Diabetes



Editor’s Pick:
Treatment of Type 2 Diabetes: A Comprehensive Review of Recent Improvements, Therapeutic Strategies, Challenges, and Future Perspectives
Interview:
EASD President Chantal Mathieu discusses the highlights of EASD 2025 and what to expect at next year’s Congress

10 Review of the European Association for the Study of Diabetes (EASD) Annual Meeting 2025, 15th–19th September 2025
Congress Features
24 The Convergence of Curative Strategies: Islet Replacement, Tolerance Induction, and the Path Beyond Immunosuppression in Type 1 Diabetes
Shareen Forbes
30 Precision Medicine in Diabetes: From Genetic Risk to Real-World Implementation
Bertie Pearcey
Abstract Reviews
36 A Comparison of Automated Insulin Delivery Systems with and Without Auto Algorithm Updates and Cloud-Integration in Children With Type 1 Diabetes
Pemberton J et al.
39 Physical Function Outcomes in People With Type 2 Diabetes: What is the Impact of Type 2 Diabetes Duration Above and Beyond Chronological Age?
Hamza M et al.
42 Glucagon-Like Peptide-1 Receptor Agonists Protect Against Hepatocyte Senescence, Driving Metabolic DysfunctionAssociated Steatotic Liver Disease/Metabolic DysfunctionAssociated Steatohepatitis
Zatterale F et al.
45 Abstract Highlights
Congress Interviews
53 Chantal Mathieu
59 Hindrik Mulder
62 Patrick Schwauren Interviews
66 Lorenzo Piemonti
70 Stephan Herzig Articles
72 Editor's Pick: Treatment of Type 2 Diabetes: A Comprehensive Review of Recent Improvements, Therapeutic Strategies, Challenges, and Future Perspectives
Islam and Uddin
83 Viral Triggers of Autoimmune Diabetes: Mechanisms, Clinical Implications, and Future Directions
Afolorunsho AD et al.
91 Severe Hypertriglyceridaemia and Uncontrolled Diabetes Presenting as Eruptive Xanthoma in a 34-Year-Old Filipino Male: A Case Report
David and Prieto
98 Enhancing Lipid Control in Patients with Type 2 Diabetes: A Critical Analysis of Care Quality in Iran
Davari M et al.
"The central theme this year was 'Rethinking Diabetes: From Classification to Personalisation’, which called for a shift in how the global diabetes community understands and treats the disease"




Dr Martin Whyte University of Surrey, UK
Dr Martin Whyte undertook his medical degree at King’s College Hospital, London, UK, after which he completed a PhD exploring the metabolic effects of insulin in critical illness at Guy’s and St Thomas’ Hospital, London, UK. He became the Associate Professor in Metabolic Medicine at the University of Surrey, UK, in 2021. His areas of research interest include nonalcoholic fatty liver disease and the effect of insulin resistance on cardiovascular disease.






Prof David Simmons Western Sydney University, Australia
Prof Gijs Goossens
Maastricht University Medical Centre+, the Netherlands
Dr Hassan Shora
Port Said University, Egypt
Mrs Anne-Marie Felton Foundation of European Nurses in Diabetes, UK
Dr Mohammad Alhadj Ali
Cardiff University School of Medicine, UK
Prof Henning Beck-Nielsen Odense University Hospital, Denmark








Dr Muthuswamy Balasubramanyam
Madras Diabetes Research Foundation (MDRF), India
Dr Yehuda Handelsman
Metabolic Institute of America, USA
Dr Sampathkumar Rangasamy
Translational Genomics Research Institute (TGen), USA
Dr Lorenzo Pasquali
Universitat Pompeu Fabra, Barcelona, Spain
Prof Nikolaos Tentolouris
National and Kapodistrian University of Athens, Greece
Dr Dario Rahelic Dubrava University Hospital, Croatia
Prof Anne Phillips Birmingham City University, UK
Dr Jonathan Bodansky Leeds Teaching Hospitals, UK
EMJ Diabetes is an open-access, peer-reviewed eJournal, committed to helping elevate the quality of healthcare in diabetes and to contribute in advancing the development of this field by informing healthcare professionals on all aspects of cardiovascular disease.
The journal is published annually, 6 weeks after the European Association for the Study of Diabetes (EASD) Annual Meeting, and features highlights from this congress, alongside interviews with experts in the field, reviews of abstracts presented at the congress, as well as in-depth features on congress sessions. The journal also covers advances within the clinical and pharmaceutical arenas by publishing sponsored content from congress symposia, which is of high educational value for healthcare professionals. This content undergoes rigorous quality control checks by independent experts and the in-house editorial team.
EMJ Diabetes also publishes peer-reviewed research papers, review articles, and case reports in the field. In addition, the journal welcomes the submission of features and opinion pieces intended to create a discussion around key topics in the field and broaden readers’ professional interests. EMJ Diabetes is managed by a dedicated editorial team that adheres to a rigorous double-blind peer-review process, maintains high standards of copy editing, and ensures timely publication.
EMJ Diabetes endeavours to increase knowledge, stimulate discussion, and contribute to a better understanding of diabetes. Our focus is on research that is relevant to all healthcare professionals. We do not publish veterinary science papers or laboratory studies not linked to patient outcomes. We have a particular interest in topical studies that advance knowledge and inform of coming trends affecting clinical practice in diabetes.
Further details on coverage can be found here: www.emjreviews.com
Editorial Expertise
EMJ is supported by various levels of expertise:
• Guidance from an Editorial Board consisting of leading authorities from a wide variety of disciplines.
• Invited contributors who are recognised authorities in their respective fields.
• Peer review, which is conducted by expert reviewers who are invited by the Editorial team and appointed based on their knowledge of a specific topic.
• An experienced team of editors and technical editors.
• A team of internal and independent medical writers.
On submission, all articles are assessed by the editorial team to determine their suitability for the journal and appropriateness for peer review.
Editorial staff, following consultation with a member of the Editorial Board if necessary, identify three appropriate reviewers, who are selected based on their specialist knowledge in the relevant area.
All peer review is double blind. Following review, papers are either accepted without modification, returned to the author(s) to incorporate required changes, or rejected.
Editorial staff have final discretion over any proposed amendments.
Submissions
We welcome contributions from professionals, consultants, academics, and industry leaders on relevant and topical subjects. We seek papers with the most current, interesting, and relevant information in each therapeutic area and accept original research, review articles, case reports, and features.
We are always keen to hear from healthcare professionals wishing to discuss potential submissions, please email: editorial.assistant@emjreviews.com
To submit a paper, use our online submission site: www.editorialmanager.com/e-m-j
Submission details can be found through our website: www.emjreviews.com/contributors/authors
Reprints
All articles included in EMJ are available as reprints (minimum order 1,000). Please contact hello@emjreviews.com if you would like to order reprints.
Distribution and Readership
EMJ is distributed through controlled circulation to healthcare professionals in the relevant fields globally.
Indexing and Availability
EMJ is indexed on DOAJ, the Royal Society of Medicine, and Google Scholar®.
EMJ is available through the websites of our leading partners and collaborating societies. EMJ journals are all available via our website: www.emjreviews.com
Open Access
This is an open-access journal in accordance with the Creative Commons Attribution-Non Commercial 4.0 (CC BY-NC 4.0) license.
Congress Notice
Staff members attend medical congresses as reporters when required.
This Publication Launch Date: 2013 Frequency: Yearly Online ISSN: 2054-6181
All information obtained by EMJ and each of the contributions from various sources is as current and accurate as possible. However, due to human or mechanical errors, EMJ and the contributors cannot guarantee the accuracy, adequacy, or completeness of any information, and cannot be held responsible for any errors or omissions. EMJ is completely independent of the review event (EASD 2025) and the use of the organisations does not constitute endorsement or media partnership in any form whatsoever. The cover photo is of Vienna, Austria the location of EASD 2025.
Front cover and contents photograph: Vienna, Austria © Tryfonov / stock.adobe.com

Managing Editor
Darcy Richards
Copy Editors
Noémie Fouarge, Sarah Jahncke
Editorial Leads
Helena Bradbury, Ada Enesco
Editorial Co-ordinator
Bertie Pearcey
Editorial Assistants
Katrina Thornber, Katie Wright,
Aleksandra Zurowska
Creative Director
Tim Uden
Design Manager
Stacey White
Senior Designers
Tamara Kondolomo, Owen Silcox
Creative Artworker
Dillon Benn Grove
Designers
Shanjok Gurung, Fabio van Paris
Junior Designer
Helena Spicer
Business Unit Lead
Kelly Byrne
Chief Executive Officer
Justin Levett
Chief Commercial Officer
Dan Healy
Founder and Chairman
Spencer Gore
Dear Readers,
We are thrilled to welcome you to the 2025 issue of EMJ Diabetes, showcasing cutting-edge research updates from the 61st European Association for the Study of Diabetes (EASD) Annual Meeting, held in Vienna, Austria. This year, key focuses at the event were obesity and lifestyle; sustainability; innovations in therapeutics, precision medicine, and monitoring; and cardiometabolic health. Additionally, the Annual Meeting built upon previous goals of focusing on patient-centred care, with a new guideline for assessing and managing distress related to diabetes.
Alongside our review of the Annual Meeting, you can find an insightful feature that provides a deeper exploration of functional cure strategies for Type 1 diabetes, discussing presented updates on islet replacement, tolerance induction, and immunosuppression. You can also discover engaging research abstracts and exclusive interviews with expert EASD members, including the President.
Among our peer-reviewed content is a comprehensive review article detailing advances in the treatment of Type 2 diabetes and providing a perspective on the current challenges and future landscape. We also have a review evaluating the viral triggers of autoimmune diabetes and a research article assessing the quality of care of patients with diabetes in Iran.
To close, we would like to take this opportunity to thank our Editorial Board, authors, peer reviewers, and interviewees for their invaluable contributions to this issue. We hope you enjoy reading and find useful takeaways for your clinical practice!
Editorial enquiries: editor@emjreviews.com
Sales opportunities: salesadmin@emjreviews.com
Permissions and copyright: accountsreceivable@emjreviews.com
Reprints: info@emjreviews.com Media enquiries: marketing@emjreviews.com



I am delighted to welcome you to this issue of EMJ Diabetes. Recently, we’ve witnessed a host of exciting developments in diabetes research and clinical care, showcased at the European Association for the Study of Diabetes (EASD) Annual Meeting 2025 in Vienna, Austria.
A major theme this year was the acceleration of beta-cell replacement and immunomodulatory approaches in Type 1 diabetes (T1D). Among these, the zimislecel stem-cell islet infusion trial was a highlight: of 12 participants treated, 10 remained insulin-independent for 1 year. Vertex is now recruiting for a next-phase trial with 50 participants, while continuing follow-up with the original cohort.
Early detection and disease modification were also heavily featured. The UK’s ongoing EarLy Surveillance for Autoimmune diabetes (ELSA) screening study (ages: 3–13 years) was discussed, aiming to identify autoantibodypositive individuals, years before clinical onset. Simultaneously, therapies designed to delay progression in early-stage T1D were spotlighted, notably teplizumab, now MHRAapproved to delay progression for up to 2 years, and low-dose anti-thymocyte globulin, which showed signals of preserved C-peptide even in new-onset disease.
EASD 2025 showcased remarkable progress, from cell therapy and immune modulation in T1D, to next-generation closedloop technology
Technological innovation featured prominently throughout the meeting. Cambridge’s CamAPS HX fully closed-loop system was featured: in adults with suboptimal control, it added roughly three extra hours of time-in-range. Hybrid and fully closed-loop systems were also applied in people with Type 2 diabetes on insulin, showing extended time-in-range and reduced management burden, including in those with renal impairment.
Another innovation was the first EASD clinical guideline on diabetes distress, a formal, evidence-based framework to help clinicians routinely assess and manage the emotional burden associated with living with diabetes.
In summary, EASD 2025 showcased remarkable progress, from cell therapy and immune modulation in T1D, to next-generation closedloop technology, and a renewed focus on the psychological dimensions of living with diabetes.



Martin Whyte
Associate Professor of Metabolic Medicine, University of Surrey, UK

The central theme this year was ‘Rethinking Diabetes: From Classification to Personalisation’, which called for a shift in how the global diabetes community understands and treats the disease
Location: Vienna, Austria
Date: 15th–19th September 2025
Citation: EMJ Diabet. 2025;13[1]:10-23. https://doi.org/10.33590/emjdiabet/KZWD7940
THE 61ST ANNUAL Meeting of the European Association for the Study of Diabetes (EASD) took place in the historic and elegant city of Vienna, Austria, from 15th–19th September. Against the backdrop of grand imperial architecture and a thriving scientific community, the Annual Meeting united researchers and clinicians in an atmosphere of discovery and collaboration.
The central theme this year was ‘Rethinking Diabetes: From Classification to Personalisation’, which called for a shift in how the global diabetes community understands and treats the disease. The opening ceremony set the tone with a warm welcome to this year’s attendees by the EASD President, Chantal Mathieu, who reflected on “how many people with diabetes do not fit neatly into a single box,” and called for a move from “types to traits” and categorisation to individualisation, in line with the EASD theme. She emphasised that true progress lies in embracing the heterogeneity of diabetes and tailoring care to each patient’s unique disease profile, a vision that aligns with the growing momentum in precision medicine.
Mirroring this philosophy, the scientific programme featured 1,354 abstracts, a record number that resulted in close to 2,000 oral and short oral presentations, with topics ranging from novel therapies and diseasemodifying treatments to implementation science and digital health innovation.
The ceremony continued with the introduction of new session formats by Programme Committee Chair Tina Vilsboll, which was met with great enthusiasm by the audience. Alongside the popular Rising Star Symposia, this year’s Meeting featured interactive Lab Talks, giving attendees a look into leading laboratories, and Early Bird Symposia, which showcased the latest breaking clinical trials.
A notable highlight was the presentation of EASD’s first-ever clinical guideline focusing on diabetes distress, a topic identified as an unmet need by both clinicians and people living with diabetes.
EASD also reaffirmed its dedication to nurturing the next generation of researchers through the Early Career Academy, led by Patrick Schrauwen, Chair of the EASD Early Career Academy Committee, and through global partnerships spearheaded by Francesco Giorgino, Chair of the EASD Global Council.
In her closing remarks, Mathieu expressed gratitude to the Executive Board, the Düsseldorf secretariat, and particularly to long-serving team member Mary Hatter, who retired after decades of service to
EASD. With humour and humility, she concluded “it has been an honour to be your president,” before offering a final wish for good health, peace, and a joyful reunion at EASD 2026 in Milan, Italy.

programme
PHYSICAL activity is a well-established component of managing Type 2 diabetes (T2D), with benefits including improved glycaemic control, better insulin sensitivity, and healthier body composition. While its protective cardiovascular effects in the general population are widely recognised, less is known about its prognostic value in people newly diagnosed with T2D. A new large-scale Danish study presented at EASD 2025 has found that higher levels of self-reported physical activity are independently associated with a significantly lower risk of cardiovascular events and all-cause mortality, even after adjusting for conventional cardiovascular risk factors.1
The prospective cohort study analysed data from 11,355 adults enrolled in the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort between 2010–2023. All participants had been diagnosed with T2D within the previous 2 years and had no history of cardiovascular disease at baseline. Physical activity levels were self-reported using the Saltin-Grimby scale and categorised as sedentary, light, or moderate-to-vigorous. Participants were followed for a median of 8.4 years, with primary outcomes being major adverse cardiovascular events (MACE) and all-cause mortality. Those with prior cardiovascular disease were excluded.
Among the participants, 18.0% were sedentary, 62.5% engaged in light activity, and 19.5% in moderate-to-vigorous physical activity. During follow-up, 1,149 MACE and 1,048 deaths occurred. After adjusting for age, sex, lifestyle factors, and central obesity, light activity was associated with a 23% reduced risk of MACE (hazard ratio [HR]: 0.77; 95% CI: 0.68–0.95) and a 27% lower risk of all-cause mortality (HR: 0.78; 95% CI: 0.65–0.94). For moderate-tovigorous physical activity, the reductions were 28% for MACE (HR: 0.70; 95% CI: 0.57–0.87) and 33% for all-cause mortality
Higher levels of self-reported physical activity are independently associated with a significantly lower risk of cardiovascular events and all-cause mortality
(HR: 0.69; 95% CI: 0.54–0.87). These associations remained significant even after adjusting for low-density lipoprotein cholesterol, HbA1c, blood pressure, kidney function, and albuminuria.
The findings highlight self-reported physical activity as a robust, independent predictor of cardiovascular outcomes and survival in people with newly diagnosed T2D. While causality cannot be established from observational data, and self-reporting may introduce measurement bias, the persistent associations suggest real-world relevance. Clinicians should consider physical activity levels not only as a modifiable risk factor, but also as a useful prognostic marker in early diabetes care. Simple assessments of activity may help identify high-risk patients who could benefit most from targeted interventions.


SWEDEN has one of the highest rates of Type 1 diabetes (T1D) globally, yet its geographical distribution remains uneven. This variation has long hinted at the role of environmental risk factors, particularly in early life. While previous research has examined where patients were born or diagnosed, new research presented at EASD 2025 took a more detailed approach, tracking residential history from birth to diagnosis to map high- and low-risk areas over time. A key finding was that areas with the highest T1D incidence were rural, while Sweden’s largest cities had significantly lower rates.2
The northern regions had the highest RRs, up to
0.1 2.7
While the lowest risk, RRs as low as was again seen in urban centres
Researchers identified all patients aged 0–30 diagnosed with T1D in Sweden between 2005–2022 using the Swedish National Diabetes Register. This cohort included 21,774 individuals, 57.7% of whom were male, and the mean age at diagnosis was 13.6 years. Address histories from birth to diagnosis were obtained from Statistics Sweden. Using spatio-temporal scan statistics, the team identified clusters of unusually high or low incidence, both at the time of diagnosis and during the first 5 years of life. Land use characteristics within these clusters were also analysed.
Four high-risk clusters were identified based on residence at diagnosis, all in central Sweden, with relative risks (RR) ranging 1.3–1.8. Notably, no high-risk clusters were found in Sweden’s major cities. In contrast, low-risk clusters were concentrated in the largest urban areas, with RRs between 0.5–0.8. When analysing residence during the first 5 years of life, the
picture became even clearer: 11 high-risk and 15 low-risk clusters were identified. The northern regions had the highest RRs (up to 2.7), while the lowest risk (RRs as low as 0.1) was again seen in urban centres. Land cover analysis showed that high-risk areas were dominated by forests and agriculture, while low-risk clusters were largely urban and open land.
This study strongly supports the hypothesis that environmental exposures in rural areas during early childhood increase the risk of developing T1D. While it cannot determine causality or identify specific environmental triggers, it provides a robust framework for future investigations. Limitations include a lack of direct environmental measurements and potential confounding by socioeconomic factors. Clinically, these findings highlight the importance of environmental context in early-life diabetes prevention strategies and warrant further attention in both research and public health planning.
CARDIOVASCULAR disease (CVD) remains the leading cause of death worldwide, with people living with Type 1 (T1D) or Type 2 diabetes (T2D) facing significantly elevated risk. While extensive research has been done on the cardiovascular implications of both diabetes types, direct comparisons of risk between T1D and T2D within each sex have been lacking. A new Swedish cohort study presented at EASD 2025 addresses this gap, revealing important sex-specific differences in cardiovascular outcomes. Notably, younger men with T2D are at higher cardiovascular risk than those with T1D, while women with T1D consistently face greater risk than those with T2D across all age groups.3
Researchers analysed data from over 400,000 adults with diabetes (38,351 with T1D and 365,675 with T2D), aged 18–84 years, using records from the Swedish National Diabetes Register. Individuals were followed for 5 years (2016–2020) to assess incidence of myocardial infarction (MI), heart failure, stroke, cardiovascular mortality, and all-cause mortality. Cox proportional hazards models were used to estimate risk, adjusting for age and diabetes type, with further adjustments for established cardiovascular and socioeconomic risk factors.
In men under 50 years of age, T2D was associated with significantly higher risk compared to T1D for all CVD (hazard ratio [HR]: 1.51), MI (HR: 2.40), and heart failure (HR: 2.16). Conversely, men over 70 years of age with T2D had lower MI risk (HR: 0.74). Overall, males with T2D had lower cardiovascular and all-cause mortality than those with T1D (HR: 0.84 and 0.90, respectively). For women, those with T2D
had consistently lower risks across all outcomes compared to those with T1D. For instance, in the 50–59-year age group, women with T2D had reduced risk of all CVD (HR: 0.75) and MI (HR: 0.59). Sexstratified analyses confirmed female sex to be protective in both diabetes types, although this protective effect was less pronounced in T1D.
These findings have important clinical implications. They suggest that younger males with T2D warrant more aggressive cardiovascular risk assessment and intervention, while women with T1D may benefit from more intensive management than current practice provides. A key limitation is that the observational nature of the study cannot establish causality, and residual confounding may remain. Nonetheless, this large-scale, sex-specific analysis highlights the need to tailor cardiovascular risk strategies not only to diabetes type, but also to sex and age.

Younger men with T2D are at higher cardiovascular risk than those with T1D, while women with T1D consistently face greater risk than those with T2D across all age groups
NEW research presented at EASD 2025 suggests that immunity to hepatitis B virus (HBV) may lower the risk of developing diabetes, highlighting an unexpected metabolic benefit of HBV vaccination.4
The study utilised data from de-identified electronic medical records within the TriNetX (Cambridge, Massachusetts, USA) Research Platform, focusing on adults with documented hepatitis B surface antibody (HBsAb) levels but no prior history of HBV infection or diabetes. Diabetes was defined based on clinical diagnosis, diabetes medication use, or HbA1c ≥6.5%. Propensity score matching was performed to balance demographic factors and comorbidities between groups with and without HBV immunity.
Results showed that individuals with HBV immunity had a 15% lower risk of developing diabetes compared to unimmunised individuals (hazard ratio [HR]: 0.85; 95% CI: 0.84–0.87). A dose-response relationship was observed, with higher antibody levels corresponding to greater reductions in diabetes risk. Participants with HBsAb levels ≥100 mIU/mL had a 19% lower risk (HR: 0.81; 95% CI: 0.80–0.83), while those with levels ≥1,000 mIU/mL experienced a 43% lower risk (HR: 0.57; 95% CI: 0.54–0.60) compared with those with antibody levels <10 mIU/mL.
Age-stratified analyses revealed consistent benefits across all age groups. Immunised individuals aged 18–44 years, 45–64 years, and ≥65 years demonstrated 20% (HR: 0.80; 95% CI: 0.78–0.82), 11% (HR: 0.89; 95% CI: 0.87–0.92), and 12% (HR: 0.88; 95% CI: 0.84–0.91) lower diabetes risks, respectively, compared with their unimmunised counterparts.
These findings suggest that HBV immunity may have protective metabolic effects independent of viral infection status. The observed association between HBV antibody levels and diabetes risk reduction supports the potential of HBV vaccination as a dual-benefit public health measure, offering both viral protection and possible metabolic advantages.
Further investigation is warranted to clarify the underlying mechanisms linking HBV immunity to improved glucose regulation and to evaluate whether enhanced HBV vaccination strategies could contribute to diabetes prevention efforts, particularly in regions with high prevalence of both conditions.

Individuals with HBV immunity had a
The observed association between HBV antibody levels and diabetes risk reduction supports the potential of HBV vaccination as a dual-benefit public health measure lower risk of developing diabetes compared to unimmunised individuals
%
A LARGE Swedish study, presented at EASD 2025, has found that people with Type 2 diabetes (T2D) who were prescribed continuous positive airway pressure (CPAP) therapy for obstructive sleep apnoea (OSA) had significantly lower long-term mortality than those who were not. The findings suggest that treating OSA may be an important, yet under-recognised component of diabetes management.5
OSA frequently coexists with T2D, affecting 50–80% of patients. Despite its association with increased cardiovascular risk and mortality, OSA often remains undiagnosed and is not currently considered a modifiable risk factor in diabetes care. While CPAP is known to improve sleep quality and daytime symptoms, its long-term effects on survival in patients with T2D have been unclear due to limited follow-up in previous studies.
Using data from five national Swedish registers, researchers followed 750,299 adults with T2D over a period of 14-year. Of these, 12,388 individuals had confirmed OSA and had been prescribed CPAP, while 737,911 individuals, whose OSA status was unknown, had never received CPAP. The CPAP-treated group tended to be younger (mean: 58 years versus 65 years) and had a higher average BMI (34.7 kg/m2 versus 30.6 kg/m2).
During follow-up, there were 212,336 deaths in the non-CPAP group and 764 in the CPAP group. After adjusting for multiple factors, including age, sex, cardiovascular history, BMI, smoking, and other clinical variables, CPAP use was associated with a 26% lower risk of death, corresponding to a hazard ratio of 0.74 (95% CI: 0.68–0.82; p<0.001).
These results provide compelling evidence that long-term CPAP use may improve survival in people with both T2D and OSA
These results provide compelling evidence that long-term CPAP use may improve survival in people with both T2D and OSA. However, further research using causal inference methods is needed to confirm whether the observed association reflects a direct protective effect of CPAP therapy.
lower risk of death CPAP use was associated with a
26 %

A NEW analysis from the BANDIT trial has reinforced the therapeutic potential of baricitinib in preserving β-cell function in individuals recently diagnosed with Type 1 diabetes (T1D). Building on earlier findings that demonstrated clinical benefits after 48 weeks of treatment, the latest data presented at EASD 2025 reveal that these effects begin to diminish once the drug is discontinued. Crucially, this is one of the few studies to demonstrate significant C-peptide preservation with an oral agent in T1D, a key marker of ongoing insulin production.6
In the double-blind RCT, 91 participants aged 10–30 years who were within 100 days of T1D diagnosis were enrolled. All had detectable C-peptide and at least one islet autoantibody. They were randomly assigned in a 2:1 ratio to receive either oral baricitinib 4 mg daily or placebo for 48 weeks. C-peptide responses were assessed using mixed-meal stimulation at baseline, during treatment (Weeks 12, 24, and 48), and at follow-up visits at Weeks 72 and 96 after treatment cessation.
At the primary endpoint of 48 weeks, baricitinib-treated participants had significantly higher median stimulated C-peptide levels compared to placebo (0.65 versus 0.43 nmol/L/min; p=0.001). However, by 96 weeks, after stopping treatment, the difference narrowed and was no longer statistically significant (0.37 versus 0.26; p=0.336). Similarly, initial improvements in glucose time-in-range and insulin requirements waned during the off-drug period. While 75% of baricitinib recipients met the quantitative response score threshold at 48 weeks versus 55% in the
placebo group (p=0.0154), this advantage was lost post-treatment. No baseline factors predicted sustained response, and there were no new safety concerns during follow-up.
These findings support the sustained, but not permanent, benefit of baricitinib in early T1D, highlighting the potential need for longer-term treatment to maintain clinical improvements. The oral administration and favourable safety profile offer a compelling case for baricitinib's use in routine care. However, the waning effects post-cessation limit the current findings and suggest further trials are needed to explore extended or maintenance dosing strategies, as well as the drug’s role in earlier stages of the disease.
The oral administration and favourable safety profile offer a compelling case for baricitinib's use in routine care
A LARGE-SCALE European study presented at EASD 2025 has confirmed a robust, two-way link between depressive symptoms and diabetes in older adults, with findings that hold true regardless of the country or most sociodemographic factors. Drawing on data from three major ageing cohort studies, the research explored whether factors like healthcare quality, income inequality, and physical activity influenced the strength of the relationship. A key finding was that depressive symptoms increased the risk of diabetes, and diabetes likewise predicted the development of depressive symptoms, independent of where participants lived.7
Researchers combined data from over 77,000 individuals aged 50 years and above from the English Longitudinal Study on Ageing, the Irish Longitudinal Study on Ageing, and the Survey on Health, Ageing and Retirement in Europe. They used a survival analysis approach to examine two pathways: from depression to diabetes and from diabetes to depression. Country-level factors included healthcare quality and socioeconomic indicators, while individuallevel variables covered age, gender, BMI, smoking, and physical activity.
The study found that elevated depressive symptoms were associated with a 15% increased risk of developing diabetes (hazard ratio [HR]: 1.15; 95% CI: 1.11–1.20), while having diabetes increased the likelihood of developing depressive symptoms by 48% (HR: 1.48; 95% CI: 1.37–1.61). All sociodemographic factors significantly predicted either diabetes or depression individually (p<0.01), but none, except BMI, altered the strength of the relationship between the two conditions. Specifically, individuals with diabetes and a higher BMI had a slightly increased risk of developing depressive symptoms (HR: 1.02; 95% CI: 1.01–1.04; p=0.006). No substantial variation in the strength of the associations was found across different European countries.
The findings suggest that the bidirectional relationship between depressive symptoms and diabetes remains largely stable across diverse European populations and healthcare systems. For clinical practice, this highlights the importance of mental health screening in diabetes care and, conversely, metabolic risk monitoring in patients with depression. While the study was comprehensive, limitations include reliance on self-reported measures and the observational design, which precludes conclusions about causality. Nonetheless, the consistent association across countries and demographic factors highlights the need for integrated approaches in managing both physical and mental health in ageing populations.
48
Having diabetes increased the likelihood of developing depressive symptoms by %


A RECENT Greek study presented at EASD 2025 has suggested that semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, provides strong protection against diabetic retinopathy, the leading cause of blindness among working-age adults.8
The study explored the impact of GLP-1 drugs, which are commonly used to treat Type 2 diabetes and obesity. These drugs mimic the action of the GLP-1 hormone, which plays a role in insulin production, digestion, appetite regulation, and increased feelings of fullness. In addition, the study highlighted the potential antioxidant and anti-inflammatory properties of GLP-1 drugs, which may be crucial in protecting the eyes from diabetic retinopathy.
The study involved lab-based tests using human retinal endothelial cells exposed to high glucose levels and oxidative stress, mimicking diabetic conditions. Semaglutide was applied at various concentrations to the retinal cells for 24 hours, followed by a series of tests. The results showed that retinal cells treated with semaglutide were up to twice as likely to survive compared to untreated cells. Additionally, three key markers of oxidative stress, apoptosis (cell death), mitochondrial superoxide production, and accumulation of advanced glycation end-products, were significantly reduced in the treated cells. Apoptosis decreased from around 50% in untreated cells to just 10% in semaglutide-treated cells, while mitochondrial superoxide production fell from about 90% to just 10%. Further analysis revealed that semaglutide
upregulated genes involved in antioxidant production, providing more evidence that the drug could repair cellular damage caused by diabetes-like conditions.
The results showed that retinal cells treated with semaglutide were up to twice as likely to survive compared to untreated cells
These promising results suggest that GLP-1 receptor agonists, such as semaglutide, have powerful antioxidant effects that could protect retinal cells from damage and possibly repair existing harm. This is particularly important given the high prevalence of diabetic retinopathy in people with diabetes, affecting up to 90% of individuals with Type 1 diabetes and 50–60% with Type 2 diabetes. Clinical trials are needed to confirm these findings in human patients and to determine whether GLP-1 receptor agonists can slow or even halt the progression of diabetic retinopathy. If proven effective, these drugs could play a vital role in clinical practice by slowing or halting the progression of diabetic retinopathy, ultimately reducing the number of people affected by vision-threatening stages of the condition.
RECENT research has suggested that Type 2 diabetes (T2D) is not a single disease, but instead comprises four distinct subtypes: two severe (severe insulin-resistant diabetes [SIRD] and severe insulin-deficient diabetes) and two milder forms (mild obesity-related diabetes and mild age-related diabetes). Understanding how lifestyle factors such as smoking affect the risk of each subtype could support more targeted prevention efforts. In a large-scale study combining Swedish and Norwegian data presented at EASD 2025, researchers found that smoking significantly increased the risk of all T2D subtypes, with the strongest association observed for SIRD.9
Data were drawn from two large populationbased studies: the Swedish case-control study ESTRID (2010–2024) and the Norwegian cohort study HUNT (1984–2008). Together, these included 3,325 incident T2D cases and 3,897 controls, with 873,349 person-years of follow-up. Participants were classified into one of the four T2D subtypes. Genetic risk scores for overall T2D, insulin resistance (IR), and insulin secretion were calculated to explore gene-environment interactions. Associations between smoking and T2D subtype risk were analysed using pooled relative risk (RR) estimates. A two-sample Mendelian randomisation (MR) approach was applied to support causal inference.
Compared with never smokers, ever smokers were at more than twice the risk of developing SIRD (RR: 2.15; 95% CI: 1.64–2.82). The association was weaker but still significant for mild obesity-related diabetes (RR: 1.29; 95% CI: 1.06–1.57), mild age-related diabetes (RR: 1.27; 95% CI: 1.12–1.44), and borderline for severe insulin-deficient diabetes (RR: 1.20; 95% CI: 0.98–1.47). Heavy smokers (≥15 packyears) were at substantially higher risk across all subtypes, with the strongest effect again observed for SIRD (RR: 2.35; 95% CI: 1.72–3.23). Population-attributable risk estimates indicated that smoking accounted for over one-third of SIRD cases (35.3%). Additive interaction was found between heavy smoking and genetic susceptibility to T2D and insulin deficiency, but not IR. MR analyses confirmed the observed associations.
These findings strengthen the evidence that smoking is a major modifiable risk factor for all forms of T2D, particularly SIRD, which is driven by IR. Importantly, individuals with a genetic predisposition to T2D or impaired insulin secretion appear to be more vulnerable to the harmful metabolic effects of smoking. While the observational nature of parts of the study limits causal certainty, the use of MR helps to mitigate this. The results support the integration of smoking cessation advice into diabetes prevention strategies, with particular attention to those at high genetic risk.
Compared with never smokers, ever smokers were at more than twice the risk of developing SIRD
2x

THE INCREASING use of semaglutide for weight loss (SEMA-WL) has raised concerns about real-world treatment persistence, particularly in patients without diabetes. While clinical trials suggest high efficacy, less is known about how long people continue taking the medication in routine clinical settings. A recent study presented at EASD 2025 used nationwide registry data from Denmark to assess discontinuation rates and identify which patient groups are more likely to stop treatment. Strikingly, one in two adults stopped SEMA-WL within the first year of use.10
Using linked Danish health registries, researchers identified 77,310 adults without diabetes who initiated SEMA-WL between 1st December 2022–1st October 2023. Individuals were considered to have discontinued treatment if there was a gap of more than 60 days between prescriptions. Poisson regression was used to calculate age- and sex-adjusted risk ratios (RR) for various demographic and clinical factors potentially associated with treatment discontinuation.
Of the 77,310 people included (median age: 50 years; 71% women), 52% discontinued semaglutide within 12 months. Discontinuation occurred rapidly for some: 18% had stopped within 3 months, 31% within 6 months, and 42% within 9 months. Younger adults aged 18–30 years were significantly more likely to stop treatment compared with those aged 45–60 years (RR: 1.48; 95% CI: 1.45–1.51). Male users were also more likely to discontinue use (RR: 1.12; 95% CI: 1.11–1.14). Other predictors of early discontinuation included prior use of psychiatric (RR: 1.12) or gastrointestinal medications (RR: 1.09), cardiovascular disease (RR: 1.11), higher comorbidity (Charlson index 3+; RR: 1.09), and residence in lower-income municipalities (RR: 1.14).
One in two adults stopped SEMA-WL within the first year of use
These findings suggest that, in realworld settings, a significant proportion of patients stop SEMA-WL relatively soon after starting, highlighting important limitations in long-term adherence. For clinical practice, this emphasises the need to assess not only eligibility for treatment but also the likelihood of persistence, particularly among younger adults, men, and those with complex health or socioeconomic challenges. Limitations of the study include lack of data on reasons for discontinuation (e.g., side effects, cost, or perceived benefit), as well as the reliance on prescription data, which may not reflect actual medication use. Nonetheless, these insights are crucial for shaping patient expectations and informing follow-up strategies in obesity care.
Younger adults aged were significantly more likely to stop treatment compared with
18–30 years


References
1. Eriksen LB et al. Association between self-reported physical activity and cardiovascular events and all- cause mortality in individuals recently diagnosed with type 2 diabetes. Abstract 6. EASD Annual Meeting, 15-19 September, 2025.
2. Sebraoui S et al. Geographical hotspots of type 1 diabetes in Sweden: are rural environments early-life risk factors? Abstract 43. EASD Annual Meeting, 15-19 September, 2025.
3. Patsoukaki V et al. Sex differences in risk for cardiovascular disease and allcause mortality: a direct comparison of type 1 with type 2 diabetes patients in a nationwide register-based study. Abstract 47. EASD Annual Meeting, 15-19 September, 2025.
4. Phan NQ et al. Potential protective effect of hepatitis B immunity against diabetes: a retrospective propensityscore-matched cohort study. Oral presentation 350. EASD Annual Meeting, 15-19 September, 2025.
5. Agholme J et al. Long-term survival associated with continuous positive airway pressure in type 2 diabetes and obstructive sleep apnoea: results from Swedish national data. Abstract 366. EASD Annual Meeting, 15-19 September, 2025.
6. Waibel M et al. Baricitinib in newonset type 1 diabetes: BANDIT 2-year outcomes. Abstract 220. EASD Annual Meeting, 15-19 September, 2025.
7. Gottfried J et al. Diabetes and depressive symptoms: examining the potential roles of country-level and individual-level factors across
European countries. Abstract 382. EASD Annual Meeting, 15-19 September, 2025.
8. Anastasiou AI et al. GLP-1 receptor agonists enhance antioxidant defense and cell viability in retinal cells under diabetic conditions. Abstract 144. EASD Annual Meeting, 15-19 September, 2025.
9. Keysendal E et al. Tobacco use, genetic susceptibility, and the risk of type 2 diabetes subtypes. Abstract 349. EASD Annual Meeting, 15-19 September, 2025.
10. Mailhac A. Disontinuation of semaglutide therapy for weight loss: population-based study of the first 77,310 users in Denmark. Abstract 681. EASD Annual Meeting, 15-19 September, 2025.
Author: *Shareen Forbes1,2
1. Islet Transplant Programme Scotland, Department of Islet Transplantation and Diabetes, Royal Infirmary of Edinburgh, UK
2. Institute for Neuroscience and Cardiovascular Research, The University of Edinburgh, UK
*Correspondence to Shareen.Forbes@ed.ac.uk
Disclosure:
Keywords:
Forbes collaborates with and is a principal investigator with Novo Nordisk Islet Stem Cell Therapy Programme; and receives funding from Breakthrough T1D, The Helmsley Foundation, Novo Nordisk, and East Bio.
ADO12 hydrogels, anti-thymocyte globulin (ATG), immunomodulation, islet transplantation, regulatory T cells (Tregs), SAB-142, VX-880 trial.
Citation: EMJ Diabet. 2025;13[1]:24-29.
https://doi.org/10.33590/emjdiabet/HYFI5286
THE THERAPEUTIC landscape for Type 1 diabetes (T1D) is undergoing a paradigm shift, transitioning from lifelong insulin management to strategies targeting a functional cure. Achieving this goal mandates the successful replacement of lost β-cell mass while establishing immunological protection against autoimmune recurrence and allograft rejection.1 Historically, these requirements have created a fundamental problem: highly effective cell replacement therapies necessitate chronic systemic immunosuppression (IS), which carries the risk of cancer and infection, limits patient applicability, and potentially compromises the islet graft.2 Updates on this topic were presented at the European Association for the Study of Diabetes (EASD) Annual Meeting 2025.
Recent data presented at the EASD symposium, titled “Guardians of the Islet Galaxy: Protect and Replace” highlighted profound advancements in both the restoration of endogenous insulin production and the development of targeted tolerance strategies. The success of allogeneic stem cell-derived islet therapy establishes a critical benchmark for functional reversal of T1D. Simultaneously, parallel advances in precision
immunomodulation and bioengineering offer viable routes to dissociate cell replacement from the systemic toxicities of chronic IS.
The early-phase β-cell transplant trials in humans should first be highlighted. The ongoing Phase I/II/III FORWARD trial (NCT04786262)3 evaluates VX-880, an allogeneic, fully differentiated embryonic stem cell-derived islet therapy, which provides definitive clinical validation of the potential for cell replacement to ‘functionally
The success of allogeneic stem cell-derived islet therapy establishes a critical benchmark for functional reversal of T1D
cure’ T1D. The abstract presented by de Koning4 outlined the enrolment of adults with established T1D and recurrent severe hypoglycaemic episodes (SHE) with impaired awareness of hypoglycaemia into the trial, a group with a high unmet medical need. Participants received a single infusion of VX-880 into the hepatic portal vein, alongside a standard IS regimen that included induction therapy with antithymocyte globulin (ATG) and IS with the calcineurin inhibitor (CNI), tacrolimus.
Analysis of the 12 participants who received a full dose and were followed for at least 1 year (as of October 2024) revealed comprehensive metabolic reversal. All 12 participants demonstrated engraftment with durable, glucose-responsive C-peptide production beginning by day 90. Critically, all participants were free of SHEs from day 90 onwards, demonstrating that functional engraftment rapidly restored the physiological counter-regulatory responses essential for patient safety in this high-risk cohort. The degree of
glycaemic control achieved confirms the transformative potential of this approach. At month 12, the mean HbA1c for the cohort dropped significantly from 7.8% at baseline to 6.0%. Furthermore, the mean time in range increased from 49.5% to 93.0%. Every participant achieved the American Diabetes Association (ADA) target HbA1c of <7%. Participants achieved a 92% mean reduction in insulin use, and 10 of 12 participants (83%) were completely free of exogenous insulin at 12 months. The median duration of insulin independence was 232 days. The overall safety profile was good; however, serious adverse events did occur in a minority of participants and
At month 12, the mean HbA1c for the cohort dropped significantly from
93.0 7.8 49.5 6.0
Furthermore, the mean time in range increased from % % % %
were associated, in all instances, with the IS, not the stem cell-derived islets. The robustness of the outcomes, especially the rapid reversal of SHEs and high rate of insulin independence, validates the scalability and functional competence of the stem cell-derived manufacturing process. However, the requirement for chronic IS remains the central clinical and logistical challenge, underscoring the necessity for advancing tolerance strategies to unlock the therapy’s universal applicability. In the short term, consideration of alternative non-T cell depleting induction agents that prevent T-lymphocyte proliferation but do not lead to absolute lymphopaenia is warranted. Stem cell-derived islets may be less immunogenic than human islets, but more research is needed in this area. A further consideration is the development of CNI-sparing IS regimens, as CNIs decrease regulatory T cells (Tregs). Strategies to eliminate CNIs and promote immune tolerance are of the utmost importance and are an area of intensive research. The VX880 trial has paved the way for alternative β-cell replacement therapies with induced pluripotent stem cell (iPSC)-derived islets, as described in a case study by Nakamura in this session.5
To bypass the requirement for chronic IS, immune-targeted therapies that aim to induce antigen-specific tolerance are being developed, thereby protecting both the allograft and any residual native β cells. This intervention is associated with the preservation or modulation of Tregs and is being achieved in a number of ways.
The functional deficiency of Tregs in T1D pathogenesis is a well-established observation.6,7 A promising therapeutic avenue involves engineering Tregs to recognise specific antigens present in the inflammatory islet microenvironment.8 Callebaut presented the successful
generation of engineered Tregs (EngTregs) specific to post-translationally modified neoepitopes, such as citrullinated and deamidated peptides, which are produced when β cells undergo endoplasmic reticulum stress.9 This strategy leverages the mechanism of disease pathogenesis itself: these EngTregs are activated only upon recognition of neoantigens formed and released by stressed human β cells, not by healthy cells.10 When cocultured with stressed iPSC-islets, these EngTregs demonstrated robust targeted suppression via a bystander effect, reducing the proliferation of CD4 T effector cells and critically mitigating CD8 T cellmediated islet cell death. The necessity of endoplasmic reticulum stress for neoantigen release means the EngTregs are activated only in the inflammatory environment of the stressed islet, ensuring localised and specific suppression precisely where inflammation is active, optimising the therapeutic index for autoimmunity.
When co-cultured with stressed iPSC-islets, these EngTregs demonstrated robust targeted suppression via a bystander effect, reducing the proliferation of CD4 T effector cells and critically mitigating CD8 T cellmediated islet cell death
Traditional rabbit-derived ATG (rATG) preserves C-peptide levels in patients with T1D but is associated with a high risk of adverse events such as serum sickness.11 Research has described a fully human ATG (hATG), SAB-142, which represents a significant refinement in immunomodulatory therapy.12 Preclinical data showed that SAB-142 achieved T cell cytotoxic effects comparable to rATG while notably sparing the Treg population. The observed
mechanism of action for SAB-142 involves promoting sustained exhaustion in both CD4 and CD8 T cell subsets. This is evidenced by increased expression of inhibitory receptors such as programmed death 1 (PD-1) and T cell immunoreceptor with Ig and ITIM domains (TIGIT), which persisted through day 120. This contrasts with rATG, which causes sustained lymphodepletion. Simultaneously, Tregs exhibited increased expression of inhibitory receptors, suggesting enhanced suppressive function. By avoiding sustained lymphodepletion and eliminating the risk of serum sickness and anti-drug antibody generation, SAB142 is positioned as a novel re-doseable immunomodulator. This therapy redefines the use of ATG, transforming it from a nonspecific depleting agent into a mechanismdriven platform capable of facilitating sustained suppression and promoting immunotolerance. It is worth noting that early-phase trials with an investigational anti-CD40L antibody immunosuppressive agent, which targets CD40L (CD154), a key mediator of the immune response, have shown encouraging results in islet transplant recipients. Funded by Breakthrough T1D, New York, USA, these studies demonstrated that this therapy could eliminate the need for tacrolimus, a drug known to reduce Treg populations.13 Importantly, it was well tolerated and associated with high rates of insulin independence. Overall, these promising findings pave the way for larger-scale trials and offer hope for
strategies that combine this therapy with other complementary approaches. These promising findings pave the way for largerscale trials and offer hope for strategies that combine this therapy with other complementary approaches.
Strategies to protect islet grafts include bioengineered hydrogels, enabling physical isolation of the cell graft using encapsulation technology. This approach offers a means to eliminate systemic IS by creating a perm-selective physical shield. Insights into the ADO12 hydrogel film were presented during the Annual Meeting.14 The hydrogel is a non-fibrotic encapsulation system designed as an easily implantable and retrievable scaffold. In preclinical studies, it successfully protected stem cellderived islets. Crucially, studies showed that the stem cell-derived islets retained in vitro functionality and, upon in vivo implantation in mouse models, underwent functional maturation over 2–3 months. This was evidenced by decreased basal insulin secretion and increased glucose stimulation indices. This observation is paramount because it confirms that the mechanical protection afforded by encapsulation does not impede the necessary post-transplant developmental maturation of stem cellderived islets, reinforcing that physical
protection and functional maturity are compatible goals. A further abstract was presented, which described advancements in bioengineering with novel, crosslinked synthetic hydrogels compatible with 3D bioprinting.15 These hydrogels effectively excluded large molecules, such as IgGs (150 kDa), responsible for triggering rejection, while ensuring rapid diffusion of necessary molecules like insulin (5.8 kDa). When transplanted into immunocompetent diabetic animals (mice, rats, and pigs), these encapsulated human and rat islets maintained functionality and achieved durable blood glucose control for >140 days without requiring systemic IS. The demonstration of efficacy in both small and large animal models validates the engineering principles and confirms that scalable, off-the-shelf physical isolation is feasible for clinical application.
The symposium data mark a clear inflection point in T1D therapy development. The functional success demonstrated by the VX-880 FORWARD trial provides the field with a high-water mark for restorative efficacy, proving that stem cell-derived islet replacement is capable of achieving a near-curative state for high-risk patients. However, the continued necessity of systemic IS dictates that parallel efforts in tolerance induction must be accelerated. The emergence of precision immunomodulation, such as antigen-specific EngTregs activated only by pathogenic neoepitopes, and the
References
1. Forbes S. β-cell benchmarks: defining predictive outcomes in islet transplantation. Diabetes. 2025;74(5):685-8.
2. Whitehouse G et al. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc Natl Acad Sci U S A. 2017;114(27):70838.
3. Reichman TW et al. Stem cellderived, fully differentiated islets for type 1 diabetes. N Engl J Med. 2025;393(9):858-68.
Early-phase trials with an investigational anti-CD40L antibody immunosuppressive agent, which targets CD40L (CD154), a key mediator of the immune response, have shown encouraging results in islet transplant recipients
refined immunomodulatory mechanism of hATG, suggests that targeted immune resetting and tolerance induction are rapidly maturing from theoretical concepts to clinical reality. Concurrently, breakthroughs in bioengineering, exemplified by the confirmed in vivo maturation of stem cellderived islets within encapsulation systems and successful long-term immune evasion in large animals, offer an entirely orthogonal solution to the IS dilemma. Initial clinical exploration of alternative delivery methods, such as the first-in-human transplantation of iPSC-derived islet cell sheets, further expands the toolkit for cell replacement, though these approaches currently remain IS-dependent. The future trajectory of T1D therapy involves the integration of these successful stem cell-derived islet platforms with strategies that genetically or physically eliminate immune rejection, or through optimised, non-toxic immunomodulation. By refining these mechanisms, investigators are closing the gap between functional cure and safe, universally accessible therapy.
4. de Koning EJ et al. Durable glycaemic control and elimination of exogenous insulin use with VX-880 in patients with type 1 diabetes: VX-880-101 (FORWARD). Abstract P166. EASD Annual Meeting, 15-19 September, 2025.
5. Nakamura T et al. First-in-human case: allogeneic transplantation of human induced pluripotent stem cell-derived islet cells in a patient with type 1 diabetes. Abstract P167. EASD Annual Meeting, 15-19 September, 2025.
6. Visperas A, Vignali DA. Are regulatory T cells defective in type 1 diabetes and can we fix them? J Immunol. 2016;197(10):3762-70.
7. Hull CM et al. Regulatory t cell dysfunction in type 1 diabetes: what's broken and how can we fix it? Diabetologia. 2017;60(10):1839-50.
8. Mitchell AM, Michels AW. Self-antigens targeted by regulatory T cells in type 1 diabetes. Int J Mol Sci. 2022;23(6)3155.
9. Callebaut A et al. Engineered Tregs specific for post-translationally modified beta cell epitopes exhibit robust bystander immune suppression upon activation by stressed iPSC-islets. Abstract P164. EASD Annual Meeting, 15-19 September, 2025.
10. ettini M, Bettini ML. Function, failure, and the future potential of tregs in type 1 diabetes. Diabetes. 2021;70(6):1211-9.
11. Haller MJ et al.; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HBA1c in new-onset type 1 diabetes. Diabetes Care. 2018;41(9):1917-25.
12. Colbert PL et al. Mechanism of action of a fully human anti-thymocyte globulin, SAB-142, for the treatment of type 1 diabetes. Abstract P163. EASD Annual Meeting, 15-19 September, 2025.
13. Wojcik N et al. 04-3: Tegoprubart as CD40L co-stimulation blockade in a non-toxic calcineurin inhibitor-free immunosuppression for beta cell replacement therapy: preliminary results from a pilot clinical study. transplantation. 2025;109(6S1):18.
14. Jouannot O et al. ADO12, a non-fibrotic encapsulation system enables stem cell derived islets in vivo maturation for type 1 diabetes treatment. Abstract P165. EASD Annual Meeting, 15-19 September, 2025.
15. Stover H et al. Bioprinting using novel hydrogels enables functional islet transplants into diabetic animals without immunosuppression. Abstract P168. EASD Annual Meeting, 15-19 September, 2025
Author: Bertie Pearcey, EMJ, London, UK
Citation: EMJ Diabet. 2025;13[1]:30-34.
https://doi.org/10.33590/emjdiabet/FXCO4643
AT A LIVELY and data-packed session titled ‘Clinical Applications of Precision Medicine in Diabetes’, delivered during the European Association for the Study of Diabetes (EASD) Annual Meeting 2025, held in Vienna, Austria, from 15th–19th September 2025, leading researchers discussed the tangible and emerging strategies to implement precision medicine in diabetes care. The session, moderated by Jordi Merino, University of Copenhagen, Denmark, highlighted not only the clinical potential of genetic and biomarker-driven treatment, but also the growing imperative to bridge the gap between research and real-world care.
Kicking off the session, Ewan Pearson, University of Dundee, UK, took the audience on a compelling ‘whistle-stop tour’ of what he termed practical precision medicine for Type 2 diabetes (T2D). He emphasised that, while the evidence base for targeted care is growing, the key challenge lies in implementation.
Pearson began with monogenic diabetes, a field that exemplifies the promise and pitfalls of precision medicine. While the clinical benefits of identifying monogenic forms of diabetes have been known for over 2 decades, large-scale data show that diagnoses are still frequently missed. In Scotland, for example, up to two-thirds of monogenic diabetes cases may go unrecognised.1
In response, Pearson and colleagues developed iDiabetes,2 a clinical decisionsupport platform designed to embed precision care into routine practice. The system automates steps like
the measurement of C-peptide and autoantibodies, critical tests often overlooked in primary care, and integrates them with algorithmic models to identify likely cases of monogenic and Type 1 diabetes.
Moving to treatment decisions in T2D, Pearson critiqued current guidelines, stating that, while comprehensive, they can lack translation to a heterogeneous population. He argued that precision tools could help identify which patients are most likely to benefit from particular drug classes, not just on the basis of clinical characteristics, but also through biomarkers and polygenic risk scores (PRS).
One striking example involved the use of a coronary artery disease PRS in middleaged men with T2D.3 Pearson showed how genetic risk could reclassify some individuals from low to high cardiovascular risk, potentially altering treatment decisions. In particular, patients with low clinical risk but high genetic risk might otherwise be
missed under conventional stratification. These risk scores, now implemented within the iDiabetes framework, offer an added layer of individualisation.
Next, Pearson described the Exeter 5-drug model,4 which uses nine routine clinical features, such as age, BMI, HbA1c, and lipid levels, to predict the likely glycaemic response to five commonly used glucoselowering medications. The model highlights substantial inter-individual variation in drug response. Notably, it reveals a sex-based divergence: women tend to respond better to glucagon-like peptide-1 receptor agonists, while men respond more favourably to sodium-glucose cotransporter-2 inhibitors and sulfonylureas.5
Genetic risk could reclassify some individuals from low to high cardiovascular risk, potentially altering treatment decisions
“When we look at the data, around one in five people are not on their best drug,” said Pearson, highlighting the clinical relevance of these findings. Through the iDiabetes platform, the model has been deployed to help clinicians select the most effective therapy, with HbA1c reduction as the guiding outcome.
Pearson also presented emerging evidence from pharmacogenetic studies linking genetic variants to cardiometabolic outcomes in commonly prescribed drugs. For example, people with loss-of-function variants in cytochrome P450 2C19 (CYP2C19), responsible for activating clopidogrel, have an increased risk of recurrent cardiovascular events. Despite guidelines recommending genotypeguided clopidogrel use, uptake remains limited.6,7 Similar findings were presented for digoxin and metformin: variants in ABCB1 and CUBN, respectively, were associated with increased hospitalisation risk and
B12 deficiency in metformin users. While these pharmacogenetic markers are not yet implemented in iDiabetes, Pearson suggested that they represent a clear opportunity to refine prescribing practices.
The final part of Pearson’s talk focused on closing the implementation gap. In Scotland, where a unified diabetes registry covers the entire population, Pearson’s team is now conducting a cluster-randomised trial
involving over 10,000 patients. General practitioner practices are assigned to either usual care, iDiabetes guideline support, or iDiabetes Plus, which integrates additional genetic and biomarker data. The platform has achieved UK Conformity Assessed certification as a medical device and returns clinical recommendations within 2 weeks of sample collection. The team aims to recruit 20,000 patients by mid-2026, with results expected within the year.

Even in cases with apparently straightforward monogenic mutations, outcomes may be influenced by polygenic background and environmental exposures
Amélie Bonnefond, National Centre for Precision Medicine in Diabetes, Lille, France, offered a molecular deep dive into the genetics of T2D, focusing on the emerging concept of oligogenic diabetes. While most T2D cases are polygenic, with small contributions from many common variants, Bonnefond highlighted that some patients may carry rare variants with moderateto-strong effects that do not meet the threshold for monogenic disease.
She contrasted monogenic and polygenic diabetes, noting that monogenic forms often involve genes crucial to β-cell development or insulin secretion. Importantly, some monogenic mutations are actionable: for example, patients with GCK mutations often require no treatment, while those with GATA4 or GATA6 mutations warrant cardiac evaluation due to associated defects.8
One of the most intriguing parts of her presentation focused on the OPRD1 gene as a novel example of oligogenic contribution to T2D. Rare gain-of-function variants in OPRD1 were associated with increased diabetes risk but decreased adiposity, mirroring the metabolic effects of chronic opium use. Conversely, loss-of-function variants reduced diabetes risk but increased adiposity.9
Bonnefond’s team showed that OPRD1 is expressed in human pancreatic β-cells and that its antagonism, using the compound naltrindole, enhances insulin secretion in vitro. They further traced the endogenous ligand for OPRD1 to β-endorphin, a cleavage product of the prohormone pro-opiomelanocortin, which is itself upregulated by agents that increase cyclic adenosine monophosphate.
In ongoing mouse studies, animals engineered to express human OPRD1 in β-cells showed increased glycaemia, supporting the pathogenic role of OPRD1
gain-of-function mutations (unpublished data). This finding opens the door to new therapeutic targets: delta-opioid receptor antagonists, perhaps modified to avoid crossing the blood–brain barrier, could be used to enhance insulin secretion in a subset of patients with genetically elevated OPRD1 activity.
RWD offers valuable insights, particularly when linked with genomic and exposomic data
Bonnefond concluded by highlighting the complexity of genetic interactions in diabetes. Even in cases with apparently straightforward monogenic mutations, outcomes may be influenced by polygenic background and environmental exposures. A study of over 500,000 people showed that carriers of GCK mutations still develop diabetes-related complications, highlighting the need for a more nuanced model that integrates monogenic, oligogenic, and polygenic contributions.10
Chirag Patel, Harvard Medical School, Boston, Massachusetts, USA, closed the session with a broader view on how realworld data (RWD) can be used to drive precision medicine. He defined RWD as observational, non-randomised data drawn from sources such as medical records, biobanks, registries, and digital health tools. While often underappreciated compared to RCTs, RWD offers valuable insights, particularly when linked with genomic and exposomic data.
One challenge with RWD, he noted, is the potential for “vibration of effects,” which is finding contradictory results depending on how data are modelled. To address this, researchers must be cautious with confounding data and apply robust designs, such as trial emulation or Mendelian randomisation.
Patel presented unpublished data exploring how environmental exposures influence phenotypic outcomes in diabetes and related diseases. In one large-scale study, 619 exposures, including heavy metals, hydrocarbons, and diet, were associated with thousands of biomarkers. Although single exposures explained a small fraction of variance (approximately 0.5%), combining 20 exposures increased explained variance to 3.5%, approaching that of genome-wide PRSs.
Proteomics analyses revealed proteins, such as leptin and growth differentiation factor 15 (GDF15), that were tightly linked to both genetic and environmental exposures. These findings were then cross validated in RCTs such as the HERITAGE study11 (exercise training) and the STEP trials12,13 (semaglutide), showing convergence between RWD and experimental interventions.
References
1. Pang L et al. Improvements in awareness and testing have led to a threefold increase over 10 years in the identification of monogenic diabetes in the U.K. Diabetes Care. 2022;45(3):642-9.
2. iDiabetes. Home. 2025. Available at: https://www.idiabetes.org.uk/. Last accessed: 13 October 2025.
3. Riveros-McKay F et al. Integrated polygenic tool substantially enhances coronary artery disease prediction. Circ Genom Precis Med. 2021;14(2):e003304.
4. Dennis JM et al. A five-drug class model using routinely available clinical features to optimise prescribing in type 2 diabetes: a prediction model development and validation study. Lancet. 2025;405(10480):701-14.
5. Cardoso P et al. Phenotype-based targeted treatment of SGLT2 inhibitors
Looking forward, Patel envisions a future where integrated, multi-modal data, including continuous glucose monitoring, dietary logs, and genomics, will enable clinicians to capture both acute and chronic responses to therapy and environment. RWD, he stressed, must be part of an integrated whole to deliver truly actionable insights.
As the field of diabetes care moves toward precision medicine, this session made one point abundantly clear: progress depends not just on the discovery of genetic variants and predictive models, but on the ability to bring these tools into routine practice. Whether through decision-support systems, the molecular dissection of rare variants, or the integration of RWD, the future of personalised diabetes care is being built, one patient, one data point, and one clinical decision at a time.
and GLP-1 receptor agonists in type 2 diabetes. Diabetologia. 2024;67(5):822-36.
6. Itkonen MK et al. Clopidogrel increases dasabuvir exposure with or without ritonavir, and ritonavir inhibits the bioactivation of clopidogrel. Clin Pharmacol Ther. 2019;105(1):219-28.
7. Bedair KF. Pharmacogenetics at scale in real-world bioresources: CYP2C19 and clopidogrel outcomes in UK Biobank. Pharmacogenet Genomics. 2024;34(3):73-82.
8. Bonnefond A, Semple RK. Achievements, prospects and challenges in precision care for monogenic insulin-deficient and insulin-resistant diabetes. Diabetologia. 2022;65(11):1782-95.
9. Meulebrouck S et al. Functional genetics reveals the contribution of delta opioid receptor to type 2 diabetes and beta-cell function. Nat Commun. 2024;15(1):6627.
10. Barrett KMS et al. Underestimated risk of secondary complications in pathogenic and glucose-elevating GCK variant carriers with type 2 diabetes. Commun Med (Lond). 2024;4(1):239.
11. Sarzynski MA et al. The HERITAGE family study: a review of the effects of exercise training on cardiometabolic health, with insights into molecular transducers. Med Sci Sports Exerc. 2022;54(5S):S1-43.
12. Wilding JPH et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
13. Davies M et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, doubleblind, double-dummy, placebocontrolled, phase 3 trial. Lancet. 2021;397(10278):971-84.
Based on highlights from the European Association for the Study of Diabetes (EASD) 2025 Annual Meeting, these abstract reviews spotlight groundbreaking research, innovative treatments, and emerging priorities shaping the future of diabetes care and metabolic disease management.

Authors: John Pemberton,¹ Louise Collins,¹ Renuka P. Dias,¹ ² Zainaba Mohamed,¹ Vrinda Saraff,¹ Suma Uday,¹,³ *Ruth Krone¹
1. Department of Diabetes & Endocrinology, Birmingham Women’s and Children’s NHS Foundation Trust, UK
2. Applied Health Sciences, University of Birmingham Institute of Cancer and Genomic Sciences, UK
3. Department of Metabolism and Systems Science, University of Birmingham, UK *Correspondence to ruthkrone@nhs.net
Disclosure: Pemberton has received consulting fees from Roche Diagnostics and Abbott Diabetes Care; honoraria for lectures from Dexcom, Insulet, and Abbott Diabetes care; is a faculty member for Exercise for Type 1 Diabetes; and is a member of the Working Group on Continuous Glucose Monitoring of the IFCC Scientific Division. Dias has received grants (NIHR304587 and NIHR206702); honoraria for an advisory board on teplizumab, with payments to the institution; and speaker fees from Sanofi and Sandoz, with payments to the author. The other authors have declared no conflicts of interest.
Acknowledgements: The authors would like to thank the Diabetes Team at Birmingham Women’s and Children’s Hospital, UK, and the children, young people, and families who participated in the programme.
Keywords: Artificial pancreas, children, cloudintegration, diabetes, equity, hybrid closed loop, insulin pumps, technology.
Citation: EMJ Diabet. 2025;13[1]:36-38. https://doi.org/10.33590/emjdiabet/IXIL9384
Do automated insulin delivery systems outperform systems requiring manual upload and adjustment?1 Automated insulin delivery (AID) systems are now widely used in paediatric diabetes care, with multiple systems recommended by international guidelines. Evidence consistently shows that AID improves HbA1c and time in range (3.9–10.0 mmol/L or 70–180 mg/dL), with systematic reviews confirming gains of 10–15% in children and young people, across both outpatient and real-world settings.2-4
The authors compared the two categories of AID systems used in their centre: AutoAID (cloud-integrated, automated algorithm updates, e.g., MiniMed 780G [Medtronic, Dublin, Ireland], CamAPS FX [CamDiab Ltd, London, UK], Omnipod 5 [Insulet, Acton, Massachusetts, USA]) and ManualAID (static algorithms requiring user uploads and adjustments, e.g., Tandem ControlIQ [Tandem Diabetes Care, San Diego, California, USA]).
Users in both categories achieved clinically meaningful outcomes, with HbA1c reduced by approximately 6 mmol/mol and time in range increased by 12–15% at 12 months, despite two-thirds of the cohort falling into the most severe category of socioeconomic deprivation. This underlines a fundamental point: AID systems work well.
However, additional benefits for AutoAID users emerged regarding higher time in tight range (TITR; 3.9–7.8 mmol/L or 70–140 mg/dL) and onboarding efficiency. TITR is a strong predictor of long-term complications, with even a 5% gain linked to reduced retinopathy risk.5 The authors’ AutoAID users demonstrated an additional TITR improvement of approximately 4%, confirmed in mixed-effects modelling (Figure 1). This suggests that automatic algorithm updates and adjustable targets may offer additional advantages.
Onboarding efficiency was another clear differentiation. AutoAID enabled 93% of families to onboard remotely compared to 52% of ManualAID, translating into a six-fold saving in both educator and family time. Automatic data uploads remove barriers such as lack of computer access, and enable remote support provision between clinic appointments, while ManualAID requires an active uploading process for this,
Figure 1: Independent predictors of time in tight range identified in mixed-effects modelling.
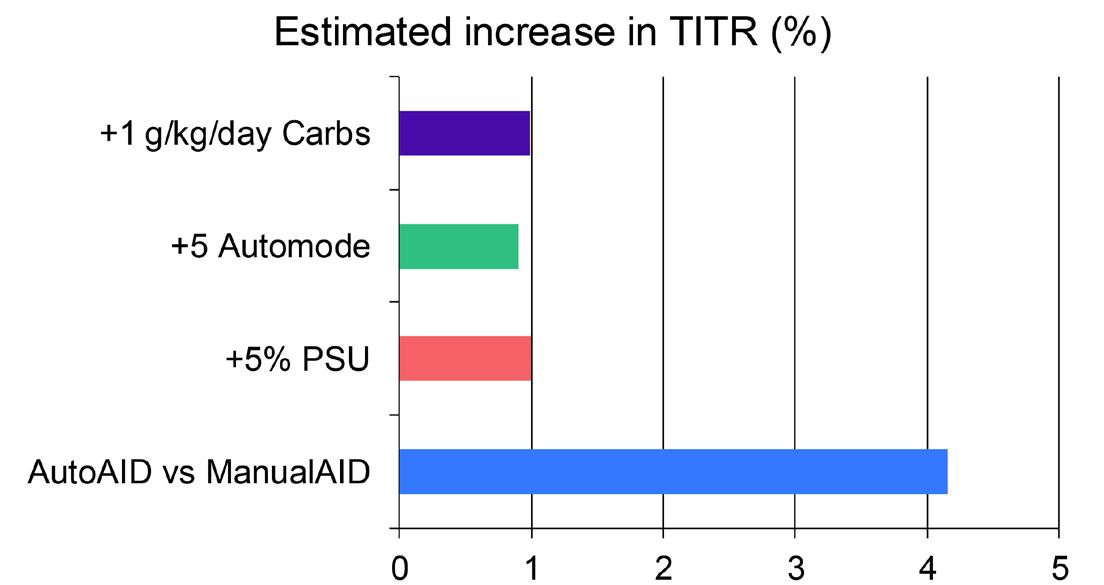
AutoAID use was associated with a 4.2% increase in TITR, while a 5% increase in sensor wear, 5% increase in automode use, and 1 g/kg/day higher carbohydrate intake were each associated with smaller, but significant gains. TITR was defined as 3.9–7.8 mmol/L (70–140 mg/dL).
PSU: percentage sensor usage; TITR: time in tight range; vs: versus.
potentially disadvantaging users like the authors’ deprived cohort. To achieve equity in diabetes care, this distinction matters: access and efficiency must not depend on socioeconomic status.
Further studies will show whether autoupdating algorithms are inherently superior to manual ones. AutoAID recalibrates automatically based on total daily insulin, reducing user burden but limiting finetuned control. In contrast, ManualAID allows clinicians and families to adjust basal rates and correction factors directly. In highly skilled hands, this flexibility may be advantageous, particularly in paediatrics, where insulin needs shift rapidly with growth, hormones, and circadian variability. Yet, as seen in the authors’ socioeconomically deprived cohort, the
need for manual upload and adjustment might prove disadvantageous.
What does this mean for clinical practice? A diabetes team confident in adjusting settings with patients able to engage regularly may achieve superior outcomes with ManualAID than automation alone, while less experienced users or those less resourced may fare worse. The power to adjust algorithms offers the potential for better control, but only if users have the required intellectual and digital capacity to upload and engage in systems.
Looking ahead, the launch of TandemSource (Tandem Diabetes Care) offers automatic
uploads for Control-IQ, addressing one major limitation. Whether this equalises outcomes, or whether algorithm update methods (automated by total daily dose versus manually tuned settings) remain a differentiator, will depend on skill, frequency of review, and the support structures surrounding families.
References
1. Pemberton J et al. Automated insulin delivery with automated algorithm updates and cloud integration: does it outperform manual adjustments and uploading? Abstract 257. EASD Annual Meeting, 15-19 September, 2025.
2. Biester T et al. ISPAD clinical practice consensus guidelines 2024: Diabetes technologies – insulin delivery. Horm Res Paediatr. 2024;97(6):636-62.
3. Zeng B et al. Automated insulin delivery systems in children and adolescents with type 1 diabetes: a systematic review and meta-analysis. Diabetes Care. 2023;46(12):2300-7.
4. Peacock S et al. A systematic review of commercial hybrid closed-loop automated insulin delivery systems. Diabetes Ther. 2023;14(5):839-55.
5. Shah VN et al. Time in range is associated with incident diabetic retinopathy in adults with type 1 diabetes: a longitudinal study. Diabetes Technol Ther. 2024;26(4):246-51.
Authors: *Malak Hamza,1,2 Dimitris
Papamargaritis,1-3 Andrew P. Hall,4 Tom Yates,1,2
Melanie J. Davies,1,2 Joseph Henson1,2
1. Diabetes Research Centre, University of Leicester, UK
2. National Institute for Health and Care Research (NIHR) Leicester Biomedical Research Centre, University Hospitals of Leicester NHS Trust and University of Leicester, UK
3. Department of Diabetes and Endocrinology, Kettering General Hospital, University Hospitals of Northamptonshire NHS Group, UK
4. Hanning Sleep Laboratory, University Hospitals of Leicester, UK
*Correspondence to Malak.hamza@leicester.ac.uk
Disclosure: Papamargaritis has received grants or contracts from Novo Nordisk, Novo Nordisk UK Research Foundation, the Academy of Medical Sciences/Diabetes UK, Health Education East Midlands, and the National Institute for Health and Care Research (NIHR), with payments to the institution; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Novo Nordisk, Eli Lilly, Boehringer Ingelheim, and Johnson and Johnson, with payments to the author; support for attending meetings and/or travel from Novo Nordisk; has participated on a data safety monitoring board or advisory board for Recordati; is an operational member for the Association for the Study of Obesity; and is a member of the Academic Subcommittee for the Association of British Clinical Diabetologists. Yates has received an investigator-initiated research grant from AstraZeneca, with payment to the institution; a contracted research grant from the Reinsurance Group of America, with payment to the institution; and consulting fees from Regeneron, with payment to the author. Davies has received grants or contracts from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk, with payments to the author; consulting fees from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Sanofi, and Eli Lilly; support for attending meetings and/or travel from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Amgen, AstraZeneca, Biomea Fusion, Regeneron, and Zealand Pharma; and has participated on a data safety monitoring board or advisory board for Amgen, AstraZeneca, Biomea Fusion, Sanofi, Zealand Pharma, Carmot/Roche, Regeneron, EktaH, AbbVie, GSK, and Daewoong
Pharmaceutical. Hamza has received support for attending meetings and/or travel from the Leicester Diabetes Research Centre, University of Leicester. The other authors have declared no conflicts of interest.
Acknowledgements: This research is funded by the National Institute for Health and Care Research (NIHR) Leicester Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The authors would like to thank the participants for taking the time to participate, as well as the members of the Leicester Diabetes Centre Patient and Public Forum for their valuable input in the study design. The authors also acknowledge the contributions of the collaborating research sites, Sahar Khodabakhsh, Leicester Diabetes Centre, University Hospitals of Leicester NHS Trust, UK, for her continued oversight of the trial, and Mike Bonar, Creative Director at the Leicester Diabetes Research Centre, UK, for designing and creating the figure.
Keywords: Frailty, physical function, Type 2 diabetes (T2D).
Citation: EMJ Diabet. 2025;13[1]:39-41. https://doi.org/10.33590/emjdiabet/JULV2437
Physical function decline is an increasingly recognised concern among individuals with long-term conditions such as Type 2 diabetes (T2D).1 This decline often coexists with obesity and is now observed at younger ages, reflecting the rising prevalence of early-onset T2D and its associated complications.2 These trends contribute to elevated risks of sarcopenia and frailty, with significant implications for long-term morbidity.3 Despite growing awareness, the relationship between the duration of T2D and physical function outcomes remains unexplored. Understanding the association is critical for identifying individuals at greatest risk and informing targeted interventions. The aim of this study was to evaluate the association between T2D duration and physical function outcomes, independent of chronological age.4
Figure 1: Five-repetition chair sit-to-stand test in association with Type 2 diabetes duration.
40-year-old man living with T2D for 20 years
60-year-old man newly diagnosed with T2D
5-STS: 12.6 seconds
*Data presented as mean.7
5-STS: 12.7 seconds
5-STS: five-repetition sit-to-stand; T2D: Type 2 diabetes.
The authors analysed data from individuals with T2D in the CODEC study, a crosssectional, multisite, observational trial exploring chronotype and health outcomes in the UK in individuals with T2D.5 Physical function was assessed using the short performance physical battery (SPPB), 4-metre gait speed, five-repetition chair sit-to-stand (5-STS), 60-second chair sit-to-stand (STS-60), handgrip strength (sex-specific), and the Duke Activity Status Index (DASI). Associations between selfreported T2D duration and physical function outcomes were evaluated using linear regression models, adjusted for age, sex, and ethnicity.
A total of 1,204 participants were included (mean age: 65±12 years; age at diagnosis: 54±14 years; HbA1c: 53±15.2 mmol/mol [7±1.4%]; T2D duration: 11±11 years; BMI: 31±7 kg/m2). The cohort was predominantly White (84%), with 12% South Asian representation. Baseline physical function scores were an SPPB score of 11±3 points, a gait speed of 4.2±2 seconds, a 5-STS
80-year-old man without T2D*
5-STS: 11.6 seconds
of 14±4 seconds, an STS-60 of 22±8 repetitions, a handgrip strength of 38±12 kg in men and 23±9 kg in women, and a DASI score of 43±24 points. Longer T2D duration was significantly associated with poorer physical function across all measures, independent of age, sex, and ethnicity. Specifically, each 10-year increase in T2D duration was associated with:
• a 0.3-point lower SPPB score (95% CI: –0.41–-0.11; p<0.001);
• a 0.2-second slower gait speed (95% CI: 0.06–0.30; p=0.003);
• a 0.6-second longer 5-STS time (95% CI: 0.12–1.01; p=0.013);
• 0.7 fewer repetitions in STS-60 (95% CI: –1.29–-0.02; p=0.043);
• a 1.02 kg lower handgrip strength in men (95% CI: –1.87–-0.16; p=0.020) and a 0.8 kg lower handgrip strength in women (95% CI: –1.64–0.08; p=0.074); and
• a 1.9-point lower DASI score (95% CI: –2.94–-0.77; p<0.001).
To contextualise, a 40-year-old man living with T2D for 20 years would be expected to have a 5-STS time of approximately 12.6 seconds (Figure 1). This indicates that, by midlife, he has already experienced a clinically significant decline in physical
function.6 This estimate is comparable to that of a 60-year-old man with newly diagnosed T2D (12.7 seconds) and both closely resemble the normative value for an 80-year-old man without T2D (11.6 seconds; Figure 1).7
The authors’ findings illustrate the cumulative impact of T2D duration on physical function, independent of chronological age. This highlights the need for greater awareness among healthcare professionals and patients regarding the long-term functional consequences of T2D. Future research should focus on evaluating the effectiveness of pharmacological, nutritional, and exercise-based interventions in preserving physical function and improving long-term outcomes in this population.
References
1. 61st EASD Annual Meeting of the European Association for the Study of Diabetes: Vienna, Austria, 15-19 September 2025. Diabetologia. 2025;68(Suppl 1):1-754.
2. Ahmad E et al. Type 2 diabetes and impaired physical function: a growing problem. Diabetology. 2022;3(1):30-45.
3. Wong E et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013;1(2):106-14.
4. Hamza et al. Physical function outcomes in people with type 2 diabetes (T2D): what is the impact of T2D duration above and beyond chronological age? Abstract 211. EASD Annual Meeting, 15-19 September, 2025.
5. Brady EM et al. Rationale and design of a crosssectional study to investigate and describe the chronotype of patients with type 2 diabetes and the effect on glycaemic control: the CODEC study. BMJ Open. 2019;9(11):e027773.
6. Jones SE et al. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax. 2013;68(11):1015-20.
7. Landi F et al. Normative values of muscle strength across ages in a ‘real world’ population: results from the longevity check‐up 7+ project. J Cachexia Sarcopenia Muscle. 2020;11(6):1562-9.
Authors: Federica Zatterale,1,2 Agnieszka Podraza-Farhanieh,1 Lorenza Zinna,2 Silvia Gogg,1 Annika Nerstedt,1 Francesco Beguinot,2 Rosa Spinelli,1,2 *Ulf Smith1
1. Lundberg Laboratory for Diabetes Research, Department of Molecular and Clinical Medicine, Sahlgrenska Academy, University of Gothenburg, Sweden
2. Department of Translational Medical Sciences, University of Naples Federico II, Italy
*Correspondence to ulf.smith@medic.gu.se
Disclosure: Zatterale has received support for attending meetings and/or travel from the European Association for the Study of Diabetes (EASD). The other authors have declared no conflicts of interest. No external funding or financial support was received for this work.
Keywords: Cellular senescence (CS), glucagon -like peptide-1 receptor agonists (GLP-1RA), hepatocyte senescence, hyperinsulinaemia, insulin resistance (IR), metabolic dysfunctionassociated steatohepatitis, metabolic dysfunction-associated steatotic liver disease, senescence-associated secretory phenotype (SASP).
Citation: EMJ Diabet. 2025;13[1]:42-44. https://doi.org/10.33590/emjdiabet/TVAJ8774
Hepatocyte senescence has emerged as a key pathogenic driver in the onset and progression of metabolic dysfunctionassociated steatotic liver disease and its advanced stage, metabolic dysfunctionassociated steatohepatitis.1 In metabolic dysfunction-associated steatotic liver disease, hepatocytes undergo senescence, losing their proliferative capacity and acquiring a senescenceassociated secretory phenotype (SASP). SASP factors, including pro-inflammatory cytokines, chemokines, and pro-fibrotic mediators, profoundly reshape the hepatic microenvironment, promoting lipid
accumulation and chronic inflammation, and activating fibrogenic pathways, thereby accelerating the transition from simple steatosis to metabolic dysfunctionassociated steatohepatitis 1-3
Recent evidence has expanded this concept, linking hyperinsulinaemia to senescence-driven metabolic dysfunction. Chronic hyperinsulinaemia directly induces cellular senescence (CS), which in turn amplifies insulin resistance (IR) and tissue dysfunction through SASP secretion, creating a self-reinforcing pathological loop.4,5 Interventions that lower insulin levels have been shown to attenuate IR, reduce hepatic steatosis, suppress inflammation, and extend lifespan. The causal role of insulin in driving CS is further supported by preclinical studies in which senolytic treatments alleviate metabolic dysfunction. Together, these results highlight a vicious cycle in which CS and hyperinsulinaemia perpetuate each other, underscoring the therapeutic value of strategies that disrupt this loop.5-7 Among these, glucagon-like peptide-1 receptor agonists (GLP-1RA) stand out as particularly promising. Beyond their glucose-lowering and weight-reducing actions, increasing evidence indicates that GLP-1RAs exert direct anti-inflammatory and anti-senescence effects. Preclinical studies show that the GLP-1RA exendin-4 reverses age-related alterations in different tissues, including liver and adipose tissue, while clinical data show that longterm GLP-1RA administration reduces systemic inflammation and mitigates metabolic decline. These observations raise the possibility that GLP-1RAs act as senotherapeutic agents.8-10
Figure 1: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 targeting protect against hepatocyte senescence.
Hyperinsulinaemia
Senescence
Senescent hepatocytes
SASP factors
Hyperinsulinaemia
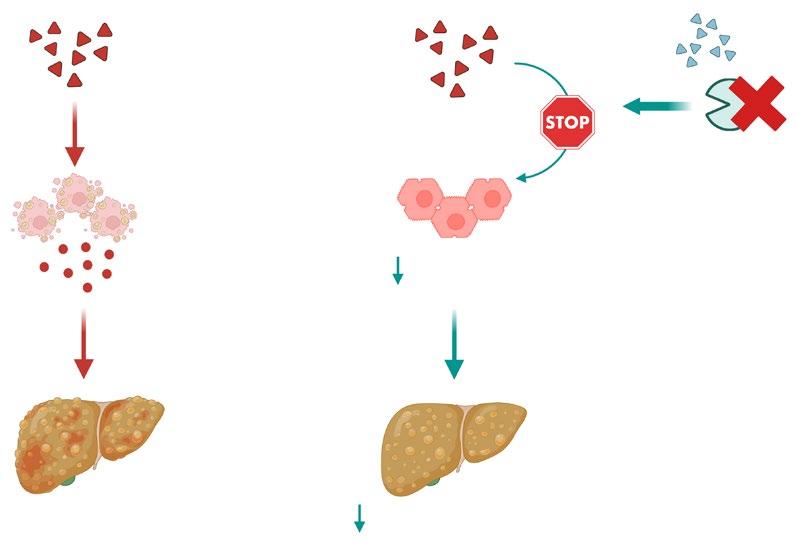
MASLD/MASH
Created using BioRender.com.
Senescent hepatocytes
Senescence
GLP-1RAs
DPP-4 inhibition
MASLD/MASH progression
DPP-4: dipeptidyl peptidase-4; GLP-1RAs: glucagon-like peptide-1 receptor agonists; MASH: metabolic dysfunctionassociated steatohepatitis; MASLD: metabolic dysfunction-associated steatotic liver disease; SASP: senescenceassociated secretory phenotype.
In this context, the authors investigated the ability of glucagon-like peptide-1 (GLP-1) and exendin-4 to counteract hepatocyte senescence induced by chronic hyperinsulinaemia. Human hepatocytes exposed to prolonged hyperinsulinaemia developed a senescent phenotype, characterised by upregulation of canonical senescence markers (ZMAT3, p53, p21, phosphorylated γH2AX) and SASP factors (CXCL8, IL-18, and MMP3). Treatment with GLP-1 or exendin-4 markedly attenuated the expression of both senescence markers and SASP mediators, demonstrating that GLP-1 and exendin-4 can suppress the senescence programme triggered by hyperinsulinaemia. Because GLP-1 metabolism is regulated by dipeptidyl peptidase-4 (DPP-4), the authors next examined whether modulating DPP-4 impacts hepatocyte senescence. Silencing
DPP-4 alone reduced senescence markers and SASP mediators. Furthermore, combining DPP-4 silencing with GLP-1 or exendin-4 treatment produced an additive reduction in senescence and SASP factors, suggesting that dual targeting of DPP-4 and GLP-1 signalling may provide synergistic protection against hepatocyte senescence.
Collectively, the authors’ findings provide evidence that GLP-1 and exendin-4 protect hepatocytes from senescence, and that lowering DPP-4 levels enhances this protective effect (Figure 1). These results uncover a previously unrecognised mechanism of GLP-1RAs and support their broader potential in reducing systemic metabolic dysfunction. Importantly, combining GLP-1RAs with lifestyle interventions and other senescence-
targeted strategies could break the vicious cycle linking IR and CS, thereby slowing disease progression and enhancing metabolic health.
References
1. Zatterale F et al. GLP-1 receptor agonists (GLP1RAs) protect against hepatocyte senescence. Presentation. EASD Annual Meeting, 15-19 September, 2025.
2. Du K et al. Targeting senescent hepatocytes for treatment of metabolic dysfunction-associated steatotic liver disease and multi-organ dysfunction. Nat Commun. 2025;16(1):4195.
3. Spinelli R et al. Increased cell senescence in human metabolic disorders. J Clin Invest. 2023;133(12):e169922.
4. Abdul-Ghani M, DeFronzo RA. Insulin resistance and hyperinsulinemia: the egg and the chicken. J Clin Endocrinol Metab. 2021;106(4):e1897-9.
5. Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: time for a conceptual framework shift. Diab Vasc Dis Res. 2019;16(2):118-27.
6. Templeman NM et al. Reduced circulating insulin enhances insulin sensitivity in old mice and extends lifespan. Cell Rep. 2017;20(2):451-63.
7. Suda M et al. Targeting cell senescence and senolytics: novel interventions for agerelated endocrine dysfunction. Endocr Rev. 2024;45(5):655-75.
8. Drucker DJ. The benefits of GLP-1 drugs beyond obesity. Science. 2024;385(6706):258-60.
9. Peng W et al. Novel insights into the roles and mechanisms of GLP-1 receptor agonists against aging-related diseases. Aging Dis. 2022;13(2): 468-90.
10. Carapeto P et al. Exercise activates AMPK in mouse and human pancreatic islets to decrease senescence. Nat Metab. 2024;6(10):1976-90.
Citation: EMJ Diabet. 2025;13[1]:45-52. https://doi.org/10.33590/emjdiabet/TIWT2484
The following highlights showcase key research presented at the European Association for the Study of Diabetes (EASD) Annual Meeting 2025. Featured studies explore innovations across the spectrum of diabetes research, from long-term real-world benefits of hybrid closed-loop systems and genetic insights into maturity onset diabetes of the young, to the predictive value of single glutamate decarboxylase autoantibody (GADA) positivity for disease progression. Other investigations reveal how rare mitochondrial mutations contribute to insulin resistance, the vascular advantages of exercise over pharmacotherapy in weight maintenance, and the distinct metabolic effects of low-carbohydrate diets on hepatic glucose handling. Together, these findings highlight the expanding frontiers of diabetes science and its growing emphasis on personalisation, prevention, and the interplay between genetics, behaviour, and technology.

A BELGIAN multi-centre study, presented at EASD 2025, revealed compelling new data on the 24-month outcomes of the Control-IQ system (Tandem Diabetes Care, Inc., San Diego, California, USA) in adults with Type 1 diabetes (T1D). The study showed persistent improvements not only in glycaemic control but also in quality of life and work participation, with over 93% of participants continuing to use the system for 2 years after initiation.¹
Hybrid closed-loop insulin delivery systems have emerged as transformative tools in the management of T1D, offering automated glucose regulation with reduced patient burden. While RCTs have demonstrated short-term efficacy, long-term real-world evidence remains crucial to understanding the sustained impact of these technologies.
This prospective observational study recruited all adults with T1D who began using Control-IQ between October 2021–December 2022 across 13 diabetes centres in Belgium. Participants underwent routine evaluations every 4 months up to 24 months post-initiation. Glycaemic metrics were collected alongside person-reported outcomes using validated questionnaires, and the incidence of severe hypoglycaemic events and work absenteeism was selfreported. Data were analysed using mean values with SDs or least-squares means with 95% CIs.
Of the 473 adults enrolled, 442 (93.4%) continued Control-IQ for the full 24 months. The cohort had a mean age of 38.5±13.1 years, with 57.3% female, and a mean diabetes duration of 20.0±12.6 years. Time in range (70–180 mg/dL) increased significantly from 58.8% at baseline to 71.0% at 12 months (p<0.001) and was maintained at 70.7% at 24 months (p<0.001). Improvements in HbA1c, time in tight range (70–140 mg/dL), and reductions in hypo- and hyperglycaemia were sustained throughout. Reported severe hypoglycaemic events dropped from 40.9 to 15.0 events per 100 person-years. Work absenteeism fell from 126 to 69 days per 100 person-years over
the same period. Participants also reported sustained reductions in diabetes distress and fear of hypoglycaemia, alongside increased treatment satisfaction.
Hybrid closed-loop insulin delivery systems have emerged as transformative tools in the management of T1D
These findings highlight the long-term benefits of Control-IQ in routine clinical practice, extending beyond glycaemic metrics to include psychosocial and functional outcomes. Limitations include the observational design, reliance on selfreported data for some endpoints, and the absence of a control group. Nonetheless, the high retention rate and consistency of benefits across multiple domains support the system’s enduring value in everyday diabetes care.
A RECENT study, presented at EASD 2025, explored the prevalence, penetrance, and mortality associated with maturity onset diabetes of the young (MODY) in a population sample of 454,275 individuals from the UK Biobank, providing insights into the broader implications for clinical practice.2
MODY is relatively prevalent in the general population and exhibits variable penetrance, with low penetrance genes being more common in non-clinical cohorts. Exome sequencing was performed on 454,275 UK Biobank participants, with a mean age of 57 years, to identify pathogenic variants in 10 known MODY genes. The prevalence of a MODY pathogenic variant was found to be 0.095%, and the prevalence increased to 0.63% among individuals with diabetes. For those diagnosed with diabetes before the age of 30 years, the prevalence rose to 2.13%. The most common MODY gene variants were GCK (37.7%), followed by RFX6 (22.0%), and HNF4A (22.0%).
Interestingly, variants in low-penetrance genes accounted for 31% of identified cases in the population, though these genes contribute less than 2% in clinical settings. GCK variants showed high penetrance, with average HbA1c 8.8 mmol/mol higher than controls, and 94.5% of carriers had prediabetes or diabetes. However, these variants did not increase all-cause mortality (hazard ratio: 0.94; p=0.79). In
contrast, non-GCK variants showed lower penetrance, with RFX6, HNF4A, and HNF1A having penetrance rates of 12.0%, 32.9%, and 60.6%, respectively, by the age of 60 years. These variants did not increase mortality risk either (hazard ratio: 0.81; p=0.32).
The findings demonstrate that MODY is more prevalent in the general population than previously recognised, with varying penetrance depending on the genetic variant. This highlights the importance of refining screening strategies for MODY, particularly for low-penetrance genes. The lack of increased mortality in individuals with GCK-MODY supports the current clinical practice of discontinuing followup for these patients, suggesting that ongoing monitoring may be unnecessary for those with this variant. Future research should focus on refining clinical guidelines to incorporate these findings, optimising management for patients with MODY based on genetic aetiology.
0.095
The prevalence of a MODY pathogenic variant was found to be % and the prevalence increased to among individuals with diabetes 0.63 %

A NEW study presented at EASD 2025 has provided new insights into diabetes risk among adults who test positive for glutamate decarboxylase autoantibodies (GADA).3
Around 80% of people diagnosed with autoimmune diabetes have detectable autoantibodies, and single GADA positivity is the most common finding in adults at diagnosis. With growing interest in diabetes prevention and immunotherapy, understanding the risk for adults identified as single GADA positive has become increasingly important.
Researchers analysed data from 6,115 adult relatives of people with autoimmune diabetes, with a median follow-up period of nearly 11 years. Among them, 199 individuals (3%) were GADA positive, and 53 of these (27%) developed diabetes during the study period. The risk of diabetes progression varied markedly depending on the number and level of autoantibodies present.
As expected, those with multiple autoantibody positivity were at the highest risk, showing a 41% chance of developing diabetes within 10 years. However, even adults who were single GADA-positive faced a substantially elevated risk compared to those testing negative for autoantibodies (12% versus 2% over 10 years). Importantly, higher GADA levels were found to significantly increase the likelihood of diabetes progression within this group. Participants with GADA levels ≥450 DK U/ mL had a 39% 10-year risk, while those with lower levels had only a 9% risk.
Overall, being single GADA positive was associated with a sixfold higher 10-year risk of developing diabetes compared with individuals who were autoantibody-negative
Overall, being single GADA positive was associated with a sixfold higher 10-year risk of developing diabetes compared with individuals who were autoantibodynegative. The study highlights that adults with high GADA levels may have a similar risk profile to those with multiple autoantibody positivity. These findings provide useful evidence for clinicians and researchers designing screening programmes and immunotherapy trials. Adults identified as single GADA positive, particularly those with high antibody levels, may benefit from closer monitoring and could be prioritised for inclusion in Type 1 diabetes prevention studies.
A PIONEERING study presented at EASD 2025 has shed new light on how mitochondrial abnormalities contribute to insulin resistance in humans. The research focused on individuals carrying a rare pathogenic mitochondrial DNA mutation, m.3243A>G, offering a unique human model to dissect the causal role of mitochondria in metabolic disease.4
Led by an international team of researchers, the case-control study compared 15 carriers of the m.3243A>G mutation with healthy participants matched for age, sex, and physical activity level. Using advanced physiological and metabolic techniques, including the hyperinsulinaemiceuglycaemic clamp, femoral arteriovenous balance, glucose tracer infusion, and muscle biopsies, the team assessed insulin sensitivity across skeletal muscle, liver, and adipose tissue, as well as β-cell function and mitochondrial integrity.
The results revealed that skeletal muscle insulin sensitivity was 45% lower in carriers of the mutation compared to controls, despite normal liver and adipose tissue insulin sensitivity. This suggests that mitochondrial dysfunction exerts a tissuespecific effect, predominantly impairing muscle glucose uptake. Carriers also exhibited a 65% reduction in β-cell function, indicating that mitochondrial defects may contribute to both insulin resistance and impaired insulin secretion.
Muscle analyses showed reduced mitochondrial content, as evidenced by lower citrate synthase activity, and diminished oxidative phosphorylation capacity per unit of tissue. However, when corrected for mitochondrial abundance, intrinsic mitochondrial function and reactive oxygen species production appeared largely preserved, suggesting that insulin resistance stems from a quantitative rather than qualitative mitochondrial deficit.
These findings point to a direct link between reduced mitochondrial content and skeletal
muscle insulin resistance, independent of systemic factors or intrinsic mitochondrial dysfunction. The study team noted that ongoing analyses of plasma and muscle samples will further clarify the molecular pathways involved.
By isolating the mitochondrial contribution to insulin resistance in humans, this research provides critical insight into the pathophysiology of Type 2 diabetes and highlights potential avenues for the development of mitochondria-targeted therapies aimed at restoring metabolic health.
The results revealed that skeletal muscle insulin sensitivity was 45% lower in carriers of the mutation compared to controls, despite normal liver and adipose tissue insulin sensitivity
EXERCISE, but not treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist, reduces the development of atherosclerosis during weight loss maintenance in adults with obesity, according to new research presented at EASD 2025.5
Obesity is a major contributor to systemic inflammation, endothelial dysfunction, and subsequent atherosclerosis, which remains the leading cause of cardiovascular disease. Whilst weight reduction strategies such as low-calorie diets, pharmacotherapy, and exercise are known to reduce cardiovascular risk, their relative effectiveness in preventing vascular changes after weight loss is not fully understood. Incretinbased therapies, such as GLP-1 receptor agonists, have gained popularity for weight management, but their direct effects on vascular health compared with exercise remain to be clarified.
This randomised, controlled, two-bytwo factorial trial enrolled 215 adults with obesity and without diabetes. Following an 8-week low-calorie diet, 195 participants who achieved an average weight loss of 13.1 kg were randomly assigned to 52 weeks of maintenance therapy with exercise and/or the GLP-1 receptor agonist liraglutide 3.0 mg/day, or placebo. Circulating biomarkers of inflammation, including IL-6 and interferon-γ (IFN-γ), and markers of endothelial function (intercellular adhesion molecule 1 [ICAM-1], vascular cell adhesion molecule 1 [VCAM-1], and tissue plasminogen activator [tPA]) were measured, along with carotid intima-media thickness as an indicator of atherosclerosis.
Participants in the exercise group demonstrated significant reductions in IL-6 by 21% (95% CI: –34–-10; p=0.012), IFN-γ by 27% (–46–-1; p=0.044), and VCAM by 6% (–12–-0.1; p=0.046). Small but favourable trends were also noted for ICAM (–8%; p=0.058) and tPA (–1.08 ng/mL; p=0.036). Carotid intima-media thickness was reduced by 0.024 mm (–0.044–-0.005; p=0.015) in exercising participants, while
no significant vascular or inflammatory changes were observed in those receiving liraglutide.
These findings demonstrate that exercise, but not incretin-based therapy, reduces atherosclerosis development during weight loss maintenance, beyond weight loss alone, improving vascular structure and inflammatory status. In clinical practice, structured exercise should remain a central component of obesity management strategies aimed at preventing atherosclerosis.
A KEY FINDING in data presented at EASD 2025 showed that a low-carbohydrate diet (LCD) led to a more effective suppression of liver-derived glucose production following a glucose load, independent of weight loss.6
The effects of LCDs on weight loss and blood glucose control are well established, but their influence on organ-specific glucose handling remains less clear, particularly when changes in body weight are accounted for. Researchers have now compared an LCD with a Mediterraneanstyle diet (MED), both energy-matched and mildly hypocaloric, to examine how each affects glucose metabolism at a physiological level in people who are overweight and without diabetes.
In the randomised cross-over study, 20 participants with a BMI over 27 kg/ m² (average age: 53.5 years; 55% female) completed two 4-week dietary interventions: an LCD (20% carbohydrate, 50% fat, 30% protein) and an energymatched MED (50% carbohydrate, 20% fat, 30% protein). Glucose metabolic fluxes were measured using dual-isotope oral glucose tolerance tests after each diet, supported by continuous glucose monitoring and food diary assessments to ensure compliance.
Both diets led to a comparable, modest weight reduction (LCD: –2.8±2.3 kg; MED: –2.7±2.4 kg; p=0.398). However, glucose metabolism differed in key areas. Suppression of endogenous glucose production was significantly greater following LCD compared to MED (area under the curve: 1,437±341 versus 1,553±250 μmol/min/kg x min; p=0.041), indicating improved hepatic insulin sensitivity. Endogenous glucose production adjusted

for insulin secretion also favoured LCD (area under the curve: 113±43 versus 125±41 μmol/min/kg x nmol/m² x min; p=0.026).
Additionally, glucose absorption was delayed on a LCD, as shown by later time to peak appearance of oral glucose (30±12 versus 21±7 min; p=0.001), although overall absorption was similar (p=0.303). There were no significant differences in peripheral glucose clearance or insulin-adjusted clearance between diets.
These findings suggest that short-term carbohydrate restriction can beneficially alter hepatic and intestinal glucose handling, potentially offering postprandial glycaemic benefits without affecting peripheral insulin sensitivity. In clinical practice, LCDs may be particularly useful in managing glucose profiles in individuals at risk of metabolic disease. However, limitations include the short study duration, small sample size, and lack of longer-term outcomes. Further research is needed to determine whether these effects persist and translate into long-term clinical benefit.
In the randomised cross-over study, 20 participants with a BMI over 27 kg/ m² (average age: 53.5 years; 55% female) completed two 4-week dietary interventions: an LCD (20% carbohydrate, 50% fat, 30% protein) and an energy-matched MED (50% carbohydrate, 20% fat, 30% protein).
References
1. De Meulemeester J et al. Long-term glycaemic management and personreported outcomes two years after Control-IQ initiation in adults with Type 1 diabetes. Abstract 54. EASD Annual Meeting, 15-19 September, 2025.
2. Sharp LN et al. Exploring MODY in the population: understanding the prevalence, variable penetrance and mortality in 454,275 people. Abstract 67. EASD Annual Meeting, 15-19 September, 2025.
3. Grace SL et al. Risk of future diabetes in single glutamate decarboxylase autoantibody positive adults. Abstract 93. EASD Annual Meeting, 15-19 September, 2025.
4. Nielsen TL et al. Harnessing a pathogenic mitochondrial DNA mutation to uncover mitochondrial mechanisms driving insulin resistance in humans. Abstract 242. EASD Annual Meeting, 15-19 September, 2025.
5. Sandsdal RM et al. Weight loss maintenance with exercise but not with GLP-1 receptor agonist
treatment decreases atherosclerosis development. Abstract 138. EASD Annual Meeting, 15-19 September, 2025.
6. Cimbalo N et al. Dietary carbohydrate restriction affects hepatic glucose production and intestinal glucose absorption independently of body weight loss. Abstract 159. EASD Annual Meeting. 15-19 September, 2025.
EMJ is delighted to present leading voices from the European Association for the Study of Diabetes (EASD), who share their insights on the future of diabetes research and care. President Chantal Mathieu discusses the association’s renewed energy and expanding educational reach; Hindrik Mulder explores the role of EASD in advancing global research and the responsible integration of AI; and Patrick Schrauwen, Chair of the EASD Early Career Academy, highlights efforts to empower the next generation of researchers and strengthen translational science for improved patient outcomes.
Featuring: Chantal Mathieu, Hindrik Mulder, and Patrick Schrauwen


Chantal Mathieu
President, European Association for the Study of Diabetes (EASD) Board; Department of Endocrinology, UZ Leuven; Department of Chronic Diseases and Metabolism, KU Leuven, Belgium
Citation: EMJ Diabet. 2025;13[1]:53-58. https://doi.org/10.33590/emjdiabet/KQFU7715
Q1As the President of the European Association for the Study of Diabetes (EASD) board, and after another successful annual meeting, what have been the most significant changes you have seen in your tenure so far? And how have they impacted patient care?
EASD is something very strange. It’s an association of individual members, including researchers, clinicians, and students; anybody involved in any type of research on diabetes can become an individual member. This association works 365 days a year, and our highlight is indeed our annual meeting, where this year, we attracted almost 14,000 people. But we’re also very active outside of these annual meetings. We have a teaching and education committee making an accredited e-learning platform with formal sit-down modules, where you need to answer evaluations to get your accreditation, but also podcasts and debates; there is a range of formats.
Face-to-face educational sessions are also important. This year, we had a technology school for early-career clinicians. Next year, we will repeat that and have an immunology school for paediatricians and endocrinologists, because the concept of screening for Type 1 diabetes and disease-modifying therapies is coming, and islet transplantation is coming. EASD is more than our annual meeting.
That has been an important lesson for me in the last 3 years of my presidency: the need to have face-to-face interactions alongside our virtual platforms
So, what is different during my presidency? I think it’s the spirit. We’re always very serious when
it comes to discussing science. We already have a flagship journal, but we just launched a new journal that is also an openaccess journal. We have a sister organisation, a foundation for supporting research, and our annual meeting. But it’s always serious. What has changed, I believe, over the last few years is the upbeat, optimistic, positive young vibe. We’re shedding a bit of our stuffy image and becoming quite the dynamic place for learning and for sharing diabetes research. This will hopefully contribute to one day being able to prevent and cure many forms of diabetes.
It is also important to consider the fact that we are recovering from COVID-19. Zoom (Zoom Communications, San Jose, California, USA) and other virtual platforms are a great medium to report research, facilitate the e-learning platform, or live stream the meeting. But what we discovered is that for research to really advance, you need to meet people and network, sit together and brainstorm, and you need to have your mind
grow bigger and bigger. Since the COVID-19 pandemic, 4,000 more people have attended our in-person meetings than our virtual meetings. Due to popular demand, especially from young people, we’re having face-to-face meetings again in our education programmes throughout the year. That has been an important lesson for me in the last 3 years of my presidency: the need to have face-to-face interactions alongside our virtual platforms.
Q2EASD has increasingly become a global platform, with strong participation from diverse groups of healthcare professionals and patient communities. How do you ensure that EASD continues to serve this broad and evolving audience?
First of all, when you talk about diversity, there is geographical diversity. We are proud to be the European Association for the Study of Diabetes, but we have participation from virtually every country in the world. To give you an idea, we received 2,170 abstracts, with a 66% acceptance rate, but the country which gave
us the most submissions was China, followed by the USA and the UK, so we have submissions from many different places. As a result, we started the global council to advise us on all things global. We just published a manuscript by the global council on global challenges to diabetes research and diabetes care,1 and we just distributed this year’s first global impact prize to a doctor from India, Viswanathan Mohan, Madras Diabetes Research Foundation, Chennai, India.
We have participation from virtually every country in the world
If you criticise one thing about our annual meeting, it’s that it’s too big. And you have a real fear of missing out, because there are so many things happening at the same time. It was extremely diverse in content and formats, so we catered for all of these stakeholders.

Q3At EASD 2025, a highlight for attendees was the discussion of results from the MELD-ATG2 and Ver-A-T1D (NCT04545151)3 clinical trials, both part of INNODIA, which you led. Could you please explain the significance of this work and how these fascinating results will benefit patients in the future?
First, SURPASS-CVOT (NCT04255433),4 which investigated tirzepatide versus dulaglutide in people with Type 2 diabetes and cardiovascular disease, was presented. Then the following day, two trials in people with new onset, clinical Stage 3, Type 1 diabetes were presented in an early bird symposium. I co-ordinated the INNODIA consortium, where we were looking for novel biomarkers and novel clinical trial designs in people with new onset, Stage 3, Type 1 diabetes. We designed the master protocol, which all of our clinical trials would run on; the two we presented, MELD-ATG and Ver-A-T1D, did just that.
We had our primary outcome at 12 months, being the area under the curve of C peptide stimulated during a 2-hour mixed meal tolerance test. MELDATG was a very complex study with an adaptive trial design, testing different doses of antithymocyte globulin (ATG) to look for the minimum effective low dose.2 First, we tested 2.5 mg/ kg in people with new onset, clinical Stage 3, Type 1 diabetes, but what was new was that we tested this dose in children as young as 5 years old to adults at 25 years of age, compared to placebo. This was a doubleblind study, and we then had three doses of ATG lower than 2.5 mg and a dose-determining committee, which was unblinded, deciding which of those to drop progressively. They decided to keep only one out of the 0.1 mg, 0.5 mg, and 1.5 mg doses. On the basis of biomarkers, for instance, the cluster of differentiation (CD) 4:CD8 ratio, they chose to drop 0.1 mg and 1.5 mg, leaving us with three arms that were well powered to allow us to conclude
something: the 0.5 mg, the 2.5 mg, and the placebo arms.
What we demonstrated was that 2.5 mg/kg ATG, just like Michael Haller, College of Medicine, University of Florida, Gainesville, USA, showed, was able to delay the destruction of β cells in people with Type 1 diabetes, as shown by stimulated C peptide.
We also showed that the 0.5 mg dose was effective, and that was accompanied by far fewer side effects
We also showed that the 0.5 mg dose was effective, and that was accompanied by far fewer side effects, meaning less cytokine


release syndrome, for instance, during the infusion, and also less serum sickness. The adaptive trial design, very low dose of ATG, and young people involved are the main sources of excitement around this trial. It’s also because ATG is an old, repurposed drug that’s been used in transplantation for more than 30 years; it opens up new venues, especially because a small company, SAB BIO, Florida, USA, is now making humanised ATG. So, by the end of this year, a new trial will start with humanised ATG, which doesn’t include serum sickness anymore.
By the end of this year, a new trial will start with humanised ATG, which doesn't include serum sickness anymore
The Ver-a-T1D, again, ran on the INNODIA master protocol. It was a simpler trial design: single arm placebo, and the other arm,
verapamil, which is another repurposed drug used to treat arrhythmia, given at a 360 mg dose. We also looked at stimulated C peptide. But this was a study run in adults above the age of 18 years, and this one did not reach its primary endpoint. It had a p value of 0.06, which was on the verge of statistical significance, but not when you looked at the curve. What was striking was that verapamil was a bit better than placebo, but the placebo didn’t move a lot. To me, the big conclusion seemed to be that, in adults, the C peptide decline isn’t very fast. When we went back to our MELD-ATG study and looked specifically at the small group of adults we had, we also didn’t see a lot of change in placebo. We probably need to reconsider the way we do our trials in Type 1 diabetes, because regulators force us to perform trials in adults first to show safety before trialling children. However, drugs are often discarded as potentially interesting agents because they don’t work in adults, even though they may have worked in children. This is really a call for regulators to allow us to perform our trials
in children more rapidly than we are now. The summary of these two trials was that adaptive trial designs allow you to make conclusions very rapidly regarding multiple things, and you can do these trials in children.
A strong theme of EASD 2025 was precision medicine. What do we need to achieve, scientifically and systematically, to reach a future where diabetes can be treated both universally, and with high precision?
To me, precision medicine is the pathway to personalised treatments. It starts with very basic variables of age, BMI, C-peptide levels, antibodies, and then biochemical biomarkers that we know, as well as all the emerging ones. I do believe in the evolution, as Andrew Hattersley, University of Exeter, UK, discussed in his Claude Bernard lecture, the way to go is what they did. For instance, they made an application where you can fill in patient characteristics and some biomarkers, and then they tell you the probability of someone having
Type 1 diabetes or a monogenic form. I believe that where we’re going is that we, as clinicians, will have a set of clinical markers and biochemical markers that we will plug in to get advice saying, for example, that this is a person with a lot of autoimmunity, but is also obese, so choose this therapy.
To me, precision medicine is the way to go, because as a clinician, you just see the heterogeneity of all the people in front of you. I think the traditional nomenclature of Type 1, Type 2, and so on will disappear. It will be an individual with components. There are some people who have typical Type 2 diabetes and some people with typical Type 1 diabetes, but many will be somewhere in between.
Q5 We've seen a surge in the adoption of AI and hybrid closed-loop systems in diabetes care. How do you see these technologies continuing to reshape both treatment and medical education?
Our hybrid closed-loop systems, automated insulin delivery systems, have changed the face of Type 1 diabetes. But these
hybrid closed-loop systems are still mostly algorithm-based. AI is just entering the stage, but it will be standard of care very, very soon in most people with Type 1 diabetes. This is what we will be using until we cure this disease. It makes such a difference for the burden of disease and education. It can be an issue because we have to educate medical students on all of these new technologies, and it’s not easy. I have 12 hours in the whole medical curriculum to talk about diabetes, from beginning to end, from Type 1 diabetes, Type 2, and everything else. Imagine that I have only half an hour to show medical students the pumps and explain what they are and how they work. I can only really show them what they are, but that’s it.
Precision medicine is the way to go, because as a clinician, you just see the heterogeneity of all the people in front of you
Q6
With so many thrilling sessions at this year’s Annual Meeting, what do you think will be the main focuses for EASD 2026?
The Programme Committee actually got together on the day before the Annual Meeting and put together a framework for the programme for next year. They will meet again in May because we have a deadline in April, when our membership can also propose topics for symposia. So, there’s a big input from the membership on the programme and where the focus will be. I do predict a lot of focus on Type 1 and Type 2 diabetes, obesity, technology, disease-modifying therapies, the incretin-based field, screening, and the early detection of Type 1 diabetes will be present. I also predict the islet transplantation field will be coming to the forefront. There will be the presentation of the new American Diabetes Association (ADA)/EASD consensus on the treatment of hyperglycaemia in people with Type 2 diabetes. The second clinical guideline by EASD, on the use of continuous glucose

monitoring in Type 2 diabetes, will also be there. There will be a lot of exciting things. Then, of course, I think the real theme throughout the conference will be precision medicine and the need for more personalised treatment.
Q7
You’ve long been a powerful advocate for supporting women in science, both in research and clinical care. Since championing this cause, what progress have you seen? And what more should the field be doing to improve gender equity in science?
Yes, I have indeed always done my best to promote women in science. It’s not on purpose, as I always choose the best person to be my PhD student or my postdoc; I like a good mix. It doesn’t work if it’s only women, and it doesn’t work if it’s only men; we need each other and very diverse profiles. A good mix of characters is the best. But indeed, it’s not easy. What we have done in EASD is to have, as much as possible, men and women as chairs in our annual meetings,
References
1. Giorgino F et al. Global challenges in diabetes research and care: which way forward? An appraisal from the EASD Global Council. Diabetologia. 2025;DOI: 10.1007/s00125-02506504-5.
2. Mathieu C et al.; INNODIA. Minimum effective low dose of antithymocyte
because it’s so important for young women to have role models and to see that it’s not always ‘Mr. Chairman’, it’s just Chair, and women can chair and lead. We shouldn’t underestimate that. It is also important to have people of all ethnicities and backgrounds. For example, my grandmother was disabled. So, as a child, I grew up in a lot of organisations with disabled people, and that was so important for me. I believe that if we have a diverse group of people on the stage, others will see that and think, ‘if they can do that, I can do that too.’ This is vitally important for ensuring that everyone gets equal opportunities.
Q8Looking back on your vast contributions in the field of diabetes, what legacy do you hope to leave for the next generation of diabetes researchers and clinicians you have inspired?
Lose all ego, lose all individualism, and collaborate. I always use Benjamin Franklin’s (founding father, USA) statement, “we must
globulin in people aged 5-25 years with recent-onset stage 3 type 1 diabetes (MELD-ATG): a phase 2, multicentre, double-blind, randomised, placebo-controlled, adaptive dose-ranging trial. Lancet. 2025;406(10510):1375-88.
3. Medical University of Graz. Verapamil SR in adults with Type 1 diabetes (Ver-
all hang together, or assuredly we shall all hang separately.” I’m a very impatient woman, and I’m sad when I think of the time we have lost in the last 20–30 years, for instance, in the domain of Type 1 diabetes, because of the egos of professors who want to cure it. Let’s share. Let’s work together. Life is too short, and our stay on this planet is too short to be individualists. We need to work together. Only when you work together do you progress, and only when you work together do you have fun. That is, I hope, my legacy. I believe that if we have a diverse group of people on the stage, others will see that and think, ‘if they can do that, I can do that too'
A-T1D). NCT04545151. https://www. clinicaltrials.gov/study/NCT04545151.
4. Eli Lilly and Company. A study of tirzepatide (LY3298176) compared with dulaglutide on major cardiovascular events in participants with Type 2 diabetes (SURPASSCVOT). NCT04255433. https:// clinicaltrials.gov/study/NCT04255433.


Hindrik Mulder
Board Member, European Association for the Study of Diabetes (EASD); Principal Investigator, Unit of Molecular Metabolism, Lund University Diabetes Centre, Sweden
Citation: EMJ Diabet. 2025;13[1]:59-61. https://doi.org/10.33590/emjdiabet/ZABN8639
Q1 Your work has significantly advanced the understanding of insulin secretion and β-cell biology. What initially sparked your interest in this area, and how has your focus evolved over the course of your career?
I began by studying neuropeptides, but then my attention was caught by one specific hormone, expressed mainly in β-cells but also in neurons and the gastrointestinal cells. Having a medical background and seeing the implications for diabetes, I decided to pursue a more β-cellfocused path. What started as a more general interest later became more specialised.
Q2
For scientists, the meeting acts as a place to present, listen, criticise, and leave with more knowledge than when they arrived
You’ve combined an active research career with clinical work in an outpatient diabetes clinic. Could you share an example of how a clinical observation shaped a research direction, or vice versa?
I would say I don’t have examples of that, because in the hospital outpatient clinic, we mainly care for patients with Type 1 diabetes, whereas my research is focused on Type 2 diabetes. So, there’s quite little concrete input from my clinical work into my research. But in general, it provides a framework to understand the situation of the patients, how severe the disease actually is, and how crucial it is to make progress, to try to find ways to treat and ultimately maybe even cure the disease. Those concepts are kept alive through my clinical work, even though there’s not a direct link.
Moving on to your role at EASD as a board member of the association, how do you see the organisation’s role in shaping the future of diabetes research and care across Europe and globally?
The main role of the association is to make research available to as many people as possible. For scientists, the meeting acts as a place to present, listen, criticise, and leave with more knowledge than when they arrived. At the same time, it’s also an opportunity to promote research on a higher level. These meetings are large in scope, and our presence is felt in the host city, something we actively use to highlight and promote diabetes research more widely.
All of this is run by EASD. It is the climax of the year for us, a process that has been worked out over the years, including the financial aspects. But the main objective, as I said before, is science. There are also other stakeholders, like pharmaceutical companies. They are here to communicate about their products and their work, but we also want them to be exposed to the science. It’s a win-win situation. And yes, we are visible all over the host city, Vienna, this year, even if the exact impact is difficult to measure.

Q4 At this year’s Congress, one major focus is on AI in diabetes care today and tomorrow. Where do you see AI having the most immediate impact in clinical diabetes care, and what should healthcare professionals do to prepare?
AI is a very broad concept, from generative models to more statistical methods trained on datasets. On a general scale, AI is everywhere already, and we don’t get to decide whether to use it or not. For example, when you’re writing on the computer, AI-based mechanisms correct your grammar and spelling.
AI is useful in many cases. You can see it as an extension of writing where we went from pencils to typewriters to word processors, and now we have programmes or apps that can write for us if prompted. But a very important thing about AI is accountability. It can, in some ways, work independently, so somebody must take responsibility. If you use AI to write an abstract, that’s fine, but you are still the author and responsible for the content, including any errors.
So, I would say we are both stakeholders and users of AI, but we need to be cautious. We must learn how to use AI responsibly, to take advantage of the benefits while protecting ourselves from misuse.
Q5Are there any sessions at this year’s Congress that particularly stood out to you in terms of impact on future research or clinical practice?
It’s always hard to know the impact in real time. You need a timeline to see if something has a real impact. But there are lectures where, retrospectively, you can see the importance. One example is the Claude Bernard Lecture. The awardee, Andrew Hattersley, University of Exeter, UK identified monogenic forms of diabetes in newborns and provided them with more suitable medication, which allowed them to stop using insulin. That has already had a huge impact on those patients’ lives.
In general, I always try to attend the prize lectures. The people receiving those awards are outstanding, and it’s a good way to gauge what the best research in diabetes looks like.
The awardee identified monogenic forms of diabetes in newborns and provided them with more suitable medication, which allowed them to stop using insulin
As for AI sessions, I’ve been to one or two. Honestly, I found them a bit underwhelming; nothing really robust beyond what we already know. AI methods are already used in many presentations based on big datasets. It’s part of the statistical toolbox now.
Q6
You were involved in the creation of the session ‘Insulin Resistance in the 21st Century: New Takes on an Old Problem’. Could you share the key ideas you discussed and what new perspectives clinicians should be aware of?
This is one of my favourite projects. A few years ago, as a journal editor, I thought that the leading diabetes journals could do something together to promote research at a higher level. So, we created an expert forum. We invite experts, senior scientists, and rising stars, with diversity in age, sex, and geography, to discuss a research area where there may be uncertainty or controversy.
We gather them in a room, provide some structure, and let them discuss. These discussions are followed by a symposium at the meeting, and then written up as a state-of-the-art review. It’s been very rewarding. The scientists really enjoy the discussions and learn from each other.
This year’s topic was insulin resistance. When I was a young scientist, this was ‘the field’, but in recent years, interest has declined. We wanted to see if there were important recent advances that could stir interest, and the response was very strong. The hall was almost full, even though it clashed with a prize lecture, so we, the editors, were very pleased.
We’ve done this twice before, first on the microbiome and last year on the heterogeneity of Type 1 diabetes. Both were successful, although attendance was lower because they were scheduled at the end of the meeting. This year’s timing worked much better.
Q7
Training and supporting the next generation of diabetes professionals is vital. What do you think are the most important skills and mindsets they should develop?
Of course, you must be smart and well-trained. But you also need ambition, not in terms of career, but ambition to solve a problem that matters, ideally one that will benefit people with diabetes. And you need passion. Passion keeps you going when things are tedious or difficult. It’s sometimes triggered by working with inspiring people; you see what’s possible, and it motivates you. Ultimately, passion and perseverance are what sustain you when logic and skills are not enough.
Q8
Finally, what do you hope attendees will take back from this year’s Congress to their clinical practice and research?
For clinicians, I hope they leave with a deeper understanding of therapies they can offer patients, information that is correct and useful, without bias or exaggeration. I also hope the
scientific framing of the meeting gives them a more critical view of what they hear in the exhibition halls. A scientific, critical attitude is extremely important, maybe more than ever, because we live in an era where facts are ignored and gut feelings are prioritised.
For researchers, I hope they have networked, established collaborations, heard inspiring things, and received valuable feedback on their work. And I hope they’ve enjoyed themselves too, meeting colleagues, sharing meals, and building relationships. Collaboration is what drives breakthroughs. It’s rare for one group alone to make major advances, a paradigm shift. When people with different perspectives come together, that’s when things move to another level.
We are privileged to have meetings like this every year. Of course, it takes a lot of work from the EASD and support from the pharmaceutical industry, but the industry also needs science, because all drugs are ultimately derived from academic discoveries.


Patrick Schrauwen Chair, European Association for the Study of Diabetes (EASD) Early Career Academy; Senior Scientist, Institute for Clinical Diabetology, German Diabetes Center, Düsseldorf, Germany
Citation: EMJ Diabet. 2025;13[1]:62-65. https://doi.org/10.33590/emjdiabet/NTJM1014
Q1
You had a session at the European Association for the Study of Diabetes (EASD) Annual Meeting focused on insulin sensitivity. Could you tell us a bit more about what that means, why it matters, and the key points you’d like people to take away?
Let me explain what that session was about. It was an expert forum organised by Diabetologia, Diabetes and Diabetes Care, where leading experts were invited to talk about a specific topic. This year, it was on insulin sensitivity.
We had a full-day meeting on the Sunday before the start of EASD discussing all aspects of insulin sensitivity: what we know, what we don’t know, where we are. We presented the outcome of our discussion in the session on the Wednesday of the EASD Annual Meeting. The major takeaway is that there are still big gaps in our knowledge.
also bring beneficial outcomes for complications and so on.
Your research has looked at broad factors like cold exposure, exercise, and circadian rhythm. From your perspective, which of these approaches shows the most promise for translation into science and clinical practice?
That’s a difficult one, as they’re all promising. Exercise, of course, is very powerful for treating and preventing diabetes. But we also know how difficult it is for people to keep up exercise, particularly with regards to motivation and time constraints.
When you push the body out of its comfort zone, it has to work and defend its homeostasis, and induces molecular mechanisms that make the body stronger
We started with the question: how do you measure insulin sensitivity? Of course, there’s whole-body sensitivity, but it’s also important to assess tissue-specific insulin sensitivity (liver, brain, adipose tissue, muscle). In experimental studies we know how to measure it, but in clinical practice there are still open questions.
Then we discussed how flexible are these tissues in improving insulin sensitivity? And does that matter for disease? If you look at diabetes medicine, hardly any drugs really target insulin sensitivity. So, the question is, should we explore that? I think the answer is yes. We believe that improving insulin sensitivity would
That connects to circadian rhythm. There’s now a lot of discussion about whether it’s better to exercise in the morning or afternoon. We and others have shown that in Type 2 diabetes, exercise in the afternoon gives better outcomes than in the morning. Why exactly, we don’t yet know. But in Type 1 diabetes, it might be the opposite. Today at the EASD meeting, data were presented that showed that morning exercise caused fewer hypoglycaemic episodes, whereas afternoon exercise caused more severe hypoglycaemia. So, recommendations really depend on the patient group.
Cold exposure is also very potent. If you expose people to cold, you get big improvements in insulin sensitivity, and beneficial cardiovascular effects, and in lipid metabolism, and blood pressure. But you need quite a lot of cold exposure, which is not easy to implement. Still, we do advise people to lower the temperature in their houses as this may help

to increase metabolism. And understanding the underlying pathways of how cold improves insulin sensitivity may help to find new targets for insulin resistance.
Collaboration is the aim: hearing from colleagues around the world and working together
Overall, my research shows that insulin resistance and diabetes are linked to “comfort”. The body needs to be challenged sometimes, such as through exercise or cold exposure. When you push the body out of its comfort zone, it has to work and defend its homeostasis, and induces molecular mechanisms that make the body stronger, including improvements in insulin sensitivity.
Q3
At this year’s Meeting, which sessions or topics do you think will have the biggest impact?
That’s difficult, but I personally like the exercise sessions. Beyond
that, I think the whole discussion on glucagon-like peptide-1 drugs and body composition is very important. Weight loss is clear, no debate there, but what does it do to body composition? We often see loss of muscle mass with weight loss, which is not good for overall health and also increases the risk of weight regain once people stop the drugs. So, we need to consider how to prevent the loss of muscle mass. Again, I think exercise will be crucial in this.
Ultimately, we need more research to understand what exactly weight loss, whether drug-induced or via other ways, does to other tissues, not just to our adipose tissue.
Q4 What role do you think EASD will play in shaping the future of diabetes research?
EASD obviously plays a role in science, that’s very clear. The Meeting is about science, presenting results, discussion, and finding analyses. But the Association does more than that.
We have strong e-learning programmes to bring science to practice and to healthcare professionals. And although
it’s the ‘European’ Association, we are moving towards a more global role. There’s now a Global Council, where we partner with other continents to ask: what are the problems there, and how can we work together to solve them? It’s an important development that will make EASD a key player in tackling diabetes worldwide. Of course, for that we also need politics. We try to influence the agenda, because you can’t solve this as one Association; we need governments too.
Collaboration is the aim: hearing from colleagues around the world and working together. And e-learning is a big part of that. If you look at the website, you’ll see many courses that are free and accessible. Some are very specific, while others are more general. They’re even helpful for medical students. They’re regularly updated, which is important. In the end, all the science we present here has to reach clinical practice and the people giving advice to patients.
Q5
You’ve chaired the EASD Early Career Academy for the past 3 years. Looking back, what do you see as the biggest achievements so far, and what do you hope to achieve next?
This Academy is really my ‘baby’, my brainchild. We started during COVID-19. Afterwards, we wanted to help young people, not only to keep them in the field, but also to help with their careers. Within Europe, there are big differences: some countries have well-funded institutes and it is easy for young people to for example find good mentors, but for others this is not the case. So, we wanted to bridge those gaps.
Therefore, we created a mentor–mentee programme, albeit with very little money. Young researchers can apply to be matched with mentors. It can be difficult for early career researchers to approach professors directly, but we bridge that gap. The mentors and mentees meet several times a year, online or in person, and the feedback has been very positive.
One achievement that I’m also proud of is giving early-career
researchers a meeting place at the meeting, among others in the academy’s social event. They can meet, network, and learn from each other. Those collaborations you form when you’re young are so valuable later in your career.
Beyond that, we organise webinars not just on science but also practical topics, such as how to apply for grants, how to publish, and how to balance work and life. Last year, for example, we had a session where established researchers talked about their careers and challenges. Young people found it very helpful.
I think this Academy fills a real gap. It gives something back to members and helps train the next generation, the stars of tomorrow.
Q6
With so many novel therapies being presented, what do you think are the key priorities for future research?
Personally, I think we need to build stronger bridges to other disease fields. Diabetes is a driver of many conditions, such as cardiovascular disease, obesity, and dementia. Ten or 15 years ago, we didn’t
link obesity or inflammation to Alzheimer’s, but now it’s becoming clear.
It’s the same with exercise: we used to look only at muscles or the liver, but now we see its effects on the brain too. So, we should learn from each other and reach out to other societies. Translational research is another priority: bridging basic and clinical science so discoveries are connected to patient care.
Q7
If you could highlight one area of diabetes care that you think will look completely different in 10 years, what would it be?
I’m a basic researcher, not a clinician, so my view is a bit different. But I think we’ll move beyond just Type 1 and Type 2 diabetes. There are many more subtypes. Whether you call it precision medicine or not, we’ll differentiate patients much more, identifying phenotypes and treating them more specifically. This has already started, with subtypes of diabetes being described, and I think it will develop rapidly. It won’t be enough to just say ‘Type 1’ or ‘Type 2’.
Another area is energy balance. Right now, a lot of new drugs focus on reducing energy intake and on weight loss. That is important, but we also need to consider energy expenditure. Even if someone is obese, if they are physically active, they can prevent many diseases. So, research and therapies should also focus on keeping the body active, not just reducing food intake.
Q8You mentioned timed exercise earlier. Could you expand a bit on your work in this area?
We’re going to have a session on timed exercise, or rather ‘time in chronobiology’, because it’s not just exercise, but also food intake. Our bodies have a biological clock. We
live in a 24-hour culture, with food and services available around the clock. Around 20% of people work night shifts, which is a risk factor for diabetes. Jet lag has similar effects. Disturbing the clock is detrimental.
The good news is that we can also use the clock to time interventions. Exercise in the afternoon seems more beneficial for insulin sensitivity in Type 2 diabetes than in the morning. Food timing is another element: most people eat over a 15-hour window. Restricting that to 10 hours has very good effects on glucose and insulin sensitivity.
Sleep is another underestimated factor. Sleep duration has gone down from 8–9 hours to 6–7 in
the past few decades. Sleep deprivation leads to insulin resistance. We underestimate these effects because we’re so used to being ‘on’ all the time. But I think there’s a lot to be gained from improving sleep and aligning behaviour with our biological clocks.
Translational research is another priority: bridging basic and clinical science so discoveries are connected to patient care
EMJ had the privilege of speaking with two leading figures in diabetes and metabolic research: Lorenzo Piemonti, San Raffaele Hospital, Milan, Italy; and Stephan Herzig, Helmholtz Center Munich, Neuherberg, Germany. Piemonti explores the evolving role of the physician-scientist, the promise of β-cell replacement therapies, and the importance of designing translational research around scalability, patient-centred outcomes, and immune tolerance. Herzig reflects on his career spanning molecular metabolic control, RNA-based therapies, and emerging complications, such as cancer cachexia, while also highlighting the need for precise diabetes subtyping and international collaboration to address the global burden of metabolic disease.
Featuring: Lorenzo Piemonti and Stephan Herzig

Lorenzo Piemonti
Director of the Diabetes Research Institute and Clinic Unit of Regenerative Medicine and Organ Transplants, IRCCS Ospedale San Raffaele, Milan, Italy
Science must be international, collaborative, and visionary, despite patents, politics, and economics
Citation: EMJ Diabet. 2025;13[1]:66-69. https://doi.org/10.33590/emjdiabet/KTFA3368
You’ve built a remarkable career between clinical care and cutting-edge scientific research. How have you managed to balance the demands of being both a practising physician and an active scientist, and how do these different perspectives help you in your day-to-day work?
This is a very good question. I think I am what we call a ‘physician scientist’, someone with a strong clinical background who completed a Doctor of Medicine degree, but also spent a lot of time doing research. This puts me in a position to examine cell cultures, study mice in a laboratory, and see patients in the clinic. Generally, there is no structured way to do this. To clinicians, you are not a clinician, but you are a scientist. To scientists, you are not a scientist, but you are a clinician. So, you are always in the middle, and this can create some problems.
If you think about how healthcare is evolving, and just see medical doctors as the interface between a guideline and patients, then it’s a role that, in my opinion, will disappear if you have no strong commitment to knowledge and research. With the coming of AI, ‘the doctor’ as we know it will probably disappear. In my opinion, evolution means that medical doctors should always also be scientists. To combine these roles, you have to work a lot, more than if you were only a scientist or only a physician.
Q2 You’ve held major roles in academic, clinical, and international research communities. How important is global collaboration in accelerating progress in diabetes care, and where do you see the biggest opportunities for synergy?
This is the only way to go. People often have an old picture of science; one genius changing the

world alone, like Einstein. That may happen once every 100 years. For us now, science is an ecosystem of technology, knowledge, and people. No one person can do everything. A good idea remains an idea without the ability to apply it, and you can’t hold all the knowledge yourself, so it’s impossible. You must create a web of collaboration, openness, and relationships. Competition exists because it’s normal, but to achieve results, we must be synergistic, which is not always easy.
So, science must be international, collaborative, and visionary, despite patents, politics, and economics. COVID-19 is a fascinating example of this. In around 1 year, we, as a scientific community, were able to gather an enormous amount of knowledge on a new disease and provide vaccines and therapies. Why? Because all those people with different roles and different knowledge supported this research for a year. Imagine if we could choose one disease every year, and all decide to work on it together. I'm sure that this could be a successful approach.
The point is that when you have a pandemic, this is easy, and everyone starts to move in the same direction, but in a normal situation, it’s difficult. COVID-19 was an example of how powerful it could be to work together for a single disease.
At the Scientific Institute of Research, Hospitalization and Healthcare (IRCCS) San Raffaele Hospital, Milan, Italy, you lead both foundational research and clinical application in diabetes and regenerative medicine. How do you prioritise the translation of research into tangible, patientcentred outcomes? What helps to bridge this gap?
This is difficult because it depends on many factors, and there is no one answer. It depends on the field; whether you're working on cell therapies, biomarkers, or screening, they each follow a different path. But now, we try to apply the quality by design approach. In my opinion, this is the best way to work in translation.
However, it isn’t easy. Scientists are always in love with their work, and they sometimes forget the final point of the research. That passion is good, as it allows innovation and vision, but if you want to reach patients, it makes things more complex. You have to start with the end goal, then work backwards to make the process feasible, scalable, and sustainable.
Treating one person versus treating one million people is very different. The same system isn’t scalable. Think of the moon landing, which involved thousands of people, years of work, and massive budgets to get one person there. But doing that every day? Completely different. The principle still applies, but the translation doesn’t. For example, gene-editing embryos to reduce future disease risk sounds promising. But to apply it, every child would need to be conceived via IVF. That’s not feasible now, and maybe it never will be, for ethical, emotional, and economic reasons. It might be used in rare, high-risk genetic conditions, but not broadly. In diabetes, we hear about building bioengineered
pancreases. I once spoke to engineers who designed a 10 cm cube to be implanted. But that’s not something you can realistically put in a person. So again, you must begin with the patient and design from the endpoint backwards.
That’s what quality by design means: bridging the gap between brilliant science and real, patientcentred outcomes. It’s a challenge, but it’s the only way forward.
Immune rejection remains a major hurdle in islet transplantation. Which emerging immune-modulating strategies show promise in extending graft survival? Are there any that might reduce or eliminate the need for long-term immunosuppression?
This is a very hot discussion in our field. In β-cell replacement, there are three main immune-modulating strategies under evaluation.
The first is the barrier strategy, like encapsulation, involving macro, micro, or other structures that can isolate cells from recognition by the immune system. This is a relatively old concept, studied for around 40 years with various biomaterials and strategies. Until now, there has been no evidence that it works in humans. Biomaterials are evolving, so maybe new ideas will emerge in the future, but I’m not a fan. As I say, ‘cells don’t like to stay in a plastic bag’: unlike drugs, cells need to be biologically integrated with the host. Endocrine hormones are messengers, and messengers need a connection, not just a presence in their host body.
The second is making the cells invisible to the immune system. This is not impossible, but the immune system is complex,
having evolved over millions of years, so it’s hard to escape. However, with gene editing, we can do a lot. The main approach is knocking out major histocompatibility complex (MHC) Class I and II molecules, which are the first messengers for immune recognition. This gives you cells that lack the usual MHC expression. It’s promising, but not enough, as immune recognition also involves natural killer cells and polymorphic markers, among others.
To combat this, we look at how tumours and viruses escape the immune system and apply those strategies to β-cells. In some candidates, it’s looking quite efficient. There is already one case of a patient for whom, after 12 weeks of observation and with only a couple of modifications, immune escape may be possible. This work was presented at the recent International Pancreas and Islet Transplantation Association (IPITA) Congress in June 2025.
The third and final approach is inducing tolerance, either central or local, to educate the immune system to avoid recognition of these foreign cells. This is complex, especially in Type 1 diabetes, where immune education has already led to disease. Some strategies exist involving the use of CAR-T regulatory cells to induce local tolerance at the level of local tissues. These strategies are showing promise, with successful trials in monkeys and the beginning of clinical trials in humans.
Overall, a regenerative approach, especially with allogeneic cells or in autoimmune settings, must address immune rejection. New antigens can also be introduced during gene editing or reprogramming, which brings regulatory challenges. This is a key frontier, and diabetes may be
the first field where these immune strategies succeed in practice.
What scientific breakthroughs are most urgently needed to integrate β-cell replacement or stem cell-derived therapies into standard diabetes care over the next decade? Is there anything ongoing at the moment that can help this to take place?
The major challenge for us now is scalability and sustainability. We already know that β-cell replacement and stem cell-derived therapies can work, so we have proof of concept. As I said before, the issue is no longer whether we can treat one person, but whether we can treat millions. To do that, we need to make the production of these therapies scalable, ensure quality control at every step, and reduce costs so that the treatment is sustainable on a large scale.
Scientifically, many solutions are already emerging, but they're still too complex and expensive for widespread clinical use. This is where the quality-by-design approach becomes essential. We need to automate differentiation and reprogramming processes, increase their efficiency, and ensure safety, because fundamentally, cells are living drugs, and unlike traditional drugs, they can survive, evolve, and interact in unpredictable ways once inside the body. This adds a layer of complexity and risk.
Cost is a major limiting factor. Even in well-resourced healthcare systems, it's becoming harder to ensure access to standard care, let alone cutting-edge therapies. Our goal is to cure all people with diabetes, not just a select few. But if we don't address cost, we risk creating systems where treatment is decided not by clinical need, but by financial or social factors.
Science can offer the tools for a cure, but it’s society that must choose how to implement them fairly. That choice of how to ensure equitable access is just as important as any scientific breakthrough.
Q6From your research into precision medicine, how is a more individualised model of diabetes care emerging? What are the expected impacts on clinical decision-making and patient outcomes?
Precision medicine in diabetes is evolving rapidly, moving far beyond a purely genetic model. Initially, it was largely about understanding which genetic variants influenced disease risk or treatment response. We now recognise that genetics alone is not enough. Diabetes is a complex condition, strongly influenced
not only by biology but also by lifestyle, culture, environment, and even socio-economic factors.
We're beginning to talk more about the exposome: everything an individual is exposed to in daily life, from what they eat and breathe, to the stressors and social dynamics that shape their health. In this context, precision medicine is less about isolated biomarkers and more about seeing the full picture of a person’s life. The same drug can produce very different outcomes depending on someone’s behaviour, education, or support network. Today, we have hundreds of therapeutic combinations available in diabetes, whereas decades ago, we only had two or three. But precision medicine only exists if you have a choice. If there’s one drug, there’s no need for a tailored approach.
With greater options comes the responsibility to make smarter, more personalised decisions.
This requires doctors to move beyond a guideline-based model. Precision care isn’t just about matching a therapy to a phenotype; it’s about understanding the person. In fact, if doctors are only applying algorithms, AI may soon outperform them. The future of medicine demands clinicians who can integrate science with empathy, biology with behaviour, and guidelines with lived experience.
Ultimately, we need to train doctors who are not just medical experts, but also humanists capable of seeing and treating the whole individual. That’s what precision medicine really means. Not just individualised treatment, but individualised care.

Stephan Herzig
Institute for Diabetes and Cancer IDC, Helmholtz Center Munich, Neuherberg, Germany; Joint Heidelberg-IDC Translational Diabetes Program, Heidelberg University Hospital, Heidelberg, Germany; German Center for Diabetes Research (DZD), Neuherberg, Germany; Chair Molecular Metabolic Control, Technical University of Munich, Germany
There are also new, what we call ‘emerging’ complications, including lung fibrosis, cancer and cancer cachexia
Citation: EMJ Diabet. 2025;13[1]:70-71. https://doi.org/10.33590/emjdiabet/KTFA3368
Q1
Can you discuss your journey to get to where you are today, as Director and Department Head of the Helmholtz Diabetes Center, Munich, Germany, and Director of the Institute for Diabetes and Cancer at Helmholtz Center Munich, Neuherberg, Germany?
After obtaining my PhD from the University of Göttingen, Germany, in 1999, I moved to the USA for postdoctoral training at the Salk Institute for Biological Studies, La Jolla, California, USA. In 2003, I relocated to Germany to establish an independent junior group at the German Cancer Research Center (DKFZ), Heidelberg, Germany, where I was promoted to Department Head in 2010. In 2012, I became a Full Professor at the University of Heidelberg, Germany, and eventually accepted an offer from Helmholtz Munich, Neuherberg, Germany, in 2015 to establish a new research institute on diabetes and cancer. In 2018, I became Director of the Helmholtz Diabetes Center, which now comprises 11 institutes and a total staff of >600, dedicated to diabetes and metabolism research. Since 2020, I have also been Research Director at Helmholtz Munich and part of the management board.
What is it about diabetes, its complications and its interactions with other metabolic disorders that initially piqued your interest, and has led you to dedicate your studies to it, publishing nearly 200 papers and receiving multiple awards, including the 2022 Werner Creutzfeldt Prize and the 2023 Camillo Golgi Prize from the European Association for the Study of Diabetes (EASD)?
While preventing obesity and diabetes is of critical importance in the first place, the reality is that millions of people develop the full-blown disease. The longterm complications eventually determine the patient’s quality of life and their life expectancy. From a research perspective, complications are an exciting topic, as it is still not possible to drive an existing complication into remission, such as an atherosclerotic plaque or tissue fibrosis. There are also new, what we call ‘emerging’ complications, including lung fibrosis, cancer and cancer cachexia, for which the mechanistic links remain largely unexplored. For these two reasons, I find the area of complications not only clinically relevant but also extremely challenging and exciting.
Q3
A highlight in your career was your discovery of the cooperative control of gluconeogenesis by glucagon and glucocorticoid hormones through the CREB–PGC1 axis, improving understanding of diabetic fasting hyperglycaemia back in 2001. Could you describe the work that went into this discovery and how it has shaped the landscape of your work since?
This initial work was driven by a long-lasting question in the field: how and why do two hormonal pathways, the glucagon and glucocorticoid pathways, synergise to induce hepatic de novo glucose production during fasting? The glucagon-dependent induction of cofactor PGC1 and its physical interaction with the glucocorticoid receptor eventually provided a molecular-level explanation for this longstanding mystery. From these studies, I learned several things that went on to shape my career and define what I now refer to as ‘molecular metabolic control’:
• Manipulating only a single molecule can significantly impact systemic metabolism.
• Proper fasting physiology is important for metabolic health. Overactivation of the PGC1 pathway, for example, triggered hyperglycaemia, as seen in diabetes.
• The liver is a powerful organ in overall systemic energy homeostasis.
These findings became guidelines for my subsequent studies, with a major emphasis on defining the molecular underpinnings of metabolic diseases, and eventually developing corresponding therapeutic modalities. Because of this early work, we now have a new class
of RNA therapies in the pipeline, targeted to restore proper fasting metabolism. Recent clinical work has shown that fasting-based lifestyle interventions, including intermittent fasting, can restore kidney function in patients with chronic kidney disease.
Q4
Do you have any exciting ongoing research? What are the expected results of it?
Beyond the mentioned ‘fastingbased’ RNA therapies, we have expanded our research in recent years to investigate conditions involving involuntary body wasting, particularly cancer cachexia. It turns out that the severity of cachexia in patients with tumours is determined by pre-existing diabetes. In this sense, cancer cachexia can be seen as one of these ‘emerging’ diabetes complications. In this context, we have just completed a study in which we defined the liver’s response to cancer cachexia, identifying secreted hepatic factors that can cause body wasting in the periphery. This again highlights the importance of the liver in metabolic health and defines new therapeutic targets for a metabolic wasting disease, for which no FDA-approved drug exists so far.
Q5 What are the key challenges in diabetes that you and your team are trying to solve? And what might be the implications for patients if you see success?
It has become clear that diabetes cannot be subdivided only into Type 1 and Type 2. There are five to six different subtypes of patients. A major challenge for the entire field in the coming years will be the precise molecularmetabolic definition of these subtypes, the understanding
of how to treat patients from different subtypes, and the characterisation of the impact of subtypes on the development of long-term complications. In this context, we will focus on the development of subtypespecific therapies and particularly aim to establish modalities to drive existing complications into remission. This would have a major impact on the life expectancy of patients and on associated healthcare costs.
Q6
Do you see any opportunities to collaborate with other teams in Europe, or further afield, to help address these challenges?
We are already engaged in multiple international, European, and worldwide collaborations at different levels, and will continue to strive to expand these networks in the future. Ultimately, the challenges can only be addressed through global cooperation, and the Helmholtz Diabetes Center, as one of the world’s largest metabolism research institutes, is well positioned to fulfil these tasks and requirements.
Q7 You have had a thrilling career so far. If you could pick out your greatest success, what would it be, and why?
In a recent international review, the Helmholtz Diabetes Center was rated ‘internationally leading’ by a high-profile panel of scientists. I am very proud of this because our team not only contributed to the scientific success, but all the work dedicated to the management of the centre, strategic planning, and coordination of all teams was also obviously recognised by our peers at a global level. So, all the work over many years has somehow paid off in the end.
Therapeutic innovation is progressing rapidly in Type 2 diabetes (T2D), driven by advances in both pharmacology and technology. New incretin-based therapies, including dual and triple agonists targeting glucagon-like peptide-1, gastric inhibitory polypeptide, and glucagon receptors, are showing remarkable benefits for glucose control, weight reduction, and cardiovascular health. Oral formulations are expanding access to these treatments for people who prefer not to use injections. Alongside drug developments, smart insulin delivery systems and continuous glucose monitoring technologies are being adapted for people with T2D, improving time-in-range and reducing treatment burden. Together, these innovations are transforming the management of T2D into more personalised, effective, and convenient care. The pace and breadth of these developments are captured in this insightful paper.
Martin Whyte
University of Surrey, UK
Authors: *Moh. Tawhidul Islam,1 Nazim Uddin2
1. New Vision University, Tbilisi, Georgia
2. Urgent Treatment Centre, Manchester Royal Infirmary, UK *Correspondence to mtislam_cu@hotmail.co.uk
Disclosure: The authors have declared no conflicts of interest.
Received: 27.12.24
Accepted: 15.10.25


Keywords: Artificial pancreas, continuous glucose monitoring (CGM), diabetes, duodenal ablation, glucagon-like peptide-1 (GLP-1), hyperglycaemia, hypoglycaemia, nanomedicine, regenerative medicine, sodium-glucose co-transporter 2 (SGLT2), treatments, Type 2 diabetes (T2D).
Citation: EMJ Diabet. 2025;13[1]:72-82. https://doi.org/10.33590/emjdiabet/RKYO7834
Abstract
Type 2 diabetes (T2D) remains a global health challenge, and ongoing advancements in therapeutic strategies are essential for its effective management. This review explores recent improvements in the treatment of T2D, including developments in pharmacotherapies, technologies, and lifestyle interventions, as well as nanomedicines and regenerative therapies. Novel glucose-lowering agents such as glucagon-like peptide-1 receptor agonists, sodium-glucose co-transporter 2 inhibitors, and their combination therapies have demonstrated improved glycaemic control and cardiovascular benefits. Dietary and lifestyle modifications, coupled with digital health tools, provide holistic approaches to long-term disease management. The integration of continuous glucose monitoring systems, insulin pumps, and artificial pancreas technologies has revolutionised personalised diabetes
management. Application of nanomedicines has been explored using nanoparticles, and has shown promise in several studies. The review also explores emerging regenerative medicine, such as gene therapy, β-cell regeneration, and microbiome-targeted treatments, which offer promising future perspectives. The evolving landscape of T2D treatment holds potential for improved patient outcomes and quality of life, with an emphasis on personalised approaches. This paper thoroughly discusses and concludes the challenges and potential future directions from the recent research findings and improvements in the management of T2D.
1. Type 2 diabetes is a significant global health challenge, affecting over 500 million adults worldwide, with a rising prevalence and associated complex comorbidities. Despite advances in pharmacotherapy and technology, durable glycaemic control and prevention of complications are difficult to achieve for many patients.
2. Recent clinical advances and trials have demonstrated meaningful progress in achieving better glycaemic control, cardiovascular protection, and patient-centred outcomes. The integration of innovative drugs, technologies, and regenerative approaches marks a shift toward personalised, evidence-driven care that continues to redefine the management of Type 2 diabetes.
3. To effectively integrate lifestyle care with emerging therapies, translational research and implementation efforts should emphasise more scalable solutions that can be used widely.
Type 2 diabetes (T2D) is a chronic metabolic disorder characterised by insulin resistance and impaired insulin secretion, leading to elevated blood glucose levels. The global prevalence of T2D has reached alarming rates, with WHO estimating over 422 million adults were living with diabetes in 2014. In 2021, the International Diabetes Federation (IDF) reported that 537 million adults had diabetes, showing a significant rise over a period of 6 years.1,2 This epidemic poses significant public health challenges, as T2D is associated with a myriad of complications, including cardiovascular disease, neuropathy, nephropathy, and retinopathy, which can severely impact patients’ quality of life and increase healthcare costs.1-3 In response to this growing crisis, there is an urgent need for innovative and effective therapeutic strategies to manage T2D. Recent advancements in pharmacotherapy, technology, and lifestyle interventions have transformed the landscape of diabetes management. Novel glucose-lowering agents, such as glucagon-like peptide-1 (GLP-1) receptor agonists, sodium-glucose co-transporter 2 (SGLT2) inhibitors, and dual-action glucose-dependent insulinotropic polypeptide (GIP) receptors have shown promise, not only in improving
glycaemic control, but also in providing weight loss and cardiovascular benefits, thereby addressing the multifaceted nature of T2D.4,5 Furthermore, the integration of continuous glucose monitoring (CGM) systems and insulin delivery technologies, including insulin pumps and artificial pancreas (AP) systems, has revolutionised personalised diabetes care, allowing for more tailored and responsive treatment approaches.4 In addition to pharmacological advancements, lifestyle modifications remain a cornerstone of T2D management. Evidence from various studies highlights the effectiveness of dietary changes and physical activity in preventing and managing diabetes.6,7 Programmes such as the Diabetes Prevention Program (DPP) have demonstrated that structured lifestyle interventions can significantly reduce the risk of developing T2D in highrisk populations.8,9 As the understanding of T2D evolves, emerging research areas, including gene therapy, β-cell regeneration, and microbiome-targeted treatments, offer exciting prospects for future therapeutic options.1 This review focuses on recent advancements in T2D treatment, emphasising the importance of a multifaceted approach that combines pharmacotherapy, nanotechnologies, regenerative medicines, and lifestyle
interventions to optimise patient outcomes and enhance quality of life. The authors’ review concludes by discussing challenges we face today in the management and treatment of T2D, potential future directions, and the need for improvements in the long-term management strategies for individuals living with T2D.
Dietary interventions remain a cornerstone of T2D management, with recent reviews advocating for structured dietary strategies aimed at achieving remission. Structured dietary interventions have been implemented in the UK to address obesity and its association with T2D. The DiRECT trial exemplifies this approach, demonstrating that a comprehensive weight management programme can lead to significant weight loss and improved glycaemic control in individuals with T2D.10,11 This programme emphasises the importance of lifestyle changes, including dietary modifications, as a cornerstone of diabetes management. Evidence suggests that specific dietary patterns can significantly improve glycaemic control and even reverse T2D in some patients.12,13 The integration of psychological support and education in diabetes management programmes has also been shown to enhance patient outcomes, emphasising the importance of a holistic approach to care.14 Recent research suggests that supplementation with certain natural products, such as chokeberry juice, which is rich in polyphenols, can positively impact glycaemic control and overall health status in patients with T2D.15,16 Additionally, lowcarbohydrate diets and ketogenic diets have shown promise in managing blood glucose levels.17 This suggests that integrating dietary strategies with conventional treatments may enhance the effectiveness of diabetes management.
Multicomponent intervention programmes like Reverse Diabetes2 Now (RD2N) in the Netherlands combine personalised dietary guidance, mainly focused on fresh and unprocessed foods, with structured exercise, behavioural coaching, and social
support. Implementing these interventions for 24 months demonstrated significant reductions in HbA1c levels, medication use, and weight, alongside improved lipid profiles.18,19 The success often hinges on sustained engagement, personalised support, and addressing social determinants of health. Strategies incorporating tailored meal planning, routine physical activity, and community involvement have proven effective for diverse populations.19 The recognition of individual patient characteristics has led to more personalised treatment strategies. This includes considering genetic factors, lifestyle, and patient preferences when designing treatment plans.20,21 The focus on personalised medicine is expected to expand even more in the near future, with an emphasis on tailoring treatment plans to individual patient needs and preferences, to optimise diabetes care.22,23 The evidence from several studies shows that lifestyle interventions are indispensable in T2D management, complementing pharmacological approaches to reduce complications and enhance patient outcomes.
Recent developments include GLP-1 receptor agonists and SGLT2 inhibitors, which not only lower blood glucose levels but also offer cardiovascular and renal protective benefits. These medications are particularly beneficial for patients who may not achieve adequate control with metformin alone, highlighting the importance of personalised treatment approaches that consider individual patient characteristics and preferences.24 Both GLP-1 receptor agonists and SGLT2 inhibitors have also been shown to be beneficial in patients with obesity for weight loss.22 However, metformin still remains the cornerstone of T2D treatment, especially for patients who are overweight or obese. Its efficacy in lowering blood glucose levels and its favourable safety profile make it a preferred choice.25,26
SGLT2 inhibitors have emerged as a major therapeutic advancement due to their glucose-lowering efficacy and established cardiovascular and renal benefits in several agents (empagliflozin and dapagliflozin) of the class. Bexagliflozin, a newer SGLT2 inhibitor, which received FDA approval in 2023, has demonstrated safety and effectiveness in glycaemic control, as highlighted in a recent systematic review.27 The BEST trial demonstrated its efficacy in improving glycaemic control with a favourable safety profile, though it did not specifically assess major adverse cardiovascular events. Consequently, unlike earlier agents such as empagliflozin and dapagliflozin, cardiovascular and renal outcome data for bexagliflozin are currently lacking, and its long-term benefits remain under investigation. The Japanese Diabetes Society (JDS) recommends SGLT2 inhibitors as a first-line option for patients with obesity, underscoring the growing importance of this class in T2D management.28 Similarly, the European Association for the Study of Diabetes (EASD) and American Diabetes Association (ADA) consensus reports now encourage the early use of SGLT2 inhibitors, especially in patients with cardiovascular and chronic kidney disease.29,30 Furthermore, the National Institute for Health and Care Excellence (NICE; UK) has updated, supporting the broader use of SGLT2 inhibitors earlier, particularly for individuals at high cardiovascular or renal risk.31
GLP-1 receptor agonists were established as effective agents in the management of T2D. Like SGLT2 inhibitors, these drugs lower blood glucose levels and promote weight loss in patients with T2D.32 Liraglutide is a well-established agent, used since its FDA approval in 2010. However, recent advancements have introduced newer and more effective incretin-based therapies, including semaglutide and dual incretin agonists such as tirzepatide (a combined GIP/GLP-1 receptor agonist), which provide much better glycaemic control and greater weight reduction compared to earlier GLP-1 agents.33,34 GLP-1 receptor agonists facilitate weight loss and improve glycaemic control (as an established medication used for weight
loss). GIP, on the other hand, is mostly used as part of dual agonists (like tirzepatide, a GIP/GLP-1 dual agonist), rather than as monotherapy. According to the British National Formulary (BNF), GLP-1 receptor agonists may be considered in combination with other antidiabetic drugs in patients with T2D when standard therapies (such as metformin and SGLT2 inhibitors) are not successful.35 Dual GIP/GLP-1 receptor agonists (e.g., tirzepatide) are recently emerging therapies, and still under evaluation, but standalone GIP agonists are not currently recommended by the BNF guidelines. The pharmacotherapy of T2D is rapidly evolving through research and clinical trials, with SGLT2 inhibitors and GLP-1 receptor agonists at the forefront.
Additionally, triple incretin agonists like retatrutide are in advanced clinical development, showing remarkable metabolic benefits in early trials.22 Novel combination drugs, like amycretin (a dual amylin/GLP-1 agonist) and semaglutide–cagrilintide co-formulation, represent the next generation of incretin-based therapies targeting both glucose regulation and body weight.36,37
Recent clinical and real-world studies indicate that the combination of SGLT2 inhibitors and GLP-1 receptor agonists may offer synergistic effects, enhancing glycaemic control while also reducing cardiovascular and renal risks.38-40 This evolving therapeutic approach exemplifies a move toward personalised medicine, where treatment regimens are tailored to individual metabolic profiles and comorbidities.41 Emerging research continues to explore additional pharmacological options, including novel agents targeting different metabolic pathways. For instance, recent findings suggest that taurine may have beneficial effects on glycaemic control and lipid profiles in patients with T2D, indicating a potential new avenue for adjunctive therapy.42 Furthermore, advancements in insulin therapy, including new analogues and delivery systems, are being developed to improve patient adherence and outcomes.43
Emerging pharmacotherapies such as glucokinase activators offer to improve
glycaemic control while protecting pancreatic β-cell function.44 Natural compounds, such as quercetagitrin, have recently been highlighted as lower-toxicity alternatives to synthetic medications.45 These agents may provide more effective and individualised management options.
Technological developments and innovations in the treatment of diabetes have shown significant transformation. One of the most notable developments is the AP, also known as a closed-loop insulin delivery system. This technology, originally developed for Type 1 diabetes (T1D), integrates CGM with automated insulin secretion to maintain target glucose levels.46-48 While most AP data relate to T1DM, recent studies have also demonstrated promising outcomes in insulin-treated patients with T2D, including improved time-in-range and reduced hypoglycaemia, proving potential applicability beyond T1DM.49-51
In the UK and other regions, the integration of wearable devices, mobile health applications, and structured lifestyle management tools reflects a shift toward more patient-centred, technology-assisted care. Emerging interventional procedures such as duodenal mucosal resurfacing have shown significant improvements in insulin sensitivity among patients with T2D. Duodenal ablation with electroporation therapy, specifically through the technique of duodenal mucosal resurfacing (DMR), has emerged as a promising intervention for patients with T2D. DMR involves the hydrothermal ablation of the duodenal mucosa, which has been shown to significantly improve glycaemic control, as evidenced by reductions in HbA1c levels in clinical trials.52-54 The mechanism behind this improvement is believed to be linked to alterations in duodenal signalling pathways that enhance insulin sensitivity, thereby addressing the underlying insulin resistance characteristic of T2D.55,56 Notably, the extent of glycaemic improvement appears to correlate positively with the length of the ablated mucosal segment, suggesting a dose-response relationship.53,54 Furthermore, the safety profile of DMR
has been established in multicentre studies, indicating that it is a minimally invasive option with favourable outcomes for metabolic control, including potential benefits for associated conditions such as non-alcoholic fatty liver disease.52,55-57 Overall, DMR represents a novel therapeutic approach that leverages the duodenum’s role in metabolic regulation, offering hope for improved management of T2D.
The integration of AI into diabetes management has opened new avenues for personalised treatment. AI-driven applications can analyse data from various sources, including CGMs and patient activity levels, to provide tailored recommendations for insulin dosing.58,59 This capability enhances patient engagement and adherence to treatment protocols, ultimately leading to better health outcomes.46,59 The use of wearable devices and smartphone applications has also facilitated more comprehensive diabetes management by allowing patients to track their glucose levels and receive real-time feedback.59,60 Research indicates that CGMs can significantly improve glycaemic control and reduce the risk of hypoglycaemia compared to traditional self-monitoring methods.60,61
CGMs have been shown to significantly improve glycaemic outcomes in patients with T2D. Clinical studies report HbA1c reductions ranging from 0.5–1.0% compared with standard self-monitoring methods.61-63 CGMs can enhance time-in-range metrics to reduce hypoglycaemic episodes, providing better glycaemic control. Despite these advantages, universal adoption remains limited due to factors like cost, lack of reimbursement in some healthcare systems, patient training requirements, and digital literacy.60 While CGMs are not currently used diagnostically, their continuous data streams are increasingly incorporated into clinical decisions and AI-based algorithms to optimise therapy adjustments.
The increasing adoption of CGMs in the UK is supported by their ability to provide continuous data, which can be crucial for making informed decisions about diet and insulin administration.61 Mobile health
applications have become important tools in the management of T2D. These applications facilitate communication between patients and healthcare providers, allowing for personalised feedback and support.64 They have significantly enhanced selfmanagement capabilities among individuals with T2D. A systematic review highlighted that these applications can effectively support diabetes self-care behaviours, leading to improved glycaemic control, as evidenced by reductions in HbA1c levels.65,66 They often include features such as medication reminders, dietary tracking, and educational resources, which empower patients to take an active role in their diabetes management.67 The integration of these applications with electronic health records enhances their utility by providing healthcare professionals with access to real-time patient data, thereby improving the quality of care.68
Innovations in technologies will play a central role in the future of diabetes care. Integrating wearable devices, AI-supported tools, CGMs, and telehealth can provide greater patient engagement, adherence, and treatment adjustments.20,58,59 They may also help address systemic barriers such as therapeutic inertia by playing a role in timely treatment intensification, hence reducing the long-term complication risks.68,69
Nanomedicine has emerged as a transformative field in drug delivery. Application of nanoparticles such as polymeric nanoparticles, polymeric nanocapsules, lipid nanoparticles, and liposomes showed several advantages, including enhanced drug solubility, controlled drug release, and targeted delivery, suggesting it can improve therapeutic efficacy and minimise side effects.70-82 Polymeric nanoparticles are characterised by their biodegradability, biocompatibility, and high drug-loading capacity, and can be carriers of therapeutic agents.70,71 They can be designed to release drugs in a controlled manner, to improve the pharmacokinetics of poorly soluble drugs.72,73
Preclinical studies have demonstrated that polymeric and lipid nanoparticles loaded with antidiabetic agents such as metformin, pioglitazone, and curcumin can improve glycaemic control and insulin sensitivity in T2D.74,75 Insulin-loaded chitosan nanoparticles have shown prolonged glucose-lowering effects and improved oral bioavailability in rats with diabetes.76 In another study, GLP-1 analogues, such as exenatide and liraglutide, have also been successfully encapsulated in poly(lacticco-glycolic acid) nanoparticles, improving half-life and enhancing pancreatic β-cell protection.77,78
Nanoparticles have also shown promise in oral and targeted drug delivery, with better absorption. Polymeric and lipidbased carriers can improve the stability and bioavailability of oral antidiabetic drugs.79,80 Lipid nanoparticles and carbon nanorods, in recent research, have enhanced glucose homeostasis and promoted wound healing in diabetic models.81,82 Nanocarriers can deliver siRNA targeting insulin resistance pathways, which is a novel strategy for addressing β-cell dysfunction in T2D.83,84 Biogenic synthesis of nanoparticles has also been seen in studies as a safe and effective method for developing antidiabetic agents.85,86
Regenerative medicine approaches, including gene therapy, stem cell therapy, and diabetic wound healing, have shown great promise in the treatment of T2D. Gene therapy aims to correct or replace defective genes responsible for disease progression, offering a potential cure rather than symptomatic treatment.87 Stem cell therapy has shown promise in regenerating damaged tissues and restoring insulin production in patients with diabetes, thereby addressing the underlying causes of the disease. Additionally, advancements in wound healing techniques, particularly for diabetic ulcers, are crucial as these complications are prevalent in T2D. Innovative strategies that incorporate stem cells and growth factors have been developed to enhance wound healing and tissue regeneration.87,88 Nanotechnologies using nanoparticles facilitate targeted and controlled drug delivery, while regenerative therapies aim to restore normal physiological
functions. Together, these strategies hold the potential to significantly improve the management and outcomes of T2D.
T2D management is likely to be shaped by personalised, technology-driven, and mechanism-based strategies. We have already seen advances in biomarker discovery, and precision medicine shows promising results. For example, glycomics, particularly the analysis of subclass-specific N-glycosylation of IgG, has the potential to enhance diagnostic precision and guide tailored therapeutic strategies.89 Recent research into the gut microbiome suggests that modulation of intestinal flora can impact insulin resistance and metabolism, which shows the way to microbiometargeted personalised therapies in future.90,91 Integrating these approaches could reveal novel therapeutic targets and optimise patient outcomes through a mechanismbased treatment.
The combination of precision medicine approaches, digital technologies, and pharmacological therapies directs toward a holistic, patient-centred model for T2D management. This model addresses physiological aspects of the disease as well as incorporates lifestyle interventions, psychological support, and patient education for overall health outcomes.92,93 These strategies can evolve toward more effective, sustainable, and personalised care in T2D.
The developments of T2D treatment reflect remarkable progress in recent years. Several pharmacological and technological advances have made the management easier. Advanced pharmacotherapies, such as GLP1 receptor agonists, SGLT2 inhibitors, and dual action GIP and GLP-1 receptor agonists, continue to enhance glycaemic control while addressing cardiovascular and renal complications. Nanomedicine has emerged as a transformative technology, enabling precision drug delivery systems that improve therapeutic efficacy and reduce side effects. Regenerative medicine approaches, including stem cell therapy and β-cell replacement strategies, offer hope for restoring
endogenous insulin production and achieving long-term disease remission. Innovative interventions like duodenal ablation with electroporation therapy target the gut’s role in metabolic regulation, presenting a minimally invasive option with the potential to reset glucose homeostasis. Collectively, these advancements signify a paradigm shift towards more personalised, effective, and comprehensive management strategies.
The future direction of treatment is driven by significant advancements in precision medicine, technological innovations, and a comprehensive understanding of the disease’s complexities. Precision medicine, which tailors treatment strategies based on individual genetic and phenotypic characteristics, is becoming increasingly important in diabetes management. Studies have shown that genetic analysis can help identify specific subgroups of patients with diabetes, allowing for more targeted and effective interventions.94-96 The integration of advanced technologies, such as CGM and AI, is transforming diabetes care. These technologies facilitate real-time data collection and analysis, enabling personalised treatment adjustments that improve glycaemic control and patient outcomes.97,98 The development of connected ecosystems for insulin delivery and glucose monitoring enhances selfmanagement and therapy adherence, effectively tackling the longstanding challenge of ‘therapeutic inertia’ in diabetes care.98,99 The exploration of combination therapies also provides evidence of improvements and suggests that these approaches can effectively manage blood glucose levels while mitigating complications associated with diabetes.100,101 The focus on holistic care, which includes lifestyle modifications alongside pharmacotherapy, is essential for preventing both microvascular and macrovascular complications.101
Recent advancements in the design of nanocarriers, such as lipid nanoparticles and carbon nanorods, have facilitated targeted delivery of therapeutic agents, which improve glucose homeostasis and promote wound healing in patients with diabetes. The ability of these nanocarriers to encapsulate and deliver genetic
materials, such as siRNA, presents a novel strategy for addressing the underlying pathophysiology of T2D, including insulin resistance and β-cell dysfunction. The biogenic synthesis of nanoparticles has emerged as a safe and effective method for developing antidiabetic agents, and may further expand the therapeutics for diabetes. The integration of nanomedicine into T2D represents an innovative approach that can enhance therapeutic efficacy while minimising adverse effects. The utilisation of nanoparticles, such as selenium and silver nanoparticles, has demonstrated significant potential in improving insulin sensitivity and alleviating diabetic complications through various mechanisms, including antioxidant activity and modulation of inflammatory markers.85,102,103 The development of oral nanodrug delivery systems for antidiabetic phytocompounds has highlighted the versatility of nanotechnology in enhancing the bioavailability.104,105 The convergence of nanotechnology and diabetes treatment not only offers a pathway to more effective management strategies but also paves the way for future research aimed at optimising these nanomedicines for clinical applications. AP systems, also known as closed-loop insulin delivery systems, are already commercially available and being used by people with diabetes, helping reduce the burden of managing diabetes by automating glucose control
References
1. Thipsawat S. Intervention for prevention of type 2 diabetes mellitus among prediabetes: a review of the literature. SAGE Open Nurs. 2023;9:237796082311755.
2. International Diabetes Federation. Facts and figures. Available at: https://idf.org/ about-diabetes/diabetes-facts-figures/. Last accessed: 30 November 2024.
3. Bekele H et al. Barriers and strategies to lifestyle and dietary pattern interventions for prevention and management of type-2 diabetes in africa, systematic review. J Diabetes Res. 2020;2020:7948712.
4. Li N et al. Effects of lifestyle intervention on long‐term risk of diabetes in women with prior gestational diabetes: a systematic review and meta‐analysis of randomized controlled trials. Obes Rev. 2021;22(1):e13122.
and reducing the risk of hypoglycaemia and hyperglycaemia. Despite these advancements, challenges remain in the widespread adoption and optimisation of these technologies. Issues such as the need for healthcare provider support, patient education, and the management of technical skills are critical barriers that must be addressed to enhance the utilisation of technologies like AP systems.106
As we look to the future, it is crucial to address the disparities in access to these advanced treatments and technologies, ensuring that all patients benefit from the latest innovations in diabetes care.107 The ongoing collaboration between healthcare providers, researchers, and policymakers will be vital in overcoming these barriers and fostering an equitable healthcare environment for individuals with diabetes. Ultimately, the convergence of precision medicine, technology, and comprehensive care strategies holds the promise of significantly improving the quality of life for those affected by diabetes. These significant advancements in technology, pharmacotherapy, and personalised care strategies hold the promise of better glycaemic control, reduced complications, and enhanced quality of life for individuals living with T2D.
5. Vajje J. Comparison of the efficacy of metformin and lifestyle modification for the primary prevention of type 2 diabetes: a meta-analysis of randomized controlled trials. Cureus. 2023;15(10):e47105.
6. Krisnadewi K et al. Health interventions and its impact on outcomes among diabetic patients: a systematic review. Pharma Sci Asia. 2022;49(4):304-11.
7. Zhang Y et al. The effectiveness of lifestyle interventions for diabetes remission on patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. Worldviews Evid Based Nurs. 2023;20(1):64-78.
8. Kriska AM et al. The impact of physical activity on the prevention of type 2 diabetes: evidence and lessons learned from the diabetes prevention program, a long-standing clinical trial incorporating subjective and objective activity measures. Diabetes Care. 2021;44(1):43-9.
9. Yamaoka K et al. Comparison of the effectiveness of lifestyle modification with other treatments on the incidence of type 2 diabetes in people at high risk: a network meta-analysis. Nutrients. 2019;11(6):1373.
10. Reynolds A et al. A primary care-led weight management intervention for adults with diabetes and obesity: quantitative results from a randomised controlled trial of total meal replacement (DiRECT). Proc Nutr Soc. 2024;83(OCE1):E120.
11. Lean M et al. Primary care-led weight management for remission of type 2 diabetes (DIRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541-51.
12. Brown A et al. Dietary strategies for remission of type 2 diabetes: a narrative review. J Hum Nutr Diet. 2022;35(1):165-78.
13. Morissette A, Mulvihill EE. Obesity management for the treatment of type 2 diabetes: emerging evidence and therapeutic approaches. J Pharm Pharm Sci. 2024;27:13065.
14. Kurita M et al. A 7 day inpatient diabetes education program improves quality of life and glycemic control 12 months after discharge. J Diabetes Investig. 2023;14(6):811-20.
15. Milutinović M et al. Chokeberry juice supplementation in type 2 diabetic patients - impact on health status. J Appl Biomed. 2019;17(4):218-24.
16. Christiansen CB et al. Aronia in the type 2 diabetes treatment regimen. Nutrients 2023;15(19):4188.
17. Raksa K et al. The ketogenic diet in the treatment of diabetes type 2. J Educ Health Sport. 2022;12(7):92-8.
18. Taheri S. Type 2 diabetes remission: a new mission in diabetes care. Diabetes Care. 2024;47(1):47-9.
19. Pot GK et al. Lifestyle medicine for type 2 diabetes: practice-based evidence for long-term efficacy of a multicomponent lifestyle intervention (Reverse Diabetes2 Now). BMJ Nutr Prev Health. 2020;3(2):188-95.
20. Kabakambira JK, Kong JM. Optimizing type 2 diabetes management in a medically complex patient: a case report of a patient with type 2 diabetes and HIV. Diabetes Metab Syndr Obes. 2023;16:2401-6.
21. Amutha A et al. Treatment regimens and glycosylated hemoglobin levels in youth with type 1 and type 2 diabetes: data from search (United States) and ydr (India) registries. Pediatr Diabetes. 2021;22(1):31-9.
22. Williams D et al. Personalized type 2 diabetes management: an update on recent advances and recommendations. Diabetes Metab Syndr Obes. 2022;15:281-95.
23. Gabbay RA et al. Addressing therapeutic inertia in 2020 and beyond: a 3-year initiative of the american diabetes association. Clin Diabetes. 2020;38(4):371-81.
24. Choudhary S et al. Transforming diabetes care: latest advancements in treatment and technology. Int J Pharm Pharm Sci. 2024;6(1):96-104.
25. Gaur RV et al. Metformin: as firstline treatment for type 2 diabetes prevention. Indian J Forensic Med Toxicol. 2020;14(4):4402-6.
26. Mannucci E et al. Italian guidelines for the treatment of type 2 diabetes. Acta Diabetol. 2022;59(5):579-622.
27. Pasqualotto E. et al. Efficacy and safety of bexagliflozin in patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. Diabetes Obes Metab. 2023;25(7):1794-802.
28. Koshizaka M et al. Urinary α1 microglobulin level is useful for selecting sodium‐glucose transporter 2 inhibitor or metformin for visceral fat reduction in patients with type 2 diabetes. Diabetes Obed Metab. 2023;25(10):3071-5.
29. Davies MJ et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-86.
30. American Diabetes Association (ADA). ADA/EASD consensus update on management of hyperglycemia in type 2 diabetes – report published in diabetes care. 2022. Available at: https://diabetes.org/newsroom/ ADA-EASD-consensus-updatemanagement-hyperglycemia-type-2diabetes-report. Last accessed: 30 November 2024.
31. NICE. Type 2 diabetes in adults: management. Updated 2023. Available at: https://www.nice.org.uk/guidance/ ng28. Last accessed: 09 Ocotber 2025.
32. Song R et al. Intensive management of obesity in people with severe chronic kidney disease: a review. Diabetes Obes Metab. 2021;23(8):1733-45.
33. Jastreboff AM et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205-16.
34. Frías JP et al. SURPASS-2 Investigators. Tirzepatide versus semaglutide once weekly in patients with Type 2 diabetes. N Engl J Med. 2021;385(6):503-15.
35. NICE. Treatment summaries: Type 2 diabetes. Available at: https:// bnf.nice.org.uk/treatment-summaries/ type-2-diabetes/. Last accessed: 09 October 2025.
36. Dahl K et al. Amycretin, a novel, unimolecular GLP-1 and amylin receptor agonist administered subcutaneously: results from a phase 1b/2a randomised controlled study. Lancet. 2025;406(10499):149-62.
37. Garvey WT et al.; REDEFINE 1 Study Group. Coadministered cagrilintide and semaglutide in adults with overweight or obesity. N Engl J Med. 2025;393(7):635-47.
38. Brown E et al. Sglt2 inhibitors and glp-1 receptor agonists: established and emerging indications. Lancet. 2021;398(10296):262-76.
39. Simms-Williams N et al. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study. BMJ. 2024;385:e078242.
40. Gourdy P et al. Combining glucagonlike peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM). Cardiovasc Diabetol. 2023;22(1):79.
41. Tan W et al. Diabetes medication recommendation system using patient similarity analytics. Sci Rep.2022;12(1):20910.
42. Caletti G et al. Effect of taurine on glycaemic, lipid and inflammatory profile in individuals with type 2 diabetes: study protocol of a randomised trial. Br J Nutr. 2022;129(11):1871-6.
43. Cheng R et al. The promising future of insulin therapy in diabetes mellitus. Am J Physiol Endocrinol Metab. 2021;320(5):E886-90.
44. Haddad D et al. New-generation glucokinase activators: potential gamechangers in type 2 diabetes treatment. Int J Mol Sci. 2023;25(1):571.
45. Gone GB et al. Exploring the antidiabetic potential of quercetagitrin through dual inhibition of ptpn6 and ptpn9. Nutrients. 2024;16(5):647.
46. Andrès E et al. Currents technologies at the service of the diabetic patients: state of the art. Diabetes Updates. 2019;DOI:10.15761/DU.1000122.
47. Kapil S et al. Artificial pancreas system for type 1 diabetes - challenges and advancements. Explor Res Hypothesis Med. 2020;5(3):110-20.
48. Boughton CK, Hovorka R. Advances in artificial pancreas systems. Sci Transl Med. 2019;11(484):eaaw4949.
49. Kudva YC et al. A randomized trial of Automated insulin delivery in Type 2 diabetes. N Engl J Med. 2025;392(18):1801-12.
50. Daly AB et al. Fully automated closedloop insulin delivery in adults with type 2 diabetes: an open-label, singlecenter, randomized crossover trial. Nat Med. 2023;29(1):203-8.
51. Ng SM et al. Long-term assessment of the NHS hybrid closed-loop real-world study on glycaemic outcomes, timein-range, and quality of life in children and young people with type 1 diabetes. BMC Med. 2024;22(1):175.
52. Haidry RJ et al. Duodenal mucosal resurfacing: proof-of-concept, procedural development, and initial implementation in the clinical setting. Gastrointest Endosc. 2019;90(4): 673-81.e2.
53. Mingrone G et al. Safety and efficacy of hydrothermal duodenal mucosal resurfacing in patients with type 2 diabetes: the randomised, doubleblind, sham-controlled, multicentre REVITA-2 feasibility trial. Gut. 2022;71(2):254-64.
54. van Baar ACG et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut. 2020;69(2):295-303.
55. van Baar ACG et al. Duodenal mucosal resurfacing: multicenter experience implementing a minimally invasive endoscopic procedure for treatment of type 2 diabetes mellitus. Endosc Int Open. 2020;8(11):E16839.
56. van Baar ACG et al. Duodenal mucosal resurfacing combined with glucagonlike peptide-1 receptor agonism to discontinue insulin in type 2 diabetes: a feasibility study. Gastrointest Endosc. 2021;94(1):111-120.e3.
57. Hadefi A et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes. Dig Dis. 2018;36(4):322-4.
58. Cambuli VM et al. Intelligent insulin vs. artificial intelligence for type 1 diabetes: will the real winner please stand up? Int J of Mol Sci. 2023;24(17):13139.
59. Zhu T et al. Deep learning for diabetes: a systematic review. IEEE J Biomed Inform. 2021;25(7):2744-57.
60. Heinemann L et al. Self-measurement of blood glucose and continuous glucose monitoring – is there only one future? Eur Endocrinol. 2018;14(2):24-9.
61. Battelino T et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-603
62. Saha T et al. Wearable electrochemical glucose sensors in diabetes management: a comprehensive review. Chem Rev. 2023;123(12):7854-89.
63. Beck RW et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Int Med. 2017;167(6):365-74.
64. Price JC et al. The effectiveness of automated digital health solutions at successfully managing obesity and obesity-associated disorders: a picostructured investigation. Digit Health. 2022;8:20552076221091351.
65. Hou C et al. Mobile phone applications and self‐management of diabetes: a systematic review with meta‐analysis, meta‐regression of 21 randomized trials and grade. Diabetes Obes Metab. 2018;20(8):2009-13.
66. Wu IXY et al. Effectiveness of smartphone technologies on glycaemic control in patients with type 2 diabetes: systematic review with meta‐analysis of 17 trials. Obes Rev. 2018;19(6):825-838.
67. Rodriguez-León C et al. Mobile and wearable technology for the monitoring
of diabetes-related parameters: systematic review. Jmir Mhealth Uhealth. 2021;9(6):e25138.
68. Okemah J et al. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther. 2018;35(11):1735-45.
69. Mata-Cases M et al. Therapeutic inertia: still a long way to go that cannot be postponed. Diabetes Spectr. 2020;33(1):50-7.
70. Gillella S et al. Polymeric nanoparticles – a review. Int J Health Care Biol. 2024;9(1):25-31.
71. Athira TR et al. Biodegradable polymeric nanoparticles: the novel carrier for controlled release drug delivery system. Int J Sci Res Arch. 2023;8(1):630-7.
72. Aryal S et al. Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Mol Pharm. 2011;8(4):1401-7.
73. Nagamani DB et al. Implementation of factorial design to optimize the formulation method of ezetimibe polymeric nanoparticle by homogenization CUM ultrasonication method. Int J Appl Pharm. 2022;14(2):151-9.
74. Mohamed HA et al. Metformin-loaded nanoparticles reduce hyperglycemiaassociated oxidative stress and induce eNOS phosphorylation in vascular endothelial cells. Sci Rep. 2024;14(1):30870.
75. Metawea MR et al. Comparative effects of curcumin versus nano-curcumin on histological, immunohistochemical expression, histomorphometric, and biochemical changes to pancreatic beta cells and lipid profile of streptozocin induced diabetes in male Sprague–Dawley rats. Environ Sci Pollut Res Int. 2023;30(22):62067-79.
76. Hunt NJ et al. Oral nanotherapeutic formulation of insulin with reduced episodes of hypoglycaemia. Nat Nanotechnol. 2024;19(4):534-44.
77. Pan S et al. Dual-phase exenatide delivery system: PLGA nanoparticles embedded in thermosensitive PLGA-PEG-PLGA hydrogel for sustained glycemic control. Expert Opin Drug Deliv. 2025;DOI: 10.1080/17425247.2025.2564870.
78. Awad A et al. The evaluation of the cytotoxicity effect of theophylline loaded with collagen nanoparticles. Damanhour J Vet Sci. 2022;8(2):5-10.
79. Deshpande PK, Gothalwal R. Formulation and evaluation of nanoparticles with neuro protective chemicals of natural and synthetic origin. PIJR. 2021;10(7):11-4.
80. Pourbadiei B et al. Ph-sensitive nanoscale polymers: highly efficient systems for dox delivery in cancer treatment. J Nanomed Res. 2017;5(3):00114.
81. Park M et al. N-doped carbon nanorods from biomass as a potential antidiabetic nanomedicine. Acs Biomater Sci Eng. 2022;8(5):2131-41.
82. Kasiewicz LN, Whitehead KA. Lipid nanoparticles silence tumor necrosis factor α to improve wound healing in diabetic mice. Bioeng Transl Med. 2018;4(1):75-82.
83. Neumann UH et al. Lipid nanoparticle delivery of glucagon receptor sirna improves glucose homeostasis in mouse models of diabetes. Mol Metab. 2017;6(10):1161-72.
84. Liu Y et al. Emerging theranostic nanomaterials in diabetes and its complications. Adv Sci (Weinh). 2022;9(3):e2102466.
85. Wahab M, Janaswamy S. A review on biogenic silver nanoparticles as efficient and effective antidiabetic agents. Funct Food Sci. 2023;3(7):93-107.
86. Alamri ES, El Rabey HA. The antioxidant and antidiabetic activity of vanillic acid and vanillic acid-coated silver nanoparticles in streptozotocin-induced diabetic male rats. Preprints. 2023;DOI: 10.20944/preprints202307.2144.v1.
87. Swapna V et al. Preparation and evaluation of lamivudine nanoparticles. Int J Res Pharm Chem. 2019;9(3): 154-60.
88. Odeniyi MA et al. Starch nanoparticles in drug delivery: a review. Polim Med. 2018;48(1):41-45.
89. Liu J et al. Glycomics for Type 2 diabetes biomarker discovery: promise of immunoglobulin G subclass-specific fragment crystallizable N-glycosylation in the Uyghur population. OMICS. 2019;23(12):640-8.
90. Liu Y et al. Intestinal flora: new perspective of type 2 diabetes. World J Clin Cases. 2024;12(11):1996-9.
91. Jia L et al. Pharmacomicrobiomics and type 2 diabetes mellitus: a novel perspective towards possible treatment. Front Endocrinol (Lausanne). 2023;14:1149256.
92. Ritholz, M et al. What helps and what hinders primary care treatment for women with type 2 diabetes and binge eating disorder? A qualitative study. Diabet Med. 2022;39(8):e14887
93. Song Y, Ren Y. Progress of clinical research on treating obese type 2 diabetes based on spleen deficiency and phlegm dampness. Meds Chin Med. 2023;5(5):45-50.
94. Xie F et al. Precision medicine in diabetes prevention, classification and management. J Diabetes Investig. 2018;9(5):998-1015.
95. Carr ALJ et al. Precision medicine in type 1 diabetes. Diabetologia. 2022;65(11):1854-66.
96. Chung W et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(7):1617-35.
97. Ahmed MG et al. A survey on personalization of diabetes treatment using artificial intelligence techniques. Benha J Appl Sci. 2023;8(5):229-36.
98. Steenkamp D et al. Adherence and persistence to insulin therapy in people with diabetes: impact of connected insulin pen delivery ecosystem. J Diabetes Sci Technol. 2022;16(4):995-1002.
99. MacLeod J et al. Technology disparities and therapeutic inertia: a call to action for the diabetes care and education specialist. ADCES in Practice. 2021;9(5):34-41.
100. Xie X et al. Benefits and risks of drug combination therapy for diabetes mellitus and its complications: a comprehensive review. Front Endocrinol (Lausanne). 2023;14:1301093.
101. Sasako T et al. Intensified multifactorial intervention in patients with Type 2 diabetes mellitus. Diabetes Metab. 2023;47(2):185-97.
102. Gutierrez RMP. Evaluation of diabetes effects of selenium nanoparticles synthesized from a mixture of luteolin and diosmin on streptozotocin-induced Type 2 diabetes in mice. Molecules. 2022;27(17):5642.
103. Abdulmalek SA, Balbaa M. Synergistic effect of nano-selenium and metformin on Type 2 diabetic rat model: diabetic complications alleviation through insulin sensitivity, oxidative mediators and inflammatory markers. PLoS One. 2019;14(8):e0220779.
104. Nie X et al. Oral nano drug delivery systems for the treatment of type
2 diabetes mellitus: an available administration strategy for antidiabetic phytocompounds. Int J Nanomedicine. 2020;15:10215-40.
105. Zolkepli H et al. A review on the delivery of plant-based antidiabetic agents using nanocarriers: current status and their role in combatting hyperglycaemia. Polymers (Basel). 2022;14(15):2991.
106. Hafez S. The path from awareness to action: exploring diabetic patients’ awareness and attitudes and barriers to utilization of artificial pancreas in the Beheira Governorate, Egypt. Cureus. 2024;16(1):e52703.
107. Leadley C et al. Following in Banting’s footsteps or straying from the path? observations from contemporary diabetes innovation. Front Endocrinol (Lausanne). 2023;14:1270517.
Authors: Adeshola Demilade Afolorunsho,1 Kareem Ademola Wahab,2 Omotayo Odunsi,1 Ridwan Opeyemi Adeniyi,3 *Hafeez Aderinsayo Adekola2
1. Olabisi Onabanjo University, Ago Iwoye, Nigeria
2. Nigerian Institute of Medical Research, Yaba, Nigeria
3. Al-Hikmah University, Ilorin, Nigeria
*Correspondence to haderinsayor@gmail.com
Disclosure: The authors have declared no conflicts of interest.
Received: 08.05.25
Accepted: 13.10.25
Keywords: Autoimmune, diabetes, infection, insulin, proteins, viral.
Citation: EMJ Diabet. 2025;13[1]:83-90.
https://doi.org/10.33590/emjdiabet/DSAE7673
Abstract
Type 1 diabetes (T1D) is an autoimmune disease where the immune system mistakenly attacks insulin-producing β-cells. Although genetics plays an important role, environmental factors, especially viral infections, are now seen as key triggers in the development of the disease. Recent studies suggest that persistent viral infections, particularly with enteroviruses, may initiate and maintain autoimmune responses that damage pancreatic β-cells. Mechanisms such as molecular mimicry and epitope spreading are well-known explanations, and while bystander activation has been proposed, it remains debated in recent studies. Viral proteins that resemble human proteins can confuse the immune system, causing it to attack the body’s own tissues. Low-level chronic infections may also disrupt normal immune regulation and increase inflammation, both of which contribute to β-cell destruction. Early detection of viral involvement through biomarkers could allow for earlier intervention and personalised treatment strategies. Moreover, antiviral therapies combined with immunomodulatory approaches may help prevent or delay the onset of T1D in at-risk individuals. Despite these advances, research gaps remain, especially regarding the long-term effects of viral persistence and the exact mechanisms of autoimmune activation. Future research using new technologies such as single-cell RNA sequencing and better imaging tools could provide deeper insights. In conclusion, understanding the role of persistent viral reservoirs in T1D could lead to better diagnostics, preventive strategies, and treatments, offering new hope for managing this complex disease.
Key Points
1. Persistent viral infections, especially enteroviruses, may trigger and sustain autoimmune attacks on pancreatic β-cells through mechanisms such as molecular mimicry, epitope spreading, and chronic inflammation.
2. Early detection of viral activity and immune responses could improve prevention and treatment, with potential for antiviral and immunomodulatory therapies to delay or prevent Type 1 diabetes in at-risk individuals.
3. Research gaps remain on how long-term viral persistence drives autoimmunity, and advanced tools like single-cell RNA sequencing and imaging may help uncover key mechanisms for better diagnosis and management.
Type 1 diabetes (T1D) is a chronic autoimmune disease that results from the destruction of insulin-producing β-cells in the pancreas.1 It typically affects children and young adults but can occur at any age.1 Although genetic risk factors, especially variations in the human leukocyte antigen (HLA) region, are important, they alone cannot fully explain the growing incidence of T1D. Environmental factors, particularly viral infections, are believed to act as triggers that initiate the autoimmune response. Enteroviruses, among others, have been strongly linked to the development of T1D.2 Persistent viral infections can interfere with immune regulation through mechanisms like molecular mimicry and epitope spreading, while the role of bystander activation is still debated.3 These processes may confuse the immune system and lead to attacks on pancreatic tissue. Early identification of viral involvement could allow for preventive interventions, while targeted therapies against persistent viruses might reduce the risk of developing the disease. This review explores how persistent viral reservoirs contribute to autoimmune mechanisms leading to T1D and discusses the clinical implications and future research directions.
T1D makes up about 10% of all diabetes cases worldwide, and it is becoming more common in younger people. A small group of patients (less than 10%) are classified as Type 1B, meaning they show no signs of autoimmunity, and the cause of their diabetes is unknown.4 T1D occurs
when the immune system destroys the insulin-producing β-cells in the islets of Langerhans. It usually occurs in people who have a genetic risk, and is likely triggered by one or more environmental factors. The disease often develops slowly over months or years, during which the individual has normal blood sugar levels and exhibits no symptoms.5
T1D often affects people who do not have a family history of the disease. Only about 10–15% of people with T1D have a close family member (first- or second-degree relative) who also has it. However, the lifetime risk is much higher in family members of affected people. About 6% of children, 5% of siblings, and 50% of identical twins develop T1D, compared to only 0.4% of the general population.6 More than 50 genetic risk areas for T1D have been found through genome-wide studies and meta-analyses.7 The most important genes are found in the major histocompatibility complex region, also called the HLA system, located on chromosome 6. Polymorphisms in the HLA region explain 40–50% of the genetic risk for developing T1D. Other important genes include the insulin gene (INS-VNTR, IDDM2) on chromosome 11 and the CTLA-4 gene on chromosome 2.7
Many other genetic areas also play smaller roles in raising the risk of T1D, either by themselves or along with other autoimmune diseases.7 In T1D, the immune system attacks the pancreatic β-cells. Mononuclear cells enter the islets in a process called ‘insulitis’, which over time leads to the loss of most β-cells.8 β-cell death during insulitis likely occurs through direct contact with activated macrophages and T cells, or through substances they release, such as cytokines, nitric oxide, and O2-free radicals.9 In laboratory studies, when β-cells
are exposed to IL-1β alone or together with interferon-γ, they show changes similar to those seen in people before they develop diabetes. These changes include higher proinsulin/insulin levels and a weaker first-phase insulin release after glucose stimulation. This happens mainly because IL-1β reduces the ability of insulin granules to dock and fuse with the β-cell membrane.10 If exposure to IL-1β and interferon-γ and/or TNF-α continues for longer, but not with each cytokine alone, the damage becomes worse and leads to β-cell death.10
Viral infections are one possible environmental cause that can lead to autoimmunity. Viruses usually trigger a strong immune response necessary to clear the infection. However, in rare cases, problems with controlling the immune response can cause the body to attack its own tissues. Because viruses can activate lymphoid cells and cause inflammation, they were suspected of causing autoimmune diseases early on.11 Chronic viral infections can lead to or maintain autoimmunity through several ways, including molecular mimicry, bystander activation, and epitope spreading. Viruses have been shown to influence how many autoimmune diseases develop, including systemic lupus erythematosus (SLE), rheumatoid arthritis, Sjögren’s syndrome, herpetic stromal keratitis, coeliac disease, and multiple sclerosis (MS). Different viruses are linked to specific autoimmune diseases. For example, Epstein–Barr virus (a herpesvirus) is often linked to SLE and MS, Coxsackie B virus and rotavirus are linked to β-cell autoimmunity in the pancreas, hepatitis C virus is associated with autoimmune hepatitis and Sjögren’s syndrome, and influenza A virus infection has been linked to autoimmune myocarditis and arthritis. Other viruses like measles, mumps, and rubella have also been studied for their possible roles in triggering or worsening autoimmune conditions.12
Viruses can promote autoimmune disease through different mechanisms, such as molecular mimicry, bystander activation, and epitope spreading.
Molecular mimicry was first described in 1964.13 This mechanism occurs when pathogen-derived peptide sequences or structures are sufficiently similar to selfproteins, causing the immune system to mistake the self-protein for the pathogen antigen.14 When this happens, the immune system might accidentally attack the body’s own cells, leading to autoimmune diseases. In T1D, molecular mimicry is believed to help trigger attacks on β-cells in the pancreas. Studies have found viral proteins in the pancreases of patients with T1D, and animal studies show viruses can trigger similar immune attacks.13 Viruses like Coxsackievirus, cytomegalovirus, enteroviruses, and rotavirus have proteins that mimic human proteins like GAD65 and IA-2. This confusion causes the immune system to mistakenly attack its own tissues.15 When viral proteins closely resemble human proteins, a strong and long-lasting immune attack can happen, and is called molecular mimicry.
The bystander activation idea suggests that immune cells can become active just because they are near an area of inflammation, even if immune cells are not recognising a pathogen-derived antigen directly. This mostly happens through chemicals called cytokines, like IL-2, which are released during inflammation. However, newer research suggests the term ‘bystander’ might not be correct. There are direct signals that activate immune cells, making it a planned and not a random reaction. Additionally, immune cells that do not recognise anything harmful usually help calm down the immune response rather than worsen it.16 This helps prevent too much inflammation and tissue damage. For example, in some mouse studies of
Coxsackievirus infection, local inflammation caused nearby immune cells to become active even without recognising viral proteins. In a study of non-obese diabetic mice infected with Coxsackievirus, high levels of insulitis (auto-reactive T cells already present) were required before infection accelerated diabetes through a bystander activation effect.17 Also, rotavirus infection in non-obese diabetic mice induces activation of bystander lymphocytes via plasmacytoid dendritic cells secreting Type I interferon, which helps activate islet-autoreactive T cells even though the initial trigger may be non-specific.18
Epitope spreading happens when the immune system starts by attacking one part of a protein and later attacks other parts or different proteins. This has been seen in autoimmune diseases like MS.19 Sometimes the immune system attacks proteins that have been changed after post-translational modifications or proteins that are normally hidden inside cells. If the thymus did not fully train immune cells to ignore these hidden proteins, there might be more cells ready to attack when they are exposed during inflammation. A clear example of epitope spreading is seen in animal models of T1D, where early immune responses to one β-cell antigen (like insulin or GAD65) later broaden to include others such as IA-2 and ZnT8, as disease develops. Studies have shown that antibodies to IA-2 and ZnT8 often appear after initial autoantibodies in human T1D and that the autoantibody profile expands over time.20-22
MOLECULAR MECHANISM LINKING PERSISTENT VIRAL RESERVOIRS TO TYPE 1 DIABETES
Studies of islets from patients with T1D where enteroviral capsid protein VP1 or RNA was found did not show signs of widespread cell death.23 However, a small number of islet cells might still break down early in infection or later during the disease. Research on small intestine and pancreas
samples from patients with new or longstanding T1D showed that enteroviral RNA and VP1 protein were found in a small number of intestinal and pancreatic cells, including pancreatic duct cells and islet β-cells.24 Low amounts of enteroviral RNA were also detected in snap-frozen pancreas samples and in the medium from enriched islet preparations, using sensitive PCR methods that needed up to 40 cycles of amplification.25 These results support the idea of a low-level enterovirus infection in the pancreas of patients with T1D.
However, live viruses could not be grown from the islets using permissive cell lines, suggesting that the viruses were either not fully active or had very slow replication.25,26 This supports the idea that persistent infection exists in the pancreas of patients with T1D.27 Besides causing direct damage to β-cells, viral infections can also lead to insulin resistance by triggering the release of interferons, mainly Type I interferons. These interferons are important for fighting viruses, but if produced for a long time during chronic or repeated infections, they can disrupt insulin signalling.
Interferons activate the JAK-STAT signalling pathway, which interferes with how the body uses glucose, affecting tissues like muscle, liver, and fat.28 Even short-term insulin resistance can add extra stress to β-cells by increasing the demand for insulin. The ‘accelerator hypothesis’ suggests that higher body weight raises insulin demand, putting more stress on β-cells and making them easier targets for autoimmune attacks.29 Additionally, fat buildup in the islets due to obesity can cause β-cell death and contribute to the onset of T1D.
Persistent infections happen when pathogens manage to avoid or weaken the immune system and stay in the body for a long time. These infections are believed to be one of the possible ways that autoimmunity can develop.30 For example, hepatitis C virus can stay in the liver, causing the immune system to attack not only the virus but also liver cells. This leads to ongoing inflammation, production of autoantibodies, liver damage, and autoimmune disease.31 Similarly, long-term
infection with Helicobacter pylori is linked to autoimmune gastritis and gastric cancer. In this case as well, the immune system attacks both the bacteria and the stomach cells, leading to chronic inflammation and autoimmunity.32
Persistent infections can also cause autoimmunity by activating many B cells at once (polyclonal activation), leading to the production of autoantibodies. For instance, chronic Epstein–Barr virus infection has been linked to SLE because the virus causes B cells to become overly active and produce harmful antibodies.33 In addition, persistent infections can activate Tolllike receptors (TLR), which detect signs of infection. Activation of TLRs causes the release of inflammatory signals like cytokines and chemokines, which leads to long-term inflammation, tissue damage, and possibly autoimmune disease.34
Mostly, these processes happen through local production of cytokines like IL-2 and other immune system activators. While it is known that inflammation can ‘license’ or activate antigen-presenting cells and trigger immune responses through pathways like TLRs, calling this a ‘bystander’ effect may not be correct in present times. First, if cells are directly activated, it is not a bystander event but a targeted response. Second, there is no strong evidence that adaptive immune cells, which do not recognise specific antigens, can accidentally get activated. Instead, studies show that T cells without matching antigens tend to work as regulators, helping to reduce inflammation.16 This makes sense because, without a driving antigen, the immune system usually shuts down instead of growing stronger. In autoimmunity, the problem is different; selfantigens wrongly become the main target. Therefore, the authors believe that random bystander activation does not play a major role in starting autoimmunity.34
The early detection of viral involvement in T1D is an important area for clinical research. Studies suggest that certain viral infections, especially those caused by enteroviruses, may play a key role in the onset of T1D. For example, finding specific viral RNA in the pancreatic tissue of patients with T1D supports the concept that persistent viral reservoirs can affect autoimmune processes.35 Identifying these viral markers could help in earlier diagnosis, allowing timely action before symptoms appear. This could involve detecting specific biomarkers linked to viral infections, such as viral RNA or antibodies against viral proteins in blood samples.36 Using these markers, doctors could monitor people at high risk more effectively and step in sooner. It is also important to develop biomarkers that can clearly show which patients are at risk because of persistent viral reservoirs. Measurements of viral load or specific immune responses to viral proteins could help classify patients and guide monitoring plans.37 These diagnostic tools could not only improve the prediction of T1D, but also support personalised treatment plans that target viral triggers of autoimmunity.
Targeting viral reservoirs offers a promising path for preventing or delaying the start of T1D. Antiviral treatments have shown potential in changing the immune response and lowering the chances of T1D in people at risk. For instance, giving antiviral medications during the early stages of infection may reduce the risk of later autoimmune reactions.38 This highlights the need to include antiviral strategies in efforts to prevent T1D. Therapies aimed at removing persistent viruses could lower the inflammation that leads to the destruction of β-cells.39 Along with antiviral treatments, immunomodulatory approaches could help manage the autoimmune processes triggered by viral infections. For example, therapies that adjust the immune response may help the body tolerate its own β-cells again, reducing autoimmune attacks.40 Immunomodulatory therapies, such as
monoclonal antibodies that target certain immune pathways, could also help calm the autoimmune reaction caused by viruses.41 A combined approach using both antiviral and immunomodulatory therapies might offer a strong new way to manage T1D.
The COVID-19 pandemic raised questions about whether SARS-CoV-2 changed the number of new diabetes cases, including T1D. Several reports from different countries found more new T1D cases in children during the pandemic years. For example, registry and hospital data from Europe and other places showed increases in T1D diagnoses and more severe presentation (diabetic ketoacidosis) in children during 2020–2021.42,43 Large cohort studies and pooled analyses also suggest an association between prior COVID-19 infection and higher risk of new diabetes (both Type 1 and 2), but results are mixed and may depend on study design and follow-up time. A pooled analysis found a higher overall risk of new diabetes after COVID-19, with subgroup data that include an increased risk estimate for T1D.44,45 The possible mechanisms are debated. Some lab and animal studies show that SARSCoV-2 can damage the pancreas and impair islets, suggesting a direct effect on β-cells.46 Other studies find low or variable expression of key viral entry molecules (ACE2) on human β-cells, so direct infection is not certain.47 Immune activation, systemic inflammation, and metabolic stress during or after infection could also trigger diabetes, or the pandemic’s social effects (delayed care, less exercise, weight gain) could change disease detection and severity. Thus, current evidence supports a possible link between COVID-19 and increased diabetes risk, but the data do not yet prove that SARS-CoV-2 directly causes sustained new T1D in most patients. More longterm, well-designed studies are needed to separate direct viral effects from indirect or social causes.46-48
Identifying which patients would benefit most from testing for viral reservoirs or viral markers is an important future direction. People who have a strong family history of T1D or who test positive for multiple islet autoantibodies but are not yet diabetic may
gain the most from such testing. Detecting viral RNA, proteins, or specific immune responses linked to enteroviruses in these high-risk individuals could help predict who is likely to develop T1D.27,35 Testing may also be useful for patients with recentonset T1D, especially within the first year after diagnosis, when viral material is more likely to be found in pancreatic or intestinal tissues.23,24 Detecting viral evidence in this group can help confirm the presence of persistent infection and guide early antiviral or immune-modifying treatments. In addition, monitoring children or adolescents with recent viral infections, such as Coxsackievirus B or rotavirus, who later develop islet autoantibodies may improve understanding of disease progression and timing of intervention.12,29 Early screening for viral markers could be most useful for (1) genetically at-risk individuals with islet autoantibodies, (2) newly diagnosed patients, and (3) children recovering from viral infections known to affect pancreatic tissue.
Even with progress, many research gaps remain in understanding how viral reservoirs relate to T1D. One major gap is the lack of long-term studies on how persistent viruses affect the immune system over time. Learning exactly how viral reservoirs cause autoimmunity is crucial for creating targeted treatments.49 New technologies, like singlecell RNA sequencing and advanced imaging, offer powerful tools to study viral reservoirs and their role in T1D. These methods could help find specific viruses involved and explain how they interact with the immune system.50 In addition, teamwork among virologists, immunologists, and doctors will be important to close these knowledge gaps and turn research into real-world treatments.
Persistent viral infections may play a central role in triggering and maintaining the autoimmune processes that lead to T1D. Through molecular mimicry, bystander
activation, and epitope spreading, viruses like enteroviruses can initiate immune attacks on pancreatic β-cells. Research has shown that viral RNA and proteins can be detected in the pancreatic tissues of patients with T1D, supporting the idea of a long-term, low-level infection. Identifying biomarkers for viral involvement could greatly improve early diagnosis and allow for preventive treatments before full disease development. In addition, combining antiviral therapies with immunomodulatory approaches offers a promising strategy
References
1. Quinn LM et al. What is type 1 diabetes? Medicine (Baltimore). 2022;50(10):619-24.
2. Knip M, Simell O. Environmental triggers of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(7):a007690.
3. Begum S et al. Molecular mimicry analyses unveiled the human herpes simplex and poxvirus epitopes as possible candidates to incite autoimmunity. Pathogens. 2022;11(11):1362.
4. Paschou SA et al. On type 1 diabetes mellitus pathogenesis. Endocr Connect. 2018;7(1):R38-46.
5. Giwa AM et al. Current understandings of the pathogenesis of type 1 diabetes: genetics to environment. World J Diabetes. 2020;11(1):13-25.
6. Fornasini S et al. The consequences of type 1 diabetes onset on family life. An integrative review. J Child Fam Stud. 2019;29:1467-83.
7. Størling J, Pociot F. Type 1 diabetes candidate genes linked to pancreatic islet cell inflammation and betacell apoptosis. Genes (Basel). 2017;8(2):72.
8. Eizirik DL et al. Why does the immune system destroy pancreatic β-cells but not α-cells in type 1 diabetes? Nat Rev Endocrinol. 2023;19:425-34.
9. Rojas J et al. Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. J Diabetes Res. 2018;2018:9601801.
10. Eizirik DL et al. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16(7):349-62.
11. Sundaresan B et al. The role of viral infections in the onset of autoimmune diseases. Viruses. 2023;15(3):782.
for stopping or delaying β-cell destruction. However, many questions remain about the exact ways viruses drive autoimmunity and how persistent infections affect the immune system over time. Future studies using advanced technologies and strong collaboration between scientists and clinicians are needed to close these gaps. Overall, a deeper understanding of viral contributions to T1D could lead to more personalised and effective prevention and treatment strategies, offering new hope for individuals at risk of developing this disease.
12. Smatti MK et al. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762.
13. Lemos JRN et al. Immunological and virological triggers of type 1 diabetes: insights and implications. Front Immunol. 2024;14:1326711.
14. Rojas M et al. Molecular mimicry and autoimmunity. J Autoimmun. 2018;95:100-23.
15. Bizimana Rukundo T. Crossreactivity in adaptive immunity: an overview. Newport Int J Biol Appl Sci. 2024;5(3):39-43.
16. Christoffersson G et al. Interference with pancreatic sympathetic signaling halts the onset of diabetes in mice. Sci Adv. 2020; 6(35):eabb2878.
17. Serreze DV et al. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes. 2000;49(5):708-11.
18. Pane JA et al. Rotavirus acceleration of type 1 diabetes in non-obese diabetic mice depends on type I interferon signalling. Sci Rep. 2016;6;29697.
19. Bretscher P. Relapsing/remitting multiple sclerosis: a speculative model and its implications for a novel treatment. Scand J Immunol. 2023;98(5):e13325.
20. Kawasaki E. Anti-islet autoantibodies in type 1 diabetes. Int J Mol Sci. 2023;24(12):10012.
21. van der Werf N et al. Viral infections as potential triggers of type 1 diabetes. Diabetes Metab Res Rev. 2007;23(3):169-83.
22. Jia X et al. Strong association of autoantibodies targeting deamidated extracellular epitopes of insulinoma antigen-2 with clinical onset of type 1 diabetes. Diabetes. 2025;74(4): 544-53.
23. Isaacs SR et al. Viruses and type 1 diabetes: from enteroviruses to the virome. Microorganisms. 2021;9(7):1519.
24. Geravandi S et al. Localization of enteroviral RNA within the pancreas in donors with T1D and T1D-associated autoantibodies. Cell Rep Med. 2012;2(8):100371.
25. Oikarinen S et al. Characterisation of enterovirus RNA detected in the pancreas and other specimens of live patients with newly diagnosed type 1 diabetes in the DiViD study. Diabetologia. 2021;64(11):2491-501.
26. Turkki P et al. Slow infection due to lowering the amount of intact versus empty particles is a characteristic feature of coxsackievirus B5 dictated by the structural proteins. J Virol. 2019;93(20):e01130-19.
27. Nekoua MP et al. Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2022;18(8):503-16.
28. Collotta D et al. Recent advances in JAK inhibitors for the treatment of metabolic syndrome. Front Pharmacol. 2023;14:1245535.
29. Houeiss P et al. Environmental triggering of type 1 diabetes autoimmunity. Front Endocrinol (Lausanne). 2022;13:933965.
30. Johnson D, Jiang W, Infectious diseases, autoantibodies, and autoimmunity. J Autoimmun. 2023;137:102962.
31. Irshad M et al. Immunopathogenesis of liver injury during hepatitis C virus infection. Viral Immunol. 2019;32(3):112-120.
32. Wang L et al. Helicobacter pylori and autoimmune diseases: involving multiple systems. Front Immunol. 2022;13:833424.
33. Jog NR, James JA. Epstein Barr Virus and autoimmune responses in systemic lupus erythematosus. Front Immunol. 2021;11:623944.
34. Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391-412.
35. Laiho JE et al. Detection of enterovirus RNA in pancreas and lymphoid tissues of organ donors with type 1 diabetes. Diabetologia. 2025;68(6):1211-25.
36. Radhakrishnan A. Biomarkers of human viral infections and their role in the diagnosis. Recent Dev Nanomater Sens Hu Pathog. 2024;DOI:10.1016/B978-0443-18574-8.00006-6.
37. Ghosh S. Diabetes: discovery of insulin, genetic, epigenetic and viral infection mediated regulation. Nucleus (Calcutta). 2022;65(2):283-97.
38. Cunningham JW et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020;181(3):379-81.
39. Morse ZJ, Horwitz MS. Virus Infection is an instigator of intestinal dysbiosis leading to type 1 diabetes. Front Immunol. 2021;12:751337.
40. Black L, Zorina T. Cell-based immunomodulatory therapy approaches for type 1 diabetes mellitus. Drug Discov Today. 2020;25(2):380-91.
41. Warshauer JT et al. New frontiers in the treatment of type 1 diabetes. Cell Metab. 2020;31(1):46-61.
42. Kamrath C et al. Incidence of type 1 diabetes in children and adolescents during the COVID-19 pandemic in Germany: results from the DPV registry. Diabetes Care. 2022;45(8):1762-71.
43. van den Boom L et al. Type 1 diabetes incidence in children and adolescents during the COVID-19 pandemic in Germany. Diabetes Res Clin Pract. 2022;193:110146.
44. Zhou J et al. Association of COVID-19 infection and the risk of new incident diabetes: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2024;15:1429848.
45. Kendall EK et al. Association of SARSCoV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Netw Open. 2022;5(9):e2233014.
46. Deng W et al. Infection with SARSCoV-2 can cause pancreatic impairment. Signal Transduct Target Ther. 2024;9(1):98.
47. El-Huneidi W et al. Expression of SARS-CoV-2 receptor “ACE2” in human pancreatic β cells: to be or not to be! Islets. 2021;13(5-6):106-14.
48. D’Souza D et al. Incidence of diabetes in children and adolescents during the COVID-19 pandemic. JAMA Netw Open. 2023;6(6):e2321281.
49. Atkinson B, Petersen E. SARS-CoV-2 shedding and infectivity, Lancet. 2020;395(10233):1339-40.
50. Lloyd RE et al. Enteroviruses and type 1 diabetes: multiple mechanisms and factors? Annu Rev Med. 2022;73: 483-99.
Authors: *Gemmy P. David,1 Elizabeth P. Prieto2
1. Department of Dermatology, Dr. Jose N. Rodriguez Memorial Hospital and Sanitarium, Caloocan, Philippines
2. Department of Dermatology, East Avenue Medical Center, Quezon City, Philippines
*Correspondence to gemmydavid@gmail.com
Disclosure: The authors have declared no conflicts of interest.
Disclaimer: Written informed consent was obtained from the patient for publishing this case report.
Received: 23.04.25
Accepted: 13.10.25
Keywords: Diabetes, eruptive xanthoma, hypertriglyceridaemia.
Citation: EMJ Diabet. 2025;13[1]:91-97. https://doi.org/10.33590/emjdiabet/UIQV4136
Abstract
Eruptive xanthomas present as a sudden eruption of multiple yellow papules and are often associated with extreme hypertriglyceridaemia. Several reports describe eruptive xanthoma as the sole presenting symptom of unrecognised hypertriglyceridaemia in adults, but only a few cases report eruptive xanthoma associated with both unrecognised hypertriglyceridaemia and undiagnosed Type 2 diabetes. This report highlights the importance of identifying a cutaneous marker suggestive of an underlying metabolic disorder. A 34-year-old Filipino male presented with a 2-month history of sudden onset of multiple skin-coloured to yellowish papules on the extremities and trunk. He reported a strong family history of diabetes, hypertension, and premature cardiovascular death. Dermoscopy and histopathology were consistent with xanthoma. Referral to endocrinology and subsequent laboratory evaluation revealed severe dyslipidaemia, with triglyceride levels elevated to 1,989 mg/dL (approximately 13 times the upper limit of normal) and a fasting blood glucose of 13.7 mmol/L. After 16 weeks of pharmacologic therapy and lifestyle modification, the patient showed complete resolution of lesions, with normalisation of lipid and glucose levels and no recurrence. This case illustrates the critical role of dermatologic findings in the early detection of systemic metabolic disorders. Early multidisciplinary intervention is key to successful treatment and prevention of life-threatening complications.
1. Recognising eruptive xanthomas can expedite the diagnosis of severe metabolic disease.
2. In skin of colour, dermoscopy is a valuable tool to aid in the diagnosis, as the typical yellow hue of xanthomas may be less evident.
3. Early initiation of lipid-lowering and antidiabetic medications combined with lifestyle modification can result in rapid resolution of skin lesions and normalisation of metabolic parameters.
Eruptive xanthoma is considered a rare disease with an incidence of 18 out of 100,000. It can only be seen in 10% of patients with severe hypertriglyceridaemia.1 There is no age or gender bias.2 It is important to promptly recognise this disease because it is a marker of severe hypertriglyceridaemia, which, if left untreated, will lead to increased risk of the potentially fatal complications of acute pancreatitis and atherosclerosis.3 A number of reports describe eruptive xanthoma as the primary presenting symptom of unrecognised hypertriglyceridaemia in adults; however, only a few reports document its association with both unrecognised hypertriglyceridaemia and undiagnosed Type 2 diabetes.4 This report aims to highlight the importance of early recognition of a cutaneous marker of severe metabolic disease.
A 34-year-old Filipino male smoker with a known history of hypertension, currently not on medication and without a known history of diabetes, presented to the dermatology outpatient department with a 2-month history of suddenly appearing, symmetrical, skin-coloured to yellowish papules on the arms, thighs, and trunk (Figure 1A). The patient had a Fitzpatrick skin Type IV. The lesions were associated with mild pruritus. There was no recent history of insect bite or contact with possible allergens. Review of systems revealed weight gain for the past 6
months, polyuria, polydipsia, polyphagia, and prickling sensation on hands and feet. There were no similar lesions in the family, but there was a strong family history of hypertension, diabetes, and death of a paternal grandfather at the early age of 50 years due to aneurysm. The patient worked as a truck driver and led a sedentary lifestyle. No prior consultation or intervention had been undertaken.
Dermoscopy of the lesions revealed a homogenous yellow centre. Some lesions showed coalescing yellow globules surrounded by erythematous to hyperpigmented halo, while some exhibited occasional serpentine vessels (Figure 1B).
Histopathological examination of a skin biopsy from the left arm was consistent with xanthoma. It showed variably sized aggregates of lipid-laden histiocytes (foamy histiocytes; Figure 1C), located in the upper third of the dermis, admixed with lymphocytes. Foci of extracellular lipid were also observed. The patient was promptly referred to an endocrinologist for further laboratory workup and confirmation of the diagnosis of eruptive xanthoma.
Laboratory results revealed severe hypertriglyceridaemia at 1,989 mg/dL, which is 13-times higher than the normal value. Grossly cloudy serum was visible upon centrifugation of the patient’s blood. Verylow-density lipoprotein was also 10-times elevated at a value of 397 mg/dL, along with other lipid parameters (Table 1), supporting the diagnosis of severe dyslipidaemia.5 Fasting plasma glucose was also twotimes elevated from the upper limit at 13.74
Figure 1: Images showing (A) lesions at baseline on the arms, thighs, and posterior trunk; (B) dermoscopy (10x); and (C) histopathology (left arm; haematoxylin and eosin stain).
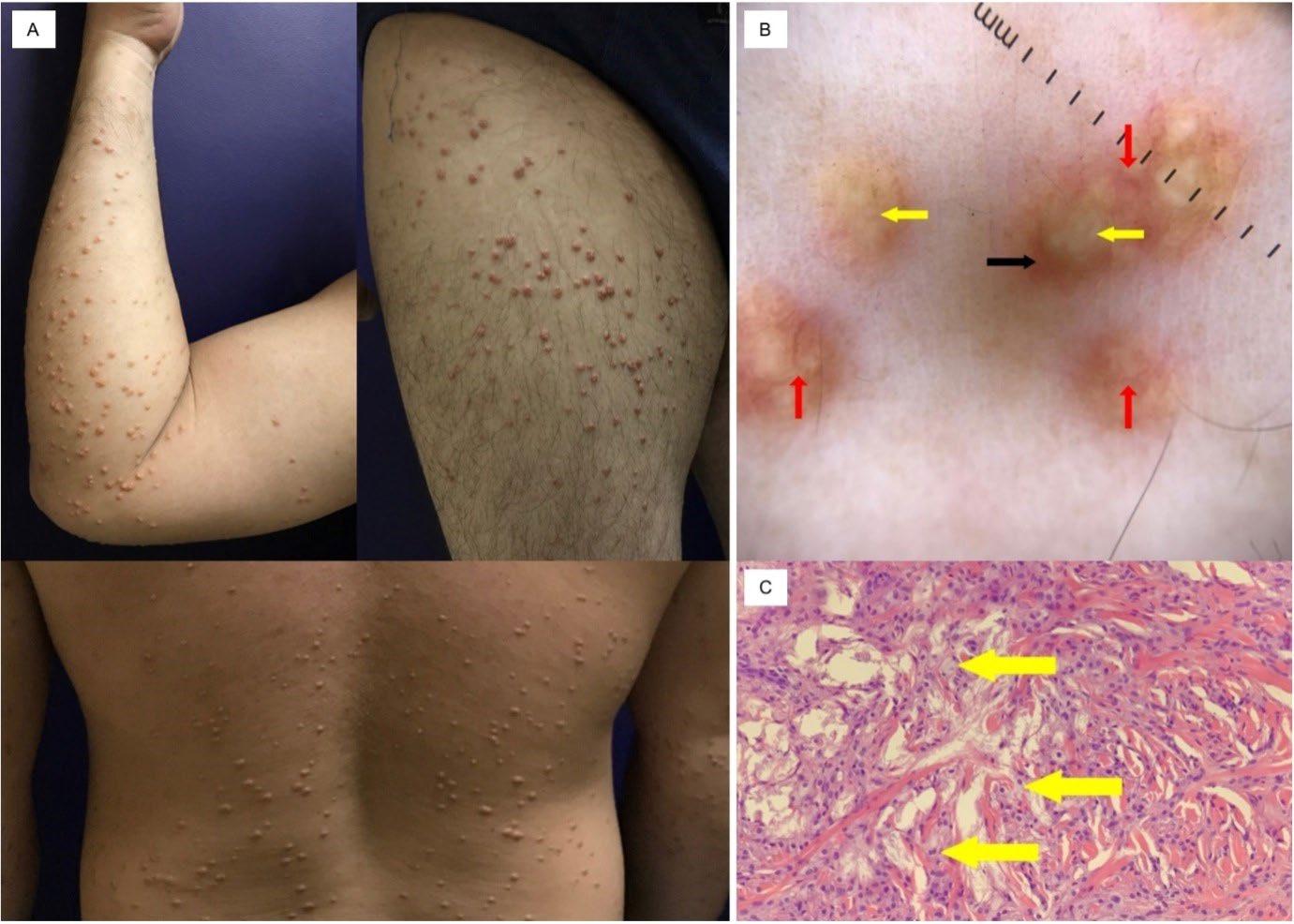
A) Clinical picture of symmetrical skin-coloured to yellowish papules on arms, thighs, and trunk. B) Dermoscopy findings of eruptive xanthoma (left arm, 10x magnification) showing a homogeneous yellow centre, some with coalescing yellow globules in clusters (yellow arrows) surrounded by erythematous to hyperpigmented halo (black arrow), some with occasional serpentine vessels (red arrows). C) Histopathology of eruptive xanthoma (left arm, H&E stain) showing variably-sized aggregates of lipid-laden histiocytes or foamy histiocytes (yellow arrows).
H&A: haematoxylin and eosin.
mmol/L, which confirmed the diagnosis of Type 2 diabetes.6
Unfortunately, HbA1c was not available at that time. Patient also satisfied four out of the five criteria of NCEP/ATP III-AHA/ NHLBI (2005) for metabolic syndrome, with a waist circumference of 104 cm, triglycerides of 1,989 mg/dL, a blood pressure of 130/90 mmHg, and fasting glucose of 275 mg/dL.7
Correlating the clinical presentation and laboratory findings confirmed the diagnosis of eruptive xanthoma.
The patient was started on the following tablets: fenofibrate 200 mg once daily, atorvastatin 80 mg once at night, metformin 500 mg three times daily, gliclazide 80 mg once daily, and linagliptin 5 mg daily. Lifestyle modifications were also advised, including a low salt, low fat, low carbohydrate diet, smoking cessation, reduced alcohol intake, regular exercise, weight monitoring, and referral to a
HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very-low-density lipoprotein.
nutritionist. The patient was also advised to avoid manipulating the lesions and to use mild emollients.
As early as 4 weeks of medical management and lifestyle modification, there was noticeable rapid improvement in the lesions, with 80% flattening and darkening of the previously yellowish papules. After 16 weeks of therapy (Figure 2), all lesions were completely resolved, with no recurrence noted. Repeat laboratory results showed a marked decrease in lipoproteins, particularly triglycerides, from 1,989 mg/dL to 776 mg/ dL. Fasting blood sugar also decreased from 13.74 mmol/L to 6.52 mmol/L. HbA1c level was 7.0%. No adverse effects were reported.
In the literature, several reports describe eruptive xanthoma as the sole presenting symptom of unrecognised hypertriglyceridaemia in adults, but only a few cases document eruptive xanthoma associated with both unrecognised hypertriglyceridaemia and undiagnosed Type 2 diabetes.4,8-10 These cases involved seemingly healthy adults whose
chief complaint was the appearance of yellowish papules, without other systemic symptoms.8-10 In contrast, the author’s case is notable because the lesions were not initially apparent due to the patient’s darker skin type with a yellow undertone.11 Although one published case report describes eruptive xanthoma in a Filipino adult, that patient initially presented with generalised body weakness and weight loss, with xanthomas appearing later in the course.12 In the author’s case, however, the primary symptom was the sudden eruption of yellowish papules without other systemic symptoms.
The pathogenesis of xanthoma begins with increased local extravasation of lipids through the blood vessel walls into the interstitial spaces of connective tissue. Macrophages subsequently engulf these lipid particles, resulting in the formation of foam cells, or lipid-laden macrophages. These foam cells then accumulate in the skin and tendons, leading to the development of xanthomas.1
According to the Frederickson Classification of Dyslipidemia, eruptive xanthoma variant is commonly seen in those with Type I, IV, and V dyslipidaemia. However, this
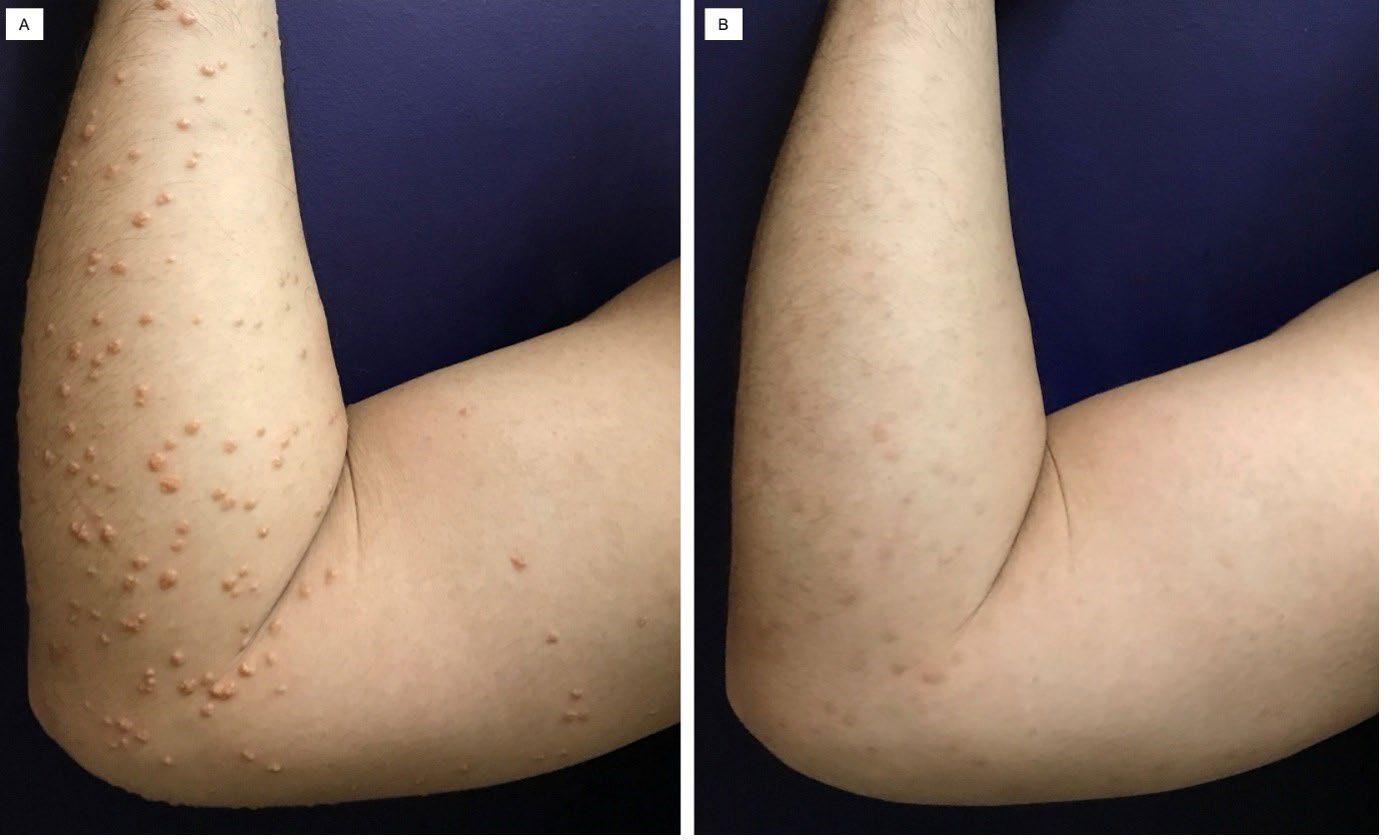
Comparison photos from baseline at Week 0 (A) and after 16 weeks (B) of medical management and lifestyle modification.
classification relies only on the biochemical profiles and does not distinguish between the genetic or acquired causes of dyslipidaemia.13 In the recent classification of Berberich and Hegele13 regarding biochemical levels for dyslipidaemia in adults, the current case can be classified as having severe deviation, with triglycerides >885 mg/dL and low-density lipoprotein >194 mg/dL.13 This cut-off is important because most clinicians observe eruptive xanthoma at a triglyceride level of >1,000 mg/dL.1 At this state, the aetiology can be due to polygenic predisposition plus secondary factors such as diabetes and metabolic syndrome. Hence, family members should be screened for mixed hyperlipidaemia, diabetes, and obesity. In recent years, there has been a growing prevalence of lipid disorders among Southeast Asian populations, such as Filipino adults. The notable increase in cases may be attributed to rapid urbanisation, lifestyle changes, and a
dietary shift toward Westernised food.14 Hence, in this case, it is more likely that both genetic and acquired causes are present.
In obesity and diabetes, the decrease in peripheral insulin resistance leads to lipolysis, decreased lipoprotein lipase, and defective chylomicron clearance. The combination of these mechanisms leads to a number of lipid disorders, including chylomicronaemia, hypertriglyceridaemia, increased low-density lipoprotein, and increased very-low-density lipoprotein, which ultimately leads to xanthoma formation.1
Aside from eruptive xanthoma, the patient is also at risk for long-term atherosclerotic cardiovascular disease, hyperlipidaemic acute pancreatitis, and lipaemia retinalis.13 Hence, hyperlipidaemia requires immediate intervention to prevent these devastating consequences.
Interestingly, it remains unclear whether the severity of dyslipidaemia correlates with the number of eruptive papules. For example, the author’s current case with a baseline triglyceride level of 1,989 mg/dL exhibited widespread lesions across the extremities and trunk. This contrasts with another report in which a patient had a significantly higher triglyceride level of 7,157 mg/dL, but presented only a few lesions localised to the extensor extremities.2 The authors think these variations suggest that, while high triglyceride levels are associated with eruptive xanthomas, the exact relationship between triglyceride levels and the number and distribution of lesions remains poorly defined.
Moreover, the authors think that the characteristic yellowish hue of the papules may be difficult to appreciate in skin of colour (Figure 1A, bottom).
With the aid of dermoscopy, the contrast between the homogeneous yellow centre and the hyperpigmented to erythematous periphery facilitates clinical diagnosis.8 Correlation of the histopathology result and metabolic panel confirmed the diagnosis of eruptive xanthoma.
In terms of management, eruptive xanthoma responds rapidly once the underlying dyslipidaemia has been addressed, often as early as 4 weeks, as seen in the authors’ case and in other reported cases (Table 2).2-3,15-17 However, if the underlying
Marogi et al.,4 2021 (Case series)
Nakamura et. al.,15 2022
Munoz et al.,16 2023
disorder is not treated, there is a high risk of developing acute pancreatitis and atherosclerosis, two potentially lifethreatening complications.17
In summary, eruptive xanthoma is an important cutaneous red flag of profound hypertriglyceridaemia, which may stem from either genetic causes of dyslipidaemia or secondary factors, such as poor glycaemic control in this patient. Improvement of dyslipidaemia and blood glucose through medications and lifestyle modification
References
1. Zak A et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(2):181-8.
2. Vangara SS et al. Severe hypertriglyceridemia presenting as eruptive xanthomatosis. J Family Med Prim Care. 2018;7(1):267-70.
3. Kashif M et al. An unusual presentation of eruptive xanthoma: a case report and literature review. Medicine (Baltimore). 2016;95(37):e4866.
4. Marogi EP et al. Eruptive xanthomas: importance of recognition to reduce delay of effective triglyceride reduction. Am J Med. 2022;135(4): 444-7.
5. Guerrero et al. 2015 Clinical practice guidelines for the management of dyslipidemia in the Philippinesexecutive summary. ASEAN Heart J. 2016;24:7.
6. Philippine Center for Diabetes Education. Philippine practice guidelines on the diagnosis and
resulted in significant improvements in both metabolic parameters and skin lesions.
Early recognition by the dermatologist and timely referrals to an endocrinologist and nutritionist played a vital role in preventing potentially severe complications.
I am glad I had my skin rashes checked because I learned that I have some hidden medical problems. As the breadwinner of our family, I got scared of the consequences if no intervention was done. So, I am very thankful the treatment worked immediately.
management of diabetes mellitus. 2014. Available at: https://www.pcdef. org/Documents/Diabetes-United-forDiabetes-Phil.pdf. Last accessed: 16 October 2025.
7. Morales DD et al. Metabolic syndrome in the Philippine general population: prevalence and risk for atherosclerotic cardiovascular disease and diabetes mellitus. Diab Vasc Res. 2008;5(1):3643.
8. Zhao C, Li H. Eruptive xanthoma associated with hypertriglyeridaemia and diabetes. Aust J Gen Pract. 2023;52(6):371-3.
9. Solak B et al. First and only symptom of undiagnosed diabetes mellitus: eruptive xanthoma. BMJ Case Rep. 2015;2015:bcr2015212160.
10. Nayak KR, Daly RG. Images in clinical medicine. Eruptive xanthomas associated with hypertriglyceridemia and new-onset diabetes mellitus. N Engl J Med. 2004;350(12):1235.
11. Goh CF. Diversity of Asian skin: a review on skin biophysical properties. Exp Dermatol. 2024;33(1):e14959.
12. Linsao MJC. Eruptive xanthoma in a young adult male with severe dyslipidemia and newly diagnosed type 1 diabetes. Metabolism. 2020;104:154055.
13. Berberich AJ and Hegele RA. A modern approach to dyslipidemia. Endocr Rev. 2022;43(4):611-53.
14. Callanta MLJ, Tantengco OAG. Dyslipidemia research landscape and socioeconomic facilitators of scientific productivity in Southeast Asia. Diabetes Metab Syndr. 2022;16(8):102583.
15. Nakamura Y et al. Eruptive xanthomas caused by primary type V hyperlipoproteinemia. Intern Med. 2022;61(9):1469-70.
16. Munoz NR et al. Eruptive xanthomas precipitated by severe hypertriglyceridemia from diabetes and alcohol use. Cureus. 2023;15(8):e43288.
17. Ohtaki S et al. Eruptive xanthomas as a marker for metabolic disorders: a specific form of xanthoma that reflects hypertriglyceridemia. Clin Case Rep. 2022;10(4):e05671.
Authors: *Majid Davari,1,2 Roya Khoshtinat,1 Yahya Bayazidi,1 Alireza Esteghamati,3 Naeemeh Dini4
1. Department of Pharmacoeconomics and Pharmaceutical Administration, Faculty of Pharmacy, Tehran University of Medical Sciences, Iran
2. Pharmaceutical Management and Economics Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Iran
3. Endocrinology and Metabolism Research Center, Vali-Asr Hospital, School of Medicine, Tehran University of Medical Sciences, Iran
4. Research Center for Rational Use of Drugs, Tehran University of Medical Sciences, Iran
*Correspondence to m-davari@tums.ac.ir
Disclosure: The authors have declared no conflicts of interest.
Received: 25.04.25
Accepted: 15.10.25
Keywords: Diabetic dyslipidemia, Iran, lipid control, quality of health care, treatment gap, Type 2 diabetes (T2D).
Citation: EMJ Diabet. 2025;13[1]:98-107. https://doi.org/10.33590/emjdiabet/RIEL1138
Abstract
Background: People with Type 2 diabetes (T2D) often have dyslipidaemia (72–85%). This study aimed to investigate the quality of care for blood lipid control in patients with T2D in Iran.
Method: In a cross-sectional retrospective study, the authors studied the quality of care provided to patients with T2D in 15 diabetes centres in five provinces of Iran. The PROCAM calculator was used to determine the low-density lipoprotein cholesterol (LDL-C) target levels based on patients' cardiovascular risk scores. The suitability and rational prescribing of lipidlowering drugs, medication errors, and achievement of target LDL-C levels based on individual patient needs were also assessed.
Results: The medical records of 2,500 patients were examined. The most commonly prescribed drug was atorvastatin (91.1%). Monotherapy was the preferred treatment option (68%). On average, 74% of the study population did not achieve individualised LDL-C target levels. The prescription of lipid-lowering drugs was not appropriate for the patients' needs in the selected provinces. Inadequacy included no medication (approximately 23%) and insufficient medication therapy (approximately 17%) for patients with high LDL-C levels.
Conclusion: The study concludes that improved evidence-based prescription practices, enhanced performance of medical staff, continued medical education, and better medication management are needed to ensure improved lipid control in patients with T2D in Iran.
1. This study reveals a critical mismatch in care, while low-risk diabetes patients had an 86% low-density lipoprotein cholesterol control rate, those at very high risk for heart attacks and strokes had a control rate of only 8–10%.
2. The vast majority (74%) of patients failed to meet their lipid targets. Crucially, this was not solely due to poor adherence; 23% of uncontrolled patients received no lipid-lowering medication at all, and another 18% were prescribed insufficient doses, pointing directly to clinical inertia and suboptimal prescribing.
3. Treatment relied heavily on atorvastatin monotherapy (91%), with negligible use of guideline-recommended combination therapies like ezetimibe. Furthermore, the low-density lipoprotein-lowering effect of atorvastatin was suboptimal and not dose-proportional, and significant medication errors (e.g., irrational drug combinations) were identified.
Diabetes is a chronic disease characterised by the body’s inability to use or produce sufficient insulin effectively. It is a growing global problem, with a higher disease burden in developing countries.1-4 In patients with Type 2 diabetes (T2D), one common group of abnormalities observed is diabetic dyslipidaemia, affecting 72–85% of these patients.5,6
Individuals with T2D are at a higher risk of developing cardiovascular diseases, stroke, and peripheral vascular diseases.1,7,8 This risk increases as low-density lipoprotein cholesterol (LDL-C) levels rise.9 Therefore, LDL-C levels are a vital aspect of treatment strategies for dyslipidaemia in patients with T2D.5,10-12
Significant clinical trials have demonstrated that lower LDL-C values are associated with a reduced risk of future cardiovascular events, with no lower limit identified for LDL-C levels.13,14 Although clinical studies have shown the safety of extremely low LDL-C levels, further monitoring is necessary.15,16
Updated guidelines, such as those of the American Diabetes Association (ADA), recommend an LDL-C goal of less than 100 mg/dL, while for patients with diabetes with established atherosclerotic cardiovascular disease, the target is less than 55 mg/dL.17
Like many other developing countries, Iran has a high prevalence of T2D, with recent
studies estimating a significant increase of 45.5% in diabetes prevalence compared to 2016.18 Furthermore, the last national survey of risk factors for non-communicable diseases revealed that 15.14% of the Iranian population over 25 years old had diabetes in 2021.10
This study aimed to assess the quality of care in managing blood lipid levels among a representative group of patients with T2D in Iran. Specifically, the authors examined various aspects of prescribed medications, including patterns, rational use, and effectiveness, with a focus on achieving target LDL-C levels based on individual patient needs.
This cross-sectional study was conducted in 15 diabetes centres across five provinces in Iran. Data were extracted from patients’ records in public, semi-public (centres related to the social security insurance organisation), and private centres. The selection of the diabetes centres and patients followed a cluster sampling method. Patients with a confirmed diagnosis of T2D, who were taking antidiabetic medications, had a comprehensive medical history from 2013–2017, and visited a doctor at least four times per year, were included in the study. The authors assumed that the adherence of these patients was in an acceptable range, >80%.19
Demographic information, laboratory examination results, prescription patterns, and treatment outcomes were extracted from the patients’ profiles in documentary and registry records.
The authors also focused on prescription patterns (the extent and profile of drug use), medication errors, achieving individualised targeting levels, and the effectiveness of lipid-lowering drugs in the T2D population.
The PROCAM calculator was used to determine LDL-C target levels based on patients’ cardiovascular risk scores. The PROCAM calculator is a reliable tool for predicting the 10-year risk of cardiovascular events based on a patient’s history and laboratory results. Eight components are checked, each with a different weight in the final calculation. These components include age, LDL-C, smoking status, highdensity lipoprotein cholesterol, systolic blood pressure, family history of myocardial infarction, diabetes, and elevated triglyceride levels.15 The PROCAM score ranges from 0–87, with higher scores indicating a higher risk of cardiovascular disease.20
One important use of the PROCAM calculator is determining the target level of LDL-C in patients with diabetes. In this analysis, the authors used target LDL-C levels based on cardiovascular risk scores.
Rational use of medicines means prescribing medications based on the patient’s needs, in appropriate doses, for an adequate period, and at the lowest cost to the community.21 In this study, the suitability and rational prescribing of lipid-lowering drugs were assessed using the defined daily dose (DDD).
Medical errors are defined as preventable adverse events or mistakes that occur during the provision of healthcare services, resulting in harm to patients.22,23 These
errors can occur at any point in the healthcare delivery process, including diagnosis, treatment, medication administration, surgery, and follow-up care.24 The authors investigated medication errors, including prescription errors such as medical interactions, duplication therapy, and dosing of LDL-C-lowering drugs.
Statistical analysis was performed using the STATA software version 14 (Stata, College Station, Texas, USA). Continuous variables, such as lipid indices, were presented as mean and standard deviation. Categorical and binary outcomes, such as achieving the LDL-C targets and family history of myocardial infarction, were presented as proportions with a 95% CI. The statistical significance of LDL-C fluctuation during the period of use was examined using MANOVA analysis.
The study included 2,500 patients, of whom 521 were excluded due to incomplete records. Among the remaining 1,979 patients, the mean age was 62.3 years, with about 34% being older than 65 years. The demographic characteristics and prescribed lipid-lowering drugs are summarised in Table 1.
Table 1 also summarises the prescription pattern of lipid-lowering drugs in these patients. The most commonly prescribed drug was atorvastatin, received by 91.1% of the patients. Monotherapy was the preferred treatment option, with 68% of the patients receiving a single drug. Combination therapy was less common, with only 7.7% of the patients receiving two drugs and less than 1% receiving three drugs.
Table 2 presents key findings on the management of LDL-C across different
Table 1: Demographic characteristics and the frequency of prescribed lipid-lowering drugs in patients with Type 2 diabetes.
(66.2)
(33.8)
(69.88)
<18.5
(68.0)
Table 2: Average low-density
DDD: defined daily dose; LDL-C: low-density lipoprotein cholesterol; PROCAM: Prospective Cardiovascular Münster Study.
provinces. It reveals that, on average, patients had an LDL-C level (88.80 mg/dL) that was higher than their recommended target (≤69.12 mg/dL). Consequently, only 26.06% of patients achieved their goal. Crucially, among those with uncontrolled LDL-C, the majority (59.7%) were already taking a sufficient daily dose of medication, indicating a potential gap in treatment efficacy or adherence.
Table 3 provides a detailed analysis of LDL-C management among 1,979 Iranian patients with diabetes over 5 years. Patients are divided into five risk groups based on their PROCAM score, ranging from ‘low’ to ‘very high’. Each year, patients are categorised as ‘controlled’ (meeting their target) or ‘uncontrolled’ (missing their target). Each risk group has a specific, progressively lower LDL-C target, from <116 mg/dL for low risk to <55 mg/dL for very high risk. The final columns display the actual average LDL-C levels for patients in each risk group, broken down by five Iranian provinces.
The comparison illustrates that none of the regions have achieved the optimal
levels of cholesterol targets. The results showed that, on average, 74% of the study population did not achieve individualised LDL-C target levels.
Medication errors can significantly affect the timely and effective treatment of patients. In this study, medication errors were investigated, including prescription errors such as medication interactions and duplication therapy. Among the study population, 1.06% had concomitant use of two fibrates or two statins without scientific justification. The consequent use of atorvastatin and lovastatin was the third most widely used drug combination in patients with T2D. Among the total research population, 9.58% of the patients simultaneously received two drugs from the drug categories of statins and fibrates. A few cases of simultaneous use of three drugs from these categories were also observed.
In addition, on average, 22.79% of patients with uncontrolled LDL-C levels did not receive any lipid-lowering medication, and
Table 3: The average low-density lipoprotein cholesterol levels in patients with diabetes over 5 years, compared to the target low-density lipoprotein cholesterol levels based on Prospective Cardiovascular Münster Study scores in the selected provinces.
Cardiovascular Risk
17.33% received insufficient daily doses, indicating errors in the prescribing decision (Table 2).
These medication errors can be attributed to improper monitoring of medication therapy by physicians.
The effectiveness of different doses of atorvastatin was evaluated in patients who had not taken any medication for their lipid disorder. The changes in LDL-C levels were compared between those who took atorvastatin and those who did not take any lipid-lowering drugs. The results are shown in Table 4
The results indicate that atorvastatin shows a reduction in LDL-C compared to the control group. However, the effects were not directly related to the prescribed doses, and the level of blood-lipid reductions was lower than expected.25,26
The authors evaluated the appropriateness and rational prescription of lipid-lowering medications using the DDD. Table 2 illustrates the prescription of lipid-lowering drugs in patients with T2D who did not reach target LDL-C levels across various provinces.
The results indicate that prescribing lipidlowering drugs was not appropriate for the patient’s needs in the selected provinces. This inadequacy included no medication and insufficient medication therapy for patients with high LDL-C levels (Table 2).
Managing dyslipidaemia in patients with T2D is crucial for reducing the risk of cardiovascular events. This study aimed to evaluate the prescription pattern of lipid-lowering drugs, identify medication errors, and assess the achievement of individualised targeting levels. The results shed light on the current state of lipid control in patients who are diabetic in Iran, and highlight areas for improvement.
The treatment of diabetic dyslipidaemia involves both pharmacological and nonpharmacological interventions.5,12,27 It is important to note that lifestyle interventions and medication therapy can reduce the risk of cardiovascular events.28 However, this article aims to evaluate the quality of medical care for blood lipid control in patients with T2D in Iran.
Drug treatments for diabetic dyslipidaemia comprise statins, cholesterol absorption inhibitors, niacin, fibrates, bile acid sequestrants, PCSK9 inhibitors, and omega-3 fatty acids.27,29,30 Statins are commonly used to prevent primary and secondary cardiovascular events. They are effective in lowering LDL-C levels and increasing high-density lipoprotein cholesterol levels.
The Achievement of Individualised Targeting
LDL-C levels across all provinces declined during the 5-year study. However, results show that with an individualised approach, only 26.06% of monitored patients reached their LDL-C targets. This is similar to the DA VINCI study, which found that only 21% of very high-risk patients (including most with diabetes) achieved the guideline LDL-C target (<55 mg/dL).31 According to the ADA guidelines, which recommend an LDL-C goal of 100 mg/dL for patients with diabetes, over 70% of patients reached the lipid control target.17
The most striking finding is the high percentage of patients failing to meet their LDL-C targets across all risk categories.
In 2017, 70.6% of all patients had uncontrolled LDL-C levels. While this is a slight improvement from 73.9% in 2013, it remains unacceptably high. The situation is most critical in the highest-risk groups. For ‘very high’ risk patients, over 92% were uncontrolled in 2013, and this improved only marginally to about 90% in 2017. This ‘treatment gap’ is a global phenomenon. A review study highlighted that despite well-established guidelines, a significant majority of high- and very high-risk patients worldwide fail to achieve LDL-C goals.32
The authors’ data highlight a clear ‘riskparadox’, showing that patients requiring the most aggressive lipid-lowering treatments are the least likely to reach their targets. In 2017, the low-risk group achieved an 86% control rate, whereas the very high-risk group had only about a 10% control rate that year. Studies that analysed data from millions of patients also found this exact paradox.33,34 These studies suggest that the more aggressive the target (e.g., <55 mg/ dL), the more challenging it is to achieve.
The data in Table 3 show that while there was a slight increase in control rates from 2013 to 2017 (overall from 26.1% to 29.4%), the progress is minimal, especially among the highest risk groups. This indicates that the management strategies used during this period were not aggressive enough.
The average LDL-C levels vary across the five provinces. For example, Mazandaran consistently shows higher average LDL-C levels across most risk categories compared to other provinces like Tehran or Kurdistan. Regional variations in cardiovascular risk factors are well-documented and are often linked to differences in diet, lifestyle, socioeconomic status, and access to healthcare services.35
This clearly demonstrates the superiority of the individualised approach in managing dyslipidaemia in people with diabetes.
The difference between provinces is strongly correlated with their population distribution in each risk group (Table 3). That means provinces with the worst cholesterol control have the highest population percentages in very high-risk groups.
In most guidelines, if the target LDL-C level is not reached with the maximum tolerable dose of statins, the addition of another drug class, such as ezetimibe or PCSK9 inhibitors, is recommended.1 However, ezetimibe was rare in the study population, and PCSK9 inhibitors were not used. This suggests that physicians have not prescribed lipid-lowering drugs based
Table 4: Efficacy of different doses of atorvastatin in reducing low-density lipoprotein cholesterol with and without a control group.
Atorvastatin doses
Average LDL-C reduction compared to the control group over 2 years (mg/dL)
Average LDL-C reduction over 2 years (mg/dL) Average LDL-C reduction compared to the control group over 5 years (mg/dL)
*The negative numbers indicate an increase in LDL-C.
LDL-C: low-density lipoprotein cholesterol.
on LDL-C levels and guidelines for patients with diabetes (Table 2). It also indicates the use of nearly identical prescription patterns for all patients, regardless of their individual needs.
Approximately 23% of patients with uncontrolled LDL-C levels received no medical treatment to lower their blood lipids. Additionally, 17.6% of the patients who did receive medication were not prescribed adequate doses (Table 2). These highlight the inadequate and low-quality care for patients with diabetes in selected provinces of the country. Therefore, it is crucial to take adequate measures to improve the performance of the medical staff, such as providing continuing medical education and developing evidencebased guidelines to enhance the quality of diabetes management.
The effectiveness of lipid-lowering drugs was assessed by comparing the LDL-C reduction in patients taking atorvastatin for 2 years with a control group. The results of the authors’ retrospective cohort showed that the mean effectiveness of atorvastatin tablets (10, 20, and 40 mg) was a reduction of 11.56%, 10.13%, and 7.76% in LDL-C levels, respectively (Table 4). However, the effects were not directly related to the prescribed doses, and the level of blood-
over the 5 years (mg/dL)
lipid reductions was lower than expected.25,26
Moreover, the findings show that about 60% of patients with diabetes in the provinces who did not meet the target LDL-C levels had been prescribed a proper DDD of atorvastatin. This indicates that patients either received fewer effective medications, needed higher doses, had to change their lifestyles, or experienced a combination of these factors. More research is necessary to verify each of these possibilities.
This study sheds light on the current state of lipid control in patients with T2D in Iran. It provides a stark, data-driven snapshot of the challenges in managing cholesterol in patients with diabetes.
Most people, especially those at the highest risk for heart attacks and strokes, are not getting enough treatment to meet evidence-based targets.
The prescribing pattern of lipid-lowering drugs was suboptimal, with a high rate of medication errors. The rate of achieving individualised LDL-C target levels was low, and the effectiveness of the prescribed medications fell short of expectations.
These findings emphasise the need for improved evidence-based prescription practices, enhanced medical staff performance, continued medical education, and better medication management to ensure improved lipid control in patients with T2D.
This study has some limitations. Initially, the authors did not directly measure
References
1. Roglic G. WHO global report on diabetes: a summary. Int J of Noncommun Dis. 2016;1(1):3-8.
2. Ilic I, Ilic M. The burden of type 2 diabetes mellitus in Latin America, 1990-2019: findings from the global burden of disease study. Public Health. 2024;233:74-82.
3. Misra A et al. Diabetes in developing countries. J Diabetes. 2019;11(7):52239.
4. Galaviz KI et al. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care. 2018;41(7):1526-34.
5. Jialal I, Singh G. Management of diabetic dyslipidemia: an update. World J Diabetes. 2019;10(5):280-90.
6. Taskinen MR. Diabetic dyslipidemia. Atheroscler Suppl. 2002;3(1):47-51.
7. Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57.
8. de Mattos Matheus AS et al. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013:653789.
9. Ference BA et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-72.
10. Azadnajafabad S et al. Noncommunicable diseases' risk factors in Iran; a review of the present status and action plans. J Diabetes Metab Disord. 2021;23(2):1-9.
11. Devaraj S, Jialal I. Optimum lipid testing for diabetic patients to enhance
the patient’s adherence or compliance. However, since they selected patients with 5 years of complete medical records and at least four doctor visits per year, the authors estimated their adherence was over 70%. The authors also did not evaluate the patients’ kidney health or the toxicity of the drugs used in this study, which could influence the medication’s effectiveness.
clinical care. Diabetes Metab Syndr. 2021;15(1):461-4.
12. Sugiyama K, Saisho Y. Management of dyslipidemia in type 2 diabetes: recent advances in nonstatin treatment. Diseases. 2018;6(2):44.
13. Liu J et al. Impact of diabetes, high triglycerides and low HDL cholesterol on risk for ischemic cardiovascular disease varies by LDL cholesterol level: a 15-year follow-up of the Chinese multi-provincial cohort study. Diabetes Res Clin Pract. 2012;96(2)217-24.
14. Navarese EP et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 2018;319(15):1566-79.
15. Assmann G et al. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105(3):310-5.
16. Kim MK et al. Diabetes duration, cholesterol levels, and risk of cardiovascular diseases in individuals with Type 2 diabetes. J Clin Endocrinol Metab. 2024;109(12):e2317-23.
17. American Diabetes Association (ADA). Standards of care in diabetes—2023 abridged for primary care providers. Clin Diabetes. 2022;41(1):4-31. Erratum in: Clin Diabetes. 2023;41(1):4-31.
18. Moradpour F et al. Prevalence of prediabetes, diabetes, diabetes awareness, treatment, and its socioeconomic inequality in west of Iran. Scientific Reports. 2022;12(1):17892.
19. Jenkins BN et al. Too much of a good thing? overexertion of self-control and dietary adherence in individuals with Type 2 diabetes. Br J Health Psychol. 2016;21(3):648-59.
20. Batsis JA, Lopez-Jimenez F. Cardiovascular risk assessment-from individual risk prediction to estimation of global risk and change in risk in the population. BMC Med. 2010;8:29.
21. World Health Organization (WHO). Promoting rational use of medicines. Available at: https://www.who.int/ activities/promoting-rational-use-ofmedicines. Last accessed: 16 January 2018.
22. World Health Organization (WHO). Technical Series on safer primary care: medication errors. Available at: https://www.who.int/publications/i/ item/9789241511643. Last accessed: 16 January 2018.
23. World Health Organization (WHO). Medication safety in transitions of care: technical report. Available at: https:// www.who.int/publications/i/item/WHOUHC-SDS-2019.9. Last accessed: 11 May 2020
24. Ferner RE, Aronson JK. Clarification of terminology in medication errors: definitions and classification. Drug safety. 2006;29:1011-22.
25. Neil HAW et al. Analysis of efficacy and safety in patients aged 65-75 years at randomization: Collaborative Atorvastatin Diabetes Study (CARDS). Diabetes Care. 2006;29(11):2378-84.
26. Collins R et al.; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterollowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005-16.
27. Wing RR et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with Type 2 diabetes. Diabetes Care. 2011;34(7):1481-6.
28. Cochrane SK et al. Association of accelerometry‐measured physical activity and cardiovascular events in mobility‐limited older adults:
the life (lifestyle interventions and independence for elders) study. J Am Heart Assoc. 2017;6(12):e007215.
29. Arrigoni E et al. Pharmacogenetic foundations of therapeutic efficacy and adverse events of statins. Int J Mol Sci. 2017;18(1):104.
30. Farhadnejad H et al. Herbal products as complementary or alternative medicine for the management of hyperglycemia and dyslipidemia in patients with type 2 diabetes: current evidence based on findings of interventional studies. J Nutr Metab. 2024;2024:8300428.
31. Ray KK et al. EU-wide cross-sectional observational study of lipid-modifying
therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. 2021;28(11):1279-89.
32. Ray KK et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: the multinational observational SANTORINI study. Lancet Reg Health Eur. 2023;29:100624.
33. Kristensen MS et al. Lipid-lowering therapy and low-density lipoprotein cholesterol goal attainment after acute coronary syndrome: a Danish population-based cohort study. BMC Cardiovasc Disord. 2020;20(1):336.
34. Wang X et al. Lipid-lowering therapy and low-density lipoprotein cholesterol (LDL-C) goal achievement in highcardiovascular-risk patients in Fuzhou, China. J Cardiovasc Pharmacol Ther. 2020;25(4):307-15.
35. Gupta R et al. Geographic epidemiology of cardiometabolic risk factors in middle class urban residents in India: cross-sectional study. J Glob Health. 2015;5(1):010411.
If you like it,why not share it...

Share with a colleague today