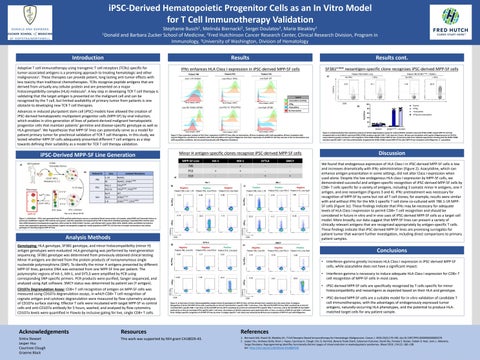
iPSC-Derived Hematopoietic Progenitor Cells as an In Vitro Model for T Cell Immunotherapy Validation Stephanie Busch1, Melinda Biernacki2, Sergei Doulatov3, Marie Bleakley2 1Donald and Barbara Zucker School of Medicine, 2Fred Hutchinson Cancer Research Center, Clinical Research Division, Program in Immunology, 3University of Washington, Division of Hematology
Introduction
Results
Results cont.
Adoptive T cell immunotherapy using transgenic T cell receptors (TCRs) specific for tumor-associated antigens is a promising approach to treating hematologic and other malignancies1. These therapies can provide potent, long lasting anti-tumor effects with less toxicity than traditional chemotherapies. TCRs recognize peptide antigens that are derived from virtually any cellular protein and are presented on a major histocompatibility complex (HLA) molecule1. A key step in developing TCR T cell therapy is validating that the target antigen is presented on the malignant cell and can be recognized by the T cell, but limited availability of primary tumor from patients is one obstacle to developing new TCR T cell therapies.
IFNg enhances HLA Class I expression in iPSC-derived MPP-5F cells
SF3B1K700E neoantigen-specific clone recognizes iPSC-derived MPP-5F cells
Line 1-1R: RUNX1
TP53R209F R209F
P53-15
TP53
Epi 9
WT
MPP-5F Line 786 P53 310
HA-1 + -
MX-1 + + -
SMCY +
Negative Patient 786, Line 1-1R
MX-1 Negative
Positive Patient P53, Line P53-2
c)
Patient 310, Line Epi9
2.24
6.06
0.68
2.19
45.5
1.13
Positive Patient 786, Line 1-1R
Positive
Negative
Patient P53, Line P53-2 Patient 310, Line Epi6
25.5
2.43
1.66
22.9
11.7
8.38
+IFN g
+IFN g
CD107a CD8 SYTL3
d)
100
b)
Control +IFNg
80
+Azacytidine
60
+IFNg and Azacytidine
40
Negative Patient 786, Line RS
Positive
e)
Patient P53, Line P53-2 Patient 310, Line Epi9 1.17
3.00
Negative
Negative
Positive
Patient 786, Line RS
Patient P53, Line P53-2
Patient 310, Line Epi6
0.51
0.76
No IFN g
1.38
+IFN g
0.99
43.6
CD8
Figure 4: a) Representative flow cytometry analysis of CD107a degranulation assays for control (RUNX1 mutation only) and SF3B1 K700E mutant MPP-5F cell lines incubated with an HLA-B40:01 restricted SF3B1 K700E mutation-specific CD8+ T cell clone for 5 hours. 48-hour pre-incubation with 1ug/mL IFNgamma prior to CD107a assay induction greatly increased T cell recognition of the SF3B1 K700E mutant MPP-5F line. b) Summary data from triplicate experiments showing that the SF3B1 K700E mutation-specific CD8+ T cell clone preferentially recognized the SF3B1 K700E mutant MPP-5F line after MPP-5F pre-incubation with IFNgamma +/- azacytidine.
Discussion We found that endogenous expression of HLA Class I in iPSC-derived MPP-5F cells is low and increases dramatically with IFNg administration (Figure 2). Azacytidine, which can enhance antigen presentation in some settings, did not alter Class I expression when used alone. Despite the low endogenous HLA class I expression by MPP-5F cells, we demonstrated successful and antigen-specific recognition of iPSC-derived MPP-5F cells by CD8+ T cells specific for a variety of antigens, including 3 somatic minor H antigens, one Y antigen, and one neoantigen (Figures 3 and 4). IFNg pretreatment was necessary for recognition of MPP-5F by some but not all T cell clones; for example, results were similar with and without IFNg for the MX-1 specific T cell clone co-cultured with 786 1-1R MPP5F cells (Figure 3c). These findings indicate that IFNg may be necessary for adequate levels of HLA Class I expression to permit CD8+ T cell recognition and should be considered in future in vitro and in vivo uses of iPSC-derived MPP-5F cells as a target cell model. More broadly, our data suggest that MPP-5F lines can present a variety of clinically relevant antigens that are recognized appropriately by antigen-specific T cells. These findings indicate that iPSC-derived MPP-5F lines are promising surrogates for patient tumor that warrant further investigation, including direct comparisons to primary patient samples.
Conclusions
3.69
No IFN g
0.80
Negative
K700E + RUNX1 SF3B1 SF3B1K700E+RUNX1
SMCY
• Interferon-gamma greatly increases HLA Class I expression in iPSC-derived MPP-5F cells, while azacytidine does not have a significant impact. 0.54
0.81
86.8
• Interferon-gamma is necessary to induce adequate HLA Class I expression for CD8+ T cell recognition of MPP-5F cells in most cases. • iPSC-derived MPP-5F cells are specifically recognized by T cells specific for minor histocompatibility and neoantigens as expected based on their HLA and genotype.
CD107a
CD107a Degranulation Assay: CD8+ T cell recognition of antigen on MPP-5F cells was measured using CD107a degranulation assays, in which CD8+ T cell recognition of cognate antigen and cytotoxic degranulation were measured by flow cytometry analysis of CD107a surface staining. Effector T cells were incubated with target MPP-5F vs control cells and anti-CD107a antibody for 5 hours, washed, and analyzed by flow cytometry. CD107a levels were quantified in FlowJo by inclusive gating for live, single CD8+ T cells.
SYTL3 +
HA-1
CD8
Genotyping: HLA genotype, SF3B1 genotype, and minor histocompatibility (minor H) antigen genotypes were evaluated. HLA genotyping was performed by next-generation sequencing. SF3B1 genotype was determined from previously obtained clinical testing. Minor H antigens are derived from the protein products of nonsynonymous single nucleotide polymorphisms (SNP). To identify the minor H antigens presented by different MPP-5F lines, genomic DNA was extracted from one MPP-5F line per patient. The polymorphic regions of HA-1, MX-1, and SYTL3 were amplified by PCR using corresponding SNP-specific primers. PCR products were purified, Sanger sequenced, and analyzed using ApE software. SMCY status was determined by patient sex (Y antigen).
62.9
CD107a HLA Class I APC
HLA Class I APC
CD107a
Analysis Methods
+Azacytidine
Minor H antigen-specific clones recognize iPSC-derived MPP-5F cells
Hsu et al, Blood 20192
Figure 1: a) Methods – iPSCs were generated from CD34-purified patient bone marrow or peripheral blood mononuclear cell samples using OSKM and Yamanaka factors as previously established. Isogenic iPSC colonies were grown, selected, expanded, and sequenced by PCR to determine individual genotypes. Expanded iPSC colonies were then differentiated to hematopoietic progenitors, FACS sorted for CD34+/CD45+ fraction, and infected with 5 genes (ERG, MYB, HOXA9, SOX4, and RORA) via lentiviral vector, then expanded to generate immortalized, isogenic hematopoietic progenitor model populations (MPP-5F). b) Overview of sample nomenclature and cellular genotypes of resulting isogenic MPP-5F lines.
Line Epi6: SF3B1
Figure 2: Flow cytometric analysis of HLA Class I expression of MPP-5F lines after no intervention, 48-hour incubation with 2nM azacytidine, 48-hour incubation with 1ug/mL IFNgamma, and 48-hour incubation with 2nM azacytidine and 1ug/mL IFNgamma. HLA Class I expression of all MPP-5F cell lines was low in the no intervention and 2nM azacytidine conditions, but increased dramatically with IFNgamma incubation.
G742D
SF3B1G742D, EZH2R685H
Line P53-15: TP53/TP53
HLA Class I APC
, TP53
1-8
1.44
RUNX1 RUNX1
R209F
SF3B1
+ IFN g
0
b)
Epi 6
Line RS: RUNX1/SF3B1
CD107a
MPP-5F
P53-2
14.1
CD8
+IFNg
No IFN g
310
RUNX1, SF3B1K700E
HLA Class I APC
+IFN g
P53
RS
No IFN g
20
a)
RUNX1
HLA Class I APC
No Intervention
Isolated Mutations
1-1R
+ IFN g
No IFN g
+Azacytidine and IFNg
HLA Class I APC
No IFN g
786
Line
Line Epi9: WT
a)
Patient 786 SF3B1K700E + RUNX1
Legend
a) Patient ID
Line P53-2: TP53
Patient 786 RUNX1 only
9.01
iPSC-Derived MPP-5F Line Generation b)
Patient 310
%CD107a+
Advances in induced pluripotent stem cell (iPSC) models have allowed the creation of iPSC-derived hematopoietic multipotent progenitor cells (MPP-5F) by viral induction, which enables in vitro generation of lines of patient-derived malignant hematopoietic progenitor cells that maintain patients’ germline and disease-specific genotype as well as HLA genotype2. We hypothesize that MPP-5F lines can potentially serve as a model for patient primary tumor for preclinical validation of TCR T cell therapies. In this study, we tested whether MPP-5F cells adequately presented different T cell antigens as a step towards defining their suitability as a model for TCR T cell therapy validation.
Patient P53
Patient 786
CD8
Figure 3: a) Overview of minor histocompatibility antigen (minor H) genotypes for MPP-5F lines. All lines derived from a patient carry the same minor H antigens. Recognition of b) the P53 MPP-5F line (HA-1 positive) by an HLA-A2 restricted HA-1 specific CD8+ T cell clone, c) the 786 and P53 MPP-5F lines (MX-1 positive) by an HLA-A2 restricted MX-1 specific CD8+ T cell clone, d) the 310 MPP-5F line (SYTL-3 positive) by an HLA-A2 restricted SYTL3 specific CD8+ T cell clone, and e) the 310 MPP-5F line (SMCY positive) by an HLA-A2 restricted SYTL3 specific CD8+ T cell clone. All analyses of CD107a expression were performed after a 5 hour co-culture of MPP-5F and CD8+ T cell clone. Note: Antigen-specific recognition of all MPP-5F lines by minor H antigen-specific T cell clones was enhanced by 48-hour pre-incubation of MPP-5F cells with IFNgamma.
• iPSC-derived MPP-5F cells are a suitable model for in vitro validation of candidate T cell immunotherapies, with the advantages of endogenously expressed tumor antigens, naturally-occurring HLA phenotypes, and the potential to produce HLAmatched target cells for any patient sample.
Acknowledgements
Resources
References
Sintra Stewart Jasper Hsu Courtnee Clough Graeme Black
This work was supported by NIH grant CA18029-43.
1. Biernacki MA, Brault M, Bleakley M.; T-Cell Receptor-Based Immunotherapy for Hematologic Malignancies. Cancer J. 2019;25(3):179-190. doi:10.1097/PPO.0000000000000378 2. Jasper Hsu, Andreea Reilly, Brian J. Hayes, Courtnee A. Clough, Eric Q. Konnick, Beverly Torok-Storb, Suleyman Gulsuner, David Wu, Pamela S. Becker, Siobán B. Keel, Janis L. Abkowitz, Sergei Doulatov; Reprogramming identifies functionally distinct stages of clonal evolution in myelodysplastic syndromes. Blood 2019; 134 (2): 186–198. doi: https://doi.org/10.1182/blood.2018884338