






The latest evidence on growth factors and how to incorporate ® into your practice
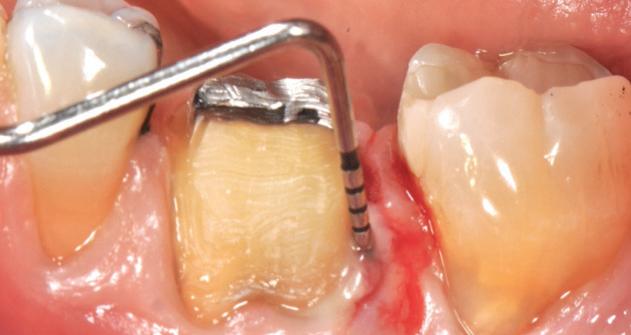
nt, GEM S® was used to fill the defect.
The evidenced based conclusions following an extensive assessment of 385 articles of which 153 met the inclusion criteria and were analyzed qualitatively with 150 studies providing data for the network meta-analysis were as follows:
Bio- Gide®.
Based on an analysis of the current evidence and expert opinion, the panel concluded that the appropriate use of biologics in periodontal practice is generally safe and provides added benefits to conventional treatment approaches.
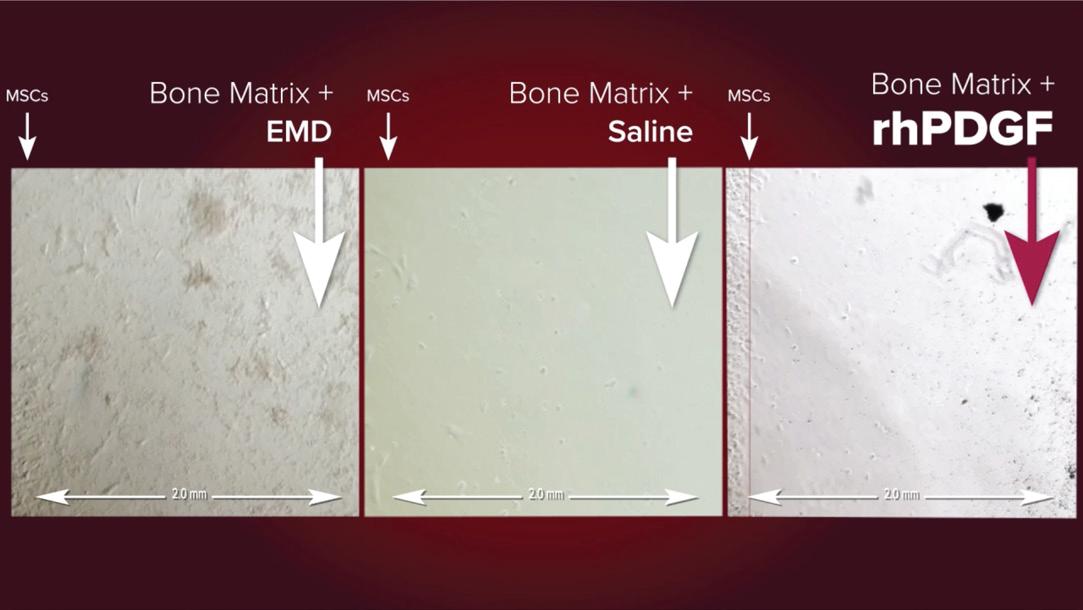
The selection of the type of Bone Graft (autogenous, allogeneic, xenogeneic or synthetic BG) and the type of biologic agent (EMD, PRF, PRP or rhPDGF-BB) plays an important role on the final results, with rhPDGF-BB [or] PRF associated with superior clinical and radiographic outcomes compared to PRP and EMD, and rhPDGF- BB exhibiting the largest effect size for most parameters.²
REV I E W
O b j e c t i v e s :
The Science Behind
Superior long-term stability, periodontal health, and esthetics after surgical treatment of infrabony defects have been demonstrated with the use of some biologic agents (i.e., EMD and rhPDGF-BB).¹
MA S T E R
“ Techniques for Achieving Optimum Soft Tissue and Bone Healing for Esthetics and Function Around Teeth and U N DER S TA N D
The latest peer-reviewed clinical evidence on the efficacy
LEA R N
Clinical Case Reports
ed the et depth ecession and
Overall, our findings revealed that the addition of biologic agents to BG (bone graft) materials significantly enhances the clinical (CAL gain, PD red, REC change) and radiographic (rBF and rLBG) outcomes of periodontal regeneration as compared to BGs alone and flap procedures.²
The goal of the procedure was the lost hard tissue suppor to al low for a significantly term prognosis. The evidenced-based usage of GEM ® as par t protocol has been documen that: hard and soft tissue regener intrabony de ec
COM MU N I C AT E
Tips for navigating patient FAQs



R E G T E
Educa Events
Generally accepted to be a mediator of tissue repair, the use of growth factors in periodontology and oral surger y is well documented over several decades of research. As a pioneer in the effort to translate regenerative growth factor research into practical applications, Dr. Samuel Lynch focused on the body’s ability to heal and what factors enabled the body to do so His breakthrough efforts resulted in the development of the recombinant human platelet-derived growth factor BB (rhPDGF-BB)
® , for the treatment of certain osseous defects and gingival recession caused by peridontal disease.¹

Dr. Lynch talks about some of his earliest experiences in the lab and what
as the only off-the-shelf source of purified rhPDGF approved for bone and soft tissue regeneration.
once the cells migrate to the site. This cellular activity of mitogenesis stimulates angiogenesis, resulting in more rapid healing and new bone formation. This cascade of events is highlighted by…










by signaling and recruiting the specific cells that the body needs to regenerate bone and the surrounding tissues (chemotaxis).
as one of the most researched growth factors in dentistr y. It has been proven to be safe and effective in regenerating bone and soft tissue in more than

With the use of biologics progressively becoming a core component of contemporar y periodontal practice, the goal of the American Academy of Periodontology (AAP) best evidence consensus (BEC) was to provide a state-of-the-art, evidence-based perspective on the therapeutic application of autologous blood-derived products (ABPs), enamel matrix derivative (EMD), recombinant human platelet-derived growth factor BB (rhPDGF-BB), and recombinant human bone morphogenetic protein 2 (rhBMP-2). The purpose was to address their safety, indications, and effectiveness in specific clinical scenarios.
The evidenced based conclusions following an extensive assessment of articles of which met the inclusion criteria and were analysed qualitatively with studies providing data for the network meta-analysis were as follows:
Based on an analysis of the current evidence and expert opinion, the panel concluded that the appropriate use of biologics in periodontal practice is generally safe and provides added benefits to conventional treatment approaches.
The selection of the type of Bone Graft (autogenous, allogeneic, xenogeneic or synthetic BG) and the type of biologic agent (EMD, PRF, PRP or rhPDGF-BB) plays an important role on the final results, with rhPDGF-BB [or] PRF associated with superior clinical and radiographic outcomes compared to PRP and EMD, and rhPDGF-BB exhibiting the largest effect size for most parameters.²
Superior long-term stability, periodontal health, and esthetics after surgical treatment of infrabony defects have been demonstrated with the use of some biologic agents (i.e., EMD and rhPDGF-BB).¹
Overall, rhPDGF-BB exhibited the largest effect size for most parameters, including clinical attachment level gain, pocket depth reduction, less gingival recession and radiographic linear bone gain.²
Overall, our findings revealed that the addition of biologic agents to BG (bone graft) materials significantly enhances the clinical (CAL gain, PD red, REC change) and radiographic (rBF and rLBG) outcomes of periodontal regeneration as compared to BGs alone and flap procedures.²
“

Discuss outcomes and how it relates to what they do in practice.
Explain what kind of attachments are they gaining.
Examine stability in the long term.
year old female patient presented with clinical and radiographic evidence of an mm.
The crown was biologically shaped and the root detoxified using Ellman burs. After flap opening and complete defect debridement, GEM S® was used to fill the defect.
The graft was then covered with Geistlich Bio-Gide®.
year old female patient presented with clinical and radiographic evidence of an mm
O bje c t i v e s :
The crown was biologically shaped and the root detoxified using Ellman burs. After flap opening and complete defect debridement, GEM S® was used to fill the defect.
The graft was then covered with Geistlich Bio-Gide®.
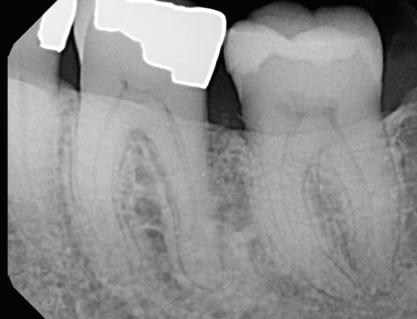
Base line
line




Post-Op



The goal of the procedure was to restore the lost hard tissue support ie. periodontal
O bje c t i v e s :
The goal of the procedure was to restore the lost hard tissue support ie. periodontal
to allow for a significantly improved longterm prognosis. The evidenced-based usage of GEM ® as part of our surgical protocol has been documented to do just that: hard and soft tissue regeneration in intrabony defects.
C on c lu s i o n s :
to allow for a significantly improved longterm prognosis. The evidenced-based usage of GEM ® as part of our surgical protocol has been documented to do just that: hard and soft tissue regeneration in intrabony defects.
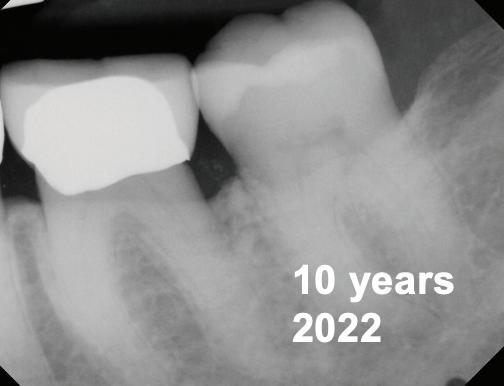
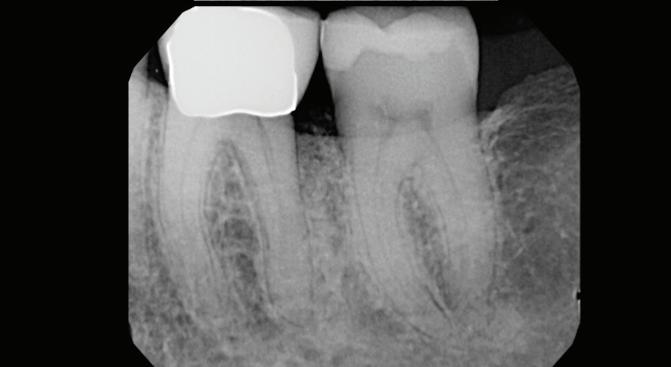
-year follow-up radiographs showed evidence of bone fill. Clinically, maturation of interdental tissue is evident as well.
C on c lu s i o n s :
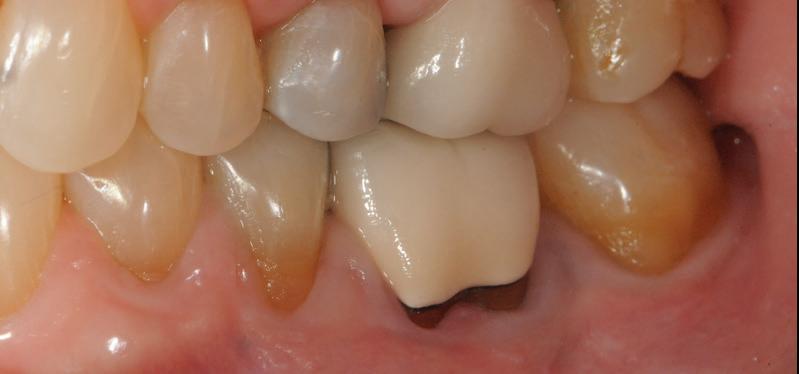
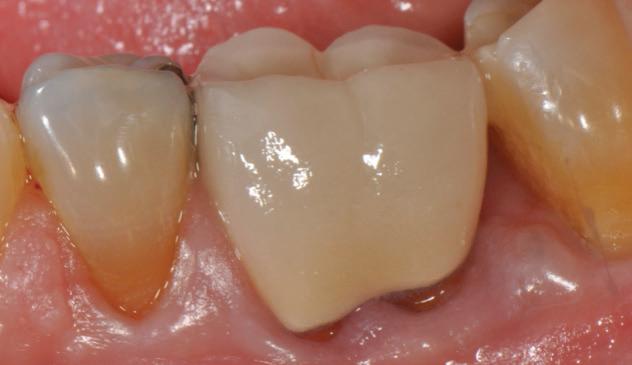
-year follow up clinical photos are demonstrating stability of bone, lack of facial and minimal interproximal recession.
-year follow-up radiographs showed evidence of bone fill. Clinically, maturation of interdental tissue is evident as well
Stability of the bone radiographically is evident as well.
-year follow up clinical photos are demonstrating stability of bone, lack of facial and minimal interproximal recession. Stability of the bone radiographically is evident as well.
® did not only enhance the outcome of this challenging case, but ensured its st ability
® did not only enhance the outcome of this challenging case, but ensured its st ability
– Dr Robert Levine
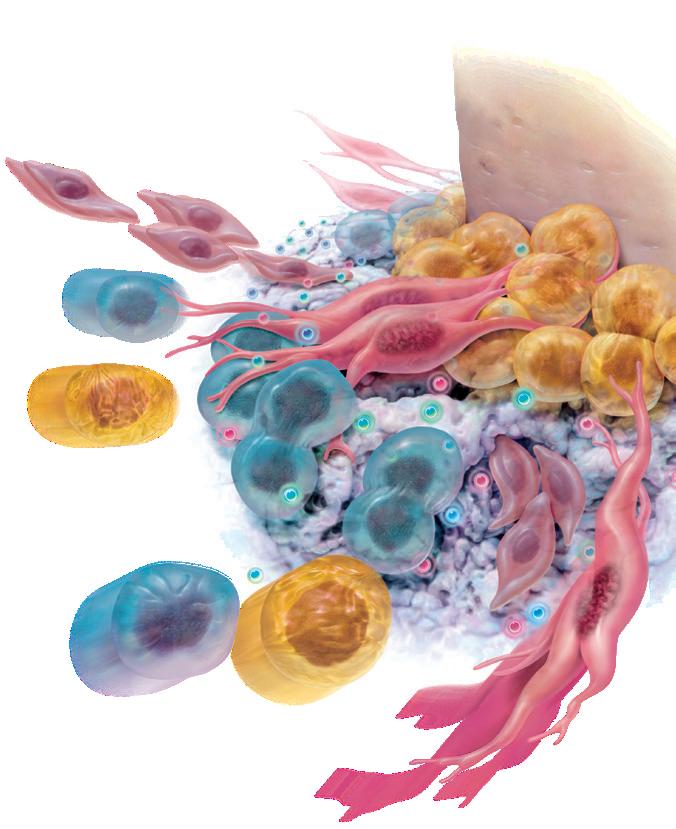
The evidenced-based usage of GEM ® as part of our surgical protocol helped the restoration of both hard and soft tissue healing and supported

The goal of the procedure was to restore the lost hard tissue support ie. periodontal
Excellent compliance to supportive periodontal therapy and plaque control by our patient ensured these lasting results held up
The evidenced-based usage of GEM ® as part of our surgical protocol helped the restoration of both hard and supported
Excellent compliance to supportive periodontal therapy and plaque control by our patient ensured these


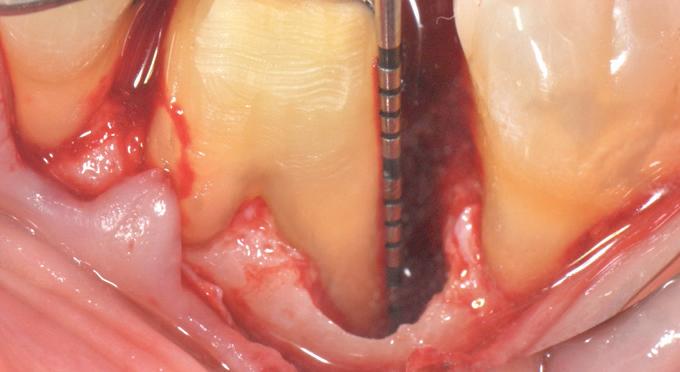
F l ap re fle c tio n sh o wing the d o n the dis t al o ≥ m m poc ket d epth

to allow for a significantly improved longterm prognosis. The evidenced-based usage of GEM ® as part of our surgical protocol has been documented to do just that: hard and soft tissue regeneration in intrabony defects. C o n cl usi o ns :
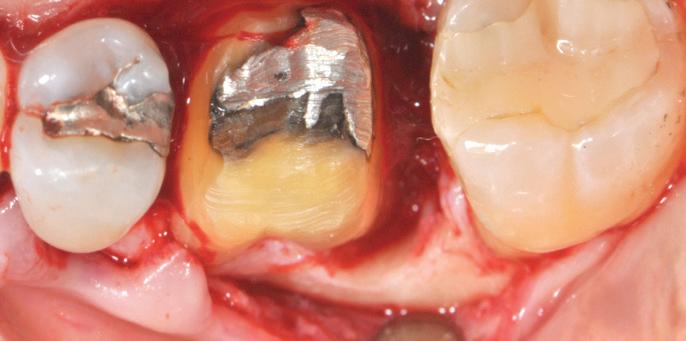
al view
-year follow-up radiographs showed evidence of bone fill. Clinically, maturation of interdental tissue is evident as well.
-year follow up clinical photos are demonstrating stability of bone, lack of facial and minimal interproximal recession. Stability of the bone radiographically is evident as well.
– Dr Rober t Levine p
® did not only enhance the outcome of this chal lenging case, but ensured its st ability

Individual results vary. This experience is specific to this patient only
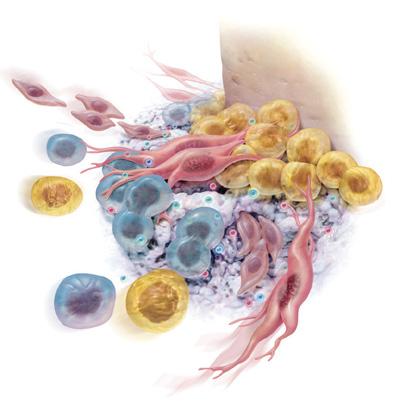
GEM S® Growth-Factor Enhanced Matrix is a synthetic bone graft composed of pure, sterile bioengineered platelet-derived growth factor (PDGF) and a bone graft. PDGF is naturally found in our bodies (present in the platelets in your blood) and is often referred to as nature’s wound healing protein. Whenever you experience a wound or surger y, your blood clots and platelets release PDGF (along with other proteins), stimulating the healing process. Unfortunately, as we grow older and with certain conditions, the amount of naturally occurring PDGF declines so we no longer heal as we did previously. GEM S® replenishes the amount of PDGF at the grafted site to aid in faster, better healing.
Since GEM S® will be implanted under your gums, you won’ t be able to see the powerful effects of the treatment as it occurs. The illustrations that follow describe how GEM S® speeds up your body’s natural healing process. (Fig )
Your doctor will begin by thoroughly cleaning the area to be treated. Once properly prepared, GEM S® is used to fill the area where bone and other supporting structures have been lost. GEM S® acts by signaling your body to actively begin to heal and regenerate healthy bone and soft tissues. (Fig. )
GEM S® attracts the specific cells that your body needs to regenerate bone and the surrounding tissues, causing them to multiply in number. This increase in cellular activity results in a more rapid healing process.* (Fig. )
GEM S® is gradually replaced with your own bone and other normal tissues. Several months after treatment with GEM S®, healing is complete. (Fig. )
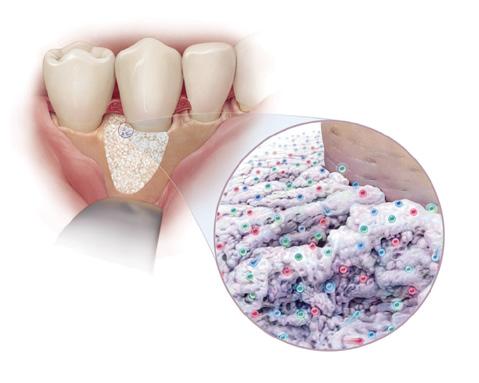
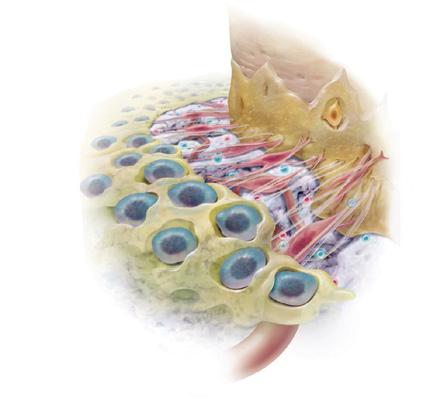
YES. GEM S® has been fully approved by FDA. In addition to being the only FDA-approved product available for use in dental surger y that contains pure PDGF (nature’s wound healing protein), GEM S® is also one of the most rigorously studied products in dentistr y over the last 20 years.
Your gums will typically heal within 1 -2 weeks. While GEM S® promotes rapid healing, it takes bone several months to mature into its final dense structure. The goal of treatment with GEM S® is restoration of a normal healthy condition (complete regeneration of the bone and soft tissues).
GEM S® will improve the health of the jaw bone and surrounding tissues that have been damaged as a result of periodontal disease. GEM S® is the only product available for use in dental surger y that contains pure PDGF to improve healing.
CONTRAINDICATIONS
As with any periodontal procedure where bone grafting material is ® is CONTRAINDICATED in the presence of one or more of the following clinical situations: untreated acute infections at the surgical site; untreated malignant neoplasm(s) at the surgical site; patients with a known hypersensitivity to any product component (ß-TCP or rhPDGF-BB); intraoperative soft tissue coverage is required for a given surgical procedure but such coverage is not possible; or conditions in which general bone grafting is not advisable.
WARNINGS
® has not been established: In patients with an active malignant neoplasm and should therefore not be used in such patients; in other non-periodontal bony locations, including other tissues of the oral and craniofacial region such as bone graft sites, tooth extraction sites, bone cavities after cystectomy, and bone defects resulting from traumatic or pathological origin. ® has also not been studied in situations where it would be AUGMENTing autogenous bone and other bone grafting materials; in pregnant and nursing women. It is not known whether rhPDGF-BB is excreted in the milk of nursing women; in pediatric patients below greater than Grade II or a Class III furcation; in patients with frequent or excessive use of tobacco products. Careful consideration should be given to alternative therapies prior to performing bone grafting in patients: who have severe endocrine-induced bone diseases (e.g hyperparathyroidism); who are receiving immunosuppressive therapy; or who have known conditions that may lead to bleeding complications (e.g. hemophilia).
® grafting material is intended to be placed into periodontally related defects. It must not be injected systemically
® is comparable to that of bone and ® ® must be considered when evaluating radiographs as it may mask underlying pathological conditions.

® contains becaplermin – a recombinantly produced, human platelet-derived growth factor, homodimer BB (rhPDGF-BB), which is a protein that has been shown to promote the formation of bone in periodontal defects. rhPDGF-BB (“PDGF”) is also the active ingredient of another FDA approved product, REGRANEX® Gel, which is a topical gel formulation, indicated for the treatment of lower extremity diabetic neuropathic ulcers.¹
An increased rate of mortality secondary to malignancy with use ® Gel) was demonstrated in a single study of its use in treatment of diabetic, neuropathic ulcers. Two subsequent studies did not demonstrate this increased rate. No relationship has been demonstrated regarding use of rhPDGF-BB in periodontal defects and malignancy or mortality secondary to malignancy
ADVERSE EVENTS
® ® may experience any of the following adverse events that have been reported in the literature with regard to periodontal surgical grafting procedures: swelling; pain; bleeding; hematoma; dizziness; fainting; difficulty breathing, eating, or speaking; sinusitis; headaches; increased tooth mobility; superficial or deep wound infection; cellulitis; wound dehiscence; neuralgia and loss of sensation locally and peripherally; and, anaphylaxis. Occurrence of one or more of these conditions may require an additional surgical procedure and may also require removal of the grafting material.
® matrix is a sterile, biocompatible, resorbable device for filling bone voids in dental, periodontal, and oral and maxillofacial surgery.
® Packet Insert
Geistlich Pharma Australia and New Zealand
The Zenith, Tower A, Level 21 821 Pacific Highway
Chatswood NSW 2067, Australia
Phone AU 1800 776 326
Phone NZ +64-(0)-800 500 043
Fax AU 1800 709 698
Fax NZ +64-(0)-800 500 044
www.geistlich.com/en-au