
Nutramedic &Cosmetics B2B No.10 / MAY/JUNE 2024 Digestive Health in Focus / Probiotics and Osteoporosis / Exploring HPMC Vcaps® and DRcaps® Capsules Advantages / Vitafoods Europe 2024 Preview / Understanding Neuro-cosmetics

Welcome to the No.10 April/May edition of B2B Nutramedic&Cosmetics Digital Magazine. In this issue, we delve into crucial topics shaping the nutraceutical and cosmetics industries.
We shine a spotlight on the upcoming Vitafoods Europe 2024, offering a preview of the latest trends and innovations set to redefine the landscape of our industry.
Our exploration extends to the realm of digestive health, uncovering ingredients tailored for dietary supplements and the versatile benefits of Sulfodyne®. Additionally, we examine the intersection of probiotics and osteoporosis, providing insights into advancements in bone health research. Revolutionary advancements in pharmaceutical and nutraceutical delivery are also on the agenda, with a focus on the advantages of HPMC Vcaps® and DRcaps® capsules.
Understanding the importance of consumer safety, we offer guidance on recognizing authentic cosmetic products amidst the proliferation of counterfeit labels. Join us as we navigate through these critical topics, seeking to inform and inspire innovation in the dynamic world of nutraceuticals and cosmetics. Explore the wealth of industry-specific content and resources awaiting you on our website portal https://nmc-magazine.com/
Best regards,

Daria Šurić, MPharm
EDITOR-IN-CHIEF
B2B Nutramedic&Cosmetics Magazine

Bimonthly digital magazine for industry professionals in health, nutrition and cosmetics sector
Bimonthly digital magazine for industry professionals in health, nutrition and cosmetics sector
Ingredients and raw materials / Contract manufacturing Equipment & Packaging / Services / Industry events
Ingredients and raw materials / Contract manufacturing Equipment & Packaging / Services / Industry events www.nmc-magazine.com
info@nmc-magazine.com
info@nmc-magazine.com
Publisher: Darmell d.o.o.
Publisher: Darmell Ltd.

Cvjetna cesta 11, 10000 Zagreb, Hrvatska Mob: + 385 91 68 12 444 darmell@protonmail.com www.dar-mell.com
Cvjetna cesta 11, 10000 Zagreb, Croatia Mob: + 385 91 68 12 444 darmell@protonmail.com www.dar-mell.com
Supported by www.inpharma.hr
The publisher does not assume responsibility for the opinions and data that the authors present in the magazine, as well as for the data and materials provided by companies for publication in texts and advertisements. It is not allowed to reuse any part of the content without the prior consent of the publisher.
provided by companies for publication in texts and advertisements. It is not allowed to reuse any part of the content without the prior consent of the publisher.

2 4 Digestive Health in Focus: Exploring Ingredients for Dietary Supplements 10 Exploring the Gut Impact of Overall Wellbeing 12 Kemin Granted Novel Food Authorization for BetaVia™ Pure in the EU 13 Darmell - Expert in the Nutritional Supplements Field 14 Sulfodyne®: an Ingredient With a Wide Range of Health Benefits 18
Know Today? 24 Oat-standing Nutrition, Oat-rageously Delicious! Nutramedic &Cosmetics The
seed extract
of
Detox Joints Women’s health Immunity ingoodbyolga.com Come Hall Pavillon Editor's word
Probiotics and Osteoporosis - What Do We
only broccoli
with the active and natural
sulforaphane
The publisher does not assume responsibility for the opinions and data that the authors present in the magazine, as well as for the data and materials
Contents
























3 Nutramedic &Cosmetics https://nmc-magazine.com/the-magazine/ 25 BGG Creates New Global Base in Switzerland 25 Review on Omega-3 Diagnostics Testing Published 26 Fine Foods: Growth With Sustainability 28 Revolutionising Pharmaceutical and Nutraceutical Delivery: Exploring HPMC Vcaps® and DRcaps® Capsules Advantages 33 Vitafoods Europe 2024 Preview 44 Understanding Neurocosmetics: an Overview 46 How to Recognize Fake or Incorrect Cosmetic Product Labels? 48 MEDICINAL PLANTS PHOTO HERBARIUM: Shepherd's Purse 49 Xylishine™ C - the Ingredient for Beautiful Hair 49 B2B Events Calendar 50 Arla Foods Ingredients and New Beverage Concept Access the archive containing all published issues of the B2B Nutramedic&Cosmetic magazine.
Digestive Health in Focus: Exploring Ingredients for Dietary Supplements
Natural ingredients offer diverse mechanisms to support digestive health, from reducing inflammation to modulating gut microbiota. Incorporating these ingredients into dietary supplements presents a promising avenue for individuals seeking to optimize their well-being and vitality through nurturing their digestive system.
AUTHOR: Amanda Gaši, MSc Nutrition
The digestive system plays a paramount role in maintaining our overall health and well-being, serving as the gateway for nutrient absorption and waste elimination. Its efficient functioning is indispensable for proper digestion, absorption of essential nutrients, and the body's ability to ward off infections. However, due to various factors such as poor dietary habits, stress, and lifestyle choices, digestive issues have become increasingly prevalent. Furthermore, some of digestive disorders exacerbate their symptoms depending on season, such as the exacerbation of chronic conditions like irritable bowel syndrome or inflammatory bowel disease in spring. In this context, supplements tailored to support digestive health have garnered significant attention. These supplements aim to optimize the digestive process, alleviate discomfort, and promote gut microbiome balance. Understanding the pivotal role of the digestive system and the benefits of supplements in enhancing its function is crucial for maintaining optimal health and vitality.
In the continuation of this article, we will introduce several potential ingredients for dietary supplements designed for digestive health and explain in more detail how they can help.
Menthapiperita
Peppermint, scientifically known as Mentha piperita , is a popular herb renowned for its refreshing flavor and various health benefits. It is a hybrid mint, a cross between watermint and spearmint, and it is native to Europe and the Middle East but is now widely cultivated worldwide. Mentha encompasses a range of compounds, notably peppermint essential oil (PEO), alongside non-essential elements like steroids, flavonoids, triterpenoids, phenolic acids, and more. PEO primarily comprises menthol, menthone, neomenthol, and iso-menthone, which are bioactive substances exhibiting diverse properties such as anti-inflammatory, antibacterial, antiviral, antitumor, immunomodulatory, scolicidal, neuroprotective, anti-fatigue, and antioxidant effects.1
Menthapiperita is predominantly used to address issues in the upper digestive tract, including indi-
gestion, cramps and nausea. Various studies indicate that PEO effectively combats gastrointestinal disorders by improving stomach and intestinal motility, as well as reducing feelings of fullness and functional dyspepsia. 2,3,4,5 It is suggested PEO can alleviate gastrointestinal spasms and abdominal pain. PEO achieves this by acting as a blocker for smooth muscle calcium channels. Essentially, PEO may reduce the contraction of gastrointestinal smooth muscle by inhibiting the entry of calcium through specific channels within the muscle cells.6 Furthermore, PEO was shown to be a safe and effective therapy for pain and global symptoms (diarrhea, constipation, bloating, gas) in adults with irritable bowel syndrome.7
Chamomile
Chamomile (Matricaria chamomilla) is one of the oldest and most widely used medicinal plants in the world whose standardized tea and herbal extracts are recommended for a variety of healing applications. Chamomile has different classes of bioactive components. The plant contains 0,24-1,9 % of volatile oil, composed of variety of separate oils. Furthermore, approximately 120 secondary metabolites have been identified in chamomile, including 28 terpenoids and 36 flavonoids.8
Traditionally, chamomile has been valued as a digestive relaxant and has been used to treat various gastrointestinal conditions, including indigestion, spasms, diarrhea, flatulence, motion sickness, nausea and vomiting.8 Studies with test animals showed that chamomile extract exhibited therapeutic gastrointestinal effects on diarrhea and gastric ulcer.9,10 Furthermore, chamomile showed a gastroprotective effect against alcohol-induced ulcer injury in rat gastric mucosa.11 One study12 showed that chamomile extract produced a significant dose-dependent protection against castor oil-induced diarrhea and intestinal fluid accumulation. Castor oil diarrhea is accompanied by an oxidative stress status assessed by an increase of malondialdehyde level and depletion of antioxidant enzyme activities as superoxide dismutase, catalase and glutathione peroxidase.
4 Nutramedic &Cosmetics
Chamomile pre-treatment abrogated all these biochemical alterations. It is believed that chamomile has potent antidiarrheal and antioxidant properties.
Mastiha
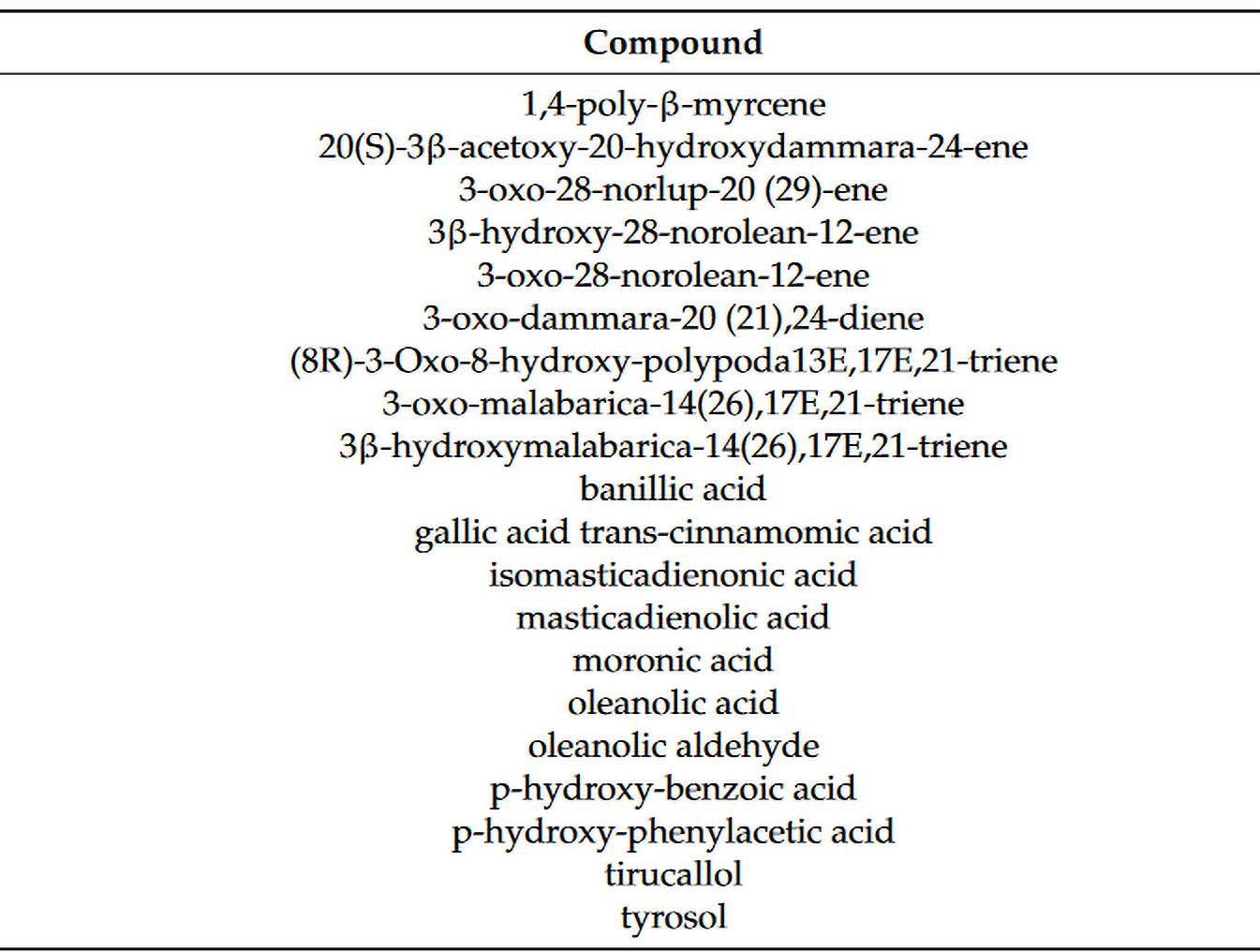
Mastiha is a natural, aromatic resin obtained from the trunk and brunches of the mastic tree (Pistacia lentiscus L. var latifolius Coss or Pistacia lentiscus var. Chia). Chios mastiha is exclusively harvested in the southern region of Chios, a picturesque Greek island nestled in the northern Aegean Sea. Its medicinal benefits have been revered since ancient times in Greece. Chios mastiha is a potent and natural reservoir of terpenes, phenolic compounds, phytosterols, arabino-galactan proteins, and organic polymers. Additionally, it contains volatile and aromatic components, showcasing a diverse array of bioactive properties, including anti-inflammatory and antioxidant properties13 (view figure 1).
Ancient Greek physicians Hippocrates, Dioscorides, and Galen recommend using Chios mastic for treating various gastrointestinal disorders such as abdominal pain, dyspepsia, gastritis, and peptic ulcer. Traditional usage of mastic is also confirmed by studies.
Due to its antibacterial properties, mastic gum can help in eradication of Helicobacter pylori , which is recognized as one of the main factors for peptic ulcer disease, gastritis and gastric cancer, with its treatment being of crucial importance for the management and prevention of prevalent digestive disorders.13 Randomized pilot study concluded that low doses and high doses of mastic gum have bactericidal activity on Helicobacter pylori (eradication of
30,8% for low dose and 38,5% for high dose of mastic gum).14 These findings are important because nowadays there has been increasing emergence of antibiotic resistance, highlighting the need to find alternative solutions. Also, it seems that mastic gum can be beneficial for patients with inflammatory bowel disease. In a pilot study, 2,8 g of mastiha a day significantly decreased in faecal lysozyme compared to patients on placebo, indicating lower disease activity. Furthermore, there was a significant improvement in Inflammatory Bowel Disease Questionnaire scores reflecting a beneficial effect on patients’ quality of life.15
In a clinical trial involving 10 individuals diagnosed with mild or moderately active Crohn's disease, participants were treated with mastic capsules (2.2 g/ day) for a duration of 4 weeks. The study revealed a notable reduction in the disease activity index and plasma levels of interleukin-6 and CRP compared to initial measurements.16
Ginger
Ginger (Zingiber officinale) is a rhizomatous plant belonging to the ginger family. Its name derives from the Sanskrit word "sihgabera," which means horn-shaped. Originating from Southeast Asia, it has been known for thousands of years in other parts of the world, not only for its fine and warm aroma but also for its medicinal properties. This medicinal exotic spice still has very high demand and is highly esteemed in Ayurvedic medicine. It is used in kitchens throughout Asia, having a fine aromatic, refreshing, and slightly spicy taste, and is best known as a remedy for nausea.
Adapted from: Soulaidopoulos,S.,Tsiogka,A.,Chrysohoou,C.,Lazarou,E.,Aznaouridis,K.,Doundoulakis,I.,Tyrovola,D.,Tousoulis,D., Tsioufis,K.,Vlachopoulos,C.,Lazaros,G.(2022).OverviewofChiosMasticGum(Pistacialentiscus)EffectsonHumanHealth.Nutrients. 14(3). 590
5 Nutramedic &Cosmetics
FIGURE 1 Compounds of Chios mastic

The constituents of ginger are numerous (there are over 100 constituents) and vary depending on the place of origin and whether the rhizomes are fresh or dry. The odor of ginger depends mainly on its volatile oil, the yield of which varies from 1% to 3%. Over 50 components of the oil have been characterized and these are mainly monoterpenoids [b-phellandrene, (+)-camphene, cineole, geraniol, curcumene, citral, terpineol, borneol] and sesquiterpenoids [a-zingiberene (30–70%), b-sesquiphellandrene (15–20%), b-bisabolene (10–15%), (E-E)-a-farnesene, ar-curcumene, zingiberol]. The pungency of fresh ginger is due primarily to the gingerols, which are a homologous series of phenols. The most abundant is [6]-gingerol. The pungency of dry ginger mainly results from shogaols, which are dehydrated forms of gingerols.17
For ages, ginger has been widely employed in Southeast Asia for its medicinal and culinary properties. It plays a pivotal role in numerous traditional medicinal practices, including Chinese, Ayurvedic, Unani, Tibetan, Sri Lankan, Korean, Arabic, Greek, Roman, and various folk medicine systems across the globe. Ginger has been essential in treating digestive issues, serving as a carminative and aiding digestion. It's also been utilized to alleviate nausea and vomiting, motion sickness, stomach discomfort, ulcers, bacterial dysentery, and dyspepsia. Mechanisms of action include scavenging free radicals, preventing oxidative damage to lipids, anti-inflammatory effect, modifying detoxifying enzymes, modulating muscarinic and 5HT receptors and enhancing gastric motility.18
Numerous studies confirm traditional usage. Oral ginger improved gastroduodenal motility in the fasting state and after a standard test meal in 12 healthy volunteers (they consumed 2 x 100 mg of ginger rhizome extract).19 A meta-analysis of 6 studies concluded that 1 g of ginger per day can improve nausea during pregnancy when given at a dose of <1000 mg/day for at least 4 days. 20 Furthermore, systematic review of 7 studies concluded that ginger extract (doses of 0,5 to 1,5 g/ day) can reduce acute chemotherapy induced nausea. 21 Ginger can also help with dysphagia. Hirata and colleagues conducted a study to assess the effectiveness of orally disintegrating tablets containing ginger, prepared by combining excipients with equal amounts of mannitol and sucrose to create a 1% ginger concentration, in improving swallowing function among eighteen healthy older adults aged 63–90. The findings revealed that 15 minutes after consuming the ginger orally disintegrating tablets, there was a notable increase in salivary substance P levels compared to pre-ingestion or after taking the placebo, approaching levels observed in healthy young adults. Additionally, there were no instances of aspiration, and a significant enhancement in swallowing function scores was observed. 22
Turmeric
Turmeric, a perennial herb belonging to the ginger family, has garnered significant attention from both the scientific and culinary communities. While its medicinal benefits, stemming from compounds like curcumin, have been recognized for millennia, recent research has delved into uncovering its precise
mechanisms of action and identifying its active constituents. Curcumin, the primary polyphenol in turmeric rhizomes, has been studied for its antioxidant, anti-inflammatory, antimicrobial, and anticancer properties, making turmeric a longstanding fixture in traditional medicine across Asian cultures. 23
It has been suggested that turmeric extract or curcumin as a potent antioxidant and natural anti-inflammatory agent may reduce the severity of IBS by relieving the IBS-associated symptoms. A study involving animals indicated that curcumin could potentially alleviate symptoms of irritable bowel syndrome by modulating neurotransmitters like serotonin, brain-derived neurotrophic factor, and cAMP response element-binding protein signaling pathways in both the brain and the intestinal system. Another study conducted on albino rats demonstrated that curcumin reduces intestinal motility, thus suggesting its therapeutic potential in various gastrointestinal disorders such as abdominal pain and irritable bowel disease. 24,25,26
A pilot study also reported that turmeric might drop the IBS symptoms by 60%. 27 Because of its limited bioavailability, inefficient absorption in the intestines, and quick elimination from the body, it is recommended to combine curcumin with enhancing agents such as other antioxidants or herbal products to potentially amplify its health benefits. Studies suggest that a blend of curcumin with substances like fish oil, peppermint oil, caraway oil, thiamine, folic acid, and vitamin D3 has been effective in alleviating various symptoms of irritable bowel system, particularly abdominal pain and discomfort. 28 Furthermore, turmeric can help in battling Helicobacter pylori infection. A study showed that turmeric extract increased IL-4 and decreased IFN-γ levels, serum gastrin levels, lipid peroxide abundance in gastric mucosal tissue and myeloperoxidase activity in gastric mucosal tissue. Also, turmeric extract lowered Helicobacterpylori counts and increased levels of anti-Helicobacter pylori IgG. In this study, there were 5 samples, but sample from Sabinsa was the most active in all of the test performed. 29
Garlic
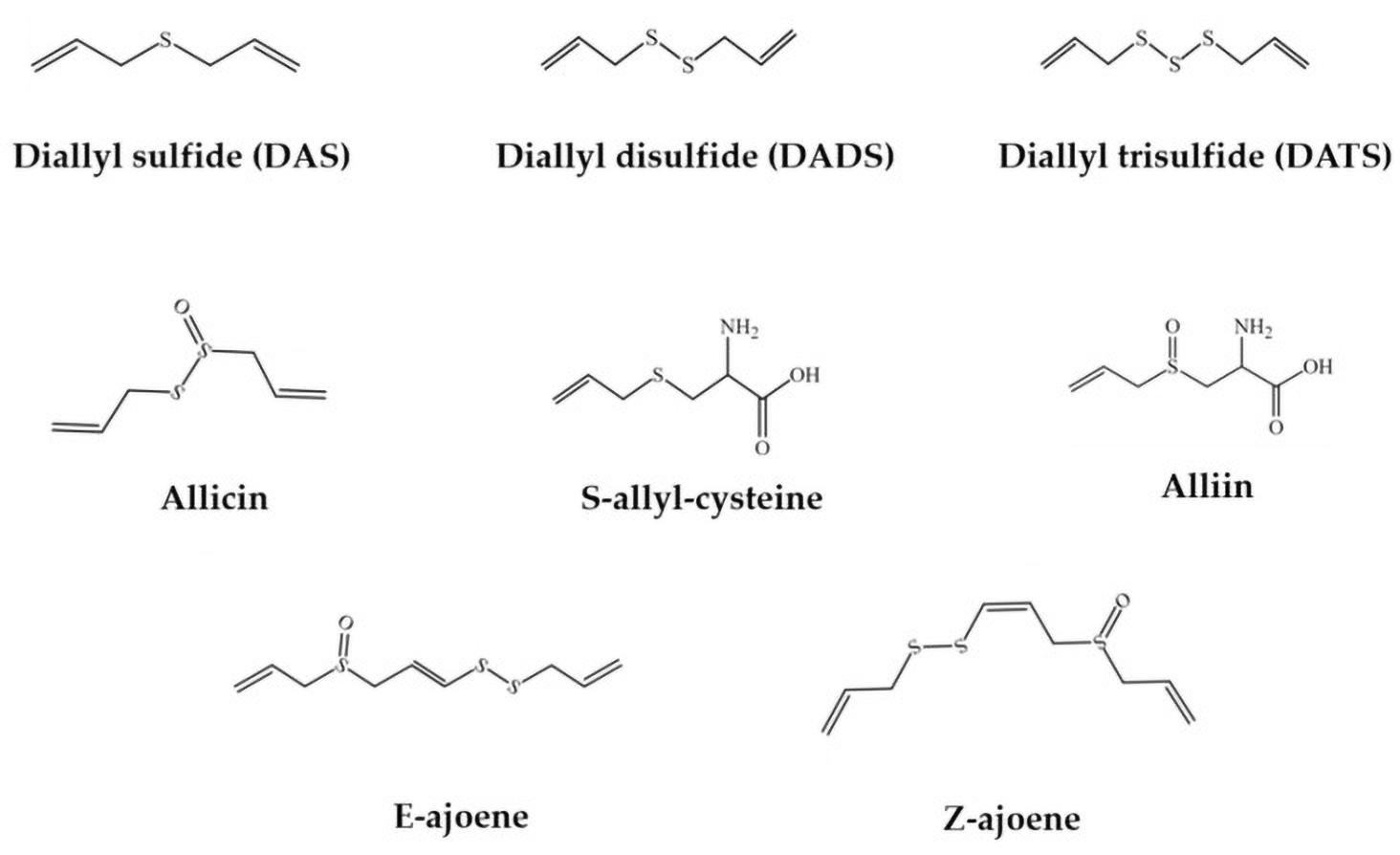
Garlic (Allium sativum), a humble yet potent member of the onion family, has a rich history deeply intertwined with human civilization for thousands of years. With its pungent aroma and distinctive flavor, garlic has been cherished not only for its culinary uses but also for its medicinal properties and cultural significance. Believed to be native to Central Asia, garlic has spread throughout the world, becoming a staple ingredient in various cuisines. Its cultivation dates back to ancient times, with evidence of garlic being used by the Egyptians, Greeks, Romans, and Chinese civilizations. Garlic comprises a diverse range of bioactive compounds, including organosulfur compounds, saponins, phenolic compounds, and polysaccharides. The primary active constituents of garlic are its organosulfur compounds, which include allicin, diallyl sulfide, diallyl disulfide, diallyl trisulfide, E/Z-ajoene, S-allyl-cysteine and alliin (view figure 2). Purple garlic contains nearly 40 times more saponins than white garlic, with unique saponin compounds exclusive to purple garlic. Garlic is rich in over 20 phenolic compounds, surpassing the
6
Nutramedic &Cosmetics
levels found in many common vegetables. The predominant phenolic compound in garlic is β-resorcylic acid, followed by pyrogallol, gallic acid, rutin, protocatechuic acid, and quercetin. 30
Garlic has many biological functions, including antioxidant, anti-inflammatory, antimicrobial, anti-hypertensive and anti-hyperlipidemic activity, as well as immunomodulatory activity. It is believed that some of these mechanisms support digestive health. 30
A review article from Wang and colleagues concluded that four main organic sulfides in garlic (diallyl disulfide, diallyl trisulfide, S-allylmercaptocysteine and allicin) may contribute to the regulation of tumor cell apoptosis, migration and the cell cycle. They identified the association between garlic intake and reduced risk of gastric and colorectal cancers and hypothesized that the active ingredients in garlic may act on multiple pathways to reduce the risk of gastrointestinal tumors. 31 Furthermore, study on animal models showed that aged garlic extract could have gastroprotective role in indomethacin induced gastric ulcer. Aged garlic extract corrected the histopathological abnormalities in gastric tissue and proved a promising gastroprotective role in gastric ulcer. Proposed mechanism of action for achieving that effect were decreasing of oxidative stress and increasing gastric level of prostaglandin E2, glutathione and nitric oxide. 32
Lactobacillus reuteri (L. reuteri)
The Lactobacillus genus encompasses a diverse group of Gram-positive, nonsporulating, facultative anaerobic bacteria, including species like L. acidophilus,L.reuteri,L.casei,L.bulgaricus, and L. rhamnosus. L. reuteri , isolated in 1962, is one of the most studied probiotics, commonly found in various mammalian tissues, offering numerous health bene-
fits such as antimicrobial production and immune system regulation. It has been identified in different body sites like breast milk, skin, urinary tract, and gastrointestinal tract. Different strains of L. reuteri have shown unique effects on conditions ranging from hypercholesterolemia to autism spectrum disorders and particularly in gastrointestinal diseases. Research suggests a close interplay between the symbiotic gut microbiota and the host immune system in maintaining gut balance with disruptions in gut microbes strongly implicated in digestive disorders. Various strains of L. reuteri have been explored for their potential in digestive and liver diseases, with promising results in conditions like inflammatory bowel disease, colorectal cancer and irritable bowel syndrome, among others. 33
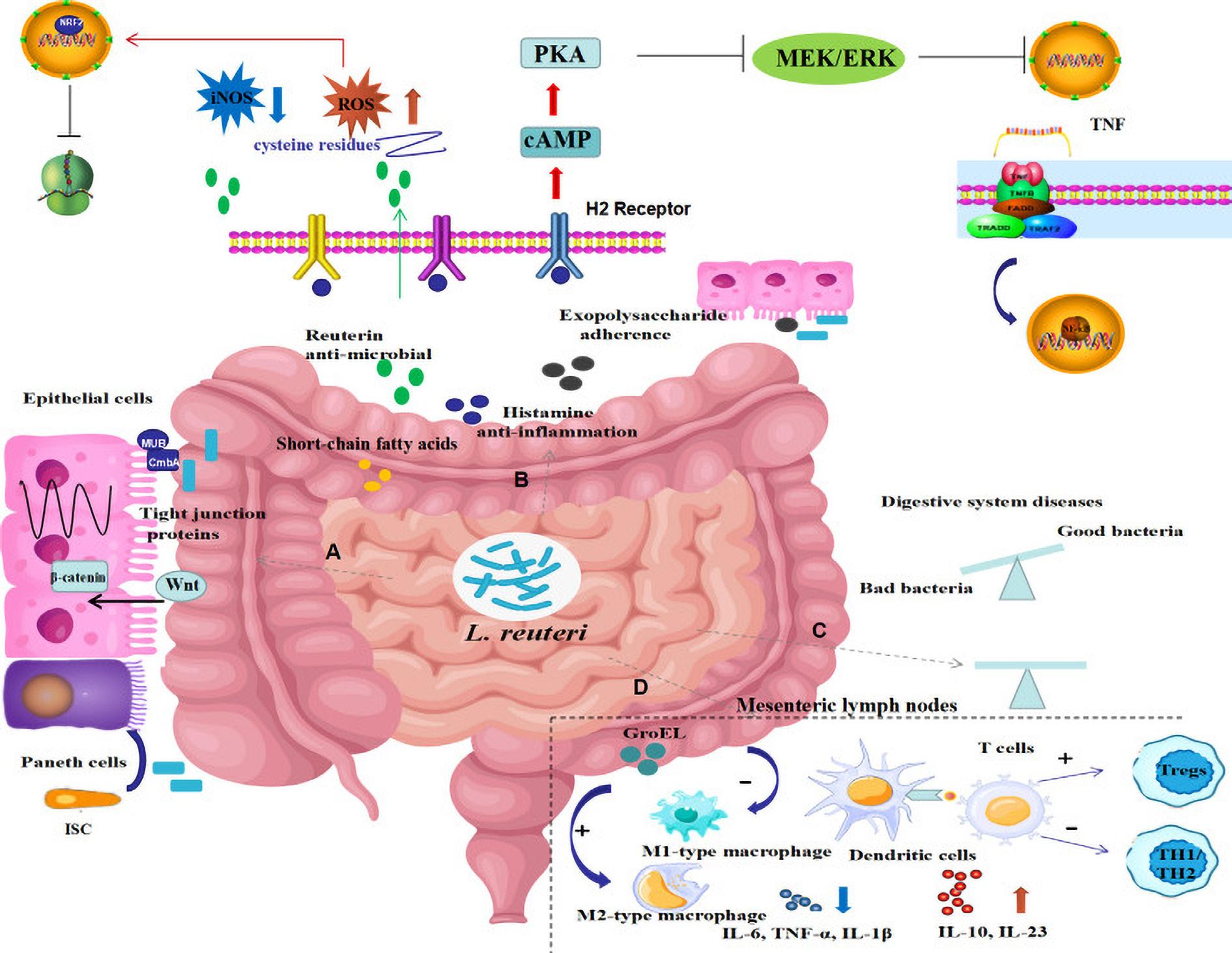
Possible mechanism of actions includes restoring gut microbiota balance, production of antimicrobial metabolites, regulation of intestinal immunity and mediation of mucosal homeostasis, thereby exhibiting protective effects on digestive system diseases (view figure 3).
Shornikova and colleagues were first to investigate the efficacy of L. reuteri DSM 17938 in pediatric acute watery diarrhea. They found that this strain could effectively reduce the duration of acute watery diarrhea in a dosage-dependent manner. 34 Since then, numerous studies demonstrated that L. reuteri DSM 17938 was capable of reducing the frequency, length and incidence of diarrhea in children and adults, especially in those with lower nutritional status.
33 Also, L. reuteri DSM 17938 can help in defecation, improved abdominal discomfort, pain and bloating in adult with functional constipation. 35 Furthermore, Research indicates that L. reuteri has the potential to decrease the load of Helicobacter pylori bacteria. However, it does not appear to significantly enhance the success rates of eradicating He-
7
Nutramedic &Cosmetics
Adapted from: Shang,A.,Cao,S.Y.,Xu,X.Y.,Gan,R.Y.,Tang,G.Y.,Corke,H.,Mavumengwana,V.,Li,H.B.(2019).BioactiveCompounds andBiologicalFunctionsofGarlic(AlliumsativumL.).Foods.8(7).246.
FIGURE 2 The chemical structures of the main organosulfur compounds in garlic
Nutramedic &Cosmetics
licobacter pylori when used alongside first-line therapy. Interestingly, various studies have highlighted that L. reuteri can alleviate symptoms such as abdominal pain, distension, and adverse events associated with eradication treatment for Helicobacterpylori 36,37,38,39
In 2013, for the first time, spray-dried cells of L. reuteri DSMZ17648 have been used in a human study and results showed reduction of Helicobacterpylori This is of special interest as spray-drying results in dead cell material, meaning that the effect of L. reuteri must be independent of its probiotic activity.40
Conclusion
In summary, natural ingredients like peppermint, chamomile, mastiha, ginger, turmeric, garlic and probiotics like Lactobacillus reuteri offer diverse mech-
anisms to support digestive health, from reducing inflammation to modulating gut microbiota. Incorporating these ingredients, along with some others like psyllium husk, fennel, glutamine, B complex vitamins and vitamin D into dietary supplements presents a promising avenue for individuals seeking to optimize their well-being and vitality through nurturing their digestive system. Embracing the synergy between traditional wisdom and modern science, we can embark on a journey towards better digestive health and overall wellness.
References:
1 Zhao, H., Ren, S., Yang, H., Tang, S., Guo, C., Liu, M., Tao, Q., Ming, T., Xu, H. (2022). Peppermint essential oil: its phytochemistry, biological activity, pharmacological effect and application. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 154, 113559.
FIGURE 3 The potential mechanisms through which L. reuteri may exert its effects on digestive system diseases
(A) Enhancement of gut barrier function by upregulating the expression of tight junction proteins, promoting the proliferation of intestinal epithelial cells, and inducing the differentiation of intestinal stem cells into Paneth cells via activation of the Wnt/β-catenin pathway; (B) Production of various metabolites such as reuterin, histamine, exopolysaccharides, and short-chain fatty acids, leading to antibacterial, anti-inflammatory, and antioxidant effects by inhibiting NF-κB signaling pathways and activating NRF2 signaling pathways; (C) Modulation of the gut microbiota composition to restore balance; (D) Regulation of the intestinal immune response by influencing macrophage phenotype selection, promoting dendritic cell differentiation, suppressing Th1/Th2 responses, and inducing regulatory T cell proliferation, ultimately leading to suppression of inflammatory responses. (ISC - intestine epithelial cell; ROS - reactive oxygen species; iNOS - inducible nitric oxide synthase; cAMP - cyclic adenosine monophosphate; PKA - Protein kinase A; TNF - tumor necrosis factor; NRF2 - nuclear factor erythroid-derived 2; H2 receptor - histamine 2 receptor; IL - interleukin; Tregs - regulatory T cells
8
Adapted from: Peng, Y., Ma, Y., Luo, Z., Jiang, Y., Xu, Z., Yu, R. (2023). Lactobacillus reuteri in digestive system diseases: focus on clinical trials and mechanisms. Frontiersincellularandinfectionmicrobiology.13.1254198.
2 Zhao, H., Ming, T., Tang, S., Ren, S., Yang, H., Liu, M., Tao, Q., Xu, H. (2022). Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Molecular cancer. 21(1). 144.
3 Grundmann, O., Yoon, S. L., Mason, S., Smith, K. (2018). Gastrointestinal symptom improvement from fiber, STW 5, peppermint oil, and probiotics use-Results from an online survey. Complementary therapies in medicine. 41. 225–230.
4 Kuchnia, A.J., Conlon, B., Greenberg, N. (2018). Natural Bioactive Food Components for Improving Enteral Tube Feeding Tolerance in Adult Patient Populations. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 33(1). 107–120.
5 Acker, B.W., Cash, B.D. (2017). Medicinal Foods for Functional GI Disorders. Current gastroenterology reports. 19(12). 62.
6 Amato, A., Liotta, R., Mulè, F. (2014). Effects of menthol on circular smooth muscle of human colon: analysis of the mechanism of action. European journal of pharmacology, 740, 295–301.
7 Alammar, N., Wang, L., Saberi, B., Nanavati, J., Holtmann, G., Shinohara, R.T., Mullin, G.E. (2019). The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC complementary and alternative medicine. 19(1). 21
8 Srivastava, J.K., Shankar, E., Gupta, S. (2010). Chamomile: A herbal medicine of the past with bright future. Molecular medicine reports. 3(6). 895–901
9 Morshedi, M., Gol, A., Mohammadzadeh, A. (2016) The effect of Matricaria chamomilla on the treatment of ibuprofen-induced gastric ulcers in male rats. Hormozgan Medical Journal. 20. 270–275.
10 Sebai, H., Jabri, M.A., Souli, A., Rtibi, K., Selmi, S., Tebourbi, O., El-Benna, J., Sakly, M. (2014) Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. Journal of Ethnopharmacology. 152. 327–332.
11 Jabri, M.A., Aissani, N., Tounsi, H., Sakly, M., Marzouki, L., Sebai, H. (2017). Protective effect of chamomile (Matricaria recutita L.) decoction extract against alcohol-induced injury in rat gastric mucosa. Pathophysiology : the official journal of the International Society for Pathophysiology. 24(1). 1–8.
12 Sebai, H., Jabri, M.A., Souli, A., Rtibi, K., Selmi, S., Tebourbi, O., El-Benna, J., Sakly, M. (2014). Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. Journal of ethnopharmacology. 152(2). 327–332.
13 Soulaidopoulos, S., Tsiogka, A., Chrysohoou, C., Lazarou, E., Aznaouridis, K., Doundoulakis, I., Tyrovola, D., Tousoulis, D., Tsioufis, K., Vlachopoulos, C., Lazaros, G. (2022). Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients. 14(3). 590.
14 Dabos, K.J., Sfika, E., Vlatta, L.J., Giannikopoulos, G. (2010). The effect of mastic gum on Helicobacter pylori: a randomized pilot study. Phytomedicine : international journal of phytotherapy and phytopharmacology. 17(3-4). 296–299
15 Papada, E., Gioxari, A., Amerikanou, C., Forbes, A., Tzavara, C., Smyrnioudis, I., Kaliora, A. C. (2019). Regulation of faecal biomarkers in inflammatory bowel disease patients treated with oral mastiha (Pistacia lentiscus) supplement: A double-blind and placebo-controlled randomised trial. Phytotherapy research. 33(2). 360–369.
16 Kaliora, A.C., Stathopoulou, M.G., Triantafillidis, J.K., Dedoussis, G.V., Andrikopoulos, N. K. (2007). Chios mastic treatment of patients with active Crohn's disease. World journal of gastroenterology. 13(5). 748–753.
17 Ali, B.H., Blunden, G., Tanira, M.O., Nemmar, A. (2008). Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 46(2). 409–420.
18 Haniadka, R., Saldanha, E., Sunita, V., Palatty, P.L., Fayad, R., Baliga, M.S. (2013). A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe). Food & function. 4(6). 845–855.
19 Micklefield, G.H., Redeker, Y., Meister, V., Jung, O., Greving, I., May, B. (1999). Effects of ginger on gastroduodenal motility. International journal of clinical pharmacology and therapeutics. 37(7). 341–346.
20 Thomson, M., Corbin, R., Leung, L. (2014). Effects of ginger for nausea and vomiting in early pregnancy: a meta-analysis. Journal of the American Board of Family Medicine. 27(1). 115–122.
21 Marx, W.M., Teleni, L., McCarthy, A.L., Vitetta, L., McKavanagh, D., Thomson, D., Isenring, E. (2013). Ginger (Zingiber officinale) and chemotherapy-induced nausea and vomiting: a systematic literature review. Nutrition reviews. 71(4). 245–254.
23 Hewlings, S.J., Kalman, D.S. (2017). Curcumin: A Review of Its Effects on Human Health. Foods. 6(10). 92.
24 Yu, Y., Wu, S., Li, J., Wang, R., Xie, X., Yu, X., Pan, J., Xu, Y., Zheng, L. (2015). The effect of curcumin on the brain-gut axis in rat model of irritable bowel syndrome: involvement of 5-HT-dependent signaling. Metabolic brain disease. 30(1). 47–55.
25 Patel, S.S., Acharya, A., Ray, R.S., Agrawal, R., Raghuwanshi, R., Jain, P. (2020). Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Critical reviews in food science and nutrition. 60(6). 887–939.
26 Sözen, S., Aziret, M., Bali, I., Emir, S., Ülgen, Y., Binnentoĝlu, K., Polat, Y., Çetinkünar, S. (2015) The Effect of Curcumin on an Animal Intestinal Ischemia/Reperfusion Model for Bacterial Translocation and Inflammatory Response. International surgery. 100(11-12). 13521359
27 Bundy, R., Walker, A.F., Middleton, R.W., Booth, J. (2004). Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. Journal of alternative and complementary medicine. 10(6). 1015–1018.
28 Alt, F., Chong, P.W., Teng, E., Uebelhack, R. (2017). Evaluation of Benefit and Tolerability of IQP-CL-101 (Xanthofen) in the Symptomatic Improvement of Irritable Bowel Syndrome: A Double-Blinded, Randomised, Placebo-Controlled Clinical Trial. Phytotherapy research. 31(7). 1056–1062.
29 Vetvicka, V., Vetvickova, J., Fernandez-Botran, R. (2016). Effects of curcumin on Helicobacter pylori infection. Annals of translational medicine. 4(24). 479.
30 Shang, A., Cao, S.Y., Xu, X.Y., Gan, R.Y., Tang, G.Y., Corke, H., Mavumengwana, V., Li, H.B. (2019). Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods. 8(7). 246.
31 Wang, Y., Huang, P., Wu, Y., Liu, D., Ji, M., Li, H., Wang, Y. (2022). Association and mechanism of garlic consumption with gastrointestinal cancer risk: A systematic review and meta-analysis. Oncology letters. 23(4). 125.
32 El-Ashmawy, N.E., Khedr, E.G., El-Bahrawy, H.A., Selim, H.M. (2016). Gastroprotective effect of garlic in indomethacin induced gastric ulcer in rats. Nutrition. 32(7-8). 849–854.
33 Peng, Y., Ma, Y., Luo, Z., Jiang, Y., Xu, Z., Yu, R. (2023). Lactobacillus reuteri in digestive system diseases: focus on clinical trials and mechanisms. Frontiers in cellular and infection microbiology. 13. 1254198.
34 Shornikova, A.V., Casas, I.A., Isolauri, E., Mykkänen, H., Vesikari, T. (1997). Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. Journal of pediatric gastroenterology and nutrition. 24(4)., 399–404
35 Riezzo, G., Orlando, A., D'Attoma, B., Linsalata, M., Martulli, M., Russo, F. (2018). Randomised double blind placebo controlled trial on Lactobacillus reuteri DSM 17938: improvement in symptoms and bowel habit in functional constipation. Beneficial microbes. 9(1). 51–60.
36 Moreno Márquez, C., Fernández Álvarez, P., Valdés Delgado, T., Castro Laria, L., Argüelles Arias, F., Caunedo Álvarez, Á., Gómez Rodríguez, B. J. (2022). Randomized, double-blind, placebo-controlled clinical trial on the usefulness of probiotic Lactobacillus reuteri in bismuth-containing quadruple eradication therapy for infection with Helicobacter pylori. Revista espanola de enfermedades digestivas. 114(2). 89–95.
37 Francavilla, R., Polimeno, L., Demichina, A., Maurogiovanni, G., Principi, B., Scaccianoce, G., Ierardi, E., Russo, F., Riezzo, G., Di Leo, A., Cavallo, L., Francavilla, A., Versalovic, J. (2014). Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. Journal of clinical gastroenterology. 48(5). 407–413.
38 Francavilla, R., Lionetti, E., Castellaneta, S.P., Magistà, A.M., Maurogiovanni, G., Bucci, N., De Canio, A., Indrio, F., Cavallo, L., Ierardi, E., Miniello, V.L. (2008). Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter. 13(2). 127–134.
39 Yang, C., Liang, L., Lv, P., Liu, L., Wang, S., Wang, Z., Chen, Y. (2021). Effects of non-viable Lactobacillus reuteri combining with 14-day standard triple therapy on Helicobacter pylori eradication: A randomized double-blind placebo-controlled trial. Helicobacter. 26(6). e12856.
40 Mehling, H., Busjahn, A. (2013). Non-viable Lactobacillus reuteri DSMZ 17648 (Pylopass™) as a new approach to Helicobacter pylori control in humans. Nutrients. 5(8). 3062–3073.
22 Hirata, A., Funato, H., Nakai, M., Iizuka, M., Abe, N., Yagi, Y., Shiraishi, H., Jobu, K., Yokota, J., Hirose, K., Hyodo, M., Miyamura, M. (2016). Ginger Orally Disintegrating Tablets to Improve Swallowing in Older People. Biological & pharmaceutical bulletin. 39(7). 1107–1111.

9
Nutramedic &Cosmetics
Exploring the Gut Impact of Overall Wellbeing
In recent years, the scientific and medical community has begun focusing more on gut health, particularly on how it is influenced by our lifestyle, dietary components, the gut microbiota and its metabolites.
Also interesting for researchers is the relationship between gut health and overall health with specific focus on immune health or cognition. One key aspect of this relationship seems to involve the characteristics and function of the microbiota.
The microbiota
“Microbiota” or “Microbiome”? People generally use these two terms interchangeably however they refer to two different things: “microbiota” describes the microorganisms including a bacteria, viruses, and fungi found in a defined environment (e.g. the gut microbiota). “Microbiome” on the other hand is a broader term which refers not only to the microorganisms, but also their genes.1 Additionally, when people talk about the microbiota, they are referring to the human gut. However, the microbiota exist in other areas of the human body, including the mouth, the skin, the urinary and reproductive tract. The gut microbiota is the delicate balance of trillions of good and bad bugs that populate the human gastrointestinal tract.
In a healthy condition, gut microorganisms are contained in the gastrointestinal tract by a structure composed of epithelial cells called the intestinal barrier. The gut microbiota relies on specific dietary components, namely dietary fibers, for survival, growth and production of crucial signaling molecules. This finely tuned combination of elements plays a vital role in various biological functions, including aiding digestion and regulating immune system functioning. 2 A well-functioning microbiota is essential for good overall health, but interestingly, no two people have the same gut environment. Instead, it is shaped by a person's DNA, environmental, and dietary exposures.
How does gut microbiota benefit us?
The microbiota plays a myriad of important roles in human health. First, through the intricate balance of good and bad bacteria, it modulates the immune system to be more effective in fighting potential pathogens. Second, it is important for various aspects of the digestion process including the breakdown of fiber humans are not able to digest. Third, its influence goes beyond the gut thanks to the release of various types of metabolites that work as signals in the body and modulate several physiolo-
gical functions. 3 The metabolic exchanges between the microbiota and the host, as well as among bacteria themselves, are essential contributors to maintaining good health. However, just like the microbiota can benefit us, it can also harm us if unbalanced. There are many internal and external factors that could contribute to this imbalance.
Gut microbiota: a major player in the function of the GI tract
The relationship between the gut microbiota and the gastrointestinal tract has been somewhat unclear. As a result, researchers have been interested in discovering key elements influencing the structure and function of the gut.
A recent review4 addresses the vast and diverse community of microorganisms arbored in our gut and the exquisite range of metabolic capacities which work in tandem with the activity of enzymes in the liver and gut mucosa. The gut microbiota plays a significant role in human metabolism by providing enzymes that we are not able to produce. The microbiota contributes to processing polyphenols, production of essential micronutrients and important vitamins, and digestion of dietary fiber. Digestion of dietary fiber by the microbiota results in the production of important molecules called short-chain fatty acids (SCFA), the most abundant being acetate, propionate, and butyrate. These SCFAs are involved in maintaining the health of the gastrointestinal tract since they are a critical energy source for the intestinal cells and are important in maintaining the integrity of the intestinal barrier which tightly regulates what enters the body and what needs to remain “out”.5
Many people struggle with poor digestion. This can express itself in numerous ways, ranging from heartburn after eating a big meal too close to bedtime to constipation and diarrhea. One often overlooked reason for this poor digestion is disruption in the gut microbiome balance or “dysbiosis” and the potential consequent changes in SCFA production. Supplementation with SCFA has been linked to the reduction of gastrointestinal symptoms and intestinal comfort. Furthermore, SCFA are crucial signaling molecules that interact with specific receptors found in the cells of the immune system located in the gut and in other organs or tissue such as the pancreas, the liver and adipose tissue.
10
Nutramedic &Cosmetics
The gut’s role in immunity
Even though many people think of the gut and the gut microbiota simply as part of the digestive system, the truth is that the gut microbiome plays a more complex and integral role in overall human health.
The link between the gut, microbiota and the immune system is complex, multifactorial and multidirectional. The gut microbiota actually conditions the immune system. Interestingly, the immune system can be modulated and/or primed by different agents or stimuli: dietary components such as beta 1,3 glucans6, host microbiota, and its metabolites, namely SCFA. Beta 1,3 glucans are considered immunomodulators7. When beta 1,3 glucans are ingested, they can directly interact with immune cells. Receptors in the immune cells detect the beta 1,3 glucans, process them, and regulate a response though the production of various signaling molecules that prime the immune system. Beta 1,3 glucans can also shape the gut microbiota overall contributing to maintain a healthy immune response in the gut and beyond.
SCFA can also directly interact with the cells of the immune system and with the microbiota itself, providing this dual support. Specific receptors for SCFA are found on the cells of the immune system. The interaction between SCFA and these receptors promotes immune balance, tissue integrity and con tributes to the response to pathogens.
Research on the gut microbiome continues
The role of the gut microbiota and its metabolites has received increasing levels of scientific interest in recent years, and a wide range of studies have been published. This research has explored a wide range of topics, such as the role of the gut biome and it’s related compounds aid in digestion and influence in the immune system. Furthermore, investigation is starting to explore other potential health benefits linked to the gut-brain axis.
Kemin explores new ways to continuously enhance health and wellness to provide everyone the opportunity to have a healthier life. To learn more about how Kemin’s ingredients may align with your products, view our portfolio of ingredients here.
References:
1 https://atlasbiomed.com/blog/whats-the-difference-betweenmicrobiome-and-microbiota/
2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5847071/ pdf/394_2017_Article_1445.pdf
3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9286904/
4 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5847071/
5 https://pubmed.ncbi.nlm.nih.gov/33764858/

Nutramedic &Cosmetics
Kemin Granted Novel Food Authorization for Immune Support Ingredient BetaVia™ Pure in the EU
The highest purity beta-glucan in the nutraceuticals market is now available in the European Union. The Novel Food authorization includes usage in food supplements for a range of age groups – from children from 3 years of age up to adulthood – and allows for BetaVia Pure to be used in diet replacements for weight control and cereal bars for the general population.
Kemin Industries, a global ingredient manufacturer that strives to sustainably transform the quality of life every day for 80 percent of the world with its products and services, receives Novel Food authorization from the European Commission for BetaVia™ Pure, along with a five-year exclusivity in the European Union.1
BetaVia Pure is manufactured through a patented process using Euglena gracilis - Kemin’s proprietary strain of the algae. The 95% beta 1,3 glucan (paramylon) ingredient for immune and gut support is the highest purity beta-glucan currently available on the market. 2-5 With its neutral odor, colour, and small recommended dose, the postbiotic can be included in numerous finished-product forms and easily included as part of a daily health routine.
Effective April 30, 2024, the Commission Implementing Regulation authorizes BetaVia Pure to be placed on the market in specific food categories. The comprehensive proprietary data generated by Kemin’s Human Nutrition and Health business unit establishing the safety of BetaVia Pure led to a fiveyear exclusivity in the European Union market.
The Novel Food authorization includes usage in food supplements for a range of age groups - from children from 3 years of age up to adulthood - and

allows for BetaVia Pure to be used in diet replacements for weight control and cereal bars for the general population.
"The European Food Safety Authority conducted an extremely rigorous evaluation of the safety data on BetaVia Pure, and now receiving approval is a tremendous achievement that demonstrates the strength of the Novel Food dossier Kemin Human Nutrition and Health submitted to the European Commission," said Pedro Vieira, Vice President - Europe, Kemin Human Nutrition and Health. "We are extremely excited to see the Commission Implementing Regulation published, which finally allows us to offer BetaVia Pure to the European Union, one of the key markets for Kemin Human Nutrition and Health."
BetaVia has an established global regulatory status, including in the U.S., Brazil, Japan, China, and many other countries. Kemin Human Nutrition and Health’s naturally sourced solutions are GRAS, kosher, halal, vegetarian, gluten free, allergen free, and non-GMO.
For more information on formulating with BetaVia for better immune and gut support visit: https:// www.kemin.com/eu/en/markets/human-nutrition/ products/betavia-complete-immune
References:
1 Commission Implementing Regulation (EU) 2024/1046 of 9 April 2024 authorising the placing on the market of beta-glucan from Euglena gracilis microalgae as a novel food and amending Implementing Regulation (EU) 2017/2470 - C/2024/2138
2 Kemin, BetaVia can prime key cells in the innate immune system. KHTL-017-150.
3 KHTL-017-160 Paramylon Improves Antioxidant Status and Metabolic Health
4 Kemin Proprietary Data TD-20-6867 “Effect of a Euglena gracilis (β-1,3-glucan) on Gut and Immune Health in Healthy Adults with Mild Gastrointestinal (GI) Issues.”
5 Patents granted a patent on beta 1,3 glucans for modulating immune function (US Patent 10,912,794). This new patent joins a previously issued patent, US 9574217, on the production of immune modulation using algae from a proprietary strain of Euglena gracilis ATCC PTA-123017
12 Nutramedic &Cosmetics
Darmell Expert in the Nutritional Supplements Field
Aholistic approach to health has always been our first choice when it comes to ways to support it. Nutritional supplements are an extremely important factor that can contribute to maintaining optimal health and are a good ally in the fight against various health disorders.
That is why professional path of Darmell is focused on developing new nutritional supplements and informing interested industry workers about ingredients, evidence of effectiveness, news, regulation and other relevant information in the field.
By combining many years of business experience, continuous acquisition of knowledge and cultivating business acquaintances, we are able to provide a variety of services.
Services we offer:
• Consulting in the elaboration of ideas and concepts for the development of new products - from the selection of ingredients to the launch of the finished product, with the help of finding the appropriate manufacturer of medicinal forms.
• Consulting related to the registration of dietary supplements.
Writing content for various purposes, in the field of dietary supplements and functional foods, related to individual ingredients and finished products.
• Help in finding a suitable distributor for finished products.
We also actively deal with:
• Representing renowned companies producing branded ingredients/raw materials for nutritional supplements.
• Publishing professional publications in the field of nutritional supplements, functional food and cosmetics.



13 sabinsa.com.pl info@sabinsa.com.pl
Darmell Ltd. 20+ years of experience in developing new concepts for food supplements Mob: + 385 91 68 12 444 darmell@protonmail.com www.dar-mell.com
Sulfodyne®: an Ingredient With a Wide Range of Health Benefits
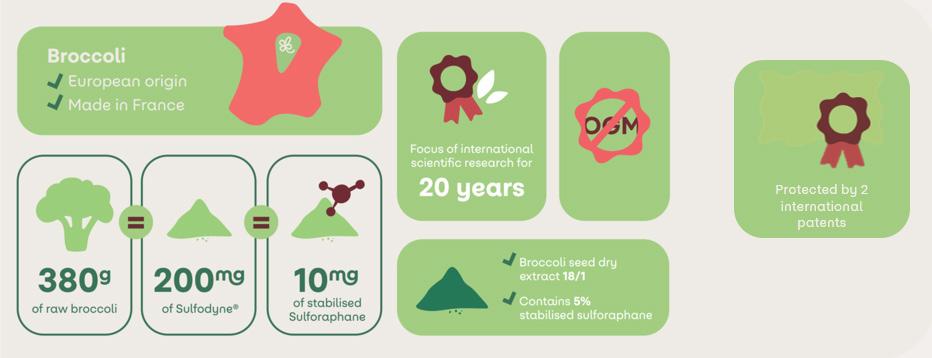
Driven by perseverance and guided by our purpose: to offer the best of our materials and after 20 years of research, we have launched Sulfodyne®, titrated to contain 5% of sulforaphane, directly bioavailable. By sulforaphane's mechanisms of actions, Sulfodyne® plays a part in 5 health benefits: detox, immunity, women's health, joint health and glucose regulation.
At Ingood by Olga we are curious by nature and we care for the others. Beyond our ingredients, we really want to provide natural and effective solutions useful to everyone. That's why we have set ourselves the challenge to develop an active ingredient directly titrated in active and stable sulforaphane, an active molecule providing many proven health benefits. Thanks to 20 years of research, we have drawn the best in broccoli.
Broccoli is known for its richness in sulfur compounds and is more specifically the richest crucifer
10 mg of sulforaphane =
mg of broccoli seed extract with SULFODYNE
641 mg of broccoli seed extract with glucoraphanin + myrosinase
mg of broccoli seed extract with glucoraphanin
in glucoraphanin, a precursor of sulforaphane. Unfortunately, eating broccoli is not enough to provide the effective dose of sulforaphane. It depends on broccoli quality, storage conditions of the broccoli, ability to hydrolyse glucoraphanin.
Sulforaphane is an isothiocyanate compound found in broccoli. It's derived from the hydrolysis of its precursor, glucoraphanin, by an enzyme called myrosinase.
Sulfodyne® is the only broccoli seed extract on the market with the stable and active bioavailable form of sulforaphane.1
amount assimilated in the body :
14 Nutramedic &Cosmetics
Expected
286
® 70% of sulforaphane
consumed
30% of glucoraphanin dose consumed
11% of glucoraphanin dose consumed
dose
1748
Sulfodyne®: 3 mechanisms of action
• Antioxidant activity: Sulforaphane is a strong indirect antioxidant thanks to the activation of Nrf2 expression2,3
• Anti-inflammatory activity: Sulforaphane reduces inflammation by inducing NF-κB inhibition and Nrf2 expression4,5
• Cellular protection: Sulforaphane has an epigenetic activity that impacts DNA methylation and histone acetylation. Some genes can be regulated by sulforaphane to induce cellular protection.6,7
These 3 main mechanisms of action explain the wide range of health benefits of Sulfodyne®
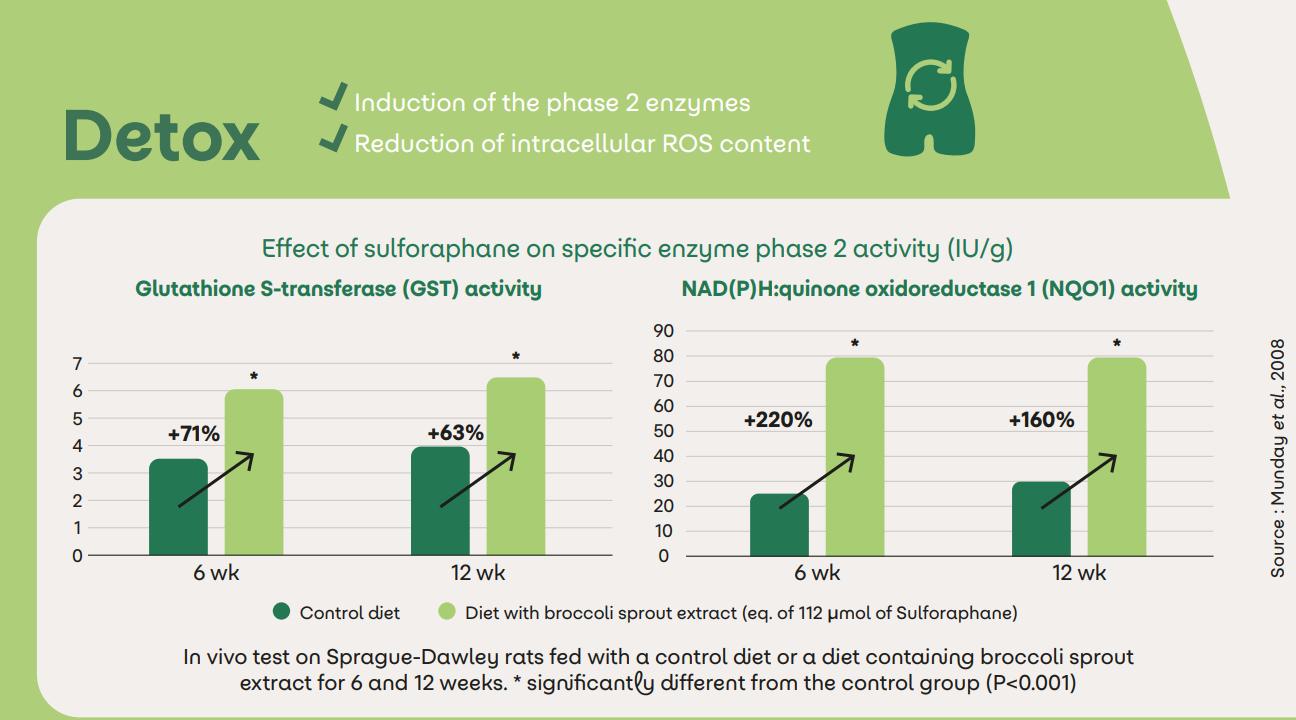
Sulfodyne® for the detox
The body naturally activates its own defense mechanisms against foreign substances that are toxic to the body, called "xenobiotics" but can only partially neutralize and eliminate them. Sulfodyne® can help the body to eliminate xenobiotics thanks to:8
Induction of the phase II enzymes
Reduction of intracellular ROS content
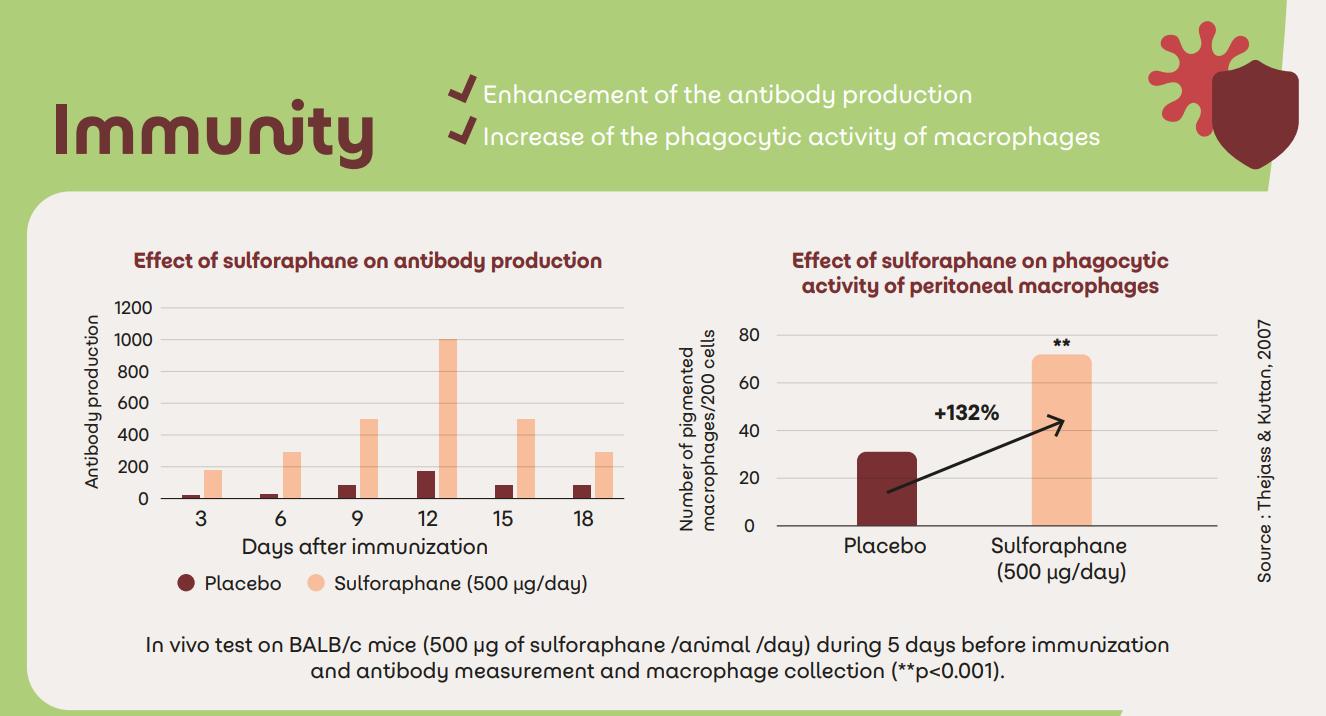
Sulfodyne® for immunity
Since Covid, immunity has been a major concern for customers. Sulfodyne® can play a role in boosting the immune system, both in the preventive phase and in the acute phases, by enhancing the body's natural defences mechanisms9,10
• Enhancement of the antibody production
• Increase of the phagocytic activity
• Antiviral activity: Reduction of the replication and the propagation of the virus and inhibition of virus both in prevention and in treatment
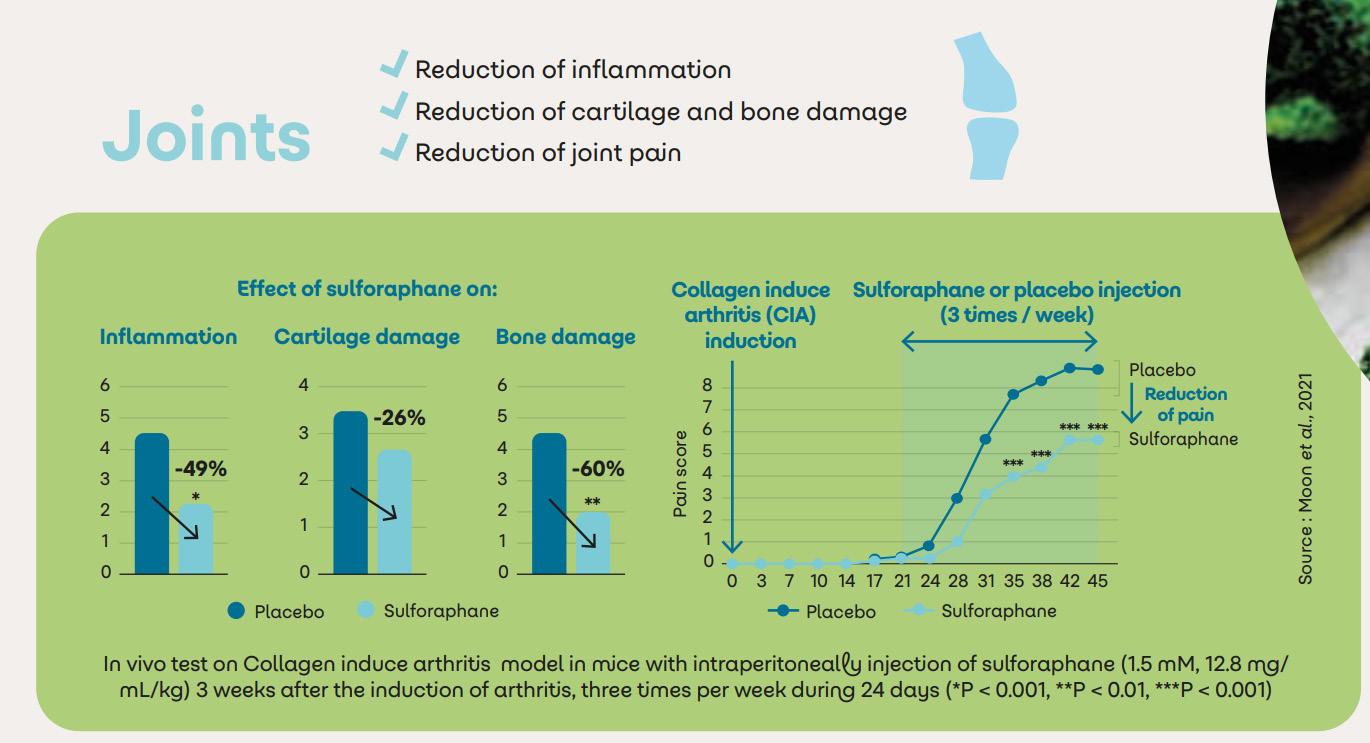
Sulfodyne® for joints
Joints are complex structures composed of bones, muscles, ligaments, tendons and other tissues. Due to ageing and chronic inflammation, 1,71 billion people are concerned by variable degrees of pain accompanied by stiffness, swelling and loss of joint function.
Sulforaphane: 3 main mechanism of action



15 Nutramedic &Cosmetics
In case of rheumatoid arthritis, Sulfodyne® can reduce inflammation: 11,12
• Decrease of the MMPs expression (cartilage degradation)
• Decrease of the COX-2 expression (joint pain)
• Decrease of the PGE2 production and NF-kB activity (inflammation pathways).
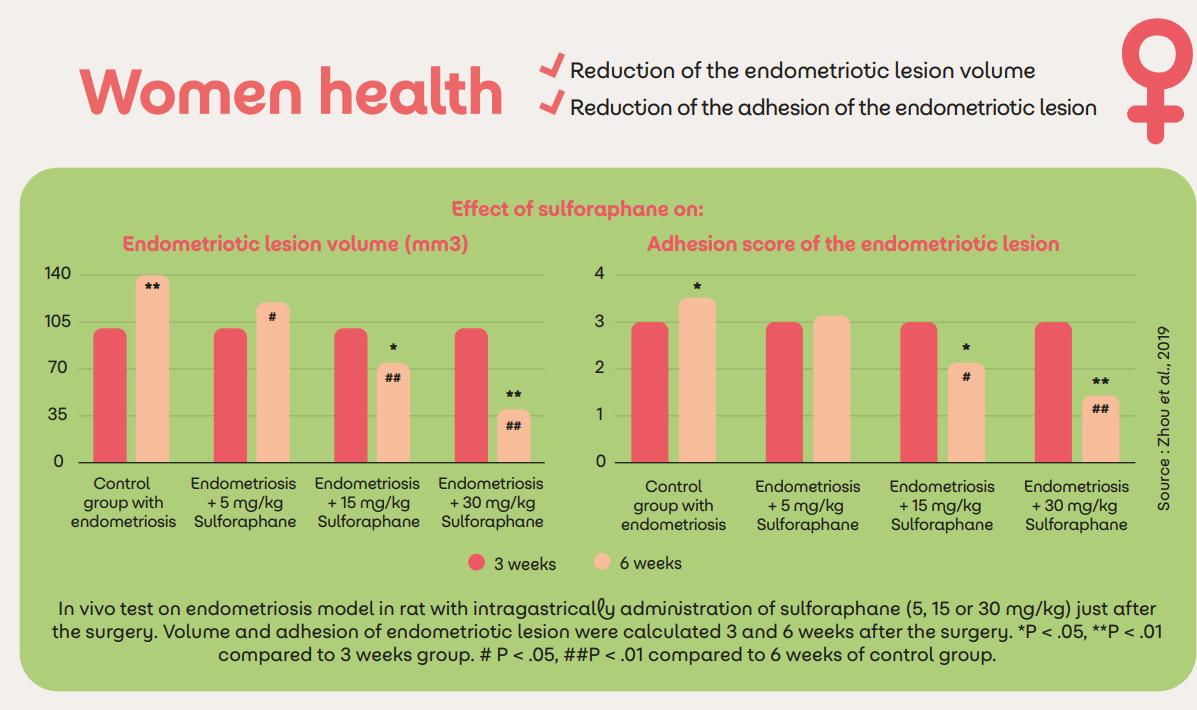
Sulfodyne® for women’s health
Women’s health is very large and encompasses a number of symptoms, time of life or diseases that have long been ignored by medical research like endometriosis, pre-menstrual syndrome, menopause, etc. Some of these conditions are linked by an uncontrolled inflammation process and the role of sulforaphane, notably through the activation of Nrf2 expression, is gaining interest in the management of some women health issues like endometriosis or premenstrual syndroms (PMS).13
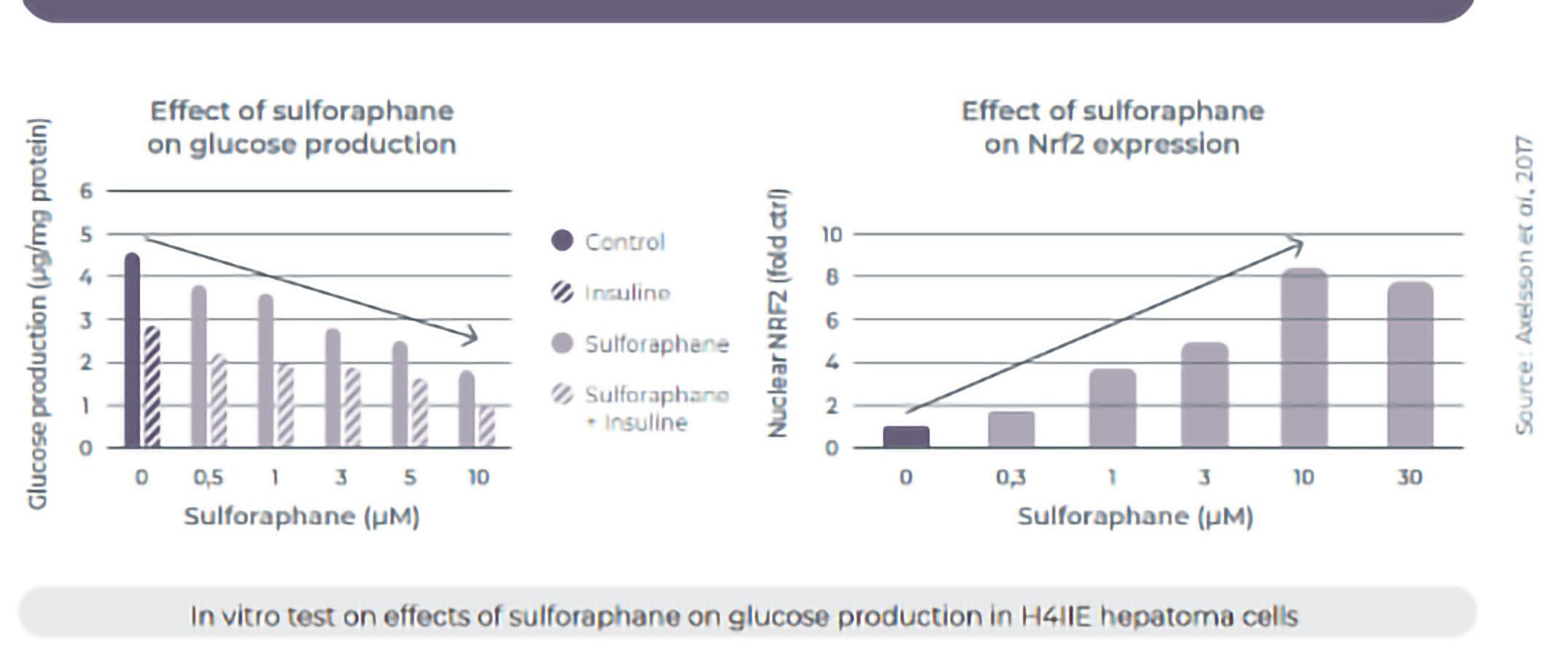
Sulfodyne® for glucose regulation
Control of blood glucose is crucial to prevent and delay diabetes. Sulforaphane was found to reduce insulin resistance, enhance glucose transport and
improve blood glucose levels. Moreover, Sulforaphane can activate the Nrf2 expression, which activation was found to prevent development of diabetes and its complications, thanks to the promotion of antioxidant pathways to mitigate oxidative stress and hyperglycemic damage..14
Induction of Nrf2 expression
Reduction in glucose production similar to metformin.
References:
1 Fahey et al., 2016
2 Briones-Herrena et al., 2018
3 Ruhee & Suzuki, 2020
4 Ahmed et al., 2016
5 Ruhee & Suzuki, 2020
6 Li, 2021; Houghton, 2019
7 Su et al., 2018
8 Munday et al., 2008
9 Thejass & Kuttan, 2006
10 Ordone z et al., 2021
11 Choi et al, 2014
12 Moon et al., 2021
13 Zhou et al., 2019
14 Axelsson et al., 2017
16 Nutramedic &Cosmetics
Ingood by Olga is the business unit dedicated to ingredients of the Olga group, a family-owned company based in Brittany. Born in 2015, we are daily guided by our purpose: To offer the best of our materials in respect of our ecosystems and with boldness. We produce and offer plant-based ingredients for health, nutrition and food markets. Convinced that everything is good in our materials, we are working for a circular model and we want to bring natural and useful ingredients to everyone promoting a more positive approach to food and societal transition. Beyond our ingredients, we believe in a whole model of sustainable development that we want to share generously around us.

Our mission supports the idea that to do good, we must first do right: for the environment, ecosystems, staff, and customers alike. This holistic approach business dovetails with that of sustainable development, a dynamic to which we are fully committed today, as evidenced by the company's exemplary ISO 26000 certification (CSR label), one of the highest certifications that can be awarded.
2 Rue Julien Neveu, 35530 Noyal-sur-Vilaine Bretagne 35530, France T. +33 (0)2 99 04 11 11 www.ingoodbyolga.com/en/ www.linkedin.com/company/ingood-by-olga/


17 The only broccoli seed extract with the active and natural form of sulforaphane Detox Joints Women’s health Glucose regulation Immunity ingoodbyolga.com Come to meet us Hall 5 - Booth N 149 Pavillon France 14 – 16 May 2024 Geneva Gosselin design & digital RCS Rennes 480 918 366Crédit photo @AdobeStock Nutramedic &Cosmetics
Probiotics and Osteoporosis - What Do We Know Today?
The gut microbiome and the host immune response play critical roles in the pathophysiology of osteoporosis. By positively influencing the gut microbiome and modulating the host immune response through various direct and indirect mechanisms, probiotics offer osteoprotective benefits. Research has demonstrated the beneficial effects of probiotic administration on bone health and osteoporosis. However, further investigation is warranted to delve into the strain-specific mechanisms of action, particularly concerning the gut microbiome and metabolite secretion.
AUTHOR: Ira Renko, MSc, Master in Molecular biotechnology
What we know today
Osteoporosis, characterized by progressive loss of bone mass and deterioration of bone tissue structure, particularly microstructure, is a prevalent skeletal disease affecting over 200 million individuals worldwide, posing a significant global public health concern (Picture 1). Fractures associated with osteoporosis stem from increased bone fragility and susceptibility to fractures. The principal pathophysiological mechanism involves an imbalance in bone remodeling, with heightened bone resorption by osteoclasts and decreased bone formation by osteoblasts, resulting in continuous bone loss, typically observed as a decline in bone mineral density (BMD) at a rate of approximately 2-5% per year, along with deterioration in bone microarchitecture.1-4
Osteoporosis can be classified as primary (type I), occurring as a natural part of aging, particularly in women with estrogen deficiency post-menopause, or secondary (senile) osteoporosis, resulting from medical conditions, diseases, or certain medical treatments. The multifactorial development of primary osteoporosis involves genetic and environmental factors, including diet, lifestyle choices (such as smoking), hygiene practices, and antibiotic use.5-7
New findings
Given the high incidence and prevalence of osteoporosis and osteopenia worldwide, the scientific community continues its pursuit of effective and safe treatment options.8 Presently, therapeutic choices remain limited, with lifestyle modifications showing low compliance rates.9 While hormonal estrogen therapy, such as red clover isoflavone supplementation, has demonstrated effectiveness in prevention and treatment, it's associated with severe adverse events.11 Similarly, antiresorptive agents like bisphosphonates, parathyroid hormone, and calcitonin face limited use due to adverse effects, high costs, and poor compliance.12 Although calcium and vitamin D offer beneficial effects on bone microarchitecture and overall bone health, they're insufficient alone for treating menopausal bone loss.13 Current treatment methods typically involve a combination of lifestyle adjustments, bone health supplements, drug intervention, and rehabilitation.
The gut microbiome's role in osteoporosis has emerged as a significant area of research.14 Studies indicate that the gut microbiome influences osteoclast and osteoblast activity through metabolite se-

18 Nutramedic &Cosmetics
Source: reference 75
PICTURE 1 Osteoporotic bone
LactobacillusplantarumNK3, BifidobacteriumlongumNK49
reuteri
LactobacillusplantarumGKM3, LactobacillusparacaseiGKS6
Bacillus subtilis, Lactococcus lactis
weeks
weeks
BMC, ↑ BMD, ↑ mineral apposition rate
serum osteocalcin,
Wnt10b expression
rate
RANKL gene expression
OPG gene expression
IL-10 gene expression
BMD
trabecular micoarchitecture
cortical microarchitecture
bone heterogeneity
Treg/Th17 balance, ↑ IL-10, IFN-Y, ↓ IL-6, IL-17, TNF-a, RANKL
serum osteocalcin
↑ bone volume, ↑ trabecular thickness
↑ Runx2 and Bmp2 expression
↓ serum C-terminal peptide
bone porosity
↑ Ca and P levels, ↑ serum osteocalcin, ↓ TNFa gene expression
BMD, ↑ anti-inflammatory cytokines
↑ trabecular number, ↑ trabecular thickness ↑ BMD, ↑ osteoblast differentiation,
RANKL
↑ osteoblast formation, ↑ bone matrix growth
↑ bone matrix mineralization
↑ Runx2, Sp7, Spp1, Col10a10a gene expression
19 Nutramedic &Cosmetics
Probiotic Form Duration Bone effect Reference Brewer’s yeast ↓ tibial dyschondroplasia 31 Lactobacillus 4 weeks ↑ Ca and P retention 32 Lactobacillus casei, Lactobacillus reuteri, Lactobacillusgasseri ↑ bone mineral content ↑ Ca absorption 33 Lactobacillus helveticus LBK-16H fermented milk 14 weeks ↑ BMD, ↑ BMC 34 Enterococcus faecium 7 weeks ↑ anti-inflammatory effects ↑ anti-arthritic effects 35 Bacillus licheniformis, Bacillus subtilis 6 weeks ↑ tibia wall thickness 36 Lactobacilus casei 393 fermented milk 6 weeks ↑ BMD, ↑ fracture strength ↑ Ca content, ↓ TRAP activity 37 LactobacillusparacaseiNTU101, LactobacillusplantarumNTU102 fermented soy milk 8 weeks ↑ bone volume fraction ↑ trabecular number 38 Lactobacillus casei, Lactobacillus acidophilus 1.5 weeks ↓ bone loss 39 Bifidobacteriumlongum fermented broccoli 12 weeks ↓ TRAP+ osteoclasts 40 Bifidobacteriumlongum 4 weeks ↑ tibial Ca, P an mg content ↑ fracture strength 41 Lactobacillus reuteri ATCC 6475 4 weeks ↑ bone volume fraction ↑ trabecular number, ↑ trabecular thickness ↑ BMC, ↑ BMD, ↑ osteocalcin ↑ bone formation rate 42 Lactobacillus reuteri ATCC 6475 4 weeks ↑ bone volume fraction, ↑ BMC, ↑ BMD, ↓ RANKL gene expression, ↓ TRAP5 expression 43 Lactobacillusparacasei, Lactobacillusplantarum 6 weeks ↑ cortical bone mineral content ↑ cortical area, ↑ OPG expression 44 Lactobacillus reuteri ATCC 6475 4 weeks ↑ bone volume fraction ↑
↑
45 Lactobacillus reuteri ATCC 6475 8 weeks ↑ bone volume fraction ↑
apposition
↑
↑
↑
46 Lactobacillus
6
↑
↑
↑
↑
47 Lactobacillus helveticus ATCC27558 16
↑
TABLE 1 Animal studies
↑
mineral
acidophilus
↑
↓
48
49
↑
50
Lactobacillus
6475
4
weeks
↓
51
52
cretion, impacting host metabolism and immune response.15,16 Dysbiosis-induced gastrointestinal inflammation and disturbances in metabolite secretion can lead to potent osteoclastogenic cytokine production, contributing to bone loss. Moreover, the gut microbiome affects the intestinal absorption of minerals crucial for bone health.17,18 Imbalances in the gut microbiome, along with estrogen deficiency, can increase gut permeability, facilitating the absorption of toxins and pathogens, thus promoting systemic inflammation detrimental to bone turnover.
The complexity and time-dependent nature of the gut microbiome's effects on bone health are evident in research. This has led to the emergence of the term "osteomicrobiology" to describe research on the gut microbiome's impact on bone health and metabolic bone diseases.19,20
Probiotics, live microorganisms with beneficial health effects for the host, offer a promising avenue for osteoporosis treatment. 21 However, not all probiotics are equally effective due to differences in food processing, strain specificity, and targeted effects. Probiotics likely exert their effects through modulation of gut microbiome metabolite secretion, influencing immune response, and improving gut epithelial barrier function, crucial for normal bone cell functioning. 22,23 While the magnitude of probiotic treatment's effect may be lower compared to anti-resorptive drugs, it's comparable to calcium and/or vitamin D treatment. 25
The discovery that probiotics can mitigate bone mass loss presents a novel approach to osteoporosis prevention and treatment. 26 In vitro studies reveal that different probiotic strains have varying effects on bone health, indicating both cell-specific and strain-specific mechanisms. For instance, Lactobacillus reuteri has been shown to inhibit osteoclast differentiation and reverse TNF-a-induced suppres-
LactobacillusparacaseiDM 13434,Lactobacillusplantarum DSM 15312, Lactobacillus plantarumDSM15313
sion of Wnt10b expression, potentially through histamine secretion. 27 Similarly, Lactobacillus casei 393 and Lactobacillus helveticus have demonstrated effects on bone cell proliferation and osteoblast differentiation, respectively, suggesting strain-specific interactions with bone metabolism. 30
Studies in animals have shown probiotic supplementation to improve BMD and have osteoprotective properties in osteoporosis. Results of several studies are shown in Table 1.
In a study by Amdekar et al. in 2012, supplementation with the probiotics Lactobacillus casei and Lactobacillus acidophilus demonstrated an osteoprotective effect, reducing bone loss caused by oxidative stress through their anti-oxidative mechanism of action. 37 Additionally, results from Rodrigues et al. showed intriguing findings, indicating that supplementation with Bifidobacterium longum ATCC15707 for 28 days increased the calcium, phosphorus, and magnesium content in the tibias of male rats, along with higher fracture strength compared to controls41 (Table 1). Furthermore, a study by Parvaneh et al. found that probiotic supplementation with Bifidobacterium longum increased bone density, trabecular thickness, and number, as well as femoral strength. This was achieved by elevating serum osteocalcin levels, promoting osteoblast genesis and bone formation parameters, while simultaneously decreasing osteoclast activity over the bone surface in the femur and reducing C-terminal telopeptide levels between ovariectomized and sham-ovariectomized mice48 (Table 1).
Positive results were seen in studies on humans too. The list of human studies on probiotics and osteoporosis are summarized in Table 2.
In a study by Narva et al., probiotic supplementation was found to decrease serum parathyroid hormone (PTH) levels while increasing serum calcium levels. Notably, this effect was observed only when
20
Nutramedic &Cosmetics
Probiotic Form Duration Bone effect Reference Year Lactobacillus helveticus randomized crossover ↓ PTH ↑ serum calcium 53 2004 Multi-strain RCT 6 months ↓ BSAP, ↓ PTH, ↓ TNF-a, ↓ CTX 54 2017 Multi-strain RCT 12 months ↑ total hip BMD 55 2017 Lactobacillus reuteri 6475 RCT 12 months ↑ total tibia BMD 56 2018 Bacillus subtilis C-3102 RCT 8 months ↑ total hip BMD ↓ uNTx level ↓ TRACP-5b level 57 2018 Multi-strain
RCT 12 months ↑ lumbar BMD 58 2019 Bifidobacterium Multi-strain Cohort 2 months ↑ BMD 59 2019 Bifidobacterium Multi-strain Cohort 6 months ↑ BMD ↑ OC 60 2019 Lactobacillus Multi-strain RCT 6 months ↑ BMD ↓ CTX ↑ OC 61 2020
TABLE 2 Human studies
the probiotic itself was administered, not when solely peptides secreted by the probiotic were applied53 (Table 2). Conversely, research by Jafarnejad et al. demonstrated that probiotic supplementation led to a decrease in bone-specific alkaline phosphatase levels, parathyroid hormone, tumor necrosis factor TNF-a, and collagen type 1 cross-linked C-telopeptide. However, spine and hip bone mineral density (BMD) remained unaffected by probiotic supplementation54 (Table 2).
Mechanisms of action
Probiotics exert their influence on bone health through various mechanisms.
The primary mechanism involves the positive alteration of gut microbiota composition by probiotics, leading to an optimal profile of gut microbiome metabolite secretion with systemic benefits to the host, including bone metabolism.62,63,64
Probiotics can enhance the utilization of calcium, phosphorus, and iron, as well as the absorption of iron and vitamin D, through the secretion of metabolites such as lactic, butyric, and acetic acid.65
Certain probiotics have been found to increase the expression of the vitamin D receptor (VDR) in both humans and animal studies, thereby increasing circulating vitamin D levels, which suggests that probiotics can enhance the positive effect of vitamin D on bone health through multiple pathways.66
Additionally, probiotic administration can lead to altered hormone levels, such as estrogen, which plays a crucial role in the pathophysiology of osteoporosis.67
By influencing gut microbiome metabolite secretion, probiotics can reduce intestinal pH, leading to increased calcium solubility and absorption in the intestinal lumen, ultimately resulting in increased bone mineral content and density. This mechanism is particularly important in the elderly population where intestinal absorption is often diminished.
Another significant mechanism of probiotics in osteoporosis involves their anti-inflammatory effects. Probiotics can mitigate the inflammatory cascade impacting bone turnover by calming gastrointestinal and systemic inflammation and producing anti-inflammatory metabolites, such as arginine deiminase in the case of Lactobacillus brevis CD2. Probiotics may also reduce the expression of proinflammatory and osteolytic cytokines, including tumor necrosis factor-alpha (TNF-α), interferon 17 (IL17), and receptor activator of nuclear factor-kappa ligand (RANKL), resulting in reduced osteoclast formation and enhanced osteoblastic activity. Furthermore, the anti-inflammatory effect of probiotics improves calcium transport across the intestinal barrier.68,69
Moreover, probiotics can influence the expression of genes crucial for bone metabolism, rendering them as antioxidants by enhancing bone cell response to oxidative stress, which is heightened in estrogen deficiency and leads to increased osteoclast over osteoblast differentiation. 39
Probiotic trials for osteoporosis
Research on the effects of probiotic supplementation on bone health has yielded promising results,
indicating potential benefits for individuals at risk of osteoporosis. Clinical trials involving both humans and animals have highlighted the positive impact of certain probiotic strains such as L. reuteri, L. casei,L.paracasei,L.bulgaricus,L.acidophilus, and B. subtilis . 70 For instance, in a study involving osteopenic postmenopausal women, a 12-week regimen of multispecies probiotics was found to potentially slow the increase in serum bone resorption marker CTX, suggesting a mechanism for reducing osteoclast-induced bone resorption without adverse effects.71 Another study observed that supplementation with probiotics containing L. reuteri 6475 over the course of a year led to a significant reduction in bone loss (osteoporosis) in women.72 This indicates a potential long-term benefit in preserving bone health. Animal studies have also provided insights, showing that strains like Lactobacillus casei, L. reuteri, L. gasseri, and B. longum (ATCC 15707) can enhance bone mineral density, calcium absorption, and fracture strength.73, 74
These findings collectively suggest that probiotic supplementation could offer a natural approach to support bone health, potentially reducing the risk of osteoporosis. However, further research, particularly randomized controlled trials with larger cohorts, is necessary to validate these findings and establish optimal dosage and duration for probiotic interventions targeting bone health.
Conclusions
The gut microbiome and the host immune response play critical roles in the pathophysiology of osteoporosis. Probiotics offer osteoprotective benefits by positively influencing the gut microbiome and modulating the host immune response through various direct and indirect mechanisms. Research across in vitro , animal, and human studies has demonstrated the beneficial effects of probiotic administration on bone health and osteoporosis. However, further investigation is warranted to delve into the strain-specific mechanisms of action, particularly concerning the gut microbiome and metabolite secretion. This emphasizes the potential of
21
Nutramedic &Cosmetics
Nutramedic &Cosmetics
probiotics as adjunctive therapeutic agents in the prevention and management of osteoporosis, while underscoring the need for continued research to elucidate their precise mechanisms of action.
References:
1 Mitlak BH, Nussbaum SR. 1993. Diagnosis and treatment of osteoporosis. Annu Rev Med. 44:265-277.
2 Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM. 2018. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 14:2029-2049.
3 aring B, Crandall CJ, Wu C, LeBlanc ES, Shikany JM, Carbone L, Orchard T, Thomas F, Wactawaski-Wende J, Li W et al. 2016. Dietary patterns and fractures in postmenopausal women: Results from the women's health initiative. JAMA Intern Med. 176(5):645-652.
4 Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME et al. 2008. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 93(3):861-868.
5 Bliuc D, Nguyen ND, Alarkawi D, Nguyen TV, Eisman JA, Center JR. 2015. Accelerated bone loss and increased post-fracture mortality in elderly women and men. Osteoporos Int. 26(4):1331-1339.
6 Kerschan-Schindl K. 2016. Prevention and rehabilitation of osteoporosis. Wien Med Wochenschr. 166(1-2):22-27.
7 Muhammad A, Mada SB, Malami I, Forcados GE, Erukainure OL, Sani H, Abubakar IB. 2018. Postmenopausal osteoporosis and breast cancer: The biochemical links and beneficial effects of functional foods. Biomed Pharmacother. 107:571-582.
8 Black DM, Rosen CJ. 2016. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 374(3):254-262.
9 Häuselmann HJ, Rizzoli R. 2003. A comprehensive review of treatments for postmenopausal osteoporosis. Osteoporos Int. 14(1):2-12.
10 Simin J, Tamimi R, Lagergren J, Adami HO, Brusselaers N. 2017. Menopausal hormone therapy and cancer risk: An overestimated risk? Eur J Cancer. 84:60-68.
11 Boonen S, Vanderschueren D, Haentjens P, Lips P. 2006. Calcium and vitamin d in the prevention and treatment of osteoporosis - a clinical update. J Intern Med. 259(6):539-552.
12 Yang CY, Chen CH, Wang HY, Hsiao HL, Hsiao YH, Chung WH. 2014. Strontium ranelate related stevens-johnson syndrome: A case report. Osteoporos Int. 25(6):1813-1816.
13 Gonnelli S, Cepollaro C, Pondrelli C, Martini S, Montagnani A, Monaco R, Gennari C. 1999. Bone turnover and the response to alendronate treatment in postmenopausal osteoporosis. Calcif Tissue Int. 65(5):359-364.
14 Ohlsson C, Sjögren K. 2015. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 26(2):69-74.
15 Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. 2015. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 161(2):264-276.
16 Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL et al. 2008. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 135(5):825-837.
17 Collins KH, Paul HA, Reimer RA, Seerattan RA, Hart DA, Herzog W. 2015. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthritis Cartilage. 23(11):1989-1998.
18 Ali T, Lam D, Bronze MS, Humphrey MB. 2009. Osteoporosis in inflammatory bowel disease. Am J Med. 122(7):599-604.
19 Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. 2016. Gut microbiota induce igf-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 113(47):E7554-e7563.
20 Ohlsson C, Sjögren K. 2018. Osteomicrobiology: A new cross-disciplinary research field. Calcif Tissue Int. 102(4):426-432.
21 Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD et al. 2017. Expert consensus document: The international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 14(8):491-502.
22 iver E, Berenbaum F, Valdes AM, Araujo de Carvalho I, Bindels LB, Brandi ML, Calder PC, Castronovo V, Cavalier E, Cherubini A et al. 2019. Gut microbiota and osteoarthritis management: An expert
consensus of the european society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (esceo). Ageing Res Rev. 55:100946.
23 Zhong Y, Zheng C, Zheng JH, Xu SC. 2020. The relationship between intestinal flora changes and osteoporosis in rats with inflammatory bowel disease and the improvement effect of probiotics. Eur Rev Med Pharmacol Sci. 24(10):5697-5702.
24 Dar HY, Azam Z, Anupam R, Mondal RK, Srivastava RK. 2018a. Osteoimmunology: The nexus between bone and immune system. Front Biosci (Landmark Ed). 23(3):464-492.
25 Biver E, Berenbaum F, Valdes AM, Araujo de Carvalho I, Bindels LB, Brandi ML, Calder PC, Castronovo V, Cavalier E, Cherubini A et al. 2019. Gut microbiota and osteoarthritis management: An expert consensus of the european society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (esceo). Ageing Res Rev. 55:100946.
26 Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. 2014. Probiotic l. Reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 229(11):1822-1830.
27 Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. 2015. Loss of bone and wnt10b expression in male type 1 diabetic mice is blocked by the probiotic lactobacillus reuteri. Endocrinology. 156(9):3169-3182.
28 homas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J. 2012. Histamine derived from probiotic lactobacillus reuteri suppresses tnf via modulation of pka and erk signaling. PLoS One. 7(2):e31951.
29 Kim DE, Kim JK, Han SK, Jang SE, Han MJ, Kim DH. 2019. Lactobacillus plantarum nk3 and bifidobacterium longum nk49 alleviate bacterial vaginosis and osteoporosis in mice by suppressing nfκb-linked tnf-α expression. J Med Food. 22(10):1022-1031.
30 Narva M, Collin M, Lamberg-Allardt C, Kärkkäinen M, Poussa T, Vapaatalo H, Korpela R. 2004. Effects of long-term intervention with lactobacillus helveticus-fermented milk on bone mineral density and bone mineral content in growing rats. Ann Nutr Metab. 48(4) :228-234.
31 I. Plavnik, M.L. Scott (1980) Effects of Additional Vitamins, Minerals, or Brewer's Yeast upon Leg Weaknesses in Broiler Chicken. Poultry Science. 59: 459-464
32 Nahashon SN, Nakaue HS, Mirosh LW. Production variables and nutrient retention in single comb White Leghorn laying pullets fed diets supplemented with direct-fed microbials. Poult Sci. 1994 Nov;73(11):1699-711
33 Ghanem, K.Z. & Badawy, I.H. & Abdel-Salam, Ahmed. (2004). Influence of yoghurt and probiotic yoghurt on the absorption of calcium, magnesium, iron and bone mineralization in rats. Milchwissenschaft. 59. 472-475.
34 Narva M, Nevala R, Poussa T, Korpela R. The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur J Nutr. 2004 Apr;43(2):61-8. doi: 10.1007/s00394-004-0441-y.
35 Rovensky, Josef & Švík, Karol & Matha, Vladimir & Istok, Richard & Ebringer, Ladislav & Ferencík, Miroslav & Stancikova, Maria. (2004). The effects of Enterococcus faecium and selenium on methotrexate treatment in rat adjuvant-induced arthritis. Clinical & developmental immunology. 11. 267-73.
36 Mutuş R, Kocabagli N, Alp M, Acar N, Eren M, Gezen SS. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult Sci. 2006 Sep;85(9):1621-5. doi: 10.1093/ps/85.9.1621
37 Kim, J. G., Lee, E., Kim, S. H., Whang, K. Y., Oh, S., & Imm, J. Y. (2009). Effects of a Lactobacillus casei 393 fermented milk product on bone metabolism in ovariectomised rats. International Dairy Journal, 19(11), 690-695. https://doi.org/10.1016/j.idairyj.2009.06.009
38 Chiang SS, Pan TM. Antiosteoporotic effects of Lactobacillus -fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem. 2011 Jul 27;59(14):7734-42. doi: 10.1021/jf2013716.
39 Amdekar S, Roy P, Singh V, Kumar A, Singh R, Sharma P. Anti-inflammatory activity of lactobacillus on carrageenan-induced paw edema in male wistar rats. Int J Inflam. 2012;2012:752015. doi: 10.1155/2012/752015
40 Tomofuji T, Ekuni D, Azuma T, Irie K, Endo Y, Yamamoto T, Ishikado A, Sato T, Harada K, Suido H, Morita M. Supplementation of broccoli or Bifidobacterium longum-fermented broccoli suppresses serum lipid peroxidation and osteoclast differentiation on alveolar bone surface in rats fed a high-cholesterol diet. Nutr Res. 2012 Apr;32(4):301-7. doi: 10.1016/j.nutres.2012.03.006.
22
41 Rodrigues FC, Castro AS, Rodrigues VC, Fernandes SA, Fontes EA, de Oliveira TT, Martino HS, de Luces Fortes Ferreira CL. Yacon flour and Bifidobacterium longum modulate bone health in rats. J Med Food. 2012 Jul;15(7):664-70. doi: 10.1089/jmf.2011.0296
42 McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013 Aug;228(8):1793-8. doi: 10.1002/jcp.24340
43 Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014 Nov;229(11):1822-30. doi: 10.1002/jcp.24636
44 Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, Movérare-Skrtic S, Islander U, Sjögren K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014 Mar 17;9(3):e92368. doi: 10.1371/journal.pone.0092368
45 Zhang R, Zhou M, Tu Y, Zhang NF, Deng KD, Ma T, Diao QY. Effect of oral administration of probiotics on growth performance, apparent nutrient digestibility and stress-related indicators in Holstein calves. J Anim Physiol Anim Nutr (Berl). 2016 Feb;100(1):33-8. doi: 10.1111/jpn.12338
46 Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, McCabe LR. Lactobacillus reuteri 6475 Increases Bone Density in Intact Females Only under an Inflammatory Setting. PLoS One. 2016 Apr 8;11(4):e0153180. doi: 10.1371/journal. pone.0153180
47 Dar HY, Shukla P, Mishra PK, Anupam R, Mondal RK, Tomar GB, Sharma V, Srivastava RK. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. 2018 Feb 5;8:46-56. doi: 10.1016/j.bonr.2018.02.001
48 Parvaneh M, Karimi G, Jamaluddin R, Ng MH, Zuriati I, Muhammad SI. Lactobacillus helveticus (ATCC 27558) upregulates Runx2 and Bmp2 and modulates bone mineral density in ovariectomy-induced bone loss rats. Clin Interv Aging. 2018 Aug 30;13:1555-1564. doi: 10.2147/CIA.S169223
49 Kim WK, Jang YJ, Han DH, Seo B, Park S, Lee CH, Ko G. Administration of Lactobacillus fermentum KBL375 Causes Taxonomic and Functional Changes in Gut Microbiota Leading to Improvement of Atopic Dermatitis. Front Mol Biosci. 2019 Sep 27;6:92. doi: 10.3389/ fmolb.2019.00092
50 Collins FL, Rios-Arce ND, Schepper JD, Jones AD, Schaefer L, Britton RA, McCabe LR, Parameswaran N. Beneficial effects of Lactobacillus reuteri 6475 on bone density in male mice is dependent on lymphocytes. Sci Rep. 2019 Oct 11;9(1):14708. doi: 10.1038/ s41598-019-51293-8
51 Yang F, Wang Y, Zhao S, Wang Y. Lactobacillus plantarum Inoculants Delay Spoilage of High Moisture Alfalfa Silages by Regulating Bacterial Community Composition. Front Microbiol. 2020 Aug 12;11:1989. doi: 10.3389/fmicb.2020.01989
52 Sojan JM, Raman R, Muller M, Carnevali O, Renn J. Probiotics Enhance Bone Growth and Rescue BMP Inhibition: New Transgenic Zebrafish Lines to Study Bone Health. Int J Mol Sci. 2022 Apr 26;23(9):4748. doi: 10.3390/ijms23094748.
53 Narva M, Nevala R, Poussa T, Korpela R. The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur J Nutr. 2004 Apr;43(2):61-8. doi: 10.1007/s00394-004-0441-y.
54 Jafarnejad S, Shab-Bidar S, Speakman JR, Parastui K, Daneshi-Maskooni M, Djafarian K. Probiotics Reduce the Risk of Antibiotic-Associated Diarrhea in Adults (18-64 Years) but Not the Elderly (>65 Years): A Meta-Analysis. Nutr Clin Pract. 2016 Aug; 31(4):502-13. doi: 10.1177/0884533616639399
55 Lambert MNT, Thybo CB, Lykkeboe S, Rasmussen LM, Frette X, Christensen LP, Jeppesen PB. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017 Sep;106(3):909-920. doi: 10.3945/ ajcn.117.153353
56 Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018 Sep;284(3):307-317. doi: 10.1111/joim.12805.
57 Takimoto T, Hatanaka M, Hoshino T, Takara T, Tanaka K, Shimizu A, Morita H, Nakamura T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized, placebo-controlled, double-blind clinical trial. Biosci Microbiota Food Health. 2018;37(4):87-96. doi: 10.12938/bmfh.18-006
58 Jansson PA, Curiac D, Lazou Ahrén I, Hansson F, Martinsson Niskanen T, Sjögren K, Ohlsson C. Probiotic treatment using a mix of
three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019 Nov;1(3):e154-e162. doi: 10.1016/S2665-9913(19)30068-2.
59 Wang H, Braun C, Murphy EF, Enck P. Bifidobacterium longum 1714™ Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am J Gastroenterol. 2019 Jul;114(7):1152-1162. doi: 10.14309/ajg.0000000000000203.
60 Liu J, Curtis EM, Cooper C, Harvey NC. State of the art in osteoporosis risk assessment and treatment. J Endocrinol Invest. 2019 Oct;42(10):1149-1164. doi: 10.1007/s40618-019-01041-6
61 Guo M, Wang H, Xu S, Zhuang Y, An J, Su C, et al.. Alteration in Gut Microbiota Is Associated With Dysregulation of Cytokines and Glucocorticoid Therapy in Systemic Lupus Erythematosus. Gut Microbes (2020) 11:1758–73. doi: 10.1080/19490976.2020.1768644
62 Druart C, Alligier M, Salazar N, Neyrinck AM, Delzenne NM. 2014. Modulation of the gut microbiota by nutrients with prebiotic and probiotic properties. Adv Nutr. 5(5):624s-633s.
63 Castaneda M, Strong JM, Alabi DA, Hernandez CJ. 2020. The gut microbiome and bone strength. Curr Osteoporos Rep. 18(6):677-683.
64 Lucas S, Omata Y, Hofmann J, Böttcher M, Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B, Krönke G et al. 2018. Shortchain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 9(1):55.
65 Anjum N, Maqsood S, Masud T, Ahmad A, Sohail A, Momin A. 2014. Lactobacillus acidophilus: Characterization of the species and application in food production. Crit Rev Food Sci Nutr. 54(9):1241-1251.
66 Rizzoli R. 2019. Nutritional influence on bone: Role of gut microbiota. Aging Clin Exp Res. 31(6):743-751.
67 Chen C, Dong B, Wang Y, Zhang Q, Wang B, Feng S, Zhu Y. 2020. The role of bacillus acidophilus in osteoporosis and its roles in proliferation and differentiation. J Clin Lab Anal. 34(11):e23471.
68 Lin YP, Thibodeaux CH, Peña JA, Ferry GD, Versalovic J. 2008. Probiotic lactobacillus reuteri suppress proinflammatory cytokines via c-jun. Inflamm Bowel Dis. 14(8):1068-1083.
69 Hemarajata P, Versalovic J. 2013. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 6(1):39-51.
70 Malmir H, Ejtahed HS, Soroush AR, Mortazavian AM, Fahimfar N, Ostovar A, Esmaillzadeh A, Larijani B, Hasani-Ranjbar S. Probiotics as a New Regulator for Bone Health: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2021 Aug 2;2021:3582989. doi: 10.1155/2021/3582989.
71 Vanitchanont, M.; Vallibhakara, S.A.-O.; Sophonsritsuk, A.; Vallibhakara, O. Effects of Multispecies Probiotic Supplementation on Serum Bone Turnover Markers in Postmenopausal Women with Osteopenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 461. https://doi.org/10.3390/nu16030461
72 Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018 Sep;284(3):307-317. doi: 10.1111/joim.12805
73 Ghanem KZ, Badawy IH, Abdel-Salam AM. 2004. Influence of yoghurt and probiotic yoghurt on the absorption of calcium, magnesium, iron and bone mineralization in rats. Milchwissenschaft 59:472–475.
74 Rodrigues FC, Castro AS, Rodrigues VC, Fernandes SA, Fontes EA, de Oliveira TT, Martino HS, de Luces Fortes Ferreira CL. 2012. Yacon flour and Bifidobacterium longum modulate bone health in rats. J Med Food 15:664–670 https://doi.org/10.1089/jmf.2011.0296.
75 Moudani, Walid & Shahin, Ahmad & Chakik, Fadi & Rajab, Dima. (2011). Intelligent Predictive Osteoporosis System. International Journal of Computer Applications. 32.

Centar Mikrobiom d.o.o. Sky Office, Toranj B R. F. Mihanovića 9, Zagreb, Croatia Mob.: + 385 98 13 73 344 Tel.: + 385 1 78 99 694 https://www.ccm.hr/

23 Nutramedic &Cosmetics
Oat-standing Nutrition, Oat-rageously Delicious!
SternLife presents three new oat-based product concepts with numerous health benefits.

SternLife will be exhibiting at the international trade fair “World of Private Label”, organised by the Private Label Manufacturers Association (PLMA). Under the slogan “Oat-standing nutrition, oat-rageously delicious”, the functional food experts will be presenting an oat shake, an apple-cinnamon porridge and an innovative oat bar in Amsterdam from May 28-29. All recipes are vegan, have a high protein content and meet the growing demand for healthy, plant-based and primarily regional products.
Oat offers numerous benefits and is increasingly on the radar of product developers. More and more foods are made from oat-based ingredients as plant-based alternatives to meat and milk. In fact, oats are the number one milk substitute. Demand for products made with this local grain is growing rapidly, particularly in Western Europe. Apart from classics such as oat flakes and oatmeal, oat milk and puffed oats are gaining in popularity in the development of new oat-based products.
Visitors to the PLMA trade show will be able to discover three oat-based product innovations at the SternLife stand. For a perfect start to the day, the German functional food supplier has developed the Vegan Oat Bowl Apple-Cinnamon. It consists of oat flour, protein and bran, and is rich in valuable fibre such as beta-glucans. These ingredients can help maintain normal cholesterol levels and, thanks to the inclusion of chia, this healthy breakfast porridge

also delivers optimal satiety.
The Vegan Protein Oat Shake is ideal for everyday use, too – whether as breakfast or a snack on the go. With wholemeal flour, protein and bran, this innovative drink includes three different oat ingredients and is available in two inspirational flavours: “Banoffee”, a combination of banana and toffee, and “Golden Milk”, an Ayurvedic-inspired spice mix.
Finally, SternLife presents the Premium Vegan Protein Bar Oat Cookie, a real protein hero amongst oat bars. It not only contains 22 per cent protein from oats, peas and soy, but is also rich in fibre and free from added sugar. The crunchy topping made from puffed oats in combination with its innovative vegan Milky-Choc coating makes this oat and cookie-flavoured bar a taste sensation.
Hans-Christian Seidel, Business Development Manager at SternLife, comments: “New product developments with oats as a key ingredient are on the rise. This is because the grain is a real all-round talent: healthy, natural, regional and highly versatile, as demonstrated by our new product range. From shakes to porridge and bars, come and discover innovative, cutting-edge products that are designed to tap into the growth potential of oat and optimize the use of health claims. Tailored for respective brands and target groups, our customers benefit from expert advice and support at every step of the journey: from concept to recipe development and production.”

24 Nutramedic &Cosmetics
BGG Creates New Global Base in Switzerland
BGG, one of China’s foremost suppliers of premium branded ingredients, has announced the establishment of a new global headquarters in Switzerland as part of a major restructuring plan.
Operating as BGG World, the HQ in Basel will be managed by newly appointed CEO Jürgen Nelis, supported by Thomas Adler, who joins as Global Commercial Director to spearhead the company’s international corporate strategy.
The opening of the BGG World HQ represents a significant leap forward for BGG’s global expansion efforts. The family-owned company, which is based in Beijing, is implementing a transformative plan to build on more than 25 years of continuous growth by fostering deeper relationships with its international customers and partners, while maintaining its strong production and R&D roots in China.
Centralizing critical international functions in Switzerland will streamline the alignment of BGG’s offer with its customers’ own strategies, meeting their needs more efficiently and enhancing risk mitigation within the global supply chain. The realignment will also leverage the significant market opportunities that exist for BGG’s innovative technology platforms and diverse product portfolio.
BGG World CEO Jürgen Nelis commented: “As we internationalize our organizational structure and operating model, we will be investing heavily in our commercial organization. We are currently hiring several sales managers in Europe and the US, and by doing so we will more than double our customer-facing resources in these key markets. At the same time, BGG will continue to invest in its asset base and in human clinical trials for its core products.”
Mr. Chunhua Li, the founder and principal shareholder of BGG, added: “We strongly believe that our three leading technology platforms – botanical extraction, enzymatic conversion, and micro-algae farming – combined with continuous investment in clinical trials, will provide our customers with high-value, sustainable business potential for the long-term future.”
Mr. Kun Li, a major shareholder and the son of Mr. Chunhua Li, shared this enthusiasm for BGG’s trajectory. “We are excited to take this logical next step to become the leading global producers of innovative natural ingredients that support human health and wellbeing,” he said.
Global Commercial Director Thomas Adler said: “This strategic overhaul underscores BGG World’s commitment to excellence, innovation, and global leadership in the natural ingredients sector, promising a future of sustained growth and international success.”
In line with its aggressive growth strategy, BGG World has also announced plans to diversify into the food ingredients sector, beginning with BGG’s existing stevia and licorice extract solutions. The company intends to broaden its product offerings further in the coming years, reinforcing its commitment to innovation in dietary supplements, cosmetics, and food ingredients.
Review on Omega-3 Diagnostics Testing Published
The review highlights the landscape of available omega-3 tests and the evidence linking LCPUFA intake to improved health.

The Global Organization for EPA and DHA Omega-3s (GOED) is pleased to announce the publication of a review the organization commissioned on the state of omega-3 diagnostics testing.
"Omega-3 Polyunsaturated Fatty Acid Status Testing in Humans: A Narrative Review of Commercially Available Options," published in The Journal of Nutrition, highlights the landscape of available omega-3 tests and the importance of diagnostics testing for improving EPA and DHA consumption.
This review provides an overview of the options available for measuring omega-3 long-chain polyunsaturated fatty acids (LCPUFA) status, including both dietary assessments and commercially available analytical tests of blood and breast milk samples. The publication also showcases the evidence linking increased omega-3 LCPUFA intake or status to improved health, focusing on cardiovascular, neurological, pregnancy and eye health outcomes. Harry B. Rice, PhD, GOED’s Vice-President of Regulatory & Scientific Affairs noted, “Seeing the overview of scientific evidence on omega-3 levels and health outcomes really gives you a sense of where the strongest evidence exists and where there might be gaps. A review like this will undoubtedly be invaluable during a discussion on dietary reference intakes for EPA/DHA.”
GOED commissioned this paper as part of its work to support diagnostics as a key tool for increasing omega-3 consumption globally and will continue to work to educate consumers about the benefits of EPA and DHA.
25 Nutramedic &Cosmetics
For more information visit: www.bggworld.com
Fine Foods: Growth With Sustainability
For 40 years, Fine Foods - Italian independent contract development & manufacturing organisation - has been establishing genuine, transparent and results-oriented relationships with its customers and is considered a reliable partner.
Founded in 1984, Fine Foods & Pharmaceuticals N.T.M. S.p.A. is an Italian independent Contract Development & Manufacturing Organisation (CDMO). It develops and manufactures contract products for the pharmaceutical, nutraceutical and cosmetics industries. It is the first Italian CDMO listed on Euronext STAR Milan. In 2021 it became a Benefit Corporation, strengthening its ethical and transparent business model.
Group revenue for 2023 was € 251.8 million and showed an increase of 21.7% compared to 2022. Adjusted EBITDA showed an increase of 64.2% in 2023 compared to the previous year.
The 2023 Sustainability Report showed a year of significant achievements. The company has strengthened its commitment to sustainability as defined in its 2025 strategy, aligning with the UN's Sustainable Development Goals - Agenda 2030. This led to substantial advancements and the attainment of targets, enhancing the company's ESG performance.
Prestigious awards were received, including the "Platinum" EcoVadis rating obtained in August 2022 and confirmed in August 2023 with a score of 83 and which placed Fine Foods in the top one per cent of
the 100,000 companies evaluated by the agency. Additionally, Morningstar-Sustainalytics rated Fine Foods’ ability to manage ESG risks as "Strong" in its annual rating.
"We are proud of our exceptional performance in 2023, with revenue growth nearly twice the usual levels. The robustness of our business model and organisation has allowed a rebound in margins, and we are on the cusp of exceeding historical benchmarks. As the market expands, we are prepared to increase our market share: the investments to increase production capacity already completed within the Cosmetic BU, and those planned for the Pharma and Nutra BUs in the next two years, will enable us to meet the growing customer interest in our Group. This results in a highly favourable outlook for the 20242028 period.” - Fine Foods & Pharmaceuticals N.T.M. S.p.A. CEO Giorgio Ferraris.
For 40 years, Fine Foods has been establishing genuine, transparent and results-oriented relationships with its customers and is considered a reliable partner. To demonstrate its approach, Fine Foods will attend Vitafoods in Geneva again this year, the main nutraceutical industry event in Europe. The

26
Nutramedic &Cosmetics

event is a platform to unveil the newest products and services and engage in dialogue on sectoral challenges, supported by an extensive schedule of events over three days.
Our reliability is rooted in a commitment to excellence and quality across all functions and stages, along with a focus on ESG issues which are increasingly integrated into different Fine Foods business processes. This includes the Research and Development department's dedication to suggesting solutions aimed at decreasing the environmental impact of products.
The Group has chosen to be certified for its carbon footprint under UNI EN ISO 14064 standards in 2023. This underscores its commitment to transparent environmental reporting and establishing foot-
print reduction goals following expert recommendations. Fine Foods supported customers in calculating the carbon footprint of their products and looked for strategies to make them more sustainable. Fine Foods is selecting and testing new materials for the circular economy.
The Group's strategy included the increasingly active and responsible involvement of players along the supply chain. It expanded the number of suppliers engaged in the process of assessing and improving their environmental and social performance, with a focus on respect for human rights.
The company’s focus on people's health continues, by participating in local programmes and adopting good health practices (cancer prevention, physical activity, healthy diet and psychological well-being).
The Group intends to continue its sustainability programme and serve as a benchmark for assessing products that align with the evolving market demands.
Fine
Foods & Pharmaceuticals N.T.M. S.p.A.
Via Berlino, 39 24040 Zingonia/Verdellino –
Bergamo – Italy
www.finefoods.it info@finefoods.it

Fine Foods develops and manufactures contract products for the pharmaceutical nutraceutical and cosmetics industries.
Nutramedic &Cosmetics
Fine Foods & Pharmaceuticals N T.M S p A COMMIT TED TO YOUR E XCELLENCE
Revolutionising Pharmaceutical and Nutraceutical Delivery: Exploring HPMC Vcaps® and DRcaps® Capsules Advantages
For pharmaceutical and nutraceutical producers, Lonza offers unrivalled productivity and performance through its Capsugel® capsules, alongside dosage form solutions tailored to optimise product performance across all stages of development. Their specialized capsules and equipment streamline pharmaceutical R&D processes, addressing industry challenges and accelerating time to market.
AUTHOR:
The origin of the word capsule comes from the Latin capsula , which means a small box.
Empty hard capsules, also referred to as hard-shell or two-piece capsules, are solid forms of medication wherein one or more medicinal substances and/or inert materials are enclosed within a compact shell. The concept of capsules dates back to the nineteenth century when both hard and soft variants were initially patented and utilised in France.
These capsules have become a staple in the pharmaceutical industry, effectively addressing numerous challenges encountered in drug delivery and the formulation of food supplements and nutraceuticals.
Two-piece hard capsules, a subset of this form, have firmly established themselves as a solution to
contemporary challenges in drug delivery and nutraceutical formulation. They serve as an essential component in facilitating innovative drug development initiatives. The increasing demand for natural alternatives to gelatin capsules, such as hydroxypropylmethyl cellulose (HPMC), has further propelled the utilisation of two-piece capsules.
Capsules, in their various forms, hold a historical significance, tracing back to their use by ancient Egyptians, making them one of the oldest known dosage forms. This well-established dosage format effectively addresses contemporary challenges in drug delivery and nutraceutical formulation.
Each hard gelatin capsule shell consists of two preformed cylindrical segments - a cap and a bodyeach featuring one rounded, sealed end and one open end. The body, slightly smaller in diameter, fits
28
Nutramedic &Cosmetics
Daria Šurić, MPharm
snugly inside the cap. These empty capsule shells are manufactured by specialised suppliers and later filled with substances during a separate production step.
During the capsule-filling unit operation, the body is filled with the substances and the shell is closed by bringing the body and the cap together.
Capsules generally are taken orally. Excipients such as opaque fillers, antimicrobial preservatives, sweetening agents, permitted flavouring agents and one or more colouring agents may be added. Capsules may be printed on the outer surface. They also may contain ingredients in solid, paste or liquid states. Additionally, empty hard capsules can be filled with different types of formulations such as powders, granules, or semi-solids, making them suitable for a wide range of drug delivery systems.
Furthermore, empty hard capsules are designed to meet the strict standards of the pharmaceutical industry, ensuring the safety and efficacy of the contained medication, but they are also suitable dosage form for food supplements.
Lonza's Capsules & Health Ingredients division stands as the trusted ally for pharmaceutical and nutraceutical companies seeking innovative solutions in capsules, dosage forms, and health ingredients.
For pharmaceutical and nutraceutical producers, Lonza offers unrivalled productivity and performance through its Capsugel® capsules, alongside dosage form solutions tailored to optimise product performance across all stages of development. Their specialized capsules and equipment streamline pharmaceutical R&D processes, addressing industry challenges and accelerating time to market.
Lonza's global network comprises 12 manufacturing sites, producing billions of capsules annually. With 2,500 innovation labs worldwide, Lonza remains at the forefront of scientific and engineering excellence, offering the broadest range of capsule polymers, sizes, and designs in the industry.
With a steadfast focus on customer success and a commitment to quality, innovation, and reliability, Lonza continues to shape the future of capsule technology.
In the production process, the quality and integrity of the empty hard capsules are of utmost importance. Quality control measures, including checks for size uniformity, mechanical strength, and resistance to tampering, are crucial steps in ensuring the reliability and performance of the capsules in pharmaceutical products. Several factors must be considered when selecting empty hard capsules for production. These factors include the compatibility of the capsules with the drug formulation, the required dosage strength, the desired release profile, and any specific handling or storage conditions. In recent years, there have been advancements in the development of empty hard capsules to address specific challenges faced during production.
When it comes to the production of food supplements and nutraceuticals, the use of empty hard capsules presents numerous opportunities and advantages. These capsules offer a versatile and customizable solution for encapsulating various types of formulations, including powders, granules, and semi-solids used in the production of dietary sup-
plements and nutraceuticals.
Capsules are capable of protecting encapsulated substances from deterioration induced by atmospheric oxygen, light, moisture etc. owing to the barrier effects of the shell. Immediate-release capsules with a simple powder filling are the best-known type of hard gelatin capsules. They require only a few manufacturing process steps. Usually, it is easy to mix the active substance with excipients and to fill the mix into the capsules. Capsule production does not require expensive and time-consuming operations like repeated mixing and sieving, or granulation and compression.
In the fluid environment of the stomach, the shell of the capsule starts to soften and dissolve within one or two minutes and comes apart. Hard gelatin capsules are fully disintegrated within about 10 minutes. Hard gelatin capsules are easier and quicker to formulate and produce, whatever the batch size, compared with other solid oral dosage forms.
Indeed, by using hard gelatin capsules it is possible to produce small or very small batch sizes on manual or semi-automatic filling machines1
The availability of empty hard capsules in different sizes and materials provides flexibility in meeting specific dosage requirements and delivery systems for food supplements and nutraceuticals. This allows for the creation of tailored products to cater to different consumer needs and preferences.
As the demand for food supplements and nutraceuticals continues to grow, harnessing the opportunities and advantages offered by empty hard capsules can contribute to the efficient and high-quality production of these products.
Unveiling consumer preferences for oral solid dosage forms: capsules in the spotlight
Lonza Capsules and Health Ingredients have made a consumer survey on oral solid dosage forms. It has found proof of the growing popularity of capsules. It provides valuable insights into the preferences and trends surrounding the use of capsules
According to this research, capsules are a popular choice among consumers due to their easy-to-swallow nature, durability, and user-friendly design. Capsules have high awareness, usage, and preference rates among consumers. The study also found that capsule purchasers are willing to invest more, indicating a strong commitment to the product. Businesses can benefit from strategically incorporating capsules into their products, as it can improve brand distinction, and customer loyalty and lead to continuous expansion. By taking advantage of the unique appeal and advantages of capsules, companies can tailor their products to meet the evolving preferences of their target demographic.
In Europe, capsules, tablets and powder sachets are the three most prevalent oral dosage forms being the most known and most likely to have been used in the past 12 months for any usage3. To European consumers, the key evaluation criteria for a good dosage form are:
1. not getting stuck in the throat
2. is easy to swallow
3. being manufactured at a high-quality standard.
29
Nutramedic &Cosmetics


Capsules and tablets don’t differentiate against each other on the key evaluation criteria according to the average European user.
HPMC (hydroxypropylmethyl cellulose) capsules explained
HPMC capsules have emerged as a popular alternative to traditional gelatin capsules in the production of food supplements and nutraceuticals1. These capsules are made from hydroxypropyl methylcellulose, a plant-based material that offers several benefits.
HPMC is widely used as an excipient in pharmaceutical, food and cosmetic formulations and is described in the “GRAS” list (Generally Recognized As Safe), the United States and European Pharmacopoeias, and the European Union as E464.
HPMC capsules provide a vegetarian and veganfriendly option, as they are free from animal-derived gelatin. Furthermore, HPMC capsules have superior stability, both in terms of physical properties and dissolution characteristics, making them ideal for the encapsulation of sensitive ingredients found in food supplements and nutraceuticals.
Moreover, the use of HPMC capsules in the production of food supplements and nutraceuticals allows for a wider range of fill materials to be used, including those with specific chemical structures, physical states, or hygroscopic properties that may not be compatible with gelatin capsules.
These properties make HPMC capsules a highly flexible and widely acceptable platform for addressing the challenges faced by the pharmaceutical and nutraceutical industries. In the disintegration test, HPMC capsules without a gelling agent demonstrated disintegration times of less than 10 min2,4
Capsugel® Vcaps® are made from HPMC, using Capsugel’s traditional dip moulding process and drying techniques. They fit all current capsule-filling machines and there are no special change parts required. Also, on-site technical service support is offered.
Lonza’s branded Capsugel® Vcaps® is a standard for vegetarian offerings worldwide. Both KO and OU Kosher and Halal certification, and certified by the
Vegetarian Society, are an appealing way to address the preferences of an important growing group of supplement users who indicate that vegetarian sources are important in their decision-making. Capsugel® Vcaps® capsules are non-GMO, gluten-free and preservative-free, a very clean-label.
Formulation benefits: the physically stable polymer is resistant to stringent heat and humidity conditions and low moisture content suitable for moisture-sensitive ingredients.
Size 00 hard HPMC capsules facts and advantages
In the production of food supplements and nutraceuticals, it is essential to consider the usage of size 00 of empty hard HPMC capsules. These capsules are made from hypromellose, a vegetarian-friendly alternative to gelatin, making them suitable for a broader consumer base, including those with dietary restrictions or preferences.
The size of 00 capsules provides a larger capacity for filling with various formulations, accommodating the nutrient diversity often found in food supplements and nutraceuticals (Table 1). Their compatibility with a wide range of ingredients and the ability to maintain the stability of sensitive compounds make them an ideal choice for encapsulating bioactive substances. TABLE 1 Vcaps Size 00 Capacity – volume in ml and weight in
For example, magnesium which is one of the most popular ingredients in food supplements needs a large volume of capsules to be able to deliver the
30 Nutramedic &Cosmetics
CAPACITY Size
Capsule volume (mL) 0.91 Powderdensitymg/mL Capsulecapacity(mg) 600 546 800 728 1000 910 1200 1092
g., depending on powder fill density
00
FIGURE 1 HPMC (hydroxypropylmethyl cellulose), transparent capsules, size 00


recommended 375 mg to 400 mg elemental magnesium. Especially if we are talking about magnesium citrate since typically, magnesium citrate ingredients contain about 11% elemental magnesium by weight. Size 00 of empty hard HPMC capsules offers better compliance in this case, compared to smaller capsules.
Also, compared to big tablet formats, 00 hard capsule is easy to swallow making it a good alternative.
Another example of 00-size capsule usage is Glucosamine sulphate since most studies are done on


1500 mg if we are talking about the standalone ingredient.
Advantages of clear capsules –clean label and more…
Clear capsules offer numerous advantages in the realm of food supplements and nutraceuticals. Their transparent nature allows consumers to see the contents of the capsules, providing a visual reassurance of the purity and quality of the ingredients.











































Nutramedic &Cosmetics
www.amacreo.com amacreo@amacreo.com
strong link that connects partners and customers for better health We connect foreign manufacturers and customers in the field of Rx and DS primary packaging with reliable and valuable information at the right time, in order to create a healthier future together.
FIGURE 2 HPMC DR (hydroxypropylmethyl cellulose delayed release), transparent capsules, size 00
A

Moreover, the use of clear capsules can enhance the overall aesthetic appeal of the supplements, creating a modern and visually appealing product presentation. This can be particularly advantageous when targeting health-conscious consumers who value transparency and a sleek product design.
From a functional perspective, clear capsules also allow for the easy identification of the encapsulated ingredients, facilitating quick recognition for users with specific dietary requirements or preferences.
In summary, clear labelling and the use of clear capsules in food supplements not only prioritize consumer trust and choice but also elevate the overall presentation and accessibility of these beneficial products.
DR (Delayed Release capsules) for food supplements and nutraceuticals
The use of Delayed Release capsules made from HPMC in the production of food supplements and nutraceuticals provides an effective way to protect sensitive bioactive substances from degradation in the harsh environment of the stomach.
These specialised capsules feature a unique coating that resists dissolution in the acidic pH of the stomach, allowing them to pass through to the intestines where they slowly release their contents. This controlled release mechanism ensures maximum absorption and bioavailability of the encapsulated ingredients, thereby enhancing the overall efficacy of food supplements and nutraceuticals.
A gamma-scintigraphy study5 confirms delayed release properties of Capsugel® DRcaps® capsules in human subjects.
Supplement manufacturers have turned to a range of dosage delivery solutions to enhance the protection of moisture and acid-sensitive ingredients. Some environments, especially the low pH of the stomach, can destroy some acid-sensitive strains.
Capsugel®DRcaps® capsule was developed to effectively address all of the various challenges and shortcomings of delivering acid-sensitive ingredients. The innovative polymer properties also mask the taste and odour of ingredients; therefore, by protecting against early disintegration, DRcaps® capsules reduce the potential for unpleasant odours, aftertaste and reflux from supplements.
In particular, the unique polymer properties were designed to resist acid to protect nutritional ingredients from full release and disintegration in the stomach and allow for complete dissolution in the intestine.
Human clinical study
To clinically assess the efficacy of Capsugel®DRcaps® capsules for resistance to stomach acids for delayed delivery properties of ingredients, Capsugel commissioned the independent laboratory, Bio-Images Research, in Glasgow, Scotland, to conduct a gamma scintigraphic invivo study. The human clinical study was designed to evaluate the disintegration behaviour of unbanded DRcaps® capsules in human subjects. Completed in spring 2013, the study documents through images and data the success of unbanded DRcaps® capsule's resistance to stomach acids for delivery of ingredients. The results of this
in vivo human clinical trial present empirical evidence of successful acid-resistance and delayed-release properties of DRcaps® capsules. The innovative DRcaps® capsule targets release after a certain period, protecting ingredients from full disintegration in the stomach5
Delayed-release capsules can play a vital role in optimising vitamin C absorption. The delayed-release technology helps protect vitamin C from being degraded by stomach acid, allowing it to bypass the acidic environment of the stomach and reach the small intestine, where it can be better absorbed by the body. This is especially beneficial for individuals with sensitive stomachs or those who have difficulty absorbing vitamin C.
By using delayed-release capsules, the bioavailability and effectiveness of vitamin C can be enhanced, ensuring that individuals can reap the full benefits of this essential nutrient.
The use of delayed-release capsules can also be beneficial for probiotics. Probiotics are sensitive to harsh stomach acids and can be destroyed before reaching the intestines where they are most effective.
The enzymes, for example, amylase enzymes need protection from denaturing in the stomach, which would render them unfit for hydrolyzing glucose.
Conclusion
From their ancient origins to modern innovations, capsules have become a cornerstone in nutraceutical delivery.
HPMC Vcaps® and DRcaps® capsules represent significant advancements in this field, offering tailored solutions that meet the highest standards of safety, efficacy, and consumer preference. These capsules provide versatile options for encapsulating sensitive ingredients and optimizing nutrient absorption, ensuring that they remain indispensable tools in pharmaceuticals, food supplements, and nutraceuticals for years to come.
References:
1 Stegemann S. Hard gelatin capsules today–and tomorrow. 2nd ed. Capsugel Library, Bornem; 2002
2 Majee, S B., Avlani, D., & Biswas, G R. (2017, October 2). HPMC as capsule shell material: physicochemical, pharmaceutical and biopharmaceutical properties.
3 Lonza OSDF Usage & Attitude Study May 2023 Europe
4 Al-Tabakha, M M. (2010, October 14). HPMC Capsules: Current Status and Future Prospects. https://doi.org/10.18433/j3k881
5 Robert Amo, Director Global OTC and Health & Nutrition Marketing, Capsugel Inc. DRcaps® Capsules Achieve Delayed Release Properties for Nutritional Ingredients in Human Clinical Study
6 Technical Reference File_v2023_Rx_HN_all prod.pdf

Amacreo d.o.o. Radnička cesta 34, Zagreb, Croatia T. +385 1 2339 767 e: amacreo@amacreo.com
32 Nutramedic &Cosmetics
www.amacreo.com
Vitafoods Europe 2024 Preview
We are just two weeks away from the start of this year's event, where over 20,000 visitors and 1,100 exhibitors are expected. In our special feature, discover the highlights you shouldn't miss and what selected exhibitors have prepared.
Informa Markets announced that the world’s nutraceutical event, Vitafoods Europe, would take place in Geneva for the final time in 2024 before heading to its new home of Barcelona. Taking place between 14-16 May, Vitafoods Europe 2024 is set to be the biggest and best edition of the show yet, with new must-see content and features, as well as returning fan favourites.
Over the course of two decades, Vitafoods Europe has become the go-to annual event in the nutraceutical calendar – and 2024 is set to be no exception. Not only does the event mark the final chapter in the show’s time in Geneva, but organisers Informa Markets expect that the 2024 event will be the biggest yet.
With new and returning show features dedicated to pushing the boundaries of innovation, encouraging knowledge sharing and providing quality networking opportunities, Vitafoods Europe is the only nutraceutical event to bring together the entire supply chain, helping key players reach new audiences
and make meaningful connections.
Alongside the debut of a new Technology & Equipment Area and R&D Pavilion, as well as an expanded Finished Products Area, the Future of Nutrition Summit (13 May, Marriott Hotel) and Vitafoods Europe Conference (14-15 May) are back by popular demand.
The latter will be free to attend for 2024 as a thank you to the nutraceutical community for its support over two decades, providing access to industry-leading content for all.
Go discover what lies ahead at the Future of Nutrition Summit
The Future of Nutrition Summit (13 May, Marriott Hotel, Geneva) is back! Designed for independent futurist thinkers, in 2024 the exclusive paid-for one-day summit will dive deep into the future of health, product development and retail.
Answering the question ‘what will the nutraceutical industry look like in 5 years’ time?’, delegates can

33
Nutramedic &Cosmetics

expect key insights on everything from artificial intelligence, personalised nutrition, precision fermentation and more. Featuring experts from the industry’s top brands, speaker highlights include Huel, Nuritas, Holland & Barrett, Kline & Company. The Summit will also provide opportunities to network with C-level experts from a range of diverse disciplines, including food science, sustainability, retail, nutrition and public health, as well as start-ups, scale-ups and investors.
Get free expert insights on the Vitafoods Europe show floor
As a heartfelt thank you to the nutraceutical community for its continued support over the event’s 26-year history, all content on the Vitafoods Europe 2024 show floor – across the Vitafoods Insights Theatre and Vitafoods Europe Conference – is free to attend this year.
Vitafoods Europe Conference
This two-day conference will see experts gather for insights and conversations across a range of health benefit areas, including sports nutrition, immune and gut health, cognitive and emotional health, and healthy ageing. Expect data-driven content, evidence-based insights and discussions on the latest research and ingredients from companies such as Mintel, TNO, Yakult, and SPINS, as well as leading academic researchers. Plus, get the chance to discover new innovations and formulation strategies to tap into the industry’s biggest trends.
Speakers featuring at the Conference this year include:
Dr Olaf Larsen, Senior Manager Science and Assistant Professor, Yakult, with a deep-dive into the importance of gut microbiota management for healthy ageing
• Dr Susan M. Kleiner, Founder and Owner of High Performance Nutrition LLC, on opportunities to optimize nutrition for female athletic performance
• Vivien Rolfe, Director of Curiosity Research, highlighting the research on the benefits of botanicals for cognitive and emotional health
• Varun Dwaraka, Head of Bioinformatics, TruDiagnostic Inc., exploring the intersection of nutrition and molecular diagnostics for health assessment.
Meanwhile, for businesses looking to keep their finger on the pulse of the health & nutrition, the renowned Vitafoods Insights Theatre will feature a host of free expert-led content exploring the major updates and consumer trends influencing the global nutraceutical market. Attendees can discover insights to help their businesses overcome the real-world challenges facing the industry today, with sessions on sustainability, regulatory, market challenges and more.
Key speaker highlights include:
James Collier, Co-Founder & Nutritionist, Huel, discussing how businesses can provide optimum nutrition while addressing environmental pressures and ethical challenges
• Elizabeth Thundow, VP Consulting, Food & Nutrition Ingredients, Kline & Company, and Simon Pet-
tman, Director of IADSA, in a panel discussion on consumer education in the influence economy
• Nicole Jansen, Account Management Team Lead, Innova Market Insights, spotlighting the beneficial ingredients making waves in the functional food & beverage landscape
• Bill Giebler, Content & Insights Director, Nutrition Business Journal, with a data-led exploration of the global dietary supplement market.
“Collaboration is key in inspiring positive change in health & nutrition – this is why we aim to ensure that there is something for everyone at Vitafoods Europe 2024. Whether you supply ingredients, manufacture products, or are a distributor or retailer, our aim is that you come away with unique insights into how you can drive your business forward. And this year, we’ve made a large amount of valuable show-floor content free to attend to further extend the learning and discovery opportunities for everyone”, says Julien Bonvallet, Group Brand Director at Informa Markets.
Fan favourite the Vitafoods Insights Theatre will also return this year, hosting a range of free expert-led content. This renowned theatre will be located on the show floor, where attendees can discover ways to help their businesses overcome the real-world challenges facing the industry today, with sessions on sustainability, regulatory, market challenges and more.
Discover innovation opportunities with new show areas
In 2024, Vitafoods Europe will be introducing two new areas, designed to tap into two of the fastestgrowing verticals in the nutrition industry. The debut of the Technology & Equipment Area marks the first ever areas exclusively dedicated to the technology and equipment innovations driving the industry forward.
Created in response to visitor demand, it will bring together leading players in technology innovation –such as IMA, OMAG and Marchesini Group – to showcase their cutting-edge solutions in action. In addition, the new R&D Pavilion will provide a platform for networking and discovering business opportunities in everything from clinical trials, laboratory testing, regulatory services, certifications and market research.
Finished products – back and bigger than ever
The expanded 2024 Finished Product Area will offer even more opportunities for manufacturers to connect with leading brands, retailers and distributors. With the likes of Nestlé, Mars, Holland & Barrett and Wholefoods attending the event each year, the Finished Products Area is the go-to place to discover the latest innovative concepts and market-ready products on offer. Visitors can scout out the industry’s latest innovations in the New Product Zone and sample some of them for themselves at the Tasting Centre.
In the following text, read an overview of the products that the selected exhibitors present to the visitors of Vitafoods Europe 2024.
34 Nutramedic &Cosmetics
Go discover world-class networking
Meet leading influencers and decision-makers from across the nutraceutical industry
14-16 May 2024 Geneva
vitafoods.eu.com

Nutramedic &Cosmetics

Aker BioMarine Human Ingredients AS BOOTH:
D96
Personalization is trending in the omega-3 space, and the Superba Krill Oil team is excited to share their insights on innovation, condition specific supplements, and all the ways they are enabling brands to develop unique, on-trend products. From women’s health and sports nutrition to healthy aging and skin health, consumers are looking for specific health benefit solutions in an easy, convenient format - the Superba team has the solutions, and krill can play a big role. From emulsions and other unique delivery formats to combination products, krill oil is on the move and is a great stand-alone supplement or perfect as the complementary ingredient for a blended multi-nutrient product.
Superba Krill oil has the purest, naturally occurring combination of phospholipids, omega-3 fatty acids (EPA & DHA), choline and astaxanthin. The advantage comes from their patented concentration of phospholipids which delivers omega-3s and choline directly into the cell membrane for whole body support. It is also a stable and sustainable source which is very important for brands and consumers alike, especially at a time when the fish oil industry is facing supply and pricing challenges.
As the leader in krill oil and expert in producing high quality, sustainable marine ingredients, Aker BioMarine is adding algae oil to its portfolio. The new B2B brand FloraMarine™ is the highest, natural concentration of DHA omega-3 oil on the market delivering health benefits for brain, eye, maternal and fetal health. The health halo combination of plantbased product benefits, paired with its environmentally friendly and sustainable practices, makes FloraMarine a unique solution in the omega-3 category.
https://www.akerbiomarine.com/


Alvinesa
BOOTH: D143
Discover Alvinesa's upcycled natural ingredients and explore their flagship products: Vintera™ grape extract and Hytolive® olive extract, each with versatile applications.
Vintera™, a grape extract standardized in proanthocyanidins, monomers, and anthocyanins, offers a myriad of health benefits. Its potent antioxidant properties support cardiovascular health, skin vitality, sport recovery and cognitive function. With applications ranging from nutraceuticals to functional foods and beverages, Vintera adds value and functionality to your products.
Hytolive®, Alviensa’s natural olive extract rich in hydroxytyrosol, is obtained through purely mechanical processes without solvents, preserving its 100% natural essence. Renowned for its powerful antioxidant properties, Hytolive combats free radicals, potentially benefiting cardiovascular health, glucose management, anti-aging, and anti-inflammatory relief. Its versatility extends to cosmetics, supplements, and functional foods.
Alviensa is introducing Hytolive® Bev, cutting-edge creation showcased in the New Ingredients Zone, perfect for sports nutrition. With zero impact on taste, Hytolive® Bev transforms functional beverages, providing athletes with a revitalizing solution to harness the health advantages of hydroxytyrosol.
Tailored for today's health-conscious athletes, it promises to redefine the sports beverage market, delivering both performance enhancement and pleasure.
Join them to learn more about how Alvinesa's sustainable and upcycled ingredients can elevate your products and meet the evolving needs of today's health-conscious consumers. Experience the power of nature with Alvinesa at Vitafoods Europe.
www.alvinesa.com

Arandovo BOOTH:
K61
Join Arandovo to discover their 100% natural functional ingredient, MKARE®. By recycling eggshell membranes and granting them a "second life," this brand actively contribute to a fully circular economy. The eggshells they sell are fresh and suitable for vegeterians.
For those unfamiliar, MKARE®, boasts a rich composition of bioactive compounds such as collagen (type I, V, X), hyaluronic acid, elastin, cysteine-rich proteins, among over 400 other proteins. Thanks to these bioactives, MKARE® serves as a nutrient for the skin, providing hydration, elasticity, and helping to reduce wrinkles. For hair, it strengthens follicles,
36
Nutramedic &Cosmetics
promotes growth, and improves texture. It also trengthens the structure of nails, reduces fragility, and fosters healthy growth.
This company also published a study in the prestigious "Journal of Functional Foods" titled "Anti-inflammatory and regenerative effects of MKARE® eggshell membrane: An in vitro osteoarthritis model and placebo-controlled clinical study." It delves into MKARE®'s anti-inflammatory properties through in vitro and clinical studies, showcasing its efficacy in alleviating symptoms of osteoarthritis (OA) and enhancing joint health. The study underscores the paramount importance of freshness in optimizing therapeutic benefits.
Vitafoods visitors will have the opportunity to engage with Arandovo team, gain valuable insights, and experience firsthand the advancements shaping our industry.
https://www.arandovo.com/

AstaReal BOOTH: H110
Double algae power for better health; AstaReal is presenting new formulation that combines different microalgae-derived ingredients - astaxanthin and omega-3 fatty acids. It was developed in cooperation with the French omega-3 expert Polaris. AstaReal will also showcase its classic retail supplement Astaxin®, which was recently rebranded with a more modern new look.
AstaReal has added a new finished formula to its range of natural astaxanthin products and will showcase it for the first time. For the microalgae combination, they partnered with the French company Polaris to create a sustainable and vegan product that contains not only astaxanthin but also omega-3 fatty acids EPA and DHA.
While astaxanthin from microalgae Haematococcus pluvialis is a strong antioxidant with cell protective power, EPA and DHA from microalgae Schizochytrium are important for flexible membranes and contribute to the normal function of the heart. DHA also contributes to the maintenance of normal brain function and vision.
AstaReal will also showcase its proven range of high-quality astaxanthin bulk products and finished formulations. Visitors can also take a look at the recently rebranded Astaxin. It contains astaxanthin, vitamin C and E – the two latter contribute to the protection of cells from oxidative stress. Company also offers astaxanthin for animals through its feed and pet food grade NOVASTA®, with products such as EB15 known for high stability.
Peter Ahlm, Head of Marketing & Sales at AstaReal, comments: “We are excited to introduce our new finished formulation that combines the most precious ingredients found in microalgae, namely astaxanthin and long-chained omega-3 fatty acids. Through this launch, we are committing to algae as a
plant-based, fast-regrowing and future-proof source which will gain further traction in the nutrition and supplement market. One pillar in the success of algae is the growing scientific research on its ingredients. With our longstanding expertise as well as through our studies and clinical trials, we contribute to a better understanding of astaxanthin and its health benefits for humans and animals.”
https://www.astareal.se/

Euromed BOOTH:
J50
Euromed, leading supplier of botanical extracts will be showcasing its latest innovation. This company presents natural ingredient solutions for healthy ageing and a holistic range of plant extracts for improved wellbeing in later life.
The company will highlight a diverse range of natural herbal extracts inspired by the Mediterranean diet. The focus will be on its new cucumber extract CuberUp®, a potentially promising natural approach to the maintenance of healthy joints.
Euromed's renowned range of Mediterranean fruit and vegetable extract, including lemon, pomegranate, olive, artichoke, fig and spinach, continue to be at the forefront of supporting cardiovascular, metabolic, digestive, immune and skeletal muscle functions. Marketed under the brand names Wellemon®, Pomanox®, Mediteanox®, Cynamed®, ABAlife® and Spisar®, they are processed with an eco-friendly, proprietary extraction technology that uses purified water as the solvent.
A major highlight of this year's presentation is CuberUp®. Backed by a recent double-blind clinical study, the cucumber extract has a positive impact on knee pain, mobility and muscle function in people over 40 years with knee function limitations.
This product offers a promising approach for all those struggling with joint problems or looking to prevent them.
To continue to provide such new medicinal botanical innovations, Euromed is committed to the advancement of scientific and research methodologies, as explained by Andrea Zangara, Head of Scientific Communication and Medical Affairs at Euromed: “We combine tradition and science, which is further supported by advanced technological applications in our research strategy.
For example, we are exploring new predictive methods, including bioinformatics, to forecast the efficacy and safety of new extracts for evaluation in subsequent clinical trials. We aim to generate new scientific knowledge through more efficient methodologies, thereby improving the quality of our science.”
www.euromedgroup.com
37 Nutramedic &Cosmetics


Fine Foods
BOOTH: J32
Fine Foods will unveil its latest products; visitors will discover the Biotic3™ range which combines prebiotics, probiotics and postbiotics in one product. The range covers new categories such as healthy ageing and metabolic system.
The Hydralibrium™ range of portable liquid gel sticks enhancing cellular hydration will receive significant focus. Nextcaps™ is the innovation unveiled during this event - the new generation of capsule products, one of the most widely used formats in supplements in Europe. This overcomes the technical limitations of hard capsules such as, the combination of powder and liquid in one product.
The exhibition will showcase new products in specific sections:
New Products Zone: Nextcaps™, innovative capsules for relaxation
• New Products Zone: Biotic3™ Gut Health, with a combination of prebiotics, probiotics, and postbiotics
Tasting Centre: Hydralibrium™ Beauty, for hydrating beauty.
On 15 May at 3 pm at the Biotics Theatre (stand K232), Fine Foods will engage in a highly anticipated sector discussion titled "Face to Face with Biotics: Exploring Trends and Regulatory Complexity in the European Market". This talk will analyse the regulatory challenges and market trends affecting biotic products, in partnership with Innova Market Insights.
Moreover, Fine Foods has been chosen as one of the exhibitors featured in the Sports and Active Nutrition Innovation Tour, organized by Vitafoods. This event is scheduled for May 14th at 11 am CET, at the Innovation Tour Meeting Point, R214.
www.finefoods.it/en/home-eng/

GC Rieber VivoMega™
BOOTH: G170
GC Rieber VivoMega™ is pleased to announce the launch of its new algal EPA+DHA omega-3 concentrate. The EPA and DHA combination is Vegan certified, non-GMO with superior triglyceride concentration (90%), bioavailability, purity, and sensory profiles. Its SuperLight™ proprietary processing technology is designed and developed exclusively for algal oil. Its process utilizes high-end technologies, allowing for gentle, non-invasive processing with superior oxidation protection. This results in a product with exceptional quality parameters and optimal resource utilization with little to zero waste.
Visit their booth to "Taste the Difference Quality Makes" and learn more about our commitment to the industry through our sustainable, traceable, and innovative technologies, which enable us to provide world-class oxidation parameters.
https://vivomega.com/
Gelita
BOOTH: E96
Gelita presents a pioneering ingredient solution in its portfolio of Bioactive Collagen Peptides (BCP®). With PeptENDURE®, the collagen innovation leader will showcase the first collagen protein for enhanced endurance performance.
As the official collagen sponsor of the show, Gelita will also present Verisol® - its Bioactive Collagen Peptides specifically optimized to support beauty from within. It stimulates fibroblasts to produce more collagen, resulting in improved hair, nail and skin health, and a reduction in wrinkles and cellulite. Scientific evidence confirms the efficacy and safety of these BCP® , which is why leading brands worldwide rely on Verisol®
The line-up will be completed by Easyseal®, a pharmaceutical grade softgel gelatin that improves capsule seams and helps reduce leakage. This results in improved production processes, cost savings and increased efficiency. Visitors can find out more about the product by attending a talk by Dr. Ulrike Braun, Manager Scientific Affairs & Communication at Gelita.
Visitors to Vitafoods will also have the opportunity to listen to a presentation about Gelita’s latest special collagen peptides formulation with exceptional binding properties for the creation of sugar-free, high-protein products such as cereal and soft bars.
Natalie Leuwer, Category Manager Food Specialities, and Sophie Brand, Application Technology and Product Development, will reveal the potential of this novel ingredient on Wednesday, 15 May, at 3pm in the New Ingredients Theatre.
https://bit.ly/3TlYAkd
38 Nutramedic &Cosmetics

Gencor
BOOTH: D134
Gencor is featuring its newest published study on its PEA ingredient, Levagen®+, for improved cognition and memory in college students. This study represents the first known cross-over PEA supplementation trial in a healthy young adult population. The results indicated that Levagen®+ revealed a direct improvement in memory and corresponding increases in circulating neurotrophic marker levels, suggesting that Levagen®+ holds promise as an innovative and practical intervention for cognitive health enhancement.
The company will also showcase its award-winning, clinically validated portfolio of women’s health ingredients, including Libifem®, Genopause®, HydroCurc®, ActivAMP®, and more.
Join Gencor’s live in-person seminar featuring Dr. Mohammed Gulrez Zariwala, Director of the Centre for Nutraceuticals at the University of Westminster in London, to present the Levagen®+ Cognitive Study health findings.
https://www.gencorpacific.com/
Givaudan
BOOTH: G110
Givaudan will showcase consumer-inspired concepts such as instant drinks and gummies that feature its branded botanical ingredients. These new concepts will demonstrate effective ways of addressing the growing demand for immune support, urinary tract health, cognition, relaxation, energy, and gut health.
Designed to ensure an optimal sensory experience and accompany consumers throughout the day, each of the concepts features solutions from Givaudan’s extensive portfolio, including health, taste, and flavours ingredients. The early morning concept presents an instant drink, providing a boost of natural caffeine with our innovative guarana extract, paired with a citrus flavour. For midday, the concept comes in a refreshing peppermint-flavoured gummy format, featuring CereboostTM Givaudan’s American ginseng that has been demonstrated to support attention and memory. Finally, the evening concept offers a relaxing experience, leveraging the power of valerian in a gummy format with a tasty black tea peach flavour.
Amandine de Santi, Ingredients Portfolio Director, Taste & Wellbeing Europe at Givaudan, said: “The nutra market is in the midst of a revolution, with a growing number of consumers seeking great-tasting food supplements supported by scientific research. Achieving a balance between efficacy and palatability is crucial. Givaudan leverages its in-depth know-
ledge of taste to successfully address off-notes in active botanicals using our extensive range of masking and flavouring solutions. By sharing our expertise in both taste and botanicals with our customers, we are able to co-create appealing consumer experiences that offer powerful benefits and that satisfy the desire for great taste backed by sound science."
Reflecting its commitment to science-backed ingredients, Givaudan will unveil the results of a ground-breaking clinical study on PacranTM during the event. Pacran™ is an extensively studied whole cranberry ingredient for urinary tract health. This study showcases the efficacy and reliability of Pacran™, further reinforcing Givaudan's expertise in developing science-backed nutra solutions. Visitors can learn more about the clinical study by attending a presentation in the New Ingredients Theatre on May 14 at 3:30pm CET.
https://www.givaudan.com/taste-wellbeing

GreenCoLab
BOOTH: J61
Visitors will have the opportunity to discover exquisite vegan caviar, algae-based beer and tasty algae burger patties.
GreenColab is focused on the valorization of micro- and macroalgae. The industry-focused research platform will showcase its newly developed algae-based prototype line-up to the nutraceutical, food and beverage industries. Featuring chlorella, tetraselmis and seaweed, the prototypes will present algae as a sustainable vegan ingredient that can enhance the nutritional, functional, sensory and visual properties of a host of product matrices.
Company will be giving new audiences an opportunity to engage with differentiating value-added algae-developed foods, that foster its agenda of advancing the use of algae as a game-changing ingredient for the global marketplace.
GreenCoLab has created three exciting prototypes that demonstrate the transformational potential of macro- and microalgae: A vividly coloured product line of microalgae-based caviar that offers a uniquely exquisite sensory experience, reinventing the traditional sturgeon roe, a microalgae-based artisan beer with exceptional organoleptic qualities, and an algae-based line of burgers that delivers on both health and excellent taste. The products have been developed using sustainable, non-GMO algae sourced locally from GreenCoLab’s associates Allmicroalgae, Necton and ALGAplus.
A nutritionally rich and high-quality source of non-animal protein, algae also provide essential amino acids, dietary fibre, omega-3, -6 and -9 fatty acids, and vitamins and minerals, including vitamin B2 and B12, iron, and zinc. Their nutritional composition enables EU health claims for blood cholesterol, vision, bone health, fatigue, cell protection and immune system support.
http://www.greencolab.com
39 Nutramedic &Cosmetics

Lonza BOOTH:
G90
With over a century of expertise in capsule innovation and the ability to help get your products to market quickly, Lonza can help brands navigate evolving consumer preferences and tap into trending market opportunities within the nutrition supplements space.
Discover how their diverse range of hard empty capsules can elevate your offering:
1. Bolster clean label and plant-based claims
With more supplement users seeking vegetarian or vegan options, their portfolio of Capsugel® vegetarian capsules can help you satisfy their needs and more. Plant-based Capsugel® Vcaps®Plus capsules are also available in titanium dioxide (TiO2)-free options, meaning you can cater to even more consumers seeking clean label claims.
2. Meet consumer demand without compromising on efficacy
Lonza’s latest capsule innovation - Capsugel® Lumishield™ TiO2-free white gelatin capsule - utilises a unique opacifying technology that enables superior UV-light protection and content masking over commonly-used calcium carbonate (CaCO3) alternatives. It provides the same whiteness, more opacity and masking capacity as TiO2 capsules, while also outperforming the common TiO2-free alternatives on the market, so you can create more efficous clean label products.
3. Develop customisable vegetarian supplements for a competitive edge
Their HMPC-only Capsugel® Vcaps Plus® capsule goes beyond standard vegetarian capsules, offering brands a gelatin-like immediate release of ingredients and protect moisture-sensitive ingredients. In addition, brands can customize Capsugel® Vcaps Plus® capsules to create colorful products that boost brand recognition.
Meet Lonza’s team, and discover how they can help you meet evolving consumer demands. www.lonza.com/capsules-health-ingredients


Probiotix
BOOTH: P56
ProBiotix Health launches new innovative InstaMelt formulation to support cardiometabolic health.
ProBiotix Health Plc (‘ProBiotix’), a life sciences company that develops finished probiotic formulations to support cardiometabolic health, has launched InstaMelt – a newly developed food supplement that offers innovative features for health brands and retailers to rebrand as a full turn-key solution.
Featuring two science-backed and natural ingredients, the InstaMelt formulation contains the company’s proprietary ingredient LPLDL®, a patented probiotic strain with proven efficacy from multiple independent human intervention studies to significantly decrease LDL and increase HDL cholesterol levels, and Thiamine (Vitamin B1), which supports heart function.
ProBiotix has a keen interest in healthy ageing solutions that not only add years to life but add life to years. With global life expectancy rising, health spans must also rise to meet that expectation.
Niels Peter Bak, Head of Product Management at ProBiotix, commented: “Our InstaMelt formulation uses InstaMelt technology, a unique dosing technology to help health brands deliver the patented strain in a novel and convenient way. Its superior organoleptic properties, combined with natural fruit flavours, make the InstaMelt formulation a great addition to any heart health and wellness portfolio.”
Available in redberry and peach flavours, visitors will be able to taste InstaMelt at stand P56, alongside viewing the company’s full product range.
https://probiotixhealth-ir.com/

Roquette
BOOTH: K148
Roquette, a global leader in plant-based ingredients and a leading provider of nutraceutical and pharmaceutical excipients, is set to showcase a new flexible addition to its LYCAGEL® hydroxypropyl pea starch excipient range, along with a fresh vision for developing vegetarian and vegan dietary supplements in softgels.
In the company’s first joint exhibition with Qualicaps, following its acquisition of the leading capsule manufacturer late last year, Roquette will demonstrate how its newly expanded portfolio can help producers craft exceptional supplements to meet any consumer health need. Attendees are invited to visit booth K148 for a first look at the company’s lat-
40 Nutramedic &Cosmetics

est excipient launch, alongside leading-edge insights for optimizing efficiency, quality, and sustainability in nutraceutical production.
Experts from Roquette’s technical, marketing and commercial teams will be available on-stand throughout the show to offer tailored support for tackling the toughest challenges in nutraceutical formulation. On the event’s opening day, Roquette’s Technical Developer Manager – Europe, Steve Amoussou-Guenou, will host a presentation on the sustainable potential of LYCOAT® modified pea starch for vegetarian and vegan dietary supplements. Attendees can join him at 12:00 p.m., May 14, 2024, in the New Ingredients Theatre, to learn about the versatile applications of LYCOAT® modified pea starch in ReadiLYCOAT® film coating premix and LYCAGEL® softgel capsules premix.
In addition to valuable knowledge sharing, visitors to the Roquette and Qualicaps booth can experience the quality of the companies’ combined portfolio firsthand through a wide range of samples – from traditional tablets and capsules to softgels and gummies.
https://www.roquette.com/

Sabinsa
BOOTH: A90
Join us at the upcoming event Vitafoods Europe, where Sabinsa will be presenting groundbreaking innovations in the realm of health and wellness. Sabinsa is presenting Curcumin C3 Reduct® curcumin as a metabolite focusing on AMP kinase. AMPK is the central regulator of energy homeostasis. Curcumin C3 Reduct® activates AMPK, and it is more potent than curcumin in activating cellular energy balance. First ever EFSA-approved tetrahydrocurcuminoids from Curcuma longa.
Cirpusins® is prepared from the dried rhizomes of Cyperus rotundus, standardised to contain a minimum of 6% total identified stilbenes. It is a breakthrough weight loss ingredient acting by preventing

the formation of fat cells in the body
Ashwagandha extract Shagandha® will be showcased, backed up with 2 clinical studies and 2 publications. It is standardised to ideal 2,5% withanolides. Increasing serotonin levels and lowering morning cholesterol can help improve stress and anxiety scores, and cognitive performance. This leads to better sleep and life quality.
LactoSpore® is the original stable probiotic backed by 6 clinical studies. It has superior gastric survival and helps with IBS symptom relief. Also, is has a 3 years stability that makes the formulation of finished products easier. Together with Cranberry extract, it makes LactoCran® a with fenumannans Lactowise® that both offer targeted symbiotic advantages.
One of the promising products, Nigellin® will also be showcased in Geneva. Prepared from the most health-promoting blessed seed – Black Cumin Seed (Nigella sativa), by solvent-free super critical fluid extraction technology, available in 3 grades: PEARL, ONYX and AMBER forms. Rich with Thymoquinone, it shown most potent and favourable effects in the context of respiratory health and general wellness.
Starmeric® insoluble turmeric fibres offer prebiotic potential. Explore these and other intriguing ingredients such as Silbinol®, Saberry® and more at our event in Geneva. Discover the future of health and wellness with Sabinsa.
https://sabinsa.com.pl

Sirio BOOTH: B90
Sirio Presents Innovative Women’s Health Solutions. Join Sirio to unveil their cutting-edge women’s health platform and the ‘Organix supplement' series for the European customer base.
Sirio introduces a game-changing portfolio of 25 meticulously developed formulations. Crafted with global R&D expertise, these solutions feature premium ingredients backed by robust efficiency data.
Sirio is presenting a number of products:
MenoBalance Gummy: Being the first to market organic EPO gummy, Sirio is debuting Novasoy® in a gummy format, an exclusive soy isoflavone from ADM, this gummy targets menopause challenges such as hot flushes and bone health, providing B2B partners with a competitive edge.
Me&Mum Post-Natal Softgel: Formulated with DHA, vitamins, minerals, and herbal extracts, this softgel addresses postpartum concerns like hormonal hair loss, offering a valuable solution for nutraceutical professionals.
InnoFolate Prenatal Softgel: Powered by Sirio's Plantegrity™ technology and Quatrefolic® from Gnosis by Lesaffre, this prenatal formulation ensures stable folate levels during pregnancy, delivering premium maternal and foetal health support.
Visit Sirio’s booth to experience these innovative products firsthand and discuss collaborations.
https://sirio-europe.com/
42 Nutramedic &Cosmetics

TriNutra
BOOTH: XX
TriNutra® will be in attendance and will be sharing its most recent announcement of its six approved structure function claims from Health Canada on its proprietary black seed (Nigella sativa) oil branded ThymoQuin®
ThymoQuin, as an oral supplement (in soft gel capsule), can be used to:
1. Help support healthy liver function*
2. Help support cardiovascular health*
3. Help support healthy glucose levels*
4. Help support stress relief*
5. Help moderate general feeling of stress*
6. Help support healthy gut microbiota*
This comes in addition to the recent grant of ThymoQuin composition patent in the USA, the same composition that also meets the USP monograph.
ThymoQuin is globally approved for use as dietary supplement. Its superior and potent composition is a full spectrum, cold pressed black seed oil standardized to 3% thymoquinone and <1.25% free fatty acids. Dr. Liki von Oppen-Bezalel, Business Development Director at TriNutra, will be walking the show and is available to discuss the company’s latest happenings. Please email her at liki@trinutra.com to schedule a meeting.
www.trinutra.com

Unique Biotech Limited
BOOTH: P100
Experts in probiotics, Unique Biotech Limited (UBL) driven by the vision of empowering 8 billion people with effective probiotic solutions for society’s most pressing problems.
Clinically-proven probiotic solutions - with over 500 well-characterized strains (Bacillus coagulans
Unique IS-2, Bacillus clausii UBBC-07, Saccharomyces boulardii Unique-28, and Lactobacillus salivarius UBLS-22, etc), 51 clinical studies, and 131 scientific studies, UBL has fueled probiotic solutions development catering to pharmaceutical, food & beverages (including gummies) and nutraceutical industries.
Gut and beyond - UBL’s solutions extend beyond gut health (B. clausii oral suspension), and address over 15 health segments including Women's Health (Provinorm), Cognitive Health (Cognisol), and Immunity (Profesorb). UBL caters to multiple preferences and age groups with various dosage forms: oral suspensions, tablets (chewable & effervescent), and more.
World-class facility that scales with you; UBL supports you in meeting your growing consumer demands for production scaling and new product launches. https://www.uniquebiotech.com/


Vidya Europe
BOOTH: G118
Vidya Europe, a subsidiary of Vidya Herbs, is an Indian group specialized in the development and production of plant extracts for various markets such as dietary supplements, animal nutrition, and cosmetics.
The company has long been specialized in plant extracts for dietary supplements, notably with flagship products such as turmeric (with a curcuminoids content > 90%) and ginger, among others. It also stands out for its extracts undergoing studies to prove their effectiveness, such as Skin-cera® (konjac extract standardized in glycosylceramides) with in vitro and in vivo tests, as well as Vi-Spo™ (saw palmetto extract with high levels of fatty acids and beta-sitosterol).
Companys range also includes natural carotenoids as well as vitamins and minerals.
The plant extracts are available in various forms (powder, granules, microbeads, emulsion, etc.) and with different standardizations. Customized solutions can also be offered to meet specific customer needs.
Vidya Europe has also opened a plant in dedicated to CO2 supercritical extraction, thus facilitating the development of a new range specifically tailored to the cosmetics market. This extraction method offers significant advantages in terms of extract purity, preservation of active compounds, and environmental friendliness due to the use of a non-toxic solvent.
This advancement has resulted in the development of five actives, with in vitro studies: Vi-Hmp® , Vi-Meric®, Vi-Dat®, Vi-Caf®, and Vi-Noa®
This initiative allows Vidya Europe to create its own product range while offering customized services to its clients.
https://www.vidyaherbs.com/
43 Nutramedic &Cosmetics
Understanding Neuro-cosmetics: an Overview
By tapping into subconscious desires and employing neuroscientific methods, neurocosmetics aims to decode how the brain processes beauty, holding vast potential for the industry. With insights from neuroscience, behavioral economics, and social psychology, neuro-cosmetics not only shapes skincare regimens but also promises to deepen our understanding of the intricate brain-skin connection.
AUTHOR: Valerija Pandža, MPharm
Recently, there has been a growing interest in the intersection between neuroscience and cosmetics. This emerging field, neuro-cosmetics, utilizes scientific knowledge about the brain to develop innovative beauty products and marketing strategies. Neuro-cosmetics aims to tap into the subconscious desires and preferences of consumers, using neuroscientific methods to analyze and understand how the brain processes beauty and aesthetic experiences. With advancements in neuroimaging techniques and the understanding of neural mechanisms, neuro-cosmetics hold great potential for revolutionizing the beauty industry.
The science behind neuro-cosmetics
Neuro-cosmetics is built upon several key scientific fields, including neuroscience, behavioural econo-
mics, and social psychology. Neuroscience provides insights into the neural mechanisms underlying beauty perception and aesthetic experiences. Through the use of neuroimaging techniques such as functional magnetic resonance imaging (fMRI) and electroencephalography, researchers can observe and measure brain activity patterns associated with beauty perception. These techniques allow researchers to identify specific brain regions and neural circuits that are activated when individuals perceive beauty or have positive aesthetic experiences. Additionally, behavioural economics helps to understand the decision-making processes and preferences of consumers when it comes to beauty products. By combining neuroscientific knowledge with behavioural economics, neuro-cosmetics aims to create products and marketing strategies that are tailored to the subconscious desires of consumers.

44 Nutramedic &Cosmetics
Exploring the brain-skin connection
One intriguing aspect of neuro-cosmetics is the exploration of the brain-skin connection. Studies have shown that there is a complex bidirectional relationship between the brain and the skin. The brain plays a crucial role in regulating skin health and appearance, while the condition of the skin can also influence the brain's perception of beauty. By studying how the brain responds to different marketing stimuli, such as packaging design, product placement, and advertising messages, neuro-cosmetics can create more effective and persuasive marketing strategies. These strategies leverage the subconscious desires and preferences of consumers, ultimately leading to increased sales and brand loyalty. Innovative research in the field of neuro-cosmetics aims to understand the complex relationship between the brain, perception of beauty, and skincare products. It is hoped that this research will not only lead to the development of more effective and personalized beauty products, but also contribute to a greater understanding of the interplay between the brain, self-perception, and aesthetic experiences.
Innovations in neurocosmetic products
The knowledge gained from neuro-cosmetics research can lead to the development of innovative beauty products. For example, neuro-cosmetics can influence the formulation of skincare products by incorporating ingredients that have been shown to positively stimulate the brain's perception of beauty. These products can be designed to target specific brain regions and neural pathways involved in beauty processing, resulting in enhanced efficacy and customer satisfaction. Neuro-cosmetics has the potential to revolutionize skincare regimens by tailoring products to individual needs based on their specific brain-skin connection. For instance, by analysing an individual's brain responses to sensory stimuli, such as temperature and texture, neuro-cosmetics can recommend personalized skincare routines that optimise the brain's impact on skin health and appearance. By incorporating neuro-cosmetic products into their skincare routines, individuals can potentially achieve better results and address their unique concerns more effectively. Neuro-cosmetics, with its focus on leveraging neuroscience and the understanding of the brain's perception of beauty, presents innovative approaches to skincare products. For example, neuro-cosmetics can incorporate virtual reality technology to enhance the customer's experience by providing immersive and realistic simulations of different skincare products and their effects on the skin. This allows consumers to make more informed decisions and have a better understanding of how a product will work for them before making a purchase. Neuro-cosmetics also has the potential to enhance brand loyalty. By providing a deeper understanding of the brain's role in beauty perception, neuro-cosmetics can create a stronger emotional connection between consumers and beauty brands
Benefits and challenges of neuro-cosmetic applications
Neuro-cosmetics offers several potential bene-
fits for the skincare industry. Firstly, it allows for more personalized and targeted skincare regimens, as products can be tailored to individual needs based on their specific brain-skin connection. This means that individuals can address their unique concerns more effectively and potentially achieve better results. Additionally, neuro-cosmetics can enhance the overall customer experience by incorporating virtual reality technology and providing immersive simulations of skincare products. This not only gives consumers a better understanding of how a product will work for them but also helps to build trust and loyalty towards beauty brands. However, some challenges come with the application of neuro-cosmetics in the skincare industry. One challenge is the need for scientific validation and evidence-based research to support the claims and benefits of neuro-cosmetic products. This is crucial to establish credibility and ensure that consumers can trust the efficacy of these products. Another challenge is the ethical consideration of manipulating consumers' brain responses and perceptions for marketing purposes. This raises concerns about consent and the potential for manipulative marketing practices.
Future prospects of neuro-cosmetics in beauty and healthcare
The prospects of neuro-cosmetics in the beauty and healthcare industries are promising. With advances in technology and neuroscience, the potential for neuro-cosmetics to revolutionize skincare and cosmetic formulations is vast. Neuro-cosmetics has the potential to enhance the efficacy and safety of antiaging cosmeceuticals, leading to improved skincare outcomes. Additionally, the application of neuro-cosmetics in healthcare holds promising possibilities. For example, neuro-cosmetics could be utilized in therapeutic skincare products that target specific skin conditions or promote mental well-being. Furthermore, the integration of neuro-cosmetics with telemedicine and remote patient monitoring could revolutionize the field of dermatology by allowing for personalized treatment plans and remote monitoring of skin health.
Conclusion
In conclusion, neuro-cosmetics represents an exciting frontier in the beauty industry. Its ability to personalize skincare regimens and enhance the customer experience has great potential. However, the development and utilization of neuro-cosmetics must be accompanied by rigorous scientific validation and ethical considerations.
References:
Oliveira, de, Caldeira, Henrique, Jorge, et al. Opening the “Black Box” in the Consumer's Mind: Understanding What is Neuromarketing. 24 Aug. 2014, https://doi.org/10.5539/ijbm.v9n9p96.
Draelos, Zoe. Neuro-cosmetics: Sensates and Smells on the Skin. 11 Apr. 2024, https://www.dermatologytimes.com/view/neuro-cosmetics-sensates-and-smells-on-the-skin.
Rizzi, Vito, et al. "Neuro-cosmetics in Skincare—The Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation." Cosmetics, vol. 8, no. 3, 16 Jul. 2021, p. 66-66. https://doi.org/10.3390/ cosmetics8030066.

45 Nutramedic &Cosmetics
How to Recognize Fake or Incorrect Cosmetic Product Labels?
Product label content should adhere to consumer rights and cosmetic regulatory obligations. While many cosmetic products on the market feature labels that are clumsy imitations of legal product labels, there are also those where illegitimacy is apparent at a glance.
 AUTHOR: Gordana Gorinšek, MSc in Phytomedicine, Expert Cosmetic Safety Assessor, Expert Regulatory Affairs Consultant
AUTHOR: Gordana Gorinšek, MSc in Phytomedicine, Expert Cosmetic Safety Assessor, Expert Regulatory Affairs Consultant
Since there is a great number of cosmetic products on the market to offer, many product labels, or most of them look very much alike, resembling each other like peas in a pod. However, we can often see cosmetic labels that differ from the majority in terms of their text, illustrations and symbols.
It does not matter if products were introduced from one European Union member country to another or imported from Third countries. If they are presented on the EU market as legal products, then product presentation and label text must follow the legal regulations. In such cases, small details can reveal the level of expertise and skills in adapting to European regulations, as well as in translating label text into the native language of the European country which places it on the market.
However, there is a quite large number of cosmetic products on the market whose labels are clumsy copies of legal products labels, but there are products that can immediately be recognised as illegal, just by quickly glancing at them.
What gives away a fake or incorrect cosmetic product label? Let's take one step at a time.
The purpose of product labelling is to identify the product and its manufacturer to distinguish it from similar products of the same or other manufacturers and to provide basic and useful information to the consumer about its purpose, quantity, shelf life,

composition, application site and method, and if necessary, precautions and storage method. All this information is necessary for the consumer to make an informed decision when purchasing.
In addition to all of the above, there is also indication of a serial number. The serial number of the product is the unique identification number of the batch to which the particular product belongs. Its role is best understood in case of supervision by the competent authority, customer complaints, withdrawal or recall of products from the market in the event of a complaint, or occurrence of undesirable or serious undesirable effects on human health.
Listing this information on the product label clearly states the Cosmetics Regulation. Article 19 states that “cosmetic products shall be made available on the market only where the container and packaging of cosmetic products bear the following information in indelible, easily legible and visible lettering” and lists the following:
1. the name or registered name and the address of the responsible person;
2. the nominal content at the time of packaging, given by weight or by volume;
3. the date until which the cosmetic product, stored under appropriate conditions, will continue to fulfil its initial function. The date of minimum durability shall be preceded by the Period-after-opening symbol (PAO) or the words “best used before the end of”;
4. particular precautions to be observed in use,
5. the batch number of manufacture,
6. the function of the cosmetic product, unless it is clear from its presentation;
7. a list of ingredients preceded by the term “ingredients” (including colourants and fragrance allergens) and labelled by their individual INCI name (International Nomenclature of Cosmetic Ingredients);
8. the country of origin must be stated in case of import from Third countries to EU.
Additionally, article 20 of the same Regulation defines the manner of advertising, determining which claims are generally allowed and which are not.
Knowing all this, what should the attention be paid to when reading the label text? I will list the most
46 Nutramedic &Cosmetics
No. TRUE
1. AROMATIČNI KUTAK Ltd., Brune Bušića 21, HR-10000 Zagreb; info@aromaticnikutak.hr | https://pif.com.hr/
FALSE
Company XY
2. Best used before the end of: Best before:
3. None Date of manufacture:
4. LOT: ABC-01
None
5. Prunus Amygdalus Dulcis Oil, Dehydroacetic Acid (and) Benzyl Alcohol (and) Aqua, Olea Europaea Fruit Oil Almond oil, Geogard 221, extra virgin olive oil
6. Ingredients: INCI: (or) Composition:
7. Ingredients: Olea Europaea Fruit Oil, Beeswax, Citrus Limon Peel Oil, Limonene.
8. Country of origin:
INCI: extra virgin olive oil, beeswax, lemon essential oil.
Made in:
9. LOT/ Best used before the end of (date): Indicated on the bottom of the packaging LOT/Best before: Indicated on the packaging
10. The product is intended for a relaxing massage of tired and heavy legs after physical and sports activities.
Improves blood circulation, anti-inflammatory effect, muscle soreness and pain
11. None The Leaping Bunny symbol
12. 21-PAP, 70-GL, 5-PP
common examples that I encounter in practice as an expert, but also as a consumer:
1. the name and the address of the manufacturer are not given or are vague;
2. misspelling phrase referring to the expiration date, e.g. „best before“;
3. indication of the date of manufacture, which is not a legal obligation;
4. the absence of a batch number;
5. the absence of a list of ingredients or ingredients that are listed by common names or by their trade name, e.g. almond oil, Geogard 221;
6. a list of ingredients preceded by the acronym “INCI” instead of “Ingredients”;
7. the absence of fragrance allergens, although the presence of fragrance is visible in the list of ingredients. With the recent expansion of the allergen list in cosmetics, it has become almost impossible to avoid listing at least one fragrance allergen in a product.
8. A fals usage of a phrase “Made in“ ; it is being used falsly, instead of stating the country of origin
9. Not specified location of the serial number and information on the expiration date are, e.g. "indicated on the packaging" instead of "indicated on the bottom of the packaging" or “on top of the lid”;
10. stating health claims from mild to brutally outspoken, e.g. from "improves blood circulation" to "cures haemorrhoid";
11. the Leaping Bunny symbol or a version thereof that suggests that the product has not been tested on animals, although emphasizing compliance with legal regulations is not allowed.
12. absence of environmental symbols on packaging materials for the purpose of recycling.
Monetary and non-monetary penalties are predicted for incorrect product labels in the national
None
laws of each member state of the European Union. A non-monetary penalty refers to the withdrawal of the product from the market and a stop of sales until correction is done. That is a more dramatic penalty for every manufacturer because it affects the reputation of the brand and business directly, which is something no responsible manufacturer should have allowed. When once damaged, reputation is difficult to restore.
The product label is the first to draw attention with the potential consumer, but also with the competent inspector. Its content should respect consumer rights and cosmetic regulatory obligations. That is why it should not be left to editing by people from fields not related to cosmetic industry, cosmetic beginners, or cosmetic experts with none or too little experience with this subject.
References:
1 Regulation (EC) No 1223/2009 of the European Parliament and the Council of 30 November 2009 on cosmetic products
2 Regulation (EU) 2023/1490 of 19 July 2023 amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as regards the use in cosmetic products of certain substances classified as carcinogenic, mutagenic or toxic for reproduction

Aromatični kutak Ltd.
Brune Bušića 21 Zagreb, Croatia https://pif.com.hr/ info@aromaticnikutak.hr
T. + 385 98 1750 934
47
TABLE 1
Nutramedic &Cosmetics
medicinal plants photo herbarium
Shepherd's Purse
Shepherd's purse (Capsellabursa-pastoris), a resilient biennial herb of the Brassicaceae family, boasts distinctive heart-shaped seedpods, earning it its common name. Beyond its botanical allure, shepherd's purse holds a rich history in traditional medicine, with its leaves and aerial parts prized for their medicinal properties, including anti-inflammatory and antimicrobial effects.

TAXONOMY
kingdom: Plantae
order: Brassicales
family: Brassicaceae
genus: Capsella
species: Capsella bursa-pastoris
common name
Lady’s purse, shepherd’s bag, mother’s heart, shovelweed, caseweed, borsa de pastor (Spanish), bouse de pasteur (French), Hirtentäschelkraut (German)
flowering time
V - IX month
Capsella bursa-pastoris, commonly known as shepherd's purse, is a flowering plant that belongs to the Brassicaceaefamily. It is a biennial herb that grows up to 40 cm in height. The leaves of Capsella bursa-pastoris are alternate and highly variable in shape, ranging from spatula-shaped to deeply lobed. The plant produces small white flowers that are arranged in clusters at the top of slender stems. The flowers of Capsella bursa-pastoris have four petals and six stamens. They also have a distinctive heart-shaped seedpod, which gives the plant its common name "shepherd's purse." The seedpods contain numerous tiny seeds that resemble miniature purses, hence the name. These seedpods can be easily identified and are often used for educational purposes in botanical studies. The stems are slender and branching. The roots of the plant are fibrous and relatively shallow, allowing it to thrive in a variety of soil types.
The plant's flowering pattern and fruiting structure make it a fascinating subject for botanical enthusiasts and researchers.
Furthermore, Capsellabursa-pastorishas both medicinal and culinary uses. In culinary applications, the young leaves of Capsella bursa-pastoris can be used in salads or cooked as a green vegetable.
Habitat & cultivation
Capsella bursa-pastoris is a resilient plant that can thrive in a variety of habitats and environmental conditions. It is commonly found in disturbed areas such as roadsides, fields, and gardens, where it quickly colonizes open spaces and establishes itself as a pioneer species. This adaptability makes it a resilient plant in both urban and rural settings.
Use for medicinal purposes
The herbal parts of Capsella bursa-pastoris used for extraction include the leaves and the aerial parts of the plant. It contains several chemical constituents that contribute to its medicinal properties. These include flavonoids, alkaloids, and glucosinolates. These chemical constituents are believed to have anti-inflammatory, antioxidant, and antimicrobial effects. In addition to its chemical constituents, Capsella bursa-pastoris also contains essential oils, tannins, and phenolic compounds. These compounds contribute to the overall therapeutic potential of
the plant and have been the subject of various phytochemical studies.
Traditional medicinal uses
Capsellabursa-pastoris has a long history of traditional use in herbal medicine. It has been used for various ailments such as urinary tract infections, gastrointestinal disorders, menstrual problems, and respiratory conditions. In some cultures, it is also believed to have diuretic and wound-healing properties.
Constituents
• flavonoids
• alkaloids
fumaric acid
glucosinolates
• tannins
• essential oil
Action and application
Recent scientific studies have further explored the potential health benefits of Capsellabursa-pastoris . These studies have shown that the plant's extracts possess antimicrobial activity against various strains of bacteria and fungi. They have also demonstrated its potential as an anti-inflammatory agent, with the ability to inhibit the production of pro-inflammatory cytokines.
Clinical trials1 have also explored the plant's efficacy in wound healing, with promising results indicating its role in promoting tissue regeneration and accelerating the healing process.
The findings from these clinical studies underscore the importance of Capsella bursa-pastoris as a valuable source of natural bioactive compounds with significant health benefits. Moreover, the antimicrobial and anti-inflammatory properties demonstrated in these scientific investigations provide a strong scientific basis for the traditional use of this botanical species in herbal medicine.
References:
1 Min, J H., Suh, W S., Lee, T H., Subedi, L., Kim, S Y., & Lee, K R. (2017, June 20). Phenolic Glycosides from Capsella bursa-pastoris (L.) Medik and Their Anti-Inflammatory Activity. Molecules/Molecules online/Molecules annual, 22(6), 1023-1023. https://doi.org/10.3390/ molecules22061023
48 Nutramedic &Cosmetics
Xylishine™ Cthe Ingredient for Beautiful Hair
Seppic completes its hair care offer with an ingredient that restores scalp and hair fiber
Inspired by the "skinification" trend (taking care of its scalp like its skin), Seppic has created Xylishine™ C, an active ingredient acting holistically on scalp and hair cuticle repair. Thanks to Xylishine™ C, hair becomes visibly more beautiful with increased shine, better curl definition and volume control.
Xylishine™ C is a natural active ingredient derived from a patented complex of sugar derivatives recognized for their moisturizing action in skin care, associated with a bio-inspired algae harvested in France, Pelvetia canaliculata. It is well-known for its exceptional resistance to dehydration.
Seppic's Research & Innovation teams have demonstrated that Xylishine™ C helps restore the scalp by increasing its ceramide content by 27%* and strengthening its skin barrier by 42%*. It also helps repair the surface appearance of damaged cuticles by 82%**. Thanks to this mode of action, XYLISHINE™ C increases the shine of all hair types, curly or straight (+7%***), curls are better defined (+16%***), and hair is softer to the touch (+7%***).
It is also efficient in different climates (tested ex vivo in standard and extreme temperature and humidity conditions) for visible anti-frizz action and hair volume control.
Compliant with Chinese IECIC and IECSC regulations, Xylishine™ C is also COSMOS and Natrue approved, with a naturality rate of 99,7% according to ISO 16128.
Audrey Bonnard, Active Product Manager at Seppic, said: "Xylishine™ C meets a strong expectation in the haircare market for ingredients with global efficacy, both on the scalp and the hair fiber. Tests carried out on different hair types and in different climates make it particularly interesting for beautifying all hair types anywhere in the world."
For more information visit: https://www.seppic.com/en
* In vivo test on 20 men and women volunteers applying a shampoo containing 3% Xylishine™ C or a placebo shampoo for 28 days.
** Ex vivo test applying a 3% Xylishine™ C solution to healthy and damaged hair.
*** In vivo test on 2 groups of volunteers with curly or straight hair applying leave-on or shampoo containing 3% Xylishine™ C or placebo
B2B Events Calendar
An overview of live B2B events that will take place during 2024.






14-16 May 2024, Geneva, Switzerland https://www.vitafoods.eu.com/
15 May 2024, Geneva, Switzerland www.nutraingredients-awards.com
10-12 June 2024, Salt Lake City, USA https://probiotaamericas.com
7-9 May 2024, Istanbul, Turkey https://chcistanbul.com/
5-6 June 2024, Munich, Germany https://www.cosmetic-business.com/en/
5-6 September 2024, Stockholm, Sweden https://www.hudochkosmetikmassan.se/

2-4 October 2024, Istanbul, Turkey https://beauty-istanbul.com/




23-24 September 2024, Lyon, France https://natexpo.com
8-10 October 2024, Milan, Italy https://www.cphi.com/europe/
9-10 October 2024, Stockholm, Sweden https://www.nordicorganicexpo.com/
19-21 November 2024, Frankfurt, Germany https://www.figlobal.com
49
Nutramedic &Cosmetics
Arla Foods Ingredients Targets Nutrition-Conscious Gamers With New Beverage Concept
Arla Foods Ingredients has launched a high-protein concept for gamers who want to level up their nutrition. Titled ‘Progamer’, the ready-to-drink solution is designed to meet the needs of e-sports enthusiasts seeking benefits for their health as well as their gaming performance.

The concept features energy-boosting ingredients alongside the game-changing whey protein isolate Lacprodan® SP-9213, which is clear, provides a refreshing taste and is high in essential and branched-chain amino acids.
Cido Silveira, Arla Foods Ingredients Marketing & Business Development Manager – South America, said: “There’s a stereotype of gamers bingeing on unhealthy snacks and guzzling down energy drinks, but a new, nutrition-focused generation is emerging. They want to maintain their energy and concentration levels over marathon sessions, but they also want the many benefits that high-protein products offer. Progamer allows manufacturers to formulate unique, refreshing, clear, high-protein solutions for gamers who want more from their energy drinks.”
Protein plays a crucial role in general health, supporting muscle growth, repair and overall body function. Research has also found that consuming essential amino acids leads to improvements in attention and cognitive flexibility. A study on esports athletes, meanwhile, showed that sufficient protein intake is associated with improved cognitive performance in gaming. 2
A 310 ml can of the ‘Progamer’ beverage concept features 15 g of protein, including 3767 mg of branched-chain amino acids. It also contains taurine, magnesium, zinc, caffeine and vitamins A, B3, B6 and B12 to support essential gamer needs such as concentration and vision. In addition, the concept is free from sugar, fat and lactose and contains only 60 kilocalories per can.
Arla Foods Ingredients showcased the ‘Progamer’ concept at NIS (the Nutri Ingredients Summit) in São Paulo, Brazil, on 23rd and 24th April. Exhibiting at Stand 3-35, it highlighted growing opportunities for e-sports nutrition products in the Latin American market – with over half of Brazilian gamers spending more than 20 hours per week playing.
Visitors to the stand were also able to discover
two clear shake concepts made with 100% whey protein isolate ingredients. Go Natural was a fruit-infused flavored water made with Lacprodan® ISO.WaterShake. Go Fresh, meanwhile, was a thirst-quenching shake created with Lacprodan® ClearShake that had a refreshing lemonade taste.
www.linkedin.com/company/arla-foods-ingredients
References:
1 Suzuki, H. et al. 'Intake of Seven Essential Amino Acids Improves Cognitive Function and Psychological and Social Function in Middle-Aged and Older Adults: A Double-Blind, Randomized, Placebo-Controlled Trial' Frontiers in Nutrition (2020)
2 Goulart, J.B. et al. 'Nutrition, lifestyle, and cognitive performance in esport athletes' Frontiers in Nutrition (2023)

50
Nutramedic &Cosmetics






































































































































 AUTHOR: Gordana Gorinšek, MSc in Phytomedicine, Expert Cosmetic Safety Assessor, Expert Regulatory Affairs Consultant
AUTHOR: Gordana Gorinšek, MSc in Phytomedicine, Expert Cosmetic Safety Assessor, Expert Regulatory Affairs Consultant
















