Low-DimensionalHalidePerovskites:Structure, Synthesis,andApplicationsYiqiangZhan
https://ebookmass.com/product/low-dimensional-halideperovskites-structure-synthesis-and-applications-yiqiangzhan/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Calculations and Simulations of Low-Dimensional Materials : Tailoring Properties for Applications 1st Edition Ying Dai
https://ebookmass.com/product/calculations-and-simulations-of-lowdimensional-materials-tailoring-properties-for-applications-1stedition-ying-dai/ ebookmass.com
Polar Organometallic Reagents - Synthesis, Structure, Properties and Applications Wheatley Andrew E.H. (Ed.)
https://ebookmass.com/product/polar-organometallic-reagents-synthesisstructure-properties-and-applications-wheatley-andrew-e-h-ed/
ebookmass.com
Chiral Building Blocks in Asymmetric Synthesis: Synthesis and Applications Elzbieta Wojaczynska
https://ebookmass.com/product/chiral-building-blocks-in-asymmetricsynthesis-synthesis-and-applications-elzbieta-wojaczynska/
ebookmass.com
Fusion of Critical Horizons in Chinese and Western Language, Poetics, Aesthetics Ming Dong Gu
https://ebookmass.com/product/fusion-of-critical-horizons-in-chineseand-western-language-poetics-aesthetics-ming-dong-gu/
ebookmass.com
(eTextbook PDF) for Principles of Managerial Finance Brief 7th by Lawrence
https://ebookmass.com/product/etextbook-pdf-for-principles-ofmanagerial-finance-brief-7th-by-lawrence/
ebookmass.com
Practical Airport Operations, Safety, and Emergency Management: Protocols for Today and the Future
https://ebookmass.com/product/practical-airport-operations-safety-andemergency-management-protocols-for-today-and-the-future/
ebookmass.com
Small Business Valuation Methods: How to Evaluate Small, Privately-Owned Businesses 1st Edition Yannick Coulon
https://ebookmass.com/product/small-business-valuation-methods-how-toevaluate-small-privately-owned-businesses-1st-edition-yannick-coulon/
ebookmass.com
Republic of Islamophobia: The Rise of Respectable Racism in France Jim Wolfreys
https://ebookmass.com/product/republic-of-islamophobia-the-rise-ofrespectable-racism-in-france-jim-wolfreys/
ebookmass.com
Women's Sexuality and Modern India: In a Rapture of Distress Amrita Narayanan
https://ebookmass.com/product/womens-sexuality-and-modern-india-in-arapture-of-distress-amrita-narayanan/
ebookmass.com
Algorithmic Modernity: Mechanizing Thought and Action, 1500-2000 1st Edition Morgan G. Ames (Editor)
https://ebookmass.com/product/algorithmic-modernity-mechanizingthought-and-action-1500-2000-1st-edition-morgan-g-ames-editor/
ebookmass.com
LOW-DIMENSIONALHALIDE PEROVSKITES
LOWDIMENSIONAL HALIDE PEROVSKITES
Structure,Synthesis, andApplications
Editedby
MOHAMMAD KHALID PAOLA VIVO
NUMAN ARSHID
YIQIANG ZHAN
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2023ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorageand retrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission, furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswith organizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,can befoundatourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribedherein. Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyandthesafety ofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors, assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproducts liability,negligenceorotherwise,orfromanyuseoroperationofanymethods,products, instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-88522-5
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionsEditor: StephenJones
EditorialProjectManager: TomMeans
ProductionProjectManager: AnithaSivaraj
CoverDesigner: VickyEsserPearson
TypesetbySTRAIVE,India
Contributorsix
1.Introduction
ManingLiu,G.KrishnamurthyGrandhi,andPaolaVivo
1.Introduction1
2.HistoryofMHPs2
3.WhatareMHPs?3
4.ChemistryofMHPs6
5.DimensionalityofMHPs7
6.Conclusionsandprospects14 References15
2.Fundamentalsandclassificationofhalideperovskites
SarahDerbali,VioricaStancu,AndreiG.Tomulescu,CristinaBesleaga, GeorgeAlexandruNemnes,IoanaPintilie,andMihaelaFlorea
1.Discoveryandfundamentalstructureofhalideperovskites19
2.Dimensionsofhalideperovskites24
3.Compositionalengineeringofperovskites28
4.Transportphenomenainlightabsorbermaterials:Impactonthe measurementprotocols36
5.Concludingremarks44 Acknowledgments45 References45
3.Structuraleffectsonhalideperovskiteproperties
IrfanAhmed,MeenakshiGussain,FatemehBehrouznejad,WaseemHaider, andYiqiangZhan
1.Introduction57
2.Compositionandcrystalstructureofhalideperovskite59
3.Optoelectronicproperties60
4.Thermaltransportinhalideperovskite72
5.Otheroutstandingproperties77
6.Conclusion81 References82
4.Synthesistechniquesofmetalhalideperovskites
IgnacioRosa-Pardo,AlejandroCortes-Villena,RaquelE.Galian, andJuliaPerez-Prieto
1.Introduction91
2.Techniquesforthepreparationofthinfilms94
3.Techniquesforthepreparationofcolloidalnanocrystals124
4.Challengesandperspectives143 5.Conclusion144 Acknowledgments144 References144
5.Abinitiostudiesonperovskites
TudorLucaMitran,RachelElizabethBrophy,MarinaCuzminschi, NicolaeFilipoiu,MovaffaqKateb,IoanaPintilie,AndreiManolescu, andGeorgeAlexandruNemnes
1.Bandgapandotherelectronicproperties153
2.Ferroelectricityofhybridperovskites157
3.Pointdefects159
4.2D-3Dperovskites161
5.Perovskite/ETLandperovskite/HTLinterfaces163
6.MDstudiesinhybridperovskites170
7.MoreapplicationsofMDmethods172
8.Concludingremarks176 Acknowledgments176 References176
6.Lead-freehalideperovskites
MohammadHatamvand,SomayehGholipour,MozhganYavari, MahboubehHadadian,MohammadSajediAlvar,BartRoose,YaserAbdi, YiqiangZhan,YonghuaChen,andWeiHuang
1.Introduction187
2.DifferentPb-freeperovskitecompositionandcrystalline structures190
3.Opticalproperties197
4.Electronicproperties201
5.Pb-freeperovskiteforoptoelectronicdevices205
6.StabilityofPb-freeperovskites214
7.Futureoutlooksandremarks222 References223 Furtherreading236
7.Low-dimensionalhalideperovskiteforsolarcellapplications
M.AtikurRahmanandFaisalIslamChowdhury
1.Introduction239
2.Perovskitesolarcellsoflow-dimensionalhalideperovskite243
3.Currentchallengesregardingcommercializationofhalideperovskites solarcellsandprospects251 4.Conclusions260 References261
8.Halideperovskiteforlight-emittingdiodes
RajanKumarSingh,Chung-HsinLu,RadhaTamrakar,NehaJain, AnupriyaSingh,MohanLalMeena,andSudiptaSom
1.Introduction267
2.TheapplicationofLEDinhalideperovskite271
3.Lead-freeperovskiteLED282
4.PerovskiteLEDstability289
5.CurrentchallengeandperspectiveofperovskiteLEDs295 References295
9.Otherapplicationsofhalideperovskites
ShivamPorwal,DineshKumar,SubrataGhosh,SakshiKansal, SurbhiPriya,AmreeshChandra,andTrilokSingh
1.Introduction301
2.Halideperovskiteforbatteriesandsupercapacitors301
3.Gassensingapplicationoforganic-inorganichybridhalide perovskitematerial307
4.Resistiveswitchingrandomaccessmemory(ReRAM)devicesusing metalhalideperovskite312
5.Piezoelectricenergyharvestingusinghalideperovskites320
6.Importanceofhalideperovskiteforpiezoelectricity321 7.Conclusions325 Acknowledgment326 References327
10.Halideperovskiteforphotodetectorapplications
C.Rajkumar,P.Vengatesh,T.S.Shyju,A.Arulraj,andR.V.Mangalaraja
1.Introduction335
2.Photodetectors336
3.Inorganichalideperovskitephotoconductor342
4.Organic-inorganichybridperovskitephotodetector343 5.Memorydevices346 6.Sensors354
7.Summary362 Acknowledgment362 References362
11.Techno-economicanalysisandtoxicityofhalideperovskites
PandiyarajanMariyappanandTowhidH.Chowdhury
1.Introduction369 2.Methods370
3.Techno-economicanalysisofdifferenttypesofperovskite-based PVmodules371
4.Toxicityofperovskitesolarcells378
5.ComparisontootherexistingPVtechnologies379
6.Challengesandfutureperspective380 References381
12.Recyclingofhalideperovskites
Md.FarhanNaseh,ChoudharyArjunSunilbhai,MohammadKhalid, andJamilurR.Ansari
1.Introduction386
2.Stabilityanddegradationofhalideperovskitesolar cells(HPSCs)391
3.ImpactofenvironmentonHPSCsandimpactofHPSCs onenvironment400
4.Recyclingmeasurestocountertheenvironmentalhazards404
5.Circulareconomyandrecyclingpolicies420
6.ImprovingtheefficiencyofHPSCs424
7.Conclusionandfuturescope428 Acknowledgments429 Declarationofcompetinginterest429 References429
13.Challengesandfutureprospects
PaulinaCarmona-Monroy,BrendaVargas,andDiegoSolis-Ibarra
1.Introduction447
2.Environmentalissuesandtoxicityofhalideperovskites448
3.Stabilityandorientationcontrol451
4.Novelorganiccations455
5.Halide-layereddoubleperovskites457
6.Low-dimensionalhalideperovskiteheterostructures465
7.Futureapplications470
8.Conclusions474 References475 Index485
Contributors
YaserAbdi DepartmentofPhysics,NanophysicsResearchLaboratory,UniversityofTehran,Tehran,Iran
IrfanAhmed StateKeyLaboratoryofASICandSystem,CentreofMicro-Nano System,SchoolofInformationScienceandTechnology,FudanUniversity, Shanghai,China;DepartmentofPhysics,GovernmentPostgraduateCollege, HigherEducationDepartment-HED,Mansehra,KhyberPakhtunkhwa, Pakistan
JamilurR.Ansari UniversitySchoolofBasicandAppliedSciences,Guru GobindSinghIndraprasthaUniversity,Dwarka,Delhi;DepartmentofApplied Science&Humanities,DronacharyaCollegeofEngineering,Gurugram, Haryana,India
A.Arulraj InstitutodeInvestigacionesCientı´ficasyTecnolo ´ gicas(IDICTEC), UniversidaddeAtacama,Copiapo ´ ;FacultyofEngineering&Sciences,UniversidadAdolfoIba ´ nez,Penalolen,Santiago,Chile
FatemehBehrouznejad StateKeyLaboratoryofASICandSystem,Centreof Micro-NanoSystem,SchoolofInformationScienceandTechnology,FudanUniversity,Shanghai,China;NanoparticlesandCoatingsLab,DepartmentofPhysics,SharifUniversityofTechnology,Tehran,Iran
CristinaBesleaga NationalInstituteofMaterialsPhysics,Magurele,Ilfov, Romania
RachelElizabethBrophy ReykjavikUniversity,IcelandicSchoolofEnergy, Reykjavik,Iceland
PaulinaCarmona-Monroy InstitutodeInvestigacionesenMateriales,UniversidadNacionalAuto ´ nomadeMexico,Mexico
AmreeshChandra DepartmentofPhysics,IndianInstituteofTechnologyKharagpur,Kharagpur,721302,India
YonghuaChen KeyLaboratoryofFlexibleElectronics(KLoFE)&Instituteof AdvancedMaterials(IAM),NanjingTechUniversity(NanjingTech),Nanjing, Jiangsu,China
FaisalIslamChowdhury DepartmentofChemistry,UniversityofChittagong, Chittagong,Bangladesh
TowhidH.Chowdhury OrganicPrintedElectronicsLaboratory(OPEL),DepartmentofChemicalEngineering,PohangUniversityofScience&Technology, Pohang,RepublicofKorea
AlejandroCortes-Villena InstituteofMolecularScience,UniversityofValencia, Valencia,Spain
MarinaCuzminschi HoriaHulubeiNationalInstituteforPhysicsandNuclear Engineering;FacultyofPhysics,MDEOResearchCenter,ResearchInstitute oftheUniversityofBucharest(ICUB),UniversityofBucharest,Magurele, Ilfov,Romania
SarahDerbali NationalInstituteofMaterialsPhysics,Magurele,Ilfov,Romania
NicolaeFilipoiu HoriaHulubeiNationalInstituteforPhysicsandNuclear Engineering;FacultyofPhysics,MDEOResearchCenter,ResearchInstitute oftheUniversityofBucharest(ICUB),UniversityofBucharest,Magurele, Ilfov,Romania
MihaelaFlorea NationalInstituteofMaterialsPhysics,Magurele,Ilfov,Romania
RaquelE.Galian InstituteofMolecularScience,UniversityofValencia,Valencia,Spain
SomayehGholipour METUGUNAMCenter,MiddleEastTechnicalUniversity,Ankara,Turkey
SubrataGhosh FunctionalMaterialsandDeviceLaboratory,SchoolofEnergy ScienceandEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur,721302,India
G.KrishnamurthyGrandhi HybridSolarCells,FacultyofEngineeringandNaturalSciences,TampereUniversity,Tampere,Finland
MeenakshiGussain StateKeyLaboratoryofASICandSystem,CentreofMicroNanoSystem,SchoolofInformationScienceandTechnology,FudanUniversity, Shanghai,China
MahboubehHadadian DepartmentofMechanicalandMaterialsEngineering, FacultyofTechnology,UniversityofTurku,Turku,Finland
WaseemHaider ScienceofAdvancedMaterials,CentralMichiganUniversity, Mt.Pleasant,MI,UnitedStates
MohammadHatamvand KeyLaboratoryofFlexibleElectronics(KLoFE)&InstituteofAdvancedMaterials(IAM),NanjingTechUniversity(NanjingTech), Nanjing,Jiangsu,China
WeiHuang KeyLaboratoryofFlexibleElectronics(KLoFE)&Instituteof AdvancedMaterials(IAM),NanjingTechUniversity(NanjingTech),Nanjing, Jiangsu,China
NehaJain DepartmentofPhysics,Dr.H.S.GourCentralUniversity,Sagar, MadhyaPradesh,India
SakshiKansal FunctionalMaterialsandDeviceLaboratory,SchoolofEnergy ScienceandEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur,721302,India
MovaffaqKateb ReykjavikUniversity,DepartmentofEngineering,Reykjavik, Iceland
MohammadKhalid Graphene&Advanced2DMaterialsResearchGroup (GAMRG),SchoolofEngineeringandTechnology,SunwayUniversity,No.5, JalanUniversiti,PetalingJaya,Selangor,Malaysia
DineshKumar FunctionalMaterialsandDeviceLaboratory,SchoolofEnergy ScienceandEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur,721302,India
ManingLiu HybridSolarCells,FacultyofEngineeringandNaturalSciences, TampereUniversity,Tampere,Finland
Chung-HsinLu DepartmentofChemicalEngineering,NationalTaiwanUniversity(NTU);DepartmentofChemicalEngineering,NationalTaiwanUniversity ScienceandTechnology(NTUST);AdvancedResearchCentreofGreenMaterialsScience&Technology,Taipei,Taiwan
R.V.Mangalaraja FacultyofEngineering&Sciences,UniversidadAdolfoIba ´nez,Penalolen,Santiago,Chile
AndreiManolescu ReykjavikUniversity,DepartmentofEngineering,Reykjavik,Iceland
PandiyarajanMariyappan DepartmentofElectricalandElectronicsEngineering,SriVenkateswaraCollegeofEngineering(SVCE),Chennai,TamilNadu, India
MohanLalMeena DepartmentofChemicalEngineering,NationalTaiwanUniversityScienceandTechnology(NTUST),Taipei,Taiwan
TudorLucaMitran HoriaHulubeiNationalInstituteforPhysicsandNuclear Engineering,Magurele,Ilfov,Romania
Md.FarhanNaseh UniversitySchoolofBasicandAppliedSciences,Guru GobindSinghIndraprasthaUniversity,Dwarka,Delhi,India
GeorgeAlexandruNemnes HoriaHulubeiNationalInstituteforPhysicsand NuclearEngineering;FacultyofPhysics,MDEOResearchCenter,Research InstituteoftheUniversityofBucharest(ICUB),UniversityofBucharest,Magurele,Ilfov,Romania
JuliaPerez-Prieto InstituteofMolecularScience,UniversityofValencia,Valencia,Spain
IoanaPintilie NationalInstituteofMaterialsPhysics,Magurele,Ilfov,Romania
ShivamPorwal FunctionalMaterialsandDeviceLaboratory,SchoolofEnergy ScienceandEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur,721302,India
SurbhiPriya FunctionalMaterialsandDeviceLaboratory,SchoolofEnergyScienceandEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur, 721302,India
M.AtikurRahman DepartmentofElectrical&ElectronicEngineering,UniversityofChittagong,Chittagong,Bangladesh
C.Rajkumar CenterforHighEnergySystemsandSciences(CHESS),DRDO, Hyderabad,India
BartRoose DepartmentofPhysics,CavendishLaboratory,UniversityofCambridge,Cambridge,UnitedKingdom
IgnacioRosa-Pardo InstituteofMolecularScience,UniversityofValencia, Valencia,Spain
MohammadSajediAlvar MaxPlanckInstituteforPolymerResearch,Mainz, Germany
T.S.Shyju CentreforNanoscienceandNanotechnology,SathyabamaInstitute ofScienceandTechnology(DeemedtobeUniversity),Chennai,India
AnupriyaSingh ResearchCenterforAppliedScience,AcademiaSinica,Taipei, Taiwan
RajanKumarSingh DepartmentofChemicalEngineering,NationalTaiwan University(NTU),Taipei,Taiwan
TrilokSingh FunctionalMaterialsandDeviceLaboratory,SchoolofEnergyScienceandEngineering,IndianInstituteofTechnologyKharagpur,Kharagpur, 721302,India
DiegoSolis-Ibarra InstitutodeInvestigacionesenMateriales,Universidad NacionalAuto ´ nomadeMexico,Mexico
SudiptaSom DepartmentofChemicalEngineering,NationalTaiwanUniversity (NTU),Taipei,Taiwan
VioricaStancu NationalInstituteofMaterialsPhysics,Magurele,Ilfov,Romania
ChoudharyArjunSunilbhai FacultyofPhysicalSciences,PDMUniversity, Bahadurgarh,Haryana,India
RadhaTamrakar DepartmentofPhysics,Govt.K.N.M.M.,Damoh,Madhya Pradesh,India
AndreiG.Tomulescu NationalInstituteofMaterialsPhysics,Magurele,Ilfov, Romania
BrendaVargas InstitutodeInvestigacionesenMateriales,UniversidadNacional Auto ´ nomadeMexico,Mexico
P.Vengatesh CentreforNanoscienceandNanotechnology,SathyabamaInstituteofScienceandTechnology(DeemedtobeUniversity),Chennai,India
PaolaVivo HybridSolarCells,FacultyofEngineeringandNaturalSciences, TampereUniversity,Tampere,Finland
MozhganYavari DepartmentofElectricalandElectronicEngineering, AdvancedTechnologyInstitute,UniversityofSurrey,Guildford,Surrey,United Kingdom
YiqiangZhan StateKeyLaboratoryofASICandSystem,CentreofMicro-Nano System,SchoolofInformationScienceandTechnology,FudanUniversity, Shanghai,China
1 Introduction
ManingLiu,G.KrishnamurthyGrandhi, andPaolaVivo
HybridSolarCells,FacultyofEngineeringandNaturalSciences, TampereUniversity,Tampere,Finland
1.Introduction
Metalhalideperovskites(MHPs)havebeenthefocusofintense researchworldwideoverthelastdecade,duetotheirenormouspotential assemiconductorsforhighlyperformingoptoelectronics,andinparticularphotovoltaics.Perovskitesolarcells(PSCs)havealreadymarkedacertifiedpowerconversionefficiency(PCE)recordabove25% [1].Suchan unprecedentedpaceofdevelopmentofPSCspromisestorevolutionize theprospectsofthird-generationphotovoltaics.TheoutstandingoptoelectronicpropertiesofMHPsincludehighabsorptioncoefficient,widetunablebandgap(400–800nm),longchargecarrierdiffusionlength( 1 μm), andhighchargecarrierconductivity(1–10cm2 V 1 s 1) [2–5].TheexceptionalinterestofthecommunityinMHPsisalsomotivatedbytheirprocessabilityinsolutionsstartingfromlow-costandeasilyavailable precursors.TherichchemistryofMHPsenablesaplethoraofstructural, optical,andelectronicpropertiesasaresultofthecomplexphysicochemicalprocessesunderlyingtheapparentlysimplesolution-basedsyntheses ofthesematerials [6–8].Finally,thetunabilityoftheoptoelectronicpropertiesofMHPshasopenedthepossibilityforotherapplicationsbeyond photovoltaics,suchaslight-emittingdiodes(LEDs),photodetectors,and lasers,justtomentionafewofthem [9–11].
Inthischapter,weintroducethereadertothetopicofMHPs,starting fromsomehighlightsonthehistoricaldevelopmentofthesematerialsand theirintenserecentinvestigation.Wealsoclarifywhichmaterialscanbe appropriatelyclassifiedashalideperovskites,giventhefrequentmisuse ofthistermintheliterature.WethenfocusonanoverviewoflowdimensionalMHPsandtheirkeyfeaturesandoptoelectronicproperties.
Finally,weprovideourviewonthekeyopenchallengesforthefuture advancementofthisclassofmaterialsandtheirdevelopmentofyet unexploredoptoelectronics.
2.HistoryofMHPs
Theterm“perovskite”wascoinedwhenthecalciumtitanate(CaTiO3) mineralwasdiscoveredbyGustavRosein1839andlaternamedinhonor oftheRussianmineralogistL.A.Perovski.Sincethen,theconceptof perovskitehasbeenexpandedtoallthecompoundsthatpossessanidenticalorsimilarcrystalstructuretothatofCaTiO3.MHPshaveatypical chemicalformulaofABX3 derivedfromtheoriginalCaTiO3 structure (Fig.1),whereArepresentseitherorganicorinorganiccationswithdifferentsizessuchasCH3NH3+ (methylammonium,MA),CH(NH2)2+ (FA),or Cs+,Bstandsformetalcations(e.g.,Pb2+ andSn2+),andXrepresents heavyhalideanions(Cl-,Br-,I-).ThefirstMHPswerepresentedbyMoller in1958andhadafullyinorganicstructure,i.e.,CsPbX3 [12].Laterin1978, organometalhybridhalideperovskiteswerediscoveredbyWeberand coworkerswithMAasA-sitecation [13].Despitesomeearlyapplications forfield-effecttransistors(FETs)andLEDsinthe1990s,MHPsdidnotinitiallycatchmuchattentionoftheresearchersowingtotheirunsatisfied deviceperformancecomparedtothatofotherorganicorinorganicsemiconductors.In2009,MiyasakaandcoworkersfirstemployedMAPbBr3 or MAPbI3 asthelightabsorberindye-sensitizedsolarcells,achievingan encouragingPCEof3.8%.Thisgroundbreakingworkquicklypromoted anenormousresearchinterestinMHPs,bothfromacademiaandindustry [14].TheskyrocketingincreaseinthePCEovertheyearshasledtovalues nowhighlycompetitivewiththoseofconventionalsilicon-basedphotovoltaics.Since2014,afewyearsafterthepioneeringsolarcellsworkby Miyasakaetal.,MHPshavebeenalsoemployedforthedevelopmentof

FIG.1 ThestandardrepresentationofacubicperovskitestructurewithanABX3 crystal structure. ReprintedwithpermissionfromQ.A.Akkerman,L.Manna,WhatDefinesaHalidePerovskite?ACSEnergyLett.(2020)604–610. https://doi.org/10.1021/acsenergylett.0c00039.Copyright (2020)AmericanChemicalSociety.
electroluminescencedevicesatroomtemperature,stemmingfromthe promisingstudybyTanandcoworkers [15] Currently,MHP-basedLEDs displayexternalquantumefficiencyvaluesof >20% [16–18].
3.WhatareMHPs?
MHPsdifferfromtheirconventionalchalcogenidecounterpartsasthe lattercontainlargebivalentcations(e.g.,Ca2+,Sr2+)attheA-site,small tetravalentcations(e.g.,Ti4+,Zr4+)attheB-site,andoxygenanionsatthe X-site.Incontrast,MHPstructuresincludemonovalentcationsatthe A-site,divalentmetalcationsattheB-site,andhalogenanionsattheX-site. Aso-calledthree-dimensional(3D)networkformsintotheABX3 perovskitestructure,inwhichthecavitybetweenfouradjacentcorner-sharing BX6 octahedraisoccupiedbyA-sitecations.BycalculatingtheGoldschmidttolerancefactor(t)andtheoctahedralfactor(μ),theprobability offormingaperovskitestructurecanbeevaluated [19] Typically, t isdeterminedbytheionicradii(r)oftheA,B,andXandisexpressedasEq. (1):
Theoctahedralfactorisdefinedas μ ¼ rB/rX,where rB and rX arethe radiiofB-siteandX-siteions,respectively.Generally,3Dnetworksofstableperovskitestructuresareformedwhen0.76 < t < 1.13.If t isoutsidethis range,otherperovskite-relatedstructures(not3D)canbealsostably formed [20].Thus,onlytheincorporationofsmallcations(e.g.,Cs+, MA+,FA+)attheA-sitecanresultintheformationofstable3Dhalide perovskitestructures.OtherpotentialalternativesattheA-siteareeither toosmall(e.g.,K+,Rb+)ortoolarge(e.g.,ethylammonium,guanidinium). SomeMHPswithaboundaryonthetolerancefactorrange,i.e.,CsPbI3 (t ¼ 0.8)andFAPbI3 (t ¼ 1),readilyinduceaphasetransitionevenatroom temperature(RT)towardmorestableorthorhombicandhexagonalphases (Fig.2A–D),respectively [21].Accordingly,thetransitedphasesareoften definedas“yellowphases” [22].ThestabilityoftheBX6 octahedraistypicallyassessedbythevalueof μ,whichisrelatedtotheradiuscomparison oftheB-siteandX-siteions.Itisempiricallyfoundthat,when μ fallsinthe rangeof0.442–0.895,stablecubicperovskitestructurescanbeformed [23]. Thus,both t and μ factorsarecommonlyusedtopredictthestabilityof newlydesignedhalideperovskitecompositions(Fig.2E).Besidesthe above-discussedABX3 stoichiometrydefininga3DnetworkofcornersharingBX6 octahedraofthetypicalMHPstructure,recently,moreand moredeviationsfromtheABX3 stoichiometryhavebeenfound,whichstill belongtothefamilyofMHPs.However,somegeneralmisunderstanding andmisuseoftheterm“halideperovskites”areacknowledgedinthe

FIG.2 Schematicrepresentativesof(A)3Dcubicstructure,observedin α-FAPbI3; (B)orthorhombicallydistorted3Dstructure,reportedforCsPbBr3;(C)one-dimensional (1D)hexagonallattice,observedintheyellowphaseofFAPbI3;(D)1Dorthorhombicstructure,foundintheyellowphaseofCsPbI3;(E)3Dand1Dstructuresreportedforvariousfully inorganicandhybridorganic-inorganicABX3 MHPs.Thelightbluesquareareahighlights theregionwherestableMHPsarelocated. ReprintedwithpermissionfromJ.Shamsi,A.S. Urban,M.Imran,L.DeTrizio,L.Manna,Metalhalideperovskitenanocrystals:synthesis,postsynthesismodifications,andtheiropticalproperties.Chem.Rev.119(5)(2019)3296–3348. https://doi.org/10.1021/acs.chemrev.8b00644.Copyright(2019)AmericanChemicalSociety.
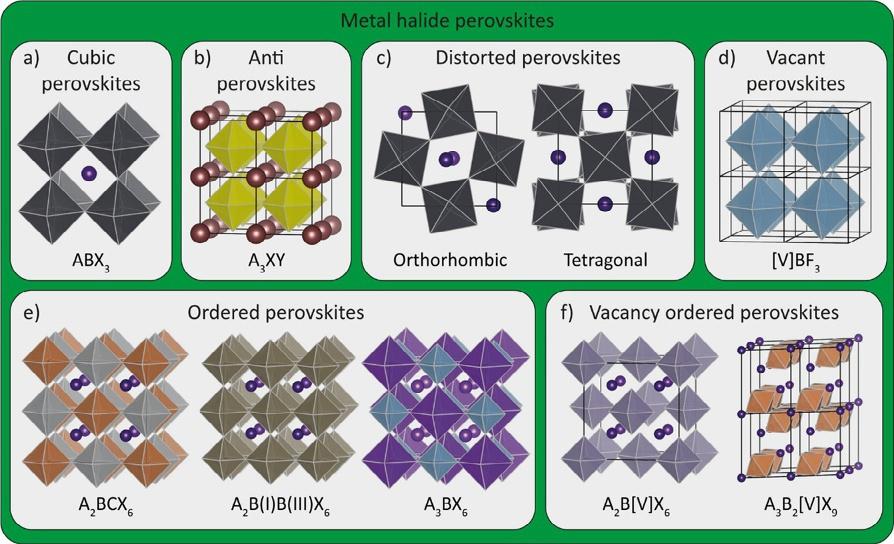
field.Thus,itisnecessarytoclearlyspecifywhichclassofmaterialscanbe termedmetalhalideperovskitesaccordingtocurrentmainstreamviewpoints. Fig3 showsanoverviewofthedifferentMHPsthathavebeen reportedintheliterature [24].
ComparedtothestandardABX3 cubicstructure(Fig.3A),theso-called halideantiperovskitescanformwhentheA-andB-sitesareoccupiedwith anions(halideandchalcogenideforAandB,respectively)andtheX-site withamonovalentcation(Fig.3B),e.g.,Li3OBrandAg3SI.Althoughthese antiperovskitesarestillclassifiedashalideperovskites,thisbookwillnot focusonthembeingthehalidenoteffectivelypartoftheBXnetworkin
FIG.3 Overviewofvariousmetalhalideperovskites.(A)StandardABX3 cubichalide perovskites,(B)Antiperovskites,(C)Commonorthorhombicandtetragonaldisordered perovskites,(D)VacantBX3 perovskites,(E)Orderedperovskites,(F)Vacancy-ordered perovskites. ReprintedwithpermissionfromQ.A.Akkerman,L.Manna,WhatDefinesaHalide Perovskite?ACSEnergyLett.(2020)604–610. https://doi.org/10.1021/acsenergylett.0c00039.Copyright(2020)AmericanChemicalSociety.
thatcase.ThesizedifferencebetweentheA-,B-,andX-siteionsresultsina distortedlattice.Thesedistortionscaninducethesingleoctahedratotilt, leadingtoeitherorthorhombicortetragonalcrystalstructures(Fig.3C). WhenABX3 stoichiometryispartiallyorfullyreplacedorevenremoved forA-and/orB-sitecations,somemorehalideperovskitederivativescan beobtained. Fig.3Dshowsaso-calledvacantBX3 crystalstructurewitha completelyvacantA-sitecation,whichisonlyapplicableinthecaseof fluorides(e.g.,AlF3 andMnF3) [25].Inaddition,anotherderivativeof theABX3 perovskite,namelyorderedperovskite,canbeattainedbyreplacingtheB-sitecationwithacombinationoftwocationsthatarepositioned atparticularcrystallographicsites(Fig.3E) [26].Thisstructureisoften termed“A2BCX6”or“A2BB’X6,”anditiscommonlycalled“double perovskite”duetothefeatureddoubleoccupancyattheB-sitecation. Recently,thistypeoforderedperovskiteshasbeenintensivelyinvestigatedasalead-freecandidate,e.g.,Cs2AgBiBr6,Cs2AgInCl6,and MA2AgSbI6 [26]. Fig.3Fshowsthelasttypeofhalideperovskites,namely vacancy-orderedperovskites,whicharesimilartoorderedperovskites, butavacancyisinvolvedtopartiallyreplacetheB-sitecation.Themost widelyinvestigatedsubgroupofthevacancy-orderedperovskiteshas anA2BX6 stoichiometry(e.g.,Cs2BX6).Inthiscase,theB-sitecationsarehalf
occupiedbytetravalentmetals(M(IV))andanotherhalfoccupiedbyvacancies(occupiedratioof1:1),e.g.,Cs2SnI6,Cs2PdBr6,andCs2TiBr6 [27,28]. Anothersubgroupofvacancy-orderedperovskitesincludesthecombinationoftrivalentmetals(e.g.,Sb3+ andBi3+)witharatioof2:1between thevacancyandtheoccupiedB-sitecations,e.g.,Cs3Sb2[V]I9 [29].More interestingly,byaddingonemoredivalentmetalcation(e.g.,Cd2+ or Cu2+)intothesevacancy-orderedperovskites,theproportionofvacancies canbefurtherreducedto25%,resultinginCs4CdSb2Cl12 orCs4CuSb2Cl12 (A4BC2[V]X12),namelyvacancy-orderedtripleperovskites [30,31].Ingeneral,thistypeofvacancy-orderedperovskitesshowpoorconductivity, owingtothehighdensityofvacanciesintheperovskitestructureandthus leadingtorestrictedapplications,i.e.,inthefieldsofphotovoltaics andLEDs.
4.ChemistryofMHPs
MostMHPstructuresareheldtogetherbyionicbonding,whichisone ofthemaincausesfortheeasyformationofhighlycrystallinestructures evenatroomtemperature.Thesesoftioniccrystalsarepronetodefects andsusceptibletostructuraldisorderatthesurface.However,thehigh defecttoleranceofMHPisthekeyresponsiblefortheircapabilityto behaveashigh-performingsemiconductormaterials.Thiscanenable themtomaintaintheelectronicstructureintheirpurestates,despite theexistenceofahighdefectdensity.Thefirst-principlesdensityfunctionaltheory(DFT)calculationsareacommonandreliabletoolto simulatethedefecttolerance,inparticularfortheformationenergyof pointdefectsandtheirassociatedeffectontheelectronicstructure [32]. TheuniquenatureoftheelectronicstructureofMHPsresultsinoneof themostattractiveproperties,i.e.,theiropticaltunability.Asanexample, theformationofenergeticbandsinaleadiodide-basedperovskiteis shownin Fig.4.ThehybridantibondingorbitalsofthePb6p orbitals andtheouter p orbitalsofthehalide(3p forCl,4p forBr,and5p forI)form theconductionbandoftheperovskite,exhibitingamore p-likestructure owingtothemajorcontributionfromtheleadsidewithahighdensityof states(DOS) [33].Similarly,thehybridantibondingstatesofthePb6s and theidenticalhalide p orbitalstogetherformthevalenceband.ThisfeaturedelectronicstructureofMHPsisdifferentfromthatofconventional semiconductormaterials,i.e.,galliumarsenide(GaAs),withtheirbandgapsforminginbetweenbondingandantibondingorbitals [25].Consequently,thevalencebandedgecanshiftatvariousenergylevelsasthe halidecomponentvaries(Cl,Br,andI).However,theirconductionband edgeshiftsrestrictedly.IncontrasttotherolesofB-siteandX-siteintuning electronicstructure,A-sitecationplaysaminorroleinbothchangingthe

FIG.4 Formationofenergeticbandsinaleadiodideperovskitethroughthehybridization ofleadandiodideorbitals. ReprintedwithpermissionfromJ.Shamsi,A.S.Urban,M.Imran,L.De Trizio,L.Manna,Metalhalideperovskitenanocrystals:synthesis,post-synthesismodifications,and theiropticalproperties.Chem.Rev.119(5)(2019)3296–3348. https://doi.org/10.1021/acs.chemrev. 8b00644.Copyright(2019)AmericanChemicalSociety.
conductionandvalencebands(accordingtoitscorrespondingDOS)and tailoringthebandgapoftheperovskite [34].
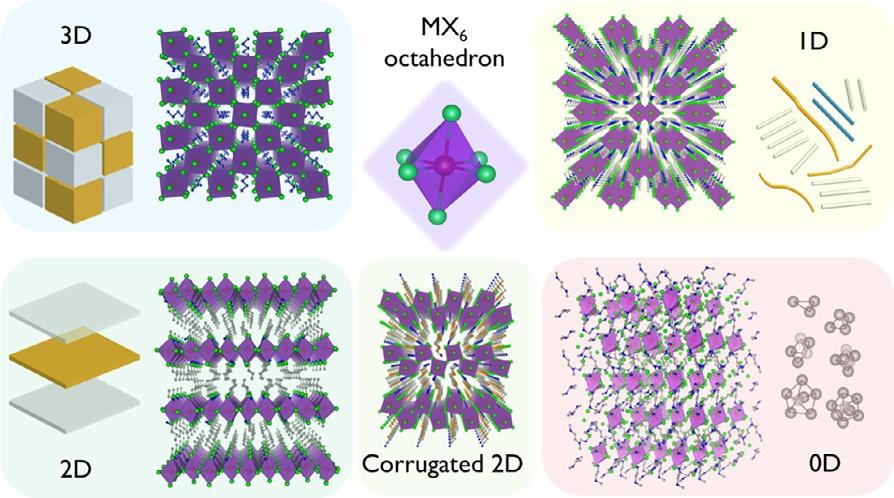
5.DimensionalityofMHPs
MHPscanbeclassifiedinto3D,quasi-2D,2D,1D,and0Dbasedonthe metalhalideoctahedraconnectivityintheircrystalstructures,asshownin Fig.5[35].Perovskiteswithdimensionalitylowerthan3Dconsistof corner-,edge-,orface-sharingBX6 octahedraandarederivedfromthereference3DABX3 structure.Itisworthnotingthatthedimensionalityhere discussedhasnothingtodowiththenanostructuredmorphologywith spatialconfinementindifferentdirections(e.g.,quantumdots,nanoplatelets/nanorods,nanowires,andnanocubes).
FIG.5 Classificationofmetalhalideperovskitesbasedonthemetalhalideoctahedraconnectivity.The2D,1D,and0Dperovskitescanbeassumedasbulkassembliesof2Dquantum wells,1Dquantumwires,and0Dclusters,respectively. ReproducedwithpermissionfromH. Lin,C.Zhou,Y.Tian,T.Siegrist,B.Ma,Low-dimensionalorganometalhalideperovskites.ACS EnergyLett.3(1)(2018)54–62. https://doi.org/10.1021/acsenergylett.7b00926
The2Dlayeredperovskitescanbeassumedasone-layerthickBX6 octahedralsheetscutfrom3DABX3 perovskitealong <100 >, < 110 >,and < 111 > crystallographicdirections,whicharelinkedwitheachothervia bulkyorganiccations.Theirgeneralformulais(RNH3)2An-1BnX3+1,where Ristheorganiclinkerand n representsthenumberofBX6 layers.For instance,atomicallythin2D(C4H9NH3)2PbX4 crystalswith n ¼ 1and R ¼ C4H9 wereobtainedbyfollowingasolution-basedprotocol [36].The thicknessofthesemonolayerperovskitesheetswasfoundtobe1.6 0.2 nm,where 0.6nmcorrespondstothelengthofthesingleBX6 layer andtheorganiclinkerspresentonbothsidesoftheinorganiclayercontributetotheremainingthickness.Thethicknessof2Dlayeredperovskites increasesbyincreasingthenumberofBX6 layersintheinorganicframework.Also,theorganicconstituentcomprisesamonolayerorbilayerof organiccations.Forinstance,inthecaseofmonoammoniumion(monolayer),NH3+ functionalgroupbindstothehalogensinoneinorganiclayer, andtheorganic(hydrophobic)groupextendsintothespacebetweenthe inorganiclayers.ThevanderWaalsinteractionbetweenthetwoorganic moietieskeepstheBX6 layerstogether.When n valueapproaches ∞,the formationofcubic3Dhalideperovskites,suchasMAPbI3 orCsPbI3,takes place.Intermediate n values(n ¼ 10,20,.…)producequasi-2Dperovskites, suchasRuddlesden-Popper(RP)structures.Inthiscase,theinorganic frameworkconsistsofafew3DABX3 layers,whichareseparatedbythe
alkylammoniumcations(vanderWaalsgap),which,inturn,forma2D+3D (quasi-2D)layeredperovskitestructure(A2BX4-type).Ontheotherhand, organiclinkerswithtwoalkylammoniumgroupsatbothendsform Dian-Jacobson(DJ)ABX4 structure [37].Forexample,while(PDA) (MA)3Pb4I13 adoptstheDJstructure,(PA)2(MA)3Pb4I13 crystallizesinRP structure [37].Otherwell-knownexamplesofRPandDJphasesare (BA)2(MA)2Pb3I10 and(3AMP)(MA)2Pb3I10,respectively [38].InRPstructures,thevanderWaalsgapswithmonoammoniumcationspromoteweak interactionsbetweentheABX3 layers,destabilizingthelayeredperovskite structurewhencomparedtotheDJstructures.DJperovskitesnotonlydisplaybetterstabilitybutalsoleadtohighersolarcellefficienciesthanthecorrespondingRPstructures [37].
The2DperovskitescanbefurtherscissoredinthedirectionperpendiculartoBX6 sheetstoensurethatthemetalhalideoctahedraremainincontactwitheachotheronlyalongoneaxis.Thisproduces1Danalogs.Onlya few1Dperovskiteshavebeenreportedsofarduetotheirlimitedoptoelectronicefficiencies.Thefirstknownfamilyof1Dhalideperovskites,[NH2C (I)¼NH2]2(CH3NH3)mSnmI3m+2,wassynthesizedinthe1990sbyMitziand coworkers [39].These2Dstructuresbreakdownintoa1Dstructure,with cornersharingoftiniodideoctahedrawhen m ¼ 1 [40].Pb-based1D perovskites,suchasC4N2H14PbBr4,arealsoknown [41].Ifthe1Dperovskitesarefurtherslicedtoobtainmetalhalideoctahedrafullyisolated fromeachother,theresultingstructuresaretermed“0Dmetalhalides.” Thisclassofstructuresiswellknownfortheirnear-unityphotoluminescenceefficienciesandrobustness,whichledtothediscoveryofaplethora oforganic-inorganicandall-inorganic0Dmetalhalides [42,43].Thetype ofcationalsoinfluencesthegeometryofthemetalhalide.Forinstance, Cu+ ionsform0Dhalidewithcopperhalidetetrahedra,whilePb2+, Sn2+,andMn2+ mostlyformstructureswithmetalhalideoctahedra [44].Withanappropriatechoiceoftheorganiclinkerandmetalhalide, low-dimensionalperovskitewiththedesireddimensionalitycanbe designed.Theexistenceofalargelibraryoforganicmoleculesthatcan fitintothevanderWaalsspaceoflow-dimensionalorganic-inorganic perovskitesmayoffertheopportunitytofurtherengineerthestructural andoptoelectronicpropertiesofthesematerials.
Tosynthesizehigh-qualitylow-dimensionalhalideperovskitefilms, themostcommonprotocolincludesaconventionalone-stepspincoating; metalandorganichalidesdissolvedinasolventaredepositedonasubstrateunderspinning [45].Inamodifiedhot-castingmethod,thesubstratesarepreheated(70-150 oC)justbeforethespin-coatingprocess [46].Insomecases,antisolvent(solventthatdissolvesthehalideprecursorsbutnottheperovskitefilm)isdrippedontothespinningsubstrate rightafterapplyingtheprecursorsolutiontoacceleratetheperovskite crystallizationbyinducingarapidsupersaturationstateinthethinfilm.
Anotherwidelyemployedapproachisthesubsequentialdepositionof metalhalideandorganichalide(two-stepmethod) [47].Low-dimensional perovskitesinglecrystalshavebeengrownusingvarioustechniques,and oneofthemisthetemperature-coolingmethod.Forexample,toprepare (CH3(CH2)3NH3)2(CH3NH3)n 1PbnI3n+1 perovskitecrystals,butylamineis addedintoaboilinghydroiodic(HI)acidsolutioncontainingtheremainingprecursorstoformatransparentyellow-coloredsolution.Thelayered perovskitecrystalsareprecipitatedoutofthesolutionuponcoolingit downtoroomtemperature [48].
5.1Stabilityoflow-dimensionalperovskites
Oneofthedrivingpointsforexploitinglow-dimensionalorganicinorganicperovskitesforoptoelectronicapplications,particularly2D andquasi-2Dstructures,istheirstrongresistancetomoisturedegradation.OneoftheearlyreportsbyKarunadasaandcoworkersdemonstrates theenhancedmoisturestabilityof2Dlayeredperovskitewhenusedasthe photo-absorbinglayerinsolarcells [45].Theyfoundthatfilmsof (PEA)2(MA)2[Pb3I10](PEA ¼ C6H5(CH2)2NH3 +,MA ¼ CH3NH3 +)are moremoisture-resistantthanMAPbI3 films,and,thus,solarcellsusing 2Dfilmscanbefabricatedevenunderhighhumidenvironment.ThefollowingreportbyLiaoetal.comparedthestabilityof3DMAPbI3 and CsPbI3 withthatofalow-dimensionalBA2CsPb2I7 (BA ¼ butylammonium; n ¼ 2)perovskite(Fig.6) [49].IntheXRDpatternoftheCsPbI3 film, thedegradationpeaksstartedappearingafter30minofairstorage.Similarly,MAPbI3 partiallydecomposestoPbI2 after48h.Onthecontrary,the BA2CsPb2I7 filmdisplayedsuperiorstabilityintheairforamonthunder 30%relativehumidity.TheBA2CsPb2I7 filmexhibitedthermalstability also.Moreover,BA2CsPb2I7-basedsolarcellsretained92%oftheirinitial efficienciesunderthesamestorageconditions.Thehydrophobicnature oflongorganiccations(linkermolecules)ofthelow-dimensional perovskitesprovidesagreatrepulsionfromthemoisture.
Also,thegeometryofthespacercationsaffectsthestabilityoflowdimensionalperovskites.Forinstance,Chenetal.comparedthestability andsolarcellefficiencywhenthespacercationchangedfromlinear-BA toiso-BA [44].The2Dperovskite(n ¼ 4)withthebranchedspacer ((iso-BA)2(MA)3Pb4I13)displaysbetterstabilityovertheonewithalinear spacercation((BA)2(MA)3Pb4I13)bypreservingitsinitialopticalabsorptionafterstorageforoveramonthat20°Cwitharelativehumidityof 60%.Here,thereducedseparationbetweentheinorganic(ABX3)layers withshorteriso-BAunitsmightbethereasonfortheimprovedstability of(iso-BA)2(MA)3Pb4I13.AbetterchargetransportfromreducedABX3 layerseparation,out-of-planecrystalorientation,andbettercrystallinity

FIG.6 XRDpatternsofthinfilmsof(A)CsPbI3,(B)BA2CsPb2I7 storedunder30%RH,(C)MAPbI3,and(D)BA2CsPb2I7 storedat85°Cinaninert environmentfor72h. ReproducedwithpermissionfromJ.-F.Liao,H.-S.Rao,B.-X.Chen,D.-B.Kuang,C.-Y.Su,Dimensionengineeringoncesiumleadiodidefor efficientandstableperovskitesolarcells,J.Mater.Chem.A.5(2017)2066–2072. https://doi.org/10.1039/c6ta09582h.
of(iso-BA)2(MA)3Pb4I13 ensuredamaximumPCEof10.63%insolarcells. Notethat,thoughlow n valuesproducelow-dimensionalperovskiteswith superiorstability,however,theirPCEvaluesarestilllowerthanthoseof 3Dperovskites.
Thestabilityof3Dperovskitescanbeenhancedbymakinga2D/3D interface.Grancinietal.mixedalongorganiccation,(HOOC(CH2)4NH3)2 (AVA),with3DMAPbI3 toformahighlystableAVAI/MAPbI3 perovskite junction(quasi-2Dwithlarge n) [50].This2D/3Dperovskitesolarcell maintainednearly60%ofinitialPCEafter300hofcontinuousAM 1.5Gilluminationunderargonatmosphere,whichprovesitshigher stabilitythandevicesemployingthestandard3DMAPbI3.The10x 10cm2 2D/3Dsolarmodulesobtainedviaafullyprintableindustrial-scale processdelivered11.2%efficiency,andtheywerestableformorethan 10,000hwithnolossintheefficiencymeasuredundercontrolled (standard)conditions,showingthestabilityenhancementduetothe2D componentofthe2D/3Dperovskite.Similarly,CA2PbI4/MAPbIxCl3 x (CA ¼ cyclopropylammonium)2D/3Dfilmexhibitedsuperiorstability: nodegradationwasobservedfor40dayswhenstoredatarelativehumidityof63%.Ontheotherhand,3DMAPbIxCl3 x fullydegradedafter8days underthesamestorageconditions [51].Inanotherreport,BAcations wereintroducedintoadouble-cationleadmixed-halideFA0.83Cs0.17Pb (IyBr1 y)3 3Dperovskite.Asaresult,layered2Dcrystalliteswereembeddedbetween3Dperovskitegrainsandproducedaquasi-2D(2D/3D) structure [52].AnoptimalBAconcentrationensured17.5 1.3%solarcell efficiencyfora1.61eVbandgapperovskite.Thesolarcellslostonly20%of theirPCEafter1000hunder1sunilluminationintheair.Sincetheexciton bindingenergyvaluesofquasi-2D(2D/3D)perovskitesareclosetothose of3Dperovskites,theyhaveahigherpotentialtogenerateefficientand stablesolarcellsthantheir2Dcounterparts.
Theadditionof2Dperovskiteshasbeenknowntoimprovethephase stabilityof3Dperovskitesaswell.Forinstance, α-CsPbI3 (or γ-CsPbI3) filmswithasuitablebandgap(1.73eV)fortandemsolarcellsareknown todegradeinstantaneouslyintoundesirable δ-CsPbI3 phaseunderambientenvironment.Zhangetal.showedthattheadditionofasmallamount of2DEDAPbI4 (EDA ¼ ethylenediaminecation)perovskiteinto3D CsPbI3 preventsitstransformationintothenonperovskite δ-CsPbI3 phase [53].Theenhancedphasestabilityof3DCsPbI3 wasascribedtothe reducedcrystallitesizeofCsPbI3 0.025EDAPbI4 (2D+3D)perovskite andtheunique(110)layeredstructureofthe2DEDAPbI4.Veryrecently, intact2D/3Dhalidejunctionperovskitesolarcellswitharecordand certifiedsolarcellefficiencyof24.35%werereported [54].Athickand highlycrystalline2D(C4H9NH3)2PbI4 filmwasgrownontopof3D (FAPbI3)0.95(MAPbBr3)0.05.Theseintact2D/3Dhalidejunctionsolarcells retained94%and98%oftheirinitialefficienciesafter 1000hat85°Cwith
arelativehumidityof85%andunder1sunillumination,respectively. Suchoutstandingthermalandmoisturestabilitiesofthedevicewere achievedviaencapsulationwith2D(C4H9NH3)2PbI4.Both2Dand quasi-2Dperovskitesexhibitbettermoisturestabilityduetothepresence ofexcesshydrophobicity,comingfromthelongorganicspacercations. However,theirthermalstabilityisreduced.Therefore,theincorporation ofinorganiccations,e.g.,Cs,in2Dor2D+3Dperovskites,improvestheir thermalstability.All-inorganic0Dmetalhalidesarewellknownfortheir robustthermalstabilities [55].
5.2Opticalpropertiesoflow-dimensionalperovskites
Animportantparameteroflow-dimensionalperovskitesistheirhigh excitonbindingenergy,whichisintheorderofafew100meVs,hence typicallyhigherthanthatof3Dperovskites.Forinstance,theexciton bindingenergyvaluesof2D(PEA)2(MA)n-1[PbnI3n+1]for n ¼ 1and n ¼ 2are220and170meV,respectively,whicharemuchhigherthanthat ofthe3DMAPbI3 perovskite(40meV) [45].Theexcitonbindingenergyof low-dimensionalperovskitesdependsonthedifferencebetweenthe dielectricconstantsofthespacercation(barrier)andtheinorganicframework(quantumwell),andthisisknownasthedielectricconfinement effect.Theinorganicframeworkhasahigherdielectricconstant( 6.1) comparedtothecationspacers(dielectricconstant 2.1).Organic spacercationswithadifferentdielectricconstantcanmodifythebinding energyvalue.
Theexcitonbindingenergyvaluesincreasewhendecreasingtheperovskitedimension;0DCs4PbBr6 hasanexcitonbindingenergyvalueof 375 meV [55].Thisshowsthattheexcitonsinlow-dimensionalperovskitesare stronglybound;i.e.,theyareFrenkelexcitons.Ontheotherhand,the bindingenergiesofquasi-2Dperovskites(forlarge n; n ¼ 40,50,60, …) aresmallerthanthoseofotherlow-dimensionalperovskitesandthus canbesuitableforachievinghighsolarcellefficienciessimilarlytothose of3Dperovskites [56].While2Dandquasi-2Dperovskitesaresuitablefor photovoltaicapplications,stronglyconfined2D(low n values),1D,and0D perovskiteshavethepotentialtoperformefficientlyinLEDs.
Sincethespacercationsusedinlow-dimensionalperovskitesareopticallyinertwhencomparedtotheinorganicsheets,theelectronic(optical) transitionenergies,andparticularlythebandgapofthesematerials,are determinedbythenumberoftheBX6 layersintheinorganicframework. Theopticalbandgapusuallyincreaseswhendecreasingthenumberof layersordimensionalityoftheperovskitematerial,duetoincreased quantumconfinement.Forinstance,Stoumposetal.synthesizedaseries of2D(CH3(CH2)3NH3)2(CH3NH3)n 1PbnI3n+1 perovskiteswithvarying