CLINICAL INITIATIVES, RESEARCH AND CURRENT UPDATES IN TREATMENT

Want Circuit straight to your inbox? Sign up for our e-newsletter here!

CLINICAL INITIATIVES, RESEARCH AND CURRENT UPDATES IN TREATMENT

Want Circuit straight to your inbox? Sign up for our e-newsletter here!
Nancy Goubran, Training Support Pharmacist, Pharmacy Operations
Respiratory Syncytial Virus (RSV) is a common cause of acute respiratory infections, particularly severe in very young children and the elderly.1
RSV infects most children by 2 years of age.1 It is a primary cause of hospitalisation in children under 6 months of age due to lower respiratory tract infections and imposes a substantial disease burden in both inpatient and outpatient settings.1 Most hospitalised infants are otherwise healthy.1
Before the COVID-19 pandemic, RSV infections followed a predictable, seasonal pattern.2 However, during the pandemic, cases of RSV and other respiratory viruses dropped significantly due to measures like travel restrictions and social distancing.3
RSV is a common and highly contagious RNA virus with two subtypes (A and B), that causes infection of the lungs and airways.6,7 The virus is spread via air-borne respiratory droplets and surfaces that have come in contact with an infected person.7 Peak season for infections in temperate regions of Australia is late autumn and throughout winter with symptoms generally appearing 3-10 days after exposure.7

In late 2020, as isolation policies eased, Australia experienced unexpected, out-of-season RSV outbreaks.4 The prolonged period of reduced viral exposure and limited social interactions likely led to a larger population of vulnerable children and adults, making them more susceptible to infection.2,4
Starting February 2025, the RSV vaccine Abrysvo® was made available for free under the National Immunisation Program (NIP) for pregnant women to protect their newborns.5 Additionally, beginning this year, infants who are not protected by antenatal RSV vaccination or who have risk factors for severe disease will be offered nirsevimab, a monoclonal antibody, through state and territory-funded programs.1
Tropical regions of Australia have a less predictable RSV season.4,6
RSV became a nationally notifiable disease in 2021, meaning health authorities must report confirmed cases to the National Notifiable Diseases Surveillance System (NNDSS).8 Last year in Australia, there were 174,764 confirmed cases of RSV as opposed to 95,778 reported cases in 2022.9
Article continued next page...
For most people, RSV presents as the common cold. Symptoms are usually mild and self-limiting, lasting 2-8 days.10
Primary risk factors for severe infection include:11,12
• Chronic lung, kidney or heart disease
• Immunocompromising conditions
• Older adults ≥ 60 years
• Children born pre-term (<32 weeks’ gestation)
• First Nations people
Infection with the virus provides short-term immunity, however individuals can be reinfected multiple times.1
In conjunction with the presence of symptoms, diagnosis is by nasal and throat swab PCR (polymerase chain reaction) test, via blood test to detect the presence of RSV antibodies or using rapid antigen tests (RATs).10
There is no specific treatment for RSV. Management focuses on symptom relief and supportive treatment including rest, hydration and analgesia.7 Individuals with chronic obstructive pulmonary disease (COPD) and asthma should continue with their prescribed treatment and ensure that they follow their action plan(s).10
Prevention of RSV includes a combination of both pharmacological and non-pharmacological methods.
As with any virus spread via respiratory droplets, maintaining good hand and respiratory hygiene is vital to minimise transmission.
Vaccination and monoclonal antibodies are available to assist with the prevention of RSV.
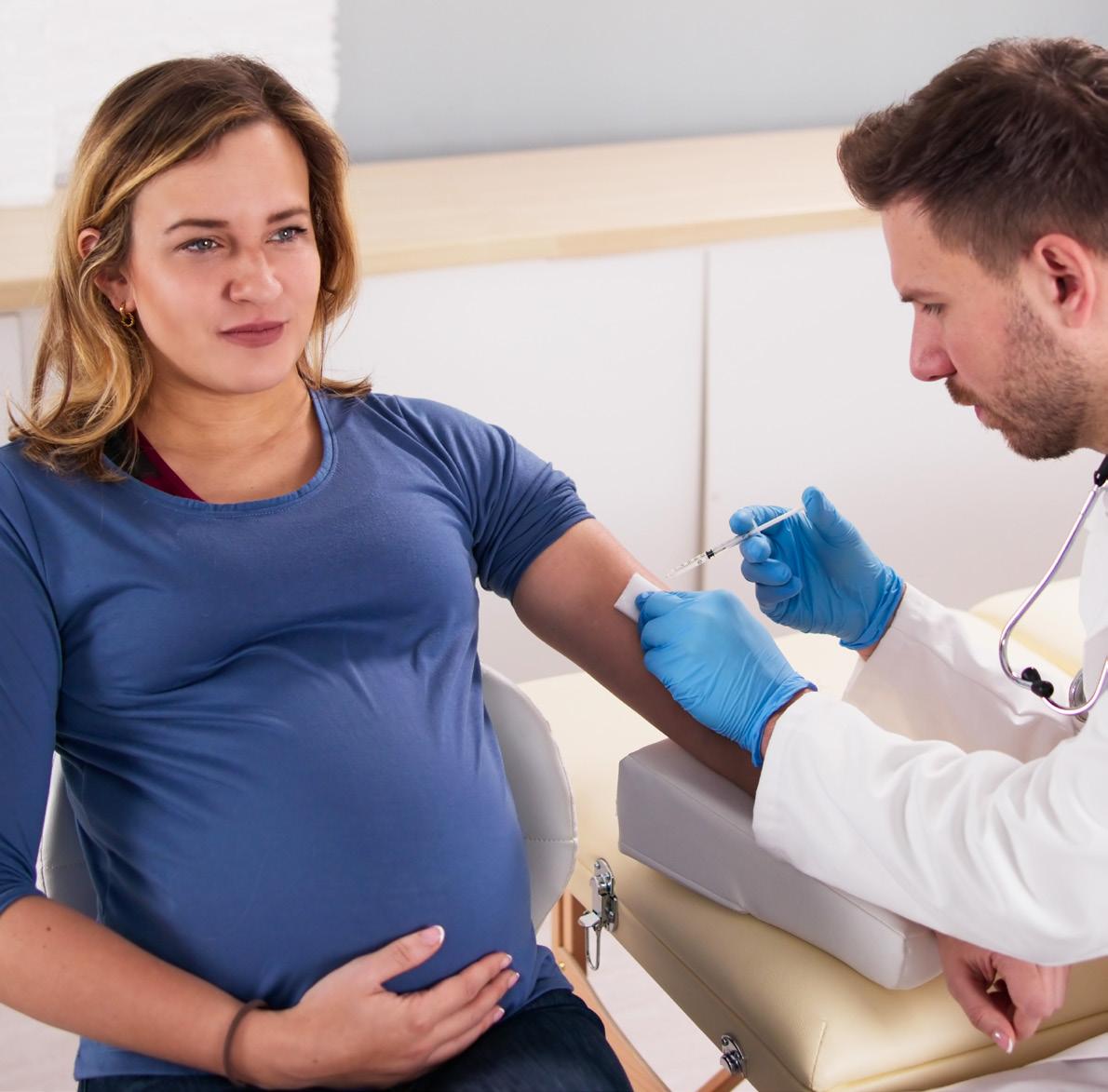
There are two brands of RSV vaccine available and approved for use in adults ≥60 years in Australia, Abrysvo® and Arexvy® 1,11 In clinical trials, both vaccines demonstrated high efficacy in adults, reducing RSV-associated lower respiratory tract disease by 83% for Arexvy® and 89% for Abrysvo® over a single RSV season.13,14
Abrysvo® is the only RSV vaccine approved for use in pregnant women.1,11 The vaccine enables pregnant women to pass RSV-specific protective antibodies to their unborn child, providing protection until six months of age when infants are most at risk.6
ATAGI (Australian Technical Advisory Group on Immunisation) recommends administering Abrysvo® between 28-36 weeks of pregnancy, differing from product information which advises 24-36 weeks.1,11 This precautionary measure is in place while awaiting further data on adverse events of special interest, including potential, rare risks of preterm birth.11 Recommendations regarding gestational age at vaccination may be updated as further data becomes available.11
Clinical trials during pregnancy showed a vaccine efficacy of 69% in preventing medically attended severe RSV-associated lower respiratory tract illness and 57% in reducing RSV-related hospitalisations among infants within their first six months.15
There are no RSV vaccines approved for use in children. Where protection is required, monoclonal antibodies offer passive immunity from RSV.1,11 Two monoclonal antibodies are available in Australia, nirsevimab and palivizumab. Nirsevimab is preferred over palivizumab due to its longacting protection.11 Only a single intramuscular dose is required to cover an RSV season, as opposed to monthly administration for palivizumab.
Immunisation programs for eligible infants will be launched in early 2025, following the success of trials conducted in Western Australia, Queensland, and New South Wales. A summary table of state and territory-funded nirsevimab for infants program can be found on the NCIRS (National Centre for Immunisation Research and Surveillance) website: https://ncirs.org.au/respiratory-syncytial-virus-rsv/respiratorysyncytial-virus-rsv-immunisation
With a government investment of $174.5 million toward vaccinating pregnant women and the funding of monoclonal antibodies in infants, it is anticipated that hospitalisation rates will be greatly reduced and 10,000 infants kept out of hospitals each year.16
Scan QR code for references
Nerida Jenkins, Quality Pharmacist, Quality and Medication Safety Unit
The Australian Commission on Safety and Quality in Health Care released the new Chronic Obstructive Pulmonary Disease (COPD) Clinical Care Standard in late 2024.1 This standard provides a framework for best practice care in both community and acute settings, aiming to improve awareness, diagnosis, and management of individuals with COPD.1
COPD affects 1.5 million Australians, including one in 13 people over 40 years of age, significantly impacting their ability to participate in work and social activities.1,2
In 2020–21, an estimated $832 million was spent on COPD, accounting for 50% of the total respiratory disease burden in Australia by 2023.1,3
There are substantial differences in care and patient outcomes across Australia, with hospitalisation rates varying by up to 18 times between local areas.1,4 Also, Indigenous Australians face a greater burden of COPD compared to non-Indigenous Australians, with approximately twice the prevalence of COPD and three times higher mortality rates.1,2 These disparities are attributed to a range of factors, including higher rates of smoking, limited access to culturally safe healthcare services, and socio-economic disadvantages.1,2
This new standard addresses the variations in COPD management and seeks to reduce potentially preventable hospitalisations associated with the condition, thereby reducing burden on the Australian healthcare system.1
It is estimated that around 50% of people living with COPD are unaware of their condition, suggesting that the actual prevalence and burden of the disease may be higher than current estimates.1,5 Additionally, COPD exacerbations are a major contributor to preventable hospital admissions among individuals with COPD.5 Each year, approximately 53,000 Australians aged 45 and over are hospitalised due to COPD, and Australia’s hospital admission rates for COPD are significantly higher than many other developed countries, being 1.7 times the average rate.3,5
Nationally, the COPD-X Guidelines by Lung Foundation Australia offer evidence-based recommendations for COPD management.4,6 However, implementing these guidelines in clinical practice can be challenging.4 One focus area is the appropriate use of inhaled corticosteroids (ICS).4 While effective in asthma, ICS have limited benefits in COPD due to different inflammation patterns.4 Overuse of ICS in COPD can increase the risk of adverse effects like pneumonia.4 The COPD-X guidelines advocate a ‘stepwise’ approach for COPD treatment, starting with short-acting bronchodilators, followed by long-acting bronchodilators, and adding ICS only after severe exacerbations.1,4,6 Regular checks of inhaler technique, appropriate use of oral corticosteroids, and limited use of antibiotics are also emphasised.1,4
The new COPD Clinical Care Standards address these issues by providing healthcare professionals and providers with a clear framework to guide clinical practice. They include ten quality statements outlining the expected care for individuals with COPD, regardless of their treatment location in Australia, and are aligned with current evidence-based Australian guidelines, including the COPD-X Guidelines and the Respiratory and Antibiotic Therapeutic Guidelines.1,7 Optimal COPD management involves early and accurate diagnosis with spirometry, patient education, personalised care plans that manage exacerbations, smoking cessation, vaccination, and pulmonary rehabilitation.1 Effective transitions from acute care to community care are crucial for coordinated COPD management.1
For a comprehensive understanding and access to the full set of quality statements and associated indicators, healthcare professionals are encouraged to consult the complete COPD Clinical Care Standard document, available at: https://www.safetyandquality.gov.au/publications-andresources/resource-library/chronic-obstructive-pulmonarydisease-clinical-care-standard-2024

In 2024, our pharmacy team proudly presented several quality improvement projects at the Advanced Pharmacy Australia and Clinical Oncology Society of Australia annual conferences. We have included one of these projects below to give you an understanding of our innovations. Scan the QR code to explore more!
Penny Lee - Epic Pharmacy Lismore
Penny Lee - Epic Pharmacy Lismore
Penny Lee -
Blister packs consist of a combination of plastic and aluminium therefore cannot be recycled through kerbside recycling programs. As such, millions of blister packs are incorrectly recycled each year contaminating the recycling stream or sent directly to landfill.
Blister packs consist of a combination of plastic and aluminium therefore cannot be recycled through kerbside recycling programs. As such, millions of blister packs are incorrectly recycled each year contaminating the recycling stream or sent directly to landfill.
Blister packs consist of a combination of plastic and aluminium therefore cannot be recycled through kerbside recycling programs. As such, millions of blister packs are incorrectly recycled each year contaminating the recycling stream or sent directly to landfill.
to collect sufficient volumes of empty blister packs which enables use of specialised recycling technology so both the aluminium and plastic can be recycled and/or diverted from landfill.
However, pharmacy can promote sustainable practice by assisting to collect sufficient volumes of empty blister packs which enables use of specialised recycling technology so both the aluminium and plastic can be recycled and/or diverted from landfill.
However, pharmacy can promote sustainable practice by assisting to collect sufficient volumes of empty blister packs which enables use of specialised recycling technology so both the aluminium and plastic can be recycled and/or diverted from landfill.



To improve recycling of blister packs and minimise the amount going into landfill by introducing a blister pack recycling program in the pharmacy.
To improve recycling of blister packs and minimise the amount going into landfill by introducing a blister pack recycling program in the pharmacy.
In the first 9 months of the program, a total of 12 thousand blisters were diverted from landfill. Following the education session, collection of blister packs increased to around 3000 every two months and currently recycling remains consistent at this level. (Figure 1) During this period, usage of return of unwanted medicines bins also decreased by 56%. (Figure 2)
In the first 9 months of the program, a total of 12 thousand blisters were diverted from landfill. Following the education session, collection of blister packs increased to around 3000 every two months and currently recycling remains consistent at this level. (Figure 1) During this period, usage of return of unwanted medicines bins also decreased by 56%. (Figure 2)
In the first 9 months of the program, a total of 12 thousand blisters were diverted from landfill. Following the education session, collection of blister packs increased to around 3000 every two months and currently recycling remains consistent at this level. (Figure 1) During this period, usage of return of unwanted medicines bins also decreased by 56%. (Figure 2)
Jun-Aug23Sep-Nov23Dec23-Feb24Mar-May24
Jun-Aug23Sep-Nov23Dec23-Feb24Mar-May24
Jun-Aug23Sep-Nov23Dec23-Feb24Mar-May24
2 - Usage of Return of Unwanted Medicines (RUM) bins over time
Figure 2 - Usage of Return of Unwanted Medicines (RUM) bins over time
who responded, 87.5% were aware of the program and actively participated in recycling blister packs. (Figure 3 & 4) Of all survey respondents, 38% recycled blister packs every time and only 12.5% never recycled a blister pack. (Figure 5)
Staff satisfaction of the program was evaluated via an anonymous survey. Of those who responded, 87.5% were aware of the program and actively participated in recycling blister packs. (Figure 3 & 4) Of all survey respondents, 38% recycled blister packs every time and only 12.5% never recycled a blister pack. (Figure 5)
Staff satisfaction of the program was evaluated via an anonymous survey. Of those who responded, 87.5% were aware of the program and actively participated in recycling blister packs. (Figure 3 & 4) Of all survey respondents, 38% recycled blister packs every time and only 12.5% never recycled a blister pack. (Figure 5)
To improve recycling of blister packs and minimise the amount going into landfill by introducing a blister pack recycling program in the pharmacy.
The pharmacy partnered with Pharmacycle, an end-to-end recycling program for household and commercial medicinal blister pack waste. Pharmacy staff were educated on the process for both internal waste and collection from patients.
The pharmacy partnered with Pharmacycle, an end-to-end pack waste. Pharmacy staff were educated on the process for both internal waste and collection from patients.
The pharmacy partnered with Pharmacycle, an end-to-end recycling program for household and commercial medicinal blister pack waste. Pharmacy staff were educated on the process for both internal waste and collection from patients.
or “sometimes”
At implementation in June 2023, the pharmacy was included on the Pharmacycle website as a participating collection point. In November 2023, an education session was provided to hospital and nursing home staff to further promote the program.
At implementation in June 2023, the pharmacy was included on November 2023, an education session was provided to hospital and nursing home staff to further promote the program.
At implementation in June 2023, the pharmacy was included on the Pharmacycle website as a participating collection point. In November 2023, an education session was provided to hospital and nursing home staff to further promote the program.
5 – Staff recycling of blister packs
This program successfully recycles blister packs and reduces landfill from return of unwanted medicines. Despite cost, it aligns with the pharmacy’s goal of increasing sustainability and is currently being investigated to expand to other pharmacies within the network.
The high uptake of staff participating in the program shows that pharmacy staff are actively aware and promoting sustainable options with positive feedback received on ways to extend the program to include hospital staff
This program successfully recycles blister packs and reduces landfill from return of unwanted medicines. Despite cost, it aligns with the pharmacy’s goal of increasing sustainability and is currently being investigated to expand to other pharmacies within the network. The high uptake of staff participating in the program shows that pharmacy staff are actively aware and promoting sustainable options with positive feedback received on ways to extend the program to include hospital staff.
This program successfully recycles blister packs and reduces landfill from return of unwanted medicines. Despite cost, it aligns with the pharmacy’s goal of increasing sustainability and is currently being investigated to expand to other pharmacies within the network.
Implementing strategies which promote recycling and minimise waste generation within pharmacies can improve sustainable pharmacy practices and ensure pharmacy is part of the solution to reducing unnecessary waste from the healthcare sector.
Implementing strategies which promote recycling and minimise waste generation within pharmacies can improve sustainable pharmacy practices and ensure pharmacy is part of the solution to reducing unnecessary waste from the healthcare sector.
The high uptake of staff participating in the program shows that pharmacy staff are actively aware and promoting sustainable options with positive feedback received on ways to extend the program to include hospital staff
Implementing strategies which promote recycling and minimise waste generation within pharmacies can improve sustainable pharmacy practices and ensure pharmacy is part of the solution to reducing unnecessary waste from the healthcare sector.
This information is intended for Australian healthcare professionals. Care has been taken to provide accurate information at the time of creation and to ensure this newsletter is free from errors or omissions. If you have any queries regarding Circuit content and authors, please contact the Quality and Medication Safety Unit via PharmacyQMSU@icon.team