Aquaculture
journal homepage: www.elsevier.com/locate/aquaculture
Differences in composition and fatty acid contents of different rainbow trout (Oncorhynchus mykiss) strains in similar and contrasting rearing conditions
Michail I. Gladyshev a, b, * , Alexander A. Makhrov c , Nadezhda N. Sushchik a, b , Olesia N. Makhutova a, b , Anastasia E. Rudchenko b , Dmitrii A. Balashov d , Evgenii V. Vinogradov d , Valentina S. Artamonova c
a Institute of Biophysics of Siberian Branch of Federal Research Center Krasnoyarsk Science Center” of Russian Academy of Sciences, Akademgorodok, 50/50, Krasnoyarsk 660036, Russia
b Siberian Federal University, Svobodny av. 79, Krasnoyarsk 660041, Russia
c A. N. Severtsov Institute of Ecology and Evolution of Russian Academy of Sciences, Leninsky prospect, 33, Moscow 119071, Russia
d Branch for the Freshwater Fisheries of VNIRO (VNIIPRKh), Rybnoe 141821, Russia
ARTICLE INFO
Keywords:
Eicosapentaenoic acid
Docosahexaenoic acid
Marker fatty acids
Nutritive value
ABSTRACT
Fatty acid (FA) composition and contents of fillets of seven strains of rainbow trout Oncorhynchus mykiss, reared in the same farm and sampled at the same time were studied. Three strains were compared with those from another farm as well as with outbreed and wild fish of the same fish species. In general, FA composition of farmed fish reflected their diet composition. Nevertheless, a genetics factor (belonging to a certain strain), also was found to contribute to fish FA composition. Rainbow trout of strain Steelhead appeared to have comparatively higher ability to regulate their FA composition, in particular, to retain high contents of physiologically important eicosapentaenoic (20:5n-3, EPA) and docosahexaenoic (22:6n-3, DHA) acids in biomass despite variations of their diet contents. Differences in contents of the EPA + DHA of different strains, found in this study, likely provided evidence for a potential of selective breeding of rainbow trout strains with a high EPA and DHA, which are of key importance for humans’ diet. Besides, FA markers, which allow differentiating wild and farmed rainbow trout, were revealed.
1. Introduction
Long-chain polyunsaturated fatty acids of omega-3 family (LCPUFA), namely eicosapentaenoic (20:5n-3, EPA) and docosahexaenoic (22:6n-3, DHA) acids have been recognized as essential components of human diet, which provide numerous health benefits, including prevention of cardiovascular diseases (Calder, 2018; Bernasconi et al., 2021). The principal dietary source of EPA and DHA for humans is fish (Robert, 2006; Tacon and Metian, 2013; Gladyshev et al., 2013, 2015; Tocher et al., 2019). Fish, reared in aquaculture, such as rainbow trout Oncorhynchus mykiss have very high contents of EPA and DHA in their edible parts – muscle tissue (filets), which determines its high nutritive value for humans (Saglik Aslan et al., 2007; Stone et al., 2011; Turchini et al., 2011, 2018).
However, in last decades, a tendency of decreasing LC-PUFA content in aquaculture of salmonids, including rainbow trout has occurred because of replacing deficient fish oil, obtained from finite wild catch by vegetable oils (Naylor et al., 2009; Turchini et al., 2009; Sprague et al., 2016; Barry and Trushenski, 2020; Qian et al., 2020; Quinones et al., 2021).
One of the reasonable ways to support the high nutritive value of fish species, reared in aquaculture, is a selection of strains that can have the high contents of LC-PUFA in their biomass in spite of the increased levels of plant oils in the feed (Naylor et al., 2009; Bell et al., 2010; Leaver et al., 2011; Turchini et al., 2011; Shepherd and Jackson, 2013; Henriques et al., 2014; Gladyshev, 2021; Gladyshev et al., 2022). However, this selection could be successful only in a case of a high heritability of LC-PUFA contents in muscle tissue.
* Corresponding author at: Institute of Biophysics of Siberian Branch of Federal Research Center “Krasnoyarsk Science Center” of Russian Academy of Sciences, Akademgorodok, 50/50, Krasnoyarsk 660036, Russia.
E-mail address: glad@ibp.ru (M.I. Gladyshev).
https://doi.org/10.1016/j.aquaculture.2022.738265
Received 16 February 2022; Received in revised form 9 April 2022; Accepted 13 April 2022
Availableonline19April2022
0044-8486/©2022ElsevierB.V.Allrightsreserved.
In literature, there are data on differences in composition of LC-PUFA between anadromous and landlocked Atlantic salmon (Salmo salar L.), reared in similar conditions (Peng et al., 2003; Rollin et al., 2003; Betancor et al., 2016). Indeed, n-3 LC-PUFA contents in muscle tissue of this species is a highly heritable trait, h2 = 0.77 ± 0.14 (Leaver et al., 2011; Horn et al., 2018), and there are differences in this trait between different family groups of Atlantic salmon (Bell et al., 2010). Thus, there is a potential of selective breeding of this species to increase LC-PUFA contents in its muscle tissue (Horn et al., 2018).
In contrast, there is no data on the heritability of LC-PUFA contents in muscle tissue of another important aquaculture species, O. mykiss, as well as data on differences of LC-PUFA contents between strains of this species. Thus, the aim of our study was a comparison of LC-PUFA contents in muscle tissue of different strains of O. mykiss. We compared different strains, reared both in similar and different conditions in three fish farms from contrasting regions of Russia. Besides, LC-PUFA content of a wild population of O. mykiss was measured for comparison.
2. Materials and methods
2.1. Sampling
The study was performed in three reputable Russian fish farms, contrasting in rearing conditions. Farm 1 was situated in the zone of the northern taiga; fish were reared in cages, placed in a large oligotrophic lake. Samples from Farm 1 were taken in November 31, 2018 and in May 19, 2019. Farm 2 was situated in the subtropical zone; fish were reared in concrete pools where water was pumped from artesian wells. Farm 2 provides fry planting material for many trout farms in Russia, including Farm 1. All samples from Farm 2 were taken in January 31, 2019. Farm 3 was situated in mountains; fish were reared in concrete pools where water was pumped from a river. Fish from Farm 3 were outbreed. Samples from Farm 3 were taken in November 03, 2019.
In the farms, specimens of rainbow trout O. mykiss of average mass 261 ± 22 g were sampled, which belonged to seven strains, common in Russian farms: Adler, Adler Amber, Steelhead, Kamloops, Augustin, Donaldson and late spawning Steelhead. A history of breeding of these strains was reviewed by Artamonova et al. (2016) In general, 82 samples of farm-raised fish were collected in three farms. Species identity of these fish were confirmed using microsatellite methods (Artamonova et al., 2016). In Farm 1, Farm 2 and Farm 3, commercial feeds, delivered by three reputable international producers, were taken, designated below as Diet 1, Diet 2 and Diet 3, respectively. Ingredients and
Table 1
Ingredients and proximate composition of three commercial diets fed to rainbow trout Oncorhynchus mykiss.
Diet 1 Diet 2 Diet 3
Ingredients Fishmeal, soy protein meal, pea protein meal, wheat gluten meal, fish oil, poultry fat, rapeseed oil, linseed oil, vitamins, minerals
Proximate composition
Fishmeal, soy protein meal, wheat gluten meal, fish oil, rapeseed oil, vitamins, minerals
Fishmeal, soy protein meal, wheat gluten meal, fish oil, rapeseed oil, vitamins, minerals
proximate composition of the diets, reported by producers, are given in Table 1.
Besides, five specimens of wild O. mykiss of 80–740 g (average mass 258 g) were caught in the Bystraia River (western part of Kamchatka Peninsula). Species identity of these fish was confirmed on the basis of sequencing of mitochondrial gene COI (Kirillova et al., 2021).
Fish were caught, euthanized using tricaine methane-sulfonate (MS222), 500 mg L 1 (Topic Popovic et al., 2012), and sampled in accordance with Federal Rules, including State Standard of Russian Federation, 33215-2014, 2016-07-01, and the protocol approved by the Institutional Animal Ethical Committee on Biomedical Ethics of Siberian Federal University (Russia).
For biochemical analyses, slices of fish white muscles of approximately 1.5–2 g, 2–3 cm below the dorsal fin were cut from an each specimen. The muscle samples were immediately placed into a volume of 3 mL of chloroform/methanol (2:1, by vol.) and kept until laboratory analysis at 20 ◦ C.
2.2. Fatty acid analyses
Fatty acid analyses are given elsewhere (Gladyshev et al., 2020). Briefly, lipids were extracted simultaneously with mechanical homogenization with chloroform/methanol mixture (2:1, v/v) three times. The dried lipids were hydrolysed under reflux at 90 ◦ C in a portion of methanolic sodium hydroxide solution (8 mg/mL). Then the mixture was added with an excess methanolic solution of 3% sulphuric acid and refluxed at 90 ◦ C for 10 min to obtain fatty acid methyl esters (FAMEs). The mixture was washed twice with portions of NaCl saturated solution, and FAMEs were extracted with a portion of hexane. FAMEs were analyzed with a gas chromatograph equipped with a mass spectrometer detector (model 7000 QQQ, or model 6890/5975C Agilent Technologies, USA) and 30-m long, 0.25-mm internal diameter capillary HP-FFAP columns installed in the both instruments. Detailed descriptions of the instrumental conditions are given in (Gladyshev et al., 2020). Data were collected and analyzed using MassHunter or Chemstation Software (Agilent Technologies, USA). Peaks of FAMEs were identified by their mass spectra, comparing them to those in the integrated database NIST 2008 MS LIB (Revision Jan2010) and to those in the standard 37-FAMEs mixture (U-47885, Supelco, USA). The mixture was also allowed to estimate response factors for various FAMEs on the two instruments. FAMEs were quantified according to a peak area of the internal standard, 19:0-FAME (Sigma-Aldrich, USA), which was added to samples prior to the lipid extraction, after addition of the first portion of chloroform/methanol mixture.
2.3. Statistical analysis
Standard errors (SE), Kolmogorov-Smirnov one-sample test for normality DK-S, ANOVA with Tukey HSD post hoc test, Kruskal-Wallis test, Pearson’s correlation coefficient and multivariate discriminant analysis (MDA) (Legendre and Legendre, 1998) were calculated conventionally, using STATISTICA software, version 9.0 (StatSoft, Inc., USA).
3. Results
Fatty acid composition of diets, i.e., mean values of levels of prominent FAs (percent of total, ≥ 0.1%) are given in Table 2 Diet 1 had the highest mean levels of 16:1n-7, 18:0, 18:2n-6 and 18:3n-3, but the lowest levels of ∑15-17BFA (sum of branched fatty acids with 15 and 17 carbon atom chains), ∑16PUFA (sum of polyunsaturated fatty acids with 16 carbon atoms), 18:1n-7, 18:4n-3, ∑20:1, 20:5n-3 and 22:5n-3 (Table 2). Diet 1 also tended to have a comparatively lower level of ∑22:1 (Table 2). Diet 2 had the highest levels of 15:0, 17:0, 20:5n-3, 22:5n-6 and especially 22:6n-3, but the lowest levels of 16:1n-7, 18:0, 18:1n-9 and 18:3n-3 (Table 2). Diet 3 had the highest levels of ∑15-
Table 2
Mean values of percentages (% of total fatty acids ± standard error) and total contents (Total FA, mg g 1 of dry weight) of fatty acids and sum of eicosapentaenoic and docosahexaenoic acids (EPA + DHA, mg g 1 of dry weight) in three diets, used in the three trout farms (n – number of samples). Normally distributed variables are compared by ANOVA and Tukey HSD post hoc test; the other variables marked with* are compared by Kruskal-Wallis test. Means labelled with the same letter are not significantly different at P < 0.05 according to the relevant test. When ANOVA or Kruskal-Wallis test are insignificant, letter labels are absent.
17BFA, ∑16PUFA, 18:1n-9, 18:1n-7 and ∑20:1, but the lowest level of 16:0 (Table 2). Diet 2 had the lowest total content (mg∙g 1 of dry weight) of fatty acids (Table 1). Diet 1 had the lowest contents (mg∙g 1) of EPA + DHA (Table 2).
The multivariate discriminant analysis (MDA), demonstrated significant differences in the FA compositions of wild and farmed trout from different farms (Fig. 1; Table 3). Root 1 discriminated best fish of Adler and Adler Amber strains of Farm 1 from wild fish and fish of
Table 3
Results of multivariate discriminant analysis of the fatty acid composition (% of total FAs) of muscle tissue of wild and farmed rainbow trout of different strains.
Fig. 1. Scatterplot of canonical scores for the two discriminant functions, Root 1 and Root 2, after multivariate discriminant analysis (MDA) of the fatty acid composition (% of total FA): open circles – Farm 1, strain Adler, open squares –Farm 1, strain Adler Amber; open triangles – Farm 1, strain Steelhead; filled circles – Farm 2, strain Adler; filled squares – Farm 2, strain Adler Amber; filled triangles – Farm 2, strain Steelhead; filled diamonds – Farm 2, strain Kamloops; filled star – Farm 2, strain Augustin; open star – Farm 2, strain Donaldson; cross – Farm 2, strain late spawning Steelhead; open diamonds – Farm 3, outbreed; oblique cross – wild fish. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Steelhead and late spawning Steelhead strains of Farm 2 (Fig. 1; Table 3). Variables that gave the highest contribution to the first discriminant function (Root 1) were 18:2n-6 and 18:1n-9, on the one hand, and 20:5n-3 and 22:6n-3, on the other (Table 3). Root 2 discriminated best fish of Augustin and Kamloops strains of Farm 2 from wild fish (Fig. 1; Table 3). These differences in Root 2 were primarily due to the contributions of 20:3n-6 and 18:2n-6 vs. 17:0, 16:1n-7 and 20:5n3 (Table 3).
Average contents of EPA + DHA varied from 1.31 ± 0.20 mg g 1 in wild fish to 2.35 ± 0.09 mg g 1 in fish from Farm 1, strain Adler Amber (Fig. 2). All fish from Farm 1, as well as fish of strain Steelhead and strain late spawning Steelhead from Farm 2, and outbred fish from Farm 3 had significantly higher contents of EPA + DHA than the wild trout (Fig. 2). Fish of strain Adler Amber from Farm 1 had significantly higher contents of EPA + DHA than fish of strains Adler, Augustin and Kamloops from Farm 2 (Fig. 2). Average content of EPA + DHA for all farmed trout was 2.03 ± 0.05 mg g 1
Three strains, Adler, Adler Amber and Steelhead, that were reared both in Farm 1 and Farm 2, were compared using two-way ANOVA regarding a number of FAs, revealed by MDA as principal contributors to the differences between farms and strains. In most cases, effects of the farms, the strains and their combinations on FA levels (%) and contents (mg g 1), were statistically significant, except effects of farm × strain combinations on EPA and DHA contents, and the effect of strains on DHA content (Table 4). Means, related to the significant effects, are given in Fig. 3 Fish, reared in Farm 1, had significantly higher levels of linoleic acid (18:2n-6, LA) than fish from Farm 2 (Fig. 3). Fish of strain Steelhead had significantly lower level of LA than fish of two other strains (Fig. 3). The highest levels of LA were characteristic of fish of strains Adler and Adler Amber from Farm 1, while the lowest levels of this FA were characteristic of fish of strains Adler Amber and Steelhead from Farm 2 (Fig. 3). In contrast, mean levels of EPA and DHA of fish, reared in Farm 1, were significantly lower, than those of fish from Farm 2 (Fig. 3). In turn, fish of strain Steelhead had significantly higher level of EPA and DHA, than fish of two other strains (Fig. 3). Fish from Farm 1 had a significantly higher content of total FA (mg g 1) as well as those of EPA and DHA (Fig. 3). Fish of strain Steelhead had the lowest content of total FA, but fish of strain Adler had the lowest content of EPA (Fig. 3). The highest content of total FA was characteristic of fish of strain Adler, reared in Farm 1 (Fig. 3).
Fatty acids, with significantly different mean levels in the farmed and
Table 4
Results of two-way ANOVA comparing levels (% of total FAs) and contents (mg g 1 wet weight) of fatty acids, selected on the basis of the multivariate discriminant analysis (see text, Fig. 1 and Table 2 for details), which provide most differences between rainbow trout of three strains, Adler, Adler Amber and Steelhead, reared in two farms, Farm 1 and Farm 2: d.f. – degree of freedom, MS – mean square effect for independent variables, F – Fisher’s test, P – significance. Variable Source of variation
Fig. 2. Average contents of sum of eicosapentaenoic and docosahexaenoic fatty acids (EPA + DHA) in rainbow trout of different strains and farms, and in wild rainbow trout: F1A – Farm 1, strain Adler; F1M – Farm 1, strain Adler Amber; F1S – Farm 1, strain Steelhead; F2A – Farm 2, strain Adler; F2M – Farm 2, strain Adler Amber; F2S – Farm 2, strain Steelhead; F2U – Farm 2, strain Augustin; F2K – Farm 2, strain Kamloops; F2D – Farm 2, strain Donaldson; F2V – Farm 2, strain late spawning Steelhead; F3o – Farm 3, outbred family; W – wild trout. Means labelled with the same letter are not significantly different at P < 0.05 after ANOVA and Tukey HSD post hoc test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
wild trout are depicted in Fig. 4. Besides of the significant mean differences, minimum levels of 18:1n-9 and 18:2n-6 in the farmed fish were higher, than their maximum levels in the wild fish (Fig. 4). In turn, minimum levels of 18:0 and 20:4n-3 in the wild fish were higher, than their maximum levels in the farmed fish (Fig. 4). Levels of 20:5n-3, 20:2n-6 and 20:3n-6 in the farmed and wild fish overlapped only marginally (Fig. 4).
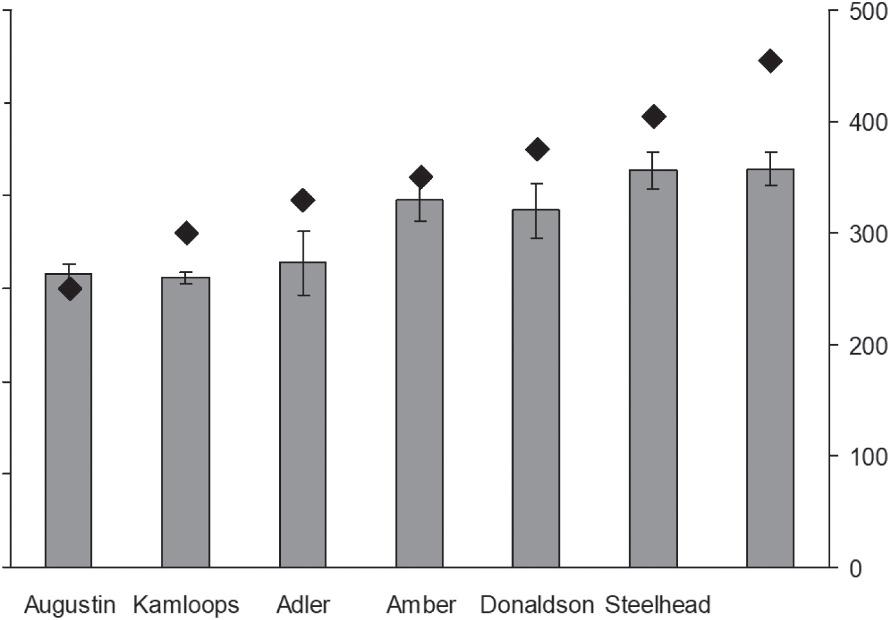
Seven strains of rainbow trout that all were reared in Farm 2 markedly varied in a season of spawning, which is genetically determined for each strain. To quantify the season of spawning, we used number of days from the beginning of year to the peak of spawning. Fish of strain Augustin had the earliest season of spawning, started in August and peaked in September. The latest season of spawning, in March, is characteristic of fish of strain late spawning Steelhead. Thus, fish of strain Augustin had the shortest period, 250 days, and fish of strain late spawning Steelhead had the longest spawning, 455 days. The fish strains also varied in their average EPA + DHA content, from 1.6 to 2.1 mg g 1 (Fig. 5). To consider likely relation between the season of spawning and PUFA content in fish muscles, we calculated Pearson’s correlation coefficient between these two parameters: r = 0.91, p < 0.01, d.f. = 5. Thus, fish strains with later season of spawning tended to accumulate more essential PUFA in their muscle tissues, when rearing in the identical conditions.
4. Discussion
In the studied farms, diets used for rainbow trout rearing, differed significantly in their constituents. Diet 1, used in Farm 1, evidently included comparatively higher proportion of vegetable oils, since it had significantly higher percentage of 18:2n-6, LA (Naylor et al., 2009; Hixson et al., 2014; Chaguri et al., 2017). In turn, Diet 2, used in Farm 2, had comparatively higher proportion of fish oil, which was indicated by significantly higher levels of 20:5n-3 (EPA) and 22:6n-3 (DHA) (Naylor
M.I. Gladyshev
et al., 2009; Chaguri et al., 2017). Besides, Diets 1 and 3 had significantly higher content of total FA (mg g 1). Nevertheless, in spite of the significantly lower content of total FA, Diet 2 had significantly higher content of EPA + DHA (mg g 1), which was evidently due to the higher proportions of these acids in total FA.
The peculiarities of FA composition and contents of diets in general
Fig. 3. Average levels (% of total FAs) and contents (mg g 1 wet weight) of fatty acids, selected on the basis of the multivariate discriminant analysis (see text, Fig. 1 and Table 2 for details), which provide most differences between rainbow trout of three strains, Adler, Adler Amber (designated as Amber) and Steelhead (designated as Steel), reared in two farms, Farm 1 and Farm 2. Means labelled with the same letter are not significantly different at P < 0.05 after two-way ANOVA and Tukey HSD post hoc test. F1 and F2 – Farm 1 and 2, respectively, A, M and S – strains Adler, Adler Amber and Steelhead, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
were reflected in FA composition of reared fish. Indeed, according to the multivariate discriminant analysis, FA composition of trout, reared in Farm 1 differed from that of trout from Farm 2 primarily due to the differences in levels of 18:2n-6, on the one hand, and 20:5n-3 and 22:6n3, on the other hand, while fish from Farm 3 had an intermediate position in the multidimensional space of FA. Moreover, mean levels of the
∑22:1 20:3n-622:5n-6
Fig. 4. Mean levels of fatty acids (% of total FA) in farmed (open rectangles, number of cases, n = 82) and wild (grey rectangles, n = 5) rainbow trout, which differed significantly (P < 0.05) according to Student’s t-test. Note different scales for major and minor FA.
Fig. 5. Average contents of sum of eicosapentaenoic and docosahexaenoic fatty acids (EPA + DHA, bars and left axis, m ± SE) in rainbow trout of different strains from Farm 2 versus number of days from the start of year to the peak of spawning (a proxy of spawning season, diamonds and right axis).
common strains, Adler and Adler Amber, reared in Farm 1 and Farm 2, had the same significant differences, as those of diets, i.e., fish from Farm 1 had in their biomass significantly higher levels of LA, originated from vegetable oils, and significantly lower levels of EPA and DHA, originated from fish oil.
However, although the well-known principle “you are what you eat”
was in general confirmed in our study, like in some other studies of rainbow trout (Barry and Trushenski, 2020; Quinones et al., 2021), the other factor, namely genetics (belonging to a strain), also was found to contribute to fish FA composition. Indeed, FA compositions of fish of two strains, Adler and Adler Amber, strictly reflected food composition, i.e., fish of these strains in Farm 1 (Diet 1) had significantly lower levels of EPA and DHA than fish of these strains in Farm 2 (Diet 2). In contrast, fish of Steelhead strain in Farm 1 and in Farm 2 had similar levels of these physiologically important acids in spite of their different levels in diets. Thus, fish of strain Steelhead likely had comparatively higher ability to regulate their FA composition regarding levels of the essential LC-PUFA. Thus, in this case an interaction of genotype and environment took place.
The ability of some fish species, including rainbow trout to maintain an optimal physiological level of LC-PUFA in muscle tissue under their dietary deficiency via selective incorporation and/or synthesis from 18:3n-3 has been described by many authors (Sushchik et al., 2006; Turchini et al., 2009; Leaver et al., 2011; Strandberg et al., 2015; Tocher, 2015; Chen et al., 2017; Gladyshev et al., 2018; Barry and Trushenski, 2020).
The studied farmed and wild trout had different nutritive values regarding content of EPA and DHA (mg g 1 of wet weight). Contrary to a widespread opinion of consumers about a higher nutritive value of wild fish compared to that of farmed species, in our study wild rainbow trout had significantly lower EPA + DHA contents than majority of farmed trout strains. However, in other studies of wild rainbow trout O. mykiss average contents of EPA + DHA in their biomass from 2.3 to 9.67 mg g 1
M.I. Gladyshev
were reported (Saglik Aslan et al., 2007; Cladis et al., 2014; Neff et al., 2014). Nevertheless, farmed rainbow trout were found to have EPA + DHA contents from 2.4 to 21.1 mg g 1 (Saglik Aslan et al., 2007; Stone et al., 2011; Turchini et al., 2011, 2018). Thus, according to our study and reports of other authors (Turchini et al., 2018), farmed rainbow trout can have the nutritive value even higher, than that of the wildcaught rainbow trout.
Most studied strains in all three farms, including the outbred fish in Farm 3, had nearly similar contents of EPA and DHA in their biomass. Nevertheless, fish of some strains, Adler, Augustin and Kamloops tended to have comparatively lower contents of EPA and DHA. The average content of EPA + DHA in farmed rainbow trout 2.03 ± 0.05 mg g 1 , found in this study, was slightly lower, than minimum values, reported by some other authors (Saglik Aslan et al., 2007; Stone et al., 2011).
The three strains, mentioned above, had spawning in late summer and autumn, in contrast to the other studied strains, which had spawning in winter and spring (Fig. 5). The correlation analysis proved a relation of EPA + DHA contents with the season of spawning. Probably, this relation took place due to a gradual decrease of water temperature in Farm 2 in August – March from 14.5–15.0 ◦ С to 7.8–9.5 ◦ С. Decreasing of water temperature is known to increase a period of incubation of eggs, and Salmoniformes with a longer period of incubation of eggs have a higher EPA and DHA contents (Artamonova et al., 2020). In any case, the difference in LC-PUFA content between the strains of rainbow trout, reared in Farm 2, demonstrated a significant genetic control of this content. Thus, a potential of selective breeding of rainbow trout strains with a high EPA and DHA contents is believed to be possible, like that of Atlantic salmon (Bell et al., 2010; Horn et al., 2018).
In this study, we regard the content of EPA + DHA as the main indicator of the nutritive value of fish for humans. Evidently, besides lipids, including LC-PUFA, fish products are valuable source of other nutrients, e.g., amino acids (proteins). Nevertheless, fish give only ca. 6% of all animal and plant protein consumed by humans (Tacon and Metian, 2013), while EPA and DHA obtained from fish constitute more than 97% of these essential nutrients in humans’ diet (Gladyshev et al., 2015). Thereby, the peculiar nutritive value of fish evidently consist in the LC-PUFA, rather than in other nutrients.
It also should be noted, that namely content of EPA and DHA in fish biomass, measured in muss units (mg g 1), rather than their level, measured in the relative units (% of total FA) should be regarded as the indicator of nutritive value. Expressing of LC-PUFA in the relative units give erroneous results concerning the real quantity of the essential nutrients, which could be obtained by humans with a certain portion of this or that fish product (Gladyshev et al., 2007; Huynh and Kitts, 2009; Joordens et al., 2014; Turchini et al., 2018; Gladyshev and Sushchik, 2019).
In our study, marker fatty acids were revealed, which potentially allow to differentiate wild and farmed rainbow trout. For instance, in the wild fish, level of oleic acid, 18:1n-9 was significantly lower, than that in the farmed fish: 13.73 ± 1.10% (maximum 16.40%) and 29.69 ± 0.71% (minimum 18.21%), respectively. Oleic acid is one of fatty acids that are heavily catabolized for energy in fish (Tocher, 2003), and it was shown to be one of the preferred lipid fuels in swimming muscles (McKenzie et al., 1998). Farmed trout, limited in their swimming, evidently have significantly lower energy demands compared to the wild trout. Thus, farmed trout preferentially accumulated ‘energetic’ oleic acid in storage lipids, rather than used it for catabolism. In other works, compared FA composition of wild and farmed rainbow trout the same tendency could be noticed: levels of 18:1n-9 in wild fish, 10.66–24.63%, were lower than those in farmed fish, 22.9–30.47%, (Fallah et al., 2011; Ozogul et al., 2013; Akpinar et al., 2015; Ural et al., 2017, but see Saglik Aslan et al., 2007).
In last decades, vegetable oils are increasingly added to farmed fish food (Turchini et al., 2009; Shepherd and Jackson, 2013; Sprague et al., 2016; Qian et al., 2020; Barry and Trushenski, 2020; Quinones et al., 2021). It is not at the least surprising, that level of the other potential
marker, 18:2n-6, which is one of the main constituent of vegetable oils (Naylor et al., 2009; Hixson et al., 2014), in our study was significantly higher in the farmed trout, than that in the wild trout: 11.47 ± 0.32% (minimum 6.17%) and 1.94 ± 0.09% (maximum 2.25%), respectively. Similarly, in other studies levels of 18:2n-6 in farmed rainbow trout, 6.2–17.15%, were higher than those in wild rainbow trout, 3.63–5.97%, (Blanchet et al., 2005; Fallah et al., 2011; Ural et al., 2017, but see Ozogul et al., 2013; Akpinar et al., 2015). In contrast, levels of another potential marker FA, 20:5n-3, in our study was significantly higher in the wild trout, than that in the farmed trout: 8.60 ± 0.44% (minimum 7.59%) and 4.11 ± 0.20% (maximum 7.85%), respectively. The same tendency could be noticed in the other studies: levels of 20:5n-3 in wild rainbow trout, 2.55–18.56%, were higher than those in farmed rainbow trout, 1.77–10.29%, (Blanchet et al., 2005; Fallah et al., 2011; Ozogul et al., 2013; Akpinar et al., 2015; Ural et al., 2017). Thus, levels of 18:1n9, 18:2n-6 and 20:5n-3 can be used as marker FA to differentiate wild and farmed rainbow trout, but quantitative ranges for these markers should be specified for each region.
5. Conclusions
As found, rainbow trout of strain Steelhead had comparatively higher ability to regulate their FA composition and thereby to retain the high contents of EPA and DHA in biomass despite variations of their contents in diet. Thus, this strain is believed to be more suitable for rearing on feeds with a high proportion of vegetable oils. In contrast, trout of strains Adler, Augustin and Kamloops tended to have lower contents of EPA + DHA than fish of the other strains, which is probably caused by their spawning in late summer and autumn, in contrast to the other strains with spawning in winter and spring.
Differences in contents of EPA + DHA of different strains, reared in the same farm, are believed to prove a potential of selective breeding of rainbow trout strains with a high EPA and DHA.
Wild and farmed rainbow trout can be differentiated using FA composition: farmed fish had 18:2n-6 more than 6% and wild fish had less than 6% of this FA in their flesh.
CRediT authorship contribution statement
Michail I. Gladyshev: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. Alexander A. Makhrov: Conceptualization, Investigation, Data collection & curation, Writing – review & editing. Nadezhda N. Sushchik: Investigation, Data collection & curation, Writing – review & editing. Olesia N. Makhutova: Investigation, Writing – review & editing. Anastasia E. Rudchenko: Investigation, Writing – review & editing. Dmitrii A. Balashov: Investigation. Evgenii V. Vinogradov: Investigation. Valentina S. Artamonova: Conceptualization, Investigation, Data collection & curation, Writing –review & editing.
Declaration of Competing Interest
No conflict of interest.
Acknowledgements
The work was supported by Federal Tasks for Institute of Biophysics SB RAS No. 0287-2021-0019, Federal Tasks for Siberian Federal University No. FSRG-2020-0019, Federal Tasks for A. N. Severtzov Institute of Ecology and Evolution RAS No. 0089-2021-0006 (АААА-А18118042490059-5) and by grant of the Krasnoyarsk Regional Science Foundation No. 2021020207159 “Development of import-substituting technologies for salmon aquaculture in the conditions of the Krasnoyarsk Territory”
M.I. Gladyshev
References
Akpinar, N., Akpinar, M.A., Gorgun, S., Akpinar, A.E., 2015. Fatty acid composition and ω3/ω6 ratios in the muscle of wild and reared Oncorhynchus mykiss Chem. Nat. Compd. 51, 22–25.
Artamonova, V.S., Yankovskaya, V.A., Golod, V.M., Makhrov, A.A., 2016. Genetic differentiation of rainbow trout (Parasalmo mykiss) strains bred in the Russian Federation (in Russian, summary in English). Trans. Papanin Inst. Biol. Inland Waters RAS 73, 25–45
Artamonova, V.S., Makhrov, A.A., Gladyshev, M.I., Sushchik, N.N., Dgebuadze, Y.Y., 2020. Polyunsaturated fatty acid content in muscle tissue is associated with the duration of embryo development in Salmonoid fishes (Salmonoidei). Dokl. Biochem. Biophys. 491, 59–61
Barry, K.J., Trushenski, J.T., 2020. Reevaluating polyunsaturated fatty acid essentiality in rainbow trout. N. Am. J. Aquac. 82, 251–264 Bell, J.G., Pratoomyot, J., Strachan, F., Henderson, R.J., Fontanillas, R., Hebard, A., Tocher, D.R., 2010. Growth, flesh adiposity and fatty acid composition of Atlantic salmon (Salmo salar) families with contrasting flesh adiposity: effects of replacement of dietary fish oil with vegetable oils. Aquaculture 306, 225–232
Bernasconi, A.A., Wiest, M.M., Lavie, C.J., Milani, R.V., Laukkanen, J.A., 2021. Effect of omega-3 dosage on cardiovascular outcomes: an updated meta-analysis and metaregression of interventional trials. Mayo Clin. Proc. 96, 304–313. https://doi.org/ 10.1016/j.mayocp.2020.08.034
Betancor, M.B., Olsen, R.E., Solstorm, D., Skulstad, O.F., Tocher, D.R., 2016. Assessment of a land-locked Atlantic salmon (Salmo salar L.) population as a potential genetic resource with a focus on long-chain polyunsaturated fatty acid biosynthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1861, 227–238
Blanchet, C., Lucas, M., Julien, P., Morin, R., Gingras, S., Dewailly, E., 2005. Fatty acid composition of wild and farmed Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Lipids 40, 529–531
Calder, P.C., 2018. Very long-chain n-3 fatty acids and human health: fact, fiction and the future. Proc. Nut. Soc. 77, 52–72
Chaguri, M.P., Maulvault, A.L., Costa, S., Gonçalves, A., Nunes, M.L., Carvalho, M.L., Marques, A., 2017. Chemometrics tools to distinguish wild and farmed meagre (Argyrosomus regius). J. Food Process. Preserv. 41, e13312
Chen, C., Chen, J., Wang, S., You, C., Li, Y., 2017. Effects of different dietary ratios of linolenic to linoleic acids or docosahexaenoic to eicosapentaenoic acids on the growth and immune indices in grouper, Epinephelus coioides Aquaculture 473, 153–160
Cladis, D.P., Kleiner, A.C., Freiser, H.H., Santerre, C.R., 2014. Fatty acid profiles of commercially available finfish fillets in the United States. Lipids 49, 1005–1018
Fallah, A.A., Saei-Dehkordi, S.S., Nematollahi, A., 2011. Comparative assessment of proximate composition, physicochemical parameters, fatty acid profile and mineral content in farmed and wild rainbow trout (Oncorhynchus mykiss). Int. J. Food Sci. Technol. 46, 767–773
Gladyshev, M.I., 2021. Terrestrial sources of polyunsaturated fatty acids for aquaculture. J. Ichthyol. 61, 471–485
Gladyshev, M.I., Sushchik, N.N., 2019. Long-chain omega-3 polyunsaturated fatty acids in natural ecosystems and the human diet: assumptions and challenges. Biomolecules 9, 485. https://doi.org/10.3390/biom9090485
Gladyshev, M.I., Sushchik, N.N., Gubanenko, G.A., Demirchieva, S.M., Kalachova, G.S., 2007. Effect of boiling and frying on the content of essential polyunsaturated fatty acids in muscle tissue of four fish species. Food Chem. 101, 1694–1700.
Gladyshev, M.I., Sushchik, N.N., Makhutova, O.N., 2013. Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Other Lipid Mediat. 107, 117–126
Gladyshev, M.I., Makhutova, O.N., Gubanenko, G.A., Rechkina, E.A., Kalachova, G.S., Sushchik, N.N., 2015. Livers of terrestrial production animals as a source of longchain polyunsaturated fatty acids for humans: an alternative to fish? Eur. J. Lipid Sci. Technol. 117, 1417–1421
Gladyshev, M.I., Glushchenko, L.A., Makhutova, O.N., Rudchenko, A.E., Shulepina, S.P., Dubovskaya, O.P., Sushchik, N.N., 2018. Comparative analysis of content of omega-3 polyunsaturated fatty acids in food and muscle tissue of fish from aquaculture and natural habitats. Contemp. Probl. Ecol. 11, 297–308
Gladyshev, M.I., Anishchenko, O.V., Makhutova, O.N., Kolmakova, O.V., Trusova, M.Y., Morgun, V.N., Sushchik, N.N., 2020. The benefit-risk analysis of omega-3 polyunsaturated fatty acids and heavy metals in seven smoked fish species from Siberia. J. Food Compos. Anal. 90, 103489
Gladyshev, M.I., Makhrov, A.A., Baydarov, I.V., Safonova, S.S., Golod, V.M., Alekseyev, S.S., Sushchik, N.N., 2022. Fatty acid composition and contents of fish of genus Salvelinus from natural ecosystems and aquaculture. Biomolecules 12, 144. https://doi.org/10.3390/biom12010144
Henriques, J., Dick, J.R., Tocher, D.R., Bell, J.G., 2014. Nutritional quality of salmon products available from major retailers in the UK: content and composition of n-3 long-chain PUFA. Br. J. Nutr. 112, 964–975
Hixson, S.M., Parrish, C.C., Anderson, D.M., 2014. Changes in tissue lipid and fatty acid composition of farmed rainbow trout in response to dietary camelina oil as a replacement of fish oil. Lipids 49, 97–111
Horn, S.S., Ruyter, B., Meuwissen, T.H.E., Hillestad, B., Sonesson, A.K., 2018. Genetic effects of fatty acid composition in muscle of Atlantic salmon. Genet. Sel. Evol. 50, 23
Huynh, M.D., Kitts, D.D., 2009. Evaluating nutritional quality of pacific fish species from fatty acid signatures. Food Chem. 114, 912–918
Joordens, J.C.A., Kuipers, R.S., Wanink, J.H., Muskiet, F.A.J., 2014. A fish is not a fish: patterns in fatty acid composition of aquatic food may have had implications for hominin evolution. J. Hum. Evol. 77, 107–116
Kirillova, E.A., Kuzishchin, K.V., Gruzdeva, M.A., Makhrov, A.A., Artamonova, V.S., Kirillov, P.I., Vinogradov, E.V., 2021. The catch of rainbow trout Parasalmo mykiss on Sakhalin island. Russ. J. Biol. Invasions 12, 350–354
Leaver, M.J., Taggart, J.B., Villeneuve, L., Bron, J.E., Guy, D.R., Bishop, S.C., Tocher, D. R., 2011. Heritability and mechanisms of n-3 long chain polyunsaturated fatty acid deposition in the flesh of Atlantic salmon. Comp. Biochem. Physiol. Part D 6, 62–69 Legendre, P., Legendre, L., 1998. Numerical Ecology. Elsevier Science, Amsterdam, p. 853
Naylor, R.L., Hardy, R.W., Bureau, D.P., Chiu, A., Elliott, M., Farrell, A.P., Nichols, P.D., 2009. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. 106, 15103–15110
Neff, M.R., Bhavsar, S.P., Ni, F.J., Carpenter, D.O., Drouillard, K., Fisk, A.T., Arts, M.T., 2014. Risk-benefit of consuming Lake Erie fish. Environ. Res. 134, 57–65
Ozogul, F., Yavuzer, E., Ozogul, Y., Kuley, E., 2013. Comparative quality loss in wild and cultured rainbow trout (Oncorhynchus mykiss) during chilling storage. Food Sci. Technol. Res. 19, 445–454
Peng, J., Larondelle, Y., Pham, D., Ackman, R.G., Rollin, X., 2003. Polyunsaturated fatty acid profiles of whole body phospholipids and triacylglycerols in anadromous and landlocked Atlantic salmon (Salmo salar L.) fry. Comp. Biochem. Phys. B 134, 335–348
Qian, C., Hart, B., Colombo, S.M., 2020. Re-evaluating the dietary requirement of EPA and DHA for Atlantic salmon in freshwater. Aquaculture 518, 734870
Quinones, J., Díaz, R., Dantagnan, P., Hernandez, A., Valdes, M., Lorenzo, J.M., Farías, J. G., 2021. Dietary inclusion of Durvillaea antarctica meal and rapeseed (Brassica napus) oil on growth, feed utilization and fillet quality of rainbow trout (Oncorhynchus mykiss). Aquaculture 530, 735882
Robert, S.S., 2006. Production of eicosapentaenoic and docosahexaenoic acid-containing oils in transgenic land plants for human and aquaculture nutrition. Mar. Biotechnol. 8, 103–109
Rollin, X., Peng, J., Pham, D., Ackman, R.G., Larondelle, Y., 2003. The effects of dietary lipid and strain difference on polyunsaturated fatty acid composition and conversion in anadromous and landlocked salmon (Salmo salar L.) parr. Comp. Biochem. Physiol. B 134, 349–366.
Saglik Aslan, S., Guven, K.C., Gezgin, T., Alpaslan, M., Tekinay, A., 2007. Comparison of fatty acid contents of wild and cultured rainbow trout Onchorhynchus mykiss in Turkey. Fish. Sci. 73, 1195–1198
Shepherd, C.J., Jackson, A.J., 2013. Global fishmeal and fish-oil supply: inputs, outputs and markets. J. Fish Biol. 83, 1046–1066
Sprague, M., Dick, J.R., Tocher, D.R., 2016. Impact of sustainable feeds on omega-3 longchain fatty acid levels in farmed Atlantic salmon, 2006-2015. Sci. Rep. 6, 21892
Stone, D.A.J., Oliveira, A.C.M., Plante, S., Smiley, S., Bechtel, P., Hardy, R.W., 2011. Enhancing highly unsaturated omega-3 fatty acids in phase-fed rainbow trout (Oncorhynchus mykiss) using Alaskan fish oils. Aquac. Nutr. 17, E501–E510
Strandberg, U., Hiltunena, M., Jelkanen, E., Taipale, S.J., Kainz, M.J., Brett, M.T., Kankaala, P., 2015. Selective transfer of polyunsaturated fatty acids from phytoplankton to planktivorous fish in large boreal lakes. Sci. Total Environ. 536, 858–865
Sushchik, N.N., Gladyshev, M.I., Kalachova, G.S., Makhutova, O.N., Ageev, A.V., 2006. Comparison of seasonal dynamics of the essential PUFA contents in benthic invertebrates and grayling Thymallus arcticus in the Yenisei river. Comp. Biochem. Physiol. B 145, 278–287
Tacon, A.G.J., Metian, M., 2013. Fish matters: importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 21, 22–38
Tocher, D.R., 2003. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 11, 107–184
Tocher, D.R., 2015. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449, 94–107.
Tocher, D.R., Betancor, M.B., Sprague, M., Olsen, R.E., Napier, J.A., 2019. Omega-3 longchain polyunsaturated fatty acids, EPA and DHA: bridging the gap between supply and demand. Nutrients 11, 89. https://doi.org/10.3390/nu11010089
Topic Popovic, N., Strunjak-Perovic, I., Coz-Rakovac, R., Barisic, J., Jadan, M., Persin Berakovic, A., Sauerborn Klobucar, R., 2012. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J. Appl. Ichthyol. 28, 553–564
Turchini, G.M., Torstensen, B.E., Ng, W.K., 2009. Fish oil replacement in finfish nutrition. Rev. Aquacult. 1, 10–57
Turchini, G.M., Francis, D.S., Keast, R.S.J., Sinclair, A.J., 2011. Transforming salmonid aquaculture from a consumer to a producer of long chain omega-3 fatty acids. Food Chem. 124, 609–614
Turchini, G.M., Hermon, K.M., Francis, D.S., 2018. Fatty acids and beyond: fillet nutritional characterisation of rainbow trout (Oncorhynchus mykiss) fed different dietary oil sources. Aquaculture 491, 391–397
Ural, M.S., Calta, M., Parlak, A.E., 2017. The comparison of fatty acids, fat-soluble vitamins and cholesterol in the muscle of wild caught, cage and pond reared rainbow trout (Oncorhynchus mykiss W., 1792). Iran. J. Fish. Sci. 16, 431–440
M.I.