patterns on body compartments of Octopus vulgaris paralarvae
M. Nande a, * , ´ O. Monroig b , A.M. Machado a , L.F.C. Castro a, c , M. Lopes-Marques c, d, e , A. Capitao a , J.C. Navarro b, *
a CIIMAR-Interdisciplinary Centre of Marine and Environmental Research, University of Porto, Terminal de Cruzeiros de Leixoes. Av., General Norton de Matos s/n, Portugal
b Instituto de Acuicultura Torre de la Sal (IATS-CSIC), 12595 Ribera de Cabanes, Castell´ on, Spain
c Department of Biology, Faculty of Sciences, University of Porto, Porto, Portugal
d i3S - Instituto de Investigaçao e Inovaçao em Saúde, Universidade do Porto, Porto, Portugal
e IBMC - Instituto de Biologia Molecular e Celular, Universidade do Porto, Porto, Portugal
ARTICLE INFO
Keywords:
Octopus vulgaris paralarvae
Fatty acids
Gene expression
Glycerophospholipid metabolism
Dietary lipids
ABSTRACT
The common octopus, Octopus vulgaris, is a promising mollusc species for marine aquaculture diversification due to its high growth rates and commercial value. Yet, the elevated mortalities mainly related to lipid-linked nutritional deficiencies during the planktonic stage (paralarvae), hinder the development of efficient protocols for a complete rearing cycle. Although the effect of dietary lipids, especially fatty acids (FA), has been the subject of intense research, the available information is essentially restricted to their impact on the composition of the whole parlarval organism. In contrast, little is known about the effects of dietary signature and the specific requirements of each anatomical structure through the paralarvae development. In addition, knowledge about the endogenous capacity in each paralarvae body structure for adaptation to different dietary scenarios is necessary. In the present work, a series of experiments were carried out based on newly hatched paralarvae (PH) and paralarvae fed (30 days post-hatch (DPH)) either with marine crustacean zoeae (PZ) or with Artemia metanauplii (PA). At the end of the trials, the paralarvae were dissected into functional (mantle, head, and arms) and digestive (digestive gland (DG)) body compartment and the FA profile, as well as the expression patterns of genes involved in the long chain polyunsaturated FA (LC-PUFA) (stearoyl-CoA desaturase (scd), ωx2 desaturase (ωx2), ωx1 desaturase (ωx1), fatty acyl desaturase (fad), elovl2/5, elovl4), and glycerophospholipid biosynthetic pathways (agpta, lpin, chpt, and dgat) were analysed. Results showed a positive effect of the PZ diet on growth and development of paralarvae as compared to PA, and distinct FA composition for the 3 experimental groups. The digestive gland was associated to 18C FA (18:3n-3, 18:4n3, 18:1n9, and 18:2n6), while n-6 and n-3 LC-PUFA (20:4n6, 20:5n3, 22:5n3, and 22:6n3) were found in a higher proportion in functional body compartments. The expression of LC-PUFA biosynthesis-linked genes increased significantly during development, with the functional body compartments of the PA treatment being up-regulated as compared to PZ, pointing at a putative compensatory mechanism. In addition, a higher amount of transcripts linked to the triggering of the phosphatidylcholine synthesis (chpt) was found in the digestive gland of PA and PZ and arms of PZ; whereas genes related with triacylglycerol (TAG) synthesis (lpin and dgat) were enhanced in the digestive gland of PA and PH. Dietary treatments affected the FA profile and the gene expression patterns in both digestive (more similar FA profile and glycerophospholipid biosynthesis) and functional (different FA profile and LC-PUFA biosynthesis) compartments of the paralarvae. Furthermore, LC-PUFA biosynthesis-related genes in the head and glycerophospholipid target genes in DG could be used as biomarkers of nutritional deficiencies in paralarvae.
* Corresponding authors.
E-mail addresses: mnande@ciimar.up.pt (M. Nande), jc.navarro@csic.es (J.C. Navarro).
https://doi.org/10.1016/j.aquaculture.2022.738293
Received 11 January 2022; Received in revised form 6 April 2022; Accepted 24 April 2022
Availableonline27April2022
0044-8486/©2022ElsevierB.V.Allrightsreserved.
1. Introduction
The expansion of the so-called “fed aquaculture” in the last decades has occurred through a remarkable optimisation of farming technology for a relatively small number of high value species, mostly fish and shrimp (FAO, 2020). Diversification of aquaculture with new species has been pointed out as a key aspect to increase the environmental sustainability and profitability of the sector (FAO, 2020). In this context, the high growth rates and commercial value of O. vulgaris has prompted enormous interest in developing appropriate culture protocols for octopus species worldwide (Iglesias et al., 2014; Dan et al., 2018, 2019; de Ortiz et al., 2021). However, high mortalities during the planktonic stage (paralarvae) still represent a bottleneck for an efficient protocol of integral rearing (Iglesias et al., 2007). Nutritional deficiencies of larval food have been suggested as one of the main causes accounting for such paralarval mortalities (Navarro and Villanueva, 2003; Navarro et al., 2014).
Certain lipids such as long-chain (≥C20) polyunsaturated fatty acids (LC-PUFA), cholesterol and phospholipids (PL) are regarded as essential nutrients for cephalopods (Almansa et al., 2006). Compared to reared individuals, wild paralarvae present fatty acid (FA) profiles that are relatively rich in LC-PUFA, especially docosahexaenoic acid (DHA, 22:6n-3), that mostly derives from diet (Navarro and Villanueva, 2003; Garrido et al., 2016)., but at present it is unknown if paralarvae can trigger specific metabolic pathways to compensate for dietary lipid deficiencies, as occurs in other aquatic animals like fish (Monroig and Kabeya, 2018; Xie et al., 2021).
Dietary lipids have been shown to regulate the expression of genes involved in the biosynthesis of LC-PUFA in aquatic animals (Monroig and Kabeya, 2018; Xie et al., 2021). Specific genes encoding key fatty acyl elongases and desaturases of the O. vulgaris LC-PUFA biosynthesis have been studied in recent years. The PUFA elongases Elovl4 and Elovl2/5, and the Δ5 front-end desaturase (Fad) and Δ9 stearoyl-CoA desaturase (Scd), were molecularly and functionally characterised in O. vulgaris (Monroig et al., 2012a, 2012b, 2016a, 2017). These studies confirmed that O. vulgaris lacks key enzymatic activities within its elongation and desaturation complement, thus making necessary the dietary supply of the physiologically important LC-PUFA, namely arachidonic (ARA, 20:4n-6), eicosapentaenoic (EPA, 20:5n-3) and docosahexaenoic (DHA, 22:6n-3) acids. More recently, two methyl-end (or ωx) desaturase genes encoding enzymes with Δ12 (ωx2) and Δ15 (ωx1) desaturase regioselectivities enabling the de novo biosynthesis of the 18C PUFA linoleic acid (LA, 18:2n-6) and α-linolenic acid (ALA, 18:3n-3), have been reported in O. vulgaris (Garrido et al., 2019). Importantly, the functional characterisation of the O. vulgaris ωx desaturases revealed that EPA can be biosynthesised through several routes and therefore should not be regarded as dietary essential, although both ARA and DHA should still be considered essential nutrients for O. vulgaris (Garrido et al., 2019).
To the best of our knowledge, and unlike LC-PUFA, the specific genes involved in PL biosynthesis (glycerophospholipid metabolism) and their regulation through diet have not been studied in O. vulgaris. A simplified view of the biosynthetic pathway of PC, one of the most abundant PL in O. vulgaris (Reis et al., 2019), includes a first step by which the acyl-snglycero-3-phosphate acyltransferase (Agpta) mediates the reaction that transforms 1-acyl-sn-glycero-3-phosphate into 1,2-diacyl-sn-glycero-3phosphate through the incorporation of a FA in the sn-2 position (Beppu et al., 2017). Next, the enzyme phosphatidate phosphatase (Lpin) catalyses the conversion of 1,2-diacyl-sn-glycero-3-phosphate into 1,2-diacyl-sn-glycerol (DAG). The final reaction, catalysed by CDP-choline and 1,2-diacylglycerol cholinephosphotransferase (Chpt), is the condensation of CDP-choline with DAG to form PC. Alternatively, DAG can be converted into TAG by the action of acyl-CoA diacylglycerol acyltransferase (Dgat). Thus, Chpt and Dgat compete for DAG for the synthesis of PC or TAG, respectively (Xu et al., 2019).
An integrative study of FA profile and lipid metabolism gene
expression may be even more revealing of the paralarvae requirements when linked to different anatomical areas of the organism. The traditional experimental approach to ascertain dietary requirements of essential nutrients such as lipids including essential FA, has been the scrutiny of the impact of food on the whole-body composition of paralarvae (Navarro and Villanueva, 2000, 2003; Seixas et al., 2008; Uriarte et al., 2011; Iglesias et al., 2014; Garrido et al., 2016). However, a design often used in adults and juveniles consists of the analysis of the main body compartments (García-Garrido et al., 2010; García et al., 2011). This strategy provides critical physiological insight on the specific needs in developmental periods, and it has not yet been attempted with paralarvae as far as we know. This is particularly interesting because different anatomical tissues, organs, or compartments may have specific requirements. Thus, the specific nutritional requirements may be different in those final accepting body compartments and with particular functions (functional) such as swimming or breathing (mantle), vision and perception (head), or development (arms) compared to those found in the digestive body compartment (digestive gland) related to the initial metabolism and storage of food (Villanueva and Norman, 2008; O’dor et al., 1984).
Thus, due to their lipid and FA composition, and for the reasons already mentioned above, the use of two live preys like Artemia and crab zoeae, offers an optimal scenario to scrutinize the effects of dietary lipids (Garrido et al., 2018; Var ´ o et al., 2017), specifically LC-PUFA, but most importantly PC and TAG. The present study aimed to investigate the growth and general performance of paralarvae fed these two live preys and further explore the dietary effects at two levels. First, analysing the dietary lipid fingerprint in different body compartment of the paralarvae. Second, associating this information to potential mechanisms of nutritional regulation through the study of the expression patterns of genes involved in LC-PUFA, PC and TAG biosynthetic pathways.
2. Materials and methods
2.1. Broodstock and spawning
During the winter of 2014 and 2015, 14 common octopuses (average weight: 1.6 ± 0.4 kg) were captured in the Ria of Vigo (NW Spain), by local fishermen. Animals were transported to the facilities of the Instituto Espanol de Oceanografía (IEO) in Vigo, Spain, in a 100 L opaque tank with seawater at 14 ◦ C and oxygen saturation. The animals were acclimatised in a 10 m3 tank (4 m L x 2 m W x 1.25 m H), with a seawater flow-through system in semidarkness conditions (<100 lx (lux)) in a 3:1 females:males ratio. The males were subsequently removed from the broodstock tank after a month, anaesthetised and humanely killed as described below. Throughout the period, the temperature was 16 ± 2 ◦ C and the salinity 34 ± 1 psu (Iglesias et al., 2016). Levels of dissolved oxygen, nitrites and ammonium were monitored daily. Several sections of PVC pipes (20 cm in diameter and 50 cm long) were placed into the broodstock tank as a shelter and spawning dens. The animals were fed ad libitum three times per week with frozen mussels (Mytilus galloprovincialis), fish (Merluccius merluccius) and crustaceans (Polybius spp.). Presence of egg batches inside the PVC dens was checked weekly. The first layings were registered in March in both years, and each female plus the spawn and den was relocated separately in 0.5 m3 tanks (1 m L x 1 m W x 1.5 m H, with 0.5 m seawater depht), with low light intensity (<100 lx), and a seawater flow-through system. The incubation period varies with temperature (Nande et al., 2017a, 2018), and was adjusted to match the highest frequency of hatched zoeae (see below).
2.2. Preys
2.2.1. Crab zoeae
From April to August (2014 and 2015) 56 ovigerous females of spider crab (Maja brachydactyla) were maintained at different stages of embryonic development according to Gonz´ alez-Gurriar´ an et al. (1995)
M. Nande
Females were kept at low light intensity (< 100 lx) and ambient temperature (14–18 ◦ C), in six 0.3 m3 flow-through seawater tanks (1 m L x 1 m W, 0.5 m H, with 0.3 m seawater depth). A 500 μm net in the outlet tube was fitted to collect the hatched zoeae. Crab females were fed three times a week with frozen mussels (M. galloprovincialis) in a proportion of 10% of their body weight. The hatched zoeae were manually harvested using a 500 μm collector and transferred to the experimental tanks to feed the O. vulgaris paralarvae.
2.2.2. Artemia metanauplii
Artemia cysts (AF 480, INVE Aquaculture, Dendermonde, Belgium) were incubated for 24 h at 28 ◦ C until hatching. The newly hatched nauplii were transferred to a 150 L tronco-conical tank at a final density of 50 ± 10 ind/mL, and kept in a closed seawater system at 25 ◦ C, 36 psu of salinity, surface light intensity from 600 to 900 lx, and constant photoperiod (24L: 0D). Artemia was grown for 8–10 days to a total length > 2 mm, and fed with a multi-specific diet of microalgae based on Isochrysis galbana and Nannochloropsis sp., at a final concentration of 250,000 and 500,000 cell/mL, respectively.
2.3.
O. vulgaris feeding experiments
Hatchling paralarvae (PH) of two different spawns (June 2014 and July 2015) were used in two indentical feeding trials, consisting of feeding paralarvae with preys, namely spider crab zoeae (PZ) and Artemia metanauplii (PA). Both dietary treatments were performed in 100 L triplicate tanks (5 paralarvae/L) with black background, soft central aeration, controlled temperature (21 ± 1 ◦ C), 14:10 (L:D) photoperiod, and a light intensity range of 300–500 lx on the seawater surface. Two microalgae, I. galbana and N. sp., were added at a final concentration of 150,000 and 250,000 cell/mL, respectively, in order to keep the paralarvae and prey in a green water environment (Iglesias and Fuentes, 2014). According to a previous estimate of food ingestion (Nande et al., 2017b), paralarvae were fed at a final prey density of 0.05 preys/mL for PZ and 0.1 preys/mL for PA, with a frequency of three shots per day (Iglesias et al., 2006), adjusting the final concentration to the preys still present in the rearing tank. Occasionally, older PZ paralarvae (20 DPH) required higher food supply, and Artemia metanauplii were used as a supplement every 3 d at a final concentration of 0.05 preys/mL.
At 0, 10, 15, 20, 25 and 30 DPH, 15 paralarvae were collected, and the number of suckers per arm was counted under a binocular microscope Leica MZ8® Then, Paralarvae were washed with distilled water, and kept for 24 h at 80 ◦ C, until weighed with an ultra-precision scale (0.000001 g) UM3 Mettler (Mettler-Toledo International Inc., Columbus, USA). The standard growth rate (SGR%) for dry weight was calculated using the formula:
SGR% = (LN DW f LN DW i tf ti ) x 100
where DWf is the final dry weight (mg), DWi is the initial dry weight (mg), tf is the final time (d), and ti is the initial time (d).
At 0 and 30 DPH, 60 paralarvae per treatment and sampling were collected (3 h after the first-morning feed) and anaesthetised with magnesium chloride (1.5% for 10 min and 3.5% for 15 min), and then dissected using entomological needles (Ento Sphinx inox 0.2 mm, Entomopraxis SCP, Barcelona, Spain) in a Petri dish placed over ice. Mantle (M), head (H) and arms (A), as representative of functional body compartments, and digestive gland (DG), representing a digestive body compartment, were either placed individually in 1.5 mL tubes filled with RNA stabilisation buffer (RNA later™ , Invitrogen Life Technologies)) for RNA extraction, or freeze-dried for lipid extraction and FA analyses. All samples were stored at 80 ◦ C.
2.4. Fatty acid analysis
Total lipids from prey samples were extracted using a modification of the method of Folch et al. (1957) Subsequently, three total lipids aliquots (250 μg) were pooled and fractionated into polar and neutral lipids by thin layer chromatography (20 × 20 plates silica-gel G60, VWR) using hexane:ethyl ether:acetic acid (75:15:1.5, in volume) as solvent system. Spots corresponding to polar and neutral lipids were scrapped off the plate and acid-catalysed transmethylated overnight (Christie, 1982), previous to analysis by gas chromatography as described in Viciano et al. (2011) The FA analyses of the body compartments of paralarvae were carried out from freeze-dried samples consisting of pools (N = 20) of samples of each body compartment (M, H, A, and DG) collected from hatchlings (PH) and 30 DPH paralarvae for both treatments, PZ and PA, and a direct transmethylation micromethod was used as described in Garrido et al. (2016)
2.5. Predicted target genes sequences
To obtain coding sequences of the O. vulgaris genes agpta, lpin, chpt, and dgat, we first identified orthologous predicted from the Octopus bimaculoides genome (Albertin et al., 2015) available at NCBI with accession numbers XM_014914065.1, XM_014912142.1, XM_014912849.1, XM_014914829.1, respectively. Next, the O. bimaculoides gene sequences were used as queries in BLASTn searches within Sequence Read Archive (SRA) databases available at NCBI for O. vulgaris (SRR2857272, SRR2857274, and SRX006887). All reads were collected and uploaded to Geneious (Geneious V7.1.9) and aligned against each predictive sequence of O. bimaculoides. Reads poorly aligned or with identity scores below 95% were removed and the consensus sequences were obtained. Each putative gene was translated to amino acid (aa) sequences using ExPASy free software (http://www. expasy.org) (Artimo et al., 2012).
2.6. Phylogenetic analysis
The aa deduced sequences for each of the target genes (agpta, lpin, chpt, and dgat) were compiled in the NCBI and Ensembl databases. The aa sequences were aligned using MAFFT v7.402 free software (Katoh et al., 2019) with the L-INS-i (Katoh and Standley, 2013). Then, gaps were deleted from the alignment using GapStrip/Squeeze v2.1.0 in any columns containing more than 95% gaps. Maximum likelihood trees were reconstructed using the PhyML 3.0 server (Guindon et al., 2010) using LG + G + I + F as an evolution model for Agpta, Chpt and Dgat, and JTT + G + I + F for Lpin. The evolutionary models were calculated automatically using built in PhyML tool Smart Model Selection (SMS) (Lefort et al., 2017). The phylogenetic trees were visualised using Dendroscope (Huson et al., 2007) and rooted with sequences from Cnidaria and Porifera species. The orthologue protein sequences of O. vulgaris, published by Zarrella et al. (2019) after the completion of our experimental and gene expression analyses, were incorporated into the phylogenetic study (XP_029655226.1, XP_029657873.1, XP_029636395.1, and XP_029647479.1) to validate ortology of our aa deduced sequences.
2.7. RNA extraction and cDNA synthesis
For the extraction of total RNA, replicate (N = 6) pools (N = 3) of body compartments (M, H, A and DG) from PH and 30 DPH PZ and PA paralarvae were homogenised in 0.5 mL of TRIzol® Reagent and using a Precellys® 24 (Bertin Technologies, France). Total RNA was extracted using total Direct-zol RNA Isolation kit (Zymo Research, Irvine, CA, USA) following the manufacturer’s instructions. The RNA integrity was checked by running an aliquot of total RNA (~500 ng) on a 1% (w/v) agarose gel stained with GelRed™ nucleic acid stain (Biotium, Hayward, CA, USA). The RNA quality was evaluated by sample absorbance
M. Nande et al.
according to the A260/280 and A260/230 ratios using a BiotTek® microplate reader. Reverse transcription was performed from 500 ng of total RNA for each group of samples, using the first-strand cDNA Synthesis Kit (NZYTech, Lisbon, Portugal) in T100 thermal cycler (Bio-Rad, Laboratories, CA, USA) according to the manufacturer’s recommendations.
2.8. Quantitative RT-PCR design
Gene expression was examined by quantitative RT-PCR (Q-PCR) in all body compartments including mantle, head, arms and, digestive gland, from PH and paralarvae fed both dietary treatments (PZ and PA). Primers of genes related to LC-PUFA biosynthesis, stearoyl-CoA desaturase (scd), ωx2 desaturase (ωx2), ωx1 desaturase (ωx1), fatty acyl desaturase (fad), elovl2/5, and elovl4, and reference genes (Ubiquitin, 18S, Ef1-1α, β-actin, β-tubulin) were compiled from the literature (Monroig et al., 2012a, 2012b, 2016a, 2017; Garrido et al., 2019; García-Fern´ andez et al., 2016) (Supplementary Table S1). Moreover, primers targeting the newly identified genes involved in glycerophospholipid biosynthesis (agpta, lpin, chpt, and dgat) were designed on the exon-exon junctions using Primer 3 software (Untergasser et al., 2012).
To quantify the relative expression of each target gene in the different samples (Q-PCR) a Mastercycler ep realplex system (Eppendorf) was used. Each 96-well plate was designed to analyse each body compartment in six replicates for each treatment (PH, PA and PZ). Each well contained 5 μL of NZYSpeedy Q-PCR Green MasterMix (2×) (NZYTech), 0.4 μL of each primer (forward and reversed), and 2 μL of diluted cDNA (250 nmol) in a final volume of 10 μL. On each plate, a four non-template control was included. The reaction was carried out with an initial denaturation at 95 ◦ C (2 min), followed by 40 cycles of amplification with denaturation at 95 ◦ C for (15 s) and combined annealing and extension to 58–62 ◦ C, depending on the set of primers (25 s) (Supplementary Table S1). A melting curve (from 55 ◦ C to 95 ◦ C) was generated at each run to confirm the specificity of the reactions. The efficiency of the PCR for both the target and the reference genes was determined by a standard curve, using six serial dilutions from 1:10 of cDNA sets of all samples. All reference gene expressions were analysed in ReFinder online platform to obtain the best comprehensive gene stability (Xie et al., 2012). Finally, Ef1-1α and 18S were selected to perform the normalisation of the relative gene expression according to the Livak method (Livak and Schmittgen, 2001).
2.9. Statistical analysis
Normality (Kolmogorov-Smirnov) and homoscedasticity (Levene) assumptions were confirmed prior to statistical analyses. The means of growth indicators (dry weight and number of suckers) for each treatment, and FA data were compared independently by means of one-way ANOVA and Tukey’s post-hoc test (P < 0.05). The FA data were compared using univariate analysis of variance (one-way ANOVA) for each body compartment as fixed factors, exposed to different treatments. Also, the FA profiles from the body compartments of the paralarvae of the different treatments were chemometrically analysed using Multi Dimensional Scaling (PROXSCAL) to establish patterns of similarities (SPSS version 15.0). Normalised relative gene expression for each target gene was analysed first for differences through the development for each body compartment and dietary treatment using a oneway analysis of variance (ANOVA, P < 0.05). Next, the dietary effect for each target gene was analysed for each body compartment between PA and PZ using a one-way analysis of variance (ANOVA, P < 0.05). Except otherwise stated, the statistical analyses above were conducted using STATISTICA 10.0© (Zar, 1999). The expression files were normalised and analysed in Partek® Genomics Suite® in order to perform the hierarchical clustering and heat map analyses. The datasets of relative gene expression for each of the treatments and body compartments were
included, and normalisation and log2 transformations were performed.
2.10. Ethics
All experiments were carried out under the Spanish legislation (RD53/2013) and the European Directive 2010/63/EU (European Parliament, Council of the European Union, 2010) for the protection of animals used for experimentation and other scientific purposes. Adults and paralarvae were anaesthetised with an initial concentration of 1.5% magnesium chloride (magnesium chloride hexahydrate, Barcelonesa©, Global Chemical Solutions, Barcelona, Spain) for 10 min, which was subsequently increased to 3.5% for 15 min to minimize pain, suffering and distress according to Fiorito et al. (2015) A humane killing method by brain destruction using a bistoury was performed at the end of the study according to the guidelines of experimentation with cephalopods (Andrews et al., 2013; Fiorito et al., 2015).
3. Results
3.1. Growth rate and development
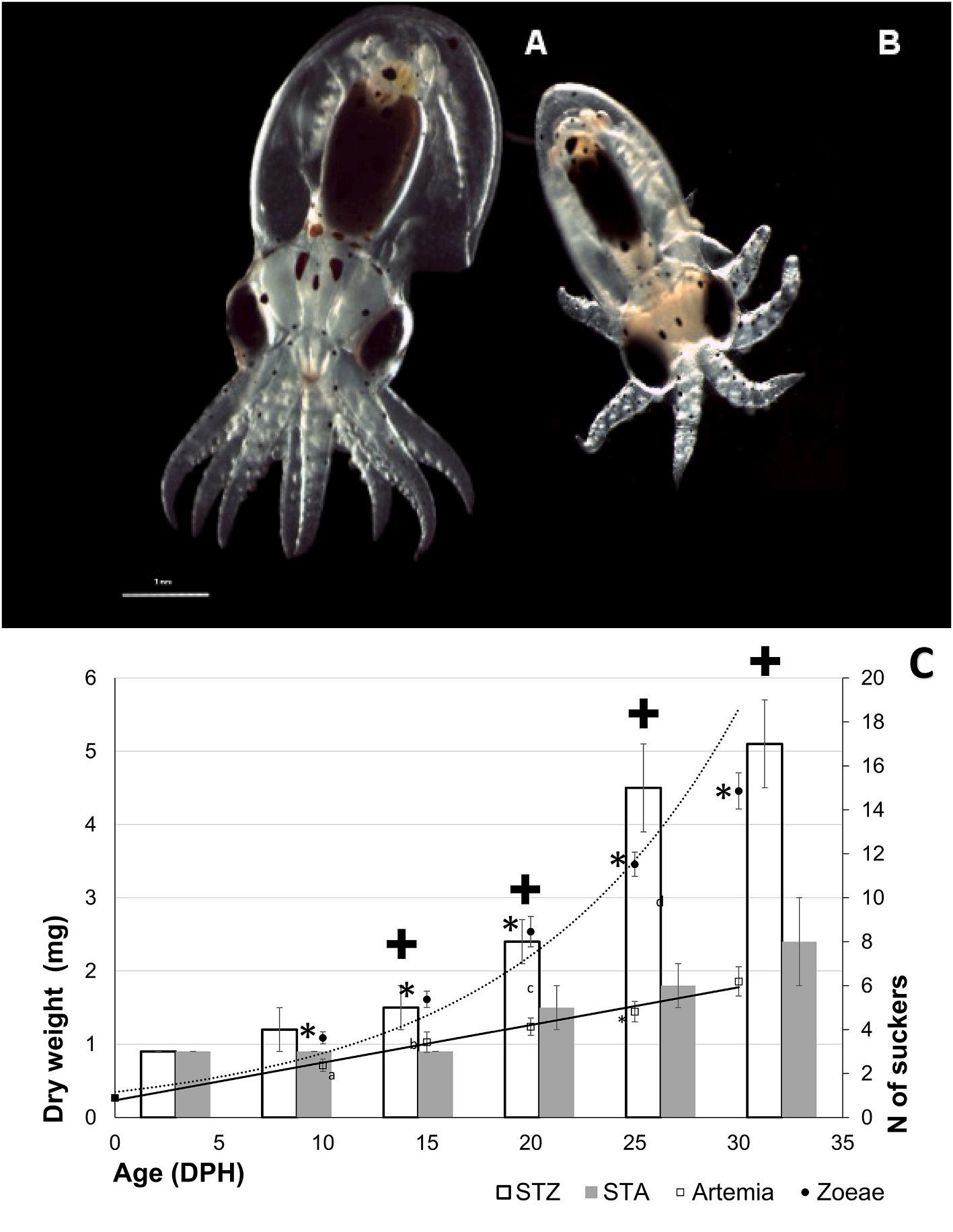
No differences in dry weight were observed in paralarvae of the same age from the two replicated experiments (P < 0.05). Thus, growth and development data were grouped by age. Average initial dry weight (PH) was 0.27 ± 0.04 mg. After 30 d of dietary treatment, average dry weight reached 1.86 ± 0.20 mg for PA and 4.46 ± 0.25 mg for PZ (P < 0.05, Fig. 1). Considering growth related to arm length, at 30 DPH it was 17 ± 2 suckers/arm for PZ, also significantly higher than that of PA (8 ± 2 suckers/arm) (P < 0.05, Fig. 1). Such difference between dietary treatments was already significant at 20 DPH, with 8 ± 1 suckers/arm for PZ, and 5 ± 1 suckers/arm for PA (P < 0.05). The SGR at 30 DPH was 9.37% for PZ, whereas PA paralarvae reached 6.46% of body increment per day.
3.2. Fatty acid composition
The FA profiles from total lipids, as well as those from the polar and neutral lipid fractions of the preys (spider crab zoeae and Artemia), are shown in Supplementary Tables S2 and S3, respectively. Also, the FA composition of the total lipids from each body compartment of the paralarvae at hatching (PH) and at 30 DPH are shown in Supplementary Table S4.
3.2.1.
Preys
The preys FA composition was significantly different (Supplementary Table S2). While Artemia metanauplii were particularly rich in 18C FA (18:1n-9, 18:1n-7, 18:2n-6, 18:3n-3, and 18:4n 3), spider crab zoeae presented a high content of ARA (20:4n-6), EPA (20:5n-3), and DHA (22:6n-3) (P < 0.05). Also, the proportion of FA in neutral and polar lipids of preys was remarkably different for most of the FA analysed (Supplementary Table S3). Besides, 18C FA like oleic acid (18:1n9), vaccenic acid (18:1n-7), and LA (18:2n-6) were present in higher proportion in the polar lipids of Artemia compared to crab zoeae, while they were more abundant in the neutral lipids from crab zoeae. However, n-6 and n-3 LC-PUFA like ARA, EPA, and DHA were found in a higher percentage in the polar lipid fraction of both preys, these being significantly more abundant in the spider crab zoeae.
3.2.2. Paralarvae
Differences were found in the FA composition of PH and fed paralarvae (Supplementary Table S4). The impact of diet on the FA composition was higher on functional (M, H, and A) than on digestive body compartment (DG). A higher proportion of palmitic acid (16:0) was found in all body compartments of PH, and it decreased significantly in both dietary treatments at 30 DPH (P < 0.05), showing the lowest amount in the DG (Table 1; Supplementary Table S4). A significantly
higher amount of 16:0 was found in the mantle and arms of PA as compared to PZ (P < 0.05).
Regarding C18-FA, the amount of stearic acid (18:0) increased during development in the mantle and digestive gland in PA and PZ compared to PH, but decreased in the arms (Table 1; Supplementary Table S4). Yet, ALA and stearidonic acid (18:4n-3) were not detected in PH and thus accumulated during development, with a higher amount found in all body compartments of PA paralarvae compared to PZ (P < 0.05). Besides, LA increased in all functiona body compartments of PA while decreasing in PZ as compared to PH (P < 0.05), and the proportion of 18C oleic (18:1n-9) and vaccenic (18:1n-7) acids, also increased in the DG of fed paralarvae (PA and PZ) as compared to that of the hatchlings (Table 1; P < 0.05). The most remarkable differences in the n-3 LC-PUFA composition between PA and PZ were found in the functional body compartments (Supplementary Table S2). Levels of eicosatrienoic acid (ETE, 20:3n-3) increased significantly in the heads of paralarvae fed both experimental diets (P < 0.05; Supplementary Table S4). The amount of ARA and EPA increased in paralarvae from both dietary treatments in all functional body compartments (Table 1; P < 0.05). Thus, the head and mantle of PZ paralarvae showed a significantly higher amount of ARA compared to PA (P < 0.05). For EPA,
Fig. 1. Image of paralarvae at 30 DPH fed with zoeae of spider crab (M. brachydactyla; A) and with Artemia metanauplii (Artemia franciscana; B). The graph represents growth in dry weight of paralarvae fed zoeae (PZ, closed circle) and Artemia (PA, open square) throughout 30 DPH. Bars indicate arms growth measured in number of suckers of paralarvae fed zoeae (SPZ), and Artemia (SPA). Within each sampling period, the asterisk (*) and symbol (✚) represent significant differences (P < 0.05) in dry weight and number of suckers respectively.
significant differences were found in the functional body compartments of the PA treatment compared to PZ (P < 0.05). Also, docosapentaenoic acid (DPA, 22:5n3) increased during development in all body compartments except in the digestive gland of PA. DHA decreased in all body compartments except in the arms and mantle of PZ during development, with the digestive gland having the lowest amounts of DHA in paralarvae from both dietary treatments (Table 1). Compared to PZ, PA showed a significantly lower proportion of DHA in all body compartments (P < 0.05).
The integration of the FA profiles in a Multidimensional Scaling Analysis (MDS) showed distinct patterns (Fig. 2). Hatchlings (PH) and on-grown paralarvae (PA and PZ) were clearly distinguishable, the latter being further segregated. Importantly, the digestive gland of both PA and PZ were separated from the rest (Fig. 2). Fatty acids of the diets also formed distinct clouds, with spider crab zoeae showing higher similarity (proximity) to the hatchlings and most functional compartments of the dietary groups, and those of Artemia being closer to the digestive gland patterns. Along the x-axis, the FA patterns were distributed following a left (more) to the right (low) gradient of FA unsaturation (i.e. LC-PUFA).
M. Nande
Table 1
Selected fatty acids (% of total fatty acids) in body compartments (mantle, head, arm and digestive gland) of hatchlings (PH) and 30 DPH paralarvae fed with zoeae of spider crab (Maja brachydactyla) (PZ) or Artemia metanauplii (Artemia franciscana) (PA). Results are the mean and standard deviation. For every body compartment and fatty acid, different letters denote significant differences (one-way ANOVA P < 0.05). ND: not detected; Sat.: saturated fatty acids; Mono.: monounsaturated fatty acids; n-3 LC-PUFA: n-3 long-chain polyunsaturated fatty acids (C ≥ 20); n-6 LC-PUFA: n-6 long-chain polyunsaturated fatty acids (C ≥ 20). Different letters within body compartments denote significant differences. Whole fatty acid results can be found in supplementary material.
Mantle Head Arm Digestive gland
3.3. Predicted sequences and phylogenetics analysis
Through the analysis of SRA from O. vulgaris, we were able to deduce complete/partial open reading frames of previously unreported genes: agpta, lpin, chpt and dgat To determine the orthology of each sequence, independent phylogenetic trees were constructed (Supplementary Fig. S1). All genes were strongly clustered within others from the phylum Mollusca with support posterior probabilties 0.99 for Agpta (Supplementary Fig. S1.A), 0.98 for Lpin (Supplementary Fig. S1.B), 1 for Chpt and Dgat (Supplementary Fig. S1.C and D). In addition, the sequences showed high homology with the sequences of O. bimaculoides (<0.99).
3.4. Gene expression
Gene expression response in each body compartment (functional and digestive) was analysed throughout development, PH to paralarvae of 30 DPH (Figs. 3 and 4). A significantly higher expression of genes involved in LC-PUFA biosynthesis was found on the functional body compartments compared to the digestive one. At hatching, paralarvae showed largely lower gene expression as compared to the 30 DPH, for scd and both for the elongases (elovl4 and elovl2/5), in all body compartments. Also, in PH, transcripts of ωx2 were only detectable in the head, and in 30 DPH paralarvae, ωx2 transcripts increased, at 30 DPH paralarvae in head. Paralleling that, expression of the desaturase fad and ωx1 was low in all body compartments of PH, and throughout development, although the gene expression increased in heads and arms for 30 DPH paralarvae.
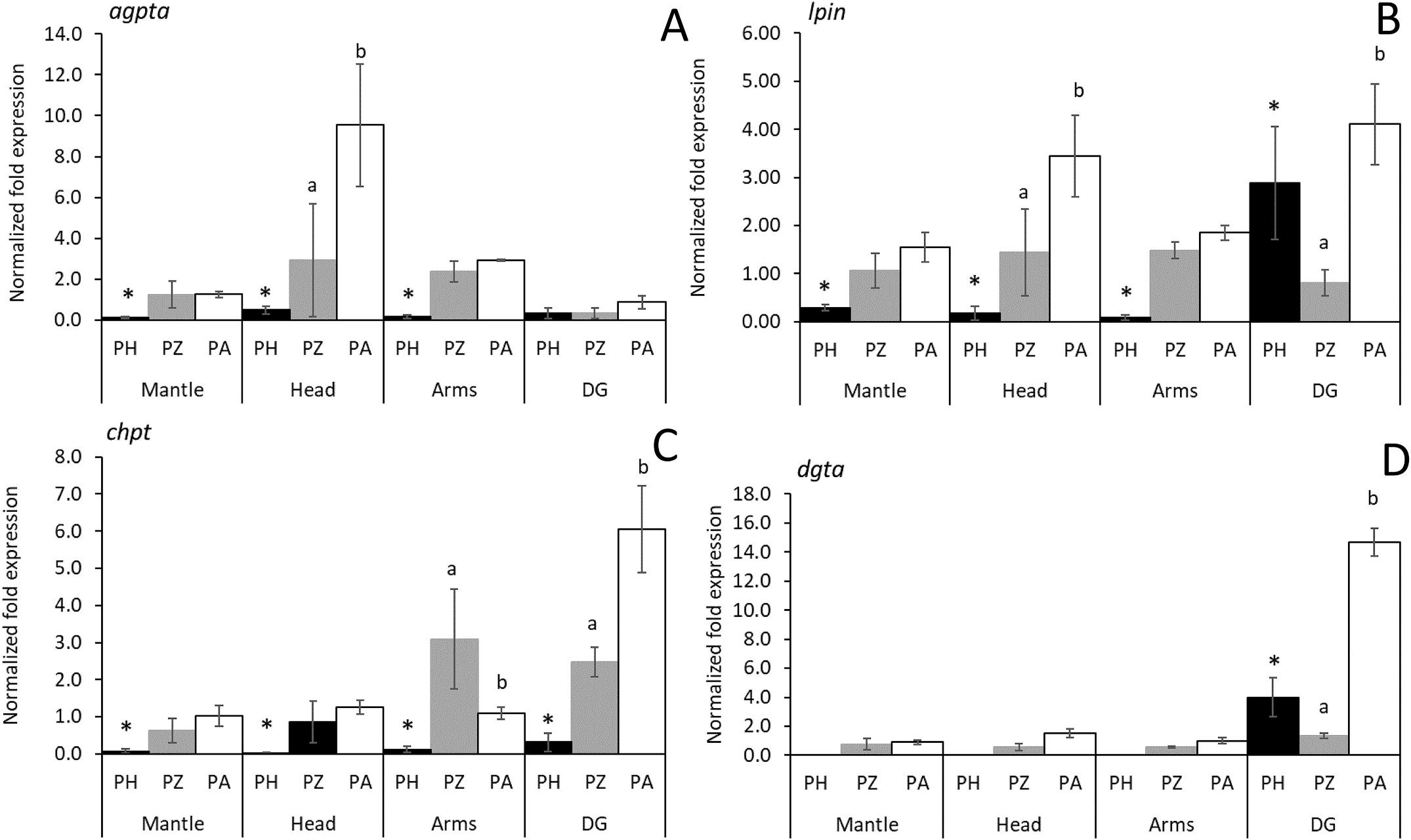
The expression of glycerophospholipid-related genes showed an increase of transcripts in the digestive gland both at hatchling (PH) and throughout development (30 DPH) except for Agpta, which was upregulated for head and arms at 30 DPH compared with PH (P < 0.05).
The lpin expression increased during development (from PH to 30 DPH) for all functional compartments of the body; however, for DG, it decreased significantly for PZ. Also, chpt showed a low number of transcripts in all body compartments of PH, increasing during development in the digestive gland and arms of PA and PZ respectively (P < 0.05). Therefore, the expression dgat in PH was higher in the digestive gland than in the functinal body compartments, and at 30 DPH it was upregulated for PA and down-regulated for PZ compared with PH (P < 0.05).
The effect of the dietary treatments (PA and PZ) on gene expression was also analysed for each body compartment (Figs. 3 and 4). Paralarvae fed Artemia (PA) showed a general pattern characterised by an upregulation of functional body compartments for LC-PUFA genes as compared to PZ, with a significant increase (P < 0.05) of ωx2 and fad transcripts in the head, and elovl4 being up-regulated in mantle and head (Fig. 3). Furthermore, PA showed an expression pattern characterised by up-regulation of genes involved in the glycerophospholipid pathway (lpin, chpt, and dgat) in the digestive gland, as well as of agpta and lpin in the head (Fig. 4, P < 0.05). However, chpt showed a higher increase of transcripts in arms of PZ as compared to PA (P < 0.05).
In order to provide a global view of these results, a hierarchical clustering and a heat map analysis of target genes were conducted. Hierarchical clustering segregated the genes into three major clusters such as elovl4, elovl2/5, and scd for the first group, agpta, fad, ωx2, ωx1 for the second, and lpin, chpt, and dgat for the third (Fig. 5). Clustering in terms of body compartments/treatments was determined by the similarity in the expression between PZ and PH in all the body compartments. In fact, the expression of PA was far from that of the other dietary treatments, and showed a tendency towards an up-regulation of most of the genes analysed. In particular, the pattern of gene expression in the digestive gland of PZ was far from that of PA and very close to that of PH (Fig. 5).

Fig. 2. Multidimensional Scaling of the fatty acid profiles of the body compartments of O. vulgaris paralarvae and preys. Green ovals group the scores of paralarvae fed zoeae (PZ), orange ovals of those fed Artemia (PA), and the blue oval hatchings (PH). Zoea (green oval) and Artemia (orange oval) identify the scores of the profiles of both preys. M, mantle; H, head; A, arms; DG, digestive gland. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)

Fig. 3. Relative expression of genes related to LC-PUFA biosynthesis (stearoyl-CoA desaturase (scd), ωx2 desaturase (ωx2), ωx1 desaturase (ωx1), fatty acyl desaturase (fad), elovl2/5, and elovl4) analysed in diffrerent body compartments (mantle, head, arms, and digestive gland) from hatchlings (PH) to 30 days post-hatch (30 DPH) paralarvae, and among dietary treatments (PZ vs PA). The results are presented as the means ± sd (N = 6) of a pool of body compartments (N = 3). Asterisks (*) indicate significant differences (P < 0.05) during development, and letters between dietary treatments (P < 0.05).
4. Discussion
This study is the first to offer comprehensible information on the effect of a diet rich in LC-PUFA (zoeae, mainly ARA and DHA) and a diet with marked content of C18-FA (Artemia) in functional body compartments compared to digestive ones, and on the compensatory
M. Nande
Fig. 4. Relative expression of genes related to glycerophospholipid biosynthetic pathway (agpta, lpin, chpt, and dgat) analysed in different body compartments (mantle, head, arms, and digestive gland) from hatchlings (PH) to 30 days post-hatch (30 DPH) paralarvae, and among dietary treatments (PZ vs PA). The results are presented as the means ± sd (N = 6) of a pool of body compartments (N = 3). Asterisks (*) indicate significant differences (P < 0.05) during development, and letters between dietary treatments (P < 0.05).

Fig. 5. Hierarchical clustering of genes detected as differentially expressed in different body compartments for the hatchlings (PH) and different dietary treatments (PA and PZ). The colour bar denotes z-score adjusted expression values, green represents lower gene expression, and red, higher. Euclidean distance and average linkage methods were used. PH: hatched paralarvae. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
M. Nande
mechanisms involved in the FA, glycerophospholipids, and glycerolipids biosynthesis during the paralarval development.
Our results show that paralarvae fed zoeae display significantly higher growth and development at 30 DPH than those fed Artemia This agrees with the results reported by several authors (Villanueva, 1995; Iglesias et al., 2004; Carrasco et al., 2006; Roo et al., 2017; Garrido et al., 2018), indicating that the decapod zoeae favor growth over Artemia Likewise, in paralarvae in co-feeding experiments (Artemia + zoeae) the SGR% varies between 7% and 8% of increment of dry weight per day (Villanueva, 1995; Iglesias et al., 2004; Carrasco et al., 2006) and reaches only values from 2% to 6% for paralarvae fed with Artemia (Navarro and Villanueva, 2000; Okumura et al., 2005; Seixas et al., 2010; Fuentes et al., 2011). It has also been reported that the number of suckers per arm reflects its growth during the transition from the planktonic stage to the benthic phase (Villanueva and Norman, 2008), and holds a good correlation with weight and development (Okumura et al., 2005), which is also agreement with the results shown in Fig. 1
The differences in growth and development of the paralarvae fed Artemia is related, among other factors, to a dietary essential FA deprivation (Navarro et al., 2014). Thus, the effect of diet on the lipid profile of paralarvae could be linked to the appearance of LA and 18:4n-3 which were retained in the metabolic organ (DG), mainly in the PA treatment, and was absent in PH. The impact of the diet in the FA composition of paralarvae has been clearly observed in co-feeding Artemia and zoea trials, or when inert diets were used as food, with the amount of LA decreasing as compared to those paralarvae fed only Artemia (Seixas et al., 2010; Roo et al., 2017). Also, our results indicate a greater amount of total n-6 LC-PUFA in energy-demanding structures, such as the mantle, involved in active swimming and breathing (Shadwick, 1995; Villanueva and Norman, 2008), and sensorial structures such as head (Villanueva et al., 2017). It has been pointed out that ARA may play pivotal roles in developmental processes (Monroig et al., 2012b). On the other hand, the head is a functional body compartment with organs such as eyes and brain that require large amounts of n-3 LC-PUFA for proper development and, interestingly, not only EPA and DHA, but for example ETE, present in high proportions in the adult eye (Monroig et al., 2012b) is also found here in the head of the paralarvae of both treatments. It is interesting that the digestive gland of fed paralarvae contained the lowest levels of n-3 LC-PUFA, as well as the arms and the digestive gland of PH (Table 1). It is tempting to hypothesize that in case of need, EPA could be quickly mobilized (Sargent et al., 1999) from the digestive gland to functional body compartments as an essential component of cell membranes (Bell and Sargent, 2003). Thus, the amount of EPA in the PA dietary treatment being higher than in the other treatments (Table 1), could account for the lower availability of DHA in Artemia and its replacement by EPA. These results agree with those obtained by several authors when feeding paralarvae with enriched Artemia (Navarro and Villanueva, 2000; Seixas et al., 2010; Fuentes et al., 2011). Although zoeae had a higher percentage of EPA than Artemia, PA retained this FA in the functional compartments pointing at the compensatory mechanism proposed before. Fish larvae fed DHA deficient diets show an increase of DPA in their FA, pointing to a similar compensatory mechanism (Furuita et al., 1996). Interestingly, functional body compartments of the paralarvae from the PA treatment showed DPA amounts similar to those of PZ (Supplementary Table S2).
Docosahexaenoic acid content was lower in the paralarvae of the PA group as compared with the others. Besides, its concentration in functional structures was notoriously higher with respect to those found in the digestive gland. The DHA dietary contribution of Artemia is very low, especially in the neutral lipids, so it can be hypothesized that the DHA present in the hatchlings was mainly functional, and was not mobilized during development because of it having a relative resistance to β-oxidation (Bell et al., 2001) in a scenario of essential FA paucity. The small percentage of DHA provided by Artemia can be counterbalanced by the incorporation of other LC-PUFA like ARA and EPA esterified into TAG (Reis et al., 2016). Compensatory mechanisms of this kind have
been reported and may be dependent on the intensity of this essential nutrient deprivation. In O. vulgaris juveniles exposed to prolonged periods of fasting it was observed that DHA was catabolized (García-Garrido et al., 2010). On the contrary, when an organ needs to increase or maintain a greater proportion of DHA, this FA can be mobilized from other body parts as occurs in wild O. vulgaris adults (Sieiro et al., 2020) and squid (Lin et al., 2019), or selectively retained as described in the cuttlefish (Castro et al., 1992). The presence of LC-PUFA, and specifically DHA, are particularly important in the head, because these FA are related to neuronal development and vision, and a deficiency could lead to serious physiological and behavioral consequences as occurs in fish (Sargent et al., 1999; Sargent et al., 2003). Also, higher DHA in the arms of the PZ paralarvae could be associated with their better performance in terms of growth and development, not to mentional axonal development and, in this sense, the fact that the arms of newly hatched paralarvae showed a lower amount of DHA can be linked to the suckers only starting to develop at 10 DPH.
The MDS analysis clearly shows the scores of the digestive gland of the dietary treatments (PA and PZ) close to each other and segregated from the rest. This points to a unique FA pattern, perhaps due to the gland being involved in nutrient storage and mobilization (Blanchier and Boucaud-Camou, 1984). Saturated FA (18:0), MUFA (18:1n-9, 18:1n-7), n6-PUFA (18:2n-6), and n3-PUFA (18:3n-3, 18:4n-3) were the main FA in the digestive gland. This points again at those found in higher proportion in functional body compartments such as 16:0, ARA, EPA, and DHA being essential (Navarro and Villanueva, 2000; Monroig et al., 2012a; Iglesias et al., 2014; Reis et al., 2014; Lourenço et al., 2017) since they would be transported and selectively retained into these structures whenever present in the diet. The MDA shows the scores of the mantle, head, and arms of the PZ group distinguished from those of PA, closer to PH, and also segregated from the composition of their prey (zoeae), reflecting the dietary effect differently expressed at the level of functional vs digestive (digestive gland) organs. The scores of the PA group distribute between the PH cluster and digestive gland, reflecting that the dietary input of a diet not fulfilling the nutritional requirements of the paralarvae, but still produces distinct functional versus digestive FA profiles. In the PH treatment, although sub-groups of organ-related scores are distinguishable, the composition of all body compartments can be grouped in the same cluster possibly reflecting the most limited “dietary” influence of yolk (Nande et al., 2017a). Finally, the scores produced by the FA patterns of Artemia are clearly separated from the rest, farther from the PH and PZ (mantle, head and arms) clusters, and next to the digestive gland ones, showing up their distinct nature.
The diet directly affects metabolic structures such as the digestive gland and has a more conservative effect on those functional body compartments that tend to keep their lipid composition constant. This effect, on the one hand, is produced by the mobilization of nutrients from the diet and, failing that, by the biosynthesis of these FA from enzymatic pathways. The ability of marine invertebrates to de novo biosynthesize saturated and monosaturated FA and their further transformation into PUFA from elongation reactions (fatty acyl elongases) and desaturations through methyl and front-end desaturases (Monroig and Kabeya, 2018) has been documented. Recently, the capacity of FA biosynthesis of cephalopods has been studied by several authors (Monroig et al., 2012a, 2012b, 2016a, 2017; Reis et al., 2014, 2016; Garrido et al., 2019; Monroig and Kabeya, 2018), as well as that of other mollusks (Pirini et al., 2007).
Our results indicate a low regulation of gene expression in PH paralarvae compared to PA and PZ (Fig. 3). This fact may be related to the ability of the yolk to meet all the nutritional needs of the paralarvae at the earliest life stages, so that the biosynthetic machinery is still on standby. Indeed, hatchlings combine endogenous and exogenous feeding and have low needs related to growth and development (Nande et al., 2017a). In any case, the digestive gland of the paralarvae from the PH group was the body compartment with the highest expression of lpin and dgat indicative of activation of glycerophospholipids synthesis
M. Nande
towards TAG. The increase in the production of TAG in this group may be related to a low concentration of this lipid class in the hatchlings (Lourenço et al., 2017) and its need in the highly demanding early stages of development as an energy source.
The degree of regulation, being inversely proportional to the suitability of the diets in providing the necessary nutrients, increased in the PA group in which most of the genes analysed were over-expressed, with the PZ treatment producing intermediate results. This is in agreement with the results of García-Fern´ andez et al. (2017) who found that the lipid biosynthesis pathways were up-regulated in paralarvae fed Artemia as compared to those fed zoeae. A higher relative gene expression of scd in functional body compartments, and mostly in the mantle of the PA treatment, was observed. The enzymatic activity of Scd, which introduces the first double bond into a saturated FA, is universally present in all organisms (Castro et al., 2011) and can be associated with the triggering of the unsaturation pathway. The presence of enzymes involved in the production of ωx2 (Δ12 activity) and ω3ωx1 (Δ15, Δ17 and Δ19 activities) methyl-end desaturations, has recently been identified in marine invertebrates (Kabeya et al., 2018). In the present results, we found a higher expression of the ωx2 methyl-end desaturations in the head and of the ωx1 in head and arms of the paralarvae of the dietary treatments, peaking in the PA and PZ dietary group, respectively. The activity of these enzymes can thus be anatomically linked in these first stages under development to the formation of structures like eyes and brain, as it has been reported in adults (Garrido et al., 2019). The activity of the Fad, supported by fad expression, was evident in the head of the PA paralarvae, perhaps due to an increase in the need for PUFA not provided by the diet. The gene that encodes this front-end desaturase has also been described in Sepia officinalis (Monroig et al., 2016b). A high expression of elongases genes was found in functional body compartments such as the mantle and head for elovl4 and head for elovl2/5. The energy demand of the mantle and the neuronal and visual maturation linked to development in head, increases the demand for LCPUFA (Monroig et al., 2017). The slower development of the arms in the PA treatment was also reflected in a lower number of transcripts, and therefore was coherent with a metabolic impairment scenario.
Hierarchical clustering helps to elucidate groupings and patterns that provide an integral vision of the results of the expression tendencies of the different genes (Bergkvist et al., 2010). In our datasets, the clustering showed first a group including elovl4, elovl2/5, and scd, that had a more widespread expression in all functional body compartments of PA and PZ, with special significance in the mantle and head. Also, these enzymes have a preference for the use of 18C and 16C saturated FA precursors. The second clustering grouped agpta, fad, ωx2, and ωx1, showing a more specific expression tendency putatively related to the development of head (Fig. 4), with visual and neuronal tissue. The clustering of different enzymes can be related to the body compartments where they are more active, as well as to the preferred FA precursor. From this point of view, ARA and EPA can be synthesized from 20:3n-6 and 20:4n-3 by the mediation of Fad (Monroig et al., 2012a), whereas Δ12ωx2 and ω3ωx1 are related to the synthesis of LA, and to the conversion of n-6 LC-PUFA to n-3 LC-PUFA, respectively (Garrido et al., 2019).
Long-chain PUFA like EPA, DHA, and ARA are an essential components of glycerophospholipid in cephalopods (Shen et al., 2020), however, the regulation of their biosynthetic involvement in these structural lipids is poorly understood in O. vulgaris Previous studies using radiotracer techniques have demonstrated de novo biosynthesis by esterification of phosphatidylethanolamine (PE) and phosphatidylcholine (PC) with a preference for ARA and EPA (Reis et al., 2014). Since the glycerophospholipid biosynthetic pathway is considered key in the production of these phospholipids and triglycerides, and PC is an abundant phospholipid in the common octopus (Reis et al., 2019), we have analysed here genes such as agpta, lpin, chpt, critical in each of the phases for the synthesis of PC, as well as dgat, involved in the synthesis of TAG. Metabolites of the glycerophospholipid pathway linked to the action of
Agpta, like phosphatidic acid, are related to neuronal development, with functions such as transmission and regulation of intracellular signaling and proliferation (Ammar et al., 2014). In our study, we found a greater amount of transcripts of this gene in the head of PA compared to the other treatments. This fact is also consistent with the hypothesis that paralarvae fed suboptimal diets tend to increase biosynthetic pathways that compensate for the effect of nutrient deficiency such as PL. Thus, Lpin plays multiple roles in the regulation of lipid metabolism and cell signaling and as a regulator in the production of PL (Reue and Brindley, 2008). Additionally, García-Fern´ andez et al. (2019) also reported upregulated gene expression of phosphatidate phosphatase (pap or lpin) in O. vulgaris paralarvae subjected to sub-optimal feeding conditions. In our study, the highest activity of Chpt was found in fed paralarvae (PA and PZ) compared to hatchlings (PH), and this fact could be due to the high content in PL of the latter, or the limited ability of de novo PL synthesis of the former, as has been documented in the liver of fish larvae (Tocher et al., 2008). Indeed, like the liver in fish, the digestive gland of cephalopods plays an important role in the digestion and metabolism of lipids, with various functions such as secretion, digestion, and absorption (O’dor et al., 1984). Furthermore, for chpt, a higher number of transcripts was found in the arms of PZ, coinciding with a significant differentiation during development, as compared to PA. The arms growth needs axons production for the formation of the nerve cord, which involves a high content of neuronal membranes rich in PC (Imperadore et al., 2019), and Chpt activity is associated with PC biosynthesis and also to the presence of synaptic membranes (Hargreaves and Clandinin, 1987).
Our results only showed overexpression of dgat in the digestive gland of PA and PH treatments. The biosynthesis of either PL or TAG in the digestive gland (involving lpin, chpt, and dgat expression) may be the result of catabolism and the re-assembly of larger molecules that facilitate their absorption. This is coherent with the absence of dgat expression in the PZ paralarvae and its overexpression in the digestive gland of the PA as a mechanism for TAG synthesis as a source of energy.
The differential anatomical gene regulation unveiled here may be of paramount importance in organisms like the paralarvae, in which a preponderant part of the body is dominated by a single organ, in this case the DG. In such cases, the analysis of the whole organism would be biased by the response of such body compartment.
In summary, the present study provides evidence of the pivotal role that diet plays in the biosynthesis of FA, glycerophospholipids, and glycerolipids in paralarvae. It shows how dietary treatments affect the expression of the related pathways, both in digestive (digestive gland) and functional (mantle, head and arms) compartments of the paralarvae, inducing unique compensatory responses. Genes related to FA biosynthesis in the head, and glycerophospholipid and glycerolipid metabolism in DG, could be used as more sensitive biomarkers of dietary effects. This anatomical approach may pave the way for further studies on the nutritional requirements of O. vulgaris and other species of cephalopods.
Author statement
MN, ´ OM, and JCN conceived, designed, and supervised the study; MN performed the rearing experiments; MN, AMM, LFCC, MLM, AC performed the Q-PCR, gene target isolation, phylogenetic analysis, and conducted data analysis. JCN performed the fatty acid profile analysis, and JCN and MN carried out the data analysis. All authors contributed to the writing and revision of the manuscript and approved the final version for submission.
Declaration of Competing Interest
We declare that all authors have no conflicts of interest in this work.
M. Nande et
Acknowledgments
This study benefited from the Short Term Scientific Missions (STSMs) include in the networking activities carried out under the COST ACTION FA1301 (STSMs), and is considered a contribution to the COST (European COoperation on Science and Technology) Action FA1301 “A network for improvement of cephalopod welfare and husbandry in research, aquaculture, and fisheries” (http://www.cephsinaction.org/). This study was funded through the project IMPROMEGA Agencia Espanola de Investigaci ´ on, Spain, grant no. RTI2018-095119-B-100, MCIU/AEI/FEDER/UE/ MCIN/AEI/10.13039/501100011033/ and FEDER “A way to make Europe.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi. org/10.1016/j.aquaculture.2022.738293.
References
Albertin, C.B., Simakov, O., Mitros, T., Wang, Z.Y., Pungor, J.R., Edsinger-Gonzales, E., Rokhsar, D.S., 2015. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 524 (7564), 220. https://doi.org/10.1038/ nature14668
Almansa, E., Domingues, P., Sykes, A., Tejera, N., Lorenzo, A., Andrade, J.P., 2006. The effects of feeding with shrimp or fish fry on growth and mantle lipid composition of juvenile and adult cuttlefish (Sepia officinalis). Aquaculture 256 (1–4), 403–413. https://doi.org/10.1016/j.aquaculture.2006.02.025
Ammar, M.R., Kassas, N., Bader, M.F., Vitale, N., 2014. Phosphatidic acid in neuronal development: a node for membrane and cytoskeleton rearrangements. Biochimie 107, 51–57. https://doi.org/10.1016/j.biochi.2014.07.026
Andrews, P.L.R., Darmaillacq, A.S., Dennison, N., Gleadall, I.G., Hawkins, P., Messenger, J.B., Smith, J.A., 2013. The identification and management of pain, suffering and distress in cephalopods, including anaesthesia, analgesia and humane killing. J. Exp. Mar. Biol. Ecol. 447, 46–64. https://doi.org/10.1016/j. jembe.2013.02.010
Artimo, P., Jonnalagedda, M., Arnold, K., Baratin, D., Csardi, G., De Castro, E., Stockinger, H., 2012. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 40 (W1), W597–W603. https://doi.org/10.1093/nar/gks400
Bell, J.G., Sargent, J.R., 2003. Arachidonic acid in aquaculture feeds: current status and future opportunities. Aquaculture 218 (1–4), 491–499. https://doi.org/10.1016/ S0044-8486(02)00370-8
Bell, J.G., McEvoy, J., Tocher, D.R., McGhee, F., Campbell, P.J., Sargent, J.R., 2001. Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. J. Nutr. 131 (5), 1535–1543. https://doi.org/10.1093/jn/131.5.1535
Beppu, F., Yasuda, K., Okada, A., Hirosaki, Y., Okazaki, M., Gotoh, N., 2017. Comparison of the distribution of unsaturated fatty acids at the sn-2 position of phospholipids and triacylglycerols in marine fishes and mammals. J. Oleo Sci. https://doi.org/ 10.5650/jos.ess17132 ess17132.
Bergkvist, A., Rusnakova, V., Sindelka, R., Garda, J.M.A., Sjogreen, B., Lindh, D., Kubista, M., 2010. Gene expression profiling–clusters of possibilities. Methods 50 (4), 323–335. https://doi.org/10.1016/j.ymeth.2010.01.009
Blanchier, B., Boucaud-Camou, E., 1984. Lipids in the digestive gland and the gonad of immature and mature Sepia officinalis (Mollusca: Cephalopoda). Mar. Biol. 80 (1), 39–43. https://doi.org/10.1007/BF00393125
Carrasco, J.F., Arronte, J.C., Rodríguez, C., 2006. Paralarval rearing of the common octopus, Octopus vulgaris (Cuvier). Aquac. Res. 37 (15), 1601–1605. https://doi.org/ 10.1111/j.1365-2109.2006.01594.x
Castro, B.G., Garrido, J.L., Sotelo, C.G., 1992. Changes in composition of digestive gland and mantle muscle of the cuttlefish Sepia officinalis during starvation. Mar. Biol. 114 (1), 11–20. https://doi.org/10.1007/BF00350851
Castro, L.F.C., Wilson, J.M., Gonçalves, O., Galante-Oliveira, S., Rocha, E., Cunha, I., 2011. The evolutionary history of the stearoyl-CoA desaturase gene family in vertebrates. BMC Evol. Biol. 11 (1), 132. https://doi.org/10.1186/1471-2148-11132
Christie, W.W., 1982. Lipid analysis, vol. 207. Pergamon Press, Oxford Dan, S., Iwasaki, H., Takasugi, A., Yamazaki, H., Hamasaki, K., 2018. An upwelling system for culturing common octopus paralarvae and its combined effect with supplying natural zooplankton on paralarval survival and growth. Aquaculture 495, 98–105. https://doi.org/10.1016/j.aquaculture.2018.05.036
Dan, S., Iwasaki, H., Takasugi, A., Shibasaki, S., Yamazaki, H., Oka, M., Hamasaki, K., 2019. Effects of co-supply ratios of swimming crab Portunus trituberculatus zoeae and Artemia on survival and growth of East Asian common octopus Octopus sinensis paralarvae under an upwelling culture system. Aquac. Res. 50 (4), 1361–1370. https://doi.org/10.1111/are.14013
de Ortiz, D.O., Gavioli, I.L., Bersano, J.G.F., Vidal, E.A.G., 2021. Feeding rates and prey preference in Octopus americanus paralarvae fed with different prey densities and types, Artemia, copepods, and zoeae. Aquac. Int. 29 (2), 779–800. https://doi.org/ 10.1007/s10499-021-00657-x
European Parliament, Council of the European Union, 2010. Directive 2010/63/EU Ofthe European Parliament and of the Council of 22 September 2010 on the ProtecTion of Animals Used for Scientific Purposes. Council of Europe, Strasbourg FAO, 2020. The State of World Fisheries and Aquaculture 2020. Sustainability in action, Rome. https://doi.org/10.4060/ca9229en
Fiorito, G., Affuso, A., Basil, J., Cole, A., de Girolamo, P., D’Angelo, Ludovic D., Andrews, P.L., 2015. Guidelines for the Care and Welfare of Cephalopods in Research–a consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 49 (2 suppl), 1–90. https://doi.org/10.1177/ 0023677215580006
Folch, J., Lees, M., Sloane Stanley, G.H., 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509. https:// doi.org/10.1016/S0021-9258(18)64849-5
Fuentes, L., S´ anchez, F.J., Lago, M.J., Iglesias, J., Pazos, G., Linares, F., 2011. Growth and survival of Octopus vulgaris (Cuvier 1797) paralarvae fed on three Artemia-based diets complemented with frozen fish flakes, crushed zooplankton and marine microalgae. Sci. Mar. 75 (4), 771–777. https://doi.org/10.3989/scimar.2011.75n4771
Furuita, H., Takeuchi, T., Toyota, M., Watanabe, T., 1996. EPA and DHA requirements in early juvenile red sea bream using LC-PUFA enriched Artemia nauplii. Fish. Sci. 62 (2), 246–251. https://doi.org/10.2331/fishsci.62.246
García, S., Domíngues, P., Navarro, J.C., Hachero, I., Garrido, D., Rosas, C., 2011. Growth, partial energy balance, mantle and digestive gland lipid composition of Octopus vulgaris (Cuvier, 1797) fed with two artificial diets. Aquac. Nutr. 17 (2), e174–e187. https://doi.org/10.1111/j.1365-2095.2009.00746.x
García-Fernandez, P., Castellanos-Martínez, S., Iglesias, J., Otero, J.J., Gestal, C., 2016. Selection of reliable reference genes for RT-qPCR studies in Octopus vulgaris paralarvae during development and immune-stimulation. J. Invertebr. Pathol. 138, 57–62. https://doi.org/10.1016/j.jip.2016.06.003
García-Fernandez, P., García-Souto, D., Almansa, E., Moran, P., Gestal, C., 2017. Epigenetic DNA methylation mediating Octopus vulgaris early development: effect of essential fatty acids enriched diet. Front. Physiol. 8, 292. https://doi.org/10.3389/ fphys.2017.00292
García-Fern´ andez, P., Prado-Alvarez, M., Nande, M., Perales-Raya, C., Almansa, E., Var ´ o, I., Gestal, C., 2019. Global impact of diet and temperature over aquaculture of Octopus vulgaris paralarvae from a transcriptomic approach. Sci. Rep. 9 (1), 1–17. https://doi.org/10.1038/s41598-019-46492-2
García-Garrido, S., Hachero-Cruzado, I., Garrido, D., Rosas, C., Domingues, P., 2010. Lipid composition of the mantle and digestive gland of Octopus vulgaris juveniles (Cuvier, 1797) exposed to prolonged starvation. Aquac. Int. 18 (6), 1223–1241. https://doi.org/10.1007/s10499-010-9335-6
Garrido, D., Navarro, J.C., Perales-Raya, C., Nande, M., Martín, M.V., Iglesias, J., Almansa, E., 2016. Fatty acid composition and age estimation of wild Octopus vulgaris paralarvae. Aquaculture 464, 564–569. https://doi.org/10.1016/j. aquaculture.2016.07.034
Garrido, D., Martín, M.V., Rodríguez, C., Iglesias, J., Navarro, J.C., Estevez, A., Varo, I., 2018. Meta-analysis approach to the effects of live prey on the growth of Octopus vulgaris paralarvae under culture conditions. Rev. Aquac. 10 (1), 3–14. https://doi. org/10.1111/raq.12142
Garrido, D., Kabeya, N., Hontoria, F., Navarro, J.C., Reis, D.B., Martín, M.V., Monroig, ´ O., 2019. Methyl-end desaturases withΔ 12 and ω3 regioselectivities enable the de novo PUFA biosynthesis in the cephalopod Octopus vulgaris BBA-Mol. Cell Biol. L. 1864 (8), 1134–1144. https://doi.org/10.1016/j.bbalip.2019.04.012
Gonzalez-Gurriaran, E., Freire, J., Parapar, J., Sampedro, M.P., Urcera, M., 1995. Growth at moult and moulting seasonality of the spider crab, Maja squinado (Herbst) (Decapoda: Majidae) in experimental conditions: implications for juvenile life history. J. Exp. Mar. Biol. Ecol. 189 (1–2), 183–203. https://doi.org/10.1016/00220981(95)00023-K
Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W., Gascuel, O., 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. https://doi.org/ 10.1093/sysbio/syq010
Hargreaves, K.M., Clandinin, M.T., 1987. Phosphocholinetransferase activity in plasma membrane: effect of diet. Biochem. Biophys. Res. Commun. 145 (1), 309–315. https://doi.org/10.1016/0006-291X(87)91322-2
Huson, D.H., Richter, D.C., Rausch, C., Dezulian, T., Franz, M., Rupp, R., 2007. Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinform 8 (1), 460. https://doi.org/10.1186/1471-2105-8-460
Iglesias, J., Fuentes, L., 2014. Octopus vulgaris Paralarval culture. In: Iglesias, J., Fuentes, L., Villanueva, R. (Eds.), Cephalopod Culture. Springer, New York, pp. 427–450. https://doi.org/10.1007/978-94-017-8648-5_23
Iglesias, J., Otero, J.J., Moxica, C., Fuentes, L., Sanchez, F.J., 2004. The completed life cycle of the octopus (Octopus vulgaris, Cuvier) under culture conditions: Paralarval rearing using Artemia and zoeae, and first data on juvenile growth up to 8 months of age. Aquac. Int. 12, 481–487. https://doi.org/10.1023/B:AQUI.0000042142.88449. bc
Iglesias, J., Fuentes, L., Sanchez, J., Otero, J.J., Moxica, C., Lago, M.J., 2006. First feeding of Octopus vulgaris Cuvier, 1797 paralarvae using Artemia: effect of prey size, prey density and feeding frequency. Aquaculture 261 (2), 817–822. https://doi. org/10.1016/j.aquaculture.2006.08.002
Iglesias, J., S´ anchez, F.J., Bersano, J.G.F., Carrasco, J.F., Dhont, J., Fuentes, L., Villanueva, R., 2007. Rearing of Octopus vulgaris paralarvae: present status, bottlenecks and trends. Aquaculture 266, 1–15. https://doi.org/10.1016/j. aquaculture.2007.02.019
Iglesias, J., Pazos, G., Fern´ andez, J., S´ anchez, F.J., Otero, J.J., Domingues, P., Linares, F., 2014. The effects of using crab zoeae (Maja brachydactyla) on growth and
M. Nande et al.
biochemical composition of Octopus vulgaris (Cuvier 1797) paralarvae. Aquac. Int. 22 (3), 1041–1051. https://doi.org/10.1007/s10499-013-9725-7
Iglesias, P., Picon, P., Nande, M., Lago, M.J., Otero, J.J., Trujillo, V., Iglesias, J., 2016. Effect of low salinity on survival and ingested food of the common octopus, Octopus vulgaris Cuvier, 1797. J. Appl. Aquac. 28 (3), 267–271. https://doi.org/10.1080/ 10454438.2016.1190953
Imperadore, P., Lepore, M.G., Ponte, G., Pflüger, H.J., Fiorito, G., 2019. Neural pathways in the pallial nerve and arm nerve cord revealed by neurobiotin backfilling in the cephalopod mollusk Octopus vulgaris Invertebr. Neurosci. 19 (2), 5. https://doi.org/ 10.1007/s10158-019-0225-y
Kabeya, N., Fonseca, M.M., Ferrier, D.E., Navarro, J.C., Bay, L.K., Francis, D.S., Monroig, O., 2018. Genes for de novo biosynthesis of omega-3 polyunsaturated fatty acids are widespread in animals. Sci. Adv. 4 (5) https://doi.org/10.1126/sciadv. aar6849 eaar6849.
Katoh, K., Standley, D.M., 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30 (4), 772–780. https://doi.org/10.1093/molbev/mst010
Katoh, K., Rozewicki, J., Yamada, K.D., 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20 (4), 1160–1166. https://doi.org/10.1093/bib/bbx108
Lefort, V., Longueville, J.E., Gascuel, O., 2017. SMS: smart model selection in PhyML. Mol. Biol. Evol. 34 (9), 2422–2424. https://doi.org/10.1093/molbev/msx149
Lin, D., Han, F., Xuan, S., Chen, X., 2019. Fatty acid composition and the evidence for mixed income–capital breeding in female Argentinean short-fin squid Illex argentinus Mar. Biol. 166 (7), 90. https://doi.org/10.1007/s00227-019-3534-0
Livak, K.J., Schmittgen, T.D., 2001. Analysis of relative gene expression data using realtime quantitative PCR and the 2 ΔΔCT method. Methods 25 (4), 402–408. https:// doi.org/10.1006/meth.2001.1262
Lourenço, S., Roura, ´ A., Fernandez-Reiriz, M.J., Narciso, L., Gonzalez, A.F., 2017. Feeding relationship between Octopus vulgaris (Cuvier, 1797) early life-cycle stages and their prey in the western Iberian upwelling system: correlation of reciprocal lipid and fatty acid contents. Front. Physiol. 8, 467. https://doi.org/10.3389/ fphys.2017.00467
Monroig, O., Kabeya, N., 2018. Desaturases and elongases involved in polyunsaturated fatty acid biosynthesis in aquatic invertebrates: a comprehensive review. Fish. Sci. 84, 911–928. https://doi.org/10.1007/s12562-018-1254-x
Monroig, O., Navarro, J.C., Dick, J.R., Alemany, F., Tocher, D.R., 2012a. Identification of a Δ5-like fatty acyl desaturase from the cephalopod Octopus vulgaris (Cuvier 1797) involved in the biosynthesis of essential fatty acids. Mar. Biotechnol. 14 (4), 411–422. https://doi.org/10.1007/s10126-011-9423-2
Monroig, O., Guinot, D., Hontoria, F., Tocher, D.R., Navarro, J.C., 2012b. Biosynthesis of essential fatty acids in Octopus vulgaris (Cuvier, 1797): molecular cloning, functional characterisation and tissue distribution of a fatty acyl elongase. Aquaculture 360, 45–53. https://doi.org/10.1016/j.aquaculture.2012.07.016
Monroig, ´ O., Lopes-Marques, M., Navarro, J.C., Hontoria, F., Ruivo, R., Santos, M.M., Castro, L.F.C., 2016a. Evolutionary functional elaboration of the Elovl2/5 gene family in chordates. Sci. Rep. 6, 20510. https://doi.org/10.1038/srep20510
Monroig, ´ O., Hontoria, F., Varo, I., Tocher, D.R., Navarro, J.C., 2016b. Investigating the essential fatty acids in the common cuttlefish Sepia officinalis (Mollusca, Cephalopoda): molecular cloning and functional characterisation of fatty acyl desaturase and elongase. Aquaculture 450, 38–47. https://doi.org/10.1016/j. aquaculture.2015.07.003
Monroig, ´ O., de Llanos, R., Varo, I., Hontoria, F., Tocher, D., Puig, S., Navarro, J.C., 2017. Biosynthesis of polyunsaturated fatty acids in Octopus vulgaris: molecular cloning and functional characterisation of a stearoyl-CoA desaturase and an elongation of very long-chain fatty acid 4 protein. Mar. Drugs 15 (3), 82. https://doi. org/10.3390/md15030082
Nande, M., Iglesias, J., Domingues, P., P´ erez, M., 2017a. Effect of temperature on energetic demands during the last stages of embryonic development and early life of Octopus vulgaris (Cuvier, 1797) paralarvae. Aquac. Res. 48 (4), 1951–1961. https:// doi.org/10.1111/are.13032
Nande, M., Presa, P., Roura, A., Andrews, P.L., P´ erez, M., 2017b. Prey capture, ingestion, and digestion dynamics of Octopus vulgaris paralarvae fed live zooplankton. Front. Physiol. 8, 573. https://doi.org/10.3389/fphys.2017.00573
Nande, M., Domingues, P., Rosas, C., 2018. Effects of temperature on the embryonic development of Octopus vulgaris J. Shellfish Res. 37 (5), 1013–1019. https://doi. org/10.2983/035.037.0512
Navarro, J.C., Villanueva, R., 2000. Lipid and fatty acid composition of early stages of cephalopods: an approach to their lipid requirements. Aquaculture 183, 161–177. https://doi.org/10.1016/S0044-8486(99)00290-2
Navarro, J.C., Villanueva, R., 2003. The fatty acid composition of Octopus vulgaris paralarvae reared with live and inert food: deviation from their natural fatty acid profile. Aquaculture 219, 613–631. https://doi.org/10.1016/S0044-8486(02) 00311-3
Navarro, J.C., Monroig, ´ O., Sykes, A.V., 2014. Nutrition as a key factor for cephalopod aquaculture. In: Cephalopod culture. Springer, Dordrecht, pp. 77–95. https://doi. org/10.1007/978-94-017-8648-5_5
O’dor, R.K., Mangold, K., Boucher-Rodoni, R., Wells, M.J., Wells, J., 1984. Nutrient absorption, storage and remobilization in Octopus vulgaris Mar. Freshw. Behav. Phy. 11 (3), 239–258. https://doi.org/10.1080/10236248409387049
Okumura, S., Kurihara, A., Iwamoto, A., Takeuchi, T., 2005. Improved survival and growth in Octopus vulgaris paralarvae by feeding large type Artemia and Pacific sandeel, Ammodytes personatus: improved survival and growth of common octopus paralarvae. Aquaculture 244 (1–4), 147–157. Lab. Anim. 49(2_suppl), 1-90. https://doi.org/10.1016/j.aquaculture.2004.11.044
Pirini, M., Manuzzi, M.P., Pagliarani, A., Trombetti, F., Borgatti, A.R., Ventrella, V., 2007. Changes in fatty acid composition of Mytilus galloprovincialis (Lmk) fed on microalgal and wheat germ diets. Comp. Biochem. Phy Part B: Biochem. Mol Biol. 147 (4), 616–626. https://doi.org/10.1016/j.cbpb.2007.04.003
Reis, D.B., Acosta, N.G., Almansa, E., Navarro, J.C., Tocher, D.R., Monroig, O., Rodriguez, C., 2014. In vivo metabolism of unsaturated fatty acids in Octopus vulgaris hatchlings determined by incubation with 14C-labelled fatty acids added directly to seawater as protein complexes. Aquaculture 431, 28–33. https://doi.org/10.1016/j. aquaculture.2014.05.016
Reis, D.B., Acosta, N.G., Almansa, E., Tocher, D.R., Andrade, J.P., Sykes, A.V., Rodriguez, C., 2016. Composition and metabolism of phospholipids in Octopus vulgaris and Sepia officinalis hatchlings. Comp. Biochem. Physiol. Part B: Biochem. Mol Biol. 200, 62–68. https://doi.org/10.1016/j.cbpb.2016.06.001
Reis, D.B., Acosta, N.G., Almansa, E., Garrido, D., Andrade, J.P., Sykes, A.V., Rodríguez, C., 2019. Effect of Artemia inherent fatty acid metabolism on the bioavailability of essential fatty acids for Octopus vulgaris paralarvae development. Aquaculture 500, 264–271. https://doi.org/10.1016/j.aquaculture.2018.10.021
Reue, K., Brindley, D.N., 2008. Thematic review series: glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 49, 2493–2503. https://doi.org/10.1194/jlr.R800019-JLR200
Roo, J., Estefanell, J., Betancor, M.B., Izquierdo, M., Fernandez-Palacios, H., Socorro, J., 2017. Effects of supplementation of decapod zoea to Artemia basal diet on fatty acid composition and digestive gland histology in common octopus (Octopus vulgaris) paralarvae. Aquac. Res. 48 (2), 633–645. https://doi.org/10.1111/are.12910
Sargent, J., McEvoy, L., Estevez, A., Bell, G., Bell, M., Henderson, J., Tocher, D., 1999. Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture 179 (1–4), 217–229. https://doi.org/10.1016/S0044-8486 (99)00191-X
Sargent, J.R., Tocher, D.R., Bell, J.G., 2003. The lipids. In: Fish Nutrition. Academic Press, pp. 181–257. https://doi.org/10.1016/B978-012319652-1/50005-7
Seixas, P., Rey-M´ endez, M., Valente, L.M., Otero, A., 2008. Producing juvenile Artemia as prey for Octopus vulgaris paralarvae with different microalgal species of controlled biochemical composition. Aquaculture 283 (1–4), 83–91. https://doi.org/10.1016/j. aquaculture.2008.06.019
Seixas, P., Rey-M´ endez, M., Valente, L.M., Otero, A., 2010. High DHA content in Artemia is ineffective to improve Octopus vulgaris paralarvae rearing. Aquaculture 300 (1–4), 156–162. https://doi.org/10.1016/j.aquaculture.2009.12.021
Shadwick, R.E., 1995. Mechanical organization of the mantle and circulatory system of cephalopods. Mar. Freshw. Behav. Phy. 25 (1–3), 69–85. https://doi.org/10.1080/ 10236249409378909
Shen, Y., Xie, H.K., Liu, Z.Y., Lu, T., Yu, Z.L., Zhang, L.H., Wang, T., 2020. Characterization of glycerophospholipid molecular species in muscles from three species of cephalopods by direct infusion-tandem mass spectrometry. Chem. Phys. Lipids 226, 104848. https://doi.org/10.1016/j.chemphyslip.2019.104848
Sieiro, P., Otero, J., Aubourg, S.P., 2020. Biochemical composition and energy strategy along the reproductive cycle of female Octopus vulgaris in Galician waters (NW Spain). Front. Physiol. 11, 760. https://doi.org/10.3389/fphys.2020.00760
Tocher, D.R., Bendiksen, E.Å., Campbell, P.J., Bell, J.G., 2008. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 280 (1–4), 21–34. https:// doi.org/10.1016/j.aquaculture.2008.04.034
Untergasser, A., Cutcutache, I., Koressaar, T., Ye, J., Faircloth, B.C., Remm, M., Rozen, S. G., 2012. Primer3 new capabilities and interfaces. Nucleic Acids Res. 40 (15), e115. https://doi.org/10.1093/nar/gks596
Uriarte, I., Iglesias, J., Domingues, P., Rosas, C., Viana, M.T., Navarro, J.C., Zuniga, O., 2011. Current status and bottle neck of octopod aquaculture: the case of American species. J. World Aquacult. Soc. 42 (6), 735–752. https://doi.org/10.1111/j.17497345.2011.00524.x
Var ´ o, I., Cardenete, G., Hontoria, F., Monroig, O., Iglesias, J., Otero, J.J., Navarro, J.C., 2017. Dietary effect on the proteome of the common octopus (Octopus vulgaris) paralarvae. Front. Physiol. 8, 309. https://doi.org/10.3389/fphys.2017.00309
Viciano, E., Iglesias, J., Lago, M.J., Sanchez, F.J., Otero, J.J., Navarro, J.C., 2011. Fatty acid composition of polar and neutral lipid fractions of Octopus vulgaris Cuvier, 1797 paralarvae reared with enriched on-grown Artemia. Aquac. Res. 42, 704–709. https://doi.org/10.1111/j.1365-2109.2010.02605.x
Villanueva, R., 1995. Experimental rearing and growth of planktonic Octopus vulgaris from hatching to settlement. Can. J. Fish. Aquat. Sci. 52 (12), 2639–2650. https:// doi.org/10.1139/f95-853
Villanueva, R., Norman, M.D., 2008. Biology of the planktonic stages of benthic octopuses. https://doi.org/10.1201/9781420065756.ch4
Villanueva, R., Perricone, V., Fiorito, G., 2017. Cephalopods as predators: a short journey among behavioral flexibilities, adaptions, and feeding habits. Front. Physiol. 8, 598. https://doi.org/10.3389/fphys.2017.00598
Xie, F., Xiao, P., Chen, D., Xu, L., Zhang, B., 2012. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 80 (1), 75–84. https://doi. org/10.1007/s11103-012-9885-2
Xie, D., Chen, C., Dong, Y., You, C., Wang, S., Monroig, ´ O., Li, Y., 2021. Regulation of long-chain polyunsaturated fatty acid biosynthesis in teleost fish. Prog. Lipid Res. 82, 101095 https://doi.org/10.1016/j.plipres.2021.101095
Xu, Y., Caldo, K.M.P., Jayawardhane, K., Ozga, J.A., Weselake, R.J., Chen, G., 2019. A transferase interactome that may facilitate channeling of polyunsaturated fatty
M. Nande et
acid moieties from phosphatidylcholine to triacylglycerol. J. Biol. Chem. 294 (41), 14838–14844. https://doi.org/10.1074/jbc.AC119.010601
Zar, J.H., 1999. In: Ryu, T. (Ed.), Biostatistical Analysis, 4th edition. Prentice-Hall Inc, Upper Saddle River, N.J, p. 663
Zarrella, I., Herten, K., Maes, G.E., Tai, S., Yang, M., Seuntjens, E., Fiorito, G., 2019. The survey and reference assisted assembly of the Octopus vulgaris genome. Sci. Data 6 (1), 1–8. https://doi.org/10.1038/s41597-019-0017-6
M. Nande et al.
Other documents randomly have different content
Supply, that since Peel’s bill of 1819 was accepted as a final settlement of the currency question, the salaries of all public servants should be cut down by 20 per cent. Though listened to attentively, he received small support, either from his own friends or the friends of the Government; but he added, by the vigour of his appeal, to the reputation which he had already acquired, and was by common consent assigned a place with Lord Althorpe, Lord John Russell, and Mr Brougham, as one of the leaders of the Opposition in the House of Commons.
Early in May 1830 the Parliamentary campaign opened in earnest, by a notice of motion by Sir James Graham for a return of all the pensions, salaries, and emoluments then receivable by members of the Privy Council. His speech was, in its own way, a telling one; and the motion was met by a proposal from the Chancellor of the Exchequer to supply the honourable member with a comprehensive enumeration of all civil and military offices and salaries under the Crown. Sir James either felt or affected great indignation, and, in rejecting Mr Goulbourne’s counter-proposal, made use of the expression, “That he was not disposed to stoop to ignoble game while flights of voracious birds of prey were floating in the upper regions of the air.” This was one of the clap-traps in which Sir James on all convenient occasions indulged, and it had its effect. Not fewer than 147 members in a House of 382 voted with him—a remarkable sign of the times, a sure proof that men’s passions had overclouded their reason on many matters, and that Government by party, as it had once existed, was for a season at least at an end.
Encouraged by the plaudits which were heaped upon him, Sir James, after remaining quiet for a few weeks, moved to reduce the grant for special diplomatic missions from £28,000 to £18,000 a year. He was again opposed with all the strength which the Government could muster, and again failed. But failure on this occasion was accepted on both sides as a triumph. In a House of 217 members, the motion was rejected by a majority of 19 only. It was a blow to the Ministers scarcely less severe than that which they received the same evening, when Sir James Mackintosh carried his clause in the Forgery Bill against them—abolishing the punishment of death in all cases except where wills were concerned.
The death of George IV., on the 20th of June 1830, was soon followed by the dissolution of Parliament. Sir James went back to his constituents with a reputation largely enhanced; and while his canvass was at its height, tidings of the revolution in Paris arrived. They set the whole country in a blaze, and two Liberal members immediately started for Cumberland. A fierce contest ensued, of the temper of which some idea may be formed when we transcribe one of the toasts which was proposed and accepted amid a tempest of applause at a public dinner given to Sir James Graham at Whitehaven—“May the heads of Don Miguel, King Ferdinand, and Charles Capet be severed from their bodies and roll in the dust, and the sooner the better.” It would be unfair to the memory of Sir James Graham if we omitted to add that he wholly disapproved of this sentiment, and that, while applauding the revolution, he expressed himself anxious that the French people should use their victory with moderation.
We have now arrived at an era into the history of which it would be out of place, while sketching Sir James Graham’s career, to enter much at length. The elections of 1830 had gone against the Government, and the country seemed to have become a prey to anarchy. There were incendiary fires in many places; and when Parliament met in November to provide a remedy, the worst spirit manifested itself in both Houses. The King’s visit to the Lord Mayor of London was postponed; and the Duke, with extraordinary rashness, gave utterance to a statement which his enemies insisted on accepting as a manifesto against all reform. A coalition between the Whigs and the ultra-Tories to expel him from power ensued, and Ministers, being defeated on a question of the civil-list, resigned their places. In bringing all this to pass Sir James had taken an active part, and he received his reward in the appointment of First Lord of the Admiralty, with a seat in Earl Grey’s Cabinet. He was placed at the Admiralty, however, rather as representing ultra opinions than from any admiration of his talents and industry; for Earl Grey, desiring above all things to throw the authority of Government into the hands of aristocrats, was too prudent to overlook the policy, situated as he then was, of having every great party in the State represented in his Cabinet. Hence the Duke of Richmond, Mr Wynn, Lord Palmerston, Lord Goderich, and Mr Charles Grant, were invited to take their seats beside Lord Lansdowne, Lord Althorpe, and Lord
Carlisle; and Lord Durham, Sir James Graham, and Lord Melbourne readily joined them. Among all these there was not one who displayed so large an amount of administrative ability as Sir James Graham, or who with so much frankness acknowledged, when the proper time came, that the improvements effected by him in the department were little more than the execution of plans which his predecessor had already arranged and determined upon.
Earl Grey had taken office pledged to three things,— Retrenchment, Non-intervention in Foreign Affairs, and Parliamentary Reform. Into all these Sir James Graham eagerly threw himself. Returned again without opposition for Cumberland, he took up his residence at the Admiralty, and worked like a slave to keep ahead of the enormous amount of business which devolved upon him. For now his real worth was discovered. What might be wanting in brilliancy he endeavoured to make up by labour; and he held his own, not without a hard fight for it, in the House of Commons. Lord Althorpe, the acknowledged leader of the Ministerial party, was slow and confused. He derived the greatest benefit from the subtle and ambitious promptings of Graham, and often sought for them. Whether the proposal in the first Whig budget to impose a tax on the transfer of stock came from this source does not appear; but the measure, in spite of the eloquence with which Sir James Graham supported it, met with no success, and was withdrawn amid the jeers of the House.
This was a bad beginning, and his speech in defence of the army estimates proved equally unfortunate. The pledge of nonintervention had been thrown over by the Government in the case of Belgium, and an increase to the army was asked for. In advocating this increase, Sir James allowed himself considerable latitude of speech in regard to the condition of Ireland, and O’Gorman Mahon, conceiving that he, among others, had been attacked, called upon the First Lord to retract, or else to give him personal satisfaction. Sir James requested Lord Althorpe to act for him on that occasion, and the quarrel was amicably settled.
The improvements introduced into the constitution of the Admiralty were chiefly these: Sir James abolished the Victualling and Navy Boards as separate establishments; he required the accounts of the office to be kept by double entry; he proposed to throw open the
great national asylum at Greenwich to seamen of the mercantile marine; and, failing to accomplish that, he relieved the mercantile marine from the special tax which it had heretofore borne. Nor was he all the while exempt from a full share of the burden of administration in other respects. Earl Grey never lost sight of the pledge which he had given to reform the representative system throughout the United Kingdom, and a committee of four was appointed to investigate the whole subject, and to report upon it to the Cabinet. Lord Durham, Lord John Russell, Lord Duncannon, and Sir James Graham composed that committee, of which no member worked more steadily and with greater zeal than Sir James.
It is not our purpose to tell over again the thrice-told tale of the bloodless revolution of 1831–32. In preparing the scheme which the Government was to bring forward, Sir James Graham appears to have been less extravagant than some of his colleagues. He desired to interfere as little as possible with existing rights in counties, except by adding copyholders and leaseholders to the ancient freeholders. In boroughs he was an advocate for occupancy as a condition to freedom, and was willing that the limit of the pecuniary qualifications should be wide. He objected to the ballot, and to anything like an attempt to establish perfect uniformity of franchise anywhere. Yet such was his infirmity of purpose that he yielded his own opinions to those of men of stronger will, and affixed his signature to a report which recommended the ballot and other arrangements of which he disapproved. It was this weakness, indeed —this apparent inability to arrive at settled convictions and to stand by them—which constituted the great flaw in Sir James Graham’s character as a public man. His biographer, we observe, commends him for the specialty, and endeavours to make what was mere irresolution stand in the light of judicial impartiality. “Half his life,” says Mr Torrens, “was spent in comparing and pondering opposite results, and determining judicially in the silence and solitude of his study on which side the balance lay. ‘Upon the whole’ again and again occurs throughout his private correspondence and public judgments, for judgments they frequently were—a phrase which a statesman of a constitutional country may well employ as eminently expressive of the true candour and humility of wisdom.” Doubtless this is true; but if we find the statesman afterwards going apart from his own conclusions, and falling in with proposals against which he
had “on the whole” decided, what can we say of him except that his humility degenerates into weakness, and that, whatever qualities he may possess, firmness of purpose is certainly not one of them?
It is a remarkable fact that not even now could Sir James Graham command the attention of the House. His great attention to business, his value on a committee, and his administrative abilities, were very generally acknowledged, but as a speaker he made little or no impression. Even his advocacy of what may be called his own measure was felt to be feeble—a strange medley of confused discussion and turgid enunciations. But the bill had other sources of strength to depend upon than the logic of its Parliamentary supporters. Political unions and conspiracies out of doors did the work, and the King and the House of Lords were forced to accept their own humiliation. First came the dissolution on the 23d of April 1831, a step into which William IV. was coerced by the overbearing insolence of Earl Grey and Lord Chancellor Brougham. Then followed elections wherein brute force bore down all opposition, and by-and-by such an assembly at Westminster as struck terror into the hearts of the Ministers who had brought it together. On the top of that wave Sir James Graham was again borne into Parliament—a colleague being given to him of opinions far more advanced than his own. So it befell in the borough of Carlisle, so also in the neighbouring county of Westmoreland. It is quite certain that Sir James Graham did not contemplate the crisis, which he had helped to bring on, without alarm. “We have ventured,” he says, speaking of himself and his colleagues, “to drive nearer the brink than any other statesman ever did before; but we did so because aware that if we let go the reins the horses would be maddened into plunging headlong into the abyss, where extrication would be impossible.”
We have alluded elsewhere to Sir James Graham’s reconstruction of the departments in the Admiralty. It is creditable to him that he disclaimed all the merit of originality in such reconstruction. He discovered, on acceding to office, that plans of practical reform were already settled, and he had the good sense to accept and act upon them as his own. He found a willing adviser likewise in Lord Melville, who kept back nothing from him when consulted. Having completed this job, he set himself next to devise some means of getting rid of the necessity of impressment, and was again fortunate enough to
have brought to him an important letter, addressed by Lord Nelson to Earl St Vincent. The letter in question suggested that there ought to be a registration of seamen, among whom, at the sudden outbreak of war, a ballot should take place, with permission, as in the militia, to find substitutes. But, anxious as he was to accomplish this object, he shrank as a Minister of the Crown from openly striking a blow at the prerogative. When, therefore, Mr Buckingham moved, “That the forcible impressment of seamen for His Majesty’s navy was unjust, cruel, inefficient, and unnecessary,” Sir James Graham resisted the motion. He fought, however, less for the evil itself than for the manner of applying a remedy, and obtained leave of the House to bring in a bill which has many admirable points in it, but which he was not destined to guide through its various stages till it became law.
The years 1833 and 1834 were seasons of sore trial to the Reform Government. They had evoked a power at home which they found themselves ill able to control. They had entered into treaties and engagements abroad, the necessity of acting up to which involved them in heavy expenses. But most of all were they hampered and annoyed by the operations of the Irish party, which, after helping them to carry their great measure, asked for its reward. The Irish Established Church must be sacrificed; and the better to insure a speedy attainment of that object, an agitation was got up for the repeal of the Union. Now, Earl Grey was not a man to endure contradiction calmly; he introduced a stern Coercion Bill into the House of Lords, which his colleagues fought inch by inch in the House of Commons. In order to conciliate their Radical supporters, they proposed at the same time to reduce the number of Irish bishops, and to substitute for church-rate in Ireland moneys to be raised by taxes imposed on all sees and benefices. Finally, after providing, as was assumed, a better method of managing episcopal and chapter lands, a clause in their bill declared “That it should be lawful to appropriate any portion thence accruing to purposes of secular utility, without regard to the religious opinions of persons to be benefited.” This famous clause (the 147th) was warmly debated, and in the end withdrawn. But neither section of the Legislature seemed to be satisfied. Indeed, in the Cabinet itself diversity of opinion was held in regard to that matter, and no great while elapsed ere diversity of opinion led to separation.
The first overt proof of schism in the Cabinet was presented by the opposite sides which Sir James Graham and his colleagues took on Mr O’Connell’s motion of censure upon the Irish judge, Sir William Smith. Sir James stoutly resisted it. Mr Stanley, Lord Althorpe, and Lord John Russell voted for it. On a division, Sir James went out with a minority of 74, and next morning tendered his resignation. He had proved himself, however, too valuable a member of the Administration to be cast adrift, and Earl Grey refused to part with him. Three nights afterwards he committed another crime by acknowledging in his place that the economy effected by his predecessors at the Admiralty was quite equal to his own. Then followed a discussion upon the Corn Laws, which he defended as they stood; whereas Mr Poulett Thompson, Vice-President of the Board of Trade, proposed to substitute a fixed duty for a slidingscale. And here an incident befell which deserves notice. Mr Poulett Thompson endeavoured to confute his opponent by reading extracts from a pamphlet which had appeared in 1830, and in which the author, under the nom de guerre of a Cumberland Landowner, advocated entire freedom of trade in corn, as in other commodities. Strange to say, Sir James Graham took no notice of the ironical cheers which followed these quotations, and which marked the conviction of the House that the pamphlet had emanated from his pen. Yet such was not the case. The pamphlet was the work of a Mr Rooke, and was acknowledged as such when, four years subsequently, the author gave to the world a volume on the science of geology. Why Sir James Graham did not decline the honour thrust upon him at the moment, we are at a loss to conceive, and his biographer certainly assigns no satisfactory reason for the proceeding.
The Cabinet worked on not very amicably, and Sir James Graham did it what service he could by taking charge of its bill for remodelling the Exchequer Office. But the time was come when he felt that he could serve it no longer. Lord Wellesley’s measure for converting tithes in Ireland into a permanent rent-charge on the land was cumbered by a question from Mr Shiel, drawing from Lord John Russell something like a pledge, that the Government might hereafter consider the propriety of applying a portion of this rentcharge to secular purposes. And a few days later Mr Ward brought forward his motion, “That the Protestant Episcopal Establishment in
Ireland exceeds the spiritual wants of the Protestant population, and that, it being the right of the State to regulate the distribution of Church property in such a manner as Parliament might determine, it is the opinion of this House ‘that the temporal possessions of the Church of Ireland, as established by law, ought to be reduced.’” There was no evading a movement like this. The Cabinet must either resist or accept Mr Ward’s motion, and a majority determined to accept it. Now, however Radical Sir James Graham’s views might be on other points, he was then, as he always had been, a consistent Churchman. On many previous occasions he had declared his determination to defend to the uttermost the inviolability of what he regarded as a fundamental institution of the Empire; and the Duke of Richmond, Lord Ripon, and Mr Stanley agreed with him. When, therefore, this Act for confiscating the property of the Church was accepted by the Cabinet as its own, the four Ministers above named felt that only one course lay open to them: they retired from the Administration, and shook it thereby to its base.
Sir James sat below the gangway, on the Ministerial side of the House, while those gyrations went on which ended in shaking Earl Grey out of the Premier’s chair, and Lord Melbourne into it. With Mr Stanley, and the half-dozen friends who adhered to him, Sir James kept aloof from each of the rival parties, becoming one of the company who, as Mr O’Connell described it, “travelled by themselves in the Derby Dilly.” It is not for us to inquire into the motives which animated the little band at that time. But considerations of delicacy towards old friends were surely rated above their just value when they induced Mr Stanley and Sir James Graham, a few months subsequently, to decline taking office under Sir Robert Peel. Had they met his advances as frankly as they deserved, the Conservative Government of 1835 would have probably stood its ground; and though it be difficult to conceive, looking both at things present and things past, how the commercial system, now in the ascendant, could have been pushed aside, still the progress of that system would have been probably more gradual; it certainly might have achieved its triumph at a sacrifice less costly than the disruption of the great Tory party, which followed on the repeal of the Corn Laws in 1846.
Sir James was coldly looked upon by the Liberals for abandoning Earl Grey’s Administration, and a cabal was got up to resist his re-
election for East Cumberland in the event of his taking office with Sir Robert Peel. He refused to take office, as we have shown, and defended himself well at the hustings against the attacks which were made upon him. East Cumberland chose him again to be its representative, and he again took his seat below the gangway on the Ministerial side of the House. As an independent member, however, he stood aloof from the struggle between Sir Robert Peel and the Whigs, till Lord John Russell brought forward his famous motion “For the appropriation to secular purposes of a portion of the Church property in Ireland.” Then Sir James Graham threw over all party scruples. He delivered against the motion one of the most telling speeches which he ever uttered in Parliament, and went out into the gallery with that gallant band which failed to keep their chief in office by twenty-five votes only. From that moment his severance from the Whigs became a mere question of time, and the bitterness with which the Municipal Reform Bill was argued hurried it on. Sir James had never desired to swamp the poorer voters, either in counties or boroughs, and voted against the extinction of the class of freemen. Having gone out with the Tories, he was preparing to cross the House to his old seat, when a storm of derisive cheering greeted him, accompanied by shouts of “Stay where you are!” He stopped, looked angrily at the benches whence the sounds proceeded, and then sat down with a smile of scorn on his lips on one of the Opposition benches.
For the part which he took in resisting the extension to Ireland of the municipal changes which were effected in England and in Scotland, Mr Torrens severely censures Sir James Graham. This is natural enough. Going far beyond his hero in Radical propensities, Mr Torrens dispenses blame where men of moderate views would award praise. He seems to forget that all legislation for Ireland was undertaken in those days with a twofold purpose only—to conciliate Mr O’Connell, and to humble the House of Lords. The Melbourne Ministry, however, rode their hobby too fast. Not a few of the most distinguished of the old reformers fell off from them. Indeed, to such a height was the spirit of alienation carried, that not Sir James Graham only, but likewise Lord Brougham, Sir Francis Burdett, and Lord Stanley, withdrew their names from Brookes’s, into which Mr O’Connell had been received as a member.
From this date up to the death of William IV. in 1837, party spirit prevailed in Parliament and out of it, with a bitterness which has no parallel in modern times. The Ministers, existing by the breath of Mr O’Connell and the Radicals, seemed indifferent to the consequences of the measures which they proposed. The great body of the Opposition, carried away by personal dislike to their opponents, fought more than one battle which it would have been wise to avoid, and compelled their more judicious leaders to fight with them. On the whole, however, the Duke in the House of Lords and Sir Robert Peel in the Commons managed matters well; and it is only just to Sir James Graham to add, that they found in him a hearty as well as a prudent coadjutor.
The accession of her present Majesty led, of course, to a dissolution, and Sir James Graham had the mortification to find himself opposed in East Cumberland by Major Aglionby, formerly his fastest friend. He received, on the other hand, but a doubtful support from the Conservatives, and on the day of nomination the mob refused to hear him. Naturally proud, and perhaps a little dissatisfied with himself, he quitted the hustings, and went to the poll in bad heart. He was defeated by a majority of upwards of 500 votes, and withdrew, full of indignation, to Netherby. He had suffered not long before this a heavy domestic affliction in the death of his mother; and mortified ambition, coming on the back of private sorrow, wellnigh broke him down. He took no further part in county business; he shut himself out from county society, and spent his time chiefly in reading every new book that came out, and corresponding on important subjects with Sir Robert Peel. It was the interval between his defeat for East Cumberland and his return to Parliament as member for the Welsh borough of Pembroke which made him, what he ever after continued to be, a Peelite to the backbone.
In 1838 Sir James Graham was elected Lord Rector of the University of Glasgow, in opposition to the Duke of Sussex. He delivered an inaugural address, which is probably still remembered in consequence of the uproar which it called forth by certain allusions to the necessity of keeping the Church in alliance with the State; for then the fever of Free-Kirk folly was at its height in Scotland. But in 1839 he had graver matters to attend to. That systematic agitation against the Corn Laws having already begun of
which Mr Charles Villiers, and not Mr Cobden, was the author. Sir James spoke in his place against interference with the sliding-scale; at the same time he guarded himself from the charge of desiring to secure a monopoly in the corn-market for the English landowner, and went out of his way to warn the House that nothing could be more perilous to English interests than that monopoly in the supply of cotton which had been conceded to America. He was anxious even then that steps should be taken to encourage the better cultivation of the plant in India, and pressed upon the President of the Board of Control the wisdom of originating such a scheme. Words of warning which, disregarded at the moment, come back upon us now with a melancholy echo!
The progress of the struggle, which ended in the withdrawal of the Appropriation Clause and the passing of the Irish Municipal Reform Bill through both Houses of Parliament, is of too recent date to require that we should speak of it in detail. So is the contest which arose about softening down some of the clauses in the New PoorLaw, of which Sir James, though advocating the law itself, was a strenuous advocate. His speech on that occasion, as well as his censure of the job which pensioned Sir John Newport and raised Mr Spring Rice to the peerage, more and more drew towards him the sympathies of the Conservative party, which indeed had already begun to look to him as one of its future leaders. He was equally efficient in his attacks on the Whig mismanagement of affairs in India and in China, and certainly did not spare his old friends when stirred by their rebukes into invective. At last the collapse came, and in 1841 the country declared against the Whigs. A new Administration was formed, with Sir Robert Peel at its head. Sir James Graham accepted the seals of the Home Office, and for five years public affairs were carried on, if not smoothly in all respects, with remarkable success upon the whole. No doubt, Lord Aberdeen’s legislation in the matter of the Church of Scotland proved unfortunate, and there was little in Sir James Graham’s manner to reconcile the discontented portion of the Scottish clergy to the law as it stood. Indeed, it is a remarkable fact, that almost all the failures in Sir Robert Peel’s policy occurred on points of which the management devolved upon Sir James Graham. To him, in a great degree, was attributed the disruption in the Scottish Church. His bill for the amendment of the Factory Act of 1833 hung fire, and was withdrawn;
while his attempt to reform the ecclesiastical courts in England utterly broke down.
In 1843 the difficulties of the Administration really began. Ireland was the rock ahead which they found it impossible to weather. They brought in one bill, which they ultimately abandoned, and were content to appoint a Commission, with Lord Devon at its head, to inquire into the state of the country with a view to future legislation. To some of their adherents, moreover, they appeared to be shaken in their adhesion to the sliding scale of duties on the importation of foreign corn; and day by day the great fact became more obvious that Parliamentary government, based on a widely-extended suffrage, is scarcely compatible with the continuance of monarchy. Fortunately, perhaps, for them, Mr O’Connell chose this moment to reawaken the demand in Ireland for the dissolution of the Union, and to inflame the passions of the people by his monster meetings. Great, we should now say unnecessary, forbearance was exhibited in dealing with this movement; but at last a manifesto appeared, which, besides calling upon the masses to assemble at Clontarf, invited the “Repeal cavalry” to attend in troops of twenty-five,—each under its own officers. The Lord-Lieutenant and Lord Chancellor of Ireland happened both to be in London at the time. They met Sir James Graham at the Home Office with the law officers of the Crown, and that decisive step was taken which not only dispersed the meeting of Clontarf, but shut up Mr O’Connell in jail.
We believe that Sir James Graham did nothing more than his duty to the Crown and to the country throughout these proceedings. He contrived, however, to concentrate all the bile of the Opposition on his own head, and a manner, not always very gracious, repelled, if it did not positively disgust, not a few of the supporters of the Administration. It so happened also, that his bill for limiting the hours of labour in the factories did not meet the views either of the mill-owners or of Lord Ashley (the present Lord Shaftesbury) and his friends. The result was, that being defeated on one point by Lord Ashley, and on another by the mill-owners, he withdrew his measure, and sustained, as unsuccessful legislators usually do, some loss of character from the process. But the most damaging event in the course of his Administration was his having authorised, by warrant, the letters of Mazzini and other refugees to be opened at the General