Dermatology



Interview:
EADV President, Branka Marinović, discusses the latest in dermatology and what’s next for the Congress
Editor's Pick:
Skin Intelligence: Advancing Cutaneous Resilience Through Biological Education, Microbial Symbiosis, and Eco-Integrated Approaches

Clinique was born in a dermatologist’s office.
Informed by decades of clinical experience, guided by dermatologists, scientists, and ophthalmologists.
Formulated with the perfect balance of ingredients for powerful efficacy with rigorous safety.
Our unwavering dedication is why millions around the world trust Clinique for solutions from skincare to makeup.
And you and your patients can, too.

Dermatologist guided solutions. Allergy tested. 100% fragrance free.


12 Review of the European Academy of Dermatology and Venereology (EADV) Congress 2025, 17th–20th September 2025
Congress Features
26 Lasers as Epigenetic Modulators: Reprogramming Skin Biology Toward Regeneration and Longevity – EADV 2025
Diala Haykal
31 Personalising Psoriasis Care: From Genetic Drivers to Dietary Interventions
Jenna Lorge
Symposium Reviews
35 Atopic Dermatitis at the European Academy of Dermatology and Venereology 2025: Updates and Insights
Poster Review
45 Two Years On: How Ruxolitinib is Shaping the Practical Management of Vitiligo
Abstract Reviews
52 Increased Incidence and Risk of Hair Loss with Glucagon-like Peptide 1 Receptor Agonists: A Real-World Multicentre Cohort Study
Akiska YM et al.
55 Classification of High- and Low-Risk Groups in Patients with Dermal Leiomyosarcoma: An Exploratory Register-Based Nationwide Cohort Study
Abebe K et al.
58 Paediatric Blaschkitis: A Rare Case of Multilinear Acquired Blaschko-Linear Dermatosis in an 11-Year-Old Girl
Aghajani M et al
61 Utilising the Modified Rintala Flap Technique for Effective Nasal Reconstruction
Tampouratzi E et al.
63 Marjolin’s Ulcer in Patients with Anogenital Lichen Planus: A Systematic Review
Branyiczky MK et al.
67 Persistent Papular Eruption in a Young Adult: Importance of Histopathology in Diagnosing Eruptive Vellus Hair Cysts
Amaral IP et al.
69 Contact Eczema of the Cheeks Due to Poppy Petal Powder
Hormi O et al.
71 Treatment of Distal Lateral Subungual Onychomycosis Due to Dermatophytes
Starace M et al.
Congress Interview
74 Branka Marinović
Interviews
78 Sara J. Brown
82 Martin Metz
85 Astrid Haaskjold Lossius
Infographic
88 Beyond the Barrier: Understanding Chronic Hand Eczema as a Multifactorial, Heterogeneous Skin Disease
Features
90 Managing Menopausal Skin: A Clinician's Review
Claudia DeGiovanni
95 Clinical Perspectives on Photosensitivity and Photodiagnostics
O’Reilly MK et al.
104 Safe Medical Management of Atopic Dermatitis in Pregnancy and Lactation
Adams PE et al.
Articles
114 Editor's Pick: Skin Intelligence: Advancing Cutaneous Resilience Through Biological Education, Microbial Symbiosis, and Eco-integrated Approaches
Haykal D et al.
124 Nail Psoriasis Treatment: A Narrative Review
El Hajj M et al.
135 Refractory Ulcerative Lupus Chilblains Treated with Deucravacitinib: A Case Report and Review of the Literature
Murase EM et al.
140 Nail Lichen Planus: A Comprehensive Review of Clinical Features, Histopathology, and Current Treatment
He and Yang
152 Atypical Koebner Response: Psoriasis Remission Following Endovenous Laser Ablation for Venous Insufficiency: A Novel Case Report and Literature Review
Arslan and Tokgoz












Prof. Simone Ribero
University of Turin, Italy
Simone Ribero trained at the University of Turin, Italy, completing a PhD on the prognostic role of histological regression in cutaneous melanoma. His training included research fellowships at prestigious institutions, including Imperial College London and King’s College London, UK, and his research field of interest is dermato-oncology with particular focus on moles and skin tumours.
Dr Jaishree Sharad
Skinfinitii Aesthetic Skin and Laser Clinic, India



Dr Jennifer Cather
Modern Research Associates, USA

Dr Michael Gold
Gold Skin Care Center, USA


Dr Hassan Galadari
United Arab Emirates University, United Arab Emirates
Prof Richard Warren University of Manchester, UK
Prof Francesca Fametani
Univeristy of Modena and Reggio Emilia, Italy
Prof Vishalakshi Viswanath
Rajiv Gandhi Medical College, India


Prof Des Tobin
University College Dublin, Ireland
Prof Alin Laurentiu Tatu
"Dunărea de Jos" University of Galați, Romania
EMJ Dermatology is an open-access, peer-reviewed eJournal committed to helping elevate the quality of healthcare for skin, hair, and nail diseases. EMJ Dermatology endeavours to increase knowledge, stimulate discussion, and contribute to a better understanding of these conditions.
The journal is published annually, six weeks after the European Academy of Dermatology and Venerology (EADV) Congress, and features highlights from this congress, alongside interviews with experts in the field, reviews of abstracts presented at the congress, as well as in-depth features on congress sessions. The journal also covers advances within the clinical and pharmaceutical arenas by publishing sponsored content from congress symposia, which is of high educational value for healthcare professionals. This undergoes rigorous quality control checks by independent experts and the in-house editorial team.
EMJ Dermatology also publishes peer-reviewed research papers, review articles, and case reports in the field. In addition, the journal welcomes the submission of features and opinion pieces intended to create a discussion around key topics in the field and broaden readers’ professional interests. The journal is managed by a dedicated editorial team that adheres to a rigorous double-blind peer-review process, maintains high standards of copy editing, and ensures timely publication.
EMJ Dermatology focuses on topics that are relevant to healthcare professionals in the field. We do not publish veterinary science papers or laboratory studies that are not linked to patient outcomes. We have a particular interest in topical studies that advance knowledge and inform of coming trends affecting clinical practice in the field.
Further details on coverage can be found here: www.emjreviews.com
Editorial Expertise
EMJ is supported by various levels of expertise:
• Guidance from an Editorial Board consisting of leading authorities from a wide variety of disciplines.
• Invited contributors who are recognised authorities in their respective fields.
• Peer review, which is conducted by expert reviewers who are invited by the Editorial team and appointed based on their knowledge of a specific topic.
• An experienced team of editors and technical editors.
Peer Review
On submission, all articles are assessed by the editorial team to determine their suitability for the journal and appropriateness for peer review.
Editorial staff, following consultation with either a member of the Editorial Board or the author(s) if necessary, identify three appropriate reviewers, who are selected based on their specialist knowledge in the relevant area.
All peer review is double blind. Following review, papers are either accepted without modification, returned to the author(s) to incorporate required changes, or rejected.
Editorial staff have final discretion over any proposed amendments.
Submissions
We welcome contributions from professionals, consultants, academics, and industry leaders on relevant and topical subjects. We seek papers with the most current, interesting, and relevant information in each therapeutic area and accept original research, review articles, case reports, and features.
We are always keen to hear from healthcare professionals wishing to discuss potential submissions, please email: editorial.assistant@emjreviews.com
To submit a paper, use our online submission site: www.editorialmanager.com/e-m-j
Submission details can be found through our website: www.emjreviews.com/contributors/authors
Reprints
All articles included in EMJ are available as reprints (minimum order 1,000). Please contact hello@emjreviews.com if you would like to order reprints.
Distribution and Readership
EMJ is distributed through controlled circulation to healthcare professionals in the relevant fields across Europe.
Indexing and Availability
EMJ is indexed on DOAJ, the Royal Society of Medicine, and Google Scholar®; selected articles are indexed in PubMed Central® .
EMJ is available through the websites of our leading partners and collaborating societies. EMJ journals are all available via our website: www.emjreviews.com
Open Access
This is an open-access journal in accordance with the Creative Commons Attribution-Non Commercial 4.0 (CC BY-NC 4.0) license.
Congress Notice
Staff members attend medical congresses as reporters when required.
This Publication Launch Date: 2025 Frequency: Yearly Online ISSN: 2054-6211
All information obtained by EMJ and each of the contributions from various sources is as current and accurate as possible. However, due to human or mechanical errors, EMJ and the contributors cannot guarantee the accuracy, adequacy, or completeness of any information, and cannot be held responsible for any errors or omissions. EMJ is completely independent of the review event (EADV 2025) and the use of the organisations does not constitute endorsement or media partnership in any form whatsoever. The cover photo is of Paris, France, the location of EADV 2025.
Front cover and contents photograph: Cathédrale Notre Dame de Paris, France © Beboy / stock.adobe.com
71% OF PEOPLE IDENTIFY AS HAVING SENSITIVE SKIN*
WaterWipes®
Break The Sensitive Skin Cycle
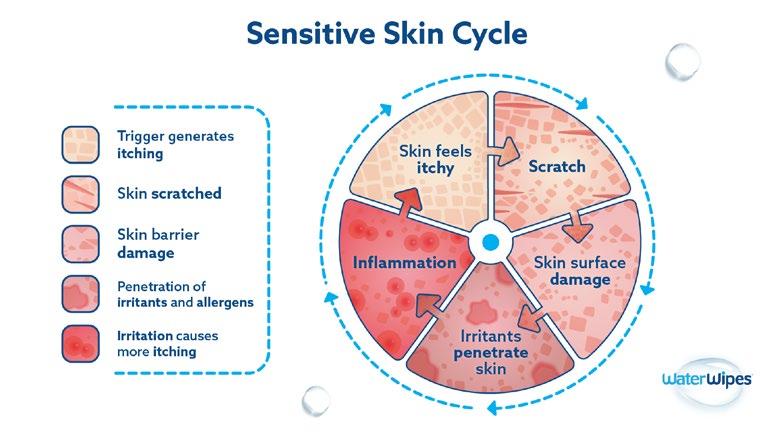

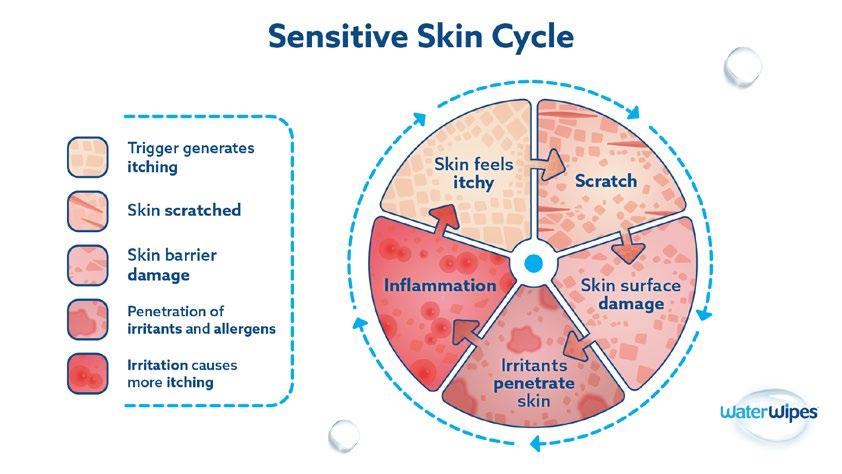
When recommending skin wipes for those with sensitive skin:

Fewer ingredients may be better than more ingredients
• Choose products that do not leave residue on the skin

Choose products with 0% alcohol and 0% perfume



Managing Editor
Darcy Richards
Copy Editors
Noémie Fouarge, Sarah Jahncke
Editorial Leads
Helena Bradbury, Ada Enesco
Editorial Co-ordinator
Bertie Pearcey
Editorial Assistants
Katrina Thornber, Katie Wright,
Aleksandra Zurowska
Creative Director
Tim Uden
Design Manager
Stacey White
Senior Designers
Tamara Kondolomo, Owen Silcox
Creative Artworker
Dillon Benn Grove
Designers
Shanjok Gurung, Fabio van Paris
Junior Designer
Helena Spicer
Head of Marketing
Stephanie Corbett
Business Unit Lead
Kelly Byrne
Chief Executive Officer
Justin Levett
Chief Commercial Officer
Dan Healy
Founder and Chairman
Spencer Gore
Dear Readers,
We are delighted to welcome you to the 2025 issue of EMJ Dermatology, bringing you the latest breakthroughs in the field through our coverage of the European Academy of Dermatology and Venereology (EADV) Congress 2025, which took place in Paris, France. This year’s event put the spotlight on early intervention to improve outcomes for patients with inflammatory skin disorders, a theme that is reflected throughout this issue.
To complement our Congress coverage, we feature an exclusive interview with EADV President, Branka Marinović, who discusses the organisation's sustainable initiatives, advocacy programme, and the EADV Games. Marinović also highlights the pressing unmet needs that the specialty must address.
Among the peer-reviewed content sits a timely feature exploring the clinical dermatological manifestations of menopause. This feature emphasises the urgent need for research to not only deepen our understanding but also to aid the development of targeted therapeutic strategies to better support women through menopause. You will also find a progressive narrative review that considers the skin as dynamic and adaptable, with the ability to be educated, and provides a proactive framework for integrating the pivotal elements of skin health and biology into disease prevention and management.
Additionally, you can learn more about the next frontier for treating atopic dermatitis, gene–environment interactions in atopic eczema, and disease-modifying therapy for chronic spontaneous urticaria in our interviews with three leading experts in the field.
We would like to take this opportunity to thank our Editorial Board, authors, peer reviewers, and interviewees for their contributions to this issue, which provides broad coverage of pertinent topics. We hope you enjoy reading and can take away useful insights for your clinical practice.
Editorial enquiries: editor@emjreviews.com
Sales opportunities: salesadmin@emjreviews.com
Permissions and copyright: accountsreceivable@emjreviews.com
Reprints: info@emjreviews.com Media enquiries: marketing@emjreviews.com
































































































We provoke conversation around healthcare trends and innovationwe also create engaging educational content for healthcare professionals. Join us for regular conversations with physician & entrepreneur, Jonathan Sackier. Listen Now


Dear Colleagues,
I am delighted to welcome you to our latest issue of EMJ Dermatology, which contains a rich selection of content, including peer-reviewed articles, a feature on managing menopausal skin, and interviews with key opinion leaders. Also included is our review of the European Academy of Dermatology and Venereology (EADV) Congress 2025, hosted in Paris, France, from 17th–20th September. This review offers a detailed overview of the most significant clinical insights presented throughout the Congress, including the impact of pregnancy on the course of vitiligo and a study that explored the trustworthiness of AI in dermatology.
EMJ once again had the delight of meeting with EADV President Branka Marinović, who shared her insights on the EADV's advocacy efforts and priorities, emphasising the unique challenge of representing a continent as diverse as Europe, where varying economic conditions, healthcare systems, and legislation create complexity. Martin Metz spoke to us about chronic spontaneous urticaria, Sara Brown on atopic dermatitis, and Astrid Haaskjold Lossius on inflammatory skin diseases.
The articles in this issue cover a wide spectrum of topics, from advances in nail psoriasis treatment and the emerging


concept of skin intelligence, highlighting cutaneous resilience through biological education, microbial symbiosis, and ecointegrated approaches, to a comprehensive review of nail lichen planus, detailing clinical features, histopathology, and current treatment strategies. Also included is a compelling example of refractory ulcerative lupus chilblains treated with deucravacitinib, as well as a novel report of an atypical Koebner response, documenting psoriasis remission following endovenous laser ablation for venous insufficiency.
This review offers a detailed overview of the most significant clinical insights presented throughout the Congress
As Editor-in-Chief, I would like to thank all the authors, reviewers, and Editorial Board members for their contributions to this issue of EMJ Dermatology, and I hope that readers find it both informative and inspiring.

Professor Simone Ribero University of Turin, Italy

With more than 180 scientific sessions and over 600 expert speakers, the Congress delivered an exceptional platform for both cutting-edge research and practical knowledge exchange
Location: Paris, France
Date: 17ᵗʰ–20ᵗʰ September 2025
Citation: EMJ Dermatol. 2025;13[1]:13-25. https://doi.org/10.33590/emjdermatol/OCPK7062
THIS AUTUMN, Paris, France, hosted the 34ᵗʰ annual European Academy of Dermatology and Venereology (EADV) Congress. For the second time in its history, the event took place in the French capital, attracting over 20,000 delegates. This is a record-breaking number, demonstrating the enduring strength and growth of the global dermatology community.
With more than 180 scientific sessions and over 600 expert speakers, the Congress delivered an exceptional platform for both cutting-edge research and practical knowledge exchange. The scientific programme, carefully curated by the Scientific Programme Committee, offered a dynamic and diverse range of session formats to engage every attendee, from residents to seasoned leaders.
The comprehensive offerings included 10 powerful plenary lectures that addressed the grand challenges facing the speciality, alongside more than 25 dedicated subspecialty tracks focusing on niche areas of dermato-venereology. In addition, abstract-based free communications and sessions devoted to late-breaking research ensured that the newest and most timesensitive findings were immediately shared with participants. Complementing these were hands-on workshops that provided practical insights into essential clinical skills.
Key scientific topics highlighted the speciality’s complexity, covering everything from the latest insights into bullous
diseases and psoriasis to advancements in dermoscopy for non-melanoma skin cancer. Attendees also engaged in deep dives into the skin microbiome and its role in conditions like acne and hidradenitis suppurativa, as well as the advanced management of nail and hair disorders. Adding an element of friendly competition, the Congress also hosted the second edition of the EADV Games, providing a spirited arena "where science meets a fun rivalry," as described by EADV President Branka Marinović.
The Opening Ceremony was launched with a warm welcome from Marinović, who reflected on the impressive turnout and expressed her profound gratitude to the Scientific Programme Committee, led by the Chair, Michel Gilliet, Lausanne University Hospital, Switzerland, for their dedication to curating such a successful and comprehensive programme. Looking to the future, Marinović optimistically expressed the hope of breaking more records when the EADV returns to Paris in 3 years.
Following the President’s address, Saskia Oro, Chair of the French Society of Dermatology (SFD), extended a gracious welcome to the thousands of international attendees. Oro highlighted the rich history of the SFD, founded in 1889, and its current membership of over 2,500 dedicated dermatologists. Oro continued to detail the SFD’s core mission: promoting public health, supporting evidence-based medical and scientific research, and advancing educational programmes across all areas of dermatology. She proudly announced that the SFD had awarded over 2 million EUR in 2024 to support strategic research and educational grants for young researchers. Furthermore, she revealed innovative projects, including the creation of a new evidence-based Dermatology Centre and the planned launch of a dermatological consultation truck, a novel project designed to bring expert care to underserved areas across France. These initiatives highlight the SFD’s commitment to improving the quality of dermatology care and promoting solidarity within the field.
The Opening Ceremony concluded with the highly anticipated keynote lecture, introduced by Gilliet, who noted that the EADV tradition is to feature crossdisciplinary topics that challenge convention, not just in medicine, but in general thinking about the world.
Zoologist, acclaimed broadcaster, and best-selling author Lucy Cooke, Honorary Senior Research Associate, University College London, UK; and Founder, Sloth Appreciation Society, took the stage to deliver her captivating lecture entitled 'Live Like a Sloth, Think Like an Octopus'. Cooke, a graduate of Oxford University, UK, and an evolutionary biologist, used her expertise to explore the profound lessons that the animal kingdom holds for humans. She demonstrated how cultural and scientific biases have long distorted our understanding of nature, and how shedding these rigid frameworks is essential for innovation.
The lecture spotlighted the naked mole rat, a biological marvel whose extraordinary longevity and cancer immunity are linked to high molecular weight hyaluronic acid in its skin, offering a powerful parallel for dermatological research into ageing.
Critically, Cooke addressed Charles Darwin’s historical bias against female biology. By highlighting the fierce, competitive nature of the female meerkat and the empathetic, post-menopausal leadership of the orca matriarchs, she powerfully dismantled long-held stereotypes about gender and power. The lecture’s overarching message was an inspiring call to action: innovation happens when old beliefs are cast aside. By embracing curiosity and flexible thinking, like the nine-brained octopus, science and the dermatology community can achieve true breakthroughs in both treatment and understanding.
Adding an element of friendly competition, the Congress also hosted the second edition of the EADV Games
EMJ had the pleasure of attending this year’s Congress, and is excited to share highlights from the diverse abstract sessions in our comprehensive review of EADV 2025 for this issue of EMJ Dermatology, alongside an exclusive interview with EADV President Branka Marinović. Continue reading for an in-depth look at the pivotal discussions and groundbreaking research from this year’s Congress.
ALTHOUGH the relationship between pregnancy and autoimmune disorders has been the subject of considerable investigation, the interaction between pregnancy and vitiligo has received little attention in the past. A recent study presented at the EADV Congress 2025 sought to address this, by conducting a systematic review evaluating the existing evidence on how pregnancy influences the course of vitiligo.1
Vitiligo, a chronic autoimmune condition characterised by the loss of skin pigmentation, affects between 0.2–1.8% of the global population. For women living with vitiligo, pregnancy may bring uncertainty about whether the disease will worsen, improve, or remain stable.
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, comprehensive searches were carried out across major databases, including MEDLINE, Embase, the Cochrane Library, and Scopus, up to December 2024. Studies were eligible if they provided data on the onset or progression of vitiligo during pregnancy. In total, seven studies were identified, though only two cross-sectional surveys specifically investigated the condition in the context of pregnancy. The remaining studies referred to vitiligo only as an incidental finding within broader analyses.
The available evidence suggests that most women with vitiligo do not experience significant changes during pregnancy. Approximately 65% of participants in the two focused surveys reported stability in their condition. Improvement and worsening of vitiligo occurred in similar proportions, affecting around 12% and 20% of women, respectively. Reports of new-onset vitiligo during pregnancy were scarce across all studies, indicating that pregnancy does not commonly trigger the condition.
These findings offer some reassurance for women with vitiligo who may be concerned about pregnancy-related disease progression. However, the small number of relevant studies and absence of prospective data limit the certainty of these conclusions. While current evidence suggests that excessive concern may not be warranted, further high-quality research is needed to provide clearer guidance and strengthen clinical recommendations.

BIOLOGIC therapies may offer an effective and safe alternative for treating severe cutaneous adverse events caused by immune checkpoint inhibitors (ICI), according to the results of a single-centre cohort study presented at the EADV Congress 2025.2
ICIs have transformed cancer treatment, improving survival outcomes in advanced disease, but they are frequently associated with immune-related adverse events, especially those affecting the skin. Approximately 40% of patients undergoing these treatments develop dermatologic complications, and while corticosteroids are

10 out of 11 patients showed significant clinical improvement
standard management, they risk diminishing the therapeutic efficacy of immunotherapy. Biologic drugs are emerging as valuable alternatives, but formal guidelines on their use are currently limited.
In this retrospective study, 252 patients with cancer receiving both ICIs and biologics were screened. Eleven of these patients had biologics prescribed specifically to treat cutaneous immune-related adverse events. The median age was 73 years, and nine out of the 11 patients were male. The most used ICIs were pembrolizumab (seven patients), followed by nivolumab (two), combination nivolumab and ipilimumab (one), and atezolizumab (one). Median latency from ICI initiation to onset of dermatologic complications was 284 days. Bullous pemphigoid was the predominant adverse event (seven patients), and one of these cases included mucous membrane pemphigoid. Dupilumab and rituximab were the most frequently used biologics, achieving control in most cases. Other biologics that were used successfully were ustekinumab and additional courses of dupilumab. Ten out of 11 patients showed significant clinical improvement, though six required temporary discontinuation of ICIs. One patient died of sepsis before effect evaluation, and three deaths occurred later due to cancer progression.
These findings suggest that biologics can be integrated into clinical practice for selected patients with ICI-related dermatologic complications, facilitating recovery without abandoning life-prolonging cancer therapy. From an oncodermatology perspective, the ability to maintain immunotherapy while effectively managing severe cutaneous events is a critical step forward.
A MULTICENTRE study, presented at the EADV Congress 2025, investigated the use of dupilumab in patients with familial benign chronic pemphigus, also known as Hailey–Hailey disease (HHD), a rare inherited skin disorder caused by mutations in the ATP2C1 gene.3 The condition is characterised by recurrent flares of vesicles, blisters, erosions, and painful fissures, predominantly affecting flexural areas.
These chronic and relapsing lesions can severely impair quality of life, and despite numerous therapeutic approaches, the management of severe or treatmentresistant cases remains challenging. Dupilumab, a monoclonal antibody targeting IL-4 and IL-13 signalling, has recently emerged as a promising new option for patients with refractory HHD.
This retrospective multicentre review, conducted between 2023–2025 across five centres, assessed the effectiveness and safety of dupilumab in eight patients with refractory HHD treated off-label. Each patient received an initial subcutaneous dose of 600 mg, followed by 300 mg every 2 weeks. Data collected included demographics, previous and concomitant treatments, lesion distribution, disease severity (evaluated using Body Surface Area [BSA] and Physician Global Assessment [PGA] scores), and quality of life, measured by the Dermatology Life Quality Index (DLQI). Clinical responses and adverse events were monitored throughout follow-up.
Complete or near-complete clinical responses were achieved in five patients, with two showing partial improvement and one showing no response. Most participants experienced substantial reductions in disease severity, supported by improvements in both BSA and PGA scores. Quality of life also improved significantly, as evidenced by lower DLQI values. Dupilumab was well tolerated, with no adverse events reported. Treatment discontinuation occurred in two cases, one due to lack of efficacy and one due to loss to follow-up.
These findings add to the growing body of evidence supporting dupilumab as a safe and effective therapeutic alternative for refractory HHD. By inhibiting Th2 cytokine activity and restoring calcium-dependent keratinocyte function, dupilumab addresses key mechanisms underlying the disease. Although limited by sample size and retrospective design, this study reinforces previous reports and highlights the need for larger, prospective trials to confirm these promising results.
AT THE EADV Congress 2025, new research comparing DFD-29 and doxycycline offered fresh insights into the management of rosacea, a chronic inflammatory skin condition that affects the central face and presents in multiple subtypes.4
Rosacea can severely impact quality of life and, if left untreated, may lead to longterm facial scarring. Oral doxycycline 40 mg, an FDA-approved anti-inflammatory therapy, has been a cornerstone of treatment for many years. However, DFD29, an extended-release 40 mg minocycline capsule, has recently emerged as a potential alternative. The study presented at the Congress aimed to evaluate and compare the efficacy and safety of DFD-29 versus doxycycline in adults with rosacea.
A systematic review of PubMed, Scopus, and Cochrane databases identified RCTs evaluating these two treatments. Eligible participants had at least moderate disease, defined by an Investigator’s Global Assessment (IGA) score of 2 or higher and a minimum of 10 facial papules and pustules. The primary outcome was treatment success, achieved when patients reached IGA Grade 0–1 with a two-grade improvement by Week 16. Secondary outcomes included serious and commonly reported adverse events such as headache, diarrhoea, and abdominal pain.
Three RCTs were analysed, including 592 patients, 298 of whom received DFD-29. The pooled data showed that patients treated with DFD-29 were more than twice as likely to achieve treatment success compared with those receiving doxycycline (odds ratio: 2.84; 95% CI: 1.98–4.09; p<0.00001). The risk of serious adverse events did not differ significantly between groups, and rates of headache and diarrhoea were similar. Although abdominal pain was reported more frequently among DFD-29 recipients, this finding did not reach statistical significance.
Overall, DFD-29 demonstrated superior efficacy, with a comparable safety profile to doxycycline 40 mg. These findings suggest that DFD-29 may represent a promising new oral therapy for rosacea, though further large-scale trials are needed to confirm its benefits across different subtypes and severities.
The primary outcome was treatment success, achieved when patients reached IGA Grade 0–1 with a twograde improvement by Week 16

NEW FINDINGS presented at the EADV Congress 2025 explore the potential use of AI in dermatology, specifically assessing the reliability of ChatGPT (OpenAI, San Francisco, California, USA) in providing information to patients about rosacea, a chronic inflammatory skin condition.5 25 11 10 4
With a growing reliance on online platforms for medical guidance, AI tools like ChatGPT offer rapid access to information. However, their accuracy and applicability in dermatology have not been extensively studied. Researchers from the Dermatology and Venereology Clinic at Ankara Bilkent Şehir Hastanesi, Türkiye, submitted 19 commonly asked questions from patients with rosacea (covering disease course, causes, differential diagnosis, treatment strategies, and prognosis) to ChatGPT version 3.5.
Responses were evaluated by 25 dermatology professionals, including 11 residents with less than 2 years of experience, 10 residents with 2–4 years of experience, and four academic staff members. Each answer was rated using a three-point scale: accurate and adequate, partially accurate or incomplete, or inaccurate/insufficient.
The study found that ChatGPT provided reliable information in 75.8% of cases. Responses to 13 of the 19 questions were
Responses were evaluated by dermatology professionals, including residents with less than 2 years of experience, residents with 2–4 years of experience, and academic staff members.

considered accurate, while six responses were insufficient. No significant agreement was observed among the majority of residents or academic evaluators, but residents with 2–4 years of experience demonstrated statistically significant concordance in their assessments, suggesting that clinical experience may influence the evaluation of AIgenerated responses.
These results indicate that ChatGPT has potential as a supportive tool for patient education in dermatology. At the same time, the findings highlight the importance of expert oversight, continuous refinement of AI outputs, and standardised evaluation methods to ensure safe and effective use in clinical settings. As patients increasingly seek digital guidance, understanding the reliability and limitations of AI tools is essential for clinicians aiming to provide accurate and responsible medical information.
CHRONIC inflammatory skin diseases are often linked with disrupted sleep, which can, in turn, worsen inflammation and negatively affect quality of life. Conditions such as psoriasis and cutaneous T cell lymphoma (CTCL) are known to be associated with sleep difficulties, yet objective evidence comparing sleep across different dermatological conditions remains limited. A recent study, presented at the EADV Congress 2025, sought to address this gap by examining the impact of these diseases on sleep using data captured through wearable technology.6
The study included 75 participants, comprising 20 patients with psoriasis, 27 with CTCL, and 28 healthy volunteers. Sleep patterns were monitored using the Withings Steel HR watch (Withings, Paris, France) over a 14-day period, with measurements including total sleep time, time in bed, sleep latency, light and deep sleep, and the number of awakenings. Data were analysed with linear mixed models to assess the influence of disease group, age, and sex on sleep outcomes.
Findings revealed that patients with psoriasis and CTCL experienced significantly poorer sleep compared to healthy controls. Both groups recorded shorter total sleep time and deep sleep, alongside more frequent awakenings. Interestingly, when comparing the two disease groups, patients with CTCL showed greater impairments than those with psoriasis, including reduced total sleep time, time in bed, and light sleep, though they experienced fewer awakenings overall. Age and sex also influenced sleep patterns, with older individuals showing longer awake periods and more awakenings, while male participants recorded shorter durations across several sleep measures.
Findings revealed that patients with psoriasis and CTCL experienced significantly poorer sleep compared to healthy controls
The study highlights the substantial impact of chronic inflammatory skin conditions on sleep quality and duration. It also spotlights the importance of recognising sleep disturbances as a clinically relevant factor in disease management. By demonstrating the utility of wearable devices in capturing real-world sleep data, the findings open the door for more personalised and holistic approaches to managing chronic skin disease.


IMPROVEMENTS in scalp symptoms and disease activity with JAK inhibitors in patients with recalcitrant folliculitis decalvans have been demonstrated in a multicentre Spanish cohort study presented at the EADV Congress 2025.7
Folliculitis decalvans is a chronic, scarring alopecia characterised by painful pustules, crusts, and tufted hairs. Long-term inflammation leads to the destruction of hair follicles and considerable psychological distress. Standard therapies frequently fail, prompting the use of off-label interventions, including targeted small-molecule agents. JAK inhibitors have gained attention for their immunomodulatory properties, though real-world data on their effectiveness are limited.
Researchers reviewed clinical records from four Spanish hospitals and included 12 patients with a histologically confirmed diagnosis of folliculitis decalvans who received oral JAK inhibitors for at least 3 months. The cohort was predominantly male, with a median age of 73 years and a history of refractory disease (having failed a median of 6.5 prior treatment courses, including adalimumab in over half). Baricitinib 4 mg was prescribed in nine cases, and upadacitinib 30 mg in three, both for a median of 6.5 months. At 3 months and the last follow-up, key disease activity parameters (pustules,
30 %
The number of patients with less than 30% of affected area with erythema and hyperkeratosis increased significantly at 3 months and the last visit (p<0.02).
crusts, polytrichia, erythema, and hyperkeratosis) and percentage of affected scalp showed improvement, although not all changes reached statistical significance. However, the number of patients with less than 30% of affected area with erythema and hyperkeratosis increased significantly at 3 months and the last visit (p<0.02). Half of the patients experienced mild adverse effects, most commonly hypercholesterolaemia (n=2) and upper respiratory tract infection (n=2), with no severe or long-lasting complications.
The findings highlight the potential utility of JAK inhibitors in clinical practice for patients with folliculitis decalvans unresponsive to traditional therapies, including biological agents. Given the favourable safety profile and observed symptom control, JAK inhibitors may be considered as an adjunct or alternative when conventional treatments fail. Further studies are needed to establish standardised outcome measures and optimal treatment regimens to guide longterm management of this rare scalp disorder.
A RECENT retrospective study, presented at the EADV Congress 2025, evaluated the impact of teledermoscopy (TD) on access to dermatological consultations for cutaneous tumours in Northern Sweden, where referral rates have risen markedly since the technology’s introduction in 2014.8
TD enables primary care centres (PCC) to send high-quality images of suspicious skin lesions to dermatologists for assessment, improving diagnostic efficiency and potentially addressing the growing demand for dermatology services amid increasing incidences of skin cancer. Despite its longstanding use, the system had not been formally assessed until now.
The study analysed 67,137 TD referrals from PCCs to a dermatology department between 2014–2024, excluding 2,384 incomplete submissions. Each case was reviewed by a dermatologist trained in dermoscopy, and diagnoses were recorded alongside patient demographics and the referring unit. In addition, a survey completed by PCCs explored local TD routines, including organisational practices, pre-assessment procedures, and the roles of healthcare professionals involved.
Over 11 years, the mean age of referred patients increased from 50 to 61 years (p<0.001), while the proportion of benign lesions decreased from 79.5% to 68.2%
(p<0.001), indicating a trend towards more targeted referrals. Referrals from private PCCs contained a higher proportion of benign lesions than those from public centres (78.4% versus 74.6%; p<0.001).
Similarly, PCCs located near the dermatology department referred more benign lesions than remote centres (77.4% versus 72.9%; p<0.001). Factors associated with increased benign referral rates included nurse-led assessments and the referral of nearly all examined lesions, whereas a lack of dermoscopy training and internal discussion of TD cases reduced benign referrals.
Overall, TD has supported earlier and more accurate tumour diagnosis, contributing to equitable access to specialist dermatology care in Northern Sweden. The findings suggest improved identification of highrisk patients, but also highlight the need to optimise TD protocols and training, particularly in private and nearby PCCs, to reduce unnecessary benign referrals.
Over 11 years, the mean age of referred patients increased from 50 to 61 years (p<0.001), while the proportion of benign lesions decreased from 79.5% to 68.2%.
DERMATOPHYTE infections are among the most common fungal diseases, affecting around 10% of the global population. Their prevalence is notably higher in individuals with Type 2 diabetes (T2D), likely due to altered immune responses and skin barrier changes. However, it remains uncertain whether these infections might serve as early clinical indicators of undiagnosed or developing diabetes. This study, presented at the EADV Congress 2025, aimed to assess whether a positive PCR test for dermatophyte infection of the feet is associated with an increased risk of subsequent T2D diagnosis.9
This large, register-based cohort study included 78,752 adults tested between 2015–2021. Among them, 19,688 individuals with a positive PCR test for dermatophytes from toenails, toe web spaces, or feet were matched 1:3 by age, sex, and geographic region to 59,064 unexposed controls. Participants with pre-existing diabetes or who were aged <20 years were excluded. The primary outcome was new-onset T2D after the date of testing. Poisson regression models were used to calculate incidence rate ratios, adjusted for age, sex, and relevant comorbidities.
The median age of participants was 49 years, and 60.3% were male. The incidence of newly diagnosed T2D was 8.2 per 100 person-years in the exposed group and 8.1 in the unexposed group. The adjusted incidence rate ratio was 1.01 (95% CI: 0.96–1.07; p=0.616), indicating no statistically significant association between dermatophyte infection and subsequent T2D. Subgroup analyses by age and sex confirmed these results, and secondary analyses showed that repeated fungal testing was also not linked to increased diabetes risk.
Overall, PCR-confirmed dermatophyte infections of the feet were not associated with an increased likelihood of developing T2D. These findings suggest that such infections do not serve as reliable early clinical indicators of undiagnosed diabetes.
Overall, PCR-confirmed dermatophyte infections of the feet were not associated with an increased likelihood of developing T2D

NEW RESEARCH presented at the EADV Congress 2025 highlights a significant association between hidradenitis suppurativa (HS), a chronic immune-mediated skin disorder, and endometriosis, a condition characterised by ectopic endometrial growth and chronic pelvic inflammation.10

The results emphasise the importance of considering gynaecologic comorbidities in the clinical management of patients with HS
The study was conducted by researchers using the US Collaborative Network within the TriNetX Research Platform (TriNetX, Cambridge, Massachusetts, USA). Female patients diagnosed with HS were compared to non-HS counterparts through propensity score matching, accounting for demographic variables (age, sex, race) and a wide range of clinical factors. These included metabolic and autoimmune comorbidities, psychiatric conditions, corticosteroid usage, hormonal status, pregnancy history, and prior gynaecologic surgeries. Time-to-event analyses using Cox regression were employed to estimate hazard ratios (HR) and 95% CIs for incident endometriosis, with additional models examining different wash-out periods (6 and 24 months) and follow-up durations (5 and 15 years).
In unadjusted analyses, results showed that individuals with HS had nearly double the risk of developing endometriosis compared to controls (HR: 1.891; 95% CI: 1.719–2.079). After adjusting for core demographic factors, the association remained statistically significant (HR: 1.278; 95% CI: 1.109–1.474). Risk estimates were consistent when early cases of endometriosis within 6 months (HR: 1.204; 95% CI: 1.017–1.424) and 24 months (HR: 1.209; 95% CI: 1.009–1.448) post-index were excluded. Stratification by follow-up duration further confirmed elevated risk over intermediate (5 years; HR: 1.306; 95% CI: 1.126–1.514) and extended periods (15 years; HR: 1.183; 95% CI: 1.072–1.306).
These findings suggest that HS is significantly associated with an increased likelihood of developing endometriosis, highlighting a potential shared pathophysiologic mechanism involving chronic inflammation and hormonal dysregulation. The results emphasise the importance of considering gynaecologic comorbidities in the clinical management of patients with HS, and may inform future research into overlapping inflammatory and hormonal pathways.
References
1. Jukema M et al. The impact of pregnancy on the course of vitiligo –a systematic review. Abstract 3151. EADV Congress, 17-20 September, 2025.
2. Tran T et al. Biologic therapies for the treatment of cutaneous adverse events induced by immune checkpoint inhibitors: oncodermatology perspective. Abstract 7318. EADV Congress, 17-20 September, 2025.
3. Andrés SB et al. Real-world experience with dupilumab in Hailey-Hailey disease: a multicenter retrospective study. Abstract 5424. EADV Congress, 17-20 September, 2025.
4. Nguyen N et al. Doxycycline 40 mg vs. DFD-29 40 mg for rosacea treatment: a meta-analysis of three randomized
controlled trials. Abstract 132. EADV Congress, 17-20 September, 2025.
5. Otlu CN et al. Rosacea meets AI: how trustworthy is ChatGPT in dermatology? Abstract 111. EADV Congress, 17-20 September, 2025.
6. Oliver M et al. Sleep disturbances in chronic inflammatory skin diseases: objective assessment using wearable devices in psoriasis and cutaneous t-cell lymphoma. Abstract 1934. EADV Congress, 17-20 September, 2025.
7. Pérez MAL et al et al. Recalcitrant folliculitis decalvans treated with JAK inhibitors: a multicenter cohort study. Abstract 762. EADV Congress, 17-20 September, 2025.
8. Zazo V et al. Evaluation of teledermoscopy for the assessment of cutaneous tumors in Northern
Sweden. Abstract 47. EADV Congress, 17-20 September, 2025.
9. Froelunde AS et al. Can the skin reveal hidden diabetes? A register-based study of dermatophyte infections and future diabetes risk. Abstract 3234. EADV Congress, 17-20 September, 2025.
10. Chang HC, Gau SY. Risk of endometriosis among women with hidradenitis suppurativa: insights from a nationwide real-world cohort. Abstract 5792. EADV Congress, 17-20 September, 2025.
Author: *Diala Haykal1
1. Centre Médical Laser Palaiseau, France
*Correspondence to docteur.haykal@gmail.com
Disclosure: The author has declared no conflicts of interest.
Keywords:
Biomarkers, dermatology, DNA methylation, epigenetics, histone modification, lasers, regenerative medicine, rejuvenation.
Citation: EMJ Dermatol. 2025;13[1]:26-30. https://doi.org/10.33590/emjdermatol/DEMN2963
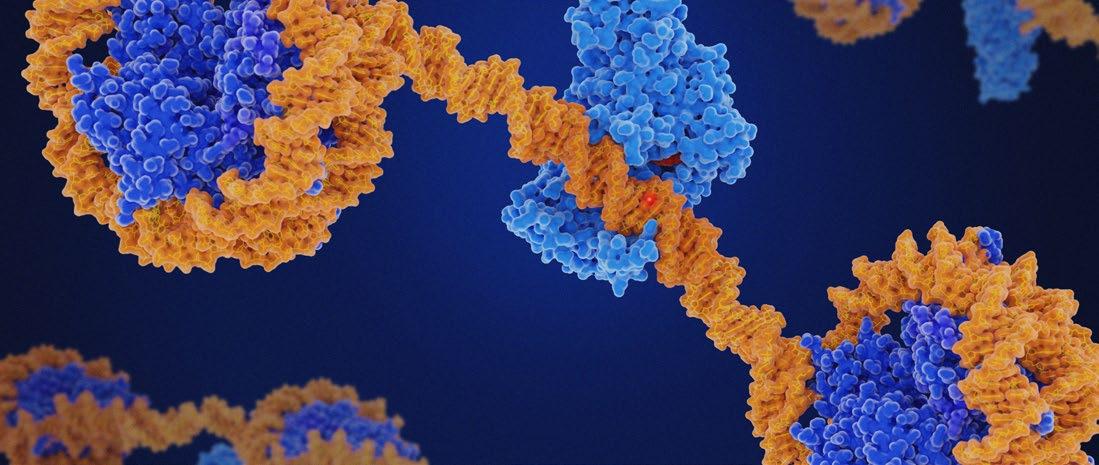
LASERS have transformed dermatology by offering effective treatments for photo-ageing, scars, and pigmentary disorders. Traditionally, their benefits were attributed to thermal injury and subsequent collagen remodelling. However, recent advances suggest that lasers exert deeper biological effects by modulating the skin’s epigenetic architecture. Epigenetics, through DNA methylation, histone modifications, and non-coding RNAs, governs cutaneous repair, ageing, and plasticity. Emerging evidence indicates that laser therapy reprogrammes these pathways, rejuvenating fibroblast function, reducing senescence, and normalising aberrant signalling. This feature, based on the Société Française des Lasers en Dermatologie (SFLD) session through the European Academy of Dermatology and Venereology (EADV), explores how lasers act as epigenetic modulators, the translational implications for personalised dermatology, and future opportunities to integrate biomarkers and AI into clinical practice.1 By reframing lasers as molecular reprogramming tools, dermatology is entering a new era of regenerative and longevity-focused care. These insights from the EADV Congress highlight a paradigm shift, positioning lasers not only as aesthetic instruments but also as drivers of regenerative dermatology and cutaneous longevity.
Dermatology is rapidly evolving from symptomatic repair to regenerative and preventive strategies. Central to this transformation is the growing recognition of the role of epigenetics, the heritable yet reversible modifications in gene expression that do not alter the DNA sequence. Epigenetic mechanisms, including DNA methylation, histone modifications, and non-coding RNAs, shape how the skin responds to intrinsic ageing and extrinsic exposures such as UV radiation, pollution, and diet. The concept of an ‘epigenetic clock’, reflecting biological rather than chronological age, has emerged as a
powerful biomarker of tissue health and longevity.2
Lasers exert effects far deeper than the dermis; they may directly reshape the epigenetic programming of skin cells
In parallel, lasers have become a cornerstone of dermatology, widely used for photo-ageing, scars, vascular and pigmentary disorders, and, more recently, functional rejuvenation. Historically, their
efficacy was explained by controlled injury leading to wound healing and neocollagenesis. Yet, accumulating data suggest that lasers exert effects far deeper than the dermis; they may directly reshape the epigenetic programming of skin cells, rejuvenating their function and shifting their molecular trajectory.3
This commentary examines the interplay between lasers and epigenetics, highlighting evidence of molecular reprogramming, the broader translational implications for dermatology, and future perspectives on integrating biomarkers and AI into clinical practice.
The skin is one of the most epigenetically dynamic organs, constantly adapting to environmental stimuli. Its regenerative capacity and ageing trajectory are determined not only by genetic predisposition but also by epigenetic regulation.4
• DNA methylation: Methylation at cytosine-phosphate-guanine islands silences transcription. With age, methylation patterns become irregular, a process known as epigenetic drift. Genes involved in collagen synthesis,
elastogenesis, and antioxidant defense are progressively silenced, while proinflammatory pathways are activated.5
• Histone modifications: Posttranslational modifications such as acetylation and methylation influence chromatin accessibility. Open chromatin promotes repair and regeneration, whereas closed conformations inhibit these functions. Altered histone states contribute to dermal thinning and impaired wound healing.6
• Non-coding RNAs: MicroRNAs (miRNA) and long non-coding RNAs regulate mRNA translation. Dysregulated miRNAs, such as upregulation of fibrotic miR-21 or downregulation of antifibrotic miR-29, drive pathological processes including scarring, pigmentation disorders, and chronic inflammation.2
Taken together, these mechanisms make the skin’s epigenome both a record of environmental exposures and a determinant of its regenerative capacity.
Lasers deliver energy that induces microthermal injury, stimulating controlled wound healing. The novelty lies in evidence that this process is mediated by epigenetic reprogramming rather than mere collagen contraction.7,8

Fractional CO₂ lasers have been shown to reduce hypermethylation of promoters controlling extracellular matrix (ECM)related genes, thereby reactivating collagen and elastin production. In fibroblasts from aged skin, post-laser treatment correlates with decreased ‘epigenetic age’, aligning cellular behaviour with a more youthful profile.9
Laser-induced stress activates histone acetyltransferases, leading to chromatin relaxation and increased transcription of pro-regenerative genes. Conversely, fibrotic genes undergo repressive histone modifications, decreasing pathological collagen deposition and improving scar remodelling.10
Several studies have demonstrated changes in miRNA profiles post-laser therapy. miR21, which promotes angiogenesis and fibroblast migration, is upregulated, while miR-29, an inhibitor of collagen synthesis, is downregulated. These coordinated shifts favour balanced ECM remodelling and tissue regeneration.11
Markers of senescence, such as p16^INK4a and β-galactosidase, decrease after fractional laser treatment. By modulating senescence-associated secretory phenotype pathways, lasers not only rejuvenate fibroblasts but also reduce chronic inflammation, creating a more favourable regenerative microenvironment.12
Transcriptome analyses post-laser reveal upregulation of growth factors, angiogenic mediators, and ECM regulators, many under epigenetic control. These findings support the hypothesis that lasers function as molecular reset devices, orchestrating global gene expression changes via epigenetic remodelling.13
These findings support the hypothesis that lasers function as molecular reset devices
The discovery that lasers modulate skin epigenome reframes their role in clinical dermatology.8,14 Several translational consequences follow:
• Objective biomarkers of efficacy: Current outcome measures rely heavily on subjective grading or patientreported improvement. Epigenetic profiling, such as changes in DNA methylation age or miRNA signatures, could provide objective, quantifiable biomarkers, enabling clinicians to measure biological as well as aesthetic outcomes.
• Patient stratification: Not all patients respond equally to laser therapy. Baseline epigenetic signatures could predict treatment response, guiding personalised protocols. For instance, patients with accelerated epigenetic ageing may require more intensive regimens, while those with youthful profiles might benefit from gentler approaches.
• Durability of results: The persistence of improvements after fractional laser therapy may be explained by longlasting epigenetic remodelling, in contrast to the transient effects of wound healing. This suggests that lasers can achieve semi-permanent molecular resets, explaining why some patients maintain benefits for years.
• Integration with preventive dermatology: If lasers can slow or reverse epigenetic ageing, they could be integrated into preventive dermatology programmes, much like sunscreens are used to prevent photoageing. This concept of ‘epigenetic dermatology’ positions lasers as tools not only for correction, but also for longterm preservation of skin health.
• Aesthetic and longevity medicine convergence: By demonstrating measurable effects on biological age, lasers could bridge the gap between aesthetic dermatology and longevity medicine. This positions them not merely as cosmetic enhancers but as regenerative therapies aligned with systemic health strategies.
While the interplay between lasers and epigenetics is promising, several challenges must be addressed:
• Evidence gap: Current studies are small and often preclinical. Large-scale, controlled trials integrating molecular endpoints are essential to validate the epigenetic effects of lasers.
• Standardisation: Consensus is needed on which epigenetic markers (e.g., DNA methylation clocks, specific miRNAs, histone states) best reflect clinical improvement.
• Longevity focus: Whether lasers truly reset the epigenetic clock or merely induce temporary changes remains unresolved. Longitudinal studies tracking patients’ epigenetic age preand post-laser are required.
• Combination therapies: The synergy between lasers and epigenetic modulators, such as exosomes and
sirtuin activators, should be investigated as potential next-generation protocols.
• Ethical and regulatory considerations: As lasers evolve into tools of molecular reprogramming, regulatory frameworks may need to redefine them as regenerative devices rather than purely cosmetic instruments.
Beyond their emerging role as epigenetic modulators, lasers continue to demonstrate remarkable versatility across a wide spectrum of dermatologic and surgical applications. The session illustrated how medical lasers are increasingly integrated into both functional and aesthetic indications, extending benefits beyond rejuvenation and resurfacing.
• Laser treatment of ingrown nails (onychocryptosis): Laser-assisted management of ingrown nails using CO₂ systems offers precise matrix ablation with minimal postoperative pain and a reduced recurrence rate. The technique enables controlled excision of the affected nail fold, promoting faster healing and superior cosmetic outcomes compared to traditional surgical methods.
• Laser therapy for adult bruises: The use of vascular lasers has proven effective in accelerating the resolution of post-
procedural bruises and vascular lesions in adults. Targeted photothermolysis of haemoglobin-rich areas enhances clearance, shortens downtime, and improves overall skin appearance. This approach highlights how lasers can refine recovery and patient satisfaction following aesthetic or medical interventions.
• Improving scars in children: Fractional ablative and non-ablative laser technologies have shown significant benefits in the treatment of paediatric scars. Careful adjustment of parameters allows effective remodelling of texture and pigmentation while preserving safety in developing skin. These interventions contribute meaningfully to improving the quality of life and selfesteem of younger patients.
Collectively, these applications underscore the multidimensional value of medical lasers, from restoring function to enhancing appearance, emphasising their place at the intersection of dermatology, surgery, and regenerative medicine.
References
1. Société Française des Lasers en Dermatologie (SFLD). 2020. Available at: https://www.sfldlaser.com. Last accessed: 17 October 2025.
2. Haykal D et al. Unlocking longevity in aesthetic dermatology: epigenetics, aging, and personalized care. Int J Dermatol. 2025;DOI:10.1111/ijd.17725.
3. Haykal D et al. Epigenetic modifications and the role of medical lasers in enhancing skin regeneration. J Cosmet Dermatol. 2025;24(1):e16780.
4. Andersen B, Millar S. Skin epigenetics. Exp Dermatol. 2021;30(8):1004-8.
5. Christensen BC et al. Aging and environmental exposures alter tissuespecific DNA methylation dependent upon CpG island context. PLOS Genet. 2009;5(8):e1000602.
6. Song H et al. Histone posttranslational modification and the DNA damage response. Genes Dis. 2022;10(4):1429-44.
Lasers are no longer simply devices of ablation or resurfacing; they emerge as epigenetic modulators capable of reprogramming skin biology. By influencing DNA methylation, histone states, and non-coding RNAs, lasers induce longlasting molecular shifts that underlie their regenerative effects.
The implications are profound: lasers may one day be guided by epigenetic biomarkers, optimised by AI, and combined with adjunctive molecular therapies to deliver personalised, preventive, and regenerative outcomes. This convergence of epigenetics and laser dermatology redefines the specialty’s role in both aesthetics and longevity medicine.
In this new paradigm, lasers should be recognised not only as instruments of cosmetic enhancement, but also as catalysts of cutaneous longevity.
7. Spandau DF et al. Randomized controlled trial of fractionated laser resurfacing on aged skin as prophylaxis against actinic neoplasia. J Clin Invest. 2021;131(19):e150972.
8. Sherrill JD et al. Transcriptomic analysis of human skin wound healing and rejuvenation following ablative fractional laser treatment. PLoS One. 2021;16(11):e0260095.
9. Kim JE et al. Gene profiling analysis of the early effects of ablative fractional carbon dioxide laser treatment on human skin. Dermatol Surg. 2013;39(7):1033-43.
10. Nascimento-Filho CHV et al. Skin wound healing triggers epigenetic modifications of histone H4. J Transl Med. 2020;18(1):138.
11. Xie J et al. Roles of MicroRNA-21 in skin wound healing: a comprehensive review. Front Pharmacol. 2022;13:828627.
12. Haykal D et al. Toward new clinical evaluation models for skin longevity: the need for predictive and accelerated approaches. GeroScience.
2025;DOI:10.1007/s11357-02501913-1.
13. Peplow PV et al. Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed Laser Surg. 2011;29(5):285-304.
14. Benson TA et al. Nonablative fractional laser treatment is associated with a decreased risk of subsequent facial keratinocyte carcinoma development. Dermatol Surg. 2023;49(2):149-54.
Author: Jenna Lorge, EMJ, London, UK
Citation: EMJ Dermatol. 2025;13[1]:31-34. https://doi.org/10.33590/emjdermatol/XZAJ5561
PSORIASIS is no longer regarded as a single, uniform condition. Once classified primarily by visible lesions or severity, it is increasingly understood as a spectrum of inflammatory disorders with distinct molecular drivers, genetic signatures, and clinical trajectories. In a session titled ‘Psoriasis’, this year’s European Academy of Dermatology and Venereology (EADV) Congress brought together leading experts to explore how insights on endotyping, early aggressive treatment, oral therapies, and dietary interventions are reshaping patient care.
Denis Jullien, Professor of Dermatology and Head of the Department of Dermatology, Hôpital Edouard Herriot, Hospices Civils de Lyon, University of Lyon, France, began the session by introducing endotypes, a framework for reclassifying psoriasis based on its molecular and immunological underpinnings rather than traditional phenotypes. “What the concept of endotypes tries to do is rationalise psoriasis diversity by stratifying the disease into subgroups that share molecular (and that’s the main word here) characteristics,” he explained. While dermatologists have long categorised patients by clinical features, Jullien emphasised that these classifications fail to capture the biological diversity underpinning disease behaviour.1
Endotyping seeks to identify subgroups of patients who share common mechanisms, genetic variants, or immunologic pathways, offering the potential to predict disease course, tailor treatment, and understand differential therapeutic responses.1 He highlighted IL-36-driven pustular psoriasis and emerging monogenic forms linked to ADAR1 mutations as examples of
molecularly distinct subtypes with clinical significance.2,3
Continuing, he explained that large-scale genomic, transcriptomic, and cellular studies are transforming our understanding, linking susceptibility variants to treatment response and classifying skin lesions into molecular subtypes. Biomarkers such as monocyte phenotypes and spatial transcriptomics could soon allow clinicians to differentiate mild from severe disease, moving beyond surface-level observation toward precision medicine.
Biomarkers such as monocyte phenotypes and spatial transcriptomics could soon allow clinicians to differentiate mild from severe disease
“The clinical translation of endotype knowledge holds immense promise for precision medicine in psoriasis,” Jullien concluded, highlighting that a move from phenotype-driven to mechanism-driven classification could revolutionise treatment selection and long-term outcomes.
Building on the concept of tailored care, Andrew Blauvelt, Blauvelt Consulting LLC, Lake Oswego, Oregon, USA, explored whether the timing and intensity of treatment could reshape disease trajectories. He proposed a provocative strategy: ‘hit early, hit hard’.4 Rather than focusing on incremental symptom control, Blauvelt argued that early, aggressive therapy could deplete pathogenic immune cells and extend remission, potentially altering the natural history of psoriasis.
Evidence increasingly supports this approach. Patients with short disease duration respond more robustly and maintain remission longer than those with long-standing disease. Studies such as STEP-IN (secukinumab)4 and GUIDE (guselkumab)5 showed higher clearance rates and delayed relapse in early-treated patients. Mechanistic studies revealed that resident memory T cells, key drivers of recurrent disease, are more effectively depleted in early-treated patients, providing a biological explanation for prolonged remission.6
Blauvelt also examined ‘hit hard’ strategies, including the KNOCKOUT study,7 which
tested high-dose risankizumab at up to four-times the standard dose. The findings showed that 83% of participants achieved Psoriasis Area and Severity Index (PASI) 100 at Week 28, and a subset remained disease-free for 2 years without additional safety concerns.7 Looking forward, he explained that next-generation IL-23 inhibitors are being designed to optimise pharmacokinetics for prolonged efficacy, testing the combined ‘hit early, hit hard’ concept in clinical practice.
He concluded with cautious optimism that early, intensive intervention may bring clinicians closer than ever to durable remission, challenging the notion that psoriasis is inevitably chronic and progressive.
Richard Warren, Professor of Dermatology, The University of Manchester, UK, shifted the focus to oral systemic therapies, exploring their evolving role in an era dominated by biologics. While injectable biologics achieve high efficacy, oral agents remain essential for many patients due to convenience, needle aversion, logistical

challenges with home-administered treatments, and accessibility issues in global healthcare settings.
Warren reviewed two key therapeutic classes. Phosphodiesterase-4 (PDE4) inhibitors offer a well-tolerated option without laboratory monitoring, though efficacy remains modest.8,9 Next-generation PDE4 agents have shown promising Phase II efficacy, particularly in achieving PASI 90 responses, but require Phase III validation.10,11 Warren explained that tyrosine kinase 2 inhibitors have been shown to have improved efficacy compared to PDE4 inhibitors, while maintaining a welltolerated safety profile and stable long-term responses. Emerging agents also show the potential to bridge the gap with biologic therapies, offering higher PASI responses and retention of effect.8,12,13
Early, aggressive therapy could deplete pathogenic immune cells and extend remission, potentially altering the natural history of psoriasis
Perhaps most striking, Warren highlighted oral peptides, including a novel, investigational IL-23 receptor antagonist developed using AI to target cytokine binding pockets. Phase III trials have demonstrated near-biologic efficacy, with particularly promising results in high-impact areas such as the scalp and genitals.14
These innovations mark a paradigm shift, combining oral convenience with biologiclevel efficacy, expanding the reach of systemic therapy, and allowing patients with high disease burden but limited body surface involvement to access meaningful treatment. Warren concluded that oral therapies will play an increasingly central role in precision, patient-centred psoriasis care.
The final speaker, Wendy Hall, Department of Nutritional Sciences, School of Life Course & Population Sciences, King’s College London, UK, provided an insightful perspective by examining the role of nutrition in psoriasis management. She explained that patients often ask whether dietary modification can improve symptoms, but robust evidence has historically been limited.
She emphasised the necessity to consider the impact of diet on disease burden in psoriasis. Excess adiposity worsens disease severity and reduces biologic responsiveness, diet influences cardiometabolic comorbidities, and sleep and mental health challenges can affect eating behaviours.15
Reviewing existing guidelines, Hall noted that advice primarily focuses on weight reduction, yet one-third of patients with psoriasis have a BMI under 25, highlighting the need for interventions beyond simple weight control.16 She also explained that observational studies suggest that higher adherence to a Mediterranean diet, dietary approaches to stop hypertension, or plantbased diets correlates with lower psoriasis severity, whereas red and processed meat, sugar, and salt intake is linked to worse outcomes.
Hall then shared results from the METRED-P pilot study,17 which tested Mediterranean and time-restricted eating approaches. Both interventions were feasible, improved quality of life, and reduced self-assessed psoriasis severity. Mediterranean diets enhanced well-being, while time-restricted eating produced modest weight loss, demonstrating that different dietary strategies may confer complementary benefits.
She emphasised the importance of individualised approaches and dietitian support, highlighting that while diet “does matter,” larger, well-powered trials are required to establish causality and refine recommendations.
Across sessions, a common theme emerged: psoriasis care must integrate molecular insights, treatment timing, therapeutic modality, and lifestyle factors. Endotyping offers the potential to classify patients according to biological mechanisms, guiding therapy selection and predicting outcomes. Early, intensive
References
1. Van Bugt LJ et al. Association of HLA-C*06:02 status with differential response to ustekinumab in patients with psoriasis: a systematic review and meta-analysis. JAMA Dermatol. 2019;155(6):708-15.
2. Uppala R et al. “Autoinflammatory psoriasis”—genetics and biology of pustular psoriasis. Cell Mol Immunol. 2020;18(2):307-17.
3. Assan F et al. 280 ADAR1 mutations drive an interferon type I dependent psoriasis subtype. JID. 2024;12(Suppl S277):S277.
4. Iversen L et al. Secukinumab treatment in new-onset psoriasis: aiming to understand the potential for disease modification - rationale and design of the randomized, multicenter STEPIn study. J Eur Acad Dermatol Venereol. 2018;32(11):1930-9.
5. Schäkel K et al. Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance ('clinical super response'): week 28 results from the ongoing phase IIIb randomized, double-blind, parallelgroup, GUIDE study. J Eur Acad Dermatol Venereol. 2023;37(10): 2016-27.
6. Oregon Medical Research Center. High dose risankizumab for psoriasis (KNOCKOUT). NCT05283135. https:// clinicaltrials.gov/study/NCT05283135.
7. Blauvelt A et al. High induction dosing of Risankizumab in patients with
treatment may alter disease trajectory, while oral therapies expand access and convenience without compromising efficacy. Nutrition and lifestyle interventions offer complementary support, particularly in mitigating comorbidities and enhancing well-being. Together, these approaches support a move toward precision, personalised, and patient-centred care.
moderate-to-severe plaque psoriasis: interim results from the phase 2 KNOCKOUT study. LB1703. ICID, 10-13 May, 2023.
8. Armstrong AW et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29-39.
9. Strober B et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40-51.
10. Warren RB et al. Orismilast in moderate-to-severe psoriasis: efficacy and safety from a 16-week, randomized, double-blinded, placebocontrolled, dose-finding, and phase 2b trial (IASOS). J Am Acad Dermatol. 2024;90(3):494-503.
11. Papp K et al. Efficacy and safety of ME3183 administered orally in subjects with moderate to severe plaque psoriasis: a multicenter, randomized, double-blind, placebocontrolled, parallel group, phase 2a study. Abstract 6624. EADV Congress, 11-14 October, 2023.
12. Papp KA. Efficacy and safety of ESK-001, a highly selective oral TYK2 inhibitor, in a phase 2 study in adults with moderate-to-severe plaque
psoriasis (STRIDE). LB1. 2024 AAD Annual Meeting, 8–12 March, 2024.
13. Blauvelt A et al. Efficacy and safety of ESK-001, a highly selective oral TYK2 inhibitor, in moderate-to-severe plaque psoriasis: phase 2 results through week 28. D3T01.3D. EADV Congress, 25-28 September, 2024.
14. Janssen Research & Development, LLC. A study of JNJ-77242113 for the treatment of participants with plaque psoriasis involving special areas (scalp, genital, and/or palms of the hands and the soles of the feet) (ICONIC-TOTAL). NCT06095102. https://clinicaltrials.gov/study/ NCT06095102.
15. Majeed-Ariss R et al. The top 10 research priorities for psoriasis in the U.K.: results of a James Lind Alliance psoriasis Priority Setting Partnership. Br J Dermatol. 2019;181(4):871-3.
16. Burchtein J et al. The association between obesity and efficacy of psoriasis therapies: an expert consensus panel. J Am Acad Dermatol. 2025;92(4):807-15.
17. King's College London. The mediterranean diet and timerestricted eating dietary intervention for psoriasis (METRED-P) study. NCT05820698. https://clinicaltrials. gov/study/NCT05820698.
This review is based on a symposium which took place on 19th September 2025 as part of the European Academy of Dermatology and Venereology (EADV) Congress held in Paris, France, and is intended for healthcare professionals only
Support: This article was funded by Incyte.
Chairperson: José Manuel Carrascosa1
Speakers: Chih-Ho Hong,2 Andreas Wollenberg,3,4 José Manuel Carrascosa1
1. Hospital Universitari Germans Trias i Pujol (IGTP), Universitat Autònoma de Barcelona (UAB), Badalona, Spain
2. University of British Columbia, Vancouver, Canada
3. Augsburg University Hospital, Germany
4. University of Luebeck, Germany
Disclosure:
Hong has served on advisory boards for AbbVie, Amgen, Arcutis, ASLAN pharmaceutics, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dermavant, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, JAMP, Janssen, Leo Pharma, Novartis, Organon, Pfizer, Regeneron, Sanofi-Genzyme, Sun Pharma, and UCB; as a lecturer for AbbVie, Amgen, Arcutis, Bausch Health, Biocon, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Eli Lilly, Galderma, Janssen, Leo Pharma, Novartis, Organon, Pfizer, Sanofi-Genzyme, Sun Pharma, and UCB; and been involved in clinical trials for AbbVie, Amgen, Arcutis, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cutanea (acquired by Biofrontera Inc), Dermira, Dermavant, DS Biopharma, Eli Lilly, Evelo Biosciences, Galderma, GlaxoSmithKline, Incyte, Janssen, Leo Pharma, Medimmune, Merck, Mirimar, Novartis, Pfizer, Regeneron, Reistone, Sanofi-Genzyme, Roche, and UCB. Wollenberg has served as an advisor, speaker, or investigator for AbbVie, Aileens Pharma, Almirall, Amgen, Beiersdorf, Bioderma, Bristol Myers Squibb, Eli Lilly, Galapagos, Galderma, Glenmark, GSK, Hans Karrer, Incyte, Janssen, LEO Pharma, L'Oréal, Maruho, Merck (MSD), Novartis, Pfizer, Pierre Fabre, Regeneron, Sanofi-Aventis, Sandoz, and UCB. Carrascosa has participated as an IP/SI, invited speaker, invited advisor, and/or member of a steering committee for Incyte, Sanofi, Gebro, Nordic Pharma, Leo Pharma, AbbVie, Janssen, Novartis, Almirall, UCB, Eli Lilly, Sandoz, Boehringer Ingelheim, Pfizer, Galderma, and Bristol Myers Squibb.
Acknowledgements: Writing assistance was provided by Allison Kirsop, Scientific Writers Ltd., Aberdeenshire, UK.
Disclaimer: The opinions expressed in this article belong solely to the named speakers and do not necessarily reflect those of Incyte. The event covered in this article was not intended for the UK and Republic of Ireland healthcare professionals.
Keywords: Atopic dermatitis (AD), eczema, flares, inflammatory skin disease, pruritus, ruxolitinib.
Citation: EMJ Dermatol. 2025;13[1]:35-44. https://doi.org/10.33590/emjdermatol/URPI2929
This article summarises the symposium on atopic dermatitis (AD) recorded at the European Academy of Dermatology and Venereology (EADV) Congress, which took place from the 17th–20th September 2025 in Paris, France. The aim was to bring a guideline perspective to the treatment of AD through presentations by leading experts in disease management. AD is driven by skin barrier dysfunction, leading to allergen penetration, chronic inflammation, and an ongoing cycle of immune activation with Th2 cytokines that perpetuate itching and further barrier damage. Treatment satisfaction remains low, so it is important to fully understand who is a ‘moderate’ patient and the treatment options that are available to improve symptoms and quality of life (QoL).
Introduction
AD is one of the most common inflammatory skin conditions,1-4 with global prevalence estimated to be 2.6% (adults: approximately 2.0%; children: approximately 4.0%), and rates vary geographically.4,5 AD is commonly classified as mild, moderate, or severe based on the extent of skin involvement, intensity of symptoms, degree of itch, course of flare-ups, and scoring systems such as the Eczema Area and Severity Index (EASI), Investigator’s Global Assessment (IGA), body surface area (BSA) affected, and Scoring Atopic Dermatitis (SCORAD). Moderate AD usually presents with more widespread lesions, persistent disease activity, and greater impact on QoL compared to mild forms.2 Key characteristics of moderate AD are widespread lesions, frequent flare-ups and exacerbations, significant itch, and sleep disturbance, with patients having impaired QoL and daily functioning.2 Recognising the key clinical characteristics of moderate AD is crucial for providing appropriate and comprehensive management of this patient population.
Topical agents for AD must penetrate the skin barrier, with optimal efficacy influenced
by characteristics including molecular size and lipophilicity. Accordingly, newer small-molecule agents offer promise for more successful topical therapy.6 Globally, treatment options are shaped by the economic context, with limited access in low-resource settings, but the recent inclusion of emollients on the WHO essential medications list is a significant advance.7 AD poses a high burden on patients’ QoL and healthcare systems, particularly in moderate-to-severe cases, and management should be tailored to disease severity, QoL impact, and patient preference using stepped-care guidelines for topical and systemic therapies.
The North American 2018 treatment algorithm for the management of AD provides information for acute and maintenance treatment.8 The Canadian guideline9 adds an emphasis on chronicity, while the European guideline10,11 expands on systemic therapy pathways.
Both the Canadian and European guidelines recommend:
• Confirming AD diagnosis and assessing severity/extent (e.g., BSA, QoL, anatomical sites).
• Starting with optimised skin care and patient education.
• Using topical therapies adjusted to severity (low-potency for mild, higher for more severe cases).
• Monitoring response - 4–6 weeks specific to Canadian guideline.
- Stepped-care approach discussed by European guideline.
• Escalating to alternative topicals, phototherapy, or systemic therapy if control is inadequate.
• Incorporating shared decision-making and regular review of adherence and goals.
Chih-Ho Hong
A well-established problem is skin barrier dysfunction, which allows allergens and pathogens to infiltrate the epidermis, creating an inflammatory response. Cytokines triggered in the pathogenesis of AD require an intracellular JAK inhibitorsignal transducer and activator of transcription proteins (JAK-STAT) pathway to mediate inflammation and transmit itch signals. It is well known that many relevant cytokines in AD are controlled through JAK1 and JAK2 signalling;12-18 for instance, the IL-13 pathway that drives AD, and IL-31 (relevant for itch), both require JAK1 and JAK2 for signalling. Many other essential cytokine pathways, such as IL-22 (relevant for epidermal hyperplasia), signal through JAK1; thymic stromal lymphopoietin (TSLP) signals through JAK1 and JAK2; IL-5, which drives eosinophils, signals through JAK2; and interferon-γ signals through JAK1 and JAK2.
There are high rates of uncontrolled AD reported, with most patients stating itch as the most burdensome symptom of AD, followed by dryness and red or inflamed skin.19 Analyses of treatment satisfaction in adults and adolescents using topical (adults: n=284; adolescents: n=114) and topical
+ systemic (adults: n=110; adolescents: n=30) agents revealed that up to 50% of physicians were less than satisfied with the current level of disease control.20 Adult and adolescent patients surveyed who were using topical (adults: n=152; adolescents: n=80) and topical + systemic (adults: n=65; adolescents: n=17) agents also reported that they were less than satisfied with their disease control (adults: up to 30.8%; adolescents: up to 35.3%).20
There is an inconsistency in the definition of who is a ‘moderate’ patient, and it is crucial to understand where the terms originate to avoid incorrect use. The approval of topical tacrolimus ointment some 20 years ago for moderate-to-severe AD relied on the Rajka and Langeland criteria for defining disease severity.21,22 Here, three domains, extent, disease course, and intensity of itch, are evaluated to produce a score where 6–7 is moderate and 8–9 is severe. However, it may be challenging to conceptualise what this looks like in the clinic.
Modern topicals such as pimecrolimus, crisaborole, and ruxolitinib cream use a more standardised global assessment, where a moderate score is 3.23-29 Additionally, for those in the patient population who are moderate-to-severe and receive systemic agents like biologics or a systemic JAK inhibitor, moderate is identified by having an EASI score of ≥16, ≥10% BSA, and an IGA score of 3 (moderate) or 4 (severe).26-28 Such inconsistency leads to conflicting and different definitions of who is termed ‘moderate’. It is essential to understand these differences when reviewing clinical trials, and for dermatologists to appreciate that it is not necessarily a uniform patient population being discussed.
A Spanish study, MODERMYS-ES, is a cross-sectional survey on the management of moderate AD post-topical corticosteroid (TCS)/topical calcineurin inhibitor (TCI) treatment (N=300). A total of 100 specialists (dermatologists and allergists) treating primarily AD completed questionnaires on
treatment shares, symptoms, and disease severity, looking at the percentage of BSA affected, IGA and EASI scores, disease onset, and comorbidities (de Frutos et al., unpublished data). Most patients were between the ages of 18–50 years (18–30 years: n=135 [45%]; 31–50 years: n=140 [47%]). Over 50% of patients were male (n=168; 56%) and most patients had had the disease since they were infants or children. A high percentage of patients had Type 2 comorbidities, including allergic rhinitis (n=145; 48%), asthma (n=139; 46%), and food allergy (n=64 [21%]; de Frutos et al., unpublished data). The face and hands were most affected (face: n=219 [73%]; hands: n=213 [71%]), almost 80% (n=169) of patients had less than 20% BSA affected, and 44% (n=133) had uncontrolled symptoms (de Frutos et al., unpublished data).
However, where BSA is less than 20%, it is difficult to have an EASI score >16. A typical patient with a similar BSA would have an EASI score of around half. Such patients would not be biologically eligible for reimbursement (in Canada). In addition to sleep disturbance, reduced productivity, and difficulties in daily activities, the visible nature of the condition can result in social stigma and emotional distress, further impacting the patient’s QoL.
Hong considered how an adult patient with chronic AD, an IGA score of 3, 11% affected BSA, and an EASI score of 10 might be currently treated, and how they might be treated post-TCS and -TCI when they are not eligible for biologic or oral JAK inhibitors.
Referring to the MODERMYS-ES study, he showed that cyclosporine was the most common treatment for patients with moderate AD after TCS and TCI, followed by systemic glucocorticosteroids (de Frutos et al., unpublished data). Of a cohort of 300 patients, the largest number were on combined therapies, which included the use of topicals with biologics (25%), conventional systemics (19%), and JAK inhibitors (9%; de Frutos et al., unpublished data).
For patients who are amenable to topical therapy, severity should be established based on clinical signs (erythema, oedema, post-inflammatory hyperpigmentation, excoriation, and lichenification), the extent of disease (%BSA), and patient-reported symptoms, notably itch.9 Initial therapy depends on whether the patient has more severe disease or mild-to-moderate disease (Figure 1). Once patients reach an optimum stage of disease control, treatment should be continued. If disease control is not achieved, patient adherence should be assessed, and the dermatologist should rule out other diagnoses. The current treatment can be adjusted or switched to a topical therapy, phototherapy, or systemic therapy.9
Andreas Wollenberg
When treating a skin disease like AD with a topical agent, the degree of penetration through the epidermal barrier is dependent on the physical characteristics of a substance. For therapeutics, the molecular weight of the agent (optimum size ≤500 Daltons) and other factors, including the extent of lipophilicity, are highly relevant.6 For example, tacrolimus (although larger, at approximately 800 Daltons) can be used topically because the epidermal barrier is disturbed in AD when compared with normal human skin or psoriasis.30,31 Newer agents, such as ruxolitinib cream or crisaborole (both approximately 300 Daltons), are much smaller, and it is reasonable to consider these molecules as an alternative strategy when considering treatment options.
The global treatment landscape for AD is influenced by several factors, the most important being economic.32,33 In Europe, the total cost of moderate-to-severe AD in adults is estimated to be approximately 30 billion EUR per year, with emotional impact and sleep deprivation adding further weight to indirect costs.34 In 2024,
Acute/intermittent/chronic mild-to-moderate AD
All affected areas should be treated at the first sign/symptom of AD, until there is resolution.
Affected body areas
• Use low- to medium-potency TCS QD/BID.*
• In sensitve areas,† use low-potency TCS BID
• Liberal use of emollients as needed.
Infants (3 months–<2 years)
TCI: Pimecrolimus 1% cream BID
Crisborole 2% ointment BID
Initial therapy
Children (2–<12 years)
TCI: Pimecrolimus 1% cream BID
TCI: Tacrolimus 0.03% ointment BID
TPDE-4i: Crisborole 2% ointment BID Roflumilast 0.15% cream QD (≥6 years)
Severe AD
All affected areas should be treated at the first sign/symptom of AD, until there is an improvement in severity
Affected body areas
• Use medium- to high-potency TCS QD/BlD * Following an improvement in severity, use of TCS should be discontinued.
• In sensitive areas,† avoid using medium- to high-potency TCS, and use crisaborole with caution, based on tolerability
• Liberal use of emollients as needed.
(12–<16 years)
TCI: Tacrolimus 0.03% ointment BID
TCI: Pimecrolimus 1% cream BID
TJAKi: Ruxolitinib 1.5% cream BID‡
TPDE-4i: Crisborole 2% ointment BID Roflumilast 0.15% cream QD
Maintenance therapy
(12–16 years)
TCI: Tacrolimus 0.1% ointment BID
TCI: Pimecrolimus 1% cream BID
TJAKi: Ruxolitinib 1.5% cream BID‡
TPDE-4i: Crisborole 2% ointment BID Roflumilast 0.15% cream QD
Maintenance therapy should continue beyond complete resolution to prevent flares and reduce the need for TCS. TCS should be limited to shor t-term use due to sa fety.
Twice-weekly application of a topical anti-inflammato ry agent will likely reduce the incidence of flares. Ruxolitinib cream can be resumed at the first sign of recurrence and stopped 3 days after signs/symptoms have resolved.
*Cochrane review has shown that QD application of TCS is as effective as BID application of TCS.
†Sensitive areas include the face (eyelids and perioral region), neck, axillary/inguinal region, and genital region.
‡Ruxolitinib (1.5% cream) can be resumed at the first sign of recurrence and stopped 3 days after signs/symptoms have resolved.9
AD: atopic dermatitis; BID: twice daily; QD: once daily; TCI: topical calcineurin inhibitor; TCS: topical corticosteroid; TJAKi: topical JAK inhibitor; TPDE-4i: topical phosphodiesterase-4 inhibitor.
a position statement reported that the AD guidelines were not adapted for lowresource settings, highlighting that the number of people to be served across different countries is highly variable.35
Treatment options in high resource settings are diverse, from emollients (humectant, occludent, non-medicated options), TCS, and TCI, 8,32,36-41 to topical preparations and phototherapy (more accepted in Europe than the USA).32,42 In low-resource settings, treatment options are emollients (humectant and occludent), TCSs, and some TCIs if affordable, topical detergents, or wet wraps. In a big step forward for global patient care, the International Society for Atopic Dermatitis (ISAD) has recently been successful in adding emollients to the WHO list of essential medications for treating AD.7 Dermatologists must, therefore, keep
in mind that the problems to be solved are different for each patient and are full of subjective burden regarding specific treatment options.
The European guideline (Figure 2) also contains useful definitions and information about treatment goals for patients with AD, including short-term and long-term therapies, reactive versus proactive treatment, and the clinical definition of flares.10
Ultimately, patient adherence is influenced by the choice of treatment and the interaction between physician and patient.43 For patients, the itch is the most burdensome part of their disease, and most care about achieving symptom relief, especially itch, before complete clearance of their physical lesions.
José-Manuel Carrascosa
There are multiple cytokines involved in AD that require the JAK-STAT pathway to mediate inflammation and transmit itch signals. This presentation reviewed some clinical trial results for ruxolitinib, a selective JAK1 and JAK2 inhibitor.
Ruxolitinib
in Atopic Dermatitis: TRuE-AD144 and TRuE-AD245
Eligible patients were at least 12 years of age with a minimum of 3 years’ history of AD, diagnosed as mild-to-moderate, and had an IGA score of 2 or 3 with BSA
3–20% (excluding scalp). Patients were randomised 2:2:1 to receive two doses of ruxolitinib, 1.5% twice daily and 0.75% twice daily for 8 weeks, with no rescue treatment permitted during this period. At 8 weeks, patients with an IGA 0–4 score could enter the long-term safety period. Patients initially randomised to ruxolitinib remained on their original regimen, and those on vehicle were randomised 1:1 to either 1.5% or 0.75% ruxolitinib twice daily. Treat-asneeded through Week 52 was performed, with treatment stopped 3 days after lesion clearance and restarting with lesion recurrence. Again, no rescue treatment was permitted, and there were site visits every 4 weeks.
The distribution of clinical characteristics was similar across treatment groups. The total population had a mean BSA of 9.8%
and select from (if appropraite): Add antiseptic/antibiotic/antiviral/antifungal treatment in cases of infections
Continue measures
Consider compliance and diagnosis, if therapy has insufficient effect
*Refer to guideline text for licensed indication, †restrictions, and ‡off-label treatment.
Dark green boxes indicate strong recommendation for the use of an intervention. Light green boxes indicate weak recommendation for the use of an intervention. For definitions of disease severity (acute, reactive, and proactive) see section VII and the ‘Introduction to Systemic Treatment’ section of the EuroGuiDerm Atopic Eczema Guideline.11 Abro: abrocitinib; AZA: azathioprine; bari: baricitinib; CyA: ciclosporin; dupi: dupilumab; lebri: lebrikizumab; MTX: methotrexate; TCI: topical calcineurin inhibitors; TCS: topical corticosteroids; tralo: tralokinumab; upa: upadicitinib; NB-UVB: narrow band UV B.
(±5.4) and a baseline EASI of 8.0% (±4.8), and 75.0% of patients had an IGA score of 3. A total of 63.9% of patients had an Itch Numeric Rating Scale (NRS) score ≥4, and many patients (38.8%) had facial involvement. Mean flares in the past 12 months were 5.9, indicating this to be a patient population that remains in need of constant therapy. Notably, 90% of patients had received prior therapies for AD, which included different potencies of TCS (low: 49.6%; medium: 42.4%; high: 32.7%), TCIs (21.5%), and systemic corticosteroids (17.5%). Most treatment benefit was observed within the first 4 weeks. Significantly more patients who applied ruxolitinib cream achieved the primary endpoint of IGA-treatment success (IGATS), defined as an IGA score of 0 (clear) or 1 (almost clear) with at least a 2-point improvement from baseline. At 8 weeks, the greatest improvements were achieved by patients administered with 1.5% and 0.75% ruxolitinib (54.0% and 51.0%, respectively; p<0.0001) when compared with the vehicle group (7.6–15.1%).
Sub-analyses were performed to identify a moderate AD profile population defined by BSA ≥10% or EASI ≥16 at baseline. At Week 8, more patients in the moderate AD group achieved IGA-TS with ruxolitinib cream compared with vehicle. Improvements in EASI-75 (defined as achieving ≥75% improvement in EASI score) and Itch NRS were consistent across both TRuE-AD1 and TRuE-AD2 studies, and significantly more patients who applied ruxolitinib cream achieved EASI-75, a clinically meaningful improvement in itch compared with vehicle (p<0.05). In patients with moderate AD (BSA ≥10% and EASI ≥16 at baseline), ruxolitinib cream appeared to be highly efficacious.
Results from the long-term safety period (IGA) showed that the proportion of patients who achieved clear or almost clear skin was maintained throughout, with ruxolitinib used when needed. After 52 weeks, between 74–78% of patients achieved IGA 0–1 when the topical therapy was used, and the data suggest that ruxolitinib cream may delay or prevent the need for systemic treatment in a subset of patients with moderate AD profile (IGA
score of 3, BSA ≥10%, and EASI ≥16 at baseline; Figure 3).
Regarding the safety profile, most adverse events were mild-to-moderate, with no marked difference between the groups. The most common treatment-emergent adverse events were upper respiratory tract infection and nasopharyngitis. Local reactions, such as burning and itching, were <1% and higher in patients treated with vehicle. Approximately 2% of patients discontinued treatment due to adverse events or serious adverse events, and in most cases, these were not considered related to the drug under the investigator period.
Itch is the most critical symptom to address in patients with moderate AD, and Carrascosa presented data from other studies, the results of which were highlighted as essential for shared decision-making.
In this small, double-blind, vehiclecontrolled, Phase 2 study of patients with facial and neck AD (N=77),47 patients were randomised to receive 1.5% ruxolitinib cream (n=54) or vehicle cream (n=23). After 4 weeks of continuous therapy, more than 40% of patients who applied ruxolitinib cream (n=48) compared with vehicle (n=18) saw improvements for facial and neck IGATS (IGA 0/1 with ≥2-point improvement from baseline) and achieved head and neck EASI-75 (37.0%; 95% CI: 24.3–51.3% versus 17.4%; 95% CI: 5.0–38.8%; p=0.091).48 Improvements in patient-oriented eczema measure (POEM) scores from baseline were observed and maintained from Week 2 (mean change from baseline: −10.4 versus −3.4) through Week 4 (–11.1 versus −3.7). When patients in the vehicle group switched to ruxolitinib cream at Week 4, similar improvements were observed at Week 8 (–11.1) in those patients randomised to ruxolitinib cream (–11.2).48
Figure 3: TRuE-AD 1 and 2 study sub-analyses: proportion of patients with moderate atopic dermatitis achieving Investigator’s Global Assessment 0/1 and their mean percentage of body surface area affected in the long-term safety period.46
These data suggest that ruxolitinib cream may delay or prevent the need for systemic therapy in a subset of patients with moderate AD (IGA=3, BSA ≥10% and EASI ≥16 at baseline)
*The VC period included up to Week 8, and the LTS period included Weeks 8–52. Data for Week 8 are from the VC period.
AD: atopic dermatitis; BSA: body surface area; EASI: Eczema Area and Severity Index; IGA: Investigator’s Global Assessment; LTS: long-term safety; RUX: ruxolitinib cream; VC: vehicle-controlled.
Evaluation of the Effect of Ruxolitinib Cream on Itch in Participants with Atopic Dermatitis (SCRATCH-AD)49
The Phase 2, open-label study SCRATCHAD49 investigated the short-term clinical benefits of this topical agent. Participants with AD (N=46) who applied ruxolitinib cream 1.5% experienced fast and considerable improvement in itch, which was sustained through 28 days of treatment. Itch reduction occurred from Day 1, as early as 15 minutes, and maximum reduction was observed at 4 hours after application of the cream. Post-treatment, patients experienced an improvement of >2 points and the effect continued through 12 hours. Mean change from baseline in modified Peak Pruritus NRS (PP-NRS) was –2.3 (after 15 minutes), peaking at –4.2 (at 4 hours) and –3.1 (at 12 hours).50
Evaluation of the Efficacy and Safety Study of Ruxolitinib Cream in Adults with Moderate Atopic Dermatitis (TRuE-AD4)51
The TRuE-AD4 study has been designed to establish the efficacy of ruxolitinib cream in participants with moderate AD who had an inadequate response to, are intolerant to, or are contraindicated to TCSs and TCIs.51 Patients will be randomised (2:1; N=225 [expected]) to receive ruxolitinib cream 1.5% twice daily (n=150) or vehicle twice daily (n=75) for up to 8 weeks. After this period, patients with no additional safety concerns will continue, and those who are not able to achieve at least EASI-50 will be switched to an escape arm and open-label ruxolitinib 1.5% twice daily as needed until the end of the study at Week 24.
Co-primary endpoints for the study are the proportion of participants with EASI-75 from baseline at Week 8 and the proportion of participants with IGA-TS at Week 8.
Key secondary objectives will include an evaluation of the impact of ruxolitinib on patients’ QoL.
Moderate AD covers a wide patient spectrum and should be defined using multiple clinical factors, including BSA, assessment scores, impact on QoL, and affected anatomical regions.9,10
Older topical agents like steroids may assist in short-term acute flare management but are less suitable for ongoing control in moderate cases, especially with extensive disease.9 Newer topical treatments, such as
1. Chu DK et al.; AAAAI/ACAAI JTF Atopic Dermatitis Guideline Panel; Patient Groups: Global Parents for Eczema Research; National Eczema Association; Evidence in Allergy Group; AAAAI/ACAAI Joint Task Force on Practice Parameters. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE- and Institute of Medicinebased recommendations. Ann Allergy Asthma Immunol. 2024;132(3): 274-312.
2. Langan SM et al. Atopic dermatitis. Lancet. 2020;396(10247):345-60.
3. Guttman-Yassky E et al. Atopic dermatitis. Lancet. 2025;405(10478):583-96.
4. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403(10440):2133-61.
5. Tian J et al. Global epidemiology of atopic dermatitis: a comprehensive systematic analysis and modelling study. Br J Dermatol. 2023;190(1): 55-61.
6. Gorzelanny C et al. Skin barriers in dermal drug delivery: which barriers
ruxolitinib cream, indicate efficacy for rapid itch relief and sustained improvement, for both acute flares and long-term disease maintenance.9 Effective topicals may reduce the need for systemic therapies in moderate AD, provided they are both efficacious and well-tolerated.9,10,11
Moderate-to-severe AD imposes substantial individual and socioeconomic burdens, with persistent symptoms and reduced QoL remaining common due to inconsistent definitions and care fragmentation.2,32-34 Best management includes shared decisionmaking, considering disease severity, QoL impact, treatment responses, and risk of relapse.1,9,52-55 Ruxolitinib cream provides a novel topical option, and ongoing studies may further define its role.29,50
have to be overcome and how can we measure them? Pharmaceutics. 2020;12(7):684.
7. World Health Organization (WHO). The selection and use of essential medicines, 2025: report of the 25th WHO Expert Committee on selection and use of essential medicines, executive summary. 2025. Available at: https://www.who.int/publications/i/ item/B09544. Last accessed: 28 September 2025.
8. Boguniewicz M et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10-22.e2.
9. Gooderham MJ et al. Canadian consensus guidelines for the management of atopic dermatitis with topical therapies. Dermatol Ther (Heidelb). 2025;15(6):1467-85.
10. Wollenberg A et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9): 1409-31.
11. Wollenberg A et al. European Guideline (EuroGuiDerm) on atopic eczema: living update. J Eur Acad Dermatol Venereol. 2025;39(9):1537-66.
12. Howell MD et al. Targeting the janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342.
13. Howell MD et al. JAK/STAT inhibitors and other small molecule cytokine antagonists for the treatment of allergic disease. Ann Allergy Asthma
Immunol. 2018;120(4):367-75.
14. Lee H et al. TSLP down-regulates S100A7 and ß-Defensin 2 via the JAK2/STAT3-dependent mechanism. J Invest Dermatol. 2016;136(12):242735.
15. Bao L et al. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: implication for atopic dermatitis. Mol Immunol. 2012;50(12):91-7.
16. Borriello F et al. IL-3 synergises with basophil-derived IL-4 and IL-13 to promote the alternative activation of human monocytes. Eur J Immunol. 2015;45(7):2042-51.
17. Howell MD et al. Past, present, and future for biologic intervention in atopic dermatitis. Allergy. 2015;70(8):887-96.
18. Solimani F et al. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847.
19. Silverberg JI et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based crosssectional study. Ann Allergy Asthma Immunol. 2018;121(3):340-7.
20. Anderson P et al. Inadequate disease control, treatment dissatisfaction, and quality-of-life impairments among US patients receiving topical therapy for atopic dermatitis. Dermatol Ther (Heidelb). 2021;11(5):1571-85.
21. Ruzicka T et al. A short-term trial of tacrolimus ointment for atopic
dermatitis. European Tacrolimus Multicenter Atopic Dermatitis Study Group. N Engl J Med. 1997;337(12):816-21.
22. Silverberg JI et al. Measurement properties of the Rajka-Langeland severity score in children and adults with atopic dermatitis. Br J Dermatol. 2021;184(1):87-95.
23. Eichenfield LF et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002;46(4):495-504.
24. Paller AS et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494-503.e6.
25. Simpson EL et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335-48.
26. Wollenberg A et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437-49.
27. Simpson EL et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255-66.
28. Guttman-Yassky E et al. Oncedaily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate doubleblind, randomised controlled phase 3 trials. Lancet. 2021;397(10290): 2151-68.
29. Papp K et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4): 863-72.
30. Cork MJ et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129(8): 1892-908.
31. De Benedetto A et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127(3):773-86.e1-7.
32. Wollenberg A et al. ETFAD/EADV Eczema task force 2020 position
paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717-44.
33. Wollenberg A et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850-78.
34. Augustin M et al. Unveiling the true costs and societal impacts of moderate-to-severe atopic dermatitis in Europe. J Eur Acad Dermatol Venereol. 2022;36(Suppl 7):3-16.
35. Faye O et al. Atopic dermatitis: a global health perspective. J Eur Acad Dermatol Venereol. 2024;38(5):801-11.
36. Wollenberg A et al. European guideline (EuroGuiDerm) on atopic eczema - part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36(11):1904-26.
37. Eichenfield LF et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4s):S49-57.
38. Mayba JN, Gooderham MJ. Review of atopic dermatitis and topical therapies. J Cutan Med Surg. 2017;21(3):227-36.
39. Eichenfield LF et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116-32.
40. Hengge UR et al. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1-15;quiz 16-8.
41. Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15(4):303-10.
42. Vangipuram R, Feldman SR. Ultraviolet phototherapy for cutaneous diseases: a concise review. Oral Dis. 2016;22(4):253-9.
43. Eicher L et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease - strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33(12): 2253-63.
44. Incyte Corporation. Topical ruxolitinib evaluation in atopic dermatitis study 1 (TRuE AD1) - an efficacy and safety study of ruxolitinib cream in adolescents and adults with atopic
dermatitis. NCT03745638. https:// clinicaltrials.gov/study/NCT03745638.
45. Incyte Corporation. TRuE AD2 - an efficacy and safety study of ruxolitinib cream in adolescents and adults with atopic dermatitis. NCT03745651. https://clinicaltrials.gov/study/ NCT03745651.
46. Simpson E. Long-term safety and disease control with ruxolitinib cream in patients with more severe atopic dermatitis: pooled results from two phase 3 studies. SKIN. 2021;5(6):s66.
47. Incyte Corporation. Ruxolitinib cream in participants with facial and/or neck atopic dermatitis involvement. NCT05127421. https://clinicaltrials.gov/ study/NCT05127421.
48. Chiesa Fuxench ZC et al. Ruxolitinib cream monotherapy for facial and/or neck atopic dermatitis: results from a decentralized, randomized phase 2 clinical trial. J Dermatolog Treat. 2025;36(1):2480744.
49. Incyte Corporation. The purpose of the study is to evaluate the effect of ruxolitinib cream on itch in participants with atopic dermatitis (SCRATCH-AD). NCT04839380. https://clinicaltrials. gov/study/NCT04839380.
50. Bissonnette R et al. 396 Rapid, substantial and sustained reduction of itch in adults with atopic dermatitis applying ruxolitinib cream 1.5% (SCRATCH-AD). Br J Dermatol. 2023;188(3):ljad162.021.
51. Incyte Corporation. A study to evaluate the efficacy, and safety study of ruxolitinib cream in adults with moderate atopic dermatitis (TRuE-AD4). NCT06238817. https:// clinicaltrials.gov/study/NCT06238817.
52. Davis DMR et al. Executive summary: guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol. 2024;90(2): 342-5.
53. Robinson L, Strowd LC. American Academy of Dermatology guidelines for managing atopic dermatitis. Adv Exp Med Biol. 2024;1447:217-25.
54. Sidbury R et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89(1):e1-20.
55. Yang YB et al. Brief academic review and clinical practice guidelines for pediatric atopic dermatitis. Curr Pediatr Rev. 2021;17(3):229-37. EU/AD/NP/25/0008 October 2025
This review is based on the Expert Hub Session that took place on the 19th September 2025 as part of the European Academy of Dermatology and Venereology (EADV) Congress held in Paris, France, and is intended for healthcare professionals only
Support: This content was funded by Incyte.
Chairperson: Alexander Zink1
Speakers: Alexander Zink,1 Angelo Marzano2,3
1. Department of Dermatology and Allergy, Technical University of Munich, Germany
2. Dermatology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
3. Dermatology and Venereology, Università degli Studi di Milano, Italy
Disclosure:
Zink has been an advisor for, received speaker’s honoraria from, received grants from, and/or participated in clinical trials for AbbVie, ALK Abelló, Almirall, Amgen, Beiersdorf Dermo Medical, Bencard Allergie, Bristol Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Incyte, Janssen Cilag, Leo Pharma, Miltenyi Biotec, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi-Aventis, Takeda Pharma, Thermo Fisher Scientific (Phadia), and UCB. Marzano declares associations with AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Sanofi, and UCB.
Acknowledgements: Writing assistance was provided by Samantha Stanbury, Stockport, UK.
Disclaimer:
The opinions expressed in this article belong solely to the named speakers. The event covered in this article was not intended for the UK and Republic of Ireland healthcare professionals.
Keywords: Facial involvement, genital involvement, non-segmental vitiligo, phototherapy, repigmentation, ruxolitinib, ruxolitinib cream, topical, vitiligo.
Citation: EMJ Dermatol. 2025;13[1]:45-50. https://doi.org/10.33590/emjdermatol/ZDBZ8505
Ruxolitinib cream was approved in Europe for the treatment of non-segmental vitiligo with facial involvement in adults and adolescents (aged ≥12 years) in 2023. Speaking at an Expert Hub session at the European Academy of Dermatology and Venereology (EADV) 2025 Congress, Alexander Zink, Professor, Department of Dermatology and Allergy, Technical University of Munich, Germany; and Angelo
Marzano, Professor of Dermatology and Head of Dermatology Unit, Fondazione
Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, discussed how the introduction of ruxolitinib cream has shaped the management of vitiligo in the 2 years since its approval in this indication, based on insights from their own clinical experience. They described positive outcomes in patients who had used ruxolitinib cream, and discussed its practical use in real-world settings, including stopping and restarting treatment. Recent data on the efficacy of ruxolitinib cream in vitiligo with genital involvement, and when used in combination with phototherapy, were also presented.
Zink opened the session by recapping key efficacy data for ruxolitinib cream in non-segmental vitiligo from the TRuE-V1/2 registration trials,1 which demonstrated significant improvements in Vitiligo Area Scoring Index (VASI) scores, with approximately half of patients achieving ≥75% improvement in facial VASI score (F-VASI75) at Week 52.2 Repigmentation was observed across body regions,3 and half of patients achieved ≥50% improvement in total VASI score (T-VASI50) at Week 52.2
Zink went on to present data from a recent Phase 2 study in patients with vitiligo involving the genital region (NCT05750823), which he described as an area of unmet need. The efficacy of ruxolitinib cream in genital regions was similar to total body efficacy, with an approximately 30% reduction in both genital VASI and T-VASI scores and an almost 20% reduction in genital body surface area involvement observed at Week 48. Over 20% of patients rated their genital vitiligo as a lot less or no longer noticeable.4
Ruxolitinib in Combination with Narrow-band UVB Phototherapy
The need for phototherapy alongside topical treatment for vitiligo is debated. Its role was explored in a recent Phase 2 open-label study (NCT03099304), in which patients who did not achieve T-VASI25 during 12 weeks of treatment with ruxolitinib cream alone were additionally treated with narrow-band UVB phototherapy three times per week for a further 36 weeks. Phototherapy appeared to accelerate the achievement of F-VASI and
T-VASI responses in patients who did not respond quickly to ruxolitinib monotherapy.5
The question of whether patients can stop treatment once they achieve satisfactory repigmentation was addressed in a long-term extension to TRuE-V1/2. A cohort of patients with an F-VASI90 response at Week 52 was randomised to continuation or withdrawal of ruxolitinib. Among patients who stopped treatment (n=56), 39% maintained response at >F-VASI75 to Week 104, but 29% relapsed (<F-VASI75) within a year of stopping (compared with 69% and 15%, respectively, among patients who continued treatment).6 Patients who relapsed could resume ruxolitinib as rescue treatment; 75% of these patients regained F-VASI75 after a median of 12 weeks’ retreatment, and approximately 70% regained F-VASI90 after a median of 15 weeks.6 In real-world practice, patients may stop and restart treatment; these data support retreatment as an effective approach.
The efficacy of ruxolitinib cream demonstrated in clinical trials is reflected in the real-world experience of patients with vitiligo, illustrated in patient cases that Zink and Marzano shared from their own clinical practice.
Zink described a series of patients with vitiligo with facial involvement and confirmed that all had responded to ruxolitinib treatment.
He described some as ‘super-responders’, who achieved complete or near complete repigmentation within a few weeks (Figure 1), while others (typically those with fairer skin) were later responders. One patient had relapsed during a treatment break, but regained a good response on retreatment.

Concurring that time to response varies between patients, Marzano highlighted the importance of managing expectations to encourage treatment adherence in patients who may require prolonged treatment to achieve a satisfactory response. He recommended continuing treatment for at least a year in these patients to ensure both adequate treatment duration and that treatment continues through the summer season, as patients may benefit from the addition of ‘natural phototherapy’.
Marzano outlined outcomes in patients participating in a trial running in his own clinic. Results to date suggest efficacy in line with that observed in the TRuE-V1/2 studies (unpublished data), and patientreported outcomes reveal very good patient perception of improvement.
Another patient had improvement in vitiligoassociated leukotrichia in his beard, as well as skin repigmentation. Zink also presented an example of a female patient with genital involvement who had a gradual response and achieved complete repigmentation after approximately 1 year of treatment (Figure 2).

He showed examples of patients who had achieved repigmentation of the face and other body areas, including a young female patient who never wore skirts/dresses because she wanted to hide vitiligo lesions on her knees. After 1 year of ruxolitinib treatment, she had a meaningful response (Figure 3) and no longer felt restricted in her choice of clothes, which had a positive impact on her mental health. Zink also described a patient who had grown his hair long to hide vitiligo lesions on his forehead but was able to cut his hair after achieving repigmentation with ruxolitinib cream. Marzano highlighted the value of talking to patients about how vitiligo impacts their lives to identify high-impact sites and support shared decision-making regarding treatment priorities.
2: Patient case: resolution of genital vitiligo with ruxolitinib cream.

Female (24) with a history of vitiligo for approximately 2 years who had prior treatment with a topical calcineurin inhibitor. She commenced ruxolitinib cream monotherapy in September 2023 (A; baseline). Response was limited for approximately 5 months of treatment (B). Substantial response was seen at approximately 8 months (C), with complete repigmentation achieved after approximately 11 months (D)
3: Patient case: meaningful response of facial and knee lesions to ruxolitinib cream.
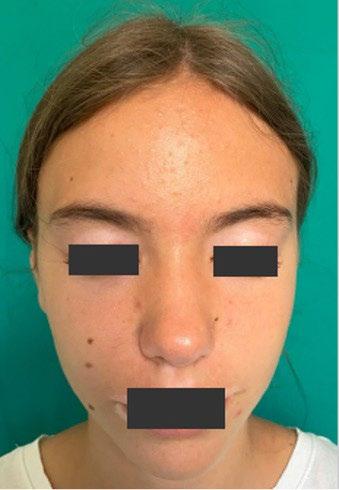
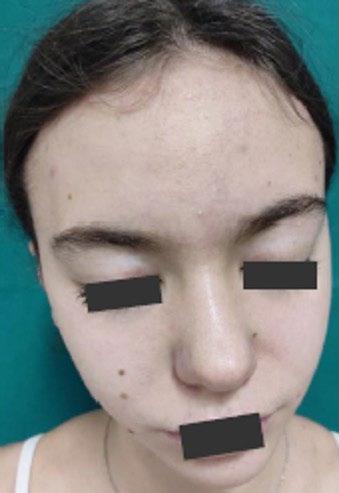




A and D) Baseline; Facial-VESplus: 0.3375; Total-VESplus: 0.76375; CDLQI: 3. B and E) 24 weeks; Facial-VESplus: 0.0785; Total-VESplus: 0.546; CDLQI: 0. C and F) 52 weeks; Facial-VESplus: 0.0785; Total-VESplus: 0.536; CDLQI: 0.
Female (15) with a 4-year history of non-segmental vitiligo. She was treated with ruxolitinib cream twice daily on the face and knees as monotherapy. A response was observed by Week 24, with further improvement of knee lesions by Week 52. Patient-reported quality of life was also substantially improved.
CDLQI: Children’s Dermatology Life Quality Index; VESplus: Vitiligo Extent Score with perifollicular scale.
Both experts concurred that the safety/ tolerability profile of ruxolitinib cream was excellent. Zink stated that he had never had a patient discontinue ruxolitinib cream due to local reactions.
More than 2 years’ experience with ruxolitinib cream in real-world clinical settings in Europe shows that the efficacy demonstrated in clinical trials is reflected
in real-world effectiveness, and produces meaningful improvements for patients with vitiligo. Recent data support a role for ruxolitinib in patients with genital involvement and suggest a possible role for narrow-band UVB phototherapy to accelerate responses. Retreatment has proven effective in patients who relapse after stopping treatment, supporting the use of a stop–start strategy. Marzano highlighted a need for consensus on an optimal postrepigmentation maintenance schedule.
References
1. Rosmarin D et al. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387(16):1445-55.
2. Wolkerstorfer A et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo: week 52 pooled analysis of the TRuE-V phase 3 studies. Abstract FC01.04. EADV Congress, 7-10 September, 2022.
3. Passeron T et al. Repigmentation by body region in patients with vitiligo treated with ruxolitinib cream over 52 weeks. J Eur Acad Dermatol Venereol. 2025;39(3):e251-4.
4. Passeron T et al. An open-label, phase 2, safety and efficacy study of ruxolitinib cream in patients with genital vitiligo. Poster 3000118. GVF Annual Scientific Symposium, 6 March, 2025.
5. Pandya AG et al. Efficacy and safety of ruxolitinib cream combined with NB-UVB phototherapy for treatment of vitiligo. Vitiligo International Symposium (VIS), 13-15 December, 2024.
6. Harris JE et al. Relapse and maintenance of clinical response in the randomized withdrawal arm of the TRuE-V long-term extension phase 3 study of ruxolitinib cream in vitiligo. Abstract 46159. AAD Annual Meeting, 17-21 March, 2023.

Based on highlights from the European Academy of Dermatology and Venereology (EADV) Congress 2025, these abstract reviews showcase significant developments and emerging priorities within the field of dermatology.
Authors: Yagiz Matthew Akiska,1,2 Savanna I. Vidal,1 Nikita Menta,1 Mana Nasseri,1 Dillon Nussbaum,1 Colleen H. Cotton,1,3 Leslie Castelo-Soccio,1,3 *Adam Friedman1
1. Department of Dermatology, George Washington University School of Medicine and Health Sciences, Washington D.C., USA
2. Milken Institute School of Public Health, George Washington University, Washington D.C., USA
3. Department of Dermatology, Children’s National Hospital, Washington D.C., USA *Correspondence to ajfriedman@mfa.gwu.edu
Disclosure: Akiska has received a travel grant from the National Alopecia Areata Foundation (NAAF) to attend the 2025 European Academy of Dermatology and Venereology (EADV) Congress in Paris, France.
Keywords: Alopecia, alopecia areata (AA), androgenic alopecia (AGA), diabetes, glucagon-like peptide 1 receptor agonists (GLP-1RA), hair loss (HL), obesity, telogen effluvium (TE).
Citation: EMJ Dermatol. 2025;13[1]:52-54. https://doi.org/10.33590/emjdermatol/TYEW1122
Glucagon-like peptide-1 receptor agonists (GLP-1RA) are widely prescribed for Type 2 diabetes and obesity owing to their robust metabolic and cardiovascular benefits. Emerging reports, however, suggest a potential link between GLP-1RA use and non-scarring hair loss (NSHL), including telogen effluvium (TE), androgenic alopecia (AGA), and alopecia areata (AA). To clarify this association, the authors conducted the first large-scale, real-world multicentre cohort study evaluating GLP-1RA exposure and risk of hair loss (HL).1
This retrospective analysis leveraged the TriNetX US Collaborative Network (2014–2024), comprising 67 healthcare organisations and >100 million patients.
Adults (18–89 years) with ≥2 instances of GLP-1RA prescriptions (liraglutide, semaglutide, dulaglutide, exenatide, lixisenatide, or tirzepatide) were included. Patients with prior alopecia or confounding conditions, such as thyroid disease, ovarian dysfunction, menopause, malnutrition, chemotherapy, connective tissue disease, scarring alopecias, bariatric surgery, or trichotillomania, were excluded. Propensity score matching was performed for age, sex, race/ethnicity, BMI, and Type 2 diabetes status. HL outcomes were defined using the 10th version of the International Classification of Diseases (ICD-10) codes, with adjusted odds ratios (aOR) estimated at 6 and 12 months.
Matched adult cohorts (n=547,993 each) were well balanced (mean age: approximately 53 years; approximately 50% female; 65% White, 17–18% Black, 10% Hispanic/Latino; Table 1). From 2014–2024, the incidence of TE, AGA, and overall NSHL rose more steeply in GLP-1RA users compared with controls. At 6 months, GLP-1RA exposure was associated with increased risk of AGA (aOR: 1.62; 95% CI: 1.24–2.12) and overall NSHL (aOR: 1.26; 95% CI: 1.15–1.38), while TE and AA were non-significant. By 12 months, risk was significantly elevated for TE (aOR: 1.76; 95% CI: 1.34–2.32), AGA (aOR: 1.64; 95% CI: 1.35–1.99), and overall NSHL (aOR: 1.40; 95% CI: 1.31–1.49), with AA remaining non-significant.
These findings align with pharmacovigilance signals from the FDA Adverse Event Reporting System (FAERS) and VigiBase, which reported increased odds of alopecia with semaglutide and tirzepatide.2,3 Proposed mechanisms include rapid weight loss precipitating TE, metabolic and hormonal shifts accelerating AGA, and possible direct follicular effects
Table 1: Baseline demographic and clinical characteristics of glucagon-like peptide-1 receptor agonist users and controls before and after propensity score matching.
GLP-1RA: glucagon-like peptide-1 receptor agonist. After matching
given GLP-1 receptor expression in hair follicles.4-6 In contrast, case reports have described improvement in scarring alopecias such as folliculitis decalvans and central centrifugal cicatricial alopecia with GLP-1RAs,7,8 suggesting divergent and complex biological pathways. Temporal trends further reinforce this signal: both cohorts experienced a spike in TE incidence around 2020, likely reflecting the impact of COVID-19, followed by a sustained rise among GLP-1RA users coinciding with the widespread adoption of semaglutide and tirzepatide.9
Together, these data support the authors’ recommendation that all patients initiating GLP-1RAs should be counselled regarding the potential HL risk. For those presenting with new-onset shedding, nutritional and endocrine evaluation should be prioritised, and early referral to dermatology may improve outcomes and adherence. Educating patients on HL risks prior to therapy initiation may help set expectations, promote timely reporting, and inspire exploration of potential methods to prevent TE in this setting.
In conclusion, GLP-1RA therapy was independently associated with increased incidence of TE, AGA, and overall NSHL in adults, but not AA. While causality cannot be established from observational data, this large, real-world dataset study provides
compelling evidence of a clinically relevant signal, highlighting the need for monitoring, patient education, and further mechanistic investigation.
References
1. Akiska YM et al. Increased incidence and risk of hair loss with GLP-1 receptor agonists: a real-world multicenter cohort study. Abstract P2948. EADV Congress 2025, 17-20 September, 2025.
2. Kim TH et al. Adverse drug reaction patterns of GLP-1 receptor agonists approved for obesity treatment: disproportionality analysis from global pharmacovigilance database. Diabetes Obes Metab. 2025;27(6):3490-502.
3. Godfrey H et al. Alopecia associated with semaglutide and tirzepatide: a disproportionality analysis from the FDA adverse event reporting system (FAERS) from 2022 to 2023. J Eur Acad Dermatol Venereol. 2025;39(2):e153-4.
4. Zhang Y et al. Glucagon-like peptide-1 receptor agonists decrease hyperinsulinemia and hyperandrogenemia in dehydroepiandrosteroneinduced polycystic ovary syndrome mice and are associated with mitigating inflammation and inducing browning of white adipose tissue. Biol Reprod. 2023;108(6):945-59.
5. Desai DD et al. GLP-1 agonists and hair loss: a call for further investigation. Int J Dermatol. 2024;63(9):1128-30.
6. Coogan PF et al. Association of type 2 diabetes with central-scalp hair loss in a large cohort study of African American women. Int J Womens Dermatol. 2019;5(4):261-6.
7. Morrissette K et al. Improvement of recalcitrant folliculitis decalvans with tirzepatide: a case report. Cureus. 2024;16(12):e76267.
8. Desir N et al. GLP-1 agonists may modulate treatment efficacy in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2025;93(3):771-3.
9. Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77.
Authors: Kiya Abebe,1 Anders Munch,2 Anne Lene Wagenblast,1 Grethe Schmidt,1 David Hebbelstrup Jensen,3 Michael M. Petersen,4,5 Anand C. Loya,6 Søren Daugaard,6 Thomas Mentzel,7 Mikkel Herly,1,8 Peter Vester-Glowinski,1,5 *Mathias Ørholt1
1. Department of Plastic Surgery and Burns Treatment, Copenhagen University Hospital, Rigshospitalet, Denmark
2. Section of Biostatistics, University of Copenhagen, Denmark
3. Department of Plastic Surgery, Odense University Hospital, Denmark
4. Department of Orthopedic Surgery, Musculoskeletal Tumor Section, Copenhagen University Hospital, Rigshospitalet, Denmark
5. Department of Clinical Medicine, University of Copenhagen, Denmark
6. Department of Pathology, Copenhagen University Hospital, Rigshospitalet, Denmark
7. Department of Pathology, University of Freiburg, Freiburg im Breisgau, Germany
8. Department of Immunology and Microbiology, Copenhagen University Hospital, Denmark
*Correspondence to mathias.oerholt.nielsen@regionh.dk
Disclosure: Loya has received support for the present manuscript from Copenhagen University Hospital Rigshospitalet and the University of Copenhagen. Wagenblast has received support for the present manuscript from Copenhagen University Hospital Rigshospitalet. Munch has received support for the present manuscript from the University of Copenhagen. Jensen has received support for the present manuscript from Odense University Hospital. Schmidt has received support for the present manuscript from Copenhagen University Hospital Rigshospitalet. Abebe has received support for the present manuscript from the Danish Medical Society and the research fund of Copenhagen University Hospital Rigshospitalet. Herly has received support for the present manuscript from Copenhagen University Hospital Rigshospitalet and the University of Copenhagen. Peterson has received support for the present manuscript from Copenhagen University Hospital Rigshospitalet and the University of Copenhagen; and travel grants from Cancers. Ørholt has received support for the present manuscript from the research fund of Copenhagen University Hospital Rigshospitalet. Vester-Glowinski has received support for the current manuscript from the University of Copenhagen and Copenhagen University Hospital Rigshospitalet. Daugaard has received support for the present manuscript from
Copenhagen University Hospital Rigshospitalet. Mentzel has received support for the present manuscript from the University of Freiburg.
Keywords: Cutaneous leiomyosarcoma, dermal leiomyosarcoma (dLMS), dermatology, epidemiology, follow-up guidelines, oncology, prognosis, prognostic factors, sarcoma, skin cancer.
Citation: EMJ Dermatol. 2025;13[1]:55-57. https://doi.org/10.33590/emjdermatol/WCYG5360
Cutaneous leiomyosarcoma is a rare smooth muscle neoplasm that can be classified as dermal or subcutaneous depending on the depth of origin. Dermal leiomyosarcoma (dLMS) is generally considered an intermediate-risk neoplasm characterised by a considerable risk of local recurrence but a low risk of metastasis. Although there is general agreement that dLMS presents a low risk of metastasis, the possibility of metastatic potential cannot be dismissed entirely, as reported metastasis rates in the literature vary from 0–14%.1-7 Furthermore, risk factors for dLMS remain poorly defined because methodological constraints, small sample sizes, and short follow-up have limited previous studies. The objective of this study was to investigate risk factors for metastasis and local recurrence, and to propose a high- and low-risk classification for dLMS.8
The data were extracted from the Danish National Health Registers. These registers, as well as individual-level linkage of data across all registers, have been described previously.9 All patients diagnosed with dLMS in Denmark between 1980–2022 were included. All tumours were diagnosed following WHO guidelines for sarcomas, and treated at specialised sarcoma centres in accordance with the Danish national
Figure 1: Forest plot of the univariable analysis of risk factors for metastasis and local recurrence.
Metastasis Local Recurrence
and Neck
Subcutaneous Invasion (AISMN)
Tissue Invasion
Mitotic Grade 3
Necrosis
Positive Margins
Perineural/Intravascular Invasion
AISMN: atypical intradermal smooth muscle neoplasms.
guidelines.¹⁰ Absolute 5-year risks were estimated with the Aalen–Johansen method, treating all-cause mortality as a competing risk. The results were presented as 5-year absolute risks and risk differences (RD) with 95% CIs. The classification of dLMS into high- and low-risk groups was based on risk factors associated with a 5-year RD of >5% for metastasis and >10% for local recurrence based on the overall 5-year risks for each outcome.
Among 381 patients (median age: 66 years; 71% male), the 5-year risks of metastasis and local recurrence were 2.4% and 10.0%, respectively (Figure 1).
The presence of tumour necrosis was associated with a 5-year metastasis risk of 9.3% (95% CI: 0.6–18.0) compared to 1.5% (95% CI: 0.2–2.8) in those without tumour necrosis (RD: 7.8%; 95% CI: –1–17).
Patients with mitotic Grade 3 had a 5-year metastasis risk of 6.5% (95% CI: 1–12) compared to 1.4% (95% CI: 0–2.8) for Grades 1 and 2 (RD: 5.1%; 95% CI: –0.6–11.0). Perineural or intravascular invasion was linked to a 25.0% (95% CI: 0–67) risk of metastasis compared to 2.1% (95% CI: 0.7–3.6) in patients without invasion (RD: 23%; 95% CI: –20–65).
For local recurrence, positive surgical margins were associated with a 5-year risk of 26.0% (95% CI: 11–41) compared to 8.4% (95% CI: 5.5–11.0) in patients with negative margins (RD: 18%; 95% CI: 3–33). Perineural or intravascular invasion was associated with a 50.0% (95% CI: 0–100) risk of local recurrence compared to 9.6% (95% CI: 6.6–13.0) in patients without invasion (RD: 40%; 95% CI: –11–92).
The authors propose that high-risk dLMS could be defined by the presence of necrosis, mitotic Grade 3, perineural/ intravascular invasion, or positive surgical margins. The authors suggest that all patients with dLMS could have clinical visits twice in the first year, followed by annual visits for 3 years, as no further relapses occurred after this time point (4 years). The follow-up of patients with high-risk dLMS could be extended to also include either PET/CT or CT of the chest in the first 2 years due to the risk of distant and pulmonary metastases. The follow-up of patients with low-risk dLMS could be limited to annual examinations with a general practitioner or dermatologist.
References
1. Bernstein SC, Roenigk RK. Leiomyosarcoma of the skin. Treatment of 34 cases. Dermatol Surg. 1996;22(7):631-5.
2. Carr MJ et al. Grade of primary cutaneous leiomyosarcoma dictates risk for metastatic spread and disease-specific mortality. Cancer Control. 2023;30:10732748231206957.
3. Dahl I, Angervall L. Cutaneous and subcutaneous leiomyosarcoma. A clinicopathologic study of 47 patients. Pathol Eur. 1974;9(4):307-15.
4. Fauth CT et al. Superficial leiomyosarcoma: a clinicopathologic review and update. J Cutan Pathol. 2010;37(2):269-76.
5. Hall BJ et al. Atypical intradermal smooth muscle neoplasms (formerly cutaneous leiomyosarcomas): case series, immunohistochemical profile and review of the literature. Appl Immunohistochem Mol Morphol. 2013;21(2):132-8.
6. Swanson P et al. Primary cutaneous leiomyosarcoma. A histological and immunohistochemical study of 9 cases, with ultrastructural correlation. J Cutan Pathol. 1988;15(3):129-41.
7. Winchester DS et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71(5):919-25.
8. Abebe K et al. Classification of high- and low-risk groups in patients with dermal leiomyosarcoma: an exploratory register-based nationwide cohort study. Abstract P0824. EADV Congress, 17-20 September, 2025.
9. Ørholt M et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89(6):1177-84.
10. Jørgensen PH et al. The Danish sarcoma database. Clin Epidemiol. 2016;8:685-90.
Authors: *Marra Aghajani,1 Matthew J. Verheyden,2-4 Adrian Cachia5
1. The Skin Hospital, Sydney, Australia
2. Department of Dermatology, The Sutherland Hospital, Caringbah, Australia
3. Sydney Medical School, University of Sydney, Australia
4. School of Medicine and Health, University of New South Wales, Sydney, Australia
5. Kossard Dermatopathologists, North Ryde, Australia
*Correspondence to marra.aghajani1@gmail.com
Disclosure: The authors have declared no conflicts of interest.
Acknowledgements: The authors would like to thank John Sullivan, The Sutherland Hospital, Caringbah; and University of New South Wales, Sydney, Australia, for his valuable support and contribution to this work.
Keywords: Blaschko-linear acquired inflammatory skin eruption, Blaschko’s lines, lichen striatus (LS), paediatric blaschkitis.
Citation: EMJ Dermatol. 2025;13[1]:58-60. https://doi.org/10.33590/emjdermatol/NAGE6319
Blaschkitis is a rare, acquired inflammatory dermatosis following the lines of Blaschko, classically seen in adults. Paediatric cases are infrequently described in the literature and may be confused with more common dermatoses such as lichen striatus or atopic dermatitis. The authors present a unique case of paediatric blaschkitis characterised by multilinear distribution and spongiotic histology, adding to the evolving spectrum of Blaschko-linear acquired inflammatory skin eruption.1
An 11-year-old girl presented with a 3-month history of a mildly pruritic papular eruption involving the left leg, buttocks, and abdomen. Past medical history included
eczema and hay fever, and family history was notable for atopy. Prior treatment with topical corticosteroids and oral antibiotics had no effect. On physical examination, numerous erythematous papules had coalesced to form plaques distributed in multiple well-defined bands, 2–5 cm in diameter, in a Blaschko linear fashion (Figure 1).
Histopathology revealed an epidermis with foci of spongiosis and patchy parakeratosis (Figure 2). Mild-to-moderate perivascular lymphocytic inflammation was evident in the upper dermis, with infrequent eosinophils. The deeper dermis appeared normal, and no fungal organisms were identified on a combined periodic acid-Schiff/Alcian blue stain. Given the time frame, distribution, and histology, a diagnosis of paediatric blaschkitis was favoured. The lesions were
Figure 1: Linear and whorled erythematous plaques following Blaschko’s lines on the posterior aspect of the left leg (A), abdomen (B), and anterior aspect of the left leg (C).

Figure 2: Histopathological features of paediatric blaschkitis.
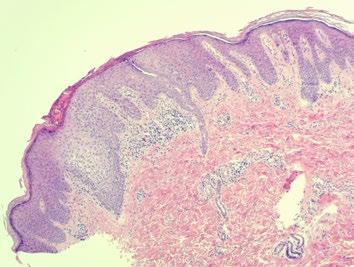
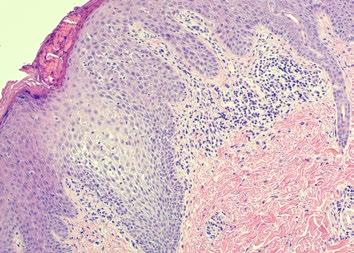
BAA) Spongiotic reaction with sparing of deeper dermis (H&E; x25). B) Detail of epidermal spongiosis and lymphocyte exocytosis, with locules of serum in surface parakeratin and no lichenoid component (H&E; x100).
H&E: haematoxylin and eosin.
Blaschko’s lines are linear patterns on the skin representing the migration of epidermal cells during embryogenesis.2 Numerous cutaneous conditions present as linear dermatoses following Blaschko’s lines and are a manifestation of cutaneous mosaicism.3 Lichen striatus (LS) is the most common acquired Blaschko-linear dermatosis, primarily affecting children 5–15 years of age.4
that coalesce to form a solitary, narrow band distributed along the extremities.4 LS is typically asymptomatic and selflimiting, with histology showing spongiosis accompanied by lichenoid and periadnexal inflammation.4 In contrast, ‘adult blaschkitis’ is an acquired Blaschko-linear dermatosis distinguished from LS by its later age of onset, truncal distribution, and multiple broad bands.5 Whilst its time course is rapid (1–4 months), relapse is commonly observed.5 On histology, blaschkitis features a primarily spongiotic rather than lichenoid dermatitis.5
Adult blaschkitis and paediatric LS have historically been regarded as distinct conditions. However, rare cases of blaschkitis in children and LS in adults have been reported.3,4 It has since been proposed that they represent two endpoints of Blaschko linear acquired inflammatory skin eruption, a spectrum of acquired dermatoses characterised by their inflammatory infiltrate and distribution along the lines of Blaschko.3,4 Clinically, the authors’ patient’s papulovesicular plaques distributed in a multilinear pattern are consistent with blaschkitis. The presence of spongiosis and focal parakeratosis in the absence of lichenoid and deep periadnexal inflammation further supports this diagnosis.
2. Molho-Pessach V, Schaffer JV. Blaschko lines and other patterns of cutaneous mosaicism. Clinics in Dermatology. 2011;29(2):205-25. successfully treated with the application of moisturiser several times daily. At the time of reporting, there have been no recurrences.
It is characterised by an eruption of hypopigmented to erythematous papules
The authors’ report reinforces the relevance of clinical–histological correlation and contributes to growing recognition of blaschkitis in the paediatric population. Improved awareness of this condition may prevent misdiagnosis and unnecessary treatment.
References
1. Aghajani M. Paediatric blaschkitis: a rare case of multilinear acquired Blaschko-linear dermatosis in an 11-year-old girl. Abstract P1856. EADV Congress, 17-20 September, 2025.
3. Darsha AK, Cohen PR. Blaschko linear acquired inflammatory skin eruption (BLAISE): case report of a young man whose dermatosis had features of lichen striatus and blaschkitis. Cureus. 2020;12(10):e10785.
4. Keegan BR et al. Pediatric blaschkitis: expanding the spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24(6):621-7.
5. Aravind M et al. Blaschkolinear acquired inflammatory skin eruption, or blaschkitis, with features of lichen nitidus. JAAD Case Rep. 2016;2(2):102-4.
Authors: Elefthria Tampouratzi,1 *Eirini-Nektaria Giagkou,1 Panagiotis Rigatos,2 Ioannis Tsimbos,1 Maria Pizimola,3 Ioannis Katsantonis1
1. Tzaneio Hospital, Piraeus, Greece
2. Private practice, Patra, Greece
3. Private practice, Rhodes, Greece *Correspondence to renatagk27@gmail.com
Disclosure: The authors have declared no conflicts of interest. Informed consent was obtained from the patient for the publication of this case report.
Keywords: Basal cell carcinoma, nose, Rintala flap.
Citation: EMJ Dermatol. 2025;13[1]:61-62. https://doi.org/10.33590/emjdermatol/YKHK2638
The nose is centrally located on the face and significantly contributes to an individual's overall appearance. Consequently, any surgical intervention involving the nose must prioritise the achievement of optimal results, which encompass skin colour matching, adequate tissue coverage, flap viability, and aesthetically acceptable outcomes.1 A modification of the Rintala glabellar linear advancement flap was used in the treatment of basal cell carcinoma located in the nasal region.2
An 87-year-old female patient was referred to the authors’ department, having a diagnosis of basal cell carcinoma situated in the middle one-third of the nasal dorsum (Figure 1A). The tumour was excised using a modified Rintala flap technique. The flap design, elevation, and transfer were carried out in accordance with the traditional Rintala flap procedure while utilising local anaesthesia. The resultant defect measured 1x1 cm (Figure 1B).3,4 A superior rectangular flap was designed, extending to the central forehead. The flap was elevated in the
supraperiosteal plane, with bilateral parallel incisions made along both sides of the nasal sidewall, from the corners of the defect to the glabellar region and into the forehead, thereby enhancing the advancement of the flap (Figure 1C–E). Burow’s triangle resection was deemed unnecessary due to the considerable elasticity of the skin, and the suturing was executed without applying increased tension.
The post-operative wound healing procedure was completed without any complications such as infections, hypertrophic scars, keloids, or delayed closure of the trauma. In addition to a rapid recovery, a highly satisfactory aesthetic result was observed during the follow-up visits at 15 and 30 days after surgery.
The Rintala flap, initially described in 1969, serves as a dependable option for the reconstruction of moderate nasal defects by utilising an advancement flap from the central forehead.3 A significant limitation of this technique is the potential for ischaemia at the distal end of the flap.5 Consequently, the acceptable range for flap transfer is defined from the glabella to the middle third of the nasal dorsum. It is crucial to recognise that the resection of Burow’s triangles, whether performed below the eyebrow or in the canthal region, may result in deformities, thereby restricting the distance that the flap can be advanced. Through the implementation of the modified Rintala technique, as previously outlined, it is possible to successfully enhance the width of the rectangle along the nasal dorsum while avoiding the creation of Burow’s triangles. This method provides aesthetically favourable outcomes without introducing distortions to the central facial region.
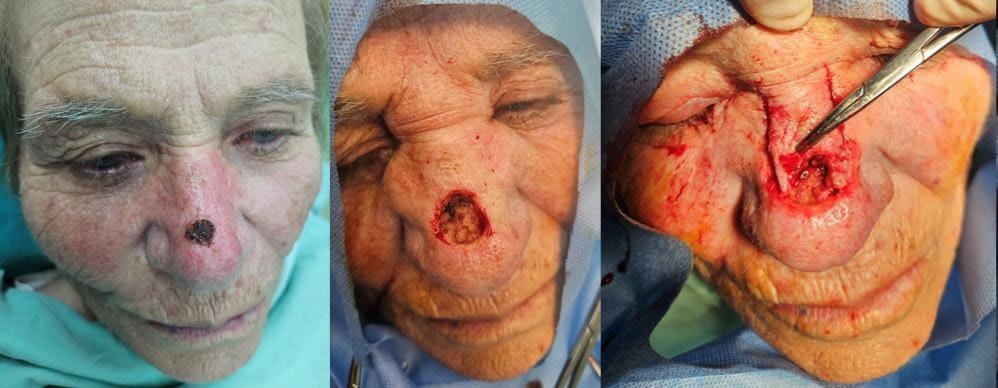
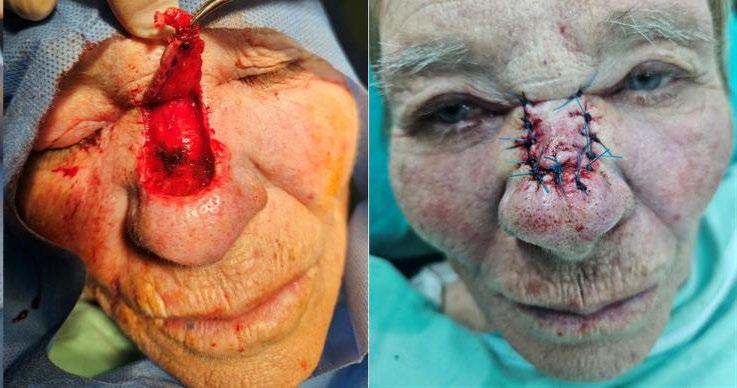
References
1. Ebrahimi A et al. Application of modified Rintala flap in nasal tip reconstruction. American J Otolaryng. 2012;33(6):685-8.
2. Tampouratzi E et al. Utilizing the modified rintala flap technique for effective nasal reconstruction. Abstract 2429. EADV Congress, 17-20 September, 2025.
3. Rintala AE, Asko-Seljavaara S. Reconstruction of the midline skin defects of the nose. Scand J Plast Reconstr Surg. 1969;3(2):105-8.
4. Girijala R et al. Revisiting the Rintala advancement flap for nasal tip reconstruction. Dermatology Online J. 2020;26(8):13030/qt5dv0s7zx.
5. Onishi K et al. The Rintala flap: a versatile procedure for nasal reconstruction. American J Otolaryng. 2014;35(5):577-81.
Authors: *Miranda K. Branyiczky,1 Megan S. Lowe,2 Mohannad Abu-Hilal,1,3 Ivan V. Litvinov4
1. Michael G. DeGroote School of Medicine, McMaster University, Hamilton, Ontario, Canada
2. Queen's School of Medicine, Queen’s University, Kingston, Ontario, Canada
3. Division of Dermatology, McMaster University, Hamilton, Ontario, Canada
4. Division of Dermatology, Department of Medicine, McGill University, Montreal, Quebec, Canada
*Correspondence to miranda.branyiczky@medportal.ca
Disclosure: Abu-Hilal has served as a consultant and received honoraria from AbbVie, Biojamp, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Galderma, Incyte, Janssen, Leo, Lilly, Medexus, Novartis, Pfizer, Recordati, Sanofi Regeneron, and Sun Pharma. The other authors have declared no conflicts of interest.
Keywords: Erosive lichen planus, human papillomavirus (HPV), lichen planus (LP), Marjolin’s ulcer (MU), squamous cell carcinoma.
Citation: EMJ Dermatol. 2025;13[1]:63-66. https://doi.org/10.33590/emjdermatol/QASI9805
Anogenital lichen planus (ALP) is a chronic inflammatory condition associated with significant morbidity, persistent symptoms, architectural changes, and reduced quality of life.1-3 ALP can give rise to cutaneous squamous cell carcinoma (cSCC), and specifically in the setting of chronic inflammation, to Marjolin’s ulcer (MU), a rare, aggressive subset of cSCC associated with a poor and potentially life-threatening prognosis.4 The true incidence of ALP is likely underestimated due to the lack of routine genital examination by healthcare providers and the potentially asymptomatic nature of lesions.5 This systematic review aims to characterise the clinical characteristics of patients developing MU within ALP and its associated outcomes.
Medline and Embase were searched from inception–November 2024, identifying English-language studies reporting MU or squamous dysplasia arising within ALP lesions in adult patients (≥18 years). Thirtytwo studies (n=176 cases) were analysed, including cohort studies (n=19), case series (n=2), and case reports (n=11), and a descriptive analysis was performed.
The mean patient age was 63.9 years, with 77.27% being female (Table 1). ALP lesions were most commonly vulval (72.2%) in females and penile (22.2%) in males. Multiple clinical types of ALP aside from the classic presentation (papules and plaques) were observed, including erosive (54.43%; n=43/79) and hypertrophic (16.45%; n=13/79) subtypes. MU or cSCC precursor prevalence in ALP cohorts ranged from 0.9–4.2%. Forms of squamous dysplasia included MU (60.23%), differentiated-vulval or penile intraepithelial neoplasia (13.64%), undifferentiatedvulval or penile intraepithelial neoplasia (5.11%), or unspecified cSCC in situ (11.36%), with 9.66% of patients exhibiting multiple overlapping subtypes. Human papillomavirus (HPV) was rarely detected (8.41%), suggesting other mechanisms underlying MU development. The mean ALP duration prior to squamous dysplasia was 47.5 months; many patients reported symptoms of anogenital pruritus, pain, erythema, and dyspareunia occurring for years prior to diagnosis. Metastases were present in 50% of cases that reported this outcome. MU treatment primarily involved surgical excision (87.13%), with a few patients requiring radiation treatment (3.96%) or chemotherapy (1.98%).
Recurrence with survival occurred in 19.74% of cases and mortality was reported in 21.05%. Of the patients who had a mortality outcome, all were female, with a mean age
Table 1: Demographic and clinical characteristics of patients with anogenital lichen planus and cutaneous squamous cell carcinoma.
Table 1: Demographic and clinical characteristics of patients with anogenital lichen planus and cutaneous squamous cell carcinoma. (Continued)
ALP: anogenital lichen planus; cSCC: cutaneous squamous cell carcinoma; d-PeIN: differentiated penile intraepithelial neoplasia; d-VIN: differentiated vulval intraepithelial neoplasia; HPV: human papillomavirus; LP: lichen planus; MU: Marjolin’s ulcer; PeIN: penile intraepithelial neoplasia; SCC: squamous cell carcinoma; VIN: vulval intraepithelial neoplasia.
of 73.1 years and a diagnosis of cSCC rather than a precursor. The majority of cases had prior erosive ALP (56.25%; n=9/16) and none had a positive HPV status. Poor disease control, erosive morphology, and long-standing lesions were associated with higher malignancy risk. Cohort studies following patients with lichen planus (which only involved female patients) demonstrated new lesion rates of 0.9–4.2% for MU and 1.0–8.2% for vulval intraepithelial neoplasia during follow-up.
This review highlights the malignant potential of ALP and underscores the importance of early detection and multidisciplinary care. Malignant transformation may be related to the immunocompromised cutaneous district seen in ALP.6-8 Routine anogenital examinations, timely biopsies of suspicious lesions, HPV vaccine administration, and aggressive management of ALP may improve outcomes.5,9 The term MU for ALP-associated cSCC is justified by high recurrence and mortality rates, and consistent use of this terminology may better reflect its aggressive nature. Further research is needed to elucidate the mechanisms underlying MU development and optimise treatment strategies for this rare but morbid complication.
1. Branyiczky MK et al. Marjolin’s Ulcer in patients with anogenital lichen planus: a systematic review. P3077. EADV Congress, 17-20 September, 2025.
2. Boch K et al. Lichen planus. Front Med (Lausanne). 2021;8:737813.
3. Gorouhi F et al. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci World J. 2014;2014:742826.
4. Olawoye O et al. Marjolin’s ulcer arising from cutaneous lichen planus. East Cent Afr J Surg. 2013;18(2):164-7.
5. Vieira-Baptista P et al. Risk of development of vulvar cancer in women with lichen sclerosus or lichen planus: a systematic review. J Low Genit Tract Dis. 2022;26(3):250-7.
6. Ruocco V et al. The immunocompromised district in dermatology: a unifying pathogenic view of the regional immune dysregulation. Clin Dermatol. 2014;32:569-76.
7. Fabbrocini G et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35(2):225-7.
8. Haenen CCP et al. Squamous cell carcinoma arising in hypertrophic lichen planus. BMJ Case Rep. 2018;2018:bcr2017224044.
9. Kherlopian A, Fischer G. Vulvar malignancy in biopsy-proven vulval lichen planus: a retrospective review of 105 cases. Australas J Dermatol. 2020;61(4):386-8.
Authors: *Inês Pereira Amaral,1,2 Ivânia Soares,1,2 Madalena Pupo Correia,1,2 Filipe Monteiro,1,2
Inês Abreu,1,2 Pedro de Vasconcelos,1,2 Joana Antunes,1,2 Paulo Filipe1,2
1. Dermatology Department, Hospital Santa Maria, Unidade Local de Saúde Santa Maria, Lisbon, Portugal
2. Dermatology University Clinic, Faculdade de Medicina da Universidade de Lisboa, Portugal *Correspondence to ines.pereiraamaral@gmail.com
Disclosure: The authors have declared no conflicts of interest.
Keywords: Eruptive vellus hair cysts (EVHC), papular eruptions, skin of colour.
Citation: EMJ Dermatol. 2025;13[1]:67-68. https://doi.org/10.33590/emjdermatol/HAIQ6985
Eruptive vellus hair cysts (EVHC) are rare, benign follicular anomalies, typically presenting as multiple small papules that may be asymptomatic or pruritic.1 Their clinical appearance can mimic acneiform, infectious, or granulomatous dermatoses, often leading to diagnostic delays and under-recognition.2 The authors aim to report a case of EVHC in a young woman with Fitzpatrick skin phototype VI, to emphasise the importance of clinicopathological correlation in persistent papular eruptions.
An otherwise healthy 29-year-old woman with Fitzpatrick skin phototype VI was referred to the Dermatology Department at Hospital Santa Maria, Unidade Local de Saúde Santa Maria, Lisbon, Portugal, for evaluation of lesions localised to the abdomen that had been present for approximately 1 year. The lesions consisted of multiple firm, erythematous-to-brownish, 2–4 mm-sized, domed papules located on
the intermammary and upper abdominal regions. Dermoscopy consisted of a central round, hypopigmented structure with a subtle pinkish hue, surrounded by a light-brown halo and fine, peripheral scale. The lesions were associated with mild pruritus and occasional drainage of yellowish material. No systemic involvement or family history of similar lesions was identified. Previous treatment with topical fusidic acid and betamethasone yielded no improvement. A skin biopsy was performed for diagnostic clarification.
Histopathological examination showed a mid-dermal cyst lined by stratified squamous epithelium with a preserved granular layer, containing laminated keratin and multiple transversely and obliquely cut vellus hair shafts. The synchronous emergence of multiple lesions and their distribution supported the diagnosis of EVHCs (Figure 1). Given the benign nature of the condition and the absence of significant symptoms, conservative management with regular follow-up was proposed.
EVHCs are thought to arise from a developmental abnormality involving infundibular blockage, follicular dilatation, and hair bulb atrophy, with K17 gene mutations implicated in familial autosomaldominant cases.3 This case underlines the relevance of considering EVHC in the differential diagnosis of chronic papular eruptions, particularly in patients with darker skin phototypes, where pigmentary changes may obscure classical features. Histopathological confirmation is key to avoiding misdiagnosis and unnecessary treatments.
Figure 1: Eruptive vellus hair cysts.

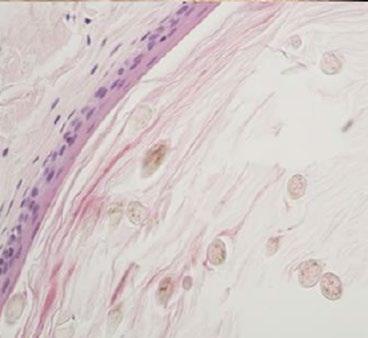
A) Clinical image: multiple dome-shaped, pink-to-brownish-coloured papules on the abdominal region. B) Dermoscopic image: central hypopigmented area with a pinkish hue, surrounded by a brown halo and fine scaling (DermLite DL4; polarised mode; 10x magnification). C) and D) Histopathology features: mid-dermal cyst cavity containing laminated keratinous material and multiple vellus hair shafts (C: H&E, 40x magnification; D: 400x magnification).
DermLite DL4: DermLite, Aliso Viejo, California, USA; H&E: haematoxylin and eosin.
References
1. Pereira Amaral I et al. Persistent papular eruption in a young adult: importance of histopathology in diagnosing eruptive vellus hair cysts. Abstract P1312. EADV Congress, 17-20 September, 2025.
2. Patokar AS et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14(1):31-3.
3. Torchia D et al. Eruptive vellus hair cysts: a systematic review. Am J Clin Dermatol. 2012;13(1):19-28.
Authors: *Ouissal Hormi,1 Moujahid Salma,1
Zerrouki Nassiba,1,2 Zizi Nada1,2
1. Department of Dermatology, Venereology, and Allergology, CHU Mohammed VI, Oujda, Morocco
2. Laboratory of Epidemiology, Clinical Research, and Public Health, Faculty of Medicine and Pharmacy, Mohammed First University, Oujda, Morocco
*Correspondence to ouissal.hormi@gmail.com
Disclosure: The authors have declared no conflicts of interest.
Keywords: Allergen, allergic contact dermatitis, herbal medicine.
Citation: EMJ Dermatol. 2025;13[1]:69-70. https://doi.org/10.33590/emjdermatol/YSXD6096
Contact eczema is an inflammatory dermatosis caused by cutaneous exposure to allergens or irritants.1 In recent years, unregulated natural products, particularly plants, have emerged as important sources of sensitisation. While often perceived as safe, these remedies can provoke allergic reactions through unconventional routes. Poppy (Papaver rhoeas) is rarely reported as an allergen,2 yet its bioactive compounds may trigger hypersensitivity.
The authors report a case of allergic contact eczema affecting the cheeks following the application of poppy petal powder as makeup, aiming to raise awareness within the allergology community about this potential new allergen.
A 25-year-old woman presented with bilateral erythematous, scaly, and pruritic lesions on her cheeks, which appeared 48 hours after the application of poppy petal powder as makeup (Figure 1). Her history revealed the recent use of this powder, purchased from a local herbalist.
Standardised patch testing with a 10% preparation of poppy powder in petrolatum demonstrated a strong positive reaction to poppy (48-hour reading: +++; 72-hour reading: +++), while all other allergens, including common cosmetic sensitisers, tested negative. Patch testing remains the gold standard for confirming allergic contact dermatitis. Management consisted of immediate discontinuation of the offending product and application of a moderatepotency topical corticosteroid for 2 weeks, leading to complete remission.
This case represents an uncommon but clinically relevant instance of allergic contact eczema induced by Papaver rhoeas petal powder used as a cosmetic. While natural remedies are often promoted as safer alternatives to industrial cosmetics, they can contain potent sensitisers. Plants are frequent culprits in allergic contact dermatitis due to their alkaloids, flavonoids, anthocyanins, and other bioactive molecules capable of acting as haptens and triggering Type IV hypersensitivity reactions. Poppy petals, rich in pigments and secondary metabolites, represent a plausible source of sensitisation.
Reports of contact sensitisation to poppies are rare, as most publications on the Papaver species address pharmacological or traditional uses rather than allergenic potential. This case adds to the limited literature, illustrating the poppy's ability to induce delayed hypersensitivity and highlighting the risks of unregulated cosmetic practices, such as the direct application of powdered petals.
Diagnosis in this case was confirmed through patch testing, which showed a clear positive reaction to poppy powder and excluded cross-sensitisation to other cosmetic allergens such as fragrance mixes, preservatives, or nickel. Patch testing
Figure 1: Bilateral erythematous, scaly, and pruritic lesions on the cheeks appearing 48 hours after the application of poppy petal powder as makeup.
remains a crucial tool in recognising novel allergens and contributes to expanding the spectrum of documented sensitisers.3
From a therapeutic perspective, management was straightforward, combining prompt elimination of the culprit agent with a short course of topical corticosteroids, resulting in complete recovery. However, prevention is essential. Raising awareness among dermatologists, allergologists, and general practitioners about the risks of ‘natural’ cosmetics is critical. Equally important is educating patients, many of whom mistakenly believe that plant-derived products are inherently safe.
In conclusion, this case highlights an emerging risk associated with the use of unregulated, plant-based cosmetics.
Beyond its originality, it contributes to broadening the spectrum of recognised plant allergens and underscores the diagnostic importance of patch testing in identifying novel sensitisers. Vigilance from healthcare professionals, combined with patient education, is required to prevent similar cases and to ensure the safer use of natural products.
References
1. Hormi O et al. Contact eczema of the cheeks due to poppy petal powder. Abstract 4712. EADV Congress, 17-20 September, 2025.
2. Gamboa PM et al. Allergic contact urticaria from poppy flowers (Papaver rhoeas). Contact Dermatitis. 1997;37(3):140-1.
3. Lin SH, Chao YC. Clinical characteristics and patch test results in 57 patients with contact dermatitis in Southern Taiwan. J Clin Med. 2025;14(7):2291.
Authors: Michela Starace,1,2 *Aurora Alessandrini,1,2 Francesca Bruni,1,2 Dionisio Franco Barattini,3 Bianca Maria Piraccini4
1. Dermatology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Policlinico S. Orsola-Malpighi, Italy
2. Department of Medical and Surgical Sciences, Alma Mater Studiorum, University of Bologna, Italy
3. Clinical Research Consultant, Genova, Italy
4. Private Dermatology Practice, Bologna, Italy
*Correspondence to aurora.alessandrini.30@gmail.com
Disclosure: The authors have declared no conflicts of interest. The patient’s parents were informed about the use of clinical information and photos for publication intent according to the Declaration of Helsinki principles. Informed consent was appropriately obtained during the medical examination.
Keywords: Dermatophytes, effectiveness, nail, onychomycosis, topical solution, treatment.
Citation: EMJ Dermatol. 2025;13[1]:71-73. https://doi.org/10.33590/emjdermatol/WFTJ7500
Onychomycosis is a prevalent and difficultto-treat nail infection. Although both oral and topical antifungals are available, systemic agents such as terbinafine and itraconazole are often limited by drug interactions and side-effects.1,2 Consequently, there is a clinical need for safer and effective topical therapies.3-5 This study evaluated the efficacy and tolerability of a novel topical antifungal formulation in patients with distal lateral subungual onychomycosis.6
This single-arm, open-label clinical trial enrolled 50 adult subjects with clinically diagnosed distal lateral subungual onychomycosis (≤30% nail involvement, at least one big toe). The tested topical solution, containing urea, lactic acid, ethoxydiglycol, decylene glycol, and polyquaternium-7, was applied twice daily for 6 months. Outcome measures included blinded investigator global assessment, mycological evaluation (potassium hydroxide microscopy and culture), and patient self-assessment of efficacy and usability. Nail growth (mm) was periodically monitored. Complete cure was defined as negative mycology and resolution of clinical signs. Safety was assessed through adverse event reporting.
Forty patients completed the study. At 3 months, 80% of participants achieved mycological negativity (direct microscopy + culture). At 6 months, the complete cure rate was 50.0% and an additional 42.5% showed significant clinical improvement (Figure 1). Mean healthy nail growth was 1.40 mm at Week 4, and after 6 months, the mean growth reached 3.55 mm. A blinded investigator assessed improvement from standardised photographs. Statistically significant changes were observed over time (p<0.001; Friedman test). At Week 4, eight patients (20.0%) showed excellent improvement and 29 (72.5%) moderate improvement. At Week 12 (Month 3), 13 (32.5%) showed excellent improvement and 24 (60.0%) moderate improvement. At Month 6, 19 patients (47.5%) showed excellent and 17 (42.5%) moderate
Figure 1: Excellent improvement (complete cure) of Trichophyton rubrum distal lateral subungual onychomycosis (A) from baseline and (B) after 3 months of treatment (Week 12).
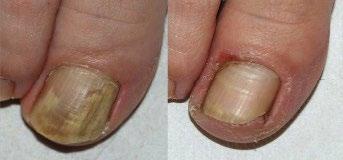
improvement (p<0.001 for all timepoints versus baseline; Wilcoxon test with Bonferroni correction).
Patient satisfaction at 6 months was 95%, and investigators rated the treatment positively in 87.5% of cases. Four patients developed mild periungual erythema due to improper application; all cases resolved without discontinuation.
Onychomycosis remains a challenging condition to manage with topical therapies, due to the nail plate’s poor permeability and slow growth rate, both of which reduce the effectiveness of antifungal agents and require prolonged treatment durations to achieve clinical resolution. This topical antifungal solution demonstrated clinically meaningful improvements in nail appearance and fungal resolution already after 12 weeks, with further benefit at 6 months. The formulation forms a protective film on the nail surface, which acts as a barrier against environmental pathogens.5,7 Moreover, polyquaternium-7, decylene glycol, and urea contribute to nail hydration and barrier integrity. Lactic acid helps to maintain an acidic pH, unfavourable for fungal growth.4,7,8 Also, a combination
ointment including urea exhibited better antifungal properties due to urea helping the penetration of active pharmacological agents.4 The formulation was safe, well tolerated, and easy to use. Its nonpharmacological mechanism of action may allow for concomitant use with systemic antifungals when indicated.
References
1. Gupta AK et al. Onychomycosis in the 21st century: an update on diagnosis, epidemiology, and treatment. J Cutan Med Surg. 2017;21(6):525-39.
2. Gupta AK et al. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 2020;34(9):1972-90 .
3. Baran R, Gupta AK. Are boosters or supplementation of antifungal drugs of any use for treating onychomycosis? J Drugs Dermatol. 2002;1(1):35-41.
4. Dars S et al. The use of urea for the treatment of onychomycosis: a systematic review. J Foot Ankle Res. 2019;12:22.
5. Piraccini BM et al. Early visible improvements during K101-03 treatment: an open-label multicenter clinical investigation in patients with onychomycosis and/or nail psoriasis. Dermatology. 2017;233(2-3):178-83.
6. Starace M et al. A 6-month, single-arm, open-label clinical study of the effects of a topical solution containing urea, lactic acid, thymus vulgaris leaf oil, used in the treatment of distal lateral subungual onychomycosis (DSLO) due to dermatophytes involving up to 30% of the nail plate of the first toe. Poster P2621. EADV Congress, 17-20 September, 2025.
7. Emtestam L et al. Treatment of distal subungual onychomycosis with a topical preparation of urea, propylene glycol and lactic acid: results of a 24week, double-blind, placebo-controlled study. Mycoses. 2012;55(6):532-40.
8. Maggioni D et al. Clinical evaluation of a topical formulation for the management of onychomycosis. J Clin Aesthet Dermatol. 2020;13(7):53-7.
EMJ is delighted to introduce Branka Marinović, President of the European Academy of Dermatology and Venereology (EADV) Congress 2025, as she shares insights into groundbreaking developments in dermatology and her vision for the future of the EADV.


Branka Marinović
President, European Academy of Dermatology and Venereology (EADV); Professor of Dermatology and Venereology, University of Zagreb School of Medicine, Croatia
Citation: EMJ Dermatol. 2025;13[1]:74-77. https://doi.org/10.33590/emjdermatol/UBHM3082
Q1In your welcome letter, you highlight the European Academy of Dermatology and Venereology (EADV)'s significant step towards sustainability at the 2025 Congress. What specific initiatives are you most proud of, and how do you envision this focus influencing other medical associations and the wider field of dermatology?
Sustainability is a very important topic in our work, outside of the scientific part of the Congress. It was started as a project by my predecessor, the Immediate Past-President, Martin Röcken. But, of course, it wasn't just him; it was also our internal Congress organisers and our office that started thinking about sustainability, because this is a truly hot topic for everybody, as it should be.
We have stopped printing, we have stopped producing Congress bags, and we try to be without papers as much as possible, though it’s not always entirely feasible.
We also have an agency to measure our food carbon footprint. We have the data for the Congress in Amsterdam, the Netherlands, and after the current Congress, we will present those data at our board meeting in Athens, Greece, at our spring symposium. We will compare how much we've improved and what more we can do.
Sustainability is a very important topic in our work
What we are doing is being very careful when choosing venues. When we select a location, one of the key points is gaining insights into the sustainability of the venue, their way of working, and the hotels we use. We try to find those who are as sustainable as possible. Of course, it's not possible to do everything today.
We also have the Climate Working Group, which issued guidance or guidelines for our members on how to improve sustainability in their offices and in their everyday work. So, we believe that we are doing some things for sustainability. Of course, you cannot change everything in one day.
The delegates’ passes also include travel, which encourages people to use public transport. We try to encourage people to travel by train. Of course, I cannot travel by train from Croatia because the trains there are not the
best; I would need a few days! But realistically, the biggest carbon footprint comes from travelling. On the other hand, during COVID-19, we thought we might move much more into the cloud and be much more online. But then we realised that we are humans, and that we like to meet each other. Maybe the high number of participants we have now is a direct result of that fundamental human need to meet with other people.
Q2 As the EADV president, what goals did you start with for the EADV's advocacy efforts, particularly considering the geopolitical and economic diversity across Europe?
When we speak about our advocacy programme, of course, we always compare ourselves with the USA, with the American Academy of Dermatology (AAD). There is a big difference, because that is one country. We involve many different countries within Europe, with truly different economic statuses, different opportunities, and different laws.
We mostly conduct our advocacy efforts in Brussels, Belgium, but we are completely aware that Brussels is the centre for only 27 countries, and there are many other European countries that are not covered. However, when we do our advocacy, we try to go with topics that can be used by our board members and representatives in their own countries, so that they can spread the message and take it to their own parliament. We don’t want to just be EU-oriented.
At the moment, we are focused on banning sunbeds for those younger than 18. This still hasn't been done in many European countries, and we are seeing an increase in melanomas. Then there was a WHO resolution about the importance of skin diseases and the burden of skin diseases, so we are also working on that.
One of the actions we are still considering how to approach is the issue of disparity. Some drugs are available in some countries, and in others, they are not. We would certainly like to address
that, but I think this is above our remit, which is trying to teach people, guide them, and put together therapeutic guidelines. Of course, it's dependent on which country will accept these.
Your clinical expertise includes the difficult management of autoimmune bullous diseases during pregnancy. How does your experience with such sensitive and complex cases inform and influence your approach to leading the EADV, such as aiming to address a wide range of diseases and public health challenges?
My main expertise is in autoimmune bullous diseases, and I really love that field of dermatology. I'm also active in our task force within the EADV dealing with autoimmune bullous diseases, and we are producing a lot of guidance and guidelines for our members. These are rare diseases, but they are very severe.

Of course, my special interest cannot entirely influence the whole EADV because the EADV programme is done by the Scientific Programming Committee. They try to cover all fields of our speciality so that everybody who comes here is satisfied, and that everybody who comes here can find something for themselves.
Q4 With the Congress offering a wide range of topics, how does the EADV ensure that emerging fields, such as the latest breakthroughs in autoimmune blistering diseases, receive the attention they deserve among a broad audience?
There is a main programme that is set by the Scientific Programming Committee with core topics each year. There are a few topics that may be highlighted a bit more, but almost all main topics are covered.
Regarding what you call 'breaking news', we have a few sessions that are designated as ‘latebreaking abstracts’. These are mostly abstracts submitted by researchers, not just industry,
but researchers working on new drugs, and this is truly very new information. It's usually done in our biggest halls, and they’re always completely full because people are very interested in what is coming next and what is on the horizon.
Q5
The EADV Games are a new and exciting format for residents. What was the inspiration behind this initiative, and how do you believe it contributes to both education and fostering a sense of community among the next generation of dermatologists?
The primary inspiration was our young members. We've seen a huge increase in young members, residents, and dermatologists. In the last 5 years, we've actually doubled the number of EADV members, and we're now at 13,300. A lot of these are young people, and we try to engage them, not only to make them colleagues but also to introduce a little bit of competition.
The concept of the EADV Games came from seeing similar formats at some French meetings and
in other countries. Then, our Scientific Committee, along with a few colleagues, started to work on the project. The Games were such a great success last year, with people standing in the corridor trying to see what was happening since it was so much fun, that this year they had to be moved to a much bigger hall!
In the interest of keeping people engaged, we have to change. I can say that we have a really excellent programme with excellent speakers, but there is always a need for some changes to attract people.
Q6 As a leader in dermatology, what do you consider to be the most pressing global challenge, or biggest unmet need, facing the speciality at the moment?
First, as a medical dermatologist, we have a lot of pressure from cosmetic dermatology. We have many dermatologists who have moved into that field. For example, in some countries, you have a high number of dermatologists, but a small percentage who are

truly interested in pure medical dermatology. I feel that we have to keep young people connected with medical dermatology, because somebody will have to treat patients. Of course, being in corrective cosmetic dermatology brings money in faster and more easily, and I can understand that people need money. But we still need to maintain interest in medical dermatology. That is one of the goals of a congress like this: to highlight the beauty of this speciality.
The second problem concerns some diseases for which we still don't have drugs, or some diseases, like certain genetic conditions, for which we only recently got drugs. They are so expensive that they will not be available in most countries because the therapy can cost somewhere between 600,000–1,000,000 EUR per year. There are not many countries that will be able to afford that. This ties into the topic of disparities, and I think this will be the issue going forward. In some countries, there are many dermatologists but not enough medical dermatologists. In other countries, there are fewer dermatologists overall.
Of course, we also have to work on the public perception of our speciality. Dermatology is not just about putting on creams. We have seen huge advances with new drugs and discoveries that have truly changed our patients' lives. I remember when I started my residency, we had a massive number of patients with psoriasis going to phototherapy and putting on various topical treatments. Today, they have biologicals; they feel much better and have a much better quality of life. But for some diseases, there is still no therapy. So, there is a lot of work to do in the future.
Q7Looking back at your time as EADV president so far, what is the one moment, whether it's a scientific breakthrough presented at a congress or a key policy win for patients, that you are/believe you will be most proud of?
Well, let's wait a year! But I have to say that I'm truly very happy today because we have reached more than 20,000 participants, and this is something that we were dreaming of. For example, for this year, I was almost sure that we wouldn’t reach that number.
The Academy was founded 38 years ago with only 21 members, so we started with very low numbers. As I mentioned previously, we are now at 13,300 members and we’re still growing, with many members from all over the world, like the USA, Canada, Mexico, South America, and Asia. We have members from about 150 countries. Of course, a majority
That is one of the goals of a congress like this: to highlight the beauty of this speciality
of members are in Europe, but we have a lot of contacts and opportunities for networking.
This is something that makes me happy. I don't have some big plan to change something huge, but I think stability and steady growth are what usually ensure that an organisation will last a little bit longer. I don't trust big changes that happen in a short time.

EMJ is pleased to present three compelling interviews with leading dermatologists, offering a glimpse into the future of inflammatory skin disease management. Sara J. Brown discusses her cuttingedge research using a human skin organoid model to map the complex interplay of genetics and environmental factors in eczema. Martin Metz sheds light on the serious systemic burden of chronic spontaneous urticaria, advocating for more effective treatment to address comorbidities like suicide risk. Finally, Astrid Haaskjold Lossius highlights the importance of consistency in care, detailing her work on harmonised treatment guidelines for childhood atopic dermatitis and discussing overcoming psychological barriers such as steroid phobia.
Featuring: Sara J. Brown, Martin Metz, and Astrid Haaskjold Lossius

Sara J. Brown
Professor & Departmental Chair of Dermatology, Wellcome Trust Senior Research Fellow, University of Edinburgh, and NHS Lothian, Edinburgh, UK
Citation: EMJ Dermatol. 2025;13[1]:78-81. https://doi.org/10.33590/emjdermatol/DLIR5470
Your research uses a human skin organoid model to investigate genetic and environmental interactions in eczema. Can you explain the advantages of this model over traditional methods, and how it's helping you pinpoint why the prevalence of eczema has increased?
The organoid model that we use is a very simplified form of human skin, but the advantage it brings is that we can isolate the specific aspects of eczema risk that we are interested in, and control these very precisely.
In the skin organoid, we can specifically alter the expression of selected genes
We know that eczema is partly a genetic disease, because it runs in families with other atopic conditions, but the fact that eczema has increased in prevalence over recent decades shows the importance of environmental factors as drivers for this disease. In human
population studies, we are limited to data that can be collected in observational epidemiology, and in clinical trials, we are obviously limited by ethical constraints over what environmental exposures can be manipulated. In the skin organoid, we can specifically alter the expression of selected genes and expose the model skin to controlled environmental effects, such as pet or food allergens, cigarette smoke, or cleansing products. We can then monitor the response using a range of functional measurements (e.g., transepidermal water loss) and molecular measurements (including gene expression, and protein and lipid levels).
We are still a long way from answering the question ‘Why has the prevalence of eczema increased?’, but in using the skin organoid, we have made a start in unpicking the complexity.
Q2
You have a background in both genetics and clinical dermatology. How does this unique combination of expertise directly influence the way you approach research questions and the design of your studies?
I love genetics because it allows us to get to the root cause, the earliest initiating mechanisms in disease. And I love clinical work because our patients are so inspiring. Patients suffer with skin disease, and clinical work raises important questions: this is what influences my approach to research. ‘Why do some patients get mild or severe eczema?’, ‘Why do some people not respond to treatment?’, ‘Why don’t we have better treatments?’ I design my studies around these big questions from the clinic and use human skin cells and human data wherever possible to address these, so the path back to the clinic is at least theoretically possible.
My approach to therapy development also brings together genetics and clinical observation, focusing on methods to improve skin barrier function to complement our current success in treating atopic inflammation.
Your recent study on gene–environment interactions in atopic eczema showed that early-life dog exposure might modify a genetic risk factor. How do you plan to build on this finding, and what are the broader implications of these gene–environment interactions for eczema prevention and treatment?
In collaboration with a group of colleagues worldwide, we analysed data from large population studies and showed that early-life dog exposure (in utero or up to 2 years of age) can reduce the risk of eczema. This is a statistical association and was most significant in a subset of the population who
carry a specific genetic risk for eczema. So, we went on to test the association using human skin cells (keratinocytes) in the lab, with genetic analysis, modulation of gene expression, and exposure of the cells to dog allergen. We showed that a dampening-down of atopic inflammation when cells are exposed to dog allergen appears to occur via the IL-7 receptor, consistent with the genetic effect. This is preliminary evidence that the IL-7 pathway may modulate the risk of eczema, but a lot more work will be needed before this can be used as a treatment or as a preventative strategy. I do love dogs, but our work does not mean that everyone who is worried about eczema should have a pet dog!
We plan to build on this work by testing further environmental effects and their interaction with other genetic risk mechanisms, because we know that different people (with a different genetic background) respond differently
to environmental factors. This may elucidate mechanisms or pathways that could be targeted to treat, or preferably prevent, eczema in the future.
Q4
You have a strong focus on drug screening using human cells to define molecular targets. How close is this work to identifying a truly novel drug or prevention strategy for eczema that goes beyond existing treatments?
The pathway to therapy development is very challenging and prone to failure at every step, so I am cautious not to over-promise. But we have made substantial progress in understanding genetic mechanisms controlling skin barrier formation and function that can be targeted by small molecules. We have identified such molecules and are working with medicinal chemists to optimise these now. This progress offers real hope for novel therapy and preventative strategies.
Q5
You have written about the need for more rigorous research into topical steroid withdrawal (TSW). What are the key unanswered questions about TSW's molecular mechanisms, and what specific research designs would you advocate for to address them?
We still have a lot to learn about the molecular mechanisms leading to TSW and a major challenge is that TSW is a very diverse condition, with changes within the skin as well as systemic features. So, the first step is for us to learn from people with lived experience of TSW, to define core features which we can use to request specific samples for molecular analysis. A powerful research design would include longitudinal sampling of skin, blood, and saliva, to track the condition from its onset to the acute, severe stage, through the longer-term effects. In some patients, these can last months or years, before full resolution. The matching of samples from
skin, blood, and saliva would allow a comprehensive analysis of mechanisms that we wish to investigate (e.g., skin or systemic steroid insufficiency) as well as ‘hypothesis-free’ multi-omic analyses. I have started this work, but it will take time and more grant funding to complete this to a high standard. Convincing our research funding bodies that TSW is an important condition, worthy of research, is therefore an essential next step.
Q6
As a clinical academic, you care for patients of all ages with a wide range of skin diseases. How does your work with patients directly inform your research questions, and what have you learned from patient and public engagement that has shaped your research priorities?
I listen to my patients in clinic, but the public engagement arena allows more open, frank discussion, and children are especially good at very honest dialogue! Probably the most

important but heart-breaking message that I have heard from patients outside the clinic is that many doctors dismiss their condition as ‘only’ skin or ‘only’ eczema. The impact on the whole of life is not fully recognised, and that is frustrating. Another learning point is the discomfort caused by many of our topical treatments, and the battles that can develop in families between children and their parents/carers. So, I have changed my practice, now recommending much simpler regimes for eczema care. I focus on pre-bedtime as an opportunity for treatment to give the best chance of sleep during the night, whilst relieving families of the burden of time-consuming and complex regimes. I have learnt that some patients prefer topical treatments, whilst others strongly prefer oral or injected systemic treatments. From a research perspective, the different modes of delivery require different pharmacokinetic characteristics of the drug compounds, and this is an important consideration for our therapy development work.
Q7Given your expertise in both eczema and ichthyoses, how does your research on genetic predispositions to dry skin conditions provide insights into other inflammatory or allergic skin diseases?
I began my research looking at the skin barrier protein filaggrin, and the genetic condition ichthyosis vulgaris, caused by loss-of-function mutations in the gene FLG. That was nearly 20 years ago; work from my group and many others over these 2 decades has shown a role for FLG in predisposition to multiple atopic disorders, indicating that primary skin barrier dysfunction can lead to multi-system disease.
The development of global dermatology is a driver for innovation
Many questions remain about exactly how filaggrin plays this role in disease predisposition, not least because more severe skin barrier defects, for example epidermolysis bullosa, do not lead to allergic or atopic disease. There is something special about the barrier defect and immune dysregulation that occurs when filaggrin is missing from the skin. There is also a damaging feedforward loop where filaggrin expression is downregulated by atopic inflammation, further worsening the skin barrier dysfunction.
Filaggrin/FLG have proven difficult to target directly and, this is where an understanding of genetic mechanisms upstream of FLG may be useful in designing treatments. Similarly, it is difficult to know where to start in modifying the vast array of environmental factors in modern life, but we know that FLG affects the skin’s response to allergens and irritants, so if we can understand these interactions then we may be able to intervene.
Q8Looking at the future of dermatology, what do you see as the most significant breakthrough we can expect in the next 5–10 years, and how might that innovation impact the way inflammatory skin conditions are managed?
Many researchers, myself included, are working towards personalised medicine as an ideal
for the future, but I don’t think this will be achieved in the next 5–10 years because of the great complexity and diversity of many skin conditions.
What I do see as an exciting opportunity is to bring together the detailed understanding that we now have of molecular mechanisms, with treatments to target multiple aspects of a complex disease simultaneously. Examples include normalising keratinocyte differentiation as well as reducing inflammation (in eczema), enhancing innate immunity as well as reducing scarring (in hidradenitis suppurativa), or targeting both active inflammation and tissue resident inflammatory memory (in psoriasis).
We are also seeing a growing number of patients who do not experience benefit, or who develop worsening skin inflammation despite novel therapies. I am hopeful that our understanding of genetic predisposition might inform treatment decision-making to rebalance the skin to a state of homeostasis for these patients.
Finally, recent progress in treating patients with inflammatory skin conditions has been exciting and rewarding, but this sadly excludes those who cannot access treatment for economic or sociopolitical reasons. The development of global dermatology is a driver for innovation to reduce cost and to combat the practical challenges of service delivery towards equitable provision of care. Success in this would represent a very significant breakthrough.

Martin Metz
Professor of Dermatology, Institute of Allergology at
Citation: EMJ Dermatol. 2025;13[1]:82-84. https://doi.org/10.33590/emjdermatol/OJBP3502
Q1
Your research highlights the increased mortality risk in patients with chronic spontaneous urticaria (CSU), particularly from comorbidities like suicide and malignant neoplasms. What are the key takeaways from your recent study on this topic, and how do you believe these findings should change clinical practice?
Every patient with CSU should receive effective and safe treatment
For me, there are two main takeaways from the study. The first is that we still don't take CSU seriously enough. It is a debilitating disease that places a significant burden on patients, sometimes even leading to suicidal thoughts, as we can see in this study. The second takeaway is directly connected to the first: every patient with CSU should receive effective and safe treatment. Too many patients continue to receive no treatment or ineffective/inadequate treatment, including those that can be harmful over time, such as high doses of oral corticosteroids.
Q2
Your work sits at the intersection of mast cell biology, neuroimmunology, and pruritus. Can you explain the complex interplay between the nervous system and mast cells in causing chronic itch, and how your research aims to disrupt this cycle?
Mast cells are long-lived cells typically located near blood vessels and sensory nerves in the skin. When activated, they can rapidly release numerous mediators. Interestingly, some of the receptors on mast cells that cause activation and
degranulation are receptors that can bind neuropeptides released from sensory nerves. Vice versa, some of the mast cell-released mediators bind to receptors on sensory nerves and cause itch. The best-known mediator in this context is histamine, but there are other itch-inducing mast cell mediators, including cytokines and proteases. Therefore, mast cells are not only the most important effector cells in allergies, but the close communication between mast cells and sensory nerves is also an important component of chronic pruritus in various skin diseases. We aim to better understand this communication and identify targets to disrupt this cycle.
Your recent publication on the treatment of CSU points to a need for more effective disease-modifying therapies beyond antihistamines and omalizumab. What emerging treatments, such as Bruton’s tyrosine kinase inhibitors or antiKIT monoclonal antibodies, show the most promise for inducing long-term remission?
As of today, we cannot say much about long-term remission through disease-modifying therapies in CSU. While these potential aspects of novel drugs are certainly interesting, none of the studies were designed to investigate them. Current standard treatment options in CSU, antihistamines and omalizumab, are symptomatic treatments that are unlikely to show any relevant disease modification. In contrast, emerging CSU treatments that target Bruton’s tyrosine kinase, KIT, and
IL-4 receptor show potential for inducing long-term remission. The mechanisms leading to disease modification may include effects on autoantibody production or changes in the inflammatory microenvironment in the skin. These are exciting new areas of investigation that we need to explore in the future.
Q4
As the head of speciality outpatient clinics for pruritus, you work with patients who have debilitating itch. What is the most misunderstood aspect of chronic pruritus from a clinical perspective, and how does your research into its neuroimmunological basis seek to correct that?
From a clinical perspective, the biggest problem with chronic pruritus is that we do not see it. In some diseases, such as atopic dermatitis, lichen planus, or urticaria, we can see the itchy skin lesions. In patients with very
From a clinical perspective, the biggest problem with chronic pruritus is that we do not see it
severe chronic pruritus, the skin can appear perfectly normal (chronic pruritus of undetermined origin) or, as is the case in chronic prurigo, we see secondary scratch lesions without any primary skin alterations. It is important to understand how much these patients suffer and how effective (or non-effective) our treatment efforts are. To support this, we have developed patient-reported outcome measures that can aid in the daily management of these patients. Additionally, understanding and explaining
the neuroimmunological basis of chronic pruritus can help physicians understand that there is a real reason for the condition and that a targeted treatment can indeed help these patients.
Q5
Beyond the skin, chronic urticaria and pruritus have significant systemic impacts. What is the next frontier of research in understanding the systemic comorbidities of these mast cell-mediated diseases, and what role do you see for biomarkers in predicting and managing these risks?
There are different aspects that need to be considered when looking for systemic comorbidities in chronic urticaria and chronic pruritus. On one hand, these comorbidities are derived from the consequences of signs and symptoms on our patients’ quality of life. For example, we see much higher rates of anxiety and depression in these patients, and

we still have to characterise this better to raise awareness for the need for highly effective and safe treatment options. Instead of classical biomarkers, patientreported outcome tools, such as quality-of-life assessment and screening for psychological comorbidities, are of importance. On the other hand, there are certain systemic comorbidities that are associated with chronic urticaria and chronic pruritus. For chronic urticaria, there is, for example, a higher rate of patients with other autoimmune diseases. In chronic pruritus, there may be systemic comorbidities that are the underlying cause of the condition. It will be important to further characterise biomarkers that identify these underlying causes. An important future area of research is to identify biomarkers that can predict treatment response and thus allow for patient-tailored treatment.
Given the significant unmet needs in treating patients with CSU, where do you believe future research should be focused? Should the priority be on refining existing treatments, identifying new molecular targets, or on a more personalised, stratified approach to therapy?
The goal of any treatment in CSU is complete control of the disease, meaning no more signs and symptoms. To achieve this, we will have to identify new molecular targets, but the refinement of existing treatments will also be necessary. For available therapies, the focus will be on identifying specific biomarkers that predict the response to treatment, therefore leading to a more personalised, stratified approach. Most importantly, with both existing and future treatment options, we need to be able to decide on the treatment options
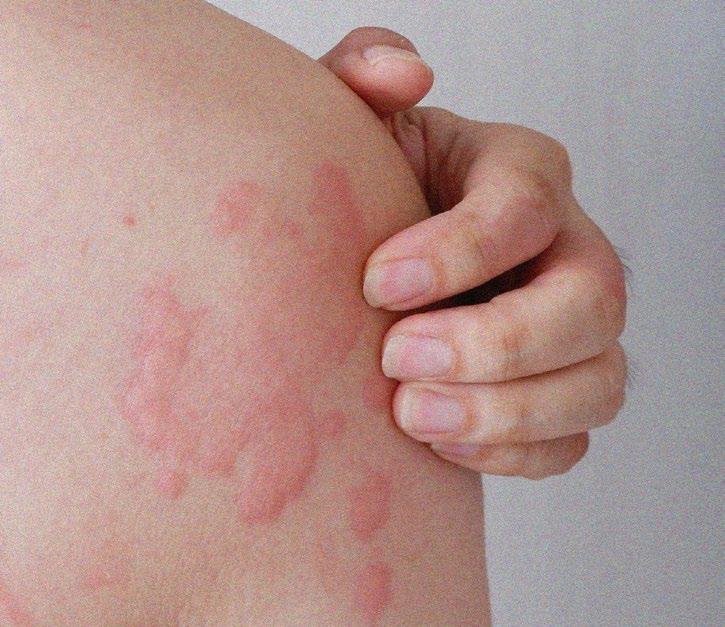
best suited for each individual patient based on shared decisionmaking processes.
In the field of mast cell-mediated diseases, which aspects of the innate immune system do you believe are most critical for future investigations, and how might that research lead to preventative strategies rather than more reactive treatments?
In the past, only a few diseases have been considered to be truly mast cell-mediated. This includes Type I allergies, urticaria, and mastocytosis. In these diseases, the main focus will be on understanding how the activation of mast cells can be best controlled and how we can prevent any signs and symptoms. While the focus here is clearly on the biology of mast cells themselves, understanding the contribution of other immune cells, including those of the adaptive system, will be crucial
for both preventative strategies and possibilities for disease modification. We anticipate, however, that there are more or less important roles for mast cells in other diseases. Here, the contribution of mast cells needs to be characterised in detail, and the interaction between cells of the innate and adaptive immune system, as well as resident cells of the respective tissues (i.e., sensory nerves, fibroblasts, etc.), needs to be explored.
An important future area of research is to identify biomarkers that can predict treatment response and thus allow for patienttailored treatment

Astrid Haaskjold Lossius Head of Department of Dermatology; Senior Dermatologist, Oslo University Hospital, Norway
Citation: EMJ Dermatol. 2025;13[1]:85-87. https://doi.org/10.33590/emjdermatol/VWRQ6617
Q1One of your recent publications focuses on harmonised topical treatment procedures for children with atopic dermatitis (AD) in Norway. What was the most significant challenge in getting consensus among an interdisciplinary group of doctors and nurses, and what do you hope will be the biggest impact of these new guidelines on patient care?
The most significant challenge in achieving consensus was bridging the differences in clinical practice philosophies. This included aligning approaches between dermatologists and paediatricians, as well as harmonising regional variations in care routines across hospitals and clinics in Norway. Differences in treatment protocols contributed to inconsistent advice and practices, making agreement complex.
Our aim was to establish a more consistent, evidence-based standard of care nationwide, hoping to reduce confusion for families and support better treatment adherence. With clearer, unified guidelines, clinicians can provide more confident and coordinated care. In turn, families are likely to experience less uncertainty, fewer disease flares, reduced stress, and ultimately a better quality of life for their children.
Our aim was to establish a more consistent, evidence-based standard of care nationwide
Q2 Your work has shown that treatment adherence is often low due to patients receiving conflicting information or having a ‘phobia’ of treatments. Beyond providing harmonised guidelines, what strategies are you exploring to improve patient education and combat these psychological barriers?
Beyond harmonised guidelines, one of our key strategies is structured education targeting healthcare personnel, general practitioners, and families. Our aim is to improve treatment adherence and address psychological barriers such as steroid phobia by increasing knowledge and confidence around AD care. We run a multidisciplinary educational programme for caregivers of children with AD, offered as a 2-day course held four times a year. The course includes sessions led by a dermatologist, a nurse, a psychologist, a social worker, and patient organisation representatives, and is designed to provide both practical guidance and emotional support. To reduce confusion around topical treatment, we have also developed a standardised written treatment plan, which helps families understand exactly how and when to apply therapies. In parallel, we offer regular training sessions for general practitioners and other healthcare professionals, helping ensure consistency of care across settings. Additionally, we have created educational videos on topical corticosteroids and emollients, which are freely available on YouTube (San Bruno, California, USA) and intended as
accessible resources for patients and their families.
Q3 You have a strong interest in systemic treatments for AD, particularly during conception, pregnancy, and breastfeeding. What was the most surprising finding from the interdisciplinary consensus you helped build, and what does this project reveal about the current limitations in evidence for treating this specific patient population?
This project really highlighted the emotional and ethical complexity of treating pregnant or breastfeeding patients with moderate-to-severe AD, especially when disease control is poor and the impact on quality of life is profound. Clearly, we are still operating with major evidence gaps. Most drug safety data come from post-marketing surveillance or registry studies, not from prospective, controlled trials in pregnant patients. As a result, we rely heavily on interdisciplinary consensus and expert judgement, rather than clear, guidelinebacked protocols. Our goal with
this project was to create a practical, risk-balanced framework for clinicians that supports shared decision-making, considers both maternal and fetal well-being, and acknowledges that untreated disease can be harmful too. In the end, the process revealed that while we're making progress, we urgently need more targeted research and long-term safety data for this specific population, who have been largely excluded from clinical trials for natural reasons.
As the head of a major tertiary referral centre for dermatology, you handle complex cases in both children and adults. What is the biggest difference in managing AD in these two groups, and how does your department's research directly address those unique challenges?
One of the biggest differences in managing AD between children and adults is the complexity of disease expression and comorbidity burden. In children, we often deal with parental
anxiety, treatment adherence, and preventing long-term disease progression. The focus is on education, early intervention, and building trust with families. In contrast, adult patients often present with chronic, treatmentresistant disease, sometimes complicated by psychological distress, sleep disruption, or occupational impacts, and they may carry years of inconsistent care or undertreatment. From a clinical standpoint, this means that our approaches must be age-specific: paediatric care needs more behavioural support and parent-led education, while adult care often requires multidisciplinary management, including psychology, allergology, and sometimes rheumatology. Our department’s research is structured to reflect this reality. For example, we’ve developed harmonised topical treatment protocols for children, aimed at reducing conflicting advice and steroid phobia. At the same time, we’re involved in clinical trials and biomarker studies in adults, particularly focusing on biologic and JAK inhibitor responses.
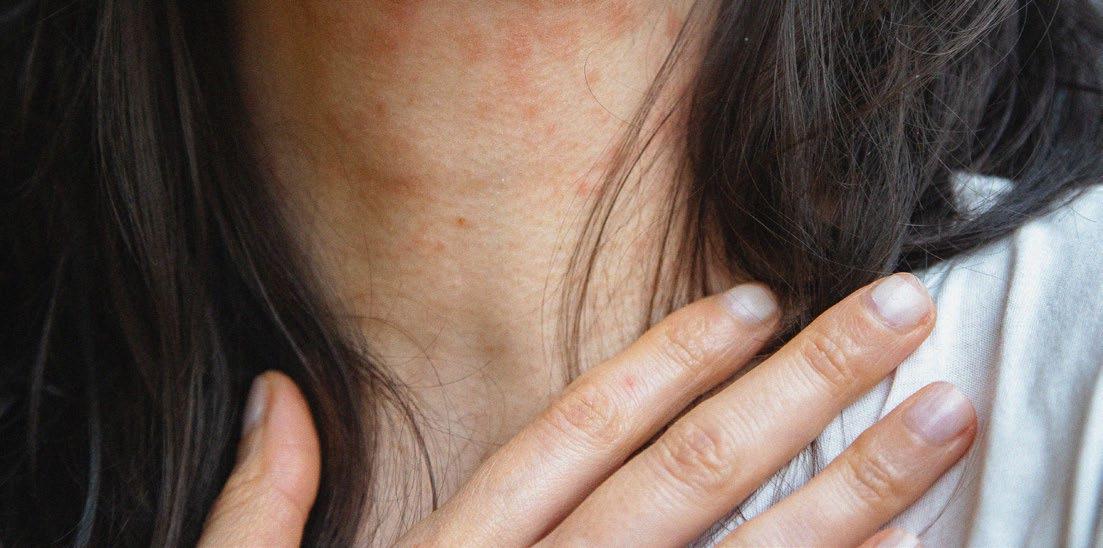
Q5 Your research group is focused on inflammatory skin disorders. Do you see a future where we can tailor topical treatments for a patient's specific genetic profile, moving beyond the current one-size-fitsall approach to emollients and corticosteroids?
Absolutely, and I think that we’re closer than we realise. AD is increasingly understood as a spectrum of endotypes rather than a single disease. Patients can have very different underlying drivers, from FLG mutations and barrier defects to distinct immune pathway activations. So yes, I believe the future lies in precision therapy, where we tailor not just systemic treatments, but even emollients and topical antiinflammatory treatments based on a patient’s genetic, molecular, or microbiome profile. The challenge will be making these approaches accessible, not just technically feasible, but practical for everyday clinical use.
Q6 Beyond the currently approved drugs, what's on the horizon for treating AD? Are there any specific new drug targets or classes that you are most optimistic about in the next 5–10 years?
There are several promising drug targets and classes in the pipeline for AD beyond what is already approved. Some are refinements of current approaches; others are novel mechanisms. I’m especially excited about the pruritusspecific targets, like the IL-31 inhibitors, and I hope these drugs will become available in Norway shortly.
Q7
The field of AD has seen significant breakthroughs with biologics and JAK inhibitors. In your opinion, where is the next frontier of innovation? Is it in prevention, personalised medicine, or finding a definitive cure?
The introduction of biologics and JAK inhibitors has been a game-changer in AD treatment, especially for patients with moderate-to-severe disease. But we're still far from a definitive solution, and in my view, the next frontier of innovation lies in personalised medicine. While biologics and small molecules have expanded our toolbox, not every patient responds the same way. The future lies in precise endotyping, understanding the distinct immunological and genetic subtypes of AD, and tailoring treatment accordingly. We're moving towards a model where biomarkers will guide therapy choice, dosing, and treatment duration, improving outcomes and reducing unnecessary exposure to systemic drugs.
Q8
Considering your work and your role as a departmental head, what advice would you give to a young dermatologist who wants to combine clinical practice with impactful research, especially in a specialised area like AD?
Be curious and authentic. If you can hold on to those two traits, surround yourself with good people, and stay rooted in patient care, your work has the potential to be both scientifically meaningful and genuinely transformative. The most impactful research often begins with something simple: a clinical observation, a recurring question, or a frustration in daily practice. Pay close attention to those moments: unmet needs,
treatment gaps, or patterns you keep seeing in your patients. Let those real-world insights shape your research agenda. It will keep your work grounded, relevant, and ultimately more impactful.
Collaboration is essential, especially early on in your career. Don’t be afraid to reach out beyond your specialty to paediatricians, nurses, psychologists, pharmacists, or even health economists. AD, like many chronic conditions, is multifaceted. Cross-disciplinary input not only strengthens your work but also helps you see the full picture of patient care.
And don't wait for the perfect big project. Start with what’s manageable: a quality improvement initiative, a retrospective review, or a simple patient survey. These smaller projects are valuable stepping stones.
The
most impactful research often begins with something simple: a clinical observation, a recurring question, or a frustration in daily practice
CHE is a long-term, heterogenous, multifactorial inflammatory skin disease on the hands and wrist. Characterised by key symptoms of itch and pain with potentially overlapping aetiological subtypes.1-5
Key signs include erythema, scaling, lichenification, hyperkeratosis, vesicles, oedema, and fissures with no reliable link between the visible signs of CHE and the underlying aetiological cause.3-6
Due to CHE, patients see increased risk of S. aureus infection. 7,8
CHE pathogenesis is multifactorial with exogenous and endogenous factors, warranting a holistic treatment approach:9
Skin barrier dysfunction is a defining feature barrier impairment facilitates the penetration
Patients with CHE have notably skin barrier with:15-19
• Increased transepidermal water
• Higher degree of Staphylococcus
• Higher IL-8 level on the skin surface
• Higher frequency of filaggrin mutations
Immune dysregulation occurs in CHE irritants, or pathogens breach the impaired inducing keratinocyte cytokine release.
Cytokines activate immune cells which pruritus, epidermal hyperplasia, and barrier dysfunction.5,14
Although CHE pathophysiology can vary cytokines through the JAK-STAT pathways.
There is considerable overlap in the pathophysiology of CHE and AD;9 however, both have distinct immune profiles that require individual targeted approaches.10-14
Symptoms localised to hands and wrist1-5
Different aetiological subtypes show different immune signatures including Th1/Th17 and Th2/Th2210-12
Broad, potentially mixed immune signature activation may require broad coverage MoA therapeutic interventions11,12
Symptoms can affect skin across the whole body10
Several immune pathways are involved in pathogenesis, with the Th2/Th22 immune signatures playing a pivotal role11-14
Narrow immune signature may require more selective treatment11,12
JAK1:JAK2
JAK1:JAK3
The JAK-STAT pathway provides a broad required to address the complex nature of
The publication of this infographic was funded by Leo Pharma A/S and is intended for healthcare professionals only.
EMJ Dermatol. 2025;13[1]:88-89. https://doi.org/10.33590/emjdermatol/ZHNR6982

feature of CHE, independent of aetiology.5,15 In healthy skin, the barrier effectively protects against external stressors.16 In CHE, penetration of irritants and allergens, eliciting immune activation that drives a self-perpetuating cycle of disease across all subtypes.15,16
Healthy skin
notably impaired
water loss
Staphylococcus colonisation surface mutations
CHE when allergens, impaired skin barrier, release.15,16 which promote and further
Damaged skin in CHE Epidermis Dermis
5,9,18,19
by subtype,8 all subtypes are driven by multiple pathways.5,18-21
JAK1:JAK2:TYK2
JAK2:TYK2
JAK1:JAK3
JAK1:JAK2:TYK2
JAK1:JAK2
JAK1:TYK2
JAK1:JAK3
JAK1:JAK2
JAK1:JAK2:TYK2 JAK1:JAK2
contact dermatitis
Atopic dermatitis contact: metals
JAK1:JAK2:TYK2
JAK2:TYK2
JAK1:TYK2
Allergic contact: rubbers/fragrance
broad target that may facilitate the holistic treatment approach of CHE.9,11,18-22
Transepidermal water loss
Structural changes to corneocytes
Cytokine release
Entry of irritants and infectious agents
Inflammation
> CHE is a distinct inflammatory skin disease marked by barrier dysfunction and immune dysregulation 1-9
> CHE and AD have unique immune profiles that require their own individual targeted approaches.9-14
> Although CHE pathophysiology can vary by subtype, all subtypes are driven by cytokine activity through JAK-STAT signalling pathways. 5,18-21
> The JAK-STAT pathway provides a broad target that may contribute to the holistic treatment approach warranted to address the complex nature of CHE.9,11,18-22
Abbreviations:
AD: Atopic dermatitis; CHE: chronic hand eczema; HR-QoL; health-related quality of life; IL: interleukin; JAK: Janus kinase; STAT: signal transducer and activator of transcription; S. Aureus: Staphylococcus Aureus; Th: T helper (cell); TYK: tyrosine kinase.
References:
1. Thyssen JP et al. Contact Dermatitis. 2022;86(5):357-78. 2. Molin S et al. Acta Derm Venereol. 2025;105:adv42596. 3. Lynde C et al. J Cutan Med Surg. 2010;14(6):267-84. Erratum in J Cutan Med Surg. 2011;15:360. 4. Menné T et al. Contact Dermatitis. 2011;65(1):3-12. 5. Lee GR et al. Dermatol Ther. 2019;32(3):e12840. 6. Haslund P et al. Br J Dermatol. 2009;161(4):772-7. 7. Mernelius S et al. Eur J Clin Microbiol Infect Dis. 2016;35(8):1355-61. 8. Dubin C et al. Ther Clin Risk Manag. 2020;16:131932. Erratum in: Ther Clin Risk Manag. 2021;17:233. 9. Rosenberg FM et al. Contact Dermatitis 2024;90(1):23-31. 10. Quaade AS et al. J Allergy Clin Immunol. 2025;155(4):1250-63. 11. Di Domizio C, Gillet C. Oral Presentation D1T09.1A. EADV Congress 2024. 25-28 September, 2024. 12. Napolitano M et al. Front Med (Lausanne) 2023;10:1165098. 13. Huang IH et al. Front Immunol. 2022;13:1068260. 14. Tauber M et al. J Eur Acad Dermatol Venereol. 2020;34(7):1529-35. 15. Smith AR et al. Curr Allergy Asthma Rep. 2017;17:6. 16. Gittler JK et al. J Allergy Clin Immunol. 2013;131:300-13. 17. Virtanen A et al. BioDrugs. 2019;33(1):15-32. 18. Pan Y et al. Front Immunol. 2022;13:911546. 19. Worm M et al. Br J Dermatol 2022;187(1):42-51. 20. Tancredi V et al. Int J Mol Sci. 2023;25(1):362. 21. Scheinman PL et al. Nat Rev Dis Primers. 2021;7:38.
Author:
*Claudia DeGiovanni1
1. University Hospitals Sussex NHS Foundation Trust, Brighton, UK *Correspondence to c.degiovanni@nhs.net
Disclosure: DeGiovanni has received the Vichy Exposome Award 2025 and an NIHR ARC IDA award to fund a current study outside the submitted work; consultancy fees from skinbetter science and UCB; speaker fees from La Roche-Posay and L’Oréal; and support for attending meetings from UCB and Galderma.
Received: 17.06.25
Accepted: 18.08.25
Keywords: Dryness, genitourinary syndrome of menopause (GSM), hair loss, hormone replacement therapy (HRT), menopause, oestrogen deficiency, perimenopause, skin, skin ageing.
Citation: EMJ Dermatol. 2025;13[1]:90-94. https://doi.org/10.33590/emjdermatol/CEVS4601
Menopause is a natural biological transition in a woman’s life, marking the permanent cessation of menstruation, and typically occurring around the age of 51 years. The preceding phase, perimenopause, is characterised by significant hormonal shifts, with fluctuating and ultimately declining levels of oestrogen and progesterone. While vasomotor and psychological symptoms are well-recognised, the impact of menopause has far-reaching consequences, including significant structural and functional alterations in the skin, hair, and vulvovaginal tissues. A comprehensive understanding of these dermatological changes is essential for clinicians supporting women through menopause, if they are to address these diverse and often distressing concerns.
Both oestrogen receptor alpha (ERα) and oestrogen receptor beta (ERβ) are widely expressed throughout the skin in epidermal keratinocytes, dermal fibroblasts, and hair follicles,1 with the highest concentrations found in the facial and genital skin.1,2 When
activated by oestrogen, they orchestrate a cascade of genetic and cellular responses, accounting for the widespread and tissuespecific changes observed.3
One of the most critical roles of oestrogen is maintaining the extracellular matrix of the dermis. Oestrogen stimulates the production of procollagen I, the precursor to collagen, and increases the levels of tropoelastin and fibrillin, which are components of elastic fibres.3 Furthermore, oestrogen downregulates the expression of matrix metalloproteinases, the enzymes that break down collagen.3 This delicate balance of synthesis and degradation is vital for maintaining skin firmness and elasticity. It is estimated that women lose approximately 30% of their cutaneous collagen in the first 5 years post-menopause, a loss more closely correlated with the duration of oestrogen deficiency than with chronological age.1,3 Oestrogen replacement has been consistently shown to reverse these changes, leading to increased epidermal thickness and a significant increase in skin collagen content, correcting but not overcorrecting collagen deficiency.1 One study demonstrated a 6.49% increase in skin collagen after 6 months of oral oestrogen, and in another trial, dermal
thickness increased by 30% after 1 year of oral oestrogen therapy.3
Oestrogen also plays a key role in skin hydration. It significantly increases the levels of glycosaminoglycans and hyaluronic acid (HA) in the dermis.4 HA, in particular, is a powerful humectant, capable of holding vast amounts of water.4 Oestrogen also increases the water-holding capacity of the stratum corneum, improving the skin’s barrier function.4 The combination of diminished collagen, elastin, and hydration leads to an increase in skin wrinkling and a reduced skin elasticity, both of which are demonstrably improved with oestrogen replacement.3,4
Beyond structural components, oestrogen is a potent antioxidant. The presence of the A-ring phenol in oestrogen allows it to directly attenuate reactive oxygen species generated by processes such as the Fenton reaction.3 Reactive oxygen species are major contributors to skin ageing, causing damage to DNA, proteins, and lipids, and oestrogen deficiency leaves the skin more susceptible to this oxidative damage.3
In wound healing, oestrogen enhances the migration of dermal fibroblasts and promotes collagen deposition.3 It also appears to increase the secretion of latent transforming growth factor-beta 1 (TGF-β1), a crucial cytokine involved in wound repair.3 Delayed re-epithelialisation and reduced collagen deposition were noted in the wounds of postmenopausal women compared to premenopausal women, and these changes improved with oestrogen administration.3
Skin-related symptoms in menopause are common and, in the author’s view, frequently under-reported by patients and inadequately addressed by healthcare professionals. A survey of women attending a menopause clinic demonstrated that 100% of respondents had at least one skin symptom, yet 48% of these women had not disclosed their skin concerns to their
medical professionals.5 Their Dermatology Quality of Life Index (DLQI) score ranged from 0–17 (median: 5), demonstrating that skin has a significant impact on quality of life for many women.5 A DLQI score >10 indicates a severe impact on quality of life.
Increased skin dryness and associated pruritus are prevalent concerns among postmenopausal women not receiving hormone replacement therapy (HRT).2 This is attributable to the decrease in glycosaminoglycans and HA, as well as compromised stratum corneum and skin barrier function.1 Oestrogen replacement has been shown to improve epidermal hydration and the water-holding capacity of the stratum corneum, as demonstrated by increased electrical capacitance.1
Hot flushes, a common vasomotor symptom, reflect impaired peripheral vascular control due to oestrogen deficiency.1,2 The resolution of these symptoms with oestrogen replacement underscores the hormone’s integral role in peripheral circulatory regulation.1
Evidence regarding the specific impact of menopause on many established skin diseases is limited, yet in one survey, 46% of women noted a deterioration in a previously diagnosed skin condition.5
Vasomotor flushing, together with increased skin sensitivity, can aggravate rosacea.2
Acne is well recognised in perimenopause, affecting approximately 25% of women.6 Its aetiology is multifactorial and involves a relative androgen excess, increased inflammation, defective skin barrier function, and possible changes to the skin microbiome.7
Management is challenging, as standard treatments can exacerbate dry menopausal skin,6 and the incidence of relapse7 and post-inflammatory hyperpigmentation is high in this population.6
Topical retinoids and azelaic acid are effective treatment options for both active acne and post-inflammatory changes, if tolerated.7 Systemic antiandrogens, such as spironolactone, have also shown benefit in persistent adult female acne,6,7 though evidence in a purely menopausal population is lacking.
The impact of menopause on other common skin conditions, including atopic eczema, psoriasis, and hidradenitis suppurativa, has not been fully evaluated, and findings from existing studies have not been consistently reproduced.2 The potential impact on the skin barrier function, reduced inhibition of the T helper 1 cell-mediated pathway, and the androgenic hormonal shift postmenopause may plausibly affect such conditions.2 Further research into these common dermatoses throughout menopause is needed.
Oestrogen exerts a notable influence on hair follicle biology, primarily affecting the hair growth cycle. Oestrogens decrease the telogen (resting) phase and prolong the anagen (growing) phase of the hair cycle in trichogram studies.3,8 Conversely, androgens (testosterone and dihydrotestosterone) can inhibit scalp hair follicles, which is a key mechanism in androgenic alopecia.9 Furthermore, women express higher levels of aromatase in scalp hair follicles than men, indicating that conversion of testosterone to oestrogen via this enzyme is protective against alopecia in women.8 Progesterone may also offer a protective effect by inhibiting the 5α-reductase enzyme that converts testosterone to the more potent dihydrotestosterone.9
The impact of hormones on hair growth is further supported by clinical observations, such as the increased proportion of anagen hairs during pregnancy (a high oestrogen state), subsequent telogen effluvium postpartum,8 and the common occurrence of scalp hair thinning in women treated with aromatase inhibitors, which suppress local oestrogen synthesis.3
Immunohistochemical studies have shown that ERβ is the predominant oestrogen receptor expressed in human non-balding scalp anagen hair follicles in both men and women.3 In vitro studies have demonstrated that 17β-oestradiol can stimulate hair shaft elongation in frontotemporal male hair follicles, indicating a direct stimulatory effect.8
It is therefore not surprising that menopausal women notice changes in hair growth and quality. In a survey of menopausal women, 82% noted at least one hair symptom, most commonly hair thinning (54%) and hair shedding (44%).5 However, evidence for the use of HRT to manage hair thinning in menopause is lacking.9
Female pattern hair loss (androgenic alopecia) incidence increases postmenopause. Circulating androgen levels are usually normal; therefore, the hypooestrogen state and relative androgen/ oestrogen ratio are the presumed causative factors.9 Recognised treatments include topical or oral minoxidil and 5α-reductase inhibitors. There is insufficient evidence to support HRT in this condition.9
Hirsutism can affect up to 50% of postmenopausal women. It may be aggravated by testosterone replacement. Again, it is the relative imbalance of androgens and oestrogen that is likely to be important in the aetiology.9
The vulvovaginal epithelium is particularly sensitive to oestrogen levels, possessing a high concentration of oestrogen receptors. In the aforementioned survey, 84% of menopausal women reported vulval symptoms, including dryness (58%), itch (54%), and soreness (34%).5
In the female genital tract, hypo-oestrogen leads to epithelial atrophy, decreased natural lubrication, decreased blood flow, and loss of collagen elastin and adipose tissue.10,11 These result in architectural changes such as loss of labial volume.10 The vulval skin becomes less elastic and more
susceptible to irritation and micro-trauma, contributing to overall discomfort and significantly impacting quality of life.10-12 These changes commonly manifest as symptoms such as vaginal dryness, itching, burning, urinary symptoms, and painful intercourse (dyspareunia). This collection of symptoms is known as genitourinary syndrome of menopause (GSM), and affects up to 84% of postmenopausal women.10-12 Co-existence with other vulval conditions, such as lichen sclerosus and vulvodynia, is not uncommon.11 GSM significantly impacts physical, sexual, and mental health, and affects quality of life.12 Correction of this oestrogen deficiency with topical or systemic oestrogen therapy can alleviate these symptoms, though early diagnosis and treatment are key.12 A significant care gap exists, as many women do not volunteer information about these symptoms, and clinicians often do not proactively inquire.12 This gap needs to be bridged to enable women to receive appropriate care early on in the condition, when treatment is more likely to be successful. Topical (vaginal) oestrogen therapy does not carry the same systemic risks as HRT,12 and it is vital to educate healthcare professionals and patients in order to alleviate unfounded fears and improve uptake.
Maintaining the pelvic floor is also important for women with GSM who are symptomatic, and the value of women’s health physiotherapy should not be overlooked.10
The global menopause market is rapidly expanding, with a recent valuation of 17.79 billion USD in 2024, and a projected growth to 24.35 billion USD by 2030.13 This proliferation of commercially available products, including cosmetics, dietary supplements, and various alternative therapies, creates a complex and challenging landscape for women navigating the symptomatic stages of menopause.
Skincare, especially, is important throughout menopause, and clinicians supporting
menopausal women are well placed to provide advice. Ensuring that high-quality products that are non-comedogenic are used on the skin, using gentle skin cleansers, and avoiding potential allergens and irritants for sensitive skin are key. Topical retinoids stimulate fibroblastmediated collagen synthesis, improve skin elasticity, and promote angiogenesis,14 and can therefore be a crucial addition to a menopausal woman’s skincare routine, though they can cause dryness and irritation. Patients should be counselled to use a high-factor UVA and UVB sunscreen meticulously to minimise such side-effects.
A critical concern is the current lack of high-quality, robust, evidence-based data regarding the efficacy and safety of many products marketed to menopausal women. This includes, but is not limited to, oral supplements, phyto-oestrogens (plantbased products with a similar structure to oestrogen), light-emitting diode therapies, and probiotics. Some products may also cause other unwanted side effects; for example, the application of topical oestrogen cream has been associated with the development of melasma.15
The author’s main concern is that menopausal women will suffer through misdirection and delayed care, missing out on evidence-based treatments and incurring significant financial costs while trialling heavily marketed products with unsubstantiated claims.
Therefore, there is a compelling need for a more rigorous evaluation of these nontraditional treatments to provide clear guidance for both clinicians and patients.
In summary, the declining oestrogen and progesterone levels in perimenopause and menopause have a profound effect on the skin, hair, and vulval tissue. These lead to distressing symptoms that are not often recognised as part of the menopausal transition.
The precise impact of menopause on the trajectory of common inflammatory skin diseases is poorly defined. Furthermore, while HRT is effective for many symptoms of oestrogen deficiency, its specific role as a primary or adjunctive therapy for dermatological conditions requires more
References
1. Brincat MP. Hormone replacement therapy and the skin. Maturitas. 2000;35(2):107-17.
2. Kamp E et al. Menopause, skin and common dermatoses. Part 2: skin disorders. Clin Exp Dermatol. 2022;47(12):2117-22.
3. Thornton MJ. Estrogens and aging skin. Dermatoendocrinol. 2013;5(2):264-70.
4. Shah MG, Maibach HI. Estrogen and skin. An overview. Am J Clin Dermatol. 2001;2(3):143-50.
5. Salih H et al. Results of a patient survey exploring skin symptoms in a menopause clinic. Post Reprod Health. 2025;DOI:10.1177/20533691251332403.
6. Khunger N, Mehrotra K. Menopausal acne - challenges and solutions. Int J Womens Health. 2019;11:555-67.
robust evaluation. There is an urgent need for further research to clarify these issues, develop targeted therapeutic strategies, and ensure clinicians are equipped with the knowledge to support women fully through their menopausal journey.
7. Bagatin E et al. Adult female acne: a guide to clinical practice. An Bras Dermatol. 2019;94(1):62-75.
8. Zouboulis CC et al. Sexual hormones in human skin. Horm Metab Res. 2007;39(2):85-95.
9. Kamp E et al. Menopause, skin and common dermatoses. Part 1: hair disorders. Clin Exp Dermatol. 2022;47(12):2110-6.
10. Musbahi E et al. Menopause, skin and common dermatosis. Part 3: genital disorders. Clin Exp Dermatol. 2022;47(12):2123-9.
11. Spring A et al. Identification and management of vulval problems of the postmenopausal woman - tips and tricks. Post Reprod Health. 2020;26(3):155-61.
12. The NAMS 2020 GSM Position Statement Editorial Panel. The
2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27(9):976-92.
13. Grand View Research (GVR). Menopause market size, share & trends analysis report by treatment (dietary supplements, OTC pharma products), by region (NA, Europe, APAC, Latin America, MEA), and segment forecasts, 20252030. Available at: https://www. grandviewresearch.com/industryanalysis/menopause-market. Last accessed: 1 August 2025.
14. Zasada M, Budzisz E. Retinoids: active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol. 2019;36(4):392-7.
15. Snyder A et al. Melasma associated with topical estrogen cream. J Clin Aesthet Dermatol. 2017;10(2):57-8.
Authors: *Marese K. O’Reilly,1 Robert Dawe,1 Sally H. Ibbotson1
1. Photobiology Unit, Ninewells Hospital and Medical School, Dundee, UK *Correspondence to marese.oreilly2@nhs.scot
Disclosure: The authors have declared no conflicts of interest.
Received: 15.09.25
Accepted: 06.10.25
Keywords: Photodermatoses, photosensitivity, phototesting, UV radiation (UVR).
Citation: EMJ Dermatol. 2025;13[1]:95-103. https://doi.org/10.33590/emjdermatol/OFRL3881
Skin photosensitivity is defined as the cutaneous response to UV radiation (UVR) and/or visible light (VL). The Fitzpatrick Skin Phototype Classification (FSPC) has been widely adopted as a method for assessing an individual’s baseline susceptibility to sunburn and tanning.1 Photosensitivity is deemed pathological when the skin’s reaction to UVR/VL deviates significantly, either qualitatively or quantitatively, from that of the general population. Clinical manifestations, including the development of rashes or exaggerated sunburn-like reactions following minimal UVR/VL exposure, indicate an underlying abnormal photosensitivity disorder.2 These photosensitivity disorders are provoked or aggravated by sunlight or artificial light sources and exhibit wide variability in both prevalence and clinical severity. Polymorphic light eruption (PLE) is among the most common, with a pooled estimated prevalence of 10.00% among the general population (ranging from 0.65% in China to 21.40% in Ireland),3 whereas solar urticaria is rare, with a point prevalence of 3.1 per 100,000 in the Tayside region of Scotland, UK.4
Management of these conditions often requires substantial lifestyle adaptations, including strict photoprotection and
reduced outdoor activity. Beyond the physical manifestations, the psychosocial impact is considerable. A systematic review by Rutter et al.5 found that 31–39% of patients reported a very large impairment in quality of life (Dermatology Life Quality Index [DLQI] >10), highlighting the broader burden imposed by photodermatoses and underscoring the importance of accurate diagnosis of abnormal cutaneous photosensitivity through thorough clinical assessment and appropriate investigations. Given the heterogeneity of the clinical presentations, patients may initially present to a range of medical specialists. This feature aims to review the clinical features, diagnostic approaches, and general management strategies for these conditions.
Although photodermatoses are traditionally classified by pathogenesis, for the purposes of this feature, the authors propose an alternative classification based on the timing of clinical presentation, which may be more practical for the general physician. Photodermatoses can be broadly classified into three categories: those with immediate reactions (seconds–minutes) following sun exposure, those with intermediate responses (minutes–hours), and those with delayed responses (hours–days), often associated with more chronic manifestations over several days–weeks.
Some causes of photosensitivity can also lead to delayed problems, including skin cancers. Regardless of the classification system used, a thorough clinical assessment is essential to identify a history of photosensitivity, characterise the nature of the reaction, and guide diagnostic evaluation.
Classification of the photodermatoses based on pathogenesis:6
• Immunological
- Polymorphic light eruption
- Juvenile spring eruption
- Solar urticaria
- Chronic actinic dermatitis
- Actinic prurigo
- Hydroa vaccinforme
• Drugs and chemicals
- Endogenous: porphyrias
- Exogenous: systemic and topical
• Photogenodermatoses
- Xeroderma pigmentosum
- Bloom syndrome
- Rothmund–Thompson syndrome and others
• Photoaggravated
- Atopic dermatitis
- Seborrhoeic dermatitis
- Connective tissue diseases, e.g., lupus, dermatomyositis
- Rosacea and others
EXPOSURE)
Solar urticaria (Figure 1) is an immediatetype hypersensitivity reaction that can occur at any age and is characterised by immediate onset (within seconds–minutes of exposure) of itching, erythema, and whealing on sun-exposed sites, which resolve in ≤1 hour in 64.1% and persist up to 24 hours in 33.5%. As with other inducible urticarias, a specific underlying cause is not identified.7

Erythropoietic protoporphyria (EPP) is an acute inherited disorder and should be suspected in patients presenting with skin pain after a few minutes of sun exposure. A typical first manifestation of EPP is a baby crying when put outdoors in daylight or when exposed to light coming through the window glass.8
A severe sunburn reaction occurring after trivial quantities of sunlight and persisting beyond the expected duration of a ‘normal sunburn’ may indicate xeroderma pigmentosum (XP), a rare autosomal recessive condition. Patients with XP may have associated features such as persistent freckles (lentigines) of sunlight-exposed skin, as well as an increased susceptibility to cutaneous malignancy from as young as 5 years of age.9
A careful drug history is of utmost importance in any patient presenting with signs or symptoms of photosensitivity. Immediate prickling, burning, or erythema is particularly associated with drugs such as
amiodarone and chlorpromazine, whereas an exaggerated sunburn-like reaction can be associated with fluoroquinolone or tetracycline antibiotics, amiodarone, or thiazide diuretics.10
PLE (Figure 2), the most common photodermatosis, usually develops in spring or summer and is typically triggered by 30 minutes–several hours of sun exposure, often occurring later that day or the next day and resolving without scarring within days, if further sun exposure is avoided. Several morphological subtypes have been described, although a pruritic, erythematous, papular, and sometimes vesicular rash on non-perennially exposed sites such as the chest and arms is the most common presentation.11 Many patients experience symptomatic improvement
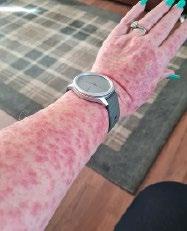
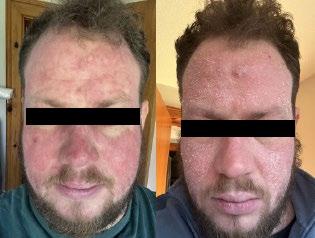
A photo-exposed site dermatitis showing acute (A) and chronic (B) dermatitic reaction. Note the relative sparing in photoprotected areas.
by late summer, likely due to a gradual ‘hardening’ or increased phototolerance of the skin. Some individuals may only exhibit symptoms during periods of intense sun exposure, such as sunny holidays, whereas those with more severe forms may experience symptoms yearround. The diagnosis of PLE is primarily clinical; however, phototesting and/ or histopathological evaluation may be necessary in atypical or unclear cases.12
PHOTODERMATOSES (TRIGGERED BY HOURS–DAYS OF EXPOSURE)
Drug-induced photosensitivity can present not only with acute reactions but also with a range of delayed manifestations. These include increased skin fragility and blistering due to trauma in pseudoporphyria (commonly linked to drugs such as naproxen and furosemide), and telangiectasia in photo-exposed areas, particularly with calcium channel blockers.10
Chronic actinic dermatitis (Figure 3) should be considered in patients presenting with eczematous eruptions in a photo-distributed pattern, or in those with pre-existing dermatitis (sometimes atopic) who develop a change in distribution or seasonal pattern of flares. As patients may not associate their symptoms with sun exposure, a high index of clinical suspicion is warranted.6
The presence of scarring in photo-exposed areas should prompt consideration of conditions such as actinic prurigo or hydroa vacciniforme. Actinic prurigo is an immunological photodermatosis, most prevalent among individuals of American Indian descent, and typically presents in childhood. In the UK, it is closely associated with human leukocyte antigen (HLA) DR4 and the subtype DRB1*0407.13 It is usually perennial and begins with sunlight-induced pruritic, patchy, and oedematous erythema accompanied by papules and occasional vesicles. Over time, excoriated prurigo nodules and papules develop, often healing with post-inflammatory changes including pitted or linear facial scars.14
In contrast, hydroa vacciniforme presents with itchy, tender papules, oedema, and haemorrhagic crusting that resolve with characteristic varioliform scarring.15 This condition is extremely rare in the UK, but needs to be distinguished from the potentially life-threatening Epstein–Barr virus-driven hydroa vacciniforme-like presentation of a lymphoproliferative disorder, which is more often reported in Asia.16
Porphyria cutanea tarda (PCT) is the most common form of cutaneous porphyria in Europe and is usually acquired. It is frequently associated with excessive alcohol consumption, chronic hepatitis C infection, autoimmune hepatitis, and haemochromatosis. Clinically, PCT typically presents with skin fragility, blistering, and scarring with milia on sun-exposed areas, particularly the dorsal hands. Early recognition is critical, as management focuses on treating the underlying hepatic pathology.17
Photoaggravated photodermatoses constitute another relatively large group of conditions, which are exacerbated but not caused by light. Connective tissue diseases such as lupus and dermatomyositis have clinical features of photodistributed rashes, and dermatoses such as atopic dermatitis, seborrhoeic dermatitis, and allergic contact dermatitis may also be aggravated by light.18-20
Patients suspected of having a photosensitivity disorder should be referred to a specialist photodiagnostic unit for comprehensive evaluation. The primary objectives of the investigation are to characterise the nature of the photosensitivity and establish a definitive diagnosis. The gold-standard diagnostic

A) Phototesting with the patient sitting comfortably. The fibre-optic light guide is connected to a filtered xenon arc source. B) Irradiation undertaken at test sites on clinically normal appearing back skin and delivered via fibre-optic light guide.
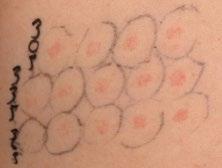
Positive monochromator testing result in a patient with chronic actinic dermatitis showing abnormal UV sensitivity
MED at 305±5 nm <1.5 (lowest normal 33) mJ/cm2; at 335±27 nm 180 (lowest normal 3,900) mJ/cm2 and at 365±27 nm 1,500 (lowest normal 18,000) mJ/cm2 .
MED: minimal erythema dose.
tool is monochromator phototesting, which uses a xenon-arc light source filtered to deliver narrow wavebands across the solar spectrum (Figure 4). This allows assessment of erythemal responses at specific wavelengths, which can then be compared with reference values from the normal population.
If monochromator phototesting is unavailable, determination of the minimal erythema dose (MED) can be attained using broadband UVB, UVA, and VL sources. MED, the lowest dose of radiation required to produce just perceptible erythema, is typically assessed 24 hours post-irradiation. MED serves as the primary endpoint in most photosensitivity disorders (Figure 5). In contrast, for conditions such as solar urticaria, the minimal urticaria dose, which is the smallest dose required to provoke detectable urticaria, serves as the principal diagnostic threshold. In some metabolic conditions, such as cutaneous porphyrias, abnormal photosensitivity is sometimes only observed at around 7 hours.
Additional investigations may include UVA provocation testing and photopatch testing when photoallergic contact dermatitis (e.g., to sunscreen chemicals or topically applied non-steroidal anti-
inflammatories) is suspected. Furthermore, MED testing (Figure 6) plays a critical role in phototherapy safety screening, identifying abnormal photosensitivity reactions and guiding the selection of accurate starting doses for treatment. Screening for connective-tissue disease, porphyrias, and rarer disorders (including HLA testing) may be indicated depending on the individual clinical presentation.21
General management principles for all photodermatoses begin with strict adherence to photoprotection, including broad-spectrum sunscreens, protective clothing, UV-filtering eyewear, and behavioural modifications to minimise sun exposure, such as seeking out shade. Patient education plays a crucial role in ensuring consistent and effective implementation of these measures.
Photohardening, a gradual increase in controlled sunlight exposure, may improve tolerance, particularly in PLE.22 Light-based therapies, such as narrowband UVB or psoralen-UVA phototherapy, may also be employed under specialist advice to induce tolerance in chronic photodermatoses and
serve as both prophylactic and therapeutic interventions.
For more severe or refractory cases, immunosuppressive agents such as methotrexate, azathioprine, or ciclosporin may be considered, especially in conditions like chronic actinic dermatitis.23 Emerging therapies include afamelanotide, a synthetic α-melanocyte-stimulating hormone analogue, which has shown efficacy in improving sunlight tolerance and quality of life in EPP.24 In PCT, effective treatment of the underlying cause, such as direct-acting antiviral therapy for hepatitis C, has been transformative.25
Biologic therapies are also being explored, with omalizumab, an anti-IgE monoclonal antibody, demonstrating benefit in selected cases of solar urticaria refractory to conventional treatments.26 These advances highlight the importance of an individualised, multidisciplinary approach that adapts to
disease severity, comorbidities, and evolving therapeutic options.
General management principles:
• Photoprotection
- Environmental factors
- Behavioural change, e.g., seeking shade
- Clothing and hat
- Broad-spectrum high protection factor sunscreens
- Window film
• Natural photohardening
• Vitamin D supplementation
• Topical treatments
- Emollients
- Topical corticosteroids
- Topical calcineurin inhibitors
• Light-based therapies
- Narrowband UVB
- Psoralen-UVA
- UVA1
A) Narrowband UVB MED testing using a handheld device with 10 fixed incremental doses of narrowband UVB.
B) UVB MED testing result showing erythema at all 10 doses, indicating abnormal photosensitivity with a MED of <0.076 (lowest normal 0.079) J/cm2. The highest dose delivered was 0.4 J/cm2, indicated by the black arrow.
MED: minimal erythema dose.
• Systemic therapies
- Immunosuppressive agents, e.g., methotrexate, ciclosporin
- Immunomodulator agents, e.g., omalizumab, dupilumab
Photosensitivity disorders encompass a diverse group of conditions that can significantly impair quality of life and often lack definitive cures. Management remains holistic, aiming to minimise sun exposure, optimise skin protection, and address comorbidities and psychosocial impact. Despite their potentially debilitating impact, photosensitivity disorders remain underserved, with significant disparities in access to diagnostic and therapeutic services. A key research priority is the Global Assessment of Photodiagnostic Services (GAPS) project, designed to
References
1. Fitzpatrick TB. Ultraviolet-induced pigmentary changes: benefits and hazards. Curr Probl Dermatol. 1986;15:25-38.
2. Ibbotson S. How should we diagnose and manage photosensitivity? J R Coll Physicians Edinb. 2014;44(4):308-12.
3. Burfield L et al. Systematic review of the prevalence and incidence of the photodermatoses with meta-analysis of the prevalence of polymorphic light eruption. J Eur Acad Dermatol Venereol. 2023;37(3):511-20.
4. Beattie PE et al. Characteristics and prognosis of idiopathic solar urticaria: a cohort of 87 cases. Arch Dermatol. 2003;139(9):1149-54.
5. Rutter KJ et al. Quality of life and psychological impact in the photodermatoses: a systematic review. Br J Dermatol. 2020;182(5):1092-102.
6. Ibbotson S, “Cutaneous photosensitivity diseases,” Griffiths C et al. (eds.), Rook’s Textbook of Dermatology (2024) 10th edition, Chichester: Wiley & Sons Ltd, pp.126.1126.21.
7. McSweeney SM et al. Systematic review of the clinical characteristics and natural history of solar urticaria. J Am Acad Dermatol. 2023;89(1): 138-40.
evaluate worldwide availability and quality of photodiagnostic services and guide targeted strategies to improve care.
Additionally, resources to support patients, ranging from specialist input to education and support groups, vary considerably, influencing both outcomes and patient experience. Emerging data, including recent updates on life expectancy in patients with XP,27 have provided critical insights into the long-term prognosis of specific photosensitivity disorders, emphasising the importance of early diagnosis, rigorous photoprotection, and coordinated care.
There remains an urgent need for greater awareness, improved access to specialist services, and continued research to refine diagnostic tools, optimise interventions, and ultimately enhance the lives of individuals affected by these conditions.
8. Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
9. Fassihi H et al. Deep phenotyping of 89 xeroderma pigmentosum patients reveals unexpected heterogeneity dependent on the precise molecular defect. Proc Natl Acad Sci U S A. 2016;113(9):e1236-45.
10. Ibbotson S. Drug and chemical induced photosensitivity from a clinical perspective. Photochem Photobiol Sci. 2018;17(12):1885-903.
11. Oakley AM et al. Polymorphic Light Eruption [Internet] (2020) Treasure Island: StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/ NBK430886/. Last accessed: 10 October 2025.
12. Ling TC et al. Treatment of polymorphic light eruption. Photodermatol Photoimmunol Photomed. 2003;19(5):217-27.
13. Dawe RS et al. Actinic prurigo and HLA-DR4. J Invest Dermatol. 1997;108(2):233-4.
14. Macfarlane L et al. Characteristics of actinic prurigo in Scotland: 24 cases seen between 2001 and 2015. Br J Dermatol. 2016;174(6):1411-4.
15. Gupta G et al. Hydroa vacciniforme: a clinical and follow-up study of 17 cases. J Am Acad Dermatol. 2000;42(2 Pt 1):208-13.
16. Ordoñez-Parra J et al. Hydroa vacciniforme-like lymphoproliferative disorder (HV-LPD) is an Epstein-Barr virus (EBV) associated disease. An Bras Dermatol. 2021;96(3):388-90.
17. Chaiyabutr C et al. Porphyria cutanea tarda in Scotland: underlying associations and treatment approaches. Int J Dermatol. 2024;63(12):1707-12.
18. Durieu C et al. Allergies de contact photoaggravées et photoallergies de contact au kétoprofène: 19 cas. Ann Dermatol Venereol. 2001;128(10 Pt 1):1020-4.
19. Aerts O et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78(4):241-5.
20. Dawe R, “Photoaggravated dermatoses,” Ferguson J, Dover J (eds.), Photodermatology (2006) 1st edition, London: Manson Publishing, pp.57-65.
21. Ibbotson SH et al. Photodiagnostic services in the UK and Republic of Ireland: a British Photodermatology Group Workshop Report. J Eur Acad Dermatol Venereol. 2021;35(12): 2448-455.
22. Kadurina M et al. Immunopathogenesis and management of polymorphic light eruption. Dermatol Ther. 2021;34(6):e15167.
23. Gozali MV et al. Update on treatment of photodermatosis. Dermatol Online J. 2016;22(2):13030/qt1rx7d228.
24. Kim ES, Garnock-Jones KP. Afamelanotide: a review in erythropoietic protoporphyria. Am J Clin Dermatol. 2016;17(2):179-85.
25. Liu J et al. Pathogenesis, prevention, and therapeutic advances in hepatitis B, C, and D. Virol J. 2025;22(1):274.
26. Morgado-Carrasco D et al. Clinical response and long-term followup of 20 patients with refractory solar urticaria under treatment with omalizumab. J Am Acad Dermatol. 2023;88(5):1110-1.
27. Paolino A et al. PD09 Life expectancy of patients with xeroderma pigmentosum: evidence from a UK study. Br J Dermatol. 2025;193(Suppl 1):ljaf085.261.
Authors: Paige E. Adams,1 Hong-An Nguyen,2 *Jenny E. Murase3,4
1. Rush University Medical Center, Chicago, Illinois, USA
2. University of California San Diego School of Medicine, La Jolla, California, USA
3. Department of Dermatology, University of California, San Francisco, USA
4. Department of Dermatology, Palo Alto Foundation Medical Group, Mountain View, California, USA *Correspondence to jemurase@gmail.com
Disclosure: Murase has served on speakers’ bureaus for Galderma, UCB, Leo Pharma, Eli Lilly, AbbVie, and Sanofi-Regeneron; served on advisory boards for UCB, Galderma, Arcutis, Eli Lilly, Leo Pharma, Sanofi-Regeneron, and Bristol Myers Squibb; and provided dermatologic consulting services for UCB, Apogee Therapeutics, Galderma, AbbVie, Attovia, Sanofi-Regeneron, and UpToDate. The other authors have declared no conflicts of interest.
Received: 30.06.25
Accepted: 06.10.25
Keywords: Atopic dermatitis (AD), drugs, eczema, medications, pregnancy, teratogenicity, toxicity.
Citation: EMJ Dermatol. 2025;13[1]:104-113. https://doi.org/10.33590/emjdermatol/QJVZ6975
Atopic dermatitis (AD) is a common and chronic skin condition that can be significantly impacted by the physiological and immunological changes of pregnancy. Hormonal changes in pregnancy induce an immunological shift to a Th2-dominant state, which may exacerbate pre-existing AD or manifest as de novo atopic eruption of pregnancy.1,2 Pregnant patients with AD face unique challenges as their condition may flare or evolve, particularly in the first two trimesters, and treatment decisions must balance maternal benefit with fetal safety. In the postpartum period, patients who are lactating encounter additional obstacles in therapeutic decision-making due to the potential excretion of drugs in breast milk that may ultimately pose risks to the infant. Given the rapid advancement of therapeutic options for AD, this feature discusses the safety considerations of dermatologic drugs during pregnancy and
lactation, and provides recommendations for clinicians treating patients of childbearing age from pre-conception through postpartum.
This section reviews the safety of topical medications for AD during pregnancy and lactation, with the authors’ recommendations summarised in Table 1
Topical corticosteroids (TCS) are a mainstay treatment for AD exacerbation and proactive maintenance.3 The local side-effect profile of TCS, such as atrophy, striae, hypertrichosis, and telangiectasias, applies to both pregnant and non-pregnant patients.2 The risk of these side-effects increases with high potency, excessive use, and application to thin skin.3 Thus, it is recommended that patients use the lowest effective potency for the shortest possible
Drug Safety in pregnancy Safety in lactation Considerations
Topicals
Mild-to-moderate potency corticosteroids (hydrocortisone, fluocinolone, triamcinolone, fluticasone)
High-potency corticosteroids (clobetasol, halobetasol)
Calcineurin inhibitors (tacrolimus, pimecrolimus)
PDE4 inhibitors (crisaborole, roflumilast)
Tapinarof
Ruxolitinib
PDE4: phosphodiesterase 4
Compatible
Compatible
Compatible
Not recommended, insufficient evidence
Not recommended, insufficient evidence
Not recommended, insufficient evidence
duration. In pregnant and breastfeeding patients, in areas of rapidly expanding skin, such as the inner thighs, gravid abdomen, or breasts, TCS predispose to striae formation, so counselling regarding judicious use is helpful to prevent the development of striae. In terms of fetal safety, intermittent use of mild-to-moderate-potency TCS, including hydrocortisone, fluocinolone, and triamcinolone, during pregnancy has not been associated with fetal adverse events (AE).4 There is some evidence that very high cumulative doses (>300 g) of potent or ultrapotent corticosteroids, such as clobetasol or halobetasol, may be associated with fetal growth restriction (FGR), though recent large studies suggest low risk.4-6 During lactation, excretion of TCS in breast milk is minimal, reflecting the low maternal systemic absorption associated with topical use.7 There was a case of iatrogenic hypertension in an infant
Compatible -
Compatible
Slight increased risk of fetal growth restriction with high cumulative doses during pregnancy.
Compatible -
Not recommended, insufficient evidence
Not recommended, insufficient evidence
Animal studies on crisaborole suggest risk of maternal toxicity and decreased fetal weight.
Animal studies suggest decreased maternal weight gain and decreased fetal weight.
Not recommended, insufficient evidence -
exposed to high-potency TCS applied directly to the nipple.7 Thus, high-potency TCS should be used with caution on the nipple and areola, where the infant may directly ingest the drug.7,8 Taken together, TCS are a safe first-line and adjunctive therapy for AD in pregnancy and lactation when used appropriately.1,2,4,7
Topical calcineurin inhibitors (TCI), such as tacrolimus and pimecrolimus, serve as steroid-sparing treatment options for AD. While there are limited data on TCI use during pregnancy or lactation, systemic tacrolimus use during pregnancy has not shown increased risk of congenital malformations. However, it has also been associated with prematurity, FGR, neonatal hyperkalaemia, and renal toxicity.2,4,9 Current evidence indicates that TCIs have poor systemic absorption, and the risk of fetal AEs is low.1,4 Pimecrolimus can be excreted
in breast milk at high maternal serum concentrations, but transfer to the infant following topical application is expected to be negligible.9 Pimecrolimus cream may be preferred for nipple application during lactation due to the mineral paraffins found in tacrolimus ointment.10 TCIs are considered safe during pregnancy and lactation when used intermittently on a limited body surface area.2,4,7,11
Phosphodiesterase 4 (PDE4) inhibitors, including crisaborole and roflumilast, are another class of steroid-sparing antiinflammatory agents used to treat AD. In animal studies, crisaborole was associated with maternal toxicity, decreased fetal viability, and low birth weight.4,12 There is no evidence to support the safety of topical PDE4 inhibitors in human pregnancy or lactation, and they are not recommended until more data become available.
Tapinarof is a novel aryl hydrocarbon modulating agent approved in the USA for the treatment of AD in children.13 Preclinical studies in rats showed decreased maternal weight gain, fetal viability, and birth weight with high doses.4,14 There is no evidence of tapinarof’s safety in human pregnancy or lactation, and it is not recommended until sufficient data emerge.
Topical ruxolitinib is a selective JAK1 and JAK2 inhibitor approved in the USA to treat AD.15 Preclinical animal studies did not demonstrate a risk of fetal malformations, though low fetal weight occurred with oral doses 22-times higher than the maximum topical human dose.16 No evidence exists regarding topical ruxolitinib safety in pregnancy or lactation, and the manufacturer recommends discontinuing 2–6 weeks before conception.4 Until further evidence emerges, it is not recommended during pregnancy or lactation.
Narrowband UVB (NB-UVB) phototherapy is an effective treatment option for AD.17 NBUVB poses no direct risk of teratogenicity; however, it can decrease serum folate levels in a dose-dependent manner in as
few as seven sessions.18,19 As maternal folic acid deficiency is highly associated with neural tube defects, pregnant patients receiving NB-UVB are considered high-risk for deficiency and should receive folic acid supplementation between 1–5 mg/day.17 Psoralen plus UVA (PUVA) is occasionally used for severe, refractory AD. PUVA is contraindicated in pregnancy due to the mutagenic and teratogenic properties of oral psoralen.20 During lactation, no AEs have been reported with NB-UVB phototherapy.21 There are limited data on the safety of PUVA in lactation, but it is recommended for mothers to avoid breastfeeding for at least 24 hours after oral psoralen due to drug excretion in breast milk and risk of photosensitivity in the breastfed infant.22 NB-UVB is a safe therapeutic option during pregnancy and lactation, particularly when TCS alone are ineffective.1
This section reviews the safety of systemic therapies for AD, including immunomodulators, JAK inhibitors, biologics, and antihistamines, during pregnancy and lactation, with the authors’ recommendations summarised in Table 2.
Oral corticosteroids, such as prednisone and prednisolone, can be useful for acute AD exacerbation. Prednisone and prednisolone at low doses (10–15 mg/day) during pregnancy confer minimal risk to the fetus, as they are converted to relatively inactive forms by 11β-hydroxysteroid dehydrogenase in the placenta.23-25 However, long-term treatment and higher doses increase the risk of fetal AEs (FGR, prematurity, and oral clefts with first-trimester exposure) and maternal AEs (premature rupture of membranes, gestational diabetes, hypertension, and pre-eclampsia with third-trimester exposure).2,4,25 The lowest effective dose for the shortest possible duration should be used in pregnancy to avoid these AEs, with a maximum dose of 0.5 mg/kg/ day.1,2 Prednisone is excreted in breast milk, with peak levels achieved 1–2 hours after ingestion.11,26 With high doses of
Table 2: Summary of systemic therapies for atopic dermatitis during pregnancy.
Drug Safety in pregnancy Safety in lactation Considerations
Systemic immunomodulators
Oral corticosteroids (prednisone, prednisolone)
Compatible Compatible
Short courses (<5 mg/kg/day) are safe.
Long-term, high doses are associated with maternal and fetal adverse effects.
Ciclosporin Compatible Compatible -
Azathioprine
Alternatives preferred Compatible
Mycophenolate mofetil
Methotrexate
Biologics
IL-4/IL-13 inhibitors (dupilumab)
IL-13 inhibitors (tralokinumab, lebrikizumab)
IL-31 inhibitors (nemolizumab)
JAK inhibitors
Systemic JAK inhibitors (tofacitinib, baricitinib, upadacitinib, abrocitinib)
Antihistamines
Contraindicated Contraindicated
Risk of asymptomatic neonatal neutropenia and thrombocytopenia with in utero exposure.
Doses <2 mg/kg, with CBC and LFT monitoring, are safe in pregnancy.
Risk of congenital malformations and spontaneous abortion.
Interferes with the efficacy of OCPs.
Contraindicated Contraindicated Risk of congenital malformations and spontaneous abortion.
Compatible, use with caution Compatible, use with caution No adverse pregnancy outcomes in animal studies and case reports.
Likely compatible, insufficient evidence Likely compatible, insufficient evidence No adverse pregnancy outcomes in animal studies.
Not recommended, insufficient evidence
Contraindicated
Not recommended, insufficient evidence Reported cases of early postnatal death at high doses in animal studies.
Not recommended, insufficient evidence Tofacitinib: animal studies resulted in decreased embryo viability.
First-generation (diphenhydramine, chlorpheniramine) Compatible Compatible -
Hydroxyzine
Second-generation (cetirizine, loratadine, fexofenadine)
Not recommended, alternatives preferred Compatible
Likely compatible Compatible
CBC: complete blood count; LFT: liver function test; OCP: oral contraceptives.
Avoid first-trimester use if possible due to a slightly increased risk of congenital malformations.
Avoid high doses in the third trimester due to reported cases of neonatal withdrawal seizures.
Fexofenadine: animal studies showed dosedependent embryofetal toxicity without evidence in human studies.
prednisone, lactating mothers should wait 4 hours before breastfeeding or pumping when maternal serum levels decrease substantially.7 Overall, oral corticosteroids are considered safe during pregnancy and lactation with appropriate use.
Ciclosporin, an oral calcineurin inhibitor, can be used off-label in the USA for treatment-resistant AD. In animal studies, high doses of ciclosporin (>10 mg/kg/day) in utero were associated with reduced nephron mass and renal insufficiency in offspring, but these findings have not been demonstrated in human studies.27-29 Data regarding ciclosporin use during pregnancy are primarily derived from organ transplant recipients and have been associated with increased risk of prematurity, FGR, Caesarean delivery, hypertension, and pre-eclampsia.4,24 However, it is difficult to discern whether these effects are due to ciclosporin exposure, underlying maternal comorbidities, or concomitant medications.6,27 Ciclosporin excretion in breast milk is highly variable, but blood levels in breastfed infants are typically undetectable.30 Case reports suggest no evidence of adverse effects on renal function or development in breastfed infants exposed to ciclosporin.11,30 With this, lowdose ciclosporin is considered safe during pregnancy and lactation when topicals or phototherapy fail, or when rapid disease control of severe AD is needed.1,2,11 During lactation, serum drug levels should be monitored in the breastfed infant.7
Azathioprine is a purine anti-metabolite that can be used off-label in the USA to treat refractory AD. Historically, azathioprine has not been recommended during pregnancy due to some evidence of increased risk of asymptomatic neonatal neutropenia and thrombocytopenia following in utero exposure.4,6,31 Multiple controlled studies suggest that azathioprine is not teratogenic, but some reports note an increased risk of FGR and prematurity.24,32 Azathioprine is found in small amounts in breast milk, and current evidence suggests no AEs in breastfed infants.33 There is concern for the potential risk of immunosuppression and carcinogenesis in breastfed infants exposed to azathioprine, but long-term follow-up
studies have not been performed.2,33,34 When necessary, azathioprine at low doses (<2 mg/kg) can be used during pregnancy; however, maternal toxicity should be monitored with complete blood counts and liver function tests to reduce the risk of fetal complications.4,34 Azathioprine is considered safe during lactation, and it is recommended to wait at least 4 hours after the last dose to reduce the amount transferred to the breastfed infant.2,7,33
Mycophenolate mofetil (MMF) inhibits purine synthesis and can be used off-label in the USA to treat AD. MMF is associated with an increased risk of spontaneous abortion and congenital malformations, such as heart defects and facial clefts, with in utero exposure.4,6,35 Patients of childbearing age should use two reliable methods of contraception while on MMF and be informed that the drug may reduce the efficacy of oral contraceptives.4,36 MMF should be discontinued for at least 6 weeks before conception, and patients should continue to use reliable contraceptive methods during this time.6 There are limited safety data on MMF use during lactation, and it is considered high risk for immunosuppression in the breastfed infant.9,37 MMF is contraindicated during pregnancy and lactation.2,4,11
Methotrexate, a dihydrofolate reductase inhibitor, can be effective for refractory AD. Methotrexate is associated with an increased risk of congenital malformations and spontaneous abortion.1,2,24 Patients of childbearing age should discontinue the drug 1–3 months before conception and start high-dose folic acid supplements during this time through the first trimester to reduce the risk of neural tube defects.4,6,38 Although small amounts of methotrexate are detected in breast milk, there is a risk of accumulation of the drug in tissues and myelosuppression in breastfed infants.9,11,39 Methotrexate is contraindicated during pregnancy and lactation.2,4,40
The advent of biologic therapy has improved patient outcomes and revolutionised the treatment of AD.41
During pregnancy, expression of placental neonatal Fc receptors allows for selective transplacental passage of maternal IgG antibodies, including therapeutic monoclonal antibodies.42 The placenta does not fully develop until approximately 12 weeks of gestation; thus, fetal exposure to monoclonal antibodies in the first trimester is negligible. Transplacental passage of monoclonal antibodies increases exponentially during the second and third trimesters as neonatal Fc receptor expression increases, with peak fetal exposure nearing parturition.4 There are a few reports of fatal tuberculosis infections following Bacillus Calmette-Guérin vaccination given at 3 months of life in infants exposed in utero to TNF inhibitors, specifically infliximab, adalimumab, and one unspecified TNF inhibitor.43,44 Due to the risk of neonatal immunosuppression following in utero biologic exposure, it may be beneficial to discontinue biologic therapy after 20 weeks of gestation. Clinicians should engage in shared decision-making with patients regarding continuation of therapy beyond 20 weeks, weighing the risk of disease flare versus the risk of neonatal immunosuppression. If biologic therapy is continued to term, decisions regarding live vaccination in exposed infants should be made in conjunction with a paediatrician or immunologist.
Dupilumab, an IgG4 monoclonal antibody, inhibits IL-4 and IL-13 signalling in AD by binding to the IL-4 receptor α subunit. Animal studies on dupilumab showed no evidence of pregnancy complications, congenital malformations, or neonatal immune dysfunction with in utero exposure.4,6 There are no large-scale human studies; however, multiple case reports and case series have not identified maternal-fetal complications, with all cases resulting in live births at term except for one premature delivery.45-51 A recent systematic review found no increased risk of congenital malformations or spontaneous abortion compared to the general population.52 Dupilumab has a high molecular weight, suggesting low transplacental passage, at least during the first two trimesters.9 Treatment in the third trimester may lead to fetal exposure, though the significance
of this exposure is unknown. Dupilumab is unlikely to be detected in breast milk due to its large molecular size.53 Taken together, these data are reassuring, and dupilumab can likely be used in pregnancy and lactation when maternal benefit outweighs potential risk.1,53
Tralokinumab and lebrikizumab are IgG4 monoclonal antibodies that bind to IL13, inhibiting its signalling pathway in the pathogenesis of AD. There is a lack of human data or case reports on tralokinumab or lebrikizumab use in pregnancy or lactation. Weekly administration of IL-13 inhibitors in preclinical animal studies did not result in adverse pregnancy outcomes, congenital malformations, or neonatal immunodeficiency.54,55 Tralokinumab and lebrikizumab are both large protein molecules, and the amount excreted in breast milk is presumably very low.56,57 IL13 inhibitors are likely safe in pregnancy and lactation; however, more evidence is needed to give definitive recommendations.
Nemolizumab is a novel IgG2 antibody that targets pruritus in AD through selective inhibition of IL-31 receptor α. There is no evidence establishing the safety of nemolizumab in pregnancy or lactation. Animal studies did not demonstrate maternal or embryofetal toxicity at doses up to 36-times the maximum human dose, though three cases resulted in postnatal death soon after birth at this dose.58 Nemolizumab is a large protein molecule, and it is likely excreted in breast milk in small amounts.59 Given the lack of data and the novelty of the drug, the safety of nemolizumab in pregnancy and lactation remains unclear.
JAK inhibitors are a relatively new class of drugs that have been shown to rapidly improve AD symptoms.60 The low molecular weight of JAK inhibitors may allow for transplacental passage early in pregnancy, and animal studies showed teratogenicity and risk of spontaneous abortion.60 For this reason, clinical trials excluded pregnant patients and required strict adherence to contraceptive methods during treatment.
Abrocitinib, a selective JAK1 inhibitor, and upadacitinib, a selective JAK1/2 inhibitor, are approved in the USA to treat AD. Other oral JAK inhibitors, such as tofacitinib and baricitinib, are occasionally used off-label to treat AD in the USA. In AD clinical trials, inadvertent exposure to abrocitinib or upadacitinib during pregnancy resulted in both healthy live births and cases of spontaneous abortion.60,61 Similarly, clinical trials and post-marketing surveillance studies on baricitinib revealed cases of healthy newborns, prematurity, and spontaneous abortion.60 Animal studies on tofacitinib resulted in decreased embryo viability and impaired structural development.62 A recent systematic review found that tofacitinib exposure in pregnancy led to healthy newborns, spontaneous abortions, and a few cases of congenital malformations; however, congenital malformations were present in cases with concomitant systemic drug exposure.63 Patients of childbearing potential should use reliable contraception while on these medications and for at least 4 weeks after stopping abrocitinib, upadacitinib, or tofacitinib, and for at least 1 week after discontinuing baricitinib.61,64 No evidence exists on the safety of abrocitinib, upadacitinib, or baricitinib in lactation. Limited data on tofacitinib suggest low concentration in breast milk without reported AEs in breastfed infants.65 Until there are sufficient data to definitively conclude the safety of systemic JAK inhibitors, these drugs are contraindicated in pregnancy and not recommended during lactation.
First-generation antihistamines (FGA), including over-the-counter options like diphenhydramine and chlorpheniramine, are considered first-line for AD-associated pruritus in pregnancy.4,6 While one study associated first-trimester diphenhydramine use with cleft palate, no other reports of teratogenicity with non-prescription FGAs exist.9,66,67 High doses are not recommended near delivery as they may cause infantile sedation.3 Hydroxyzine, a prescription FGA, crosses the placenta and has been associated with a slightly increased risk
of congenital malformations (5.8%) with no specific pattern of anomaly when used in the first trimester, as well as neonatal withdrawal seizures following prolonged maternal use in the third trimester.4,9,68 FGAs are transferred in breast milk, and high doses may decrease milk supply and cause drowsiness and irritability in the breastfed infant.69-71 Overall, diphenhydramine and chlorpheniramine are safe in pregnancy, and hydroxyzine should be used with caution.4,6 FGAs are safe during lactation, but low doses at bedtime are preferred to reduce AEs in the infant.69
Second-generation antihistamines, including cetirizine and loratadine, are considered acceptable alternatives to FGAs after the first trimester based on animal and human studies showing no significant maternalfetal toxicity.6,72 While there are minimal data on the safety of fexofenadine in human pregnancy, preclinical studies found a dose-dependent embryofetal toxicity with first-trimester exposure in rats.9 Loratadine and fexofenadine are found in low levels in breast milk and are non-sedating.73,74 Large doses of cetirizine may cause drowsiness in the breastfed infant.75 Due to more comprehensive safety data, FGAs are preferred in pregnancy.9 Second-generation antihistamines, particularly loratadine and fexofenadine, are safe in lactation.39,73-75
Effective management of AD during pregnancy and lactation requires careful consideration of maternal benefits and the wellbeing of the child. Evaluating the risk–benefit ratios of available dermatologic therapeutics is crucial in the shared decision-making for this patient population. Treatment of AD during pregnancy and lactation should be multidisciplinary, weighing the expert opinion of dermatologists, obstetricians, and paediatricians. Continued clinical trials, patient registries, and pharmacovigilance studies are essential to refine treatment strategies and ensure optimal outcomes for both mother and child.
References
1. Balakirski G, Novak N. Atopic dermatitis and pregnancy. J Allergy Clin Immunol. 2022;149(4):1185-94.
2. Vestergaard C et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33(9):1644-59.
3. Sidbury R et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89(1):e1-20.
4. McMullan P et al. Safety of dermatologic medications in pregnancy and lactation: an update - part I: pregnancy. J Am Acad Dermatol. 2024;91(4):619-48.
5. Ragi SD et al., “Safety of dermatological drugs in pregnancy and in lactation,” ‘Sarkar R et al. (eds.), Skin and pregnancy: a comprehensive guide for clinical practice (2025) 1st edition, CRC Press, pp.172-95.
6. Andersson NW et al. Evaluation of topical corticosteroid use in pregnancy and risk of newborns being small for gestational age and having low birth weight. JAMA Dermatol. 2021;157(7):788-95.
7. Yaghi M et al. Safety of dermatologic medications in pregnancy and lactation: an update-part II: lactation. J Am Acad Dermatol. 2024;91(4): 651-68.
8. “Halobetasol,” Drugs and Lactation Database (LactMed®) [Internet] (2021) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK500899/. Last accessed: 12 September 2025.
9. Briggs GG et al., Briggs drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk [Internet] (2021) Philadelphia: Lippincott Williams & Wilkins (LWW). Wolters Kluwer Health. Last accessed: 29 September 2025.
10. “Tacrolimus,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK501104/. Last accessed: 12 September 2025.
11. Yaghi M et al. Dermatologic drug safety in lactation. J Dermatol Physician Assist. 2024;18(2):4-10; DOI:10.1097/jdpa.0000000000000016.
12. DailyMed. EUCRISA- crisaborole ointment. 2025. Available at: https:// dailymed.nlm.nih.gov/dailymed/
drugInfo.cfm?setid=609b77de1ca3-4783-b8f5-01a9c0f1d77d. Last accessed: 13 October 2025.
13. Bobonich M et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22(8):779-84.
14. DailyMed. VTAMA- tapinarof cream. 2025. Available at: https://dailymed. nlm.nih.gov/dailymed/drugInfo. cfm?setid=9309d20f-8cd4-4c9693fa-7f730e83c7ab. Last accessed: 13 October 2025.
15. Tegtmeyer K et al. Off-label studies on the use of ruxolitinib in dermatology. Dermatitis. 2021;32(3):164-72.
16. DailyMed. OPZELURA- ruxolitinib cream. 2024. Available at: https:// dailymed.nlm.nih.gov/dailymed/ drugInfo.cfm?setid=24da5509-66314795-9d42-273faecd08e7. Last accessed: 13 October 2025.
17. Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148(1):132-3.
18. Juzeniene A et al. Pilot study of folate status in healthy volunteers and in patients with psoriasis before and after UV exposure. J Photochem Photobiol B. 2010;101(2):111-6.
19. El-Saie LT et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26(4):481-5.
20. Gottlieb AB et al. Clinical considerations for the management of psoriasis in women. Int J Womens Dermatol. 2019;5(3):141-50.
21. Butler DC et al. Safety of dermatologic medications in pregnancy and lactation: Part II. Lactation. J Am Acad Dermatol. 2014;70(3):417.e1-10.
22. “Methoxsalen,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK500736/. Last accessed: 25 September 2025.
23. Palmsten K et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27(4):430-8.
24. ACOG Committee Opinion No. 776: Immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133(4):e287-95.
25. Bandoli G et al. A review of systemic corticosteroid use in pregnancy and
the risk of select pregnancy and birth outcomes. Rheum Dis Clin North Am. 2017;43(3):489-502.
26. “Prednisone,” Drugs and Lactation Database (LactMed®) [Internet] (2024) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK501077/. Last accessed: 12 September 2025.
27. Menter A et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445-86.
28. Tendron A et al. Cyclosporin A administration during pregnancy induces a permanent nephron deficit in young rabbits. J Am Soc Nephrol. 2003;14(12):3188-96.
29. Tendron-Franzin A et al. Longterm effects of in utero exposure to cyclosporin A on renal function in the rabbit. J Am Soc Nephrol. 2004;15(10):2687-93.
30. “Cyclosporine,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK501683/. Last accessed: 13 October 2025.
31. DailyMed. IMURAN- azathioprine tablet. 2014. Available at: https:// dailymed.nlm.nih.gov/dailymed/ drugInfo.cfm?setid=aaa6c540-4c8448a0-939c-cd423134fa2a. Last accessed: 13 October 2025.
32. Sarkar M et al. Reproductive health and liver disease: practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73(1):318-65.
33. “Azathioprine,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK501050/. Last accessed: 12 September 2025.
34. Wollenberg A et al. European guideline (EuroGuiDerm) on atopic eczema: living update. J Eur Acad Dermatol Venereol. 2025;39(9):1537-66.
35. DailyMed. MYCOPHENOLATE MOFETIL capsule. 2025. Available at: https://dailymed.nlm.nih.gov/dailymed/ drugInfo.cfm?setid=3eb584dc-f72d4545-96fa-ab3d47be41ce. Last accessed: 13 October 2025.
36. Orvis AK et al. Mycophenolate mofetil in dermatology. J Am Acad Dermatol. 2009;60(2):183-99.
37. “Mycophenolate,” Drugs and Lactation Database (LactMed®) [Internet] (2023) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK501638/. Last accessed: 12 September 2025.
38. Mahadevan U et al.; Global Consensus Group for Pregnancy and IBD. Global consensus statement on the management of pregnancy in inflammatory bowel disease. Inflamm Bowel Dis. 2025:izaf171; DOI: 10.1093/ ibd/izaf171.
39. Hale TW, Krutsch K, Hale’s Medications and Mothers’ Milk 2025-2026: A Manual of Lactational Pharmacology (2024) 21st edition, New York: Springer Publishing Company.
40. “Methotrexate,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK501341/. Last accessed: 12 September 2025.
41. Snast I et al. Are biologics efficacious in atopic dermatitis? A systematic review and meta-analysis. Am J Clin Dermatol. 2018;19(2):145-65.
42. Sand KMK et al. Contribution of the ex vivo placental perfusion model in understanding transplacental immunoglobulin G transfer. Placenta. 2022;127:77-87.
43. Gisbert JP, Chaparro M. Vaccines in children exposed to biological agents in utero and/or during breastfeeding: are they effective and safe? J Crohns Colitis. 2023;17(6):995-1009.
44. Goulden B et al. A systematic review of live vaccine outcomes in infants exposed to biologic disease modifying anti-rheumatic drugs in utero. Rheumatology (Oxford). 2022;61(10):3902-6.
45. Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47(5):960-1.
46. Gracia-Darder I et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35(2):e15237.
47. Kage P et al. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48(10):e484-5.
48. Kage P et al. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34(6): e256-7.
49. Lobo Y et al. Atopic dermatitis treated safely with dupilumab during
pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13(2):248-56.
50. Mian M et al. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6(10):1051-2.
51. Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46(8):1578-9.
52. Sánchez-García V et al. Safety of dupilumab therapy for atopic dermatitis during pregnancy: a systematic review and metaanalysis. Acta Derm Venereol. 2025;105:adv41307.
53. “Dupilumab,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK500904/. Last accessed: 12 September 2025.
54. DailyMed. ADBRY- tralokinumab-ldrm injection, solution. 2024. Available at: https://dailymed.nlm.nih.gov/dailymed/ drugInfo.cfm?setid=d8020b69-300144e2-9b5d-5f93d9aaf6e1. Last accessed: 13 October 2025.
55. DailyMed. EBGLYSS- lebrikizumablbkz injection, solution. 2025. Available at: https://dailymed. nlm.nih.gov/dailymed/drugInfo. cfm?setid=7a774ea2-acde-4aaa9a8f-ccba5a5b1d5f. Last accessed: 13 October 2025.
56. “Tralokinumab,” Drugs and Lactation Database (LactMed®) [Internet] (2024) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK576870/. Last accessed: 12 September 2025.
57. “Lebrikizumab,” Drugs and Lactation Database (LactMed®) [Internet] (2024) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK608176/. Last accessed: 12 September 2025.
58. DailyMed. NEMLUVIO- nemolizumabilto injection, powder, lyophilized, for solution. 2024. Available at: https:// dailymed.nlm.nih.gov/dailymed/ drugInfo.cfm?setid=e9229ef1-ac604c24-afb6-009d3c781687. Last accessed: 13 October 2025.
59. “Nemolizumab,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK611168/. Last accessed: 12 September 2025.
60. Haag C et al. A practical guide to using oral Janus kinase inhibitors for atopic dermatitis from the International
Eczema Council. Br J Dermatol. 2024;192(1):135-43.
61. Dulai AS et al. Maternal JAK-inhibitor usage associated with increased risk of aborted pregnancy: a systematic review and meta-analysis. Br J Dermatol. 2025;193(1):187-9.
62. XELJANZ XR- tofacitinib tablet, extended release. 2025. Available at: https://dailymed.nlm.nih.gov/dailymed/ drugInfo.cfm?setid=68e3d6b2-78384d2d-a417-09d919b43e13. Last accessed: 13 October 2025.
63. Gordon ER et al. Current evidence on safety of Janus kinase inhibitors in pregnancy and lactation. J Am Acad Dermatol. 2025;92(5):1082-4.
64. Laube R et al. Australian inflammatory bowel disease consensus statements for preconception, pregnancy and breast feeding. Gut. 2023;72(6):104053.
65. “Tofacitinib,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK500664/. Last accessed: 12 September 2025.
66. Li Q et al. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract. 2013;1(6):666-74.e1.
67. Saxén I. Letter: Cleft palate and maternal diphenhydramine intake. Lancet. 1974;1(7854):407-8.
68. Serreau R et al. Neonatal seizures associated with maternal hydroxyzine hydrochloride in late pregnancy. Reprod Toxicol. 2005;20(4):573-4.
69. “Diphenhydramine,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www. ncbi.nlm.nih.gov/books/NBK501878/. Last accessed: 12 September 2025.
70. “Chlorpheniramine,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www. ncbi.nlm.nih.gov/books/NBK501530/. Last accessed: 12 September 2025.
71. “Hydroxyzine,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK500985/. Last accessed: 12 September 2025.
72. Golembesky A et al. Safety of cetirizine in pregnancy. J Obstet Gynaecol. 2018;38(7):940-5.
73. “Loratadine,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of
Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK501009/. Last accessed: 12 September 2025.
74. “Fexofenadine,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of
Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK500677/. Last accessed: 12 September 2025.
75. “Cetirizine,” Drugs and Lactation Database (LactMed®) [Internet] (2025) Bethesda: National Institute of
Child Health and Human Development. Available at: http://www.ncbi.nlm. nih.gov/books/NBK501509/. Last accessed: 12 September 2025.
Editor's Pick
This review offers a forward-looking approach to dermatology, framing the skin as a dynamic and adaptive organ. By exploring barrier function, immune modulation, neuroimmune pathways, and microbial symbiosis, it introduces ‘educating the skin’ as a strategy to enhance resilience and long-term health. Clinicians will be able to gain practical insights into preventive and integrative care that address both biology and the environment.
Simone Ribero
University of Turin, Italy


Authors: *Diala Haykal,1 Brigitte Dréno,2 Hugues Cartier,3 Serge Dahan4
1. Centre Laser Palaiseau, France
2. Department of Dermato-Cancerology, CHU Nantes-Hôtel-Dieu, France
3. Centre Médical Laser Saint-Jean, Arras, France
4. Private Dermatology Practice, Toulouse, France
*Correspondence to docteur.haykal@gmail.com
Disclosure: The authors have declared no conflicts of interest.
Received: 03.03.25
Accepted: 26.08.25
Keywords: AI, dermatology, environmental stressors, microbiome, neural regulation, skin resilience.
Citation: EMJ Dermatol. 2025;13[1]:114-123.
https://doi.org/10.33590/emjdermatol/XXBW5167
Abstract
The skin functions as a dynamic organ that integrates biological, microbial, environmental, and neural inputs to maintain resilience. Traditional dermatology has often prioritised treatment of symptoms rather than fostering the underlying mechanisms of cutaneous health. This review introduces the paradigm of ‘educating the skin,’ a proactive approach that emphasises prevention, adaptability, and long-term health through an integrative model. Key elements include barrier function, immune modulation, neural dynamics, microbial symbiosis, and environmental adaptation. Evidence demonstrates the importance of lipid replenishment, circadian regulation, microbiome-targeted therapies, and neuroimmune pathways in enhancing skin integrity and mitigating inflammatory disorders. Environmental challenges such as ultraviolet radiation, pollution, and psychological stress further underscore the need for pre-emptive and barrier-focused strategies. Advances in AI and biotechnology provide opportunities for precision diagnostics, personalised care, and patient empowerment, shifting dermatology towards preventive rather than reactive practice. By integrating biological insights, microbial ecology, neuroendocrine regulation, and environmental adaptation, this
framework supports resilient, adaptable skin health. Educating patients about skin biology and daily practices reinforces long-term outcomes. Looking forward, interdisciplinary research and AI-driven tools will refine personalised interventions, paving the way for proactive, sustainable strategies in dermatology. Through this lens, the skin is not merely treated but empowered to thrive in harmony with its environment.
1. Chronic skin diseases are influenced by barrier dysfunction, microbiome imbalance, and environmental stressors. Clinicians must understand resilience mechanisms to move beyond symptom-based treatment and promote longterm cutaneous health.
2. This narrative review synthesises evidence on ‘educating the skin’, integrating barrier science, microbial symbiosis, environmental adaptation, and neurocutaneous pathways to provide clinicians with a proactive, preventive framework for dermatological care.
3. Clinicians should adopt proactive strategies, barrier reinforcement, microbiome modulation, stress management, and AI-personalised approaches to strengthen resilience, prevent chronic conditions, and improve patient outcomes in daily dermatological practice.
The skin, as the body’s largest organ, serves as a dynamic interface between internal systems and environmental influences. Beyond its role as a protective barrier, it interacts with microbial ecosystems, immune pathways, and neural networks, while responding to external challenges such as UV radiation and pollution.1 Despite its complexity, dermatology has traditionally emphasised the treatment of visible symptoms rather than fully addressing the underlying mechanisms that sustain skin health and adaptability. While the biological processes governing skin resilience are still being unravelled, emerging evidence provides actionable insights into strategies that strengthen the skin’s capacity to adapt and thrive.2 Recent advancements in skin biology research, microbiome research, environmental science, and neurodermatological studies have laid the foundation for a new framework called ‘educating the skin’.
This paradigm emphasises enhancing the skin’s ability to adapt, repair, and thrive by addressing its biological, microbial, environmental, and neural interactions.3,4 It proposes a shift from reactive to proactive treatments, leading the way
to comprehensive strategies that foster resilience and long-term health.5 The objective of this review is to synthesise current evidence supporting the approach of educating the skin, focusing on key components such as the epidermal barrier, microbial balance, environmental adaptation, and neural regulation.6 By adopting an integrative model, this review combines molecular and clinical insights to provide actionable strategies for implementing this framework. To educate the skin with the aim of preventing conditions and reducing the need for treatment, it is essential to highlight the critical factors that contribute to maintaining healthy skin. This review explores the interconnected roles of skin biology, the microbiome, and environmental stressors, shedding light on their impact on skin resilience. By redefining dermatological care to emphasise prevention and adaptability, educating the skin offers a transformative, holistic approach to advancing the field and improving long-term skin health. While our understanding of the underlying biological mechanisms continues to evolve, this review emphasises the evidence-backed strategies currently available, paving the way for innovative and personalised interventions in dermatology.
The skin’s biological integrity is fundamentally rooted in its barrier function, immune system, neural networks, and circadian rhythms, which work in harmony to maintain homeostasis and protect against external and internal challenges. Circadian rhythms regulate key processes, such as barrier repair, immune cell activity, and hydration, thereby adapting the skin to daily environmental changes. The skin also produces and metabolises melatonin. This hormone is made by keratinocytes, melanocytes, and fibroblasts. It protects the skin from UV damage, oxidative stress, and inflammation. A recent review confirmed its importance for skin health.7 Earlier studies showed that serotonin and N-acetylserotonin are also present in the skin.8 Together, they form a local serotonergic–melatoninergic system that supports circadian regulation, repair, and immune balance. Notably, serotonin is also synthesised by simple microorganisms, including many skin-resident species. This further supports the idea that the skin microbiome contributes not only to immune and barrier functions, but also to local neuroendocrine signalling.9 In addition, melanocytes also function as sensory and regulatory units within the epidermis. This concept was first proposed in 1993 and has since been supported by in-depth reviews of the pigmentary system’s physiological and immunoregulatory functions.10,11
Disruptions in these rhythms, caused by ageing or environmental stress, can compromise skin integrity and exacerbate conditions such as dermatitis or psoriasis, highlighting their crucial role in skin health.6
The epidermal barrier, composed primarily of lipids such as ceramides, cholesterol, and fatty acids, plays a vital role in preventing transepidermal water loss (TEWL) and protecting against microbial invasion.12 Research by Elias et al.13 has shown that ceramide depletion compromises barrier function, leading to conditions such as atopic dermatitis (AD). In their study of patients with paediatric AD, ceramidebased moisturisers were found to reduce
TEWL by 50% within 8 weeks, significantly alleviating inflammation and pruritus. These findings underscore the critical importance of lipid replenishment in maintaining optimal skin barrier function on a daily basis.13 These findings were confirmed by Chamlin et al.14 and Luger et al.,15 who demonstrated that ceramidecontaining formulations not only improve barrier function but also reduce the clinical severity of AD. Their study highlighted the ability of ceramides to enhance stratum corneum integrity, restoring hydration and reducing inflammatory markers. The longterm benefits of constant ceramide-based interventions were evident in sustained symptom relief and a significant decrease in the need for topical corticosteroids.
Another study emphasised the importance of consistently applying emollients to achieve optimal results, regardless of the formulation used. Regular use of emollients strengthens the skin barrier, reduces TEWL, and minimises flare-ups, reinforcing their critical role in long-term eczema management.16 Along with these findings, the study by Lefèvre-Utile et al.17 explores the multifaceted roles of the epidermis in maintaining skin health and integrity. The skin’s protective and functional roles are supported by six interconnected barriers. The physical barrier defends against environmental insults, like pathogens and UV radiation, through the structure of the stratum corneum. In contrast, the chemical barrier, maintained by the skin’s acidic pH, regulates enzymatic activities and microbiome balance. The microbial barrier supports a protective and diverse microbiota, playing a crucial role in immune modulation and pathogen competition. The immunological barrier, involving Langerhans cells and other immune mediators (innate immunity in the epidermis and the dermis), prevents infections and controls inflammation. The sensory barrier detects mechanical, thermal, and chemical stimuli contributing to homeostasis. In addition to its physical, chemical, and immunological functions, the skin also acts as a neuroendocrine and stress organ.
This concept, first introduced over two decades ago, revolutionised our
understanding of the skin’s integrative role in homeostasis and adaptation to environmental stressors.18 Subsequent reviews have further elaborated on how the skin possesses a functional equivalent of the hypothalamic–pituitary–adrenal axis, capable of producing neuropeptides, neurotransmitters, and hormones that influence both local and systemic physiology.19 This perspective highlights the skin’s capacity to respond to stress via neuroendocrine signalling pathways that affect immune modulation, inflammation, and even pigmentation. Moreover, this neuroendocrine identity positions the skin as both a sensor and effector organ, capable of computing complex environmental inputs to maintain internal equilibrium.20
The sixth component, the endocrine and neuroendocrine barrier, synthesises hormones and neuropeptides that regulate immune responses, repair mechanisms, and stress adaptation. Disruptions in any of these barriers, whether caused by genetic factors, environmental stressors, or disease, can result in various dermatological conditions. These insights underscore the importance of therapeutic strategies aimed at restoring skin barrier functions to improve overall skin health. In addition to these barrier systems, the immune system plays a critical role in maintaining cutaneous homeostasis. Keratinocytes, Langerhans cells, and T cells regulate immune responses by balancing defence mechanisms with tolerance to benign stimuli.
By targeting these immune pathways, DNA repair enzymes have shown promise in reversing damage and restoring skin resilience. Peripheral nerves also contribute to the skin’s sensory and regulatory functions, influencing both immunity and barrier dynamics.21,22 Takahashi et al.23 explored how mechanical stress and oxidative damage disrupt nerve signalling, leading to sensory abnormalities and inflammatory skin conditions. Their study demonstrates how disrupted epidermal nerve pruning and prolonged calcium ion signalling contribute to sensory abnormalities, such as chronic itch, in
conditions like AD. By identifying aberrant transient receptor potential ankyrin 1 (TRPA1) activity as a key driver of these issues, the research underscores the therapeutic potential of restoring neural pathways to manage sensory dysfunction and improve patient outcomes.23 Nguyen et al.24 performed a single-nucleus transcriptomic analysis of human dorsal root ganglion neurons, revealing how neural circuits contribute to the sensory and inflammatory pathways involved in skin conditions.24 This research provides a deeper understanding of how neural mechanisms, such as pain perception and itch, are intricately linked to inflammatory processes. These findings suggest potential therapeutic interventions, including neuromodulating agents that target specific pathways to reduce chronic itch and pain, or the use of neuroprotective peptides to support nerve health and mitigate inflammation. Such approaches could be transformative for conditions like eczema, psoriasis, and chronic pruritus, where neural contributions to symptom persistence are significant. This highlights the interconnectedness of neural health and skin outcomes, suggesting that therapies targeting nerve pathways can provide holistic benefits in conditions involving sensory impairment or chronic itch.
These findings underscore the complex interplay between skin integrity, immune function, and neural health, emphasising that therapies addressing these interconnected systems can provide holistic benefits in preventing inflammatory conditions, sensory disturbances, and maintaining overall skin resilience.
The skin microbiome is an essential component of its defence system, supporting barrier function, modulating immunity, and preventing pathogen overgrowth.25 Dysbiosis, an imbalance in microbial populations, has been implicated in inflammatory skin conditions such as acne, rosacea, and AD.26 Conversely, a balanced skin microbiome plays a significant
role in educating the skin to maintain its natural balance and resilience. Research by Dréno27 demonstrated the efficacy of microbiome-targeted therapies in restoring microbial equilibrium and alleviating symptoms. Recent research highlights the skin microbiome’s pivotal role in maintaining cutaneous health by regulating inflammatory processes and microbial stasis.27 Studies on inflammatory acne have revealed that shifts in Cutibacterium acnes phylotypes, particularly an overabundance of virulent strains like IA1, contribute to inflammation and immune activation. The dynamic interplay between C. acnes and commensal organisms, such as Staphylococcus epidermidis, underscores the importance of microbial diversity in preventing dysbiosis-related conditions. Innovative therapies focusing on restoring microbial equilibrium, including probiotics, postbiotics, and bacteriophage applications, show promise in reducing inflammation and addressing underlying microbial imbalances.28,29 Another study by Bae et al.30 highlighted the need for tailored treatments that address microbiome imbalances, which are often linked to acne severity.30 Therapeutic interventions in this context could focus on strategies that enhance the functionality and diversity of the skin’s commensal microbiome. Postbiotic creams can support the existing microbial ecosystem, while bacteriophage therapies offer a targeted approach to reducing pathogenic strains, such as C. acnes. These interventions aim to restore the balance of microbial phylotypes rather than introducing non-native bacteria, aligning with the skin’s natural ecological preferences and its capacity to maintain homeostasis.
These interventions align with a precisionmedicine approach, ensuring that treatments are both personalised and effective in restoring the skin’s natural microbial balance. These approaches emphasise maintaining the skin’s natural microbial ecosystem rather than eradicating bacteria, paving the way for personalised and sustainable microbiome-targeted preventive procedures.30 Similarly, other findings suggest that maintaining a balanced population of Staphylococcus epidermidis and C. acnes on the skin is
crucial for reducing erythema and managing inflammatory skin conditions, highlighting the therapeutic potential of targeting specific bacterial strains.31,32 Furthermore, emerging research highlights the role of microbiome modulation in managing rosacea, with treatments targeting bacterial balance showing promise in reducing inflammation and redness. Although evidence is still emerging, some studies suggest that topical applications of probiotics and postbiotics may help support a healthy skin microbiome. While these approaches show promise as potential noninvasive alternatives to traditional therapies, further research is needed to establish their efficacy and long-term benefits.33 Recent studies highlight the potential of topical probiotics in managing AD. For instance, Frankel et al.34 conducted a systematic review that evaluated 12 studies and four clinical trials, demonstrating that topical probiotics improved skin barrier function and symptom severity in patients with AD.34 Similarly, Flint et al.35 reported significant reductions in AD severity, as measured by the Severity Scoring of Atopic Dermatitis (SCORAD) index, in RCTs using topical probiotic formulations. These findings underscore the therapeutic potential of microbiome-targeted strategies in dermatology, particularly in addressing inflammatory skin conditions.35 Moreover, research by Liu et al.36 on microbial metabolites such as short-chain fatty acids sheds light on how these by-products influence neural pathways regulating itch and pain.36
By addressing both microbial imbalances and neural dysfunction, dermatologists can offer comprehensive management strategies for conditions like chronic pruritus.
Environmental stressors, including UV radiation, pollution, stress, and climate variability, significantly influence skin health and resilience. Chronic exposure to these factors disrupts cellular homeostasis, accelerates oxidative damage, and
weakens the skin’s barrier.37 Another study highlighted the detrimental effects of urban pollution, showing that it increases oxidative stress markers and compromises barrier function.38 Choi and Kang39 demonstrated that skin barrier function is influenced by both intrinsic factors (such as genetics, age, and hydration levels) and extrinsic factors (such as climate, skincare products, and irritants). Their research emphasises the importance of maintaining the acidic pH of AD and senile xerosis. Their findings underline how disruptions in stratum corneum acidity caused by environmental or biological factors can lead to skin barrier dysfunction and increased susceptibility to infections and inflammatory conditions.39 Similarly, another study conducted by Soares et al.40 explores the bilateral relationship between psychological stress and inflammatory skin diseases such as psoriasis, AD, and acne. It highlights how stress exacerbates skin inflammation through neuroimmune pathways, while chronic skin conditions can, in turn, increase psychological distress, creating a cycle that impacts overall health and quality of life.40 They highlight that stress triggers a cascade of neuroimmunological responses, such as the activation of the hypothalamic–pituitary–adrenal axis and the release of pro-inflammatory cytokines, which can impair the skin barrier, worsen inflammation, and delay healing processes. Furthermore, they discuss the bilateral nature of this relationship, where inflammatory skin diseases can themselves lead to increased psychological stress, creating a vicious cycle. Their review also underscores the importance of integrative approaches that combine dermatological and psychological therapies, including mindfulness, cognitive behavioural therapy, and stress management techniques, to effectively manage these conditions.40 By examining these ways, Lefèvre-Utile et al.17 highlight how external factors such as UV radiation, pollution, temperature changes, and pathogens can compromise the physical integrity of the barrier, disrupt its chemical pH balance, and alter the skin’s microbiota, leading to dysbiosis. These stressors also impact the immunological barrier by triggering inflammatory responses and impairing the skin’s sensory
function, which can exacerbate conditions like eczema, psoriasis, and premature ageing. By understanding the mechanisms through which environmental stressors affect these five functional aspects, the review underscores the need for barriertargeted therapies and preventative strategies to mitigate their impact and maintain skin health.16 Similarly, the work by Nomura41 on the cellular effects of UV exposure revealed its role in inducing inflammation and barrier degradation. Photoprotective strategies, including broadspectrum sunscreens and antioxidant serums, are essential for mitigating these impacts.41 Environmental stressors affect not only cellular homeostasis but also the neurodermatological mechanisms that respond to environmental challenges.
UV radiation, particularly UVB, plays a dual role in skin physiology, acting both as a stressor and as a modulator of local and systemic homeostasis. While excessive exposure can lead to DNA damage, inflammation, and carcinogenesis, controlled UV exposure activates essential phototransduction pathways that regulate immune, endocrine, and neural responses. Notably, UVB stimulates cutaneous production of vitamin D3 and its metabolites, including lumisterol and tachysterol, which contribute to immune regulation and systemic calcium homeostasis. Recent research highlights a broader framework termed photo-neuroimmuno-endocrinology, which describes how UV radiation influences not only the skin but also neuroendocrine pathways affecting the brain and immune system.42 In addition, UVB exposure induces the production of secosteroids such as vitamin D, lumisterol, and tachysterol. These molecules, particularly via cytochrome P450 (CYP)11A1-derived metabolic pathways, contribute not only to local skin homeostasis but may also have systemic regulatory effects.43 Research by Takahashi23 showed that oxidative stress, as a part of environmental stressors, alters nerve conduction, leading to sensory dysfunction. In the same way, this finding suggests that inhibiting TRPA1 activity could serve as a therapeutic strategy for managing chronic itch and sensory
dysfunction associated with conditions like AD, thus broadening the insight in terms of preventive measures.23
Educating the skin involves a holistic approach that addresses its biological and environmental components, fostering longterm skin health and resilience. Advances in diagnostic technologies, such as AIdriven analyses, enable the identification of unique patient needs, allowing for more personalised and effective care.44 AI tools are increasingly used in both clinical and consumer skincare. For example, deep learning algorithms can analyse highresolution skin images to detect early signs of ageing, pigmentation, or inflammation. Computer vision and convolutional neural networks are applied to evaluate texture, tone, and pore structure in real time. Mobile-based AI applications, such as SkinVision® (SkinVision, Amsterdam, the Netherlands), or DermAITM (Proscia, Philadelphia, Pennsylvania, USA), help track skin changes and suggest product routines. In dermatology clinics, AIdriven platforms integrate skin imaging, microbiome profiling, and patient-reported outcomes to generate tailored treatment plans. These technologies support more precise, proactive, and data-informed skin management strategies.45
Nicolau and Kendall demonstrated the utility of combining skin condition assessments with microbiome profiles and neural sensitivities to tailor treatment strategies, showing how these insights can guide precise, individualised interventions.46 These studies illustrate the transformative potential of personalised interventions. Biotechnology further supports this framework by developing innovative tools for skin health. The study by Usui et al.47 investigates the role of transient receptor potential vanilloid 1 (TRPV1)-positive sensory nerves and neuropeptides in the epidermal barrier. Their findings suggest that activation of these sensory nerves and the release of neuropeptides are crucial for promoting skin barrier recovery
and reducing inflammation after barrier disruption.47 Combining these neural therapies with microbiome-focused treatments provides a comprehensive approach for complex dermatological conditions. This integrated framework emphasises prevention over reactive treatments, empowering dermatologists and patients to focus on maintaining skin health and addressing issues before they become chronic.
With advancements in dermatological science and personalised medicine, it is now possible to delve deeper into a patient’s skin biology, analysing individual gene expression profiles and identifying vulnerabilities influenced by genetic predisposition. These insights, coupled with a detailed understanding of potential environmental stressors such as UV radiation, pollution, or lifestyle-related oxidative damage, allow dermatologists to predict the skin’s susceptibility to various conditions, including inflammatory disorders, neuropathic pain, and premature ageing. By incorporating this knowledge into care strategies, dermatology can transition from a reactive to a proactive discipline. The concept of ‘educating the skin’ as a preventive measure is built on this foundation. By focusing on early risk assessment and targeted interventions, we can take initial steps toward bolstering the skin’s resilience and potentially delaying the onset of chronic or multifactorial conditions. While the ability to fully predict and preempt such issues remains a future goal, emerging research on genetic markers and skin profiling shows promise. For instance, understanding tendencies towards impaired barrier function or heightened inflammation could, in the future, guide the introduction of ceramide-enriched therapies and immune-modulating treatments tailored to individual needs. Similarly, by analysing DNA markers linked to microbial imbalances or heightened sensory nerve responses, clinicians can tailor microbiome-supportive and neuroprotective interventions, reducing the risk of conditions such as
rosacea or chronic pruritus. This predictive approach not only safeguards against the development of debilitating skin conditions but also enhances overall skin health and quality of life. By integrating data on skin biology, environmental exposures, and patient-specific vulnerabilities, dermatologists can design personalised regimens that reinforce the skin’s natural defences. This comprehensive strategy supports the idea that educating the skin is not only a therapeutic tool but also a cornerstone of preventive dermatological care, ensuring long-term adaptation and functionality in a rapidly changing environment.
Patient empowerment and education play a crucial role in the success of the ‘educating the skin’ approach, particularly as it shifts dermatology from a reactive, treatment-focused discipline to one that emphasises prevention and long-term skin health.48-50 By educating patients about the underlying mechanisms that contribute to their skin conditions, including the importance of maintaining a healthy skin barrier, managing the skin microbiome, and adapting to environmental factors, patients can become more active participants in their care.51 A key component of patient empowerment is providing individuals with the tools and knowledge they need to make informed decisions about their skincare routines.52 This could involve educating patients on how to select the appropriate skincare products, such as emollients, moisturisers, and sunscreens that support their skin’s natural barrier function. It also involves teaching patients how to recognise early signs of skin imbalance or irritation, enabling them to take proactive measures before symptoms escalate into more serious conditions.53 Moreover, patient education about lifestyle factors such as diet, stress management, and sleep hygiene can enhance the skin’s ability to adapt and repair itself.54 For example, patients could be educated about the benefits of anti-inflammatory diets or mindfulness practices to reduce the impact of stress
on their skin.55 Additionally, understanding the influence of environmental factors, such as pollution and UV radiation, could help patients make informed choices about how to protect their skin on a daily basis. Empowering patients also involves ensuring that they understand the importance of consistent skincare practices. Educating patients about the significance of daily routines and how they directly contribute to skin health can encourage adherence to long-term preventive measures.56 As patients gain more knowledge, they may feel more confident in managing their skin conditions, reducing their reliance on medications and invasive treatments. Overall, patient empowerment and education are fundamental to successfully implementing the ‘educating the skin’ framework. When patients understand the science behind their skin’s health, they are more likely to take ownership of their well-being, ultimately leading to improved outcomes and quality of life. Through continued education and support, dermatologists can foster a partnership with patients that promotes self-care, prevention, and long-term resilience.57
The concept of educating the skin would necessitate further research to validate its long-term efficacy and refine its implementation.58 Longitudinal studies are crucial for exploring the complex interactions between microbiome metabolites, neural pathways, and environmental stressors, which will uncover new preventive targets for skin health.58 These studies should focus on the dynamic relationship between these factors and their collective impact on the skin’s resilience and immune response. Interdisciplinary collaborations between dermatologists, neurologists, microbiologists, and environmental scientists will be essential for advancing this integrated framework. By combining expertise from these fields, more effective strategies can be developed to address the root causes of skin conditions, rather than merely treating symptoms. Additionally, partnerships with biotechnology companies
will be instrumental in accelerating the development of innovative products that harness these diverse insights. With the increasing use of AI and deep learning, the potential for personalised skincare solutions will be significantly enhanced. AI-driven tools, such as biosensors that monitor real-time changes in skin conditions, can provide valuable data that inform more precise and targeted treatments. These technologies could allow for adaptive, ondemand therapies that respond to the skin’s immediate needs, improving both short-term and long-term outcomes. By integrating AI with the expertise of dermatologists and other specialists, the future of skincare can become more proactive, personalised, and data-driven, ensuring accessibility to cutting-edge treatments while improving patient care and skin health.
The concept of ‘educating the skin’ offers a groundbreaking, proactive framework for dermatology, integrating the fundamental pillars of skin health: biological mechanisms, microbial symbiosis, environmental adaptation, and neural function. By shifting the focus from reactive symptom management to prevention, adaptability, and resilience, this paradigm has the potential to transform dermatological care. Current evidence underscores the
References
1. Eyerich S et al. Cutaneous barriers and skin immunity: differentiating a connected network. Trends Immunol. 2018;39(4):315-27.
2. Cope H et al. Transcriptomics analysis reveals molecular alterations underpinning spaceflight dermatology. Commun Med. 2024;4(1):1-18.
3. Inchingolo AD et al. The integumentary system and its microbiota between health and disease. J Biol Regul Homeost Agents. 2021;35(2 Suppl_1):303-21.
4. Ongoro G et al. Skin inclusion: addressing deficits in medical education to promote diversity in dermatological diagnosis and treatment. Clin Cosmet Investig Dermatol. 2023;16:3481-5.
5. Agostini L et al. A management
importance of addressing skin health at multiple levels, from fortifying the epidermal barrier and balancing the microbiome to mitigating environmental stressors and enhancing neural interactions.
The integration of advanced technologies and scientific insights into skin biology is essential for realising the full potential of this approach. The role of AI is pivotal, enabling precise analysis of inter-individual variability and supporting the personalisation of interventions. By identifying genetic, environmental, and microbial factors that contribute to chronic skin conditions, AI tools can facilitate targeted strategies, ensuring interventions are tailored to the unique needs of each patient. Looking forward, advancements in AI and personalised medicine will continue to propel this approach, fostering interdisciplinary collaboration between dermatologists, researchers, and technologists. By connecting these elements, educating the skin not only strengthens resilience but also redefines the standard of care, emphasising prevention, patient-centred strategies, and long-term well-being. This innovative framework has the potential to elevate dermatological outcomes and improve quality of life, making it a cornerstone for the future of dermatology. Through this lens, the skin is no longer just treated; it is empowered to thrive in harmony with its environment.
perspective on resilience in healthcare: a framework and avenues for future research. BMC Health Serv Res. 2023;23:774.
6. Salazar A, von Hagen J. Circadian oscillations in skin and their interconnection with the cycle of life. Int J Mol Sci. 2023;24(6):5635.
7. Slominski AT et al. Melatonin and the skin: current progress and perspectives for human health. J Invest Dermatol. 2025;145(6):1345-60. e2.
8. Slominski RM et al. Neuro–immuno–endocrinology of the skin: how environment regulates body homeostasis. Nat Rev Endocrinol. 2025;21(8):495-509.
9. Slominski AT et al. Melatonin, mitochondria, and the skin. Cell Mol Life Sci. 2017;74(21):3913-25.
10. Slominski A et al. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993;164(1):103-20.
11. Guo L et al. Recent advances and progress on melanin: from source to application. Int J Mol Sci. 2023;24(5):4360.
12. Baker P et al. Skin barrier function: the interplay of physical, chemical, and immunologic properties. Cells. 2023;12(23):2745.
13. Elias PM. Optimizing emollient therapy for skin barrier repair in atopic dermatitis. Ann Allergy Asthma Immunol. 2022;128(5):505-11.
14. Chamlin SL et al. Ceramide-dominant, barrier-repair lipids improve childhood atopic dermatitis. Arch Dermatol. 2001;137(8):1110-2.
15. Luger T et al. Atopic dermatitis: role
of the skin barrier, environment, microbiome, and therapeutic agents. J Dermatol Sci. 2021;102(3):142-57.
16. Ridd MJ et al. Effectiveness and safety of lotion, cream, gel, and ointment emollients for childhood eczema: a pragmatic, randomised, phase 4, superiority trial. Lancet Child Adolesc Health. 2022;6(8):522-32.
17. Lefèvre-Utile A et al. Five functional aspects of the epidermal barrier. Int J Mol Sci. 2021;22(21):11676.
18. Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457-87.
19. Slominski AT et al. Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. Am J Physiol Cell Physiol. 2022;323(6):C1757-76.
20. Slominski AT et al. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v,vii,1-115.
21. Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4(3):211.
22. Zhou L et al. The roles of skin langerhans cells in immune tolerance and cancer immunity. Vaccines (Basel). 2022;10(9):1380.
23. Takahashi S et al. Homeostatic pruning and activity of epidermal nerves are dysregulated in barrier-impaired skin during chronic itch development. Sci Rep. 2019;9(1):8625.
24. Nguyen MQ et al. Single-nucleus transcriptomic analysis of human dorsal root ganglion neurons. eLife. 2021;10:e71752.
25. Theodorou IM et al. Cosmeceuticals: a review of clinical studies claiming to contain specific, well-characterized strains of probiotics or postbiotics. Nutrients. 2024;16(15):2526.
26. Haykal D et al. Dermatological health in the light of skin microbiome evolution. J Cosmet Dermatol. 2024;23(12):3836-46.
27. Dréno B et al. Microbiome in healthy skin, update for dermatologists. J Eur Acad Dermatol Venereol. 2016;30(12):2038-47.
28. Dréno B et al. The skin microbiome: a new actor in inflammatory acne. Am J Clin Dermatol. 2020;21(Suppl 1):18-24.
29. Bowe W et al. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5(2):185-99.
30. Bae IH et al. A comprehensive review of the acne grading scale in 2023. Ann Dermatol. 2024;36(2):65-73.
31. Landemaine L et al. Staphylococcus epidermidis isolates from atopic or healthy skin have opposite effect
on skin cells: potential implication of the AHR pathway modulation. Front Immunol. 2023;14:1098160.
32. Brown MM, Horswill AR. Staphylococcus epidermidisskin friend or foe? PLoS Pathog. 2020;16(11):e1009026.
33. Grinnell M et al. Probiotics in acne and rosacea: a systematic review. J Am Acad Dermatol. 2019;81(4):AB258.
34. Frankel D, Lio P. The role of topical probiotics for atopic dermatitis: a systematic review. J Integr Dermatol. 2023;DOI:10.64550/joid.cfqv4r95.
35. Flint E et al. Topical probiotics decrease the severity of atopic dermatitis in children and adults: a meta-analysis of double-blind, randomized, placebo-controlled trials. Cureus. 2024;16(9):e70001.
36. Liu XF et al. Regulation of short-chain fatty acids in the immune system. Front Immunol. 2023;14:1186892.
37. Kortekaas Krohn I et al. The influence of lifestyle and environmental factors on host resilience through a homeostatic skin microbiota: an EAACI Task Force Report. Allergy. 2024;79(12):3269-84.
38. Belzer A, Parker ER. Climate change, skin health, and dermatologic disease: a guide for the dermatologist. Am J Clin Dermatol. 2023;24(4):577-93.
39. Choi EH, Kang H. Importance of stratum corneum acidification to restore skin barrier function in eczematous diseases. Ann Dermatol. 2024;36(1):1-8.
40. Biazus Soares G et al. The mindskin connection: a narrative review exploring the link between inflammatory skin diseases and psychological stress. J Eur Acad Dermatol Venereol. 2024;38(5):821-34.
41. Nomura T, Kabashima K. Advances in atopic dermatitis in 2019-2020: endotypes from skin barrier, ethnicity, properties of antigen, cytokine profiles, microbiome, and engagement of immune cells. J Allergy Clin Immunol. 2021;148(6):1451-62.
42. Slominski RM et al. Photo-neuroimmuno-endocrinology: how the ultraviolet radiation regulates the body, brain, and immune system. Proc Natl Acad Sci U S A. 2024;121(14):e2308374121.
43. Slominski AT et al. Biological Effects of CYP11A1-derived vitamin D and lumisterol metabolites in the skin. J Invest Dermatol. 2024;144(10):214561.
44. Haykal D et al. What happens when simulations get real and cosmetic dermatology goes virtual? J Cosmet Dermatol. 2023;22(10):2682-4.
45. Haykal D. Emerging and pioneering AI technologies in aesthetic dermatology: sketching a path toward personalized,
predictive, and proactive care. Cosmetics. 2024;11(6):206.
46. Nicolaou A, Kendall AC. Current insights into skin lipids and their roles in cutaneous health and disease. Curr Opin Clin Nutr Metab Care. 2023;26(2):83-90.
47. Usui K et al. TRPV1-positive sensory nerves and neuropeptides are involved in epidermal barrier repair after tape stripping in mice. J Allergy Clin Immunol. 2024;153(3):868-73.e4.
48. Bhattad PB, Pacifico L. Empowering patients: promoting patient education and health literacy. Cureus. 2022;14(7):e27336.
49. Zirwas MJ, Holder JL. Patient education strategies in dermatology: part 1: benefits and challenges. J Clin Aesthet Dermatol. 2009;2(12):24-7.
50. de Bes J et al., ‘’Patient education in chronic skin diseases: a systematic review,’’ Database of abstracts of reviews of effects (DARE): qualityassessed reviews. [Internet] (2011) The University of York. Available at: https://www.ncbi.nlm.nih.gov/ books/NBK81420/. Last accessed: 18 November 2024.
51. Swaney MH, Kalan LR. Living in your skin: microbes, molecules, and mechanisms. Infect Immun. 2021;89(4):e00695-20.
52. Vainauskienė V, Vaitkienė R. Enablers of patient knowledge empowerment for self-management of chronic disease: an integrative review. Int J Environ Res Public Health. 2021;18(5):2247.
53. Barnes TM et al. Vehicles for drug delivery and cosmetic moisturizers: review and comparison. Pharmaceutics. 2021;13(12):2012.
54. Lippman D et al. Foundations of lifestyle medicine and its evolution. Mayo Clin Proc Innov Qual Outcomes. 2024;8(1):97-111.
55. Cowan S et al. Inflaming public interest: a qualitative study of adult learners’ perceptions on nutrition and inflammation. Nutrients. 2020;12(2):345.
56. Arlinghaus KR, Johnston CA. The importance of creating habits and routine. Am J Lifestyle Med. 2018;13(2):142-4.
57. Haykal D et al. The art of a successful cosmetic consultation in 2023. J Eur Acad Dermatol Venereol. 2023;37(9):e1166-7.
58. Price K et al. Education and process change to improve skin health in a residential aged care facility. Int Wound J. 2017;14(6):1140-7.
Authors: Myriam El Hajj,1 Joy Naba,1 Jean El Hajj,1 Claudia Chidiac,1 *Boutros Soutou1
1. Dermatology Department, Faculté de Médecine, Hôtel-Dieu de France Hospital, Université Saint-Joseph, Beirut, Lebanon *Correspondence to boutros.soutou@usj.edu.lb
Disclosure: The authors have declared no conflicts of interest.
Acknowledgements: M. El Hajj, Naba, J. El Hajj, and C. Chidiac have equally contributed to this work.
Received: 01.07.25
Accepted: 19.08.25
Keywords: Biologic, corticosteroids, intralesional, laser, methotrexate (MTX), nail, psoriasis.
Citation: EMJ Dermatol. 2025;13[1]:124-134. https://doi.org/10.33590/emjdermatol/XIMA3955
Abstract
Nail psoriasis remains a therapeutic challenge that is insufficiently addressed compared to skin and joint involvement. Treatment choices should be personalised and informed by the number of nails affected, the severity of the disease, the impact on daily life, skin or joint involvement, the molecule’s safety profile, patient preferences, adherence to treatment, and accessibility or cost-related issues. Researched treatments include intralesional and topical therapies, laser and light therapies alone or in combination, oral molecules, and biologics. No single intervention fits all patients. First-line treatment consists of topical steroids for nail bed involvement and intralesional steroids for limited disease or nail matrix involvement. When these fail or the disease severity warrants escalation, systemic options, such as biologics, are preferred. When biologics are neither feasible nor desired, alternatives include methotrexate, apremilast, pulsed dye laser, or other therapies.
1. Nail psoriasis highlights the need for personalised medicine. Treatment decisions should take into account the number of nails affected, disease severity, its impact on daily life, involvement of the skin or joints, the treatment’s safety profile, patient preferences, likelihood of adherence, and issues related to accessibility or cost.
2. Many treatments have been researched, including topical therapies, light and laser modalities, used alone or in combination, as well as conventional systemic agents and, more recently, biologic and targeted therapies.
3. No single intervention fits all patients. First-line treatment consists of topical steroids in nail bed involvement and intralesional steroids for limited disease or nail matrix involvement.
Nail psoriasis affects up to 40% of individuals with psoriasis and up to 80% of those with psoriatic arthritis.1 It presents a variety of signs involving the nail matrix (pitting, leukonychia, red lunulae, crumbling) and the nail bed (onycholysis, subungual hyperkeratosis, salmon patches, splinter haemorrhages), leading to pain, functional impairment, disfigurement, stigmatisation, and a significant psychosocial burden.2,3 It remains a therapeutic challenge that is insufficiently addressed compared with skin and joint involvement.
Nail psoriasis underscores the necessity for personalised medicine. Treatment choices should be informed by the number of nails affected, the severity of disease (e.g., Nail Psoriasis Severity Index [NAPSI] score), the impact on daily life, skin or joint involvement, and the risk of progression to psoriatic arthritis. Additional factors to consider include the treatment’s safety profile, patient preferences, adherence to treatment, and accessibility or cost-related issues. Furthermore, the unique anatomy of the nail unit and the involvement of both the matrix and the bed create therapeutic challenges, such as limited drug penetration and variable treatment responses, highlighting the importance of individualised and multifaceted approaches.4
Many treatments have been researched, including topical therapies, light and laser modalities, used alone or in combination, as well as conventional systemic agents and, more recently, biologic and targeted therapies.4 This article provides a comprehensive overview of treatment options for nail psoriasis, emphasising their mechanism, efficacy, safety, and role in personalised management strategies.
Despite numerous treatments, standardised guidelines have been lacking.5 However, Rigopoulos et al.2 recently proposed recommendations based on the number of affected nails and the site of psoriatic lesions. Intralesional steroid injections are considered the first-line treatment for limited nail disease (≤3 nails affected; NAPSI: ≤20) with nail matrix involvement.
Second-line options include topical corticosteroids, vitamin D analogues, retinoids, keratolytics, and tacrolimus.2,6 For nail bed involvement, first-line treatment consists of topical corticosteroids, either alone or in combination with vitamin D analogues, although evidence supporting the effectiveness of topical therapy remains limited.2
Triamcinolone acetonide (TA) is the most studied. When injected into the nail bed or matrix, it significantly improves many nail abnormalities, like pitting, hyperkeratosis, ridging, and onycholysis, with the most notable improvement observed in hyperkeratosis.7-9 Boontaveeyuwat et al.9 showed the superiority of intralesional TA over topical clobetasol in nail bed involvement, although superficial injections carry a risk of skin atrophy and telangiectasia.
Methotrexate (MTX) is a more recent option. Mittal and Mahajan11 found that intramatricial MTX had slightly higher efficacy on the NAPSI than intramatricial TA and ciclosporin, though not statistically significant.10 Other studies have shown significant clinical improvement, including a reduction in NAPSI scores, with minimal side effects.11-13 5-fluorouracil has been explored in one study; it was less effective than TA and MTX injections.14
Secukinumab, an IL-17A inhibitor, has been trialled intralesionally for resistant cases. He et al.15 reported a >90% NAPSI reduction in some patients by Week 24 for both nail matrix and nail bed features, along with good tolerability.15
Botulinum toxin Type A (BoNT-A) is the most recently studied agent. A recent RCT revealed that BoNT-A was as effective as TA at Week 16, and continued to show improvement beyond Week 16, particularly in nail bed psoriasis. Smaller case studies corroborate its efficacy and tolerability.16
Topical corticosteroids, particularly clobetasol, remain the first-line treatment for nail bed involvement. According to Sánchez Regaña et al.,17 onycholysis, pitting, and salmon patches are the clinical signs that showed the best improvement.
Vitamin D analogues, including calcipotriol, calcitriol, and tacalcitol, have shown varying success. Kokelj et al.18 and Zakeri et al.19 demonstrated efficacy in reducing hyperkeratosis and onycholysis; however, there was no statistical difference when compared with betamethasone dipropionate ointment alone or combined with salicylic acid.20,21 Combination therapies of vitamin D analogues with corticosteroids enhance effectiveness. Rigopoulos et al.22 and Sánchez Regaña et al.17 showed synergistic effects on both matrix and bed lesions over 6–12 months. On the other hand, the RCT conducted by Tzung et al.23 reported no difference between this combination and the use of calcipotriol alone.
Tazarotene, a topical retinoid, presents no significant difference in efficacy compared with topical clobetasol in terms of pitting, onycholysis, hyperkeratosis, and salmon patches; however, it yields a more sustained improvement in subungual hyperkeratosis at Week 12.24 In the study by Fischer-Levancini et al.,25 the NAPSI improved by up to 88% at 6 months, but some cases of irritation were reported. Tretinoin 0.025% cream showed a promising result in a case report; however, further investigation is needed.6
The phosphodiesterase-4 inhibitor roflumilast 0.3% cream was tried in two patients, showing complete lesion clearance and good tolerability after daily use for 4–5 months.26,27 Johnston and Poelman27 reported a case of nail psoriasis treated with daily roflumilast 0.3% cream. After 5 months, the patient had complete resolution of pain, erythema, and periungual scaling, along with full nail regrowth without onychodystrophy. The treatment was highly effective with no adverse effects.27 JassoOlivares et al.26 conducted a retrospective study on seven treatment-resistant patients using daily roflumilast 0.3% cream for 16
weeks. The mean NAPSI score significantly dropped from 19 to 6.8. Most patients achieved near-complete clearance, improved quality of life, and reported no adverse effects.26 Apremilast lacquer also showed promise but still lacks robust clinical trial data.28
Topical calcineurin inhibitor, tacrolimus 0.1%, applied without occlusion, proved effective for both nail matrix and nail bed signs in an RCT reporting >50% NAPSI reduction.29
Ciclosporin was first topically investigated in a case report by Tosti et al.30 using a 10% concentration, with 0.2 mL applied once daily. A marked improvement was observed after 2 months, with nearcomplete lesion clearance after 3 months.30 An RCT conducted in 2003 utilised a 70% ciclosporin emulsion, resulting in a 77% improvement in some patients.31 It was later mentioned that the instability of the emulsion might contribute to treatment failure.32
5-fluorouracil topical formulation has mixed evidence. While early studies showed some improvement, especially when combined with urea for better penetration, others failed to demonstrate superiority over placebo.33,34 The risk of onycholysis or irritation limits its use.
‘Natural products’ such as indigo naturalis were mostly investigated by Lin et al.35 in RCTs and case reports. The mean reduction in the NAPSI score was 51.2% after a 3-month treatment consisting of a twice-daily application over 24 weeks, thus outperforming calcipotriol.35 All articles discussing intralesional and topical treatment in nail psoriasis are compiled in Supplementary Table 1.
Lasers have been explored as potential treatments for nail psoriasis, showing varying degrees of efficacy.
The pulsed dye laser (PDL) is the most studied. It afforded a significant reduction in total, matrix, and bed NAPSI scores, though fluence higher than 8 J/cm2 did not yield a
superior outcome.36 Nevertheless, results remain heterogeneous due to variations in pulse durations, study design, and baseline NAPSI scores. Comparative studies have demonstrated that PDL is as effective and safe as intralesional corticosteroids, intense pulsed light (IPL), neodymium:yttriumaluminium-garnet (Nd:YAG) laser, and photodynamic therapy (PDT).37-40 Only one study found superior outcomes with topical calcipotriol/betamethasone dipropionate compared with PDL alone.41 PDL caused less pain compared with IPL and Nd:YAG laser. Reported side-effects were generally mild and transient, including pain, purpura, and petechiae.36,39,40 Given its tolerability, PDL may be considered a reliable treatment for nail psoriasis, but larger RCTs must confirm its long-term efficacy before establishing standardised treatment protocols.
The long-pulsed Nd:YAG laser has shown conflicting results.42 Some studies reported an improvement in matrix and nail bed parameters, like subungual hyperkeratosis and onycholysis, and other studies have shown no change in total or nail bed NAPSI scores, limiting benefits to matrix involvement.43,44
The 10,600-nm fractional CO2 laser (FCL) is a promising treatment for nail psoriasis, particularly in laser-assisted drug delivery. It improves nail bed remodelling and enhances the penetration of topical agents. Multiple studies have shown that FCL, either alone or in combination with topical treatments such as calcipotriol/betamethasone or MTX, significantly reduces total NAPSI scores and improves symptoms like pitting, subungual hyperkeratosis, and onycholysis. Combination therapies often showed better clinical outcomes than monotherapies, but most differences were not statistically significant.45,46 A recent case report even described resolution of refractory single-nail psoriasis after one session of FCL-assisted MTX delivery.47 Despite these encouraging results, there is a lack of rigorous trials comparing FCL to established treatments such as intralesional MTX or corticosteroids in resistant cases.
PDT and IPL have surfaced as risk-free and encouraging approaches for treating nail psoriasis. Aminolevulinic acid, activated by red light, demonstrated superior results at the 24-week follow-up compared with clobetasol 0.05% ointment.48 Variants of PDT have also been explored: a combination of PDL with methyl-aminolevulinic acid showed no additional benefit compared with PDL alone,37 methylene blue-assisted PDT was more effective in nail bed lesions than IPL.49
Non-ablative bipolar radiofrequency, generating dermal heat with antiinflammatory effects, and microneedling with enhanced topical drug delivery via the microchannels, have emerged as potential treatments, though each has so far been explored in a single study.50,51
Excimer laser showed limited efficacy compared with PDL.52
All articles discussing laser and light treatment in nail psoriasis are compiled in Supplementary Table 2
Several systemic treatments are available and effective for managing nail psoriasis.
MTX inhibits dihydrofolate reductase, thereby reducing lymphocyte proliferation.53 It is, however, associated with many side effects that sometimes limit its use. It is contraindicated in pregnant women, individuals with severe liver disease or renal impairment, and patients with HIV. MTX has moderate efficacy in nail psoriasis. In the study by Warren et al.,54 which compared MTX (17.5 mg weekly, increased to 22.5 mg for non-responders) with placebo, total nail clearance was achieved by 5% of patients in the MTX group versus 0% in the placebo group at Week 16, and 14% achieved clearance by Week 52. Another study by Gümüşel et al.55 compared MTX with cyclosporine in a 24-week single-blind randomized trial, showing a NAPSI reduction of 43% in the MTX group versus 37.2% in the ciclosporin group. Several side effects, such as myelosuppression, hepatotoxicity, and nephrotoxicity, have been reported.
Pulmonary fibrosis should also be kept in the prescriber’s sight, especially if the patient uses other medications known to cause such a side effect, like nitrofurantoin.
Ciclosporin is a calcineurin inhibitor that blocks T cell activation and IL-2. It is effective in treating nail psoriasis and is given for short-term use.2 Ciclosporin is not contraindicated during pregnancy, although it has been associated with low birth weight. In an open-label study of 18 patients, 44% had ≥50% NAPSI improvement after 12 months.56 In patients with psoriatic arthritis and nail psoriasis, ciclosporin achieved a 44% improvement in NAPSI score, compared with 56% for adalimumab and 100% for combination treatment.56 One study showed a 79% improvement in nail features when ciclosporin was combined with calcipotriene, while 47.6% improved with ciclosporin alone.57 Despite its effectiveness, ciclosporin’s use is limited by its high side effect profile and potential kidney damage in the medium and long terms.58
Acitretin is a retinoic acid with antiinflammatory and antiproliferative effects. It is contraindicated in individuals with severe liver disease and pregnancy, requiring 3 years of contraception after discontinuation. It has moderate efficacy in nail psoriasis. In a study by Tosti et al.,59 low-dose acitretin (0.2–0.3 mg/kg/day) given for 6 months led to a 41% NAPSI improvement and complete or near-complete nail clearance in 25% of patients.59 Another study involving 41 patients treated with acitretin (0.6–0.8 mg/ kg) for 6 months showed improvements in nail matrix and bed thickness, although with no effect on enthesopathy and vascular inflammation.60 A case report described a patient with severe, treatment-resistant nail psoriasis who improved significantly after taking 25 mg/day of acitretin for 2 months, with continued improvement over 6 months.61
Alitretinoin, or 9-cis-retinoic acid, did not show a significant difference at Weeks 12 and 24, when compared with placebo in a Phase II randomised, double-blind, placebocontrolled, multicentre study.62
Apremilast is an oral phosphodiesterase-4 inhibitor that increases intracellular cyclic adenosine monophosphate and modulates immune responses by blocking proinflammatory cytokines and promoting anti-inflammatory mediators such as IL10.63 It does not require baseline laboratory tests or monitoring during treatment. It should be avoided in pregnancy, in children <6 years, and in patients treated with strong cytochrome P450 inducers. It is a convenient, non-immunosuppressive oral treatment often preferred by patients seeking non-injectable options. The ESTEEM 1 and 2 trials included 1,255 patients with nail psoriasis, comparing apremilast 30 mg twice daily with placebo. By Week 16, apremilast reduced nail psoriasis severity scores by about 22.5–29.0%, while the placebo showed little to no improvement. Around one-third to nearly half of patients achieved at least 50% improvement by then. Benefits continued through Week 32 and Week 52.64,65 The LIBERATE trial showed a maintained response up to 2 years.66
Tofacitinib is a JAK-1/2/3 and tyrosine kinase 2 inhibitor. The higher dose of tofacitinib (10 mg twice daily) showed better improvement in nail psoriasis than the lower dose (5 mg twice daily), observed as early as Week 12, with a sustained response through Week 52.67,68 Upadacitinib is a selective JAK1 inhibitor with a promising effect on nail psoriasis.69 All JAK inhibitors carry black box warnings for serious adverse events, including infections, malignancies, thrombosis, and fetal toxicities, requiring careful risk–benefit assessment. Deucravacitinib is a new selective tyrosine kinase 2 inhibitor with a promising effect on nail psoriasis and a favourable safety profile.70 Real-world studies demonstrated significant improvement and sustained clearance rates up to 52 weeks.70,71 A case report highlighted its potential in refractory nail psoriasis but also noted some adverse effects that required discontinuation.72 While generally well tolerated, some adverse events, including oral ulcers and pruritus, have been reported, highlighting the need for continued safety monitoring. Further large-scale studies are needed to confirm its safety and efficacy.
All articles discussing oral treatments in nail psoriasis are compiled in Supplementary Table 3.
Biologics are an option for cases involving multiple nails, treatment-resistant disease, extensive skin or joint involvement, specific patient preferences, or a significant impact on quality of life.73,74 Several agents are effective, including the following: TNF-α inhibitors (infliximab, adalimumab, etanercept, certolizumab, golimumab), IL-12/23 inhibitor (ustekinumab), IL-23 inhibitors (guselkumab, risankizumab, tildrakizumab), and IL-17 inhibitors (secukinumab, ixekizumab, brodalumab, bimekizumab, netakimab). Improvement tends to be slower than in cutaneous psoriasis, closely linked to the skin and joint response, and is typically faster in fingernails than in toenails.75
TNF-α inhibitors were the first biologic agents approved for the treatment of nail psoriasis.76 Etanercept, a TNF-α inhibitor, reduced the NAPSI score by 50–90%, with a significant improvement in quality of life. Notably, etanercept combined with MTX resulted in greater NAPSI score reductions than MTX alone.73 On the other hand, combining adalimumab and ciclosporin can achieve a 100% NAPSI score improvement.77 A prospective study demonstrated that infliximab was more effective than adalimumab and etanercept by Week 14.78 Simultaneously, a network meta-analysis showed the superiority of infliximab among TNF-α inhibitors in improving nail psoriasis both in the short and long term, likely due to higher blood concentrations achieved through its intravenous administration.79,80 Among IL-17 inhibitors, a network meta-analysis found that ixekizumab was superior to brodalumab and bimekizumab. Additionally, the rates of complete nail cure followed the order of efficacy: ixekizumab, adalimumab, brodalumab, bimekizumab, secukinumab, and ustekinumab.81 Multiple trials have shown that ixekizumab yields higher response rates in nail psoriasis compared to etanercept, guselkumab, ustekinumab,
and adalimumab.82-85 Similarly, a systematic review emphasised the superiority of ixekizumab over other biologics.86 Huang et al.79 found that infliximab ranked first among biologics for short-term nail psoriasis treatment (10–16 weeks), while ixekizumab outperformed other agents in the long term (24–26 weeks). A network meta-analysis by Khan et al.87 in 2024 reported the greatest mean improvements in NAPSI scores at 24 weeks with brodalumab and etanercept, while complete nail psoriasis resolution was achieved with ixekizumab and adalimumab. These findings suggest that ixekizumab may be the most effective biologic for achieving complete nail psoriasis resolution over the long term. Nail psoriasis and psoriatic arthritis are closely related both anatomically and clinically, a connection further supported by the superior efficacy of ixekizumab and infliximab, leading agents among IL-17 and TNF-α inhibitors, respectively. The TRANSFIGURE trial studied secukinumab, an IL-17A inhibitor, specifically in patients with nail psoriasis, and found sustained long-term efficacy.76 Both secukinumab and brodalumab were considered superior to ustekinumab.88
Biologic treatments carry many risks; therefore, the choice should be tailored to the patient’s comorbidities and medical history. Common adverse events range from mild signs, such as injection site reactions and upper respiratory tract infections, to serious complications like severe infections or allergic reactions. Special consideration should be given to the relative safety of TNF-α inhibitors during pregnancy, while caution is advised with IL-17 inhibitors because of their potential to exacerbate inflammatory bowel disease. In summary, biologics represent an effective treatment option with an acceptable safety profile and a remarkable improvement in quality of life. All articles discussing biologic treatments in nail psoriasis are compiled in Supplementary Table 4
As the authors’ understanding of the condition evolves, it becomes increasingly clear that no single intervention is suitable
Figure 1: Suggestions for nail psoriasis treatment in daily practice.
One or two nails involved
Nail bed involved
Many nails involved
Matrix involved
Topical: steroid or vitamin D analogue, or both combined; then switch to tazarotene if hyperkeratosis; if no response in 8 weeks in fingers or 12 weeks in toes, try tacrolimus 0.1%, a PDE4 inhibitor, or cyclosporin, or 10,600 nm fractional CO2 laser in laser-assisted drug delivery of calcipotriol/betamethasone or methotrexate.
To consider: bad compliance, risk of skin atrophy, and pain tolerance.
Intralesional triamcinolone acetonide or methotrexate; if no response in 8 weeks in fingers or 12 weeks in toes, try intralesional secukinumab, or botulinum toxin A, or pulsed dye laser (best device with efficacy/tolerance ratio).
To consider: pain, haematoma, transitory numbness, nail unit/skin atrophy.
Mild disease (NAPSI: <20)
Joints not involved, skin not or limitedly involved
If low impact on quality of life, topical treatment can be initiated. If high impact on quality of life, intralesional injections, lasers, oral methotrexate, or apremilast are indicated according to the patient’s preferences.
Large skin areas or joints involved
A systemic treatment can be initiated according to the best nail efficacy, the patient’s preferences (needle phobia, travel frequency, cost, accessibility, etc.), and comorbidities: oral methotrexate, apremilast, and biologics.
Moderate-to-severe disease (NAPSI: >20)
Joints not involved, skin not or limitedly involved
Intralesional injections, lasers, oral methotrexate, apremilast, and biologics. Skin or joints involved
Oral methotrexate, apremilast, and biologics.
To consider: myelosuppression, hepato-nephrotoxicities, and pulmonary fibrosis with methotrexate; risk of infections and malignancies with biologics.
Notes:
1. In pregnancy:
Topical steroids, vitamin D analogues, alone or combined with devices
Certolizumab
Oral cyclosporine: a short-term treatment, if the benefits outweigh the risks
2. In children:
Topical steroids, vitamin D analogues, tazarotene
Oral methotrexate, apremilast (if >6 years), or biologics
3. HIV infection:
Topical steroids, vitamin D analogues, tazarotene
Non-invasive devices (pulsed dye laser)
Oral acitretin
Caution with oral methotrexate, apremilast, and biologics
4. Biologics are an option for cases involving multiple nails, treatment-resistant disease, extensive cutaneous or joint involvement, or significant impact on quality of life. IL-17 inhibitors are the fastest; caution is needed if there is a risk of inflammatory bowel disease.
5. Lasers should be used more as an adjuvant or an alternative treatment than an escalating option.
6. Some of the cited options should still be considered experimental.
NAPSI: nail psoriasis severity index; PDE4: phosphodiesterase-4.
for all patients. A tailored treatment approach, guided by disease severity, number and location of affected nails, joint or skin involvement, impact on quality of life, and patient-specific factors such as comorbidities, preferences, and treatment access, is crucial (Figure 1).
First-line treatment usually involves topical agents such as calcipotriol, high-potency corticosteroids, or tacrolimus. If these fail or if the disease severity requires escalation, systemic options like biologics (TNF-α
1. Lipner SR. Nail disorders: diagnosis and management. Dermatol Clin. 2021;39(2):xi.
2. Rigopoulos D et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81(1):228-40.
3. Stewart CR et al. The impact of nail psoriasis and treatment on quality of life: a systematic review. Skin Appendage Disord. 2021;7(2):83-9.
4. Ricardo JW, Lipner SR. Nail psoriasis in older adults. Dermatol Clin. 2021;39(2):195-210.
5. de Vries AC et al. Interventions for nail psoriasis. Cochrane Database Syst Rev. 2013;2013(1):CD007633.
6. Grover C, Daulatabad D. Topical tretinoin in the treatment of nail psoriasis. Indian Dermatol Online J. 2022;13(1):126-7.
7. Saleem K, Azim W. Treatment of nail psoriasis with a modified regimen of steroid injections. J Coll Physicians Surg Pak. 2008;18(2):78-81.
8. de Berker DA, Lawrence CM. A simplified protocol of steroid injection for psoriatic nail dystrophy. Br J Dermatol. 1998;138(1):90-5.
9. Boontaveeyuwat E et al. A randomized comparison of efficacy and safety of intralesional triamcinolone injection and clobetasol propionate ointment for psoriatic nails. J Dermatolog Treat. 2019;30(2):117-22.
10. Mittal J, Mahajan BB. Intramatricial injections for nail psoriasis: an open-label comparative study of triamcinolone, methotrexate, and cyclosporine. Indian J Dermatol Venereol Leprol. 2018;84:419-23.
11. Sarıcaoglu H et al. Nail psoriasis
inhibitors, ustekinumab, secukinumab, or ixekizumab) are preferred. When biologics are neither feasible nor desired, alternatives include MTX, apremilast, intralesional corticosteroid injections, PDL, or other therapies. In this context, personalised medicine is not just ideal; it is crucial for optimising clinical outcomes, reducing adverse effects, and enhancing both functional and psychosocial well-being.
successfully treated with intralesional methotrexate: case report. Dermatol Basel Switz. 2011;222(1):5-7.
12. Grover C et al. Role of nail bed methotrexate injections in isolated nail psoriasis: conventional drug via an unconventional route. Clin Exp Dermatol. 2017;42(4):420-3.
13. Choudhary P et al. Treatment of nail psoriasis with intramatrical methotrexate: an uncontrolled prospective study of 20 patients. J Am Acad Dermatol. 2021;84(2):526-8.
14. Abdelmeniem IM et al. Topical calcipotriol combined with urea 20% versus intralesional injection of triamcinolone acetonide, 5‐fluorouracil, and methotrexate in the treatment of nail psoriasis: a comparative study. Dermatol Ther. 2022;35(9):e15660.
15. He F et al. Intramatricial low-dose secukinumab injection for nail psoriasis. Indian J Dermatol Venereol Leprol. 2021;87:116-9.
16. Juntongjin P et al. Botulinum toxin injection shows promise in nail psoriasis: a comparative randomized controlled trial. JAAD Int. 2024;16: 105-11.
17. Sánchez Regaña M et al. Nail psoriasis: a combined treatment with 8% clobetasol nail lacquer and tacalcitol ointment. J Eur Acad Dermatol Venereol. 2008;22(8):963-9.
18. Kokelj F et al. Nail psoriasis treated with calcipotriol (MC 903): an open study. J Dermatolog Treat. 1994;5(3):149-50.
19. Zakeri M et al. Topical calcipotriol therapy in nail psoriasis: a study of 24 cases. Dermatol Online J. 2005;11(3):5.
20. Tosti A et al. Calcipotriol ointment in nail psoriasis: a controlled doubleblind comparison with betamethasone dipropionate and salicylic acid. Br J Dermatol. 1998;139(4):655-9.
21. Kole L et al. A randomized, double-
blinded trial evaluating the efficacy and tolerability of vectical ointment (calcitriol 3 mcg/g ointment) when compared to betamethasone diproprionate ointment (64 mg/g) in patients with nail psoriasis. J Drugs Dermatol. 2014;13(8):912-5.
22. Rigopoulos D et al. Nail psoriasis: a combined treatment using calcipotriol cream and clobetasol propionate cream. Acta Derm Venereol. 2002;82(2):140.
23. Tzung T et al. Calcipotriol used as monotherapy or combination therapy with betamethasone dipropionate in the treatment of nail psoriasis. Acta Derm Venereol. 2008;88(3):279-80.
24. Rigopoulos A et al. Treatment of psoriatic nails with tazarotene cream 0.1% vs. clobetasol propionate 0.05% cream: a double-blind study. Acta Derm Venereol. 2007;87(2):167-8.
25. Fischer-Levancini C et al. Nail psoriasis: treatment with tazarotene 0.1% hydrophilic ointment. Actas Dermosifiliogr. 2012;103(8):725-8.
26. Jasso-Olivares J. Outcomes of isolated nail psoriasis treatment with roflumilast 0.3% cream: a case series of 7 patients. J Cutan Med Surg. 2025;17:12034754251339106.
27. Johnston LA, Poelman SM. Successful treatment of nail psoriasis with topical roflumilast: a case report. SAGE Open Med Case Rep. 2024;12:2050313X241289594.
28. Kushwaha AS et al. A novel apremilast nail lacquer formulation for the treatment of nail psoriasis. AAPS PharmSciTech. 2017;18(8):2949-56.
29. De Simone C et al. Tacrolimus 0.1% ointment in nail psoriasis: a randomized controlled open‐label study. J Eur Acad Dermatol Venereol. 2013;27(8):1003-6.
30. Tosti A et al. Topical ciclosporin in nail psoriasis. Dermatologica.
1990;180(2):110.
31. Cannavò SP et al. Treatment of psoriatic nails with topical cyclosporin: a prospective, randomized placebocontrolled study. Dermatology. 2003;206(2):153-6.
32. Prins AM et al. Instability of topical ciclosporin emulsion for nail psoriasis. Dermatology. 2007;215(4):362-3.
33. De Jong EM et al. Dystrophic psoriatic fingernails treated with 1% 5-fluorouracil in a nail penetrationenhancing vehicle: a double-blind study. Dermatology. 1999;199(4): 313-8.
34. Fritz K. [Successful local treatment of nail psoriasis with 5-fluorouracil]. Z Hautkr. 1989;64(12):1083-8. (In German).
35. Lin YK et al. A Chinese herb, indigo naturalis, extracted in oil (lindioil) used topically to treat psoriatic nails: a randomized clinical trial. JAMA Dermatol. 2015;151(6):672.
36. Treewittayapoom C et al. The effect of different pulse durations in the treatment of nail psoriasis with 595nm pulsed dye laser: a randomized, double-blind, intrapatient left-toright study. J Am Acad Dermatol. 2012;66(5):807-12.
37. Fernández‐Guarino M et al. Pulsed dye laser vs. photodynamic therapy in the treatment of refractory nail psoriasis: a comparative pilot study. J Eur Acad Dermatol Venereol. 2009;23(8):891-5.
38. Soliman M et al. Pulsed-dye laser versus intralesional steroid in the management of nail psoriasis: a randomized, intra-patient, comparative, controlled study. J Clin Aesthetic Dermatol. 2021;14(9):45-9.
39. Morsy EE et al. Intense pulsed light versus pulsed dye laser in the treatment of nail psoriasis: Intrapatient left to right comparative controlled study. Indian J Dermatol Venereol Leprol. 2024;90:713-21.
40. Arango-Duque LC et al. Treatment of nail psoriasis with pulse dye laser plus calcipotriol betametasona gel vs. Nd:YAG plus calcipotriol betamethasone gel: an intrapatient left-to-right controlled study. Actas Dermosifiliogr. 2017;108(2):140-4.
41. Gregoriou S et al. Treatment of nail psoriasis with calcipotriol/ betamethasone dipropionate foam versus pulse dye laser: an unblinded, intra‐patient, left‐to‐right prospective study. J Eur Acad Dermatol Venereol. 2020;34(9):e519-20.
42. Hesham Ali Elwan Y et al. Nd:YAG laser in the treatment of nail psoriasis: clinical and dermoscopic assessment. Dermatol Pract Concept. 2021;11(2):e2021140.
43. Kartal SP et al. Long-pulsed Nd:YAG laser treatment for nail psoriasis. Dermatol Surg. 2018;44(2):227-33.
44. Khashaba SA et al. Efficacy of longpulsed Nd-YAG laser in the treatment of nail psoriasis: a clinical and dermoscopic evaluation. J Dermatolog Treat. 2021;32(4):446-52.
45. Afify AA et al. Fractional CO2 laser in the treatment of nail psoriasis: how can it help? Arch Dermatol Res. 2023;315(6):1705-15.
46. Hassan Moftah N et al. Combined fractional CO2 laser 10,600 nm with methotrexate 1% gel versus methotrexate 1% gel alone in the treatment of nail psoriasis: a randomized comparative study. Arch Dermatol Res. 2024;317(1):153.
47. Soutou B et al. Resolution of refractory single-nail psoriasis through a single session of fractional CO2 laser-asssited methotrexate delivery. Ann Dermatol Venereol. 2024;151(2):103255.
48. Tehranchinia Z et al. A Comparison of the effects of clobetasol 0.05% and photodynamic therapy using aminolevulinic acid with red light in the treatment of severe nail psoriasis. J Lasers Med Sci. 2020;11(1):3-7.
49. Shaheen MA et al. Comparison between the efficacy of intense pulsed light (I.P.L.) versus photo-dynamic therapy (P.D.T.) with methylene-blue in the treatment of psoriatic nails. Photodiagnosis Photodyn Ther. 2023;41:103298.
50. Yew YW et al. Novel transdermal device for delivery of triamcinolone fornail psoriasis treatment. Ann Acad Med Singap. 2022;51(1):16-23.
51. El-Basiony MAS et al. Efficacy of nonablative bipolar radiofrequency in the treatment of fingernail psoriasis. Dermatol Surg. 2025;51(5):522-6.
52. Al-Mutairi N et al. Single blinded leftto-right comparison study of excimer laser versus pulsed dye laser for the treatment of nail psoriasis. Dermatol Ther (Heidelb). 2014;4(2):197-205.
53. Menter A et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445-86.
54. Warren RB et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;389(10068):528-37.
55. Gümüşel M et al. Evaluation of the efficacy of methotrexate and
cyclosporine therapies on psoriatic nails: a one‐blind, randomized study. J Eur Acad Dermatol Venereol. 2011;25(9):1080-4.
56. Karanikolas GN et al. Adalimumab or cyclosporine as monotherapy and in combination in severe psoriatic arthritis: results from a prospective 12-month nonrandomized unblinded clinical trial. J Rheumatol. 2011;38(11):2466-74.
57. Feliciani C et al. Nail psoriasis: combined therapy with systemic cyclosporin and topical calcipotriol. J Cutan Med Surg. 2004;8(2):122-5.
58. Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18(8): 897-903.
59. Tosti A et al. Evaluation of the efficacy of acitretin therapy for nail psoriasis. Arch Dermatol. 2009;145(3):269-71.
60. Krajewska-Włodarczyk M et al. Ultrasound evaluation of the effectiveness of the use of acitretin in the treatment of nail psoriasis. J Clin Med. 2021;10(10):2122.
61. Ricceri F et al. Treatment of severe nail psoriasis with acitretin: an impressive therapeutic result: efficacy of acitretin in nail psoriasis. Dermatol Ther. 2013;26(1):77-8.
62. Reich K et al. Oral alitretinoin treatment in patients with palmoplantar pustulosis inadequately responding to standard topical treatment: a randomized phase II study. Br J Dermatol. 2016;174(6):1277-81.
63. Lanna C et al. Apremilast as a target therapy for nail psoriasis: a reallife observational study proving its efficacy in restoring the nail unit. J Dermatol Treat. 2022;33(2):1097-101.
64. Papp K et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (efficacy and safety trial evaluating the effects of apremilast in psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1): 37-49.
65. Paul C et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387-99.
66. Reich K et al. Safety and efficacy of apremilast through 104 weeks in patients with moderate to severe psoriasis who continued on apremilast or switched from etanercept treatment: findings from the LIBERATE study. J Eur Acad Dermatol Venereol. 2018;32(3):397-402.
67. Merola JF et al. Efficacy of tofacitinib for the treatment of nail psoriasis: two 52-week, randomized, controlled phase 3 studies in patients with moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2017;77(1):79-87. e1.
68. Zhang J et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol Sci. 2017;88(1): 36-45.
69. Werner SG et al. Treatment with upadacitinib in active psoriatic arthritis: efficacy and safety data of the first 192 patients from the UPJOINT study, a multicentre, observational study in clinical practice. Rheumatol Ther. 2023;10(6):1503-18.
70. Okubo Y et al. Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, in Japanese patients with plaque psoriasis: in‐depth analysis of efficacy and safety in the phase 3 POETYK PSO ‐4 trial. J Dermatol. 2025;52(6):953-66.
71. Hagino T et al. Long-term effectiveness and safety of deucravacitinib for psoriasis: a 52week real-world study of genital, scalp and nail lesions. Clin Exp Dermatol. 2025;50(5):952-9.
72. Wang J. Successful treatment of severe dystrophic nail psoriasis with deucravacitinib. Cutis. 2024;114(6):196-8.
73. Battista T et al. Nail psoriasis: an updated review of currently available systemic treatments. Clin Cosmet Investig Dermatol. 2023;16:1899-932.
74. Hwang JK, Lipner SR. Treatment of nail psoriasis. Dermatol Clin. 2024;42(3):387-98.
75. Canal-García E et al. Nail psoriasis. Actas Dermosifiliogr. 2022;113(5): 481-90.
76. Bardazzi F et al. Nail psoriasis: an updated review and expert opinion on available treatments, including biologics. Acta Derm Venereol. 2019;99(6):516-23.
77. Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51-63.
78. Saraceno R et al. TNF-α antagonists and nail psoriasis: an open, 24-week, prospective cohort study in adult patients with psoriasis. Expert Opin Biol Ther. 2013;13(4):469-73.
79. Huang IH et al. Small molecule inhibitors and biologics in treating nail psoriasis: a systematic review and network meta-analysis. J Am Acad Dermatol. 2021;85(1):135-43.
80. Satoh M, Yamamoto T. Case of recalcitrant nail psoriasis unresponsive to adalimumab but successfully treated with infliximab. J Dermatol. 2017;44(11):e288-9.
81. Egeberg A et al. Network metaanalyses comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis at 24–28 and 48–52 weeks. J Dermatolog Treat. 2023;34(1):2263108.
82. Mease PJ et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologicnaive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebocontrolled and active (adalimumab)controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79-87.
83. Wasel N et al. Ixekizumab and ustekinumab efficacy in nail psoriasis in patients with moderate-to-severe psoriasis: 52-week results from a phase 3, head-to-head study (IXORA-S). Dermatol Ther (Heidelb). 2020;10(4):663-70.
84. Mease PJ et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blindedassessor trial. Ann Rheum Dis. 2020;79(1):123-31.
85. Blauvelt A et al.; IXORA-R Study Group. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 24‐week efficacy and safety results from a randomized, double‐blinded trial. Br J Dermatol. 2021;184(6):1047-58.
86. Hwang JK et al. Efficacy and safety of nail psoriasis targeted therapies: a systematic review. Am J Clin Dermatol. 2023;24(5):695-720.
87. Khan M et al. Assessing comparative efficacy of biologics for the treatment of psoriasis with nail involvement: a systematic review. J Psoriasis Psoriatic Arthritis. 2024;9(2):61-8.
88. Conrad C et al. Nail involvement as a predictor of differential treatment effects of secukinumab versus ustekinumab in patients with moderate to severe psoriasis. Dermatol Ther (Heidelb). 2022;12(1):233-41.
89. Nantel-Battista M et al. Treatment of nail psoriasis with intralesional triamcinolone acetonide using a needle-free jet injector: a prospective trial. J Cutan Med Surg. 2014;18(1): 38-42.
90. Bleeker JJ. Intralesional triamcinolone acetonide using the Port-O-Jet and needle injections in localized
dermatoses. Br J Dermatol. 1974;91(1):97-101.
91. Mascaró JM. Epidermoid cyst formation after jet injection of triamcinolone for nail psoriasis. In Sevilla; 2012.
92. Botsali A, Erbil H. Management of nail psoriasis with a single injection of abobotulinum toxin. J Cosmet Dermatol. 2021;20(5):1418-20.
93. Nakamura RC et al. Comparison of nail lacquer clobetasol efficacy at 0.05%, 1% and 8% in nail psoriasis treatment: prospective, controlled and randomized pilot study. An Bras Dermatol. 2012;87(2):203-11.
94. Márquez Balbás G et al. Tacalcitol ointment for the treatment of nail psoriasis. J Dermatolog Treat. 2009;20(5):308-10.
95. Usmani N, Wilson C. A case of nail psoriasis treated with topical calcitriol. Clin Exp Dermatol. 2006;31(5):712-3.
96. Bianchi L et al. Tazarotene 0.1% gel for psoriasis of the fingernails and toenails: an open, prospective study. Br J Dermatol. 2003;149(1):207-9.
97. Scher RK et al. Tazarotene 0.1% gel in the treatment of fingernail psoriasis: a double-blind, randomized, vehicle-controlled study. Cutis. 2001;68(5):355-8.
98. Fiallo P. Yellow nails as an adverse reaction to the topical use of 5-fluorouracil for the treatment of nail psoriasis. J Dermatolog Treat. 2009;20(5):299-301.
99. Fredriksson T. Topically applied fluorouracil in the treatment of psoriatic nails. Arch Dermatol. 1974;110(5):735-6.
100. Yamamoto T et al. Topical anthralin therapy for refractory nail psoriasis. J Dermatol. 1998;25(4):231-3.
101. Lin YK et al. Efficacy and safety of Indigo naturalis extract in oil (Lindioil) in treating nail psoriasis: a randomized, observer-blind, vehicle-controlled trial. Phytomedicine. 2014;21(7):1015-20.
102. Liang CY et al. Successful treatment of pediatric nail psoriasis with periodic pustular eruption using topical indigo naturalis oil extract. Pediatr Dermatol. 2013;30(1):117-9.
103. Lin YK. Indigo naturalis oil extract drops in the treatment of moderate to severe nail psoriasis: a small case series. Arch Dermatol. 2011;147(5):627-9.
104. El-Basiony MAS et al. Long-pulsed nd: YAG laser treatment of nail psoriasis: clinical and ultrasonographic assessment. Arch Dermatol Res. 2024;316(7):365.
105. Roter G et al. Treatment of nail
psoriasis with pulsed dye laser versus combined pulsed dye and Nd:YAG lasers-an intrapatient leftto-right study. Lasers Surg Med. 2022;54(5):688-92.
106. Oram Y et al. Pulsed dye laser in the treatment of nail psoriasis. Dermatol Surg. 2010;36(3):377-81.
107. Peruzzo J et al. Nail psoriasis treated with pulsed dye laser. An Bras Dermatol. 2017;92(6):885-7.
108. Huang Y et al. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: A single‐blind, intrapatient left‐to‐right controlled study. Lasers Surg Med. 2013;45(2):102-7.
109. Goldust M, Raghifar R. Clinical trial study in the treatment of nail psoriasis with pulsed dye laser. J Cosmet Laser Ther. 2013;DOI:10.3109/14764172.201 3.854627.
110. Youssef NY et al. Pulsed dye laser in the treatment of psoriatic nails: a controlled study. J Eur Acad Dermatol Venereol. 2017;31(1):e49-50.
111. Shehadeh W et al. Pulse-dye laser followed by betamethasonecalcipotriol and fractional ablative CO2-laser-assisted delivery for nail psoriasis. Dermatol Surg. 2021;47(4):e111-6.
112. Alakad R et al. Fractional CO2 laserassisted delivery versus intralesional injection of methotrexate in psoriatic nails. Dermatol Surg. 2022;48(5):53944.
113. Essa Abd Elazim N et al. Efficacy of combined fractional carbon dioxide laser and topical tazarotene in nail psoriasis treatment: a randomized intrapatient left‐to‐right study. J Cosmet Dermatol. 2022;21(7):2808-16.
114. Nassar A et al. Comparison of fractional laser‐assisted drug delivery and intralesional injection of triamcinolone acetonide in nail psoriasis. J Dtsch Dermatol Ges. 2022;20(6):788-96.
115. Ortner VK et al. Investigating the efficacy and safety of calcipotriol/ betamethasone dipropionate foam and laser microporation for psoriatic nail disease-A hybrid trial using a smartphone application, optical coherence tomography, and patientreported outcome measures. Dermatol Ther. 2022;35(12):e15965.
116. Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40(7):763-8.
117. Asahina A et al. Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: a randomized, double‐blind, phase 3 study. J Dermatol.
2016;43(8):869-80.
118. Wang N et al. Upadacitinib in nail psoriasis: a case report. J Dermatolog Treat. 2023;34(1):2246604.
119. Hagino T et al. Effectiveness of deucravacitinib for genital, nail and scalp lesions in patients with psoriasis: a 24-week real-world study. Clin Exp Dermatol. 2024;50(1):130-3.
120. Reich K et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebocontrolled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507-17.
121. Ortonne JP et al. A 24-week randomized clinical trial investigating the efficacy and safety of two doses of etanercept in nail psoriasis. Br J Dermatol. 2013;168(5):1080-7.
122. Fabroni C et al. Infliximab efficacy in nail psoriasis. A retrospective study in 48 patients. J Eur Acad Dermatol Venereol. 2011;25(5):549-53.
123. Bianchi L et al. Remission and time of resolution of nail psoriasis during infliximab therapy. J Am Acad Dermatol. 2005;52(4):736-7.
124. Reich K et al. Infliximab induction and maintenance therapy for moderateto-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet Lond Engl. 2005;366(9494):1367-74.
125. Kokolakis G et al. Efficacy of adalimumab for nail psoriasis during 24 months of continuous therapy. Acta Derm Venereol. 2020;100(14):adv00214.
126. Elewski BE et al. Adalimumab for nail psoriasis: efficacy and safety over 52 weeks from a phase‐3, randomized, placebo‐controlled trial. J Eur Acad Dermatol Venereol. 2019;33(11):21682178.
127. Dattola A et al. Certolizumab for the treatment of psoriasis and psoriatic arthritis: a real-world multicentre Italian study. J Eur Acad Dermatol Venereol. 2020;34(12):2839-45.
128. van der Heijde D et al. 4-year results from the RAPID-PsA phase 3 randomised placebo-controlled trial of certolizumab pegol in psoriatic arthritis. RMD Open. 2018;4(1):e000582.
129. Mease P et al. Evaluation of improvement in skin and nail psoriasis in bio-naïve patients with active psoriatic arthritis treated with golimumab: results through week 52 of the GO-VIBRANT study. ACR Open Rheumatol. 2020;2(11):640-7.
130. Kavanaugh A et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection
in psoriatic arthritis: twenty-fourweek efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60(4):976-86.
131. Yang S et al. Toenail psoriasis during ustekinumab therapy: results and limitations. Ann Dermatol. 2021;33(2):131-7.
132. Youn SW et al. Determination of the Nail Psoriasis Severity Index improvement rate standards for nail psoriasis treatment in a phase IV clinical trial of ustekinumab: the MARCOPOLO study. J Eur Acad Dermatol Venereol. 2017;31(6):e298-9.
133. Kristensen LE et al. Effects of risankizumab on nail psoriasis in patients with active psoriatic arthritis: results from KEEPsAKE 1. J Eur Acad Dermatol Venereol. 2022;36(5):e38992.
134. Megna M et al. Risankizumab treatment in psoriasis patients who failed anti-IL17: a 52-week real-life study. Dermatol Ther. 2022;35(7):e15524.
135. Galluzzo M et al. Efficacy of tildrakizumab for the treatment of difficult-to-treat areas: scalp, nail, palmoplantar and genital psoriasis. J Clin Med. 2022;11(9):2631.
136. Brunasso A. Nail psoriasis improvement during tildrakizumab therapy: a real-life experience. J Drugs Dermatol. 2022;21(8):914-6.
137. van de Kerkhof P et al. Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: results from the randomized, controlled and open-label phases of UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31(3):477-82.
138. Reich K et al. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. Br J Dermatol. 2019;181(5):954-66.
139. Nash P et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40(5):952-9.
140. Campione E et al. Fast clinical response of bimekizumab in nail psoriasis: a retrospective multicenter 36-week real-life study. Pharmaceuticals (Basel). 2024;17(10):1378.
141. Hagino T et al. Effectiveness of bimekizumab for genital, nail, and scalp lesions with psoriasis: a 24week real-world study. J Dermatol. 2024;51(12):1658-64.
Authors: *Emi M. Murase,1 Paige E. Adams,2 Jenny E. Murase3,4
1. Department of Biological Sciences, University of California, Davis, USA
2. Rush Medical College, Chicago, Illinois, USA
3. Department of Dermatology, University of California, San Francisco (UCSF), USA
4. Department of Dermatology, Palo Alto Foundation Medical Group, Mountain View, California, USA
*Correspondence to emimaymurase@gmail.com
Disclosure: J.E. Murase serves on the speakers’ board for Galderma, UCB, LeoPharma, Eli Lilly, AbbVie, and Sanofi-Regeneron; has served on advisory boards for UCB, Galderma, Arcutis, Eli Lilly, LeoPharma, Sanofi-Regeneron, and Bristol Myers Squibb; and has provided dermatologic consulting services for UCB, Apogee Therapeutics, Galderma, AbbVie, Attovia, Sanofi-Regeneron, and UpToDate. The other authors have declared no conflicts of interest.
Acknowledgements: E.M. Murase and Adams share co-first authorship for this work. Written informed consent was obtained from the patient for the publication of this case report.
Received: 02.07.25
Accepted: 16.09.25
Keywords: Case report, cutaneous lupus erythematosus (CLE), deucravacitinib, lupus chilblains, systemic lupus erythematosus (SLE).
Citation: EMJ Dermatol. 2025;13[1]:135-139. https://doi.org/10.33590/emjdermatol/ZOFM4548
Abstract
Cutaneous lupus erythematosus (CLE) encompasses a group of autoimmune skin disorders that may occur independently or in association with systemic lupus erythematosus. Deucravacitinib, a selective tyrosine kinase 2 inhibitor approved for plaque psoriasis, is currently under investigation in clinical trials for the treatment of systemic lupus erythematosus. This article presents a case of lupus chilblains, a rare subtype of CLE, successfully treated with deucravacitinib, and reviews the existing literature surrounding its use in lupus. The patient is a 72-year-old woman with a history of chronic toe pernio who presented with painful, erythematous ulcerations on their fingertips with associated onycholysis. Serologic studies were negative for autoimmune markers, and a prior biopsy showed vacuolar interface dermatitis. The patient failed multiple therapies including topical clobetasol, nifedipine, doxycycline, and hydroxychloroquine. The initiation of daily deucravacitinib monotherapy resulted in marked clinical improvement within 4 weeks and sustained remission over the subsequent 2 years. This case highlights the potential role of deucravacitinib in refractory chilblain lupus and contributes to the emerging evidence to support its efficacy in CLE.
1. Deucravacitinib, a selective tyrosine kinase 2 inhibitor, shows promising efficacy in the treatment of refractory chilblain lupus, a rare subtype of cutaneous lupus erythematosus (CLE), offering a potential therapeutic option for patients unresponsive to conventional therapies.
2. The patient experienced significant clinical improvement within 4 weeks of initiating deucravacitinib monotherapy, with sustained remission over 2 years, demonstrating the drug's potential for rapid symptom control and long-term disease management in CLE.
3. This report contributes to the growing body of literature supporting deucravacitinib's efficacy across CLE subtypes and suggests that further studies are warranted to establish its role as a targeted therapy in autoimmune skin diseases beyond psoriasis.
Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects multiple organ systems, with a strong predilection for women of childbearing age. This female predominance is thought to be influenced by hormonal factors, including the immunomodulatory effects of oestrogen, which may contribute to heightened autoimmune susceptibility. Cutaneous manifestations occur in up to 85% of patients with SLE.1 Conversely, cutaneous lupus erythematosus (CLE) is defined by disease limited to the skin without systemic involvement, and can occur as an isolated entity or as the initial manifestation of SLE. Chilblain lupus is a rare, chronic subtype of CLE characterised by painful, violaceous nodules with central ulceration, which are often triggered or exacerbated by cold exposure.2 Deucravacitinib is a selective tyrosine kinase 2 (TYK2) inhibitor approved for moderate-to-severe plaque psoriasis with ongoing clinical trials for SLE.3-5 Current data from both clinical trials and case reports in the literature suggest that deucravacitinib holds promise as an emerging therapeutic option in SLE and CLE. This article describes a case of lupus chilblains treated with deucravacitinib and reviews the promising role of deucravacitinib in CLE and SLE.
A 72-year-old woman with a past medical history of hypertension, asthma, coronary artery disease, prediabetes, and pernio of the toes presented to the dermatologist with painful, erythematous-to-violaceous acral lesions with tender fingertip ulcerations and onycholysis. The patient denied associated joint pain, fatigue, myalgia, or photosensitivity.
The patient underwent an extensive diagnostic work-up over the course of 6 years. Complete blood count revealed mild anaemia and baseline thrombocytosis. Autoimmune serologic studies revealed negative antinuclear, anti-double-stranded DNA, anti-ribosomal P, anti-topoisomerase I, thyroid peroxidase, anti-Smith, antiribonucleoprotein, anti-Sjögren’s-syndromerelated antigen A, anti-Sjögren’s syndrome B, and myositis antibody panels, as well as negative rheumatoid factor and normal complements (C3/C4). Serum protein electrophoresis and immunofixation were normal. A prior skin biopsy of the toes showed vacuolar interface dermatitis.
The patient had minimal relief with a range of topical therapies in the past, including clobetasol, mupirocin, nitroglycerin, betamethasone, and minoxidil. Systemic therapies such as nifedipine, doxycycline, and hydroxychloroquine were ineffective, and the patient was unable to tolerate pentoxifylline therapy due to adverse gastrointestinal effects.
Table 1: Documented cases of cutaneous lupus erythematosus successfully treated with deucravacitinib.
1 65-year-old female DLE with concomitant palmoplantar pustular psoriasis
Hydroxychloroquine 200 mg BID
2 66-year-old female Concomitant SCLE and DLE Topical ruxolitinib cream
3 56-year-old female SCLE with overlapping features of Sjögren’s disease
4 51-year-old female SCLE
5 51-year-old male Tumid lupus erythematosus
6 74-year-old male CLE
7 51-year-old male DLE
8 48-year-old female DLE scarring alopecia
9 57-year-old female SCLE with concomitant psoriasis vulgaris
Hydroxychloroquine
200 mg BID, prednisone 5 mg QD
None
Chloroquine 250 mg QD
None
None
Hydroxychloroquine
200 mg daily 5 days/ week, 200 mg BID 2 days/week
None
Complete remission after 1 month
Improvement with PIH after 5 months; complete remission after 8 months
Improvement in disease severity after 4 weeks
50% improvement after 8 weeks; near clearance after 4 months
Ezeh et al.8
None Matthew et al.9
None Kurz et al.10
None Bouché et al.11
Significant improvement after 3 months None Zhang et al.12
Significant improvement with mild PIH after 2 months None Hren et al.13
Mild improvement after 1 month; complete resolution with PIH after 11 months
Symptomatic improvement after 3 months; significant hair regrowth and reduction in plaque size after 6 months
Resolution of skin changes with residual psoriatic nail changes after 4 months; sustained disease control with one stable psoriatic plaque after 7 months
None Hren et al.13
None Aw and Gavigan14
None Assaf et al.15
BID: twice daily; CLE: cutaneous lupus erythematosus; DLE: discoid lupus erythematosus; PIH: post-inflammatory hyperpigmentation; QD: once daily; SCLE: subacute cutaneous lupus erythematosus.
Development of onychodystrophy prompted potassium hydroxide testing of the nails, which revealed fungal hyphae. The patient was prescribed oral fluconazole 100 mg daily for the first 7 days of each month, daily vinegar soaks, and topical tacrolimus on weekdays and clobetasol on weekends. The patient’s condition remained refractory, and the ulcerations on their hands interfered with activities of daily living.
At initial dermatologic consultation, bacterial cultures of the fingertips grew Pseudomonas aeruginosa and
Serratia marcescens. A 10-day course of ciprofloxacin 500 mg twice daily provided modest improvement. The patient was started on a trial of deucravacitinib 6 mg daily. Within 4 weeks, the ulcerations on their fingertips fully re-epithelialised with marked improvement of onychodystrophy. At this point, the patient reported a 90% reduction in associated pain and swelling of the fingers. They remained on deucravacitinib for the subsequent 2 years without ulcer recurrence, pain, or adverse effects. The patient’s quality of life improved substantially, and no further complications were noted.
Lupus chilblains, also known as pernio, is a chronic inflammatory skin condition characterised by painful, erythematous, or violaceous lesions typically affecting acral sites including the toes, fingers, and ears.2 It is thought to be an abnormal vascular response to cold, leading to localised vasculitis. Lupus chilblains can occur sporadically or secondary to autoimmune conditions like SLE. Like other forms of connective tissue diseases, lupus chilblains is seen more frequently in women, likely due to the complex interplay between sex hormones, immune regulation, and vascular reactivity. Management is often challenging, and first-line management focuses on lifestyle modifications such as strict avoidance of cold exposure, photoprotection, and smoking cessation. Topical corticosteroids and systemic vasodilators such as nifedipine can provide symptomatic relief by reducing inflammation and improving peripheral circulation. Hydroxychloroquine is frequently used to treat chronic or autoimmune-related lupus chilblains, though outcomes are variable.6 Patients may see some improvement with these therapies, but relapse rates remain high.
Deucravacitinib, an oral, selective TYK2 inhibitor, is approved for the treatment of moderate-to-severe plaque psoriasis. TYK2 is involved in the regulation of IL-12, IL-23, and Type 1 interferons. Deucravacitinib is currently under investigation for the treatment of CLE and SLE. In the Phase II PAISLEY trial on adults with active SLE, deucravacitinib treatment resulted in higher response rates on the SLE Responder Index 4 (SRI4) compared to placebo, with a favourable side-effect profile.3-5 In addition to this, in a recent systematic review, deucravacitinib demonstrated superior efficacy and safety
References
1. Stull C et al. Cutaneous involvement in systemic lupus erythematous: a review for the rheumatologist. J Rheumatol. 2023;50(1):27-35.
with fewer side effects compared to anifrolumab in CLE.7
To date, nine cases of treatmentresistant CLE successfully treated with deucravacitinib have been documented in the literature Table 1, with most cases reported in female patients.8-15 Four cases describe the efficacy of deucravacitinib as adjunctive therapy to concomitant hydroxychloroquine or chloroquine, while five cases demonstrated significant clinical improvement with deucravacitinib as systemic monotherapy. In all cases, patients achieved rapid symptomatic relief and either complete clearance or minimal residual disease within a year of initiating deucravacitinib. The drug was well-tolerated across reports, with one case noting a mild inflammatory acneiform eruption that was managed with topical clindamycin.8 The literature indicates efficacy for multiple CLE subtypes, including discoid and tumid lupus erythematosus.8,9,12-14 Two cases, presented in Table 1 as Case 1 and Case 3, underscore the utility of deucravacitinib in patients with CLE with concurrent psoriatic disease, reinforcing its FDA-approved indication.8,15 The growing number of cases reporting successful use of deucravacitinib in treatment-refractory cutaneous lupus support its potential as an effective option for refractory cases.
To the authors’ knowledge, this article reports the first case of recalcitrant lupus chilblains successfully treated with deucravacitinib monotherapy. This case adds to the emerging literature on the promising use of deucravacitinib in CLE and highlights its potential as a targeted therapy for chilblain lupus, a rare and difficult-to-treat subtype of CLE. Further prospective studies are warranted to assess the long-term efficacy and safety of deucravacitinib in CLE.
2. Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10(6):365-81.
3. Morand EF et al. Plain language summary of the PAISLEY study: deucravacitinib as a treatment for people with systemic lupus erythematosus. Expert Opin Investig Drugs. 2024;33(5):431-40.
4. Morand E et al. Deucravacitinib, a tyrosine kinase 2 inhibitor, in systemic
lupus erythematosus: a phase II, randomized, double-blind, placebocontrolled trial. Arthritis Rheumatol. 2023;75(2):242-52.
5. Merola JF et al. Efficacy and safety of oral deucravacitinib in patients with cutaneous manifestations of lupus erythematosus: results from PAISLEY CLE, a global, randomized, placebocontrolled, phase 2 trial. Ann Rheum Dis. 2025;84(Suppl 1):940-1.
6. Chasset F et al. Efficacy and comparison of antimalarials in cutaneous lupus erythematosus subtypes: a systematic review and meta-analysis. Br J Dermatol. 2017;177(1):188-96.
7. Bokor LA et al. Deucravacitinib shows superior efficacy and safety in cutaneous lupus erythematosus compared to various biologics and small molecules - a systematic review and meta-analysis. Autoimmun Rev. 2025;24(3):103723.
8. Ezeh N et al. Discoid lupus erythematosus successfully treated with deucravacitinib. JAAD Case Rep. 2024;49:59-61.
9. Matthew EJ et al. Oral deucravacitinib for treatment-refractory cutaneous lupus erythematosus. Cureus. 2025;17(3):e81539.
10. Kurz B et al. Rapid clinical improvement of refractory subacute cutaneous lupus erythematosus with oral tyrosine kinase 2 inhibitor deucravacitinib: a case report. J Eur Acad Dermatol Venereol. 2024;38(5):e434-6.
11. Bouché N et al. Successful treatment of refractory subacute cutaneous lupus erythematosus with deucravacitinib. JAAD Case Rep. 2023;39:93-5.
12. Zhang A et al. Treatment of recalcitrant lupus erythematosus tumidus with deucravacitinib. JAAD Case Rep. 2023;45:110-2.
13. Hren GM et al. Use of deucravacitinib in cutaneous lupus erythematosus: two patient experiences at a combined rheumatology-dermatology clinic. Int J Dermatol. 2025;DOI:10.1111/ijd.17817.
14. Aw K, Gavigan G. Treatment of discoid lupus erythematosus scarring alopecia with deucravacitinib: a case report. SAGE Open Med Case Rep. 2025;13:2050313X241304891.
15. Assaf K et al. Successful treatment of subacute cutaneous lupus erythematosus with the TYK-2 inhibitor deucravacitinib in a patient with concomitant psoriasis vulgaris. J Dtsch Dermatol Ges. 2025;23(7): 874-7.
Authors: Juan He,1,2 *Yi Yang2
1. School of Medicine, Nankai University, Tianjin, China
2. Department of Dermatology, The First Medical Center of Chinese People's Liberation Army (PLA) General Hospital, Beijing, China *Correspondence to sydyy@163.com
Disclosure: The authors have declared no conflicts of interest.
Acknowledgements: AI was used to aid in the improvement of article fluency and formatting.
Received: 05.07.25
Accepted: 23.09.25
Keywords: Clinical feature, nail lichen planus (NLP), treatment outcomes.
Citation: EMJ Dermatol. 2025;13[1]:140-151. https://doi.org/10.33590/emjdermatol/GGVF8668
Abstract
Nail lichen planus (NLP) is a chronic inflammatory disorder that can lead to irreversible damage, such as pterygium and anonychia. While NLP may initially present with subtle nail matrix changes, its unpredictable course and potential for permanent dystrophy highlight the need for early recognition and timely intervention. However, the evidence guiding NLP management remains limited, with most recommendations based on case reports and retrospective studies. This review summarises the current understanding of NLP, including recent insights into its pathogenesis, epidemiology, clinical features, diagnosis, and therapeutic strategies. Topical treatments alone have limited efficacy due to poor drug penetration and are not recommended as monotherapy. Intralesional corticosteroids remain the mainstay of treatment and should be considered early. Systemic corticosteroids, either via intramuscular injection or oral administration, can be considered in severe NLP, but longterm use may be limited by relapse and adverse effects. Oral retinoids, particularly alitretinoin, offer moderate benefit in mild or early-stage disease. Immunosuppressive agents may be reserved for refractory cases. Notably, while most recent findings regarding the JAK-signal transducer and activator of transcription pathway have emerged from studies on cutaneous or mucosal lichen planus, early reports of successful treatment in NLP using JAK inhibitors offer promising new insights for this challenging condition.
1. Nail lichen planus (NLP) is a rare but potentially disfiguring nail disorder that can lead to permanent nail damage. This review provides a comprehensive synthesis of current knowledge on NLP, addressing critical gaps in diagnosis, classification, and treatment strategies, based on recent clinical insights and expert consensus.
2. This review outlines the clinical, histopathological, and immunopathogenic features of NLP and introduces a newly proposed clinical severity grading system (Typical Nail Lichen Planus Severity Index [tNLPSI]). It also compares available treatment options, from topical therapies to systemic immunosuppressants and JAK inhibitors, emphasising both practical application and emerging trends.
3. Timely recognition and intervention are crucial in preventing permanent nail damage in NLP. Intralesional corticosteroids remain a cornerstone of early treatment, while systemic options, such as intramuscular or oral corticosteroids, may be effective in more extensive disease, but are limited by relapse and adverse events. Retinoids offer modest benefit in milder cases. Encouragingly, emerging evidence from lichen planus research suggests that JAK inhibitors may represent a promising therapeutic option in difficult-to-treat NLP.
Lichen planus (LP) is a chronic, immunemediated inflammatory disorder that affects the skin, mucous membranes, hair, and nails.1 Among these, nail lichen planus (NLP) is an uncommon but potentially destructive variant that may lead to permanent nail deformities, such as pterygium and anonychia.2 NLP can occur in isolation or alongside other LP subtypes, and its clinical spectrum ranges from subtle longitudinal ridging to complete nail plate loss, often mimicking other nail disorders and resulting in delayed diagnosis.3 Although NLP has gained increasing recognition in recent years, high-quality data remain limited. Most literature consists of small cohorts, case reports, or expert consensus, with limited representation across ethnicities and long-term follow-up.2 Diagnostic challenges persist due to the non-specific clinical features of NLP, which may overlap with other dystrophic nail conditions.4 Despite a range of available treatments, from topical corticosteroids to systemic immunosuppressants, managing NLP remains challenging due to its recurrent nature.2 Recent developments, including insights into the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway and the emerging use of JAK inhibitors, offer promising new therapeutic avenues for the management of NLP.5 This review aims to summarise the current understanding of NLP, encompassing its pathogenesis, epidemiology, clinical and histopathological features, diagnostic approaches, and evolving therapeutic strategies, to guide clinicians and inform future research directions.
A comprehensive literature search was conducted to identify peer-reviewed articles on LP, with a specific focus on isolated NLP. Searches were performed using the PubMed/MEDLINE and Web of Science databases for articles published up to January 2025. The following search terms were used alone or in combination: “lichen planus,” “nail lichen planus,” “isolated nail lichen planus,” “onycholichen planus,” and “nail dystrophy.” Reference lists from key articles and relevant reviews were also manually screened to identify additional studies. Only studies published in English were included. The review aimed to synthesise the current evidence base and identify recent advancements in understanding and managing NLP.
LP, including NLP, is considered a multifactorial inflammatory disorder, involving a complex interplay of genetic susceptibility, environmental triggers, and immune dysregulation.1 Genetic predisposition has been proposed based on familial cases and certain human leukocyte antigen (HLA) associations, such as HLA-A3, HLA-A5, HLA-A28, HLA-B8, HLA-B16, HLA-Bw35, HLA-B7, HLA-B18, HLA-Aw19, and HLA-Cw8, though specific genes remain undefined.6 Environmental factors, including drug exposure (e.g., antimalarial and antihypertensive agents),7 metal allergy (especially nickel and amalgam),8 and viral infections such as human papillomavirus,9 human herpesvirus,10 and hepatitis C virus,11 as well as, in rare cases, hepatitis B vaccination,11 have been reported as potential triggers in susceptible individuals. There is evidence that cellmediated immune response plays a major
role in the development of the disease.12 Current understanding indicates that the immunopathogenesis of LP is primarily driven by CD8+ cytotoxic T lymphocytes and CD45RO+ T cells, with their activity being modulated by the Th1 and IL-23/ Th17 axes.12 Shao et al.13 demonstrated that CD8+ cytotoxic T cells induce keratinocyte apoptosis, possibly triggered by overexpression of major histocompatibility complex Class I molecules on keratinocytes, and interferon-γ plays a pivotal role in this process by upregulating major histocompatibility complex expression and activating the JAK2-STAT1 pathway. Inhibition of the JAK-STAT pathway has shown therapeutic efficacy in different subtypes of LP.5 Taken together, LP is increasingly understood as a complex immune-mediated condition involving coordinated actions of cytotoxic T cells, helper T cell subsets, cytokines, and innate immune elements.
LP affects approximately 0.5–1.0% of the general population, with nail involvement reported in 10.0–15.0% of cases.14 NLP may occur in isolation or alongside cutaneous or mucosal LP, and although it can affect individuals of any age, it most commonly presents during the fifth and sixth decades of life.4 NLP appears to be slightly more prevalent in males, though some studies have shown no significant sex predilection.4,15 Adult patients are more frequently affected than children, in whom NLP remains relatively rare and often underdiagnosed.16 Fingernails are more frequently involved than toenails, with isolated toenail involvement being distinctly uncommon. Involvement may be asymmetric or symmetric, and while single-nail presentations do occur, multi-nail dystrophy is more typical in moderate-tosevere disease.
NLP presents with a broad spectrum of changes affecting the nail matrix, nail bed, and nail folds (Figure 1), with nail matrix
involvement being the most predominant and diagnostically significant feature (Table 1).2,17 Common matrix-associated findings include longitudinal ridging, splitting, onychoschizia, trachyonychia, thinning of the nails, red or ‘mottled’ lunula, longitudinal melanonychia, and pterygium.15 Matrixrelated changes were observed in 97.53% of patients in the authors’ unpublished cohort, consistent with prior reports of approximately 91.00% by Goettmann et al.,4 underscoring its central role in NLP pathogenesis. Among nail matrix involvement symptoms, longitudinal ridging (69.14%), splitting (48.15%), and nail plate thinning (43.21%) were some the most common findings in the authors’ cohort. Erythronychia, or red lunula, was observed in 29.63–31.30% of patients, which can be histopathologically correlated with distal nail matrix inflammation. Longitudinal melanonychia, while less frequent, is observed more often in individuals with skin of colour.18 Dorsal pterygium, defined as an irreversible alteration secondary to chronic inflammation-induced matrix scarring, was observed in 7.41–17.90% of cases. Interestingly, the occurrence of pterygium didn’t correlate with the duration of the disease.4 Changes in the nail bed may cause onycholysis, subungual hyperkeratosis, and splinter haemorrhages,15 which were documented in 29.63–43.30% of patients.4 Inflammation of the nail fold can result in periungual hyperkeratosis, erythematous lesions, and scaling of the proximal nail fold, which were identified in 17.28–18.00% of patients. Besides, an acute onset of pain may be indicative of bullous NLP.19
In most cases, the diagnosis of NLP is primarily clinical, guided by the recognition of characteristic patterns of nail matrix and nail bed changes, as the authors mentioned above. A detailed history and physical examination of other mucocutaneous sites should be performed to identify associated skin, oral, or genital LP, though NLP may occur in isolation. Dermoscopy serves as a useful adjunctive tool in identifying subtle features of NLP. For instance, NLP may manifest as linear discolouration of
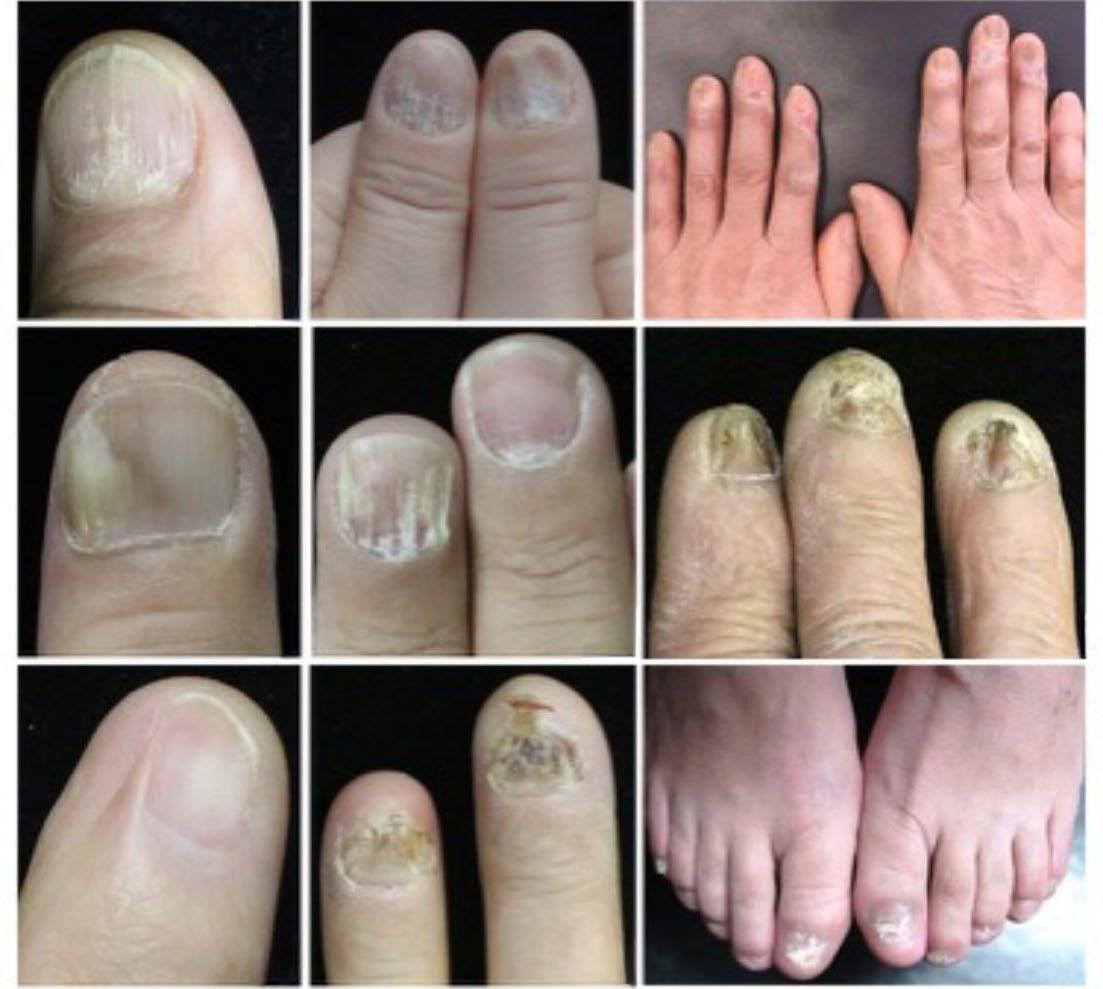
A) and B) Longitudinal ridging and splitting are among the most common features of matrix involvement in NLP. C) A representative case of typical NLP showing nail plate thinning, prominent longitudinal melanonychia, and periungual erythema with scaling. D) and E) Nail bed involvement characterised by distal onycholysis. F) Early stage of dorsal pterygium formation with adhesion of the proximal nail fold to the matrix, and partial loss of nail plate continuity.
G) Fully developed dorsal pterygium presenting as a V-shaped extension of the proximal nail fold onto the nail bed, replacing the nail plate. H) and I) Atrophic NLP with severe nail plate thinning, scarring, and permanent anonychia, reflecting irreversible damage in advanced stages of the disease.
NLP: nail lichen planus.
the nail bed, displaying alternating hues of blue, brown, red, or white while sparing the lunula. Although these subtle colour bands are often imperceptible to the naked eye, they can be readily detected using contact dermoscopy with ultrasound gel.20 Characteristic dermoscopic findings include longitudinal striae, red lunula, and trachyonychia-like surface roughness.21 However, dermoscopy remains underutilised in routine practice and may not replace histologic confirmation in uncertain presentations. Histopathological examination remains the gold standard
for definitive diagnosis of NLP.22 The classic histologic presentations include hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis with band-like lymphocytic infiltrate at the dermoepidermal junction. Basal layer degeneration and Civatte bodies may also be observed.23 However, these histologic features may not be evident in more chronic NLP, which presents with nail matrix hypergranulosis and thin/atrophic nail unit epithelium.24 Longitudinal nail biopsies, particularly those that include both the nail matrix and ventral proximal nail fold, are recommended
to ensure sufficient sampling. However, the risk of post-biopsy nail dystrophy necessitates careful patient selection and procedural planning.2
Differential diagnoses of NLP include onychomycosis, psoriasis, trachyonychia, alopecia areata-associated nail changes, and traumatic nail dystrophy.2 Fungal stains and cultures, along with histology, are crucial in distinguishing these entities. To support clinical assessment and standardised outcome measurement, the authors’ group recently proposed the Typical Nail Lichen Planus Severity Index (tNLPSI), a grading tool specifically designed for classical NLP.25 The tNLPSI evaluates both disease activity and irreversible damage by scoring key features of the nail matrix, nail bed, and nail fold. The
tNLPSI activity scale was demonstrated to be consistent, reliable, reproducible, and feasible. It may prove to be a valuable tool in evaluating the treatment response in typical NLP clinical trials. Further validation and longitudinal testing are essential to establish the robustness and reliability of the tNLPSI scale.
Due to the unpredictable nature of NLP progression, early therapeutic management is essential.26 Despite this clinical necessity, the current research landscape on NLP remains limited and fragmented. Most existing evidence stems from isolated case reports and retrospective studies (Table 2), with a notable lack of highquality, prospective, and randomised controlled trials. Furthermore, the available research is characterised by considerable heterogeneity in treatment regimens, outcome measures, and follow-up durations, thereby reducing the comparability and generalisability of findings. This variability presents a significant barrier to the development of standardised, evidence-based treatment protocols.
Based on the published literature and combined with the authors’ clinical observations, the authors found that topical therapies are largely ineffective for NLP, primarily due to the limited drug penetration. In contrast, localised interventions such as intralesional triamcinolone acetonide (TAC) injections, as well as systemic treatments including intramuscular (IM) and oral corticosteroids, have demonstrated more reliable therapeutic efficacy. However, these conventional approaches are frequently associated with high relapse rates following treatment cessation. Notably, emerging therapies, such as JAK inhibitors, represent a promising advancement, offering new hope for patients with refractory NLP by targeting key inflammatory pathways involved in disease pathogenesis.
Overall, topical treatments are not recommended as monotherapy therapy for NLP due to their limited drug penetration and the potential adverse effects associated with prolonged use. Both nail matrix and nail bed involvement in NLP typically respond poorly to topical agents, resulting in minimal clinical improvement. However, in cases where the periungual tissue is affected, particularly with erythema and scaling of the proximal nail fold, topical corticosteroids and topical calcineurin inhibitors, such as tacrolimus, may offer a clear therapeutic benefit by reducing local inflammation.
As outlined in the expert consensus by Iorizzo et al.,26 intralesional TAC injection is regarded as a first-line therapy for NLP. This approach allows for localised drug administration directly to the target tissue, offering enhanced therapeutic concentrations with reduced systemic exposure. While an optimal dosing regimen has not been standardised, TAC is most frequently used at concentrations of 2.5, 5.0, or 10.0 mg/mL. Injections are typically administered every 4–6 weeks, and clinical response is evaluated after a minimum of 4–6 months of continuous treatment. If significant improvement is observed, injection intervals may be extended to every 6–8 weeks. In contrast, a lack of response after six sessions should prompt reconsideration of the therapeutic approach.
However, patient discomfort during injections remains a common challenge. To enhance tolerability, various anaesthetic strategies can be employed. These include the pre-application of topical lidocaine cream 60 minutes before injection and the use of ethyl chloride spray.49 Distraction techniques, such as ‘talkesthesia’, vibratory devices, and percussive methods, have also proven helpful.49 For nail-bed involvement, digital nerve block anaesthesia is typically necessary.26 Although dermal atrophy is relatively rare, transient adverse effects such as pain, haematoma formation, and temporary distal numbness are
Topical therapy
Clobetasol propionate
Topical
0.05%+tazarotene 0.10% twice/ day with occlusion
Clobetasol propionate
Topical
0.05% once/day
Tacrolimus
Topical 0.10% ointment twice/day
Prevost and English27
Goettmann et al.4
Ujiie et al.28
Intralesional corticosteroid injections
Triamcinolone acetonide
Intralesional
10 mg/mL a month
Triamcinolone acetonide
Intralesional
5 mg/mL a month
Triamcinolone acetonide
Intralesional 5 mg/mL a month
Triamcinolone acetonide
Intralesional 10 mg/mL a month
Triamcinolone acetonide
Intralesional 10 mg/mL a month
Triamcinolone acetonide
Intralesional 10 mg/mL a month
Triamcinolone acetonide
Intralesional 5 mg/mL a month
Gerstein29
Abell and Samman30
Tosti et al.24
Grover et al.31
Piraccini et al.32
Brauns et al.33
Goettmann et al.4
Intramuscular corticosteroid injections
Triamcinolone acetonide
Intramuscular (to taper)
0.5 mg/kg a month
Triamcinolone acetonide
Intramuscular
0.5–1.0 mg/kg a month
Triamcinolone acetonide
Intramuscular (to taper)
0.5 mg/kg a month
Triamcinolone acetonide
Intramuscular
0.5–1.0 mg/kg a month
Tosti et al.24
Tosti et al.16
Piraccini et al.32
Goettmann et al.4
• 1 female aged 72 years
• All fingernails
• 2 males, 4 females, aged 8–69 years
• Multiple finger/toenails
• 4 males, 1 female, aged 11–58 years
• Multiple finger/toenails
• Cured after 7 months
• Recurrence after discontinuation
• Five improved considerably
• One slow amelioration
• Recurrence after discontinuation
• Improved after 6 months
• 3 males/NK age
• Multiple finger/toenails
• 9 males, 2 females, aged 7–63 years
• Multiple finger/toenails
• 4 patients (NK age/sex)
• Few fingernails
• 7 patients (NK age/sex)
• Multiple finger/toenails
• 3 males, 5 females, aged 10–78 years
• Multiple finger/toenails
• 1 female aged 52 years
• Multiple fingernails
• 13 males, 2 females, aged 6–68 years
• Multiple finger/toenails
• 2 patients (NK age/sex)
• Multiple finger/toenails
• 7 males, 3 females, aged 6–12 years
• Multiple finger/toenails
• 36 males, 31 females, aged 7–80 years
• Multiple finger/toenails
• 6 males, 30 females, aged 9–72 years
• Multiple finger/toenails
• 2/3 improved after 2 months
• Recurrence after discont. (1 pts)
• 7 cured
• Recurrence after discont. (1 pts)
• Cured after 3 months
• 4/7 cured after 6 months
• Recurrence after discontinuation
• Cured after 4–7 months (seven pts)
• Cured after 4 months
• Recurrences after 2 years
• 3 cured, 6 improved considerably
• 2 slow ameliorations
• Recurrence after discontinuation
• Cured after 3 months
• Cured after 6–9 months (9 pts)
• Recurrence after discont. (2 pts)
• Cured after 4 months (44 pts)
• Improved at 4 months (9 pts)
• Recurrence after discont. (11 pts)
• 11 cured, 15 improved considerably
• 4 slow ameliorations
• Recurrence after discontinuation
Oral corticosteroids
Prednisone
Oral 0.5 mg/kg (to taper)
Prednisolone
Oral 20 mg/day (to taper)
Prednisolone
Oral 40 mg/day (to taper)
Prednisone
Oral 0.5 mg/kg (to taper)
Oral retinoids
Etretinate
Oral 30 mg/day (to taper)
Alitretinoin
Oral 30 mg/day (to taper)
Alitretinoin
Oral 30 mg/day (to taper)
Alitretinoin
Oral 30 mg/day (to taper)
Tosti et al.24
Takeuchi34
Evans et al.35
Goettmann et al.4
Kato and Ueno36
Pinter et al.37
Iorizzo38
Alsenaid et al.39
Other systemic immunosuppressants
Methotrexate
Subcutaneous 10–20 mg/week
Cyclosporine
Oral 100 mg twice daily for 10 months
Advanced therapies
Etanercept
Subcutaneous 25 mg twice/ week to 50 mg/week
Tofacitinib
Oral 5 mg BID
Baricitinib
Oral 4 mg QD (to taper)
Tofacitinib
Oral 5 mg BID
Baricitinib
Oral 4 mg QD (to taper)
Abrocitinib
Oral 100 mg QD (to taper)
Manousaridis et al.40
Florian et al.41
Irla et al.42
Iorizzo and Haneke43
Pünchera and Laffitte44
Huang and Shi45
He et al.46
He and Yang47
• 15 patients (NK age/sex)
• Multiple finger/toenails
• 57 males
• 20 nails
• 57 males
• Multiple toenails
• 8 males, 2 females, aged 18–71 years
• Multiple finger/toenails
• 46 males
• All fingernails and four toenails
• 43 males
• All fingernails
• 2 males aged 50 and 56 years, 1 female aged 42 years
• Multiple finger/toenails
• 1 male aged 50 years, 1 female aged 40 years
• 20 nails
• 2 patients (NK sex/age)
• NK affected nails
• 1 female aged 39 years
• 20 nails
• 1 female aged 53 years
• Multiple finger/toenails
• 1 female aged 57 years
• All fingernails
• 1 female aged 60 years
• Multiple fingernails
• 1 female aged 41 years
• All fingernails
• 1 male aged 30 years
• Multiple fingernails
• 1 female aged 39 years
• Multiple fingernails
• Improved after 0.5–1.5 months
• Cured after 3–6 months
• Recurrence after 2 years (8 pts)
• Improved after 3 months
• Cured despite treatment suspension after
• Improved after 1 month
• 1 cured, one improved considerably
• 1 slow amelioration
• Recurrence after discontinuation
• Cured after 12 months
• Cured after 6 months
• Improved after 3 months
• Cured in 6 months with 10 mg/day
• Cured after 8–9 months
• Improved after a few weeks
• Improvement after 10 months
• Recurrence after discontinuation
• Marked improvement after 6–9 months
• Improved after 6 months
• Cured in 6 months
• Recurrence with dose reduction
• Improved after 6 months
• Cured in 6 months
• Improved after 6 months
BID: twice daily; discont: discontinued; NK: not known; pts: patients; QD: once daily.
more frequently reported.50 Despite these limitations, when administered with appropriate technique, intralesional injections remain a highly effective therapeutic option in the management of NLP.50
IM triamcinolone offers a practical and effective therapeutic option for patients with NLP, particularly in cases involving multiple nails.51 TAC is the most commonly used agent, typically administered at a dose of 0.5–1.0 mg/kg every 4 weeks for a duration of at least 3–6 months. Doses of up to 1 mg/kg daily warrant consideration in patients with active or rapidly progressive disease.26 Iorizzo et al.26 recommend IM corticosteroids as an adjunct to intralesional administration in case of severe disease, especially when more than three nails are involved. Nonetheless, systemic therapy warrants consideration even with limited nail involvement when digitally critical nails (thumb, index, and middle fingers) exhibit clinically significant functional impairment.2 Besides, IM therapy is necessary and useful when intralesional injections are not feasible, whether due to anatomical challenges, physician inexperience, or patient discomfort.26 However, clinicians must remain mindful of potential contraindications, including uncontrolled diabetes, severe osteoporosis, active infections, peptic ulcer disease, and unstable psychiatric conditions.52
Oral corticosteroids may also serve as an adjunctive option in the treatment of NLP. The indications for oral corticosteroid therapy are generally the same as those of IM use, particularly in cases involving extensive nail disease or functional impairment. While oral corticosteroids can achieve similar systemic effects, their longterm administration is often limited by a higher risk of cumulative side-effects, such as metabolic disturbances, mood changes, and gastrointestinal complications.52 Compared to oral administration, IM corticosteroid injections provide a more controlled and sustained drug release, potentially improving adherence and minimising daily fluctuations in serum drug levels. Iorizzo et al.26 have expressed a preference for IM corticosteroids over oral routes in NLP. Nonetheless, in certain cases, particularly when rapid dose adjustments are needed, or where oral administration allows for greater flexibility and physician control over tapering, oral corticosteroids may offer practical advantages over IM injections. Therefore, a carefully monitored course of oral corticosteroids remains a viable option, especially when IM delivery is not feasible or preferred by the patient.
For patients who decline systemic corticosteroids, oral retinoids represent a viable alternative for NLP, particularly in mild-to-moderate cases. Acitretin, typically administered at 0.2–0.3 mg/kg/ day, is known to be effective in patients with cutaneous LP,53 which functions by regulating keratinocyte proliferation and
differentiation. Alitretinoin, used at a dose of 30 mg/day, offers several advantages over acitretin, including fewer side-effects, greater anti-inflammatory properties, and better regulation of keratinocyte differentiation and proliferation.54 In clinical practice, retinoid therapy is generally continued for several months, with gradual tapering considered upon achieving significant clinical remission. If no meaningful improvement is observed within 6 months, alternative treatments should be explored. Despite their efficacy, retinoids are associated with notable adverse effects, such as skin dryness, lip inflammation, hyperlipidaemia, hepatotoxicity, and teratogenicity, necessitating close patient monitoring.53
Immunosuppressive agents such as azathioprine (100 mg/day), cyclosporine (3–5 mg/kg/day), and mycophenolate mofetil (1,000 mg twice daily) have been proposed as third-line options for NLP, particularly in patients who show inadequate response to first-line therapies.40 However, these agents are typically reserved for refractory cases due to their systemic immunosuppressive effects and limited supporting evidence. Clinical experience from both the authors’ team and the Iorizzo et al.26 group suggests that hydroxychloroquine and methotrexate, though commonly used in other inflammatory dermatoses, appear to lack therapeutic benefit in NLP and are therefore not recommended.
Recent advances in targeted immunotherapy have introduced JAK inhibitors as promising agents in the management of NLP,43-48 particularly in patients with recalcitrant or isolated nail involvement unresponsive to conventional therapies. The rationale for their use is
grounded in the immunopathogenesis of LP, in which interferon-γ-driven cytotoxic CD8+ T cell responses are believed to play a central role.12 These T cells target basal keratinocytes via the JAK-STAT signalling axis, triggering apoptosis and interface dermatitis.13 JAK inhibitors disrupt this signalling cascade, thereby reducing pro-inflammatory cytokine production, inhibiting T cell activation, and suppressing keratinocyte damage. Several case reports have supported the potential efficacy of JAK inhibitors in NLP. Baricitinib (a JAK1/2 inhibitor),44,46 abrocitinib (a selective JAK1 inhibitor),47 and tofacitinib (a pan JAK inhibitor)43 have been reported to induce marked improvements in NLP. In the authors’ own experience, abrocitinib monotherapy led to a significant resolution in a patient with isolated NLP, with a favourable safety profile. Compared to other JAK inhibitors, selective JAK1 inhibition may offer an advantage by minimising haematological and metabolic side-effects often seen with broader JAK blockade.47 While these initial findings are encouraging, they remain limited to anecdotal evidence. Larger prospective studies are urgently needed to validate efficacy, establish optimal dosing regimens, and ensure longterm safety, particularly given the potential for systemic immunomodulation.
NLP is a rare but potentially scarring inflammatory nail disorder with unpredictable progression. Early recognition and intervention are crucial to prevent irreversible damage. While evidencebased treatment strategies remain limited, intralesional and systemic corticosteroids are currently the most effective options. Retinoids and immunosuppressants may serve as alternatives in select cases. Emerging therapies, such as JAK inhibitors, show promising potential and warrant further investigation.
References
1. Ioannides D et al. European S1 guidelines on the management of
lichen planus: a cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. 2020;34:1403-14.
2. Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39(2):221-30.
3. Singal A et al. Clinical characteristics and management outcomes in isolated nail lichen planus: a retrospective case series. Indian J Dermatol Venereol Leprol. 2023;DOI:10.25259/ IJDVL_449_2023.
4. Goettmann S et al. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. J Eur Acad Dermatol Venereol. 2012;26(10):1304-9.
5. Motamed-Sanaye A et al. JAK inhibitors in lichen planus: a review of pathogenesis and treatments. J Dermatol Treat. 2022;33(8):3098-103.
6. Pittelkow MR, Daoud MS, “Lichen planus,” Wolff K et al. (eds.), Fitzpatrick’s Dermatology in General Medicine (2008) 7th edition, New York: McGraw-Hill, pp.244-55.
7. Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-71.
8. Nishizawa A et al. Close association between metal allergy and nail lichen planus: detection of causative metals in nail lesions. J Eur Acad Dermatol Venereol. 2013;27(2):e231-4.
9. Ma J et al. The magnitude of the association between human papillomavirus and oral lichen planus: a meta-analysis. PloS One. 2016;11(8):e0161339.
10. Nahidi Y et al. Association of classic lichen planus with human herpesvirus-7 infection. Int J Dermatol. 2017;56(1):49-53.
11. Todorov B et al. The potential association between oral lichen planus and vaccination against hepatitis B. A real-world study analyzing data from approximately 200,000 patients. BMC Oral Health. 2025;25(1):1378.
12. Vičić M et al. Comprehensive insight into lichen planus immunopathogenesis. Int J Mol Sci. 2023;24(3):3038.
13. Shao S et al. IFN-γ enhances cellmediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11(511):eaav7561.
14. Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1(3): 140-9.
15. Wechsuruk P et al. Clinical features and treatment outcomes of nail lichen planus: a retrospective study. JAAD Case Rep. 2021;17:43-8.
16. Tosti A et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137(8):1027-32.
17. Hwang JK et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90(3):585-96.
18. Kharghoria G et al. Nail dermatoscopic (onychoscopic) features of nail lichen planus: a cross-sectional study. Australas J Dermatol. 2021;62(1): e79-82.
19. Hwang JK et al. Bullous lichen planus of the nails: a case report and review of the literature. Case Rep Dermatol. 2023;15(1):133-41.
20. Grover C et al. Linear nail bed dyschromia: a distinctive dermoscopic feature of nail lichen planus. Clin Exp Dermatol. 2019;44(6):697-9.
21. Grover C et al. Nail lichen planus: a review of clinical presentation, diagnosis and therapy. Ann Dermatol Venereol. 2022;149(3):150-64.
22. Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9(1):3-15.
23. Kharghoria G et al. Histopathological evaluation of nail lichen planus: a cross‐sectional study. J Cutan Pathol. 2021;48(1):11-7.
24. Tosti A et al. Nail lichen planus: clinical and pathologic study of twentyfour patients. J Am Acad Dermatol. 1993;28(5 Pt 1):724-30.
25. He J et al. The typical nail lichen planus severity index: an outcome instrument for typical nail lichen planus. Dermatology. 2024;240(56):758-66.
26. Iorizzo M et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83(6):1717-23.
27. Prevost NM, English JC 3rd. Palliative treatment of fingernail lichen planus. J Drugs Dermatol. 2007;6(2):202-4.
28. Ujiie H et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90(2):218-9.
29. Gerstein W. Psoriasis and lichen planus of nails. Treatment with triamcinolone. Arch Dermatol. 1962;86:419-21.
30. Abell E, Samman PD. Intradermal triamcinolone treatment of nail dystrophies. Br J Dermatol. 1973;89(2):191-7.
31. Grover C et al. Efficacy of triamcinolone acetonide in various acquired nail dystrophies. J Dermatol. 2005;32(12):963-8.
32. Piraccini BM et al. Nail lichen planus: response to treatment and long
term follow-up. Eur J Dermatol. 2010;20(4):489-96.
33. Brauns B et al. Intralesional steroid injection alleviates nail lichen planus. Int J Dermatol. 2011;50(5):626-7.
34. Takeuchi Y et al. Lichen planus with involvement of all twenty nails and the oral mucous membrane. J Dermatol. 2000;27(2):94-8.
35. Evans AV et al. Isolated lichen planus of the toe nails treated with oral prednisolone. Clin Exp Dermatol. 2001;26(5):412-4.
36. Kato N, Ueno H. Isolated lichen planus of the nails treated with etretinate. J Dermatol. 1993;20(9):577-80.
37. Pinter A et al. Lichen planus of nails - successful treatment with alitretinoin. J Dtsch Dermatol Ges. 2011;9(12):1033-4.
38. Iorizzo M. Nail lichen planus - a possible new indication for oral alitretinoin. J Eur Acad Dermatol Venereol. 2016;30(3):509-10.
39. Alsenaid A et al. Successful treatment of nail lichen planus with alitretinoin: report of 2 cases and review of the literature. Dermatology. 2014;229(4):293-6.
40. Manousaridis I et al. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges. 2013;11(10):981-91.
41. Florian B et al. Successful treatment of palmoplantar nail lichen planus with cyclosporine. J Dtsch Dermatol Ges. 2014;12(8):724-5.
42. Irla N et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2(3):173-6.
43. Iorizzo M, Haneke E. Tofacitinib as treatment for nail lichen planus associated with alopecia universalis. JAMA Dermatol. 2021;157(3):352-3.
44. Pünchera J, Laffitte E. Treatment of severe nail lichen planus with baricitinib. JAMA Dermatol. 2022;158(1):107-8.
45. Huang J, Shi W. Successful treatment of nail lichen planus with tofacitinib: a case report and review of the literature. Front Med (Lausanne). 2023;10:1301123.
46. He J et al. Alleviation of isolated nail lichen planus by the JAK1/2 inhibitor baricitinib: a case report. J Dermatolog Treat. 2023;34(1):2274816.
47. He J, Yang Y. Janus kinase 1 inhibitor abrocitinib for isolated nail lichen planus: a case report and literature review. J Dermatolog Treat. 2024;35(1):2434094.
48. Luo Y et al. Successful treatment of severe nail lichen planus with
janus kinase 1 inhibitor abrocitinib. Clin Cosmet Investig Dermatol. 2025;18:1095-100.
49. Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80(6):e177-8.
50. Clark A, Jellinek NJ. Intralesional injection for inflammatory nail diseases. Dermatol Surg. 2016;42(2):257-60.
51. Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366(8):723-32.
52. Caplan A et al. Prevention and management of glucocorticoidinduced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76(1):1-9.
53. Laurberg G et al. Treatment of lichen planus with acitretin. A double-
blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24(3):434-7.
54. Cheng C et al. Alitretinoin: a comprehensive review. Expert Opin Investig Drugs. 2008;17(3):437-43.
Authors: Gokhan Arslan,1 Yigit Tokgoz1
1. Department of Cardiovascular Surgery, Gülhane Faculty of Medicine, University of Health Sciences, Ankara, Türkiye *Correspondence to gokhan.arslan@sbu.edu.tr
Disclosure: The authors have declared no conflicts of interest. Informed written consent was obtained from the patient for his participation and the publication of this case report.
Received: 25.03.25
Accepted: 15.09.25
Keywords: Endovenous laser ablation (EVLA), Koebner phenomenon, lower extremity, management, psoriasis.
Citation: EMJ Dermatol. 2025;13[1]:152-155. https://doi.org/10.33590/emjdermatol/LRJI6587
Abstract
Psoriasis, a chronic inflammatory skin disease, presents treatment challenges, especially when complicated by conditions like venous insufficiency. While the Koebner phenomenon suggests trauma can worsen psoriasis, this case report describes a 41-year-old male patient with psoriasis vulgaris and venous insufficiency, who experienced complete remission of his psoriasis following endovenous laser ablation for his varicose veins. This unexpected outcome, beginning within weeks of the procedure and culminating in complete remission within 6 months, highlights the complex relationship between trauma and psoriasis. Potential explanations include a localised immune response, improved circulation, or a direct laser effect. This case underscores the need for further research into the interplay between venous insufficiency treatments and psoriasis activity, as understanding these mechanisms could inform future treatment strategies.
1. This case highlights the potential for endovenous laser ablation (EVLA) to induce unexpected remission of psoriasis in patients with concurrent venous insufficiency. Clinicians should be aware that EVLA, while addressing vascular concerns, may offer unanticipated dermatological benefits, challenging the traditional view of traumainduced psoriasis flares.
2. While the Koebner phenomenon suggests trauma can exacerbate psoriasis, this case demonstrates a paradoxical outcome. EVLA, a controlled form of trauma, resulted in complete psoriasis remission, suggesting complex immunomodulatory effects. This case prompts clinicians to consider the nuanced relationship between trauma and psoriasis activity in vascular interventions.
3. This report underscores the need for further research into the interaction between venous insufficiency treatments and psoriasis. Clinicians should be cognisant of the potential for EVLA to positively influence psoriasis. Further studies are warranted to explore the mechanisms behind this observation, potentially leading to novel therapeutic strategies for both conditions.
Psoriasis, a prevalent chronic inflammatory skin disease affecting 2–3% of the global population,1 poses a significant therapeutic challenge. Characterised by well-demarcated erythematous plaques with adherent silvery scales, psoriasis vulgaris, the most common variant (85–90% of cases),2,3 significantly impacts patients’ quality of life.4 While topical corticosteroids remain a common treatment, long-term safety concerns limit their sustained efficacy.5 The complex, multifactorial pathogenesis of psoriasis involves genetic predisposition, environmental triggers, and immune dysregulation.6 Recent advances have led to targeted therapies for moderate-tosevere disease,7 but challenges persist, particularly in the lower extremities, where treatment is complicated by factors such as friction, dependent oedema, and potentially altered drug absorption.8 Furthermore, psoriasis can coexist with venous insufficiency, a common disorder. Venous insufficiency treatments, including thermal ablation, may induce the Koebner phenomenon (isomorphic response), exacerbating psoriasis.9 However, the relationship between trauma and psoriasis is not always linear. While trauma can trigger flares, controlled trauma may have therapeutic potential in certain contexts. For instance, Krefting et al.10 demonstrated that compression therapy in patients with psoriasis and lower extremity oedema did not worsen the skin condition and even suggested potential benefit, highlighting the complex interplay between pressure and psoriasis. In this case report, the authors present the unexpected remission of psoriasis following endovenous laser ablation (EVLA) for venous insufficiency in a patient with both conditions, and discuss the potential mechanisms underlying this observation.
A 41-year-old male patient presented with prominent, localised psoriasis vulgaris on his left lower extremity and scalp, accompanied
by symptoms of venous insufficiency. Clinically, his psoriasis was characterised by well-defined, erythematous plaques with silvery scales. Concurrently, he exhibited classic signs of venous insufficiency, including oedema, pain, and visible varicose veins. Psoriasis vulgaris had been previously diagnosed based on its characteristic clinical presentation. Venous insufficiency was confirmed by both clinical signs and symptoms, with duplex ultrasound imaging further corroborating reflux in the great saphenous vein. No significant diagnostic challenges were encountered.
The patient had a 25-year history of psoriasis, during which he had undergone various topical and systemic therapies without achieving complete resolution. Notably, he was not receiving any specific treatment for his psoriasis immediately before or during the EVLA procedure. Given his dual diagnosis, treatment options for his varicose veins were carefully considered, particularly regarding the potential risk of the Koebner phenomenon. Non-thermal treatments, such as sclerotherapy and glue ablation, which carry a lower trauma potential, were thoroughly discussed. Ultimately, after a comprehensive review of the risks and benefits, the patient opted for EVLA, a thermal treatment, for his venous insufficiency. The procedure was performed using a Biolitec® ELVeS Radial 2ring fiber (Integrated Connection [IC], 1,470 nm; Biolitec AG, Jena, Germany).
Surprisingly, following the EVLA procedure, the psoriatic lesions on his left lower extremity achieved complete remission (Figure 1). This unexpected improvement began within a few weeks post-procedure and progressively advanced to full remission by the 6-month follow-up mark. During this period, the patient received a 3-month course of calcium dobesilate 500 mg twice daily for his venous insufficiency, after which the medication was discontinued. No changes were observed in the psoriatic plaques on the patient’s scalp throughout the follow-up period, indicating that the effect was localised to the treated area rather than systemic. The patient reported excellent tolerability to the EVLA procedure
A) Post-operative Day 1. This image shows the localised psoriasis (indicated by the red arrow) on the patient's left lower extremity.
B) Six months post-operative. This image demonstrates complete resolution of the patient's localised psoriasis on the left lower extremity.
and adhered well to post-procedural care instructions. No adverse or unanticipated events directly related to the EVLA procedure were observed, apart from the remarkable and unexpected psoriasis remission itself.
To contextualise this case, a comprehensive literature search of PubMed, Embase, and Scopus using the keywords “Koebner phenomenon,” “lower extremity,” “psoriasis,” “EVLA,” and “management” revealed a notable absence of prior reports directly linking psoriasis remission to EVLA for
venous insufficiency or similar atypical Koebner responses. This lack of literature underscores the novelty and potential significance of this observation as a rare, possibly first-documented instance. While the exact mechanisms remain unclear, EVLA may have exerted local immunomodulatory effects via reduced venous stasis. Interestingly, while the authors’ case demonstrates a surprising remission of psoriasis following EVLA, a recent systematic review and meta-analysis suggests that psoriasis is associated with an increased risk for incident venous thromboembolism and peripheral vascular disease.11 This highlights the complex and potentially bidirectional relationship
between psoriasis and the vascular system, further emphasising the need for comprehensive research in this area.
This case shows the complex and sometimes counterintuitive relationship between trauma and psoriasis activity. While the Koebner phenomenon9 describes the risk of psoriasis flares following trauma, this patient experienced complete remission of his psoriasis after EVLA for venous insufficiency, a paradoxical outcome. Several potential mechanisms may explain this observation. EVLA, although a controlled form of trauma, may have triggered a localised immune response that inadvertently modulated the patient’s psoriasis. Alternatively, the improved venous circulation resulting from EVLA may have indirectly influenced the psoriasis, perhaps by reducing local inflammation or enhancing the delivery of topical or systemic medications. This concept aligns with findings from Krefting et al.,10 whose study on compression therapy suggests that controlled pressure or trauma may have a therapeutic role in some patients
References
1. Adamski Z et al. The link between psoriasis and other diseases based on epidemiological and genetic analyses. Postepy Dermatol Alergol. 2023;40(4):496-503.
2. Lambert J et al. A descriptive study of psoriasis characteristics, severity and impact among 3,269 patients: results of a Belgian cross sectional study (BELPSO). Eur J Dermatol. 2012;22(2):231-7.
3. Rakkhit T et al. Plaque thickness and morphology in psoriasis vulgaris associated with therapeutic response. Br J Dermatol. 2009;160(5):1083-9.
4. Šmejkalová J et al. Quality of life of patients with psoriasis. Cent Eur J Public Health. 2020;28(3):219-25.
with psoriasis, supporting the notion that the relationship between trauma and psoriasis is not universally detrimental. Another possibility is a direct effect of the laser energy on the psoriasis. Given the use of laser therapy in treating psoriasis, the EVLA laser may have exerted a similar, albeit unintended, therapeutic effect.12,13 While non-thermal treatments for venous insufficiency, such as sclerotherapy, are available and also induce some degree of intentional vascular trauma, the literature regarding their impact on concurrent psoriasis is limited. The limited existing literature on this specific clinical scenario underscores the importance of future research to comprehensively evaluate the impact of venous insufficiency treatments on psoriasis activity. Well-designed studies are needed to determine the safety and efficacy of these interventions in patients with both conditions, and to clarify the biological pathways that may lead to atypical responses like the psoriasis remission seen in the authors’ case, ultimately guiding clinical decision-making.
5. Mason A et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69(5):799-807.
6. Singh R et al. The cytokine mediated molecular pathophysiology of psoriasis and its clinical implications. Int J Mol Sci. 2021;22(23):12793.
7. Azuaga AB et al. Psoriatic arthritis: pathogenesis and targeted therapies. Int J Mol Sci. 2023;24(5):4901.
8. Fülle M et al. Psoriasis on the leg: site-specific histopathological and immuno-histochemical features and diagnostic difficulties. Acta Derm Venereol. 2021;101(5):adv00453.
9. Liu S et al. Triggers for the onset and recurrence of psoriasis: a review and update. Cell Commun Signal. 2024;22(1):108.
10. Krefting F et al. [Randomized clinical trial of compression therapy of the lower legs in patients with psoriasis]. Dermatologie (Heidelb). 2023;74(8):605-13. (In German).
11. Chen TL et al. Association of psoriasis with ıncident venous thromboembolism and peripheral vascular disease: a systematic review and meta-analysis. JAMA Dermatol. 2022;158(1):59-67.
12. Abrouk M et al. Excimer laser for the treatment of psoriasis: safety, efficacy, and patient acceptability. Psoriasis (Auckl). 2016;6:165-73.
13. Sarda A et al. Laser and lights in psoriasis. Indian J Dermatol. 2024;69(2):159-64.
If you like it,why not share it...

Share with a colleague today