Disease Attenuative Effects of Synovial Mesenchymal Stem CellDerived Exosomes on Osteoarthritis induced Chondrocytes Adam Kiridly , Dan Li , Pablo Palacios , Jedidiah Bondy , Christopher Antonacci , Hudson Liang , Michael Sayegh , Pooja Swami , Andrew Wong, 2
1
2
1
3
1
2
1
Dr. Nicholas Sgaglione2, Daniel A. Grande1 1Orthopaedic Research Laboratory, The Feinstein Institute for Medical Research, Manhasset, NY, 2Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, 3Rothman Orthopaedic Institute, Philadelphia, PA.
Background and hypothesis
A
FC
0.2
0.1
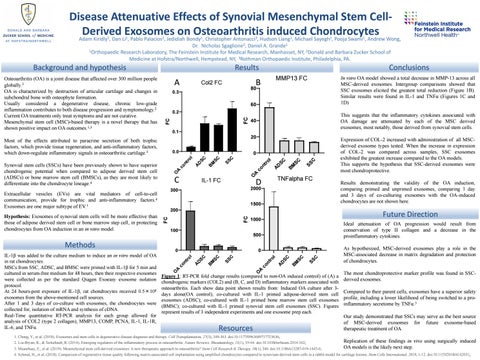
This suggests that the inflammatory cytokines associated with OA damage are attenuated by each of the MSC derived exosomes, most notably, those derived from synovial stem cells.
40 20
0.0
O
IL-1 FC
C SS
SC B M
D
A
A
nt ro co
A O
SC
l
C SS
SC
SC D
B M
co
A
nt ro
l
0
C
TNFalpha FC
D
2000
300
1500
FC
FC
200
1000
100
C SS
SC B M
D
As hypothesized, MSC-derived exosomes play a role in the MSC-associated decrease in matrix degradation and protection of chondrocytes.
A
A
co
SC
l
C SS
SC B M
SC D
A
co
nt ro
l
0
A
Results demonstrating the validity of the OA induction, comparing primed and unprimed exosomes, comparing 1 day and 3 days of co-culturing exosomes with the OA-induced chondrocytes are not shown here.
Ideal attenuation of OA progression would result from conservation of type II collagen and a decrease in the proinflammatory cytokines.
500
0
Expression of COL-2 increased with administration of all MSCderived exosome types tested. When the increase in expression of COL-2 was compared across samples, SSC exosomes exhibited the greatest increase compared to the OA models. This supports the hypothesis that SSC-derived exosomes were most chondroprotective.
Future Direction
nt ro
Extracellular vesicles (EVs) are vital mediators of cell-to-cell communication, provide for trophic and anti-inflammatory factors.4 Exosomes are one major subtype of EV.1
The most chondroprotective marker profile was found in SSCFigure 1: RT-PCR fold change results (compared to non-OA induced control) of (A) a derived exosomes. chondrogenic markers (COL2) and (B, C, and D) inflammatory markers associated with osteoarthritis. Each show data point shown results from: Induced OA culture after 3Compared to their parent cells, exosomes have a superior safety days alone(OA control); co-cultured with IL-1 primed adipose-derived stem cell profile, including a lower likelihood of being switched to a proexosomes (ADSC); co-cultured with IL-1 primed bone marrow stem cell exosomes 3 inflammatory secretome by TNFα. (BMSC); co-cultured with IL-1 primed synovial stem cell exosomes (SSC). Figures represent results of 3 independent experiments and one exosome prep each. Our study demonstrated that SSCs may serve as the best source of MSC-derived exosomes for future exosome-based therapeutic treatment of OA. O
O
IL-1β was added to the culture medium to induce an in vitro model of OA in rat chondrocytes. MSCs from SSC, ADSC, and BMSC were primed with IL-1β for 5 min and cultured in serum-free medium for 48 hours, then their respective exosomes were collected as per the standard Qiagen Exoeasy exosome isolation protocol. At 24 hours-post exposure of IL-1β, rat chondrocytes received 0.5×108 exosomes from the above-mentioned cell sources. After 1 and 3 days of co-culture with exosomes, the chondrocytes were collected for, isolation of mRNA and synthesis of cDNA. Real-Time quantitative RT-PCR analysis for each group allowed for analysis of COL2 (type 2 collagen), MMP13, COMP, PCNA, IL-1, IL-1R, IL-6, and TNFα.
In vitro OA model showed a total decrease in MMP-13 across all MSC-derived exosomes. Intergroup comparisons showed that SSC exosomes elicited the greatest total reduction (Figure 1B). Similar results were found in IL-1 and TNFα (Figures 1C and 1D)
60
Synovial stem cells (SSCs) have been previously shown to have superior chondrogenic potential when compared to adipose derived stem cell (ADSCs) or bone marrow stem cell (BMSCs), as they are most likely to differentiate into the chondrocyte lineage.4
Methods
MMP13 FC
80
0.3
Most of the effects attributed to paracrine secretion of both trophic factors, which provide tissue regeneration, and anti-inflammatory factors, which down-regulate inflammatory signals in osteoarthritic cartilage.3
Hypothesis: Exosomes of synovial stem cells will be more effective than those of adipose derived stem cell or bone marrow step cell, in protecting chondrocytes from OA induction in an in vitro model.
Conclusions
B
Col2 FC
FC
Osteoarthritis (OA) is a joint disease that affected over 300 million people globally.3 OA is characterized by destruction of articular cartilage and changes in subchondral bone with osteophyte formation. Usually considered a degenerative disease, chronic low-grade inflammation contributes to both disease progression and symptomology.2 Current OA treatments only treat symptoms and are not curative. Mesenchymal stem cell (MSC)-based therapy is a novel therapy that has shown positive impact on OA outcomes.1,3
Results
Resources
1. Chang, Y., et al. (2018). Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transplantation, 27(3), 349-363. doi:10.1177/0963689717723636; 2. Liu-Bryan, R., & Terkeltaub, R. (2014). Emerging regulators of the inflammatory process in osteoarthritis. Nature Reviews. Rheumatology, 11(1), 35-44. doi:10.1038/nrrheum.2014.162; 3. Mianehsaz, E., et al. (2019). Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Research & Therapy, 10(1), 340. doi:10.1186/s13287-019-1445-0;
Replication of these findings in vivo using surgically induced OA models is the likely next step.
4. Schmal, H., et al. (2018). Comparison of regenerative tissue quality following matrix-associated cell implantation using amplified chondrocytes compared to synovium-derived stem cells in a rabbit model for cartilage lesions. Stem Cells International, 2018, 1-12. doi:10.1155/2018/4142031;