My Blood My Health


Welcome to My Blood, My Health Digital Magazine
We would like to dedicate this issue to Debbie Hanlon, a remarkable woman who sadly lost her battle with non-Hodgkin lymphoma this month. Debbie, a longtime St. John's city councillor and real estate agent, was a force of nature. Her energy, passion, and larger-than-life spirit left a lasting impression on everyone who knew her. She was a fighter, and we would like to acknowledge all persons who live with lymphoma. In this issue will find some new treatments available, providing some hope.
With this magazine, we aim to pursue Heal Canada's mission to empower patients to access better and equitable services. We're deeply grateful to our readers for their continued interest and support. Your engagement drives us to produce insightful and valuable content that encourages patient-centricity in healthcare.
Thank you for participating in our journey, and welcome to this enlightening issue!
From your dedicated team, Brigitte and Cheryl.


Cheryl Petruk, MBA B.Mgt. CEO & Founder of Heal Canada

Brigitte Leonard, Ph.D Chief Scientific Officer
My Blood, My Health Director
Heal Canada is a registered not-for-profit organization in Canada. healcanada.org
Disclaimer: The Patient Advocacy Digital Magazine provides general information and resources to promote patient empowerment and awareness The content is not a substitute for professional medical advice or treatment Always consult with qualified healthcare professionals for personalized guidance regarding your specific medical condition or situation


Critical PEGASYS Shortage, BESREMI Is The Alternative!
Brigitte Leonard, Ph.D

Recently, due to manufacturing disruptions, health authorities worldwide have faced a critical shortage of Pegasys, a peginterferon alfa-2a injection.
Canada is no exception to this stressful situation. This shortage presents serious risks to patients who depend on interferon-based therapy for treating myeloproliferative neoplasms (MPNs).
Health Canada has permitted the exceptional importation of another interferon-based therapy, Besremi, a ropeginterferon alfa-2b injection [1]. Besremi can be used instead of Pegasys to treat MPN. BESREMi® will be available to you regardless of your MPN form. Speak to your doctor to formulate a transition plan that is suitable for you.
Besremi is not an experimental drug and has proven efficient in MPN. The FDA approved Besremi as a PV treatment in 2021[2]. Besremi is still studied extensively in several phase III trials in Polycythemia vera (PV), Essential Thrombocytopenia (ET), and Myelofibrosis. Also, Besremi is part of the treatment recommendation in the USA (NCCN guidelines)[3].
You can find more information on the FORUS website: www.besremiei.com. This website provides information on exceptional importation and is easy to navigate. 0:42min.
Why is the MPN community facing a Pegasys shortage?
When a new company acquired the rights to sell Pegasys in 2017, a 10-year global supply stockpile was planned to allow the new manufacturer time to establish the new production line. Due to increased demand across many diseases, the stockpile has run out much earlier than anticipated. There is a minimal supply of Pegasys® in Canada. If you are experiencing difficulty filling your prescription, work with your doctor and pharmacist to find a pharmacy that still has a supply. Your pharmacist can initiate a pharmacy-topharmacy transfer.
Why can Besrmi be used instead of Pegasys?
Besremi (ropeginterferon alfa-2b) and Pegasys (peginterferon alfa-2a) are interferon therapies used in treating MPN, such as PV. Both Besremi and Pegasys are long-acting forms of interferon alfa, which stimulate the immune system, inhibit the production of abnormal blood cells, and reduce the production of red blood cells in PV. They both target similar pathways but differ in formulation and specific pharmacokinetic properties.
Formulation and Dosing:
Besremi (Ropeginterferon alfa-2b):
Besremi is a longer-acting interferon form, allowing for less frequent dosing. It is typically administered once every 2 weeks during the initial treatment phase and then once a month after the maintenance phase. Its extended duration of action means fewer injections than Pegasys.
Pegasys (Peginterferon alfa-2a):
Pegasys is also a long-acting interferon but requires more frequent administration than Besremi. It is typically injected once a week for the treatment of PV.

The dosage must be adjusted when a patient switches from Pegasys to Besremi. Your healthcareprofessionalwillbeabletoassistyouduringthetransition.
Besremi:
A Proven
Interferon Formulation For Myeloproliferative Neoplasms (MPNs)
Interferon (IFN) has been known for decades to provide interesting results in PV. However, the poor tolerability of the old formulation impacted its adoption in clinical practices. RopegIFN (BESREMI) has been studied in low-risk and high-risk patient populations.
In low-risk patients, the dose can be increased from 100 µg every 2 weeks to 250 → 350 → 500 microgramme every two weeks if the clinical benefits are not observed [4]. RopegIFN provides superior results in hydroxyurea-naive patients Avoiding HU and initiating patients with RopegIFN could improve long-term care. Based on these findings, the National Cancer Network (NCCN) recommended RopegIFN as the preferred cytoreductive regimen in the first-line setting for patients with low-risk PV and a preferred interferon regimen in high-risk PV.

Besremi:
1) Reduces the need for phlebotomy (Figure 1a).
2) Reduces the number of phlebotomy from 3 to 0.5 (Figure 1b), with patient who receive Hydroxyurea (HU) the number of phlebotomy are reduced to 2.4 to 0.5.
3) Normalizes leucocyte and platelet counts, resulting in no thrombotic events (Figure 2).
4) Reduces the JAK2 mutation burden and is considered a disease modifier treatment (Figure 3).
5) Improved the quality of life for PV patients, addressing concerns about drug tolerability and toxicity.






Figure 1a
Figure 1b
Figure 2
Figure 3
Figure 4

The Role of Real-World Evidence in Blood Cancer Advocacy
Cheryl Petruk, MBA
How Patient-Reported Outcomes and Real-World Data Can Shape Better Treatments and Policies for Blood Cancer Patients.
Blood cancers, including leukemia, lymphoma, myeloma, and myeloproliferative neoplasms, represent a significant health burden, affecting thousands of patients annually. While clinical trials remain the gold standard for evaluating new therapies, they often fail to capture the full spectrum of patient experiences in real-world settings. This is where Real-World Evidence (RWE) and Patient-Reported Outcomes (PROs) play a crucial role in blood cancer advocacy. By leveraging data from diverse sources outside controlled clinical environments, RWE provides a more comprehensive understanding of disease progression, treatment effectiveness, and patient needs. In the healthcare landscape, where public health policies and reimbursement decisions impact patient access to therapies, RWE and PROs can be instrumental in shaping better treatments and policies.
Understanding Real-World Evidence and Patient-Reported Outcomes
Real-world evidence (RWE) is derived from Real-World Data (RWD), which includes patient registries, electronic health records (EHRs), insurance claims, and patient-reported information. Unlike traditional clinical trial data, RWE reflects the experiences of a broader population, encompassing factors like comorbidities, socioeconomic status, and long-term treatment effects.
Patient-reported outcomes (PROs) are a subset of RWE that focus on patients’ self-reported experiences with their disease and treatments. PROs capture elements such as quality of life, symptom burden, treatment side effects, and emotional well-being. PROs are increasingly used to assess patient satisfaction and treatment efficacy in real-world conditions.
The Importance of RWE in Blood Cancer Advocacy
Patient advocacy groups and healthcare policymakers have started to recognize the power of RWE in influencing better care and treatment outcomes for blood cancer patients. Key areas where RWE has an impact include improving access to innovative therapies and enhancing patient-centered Care.


Improving Access to Innovative Therapies
One of the biggest challenges in blood cancer treatment is ensuring timely and equitable access to novel therapies The drug approval and reimbursement process involves multiple stakeholders, including regulatory and health technology assessment agencies. While clinical trial data is crucial for initial approvals, RWE strengthens the case for coverage and reimbursement by demonstrating real-world efficacy and patient benefits beyond clinical trial settings.
For example, RWE has been pivotal in expanding access to targeted therapies, such as CAR-T cell therapy, for patients with relapsed or refractory blood cancers. By showing survival benefits, fewer hospitalizations, and improved quality of life compared to standard chemotherapy, RWE has influenced decisionmakers to prioritize funding for these cuttingedge treatments

Enhancing Patient-Centered Care
The patient’s journey in blood cancer treatment varies significantly depending on factors such as age, genetic mutations, and co-existing conditions. Traditional clinical trials often exclude older patients or those with complex medical histories, leading to gaps in treatment guidance. RWE bridges these gaps by providing insights into how different patient populations respond to treatment in real-world settings.

Influencing Policy and Healthcare Planning
In Canada, where healthcare is publicly funded, policymakers rely on data to make informed decisions about resource allocation and funding priorities. RWE plays a crucial role in shaping policies by providing evidence of unmet needs, treatment gaps, and the costeffectiveness of different interventions. For instance, a study using real-world data may reveal that blood cancer patients in rural communities experience poorer outcomes due to limited access to specialized care. This evidence can drive policy changes such as increased telemedicine services, funding for regional
PROs (patient-reported outcomes) also help healthcare providers tailor treatment approaches based on patient preferences. For example, some patients may prioritize treatments with fewer side effects over those with a slightly higher survival rate. By incorporating PROs into clinical decisionmaking, oncologists can better align treatment plans with individual patient goals.

cancer centers, or improved transportation support for patients. Similarly, RWE can support value-based healthcare models, where reimbursement is linked to patient outcomes rather than the volume of services provided. By demonstrating the long-term benefits of specific treatments, RWE helps policymakers optimize healthcare spending for maximum patient benefit.

Advancing Research and Drug Development
Pharmaceutical companies and researchers increasingly rely on RWE to complement traditional clinical trials. In blood cancer research, RWE helps identify treatment patterns, assess long-term safety, and discover new therapeutic targets. For example, post-market surveillance studies use RWE to track side effects and treatment adherence among blood cancer patients. This real-world insight allows researchers to refine therapies and develop better treatment protocols. Furthermore, RWE can help identify subpopulations that respond exceptionally well to specific treatments, leading to more personalized and effective therapies.

Challenges and Considerations in Implementing RWE and PROs in Canada
Despite the many benefits, incorporating RWE and PROs into healthcare decision-making in Canada presents several challenges:
Data Integration and Standardization
Real-world data comes from various sources, including hospital records, patient surveys, and pharmacy databases However, the lack of standardized data collection and integration across provinces creates barriers to its practical use Efforts like the Pan-Canadian Health Data Strategy aim to address these challenges by improving data interoperability and accessibility
Privacy and Ethical Concerns
Patient data must be handled with strict privacy protections under laws such as the Personal Information Protection and Electronic Documents Act (PIPEDA). Patient Advocacy groups must work with policymakers to ensure RWE is collected and shared ethically while maintaining patient confidentiality
Stakeholder Engagement
For RWE to have a meaningful impact, collaboration between patients, researchers, healthcare providers, and policymakers is essential. Patient advocacy organizations play a crucial role in educating stakeholders about the value of RWE and advocating for policies that support its integration into healthcare decision-making.

The Future of RWE in Canadian Blood Cancer Advocacy
The future of RWE in blood cancer advocacy looks promising, with several emerging trends set to enhance its role in improving patient outcomes:


Expanded Use in Health Policy
Artificial Intelligence and Big Data
AI-driven analytics can process vast amounts of RWD to identify patterns, predict treatment responses, and optimize care pathways. Machine learning models can detect early signs of treatment resistance, helping physicians adjust therapies before patients experience severe progression. AI also aids in analyzing large-scale patient-reported outcomes to personalize treatments based on symptom management and quality of life indicators.
Patient-Generated Health Data
Wearable devices and mobile health apps enable patients to contribute real-time data on symptoms and treatment effects, enriching RWE datasets. This allows for continuous monitoring of patient health beyond clinical visits, providing a more holistic view of treatment impact. In Canada, integrating patientgenerated data with electronic health records could bridge gaps in patient care and improve personalized treatment strategies.
As Canadian healthcare moves towards personalized and value-based care models, RWE will become a cornerstone of decision-making. Government agencies are increasingly incorporating RWE into health technology assessments, ensuring policies reflect real-world patient experiences. Additionally, RWE-driven policy decisions will help identify disparities in healthcare access, leading to targeted interventions that improve outcomes for marginalized and underserved patient populations.
Real-world Evidence and Patient-Reported Outcomes are transforming blood cancer advocacy in Canada by providing a deeper understanding of patient needs, treatment effectiveness, and healthcare gaps. By leveraging RWE, advocacy organizations can drive policy changes, improve access to innovative treatments, and promote patientcentered care. As technology and data integration continue to evolve, RWE will play an increasingly vital role in shaping better treatments and policies for blood cancer patients, ultimately improving outcomes and quality of life for those affected by these devastating diseases.


Legislative Advocacy for Blood Health: How Policy Changes Can Improve Patient Outcomes
Cheryl Petruk, MBA
Blood health policies play a critical role in ensuring that patients with hematological malignancies and blood disorders receive timely diagnoses, effective treatments, and comprehensive care. From funding for research to equitable access to life-saving therapies, legislative decisions shape the healthcare landscape for millions of patients worldwide. Legislative advocacy is a powerful tool for influencing these policies, ensuring that patient voices are heard and that systemic improvements lead to better outcomes for blood cancer and blood disorder patients.

In recent years, significant policy advancements have been made in blood health, including increased investment in rare disease research, improved access to new therapies, and strengthened patient protections. However, continued advocacy is essential to bridge existing gaps, especially for underserved populations. This article explores recent legislative wins, the ongoing challenges in blood health policy, and the ways in which patients and advocates can drive meaningful change.
ThePowerofLegislativeAdvocacyinBloodHealth
Advocacy is a key driver of change in healthcare By influencing policymakers, patient advocatescanhelpsecurefundingforresearch,improvedrugaccessibility,andpushfor policies that protect patient rights Legislative advocacy involves engaging with governmentrepresentatives,regulatoryagencies,andstakeholderstoshapepoliciesthat improvepatientcare
Effectiveadvocacyhasledtomajorpolicymilestonesinbloodhealth,suchas:
Expandedscreeningprogramsforblooddisorders
Fasterapprovalprocessesforinnovativetherapies
Improvedreimbursementpoliciesforblooddiseasetreatments
Thesechangesunderscorethepowerofcollectiveactioninshapingthefutureofblood healthcare.
Recent Legislative Wins in Blood Health
Several key legislative advancements in recent years have positively impacted patients with blood disorders. Below are some noteworthy developments:
The 21st Century Cures Act (United States)
The 21st Century Cures Act passed in 2016 and expanded in subsequent years, has accelerated medical innovation by streamlining the FDA approval process for new treatments, including gene and cell therapies for blood disorders like sickle cell disease and hemophilia. The law has also provided funding for biomedical research, enhancing efforts to develop cures for blood cancers and rare hematologic conditions.
The Rare Disease Drug Development Initiatives (Canada & U.S.)
Canada and the United States have strengthened rare disease frameworks to improve access to life-saving treatments. In Canada, the National Strategy for Drugs for Rare Diseases announced in 2023 that it would allocate significant funding to improve access to new treatments. In the U.S., the Orphan Drug Act continues to incentivize pharmaceutical companies to develop therapies for rare blood disorders.



Expanded Newborn Screening Programs
Many countries have expanded newborn screening programs to include genetic testing for inherited blood disorders such as sickle cell disease and thalassemia. Early detection can lead to timely interventions, significantly improving long-term health outcomes.
Advances in CAR T-Cell Therapy Policy & Reimbursement
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized treatment for blood cancers like leukemia and lymphoma. Legislative efforts in multiple countries have focused on making these therapies more accessible through improved insurance reimbursement and public healthcare coverage. The Ontario government’s recent decision to expand coverage for CAR T-cell therapy is a prime example of policy-driven progress.
Insurance and Affordability Protections
Countries such as the U.K., Canada, and the U.S. have enacted policies to prevent discrimination in insurance coverage for individuals with genetic predispositions to blood disorders. The Genetic Information Nondiscrimination Act (GINA) in the U.S. prevents insurers from using genetic test results to deny coverage, a crucial protection for people at risk of developing blood conditions.
ChallengesThatStillExistinBloodHealthPolicy



Despitetheseadvancements,manychallengesremaininachievingequitableaccessto careforblooddisorderpatients:
DelaysinDrugApprovals:Whileregulatoryagencieshaveacceleratedtheapprovalof breakthrough therapies, some countries still face significant bureaucratic delays, preventingtimelyaccesstolife-savingtreatments.
AffordabilityIssues:Evenwithimprovedreimbursementpolicies,highout-of-pocketcosts continuetoburdenpatients,particularlythoseneedingspecializedtherapieslikeCARTcelltreatmentorgenetherapies.
Inequities in Access: Rural and underserved communities often struggle to access cutting-edge treatments due to geographic barriers and limitations in healthcare infrastructure.
LackofResearchFunding:Whilerarediseaseframeworkshaveimproved,someblood disordersstillreceiveinadequateresearchanddrugdevelopmentfunding.
BloodDonationPolicies:Policiesaroundblooddonationeligibilityhavebeenhistorically restrictiveforcertaingroups,includingindividualsfromLGBTQ+communitiesandthose withspecificmedicalhistories
To address these challenges, sustained advocacy efforts are required to push for policy changesthatprioritizepatientneeds

How Patients and Advocates Can Influence Policy Change
Patients, caregivers, and advocates play a crucial role in shaping blood health policies
Here are some key strategies for influencing legislative change:
Engage with Policymakers
One of the most effective ways to advocate for policy change is to directly engage with lawmakers Patients and advocacy groups can: Request meetings with legislators to share personal stories and highlight policy needs
Write letters or emails advocating for specific bills or funding initiatives
Attend public hearings or town halls to voice concerns about blood health policy gaps
Join or Support Patient Advocacy Groups
Organizations such as the My Blood My Health program, Canadian Organization for Rare Disorders (CORD), National Hemophilia Foundation (NHF), and Leukemia & Lymphoma Society actively work to influence legislative decisions. Becoming a member or supporting their efforts can amplify the collective voice of the patient community.
Leverage Social Media and Public Awareness Campaigns
Social media platforms provide a powerful tool for advocacy. Patients and supporters can: Share personal experiences to humanize policy issues.
Use hashtags like #BloodHealthMatters and #AdvocateForPatients to raise awareness.
Organize online petitions to push for specific legislative changes.
Advocate for Clinical Trial Access and Equity
Many blood disorder patients rely on clinical trials for access to experimental therapies. Advocacy efforts can focus on:
Expanding trial availability in underserved areas.
Ensuring inclusive recruitment practices to represent diverse patient populations. Pushing for policies that improve insurance coverage for trial participants.
Participate in Legislative Advocacy Days
Several organizations host annual advocacy days, where patients and advocates can meet with policymakers to discuss pressing issues in blood health. Events like Rare Disease Day, Blood Cancer Awareness Month, and National Hemophilia Advocacy Days provide key opportunities to influence policy.





LookingAhead:TheFutureofBloodHealthPolicy
As science and medicine advance, so must public policy. Thefutureofbloodhealthadvocacywilllikelyfocuson:
Expanding gene therapy access and ensuring affordability.
Strengthening rare disease frameworks for faster treatmentapprovals.
Promoting global collaborations to align blood health policiesacrossdifferentcountries.

Improving telemedicine services for patients with mobility oraccessibilitychallenges.
Advocating for a patient-first approach in healthcare decision-making.
With continued efforts from the patient community, advocacy groups, and healthcare professionals, future policies can drive more equitable, effective, and innovative solutions for blood healthcare.
Legislative advocacy is a powerful force for improving patient outcomes in blood health While significant progress has been made, continued efforts are necessary to address remaining challenges, such as drug affordability, access inequities, and research funding gaps
By engaging with policymakers, supporting advocacy organizations, raising public awareness, and championing equitable policies, patients and advocates can help shape a future where everyone with a blood disorder has access to the care, treatment, and support they deserve. Now more than ever, your voice matters—join the movement to advocate for better blood health policies today.





CACHEducation is evolving to better serve the needs of patient advocates and healthcare professionals with its rebrand to CACHEducation Academy. This transformation reflects an expanded commitment to delivering high-quality, structured learning experiences tailored to the ever-changing landscape of patient advocacy and healthcare education. As part of this rebrand, CACHEducation Academy will introduce Advanced Curriculum offerings starting in April 2025, providing deeper insights, specialized training, and enhanced skill development for those looking to elevate their expertise. This next phase marks a significant step forward in strengthening the capacity and impact of patient advocates through comprehensive and innovative education.
"Enrolling in CACHEducation was a game-changer for me as a patient advocate. The program provided invaluable knowledge, practical skills, and a supportive community that empowered me to make a real impact in healthcare advocacy."


Evoluation of Immunotherapy in B Cell Lymphoma
Brigitte Leonard, Ph.D
We are entering an exciting new phase in the history of B-cell nonHodgkin lymphoma treatment (B-NHL), witnessing a dramatic shift in the approach to treating this cancer. Several non-chemotherapeutic agents, even when used as monotherapies or combined with standard treatment, can confer robust and long-lasting remissions and, in some cases, even hold the promise of potential cures. This scientific advancement represents a remarkable opportunity, especially for patients who received extensive prior treatments. This possibility would have been inconceivable until a short while ago.

Unlock immune system potential to attack cancer cells. B-NHL was one of the 1st blood cancers to be treated with antibodies, initiating the era of cancer immunochemotherapy more than two decades ago. Rituxan (rituximab) is a monoclonal antibody (MAB) that binds to the B-cell surface protein CD20. When Rituxan binds to normal and malignant B-cells, it allows the immune system to recognize and destroy them.
There are two mechanisms by which B-cells are destroyed. The first one is called CDD and involves the activation of a series (cascade) of molecules called complement. These molecules will facilitate the lysis of the cells The second mechanism, ADCC, involves another cell from the immune system that recognizes the antibody and secretes molecules called cytokines that destroy B-cells.
The World Health Organization (WHO) includes rituximab in its list of essential medicines. Combining Rituxan with standard chemotherapy significantly improved response rates and long-term disease-free survival across all B-cell lymphoma subtypes. However, a subset of patients with recurrent or relapsed (R/R) disease have proven more challenging to treat, showing lower responses to salvage therapies.
Focus on new targets
Malignant B-cells can escape rituximab by reducing the expression of CD20 at their surface. The scientific community focused on additional targets such as CD19. Like CD20, CD19 is essential in normal and malignant Bcell development and proliferation. Minjuvi, tafasitamab, is the first-in-class, humanized monoclonal antibody engineered to target CD19. His design allows a more robust immune response than rituximab
Another molecule that has been proven efficient in targeting is CD79b. Specific to Bcells, CD79b is an essential component of the B-cell signalling pathway. It is also present in lymphoma cells.


Push immunotherapy Boundaries
The scientific community has started to use two strategies to push immunotherapy boundaries. One of these strategies is to attach a toxic molecule to the antibody These constructs are called ADC, for antibody-drug conjugates. ADCs combine the targeting precision of MABs with the potent cancer-killing capabilities of drugs, allowing them to concentrate on only one type of cell in the body, reducing potential general side effects. Two ADC treatments have been evaluated and approved by health authorities: Polivy (polatuzumab vedotin) and Zynlonta (loncastuximab tesirine).
The second strategy is called bispecific antibodies (bsAbs) Bispecific antibodies recognize markers on two different cells. They allow normal immune cells (T cells) to be linked physically with cancer cells, increasing the destruction capabilities of the immune system cells. Four bsAbs treatments have been evaluated and approved by health authorities: Lunsumio (mosunetuzumab), Columvi (glofitamab), Epkinly (epcoritamab) and Ordspono (odronextamab).
In Conclusion


Overall, the availability of improved drugs marks a significant step forward in the quest to cure lymphoma However, this progress does not make this mission any more straightforward. Several questions remain about the optimal sequencing and usage of all immunotherapies, including CAR-T cells. Doctors, nurses, pharmaceutical companies, regulators, policymakers, patients and their organizations must know these historic opportunities and the associated challenges. Together, they should collaborate to ensure the rapid and comprehensive use of the novel opportunities that will become available in the coming years.




Can Hemoglobin Diseases Be Cured Now?
Brigitte Leonard, Ph.D
More than 330,000 newborns every year are affected by thalassemia and sickle cell diseases. These hemoglobin disorders kill 3% of these children before their 5th birthday [1]. The malformation of hemoglobin impacts red blood cells, reducing their number and capability to transport oxygen from the lungs to body organs.
Carriers of abnormal hemoglobin genes are higher in malariaendemic regions, causing 2.5% to 15% of children to be born with the disorder. However, the distribution has undergone profound changes in recent decades with migration, making hemoglobin disorders a global issue that is no longer limited to this part of the world.
Hemoglobin disorders are managed by supportive care rather than curative treatment, consisting of periodic blood transfusions for life and iron chelation, which puts a lot of pressure on the healthcare system without addressing real patient problems. Now, curative options are available, offering hope to people touched by these disorders.
Sickle-Cell Disease
Hemoglobin 101
Hemoglobin is the major component of red blood cells Its composition consists of four globin subunit chains, which are typically composed of two alpha (α) chains and two beta (β) chains in adult humans (HbA)(Figure 1)[1]. Both types of chains and their structure are essential for the function of hemoglobin. These chains fold into a specific three-dimensional shape containing a heme group, a small molecule and iron that binds to oxygen This structure allows hemoglobin to transport oxygen from the lungs to tissues and organs (Figure 2). When red blood cells release oxygen into the organs and tissues, the hem group binds carbon dioxide to return it to the lungs for exhalation.

During a lifetime, the body produces several variants of the hemoglobin chain. In the fetus, the predominant form of hemoglobin (Hemoglobin F (HbF)) contains four chains: two alpha and two gamma. Twelve weeks after birth, the body will stop producing HbF and start to produce HbA variants (Figure 3)[2].
Patients with sickle-cell disease or betathalassemia have a mutation in the beta chain gene on chromosome 11 (Figure 4). The mutation produces a defective beta chain, impacting the efficacy of hemoglobin.
Gene therapy, Casgevry, is available now to treat sickle cell disease and beta-thalassemia [3]. Casgevry forces the body to produce fetal gamma chains instead of defective beta chains, restoring functional hemoglobin production.




Figure1 Figure2
Figure3
Figure4
Beta-thalassemia (β-thalassemia)
Beta-thalassemia is an inherited blood disorder and a form of thalassemia resulting in variable outcomes ranging from silent to severe anemia in individuals.[3] It is caused by reduced or absent production of the beta chains of hemoglobin.[4] The clinical presentation varies based on the type of mutation and the copy of the mutated chromosome received (Figure 5). When a child inherits only one mutated chromosome, the anemia can be mild (Figure 6a)
When a child inherits two mutated chromosomes, anemia can be moderate or severe (Figures 6b and 6c). In severe cases, the child will require lifelong blood transfusion. Half of beta-thalassemiaaffected infants are transfusion-dependent [5] Blood transfusions must be initiated immediately in a severe form of betathalassemia. Otherwise, the disease is fatal.
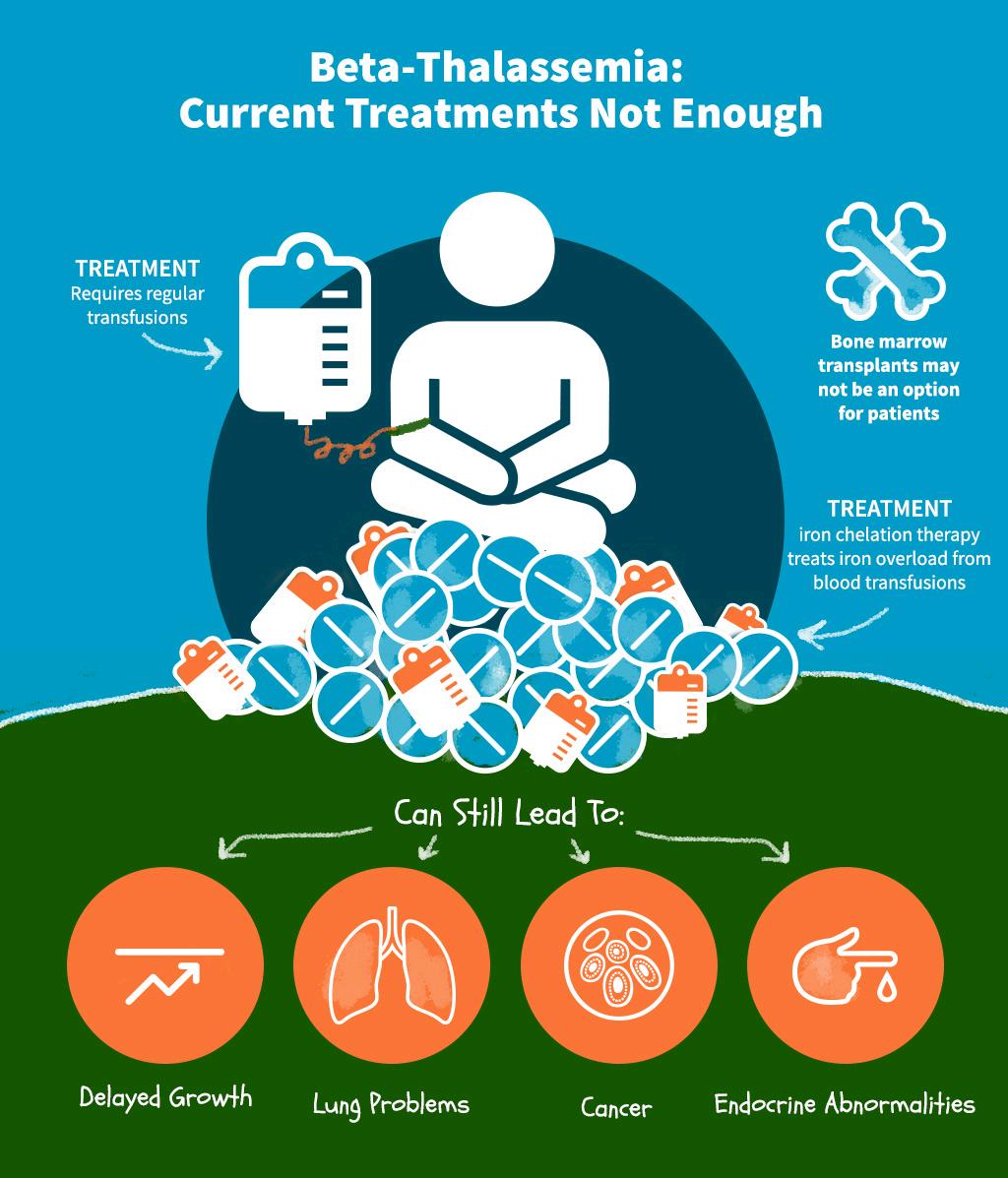
Figure5


Figure6


C B
Depending on how much hemoglobin is deficient, moderate and severe cases will experience symptoms. These include pallor, tiredness, enlargement of the spleen, jaundice, and gallstones.
The number of affected childbirths has declined in developed countries with substantial resources and prevention programs. Improvement in access to periodic blood transfusion and iron chelation has increased their odds of reaching adulthood [4] In developing countries with limited healthcare resources, preventing beta thalassemia remains challenging, and the survival rate is much lower than that of wealthier nations [5].

What is Sickle Cell Disease (SCD)?
The number of people living with SCD reached 7,7 million in 2021, a 41% increase from 2001. It is estimated that 376 thousand persons die every year due to SCD or its complications [8].
In SCD, the red blood cells (RBC) have an abnormal shape. Instead of the typical sympathetic donut shape, they adopt a sickle-like (banana) shape (Figure 7). The production of abnormal hemoglobins causes this shape [7]. There are different types of hemoglobin, including hemoglobin A (standard) and S (abnormal sickle) (Figure 7). Hemoglobin S can form stiff fibres inside the red blood cells, causing them to reshape.
Normal RBCs have mostly hemoglobin A, which helps to keep them soft and round so they flow easily through small blood vessels. However, sickle RBCs cannot move through blood vessels easily. Sometimes, they even get stuck and cannot deliver oxygen to some tissues (Figure 8).
The disease occurs when a person inherits two abnormal copies of the gene that makes hemoglobin, one from each parent (Figure 9). Depending on the exact mutation in each hemoglobin gene, several subtypes exist.
Signs of SCD usually begin in early childhood. The disease leads to various complications, several of which have a high mortality rate. Because sickle red blood cells get stuck and impede blood flow, it results in painful vaso-occlusive crisis (VOC) and organ damage Over time, the spleen, kidney, lungs, heart, and eyes become damaged People can also have strokes, increased infection rates, leg ulcers and anemia (Figure 10).


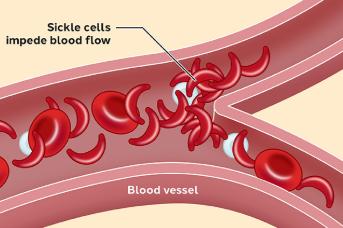
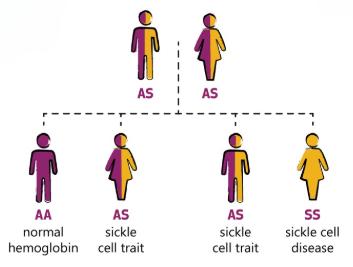


Figure7
Figure8
Figure10
Figure9



C l i n i c a l T r i a l s a n d S u r v e y s











Heal Canada and Pat ADV Hub in the USA have embarked on a collaborative journey, aiming to revolutionize the realm of patient advocacy across North America. This pioneering partnership brings together two influential organizations from neighbouring countries, combining their extensive expertise and resources.
The objective is to expand and enhance the access to critical information for patient advocates, ensuring that individuals across the continent receive the best possible support and guidance in their healthcare journeys.
By bridging the gap between Canadian and American healthcare advocacy, this alliance promises to foster a more informed, empowered, and connected community of patient advocates, significantly contributing to the improvement of healthcare experiences for countless individuals.

patadvhub@gmail.com www.patadvhub.org



References
Besremi
1)
English:https://recalls-rappels.canada.ca/en/alert-recall/importation-usauthorized-besremi-ropeginterferon-alfa-2b-njft-injection-due-current
French:https://recalls-rappels.canada.ca/fr/avis-rappel/importation-besremiropeginterferon-alfa-2b-njft-en-solution-injectable-autorise-etats
2)https://www.fda.gov/news-events/press-announcements/fda-approves-treatmentrare-blood-disease
3)https://www.onclive.com/view/nccn-lists-ropeginterferon-alfa-2b-as-preferred-firstline-cytoreductive-therapy-for-pv
4) Ji Yun Lee, Ropeginterferon in Low-Risk Patients with Polycythemia Vera ASH 2024 Abstracts 1799
Hemoglobin diseases
1) David J Weatherall, The inherited diseases of hemoglobin are an emerging global health burden, Blood, 2010 Mar 16;115(22):4331–4336.
2) Hemoglobin - Wikipedia
3) Patient Information | CASGEVY® (exagamglogene autotemcel)
4) Beta thalassemia - Wikipedia
5) Lanzkowsky's Manual Of Pediatric Hematology And Oncology 6th Edition ( 2016) 6)"Beta Thalassemia". John Hopkins Medicine. Archived from the original on 2025-0125. Retrieved 2025-02-18.
7) https://en.wikipedia.org/wiki/Sickle cell disease
8)GBD 2021 Sickle Cell Disease Collaborators, Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: a systematic analysis from the Global Burden of Disease Study 2021, Lancet Haematol 2023; 10: e585–99

Alliances

























Partners







My Blood, My Health Team
Brigitte Leonard, Ph.D

Brigitte Léonard is the Chief Scientific Officer at My Blood, My Health, a registered not-for-profit organization advocating equitable access to quality healthcare across Canada. She had the privilege of working in Pharma for over 20 years, contributing to bringing lifechanging treatments to patients with the highest ethical standards. Now, she wants to share her knowledge and utilize her scientific, strategic, and communication skills to help the patients' community.
She obtained her Ph.D. in Biomedical Sciences from Université de Montréal in 2003. Her doctoral research was conducted under the supervision of Dr. Denis-Claude Roy at Guy-Bernier, MaisonneuveRosemont Hospital Research Center. She developed a quantitative diagnostic assay in non-Hodgkin's lymphoma and evaluated the relevance of this marker in the patient's outcome.
Cheryl Petruk, MBA B.Mgt.

Cheryl A. Petruk is a multifaceted professional whose career spans across patient advocacy, business, and post-secondary education, showcasing her dedication to making a significant impact in each of these areas.
Cheryl's transition into patient advocacy was driven by a passion from her family circumstances and a deep commitment to ensuring patients' rights and access to care Cheryl has worked tirelessly to bridge the gap between the healthcare system, patients, and pharma stakeholders, ensuring that patients' voices are heard and their needs are met. Her work involves collaborating with Stakeholders and Patient Advocacy Organizations, lobbying for patient centricity, and providing patient support and guidance. Cheryl's empathetic approach and dedication to advocacy have made her a respected figure in this field, admired by patients, healthcare professionals, and fellow advocates.
Cheryl also leads and is the lead faculty member at CACHEducation, Patient Advocacy Training.