Identifying Long-term Hemodynamic Adaptations Following Pipeline Embolization with Quantitative Magnetic Resonance Angiography Brendan Ryu BS1, Timothy G White MD2, Kevin Shah MD2, Justin Turpin MD2, Henry Woo MD2 1Donald
and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA 2Department of Neurosurgery, North Shore University Hospital, Northwell Health, Manhasset, NY, USA
INTRODUCTION
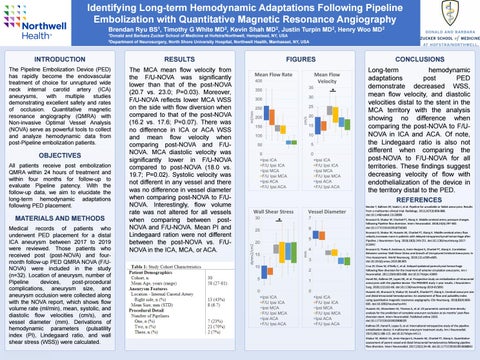
RESULTS
The Pipeline Embolization Device (PED) has rapidly become the endovascular treatment of choice for unruptured wide neck internal carotid artery (ICA) aneurysms, with multiple studies demonstrating excellent safety and rates of occlusion. Quantitative magnetic resonance angiography (QMRA) with Non-invasive Optimal Vessel Analysis (NOVA) serve as powerful tools to collect and analyze hemodynamic data from post-Pipeline embolization patients.
The MCA mean flow velocity from the F/U-NOVA was significantly lower than that of the post-NOVA (20.7 vs. 23.0; P=0.03). Moreover, F/U-NOVA reflects lower MCA WSS on the side with flow diversion when compared to that of the post-NOVA (16.2 vs. 17.6; P=0.07). There was no difference in ICA or ACA WSS and mean flow velocity when comparing post-NOVA and F/UNOVA. MCA diastolic velocity was significantly lower in F/U-NOVA compared to post-NOVA (18.0 vs. 19.7; P=0.02). Systolic velocity was not different in any vessel and there was no difference in vessel diameter when comparing post-NOVA to F/UNOVA. Interestingly, flow volume rate was not altered for all vessels when comparing between postNOVA and F/U-NOVA. Mean PI and Lindegaard ration were not different between the post-NOVA vs. F/UNOVA in the ICA, MCA, or ACA.
OBJECTIVES All patients receive post embolization QMRA within 24 hours of treatment and within four months for follow-up to evaluate Pipeline patency. With the follow-up data, we aim to elucidate the long-term hemodynamic adaptations following PED placement.
MATERIALS AND METHODS Medical records of patients who underwent PED placement for a distal ICA aneurysm between 2017 to 2019 were reviewed. Those patients who received post (post-NOVA) and fourmonth follow-up PED QMRA NOVA (F/UNOVA) were included in the study (n=32). Location of aneurysm, number of Pipeline devices, post-procedural complications, aneurysm size, and aneurysm occlusion were collected along with the NOVA report, which shows flow volume rate (ml/min), mean, systolic, and diastolic flow velocities (cm/s), and vessel diameter (mm). Derivations of hemodynamic parameters (pulsatility index (PI), Lindegaard ratio, and wall shear stress (WSS)) were calculated. RESEARCH POSTER PRESENTATION TEMPLATE © 2019
www.PosterPresentations.com
FIGURES
CONCLUSIONS
*
Long-term hemodynamic adaptations post PED demonstrate decreased WSS, mean flow velocity, and diastolic velocities distal to the stent in the MCA territory with the analysis showing no difference when comparing the post-NOVA to F/UNOVA in ICA and ACA. Of note, the Lindegaard ratio is also not different when comparing the post-NOVA to F/U-NOVA for all territories. These findings suggest decreasing velocity of flow with endothelialization of the device in the territory distal to the PED. REFERENCES Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology. 2013;267(3):858-868. doi:10.1148/radiol.13120099
*
Brunozzi D, Shakur SF, Charbel FT, Alaraj A. Middle cerebral artery pressure changes following Pipeline flow diversion. Interv Neuroradiol. 2018;24(3):297-302. doi:10.1177/1591019918756583 Brunozzi D, Shakur SF, Hussein AE, Charbel FT, Alaraj A. Middle cerebral artery flow velocity increases more in patients with delayed intraparenchymal hemorrhage after Pipeline. J Neurointerv Surg. 2018;10(3):249-251. doi:10.1136/neurintsurg-2017013042 Brunozzi D, Theiss P, Andrews A, Amin-Hanjani S, Charbel FT, Alaraj A. Correlation Between Laminar Wall Shear Stress and Growth of Unruptured Cerebral Aneurysms: In Vivo Assessment. World Neurosurg. 2019;131:e599-e605. doi:10.1016/j.wneu.2019.08.005 Cruz JP, Chow M, O’Kelly C, et al. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. Am J Neuroradiol. 2012;33(4):603-608. doi:10.3174/ajnr.A3065 Hanel RA, Kallmes DF, Lopes DK, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: The PREMIER study 1 year results. J Neurointerv Surg. 2020;12(1):62-66. doi:10.1136/neurintsurg-2019-015091 Hussein AE, Brunozzi D, Shakur SF, Ismail R, Charbel FT, Alaraj A. Cerebral aneurysm size and distal intracranial hemodynamics: An assessment of flow and pulsatility index using quantitative magnetic resonance angiography. Clin Neurosurg. 2018;83(4):660665. doi:10.1093/neuros/nyx441 Hussein AE, Shownkeen M, Thomas A, et al. 2D parametric contrast time-density analysis for the prediction of complete aneurysm occlusion at six months’ post-flow diversion stent. Interv Neuroradiol. Published online 2020. doi:10.1177/1591019920908205 Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: A multicenter aneurysm treatment study. Am J Neuroradiol. 2015;36(1):108-115. doi:10.3174/ajnr.A4111 Shakur SF, Aletich VA, Amin-Hanjani S, Hussein AE, Charbel FT, Alaraj A. Quantitative assessment of parent vessel and distal intracranial hemodynamics following pipeline flow diversion. Interv Neuroradiol. 2017;23(1):34-40. doi:10.1177/1591019916668842