@greymattersjournalvc



@greymattersjournalvc
Beyond the Billboards: Uncovering the Hidden Potential of GLP-1 Medications
It Takes a Microbial Village: Maternal Microbiota and Neonatal Neurodevelopment
PANDAS: A Not so Fluffy Disorder
FEATURED ARTICLE
8
BEYOND THE BILLBOARDS: UNCOVERING THE HIDDEN POTENTIAL OF GLP-1 MEDICATIONS
by Jacqueline Rosenblum | art by Grace Buckles
13
BRAIN UNDER FIRE: WHEN FEVER TURNS TO SEIZURE
by Lucy Gaffneyboro | art by Cora Thompson
17
UNDER THE SURFACE: REVEALING THE MECHANISMS BEHIND EATING DISORDERS
by Sam Jacobs | art by Emily Holtz
23
FROM DEAN'S LIST TO DRAINED LIST: THE BRAIN SCIENCE OF BURNOUT
by Joseph Lippman | art by Racine Rieke
FEATURED ARTICLE
27
IT TAKES A MICROBIAL VILLAGE: MATERNAL MICROBIOTA AND NEONATAL NEURODEVELOPMENT
by Jannessa Ya | art by Elizabeth Catizone
34
38
PICTURE PERFECT: THE NEW REALITY OF REHABILITATION IS VIRTUAL
by Sushama Gadiyaram | art by Alexandra Tapia
WAKE-UP CALL: THE DISASTROUS CONSEQUENCES OF SLEEP DEPRIVATION
by Daniel Bader | art by Erica Langlais
44
COMPASSION DIVIDED: HOW RACIAL BIAS IMPACTS EMPATHY
by Yasmine Alami | art by Ella Manhardt
FEATURED ARTICLE
48
53
PANDAS: A NOT SO FLUFFY DISORDER
by Cailey Metter | art by Leo Malkhe
COMPARING MINDS AND MACHINES: WHAT WE’VE LEARNED ABOUT LEARNING by Jaron Ezekiel | art by Sarah McDonald
Art by Alexandra Tapia
If you have any questions or comments regarding this Issue 11, please write a letter to the editor at brainstorm.vassar@gmail.com.
Check out our website to read our articles, find out how to get involved, and more at greymattersjournalvc.org.


















Alexandra Tapia
Cora Thompson
Elizabeth Catizone
Ella Manhardt
Emily Holtz
Erica Langlais
Grace Buckles
Leo Malhke
Racine Rieke
Sarah McDonald
Anoushka Bhatt
Ashley Hong
Caleb Joyce
Caroline Martin
Hannah Lee
Matthew Rawson
Max Tuz
Nika Jalali
Paige King
Sydney Keenan
Talia Mohideen
TJ Schully
Veronica Crenshaw
Cailey Metter
Dan Bader
Jacqueline Rosenblum
Jannessa Ya
Jaron Ezekiel
Joseph Lippman
Lucy Gaffneyboro
Sam Jacobs
Sushama Gadiyaram
Yasmine Alami
Alex Astalos
Alex Orellana Rico
Alexis Lazarte
Alma Sutherland-Roth
Ashton Spradling
Bailey Mann
Charlotte Tobin
Emily Nothdurft
Jadyn Smith
Kavi Agnihotri
Malathi Kalluri
Ren Nicolau
Stella Petersen
Tara Dacey
Bojana Zupan PhD
Evan Howard PhD
Hadley Bergstrom PhD
John Long PhD
Lori Newman PhD
Stephanie Jackvony PhD
James Hatch — Layout
Summer Stern Layout
Catherine Zhang Layout
Anna Cohen
Basma Sultan
Chloe Bilger
Claire Paris
Cooper Jaffe
Jenais Panday
Julian Cardenas-Moncada
Kaitlin Raskin
Lea Repovic
Lena Lynch
Lila Horberg Decter
Lily Paine
Mercer Colby
Nazwa Rahman
Shayni Richter
Sophia Lorens
Stephanie Norris
Susie Osborne
Tyler Lawton
Zachary Cahn
Zoe Rodriguez
In a world where we are constantly inundated by flashing headlines, phones buzzing with notifications, and an endless stream of social media posts, it can feel overwhelming to wade through the sheer amount of information that surrounds us. Attempting to find reliable scientific news online often feels futile: a simple Google search might return links to academic publications filled with complicated terminology, inaccurate social media posts, or politicized news articles that undermine the integrity of scientific research — everything but a clear explanation. Grey Matters Journal at Vassar College aims to cut through the noise by remaining dedicated to our mission of providing our readers with reliable scientific articles that are approachable for readers of all backgrounds.
Throughout my first publication cycle as Editor-in-Chief, I have been endlessly inspired by the efforts of the GMJvc team to make neuroscience accessible. I have watched the articles of Issue 11 truly come to life over the past semester, growing from proposals to fully developed pieces that are creatively crafted and rigorously researched. Each article reflects the collaborative effort of students who came together to engage in meaningful conversations about science with the intention of opening the world of neuroscience to the public. Witnessing these conversations encourages me to continue pursuing the goal that motivated me to join GMJvc in the first place — making scientific research accessible to every reader — and makes me feel optimistic about our generation’s commitment to accessibility within science.
I am honored to have had the opportunity to lead our incredible team this semester, and am beyond excited to turn Issue 11 over to our readers. Upon opening this newest issue, you can expect to find a variety of articles that span the breadth of the neuroscientific field. I invite you to learn about neuroscientific breakthroughs, such as the growing neurological applications of GLP-1 drugs in ‘Beyond the Billboards: Uncovering the Hidden Potential of GLP-1 Medications’ or the use of virtual reality in neurorehabilitation in ‘Picture Perfect: The New Reality of Rehabilitation is Virtual’. Readers can also discover the neuroscientific basis of issues such as work-related burnout or lack of sleep in ‘From Dean’s List to Drained List: The Brain Science of Burnout’ or ‘Wake-Up Call: The Disastrous Consequences of Sleep Deprivation’.
Upon publishing Issue 11, I am immensely proud of how much GMJvc has grown as an initiative, and would like to thank our readers for enabling our journal to flourish. Thank you for your support, and I hope you enjoy reading Issue 11 as much as we have enjoyed working on it.
Sincerely,

Evelynn Bagade Editor-in-Chief
by Jacqueline Rosenblum | art by Grace Buckles
Whether you’re watching the morning news on cable or streaming your favorite movie, odds are that you’ve seen advertisements for Ozempic, Trulicity, and Mounjaro — medications used to treat diabetes. Now, these pharmaceuticals have rapidly entered public awareness as weight-loss drugs, becoming major cultural talking points on social media, in celebrity interviews, and across medical headlines. Behind this growing buzz lies a complex biological story about how these medications actually work. Originally, GLP-1 medications were prescribed to people with type 2 diabetes, a disorder in which the body is unable to properly regulate energy use and storage, specifically involving how the body’s metabolic processes break down the simple carbohydrate, glucose [1, 2, 3, 4, 5]. The key mechanism of these medications is glucagon-like peptide-1 (GLP1), a hormone naturally produced in the intestines, pancreas, and certain brain regions [6]. GLP-1 plays a critical role in maintaining energy balance by stimulating metabolism, slowing digestion, and signalling fullness after eating [7, 8]. Today, GLP-1s have hit mainstream media following the discovery of their powerful weight-loss effects, but this is not the full story [8]. What’s left out of the advertisements you’ve seen is that GLP-1 also communicates with the brain by interacting with neurons — specialized cells that send and receive the electrical and chemical signals within the nervous system [9]. Though initially formulated and prescribed for the treatment of metabolic conditions like type 2 diabetes, evidence is growing regarding how GLP-1 agents may be repurposed for the treatment of brain conditions that threaten neuron survival [4].
In the human body, an organ called the pancreas releases two important signaling molecules: insulin, which lowers blood sugar levels, and glucagon, which raises them [10, 11]. When you have not eaten for a while, such as while sleeping overnight, your blood sugar drops [11]. In response, the pancreas releases glucagon, which acts as a key to directly ‘unlock’ energy reserves in the liver, releasing glucose into the blood [11, 12]. Additionally, glucagon acts on fat cells to release fatty acids and on muscle cells to break down glycogen — the body’s stored form of glucose — thereby increasing the availability of glucose for energy conversion [11,12]. As you eat breakfast, your blood sugar rises, and the pancreas releases insulin, which becomes the new key that fits into a different

set of ‘locks’ [11]. This set of locks opens specialized ‘doorways’ in the cell membrane through which glucose can be brought into the cell by transporters for use or storage [11]. If a person’s cells stop responding properly to insulin, they are said to have insulin resistance — a hallmark symptom of type 2 diabetes, where the ‘locks’ become jammed or the ‘keys’ are missing, leaving glucose stranded outside the ‘doors’ [3]. Before a patient with type 2 diabetes receives a management regimen to address chronic high blood sugar, they may notice symptoms such as increased urination, blurry vision, a drastic increase in thirst, and unexplained nausea [13]. Additionally, when left untreated or managed poorly, type 2 diabetes significantly increases one’s risk for other long-term health consequences [14]. Such complications include high blood pressure, heart disease, liver disease, stroke, loss of vision, kidney failure, painful nerve damage, and sores or ulcers [14]. Additionally, type 2 diabetes is associated with a greater vulnerability to infection, bone loss, joint issues, and muscle problems — all of which can become severe enough such that amputation is necessary to address the immediate issue [14]. Together, these complications can greatly reduce quality of life, making the management and reduction of insulin resistance crucial in treating type 2 diabetes [8].
GLP-1-based therapies act by binding to specific receptors, thereby enhancing insulin release and suppressing glucagon secretion in response to blood glucose levels [15]. While this mechanism regulates blood sugar and weight by slowing the passage of food from the stomach to the intestines, the impact of these drugs extends far beyond the digestive system [8]. GLP-1, in both natural and synthetic forms, plays a critical role in the gut-brain axis, a complex
communication network linking metabolic function with the nervous system [16]. Because GLP-1 receptors are found in both the brain and throughout the rest of the body, GLP-1-based medications exert effects on various biological systems [16]. Interestingly, these drugs may offer neuroprotective effects by reducing neuroinflammation associated with both type 2 diabetes and neurodegenerative diseases [17]. Neuroinflammation is the brain’s response to injuries, toxins, and pathogens, which helps control damage, clear debris, and initiate healing [18, 19]. In neurodegenerative diseases, neuroinflammation becomes chronic as cells that regulate brain metabolism and immune response release inflammatory proteins, causing damage and leading to progressive neuronal degeneration [18]. The neuroprotective effects of GLP-1 medications are inspiring further research that could eventually position these drugs as promising agents for the treatment and prevention of neurodegenerative diseases [17].
Dementia is a well-known umbrella term for several neurodegenerative conditions [20]. In fact, dementia is actually considered to be a pandemic condition among the aging population, with cases expected to double in the US and Europe over the next 25 years [21, 22, 23]. One disorder that falls under this umbrella is Alzheimer’s disease [20]. In its early stages, Alzheimer’s disease typically manifests as subtle changes in a person’s memory and thinking, such as forgetting recent conversations, struggling to make decisions, or becoming easily confused [24]. Over time, individuals may also experience shifts in mood or behavior, including increased irritability, episodes of verbal or physical aggression, and symptoms of depression [24]. Though there are no definitive conclusions regarding the causes of Alzheimer’s, there are two possible mechanisms that GLP-1s may manipulate to counteract this neurodegenerative condition [24]. The first mechanism is the buildup of two abnormal proteins: amyloid-beta proteins and phosphorylated tau proteins [24]. Amyloid-beta plaques are clumps of irregularly folded proteins that accumulate on the outer surfaces of neurons and disrupt cell-to-cell communication [24]. Additionally, the accumulation of abnormal tau proteins blocks the neuron’s internal transport system, impairing the movement of materials crucial for energy production and other cell functions [25]. The second mechanism contributing to Alzheimer’s development is impaired glucose
metabolism, in which neurons cannot properly absorb and break down enough glucose to produce adequate energy [18]. The brain has two types of cells: neurons and glial cells [26]. Glial cells are neuron-supporting cells that regulate neural immune response and metabolism [26]. One key function of glial cells is their ability to turn glucose into fuel that neurons can use [26]. While the role of glucose metabolism in Alzheimer’s is debated, it is theorized that the amyloid plaques associated with the condition may prevent glial cells from converting enough glucose into sufficient fuel. This inconsistency reduces the energy available to neurons and may therefore reduce the production of chemical messenger molecules necessary for neuronal communication [18, 27, 28]. Conventional Alzheimer’s treatments aim to reduce the cognitive impairments that result from abnormal protein accumulation and deficiencies in glucose metabolism [18].
Synthetic GLP-1s are being considered as a new avenue for potential neurodegenerative disease treatments due to the growing evidence in clinical studies and animal models that type 2 diabetes and Alzheimer’s exacerbate one another [29]. People with type 2 diabetes are twice as likely to develop dementia, including Alzheimer’s [30]. In people with type 2
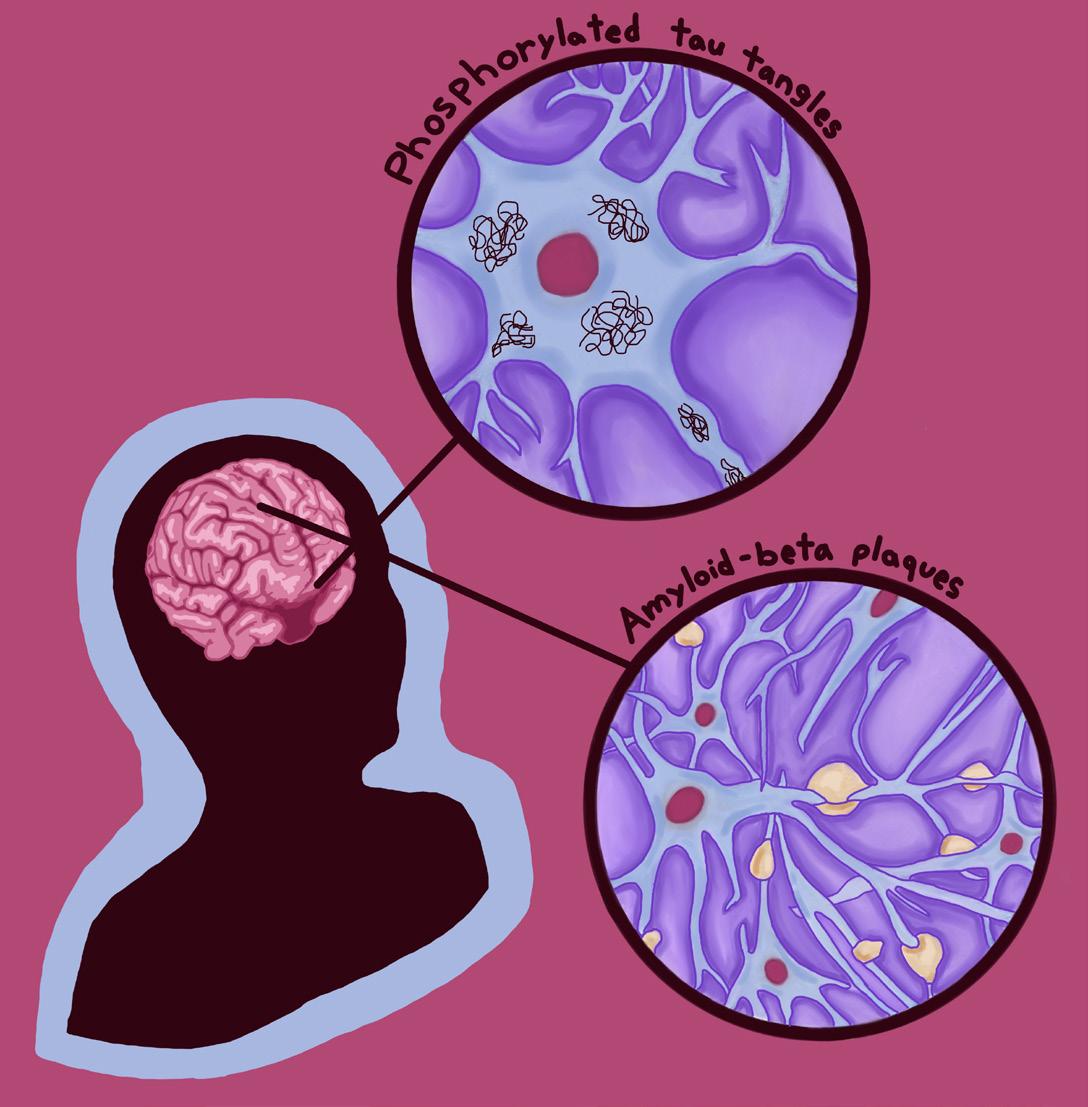
diabetes, neurons become less responsive to insulin due to a lack of insulin receptors and/or interrupted insulin signaling [31]. Because insufficient insulin response impairs glucose utilization, neural insulin resistance causes an energy deficit among brain cells [31]. The resulting metabolic irregularity could disrupt the processes that maintain a healthy balance of functional proteins within a neuron as well as its waste-clearing mechanisms, leading to the accumulation of amyloid-beta plaques and phosphorylated tau [29, 31]. These protein abnormalities may subsequently disrupt communication to insulin-producing cells in the pancreas, thus reducing insulin production and insulin sensitivity throughout the body, worsening neurodegeneration [29]. Because both Alzheimer’s and type 2 diabetes involve the buildup of misfolded proteins that interfere with cellular communication and metabolic stability, GLP-1-based therapies traditionally used to treat the latter may be a new way to slow — or possibly prevent — the progression of Alzheimer’s [17].
The synthetic GLP-1 drug, liraglutide, has been shown to demonstrate this benefit in animal models by preventing abnormal protein accumulation that would otherwise impair glucose metabolism in neurons, contributing to the presentation of Alzheimer’s [4]. As a result, classic Alzheimer’s symptoms seem to be reduced by mitigating learning problems, restoring brain signaling, and potentially even reversing memory loss [4]. One example of how these mechanisms counteract the symptoms of Alzheimer’s is evident in the way that connections between neurons in the hippocampus — a brain region important for memory formation — strengthen in response to GLP-1 treatment [6, 32]. As a result, the brain is better equipped to store new memories, retain information for longer periods, and organize information more effectively [32]. People with Alzheimer’s who take synthetic GLP-1s seem to maintain normal brain glucose metabolism and have more gradual cognitive decline, delaying the progression of Alzheimer’s symptoms [33]. By reducing inflammation and improving glucose metabolism in the brain, neurons can generate the energy needed to support the growth of stronger, more stable connections, thereby delaying the deterioration of cognitive function [6].
Another potential application for synthetic GLP-1s is in the treatment of the neurodegenerative condition Parkinson’s disease [34]. Parkinson’s is characterized by motor and non-motor symptoms [34]. Motor
symptoms can present as muscle rigidity, involuntary rhythmic shaking at rest, and difficulty maintaining balance [34]. On the other hand, non-motor symptoms may manifest as cognitive decline, sleep disturbances, and difficulty concentrating, along with dysfunctions that cause an abnormal heart rate and low blood pressure [34]. These symptoms are a direct byproduct of the degeneration of neurons within the substantia nigra, a brain region associated with motor control and coordination [35]. The substantia nigra contains cells that produce dopamine, a chemical signal that allows the brain to initiate and regulate smooth, intentional muscle movement [35, 36]. When dopamine levels drop, the brain’s communication with the body becomes impaired, leading to the motor symptoms that characterize Parkinson’s [35, 36]. Research into novel treatments for Parkinson’s disease is crucial, as it is the fastest-growing neurological disorder in the world [37]. Currently, Parkinson’s treatments are comparable to patches on a crack in the foundation of a house — current therapies manage symptoms and delay progression, but ultimately do not prevent the underlying disease [38]. As the number of people affected grows and neurodegeneration progresses, developing new approaches is essential to truly protect brain health [37, 38].
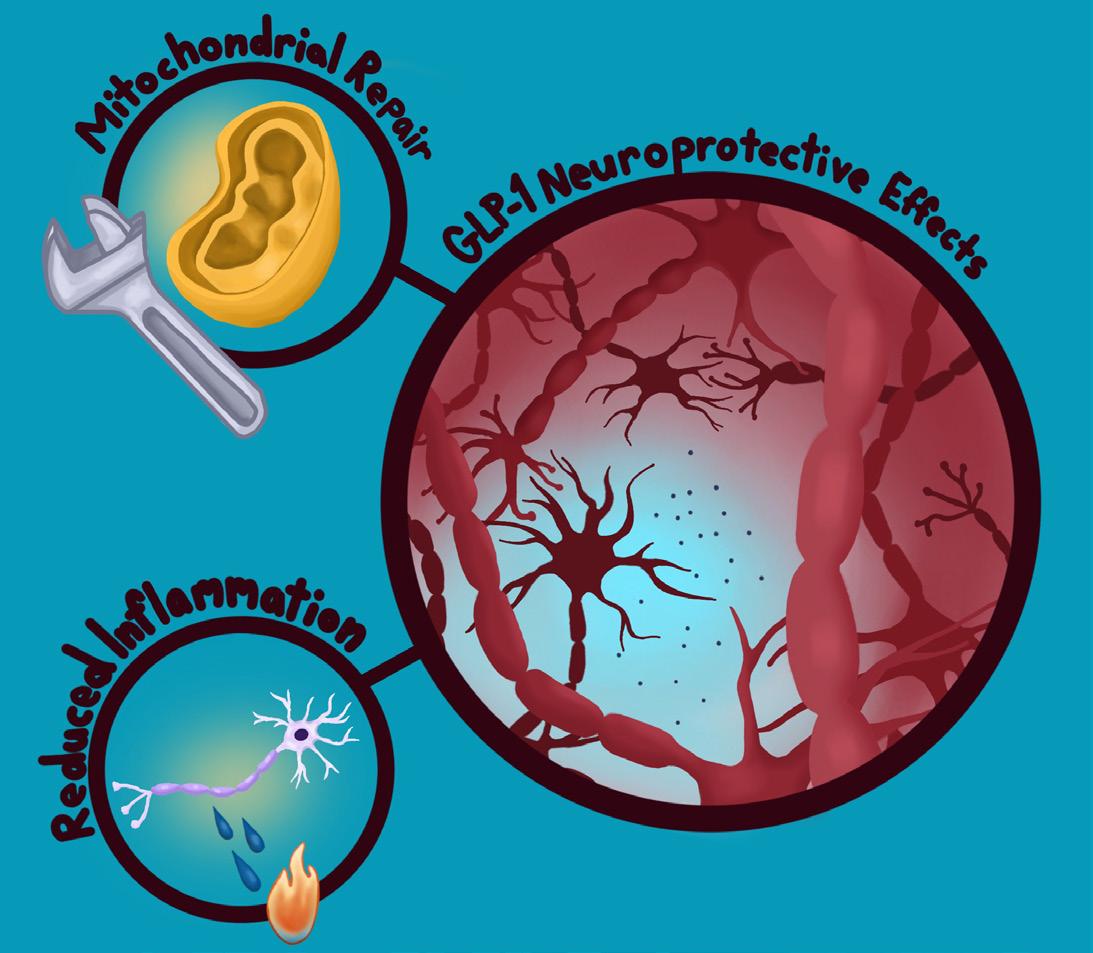
Fortunately, synthetic GLP-1s may offer new hope by protecting the brain in multiple ways [39]. One of their most promising effects is helping preserve the dopamine neurotransmitter system, as has been found in mouse models [39]. In Parkinson’s, the cells that produce dopamine slowly die off, leading to motor symptoms that impede daily functioning [39].
Because GLP-1s seem to shield dopamine-producing neurons to some degree, they can help those important neurons survive longer and function more effectively [39]. Beyond protecting the dopamine system, GLP-1s also appear to reduce harmful inflammation in the brain, as seen in Alzheimer’s mouse models [39]. Essentially, these drugs act as ‘firefighters’ containing a chronic ‘fire’ that gradually damages neurons [39]. GLP-1s support mitochondria, the cell’s energy factories, which become vulnerable in individuals with Parkinson’s disease [39]. In addition, GLP-1s may prevent misfolded proteins from building up and contributing to degeneration [39]. Because neuroinflammation, insulin resistance, and other biological mechanisms overlap across type 2 diabetes, Alzheimer’s, and Parkinson’s, GLP-1s have the potential to offer meaningful effects beyond just symptom management for individuals with any of these conditions [17, 39].
NEXT GENERATION NEUROPROTECTION: DUAL AGONISTS AGAINST PARKINSON’S
The use of synthetic GLP-1s as a treatment for Parkinson’s shows promise through the use of dual agonists — drugs that act upon two receptors rather than one [40]. Specifically, GLP-1s work closely with glucose-dependent insulinotropic polypeptide (GIP), a gut hormone that also stimulates insulin secretion and regulates appetite [41]. This dual-receptor activation mimics a more natural hormone response in the body, similar to how the gut normally uses GLP1 and GIP signals together to regulate metabolism and cellular health [42]. In preliminary mouse model studies, DA-CH5 — a dual agonist drug designed to activate both GLP-1 and GIP receptors — stood out for its ability to provide therapeutic effects from the activation of both GLP-1- and GIP-related signaling pathways [43, 44, 45]. Once inside the brain, DA-CH5 appears to act like a ‘smart key’ by turning on only the GLP-1 and GIP switches without inadvertently activating any other systems, unlike insulin and glucagon, which act on systems throughout the whole body [45, 46]. DA-CH5 treatment may also help preserve neurons in the brain region most affected by Parkinson’s, delaying degeneration at least as effectively as existing treatments [45]. Beyond protecting neurons, DA-CH5 promotes processes that clear dysfunctional glial cells from the brain [45]. Typically, specific glial cells called microglia regulate neuroinflammation pathways by increasing inflammation in response to pathogens and by initiating cell death to clear abnormal or damaged cells from the brain
[45]. In Parkinson’s, however, chronic hyperactivity of microglia induces long-term neuroinflammation and triggers a process in which the immune system mistakenly attacks the fatty membranes of neurons [45]. It is suggested that by activating both GLP-1 and GIP receptors, dual agonists initiate signaling processes that protect vulnerable neurons from immune attack, improve mitochondrial energy production, and normalize the breakdown of cellular material, ultimately providing broader neuroprotection than solely GLP-1based drugs [45]. With their wide range of promising effects, dual agonists represent a powerful new direction for Parkinson’s treatment [43, 45].
Although GLP-1 medications were originally developed to treat metabolic disorders like type 2 diabetes, emerging research suggests they may also play a valuable role in addressing neurodegenerative diseases [8]. Since they act on the gut-brain axis, reduce neuroinflammation, and protect vulnerable neurons, GLP-1s are being explored as potential disease-modifying therapies for various conditions, including Alzheimer’s and Parkinson’s diseases, where current FDA-approved treatments remain largely limited to symptom management [4, 16, 17]. Despite this growing scientific interest, GLP-1 drugs remain widely known for their effects on blood sugar regulation and weight loss, yielding results that have fueled their rapid rise in popularity. Their presence in advertising, social media, and celebrity culture has transformed GLP-1s into a highly commercialized medical phenomenon — often overshadowing their broader, and still largely untapped, neuroprotective potential.
References on page 59.

by Lucy Gaffneyboro | art by Cora Thompson
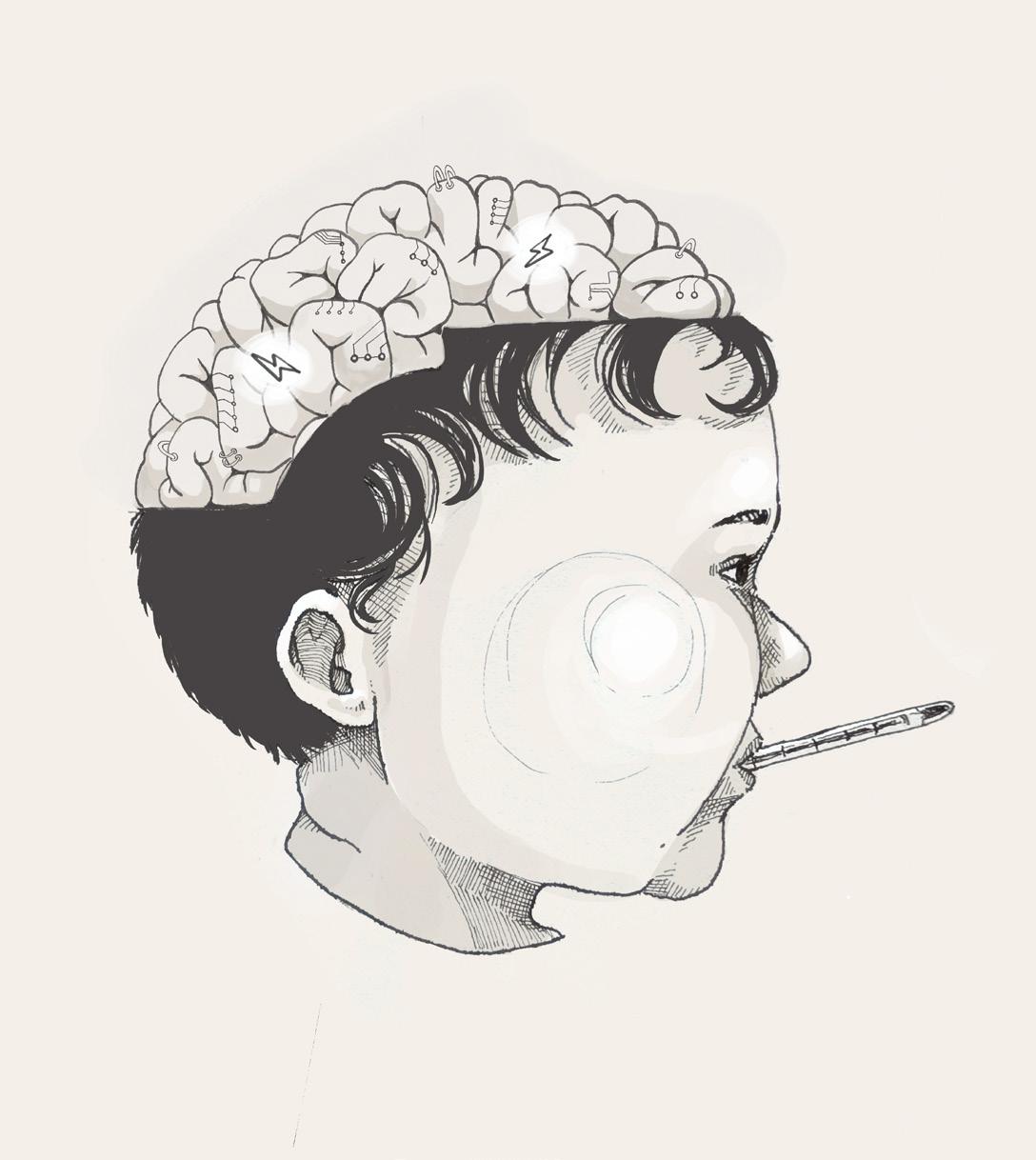
Amy Brown has been out sick from preschool for the past two days. Her parents assumed that her fever and runny nose were due to a common cold. But last night, as Amy’s dad finished tucking her into bed, she suddenly began to twitch violently and lost consciousness. Unbeknownst to her parents, Amy had suffered from a seizure: a transient episode of excessive and often synchronized electrical activity in the brain [1, 2]. When a seizure is provoked by a fever, it is referred to as a febrile seizure, an often benign phenomenon that commonly occurs in children under the age of five [3]. Even though young children frequently get fevers, only 2-5% develop febrile seizures [4]. Most children who have a febrile seizure will fully recover and have no further complications, but others may go on to experience recurrent episodes or chronic symptoms. In some cases, a febrile seizure can evolve into a more serious lifelong condition that impacts everyday life, begging the question:
What are the underlying physiological and genetic factors that can predispose certain children to these chronic disorders?
Every brain is constantly thrumming with electrical activity that strives to regulate the body. Within the brain, cells called neurons transmit information through electrical signals [5]. Every neuron has a membrane potential, a voltage created by the unequal distribution of charged particles called ions across the neuronal membrane [6]. Typically, inactive neurons have a constant voltage called the resting membrane potential [7]. When a neuron is sufficiently stimulated by incoming signals from surrounding neurons, the flow of ions across the membrane changes [8]. If there is enough ion movement to significantly alter the voltage of the neuronal membrane, the cell becomes activated. Signals are then sent to adjacent neurons by chemical messengers called neurotransmitters [8, 9].
During a seizure, like the one that Amy experienced, the normal pattern of electrical signalling is disrupted [10]. Neurons become hyperexcitable, or overreactive, and are more likely to send out extraneous signals, leading to excessive and irregular electrical firing in the brain that is characteristic of a seizure [11]. All seizures are caused by the disruption of typical neuronal function, but different types of seizures can present with varied symptoms [12, 13, 14]. Some seizures appear more pronounced, potentially including a loss of consciousness, rapid jerking movements, or the body becoming either stiff or floppy [15]. These types of seizures are often seen in medical dramas. However, some seizures present more subtly, manifesting as occasional slight muscle twitching, a prolonged blank stare into space, or both [15]. Most seizures only last a few minutes [15]. If a seizure lasts longer than five minutes or consists of multiple recurrent seizure episodes without regaining full consciousness, it is classified as status epilepticus, a serious condition that requires immediate hospitalization [16, 17]. During a viral infection, such as the flu, proteins called
antibodies detect a virus in the body and trigger the immune system to respond [19]. Through a process called inflammation, immune cells are sent to attack the virus [20]. Inflammation stimulates the release of cytokines, which are small proteins that play many roles in the body and can indirectly affect how likely neurons are to become activated [20]. Pro-inflammatory cytokines ramp up immune activity to destroy the pathogen, whereas anti-inflammatory cytokines promote healing and inhibit pro-inflammatory cytokine pathways [20, 21]. Both pro- and anti-inflammatory cytokines are present in every immune response, and an equilibrium of cytokines is crucial to a healthy and functional immune system [20, 22]. Equilibrium does not necessarily mean completely equal levels of each type, but rather an ever-changing harmonization between the two [22].
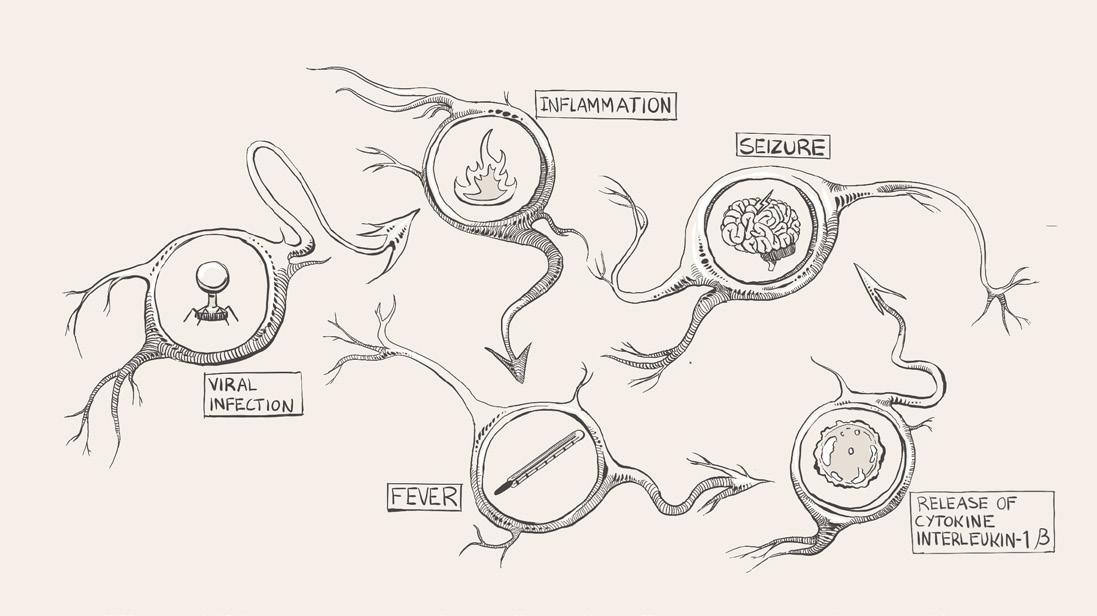
A common byproduct of inflammation is fever, an immunological defense that limits bacterial and viral proliferation [23]. Although a fever defends against unwelcome pathogens, over time, the continuous immune response can wreak havoc by disrupting the body's carefully calibrated equilibrium [24]. During a fever, pro-inflammatory cytokines are released throughout the body [25]. For example, interleukin-1β increases the production and release of glutamate, a neurotransmitter that increases the likelihood of a neuron becoming activated [26, 27]. As glutamate levels rise, weak stimuli that do not typically change the membrane voltage end up prompting a neuronal response [14]. If enough hyperexcitable neurons are concentrated in one area of the brain, erratic electrical activity can compound and lead to a febrile seizure [28, 29]. If a febrile seizure lasts longer than five minutes, it is referred to as febrile status epilepticus (FSE) [30]. Keeping the possibility of FSE in mind, Dr. Bird walks into the exam room and consults Amy's chart. He sees that she had a high fever and tested positive for a viral infection. He considers her age and concludes that she had a febrile seizure, a
seizure brought on by a fever greater than 100.4°F [13, 31]. Young children are more vulnerable to febrile seizures than other age groups since their nervous systems are still developing [29]. Given that this was Amy's first seizure, Dr. Bird explains to the Browns that this seizure increases her likelihood of having another.
Febrile seizures and their associated conditions sometimes have no known cause, but they can also be the result of a constellation of factors that make one brain more susceptible than another [13]. A family history of febrile seizures increases an individual’s chance of having one themselves, suggesting an underlying genetic basis for febrile seizure development [25, 32, 33]. Amy's mother also had a febrile seizure as a child, which Dr. Bird noted in his assessment [34]. One gene likely responsible for some of the hereditary patterns of febrile seizures is SCN1A, which helps control the construction of voltage-gated channels: special proteins that help modulate the membrane potential of a neuron [35]. A mutation in the SCN1A gene disrupts the flow of sodium ions through these channels, decreasing the brain’s ability to inhibit neurons and subsequently increasing the potential for neuronal hyperexcitability [35, 36, 37]. SCN1A mutations, therefore, elevate a person’s likelihood to experience a febrile seizure during a fever [36, 37]. Genetic epilepsy with febrile seizures plus (GEFS+) is associated with mutations in the SCN1A gene and is an inherited disorder that encompasses several febrile seizure-related conditions [38, 39]. Around half of all children with GEFS+ have at least one parent with the same condition [39]. In addition to febrile seizures, individuals with GEFS+ experience febrile seizures plus (FS+), which are seizures that occur outside of the typical febrile seizure age range and are not caused by fevers [33, 40]. While most children will outgrow febrile seizures, children with GEFS+ will continue to have both recurrent FS+ and non-febrile seizures past age six [39]. In the next room over from Amy, Dr. Bird sees another one of his patients: Luke, an eight-month-old recently admitted for Dravet syndrome. A severe subtype of GEFS+, Dravet syndrome, affects one in 15,700 people, and its onset is often provoked by fevers in infancy [35, 41, 42]. The SCN1A mutation predisposes Luke to unnecessary pro-inflammatory responses; during a fever, his brain releases more pro-inflammatory cytokines than necessary [43]. In addition to fever, Dravet syndrome seizures can also be triggered by acute stress, sudden changes in temperature, or excitement as a
result of SCN1A gene dysfunction [44]. Despite similarities in the underlying mechanisms of febrile seizure and Dravet syndrome, the latter seizures are harder to regulate and treat because the threshold for neuronal excitement is lower [45, 46].
When a status epilepticus seizure or an FSE rages for several minutes, there is a chance that the harm caused to the brain can be irreversible, leading to lifelong medical complications [47, 48]. For example, temporal lobe epilepsy (TLE) is caused by abnormal electrical signaling in the temporal lobe, a brain region crucial to language, emotion, visual recognition, and memory [49, 50, 51]. In the long term, TLE can cause cognitive decline and even the development of disorders such as depression and anxiety [52]. Additionally, TLE can negatively affect executive functions such as memory, planning, and attention, making it more challenging to maintain social relationships, manage time, and control impulses [52, 53]. Within the temporal lobe is the hippocampus, a structure integral to short and long-term memory, spatial awareness, and emotional memory [54]. Damage to the hippocampus can result in memory impairment [55]. Recent research has investigated the relationship between the structure and orientation of the hippocampus and the occurrence of FSE [56]. Hippocampal malrotation (HIMAL) is a condition that describes an abnormally positioned hippocampus, and it can be an indicator of abnormal brain development that can predispose individuals to seizures [57]. Individuals could be born
with HIMAL, or HIMAL could arise after a seizure [57]. HIMAL is predominantly observed in patients who have had an FSE as opposed to those who have had a febrile seizure, and it may play a role in the development of TLE [57]. Reduced hippocampal size also predisposes individuals to febrile seizures and FSE development, and children with FSE have higher rates of internal hippocampal injury; however, it is unclear whether the damage preceded the seizure or whether the seizure caused the damage [25, 58].
Dr. Bird's schedule is packed. His next patient is a seven-year-old boy named Harry, who was previously healthy without any neurological illnesses. One week ago, Harry developed a fever and a sore throat, so his parents brought him to be seen by his primary care doctor, who gave him medicine to treat his respiratory infection. After many days, some of his symptoms resolved, but his fatigue and fever lingered, and Harry was admitted to the hospital. Several days into his hospital stay, he started having refractory status epilepticus, which are seizures that cannot be stopped after treatment with two or more medications [33]. Dr. Bird and his team concluded that Harry was suffering from febrile-infection-related epilepsy syndrome (FIRES). FIRES is a chronic, sudden-onset epilepsy condition that arises after a fever caused by an infection [59]. The key difference between FIRES and an FSE or febrile seizures is that FIRES is often accompanied by lifelong after-effects [60]. FIRES can cause refractory status epilepticus that arises between twenty-four hours to two weeks after the onset of a fever, just like Harry experienced [61]. A fever is a marker of the body’s immune system protecting against pathogens with inflammation, but excessive inflammation in the brain can induce seizures, which in turn provoke more inflammation [23, 62]. Individuals with FIRES are often found to have increased levels of pro-inflammatory cytokines, such as IL-1β [63]. These pro-inflammatory cytokines trigger the release of even more pro-inflammatory cytokines, leading to a cytokine storm [64]. The massive increase of pro-inflammatory cytokines causes increased excitability of neurons and subsequent exacerbation of seizures [65, 66]. FIRES is most commonly observed in children of elementary school age, like Harry, but it can affect individuals from infancy to adolescence [33, 67]. Patients continue to suffer from progressively debilitating seizures throughout their lives; damage to their brains is significant [68]. As a result, FIRES can cause neurological symptoms like memory loss and behavioral problems [69].
When Amy arrived at the hospital, she was first given medication, such as acetaminophen or ibuprofen, to control her fever [4, 42, 70]. If the seizure had been an FSE, the dose of acetaminophen would have been followed by a depressant medication such as a benzodiazepine to slow the nervous system by preventing hyperexcitable neuronal firing, which terminates the seizure [30]. If seizure activity hadn’t stopped after the first dose of benzodiazepine, additional doses would have been given [30]. Most children who have a seizure will experience no long-term complications, but immediate medical attention is crucial to ensure a full recovery [3, 71]. Unlike febrile seizures and FSE, FIRES has a different treatment plan: cytokine-directed immunotherapy (CDI), which uses the body's own molecular components and processes to modulate cytokine activity [28, 72]. CDI is individualized: One person’s treatment could focus on increasing levels of anti-inflammatory cytokines while another person’s could focus on increasing levels of pro-inflammatory cytokines [72]. CDI is used for a variety of conditions, and it can be incredibly successful in treating FIRES by targeting specific cytokines that are key to regulating inflammation [28, 72]. During Harry's hospital stay, he was treated with a CDI drug called Anakinra IL-1 to block pro-inflammatory IL-1 receptors [28, 72]. When IL-1 receptors are blocked, inflammatory cytokines cannot bind to them, which reduces seizure propagation [73]. Anakinra IL-1 can be used over long periods of time for chronic inflammatory conditions, and Harry will likely receive this medication for up to a year [74]. Another common drug used for CDI is Tocilizumab, an antibody that binds to a different pro-inflammatory receptor site and prevents that pro-inflammatory cytokine from further activating more immune cells [28].
Amy, Luke, and Harry all had fevers, and their haywire immune responses produced a myriad of side effects, ultimately resulting in febrile seizures. Dr. Bird's patients showcase how febrile seizures range from benign and temporary to life-threatening and chronic. Pronounced seizures like the ones that Luke and Harry experienced are well-documented in the media, but their prevalence on page and screen belies their relative rarity. More common instances of seizures, such as Amy's febrile seizure, are shorter, less consequential, and less dramatic; however, they can still be traumatic and alarming to both patients and their family members. All seizures are the result of the
same core physiological vulnerability: a brain temporarily pushed to the brink by neuronal hyperexcitability. In the cases of Amy, Luke, and Harry, their brains were also the victims of fever and inflammation, but the extent to which genetics and family history contributed to their conditions remains unknown. Although Dr. Bird is fictional, real-life physicians treat people who experience brief febrile seizures and others with lifelong neurological challenges. These physicians must effectively communicate with patients and families while balancing the nuances between each condition to provide the best treatment.

References on page 60.

by Samuel Jacobs | art by Emily Holtz

Eating disorders such as anorexia nervosa and bulimia nervosa are serious, life-threatening medical conditions that are often misunderstood as mere ‘vanity disorders’ due to an excessive preoccupation with body shape and weight [1, 2]. The most widely known kind of anorexia nervosa is the restrictive subtype, characterized by the persistent restriction
of food relative to what an individual’s body needs [2].
On the other hand, bulimia and the binge-purge subtype of anorexia nervosa (AN-BP) are characterized by recurrent binge-eating episodes accompanied by purging behaviors, including vomiting, fasting, excessive exercise, or misuse of laxatives and diuretics [2].
The cycle of bingeing and purging is a behavioral loop that briefly relieves emotional distress, but ultimately reinforces harmful eating patterns [3, 4]. Individuals with bulimia may eat large amounts of food over a short period and feel unable to stop [3]. These binges are often followed by intense guilt or discomfort that compels the individual to engage in purging behaviors aimed at preventing weight gain [3, 5]. Patterns of restriction in anorexia nervosa and binge-purge in bulimia/AN-BP can both cause distinct structural alterations within the brain [6]. Specifically, physical changes can occur within neural circuits, the pathways between different brain regions [6]. Through the modification of neural circuits that underlie eating habits, behaviors can become permanently altered, contributing to the dangerous long-term nature of eating disorders [6]. Even after recovery, many individuals continue to experience residual symptoms, rather than a definitive return to a pre-disorder baseline [7]. Due to this, eating disorders have high relapse rates, and individuals in recovery remain vulnerable to returning to destructive behaviors [8, 9]. As a result, they could suffer from the long-lasting psychological and physical effects of these disorders for the rest of their lives [10, 11, 12].
Individuals with bulimia have immense difficulty shifting their thinking away from binge-purge behaviors, even when these actions no longer provide a sense of reward or satisfaction [13]. In fact, these behaviors can persist despite the unpleasant consequences, ranging from emotional distress to dental decay [3, 14]. The brain relies more on ingrained harmful behaviors than flexible responses, such as recognizing
hunger and fullness cues, eating regularly, or seeking emotional support [15]. The flexible responses require conscious reflection rather than thoughtless reaction [15]. Over time, repeated responses strengthen the specific neural connections responsible for these behavioral reactions, creating a mental ‘shortcut’ that makes binge-purge routines habitual and difficult to interrupt [15]. So, when feelings of shame or loss of control hit after bingeing, a person might automatically purge as a way to ‘undo’ the experience, even if they no longer believe it actually helps [3, 13]. If purging is not an option, individuals may struggle to figure out what to do next, since the brain pathways that normally help them move from emotional distress to flexible problem-solving are not communicating efficiently [15].
While bulimia is driven by compulsive reward-seeking through binge-purge behaviors, anorexia nervosa is driven by reward-seeking through rigid control and avoidance of foods [16, 17]. However, both share a fundamental disruption in the neural circuits underlying reward learning and habit formation [16, 18].
Similar to bulimia, the reward system of an individual with anorexia nervosa becomes less responsive to ordinary pleasures and more attuned to the sense of control that comes from restriction and the pleasure created from this control [19]. For example, a person with anorexia nervosa may avoid high-calorie and high-fat foods or adhere to strict meal rituals [17, 20]. The more a person restricts their food intake, the more distressing normal eating patterns can feel, whereas avoiding food can feel calming or purposeful [19, 21]. Once a particular behavior has been reinforced, such as food restriction, individuals with anorexia nervosa tend to persist in that pattern even when it starts causing life-threatening health effects like heart and blood-vessel complications or bone deterioration [21, 22, 23, 24]. This inflexibility is a characteristic of the restrictive and ritualized nature of eating behaviors in anorexia nervosa [19].

In a healthy brain, dopamine serves as a chemical messenger that signals which experiences are rewarding, thereby motivating repeated behaviors [13]. The brain’s reward circuit relies on dopamine as a central component that guides decision-making and reinforces learning [25]. When a behavior, such as maintaining a daily calorie deficit, yields a more positive result than expected — for example, alleviating discomfort or expediting weight loss — dopamine levels increase, leading to behavior reinforcement [26]. Conversely, when a behavior, such as unintentionally overeating, turns out worse than expected and prompts feelings of discomfort, dopamine levels drop, teaching the brain to avoid this behavior in the future [13, 25]. A ‘two-stage’ model can be used to describe dopamine’s integral role in reinforcing anorexia nervosa [27]. First, dopamine activity may increase in response to dieting and excessive exercise, producing a sense of control and success that reinforces food restriction [27]. In the second stage, prolonged starvation lowers overall brain dopamine levels, making the dopamine receptors hypersensitive [27]. Consequently, a normal burst of dopamine from regular eating can feel abnormally intense, triggering an anxiety response instead of a pleasurable one [13, 27]. This heightened dopamine sensitivity in anorexia nervosa illustrates why normal eating can feel uncomfortable or anxiety-provoking, helping explain how restriction becomes reinforced even though it is ultimately harmful [26]. In this model, dopamine not only contributes to the onset of anorexia nervosa but also sustains the duration of the disorder by transforming short-term relief into unhealthy routines [27]. Additionally, binge-purge behaviors in bulimia may also engage dopamine signaling in a similar pattern to anorexia nervosa [28]. In bulimia, habit and reward-related brain circuits are dysregulated in a manner that promotes impulsive, reward-seeking behavior like bingeing, associated with decreased dopamine receptor binding [13]. Bulimia appears to be linked with lower dopamine sensitivity, so larger amounts of food are often needed to generate a noticeable sense of reward or satisfaction [26]. Thus, both disorders exhibit maladaptive patterns in the same underlying reward circuitry [30].
Eating disorders leave their mark not only on behavior but within the brain itself, where structural changes leave a lasting neurobiological impact [29, 30, 31]. The brain is composed of two main types of tissue: gray matter and white matter [32, 33]. Gray matter contains neurons that handle thinking and processing, while white matter consists of bundles of axons — long, slender projections of neurons that conduct electrical impulses away from the cell body — that are coated in an insulating fatty sheath, fostering quick communication between distant brain regions [32, 34, 35]. In anorexia nervosa, the white matter begins to thin due to malnutrition, which reduces coordination between areas that regulate reward, emotion, and self-perception [36, 37]. Think of white matter as a network of highways connecting different cities: when the roads are well-maintained, traffic flows smoothly, and messages are delivered efficiently to their intended destinations. Thinning of axons in these pathways is similar to potholes, lane closures, or damaged bridges on the highways — signals slow down, get misdirected, or fail to reach their destination, which can disrupt coordination between brain regions that regulate reward, emotions, and self-perception [37]. In the brain, these disruptions in physical wiring and coordinated regional activity represent a breakdown in overall connectivity [6]. People with anorexia nervosa experience reduced connectivity, meaning weaker communication between brain circuits like the default mode
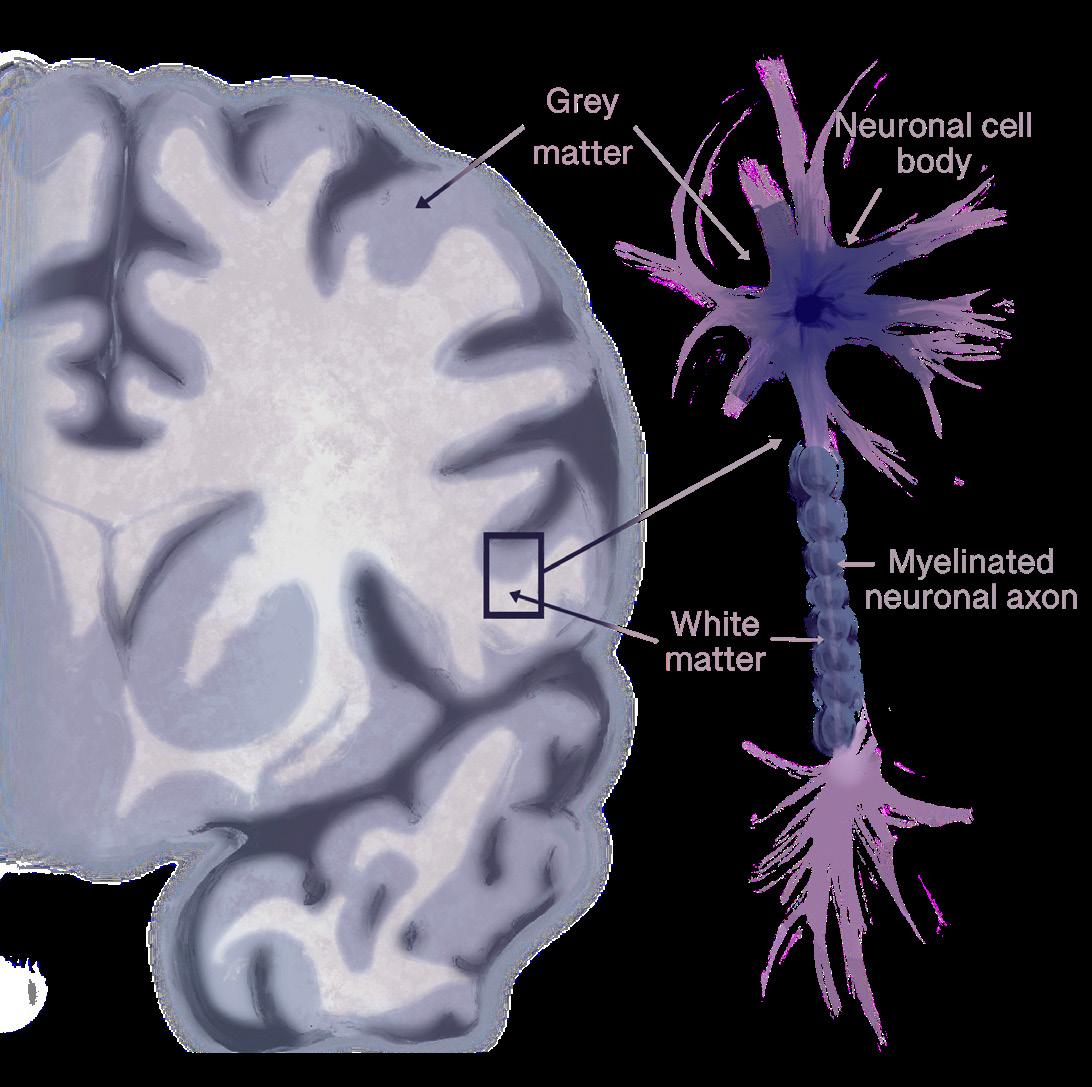
network, which is involved in self-focused thinking, and the salience network, which helps the brain detect important emotional or bodily signals like hunger or fullness [37]. Furthermore, loss of white matter fibers has been found in pathways connecting emotional and decision-making centers of the brain [37]. Reliance on rigid, well-worn habits over flexible decision-making can lead to sudden spikes of fear or distress around eating, making even small changes — like adding one new food to a meal — feel impossible [17]. Some white matter abnormalities improve during long-term weight restoration, while others persist and are associated with illness duration [18]. A longer illness duration predicts enduring white matter deficits, suggesting that the longer anorexia nervosa remains untreated, the more persistent these changes become [38]. Over time, structural disruptions may deepen and become harder to reverse, making early intervention critical to recovery [39].
In contrast, bulimia is characterized by abnormalities in brain regions that support habit formation and reward regulation [18, 38]. Disrupted communication within those regions can harm reward evaluation abilities and behavioral control [38]. Structural disturbances may contribute to the compulsive nature of binge-purge cycles and the difficulty of disengaging from them [40]. Moreover, bulimia is linked to structural alterations in brain regions that regulate reward, self-control, and emotional processing [18]. Abnormalities include reduced gray-matter volume or altered connectivity in key areas involved in evaluating rewards and regulating internal bodily sensations like hunger, fullness, and taste [18, 41]. Changes in underlying brain structure may reinforce maladaptive patterns of eating behavior and make it harder to resist urges to binge or purge [18]. Taken together, both anorexia nervosa and bulimia are marked by structural and connectivity disturbances that reinforce maladaptive behaviors; anorexia nervosa shows disrupted communication between self-perception and control networks, while bulimia shows dysfunctional integration within reward and habit systems [18, 23, 38].
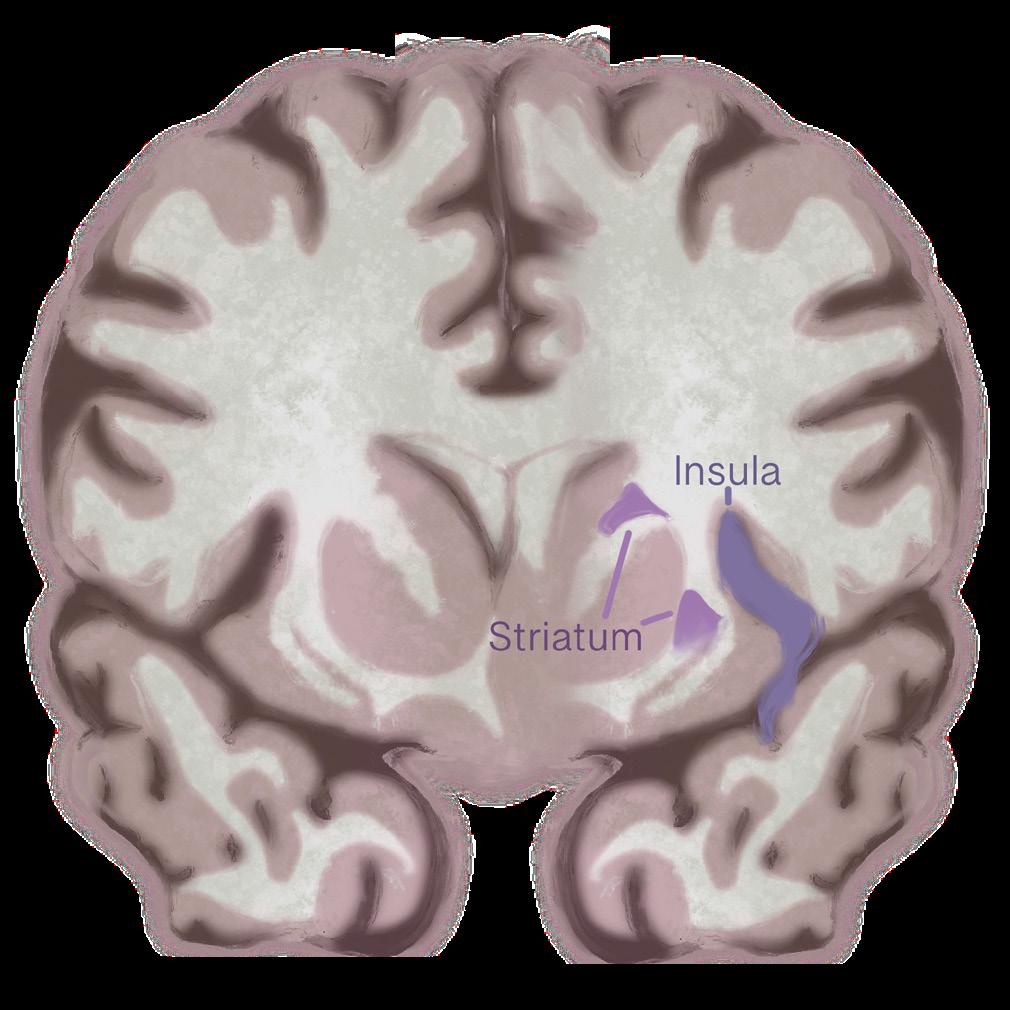
Structural changes in a brain region called the insula may drive binge-purge behavior in bulimia. Similar to anorexia nervosa, bulimia also affects the insula [13]. People with bulimia have less gray matter in the front part of the insula. Reductions in gray matter may
indicate a decrease in healthy neurons in a given area, and that the insula does not function as efficiently when integrating emotions with physical urges or sensations in bulimia. Weakened integration could make it harder to regulate powerful urges to binge or purge, since the brain struggles to align emotional experiences with body signals. Disrupted activity in different subregions of the insula also suggests that cognition-emotion coordination is impaired, contributing to the impulsive, emotionally driven eating behaviors seen in bulimia. These findings highlight the insula’s key role in linking emotional states with bodily urges, showing how its dysfunction may fuel the binge-purge cycle [13]. Beyond structural changes in the insula, bulimia also disrupts communication between brain re gions, particularly within circuits that link motivation, habits, and decision-making [38]. A key structure in this process is the striatum, a deep-brain region involved in learning what feels rewarding and shaping habitual behaviors [38]. In people with bulimia, the striatum shows abnormal communication with other regions such as the prefrontal cortex, thalamus, and sensorimotor areas — regions responsible for planning, emotional regulation, and body awareness. A miscommunication appears to dis rupt the balance between the brain’s reward and control systems, which may underlie the recurring binge–purge cycle. When these systems fail to coordinate properly, urges to binge or purge can override rational decision-making, making the behavior feel automatic and difficult to stop [38].
symptoms: those with more pronounced connectivity issues tend to experience stronger binge–purge urges and more emotional instability [42]. These findings suggest that bulimia involves widespread disruptions not only in reward and control circuits, but also in the broader networks that govern attention, self-control, and emotional regulation [38, 42].
Large-scale brain region networks that coordinate attention, emotion regulation, and self-control also appear to function differently in bulimia [42]. These networks, such as the fronto-parietal network (FPN) and the cingulo-opercular network (CON), are like the brain’s ‘command centers’ that help people stay focused on goals, manage impulses, and adapt their behavior [42]. The FPN supports flexible control and attention, while the CON helps maintain task focus and emotional stability over time [42, 43]. In bulimia, these large-scale networks show decreased connectivity, indicating weaker functional communication within and between them [42]. The degree of this disruption is linked to the severity of a person’s
Just as the brain’s wiring patterns can shape habits and emotions, its chemical composition also plays a crucial role in the development and persistence of eating disorders [13]. Chemicals known as metabolites are small molecules that help neurons produce energy, communicate effectively, and repair themselves [13]. When these chemical systems are disrupted, the brain may struggle to regulate hunger, reward, and emotional balance [13]. Reduced levels of metabolites in the insula may indicate that the neurons are functioning, but not at full capacity [41]. Weakened neural signaling in the insula may contribute to the disconnect that many people with anorexia nervosa experience from their own bodily cues, making hunger feel confusing or even anxiety-provoking rather than natural [41]. Lower levels of the metabolite N-acetylaspartate (NAA) are strongly linked to greater concern about weight and body image, and therefore, these chemical changes may reinforce obsessive self-focus and distorted body perception [41]. Importantly, these chemical changes may not be permanent. As individuals begin the recovery process, metabolite levels in some regions, such as the insula, begin to return to typical ranges [41]. At this point, the brain can regain chemical balance with appropriate treatment and nutrition, thereby promoting the restoration of hunger cues [44]. However, many individuals still report lingering difficulties with body image, suggesting that emotional and cognitive patterns often return to baseline more slowly than brain chemistry [41]. Recovery from anorexia nervosa is both physical and psychological, requiring time for both the brain and self-perception to return to baseline [41].
In bulimia and AN-BP, brain chemistry follows a somewhat different pattern from anorexia nervosa [45]. Both disorders involve abnormal activity in brain regions responsible for reward, self-control, and emotional awareness, but the underlying neurochemistry is not identical. People with AN-BP showed reduced levels of NAA and myo-inositol — chemicals that support healthy brain cell membranes and energy use — in the prefrontal cortex, a region involved in impulse regulation, and the occipital lobe, an area involved in visual information. This could mean that the brains of individuals with AN-BP are less efficient at regulating impulses and integrating visual information related to body image [45]. Conversely, people with bulimia do not show the same reductions in certain brain chemicals seen in anorexia nervosa, suggesting that their symptoms may arise from a different pattern of brain activity related to how rewards are processed, rather than from widespread reductions in brain efficiency [45]. Together, these findings suggest that while both anorexia nervosa and bulimia affect overlapping networks in the brain, each disorder uniquely disrupts the brain chemistry; therefore, effective treatment might need to target not just behavior, but also the specific neurochemical imbalances underlying each condition [41, 45].
Eating disorders can be treated through pharmacological, psychological, or neurological methods [46, 47, 48]. Anti-depressant and anti-anxiety medications treat anorexia nervosa and bulimia by reducing co-occurring depression or anxiety that reinforce restrictive behaviors, and are often used in tandem with psychotherapy for further symptom reduction [28, 46, 49]. Cognitive Behavioral Therapy for Eating Disorders (CBT-ED) is the most widely supported psychological approach across eating disorder diagnoses [47]. CBTED targets the cognitive and behavioral mechanisms that sustain disordered eating — such as strict dieting, over-valuation of shape and weight, and maladaptive emotion regulation [47]. Cognitive Behavioral Therapy for anorexia nervosa (CBT-AN) helps individuals recognize and modify patterns that maintain restriction or obsessive focus on weight [50]. Patients who receive CBT-AN show greater weight gain and larger reductions in eating disorder symptoms compared to standard care [50]. Cognitive-training programs that aim to dissuade urges to binge-purge and strengthen inhibitory control are showing promise in reducing compulsive eating patterns in bulimia as well [16, 45]. CBT-ED protocols address the specific psychological and neurobehavioral mechanisms that
maintain anorexia nervosa and bulimia, regardless of whether the presentation is restrictive or bingepurge [51].
Similarly, emerging therapies that directly target brain circuits offer another avenue for treating anorexia nervosa and bulimia [48, 52]. Transcranial direct current stimulation (tDCS) is a non-invasive technique that applies a mild electrical current to specific brain areas, aiming to improve cognitive control, emotional regulation, and food intake [49, 53, 54]. Depressive symptoms, which often co-occur with anorexia nervosa and bulimia, may be reduced by tDCS [48]. While still experimental, these interventions aim to strengthen the brain circuits that are disrupted in anorexia nervosa and bulimia [16, 48, 52]. In anorexia nervosa, tDCS helps patients regain more flexible responses to food and emotional cues, similar to findings reported in bulimia [48, 52]. After only a few sessions of tDCS, individuals with bulimia reported suppression of binge-purge urges and increases in self-regulatory control [52]. Multi-session trials would be necessary to validate this approach as a clinical treatment method for bulimia; however, the findings to date have been positive [52]. By targeting the neural underpinnings of the disorder, neurofeedback and brain-stimulation techniques could eventually complement traditional therapy and behavioral interventions.
While both anorexia nervosa and bulimia share core features of disrupted eating behaviors, body-image disturbance, and altered brain processes, the neurobiological pathways that sustain each disorder diverge in meaningful ways [13, 19]. Anorexia nervosa is characterized by extreme food restriction, heightened dopamine sensitivity, rigid habit formation, and structural white-matter and connectivity changes that reflect a brain entrenched in avoidance and control [25, 27, 37]. Bulimia and AN-BP, however, are marked by binge-purge cycles, reduced dopamine responsiveness, impulsive reward-seeking, and alterations in habit and reward-based circuitry [13, 28, 30]. Structural and functional brain alterations in eating disorders continue even after physical recovery, indicating persistent neurobiological vulnerability even after symptomatic improvement [29, 30]. Therefore, recovery not only involves returning to normalized eating behaviors but also restoring healthier brain circuit function and encouraging flexible behavioral patterns [41, 44]. Treatment should be tailored to these neural profiles: for bulimia, interventions may
focus on disrupting maladaptive habit loops and retraining reward sensitivity, while for anorexia nervosa, strategies may focus on targeting dopamine or metabolite-related pathways to rewire the brain’s rigid control systems toward adaptive regulation [16, 26, 27, 42]. Early intervention is critical because the longer pathological reward or habit patterns persist, the more entrenched and difficult they become to reverse [38, 39]. By viewing anorexia nervosa and bulimia not just as psychiatric disorders, but as disorders of neural circuitry and learned behavior, we gain a clearer framework for precise treatments and innovative therapeutic approaches [18, 52].
References on page 64.


by Joseph Lippman | art by Racine Rieke
When you picture somebody burning the midnight oil, what comes to mind? An exhausted college student? A nurse during the COVID-19 pandemic, overworked and underappreciated? Chances are, if you have ever overexerted yourself for a period, you are probably familiar with the feeling of burnout. Burnout is commonly thought of as psychological — a way to describe extreme mental exhaustion from being overly stressed [1, 2]. Prolonged exposure to stress, known as chronic stress, can result in feeling burnt out and affects much more than simply our mental state [1, 3]. The impacts of this exhaustion extend to physical ailments such as high blood pressure and obesity [4, 5, 6]. It is vital to understand how burnout affects the mind and body in order to combat its debilitating effects, especially for those who work in consistently high-stress environments.
Imagine a student who is nervous about their upcoming midterm exam. The content feels difficult to understand, they have little time to study, and the exam accounts for a massive percentage of their grade. Needless to say, the student is experiencing a good amount of stress. Stress is a physiological and psychological response that is prompted by a perceived threat, causing worry and increased focus on a given task [7, 8, 9]. While it has an unpleasant reputation, stress in moderation allows us to function efficiently [10, 11]. For example, stress will compel the student to manage their time and focus on studying for their midterm [12, 13, 14]. Studying for a midterm is an example of an acute stress response, characterized by short bursts of stress that allow the individual to push past the stressor [10, 12, 14]. Healthy and unhealthy amounts of stress are best measured by utilizing a principle called the Yerkes-Dodson law, which stipulates that a moderate amount of stress is ideal for optimal performance [15, 16]. This principle suggests that too little stress can lead to insufficient motivation, while excessive stress can lead to anxiety and a decline in performance [15, 16].

Chronic stress occurs when the body is overloaded with a repeated or constant stress response [17, 18, 19]. A student may be struggling with chronic stress if they are constantly worrying about their exam to the extent that they are unable to focus or commit to productive studying [20, 21]. Individuals working under excessive workloads and high emotional demands, such as healthcare workers in hospital settings, commonly experience chronic stress [3, 22, 23]. Chronic stress overwhelms the body’s response to acute stressors, interrupting the process of returning to a resting state and prolonging the symptoms of stress [17, 18]. For instance, if the student experiences chronic stress from being spread too thin
with commitments and is unable to study efficiently, their chronic stress can result in burnout [3, 23]. In burnout, an individual might feel severe emotional exhaustion, lack of personal achievement, and general cynicism [3, 23, 24]. Historically, the association of burnout with stressful work environments has led many to believe that burnout is simply psychological [1, 2, 14]. This is, however, an incorrect assumption, as burnout is also physiological [14, 25, 26]. In the case of burnout, the physiological symptoms of the typical stress response, such as increased blood pressure and exhaustion, become prolonged and can potentially lead to harmful long-term health consequences [14, 25, 26]. Therefore, it is important to understand the mechanism of the typical stress response and what changes when it becomes chronic [26, 27].
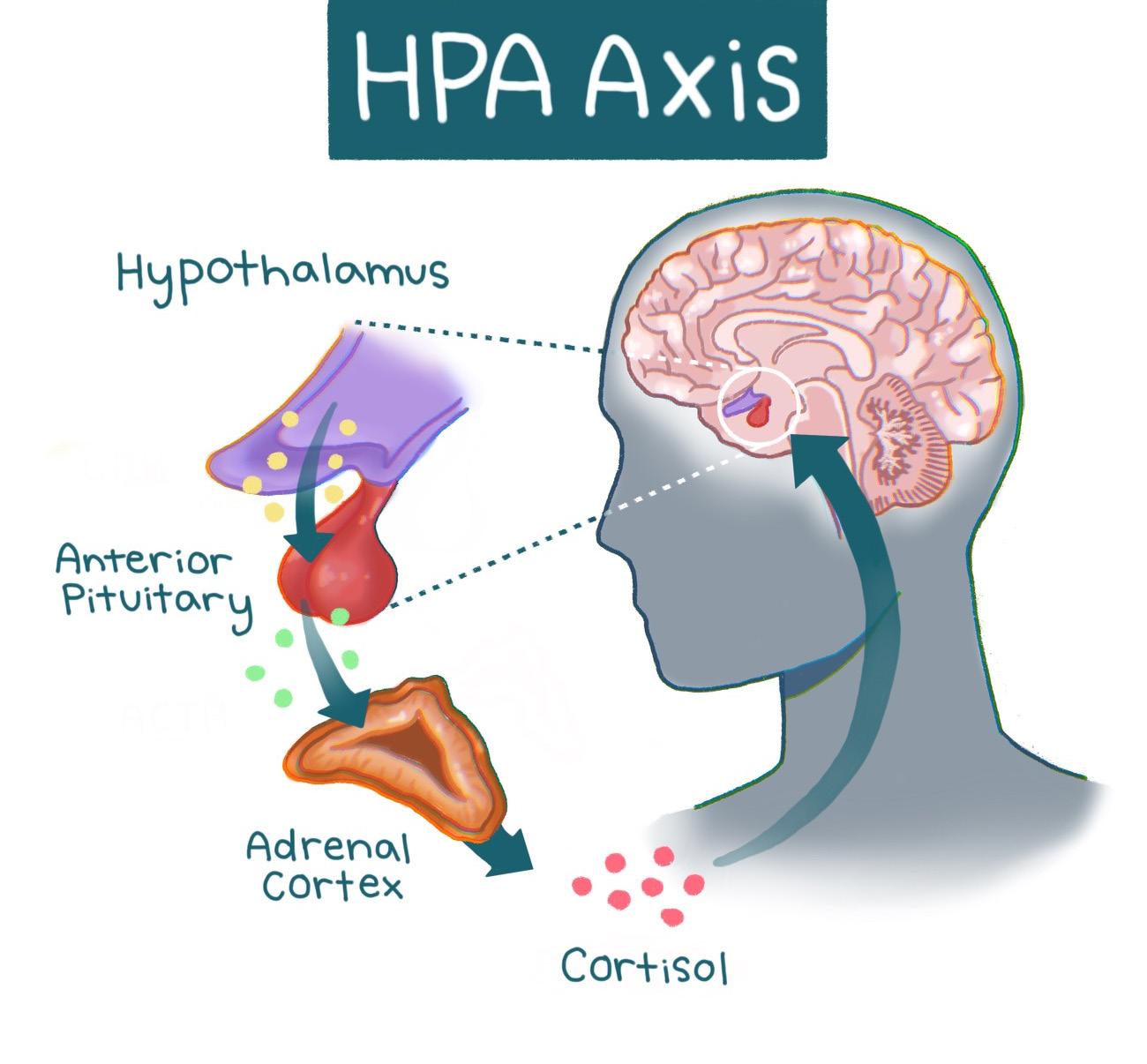
Stress responses are mediated by a pathway in our brain and body known as the hypothalamic pituitary adrenal (HPA) axis [11, 28]. At the onset of a stressful event, the hypothalamus is activated [10, 11]. The hypothalamus is a structure in the brain responsible for multiple regulatory functions and links the brain’s emotional centers with the major structures involved in the stress response [28, 29]. Activation of the hypothalamus triggers a cascade of hormones — chemical messengers that mobilize the energy and resources needed for the body to overcome a stressor [11, 28]. The cascade is similar to a relay race, with different hormones acting as runners that pass the ‘stress response’ baton. In the first leg of the race, hormones carry the baton from the hypothalamus to the brain structure that acts as the hormonal control center
called the pituitary gland, which releases hormones that control metabolism, blood pressure, and other physiological responses [11, 28, 30]. The second runner — the next set of hormones — carries the baton to the adrenal gland, which produces one particularly important hormone released in this process: cortisol, commonly known as the ‘stress hormone’ [10, 31]. Cortisol is also involved in immune function, as well as helping to regulate metabolism and blood pressure [10, 31]. Following its release, cortisol prompts the hypothalamus to cease the cortisol-producing hormone cascade [11, 32]. Similarly, once the relay runners cross the finish line, the race is over. The inhibition of the hormone cascade restores the body to a neutral state [10, 11]. Think of the HPA axis as a thermostat that adjusts to changing temperatures. When the thermostat senses that the temperature is warmer than the set temperature — such as when cortisol levels are elevated — the thermostat will turn off the heat. Once cortisol signals to the hypothalamus to stop sending signals to the pituitary gland, overall cortisol production will be reduced [10, 11]. The restoration of the body to a neutral state is key in acute stress responses, as it allows a person to focus on a stressor or task for the duration of the response and then return to regular functioning [10, 33]. When cortisol is regulated correctly, the HPA axis maintains the ability to utilize stress constructively [10, 11]. However, as most college students will report, stress does not always feel healthy [33, 34].
During states of chronic stress, prolonged activation of the HPA axis leads to hypercortisolism, a condition characterized by excess cortisol production [11, 26, 35]. In the early stages of burnout, symptoms have been heavily associated with hypercortisolism — excess cortisol release — highlighting a link between overactivation of the HPA axis and burnout [26, 35, 36]. With that said, severe burnout has also paradoxically been associated with hypocortisolism, a condition characterized by reduced cortisol release from the HPA axis [36, 37]. Hypercortisolism eventually transforms into hypocortisolism as the body stops being able to produce cortisol efficiently in response to constant stress [37, 38, 39]. After experiencing heightened levels of cortisol, individuals may experience a blunted stress response caused by hypocortisolism that can manifest as negative feelings, depressed mood, and reduced task-related motivation [4, 5, 40]. For a burnt-out student, a blunted stress response during burnout might look like having significantly lower motivation to study or perform well and believing they will get a bad grade regardless of their efforts.
While the physiological ramifications of hypocortisolism remain understudied, hypercortisolism has been connected to negative health outcomes such as altered cardiovascular function and impaired stress response [6, 41, 42]. During a stress response, hypercortisolism can additionally lead to the dysregulation of metabolic processes, wherein the body struggles to process carbohydrates, fats, and sugars [6, 43, 44]. Excess levels of cortisol from the chronic stress response can make it harder for the body to manage blood sugar, sometimes leading to hyperglycemia, or an excess of sugar in the blood [45, 46, 47]. Chronic stress also activates the body’s immune system, releasing inflammatory molecules called cytokines in order to attempt to attack the stressor and stop the endless stress response cycle [48, 51]. While inflammation is helpful in short bursts, persistent inflammation, caused by the sustained activation of the HPA axis, can disrupt metabolism, making it harder to control blood sugar and contributing to weight gain as well as other health risks [47, 49, 50]. Usually, when blood sugar rises, the body signals the pancreas to release insulin, the hormone that allows cells to absorb energy from sugar [52]. Insulin, however, becomes less effective during chronic inflammation due to the cytokines disrupting the insulin signaling pathway [47]. When cells cannot properly take in sugar, it remains in the bloodstream, increasing the risk of type 2 diabetes and other metabolic problems [53, 54, 55]. In this way, chronic stress can create a cycle where elevated cortisol and inflammation interfere with the body’s ability to handle sugar and fat, putting longterm health at risk [56, 57]. There are often physiological consequences to psychological processes, such as stress. The HPA axis is a significant example of that brain-body connection [58, 59]. For instance, people with neuropsychiatric disorders such as schizophrenia, bipolar disorder, depression, and generalized anxiety disorder experience more physical health issues than the general population [60, 61]. These disorders are all associated with chronically high levels of psychological distress, similar to burnout, which may explain the equivalent health risks [60, 62, 63]. Long-term physiological changes, such as increased inflammation due to chronic stress, can in turn result in the deterioration of tissue in the nervous system [11, 64]. Psychological symptoms of burnout such as cynicism and emotional exhaustion are also coupled with structural changes in the brain, specifically in the ventromedial prefrontal cortex (vmPFC), which is involved in
regulating stressors [24, 65, 66]. Shrinkage of the vmPFC has been linked to dysregulated HPA axis responsivity and it is possible that this impairment could be due to burnout [65, 66, 67]. Furthermore, burnout may lead to more serious physical conditions culminating in increased mortality below age 45 [85, 86].

Burnout, as well as hypercortisolism, often accompanies symptoms such as higher levels of depression, lack of satisfaction with job performance, and substantially altered HPA axis activity [10, 68, 69]. At a societal level, burnout has been concerningly correlated with decreased job performance in critical professions such as nursing, causing a lower quality of patient care [70, 71]. Similar to nurses, other medical professionals are at high risk for burnout due to their high-stakes work environment, which should be reason enough to sound the alarm for access to reliable burnout prevention [69, 72]. Knowing that burnout can be detrimental to long-term psychological and physical well-being, the logical next step is to investigate the ways that burnout could be prevented or mitigated. Physical exercise, including yoga as well as aerobic and strength exercises, has been shown to
reduce burnout symptoms and overall stress [73, 74]. Physical exercise can reduce stress via increased production of serotonin — a hormone involved in both emotional regulation and cognitive function [73, 75]. Interestingly, mindfulness-based practices do not show a decrease in burnout, but they do seem to alleviate symptoms such as anxiety and poor mood [76, 77, 78]. If a stressed student is feeling burned out after their midterm and tries to help themselves by engaging in a mindfulness-based practice like meditation, they might feel a short-term reduction in anxiety and be in a better mood. However, while these shortterm effects may be achieved, meditative practices are unlikely to improve cortisol levels in the body and will not change how burned-out the student actually feels [74, 78]. It is critical to consider the effects that burnout has on society to prioritize reliable burnout prevention and reduction strategies. As established, healthcare workers are particularly vulnerable to burnout due to their intense work schedules and the stakes of their jobs [69, 70]. Strategies that focus on reducing burnout in a particular individual include reducing monotony in the workday by varying everyday responsibilities as well as receiving guidance from peers and counselors to mediate the effects of burnout before they escalate [79, 80, 81]. On a larger scale, those working high-demand jobs, like doctors, benefitted greatly from structural and policy change such as the alteration of work schedules for more reasonable hours, and the introduction of medical scribes [71, 82, 83]. Structural changes in the healthcare system are needed to reduce physician burnout, an issue that is affecting quality of healthcare [70, 71, 82].

Given the prevalence of burnout today, understanding the complex effects of burnout and chronic stress on the body is profoundly important [69, 70]. More than half of US physicians are now experiencing professional burnout [84]. High-risk populations, like healthcare professionals, require reliable ways to prevent and reduce burnout symptoms to improve both their health and their service to patients [69, 70]. Investigations of burnout prevention strategies have proven that not only is individual treatment required in the form of physical stress-busters, but importantly, that large-scale change is needed to refine the systems that generate lasting burnout for healthcare workers [87, 88]. Burnout has been proven to be a physiological phenomenon in addition to a psychological one, as evidenced by the effects of chronic stress on both the brain and body [4, 5, 6]. With the knowledge that acute stress is a normal and helpful response, we should strive to embrace stress in a healthy manner while also being mindful of the possible consequences of chronic stress [10, 12, 14].
References on page 67.

by
*Note: This article uses female-gendered language to refer to pregnant and postpartum people due to the vast majority of cited literature being focused on female-identifying subjects. The editors wish to acknowledge that pregnancy is independent of gender identity.
What if someone told you that since the moment you were born, and possibly even before that, tiny creatures have been living throughout your body, quietly helping you go about your life? No, it’s not your fairy godmother; it’s microbes! Microbes are microscopic organisms that are undetectable to the naked eye, yet have an enormous impact on human lives [1, 2]. You may know microbes as the cause of some diseases, but the majority of microbes are harmless and are actually beneficial [3, 4]. In particular, microbes residing in the gut are integral to human health [5, 6]. However, the impacts of microbes are not limited to the host they inhabit; they can even shape the health of future generations through the maternal-fetal relationship [7]. Maternal microbiota — referring to all of the microbes of a mother — can influence neurodevelopment both before and after birth, highlighting how bacteria support human function from the earliest stages of development [8]. To understand how maternal microbiota may affect neonatal brain development, it is essential first to become familiar with the microbial communities that inhabit the human body and their unique characteristics.
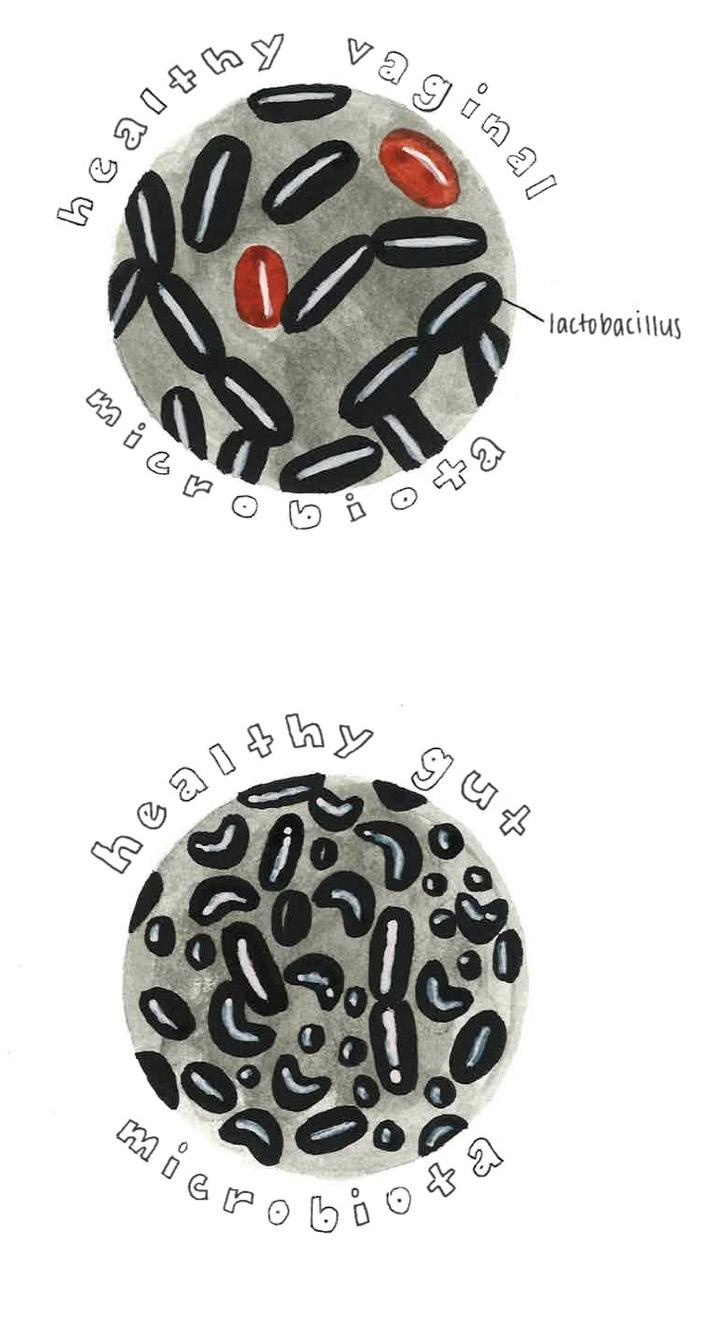
When you eat, you are not only providing sustenance for your body, but also for the trillions of microbes that live inside you [9]. The human body harbors various communities of microbes, and the gut microbiome is home to the most concentrated one [2]. The human gut microbiome refers to the collection of microorganisms that live in the gastrointestinal (GI) tract, which consists of the stomach, small intestine, and large intestine [10]. Much like a natural ecosystem, the gut microbiome relies on intricate interactions among its microbes to maintain balance and function [11]. Microbes live together in much the same way that organisms coexist in a coral reef: coral rely on algae for energy, algae rely on fish waste for nutrients, and fish rely on coral for shelter, maintaining an optimal and delicate harmony [12]. If an invasive species, such as a lionfish, were introduced to the reef, the balance would unravel — smaller fish would vanish, algae would overgrow, and the coral would
begin to die off, destabilizing the entire ecosystem [13]. Similarly, proper functioning of the gut microbiome depends on symbiotic interactions between the host and other microbes [5, 14]. For example, the bacteria Oxalobacter formigenes break down oxalate, a compound that can lead to kidney stone development, and in return, the bacteria gain nutrients and energy from the host [15, 16]. The gut microbiome is both dynamic and interdependent, and even minor disturbances can contribute to extremely detrimental effects [5].
Despite the misconception that all bacteria are harmful, microbes throughout the body have developed mutually beneficial relationships with their hosts that are crucial for the survival of both [ 4,9]. Gut microbiota help maintain a stable internal environment in the body, defend against foreign invaders, and synthesize metabolites — small molecules that support digestion, immunity, and communication between cells [17, 18]. In addition, gut microbiota are involved in regulating the immune response, wherein the body recognizes, identifies, and eliminates foreign substances while supplying essential nutrients like vitamins [9,19]. The specific composition of microbial species in the gut varies considerably between individuals and is influenced by factors like diet, environment, and lifestyle, each of which introduces new microbes and shapes the microbial community of the GI tract [9, 20]. Although no two people share the same microbial species composition, the bacteria generally perform similar functions across all individuals [9, 21].
A balanced gut microbiome facilitates proper bodily functions, while deviations can be detrimental to overall health [22]. Disruption of this balance can lead to gut dysbiosis, a condition in which the composition or function of the gut microbiome is altered due to a loss of beneficial bacteria, an overgrowth of pathogenic bacteria, or a reduction in the variety of microorganisms present [22, 23]. Specifically, pathogenic bacteria cause disease, whereas non-pathogenic bacteria generally do not harm their host [24, 25]. One pathogenic bacterium that can cause
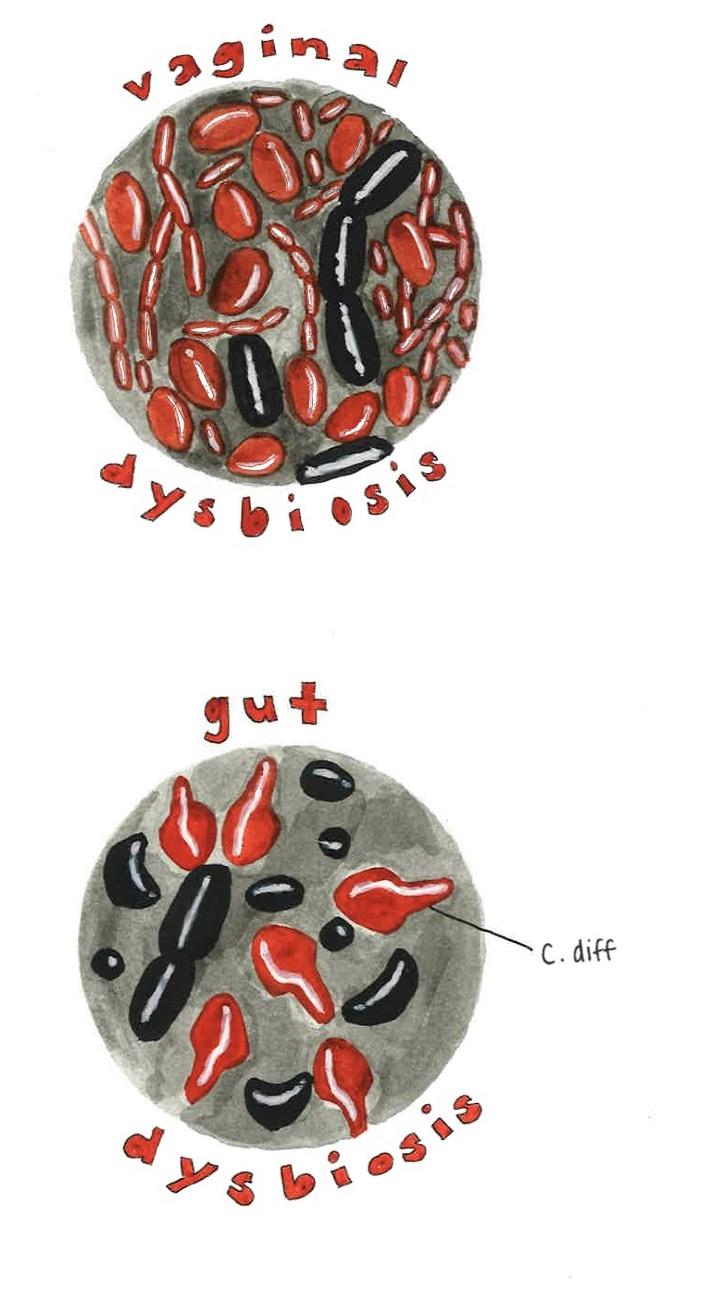
infection is Clostridiodes difficile, which infects the gut microbiome and induces diarrhea and other severe intestinal issues [26, 27]. However, when the host is in a weakened state, even non-pathogenic bacteria can become dangerous by attacking the host and causing illness [25, 28]. Maintaining microbial balance requires several physical and chemical defenses. For example, the mucus layer, which coats the intestinal cell surface, serves as the first line of defense against pathogenic bacteria and protects the gut lining [29, 30]. Bacteroides thetaiotaomicron (B. theta) inhabits the mucus layer and typically aids in the processing and digestion of carbohydrates like fiber, starches, and sugars [31, 32]. During periods of prolonged carbohydrate deficiency, B. theta may begin degrading the gut’s sugar-composed mucus layer, making the host more susceptible to infection [30]. Therefore, even beneficial bacteria like B. theta can contribute to dysbiosis when environmental or nutritional imbalances disrupt the gut microbiome [30].
Microbiomes exist in multiple locations throughout the body, not just the gut. The vaginal microbiome is another finely tuned ecosystem where microbial balance plays a crucial protective role [33]. In both pregnant and non-pregnant women, the vaginal microbiome is typically dominated by the Lactobacillus genus, which is the primary producer of lactic acid, thereby lowering vaginal pH and limiting the growth of pathogenic bacteria [33, 34, 35]. Beneficial vaginal microbes thrive in acidic conditions, whereas many pathogenic bacteria struggle to survive [35].
In addition to acidifying the environment, lactic acid possesses antimicrobial properties that selectively target and eliminate pathogenic bacteria [36]. Together, conditions created by lactic acid cultivate an inhospitable environment that prevents pathogenic bacteria from growing, thereby protecting vaginal health [37]. Just like the gut microbiome, the vaginal microbiome can experience dysbiosis [38]. Vaginal dysbiosis can manifest as bacterial vaginosis (BV), an infection that causes pain and discomfort in the vagina [39]. BV, characterized by a reduction in Lactobacillus, lowers vaginal acidity, which enables potentially pathogenic microbes to proliferate within the vagina [33, 40]. BV increases the risk for sexually transmitted infections and pelvic inflammatory disease, an infection in the upper genital tract [33, 41]. For pregnant women, BV has been linked to detrimental birth outcomes, including pre-term birth, low birth weight, and miscarriage [33].
The delicate equilibrium of the vaginal microbiome mirrors that of the gut, and the two systems are more closely connected than they might appear. Emerging evidence indicates that there is continuous microbial exchange and communication between the gut and vagina [42]. Picture the Galápagos Islands: each island has its unique ecosystem, but microbes — like the Galápagos birds — can travel between them [36]. For instance, evidence suggests that vaginal Lactobacillus may have originated in the gut [36]. Vaginal dysbiosis and gut dysbiosis work in tandem to trigger an inflammatory immune response that can contribute to serious pregnancy complications: gut dysbiosis allows bacteria to enter the bloodstream, while vaginal dysbiosis enables bacterial infections to reach the uterus [36, 43, 44]. Preeclampsia is one such complication where mothers experience severe high blood pressure and damage to organs like the liver or kidneys during or after pregnancy [45]. A significant cause of birth-related complications, preeclampsia greatly increases maternal, fetal, and infant morbidity and mortality worldwide [44]. Microbiota dysbiosis contributes to preeclampsia through inflammation, immune disruption, and dysfunction of the placenta — an organ that provides nutrients to the fetus from the mother. The convergence of gut and vaginal dysbiosis illustrates how the gut and vaginal microbiomes can have concerted effects on human health, particularly in pregnant women [44].
Maternal microbiome interactions between the gut and vagina not only influence pregnancy outcomes but also help determine the microbial communities that are first transmitted to the newborn. The initial colonization of the infant’s gut is crucial for its health and long-term development [46, 47]. As the gut microbiome develops, the infant acquires species that aid in nutrient utilization, vitamin production, and the breakdown of foreign compounds [48, 49]. Postnatally, the bacteria obtained by the infant support the digestion of breast milk, formula, and solid foods, without which infants could not digest food [48, 49]. For instance, Bifidobacterium aids in the processing of breast milk and enables infants to absorb essential nutrients for growth and development [47, 50, 51]. The gut microbiomes of infants delivered vaginally are colonized by maternal vaginal and fecal microbes [47, 52]. Hence, vaginally delivered babies have a high abundance of vaginal microbes like Lactobacillus during the first few days of life [9]. However, infants born via C-section tend to have gut microbiomes that are colonized by maternal skin and oral microbes, along with hospital-acquired bacteria [48, 52, 54]. Consequently, the gut microbiome of babies born via C-section does not mirror the maternal microbiome as closely as the gut microbiomes of vaginally born babies [9]. C-sections can be life-saving interventions in case of pregnancy, labor, or delivery complications [54]. However, the rate of C-section use has increased in recent decades, largely due to an uptick in non-medically suggested C-sections [54, 55, 56]. C-sections disrupt the microbial transmission process from mother to infant, with C-section babies often experiencing a decreased and delayed

colonization of Bacteroides, thus reducing the diversity of bacterial composition [9, 47]. Bacteroides, along with Bifidobacterium, are considered health-protective, and the absence of one or the other could lead to adverse health consequences, such as an increased risk of obesity or diabetes [51, 57]. Therefore, high diversity in the gut microbiome serves a protective function and promotes overall health during the early phases of an infant’s life [51].
Health complications during early infant development, such as malnutrition or infection, can have a long-term influence on physiology and behavior in adulthood [48, 58, 59]. Within this framework, the initial microbial colonization of the infant gut plays a crucial role in neural function and brain development [51, 60, 61]. For that reason, disruption of infant gut colonization can affect neonatal neurodevelopment and may even lead to adverse health outcomes later in life [62]. Research on how the maternal microbiome influences development is limited due to the ethics of conducting experiments on infants and pregnant women [63]. Additionally, the relative novelty of gut microbiome research, coupled with a lack of research on women’s health, means that longitudinal studies in humans are scarce [64]. Much of the knowledge in this area is therefore dependent on rodent studies [65]. Despite these limitations, researchers have formulated several hypotheses on how the maternal microbiome may influence neonatal neurodevelopment. One hypothesis concerns the prenatal period: during this time, metabolites and other substances produced by maternal microbiota may assist in the formation of the fetus’s neural circuits [48]. Neural circuits are


pathways of interconnected neurons — cells in the nervous system responsible for transmitting messages throughout the body — and other cells that work together to accomplish specific functions [66, 67]. Microbial metabolites, like short-chain fatty acids (SCFAs), aid in neural development [68]. After crossing the placenta, SCFAs facilitate the development of neural cells and serve as an energy source for neurons, playing an important role in postnatal neurodevelopment [69, 70, 71]. Moreover, gut microbes have been found to produce enzymes, which are important proteins that transform biological materials into different forms [69]. For example, Lactobacillus reuteri causes an enzyme to change one form of histidine to histamine, a chemical that regulates neurons and behaviors such as wakefulness, attention, and memory [69, 72]. The depletion of the maternal gut microbiome through antibiotic treatment can lead to decreased levels in maternal blood and, in turn, leads to decreased levels in the fetal brain [18, 73]. Collectively, these findings indicate that maternal microbial metabolites act as biochemical mediators between the maternal gut and the developing fetal brain.
A second hypothesis focuses on the postnatal period, proposing that after birth, the microbes an infant acquires from its mother and environment help shape neural circuit formation and regulate molecules involved in brain development [48, 74]. A potential pathway for this mechanism is the gut-brain axis, a system that communicates between the brain and the gut [75, 76]. The vagus nerve extends from the brain to the gut, enabling the relay of signals about the gut’s chemical composition, intestinal conditions, and hormone release [75, 77]. The brain and the gut are like two people talking on the phone, with the vagus nerve as the telephone line that connects them. The vagus nerve promotes growth and reproduction of enteroendocrine cells, which are specialized intestinal cells that can sense microbes and relay information to the brain [75, 78]. SCFAs continue to play a role in neurodevelopment after birth by mediating messages between gut microbiota and the vagus nerve [79]. Therefore, the gut-brain axis represents a direct physiological pathway through which microbiota acquired from the mother may influence neurodevelopment [75]. Both theories are not mutually exclusive and may operate simultaneously, and further research is necessary to clarify exactly how these processes occur [48, 80].
Knowing that the maternal microbiome impacts neonatal neurodevelopment raises the question of whether maternal dysbiosis can impact the development of neural circuits in infants [79]. Maternal microbial dysbiosis caused by infection or antibiotic use can affect pre- and postnatal development and increase the offspring’s risk of developing neuropsychiatric or neurodevelopmental disorders [81, 82]. For example, getting an infection during pregnancy can trigger the maternal inflammatory response, which is one way fetal and neonatal neurodevelopment is influenced [83, 84]. An inflammatory response in the mother exposes the fetus to an inflammatory environment, since signals can cross the placental barrier and reach the fetus [61]. Inflammatory signals influence fetal brain development by indirectly affecting the function of microglia in the infant [61]. Microglia are immune cells that support the central nervous system (CNS), which encompasses the brain and spinal cord, and influence brain development, maturation, and maintenance [85, 86]. Inflammatory signals cross the fetus’s blood-brain barrier, the highly selective membrane that separates the brain from the rest of the body, and activate microglia, causing neuroinflammation [87]. The maternal inflammatory response can lead to maternal immune activation (MIA), which may disturb signaling between microglia and neurons and contribute to neuroinflammation [83, 84, 88]. MIA is thought to be linked to an offspring’s likelihood of developing neurodevelopmental disorders [85]. MIA also influences brain development at the embryonic stage by impacting the creation and movement of neurons, crucial processes for brain development [89]. There is a decreased production of neurons in the fetal brain, possibly due to cells being forced to exit the cell cycle prematurely [89]. Reduced neuronal production delays neuronal migration, a critical process for CNS development [89, 90].
There is some evidence that suggests MIA can also be induced by maternal gut dysbiosis and lead to behavioral changes associated with autism spectrum disorder (ASD) in offspring [91]. ASD is a neurodevelopmental disorder with varying manifestations, but is often characterized by difficulty with social interaction or communication, restricted or repetitive behaviors, and reduced ability to process and respond to external stimuli [61]. Disruption of the gut-brain axis is also associated with ASD-related behaviors [92, 93]. Bifidobacterium and Lactobacillus support
the production and balance of SCFAs that regulate microglia maturation and activation [91, 94]. It is possible that the loss of these microbes through gut dysbiosis may disrupt microglia function and lead to an impaired neuroimmunological response, correlating with an increased risk of developing ASD behaviors [91]. Additionally, altered SCFA levels are thought to underlie some of the neural and behavioral symptoms observed in ASD due to neuroinflammation [91]. Weakening of the gut barrier, due to a condition known as ‘leaky gut,’ or excess intestinal permeability, has also been suggested to increase rates of ASD characteristics — though this research is based on correlations, and is not definitive [93, 95]. Microbial products can leak into circulation and invade the CNS, which may trigger a neuroinflammatory response [61]. Despite these connections, it is difficult to draw any definitive conclusions on whether or not ASD is the cause or a result of a dysbiotic gut microbiome [61].
Using antibiotics during pregnancy can alter the maternal microbiota composition by reducing bacterial diversity [48]. While antibiotics are often used as a preventative and necessary measure against infection, antibiotic overuse can cause maternal dysbiosis and affect the infant’s initial gut colonization [69, 81]. Lactobacillus and Bifidobacterium produce GABA, a chemical messenger between neurons essential for controlling stress, anxiety, and sleep, as well as regulating gut motility and inflammation [96, 97]. By depleting these beneficial bacteria, antibiotic treatment alters the production of SCFAs and chemical messengers like GABA, which may lead to dysfunction of the gut barrier, blood-brain barrier, and neuroimmune interactions [91, 96, 98]. Overuse of antibiotics may even alter the expression of molecules involved in learning and memory through changes in gut microbiota composition. [98, 99]. For example, brain-derived neurotrophic factor, a protein that promotes brain health, showed altered levels in response to changes in microbial composition caused by antibiotics [100]. Furthermore, exposure to antibiotics can result in delayed maturation of inhibitory neural circuits, or more specifically, parvalbumin-expressing inhibitory interneurons (PV+ INS) [98]. PV+ INS are neurons that serve as intermediaries between sensory and motor neurons [98, 101]. PV+ INS mature after birth and are involved in processing external stimuli and feedforward inhibition — a process that acts as a warning system, to dampen a target neuron's response to a messenger [98, 101]. Exposure to antibiotics slows the maturation of PV+ INS, causing physical changes in the brain and impairing sensory
perception [102]. Altogether, maternal gut dysbiosis caused by infection or antibiotic use can lead to maternal immune activation that can disturb the brain development process.
The mother-infant relationship extends beyond genetics and environment to the microbial level, where maternal microbiota play a powerful and lasting role in shaping neonatal neurodevelopment. The microbial link between mother and infant can be leveraged to positively influence health outcomes for both. Pregnant people can be more susceptible to infection, so it is particularly important to maintain maternal health and a balanced microbiome for the sake of both the mother and the infant [103, 104]. Probiotics are live microorganisms that provide health benefits, such as strengthening the immune system or improving digestive health [105, 106]. When administered during pregnancy, probiotics can support the infant microbiome and overall health [105, 107]. Probiotics can also be given directly to infants who experience a disruption in initial gut colonization, though they are not effective in every scenario [108]. More research is needed to determine the exact mechanisms by which maternal microbiota influence neonatal neurodevelopment. Nevertheless, it is undeniable that microbes are essential in shaping the mother-child relationship and human health.
References on page 71.

by Sushama Gadiyaram | art by Alexandra Tapia

What happens when fundamental abilities, such as walking, speaking, and remembering, suddenly disappear after a neurological injury? For individuals suffering from stroke, traumatic brain injury (TBI), or Parkinson’s Disease (PD), the loss of these essential skills transforms everyday life into a series of obstacles [1]. Individuals may turn to neurorehabilitation — therapeutic practices used to restore lost functions or help the brain adapt to lasting damage [2]. Traditional neurorehabilitation usually involves repetitive cognitive drills, structured physical training, and various therapist-guided exercises, which all aim to retrain the brain through consistent practice [3]. While clinically effective, many individuals struggle with decreased engagement, slower progress, and repetitiveness of traditional rehabilitation tasks, which can undermine recovery [4]. This is where
virtual reality (VR) offers a completely new advantage. VR refers to immersive, digitally generated environments that allow individuals to interact with both realistic or fictional virtual worlds using visual, auditory, and sensory feedback [5]. Compared to traditional therapy at an office, VR can simulate everyday activities such as navigating a busy grocery store, preparing a meal, and driving to work [6]. In these guided and safe simulations, VR excites cognitive, motor, and emotional neural pathways that are key for long-term neurological changes [6]. Immersive and interactive therapeutic approaches like VR-based therapies are very effective as they increase feedback, consistency, and motivation [7]. VR therapy has a promising future in neurorehabilitation, offering innovative ways to restore cognitive and motor function through the power of generative images and immersive worlds [8].
VR is a valuable tool for improving impaired cognitive abilities from brain injury or cognitive decline [9]. Cognitive abilities can be thought of as a mental toolkit that allows us to take in information, understand it, and respond appropriately [10]. They are the foundation of how we think and interact with the world, including abilities such as attention, memory, and problem-solving [11, 12]. When cognitive skills are disrupted, neurorehabilitation is required in order to get them working properly again [11]. VR offers an exciting alternative to traditional cognitive therapy, which can be slow due to inconsistency or a lack of engagement [13]. By simulating real-world situations, VR allows the brain to practice cognitive abilities in dynamic and meaningful ways [6]. Think of VR as a flight simulator: a controlled environment that enables users to safely gain a realistic experience flying and maneuvering planes that would otherwise be impossible [14]. Therapists are therefore able to recommend VR regimens that offer functions unavailable in reality, such as seeing a virtual version of one's body from a third-person perspective or instantly teleporting to a brand new environment [6].
In addition, the highly controllable nature of VR allows it to be tailored to the specific conditions or interests of individuals [7, 15]. The cognitive decline experienced by a person with PD, for example, differs from the cognitive impairments of a person suffering from a TBI [16]. With PD, there is an overall decline in cognitive function, affecting abilities like problem-solving, whereas a TBI often involves attention impairment or localized brain damage [17, 18]. Using VR, an individual with PD can solve puzzles, fingerpaint, or switch between Earth and zero gravity simulations to train the brain to handle novel demands [19]. On the other hand, VR can improve TBI symptoms by challenging individuals to perform important tasks such as planning a bus route or evacuating a burning building [20].
Treatment of cognitive deficits with VR results in tangible changes in both the brain’s structure and activity [7]. This impact is structurally visible in increased volume of gray matter — brain tissue composed primarily of cell bodies and neural circuits that foster cognitive functions — following long-term VR therapy [21, 22]. Along with structural changes, there are changes in brain activity that can be measured with electroencephalography (EEG), a technology that monitors the electrical signals in the brain and determines how different brain regions activate and interact with one another, correlating with the recovery of cognitive function [23, 24]. Specifically, beta-wave activity — a type of electrical signal measured with an EEG that reflects active thinking and alertness — tends to increase when these individuals participate in VR tasks that require attention, memory, or quick decision-making [25, 26]. These changes in the brain correlate with subjective reports: VR makes it easier to focus, recall information, and solve complex problems [27]. Furthermore, individuals who train with VR often demonstrate an improvement in cognitive skills that carry over into real-life scenarios [28]. By creating experiences that are both challenging and enjoyable, VR creates an environment where the brain can restore impaired cognitive skills, which is foundational for long-term functional improvements [13].
VR technology is also a valuable tool for enhancing motor functions, the abilities that control movement, balance, and coordination [29]. Traditionally, individuals in pain or experiencing limited motor skills may turn to physical therapy (PT) for physical
rehabilitation [30]. Traditional PT helps individuals create new motor pathways effectively or strengthen existing ones [31]. Motor pathways refer to a system of neural circuits — the web of communication between neurons, or brain cells — that transmit signals from the brain to the muscles, enabling movement and controlling posture, balance, and reflexes [32]. When these motor circuits are weakened, by injury or disease, an individual experiences limited motor function [33, 34]. VR can better address these deficits by immersing individuals in a simulated world and actively engaging the same brain regions used for real-world movement [31]. Furthermore, traditional PT often involves physical exercises in a contained environment, making the experience feel clinical or overly repetitive [3]. Conversely, VR’s limitless visuals allow individuals to experience physical therapy in various environments [35]. With VR-based therapy as a compelling alternative, the familiar PT office falls away as an individual puts on a VR headset. Engagement is heightened in this new world of therapy, where one could walk across a virtual canyon or navigate a busy intersection all from the safety of the PT office [36].
In this way, VR provides individuals with motor loss a diverse and exciting environment to pursue rehabilitation [31].
The efficacy of VR training depends heavily on the combination of physical effort with instant, multi-sensory guidance [21]. Immersive VR tasks enhance motor function by transforming abstract movement into visual and sensory feedback that informs coordination [21]. For example, during a task like lifting one’s arm to a specific height, the VR environment can instantly offer feedback in multisensory cues: a visual score will increase, and a positive tone will sound
[37]. Immediate feedback is paramount to long-term learning in the brain because it allows the brain to quickly recognize errors and correct movement patterns, strengthening neural circuits [7]. Strengthening motor pathways can be facilitated by many types of VR motor function therapy, including virtual mirror therapy and touch-enhanced feedback [38, 39]. Virtual mirror therapy enables motor learning by displaying a real-time reflection of one’s impaired limb [40]. By creating the illusion of a fully functioning limb through visual feedback, this technique activates the motor cortex — the brain region primarily responsible for motor coordination — and strengthens the connection between visual and motor processing [38]. The mirroring essentially tricks the brain into reactivating dormant pathways and restoring motor function [38]. Furthermore, some VR models include touch enhancement, which allows the individual to experience the sensation of other objects, such as simulating the weight of a coffee cup [39]. This version of highly engaging, feedback-rich rehabilitation effectively ‘sets the scene,’ allowing individuals to have a fulfilling experience, even if it’s virtual [35]

Recall the possibility of walking across a virtual canyon: the emotional excitement of this novel experience highlights VR’s unique capability to activate the brain's reward and motivation systems [15, 41]. When interacting with an object or completing a task in VR, the brain still responds as if the experience is real [42, 43]. These experiences can be expanded to positive feelings of success; for example, winning a boxing game in VR can trigger genuine physical and emotional reactions of success and achievement [42, 43]. VR has been shown to trigger the release of the reward chemical dopamine, activating the reward pathways that influence us to repeat the
rewarding activity [44, 45]. Dopamine release is critical for motivation, learning, and the rewiring of the brain — a process known as dopamine-modulated neuroplasticity [45, 46]. All forms of neurorehabilitation rely on neuroplasticity, which is the brain's ability to reshape its own wiring by strengthening existing connections and building new ones [46]. Neuroplasticity facilitates the learning of new skills, recovery of lost functions, and adaptation to changing physical or cognitive demands. Hence, neuroplasticity is extremely beneficial for neurorehabilitation because it facilitates motor learning, increases motivation, and helps restore damaged neural circuits. By activating these reward pathways and stimulating the motor cortex through feedback-driven tasks, neuroplasticity increases — new neural connections are formed, some are strengthened, and others are rewired. This, in turn, leads to both short-term and long-term positive behavioral changes. In the short term, one could experience improved attention, motor control, and task performance; if one continues this VR therapy long term, they could experience cognitive improvement, behavioral adaptations, and emotional resilience. These beneficial effects contribute to a positive feedback loop, where the response to a behavior increases the behavior. Due to these encouraging associations, the individual would continue using VR, increasing the beneficial long-term effects of neurorehabilitation [46].
Crucially, due to novelty and motivational engagement, VR-based rehabilitation can create emotional investment in one’s recovery [47, 48]. A key issue with current neurorehabilitation practices is a lack of motivation and long-term continuation, which is critical for maintaining improved cognitive skills [21, 49]. Motivation can wane due to its emotionally overwhelming nature, its tediousness, or avoidance of pain [49, 50]. Although difficult to maintain, emotional engagement is necessary to reap the positive benefits of rehabilitation by leading individuals to continue, as shown by the high connection between engagement and successful therapeutic results [48, 51]. Emotional engagement further contributes to the formation and strengthening of neural pathways over time by encouraging the individuals to continue therapy, ensuring it takes full effect in the brain [7, 48]. While traditional methods may allow motivation to decrease, VR’s engaging nature increases motivation and can dramatically increase the number of therapeutic exercises an individual completes [7]. VR encourages individuals to stay motivated through emotional engagement, increasing learning and neuroplasticity, and furthering long-term rehabilitation [7].
VR aids in the strengthening of neural pathways by recreating a feeling of embodiment — the deep connection between the brain, body, and the environment that defines how we experience movement and selfhood [52]. Similar to virtual mirror feedback, embodiment enables individuals to perceive virtual limbs or avatars as part of their own body [53]. The illusion of ownership activates and strengthens previously weakened neural pathways that support motor and sensory functions [53]. For example, a person recovering from a stroke may see their virtual leg move smoothly in coordination with their thoughts, and over time, the brain can begin to regain function in their physical leg [54]. While PT is limited to the external repetition of movement, VR can simulate embodiment by combining visual displays, sensorimotor feedback, and emotional engagement to stimulate the neural circuits involved in body control and awareness [55].
Furthermore, VR therapy can be applied to a variety of neurological conditions [56]. People with PD experience a loss of dopamine-producing neurons, leading to disruption of the brain’s motor functions [57]. For these individuals, VR can significantly improve gait, balance, and coordination while also encouraging the brain to rewire itself through rewarding and repetitive tasks [56, 58]. Moreover, VR-based rehabilitation can increase dopamine release from remaining neurons and reinforce adaptive neural networks, which may help slow the progression of motor or cognitive deficits seen with PD [15, 59]. The adaptability of VR allows exercises to be modified based on individual performance, pushing participants to achieve cognitive engagement and promoting recovery [15, 60]. This VR technology is generally safe for continuous use, can be adapted for sev eral age groups, and is much more accessi ble than traditional therapy [51]. Addition ally, the reduced rehabilitation costs and flexibility of VR make it more convenient than conventional therapy [51].
VR-based rehabilitation has the power to assist disrupted relationships between the brain and body by using the brain’s capacity for plasticity [6, 8]. While traditional therapies have been indispensable, VR introduces a method that combines embodiment, engagement, and personalization that is difficult to replicate in a clinical setting [14, 15]. Although VR-based therapy isn’t the only path to recovery, it is an evidence-based, rapidly evolving technology that should be more widely available to all individuals who might benefit from its therapeutic strengths [35]. VR illustrates the ties between cognition, movement, and perception, which is especially crucial for the treatment of neurodegenerative diseases such as PD or injuries that disrupt the brain in multiple ways [7]. With VR, the motions of PT can now be embedded in an emotionally rich scenario such as catching a firefly in a forest, playing beautiful music on a piano, or throwing pottery on a wheel [15, 41]. The possibilities with VR are essentially limitless. Clinicians can create environments that could reduce anxiety, increase emotional engagement, and stimulate real-world challenges that can be faced outside the clinic [6].
The flexibility that VR provides allows it to create a positive feedback loop; increased engagement leads to more meaningful usage, which strengthens neural networks, in turn leading to further motivation and functional improvement [46]. VR represents an impactful therapeutic modality that combines embodiment, engagement, and emotions in ways that are beyond traditional therapy [46]. VR technology makes personalized, immersive, and transformative neurorehabilitation more achievable [6, 46].

References on page 73.


by Daniel Bader | art by Erica Langlais
Have you ever felt like you’ve had a good night’s sleep, but woke up feeling tired and unable to concentrate? Or has the reverse happened, where you wake up feeling particularly refreshed after only a few hours of rest? You might wonder, exactly what function does sleep serve? Identifying a single reason why humans need sleep is difficult, but quantifying the benefits of sleep and the dangers of sleep deprivation is easy [1]. Sleep assists physical recovery, emotional stability, immune function, and cognitive processes such as learning and memory [1, 2, 3]. Consequently, a lack of sleep can have severe impacts on emotional states and cognition — even mild sleep deprivation is associated with lapses in attention, feelings of sleepiness, and slower reaction times [4]. The physical effects of sleep deprivation are also profound, including increased levels of stress hormones, decreased physical recovery after exercise due to inefficient muscle repair, and weight gain
and obesity [3, 5, 6]. Despite the increasingly understood value of sleep, the modern world has gradually undergone a decrease in sleep duration [7]. In fact, more than 30% of Americans now receive less than the recommended minimum of seven to eight hours of sleep per night, which is considered to be a public health crisis [8]. Routine aspects of our daily life, like exposure to blue light from electronics, use of drugs and alcohol, and improper dietary habits, can decrease sleep performance and ultimately lead to sleep deprivation [9, 10, 11].

Sleep is primarily controlled by circadian rhythms — biological processes that are influenced by external and internal factors that restart approximately every 24 hours [12]. Circadian rhythms, in conjunction with light availability, regulate when we go to bed and when we wake up through a process called the sleep-wake cycle [12]. Melatonin, a sleep-promoting hormone, is secreted throughout the circadian cycle, reaching its highest concentrations during nighttime hours [12, 13]. When sleep is misaligned with circadian rhythms, sleep tends to be of poor quality or insufficient [14]. Disruption of circadian rhythms may have long-lasting effects on sleep and lead to other health
issues [15]. Therefore, an eight-hour sleep during the nighttime is more restorative than an eight-hour sleep during the day after staying up all night [14, 15]. Sleep is categorized into stages, each featuring different brain activity that can be visualized as electrical waves [16]. Brain waves are characterized by their frequency, and different types of waves are associated with varying levels of mental processing [17]. The sleep-wake cycle is generally divided into five stages, which are classified as either rapid eye movement (REM) or non-rapid eye movement (NREM) [18, 19]. Stage one of the sleep-wake cycle is wakefulness, when a person can move voluntarily and respond to stimuli such as someone speaking [16]. Wakefulness involves high-frequency brain waves, which are associated with complex mental tasks like logical reasoning [18, 19]. If we imagine brain wave frequencies as different forms of movement, wakefulness is running — the body is in an active and alert state [20]. The second stage, N1 (non-rapid eye movement stage 1), involves the transition from wakefulness to sleep and features slower frequency brain waves called theta waves [18, 19]. N1 is like jogging — your brain is still relatively active and working fairly hard, but not as hard as it was when you were fully awake. N1 progresses into N2, which describes a deeper stage of sleep where the body further relaxes and theta waves continue [19]. N2 accounts for the majority of time spent asleep, and might resemble speed walking: the brain continues to experience theta waves, but with greater body relaxation [19]. The fourth stage of sleep is N3, more commonly known as slow-wave sleep (SWS) [21]. Characterized by delta waves, which have a lower frequency compared to theta waves, SWS is considered ‘deep sleep’ as individuals are essentially unresponsive to mild external stimuli, such as footsteps or soft noises [21, 22]. SWS is like slow walking; brain activity is low and accompanied by bodily relaxation [21]. Following SWS is Stage 5, or REM sleep, which, unlike NREM sleep stages, is physiologically very similar to wakefulness [18, 23]. During the REM stage, respiratory rate, heart rate, and brain wave frequencies increase to levels resembling wakefulness, while the body becomes paralyzed except for rapidly moving eyes [1, 23]. REM is like running on a treadmill — your brain is highly active, almost as if you were awake, but your body remains in place [23]. The contrast of the hyperactive brain with the completely inactive body is why REM is often referred to as ‘paradoxical sleep’ [23]. REM and SWS are extremely valuable for mental and physical recovery, and together they account for about 50% of total sleep duration [18, 23]. Most sleep cycles last around 90 minutes, but the composition of each
cycle varies [18]. Sleep cycles are initially composed of large amounts of SWS that decrease in subsequent cycles, while REM sleep dominates the later sleep cycles [18]. Thus, for any given night, individuals who do not sleep for long enough or whose sleep gets interrupted risk losing REM sleep [18, 24]. When we are short on REM sleep, our bodies attempt to ‘catch up’ by altering our sleep to have longer stages of REM [23]. This process, called REM rebound, ensures that REM sleep is properly regulated long-term [23]. Continuous REM sleep is also important, as interruptions, such as waking up or temporarily entering non-REM sleep stages, can have adverse effects on sleep quality [25, 26].
Sleep quality is key to reaping the cognitive and physical benefits of SWS and REM sleep [18, 21]. SWS is heavily involved in memory, cognition, and especially physical restoration [21]. It is the only stage of sleep associated with subjective feelings of being well rested, which is largely attributed to the release of human growth hormone (hGH) during this stage [18, 21]. While hGH is commonly associated with physical growth in children, it is also involved in rebuilding muscular tissue after exercise or general strain [3, 21, 27]. In addition to promoting physical restoration, SWS plays a valuable role in consolidating consciously recalled information such as facts and memories of events [21, 28]. Therefore, if you stay up late and sacrifice SWS to study for a big exam, you may actually be harming your ability to store information.
REM also plays a key role in memory formation [18]. REM sleep ‘refines’ memories by minimizing background activity in the brain, while increasing the activity of brain cells called neurons that are necessary for storing learned or trained information [18]. Just like turning down the volume of the TV when you answer a phone call, decreasing the overall noise allows the subject of focus to be better understood. Furthermore, REM sleep ‘rescues’ memories that may otherwise have been forgotten by making strong and weak memories equally retrievable and less vulnerable to interference [18]. Memory interference occurs when memories with similar features overlap [29]. One might inadvertently recall details of similar experiences instead of the desired event. For example, if you are trying to remember specific details from your birthday five years ago, events from different birthdays may distort your recollection. Decreased
interference is likely due to the selective activation of neurons during REM sleep, which limits the activity of neurons that are not necessary for prioritized information [18]. In this process, REM sleep limits neural overlap, storing only the important details of each memory [18]. The retrieval and consolidation of memories influence subconscious experiences that, upon waking, we remember as dreams [30]. From dreaming to remembering, REM sleep is vital for our cognitive functioning and learning processes [18].

Pulling an all-nighter to finish an assignment, working a night shift, or staying up to finish binge-watching a TV series are just some of the ways we can end up with insufficient sleep. The cognitive, physical, and mental benefits of sleep are necessary for a healthy lifestyle, which explains why sleep deprivation — consistent insufficient sleep leading to difficulty staying awake during the day — can have such catastrophic effects [4, 14, 31]. In the workplace, sleep deprivation has been found to substantially decrease efficiency and increase accident risks, with daytime sleepiness directly linked to injuries and task mistakes [4, 14]. In fact, employee exhaustion is actually more predictive of fatal accidents than the hectic nature of the job or the amount of physical labor required [6]. Disruption of the circadian rhythm and subsequent sleep deprivation are thought to be indirectly responsible for some of the most disastrous workplace mistakes in history, such as the nuclear accident at Chernobyl and the Exxon Valdez oil spill [14].
Beyond the workplace, many undesirable behaviors are connected to sleep deprivation; the severity of sleep deprivation is associated with proportional decreases in reaction time and increased instances of lapses in attention [6]. Inadequate sleep can also have detrimental emotional effects, such as a lack of social and emotional awareness, while predisposing individuals toward impulsive and risk-taking behaviors [2]. Even a single night of insufficient sleep can lead to depressed mood, anxiety, anger, and confusion the following day [32]. Lack of sleep is also responsible for accidents involving everyday tasks like driving; 20% of injuries from car accidents can be attributed to driver sleepiness [33]. Whether long-term or short-term, the cognitive, behavioral, and emotional consequences of sleep deprivation are broad and severe [6, 32].
Consistent sleep deprivation causes hormonal imbalances in the body, which can lead to elevated levels of stress hormones and emotional volatility [33]. Thus, mood disorders such as depression are common among individuals with chronic sleeping issues [33]. Sleep deprivation alters perception of social events, often leading to negative outlooks and voluntary isolation [34]. People who are sleep deprived are more likely to enter social events with negative expectations or even avoid them entirely [34]. These effects are especially detrimental in adolescents, whose brains are not yet fully developed [34, 35]. Adolescents suffering from sleep deprivation report feeling less connected to their peers compared to people with proper sleeping habits [34]. Furthermore, they are more likely to have an enhanced perception of loneliness following social activity [34]. Many adolescents struggle with emotional disorders resulting from a lack of sleep, as sleeping less than 6 hours every school night substantially increases the risk of developing anxiety and depression just one year later [35]. Sleep deprivation is also associated with self-harm and suicidal ideation; the likelihood of an individual planning to commit suicide increases by 11% for every hour of sleep below the recommended 8-9 hours [35]. The relationship between depressive symptoms and sleep deprivation elucidates the value of sleep in promoting mental health [34, 35].
In addition to the behavioral consequences of sleep deprivation, failure to get sufficient sleep can lead to the development of various physical health issues [33, 36]. Sleep deprivation is a known risk factor for high blood pressure, which can predispose individuals to cardiovascular disease and heart failure [37]. Extended periods of wakefulness cause increased activity
of the sympathetic nervous system, which is perhaps best known for activating the fight-or-flight response and the release of adrenaline [36, 38]. Enhanced release of adrenaline as a result of prolonged wakefulness not only increases blood pressure, but can also cause elevated heart rate and respiratory frequency [37]. Weight gain is another potential consequence of sleep deprivation, as lack of sleep decreases the secretion of leptin, a hormone that promotes satiety, and increases levels of ghrelin, a hormone that promotes feeling hungry [39, 40]. Frequently interrupted sleep is thought to be associated with increased unhealthy eating habits and weight gain, further supporting how lack of sleep can have detrimental health effects [33].
Habitual sleep deprivation can also predispose individuals to neurodegenerative diseases [12, 41]. With enough SWS, your brain is better able to remove waste proteins called amyloid-beta proteins and prevent them from forming harmful clusters [42]. Amyloid-beta proteins are naturally occurring and accumulate as a byproduct of brain activity when individuals are awake [43, 44]. Importantly, the buildup of amyloid-beta proteins can lead to structural impairments in the brain associated with Alzheimer’s Disease [43]. By creating an environment with consistently reduced neuronal activity, sleep plays a crucial role in decreasing amyloid-beta protein levels in the cerebral spinal fluid (CSF) — the primary fluid of the brain and spinal cord responsible for protecting the brain from physical stress and draining waste [43, 44]. In particular, SWS increases CSF to the glymphatic system, which is responsible for removing neurotoxic waste such as amyloid-beta proteins [42, 45]. However, if sleep cycles are frequently interrupted, this removal process cannot occur, and amyloid-beta protein can build up in the CSF, which can increase the risk for neurodegenerative diseases like Alzheimer's Disease [43]. Thus, sleep is essential to perform regular maintenance that may prevent the development of diseases [33].
Patterns of insufficient sleep are also implicated in the development of serious diseases by weakening the immune system (46). In animal models, sleep deprivation leads to a decrease in immune cells that are responsible for recognizing and terminating diseased or cancerous cells in the body (46). Thus, sleep deprivation in humans is associated with higher cancer risk, but causality is uncertain [33]. Another contributing factor for cancer risk is impaired melatonin regulation, which results from circadian misalignment [33, 46]. Although primarily known as the
sleep-promoting hormone, melatonin is also implicated in DNA repair and tumor growth suppression [33]. This association may explain why nightshift workers who consistently sleep outside of their circadian rhythms are at a higher risk of developing cancer [33, 46]. Decreased levels of melatonin secretion may contribute to the immunosuppressant effects of sleep deprivation, potentially leading to the development of cancer and the acceleration of tumor growth [33].
We are often told to ‘get our eight hours,’ yet we rarely consider the daily factors that can affect our sleep health. For example, caffeine reduces the production of 6-sulfatoxymelatonin, one of the main compounds necessary to make melatonin, thereby decreasing melatonin production [47]. Caffeine promotes wakefulness by blocking the effects of adenosine, which is a byproduct of energy use in the brain [48]. Typically, adenosine binds to proteins that recognize and bind to specific target molecules, called receptors, which is thought to result in increased sleepiness [48, 49]. Caffeine inhibits the ability of adenosine to bind to receptors, negating the effectiveness of feedback loops in the brain that associate long periods of wakefulness with the need to sleep [48]. Screen use is another daily habit that can significantly impact sleep health [9]. You may have learned that screens emit blue light, possibly even while scrolling on your phone at night, but its negative effects are often misunderstood [9]. Blue light is a natural component of the light spectrum and is even beneficial in the sleep-wake cycle during the beginning and middle of the day to promote wakefulness [50]. However, nighttime exposure to common electronics such as smartphones and computers can disrupt circadian rhythms by suppressing melatonin release and increasing wakefulness [9]. Hence, using electronic devices before going to bed can increase the time it takes to fall asleep and negatively affect sleep quality [51]. Decreasing blue light exposure 90 minutes before bedtime has been linked to decreased time to fall asleep and increased total sleep duration [51]. Thus, caffeine consumption and screen usage are two daily habits that have adverse effects on sleeping ability due to their negative effects on circadian rhythms [47, 51]. It probably comes as no surprise that consuming drugs and alcohol can significantly decrease sleep duration and quality [10]. Even moderate alcohol consumption has been found to decrease REM sleep,
overall sleep duration, and sleep quality, and these effects are even more pronounced after drinking heavily [10]. In the first one or two sleep cycles, which typically occur while the individual is still intoxicated, SWS and REM levels usually appear relatively normal [10]. However, as blood alcohol content decreases, the individual spends significantly more time in light sleep phases than normal, and REM sleep is frequently interrupted by wake events [10]. Therefore, in addition to experiencing less total sleep, alcohol intoxication causes individuals to experience more light sleep instead of other stages associated with more cognitive and physical restoration, such as REM and SWS [10]. The effects of cannabinoids such as tetrahydrocannabinol (THC) are more complex; chronic usage is detrimental, but short-term use is not as overwhelmingly negative [52]. While short-term THC use is thought to increase levels of SWS and reduce wake events, functioning as somewhat of a sedative, it also decreases REM duration [52]. Conversely, chronic THC use has been associated with decreases in both SWS and REM duration, as well as decreased overall sleep time [52]. Thus, although cannabinoids may promote sleep in the short term, their beneficial effects are negated when a tolerance is developed, and sleeping ultimately becomes more difficult [52, 53].
By contrast, sleep performance can be improved through healthy eating and exercise habits [9, 11]. Melatonin promotes sleep by expediting the transition from wakefulness to sleep, increasing overall sleep duration by allowing individuals to fall asleep faster [13]. Foods rich in melatonin, such as cherries, fish, and eggs, as well as supplements, can be beneficial for sleep [11, 13, 54]. However, the safety of longterm supplemental melatonin use is unclear — everyday use in adolescence is suspected to have mild, adverse effects on hormonal balance during puberty

[55]. Exercise is also known to affect sleep by altering the composition of sleep cycles and promoting increased total sleep duration [12]. Since physical activity induces exhaustion and muscular fatigue, it has also been associated with an increased percentage of SWS in the following night [56, 57]. This connection is likely due to the necessity of hGH to promote recovery in muscles exercised during strenuous activity [56]. As a result of the increase in SWS, it follows that exercise can lead to subjective feelings of being well rested the following day [18, 56]. Thus, regular exercise and consuming melatonin-rich foods are both beneficial lifestyle choices for maximizing sleep health [11, 12].
Sufficient sleep is an essential component of cognition, physical recovery, emotional balance, and disease prevention [18, 33, 36]. Decrease in sleep can be attributed to consumption of caffeine, drugs, and alcohol, as well as screen usage [10, 47, 51]. As contemporary research continues to reveal the importance of proper sleep habits, sleep deprivation and excessive daytime sleepiness remain significant problems in the United States [58]. The consequences of this sleep shortage are staggering: sleep deprivation contributes to impaired judgement and memory, increased risk of neurodegenerative diseases, and weakening of the immune system [43, 46, 59]. The value of proper sleep health must be emphasized now more than ever, as our present habits seem to be in complete contradiction with prioritizing sleep. You might think you are ‘surviving on five,’ but imagine what you could do if you were properly rested.
References on page 75.

by Yasmine Alami | art by Ella Manhardt
‘You ask, ‘Where do I sign in?’ You get dismissed. They’re like, ‘I’m on the phone,’ or whatever. Then you turn around for a second and you have a Caucasian that comes in and they are like, ‘Hello, how can we help you?’’ [1].
Racial bias has far-reaching effects throughout society and is especially prevalent in medical settings [2]. Documented patient experiences show us how these biases manifest for different marginalized groups [1]. When Black women were asked about their experiences with the health care system, one individual spoke about how doctors continuously ignored her symptoms, brushing past her as if she didn’t exist [1]. Other instances have occurred where Black women were told they must leave the hospital right after surgery, despite expressing their pain [1]. Similarly, a Latino man spoke out about his experience in an emergency room, where, upon arrival, he was immediately spoken to in Spanish, solely based on his Latino features [1]. Upon revealing he was a
doctor with health insurance, medical professionals surrounded him, eager to treat the sick doctor [1]. Later, he retold the story, noting how shocked the nurse was, as the ‘color on her face went completely white, like whiter than it already was’ [1]. These experiences of mistreatment, neglect, or even something as simple as an assumed narrative are derived from subconscious bias [3]. This form of discrimination is known as implicit bias and heavily impacts minorities, immigrants, women, and children [4]. Specifically, racial biases can have a profound effect on our ability to empathize with others, leading to unequal treatment from patient to patient, with racial minorities facing medical neglect, which forces them to self-advocate or chase down health care professionals in order to be treated [1,5]. Implicit bias greatly affects empathy and can be detrimental in a clinical setting, creating unequal medical treatment [5].
Empathy is the ability to understand other individuals' emotional and psychological states — to feel what another person feels [6]. However, empathy is more than just a feeling; it informs our actions and decisions [7]. When we perceive others to be in some form of distress, our empathy motivates us to perform voluntary actions to help them, creating more caring and selfless environments [6, 8]. An action performed to help another is known as a prosocial behavior and typically improves one’s own feelings of mood and self-worth [8]. A subtype of prosocial behavior includes altruistic behaviors, which are completely selfless actions [8]. Altruistic behaviors are performed expecting no personal gain, even ignoring personal consequences to perform the selfless act [9, 10]. While prosocial behaviors can be motivated by positive feelings of self-worth, altruistic behaviors may result in a similar boost in self-worth, but these feelings are not motivators behind altruistic actions [11]. Prosocial behavior and altruism are fundamental for the creation of caring environments, especially in healthcare [12]. Ultimately, when observing someone in distress, our empathy responses are ignited,
catalyzing prosocial and sometimes altruistic behaviors [6]. Therefore, as empathy is impacted by biases, so are the behaviors that are essential in helping others [13]. In a medical environment, health workers' ability to empathize with their patients is essential for providing adequate care [14]. When physicians identify with a patient’s emotional state, it enables them to see the patient as a human being and prioritize the patient’s well-being, values, and dignity [15, 16].

Empathetic responses are formed by an intricate neural mechanism composed of different neural networks within the brain [17]. These networks are complex systems made of neurons — which are specialized cells that communicate through chemical and electrical signals within the nervous system [18]. Different networks are present across the brain and have different purposes. Multiple networks of neurons reside in the somatosensory cortex, a section of the brain that processes sensory information in tandem with other brain regions, such as the amygdala and the insula [19]. While the amygdala is involved in emotional processing, the insula is involved in the perception and interpretation of social cues [19]. Specifically, the mirror neuron system, within the somatosensory cortex, is a system implicated in understanding the actions of others — allowing an individual to ‘feel what another person feels’ [20]. Activity in the mirror neuron system increases both when observing an action performed by someone else and when performing the same action [21]. Observing other people’s pain automatically activates the mirror neuron system in
the somatosensory cortex because the mirror neuron system is activated along the same networks as an individual personally experiencing pain [19]. The mirror neuron system is intricately connected to the mentalizing network, a network involved in interpreting and responding to others' thoughts, perceptions, and experiences [19, 22]. Communication between the mirror neurons within the somatosensory system and the mentalizing network allows the brain to interpret the experience of others’ pain to better understand the situation and form an appropriate response [19]. Stronger communication enables the processing of understanding other individuals, further providing the foundation of the underlying mechanisms of pain empathy [23].
The ability to empathize with pain is further contributed to other central brain regions: the anterior insula cortex (AIC) — a region involved in the integration of internal bodily sensations, emotions, and cognitive processing — and the anterior cingulate cortex (ACC) — a region implicated in pain perception and emotional regulation [24, 25, 26]. Overlapping activity in neural networks in the AIC and ACC subsequently lays a foundation for pain-based empathy, allowing individuals to consciously perceive the pain of others based on their emotional states and other cues without directly experiencing the pain themselves [24, 27]. Specifically, the AIC communicates with a subregion of the ACC known as the anterior midcingulate cortex (aMCC), which is involved in understanding information, formulating responses to these events, and empathizing with others' pain [26, 28]. However, activity in these regions is impacted by multiple factors, including subconscious bias [29].
The activation of the aMCC can be influenced by different forms of implicit biases, including ingroup and outgroup biases [29]. Individuals tend to favor people they consider part of their own social group — a behavior known as ‘ingroup bias’ [29, 30]. Ingroup bias can be shaped by social categories such as race, ethnicity, or even sports team affiliations [30]. Say you are watching your favorite soccer team, and a player on the opposing team gets injured. Since the player is part of the outgroup, you would feel less empathy towards them [31]. Whereas if a player on your team gets injured, you would feel more empathy towards them because they are part of the ingroup [30]. Ingroup bias has consequences that reach beyond sports rivalries; ingroup bias affects how we view individuals of other races [32]. ACC activity is
increased when observing pain in own-race individuals compared to other-race individuals [29]. Such bias is known as racial ingroup bias, where one views people of the same race as their ingroup — on the same team [32]. Implicit bias shapes this response depending on the situation; individuals with higher bias exhibit greater ACC activation for own-race individuals regardless of whether there is pain present [29]. The difference in ACC activation to ingroup vs. outgroup members suggests that the ACC plays a key role in empathetic processing that may also be heavily influenced by racial and other social factors [29].
It is crucial to understand that empathy is influenced by ingroup and outgroup biases, which, in clinical settings, contribute to inequalities within patient care [30]. Racial empathy is shaped by social group membership — namely, by who is perceived as an ingroup or outgroup member [30]. Social group membership influences neural activation related to empathy, where individuals have stronger neural responses for same-race pain compared to other-race pain [30]. When witnessing others in pain, people connect more deeply and feel greater emotional understanding towards same-race individuals [33]. Thus, in clinical settings, physicians’ empathetic responses are shaped by their implicit biases, potentially leading them to favor patients of the same race and provide lower-quality care to other races; such bias is seen when Black patients are prescribed insufficient pain medication by White doctors [34, 35]. Unequal treatment also can affect patient-physician communication across the board, as communication, influenced
by bias, systematically allows more engagement with White patients [29]. Implicit bias correlates with unconscious differences in treatment, decisions, and judgments that contribute to healthcare disparities [14]. Therefore, pain assessment, treatment recommendations, and prioritization of attention and care, as well as a patient's willingness to share concerns surrounding their health, are not given the same attention and care that same-race patients receive [1, 14, 36].
The impact of reduced empathy further extends to systemic disparities, creating a lack of trust between patients and physicians, causing an overall harmful psychological impact [37, 38, 39]. Systemic racism — racial inequalities built into societal structures and opportunities — is reinforced through the normalization of decreased empathy caused by racial bias, alienating minority patients who feel less empathy from providers [37, 38, 40]. Further, physicians are less likely to communicate with or understand minority patients, due to said lack of empathy, reinforcing these patterns of inequitable care [37]. In one instance, a Pakistani patient in the US often felt unwanted, claiming that ‘those [patients] who cannot speak English get into trouble, and they get a bit bullied as well’ [37]. Similar stories have been seen across the US, where minority groups felt they were treated harshly, given less priority, and experienced hostile conversations [37]. This mistreatment leads to a lack of trust between patients and health care providers [37]. Moreover, physicians who believe racial stereotypes further perpetuate this cycle of mistreatment and mistrust [41]. For example, some providers think Black patients are less likely to follow medical instructions than their White counterparts, and therefore may not provide adequate medical information to Black patients in the first place [38]. Consequently, Black patients are significantly less likely to be notified of their diagnosis than White patients [38]. Implicit bias often leads physicians to favor White patients over Black patients, which can lead to detrimental effects on the mental and physical health of minority patients [39]. Racial discrimination can be a chronic stressor, often linked to an increased risk for depression, anxiety, and psychiatric disorders [39]. Discrimination can further lead to self-condemnation and exclusion from others, causing individuals to feel on edge when they enter the patient setting as they anticipate discrimination [39]. Ultimately, health care providers’ racial and implicit bias lead to a lack of empathetic behaviors that result in not only diminished care but increased risk for additional health concerns [39].
Investigating the neural underpinnings of empathy is essential in clinical practice as it provides insight into racial disparities within healthcare [36]. Implicit bias also expands to social class, affecting the treatment of individuals across social classes [42]. In addition to pre-existing financial barriers to treatment, patients with lower socio-economic status often face lower standards of care from providers with implicit bias [42]. Bias towards people of lower socio-economic status can manifest as health care providers offering lower quality treatment or viewing low-income patients as either incapable or noncompliant with medication instructions [42]. However, because medication may be prohibitively expensive for individuals with low incomes, compliance with medical treatment is inherently unattainable [43]. Mistreatment of patients based on their low socioeconomic status also leads to lower life expectancy, as physicians often misinterpret patient needs, basing physician-patient communication on preconceived stereotypes [44]. Reduced empathy from implicit racial bias further contributes to healthcare inequality for individuals with low income, as care becomes less equitable and accessible [44, 45]. African American patients on Medicare received worse medical care than Caucasian patients on Medicare who had the same condition [45]. Low-quality care makes patients question whether they should seek medical attention, forcing them to struggle with their health issues alone — including dealing with a lack of treatment, potential disease progression, and having poor health overall [46].
The effects of racial bias expand to medical education, where Black and Latine student trainees are often averse to challenging their resident or attending, who are authoritative figures in the medical hierarchy [47]. Some students are concerned with being misjudged, having experienced microaggressions previously during their training [47]. In a severe example, one group of students had concerns about their non-Black resident, who treated a Black patient with sickle cell disease, all while the patient continued to experience pain [47]. The non-Black resident acted as though the Black patient’s pain was made up [47].
The Black and Latine students struggled with advocating for their Black patient, worried they may be at risk of discrimination themselves [47]. Furthermore, while race has no scientific basis, medical education still harbors the idea that people are biologically different based on their ethnicity [48]. This false idea continues to be used within teaching practices, perpetuating unequal treatment within the health care system [48].
Total elimination of bias within clinical settings is unrealistic [1]. However, recognizing these treatment disparities highlights the need for intervention aimed at reducing bias and improving empathetic responses in healthcare. Perspective taking, a method that has a mindfulness-based approach, allows individuals to consider how others think and feel [49]. Understanding others from a different perspective allows for an increase in empathy for individuals of other races by more strongly identifying with them, effectively reducing bias in decision-making [50]. In addition, exposure to outgroup members through interracial and sociocultural interactions — spending time with people from different racial or social groups — can also lead to stronger empathetic neural responses for pain [51]. There are many ways we are taught to discriminate; some are benign, like favoring one sports team over another, and others are far more insidious, like racial bias. There are also many ways that we can learn to overcome our biases and practice empathy for members of both ingroups and outgroups. Implementing training systems and real-world experience can help mitigate implicit biases in healthcare and move towards providing equitable care for all [5, 51].
References on page 78.


by Cailey Metter | art by Leo Mahlke
June B. Kelly is six years old. Like many children her age, she enjoys playing pretend, reading fanciful stories, and performing magic tricks for her friends and family. June’s life has changed after contracting strep throat multiple times. Following her illnesses, June’s mother noticed some concerning changes in her child. June became preoccupied with germs and contamination, causing her extreme stress. She began washing her hands excessively throughout the day, to the point of developing painful cuts and abrasions on her skin. June developed other unusual habits, such as tapping objects three times before picking them up, only eating food she knew for certain was not contaminated by germs, and flinching with uncontrollable movements. Her mother was dumbfounded, unable to think of a reason for June’s new behaviours. One day, June came home in tears after a classmate touched her sandwich during lunch. Despite her mother’s attempts to comfort her, June was inconsolable. She called June’s pediatrician to find out what was behind June’s new behaviors, and following a visit to a psychiatrist, June was diagnosed with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). June’s mother was shocked to discover that PANDAS is a rare case where a common strep infection can have long-lasting effects on the brain. June’s strep infections, like all strep infections, were caused by the Streptococcus pyogenes bacteria, which spreads through coughing or skin contact [1]. Antibiotics, such as amoxicillin or penicillin, are commonly used to treat strep infections [2]. However, in rare cases, even after antibiotic treatment, strep bacteria can leave behind physical and psychological damage that may persist throughout a child’s life [2, 3, 4]. Though June is fictional, her case is based on the true stories of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) and their known symptoms.
After June’s most recent strep infection, she began to experience symptoms comparable to obsessive-compulsive disorder (OCD), a psychological condition characterized by obsessions and compulsions [5]. Obsessions are recurring, unwanted thoughts or urges that can cause intense distress to the individual experiencing them [5]. Common obsessions include a preoccupation with germs, organization, or thoughts of harm [5]. Compulsions are ritualistic, repetitive behaviors performed in response to the
anxiety caused by an obsession [5, 6]. Compulsions can include excessive cleaning, repetitively checking or touching a surface, or fixating on the exact arrangement of objects [5, 6]. A person must have obsessive thoughts, compulsive behaviors, or both to be diagnosed with OCD [5, 7]. June personally experiences germ-related obsessions and compulsions. Her hand-washing compulsion is an attempt to soothe her contamination obsession. Additionally, she experiences uncontrollable facial and arm movements, which are comparable to tics. Tics are quick, uncontrollable movements or sounds that manifest from physical urges, while compulsions stem from a desire to soothe mental distress [8, 9, 10]. She is one of the rare cases in which a strep infection has led to a child developing tics as well as OCD symptoms [11]. June’s symptoms are distinct from OCD because they were brought on suddenly following her strep infections [12].

After June’s psychiatrist diagnosed her with PANDAS, June’s mother spent hours researching her daughter’s condition. PANDAS describes the onset or worsening of OCD-like symptoms and/or tic-related disorder symptoms in a prepubescent child following a strep infection [12]. Receiving a PANDAS diagnosis also typically requires the presence of another neurological symptom, such as excessive physical movement, impulsivity, restrictive eating, separation anxiety, or a deterioration in handwriting, which are not necessarily symptoms of OCD [11, 12, 13]. Contracting
strep multiple times increases the risk of a child developing PANDAS [14]. The physiological mechanism underlying PANDAS is still uncertain, and its symptoms overlap extensively with other neuropsychiatric disorders [15]. As a result, PANDAS remains a heavily disputed diagnosis and is an exclusionary diagnosis, meaning it is only diagnosed when no other diagnosis matches the symptoms [15, 16, 17].
The leading explanation for the symptoms observed in PANDAS is molecular mimicry [18]. Molecular mimicry is when proteins from foreign bodies, such as bacteria and viruses, resemble proteins already present in the human body [19, 20, 21]. As a result, the immune system mistakenly attacks the body’s own proteins, a phenomenon called cross-reactivity [18]. When infected by strep bacteria, a healthy immune system releases proteins called antibodies, which work to eliminate foreign pathogens [22]. In the case of PANDAS, these antibodies also cross-react with proteins in the brain that share structural similarities with the proteins on strep bacteria [23, 24]. The immune system also releases small proteins called cytokines that respond to infection by regulating inflammation, the process by which the body eliminates foreign substances, like strep bacteria [16, 25]. There is an overabundance of these cytokines, leading to more severe inflammation in the brain than is typical, which has the potential to damage healthy tissues [16, 25, 26]. Normally, a tightly regulated border of cells called the blood-brain barrier (BBB) controls which substances from the blood are allowed to enter the brain, and in this case, shields the brain from antibodies [22, 27]. In PANDAS, it is proposed that the inflammation triggers the release of a specific cytokine called IL-17, which disrupts proteins that seal the BBB [28, 29]. The damage caused by the IL-17 cytokines renders the BBB more permeable, allowing immune cells to pass through the BBB and cross-react with a part of the brain called the basal ganglia [15, 16]. The basal ganglia are a collection of brain structures associated with emotional processing and movement [24, 30]. Proteins in strep bacteria have structural similarities to a type of protein in the basal ganglia called dopamine receptors [23, 31, 32]. When strep bacteria enter the body, antibodies cross-react with dopamine receptors in the basal ganglia [11, 33]. Dopamine is a chemical in the brain that regulates motor control, motivation, and emotion [34, 35, 36]. In the proposed molecular mimicry mechanism, it is
thought that antibodies bind to dopamine receptors in the basal ganglia, leading to increased dopamine signaling [37]. Increased dopamine signaling may lead to symptoms associated with PANDAS, such as tics and emotional dysregulation [33]. While there are excellent theories on the true mechanism of PANDAS, more research is needed to know for sure which explanation is accurate [33].
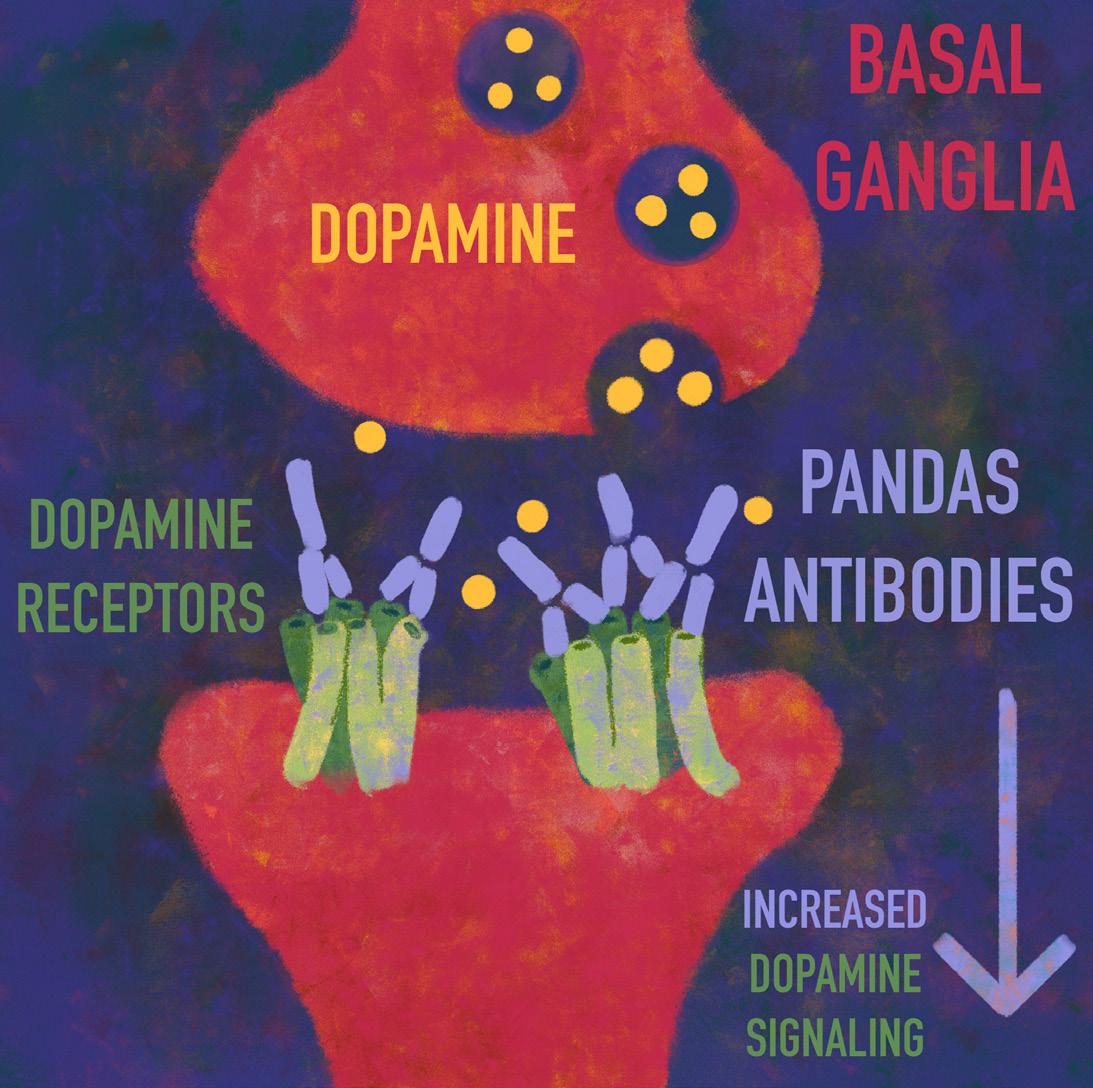
Little June may not be able to say for certain whether her OCD and tic-like symptoms are a direct result of her multiple strep infections, but either way, there are options to help relieve her symptoms. When June initially developed strep, she was treated using antibiotics [38, 39]. Another round of antibiotics successfully addressed all of June’s PANDAS symptoms for some time, and her mother was overjoyed to see her daughter feel somewhat better. Antibiotics have the potential to reduce PANDAS symptoms to a manageable level or even eliminate them [13, 40]. Unfortunately, the long-term use of antibiotics has several negative consequences, such as bacteria growing resistant to the medication, thereby causing the antibiotic treatment to lose efficacy [40, 41, 42]. June’s mother needed to find a different treatment for her daughter. One alternative is intravenous immunoglobulins (IVIG), where blood is infused with thousands of healthy donor antibodies, reducing inflammation and increasing immunity to illness [43, 44]. IVIG appears
to have the strongest long-term success in treating PANDAS symptoms, despite its invasive nature [45, 46]. Though the long-term results of IVIG can be unreliable, as PANDAS symptoms tend to wax and wane over time [46]. IVIG has been shown to reduce the symptoms of children with PANDAS drastically, but June’s mother was hesitant to put her daughter through IVIG treatment as it is underresearched [47]. Distressed, June’s mother turned to exposure and response prevention (ERP) therapy to help her child find peace again.
For many people with PANDAS, behavioral therapy helps to alleviate symptoms [13]. ERP is a common treatment for the OCD-like symptoms associated with PANDAS [13]. In ERP, individuals are placed in situations that trigger their compulsions and asked to resist engaging in their desired action [13, 48]. For example, an individual with a contamination obsession may be instructed to touch something they perceive as ‘dirty’ and not wash their hands immediately afterward [49]. Many people are reluctant to engage in ERP since it deliberately places them in stressful situations, and the individual’s reluctance usually depends on the severity of their OCD [13]. June did her best to follow her ERP therapist's instructions, but when she was asked to touch a garbage can and not wash her hands afterwards, she was too terrified to complete the task. June was making little progress in ERP therapy, and she begged her mother to find another course of treatment. For individuals with PANDAS who develop tic-related symptoms, a common behavioral treatment is habit reversal training, where doctors encourage individuals to identify when a tic is about to occur and then engage in a voluntary action to suppress it [13, 50]. There are many ways to treat the symptoms of PANDAS individually, and the correct course of treatment depends on symptom severity and one’s individual needs [13]. Though ERP is effective for many individuals with PANDAS, the discomfort of being placed in stressful situations regularly ultimately proved too much for June, and she was not able to complete ERP treatment. June’s mother would have to find another course of treatment for her daughter.
Having exhausted clinical treatment options, which were either ineffective or made June too distressed to continue, June’s mother decided to have her try a different approach involving herbs and vitamins. Approximately 40% of individuals with OCD end up dissatisfied with outcomes using traditional medicine, and so they may turn to herbal medicine or vitamin supplements as a result [51]. Although more research
is needed on these alternative forms of medicine, individuals seek out herbal treatments due to their less severe side effects [52, 53]. For example, some tic-disorder pharmaceuticals have negative side effects such as weakness, stomach problems, and cognitive issues, depending on the prescribed medication [54, 55]. Many people do not see a benefit from using pharmaceuticals to treat their OCD [56]. Antidepressants that are used to treat OCD can have negative side effects such as anxiety, aggression, low blood sodium levels, and restlessness [13, 56]. There is some evidence to indicate that herbal medicine and vitamin supplements may effectively relieve symptoms for people with PANDAS [57]. For instance, individuals with severe PANDAS-related symptoms tend to have lower levels of vitamin D, which is important to the functioning of the immune system and metabolic pathways [57, 58, 59]. Increasing a child’s intake of vitamin D through increased sun exposure, certain foods, or supplements may decrease the severity of neuropsychiatric PANDAS symptoms [57, 60]. People with OCD may also have lower levels of vitamin B12, which helps the nervous system function properly, plays a part in metabolism, and creates red blood cells and DNA [58, 59, 61]. Due to vitamin B12’s many roles, especially its function in the nervous system, increased vitamin B12 intake could improve symptoms of OCD when used with some other form of treatment, such as an antidepressant [59, 61, 62]. Once June’s mother thoroughly researched the potential role of vitamins in OCD and PANDAS,

she made sure her daughter got enough sunlight and had a healthy breakfast each day consisting of meat, eggs, and a supplement of cod liver oil to increase her daughter’s intake of vitamin D and vitamin B12 [63, 64].
Through further research, June’s mother found another alternative to pharmaceuticals for the treatment of June’s OCD-like symptoms: saffron [65, 66]. Saffron is a spice used in herbal medicine, and it can function similarly to pharmaceutical treatments for OCD [65, 67]. There are also potential herbal treatments for tic-related disorders [68]. One promising candidate is 5-Ling Granule, a patented herbal medicine with fewer side effects than traditional psychiatric medications [68]. 5-Ling Granule is essentially an herbal cocktail made from 11 different herbs commonly used in Chinese medicine [68]. The herbs in the medicine can subdue emotional hyperactivity, have sedative qualities for excessive movement, and help to combat insomnia, among other functions [68]. Other traditional Chinese medicines have also been used to treat tic-related disorders, including Bai Shao — white peony root — and Fu Ling — a medicinal fungus [53, 69, 70]. Using herbs to try to decrease PANDAS symptoms could be a valid option for parents concerned about the side effects of pharmaceuticals for their children [52].
Following a combination of pharmaceutical and herbal treatments, June saw a reduction in her PANDAS symptoms. June was now finally able to play tag at recess with her best friends without obsessing over the thought of being contaminated by germs. PANDAS remains an unexplored disorder that affects the availability of long-term treatment options [46, 71]. Due to the lack of research around the disorder, many people affected by PANDAS have grown frustrated [46]. Some have created support groups for people with PANDAS and their loved ones, including the PANDAS Network in the United States and Canada and the PANDASHELP organization [46]. June may now have decreased PANDAS symptoms, but she still has a disorder that she must endure throughout her life. Although June experienced a decrease in her symptoms, the same can not be said for all children with the PANDAS disorder. With time, PANDAS symptoms can increase in severity and may be related to increases in suicidal ideation, but further research and more treatment options could lead to better
outcomes for individuals with PANDAS [72]. Although PANDAS is currently understudied, the future may be a beacon of hope for June and others like her if time and materials are devoted to studying PANDAS.
References on page 81.

by Jaron Ezekiel | art by Sarah McDonald

Do you think you could tell whether you were conversing with an artificial intelligence (AI) or a human? In 1950, mathematician Alan Turing proposed the Turing Test, a hypothetical assessment of the intelligence of AI [1]. In the test, an observer reads through a conversation between an AI and another human [1]. If they cannot tell which conversant is the real person, then the AI is considered to have achieved human-like intelligence [1]. While the Turing Test remains controversial as a measure of intelligence, some find that AI can now pass what was once considered a benchmark only achievable by human intelligence [2]. But how does human-like intelligence come to be? It seems to be made possible by biological neural networks (BNNs), which are collections of interconnected neurons — the basic cell units of the brain [3]. Neurons extend throughout our entire bodies, from head to toe, comprising the complex information-processing systems collectively known as the nervous system [4]. Working together, neurons allow eyes to see, tongues to taste, and fingers to feel. Using the nervous system as a blueprint,
AI designs have strived to imitate the structure of connected units through nodes, which serve as analogs to neurons [5, 6, 7, 8, 9]. The story began in the 1940s, when researchers built the first mathematical model of a neuron, showing that simple units could interact to perform logical reasoning [5]. These types of AI, in which connected units process inputs to produce outputs, are called artificial neural networks (ANNs) — a term inspired by their similarities to BNNs — and are the systems that underlie leading AI models, such as ChatGPT, Gemini, and Siri [3]. However, ANNs are fundamentally different from BNNs in numerous ways. ANNs are far less efficient and have distinct structures and activation mechanisms, but one of their most significant differences is how they learn new information [10, 11]. The fundamental differences in learning mechanisms between ANNs and BNNs demonstrate that ANNs function more as loose analogies than as accurate models of biological cognition.
To fully understand the similarities and differences between BNNs and ANNs, we first must grasp their basic anatomy and how they function and learn. In a broad sense, both ANNs and BNNs function through a structure of interconnected units that contribute to the output produced [12]. However, upon closer examination, an overwhelming number of differences emerge [12]. While BNNs and their units are physical structures that have evolved over more than half a billion years to process and react advantageously to their environment, ANNs are virtual, human-made structures whose primary goal is not to survive, but instead to perform specific, preconfigured tasks [12, 13, 14]. BNNs are composed of up to hundreds of billions of neurons, which support information processing and are interwoven in elaborate ways that are not yet fully understood [12]. ANNs, on the other hand, are composed of layers of up to billions of virtual nodes that use mathematical functions to pass signals from layer to layer [3]. Each connection between nodes has an associated weight depending on its importance and projected influence on the output.
The number, organization, and unique connections of units in BNNs and ANNs play a large role in determining the degree of complexity to which the overall network can process and respond. [15, 16]. For example, the simplest organisms with sensory systems have neuronal counts ranging into the hundreds and are limited to basic survival instincts [17]. However, complex organisms like humans can have billions of neurons, fostering elaborate thought and learning [17]. Similarly, ANNs are limited by the number and degree of interconnectedness of their nodes [11]. For example, a simple ANN designed to recognize handwritten numbers may contain around 450 nodes, whereas a more complicated ANN, such as the one underlying ChatGPT, may contain trillions of nodes [18]. Although these individual units are meaningless devoid of the larger structure, in combination, they can produce an output that is both complex and substantive [19].
Despite the similarity in general structure between ANNs and BNNs, the actual mechanisms involved in the activation of these two networks differ significantly [3]. At the most basic level, neurons in BNNs are ‘all or nothing,’ meaning that they cannot be partially activated — they either send a signal or they do not [20]. The process of sending a signal from one neuron to the next is called neurotransmission, and a neuron will only fire when the combined incoming
signals push it past its threshold potential [21]. Additionally, when a neuron fires, the signal it sends is always the same strength. [21]. On the other hand, ANNs have no equivalent to threshold potentials and do not follow an ‘all or nothing’ principle [11]. Instead, most ANNs involve every node sending a signal to all of its connected nodes, and the degree of the signal is subject to fluctuation [11]. A node's outgoing signal strength is dependent on that of the prior nodes in the network [11]. The actual calculation of how much one node affects another and the value, or signal strength, that node will pass on is highly complicated and involves complex mathematical functions [22]. The mechanism of ANNs differs significantly from the ‘all or nothing’ signaling of BNNs, and is one key difference between the two types of networks [11].
Another difference between ANNs and BNNs is unit connectivity and the structural organization of the connections [23, 24]. In BNNs, each neuron is connected to only a small fraction of the other neurons in the system [23]. In most ANNs, however, each node in a given layer is connected to every other node in the layers next to it [11]. ANN nodes are organized neatly into three layers: input, hidden, and output [24]. BNNs, specifically brains, are much more intricate and not as neatly partitioned [25]. The brain is roughly sectioned into areas of specialized function, but this specialization is flexible, and the boundaries between functional regions are fuzzy [25]. That being said, certain types of information flow in predictable neural pathways, as is the case with the sensory processing of visual and auditory stimuli [14, 26].
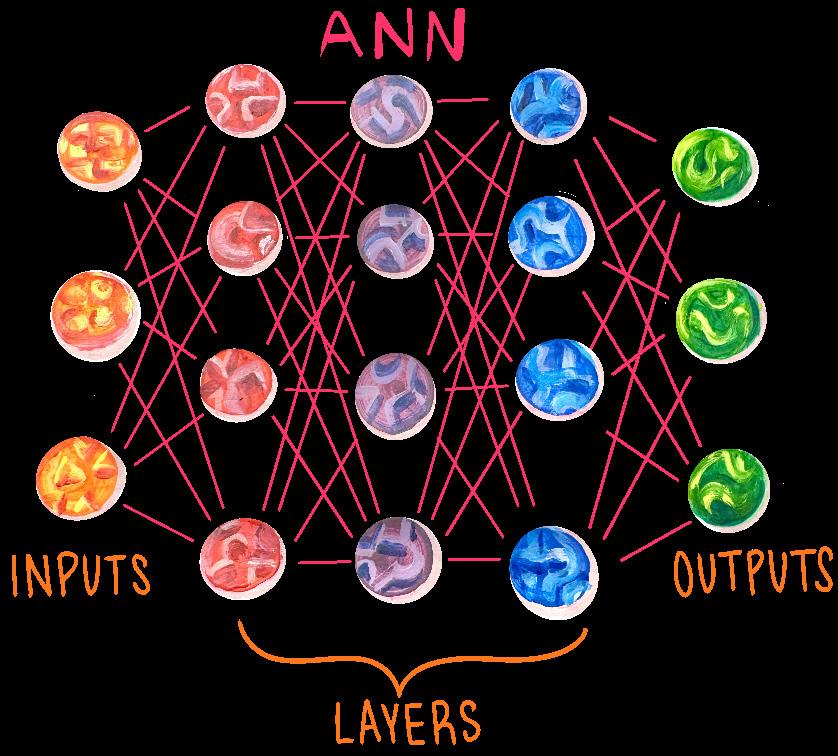
The best way to break down the structure of an ANN is with an example. Let’s say you get curious and ask ChatGPT, ‘What’s the capitol of France?’ The first thing ChatGPT does is break down the prompt using its first layer of nodes — the input layer [27, 28, 29]. Each node of an input layer represents some detail about the input, typically text, images, or audio [30]. If the input is an image, then input nodes may represent individual pixels. Many ANNs rely on a process called tokenization to break the larger input up into smaller, more manageable data [27, 28]. In the case of written text, the input nodes are tokenized into smaller building blocks of text [27]. For example, the word ‘What’s’ is broken down — tokenized — into two tokens, ‘What’ and ‘’s’.
The main reason ANNs use tokenization is due to the sheer amount of storage required to process every version of a word as a separate unit [28]. While babies only need a couple of ounces of food to learn up to ten new words per day, training the most recent version of ChatGPT required the energy equivalent of multiple neighborhoods’ annual energy consumption [10, 31]. Thus, maximizing energy and storage efficiency through tokenization is vital to the performance of an ANN [10]. If you tell an ANN, ‘rewrite my lab report, but make it more science-y’, the network uses the pattern ‘[word]-y’ as a set of tokens meaning ‘like that word’. Hence, AI neural networks do not process ‘science-y’ as a term meaning ‘in a scientific way’ — the ANN does not have ‘science-y’ saved with its meaning. As such, tokenization can both help an ANN save energy and deal with words it has never encountered before [28]. Interestingly, children perform a process similar to tokenization when learning to speak [32]. If you have ever heard a toddler say ‘goed’ instead of ‘went,’ or ‘gooder’ instead of ‘better,’ it is because they have recognized patterns in language before they have learned the accurate vocabulary [32]. Similarly, ChatGPT will see that the word ‘capitol’ in the prompt is almost certainly a typo, and that the user probably meant ‘capital.’ By utilizing tokenization, ChatGPT can determine how to respond accurately from context clues rather than having an infinite internal dictionary.
Once the prompt has been tokenized into chunks that ChatGPT can digest, the processing moves into the second part of the network: the hidden layers [33]. Unlike the input layer, any one node in these hidden layers does not explicitly represent any decipherable information, hence the name ‘hidden’
[34]. The meaning in the hidden layers emerges from an intricate interaction of nodes that activate with varying intensities [3]. Every ANN has specific activation patterns that each correlate with a concept [35]. There is no specific node that corresponds to ‘Paris,’ but rather a certain pattern of activation [35]. It would be impossible to look at a node and understand exactly what it ‘means,’ because individual nodes do not carry specific meanings [34]. We cannot understand what a house looks like by staring at a single brick; a house looks like a house because all of its parts have been arranged together in a particular way. In the hidden layers, ChatGPT does not just look up the capital of France. Instead, it recognizes a relationship between ‘Capital of X’ and a country-city pair, and then looks for the strongest match for the country-city pair whose country is France: Paris. In doing so, its nodes activate in a seemingly patternless arrangement that collectively correlates to the concept of ‘Paris.’ You can think of the activation pattern as the pattern of ridges on a key, while the respective concept is the door that the key opens. Each ridge in the key has a different height, like the various intensities of individual nodes, but any one ridge is not enough to open the door, or even to know which door the key will open. You need to look at the pattern the ridges make, similar to how you need to look at the activation pattern of the whole ANN. Finally, once the ANN has done its ‘thinking’ in the hidden layers, the last step is to convert its findings into a digestible form for the human user, which is accomplished by the final section of nodes: the output layer [11]. The nodes of the output layer, like the input layer, have explicit meanings, typically in the form of tokens or words [36]. In this example, ChatGPT may convert all its computations into tokens and then sequentially link them together to create the final output: ‘The capital of France is Paris.’
Let’s say you trust ChatGPT and commit to memory that Paris is the capital of France. How exactly does this process of remembering something work? What modifications occur in a BNN, such as your brain, that allow for the storage of this fact and its retrieval later on? The truth is that we just don’t know [20]. We have discovered some of the neural mechanisms involved, but how they give rise to the full process of remembering a new fact or learning a new skill remains unknown [20]. That being said, it is widely accepted that learning is made possible through neuroplasticity: the brain’s ability to create, remove, strengthen, and
weaken the connections between its neurons, called synapses [37]. Neuroplasticity is achieved through a principle called Hebbian learning, summed up by the phrase, ‘cells that fire together, wire together’ [37]. Importantly, the converse is also true: cells that do not fire together do not wire together [12]. If a presynaptic neuron, the neuron sending the signal, and a postsynaptic neuron, the neuron receiving the signal, fire together repeatedly, their connection is strengthened, and the postsynaptic neuron becomes more responsive to signals from the presynaptic neuron [37]. This phenomenon is known as long-term potentiation (LTP) [38]. Long-term depression (LTD), on the other hand, is the weakening of connections between neurons when they do not fire together [39]. Some theories suggest that the Hebbian learning model — including both LTP and LTD — alone enables learning, while other theories suggest that it is the first step in a long chain of neural events [20]. Regardless, it is accepted that the strengthening and weakening of synapses is a crucial piece of the learning puzzle [40]. Another neural mechanism associated with learning is neurogenesis: the birth of new neurons [41]. Some evidence suggests that new neurons are constantly created in the adult brain, but that they die unless they are integrated into existing neural pathways [41]. Learning, especially through conscious effort, seems to be the way that these new neurons are integrated and thus rescued from death [41]. Although the exact mechanisms behind learning are debated, some aspects, such as neuroplasticity, Hebbian learning, and neurogenesis, are widely recognized as essential factors [20, 42].

A deeper understanding of the mechanisms behind learning gives us a better picture of how an organism modifies its future actions appropriately. To do this, organisms rely on feedback from chemical messengers called neuromodulators to decide whether or not to repeat an action [43]. Neuromodulators have widespread effects across large regions of the brain, impacting many neurons simultaneously [43]. As far as we can tell, the conscious experience of emotions is largely due to the release of neuromodulators within the brain [44]. Learning, in which feedback, such as emotions, informs future decisions, is called reinforcement learning [45]. When a curious toddler touches a lit stove, they will recoil in pain. Neurochemically speaking, this happens because touching a hot object sends signals from heat sensors in the toddler’s fingers to the brain that stimulate the release of chemicals that make the toddler feel pain [46]. Hebbian learning comes into play here by helping the toddler learn to associate a hot stove with pain [47]. While Hebbian learning may be responsible for the neural association between touching the stove and the pain signal, this will not actually change the toddler’s future behavior [48]. The toddler has to feel the pain to learn not to produce that behavior again [48]. In this way, Hebbian learning establishes the association, and reinforcement learning teaches the toddler that this association is bad and thus the action should not be repeated [48, 49].
Unlike a toddler touching a hot stove, ANNs cannot feel whether their action was ‘right’ or ‘wrong’ [50]. There are no chemicals that produce the feeling of being rewarded or punished in the world of computers. Instead, there are many other methods of ANN learning. The two most popular are supervised and unsupervised learning [50]. To demonstrate supervised learning, imagine ChatGPT is still in training when it’s asked, ‘What’s the capital of France?’ If it responds with ‘Paris,’ great. However, since ChatGPT is still being trained, it will likely instead come up with an incorrect answer, like ‘London,’ or perhaps just plain gibberish. The important catch with supervised learning is that the ANN is corrected by an external agent [51]. Once the correct answer is provided, the ANN can update its node weights, or the connection between nodes that depends on the importance and projected influence on the output, to be slightly more accurate next time [11, 51]. The most widespread learning mechanism in ANNs is the backpropagation algorithm, which updates connection weights after receiving feedback in order to
increase response accuracy [52]. If ChatGPT responds with ‘London,’ then the backpropagation algorithm will backtrack through the network by starting with the output layer, and assessing how much each node contributed to the incorrect answer, and then adjusting their weights to minimize the future prediction of ‘London,’ while maximizing the future prediction of ‘Paris’ [52]. Interestingly, there is no known learning mechanism equivalent to backpropagation in BNNs [13]. In other words, the primary method that ANNs use to learn is absent in nature as far as we can currently tell. Instead, the major learning mechanisms employed in nature seem to be local — that is, a neuron firing will only affect the connections between that neuron and imme diately subsequent neu rons [53]. Unlike a tod dler learning the valuable lesson of not touching a lit stove through just one experience, backpropa gation must be repeated a vast number of times to reliably minimize error [8, 54].
In unsupervised learn ing, the ANN still produc es outputs, but it is not subsequently corrected [51]. Unsupervised learning usually involves finding patterns in large amounts of data, such as classifying faces or predicting words [51]. In some ways, unsupervised learning is similar to the learning of BBNs [55]. For the most part, babies learn to recognize words, faces, sounds, and objects, often without anyone explicitly labeling them [55]. Hearing the word ‘apple’ when an apple is present may teach a baby that the sound ‘apple’ means the shiny red fruit in front of them [55]. This connection of the word ‘apple’ to the physical object, flavor, and scent is made possible by Hebbian learning [37]. However, unsupervised learning in ANNs lacks the physical nature of biological learning and is far more specialized than the learning of a toddler who must navigate the complexities of both the physical and emotional world [51]. In its initial training phase, ChatGPT was left unsupervised to predict ensuing words by identifying patterns across a vast number of books, websites, and articles, ultimately processing hundreds of billions of tokens over the span of several months [29].
Certain features of BNNs, such as their impressive adaptability and efficiency, make them a useful model for the future growth and development of many kinds of ANNs [19]. Recent advancements have further bridged the gap between ANNs and BNNs. For example, one biological factor that differentiates the two is that during development, there is a genetic constraint; the genome — an organism’s complete genetic material — is nowhere near complex enough to encompass all the intricacies of the brain, so this information must somehow be condensed [23, 56].

To mirror this biological constraint in their ANN, researchers introduced ‘innate,’ pretrained wiring to their ANN, and this ANN performed exceptionally well, even before the regular training phase [23]. Like a giraffe being able to walk almost immediately after being born, this ANN seemingly came into the world with predisposed capacities, namely to classify images and recognize patterns in
Other ANN models have bridged the gap to BNNs using different methods. Spiking Neural Networks (SNNs), an exciting branch of ANNs, have risen in popularity because of their biological plausibility: they mirror neural signaling much more closely than other ANNs, and they rarely use backpropagation [57, 6]. SNNs are also more energetically efficient than other ANNs, further minimizing the energy disparity between ANNs and BNNs [6, 58]. Other models have been able to approximate backpropagation-like learning solely with Hebbian learning mechanisms [9]. Using local weight adjustments, one ANN model was able to learn solely via Hebbian learning in a manner very similar to using backpropagation [9]. Liquid Neural Networks, another branch of ANNs, can constantly reconfigure themselves by changing the mathematical equations behind their weights, somewhat resembling the adaptive nature of BNNs [59]. Regardless, ANN learning has a long way to go before it can accurately mirror the energy consumption, mechanisms, and adaptability of BNN learning [60, 61].
Insights into how brains work have been instrumental in developing more accurate and efficient ANNs [62]. But what about the reverse: what can ANNs teach us about the human mind? For one, contemporary ANN models demonstrate that information can be stored and retrieved in the organization of connected units [19]. They support the idea that knowing a concept does not mean having a neuron in your brain that explicitly codes for that concept, but rather an activation pattern of interconnected neurons that collectively represent the concept [35]. By highlighting this parallel, the development of ANNs has given us valuable insight into biological pattern recognition as well as language processing. Although it may seem intuitive to us today, the notion that organisms process information with many individual units working together was not a mainstream viewpoint until the first AI system was proposed in the 1940s [63]. That being said, AI is still in its infancy compared to its biological counterparts. While BNN learning involves neuroplasticity, Hebbian mechanisms, and emotional feedback, ANNs learn mathematically, primarily through backpropagation — a process biology has never been known to use [12, 13, 14]. The differences between the two kinds of networks are striking, and they demonstrate that, no matter how human an ANN may seem in conversation or how well it appears to pass the Turing Test, many argue its learning and functioning remain fundamentally unlike those of our brains [12].
References on page 84.

1. Lu, X., Xie, Q., Pan, X., Zhang, R., Zhang, X., Peng, G., Zhang, Y., Shen, S., & Tong, N. (2024). Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduction and Targeted Therapy, 9(1). doi:10.1038/s41392-024-01951-9.
2. Galicia-Garcia, U., Benito-Vicente, A., Jebari, S., Larrea-Sebal, A., Siddiqi, H., Uribe, K. B., Ostolaza, H., & Martín, C. (2020). Pathophysiology of type 2 diabetes mellitus. International Journal of Molecular Sciences, 21(17), 6275. doi:10.3390/ijms21176275.
3. Holst, J. J. (2019). The incretin system in healthy humans: The role of GIP and GLP-1. Metabolism: Clinical and Experimental, 96, 46–55. doi:10.1016/j.metabol.2019.04.014.
4. Nowell, J., Blunt, E., & Edison, P. (2023). Incretin and insulin signaling as novel therapeutic targets for Alzheimer’s and Parkinson’s disease. Mol Psychiatry, 28, 217–229. doi:10.1038/s41380-022-01792-4.
5. Shendurse, A. M., & Khedkar, C. D. (2016). Glucose: Properties and analysis. Encyclopedia of Food and Health, 239–247. doi:10.1016/b978-0-12-3849472.00353-6.
6. Zheng, Z., Zong, Y., Ma, Y., Tian, Y., Pang, Y., Zhang, C., & Gao, J. (2024). Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduction and Targeted Therapy, 9, 234. doi:10.1038/ s41392-024-01931-z.
7. Salehi, M., & Purnell, J. Q. (2019). The role of glucagon-like peptide-1 in energy homeostasis. Metabolic Syndrome and Related Disorders, 17(4). doi:10.1089/ met.2018.0088.
8. Drucker, D. J. (2018). Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabolism, 27(4), 740-756. doi:10.1016/j.cmet.2018.03.001.
9. Lovinger, D. M. (2008). Communication networks in the brain. Alcohol Research and Health, 31(3), 196-214. PMID: 23584863.
10. Karpińska, M., & Czauderna, M. (2022). Pancreas—its functions, disorders, and physiological impact on the mammals’ organism. Frontiers in Physiology, 13. doi:10.3389/fphys.2022.807632.
11. Röder, P. V., Wu, B., Liu, Y., & Han, W. (2016). Pancreatic regulation of glucose homeostasis. Experimental & Molecular Medicine, 48. doi:10.1038/emm.2016.6.
12. Rix, I., Nexøe-Larsen, C., Bergmann, N. C., Lund, A., & Knop, F. K. (2019, July 16). Glucagon Physiology. National Library of Medicine; MDText.com, Inc. https:// www.ncbi.nlm.nih.gov/books/NBK279127/
13. Brady, V., Whisenant, M., Wang, X., Ly, V. K., Zhu, G., Aguilar, D., & Wu, H. (2022). Characterization of symptoms and symptom clusters for type 2 diabetes using a large nationwide electronic health record database. Diabetes Spectrum, 35(2), 159–170. doi:10.2337/ds210064.
14. Farmaki, P., Damaskos, C., Garmpis, N., Garmpi, A., Savvanis, S., & Diamantis, E. (2020). Complications of the type 2 diabetes mellitus. Current Cardiology Reviews, 16(4), 249–251. doi:10.2174/157340 3X1604201229115531.
15. Nauck, M. A., & Meier, J. J. (2016). The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. The Lancet, Diabetes & Endocrinology, 4(6), 525–536. doi:10.1016/S22138587(15)00482-9.
16. Wachsmuth, H.R., Weninger, S.N. & Duca, F.A. (2022). Role of the gut–brain axis in energy and glucose metabolism. Experimental and Molecular Medicine, 54, 377–392. doi:10.1038/s12276-021-00677-w.
17. Green, C., Zaman, V., Blumenstock, K., Banik, N. L., & Haque, A. (2025). Dysregulation of metabolic peptides in the gut-brain axis promotes hyperinsulinemia, obesity, and neurodegeneration. Biomedicines, 13(1), 132. doi:10.3390/biomedicines13010132.
18. Adamu, A., Li, S., Gao, F., & Xue, G. (2024). The role of neuroinflammation in neurodegenerative diseases: current understanding and future therapeutic targets. Frontiers in Aging Neuroscience, 16. doi:10.3389/ fnagi.2024.1347987.
19. Cheataini, F., Ballout, N., & Al Sagheer, T. (2023). The effect of neuroinflammation on the cerebral metabolism at baseline and after neural stimulation in neurodegenerative diseases. Journal of Neuroscience Research, 101, 1360–1379. doi:10.1002/jnr.25198.
20. DeLozier, S. J., & Davalos, D. (2015). A systematic review of metacognitive differences between Alzheimer’s disease and frontotemporal dementia. American Journal of Alzheimer’s Disease & Other Dementias, 31(5), 381-388. doi:10.1177/1533317515618899.
21. Sadigh-Eteghad, S., Sahebari, S. S., & Naseri, A. (2020). Dementia and COVID-19: complications of managing a pandemic during another pandemic. Dementia & Neuropsychologia, 14(4), 438–439. doi:10.1590/198057642020dn14-040017.
22. Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., Cummings, J., & van der Flier, W. Alzheimer's disease. (2021). The Lancet, 397(10284), 1577-1590. doi:10.1016/S01406736(20)32205-4.
23. Singer, M. E., Dorrance, K. A., Oxenreiter, M. M., Yan, K. R., & Close, K. L. (2022). The type 2 diabetes 'modern preventable pandemic' and replicable lessons from the COVID-19 crisis. Preventive Medicine Reports, 25. doi:10.1016/j.pmedr.2021.101636.
24. McGirr, S., Venegas, C., & Swaminathan, A. (2020). Alzheimer's disease: a brief review. Journal of Experimental Neurology, 1(3), 89-98. doi:10.33696/Neurol.1.015.
25. Zhang, X., Wang, J., Zhang, Z., & Ye, K. (2024). Tau in neurodegenerative diseases: molecular mechanisms, biomarkers, and therapeutic strategies. Translational Neurodegeneration, 13. doi:10.1186/s40035-024-004296.
26. Jha, M. K., & Morrison, B. M. (2020). Lactate transporters mediate glia-neuron metabolic crosstalk in homeostasis and disease. Frontiers in Cellular Neuroscience, 14. doi:10.3389/fncel.2020.589582.
27. Liu, L., MacKenzie, K. R., Putluri, N., Maletić-Savatić, M., & Bellen, H. J. (2017). The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metabolism, 26(5), 719-737. doi:10.1016/j. cmet.2017.08.024.
28. Yu, L., Jin, J., Xu, Y., & Zhu, X. (2022). Aberrant energy metabolism in Alzheimer's disease. Journal of Translational Internal Medicine, 10(3), 197–206. doi:10.2478/ jtim-2022-0024.
29. Bharadwaj, P., Wijesekara, N., Liyanapathirana, M., Newsholme, P., Ittner, L., Fraser, P., & Verdile, G. (2017). The link between type 2 diabetes and neurodegeneration: roles for amyloid-ß, amylin, and tau proteins. Journal of Alzheimer's Disease, 59(2), 421–432. doi:10.3233/JAD-161192.
30. Pruzin, J. J., Nelson, P. T., Abner, E. L., & Arvanitakis, Z. (2018). Review: relationship of type 2 diabetes to human brain pathology. Neuropathology and Applied Neurobiology, 44(4), 347–362. doi:10.1111/nan.12476.
31. Arnold, S. E., Arvanitakis, Z., Macauley-Rambach, S. L., Koenig, A. M., Wang, H. Y., Ahima, R. S., Craft, S., Gandy, S., Buettner, C., Stoeckel, L. E., Holtzman, D. M., & Nathan, D. M. (2018). Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nature Reviews Neurology, 14(3), 168–181. doi:10.1038/nrneurol.2017.185.
32. Li, T., Jiao, J. J., Hölscher, C., Wu, M. N., Zhang, J., Tong, J. Q., Dong, X. F., Qu, X. S., Cao, Y., Cai, H. Y., Su, Q., & Qi, J. S. (2018). A novel GLP-1/GIP/Gcg triagonist reduces cognitive deficits and pathology in the 3xTg mouse model of Alzheimer's disease. Hippocampus, 28(5), 358–372. doi:10.1002/hipo.22837.
33. Gejl, M., Gjedde, A., Egefjord, L., Møller, A., Hansen, S. B., Vang, K., Rodell, A., Brændgaard, H., Gottrup, H., Schacht, A., Møller, N., Brock, B., & Rungby, J. (2016). In Alzheimer's disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: Randomized, placebo-controlled, double-blind clinical trial. Frontiers in Aging Neuroscience, 8, 108. doi:10.3389/fnagi.2016.00108.
34. Kalinderi, K., Papaliagkas, V., & Fidani, L. (2024). GLP-1 receptor agonists: A new treatment in Parkinson's disease. International Journal of Molecular Sciences, 25(7), 3812. doi:10.3390/ijms25073812.
35. Costa, K. M., & Schoenbaum, G. (2022). Dopamine. Current Biology, 32(15). doi:10.1016/j.cub.2022.06.060.
36. Zhou, Z.D., Yi, L.X., Wang, D.Q., Lim, T. M., & Tan, E. K. (2023). Role of dopamine in the pathophysiology of Parkinson’s disease. Translational Neurodegeneration, 12. doi:10.1186/s40035-023-00378-6.
37. Dorsey, E. R., Sherer, T., Okun, M. S., & Bloem, B. R. (2018). The emerging evidence of the Parkinson pandemic. Journal of Parkinson's Disease, 8. doi:10.3233/ JPD-181474.
38. Mahlknecht, P., & Poewe, W. (2024). Pharmacotherapy for disease modification in early Parkinson's disease: How early should we be?. Journal of Parkinson's Disease, 14. doi:10.3233/JPD-230354.
39. Lv, D., Feng, P., Guan, X., Liu, Z., Li, D., Xue, C., Bai, B., & Hölscher, C. (2024). Neuroprotective effects of GLP-1 class drugs in Parkinson's disease. Frontiers in Neurology, 15. doi:10.3389/fneur.2024.1462240.
40. Spezani, R., & Mandarim-de-Lacerda, C. A. (2022). The current significance and prospects for the use of dual receptor agonism GLP-1/glucagon. Life Sciences, 288. doi:10.1016/j.lfs.2021.120188.
41. Fukuda, M. (2021). The role of GIP receptor in the CNS for the pathogenesis of obesity. Diabetes, 70(9), 1929–1937. doi:10.2337/dbi21-0001.
42. Liu, Q. K. (2024). Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Frontiers in Endocrinology, 15. doi:10.3389/ fendo.2024.1431292.
43. Feng, P., Zhang, X., Li, D., Ji, C., Yuan, Z., Wang, R., Xue, G., Li, G., & Hölscher, C. (2018). Two novel dual GLP-1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson's disease. Neuropharmacology, 133, 385–394. doi:10.1016/j.neuropharm.2018.02.012.
44. Rizvi, A. A., & Rizzo, M. (2022). The emerging role of dual GLP-1 and GIP receptor agonists in glycemic management and cardiovascular risk reduction. Diabetes, Metabolic Syndrome and Obesity, 15, 1023–1030. doi:10.2147/DMSO.S351982.
45. Zhang, L., Zhang, L., Li, Y., Li, L., Melchiorsen, J. U., Rosenkilde, M., & Hölscher, C. (2020). The novel dual GLP-1/GIP receptor agonist DA-CH5 is superior to single GLP-1 receptor agonists in the MPTP model of Parkinson's disease. Journal of Parkinson's Disease, 10(2), 523–542. doi:10.3233/JPD-191768.
46. Petersen, M. C., & Shulman, G. I. (2018). Mechanisms of insulin action and insulin resistance. Physiological Reviews, 98(4), 2133–2223. doi:10.1152/physrev.00063.2017.
1. Fisher, R. S., Cross, J. H., French, J. A., Higurashi, N., Hirsch, E., Jansen, F. E., Lagae, L., Moshé, S. L., Peltola, J., Roulet Perez, E., Scheffer, I. E., & Zuberi, S. M. (2017). Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia, 58(4), 522–530. https://doi.org/10.1111/ epi.13670
2. Stafstrom, C. E., & Carmant, L. (2015). Seizures and Epilepsy: an Overview for Neuroscientists. Cold Spring Harbor Perspectives in Medicine, 5(6), a022426–a022426. https://doi.org/10.1101/cshperspect.a022426
3. Eilbert, W., & Chan, C. (2022). Febrile seizures: A review. Journal of the American College of Emergency Physicians Open, 3(4). https://doi.org/10.1002/emp2.12769
4. Tiwari, A., Meshram, R. J., & Kumar Singh, R. (2022). Febrile Seizures in children: a Review. Cureus, 14(11). https://doi.org/10.7759/cureus.31509
5. Faber, D. S., & Pereda, A. E. (2018). Two Forms of Electrical Transmission Between Neurons. Frontiers in Molecular Neuroscience, 11. https://doi.org/10.3389/fnmol.2018.00427
6. Raghavan, M., Fee, D., & Barkhau, P. (2019). Generation and propagation of the action potential. In K. Levin & P. Chauvel (Eds.), Clinical neurophysiology : basis and technical aspects (pp. 3–22). Amsterdam Elsevier.
7. Khadria, A. (2022). Tools to measure membrane potential of neurons. Biomedical Journal, 45(5), 749–762. https://doi.org/10.1016/j.bj.2022.05.007
8. Drukarch, B., & Micha M.M. Wilhelmus. (2023). Thinking about the action potential: the nerve signal as a window to the physical principles guiding neuronal excitability. Frontiers in Cellular Neuroscience, 17. https:// doi.org/10.3389/fncel.2023.1232020
9. Zbili, M., Rama, S., & Debanne, D. (2016). Dynamic Control of Neurotransmitter Release by Presynaptic Potential. Frontiers in Cellular Neuroscience, 10. https://doi. org/10.3389/fncel.2016.00278
10. Doubovikov, E. D., Serdyukova, N. A., Greenberg, S. B., Gascoigne, D. A., Minhaj, M. M., & Aksenov, D. P. (2023). Electric Field Effects on Brain Activity: Implications for Epilepsy and Burst Suppression. Cells, 12(18), 2229–2229. https://doi.org/10.3390/cells12182229
11. Kopf, M., Martini, J., Stier, C., Ethofer, S., Braun, C., Li Hegner, Y., Focke, N. K., Marquetand, J., & Helfrich, R. F. (2024). Aperiodic activity indexes neural hyperexcitability in generalized epilepsy. Eneuro, 11(9), ENEURO.0242-24.2024. https://doi.org/10.1523/eneuro.0242-24.2024
12. Smith, D. K., Sadler, K. P., & Benedum, M. (2019). Febrile Seizures: Risks, Evaluation, and Prognosis. American Family Physician, 99(7), 445–450. https://pubmed.ncbi. nlm.nih.gov/30932454/
13. Leung, A. K., Hon, K. L., & Leung, T. N. (2018). Febrile seizures: an overview. Drugs in Context, 7(1). https://doi. org/10.7573/dic.212536
14. Mosili, P., Maikoo, S., Mabandla, M., Vuyisile, & Qulu, L. (2020). The Pathogenesis of Fever-Induced Febrile Seizures and Its Current State. Neuroscience Insights, 15(15). https://doi.org/10.1177/2633105520956973
15. Chang, R. S., Leung, C. Y. W., Ho, C. C. A., & Yung, A. (2017). Classifications of seizures and epilepsies, where are we? – A brief historical review and update. Journal of the Formosan Medical Association, 116(10), 736–741. https://doi.org/10.1016/j.jfma.2017.06.001
16. Horváth, L., Fekete, I., Molnár, M., Válóczy, R., Márton, S., & Fekete, K. (2019). The Outcome of Status Epilepticus and Long-Term Follow-Up. Frontiers in Neurology, 10. https://doi.org/10.3389/fneur.2019.00427
17. Trinka, E., Cock, H., Hesdorffer, D., Rossetti, A. O., Scheffer, I. E., Shinnar, S., Shorvon, S., & Lowenstein, D. H. (2015). A definition and classification of status epilepticus - Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia, 56(10), 1515–1523. https:// doi.org/10.1111/epi.13121
18. Corsello, A., Maria Beatrice Marangoni, Macchi, M., Cozzi, L., Agostoni, C., Gregorio Paolo Milani, & Dilena, R. (2024). Febrile Seizures: A Systematic Review of Different Guidelines. Pediatric Neurology, 155, 141–148. https://doi.org/10.1016/j.pediatrneurol.2024.03.024
19. Murin, C. D., Wilson, I. A., & Ward, A. B. (2019). Antibody responses to viral infections: a structural perspective across three different enveloped viruses. Nature Microbiology, 4(5), 734–747. https://doi.org/10.1038/s41564019-0392-y
20. Kany, S., Vollrath, J. T., & Relja, B. (2019). Cytokines in Inflammatory Disease. International Journal of Molecular Sciences, 20(23), 6008. https://doi.org/10.3390/ ijms20236008
21. Al-Qahtani, A. A., Alhamlan, F. S., & Al-Qahtani, A. A. (2024). Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Tropical Medicine and Infectious Disease, 9(1), 13. https://doi.org/10.3390/tropicalmed9010013
22. Cicchese, J. M., Evans, S., Hult, C., Joslyn, L. R., Wessler, T., Millar, J. A., Marino, S., Cilfone, N. A., Mattila, J. T., Linderman, J. J., & Kirschner, D. E. (2018). Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunological Reviews, 285(1), 147–167. https://doi.org/10.1111/imr.12671
23. Evans, S. S., Repasky, E. A., & Fisher, D. T. (2015). Fever and the thermal regulation of immunity: the immune system feels the heat. Nature Reviews Immunology, 15(6), 335–349. https://doi.org/10.1038/nri3843
24. Han, L., Wu, T., Zhang, Q., Qi, A., & Zhou, X. (2025). Immune Tolerance Regulation Is Critical to Immune Homeostasis. Journal of Immunology Research, 2025(1). https://doi.org/10.1155/jimr/5006201
25. Sawires, R., Buttery, J., & Fahey, M. (2022). A Review of Febrile Seizures: Recent Advances in Understanding of Febrile Seizure Pathophysiology and Commonly Implicated Viral Triggers. Frontiers in Pediatrics, 9(1), 801321. https://doi.org/10.3389/fped.2021.801321
26. Pal, M. M. (2021). Glutamate: the Master Neurotransmitter and Its Implications in Chronic Stress and Mood Disorders. Frontiers in Human Neuroscience, 15(15). https://doi.org/10.3389/fnhum.2021.722323
27. Zhang, N., Song, Y., Wang, H., Li, X., Lyu, Y., Liu, J., Mu, Y., Wang, Y., Lu, Y., Li, G., Fan, Z., Wang, H., Zhang, D., & Li, N. (2024). IL-1ß promotes glutamate excitotoxicity: indications for the link between inflammatory and synaptic vesicle cycle in Ménière’s disease. Cell Death Discovery, 10(1). https://doi.org/10.1038/s41420-024-02246-2
28. Goh, Y., Sen Hee Tay, Leong, L., & Rahul Rathakrishnan. (2023). Bridging the Gap: Tailoring an Approach to Treatment in Febrile Infection-Related Epilepsy Syndrome. Neurology, 100(24), 1151–1155. https://doi.org/10.1212/ wnl.0000000000207068
29. Han, J. Y., & Han, S. B. (2023). Pathogenetic and etiologic considerations of febrile seizures. Clinical and Experimental Pediatrics, 66(2), 46–53. https://doi.org/10.3345/ cep.2021.01039
30. Ferretti, A., Riva, A., Fabrizio, A., Bruni, O., Capovilla, G., Foiadelli, T., Orsini, A., Raucci, U., Romeo, A., Striano, P., & Parisi, P. (2024). Best practices for the management of febrile seizures in children. The Italian Journal of Pediatrics/Italian Journal of Pediatrics, 50(1). https://doi. org/10.1186/s13052-024-01666-1
31. Wang, Y., Trevelyan, A. J., Valentin, A., Alarcon, G., Taylor, P. N., & Kaiser, M. (2017). Mechanisms underlying different onset patterns of focal seizures. PLOS Computational Biology, 13(5), e1005475. https://doi.org/10.1371/ journal.pcbi.1005475
32. Kumar, N., Midha, T., & Rao, Y. K. (2019). Risk Factors of Recurrence of Febrile Seizures in Children in a Tertiary Care Hospital in Kanpur: A One Year Follow Up Study. Annals of Indian Academy of Neurology, 22(1), 31–36. https://doi.org/10.4103/aian.AIAN_472_17
33. Pavone, P., Pappalardo, X. G., Parano, E., Falsaperla, R., Marino, S. D., Fink, J. K., & Ruggieri, M. (2022). Fever-Associated Seizures or Epilepsy: An Overview of Old and Recent Literature Acquisitions. Frontiers in Pediatrics, 10. https://doi.org/10.3389/fped.2022.858945
34. Permana, I., Meyrisa, N., & Brajadenta, G. (2025). The Effect of Family History of Seizures as A Risk Factor for The Inci-dence of Recurrent Febrile Seizures and Types of Febrile Seizures in Children at Waled Cirebon Hospital. Eduvest, 5(4). https://doi.org/10.59188/eduvest. v5i4.25530
35. Ding, J., Li, X., Tian, H., Wang, L., Guo, B., Wang, Y., Li, W., Wang, F., & Sun, T. (2021). SCN1A Mutation—Beyond Dravet Syndrome: A Systematic Review and Narrative Synthesis. Frontiers in Neurology, 12, 743726. https:// doi.org/10.3389/fneur.2021.743726
36. Bender, A. C., Morse, R. P., Scott, R. C., Holmes, G. L., & Lenck-Santini, P.-P. (2012). SCN1A mutations in Dravet syndrome: Impact of interneuron dysfunction on neural networks and cognitive outcome. Epilepsy & Behavior, 23(3), 177–186. https://doi.org/10.1016/j.yebeh.2011.11.022
37. Ademuwagun, I. A., Rotimi, S. O., Syrbe, S., Ajamma, Y. U., & Adebiyi, E. (2021). Voltage Gated Sodium Channel Genes in Epilepsy: Mutations, Functional Studies, and Treatment Dimensions. Frontiers in Neurology, 12. https://doi.org/10.3389/fneur.2021.600050
38. Wu, Y. W., Sullivan, J., McDaniel, S. S., Meisler, M. H., Walsh, E. M., Li, S. X., & Kuzniewicz, M. W. (2015). Incidence of Dravet Syndrome in a US Population. Pediatrics, 136(5), e1310–e1315. https://doi.org/10.1542/ peds.2015-1807
39. Camfield, P., & Camfield, C. (2015). Febrile seizures and genetic epilepsy with febrile seizures plus (GEFS+). Epileptic Disorders, 17(2), 124–133. https://doi.org/10.1684/ epd.2015.0737
40. Myers, K. A., Scheffer, I. E., & Berkovic, S. F. (2018). Genetic literacy series: genetic epilepsy with febrile seizures plus. Epileptic Disorders, 20(4), 232–238. https:// doi.org/10.1684/epd.2018.0985
41. Ram, D., & Newton, R. (2015). The Genetics of Febrile Seizures. Pediatric Neurology Briefs, 29(12), 90. https:// doi.org/10.15844/pedneurbriefs-29-12-1
42. Wu, C., Wang, Q., Li, W., Han, M., Zhao, H., & Xu, Z. (2024). Research progress on pathogenesis and treatment of febrile seizures. Life Sciences, 362, 123360. https://doi. org/10.1016/j.lfs.2024.123360
43. Kwon, A., Kwak, B. O., Kim, K., Ha, J., Kim, S.-J., Bae, S. H., Son, J. S., Kim, S.-N., & Lee, R. (2018). Cytokine levels in febrile seizure patients: A systematic review and meta-analysis. Seizure, 59, 5–10. https://doi.org/10.1016/j. seizure.2018.04.023
44. Okudan, Z. V., & Ozkara, C. (2018). Reflex epilepsy: triggers and management strategies. Neuropsychiatric Disease and Treatment, Volume 14, 327–337. https://doi. org/10.2147/ndt.s107669
45. Zhang, G., Huang, S., Wei, M., Wu, Y., Xie, Z., & Wang, J. (2025). Dravet syndrome: novel insights into SCN1A-mediated epileptic neurodevelopmental disorders within the molecular diagnostic-therapeutic framework. Frontiers in Neuroscience, 19. https://doi.org/10.3389/ fnins.2025.1634718
46. Ballesteros-Sayas, C., Muñoz-Montero, A., Giorgi, S., Cardenal-Muñoz, E., Turón-Viñas, E., Pallardó, F., & José Ángel Aibar. (2024). Non-pharmacological therapeutic needs in people with Dravet syndrome. Epilepsy & Behavior, 150, 109553–109553. https://doi.org/10.1016/j.yebeh.2023.109553
47. Heuser, K., Horn, M., Samsonsen, C., Ulvin, L. B., Olsen, K. B., Power, K. N., Veiby, G., Molteberg, E., Engelsen, B., & Taubøll, E. (2024). Treating status epilepticus in adults. Tidsskrift for Den Norske Legeforening, 144. https://doi.org/10.4045/tidsskr.23.0782
48. Specchio, N., & Auvin, S. (2025). To what extent does status epilepticus contribute to brain damage in the developmental and epileptic Encephalopathies. Epilepsy & Behavior, 164, 110271. https://doi.org/10.1016/j. yebeh.2025.110271
49. Pack, A. M. (2019). Epilepsy Overview and Revised Classification of Seizures and Epilepsies. CONTINUUM: Lifelong Learning in Neurology, 25(2), 306–321. https://doi. org/10.1212/con.0000000000000707
50. Zachlod, D., Kedo, O., & Amunts, K. (2022). Anatomy of the temporal lobe: From macro to micro. Handbook of Clinical Neurology, 187, 17–51. https://doi.org/10.1016/ B978-0-12-823493-8.00009-2
51. Gao, Y., Su, Q., Liang, L., Yan, H., & Zhang, F. (2022). Editorial: Temporal lobe dysfunction in neuropsychiatric disorder. Frontiers in Psychiatry, 13. https://doi. org/10.3389/fpsyt.2022.1077398
52. Vinti, V., Dell’Isola, G. B., Tascini, G., Mencaroni, E., Cara, G. D., Striano, P., & Verrotti, A. (2021). Temporal Lobe Epilepsy and Psychiatric Comorbidity. Frontiers in Neurology, 12. https://doi.org/10.3389/fneur.2021.775781
53. Cristofori, I., Cohen-Zimerman, S., & Grafman, J. (2019). Executive functions. Handbook of Clinical Neurology, 163(3), 197–219. https://doi.org/10.1016/B978-0-12804281-6.00011-2
54. Chauhan, P., Jethwa, K., Rathawa, A., Chauhan, G., & Mehra, S. (2021). Cerebral Ischemia. In Exon Publications eBooks. Exon Publications. https://doi.org/10.36255/exonpublications.cerebralischemia.2021
55. Cutsuridis, V., & Yoshida, M. (2017). Editorial: Memory Processes in Medial Temporal Lobe: Experimental, Theoretical and Computational Approaches. Frontiers in Systems Neuroscience, 11. https://doi.org/10.3389/ fnsys.2017.00019
56. Chan, S., Bello, J. A., Shinnar, S., Hesdorffer, D. C., Lewis, D. V., MacFall, J., Shinnar, R. C., Gomes, W., Litherland, C., Xu, Y., Nordli, D. R., Pellock, J. M., Frank, L. M., Moshé, S. L., Sun, S., & FEBSTAT Study Team. (2015). Hippocampal Malrotation Is Associated With Prolonged Febrile Seizures: Results of the FEBSTAT Study. AJR. American Journal of Roentgenology, 205(5), 1068–1074. https://doi.org/10.2214/AJR.14.13330
57. Fu, T.-Y., Ho, C.-R., Lin, C.-H., Lu, Y.-T., Lin, W.-C., & Tsai, M.-H. (2021). Hippocampal Malrotation: A Genetic Developmental Anomaly Related to Epilepsy? Brain Sciences, 11(4), 463–463. https://doi.org/10.3390/brainsci11040463
58. Feyissa, A. M. (2024). Have We Solved the Chickenand-Egg Conundrum? Hippocampal Sclerosis and Temporal Lobe Epilepsy Following Febrile Status Epilepticus: Results of the 10-Year Follow-up FEBSTAT Study. Epilepsy Currents, 24(6), 400–402. https://doi. org/10.1177/15357597241279746
59. deCampo, D., Xian, J., Karlin, A., Katie Rose Sullivan, Ruggiero, S. M., Galer, P. D., Ramos, M., Abend, N. S., Gonzalez, A., & Helbig, I. (2023). Investigating the genetic contribution in febrile infection-related epilepsy syndrome and refractory status epilepticus. Frontiers in Neurology, 14. https://doi.org/10.3389/fneur.2023.1161161
60. Shi, X., Wang, Y., Wang, X., Kang, X., Yang, F., Yuan, F., & Jiang, W. (2023). Long-term outcomes of adult cryptogenic febrile infection–related epilepsy syndrome (FIRES). Frontiers in Neurology, 13. https://doi. org/10.3389/fneur.2022.1081388
61. Pavone, P., Corsello, G., Raucci, U., Lubrano, R., Enrico Parano, Ruggieri, M., Greco, F., Marino, S., & Raffaele Falsaperla. (2022). Febrile infection-related Epilepsy Syndrome (FIRES): a severe encephalopathy with status epilepticus. Literature review and presentation of two new cases. Italian Journal of Pediatrics, 48(1). https:// doi.org/10.1186/s13052-022-01389-1
62. Li, W., Wu, J., Zeng, Y., & Zheng, W. (2023). Neuroinflammation in epileptogenesis: from pathophysiology to therapeutic strategies. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1269241
63. Clarkson, B. D., LaFrance-Corey, R. G., Kahoud, R. J., Farias-Moeller, R., Payne, E. T., & Howe, C. L. (2019). Functional deficiency in endogenous interleukin-1 receptor antagonist in patients with febrile infection-related epilepsy syndrome. Annals of Neurology, 85(4), 526–537. https://doi.org/10.1002/ana.25439
64. Jarczak, D., & Nierhaus, A. (2022). Cytokine Storm— Definition, Causes, and Implications. International Journal of Molecular Sciences, 23(19), 11740. https://doi. org/10.3390/ijms231911740
65. Wolinski, P., Ksiazek-Winiarek, D., & Glabinski, A. (2022). Cytokines and Neurodegeneration in Epileptogenesis. Brain Sciences, 12(3), 380. https://doi.org/10.3390/ brainsci12030380
66. Liu, L., Han, X., Huang, Q., Zhu, X., Yang, J., & Liu, H. (2016). Increased neuronal seizure activity correlates with excessive systemic inflammation in a rat model of severe preeclampsia. Hypertension Research, 39(10), 701–708. https://doi.org/10.1038/hr.2016.53
67. van Baalen, A. (2023). Febrile infection-related epilepsy syndrome in childhood: A clinical review and practical approach. Seizure, 111, 215–222. https://doi.org/10.1016/j. seizure.2023.09.008
68. Serino, D., Santarone, M., Caputo, D., & Fusco, L. (2019). Febrile infection-related epilepsy syndrome (FIRES): prevalence, impact and management strategies. Neuropsychiatric Disease and Treatment, Volume 15, 1897–1903. https://doi.org/10.2147/ndt.s177803
69. Khair, A. M., & Elmagrabi, D. (2015). Febrile Seizures and Febrile Seizure Syndromes: An Updated Overview of Old and Current Knowledge. Neurology Research International, 2015, 1–7. https://doi.org/10.1155/2015/849341
70. Tan, E., Braithwaite, I., McKinlay, C. J. D., & Dalziel, S. R. (2020). Comparison of Acetaminophen (Paracetamol) With Ibuprofen for Treatment of Fever or Pain in Children Younger Than 2 Years. JAMA Network Open, 3(10). https://doi.org/10.1001/jamanetworkopen.2020.22398
71. Peng, P., Peng, J., Yin, F., Deng, X., Chen, C., He, F., Wang, X., Guang, S., & Mao, L. (2019). Ketogenic Diet as a Treatment for Super-Refractory Status Epilepticus in Febrile Infection-Related Epilepsy Syndrome. Frontiers in Neurology, 10. https://doi.org/10.3389/fneur.2019.00423
72. Harrar, D. B., Genser, I., Najjar, M., Davies, E., Sule, S., Wistinghausen, B., Goldbach-Mansky, R., & Wells, E. (2024). Successful Management of Febrile Infection–Related Epilepsy Syndrome Using Cytokine-Directed Therapy. Journal of Child Neurology, 39(11-12), 440–445. https://doi.org/10.1177/08830738241273448
73. Lai, Y., Muscal, E., Wells, E., Shukla, N., Eschbach, K., Hyeong Lee, K., Kaliakatsos, M., Desai, N., Wickström, R., Viri, M., Freri, E., Granata, T., Nangia, S., Dilena, R., Brunklaus, A., Wainwright, M. S., Gorman, M. P., Stredny, C. M., Asiri, A., & Hundallah, K. (2020). Anakinra usage in febrile infection related epilepsy syndrome: an international cohort. Annals of Clinical and Translational Neurology, 7(12), 2467–2474. https://doi.org/10.1002/ acn3.51229
74. Westbrook, C., Subramaniam, T., Seagren, R. M., Tarula, E., Co, D., Furstenberg-Knauff, M., Wallace, A., & Hsu, D. (2019). Febrile Infection-Related Epilepsy Syndrome (FIRES) Treated Successfully With Anakinra In A 21-Year-Old Woman. WMJ : Official Publication of the State Medical Society of Wisconsin, 118(3), 135. https:// pmc.ncbi.nlm.nih.gov/articles/PMC7082129/
1. Aird, C. S., Reisinger, B. A., Webb, S. N., & Gleaves, D. H. (2025). Comparing social stigma of anorexia nervosa, bulimia nervosa, and binge-eating disorder: A quantitative experimental study. Journal of Eating Disorders, 13(1). https://doi.org/10.1186/s40337-025-01198-x
2. American Psychiatric Association. (2022). Feeding and eating disorders. In Diagnostic and Statistical Manual of Mental Disorders (5th ed., text rev.). https://doi. org/10.1176/appi.books.9780890425596
3. Rouhani, N., Grossman, C. D., Feusner, J., & Tusche, A. (2025). Eating disorder symptoms and emotional arousal modulate food biases during reward learning in females. Nature Communications, 16(1), 2938. https:// doi.org/10.1038/s41467-025-57872-w
4. Serra, R., Di Nicolantonio, C., Di Febo, R., De Crescenzo, F., Vanderlinden, J., Vrieze, E., Bruffaerts, R., Loriedo, C., Pasquini, M., & Tarsitani, L. (2022). The transition from restrictive anorexia nervosa to binging and purging: A systematic review and meta-analysis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity, 27(3), 857–865. https://doi.org/10.1007/s40519021-01226-0
5. Wilson, K., & Kagabo, R. (2024). Bulimia nervosa and treatment-related disparities: A review. Frontiers in Psychology, 15. https://doi.org/10.3389/fpsyg.2024.1386347
6. Frank, G. K. W. (2019). Neuroimaging and eating disorders. Current Opinion in Psychiatry, 32(6), 478–483. https://doi.org/10.1097/YCO.0000000000000544
7. Adame, A. L., Pierce, E., Jimenez, A., Shelby, T., & Parks, D. (2024). How Does Self-Identity Change in Eating Disorder Recovery? Journal of Humanistic Psychology, 00221678241255264. https://doi. org/10.1177/00221678241255264
8. Miskovic-Wheatley, J., Bryant, E., Ong, S. H., Vatter, S., Le, A., Aouad, P., Barakat, S., Boakes, R., Brennan, L., Bryant, E., Byrne, S., Caldwell, B., Calvert, S., Carroll, B., Castle, D., Caterson, I., Chelius, B., Chiem, L., Clarke, S., … National Eating Disorder Research Consortium. (2023). Eating disorder outcomes: Findings from a rapid review of over a decade of research. Journal of Eating Disorders, 11(1), 85. https://doi.org/10.1186/s40337-02300801-3
9. Khalsa, S. S., Portnoff, L. C., McCurdy-McKinnon, D., & Feusner, J. D. (2017). What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. Journal of Eating Disorders, 5(1), 20. https://doi.org/10.1186/s40337-017-0145-3
10. Bardone-Cone, A. M., Hunt, R. A., & Watson, H. J. (2018). An Overview of Conceptualizations of Eating Disorder Recovery, Recent Findings, and Future Directions. Current Psychiatry Reports, 20(9), 79. https://doi. org/10.1007/s11920-018-0932-9
11. McDonald, S., Williams, A. J., Barr, P., McNamara, N., & Marriott, M. (2021). Service user and eating disorder therapist views on anorexia nervosa recovery criteria. Psychology and Psychotherapy: Theory, Research and Practice, 94(3), 721–736. https://doi.org/10.1111/ papt.12340
12. Kenny, T. E., Trottier, K., & Lewis, S. P. (2022). Lived experience perspectives on a definition of eating disorder recovery in a sample of predominantly white women: A mixed-method study. Journal of Eating Disorders, 10(1), 149. https://doi.org/10.1186/s40337-022-00670-2
13. Li, W., Wang, Y., Wang, J., Wang, M., Liu, J., Chen, Q., Yang, Z., Li, Z., Wu, G., Wang, Z., Zhang, P., & Tang, L. (2024). Bulimia nervosa selectively reshapes the structure and intrinsic function of anterior insula subregions associated with cognition-emotion integration. Journal of Affective Disorders, 362, 529–535. https://doi. org/10.1016/j.jad.2024.07.051
14. Nijakowski, K., Jankowski, J., Gruszczyński, D., & Surdacka, A. (2023). Eating Disorders and Dental Erosion: A Systematic Review. Journal of Clinical Medicine, 12(19), 6161. https://doi.org/10.3390/jcm12196161
15. Dougherty, E. N., Wildes, J. E., & Haedt-Matt, A. A. (2024). The role of habit in maintaining binge/purge behaviors: An ecological momentary assessment study. International Journal of Eating Disorders, 57(5), 1160–1171. https://doi.org/10.1002/eat.24070
16. Berner, L. A., Fiore, V. G., Chen, J. Y., Krueger, A., Kaye, W. H., Viranda, T., & de Wit, S. (2023). Impaired belief updating and devaluation in adult women with bulimia nervosa. Translational Psychiatry, 13(1), 2. https://doi. org/10.1038/s41398-022-02257-6
17. Conceição, I. S. R., Garcia-Burgos, D., de Macêdo, P. F. C., Nepomuceno, C. M. M., Pereira, E. M., Cunha, C. de M., Ribeiro, C. D. F., & de Santana, M. L. P. (2023). Habits and Persistent Food Restriction in Patients with Anorexia Nervosa: A Scoping Review. Behavioral Sciences, 13(11), 883. https://doi.org/10.3390/bs13110883
18. Berner, L. A., Wang, Z., Stefan, M., Lee, S., Huo, Z., Cyr, M., & Marsh, R. (2019). Subcortical shape abnormalities in bulimia nervosa. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(12), 1070–1079. https://doi.org/10.1016/j.bpsc.2018.12.011
19. Haynos, A. F., Anderson, L. M., Askew, A. J., Craske, M. G., & Peterson, C. B. (2021). Adapting a neuroscience-informed intervention to alter reward mechanisms of anorexia nervosa: A novel direction for future research. Journal of Eating Disorders, 9(1), 63. https:// doi.org/10.1186/s40337-021-00417-5
20. Foerde, K., Schebendach, J. E., Davis, L., Daw, N., Walsh, B. T., Shohamy, D., & Steinglass, J. E. (2022). Restrictive eating across a spectrum from healthy to unhealthy: Behavioral and neural mechanisms. Psychological Medicine, 52(9), 1755–1764. https://doi.org/10.1017/ S0033291720003542
21. Wierenga, C. E., Reilly, E., Bischoff-Grethe, A., Kaye, W. H., & Brown, G. G. (2022). Altered Reinforcement Learning from Reward and Punishment in Anorexia Nervosa: Evidence from Computational Modeling. Journal of the International Neuropsychological Society, 28(10), 1003–1015. https://doi.org/10.1017/S1355617721001326
22. Springall, G. A. C., Caughey, M., Zannino, D., Kyprianou, K., Mynard, J. P., Rudolph, S., Cheong, J., Yeo, M., & Cheung, M. M. H. (2023). Long-term cardiovascular consequences of adolescent anorexia nervosa. Pediatric Research, 94(4), 1457–1464. https://doi.org/10.1038/ s41390-023-02521-5
23. Friars, D., Walsh, O., & McNicholas, F. (2023). Assessment and management of cardiovascular complications in eating disorders. Journal of Eating Disorders, 11(1), 13. https://doi.org/10.1186/s40337-022-00724-5
24. Gibson, D., Filan, Z., Westmoreland, P., & Mehler, P. S. (2024). Loss of Bone Density in Patients with Anorexia Nervosa Food That Alone Will Not Cure. Nutrients, 16(21), 3593. https://doi.org/10.3390/nu16213593
25. Hart, G., Burton, T. J., & Balleine, B. W. (2024). What Role Does Striatal Dopamine Play in Goal-directed Action? Neuroscience, 546, 20–32. https://doi.org/10.1016/j.neuroscience.2024.03.020
26. Lopez, G. C., & Lerner, T. N. (2025). How dopamine enables learning from aversion. Current Opinion in Behavioral Sciences, 61, 101476. https://doi.org/10.1016/j. cobeha.2024.101476
27. Beeler, J. A., & Burghardt, N. S. (2022). The rise and fall of dopamine: A two-stage model of the development and entrenchment of anorexia nervosa. Frontiers in Psychiatry, 12, 799548. https://doi.org/10.3389/fpsyt.2021.799548
28. Yu, Y., Miller, R., & Groth, S. W. (2022). A literature review of dopamine in binge eating. Journal of Eating Disorders, 10(1), 11. https://doi.org/10.1186/s40337-02200531-y
29. Hagan, K., Lloyd, E. C., & Gorrell, S. (n.d.). Annual Research Review: Neural mechanisms of eating disorders in youth – from current theory and findings to future directions. Journal of Child Psychology and Psychiatry, n/a(n/a). https://doi.org/10.1111/jcpp.70029
30. Steinglass, J. E., & Walsh, B. T. (2016). Neurobiological model of the persistence of anorexia nervosa. Journal of Eating Disorders, 4, 19. https://doi.org/10.1186/ s40337-016-0106-2
31. Su, T., Gong, J., Tang, G., Qiu, S., Chen, P., Chen, G., Wang, J., Huang, L., & Wang, Y. (2021). Structural and functional brain alterations in anorexia nervosa:A multimodal meta-analysis of neuroimaging studies. Human Brain Mapping, 42(15), 5154–5169. https://doi.org/10.1002/ hbm.25602
32. Timmler, S., & Simons, M. (2019). Grey matter myelination. Glia, 67(11), 2063–2070. https://doi.org/10.1002/ glia.23614
33. Sampaio-Baptista, C., & Johansen-Berg, H. (2017). White Matter Plasticity in the Adult Brain. Neuron, 96(6), 1239–1251. https://doi.org/10.1016/j.neuron.2017.11.026
34. Alcami, P., & El Hady, A. (2019). Axonal Computations. Frontiers in Cellular Neuroscience, 13. https://doi. org/10.3389/fncel.2019.00413
35. Chorghay, Z., Káradóttir, R. T., & Ruthazer, E. S. (2018). White Matter Plasticity Keeps the Brain in Tune: Axons Conduct While Glia Wrap. Frontiers in Cellular Neuroscience, 12. https://doi.org/10.3389/fncel.2018.00428
36. Krasner, H., Ong, C. V., Hewitt, P., & Vida, T. A. (2025). From Stress to Synapse: The Neuronal Atrophy Pathway to Mood Dysregulation. International Journal of Molecular Sciences, 26(7), 3219. https://doi.org/10.3390/ ijms26073219
37. de la Cruz, F., Schumann, A., Rieger, K., Giuliano, M. D., & Bär, K. J. (2023). Fibre-specific white matter changes in anorexia nervosa. Psychiatry Research: Neuroimaging, 336, 111736. https://doi.org/10.1016/j.pscychresns.2023.111736
38. Wang, L., Bi, K., Song, Z., Zhang, Z., Li, K., Kong, Q. M., Li, X. N., Lu, Q., & Si, T. M. (2020). Disturbed resting-state whole-brain functional connectivity of striatal subregions in bulimia nervosa. The International Journal of Neuropsychopharmacology, 23(6), 356–365. https://doi. org/10.1093/ijnp/pyaa023
39. Geisler, D., Roessner, V., Biemann, R., Marxen, M., & Ehrlich, S. (2019). Dynamic changes in white matter microstructure in anorexia nervosa: Findings from a longitudinal study. Psychological Medicine, 49(9), 1555–1564. https://doi.org/10.1017/S003329171800212X
40. Donnelly, B., Touyz, S., Hay, P., Burton, A., Russell, J., & Caterson, I. (2018). Neuroimaging in bulimia nervosa and binge eating disorder: A systematic review. Journal of Eating Disorders, 6(1), 3. https://doi.org/10.1186/ s40337-018-0187-1
41. Maier, S., Nickel, K., Perlov, E., Kukies, A., Zeeck, A., van Elst, L. T., Endres, D., Spieler, D., Holovics, L., Hartmann, A., Dacko, M., Lange, T., & Joos, A. (2020). Insular cell integrity markers linked to weight concern in anorexia nervosa: An MR-spectroscopy study. Journal of Clinical Medicine, 9(5), 1292. https://doi.org/10.3390/ jcm9051292
42. Lan, Z., Zhu, L. L., Wu, Y. K., Yang, J. J., Li, J. T., Zeng, Y. W., Li, K., Kong, Q. M., Su, Y. A., & Si, T. (2023). Aberrant modular segregation of brain networks in female patients with bulimia nervosa. The International Journal of Eating Disorders, 56(7), 1353–1364. https://doi. org/10.1002/eat.23939
43. D’Andrea, C. B., Laumann, T. O., Newbold, D. J., Nelson, S. M., Nielsen, A. N., Chauvin, R., Marek, S., Greene, D. J., Dosenbach, N. U. F., & Gordon, E. M. (2023). Substructure of the Brain’s CINGULO-Opercular Network. https://doi.org/10.1101/2023.10.10.561772
44. Seidel, M., Markmann Jensen, S., Healy, D., Dureja, A., Watson, H. J., Holst, B., Bulik, C. M., & Sjögren, J. M. (2021). A systematic review and meta-analysis finds increased blood levels of all forms of ghrelin in both restricting and binge-eating/purging subtypes of anorexia nervosa. Nutrients, 13(2), 709. https://doi.org/10.3390/ nu13020709
45. Westwater, M. L., Murley, A. G., Diederen, K. M. J., et al. (2022). Characterizing cerebral metabolite profiles in anorexia and bulimia nervosa and their associations with habitual behavior. Translational Psychiatry, 12, 103. https://doi.org/10.1038/s41398-022-01872-7
46. Chiu, H.-P., Huang, M.-W., Tsai, S.-Y., & Hsu, C.-Y. (2023). A retrospective study of pharmacological treatment in anorexia nervosa: 6-month and 12-month follow-up. BMC Psychiatry, 23(1), 126. https://doi.org/10.1186/ s12888-023-04604-3
47. Agras, W. S., & Bohon, C. (2021). Cognitive Behavioral Therapy for the Eating Disorders. Annual Review of Clinical Psychology, 17(Volume 17, 2021), 417–438. https:// doi.org/10.1146/annurev-clinpsy-081219-110907
48. Chmiel, J., Gladka, A., & Leszek, J. (2023). The Effect of Transcranial Direct Current Stimulation (tDCS) on Anorexia Nervosa: A Narrative Review. Nutrients, 15(20), 4455. https://doi.org/10.3390/nu15204455
49. Kirchberg, M. C., Pinson, C., & Frank, G. K. W. (2024). Pharmacotherapeutic strategies for the treatment of anorexia nervosa – novel targets to break a vicious cycle. Expert Opinion on Pharmacotherapy, 25(17), 2253–2265. https://doi.org/10.1080/14656566.2024.2424316
50. Nohara, N., Yamanaka, Y., Matsuoka, M., Yamazaki, T., Kawai, K., Takakura, S., Sudo, N., Ando, T., Matsuyama, Y., Byrne, S., Dalle Grave, R., Cooper, Z., & Yoshiuchi, K. (2023). A multi-center, randomized, parallel-group study to compare the efficacy of enhanced cognitive behavior therapy (CBT-E) with treatment as usual (TAU) for anorexia nervosa: Study protocol. BioPsychoSocial Medicine, 17(1), 20. https://doi.org/10.1186/s13030-02300277-2
51. Murphy, R., Bailey-Straebler, S., Dalle Grave, R., Calugi, S., Osborne, E. L., & Cooper, Z. (2025). Evolving perspectives on CBT-E for eating disorders: Clarifying ten key points – misconceptions and communication gaps explored. The Cognitive Behaviour Therapist, 18. https:// doi.org/10.1017/s1754470x25100299
52. Kekic, M., McClelland, J., Bartholdy, S., Boysen, E., Musiat, P., Dalton, B., Tiza, M., David, A. S., Campbell, I. C., & Schmidt, U. (2017). Single-Session Transcranial Direct Current Stimulation Temporarily Improves Symptoms, Mood, and Self-Regulatory Control in Bulimia Nervosa: A Randomised Controlled Trial. PLOS ONE, 12(1), e0167606. https://doi.org/10.1371/journal.pone.0167606
53. Phillipou, A., Kirkovski, M., Castle, D. J., Gurvich, C., Abel, L. A., Miles, S., & Rossell, S. L. (2019). High-definition transcranial direct current stimulation in anorexia nervosa: A pilot study. International Journal of Eating Disorders, 52(11), 1274–1280. https://doi.org/10.1002/ eat.23146
54. Baumann, S., Mareš, T., Albrecht, J., Anders, M., Vochosková, K., Hill, M., Bulant, J., Yamamotová, A., Štastný, O., Novák, T., Holanová, P., Lambertová, A., & Papežová, H. (2021). Effects of Transcranial Direct Current Stimulation Treatment for Anorexia Nervosa. Frontiers in Psychiatry, 12. https://doi.org/10.3389/fpsyt.2021.717255
1. de Vente, W., van Amsterdam, J. G., Olff, M., Kamphuis, J. H., & Emmelkamp, P. M. (2015). Burnout is associated with reduced parasympathetic activity and reduced HPA axis responsiveness, predominantly in males. BioMed Research International, 1–13. doi:10.1155/2015/431725
2. Pihlaja, M., Tuominen, P. P., Peräkylä, J., & Hartikainen, K. M. (2022). Occupational burnout is linked with inefficient executive functioning, elevated average heart rate, and decreased physical activity in daily life - Initial evidence from teaching professionals. Brain Sciences, 12(12), 1723. doi:10.3390/brainsci12121723
3. Doulougeri, K., Georganta, K., & Montgomery, A. (2016). “Diagnosing” burnout among healthcare professionals: Can we find consensus? Cogent Medicine, 3(1). doi:10.10 80/2331205X.2016.1237605
4. Carroll, D., Ginty, A. T., Whittaker, A. C., Lovallo, W. R., & de Rooij, S. R. (2017). The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neuroscience & Biobehavioral Reviews, 77, 74–86. doi:10.1016/j. neubiorev.2017.02.025
5. Chen, C., Xiong, B., Tan, W., Tian, Y., Zhang, S., Wu, J., Song, P., & Qin, S. (2025). Setting the tone for the day: Cortisol awakening response proactively modulates fronto-limbic circuitry for emotion processing. NeuroImage, 315, 121251. doi:10.1016/j.neuroimage.2025.121251
6. Li, D., El Kawkgi, O. M., Henriquez, A. F., & Bancos, I. (2020). Cardiovascular risk and mortality in patients with active and treated hypercortisolism. Gland Surgery, 9(1), 43–58. doi:10.21037/gs.2019.11.03
7. Laferton, J. A. C., Bartsch, L. M., Möschinger, T., Baldelli, L., Frick, S., Breitenstein, C. J., Züger, R., Annen, H., & Fischer, S. (2023). Effects of stress beliefs on the emotional and biological response to acute psychosocial stress in Healthy Men. Psychoneuroendocrinology, 152, 106091. doi:10.1016/j.psyneuen.2023.106091
8. Rojas-Thomas, F., Artigas, C., Wainstein, G., Morales, J.P., Arriagada, M., Soto, D., Dagnino-Subiabre, A., Silva, J., & Lopez, V. (2023). Impact of acute psychosocial stress on attentional control in humans. A study of evoked potentials and pupillary response. Neurobiology of Stress, 25, 100551. doi:10.1016/j.ynstr.2023.100551
9. James, K. A., Stromin, J. I., Steenkamp, N., & Combrinck, M. I. (2023). Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Frontiers in Endocrinology, 14. doi:10.3389/fendo.2023.1085950
10. Isakovic, B., Bertoldi, B., Tuvblad, C., Cucurachi, S., Raine, A., Baker, L., Ling, S., & Evans, B. E. (2023). Hypothalamic-pituitary-adrenal axis responsivity during adolescence in relation to psychopathic personality traits later in life. Acta Psychologica, 241, 104055. doi:10.1016/j. actpsy.2023.104055
11. P, S., & Vellapandian, C. (2024). Hypothalamic-pituitary-adrenal (HPA) axis: Unveiling the potential mechanisms involved in stress-induced alzheimer’s disease and depression. Cureus. doi:10.7759/cureus.67595
12. Voulgaropoulou, S. D., Fauzani, F., Pfirrmann, J., Vingerhoets, C., van Amelsvoort, T., & Hernaus, D. (2022). Asymmetric effects of acute stress on cost and benefit learning. Psychoneuroendocrinology, 138, 105646. doi:10.1016/j.psyneuen.2021.105646
13. Pluut, H., Curșeu, P. L., & Fodor, O. C. (2022). Development and validation of a short measure of emotional, physical, and behavioral markers of eustress and distress (MEDS). Healthcare, 10(2), 339. doi:10.3390/ healthcare10020339
14. Ahmed, F., Dubey, D. K., Garg, R., & Srivastava, R. (2023). Effects of examination-induced stress on memory and blood pressure. Journal of Family Medicine and Primary Care, 12(11), 2757–2762. doi:10.4103/jfmpc.jfmpc_925_23
15. McGinley, M. J., Vinck, M., Reimer, J., Batista-Brito, R., Zagha, E., Cadwell, C. R., Tolias, A. S., Cardin, J. A., & McCormick, D. A. (2015). Waking state: Rapid variations modulate neural and behavioral responses. Neuron, 87(6), 1143–1161. doi:10.1016/j.neuron.2015.09.012
16. Bray, E. E., MacLean, E. L., & Hare, B. A. (2015). Increasing arousal enhances inhibitory control in calm but not excitable dogs. Animal Cognition, 18(6), 1317–1329. doi:10.1007/s10071-015-0901-1
17. Mariotti, A. (2015). The effects of chronic stress on health: New insights into the molecular mechanisms of brain–body communication. Future Science OA, 1(3). doi:10.4155/fso.15.21
18. khan, A., Song, M., & Dong, Z. (2025). Chronic stress: A fourth etiology in tumorigenesis? Molecular Cancer, 24(1). doi:10.1186/s12943-025-02402-x
19. Atrooz, F., Alkadhi, K. A., & Salim, S. (2021). Understanding stress: Insights from rodent models. Current Research in Neurobiology, 2, 100013. doi:10.1016/j. crneur.2021.100013
20. Zeeman, J. M., Anderson, E. B., Matt, I. C., Jarstfer, M. B., & Harris, S. C. (2025). Assessing factors that influence graduate student burnout in health professions education and identifying recommendations to support their well-being. PLOS ONE, 20(4). doi:10.1371/journal. pone.0319857
21. Szwamel, K., Kowalska, W., Mazur, E., Janus, A., Bonikowska, I., & Jasik-Pyzdrowska, J. (2025). Determinants of burnout syndrome among undergraduate nursing students in poland: A cross-sectional study. BMC Medical Education, 25(1). doi:10.1186/s12909-025-06777-9
22. Brindley, P. G., Olusanya, S., Wong, A., Crowe, L., & Hawryluck, L. (2019). Psychological ‘burnout’ in healthcare professionals: Updating our understanding, and not making it worse. Journal of the Intensive Care Society, 20(4), 358–362. doi:10.1177/1751143719842794
23. West, C. P., Dyrbye, L. N., & Shanafelt, T. D. (2018). Physician burnout: Contributors, consequences and solutions. Journal of Internal Medicine, 283(6), 516–529. doi:10.1111/joim.12752
24. Maslach, C., & Leiter, M. P. (2016). Understanding the burnout experience: Recent research and its implications for psychiatry. World Psychiatry, 15(2), 103–111. doi:10.1002/wps.20311
25. Andelic, N., Allan, J., Bender, K. A., Powell, D., & Theodossiou, I. (2023). Stress in performance-related pay: The effect of payment contracts and social-evaluative threat. Stress, 26(1). doi:10.1080/10253890.2023.228343
5
26. Ibar, C., Fortuna, F., Gonzalez, D., Jamardo, J., Jacobsen, D., Pugliese, L., Giraudo, L., Ceres, V., Mendoza, C., Repetto, E. M., Reboredo, G., Iglesias, S., Azzara, S., Berg, G., Zopatti, D., & Fabre, B. (2021). Evaluation of stress, burnout and hair cortisol levels in health workers at a university hospital during COVID-19 pandemic. Psychoneuroendocrinology, 128, 105213. doi:10.1016/j. psyneuen.2021.105213
27. Rohleder, N. (2019). Stress and inflammation – The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology, 105, 164–171. doi:10.1016/j.psyneuen.2019.02.021
28. Herman, J. P., McKlveen, J. M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., Scheimann, J., & Myers, B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology, 6(2), 603–621. doi:10.1002/j.2040-4603.2016.tb00694.x
29. Schindler, S., Schmidt, L., Stroske, M., Storch, M., Anwander, A., Trampel, R., Strauß, M., Hegerl, U., Geyer, S., & Schönknecht, P. (2018). Hypothalamus enlargement in mood disorders. Acta Psychiatrica Scandinavica, 139(1), 56–67. doi:10.1111/acps.12958
30. Al salman, J. M., Al Agha, R. A., & Helmy, M. (2017). Pituitary abscess. BMJ Case Reports. doi:10.1136/bcr-2016217912
31. Johnson, K., Peterson, J., Kopper, J., & Dembek, K. (2023). The hypothalamic-pituitary-adrenal axis response to ovine corticotropin-releasing-hormone stimulation tests in healthy and hospitalized foals. Journal of Veterinary Internal Medicine, 37(1), 292–301. doi:10.1111/jvim.16604
32. Gjerstad, J. K., Lightman, S. L., & Spiga, F. (2018). Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress, 21(5), 403–416. doi:10.1080/ 10253890.2018.1470238
33. Wadsworth, M. E., Broderick, A. V., Loughlin-Presnal, J. E., Bendezu, J. J., Joos, C. M., Ahlkvist, J. A., Perzow, S. E., & McDonald, A. (2019). Co-activation of SAM and HPA responses to acute stress: A review of the literature and test of differential associations with preadolescents’ internalizing and externalizing. Developmental Psychobiology, 61(7), 1079–1093. doi:10.1002/dev.21866
34. Barbayannis, G., Bandari, M., Zheng, X., Baquerizo, H., Pecor, K. W., & Ming, X. (2022). Academic stress and mental well-being in college students: Correlations, affected groups, and covid-19. Frontiers in Psychology, 13. doi:10.3389/fpsyg.2022.886344
35. Penz, M., Stalder, T., Miller, R., Ludwig, V. M., Kanthak, M. K., & Kirschbaum, C. (2018). Hair cortisol as a biological marker for burnout symptomatology. Psychoneuroendocrinology, 87, 218–221. doi:10.1016/j.psyneuen.2017.07.485
36. Pilger, A., Haslacher, H., Meyer, B. M., Lackner, A., Nassan-Agha, S., Nistler, S., Stangelmaier, C., Endler, G., Mikulits, A., Priemer, I., Ratzinger, F., Ponocny-Seliger, E., Wohlschläger-Krenn, E., Teufelhart, M., Täuber, H., Scherzer, T. M., Perkmann, T., Jordakieva, G., Pezawas, L., & Winker, R. (2018). Midday and nadir salivary cortisol appear superior to cortisol awakening response in burnout assessment and monitoring. Scientific Reports, 8(1). doi:10.1038/s41598-018-27386-1
37. Penz, M., Siegrist, J., Wekenborg, M. K., Rothe, N., Walther, A., & Kirschbaum, C. (2019). Effort-reward imbalance at work is associated with hair cortisol concentrations: Prospective evidence from the dresden burnout study. Psychoneuroendocrinology, 109, 104399. doi:10.1016/j.psyneuen.2019.104399
38. Rohleder, N. (2018). Burnout, hair cortisol, and timing: Hyper- or hypocortisolism? Psychoneuroendocrinology, 87, 215–217. doi:10.1016/j.psyneuen.2017.10.008
39. Kaltenegger, H. C., Marques, M. D., Becker, L., Rohleder, N., Nowak, D., Wright, B. J., & Weigl, M. (2024). Prospective associations of technostress at work, burnout symptoms, hair cortisol, and chronic low-grade inflammation. Brain, Behavior, and Immunity, 117, 320–329. doi:10.1016/j.bbi.2024.01.222
40. Counts, C. J., Ginty, A. T., Larsen, J. M., Kampf, T. D., & John-Henderson, N. A. (2022). Childhood trauma and cortisol reactivity: An investigation of the role of task appraisals. Frontiers in Psychology, 13. doi:10.3389/ fpsyg.2022.803339
41. Wekenborg, M. K., von Dawans, B., Hill, L. K., Thayer, J. F., Penz, M., & Kirschbaum, C. (2019). Examining reactivity patterns in burnout and other indicators of chronic stress. Psychoneuroendocrinology, 106, 195–205. doi:10.1016/j.psyneuen.2019.04.002
42. Sahiti, F., Detomas, M., Cejka, V., Hoffmann, K., Gelbrich, G., Frantz, S., Kroiss, M., Heuschmann, P. U., Hahner, S., Fassnacht, M., Deutschbein, T., Störk, S., & Morbach, C. (2025). The impact of hypercortisolism beyond metabolic syndrome on left ventricular performance: A myocardial work analysis. Cardiovascular Diabetology, 24(1). doi:10.1186/s12933-025-02680-1
43. Crawford, A. A., Soderberg, S., Kirschbaum, C., Murphy, L., Eliasson, M., Ebrahim, S., Davey Smith, G., Olsson, T., Sattar, N., Lawlor, D. A., Timpson, N. J., Reynolds, R. M., & Walker, B. R. (2019). Morning plasma cortisol as a cardiovascular risk factor: Findings from prospective cohort and mendelian randomization studies. European Journal of Endocrinology, 181(4), 429–438. doi:10.1530/ eje-19-0161
44. Willette, A. A., Bendlin, B. B., Starks, E. J., Birdsill, A. C., Johnson, S. C., Christian, B. T., Okonkwo, O. C., La Rue, A., Hermann, B. P., Koscik, R. L., Jonaitis, E. M., Sager, M. A., & Asthana, S. (2015). Association of insulin resistance with cerebral glucose uptake in late middle–aged adults at risk for alzheimer disease. JAMA Neurology, 72(9), 1013. doi:10.1001/jamaneurol.2015.0613
45. Fan, H.-P., Zhou, Y., Zhou, Y., Jin, J., & Hu, T.-Y. (2023). Association between short-term systemic use of glucocorticoids and prognosis of cardiogenic shock: A retrospective analysis. BMC Anesthesiology, 23(1). doi:10.1186/s12871-023-02131-y
46. Scheen, M., Giraud, R., & Bendjelid, K. (2021). Stress hyperglycemia, cardiac glucotoxicity, and critically ill patient outcomes current clinical and pathophysiological evidence. Physiological Reports, 9(2). doi:10.14814/ phy2.14713
47. Song, G., Liu, X., Lu, Z., Guan, J., Chen, X., Li, Y., Liu, G., Wang, G., & Ma, F. (2025). Relationship between stress hyperglycaemic ratio (SHR) and critical illness: A systematic review. Cardiovascular Diabetology, 24(1). doi:10.1186/s12933-025-02751-3
48. Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L. (2017). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), 7204–7218. doi:10.18632/oncotarget.23208
49. Fritsche, K. L. (2015). The science of fatty acids and inflammation. Advances in Nutrition, 6(3). doi:10.3945/ an.114.006940
50. Ma, X., Nan, F., Liang, H., Shu, P., Fan, X., Song, X., Hou, Y., & Zhang, D. (2022). Excessive intake of sugar: An accomplice of inflammation. Frontiers in Immunology, 13. doi:10.3389/fimmu.2022.988481
51. de Punder, K., & Pruimboom, L. (2015). Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Frontiers in Immunology, 6. doi:10.3389/fimmu.2015.00223
52. Röder, P. V., Wu, B., Liu, Y., & Han, W. (2016). Pancreatic Regulation of Glucose Homeostasis. Experimental & Molecular Medicine, 48(3), 1–19. https://doi.org/10.1038/ emm.2016.6
53. Li, M., Chi, X., Wang, Y., Setrerrahmane, S., Xie, W., & Xu, H. (2022). Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduction and Targeted Therapy, 7(1). doi:10.1038/s41392022-01073-0
54. Khodabandehloo, H., Gorgani-Firuzjaee, S., Panahi, G., & Meshkani, R. (2016). Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Translational Research, 167(1), 228–256. doi:10.1016/j.trsl.2015.08.011
55. Fernandez-Montero, A., García-Ros, D., Sánchez-Tainta, A., Rodriguez-Mourille, A., Vela, A., & Kales, S. N. (2019). Burnout syndrome and increased insulin resistance. Journal of Occupational & Environmental Medicine, 61(9), 729–734. doi:10.1097/jom.0000000000001645
56. Beaupere, C., Liboz, A., Fève, B., Blondeau, B., & Guillemain, G. (2021). Molecular mechanisms of glucocorticoid-induced insulin resistance. International Journal of Molecular Sciences, 22(2), 623. doi:10.3390/ ijms22020623
57. Hackett, R. A., Kivimäki, M., Kumari, M., & Steptoe, A. (2016). Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the whitehall II cohort study. The Journal of Clinical Endocrinology & Metabolism, 101(2), 619–625. doi:10.1210/jc.2015-2853
58. Liyanarachchi, K., Ross, R., & Debono, M. (2017). Human studies on hypothalamo-pituitary-adrenal (HPA) axis. Best Practice & Research Clinical Endocrinology & Metabolism, 31(5), 459–473. doi:10.1016/j.beem.2017.10.011
59. Hinds, J. A., & Sanchez, E. R. (2022). The role of the hypothalamus–pituitary–adrenal (HPA) axis in test-induced anxiety: Assessments, physiological responses, and molecular details. Stresses, 2(1), 146–155. doi:10.3390/stresses2010011
60. Tian, Y. E., Di Biase, M. A., Mosley, P. E., Lupton, M. K., Xia, Y., Fripp, J., Breakspear, M., Cropley, V., & Zalesky, A. (2023). Evaluation of brain-body health in individuals with common neuropsychiatric disorders. JAMA Psychiatry, 80(6), 567. doi:10.1001/jamapsychiatry.2023.0791
61. Reilly, S., Olier, I., Planner, C., Doran, T., Reeves, D., Ashcroft, D. M., Gask, L., & Kontopantelis, E. (2015). Inequalities in physical comorbidity: A longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open, 5(12). doi:10.1136/bmjopen-2015-009010
62. Cyr, S., Marcil, M.-J., Houchi, C., Marin, M.-F., Rosa, C., Tardif, J.-C., Guay, S., Guertin, M.-C., Genest, C., Forest, J., Lavoie, P., Labrosse, M., Vadeboncoeur, A., Selcer, S., Ducharme, S., & Brouillette, J. (2022). Evolution of burnout and psychological distress in healthcare workers during the COVID-19 pandemic: A 1-year observational study. BMC Psychiatry, 22(1). doi:10.1186/s12888022-04457-2
63. Sato, A., Hashimoto, T., Kimura, A., Niitsu, T., & Iyo, M. (2018). Psychological distress symptoms associated with life events in patients with bipolar disorder: A cross-sectional study. Frontiers in Psychiatry, 9. doi:10.3389/fpsyt.2018.00200
64. Yang, J., Jia, Y., Guo, T., Zhang, S., Huang, J., Lu, H., Li, L., Xu, J., Liu, G., & Xiao, K. (2025). Comparative analysis of hpa-axis dysregulation and dynamic molecular mechanisms in acute versus chronic social defeat stress. International Journal of Molecular Sciences, 26(13), 6063. doi:10.3390/ijms26136063
65. Valli, I., Crossley, N. A., Day, F., Stone, J., Tognin, S., Mondelli, V., Howes, O., Valmaggia, L., Pariante, C., & McGuire, P. (2016). HPA-axis function and grey matter volume reductions: Imaging the diathesis-stress model in individuals at ultra-high risk of psychosis. Translational Psychiatry, 6(5). doi:10.1038/tp.2016.68
66. Abe, K., Tei, S., Takahashi, H., & Fujino, J. (2022). Structural brain correlates of burnout severity in medical professionals: A voxel-based morphometric study. Neuroscience Letters, 772, 136484. doi:10.1016/j. neulet.2022.136484
67. White, S., Mauer, R., Lange, C., Klimecki, O., Huijbers, W., & Wirth, M. (2023). The effect of plasma cortisol on hippocampal atrophy and clinical progression in mild cognitive impairment. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 15(3). doi:10.1002/ dad2.12463
68. Tao, N., Zhang, J., Song, Z., Tang, J., & Liu, J. (2015). Relationship between job burnout and neuroendocrine indicators in soldiers in the xinjiang arid desert: A cross-sectional study. International Journal of Environmental Research and Public Health, 12(12), 15154–15161. doi: 10.3390/ijerph121214977
69. Serrano, F. T., Calderón Nossa, L. T., Gualdrón Frías, C. A., Mogollón G., J. D., & Mejía, C. R. (2023). Burnout syndrome and depression in students of a colombian medical school, 2018. Revista Colombiana de Psiquiatría (English Ed.), 52(4), 345–351. doi:10.1016/j. rcpeng.2021.09.001
70. Richemond, D., Needham, M., & Jean, K. (2022). The effects of nurse burnout on patient experiences. Open Journal of Business and Management, 10(05), 2805–2828. doi:10.4236/ojbm.2022.105139
71. Kiratipaisarl, W., Surawattanasakul, V., & Sirikul, W. (2024). Individual and organizational interventions to reduce burnout in resident physicians: A systematic review and meta-analysis. BMC Medical Education, 24(1). doi:10.1186/s12909-024-06195-3
72. Gómez-Polo, C., Casado, A. M., & Montero, J. (2022). Burnout syndrome in dentists: Work-related factors. Journal of Dentistry, 121, 104143. doi:10.1016/j. jdent.2022.104143
73. Rosales-Ricardo, Y., & Ferreira, J. P. (2022). Effects of physical exercise on burnout syndrome in university students. MEDICC Review, 24(1), 36. doi:10.37757/ mr2022.v24.n1.7
74. Hilcove, K., Marceau, C., Thekdi, P., Larkey, L., Brewer, M. A., & Jones, K. (2020). Holistic nursing in practice: Mindfulness-based yoga as an intervention to manage stress and burnout. Journal of Holistic Nursing, 39(1), 29–42. doi:10.1177/0898010120921587
75. Pietrelli, A., Matković, L., Vacotto, M., Lopez-Costa, J. J., Basso, N., & Brusco, A. (2018). Aerobic exercise upregulates the BDNF-serotonin systems and improves the cognitive function in rats. Neurobiology of Learning and Memory, 155, 528–542. doi:10.1016/j.nlm.2018.05.007
76. Taylor, H., Cavanagh, K., Field, A. P., & Strauss, C. (2022). Health care workers’ need for headspace: Findings from a multisite definitive randomized controlled trial of an unguided digital mindfulness-based self-help app to reduce healthcare worker stress. JMIR mHealth and uHealth, 10(8). doi:10.2196/31744
77. Ameli, R., Sinaii, N., West, C. P., Luna, M. J., Panahi, S., Zoosman, M., Rusch, H. L., & Berger, A. (2020). Effect of a brief mindfulness-based program on stress in health care professionals at a US biomedical research hospital. JAMA Network Open, 3(8). doi:10.1001/jamanetworkopen.2020.13424
78. Lamothe, M., Rondeau, É., Duval, M., McDuff, P., Pastore, Y. D., & Sultan, S. (2020). Changes in hair cortisol and self-reported stress measures following mindfulness-based stress reduction (MBSR): A proof-of-concept study in pediatric hematology-oncology professionals. Complementary Therapies in Clinical Practice, 41, 101249. doi:10.1016/j.ctcp.2020.101249
79. Profit, J., Adair, K. C., Cui, X., Mitchell, B., Brandon, D., Tawfik, D. S., Rigdon, J., Gould, J. B., Lee, H. C., Timpson, W. L., McCaffrey, M. J., Davis, A. S., Pammi, M., Matthews, M., Stark, A. R., Papile, L.-A., Thomas, E., Cotten, M., Khan, A., & Sexton, J. B. (2021). Randomized controlled trial of the “wiser” intervention to reduce healthcare worker burnout. Journal of Perinatology, 41(9), 2225–2234. doi:10.1038/s41372-021-01100-y
80. Linzer, M., Poplau, S., Grossman, E., Varkey, A., Yale, S., Williams, E., Hicks, L., Brown, R. L., Wallock, J., Kohnhorst, D., & Barbouche, M. (2015). A cluster randomized trial of interventions to improve work conditions and clinician burnout in primary care: Results from the healthy work place (HWP) study. Journal of General Internal Medicine, 30(8), 1105–1111. doi:10.1007/s11606015-3235-4
81. Kiser, S. B., Sterns, J. D., Lai, P. Y., Horick, N. K., & Palamara, K. (2024). Physician coaching by professionally trained peers for burnout and well-being. JAMA Network Open, 7(4). doi:10.1001/jamanetworkopen.2024.5645
82. Linzer, M., Jin, J. O., Shah, P., Stillman, M., Brown, R., Poplau, S., Nankivil, N., Cappelucci, K., & Sinsky, C. A. (2022). Trends in clinician burnout with associated mitigating and aggravating factors during the COVID-19 pandemic. JAMA Health Forum, 3(11). doi:10.1001/jamahealthforum.2022.4163
83. DeChant, P. F., Acs, A., Rhee, K. B., Boulanger, T. S., Snowdon, J. L., Tutty, M. A., Sinsky, C. A., & Thomas Craig, K. J. (2019). Effect of organization-directed workplace interventions on physician burnout: A systematic review. Mayo Clinic Proceedings: Innovations, Quality & Outcomes, 3(4), 384–408. doi:10.1016/j. mayocpiqo.2019.07.006
84. Shanafelt, T. D., Hasan, O., Dyrbye, L. N., Sinsky, C., Satele, D., Sloan, J., & West, C. P. (2015). Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clinic Proceedings, 90(12), 1600–1613. doi:10.1016/j.mayocp.2015.08.023
85. Salvagioni, D. A., Melanda, F. N., Mesas, A. E., González, A. D., Gabani, F. L., & Andrade, S. M. (2017). Physical, psychological and occupational consequences of job burnout: A systematic review of prospective studies. PLOS ONE, 12(10). doi:10.1371/journal.pone.0185781
86. Liyanarachchi, K., Ross, R., & Debono, M. (2017). Human studies on hypothalamo-pituitary-adrenal (HPA) axis. Best Practice & Research Clinical Endocrinology & Metabolism, 31(5), 459–473. doi:10.1016/j.beem.2017.10.011
87. Adam, D., Berschick, J., Schiele, J. K., Bogdanski, M., Schröter, M., Steinmetz, M., Koch, A. K., Sehouli, J., Reschke, S., Stritter, W., Kessler, C. S., & Seifert, G. (2023). Interventions to reduce stress and prevent burnout in healthcare professionals supported by digital applications: A scoping review. Frontiers in Public Health, 11. doi:10.3389/fpubh.2023.1231266
88. Hsu, H.-C., Lee, H.-F., Hung, H.-M., Chen, Y.-L., Yen, M., Chiang, H.-Y., Chow, L.-H., Fetzer, S. J., & Mu, P.-F. (2024). Effectiveness of individual-based strategies to reduce nurse burnout: An umbrella review. Journal of Nursing Management, 2024, 1–13. doi:10.1155/2024/8544725
1. Biagioli, V., Matera, M., Ramenghi, L. A., Falsaperla, R., & Striano, P. (2025). Microbiome and Pregnancy Dysbiosis: A Narrative Review on Offspring Health. Nutrients, 17(6), 1033. https://doi.org/10.3390/nu17061033
2. Pahirah, N., Narkwichean, A., Taweechotipatr, M., Wannaiampikul, S., Duang-Udom, C., Laosooksathit, W. (2024). Comparison of Gut Microbiomes Between Neonates Born by Cesarean Section and Vaginal Delivery: Prospective Observational Study. Biomed Research International. 10.1155/bmri/8302361
3. Deady, C., FitzGerald, J., Kara, N., Mazzocchi, M., O’Mahony, A., Ionescu, M. I., Shanahan, R., Kelly, S., Crispie, F., Cotter, P. D., McCarthy, F. P., McCarthy, C., O’Keeffe, G. W., & O’Mahony, S. M. (2025). Maternal immune activation and antibiotics affect offspring neurodevelopment, behaviour, and microbiome. Brain, Behavior, & Immunity - Health, 48, 101065. https://doi.org/10.1016/j. bbih.2025.101065
4. Faysal, M., Zehravi, M., Sutradhar, B., Al Amin, M., Shanmugarajan, T. S., Arjun, U. V. N. V., Ethiraj, S., Durairaj, A., Dayalan, G., Ahamad, S. K., Rab, S. O., Raman, K., & Emran, T. B. (2025). The Microbiota-Gut-Brain Connection: A New Horizon in Neurological and Neuropsychiatric Disorders. CNS Neuroscience & Therapeutics, 31(9), e70593. https://doi.org/10.1111/cns.70593
5. Forsythe, P., Sudo, N., Dinan, T., Taylor, V. H., & Bienenstock, J. (2010). Mood and gut feelings. Brain, Behavior, and Immunity, 24(1), 9–16. https://doi.org/10.1016/j. bbi.2009.05.058
6. Chen, X., Lu, Y., Chen, T., Li, R. (2021). The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Frontiers in Cellular and Infection Microbiology, 11. https:// doi.org/10.3389/fcimb.2021.631972
7. Gur, T. L., Worly, B. L., & Bailey, M. T. (2015). Stress and the commensal microbiota: Importance in parturition and infant neurodevelopment. Frontiers in Psychiatry, 6, 5. https://doi.org/10.3389/fpsyt.2015.00005
8. Hasan, A., Scuderi, S.A., Capra, A.P., Giosa, D., Bonomo, A., Ardissone, A., Esposito, E. (2025). An Updated and Comprehensive Review Exploring the Gut–Brain Axis in Neurodegenerative Disorders and Neurotraumas: Implications for Therapeutic Strategies. Brain Sciences, 15(6), 654. https://doi.org/10.3390/brainsci15060654
9. Hu, J., Ly, J., Zhang, W., Huang, Y., Glover, V., Peter, I., Hurd, Y. L., & Nomura, Y. (2019). Microbiota of newborn meconium is associated with maternal anxiety experienced during pregnancy. Developmental Psychobiology, 61(5), 640–649. https://doi.org/10.1002/dev.21837
10. Vuong, H., Coley, E.J.L., Kazantsev, M., Cooke, M.E., Rendon, T.K., Paramo, J., Hsiao, E.Y. (2021). Interactions between maternal fluoxetine exposure, the maternal gut microbiome and fetal neurodevelopment in mice. Behavioral Brain Research. 10.1016/j.bbr.2021.113353
11. Jameson, K. G., Kazmi, S. A., Ohara, T. E., Son, C., Yu, K. B., Mazdeyasnan, D., Leshan, E., Vuong, H. E., Paramo, J., Lopez-Romero, A., Yang, L., Schweizer, F. E., & Hsiao, E. Y. (2025). Select microbial metabolites in the small intestinal lumen regulate vagal activity via receptor-mediated signaling. iScience, 28(3), 112059. https:// doi.org/10.1016/j.isci.2025.112059
12. Jašarević, E., Rodgers, A. B., & Bale, T. L. (2014). A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiology of Stress, 1, 81–88. https://doi.org/10.1016/j.ynstr.2014.10.005
13. Liu, S., Chen, Y., Zhang, K., Tang, D., Zhang, J., Wang, Y., Zhao, J., Li, D., & Wang, T. (2025). Exploring vaginal microbiome: From traditional methods to metagenomic next-generation sequencing—a systematic review. Frontiers in Microbiology, 16. https://doi.org/10.3389/ fmicb.2025.1578681
14. Ma, G., Chen, Z., Li, Z., & Xiao, X. (2024). Unveiling the neonatal gut microbiota: Exploring the influence of delivery mode on early microbial colonization and intervention strategies. Archives of Gynecology and Obstetrics, 310(6), 2853–2861. https://doi.org/10.1007/ s00404-024-07843-1
15. Matharu, D., Ponsero, A. J., Dikareva, E., Korpela, K., Kolho, K.-L., de Vos, W. M., & Salonen, A. (2022). Bacteroides abundance drives birth mode dependent infant gut microbiota developmental trajectories. Frontiers in Microbiology, 13, 953475. https://doi.org/10.3389/ fmicb.2022.953475
16. Mavel, S., Pellé, L., & Andres, C. R. (2025). Impact of maternal microbiota imbalance during pregnancy on fetal cerebral neurodevelopment: Is there a link to certain autistic disorders? Brain, Behavior, & Immunity - Health, 48, 101074. https://doi.org/10.1016/j.bbih.2025.101074
17. Ahmed, U., Fatima, F., Farooq, H.A. (2025). Microbial dysbiosis and associated disease mechanisms in maternal and child health. Infection and Immunity, 93(8), e0017925. 10.1128/iai.00179-25
18. Murine maternal microbiome modifies adverse effects of protein undernutrition on offspring neurobehaviour | Nature Microbiology. (n.d.). Retrieved September 14, 2025, from https://www.nature.com/articles/s41564025-02022-7
19. O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., & Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Research, 277, 32–48. https://doi.org/10.1016/j. bbr.2014.07.027
20. Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: A pilot randomised placebo-controlled trial—PubMed. (n.d.). Retrieved September 14, 2025, from https://pubmed.ncbi.nlm.nih. gov/34186487/
21. Radford-Smith, D. E., & Anthony, D. C. (2023). Mechanisms of Maternal Diet-Induced Obesity Affecting the Offspring Brain and Development of Affective Disorders. Metabolites, 13(3), 455. https://doi.org/10.3390/ metabo13030455
22. Shekarabi, A., Qureishy, I., Puglisi, C. H., Dalseth, M., & Vuong, H. E. (2024). Host–microbe interactions: Communication in the microbiota–gut–brain axis. Current Opinion in Microbiology, 80, 102494. https://doi. org/10.1016/j.mib.2024.102494
23. Coley-O’Rourke, E.J., Lum, G.R., Pronovost, G.N., Yu, L.W., Özcan, E., Yu, K.B., McDermott, J., Chakhoyan, A., Golman, E., Vuong, H.E., Paramo, J., McCune, S., Sejane, K., Renwick, S., Bode, L., Chu, A., Calkins, K.L., Hsiao, E.Y. (2025). The influence of maternal gut and vaginal microbiota on gastrointestinal colonization of neonates born vaginally and per caesarean section. Nature Microbiology, 10, 1648-1664. https://doi.org/10.1038/ s41564-025-02022-7
24. Thursby, E., & Juge, N. (2017). Introduction to the human gut microbiota. Biochemical Journal, 474(11), 1823–1836. https://doi.org/10.1042/BCJ20160510
25. Tochitani, S., Tsukahara, T., & Inoue, R. (2024). Perturbed maternal microbiota shapes offspring microbiota during early colonization period in mice. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences, 100(6), 335–352. https://doi.org/10.2183/ pjab.100.020
26. Vuong, H. E. (2022). Chapter One—Intersections of the microbiome and early neurodevelopment. In T. R. Sampson (Ed.), International Review of Neurobiology (Vol. 167, pp. 1–23). Academic Press. https://doi.org/10.1016/ bs.irn.2022.06.004
27. Vuong, H. E., Pronovost, G. N., Williams, D. W., Coley, E. J. L., Siegler, E. L., Qiu, A., Kazantsev, M., Wilson, C. J., Rendon, T., & Hsiao, E. Y. (2020). The maternal microbiome modulates fetal neurodevelopment in mice. Nature, 586(7828), 281–286. https://doi.org/10.1038/ s41586-020-2745-3
28. Xie, H., Meng, L., Duan, X., Liang, X., Huang, T., Ma, G., Luo, H., Tang, X., & Xiao, X. (2025). Establishment of the early gut microbiota in vaginally delivered infants: The influence of maternal gut microbiota outweighs vaginal microbiota. Microbiology Spectrum, 13(9), e0177525. https://doi.org/10.1128/spectrum.01775-25
PICTURE PERFECT: THE NEW REALITY OF REHABILITATION IS VIRTUAL
1. Alessandro, C., Beckers, N., Goebel, P., Resquin, F., González, J., & Osu, R. (2015). Motor Control and Learning Theories. Biosystems & Biorobotics, 225-250. doi: 10.1007/978-3-319-24901-8_9
2. Shen, Y., Jiang, L., Lai, J., Hu, J., Liang, F., Zhang, X., & Ma, F. (2025). A comprehensive review of rehabilitation approaches for traumatic brain injury: Efficacy and outcomes. Frontiers in Neurology, 16. doi: 10.3389/ fneur.2025.1608645
3. Bateni, H., Carruthers, J., Mohan, R., & Pishva, S. (2024). Use of virtual reality in physical therapy as an intervention and diagnostic tool. Rehabilitation Research and Practice, 1-9. doi: 10.1155/2024/1122286
4. Wojciechowski, A., & Korjonen-Kuusipuro, K. (2022). Rehabilitation in digital environments – biophysiologically motivated gamification. Human Technology, 18(3), 209212. doi: 10.14254/1795-6889.2022.18-3.1
5. Hamad, A., & Jia, B. (2022). How Virtual Reality Technology Has Changed Our Lives: An Overview of the Current and Potential Applications and Limitations. International Journal of Environmental Research and Public Health, 19(18). doi: 10.3390/ijerph191811278
6. Catania, V., Rundo, F., Panerai, S., & Ferri, R. (2024). Virtual Reality for the Rehabilitation of Acquired Cognitive Disorders: A Narrative Review. Bioengineering, 11(1), 35. doi:10.3390/bioengineering11010035
7. Drigas, A., & Sideraki, A. (2024). Brain Neuroplasticity Leveraging Virtual Reality and Brain-Computer Interface Technologies. Sensors (Basel, Switzerland), 24(17), 5725. doi:10.3390/s24175725
8. Georgiev, D., Georgieva, I., Gong, Z., Nanjappan, V., & Georgiev, G. (2021). Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sciences, 11(2), 221. doi: 10.3390/brainsci11020221
9. Rabinovici, G. D., Stephens, M. L., & Possin, K. L. (2015). Executive dysfunction. Continuum (Minneapolis, Minn.), 21(3), 646–659. doi: 10.1212/01. CON.0000466658.05156.54
10. Cowan, N., Bao, C., Bishop-Chrzanowski, B. M.Costa, A., Greene, N. R., Guitard, D., Li, C., Musich, M., & Ünal, Z. E. (2023). The Relation Between Attention and Memory. Annual Review of Psychology, 75(1). doi: 10.1146/annurev-psych-040723-012736
11. Harvey, P. (2019). Cognition in Mental Health. Dialogues in Clinical Neuroscience, 21(3). doi: 10.31887/ dcns.2019.21.3
12. Shi, Y., & Qu, S. (2021). Cognitive Ability and Self-Control’s Influence on High School Students’ Comprehensive Academic Performance. Frontiers in Psychology, 12. doi: 10.3389/fpsyg.2021.783673
13. Shi, L. (2024). Research on the impact of virtual reality games on cognitive abilities. Transactions on Computer Science and Intelligent Systems Research, 7, 44–48. doi: 10.62051/qkn7tx27
14. Keshner, E. A., & Lamontagne, A. (2021). The Untapped Potential of Virtual Reality in Rehabilitation of Balance and Gait in Neurological Disorders. Frontiers in Virtual Reality, 2. doi: 10.3389/frvir.2021.641650
15. Maggio, M. G., Bonanno, L., Rizzo, A., Barbera, M., Benenati, A., Impellizzeri, F., Corallo, F., De Luca, R., Quartarone, A., & Calabrò, R. S. (2025). The role of virtual reality-based cognitive training in enhancing motivation and cognitive functions in individuals with chronic stroke. Scientific Reports, 15(1). doi: 10.1038/s41598025-08173-1
16. Quan, W., Liu, S., Cao, M., & Zhao, J. (2024). A Comprehensive Review of Virtual Reality Technology for Cognitive Rehabilitation in Patients with Neurological Conditions. Applied Sciences, 14(14), 6285. doi: 10.3390/ app14146285
17. Fang, C., Lv, L., Mao, S., Dong, H., & Liu, B. (2020). Cognition deficits in parkinson’s disease: Mechanisms and treatment. Parkinson’s Disease, 2020(1), 1-11. doi: 10.1155/2020/2076942
18. Chan, A., Ouyang, J., Nguyen, K., Jones, A., Basso, S., & Karasik, R. (2024). Traumatic brain injuries: a neuropsychological review. Frontiers in Behavioral Neuroscience, 18. doi: 10.3389/fnbeh.2024.1326115
19. Muñoz, D., Barria, P., Cifuentes, C. A., Aguilar, R., Baleta, K., Azorín, J. M., & Múnera, M. (2022). EEG Evaluation in a Neuropsychological Intervention Program Based on Virtual Reality in Adults with Parkinson's Disease. Biosensors, 12(9), 751. doi: 10.3390/bios12090751
20. Zanier, E. R., Zoerle, T., Di Lernia, D., & Riva, G. (2018). Virtual Reality for Traumatic Brain Injury. Frontiers in Neurology, 9. doi: 10.3389/fneur.2018.00345
21. Teo, W.-P., Muthalib, M., Yamin, S., Hendy, A. M., Bramstedt, K., Kotsopoulos, E., Perrey, S., & Ayaz, H. (2016). Does a combination of virtual reality, neuromodulation and neuroimaging provide a comprehensive platform for neurorehabilitation? – A narrative review of the literature. Frontiers in Human Neuroscience, 10. doi: 10.3389/fnhum.2016.00284
22. Richlan, F. (2025). Behavioral and neuroanatomical effects of soccer heading training in virtual reality: A longitudinal fMRI case study. Neuropsychologia, 211, 109124. doi: 10.1016/j.neuropsychologia.2025.109124
23. Tremmel, C., Herff, C., Sato, T., Rechowicz, K., Yamani, Y., & Krusienski, D. J. (2019). Estimating Cognitive Workload in an Interactive Virtual Reality Environment Using EEG. Frontiers in Human Neuroscience, 13. doi: 10.3389/ fnhum.2019.00401
24. Liu, X.-Y., Wang, W.-L., Liu, M., Chen, M.-Y., Pereira, T., Doda, D. Y., Ke, Y.-F., Wang, S.-Y., Wen, D., Tong, X.-G., Li, W.-G., Yang, Y., Han, X.-D., Sun, Y.-L., Song, X., Hao, C.-Y., Zhang, Z.-H., Liu, X.-Y., Li, C.-Y., … Ming, D. (2025). Recent applications of EEG-based brain-computer-interface in the medical field. Military Medical Research, 12(1). doi: 10.1186/s40779-025-00598-z
25. Wang, Y., Weng, T., Tsai, I., Kao, J., & Chang, Y. (2022). Effects of virtual reality on creativity performance and perceived immersion: A study of brain waves. British Journal of Educational Technology. doi: 10.1111/bjet.13264
26. Rueda-Castro, V., Chacon, J., Azofeifa, J. D., Caratozzolo, P., & Camacho-Leon, S. (2025). Modulation of alpha and theta waves by social stimuli in virtual educational environments. Scientific Reports, 15(1). doi: 10.1038/ s41598-025-13027-x
27. Moulaei, K., Sharifi, H., Bahaadinbeigy, K., & Dinari, F. (2024). Efficacy of virtual reality-based training programs and games on the improvement of cognitive disorders in patients: A systematic review and meta-analysis. BMC Psychiatry, 24(1). doi: 10.1186/s12888024-05563-z
28. Eggenberger, P., Schumacher, V., Angst, M., Theill, N., & de Bruin, E. (2015). Does multicomponent physical exercise with simultaneous cognitive training boost cognitive performance in older adults? A 6-month randomized controlled trial with a 1-year follow-up. Clinical Interventions in Aging, 10, 1335-1349. doi: 10.2147/cia. s87732
29. Zhang, B., Li, D., Liu, Y., Wang, J., & Xiao, Q. (2021). Virtual reality for limb motor function, balance, gait, cognition and daily function of stroke patients: A systematic review and meta-analysis. Journal of advanced nursing, 77(8), 3255–3273. doi: 10.1111/jan.14800
30. Nettles, G., Greene, R., Cancer, A., & Stasher-Booker, B. (2023). Physical Therapy. Springer EBooks, 85-97. doi: 10.1007/978-3-031-40889-2_6
31. Calabrò, R. S., Naro, A., Russo, M., Leo, A., De Luca, R., Balletta, T., Buda, A., La Rosa, G., Bramanti, A., & Bramanti, P. (2017). The role of virtual reality in improving motor performance as revealed by EEG: A randomized clinical trial. Journal of NeuroEngineering and Rehabilitation, 14(1). doi: 10.1186/s12984-017-0268-4
32. Jacobson, S., Marcus, E. M., & Pugsley, S. (2017). Motor System, Movement, and Motor Pathways. Springer EBooks, 331-360. doi: 10.1007/978-3-319-60187-8_11
33. Fink, K. L., & Cafferty, W. B. J. (2016). Reorganization of Intact Descending Motor Circuits to Replace Lost Connections After Injury. Neurotherapeutics, 13(2), 370-381. doi: 10.1007/s13311-016-0422-x
34. Inoue, T., & Ueno, M. (2025). The diversity and plasticity of descending motor pathways rewired after stroke and trauma in rodents. Frontiers in Neural Circuits, 19. doi: 10.3389/fncir.2025.1566562
35. Naqvi, W. M., Naqvi, I., Mishra, G. V., & Vishnu Vardhan. (2024). The Dual Importance of Virtual Reality Usability in Rehabilitation: A Focus on Therapists and Patients. Curēus, 16(3). doi: 10.7759/cureus.56724
36. Schalbetter, L., Grêt-Regamey, A., Gutscher, F., & Wissen Hayek, U. (2025). High-fidelity immersive virtual reality environments for gait rehabilitation exergames. Frontiers in Virtual Reality, 5. doi: 10.3389/frvir.2024.1502802
37. Atilla, F., Postma, M., & Alimardani, M. (2024). Gamification of Motor Imagery Brain-Computer Interface Training Protocols: a systematic review. Computers in Human Behavior Reports, 100508-100508. doi: 10.1016/j. chbr.2024.100508
38. Wen, X., Li, L., Li, X., Zha, H., Liu, Z., Peng, Y., Liu, X., Liu, H., Yang, Q., & Wang, J. (2022). Therapeutic role of additional mirror therapy on the recovery of upper extremity motor function after stroke: A single-blind, randomized controlled trial. Neural Plasticity, 2022, 1–9. doi: 10.1155/2022/8966920
39. Özen, Ö., Buetler, K. A., & Marchal-Crespo, L. (2022). Towards functional robotic training: motor learning of dynamic tasks is enhanced by haptic rendering but hampered by arm weight support. Journal of NeuroEngineering and Rehabilitation, 19(1). doi: 10.1186/s12984022-00993-w
40. Jo, S., Jang, H., Kim, H., & Song, C. (2024). 360° immersive virtual reality-based mirror therapy for upper extremity function and satisfaction among stroke patients: a randomized controlled trial. European Journal of Physical and Rehabilitation Medicine, 60(2), 207-215. doi: 10.23736/S1973-9087.24.08275-3
41. Nagamine, T. (2025). Challenges in using virtual reality technology for pain relief. World Journal of Clinical Cases, 13(16). doi: 10.12998/wjcc.v13.i16.103372
42. Sokołowska, B. (2024). Being in Virtual Reality and Its Influence on Brain Health—An Overview of Benefits, Limitations and Prospects. Brain Sciences, 14(1), 72. doi: 10.3390/brainsci14010072
43. Riva, G., Wiederhold, B. K., & Mantovani, F. (2019). Neuroscience of virtual reality: From virtual exposure to embodied medicine. Cyberpsychology, Behavior, and Social Networking, 22(1). doi: 10.1089/cyber.2017.29099. gri
44. Park, S. Y., Kim, S. M., Roh, S., Soh, M.-A., Lee, S. H., Kim, H., Lee, Y. S., & Han, D. H. (2016). The effects of a virtual reality treatment program for online gaming addiction. Computer Methods and Programs in Biomedicine, 129, 99–108. doi: 10.1016/j.cmpb.2016.01.015
45. Speranza, L., di Porzio, U., Viggiano, D., de Donato, A., & Volpicelli, F. (2021). Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells, 10(4), 735. doi: 10.3390/cells10040735
46. Wankhede, N. L., Sushruta Koppula, Bhalla, S., Doshi, H., Rohit Kumawat, Raju, Ss., Arora, I., Sammeta, S. S., Khalid, M., Zafar, A., Taksande, B. G., Upaganlawar, A. B., Gulati, M., Umekar, M. J., Spandana Rajendra Kopalli, & Kale, M. B. (2024). Virtual reality modulating dynamics of neuroplasticity: Innovations in neuro-motor rehabilitation. Neuroscience. doi: 10.1016/j.neuroscience.2024.12.040
47. Sramka, M., Lacko, J., Ruzicky, E., & Masan, J. (2020). Combined methods of rehabilitation of patients after stroke: virtual reality and traditional approach. Neuro Endocrinology Letters, 41(3), 123–133. PMID: 33201645
48. Zhao, J., Guo, J., Chen, Y., Li, W., Zhou, P., Zhu, G., Han, P., & Xu, D. (2024). Improving rehabilitation motivation and motor learning ability of stroke patients using different reward strategies: study protocol for a single-center, randomized controlled trial. Frontiers in Neurology, 15. doi: 10.3389/fneur.2024.1418247
49. Navas-Otero, A., Canal-Pérez, A., Martín-Núñez, J., Ortiz-Rubio, A., Raya-Benítez, J., Valenza, M. C., & Cabrera-Martos, I. (2025). Rehabilitation applied with virtual reality improves functional capacity in post-stroke patients. A systematic review and meta-analysis. Rehabilitación, 59(2), 100907. doi: 10.1016/j.rh.2025.100907
50. Jehl, N. M., Hess, C. W., Choate, E. S., Nguyen, H. T., Yang, Y., & Simons, L. E. (2024). Navigating virtual realities: identifying barriers and facilitators to implementing VR-enhanced PT for youth with chronic pain. Journal of Pediatric Psychology. doi: 10.1093/jpepsy/jsae056
51. Capriotti, A., Moret, S., Del Bello, E., Federici, A., & Lucertini, F. (2025). Virtual Reality: A New Frontier of Physical Rehabilitation. Sensors, 25(10), 3080. doi: 10.3390/ s25103080
52. Wiederhold, B. K. (2020). Embodiment Empowers Empathy in Virtual Reality. Cyberpsychology, Behavior, and Social Networking, 23(11), 725–726. doi: 10.1089/cyber.2020.29199.editorial
53. Matamala-Gomez, M., Donegan, T., Bottiroli, S., Sandrini, G., Sanchez-Vives, M. V., & Tassorelli, C. (2019). Immersive Virtual Reality and Virtual Embodiment for Pain Relief. Frontiers in Human Neuroscience, 13. doi: 10.3389/fnhum.2019.00279
54. Ventura, S., Marchetti, P., Baños, R., & Tessari, A. (2023). Body ownership illusion through virtual reality as modulator variable for limbs rehabilitation after stroke: A systematic review. Virtual Reality, 27(3), 2481–2492. doi: 10.1007/s10055-023-00820-0
55. Tong, Z. (2016). Virtual Reality in Neurorehabilitation. International Journal of Neurorehabilitation, 3(1). doi: 10.4172/2376-0281.1000e117
56. Kwon, S. H., Park, J. K., & Koh, Y. H. (2023). A systematic review and meta-analysis on the effect of virtual reality-based rehabilitation for people with Parkinson’s disease. Journal of Neuroengineering and Rehabilitation, 20(1). doi: 10.1186/s12984-023-01219-3
57. Ramesh, S., & Arachchige, A. S. (2023). Depletion of dopamine in parkinson’s disease and relevant therapeutic options: A review of the literature. AIMS Neuroscience, 10(3), 200–231. doi: 10.3934/neuroscience.2023017
58. Triegaardt, J., Han, T. S., Sada, C., Sharma, S., & Sharma, P. (2019). The role of virtual reality on outcomes in rehabilitation of Parkinson’s disease: meta-analysis and systematic review in 1031 participants. Neurological Sciences, 41(3), 529-536. doi: 10.1007/s10072-01904144-3
59. Pezzetta, R., Ozkan, D. G., Era, V., Tieri, G., Zabberoni, S., Taglieri, S., Costa, A., Peppe, A., Caltagirone, C., & Aglioti, S. M. (2023). Combined EEG and immersive virtual reality unveil dopaminergic modulation of error monitoring in Parkinson's Disease. NPJ Parkinson's disease, 9(1), 3. doi: 10.1038/s41531-022-00441-5
60. Cristofori, I., & Levin, H. S. (2015). Traumatic brain injury and cognition. Handbook of Clinical Neurology, 128, 579-611. doi: 10.1016/B978-0-444-63521-1.00037-6
1. Freiberg A. S. (2020). Why We Sleep: A Hypothesis for an Ultimate or Evolutionary Origin for Sleep and Other Physiological Rhythms. Journal of circadian rhythms, 18, 2. doi:10.5334/jcr.189.
2. Krause, A. J., Simon, E. B., Mander, B. A., Greer, S. M., Saletin, J. M., Goldstein-Piekarski, A. N., & Walker, M. P. (2017). The sleep-deprived human brain. Nature Reviews Neuroscience, 18(7), 404–418. doi:10.1038/nrn.2017.55.
3. Doherty, R., Madigan, S. M., Nevill, A., Warrington, G., & Ellis, J. G. (2021). The Sleep and Recovery Practices of Athletes. Nutrients, 13(4), 1330. doi:10.3390/nu13041330.
4. Grant, L. K., Gonsalvez, I., Cohn, A. Y., Nathan, M. D., Harder, J. A., Klerman, E. B., Scheer, F. A. J. L., Kaiser, U. B., Crawford, S., Luo, T., Wiley, A., Rahman, S. A., & Joffe, H. (2024). The effect of experimentally induced sleep fragmentation and estradiol suppression on neurobehavioral performance and subjective sleepiness in premenopausal women. Sleep, 47(8), zsae130. doi:10.1093/ sleep/zsae130.
5. Depner, C. M., Melanson, E. L., Eckel, R. H., Higgins, J. A., Bergman, B. C., Perreault, L., Knauer, O. A., Birks, B. R., & Wright, K. P. (2021). Effects of ad libitum food intake, insufficient sleep and weekend recovery sleep on energy balance. Sleep, 44(11), zsab136. doi:10.1093/ sleep/zsab136.
6. Pilcher, J. J., & Morris, D. M. (2020). Sleep and Organizational Behavior: Implications for Workplace Productivity and Safety. Frontiers in psychology, 11, 45. doi:10.3389/ fpsyg.2020.00045.
7. Hoyos, C., Glozier, N. & Marshall, N.S. Recent Evidence on Worldwide Trends on Sleep Duration. Curr Sleep Medicine Rep 1, 195–204 (2015). doi:10.1007/s40675015-0024-x.
8. Barnes, C. M., & Drake, C. L. (2015). Prioritizing Sleep Health: Public Health Policy Recommendations: Public Health Policy Recommendations. Perspectives on Psychological Science, 10(6), 733-737. doi:10.1177/1745691615598509.
9. Vethe, D., Drews, H. J., Scott, J., Engstrøm, M., Heglum, H. S. A., Grønli, J., Wisor, J. P., Sand, T., Lydersen, S., Kjørstad, K., Faaland, P. M. P., Vestergaard, C. L., Langsrud, K., & Kallestad, H. (2022). Evening light environments can be designed to consolidate and increase the duration of REM-sleep. Scientific reports, 12(1), 8719. doi:10.1038/s41598-022-12408-w.
10. He, S., Hasler, B. P., & Chakravorty, S. (2019). Alcohol and sleep-related problems. Current opinion in psychology, 30, 117–122. doi:10.1016/j.copsyc.2019.03.007.
11. Binks, H., E Vincent, G., Gupta, C., Irwin, C., & Khalesi, S. (2020). Effects of Diet on Sleep: A Narrative Review. Nutrients, 12(4), 936. doi:10.3390/nu12040936.
12. Shen, Y., Lv, Q. K., Xie, W. Y., Gong, S. Y., Zhuang, S., Liu, J. Y., Mao, C. J., & Liu, C. F. (2023). Circadian disruption and sleep disorders in neurodegeneration. Translational neurodegeneration, 12(1), 8. doi:10.1186/s40035-02300340-6.
13. Cruz-Sanabria, F., Carmassi, C., Bruno, S., Bazzani, A., Carli, M., Scarselli, M., & Faraguna, U. (2023). Melatonin as a Chronobiotic with Sleep-promoting Properties. Current neuropharmacology, 21(4), 951–987. doi:10.2174 /1570159X20666220217152617.
14. Ruggiero, J. S., & Redeker, N. S. (2014). Effects of napping on sleepiness and sleep-related performance deficits in night-shift workers: a systematic review. Biological research for nursing, 16(2), 134–142. doi:10.1177/1099800413476571.
15. Hasan, S., Foster, R. G., Vyazovskiy, V. V., & Peirson, S. N. (2018). Effects of circadian misalignment on sleep in mice. Scientific Reports, 8(1), 15343. doi:10.1038/ s41598-018-33480-1.
16. Scammell, T. E., Arrigoni, E., & Lipton, J. O. (2017). Neural Circuitry of Wakefulness and Sleep. Neuron, 93(4), 747–765. doi:10.1016/j.neuron.2017.01.014.
17. Gong, Z.-Q., & Zuo, X.-N. (2024). Cortical activations in cognitive task performance at multiple frequency bands. Cerebral Cortex, 34(12). doi:10.1093/cercor/ bhae489.
18. Shuster, A. E., Morehouse, A., McDevitt, E. A., Chen, P. C., Whitehurst, L. N., Zhang, J., Sattari, N., Uzoigwe, T., Ekhlasi, A., Cai, D., Simon, K., Niethard, N., & Mednick, S. C. (2025). REM refines and rescues memory representations: a new theory. Sleep advances : a journal of the Sleep Research Society, 6(1), zpaf004. doi:10.1093/ sleepadvances/zpaf004.
19. Wara, T. U., Fahad, A. H., Das, A. S., & Shawon, M. M. H. (2025). A systematic review on sleep stage classification and sleep disorder detection using artificial intelligence. Heliyon, 11(12), e43576. doi:10.1016/j.heliyon.2025.e43576.
20. Chiu, C.-A., Lu, M.-C., Zhong, Y.-L., Tsai, T.-Y., Liu, C.-J., & Ho, M.-C. (2023). Quantifying and Analyzing Brainwave Electroencephalography with Welch’s Method. Sensors and Materials, 35(5), 1579. doi:10.18494/sam4065.
21. Ishii, T., Pahnwat Tonya Taweesedt, Chick, C. F., O’Hara, R., & Kawai, M. (2024). From macro to micro: slow-wave sleep and its pivotal health implications. Frontiers in Sleep, 3. doi:10.3389/frsle.2024.1322995.
22. Coenen, A. (2024). Sensory gating and gaining in sleep: the balance between the protection of sleep and the safeness of life (a review). Journal of Sleep Research, 33(5). doi:10.1111/jsr.14152.
23. Park, S. H., & Weber, F. (2020). Neural and Homeostatic Regulation of REM Sleep. Frontiers in psychology, 11, 1662. doi:10.3389/fpsyg.2020.01662.
24. Barbato, G. (2021). REM Sleep: An Unknown Indicator of Sleep Quality. International Journal of Environmental Research and Public Health, 18(24), 12976. doi:10.3390/ ijerph182412976.
25. Grafe, L., Miller, K. E., Ross, R. J., & Bhatnagar, S. (2023). The importance of REM sleep fragmentation in the effects of stress on sleep: Perspectives from preclinical studies. Neurobiology of stress, 28, 100588. doi:10.1016/j. ynstr.2023.100588.
26. Habukawa, M., Uchimura, N., Maeda, M., Ogi, K., Hiejima, H., & Kakuma, T. (2018). Differences in rapid eye movement (REM) sleep abnormalities between posttraumatic stress disorder (PTSD) and major depressive disorder patients: REM interruption correlated with nightmare complaints in PTSD. Sleep Medicine, 43, 34–39. doi:10.1016/j.sleep.2017.10.012.
27. Erlacher, D., & Vorster, A. (2023). Sleep and muscle recovery – Current concepts and empirical evidence. Current Issues in Sport Science (CISS), 8(2), 058. doi:10.36950/2023.2ciss058.
28. Bird, C. M. (2017). The role of the hippocampus in recognition memory. Cortex, 93, 155–165. doi:10.1016/j.cortex.2017.05.016.
29. Chanales, A. J. H., Dudukovic, N. M., Richter, F. R., & Kuhl, B. A. (2019). Interference between overlapping memories is predicted by neural states during learning. Nature Communications, 10(1), 5363. doi:10.1038/ s41467-019-13377-x.
30. Chen, Z., & Wilson, M. A. (2017). Deciphering Neural Codes of Memory during Sleep. Trends in Neurosciences, 40(5), 260–275. doi:10.1016/j.tins.2017.03.005.
31. Liew, S. C., & Aung, T. (2021). Sleep deprivation and its association with diseases- a review. Sleep Medicine, 77, 192-204. doi:10.1016/j.sleep.2020.07.048.
32. Short, M. A., & Louca, M. (2015). Sleep deprivation leads to mood deficits in healthy adolescents. Sleep Medicine, 16(8), 987-993. doi:10.1016/j.sleep.2015.03.007.
33. Medic, G., Wille, M., & Hemels, M. E. (2017). Short- and long-term health consequences of sleep disruption. Nature and Science of Sleep, 9, 151. doi:10.2147/NSS. S134864.
34. Mi, Y., & Lei, X. (2023). Sleep loss and lack of social interaction: a summary interview. Brain-Apparatus Communication: A Journal of Bacomics, 2(1). doi:10.1080/27 706710.2022.2163593.
35. Uccella, S., Cordani, R., Salfi, F., Gorgoni, M., Scarpelli, S., Gemignani, A., Geoffroy, P. A., De Gennaro, L., Palagini, L., Ferrara, M., & Nobili, L. (2023). Sleep Deprivation and Insomnia in Adolescence: Implications for Mental Health. Brain Sciences, 13(4), 569. doi:10.3390/brainsci13040569.
36. Makarem, N., Alcántara, C., Williams, D. R., Bello, N. T., & Abdalla, S. (2021). Effect of sleep disturbances on blood pressure. Hypertension, 77(3), 692-699. doi:10.1161/HYPERTENSIONAHA.120.14479.
37. Sá Gomes e Farias, A. V., De Lima Cavalcanti, M. P., De Passos Junior, M. A., & Vechio Koike, B. D. (2022). The association between sleep deprivation and arterial pressure variations: A systematic literature review. Sleep Medicine: X, 4, 100042. doi:10.1016/j.sleepx.2022.100042.
38. M Verberne, A. J., Korim, W. S., Sabetghadam, A., & Llewellyn-Smith, I. J. (2016). Adrenaline: Insights into its metabolic roles in hypoglycaemia and diabetes. British Journal of Pharmacology, 173(9), 1425-1437. doi:10.1111/ bph.13458.
39. Kim, T. W., Jeong, H., & Hong, C. (2015). The Impact of Sleep and Circadian Disturbance on Hormones and Metabolism. International Journal of Endocrinology, 2015, 591729. doi:10.1155/2015/591729,
40. Van Egmond, L. T., S. Meth, E. M., Engström, J., Ilemosoglou, M., Keller, J. A., Vogel, H., & Benedict, C. (2023). Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: A laboratory study. Obesity, 31(3), 635-641. doi:10.1002/ oby.23616.
41. Ooms, S., Bollen, J., van der Meij, A., van Waalwijk, V., Prins, N., Clare, L., Yaffe, K., van der Flier, W. M., Scheltens, P., & Swaab, D. F. (2014). Effect of 1 night of total sleep deprivation on cerebrospinal fluid ß-amyloid 42 in healthy middle-aged men: A randomized clinical trial. JAMA Neurology, 71(8), 971–977. doi:10.1001/jamaneurol.2014.1875833.
42. Lyckenvik, T., Olsson, M., Forsberg, M., Wasling, P., Zetterberg, H., Hedner, J., & Hanse, E. (2025). Sleep reduces CSF concentrations of beta-amyloid and tau: A randomized crossover study in healthy adults. Fluids & Barriers of the CNS, 22(1), 84. doi:10.1186/s12987-02500698-x.
43. Lucey, B. P., Hicks, T. J., McLeland, J. S., Toedebusch, C. D., Boyd, J., Elbert, D. L., Patterson, B. W., Baty, J., Morris, J. C., Ovod, V., Mawuenyega, K. G., & Bateman, R. J. (2018). Effect of sleep on overnight cerebrospinal fluid amyloid ß kinetics. Annals of neurology, 83(1), 197–204. doi:10.1002/ana.25117.
44. Winer, J. R., Mander, B. A., Kumar, S., Reed, M., Baker, S. L., Jagust, W. J., & Walker, M. P. (2020). Sleep disturbance forecasts ß-amyloid accumulation across subsequent years. Current Biology, 30(19), R1234–R1235. doi:10.1016/j.cub.2020.08.017.
45. Verghese, J. P., Terry, A., De Natale, E. R., & Politis, M. (2022). Research Evidence of the Role of the Glymphatic System and Its Potential Pharmacological Modulation in Neurodegenerative Diseases. Journal of Clinical Medicine, 11(23), 6964. doi:10.3390/jcm11236964.
46. Berisha, A., Shutkind, K., & Borniger, J. C. (2022). Sleep Disruption and Cancer: Chicken or the Egg? Frontiers in Neuroscience, 16, 856235. doi:10.3389/ fnins.2022.856235.
47. O’Callaghan, F., Muurlink, O., & Reid, N. (2018). Effects of caffeine on sleep quality and daytime functioning. Risk Management and Healthcare Policy, 11, 263–271. doi:10.2147/RMHP.S156404.
48. Reichert, C. F., Deboer, T., & Landolt, P. (2022). Adenosine, caffeine, and sleep–wake regulation: State of the science and perspectives. Journal of Sleep Research, 31(4), e13597. doi:10.1111/jsr.13597.
49. Harris, J. R., & Marles-Wright, J. (Eds.). (2019). Macromolecular Protein Complexes II: Structure and Function (Subcellular Biochemistry, Vol. 93). Springer. doi:10.1007/978-3-030-28151-9.
50. Mainster, M. A., Findl, O., Dick, H. B., Desmettre, T., Ledesma-Gil, G., Curcio, C. A., & Turner, P. L. (2022). The Blue Light Hazard Versus Blue Light Hype. American Journal of Ophthalmology, 240, 51-57. doi:10.1016/j. ajo.2022.02.016.
51. Janků, K., Šmotek, M., Fárková, E., & Kopřivová, J. (2019). Block the light and sleep well: Evening blue light filtration as a part of cognitive behavioral therapy for insomnia. Chronobiology International, 37(2), 248–259. doi:10. 1080/07420528.2019.1692859.
52. Kaul, M., Zee, P. C., & Sahni, A. S. (2021). Effects of Cannabinoids on Sleep and their Therapeutic Potential for Sleep Disorders. Neurotherapeutics, 18(1), 217. doi:10.1007/s13311-021-01013-w.
53. Babson, K. A., Sottile, J., & Morabito, D. (2017). Cannabis, cannabinoids, and sleep: A review of the literature. Current Psychiatry Reports, 19, 23. doi:10.1007/s11920017-0775-9.
54. Meng, X., Li, Y., Li, S., Zhou, Y., Gan, R., Xu, D., & Li, H. (2017). Dietary Sources and Bioactivities of Melatonin. Nutrients, 9(4), 367. doi:10.3390/nu9040367.
55. Händel, M. N., Andersen, H. K., Ussing, A., Virring, A., Jennum, P., Debes, N. M., Laursen, T., Baandrup, L., Gade, C., Dettmann, J., Holm, J., Krogh, C., Birkefoss, K., Tarp, S., Bliddal, M., & Edemann-Callesen, H. (2023). The short-term and long-term adverse effects of melatonin treatment in children and adolescents: A systematic review and GRADE assessment. EClinicalMedicine, 61, 102083. doi:10.1016/j.eclinm.2023.102083.
56. Chennaoui, M., Arnal, P. J., Sauvet, F., & Léger, D. (2015). Sleep and exercise: A reciprocal issue? Sleep Medicine Reviews, 20, 59-72. doi:10.1016/j.smrv.2014.06.008.
57. Park, I., Díaz, J., Matsumoto, S., Iwayama, K., Nabekura, Y., Ogata, H., Kayaba, M., Aoyagi, A., Yajima, K., Satoh, M., Tokuyama, K., & Vogt, K. E. (2021). Exercise improves the quality of slow-wave sleep by increasing slowwave stability. Scientific Reports, 11, 4410. doi:10.1038/ s41598-021-83817-6.
58. Rubin, A., Mangal, R., Stead, T. S., Walker, J., & Ganti, L. (2023). The extent of sleep deprivation and daytime sleepiness in young adults. Health Psychology Research, 11. https://doi.org/10.52965/001c.74555
59. Khan, M. A., & Al-Jahdali, H. (2023). The consequences of sleep deprivation on cognitive performance. Neurosciences, 28(2), 91–99. 10.17712/nsj.2023.2.20220108.
1. Gonzalez, C. M., Deno, M. L., Kintzer, E., Marantz, P. R., Lypson, M. L., & McKee, M. D. (2018). Patient perspectives on racial and ethnic implicit bias in clinical encounters: Implications for curriculum development. Patient Education and Counseling, 101(9), 1669–1675.
2. Ramsoondar, N., Anawati, A., & Cameron, E. (2023). Racism as a determinant of health and health care: Rapid evidence narrative from the SAFE for Health Institutions project. Canadian Family Physician Medecin de Famille Canadien, 69(9), 594–598. https://doi.org/10.46747/ cfp.6909594
3. Thompson, R. V., Barbee, J., Stancato, N., & Pfeil, S. (2025). The effectiveness of implicit bias training for simulated participants. Clinical Simulation in Nursing, 105, 101781. https://doi.org/10.1016/j.ecns.2025.101781
4. Dahlen, B., McGraw, R., & Vora, S. (2024). Evaluation of simulation-based intervention for implicit bias mitigation: A response to systemic racism. Clinical Simulation in Nursing, 95, 101596. https://doi.org/10.1016/j. ecns.2024.101596
5. Mei, S., Deng, Y., Zheng, G., & Han, S. (2025). Reducing racial ingroup biases in empathy and altruistic decision-making by shifting racial identification. Science Advances, 11(17). https://doi.org/10.1126/sciadv.adt6207
6. Decety, J., Bartal, I. B.-A., Uzefovsky, F., & Knafo-Noam, A. (2016). Empathy as a driver of prosocial behaviour: Highly conserved neurobehavioural mechanisms across species. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1686), 20150077. https://doi. org/10.1098/rstb.2015.0077
7. Decety, J., & Cowell, J. M. (2014). Friends or Foes: Is Empathy Necessary for Moral Behavior?. Perspectives on psychological science : a journal of the Association for Psychological Science, 9(5), 525–537. https://doi. org/10.1177/1745691614545130
8. Filkowski, M., Cochran, R. N., & Haas, B. (2016). Altruistic behavior: Mapping responses in the brain. Neuroscience and Neuroeconomics, Volume 5, 65–75. https:// doi.org/10.2147/nan.s87718
9. Bhuvana, M. L., Pavithra, M. B., & Suresha, D. S. (2021). Altruism, an attitude of unselfish concern for othersan analytical cross sectional study among the Medical and Engineering students in Bangalore. Journal of Family Medicine and Primary Care, 10(2), 706–711. https:// doi.org/10.4103/jfmpc.jfmpc_834_20
10. Pfattheicher, S., Nielsen, Y. A., & Thielmann, I. (2022). Prosocial behavior and altruism: A review of concepts and definitions. Current Opinion in Psychology, 44, 124–129. https://doi.org/10.1016/j.copsyc.2021.08.021
11. Weiss-Sidi, M., & Riemer, H. (2023). Help others-be happy? The effect of altruistic behavior on happiness across cultures. Frontiers in Psychology, 14, 1156661. https://doi.org/10.3389/fpsyg.2023.1156661
12. Hart R. (2024). Prosocial behaviors at work: Key concepts, measures, interventions, antecedents, and outcomes. Behavioral Sciences (Basel, Switzerland), 14(1), 78. https://doi.org/10.3390/bs14010078
13. Fowler, Z., Law, K. F., & Gaesser, B. (2021). Against empathy bias: The moral value of equitable empathy. Psychological Science, 32(5), 766–779. https://doi. org/10.1177/0956797620979965
14. Moudatsou, M., Stavropoulou, A., Philalithis, A., & Koukouli, S. (2020). The role of empathy in health and social care professionals. Healthcare (Basel, Switzerland), 8(1), 26. https://doi.org/10.3390/healthcare8010026
15. Des Ordons, A. L., De Groot, J. M., Rosenal, T., Viceer, N., & Nixon, L. (2018). How clinicians integrate humanism in their clinical workplace—‘just trying to put myself in their human being shoes.’ Perspectives on Medical Education, 7(5), 318–324. https://doi.org/10.1007/s40037018-0455-4
16. Thibault G. E. (2019). Humanism in medicine: What does it mean and why is it more important than ever?. Academic Medicine : Journal of the Association of American Medical Colleges, 94(8), 1074–1077. https://doi. org/10.1097/ACM.0000000000002796
17. Ebisch, S. J. H., Scalabrini, A., Northoff, G., Mucci, C., Sergi, M. R., Saggino, A., Aquino, A., Alparone, F. R., Perrucci, M. G., Gallese, V., & Di Plinio, S. (2022). Intrinsic shapes of empathy: Functional brain network topology encodes intersubjective experience and awareness traits. Brain Sciences, 12(4), 477. https://doi.org/10.3390/ brainsci12040477
18. Bile, A. (2024). Solitonic neural networks. Machine Intelligence for Materials Science. https://doi. org/10.1007/978-3-031-48655-5
19. Zhang, P., Ding, L., Wang, M., & Qiu, S. (2025). Neural mechanisms of physical and social pain empathy: An activation likelihood estimation meta-analysis of fmri studies. Cerebral Cortex, 35(8). https://doi.org/10.1093/ cercor/bhaf227
20. Lamm, C., & Majdandžić, J. (2015). The role of shared neural activations, mirror neurons, and morality in empathy – a critical comment. Neuroscience Research, 90, 15–24. https://doi.org/10.1016/j.neures.2014.10.008
21. Patel, J. (2024). Advances in the study of mirror neurons and their impact on neuroscience: An Editorial. Cureus. https://doi.org/10.7759/cureus.61299
22. Fu, X., Hung, A., de Silva, A. D., Busch, T., Mattson, W. I., Hoskinson, K. R., Taylor, H. G., & Nelson, E. E. (2022). Development of the mentalizing network structures and theory of mind in extremely preterm youth. Social Cognitive and Affective Neuroscience, 17(11), 977–985. https://doi.org/10.1093/scan/nsac027
23. Wagner, I. C., Rütgen, M., & Lamm, C. (2020). Pattern similarity and connectivity of hippocampal-neocortical regions support empathy for pain. Social Cognitive and Affective Neuroscience, 15(3), 273–284. https://doi. org/10.1093/scan/nsaa045
24. Fallon, N., Roberts, C., & Stancak, A. (2020). Shared and distinct functional networks for empathy and pain processing: A systematic review and meta-analysis of fmri studies. Social Cognitive and Affective Neuroscience, 15(7), 709–723. https://doi.org/10.1093/scan/nsaa090
25. Cox, S. S., Kearns, A. M., Woods, S. K., Brown, B. J., Brown, S. J., & Reichel, C. M. (2022). The role of the anterior insula during targeted helping behavior in male rats. Scientific Reports, 12(1). https://doi.org/10.1038/ s41598-022-07365-3
26. Namkung, H., Kim, S. H., & Sawa, A. (2017). The Insula: An underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends in Neurosciences, 40(4), 200–207. https://doi.org/10.1016/j.tins.2017.02.002
27. Xiang, Y., Wang, Y., Gao, S., Zhang, X., & Cui, R. (2018). Neural mechanisms with respect to different paradigms and relevant regulatory factors in empathy for pain. Frontiers in Neuroscience, 12. https://doi.org/10.3389/ fnins.2018.00507
28. Chen, W. G., Schloesser, D., Arensdorf, A. M., Simmons, J. M., Cui, C., Valentino, R., Gnadt, J. W., Nielsen, L., Hillaire-Clarke, C. St., Spruance, V., Horowitz, T. S., Vallejo, Y. F., & Langevin, H. M. (2021). The emerging science of interoception: Sensing, integrating, interpreting, and regulating signals within the self. Trends in Neurosciences, 44(1), 3–16. https://doi.org/10.1016/j. tins.2020.10.007
29. Shen, F., Hu, Y., Fan, M., Wang, H., & Wang, Z. (2018). Racial bias in neural response for pain is modulated by minimal group. Frontiers in Human Neuroscience, 11. https://doi.org/10.3389/fnhum.2017.00661
30. Han, S. (2018). Neurocognitive basis of racial ingroup bias in empathy. Trends in Cognitive Sciences, 22(5), 400–421. https://doi.org/10.1016/j.tics.2018.02.013
31. Arceneaux, K. (2017). Anxiety reduces empathy toward outgroup members but not in ingroup members. Journal of Experimental Political Science, 4(1), 68–80. doi:10.1017/XPS.2017.12
32. Yu, J., Wang, Y., Yu, J., & Zeng, J. (2021). Racial ingroup bias and efficiency consideration influence distributive decisions: A dynamic analysis of Time Domain and time frequency. Frontiers in Neuroscience, 15. https://doi. org/10.3389/fnins.2021.630811
33. Pang, C., Zhou, Y., & Han, S. (2024). Temporal unfolding of racial ingroup bias in neural responses to perceived dynamic pain in others. Neuroscience Bulletin, 40(2), 157–170. https://doi.org/10.1007/s12264-023-01102-0
34. Rojas, C. R., Badolato, G. M., Marshall, J., Boyle, M., Zimber, D., Sabin, J., Chamberlain, J. M., Hinds, P., Schultz, T. R., Bost, J. E., McCarter, R., & Goyal, M. K. (2025). Inequities in timeliness of pain management of fractures in the pediatric emergency department. Pediatrics Open Science, 1(3), 1–8. https://doi.org/10.1542/pedsos.2025-000617
35. Vela, M. B., Erondu, A. I., Smith, N. A., Peek, M. E., Woodruff, J. N., & Chin, M. H. (2022). Eliminating explicit and implicit biases in health care: Evidence and research needs. Annual Review of Public Health, 43(1), 477–501. https:// doi.org/10.1146/annurev-publhealth-052620-103528
36. Hoffman, K. M., Trawalter, S., Axt, J. R., & Oliver, M. N. (2016). Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proceedings of the National Academy of Sciences, 113(16), 4296–4301. https://doi.org/10.1073/pnas.1516047113
37. Sim, W., Lim, W. H., Ng, C. H., Chin, Y. H., Yaow, C. Y., Cheong, C. W., Khoo, C. M., Samarasekera, D. D., Devi, M. K., & Chong, C. S. (2021). The perspectives of health professionals and patients on racism in Healthcare: A qualitative systematic review. PLOS ONE, 16(8). https:// doi.org/10.1371/journal.pone.0255936
38. Schut, R. A. (2021). Racial disparities in provider-patient communication of incidental medical findings. Social Science & Medicine, 277, 113901. https://doi. org/10.1016/j.socscimed.2021.113901
39. Williams, D. R. (2018). Stress and the mental health of populations of color: Advancing our understanding of race-related stressors. Journal of Health and Social Behavior, 59(4), 466–485. https://doi. org/10.1177/0022146518814251
40. Banaji, M. R., Fiske, S. T., & Massey, D. S. (2021). Systemic racism: Individuals and interactions, institutions and Society. Cognitive Research: Principles and Implications, 6(1). https://doi.org/10.1186/s41235-021-00349-3
41. Sorice, V., Mortimore, G., Faghy, M., Sorice, R., & Tegally, D. (2025). The perpetual cycle of racial bias in healthcare and Healthcare Education: A systematic review. Journal of Racial and Ethnic Health Disparities. https:// doi.org/10.1007/s40615-025-02417-6
42. Atkins, N., & Mukhida, K. (2022). The relationship between patients’ income and education and their access to pharmacological chronic pain management: A scoping review. Canadian Journal of Pain, 6(1), 142–170. https://doi.org/10.1080/24740527.2022.2104699
43. Fernandez-Lazaro, C. I., Adams, D. P., Fernandez-Lazaro, D., Garcia-González, J. M., Caballero-Garcia, A., & Miron-Canelo, J. A. (2019b). Medication adherence and barriers among low-income, uninsured patients with multiple chronic conditions. Research in Social and Administrative Pharmacy, 15(6), 744–753. https://doi. org/10.1016/j.sapharm.2018.09.006
44. Job, C., Adenipekun, B., Cleves, A., & Samuriwo, R. (2022). Health professional’s implicit bias of adult patients with low socioeconomic status (SES) and its effects on clinical decision-making: A scoping review protocol. BMJ Open, 12(12). https://doi.org/10.1136/bmjopen-2021-059837
45. Yearby, R. (2018). Racial disparities in health status and access to healthcare: The continuation of inequality in the United States due to structural racism. The American Journal of Economics and Sociology, 77(3–4), 1113–1152. https://doi.org/10.1111/ajes.12230
46. Campbell, C. N. (2025). Healthcare inequities and healthcare providers: We are part of the problem. International Journal for Equity in Health, 24(1). https:// doi.org/10.1186/s12939-025-02464-9
47. Plaisime, M. V., Jipguep-Akhtar, M.-C., & Belcher, H. M. E. (2023). ‘white people are the default’: A qualitative analysis of medical trainees’ perceptions of cultural competency, medical culture, and racial bias. SSM - Qualitative Research in Health, 4, 100312. https://doi. org/10.1016/j.ssmqr.2023.100312
48. Mouhab, A., Radjack, R., Moro, M. R., & Lambert, M. (2024). Racial biases in clinical practice and medical education: A scoping review. BMC Medical Education, 24(1). https://doi.org/10.1186/s12909-024-06119-1
49. Beck, C., García, Y., & Catagnus, R. (2023). Effects of perspective taking and values consistency in reducing implicit racial bias. Universitas Psychologica, 21. https:// doi.org/10.11144/javeriana.upsy21.eptv
50. Gutsell, J. N., Simon, J. C., & Jiang, Y. (2020). Perspective taking reduces group biases in sensorimotor resonance. Cortex, 131, 42–53. https://doi.org/10.1016/j.cortex.2020.04.037
51. Zhou, Y., Pang, C., Pu, Y., & Han, S. (2022). Racial outgroup favoritism in neural responses to others’ pain emerges during sociocultural interactions. Neuropsychologia, 174, 108321. https://doi.org/10.1016/j.neuropsychologia.2022.108321
1. Brouwer, S., Rivera-Hernandez, T., Curren, B. F., Harbison-Price, N., De Oliveira, D. M. P., Jespersen, M. G., Davies, M. R., & Walker, M. J. (2023). Pathogenesis, epidemiology and control of group a streptococcus infection. Nature Reviews Microbiology, 21(7), 1–17. https:// doi.org/10.1038/s41579-023-00865-7
2. Biała, M., Babicki, M., Malchrzak, W., Janiak, S., Gajowiak, D., Żak, A., Kłoda, K., Gibas, P., Ledwoch, J., Myśliwiec, A., Kopyt, D., Węgrzyn, A., Knysz, B., & Leśnik, P. (2024). Frequency of Group A streptococcus infection and analysis of antibiotic use in patients with pharyngitis—a retrospective, Multicenter Study. Pathogens, 13(10), 846. https://doi.org/10.3390/pathogens13100846
3. Grandinetti, R., Mussi, N., Pilloni, S., Ramundo, G., Miniaci, A., Turco, E., Piccolo, B., Capra, M. E., Forestiero, R., Laudisio, S., Boscarino, G., Pedretti, L., Menoni, M., Pellino, G., Tagliani, S., Bergomi, A., Antodaro, F., Cantù, M. C., Bersini, M. T., . . . Esposito, S. (2024). Pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections: a delphi study and consensus document about definition, diagnostic criteria, treatment and follow-up. Frontiers in Immunology, 15, 1420663. https://doi.org/10.3389/fimmu.2024.1420663
4. Prato, A., Gulisano, M., Scerbo, M., Barone, R., Vicario, C. M., & Rizzo, R. (2021). Diagnostic Approach to Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): A Narrative Review of literature data. Frontiers in Pediatrics, 9, 746639. https://doi.org/10.3389/fped.2021.746639
5. Jalal, B., Chamberlain, S. R., & Sahakian, B. J. (2023). Obsessive-compulsive disorder: Etiology, neuropathology, and cognitive dysfunction. Brain and Behavior, 13(6), e3000. https://doi.org/10.1002/brb3.3000
6. Moreno-Amador, B., Piqueras, J. A., Rodríguez-Jiménez, T., Martínez-González, A. E., & Cervin, M. (2023). Measuring symptoms of obsessive-compulsive and related disorders using a single dimensional self-report scale. Frontiers in Psychiatry, 14, 958015. https://doi. org/10.3389/fpsyt.2023.958015
7. American Psychiatric Association. (2022). Obsessive-compulsive and related disorders. In Diagnostic and statistical manual of mental disorders (5th ed., text rev.).
8. Billnitzer, A., & Jankovic, J. (2020). Current Management of Tics and Tourette Syndrome: Behavioral, Pharmacologic, and Surgical Treatments. Neurotherapeutics, 17(4), 1681–1693. https://doi.org/10.1007/s13311-02000914-6
9. Katz, T. C., Khan, T. R., Chaponis, O., & Tomczak, K. K. (2024). Repetitive but not interchangeable. Psychiatric Clinics of North America, 48(1), 165–180. https://doi. org/10.1016/j.psc.2024.09.002
10. Mittal, S. O. (2020). Tics and Tourette’s syndrome. Drugs in Context, 9, 1–7. https://doi.org/10.7573/dic.2019-12-2
11. La Bella, S., Scorrano, G., Rinaldi, M., Di Ludovico, A., Mainieri, F., Attanasi, M., Spalice, A., Chiarelli, F., & Breda, L. (2023). Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Myth or Reality? The State of the Art on a Controversial Disease. Microorganisms, 11(10), 2549. https://doi.org/10.3390/microorganisms11102549
12. Grandinetti, R., Mussi, N., Pilloni, S., Ramundo, G., Miniaci, A., Turco, E., Piccolo, B., Capra, M. E., Forestiero, R., Laudisio, S., Boscarino, G., Pedretti, L., Menoni, M., Pellino, G., Tagliani, S., Bergomi, A., Antodaro, F., Cantù, M. C., Bersini, M. T., . . . Esposito, S. (2024). Pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections: a delphi study and consensus document about definition, diagnostic criteria, treatment and follow-up. Frontiers in Immunology, 15, 1420663. https://doi.org/10.3389/fimmu.2024.1420663
13. Thienemann, M., Murphy, T., Leckman, J., Shaw, R., Williams, K., Kapphahn, C., Frankovich, J., Geller, D., Bernstein, G., Chang, K., Elia, J., & Swedo, S. (2017). Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part I—Psychiatric and Behavioral interventions. Journal of Child and Adolescent Psychopharmacology, 27(7), 566–573. https://doi.org/10.1089/ cap.2016.0145
14. Orlovska, S., Vestergaard, C. H., Bech, B. H., Nordentoft, M., Vestergaard, M., & Benros, M. E. (2017). Association of Streptococcal Throat Infection With Mental Disorders.JAMAPsychiatry,74(7),740–746. https://doi. org/10.1001/jamapsychiatry.2017.0995
15. Leonardi, L., Perna, C., Bernabei, I., Fiore, M., Ma, M., Frankovich, J., Tarani, L., & Spalice, A. (2024). Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Immunological Features Underpinning Controversial Entities. Children, 11(9), 1043. https://doi.org/10.3390/ children11091043
16. Foiadelli, T., Loddo, N., Sacchi, L., Santi, V., D’Imporzano, G., Spreafico, E., Orsini, A., Ferretti, A., De Amici, M., Testa, G., Marseglia, G. L., & Savasta, S. (2024). IL-17 in serum and cerebrospinal fluid of pediatric patients with acute neuropsychiatric disorders: Implications for PANDAS and PANS. European Journal of Paediatric Neurology, 54, 1–7. https://doi.org/10.1016/j.ejpn.2024.11.004
17. Frånlund, K., & Talani, C. (2023). PANDAS – a rare but severe disorder associated with streptococcal infections; Awareness is needed. Acta Oto-Laryngologica Case Reports, 8(1), 104–107. https://doi.org/10.1080/2377 2484.2023.2231146
18. Hutanu, A., Reddy, L. N., Mathew, J., Avanthika, C., Jhaveri, S., & Tummala, N. (2022). Pediatric autoimmune neuropsychiatric disorders associated with Group A streptococci: etiopathology and diagnostic challenges. Cureus, 14(8), e27729. https://doi.org/10.7759/cureus.27729
19. Cunningham, M. W. (2019). Molecular Mimicry, Autoimmunity, and Infection: The Cross-Reactive Antigens of Group A Streptococci and their Sequelae. Microbiology Spectrum, 7(4). https://doi.org/10.1128/microbiolspec. gpp3-0045-2018
20. Gagliano, A., Carta, A., Tanca, M. G., & Sotgiu, S. (2023). Pediatric Acute-Onset Neuropsychiatric Syndrome: Current Perspectives. Neuropsychiatric Disease and Treatment, Volume 19, 1221–1250. https://doi.org/10.2147/ndt. s362202
21. Thaper, D., & Prabha, V. (2018). Molecular mimicry: An explanation for autoimmune diseases and infertility. Scandinavian Journal of Immunology, 88(2), e12697. https://doi.org/10.1111/sji.12697
22. Tien, J., Leonoudakis, D., Petrova, R., Trinh, V., Taura, T., Sengupta, D., Jo, L., Sho, A., Yun, Y., Doan, E., Jamin, A., Hallak, H., Wilson, D. S., & Stratton, J. R. (2023). Modifying antibody-FcRn interactions to increase the transport of antibodies through the blood-brain barrier. mAbs, 15(1), 2229098. https://doi.org/10.1080/19420862. 2023.2229098
23. Frick, L. R., Rapanelli, M., Jindachomthong, K., Grant, P., Leckman, J. F., Swedo, S., Williams, K., & Pittenger, C. (2018). Differential binding of antibodies in PANDAS patients to cholinergic interneurons in the striatum. Brain, Behavior, and Immunity, 69, 304–311. https://doi. org/10.1016/j.bbi.2017.12.004
24. Grant, J. E., & Chamberlain, S. R. (2020). Exploring the neurobiology of OCD: clinical implications. The Psychiatric Times, 2020, exploring-neurobiology-ocd-clinical-implications. https://pmc.ncbi.nlm.nih.gov/articles/ PMC7334048/
25. McGeachy, M. J., Cua, D. J., & Gaffen, S. L. (2019). The IL-17 family of cytokines in health and disease. Immunity, 50(4), 892–906. https://doi.org/10.1016/j.immuni.2019.03.021
26. Schnell, A., Littman, D. R., & Kuchroo, V. K. (2023). TH17 cell heterogeneity and its role in tissue inflammation. Nature Immunology, 24(1), 19–29. https://doi. org/10.1038/s41590-022-01387-9
27. Alahmari, A. (2021). Blood-Brain Barrier Overview: Structural and functional correlation. Neural Plasticity, 2021, 1–10. https://doi.org/10.1155/2021/6564585
28. Castillo, E. F., Zheng, H., Van Cabanlong, C., Dong, F., Luo, Y., Yang, Y., Liu, M., Kao, W. W., & Yang, X. O. (2016). Lumican negatively controls the pathogenicity of murine encephalitic TH17 cells. European Journal of Immunology, 46(12), 2852–2861. https://doi.org/10.1002/ eji.201646507
29. Milovanovic, J., Arsenijevic, A., Stojanovic, B., Kanjevac, T., Arsenijevic, D., Radosavljevic, G., Milovanovic, M., & Arsenijevic, N. (2020). Interleukin-17 in chronic inflammatory neurological diseases. Frontiers in Immunology, 11, 947. https://doi.org/10.3389/fimmu.2020.00947
30. Simonyan, K. (2019). Recent advances in understanding the role of the basal ganglia. F1000Research, 8, 122. https://doi.org/10.12688/f1000research.16524.1
31. Negi, S. S., & Braun, W. (2016). Cross-React: a new structural bioinformatics method for predicting allergen cross-reactivity. Bioinformatics, 33(7), 1014–1020. https://doi.org/10.1093/bioinformatics/btw767
32. Trier, N. H., & Houen, G. (2023). Antibody Cross-Reactivity in Auto-Immune diseases. International Journal of Molecular Sciences, 24(17), 13609. https://doi. org/10.3390/ijms241713609
33. Menendez, C. M., Zuccolo, J., Swedo, S. E., Reim, S., Richmand, B., Ben-Pazi, H., Kovoor, A., & Cunningham, M. W. (2024). Dopamine receptor autoantibody signaling in infectious sequelae differentiates movement versus neuropsychiatric disorders. JCI Insight, 9(21). https:// doi.org/10.1172/jci.insight.164762
34. Dong, M., Chen, G., & Hu, L. (2020). Dopaminergic System Alteration in anxiety and Compulsive Disorders: A Systematic Review of Neuroimaging studies. Frontiers in Neuroscience, 14, 608520. https://doi.org/10.3389/ fnins.2020.608520
35. Lindahl, M., & Kotaleski, J. H. (2016). Untangling basal ganglia network dynamics and function: Role of dopamine depletion and inhibition investigated in a spiking network model. eNeuro, 3(6), ENEURO.0156-16.2016. https://doi.org/10.1523/eneuro.0156-16.2016
36. Schultz, W. (2016). Reward functions of the basal ganglia. Journal of Neural Transmission, 123(7), 679–693. https://doi.org/10.1007/s00702-016-1510-0
37. Cunningham, M. W., & Cox, C. J. (2016). Autoimmunity against dopamine receptors in neuropsychiatric and movement disorders: a review of Sydenham chorea and beyond. Acta physiologica (Oxford, England), 216(1), 90–100. https://doi.org/10.1111/apha.12614
38. Cooperstock, M. S., Swedo, S. E., Pasternack, M. S., & Murphy, T. K. (2017). Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part III-Treatment and Prevention of Infections. Journal of child and adolescent psychopharmacology, 27(7), 594–606. https://doi.org/10.1089/cap.2016.0151
39. Gilbert, D. L. (2019). Inflammation in Tic Disorders and Obsessive-Compulsive Disorder: Are PANS and PANDAS a Path Forward? Journal of Child Neurology. https://doi. org/10.1177/0883073819848635
40. Horne, M., Woolley, I., & Lau, J. S. (2024). The Use of Long-term Antibiotics for Suppression of Bacterial Infections. Clinical Infectious Diseases, 79(4), 848-854. https://doi.org/10.1093/cid/ciae302
41. Frieri, M., Kumar, K., & Boutin, A. (2017). Antibiotic resistance. Journal of Infection and Public Health, 10(4), 369-378. https://doi.org/10.1016/j.jiph.2016.08.007
42. Patangia, D. V., Anthony Ryan, C., Dempsey, E., Paul Ross, R., & Stanton, C. (2022). Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen, 11(1), e1260. https://doi.org/10.1002/ mbo3.1260
43. Dalakas M. C. (2021). Update on Intravenous Immunoglobulin in Neurology: Modulating Neuro-autoimmunity, Evolving Factors on Efficacy and Dosing and Challenges on Stopping Chronic IVIg Therapy. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics, 18(4), 2397–2418. https://doi. org/10.1007/s13311-021-01108-4
44. Melamed, I., Rahman, S., Pein, H., Heffron, M., Frankovich, J., Kreuwel, H., & Mellins, E. D. (2024). IVIG response in pediatric acute-onset neuropsychiatric syndrome correlates with reduction in pro-inflammatory monocytes and neuropsychiatric measures. Frontiers in Immunology, 15, 1383973. https://doi.org/10.3389/fimmu.2024.1383973
45. Leon, J., Hommer, R., Grant, P. et al. Longitudinal outcomes of children with pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS). Eur Child Adolesc Psychiatry 27, 637–643 (2018). https://doi.org/10.1007/s00787-0171077-9
46. Wilbur, C., Bitnun, A., Kronenberg, S., Laxer, R. M., Levy, D. M., Logan, W. J., Shouldice, M., & Yeh, E. A. (2018). PANDAS/PANS in childhood: Controversies and evidence. Paediatrics & child health, 24(2), 85–91. https:// doi.org/10.1093/pch/pxy145
47. Eremija, J., Patel, S., Rice, S., & Daines, M. (2023). Intravenous immunoglobulin treatment improves multiple neuropsychiatric outcomes in patients with pediatric acute-onset neuropsychiatric syndrome. Frontiers in pediatrics, 11, 1229150. https://doi.org/10.3389/ fped.2023.1229150
48. Law, C., & Boisseau, C. L. (2019). Exposure and Response Prevention in the Treatment of Obsessive-Compulsive Disorder: Current Perspectives. Psychology research and behavior management, 12, 1167–1174. https://doi. org/10.2147/PRBM.S211117
49. Hezel, D. M., & Simpson, H. B. (2019). Exposure and response prevention for obsessive-compulsive disorder: A review and new directions. Indian journal of psychiatry, 61(Suppl 1), S85–S92. https://doi.org/10.4103/psychiatry.IndianJPsychiatry_516_18
50. Chen, X. M., Zhang, S., & Xu, M. (2025). Integrating behavioral interventions for Tourette's syndrome: Current status and prospective. World journal of psychiatry, 15(3), 99045. https://doi.org/10.5498/wjp.v15.i3.99045
51. Khan, I., Jaura, T. A., Tukruna, A., Arif, A., Tebha, S. S., Nasir, S., Mukherjee, D., Masroor, N., & Yosufi, A. (2023). Use of Selective Alternative Therapies for Treatment of OCD. Neuropsychiatric disease and treatment, 19, 721–732. https://doi.org/10.2147/NDT.S403997
52. Salm, S., Rutz, J., van den Akker, M., Blaheta, R. A., & Bachmeier, B. E. (2023). Current state of research on the clinical benefits of herbal medicines for non-life-threatening ailments. Frontiers in pharmacology, 14, 1234701. https://doi.org/10.3389/fphar.2023.1234701
53. Zhao, Q., Hu, Y., Yan, Y., Song, X., Yu, J., Wang, W., Zhou, S., Su, X., Bloch, M. H., Leckman, J. F., Chen, Y., & Sun, H. (2024). The effects of Shaoma Zhijing granules and its main components on Tourette syndrome. Phytomedicine, 129, 155686. https://doi.org/10.1016/j. phymed.2024.155686
54. Alharthi, M. S. (2025). A narrative review of Phase III and IV clinical trials for the pharmacological treatment of Tourette's syndrome in children, adults, and older adults. Medicine, 104(23), e42760. https://doi. org/10.1097/MD.0000000000042760
55. Quezada, J., & Coffman, K. A. (2018). Current Approaches and New Developments in the Pharmacological Management of Tourette Syndrome. CNS drugs, 32(1), 33–45. https://doi.org/10.1007/s40263-017-0486-0
56. van Roessel, P. J., Grassi, G., Aboujaoude, E. N., Menchón, J. M., Van Ameringen, M., & Rodríguez, C. I. (2023). Treatment-resistant OCD: Pharmacotherapies in adults. Comprehensive Psychiatry, 120, 152352. https:// doi.org/10.1016/j.comppsych.2022.152352
57. Celik, G., Taş, D., Tahiroglu, A., Avci, A., Yuksel, B., & Cam, P. (2016). Vitamin D Deficiency in Obsessive-Compulsive Disorder Patients with Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections: A Case Control Study. Archives of Neuropsychiatry, 53(1), 33–37. https://doi.org/10.5152/ npa.2015.8763
58. Kuygun Karci, C., & Gul Celik, G. (2020). Nutritional and herbal supplements in the treatment of obsessive compulsive disorder. General psychiatry, 33(2), e100159. https://doi.org/10.1136/gpsych-2019-100159
59. Sathvika, V. P., Subhas, P. G., Bhattacharjee, D., Koppad, V. N., Samrat, U., Karibasappa, S. B., & Sagar, K. M. (2024). Review of Case Study Results: Assessing the Effectiveness of Curcumin, St. John’s Wort, Valerian Root, Milk Thistle, and Ashwagandha in the Intervention for Obsessive-Compulsive Disorder. Drugs and Drug Candidates, 3(4), 838-859. https://doi.org/10.3390/ ddc3040047
60. Wimalawansa S. J. (2023). Physiological Basis for Using Vitamin D to Improve Health. Biomedicines, 11(6), 1542. https://doi.org/10.3390/biomedicines11061542
61. Wang, S., Zhang, X., Ding, Y., Wang, Y., Wu, C., Lu, S., & Fang, J. (2024). From OCD Symptoms to Sleep Disorders: The Crucial Role of Vitamin B12. Neuropsychiatric disease and treatment, 20, 2193–2201. https://doi. org/10.2147/NDT.S489021
62. Algin, S., Ahmed, T., Reza, M. M., Akter, A., Tanzilla, N. J., Haq, M. A., Ahmad, R., Mehta, M., & Haque, M. (2025). Evaluating Treatment Outcomes of Vitamin B12 and Folic Acid Supplementation in Obsessive-Compulsive Disorder Patients With Deficiencies: A Comparative Analysis. Cureus, 17(4), e82420. https://doi.org/10.7759/ cureus.82420
63. Dominguez, L. J., Farruggia, M., Veronese, N., & Barbagallo, M. (2021). Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites, 11(4), 255. https://doi. org/10.3390/metabo11040255
64. Obeid, R., Heil, S. G., Verhoeven, M. M. A., van den Heuvel, E. G. H. M., de Groot, L. C. P. G. M., & Eussen, S. J. P. M. (2019). Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Frontiers in nutrition, 6, 93. https://doi.org/10.3389/fnut.2019.00093
65. Esalatmanesh, S., Biuseh, M., Noorbala, A. A., Mostafavi, S. A., Rezaei, F., Mesgarpour, B., Mohammadinejad, P., & Akhondzadeh, S. (2017). Comparison of Saffron and Fluvoxamine in the Treatment of Mild to Moderate Obsessive-Compulsive Disorder: A Double Blind Randomized Clinical Trial. Iranian journal of psychiatry, 12(3), 154–162.
66. Han, S., Cao, Y., Wu, X., Xu, J., Nie, Z., & Qiu, Y. (2024). New Horizons for the study of saffron (Crocus sativus L.) and its active ingredients in the management of neurological and psychiatric disorders: A systematic review of clinical evidence and mechanisms. Phytotherapy Research, 38(5), 2276–2302. https://doi.org/10.1002/ ptr.8110
67. Kell, G., Rao, A., Beccaria, G., Clayton, P., Inarejos-García, A. M., & Prodanov, M. (2017). AFFRON® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complementary Therapies in Medicine, 33, 58–64. https://doi. org/10.1016/j.ctim.2017.06.001
68. Zheng, Y., Zhang, Z.-J., Han, X.-M., Ding, Y., Chen, Y.-Y., Wang, X.-F., Wei, X.-W., Wang, M.-J., Chen, Y., Nie, Z.H., Zhao, M., & Zheng, X.-X. (2015). A proprietary herbal medicine (5-Ling Granule) for Tourette syndrome: a randomized controlled trial. The Journal of Child Psychology and Psychiatry , 57(1), 74–83. https://doi.org/10.1111/ jcpp.12432
69. Li, X., He, Y., Zeng, P., Liu, Y., Zhang, M., Hao, C., Wang, H., Lv, Z., & Zhang, L. (2019). Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. Journal of cellular and molecular medicine, 23(1), 4–20. https://doi.org/10.1111/jcmm.13564
70. Yan, B., Shen, M., Fang, J., Wei, D., & Qin, L. (2018). Advancement in the chemical analysis of Paeoniae Radix (shaoyao). Journal of Pharmaceutical and Biomedical Analysis, 160, 276–288. https://doi.org/10.1016/j. jpba.2018.08.009
71. Gilbert DL. Inflammation in Tic Disorders and Obsessive-Compulsive Disorder: Are PANS and PANDAS a Path Forward? Journal of Child Neurology. 2019;34(10):598611. doi:10.1177/0883073819848635
72. Colvin, M. K., Erwin, S., Alluri, P. R., Laffer, A., Pasquariello, K., & Williams, K. A. (2021). Cognitive, graphomotor, and psychosocial challenges in pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (pandas). The Journal of Neuropsychiatry and Clinical Neurosciences, 33(2), 90–97. https://doi. org/10.1176/appi.neuropsych.20030065
1. Mitchell, M. (2024). The Turing Test and our shifting conceptions of intelligence. Science, 385(6710). https:// doi.org/10.1126/science.adq9356
2. Jones, C. R., & Bergen, B. K. (2025). Large Language Models Pass the Turing Test. ArXiv (Cornell University). https://doi.org/10.48550/arxiv.2503.23674
3. Han, S.-H., Kim, K. W., Kim, S., & Youn, Y. C. (2018). Artificial Neural Network: Understanding the Basic Concepts without Mathematics. Dementia and Neurocognitive Disorders, 17(3), 83–89. https://doi.org/10.12779/ dnd.2018.17.3.83
4. Murtazina, A., & Adameyko, I. (2023). The peripheral nervous system. Development, 150(9). https://doi. org/10.1242/dev.201164
5. He, J., Yang, H., He, L., & Zhao, L. (2021). Neural networks based on vectorized neurons. Neurocomputing, 465, 63–70. https://doi.org/10.1016/j.neucom.2021.09.006
6. Yamazaki, K., Vo-Ho, V.-K., Bulsara, D., & Le, N. (2022). Spiking Neural Networks and Their Applications: A Review. Brain Sciences, 12(7), 863. https://doi.org/10.3390/ brainsci12070863
7. Kell, A. J. E., Yamins, D. L. K., Shook, E. N., Norman-Haignere, S. V., & McDermott, J. H. (2018). A Task-Optimized Neural Network Replicates Human Auditory Behavior, Predicts Brain Responses, and Reveals a Cortical Processing Hierarchy. Neuron, 98(3), 630-644.e16. https:// doi.org/10.1016/j.neuron.2018.03.044
8. Zador, A. M. (2019). A critique of pure learning and what artificial neural networks can learn from animal brains. Nature Communications, 10(1). https://doi.org/10.1038/ s41467-019-11786-6
9. Whittington, J. C. R., & Bogacz, R. (2017). An Approximation of the Error Backpropagation Algorithm in a Predictive Coding Network with Local Hebbian Synaptic Plasticity. Neural Computation, 29(5), 1229–1262. https:// doi.org/10.1162/neco_a_00949
10. Jiang, P., Sonne, C., Li, W., You, F., & You, S. (2024). Preventing the Immense Increase in the Life-Cycle Energy and Carbon Footprints of LLM-Powered Intelligent Chatbots. Engineering, 40. https://doi.org/10.1016/j. eng.2024.04.002
11. Zhang, Z. (2016). A gentle introduction to artificial neural networks. Annals of Translational Medicine, 4(19), 370–370. https://doi.org/10.21037/atm.2016.06.20
12. Schmidgall, S., Ziaei, R., Achterberg, J., Kirsch, L., Hajiseyedrazi, S. P., & Eshraghian, J. (2024). Brain-inspired learning in artificial neural networks: A review. APL Machine Learning, 2(2). https://doi.org/10.1063/5.0186054
13. Lillicrap, T. P., Santoro, A., Marris, L., Akerman, C. J., & Hinton, G. (2020). Backpropagation and the brain. Nature Reviews Neuroscience, 21(6), 335–346. https://doi. org/10.1038/s41583-020-0277-3
14. Pham, T. Q., Matsui, T., & Chikazoe, J. (2023). Evaluation of the Hierarchical Correspondence between the Human Brain and Artificial Neural Networks: A Review. Biology, 12(10), 1330–1330. https://doi.org/10.3390/biology12101330
15. Bertolero, M. A., Yeo, B. T. T., Bassett, D. S., & D’Esposito, M. (2018). A mechanistic model of connector hubs, modularity and cognition. Nature Human Behaviour, 2(10), 765–777. https://doi.org/10.1038/s41562-0180420-6
16. Raghu, M., Poole, B., Kleinberg, J., Ganguli, S., & Sohl-Dickstein, J. (2016). On the Expressive Power of Deep Neural Networks. ArXiv. https://doi.org/10.48550/ arxiv.1606.05336
17. Herculano-Houzel, S. (2017). Numbers of neurons as biological correlates of cognitive capability. Current Opinion in Behavioral Sciences, 16, 1–7. https://doi. org/10.1016/j.cobeha.2017.02.004
18. Cherny, S. N., & Gibadullin, R. F. (2022). The Recognition of Handwritten Digits Using Neural Network Technology. 2022 International Conference on Industrial Engineering, Applications and Manufacturing (ICIEAM). https:// doi.org/10.1109/icieam54945.2022.9787104
19. Jeon, I., & Kim, T.-G. (2023). Distinctive properties of biological neural networks and recent advances in bottom-up approaches toward a better biologically plausible neural network. Frontiers in Computational Neuroscience, 17. https://doi.org/10.3389/fncom.2023.1092185
20. Langille, J. J., & Brown, R. E. (2018). The Synaptic Theory of Memory: A Historical Survey and Reconciliation of Recent Opposition. Frontiers in Systems Neuroscience, 12. https://doi.org/10.3389/fnsys.2018.00052
21. Kavalali, E. T. (2017). Spontaneous neurotransmission: A form of neural communication comes of age. Journal of Neuroscience Research, 96(3), 331–334. https://doi. org/10.1002/jnr.24207
22. Zhang, Q., Shuai, B., & Lü, M. (2022). A novel method to identify influential nodes in complex networks based on gravity centrality. Information Sciences, 618, 98–117. https://doi.org/10.1016/j.ins.2022.10.070
23. Shuvaev, S., Lachi, D., Koulakov, A., & Zador, A. (2024). Encoding innate ability through a genomic bottleneck. Proceedings of the National Academy of Sciences, 121(38). https://doi.org/10.1073/pnas.2409160121
24. Zivich, P. N., & Naimi, A. I. (2024). A primer on neural networks. American Journal of Epidemiology, 194(6), 1473–1475. https://doi.org/10.1093/aje/kwae380
25. Fan, Y., Wang, R., Yi, C., Zhou, L., & Wu, Y. (2023). Hierarchical overlapping modular structure in the human cerebral cortex improves individual identification. IScience, 26(5), 106575–106575. https://doi.org/10.1016/j. isci.2023.106575
26. Horikawa, T., & Kamitani, Y. (2017). Generic decoding of seen and imagined objects using hierarchical visual features. Nature Communications, 8(1). https://doi. org/10.1038/ncomms15037
27. Rajaraman, N., Jiao, J., & Ramchandran, K. (2024). Toward a Theory of Tokenization in LLMs. ArXiv (Cornell University). https://doi.org/10.48550/arxiv.2404.08335
28. Kaplan, G., Oren, M., Reif, Y., & Schwartz, R. (2024). From Tokens to Words: On the Inner Lexicon of LLMs. ArXiv. https://doi.org/10.48550/arxiv.2410.05864
29. Koubâa, A., Boulila, W., Ghouti, L., Alzahem, A., & Latif, S. (2023). Exploring ChatGPT Capabilities and Limitations: A Survey. IEEE Access, 11. https://doi.org/10.1109/ access.2023.3326474
30. Kong, Q., Cao, Y., Iqbal, T., Wang, Y., Wang, W., & Plumbley, M. D. (2020). PANNs: Large-Scale Pretrained Audio Neural Networks for Audio Pattern Recognition. IEEE/ ACM Transactions on Audio, Speech, and Language Processing, 28, 2880–2894. https://doi.org/10.1109/ taslp.2020.3030497
31. DiMaggio, D. M., Cox, A., & Porto, A. F. (2017). Updates in Infant Nutrition. Pediatrics in Review, 38(10), 449–462. https://doi.org/10.1542/pir.2016-0239
32. Capone Singleton, N., & Saks, J. (2023). Object Shape and Depth of Word Representations in Preschoolers. Journal of Child Language, 51(1), 1–23. https://doi. org/10.1017/s0305000922000630
33. Vaswani, A., Shazeer, N., Parmar, N., Uszkoreit, J., Jones, L., Gomez, A. N., Kaiser, L., & Polosukhin, I. (2017). Attention Is All You Need. ArXiv. https://doi.org/10.48550/ arXiv.1706.03762
34. Xu, X., Huang, S.-L., Zheng, L., & Wornell, G. W. (2022). An Information Theoretic Interpretation to Deep Neural Networks. Entropy, 24(1), 135. https://doi.org/10.3390/ e24010135
35. Xiao, P., Toivonen, H., Gross, O., Cardoso, A., Correia, J., Machado, P., Martins, P., Oliveira, H. G., Sharma, R., Pinto, A. M., Díaz, A., Francisco, V., Gervás, P., Hervás, R., León, C., Forth, J., Purver, M., Wiggins, G. A., Miljković, D., & Podpečan, V. (2020). Conceptual Representations for Computational Concept Creation. ACM Computing Surveys, 52(1), 1–33. https://doi.org/10.1145/3186729
36. Chen, P. H., Si, S., Li, Y., Chelba, C., & Hsieh, C. (2018). GroupReduce: Block-Wise Low-Rank Approximation for Neural Language Model Shrinking. ArXiv. https://doi. org/10.48550/arxiv.1806.06950
37. Sumner, R. L., Spriggs, M. J., Muthukumaraswamy, S. D., & Kirk, I. J. (2020). The role of Hebbian learning in human perception: a methodological and theoretical review of the human Visual Long-Term Potentiation paradigm. Neuroscience & Biobehavioral Reviews, 115, 220–237. https://doi.org/10.1016/j.neubiorev.2020.03.013
38. He, K., Huertas, M., Hong, Su Z., Tie, X., Hell, Johannes W., Shouval, H., & Kirkwood, A. (2015). Distinct Eligibility Traces for LTP and LTD in Cortical Synapses. Neuron, 88(3), 528–538. https://doi.org/10.1016/j.neuron.2015.09.037
39. Fox, K., & Stryker, M. (2017). Integrating Hebbian and homeostatic plasticity: introduction. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1715), 20160413. https://doi.org/10.1098/ rstb.2016.0413
40. Kelvington, B. A., Nickl-Jockschat, T., & Abel, T. (2022). Neurobiological insights into twice-exceptionality: Circuits, cells, and molecules. Neurobiology of Learning and Memory, 195(107684), 107684. https://doi. org/10.1016/j.nlm.2022.107684
41. Song, J., Olsen, R. H. J., Sun, J., Ming, G., & Song, H. (2016). Neuronal Circuitry Mechanisms Regulating Adult Mammalian Neurogenesis. Cold Spring Harbor Perspectives in Biology, 8(8), a018937. https://doi.org/10.1101/ cshperspect.a018937
42. Kempermann, G. (2022). What Is Adult Hippocampal Neurogenesis Good for? Frontiers in Neuroscience, 16. https://doi.org/10.3389/fnins.2022.852680
43. Bazzari, A. H., & Parri, H. R. (2019). Neuromodulators and Long-Term Synaptic Plasticity in Learning and Memory: A Steered-Glutamatergic Perspective. Brain Sciences, 9(11), 300. https://doi.org/10.3390/brainsci9110300
44. Pereira, A., & Wang, F. (2016). Neuromodulation, Emotional Feelings and Affective Disorders. Mens Sana Monographs, 14(1), 5. https://doi.org/10.4103/09731229.154533
45. Recht, B. (2018). A Tour of Reinforcement Learning: The View from Continuous Control. Annual Review of Control, Robotics, and Autonomous Systems, 2(1). https:// doi.org/10.1146/annurev-control-053018-023825
46. Pinho-Ribeiro, F. A., Verri, W. A., & Chiu, I. M. (2017). Nociceptor Sensory Neuron–Immune Interactions in Pain and Inflammation. Trends in Immunology, 38(1), 5–19. https://doi.org/10.1016/j.it.2016.10.001
47. Fuchsberger, T., Stockwell, I., Woods, M., Brzosko, Z., Greger, I. H., & Paulsen, O. (2025). Dopamine increases protein synthesis in hippocampal neurons enabling dopamine-dependent LTP. ELife, 13. https://doi. org/10.7554/elife.100822.2
48. Frémaux, N., & Gerstner, W. (2016). Neuromodulated Spike-Timing-Dependent Plasticity, and Theory of Three-Factor Learning Rules. Frontiers in Neural Circuits, 9. https://doi.org/10.3389/fncir.2015.00085
49. Brzosko, Z., Zannone, S., Schultz, W., Clopath, C., & Paulsen, O. (2017). Sequential neuromodulation of Hebbian plasticity offers mechanism for effective reward-based navigation. ELife, 6. https://doi.org/10.7554/ elife.27756
50. Alzubaidi, L., Zhang, J., Humaidi, A. J., Al-Dujaili, A., Duan, Y., Al-Shamma, O., Santamaría, J., Fadhel, M. A., Al-Amidie, M., & Farhan, L. (2021). Review of deep learning: concepts, CNN architectures, challenges, applications, future directions. Journal of Big Data, 8(1), 1–74. https://doi.org/10.1186/s40537-021-00444-8
51. Sarker, I. H. (2021). Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Computer Science, 2(3), 1–21. Springer. https://link. springer.com/article/10.1007/s42979-021-00592-x
52. Li, M. (2024). Comprehensive Review of Backpropagation Neural Networks. Academic Journal of Science and Technology, 9(1), 150–154. https://doi. org/10.54097/51y16r47
53. Speranza, L., di Porzio, U., Viggiano, D., de Donato, A., & Volpicelli, F. (2021). Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells, 10(4), 735. https://doi.org/10.3390/ cells10040735
54. Fan, Q., Wu, W., & Zurada, J. M. (2016). Convergence of batch gradient learning with smoothing regularization and adaptive momentum for neural networks. SpringerPlus, 5(1). https://doi.org/10.1186/s40064-016-1931-0
55. Twomey, K. E., & Westermann, G. (2017). Learned Labels Shape Pre-speech Infants’ Object Representations. Infancy, 23(1), 61–73. https://doi.org/10.1111/infa.12201
56. Goldman, A. D., & Landweber, L. F. (2016). What Is a Genome? PLOS Genetics, 12(7), e1006181. https://doi. org/10.1371/journal.pgen.1006181
57. Zeng, Y., Zhao, D., Zhao, F., Shen, G., Dong, Y., Lu, E., Zhang, Q., Sun, Y., Liang, Q., Zhao, Y., Zhao, Z., Fang, H., Wang, Y., Li, Y., Liu, X., Du, C., Kong, Q., Ruan, Z., & Bi, W. (2023). BrainCog: A spiking neural network based, brain-inspired cognitive intelligence engine for brain-inspired AI and brain simulation. Patterns, 4(8), 100789–100789. https://doi.org/10.1016/j.patter.2023.100789
58. Dutta, S., Kumar, V., Shukla, A., Mohapatra, N. R., & Ganguly, U. (2017). Leaky Integrate and Fire Neuron by Charge-Discharge Dynamics in Floating-Body MOSFET. Scientific Reports, 7(1). https://doi.org/10.1038/s41598017-07418-y
59. Hasani, R., Lechner, M., Amini, A., Rus, D., & Radu Grosu. (2020). Liquid Time-constant Networks. ArXiv. https:// doi.org/10.48550/arxiv.2006.04439
60. Maass, W. (2023). How can neuromorphic hardware attain brain-like functional capabilities? National Science Review, 11(5). https://doi.org/10.1093/nsr/nwad301
61. Ostrau, C., Klarhorst, C., Thies, M., & Rückert, U. (2022). Benchmarking Neuromorphic Hardware and Its Energy Expenditure. Frontiers in Neuroscience, 16. https://doi. org/10.3389/fnins.2022.873935
62. Abiodun, O. I., Jantan, A., Omolara, A. E., Dada, K. V., Mohamed, N. A., & Arshad, H. (2018). State-of-the-art in artificial neural network applications: A survey. Heliyon, 4(11), e00938. https://doi.org/10.1016/j.heliyon.2018. e00938
63. Doroudi, S. (2022). The intertwined histories of artificial intelligence and education. International Journal of Artificial Intelligence in Education, 33, 885–928. https:// doi.org/10.1007/s40593-022-00313-2