combination group is higher. The results are as follows : WMD = 2.52, 95%CI: 8.32 13.37; I2 = 0.0%, p = 0.923; Z = 0.46, p = 0.648. There was no significant difference in baseline values between groups. Compared with denosumab, combination can improve 25(OH)D levels but has no statistical significance: WMD = 12.27, 95%CI: 8.91∼33.45; I2 = 27.2%, p = 0.241; Z = 1.14, p = 0.256.
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE 36
Figure 2
ABBREVIATED PRESCRIBING INFORMATION

































Please refer to the Summary of Product Characteristics (SmPC) before prescribing Pelgraz▼(peg lgrastim) 6 mg solution for injection in pre- lled syringe or prelled injector. Presentation: Each pre- lled syringe or pre- lled injector contains 6 mg of peg lgrastim* in 0.6 mL solution for injection. The concentration is 10 mg/mL based on protein only**. *Produced in Escherichia coli cells by recombinant DNA technology followed by conjugation with polyethylene glycol (PEG). ** The concentration is 20 mg/mL if the PEG moiety is included. Indications: Reduction in the duration of neutropenia and the incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancy (with the exception of chronic myeloid leukaemia and myelodysplastic syndromes). Dosage and Administration: Pelgraz therapy should be initiated and supervised by physicians experienced in oncology and/or haematology.





Posology: One 6 mg dose (a single pre- lled syringe or pre- lled injector) of Pelgraz is recommended for each chemotherapy cycle, given at least 24 hours after cytotoxic chemotherapy. Safety and e cacy of Pelgraz in children and adolescents has not yet been established and no recommendation on a posology can be made. No dose change is recommended in patients with renal impairment, including those with end-stage renal disease. Method of administration: Pelgraz is for subcutaneous use. The injections should be given subcutaneously into the thigh, abdomen or upper arm. See SmPC for instructions on handling of the medicinal product before administration. Contraindications: Hypersensitivity to peg lgrastim or any of the excipients in Pelgraz. Warnings and precautions: To improve the traceability of biological medicinal products, the trade name of the administered product should be clearly recorded. The long-term e ects of peg lgrastim have not been established in acute myeloid leukaemia (AML); therefore, it should be used with caution in this patient population. Granulocytecolony stimulating factor (G-CSF) can promote growth of myeloid cells in vitro and similar e ects may be seen on some non-myeloid cells in vitro. The safety and e cacy of peg lgrastim have not been investigated in patients with myelodysplastic syndrome, chronic myelogenous leukaemia, and in patients with secondary AML; therefore, it should not be used in such patients. Particular care should be taken to distinguish the diagnosis of blast transformation of chronic myeloid leukaemia from AML. The safety and e cacy of peg lgrastim administration in de novo AML patients aged < 55 years with cytogenetics t(15;17) have not been established. The safety and e cacy of peg lgrastim have not been investigated in patients receiving high dose chemotherapy. This medicinal product should not be used to increase the dose of cytotoxic chemotherapy beyond established dose regimens. Pulmonary adverse reactions, in particular interstitial pneumonia, have been reported after G-CSF administration. Patients with a recent history of pulmonary in ltrates or pneumonia may be at higher risk. The onset of pulmonary signs such as cough, fever, and dyspnoea in association with radiological signs of pulmonary in ltrates, and deterioration in pulmonary function along with increased neutrophil count may be preliminary signs of adult respiratory distress syndrome (ARDS). In such circumstances peg lgrastim should be discontinued at the discretion of the physician and the appropriate treatment given. Glomerulonephritis has been reported in patients receiving lgrastim and peg lgrastim. Generally, glomerulonephritis resolved after dose reduction
or withdrawal of lgrastim and peg lgrastim. Urinalysis monitoring is recommended. Capillary leak syndrome has been reported after G-CSF administration and is characterised by hypotension, hypoalbuminaemia, oedema and haemoconcentration. Patients who develop symptoms of capillary leak syndrome should be closely monitored and receive standard symptomatic treatment, which may include a need for intensive care. Generally asymptomatic cases of splenomegaly and cases of splenic rupture, including some fatal cases, have been reported following administration of peg lgrastim. Spleen size should be carefully monitored (e.g. clinical examination, ultrasound). A diagnosis of splenic rupture should be considered in patients reporting left upper abdominal pain or shoulder tip pain. Treatment with peg lgrastim alone does not preclude thrombocytopenia and anaemia because full dose myelosuppressive chemotherapy is maintained on the prescribed schedule. Regular monitoring of platelet count and haematocrit is recommended. Special care should be taken when administering single or combination chemotherapeutic medicinal products which are known to cause severe thrombocytopenia. Peg lgrastim in conjunction with chemotherapy and/or radiotherapy has been associated with development of myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML) in breast and lung cancer patients. Patients treated in these settings should be monitored for signs and symptoms of MDS/AML. Sickle cell crises have been associated with the use of peg lgrastim in patients with sickle cell trait or sickle cell disease. Therefore, use caution when prescribing peg lgrastim in patients with sickle cell trait or sickle cell disease, monitor appropriate clinical parameters and laboratory status and be attentive to the possible association of this medicinal product with splenic enlargement and vasoocclusive crisis. White blood cell (WBC) counts of 100 × 109/L or greater have been observed in less than 1% of patients receiving peg lgrastim. No adverse reactions directly attributable to this degree of leukocytosis have been reported. Such elevation in WBCs is transient, typically seen 24 to 48 hours after administration and is consistent with the pharmacodynamic e ects of this medicinal product. Consistent with the clinical e ects and the potential for leukocytosis, a WBC count should be performed at regular intervals during therapy. If leukocyte counts exceed 50 × 109/L after the expected nadir, this medicinal product should be discontinued immediately. Hypersensitivity, including anaphylactic reactions, have been reported with peg lgrastim. Permanently discontinue peg lgrastim in patients with clinically signi cant hypersensitivity. Do not administer peg lgrastim to patients with a history of hypersensitivity to peg lgrastim or lgrastim. If a serious allergic reaction occurs, appropriate therapy should be administered, with close patient follow-up over several days. Stevens-Johnson syndrome (SJS), which can be life-threatening or fatal, has been reported rarely in association with peg lgrastim treatment. If the patient has developed SJS with the use of peg lgrastim, treatment must not be restarted at any time. As with all therapeutic proteins, there is a potential for immunogenicity. Rates of generation of antibodies against peg lgrastim is generally low. Binding antibodies do occur as expected with all biologics; however, they have not been associated with neutralising activity at present. Aortitis has been reported after lgrastim or peg lgrastim administration in healthy subjects and in cancer patients. The symptoms experienced included fever, abdominal pain, malaise, back pain and increased in ammatory markers (e.g. C-reactive protein and WBC count). In most cases aortitis was
diagnosed by CT scan and generally resolved after withdrawal of lgrastim or peg lgrastim. The safety and e cacy of Pelgraz for the mobilisation of blood progenitor cells in patients or healthy donors has not been adequately evaluated. Increased haematopoietic activity of the bone marrow in response to growth factor therapy has been associated with transient positive bone-imaging ndings. This should be considered when interpreting bone-imaging results. The additive e ect of concomitantly administered products containing sorbitol (or fructose) and dietary intake of sorbitol (or fructose) should be taken into account. Pelgraz contains less than 1 mmol sodium (23 mg) per 6 mg dose, that is to say essentially ‘sodium-free’. The needle cover contains dry natural rubber (a derivative of latex), which may cause allergic reactions. Pregnancy and Lactation: Peg lgrastim is not recommended during pregnancy and in women of childbearing potential not using contraception. A decision must be made whether to discontinue breastfeeding or to discontinue/abstain from peg lgrastim therapy taking into account the bene t of breastfeeding for the child and the bene t of therapy for the woman. Adverse Events include: Adverse events which could be considered serious include: Common: Thrombocytopenia. Uncommon: Myelodysplastic syndrome, acute myeloid leukaemia, sickle cell anaemia with crisis, capillary leak syndrome, glomerulonephritis, hypersensitivity reactions (including angioedema, dyspnoea, anaphylaxis), splenic rupture (including some fatal cases), Sweet’s syndrome (acute febrile neutrophilic dermatosis), pulmonary adverse reactions including interstitial pneumonia, pulmonary oedema and pulmonary brosis have been reported. Uncommonly cases have resulted in respiratory failure or ARDS which may be fatal. Rare: Aortitis, pulmonary haemorrhage, Stevens-Johnson syndrome. Other Very Common adverse events: Headache, nausea, bone pain. Other Common adverse events: Leukocytosis, musculoskeletal pain (myalgia, arthralgia, pain in extremity, back pain, musculoskeletal pain, neck pain), injection site pain, non-cardiac chest pain. See SmPC for details of other adverse events. Shelf Life: 3 years. Store in a refrigerator (2∞C – 8∞C). Pelgraz may be exposed to room temperature (not above 25°C ± 2°C) for a maximum single period of up to 72 hours. Pelgraz left at room temperature for more than 72 hours should be discarded. Do not freeze. Accidental exposure to freezing temperatures for a single period of less than 24 hours does not adversely a ect the stability of Pelgraz. Keep the container in the outer carton in order to protect from light. Pack Size: One pre lled syringe or pre lled syringe injector with one alcohol swab, in a blistered packaging. Marketing Authorisation Numbers: Pre- lled syringe: EU/1/18/1313/001, Prelled injector: EU/1/18/1313/002. Marketing Authorisation Holder (MAH): Accord Healthcare S.L.U, World Trade Center, Moll de Barcelona, s/n, Edi ci Est, 6a planta, Barcelona, 08039 Spain. Legal Category: POM. Full prescribing information including the SmPC is available on request from Accord Healthcare Ireland Ltd, Euro House, Little Island, Co. Cork, Tel: 021-4619040 or www.accord-healthcare.ie/products Adverse reactions can be reported to Medical Information at Accord Healthcare Ltd. via E-mail: medinfo@accord-healthcare.com or Tel: +44(0)1271385257. Date of Generation of API: May 2021. IE-01426
Adverse events should be reported. Reporting forms and information can be found on the HPRA website (www.hpra.ie), or by e-mailing medsafety@hpra.ie. Adverse events should also be reported to Medical Information via email; medinfo@accord-healthcare.com or tel:0044 (0) 1271 385257






July 2021. IE-01663
Con dence, Convenience, Compliance Download the Pelgraz® PFI App in the App store, or Google Play store, today Use your smart phone to scan the QR code and be directed straight to the Pelgraz® PFI App www.accord-healthcare.ie Associated member of: Oncology & Haematology Pelgraz® PFI Patient Support App The Pelgraz® PFI App is designed to support patients self-administering Pelgraz® PFI at home Self-inject support toolPre-injection preparation Injecting the solution step-by-step Injection instructions Always administer Pelgraz® exactly as your doctorThe following instructions shouldact as a helpful reminder, but if you are unsureabout anything you should check with your doctor SER More ation has told you to. reminder doctor pharmacist. Mon 8 Jul 7 5 Tue 9 Jul Wed 10 Jul Fri 12 Jul GUIDE SER DASHBOARD Wellness Se verity scale 10 8 6 4 2 0 Mon 8 Tue 9 Wed 10 Thu 11 Fri 12 Sat 13 Sun 14 Thu 11 July Chemotherapy session Pelgraz dose Mon 8 Jul Sun 7 Jul Entries Hide 3-day7-day14-day 1-day Changes in libido & sexual function Loss of appetite Muscle or joint pain S h GUIDE SER DASHBOARD MORE Wellness 10:15 am Don’t forget to keep your Pelgraz® in the fridge Your next Pelgraz® injection is due Thursday 11th July Last chemotherapy session Wed 10 h Jul at 10:15am Edit your reminder Mild Moder 5 How severe is your Fatigue Feeling weary or exhausted 1 2 3 4 5 Select your symptom Close Wed 10 July Fatigue Loss of appetite Further symptoms Nausea Diarrhoea Swelling of hands and feet Vomiting Difficulty swallowing Allergic reaction Muscle or joint pain Shortness of breath Fever or chills Sore mouth Numbing/tingling of hands or feet Itching or rash Constipation One Dose for ANC Pre-FilledRecovery Injector
2 trials (Nakamura et al., 2017; Suzuki et al., 2019) with 67 patients provided the PTH data and were included in the analysis. Before conducting the meta-analysis, it is important to ensure that there is no difference between the baseline values of the combination therapy and control therapy. The positive values indicates that the combination group is higher. The results are as follows : WMD = 6.15, 95%CI: 13.36∼1.06; I2 = 0.0%, p = 0.726; Z = 1.67, p = 0.094 (Supplementary Figure S9). There was no significant difference in baseline values between groups. Compared with denosumab, combination can significantly improve PTH levels: WMD = 22.28, 95%CI: 2.17 42.39; I2 = 0.0%, p = 0.876; Z = 2.17, p = 0.03.
Subgroup Analysis
Subgroup Analysis of Mean Percent Change of BMD in Lumbar Spine
Although the heterogeneity among the five trials (Tsai et al., 2013; Leder et al., 2015; Idolazzi et al., 2016; Nakamura et al., 2017; Suzuki et al., 2019) included in this meta-analysis was low (I2 = 38.7%), we still perform
specific monotherapy. Patients in the combination group had a statistically significant 3.57% increase in lumbar BMD compared to patients in the denosumab alone group. Similarly, patients in the combination group had a statistically significant 2.00% increase in lumbar BMD compared to patients in the teriparatide alone group. The specific results are shown in Table 3
ubgroup Analysis of Mean Percent Change of BMD in Hip
Since there was moderate heterogeneity (I2 = 59.9%) among the 5 trials (Tsai et al., 2013; Leder et al., 2015; Idolazzi et al., 2016; Nakamura et al., 2017; Suzuki et al., 2019) included in this meta-analysis, we took a subgroup analysis to explore the sources of heterogeneity. Meta-regression analyses found a significant effect of control measures (t = 2.57, p = 0.042) on the mean percent change of BMD in hip. Based on this, we performed subgroup analyses based on different control measures and found the heterogeneity decreases significantly. The combined results suggest that patients in the combination group had a
WOMEN’S HEALTH OSTEOPOROSIS

statistically significant 2.28% increase in hip BMD compared to patients in the denosumab alone group. Similarly, patients in the combination group had a statistically significant 4.10% increase in hip BMD compared to patients in the teriparatide alone group. The specific results are shown in Table 3

Subgroup Analysis of Mean Percent Change of CTX in Serum
Since there was strong heterogeneity (I2 = 95.5%) among the 3 trials (Tsai et al., 2013; Leder et al., 2015; Idolazzi et al., 2016) included in this meta-analysis compared combination with denosumab, we took a subgroup analysis to explore the sources of heterogeneity. Based on this, we performed subgroup analyses based on different control measures. Compared with denosumab, combination can improve CTX levels but has no statistical significance: WMD = 3.19, 95%CI: 8.32∼14.71; I2 = 87.5%, p = 0.000; Z = 0.54, p = 0.587; compared with teriparatide, combination can significantly decrease CTX levels: WMD = 116.50, 95%CI: 137.90 95.11; I2 = 0.0%, p = 0.385; Z = 10.67, p = 0.00. The specific results are shown in Table 3
Discussion
We discovered in this meta-
analysis of 6 RCTs that the combination of teriparatide and denosumab was superior to monotherapy with these two drugs in improving BMD in lumbar spine and hip for patients with postmenopausal osteoporosis. This result is congruent with a recently published metaanalysis by Lou et al. (Lou et al., 2019). However, in Lou’s meta-analysis, the subjects were osteoporosis rather than postmenopausal osteoporosis patients, and the interventions were similarly expanded to combination treatment with parathyroid peptide analogues and antiresorptive agents. Considering the differences between the 2 meta-analyses, we compared the results with caution. Besides, to clarify the specific differences between combination treatment and denosumab or teriparatide, we performed further subgroup analyses. We found that the combination group could improve lumbar spine BMD and hip BMD by 3.57 and 2.0%, respectively, compared with denosumab. Compared with teriparatide, the combination group could improve 2.28 and 4.10%, respectively. Interestingly, the comparison of the combination group with denosumab or teriparatide showed opposite results in the metaanalysis of CTX. The combination group was elevated by 16.49% compared to denosumab and decreased by 116.50% compared to teriparatide. This phenomenon may be associated with the mechanism of action of both medicines. Denosumab inhibits bone resorption by antagonizing osteoclasts but teriparatide promotes bone resorption at the same time as it promotes bone synthesis (Cummings et al., 2009; Tsai et al., 2019). In terms of safety, this meta-analysis showed that the incidence of adverse events in the combination group was only 82% of that of the monotherapy modalities, but the results were not statistically significant. We know that the side effect of teriparatide is to increase serum calcium while causing dizziness, poor appetite, muscle, and joint pain (Langdahl et al., 2017), while the side effect of denosumab is to reduce blood calcium (Lecoq et al., 2021), if the combination of the two drugs will
Figure 3 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
38
TABLE 3
Subgroup analysis.
Subgroup WMD (95% CI)
Mean percent change of BMD in lumbar spine
Combination therapy vs. denosumab monotherapy 3.57 (2.35, 4.79)
Combination therapy vs. teriparatide monotherapy 2.00 (0.59, 3.42)
Mean percent change of BMD in hip
Combination therapy vs. denosumab monotherapy 2.28 (1.53, 3.03) 36.90 0.000
Combination therapy vs. teriparatide monotherapy 4.10 (3.30, 4.90) 0.00 0.000
Mean percent change of CTX in serum
Combination therapy vs. denosumab monotherapy 3.19 ( 8.32, 14.71) 87.50 0.587
Combination therapy vs. teriparatide monotherapy 116.50 ( 137.90, 95.11) 0.00 0.000
BMD, Bone Mineral Density; CTX, Serum β-C-terminal telopeptide of type 1 collagen; CI, Confidence Interval.
reduce the side effect in some aspects? This requires more research to further explore.

Osteoporotic fractures have a direct patient burden in terms of morbidity and mortality, as well as a substantial societal economic burden due to direct healthcare resource consumption, direct nonmedical expenses, and indirect expenses (Burge et al., 2007; Pike et al., 2011; Bliuc et al., 2013; Tajeu et al., 2014). The vast majority of patients suffering from osteoporosis are postmenopausal women (Selby, 2004). Bisphosphonates are recommended as the first line of medication for postmenopausal osteoporosis, but there are growing concerns about their increasingly obvious side effects (Black and Rosen, 2016b; Vargas-
Franco et al., 2018). Along with clinical trials, teriparatide and denosumab are gradually gaining public recognition for their use in postmenopausal osteoporosis (Chen et al., 2006; Cummings et al., 2009). In particular, attention has been drawn to the efficacy produced by the combination of the two drugs. The combination of teriparatide and denosumab, on the other hand, enhanced lumbar spine and hip BMD more than either drug alone, and to a greater extent than any of the currently available drugs (Tsai et al., 2013). The capability of denosumab to completely inhibit teriparatideinduced bone resorption but only partially inhibit teriparatide-induced bone formation seems to be the cause of the superimposed effect (Tsai et al., 2013). Denosumab could regulate the canonical wnt
signaling pathway by upregulating sclerostin and inhibiting DKK1 expression (Glass et al., 2005; Diarra et al., 2007; Gatti et al., 2012). This mechanism may have important implications for the regulation of bone metabolism during combination drug administration. These findings are consistent with findings from other animal models, such as the ovariectomized rat, in which the combination of osteoprotegerin (an innate chemical with properties similar to denosumab) and teriparatide greatly reduced osteoclast quantity, did not decrease osteoblast quantity, and improved trabecular and cortical BMD more than either agent alone (Kostenuik et al., 2001). Correspondingly, in a mouse model where osteoprotegerin and teriparatide were co-
the detailed mechanisms have not been illuminated, these findings demonstrate the significant efficacy of the combination of teriparatide and denosumab in postmenopausal osteoporosis.
Our meta-analysis has several strengths. Previously, there was a meta-review on the combination treatment of parathyroid peptide analogues and antiresorptive medications for osteoporosis. However, this meta-analysis is the first to review the combination of teriparatide and denosumab in postmenopausal osteoporosis. Second, the quality of RCTs included in this meta-analysis was generally high. Third, although there was certain heterogeneity in some results, we reduced or even eliminated the heterogeneity by conducting subgroup analyses with various stratification factors. On the other hand, our meta-analysis also has several limitations. First, the original studies had methodological flaws, such as ambiguous randomization methods and insufficient treatment allocation concealment. Second, the sample size was relatively small in our meta-analysis. Third, with the language limited to English, some potential studies may be missed.
Launch of Major Trauma Audit Report
Professor Conor Deasy, Clinical Lead for Major Trauma Audit (MTA) and Louise Brent, Major Trauma Audit Manager, have launched the Major Trauma Audit Report 20192020 at the Major Trauma Audit Webinar 2022. The report focuses on patients who have suffered a major trauma accident over 2019 and 2020 with a focus on the early impact of the COVID-19 pandemic on trauma activity and care. Falls in the home account for the majority of these injuries and present an obvious opportunity for injury prevention.
From 2019-2020, data was collected on 8,764 cases from the majority of the 26 hospitals involved in the audit.
Launching this year’s report, Professor Deasy commented, “The publication of this report presents the first picture of how trauma activity, care and outcomes were effected during a very tumultuous time in the Health Service due to the COVID-19 pandemic. The significance of this data can help to inform future public health strategies and the reconfiguration of the Trauma System when such events like a pandemic or cyberattack occur.”
Key Findings
• Major Trauma Audit (MTA) data coverage was 83% in 2019 and 73% in 2020.
• The mean age of major trauma patients increased from 58 years in 2019 to 61 years in 2020.
• The percentage of falls of less than 2 m increased from 58% in 2019 to 62% in 2020.
• The proportion of patients injured at home increased from 48% in 2019 to 56% in 2020.
• There was an approximately 10% reduction in the number of major trauma admissions during 2020, compared with 2019.
• Both 2019 and 2020 had a lowlevel pre-alert rate of 12%.
• The overall percentage of major trauma patients received by a
trauma team remains extremely low, at 8% in 2019 and 9% in 2020.
• As patients get older they are less likely to be pre-alerted, met by a trauma team or received by a senior clinician.
• There was an increase in the proportion of major trauma patients who died from falls. The percentage of those who died from falls less than 2 m increased from 59% in 2019 to 64% in 2020, while the percentage of those who died from falls more than 2 m increased from 11% in 2019 to 16% in 2020.
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
News
39
WOMEN’S HEALTH MENOPAUSE
Navigating Menopause in Thrombosis Patients
Written by Dr Deirdre Lundy, Clinical Lead, Complex Menopause Service, NMH, Holles St Picture: Gerry Mooney

Levels of female reproductive hormone will start to become less predictable and then drop; sometimes gradually with age, sometimes abruptly as a result of disease or medical treatments.

The transition typically kicks off naturally from about the mid- 40’s and the symptoms resulting from hormone derangement or loss are commonly referred to as “The Menopause” by patients and HC (even though we know that the word ‘menopause’ actually means “end of the monthly”). So while menopause officially is defined as the last ever natural menses and most commonly occurs in the early 50’s, the symptomatic phase of the lead into sex hormone changes - more accurately referred to as ‘perimenopause’, often precede the final menstrual period by many months or even years. We use those terms interchangeably. This transitional time (‘Perimenopause’) puts some patients in the unenviable position of having to juggle midlife hormonal symptoms as well as contraceptive concernsfertility may decline in your 40’s & 50’s but it’s not gone!
Some but thankfully not all patients struggle with perimenopausal symptoms and seek our advice in primary care. The amount of issues that may be uncovered in a first menopause visit are legion.

The classic physical symptoms are easily recognized: night sweats, hot flushes, heavy and/ or irregular periods, loss of vaginal elasticity and lubrication, decrease in metabolism (+/- weight gain), hair
and skin changes, joint complaints, bladder complaints, et al.
The psychological symptoms are just as prevalent and possibly more likely to be misinterpreted. They include; depression, mood swings, anxiety, irritability/ rage, tiredness, memory loss, poor concentration, loss of libido, PMStype symptoms, et al.
The first thing to do is confirm the diagnosis. Easier said than done as hormone blood tests are often within normal ranges for perimenopausal patients and so are not recommended as having any part in the diagnostic process. In addition, not everything a patient experience in their middle life is related to perimenopause hormones changes. It can be challenging to identify whose mood symptoms are arising directly from the perimenopause vs. pre-existing mood issues being exacerbated by the approach of menopause. A thorough history - maybe even expedited by pre consult symptom self-assessment questionnaires- may be of use. The most effective treatment for menopause related symptoms is HRT but it is not a ‘miracle cure’ and some people won’t need it, won’t want it or have been advised to avoid it. Clinicians are reminded that menopause care should be individualised- using a comprehensive approach that pays attention to advice on exercise, optimising weight, stopping smoking, and reducing alcohol consumption as well as management options such as HRT.
Talking about Thrombosis
One of the more important aspects of the history taking (apart from a kind ear which is of immeasurable benefit) is the need to exclude comorbidities that might affect the range of treatment options available to that patient. Some people will be keen to consider menopause hormone balancing or replacement therapy for their menopause symptoms (MHT or HRT). MHT involves providing small dose of estrogen in all cases and usually some low dose progestagen for the patient with a uterus. Occasionally, in cases of hypoactive sexual desire or arousal, some testosterone may also be offered. Each of these individual hormones come with their own lists of contraindications and special precautions. Some contraindications are absolute but some special situations mainly require a conversation with either a menopause specialist or the consultant team that manages
the patient’s medical condition; this is according to the British Menopause Society. One such situation is in the case of patients with a personal past history of thrombosis. If a perimenopausal patient who has had a VTE has considered and/ or explored nonhormonal options for managing their symptoms and now is keen to consider HRT, they can but we need to be much more cautious when prescribing for them. Our first job is to make sure as much is being done to optimise any modifiable risks that they carry. We know venous thrombosis particularly can be liked to modifiable issues such as:
Immobilization
Smoking
Long plane or car trips of more than 4 hours &
- Certain contraceptives – any high-dose, strong estrogen – like the ones in all the combined pills, rings and patches – can increase thrombosis risk; similar to the elevated risk linked to being pregnant. Some progestagens have been linked to increased VTE risk too, but not at the typical doses we see in progestagenonly contraceptives.
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
40
Time is essential . So is starting with ENTRESTO®1-4 For patients living with heart failure,
Change the heart. Change heart failure 5-9

Reverse adverse cardiac remodelling, improve cardiac structure and pumping function, and target HF via a unique dual MOA that inhibits neprilysin and RAAS
Safety and tolerability profile similar to ACEi 5,10-12
In all stages of the HFrEF patient journey whether initiated in the hospital or outpatient setting
Make a lasting difference patients can feel 5,10,11,13-15
Help your patients stay out of the hospital, live longer, and feel better, so they have more time for what matters most
Start ENTRESTO ® as early as possible in your HFrEF patients to help keep them at home and better protected † 1-4

Abbreviated Prescribing Information
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Entresto® (sacubitril valsartan) 24 mg/26 mg film-coated tablets, 49 mg/51mg film-coated tablets and 97mg/103mg film-coated tablets Presentation: Film-coated tablets of 24 mg/26 mg, 49 mg/51mg and 97 mg/103 mg of sacubitril and valsartan respectively (as sacubitril valsartan sodium salt complex). Indications: In adult patients for treatment of symptomatic chronic heart failure with reduced ejection fraction. Dosage and administration: The recommended starting dose of Entresto is one tablet of 49 mg/51 mg twice daily, doubled at 2-4 weeks to the target dose of one tablet of 97 mg/103 mg twice daily, as tolerated by the patient. In patients not currently taking an ACE inhibitor or an ARB, or taking low doses of these medicinal products, a starting dose of 24 mg/26 mg twice daily and slow dose titration (doubling every 3 - 4 weeks) are recommended. A starting dose of 24 mg/26 mg twice daily should be considered for patients with SBP ≥100 to 110 mmHg, moderate or severe renal impairment (use with caution in severe renal impairment) and moderate hepatic impairment. Do not co-administer with an ACE inhibitor or an ARB. Do not start treatment for at least 36 hours after discontinuing ACE inhibitor therapy. Entresto may be administered with or without food. The tablets must be swallowed with a glass of water. Splitting or crushing the tablets is not recommended. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Concomitant use with ACE inhibitors. Do not administer until 36 hours after discontinuing ACE inhibitor therapy. Known history of angioedema related to previous ACE inhibitor or ARB therapy. Hereditary or idiopathic angioedema. Concomitant use with aliskiren-containing medicinal products in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2). Severe hepatic impairment, biliary cirrhosis and cholestasis. Second and third trimesters of pregnancy. Warnings/Precautions: Dual blockade of the renin angiotensin-aldosterone system (RAAS): Combination with an ACE inhibitor is contraindicated due to the increased risk of angioedema. Sacubitril/valsartan must not be initiated until 36 hours after taking the last dose of ACE inhibitor therapy. If treatment with sacubitril/valsartan is stopped, ACE inhibitor therapy must not be initiated until 36 hours after the last dose of sacubitril/valsartan. Combination of sacubitril/valsartan with direct renin inhibitors such as aliskiren is not recommended. Sacubitril/valsartan should not be co-administered with another ARB containing product. Hypotension: Treatment should not be initiated unless SBP is ≥100 mmHg. Patients with SBP <100 mmHg were not studied. Cases of symptomatic hypotension have been reported in patients treated with sacubitril/valsartan during clinical studies, especially in patients ≥65 years old, patients with renal disease and patients with low SBP (<112 mmHg). Blood pressure should be monitored routinely when initiating or during dose titration with sacubitril/valsartan. If hypotension occurs, temporary down-titration or discontinuation of sacubitril/valsartan is recommended. Impaired or worsening renal function: Limited clinical experience in patients with severe renal impairment (estimated GFR <30 ml/min/1.73m2). There is no experience in patients with end-stage renal disease and use of sacubitril/valsartan is not recommended. Use of sacubitril/valsartan may be associated with decreased renal function, and down-titration should be considered in these patients. Hyperkalaemia: Treatment should not be initiated if the serum potassium level is >5.4 mmol/l. Monitoring of serum potassium is recommended, especially in patients who have risk factors such as renal impairment, diabetes mellitus or hypoaldosteronism or who are on a high potassium diet or on mineralocorticoid antagonists. If clinically significant hyperkalaemia occurs, consider adjustment of concomitant medicinal products or temporary down-titration or discontinuation of sacubitril/valsartan. If serum potassium level is >5.4 mmol/l discontinuation should be considered. Angioedema: Angioedema has been reported with sacubitril/valsartan. If angioedema occurs, discontinue sacubitril/valsartan immediately and provide appropriate therapy and monitoring until complete and sustained resolution of signs and symptoms has occurred. Sacubitril/valsartan must not be re-administered. Patients with a prior history of angioedema were not studied. As they may be at higher risk for angioedema, caution is recommended if sacubitril/valsartan is used in these patients. Black patients have an increased susceptibility to develop angioedema. Patients with renal artery stenosis: Caution is required and monitoring of renal function is recommended. Patients with NYHA functional classification IV: Caution should be exercised due to limited clinical experience in this population. Patients with hepatic impairment: There is limited clinical
experience in patients with moderate hepatic impairment (Child Pugh B classification) or with AST/ALT values more than twice the upper limit of the normal range. Caution is therefore recommended in these patients. B-type natriuretic peptide (BNP): BNP is not a suitable biomarker of heart failure in patients treated with sacubitril/valsartan because it is a neprilysin substrate. Psychiatric disorders: hallucinations, paranoia and sleep disorders, in context of psychotic events, have been associated with sacubitril/valsartan use. Consider discontinuation if patient experiences such events. Interactions: Contraindicated with ACE inhibitors, 36 hours washout is required. Use with aliskiren contraindicated in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2). Should not be co-administered with another ARB. Use with caution when co-administering sacubitril/ valsartan with statins or PDE5 inhibitors. No clinically relevant drug-drug interaction was observed when simvastatin and sacubitril/valsartan were coadministered. Monitoring serum potassium is recommended if sacubitril/valsartan is co-administered with potassium-sparing diuretics or substances containing potassium (such as heparin). Monitoring renal function is recommended when initiating or modifying treatment in patients on sacubitril/ valsartan who are taking NSAIDs concomitantly. The combination of sacubitril/valsartan and lithium is not recommended. If the combination proves necessary, careful monitoring of serum lithium levels is recommended. Co-administration of sacubitril/valsartan and furosemide reduced Cmax and AUC of furosemide by 50% and 28%, respectively, with reduced urinary excretion of sodium. Co-administration of nitroglycerin and sacubitril/valsartan was associated with a treatment difference of 5 bpm in heart rate compared to the administration of nitroglycerine alone, no dose adjustment is required. Coadministration of sacubitril/valsartan with inhibitors of OATP1B1, OATP1B3, OAT3 (e.g. rifampicin, ciclosporin), OAT1 (e.g. tenofovir, cidofovir) or MRP2 (e.g. ritonavir) may increase the systemic exposure of sacubitril or valsartan. Appropriate care should be exercised. Co-administration of sacubitril/valsartan with metformin reduced both C max and AUC of metformin by 23%. When initiating therapy with sacubitril/valsartan in patients receiving metformin, the clinical status of the patient should be evaluated. Fertility, pregnancy and lactation: The use of sacubitril/valsartan is not recommended during the first trimester of pregnancy and is contraindicated during the second and third trimesters of pregnancy. It is not known whether sacubitril/valsartan is excreted in human milk, but components were excreted in the milk of rats. Sacubitril/valsartan is not recommended during breast-feeding. A decision should be made whether to abstain from breast-feeding or to discontinue sacubitril/valsartan while breast-feeding, taking into account the importance of sacubitril/ valsartan to the mother. No available data on the effect of sacubitril/valsartan on human fertility. Undesirable effects: Very common: Hyperkalaemia, hypotension, renal impairment. Common: Anaemia, hypokalaemia, hypoglycaemia, dizziness, headache, syncope, vertigo, orthostatic hypotension, cough, diarrhoea, nausea, gastritis, renal failure, acute renal failure, fatigue, asthenia. Uncommon:Hypersensitivity, postural dizziness, pruritus, rash, angioedema. Rare: Hallucinations, sleep disorders. Very rare: Paranoia. Please refer to SmPC for a full list of adverse events for Entresto. Pack sizes: Entresto 24 mg/ 26 mg - 28 tablet pack; Entresto 49 mg/51 mg - 28 and 56 tablet pack; Entresto 97 mg/103 - 56 tablet pack. Legal Category: POM. Marketing Authorisation
Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road Dublin 4, Ireland.
EU/1/15/1058/002-003; Entresto
Numbers: Entresto
and reduced ejection fraction; a randomized clinical trial. JAMA. 2019;322(11):1077-1084.
8. Ramani GV, Uber PA, Mehra MR. Chronic heart failure: contemporary diagnosis and management. Mayo Clin Proc. 2010;85(2):180-195. 9. McDonagh T, Metra M et al ESC Scientific Document Group, 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the ESC With the special contribution of the HFA of the ESC, European Heart Journal, 2021;ehab368, https://doi.org/10.1093/eurheartj/ehab368 10. McMurray JJV, Packer M, Desai AS, et al; for the PARADIGM-HF Investigators and Committees; Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
11. Velazquez EJ, Morrow DA, DeVore AD, et al; for the PIONEER-HF Investigators. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539-548.
12. Wachter R, Senni M, Belohlavek J, et al; on behalf of the TRANSITION Investigators. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in-hospital or early after discharge: primary results of the randomised TRANSITION per approved citation: study [published online ahead of print May 27, 2019]. Eur J Heart Fail. 2019. doi:10.1002/ehjf.1498. 13. Claggett B, Packer M, McMurray JJV, et al; for the PARADIGM-HF Investigators. Estimating the long-term treatment benefits of sacubitril–valsartan. N Engl J Med. 2015;373(23):22892290.
14. Lewis EF, Claggett BL, McMurray JJV, et al. Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail. 2017;10(8):e003430. 15. Chandra A, Lewis EF, Claggett BL, et al. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure. A secondary analysis of the PARADIGM-HF trial. JAMA Cardiol. 2018;3(6):498-505.
MOA=mechanism of action; RAAS=Renin-Angiotensin-Aldosterone System; HFrEF=heart failure with reduced ejection fraction. *In place of an ACEi or ARB. † For PCRS reimbursement of ENTRESTO ®, HF patients must meet the following criteria: HFrEF ≤ 35%, currently prescribed ACEi/ARB therapy, symptomatic (NYHA Class II-IV), Systolic Blood Pressure ≥ 100 mmHG, Potassium ≥ 5.4mmol/L.
ENTRESTO ® is indicated in adult patients for treatment of symptomatic chronic heart failure with reduced ejection fraction.
Holder:
Marketing Authorisation
24 mg/26 mg film coated tablets EU/1/15/1058/001; Entresto 49 mg/51 mg film coated tablets
97 mg/103 mg film coated tablets EU/1/15/1058/006. Full prescribing information is available on request from Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Merrion Road, Dublin 4. Tel: 01 2601255 or at www.medicines.ie. Detailed information on this product is also available on the website of the European Medicines Agency http://www.ema.europa.eu. Prescribing Information last revised: May 2021 Reporting suspected adverse reactions of the medicinal product is important to Novartis and the HPRA. It allows continued monitoring of the benefit/risk profile of the medicinal product. All suspected adverse reactions should be reported via HPRA Pharmacovigilance, website www.hpra.ie. Adverse events could also be reported to Novartis preferably via www.report.novartis.com or by email: drugsafety.dublin@novartis.com or by calling 01 2080 612. REFERENCES: 1. Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(10):1169-1186. 2. Hollenberg SM, Stevenson LW, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patient hospitalized with heart failure: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74(15):1966-2011. 3. Maddox TM, Januzzi JL, Allen, LA, et al; 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2021 Feb, 77 (6) 772–810. 4. CaReMe Heart Failure in Adults: Diagnosis & Management Algorithm. Available at: cardionewsuk.org. (Accessed: November 2021). 5. ENTRESTO Summary of product characteristics. Available at www.medicines.ie 6. Januzzi JL Jr, Prescott MF, Butler J, et al; for the PROVE-HF Investigators. Association of change in N-terminal pro–b-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction [published online ahead of print September 2, 2019]. JAMA. doi:10.1001/jama.2019.12821. 7. Desai AS, Solomon SD, Shah AM, et al; for the EVALUATE-HF Investigators. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure
October 2021 | IE167236
avoidable triggers is an important part of a menopausal chat for someone with a past history of VTE. Now traditionally the estrogen in HRT was considered contraindicated for people with a past history of VTE as we expected the estrogen in HRT to have a similar effect on clotting mechanisms to the estrogens in contraceptives. High levels of estrogen (and certain progestagens) in the blood can be ‘Pro- thrombotic’. Oral oestrogen goes straight from the GI tract to the liver, where it is metabolized. During this first run through the liver is where the impact on clotting molecules occurs. But even non oral estrogens can impact clotting risk if the doses are high or the estrogen molecule is particularly potent - so the contraceptive patch and vaginal ring are just as likely to promote VTE as oral contraceptive pills. Risk is maximally elevated at initiation -
staying on them for months or years. In addition, every time a patient switches brands or comes off for a few weeks or months ‘to see if everything is still working in there’, they increase their clot risk when they restart.

So, knowing all this it was only logical that we assume HRT - type estrogen would also be risky for women with elevated VTE risk?
Well, apparently this is not the case.
While it is true that the estrogens in ORAL HRT can increase clot risk, there is an abundance of research to confirm that lowdose, ovarian - similar HRT oestrogen does not affect clot risk when used transdermally. This is supported by all the menopause guideline groups as well as by the gynaecology and haematology guidance groups.
The go-to data to support this is the nested case-control study
WOMEN’S HEALTH MENOPAUSE
by Yana Vinogradova, et al that looked at women diagnosed with VTE between 1998 and 2017. Ref 2 With regard to progestagens, this study did not actually review micronized progesterone for some reason, but it did look at dydrogesterone and found it also to be a safer option when there is concern about blood clots.
So we tell patients this: while people who are smoking, overweight or who have had a blood clot in the past should not use estrogen contraceptives nor should they use oral estrogen in HRT, they can safely try transdermal estrogen HRT products, in modest doses after discussion with their haematologist or menopause specialist.
According to the UK’s National Institute for Health and Care Excellence: ‘The risk of venous thromboembolism (VTE) is increased by use of an oral oestrogen-containing HRT product compared with baseline population risk, but the risk associated with transdermal HRT given at standard therapeutic doses is no greater than baseline population risk.’
Be warned though; the information leaflets that come with transdermal HRT estrogen products (and even with local vaginal oestrogen, which has never been suspected of increasing DVT risk) say: May cause blood clots or Do not use if you are at risk of blood clots. So patient counselling is key.
If someone is already on an anticoagulant, they may also consider low dose transdermal HRT oestrogen.
1. National Institute for Health and Care Excellence. Menopause: diagnosis and Management. NICE, 2015
2. Vinogradova Y, et al. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019 Jan 9;364:k4810. doi: 10.1136/ bmj.k4810. Erratum in: BMJ. 2019 Jan 15;364:l162. PMID: 30626577; PMCID: PMC6326068.
“Support the Kaftrio 35” campaign Mounts Dáil Protest
Families affected by the decision not to provide the ground-breaking Kaftrio therapy to 35 children with cystic fibrosis (CF) between 6 and 11 years have mounted a protest at the gates of the Dáil and Seanad. The families are urging the support of TDs and Senators amid their ongoing anger and frustration at being caught in the middle of a pricing dispute that shows no signs of resolution.
With negotiations seemingly stalled, Cystic Fibrosis Ireland has called on the Minister for Health to intervene to break the impasse and continues to make itself available, along with parents of the children affected, to discuss their concerns. The CF community wishes to contribute in a constructive way to seeking a solution for the 35 children and to futureproof approval/reimbursement arrangements to ensure that such disputes do not happen again.
Cystic Fibrosis Ireland believes that if there is little prospect of a
resolution then the Minister and Vertex should agree to arbitration, with an independent arbitrator being appointed to adjudicate on the parties’ stated positions in a time-defined process.
Purple roses, the emblem of the cystic fibrosis community, were presented to the Head Usher of Leinster House – who received them on behalf of the Ceann Comhairle – to highlight the plight of the 35 children who continue to be deprived of Kaftrio.
This is notwithstanding the fact that 140 other children in the same age group are accessing Kaftrio right now in Ireland – they just happen to have a different genotype, with the HSE and Vertex arguing over which genotype was included in the original deal.
The protest saw children with CF, their mums and dads, brothers and sisters, as well as supporters from the wider CF community and Cystic Fibrosis Ireland, come together to highlight:
• The apparent lack of progress being made following over four months of negotiations
• The major ethical and human rights issues in providing Kaftrio to all eligible children and adults with CF, but not the 35 children affected by this dispute
• The need to consider fresh approaches to dispute resolution, including appointing an independent arbitrator, if the parties continue to be unable to find a solution
Philip Watt, CEO of Cystic Fibrosis Ireland, comments, “Until there is a resolution to this Boston/Dublin stand-off, the healthcare of the ‘Kaftrio 35’ remains compromised in a dispute not of their making and which is increasingly raising major ethical and human rights concerns as 140 children gained access in May 2022 and 35 children remain excluded. Healthcare delayed is healthcare denied.
“It raises the unwelcome prospect of parents being forced to consider taking legal action in future because their children are being denied access to healthcare available to all other children and adults with CF, including those in the same age group in Ireland.
“The suggestion of referring this issue to the National Centre for Pharmacoeconomics for a Health Technology Assessment is patently absurd. Health Technology Assessments are undertaken before new drugs are approved/reimbursed, but this therapy has already been approved/reimbursed for use in Ireland and is also recognised by the Cystic Fibrosis Registry of Ireland as ‘revolutionising CF treatment’ here. Such a move would appear to many as ‘kicking the can down the road’, cause months’ more delay, and ratchet up angst for the families in the process, only to arrive right back where we are now.”
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
News
42
HEALTH VTE IN PREGNANCY

Treatment, diagnosis and prevention of pregnancy-associated venous thromboembolism
 Written by: Osas Edebiri1, Fergal O’Shaughnessy2,3, Brian Cleary2,3 and Fionnuala Ní Áinle4,5,6
Written by: Osas Edebiri1, Fergal O’Shaughnessy2,3, Brian Cleary2,3 and Fionnuala Ní Áinle4,5,6


1. Dept of Internal Medicine, Darlington Memorial Hospital, United Kingdom
2. Pharmacy Department, Rotunda Hospital, Dublin 1, Ireland
3. Division of Population Health Sciences, Royal College of Surgeons in Ireland, Dublin 2, Ireland
4. School of Medicine, University College Dublin, Dublin 4, Ireland
Department of Haematology, Rotunda Hospital, Dublin 1, Ireland
Department of Haematology, Mater University Hospital, Dublin 7, Ireland
Background
Venous Thromboembolism (VTE) is a leading cause of maternal death in the developed world.1 Although a rare event, occurring in approximately 1-2 per 1,000 pregnancies,2 every pregnant woman is potentially at risk, and every event is potentially fatal. The death of a mother is a devastating event, with far-reaching impact on an individual, societal and health systems level. Moreover, non-fatal consequences may be associated with significant short- and longterm morbidity.3
Prevention, diagnosis and treatment of pregnancy-associated VTE (PA-VTE) should therefore be a priority for health services globally.
Risk factors for PA-VTE
The risk of VTE is estimated to be 5-10 times higher in pregnant and postpartum women compared with non-pregnant women.4-6 Risk factors for PA-VTE differ in the antenatal and postnatal periods. Maternal VTE risk factors include advanced age and parity, Body
Mass Index (BMI), smoking status and medical co-morbidities.7 VTE risk is dynamic and may change during pregnancy and delivery. For example, the risk of VTE following an emergency caesarean delivery is reported to be twice that of a planned caesarean and four times that of a vaginal delivery.8,9 Other VTE risk factors may only arise, or may only be identifiable, in the postpartum period, including stillbirth, systemic infection, postpartum haemorrhage or receipt of a blood transfusion.10
VTE risk factors are common among pregnant and postpartum populations. In a recent analysis of risk factors for postpartum VTE among 21,000 consecutively sampled women, we found that more than 75% of women had at least one risk factor for VTE, while more than 40% carried at least two risk factors.11 This high prevalence suggests a considerable burden of VTE risk at a population level, even in the absence of well defined ‘high-risk’ characteristics. However, little is known on the

interaction between individual risk factors and the cumulative risk of VTE when two or more risk factors co-occur.
Together, the high and increasing prevalence of VTE risk factors emphasise the need for interventions towards effective management of VTE risk to avoid associated morbidity and mortality.
In some cases, PA-VTE may be preventable.12 Low Molecular Weight Heparin (LMWH) is the preferred agent for pharmacological thromboprophylaxis.10,13-15 LMWH is administered as a subcutaneous injection, and it is safe to use in pregnancy and breastfeeding.16 For women with prior VTE whose risk warrants antenatal pharmacological thromboprophylaxis, the optimal dose is unknown, and guidelines have previously advocated strategies including a low prophylactic or an intermediate (half-therapeutic) dose.15,17 The recently completed Highlow study (NCT 01828697; clinicaltrials.gov),
has addressed this question in an investigator-initiated, multicentre, multinational RCT comparing a fixed low dose of LMWH with an intermediate weight-adjusted dose in the prevention of pregnancy-related VTE recurrence in women ≥18 years with a history of VTE and an indication for ante- and postpartum thromboprophylaxis who are recruited ≤14 weeks gestation.
Direct oral anticoagulant agents (DOACs) are not routinely recommended in the context of PA-VTE due to insufficient evidence to evaluate safety for the mother, the fetus or the breastfeeding infant.
Previous studies have also demonstrated the effectiveness of low-dose aspirin (ASA) in the prevention of VTE in other populations,18-21 and its potential role in PA-VTE is also being considered. The pilot PARTUM trial (Postpartum Aspirin to Reduce Thromboembolism Undue Morbidity) will assess the feasibility of a randomised, multicentre,
Fionnuala Ní Áinle
Fergal O’Shaughnessy
Osas Edebiri Brian Cleary
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
WOMEN’S
5.
6.
43
WOMEN’S HEALTH VTE IN PREGNANCY

at moderate risk of VTE (NCT: NCT04153760, Clinicaltrials.gov).
Several prominent international organisations have published clinical recommendations to guide VTE prevention in clinical practice.10,15,17,22-24 The most widely cited are from the Royal College of Obstetrics and Gynaecology (RCOG) in the UK10, and the American College of Chest Physicians (ACCP)15 and the American College of Obstetrics and Gynecology (ACOG)17 in North America. There is a lack of high-quality evidence to guide optimal prevention of PA-VTE in women with intermediate- and low-risk characteristics.22,24,25 In the absence of robust evidence, clinical recommendations have been based on expert opinion and consensus.26 This has resulted in striking variation in recommendations on the prevention of PA-VTE.11,27 We have recently reported a five-fold difference in the number of women who would theoretically receive a recommendation for postpartum thromboprophylaxis by various international guidelines, which ranged from 7% under ACOG to 37% under RCOG guidelines.11
This highlights the need for large-scale, prospective, high-quality research to determine best practices for effective prevention of PA-VTE and to guide clinical decision making regarding the use of pharmacological thromboprophylaxis.
Promoting a systematic approach to VTE prevention
Irrespective of the approach taken to prevent VTE in individual hospitals or countries, VTE prevention is based on the accurate identification of women who are at risk of VTE and subsequent implementation of risk-appropriate preventative interventions. Despite the potentially devastating consequences associated with PAVTE, and the availability of nationallevel recommendations to guide its prevention, an implementation gap regarding utilisation of riskappropriate thromboprophylaxis remains suboptimal in many developed countries.28-33
The introduction of VTE risk scores and harnessing electronic health
A number of VTE risk scores intended to guide PA-VTE prevention strategies have been reported in the literature and recommended in clinical guidelines, reflecting trends towards efficiency and algorithmic approaches seen elsewhere in healthcare.34 There is considerable variation among these risk scores, in their development, target population, risk factors included and the weight of risk assigned to each risk factor. Critically, few have been validated, and the ability of each risk score to identify women at risk of VTE remains unknown.
The previously mentioned implementation gap’ has led to various other systemslevel interventions aimed at improving thromboprophylaxis.35 These interventions are primarily implemented in nonobstetric settings and include the introduction of automatic computerised or human alerts, or multifaceted interventions.36
Many interventions have also been described which are embedded in electronic health records (EHR) or employ Computerised Clinical Decision Support Systems (CCDSS) to stratify the patient according to VTE risk and make suggestions for thromboprophylaxis. Examples of advanced CCDSS functionality include the auto-population of clinical information into risk assessment checklists and translation of thromboprophylaxis recommendations into an order.37
In a systematic review of clinical trials and observational studies, the use of CCDSS was associated with a two-fold increase in the rate of ordering prophylaxis for VTE when compared with controls (OR 2.35, 95% CI: 1.78-3.10, p<0.001). As yet, there are no large published studies reporting the use of CCDSS in obstetric settings.
Diagnosis of pulmonary embolism (PE) in pregnancy
Evaluating a pregnant woman with suspected PE in pregnancy can be challenging because symptoms that can suggest PE can be common during normal pregnancy, particularly as the pregnancy advances.38,39
Both maternal and fetal radiation exposure is low using modern
imaging techniques.38 Perfusion scintigraphy and computed tomography pulmonary angiography (CTPA) confer extremely low fetal radiation doses, well below the threshold associated with fetal radiation complications (50-100mSv).40,41 Moreover, advances in CT technology have greatly reduced radiation exposure to the mother using a number of new methods that do not simultaneously reduce image quality. These include: reduced kilovoltage, reduced anatomic coverage of the scan (reported to reduce radiation dose by up to ~70%42), using iterative reconstructive techniques and, of particular relevance to breast radiation dose, reducing the CTPA contrast-monitoring component.42,43 It is now possible to achieve such low radiation dose that the breast tissue may only be exposed to doses as low as 3-4 mGy.38
A normal perfusion scan and a negative CTPA appear equally safe for ruling out PE in pregnancy.44-46 Inconclusive or indeterminate results are common47-49 and the chance of an inconclusive or suboptimal CTPA scan increases with advancing gestation.49 Decreased arterial enhancement in pregnancy is a contributing factor to the high proportion of inconclusive CTPA scans. Nevertheless, sensitivity and negative predictive value of lung scintigraphy and CTPA are reported to be high.
In light of these data, recently published 2019 guidelines of the European Society of Cardiology recommend that “Perfusion scintigraphy or CTPA (with a low-radiation dose protocol) should be considered to rule out suspected PE during pregnancy; CTPA should be considered as the first-line option if the chest X-ray is abnormal”.50 In contrast, 2018 ASH guidelines suggest “V/Q lung scanning over CT pulmonary angiography (conditional recommendation, low certainty in evidence about effects)”.13 Either imaging approach seems reasonable as a first-line diagnostic test, and is, in practice, driven by local availability.
The ongoing OPTICA Study (Optimised Computed Tomography Pulmonary Angiography (CTPA) in Pregnancy, Quality and Safety; NCT04179487, Clinicaltrials.gov) is a prospective multicentre study aiming to validate the clinical utility and safety of an optimised lowdose CTPA protocol for suspected pulmonary embolism in pregnancy.
Pregnant women undergoing the specified low-dose CTPA protocol for suspected PE at study sites with equivalent CT capabilities will be recruited. The primary outcome is the incidence of VTE at 3 months in women in whom PE was excluded at baseline CTPA.51
Until recently, clinical prediction rules and D-dimer levels had not been clinically validated to exclude PE during pregnancy. Therefore, the overall prevalence of PE during pregnancy was low, approximately 2-5%.47,48,52 A combination of data from clinical prediction rules and D-dimers may be used to rule out PE in non-pregnant patients without the need for radiological imaging. However, D-dimers continuously increase during pregnancy53,54 and levels are above the threshold for VTE “rule-out” of 500 µg/L in almost one-quarter of pregnant women in the third trimester.54 A negative D-dimer has not been clinically validated as a stand-alone rule-out test for PE during pregnancy.
The results of two recently published multicentre, multinational prospective diagnostic management outcome studies have, for the first time, provided evidence supporting the incorporation of D-dimers and clinical prediction rules into diagnostic algorithms for women with suspected PE during pregnancy. The first was published by Righini et al in 2019.55 In this study, PE was deemed excluded without CT pulmonary angiography (CTPA) in pregnant women who had a low or intermediate pretest clinical probability assessment (using the revised Geneva score) and a negative D-dimer (defined as <500 µg/L). Women fulfilling these criteria did not undergo diagnostic imaging, were not treated and were followed up at 3 months. PE was diagnosed in 7.1% of women and the rate of symptomatic VTE events at 3 months was 0.0% (95% CI: 0.0-1.0%) among untreated women. Among the 392 women who did not have a high pretest probability, 11.7% had a negative D-dimer result and did not require diagnostic imaging according to this algorithm. Bilateral CUS was mandated in women with a high pretest probability or low/ intermediate pretest probability with a positive D-dimer because CTPA could be avoided if the CUS revealed DVT. Of these 397 women undergoing CUS, only 7 had a positive result (an overall diagnostic yield of only 2%).
A second multicentre, international prospective management study
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
44
FOR YOURSELF, WOULD YOU CONSIDER BOTH EFFICACY AND SAFETY?
Choose both efficacy and safety with ELIQUIS®

ELIQUIS is a factor Xa inhibitor that offers superior risk reduction in stroke and systemic embolism, with significantly less major bleeding vs. warfarin in non-valvular AF patients.1,*
• Superiority demonstrated on stroke / systemic embolism vs. warfarin 1

• Superiority demonstrated on major bleeding vs. warfarin 1





DOSAGE AND ADMINISTRATION (SPC section 4.2): Oral. Taken with water, with or without food. Prevention of stroke and systemic embolism in patients with NVAF: The recommended dose is 5 mg twice a day. In patients who meet at least two of the following criteria: serum creatinine ≥ 1.5 mg/dL (133 micromole/L), age ≥ 80 years, or body weight ≤ 60 kg the recommended dose is Eliquis, 2.5 mg twice daily. Patients with severe renal impairment (creatinine clearance 15-29 ml/min) should receive Eliquis 2.5 mg twice daily. Therapy should be continued long term. Treatment of DVT, treatment of PE and prevention of recurrent DVT and PE (VTEt): The recommended dose for the treatment of acute DVT and treatment of PE is 10 mg twice daily for the first 7 days followed by 5 mg twice daily. As per available medical guidelines, short duration of treatment (at least 3 months) should be based on transient risk factors (e.g. recent surgery, trauma, immobilisation). The recommended dose for the prevention of recurrent DVT and PE is 2.5 mg twice daily. When prevention of recurrent DVT and PE is indicated, the 2.5 mg twice daily dose should be initiated following completion of 6 months of treatment with Eliquis 5 mg twice daily or with another anticoagulant. The duration of overall therapy should be individualised after careful assessment of the treatment benefit against the risk for bleeding. Prevention of VTE (VTEp): elective hip or knee replacement surgery: The recommended dose is 2.5 mg twice a day. The initial dose should be taken 12 to 24 hours after surgery. Hip replacement surgery, the recommended duration of treatment is 32 to 38 days. Knee replacement surgery, the recommended duration of treatment is 10 to 14 days. Missed Dose for All Indications: If a dose is missed, Eliquis should be taken immediately and then continue with twice daily dose as before. Switching: Switching treatment from parenteral anticoagulants to Eliquis (and vice versa) can be done at the next scheduled dose. These medicinal products should not be administered simultaneously. Switching treatment from VKA therapy to Eliquis: Warfarin or other VKA therapy should be discontinued and Eliquis started when the international normalized ratio (INR) is < 2. Switching treatment from Eliquis to VKA therapy: Administration of Eliquis should be continued for at least 2 days after beginning VKA therapy. After 2 days of co- administration of Eliquis with VKA therapy, an INR should be obtained prior to next scheduled dose of Eliquis. Co-administration of Eliquis and VKA therapy should be continued until the INR is ≥2. Renal Impairment - mild or moderate renal impairment: For the prevention of VTE in elective hip or knee replacement surgery (VTEp), for the treatment of DVT, treatment of PE and prevention of recurrent DVT and PE (VTEt), no dose adjustment is necessary. For the prevention of stroke and systemic embolism in patients with NVAF and serum creatinine ≥ 1.5 mg/dL (133 micromole/L) associated with age ≥ 80 years or body weight ≤ 60 kg, a dose reduction is necessary. In the absence of other criteria for dose reduction (age, body weight), no dose adjustment is necessary. Severe renal impairment (creatinine clearance 15-29 mL/min): For the prevention of VTE in elective hip or knee replacement surgery (VTEp), for the treatment of DVT, treatment of PE and prevention of recurrent DVT and PE (VTEt), Eliquis is to be used with caution. For the prevention of stroke and systemic embolism in patients with NVAF, patients should receive the lower dose of Eliquis 2.5mg twice daily. In patients with creatinine clearance < 15 mL/min, or in patients undergoing dialysis, there is no clinical experience therefore Eliquis is not recommended. See SmPC for further details. Hepatic impairment: Contraindicated in patients with hepatic disease associated with coagulopathy and clinically relevant bleeding risk. Not recommended in patients with severe hepatic impairment. Use with caution in patients with mild or moderate hepatic impairment (Child Pugh A or B). No dose adjustment is required in patients with mild or moderate hepatic impairment. Use with caution in patients with elevated liver enzymes (ALT/AST >2 x ULN) or total bilirubin ≥ 1.5 x ULN. Prior to initiating Eliquis, liver function testing should be performed. Catheter ablation (NVAF): Patients can continue Eliquis use while undergoing catheter ablation. Cardioversion (NVAF): Eliquis can be initiated or continued in NVAF patients who may require cardioversion. See SmPC for further details. Patients with NVAF and acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI): There is limited experience of treatment with apixaban at the recommended dose for NVAF patients when used in combination with antiplatelet agents in patients with ACS and/or undergoing PCI after haemostasis is achieved. See SmPC for further details. Paediatric population: Eliquis is not recommended in children and adolescents below the age of 18. CONTRAINDICATIONS (SPC section 4.3): Hypersensitivity to active substance or to excipients, active clinically significant bleeding, hepatic disease associated with coagulopathy and clinically relevant bleeding risk, lesion or condition if considered a significant risk factor for major bleeding, see SmPC for further details. Concomitant treatment with any other anticoagulant agent except under specific circumstances of switching anticoagulant therapy or when unfractionated heparin (UFH) is given at doses necessary to maintain an open central venous or arterial catheter or when UFH is given during catheter ablation for atrial fibrillation, see SmPC for further details. WARNINGS AND PRECAUTIONS (SPC section 4.4): Haemorrhage risk: Carefully observe for signs of bleeding. Use with caution in conditions with increased risk of haemorrhage. Discontinue administration if severe haemorrhage occurs. An agent to reverse the anti-factor Xa activity of apixaban is available. For information on reversal and managing bleeding, see SmPC for further details. Interaction with other medicinal products affecting haemostasis: Concomitant treatment with any other anticoagulant is contraindicated (see contraindications). Concomitant use of Eliquis with antiplatelet agents increases the risk of bleeding. Care with concomitant SSRIs, SNRIs or NSAIDs, including acetylsalicylic acid. Following surgery, other platelet aggregation inhibitors are not recommended concomitantly with Eliquis. In patients with atrial fibrillation and conditions that warrant mono or dual antiplatelet therapy, a careful assessment of the potential benefits against the potential risks should be made before combining this therapy with Eliquis. A clinical trial enrolled patients with atrial fibrillation with ACS and/or undergoing PCI and a planned treatment period with a P2Y12 inhibitor, with or without ASA, and oral anticoagulant (either apixaban or VKA) for 6 months. Concomitant use of ASA increased the risk of ISTH (International Society on Thrombosis and Hemostasis) major or CRNM (Clinically Relevant Non-Major) bleeding in apixaban-treated subjects. See SmPC for further details. Use of thrombolytic agents for the treatment of acute ischemic stroke: Limited experience. Patients with prosthetic heart valves: safety and efficacy of Eliquis have not been studied in patients with prosthetic heart valves, with or without atrial fibrillation. Therefore, the use of Eliquis is not recommended in this setting. Patients with antiphospholipid syndrome: Direct acting Oral Anticoagulants (DOACs), including Eliquis, are not recommended for patients with a history of thrombosis who are diagnosed with antiphospholipid syndrome (see SmPC for further details). Surgery and invasive procedures: Discontinue at least 48 hours prior to elective surgery or invasive procedures with a moderate or high risk of bleeding. Discontinue at least 24 hours prior to elective surgery or invasive procedures with a low risk of bleeding. If surgery or invasive procedures cannot be delayed, appropriate caution should be exercised, taking into consideration an increased risk of bleeding. Eliquis should be restarted after the invasive procedure or surgical intervention as soon as possible provided the clinical situation allows and adequate haemostasis has been established. For patients undergoing catheter ablation for atrial fibrillation, Eliquis treatment does not need to be interrupted. Temporary discontinuation: Discontinuing anticoagulants, including Eliquis, for active bleeding, elective surgery, or invasive procedures places patients at an increased risk of thrombosis. Lapses in therapy should be avoided and if anticoagulation with Eliquis must be temporarily discontinued for any reason, therapy should be restarted as soon as possible. Spinal/ epidural anaesthesia or puncture Patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal haematoma which can result in long- term or permanent paralysis. The risk of these events may be increased by the post- operative use of indwelling epidural catheters or the concomitant use of medicinal products affecting haemostasis. Indwelling epidural or intrathecal catheters must be removed at least 5 hours prior to the first dose of Eliquis. The risk may also be increased by traumatic or repeated epidural or spinal puncture. Patients are to be frequently monitored for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, bowel or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis. There is no clinical experience with the use of Eliquis with indwelling intrathecal or epidural catheters. See SmPC for further details. Haemodynamically unstable PE patients or patients who require thrombolysis or pulmonary embolectomy: Eliquis is not recommended as an alternative to unfractionated heparin in patients with pulmonary embolism who are haemodynamically unstable or may receive thrombolysis or pulmonary embolectomy since the safety and efficacy of Eliquis have not been established. Patients with active cancer: Patients with active cancer can be at high risk of both venous thromboembolism and bleeding events. When apixaban is considered for DVT or PE treatment in cancer patients, a careful assessment of the benefits against the risks should be made. Renal impairment: see dosage and administration section. Elderly patients: Increasing age may increase haemorrhagic risk. Also, the co-administration of Eliquis with ASA in elderly patients should be used cautiously because of a potentially higher bleeding risk. Body weight: Low body weight (< 60 kg) may increase haemorrhagic risk. Hepatic impairment: see dosage and administration section. Interaction with Inhibitors of CYP3A4 and P-gp: Not recommended with strong inhibitors of both CYP3A4 and P-gp. These medicinal products may increase Eliquis exposure by 2-fold or greater in the presence of additional factors that increase Eliquis exposure (e.g. severe renal impairment) see SmPC for further details. Interaction with Inducers of CYP3A4 and P-gp: Eliquis should not be used for the treatment of DVT and PE in patients receiving concomitant systemic treatment with strong inducers of both CYP3A4 and P-gp since efficacy may be compromised. Concomitant systemic treatment with strong inducers of both CYP3A4 and P-gp, Eliquis should be used with caution for the prevention of VTE in elective hip or knee replacement surgery, for the prevention of stroke and systemic embolism in patients with NVAF and for the prevention of recurrent DVT and PE, though no dose adjustment for Eliquis is required during concomitant therapy with such medicinal products. Hip fracture surgery: Eliquis has not been studied in clinical trials in patients undergoing hip fracture surgery. Therefore, it is not recommended in these patients. Laboratory parameters Clotting tests (PT, INR, and aPTT) are affected by the mechanism of action of apixaban. Changes observed at the expected therapeutic dose are small and subject to a high degree of variability, see SmPC for further details. Information about excipients: Eliquis contains lactose. Patients with galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take Eliquis. DRUG INTERACTIONS (SPC Section 4.5): Eliquis should be used with caution when co-administered with SSRIs/SNRIs, NSAIDs, ASA and/or P2Y12 inhibitors because these medicinal products typically increase the bleeding risk. There is limited experience of co-administration with other platelet aggregation inhibitors (such as GPIIb/IIIa receptor antagonists, dipyridamole, dextran or sulfinpyrazone) or thrombolytic agents. As such agents increase the bleeding risk, co-administration of these products with Eliquis is not recommended. See SmPC for further details.Due to an increased bleeding risk, concomitant
 Prevention
and systemic embolism in adults with
symptomatic heart failure (NYHA Class ≥ II). Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent
in adults who
Prevention
and systemic embolism in adults with
symptomatic heart failure (NYHA Class ≥ II). Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent
in adults who
ELIQUIS® (apixaban) PRESCRIBING INFORMATION Ireland Consult Summary of Product Characteristics (SmPC) before prescribing PRESENTATION: Film-coated tablets; 5 mg and 2.5 mg apixaban. INDICATION (SPC section 4.1):
of stroke
non-valvular atrial fibrillation (NVAF) with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA), age ≥ 75 years, hypertension, diabetes mellitus or
DVT and PE in adults (see Special warnings and precautions for information on haemodynamically unstable PE patients). Prevention of venous thromboembolic events (VTE)
have undergone elective hip or knee replacement surgery (2.5 mg only).
treatment with any other anticoagulants is contraindicated, except under specific circumstances of switching anticoagulant therapy, when UFH is given at doses necessary to maintain an open central venous or arterial catheter or when UFH is given during catheter ablation for atrial fibrillation. Administration of activated charcoal reduces Eliquis exposure. Also see contraindications and special warnings and precautions section; Consult SmPC (contraindications, special warnings and precautions and drug interactions) for full details on interactions. PREGNANCY AND LACTATION (SPC section 4.6): Pregnancy: As a precautionary measure, it is preferable to avoid the use of apixaban during pregnancy. Breastfeeding A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from apixaban therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman. UNDESIRABLE EFFECTS (SPC section 4.8): Increased risk of occult or overt bleeding from any tissue or organ, which may result in post haemorrhagic anaemia. The signs, symptoms, and severity will vary according to the location and degree or extent of the bleeding. Frequencies: common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000); not known (cannot be estimated from the available data). Prevention of VTE in adult patients who have undergone elective hip or knee replacement surgery (VTEp): Common: anaemia; haemorrhage*; haematoma*; nausea; contusion. Uncommon: thrombocytopenia*; epistaxis*; haematochezia*; liver function test abnormal (including blood bilirubin increased*); haematuria*; specific haemorrhage such as gastrointestinal*, abnormal vaginal*, urogenital*, post procedural*, wound secretion*, incision site*, operative*. Rare: hypersensitivity*; anaphylaxis*; haemoptysis*; gingival bleeding*; specific haemorrhage such as eye (including conjunctival)*, rectal*, muscle*. Not known: angioedema*; specific haemorrhage such as brain (encompassing intracranial, intraspinal)*, intra-abdominal*, respiratory tract*, haemorrhoidal*, mouth*, retroperitoneal*, traumatic*, erythema multiforme*. Prevention of stroke and systemic embolism in adult patients with NVAF, with one or more risk factors (NVAF): Common: anaemia; haemorrhage*; haematoma*; hypotension (including procedural hypotension); epistaxis*; nausea; gingival bleeding*; gamma-glutamyltransferase increased; haematuria*; contusion; specific haemorrhage such as eye (including conjunctival)*, gastrointestinal*, rectal*. Uncommon: thrombocytopenia*; hypersensitivity*; anaphylaxis*; haemoptysis*; haematochezia*; liver function test abnormal (including blood bilirubin increased*); specific haemorrhage such as brain (encompassing intracranial, intraspinal)*, intra-abdominal*, haemorrhoidal*, mouth*, abnormal vaginal*, urogenital*, post procedural*, wound secretion*, incision site*, operative*, traumatic*. Rare: specific haemorrhage such as respiratory tract*, retroperitoneal*, muscle*. Very Rare: erythema multiforme*. Not known: angioedema*. Treatment of DVT and PE, and prevention of recurrent DVT and PE (VTEt): Common: anaemia; thrombocytopenia*; haemorrhage*; haematoma*; epistaxis*; nausea; gingival bleeding*; gamma-glutamyltransferase increased; alanine aminotransferase increased; skin rash; haematuria*; contusion; specific haemorrhage such as gastrointestinal*, mouth*, rectal*, abnormal vaginal*, urogenital*. Uncommon: hypersensitivity*; anaphylaxis*; haemoptysis*; haematochezia*; liver function test abnormal (including blood bilirubin increased*); specific haemorrhage such as eye (including conjunctival)*, haemorrhoidal*, muscle*, post procedural*, wound secretion*, incision site*, operative*, traumatic*. Rare: specific haemorrhage such as brain (encompassing intracranial, intraspinal)*, respiratory tract*. Not Known: angioedema*; specific haemorrhage such as intra-abdominal* and retroperitoneal*, erythema multiforme*. *Denotes serious adverse reaction Refer to SmPC for all other adverse events LEGAL CATEGORY: POM. MARKETING AUTHORISATION NUMBER (SPC section 8): EU/1/11/691/002-3, EU/1/11/691/008, EU/1/11/691/014 PACKAGE QUANTITIES: Carton of 20 film-coated tablets 2.5 mg, 60 film-coated tablets 2.5 mg, 56 film-coated tablets 5 mg, 28 film- coated tablets 5 mg. MARKETING AUTHORISATION HOLDER (SPC section 7): Bristol-Myers Squibb/Pfizer EEIG, Plaza 254, Blanchardstown Corporate Park 2, Dublin 15, D15 T867, Ireland FOR FURTHER INFORMATION CONTACT: medical.information@bms.com or 1 800 749 749 (Ireland) DATE OF PREPARATION: April 2021 ADDITIONAL INFORMATION AVAILABLE ON REQUEST Approval Code: 432-IE-2100041 Adverse events should be reported. Reporting forms and information can be found at: Ireland - via HPRA Pharmacovigilance at www.hpra.ie Adverse events should also be reported to Bristol-Myers Squibb via medical.information@bms.com or 1 800 749 749 (Ireland) AF = Atrial Fibrillation. Reference: 1. Granger CB et al. N Engl J Med 2011; 365: 981–992. Date of approval: May 2021 Job code: PP-ELI-IRL-0497 www.eliquis.ie *with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA); age≥ 75 years; hypertension; diabetes mellitus; symptomatic heart failure (NYHA Class ≥ II)
WOMEN’S HEALTH VTE IN PREGNANCY


threshold and clinical prediction rule in the evaluation of a pregnant woman with a suspected PE.
Treatment of VTE in pregnancy
3 months evaluated a pregnancyadapted YEARS algorithm56 with parallel assessment of the three YEARS criteria (clinical signs of DVT, haemoptysis and pulmonary embolism as the most likely diagnosis) and D-dimer levels followed by selective CTPA in 498 women with suspected PE during pregnancy.57 In women with no YEARS items and a D-dimer <1000ng/mL, or in patients with ≥1 YEARS item and D-dimer <500ng/ mL, PE was deemed excluded. All other patients underwent diagnostic imaging, consisting of CTPA (or CUS if women had clinical signs of DVT, without further imaging if the CUS revealed DVT). The primary outcome was the cumulative incidence of symptomatic VTE, with confirmation by objective tests, during a 3 month follow up period in women in whom anticoagulation had been withheld on the basis of a negative algorithm result. In 510 consecutive patients screened, 4.0% were diagnosed with PE.
pregnancy. Exposure to diagnostic imaging could be avoided in 39% (95% CI: 35-44%) of patients. D-dimers increased throughout pregnancy. Consequently, the proportion of women in whom PE could be excluded without diagnostic imaging was lower in the first trimester (32%) and higher in the third trimester (65%). The diagnostic yield of targeted CUS was higher than that reported in the Righini et al study, at 7%.
Collectively, these prospective data derived from high-quality clinical management studies prompted a change in the recommendation given in the 2019 ESC Guidelines on Diagnosis and Management of Acute PE38: that D-dimer measurement in conjunction with clinical prediction rules “should be considered” during investigation of suspected PE in pregnancy. Further prospective clinical trials and studies may consider further evaluating the optimal d-dimer
The management of VTE during pregnancy should be delivered by an experienced multidisciplinary team38 and ideally, written care pathways should be agreed and discussed with the woman who has experienced the VTE event. LMWH is recommended by international guidelines for the treatment of VTE during pregnancy, with a more favourable risk profile and more predictable pharmacokinetics compared with unfractionated heparin.13,15,38 Unlike vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs), LMWH does not cross the placenta and there is no associated risk of fetal haemorrhage or teratogenicity.
DOACs are contraindicated in pregnant patients.58 For most women receiving LMWH treatment for VTE during pregnancy (except in exceptional circumstances including renal impairment and at extremes of body weight59,60), the measurement of plasma antiFXa activity to guide dosing is not recommended in view of the predictable pharmacokinetic profile of LMWH, lack of data on optimal anti-FXa levels and limitations of the assay 61. Unfractionated Heparin (UFH) use in previous decades was associated with heparin-induced thrombocytopenia and bone loss resulting in a reported 2-9% risk of osteoporotic fracture following prolonged UFH use during pregnancy (summarized in62). However it remains uncertain whether the risk is increased with LMWH use: in a recent observational cohort study, lumbar spine bone mineral density was similar in LWMH-treated women to controls following adjusting for potential confounders. No osteoporosis or osteoporotic fractures were reported.62
Management of LMWH in the peripartum period for women diagnosed with VTE during pregnancy is not supported by high-quality data. The incidence of spinal haematoma after regional analgesia in pregnant women is uncertain. Moreover, pregnant women are in a procoagulant state at the time of delivery. International guideline recommendations vary on timing of peripartum regional analgesia63,64 and it is very important to consider the competing risk of performing
a caesarean section under general anaesthesia. In general, regional analgesia should be avoided unless LMWH has been discontinued at least 24 hours before delivery (assuming normal renal function and including risk assessment at extremes of body weight). Current guidelines remind us that data are limited on the optimal timing of postpartum LMWH re-initiation and should be guided by a personalized risk assessment, taking into account mode of deliver and thrombotic versus bleeding risks.64,65 Recent European Society of Cardiology guidelines recommend that “LMWH should not be given for at least 4 hours after removal of the epidural catheter; the decision on timing and dose should consider whether the epidural insertion was traumatic and take into account the risk profile of the woman. For example, an interim dose of a prophylactic LMWH dose may be considered postoperatively (after caesarean section), once at least 4 hours have elapsed since epidural catheter removal and allowing for an interval of at least 8–12 hours between the prophylactic and the next therapeutic dose”66,67 ENREF_119
Close collaboration between the obstetrician, the anaesthesiologist, and the attending physician is important. After delivery, a planned transition from LMWH to VKA therapy can be considered by the multidisciplinary team. Anticoagulant treatment should be administered for at least 6 weeks after delivery and with a minimum overall treatment duration of 3 months. LMWH and VKAs can be given to breast-feeding mothers.
Summary
Excellent prevention, diagnosis and treatment strategies should be a priority for health service providers globally. Preventing PA-VTE is reported to be the most readily implementable means of systematically reducing the rate of maternal death at a population level.68,69 However, effective prevention is limited by a paucity of evidence to support effective interventions and challenges with implementing of VTE prevention strategies in clinical practice. A systematic approach to VTE prevention should be adopted to reliably identify women at increased risk and ensure consistency of VTE prevention where indicated.
References available on request
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
46
WOMEN’S HEALTH BONE HEALTH
Exercise for Bone Health in Women

Osteoporosis and Fracture Risk
Osteoporosis is a disease characterised by low bone mass which affects both men and women but with a higher prevalence among women. It is estimated that 1 in 3 women over age 50 will experience fractures due to osteoporosis, as will 1 in 5 men. With people living longer, the lifetime risk of a fracture related to bone fragility is heightened.
Bone Health across the Lifespan
Childhood and adolescence represent a critical period for the development of strong healthy bones with peak bone mass usually achieved between age 25 to 30. Bone density remains relatively stable until age 50 but begins to decline thereafter. Adopting and sustaining healthy lifestyle behaviours including exercise, a healthy diet (adequate calcium, Vitamin D, Protein intake) and getting sufficient sun exposure (Vitamin D) are advocated to promote bone health across the lifespan. At menopause bone loss may accelerate significantly in women due to the reduction in oestrogen, a key regulator of bone metabolism. Women should actively seek a DXA scan at that time. Where bone loss issues are identified, pharmacological therapy may be required which is based on a number of factors including DXA findings, prior fragility fracture and the risk of future fracture.
Exercise and Bone Health
Exercise for bone health should start in the childhood years with the goal of optimising peak bone mass. In the middle years maintaining bone mass is the goal and in later life, the emphasis is more on slowing down bone loss.
As for all exercise programmes achieving an effective exercise dose is critical with consideration of frequency, intensity, type and time (FITT). Some bone health exercise programmes aim to prevent osteoporosis in healthy populations whereas exercise prescription for individuals with osteoporosis requires risk stratification.
What type of exercise is optimal to build bone?
Bone responds to mechanical stimuli with adjustment of skeletal mass and architecture in response to changing mechanical environments. The best types of exercise to build bone (osteogenic exercise) are impact (involves running, hopping and jumping) and resistance (strength) training with a combination of both the most effective. Strength training is also critical to slow down age related loss of muscle mass and function.
Walking is a form of impact exercise but higher impact, more unusual activity will likely induce an even better bone formation response. Recent research places more emphasis on the need for higher loads to enhance bone when engaging in resistance training. Most of the studies
Associate Professor at UCD School of Public Health, Physiotherapy and Sports Science. A Chartered Physiotherapist, she and colleagues at UCD cofounded UCD’s Better Bones programme in 2012, which focused on empowering individuals (age 55+) to adopt positive bone building and fracture prevention behaviours

regarding exercise interventions focusing on enhancing bone have been conducted in post -menopausal women. Mixed loading programmes (impact+ resistance training) conducted two to three times per week for approximately one hour for over six to nine months have shown positive changes in bone mineral density (BMD) on DXA scan. However, the minimal effective exercise dose to enhance BMD has not been established to date.
Exercising if have osteoporosis
Maintaining an active lifestyle is always positive and it is important that individuals with osteoporosis do not become fearful about exercise participation but adapt their exercise as required. ‘Bone building’ may continue to be a key goal of exercise for people with osteoporosis. However, when

osteoporosis is more severe the main focus switches to improving muscle strength and balance to maintain and enhance function and reduce the risk of a fall and fracture, rather than aiming for an improvement in bone density. For example, where a person has severe osteoporosis of the spine and/or a history of a fragility fracture, exercises which involve lifting heavier weights with twisting of the spine are to be avoided, consistent with manual handling principles. In addition, higher impact exercises may not be appropriate. A risk benefit assessment and exercise prescription by a physiotherapist is recommended, bearing in mind that maintaining cardiovascular fitness through exercise is always important for health.
Key Messages
• Exercise for bone health should start in the childhood years and continue through all life stages
• A combination of strength and impact exercise is optimal for building bone
• A DXA scan is recommended for women around the time of menopause, with repeat scans thereafter.
• Consultation with healthcare professionals is critical to decide on the need for initiation of pharmacological therapy to enhance bone, a decision which is based on a number of factors including DXA findings, prior fragility fracture and the risk of future fracture.
• Exercise continues to be very important for women with osteoporosis and consultation with a physiotherapist is recommended
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
47
WOMEN’S HEALTH MENOPAUSE
The Complex Menopause Clinic
Written by Dr Deirdre Lundy, Clinical Lead, Complex Menopause Service, NMH, Holles St
 Picture: Gerry Mooney
Picture: Gerry Mooney
HRT, its risks and benefits. Thus began the renaissance of menopause consultations and requests for HRT.
Our guidelines for prescribing in the community are fairly clear. Women under 60 yrs. of age without certain & specific comorbidities CAN almost always try HRT- if they like. If it doesn’t help, they stop. If it does, they can choose to carry on. The choice is theirs and for women under 60, benefits almost always outweigh risks.
HRT. Last but never least, we address pelvic floor and vaginal issues with local vaginal estrogen. Genitourinary issues are typically resistant to systemic estrogen supplements and need their own targeted therapies.
Doing a Menopause ‘MOT’ takes time
Lately the more positive media coverage of menopausal experiences and the benefits of hormone replacement therapies (HRT) has created a tsunami of interest from patients. Maybe an overstretched health service working through a pandemic is not the ideal setting for a resurgence in menopause complaints and HRT requests – but that is where we are in 2022. Moreover, many common comorbidities can make it difficult for colleagues in primary care to offer treatment. The various Menopause guidance bodies will say, ‘referral to a menopause specialist is advisable’. Well up to this year, no such clinic existed in the public system in Ireland. The new “Complex Menopause Service” in the National Maternity Hospital, Holles ST is the first of many promised clinics.
We are a team of three part- time GP Menopause Specialists and an extraordinarily dedicated full time Clinic Nurse Manager have been accepting referrals and advising patients since late 2021. It is not possible for us to offer a service to everyone going into menopause. This is meant to be a GP skill in any event. We only accept patients meeting specific criteria that can make managing their menopause in the community more challenging. We have a dedicated referral form on the NMH website that lays out those criteria. In the event that a patient is not suitable for our clinic and HRT could be offered in primary care, we try to write back to the
referring doctor with suggestions and advice rather than leave them with nowhere to turn.
Some background on Perimenopause and Menopause

As far as controlling perimenopausal (still ovulating sometimes) & menopausal (no longer ovulating) symptoms are concerned, there is only one remedy that will improve most if not all the symptoms with any proven efficacy and that is HRT.
The management of peri/ menopause symptoms and the prescribing of HRT is expected to be part of a GP skillset. Lack of interest in HRT use on the part of our patients can be dated from 2002 when a deeply flawed US study (the Women’s Health Initiative) linked HRT use to breast cancer. The fear generated by this publication was deeply felt by patients and their healthcare providers. Lack of demand for menopause hormonal relief resulted in widespread deskilling among colleagues in both primary and secondary care and even women who requested medical therapy were frequently advised, ‘you don’t want HRT, it causes breast cancer’. Things finally started to swing back toward sanity and a more evidence based approach when in 2015, the UK’s National Institute of Health and Care Excellence (NICE) published their guideline (NG23) on Menopause Care and HRT use; a very positive and reassuring review of modern
What is in modern HRT? HRT is not the Pill. The horse urine estrogen(s) offered in the WHI study are rarely used in Ireland anymore and modern HRT contains low doses of estrogen that is molecularly identical to the 17-beta Estradiol created in a functioning ovary. We often reassure patients, ‘you already have this hormone in your body, we are just going to ‘smooth out’ the peaks and troughs’ of estrogen levels that can cause meno symptoms to flare. Modern progestagens also appear to be ‘kinder’ than the high dose medroxyprogesterone acetate used in the WHI study. We typically offer micronised progesterone (another ‘ovarian - similar’ molecule) or even the synthetic progestagen dydrogesterone to oppose and balance the additional estrogen. These have been shown to have a neutral impact on clotting mechanisms and are less likely to cause side effects than their older, stronger predecessors. For women with contraceptive need or reactive/heavy baseline uterine bleeding we recommend our most loyal of servants, the Mirena IUS. The levonorgestrel molecule carried by the IUS is a strong, synthetic progestagen but very little of it is absorbed systemically and so most patients tolerate it quite well. Sometimes, we turn to testosterone. Androgens are important during the menopause- for some more than others- and the use of supplemental testosterone - in low female doses- is indicated for patients with libido issues that have not been improved by standard estrogen + progestagen
We in GP often find a menopause consultation becomes more like a mid-life pit stop which will not be squeezed into a 15-minute encounter no matter how fast you talk. We are often faced with multiple complaints that require investigation and advice. We strive to tweak those modifiable risk factors, giving advice on smoking cessation, physical activity, living with and addressing obesity, diet, etc. We encourage screening uptake, we measure BP and correct hypertension when needed. We often do bloods for lipids, thyroid, etc. and act on the results. We address disorders of mood which can frequently come to the fore around menopause and attempt to intervene if we can. We discuss contraception and sexual health where relevant, we discuss menstrual blood loss and intervene if necessary - I could go on. Thorough menopause consultations can be very time consuming and the HSE does not yet ring fence funding for these visits which in my opinion is unfortunate. Somewhere in that patient visit, we need to provide balanced advice and signpost evidence-based information on menopause strategies including HRT use.
How safe is HRT & with whom should we be more cautious?
Should everyone be using HRT? The current recommendations say no. We do not have enough data to say all women should keep their estrogen levels high – there are many unknowns or “not too sures” surrounding risks of ongoing HRT use in people who do not need HRT for symptom relief.
HRT is not for everyone and is certainly not to be used as a salve for people who know they ought to move more, eat better, drink less and stop smoking! But if a patient is struggling, if they are not well
The Complex Menopause Clinic in the National Maternity Hospital - The Story so Far!
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
48

NOW RINVOQ® (upadacitinib) INDICATED FOR ADULTS WITH MODERATE TO SEVERELY ACTIVE ULCERATIVE COLITIS1 *RINVOQ® is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1 AbbVie® is a registered trademark of AbbVie Inc. RINVOQ® and its design are registered trademarks of AbbVie Inc. REFERENCES: 1. RINVOQ® summary of product characteristics, available at www.medicines.ie. JAK: Janus kinase; UC: ulcerative colitis. Further information is available from AbbVie Limited, 14 Riverwalk, Citywest Business Campus, Dublin 24, Ireland. Legal Classification: POM(S1A). IE-RNQG-220007 | August 2022 Full Summary of Product Characteristics is available at medicines.ie ▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; website: www.hpra.ie. Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
and if they are likely to get more benefits from using HRT than to be exposed to risk, then we offer HRT.
If someone is experiencing classic symptoms of ovarian hormone fluctuation/decline, who is also generally healthy and in the typical perimenopause/menopause age group and who – most importantly – would like to try a menopause hormone therapy of some kind, then why not?

And the most important thing to remember is that while individual doctors and attitudes may vary and are worthy of discussion, we have the benefit of UK, European and International guidelines on menopause care. And they all sing
News
from the same hymn sheet – the title of which is individualisation.
Co-Morbidities
There are some cases where the risk/benefit profile of HRT use may be less obvious. The British Menopause Society suggests referral to a menopause speciality for patients with a personal past history; note: NOT family history, of:
• Cardiovascular & Cerebrovascular disease; especially ischemic stroke or MI
• Venous thromboembolism
• Sex- Hormone Sensitive Cancers; especially breast, ovary and uterine lining
WOMEN’S HEALTH MENOPAUSE
Immunological Diseases including HIV
In these situations, use of HRT is usually allowed if the comorbidity is well managed and the HRT is confined to certain products and used at modest doses. Patients with a background of VTE are arguably the most straightforward & are all offered HRT if they wish to try it but the estrogen component is given at low doses, only through the skin (thus avoiding any impact on clotting activity) and with judicious choice of a nonthrombogenic progestagen.
The most challenging of this group are people who have been diagnosed with
• Breast Cancer
There seems to be a lack of follow on care for women who have been treated for breast cancer with over 50% of our referrals this year coming from GP’s trying to help patients with menopause symptoms after breast cancer. Sometimes the symptoms are coincidental, sometimes the menopause has been induced iatrogenically as part of their breast cancer management but either way there can be ongoing suffering with little support apart from ‘talk to your GP’. This needs to be addressed as more and more of us are being diagnosed with and treated for breast cancer. We are reaching out to colleagues to try and improve this situation. Non-HRT prescription medications and liberal access
to Cognitive behavioural Therapy techniques has helped us offer some relief to these patients but it is far from ideal.
What’s next?
These first few months of our Complex Menopause Service have been both challenging and highly rewarding. The feedback from our patents has been very positive- gratitude for a listening ear seems to be the overriding theme. Our ‘to do list’ is endless but key goals include: getting as much information on the NMH. ie website as we can, developing standards of practice for our own Menopause Specialists when it comes to prescribing HRT for patients with comorbidities (and hopefully engaging with the other services around Ireland to keep practices consistent) and inviting colleagues in other hospital specialities to connect with us and help us better serve all of our patients. We are doing some basic research on all of the above so we can get a sense of what might be needed as we go forward. As the next wave of Dublin, Cork, Galway and Limerick Menopause Specialist clinics start to open their doors and enrol patients, we in the NMH hope to expand our referral criteria to include local patients without comorbidities who are struggling to find symptom relief in spite of good GP support. Upward and onward.

For further information, please visit www.nmh.ie
Healthy Ireland – Healthy Weight
The Minister for Health Stephen Donnelly and the Minister for Public Health, Well Being and National Drugs Strategy, Frank Feighan, have launched a new Healthy Weight campaign, developed under the Healthy Ireland Framework.
Research has shown that weightgain is influenced by multiple factors such as environment, access to healthy and affordable food, physical activity, genetic make-up, and lifestyle. This campaign will support people to prevent weight gain in their 20s and 30s by focussing on four pillars – sleep, stress, physical activity and nutrition.
Minister Donnelly said, “All of us in Government have a role in addressing overweight and obesity, the underlying causes of which are impacted by national policy and action across a wide range of areas. Many of the influencing factors lie outside our own individual control, such as accessibility and affordability of healthy food, socio-economic stressors and the built environment.
“This campaign forms part of a series of initiatives by Healthy Ireland to address overweight and obesity in the adult population, with this particular element raising awareness of the heightened risk of developing overweight and
obesity as people move through young adulthood.”
The HSE's Clinical Lead for Obesity, Professor Donal O’Shea added, “Today is an important day as we launch this campaign, which, in many ways is a world first –because the emphasis is so clearly on prevention of weight gain.
“We want to communicate to people in their mid-twenties the risks and opportunities that the following decade presents when it comes to maintaining the healthiest weight you can. Eating healthily, keeping active, managing stress and sleeping well are core habits that can help us prevent
weight gain as we move through this stage of life – and that would be a massive investment in your future self.”
The Healthy Weight campaign will share practical, expert information about behaviours that can affect weight, in order to support adults to be and feel healthier. These behaviours include good nutrition and eating well, managing stress, sleeping well and engaging in regular physical activity.
The campaign is aimed at 25 to 34-year-olds, and will be hosted on social media including Instagram, Facebook and TikTok.
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
50
MIGRAINE IN WOMEN
Migraine in Women
Introduction: Migraine is a neurological disorder characterised by recurrent bouts of severe headaches with autonomic and neurological symptoms. Scientific advances in recent decades have provided us with a clear understanding of the pathophysiology of migraine and enabled the development of migraine specific acute and preventative therapies. Migraine occurs in 10-12% of the population and there is a 3:1 female to male ratio. It is particularly common, in women of all ages, and female preponderance begins around the time of the menarche. The World Health Organisation Global Burden of Disease study ranked migraine as the 2nd leading cause of disability and the leading cause in women under the age of 50 years.
Diagnosis: The International Headache Society has standardised the diagnosis of migraine using universally accepted diagnostic criteria (ICHD3) and these guidelines have provided us with a uniform approach to diagnosis in clinical practice (Fig 1,2,3,4).
Migraine Without Aura: 1.1
A. At least 5 attacks fulfilling criteria B-D
B. Headache attacks lasting 4-72 hours (untreated or unsuccessfully treated)
C. Headache has > 2 of the following characteristics
1. Unilateral Location
2. Pulsating Quality
3. Moderate or severe pain intensity
4. Aggravated by or causing avoidance of routine physical activity.
D. During Headache > 1 of the following.
1. Nausea and or vomiting
2. Photophobia and Phonophobia
E. Not better accounted for by another ICHD-3 diagnosis
Fig: 1
Migraine With Typical Aura: 1.2.1
A. At least 2 attacks fulfilling B and C
B. Aura of visual, sensory and /or speech /language symptoms, each fully reversible, but no motor, brainstem or retinal symptoms.
C. > 2 of the following 4 characteristics
1. > aura symptom spreads gradually over 5 minutes and /or > 2 symptoms occur in succession.
2. Each individual aura symptom lasts 5-60 minutes
3. > 1 aura symptom is unilateral
4. Aura accompanied by or followed in < 60 minutes by headache
D. Not better accounted for by another ICHD-3 diagnosis, and TIA excluded.
Fig: 2
Chronic Migraine: 1.3
A. Headache (TTH-like and /or Migraine-like on >15 d/mo for > 3 month and fulfilling criteria B and C.
B. In a patient who has had > 5 attacks of fulfilling criteria B-D For 1.1 Migraine Without Aura and /or criteria B and C for 1.2 Migraine With Aura
C. On > 8 d/month for > 3 month fulfilling any of the following:
1. Criteria C and D for Migraine Without Aura
2. Criteria B and C for Migraine With Aura
3. Believed by the patient to be migraine at onset and relieved by a triptan or ergot derivative
D. Not better accounted for by another ICHD-3 diagnosis

Fig: 3
Hemiplegic Migraine: 1.2.3
A. Attacks fulfilling criteria for 1.2 Migraine With Aura and criterion B below.
B. Aura consists of both of the following
1. Fully reversible motor weakness
2. Fully reversible visual, sensory, and or speech / language symptoms
Fig: 4
Many migraine patients also experience other symptoms which are disabling but are not incorporated into the diagnostic guidelines. These symptoms vary and include vertigo, dizziness,
The natural history of migraine in women is that it begins around the time of the menarche and early teenage years, peaks in the 30’s and declines rapidly in the post-menopausal years. The average patient gets 2-3 attacks per month and for 10% of sufferers migraine is a progressive disorder leading to Chronic Migraine (>15 days per month) at a time in life when individual demands and commitments are greatest.
Migraine and Women: Throughout a woman’s life hormonal factors have a significant influence in the threshold, susceptibility, risks, and management of migraine (Fig:5).
1. Migraine and the menstrual cycle

2. Migraine and the oral contraceptive
3. Migraine and Pregnancy
4. Migraine and HRT / Menopause
Fig:5
1. Migraine and Menstrual Cycle: Women are vulnerable to migraine attacks at the time of menses and less commonly around the time of ovulation. Attacks, however, are not exclusive to these times and many other migraines may occur either spontaneously or on exposure to a trigger (e.g. diet factors, alcohol, stress and lifestyle factors). Menstrual migraines most frequently occur between days -2 to + 3 of menses. Attacks are more likely to be migraine without aura and are longer in duration (> 72 hours). It is also considered more disabling and more difficult to treat. In the perimenstrual period, and to a lesser extent at the time of ovulation, oestrogen levels decline and fluctuate. This decline activates the trigeminovascular system resulting in an acute attack. Many patients are able to anticipate menstrual migraines enabling them to treat early, particularly with longer acting triptans (e.g. frovatriptan 2.5mg or naratriptan 2.5mg) with or without an NSAID (e.g. naproxyn 500mg).
2. Migraine and Oral Contraceptive: The combined oral contraceptives are ethinyloestradiol / progestogen preparations (COCP). The newer versions contain lower doses of the oestrogen component.
The combined pill is a risk factor for cardiovascular and cerebrovascular disease and careful consideration needs to be given when prescribing in migraine patients. Migraine With Aura is an independent risk factor for stroke as are the COCP, cigarette smoking and hypertension. This risk is accumulative and migraine patients need to be advised to stop smoking. The use of a headache diary is invaluable in this supervision as the pill is known to:
• Trigger headache and first attack of migraine without aura.
• Trigger aura symptoms for the first time in patients already diagnosed with migraine without aura.
• Worsen and increase the frequency of migraine in patients with an established diagnosis.
• It can improve migraine or in others it has no impact on the frequency, severity and duration attacks.
• Continuation of the pill without break through 3 cycles may prevent menstrual migraine attacks.
In patients with an established diagnosis of Migraine With Aura the combined pill is contraindicated and patients who develop aura symptoms
Written by Dr Edward M. O’ Sullivan, Clinical Director Headache Migraine Clinic Department of Neurology Cork University Hospital
General Practitioner in Cork Medical advisor to the Migraine Association of Ireland
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
51
MIGRAINE IN WOMEN
after commencing the COCP need to discontinue the pill immediately due to stroke risk. The headache diary will identify those patients in whom the COCP worsens their migraine and for whom it must be stopped or is contraindicated in the first place. The diary, paradoxically, will also demonstrate those patients in whom the COCP has benefited their migraine and reduced the frequency of attacks.
The protestrogen-only-preparations are an alternative in women for whom the COCP is contraindicated. The POP’s are safe and do not trigger or worsen migraine.

3. Migraine and Pregnancy:
Pregnancy is a positive time for migraine patients and up to 6070% of migraneurs improve during pregnancy, particularly after the first trimester, with many achieving a remission. This is due to the sustained high levels of oestrogen, which no longer fluctuate, providing a preventative benefit to triggering attacks. After childbirth the migraine pattern tends to re-emerge within the first month. Breast-feeding may delay the re-emergence in the puerperium.
7% of women, however, experience their first migraine attack during pregnancy and women who present with new onset headache may have other causes such as cerebral venous thrombosis (CVS), preeclampsia or subarachnoid haemorrhage.
Many of the acute and preventative therapies are contraindicated during pregnancy due to their potential for harm. These include the triptans, paracetamol / codeine preparations, topiramate, sodium valproate and the latest preventative therapies the CGRP monoclonal anti-body antagonists. Patients are limited to the use of simple analgesics such as paracetamol combined with
metoclopramide for the treatments of acute attacks.
4. Migraine HRT and the menopause: The perimenopausal years frequently sees a rise in the frequency and disability of migraine attacks with many women progressing to chronic migraine (> 15 headache days per month) along with the vasomotor symptoms of the menopause. This can be a very challenging time and it is due to the fluctuating and erratic oestrogen levels during this time.
The use of hormone replacement therapy (HRT) is NOT contraindicated in either Migraine With Aura or Without Aura . Oestrogen replacement therapy administered transdermally (with progestogen) in physiological doses can be very efficacious as a preventative migraine therapy and also for the troublesome menopausal vasomotor symptoms.
The natural history of history of migraine is that migraine prevalence goes into a rapid decline after the menopause. It is rare, but not unheard of, for the elderly to continue to complain of migraine in their advancing years.

Management of Migraine: The guiding principles in the management and treatment of migraine are outlined in fig:6.
1. Use of a Headache Diary.
2. Acute Therapies.
3. Preventative Therapies.
4. Non Drug Therapies.
Fig:6
1.Use of a Headache Diary: Migraine is a recurrent disorder and a headache diary is a useful guide and aid memoir in chronicling the frequency, severity, duration and impact of attacks. It helps identify trigger factors and
evaluate the efficacy of acute and preventative therapies.
1. Acute Therapies: One third of migraine patients can effectively manage their acute attacks with over-the-counter simple analgesics such as soluble aspirin, paracetamol or ibuprofen. Prescribed medications, the triptans, are now the gold standard in the management of the acute attack and these include: sumatriptan, zolmitriptan, almotriptan, eletriptan, frovatriptan and naratriptan. Ideally patients should get meaningful relief from their headache and most bothersome symptoms within 2 hours. These medications can be combined with anti-nausea medication and NSAIDS (e.g. naproxyn) which may enhance efficacy. Acute therapies which provide no relief within 4 hours are deemed to be lacking in efficacy. Compound analgesics (e.g. paracetamol / codeine, tramadol) should be avoided or prescribed with caution due to risk of developing Medication-OveruseHeadache.
Newer treatments, the small molecule ‘’gepants’’, CGRP antagonists, are on the horizon and soon to be approved. They will add to armamentarium for the treatment of acute attacks and also as a preventative therapy.
2. Preventative Therapies: Preventative therapies are indicated when patients experience 4 or more migraine days per months, and are poor responders to acute therapies. The conventional preventative therapies are listed in fig:7
1. B-blockers: propranolol, metoprolol, atenolol.
2. Anti-depressants: amitriptyline, venlafaxine.
3. Anti-epileptics: topiramate, sodium valproate.
4. Anti-hypertensives: candesartan.
5. Flunarizine.
6. Pizotifen.
7. Botulinun Toxin.
Fig: 7
The benchmark in evaluating the efficacy of preventative therapies is the achievement of at least a
50% reduction in the frequency of headaches in 50% of patients. To determine this end point patients need to remain on their medication for 3 months before a determination is made. Many patients in clinical practice discontinue their preventative therapy within weeks either due to side-effects or a perceived lack of efficacy.
New Preventative Therapies: C.G.R.P. (Calcitonin Gene Related Peptides) Monoclonal Antibody Antagonists:
The CGRP monoclonal antibody antagonists are the first specifically developed migraine preventative therapies targeting the underlying mechanisms of migraine (Fig:8).
1. Erenumab 70mg or140mg s.c monthly injection.
2. Fremanezumab 225mg s.c monthly or 3 monthly
3. Galcanezumab s.c monthly
4. Eptinezumab 100mg or 300mg IV every 3 months (awaiting approval)
Fig: 8
Erenumab, fremanezumab and galcanezumab are approved by the HSE on the Hi-Tech hub for patients who have Chronic Migraine (>15 headache days per month) and have failed to benefit from 3 previously prescribed conventional preventative therapies. They are licenced for patients aged between 18 and 65 years. In clinical trials benefits and efficacy are seen within 1 week and 25% of patients achieve up to 75% reduction in the frequency of headaches.
Conclusions: Migraine is a common disabling disorder which significantly impacts on the quality of life of women at pivotal times throughout their adult lives. In the past, it has traditionally been underdiagnosed and undertreated. Many patients have fatalistic expectations regarding the management of migraine as they have had to endure migraine attacks for many years. Others may have witnessed a family member such as a parent or aunt or uncle suffer and endure a similar fate.
Today we are much better at diagnosing, understanding and treating migraine. Migraine specific acute and preventative are now available enabling us to better manage migraine. On going research is developing additional treatments (small molecule 'gpants' and ditans) which will further advance our ability to manage migraine and improve the quality of life for many migraneurs.
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
52
WOMEN’S HEALTH SEPSIS IN PREGNANCY



Sepsis and Pregnancy
 Written by Farren M1, Houlihan E2, Kiafar S3, O’Reilly D
Written by Farren M1, Houlihan E2, Kiafar S3, O’Reilly D
Infection, sepsis and septic shock are a spectrum of disease from “mild” to severe, from a diagnosis that we have all had at different times of our lives, if even only due to the common cold, to severe disease. Take these challenges – common conditions, range in severity from mild to severe, and multiple different presentations – and then add in pregnancy, where we must think of both an adult and a baby, and where sepsis may be masked by the common symptoms of pregnancy, making the diagnosis and treatment potentially more difficult. The aim of this review is to support healthcare professionals and educate regarding sepsis in pregnancy. As with the clinical care that we provide, this review was written with the input of a multidisciplinary team. Here we have included nurses, midwives, obstetricians, microbiologists and anaesthesiologists, but we also recognise that other specialities may be required to provide care when pregnant people have sepsis, including pharmacy, infectious diseases, neonatology, general medical physicians and the medical specialities, surgery and the surgical specialities, allied health including physiotherapists, occupational therapists and dietetics; high dependency, intensive care, peri-operative teams and (as always) the wonderful medical scientists that help us make these diagnoses.
Firstly, to review the difference between infection, sepsis and septic shock. Infection is defined as the invasion and multiplication of microorganisms such as bacteria, viruses, fungi or parasites that are not normally present within the body.
An infection may cause no symptoms and be subclinical, only recognised years later or not at all – we sometimes see this when testing for toxoplasmosis or cytomegalovirus, when we tell people they have had it in the past and they had no memory of this. Infections may cause symptoms and be clinically apparent – people

may say “I have a cold” or “I have COVID”. An infection may remain localized, or it may spread through the blood or lymphatic vessels to become systemic.
Microorganisms that live naturally in the body are not considered infections. For example, bacteria that normally live within the mouth and intestine are not infections, they are the commensal microbiota. If normal flora migrate to another part of the body, they may cause infection e.g. E. coli is normally resident in the bowel and it is the commonest cause of urinary tract infection.
Sepsis is defined as new organ dysfunction due to infection. Here the diagnosis becomes more technical, relying on vital sign changes or blood test results. Sepsis may be based on vital signs (high temperature, heart rate or respiratory rate, low blood pressure or low temperature, low oxygen saturations) or tests (glucose, white cell counts). It should be noted for pregnancy that the upper limit of normal for heart rate is 100 beats per minute rather than 90 beats per minute in the non-pregnant population due to the physiological changes of pregnancy.
In pregnancy and the postpartum period (six weeks after the birth) the monitoring system of IMEWS (Irish Maternity Early Warning System) is recommended. The presence of two persistent abnormal vital signs necessitates a review to consider sepsis; if this is suspected then use of the “Sepsis Six + 1” should be used; this will be discussed in more detail later in the review.
Septic Shock is a subset of sepsis defined as a requirement for vasopressors/ inotropes to achieve a mean arterial pressure of ≥ 65mmHg AND a lactate > 2mmols/l despite adequate fluid resuscitation. The diagnosis of septic shock mandates an escalation of care – if not already done- to consultant level, multidisciplinary care and immediate consult to critical care and anaesthesia.
Table: Definition of Mean Arterial Pressure
Mean Arterial Pressure (MAP): the average arterial pressure throughout one cardiac cycle, systole, and diastole.
Morbidity and mortality

While infections are reasonably common in pregnancy, sepsis is less common and septic shock is incredibly rare. All women who experience a severe maternal morbidity in the Republic of Ireland must be reported anonymously to the National Perinatal Epidemiology Centre in Cork. They produce an annual report of this audit, which are freely available on line. In 2020, for example, in the first few waves of the SARS COV2 pandemic, sixteen women were diagnosed with septic shock, in comparison to 329 women who had a major obstetric haemorrhage (the most common cause of maternal morbidity in Ireland). These may seem like small numbers, but if the degree of morbidity is considered, this is significant. In addition, it was noted that pregnant women were disproportionally represented in
intensive care admissions due to SARS COV2 infection.
A second mandatory reporting audit in the UK, the United Kingdom Obstetric Surveillance System (UKOSS) report, showed common themes for maternal admission to ICU and maternal deaths in the United Kingdom (there have thankfully been no maternal deaths due to SARS CoV2 in the Republic of Ireland).

From February to September 2021, when the COVID vaccines became available, 1,714 pregnant women were admitted to hospital in the UK with symptomatic COVID infection, of which 235 (14%) required intensive care admission. The vast majority (98%) were unvaccinated against COVID. Thirty three women died in pregnancy or the postnatal period – thirteen of these women died between July to September 2021 died during the “Delta” wave, when vaccination was recommended to all adults; of these eleven were known to be unvaccinated. UKOSS also showed the risk factors for more severe infection, sepsis and shock in pregnancy, including higher BMI, age over 35 years, diabetes and co-existing medical disease.
 Mary Higgins
Mary Higgins
1Obstetrics and Gynaecology 2Microbiology 3Infection Control, Nursing and Midwifery Anaesthesiology
The National Maternity Hospital, Holles Street, Centre of Excellence for Maternal, Neonatal and Women’s Health. For further information, please visit www.nmh.ie
Shideh Kiafar Elaine Houlihan Maria Farren
Susan Knowles Darragh O’Reilly Siaghal MacColgain
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
53
WOMEN’S HEALTH SEPSIS IN PREGNANCY
Listeria monocytogenes
History of pelvic infection
Group A Strep infection in a close contact
Recent amniocentesis
background Obesity
Diabetes, including gestational Recent surgery
Symptoms of infection in the past week
Immunocompromise
Chronic renal/liver/heart failure
A third mandatory reporting audit, for both the UK and Republic of Ireland, is the MBRAACE report (Mothers and Babies: reducing risk though audits and confidential enquires across the UK). The 2021 report reviewed cases from 20172019, so did not include the SARS CoV2 pandemic, and concentrated on specific causes of mortality: cancer, adversity, older mothers, venous thromboembolism and mental health. Previous reports which focused on sepsis (again, prior to the SARS CoV2 pandemic, but with learning from the influenza pandemic) highlight the importance of suspecting sepsis, early diagnosis and treatment, multidisciplinary care, early antibiotics and ongoing education. One study showed that for “each maternal sepsis death, approximately 50 women have life threatening morbidity from sepsis” and that “follow-up to ensure infection is eradicated is important”.
What are the most common causes of maternal septic shock?
Chorioamnionitis
Chorioamnionitis is an infection of the chorion and amnion, the two layers of the placenta, which can occur after spontaneous rupture of the membranes (SROM, or “waters breaking”). After 37 weeks’ gestation women will usually go into labour themselves, or induction of labour will be recommended if they have prolonged SROM or risk factors for vertical infection, such as infection with HIV, Hepatitis B or C, or Group B Strep colonisation.
When SROM occurs at a preterm gestation – under 36-37 weeks’ gestation – then the risk of infection needs to be balanced against the risk of prematurity.
Women are usually recommended to be admitted to hospital for support and intensive surveillance for complications. Treatment for chorioamnionitis is the same as treatment for any infection: it starts with recognition (which can be hard in the early stages), source control (starting intravenous antibiotics while making rapid plans for birth) and then monitoring for further complications. Even after the birth there can be some complications, including for endometritis (infection of the lining of the womb) which may present with heavier vaginal bleeding than normal or a malodorous discharge. If pre-viable the decision to deliver can be difficult to make but the principles of source control remain.
Pyelonephritis
Pyelonephritis is an infection of the kidneys or upper urinary tract. Pyelonephritis can arise from cystitis (infection of the bladder). It may surprise clinicians that pyelonephritis can cause septic shock in pregnancy, but nearly every year in our hospital one or two women will need High Dependency care with pyelonephritis or urogenic (“from the urinary tract”) septic shock. Treatment starts with recognition, then source control (high dose intravenous antibiotics, ultrasound for renal abscess is occasionally required if no response) and referral to urology may be required. Remember that pyelonephritis can cause preterm labour so the aim is to treat early and hard!
SARS CoV2
Multitudes have been written about COVID infection in pregnancy. While vaccination has undoubtedly reduced maternal and fetal complications, there are still many pregnant women who get a COVID infection in pregnancy.
There are also pregnant women who have chosen not to get a COVID vaccine. The advice remains that non vaccination confers risks of increased severity of infection and maternal and fetal complications, including requiring oxygen care, admission to critical/ intensive care and preterm birth. It is now recommended that women consider a second COVID booster vaccine during pregnancy and this programme is currently rolling out. There is minimal safety information currently available for use of antiviral treatment against COVID in pregnancy.
Influenza
Infection, sepsis and septic shock due to maternal influenza infection usually occurs during the Flu season. Pregnant woman, particularly those in the third trimester, are at increased risk of developing Influenza-related sepsis. While most people can self-care at home with infection, as per the normal practice we would recommend admission if there are symptoms or signs suggestive of more severe infection (for example, an abnormal respiratory rate, O2 saturations) or any maternal or fetal concerns. Oseltamivir is commonly prescribed for influenza infection with pregnancy.
Puerperal Sepsis
The puerperium is the six weeks postnatal period after the birth, where there are risks of complications such as infection, deep venous thrombosis and mental health issues. With regard to infections, Group A Streptococcus (GAS) most commonly occurs postnatally. Infection usually occurs in patients delivered vaginally. Onset of symptoms and deterioration can be rapid. Group A Strep is generally the most virulent maternal infection with a high rate of critical care admission. It is the most common cause of maternal death due to infection. In a recent maternal mortality report, women who died from GAS sepsis were often in a caring role for children (suggesting GAS infection in the child, passed onto the mother). After this report, maternity antenatal and postnatal education recommended that women should wash their hands both before and after using the bathroom.
Listeria is uncommon in Ireland with <20 cases occurring per annum. Pregnant women and new-born infants are more likely to develop serious infection if they come into contact with Listeria. Listeria is a foodborne illness in the mother and can spread via the blood stream and placenta, from mother to her unborn baby. Listeria may cause meningitis in the baby.
No organism identified
Sepsis and septic shock can occur if infection is clinically diagnosed, even if no organism is identified in blood cultures, other cultures or PCR tests.
Prevention: vaccination
Three vaccines are currently recommended in pregnancy in the Republic of Ireland. These are based on the principles of reduction in severity of maternal complications, and thus fetal complications, as well as neonatal protection against infection. As per the HSE: “The immunity developed by a mother after vaccination during pregnancy is passed on to her baby in the womb. This immunity helps protect the baby during the first few months of life.
The flu vaccine is inactive and can be given safely at any time during pregnancy. A pregnant woman who gets the flu is at risk for serious respiratory illness and complications. Getting flu in pregnancy can also so lead to premature birth and smaller babies. Flu vaccination during pregnancy provides immunity against influenza infection to babies in the first six months of life.”
For the COVID vaccines, the HSE state that if a woman “has not had any COVID-19 vaccines then you can get your first round of COVID-19 vaccination or first booster at any stage of pregnancy.
If you had a booster done during your current pregnancy then a second booster is not required.

If you had a booster done before this pregnancy, you can get your second booster after 16 weeks of your pregnancy” (All above as of September 2022, please refer to HSE website for more current guidance).
Pertussis vaccine is also recommended during pregnancy, from 16 weeks gestation onwards and maternal immunity helps protect the newborn baby against severe whooping cough during the early months of life.
Table: Risk factors for Sepsis in
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
54
Principles of sepsis management
Timely recognition and appropriate treatment by the right team in the right place are the key principles for sepsis management.
A favourable clinical outcome is dependent upon early recognition and a rapid response to changes in the clinical picture or triggers on the IMEWS. This should prompt a clinical review with history and examination focused on trying to identify a likely source.

The Sepsis Six (“take three” and “give three”) has widely been adopted as a framework for sepsis management. This should be commenced as soon as possible. Blood is taken to include a full blood count, urea and electrolytes, c reactive protein, liver function and lactate, the urine output is monitored and samples (including viral swabs) are taken to help determine the cause (“take three”). Fluids should be given in accordance with weight (30mls/ kg) to ensure adequate volume resuscitation (with the exception of maternal pre-eclampsia); oxygen is given if required and appropriate antibiotics are commenced (“give three”). These should be specific to the presumed source and given within one hour. If no source is identified, antibiotics suitable for pyrexia of unknown origin are commenced.
Antibiotic therapy should be guided by local guidelines and microbiology input. Most hospitals or hospital groups have consolidated their antimicrobial guidelines in an app format, and these should be used as routine. A sepsis decision tool should be used to guide care and management, and this should be included within the patient notes or electronic chart.
If there is not sufficient improvement with antimicrobial therapy, other avenues for source control should be considered e.g., imaging to help identify a focus amenable to drainage.
TAKE THREE
Sepsis should be managed by appropriately trained personnel. A multidisciplinary team approach is needed and early escalation to senior level is essential. Care at ward level may not be appropriate and an assessment should be made to determine if the patient requires transfer to a high dependency unit (HDU) or intensive care (ICU) with the escalated nursing, midwifery and medical care inherent to level two/ three care.
The documentation of sepsis should include a sepsis-specific form. This serves as an aide de memoir, ensuring that essential elements do not get forgotten. It also allows for de-escalation of care when appropriate. In hospitals using an electronic chart this has been embedded in the electronic health record.
Sepsis and Infection Prevention and Control
As with all patients, please don’t forget the basics. Standard precautions are a series of routine measures that should be used for all patients regardless of the perceived risk of infection. These measures include hand hygiene, use of personal protective equipment (PPE), respiratory etiquette, aseptic technique, sharps & safe injection practices, environmental hygiene and appropriate waste disposal. You must assess the risk for each patient and decide what level of PPE is required. If there is likely to be contact with blood or body fluids but there is a low risk of splashing, you should wear an apron and gloves; if there is a high risk of splashing, you should wear a gown, gloves and eye protection. Some patients require isolation in a single room with an en-suite e.g., patients with gastro-enteritis or if the patient is at risk of having a multi-drug resistant organism (MDRO) such as MRSA, VRE, CPE, etc. Other patients require airborne precautions and isolation in a
Measure glucose (commonly forgotten as part of the assessment!)
Remember
How to get source control?
Aim for appropriate antibiotics within the first
Ask for help
GIVE
PLUS
negative pressure room or a room with a positive pressure ventilated lobby (PPVL) e.g., COVID, open pulmonary TB. An FFP-2 or FFP-3 mask should be worn if airborne isolation is required.
Education in sepsis
Ongoing education in infection, sepsis and septic shock is crucial for all healthcare professionals. This education needs to be evidence based, appropriate, meaningful and relevant. In September 2022, the NMH ran a “Sepsis Education” week for all healthcare workers and for women and families attending the hospital. Daily emails were sent out, highlighting the issues reported in this review, with daily posters and reminders of key learning points for sepsis in pregnancy. Sepsis was highlighted at daily multidisciplinary huddles, multidisciplinary team meetings and team/ward handovers. At grand rounds we reviewed case reports of relevance to sepsis in maternity care,.
We ran face to face practical drill training in the Emergency Department, the Labour and Birthing Unit, Operating theatre, Antenatal and Postnatal wards and Antenatal clinics. Sepsis is always included in our core training for practical emergency skills. As with other maternity units and departments, multiple staff members have been trained as PROMPT (Obstetrics Emergency) trainers, which includes sepsis training, and we are grateful to the support of the National Women and Infants Health Programme for their support in this. Ongoing training in infection, infection
of
Table: Key Points to consider for Sepsis in Pregnancy
control, sepsis and septic shock is provided to staff at all levels of training and speciality.
Further Recommended Reading: National Perinatal Epidemiology Centre Severe Maternal Morbidity
https://www.ucc.ie/en/npec/npecclinical-audits/severematernalmor bidity/severematernalmorbidityre portsandforms/
MBRAACE reports
https://www.npeu.ox.ac.uk/assets/ downloads/mbrrace-uk/reports/ maternal-report-2021/MBRRACEUK_Maternal_Report_2021_-_Lay_ Summary_v10.pdf
https://www.npeu.ox.ac.uk/mbr race-uk/news/2173-covid-19-inpregnancy
https://www.npeu.ox.ac.uk/re search/projects/75-ukoss-sepsis
Sepsis decision tool in maternity care
https://www.hse.ie/eng/services/ publications/clinical-strategy-andprogrammes/maternity-sepsisscreening-algorithm.pdf
https://www.hse.ie/eng/about/ who/cspd/ncps/sepsis/resources/ maternity-sepsis-form-2020.pdf
Royal College of Physicians of Ire land COVID vaccine decision tool https://www.rcpi.ie/news/releases/ information-for-women-who-arepregnant-or-breastfeeding-aboutthe-covid-19-vaccine-update/
HSE: Vaccinations in pregnancy
https://www.hse.ie/eng/health/im munisation/pubinfo/pregvaccs/ https://www2.hse.ie/screeningand-vaccinations/covid-19-vac cine/get-the-vaccine/pregnancy/
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
Take blood (FBC and lactate and other bloods as indicated Take/monitor urine output Take samples (to find a cause) Table: Sepsis Six plus one
THREE Give IV fluids (Hartman’s 30mls/kg = for a 70kg woman that’s two litres!) Give oxygen if required Give appropriate antibiotics (what’s the most likely cause clinically?)
ONE Think of the baby! Check fetal heart rate (may have reactive tachycardia) If the baby has been born and their mother has a significant infection - e.g. blood stream infection, flu, SARS COV2-19, MRSA, group A Strep – inform the paediatricians
fluid resuscitation with an appropriate fluid volume for the patient
hour
diagnosis ; the “golden hour”
55
WOMEN’S HEALTH FERTILITY
The National Maternity Hospital Fertility Hub
Drinking no more than one to two units of alcohol once or twice per week and avoiding episodes of intoxication can improve fertility. Those who smoke should be informed that this is likely to reduce their fertility. In addition, passive smoking is likely to affect their chance of conceiving. There is currently no consistent evidence of an association between consumption of caffeinated beverages and fertility problems.
Health Programme (NWIHP). Our vision is to continually strive to provide high quality care that places our patients at the centre of their management. Our approach is to promote a culture of evidence based practice to assist patients to navigate their fertility journey.
Our hub accepts direct consultant and GP referrals from within the Ireland East Hospital Group for patients who meet the following criteria as defined by NWIHP:
Background
Infertility is the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse or due to an impairment of a person’s capacity to reproduce either as an individual or with his or her partner. The World Health Organisation (WHO) has characterised infertility as a disease affecting 48 million couples and 186 million individuals globally. Over 80% of couples in the general population will conceive within one year if the woman is aged under 40 years. Of those who do not conceive in the first year, about half will do so in the second year (cumulative pregnancy rate over 90%).
The Health Service Executive (HSE) has estimated that one in six heterosexual couples will experience infertility in Ireland.

The causes of infertility can be due to problems with the female and/ or male reproductive systems. Female causes can relate to abnormal ovulation (polycystic ovarian syndrome, premature ovarian failure, hypothalamic or pituitary disease), fallopian

tube damage (pelvic surgery or infection) and uterine disorders (endometriosis, polyps or fibroids, congenital disease). Male causes can relate to reproductive tract obstruction (trauma, infection), testicular failure (chemotherapy), genetic disorders (cystic fibrosis), hormonal disorders (hypothalamic, pituitary) and medications (steroid use). In approximately one quarter of all cases, the cause is unknown or ‘unexplained’.
Advice
It is important that people who are concerned about their fertility know that both female and male fertility decline with age. Couples should be informed that they should have sexual intercourse every two to three days to optimise the chance of pregnancy. Lifestyle factors may negatively impact fertility and should be optimised for anyone trying or planning to try to conceive prior to any investigations or treatment.
Time to conception can be delayed in people with a body mass index (BMI) of 30kg/m2 or over. Group programmes involving exercise and diet are the most optimal ways to achieve weight loss which may also improve ovulation in women.
Women intending to become pregnant should be informed that dietary supplementation with folic acid before conception and up to 12 weeks gestation reduces the risk of having a baby with neural tube defects. Some patients require 5mg folic acid as they are higher risk (e.g epilepsy or BMI ≥30kg/m2). Women should ensure their cervical cancer screening is up to date, that they have been vaccinated against rubella if they are susceptible and that sexually transmitted disease is performed if applicable.
Investigation and Management
Regional fertility hubs within the six hospital groups are being rolled out across Ireland. These hubs allow for investigation and management of a large proportion of fertility patients.
The National Maternity Hospital is delighted to announce the launch of its new Ireland East Fertility Hub supported by funding from the National Women and Infants
If they have been unable to achieve a pregnancy after 6 months of regular unprotected sexual intercourse and are 36 years of age and older
If they have been unable to achieve a pregnancy after 12 months of regular unprotected sexual intercourse and are under 36 years of age
If there is a known clinical cause of infertility or a history of predisposing factors for infertility i.e. endometriosis, previous fertility treatment to conceive, not having periods or very few periods per year, pelvic inflammatory disease or undescended testes.
Investigations prior to referral can be extremely helpful in the early identification of fertility problems. Women with regular monthly menstrual cycles should be informed that they are likely to be ovulating. Women should be offered a blood test to measure serum progesterone in the midluteal phase of their cycle (day 21 of a 28-day cycle) to confirm ovulation. Women with irregular menstrual cycles should be offered a blood tests to measure serum gonadotrophins (Follicular stimulating hormone (FSH) and
“The National Maternity Hospital is delighted to announce the launch of its new Ireland East Fertility Hub supported by funding from the National Women and Infants Health Programme (NWIHP)”
Dr David Crosby
Written by Dr David Crosby, Consultant Obstetrician & Gynaecologist, Subspecialist in Reproductive Medicine, Surgery & Genetics, Clinical Lead Fertility Hub, Head of Department of Reproductive Medicine, The National Maternity Hospital, Dublin, Assistant Clinical Professor, University College Dublin
Dr Andrew Downey, Clinical Research Fellow and Specialist Registrar, The National Maternity Hospital & Merrion Fertility Clinic, Dublin
Ms. Michelle Barry, Clinical Nurse Specialist, Fertility Hub, The National Maternity Hospital, Dublin
Ms Catherine Dunne, Administrative Lead, Fertility Hub, The National Maternity Hospital, Dublin
Dr Sorca O’Brien, Clinical Aspire Fellow in Fertility, The National Maternity Hospital and Merrion Fertility Clinic
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
56
This
For the treatment of moderate to severe atopic dermatitis in adult patients who are candidates for systemic therapy1
TIME TO PRESS PLAY
Introducing Adtralza®

Not
The first licensed biologic that inhibits IL-13 alone
a key driver of atopic dermatitis signs and symptoms.2


at www.adtralza.ie
Please refer to the full Summary of Product Characteristics (SmPC) (www.medicines.ie) before prescribing.
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. Indications: Treatment of moderate-tosevere atopic dermatitis in adult patients who are candidates for systemic therapy.
Active ingredients: Each pre-filled syringe contains 150 mg of tralokinumab in 1 mL solution (150 mg/mL). Dosage and administration: Posology: The recommended dose of tralokinumab is an initial dose of 600 mg (four 150 mg injections) followed by 300 mg (two 150 mg injections) administered every other week as subcutaneous injection. Every fourth week dosing may be considered for patients who achieve clear or almost clear skin after 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve further with continued treatment every other week beyond 16 weeks. Tralokinumab can be used with or without topical corticosteroids. The use of topical corticosteroids, when appropriate, may provide an additional effect to the overall efficacy of tralokinumab. Topical calcineurin inhibitors may be used, but should be reserved for problem areas only, such as the face, neck, intertriginous and genital areas. If a dose is missed, the dose should be administered as soon as possible and then dosing should be resumed at the regular scheduled time. No dose adjustment is recommended for elderly patients, patients with renal impairment or patients with hepatic impairment. For patients with high body weight (>100 kg), who achieve clear or almost clear skin after 16 weeks of treatment, reducing the dosage to every fourth week might not be appropriate. The safety and efficacy of tralokinumab in children below the age of 18 years have not yet been established. Method of administration: Subcutaneous use. The pre-filled syringe should be not shaken. After removing the pre-filled syringes from the refrigerator, they should be allowed to reach room temperature by waiting for 30 minutes before injecting. Tralokinumab is administered by subcutaneous injection into the thigh or abdomen, except the 5 cm around the navel. If somebody else administers the injection, the upper arm can also be used. For the initial 600 mg dose, four 150 mg tralokinumab injections should be administered consecutively in different injection sites. It is recommended to rotate the
injection site with each dose. Tralokinumab should not be injected into skin that is tender, damaged or has bruises or scars. A patient may self-inject tralokinumab or the patient’s caregiver may administer tralokinumab if their healthcare professional determines that this is appropriate. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Precautions and warnings: If a systemic hypersensitivity reaction (immediate or delayed) occurs, administration of tralokinumab should be discontinued and appropriate therapy initiated. Patients treated with tralokinumab who develop conjunctivitis that does not resolve following standard treatment should undergo ophthalmological examination. Patients with pre-existing helminth infections should be treated before initiating treatment with tralokinumab. If patients become infected while receiving tralokinumab and do not respond to antihelminth treatment, treatment with tralokinumab should be discontinued until infection resolves. Live and live attenuated vaccines should not be given concurrently with tralokinumab. Fertility, pregnancy and lactation: There is limited data from the use of tralokinumab in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity. As a precautionary measure, it is preferable to avoid the use of tralokinumab during pregnancy. It is unknown whether tralokinumab is excreted in human milk or absorbed systemically after ingestion. Animal studies did not show any effects on male and female reproductive organs and on sperm count, motility and morphology. Side effects: Very common (≥1/10): Upper respiratory tract infections. Common (≥1/100 to <1/10): conjunctivitis, conjunctivitis allergic, eosinophilia, injection site reaction. Uncommon (≥1/1,000 to <1/100): keratitis. Precautions for storage: Store in a refrigerator (2°C-8°C). Do not freeze. Store in the original package in order to protect from light. Legal category: POM. Marketing authorisation number and holder: EU/1/21/1554/002. LEO Pharma A/S, Ballerup, Denmark. Last revised: June 2021 Reference number: REF-19238(1)
Reporting of Suspected Adverse Reactions
Adverse events should be reported.
Reporting forms and information can be obtained from: HPRA Pharmacovigilance, Website: www.hpra.ie
Adverse events should also be reported to Drug Safety at LEO Pharma by calling +353 1 4908924 or e-mail medical-info.ie@leo-pharma.com
® Registered trademark IL, interleukin.
References: 1. Adtralza® SPC. 2. Bieber T. Allergy 2020;75:54–62.
Further information can be found in the Summary of Product Characteristics or from: LEO Pharma, Cashel Road, Dublin 12, Ireland.
E-mail: medical-info.ie@leo-pharma.com
Date of preparation: February 2022
 Prescribing Information for Adtralza® (tralokinumab) 150 mg solution for injection in pre-filled syringe
Prescribing Information for Adtralza® (tralokinumab) 150 mg solution for injection in pre-filled syringe
NEW
is a promotional advertisement from LEO Pharma for IE healthcare professionals. Learn more
, 1
IE/MAT-54463 V1
an actual patient. For illustrative purposes only.
WOMEN’S
Luteinising Hormone (LH)). If polycystic ovarian syndrome (PCOS) is suspected, a hormone profile including FSH and LH, testosterone, sex hormone binding globulin, and free testosterone index levels should be assessed. Women should have thyroid function tests performed and optimised if there is presence of subclinical or clinical thyroid dysfunction.
Once a referral has been accepted, a fertility hub pack is posted to the patient/s for their completion and following completion of further investigations, an initial consultation is offered to ensure that all the necessary investigations have been completed and to advise on an individualised management plan. The range of services provided by our hub include investigative support to GPs (semen analysis and pelvic ultrasound), and secondary level investigations including testing of tubal patency, hysteroscopy, laparoscopy, fertility related surgeries, ovulation induction and follicle tracking.
There are a number of quantitative measures that can be performed as an assessment of ovarian reserve. Serum Anti-Müllerian hormone (AMH) testing may identify women with a lower ovarian reserve than expected for age. These women can be
provided with fertility management options in a timely manner.
AMH testing can also be a predictor for ovarian response in in vitro fertilization (IVF). AMH testing can be offered through GP practices or through our hub. Another measure of ovarian reserve is the antral follicle count (AFC), which can be assessed by transvaginal ultrasound. Transvaginal scanning can also assess the endometrium for the presence of polyps or fibroids.
Fallopian tubal patency can be checked by hysterosalpingography (HSG: pelvic x-ray with dye injected through cervix) or hysterosalpingo-Contrast Sonography (HyCoSy: pelvic ultrasound with dye injected through cervix). If there is a suspicion of tubal damage (endometriosis or pelvic inflammatory disease), a diagnostic laparoscopy and dye test can be performed as it allows for a both diagnosis and treatment. A diagnostic or operative hysteroscopy can be performed if endometrial pathology, such as fibroids and polyps, is suspected. These services are provided on site at the National Maternity Hospital. Men experiencing fertility problems should have a semen analysis performed assessing the sperm concentration, count,
morphology, motility, and volume as defined by the WHO. Repeat samples or further investigation and work up may be required if the first result is abnormal.
Ovulation induction with oral and injectable ovulation agents and follicle tracking is offered through our service to patients with suspected anovulation. Letrozole, an aromatase inhibitor, stops oestrogen synthesis resulting in increased ovarian stimulation via the pituitary gland. Other second line oral medications that are used include Clomid and Tamoxifen. Injectable ovulation induction agents, including FSH, LH and human menopausal gonadotropin (hMG) are available. It is necessary that ultrasound follicle tracking is performed to monitor follicle growth, ensure that the response is optimal and to significantly reduce the risk of multiple pregnancy. Metformin can be used in anovulatory women with PCOS as an adjunctive therapy, with evidence for women with good evidence of restoration of ovulation in women, particularly with BMI of 30kg/m2 or over. If a male sperm factor is identified, adverse lifestyle measures should be addressed and treatment of the underlying disorder may be required.
Some patients may need to be referred for further treatment with assisted reproduction such as Intrauterine Insemination (IUI),


In Vitro Fertilisation (IVF), or Intracytoplasmic Sperm Injection (ICSI). IUI involves a semen sample being produced and then placed into a woman’s uterus around the time of ovulation after laboratory preparation. In IVF, the eggs are removed from the ovary before ovulation and fertilised with sperm in a laboratory with the embryo transferred to the uterus a few days later. ICSI is a specialised form of IVF where a single sperm is directly injected into the egg to allow fertilisation. These treatments require referral to a specialist fertility clinic after initial workup and management in the regional fertility hubs. We are continuously auditing these referral rates to ensure that we provide NWIHP with the most up to date information to facilitate planning for publicly funded assisted reproduction.
Conclusion
Infertility is a common disorder that affects many individuals and couples. The newly established regional fertility hubs provide evidence based fertility work up and management. Through the National Maternity Hospital fertility hub and with its network of regional hubs, we will continue to advocate for the equitable evolution of comprehensive public fertility services, including IVF, in Ireland in the future.
For more information visit https://www.nmh.ie/
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
HEALTH FERTILITY
58

Our annual listing celebrating innovation and excellence in healthcare is coming soon. PROFESSIONAL 100 PROFESSIONAL 100 To request an entry or nomination form please contact: sarah@hospitalprofessionalnews.ie 2 0 2 2 NO M I N A T I O N S NO W O P E N HOSPITAL PROFESSIONAL NEWS IRELAND Ireland’s Dedicated Hospital Professional Publication To request an entry or nomination form please contact: kelly-jo@ipn.ie or danielle@hospitalprofessionalnews.ie
Her Heart Matters – Women’s Cardiovascular Health
Janis Morrissey- HPIT-IHF

disparity between the experiences of male and female patients, with female patients waiting longer to be diagnosed with heart failure than men.
Ireland, to increase awareness of the risk of heart disease and stroke in women and the fact that this risk increases as women enter menopause.
The Her Heart Matters Campaign will unapologetically focus on women’s hearts because research has shown that heart disease in women has been underresearched, under-diagnosed, and under-treated for far too long.
On average, women have to wait five weeks to receive a formal diagnosis of heart failure, compared to men who have to wait three weeks. Women are also more likely to delay seeking help from health professionals after first developing symptoms, with females making appointments at four weeks – almost twice as long as males.
anxiety, what is less well known is that the menopause also affects the heart and in particular puts women at increased risk of heart disease and stroke.

While the menopause itself doesn’t cause cardiovascular disease, lowering hormone levels coupled with the ageing process, increase your chances of developing risk factors for heart disease and stroke such as high blood pressure and high cholesterol.
September is Heart Month and this year the Irish Heart Foundation is running a new campaign focusing on women’s cardiovascular health.
Women are five times more likely to die from heart disease and stroke than breast cancer yet new research has shown that the majority of people are not aware of this.
According to a survey carried out by Ipsos MRBI on behalf of the Irish Heart Foundation more than half or 59 percent of people surveyed were surprised that the risk of death from heart disease and stroke was so high for women compared to breast cancer. They stated that the five-time risk was “higher than they thought.”
This September the Irish Heart Foundation is running the ‘Her Heart Matters’ campaign with support of the HSE and Healthy
According to the Lancet women and cardiovascular disease commission published in May last year “despite being responsible for causing 35% of deaths in women each year, cardiovascular disease (CVD) in women remains understudied, under-recognised, under-diagnosed, and undertreated, with women underrepresented in clinical trials.”
A report launched earlier this year found that female patients in Ireland were waiting almost twice as long as men to be diagnosed with heart failure, with delays to diagnosis associated with poorer quality of life, mental health issues, and impact on relationships.
The report State of the Heart: Heart Failure in Ireland, by Roche Diagnostics and the Irish Heart Foundation, analysed data from a first-of-its-kind survey of heart failure patients in Ireland, conducted by Censuswide, which identified a significant gender
Furthermore, studies from the Polish Registry of Acute Coronary Syndromes (PL-ACS) presented at Acute Cardiovascular Care 2019 –a European Society of Cardiology (ESC) congress revealed that women call an ambulance for husbands, fathers, and brothers with heart attack symptoms but not for themselves. Further confirmation that women tend to play down their symptoms and are less likely to call an ambulance for themselves, putting the pain down to “indigestion.”
The Menopause and heart health

While the Irish Heart Foundation’s Her Heart Matters campaign aims to raise awareness of heart health among all women, part of the campaign will focus on menopause as this is a particular time in a woman’s life when their cardiovascular health is most at risk.
While a lot of people have heard about menopausal symptoms such as night sweats, hot flushes, vaginal dryness and low mood or
In general women experience menopause between 45 and 55 years of age however, the average age for women to enter the menopause is 51. Some women experience what is known as early or premature menopause before the age of 40
As we age and particularly around the time of menopause, the level of cholesterol in our blood increases, in particular the bad cholesterol or LDL, often triglycerides also increase and the good cholesterol or HDL decreases. This leads to an increased risk of cardiovascular disease – heart attack and stroke.
Lower oestrogen levels as a result of the menopause lead to a stiffening of the arteries of the heart which can increase blood pressure. Furthermore, as its easier to gain weight and harder to lose it as we age, and this can also lead to an increase in blood pressure- another risk factor for heart disease.
The chances of a heart attack increase in menopause as a result of lower oestrogen levels which adversely affect your cholesterol
WOMEN’S HEART HEALTH OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
60
Life-threatening,

condition


heart failure with preserved ejection fraction
typically over
years old5-7
to standard heart failure therapies (ACEi, ARBs, and beta blockers)8-10
QRS voltage and left ventricular (LV) wall thickness11-13
tunnel syndrome or lumbar spinal stenosis3,8,14-20
 T, Ueda M, Jono H, et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons.
Pathol.
is both
and
for the
T, Ueda M, Jono H, et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons.
Pathol.
is both
and
for the
The health information contained in this ad is provided for educational purposes only. Date of Preparation: February 2021 GCMA code: PP-RDP-IRL-0105
in patients
60
underrecognized, and underdiagnosed, ATTR-CM is a rare
found in mostly older patients in which misfolded transthyretin proteins deposit in the heart.1-7 It is vital to recognize the diagnostic clues so you can identify this disease.
between
of carpal
showing increased LV wall thickness6,13,16,21,22 —autonomic nervous system dysfunction-including gastrointestinal complaints or unexplained weight loss6,16,23,24 References 1. Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23(4):209-213. 2. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357-1377. 3. Connors LH, Sam F, Skinner M, et al. Heart failure due to age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282-290. 4. Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098. 5. Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2(2):113-122. 6. Maurer MS, Hanna M, Grogan M, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68(2):161-172. 7. González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585-2594. 8. Narotsky DL, Castano A, Weinsaft JW, Bokhari S, Maurer MS. Wild-type transthyretin cardiac amyloidosis: novel insights from advanced imaging. Can J Cardiol. 2016;32(9):1166.e1-1166.e10. 9. Brunjes DL, Castano A, Clemons A, Rubin J, Maurer MS. Transthyretin cardiac amyloidosis in older Americans. J Card Fail. 2016;22(12):996-1003. 10. Castaño A, Drach BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163-178. 11. Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49:9-13. 12. Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114(7):1089-1093. 13. Quarta CC, Solomon D, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840-1849. 14. Connors LH, Prokaeva T, Lim A, et al. Cardiac amyloidosis in African Americans: Comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158(4):607-614. 15. Nakagawa M, Sekijima Y, Yazaki M, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23(1):58-63. 16. Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203-1212. 17. Sperry BW, Reyes BA, Ikram A, et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018;72(17): 2040-2050. 18. Westermark P, Westermark GT, Suhr OB, Berg S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. 2014;119(3):223-228. 19. Yanagisawa A, Ueda M, Sueyoshi T, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28(2):201-207. 20. Sueyoshi
Hum
2011;42(9):1259-1264. 21. Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography
sensitive
specific
diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442-1448. 22. Ternacle J, Bodez D, Guellich A, et al. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging 2016;9(2):126-138. 23. Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63-76. 24. Swiecicki PL, Zhen DB, Mauermann ML, et al. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid. 2015;22(2):123-131. LEARN HOW TO RECOGNIZE THE CLUES OF ATTR-CM AT: S U S P E C T A N D D E T E C T . I E CONSIDER THE FOLLOWING CLINICAL CLUES, ESPECIALLY IN COMBINATION, TO RAISE SUSPICION FOR ATTR-CM AND THE NEED FOR FURTHER TESTING SUSPECT ATTR-CM (TRANSTHYRETIN AMYLOID CARDIOMYOPATHY) A LIFE-THREATENING DISEASE THAT CAN GO UNDETECTED H FpEF I NTOLERANCE DISCORDANCE DIAGNOSIS E CHO N ERVOUS SYSTEM
WOMEN’S
levels, arteries, and blood pressure all of which increasing the risk of a heart attack.
Her Heart Matters
The demands of a busy lifestyle mean that for many women, taking care of their own wellbeing can fall by the wayside. That’s why the Irish Heart Foundation aims to inform, empower, and support women in taking care of their heart health this September.
The Her Heart Matters campaign will support women by sharing information about lifestyle factors that contribute to the risk of cardiovascular disease and how to address them. It will also include an online self-assessment tool of cardiovascular risk factors so that women can take stock of their heart health, and plan to develop more heart-healthy habits.
The Irish Heart Foundation wants to empower women to share information and stories with each other. To help with this a number of women have generously shared their experiences of stroke and heart disease which are available to read on irishheart.ie
The campaign also includes a selfcare and wellbeing journal that can be downloaded for free, which is full of information and activities that will support women in protecting their heart health every day.
The Irish Heart Foundation was keen to address the issues that
concerned real women; therefore, the Her Heart Matters campaign was developed with collaboration and insight from polls, focus groups, and representative groups including Travellers, ethnic minorities, people with disabilities, the trans communities, and academics.
Speaking about the importance of the Her Heart Matters campaign, Janis Morrissey, Director of Health Promotion, Information and Training at the Irish Heart Foundation said cardiovascular disease (CVD) is a leading cause of death in women.
“In Ireland, women are 5 times more likely to die from heart disease and stroke than breast cancer, yet only a third (34%) of women aware of this. Sadly, one in four women in Ireland die from heart disease and stroke.
So, it’s a big problem, but we don’t hear enough about it because cardiovascular disease in women is under-researched, underdiagnosed and under-treated. There is a false belief that CVD is a man’s disease, but there are important women-specific risk factors throughout women’s lives, like menopause.”
“We are encouraging women, particularly those in their 40s and 50s or those entering menopause, to take stock of their heart health in September by identifying what small changes they can make now to benefit their heart health into the

future. The Irish Heart Foundation has a range of information and resources to support women on their heart health journey,” she said.
Ms Morrissey said that all women are different and unique, so what one woman needs most to protect her heart health was not necessarily what another woman needs.
“We have created a holistic selfreflection tool to support women to find out where what areas of their life they could benefit from extra support in – be that stress and mental wellbeing, physical activity, or healthy eating. Visit irishheart.ie and use the tool there or download our Self-care and wellbeing journal,” she explained.
Ms Morrissey also said that the Her Heart Matters campaign included a focus on the menopause as this was a time in a woman’s life when she is at high risk for heart disease and stroke.
“Some of the most important long-term effects of menopause often go unnoticed. The loss of oestrogen means less protection for heart and bone health. So, it’s important for women as they approach menopause to really take stock of their health.”
Research has shown that women from more socio-deprived backgrounds are more at risk from heart disease, and according to Ms Morrissey it is wrong to say that our risk of heart disease and stroke is our individual
responsibility and solely under our own control.
“Many people from marginalised groups are at higher risk of heart disease because of the influence of the wider determinants of health – forces around them that they can’t control. These increase biological, behavioural, and psychosocial risk factors for heart disease and stroke. Women are known to be more affected by these factors than men.
To support these women, we need to make health promotion interventions available and accessible and responsive to their specific needs. The Irish Heart Foundation has a range of programmes targeted at underserved communities for example of Mobile Health Unit which offers free blood pressure checks,” she said.
Finally, Ms Morrissey said the good news was that 8% of premature heart disease and stroke can be prevented through very simple changes to lifestyle changes.
“Most women know that makes a healthy lifestyle, but life can get in the way. 1 in 3 women feel they don’t get enough time to focus on their health every day. That’s very understandable when you consider how busy many women’s lives are as they juggle careers, family, caring for elderly parents maybe financial pressures. This is a time when so many women are busy looking after everyone else in their lives, that it’s hard to look after themselves.”
“We are on a mission to help women in Ireland, but we can’t do it alone. Let’s get talking about menopause and heart health – with your sisters, mothers, friends, and daughters. Let’s talk – share –empower,” she concluded.
On World Heart Day, the 29th of September, the Irish Heart Foundation will host a free webinar about women’s heart health with insights from health professionals including menopause specialist Dr Deirdre Lundy and a woman living with heart disease.

Throughout the month of September, the Irish Heart Foundation will also be encouraging donations to help support its valuable and lifesaving work.
For more information on the Irish Heart Foundation’s September campaign Her Heart Matters please see www.irishheart.ie for more information or to donate.
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
HEART HEALTH
62
WOMEN’S HEALTH MENOPAUSE
Menopause, Vaginal Atrophy and Urinary Incontinence
Almost every tissue in the body has estrogen receptors, which suggests that estrogen has a role to play in their function. This is true of the genitourinary system, which includes the external genitalia, vagina, bladder and urethra. The loss of estrogen which comes with menopause has several effects on these tissues, which used to come under terms such as atrophic vaginitis, vulvovaginal atrophy, and various urinary conditions. More recently, the term Genitourinary Syndrome of Menopause (GSM) is used to refer to these issues as a group.
“Genitourinary syndrome of menopause (GSM) is a more accurate and inclusive term that describes the multiple changes occurring in the external genitalia, pelvic floor tissues, bladder and urethra, and the sexual sequelae of loss of sexual function and libido, caused by hypoestrogenism during the menopause transition and post menopause. These genitourinary changes primarily occur in response to reduced estrogen levels and aging, and do not settle with time”. (Kim et.al, 2015)
Vaginal atrophy
Vaginal atrophy is one consequence of low estrogen. Prior to menopause, when there is plenty of estrogen in circulation, the lining of the vagina is quite thick, glandular, elastic, muscular and folded into rugae. During intercourse, childbirth and, indeed the insertion of a speculum, the vagina can therefore stretch to a great degree.
The vagina contains a diverse but stable community of microorganisms. Pre-menopause, Lactobacilli make up approx 53% of the microbiome, with multiple other organisms making up the balance. These include some potentially pathogenic organisms such as Gardnerella vaginalis, which typically makes up approx 17% of the healthy vaginal microbiome. Vaginal epithelial glands produce constant secretions and clear-white odourless vaginal discharge is normal. These secretions contain glycogen from which the Lactobacilli produce lactic acid, thus maintaining an acidic pH between 3.6 and 4.5. They also produce other antimicrobial substances such as hydrogen
peroxide and bacteriocins which suppress the growth of other organisms, maintaining a healthy balance. During sexual intercourse, further lubrication is produced.
When estrogen levels decline, the vaginal epithelium loses thickness, elasticity and glandularity. It can, therefore, become dry, tight and friable. Symptoms include vaginal dryness, burning, irritation, itching, dysuria, urinary frequency, discharge/infections, painful intercourse and postcoital bleeding. There can also be discomfort when wearing jeans or tight clothes or during exercise as simple as walking, or even just while sitting.
A decrease in glandular secretions containing glycogen leads to a decline in Lactobacillus colonies and a subsequent increase in pH. Post menopause the lactobacilli may fall to just 11% of the microbiome which leaves the field open to growth of some pathogenic organisms such as E.Coli, Enterobacter and Gardnerella. Indeed, Gardnerella can increase to 42%, thus bacterial vaginosis is more common in post menopausal women. Vulvovaginal candidiasis (VVC) is uncommon in a hypoestrogenic environment. However, high estrogen can be associated with vulvovaginal candidiasis and some women, particularly those who had a tendency to VVC pre-menopause, may find that HRT brings their thrush back.
Approx 60% of women will suffer from these issues at some point. Of these, 13% notice symptoms pre-menopause, 30% in the first year after their last menstrual period and 67% after menopause. What is concerning is that there is a low awareness among women that these are symptoms of menopause. Most women see them as a normal part of aging which should just be accepted and ignored if possible. They are also often embarrassed to bring them up with their GP. It is, therefore, very important for healthcare professionals to bear this in mind and to ask direct questions.
Once the symptoms have been brought up, treatment options must be explored. It is of course, important to always bear in mind that many of the
Written by Dr Catherine Riordan, The Menopause Hub
Dr Riordan is a Fellow of the Royal College of Surgeons, England. She spent several years as a fertility specialist at both Sims and ReproMed IVF clinics. She is a member of the Urology team at St Michael’s Hospital, Dun Laoghaire and a Surgeon Prosector in the anatomy department of the RCSI.
symptoms may be caused by or associated with other conditions. A differential diagnosis may include dermatological conditions such as lichen sclerosus/planus, inflammatory vaginitis, vulvodynia, vaginismus, chronic pelvic pain, interstitial cystitis, malignancy, trauma, diabetes and autoimmune conditions such as lupus. Initially, it is important to ask about products which may exacerbate the symptoms, such as soaps, bath products, lubricants, condoms, douches, perfumed pantyliners etc.
Clearly, one way of addressing the problem is to correct the cause and replace the lost estrogen. This can be done using local estrogen in the form of pessaries, creams or rings which deliver low dose estrogen. Response is usually quite rapid, with improved blood flow and increased thickness of the epithelium of the vagina, bladder and urethra. Lactobacillus spp colonisation returns to healthy levels, with a corresponding reduction in the proportion of other organisms.
There is minimal systemic absorption of vaginal estrogen, with an initial peak, then almost no further absorption. Hence, it is perfectly safe for the vast majority of women to use and, indeed, has recently become available over the counter in the UK. Products such as Vagifem deliver 10mcg of estradiol and the annual absorption is estimated to be just 1.14mcg. Alternatives are estriol in the form of products such as Ovestin and Imvaggis. Estriol is a less potent estrogen and sometimes easier for older women to tolerate. For elderly women suffering from recurrent UTIs, a cream may be easier to use and can be quite effective
even if not inserted fully into the vagina, thus making it easier both for self-administration and for application by a carer. Other alternatives are vaginal DHEA and the SERM, Ospemifene, which has an estrogen agonist effect on the vaginal epithelium.
For those who cannot or prefer not to use hormonal treatment, vaginal lubricants and moisturisers are very useful and can be used in combination with local estrogen products. Women using condoms for contraception or protection from STIs, should avoid oil-based products due to their propensity to damage latex. A water-based product is the best first line option as it is less likely to cause irritation, but silicone products do provide longer lasting results and better glide. There are several on the market, such as YES, Sylk, Regelle and Replens.
Menopause and Urinary Incontinence


There are two main types of incontinence; stress urinary incontinence (SUI) and urge urinary incontinence (UUI). Aging is a large factor in the development of urinary incontinence, but menopause can have an added impact. By the age of 70, 1 in 2 women has a degree of urinary incontinence and it has an enormous impact on quality of life.
SUI is leakage of urine when the pressure on the bladder is increased from within the abdomen, as happens when the abdominal muscles are tightened during coughing, laughing, sneezing or lifting something heavy. It can also happen when there is extra pressure applied to the pelvic floor while running,
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
63
WOMEN’S HEALTH MENOPAUSE
SUI from 1 in 10 to 1 in 3.
UUI is leakage which happens when you have the sudden urge to pee and can’t quite hold on until you get to the toilet. It is less common than SUI, affecting approx 1 in 30 women, but becomes more common with age and with the onset of menopause. It is caused by overactivity of the muscle of the bladder wall, also known as overactive bladder syndrome (OAB) and is associated with reduced bladder capacity, urinary frequency, urgency and the need to get up multiple times at night to pass urine. You may have OAB without leakage.
The bony pelvis is like a bowl without a bottom, into the top of which rest the abdominal contents. The pelvis itself contains the bladder, uterus and rectum, all of which would fall out through the bottom of the bowl were it not for a muscular sheet or diaphragm stretched across it. This group of muscles is collectively referred to as the pelvic floor. It contains condensations of muscle fibres around the bladder neck and a sling around the lower rectum which act as sphincters to prevent unwanted escape of their contents. When you are about to cough or sneeze etc, there is a reflex tightening of the pelvic floor muscles a split second beforehand, so that when the cough occurs, everything is sealed tight and no urine or faeces are squeezed out. If the pelvic floor is weak, this may not happen strongly or quickly enough and leakage occurs. If the pelvic floor is significantly weak, over time the pelvic organs, ie the bladder,

pelvic floor, this is known as a cystocele.
There are many factors that can weaken the pelvic floor, such as a chronic cough or excess intra abdominal fat. Women are at higher risk than men because of their childbearing potential. Firstly, the birth canal passes through the pelvic floor which necessitates a third opening and thus a further potential weakness. Secondly, the pelvic floor is subjected to stretching during pregnancy and childbirth. Even carrying a baby for 9 months puts pressure on the pelvic floor and will stretch it. Pushing a baby through it during vaginal delivery, particularly if the passage is rapid or if instruments are required, stretches it even more dramatically. Indeed, the muscles can tear. Not only are the muscle fibres stretched, but the elastic and collagen fibres are also stretched or broken. The result is a loss of muscle tone and strength of the pelvic floor as a whole. Pelvic floor exercises are extremely important in the post delivery phase, to restore as much muscle tone as possible, but the pelvic floor will never be as taught as before pregnancy. Awareness and exercise of the pelvic floor before pregnancy is very beneficial in terms of minimising any damage during pregnancy and childbirth.
As we get older, our muscle tone in general will tend to reduce. A sedentary lifestyle or reduced participation in exercise contributes to this loss of muscle tone. With menopause, when estrogen levels fall, this reduction

accelerates because low estrogen leads to loss of muscle tone and strength, including that of the pelvic floor muscles. A fall in estrogen results in a loss of collagen of up to 30% in the first 5 years of menopause. On top of that, with aging, our metabolism slows and lower estrogen levels make women prone to gaining weight and to store any extra fat around their abdomen. This additional abdominal fat adds to the pressure on their pelvic floor. As a result, women who previously had things under control, may find that leakage now becomes a problem.
Another factor is the atrophy of the vaginal and urethral epithelium, which comes with a decline in estrogen. From a continence point of view, if the urethral lining is less puffy, it is less able to hold in any urine which may try to escape. The cells of the urethra and the trigone of the bladder may become more sensitive, leading to increased irritability, urinary frequency and discomfort. Finally, as mentioned above, there may be

an increased tendency to UTI due to the disturbance of the vaginal microbiome, allowing infection with organisms such as E.Coli. Anyone with even slight SUI or UUI, may find it worsened in the presence of a UTI.
Treatment
As with vaginal atrophy, most women are reluctant to bring incontinence up with their doctor, either because they think it is a normal part of aging and something to be put up with, or because they are embarrassed. Without direct questioning, the problem may not be revealed. Secondly, an accurate diagnosis is important. It can be very clear which type of incontinence someone has, but quite commonly, women have a combination of both SUI and UUI, known as Mixed Urinary Incontinence. It can sometimes be difficult to determine how much each contributes to the problem and, therefore, which is the best treatment option. In this case, a urodynamic study may be helpful.
Types of pelvic organ prolapse
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
64
SUI
Pelvic floor exercises (PFE) can achieve significant improvement in up to 75% of women with SUI and may well avoid the need for any further treatments. However, 1 in 2 women have difficulty identifying and contracting their pelvic floor as the contractions are internal and the effect unseen. Assessment by a specialist pelvic floor physiotherapist can be invaluable, both to identify an individual’s particular muscle weakness and ensure the most appropriate exercises are done, using the correct technique. Some improvement may be seen within weeks, but maximal benefit may not be seen for up to 6 months. As with all muscle exercise, continued exercise is necessary to maintain the effect, of course. Physiotherapists may use tools such as biofeedback and electrostimulation for women who have difficulty in contracting their pelvic floor.
The avoidance of constipation is important, as any loading of the rectum will increase pressure on the bladder and straining to open the bowel further increases pressure on the pelvic floor. Stool softening agents and plenty of fluid intake is helpful. The use of a footstool to raise the knees above hip height relaxes the abdomen and pelvic floor and helps with defaecation. Weight loss is essential for those with increased abdominal fat. Many exercises such as those which are part of a pilates or yoga programme, can help to restore muscle tone. Vaginal pessaries may be useful for support of the bladder neck.
When conservative measures have failed, surgical options may
be required. These procedures are generally only provided by urogynaecologists or urologists with a special interest in this field and referral to a general urologist may delay accessing the appropriate treatment. Procedures include the injection of bulking agents around the bladder neck, mid-urethral slings to elevate and support the bladder neck or colposuspension. It is important to rule out UUI as surgery for SUI may unmask or make UUI worse, so a urodynamic study may be required pre-operatively.
UUI
Vaginal estrogen reverses the atrophy of the vaginal and urethral epithelium, making them thicker, less sensitive and reducing the incidence of UTI. It may also reduce the sensitivity of the bladder itself, thus reducing overactivity of the muscle. As mentioned earlier, it is safe for almost all women of any age.
Pelvic floor physiotherapy is useful for both rehabilitation of the pelvic floor and retraining of the bladder to hold larger amounts of urine.
As with SUI, weight loss and avoidance of constipation is beneficial.
Bladder irritants such as fizzy drinks, caffeine and alcohol are best avoided. Although most people with OAB tend to instinctively restrict their fluid intake, this can be counter productive as concentrated urine is an irritant to the bladder and increases the risk of UTI.
Good diabetic control is vital as glucosuria is a bladder irritant and
treatment has failed, other options again require referral to a urogynaecologist or a urologist with a special interest. Treatments include intravesical Botulinum toxin (Botox) injections, percutaneous tibial nerve stimulation or sacral nerve stimulator implants.
It is important to bear in mind that rapid onset of symptoms of bladder irritation, particularly in older women or those with a history of smoking and also the presence of haematuria, may signify a bladder neoplasm and such symptoms should be carefully monitored. Lack of response to conservative management, should trigger referral to a urologist for possible cystoscopy.
Although SUI and UUI are the main types of urinary incontinence, one can also have overflow incontinence. This can be caused by obstruction which prevents the bladder emptying adequately, such as happens in men with enlarged prostates. Symptoms may begin with issues similar to OAB but develop to include weak urinary flow or a dribble, small amounts of urine passed, a feeling of incomplete emptying, having to get up multiple times at night to pass urine, incontinence and recurrent UTIs. In women, a significant cystocele may
position on the toilet and it is important that they do try to empty completely, if possible. Another cause of overflow incontinence is a neurogenic bladder, as can happen with certain neurological conditions such as Parkinson’s or MS. Neuropathy can also be the result of poorly controlled diabetes. Treatment of overflow incontinence depends on the cause and may entail surgery to elevate the bladder neck, learning how to self-catheterise or using an indwelling urinary catheter.
Finally, functional incontinence occurs when an issue such as poor mobility means you can’t make it to the toilet in time or arthritis means you cannot undo your clothes quickly enough. This increases with age and is not a direct result of menopause. In this situation, products such as pads and absorbent underwear may be the best option. For some, an indwelling urinary catheter can be useful.
With all aspects of GSM, it is important for healthcare professionals to bring the subject up. Awareness among the general public is increasing, but women still tend to be reluctant to do it themselves. Once out in the open, treatment can be simple, rapid and life-changing.
Free Emergency Contraception
The HSE has launched a new service to provide free prescription and emergency contraception for 17 to 25 year old women and people.
The free contraception service will cover the full cost of prescription contraception, including the cost of:
GP or doctor’s appointments - to talk about contraception options and for repeat prescriptions when needed
any prescriptions given by your doctor - these will be given free of charge at participating pharmacies
your choice of contraceptionfrom a wide range of short-term and long-term contraceptives such as the patch, pill, ring, implant, injection and IUDs
fittings and removals of implants and IUDs or IUSs (coils)

any check-ups or other follow up care needed, relating to your implant or coil
emergency contraception (morning after pill).
Maeve O'Brien, Interim Programme Lead for the HSE Sexual Health and Crisis Pregnancy Programme commented, “This new service
is a welcome development. As part of the service, the HSE is launching a campaign to promote the free contraception service and the range of contraceptive options available over the coming weeks. As a first step, we are encouraging those who may be eligible to visit SexualWellbeing. ie to learn more about the service, the contraception options available and how they work. This information will help to support discussions with your GP.”
Dr Ciara McCarthy, ICGP/HSE GP Clinical Lead in Women's Health added, “Finding a suitable method of contraception will depend on
your needs, your medical history and your own preferences. I would encourage anyone thinking about contraception for the first time, or thinking about changing their contraception, to speak to their GP about the options available. GPs are in a good position to discuss the options and advise about side effects or the method that will suit your needs best. The free GP consultations covered under the new service will give anyone considering availing of free contraception the chance to discuss these questions in full before making an informed decision.”
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
News
65
Importance of Sleep in Women’s Health
How does stress affect the female body and why a quality sleep is so important
Written by Tatiana Iarmak – http:// orcid.org/0000-0001-5371-2958.

Lecturer of the Department of Advanced Training of Junior Medical Specialists at the Municipal Health Care Institution “Kharkiv Regional Medical Vocational College”, Kharkiv, Ukraine. An independent trainer, consultant to medical facilities and non-medical facilities to instruct medical staff on how to safely provide services to patients/clients.
Corresponding Author details: iarmak.tat@gmail.com
The aim of the study:
Show the role of a healthy lifestyle in maintaining and strengthening women's health: the impact of stress; the need and importance of an adequate sleep; the significance of the sleep hormone - melatonin for the female body, its effect on the functioning of all organs and systems of the body; the importance of women's positive and responsible attitude towards their health.
Brief summary:
This article explores the impact of a healthy lifestyle on the women's health: the quality and quantity of sleep, psychological and physical stress, social and other factors which have a direct impact on the female body. It studies the significance of sleep and the sleep hormone - melatonin, as well as the role of chronotypes and circadian rhythms in more detail. Healthy sleep makes it possible for a woman to be active, productive, successful, look good, and most importantly be healthy. Deprivation - lack of sleep or a prolonged lack of sleep, creates a whole bunch of health problems for women: weakens the immune system, decreases the physical and mental capabilities of the body, deteriorates vision, impairs cognitive functions, leads to a loss of attractiveness, contributes to the emergence of bad habits, for instance, overeating, leading to obesity and much more.

Unhealthy sleep is often caused by stress. Stress factors can
lead to heart attacks, strokes, neurosis, depression, increased fatigue; deterioration of the endocrine, hormonal, reproductive systems of a female body and other negative consequences.
Background:
Philosophers such as Avicenna, Hippocrates, Helvetius, Socrates, later J. Locke, A. Smith and others have been studying the notion of a healthy lifestyle since ancient times. In their opinion, a healthy lifestyle played a decisive role in the person’s wellbeing and, therefore, health was the main value of life. ‘Health is not everything, but everything without health is nothing’, Socrates believed. The outstanding Ukrainian surgeon Nikolai Amosov said: "A doctor treats diseases, but health must be acquired by oneself." In that respect, modern conditions of life may influence women's health, but women themselves should acquire their health.
In the past centuries, the sleep researches have been conducted by scientists from all over the world. The teaching of the an American neurophysiologist Nathaniel Kleitman, ‘the Father of Sleep Research’ and the founder of the scientific study of sleep in the early 20th century, has grown into a science of sleep - somnology, which has played a fundamental role in the development of neuroscience and modern medicine in the 21st century. Sleep is a prerequisite for the survival of any biological
species and one of the most important components of the human life. Sleep is a natural physiological process during which the female body recovers thus, sleep is vital for the female body. It is impossible to overestimate the importance of sleep. Sleep is more important than food. One can live on average 20-25 days without food, but without sleep, health problems start to become already visible on the first day and serious consequences for the body appear on the seventh day of continuous wakefulness, according to scientists from the University of California. Sleep regulates the process of metabolism, helps restore physical and mental activity, preserves and develops human cognitive abilities (memory, speech, intelligence); supports the functioning of the immune, nervous, skeletal and muscular, the endocrine systems; affects the psycho-emotional state and the speed of reactions in the body; increases the stress resistance, has a rejuvenating effect, and much more. A person spends one third of life in a dream, therefore a full-fledged sleep determines a person’s vital activity, the quality of life. The world-renowned Professor of Experimental Brain Research, a Director of the Institute of Neuroscience at Trinity College Dublin, Ireland - Shane O'Mara believes that during the day toxic substances accumulate in the brain, which are neutralized during sleep. He notes when sleep is insufficient, the person’s state resembles ‘a mild concussion’. Longer sleep disrupts brain activity. In that respect, sleep and health are interconnected.
According to the World Health Organization (WHO) studies, every second adult suffers from insomnia. Only 55% of the world's population are satisfied with their sleep, 25% have problems, and 20% have serious sleep-related health problems. According to the studies of the scientists from different countries, women suffer from insomnia twice as often as men: they cannot fall asleep for longer, often wake up at night, and feel tired in the morning. Women aged 40-80 and older are more susceptible to insomnia. One of the potential causes of
female insomnia are poor sleep hygiene and sleep patterns, such as going to bed at different times, sleeping less than 7 hours, low or high temperature in the bedroom; uncomfortable sleeping place; bright light and use of gadgets, hearty meals, drinking caffeinated or alcoholic beverages, smoking, physical and mental activity shortly before bedtime and more. Frequently, female insomnia can be caused by acute stress associated with a negative event, such as a conflict, a loss of a loved one, war, physical or mental trauma, etc. Respectively, women cannot relax for a long time, ruminating thoughts do not allow them to fall asleep, and as a result they are tired, sleepy, irritable. Thus, stress leads to poor sleep and poor sleep leads to stress. Stress is an integral part of life - the body's reaction to the impact of various adverse factors - stressors (physical and mental trauma, conflicts, illnesses, war, etc.). Stress can be beneficial - eustress - causes positive emotions and negative - distress, posing a threat to health. Stress can be mild or severe; acute and chronic. Insomnia for more than three months is a direct result of chronic stress. Stress significantly affects the female body, therefore, with age, stress resistance decreases, self-regulation processes are disrupted, leading to changes in the body and appearance of a woman. Scientists at the Karolinska Institute in Stockholm have found that sleep has a major impact on the women's appearance. Lack of sleep or poor sleep make women less healthy and attractive due to increased levels of the stress hormone cortisol. As a resultbags and dark circles under the eyes; puffy face, dull complexion, rash, brittle nails and hair; the firmness and elasticity of the skin decreases, excess weight; the aging process progresses, a woman looks older than her years. As reported by the WHO, around 32% of women's population suffer from neurological diseases such as tension-type headache (THT) or chronic tension-type headache (CTTH), provoked by stress and sleep disturbance. A research led by the scientists of Pennsylvania State University has shown that
WOMEN’S HEALTH SLEEP OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
66
quite often women experience headaches associated with a lack of production of the hormone vasopressin, which is responsible for retaining the fluid in the body, which causes dehydration if a woman sleeps less than 6 hours a day. Sleep deprivation can also cause restless legs syndrome (RLS), a neurological disorder characterized by discomfort in the legs. Restless legs syndrome in pregnant women is associated with iron deficiency and impaired dopamine metabolism and usually stops after childbirth. Sleeping less than 7 hours a day for women leads to serious health problems like hypertension, risk of heart attack or stroke, according to research by scientists at Tulane University in New Orleans; type 2 diabetes, obesity, breast cancer; weakened immune system; developing depression; anxiety, irritability or, conversely, apathy, impotence; inability to enjoy life; weakened sense of humor, impaired memory, concentration, reaction; decreased libido and even death.
The human body is a wellestablished mechanism that works according to certain laws and rules. A person has an internal biological clock - circadian rhythms, introduced in the mid60s of the twentieth century by the American scientist F. Halberg, associated with the change of day and night and are close to 24 hours. Circadian rhythms work differently for everyone and affect the psycho-emotional state of a person. All people are divided into chronotypes - these are individual characteristics of the activity of the human body at different times of the day (daily rhythms), therefore it is so important to know your chronotype in order to be
healthy. The Swedish psychologist O. Okvist in 1970 compiled a questionnaire to determine the chronotype, the results of which revealed three chronotypes: morning - "larks" (15%) - wake up early in the morning, active in the morning, evening - owls (20%)wake up late, closer to noon, are active in the evening-nighttime and intermediate type - arrhythmic - "pigeons" (65%) - get up a little later than "larks", are active during the day, go to bed at 23.00-24.00 hours. Michael J. Breus, Ph.D., clinical psychologist, member of the American Council and the American Academy of Sleep Medicine, believes that "humans are mammals, not birds and so behave like other mammals." His research is based on the natural biorhythms of each person. In his book ‘Always on Time’ (2016), he described the new human chronotypes: a. Bears (55%) have a normal sleep pattern. b. Lions (15%) - easy to wake up early in the morning without an alarm clock. c. Wolves 15-20% - hate mornings and the alarm clock. d. Dolphins (10%) - irregular sleep patterns, insomniacs, can work all night. For each chronotype, Breus developed a daily routine which showed positive results for sleep improvement, general well-being, mood, memory, concentration, weight normalization and emphasized that living according to one's own biorhythms, can make one healthier, help manage time better and be happier.
In recent years, the scientists have discovered that social factors also have a great impact on the chronotype. A life in cities, especially in megacities, has changed the lifestyles of a large number of people who live and work on a night or shift

work schedule (day - night), such as doctors and nurses, call center operators, rescue service dispatchers, police officers, railroad workers; airport employees, pilots and stewards; transport drivers and employees of gas stations, waiters and others, many of whom are women. Working at night is a serious psychological and physical load, especially for women, which affects the entire female body. It leads to metabolic disorders and as a resultobesity, atherosclerosis, diabetes mellitus; wrong diet resulting in diseases of the gastrointestinal tract; not to mention menstrual disorders, endometriosis, infertility, dysfunction of the hormonal, immune, nervous systems; in pregnant women – an increased risk of miscarriages, complicated and premature births. The results of the research at the University of Michigan showed that night or shift work (day - night) - leads to constant disruptions in circadian rhythms; increases the risk of ischemic stroke by 4% every five years; colorectal cancer and breast cancer by 50% among women. The WHO has classified night and shift work as one of the potential carcinogen factors. Moreover, heart attacks, strokes, hypertensive crises often occur at night. The recommendations of sleep researchers for those, who work at night or in shifts: to create conditions as close as possible to the night sleep regimen: turn off all kinds of gadgets (smartphones, tablets, computers, etc.); ensure silence, complete darkness (blackout curtains or blinds; sleep masks), sleep hygiene (go to bed and wake up at the same time, regardless of the time of work), comfortable temperature, ventilated room, comfortable
American professor of dermatology Aaron Lerner discovered the hormone melatonin and in 1958 he established its structure.
Melatonin is a sleep hormone, the only hormone which is produced directly at night on 80% by the pineal gland and on 20% in special cells located in the retina, prostate, ovaries, bronchi, lungs, digestive tract, kidneys, pancreas and others. The level of the hormone in the blood depends on the quality of sleep, sex, age, season, biorhythms and other indicators.
The human body needs melatonin all the time. It can be detected in the blood only at night, when it reaches its highest concentration. The peak of its content in the blood is at 2 am. The hormone has a huge number of properties which help improve women’s health, prolong the lifespan, maintain and preserve female beauty, and youth. Melatonin has an antistress effect, it regulates sleep, metabolic processes, the work of the endocrine, cardiovascular, reproductive systems; contributes to the body's adaptation to changing time zones and climatic and geographical zones, optimizes the cognitive functions of the brain, slows down the aging process, normalizes blood pressure and much more. Thus, being famous for its miraculous effect on women’s health, melatonin is called a ‘magic hormone’.
Conclusions:
Lifestyle is a dynamic system which is influenced not only by the life situations, but also by the tasks and goals women set for themselves. A healthy lifestyle is the basis of a quality happy life for women. Modern fast-paced life often upsets the balance of a healthy lifestyle, therefore, it is paramount for women to maintain and strengthen their health by assuring a good healthy sleep, reducing stress and adopting an attentive caring attitude towards their own body. Sleep is one of the most effective and cost-effective rejuvenation methods. It is at night that the cells of the body are actively regenerating. Observing circadian rhythms and sleep hygiene helps women restore their psycho-emotional and physical health, enjoy life, remain young and be attractive for many years.
A healthy lifestyle gives freedom in all areas of life and a healthy woman is not limited by anything.

HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
67
Metastatic Breast Cancer: An overview of


1School of Nursing & Midwifery, University College Cork
2School of Medicine, University College Cork
3Cancer Research @UCC, College of Medicine & Health, University College Cork

4Department of Medical Oncology, Cork University Hospital, Cork
Background
Globally, breast cancer is the most common diagnosis in new cases of cancer, with 2.26 million new cases diagnosed in 2020.1 Breast cancer treatment can be highly effective, especially when the disease is diagnosed early. Mortality rates have been on a steady decline in recent years in high-income countries, largely due to advancements in population screening programmes and early-stage treatments.2 Metastatic breast cancer, also
known as stage IV or advanced breast cancer, is rare as a primary diagnosis, making up 6-10% of new cancer diagnoses. Metastatic breast cancer has a current 5-year survival rate of 25-27%3,4, and is considered an incurable disease caused by the spread of breast cancer to other areas beyond the lymph nodes of the axilla to other sites in the body.5,6 However some patients (estimated at 10%) will attain long-term disease control, or undetectable disease, and thus achieve a prolonged survival.7
Improved survival rates for metastatic breast cancer have come predominantly as a result of advances in therapeutic options including targeted therapies, immune checkpoint inhibitors and antibody-drug conjugates.2,5 Novel agents targeting the oestrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) pathways, and the tumour microenvironment have been associated with improved survival outcomes; although adverse event profiles and response to therapy can vary between individuals 8,9. As such, there has been a notable extension in survival time for patients with metastatic breast cancer, though it varies by the clinical, pathologic, and disease characteristics and extent of metastasis.
Management of Metastatic Breast Cancer
For metastatic breast cancer, the intention of treatment is to achieve longer-term disease control, manage tumour-related symptoms, extend survival and importantly, preserve quality of life.4 Supportive care for patients is usually led by the primary oncology team, often with support



from primary care, palliative care and other experts. The treatment approach is multidisciplinary and can include local therapy options (surgery, radiation therapy) and systemic therapies including endocrine therapy, chemotherapy and targeted therapies to extend survival. However, such treatments can be associated with bothersome short- and longer-term side effects. These can include the more recognized physical effects (e.g., nausea, fatigue, hair loss), but also emotional and social, such as the psychological burden or feelings of social isolation due to fatigue. 10
Studies focusing on metastatic breast cancer have tended to focus on the efficacy of various medical treatments and their impact on quality of life,10 yet few studies focus on supportive care interventions to improve the quality of life of patients with metastatic breast cancer undergoing such treatments. In recent years, this area of supportive care has been gaining attention internationally as ideas of survivorship and what it means to ‘survive’ cancer have evolved.11 Health care professionals have also sought to integrate acute oncology, palliative care and community services more effectively. Research supporting the benefits of early integration of palliative care services with standard oncology care is emerging. 12
Cancer survivorship is broadly defined as living with, through and beyond cancer, beginning at the time of diagnosis until the end of life.13 Survivorship services have been identified as a priority area as they are underdeveloped and under-resourced.14,15 The dominant model of care is specialist-led follow up care that focuses primarily on the
Danielle Keane
Written by Danielle Keane, BA Hons MPH1; Grace Phillips, BSc MPH1; Nicola Mitchell2; Roisin M Connolly, MB BCh MD3,4; Josephine Hegarty, PhD MSc BSc RGN RNT1
Roisin Connolly
Grace Phillips
Josephine Hegarty
Nicola Mitchell
WOMEN’S HEALTH BREAST CANCER OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
68
KISQALI—the longest median overall survival reported for a CDK4/6 inhibitor in HR+/HER2- aBC 1,5,6
MEDIAN
KISQALI + ET vs ET alone
MONALEESA-2: N=668, 1:1 randomization. As 1L in advanced disease. KISQALI 600 mg or placebo once
(3 weeks on/1 week off) + letrozole 2.5 mg4
MONALEESA-7: N=672, 1:1 randomization. As 1L in advanced disease. KISQALI 600 mg or placebo once daily (3 weeks on/1 week off) + ET (letrozole 2.5 mg or anastrozole 1 mg, or tamoxifen 20 mg orally) + LHRH agonist 3.6 mg4
MONALEESA-3: N=726, 2:1 randomization. As 1L or after 1L progression for advanced disease. KISQALI 600 mg or placebo once daily (3 weeks on/1 week off) + fulvestrant 500 mg4
KISQALI is not indicated for concomitant use with tamoxifen 4

1L,
line; 2L, second line;
REFERENCES:
therapy; LHRH, luteinizing hormone-releasing hormone,
,
breast cancer.
1. ESMO - European society of medical oncology
2. SABC - San Antonio Breast Cancer Conference
1. Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival results from the phase III MONALEESA-2 trial of postmenopausal patients with HR+/HER2− advanced breast cancer treated with endocrine therapy ± ribociclib. Presented at: European Society of Medical Oncology; September 16-21, 2021.
2. Slamon DJ, Neven P, Chia S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32(8):1015- 1024.
3. Tripathy D, Im S-A, Colleoni M, et al. Updated overall survival (OS) results from the phase III MONALEESA-7 trial of pre- or perimenopausal patients with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/ HER2–) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib. Presented at: San Antonio Breast Cancer Symposium; December 8-12, 2020; San Antonio, TX. Poster PD2-04.
4. KISQALI [Summary of Product Characteristics]. Novartis, available at www.medicines.ie.
ABBREVIATED PRESCRIBING INFORMATION
Please refer to Summary of Product Characteristics (SmPC) before prescribing. Kisqali (ribociclib) 200 mg film-coated tablets
Presentation: Film coated tablets (FCT) containing 200 mg of ribociclib and 0.344 mg soya lecithin.
Indications: Kisqali is indicated for the treatment of women with hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy In pre or perimenopausal women, the endocrine therapy should be combined with a luteinising hormone releasing hormone (LHRH) agonist.
Dosage and administration:
Adults: The recommended dose is 600 mg (3 x 200 mg FCT) taken orally, once daily for 21 consecutive days followed by 7 days off treatment, resulting in a complete cycle of 28 days.
Kisqali should be used together with 2.5 mg letrozole or another aromatase inhibitor or with 500 mg fulvestrant.
When Kisqali is used in combination with an aromatase inhibitor, the aromatase inhibitor should be taken orally once daily continuously throughout the 28 day cycle. Please refer to the Summary of Product Characteristics (SmPC) of the aromatase inhibitor for additional details.
When Kisqali is used in combination with fulvestrant, fulvestrant is administered intramuscularly on days 1, 15 and 29, and once monthly thereafter. Please refer to the SmPC of fulvestrant for additional details.
Treatment of pre and perimenopausal women with the approved Kisqali combinations should also include an LHRH agonist in accordance with local clinical practice. Management of severe or intolerable adverse reactions (ARs) may require temporary dose interruption, reduction or discontinuation of Kisqali. Please see section 4.2 of SmPC for recommended dose modification guidelines.
Kisqali can be taken with or without food (see section 4.5).The tablets should be swallowed whole and should not be chewed, crushed or split prior to swallowing.
Special populations: ♦Renal impairment: Mild or moderate: No dose adjustment is necessary. Severe: A starting dose of 200 mg is recommended in patients with severe renal impairment. Kisqali has not been studied in breast cancer patients with severe renal impairment. Caution should be used in patients with severe renal impairment with close monitoring for signs of toxicity.
transaminases have been reported. Liver function tests (LFTs) should be performed before initiating treatment. LFTs should be monitored every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. If grade ≥ 2 abnormalities are noted, more frequent monitoring is recommended. Recommendations for patients who have elevated AST/ALT grade ≥ 3 at baseline have not been established. Based on the severity of transaminase elevations, Kisqali may require dose interruption, reduction, or discontinuation. ♦QT interval prolongation has been reported with Kisqali. The use of Kisqali should be avoided in patients who have already or who are at significant risk of developing QTc prolongation. The ECG should be assessed prior to initiation of treatment. Treatment with Kisqali should be initiated only in patients with QTcF values <450 msec. The ECG should be repeated at approximately Day 14 of the first cycle and at the beginning of the second cycle, then as clinically indicated. In case of QTcF prolongation during treatment, more frequent ECG monitoring is recommended Appropriate monitoring of serum electrolytes (including potassium, calcium, phosphorous, and magnesium) should be performed prior to initiation of treatment, at the beginning of the first 6 cycles, and then as clinically indicated. Any abnormality should be corrected before the start of Kisqali treatment. Based on the observed QT prolongation during treatment, Kisqali may require dose interruption, reduction, or discontinuation. Based on the E2301 study QTcF interval data, Kisqali is not recommended for use in combination with tamoxifen. ♦Critical visceral disease. The efficacy and safety of ribociclib have not been studied in patients with critical visceral disease. ♦Severe cutaneous reactions Toxic epidermal necrolysis (TEN) has been reported with Kisqali treatment. If signs and symptoms suggestive of severe cutaneous reactions (e.g. progressive widespread skin rash often with blisters or mucosal lesions) appear, Kisqali should be discontinued immediately. ♦Interstitial lung disease/pneumonitis ILD/pneumonitis has been reported with CDK4/6 inhibitors including Kisqali. Based on the severity of the ILD/pneumonitis, which may be fatal, Kisqali may require dose interruption, reduction or discontinuation as described in SmPC. Patients should be monitored for pulmonary symptoms indicative of ILD/pneumonitis which may include hypoxia, cough and dyspnoea and dose modifications should be managed in accordance with Table 5 (see section 4.2)
♦Blood creatinine increase ribociclib may cause blood creatinine increase – if this occurs it is recommended that further assessment of the renal function be performed to exclude renal impairment.
♦Effects on ability to drive and use machines Patients should be advised to be cautious when driving or using machines in case they experience fatigue, dizziness or vertigo during treatment with Kisqali.
Interactions: ♦Concomitant use of strong CYP3A4 inhibitors should be avoided, including, but not limited to, clarithromycin, indinavir, itraconazole, ketoconazole, lopinavir, ritonavir, nefazodone, nelfinavir, posaconazole, saquinavir, telaprevir, telithromycin, verapamil, and voriconazole. Alternative concomitant medicinal products with less potential to inhibit CYP3A4 should be considered. Patients should be monitored for ARs. If concomitant use of a strong CYP3A4 inhibitor cannot be avoided, the dose of Kisqali should be reduced (see section 4.2 of SmPC). ♦Grapefruit or grapefruit juice should be avoided. ♦Concomitant use of strong CYP3A4 inducers should be avoided, including, but not limited to, phenytoin, rifampicin, carbamazepine and St John’s Wort (Hypericum perforatum). An alternative medicinal product with no or minimal potential to induce CYP3A4 should be considered. ♦Caution is recommended when Kisqali is administered with sensitive CYP3A4 substrates with narrow therapeutic index (including, but not limited to, alfentanil, ciclosporin, everolimus, fentanyl, sirolimus, and tacrolimus), and their dose may need to be reduced. ♦Concomitant administration of Kisqali at the 600 mg dose with the following CYP3A4 substrates should be avoided: alfuzosin, amiodarone, cisapride, pimozide, quinidine, ergotamine, dihydroergotamine, quetiapine, lovastatin, simvastatin, sildenafil, midazolam, triazolam. ♦Caution and monitoring for toxicity are advised during concomitant treatment with sensitive substrates of drug transporters P-gp, BCRP, OATP1B1/1B3, OCT1, OCT2, MATE1 and BSEP which exhibit a narrow therapeutic index, including but not limited to digoxin, pitavastatin, pravastatin, rosuvastatin and metformin. ♦Co-administration of Kisqali with medicinal products with known potential to prolong the QT interval should be avoided such as anti-arrhythmic medicinal products (including, but not limited to, amiodarone, disopyramide, procainamide, quinidine and sotalol) and other medicinal products known to prolong the QT interval including, but not limited to, chloroquine, halofantrine, clarithromycin, ciprofloxacin, levofloxacin, azithromycin, haloperidol, methadone, moxifloxacin, bepridil, pimozide and intravenous ondansetron. Kisqali is not recommended for use in combination with tamoxifen.
♦Elderly (>65 years): No dose adjustment is required.
♦Hepatic impairment: Mild: No dose adjustment is necessary. Moderate or severe: Dose adjustment is required, and the starting dose of 400 mg once daily is recommended.
♦Pediatrics(<18 years): Safety and efficacy have not been established.
Contraindications: Hypersensitivity to the active substance or to peanut, soya or any of the excipients.
Warnings/Precautions: ♦Neutropenia was most frequently reported AR. A complete blood count (CBC) should be performed before initiating treatment. CBC should be monitored every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, then as clinically indicated. Febrile neutropenia was reported in 1.4% of patients exposed to Kisqali in the phase III clinical studies. Patients should be instructed to report any fever promptly.Based on the severity of the neutropenia, Kisqali may require dose interruption, reduction, or discontinuation. ♦Hepatobiliary toxicity - increases in
♦CYP3A4 substrates. ribociclib may interact with medicinal products which are metabolised via CYP3A4, which may lead to increased serum concentrations of CYP3A4 substrates (see section 4.5). Caution is recommended in case of concomitant use with sensitive CYP3A4 substrates with a narrow therapeutic index and the SmPC of the other product should be consulted for the recommendations regarding co administration with CYP3A4 inhibitors.
Pregnancy, Fertility and Lacation
♦Pregnancy: Pregnancy status should be verified prior to starting treatment as Kisqali can cause foetal harm when administered to a pregnant woman.
♦Women of childbearing potential who are receiving Kisqali should use effective contraception (e.g. double-barrier contraception) during therapy and for at least 21 days after stopping treatment with Kisqali. ♦Breast feeding: Patients receiving Kisqali should not breast feed for at least 21 days after the last dose. ♦Fertility: There are no clinical data available regarding effects of ribociclib on fertility. Based on animal studies, ribociclib may impair fertility in males of reproductive potential.
Adverse reactions: ♦Very common: Infections, neutropenia, leukopenia, anaemia lymphopenia, decreased appetite, headache, dizziness, dyspnoea, cough, nausea, diarrhoea, vomiting, constipation, stomatitis, abdominal pain, dyspepsia alopecia, rash, pruritus, back pain, fatigue, peripheral oedema, asthenia, pyrexia, abnormal liver function tests. ♦Common:, thrombocytopenia, febrile neutropenia, hypocalcaemia, hypokalaemia, hypophosphataemia, vertigo, lacrimation increased, dry eye, syncope, dysgeusia, , hepatotoxicity, erythema, dry skin, vitiligo, dry mouth, oropharyngeal pain, blood creatinine increased, electrocardiogram QT prolonged. ♦Please refer to SmPC for a full list of adverse reactions.
Legal Category: POM
Pack sizes: Unit packs containing 21, 42 or 63 FCTs. Not all pack sizes may be marketed.
Marketing Authorisation Holder: Novartis Europharm Limited Vista Building, Elm Park, Merrion Road, Dublin 4 Ireland
Marketing Authorisation Numbers: EU/1/17/1221/003 & 005.
Full prescribing information is available on request from Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Dublin 4. Tel: 01 2601255 or at www.medicines.ie
Prescribing information last revised: April 2022
Novartis Ireland Ltd, Vista Building, Elm Park Business Park, Merrion Road, Dublin 4, D04 A9N6
overall
across
The only CDK4/6 inhibitor with statistically significant
survival
all 3 phase III trials1,5,6 5 YEARS
first
ET, endocrine
aBC
advanced
daily
OVER 5 YEARS KISQALI + AI in 1L postmenopausal patients MONALEESA-21 ESMO 2021 4.5 YEARS KISQALI + fulvestrant in 1L/2L postmenopausal patients MONALEESA-32 ASCO2021NEARLY 5 YEARS KISQALI + ET in 1L premenopausal patients MONALEESA-73 SABC 2020
OS
September 2022 | IE 237611© 2022 Novartis
detection of recurrences. This model of care may result in unmet supportive care needs, an issue that is becoming more salient as the prognosis of metastatic breast cancer improves and life postdiagnosis is extended. 8,14,16,17
Quality of life is typically assessed based on functional status of the individual and how the patient perceives their health status to affect their life, usually through patient reported outcomes. 18
Supportive Care Needs
Research has shown that unmet informational needs on treatment side effects, managing pain and alternative treatments are among the top concerns of patients with metastatic breast cancer.1 Other concerns outlined include financial concerns over the high cost of treatment and the ability to continue employment. Individuals also report feeling under supported by healthcare providers and a lack of support outside of the hospital setting. 19 The concerns, issues and side effects associated with living with metastatic breast cancer are significant, yet interventions to address the
issues raised are understudied. Indeed, it is suggested that minimising the physical and psychosocial impact of the disease and treatments should be considered an endpoint in itself, and that quality of life should be a primary focus of healthcare for those with metastatic disease. As such, it is essential that the focus is shifted to addressing the unmet supportive care needs of patients with metastatic breast cancer through more focused interaction of primary teams with palliative, supportive care, and allied health experts. Patients living with metastatic disease have unique needs that are found to diverge from those of patients with early breast cancer, specifically an increased need for support services.10 Additionally, the rarity of male breast cancer means the gender dimension of the supportive care experience has been understudied and requires increased attention.
Supportive Care Interventions
Supportive care refers to the care provided to improve the quality of life for people with metastatic
WOMEN’S HEALTH BREAST CANCER

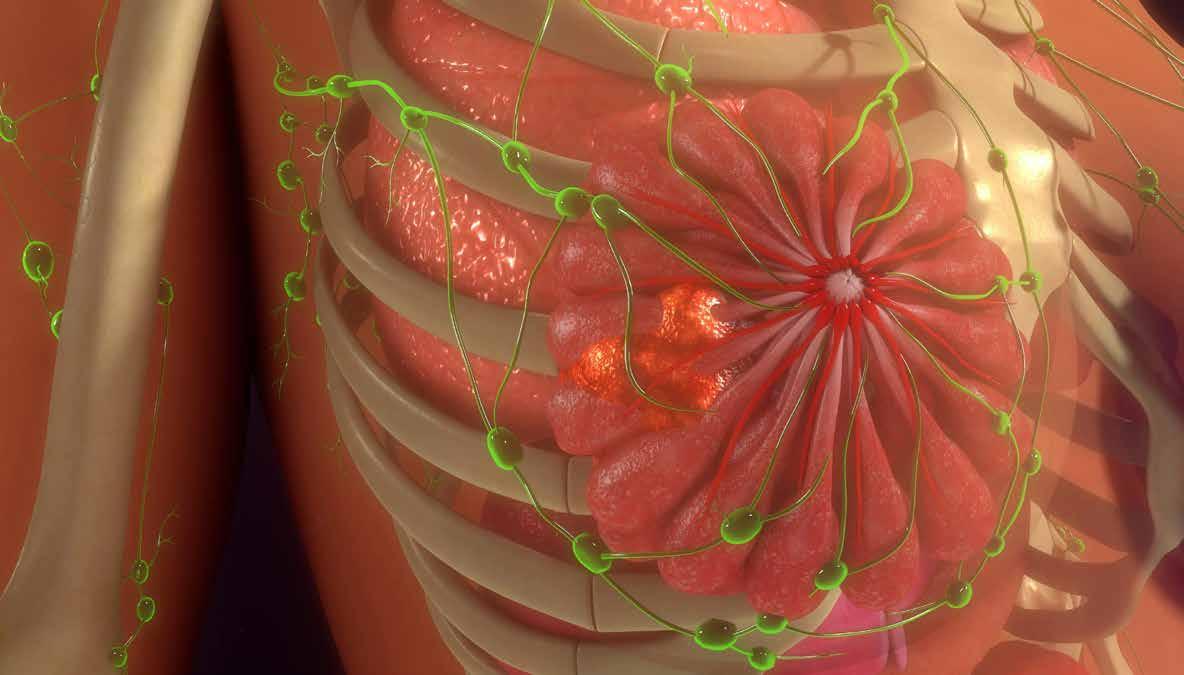
breast cancer, including palliative care, which aims to reduce the burden of symptoms of treatment or cancer itself.20 Although research has shown that supportive care interventions improve quality of life, research in patients with metastatic breast cancer is limited.2 Financial, informational, psychological, and social support are identified consistently as areas that patients require additional support; the provision of which was reported to have a positive impact on patients.22,23
Psychosocial Interventions
Psychological interventions such as cognitive behavioural therapy or psychotherapy can improve psychosocial outcomes like mood and quality of life but there is little evidence that such improvements are sustained or if interventions address the underlying causes of ongoing distress.21 In a randomised controlled trial involving 64 women with metastatic breast cancer, greater mindfulness was associated with lower levels of symptoms (i.e. pain, fatigue, anxiety, depression,
and sleep disturbance).24
Psychological and physical problems have been managed with mindfulness meditation, which is increasingly being used in non-spiritual settings. Similarly, psychosocial interventions have led to improved sexual and relationship satisfaction.25 Coping and communication skills training, mindfulness training, and sensate focusing are key components of effective psychosocial interventions to improve sexual relationships and function. Despite the availability of such interventions, many women find it difficult to discuss sexual concerns with their healthcare teams.25
Increased research into internetbased interventions may aid in overcoming these barriers.25
Technology Enabled Interventions
Technology enabled interventions could potentially aid in symptom self-management for patients with metastatic breast cancer. Given the time constraints that patients face when visiting their clinical care team, technologybased interventions for symptom
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
70
management are being rolled out in this population. Research using a technology-based approach (Nurse AMIE intervention) has been shown to have great potential to address disparities in the provision of supportive care by reaching poor and medically underserved women with metastatic breast cancer.26 Technology-based health interventions have the potential to reach a wide and diverse audience, having a significant and positive impact on quality of life, health selfefficacy, levels of psychological distress, and fatigue.27 Similarly, a feasibility study based in Cork (the LYSA trial), is introducing a women’s malignancy survivorship clinic incorporating symptom management through ePRO into routine follow up care in patients with early-stage hormone receptor (HR)-positive breast cancer post primary curative therapy; however additional research is needed in the area of metastatic breast cancer. 28 While technology has huge potential and reach, as healthcare professionals, we must be mindful of segments of the population that may not have access to or the literacy to use such technology.
Diet and Exercise Interventions
Physical activity and nutrition are potentially modifiable aspects of a patient’s journey, which through self-management support, can be particularly empowering in the face of uncertainties surrounding their cancer journey.29 Evidence has shown that walking interventions are beneficial for women with metastatic breast cancer, with improvements in walking distance, strength, and body mass index.30 A meta-analysis of randomised controlled trials in participants with advanced cancers, found that supervised aerobic or resistance interventions could improve physical fitness and sleep quality.31 According to epidemiological studies, a poor diet quality may have a negative impact on cancer recurrence and survival in patients with breast cancer; however, research in patients with metastatic breast cancer is limited.32
Informational Support Interventions
Many patients with metastatic breast cancer have expressed a desire for more information relating to their disease. In a systematic review involving 11 studies, ten studies reported a need for informational support.32 Women expressed a need for information about various aspects of their disease, such as treatment,
disease, symptoms, and side effects.33 Similarly, interviews with patients with metastatic breast cancer revealed that they wanted more information about their future, including how the illness might affect their daily lives, with less emphasis on survival estimates. One-third of those surveyed desired information on symptoms and side effects.3 Despite this, there is limited research around the efficacy of informational interventions.
Limitations
There are many limitations to the research surrounding supportive care interventions for patients with metastatic breast cancer. Sample sizes are often small, and patients may be lost-to-follow-up leading to incomplete study data and associated regulatory issues. These issues may be reflective of the challenges associated with living with metastatic breast cancer. This may result in extended accrual periods for clinical trials and associated higher monetary expenditures as additional resources may need to be dedicated to recruitment efforts. Furthermore, men with metastatic breast cancer are not represented in trials investigating supportive care interventions; a challenge observed across breast cancer stages.
Clinical trial recruitment may also be a challenge as some patients may be too unwell or too fatigued to participate, or travel may be challenging. Incomplete accrual may result in an inability to meet the primary objective of the study and thus inhibit the attainment


challenges.33 The development of clinical trials of supportive care interventions will both cater to an unmet clinical need in patients with metastatic breast cancer, and assist Irish cancer centres in reaching target clinical trial accrual goals provided by the National Cancer Strategy of 6%.35
Many interventional studies in the field of physical or psychological support services report an improvement in patients’ quality of life. However, long-term adherence to behavioural interventions may be difficult without ongoing support. Permanent lifestyle changes are required for long-term health benefits; however, this is not always possible for patients with metastatic breast cancer.36
Conclusion
It is critical to recognise the unique challenges affecting patients with metastatic breast cancer and subsequently the need for supportive care to optimise quality of life in this cohort. Further studies to validate efficacy and provide evidence for best supportive care interventions are needed. To address this gap, our team are currently conducting a needs assessment study for patients with metastatic breast cancer. Through focus group
in this area of care, our study aims to investigate and identify how supportive care services could be developed to meet the unmet needs of this cohort. We anticipate that results from this needs assessment will guide the development of dedicated services in the future.
With the continued support of the National Cancer Control Programme, designated cancer centres, HRB-funded cancer trials networks nationally and Cancer Trials Ireland; alongside charities and grant bodies we can conduct research to minimize disparities in care, improve the patient experience, enhance symptom management and quality of life.
Acknowledgment
The needs assessment study for patients with metastatic breast cancer referenced in this paper is supported by Irish Cancer Society and Pfizer through the Research Grant WHI21COHE. The opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Irish Cancer Society or Pfizer.
References available on request
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
71
What means everything to the patient? The potential for nothing left on their skin.1,2
* Nothing on the skin: Defined as 75% achievement of PASI90 at Week 16 and ≥50% achievement of PASI 100 at Week 52 in UltIMMa-1 and UltIMMa-2.1
High skin clearance matters to patients: Patients who achieve and maintain high levels of skin clearance (PASI 90-99 or PASI 100) have significantly better HRQoL than those with lower levels of skin clearance (PASI 75-89).2
Sustaining high skin clearance or complete skin clearance is associated with incremental and durable benefits in HRQoL and mental health of psoriasis patients.2
PRESCRIBING INFORMATION (PI). SKYRIZI®▼ (risankizumab) 75 mg solution for injection in prefilled syringe; Skyrizi 150 mg solution for injection in pre-filled pen and Skyrizi 150 mg solution for injection in prefilled syringe. Refer to Summary of Product Characteristics (SmPC) for full information before prescribing. PRESENTATION: Skyrizi 150 mg solution for injection in pre-filled pen/syringe: each prefilled pen/syringe contains 150 mg risankizumab in 1 mL solution. Skyrizi 75 mg solution for injection in pre-filled syringe: each pre-filled syringe contains 75 mg risankizumab in 0.83 ml mL solution. INDICATION: Plaque Psoriasis: For treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy. Psoriatic Arthritis: alone or in combination with methotrexate (MTX), is indicated for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more disease-modifying antirheumatic drugs (DMARDs). DOSAGE AND ADMINISTRATION: Intended for use under guidance and supervision of a physician experienced in diagnosis and treatment of psoriasis. Dosage: The recommended dose of Skyrizi is 150 mg by subcutaneous injection at weeks 0, 4, and every 12 weeks thereafter (either as two 75 mg pre-filled syringe injections or one 150 mg pre-filled pen or pre-filled syringe injection). Two 75mg pre-filled syringes should be injected for the full 150 mg dose. Consider discontinuation of treatment in patients showing no response after 16 weeks of treatment. Some patients with initial partial response may subsequently improve with continued treatment beyond 16 weeks. Special Populations: Elderly: No dose adjustment required. Renal or hepatic impairment: No dose adjustment required. Paediatric Population: No data available. Overweight patients: No dose adjustment required. CONTRAINDICATIONS: Hypersensitivity to any of the active substances or excipients. Clinically important active infections (e.g. active tuberculosis). SPECIAL WARNINGS AND PRECAUTIONS: See SmPC for full details. In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Skyrizi may increase the risk of infections. In patients with a chronic infection or history of recurrent infections, or known risk factors for infection, Skyrizi should be used with caution. Treatment with Skyrizi should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated. Patients should be evaluated for tuberculosis infection prior to initiating treatment. Anti-TB therapy should be considered prior to initiating Skyrizi in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Completion of all appropriate immunisations should be considered prior to initiating therapy. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with Skyrizi. Patients treated with Skyrizi should not receive live vaccines during treatment and for at least 21 weeks after treatment. If a serious hypersensivity reaction occurs, administration of Skyrizi should be discontinued immediately and appropriate therapy initiated. Excipients with known effect (75
mg solution for injection only): Skyrizi contains 68.0 mg sorbitol and less than 1 mmol sodium (23 mg) per 150 mg dose. INTERACTIONS: The safety and efficacy of Skyrizi in combination with immunosuppressants, including biologics or phototherapy have not been evaluated.
PREGNANCY AND LACTATION: Women of Childbearing potential: An effective method of contraception during treatment and for at least 21 weeks after treatment should be used. Pregnancy: Limited data available. It is preferable to avoid the use of Skyrizi during pregnancy as a precautionary measure. Lactation: It is not known whether Skyrizi is excreted in breast milk. Human IgGs are known to be excreted in breast milk during the first few days after birth, which decreases to low concentrations soon afterwards; consequently, a risk to the breast-fed infant cannot be excluded during this short period. A decision should be made whether to discontinue/abstain from Skyrizi therapy, taking into account the benefit of breastfeeding to the child and the benefit of Skyrizi therapy to the woman. Fertility: The effect of Skyrizi on human fertility has not been evaluated. ADVERSE REACTIONS: See SmPC for full details on adverse reactions. Very common adverse reactions (≥1/10): Upper respiratory infections. Common adverse reactions (≥1/100 to <1/10): Tinea infections, headache, pruritus, fatigue and injection site reactions.
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance; Website: www.hpra.ie. Suspected adverse events should also be reported to AbbVie Limited on 01-4287900.
LEGAL CLASSIFICATION: POM (S1A). MARKETING AUTHORISATION NUMBERS/PRESENTATIONS: Skyrizi 75 mg solution for injection in pre-filled syringe (Pack of 2 pre-filled syringes): EU/1/19/1361/001; Skyrizi 150 mg solution for injection in pre-filled pen: EU/1/19/1361/002; Skyrizi 150 mg solution for injection in prefilled syringe: EU/1/19/1361/003. Not all presentations may be marketed. Further information is available from: AbbVie Ltd., 14 Riverwalk, Citywest Business Campus, Dublin 24, D24XN32. DATE OF REVISION: November 2021 PI/1361/004
HRQoL, Health-Related Quality of Life; PASI, Psoriasis Area Severity Index.


REFERENCES: 1. Gordon KB, et al. Lancet 2018; 392: 650-661. 2. Ryan C et al. Poster presented at the 27th European Academy of Dermatology & Venerology (EADV) Congress 2018; September 12–16; Paris, France.
Skyrizi® Summary of Product Characteristics, available on www.medicines.ie. Date of preparation: April 2022 | IE-RISN-220005
When it comes to your patient’s psoriasis treatment goals
WOMENS HEALTH: PELVIC FLOOR
Can Diet Impact our Pelvic Floor Health?

Pelvic organ prolapse (POP), or urogenital prolapse, is a common health problem associated with pelvic floor dysfunction amongst women1 and was found to be more prevalent in low-income countries.2 It is estimated that POP can affect as many as 50% of adult women over the age of 40 years3 with a 13% lifetime risk of undergoing surgery.4–6
Well-documented risk factors for all three POP compartments include older age, obesity, pregnancy, vaginal delivery, high parity, previous hysterectomy, and chronic obstructive defecation. It has been established that prolapse of the anterior compartment is the most common type, being two times more prevalent than posterior prolapse and three times more prevalent than apical prolapse.7 However, it has been determined that posterior wall prolapse has a different prevalence among different countries, with a significantly higher prevalence (48.2%) in Ireland than in other Western countries, such as the United States of America (38.4%) and Germany (33.2%).8
The reasons behind this remain unclear in the current literature; however, these associations seem to be linked9–11 and are increasingly studied worldwide as global diets change. With a rise in carbohydrate and salt-rich foods and a

widespread decrease in grains, cereals, and other fibrous foods, there seems to be a "nutrition transition" rapidly evolving with the rise in urbanization.12 The traditional Mediterranean diet (MD) is inversely associated with obesity risk or weight gain13–15 and is well known to improve bowel function.16
Low adherence Mediterranean diet and obesity

Numerous studies14,17,18 showed that MD adherence was associated with a significantly lower likelihood of becoming obese which remains similar amongst (odds ratios with 95% CI) women (0.69, 0.54-0.89) and men (0.68, 0.53-0.89).14
Obesity and pelvic organ prolapse
Pandey and Bhattacharya19 found a significant correlation between female POP and the severity of obesity. POP was most prevalent amongst morbidly obese (53%) participants (BMI >39.9) and considerably higher (44%) in severely obese (BMI 35) than obese (BMI 30) women.
Mediterranean diet, constipation, and pelvic organ prolapse
While research on the associations between chronic constipation and POP in women has increased in recent years,
there has yet to be a conclusive finding as to the direction of this causal relationship. It is unclear whether chronic constipation and straining predispose an individual to POP20 or if the low Adherence to the Mediterranean diet (LAMD) results in defecatory obstructions and eventual chronic constipation. Varma and colleagues20 determined that women who had constipation symptoms on a weekly basis were significantly more likely to have POP. Women who had undergone hysterectomies or POP surgeries had a two times greater risk of developing weekly obstructive defecation.
Numerous studies9–11,21 focused on the relation of a low fiber diet and constipation to pelvic organ prolapse and the link to the posterior compartment. Furthermore, it was determined that the prevalence of posterior prolapse is much greater in constipated women than in prolapse of any other compartment.22 In contrast, Handa et al.23 found that defecatory symptoms are not significantly more likely to develop among asymptomatic women with posterior vaginal prolapse than in asymptomatic women without such prolapse. Although, defecatory symptoms were
associated with a more rapid worsening of posterior support (difficulty with bowel movements, P=.005; splinting, P=.057).23
Soligo and colleagues22 determined that anterior prolapse was less common in constipated women than posterior prolapse.
Clinical Implications
Current data from the literature support the correlation between LAMD and pelvic organ prolapse, especially the posterior compartment's prolapse. As there is a direct relationship between impaired bowel function and POP, it is requested that diet habits will also influence this problem.
We have recently finished a largescale study that shows this direct relationship between posterior prolapse which was linked to LAMD. In this study, 73% of the Irish population in the study cohort showed low adherence to the Mediterranean diet, and almost 50% had some degree of impaired bowel function.


Women should be educated about this relationship, as adherence to the Mediterranean diet may decrease POP prevalence in this population.
References available on request
Yair Daykan, The department of Obstetrics and Gynecology, Cork University Maternity Hospital, Cork, Ireland
Written by
Barry O'Reilly, The department of Obstetrics and Gynecology, Cork University Maternity Hospital, Cork, Ireland
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
73
Pelvic Health – Top tips for a fun and active life
Written by Aoife Ni Eochaidh, Chartered Physiotherapist, Clinical Specialist Pelvic, Women's & Men's Health Physiotherapist

International Pelvic Physiotherapy Management Ltd Suite 16, Bon Secours Consultant Clinic, Renmore, Galway
dysfunction our quality of life is affected. We can’t live our lives, go to work travel or exercise without worries about bladder or bowel leaks or we worry if we night need the bathroom urgently to empty our bladder or bowel. We won’t feel well if we can’t empty or bowels or our bladders and we might not sleep well either and our energy levels will be poor, and we will feel anxious. We might not be able to run around after our little ones, without the fear of a leak too!
We all know the dreadful feeling of needing the bathroom suddenly to empty the bladder while having coffee with friends or while out shopping and how humiliating and embarrassing it is to be caught short and leak urine. Imagine the awful feeling with an over whelming urge to empty your bladder and not making it to the bathroom on time. Worse still is the fear of not being able to control stool or gas. New Mums (and some not so new mums!) up and down the country are dealing with these awful problems. Help is available to give yourself back your freedom without fear of bladder and bowel leaks, for normal sexual function (to be able to orgasm or climax correctly) and to ensure optimal bowel and bladder emptying and solve constipation!
Progressive pelvic floor muscle training is recommended as a first line treatment for incontinence (CG171 NICE Guideline 2016). Two thirds of women with any type of incontinence who have pelvic floor muscle training see improvements or cure with 75% of women reporting resolution of symptoms such as episodes of incontinence (National Institute of Research doi:10.33310/signal-000702.
That’s 2 out of 3 women will be cured of incontinence with pelvic floor muscle training and the third woman will be improved! This is also reflected in women
and men for faecal incontinence, constipation, pelvic organ prolapse, and sexual and erectile dysfunction
What about your pelvic floor and mothers? What are the pelvic floor problems that can arise, that can be resolved? There are hormonal changes that occur during pregnancy and the postnatal period that affect the pelvic floor muscle function. In the pelvis and in the pelvic floor, hormonal changes occur that cause laxity of the pelvic soft tissues. The bladder and bowel muscles themselves can be affected with the hormonal changes too. Sexual function can be affected too with the hormonal changes, there can be pain and reduced libido. Pelvic Organ Prolapse can occur, (the pelvic organs, the bladder, rectum, womb and others coming down in the pelvic floor). Keeping your pelvic floor muscle function in the normal range of function is so important before, during and after pregnancy and it will prevent and treat these bladder, bowel, pelvic prolapse, pain and sexual problems.
If you are a complete beginner and don’t have a clue what a pelvic floor is or where it is, lets talk about where these muscles are located in the body. Firstly, think of the area of your body between your waist and the top of your legs. This is where your Pelvis is located, think of it as a basin
shaped region. Your tummy area is where your core muscles are located. The pelvic floor muscles form the base of the pelvic basin and are really the underneath part of your core. The pelvic floor muscles hold up or supports your organs correctly in the pelvic cavity. These organs are your bladder and your bowel and in ladies your vaginal walls and your womb too.
How important are they? The pelvic floor muscles are among some of the most important muscles in the body because they are responsible for the normal filling, storing, and emptying of our bladder and our bowels. They are important too for normal sexual function (to be able to orgasm or climax correctly) and to hold our pelvic organs in place correctly and to guard as pelvic organ prolapse, the organs coming down (the bladder, bowel, and womb in women). If the pelvic floor muscles are not in the normal range of function, then our bladder and our bowel may not fill, store and empty urine (our wee) and stool (our poo) correctly. If we are not eliminating our waste material (wee and poo) correctly we can become unwell, with urinary tract infection or obstructed bowel or constipation.
Why are they important? If our bladders and our bowels are not working correctly or if we have pelvic organ prolapse, or sexual

How do we exercise the pelvic floor muscles? We need to train our muscles to be in the normal range or function. This involves a series of exercises and training routines. It can take a 3-to-6month period of time to get the bulk, endurance, fitness, movement, and automatic function working in the normal range. The bladder and the bowel muscle themselves need to be trained too with lifestyle changes and some other exercises. The exercises need to be targeted to the different muscle fibers, and you need to progress them and keep doing them for life (at a maintenance dose but much more frequently in the beginning). It is not a quick fix, but it works, and it is so worth it! It is so much easier when the muscle is in the normal range to maintain then, it is harder for sure in the beginning, but it is possible! Post-natal Mums also need to train their abdominal muscles, training the pelvic floor will help zip up the tummy and gap or muscle separation (Diastasis Rectus Abdominal Muscle: DRAM) and is done as part of the nose to toes approach that is needed in getting the pelvic floor fully trained and back in shape after giving birth.
What age should we be starting pelvic floor exercises? Is it ever too late and should it be done before or during Pregnancy (best from 20 weeks in first pregnancy) and the Menopause? The largest group of people that have pelvic floor muscle weakness and bladder leakage is mothers in their 20’s, 30’s, 40’s, 50’s 60’s and 70’s. Before or during or after pregnancy
WOMEN’S HEALTH PELVIC HEALTH OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE
74
and Menopause a great time to start training your pelvic floor muscles, but you will need to do it sooner if you have any bladder or bowel problems.
Home Pelvic Routines are an online way of doing the pelvic floor muscle training, without even having to come and see the pelvic physiotherapist! They are an affordable, convenience and quick way of getting you pelvic floor muscle trained, in privacy, using any device, phone, tablet or laptop. All you need is an email address to log on and create a password enabled account. They are available to purchase from our platform, course. homepelvicroutines.com or from our website www.ippm.ie. I have seen thousands of women and men through my hands every year, in the last 25 years, with pelvic floor muscle dysfunction. My at home pelvic floor muscle training program, Home Pelvic Routine, is designed to that even the weakest person can do this program of training and routines
successfully, will feel that they are doing the exercises correctly and will be progressed through the exercises that are needed, at the right pace for optimal cost effective, convenient and quick training results!

How long can it take to see the benefits? In 1 week, you will see improvements, but it can take 3 -6 months to fully train the pelvic floor muscles and then you need to keep up a maintenance amount up for life.

What issues can it pose for Women and Mothers and Sex?
Pelvic Floor Muscle Dysfunction can affect mothers and women from a pain, a climax and arousal side of things. Many people might think it’s just women that have a pelvic floor but there are many benefits for men too for having a strong pelvic floor, what are those? Men also need to train their pelvic floors for exactly the same reasons
as women. Men too unfortunately get the very same bladder, bowel, and sexual dysfunction, especially, erectile dysfunction as women.
In summary if you are thinking of starting a family, if you are pregnant or post-natal, train your
pelvic floor muscles as an essential of preventative therapy for optimal pelvic health! Live your best life without our fear of bladder leaks or other pelvic floor problems! Please send me any questions or queries to aoife@ippm.ie
TILDA publishes research on Brain Blood Oxygen
New research from The Irish Longitudinal Study on Ageing (TILDA) examined how blood oxygen levels in the brain (“cerebral oxygenation”) are affected when a person stands up. Blood flow to the brain provides oxygen and nutrients for normal function. A reduced supply has been associated with adverse events such as falls, depression and cognitive decline.
The group has previously studied the blood pressure response to standing, but in this study - published in the journal Experimental Gerontology - cerebral oxygenation was examined.
The study used data from 2,764 community-dwelling participants. The size of the study enabled researchers to account for a range of confounding factors.
Key Findings:
• Women experienced a smaller drop in cerebral oxygenation compared to men.
• Women took longer to return to their normal level.
• Older age groups had a larger initial drop in cerebral oxygenation and impaired stabilisation.
• Those taking blood pressure lowering medications took longer to recover.
• Blood pressure levels also only partly explained some of the differences that we found.
Louise Newman is the lead author of the study and Research Assistant in Medical Gerontology at Trinity. She said, “We know that age affects how quickly blood pressure recovers when a person stands up, but we didn’t know if it was the same for cerebral oxygenation.

"We used novel equipment, nearinfrared spectroscopy, to measure the change in blood oxygen in the brain during a standing task. We found that women experienced a smaller drop in cerebral oxygenation compared to men, but women took longer to return to their normal level. We also found that those taking antihypertensive medications took longer to recover.
“However, there were no differences in the cerebral oxygenation response in those who told us they felt dizzy, light-headed or unsteady when standing up. Blood pressure levels also only partly explained some of the differences that we found.”
She explained, “The study highlights the need for standing cerebral blood measures to be assessed in older patients, regardless of symptoms.”
Professor Rose Anne Kenny, TILDA’s Principal Investigator, added, “Brain cells survive and function according to how much oxygen they receive and how quickly the cells and circulation can clear toxins from the brain cells. We stand up 30-50 times per day and each time our bodies must react quickly to ensure that the flow of blood and therefore oxygen is kept constant. For the first time in such a large adult study we have measured such brain blood flow when TILDA participants stood up and demonstrated that the ability to react quickly and maintain flow is impaired year on year over 50 years.
“Furthermore, men and women react differently. Muscle strengthening exercises and other interventions can change in a beneficial way these responses, so early recognition of problems, using this novel technology, should trigger treatments and lifestyle behaviour changes. As a result of this research, we have started to use the new technology in
clinical settings to improve patient management.”
The study, Age and sex related differences in orthostatic cerebral oxygenation: Findings from 2764 older adults in the Irish
Longitudinal Study on Ageing, is published in the journal
Experimental Gerontology and is freely available to all online.
Professor Rose Anne Kenny, TILDA
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
News
75
Management of Herpes Simplex Virus in Women
HSV infection: How to approach diagnosis and management in pregnancy
Written by Dr Elaine Houlihan, Microbiology Specialist Registrar, The National Maternity Hospital and Louise Delany, Antimicrobial Pharmacist, The National Maternity Hospital
Introduction
Herpes simplex virus (HSV) 1 and 2 are members of the Herpesviridae group which is a group of double-stranded, enveloped DNA viruses. The Herpesviridae group also includes Varicella Zoster Virus (VZV), Cytomegalovirus (CMV), EpsteinBarr virus (EBV) and Human Herpesvirus (HHV) 6, 7 and 8.
Classically, HSV1 causes oral infection i.e. cold sores and HSV2 causes genital infection, but this can be interchangeable. Both HSV1 and HSV2 can cause neonatal herpes simplex encephalitis, while HSV1 is the most common cause of childhood herpes disease. Spread of the virus is usually via the orolabial route and mucous membranes, and following primary infection, HSV develops latent infection in neurons. Latent infection is maintained in latency by cellmediated immune response, but the virus can reactivate and cause recurrent disease, usually near the site of within the dermatome of the affected ganglion, at times of stress, illness, immunosuppression and after UV exposure.
Infectious Syndromes:
• Asymptomatic – in approximately two-thirds of individuals. US figures report that 50-80% of adults are seropositive for HSV1 and 2040% for HSV2, and European figures report a 50-80% seropositivity for HSV1 with 4-25% seropositivity for HSV2.1, 2
• Genital herpes infection small number of painful, clustered vesicles with an erythematous base associated with pain on rupture of lesion leaving shallow ulcers that heal without treatment. Lesions in primary infections typically last 4-10 days, with recurrent infections resolving sooner. Lesions are classically located around vaginal region including vulva, cervix, perianal region, buttocks or thighs. This occurs with or without associated dysuria or difficulty with
urination secondary to pain, and with or without systemic symptoms including fever. HSV2 accounts for 70-80% of cases and HSV1 accounts for 20-30%.
• Neonatal herpes – Symptoms include fever, lethargy, poor feeding, seizures, bulging fontanelle and neurological signs and symptoms. 70% of neonatal herpes have CNS involvement or are disseminated, while 30% have localised skin/eye/mouth disease.
Neonatal encephalitis presents with fever, headache, ataxia, confusion, visual disturbance, seizures, photophobia, and fluctuating consciousness.
Other HSV related infectious syndromes include herpes labialis, Mollaret’s meningitis, ocular herpes and herpetic whitlow.
HSV in pregnancy:
There are many factors associated with HSV transmission causing neonatal herpes including the type of maternal infection (primary or non-primary infection or recurrent infection), the presence of maternal antibodies (which would
be expected to cross the placenta to offer neonatal protection), the duration of rupture of membranes prior to delivery, and the mode of delivery. The greatest risk of neonatal infection is when a woman acquires a new infection (i.e. primary genital herpes) in the third trimester, especially within 6 weeks of delivery as the neonate will be unlikely to have acquired protective antibodies in this time. For these reasons, it is vital to be familiar with the important definitions of HSV infections in order to assess risk and direct management.
• First episode genital HSV infection - primary: First acquisition of HSV causing genital herpes in pregnancy. This is usually HSV2, but not always. Maternal antibodies for HSV1 and 2 should be checked; these will be negative in primary infection.
herpes. The patient will usually have previous HSV1 infection i.e. cold sores and will have positive antibodies to HSV1, and then acquires HSV2 genital herpes infection during pregnancy.
• Recurrent HSV infection: The HSV type recovered from the genital lesion is the same type as pre-existing antibodies in the serum. Recurrence risk is greater with HSV2 than with HSV1, and the likelihood of recurrences reduces over time.3
Diagnosis:
Diagnosis is based on clinical suspicion for HSV infection, including vesicular or ulcerated lesions. HSV DNA PCR from lesions is 98-100% sensitive. Viral shedding is intermittent so negative PCR does not exclude diagnosis.
• First episode genital HSV infectionnon-primary: First acquisition of genital herpes in pregnancy, but with a background history of oral
Patients should be advised to avoid sexual contact when they have symptoms of HSV and should be offered testing for other STIs including HIV, hepatitis B, syphilis, chlamydia and gonorrhoea.

Table 1. Management options for HSV in pregnancy
Infection type Management Mode of delivery
Primary infection in trimester 1 and 2
Aciclovir 400mg TDS PO for five days. From 36 weeks gestation suppression with aciclovir 400mg TDS PO. A possible alternative antiviral agent is valaciclovir 500mg PO BD for treatment and OD for suppression.
Should offer SVD (spontaneous vaginal delivery)
Low risk to baby.
Primary infection in trimester 3
Aciclovir 400mg TDS PO for five days and continue until delivery as suppression (aciclovir 400mg TDS until delivery)
Recurrent infection Supportive management i.e. analgesia. Recurrent episodes are usually short lasting and resolve within 7 10 days without antiviral treatment.
From 36 weeks gestation consider suppression with aciclovir 400mg TDS PO until delivery
Table 1. Management options for HSV in pregnancy Labour management
CS (Caesarean section)
recommended mode of delivery, especially if symptoms within 6 weeks of delivery.
Team to inform neonatologist of any neonate born to a mother who had HSV during pregnancy.
Should offer SVD Very low risk to baby.
OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE WOMEN’S HEALTH HSV INFECTION
[4]
76
Type-specific HSV serology should be used with caution and under specialist advice.
For women presenting with first episode genital herpes in the third trimester, particularly within 6 weeks of expected delivery, type-specific HSV antibody testing (immunoglobulin G [IgG] antibodies to HSV1 and HSV2) may be helpful. For these women, characterising the infection will influence the advice given regarding mode of delivery and risk of neonatal herpes infection. The presence of antibodies of the same type as the HSV isolated from genital swabs would confirm this episode to be a recurrence rather than a primary infection and elective caesarean section would not be indicated to prevent neonatal transmission.
Management4
Management of HSV in pregnancy is divided into the treatment of infection during pregnancy, decisions regarding delivery, and management of the neonate (Table 1). Pregnant patients with a history of HSV should inform their obstetrician and a referral to GUM clinic should be considered. Genital HSV is a notifiable disease and should be reported via the HPSC (https:// www.hpsc.ie/notifiablediseases/ notifyinginfectiousdiseases/).
Aciclovir is first line antiviral therapy for managing HSV in pregnancy. Aciclovir is well tolerated and does not require clinical or laboratory monitoring. Aciclovir is appropriate to use in all trimesters of pregnancy. The use of aciclovir is associated with a reduction in the duration and severity of symptoms and a decrease in the duration of viral
Table 2. Management of labour for patients with HSV at time of labour
Mode of delivery / infection type
Born via Elective Caesarean Section (CS) with intact membranes in mothers with primary HSV in 3rd trimester
Born via SVD with primary HSV within the last 6 weeks
High risk of vertically transmitted HSV infection to baby.
Swab the skin, conjunctiva, oropharynx, rectum and send for HSV PCR.
If baby is well empiric treatment with aciclovir IV 20mg/kg TDS until infection ruled out
If baby is unwell as above PLUS perform lumbar puncture and send CSF for HSV PCR.
Born to mothers with recurrent HSV Infection risk to baby is low. Maternal IgG will be protective for baby. Swabs not indicated.
Antivirals NOT recommended.
shedding. Oral paracetamol and topical lidocaine 2% gel can be offered as symptomatic relief for patients with HSV.
Patients should be advised to avoid sexual contact when they have symptoms of HSV and should be offered testing for other STIs including HIV, hepatitis B, syphilis, chlamydia and gonorrhoea.

Primary infection at the onset of labour (including genital herpes in preterm prelabour rupture of membranes before 37+0 weeks of gestation (PPROM))
Recurrent infection at onset of labour
Table 3. Neonatal management of neonates born to mothers who have had HSV during pregnancy
References available on request.
Labour management4
Patients who have a primary infection at the onset of labour should be given IV aciclovir (Table 2).
Neonatal infection:
Neonatal HSV is rare but has been associated with high morbidity and mortality. It is defined as HSV infection in any infant ≤42 days of age. A confirmed case is a laboratory confirmed infection, a probable case is a clinically compatible illness without another known cause of infection. 50% of neonatal herpes infection is due to HSV1 and 50% due to HSV2. Neonatal infection is
classified into three subgroups depending on site of infection - disease localised to skin/eye/ mouth, local central nervous system disease (encephalitis) and disseminated infection with multi-organ involvement. Infants with disease localised to the skin/eye/mouth have the best prognosis, and represent 30% of neonatal herpes infections. Most cases of neonatal herpes occur as a result of direct contact with infected maternal secretions, and vertical transmission is most likely to occur during passage through the birth canal among women with active lesions. The highest risk for neonatal infection occurs in women with a primary genital HSV infection, which may be asymptomatic, acquired near the time of delivery. Congenital infection, i.e. transfer of infection in utero, is extremely rare. Post-natal acquisition from another person can also occur in approximately 10% of cases. Aside from timing (within 6 weeks of delivery) and type of (primary/
Table 3. Neonatal management of neonates born to mothers who have had HSV during pregnancy
Many thanks to Dr Susan Knowles, Consultant Microbiologist and Dr Niamh Lynn, Consultant GU/HIV Medicine for their input with this article.
non-primary) infection, other factors that increase the risk of neonatal transmission include the use of foetal scalp electrodes and duration of membrane rupture. Neonatal HSV became a notifiable disease in Ireland in 2018.
Neonatal care
The neonatal team should be informed of all babies born to mothers who have HSV during their pregnancy. If a baby is born via SVD to a woman who has third trimester primary genital herpes, the baby should be commenced on aciclovir 20mg/kg TDS for 10 days (Table 3) while awaiting results if well, and if baby is unwell to perform lumbar puncture. Breastfeeding is recommended unless there are herpetic lesions around the nipples.
Infection at onset of delivery Management Mode of delivery
Aciclovir IV 5mg/kg TDS intrapartum to mother.
Prophylactic steroids should be considered to reduce the implications of preterm delivery upon the infant in PPROM.
Consider treatment with acyclovir 400mg TDS PO for five days. Prophylactic acyclovir 400mg TDS PO may be indicated until delivery.
Avoid SVD if possible.
Parents and other caregivers with active lesions, regardless of site, should be careful when handling the infant: lesions should be covered and hands should be washed before touching the baby. Parents should be advised to monitor the baby and look for; skin, eye and mucous membrane lesions, lethargy/ irritability and poor feeding. If the baby develops any of these, parents should be advised to seek medical help.
Offer vaginal delivery.
Many thanks to Dr Susan Knowles, Consultant Microbiologist and Dr Niamh Lynn, Consultant GU/HIV Medicine for their input with this article.
References available on request
Table 2. Management of labour for patients with HSV at time of labour
Neonatal infection:
HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
77
Management of Endometriosis
Interview with Theresa LowryLehnen (PhD), Clinical Nurse Specialist and Associate Lecturer, South East Technological University

only in presentation but also in duration, Theresa notes adding, “Symptoms of endometriosis can begin prior to the first menstrual period, and for many it can persist into menopause and throughout their lives. It has been found in cis males, Tran’s males, pre-menarche girls and postmenopausal women. Endometriosis triggers a chronic inflammatory reaction resulting in pain and adhesions, and can have a profound effect on an individual’s quality of life. Adhesions develop when scar tissue attaches separate structures or organs together. Pain and symptoms due to endometriosis may vary during the menstrual cycle as hormone levels fluctuate.
diagnosis of endometriosis in individuals presenting with cyclical and non-cyclical signs and symptoms; dysmenorrhea, deep dyspareunia, dysuria, dyschezia, painful rectal bleeding or haematuria, shoulder tip pain, catamenial pneumothorax, cyclical cough/haemoptysis/ chest pain, cyclical scar swelling and pain, fatigue, and infertility.”
The American Society of Reproductive Medicine (ASRM) developed a staging system, to stage endometriosis and adhesions due to endometriosis.
Endometriosis is a word that 1 in 10 women hear from their gynaecologist, relating to their ongoing pain or as an explanation for their fertility problems. It is one of the most commonly seen gynaecological diseases, yet is poorly understood and not commonly talked about.
We recently spoke to Theresa Lowry Lehnen, RGN, RNP, BSc, MSc, PG. Dip. Ed (QTS), M. Ed, PhD, Clinical Nurse Specialist and Associate Lecturer South East Technological University to gain a further understanding of this common, chronic condition.
Endometriosis is a chronic gynaecological condition characterised by the presence of endometrial-like tissue in anatomical positions and organs outside of the uterine cavity.
“Establishment and growth of such endometriotic tissue is oestrogendependent,” Theresa told us.
The exact prevalence of endometriosis is unknown, but estimates range from 2 to 10% within the general female population, and up to 50% in infertile women. It is estimated that at least 190 million women and adolescent girls worldwide are currently affected by the disease during reproductive age, although some women are affected beyond menopause.
“Most endometriotic disease is located on the pelvic peritoneum including the ovaries, fallopian tubes, the bowel, and the areas in front, on the back, and to the sides of the uterus while a smaller percentage involves the ureters, bladder, urethra and the upper abdomen,” she explains.
“The condition is not limited to the pelvis, and occasionally can cause damage to extra pelvic structures such as the pleura and the pericardium, however, it rarely extends beyond the peritoneal cavity.”
Pathophysiology
Theresa adds, “The pathophysiology of endometriosis is strongly influenced by factors such as genetic predisposition and hormonal factors such as resistance to progesterone, oestrogen dependence; inflammation, angiogenesis, and vascularisation processes.
Oxidative stress, resistance to apoptosis and immunological factors are also involved to various degrees in lesion development.
Several predisposing factors have been linked with the risk of developing endometriosis. Early age at menarche (below 11 years old), shorter duration of menstrual periods of less than 27 days, menorrhagia, and nulliparity increase the risk for endometriosis, indicating that it is closely linked with the female hormonal.
“On the contrary, there are protective factors against endometriosis, mainly acting via lowering the inflammatory process, or by decreasing the levels of oestrogen in the body. Protracted breastfeeding, current oral contraceptive use, tubal ligation, and smoking are related to a decreased risk for endometriosis. Although the mechanism is still not clear, women who smoke have lower levels of oestrogen in the body.”
The clinical presentation of endometrial disease differs not
“Symptoms may be worse at certain times in the menstrual cycle, with ovulation and prior to and during menstruation being the most severe for many. While some experience severe pelvic, bladder and/or bowel pain, others may have few or no symptoms or regard the symptoms as simply period pain or cramps.”
Diagnosis
Diagnosis is often delayed on average of 4 to 11 years from the onset of symptoms,” Theresa told us. “According to the endometriosis association of Ireland, the average delay in Ireland is 9 years. This phenomenon is attributed to the non-existence of a pathognomonic test or biomarker to detect the disease; to the diversity of symptoms being considered physiologic responses during menstruation, and to the wide range of reported symptoms that overlap with other gastrointestinal or gynaecological conditions.
“The European Society of Human Reproduction and Embryology (ESHRE) Guidelines on Endometriosis were updated in 2022, and offer best practice advice on the care of women with endometriosis, including recommendations on the diagnostic approach and treatments for endometriosis for both the relief of painful symptoms and infertility due to endometriosis
“Laparoscopy is no longer the diagnostic gold standard, and is now only recommended in patients with negative imaging results and / or where empirical treatment was unsuccessful or inappropriate. The ESHRE guideline development group (GDG) recommends that clinicians should consider the
• Stage 1 and 2 (Minimal to mild disease): Superficial peritoneal endometriosis. Possible presence of small deep lesions. No endometrioma. Mild filmy adhesions, if present.4
• Stages 3 and 4 (Moderate to severe disease): The presence of superficial peritoneal endometriosis, deeply invasive endometriosis with moderate to extensive adhesions between the uterus and bowels and/ or endometrioma cysts with moderate to extensive adhesions involving the ovaries and tubes.
Theresa adds, “The classification was originally developed to predict impairment to fertility, and is therefore focused on ovarian disease and adhesions. Patients with the same ‘stage’ of disease may have different disease presentations and types, and some forms of severe disease such as invasive disease of the bowel, bladder and diaphragm are not included.
“Apart from the classification system, 3 subtypes of endometriosis can be distinguished according to localisation: superficial peritoneal endometriosis, cystic ovarian endometriosis (endometrioma or ‘chocolate cysts’) and deep endometriosis also referred to as deeply infiltrating endometriosis.
“The different types of disease may co-occur.
• Superficial peritoneal endometriosis is the most common type. Lesions involve the peritoneum, are flat, shallow, and do not invade the space underlying the peritoneum.
• Cystic ovarian endometriosis (ovarian endometrioma) occurs less commonly. The cyst is filled with old blood, and because
78 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE Endometriosis
of the colour are referred to as ‘chocolate cysts’. Most people with endometrioma cysts will also have superficial and/or deep disease present elsewhere in the pelvis.
• Deep endometriosis is the least common subtype. An endometriosis lesion is defined as deep if it has invaded at least 5mm beyond the surface of the peritoneum. Deep lesions involve tissue underlying the retroperitoneal space.”
Treatment
There is no cure for endometriosis, and treatment is broadly divided into two main categories; pharmacological and surgical, says Theresa. Currently, there is no specific drug that can inhibit the progress of the disease, other than hormonal and non-hormonal agents used to alleviate the symptoms and increase fertility rates.
“Treatment of endometriosis depends on the severity of symptoms, reproductive plans, patient’s age, medical history and side effects of both surgical and medical treatments. Regarding treatment of symptoms in postmenopausal women, the potential increased risk of underlying malignancy in this population should be keep in mind and the uncertainty of the diagnosis, as pain symptoms may present differently in this group compared to premenopausal women.
“NSAIDs or other analgesics, either alone or in combination with other treatments, can be used to reduce endometriosis-associated pain. ESHRE guidelines recommend offering women hormone treatment; combined hormonal contraceptives, progestogens, GnRH agonists or GnRH antagonists, as one of the options to reduce endometriosisassociated pain.
“Ovarian suppression can reduce disease activity and pain. Systematic review has confirmed the efficacy of combined hormonal contraceptives and continuous progestogens, including medroxyprogesterone acetate, norethisterone, cyproterone acetate, or dienogest, for pain associated with endometriosis.
Second line medical treatments include GnRH agonists and the levonorgestrel releasing intrauterine device (IUD). Danazol and the antiprogesterone gestrinone should not be used, as androgenic side effects outweigh benefits.
“Ovarian suppression with GnRH agonists improves symptoms but induces vasomotor symptoms in most women, and prolonged use of more than six months can

TREATME NTS FOOR E ND OME TRIOSIS
TREATME NTS FOR E ND OME TRIOSIS
INFERTILITY
TREATME NTS
INFERTILITY
INFERTILITY
OME TRIOSIS
URGERY
S URGERY
lead to bone demineralisation.
Prospective studies have shown that this bone loss is reversible and that concurrent treatment with a low dose oestrogen and progestogen hormone replacement therapy (HRT) regimen or tibolone can extend use without reducing treatment efficacy.
“There is limited evidence from randomised trials to show superior efficacy of one ovulation suppression treatment for pain over another, and, in clinical practice, choice of treatment is commonly guided by the tolerability of available treatments.
GnRH agonists are sometimes used to “trial” how a patient might respond to surgical menopause, but the predictive value of this approach is not known,” she adds.
“In women with endometriosisassociated pain refractory to other
medical or surgical treatment, it is recommended to prescribe aromatase inhibitors, as they reduce endometriosis-associated pain. Aromatase inhibitors may be prescribed in combination with oral contraceptives, progestogens, GnRH agonists or GnRH antagonists.
“Surgical treatment to eliminate endometriotic lesions and divide adhesions has long been an important part of the management of endometriosis. Historically, surgical approaches were achieved at open surgery, but in recent decades, laparoscopy has dominated. Elimination of endometriosis may be achieved by excision, diathermy, or ablation/ vaporisation. Division of adhesions aims to restore pelvic anatomy. Some clinicians use interruption of pelvic nerve pathways with the intention of improving pain control.
Surgery
“Surgery is recommended as one of the options to reduce endometriosis associated pain. When surgery is performed, clinicians may consider excision instead of ablation to reduce endometriosisassociated pain. Clinicians can consider hysterectomy with or without removal of the ovaries, with removal of all visible endometriosis lesions in women who no longer wish to conceive, and who failed to respond to more conservative treatments.
“Oestrogen as HRT is advised for those aged under 45 and/ or symptomatic women after oophorectomy for endometriosis, but HRT or tibolone can potentially lead to recurrence. There is, however, no indication to use combined HRT after hysterectomy for endometriosis.
H
DI F F ER EN T O PT I O N S
A p p l y sh a re d d e c i si o n m a k i n g
S PO N TAN EO U S CO N C EPT IO N MAR
EFI could be used
79 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022 Guideline Endometriosis 2022 189 EFI,EndometriosisFertilityIndex;MAR,medicallyassistedreproduction
O R M O N A L T R EAT M EN T Not recommended MA R
Guideline Endometriosis 2022 189 EFI,EndometriosisFertilityIndex;MAR,medicallyassistedreproduction
H O R MO N A L T R EAT MEN T Not recommended MA R
FOOR E ND
DIF F ER EN T O PT IO N S A p p l y sh a re d d e ci si o n m a k i n g S PONTANEOU S CONC EPT ION MAR EFI could be used S URGERY Guideline Endometriosis 2022 189 EFI,EndometriosisFertilityIndex;MAR,medicallyassistedreproduction
H O R MO N A L T R EAT MEN T Not recommended MA R
DIF F ER EN T O PT IO N S A p p l y sh a re d d e ci si o n m a k i n g S PONTANEOU S CONC EPT ION MAR EFI could be used S
CERAMENT®G SHOWING OUTSTANDING LONG-TERM RESULTS
BONESUPPORT™, an emerging leader in orthobiologics for the management of bone injuries, has announced the publication of mid- to long-term data confirming the sustained clinical effectiveness of CERAMENT G in a single-stage protocol to manage chronic osteomyelitis.
The study followed 100 patients treated at the Nuffield Orthopaedic Centre, Oxford University Hospitals. Results at a mean follow-up of 6.05 years [range: 4.2 to 8.4 years] were reported:
• 94% of patients remained infection-free
• 3% fracture rate and no further pathologic fractures beyond the first year after surgery
“These results over a long follow-up period confirm that our protocol using CERAMENT G remains highly effective over several years. There are hardly any prospective studies on bone graft substitutes with a follow-up over two years. Additionally, we were able to achieve these results in single-stage operation due to CERAMENT G’s unique properties of eluting high concentrations of local antibiotics and providing bone remodeling. Reducing the number of surgical procedures is more patient-friendly and allows for a cost-effective strategy to tackle chronic osteomyelitis,” said Professor Martin McNally, lead author and lead consultant in limb reconstruction surgery at Oxford University Hospital.
The study was published in the September issue of The Bone and Joint Journal (see reference below). The long-term results will be presented, and has been selected as one of the ten best papers, at this year’s annual meeting of the European Bone and Joint Infection Society (EBJIS) held in Graz, Austria on 8-10 September. Approximately 500 surgeons are expected to attend.
“Professor McNally’s group show long-term results that have not been displayed with any other orthobiologics product. This study will be influential in transforming the standard of care,” said Emil Billbäck, CEO of BONESUPPORT.
CERAMENT G is implanted to promote bone healing in bone voids or defects and simultaneously to protect it from infection. More than 70,000 patients have been treated thus
far using the CERAMENT bone healing technology.
Reference
McNally, M, et al., ‘Mid- to Long-Term Results of SingleStage Surgery for Patients with Chronic Osteomyelitis Using a Bioabsorbable GentamicinLoaded Ceramic Carrier’, The Bone & Joint Journal, 104-B.9 (2022), 1095–1100 <https://doi. org/10.1302/0301-620X.104B9. BJJ-2022-0396.R1>
DANTE GENOMICS TO INNOVATE UNIVERSAL GENOMICS
Dante Genomics, a global leader in genomics and precision medicine, announced today that the Company has begun using needleless, athome blood collection kits for its premium clinical whole genome sequencing test, thus bringing the high quality of concierge clinics to at home testing.
Dante Genomics is utilizing these at-home blood collection kits for its whole genome sequencing on the www.dantelabs.com website, making it easier than ever and virtually painless for patients to collect their blood sample on their own without having to travel to a hospital or have direct interaction with a mobile phlebotomist.
“This groundbreaking innovation comes from our relentless focus on providing universal access to the highest quality genomics technologies,” said Andrea Riposati, CEO of Dante Genomics. “Anyone, anywhere in the world, deserves to receive the most advanced genomic care. Anyone. Anywhere.”
The collection of blood samples for the use of genomics sequencing has key advantages over the collection of saliva:
• Saliva-derived DNA contains significantly less DNA than blood-derived DNA.
• There is far less risk of DNA contamination due to user error.
• Blood samples have a higher percentage of passing Quality Control standards, avoiding the need for the customer to supply an additional sample to be retested.
• As a result, blood collection lowers turnaround times significantly for the customer.
With this innovation, individuals anywhere in the world get access to the highest quality of genomic testing and now also the best options for sample collection.
Dante has deployed two products for blood collection:
• For the US market, an FDA registered device that collects whole liquid blood samples that are ready to be used for genomic DNA isolation and sequencing. Users can successfully collect blood samples at home without direct interaction at a lab or with a mobile phlebotomist.
• For the European market, a CE-IVD marked device delivers whole dried blood samples from the patient to the lab. Subjects can successfully collect volumetrically precise samples.
For our DNA wellness programs and other specific applications, Dante will continue to offer its saliva collection kit as an option.
IRISH WHEELCHAIR ASSOCIATION RUN ‘N’ ROLL

In excess of 380 participants competed in the family fun run (Sunday, 4 September) in Saint Annes Park, Raheny, Dublin 5. The event was designed to support and promote a more inclusive society for people with disabilities. Participants included disability advocates Joanne O’Riordan and Jack Kavanagh.
Liberty is committed to greater equity, diversity and inclusion within society, and central to this commitment is greater support and allyship with people with functional disabilities. Due to Liberty’s support, 100% of race registration fees went to the IWA. The event raised over ¤17,000 for the IWA including an additional donation of ¤10,000 from Liberty.
REBOUND WEIGHT GAIN IN CHILDREN WITH OBESITY LINKED TO DISCONNECT BETWEEN BRAIN AND GUT
Children with obesity, who have recently lost weight, are more likely to show hunger-related activity in their brains after a meal, according to research presented at the 60th Annual European Society for Paediatric Endocrinology Meeting. This brain activity, reflecting that they were unsatisfied by their meal, happens even though their gut hormone levels have changed, as expected, to reduce hunger and indicate fullness. This disconnect between food satisfaction in their brain versus their digestive system may underlie why many people regain weight, particularly after a strict diet. Understanding and addressing this persistence of hunger-promoting brain activity could lead to better and more sustainable treatments for obesity in children and adults.
Obesity is a growing worldwide health crisis with an estimated 124 million children affected globally. Obesity increases the risk of many other health problems including type 2 diabetes, heart disease and cancer. Obesity in children is often managed through family-based behavioural therapy involving regular outpatient sessions that focus on dietary and physical activity education.
Jack Kavanagh, Disability Advocate and Pharmacist
In the USA, the gold standard for such programmes is a minimum of 26 contact hours over a 6 month period, however, many children regain weight soon after programme completion. It is poorly understood why the success rate is so low. Appetite and metabolism, and therefore weight gain, is regulated by activity in both the brain and the digestive system. Understanding how these processes are affected by weight loss may help us better understand the mechanisms that
80 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE Clinical R&D
predispose children to rebound weight gain.
In this study, Professor Roth and colleagues at Seattle Children’s Hospital in the USA, compared brain appetite regulation activity with gut hormone responses in children with obesity before and after a 24-week weight loss programme. Using functional MRI, they assessed activation patterns in appetite-regulating brain areas in response to high- vs. low-calorie images, after a meal. Gut hormone levels were also assessed before and after meals, at the beginning and end of the programme. At the end of the programme, children still showed high levels of activation in brain areas related to appetite, after a meal, in response to food images, indicating that they were hungry. However, their levels of appetite-regulating gut hormones indicated fullness and satiety. Strikingly, the children who lost the most weight, showed the strongest activation in their brains to food cues after a meal, at the end of the programme.
Professor Roth comments, “Our results imply that during weight loss intervention, your body acts to conserve fat through maintaining hunger responses in the brain, and that this needs to be addressed, perhaps through drug treatment, for successful and sustained weight loss in children with obesity.”
Although Professor Roth cautions, “These findings are from a small group of children tested only at the start and end of the intervention programme, so larger and more detailed studies would be needed to confirm this central effect. It would also be useful to investigate how long the disconnect between central and local appetite regulation persists after maintained weight loss, to guide intervention plans.”
Professor Roth suggests, “For more successful treatment of obesity in children, we should avoid interventions that lead to fast body weight reductions and instead aim for more gradual and consistent lifestyle changes, over years rather than months, which will lead to sustained and longterm improvements in weight loss and health.”
IMMUNOCORE PRESENTS
PROMISING INITIAL PHASE
1 DATA FOR FIRST OFFTHE-SHELF TCR THERAPY TARGETING PRAME AT THE ESMO 2022 CONGRESS
Immunocore Holdings plc (Nasdaq: IMCR), a commercialstage biotechnology company pioneering the development of a novel class of T cell receptor
(TCR) bispecific immunotherapies designed to treat a broad range of diseases, including cancer, autoimmune and infectious diseases, has released initial Phase 1 data for the first off-theshelf ImmTAC® targeting PRAME, demonstrating that IMC-F106C is well tolerated and resulted in durable responses across multiple solid tumor types.
The initial data from the ongoing Phase 1 dose escalation trial of IMC-F106C is the subject of a presentation today at 4:40 PM CEST/10:40 AM EDT, in the Investigational Immunotherapy Proffered Paper session at the European Society for Medical Oncology (ESMO) Congress. The presentation can be accessed in the ‘News & Events’ section of the Investor Relations section of the Company’s website.
“The durable responses in heavily pre-treated patients show that our PRAME-targeted bispecific therapy, IMC-F106C, can deliver meaningful benefits to patients across a range of cancer types,” said Bahija Jallal, Chief Executive Officer of Immunocore. “Based on this promising data, we have initiated expansion arms in multiple tumor types to further assess the efficacy.”
Initial Phase 1 Clinical Data
As of 18 July 2022, 55 patients have been treated across 10 dose cohorts. IMC-F106C was welltolerated, with treatment-related adverse events (AEs) that were manageable and consistent with the mechanism of action. The most frequent treatment-related AE reported was cytokine release syndrome (CRS), which was mostly Grade 1 (none were Grade ≥3) and occurred predominantly during the initial three doses. None of the related AEs led to treatment discontinuation or patient death.
Dr. Omid Hamid, Chief, Translational Research and Immunotherapy, Co-Director, Melanoma Therapeutics at Cedars-Sinai Cancer at the Angeles Clinic and Research Institute, said: “ImmTAC therapies are designed to provide potent and target-specific T-cell response, overcoming resistance in immune excluded tumors. Through redirection and activation of nontumor-specific T cells, as shown in this trial with IMC-F106C, we can influence a diverse range of tumors leading to durable response. This trial shows tolerability and activity in a wide range of tumors, including checkpoint inhibitor pretreated patients. I look forward to
upcoming cohorts in combination with checkpoint inhibitors and chemotherapy.”
Doses of ≥ 20 mcg were clinically active and had consistent and robust interferon gamma induction, a specific marker of T cell activation. Most of the patients in these active dose cohorts were enrolled without prospective PRAME testing. In these patients, PRAME expression was analyzed retrospectively; the vast majority were positive, and the average expression was high (median H score 188).
In the clinically active dose cohorts, durable partial responses (PR) were observed in 2/6 patients with cutaneous melanoma, 2/4 with ovarian cancer and 3/6 with tebentafusp-naïve uveal melanoma (UM) (0/5 response in patients with UM who had progressed on prior tebentafusp). All ovarian patients were platinum-resistant, and all cutaneous melanoma patients had progressed on prior anti-PD1 and anti-CTLA4. Six of the seven PRs are still ongoing, including two for over seven months. Ten additional efficacy evaluable patients across four other tumor types had a best RECIST response of stable disease or progressive disease. A majority of patients evaluable for circulating tumor DNA (ctDNA) had at least a 50% reduction.
PORTIUNCULA UNIVERSITY HOSPITAL LAUNCH ‘SEO IS MISE - THIS IS ME’
Portiuncula University Hospital (PUH) with support from the National Dementia Office launched ‘Seo Is Mise - This is Me’ in the hospital recently.
‘Seo Is Mise – This is Me’ is a quality improvement initiative developed by the Occupational Therapy and Medical Social Work Departments in PUH. It includes a Dementia Resource Pack which

has been designed to empower patients and families to make effective decisions and choices regarding their future care. The four medical and surgical wards at the hospital will also have access to a Dementia Activity Box suited to individuals requiring stimulation with therapeutic intervention.
Siobhan Coen, Occupational Therapist at Portiuncula University Hospital said, “Approximately one in three patients aged 70 and older admitted to Irish hospitals have dementia and this figure will increase annually as the population ages. People living with dementia are among the most vulnerable patients in hospital. They are often older, frail, and very prone to delirium, falls, pressure ulcers, and malnutrition which are key safety issues in hospitals.
“Included in the information pack is the ‘Seo Is Mise – This is Me’ booklet containing information for service users and their families on the availability of support services in Ballinasloe and surrounding areas. It also includes the ‘Think Ahead’ form developed by the Irish Hospice Foundation which encourages service users and their families to discuss and record their wishes in regards to all aspects of their future care.”
Another part of the initiative is the introduction of a Dementia Activity Box to enhance the patient’s experience at the hospital.
From left: Ciara Mulkerns, Medical Social Worker; Rosaleen Leonard, Clinical Nurse Specialist Older Persons; Caroline McInerney Layng, Principal Medical Social Worker; James Keane, General Manager; Siobhan Coen, Occupational Therapist; Bryan Dixon, Medical Social Worker; Roisin McHugh, Medical Social Worker; and Mary Mahon, Director of Nursing
81 HOSPITALPROFESSIONALNEWS.IE | HPN • OCTOBER 2022
ASTELLAS’ EVRENZO™ (ROXADUSTAT) TO BE REIMBURSED UNDER THE HIGH TECH DRUGS SCHEME IN IRELAND FOR ADULTS WITH SYMPTOMATIC ANAEMIA ASSOCIATED WITH CHRONIC KIDNEY DISEASE (CKD)
Astellas Pharma Company Ltd. (part of the Astellas Pharma Inc group TSE: 4503, President and CEO: Kenji Yasukawa, Ph.D., “Astellas”) is pleased to announce that EVRENZO™ (roxadustat) has been granted reimbursement, in line with its marketing authorisation under the High Tech Drugs Scheme in Ireland from 1st September, 2022, as an option for treating adult patients with symptomatic anaemia associated with CKD.
Roxadustat is the first and only HIF-PH inhibitor licensed and reimbursed for use in Ireland. The licence was based on results from a comprehensive pivotal Phase 3 programme comprising of eight multicentre and randomised studies, which involved 9,600 patients worldwide.6-11 The results of this programme support roxadustat as efficacious in achieving and maintaining target haemoglobin (Hb) levels (10–12 g/ dL) in patients with symptomatic anaemia of CKD regardless of dialysis status and irrespective of prior erythropoiesis-stimulating agent (ESA) treatment.6-10
The safety profile observed in the roxadustat development programme is reflective of the CKD populations studied and comparable to ESAs.6-11
‘This is an excellent demonstration of how a ground-breaking, Nobel Prize winning, scientific discovery can inform and pave the way for innovative medicines that can benefit patients.’ said Dr. Timir Patel, Medical Director, Astellas UK and Ireland. ‘Through its novel mechanism of action, roxadustat offers physicians an orally administered option to tackle anaemia of CKD, a complex and multi-faceted disease that can be hugely debilitating.’
Roxadustat activates the body’s natural response to reduced oxygen levels in the blood via inhibition of the HIF-PH pathway. The response involves the regulation of multiple, coordinated processes that improve iron absorption and mobilisation, and increase red cell production, and can therefore assist in the management of anaemia of CKD.
According to The WHO, around 20% of people living with diagnosed CKD suffer from
anaemia.3,4 Anaemia of CKD symptoms include dizziness, fatigue and headaches, and can be associated with significant impairment in quality of life.12-15 Additionally, patients with CKD that also suffer from anaemia have impaired health-related quality of life compared to patients without the added complication of anaemia.5
‘Chronic kidney disease patients suffer frequently from anaemia and this complication of CKD has greater impact as CKD worsens. Anaemia significantly impacts patient’s quality of life and vitality’, said Prof. Donal Reddan, Consultant Nephrologist, Galway University Hospital.
‘This licence enhances patient care by providing a novel alternative option for managing anaemia and improving patient’s quality of life’.
PATIENT GROUP CALLS FOR EXPANDED ACCESS TO MEDICAL CANNABIS ON ‘NATIONAL DAY OF ACTION’
A leading patient advocacy group has called for a major overhaul of Ireland’s cannabis laws on the day that campaigners rally outside the Department of Health.
The Patients For Safe Access (PFSA) group said little progress has been made since the Medical Cannabis Access Programme (MCAP) was introduced here, and they insisted that more action is needed immediately.
PFSA organised a large-scale event outside the Department of Health’s offices in Dublin where speakers called for changes to be made to expand access to medical cannabis.
Speakers at the event included Martin O’Brien and Alicia Maher of PFSA, Deputy Gino Kenny, Peter Reynolds of the Cannabis Industry Council and the activist Martin Condon.
Martin O’Brien, the PFSA director, said that until major changes are made to Ireland’s drugs, barriers to access would continue to inflict real suffering on patients, some of whom are emigrating to escape needless constraints.
“Many patients and those who care about this issue hoped that the limited MCAP reforms would represent a new dawn.
Unfortunately, three years on, it has become abundantly clear that the existing law is not fit for purpose.
“People with a range of serious medical conditions are not able to access cannabis legally. In spite of the evidence showing the benefits
of cannabis in treating the chronic pain which so many people suffer from, the Government has slammed the door shut.
“Under MCAP, patients have to exhaust several other medical avenues before being allowed onto it, but we firmly believe that the Programme should be open to any patient with a condition that can be treated with medical cannabis,” O’Brien said.
LATE-BREAKING DUPIXENT® (DUPILUMAB) DATA
Results from a Phase 3 open-label extension trial demonstrated the efficacy and safety profile of Dupixent® (dupilumab) as a maintenance therapy when added to other asthma medications was consistent for up to two years in children aged 6 to 11 years with uncontrolled moderate-to-severe asthma with evidence of type 2 inflammation. These results were presented today in a late-breaking session at the 2022 European Respiratory Society (ERS) International Congress, which coincides with the milestone that more than 500,000 people around the world have been treated with Dupixent in its approved indications.
The results are from data in children who entered the extension trial after finishing active treatment or placebo in the Phase 3 trial (pivotal trial). Children in the extension trial were treated for up to an additional year with Dupixent, providing up to two years of data in total. Children treated with Dupixent in the extension trial experienced a:
• Low rate of severe asthma attacks with an average of 0.118-0.124 events per year, compared to 2.16-2.56 events per year at baseline in the pivotal trial.
• Sustained improvement in lung function at 52 weeks of 9.43-12.6 percentage points from baseline in the pivotal trial, measured by percent predicted FEV1 (FEV1pp). FEV1pp seeks to evaluate a patient's change in lung function compared to their predicted lung function based on age, height, sex and ethnicity to account for children's growing lung capacity at different stages of development.
o Children who switched from placebo in the pivotal trial to Dupixent in the extension trial demonstrated improvement of 8.71 percentage points in lung function at two weeks.
Accord Healthcare is delighted to announce the launch of another medicine to their already extensive high-tech portfolio: Abiraterone Accord 500 mg film-coated tablets which comes in a pack of 56 tablets.
Abiraterone Accord is indicated with prednisone or prednisolone for:
• the treatment of newly diagnosed high risk metastatic hormone sensitive prostate cancer (mHSPC) in adult men in combination with androgen deprivation therapy (ADT).
• the treatment of metastatic castration resistant prostate cancer (mCRPC) in adult men who are asymptomatic or mildly symptomatic after failure of androgen deprivation therapy in whom chemotherapy is not yet clinically indicated.
• the treatment of mCRPC in adult men whose disease has progressed on or after a docetaxel-based chemotherapy regimen.
Please refer to the Summary of Product Characteristics (SPC) for further information. The SPC will be available from the launch date at www.hpra.ie and for Healthcare Professionals at www.accord-healthcare.ie.
Abiraterone Accord 250 mg filmcoated tablets will be available from both full line wholesalers from launch. For further information please contact Accord in Cork on 021 461 9040 or visit www.accord-healthcare.ie
Think High-Techs, Think Accord
 ACCORD HEALTHCARE LAUNCH ABIRATERONE ACCORD 500MG FILM-COATED TABLETS
ACCORD HEALTHCARE LAUNCH ABIRATERONE ACCORD 500MG FILM-COATED TABLETS
82 OCTOBER 2022 • HPN | HOSPITALPROFESSIONALNEWS.IE Clinical R&D
In the FLAURA Study,
showed GROUNDBREAKING EFFICACY
1st-generation EGFR TKIs* in the FLAURA study (HR: 0.799; 95.05% CI, 0.641 to 0.997; P=0.0462)1

IN THE FLAURA STUDY
Progression-free survival was the primary endpoint. Overall survival was a prespecified secondary endpoint.2
TAGRISSO AS MONOTHERAPY IS INDICATED FOR:
• the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.† • the first-line treatment of adult patients with locally advanced or metastatic NSCLC with activating EGFR mutations.
• the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC.
Adapted from: TAGRISSO. Summary of Product Characteristics. *gefitinib and erlotinib Reference:
TAGRISSO® (osimertinib).
TAGRISSOTM (osimertinib) 40mg and 80mg FILM-COATED TABLETS. Consult Summary of Product Characteristics (SmPC) before prescribing. Indication: TAGRISSO as monotherapy is indicated for: the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations; the first-line treatment of adult patients with locally advanced or metastatic non-small-cell lung cancer (NSCLC) with activating EGFR mutations and the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC. Presentation: 40mg and 80mg osimertinib (as mesylate) film-coated tablets. Dosage and Administration: Treatment should be initiated by a physician experienced in the use of anticancer therapies. When considering the use of TAGRISSO, EGFR mutation status (in tumour specimens for adjuvant treatment and tumour or plasma specimens for locally advanced or metastatic setting) should be determined using a validated test method. The recommended dose is 80mg once a day, taken with or without food at the same time each day. Patients in the adjuvant setting should receive treatment until disease recurrence or unacceptable toxicity. Treatment duration for more than 3 years was not studied. Patients with locally advanced or metastatic lung cancer should receive treatment until disease progression or unacceptable toxicity. If a dose is missed, the dose should be made up unless the next dose is due within 12 hours. Should be swallowed whole with water and it should not be crushed, split or chewed. If patients are unable to swallow, the tablet may be dispersed in water (non-carbonated). No other liquids should be used. The dispersion can also be administered through a nasogastric tube. Refer to SmPC for full instructions. Dose adjustments: If required to manage safety and tolerability, then dose should be reduced to 40mg taken once daily. Special populations: Hepatic impairment: No dose adjustments are necessary in patients with mild hepatic impairment (Child Pugh A) (total bilirubin ≤upper limit of normal (ULN) and aspartate aminotransferase (AST) >ULN or total bilirubin >1.0 to 1.5x ULN and any AST) or moderate hepatic impairment (Child Pugh B) (total bilirubin between 1.5 to 3 times ULN and any AST). Use in patients with severe hepatic impairment is not recommended. Renal impairment: No dose adjustments are necessary in patients with mild, moderate, or severe renal impairment. Caution should be exercised when treating patients with severe and end stage renal impairment. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Should not be used with St. John’s Wort. Warnings and Precautions: Interstitial Lung Disease (ILD): Patients with acute onset and/or unexplained worsening of pulmonary symptoms (dyspnoea, cough, fever) should have their treatment interrupted while investigations are performed to exclude ILD. If ILD is diagnosed, TAGRISSO should be discontinued and appropriate treatment initiated as necessary. Reintroduction should be considered only after careful consideration of the individual patient’s benefits and risk. Stevens-Johnson syndrome (SJS): Before initiating treatment, patients should be advised of signs and symptoms of SJS. If suggestive, TAGRISSO should be interrupted or discontinued immediately. QTc interval prolongation: May lead to an increased risk for ventricular tachyarrhythmias (e.g. torsade de pointes) or sudden death. When possible, TAGRISSO should be avoided in patients with congenital long QT syndrome. Periodic monitoring with electrocardiograms and electrolytes should be considered in patients with congestive heart failure, electrolyte abnormalities, or those who are taking medicinal products that are known to prolong the QTc interval. Treatment should be withheld in patients who develop a QTc interval greater than 500 msec on at least 2 separate ECGs until the QTc interval is less than 481 msec or recovery to baseline if the QTc interval is greater than or equal to
481 msec, then resume TAGRISSO at a reduced dose. TAGRISSO should be permanently discontinued in patients who develop QTc interval prolongation in combination with any of the following: torsade de pointes, polymorphic ventricular tachycardia, signs/symptoms of serious arrhythmia. Changes in cardiac contractility: In patients with cardiac risk factors and those with conditions that can affect LVEF, cardiac monitoring, including an assessment of LVEF at baseline and during treatment, should be considered. In patients who develop relevant cardiac signs/symptoms during treatment, cardiac monitoring including LVEF assessment should be considered. Keratitis: Patients with signs and symptoms suggestive of keratitis such as acute or worsening: eye inflammation, lacrimation, light sensitivity, blurred vision, eye pain and/or red eye should be referred promptly to an ophthalmology specialist. Age and body weight: Elderly patients (>65 years) or patients with low body weight (<50 kg) may be at increased risk of developing adverse events of Grade 3 or higher. Close monitoring is recommended. Drug Interactions: Concomitant use of strong CYP3A inducers (e.g. phenytoin, rifampicin and carbamazepine) should be avoided. Moderate CYP3A4 inducers (e.g. bosentan, efavirenz, etravirine, modafinil) should be used with caution, or avoided when possible. Closely monitor patients taking concomitant medicinal products with disposition dependent upon breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) and with narrow therapeutic index. Concomitant use of St. John’s Wort is contraindicated. Pregnancy and Lactation: TAGRISSO should not be used during pregnancy unless the clinical condition of the woman requires treatment with TAGRISSO. Discontinue breast-feeding during treatment with TAGRISSO. Women of childbearing potential should be advised to avoid becoming pregnant if they or their partner are being treated with TAGRISSO. Undesirable Events: Consult SmPC for full list of adverse events. Very common (all grades): Decreased appetite, diarrhoea, stomatitis, rash, dry skin, paronychia, pruritus, platelet count decreased, leucocytes decreased, lymphocytes decreased, neutrophils decreased. Common (all grades): Epistaxis, interstitial lung disease, alopecia, urticaria, palmar-plantar erythrodysaesthesia syndrome, blood creatine phosphokinase increased, blood creatinine increased. Uncommon (all grades): Keratitis, QTc interval prolongation, erythema multiforme, cutaneous vasculitis. Rare (all grades): Stevens-Johnson syndrome, myositis. Legal Category: Product subject to prescription which may not be renewed (A). Marketing Authorisation Number: EU/1/16/1086/001 and EU/1/16/1086/002. Marketing Authorisation Holder: AstraZeneca AB, SE-151 85, Södertälje,
+353 1 609 7100.
from: AstraZeneca
North,
AstraZeneca.
reserved. Date of Preparation:
Veeva ID: IE-3780
©2022
All rights
April 2022 |
1.
Summary of Product Characteristics. 2022 38.6 vs 31.8 months median OS vs.
FIRST-LINE TAGRISSOTM
delivering statistically significant and clinically meaningful OS; †This indication is not currently reimbursed in Ireland.
Sweden. Further information available on request
Pharmaceuticals (Ireland) DAC, College Business and Technology Park, Blanchardstown Road
Dublin 15. Tel:
TAGRISSO is a trade mark of the AstraZeneca group of companies. Date of API Preparation: 03/2022 Veeva ID: IE-3782 Adverse events should be reported directly to: HPRA Pharmacovigilance. Website: www.hpra.ie. Adverse events should also be reported to AstraZeneca Patient Safety on Freephone 1800 800 899. ABRIDGED PRESCRIBING INFORMATION
Significant skin clearance at week 12, with sustained control at week 481,5



Rapid itch relief, superior to dupilumab + TCS at week 2 for CIBINQO 200 mg + TCS, with significant results as early as day 41,6


A once-daily pill available in multiple doses that can be used with or without medicated topical therapies so you can tailor treatment to meet the individual needs of your patients1-3,7,8
CONSISTENT SAFETY PROFILE: Rigorously studied in >3100 patients across 7 clinical trials, including one ongoing LTE 9


PRESCRIBING INFORMATION CIBINQO® ▼ (ABROCITINIB)












Please refer to full Summary of Product Characteristics (SmPC) before prescribing Cibinqo®.

Presentation: Film-coated tablets containing 50 mg, 100 mg, or 200 mg abrocitinib. Each tablet contains 1.37 mg, 2.73 mg, and 5.46 mg of lactose monohydrate, respectively.

Indications: For the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy. Dosage: Treatment should be initiated and supervised by a healthcare professional experienced in the diagnosis and treatment of atopic dermatitis. Posology: The recommended starting dose is 200 mg once daily. A starting dose of 100 mg once daily is recommended for patients ≥ 65 years of age. For other patients who may benefit from a starting dose of 100 mg, see sections 4.4 and 4.8 of the SmPC. During treatment, the dose may be decreased or increased based on tolerability and e cacy. The lowest e ective dose for maintenance should be considered. The maximum daily dose is 200 mg. Can be used with or without medicated topical therapies for atopic dermatitis. Discontinuation of treatment should be considered in patients who show no evidence of therapeutic benefit after 24 weeks. Treatment initiation: Treatment should not be initiated in patients with a platelet count < 150 × 103/mm3, an absolute lymphocyte count (ALC) < 0.5 × 103/mm3, an absolute neutrophil count (ANC) < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Dose interruption: If a patient develops a serious infection, sepsis or opportunistic infection, dose interruption should be considered until the infection is controlled. Interruption of dosing may be needed for management of laboratory abnormalities. Missed doses: If a dose is missed, patients should be advised to take the dose as soon as possible unless it is less than 12 hours before the next dose, in which case the patient should not take the missed dose. Thereafter, dosing should be resumed at the regular scheduled time. Interactions: In patients receiving dual strong inhibitors of cytochrome CYP2C19 and moderate inhibitors of CYP2C9, or strong inhibitors of CYP2C19 alone (e.g., fluvoxamine, fluconazole, fluoxetine and ticlopidine), the recommended starting dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. Treatment is not recommended concomitantly with moderate or strong inducers of CYP2C19/CYP2C9 enzymes (e.g., rifampicin, apalutamide, efavirenz, enzalutamide, phenytoin). Renal impairment: No dose adjustment is required in patients with mild renal impairment, i.e., estimated glomerular filtration rate (eGFR) of 60 to < 90 mL/min. In patients with moderate (eGFR 30 to < 60 mL/min) renal impairment, the recommended dose of Cibinqo should be reduced by half to 100 mg or 50 mg once daily. In patients with severe (eGFR < 30 mL/min) renal impairment, 50 mg once daily is the recommended starting dose. The maximum daily dose is 100 mg. Cibinqo has not been studied in patients with end-stage renal disease (ESRD) on renal replacement therapy. Hepatic impairment: No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. Cibinqo must not be used in patients with severe (Child Pugh C) hepatic impairment. Elderly: The recommended starting dose for patients aged 65 years or more 100 mg once daily. Paediatric population: The safety and e cacy of Cibinqo in children under 12 years of age have not yet been established. No data are available. Cibinqo has been studied in adolescents 12 to < 18 years of age. However, because of bone findings in juvenile rats (comparable to a 3 month old human), additional long-term data in growing adolescents is needed to conclude that the benefits outweigh the risks. Method of administration: This medicinal product is to be taken orally once daily with or without food at approximately the same time each day. In patients who experience nausea, taking Cibinqo with food may improve nausea. Tablets should be swallowed whole with water and should not be split, crushed, or chewed because these methods have not been studied in clinical trials. Contra-indications: Hypersensitivity to the active substance or to any of the excipients. Active serious systemic infections, including tuberculosis (TB), severe hepatic impairment, pregnancy, and breast-feeding. Warnings and Precautions: Serious infections: Serious infections have been reported in patients receiving Cibinqo. The most frequent serious infections in clinical studies were herpes simplex, herpes zoster and pneumonia. Treatment must not be initiated in patients with an active, serious systemic infection. Risks and benefits of treatment prior to initiating Cibinqo should be considered. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with abrocitinib. A patient who develops a new infection during treatment should undergo prompt and complete diagnostic testing and appropriate antimicrobial therapy should be initiated. The patient should be closely monitored, and therapy should be temporarily interrupted if the patient is not responding to standard therapy. Tuberculosis: Patients should be screened for TB before starting treatment and yearly screening for patients in highly endemic areas for TB should be considered. Abrocitinib must not be given to patients with active TB. For patients with a new diagnosis of latent TB or prior untreated latent TB, preventive therapy for latent TB should be started prior to initiation of treatment. Viral reactivation: Viral reactivation, including herpes virus reactivation (e.g., herpes zoster, herpes simplex), was reported in clinical studies. The rate of herpes zoster infections was higher in patients 65 years of age and older and patients with severe atopic dermatitis at baseline. If a patient develops herpes zoster, temporary interruption of treatment should be considered until the episode resolves. Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting therapy and during therapy. Patients with evidence of active hepatitis B or hepatitis C (positive hepatitis C PCR) infection were excluded from clinical studies. Patients who were hepatitis B surface antigen negative, hepatitis B core antibody positive, and hepatitis B surface antibody positive had testing for hepatitis B virus (HBV) DNA. Patients who had HBV DNA above the lower limit of quantification (LLQ) were excluded. Patients who had HBV DNA negative or below LLQ could initiate treatment; such patients had HBV DNA monitored. If HBV DNA is detected, a liver specialist should be consulted. Vaccination: No data are available on the response to vaccination in patients receiving Cibinqo. Use of live, attenuated vaccines should be avoided during or immediately prior to treatment. Prior to initiating treatment with this medicinal product, it is recommended that patients be brought up to date with all immunisations, including prophylactic herpes zoster vaccinations, in agreement with current immunisation guidelines. Thrombotic events including pulmonary embolism: Events of deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving abrocitinib. Cibinqo should be used with caution in patients at high risk for DVT/PE. Risk factors that should be considered in determining the patient’s risk for DVT/PE include older age, obesity, a medical history of DVT/PE, prothrombotic disorder, use of combined hormonal contraceptives or hormone replacement therapy, patients undergoing major surgery or prolonged immobilisation. If clinical features of DVT/PE occur, treatment should be discontinued and patients should be evaluated promptly, followed by appropriate treatment. Malignancy (including non-melanoma skin cancers): Malignancies, including non-melanoma skin cancer (NMSC), were observed in clinical
studies with abrocitinib. Clinical data are insu cient to assess the potential relationship of exposure to abrocitinib and the development of malignancies. Long-term safety evaluations are ongoing. The risks and benefits of Cibinqo treatment should be considered prior to initiating in patients with a known malignancy other than a successfully treated NMSC or cervical cancer in situ or when considering continuing Cibinqo therapy in patients who develop a malignancy. Periodic skin examination is recommended for patients who are at increased risk for skin cancer. Haematologic abnormalities: Confirmed ALC < 0.5 × 103/mm3 and platelet count < 50 × 103/mm3 were observed in less than 0.5% of patients in clinical studies. Treatment with abrocitinib should not be initiated in patients with a platelet count < 150 × 103/mm3, an ALC < 0.5 × 103/mm3, an ANC < 1.2 × 103/mm3 or who have a haemoglobin value < 10 g/dL. Complete blood count should be monitored 4 weeks after initiation of therapy and thereafter according to routine patient management. Lipids: Dose dependent increases in blood lipid parameters were reported in patients treated with abrocitinib compared to placebo. Lipid parameters should be assessed approximately 4 weeks following initiation of therapy and thereafter according to the patient’s risk for cardiovascular disease. The e ect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined. Patients with abnormal lipid parameters should be further monitored and managed according to clinical guidelines, due to the known cardiovascular risks associated with hyperlipidaemia. In patients with a high burden of cardiovascular risk factors, the risks, and benefits of abrocitinib compared to that of other available therapies for atopic dermatitis should be considered. If abrocitinib is chosen, interventions to manage lipid concentrations should be implemented according to clinical guidelines. Elderly: The safety profile observed in elderly patients was similar to that of the adult population with the following exceptions: a higher proportion of patients 65 years of age and older discontinued from clinical studies and were more likely to have serious adverse reactions compared to younger patients; patients 65 years and older were more likely to develop low platelet and ALC values; the incidence rate of herpes zoster in patients 65 years of age and older was higher than that of younger patients. There are limited data in patients above 75 years of age. Immunosuppressive conditions or medicinal products: Patients with immunodeficiency disorders or a first-degree relative with a hereditary immunodeficiency were excluded from clinical studies and no information on these patients is available. Combination with biologic immunomodulators, potent immunosuppressants such as ciclosporin or other Janus kinase (JAK) inhibitors has not been studied. Their concomitant use with abrocitinib is not recommended as a risk of additive immunosuppression cannot be excluded. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Drug Interactions: Potential for other medicines to a ect pharmacokinetics of abrocitinib: Abrocitinib is metabolised predominantly by CYP2C19 and CYP2C9 enzymes, and to a lesser extent by CYP3A4 and CYP2B6 enzymes, and its active metabolites are renally excreted and are substrates of the organic anion transporter 3 (OAT3). Therefore, exposures of abrocitinib and/or its active metabolites may be a ected by medicinal products that strongly inhibit or induce theses enzymes and transporter. Dose adjustments, as appropriate, may be required. Co-administration with products which increase gastric pH: The e ect of elevating gastric pH with antacids, H2-receptor antagonists (famotidine), or proton pump inhibitors (omeprazole) on the pharmacokinetics of abrocitinib has not been studied and may reduce the absorption of abrocitinib due to the low solubility of abrocitinib at pH above 4. Potential for Cibinqo to a ect pharmacokinetics of other medicinal products: No clinically significant e ects of Cibinqo were observed in drug interaction studies with oral contraceptives. Caution should be exercised for concomitant use of abrocitinib with dabigatran. Caution should be exercised as the levels of P-gp substrates with a narrow therapeutic index, such as digoxin, may increase. The exposures of medicinal products metabolised by CYP2B6 (e.g., bupropion, efavirenz) and CYP1A2 (e.g., alosetron, duloxetine, ramelteon, tizanidine) may be decreased and of those metabolised by CYP2C19 (e.g., S mephenytoin) may be increased initially and then decreased, when used concomitantly with abrocitinib. Fertility, pregnancy, and lactation: Women of childbearing potential: Women of reproductive potential should be advised to use e ective contraception during treatment and for 1 month following the final dose of Cibinqo. Pregnancy planning and prevention for females of reproductive potential should be encouraged. Pregnancy: There are no or limited amount of data on the use of abrocitinib in pregnant women. Studies in animals have shown reproductive toxicity. Abrocitinib has been shown to cause embryo-foetal lethality in pregnant rats and rabbits, skeletal variations in the foetuses of pregnant rats and rabbits and to a ect parturition and peri/postnatal development in rats. Cibinqo is contraindicated during pregnancy. Breast-feeding: There are no data on the presence of abrocitinib in human milk, the e ects on the breast fed infant, or the e ects on milk production. Abrocitinib was secreted in milk of lactating rats. A risk to newborns/infants cannot be excluded and Cibinqo is contraindicated during breast feeding. Fertility: Based on the findings in rats, oral administration of Cibinqo may result in temporary reduced fertility in females of reproductive potential. The e ects on female rat fertility were reversible 1 month after cessation of abrocitinib oral administration. Driving and operating machinery: Cibinqo has no e ect on the ability to drive or use machines. Side E ects: The most commonly reported adverse reactions are nausea (15.1%), headache (7.9%), acne (4.8%), herpes simplex (4.2%), blood creatine phosphokinase increased (3.8%), vomiting (3.5%), dizziness (3.4%) and abdominal pain upper (2.2%). The most frequent serious adverse reactions are infections (0.3%) including herpes simplex, herpes zoster, and pneumonia. Refer to SmPC
EU/1/21/1593/002 - Cibinqo 50 mg (28 film-coated tablets), EU/1/21/1593/007
regarding
J Med. 2021;384(12):1101-1112.
2. Simpson EL, Sinclair R, Forman S, et al. Lancet. 2020;396(10246):255-266.
3. Silverberg JI, Simpson EL, Thyssen JP, et al. JAMA Dermatol 2020;156(8):863-873.
4. Boeri M, Sutphin J, Hauber B, et al. J Dermatolog Treat. 2020 Nov 2:1-10. doi:10.1080/09546634.1832185.





5. Reich K, Silverberg JI, Papp K, et al. Presented at the Revolutionizing Atopic Dermatitis Virtual Conference; 13 June 2021. 6. Ständer S, Yosipovitch G, Simpson EL, et al. Presented at the American Academy of Dermatology Virtual Meeting Experience 2021; 23-25 April 2021.
Cibinqo Summary of Product Characteristics.
Cork MJ, Deleuran MS, Geng B, et al. Presented at the European Academy of Dermatology and Venereology Virtual Congress 2021; 29 September-2 October 2021.




8. Blauvelt A, Silverberg JI, Lynde CW, et al. J Am Acad Dermatol. Published online 17 August 2021. doi: 10.1016/j.jaad.2021.05.075.












Although some clinical trials included adolescents, please note that CIBINQO is indicated for the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy.

TCS includes low- to medium-potency topical corticosteroids, topical calcineurin inhibitors, or topical phosphodiesterase 4 inhibitors, per protocol guidance in JADE COMPARE. Nonmedicated topicals were also required.1 AD=atopic dermatitis; JAK=Janus kinase; LTE=long-term extension.
July 2022. PP-CIB-IRL-0035

CIBINQO is a convenient once-daily oral JAK1 inhibitor for moderate-to-severe atopic dermatitis (AD) that o ers1-4
Whether your patients’ moderate-to-severe AD is FIERCE or TAMER,
for further information on side e ects. Legal Category: S1A. Marketing Authorisation Numbers:
- Cibinqo 100 mg (28 film-coated tablets); EU/1/21/1593/012 - Cibinqo 200 mg (28 film-coated tablets). Marketing Authorisation Holder: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium. For further information on this medicine please contact Pfizer Medical Information on 1800 633 363 or at medical.information@pfizer.com. For queries
product availability please contact: Pfizer Healthcare Ireland, Pfizer Building 9, Riverwalk, National Digital Park, Citywest Business Campus, Dublin 24 + 353 1 467 6500. ▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 of the SmPC for how to report adverse reactions. Last revised: December 2021. Ref: CQ 1_0 IE. References: 1. Bieber T, Simpson EL, Silverberg JI, et al. N Engl
7.
9.
© 2022 Pfizer Inc. All rights reserved.































