FALL 2024



Brain Breakup: What Happens When the Left and Right Hemispheres Stop Talking
The Brain Tsunami: Implications and Mechanisms of Migraine Aura
The Stakes of Stimulants: How Amphetamine Misuse can Induce Psychosis in a Collegiate Environment

14 18 23 28 8
FEATURED ARTICLE
BRAIN BREAKUP: WHAT HAPPENS WHEN THE LEFT AND RIGHT HEMISPHERES STOP TALKING
by Maxx Martinez | art by Emily Holtz
34
ME, MYSELF, AND MY INNER VOICE: THE VARIOUS VALUES OF THE INNER MONOLOGUE
by Kanyinsola Arowolo | art by Zhengqiu Ge
BEAUTY IS IN THE BRAIN OF THE BEHOLDER: THE NEUROSCIENCE BEHIND AESTHETIC PERCEPTION
by Alya Bagdas | art by Iona Duncan
BEYOND THE MOZART EFFECT: TUNING INTO THE COGNITIVE BENEFITS OF MUSIC
by Daniel Bader | art by Michelle Schaffer
FEATURED ARTICLE
THE BRAIN TSUNAMI: IMPLICATIONS AND MECHANISMS OF MIGRAINE AURA
by Eli Kanetsky | art by Alexandra Adsit
38 42 48
THE BRAIN IN THE OPERATING ROOM: UNDERSTANDING THE LOSS OF CONSCIOUSNESS DURING GENERAL ANESTHESIA
by Katerina Hristova | art by Anna Bishop & Iris Li
BRIDGING THE PHYSICAL AND THE MENTAL: THE EFFECTS OF CHRONIC STRESS ON RHEUMATOID ARTHRITIS
by Evan Seker | art by Elizabeth Catizone
HEY SIRI, WILL YOU BE MY THERAPIST? THE USE OF AI CHATBOTS IN PSYCHOTHERAPY by Lilah Lichtman | art by Victoria Xia
FEATURED ARTICLE
THE STAKES OF PSYCHOSTIMULANTS: HOW AMPHETAMINE MISUSE CAN INDUCE PSYCHOSIS IN A COLLEGIATE ENVIRONMENT
by Michael Silva | art by Michael Silva
Art by Iona Duncan
If you have any questions or comments regarding this Issue 9, please write a letter to the editor at brainstorm.vassar@gmail.com.
Check out our website to read our articles, find out how to get involved, and more at greymattersjournalvc.org.

SHAWN BABITSKY Editor-in-Chief

FREDERICA VON SIEMENS Senior Editor, General Editing

ALEXIS EARP Outreach Coordinator

ANSHUMAN DAS Senior Managing Editor & Production Manager

LAUREL OBERMUELLER Senior Editor, General Editing

TALIA ROMAN Assistant Outreach Coordinator

LORMAN Graduate Student Executive

EVE ANDERSEN Senior Managing Editor & Treasurer

RILEIGH CHINN Senior Editor, Scientific Review

SOPHIA SKLAR Layout Executive & Website Manager

LI Graduate Student Executive

BISHOP Art Executive

EVELYNN BAGADE Senior Editor, Scientific Review & Assistant Outreach Coordinator

OLIVIA POCAT Assistant Layout Executive

DUNCAN Graduate Art Executive

LI Art Executive

GU Senior Editor, Lay Review

BERBECO Social Media Coordinator
Alexandra Adsit
Elizabeth Catizone
Emily Holtz
Michael Silva
Michelle Shaffer
Victoria Xia
Zhengqiu Ge
Alya Bagdas
Daniel Bader
Eli Kanetsky
Evan Seker
Kanyinsola Arowolo
Katerina Hristova
Lilah Lichtman
Maxx Martinez
Michael Silva
Cailey Metter
Chloe Ahn
Dimple Kangriwala
Eden Lanham
Jack Matter
Jannessa Ya
Kaia Takahashi
Krisha Jeevarathnam
Naomi Meyers
Nidhi Pandruvada
Nika Jalali
Paige King
Posey Whidden
Sarah Boucher-Rowe
Sushama Gadiyaram
Talia Roman
Alexandra Astalos
Bertha Shipper
Gordon Zhang
Julia Haggerty-DeGiorgis
John Hurly
Joseph Lippman
Lucy Gaffneyboro
Malathi Kalluri
Munashe Mupunga
Margot Vaughan
Evan Howard PhD
Lori Newman PhD
Kathleen Susman PhD
Bojana Zupan PhD
Lauren Gracie - Layout
Chloe Bilger - Production
Amelie Grube
Anisha Azizi
Chloe Bilger
Claire Bennett
Erin Thatcher
Grace Cabasco
Jenais Panday
Julia Fallon
Kaitlin Raskin
Kyle Benson
Lauren Gracie
Lea Repovic
Mihika Hete
Neha Dhakal
Nico Silverman-Lloyd
Owen Raiche
Quincey Dern
Shayni Richter
Susanna Osborne
Tyler Lawton
Zachary Cahn
Zachary Garfinkle
Zayn Cheema
Zoe Polinsky
The rapid pace of scientific discovery has never been more evident, yet as we continue to push the boundaries of knowledge, we are often met with a stark divide between the research community and the public. In an age of information overload, where scientific findings are published faster than ever before, it can be overwhelming to stay informed. More troubling still is the growing skepticism around science — often fueled by misinformation, political agendas, and a general distrust in experts.
In our current climate, the need for clear, honest, and open communication has never been more urgent. Scientific progress must go hand in hand with public trust, and this can only be achieved by breaking down the walls that separate the two. Scientists have an obligation not only to advance knowledge but to ensure that their discoveries are accessible to all, and to foster understanding in a world increasingly influenced by rhetoric over evidence.
At Grey Matters, we believe that bridging this gap begins with accessible, transparent science communication. It’s time to rethink the way we communicate research. Science should not be a conversation held behind closed doors in academic journals or complicated papers; it should be a dialogue, open to everyone. And it is with this goal in mind that we approach every issue of the journal.
As we publish Issue 9, we remain committed to creating a space where neuroscience can be understood, appreciated, and discussed by a broader audience. In this issue, we hope you encounter a wide variety of topics by uncovering how stress can leave its mark in “Bridging the Physical and the Mental: The Effects of Chronic Stress on Rheumatoid Arthritis” and by delving into the beautiful art of perception through “Beauty is in the Brain of the Beholder: The Neuroscience Behind Aesthetic Perception”.
By providing accessible scientific articles, we hope to create a deeper, more inclusive conversation about neuroscience — one that involves not just researchers and clinicians, but everyone who is curious about the complexities of the brain.
As always, we’re most grateful for our readers, whose curiosity and enthusiasm drive us to continue our work. Your engagement encourages us to explore the inner workings of the mind and share that exploration with you. We hope this issue sparks new questions and insights, and that it continues to inspire you to be part of the conversation.
Let’s keep the dialogue going.
Shawn Babitsky Editor-in-Chief

by Maxx Martinez | art by Emily Holtz
As you read these words, the over 100 billion neurons that make up your brain are com municating through a complex network of more than 60 trillion neuronal connections [1]. Neurons and the connections between them enable you to do everything from identifying words out of com binations of letters to deciphering their meanings [2]. Of the trillions of connections that exist, one that holds crucial importance is the corpus callosum: a bundle of nerves that links the left and right hemispheres of the brain [3, 4]. Through linking the neural hemispheres, the corpus callosum allows for information to be ex changed across the brain and used to execute co ordinated thought and movement [3, 4]. When the corpus callosum is severed, the degree to which neural hemispheres can communicate is significantly impacted [5, 6]. Severing the structure is beneficial to alleviate dangerous symptoms of epilepsy, and is done through a surgical procedure known as a corpus callosotomy [5, 6]. Cutting the corpus callosum causes a variety of unique symptoms, ranging from uncontrolled hand movements to a reduced understanding of one’s own emotions [5, 7, 8]. Despite the procedure’s observable symptoms, the full range of consequences that disrupting the connections within the corpus callosum has on consciousness and thought processes remain largely unknown [5, 7, 8]. Although there is a lack of certainty surrounding the cognitive effects of cutting the corpus callosum, the procedure provides an important perspective on current theories of consciousness [5, 9]. The corpus callosotomy offers a unique opportunity to observe how the brain’s hemispheres function when disconnected from each other, shedding light on how consciousness works in the brain [5].

To understand the effects that splitting the corpus callosum has on a person and their consciousness, it is essential to first explore some basic information about the corpus callosum and its role in brain function [5, 10]. Many brain functions are lateralized, meaning they are controlled primarily by either the left or right hemisphere [11, 12, 13]. Speech is an example of a lateralized function, as its production is localized to Broca’s area, a region in the brain’s left hemisphere [13, 14, 15, 16]. Speech, language processing, and right-hand control are also lateralized functions of the left hemisphere [13, 16, 17, 18]. The brain’s right hemisphere, in contrast, is the primary location for spatial reasoning and awareness, musical ability, and left-hand control [13, 15, 16] . Despite the lateralization of the brain, many activities require input from both hemispheres and often rely on the corpus callosum — a bridge composed of millions of nerve fibers that link the hemispheres together with a near-seamless exchange of information — to achieve cross-hemispheric communication [4, 19, 20]. Cross-hemispheric communication via the corpus callosum allows for the execution of coordinated full-body movement and whole-brain processing of sensory input [4, 19].
Due to the brain’s lateralization, each hemisphere controls the opposite side of the body; the left hemisphere controls the right side and the right hemisphere controls the left side [14, 21]. Cross-body hemispheric control encompasses everything from
arm and leg movement to the intake of visual information from the periphery of each eye [22, 23].
The corpus callosum enables both hemispheres to share and utilize information, leading to a cohesive experience of vision and movement [24, 25, 26]. The interconnectedness of the brain’s hemispheres suggests that neither is truly dominant, as each relies on the other for many functions [11, 12]. The reliance between the brain’s hemispheres debunks the common myth that individuals can be either left- or rightbrained, as both the left and right hemispheres must work in unison to execute cognitive and motor functions [11, 12]. Although the brain is lateralized, certain neurological processes arise from activity located in structures that are bilateral, meaning the structures extend across, or independently exist on, both hemispheres [27]. Brain structures that present bilaterally are able to execute their functions in either hemisphere of the brain and often communicate across hemispheres through other neural pathways to an increased degree following a corpus callosotomy [27].
Two examples of regions that are bilateral are the superior temporal gyrus, which is associated with audi tory processing, and the posterior cerebellum, which is associated with tasks such as balance and walking [28, 29, 30].
“NO CONTACT:” IS SPLITTING UP FOR THE BEST?
Given the importance of the corpus callosum, it is reasonable to wonder why one would sever the struc ture, and how the procedure may affect one’s quality of life and mental processes. In some extreme cases of epilepsy, where seizures arise from uncontrolled neuronal firing, seizures localized in one hemisphere
can spread to the other hemisphere via the corpus callosum, resulting in an atonic seizure [6]. An atonic seizure occurs when the regular neuronal firing that contributes to muscle tone — or the involuntary tension within muscles activated during activities such as standing — becomes inconsistent, resulting in a loss of normal motor functioning. When individuals lose muscle tone, they lose their ability to stand unassisted, potentially resulting in broken bones and concussions from uncontrolled falls [6]. In rare cases of extreme epilepsy, such as those resulting in atonic seizures where medications fail to reduce seizure severity and frequency, a procedure known as a corpus callosotomy can be performed [10]. A corpus callosotomy severs the corpus callosum and prevents seizures from spreading to the other side of the brain, restricting dysfunctional firing to one area and therefore reducing seizure severity and risk [6]. Following a corpus callosotomy, several people report no longer experiencing atonic seizures, and several others report no longer experiencing seizures of any kind [6].

Though a corpus callosotomy successfully alleviates seizure symptoms, there are notable side effects individuals experience following the procedure [6]. Interestingly, many people who receive a corpus callosotomy report feeling and behaving ‘normally’ [9, 31]. Most people who undergo the operation spend only a few days in the hospital, and report increased independence in daily tasks following their recovery [32, 33, 34]. However, despite a reported general sense of normalcy, many experience distinct side effects following a corpus callosotomy [5, 8]. One common and noticeable side effect is referred to as ‘alien hand syndrome,’ a condition in which one limb acts involuntarily, leading to a feeling of estrangement with the affected limb [5, 7, 35]. For example, one hand may pull a candle away as the other tries to light it, or one hand may button a shirt only for the other to undo each button [7, 36]. An often overlooked consequence of a corpus callosotomy is that, following the procedure, individuals lose their ability to correctly link their emotional responses with stimuli processed by the left hemisphere [8]. In one study, a visually frightening scene was shown to the peripheral right eye of participants who had undergone a corpus callosotomy, leading to the scene being processed solely by the left hemisphere of the brain. The sight of this scene made the person feel uneasy, but when asked what was wrong, the person attributed their discomfort to the room they were in, rather than the scene they had observed. When the experiment was repeated, this time presenting the scene to participants’ left periphery — which was processed by their right hemisphere — participants immediately attributed their unease to the scene they were shown. The participants’ inability to properly attribute the emotional response experienced to the scene processed by the left hemisphere may stem from the fact that most emotional comprehension occurs in the right hemisphere. Since the link between the hemispheres is now severed, the left hemisphere no longer receives the right hemisphere’s emotional interpretations. As a result, when asked about their feelings, participants who received a corpus callosotomy were unable to access relevant information to answer the question, and could only rely on the logically thinking left brain to ‘guess’ the cause of their discomfort [8].
In another well-known split-brain experiment, the notion that a corpus callosotomy leads to a lack of communication between the hemispheres of the brain is further supported [5, 9, 37] . In the experiment, participants were told to stare at a dot in the middle of a screen. Different images were then flashed on both sides of the dot, and, due to the brain’s
lateralization, each image was processed by one hemisphere of the brain. When asked what they saw, participants were able to name the stimulus shown on the right side of the screen, as the image was processed by the left side of the brain, where the speech centers are predominantly located. Because the left hand is controlled by the right side of the brain, which processes the image shown to the left side of the dot, when instructed to draw what they saw using their left hand, participants drew the image shown on the left side. When asked why they drew something other than what they reported seeing, participants were often confused and provided rationales that had nothing to do with the circumstances of the experiment [5, 9, 37]. For example, in one rendition of the study, a person was shown the words ‘bell’ and ‘music,’ to the left and right sides of the dot respectively, and was then asked to point out what they saw using their left hand [9, 38]. Out of a group of images depicting music-related items, including someone hitting a drum and someone playing a trumpet, the person selected a picture of a bell. When asked why they chose that specific image, the participant insisted that they ‘must have heard a bell ringing on [their] way into the lab’ and that this was the last time they had heard music [9, 38]. Although it is possible that the participant heard a bell before engaging in the study, it is far more likely that the person’s left hemisphere, which has an essential role in speech control, was trying to develop a rationale for the actions of their left hand [5, 38] . Due to the split in the corpus callosum, the two hemispheres of the brain cannot communicate, and the left brain is therefore unable to access the reason for the right brain’s actions [5, 38].
Decades of research on the effects of a corpus callosotomy has sparked debate as to whether severing the corpus callosum splits one’s consciousness into two distinct conscious agents, or whether one’s consciousness remains unified [5, 9, 39, 40]. A unified consciousness is one in which all experiences generated by the body are perceived through one perspective and sense of self [5, 9]. A split consciousness is one in which each hemisphere contains its own conscious agent which operates separately from the other. The belief that consciousness would split arose from the theory that each hemisphere of the brain — the left side represented by speech and right side represented by left-hand movements — could form conflicting conclusions based on the same stimulus, without being aware of the other hemisphere’s
response [5, 9]. What this theory fails to account for, however, is that the inability to compare visual stimuli from each hemisphere is not consistent across all cases in which people receive a corpus callosotomy; many people who have undergone a corpus callosotomy retain their ability to name an object no matter where in the visual field it is presented, indicating that individual difference may play a large role in whether consciousness is split [5, 9, 40]. Some experiments show evidence supporting the existence of a split consciousness when comparing some stimuli, but a unified consciousness when comparing others [5, 9]. In another experiment, two shapes were presented to participants in a similar manner to the previous study. When asked to identify whether the shapes were the same or different, most participants were unable to do so. However, when the shapes were replaced with tilted lines that were either parallel to or identical to each other, participants were able to consistently identify the relationship between the lines accurately. The ability to successfully compare the tilted lines, but not the shapes, suggests that cross-hemisphere communication takes place at varying degrees depending on the task [5, 9].
Ultimately, the answer to whether consciousness is split into multiple agents is dependent on what theory one uses to define consciousness [5]. One theory of consciousness, known as the global neuronal workspace theory, suggests that all subconscious information processing occurs in different subsections of the brain called cortical modules, each of which sends signals to a central ‘headquarters’ [5, 41]. At these cortical ‘headquarters,’ the strongest signals, which are determined by signal frequency and number, are broadcast throughout the brain and become conscious, coordinated thoughts. Under this system of consciousness, it can be argued that those who have undergone a corpus callosotomy do display split consciousness, as the ability for modules to communicate with cortical headquarters across hemispheres is lost when connections between hemispheres are severed. Therefore, each hemisphere’s cortical ‘headquarters’ is only able to broadcast information within its own hemisphere, leading to two separate conscious broadcasts occurring simultaneously. The global neuronal workspace theory indicates that depending on the level of hemispheric separation — or what percent of the corpus callosum is severed and where it is severed — different types and amounts of information may be able to cross to the ‘cortical headquarters’ within the other hemisphere of the brain. Differing levels of hemispheric separation explain why some people are able to communicate
across visual fields while others cannot, and why some stimuli, such as the tilted lines discussed in the previous experiment, can almost always be compared across visual fields. Varying degrees of hemispheric separation leads neuroscientists who subscribe to the global neuronal workspace theory to view consciousness as a spectrum of connectedness, rather than completely split or completely unified [5, 41].
Another popular model used for defining consciousness is recurrent processing theory [5, 42]. Recurrent processing theory argues that the brain, and therefore one’s consciousness, can intake information passively, or without one actively paying attention to the input [5, 9, 42]. Information absorbed passively is not always readily available to be recalled consciously, but still factors into decision-making [5, 9, 42]. In one experiment, people who had not undergone a corpus callosotomy were shown different moving objects to the outer periphery of each eye [5, 9]. The act of watching a different moving object with each eye caused a person’s attention to be split between the two objects, and led each hemisphere to independently process the information from one of the eyes. Despite the participant’s divided attention, when asked to recall the moving objects afterward, it seemed clear that their perception of viewing the moving objects was unified [5, 9]. In another experiment, known as a partial report paradigm, participants were instructed to stare at a cross in the middle of a screen, at which point several letters appeared in a circle around the cross for less than a second [5, 43]. Next, a blue dot appeared on the screen in a location that was previously occupied by

a letter, at which point the participant was instructed to quickly state the letter that was at that location [5, 43]. Participants consistently performed better when asked to recall a letter that was at a specific location versus when simply asked to list the letters they saw during the experiment [5, 9, 43]. The inability to recall the information until specifically prompted suggests that acquisition of information does not equate to the ability to convey said information to others [5, 43]. In other words, the brain subconsciously takes in information that is made available to the conscious mind after being prompted with specific cues, such as instructions. Recurrent processing theory argues that people who have undergone a corpus callosotomy do not have a split consciousness, but rather have a greater disassociation between their consciousness and what they are able to communicate to others. The theory suggests that due to a lack of understanding of the disjointment, it appears as if there are two existing conscious agents, but in reality, there exists only one that is not fully capable of integrating and reporting the information it has access to. Recurrent processing theory can also explain observed personal differences in people’s ability to report information; it suggests that their ability to do so is dependent on the percentage of the corpus callosum that is severed and where the severance occurs. As a result of differences between individual patients, each person may be able to integrate and report varying amounts of information, leading to a range of reportability in lieu of the split versus unified consciousness dichotomy or the spectrum of consciousness previously mentioned [5, 43].

Whether one views a corpus callosotomy as a split in consciousness or a shift in ability to report known information, it is clear that the procedure alters the brain’s inner workings by restricting information flow between neural hemispheres [5]. Despite the lack of knowledge about the procedure’s full implications, a corpus callosotomy remains a crucial intervention that alleviates otherwise untreatable and debilitating symptoms of epilepsy [10]. While less invasive alternatives to the procedure are being explored, the corpus callosotomy provides valuable insight into the mechanisms underlying consciousness and thought, deepening our understanding of the interconnected nature of the brain’s hemispheres and their impact on consciousness as a whole [5, 10].
References on page 54.

by Kanyinsola Arowolo | art by Zhengqiu Ge

Have you ever noticed that little voice in your head? The one that helps you make decisions and dissect the world around you? That’s your inner voice. It’s a constant presence that helps you organize your daily thoughts [1]. The inner voice impacts everything from our confidence to our problem-solving abilities, making it a key player in our mental and emotional well-being [2, 3]. Over the course of development, baby babbles evolve into coherent private musings that ultimately give rise to the powerful inner voice [1]. The progression of the inner voice reveals a fascinating capacity of the mind to link memory,
focus, and emotional resilience to the creation of an internal guide [3, 4]. Take a moment to reflect: how have you used your inner voice today? Perhaps you leaned on it to weigh the pros and cons of a tough decision, maintain focus during a challenging task, or find calm in a stressful moment. By consciously employing internal dialogue, you tap into a powerful tool that helps you navigate the world with clarity and intention.
Even though the inner voice is ingrained in our daily functioning, it has not always been a present force in our lives. Young children use private speech — the act of speaking aloud to oneself while engaged in a task — which serves as a major building block towards the internalization of language [1, 5]. Imagine a two-year-old named Johnny who has recently begun to speak aloud to himself in simple sentences; this marks the beginning of his private speech [6, 7]. While every individual develops at a unique pace, most children begin using private speech during early childhood [8]. Children in early childhood tend to struggle with switching between tasks that have different rules which is normally facilitated by inner voice. For example, a child is shown an image and asked to identify whether the animal depicted in the image swims or flies. Then the child is immediately asked to identify whether the image in front of them is in color or not. Younger children tend to perform worse on identification tasks like these when they are not given verbal cues. However, when they are encouraged to use verbal labels to guide their thinking, such as a reminder to focus on color identification, a young child’s performance improves. The need for assistance suggests that young children may not yet have an inner voice to help them switch between tasks, a skill that develops as they get older [1].
Use of private speech generally peaks around age five and gradually decreases afterward [1, 9, 10]. Fiveyear-olds benefit significantly from talking out loud
when they’re learning something new and perform better on tasks with the help of private speech [1, 11]. Meanwhile, older children do not benefit from using private speech, indicating that their inner voice is likely more developed [1, 12]. Johnny’s overt private speech fades with age as he needs it less and less to perform tasks or switch between actions [13]. While he solves a puzzle, instead of voicing his train of thought in spoken musings like ‘Does my piece fit here?’, he approaches the logic problem through an internal monologue [14]. Overall, speech gradually turns inward, as private speech becomes less necessary and the ‘inner voice’ strengthens [15].
By about the age of eight, Johnny begins to utilize this newfound introspection to plan, re member, and solve problems in his mind [16]. While one’s external and internal voice is distinct, a num ber of brain regions are used in the production of both forms of language [17]. Broca’s area is essential for producing speech and it is activated during both verbal and inner speech [1, 18]. Additionally, the supplementary motor area (SMA) — a brain region involved in language processing — is activated during verbal and inner speech production, demonstrating its importance in both processes [19]. The overlapping activation of Broca’s area and the SMA in both inner and verbal speech indicate connections between the forms of language production [3, 19, 20]. As he grows, Johnny’s inner voice transforms from childhood chatter into an invaluable companion [3]. While our private speech never truly stops supporting us throughout our lives, the inner voice becomes the primary cognitive support throughout our lives.

‘E-L-E-P-H-A-N-T, elephant.’ This inner verbal rehearsal keeps the letters fresh in his memory and ensures that he can recall them when he needs to [1]. Johnny’s hippocampus — a brain area crucial for forming and retrieving memories — organizes and stores the information that he rehearses [21]. The hippocampus is like a library where all of Johnny’s memories are cataloged [1, 22]. When he uses his inner voice to rehearse, it’s like reshelving books in the right order. The more Johnny practices, the easier it becomes for his hippocampus to retrieve those memories later, whether he’s answering questions in class or spelling the word ‘ELEPHANT’ [23, 24]. This collaboration between inner speech and the hippocampus shows that verbal rehearsal strengthens memory retention, making it easier for Johnny to access the information when it’s needed. [1, 22, 25, 26]. As Johnny prepares for his spelling test, his inner voice helps him stay focused [27]. Imagine him at his desk, distractions all around — the TV is on, and his dad is playing fetch with his dog. To drown out these distractions, Johnny mentally rehearses the spelling words: ‘E-L-E-P-H-A-N-T’, elephant; L-A-M-B, lamb.’ The engagement of his inner voice boosts his attention and increases concentration. Strategic use of inner speech can improve learning and retention [28].
One of the vital functions of the inner voice is its role in memory storage and retrieval. Johnny is now a young student of age nine preparing for a spelling test. His inner voice can serve as a personal assistant in navigating his learning experiences [3]. Each night, he sits at his desk with a pen in hand, ready to commit new words to memory. With the inner voice as his trusted companion, he whispers the letters of each word in his mind. When he practices the word ‘elephant,’ he repeats to himself internally:
As he enters his early 20s, Johnny’s inner voice rises to meet the ever-increasing challenges of his adult life, helping him to process information effectively while exerting minimal effort [1, 4, 29, 30]. As a businessman living alone in the bustling heart of New York City, Johnny needs to use effective decision-making and quick thinking to meet the demands of the concrete jungle. As he navigates the high-stakes world of Wall Street, Johnny finds himself managing the everyday complexities of adulthood — meal planning, social commitments, and budgeting. He sits at his kitchen table, laptop open and notepad ready, mentally preparing for the week ahead. ‘Alright, what’s for dinner this week?’ he prompts himself, activating his inner voice to prioritize his choices. Johnny’s inner voice guides his thoughts as he relies on his frontal cortex, the brain region responsible for critical
thinking and problem-solving higher-order thinking [31, 32]. He reflects, ‘If I meal prep on Sunday, I’ll save time during the week,’ mapping out his schedule. By mentally rehearsing his week, Johnny streamlines the decision-making process and ensures proper preparation for what’s to come [33]. As he contemplates his options, Broca’s area engages and helps him to articulate his inner thoughts clearly [15, 34]. Johnny structures his ideas: ‘Pasta is quick, but I should also add some veggies.’ Broca’s area selects the right words and proper grammatical structures, which allows Johnny to mentally articulate his plans for a balanced diet [15, 35, 36].
Not only does the inner voice help in planning, it also plays an important role in helping us pivot between different tasks. As the day unfolds, Johnny must manage a number of errands such as finalizing his grocery list, coordinating lunch plans with friends, and checking his budget. In this hectic environment, his inner voice becomes an invaluable asset. He might say to himself, ‘after grocery shopping, I need to call Sarah to confirm our dinner plans.’ Inner speech is particularly helpful in tasks that require switching between different responses and rules [1]. For instance, Johnny uses inner speech to enhance his ability to shift attention and manage multiple responsibilities [1]. When faced with distractions, his inner voice helps him maintain focus. Each decision — from planning meals to strategizing in work challenges and navigating social interactions—is influenced by his inner voice.
As Johnny navigates adult life, emotional regulation becomes essential for managing his interpersonal relationships and the feelings that arise from them [1]. Emotional regulation refers to the processes that influence which emotions we have, when we have them, and how we experience and express them [37]. Emotional regulation allows Johnny to respond to challenging situations with resilience. The inner voice plays a pivotal role in the emotional regulatory processes, engaging various brain regions to help Johnny reflect and reframe his emotional experiences [2]. Imagine it’s Monday morning, and Johnny is running late for work. He has barely had any of his coffee when his phone buzzes with a text from his boss, asking for an update on the big project — one he thought was still a week away. Panic sets in as he rushes to the subway, dodging tourists and pigeons that seem intent on thwarting his progress. ‘Why do I
always forget to check my calendar?’ he thinks. In this moment of chaos, his inner voice becomes a critical tool for processing his feelings and thoughts. Johnny is able to reflect on his past oversights and evaluate his options through the internal verbalization of his frustration. Using self-talk, Johnny identifies the urgency of his situation and reminds himself to make a mental note to check his calendar more regularly in the future. Engaging with his inner voice allows him to gain clarity amid the panic, guiding him toward a more organized approach to his day [38].
In stressful times, Johnny actively engages his inner voice to reflect on and reframe his emotions [21]. He takes a mental pause to assess the situation. ‘Okay, I don’t have to panic yet,’ he thinks. While his frontal cortex plays a significant role in evaluating his feelings and considering the consequences of his actions, this process also involves other interconnected brain regions [31]. The integration of areas responsible for emotional regulation, such as the amygdala, with other areas involved in language and thought supports his ability to think about his situation [5, 39]. This combined effort allows him to manage his emotional state more effectively. ‘If I can present a few solid points, I’ll be fine.’ This thought acts as a calming mantra and allows him to regain some composure. As he enters the conference room, he feels the familiar knot of anxiety tightening in his stomach; Instead of letting that anxiety dictate his actions, he channels his inner voice to articulate his feelings. ‘My boss can be a bit intense, but I’m not in the Hunger Games,’ he reassures himself.
When a challenging question comes from his boss — ‘Johnny, can you explain why this strategy will work?’ — Johnny’s brain jumps into action [18]. His Broca’s area is activated, and begins forming coherent sentences, allowing him to communicate effectively even

as his heart races with anxiety [18]. The pressure of the moment can make it challenging to express ideas clearly, but this brain region transforms Johnny’s nervous energy into a well-thought-out response that he is able to articulate while under stress [2, 40]. As worry blossoms into clarity, he can see his boss nodding. Each decision — from managing a tricky conversation with his boss to handling his daily dose of office chaos — is influenced by his inner voice.
While the experiences of Johnny are relatable to most, in certain ‘atypical populations,’ the development and function of the inner voice can vary significantly, creating distinct differences in how individuals process and interact with information [6, 42]. Although the frequency at which people use their inner voice can differ in the general population, those with certain disorders may face unique challenges with inner speech [43, 44]. For example, people with diminished inner speech often perform worse in verbal recall tasks, including rhyme judgment and word list memory tasks [43]. The lack of an inner voice, known as anendophasia, points to possible memory and recall limitations when inner speech is less accessible [43]. One group of people who may experience diminished inner voice are those with Autism Spectrum Disorder (ASD). ASD can be defined as a set of neurodevelopmental disorders characterized by a wide variety of communicative and interpersonal behaviors often labeled as divergent from social norms [1, 45]. Children with ASD frequently experience delays in early language development that may extend to disruptions in the maturation of inner speech [46, 47]. Given the role of the inner voice as an internal form of verbal communication, people with ASD may exhibit differences in their inner voice [42]. While little is known about ASD’s influence on inner speech, one

study infers that some people with ASD may have a diminished inner voice [42]. When asked to perform a complicated logic task, people with ASD took longer than people without ASD to complete the task. Interestingly, when people without ASD were required to speak continuously while performing the task — an action that suppressed their inner voice — they took longer, increasing their completion times to a level comparable to people with ASD. The results of those with ASD remained largely unchanged during the suppression of inner voice. A decrease in performance when people without ASD suppressed their inner voice indicates that the inner voice is useful in puzzle completion. Furthermore, these results support the hypothesis that some people with ASD may have a diminished inner voice, which could explain why their task completion times were unaltered by inner voice suppression [48]. Ultimately, these findings indicate inner voice is a highly variable factor of people’s internal lives.
In examining our cognitive abilities, the inner voice stands out as a powerful internal guide — a tool that enhances memory, aids in problem-solving, and plays a critical role in emotional regulation. As we’ve seen through Johnny’s development, this inner voice is more than meaningless meandering; it’s an evolving companion, steering us through life’s complexities efficiently. From early childhood through adulthood, the inner voice transitions from private speech into an essential cognitive asset, driving both thought and self-awareness [6]. Given its importance in various cognitive functions, furthering our understanding of the inner voice is crucial [4]. Future research on inner voice may open new doors to understanding mental health, brain function, and individualized cognitive experiences [1]. Increased dedication to investigating this topic can help us answer questions such as: why do some people seem to lack an inner voice and how do variations in brain function and emotional disorders affect inner speech? This research will not only advance knowledge but also pave the way for therapeutic approaches to support brain health and resilience [49]. A greater understanding of the inner voice can illuminate how this powerful cognitive tool shapes our thoughts, behaviors, and emotional resilience, offering new insights into mental health and the human mind.
References on page 56.

by Alya Bagdas | art by Iona Duncan

As you sit on your couch with the TV glaring in front of you, clips of a beauty pageant flash on the screen. You watch as contestants walk across the stage; they are dressed in outfits meticulously chosen to highlight their eyes or wearing makeup chosen to accentuate their sharp cheekbones. What makes the contestant with a symmetrical face and confident
strut stand out? How will the contestants on TV influence the clothes you pick out to wear tomorrow or the way you consider your own attractiveness? While questions like these may seem obvious in the context of beauty pageants, they can be applied to many different daily experiences [1, 2]. Everything we see, taste, smell, hear, and touch manifests into experiences [1, 2, 3, 4]. As we process sensory information, we form subjective judgments, create emotional connections, and assign values that influence individual behavior [2, 5, 6]. For example, if you find people with full lips and brown hair more attractive, the contestants in the beauty pageant who have these features may appear more sympathetic and beautiful because of your preconceived notions of beauty [1, 2, 7].
Aesthetics as a philosophical field explores how each person cultivates their own aesthetic tastes, why tastes differ, and if one aesthetic taste is better or more ‘correct’ than another [8]. Neuroaesthetics, which is an interdisciplinary field, applies these complex questions to the brain, investigating the neurological and behavioral processes that impact aesthetic taste and experience. Though these processes are not fully understood, previous life experiences, emotions, and perceptions all guide and influence our interpretation of the present [2, 9]. Neuroaesthetics allows us to examine the neurological underpinnings of aesthetic experiences and preferences from the individual level to our broader society [2].
When you meet someone for the first time, you immediately begin to form an impression of them based on your aesthetic evaluation [10]. The symmetry of their face, the brightness of their eyes, the contour of their jawline, and even the shifting of their facial expressions can contribute to your subconscious assessment of their appearance. In fact, it only takes about 100 milliseconds of exposure to a face to judge whether or not it is attractive. However, it is important to note that this first impression is malleable
[10]. The visual processing of a face begins when light enters the eye and hits the retina, which transmits light into an electrical signal that is understood by the brain [2, 11, 12]. Electrical signals are the means through which neurons, the fundamental cells of the nervous system, communicate with one another [13]. In the case of our visual system, neurons in the optic nerve carry electrical signals from the retina to the brain [14]. From the optic nerve, electrical signals pass through the thalamus — the relay center, similar to a train station — to be directed to different regions of the brain. For visual processing, information is sent to the occipital lobe [11, 12]. The occipital lobe forms a basic image of the person’s face, accounting for details like the edges of their jaw, the orientation of their head, the placement of their eyebrows, and the color of their hair [11, 12]. Electrical signals then travel to different parts of the brain via networks of neurons to complete higher-order processes, which are cognitive functions that integrate sensory information into thoughts and actions [11, 12, 15].
To carry out higher-order processing, visual information from the occipital lobe needs to be delivered to crucial brain regions [16, 17]. One of these regions is the fusiform face area (FFA), which allows us to recognize and distinguish different faces by processing the location of facial features and their variation across people [16, 17]. When we encounter an unfamiliar face for the first time, we unconsciously perform distinct eye movements that scan the face to gather visual information [15, 18]. We distinguish individual differences between people’s faces in the FFA, such as the distance between their eyes, the angle of their brows, and the spacing of their cheeks [15, 17]. The orbitofrontal cortex (OFC) integrates sensory information from the occipital lobe, the FFA, and the limbic system — a group of brain structures that regulate emotions, memory, and behavior [15, 17, 19, 20]. Integration of sensory information allows the OFC to associate certain visual aspects with rewarding properties [15, 17, 19, 20]. The OFC plays a critical role in assigning value to aesthetic stimuli, labeling any

received input as positive, neutral, or negative [2, 21, 22]. Neurons from the OFC transmit signals to the nucleus accumbens (NAc), causing altering the release of a chemical messenger called dopamine, which is critical in regulating our reward system [2, 15, 20, 23, 24]. When we look at attractive faces or other rewarding stimuli, the OFC sends excitatory signals to the NAc, which leads to increased dopamine release in the NAc [15, 25].
The dopamine reward system is activated in response to seeing beautiful stimuli, contributing to a feeling of reward and reinforcing that certain objects and faces are beautiful [26, 27, 28]. When a certain cue results in an increase of dopamine release in the NAc, that cue becomes associated with a reward and that person is more motivated to seek out that particular cue in the future or carry out a certain behavior that will allow them to experience another rewarding feeling [29, 30, 31]. For example, if we have more positive interactions with people who look a certain way, dopamine reinforces that it was a rewarding experience, encouraging us to continue to seek out other individuals who look similar to them [31, 32, 33, 34]. Additionally, dopamine release in the NAc can impact the prominence of the cue being processed [26]. An increase in dopamine can essentially ‘tag’ certain faces or aesthetic experiences as more significant, and therefore they are more likely to be remembered [29, 30, 31].
Another part of aesthetic evaluation unfolds in the middle temporal gyrus (MTG) [2, 30, 35]. Although the MTG is not traditionally considered one of the core areas of facial processing, it plays a crucial role in integrating inputs from our visual system, memory, emotions, and social context [2, 30, 35, 36]. When we judge someone’s attractiveness, the MTG helps us synthesize not only what we see but also how we feel about them based on prior experiences and learned information [2, 37, 38]. In essence, the MTG allows us to interpret faces beyond simple visual features by incorporating emotional significance and social meaning into our judgment of attractiveness [2]. For example, when you’re looking at someone, the MTG
is activated during your evaluation of their attractiveness, and the MTG may be drawing from memories of previous encounters with individuals who share similar traits. Perhaps those past experiences were positive, causing the brain’s reward systems to link those features with a rewarding feeling. Therefore, the MTG may help encode learned social and cultural values about beauty, combining objective visual stimuli with subjective emotional and social context [2].

Various theories link the reward system’s role in aesthetic experiences to specific behaviors. One popular theory focuses on the evolutionary and reproductive benefits of finding certain features more rewarding than others [39, 40, 41]. Perceived physical and facial attractiveness can symbolize fertility, health, and genetic favorability, impacting the initiation of sexual relations and the continued motivation for parental behavior [39, 40, 41]. For example, facial masculinity in men, like sharp jaws and thick eyebrows, is perceived as a sign of good health and genes [42]. Perceived notions of favorability, however, do not always have a direct link to health; a common example is that facial structures, like symmetry, have no identifiable correlations to one’s health status, but we still utilize these structures to judge other’s health status and attractiveness [42, 43]. People of different sexualities demonstrated greater activation of their OFC when shown faces of individuals of the sex they were attracted to compared to faces of individuals of the sex they were not attracted to [39, 41, 44]. Heterosexual people demonstrated greater activation of components of the brain’s reward systems — namely the NAc, OFC, and the prefrontal cortex — when shown faces of members of the opposite sex that they categorized as attractive [39]. This indicates that the neurophysiology underlying our romantic choices and the reward value of attractiveness is similar across sexes [39, 41]. In addition to mate-choice behaviors, attractiveness also influences caregiver behavior toward infants [39, 41]. ‘Cuter’ infants are more likely to

receive care and positive attention from others [41]. Perception of cuteness leading to increased attention might be the result of cuteness being associated with health and viability, where the caregiver’s attention to cuter babies is more likely to ‘pay off’ in the survival of the child [39]. For example, when a woman looks at a baby they consider ‘cute’, their NAc, and reward system, becomes activated, resulting in the reinforcement of those ‘cute’ features as favorable [39]. Differences in aesthetic processing between sexes are of great interest in the field of neuroaesthetics, with ongoing efforts to uncover the neural pathways, differences, and behaviors underlying mate choice and infant care choices [15, 45, 46, 47].
Another large focus within the field of neuroaesthetics is the cycle of influence between individual aesthetic ideals and larger societal ideals [48]. Societal standards of beauty directly influence how our brains process and evaluate attractiveness [48, 49]. Western beauty standards often emphasize extreme thinness for women and hypermuscularity for men while emphasizing Anglo-European features, creating a narrow definition of what is deemed attractive [48, 49]. Society’s fixation on specific body types is evident within the media, which frequently presents disproportionate representations of appearance and physique. Different forms of entertainment and social media bombard people with idealized images of physical appearance [48, 49, 50, 51, 52]. Photoshopped images present even more unrealistic representations of beauty, creating an environment where individuals increasingly endorse socially prescribed appearance
ideals. Repeated exposure to these ideals can condition the brain to associate those traits with higher social value and reward [30, 31, 48, 49]. Over time, the brain may develop a preference for traits that are constantly being portrayed as desirable, driven by the release of dopamine and memory processing [2, 30, 31]. The internalization of societal beauty ideals leads people to engage in behaviors aimed at conforming to these standards, such as dieting, excessive exercise, and cosmetic surgery [49, 50, 51, 52]. Dissatisfaction with one’s own attractiveness is particularly prevalent among women, who are subject to stricter and more rigid social standards than men [53]. Deviations from societal beauty standards can trigger feelings of social rejection or inadequacy, which may activate brain regions associated with negative emotional states, such as the anterior insula or amygdala, intensifying the pressure we feel to conform [54, 55].
Cultural and social backgrounds can also impact our perception of what is beautiful [49]. While many socioeconomic groups may share similar standards of facial attractiveness — such as symmetry and youth — notable differences in what others deem attractive persist and are shaped by their race, ethnicity, and gender [42, 43, 49]. For instance, Black women tend to be more accepting of body diversity, despite pressures they may face linked to colorism — bias favoring lighter skin tones — in determining beauty [49]. Factors such as colorism and hair texture play crucial roles in shaping perceptions of beauty within different communities; women’s attractiveness ratings are often influenced by these characteristics
[49, 53]. Furthermore, societal implications of beauty extend beyond personal perception; they influence social interactions, professional opportunities, and social standing [49, 56]. The ‘beautiful-is-good’ stereotype suggests that attractive individuals are often perceived as more intelligent, competent, and cooperative, leading to biases in various settings, including hiring practices and legal judgments [49, 56]. For example, women are likely to offer more resources to attractive males in behavioral games, demonstrating that physical appearance can affect social dynamics and decision-making [56]. The ‘beautiful-is-good’ attractiveness bias reinforces the notion that those who align more closely with conventional beauty standards tend to receive advantages in employment opportunities and income, perpetuating a cycle in which beauty equates to social and economic capital [49, 56].
Watching a beauty pageant may seem like a simple action on the surface, but on a deeper level, it involves the merging of all the aesthetic values that surround you and the intricate interactions of neurons within your brain [2, 48]. Neuroaesthetics uncovers the hidden choreography of the social, individual, and neural relationships that are behind the judgments and actions that inform our daily experiences. Our personal notions of beauty are behind our daily choices and perceptions. Every brushstroke in a painting, every note in a song, every face in a crowd becomes part of the mental landscape where aesthetics and our brains intertwine. By understanding the neuroscience behind our aesthetic experiences, we gain insight into the art of our own perceptions, an art we are painting in every choice we make, every moment we savor, and every beautiful thing we see [2, 3, 4, 9, 48].
References on page 58.

by Daniel Bader | art by Michelle Schaffer

What if the key to boosting your child’s intelligence wasn’t in a Mozart CD, but in a more nuanced understanding of how music affects the brain? For years, parents have been playing classical music for their infants, hoping it will make them smarter [1, 2]. This popular belief has led many to assume that exposure to classical music could significantly enhance a child’s cognitive abilities. But is there really scientific evidence to support this claim? This fallacy stems from a highly-referenced study that claimed that listening to Mozart temporarily increases spatial intelligence in college students [3, 4].
Spatial intelligence describes the ability to analyze objects in three-dimensional space and draw conclusions based on limited information [5]. Tasks such as envisioning molecules in chemistry class and navigating the streets of New York City utilize spatial intelligence [6]. Although the initial study only concerned spatial intelligence, popular media overgeneralized the results and reported that listening to classical music permanently enhances general intelligence [7, 8]. The public misconception that music directly correlates with intelligence was labeled the ‘Mozart Effect’. Many parents were led to believe that
exposure to Mozart’s music would improve their child’s intelligence. As a result countless parents purchased Mozart CDs to expose their infant children and developing fetuses to classical music, hoping that their purchase would yield cognitive benefits for their children [7, 8]. However, there is no direct link between classical music and intelligence [7, 9]. While there are cognitive benefits to listening to music, these benefits are not exclusive to listening to solely classical music [3, 10]. How music impacts cognitive performance extends beyond the erroneous ‘Mozart Effect.’
Does a particular song or genre increase your productivity when studying? Contrary to the Mozart effect, listening to music does not directly enhance general intelligence. Instead, listening to music can indirectly boost cognitive function, which is a key idea of the Arousal Mood Hypothesis (AMH) [3, 11]. The AMH suggests that listening to music induces arousal, leading to improvements in reading comprehension and problem-solving abilities. Arousal is the degree of excitement and alertness experienced by an individual in response to external stimuli [3, 12, 13]. Listening to pleasant music improves mood and increases arousal, which can, in turn, improve cognitive function while actively performing tasks [3, 14]. The AMH also asserts that too much arousal can distract individuals, negatively impacting their performance on select tasks [3]. Although a heavy metal fan may find rock music to be very pleasurable, they might not listen to it while doing homework; the presence of lyrics and loud, fast melodies may have a distracting effect on listeners, counteracting the potential positive impact of music on cognitive tasks like reading and writing. Hence, the optimal music to boost cognitive performance is music that the listener enjoys but is not so loud or distracting as to impede their concentration [3].
Listening to music can contribute to feelings of immense pleasure through dopamine release [15]. Dopamine is a neurotransmitter — or a chemical messenger of the nervous system — that is involved in reward-based learning [15, 16]. Dopamine release has been found to improve our mood and increase arousal, which can boost cognitive performance [15, 16, 17]. However, the relationship between dopamine and cognitive performance is not linear; dopamine levels that are too low or too high can impede cognitive performance [18]. Memory plays a crucial role

in our ability to complete cognitive tasks [19]. Different types of information are stored within different categories of memories, and our episodic memory is responsible for storing our personal history and is invoked when we recall our life experiences [20]. Our ability to create new episodic memories is heightened following exposure to a stimulus that induces dopamine secretion, such as music [15]. Additionally, dopamine is linked to improvements in working memory performance, relating arousal and dopamine to learning [21]. Working memory refers to the small amount of task-relevant information that is readily accessible to us, and is instrumental to our ability to plan, problem-solve, and comprehend information [19]. For example, working memory is crucial for our ability to navigate; we utilize our working memory when we drive to a familiar destination for the first time without consulting a GPS [22]. Increased arousal also leads to an increase in working memory capacity, further suggesting that stimuli triggering dopamine secretion — such as music — can boost cognitive function [21, 23, 24].
Although recent studies refute overgeneralizing the Mozart Effect, listening to classical music can lead to cognitive benefits, like improvements in sustained attention [3, 25]. Pop, rock, and jazz music have been shown to impede our attention. Slow, non-vocal classical music, however, does not impede our attention [3, 26]. Music with lyrics can be distracting, as the presence of words hinders our ability to comprehend the words we read and encode them to our memory [3, 25, 26]. Instrumental music lowers our heart rate and blood pressure and allows us to relax, which may explain why non-lyrical genres of music — such as classical and lo-fi — support our ability to execute cognitive processes like reading and completing homework [26, 27]. Additionally, classical music is effective in reducing the stress hormone cortisol. Reductions in cortisol levels can lead to feelings of relaxation, which could explain why classical music may relax listeners [27]. Lyrical music may be less relaxing because lyrics require attention to process [3, 28]. Additionally, lyrics of a familiar language interfere with cognitive performance more than lyrics of an unfamiliar language, suggesting that lyrics themselves are not inherently distracting. Rather, one’s degree of familiarity with the lyrical language they are listening to may determine how distracting the lyrics are on their ability to complete a task [3, 28]. As reading and listening to lyrical music both require language processing, doing both at the same time limits our reading comprehension ability [29, 30]. In essence, listening to music with lyrics while reading is a form of multitasking, and because of the limitations in our brain’s ability to switch our attention, multitasking can be very challenging [31]. Most attempts at multitasking involve rapidly switching from one task to another because the brain most effectively operates with a single goal in mind. Switching between tasks leads to a decreased ability to focus on tasks and efficiently complete them [31]. Listening to lyrical music has been found to be detrimental when performing verbal recall tasks as well; lyrics are subconsciously committed to our long-term memory instead of the subject we are trying to learn, interfering with future retrieval of the content we intend to commit to memory [29]. Classical music is an excellent choice of background music because it optimizes arousal while mitigating the potential distracting effect of lyrics [3, 25].
Other characteristics of music — such as tempo, volume, and tonal changes — can also impede our ability to perform tasks [32]. Simple music, which is characterized by consistent, narrow tonal ranges, is recommended to support our ability to study and read [32, 33]. Imagine you are doing homework in a quiet classroom when the squeak of a chair sliding across the floor draws your attention. The tone change involuntarily distracts you, impeding your ability to concentrate. Changes in tone require our attention and utilize cognitive resources that could be directed toward tasks we are trying to complete [32]. Likewise, music characterized by a fast tempo is theorized to be more distracting than slow-tempo music due to the presence of more auditory stimuli, which each require attentional resources to process [17, 34]. The sounds of a woodpecker’s beak hitting a tree are frequent and distracting, with each auditory peck demanding your attention.

Similarly, fast-tempo music is more distracting due to its quicker pace and greater frequency of beats [17, 34]. The sounds of a woodpecker’s beak hitting a tree are frequent and distracting, with each auditory peck demanding your attention. Similarly, fast-tempo music is more distracting due to its quicker pace and greater frequency of beats [17, 34]. Music volume also affects our ability to concentrate on a task, as loud music can be overly arousing and impede our ability to focus our attention [34, 35]. Classical music often exhibits slow tempos, consistent tonal ranges, and low volumes, providing benefits when performing cognitive tasks by limiting distraction [32, 36].
The slow tempo, repetitive tonal ranges, and low volume characteristic of classical music affect the activity of our autonomic nervous system (ANS), which regulates involuntary physiological processes such as our heart rate, blood pressure, and ability to breathe [25, 37]. In response to music with a slow tempo, a part of our ANS called the vagus nerve decreases blood pressure after detecting vibrations, which contributes to a feeling of relaxation [25, 37]. Conversely, faster music increases our blood pressure and heart rate, inducing arousal rather than relaxation [25]. In addition to impacting blood pressure, classical music has been used therapeutically to alleviate stress [37]. Classical music significantly lowers the stress hormone corticosterone in rodents, which is comparable to the stress hormone cortisol in humans [37]. As a result, classical music has been implemented as background music in many hospitals to reduce people’s stress, anxiety, and pain levels [38]. While classical music may not be unique in its ability to relieve stress, not all genres of music decrease stress levels [37, 39]. Rock music and atonal composition music — or music without a central tone, harmonies, or keys — are ineffective in reducing stress [37, 39]. Ultimately, the stress-relieving effect of classical music can likely be attributed to its rhythmic structure and emotional impact, further cementing how properties of classical music support cognition [37].
People can use music to enhance their enjoyment and capacity to perform long and tedious tasks [40]. When performing tasks that are monotonous or those that do not require a high level of focus, listening to pleasurable music can be used to improve mood and well-being, which can lead to improved task performance [40]. For example, listening to music while driving can be extremely beneficial by moderating the

driver’s arousal, which in turn may limit the stress they experience and prevent drowsiness [41, 42]. Additionally, pleasurable music can limit mind-wandering when driving long distances by contributing to enhanced and sustained focus [17, 42]. While listening to slower music may not increase arousal enough to sustain prolonged attention, listening to fast-tempo music can be overstimulating, and has been correlated with an increase in driving speed and number of lane crossings [42, 43]. Medium-tempo music is theorized to be best to support driving; while it increases arousal and miniimizes fatigue, medium-tempo music is not overly stimulating to the point of inhibiting our attention and ability to reach our intended destination safely [42]. Music volume has also been associated with driving speed: high volume tends to increase our driving speed while low volume decreases it, highlighting how music can impact arousal [17, 41, 42].
Listening to music can also distract us from exertion during exercise by stimulating arousal and distracting us from strenuous physical efforts [44]. While fastpaced music is energizing, slower and less distracting music can lead to quicker fatigue as it causes us to focus more on the effort we exert [44]. Fast-paced music has also been theorized to increase power output and decrease muscular fatigue, which are both beneficial when performing resistance-based and weight-training exercises [45]. High-volume alerts — like fire alarms or amber alerts — are used to maximize reaction time efficiency by attracting our
attention [46]. When your phone receives a notification, you may not hear the buzz since the quiet noise is easy to ignore. However, if a fire alarm goes off, you will hear it and react instantly because the loud noise demands your attention. Increased music volume has also been shown to support enhanced exercise performance; as a higher volume grasps our attention, directing it away from the difficulty of the exercise we are performing [46]. The role of music in exercise and driving conveys music’s versatility and emphasizes how it can be utilized as a resource to support our ability to conquer daily tasks [3, 44].
Music therapy has been utilized as an accessible and effective sleep aid due to its physical benefits and ability to create a stable auditory environment [32, 47]. Specific attributes of music can enhance the ability to fall asleep and improve overall sleep quality; low, gentle, and calm music is thought to be an effective sleep aid because it reduces heart rate and blood pressure, contributing to overall muscle relaxation [25, 32]. Music that decreases arousal has been implemented as a sleep aid due to its ability to distract people from stressful thoughts, which may limit their ability to fall asleep [32, 47]. Additionally, music mimics the effect of white noise by drowning out external sounds, creating a stable and calm auditory environment and improving sleep [17, 32, 34, 47, 48]. As illustrated by the squeak of the chair moving in a quiet room, auditory changes unconsciously utilize cognitive resources [32]. Music is affordable and accessible, and has been implemented as a treatment for people with insomnia, a condition marked by difficulty sleeping [23, 50, 55].
Applications of music have also extended far beyond sleep aids [51]. Music therapy could benefit people with Alzheimer’s Disease (AD), an incurable condition characterized by cognitive decline and memory loss that affects over seven million Americans — a number expected to double by 2050 [51, 52]. Music allows people with AD to learn new melodies, understand words through lyrics, and react emotionally to songs [51]. Through connecting individuals with AD who are experiencing cognitive decline with familiar memories, music therapy can be an effective tool to improve the lives of patients with AD [53]. For example, listening to classical music may help patients with mild AD recall important moments from their past — AD patients who were played the music of Vivaldi were more capable of recalling personal memories than those who were asked to recall memories without Vivaldi playing. Therefore, in some cases, music therapy may boost cognitive performance in patients
with AD, potentially improving quality of life [53]. Music has been a staple of human culture for ages, and contemporary applications of music therapy demonstrate its continued relevance in the future as AD and related disorders continue to become more prevalent [49, 50, 51, 52].
Listening to music is a universal experience that can evoke strong emotions in listeners regardless of what genre they listen to [54]. In fact, arousal arising from listening to music has been associated with improvements in performance on cognitive tasks [3]. While the Mozart Effect overgeneralized the impact of classical music on intelligence, listening to pleasure music can improve our mood and increase our arousal, which can boost our ability to execute various tasks [3, 10]. Additionally, several characteristics of music — such as music volume, tonality, and tempo — must be considered when deciding on the effective background music for different tasks [25, 32, 36, 46]. Music can be implemented to increase task performance when its properties and effects provide the optimal level of energy and focus for a specific task [32, 37]. Folding clothes and doing the dishes are boring tasks, so moderately arousing and uplifting music may improve mood and enhance enjoyment and efficiency [40]. Slow, quiet, and relaxing background music can be utilized when studying and reading to improve mood and arousal while avoiding distraction [3, 37]. As we continue to study music’s capacity to influence our emotional states and support cognition and task performance, it is clear that the applications of music extend far beyond listening pleasure [49, 50].
References on page 62.

by Eli Kanetsky | art by Alexandra Adsit

Everyone is bound to experience a headache at some point, whether it be from poor sleep the night before or from seasonal allergies. However, only about 10% of people experience migraines, a subset of headaches characterized by a unique set of intense symptoms [1]. Imagine you have a friend named Maya, who has been plagued by migraines her entire life. When she experiences a migraine attack, Maya feels a strong, pulsating pain on one side of her face that lasts for several hours [2]. Her throbbing headache is often combined with nausea, dizziness, and a sensitivity to light, differentiating it from what is considered a typical headache [2]. Interestingly, Maya can occasionally predict that a migraine attack will occur when she notices peculiar changes in her vision, like a blurry patch or floating bright dots. These bizarre visual manifestations that Maya experiences are part of a collection of symptoms referred to as migraine aura [1]. Unfortunately, the discomfort caused by migraine aura symptoms may not be easily relieved as there are no current treatments that specifically target aura. Nevertheless, numerous aura-specific treatments are undergoing testing and show promise in reducing migraine aura symptoms [1].
Migraine aura is defined as a collection of temporary, neurological symptoms that can precede migraine headaches, affecting approximately a third of those who experience migraines [1, 3]. These symptoms can manifest in various ways, affecting linguistic, motor, or sensory functions, including difficulty speaking, motor fatigue, numbness of the limbs, or visual disturbances [4]. Our friend Maya experiences visual disturbances, which are the most common group of symptoms associated with migraine aura [2, 5]. Visual symptoms can be defined as positive or negative, depending on whether the visual aura ‘adds’ a hallucination or ‘takes away’ part of one’s vision, respectively [2, 3, 5, 6]. One instance of a positive symptom that Maya experienced was the appearance of colorful zigzags in her left visual field one morning. The zigzag pattern Maya saw is one of the most common forms of positive aura symptoms [5]. During another time, Maya stopped playing basketball with her friends after she noticed a blind spot that began interfering with her vision. The temporary blindness Maya experienced within a concentrated area of her vision is called scotoma, a common negative aura symptom [5]. In addition to zigzags and scotomas, other geometric shapes or light disturbances — including foggy vision, flashing lights, and black or white spots — are

commonly experienced by individuals with migraine aura [5, 6]. Still, some individuals may hallucinate ‘complex aura,’ which means they picture entire people or objects, although this phenomenon is far less researched and understood [6].
The assortment of symptoms a person may experience is not the only unpredictable aspect of migraine aura [6, 7]. Aura symptoms can also vary from episode to episode in terms of their onset and duration [7]. Aura typically begins ten minutes before a migraine headache sets in, and lasts between five minutes and an hour, meaning that aura can occur before the start of a headache as well as continue during the headache [6]. The severity of each experience with aura may also differ — for instance, when Maya saw zigzags, they vanished shortly afterward and were not accompanied by a migraine headache, allowing her to go about the rest of her day as usual. Contrarily, during her less fortunate experience playing basketball, the blind spot continued for an hour and overlapped with a painful headache, forcing Maya to recuperate at home for the rest of the day. Evidently, experiencing aura can be stressful and disorienting, potentially interfering with one’s daily activities [6, 8]. As distressing as aura symptoms may be, the causes of migraine headaches and aura are not fully understood [1, 2]. Thus, working to understand the mechanisms underlying aura may help narrow this gap in knowledge and reveal more about migraines as a whole [1, 2].
While there are multiple theories surrounding the processes that generate migraine aura, the leading explanation is that cortical spreading depression (CSD) may be the underlying mechanism [3, 4, 6]. CSD can be understood when broken down into a few different stages. The first stage of CSD involves a wave of activity in the outermost layer of the brain, called the cerebral cortex [6, 9]. Brain cells that specialize in receiving and transmitting information, called neurons, are found throughout the brain, including within the cerebral cortex [10]. At rest, neurons have a more negative charge inside compared to their more positive surroundings [11]. This resting state can be changed through a process called depolarization, which alters the distribution of electrical charges and makes the inside of the cell more positive. Typically, when depolarization occurs and the neuron reaches a certain threshold of positivity, the neuron will ‘turn on’ to communicate with other cells, which is referred to as an action potential. The relationship between depolarization and an action potential is analogous to flushing a toilet: to flush a toilet, the handle must first be pushed down enough. Similarly, to cause an action potential, a neuron’s voltage must be made positive enough. Then, once the toilet is flushed, it cannot un-flush. Action potentials work the same way — they either happen fully or do not happen at all [11].
While depolarization and action potentials are important functional mechanisms in the brain, these processes occur at an abnormally large scale in CSD [6]. Once one neuron is depolarized during CSD, the
change in the distribution of charges also affects neighboring neurons, inducing depolarization in those neurons as well [6]. The activation of surrounding neurons then leads to more depolarization, causing a self-sustaining wave of spreading neuron activation [12]. This wave of activity that passes through the cerebral cortex constitutes the first stage, or the spreading stage, of ‘cortical spreading depression’ [6]. Therefore, CSD can be thought of as a ‘brain tsunami;’ each activated neuron acts like a droplet of water, accumulating to form a massive tsunami of neural activation. After the ‘brain tsunami’ occurs, however, it takes a prolonged amount of time for the affected area of the brain to return to baseline neural activity [6].
The disruption following the ‘brain tsunami’ constitutes the second stage of CSD — a period of suppressed neural activity across the cerebral cortex caused by the massive redistribution of charges in the first stage of CSD [6, 12]. Typically, depolarization and action potentials are followed by repolarization, during which the inside of a neuron returns to its resting, negative state before another action potential can occur [11]. The restoration of a negative charge via repolarization is similar to what happens after a toilet is flushed. Once water is drained from the toilet bowl, the water has to return before the toilet can be flushed again. If the water does not return properly, the toilet is ‘stuck.’ The toilet getting stuck is symbolic of what goes wrong during CSD: neurons do not undergo repolarization and thus cannot return to their normal functions, inducing a period of inactivity during which they are unable to fire [12]. As hinted at through the name ‘cortical spreading depression,’ the

period of activity suppression constitutes the stage of CSD that is an inhibition, or depression, in neural activity [6, 12]. Fortunately, due to the temporary nature of CSD, inhibited neurons eventually repolarize and return to normal function [12]. All in all, CSD can be conceptualized as a wave of depolarization across the cerebral cortex, followed by a period of neural inhibition in the same region [1, 6].
So, what’s the connection between CSD and migraine aura? Establishing a relationship between the two is done primarily by observing blood flow changes in the brain during migraine attacks with aura [3, 13]. Tracking the movement of blood flow can be used to visualize neural activity, elucidating similarities between neural activity seen in migraine aura and in CSD [13]. In CSD, cerebral blood flow initially increases before subsequently decreasing below its baseline level [3, 14]. The ‘rise and fall’ pattern of blood flow observed in CSD meets our brains’ demand for oxygen and nutrients — a wave of depolarization calls for more blood flow to sustain neural activity, whereas a period of inactivity that follows depolarization requires fewer resources [3]. Functional MRI (fMRI) is used to observe the relationship between the characteristic blood flow pattern in CSD and the blood flow pattern seen during instances of migraine aura. fMRI often uses Blood Oxygen Level Dependent (BOLD) signals to map brain activity by measuring changes in blood flow that reflect neuronal activity [13]. When a person experiences an instance of visual migraine aura and simultaneously receives an fMRI scan, an initial increase and subsequent decrease in BOLD signals is observed, indicating a rise and successive decline of cerebral blood flow [7, 14]. Therefore, cerebral blood flow increases and decreases during aura in a way that parallels neuronal activity and blood flow in CSD [7]. Additionally, the BOLD signal spreads at the same rate as the spread of CSD, which supports parallels observed between migraine aura and CSD [3].
Another similarity between blood flow in CSD and instances of migraine aura is the distinction between positive and negative aura symptoms [2, 6, 15]. The specific phases of activity in CSD are related to the occurrence of positive and negative symptoms — positive symptoms correspond with the wave of depolarization, and negative symptoms with the period of inactivity — and the blood flow patterns of each phase reflect this connection [2, 6, 15]. Accordingly, BOLD signals of individuals experiencing positive aura symptoms increase, but decrease while they
experience negative aura symptoms [15]. An association can therefore be drawn between specific types of aura symptoms and different neural responses during CSD [15]. Beyond the correlation between CSD and migraine aura, a connection between CSD and the type of pain experienced during migraine headaches may also exist [3, 16]. Migraine headaches can be triggered by the activation of a group of neurons that form the trigeminal nerve, a cranial nerve involved in pain transmission and blood flow regulation [3, 16, 17]. CSD can trigger the activation of the trigeminal nerve, which could result in the pain associated with migraine headaches [3, 16]. If CSD is involved in both migraine headaches and aura, CSD may be a crucial connection for clarifying the physiological processes of both these aspects of migraine attacks.
In addition to CSD’s implications for migraine with and without aura, CSD may serve as an overall connection between other conditions that are comorbid with, or that typically coexist with, migraine [3, 18, 19, 20]. Individuals who experience migraine with aura are at a higher risk of experiencing ischemic stroke, a type of stroke characterized by reduced cerebral blood due to blockage of blood flow to the brain [3, 21]. People who experience migraine with aura are at double the risk of developing ischemic stroke in their lifetime compared to people who do not have migraines at all. In rare circumstances, an episode of aura may be severe enough to cause an ischemic stroke [12, 22]. In contrast, there is no established connection between experiencing migraine without aura and the occurrence of ischemic stroke [3, 22]. However, the exact nature of the relationship between ischemic stroke and migraine with aura is also difficult to interpret due to confusion as to whether stroke or aura occurs first [22, 23]. Furthermore, complications in distinguishing ischemic stroke from migraine with aura arise from the misdiagnosis of migraine aura with a transient ischemic attack — a separate brain dysfunction caused by reduced blood flow that can be a sign of an oncoming ischemic stroke [22, 23]. Nevertheless, migraine with aura and ischemic stroke may be connected through the decrease of blood flow during the period of inactivity in CSD [24]. The decrease in blood flow may not only lead to ischemic stroke but can also increase the intensity of the stroke experienced [12, 22, 24].
A comorbidity between migraine both with and without aura and epilepsy is also observed [24]. Epilepsy, a disorder characterized by recurring seizures, is considered related to migraine disorders because the two share many similarities, which may cause difficulty in distinguishing them from one another [19, 20]. Both epilepsy and migraine with aura seem to share underlying pathophysiological mechanisms that can lead to hyperexcitability, a state in which neurons are more likely to activate than usual [18, 19, 20, 25].
In epilepsy, hyperexcitability leads to unrestricted depolarization and may cause groups of neurons in the cortex to fire action potentials simultaneously, which is a crucial part of the development of seizures in epilepsy [24]. In migraines, the depolarizing effects of hyperexcitability may progress into CSD [19, 24].

Hyperexcitability’s possible role in migraines is supported by the fact that individuals who experience both migraines with or without aura demonstrate increased hyperexcitability in the visual processing area of their cerebral cortex compared to individuals without migraine at all [26]. In addition to similarities in epilepsy and migraine aura initiation, the two phenomena are also connected through migraine aura-triggered seizures, which are characterized by an epileptic attack during or within an hour after the occurrence of migraine with aura [2, 19, 24]. Due to similarities in epilepsy and migraine aura — including resemblances in visual aura and possible sensory disturbances in epilepsy — misdiagnosis may occur when identifying migraine aura-triggered seizures since it is difficult to differentiate which characteristics may be attributed to epilepsy and which to migraine aura [19, 20].
While it becomes apparent that CSD may underlie migraine with aura and other related disorders, there is no current treatment that targets aura [1]. A potential aura-specific treatment may aim to suppress CSD by targeting its mechanism of initiation [1]. The initiation of CSD involves the release of glutamate, an excitatory neurotransmitter that is often released by neurons after firing an action potential [1, 4, 27, 28]. After being released, glutamate binds to receptors on neurons that stimulate depolarization and trigger action potentials [28]. If an action potential is like a toilet flushing, excitatory neurotransmitters are like your hand — the force that increases the pressure put on the toilet handle. When pressure pushes the handle down enough, the toilet flushes. Glutamate’s excitatory role may be a significant aspect of the initiation and spreading of CSD [4, 27].
Glutamate can notably ‘turn on’ certain receptors that play a crucial role in the generation of CSD [4, 27]. The specific type of glutamate receptors involved in the stimulation of CSD are called NMDA receptors [29, 30]. When NMDA receptors are activated, they provide a channel for positive charges to travel into the cell, making the cell more likely to depolarize [31]. So, when a stimulus triggers the release of glutamate, glutamate binds to and activates NMDA receptors, and this contributes to depolarization [27]. NMDA receptor activity likely plays a role in the prolongation and spreading of depolarization
in CSD, as well [27, 30]. By creating a way for positive charges to flow into neurons continuously, active NMDA receptors initiate the self-propagating nature of depolarization in CSD [30]. Furthermore, NMDA receptors are necessary for CSD to occur because CSD can be completely inhibited when NMDA receptors are blocked [1, 30].
Due to the role of NMDA receptors in CSD, NMDA receptors may be a useful target for managing migraine aura symptoms [1, 4]. Ketamine, a quick-acting general anesthetic, has been explored as a potential treatment for migraine aura because of its ability to block the action of NMDA receptors [1, 4, 32]. Ketamine can reduce the severity of aura symptoms, but does not always impact the duration of aura [1, 29]. In a small subset of people, the use of ketamine has even stopped aura symptoms altogether [29]. Ketamine is an effective NMDA receptor blocker, but it has potential adverse side effects including nausea, high blood pressure, abnormally high heart rate, and dissociation [1, 29, 33]. However, ketamine’s ability to reduce aura symptoms illuminates the utility of targeting NMDA receptors as a method of regulating migraine aura.
Gaining a greater understanding of migraine aura and its mechanisms may elucidate the functions of a myriad of neural systems. Currently, however, most studies regarding migraine treatments include a variety of people both with and without aura, making it difficult to develop a treatment based on one subtype of migraine [1]. Differentiating experiments to focus on either people experiencing migraine with aura or people experiencing migraine without aura may be a promising direction for managing specific aspects of migraine attacks [1]. Using such an approach to develop an aura-specific treatment may help in alleviating harrowing aura symptoms, and reveal how migraine with aura relates to debilitating phenomena such as ischemic stroke and epilepsy [3, 18, 19]. Fortunately, applying the knowledge that blocking NMDA receptors can inhibit CSD means that continuing research on the effects of drugs on NMDA receptors may eventually illuminate a successful way to suppress CSD and manage migraine aura symptoms [1, 29]. Future research should build off of current knowledge and pursue separate studies between migraine with and without aura to solidify our understanding of migraine aura and lead to the development of aura-specific treatments [1].
References on page 63.

by Katerina Hristova | art by Anna Bishop, Iris Li

Imagine that you are being wheeled into the operating room for surgery, your mind racing with anxious questions. Will you remember anything? Will you feel pain? Will you be able to see or hear the doctors and nurses? Amidst your swirling thoughts, the nurse attaches an IV to the top of your hand. She tells you to begin counting down from ten. You feel drowsy and begin counting: ten, nine, eight, seven. When you open your eyes, the operation is over. You have no recollection of falling asleep at all. Most people have no sense that any time has passed between the moments of anesthesia administration and waking up [1, 2, 3]. General anesthesia is a drug-induced loss of consciousness that is characterized by amnesia, immobility, hypnosis, unresponsiveness to painful
stimuli, and the absence of reflexes [4]. Anesthetics, such as propofol, are medications used to put people in a controlled state of unconsciousness so that invasive surgical procedures may be completed without physical or psychological discomfort [5]. While people are sedated under anesthesia, their heart rate, blood pressure, oxygen levels, and other vital signs must be monitored because some bodily functions temporarily slow down [6]. At the end of a surgical procedure, the sedation is reversed and the person wakes up. What occurs inside the brain when someone is under general anesthesia remains enigmatic, however the neurological mechanisms behind how anesthesia operates have been explored [7].
You may think that you were ‘put to sleep’ for your surgery, but a myriad of neurological and physiological differences between sleep and anesthesia-induced unconsciousness exist [8]. Both states are characterized by immobility and one’s reduced responsiveness to their environment [9]. However, while sleep is a naturally altered state of consciousness, anesthesia is drug-induced, and is considered a reversible loss of consciousness [4, 10]. Additionally, sleep is a restorative and active process that is vital to maintaining optimal brain function, facilitating hormonal and metabolic regulation as well as memory consolidation processes [11]. Via consolidating memories, we cement the knowledge and experiences we gain while we are awake into our long-term memory [11]. In contrast, anesthetics block the use of working-memory, which is the temporary storage of limited information [12, 13]. Undergoing general anesthesia is therefore not the same as just falling asleep.
Before you start to feel drowsy, propofol has to find its way to where consciousness arises: the brain. For this to happen, the drug must pass through the blood-brain barrier, which regulates the movement of blood and other fluids into the brain [14]. Once past the blood-brain barrier and into the brain, propofol can begin to affect neurons, or communicative brain cells [15]. In order to transmit information, neurons utilize chemical and electrical signaling [16]. At rest, the inside of a neuron is negatively charged relative to the outside of the cell, a difference called membrane potential [17]. An increase in the movement of positively charged particles called ions into the cell changes the membrane potential. When the interior charge of a neuron is increased to a certain threshold, the neuron is able to fire signals to other neurons [17]. GABA is a neurotransmitter that generally inhibits neuronal activity; when GABA binds to its respective receptor, negatively charged ions will enter the neurons. An influx of negative ions causes the charge of the cell to become more negative, which prevents activation and therefore communication with other neurons [18]. GABA can bind to a variety of receptors, including the GABAA receptor subtype [19, 20]. GABAA receptors play a key role in facilitating the loss of spinal and muscle reflexes and amnesia in general anesthesia [21, 22]. Propofol imitates GABA and binds directly to the GABAA receptor, allowing negative ions
to enter the cell. The increasingly negative membrane potential prevents the neuron from being activated. Therefore, propofol prevents neurons from sending electrical signals to communicate with other cells [9]. Propofol can also bind to another part of the GABAA receptor and enhance the existing activity of GABA [20, 23]. Unlike other modulators that potentiate, or intensify, the response of the GABAA receptor, propofol binds to a subunit that is seen in all GABAA subtypes, allowing it to bind to GABAA receptors, quickly and efficiently targeting various regions of the brain where the receptor is abundant [19].

Propofol, likely through its action on GABA receptors, significantly decreases overall activity in the brain [24, 25]. One way to measure levels of brain activity is to measure rates of metabolism in the brain known as cerebral metabolic rate (CMR) [26]. Metabolism is the process by which food is transformed into a form of energy that our body can use to fuel its functions [27]. Therefore, a higher cerebral metabolic rate can be indicative of higher brain activity [26]. The injection of propofol has been shown to decrease CMR in every region of the brain by amounts ranging from 30-70% [24, 25]. Metabolic suppression is also observed with other anesthetic agents, and may be responsible for unconsciousness [28, 29]. However, ketamine, which is another agent known to disrupt consciousness, has been shown to increase the CMR in several brain regions [24, 30].
The effects of anesthetics, including propofol, on consciousness also vary across regions of the brain [24, 31, 32, 33]. Propofol’s impact on the thalamus, a region in the brain responsible for relaying sensory and motor information from the body to the brain for processing, has been well studied [31, 34]. The thalamus plays an important role in regulating alertness and consciousness [34, 35]. Nuclei, or clusters of neurons, each with different functions, can be found through out the thalamus [36, 37]. Notably, thalamic nuclei can be either specific or nonspecific [34, 38]. Most sensory information, like the chirping of birds or the taste of coffee, initially passes through specific tha lamic nuclei and is then sent on to the other areas in the brain for processing [39]. Meanwhile, nonspecif ic thalamic nuclei transmit information between the thalamus and the cerebral cortex, the outermost layer of the brain that contains several distinct sub-regions involved in
a variety of processes such as attention and cognition [38, 40]. Nonspecific thalamic nuclei are important in regulating cortical arousal, the level of neural activity within the cerebral cortex [38]. Additionally, nonspecific nuclei are vital for integrating information across the different functional regions that make up the cortex [34, 41]. Unlike specific thalamic nuclei, which solely relay sensory information, nonspecific thalamic nuclei synchronize information between different brain regions, allowing the thalamus to modulate and coordinate neural activity across brain regions [38].
The effect of propofol on the activity of specific and nonspecific nuclei can be measured using a blood oxygen level-dependent (BOLD) fMRI, which measures changes in blood oxygenation [42]. When neurons in a particular brain region become more active, they expend more energy and, therefore, consume more oxygen [43]. To meet this increased demand, blood flow to the active area increases, and BOLD fMRI signals reflect an increase in the concentration of oxygenated blood [42]. BOLD fMRI can be used to analyze changes in functional connectivity, or the temporal connection — how in sync or coordinated the activity is — between brain regions [34].

When distant brain regions synchronize their activity, they are said to be functionally connected, indicating substantial communication between those regions [44]. Propofol decreases the functional connectivity of both specific and nonspecific nuclei [31, 45, 41]. While functional connectivity in specific thalamic nuclei is moderately reduced by propofol, propofol significantly suppresses functional connectivity in nonspecific thalamic nuclei [31, 34]. The significant decrease in the functional connectivity of nonspecific nuclei, and subsequent disruption of arousal and information integration, may be how propofol results in a loss of consciousness [34, 41]. Specific thalamic nuclei, however, remain mostly active after propofol is administered. In fact, during propofol-induced unconsciousness, the sensory cortices — or the parts of the brain responsible for processing sensory information — are still reactive. Therefore, the brain can still receive information during propofol-induced unconsciousness, but it cannot consciously perceive this sensory information. As shocking as it may seem, you can hear during anesthesia, but your brain is unable to consciously recall auditory signals. A lack of conscious perception of sensory stimuli may be due to a decrease of functional activity in nonspecific thalamic nuclei, since information across sensory systems is unable to be integrated and utilized to support higher cognitive functions [34, 41]. A disruption in functional connectivity within nonspecific thalamic nuclei may explain why sensory input continues to reach the cortex during anesthesia, yet we are not consciously aware of it.
The study of anesthesia-induced unconsciousness, particularly through the lens of propofol’s effects on the brain, offers a valuable perspective on the mechanisms underlying consciousness itself [46]. The suppression of functional connectivity in nonspecific thalamic nuclei provides critical insights into how the brain orchestrates integrated states of awareness, highlighting the importance of dynamic communication between brain regions in maintaining consciousness [34, 41, 47]. Furthermore, understanding how anesthetics like propofol interfere with these processes could not only advance medical practices by improving anesthetic techniques and safety, but also deepen our understanding of what it means to be conscious [46, 48]. Moreover, by identifying the specific neural circuits that underlie conscious experience, we may uncover new avenues of research for further understanding other conditions, such as coma [47]. Anesthesia-induced unconsciousness serves as a compelling tool for unraveling the complex neurological basis of consciousness, offering both clinical and philosophical avenues for our understanding of the mind [47].
References on page 65.


by Evan Seker | art by Elizabeth Catizone

Susan, a 25-year-old digital media manager, spends hours every week typing on her laptop. After a while, her hands begin to experience an unfamiliar ache, affecting her performance at work; tasks that once took Susan 30 minutes to complete now take over an hour. In her quest to identify the problem,
Susan consults different doctors and takes their recommendations for various pain medications. After months of visits to several specialists, a blood test finally provides a diagnosis. Susan has rheumatoid arthritis, an autoimmune disorder that causes her immune system to attack her joints.
Let’s follow Susan through a day in her life with her new diagnosis. Getting out of bed takes longer than before, so now she sets an earlier alarm. Susan takes her coffee with prescribed painkillers and sits at her desk, which has been modified to accommodate her new physical limitations. Today is particularly stressful because she needs to submit an important report to her boss, and the stress Susan experiences causes her symptoms to flare up and she feels her joint pain more acutely. Despite her best efforts to manage her symptoms, the condition continues to worsen and Susan feels helpless.
Susan comes across an internet article on the link between chronic stress and certain autoimmune disorders, including rheumatoid arthritis. She is initially skeptical as ‘cure-alls’ are exceedingly rare and unlikely to be promising, but all the article asks her to do is meditate for 30 minutes a day, have something to look forward to at the end of each week, and take steps to make her life more enjoyable. With these suggestions in mind, Susan puts forth a significant effort towards living mindfully, and in a month, her symptoms have decreased and her pain has improved. While managing stress did not cure Susan’s rheumatoid arthritis, it did help reduce her painful symptoms. But, how are rheumatoid arthritis and stress connected?
Whether it be submitting an assignment at the last minute or rushing to catch a train, we have all experienced stress. While uncomfortable, short-term stress is a normal part of the human experience and can be beneficial, boosting our focus and enhancing our physical performance [1, 2]. In a state of stress, our body goes into overdrive to address a challenge. Typically, when the challenge — or the stressor — is removed, the stress subsides and our body returns to normal [2]. While short-term stress is adaptive, longterm or chronic stress is maladaptive and has several negative effects on the body [3, 4]. When someone experiences long-term stress, their body fails to return to baseline after the stressor disappears [1, 5]. Chronic stress can affect a variety of organs: including the heart, gut, and brain [6, 7, 8]. The effects of chronic stress can also have ramifications on body systems, most notably the immune system.
The immune system protects the body by coordinating attacks against possible threats, which include harmful external substances known as pathogens as well as the body’s own damaged cells [9]. When the body identifies a possible pathogen or experiences tissue damage, such as from a paper cut, the immune system dispatches small messenger molecules called cytokines [10]. Cytokine release triggers inflammation, during which blood vessels widen to increase blood flow and send an influx of specialized immune cells to the site of infection [11, 12]. The inflammatory response — one of the body’s first lines of self-defense against pathogens — is a quick and effective component of immunity that is heavily regulated by the brain [13, 14, 15].
Typically, the brain modulates the immune system in response to environmental factors, like stress [15, 16, 17]. The stress response is initiated in the hypothalamus, a brain region responsible for regulating bodily functions such as temperature control, our sense of hunger, and hormone release [18, 19]. The hypothalamus sends a chemical signal to the pituitary gland, located at the base of the brain, which in turn signals the adrenal glands that are located above the kidneys [18]. The signal cascade culminates in the release of the hormone cortisol from the adrenal glands [18, 20, 21]. Cortisol inhibits the release of proinflammatory cytokines — much like a skilled referee prevents players from committing fouls — and contributes to the initial downregulation of the inflammatory response [20, 21]. The suppression of immune processes allows
for the reallocation of energy toward other, more urgent bodily functions, such as fighting off a threat or fleeing from it [20, 22]. This short-term stress response is not only typical, but necessary for survival [2]. However, chronic stress decreases the efficacy of cortisol, leading to dysregulation of the immune system, which can worsen pre-existing symptoms of immune-related disorders [3, 21, 23, 24].


Normally, the immune system distinguishes the familiar — that which belongs to the body — from the foreign — that which comes from outside the body [24, 25]. The ability to recognize the body’s tissues and spare them from an immune attack is known as self-tolerance, and is a crucial component of the immune response [26]. Sometimes, the highly coordinated self-tolerance response goes awry, and the immune system’s ability to recognize its own immune cells fails [24]. When self-tolerance is lost, autoimmune disorders arise [24]. Autoimmune disorders are a type of disease in which the immune system misidentifies the body’s own cells as foreign entities, prompting the attack of healthy cells, tissues, and organs [26, 27]. There is no single cause of an autoimmune disease that has been isolated; rather, autoimmune diseases develop as a result of both genetic and environmental factors [28].
Rheumatoid arthritis (RA) is one autoimmune disease that involves the loss of self-tolerance in the joints [25]. In RA, the immune system erroneously identifies the joints as foreign and mounts an attack against them [29]. The misrecognition of benign joint proteins as foreign stimulates local inflammation, leading to pain, swelling, and tissue damage in the area [30]. These attacks weaken joint tissue, impacting mobility and fine motor skills such as typing, writing, and finger-gripping [31]. The immune system then recognizes the resulting tissue damage and prompts an inflammatory response to bring more immune cells to the damaged site [32]. In doing so, a devastating positive feedback loop is created as the immune system continues to target the damaged area. Therefore, the inflammation in Susan’s joints remains elevated, and the condition worsens. [32]. Over time, the constant barrage of immune attacks one experiences can lead to irreversible disability [33, 34].
Chronic stress can negatively affect the prognosis and induce an earlier onset of RA by continuously stimulating the release of cortisol [35, 36, 20]. Over time, the body develops resistance to cortisol and no longer responds as strongly to its signals, diminishing its immunosuppressive effects [37, 20]. Now, cortisol is like a referee who has lost control of its players. With cortisol’s ability to regulate the immune system dampened, immune activators are able to promote rampant inflammation [20, 37, 38]. Unchecked inflammation accelerates the degradation of joint tissues, thereby exacerbating RA symptoms, much like a game of soccer without a referee in which players would become more erratic and fouls more frequent [35, 39, 20, 38]. Chronic stress enhances immune dysfunction, significantly impacting the prognosis of people living with RA [2]. Both RA and stress aggravate each other in a vicious circle [40, 37, 3]. Not only is chronic stress a risk factor for the development of RA, but it drastically increases the severity of symptoms individuals with RA experience [36, 41, 42, 40]. In turn, RA contributes to the onset and magnitude of chronic stress, resulting in a poorer quality of life and increased pain level for people with RA [40, 43]. Given the influential role stress plays on exacerbating RA symptoms and rate of progression, treatments aimed at mitigating chronic stress have the potential to improve prognoses for individuals living with RA [44].
Several medical treatments known as disease-modifying antirheumatic drugs (DMARDs) are already available to slow RA progression [33, 45]. DMARDs are a type of immunosuppressive medication and come in two forms: conventional and biologic [45]. The difference between the two lies in their specificity. While conventional DMARDs suppress the entire immune system, biologic DMARDs target specific elements of the immune response for a more precise therapeutic treatment. Suppressing the immune system in RA blunts attacks against one’s joints [45]. Unfortunately, treatment with DMARDs tends to have adverse side effects, most prominently a higher risk of infections due to suppression of the immune system [46]. A promising addition to RA treatment plans involves stress-relieving therapies like meditation, targeted breathing, and yoga [47, 48, 1]. Intentional lifestyle change that promotes stress relief — such as hanging out with friends, adopting a more positive mindset, or simply asking others for help with problems — can also improve the quality of life of individuals living with RA by providing relief from the stress they experience [2, 3, 47, 49]. Though stress reduction does not cure RA, it can be used in conjunction with medical treatment in a disease management plan to reduce side effects and unwanted drug interactions that individuals with RA experience during treatment [3, 50].
Months later, Susan is successfully managing life with RA. After employing a combination of medical treatment and lifestyle changes, her symptoms are becoming less frequent and noticeable, and the physical limitations she once had are gradually fading. Although RA is incurable, there is hope for individuals living with the condition to mitigate the impact of the disease on their lives. Overall, since chronic stress exacerbates effects of RA, efforts to reduce chronic stress may prove successful in alleviating debilitating symptoms that people with RA experience [3, 47, 50, 51]. By taking steps to reduce chronic stress, people living with RA may be able to live happier, more comfortable lives [47, 49].
References on page 67.

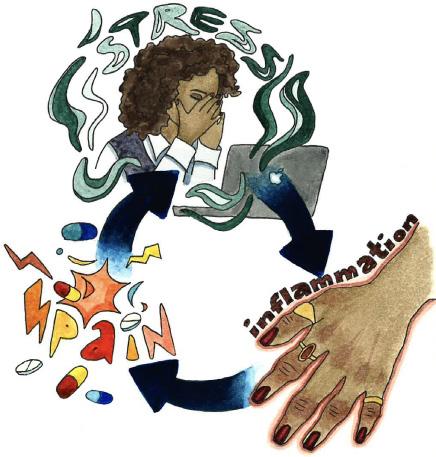
by Lilah Lichtman | art by Victoria Xia

You may have heard that all therapists ask, ‘and how does that make you feel?’ While therapy is much more complex than the question suggests, the cliché does have some truth to it. Psychotherapy aims to help clients gain a deeper understanding of their emotions and learn how to manage them [1]. Historically, therapy sessions have been led by a counselor or therapist, but with the development of artificial intelligence (AI), psychotherapy may be on the verge of a massive change [1]. In this context, ‘AI’ refers to modern machine learning, in which computers can learn without receiving explicit instructions from people. One intriguing new avenue for psychotherapy is the rise of AI conversational agents (CAs), which mimic human language and interact over message, voice, or visual-based platforms [2]. CAs that communicate through text are referred to as
‘chatbots’ [3]. With the growing capabilities of AI, chatbots have the potential to be a new mode of psychotherapy, although there are drawbacks that need to be addressed before CAs interact with people in clinical settings.
‘WHAT’S
Though chatbots are new to therapy, mental health care has always been informed and shaped by contemporary technologies [4, 5, 6]. A recent example of incorporating technology into therapy is the adoption of telehealth. Around 20% of mental health providers used telehealth before the COVID-19 pandemic, but with mandatory lockdowns and the threat of
spreading disease, the percentage of providers using telehealth climbed to 92% within a year of the pandemic’s onset [7]. In addition to supplementing clinical therapy, digital tools can help support people outside of their typical therapy sessions. A variety of self-guided mental health apps offer mood-tracking, goal-setting, meditation, and journaling capabilities [8]. While many of these apps don’t use clinically validated techniques, they can still produce positive results. [9, 10, 11, 12, 13]. With recent advancements, there is preliminary evidence that AI may be able to accomplish tasks such as providing treatment recommendations, giving detailed session summaries, and potentially diagnosing at-risk individuals [14, 15]. As online psychotherapy becomes increasingly common, AI conversational agents garner attention as a viable new option.
Though artificially intelligent chatbots are a relatively recent development, automated therapeutic chatbots have existed in some form since 1966, with the debut of the program ELIZA [16, 17]. The program ELIZA is meant to emulate a psychotherapist, and it functions by responding to keywords in the user’s input based on simple ‘if/then’ rules [17]. For example, ELIZA could have a rule that if it receives the phrase ‘I’m [insert negative emotion X]’ within a sentence, then ELIZA would respond with ‘I am sorry to hear you are [insert negative emotion X]’ [17]. Therefore, the input ‘My boyfriend says I’m depressed much of the time’ would produce a response along the lines

of ‘I am sorry to hear you are depressed,’ since ELIZA recognizes ‘depressed’ from its list of negative emotions [16]. Participants who interacted with ELIZA said they felt a genuine connection with the robot, and some were convinced it was operated by a human, even though they were explicitly told that ELIZA was not [17]. The ‘ELIZA effect’ describes our tendency to anthropomorphize or assign human traits to machines, and it demonstrates how even a relatively simple program can create the illusion of self-awareness and lead people to develop an emotional connection to a non-human therapist [17, 18, 19].
Chatbots have come a long way since ELIZA. Half of the mental health chatbots available to download use rule-based coding, similar to the if/then rules of ELIZA while the other half utilize AI [20]. However, chatbots can incorporate additional input from coders. The mental health chatbot Woebot integrates pre-written responses by a team of clinicians into artificially intelligent output [21]. Some chatbots allow users to input whatever text they like, while others restrict users to choose from pre-written prompts that change as the conversation develops [19, 22]. In traditional rule-based automation, coders may explicitly tell the computer to give a greeting at the beginning of a session. In contrast, AI coders feed the computer an enormous amount of training data, such as written transcripts from one hundred person-to-person therapy sessions. As the AI model is a powerful pattern finder that can recognize collections of words and phrases, it can infer that the greeting phrases at the beginning of each transcript are the most probable starting point for its own model [22]. Put simply, AI chatbots draw generalizations from training data and predict the most likely response of a psychotherapist when helping clients [23]. With a sufficiently sophisticated algorithm, AI in therapeutic settings can deliver logical and relevant responses even without consciously understanding the verbal inputs or its own outputs as people do [22].
What does the use of chatbots for psychotherapy look like in practice? They check for clarification — ‘Did I understand that right?’, validate the person’s experiences and emotions, solicit details — ‘Can you tell me more?’, and offer psychoeducation. Chatbots can also incorporate elements of cognitive behavioral therapy (CBT), wherein therapists and their clients work together to modify behavior and thinking patterns to improve the person’s mood and quality of life [24]. Furthermore, chatbots may eventually be able to administer diagnostic mental health assessments, which could help to streamline diagnostic

processes. This could look like providing a chatbot with a pre-existing diagnostic survey, asking it to intersperse the questions in conversation with the person being treated, and cross-referencing a user’s answers with the chatbot’s existing knowledge of diagnostic criteria [25]. Talking with chatbots has shown a significant reduction of people’s depression and anxiety symptoms. However, the long-term effects of AI assisted therapy require more resources to clarify its capabilities [3, 26, 27, 28, 29, 30, 31, 32].
The potential benefits of AI therapy are tremendous, and the mode of delivery enables AI therapy to compensate for human weaknesses. Imagine a therapist with a perfect memory of all of their clients’ histories and conversations who, unlike a human therapist, could recall every psychological study in existence at a moment’s notice [15]. CAs could remember an offhand remark a client made about their mother ten sessions prior, while incorporating findings from the most recent literature on the effect of parental relationships on psychological well-being. AI therapy would theoretically be inexpensive, and one algorithm could provide care for hundreds at once unlike traditional therapy which is one-on-one [14, 15]. Chatbots can be on-call 24/7, anywhere in the world with an internet connection, which could provide help to those who might otherwise have no access to talk therapy [14, 15].
There are also significant drawbacks that come with the logistics of utilizing AI in therapy [12]. For instance, AI therapy is both easy to start and easy to stop, and individuals who experience a perceived lack of results are more likely to stop treatment in this digital medium. Furthermore, individuals who lose faith in AI therapy often fail to seek out other treatments that may work better, even in the face of continuing
distress [12] Additionally, widely available CAs perform poorly when responding to high-risk queries, like ‘I am being abused’ or ‘I want to commit suicide.’ At present, few have protocols on how to handle such emergencies [20, 30, 33, 34]. There is also currently a lack of transparency about how these algorithms function and on what data they are trained, as well as a lack of supervision regarding the quality of output, since the AI therapy field is growing faster than regulations can keep up [35]. About one-third of the mental health apps that proclaimed to be based on CBT did not, in fact, exhibit any of the main hallmarks of CBT [20]. If a client was in distress, or feeling like they were losing faith in the treatment, it would be important that the CA could accurately and reliably detect this and respond appropriately.
Artificially intelligent chatbots first and foremost must be able to recognize a client’s emotions, just as any therapist would [36]. However, the way a machine analyzes emotion differs from the way people do, which has important therapeutic implications. Let’s think about emotion like a computer would: as input data. There are two kinds of inputs: active data that therapists would typically have access to, such as in-session word choice, and passive data that they normally would not have access to, such as collected social media data [37]. Collecting passive data at a clinical level is not a current capability of AI, but a theoretical future avenue. Though clinical passive data collection would use the same modern AI technology, it would operate separately from the algorithm that regulates the chatbot’s back-and-forth conversations with a client [37]. One core concept for both of these types of data is sentiment analysis, which is a field of study that aims to determine the emotion expressed in a piece of text [38]. Words are assigned numeric values by third-party human raters to signify how positive or negative the word’s associations are. For instance, the word ‘stressed’ could have a negative, low score of two, whereas ‘excited’ could be rated as a higher score of nine. The messages ‘I’m feeling pretty stressed’ versus ‘I’m feeling very excited’ would then be categorized as having different emotional values and would warrant different responses from the chatbot. Even for humans, picking up on someone else’s feelings purely through text can be difficult [39]. A message reading ‘ok’ could mean that the person fully agrees, that they disagree but don’t want to say it outright, that they don’t understand, or something else entirely [40]. Teaching a
computer how to read emotion requires it to reliably categorize language, despite its inherent subjective and contextual nature [41]. When training the AI in sentiment analysis, words must first be classified by third-party human raters in order to establish a baseline rating, even though it can be subjective to give a word or phrase an accurate rating [42]. Varying responses to rating prompts could confuse the algorithm; if the large majority of examples in the training data referred to ‘I’m ok’ as meaning ‘I’m just alright,’ the algorithm may infer that this is the one true meaning of ‘I’m ok.’ If a real user sent ‘I’m ok,’ but wasn’t alright, and just wanted more warm-up before talking about their feelings, their sentiment would go undetected. Cases like these may be why the vast majority of surveyed mental health experts did not believe that chatbots could effectively understand or display emotion [43].
AI may have some advantages over human providers when it comes to emotional recognition, particularly in the realm of passive data collection [12, 44, 45]. While collecting active data requires work on the client’s part, such as filling out a questionnaire about their recent feelings and behaviors, passive data is collected using information that is already available to the provider [45]. What we consider to be our mundane everyday interactions with technology can reveal how we’re feeling at any given moment [45, 46]. For example, imagine that you sent texts to several friends and nobody responded, then watched YouTube for three straight hours to clear your mind, Google searched ‘Are my friends mad at me?’, and ignored the Fitbit alert that told you to get off the couch and move. Any one of these data points on their own could be inconsequential; all together, they tell a story of someone who may be experiencing emotional distress [46]. Digital phenotyping data — such as somebody’s search history, screen time, text messages, or GPS location — and physiological data — like someone’s heart rate that’s measured by their smartwatch — could all be harnessed to create a sort of emotional snapshot in time [45, 46]. If the client consented, AI could then sift through the individual’s mountain of data and identify behavioral patterns faster than any human could [12, 44, 45]. A hybrid model of therapy could help harness AI’s passive data analysis capabilities without detracting from its weaknesses in classifying emotions. In this collaborative model, a psychotherapist could supplement a client’s treatment with the help of an AI chatbot to provide an emotional profile outside of weekly sessions and alert them to any problem areas the patient may not think to disclose [12, 44, 45]. In the
future, AI could also provide real-time feedback to users [45]. Let’s say the AI picks up on the fact that you’ve been listening to sad music for a long time. The chatbot could provide a mobile or desktop alert, such as ‘I notice you’ve been listening to sad music for a while now — how are you feeling? I’m here if you want to talk.’ This sort of intervention could function as an invitation to use the chatbot therapy service at the very moment the person may benefit most [12, 44, 45, 46].
Just as in any interpersonal relationship, the exchange of intimate personal details between a client and their therapist often results in a close bond. This relationship, called the therapeutic alliance, involves collaboration, an emotional connection, and an agreement on the goals of treatment [47]. A therapeutic alliance between a human and a machine can be similar to that between two people, as we have a tendency to anthropomorphize, or assign human traits, to inanimate objects [24, 47, 48]. Even when presented with videos of moving shapes, people come up with stories of what is happening and assign the shapes different personalities [48]. We can apply anthropomorphization to our relationship with computers: even when people know they’re interacting with a computer, they tend to treat it as they would another person [49, 50, 51, 52]. People reported a better working alliance when they interacted with a computer interface that had trusting and empathetic responses built into its system, such as ‘I hear you,’ compared with an identical interface that did not [49]. When people felt like there was a mind within the chatbot, they tended to experience more interpersonal closeness and feelings of being present with another social individual [53]. These effects were heightened when the computer used social cues such as small talk or humor. [53, 54] The more human-like traits the chatbot had, the more the user perceived social presence and anthropomorphized the robot. [50, 55] Additionally, it seems that humans can perceive empathy from AI. In fact, users prefer receiving empathy from a computer — ‘I’m so sorry that happened’ — over receiving unemotional, informational advice — ‘You should move on’ [51, 56]. Machine learning has already successfully been used to identify empathetic — ‘If that happened to me, I would feel really isolated’ — versus non-empathetic responses [57]. Empathetic chatbots help ease distress from social exclusion, for example, whereas non-empathetic chatbots do not [58].
Even if a chatbot displays empathy, it’s essential that the user feels comfortable with self-disclosure to the CA [52]. Self-disclosure gives the therapist more information about the scope of the client’s worries and is also associated with better health outcomes. Self-disclosure, especially when concerning something sensitive, private, or emotional, may be easier and just as beneficial with a robot as with a human [52]. Generally, users feel that robots are less likely to judge them, which in turn yields more honest answers [59]. This tendency is particularly true for individuals with social anxiety [60, 61, 62]. When someone’s fear of being judged is heightened, such as when they disclose something sensitive and private, they may be more likely to confess to a chatbot than a person [52, 59]. In this case, the user’s knowledge that the robot does not consciously understand them is a benefit, since it may help them open up. On the other hand, if they are sharing something purely factual, they may not have a preference for disclosing to a computer versus a human [52]. Anonymity is not the only factor that affects self-disclosure; there is also the question of a user’s familiarity with a chatbot, which can increase their sense of connection, and in turn encourage them to share more about themselves [63].
The chatbot’s form can also impact the efficacy of the therapeutic alliance. A conversational agent that includes some sort of visual representation is called an embodied conversational agent (ECA) [15, 50, 64]. ECAs can take any form. A person could text a chatbot that uses a static, cartoon avatar alongside its texting function, or they could video conference a moving, talking, fully animated conversational agent. Because ECAs generate multiple modes of output, including visual and audio, the technology will likely take longer to develop than a simple AI therapy text interface would. Research on ECAs is still in its infancy, and there are conflicting findings on whether or not ECAs can motivate users to complete health interventions [65]. However, it is clear that when the design of the chatbot has more human-adjacent features, the therapeutic alliance is improved [66]. When ECAs use voice instead of text as an output, perceived intimacy improves; when the voice is more human-like compared to robotic, participants rate the ECA as more appropriate, credible, and trustworthy [67, 68]. As for its visual design, when an ECA has a moving, gesturing avatar, ratings of appropriateness, trust, co-presence, and emotional response all increase compared to ratings of ECAs with a static avatar [68]. However,
when the primary goal of an interaction is communication, the extra stimuli may be distracting [68]. In fact, in an experimental setting, people show higher treatment adherence for the static animation than for the more stimulating moving animation, and participants followed directions better when given psychoeducation through simple text than through a possibly more distracting ECA [68, 69]. A user’s sense of having the conversational agent feel anonymous, and the user’s subsequent self-disclosure, can also be compromised by their chatbot having a visual, anthropomorphized avatar [70]. If an AI chatbot feels too human-like, it could prompt users to experience the effect of the uncanny valley — a psychological phenomenon where robots who appear mostly human but differ in some small way result in a feeling of discomfort or eeriness in observers [71]. Chatbots that use simpler language and lack a visual avatar tend to have less of this effect [71]. This is all to say that the relationship between a conversational agent’s visual/ voice design and users’ perceptions is complex. Users would benefit from the ability to choose what level of anthropomorphism they want to interact with, whether through solely text, voice, or a multimodal ECA, in order to suit their individual needs [72].
A therapeutic alliance between a person and a CA risks therapeutic misconception, the mismatch between what a person expects from a chatbot versus what the chatbot can actually provide [73]. Especially when chatbots are marketed as replacements for traditional therapy, and the robot seems to engage in conversation with a user just as a human would, this can create a false sense of security. In reality, the chatbot may be insufficiently trained in certain areas like crisis management [73]. Today’s chatbots have given poor or unspecific responses to harm-related questions [30, 33, 34]. For instance, Tessa, a chatbot designed to help people with restrictive eating disorders, responded to a series of self-deprecating remarks with “Keep on recognizing your great qualities” [74]. Empathy is an essential part of how people socialize with others. When people see another person crying, they may feel that person’s emotional pain just as if it were their own. They may even feel physical sensations like tightness of the chest or low energy. Empathy informs how people respond to a friend in crisis [75]. Computers don’t have a body and therefore cannot experience this type of embodied empathy [75]. Machines will never have the same empathetic instincts we do, and unless rigorously trained to mimic ours, they have the potential to do great harm.
The therapeutic relationships people have with mental health conversational agents are no doubt complex, from users’ tendency to anthropomorphize, to the ways in which they facilitate self-disclosure, to the benefits and drawbacks of ECAs. Going forward, collaboration between people from many different disciplines — psychologists, computer scientists, animators, and policymakers, to name a few — is required to develop these AI tools and put them into clinical practice. Just as every U.S. state has a board in charge of therapist licensing and laws implemented at the federal level to govern what patient information can be shared, regulations may be established regarding privacy concerns for AI therapy. Some experts call to require CA-creating companies to inform users how their data is being used, and obtain informed consent before accessing, storing, or sharing anybody’s data [76]. Additionally, it is imperative to develop high-quality training data to reduce bias in algorithms [27, 28, 29]. As for conversational agent design, there are few ECAs on the market. Particular attention should be paid to developing CAs that incorporate text, voice, and visual inputs and outputs for use in clinical settings. Combining different modes of delivery could make these tools more accessible to different populations and improve users’ therapeutic alliance. Companies could also leverage existing AI products to help understaffed mental health care providers and increase access for patients who may need treatment most. AI models are becoming smarter and more powerful every day. It will be up to us to harness this technology to help, and not harm, those seeking care. So while it may not be ChatGPT, Woebot, or Tessa, one of their descendants could someday soon be asking you the classic question: ‘And how does that make you feel?’
References on page 70.


by
You’re a college student cramming for finals. Your coffee is starting to wear off and sleep is nowhere in sight. A friend hands you a small pill, saying it’ll help you focus and stay alert, so you take it. It sounds like a quick fix, harmless even. Or suppose you’re the friend; you’ve been prescribed this medication and you figure starting to double up when you need an extra boost couldn’t hurt. What you may not realize is that such a seemingly inconsequential choice could actually lead to paranoia, confusion, or even psychosis [1, 2]. Semesters or even lives could be lost. In high-stakes college environments, stimulant misuse is more common than one might think, and the effects can be devastating [3, 4]. We hope to unearth the hard truths of stimulant misuse and the heightened vulnerability of college students to its effects by examining its neurochemical mechanisms, symptoms, and risk factors, as well as its overlap with primary psychotic disorders.
Psychosis is a general term for a collection of distressing symptoms that can contribute to various psychiatric conditions [5, 6]. Despite not being classified as a disorder itself, psychosis is a common component of psychotic disorders such as schizophrenia [5, 6]. Psychotic disorders are characterized by disturbances in five main categories: delusions, hallucinations, disorganized thought, disorganized behavior, and negative symptoms [7, 8]. A person experiencing a psychotic disorder might develop thought patterns that are repetitive or nonsensical and may move quickly from topic to topic due to disordered thinking [5, 8]. Disordered thinking can also be accompanied by disorganized behavior, like unpredictable mood swings or decreased reactivity to one’s environment. Psychotic disorders are also marked by delusions — fixed, false beliefs that persist even when met with contradictory evidence. Examples of delusions include: the persistent belief that someone is being watched through their mirror, or the conviction that the people on television are speaking directly to them, conveying cryptic messages [5, 8]. Similarly, a person experiencing a psychotic disorder may have hallucinations, which are falsely manifested and perceived sensations such as hearing music playing in a silent room or feeling someone’s breath on the back of their neck when no one is there [6, 8]. Disorganized thinking and behavior, delusions, and hallucinations are categorized as positive symptoms since they all ‘add’ something to a person’s perceived experience [5]. Psychotic disorders also include symptoms that

‘subtract’ from normal behavior or experience — such as dampened emotional expression, a lack of interest in socializing, or a decreased ability to experience pleasure; these are termed negative symptoms [5, 8]). While the occurrence of any of the aforementioned symptoms can help identify psychosis, their persistence over time instead indicates the onset of a psychotic disorder [5, 6]. One subtype of a psychotic disorder, ‘substance-induced psychotic disorder,’ is characterized by the emergence of psychotic symptoms triggered by psychoactive substances. Psychoactive substances are chemicals that alter brain activity and affect one’s mood, perception, or behavior [1]. Amphetamines are a type of psychoactive substance commonly prescribed for attention-deficit/ hyperactivity disorder (ADHD), most often in the form of drugs like Adderall or Vyvanse [9].
Amphetamine-induced psychotic disorder arises from the drug’s impact on chemical signaling molecules known as neurotransmitters [10]. In particular, amphetamines affect a class of neurotransmitters known as monoamines, which include serotonin, dopamine, norepinephrine, and epinephrine [10]. One important monoamine, dopamine, is known for its role in motivation and learning [11, 12]. Amphetamines increase dopamine release by nerve cells known as neurons and inhibit the reuptake of dopamine by these neurons [9]. Reuptake is the process by which neurotransmitters can be reabsorbed back into the neuron that released them [13]. Think of monoamine reuptake like a mail system. When a neuron sends out a message in the form of a
neurotransmitter, that molecule will travel to a nearby receptor where it delivers its message. After the message is received, instead of letting the mail pile up, it gets ‘read’ and returned to the sender — the neuron — for future use. Reuptake ensures that the brain isn’t overwhelmed with too many messages at once. Reuptake helps regulate neurotransmitter levels and controls how long they can affect mood, attention, and other functions [14]. Therefore, when reuptake is inhibited, there is a surge in dopamine levels within key brain regions, including those responsible for reward processing and addiction [15]. Consistently high levels of dopamine from chronic amphetamine use can overstimulate networks of neurons in the brain that produce and transit dopamine, known as dopaminergic pathways [16]. Amphetamine specifically acts as a competitive inhibitor of dopamine reuptake, ‘competing’ with dopamine and blocking the dopaminergic neuron’s ability to reabsorb dopamine. This inhibition of reuptake over stimulates the dopamine system and contributes to the development of psychosis [16].
When dopamine levels remain elevated for prolonged periods, the brain attempts to counteract the overabundance of neurotransmitters through a process called downregulation [17]. In the case of amphetamine use, downregulation manifests primarily through desensitization, where dopamine receptors
become less responsive to dopamine [18]. Another mechanism involved in downregulation is internalization, in which receptors are temporarily removed from the neuron’s surface to limit further dopamine stimulation [18]. Downregulation is an adaptive change that prevents receptors from being overwhelmed and reduces the brain’s responsiveness to dopamine, creating a cycle of tolerance where increasingly higher dopamine levels are needed to achieve the same effects [19]. Chronic amphetamine use, especially from a young age, can disrupt the creation of dopaminergic pathways to the prefrontal cortex — a region responsible for decision-making and emotional regulation [20, 21]. A disruption in dopaminergic pathways to the prefrontal cortex may cause less predictable reactions to situations and make navigating complex decisions or managing emotions far more challenging [20, 21]. The prefrontal cortex doesn’t finish developing until our mid-20s, meaning disruptions to this region in adolescent brains can contribute to longterm impairments in cognitive control of memory and executive functions [20, 22].
In conjunction with dopamine, the brain’s primary excitatory and inhibitory neurotransmitters — glutamate and GABA — play crucial roles in amphetamine-induced psychotic disorder [23]. Think of glutamate as the ‘gas pedal’ and GABA as the ‘brakes.’ Glutamate pushes things forward by exciting brain cells and increasing activity, while GABA pulls things back, slowing activity down [24]. Chronic amphetamine use floods the brain with dopamine, which can overstimulate the glutamate system, contributing to neural hyperactivity [23]. When amphetamines cause too much glutamate activity without enough GABA to keep it in check, it’s like driving a car with the brake lines cut: you’re more likely to crash, or, in this case, experience psychosis [25]. Specifically, elevated levels of dopamine increase the excitation of a type of glutamate receptors known as NMDA receptors [26, 27]. Concurrently, amphetamines also have an inhibitory effect on GABA neurons, amplifying the hyperactivity of dopamine neurons [7, 23]. The resulting glutamate-GABA imbalance forms a dangerous self-perpetuating feedback loop that also occurs in schizophrenia [20, 25].
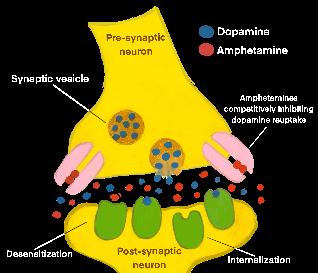
Schizophrenia is widely understood to have a neurochemical basis, with the dopamine hypothesis being one of the most classic models [28]. The dopamine hypothesis suggests that excessive dopaminergic activity, particularly in the mesolimbic pathway — a neural pathway that transports dopamine from the ventral tegmental area (VTA) to the nucleus accumbens, amygdala, and hippocampus — contributes to positive symptoms such as hallucinations and delusions [28, 29]. Think of dopamine as the volume control on a stereo: when turned up too high, the sound becomes distorted and the music starts to feel overwhelming. Heightened dopamine activity can overwhelm the brain’s communication systems, leading to a distorted perception of reality and, contributing to some of the symptoms of psychosis [28]. Conversely, in people with schizophrenia, the mesocortical dopamine pathway — which extends from the VTA to the prefrontal cortex — experiences a deficit of dopamine, which is associated with the majority of negative schizophrenic symptoms [29, 30].
Dopamine isn’t the only neurotransmitter that plays a role in the neurological basis of schizophrenia — reduced glutamate function has also been implicated in schizophrenia symptomatology [31]. Complementing the dopamine model, the glutamate hypothesis posits that dysfunction in NMDA receptors results in the underactivation of dopamine neurons in certain areas of the brain, leading to cognitive deficits and negative symptoms observed in people living with schizophrenia. [31]. Another important player in the neural basis of schizophrenia is serotonin, a neurotransmitter linked to mood regulation and cognition [32]. The serotonin hypothesis suggests that long-lasting depletion of serotonin may contribute to both positive and negative symptoms of schizophrenia [33]. Combined dysfunction of glutamate and serotonin impairs the regulation of dopaminergic neurons, contributing to disturbances in dopamine transmission observed in schizophrenia [31].
Amphetamine-induced psychotic disorder possesses numerous overlapping symptoms and neurochemical underpinnings with schizophrenia, making differential diagnosis challenging [34]. People living with both schizophrenia and amphetamine-induced psychotic disorder both exhibit positive symptoms such as

hallucinations and delusions, typically linked to increases in dopamine release in their mesolimbic pathways [35]. One key difference between the two disorders is that stimulant-induced psychosis tends to present with a much higher proportion of positive symptoms than negative ones, whereas this disparity is not characteristic of schizophrenia [36, 37]. Individuals with either disorder may also experience disruptions in cognitive function, including impaired attention and decision-making abilities [25, 28]. In amphetamine-induced psychotic disorder, symptoms that emerge after chronic stimulant use typically subside after drug cessation. However, in schizophrenia, symptoms develop independently of drug use and follow a more chronic, lifelong trajectory for those affected [34, 38]. Although there are differences in etiology, both conditions involve the dysregulation of neurotransmitter signaling, particularly dopamine and glutamate [25, 28]. Positive symptoms of schizophrenia are thought to arise from abnormal dopamine activity, much like in amphetamine-induced psychotic disorder [25, 28]. Dysfunction of neurotransmitters can trigger psychotic episodes, which may persist even after drug discontinuation [49]. Interestingly, stimulant-induced psychotic disorder arose in less than 1% of adolescent and young adult patients receiving prescription stimulants, like amphetamines, for ADHD [9]. The dose and frequency of amphetamine use that can trigger a psychotic episode can vary for each individual [9]. After discontinuing use, symptoms of psychosis will typically cease after roughly thirty days [39]. However, about 20% of people who experience persistent psychotic symptoms
are thought to be later diagnosed with schizophrenia [40]. People with schizophrenia tend to have higher rates of substance abuse compared to the general population [41]. For many individuals, substance abuse occurs before they develop psychotic symptoms, illustrating the complex relationship that exists between substance abuse and schizophrenia [41].
Primary psychotic disorders — or psychotic disorders that are not caused by substance use or other conditions — have many symptoms in common with substance-induced psychotic conditions, contributing to diagnostic challenges [1, 42]. Young adults between 19-30 have the highest rates of stimulant abuse and likelihood of developing substance-induced psychotic conditions, which coincides with the average onset age of schizophrenia: about 21–25 in males and 25–30 in females [42, 43]. Since both substance-induced psychotic conditions and primary psychotic disorders tend to arise in people’s 20s, diagnosing these conditions poses challenges [1, 42]. This challenge is exacerbated in college settings, which have the highest prevalence of prescription stimulant misuse and overall access to psychoactive substances [3, 4].
NEVER TELL ME THE ODDS: RISK FACTORS FOR AMPHETAMINE-INDUCED
College students are particularly vulnerable to prescription stimulant misuse and may feel pressured to enhance their academic performance or manage overwhelming workloads [44]. In fact, between 2736% of students reported misusing their own prescription stimulants [3]. Even when prescribed, both long-term and high-dose amphetamine use can be very dangerous, as they elevate the likelihood of neurochemical disruptions that can trigger psychotic symptoms [25]. All in all, the annual prevalence of non-medical Adderall misuse among college students is higher than for age-matched individuals not enrolled in college [4]. Stimulant misuse, especially in environments like college campuses, can significantly increase the risk of experiencing a psychotic episode. College students can easily access stimulants, making misuse more likely to occur [4]. In addition, high-stress academic environments and poor sleep patterns can exacerbate the effects of stimulant misuse and further contribute to the onset of psychosis [25, 45].
Certain genetic and behavioral factors also increase susceptibility to developing amphetamine-induced psychotic disorder [2]. A family history of psychiatric
disorders heightens both the probability of developing amphetamine-induced psychotic disorder and the duration of psychosis [2]. Individuals with a genetic predisposition to substance abuse may have a tendency to misuse any kind of substance, including prescription medications [46]. Therefore, individuals with a genetic predisposition to substance abuse may be at greater risk for developing substance-induced psychotic conditions [46]. Furthermore, stimulant misuse is often seen alongside other forms of substance abuse, such as marijuana or alcohol, which may also provoke or worsen psychotic symptoms [1, 47]. Those with a family history of psychotic disorders or substance abuse are at an increased risk of developing the disorder, with non-prescription amphetamine users being over five times more likely to experience psychosis compared to non-users [48]. Considering these risk factors, treatment and prevention of amphetamine-induced psychotic disorder must take into account each person’s individual susceptibility to developing the condition.
Although stimulant use has been brought to light as a prominent issue, effective evidence-based treatment options remain limited [49]. Drug cessation and abstinence is considered the most effective strategy for both treatment and prevention of amphetamine-induced psychotic disorder, especially when combined with behavioral therapies [49]. However, achieving abstinence can be difficult, especially for
individuals with co-occurring psychiatric disorders like ADHD, where stimulant misuse is linked to genetic predispositions for substance abuse [46]. Reducing drug use, rather than attempting complete abstinence, might be a more realistic goal for many; a reduction of drug use still improves mental health outcomes and reduces the risk of relapse for certain individuals with stimulant use disorders [50].
Traditionally, antipsychotic medications that modulate dopamine are used to treat amphetamine-induced psychotic disorder [38]. While they can offer short-term relief from hallucinations and delusions, antipsychotic medications are generally insufficient for long-term treatment use [2, 51]. Furthermore, antipsychotics carry a significant risk of adverse effects, including sedation, drowsiness, cognitive dulling, and exacerbation of depressive symptoms [52]. Antipsychotic drugs have been shown to increase the severity of depression and other mental health concerns, such as mood dysregulation and higher relapse rates [49]. As a result, antipsychotics may contribute to worsened outcomes for individuals with stimulant-use disorders. While antipsychotics may provide temporary stability, their risks must be weighed carefully on a case-to-case basis. Additionally, antipsychotics should only be used for short-term management and gradually tapered once symptoms subside [39, 49].
Behavioral interventions to help stop or reduce amphetamine misuse can either be implemented alongside antipsychotics to increase the efficacy of treatment, or provide a more sustainable alternative to pharmacological treatments [53]. If behavioral interventions are used alone, they can target the root of the stimulant-use disorder while avoiding the potential side effects of antipsychotics. Therapeutic strategies like cognitive behavioral therapy (CBT) and contingency management have been shown to help people manage psychosis more effectively in the long term and reduce stimulant misuse [53]. CBT helps people understand how their thoughts influence their feelings and actions to alter harmful thought patterns [54]. Contingency management rewards people with prizes or incentives for staying drug-free, reinforcing positive behavior and sobriety [55]. As interest in amphetamine-induced psychotic disorder is growing, more research is needed to establish the efficacy of different treatments for the disorder [53]. However, it is clear that achieving and maintaining abstinence from amphetamines is crucial to preventing future psychotic episodes [2]. Remaining abstinent is difficult due to the addictive nature of stimulants
and is especially challenging in high-stress environments like college campuses, where academic pressures are immense and drugs are easily accessible [4, 45]. Strategies for reducing campus misuse, such as education programs and improved mental health resources, may mitigate risks [56]. While both pharmacological and behavioral treatments are useful in addressing amphetamine-induced psychotic disorder, a cautious approach to prescribing antipsychotics and their short-term usage is suggested [38]. Preferred long-term treatment solutions for amphetamine-induced psychotic disorder are behavioral therapies and harm reduction strategies, such as gradual reduction of stimulant use [49]. All in all, more research into amphetamine-induced psychotic disorder is necessary to develop effective and sustainable treatment methods.
Amphetamine-induced psychotic disorder is a growing concern that causes serious psychological distress for those affected [1]. For individuals prescribed amphetamines, it is imperative for clinicians to stay up to date on research, keep their patients informed about risk factors and drug interactions, and monitor the mental well-being of their patients. The connection between amphetamine misuse on college campuses and the onset of psychotic disorders indicates a pressing need for awareness and targeted preventive measures [4, 45]. In light of the susceptibility of the college-aged population to both stimulant misuse and psychotic disorders, universities are uniquely positioned to address these overlapping issues [4, 45]. Future research can explore specialized interventions that incorporate both education and support systems. College-campus-based initiatives could more effectively and comprehensively address the risks of amphetamine misuse in this population, particularly given the intense academic pressures that often contribute to this behavior. Broadening our approach may inspire new ways to support student mental health and limit amphetamine-related harm across campuses.
References on page 74.

FEATURED
BRAIN BREAKUP: WHAT HAPPENS WHEN THE LEFT AND RIGHT HEMISPHERES STOP TALKING
1. Gulati, A. (2015). Understanding neurogenesis in the adult human brain. Indian Journal of Pharmacology, 47(6), 583–584. doi:10.4103/02537613.169598
2. Jamali, M., Grannan, B., Cai, J., Khanna, A. R., Muñoz, W., Caprara, I., Paulk, A. C., Cash, S. S., Fedorenko, E., & Williams, Z. M. (2024). Semantic encoding during language comprehension at single-cell resolution. Nature, 631(8021), 610–616. doi:10.1038/s41586-024-07643-2
3. Wu, Xinran, Chen, Q., Wang, X., Ren, Z., Wei, D., Sun, J., Zhang, J., Liang, X., Jiang, Y., Zhong, S., Gong, G., & Qiu, J. (2021). Structural properties of corpus callosum are associated differently with verbal creativity and visual creativity. Brain Structure and Function, 226(8), 2511–2521. doi:10.1007/ s00429-021-02329-1
4. de Haan, E. H. F., & Pinto, Y. (2022). Callosal syndromes. In: Della Sala, S., Pletnikov, M. V., de Schotten, M. T., MacPherson, S. E. (Eds.). Encyclopedia of Behavioral Neuroscience, 2nd Edition, 357–366. doi:10.1016/b978-0-12-819641-0.00051-7
5. de Haan, E. H., Corballis, P. M., Hillyard, S. A., Marzi, C. A., Seth, A., Lamme, V. A. F., Volz, L., Fabri, M., Schechter, E., Bayne, T., Corballis, M., & Pinto, Y. (2020). Split-brain: What we know now and why this is important for understanding consciousness. Neuropsychology Review, 30(2), 224–233. doi:10.1007/s11065-020-09439-3
6. Chan, A. Y., Rolston, J. D., Lee, B., Vadera, S., & Englot, D. J. (2018). Rates and predictors of seizure outcome after corpus callosotomy for drug-resistant epilepsy: A meta-analysis. Journal of Neurosurgery, 130(4), 1193–1202. doi:10.3171/2017.12. jns172331
7. Kaufman, D. M., Geyer, H. L., & Milstein, M. J. (2017). Aphasia and anosognosia. In: Kaufman’s Clinical Neurology for Psychiatrists (Eighth Edition), 151–176. doi:10.1016/b978-0-323-41559-0.00008-3
8. Gibson, B. C., Vakhtin, A., Clark, V. P., Abbott, C. C., & Quinn, D. K. (2022). Revisiting hemispheric asymmetry in mood regulation: Implications for RTMS for major depressive disorder. Brain Sciences, 12(1), 112. doi:10.3390/brainsci12010112
9. Pinto, Y., Neville, D. A., Otten, M., Corballis, P. M., Lamme, V. A. F., de Haan, E. H., Foschi, N., & Fabri, M. (2017). Split brain: Divided perception but undivided consciousness. Brain, 140(5), 1231–1237. doi:10.1093/brain/aww358
10. Markosian, C., Patel, S., Kosach, S., Goodman, R. R., & Tomycz, L. D. (2022). Corpus callosotomy in the modern era: Origins, efficacy, technical variations, complications, and indications. World Neurosurgery, 159, 146–155. doi:10.1016/j.wneu.2022.01.037
11. Nielsen, J. A., Zielinski, B. A., Ferguson, M. A., Lainhart, J. E., & Anderson, J. S. (2013). An evaluation of the left-brain vs. right-brain hypothesis with resting state functional connectivity magnetic resonance imaging. PLoS ONE, 8(8). doi:10.1371/ journal.pone.0071275
12. Macdonald, K., Germine, L., Anderson, A., Christodoulou, J., & McGrath, L. M. (2017). Dispelling the myth: Training in education or neuroscience decreases but does not eliminate beliefs in neuromyths. Frontiers in Psychology, 8. doi:10.3389/ fpsyg.2017.01314
13. Ocklenburg, S., El Basbasse, Y., Ströckens, F., & Müller-Alcazar, A. (2023). Hemispheric asymmetries and brain size in mammals. Communications Biology, 6(1). doi:10.1038/s42003-023-04894-z
14. Corballis, M. C. (2014). Left Brain, right brain: Facts and fantasies. PLoS Biology, 12(1). doi:10.1371/journal.pbio.1001767
15. Gainotti, G. (2018). Emotions and the right hemisphere: Can new data clarify old models? The Neuroscientist, 25(3), 258–270. doi:10.1177/1073858418785342
16. Duboc, V., Dufourcq, P., Blader, P., & Roussigné, M. (2015). Asymmetry of the brain: Development and implications. Annual Review of Genetics, 49(1), 647–672. doi:10.1146/annurev-genet-112414-055322
17. Arsalidou, M., Pawliw-Levac, M., Sadeghi, M., & Pascual-Leone, J. (2018). Brain areas associated with numbers and calculations in children: Meta-analyses of fmri studies. Developmental Cognitive Neuroscience, 30, 239–250. doi:10.1016/j. dcn.2017.08.002
18. Popescu, T., Sader, E., Schaer, M., Thomas, A., Terhune, D. B., Dowker, A., Mars, R. B., & Cohen Kadosh, R. (2019). The brain-structural correlates of mathematical expertise. Cortex, 114, 140–150. doi:10.1016/j.cortex.2018.10.009
19. Ocklenburg, S., & Guo, Z. V. (2024). Cross-hemispheric communication: Insights on lateralized brain functions. Neuron, 112(8), 1222–1234. doi:10.1016/j.neuron.2024.02.010
20. Hartwigsen, G., Bengio, Y., & Bzdok, D. (2021b). How does hemispheric specialization contribute to human-defining cognition? Neuron, 109(13), 2075–2090. doi:10.1016/j.neuron.2021.04.024
21. Rogers, L. J. (2021). Brain lateralization and cognitive capacity. Animals, 11(7), 1996. doi:10.3390/ ani11071996
22. Strong, A., Grip, H., Arumugam, A., Boraxbekk, C. J., Selling, J., & Häger, C. K. (2023). Right hemisphere brain lateralization for knee proprioception among right-limb dominant individuals. Frontiers in Human Neuroscience, 17. doi:10.3389/ fnhum.2023.969101
23. Goodarzi, N., Dabbaghi, P., Valipour, H., & Vafadari, B. (2015). Pilot study: The role of the hemispheric lateralization in mental disorders by use of the limb (eye, hand, foot) dominance. Basic and Clinical Neuroscience, 6(2), 101–106. PMID:27307954
24. Gooijers, J., & Swinnen, S. P. (2014). Interactions between brain structure and behavior: The corpus callosum and bimanual coordination. Neuroscience & Biobehavioral Reviews, 43, 1–19. doi:10.1016/j.neubiorev.2014.03.008
25. Hung, Y.-C., Robert, M. T., Friel, K. M., & Gordon, A. M. (2019). Relationship between integrity of the corpus callosum and bimanual coordination in children with unilateral spastic cerebral palsy. Frontiers in Human Neuroscience, 13. doi:10.3389/ fnhum.2019.00334
26. Ekanem, U.-O. I., & Tubbs, R. S. (2023). Role of the corpus callosum in decision-making. The Corpus Callosum, 147–149. doi:10.1007/978-3-031-381140_16
27. Karolis, V. R., Corbetta, M., & Thiebaut de Schotten, M. (2019). The architecture of functional lateralisation and its relationship to callosal connectivity in the human brain. Nature Communications, 10(1). doi:10.1038/s41467-019-09344-1
28. Luo, X., Mao, Q., Shi, J., Wang, X., & Li, C. R. (2019). Putamen gray matter volumes in neuropsychiatric and neurodegenerative disorders. World Journal of Psychiatry and Mental Health Research, 3(1), 1020. PMID:31328186
29. Li, L., Zhang, Y., Zhao, Y., Li, Z., Kemp, G. J., Wu, M., & Gong, Q. (2022). Cortical thickness abnormalities in patients with post-traumatic stress disorder: A vertex-based meta-analysis. Neuroscience & Biobehavioral Reviews, 134. doi:10.1016/j.neubiorev.2021.104519
30. Montgomery, J. C. (2023). Roles for cerebellum and subsumption architecture in central pattern generation. Journal of Comparative Physiology A, 210(2), 315–324. doi:10.1007/s00359-023-01634-w
31. Tsytsarev, V. (2022). Methodological aspects of studying the mechanisms of consciousness. Behavioural Brain Research, 419, 113684. doi:10.1016/j. bbr.2021.113684
32. Roland, J. L., Akbari, S. H., Salehi, A., & Smyth, M. D. (2019). Corpus callosotomy performed with laser interstitial thermal therapy. Journal of Neurosurgery, 134(1), 314–322. doi:10.3171/2019.9.jns191769
33. Wu, X., Ou, S., Zhang, H., Zhen, Y., Huang, Y., Wei, P., & Shan, Y. (2023). Long-term follow-up seizure outcomes after corpus callosotomy: A systematic review with meta-analysis. Brain and Behavior, 13(4). doi:10.1002/brb3.2964
34. Tsuchiya, H., Shibata, T., Sasaki, T., Akiyama, M., Akiyama, T., & Kobayashi, K. (2024a). A retrospective study on post-operative recovery of daily living activity after total corpus callosotomy. Brain and Development, 46(10), 339–343. doi:10.1016/j. braindev.2024.09.006
35. Ma, Y., Liu, Y., Yan, X., & Ouyang, Y. (2023). Alien hand syndrome, a rare presentation of corpus callosum and cingulate infarction. Journal of the Neurological Sciences, 452, 120739. doi:10.1016/j. jns.2023.120739
36. Thomas, J. O., & Barrett, A. M. (2019). Right brain stroke syndromes. Stroke Rehabilitation, 71–89. doi:10.1016/b978-0-323-55381-0.00005-6
37. Rosen V. (2018). One brain. Two minds? Many questions. Journal of Undergraduate Neuroscience Education, 16(2), R48–R50. PMID:30057510
38. Gazzaniga, M. S. (2000). Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain, 123(7), 1293–1326. doi:10.1093/brain/123.7.1293
39. de Haan, E. H., Scholte, H. S., Pinto, Y., Foschi, N., Polonara, G., & Fabri, M. (2021). Singularity and consciousness: A neuropsychological contribution. Journal of Neuropsychology, 15(1), 1–19. doi:10.1111/jnp.12234
40. Marinsek, N. L., Gazzaniga, M. S., & Miller, M. B. (2016). Split-Brain, split-mind. The Neurology of Consciousness, 271–279. doi:10.1016/b978-0-12800948-2.00017-0
41. Mashour, G. A., Roelfsema, P., Changeux, J.-P., & Dehaene, S. (2020). Conscious processing and the global neuronal workspace hypothesis. Neuron, 105(5), 776–798. doi:10.1016/j.neuron.2020.01.026
42. Lamme, V. A. F. (2020). Visual functions generating conscious seeing. Frontiers in Psychology, 11. doi:10.3389/fpsyg.2020.00083
43. Graziano, M. S. (2018). The temporoparietal junction and Awareness. Neuroscience of Consciousness, 2018(1). doi:10.1093/nc/niy005
1. Alderson-Day, B., & Fernyhough, C. (2015). Inner speech: Development, cognitive functions, phenomenology, and neurobiology. Psychological Bulletin, 141(5), 931–965. https://doi.org/10.1037/ bul0000021
2. Jo, H., Park, C., Lee, E., Lee, J. H., Kim, J., Han, S., Kim, J., Kim, E. J., Kim, E., & Kim, J. (2024). Neural effects of one’s own voice on Self-Talk for emotion regulation. Brain Sciences, 14(7), 637. https:// doi.org/10.3390/brainsci14070637
3. Perrone-Bertolotti, M., Rapin, L., Lachaux, J., Baciu, M., & Lœvenbruck, H. (2014). What is that little voice inside my head? Inner speech phenomenology, its role in cognitive performance, and its relation to self-monitoring. Behavioural Brain Research, 261, 220–239. https://doi.org/10.1016/j. bbr.2013.12.034
4. Hoffman, B., Schraw, G., & McCrudden, M. T. (2012). Cognitive efficiency. In Springer eBooks (pp. 590–593). https://doi.org/10.1007/978-1-44191428-6_353
5. Viacheslav, I., Vartanov, A., Bueva, A., & Bronov, O. (2022). The emotional component of inner speech: A pilot exploratory fMRI study. Brain and Cognition, 165, 105939. https://doi.org/10.1016/j. bandc.2022.105939
6. Vissers, C. T. W. M., Tomas, E., & Law, J. (2020). The emergence of inner speech and its measurement in atypically developing children. Frontiers in Psychology, 11. https://doi.org/10.3389/ fpsyg.2020.00279
7. Prathanee, B., Pongjanyakul, A., & Chano, J. (2008). Thai Speech and Language Test for children between 1 and 2 years of age. International Journal of Language & Communication Disorders, 43(1), 125–140. https://doi.org/10.1080/13682820601181208
8. Vygotsky, L. (1986). Thought and Language. The Massachusetts Institute of Technology.
9. Kohlberg, L., Yaeger, J., & Hjertholm, E. (1968). Private Speech: Four studies and a Review of Theories. Child Development, 39(3), 691. https://doi. org/10.2307/1126979
10. Montazeri, M., Hamidi, H., & Hamidi, B. (2015). A closer look at different aspects of private speech in SLA. Theory and Practice in Language Studies, 5(3), 478. https://doi.org/10.17507/tpls.0503.04
11. Sawyer, J. E., & Brooks, P. J. (2021). Sociodramatic play enhances preschoolers’ private speech and motivation across activities. Cognitive Development, 59, 101073. https://doi.org/10.1016/j.cogdev.2021.101073
12. Geva, S., & Fernyhough, C. (2019). A penny for your thoughts: Children’s inner speech and its Neuro-Development. Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.01708
13. Doebel, S., & Munakata, Y. (2022). Unraveling the nature of children’s self-directed speech: correlates of five- and six-year-olds’ overt and partially covert speech on three tasks. Collabra Psychology, 8(1). https://doi.org/10.1525/collabra.57543
14. Winsler, A., De León, J. R., Wallace, B. A., Carlton, M. P., & Willson-Quayle, A. (2003). Private speech in preschool children: developmental stability and change, across-task consistency, and relations with classroom behaviour. Journal of Child Language, 30(3), 583–608. https://doi.org/10.1017/ s0305000903005671
15. Alarcón-Rubio, D., Sánchez-Medina, J. A., & Prieto-García, J. R. (2013). Executive function and verbal self-regulation in childhood: Developmental linkages between partially internalized private speech and cognitive flexibility. Early Childhood Research Quarterly, 29(2), 95–105. https://doi. org/10.1016/j.ecresq.2013.11.002
16. Sawyer, J. (2016). I think I can: Preschoolers’ private speech and motivation in playful versus non-playful contexts. Early Childhood Research Quarterly, 38, 84–96. https://doi.org/10.1016/j. ecresq.2016.09.004
17. Stephan, F., Saalbach, H., & Rossi, S. (2020). Inner versus Overt Speech Production: Does This Make a Difference in the Developing Brain? Brain Sciences, 10(12), 939. https://doi.org/10.3390/brainsci10120939
18. Flinker, A., Korzeniewska, A., Shestyuk, A. Y., Franaszczuk, P. J., Dronkers, N. F., Knight, R. T., & Crone, N. E. (2015). Redefining the role of Broca’s area in speech. Proceedings of the National Academy of Sciences, 112(9), 2871–2875. https:// doi.org/10.1073/pnas.1414491112
19. Hertrich, I., Dietrich, S., & Ackermann, H. (2016). The role of the supplementary motor area for speech and language processing. Neuroscience & Biobehavioral Reviews, 68, 602–610. https://doi. org/10.1016/j.neubiorev.2016.06.030
20. Barber, L., Reniers, R., & Upthegrove, R. (2021). A review of functional and structural neuroimaging studies to investigate the inner speech model of auditory verbal hallucinations in schizophrenia. Translational Psychiatry, 11(1). https://doi. org/10.1038/s41398-021-01670-7
21. Ólafsdóttir, H. F., Bush, D., & Barry, C. (2018). The role of hippocampal replay in memory and planning. Current Biology, 28(1), R37–R50. https://doi. org/10.1016/j.cub.2017.10.073
22. Van De Ven, V., Waldorp, L., & Christoffels, I. (2020). Hippocampus plays a role in speech feedback processing. NeuroImage, 223, 117319. https://doi. org/10.1016/j.neuroimage.2020.117319
23. Zhan, L., Guo, D., Chen, G., & Yang, J. (2018). Effects of repetition learning on associative recognition over time: role of the hippocampus and prefrontal cortex. Frontiers in Human Neuroscience, 12. https://doi.org/10.3389/fnhum.2018.00277
24. Himmer, L., Schönauer, M., Heib, D. P. J., Schabus, M., & Gais, S. (2019). Rehearsal initiates systems memory consolidation, sleep makes it last. Science Advances, 5(4). https://doi.org/10.1126/sciadv. aav1695
25. Jun, S., Kim, J. S., & Chung, C. K. (2019). Direct stimulation of human hippocampus during verbal associative encoding enhances subsequent memory recollection. Frontiers in Human Neuroscience, 13. https://doi.org/10.3389/fnhum.2019.00023
26. Alderson-Day, B., Weis, S., McCarthy-Jones, S., Moseley, P., Smailes, D., & Fernyhough, C. (2015). The brain’s conversation with itself: neural substrates of dialogic inner speech. Social Cognitive and Affective Neuroscience, 11(1), 110–120. https:// doi.org/10.1093/scan/nsv094
27. Tullett, A. M., & Inzlicht, M. (2010). The voice of self-control: Blocking the inner voice increases impulsive responding. Acta Psychologica, 135(2), 252–256. https://doi.org/10.1016/j.actpsy.2010.07.008
28. Fernyhough, C., & Borghi, A. M. (2023). Inner speech as language process and cognitive tool. Trends in Cognitive Sciences, 27(12), 1180–1193. https://doi. org/10.1016/j.tics.2023.08.014
29. Grandchamp, R., Rapin, L., Perrone-Bertolotti, M., Pichat, C., Haldin, C., Cousin, E., Lachaux, J., Dohen, M., Perrier, P., Garnier, M., Baciu, M., & Lœvenbruck, H. (2019). The ConDiallnt model: condensation, dialogality, and intentionality dimensions of inner speech within a hierarchical predictive control framework. Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.02019
30. Tilsen, S. (2024). Internal speech is faster than external speech: Evidence for feedback-based temporal control. Cognition, 244, 105713. https://doi. org/10.1016/j.cognition.2023.105713
31. Hertrich, I., Dietrich, S., Blum, C., & Ackermann, H. (2021). The role of the dorsolateral prefrontal cortex for speech and language processing. Frontiers in Human Neuroscience, 15. https://doi. org/10.3389/fnhum.2021.645209
32. Pratts, J., Pobric, G., & Yao, B. (2023). Bridging phenomenology and neural mechanisms of inner speech: ALE meta-analysis on egocentricity and spontaneity in a dual-mechanistic framework. NeuroImage, 282, 120399. https://doi.org/10.1016/j. neuroimage.2023.120399
33. Gabbott, B., Tennent, D., & Snelgrove, H. (2020). Effect of mental rehearsal on team performance and non-technical skills in surgical teams: systematic review. BJS Open, 4(6), 1062–1071. https:// doi.org/10.1002/bjs5.50343
34. Stephane, M., Dzemidzic, M., & Yoon, G. (2021). Keeping the inner voice inside the head, a pilot fMRI study. Brain and Behavior, 11(4). https://doi. org/10.1002/brb3.2042
35. Hickok, G. (2013). The role of Broca’s area in language function. In Cambridge University Press eBooks (pp. 341–349). https://doi.org/10.1017/ cbo9780511980435.020
36. Rogalsky, C. (2008). Broca’s area, sentence comprehension, and working memory: an fMRI study. Frontiers in Human Neuroscience, 2. https://doi. org/10.3389/neuro.09.014.2008
37. Kozubal, M., Szuster, A., & Wielgopolan, A. (2023). Emotional regulation strategies in daily life: the intensity of emotions and regulation choice. Frontiers in Psychology, 14. https://doi.org/10.3389/ fpsyg.2023.1218694
38. Racy, F., & Morin, A. (2024). Relationships between Self-Talk, Inner Speech, Mind Wandering, Mindfulness, Self-Concept Clarity, and Self-Regulation in University Students. Behavioral Sciences, 14(1), 55. https://doi.org/10.3390/bs14010055
39. Šimić, G., Tkalčić, M., Vukić, V., Mulc, D., Španić, E., Šagud, M., Olucha-Bordonau, F. E., Vukšić, M., & Hof, P. R. (2021). Understanding Emotions: Origins and roles of the amygdala. Biomolecules, 11(6), 823. https://doi.org/10.3390/biom11060823
40. Flinker, A., Korzeniewska, A., Shestyuk, A. Y., Franaszczuk, P. J., Dronkers, N. F., Knight, R. T., & Crone, N. E. (2015b). Redefining the role of Broca’s area in speech. Proceedings of the National Academy of Sciences, 112(9), 2871–2875. https:// doi.org/10.1073/pnas.1414491112
41. Okon-Singer, H., Hendler, T., Pessoa, L., & Shackman, A. J. (2015). The neurobiology of emotion–cognition interactions: fundamental questions and strategies for future research. Frontiers in Human Neuroscience, 9. https://doi.org/10.3389/ fnhum.2015.00058
42. Alderson-Day, B., & Pearson, A. (2023). What can neurodiversity tell us about inner speech, and vice versa? A theoretical perspective. Cortex, 168, 193–202. https://doi.org/10.1016/j.cortex.2023.08.008
43. Nedergaard, J. S. K., & Lupyan, G. (2024). Not everybody has an inner voice: Behavioral consequences of anendophasia. Psychological Science. https://doi.org/10.1177/09567976241243004
44. Ren, X., Wang, T., & Jarrold, C. (2016). Individual Differences in Frequency of Inner Speech: Differential Relations with Cognitive and Non-cognitive Factors. Frontiers in Psychology, 7. https://doi. org/10.3389/fpsyg.2016.01675
45. Park, H. R., Lee, J. M., Moon, H. E., Lee, D. S., Kim, B., Kim, J., Kim, D. G., & Paek, S. H. (2016). A short review on the current understanding of autism spectrum disorders. Experimental Neurobiology, 25(1), 1–13. https://doi.org/10.5607/en.2016.25.1.1
46. Lartseva, A., Dijkstra, T., & Buitelaar, J. K. (2015). Emotional language processing in autism spectrum disorders: a systematic review. Frontiers in Human Neuroscience, 8. https://doi.org/10.3389/ fnhum.2014.00991
47. Xie, F., Pascual, E., & Oakley, T. (2023). Functional echolalia in autism speech: Verbal formulae and repeated prior utterances as communicative and cognitive strategies. Frontiers in Psychology, 14. https://doi.org/10.3389/fpsyg.2023.1010615
48. Wallace, G. L., Silvers, J. A., Martin, A., & Kenworthy, L. E. (2009). Brief Report: Further evidence for inner Speech Deficits in autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 39(12), 1735–1739. https://doi.org/10.1007/ s10803-009-0802-8
49. Smailes, D., Alderson-Day, B., Fernyhough, C., McCarthy-Jones, S., & Dodgson, G. (2015). Tailoring cognitive behavioral therapy to subtypes of Voice-Hearing. Frontiers in Psychology, 6. https:// doi.org/10.3389/fpsyg.2015.01933
1. Aparicio-Martinez, P., Perea-Moreno, A. J., Martinez-Jimenez, M. P., Redel-Macías, M. D., Pagliari, C., & Vaquero-Abellan, M. (2019). Social Media, Thin-Ideal, Body Dissatisfaction and Disordered Eating Attitudes: An Exploratory Analysis. International Journal of Environmental Research and Public Health 2019, Vol. 16, Page 4177, 16(21), 4177. https://doi.org/10.3390/IJERPH16214177
2. Brown, S., Gao, X., Tisdelle, L., Eickhoff, S. B., & Liotti, M. (2011). Naturalizing aesthetics: brain areas for aesthetic appraisal across sensory modalities. NeuroImage, 58(1), 250–258. https://doi. org/10.1016/J.NEUROIMAGE.2011.06.012
3. Calvo-Merino, B., Jola, C., Glaser, D. E., & Haggard, P. (2008). Towards a sensorimotor aesthetics of performing art. Consciousness and Cognition, 17(3), 911–922. https://doi.org/10.1016/j. concog.2007.11.003
4. Campagna, M., & Chamberlain, R. (2024). How material sensory properties and individual differences influence the haptic aesthetic appeal of visually presented stimuli. Scientific Reports 2024 14:1, 14(1), 1–15. https://doi.org/10.1038/ s41598-024-63925-9
5. Chatterjee, A., & Vartanian, O. (2014). Neuroaesthetics. Trends in Cognitive Sciences, 18(7), 370–375. https://doi.org/10.1016/J.TICS.2014.03.003
6. Christensen, A. P., Cardillo, E. R., & Chatterjee, A. (2023). Can Art Promote Understanding? A Review of the Psychology and Neuroscience of Aesthetic Cognitivism. Psychology of Aesthetics, Creativity, and the Arts. https://doi.org/10.1037/ ACA0000541
7. Cloutier, J., Heatherton, T. F., Whalen, P. J., & Kelley, W. M. (2008). Are Attractive People Rewarding? Sex Differences in the Neural Substrates of Facial Attractiveness. Journal of Cognitive Neuroscience, 20(6), 941–951. https://doi.org/10.1162/ JOCN.2008.20062
8. Estrada Gonzalez, V., Meletaki, V., Walker, M., Payano Sosa, J., Stamper, A., Srikanchana, R., King, J. L., Scott, K., Cardillo, E. R., Rhodes, C. S., Christensen, A. P., Darda, K. M., Workman, C. I., & Chatterjee, A. (2024). Art therapy masks reflect emotional changes in military personnel with PTSS. Scientific Reports 2024 14:1, 14(1), 1–12. https://doi.org/10.1038/s41598-024-57128-5
9. Humphries, S., Rick, J., Weintraub, D., & Chatterjee, A. (2021). Movement in Aesthetic Experiences: What We Can Learn from Parkinson Disease. Journal of Cognitive Neuroscience, 33(7), 1329–1342. https://doi.org/10.1162/JOCN_A_01718
10. Hung, S. M., Nieh, C. H., & Hsieh, P. J. (2016). Unconscious processing of facial attractiveness: invisible attractive faces orient visual attention. Scientific Reports 2016 6:1, 6(1), 1–8. https://doi. org/10.1038/srep37117
11. Isik, A. I., & Vessel, E. A. (2021). From Visual Perception to Aesthetic Appeal: Brain Responses to Aesthetically Appealing Natural Landscape Movies. Frontiers in Human Neuroscience, 15, 676032. https://doi.org/10.3389/FNHUM.2021.676032/BIBTEX
12. Kaziga, R., Muchunguzi, C., Achen, D., & Kools, S. (2021). Beauty Is Skin Deep; The Self Perception of Adolescents and Young Women in Construction of Body Image within the Ankole Society. International Journal of Environmental Research and Public Health, 18(15). https://doi.org/10.3390/ IJERPH18157840
13. Kenett, Y. N., Cardillo, E. R., Christensen, A. P., & Chatterjee, A. (2023). Aesthetic emotions are affected by context: a psychometric network analysis. Scientific Reports 2023 13:1, 13(1), 1–16. https://doi.org/10.1038/s41598-023-48219-w
14. Kirsch, L. P., Urgesi, C., & Cross, E. S. (2016). Shaping and reshaping the aesthetic brain: Emerging perspectives on the neurobiology of embodied aesthetics. Neuroscience & Biobehavioral Reviews, 62, 56–68. https://doi. org/10.1016/J.NEUBIOREV.2015.12.005
15. Little, A. C., Jones, B. C., & Debruine, L. M. (2011). Facial attractiveness: evolutionary based research. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1571), 1638–1659. https://doi.org/10.1098/RSTB.2010.0404
16. Luo, Q., Yu, M., Li, Y., & Mo, L. (2019). The neural correlates of integrated aesthetics between moral and facial beauty. Scientific Reports 2019 9:1, 9(1), 1–10. https://doi.org/10.1038/s41598-01938553-3
17. MacCallum, F., & Widdows, H. (2018). Altered Images: Understanding the Influence of Unrealistic Images and Beauty Aspirations. Health Care Analysis, 26(3), 235–245. https://doi.org/10.1007/ S10728-016-0327-1/METRICS
18. Magsamen, S. (2019). Your Brain on Art: The Case for Neuroaesthetics. Cerebrum: The Dana Forum on Brain Science, 2019. /pmc/articles/ PMC7075503/
19. Meghani, S. H., Peterson, C., Kaiser, D. H., Rhodes, J., Rao, H., Chittams, J., & Chatterjee, A. (2018). A Pilot Study of a Mindfulness-Based Art Therapy Intervention in Outpatients With Cancer. Https:// Doi.Org/10.1177/1049909118760304, 35(9), 1195–1200. https://doi.org/10.1177/1049909118760304
20. Mutter, Y., & Hübner, R. (2024). The effect of expertise on the creation and evaluation of visual compositions in terms of creativity and beauty. Scientific Reports 2024 14:1, 14(1), 1–19. https:// doi.org/10.1038/s41598-024-64494-7
21. Osaka, N. (2022). Emotional neuroaesthetics of color experience: Views from single, paired, and complex color combinations. PsyCh Journal, 11(5), 628–635. https://doi.org/10.1002/PCHJ.577 Pearce, M. T., Zaidel, D. W., Vartanian, O., Skov, M., Leder, H., Chatterjee, A., & Nadal, M. (2016). Neuroaesthetics: The Cognitive Neuroscience of Aesthetic Experience. Perspectives on Psychological Science, 11(2), 265–279. https://doi. org/10.1177/1745691615621274 Pedalino, F., & Camerini, A. L. (2022). Instagram Use and Body Dissatisfaction: The Mediating Role of Upward Social Comparison with Peers and Influencers among Young Females. International Journal of Environmental Research and Public Health, 19(3).
22. https://doi.org/10.3390/IJERPH19031543
23. Rodgers, R. F., Campagna, J., & Attawala, R. (2019). Stereotypes of physical attractiveness and social influences: The heritage and vision of Dr. Thomas Cash. Body Image, 31, 273–279. https://doi.org/10.1016/J.BODYIM.2019.01.010
24. Rolls, E. T. (2023). Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala. Brain Structure and Function 2023 228:5, 228(5), 1201–1257. https://doi.org/10.1007/S00429-023-02644-9
25. Sakano, Y., Wada, A., Ikeda, H., Saheki, Y., Tagai, K., & Ando, H. (2021). Human brain activity reflecting facial attractiveness from skin reflection. Scientific Reports 2021 11:1, 11(1), 1–13. https:// doi.org/10.1038/s41598-021-82601-w
26. Salgues, S., Jacquot, A., Makowski, D., Tahar, C., Baekeland, J., Arcangeli, M., Dokic, J., Piolino, P., & Sperduti, M. (2024). Self-reference and emotional reaction drive aesthetic judgment. Scientific Reports 2024 14:1, 14(1), 1–11. https://doi. org/10.1038/s41598-024-68331-9
27. Shang, J., & Zhang, Y. (2024). Influence of male’s facial attractiveness, vocal attractiveness and social interest on female’s decisions of fairness. Scientific Reports 2024 14:1, 14(1), 1–11. https:// doi.org/10.1038/s41598-024-67841-w
28. Świątek, A. H., Szcześniak, M., Stempień, M., Wojtkowiak, K., & Chmiel, M. (2024). The mediating effect of the need for cognition between aesthetic experiences and aesthetic competence in art. Scientific Reports 2024 14:1, 14(1), 1–9. https://doi.org/10.1038/s41598-024-53957-6
29. Talamas, S. N., Mavor, K. I., & Perrett, D. I. (2016). Blinded by Beauty: Attractiveness Bias and Accurate Perceptions of Academic Performance. PLOS ONE, 11(2), e0148284. https://doi. org/10.1371/JOURNAL.PONE.0148284
30. Villavisanis, D. F., Workman, C. I., Zapatero, Z. D., Vu, G. H., Humphries, S. A., Blum, J. D., Cho, D. Y., Swanson, J. W., Bartlett, S. P., Chatterjee, A., & Taylor, J. A. (2023). Visual Attention, Bias, and Social Dispositions Toward People With Facial Anomalies: A Prospective Study With Eye Tracking Technology. Annals of Plastic Surgery, 90(5), 482–486. https://doi.org/10.1097/ SAP.0000000000003435
31. Villavisanis, D., Workman, C., Zapatero, Z. D., Vu, G., Humphries, S., Blum, J. D., Cho, D. Y., Paul Bartlett, S., Swanson, J. W., Chatterjee, A., & Taylor, J. A. (2022). Layperson Bias and Empathy Influence Visual Attention Toward Patients with Hemifacial Microsomia: A Prospective Eye tracking Study. Plastic and Reconstructive Surgery - Global Open, 10(10S), 128–129. https://doi. org/10.1097/01.GOX.0000898996.44696.95
32. Wald, C. (2015). Neuroscience: The aesthetic brain. Nature 2015 526:7572, 526(7572), S2–S3. https://doi.org/10.1038/526s2a
33. Wang, L., Ma, L., Yang, J., & Wu, J. (2021). Human Somatosensory Processing and Artificial Somatosensation. Cyborg and Bionic Systems, 2021. https://doi.org/10.34133/2021/9843259 Weinberger, A. B., Garside, E. W., Christensen, A. P., & Chatterjee, A. (2022a). Effects of expertise on psychological responses to buildings and natural landscapes. Journal of Environmental Psychology, 84, 101903. https://doi.org/10.1016/J.JENVP.2022.101903
34. Weinberger, A. B., Garside, E. W., Christensen, A. P., & Chatterjee, A. (2022b). Effects of expertise on psychological responses to buildings and natural landscapes. Journal of Environmental Psychology, 84, 101903. https://doi.org/10.1016/J. JENVP.2022.101903
35. Wu, E. G., Brackbill, N., Rhoades, C., Kling, A., Gogliettino, A. R., Shah, N. P., Sher, A., Litke, A. M., Simoncelli, E. P., & Chichilnisky, E. J. (2024). Fixational eye movements enhance the precision of visual information transmitted by the primate retina. Nature Communications 2024 15:1, 15(1), 1–15. https://doi.org/10.1038/s41467024-52304-7
36. Wu, Y. H., Podvalny, E., Levinson, M., & He, B. J. (2024). Network mechanisms of ongoing brain activity’s influence on conscious visual perception. Nature Communications 2024 15:1, 15(1), 1–18. https://doi.org/10.1038/s41467-024-50102-9
37. Yarosh, D. B. (2019). Perception and Deception: Human Beauty and the Brain. Behavioral Sciences 2019, Vol. 9, Page 34, 9(4), 34. https://doi. org/10.3390/BS9040034
38. Yen, C., Lin, C. L., & Chiang, M. C. (2023). Exploring the Frontiers of Neuroimaging: A Review of Recent Advances in Understanding Brain Functioning and Disorders. Life 2023, Vol. 13, Page 1472, 13(7), 1472. https://doi.org/10.3390/ LIFE13071472
39. Zhang, W., He, X., Lai, S., Wan, J., Lai, S., Zhao, X., & Li, D. (2017). Neural substrates of embodied natural beauty and social endowed beauty: An fMRI study. Scientific Reports 2017 7:1, 7(1), 1– 12. https://doi.org/10.1038/s41598-017-07608-8
40. Zhang, W., Lai, S., He, X., Zhao, X., & Lai, S. (2016). Neural correlates for aesthetic appraisal of pictograph and its referent: An fMRI study. Behavioural Brain Research, 305, 229–238. https:// doi.org/10.1016/J.BBR.2016.02.029
41. Zhao, X., Wang, J., Li, J., Luo, G., Li, T., Chatterjee, A., Zhang, W., & He, X. (2020). The neural mechanism of aesthetic judgments of dynamic landscapes: an fMRI study. Scientific Reports 2020 10:1, 10(1), 1–11. https://doi.org/10.1038/s41598020-77658-y
42. Zhou, C., & Li, J. Q. (2024). The development of aesthetic experience through virtual and augmented reality. Scientific Reports 2024 14:1, 14(1), 1–12. https://doi.org/10.1038/s41598-02453840-4
1. Barrett, M. S., Flynn, L. M., Brown, J. E., & Welch, G. F. (2019). Beliefs and values about music in early childhood education and care: perspectives from practitioners. Frontiers in Psychology, 10. doi:10.3389/fpsyg.2019.00724
2. Ticker, C. S. (2017). Music and the mind: music’s healing powers. Musical Offerings, 8(1), 1–12. doi:10.15385/jmo.2017.8.1.1
3. Goltz, F., & Sadakata, M. (2021). Do you listen to music while studying? A portrait of how people use music to optimize their cognitive performance. Acta Psychologica, 220. doi:10.1016/j. actpsy.2021.103417
4. Rauscher, F. H., Shaw, G. L., & Ky, C. N. (1993). Music and spatial task performance. Nature, 365(6447), 611. doi:10.1038/365611a0
5. Jamil, Z., Saeed, A. A., Madhani, S., Baig, S., Cheema, Z., & Fatima, S. S. (2018). Three-dimensional visualization software assists learning in students with diverse spatial intelligence in medical education. Anatomical Sciences Education, 12(5), 550–560. doi:10.1002/ase.1828
6. Ishikawa, T., & Newcombe, N. S. (2021). Why spatial is special in education, learning, and everyday activities. Cognitive Research: Principles and Implications, 6(1). doi:10.1186/s41235-021-00274-5
7. Pietschnig, J., Voracek, M., & Formann, A. K. (2010). Mozart effect–Shmozart effect: A meta-analysis. Intelligence, 38(3), 314–323. doi:10.1016/j.intell.2010.03.001
8. Mehr S. A. (2015). Miscommunication of science: Music cognition research in the popular press. Frontiers in Psychology, 6, 988. doi: 10.3389/ fpsyg.2015.00988
9. Zaatar, M. T., Alhakim, K., Enayeh, M., & Tamer, R. (2023). The transformative power of music: insights into neuroplasticity, health, and disease. Brain, Behavior, & Immunity - Health, 35. doi:10.1016/j.bbih.2023.100716
10. Silva, S., Belim, F., & Castro, S. L. (2020). The Mozart effect on the episodic memory of healthy adults is null, but low-functioning older adults may be an exception. Frontiers in Psychology, 11. doi:10.3389/fpsyg.2020.538194
11. He, W. J., Wong, W. C., & Hui, A. N. N. (2017). Emotional reactions mediate the effect of music listening on creative thinking: Perspective of the arousal-and-mood hypothesis. Frontiers in Psychology, 8. doi:10.3389/fpsyg.2017.01680
12. Deckert, M., Schmoeger, M., Auff, E., & Willinger, U. (2019). Subjective emotional arousal: An explorative study on the role of gender, age, intensity, emotion regulation difficulties, depression and anxiety symptoms, and meta-emotion. Psychological Research, 84(7), 1857–1876. doi:10.1007/s00426-019-01197-z
13. Cudo, A., Francuz, P., Augustynowicz, P., & Stróżak, P. (2018). The effects of arousal and approach motivated positive affect on cognitive control. An ERP study. Frontiers in Human Neuroscience, 12. doi:10.3389/fnhum.2018.00320
14. Nadon, É., Tillmann, B., Saj, A., & Gosselin, N. (2021). The emotional effect of background music on selective attention of adults. Frontiers in Psychology, 12. doi:10.3389/fpsyg.2021.729037.
15. Ferreri, L., Mas-Herrero, E., Zatorre, R. J., Ripollés, P., Gomez-Andres, A., Alicart, H., Olivé, G., Marco-Pallarés, J., Antonijoan, R. M., Valle, M., Riba, J., & Rodriguez-Fornells, A. (2019). Dopamine modulates the reward experiences elicited by music. Proceedings of the National Academy of Sciences, 116(9), 3793–3798. doi:10.1073/ pnas.1811878116
16. Schultz, W. (2024). A dopamine mechanism for reward maximization. Proceedings of the National Academy of Sciences, 121(20). doi:10.1073/ pnas.2316658121.
17. Kiss, L., Szikora, B., & Linnell, K. J. (2024). Music in the eye of the beholder: A pupillometric study on preferred background music, attentional state, and arousal. Psychological Research, 88(5), 1616–1628. doi:10.1007/s00426-024-01963-8
18. Swart, E. K., & Sikkema-de Jong, M. T. (2021). The effects of increased dopamine-levels on attentional control during reading and reading comprehension. Current Psychology, 42(13), 11009–11025. doi:10.1007/s12144-021-02363-6
19. Boag, R. J., Stevenson, N., van Dooren, R., Trutti, A. C., Sjoerds, Z., & Forstmann, B. U. (2021). Cognitive control of working memory: A model-based approach. Brain Sciences, 11(6). doi: 10.3390/brainsci11060721
20. Huston, J. P., & Chao, O. Y. (2023). Probing the nature of episodic memory in rodents. Neuroscience & Biobehavioral Reviews, 144. doi:10.1016/j. neubiorev.2022.104930
21. Westbrook, A., & Braver, T. S. (2016). Dopamine does double duty in motivating cognitive effort. Neuron, 91(3), 695–710. doi:10.1016/j.neuron.2016.07.020
22. Meneghetti, C., Labate, E., Toffalini, E., & Pazzaglia, F. (2021). Successful navigation: The influence of task goals and working memory. Psychological Research, 85(2), 634–648. doi:10.1007/ s00426-019-01270-7
23. Zsidó A. N. (2024). The effect of emotional arousal on visual attentional performance: A systematic review. Psychological Research, 88(1), 1–24. doi:10.1007/s00426-023-01852-6
24. LaLumiere, R. T. (2014). Dopamine and memory. In: Menses, A. (Eds.). Identification of Neural Markers Accompanying Memory. Elsevier. doi:10.1016/B978-0-12-408139-0.00005-5
25. Darki, C., Riley, J., Dadabhoy, D. P., Darki, A., & Garetto, J. (2022). The effect of classical music on heart rate, blood pressure, and mood. Cureus, 14(7). doi:10.7759/cureus.27348
26. Souza, A. S., & Leal Barbosa, L. C. (2023). Should we turn off the music? Music with lyrics interferes with cognitive tasks. Journal of Cognition, 6(1), 24. doi:10.5334/joc.273
27. Trappe, H. J., & Voit, G. (2016). The cardiovascular effect of musical genres. Deutsches Ärzteblatt International, 113(20), 347–352. doi:10.3238/arztebl.2016.0347
28. De Groot, A. M. B., & Smedinga, H. E. (2014). LET THE MUSIC PLAY! Studies in Second Language Acquisition, 36(4), 681–707. doi:10.1017/ s0272263114000059
29. Vasilev, M. R., Hitching, L., & Tyrrell, S. (2023). What makes background music distracting? Investigating the role of song lyrics using selfpaced reading. Journal of Cognitive Psychology, 36(1), 138–164. doi:10.1080/20445911.2023.22093 46
30. Weiss, Y., Cweigenberg, H. G., & Booth, J. R. (2018). Neural specialization of phonological and semantic processing in young children. Human brain mapping, 39(11), 4334–4348. doi:10.1002/ hbm.24274
31. Madore, K. P., & Wagner, A. D. (2019). Multicosts of multitasking. Cerebrum, 2019. PMID: 32206165
32. Scarratt, R. J., Heggli, O. A., Vuust, P., & Sadakata, M. (2023). Music that is used while studying and music that is used for sleep share similar musical features, genres and subgroups. Scientific Reports, 13(1). doi:0.1038/s41598-023-31692-8
33. Verrusio, W., Ettorre, E., Vicenzini, E., Vanacore, N., Cacciafesta, M., & Mecarelli, O. (2015). The Mozart effect: A quantitative EEG study. Consciousness and Cognition, 35, 150–155. doi:10.1016/j.concog.2015.05.005
34. Bottiroli, S., Rosi, A., Russo, R., Vecchi, T., & Cavallini, E. (2014). The cognitive effects of listening to background music on older adults: processing speed improves with upbeat music, while memory seems to benefit from both upbeat and downbeat music. Frontiers in aging neuroscience, 6, 284. doi: 10.3389/fnagi.2014.00284
35. Jafari, M. J., Khosrowabadi, R., Khodakarim, S., & Mohammadian, F. (2019). The effect of noise exposure on cognitive performance and brain activity patterns. Open Access Macedonian Journal of Medical Sciences, 7(17), 2924–2931. doi:10.3889/oamjms.2019.742
36. Young, J. O. (2016). How classical music is better than popular music. Philosophy, 91(4), 523–540. doi:10.1017/S0031819116000334
37. Cheng, H. Y., Xie, H. X., Tang, Q. L., Yi, L. T., & Zhu, J. X. (2024). Light and classical music therapies attenuate chronic unpredictable mild stress-induced depression via BDNF signaling pathway in mice. Heliyon, 10(13). doi:10.1016/j.heliyon.2024. e34196
38. Finn, S., & Fancourt, D. (2018). The biological impact of listening to music in clinical and nonclinical settings: A systematic review. Progress in Brain Research, 237, 173–200. doi:10.1016/ bs.pbr.2018.03.007
39. Mencke, I., Omigie, D., Wald-Fuhrmann, M., & Brattico, E. (2019). Atonal music: Can uncertainty lead to pleasure? Frontiers in Neuroscience, 12. doi:10.3389/fnins.2018.00979
40. Sanseverino, D., Caputo, A., Cortese, C. G., & Ghislieri, C. (2022). “Don’t Stop the Music,” please: The relationship between music use at work, satisfaction, and performance. Behavioral Sciences, 13(1). doi:10.3390/bs13010015
41. Ghojazadeh, M., Farhoudi, M., Rezaei, M., Rahnemayan, S., Narimani, M., & Sadeghi-Bazargani, H. (2023). Effect of music on driving performance and physiological and psychological indicators: A systematic review and meta-analysis study. Health Promotion Perspectives, 13(4), 267–279. doi:10.34172/hpp.2023.32
42. Li, R., Chen, Y. V., & Zhang, L. (2019). Effect of music tempo on long-distance driving: Which tempo is the most effective at reducing fatigue? i-Perception, 10(4). doi:10.1177/2041669519861982
43. Wen, H., Sze, N. N., Zeng, Q., & Hu, S. (2019). Effect of music listening on physiological condition, mental workload, and driving performance with consideration of driver temperament. International Journal of Environmental Research And Public Health, 16(15). doi:10.3390/ijerph16152766
44. Wu, J., Zhang, L., Yang, H., Lu, C., Jiang, L., & Chen, Y. (2022). The effect of music tempo on fatigue perception at different exercise intensities. International Journal of Environmental Research and Public Health, 19(7), 3869. doi:10.3390/ ijerph19073869
45. Centala, J., Pogorel, C., Pummill, S. W., & Malek, M. H. (2020). Listening to fast-tempo music delays the onset of neuromuscular fatigue. Journal of Strength and Conditioning Research, 34(3), 617–622. doi:10.1519/jsc.0000000000003417
46. Van Dyck, E. (2019). Musical intensity applied in the sports and exercise domain: An effective strategy to boost performance? Frontiers in Psychology, 10. doi:10.3389/fpsyg.2019.01145
47. Trahan, T., Durrant, S. J., Müllensiefen, D., & Williamson, V. J. (2018). The music that helps people sleep and the reasons they believe it works: A mixed methods analysis of online survey reports. PLoS ONE, 13(11). doi:10.1371/journal.pone.0206531
48. Dickson, G. T., & Schubert, E. (2019). How does music aid sleep? lLiterature review. Sleep Medicine, 63, 142–150. doi:10.1016/j.sleep.2019.05.016
49. Feng, F., Zhang, Y., Hou, J., Cai, J., Jiang, Q., Li, X., Zhao, Q., & Li, B. (2018). Can music improve sleep quality in adults with primary insomnia? A systematic review and network meta-analysis. International Journal of Nursing Studies, 77, 189–196. doi:10.1016/j.ijnurstu.2017.10.011
50. Lai, H. L., Chang, E. T., Li, Y. M., Huang, C. Y., Lee, L. H., & Wang, H. M. (2014). Effects of music videos on sleep quality in middle-aged and older adults with chronic insomnia. Biological Research For Nursing, 17(3), 340–347. doi:10.1177/1099800414549237
51. Matziorinis, A. M., & Koelsch, S. (2022). The promise of music therapy for Alzheimer’s disease: A review. Annals of the New York Academy of Sciences, 1516(1), 11–17. doi:10.1111/nyas.14864
52. Fernandez, M. V., Liu, M., Beric, A., Johnson, M., Cetin, A., Patel, M., Budde, J., Kohlfeld, P., Bergmann, K., Lowery, J., Flynn, A., Brock, W., Sanchez Montejo, B., Gentsch, J., Sykora, N., Norton, J., Gentsch, J., Valdez, O., Gorijala, P., Sanford, J., Sun, Y., Wang, C., Western, D., Timsina, T., Goncalves, T. M., Do, A. N., Sung, Y. J., Zhao, G., Morris, J. C., Moulder, K., Holztman, D. M., Bateman, R. J., Karch, C., Hassenstab, J., Xiong, C., Schindler, S. E., Balls-Berry, J., Benzinger, T. L. S., Perrin, R. J., Denny, A., Snider, B. J., Stark, S. L., Ibanez, L. & Cruchaga, C. (2024). Genetic and multi-omic resources for Alzheimer disease and related dementia from the Knight Alzheimer Disease Research Center. Scientific Data, 11(1). doi:10.1038/s41597-024-03485-9
53. Bleibel, M., El Cheikh, A., Sadier, N. S., & Abou-Abbas, L. (2023). The effect of music therapy on cognitive functions in patients with Alzheimer’s disease: A systematic review of randomized controlled trials. Alzheimer’s research & therapy, 15(1), 65. doi:10.1186/s13195-023-01214-9
54. Mavridis I. N. (2015). Music and the nucleus accumbens. Surgical and Radiologic Anatomy, 37(2), 121–125. doi: 10.1007/s00276-014-1360-0
55. Jespersen, K. V., Pando-Naude, V., Koenig, J., Jennum, P., & Vuust, P. (2022). Listening to music for insomnia in adults. The Cochrane database of systematic reviews, 8(8), CD010459. https://doi. org/10.1002/14651858.CD010459.pub3
1. Hansen, J. M., & Charles, A. (2019). Differences in treatment response between migraine with aura and migraine without aura: Lessons from clinical practice and RCTS. The Journal of Headache and Pain, 20(1). doi:10.1186/s10194-019-1046-4
2. Headache classification committee of the International Headache Society (IHS) (2018). The international classification of headache disorders, 3rd Edition. Cephalalgia, 38(1), 1–211. doi:10.1177/0333102417738202
3. Goadsby, P. J., Holland, P. R., Martins-Oliveira, M., Hoffmann, J., Schankin, C., & Akerman, S. (2017). Pathophysiology of migraine: A disorder of sensory processing. Physiological Reviews, 97(2), 553–622. doi:10.1152/physrev.00034.2015
4. Frimpong-Manson, K., Ortiz, Y. T., McMahon, L. R., & Wilkerson, J. L. (2024). Advances in understanding migraine pathophysiology: A bench to bedside review of research insights and therapeutics. Frontiers in Molecular Neuroscience, 17. doi:10.3389/fnmol.2024.1355281
5. Viana, M., Tronvik, E. A., Do, T. P., Zecca, C., & Hougaard, A. (2019). Clinical features of visual migraine aura: A systematic review. The Journal of Headache and Pain, 20(1). doi:10.1186/s10194019-1008-x
6. O’Hare, L., Asher, J. M., & Hibbard, P. B. (2021). Migraine visual aura and cortical spreading depression—linking mathematical models to empirical evidence. Vision, 5(2), 30. doi:10.3390/ vision5020030
7. Hadjikhani, N., & Vincent, M. (2019). Neuroimaging clues of Migraine Aura. The Journal of Headache and Pain, 20(1). doi:10.1186/s10194-019-0983-2
8. Petrusic, I., Viana, M., Dakovic, M., & Zidverc-Trajkovic, J. (2019). Application of the migraine aura complexity score (macs): Clinical and neuroimaging study. Frontiers in Neurology, 10. doi:10.3389/ fneur.2019.01112
9. Cadwell, C. R., Bhaduri, A., Mostajo-Radji, M. A., Keefe, M. G., & Nowakowski, T. J. (2019). Development and arealization of the cerebral cortex. Neuron, 103(6), 980–1004. doi:10.1016/j.neuron.2019.07.009
10. Yuste, R. (2015). From the neuron doctrine to neural networks. Nature Reviews Neuroscience, 16(8), 487–497. doi:10.1038/nrn3962
11. Khodagholy, D., Gelinas, J. N., Thesen, T., Doyle, W., Devinsky, O., Malliaras, G. G., & Buzsáki, G. (2014). NeuroGrid: Recording action potentials from the surface of the brain. Nature Neuroscience, 18(2), 310–315. doi:10.1038/nn.3905
12. Iftikhar, W., Cheema, F. F., Khanal, S., & Khan, Q. U. (2020). Migrainous infarction and cortical spreading depression. Discoveries, 8(3). doi:10.15190/d.2020.9
13. Chow, M. S., Wu, S. L., Webb, S. E., Gluskin, K., & Yew, D. (2017). Functional magnetic resonance imaging and the brain: A brief review. World Journal of Radiology, 9(1), 5. doi:10.4329/wjr. v9.i1.5
14. Russo, A., Silvestro, M., Tessitore, A., & Tedeschi, G. (2019). Recent insights in migraine with aura: A narrative review of advanced neuroimaging. Headache: The Journal of Head and Face Pain, 59(4), 637–649. doi:10.1111/head.13512
15. Arngrim, N., Hougaard, A., Ahmadi, K., Vestergaard, M. B., Schytz, H. W., Amin, F. M., Larsson, H. B., Olesen, J., Hoffmann, M. B., & Ashina, M. (2017). Heterogenous migraine aura symptoms correlate with visual cortex functional magnetic resonance imaging responses. Annals of Neurology, 82(6), 925–939. doi:10.1002/ana.25096
16. Harriott, A. M., Takizawa, T., Chung, D. Y., & Chen, S.-P. (2019). Spreading depression as a preclinical model of migraine. The Journal of Headache and Pain, 20(1). doi:10.1186/s10194-019-1001-4
17. Rasmussen, M. K., Møllgård, K., Bork, P. A. R., Wiekop, P., Esmail, T., Drici, L., Wewer Albrechtsen, N. J, Carlsen, J. F., Huynh, N. P. T., Ghitani, N., Mann, M., Goldman, S. A., Mori, Y., Chesler, A. T., & Nedergaard, M. (2024). Trigeminal ganglion neurons are directly activated by influx of CSF solutes in a migraine model. Science, 385(6704), 80-86. doi:10.1126/science.adl0544
18. Sowell, M. K., & Youssef, P. E. (2016). The comorbidity of migraine and epilepsy in children and adolescents. Seminars in Pediatric Neurology, 23(1), 83–91. doi:10.1016/j.spen.2016.01.012
19. Garg, D., & Tripathi, M. (2021). Borderlands of migraine and epilepsy. Neurology India, 69(Suppl 1). doi:10.4103/0028-3886.315994
20. Paungarttner, J., Quartana, M., Patti, L., Sklenárová, B., Farham, F., Jiménez, I. H., Soylu, M. G., Vlad, I. M., Tasdelen, S., Mateu, T., Marsico, O., Reina, F., Tischler, V., & Lampl, C. (2024). Migraine - a borderland disease to epilepsy: Near it but not of it. The Journal of Headache and Pain, 25(1). doi:10.1186/s10194-024-01719-0
21. Feske, S. K. (2021). Ischemic stroke. The American Journal of Medicine, 134(12), 1457–1464. doi:10.1016/j.amjmed.2021.07.027
22. Øie, L. R., Kurth, T., Gulati, S., & Dodick, D. W. (2020). Migraine and risk of stroke. Journal of Neurology, Neurosurgery & Psychiatry, 91(6), 593–604. doi:10.1136/jnnp-2018-318254
23. Coutts, S. B. (2017). Diagnosis and management of transient ischemic attack. Continuum, 23(1), 82–92. doi:10.1212/con.0000000000000424
24. Pelzer, N., de Boer, I., van den Maagdenberg, A. M. J. M., & Terwindt, G. M. (2023). Neurological and psychiatric comorbidities of migraine: Concepts and future perspectives. Cephalalgia, 43(6). doi:10.1177/03331024231180564
25. Anastacio, H. T. G., Matosin, N., & Ooi, L. (2022). Neuronal hyperexcitability in Alzheimer’s disease: What are the drivers behind this aberrant phenotype? Translational Psychiatry, 12(1). doi:10.1038/ s41398-022-02024-7
26. Hadjikhani, N., & Vincent, M. (2021). Visual perception in migraine: A narrative review. Vision, 5(2). doi:10.3390/vision5020020
27. Close, L. N., Eftekhari, S., Wang, M., Charles, A. C., & Russo, A. F. (2018). Cortical spreading depression as a site of origin for migraine: Role of CGRP. Cephalalgia, 39(3), 428–434. doi:10.1177/0333102418774299
28. Gasmi, A., Nasreen, A., Menzel, A., Gasmi Benahmed, A., Pivina, L., Noor, S., Peana, M., Chirumbolo, S., & Bjørklund, G. (2022). Neurotransmitters regulation and food intake: The role of dietary sources in neurotransmission. Molecules, 28(1), 210. doi:10.3390/molecules28010210
29. Hoffmann, J., & Charles, A. (2018). Glutamate and its receptors as therapeutic targets for migraine. Neurotherapeutics, 15(2), 361–370. doi:10.1007/ s13311-018-0616-5
30. Vitale, M., Tottene, A., Zarin Zadeh, M., Brennan, K., & Pietrobon, D. (2023). Mechanisms of initiation of cortical spreading depression. The Journal of Headache and Pain, 24(1). doi:10.1186/s10194023-01643-9
31. Hansen, K. B., Yi, F., Perszyk, R. E., Furukawa, H., Wollmuth, L. P., Gibb, A. J., & Traynelis, S. F. (2018). Structure, function, and allosteric modulation of NMDA receptors. Journal of General Physiology, 150(8), 1081–1105. doi:10.1085/ jgp.201812032
32. Li, L., & Vlisides, P. E. (2016). Ketamine: 50 years of modulating the mind. Frontiers in Human Neuroscience, 10. doi:10.3389/fnhum.2016.00612
33. Yavi, M., Lee, H., Henter, I. D., Park, L. T., & Zarate, C. A. (2022). Ketamine treatment for depression: A review. Discover Mental Health, 2(1). doi:10.1007/s44192-022-00012-3
1. Linassi, F., Obert, D. P., Maran, E., Tellaroli, P., Kreuzer, M., Sanders, R. D., & Carron, M. (2021). Implicit memory and anesthesia: A systemic review and meta-analysis. Life, 11(8), 850. doi:10.3390/life11080850
2. Bonhomme, V., Staquet, C., Montupil, J., Defresne, A., Kirsch, M., Martial, C., Vanhaudenhuyse, A., Chatelle, C.,, Larroque, S., Raimondo, F., Demertzi, A., Bodart, O., Laureys, S., & Gosseries, O. (2019). General anesthesia: A probe to explore consciousness. Frontiers in Systems Neuroscience, 13(36). doi.10.3389/fnsys.2019.00036
3. Perrott, C., Lee, C. A., Griffiths, S., & Sury, M. R. J. (2017). Perioperative experiences of anesthesia reported by children and parents. Pediatric Anesthesia, 28(2), 149-156. doi:0.1111/pan.13300
4. Shin, T. J., Kim, P. J., & Choi, B. (2022). How general anesthetics work: From the perspective of reorganized connections within the brain. Korean Journal of Anesthesiology, 75(2), 124-138. doi:10.4097/kja.22078
5. Mashour, G. A., (2024). Anesthesia and the neurobiology of consciousness. Neuron, 112(10), 15531567. doi:10.1016/j.neuron.2024.03.002
6. Anderson, J. A. (2024). Respiratory monitoring for anesthesia and sedation. Anesthesia Progress, 70(4), 198-201. doi:10.2344/862700
7. Eikermann, M., Akeju, O., & Chamberlin, N. L. (2020). Sleep and anesthesia, The shared circuit hypothesis has been put to bed. Current Biology, 30(5), R219-R221. doi:10.1016/j.cub.2020.01.057
8. Moody, O. A., hang, E. R., Vincent, K. F., Kato, R., Melonakos, E. D., Nehs, C. J., & Solt, K. (2021). The neural circuits underlying general anesthesia and sleep. Anesthesia & Analgesia, 132(5), 12541264. doi:10.1213/ANE.0000000000005361
9. Jung, J., & Kim, T. (2022). General anesthesia and sleep: Like and unlike. Anesthesia & Pain Medicine, 17(4), 343-351. doi:10.17085/apm.22227
10. Bao, W. W., Xu, W., Pan, G. J., Wang, T. X., Han, Y., Qu, W. M., Li, W. X., & Huang, Z. L. (2021). Nucleus accumbens neurons expressing dopamine D1 receptors modulate states of consciousness in sevoflurane anesthesia. Current Biology, 31(9), 1893-1902. doi:10.1016/j.cub.2021.02.011
11. Desai, D., Momin, A., Hirpara, P., Jha, H., Thaker, R., & Patel, J. (2024). Exploring the role of circadian rhythms in sleep and recovery: A review article. Cureus, 16(6). doi:10.7759/cureus.61568
12. Chai, W. J., Abd Hamid, A. I., & Abdullah, J. M. (2018). Working memory from the psychological and neurosciences perspectives: A review. Frontiers in Psychology, 9. doi:10.3389/ fpsyg.2018.00401
13. Amiri, A. A., Karvandian, K., Ramezani, N., Ahmadzadeh, A. (2020). Short-term memory impairment in patients undergoing general anesthesia and its contributing factors. Saudi Journal of Anesthesia, 14(4), 454-458. doi:10.4103/sja. SJA_651_19
14. Dong, X. (2018). Current strategies for brain drug delivery. Theranostics, 8(6), 1481-1493. doi:10.7150/thno.21254
15. Längrich, T., Bork, J., Horstkorte, R., Weber, V., Hofmann, B., Fuszard, M., & Olzscha, H. (2022). Disturbance of key cellular subproteomes upon propofol treatment is associated with increased permeability of the blood-brain barrier. Proteomes, 10(3). doi:10.3390/proteomes10030028
16. Yuste, R. (2015). From the neuron doctrine to neural networks. Nature Reviews Neuroscience, 16, 487-497. doi:10.1038/nrn3962
17. Khodagholy, D., Gelinas, J. N., Thesen, T., Doyle, W., Devinsky, O., Malliaras, G. G., & Buzsáski, G. (2015). NeuroGrid: Recording action potentials from the surface of the brain. Nature Neuroscience, 18, 310-315. doi:10.1038/nn.3905
18. Rienecker, K. D. A., Poston, R. G., & Saha, R. N. (2020). Merits and limitations of studying neuronal depolarization-dependent processes using elevated external potassium. ASN Neuro, 12. doi:10.1177/1759091420974807
19. Brickley, S. G., Franks, N. P., & Wisden, W. (2018). Modulation of GABAA receptor function and sleep. Current Opinion in Physiology, 2, 51-57. doi:10.1016/j.cophys.2017.12.011
20. Shin, D. J., Germann, A. L., Johnson, A. D., Forman, S. A., Steinbach, J. H., & Akk, G. (2018). Propofol is an allosteric agonist with multiple binding sites on concatemeric ternary GABAA receptors. Molecular Pharmacology, 93(2), 178-189. doi:10.1124/mol.117.110403
21. Du, W. J., Zhang, R. W., Li, J., Zhang, B. B., Peng, X. L., Cao, S., Yuan, J., Yuan, C. D., Yu, T., & Du, J. L. (2018). The locus coeruleus modulates intravenous general anesthesia of zebrafish via a cooperative mechanism. Cell Reports, 24(12), 31463155. doi:10.1016/j.celrep.2018.08.046
22. Kreuzer, M., Butovas, S., García, P. S., Schneider, G., Schwarz, C., Rudolph, W., Antkowiak, B. & Drexler, B. (2020). Propofol affects cortico-hippocampal interactions via β3 subunit-containing GABAA receptors. International Journal of Molecular Sciences, 21(16). doi:10.3390/ijms21165844
23. Sahinovic, M. M., Struys, M. M. R. F., & Absalom, A. R., (2018). Clinical pharmacokinetics and pharmacodynamics of propofol. Clinical Pharmacokinetics, 57(12), 1539-1558. doi:10.1007/s40262-0180672-3
24. Hudetz, A. G. (2012). General anesthesia and human brain connectivity. Brain Connectivity, 2(6). doi:10.1089/brain.2012.0107
25. Tagliabue, S., Lindner, C., Chochron da Prat, I., Sanchez-Guerrero, A., Serra, I., Kacprzak, M., Maruccia, F., Martinez Silva, O., Weigel, U. M., de Nadal, M., Poca, M. A., & Durduran, T. (2023). Comparison of cerebral metabolic rate of oxygen, blood flow, and bispectral index under general anesthesia. Neurophotonics, 10(1). doi:10.1117/1. NPh.10.1.015006
26. Rodgers, Z. B., Detre, J. A., & Wehrli, F. W. (2016). MRI-based methods for quantification of the cerebral metabolic rate of oxygen. Journal of Cerebral Blood Flow & Metabolism, 36(7). doi:10.1177/0271678X16643090
27. Judge, A., & Dodd, M. S. (2020). Metabolism. Essays in Biochemistry, 64(4), 607-647. doi:10.1042/ EBC20190041
28. Stender, J., Kupers, R., Rodell, A., Thibaut, A., Chatelle, C., Bruno, M. A., Gejl, M., Bernard, C., Hustinx, R., Laureys, S., & Gjedde, A. (2014). Quantitative rates of brain glucose metabolism distinguish minimally conscious from vegetative state patients. Journal of Cerebral Blood Flow & Metabolism, 35(1), doi:10.1038/jcbfm.2014.169
29. Chen, Y., & Zhang, J. (2021). How energy supports our brain to yield consciousness: Insights from neuroimaging based on the neuroenergetics hypothesis. Frontiers in Systems Neuroscience, 15. doi:10.3389/fnsys.2021.648860
30. Laaksonen, L., Kallioinen, M., Långsjö, J., Laitio, T., Scheinin, A., Scheinin, J., Kaisti, K., Maksimow, A., Kallionpää, R. E., Rajala, V., Johansson, J., Kantonen, O., Nyman, M., Sirén, S., Valli, K., Revonsuo, A., Solin, O., Vahlberg, T., Alkire, M., & Scheinin, H. (2018). Comparative effects of dexmedetomidine, propofol, sevoflurane, and S-ketamine on regional cerebral glucose metabolism in humans: A positron emission tomography study. British Journal of Anaesthesia, 121(1), 281290. doi:10.1016/j.bja.2018.04.008
31. Song, X. X., & Yu, B. W. (2014). Anesthetic effects of propofol in the healthy human brain: Functional imaging evidence. Journal of Anesthesia, 29, 279-288. doi:10.1007/s00540-014-1889-4
32. Leung, L. S., Luo, T., Ma, J., & Herrick, I. (2014). Brain areas that influence general anesthesia. Progress in Neurobiology, 122, 24-44. doi.10.1016/j. pneurobio.2014.08.001
33. Gruenbaum, B. F. (2020). Comparison of anaesthetic- and seizure-induced states of unconsciousness: A narrative review. British Journal of Anesthesia, 126(1), 219-229. doi:10.1016/j. bja.2020.07.056
34. Liu, X., Lauer, K. K., Ward, B. D., Li, S. J., Hudetz, A. G. (2013). Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: A functional magnetic resonance imaging study. Anesthesiology, 118, 59-69. doi:10.1097/ALN.0b013e318277a801
35. Wang, X., Zhao, X., Xue, G., & Chen, A. (2016). Alertness function of thalamus in conflict adaptation. NeuroImage, 132, 274-282. doi:10.1016/j. neuroimage.2016.02.048
36. Zhou, J., Liu, X., Song, W., Yang, Y., Zhao, Z., Ling, F., Hudetz, A. G., & Li, S. J. (2010). Specific and nonspecific thalamocortical functional connectivity in normal and vegetative states. Conscious and Cognition, 20(2), 257-268. doi:10.1016/j.concog.2010.08.003
37. Grodd, W., Kumar, V. J., Schüz, A., Lindig, T., & Scheffler, K. (2020). The anterior and medial thalamic nuclei and the human limbic system: Tracing the structural connectivity using diffusion-weighted imaging. Scientific Reports, 10. doi:10.1038/s41598-020-67770-4
38. Pereira de Vasconcelos, A., & Cassel, J. C. (2015). The nonspecific thalamus: A place in a wedding bed for making memories last? Neuroscience & Behavioral Reviews, 54, 175-196. doi:10.1016/j.neubiorev.2014.10.021
39. Kobayashi, K., Chien, J. H., Kim, J. H., & Lenz, F. A. (2017). Sensory, motor, and intrinsic mechanisms of thalamic activity related to organic and psychogenic dystonia. Journal of Alzheimer’s Disease & Parkinsonism, 7(3). doi:10.4172/21610460.1000324
40. Cadwell, C. R., Bhaduri, A., Mostajo-Radji, M. A., Keefe, M. G., & Nowakowski, T. J. (2019). Development and arealization of the cerebral cortex. Neuron, 103(6), 980-1004. doi:10.1016/j.neuron.2019.07.009
41. Malekmohammadi, M., Price, C. M., Hudson, A. E., DiCesare, J. A. T., & Pouratian, N. (2019). Propofol-induced loss of consciousness is associated with a decrease in thalamocortical connectivity in humans. Brain, 142(8), 2288-2302. doi:10.1093/ brain/awz169
42. Germuska, M., & Wise, R. G. (2019). Calibrated fMRI for mapping absolute CMRO2: Practicalities and prospects. NeuroImage, 187, 145-153. doi:10.1016/j.neuroimage.2018.03.068
43. Gauthier, C. J. & Fan, A. P. (2019). BOLD signal physiology: Models and applications. Neuroimage, 187, 116-127. doi:0.1016/j.neuroimage.2018.03.018
44. Sala-Llonch, R., Bartrés-Faz, D., & Junqué, C. (2015). Reorganization of brain networks in aging: A review of functional connectivity studies. Frontiers in Psychology, 6. doi:10.3389/ fpsyg.2015.00663
45. Flores, F. J., Hartnack, K. E., Fath, A. B., Kim, S. E., Wilson, M. A., Brown, E. N., & Purdon, P. L. (2017). Thalamocortical synchronization during induction and emergence from propofol-induced unconsciousness. PNAS, 114(32), E6660-E6668. doi:10.1073/pnas.1700148114
46. Bastos, A. M., Donoghue, J. A., Brincat, S. L., Mahnke, M., Yanar, J., Correa, J., Waite, A. S., Lundqvist, M., Roy, J., Brown, E. N., Miller, E. K. (2021). Neural effects of propofol-induced unconsciousness and its reversal using thalamic stimulation. eLife, 10. doi:10.7554/eLife.60824
47. Montupil, J., Cardone, P., Staquet, C., Bonhomme, A., Defresne, A., Martial, C., Alnagger, N. L. N., Gosseries, O., & Bonhomme, V. (2023). The nature of consciousness in anesthesia. BJA Open, 8. doi:10.1016/j.bjao.2023.100224
48. Degrandi Oliveira, C. R., Marques bernardo, W., & Nunes, V. M. (2017). Benefit of general anesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta-analysis. Brazilian Journal of Anesthesiology, 67(1), 72-84. doi:10.1016/j.bjane.2015.09.001
AND THE MENTAL: THE EFFECTS OF CHRONIC STRESS ON RHEUMATOID ARTHRITIS
1. Dhabhar, F. S. (2014). Effects of stress on immune function: The good, the bad, and the beautiful. Immunological Research, 58, 193-210. doi:10.1007/s12026-014-8517-0.
2. Dhabhar, F. S. (2018). The short-term stress response – Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Frontiers in Neuroendocrinology, 49, 175–192. doi:10.1016/j.yfrne.2018.03.004.
3. Alotiby, A. (2024). Immunology of stress: A review article. Journal of Clinical Medicine, 13(21), 6394. doi:10.3390/jcm13216394.
4. Russell, G., & Lightman, S. (2019). The human stress response. Nature Reviews Endocrinology, 15, 525-534. doi:10.1038/s41574-0190228-0.
5. Lehman, H. K. (2015). Autoimmunity and immune dysregulation in primary immune deficiency disorders. Current Allergy and Asthma Reports, 15, 53. doi:10.1007/s11882015-0553-x.
6. Golbidi, S., Frisbee, J. C., & Laher, I. (2015). Chronic stress impacts the cardiovascular system: Animal models and clinical outcomes. American Journal of Physiology, 308(12), 1476-1498. doi:10.1152/ ajpheart.00859.2014.
7. Labanski, A., Langhorst, J., Engler, H., & Elsenbruch, S. (2020). Stress and the braingut axis in functional and chronic-inflammatory gastrointestinal diseases: A transdisciplinary challenge. Psychoneuroendocrinology, 111. doi:10.1016/j.psyneuen.2019.104501.
8. Hassamal, S. (2023). Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Frontiers in Psychiatry, 14. doi:10.3389/fpsyt.2023.1130989.
9. Spiering, M.J. (2015). Primer on the immune system. Alcohol Research: Current Reviews, 37(2), 171-175. PMID: 26695756.
10. Kany, S., Vollrath, J. T., & Relja, B. (2019). Cytokines in inflammatory disease. International Journal of Molecular Sciences, 20(23). doi:10.3390/ijms20236008.
11. Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L. (2017). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), 7204-7218. doi:10.18632/oncotarget.23208.
12. Schwager, S., & Detmar, M. (2019). Inflammation and lymphatic function. Frontiers in Immunology, 10. doi:10.3389/fimmu.2019.00308.
13. Xiao, T. S. (2016). Innate immunity and inflammation. Cellular and Molecular Immunology, 14, 1-3. doi:10.1038/cmi.2016.45.
14. Handfield, C., Kwock, J., & Macleod, A.S. (2018). Innate antiviral immunity in the skin. Trends in Immunology, 39(4), 328-340. doi:10.1016/j. it.2018.02.003.
15. Dantzer, R. (2017). Neuroimmune interactions: From the brain to the immune system and vice versa. Physiological Reviews, 98(1), 477-501. doi:10.1152/physrev.00039.2016.
16. Pang, T.Y., Yaeger, J. DW., Summers, C.H., & Mitra, R. (2021). Cardinal role of the environment in stress induced changes across life stages and generations. Neuroscience & Biobehavioral Reviews, 124, 137-150. doi:10.1016/j.neubiorev.2021.01.012.
17. Herman, J.P., McKlveen, J.M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., Scheimann, J., & Myers, B. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology, 6(2), 603-621. doi:10.1002/ cphy.c150015.
18. Kinlein, S.A., & Karatsoreos, I.N. (2019). The hypothalamic-pituitary-adrenal axis as a substrate for stress resilience: Interactions with the circadian clock. Frontiers in Neuroendocrinology, 56. doi:10.1016/j.yfrne.2019.100819.
19. Xie, Y., & Dorsky, R.I. (2017). Development of the hypothalamus: Conservation, modification and innovation. Development, 144(9), 1588-1599. doi:10.1242/dev.139055.
20. Knezevic, E., Nenic, K., Milanovic, V., & Knezevic, N.N. (2023). The role of cortisol in chronic stress, neurodegenerative diseases, and psychological disorders. Cells, 12(23), 2726. doi:10.3390/ cells12232726.
21. Morey, J. N., Boggero, I. A., Scott, A. B., & Segerstrom, S. C. (2015). Current directions in stress and human immune function. Current Opinion in Psychology, 5, 13-17. doi:10.1016/j.copsyc.2015.03.007
22. Yaribeygi, H., Panahi, Y., Sahraei, H., Johnston, T.P., & Sahebkhar, A. (2017). The impact of stress on body function: A review. Excli Journal: Experimental and Clinical Sciences, 16, 1057-1072. doi:10.17179/excli2017-480
23. Liu, Y., Tian, S., Ning, B., Huang, T., Li, Y., & Wei, Y. (2022). Stress and cancer: The mechanisms of immune dysregulation and management. Frontiers in Immunology, 13. doi:10.3389/fimmu.2022.1032294.
24. Kumar, P., Saini, S., Khan, S., Lele, S.S., & Prabhakar, B.S. (2019). Restoring self-tolerance in autoimmune diseases by enhancing regulatory T-cells. Cellular Immunology, 339, 41-49. doi:10.1016/j.cellimm.2018.09.008.
25. Sattler, S. (2017). The role of the immune system beyond the fight against infection. Advances in Experimental Medicine and Biology, 1003, 3-14. doi:10.1007/978-3-319-57613-8_1.
26. Wang, L., Wang, F., & Gershwin, M. E. (2015). Human autoimmune diseases: A comprehensive update. Journal of Internal Medicine, 278(4), 369395. doi:10.1111/joim.12395
27. Richard-Eaglin, A., & Smallheer, B. A. (2018). Immunosuppressive/autoimmune disorders. Nursing Clinics of North America, 53(3), 319-334. doi:10.1016/j.cnur.2018.04.002
28. Ellis, J.A., Kemp, A.S., & Ponsonby, A. (2014). Gene-environment interaction in autoimmune disease. Expert Reviews in Molecular Medicine, 16(4). doi:10.1017/erm.2014.5.
29. Weyand, C.M., & Goronzy, J.J. (2020). The immunology of rheumatoid arthritis. Nature Immunology, 22(1), 10-18. doi:10.1038/s41590-020-00816-x
30. Page, A., Fusil, F., & Cosset, F. (2021). Antigen-specific tolerance approach for rheumatoid arthritis: Past, present, and future. Joint Bone Spine, 88(4). doi:10.1016/j.jbspin.2021.105164
31. Cavalheiro do Espírito Santo, R., Baker, J.F., dos Santos, L.P., de Souza Silva, J.M., Filippin, L.I., Portes, J.K.S., Brenol, C.V., da Silva Chakr, R.M., & Xavier, R.M. (2023). Changes in physical function over time in rheumatoid arthritis. PLoS One, 18(1). doi:10.1371/journal.pone.0280846
32. Saha, S., Müller, D., & Clark, A.G. (2023). Mechanosensory feedback loops during chronic inflammation. Frontiers in Cell and Developmental Biology, 11. doi:10.3389/fcell.2023.1225677
33. Lin, Y., Anzaghe, M., & Schülke, S. (2020). Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells, 9(4), 880. doi:10.3390/cells9040880.
34. Maeda, K., Yoshida, K., Nishizawa, T., Otani, K., Yamashita, Y., Okabe, H., Hadano, Y., Kayama, T., Kurosaka, D., & Saito, M. (2022). Inflammation and bone metabolism in rheumatoid arthritis: Molecular mechanisms of joint destruction and pharmacological treatments. International Journal of Molecular Sciences, 23(5). doi:10.3390/ ijms23052871
35. Sharif, K., Watad, A., Coplan, L., Lichtbroun, B., Krosser, A., Lichtbroun, M., Bragazzi, N. L., Amital, H., Afek, A., & Shoenfeld, Y. (2018). The role of stress in the mosaic of autoimmunity: An overlooked association. Autoimmunity Reviews, 17(10), 967-983. doi:10.1016/j.autrev.2018.04.005.
36. Germain, V., Scherlinger, M., Barnetche, T., Pichon, C., Balageas, A., Lequen, L., Shipley, E., Foret, J., Dublanc, S., Capuron, L., & Schaeverbeke, T. (2020). Role of stress in the development of rheumatoid arthritis: A case-control study. Rheumatology, 60(2), 629-637. doi:10.1093/ rheumatology/keaa216
37. Mariotti, A. (2015). The effects of chronic stress on health: New insights into the molecular mechanisms of brain-body communication. Future Science OA, 1(3). doi:10.4155/fso.15.21
38. Kulakova, K., Lawal, T.P., Mccarthy, E., & Floudas, A. (2024). The contribution of macrophage plasticity to inflammatory arthritis and their potential as therapeutic targets. Cells, 13(18), 1586. doi:10.3390/cells13181586.
39. Di Matteo, A., Bathon, J.M., & Emery, P. (2023). Rheumatoid arthritis. The Lancet, 402(10416), 2019-2033. doi:10.1016/S0140-6736(23)01525-8.
40. Lwin, M.N., Serhal, L., Holroyd, C., & Edwards, C.J. (2020). Rheumatoid arthritis:The impact of mental health on disease: A narrative review. Rheumatology and Therapy, 7, 457-471. doi:10.1007/ s40744-020-00217-4
41. Polinski, K. J., Bemis, E. A., Feser, M., Seifert, J., Demoruelle, M. K., Striebich, C. C., Brake, S., O’dell, J. R., Mikuls, T. R., Weisman, M. H., Gregersen, P. K., Keating, R. M., Buckner, J., Nicassio, P., Holers, V. M., Deane, K. D., & Norris, J. M. (2020). Perceived stress and inflammatory arthritis: A prospective investigation in the Studies of the Etiologies of Rheumatoid Arthritis (SERA) cohort. Arthritis Care & Research, 72(12), 17661771. doi:10.1002/acr.24085
42. Chaudhary, R., Prasad, A., Agarwal, V., Rehman, M., Kumar, A., Kaushik, A.S., Srivastava, S., Srivastava, S., & Mishra, V. (2023). Chronic stress predisposes to the aggravation of inflammation in autoimmune diseases with focus on rheumatoid arthritis and psoriasis. International Immunopharmacology, 125. doi:10.1016/j.intimp.2023.111046.
43. Khadour, F.A., Khadour, Y.A., & Ebrahem, B.M. (2024). A qualitative survey on factors affecting depression and anxiety in patients with rheumatoid arthritis: A cross-sectional study in Syria. Scientific Reports, 14(1). doi:10.1038/s41598-02461523-3
44. Zielinski, M.R., Systrom, D.M., & Rose, N.R. (2019). Fatigue, sleep, and autoimmune and related disorders. Frontiers in Immunology, 10. doi:10.3389/ fimmu.2019.01827.
45. Radu, A., & Bungau, S.G. (2021). Management of rheumatoid arthritis: An overview. Cells, 23(10), 2857. doi:10.3390/cells10112857.
46. Bullock, J., Rizvi, S.AA., Saleh, A.M., Ahmed, S.S., Do, D.P., Ansari, R.A., & Ahmed, J. (2018). Rheumatoid arthritis: A brief overview of the treatment. Medical Principles and Practice, 27(6), 501-507. doi:10.1159/000493390
47. Wróbel, A., Baranska, I., Szklarczyk, J., Majda, A., & Jaworek, J. (2023). Relationship between perceived stress, stress coping strategies, and clinical status in patients with rheumatoid arthritis. Rheumatology International, 43, 16651674. doi:10.1007/s00296-023-05367-6.
48. Black, D. S., & Slavich, G. M. (2016). Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Annals of the New York Academy of Sciences, 1373(1), 13-24. doi:10.1111/nyas.12998
49. Nagy, Z., Szigedi, E., Takács, S., & Császár-Nagy, N. (2023). The effectiveness of psychological interventions for rheumatoid arthritis (RA): A systematic review and meta-analysis. Life, 13(3). doi:10.3390/life13030849
50. de Brouwer, S.J.M., van Middendorp, H., Kraaimaat, F.W., Radstake, T.R.D., Joosten, I., Donders, A.R., Eijsbouts, A., Spillekom-van Koulil, S., van Riel, P.L., & Evers, A.W.M. (2013). Immune responses to stress after stress management training in patients with rheumatoid arthritis. Arthritis Research & Therapy, 15(6). doi:10.1186/ ar4390.
51. Sturgeon, J. A., Finan, P. H., & Zautra, A. J. (2016). Affective disturbance in rheumatoid arthritis: Psychological and disease-related pathways. Nature Reviews Rheumatology, 12(9), 532-542. doi: 10.1038/nrrheum.2016.112
WILL YOU BE MY THERAPIST? THE USE OF AI
1. Locher, C., Meier, S., & Gaab, J. (2019). Psychotherapy: A world of meanings. Frontiers in Psychology, 10. doi.10.3389/fpsyg.2019.00460
2. Adamopoulou, E., & Moussiades, L. (2020). An overview of chatbot technology. In: Maglogiannis, I., Iliadis, L., Pimenidis, E. (Eds.). Artificial Intelligence Applications and Innovations. Springer, Cham. doi:10.1007/978-3-030-49186-4_31
3. He, Y., Yang, L., Qian, C., Li, T., Su, Z., Zhang, Q., & Hou, X. (2023). Conversational agent interventions for mental health problems: Systematic review and meta-analysis of randomized controlled trials. Journal of Medical Internet Research, 25. doi:10.2196/43862
4. Feijt, M., de Kort, Y., Westerink, J., Bierbooms, J., Bongers, I., & IJsselsteijn, W. (2023). Integrating technology in mental healthcare practice: A repeated cross-sectional survey study on professionals’ adoption of digital mental health before and during COVID-19. Frontiers in Psychiatry, 13. doi:10.3389/fpsyt.2022.1040023
5. Imel, Z. E., Caperton, D. D., Tanana, M., & Atkins, D. C. (2017). Technology-enhanced human interaction in psychotherapy. Journal of Counseling Psychology, 64(4), 385-393. doi:10.1037/ cou0000213
6. Fairburn, C. G., & Patel, V. (2017). The impact of digital technology on psychological treatments and their dissemination. Behaviour Research and Therapy, 88, 19-25. doi:10.1016/J.BRAT.2016.08.012
7. Kris, J. (2023). Telehealth implementation, treatment attendance, and socioeconomic disparities in treatment utilization in a community mental health setting during the COVID-19 pandemic: A retrospective analysis of electronic health record data. Telemedicine Reports, 4(1), 55-60. doi:10.1089/tmr.2022.0005
8. Neary, M., & Schueller, S. M. (2018). State of the Field of Mental Health Apps. Cognitive and Behavioral Practice, 25(4), 531-537. doi:10.1016/j. cbpra.2018.01.002
9. Huguet, A., Rao, S., McGrath, P. J., Wozney, L., Wheaton, M., Conrod, J., & Rozario, S. (2016). A systematic review of cognitive behavioral therapy and behavioral activation apps for depression. PLoS ONE, 11(5). doi:10.1371/journal.pone.0154248
10. Firth, J., Torous, J., Nicholas, J., Carney, R., Pratap, A., Rosenbaum, S., & Sarris, J. (2017). The efficacy of smartphone-based mental health interventions for depressive symptoms: A metaanalysis of randomized controlled trials. World Psychiatry, 16(3), 287-298. doi:10.1002/wps.20472
11. Sharma, A., Rushton, K., Lin, I. W., Nguyen, T., & Althoff, T. (2024). Facilitating self-guided mental health interventions through human-language model interaction: A case study of cognitive restructuring. In: Mueller, F.F., Kyburz, P., Williamson, J.R., Sas, C., Wilson, M.L., Dugas, P.T., & Shklovski, P. (Eds.) Proceedings of the CHI Conference on Human Factors in Computing Systems, 1-29. doi:10.1145/3613904.3642761
12. Alfano, L., Malcotti, I., & Ciliberti, R. (2024). Psychotherapy, artificial intelligence and adolescents: ethical aspects. Journal of Preventive Medicine and Hygiene, 64(4), E438-E442. doi:10.15167/2421-4248/jpmh2023.64.4.3135
13. Linardon, J., Cuijpers, P., Carlbring, P., Messer, M., & Fuller-Tyszkiewicz, M. (2019). The efficacy of app-supported smartphone interventions for mental health problems: A metaanalysis of randomized controlled trials. World Psychiatry, 18(3), 325-336. doi:10.1002/wps.20673
14. Miner, A. S., Shah, N., Bullock, K. D., Arnow, B. A., Bailenson, J., & Hancock, J. (2019). Key considerations for incorporating conversational AI in psychotherapy. Frontiers in Psychiatry, 10. doi:10.3389/fpsyt.2019.00746
15. Fiske, A., Henningsen, P., & Buyx, A. (2019). Your robot therapist will see you now: Ethical implications of embodied artificial intelligence in psychiatry, psychology, and psychotherapy. Journal of Medical Internet Research, 21(5). doi:10.2196/13216
16. Weizenbaum, J. (1966). ELIZA—A computer program for the study of natural language communication between man and machine. Communications of the ACM, 9(1), 36-45. doi:10.1145/365153.365168
17. Coheur L. (2020). From Eliza to Siri and beyond. In: Lesot, M.J., Veira, S., Reformat, M.Z., Carvalho, J.P., Wilbik, A., Bouchon-Meunier, B., & Yager, R.R. (Eds.). Information Processing and Management of Uncertainty in Knowledge-Based Systems. IPMU 2020. Communications in Computer and Information Science, vol 1237, 29-41. Springer. doi:10.1007/978-3-030-50146-4_3
18. Kim, S. Y., Schmitt, B. H., & Thalmann, N. M. (2019). Eliza in the uncanny valley: Anthropomorphizing consumer robots increases their perceived warmth but decreases liking. Marketing Letters, 30(1), 1-12. doi:10.1007/s11002-019-094859
19. Coghlan, S., Leins, K., Sheldrick, S., Cheong, M., Gooding, P., & D’Alfonso, S. (2023). To chat or bot to chat: Ethical issues with using chatbots in mental health. DIGITAL HEALTH, 9. doi:10.1177/20552076231183542
20. Lin, X., Martinengo, L., Jabir, A. I., Ho, A. H., Car, J., Atun, R., & Tudor Car, L. (2023). Scope, characteristics, behavior change techniques, and quality of conversational agents for mental health and well-being: Systematic assessment of apps. Journal of Medical Internet Research, 25. doi:10.2196/45984
21. Fitzpatrick, K. K., Darcy, A., & Vierhile, M. (2017). Delivering cognitive behavior therapy to young adults with symptoms of depression and anxiety using a fully automated conversational agent (Woebot): A randomized controlled trial. JMIR Mental Health, 4(2). doi:10.2196/mental.7785
22. Kiuchi, K., Otsu, K., & Hayashi, Y. (2023). Psychological insights into the research and practice of embodied conversational agents, chatbots and social assistive robots: A systematic meta-review. Behaviour & Information Technology, 1-41. doi:10.1080/0144929X.2023.2286528
23. Jindal, J. A., Lungren, M. P., & Shah, N. H. (2024). Ensuring useful adoption of generative artificial intelligence in healthcare. Journal of the American Medical Informatics Association, 31(6), 14411444. doi:10.1093/jamia/ocae043
24. Grodniewicz, J. P., & Hohol, M. (2023). Waiting for a digital therapist: Three challenges on the path to psychotherapy delivered by artificial intelligence. Frontiers in Psychiatry, 14. doi:10.3389/ fpsyt.2023.1190084
25. Schick, A., Feine, J., Morana, S., Maedche, A., & Reininghaus, U. (2022). Validity of chatbot use for mental health assessment: experimental study. JMIR mHealth and uHealth, 10(10), e28082. doi:10.2196/28082
26. Liu, H., Peng, H., Song, X., Xu, C., & Zhang, M. (2022). Using AI chatbots to provide self-help depression interventions for university students: A randomized trial of effectiveness. Internet Interventions, 27. doi:10.1016/j.invent.2022.100495
27. Vaidyam, A. N., Linggonegoro, D., & Torous, J. (2021). Changes to the psychiatric chatbot landscape: a systematic review of conversational agents in serious mental illness: Changements du paysage psychiatrique des chatbots: Une revue systématique des agents conversationnels dans la maladie mentale sérieuse. The Canadian Journal of Psychiatry, 66(4), 339-348. doi:10.1177/0706743720966429
28. Koulouri, T., Macredie, R. D., & Olakitan, D. (2022). Chatbots to support young adults’ mental health: An exploratory study of acceptability. ACM Transactions on Interactive Intelligence Systems, 12(2), 1-39. doi:10.1145/3485874
29. Vaidyam, A. N., Wisniewski, H., Halamka, J. D., Kashavan, M. S., & Torous, J. B. (2019). Chatbots and conversational agents in mental health: A review of the psychiatric landscape. The Canadian Journal of Psychiatry, 64(7), 456-464. doi:10.1177/0706743719828977
30. Martinengo, L., Lum, E., & Car, J. (2022). Evaluation of chatbot-delivered interventions for self-management of depression: Content analysis. Journal of Affective Disorders, 319, 598-607. doi:10.1016/j.jad.2022.09.028
31. Bendig, E., Erb, B., Schulze-Thuesing, L., & Baumeister, H. (2019). The next generation: Chatbots in clinical psychology and psychotherapy to foster mental health - A scoping review. Verhaltenstherapie, 32(Suppl. 1), 64-76. doi:10.1159/000501812
32. Lim, S. M., Shiau, C. W. C., Cheng, L. J., & Lau, Y. (2022). Chatbot-delivered psychotherapy for adults with depressive and anxiety symptoms: A systematic review and meta-regression. Behavior Therapy, 53(2), 334-347. doi:10.1016/j. beth.2021.09.007
33. Kocaballi, A. B., Quiroz, J. C., Rezazadegan, D., Berkovsky, S., Magrabi, F., Coiera, E., & Laranjo, L. (2020). Responses of conversational agents to health and lifestyle prompts: Investigation of appropriateness and presentation structures. Journal of Medical Internet Research, 22(2). doi:10.2196/15823
34. Miner, A. S., Milstein, A., Schueller, S., Hegde, R., Mangurian, C., & Linos, E. (2016). Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Internal Medicine, 176(5). doi:10.1001/jamainternmed.2016.0400
35. Sakurai, Y., Ikegami, Y., Sakai, M., Fujikawa, H., Tsuruta, S., Gonzalez, A. J., Sakurai, E., Damiani, E., Kutics, A., Knauf, R., & Frati, F. (2019). VICA, a visual counseling agent for emotional distress. Journal of Ambient Intelligence and Humanized Computing, 10(12), 4993-5005. doi:10.1007/ s12652-019-01180-x
36. Sharma, A., Lin, I. W., Miner, A. S., Atkins, D. C., & Althoff, T. (2023). Human-AI collaboration enables more empathic conversations in text-based peer-to-peer mental health support. Nature Machine Intelligence, 5(1), 46-57. doi:10.1038/s42256-022-00593-2
37. Bloodgood, M. & Vijay-Shanker K. (2014). Taking into account the differences between actively and passively acquired data: The case of active learning with support vector machines for imbalance datasets. CoRR. doi:10.48550/arXiv.1409.4835
38. Lossio-Ventura, J. A., Weger, R., Lee, A. Y., Guinee, E. P., Chung, J., Atlas, L., Linos, E., & Pereira, F. (2024). A comparison of ChatGPT and fine-tuned open pre-trained transformers (OPT) against widely used sentiment analysis tools: Sentiment analysis of COVID-19 survey data. JMIR Mental Health, 11. doi:10.2196/50150
39. Akçay, M. B., & Oğuz, K. (2020). Speech emotion recognition: Emotional models, databases, features, preprocessing methods, supporting modalities, and classifiers. Speech Communication, 116, 56-76. doi:10.1016/j.specom.2019.12.001
40. Masson, A., Cazenave, G., Trombini, J., & Batt, M. (2020). The current challenges of automatic recognition of facial expressions: A systematic review. AI Communications, 33(3-6), 113-138. doi:10.3233/aic-200631
41. Kang, E. B. (2023). On the Praxes and politics of Ai Speech Emotion Recognition. 2023 ACM Conference on Fairness, Accountability, and Transparency, 8, 455-466. doi:10.1145/3593013.3594011
42. Malgaroli, M., Hull, T. D., Zech, J. M., & Althoff, T. (2023). Natural language processing for mental health interventions: A systematic review and research framework. Translational Psychiatry, 13(1), 309. doi:10.1038/s41398-023-02592-2
43. Sweeney, C., Potts, C., Ennis, E., Bond, R., Mulvenna, M. D., O’neill, S., Malcolm, M., Kuosmanen, L., Kostenius, C., Vakaloudis, A., Mcconvey, G., Turkington, R., Hanna, D., Nieminen, H., Vartiainen, A.-K., Robertson, A., & Mctear, M. F. (2021). Can chatbots help support a person’s mental health? Perceptions and views from mental healthcare professionals and experts. ACM Transactions on Computing for Healthcare, 2(3), 1-15. doi:10.1145/3453175
44. Minerva, F., & Giubilini, A. (2023). Is AI the future of mental healthcare? Topoi, 42(3), 809-817. doi:10.1007/s11245-023-09932-3
45. Torous, J., Bucci, S., Bell, I. H., Kessing, L. V., Faurholt Jepsen, M., Whelan, P., Carvalho, A. F., Keshavan, M., Linardon, J., & Firth, J. (2021). The growing field of digital psychiatry: Current evidence and the future of apps, social media, chatbots, and virtual reality. World Psychiatry, 20(3), 318-335. doi:10.1002/wps.20883
46. Meadows, R., Hine, C., & Suddaby, E. (2020). Conversational agents and the making of mental health recovery. Digital Health, 6. doi.10.1177/2055207620966170
47. Stubbe, D. E. (2018). The therapeutic alliance: The fundamental element of psychotherapy. Focus, 16(4), 402-403. doi:10.1176/appi.focus.20180022
48. Ratajska, A., Brown, M. I., & Chabris, C. F. (2020). Attributing social meaning to animated shapes: A new experimental study of apparent behavior. The American Journal of Psychology, 133(3), 295312. doi:10.5406/amerjpsyc.133.3.0295
49. Bickmore, T. W., Mitchell, S. E., Jack, B. W., Paasche-Orlow, M. K., Pfeifer, L. M., & O’Donnell, J. (2010). Response to a relational agent by hospital patients with depressive symptoms. Interacting with Computers, 22(4), 289-298. doi:10.1016/j.intcom.2009.12.001
50. Araujo, T. (2018). Living up to the chatbot hype: The influence of anthropomorphic design cues and communicative agency framing on conversational agent and company perceptions. Computers in Human Behavior, 85, 183-189. doi:10.1016/j. chb.2018.03.051
51. Liu, B., & Sundar, S. S. (2018). Should machines express sympathy and empathy? Experiments with a health advice chatbot. Cyberpsychology, Behavior, and Social Networking, 21(10), 625-636. doi:10.1089/cyber.2018.0110
52. Ho, A., Hancock, J., & Miner, A. S. (2018). Psychological, relational, and emotional effects of self-disclosure after conversations with a chatbot. Journal of Communication, 68(4), 712-733. doi:10.1093/joc/jqy026
53. Lee, S., Lee, N., & Sah, Y. J. (2019). Perceiving a mind in a chatbot: Effect of mind perception and social cues on co-presence, closeness, and intention to use. International Journal of Human-Computer Interaction, 36(10), 930-940. doi:1 0.1080/10447318.2019.1699748
54. Heppner, H., Schiffhauer, B., & Seelmeyer, U. (2024). Conveying chatbot personality through conversational cues in social media messages. Computers in Human Behavior: Artificial Humans, 2(1). doi:10.1016/j.chbah.2024.100044
55. Janson, A. (2023). How to leverage anthropomorphism for chatbot service interfaces: The interplay of communication style and personification. Computers in Human Behavior, 149, 1-17. doi:10.1016/j.chb.2023.107954
56. Rubin, M., Arnon, H., Huppert, J. D., & Perry, A. (2024). Considering the Role of Human Empathy in AI-Driven Therapy. JMIR Mental Health, 11, e56529. doi:10.2196/56529
57. Sharma, A., Miner, A., Atkins, D., & Althoff, T. (2020). A computational approach to understanding empathy expressed in text-based mental health support. In: Webber, B., Cohn, T., He, Y., & Liu, Y. (Eds.). Proceedings of the 2020 Conference on Empirical Methods in Natural Language Processing (EMNLP), 5263-5276. Association for Computational Linguistics. doi:10.18653/v1/2020. emnlp-main.425
58. de Gennaro, M., Krumhuber, E. G., & Lucas, G. (2020). Effectiveness of an empathic chatbot in combating adverse effects of social exclusion on mood. Frontiers in Psychology, 10. doi:10.3389/ fpsyg.2019.03061
59. Lucas, G. M., Gratch, J., King, A., & Morency, L.P. (2014). It’s only a computer: Virtual humans increase willingness to disclose. Computers in Human Behavior, 37, 94-100. doi:10.1016/j. chb.2014.04.043
60. Kang, S., & Gratch, J. (2010). Virtual humans elicit socially anxious interactants’ verbal self disclosure. Computer Animation and Virtual Worlds, 21(3-4), 473-482. doi:10.1002/cav.345
61. Tian, Q. (2013). Social anxiety, motivation, self-disclosure, and computer-mediated friendship: A path analysis of the social interaction in the blogosphere. Communication Research, 40(2), 237-260. doi:10.1177/0093650211420137
62. Laban, G., Kappas, A., Morrison, V., & Cross, E. S. (2023). Opening up to social robots: How emotions drive self-disclosure behavior. In: 2023 32nd IEEE International Conference on Robot and Human Interactive Communication, 1697-1704. IEEE. doi:10.1109/ROMAN57019.2023.10309551
63. Lee, J., Lee, J., & Lee, D. (2022). Influence of rapport and social presence with an AI psychotherapy chatbot on users’ self-disclosure. SSRN Electronic Journal. doi:10.2139/ssrn.4063508
64. Provoost, S., Lau, H. M., Ruwaard, J., & Riper, H. (2017). Embodied conversational agents in clinical psychology: A scoping review. Journal of Medical Internet Research, 19(5). doi:10.2196/ jmir.6553
65. Scholten, M. R., Kelders, S. M., & Van Gemert-Pijnen, J. E. (2017). Self-Guided web-based interventions: Scoping review on user needs and the potential of embodied conversational agents to address them. Journal of Medical Internet Research, 19(11). doi:10.2196/jmir.7351
66. D’Alfonso S., Lederman R., Bucci S., Berry K. (2020). The digital therapeutic alliance and human-computer interaction. JMIR Mental Health, 7(12). doi:10.2196/21895
67. Potdevin, D., Clavel, C., & Sabouret, N. (2021). Virtual intimacy in human-embodied conversational agent interactions: The influence of multimodality on its perception. Journal on Multimodal User Interfaces, 15(1), 25-43. doi:10.1007/s12193-02000337-9
68. Parmar, D., Olafsson, S., Utami, D., Murali, P., & Bickmore, T. (2022). Designing empathic virtual agents: Manipulating animation, voice, rendering, and empathy to create persuasive agents. Autonomous Agents and Multi-Agent Systems, 36(1), 17. doi:10.1007/s10458-021-09539-1
69. Tielman, M. L., Neerincx, M.A., van Meggelen, M., Franken, I., & Brinkman, W. P. (2017). How should a virtual agent present psychoeducation? Influence of verbal and textual presentation on adherence. Technology and Health Care, 25(6), 1081-1096. doi:10.3233/THC-170899
70. Kang, E., & Kang, Y. A. (2023). Counseling chatbot design: The effect of anthropomorphic chatbot characteristics on user self-disclosure and companionship. International Journal of Human-Computer Interaction, 40(11), 2781-2795. doi :10.1080/10447318.2022.2163775
71. Ciechanowski, L., Przegalinska, A., Magnuski, M., & Gloor, P. (2019). In the shades of the uncanny valley: An experimental study of human-chatbot interaction. Future Generation Computer Systems, 92, 539-548. doi:10.1016/j. future.2018.01.055
72. Ahmad, R., Siemon, D., Gnewuch, U., & Robra-Bissantz, S. (2022). Designing personality-adaptive conversational agents for mental health care. Information Systems Frontiers, 24(3), 923-943. doi:10.1007/s10796-022-10254-9
73. Khawaja, Z., & Bélisle-Pipon, J. C. (2023). Your robot therapist is not your therapist: Understanding the role of AI-powered mental health chatbots. Frontiers in Digital Health, 5. doi:10.3389/ fdgth.2023.1278186
74. Gabriel, S., Puri, I., Xu, X., Malgaroli, M., & Ghassemi, M. (2024). Can AI relate: Testing large language model response for mental health support. arXiv [Cs.CL]. doi:10.48550/arXiv.2405.12021
75. Montemayor, C., Halpern, J., & Fairweather, A. (2021). In principle obstacles for empathic AI: Why we can’t replace human empathy in healthcare. AI & Society, 37(4), 1353-1359. doi:10.1007/ s00146-021-01230-z
76. Sağlam, R. B., & Nurse, J. R. C. (2020). Is your chatbot GDPR compliant? Open issues in agent design. In: CUI ‘20: Proceedings of the 2nd Conference on Conversational User Interfaces, 1-3. Association for Computing Machinery. doi:10.1145/3405755.3406131
FEATURED THE STAKES OF STIMULANTS: HOW AMPHETAMINE MISUSE CAN INDUCE PSYCHOSIS IN A COLLEGIATE ENVIRONMENT
1. Fiorentini, A., Cantù, F., Crisanti, C., Cereda, G., Oldani, L., & Brambilla, P. (2021). Substance-induced psychoses: An updated literature review. Frontiers in Psychiatry, 12, 694863. doi:10.3389/ fpsyt.2021.694863
2. Rognli, E.B., Bramness, J.G. (2015) Understanding the relationship between amphetamines and psychosis. Current Addiction Reports, 2, 285–292. doi:10.1007/s40429-015-0077-4
3. Edinoff, A. N., Nix, C. A., McNeil, S. E., Wagner, S. E., Johnson, C. A., Williams, B. C., Cornett, E. M., Murnane, K. S,; Kaye, A. M., & Kaye, A. D. (2022) Prescription stimulants in college and medical students: A narrative review of misuse, cognitive impact, and adverse effects. Psychiatry International, 3(3), 221-235. doi:10.3390/psychiatryint3030018
4. Welsh, J. W., Shentu, Y., & Sarvey, D. B. (2019). Substance use among college students. Focus,17(2), 117–127. doi:10.1176/appi.focus.20180037
5. Rosenthal, E.,& Ahmed, A.O. (2020). Psychosis. In: Zeigler-Hill, V., Shackelford, T.K. (Eds.) Encyclopedia of Personality and Individual Differences, 4196-4201. Springer, Cham. doi:10.1007/978-3319-24612-3_934
6. Gaebel, W., & Zielasek, J. (2015). Focus on psychosis. Dialogues in Clinical Neuroscience, 17(1), 9–18. doi:10.31887/DCNS.2015.17.1/wgaebel
7. Heckers, S., & Konradi, C. (2015). GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophrenia Research, 167(1-3), 4–11. https://doi.org/10.1016/j.schres.2014.09.041
8. American Psychiatric Association. (2022). Schizophrenia spectrum and other psychotic disorders. In: Diagnostic and Statistical Manual of Mental Disorders (5th ed., text rev.). doi:10.1176/appi. books.9780890425787
9. Moran, L. V., Ongur, D., Hsu, J., Castro, V. M., Perlis, R. H., & Schneeweiss, S. (2019). Psychosis with methylphenidate or amphetamine in Patients with ADHD. The New England Journal of Medicine, 380(12), 1128–1138. doi:10.1056/NEJMoa1813751
10. Hasenhuetl, P. S., Bhat, S. ,Freissmuth, M., & Sandtner, W. (2019). A Concept for atypical monoamine transporter pharmacology. Molecular Pharmacology, 95(3) 303-312; doi:doi:10.1124/ mol.118.114793
11. Mohebi, A, & Berke, J.D. (2020) Dopamine release drives motivation, independently from dopamine cell firing. Neuropsychopharmacology, 45(1). doi:10.1038/s41386-019-0492-7.
12. Juárez Olguín, H., Calderón Guzmán, D., Hernández García, E., & Barragán Mejía, G. (2016). The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxidative Medicine and Cellular Longevity, 2016(1). doi:10.1155/2016/9730467
13. Ilipilla, G., & Arnold, L. E. (2024). The role of adrenergic neurotransmitter reuptake inhibitors in the ADHD armamentarium. Expert Opinion on Pharmacotherapy, 25(8), 945–956. https://doi.org /10.1080/14656566.2024.2369197
14. Basmadjian, O. M., Occhieppo, V. B., Montemerlo, A. E., Rivas, G. A., Rubianes, M. D., Baiardi, G., & Bregonzio, C. (2024). Angiotensin II involvement in the development and persistence of amphetamine-induced sensitization: Striatal dopamine reuptake implications. European Journal of Neuroscience, 59(10), 2450–2464. doi:10.1111/ejn.16312
15. Harris, H. N., & Peng, Y. B. (2020). Evidence and explanation for the involvement of the nucleus accumbens in pain processing. Neural Regeneration Research, 15(4), 597–605. doi:10.4103/16735374.266909
16. Ashok, A. H., Mizuno, Y., Volkow, N. D., & Howes, O. D. (2017) Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: A systematic review and meta-analysis. JAMA Psychiatry, 74(5), 511–519. doi:10.1001/jamapsychiatry.2017.0135
17. Kohno, M., Dennis, L. E., McCready, H., & Hoffman, W. F. (2022). Dopamine dysfunction in stimulant use disorders: Mechanistic comparisons and implications for treatment. Molecular Psychiatry, 27(1), 220–229. doi:10.1038/s41380021-01180-4
18. Nawaratne, V., McLaughlin, S. P., Mayer, F. P., Gichi, Z., Mastriano, A., & Carvelli, L. (2021). Prolonged amphetamine exposures increase the endogenous human dopamine receptors 2 at the cellular membrane in cells lacking the dopamine transporter. Frontiers in Cellular Neuroscience, 15. doi>10.3389/fncel.2021.681539
19. Koob, G. F., & Volkow, N. D. (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760–773. doi:10.1016/S22150366(16)00104-8
20. Sherrill, L. K., & Gulley, J. M. (2018). Effects of amphetamine exposure during adolescence on behavior and prelimbic cortex neuron activity in adulthood. Brain Research, 1694, 111–120. doi:10.1016/j.brainres.2018.05.028
21. Reynolds, L. M., Makowski, C. S., Yogendran, S. V., Kiessling, S., Cermakian, N., & Flores, C. (2015). Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a DCC-dependent manner. Neuropsychopharmacology, 40(5), 1101–1112. doi:10.1038/npp.2014.287
22. Orsini, C. A., Heshmati, S. C., Garman, T. S., Wall, S. C., Bizon, J. L., & Setlow, B. (2018). Contributions of medial prefrontal cortex to decision making involving risk of punishment. Neuropharmacology, 139, 205–216. doi:10.1016/j.neuropharm.2018.07.018
23. Jiao, D., Liu, Y., Li, X., Liu, J., & Zhao, M. (2015). The role of the GABA system in amphetamine-type stimulant use disorders. Frontiers in Cellular Neuroscience, 9, 162. doi:10.3389/fncel.2015.00162
24. Wu, C., & Sun, D. (2015). GABA receptors in brain development, function, and injury. Metabolic Brain Disease, 30(2), 367–379. doi:10.1007/s11011014-9560-1
25. AlOtaibi, S., Emara, A., & Elsisi, H. (2024). Mechanisms of psychiatric disorders induced by amphetamines: A comprehensive review. International Journal of Science and Research Archive, 11(1), 260-274. doi:10.30574/ijsra.2024.11.1.0007.
26. Javitt D. C. (2023). Cognitive impairment associated with schizophrenia: From pathophysiology to treatment. Annual Review of Pharmacology and Toxicology, 63, 119–141. doi:10.1146/annurev-pharmtox-051921-093250
27. Li, M. H., Underhill, S. M., Reed, C., Phillips, T. J., Amara, S. G., & Ingram, S. L. (2017). Amphetamine and methamphetamine increase NMDAR-GluN2B synaptic currents in midbrain dopamine neurons. Neuropsychopharmacology, 42(7), 1539–1547. doi:10.1038/npp.2016.278
28. Brisch, R., Saniotis, A., Wolf, R., Bielau, H., Bernstein, H. G., Steiner, J., Bogerts, B., Braun, K., Jankowski, Z., Kumaratilake, J., Henneberg, M., & Gos, T. (2014). The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Frontiers in Psychiatry, 5, 47. doi:10.3389/fpsyt.2014.00047
29. Doyle, C. A., & McDougle, C. J. (2021). Dopamine. In: Volkmar, F.R. (Eds.). Encyclopedia of Autism Spectrum Disorders. Springer, Cham. doi:10.1007/978-3-319-91280-6_823
30. McCutcheon, R. A., Abi-Dargham, A., & Howes, O. D. (2019). Schizophrenia, dopamine and the striatum: From biology to symptoms. Trends in Neurosciences, 42(3), 205–220. doi:10.1016/j. tins.2018.12.004
31. Sotoyama, H. (2024). Putative neural mechanisms underlying release-mode-specific abnormalities in dopamine neural activity in a schizophrenia-like model: The distinct roles of glutamate and serotonin in the impaired regulation of dopamine neurons. European Journal of Neuroscience, 59(6), 1194–1212. doi:10.1111/ejn.16123
32. Lin, S. H., Lee, L. T., & Yang, Y. K. (2014). Serotonin and mental disorders: A concise review on molecular neuroimaging evidence. Clinical Psychopharmacology and Neuroscience, 12(3), 196–202. doi:10.9758/cpn.2014.12.3.196
33. Quednow, B. B., Geyer, M. A., Halberstadt, A. L. (2020). Chapter 39 - Serotonin and schizophrenia. In: Müller, C. P., & Cunningham, K. A. (Eds.). Handbook of Behavioral Neuroscience, 711-743. Elsevier. doi:10.1016/B978-0-444-64125-0.000396.
34. Ham, S., Kim, T. K., Chung, S., & Im, H. I. (2017). Drug abuse and psychosis: New insights into drug-induced psychosis. Experimental Neurobiology, 26(1), 11–24. doi:10.5607/en.2017.26.1.11
35. Howes, O., McCutcheon, R., & Stone, J. (2015). Glutamate and dopamine in schizophrenia: An update for the 21st century. Journal of Psychopharmacology (Oxford, England), 29(2), 97–115. doi:10.1177/0269881114563634
36. Correll, C. U., & Schooler, N. R. (2020). Negative symptoms in schizophrenia: A review and clinical guide for recognition, assessment, and treatment. Neuropsychiatric Disease and Treatment, 16, 519–534. doi:10.2147/NDT.S225643
37. Alexander, P. D., Gicas, K. M., Cheng, A. Lang, D. J., Procyshyn, R. M., Vertinsky, A. T., Panenka, W. J., Thornton, A. E., Rauscher, A., Wong, J. Y. X., Chan, T., Jones, A. A., Vila-Rodriguez, F., Honer, W. G., & Barr, A. M. (2019). A comparison of regional brain volumes and white matter connectivity in subjects with stimulant induced psychosis versus schizophrenia. Psychopharmacology, 236, 3385–3399. doi:10.1007/s00213-019-05298-w
38. Fluyau, D., Mitra, P., & Lorthe, K. (2019). Antipsychotics for amphetamine psychosis. A systematic review. Frontiers in Psychiatry, 10, 740. doi:10.3389/fpsyt.2019.00740
39. Baldaçara, L., Ramos, A., & Castaldelli-Maia, J. M. (2023). Managing drug-induced psychosis. International Review of Psychiatry, 35(5–6), 496–502. doi:10.1080/09540261.2023.2261544
40. Murrie, B., Lappin, J., Large, M., & Sara, G. (2020). Transition of substance-induced, brief, and atypical psychoses to schizophrenia: A systematic review and meta-analysis. Schizophrenia Bulletin, 46(3), 505–516. doi:10.1093/schbul/sbz102
41. Khokhar, J. Y., Dwiel, L. L., Henricks, A. M., Doucette, W. T., & Green, A. I. (2018). The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophrenia Research, 194, 78–85. doi:10.1016/j. schres.2017.04.016
42. Zhan, N., Sham, P. C., So, H. C., & Lui, S. S. Y. (2023). The genetic basis of onset age in schizophrenia: Evidence and models. Frontiers in Genetics, 14. doi:10.3389/fgene.2023.1163361
43. Board, A. R., Guy, G., Jones, C. M., & Hoots, B. (2020). Trends in stimulant dispensing by age, sex, state of residence, and prescriber specialty - United States, 2014–2019. Drug and Alcohol Dependence, 217(1). doi:10.1016/j.drugalcdep.2020.108297
44. Arria, A. M., Caldeira, K. M., Vincent, K. B., O’Grady, K. E., Cimini, M. D., Geisner, I. M., Fossos-Wong, N., Kilmer, J. R., & Larimer, M. E. (2017). Do college students improve their grades by using prescription stimulants nonmedically? Addictive Behaviors, 65, 245–249. doi:10.1016/j. addbeh.2016.07.016
45. Hajdúk, M., Dančík, D., Januška, J., Svetský, V., Straková, A., Turček, M., Vašečková, B., Forgáčová, Ľ., Heretik, A., & Pečeňák, J. (2020). Psychotic experiences in student population during the COVID-19 pandemic. Schizophrenia Research, 222, 520–521. doi:10.1016/j.schres.2020.05.023
46. Hatoum, A. S., Colbert, S. M. C., Johnson, E. C. Huggett, S. B., Deak, J. D., Pathak, G. A., Jennings, M. V., Paul, S. E., Karcher, N. R., Hansen, I., Baranger, D. A. A., Edwards, A., Grotzinger, A. D., Tucker-Drob, E. M., Kranzler, H. R., Davis, L. K., Sanchez-Roige, S., Polimanti, R., Gelernter, J., Edenberg, H. J., Bogdan, R., & Agrawal, A. (2023). Multivariate genome-wide association meta-analysis of over 1 million subjects identifies loci underlying multiple substance use disorders. Nature Mental Health, 1, 210-223. doi:10.1038/ s44220-023-00034-y
47. Cole, V. T., & Hussong, A. M. (2020). Psychosocial functioning among college students who misuse stimulants versus other drugs. Addictive Behaviors, 105. doi:10.1016/j.addbeh.2020.106290
48. Huang, C. L., Tsai, I. J., & Lee, C. W. (2022). Risk of psychosis in illicit amphetamine users: a 10 year retrospective cohort study. Evidence-based Mental Health, 25(4), 163–168. doi:10.1136/ebmental-2021-300300
49. Ronsley, C., Nolan, S., Knight, R., Hayashi, K., Klimas, J., Walley, A., Wood, E., & Fairbairn, N. (2020) Treatment of stimulant use disorder: A systematic review of reviews. PLoS ONE, 15(6). doi:10.1371/journal.pone.0234809
50. Amin-Esmaeili, M., Farokhnia, M., Susukida, R., Leggio, L., Johnson, R. M., Crum, R. M., & Mojtabai, R. (2024). Reduced drug use as an alternative valid outcome in individuals with stimulant use disorders: Findings from 13 multisite randomized clinical trials. Addiction, 119(5), 833–843. doi:10.1111/add.16409
51. Servonnet, A., & Samaha, A. N. (2020). Antipsychotic-evoked dopamine supersensitivity. Neuropharmacology, 163. doi:10.1016/j.neuropharm.2019.05.007
52. Groom, M. J., & Cortese, S. (2022). Current pharmacological treatments for ADHD. In: Stanford, S. C., & Sciberras, E. (Eds.). New Discoveries in the Behavioral Neuroscience of Attention-Deficit Hyperactivity Disorder. Springer, Cham. doi:10.1007/7854_2022_330
53. Harada, T., Tsutomi, H., Mori, R., & Wilson, D. B. (2018). Cognitive-behavioural treatment for amphetamine-type stimulants (ATS)-use disorders. The Cochrane Database of Systematic Reviews, 12(12). doi:10.1002/14651858.CD011315.pub2
54. Nakao, M., Shirotsuki, K., & Sugaya, N. (2021). Cognitive-behavioral therapy for management of mental health and stress-related disorders: Recent advances in techniques and technologies. BioPsychoSocial Medicine, 15(1). doi:10.1186/ s13030-021-00219-w
55. Rash, C. J., Stitzer, M., & Weinstock, J. (2017). Contingency management: New directions and remaining challenges for an evidence-based intervention. Journal of Substance Abuse Treatment, 72, 10–18. doi:10.1016/j.jsat.2016.09.008
56. Kennedy S. (2018). Raising awareness about prescription and stimulant abuse in college students through on-campus community involvement projects. Journal of Undergraduate Neuroscience Education, 17(1), A50–A53. PMID:30618499

