Pincer-MetalComplexesApplicationsinCatalytic DehydrogenationChemistryAkshaiKumar
https://ebookmass.com/product/pincer-metal-complexesapplications-in-catalytic-dehydrogenation-chemistry-akshaikumar/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Pincer Compounds: Chemistry and Applications 1st Edition
David Morales-Morales
https://ebookmass.com/product/pincer-compounds-chemistry-andapplications-1st-edition-david-morales-morales/
ebookmass.com
Biopolymer-Based Metal Nanoparticle Chemistry for Sustainable Applications Mahmoud Nasrollahzadeh
https://ebookmass.com/product/biopolymer-based-metal-nanoparticlechemistry-for-sustainable-applications-mahmoud-nasrollahzadeh/
ebookmass.com
Catalytic Kinetics. Chemistry and Engineering 2nd Edition
Dmitry Yu Murzin
https://ebookmass.com/product/catalytic-kinetics-chemistry-andengineering-2nd-edition-dmitry-yu-murzin/
ebookmass.com
Evidence■Based Neurological Disorders-Symptoms, Causes, and Therapy 1st Edition Upadhyay
https://ebookmass.com/product/evidence%e2%80%90based-neurologicaldisorders-symptoms-causes-and-therapy-1st-edition-upadhyay/
ebookmass.com
Re-Imagining Democracy in the Mediterranean, 1780-1860
Joanna Innes
https://ebookmass.com/product/re-imagining-democracy-in-themediterranean-1780-1860-joanna-innes/
ebookmass.com
Foundations of Materials Science and Engineering, 7th Edition William Fortune Smith
https://ebookmass.com/product/foundations-of-materials-science-andengineering-7th-edition-william-fortune-smith/
ebookmass.com
Before We Are Born E Book: Essentials of Embryology and Birth Defects With STUDENT CONSULT Online Access 9th Edition, (Ebook PDF)
https://ebookmass.com/product/before-we-are-born-e-book-essentials-ofembryology-and-birth-defects-with-student-consult-online-access-9thedition-ebook-pdf/ ebookmass.com
Digital SAT Math Practice Questions Vibrant Publishers
https://ebookmass.com/product/digital-sat-math-practice-questionsvibrant-publishers/
ebookmass.com
The Artful Pie Project: A Sweet and Savoury Book of Recipes
Denise Marchessault
https://ebookmass.com/product/the-artful-pie-project-a-sweet-andsavoury-book-of-recipes-denise-marchessault/
ebookmass.com
Exam Guide (Exam 1Z0-1072) Roopesh Ramklass
https://ebookmass.com/product/oracle-cloud-infrastructure-architectassociate-all-in-one-exam-guide-exam-1z0-1072-roopesh-ramklass/
ebookmass.com
Pincer Metal Complexes Pincer Metal Complexes ApplicationsinCatalytic DehydrogenationChemistry Editedby AKSHAIKUMAR
DepartmentofChemistry,CentreforNanotechnology, SchoolofHealthScienceandTechnology,Indian InstituteofTechnology,Guwahati,Guwahati,India
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorageand retrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseek permission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyright LicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightby thePublisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professionalpractices, ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribed herein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafety andthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,or editors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatter ofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress ISBN:978-0-12-822091-7
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: EmilyM.McCloskey
EditorialProjectManager: LenaSparks
ProductionProjectManager: PaulPrasadChandramohan
CoverDesigner: VictoriaPearson
TypesetbyMPSLimited,Chennai,India
1.Applicationofpincermetalcomplexesincatalytictransformations1
AisaMohanty,RajuSharmaandProsenjitDaw
1.1 Introduction1
1.2 Dehydrogenationofammoniaboraneanditsderivatives3
1.3 Dinitrogenactivationusingpincerligands13
1.3.1 CatalyticdinitrogenactivationusingMopincercatalyst14
1.3.2 Catalyticdinitrogenactivationusing3dmetalpincercatalyst19
1.3.3 Catalyticdinitrogenfixationusingearliertransitionmetals(V,Ti,Zr)21
1.3.4 N Xbondformationwithmetalnitrideinpincercomplexes23
1.3.5 Catalyticsilylationofdinitrogenusingtransitionmetalpincer complexes26
1.4 Pincercomplexesashydrogenationcatalyst27
1.4.1 Esterhydrogenation27
1.4.2 Amidehydrogenation28
1.4.3 Hydrogenationofurea,carbamate,carbonate,and imidesderivatives31
1.4.4 Nitrilehydrogenation32
1.4.5 Hydrogenationofalkynes33
1.5 Couplingreaction:C Cbondformationreaction34
1.5.1 Mizoroki-Heckreaction34
1.5.2 Suzuki Miyaurareaction35
1.5.3 Sonogashira,Negishi,Kumada-Corriu,Stillecross-coupling36
1.6 Redox-ActivePincerComplexes38
1.7 Conclusion52 References53
2.Pincer-group(8)andpincer-group(9)metalcomplexesfor catalyticalkanedehydrogenationreactions69
PranGobindaNandi,VinayArora,EileenYasminandAkshaiKumar
2.1 Introduction69
2.1.1 Alkanedehydrogenation70
2.2 Dehydrogenationreactionsofalkaneusingpincer Ircomplexes72
2.2.1 Initialreportsbasedonpincer Ircatalysts72
2.2.2 AlkanedehydrogenationbyPC(sp2)P-Irsystems75
2.2.3 AlkanedehydrogenationbyPYC(sp2)ZP-Ir(Y 5 O,S,CH2)systems79
2.2.4 Mechanismofpincer Ir-catalyzedalkanedehydrogenation80
2.2.5 Solid/gas-phasealkanedehydrogenation83
2.2.6 Continuous-flowgas-phasealkanedehydrogenation84
2.2.7 AlkanedehydrogenationbyPC(sp3)P Ircomplexes86
2.2.8 AlkanedehydrogenationbyPOCN Ir,PBP Ir,PNP Ir,andPAlP complexes88
2.2.9 AlkanedehydrogenationbyPXC(sp2)NP-Ir-HCl (X 5 O,S)complexes89
2.2.10 Alkanedehydrogenationbynon-phosphine-basediridiumpincer complexes90
2.3 Dehydrogenationofalkanesbypincer metalcomplexesother thaniridium94
2.3.1 Rutheniumpincercomplexesforalkanedehydrogenation94
2.3.2 Osmiumpincercomplexesforalkanedehydrogenation97
2.3.3 Rhodiumpincercomplexesforalkanedehydrogenation98
2.4 Applicationsofalkanedehydrogenation100
2.4.1 Alkanemetathesis100
2.4.2 Alkanecoupling102
2.4.3 Synthesisofaromatics104
2.4.4 Functionalizationofalkanes108
2.5 Summaryandoutlook112 References113
3.Transitionmetal-catalyzeddehydrogenationofmethanol andrelatedtransformations123 SujanShee,BhaskarPaulandSabujKundu
3.1 Introduction123
3.2 Hydrogenproduction125
3.3 N-Methylationreactions127
3.3.1 N-Methylationofamines127
3.3.2 N-Methylationofnitro,nitrile,andazidecompounds130
3.3.3 N-Methylationofamidesandoximes132
3.4 N-Formylationreactions134
3.5 C-Methylationreactions134
3.5.1 α-Methylationofketones134
3.5.2 C-Methylationofalcohols139
3.5.3 C3-Methylationofindoles143
3.5.4 α-Methylationofarylacetonitriles144
3.5.5 Aminomethylations146
3.6 N-Heterocyclessynthesis147
3.7 Miscellaneoustransformations150
3.8 Conclusion154 References154
4.Transitionmetalpincercomplexesinacceptorless dehydrogenationreactions163
VinitaYadav,GanesanSivakumarandEkambaramBalaraman
4.1 Introduction163
4.2 Acceptorlessdehydrogenation164
4.2.1 Acceptorlessalcoholdehydrogenation164
4.2.2 Dehydrogenationofalcoholstocarboxylicacids172
4.2.3 Dehydrogenationofamines179
4.2.4 DehydrogenationofN-heterocycles182
4.3 Conclusionandfutureperspective184 Acknowledgments185 References185
5.Anoutlookontheapplicationsofpincer-metalcomplexes incatalyticdehydrogenationchemistry191
EileenYasmin,VinayAroraandAkshaiKumar References202
Listofcontributors VinayArora
DepartmentofChemistry,IndianInstituteofTechnologyGuwahati,Guwahati,India
EkambaramBalaraman
DepartmentofChemistry,IndianInstituteofScienceEducationandResearch(IISER), Tirupati,India
ProsenjitDaw
DepartmentofChemicalSciences,IndianInstituteofScienceEducationandResearch Berhampur,Berhampur,India
AkshaiKumar
DepartmentofChemistry,IndianInstituteofTechnologyGuwahati,Guwahati,India; CenterforNanotechnology,IndianInstituteofTechnologyGuwahati,Guwahati,India; SchoolofHealthScience&Technology,IndianInstituteofTechnologyGuwahati, Guwahati,India
SabujKundu
DepartmentofChemistry,IndianInstituteofTechnologyKanpur,Kanpur,India
AisaMohanty
DepartmentofChemicalSciences,IndianInstituteofScienceEducationandResearch Berhampur,Berhampur,India
PranGobindaNandi
DepartmentofChemistry,IndianInstituteofTechnologyGuwahati,Guwahati,India
BhaskarPaul
DepartmentofChemistry,IndianInstituteofTechnologyKanpur,Kanpur,India; UniversityofCaliforniaatRiverside,Riverside,CA,UnitedStates
RajuSharma
DepartmentofChemicalSciences,IndianInstituteofScienceEducationandResearch Berhampur,Berhampur,India
SujanShee
DepartmentofChemistry,IndianInstituteofTechnologyKanpur,Kanpur,India
GanesanSivakumar
DepartmentofChemistry,IndianInstituteofScienceEducationandResearch(IISER), Tirupati,India
VinitaYadav
OrganicChemistryDivision,CSIR NationalChemicalLaboratory(CSIR NCL), Pune,India;AcademyofScientificandInnovativeResearch(AcSIR),Ghaziabad,India
EileenYasmin
DepartmentofChemistry,IndianInstituteofTechnologyGuwahati,Guwahati,India
Foreword Atypicalpincerligandconsistsofthreedonoratomsstabilizingaplanar, meridional,oroccasionallyfacialmetalscaffold,withthecentraldonor moietybeingneutraloranionic,resultingintwofive-memberedchelate rings.AlthoughPCPtypeofpincerligandswithcentralanioniccarbondonoratomflankedbysoftphosphorusdonorswasfirstestablishedby Shawinthe1970s,ittookcenterstagemuchlaterandwassubsequently establishedasanovelligandsystemtopromoteseveralchallengingcatalyticreactionsandshoweditssuperiorityinmetal-mediatedorganicsynthesis.Asaresult,pincerframeworkwitnessedtremendousgrowthinthe lasttwodecadeswithavarietyofcombinationsofdonoratoms,preferably havinganaromaticorpyridylbridgingunitsandbecameanintegralpart ofinorganicandorganometallicchemistryandhomogeneouscatalysis. Relativelyeasysyntheticmethodologies,six-electrondonorcapability, availabilityofnumerousP,N,O,andSdonormoietiesforincorporation, optionsfortuningstericandelectronicproperties,androbustnaturemade pincerligandsoneofthemostinvinciblesystemsinthefieldofapplied science.
Presentlyresearchersareusingavarietyofpincerplatformstoperform challengingandunusualorganictransformationsundermildconditions, forexample,ammoniaproductionfromdinitrogen,conversionofCO2 intousefulorganicmolecules,andalkanedehydrogenation.Metalmediateddehydrogenationisagreenandenvironmentallybenignsystem valuableinseveralsyntheticprocessesandpossiblyforhydrogengenerationandstorageforfutureenergyrequirements.
Inthiscontext,AkshaiKumar’seffortstobringaconsolidatedmonographonvariousdehydrogenationprocessesutilizingpincerligandsare commendable.Iamsurethatthisbookwillreceiveaverygoodresponse fromsyntheticchemistsandallreaders.
M.S.Balakrishna
PhosphorusLaboratory,DepartmentofChemistry,IITBombay,Mumbai,India
Preface Eversincethediscoveryofthefirstorganometalliccomplex,ligandshave beensystematicallymodifiedtoattainbettercontrolonthestabilityand reactivityoforganometallicsystems.Thishasledtointerestingfindings thatmetalcomplexescontainingmonodentateligandsarelessstablethan bidentate,whichinturnarelessstablethantridentatesystemsandsoon. Nosurprisingly,theenhancedstabilitycomesasatrade-offtothereduced reactivity.Ithasbeenwidelyacceptedthattridentatesystems(thosebindingthemetalcenterinameridionalfashion,inparticular)strikeanoptimalbalancebetweenthestabilityandreactivity.These “Pincer” systems offernumerousavenuestotailorthereactivityviathestericandtheelectroniccontrol.Thetypicallyrigidframeworkprovidesrobustnesstothe complextherebyenhancingitsthermalstability.Ontheotherhand,the catalyticversatilityisprovidedbytheeaseofligandtailoringtosuitone’ s stericandelectronicrequirements.
Severalmodificationsofthepincermotifhavebeenreportedowingto theeasilytailorableligandbackboneleadingtofive-orsix-membered chelateringsthathaveacommonmetal-heteroatombond.Pincercomplexeswithdesiredcatalyticpropertieshavebeenobtainedbyvarying eithertheligatinggroupsorbychangingthelinkeratomsapartfromthe possibilityofhavingthepincerplatformitselfasanoninnocentligand. Furthermore,thearylbackboneoffersthepossibilityofintroducingseveralpolarfunctionalities,whichnotonlyhelpsincontrollingtheelectronicsremotelybutalsofacilitatestheheterogenizationofthemolecular catalystsonmanysolidsupports.Thechemistryofpincercomplexesis veryvastandowesalottotheoutstandingcontributionstopioneerssuch asShaw,van-Koten,Kaska,Jensen,Goldman,Milstein,andBrookhart amongseveralothers.Rightly,thisinterestingchemistryhasbeen reviewedseveraltimesinvariouscontextsandforscatteredapplications. Thisbookdiscussesthechemistryofpincer metalcomplexesinthecontextofasingleapplicationofdehydrogenationofvarioushydrocarbon derivativescatalyzedbypincer metalcomplexes.
Hitherto,thedehydrogenationofhydrocarbonsandtheirderivatives havebeendiscussedseparately.Thisstemsfromthefactthattheoperating mechanismsandcatalystcompatibilityforpurehydrocarbonsaredistinctly differentfromtheirfunctionalizedanalogs.However,inrecentyears,
therehasbeenasurgeinthelookoutforhydrocarbondehydrogenation catalyticsystemsthatarecompatiblewithpolarsubstituents.Thiswould facilitateformulationoftandemprocessesthatarenotonlylimitedto hydrocarbontransformationbutalsotohydrocarbonfunctionalizationina singlepot.Inthiscontext,thisbookprovidesagoodunderstandingon theoperatingmechanismsanddehydrogenationcatalystcompatibilityin both(functionalizedandunfunctionalized)hydrocarbonsystems.This unifiedapproachwouldnotonlyshedlightonthedistinctdifferencesof thesecatalyticsystemsbutalsowouldrevealthesubtlesimilaritiesinthe reactivity.
Accordingly,itisagreatpleasuretostartthebookwithChapter1on ApplicationofPincer MetalComplexesinCatalyticTransformationsby Daw.Chapter2,Pincer-Group(8)andPincer-Group(9)MetalComplexes forCatalyticAlkaneDehydrogenationReactions,reviewsthechemistry ofgroup-8andgroup-9pincer metalcomplexesinalkanedehydrogenation.InChapter3,TransitionMetalCatalyzedDehydrogenationof MethanolandRelatedTransformations,Kunduprovidesanoverviewon thepincer metalcatalyzeddehydrogenationofmethanolandrelated reactions.ThisisfollowedbyChapter4,TransitionMetalPincer ComplexesinAcceptorlessDehydrogenationReactions,fromBalaraman whocommentontheapplicabilityofpincer metalcomplexesinacceptorlessdehydrogenationoffunctionalizedhydrocarbons.Chapter5,An OutlookontheApplicationsofPincer MetalComplexesinCatalytic DehydrogenationChemistry,summarizesthecurrentstate-of-artinthe pincer metalcatalyzeddehydrogenationchemistryofhydrocarbonsand theirderivatives.Itishopedthattheknowledgechurnedoutfromthis wholeexercisewouldbebeneficialandenjoyablefortheauthorsandthe audiencealike.Thisconceptoffersimmensepotentialofleadingtoexcitingfutureinnovationsinthedehydrogenationchemistry.Itisenvisaged thattheaudiencewhoreadthisbookwoulddeveloptheirownperceptiononthisfascinatingchemistry,whichcouldresultindiversenew researchendeavorsandexcitingpossibilities.
Myheartfeltappreciationtotheauthorsfortheirexcellentcontributionofscience.Ihavebeenfortunatetohavethesupportfromavery able,professional,andcooperativeeditorialteamcomprisingEmilyM. McCloskeyandLenaSparks.TheperiodicdiscussionswithLenahave beenofimmensehelpinproducingthisvolumeinatimelymanner.
Applicationofpincermetal complexesincatalytic transformations AisaMohanty,RajuSharmaandProsenjitDaw
DepartmentofChemicalSciences,IndianInstituteofScienceEducationandResearchBerhampur, Berhampur,India
Correspondingauthor.e-mailaddress: pdaw@iiserbpr.ac.in
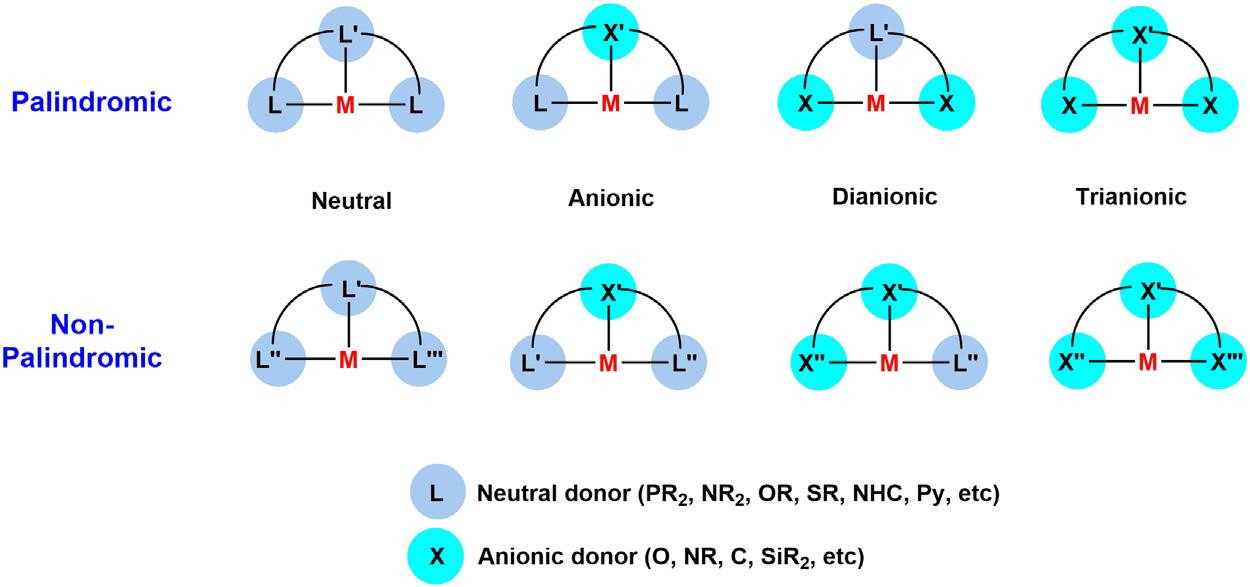
1.1Introduction Ligand’sversatilityplaysasignificantroleinthesensitivedesignofmetal complexestodisplayinterestingreactivitytowardthefieldofcatalysis. Awidevarietyofligandsaredevelopedthattunethemetalcenterreactivitybasedonstericandelectronicproperties.Pincerligandshavegained specialinterestsincatalysisduetotheirspecificproperties [1].Theterm “Pincer” wasfirstcoinedbyG.VanKotenin1989,anditwasnotedthat therigidityofsuchligandscantunethestabilityofthemetalcomplexes [2].Thesynthesisofpincercomplexeshasbeenstartedintheearly1970s byMoultonandShaw [3],andinpresentdays,variousnewarchitectural designscontinuetocomeout.Themoderndefinitionofpincercomplexesisassociatedwithrigidbindingwiththethreeadjacentcoplanar sitesofmetalinameridionalconfiguration [4].Ingeneral,acentralaromaticbackbonewithtwoLewisbasicdonorgroupsattachedinthesidearmbyspacersgroupisthemorefrequentlyobservedsystemthatmakesa tridentateligand.Althoughvariouskindsofpincersystemsarealsoknown withouthavingthearomaticbackbone.Pincerligandsofferavaluable scopetowardstericandelectronicpropertiesofthecomplexes,therefore providinghighstabilityduetoitschelatingnatureandcanincreaseitspotential valuetowardcatalysis.CrabtreeandPerisdescribedtheclassificationofthe pincerligandsbasedontheirsymmetry(palindromicornonpalindromic)and neutralorionicnatureofitsbindingmotifs,whichisdepictedin Fig.1.1[5]
Duetothethermalstability,thepincercomplexesareusedascatalysts invariouskindsoforganictransformations [6].Thespecificarchitectural designandtheirdifferentpropertieslikenon-innocentnature,outer
sphereeffectsonligandframeworks [7],metal ligandcooperation [8], hemilabilityofpendantarms [9],andredox-activebehavior,whichfacilitatessingleelectrontransfer [10] ofsuchcomplexes,serveasanunique platformforintroducingtheirroleinhomogeneouscatalysis.Alongwith pincersystemsareenabletoactivatethesmallorganicmoleculesuchas CO2,H2,N2,water,acids,dihalides,oxidizing,andreducingagentsat elevatedtemperaturewithouthamperingthepincercoordinationgeometry [11].Exceptforcatalysis,somespeciesarealsousedformultiplepurposesrangingfromapplicationinnanosciencetothedevelopmentof chemicalsensorsandchemicalswitches [12].Moreover,pincercomplexes havebeenusedtoinvestigateC C,C H,andC Obondactivation processes [13] orserveasbuildingblocksforthesynthesisofselfassembledsupramolecularstructures [14].Themajorapplicationofsuch kindofpincercomplexesisinthefieldofhomogeneouscatalysisreaction involvinghydrogenationanddehydrogenationreactionoforganicmolecules.Avastliteraturepresentsonthedehydrogenationoforganicsubstrates [15],transferhydrogenation [16],acceptorlessdehydrogenation [17],dehydrogenativecouplingreaction [18],borrowinghydrogenation reaction [19],andalkanedehydrogenationreactionusingsuchpincer complexes [20]
Inthisbook,otherchaptersarededicatedtothetopicsofolefindehydrogenation,alcoholdehydrogenativecouplingreaction,andmethanol dehydrogenationreaction.Inthischapter,wepresentgeneralapplications ofthepincercomplexesinthehomogeneouscatalysisreactionsuchas ammoniaboranedehydrogenationanditsapplicationinthehydrogen storage,dinitrogenactivationanditsapplicationtotheammoniasynthesis,
Figure1.1 Commontypesofpincerligandsandtheirclassification.
couplingreactions,hydrogenationofchallengingorganicmoleculeslike urea,carbonate,carbamate,amide,ester,andtheredox-activepincer ligandanditsapplicationintheorganictransformation.
1.2Dehydrogenationofammoniaboraneandits derivatives Insearchofalternativestofossilfuels,hydrogenisprobablythemost promisingastheenergysourceforthefuture,whichdoesnotproduce anycommonpollutantofgreenhousegases [21 24].Duetoitslowvolumetricenergydensity,thestorageofhydrogenisthemajorchallengein thehydrogeneconomy.Hydrogencanbestoredintwoforms:physical andchemical.Apartfromthephysicalformofstorage(likecompressed gastoliquidhydrogenandadsorbentmaterials,havingsomemajorlimitation),thechemicalformhasemergedasagoodalternative,consistingof thereleasingofmolecularhydrogenfromorganiccompoundswithhigh hydrogendensity,suchasHCOOH,CH3OH,organicheterocycles [25 27]
Ammoniaboraneisoftenconsideredasapromisingchemicalhydrogenstoragematerialduetoitshighhydrogencontentaslowmolecular weightammoniaboranescanofferupto19.6wt%storagecapacitytheoretically.Aproticandhydridichydrogenatomsadjacenttoeachother presentintheammoniaboranesthatareLewisacid-baseadductsand makethereleaseofhydrogengasfavorable.Ammoniaboranescandehydrogenatethermallyintheabsenceofacatalyst,althoughtheuseofacatalystaffordsthedehydrogenationprocessundermildconditions.The hydrogenstoragecapacitydependsonthefinaldehydrogenatedproduct distributionandthecatalystplaysavitalrolehere.Poly(aminoborane) [H2BNH2]n,borazine[HBNH]3,orpolyborazylene(hydrogenstorage capacity6.8 12.96wt%)canbeformedbythedehydropolymerization processofammoniaboraneH3BUNH3 byeliminatingmorethan1equivalentofhydrogengas.Whereasthesubstitutedamine-boraneslike H3B NMeH2,H3B NMe2H,andMeH2B NH2Rexhibitarelatively lowerhydrogenstoragecapacityafterthedehydrocouplingtoformpoly (aminoborane)derivative.Thevastamountofresearchattemptstomaximizethehydrogenyieldtoimplementthesecompoundsashydrogen storagematerialalongwithdetailedmechanisticstudieswereperformed tounderstandthetransitionmetalcomplexesinteractionwithsuchsubstratescontainingproticandhydridichydrogens [28 37].Severalpincer
complexeshavealsobeenusedforthedehydrogenationofammonia boranederivativesasdescribehere.
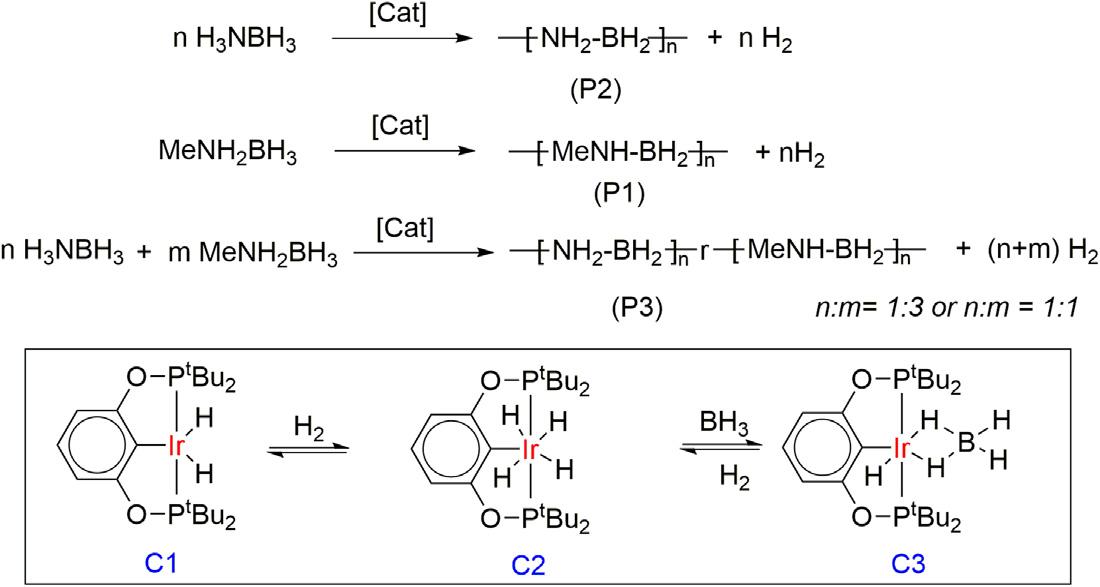
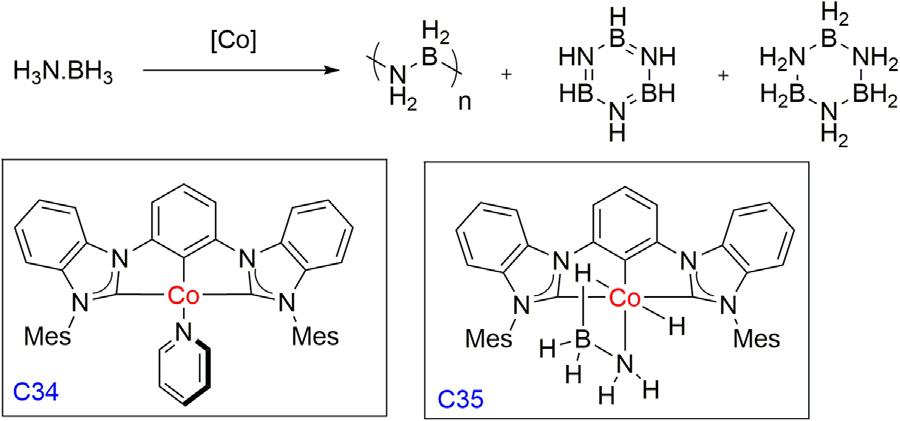
In2006,theGoldberggroupfirstexploredthe(POCOP)Ir(H)2 (C1) pincercomplexasacatalystfortheammoniaboranedehydrogenation [38].Treatmentof0.5Mammoniaboranesolutionwitha0.5mol%catalystaffordedquantitativeconversionand1equivalentofhydrogengas wasreleased.Duringthereaction,awhitesolidwasdepositedthatafter analyzedbyNMR,IR,andX-raypowderdiffractionidentifiedasthe cyclicpentamer,[H2NBH2]5.Todescribethemechanisticinsight,they reportedthatatetrahydridecomplex(POCOP)Ir(H)4 (C2)wasobserved initiallyfromcomplexC1duringthedehydrogenationofH3NBH3,and afterlongreactiontimes,anewBH3 adductiridiumdihydridecomplex (C3)wasformed.
Later,theMannergroupreportedthesameIrpincerC1catalystfor thedehydropolymerizationoftheN-methylamine-borane,which affordedapoly(N-methylaminoborane)withweightaveragemolecular weightof160,000(P1)asanoff-whitesolidinhighyield(60%)withthe vigorousbubblingofhydrogengeneration(Fig.1.2) [39].Parallelly,the samecatalystwasalsousedforthecatalyticdehydrocouplingreactionof ammoniaboraneandaffordedapolymericwhitesolidproduct(P2)and correspondingcopolymer(P3)wassynthesizedbyamixtureofNmethylamine-boraneandammoniaborane.Thelinearpolymericstructure wasreportedbydetailedstructuralanalysisofpolymersP1,usingvarious massspectrometrictechniques,suchashigh-resolutionESIandnanospray MS,solutionandsolid-stateNMR,andIRspectroscopymethods.
Dehydropolymerizationofamine-boranederivativesusinglr(POCOP)pincercomplex.
Figure1.2
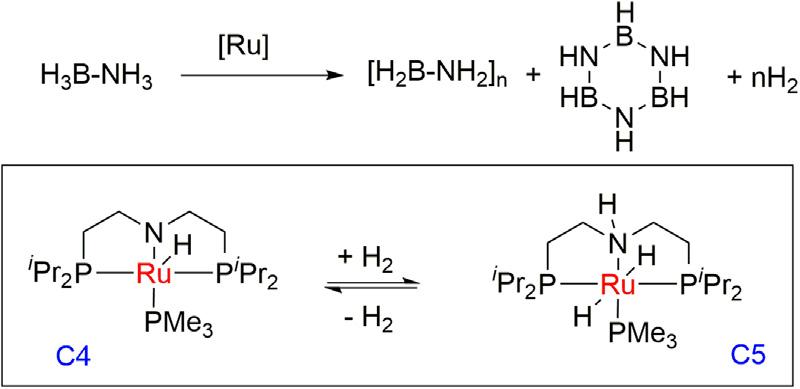
SchneidergroupreportedRu amidocomplex[Ru(PNP)(H)(PMe3)] (PNP 5 N(CH2CH2PiPr2)2)C4(0.01mol%)ascatalystinthedehydrogenationofammoniaboranewith0.83equivalentofH2 formation(TON 5 8300)andapseudo-first-orderreactionwith21s 1 TOFvalue (Fig.1.3) [40] whereascatalystloadingsof0.1mol%(C4)produceslightly morethanoneequivalentofH2.AMAS-11BNMRandIRspectrapredictedtheformationofapolymericdehydrocouplingproduct (BH2NH2)n alongwithsmallamountsofborazine.AconcertedmechanismoftheNHandBHbondcleavagesispredictedintheratedeterminingstep.Themechanisticstudiesdisclosedthatattheearlystage ofthereactiononlytrans-dihydroaminocomplex(C5)wasformed.The samegrouplaterexploredthedetailsofthemechanismofthereaction basedonthekineticstudies,controlexperiments,andtheDFTcalculation [41].
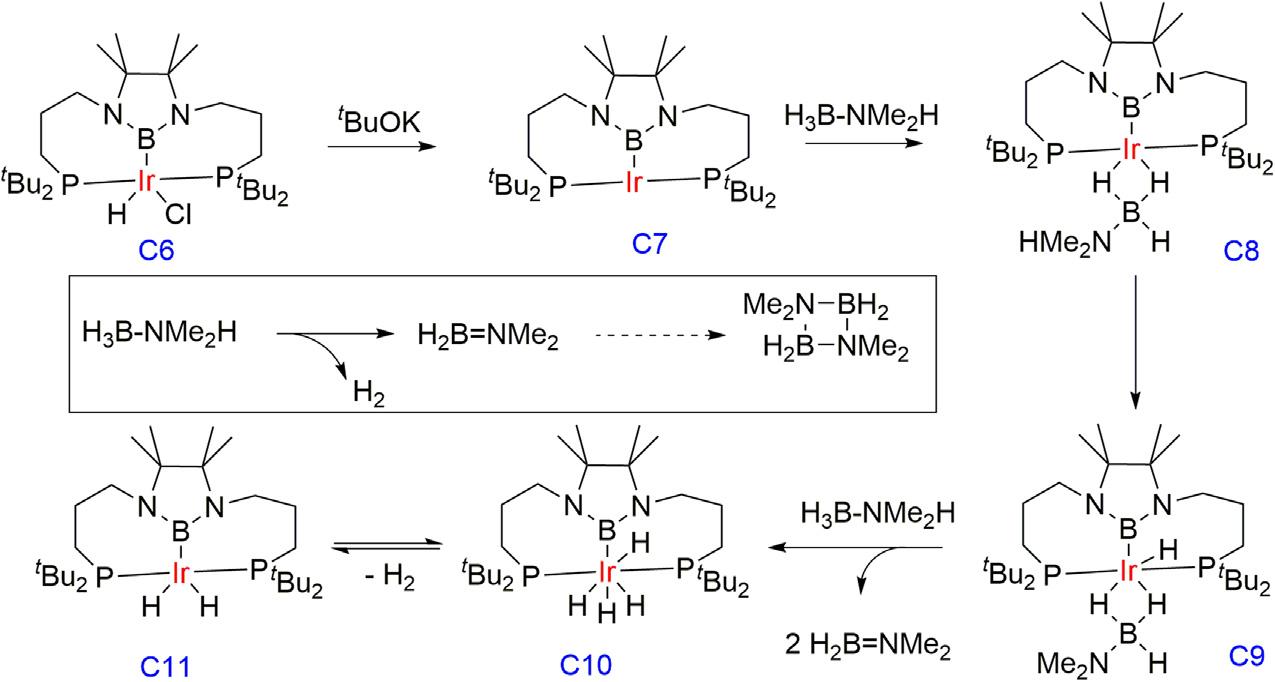
Yamashitagroupdevelopedaboron-basedpincerligandwithiridium catalystfortheABdehydrogenationreaction [42].Inthepresenceof KOtBu,astoichiometricamountofdimethylamine-borane(DMAB) convertedintocyclicdimer(Me2N BH2)2,withatraceamountofBH (NMe2)2.Undercatalyticcondition2mol%ofC6/KOtBu(1/1.5)undergoesdehydrogenationofDMABwithquantitativeconversionwithin 10minandH2 wasevolvedimmediatelyuponmixingthesubstrateand catalyst.Reducingthecatalystloadingto0.5mol%resultedin65%ofthe conversionafter3hat23°Cwhereasat60°Cimprovedto83%. AlthoughthequantitativeconversionofDMABtoacyclicproductat 60°Cafter3hwasobservedinaconcentratedreactionmediumbyreducingthevolumeofTHFsolvent.Monitoringthecatalysisreactionby 11B NMRspectroscopydepictedthatDMAB,dehydrogenatedcyclicdimer, lineardimer(HMe2N BH2 NMe2 BH3),monomer(Me2N 5 BH2),
Figure1.3 Dehydrogenationofammoniaboranetothepolymericdehydrocoupling productusingRu(PNP)pincercomplex.
andasmallamountofBH(NMe2)2 wereformed.Inthefirst5min,conversionofDMABtothelineardimerandsubsequentcyclizationtothe cyclicdimerwasobservedwith77%,andalmostfullconversionwas achievedafter3h.ThecomplexC6showsaninitialTOFof3400h 1 in 2minusing0.5mol%catalystloading.
AprobablemechanismwasproposedwherecomplexC6wasactivated byKOtButogeneratethethree-coordinated “T-shaped” (PBP)iridium(I) species(C7)andfollowedbycoordinationtoaDMABmoleculethrough itsnucleophilicB Hendedina η 2 fashionformingC8.TheN HprotonincoordinatedDMABwastransferredtotheiridiumcenterformed aniridiumhydrideintermediate(C9)andfollowedbydissociationofthe dehydrogenatedmonomerMe2N 5 BH2 andgeneratedthedihydride complexC11viatetrahydrideintermediateC10.Acouplingreactionof DMABandthedehydrogenatedmonomertoproducethelineardimer (HMe2N BH2 NMe2 BH3)andspontaneouslytransformedintothe cyclicdimerbyreleasinganadditionalequivalentofH2 wasproposed (Fig.1.4).
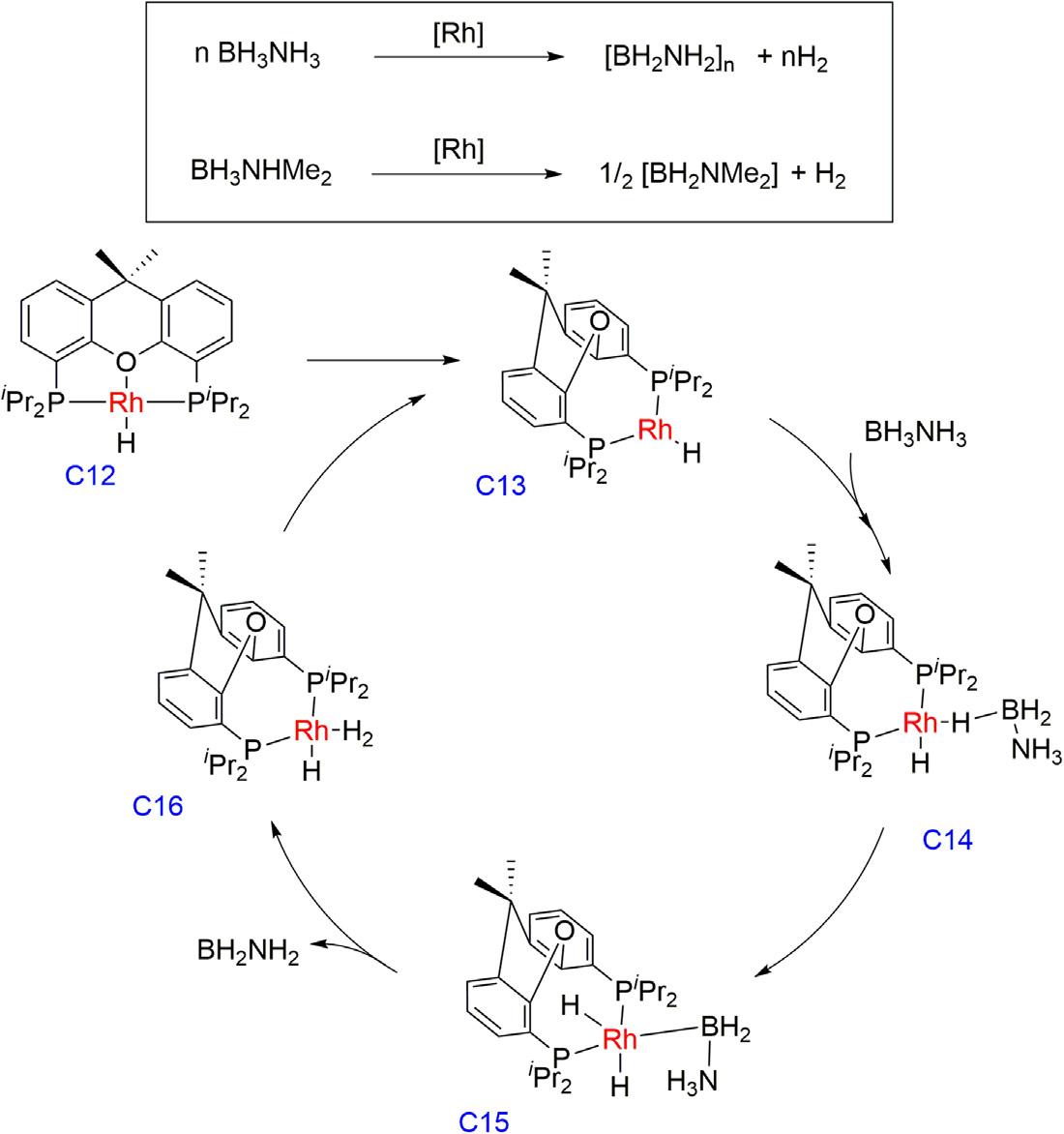
In2016,theVelezgroupreportedRhH{xant(PiPr2)2}complex(C12) forthesamereactionwhere1molofmolecularhydrogenwasreleased permoleofammoniaboraneanddimethylamineboranewithTOFat 50%conversionof3150h 1 and1725h 1,respectively [43].Thedehydrogenationofammoniaboraneyieldedpoly(aminoborane)asawhite, insolubleproductwhereasdimethylamineboranedehydrogenation
Figure1.4 Dehydrogenationofdimethylamineboranetoacyclicproductusinglr (PBP)complex.
affordedthedimer[H2BNMe2]2 andnotracesofthelineardiborazane H3B NMe2 BH2 NHMe2 wereobservedatanypointofthereaction, whichsuggestsanoff-metaldimerizationofH2B 5 NMe2.Additionally, thesamecatalystalsousedforthetransferhydrogenationreactionwhere 1:1ammoniaborane/cyclohexenemixtureinitiallyundergoestheselectivedehydrocouplingofammoniaborane,andsubsequentlythegenerated molecularhydrogenreducestheolefinwithaTOF50%valueof12h 1 . Duringthetandemprocess,noformationofanyborylationproductwas observed,whichisalsoconsistentwiththereleaseofH2BNH2 and experiencespolymerizationoutofthecoordinationsphereofthemetal, initiatedbyanucleophile.
ThedetailedDFTcalculationrevealedthemechanismwhereintheinitial stepcomplexC12dissociatedtheoxygenatomofthediphosphineligandto affordthetricoordinatedT-shapedintermediateC13followedbythesubsequentcoordinationofammoniaboranetoformC14,whichhomolytically addedthecoordinatedB Hbond,toaffordthedihydrideintermediateC15, andcontainedastabilizedborylligand.Thefivecoordinatedrhodium(III) complexC15evolvedintothehydride dihydrogenintermediateC16, whichuponreleaseofthedihydrogenligandregeneratesC13tocompletethe cycle(Fig.1.5).
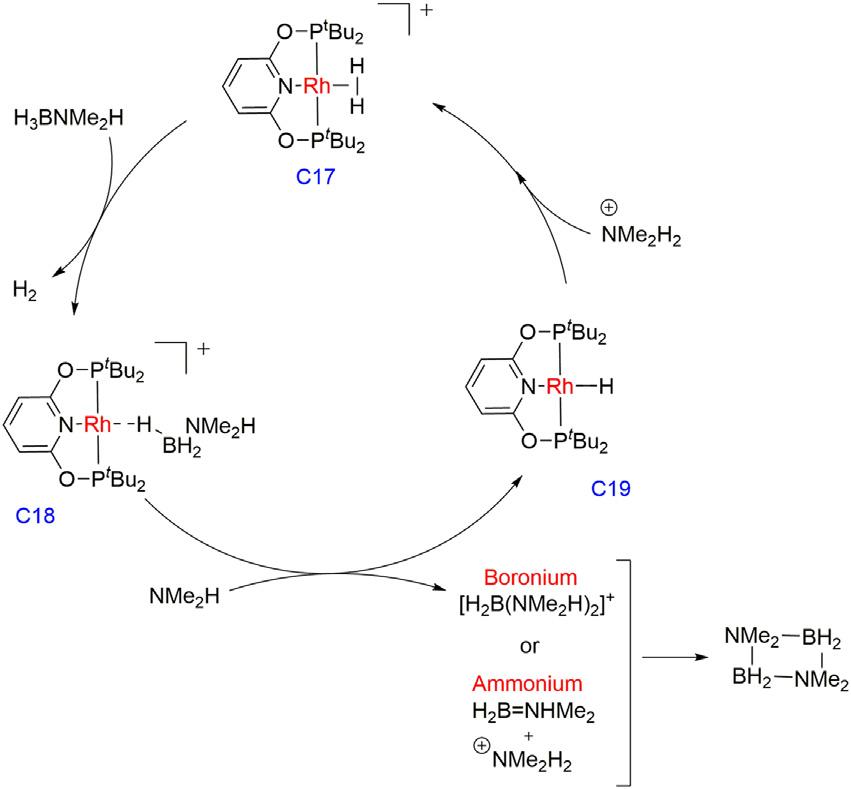
Wellergroupin2019showedthatthe[Rh(PONOP)(η2-H2)][BArF4] precatalystC17(2mol%)canquantitativelydehydrogenateH3B-NMe2H to[H2BNMe2]2 [44].Intheproposedmechanism,firstthedeprotonation oftheShimoi-typeamine-borane σ-complex(C18)occurredbyamines toformametal hydrideintermediate(C19)andammoniumsaltalong withthedehydrogenatedaminoboraneproduct,whichthenformeda cyclicfinalproductineitheroftwopathways,whichincludesanucleophilicattackonboronviaaboroniumrouteordeprotonationofthe σ-boundamine-boraneviaanammoniumroute.Lastly,theammonium salttransferredtheprotontoRh-Hintermediate(C19)toformdihydrogencomplexC17(Fig.1.6).
Inadditiontocatalystsof2nd-and3rd-rowtransitionmetals,avariety ofearth-abundantmetal-basedcatalystshavealsobeenemployedforthe dehydrogenationofammoniaborane.
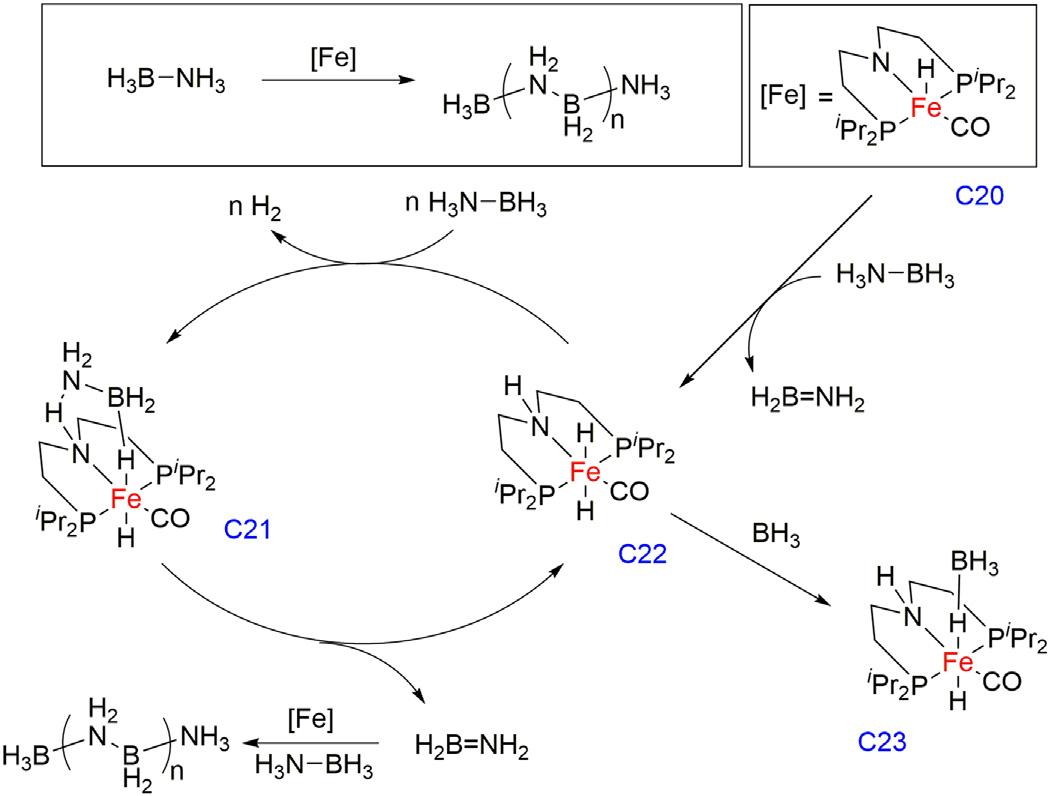
SchneiderGroupin2015reportedironpincercomplex[FeH(CO) (PNP)](PNP 5 N-(CH2CH2PiPr2)2)C20forammoniaboranedehydrocouplingreaction [45].OneequivalentofH2 wasreleasedwithTOFof 30h 1 fromABatroomtemperaturewithoutanyadditionalactivation, suchasbaseorirradiation.Thefullconversionwasobtainedwithcatalyst
Figure1.5 DehydrogenationofammoniaboraneusingRh(xantphos)pincercomplex andproposedmechanism.
loadingsof0.5mol%andamaximumTONof200wasachievedwith 0.1mol%catalystloadingwherepoly(aminoborane)wasobtainedasa majorproductalongwithsmallamountsofborazine,polyborazylene, cyclotriaminoborane,cyclodiaminoborane,andB-(cyclotriborazanyl)amine-borane. 31Pand 11BNMRspectroscopyrevealedthatfreeboron moietyiscoordinatedbythedihydrideintermediate(C23),whichundergoesthecatalystdeactivationprocess.Toimprovethecatalystactivities, additionalNMe2Et(Cat/NMe2Et/AB 5 1/4/500)wasaddedanda higherTONof330wasobservedwithoutaffectingtheselectivityofthe reaction.ComplexC20reactedwithABandformedrestingstate iron dihydridecomplexC22,whichfurtherdehydrogenatedtheammoniaboranetoH2,andtransientaminoborane(H2B 5 NH2),which underwentthepolymerizationviatheintermediateC21.CatalystC22 wasalsoobservedtoformadeactivatedproductC23withtheadditionof freeBH3 (Fig.1.7).
Applicationofpincermetalcomplexesincatalytictransformations
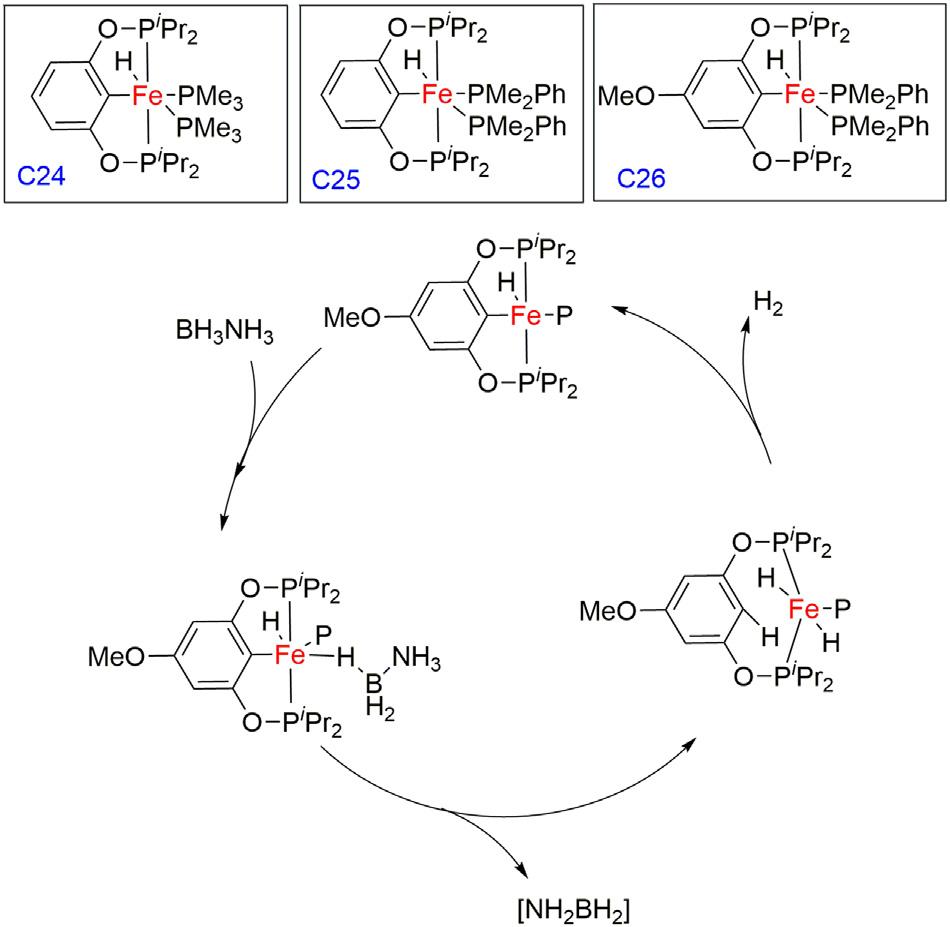
Guangroupreportedaseriesofironbis(phosphinite)pincercomplexes employedforthesamereactionandobservedanimmediategasevolution whenasolutionofcatalystC24inTHFwasmixedwithasolutionofAB (20equivalents)indiglymeat60°C(Fig.1.8) [46].After24h,2.5
Figure1.6 Dehydrogenationofamine-boraneusingRh(PONOP)pincercomplexand proposedmechanism.
Figure1.7 AmmoniaboranedehydrogenationusingFe(PNP)pincercomplex.
equivalentsofH2 perABwasreleased.ComplexC25showsimproved catalyticactivitywhereascomplexC26isasuperiorcatalystintermsof bothrateandtheextentofH2 released.The 11BNMRspectrumofthe solublematerialsshowstheformationofborazineandpolyborazyleneas productswithcyclotriborazane(CTB)andB-(cyclodiborazanyl)aminoborohydride(BCDB)inminorportions.TheH2 evolutionfromthisprocess usingcatalystC26gives1.0,2.0,and2.5equivalentsofH2 perABin1.5, 7,and16h,respectively.However,theamountofreleasedH2 still increasesafter12h,suggestingthatthelifetimeofthecatalyticallyactive speciesislonger.Itwasproposedthattheactiveintermediatewasformed byremovingonePMe2PhunitfromcatalystC26.Comparedtothe uncatalyzedthermalreaction,therateforthedehydrogenationofABcatalyzedbyC26isabout50timesfaster.Theproposedrate-determining stepinvolvessimultaneouslybreakingtheN HandB Hbondsof AB,whichresultsintheprotonationoftheipsocarbonandthetransfer ofahydrideligandfromBtoFe,whichisfollowedbytheformationofa dihydridespecies.Atthefinalstage,thesimultaneousC Hbondactivationtorestorethepincerframeworkcompletesthecyclebyeliminating onemoleculeofhydrogen,whichisfacileevenatroomtemperature.
Figure1.8 AmmoniaboranedehydrogenationusingFe(POCOP)pincercomplexand proposedmechanism.
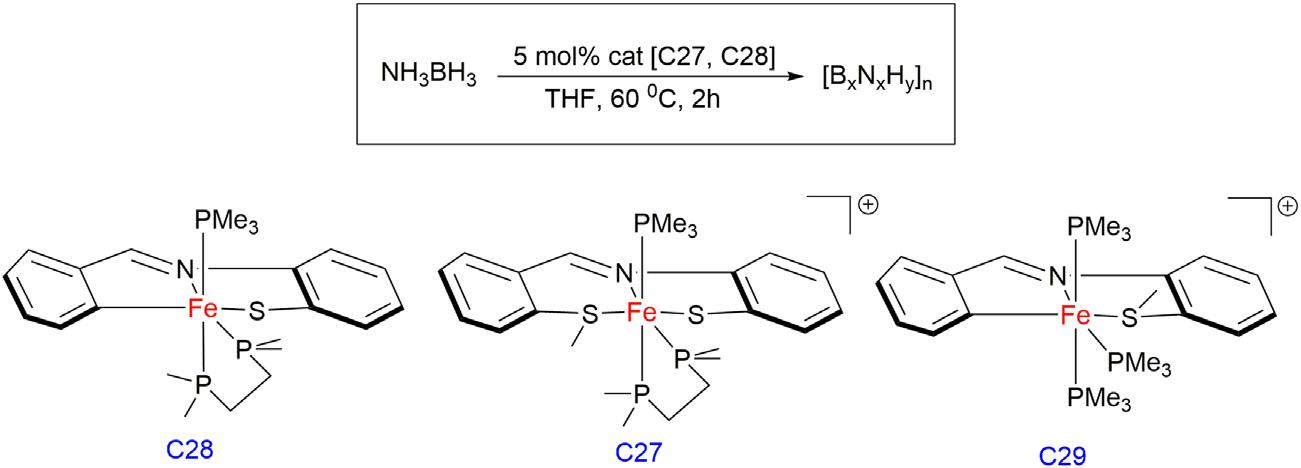
Bakergroupin2019reportedthatthereactionofFepincercomplex C27with20equivalentsofABinTHFat60°Cresultedintheimmediate observationofgasevolutionandformationofinsolublepoly(aminoborane) [47].After2h,the 11B{1H}NMRspectrumrevealedtheformation ofborazineandpolyborazylenealongwithamixtureoflinearand branchedaminoboraneoligomers.Dehydrogenationofmethylamineborane(MeAB)alsoproceededsmoothlywiththeC27precatalyst,albeit withpoorselectivity.Interestingly,ammoniaboranedehydrogenation reactionsusingprecatalystC28showedloweractivitybutmuchgreater selectivity,yieldingonlythebranchedcyclicaminoboranetetramer [B-(cyclotriborazanyl)amine-borane]inadditiontoborazineandpolyborazylene.Furthermore,C28wasalsoreusedformultipletimes,although longerreactiontimeswererequiredtoconsumethesecondandthird batchesofABwhilemaintaininghighselectivityofthecyclicproducts. Also,thesamewasemployedfordehydrogenationofMeABandtrimethylamineborane(TMAB),whichwasselectiveforthecyclicproducts withprimarilyCTB[(MeNHBH2)3],whereasnoreactionwasobserved withTMAB,suggestingthatbifunctionalN Hactivationisintegralto thedehydrogenationpathway.ThereactionofC29with20equivalents ofABatroomtemperatureshowedthesamereactivity;however,after 1h,ablackprecipitatewasobserved,indicativeofcatalystdecomposition. OverallamidocomplexC29wasthemostactivecatalystbutleast stableandcomplexC27alsoshowedlimitedcatalystlifetime,whileless activeFe thiolatecomplexC28provedtobethemostrobustunderthe optimizedconditionsofamine-boranedehydrogenation(Fig.1.9).
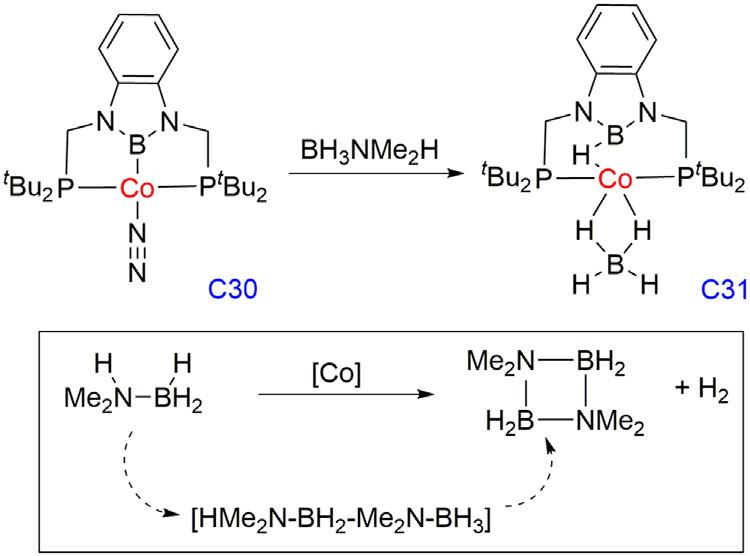
PetergroupreportedthedehydrogenationofHMe2N BH3 catalyzedbytheCopincercomplex [48].ThereactionofC30with Me2NH BH3 ina1:2stoichiometricratioaffordedthequantitative
Figure1.9 AmmoniaboranedehydrogenationusingFe(SNS)pincercomplexes.
formationofahydridoboranetetrahydridoboratecobaltcomplex(C31) accommodatingabridginghydrideandan η2-BH4 ligandsconfirmedby NMRandX-raycrystallography.Me2NH BH3 stirredinC6D6 with2 mol%ofC30orC31underinertatmosphereunderwentinitialdehydrogenationtoalinearproduct(HMe2N BH2 Me2N BH3),which fullyconvertedinto(Me2N BH2)2 in6h(TOF 8h 1).C31effectivelycatalyzedbotholefinhydrogenationandamine boranedehydrogenationandtogetheraffordedatransferhydrogenationreaction.Equal amountsofstyreneandHMe2N BH3 werereactedinC6D6 at25°C with2mol%ofcatalystandafter24h,styrenewashydrogenatedtoethylbenzenequantitatively,andthedehydrogenatedproduct(Me2N BH2)2 wasobserved(Fig.1.10).
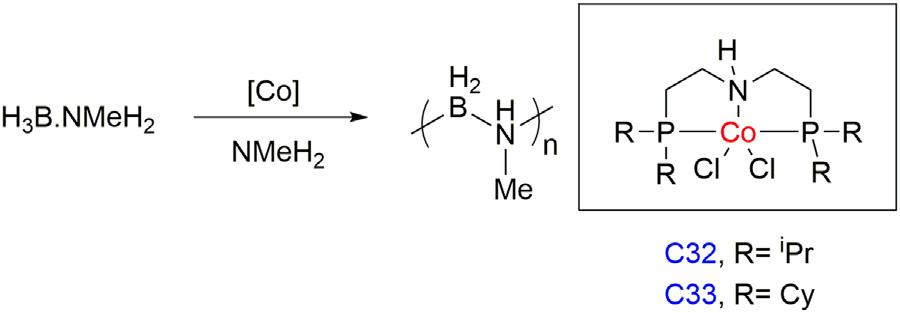
WellergroupreportedthattheCoCl2(R2PCH2CH2)2NH(R 5 iPr, Cy)C32,C33respectivelycomplexesusedfortheH3B-NMeH2 dehydrogenationundermildcondition(0.4mol%catalyst,25°C,1,2-F2C6H4 assolvent)inpresenceof2equivalentsofanamine(NMeH2)with respectto[Co],alinear(H2BNMeH)n,mediummolecularweightpolymerwasformed(47600gmol 1 andÐ 5 1.5)in60%yieldwithTOF of750h 1 forcomplexC32and3700h 1 forC33 [49].A10gscale reactionwasperformedundertheoptimizedconditionusingprecatalyst C33whichaffordedanoff-whitepolymerofmolecularweightof 74200gmol 1.Themechanismwasproposedwheremetal ligandcooperativityplayedasignificantroleinthesubstrateactivationanddehydrogenationprocess(Fig.1.11).
FoutandMelchorgroupexploredaCocomplex(C34)withmonoanionicbis(carbene)arylpincerligandforthedehydrogenationofAB
Figure1.10 Amine-boranedehydrogenationusingCo(PBP)pincercomplex.
Figure1.11 Amine-boranedehydrogenationusingCo(PNP)pincercomplexandproposedmechanism.
Figure1.12 AmmoniaboranedehydrogenationusingCo(CCC)pincercomplexand proposedmechanism.
catalyticallyandreportedthat1.7equivalentsofH2 perABwasreleased inTHFat60°Candaffordedbothsoluble(borazineandpolyborazylene) andinsoluble(poly(aminoborane))BN-containingspecies [50].ThecontrolledreactionofC34withastoichiometricamountofABinTHF yieldedahydride amidoboraneCocomplex(MesCCC)CoH(NH2BH3) (C35),whichwasisolatedandcharacterized(Fig.1.12).
1.3Dinitrogenactivationusingpincerligands Nitrogenisoneofthemostabundantelementsintheearth’satmosphere; however,duetothechemicalinertness,itshighbonddissociationenergy (945KJ/mol),lowprotonaffinity,nonpolarcharacterandhighHOMOLUMOgap,itsfixationortransformationtofunctionalizedmolecules suchasammonia,hydrazineorotherfinechemicalsarethermodynamicallyverydemanding [51,52].Ammoniaproductionfromdinitrogenis oneofthemostimportantchemicaltransformationsthathaveadirect impactonhumansociety.Ammoniaisusedfortheproductionoffertilizers,whichareessentialtoprovidefeedstocksfortheworld’sexpanding
population [53,54].Industrially,ammoniaissynthesizedbythe Haber Boschprocess,fromthereactionofdinitrogenanddihydrogen gasesinthepresenceofaheterogeneouscatalystinharshreactionconditions(hightemperatureandhighpressure) [55].Thereforethedevelopmentofmoreenergy-efficientandambientconditionsforthereduction ofdinitrogentoammoniaisoneofthemostimportantandchallenging chemicaltransformationsinthecentury.
Incontrasttothemanyreportsonthestoichiometricreactivityof transitionmetal-bounddinitrogencomplexes,thereareonlyafewexamplesreportingthecatalytictransformationofdinitrogenusingthesecomplexesunderambientreactionconditions [52,56 70].In2003,the Schrockgroupreportedthesuccessfulcatalyticreductionofdinitrogen intoammoniaunderambientconditionsusingamolybdenum dinitrogen complexbearingabulkyHIPT-substitutedtriamidoamineligandasacatalyst,alongwith2,6-lutidiniumtetrakis[3,5-bis(trifluromethyl)phenylborate]([LutH]BArF4)asaprotonsourceandCrCp2 asareducingagent [71].Recently,pincer-basedmetalcompoundshaveattractedtheirapplicationindinitrogenactivationchemistry,whichisdevelopingwithtime. Inthissection,wehavedisclosedtheimportantdinitrogenactivation reactionsbyusingseveralreportedtransitionmetalpincercomplexes.
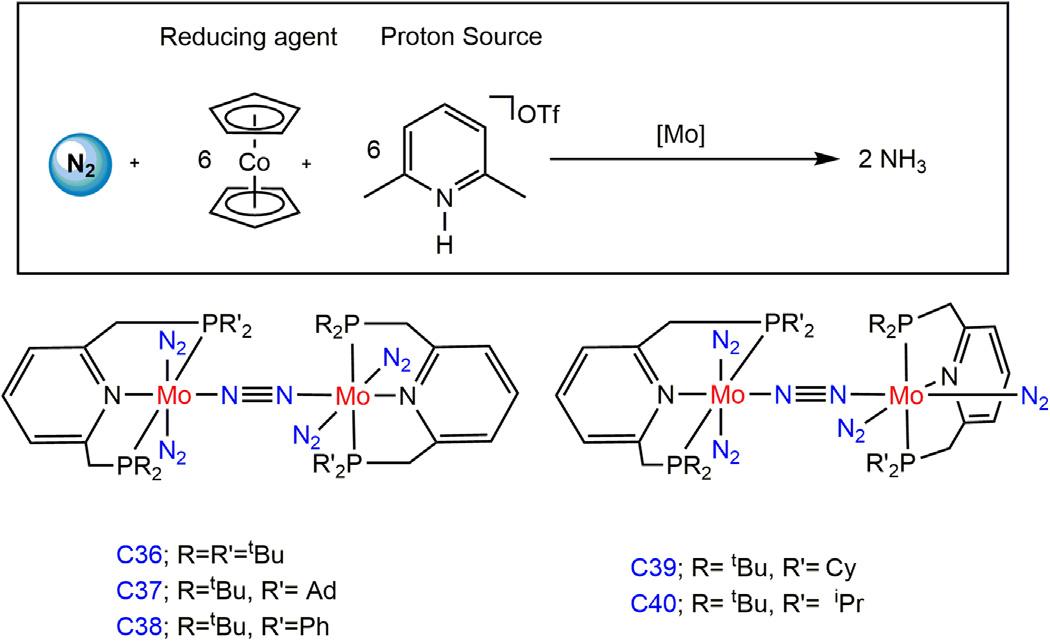
1.3.1CatalyticdinitrogenactivationusingMopincercatalyst AftertheSchrock’sreport,in2010,asignificantbreakthroughhasbeen achievedinthisfieldbyNishibayashiandco-workersusingMo PNP-based pincerligand [72].Mo PNP N2 bridgedbinuclearpincercatalystC36 (0.01mmol)(PNP 5 2,6-bis(di-tert-butylphosphinomethyl)pyridine),under excessprotonsourceas[LutH]OTf(0.96mmol)andreductantasCoCp2 (0.72mmol)afforded12equivalentsofammoniawithrespecttocatalyst whereasbyincreasingthe[LutH]OTf(2.88mmol)andCoCp2 (2.16mmol), itafforded23equivalentsofammonia;however,dihydrogenisalsodetected asasideproduct.In2012,thesamegroupreportedthatsubstitutionofthe tert-butyl(tBu)groupbyadamantyl(Ad)grouptooneoftheP-armofcatalystC37afforded14equivalentsofammoniaundersimilarcondition[[LutH] OTf(0.96mmol)andCoCp2 (0.72mmol)]whilewithalargeexcessof reagents[LutH]OTf(288equivalents)andCoCp2 (216equivalents),it affordedonly16equivalentsofammoniabasedonthecatalystC37.Other systemcontaining Ph(C38), Cy(C39),and iPr(C40)substituentswere foundtobealesseffectivecatalyst(Fig.1.13) [73].
Figure1.13 N2 bridgedbinuclearMo(PNP)pincercomplexes.
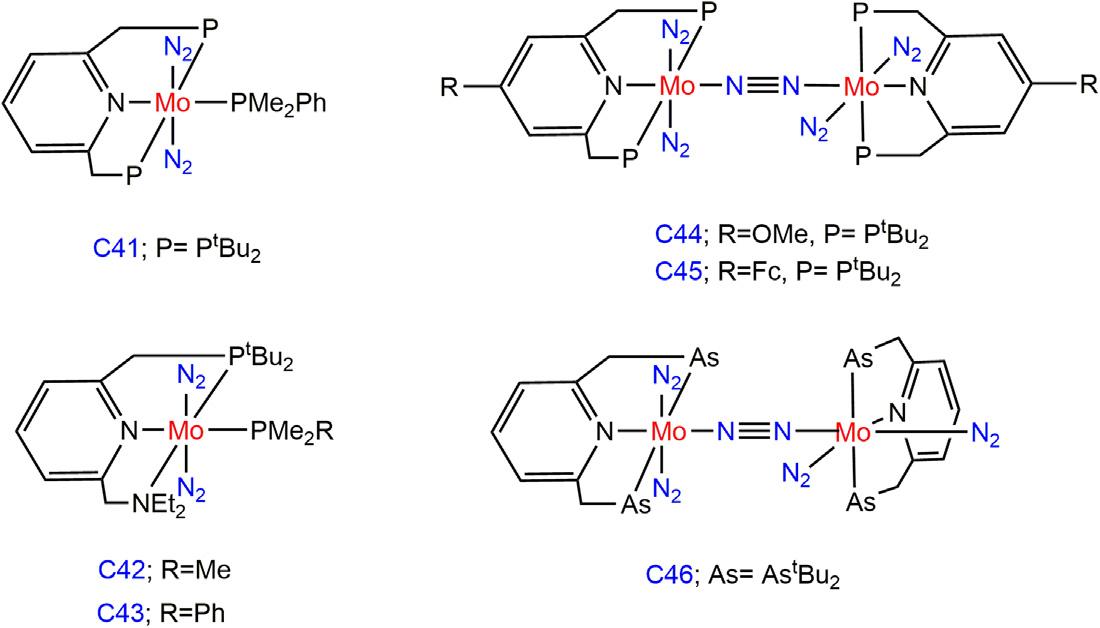
Figure1.14 MononuclearN2 boundMopincercomplexesandN2 bridgedbinuclear Mopincercomplexes.
Themononuclear molybdenumcomplexbearingPNPpincerligand C41(0.04mmol)reportedbytheNishibayashigroupyielded1.38 equivalentsofNH3 basedonMousingH2SO4 asprotonsourceatroom temperature(Fig.1.14) [74].ThecomplexesC42andC43bearingPNN ligandalsoaffordedammoniainaloweramount(0.20,0.25equivalents/ Morespectively)comparedtoC41underthesimilarcondition [75]. Nishibayashigroupobservedthattheintroductionofelectron-donating methoxy(-OMe)groupatthe4-positionofpyridinemoietyofcatalyst C36dramaticallyincreasedtheyieldofammonia [76].Upto52equivalentsofammoniawereproducedbasedonthecatalystC44togetherwith