Aquaculture
journal homepage: www.elsevier.com/locate/aquaculture
Comparative transcriptome analysis revealed the role and mechanism of a FeoC-like LuxR-type regulator in intracellular survival
of Aeromonas hydrophila
Zhe Zhang 1 , Leilei Mao 1 , Yingxue Qin * , Lingmin Zhao, Lixing Huang, Xiaojin Xu, Qingpi Yan *
Fisheries College, Key Laboratory of Health Mariculture for the East China, Ministry of Agriculture, Jimei University, Xiamen, China
ARTICLE INFO
Keywords:
LuxR-type regulator
FeoC regulator
Intracellular survival
Macrophage
Aeromonas hydrophila
ABSTRACT
A. hydrophila is one of the most important pathogens in the aquaculture industry, and its drug-resistant strains continue to emerge due to the irregular use of drugs. Previous studies found A. hydrophila could survive in fish macrophages, so it was speculated that A. hydrophila may hide in host macrophages to escape the killing of drugs and other antibacterial factors. The mechanism of A. hydrophila survival in fish macrophages is worthy of further study. In this study, the expression of a FeoC-like LuxR-type regulator was stable silenced by shRNA to construct the silencing strain feoC-RNAi. And the intracellular survival rate of feoC-RNAi decreased by about 67.5% compared with that of the wild-type strain. Comprehensive transcriptome analysis suggested that this FeoC-like LuxR-type regulator may regulate at least 1286 genes. According to GO and KEGG analysis of these 1286 DEGs, it can be speculated that this regulator may affect A. hydrophila intracellular survival by regulating bacterial metal ion metabolism, chemotaxis, type IV pilus biogenesis and binding, then further regulate the bacterial intracellular survival. Further comparison on biological phenotype of A. hydrophila B11 and feoC-RNAi revealed this FeoC-like LuxR-type regulator could regulate the expression of genes related to iron and magnesium metabolism and greatly affect bacterial growth under the limited magnesium concentration, but has no effect on bacterial growth under the limited iron concentration. And inhibited expression of this FeoC-like LuxR-type regulator led to a decrease in chemotactic ability of A. hydrophila by about 60%, disappearance of pilus, and a decrease in adhesion by about 50%. So a conclusion could be made that this FeoC-like LuxR-type regulator might play a vital role in regulating the uptake of magnesium and the homeostasis of magnesium and iron, and chemotaxis, type IV pilus biogenesis and adhesion of A. hydrophila, then further protect bacteria from the microbicidal actions and keep survival in host macrophages.
1. Introduction
A. hydrophila is an opportunist pathogen widely distributed throughout the world, which not only causes aquatic animal diseases, but also has high pathogenicity to other terrestrial animals including human (Awan et al., 2018; Daskalov, 2006; Elwitigala et al., 2005; Lin et al., 2017). As one of the most common pathogens in the aquaculture, A. hydrophila has been regarded as a serious threat to the aquaculture industry. At present, the measures to control the diseases caused by A. hydrophila mainly relies on antibacterial drugs, and the irregular use of antibiotics easily lead to the emergence of drug-resistant strains (Kusdarwati and Rozi, 2017). Previous studies have shown that
* Corresponding authors.
E-mail addresses: yxqin@jmu.edu.cn (Y. Qin), yanqp@jmu.edu.cn (Q. Yan).
A. hydrophila can survive in fish macrophages, which means that A. hydrophila can keep host macrophage as a shelter to escape the killing of drugs and other antibacterial factors, making the bacteria more difficult to control (Qin et al., 2014; Wang et al., 2019; Zhang et al., 2019; Zhang et al., 2018). These studies also suggested that A. hydrophila may be a facultative intracellular bacterium.
It has been known that phagocytes have developed various antimicrobial defense mechanisms to eliminate pathogens, including the oxidative burst, acidification of phagosomes, or fusion of phagosomes with lysosomes (Kohler et al., 2002). At the same time, facultative intracellular bacteria, in return, have developed strategies counteracting the phagocytes defense to keep survive. Therefore, to elucidate the
1 These authors contributed equally to this work and should be considered co-first authors
https://doi.org/10.1016/j.aquaculture.2022.738287
Received 7 February 2022; Received in revised form 14 April 2022; Accepted 20 April 2022
Availableonline25April2022
0044-8486/©2022ElsevierB.V.Allrightsreserved.
mechanism of intracellular survival of A. hydrophila will help to find more effective treatments to control this bacterium.
The genome analysis of the pathogenic A. hydrophila B11 isolated from diseased eel revealed that six LuxR family proteins were found in this bacterium (Mao et al., 2020). Among them, LuxR05735 is the smallest one with only 243 bp, but it had the greatest impact on the intracellular survival of A. hydrophila B11 (Accession Number: MN327964) (Mao, 2019). Furthermore, LuxR05735 is also annotated as FeoC-like transcription regulator in databases. It means that LuxR05735 has both the structure and function of LuxR-type family and FeoC transcriptional regulatory factors. FeoC is regarded as a member of Feo system which is the first transport system commonly used by bacteria to acquire environment Fe2+ under anaerobic and low pH conditions, but the function of FeoC is still controversial (Hung et al., 2012; Lau et al., 2016). LuxR family proteins have been known to distribute in a variety of bacteria, which can regulate various physiological functions of bacteria (Zeng and Xie, 2011). Studies have also suggested that LuxR family proteins involve in the adaptation of bacteria to the host environment, and have a regulatory effect on the intracellular viability of bacteria (Ahmer, 2004; Fang et al., 2013). At the same time, the host cells also evolve several mechanisms to limit the survival and growth of bacteria in them, including generating reactive oxygen and nitrogen species, lowering the pH of the compartment harboring and invading microbe, limiting pathogenic microbes access to metal ion and so on (Blanc-Potard and Groisman, 2021; Gan et al., 2019; Nunez et al., 2018).As transition metals such as iron, manganese, and zinc play a key role in nutrition for both microbes and their hosts, intracellular bacteria must regulate the uptake and metabolism of metal ion in the intracellular environment and help bacteria resist cell killing, which may be the key factor in determining the survival of intracellular bacteria. What is worthy of further study is how this regulator, which has both the LuxR and FeoC characteristics, regulates the expression of A. hydrophila and further help bacteria survive in host macrophages.
In this study, silencing strain feoC-RNAi was constructed and its intracellular survival ability was evaluated. Then, comparative transcriptome was used to analyze the difference in expression between the wild-type strain and silencing strain. Finally, the biological phenotype was verified to explore the role and mechanism of this FeoC-like LuxRtype regulator in the intracellular survival of A. hydrophila
2. Materials and methods
2.1. Bacterial strains and growth conditions
The pathogenic A. hydrophila strain B11 was routinely grown in LB at 28 ◦ C, Escherichia coli was grown at 37 ◦ C in LB. The medium was supplemented with the appropriate antibiotics at the following concentrations: 50 μg/mL streptomycin (Sm) and 25 μg/mL chloromycetin (Cm). Table 1 listed the bacterial strains and plasmids used in this study.
2.2. Construction of feoC-RNAi
The gene of this FeoC-like LuxR-type regulator of A. hydrophila B11 was stably silenced according to the protocol described before (Tokunaga et al., 2015; Zhang et al., 2019; Zhang et al., 2018). Briefly, three short hairpin RNA sequences targeting different part of mRNA coding regions were designed and synthesized by Shanghai Generay Biotech Co., Ltd. (Shanghai, China) (Table 2). These annealed shRNA were ligated to the pACYC184 vector digested with SphI and BamHI (Takara, Kusatsu, Japan) usingT4 DNA ligase (Takara, Kusatsu, Japan). The recombinant plasmids were identified and transformed into E.coli DH5α to amplification. And then, the recombinant plasmids were transformed into A. hydrophila B11 by electroporation. The stable silencing strains were first screened by Chloramphenicol and Streptomycin, identified by PCR and sequencing. Finally, the expression of this FeoC-like LuxR-type regulator in each silencing strains were evaluated by qRT-PCR and the
Table 1
Strains and plasmids used in this study.
Strains or plasmids
Plasmids
Characteristic(s)
pACYC184 (CmR TcR)
pACYC184feoC-68
pACYC184 with shRNA- pACYC184feoC 68 (CmR)
pACYC184feoC 78 pACYC184 with shRNA- pACYC184feoC 78 (CmR)
pACYC184feoC 175
Strains
pACYC184 with shRNA- pACYC184feoC 175(CmR)
B11 A. hydrophila isolated from diseased Anguilla japonica (SmR)
feoC68-RNAi feoC of B11 was silenced by shRNA- feoC68 (SmR, CmR)
feoC78-RNAi feoC of B11was silenced by shRNA- feoC78 (SmR, CmR)
feoC175-RNAi feoC of B11 was silenced by shRNA- feoC175 (SmR, CmR)
E.coli DH5α deoR,recA1 endA1,hsdR17(rK ,mK+),phoA, supE44,λ ,thi-1,gyrA96,relA1
Source or reference
provided by Prof. Nie
This study
This study
This study
Provided by Prof. Guo
This study
This study
This study
Takara
strain exhibited the best efficiency of gene silencing was designated as feoC-RNAi and chosen for further studies.
2.3. Preparation of fish macrophages
Healthy tilapia (Oreochromis spp) (1068.1 ± 40.2 g) from aquaculture farm was used to prepare fish macrophages according to the method described by Isla et al. and Mccarthy et al (McCarthy et al., 2008). Briefly, fish were anesthetized with 4-ethyl-amino-benzocaine, the head kidneys were removed and pooled under sterile conditions. The tissue was then pushed through a 100 mesh nylon mesh and suspended in L-15 medium (Biological Industries, Israel) with 100 IU streptomycin/penicillin (S/P)/mL and 2% fetal bovine serum (FBS) (Thermo Fisher, USA). The cell suspension was slowly added to a 34%/51% discontinuous Percoll (Amersham Pharmacia Biotech, UK) separation solution with a syringe and centrifuged at 400 ×g for 30 min at 4 ◦ C. Cell bands were collected in the middle of the 34%/51% interface and the cells were washed twice and resuspended in L-15 medium containing 10% FBS, 100 IU S/P/mL. After that, the cells were incubated at 28 ◦ C for 4 h, nonadherent cells were removed by washing with L-15 medium and adherent cells were collected by centrifugation. Then the cells were resuspended in L-15 medium containing 10% FBS, 100 IU S/P/mL and adjusted to approximately 2 × 106 cells/mL.
2.4. Survival of A. hydrophila in fish macrophages in vitro
Bacterial survival in fish macrophages was performed according to the methods described by Larsen et al (Boesen et al., 2001) with some modifications. Briefly, the assay was performed according to the following procedure: 1 mL of prepared fish macrophage suspension and 1 mL of prepared bacteria suspension was added to a 6-well culture plate [multiplicity of infection (MOI) = 100 (100 bacteria per macrophage)] and incubated for 1 h at 28 ◦ C for cell phagocytosis. After that, the macrophages were collected and pooled in sterile tubes and centrifuged at 100 ×g for 5 min at 28 ◦ C. Then the supernatant was carefully removed without disturbing the packed cells. After washing the packed cells twice with cold PBS, the cells were resuspended in 2 mL PBS. The cell suspension was treated with 2500 mg/mL gentamicin at 28 ◦ C for 20 min to kill extracellular bacteria, and then washed twice with icecold PBS. After centrifugation to collect the cells, the supernatant was removed and tested for sterility by plate count. The packed cells were resuspended in fresh L-15 medium containing 10% FBS and 100 units S/ P. The cell suspension was incubated at 28 ◦ C in 5% CO2, the cell
Z.
Table 2
Oligonucleotides used in producing shRNA for stable gene silencing.
Target gene shRNA sequence for stable gene silence
feoC-RNAi-68
feoC-RNAi-78
feoC-RNAi-175
F:5’-GATCCGCCACTTCCACACCTCGGAAGTTCAAGAGACTTCCGAGGTGTGGAAGTGGCTTTTTTGCATG-3′
R:5′ - CAAAAAAGCCACTTCCACACCTCGGAAGTCTCTTGAACTTCCGAGGTGTGGAAGTGGCG 3’
F:5’ - GATCCCGGTCCCATCGTCATGCTCATTCAAGAGATGAGCATGACGATGGGACCGTTTTTTGCATG 3′
R:5′ - CAAAAAACGGTCCCATCGTCATGCTCATCTCTTGAATGAGCATGACGATGGGACCGG 3’
F:5 - GATCCGGCAAGAGCCTGGAGGTGATGTTCAAGAGACATCACCTCCAGGCTCTTGCCTTTTTTGCATG 3′
R:5′ - CAAAAAAGGCAAGAGCCTGGAGGTGATGTCTCTTGAACATCACCTCCAGGCTCTTGCCG 3’
samples were taken out after incubation for 0 h and 1 h, then centrifuged for 5 min at 100 ×g at 28 ◦ C. For the samples of 0 h and 1 h, the supernatant was discarded, and 1 mL of sterile distilled water was added to incubate for 30 min to lyse the cells. After that, the cell lysis was centrifuged and the CFU of the precipitate was determined by plate counting. Intracellular survival rate was defined as the number of CFU of 1 h cell lysate sample divided by the number of CFU of 0 h cell lysate sample.
2.5. Transcriptomic analysis
Total RNA were extracted from B11 and feoC-RNAi strains from three independent cultures. The RNA-seq libraries were constructed using TruSeq™ Stranded Total RNA Library Prep Kit (Illumina, San Diego, CA, USA). dUTP was used instead of dTTP in the dNTPs reagent for the second strand of cDNA to make the base in the second strand of cDNA contain A/U/C/G. Prior to PCR amplification, the second strand of the cDNA was digested with the UNG enzyme such that the library contained only the first strand of cDNA. After that, sequencing was carried out on the Illumina HiSeq4000 platforms at Majorbio Biotech Co., Ltd. (Shanghai, China).
In order to ensure the accuracy of subsequent bioinformatics analysis, the original sequencing data is first filtered to obtain high quality sequencing data (clean data). High quality sequences were aligned to the specified reference genome by Bowtie software. The differentially expressed genes were screened under the condition of P ≤ 0.05, |logFC| ≥ 1, which means that P ≤ 0.05 after correction, and the difference multiples more than 2 times were identified as significant differences. Significant enrichment analysis of GO functions was performed on differential genes to illustrate the functional enrichment of differential genes using the Blast2GO software, and the differences between samples were clarified at the level of gene function. KOBAS software was used to perform KEGG pathway enrichment analysis.
The transcriptome data were submitted to NCBI (Accession Number: SRR10681608, SRR10681622, SRR10681623, SRR10681612, SRR10681613, SRR10681614).
2.6. RNA extraction and reverse transcription
The RNA samples returned by the sequencing company was used for qPCR verification to confirm the transcriptome data. The other RNA samples was extracted from freshly cultured bacteria and first-strand cDNA synthesis were performed using EasyPure RNA Kit (TransGen Biotech, China) and TransScript® ALL-in-one First-Strand cDNA Synthesis SuperMix (TransGen Biotech, China) according to the manufacturer’s recommended protocol, respectively.
2.7. qRT-PCR
qRT-PCR was performed using QuantStudio 6 Flex PCR system (ABI, Garlsbad, CA, USA) with SYBR Green qPCR Mix (TransGen Biotech, China) according to the manufacturer’s instructions. 16S rRNA was used as an internal control. The reaction was carried out in a 10 μL volume mixture containing 0.2 μL PowerSYBR Green PCR Master Mix, 5 pmol L1 specific primers and approximately 50 ng cDNA. The cycling
parameters were 95 ◦ C for 10 min, followed by 45 cycles of 95 ◦ C for 20 s, 56 ◦ C for 20 s, and 72 ◦ C for 20 s. Threshold cycling and dissociation curves were determined using QuantStudioTM 6 Flex software to confirm that only one PCR product was amplified and detected. The relative expression of genes in qRT-PCR was calculated by Pair Wise Fixed Reallocation Randomization Test (Pfaffl et al., 2002) using a relative expression software tool (version 2, REST 2008). Primer sequences are listed in Table 3
2.8. Effect of iron and magnesium on the growth and related genes expression of B11 and feoC-RNAi
Iron chelate 2,2′ -bipyridine and magnesium chelate ammonium polyphosphate was added to LB medium respectively. And the concentration of iron chelate in LB was 0, 50, 100, 200, 300 and 400 μmol/L respectively. The concentration of magnesium chelate in LB was 10, 20, 30, 40, 50 mmol/L respectively. A. hydrophila B11 was inoculated into the medium for 36 h and the growth curves of bacteria in different concentrations of chelate was draw. The lowest chelate concentration that completely inhibited bacterial growth was screen out.
According to the above screening results, the chelate that can completely inhibited the growth of A. hydrophila B11 was added to the LB medium. And different concentrations of Fe2+ , Fe3+ and Mg2+ were added. Then, the medium with Fe2+ or Fe3+ concentration of 10 3 , 10 4 , 10 5 , 10 6 mol/L and the medium with Mg2+ concentration of 10 2 , 10 3 , 10 4 , 10 5 mol/L was prepared. A. hydrophila B11 and feoC-RNAi was inoculated in these medium and cultured at 28 ◦ C, the values of
Table 3
Primer sequences for PCR and qRT-PCR.
Gene name Sequence
AHA_2496F / AHA_2496R 5′ - GGACAGCCATTTTCAGCC- 3’ / 5’ -CCTCTTTCACCACCACCG 3’
AHA_3833F / AHA_3833R 5′ - CCCAAGATCATTGCCCACC- 3’ / 5’ -CTTCCAGAAACAGCACCCG- 3’
AHA_2558F / AHA_2558R 5′ - CTGCTGGGGCTGGAAAG- 3′ / 5’AACAGGTCGGGAATGGG- 3’
AHA_2559F /AHA_2559R 5’ - TTTTGCGAGCGGGTGTTC- 3′ / 5’CCTGGCTGATGTGGGGAT- 3’
AHA_2831 F / AHA_2831 R 5’ - CAGGGCAAACTGGTGGGG- 3′ / 5′TGTCGAGCGGCGAGGAAC- 3’
AHA_4206 F / AHA_4206 R 5′ - GTTCCCTTCCAAACACAT- 3′ / 5′CAGTTCGCCTAAAAATTC- 3’
AHA_3869 F / AHA_3869 R 5′ - CGCCCTGCGTTCTTTCTT- 3′ / 5’CATCCGGGTCAGCGTTTC- 3’
AHA_0691 F / AHA_0691 R 5′ - ACTGATGATTGCGGTAGCC- 3′ / 5’CATTGAGCGCCAGATTGTA- 3’
AHA_3193 F / AHA_3193 R 5′ - CGGCTTCACTCGGCTTT- 3′ / 5’TCCTTGCGTCGCTCCTT- 3’
AHA_0686 F / AHA_0686 R 5’-GTTTTTGCGTTCAGGTTT- 3 / 5’GTTCTGGGTCAGGTTGCT- 3
AHA_4123 F / AHA_4123 R 5’-GGGCTTTCGCCACCTCAT- 3 / 5’CCCCTCCATCGCCTTGTT- 3
AHA_0937 F / AHA_0937 R 5 -GCTGGAATCCTTCATCG- 3 / 5CTCCCACTCCTCCTCGT- 3
AHA_2529 F / AHA_2529 R 5’-GACCGAGACCCAGCAGAT- 3 / 5’AGAGGGCGGGATAAAAGC- 3
AHA_1036 F / AHA_1036 R 5’-CAAAAACAAGGAGGGACG- 3 / 5’TGGAAGCGGTTGAAGGTA- 3
OD600 nm were measured every hour, and growth curves were plotted. According to the growth curve, the bacteria that grew in the medium with Fe2+ or Fe3+ concentration of 10 3 , 10 4 mol/L and Mg2+ concentration of 10 2 , 10 3 , 10 4 , 10 5 mol/L were collected and evaluated the expression of related genes by RT-PCR.
2.9. Chemotaxis of bacteira to the Surface Mucus of fish
Fish mucus was prepared according to the method described previously (Huang et al., 2015). Skin mucus of healthy tilapia (Oreochromis spp.) was carefully collected by a soft scraper, then the skin mucus was mixed with PBS and centrifuged at 20000 g for 30 min at 4 ◦ C. The supernatant was collected and filtered by 0.45 μm and 0.22 μm filters, respectively. The overnight cultured bacteria were centrifuged at 2000 rpm for 10 min at 4 ◦ C, and the collected bacteria were washed twice with PBS. Then the bacteria were resuspended with PBS and the concentration was adjusted to OD600 = 1.0.
The capillary tubes with an inner diameter of 0.1 mm were sealed at one end and filled with the fish skin mucus. A syringe was filled with the bacterial suspension prepared above. Then, the unclosed end with fish mucus of the capillary was dipped into the bacterial suspension in the syringe, and incubated at 28 ◦ C for 1 h. During this process, the capillary remains horizontal. Then the liquid in the capillary tubes was blown into PBS in the eppendorf tubes, and was diluted to evaluate the number of chemotactic bacteria by plate counting. Chemotactic index = chemotactic cell of each strains / chemotactic cell of the wild-type strain.
2.10. Adhesion of bacteria to fish mucus
The bacteria adhesion ability was measured according to the method of Huang et al. (Huang et al., 2015). Briefly, 20 μL of tilapia surface mucus was evenly spread on a glass slide with a size of 22 mm × 22 mm, and fix with methanol at room temperature for 20 min. Then 200 μL of bacterial suspension (OD600 = 0.3) was evenly spread on the mucuscoated glass slide and incubated at 28 ◦ C for 2 h, the PBS-coated glass slide was used as a negative control. Then the slides were gently washed 5 times with PBS to remove unattached bacteria, and then the adhered bacteria were fixed with 4% methanol for 30 min and stained with crystal violet for 3 min. The number of adhered bacteria was counted under microscope (Leica) (×1000). 20 fields of view of each sample were randomly selected for counting.
2.11. Transmission electron micrographs
Formvar-coated grids were floated on 20 μL drops of bacterial suspensions for 1 min. Excess sample was withdrawn by touching the edge of the grid to a cut edge of Whatman filter paper. Then, the grids were washed three times in sterile deionized water to remove the medium, and the cells were negatively stained by floating the grids on a drop of 1% phosphotungstic acid. At last, the bacteria were observed with a transmission electron microscope (Philips, Tecnai F20, Amsterdam, Holland).
2.12. Data processing
Unless otherwise stated, all experiments were repeated at least three times in triplicates. Data are presented as mean ± SD. Statistical analysis was performed by one-way analysis of variance with Dunnett’s test using SPSS 22.0 software (Chicago, IL, USA). P < 0.05 was considered statistically significant.
3. Results
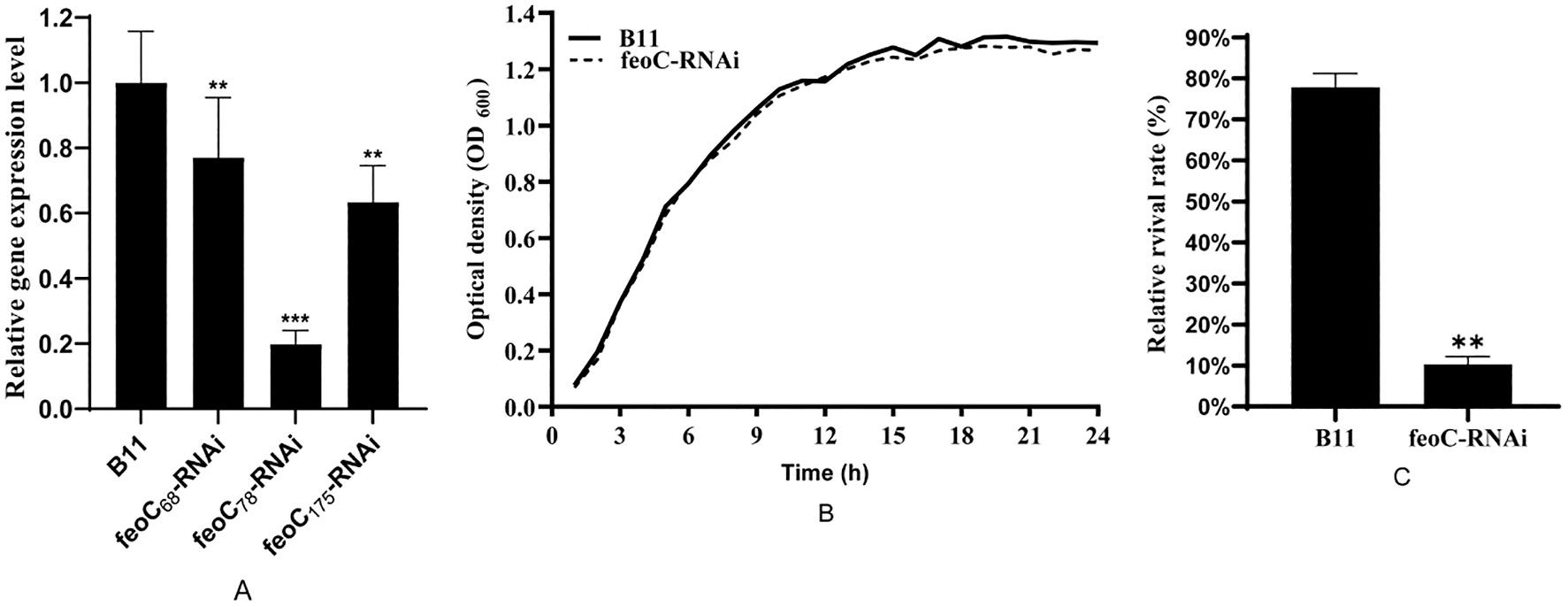
3.1. feoC-RNAi construction and intracellular survival in fish macrophages
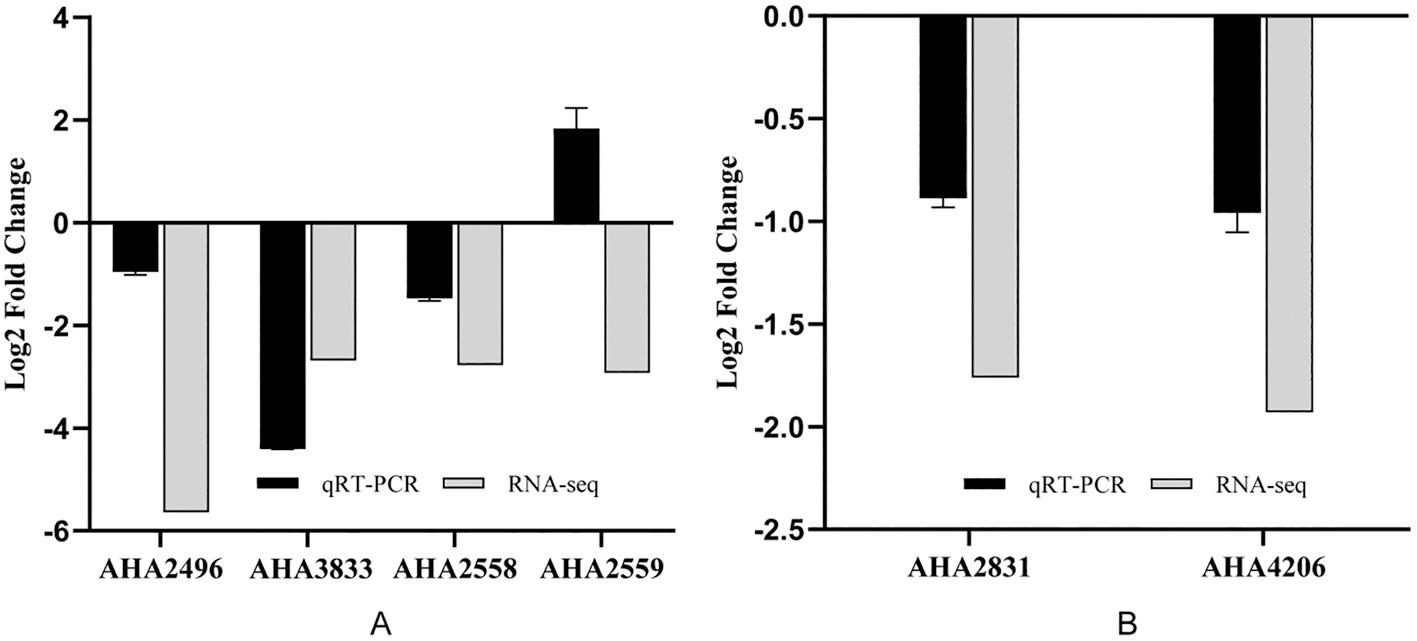
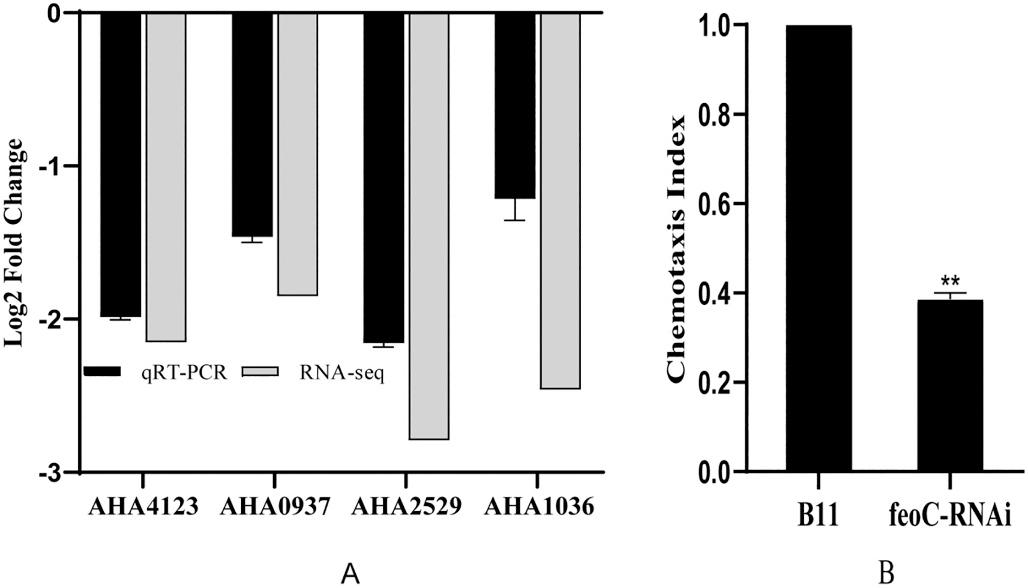
The efficiency of feoC silencing in three silencing strains showed that feoC78-RNAi exhibited the highest efficiency of gene silencing (Fig. 1A). Furthermore, the wild-type strain and feoC78-RNAi displayed almost the same growth curves (Fig. 1B). Therefore, feoC78-RNAi was selected for further studies and designated as feoC-RNAi.
The intracellular survival rates of the wild-type and feoC-RNAi (Fig. 1C) displayed that the intracellular survival rate of feoC-RNAi decreased by about 67.5% compared with that of the wild-type strain. This result indicates that the expression of the FeoC-like LuxR-type regulator could greatly affect intracellular survival of A. hydrophila
3.2. Transcriptomic analysis
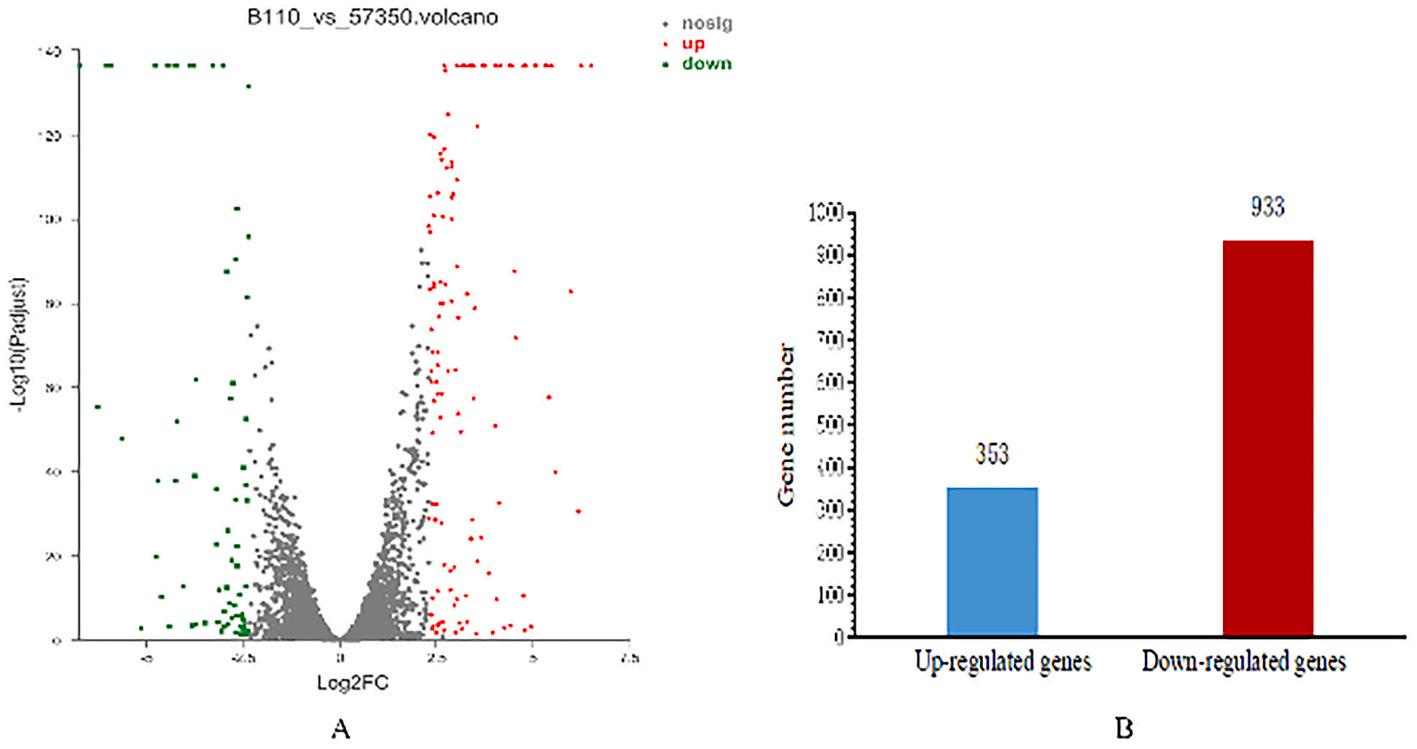
The transcriptome of B11 and feoC-RNAi were compared and analyzed. The gene expression profile was calculated and the changes in the expression level met FDR < 0.05 & |log2FC| ≥ 1 were considered statistically significant difference. According to this standard, there are 1286 differentially expressed genes (DEGs), of which 353 genes are significantly upregulated and 933 genes are significantly downregulated (Fig. 2). These data suggested that the FeoC-like LuxR-type regulator can regulate the expression of a large number of genes and has an extremely important function in A. hydrophila.
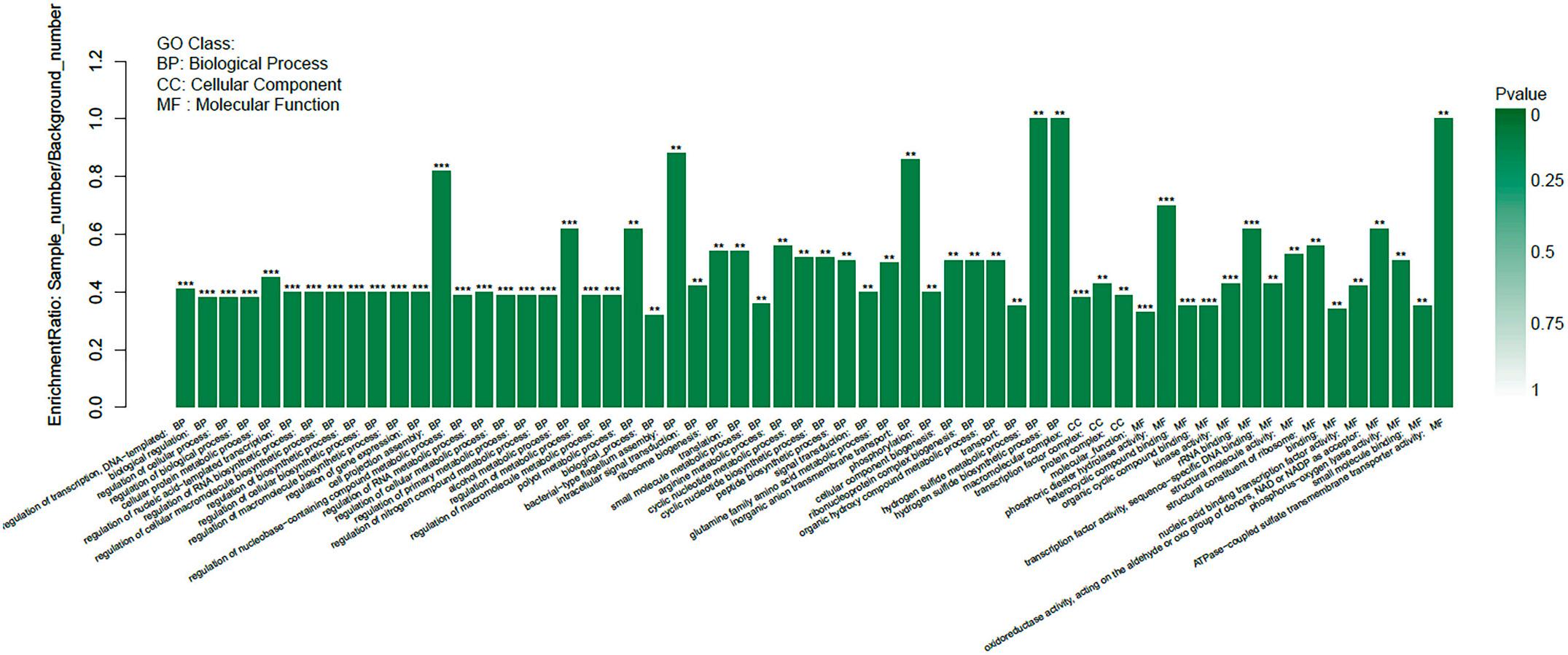
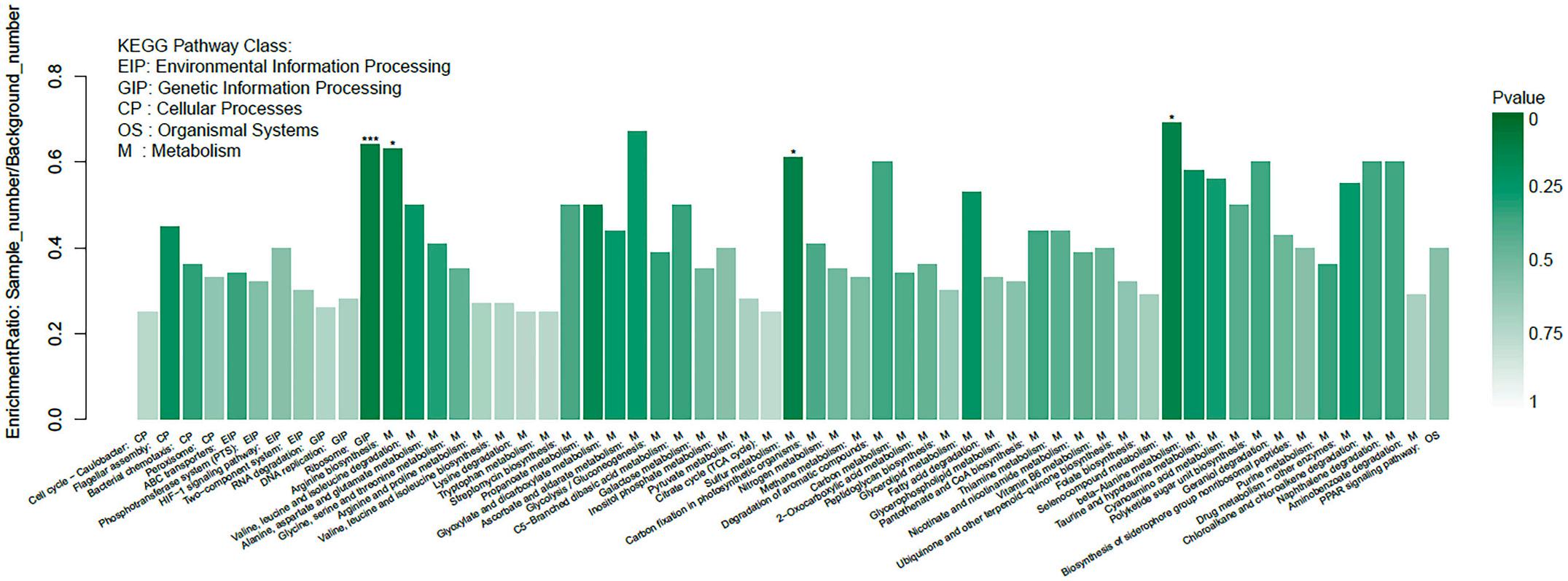
The functions of the 1286 DEGs were analyzed by GO and categorized into different enriched functional groups, among which the important functions included cell projection assembly, small molecule metabolic process, binding, inorganic anion transmembrane transport and so on (Fig. 3). The 1286 DEGs were also performed KEGG pathway enrichment analysis and enriched in 59 KEGG pathways, including bacterial chemotaxis, ABC transporters, two-component system, ribosome and so on (Fig. 4). Comprehensive transcriptome analysis suggested that the FeoC-like LuxR-type regulator may regulate metal ion metabolism, chemotaxis, type IV pilus biogenesis and binding of A. hydrophila, then further regulate the bacterial intracellular survival. According to the GO and KEGG analysis, the functional genes that may be directly and closely related to the intracellular survival of bacteria were screened out(Table 4).
3.3. Effects of the FeoC-like LuxR-type regulator expression on Fe and Mg metabolism in A. hydrophila
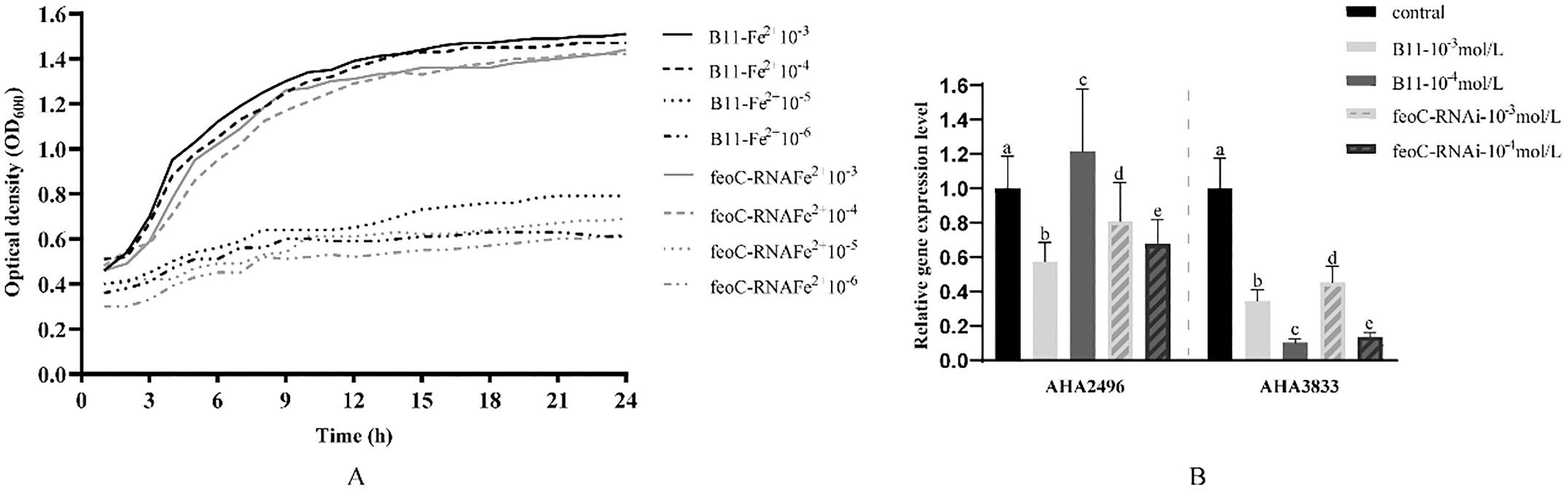
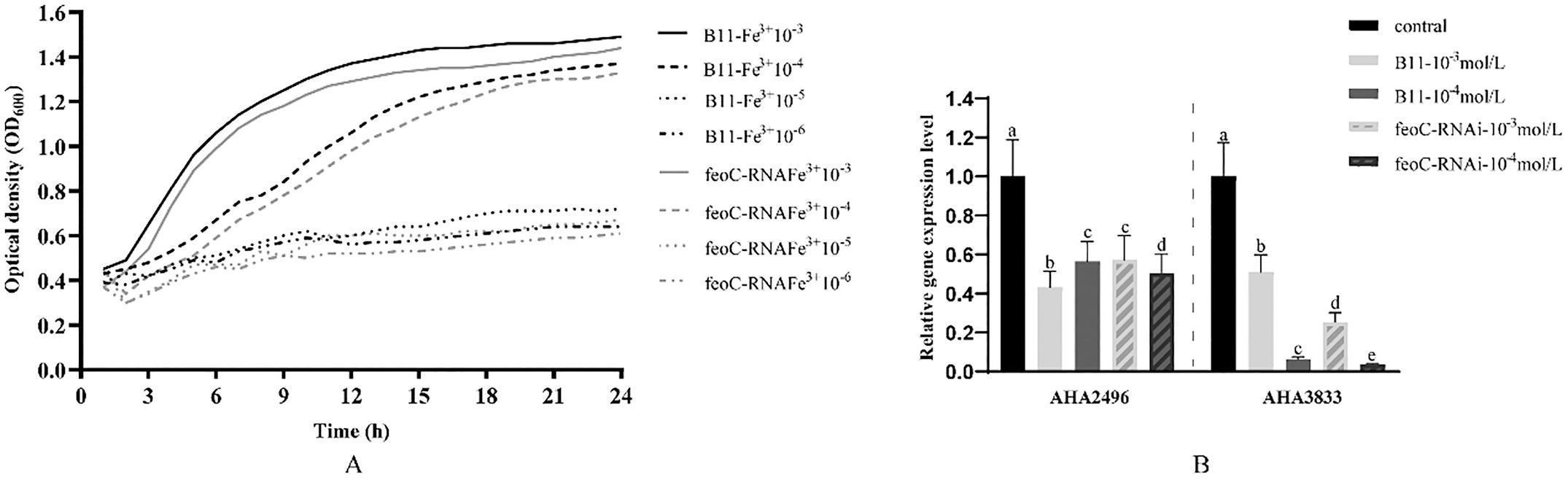
qPCR verification confirmed the transcriptome data met the requirements (Figure 5). And the trend of the effects of Fe2+ and Fe3+ on bacterial growth and the expression of genes related to iron metabolism almost the same (Figure 6 and 7). The growth curves of the wild-type strain and the silent strain basically coincide. When the iron ion concentration is lower than 10 4 mol/L, the wild-type strain and silent strain only grew at a very low level. As the iron ion concentration decreases, the expression of the gene AHA2496 encoded a (Fe–S)-binding protein is up-regulated or maintained the original level, while the expression of gene AHA3833 encoded bacterioferritin (bfr) is significantly down-regulated. These results suggested that the FeoC-like LuxRtype regulator can regulate the expression of genes related to iron metabolism, but has no effect on the growth of bacteria, indicating that A. hydrophila may have other pathways to obtain iron, and those pathways are not regulated by the FeoC-like LuxR-type regulator.
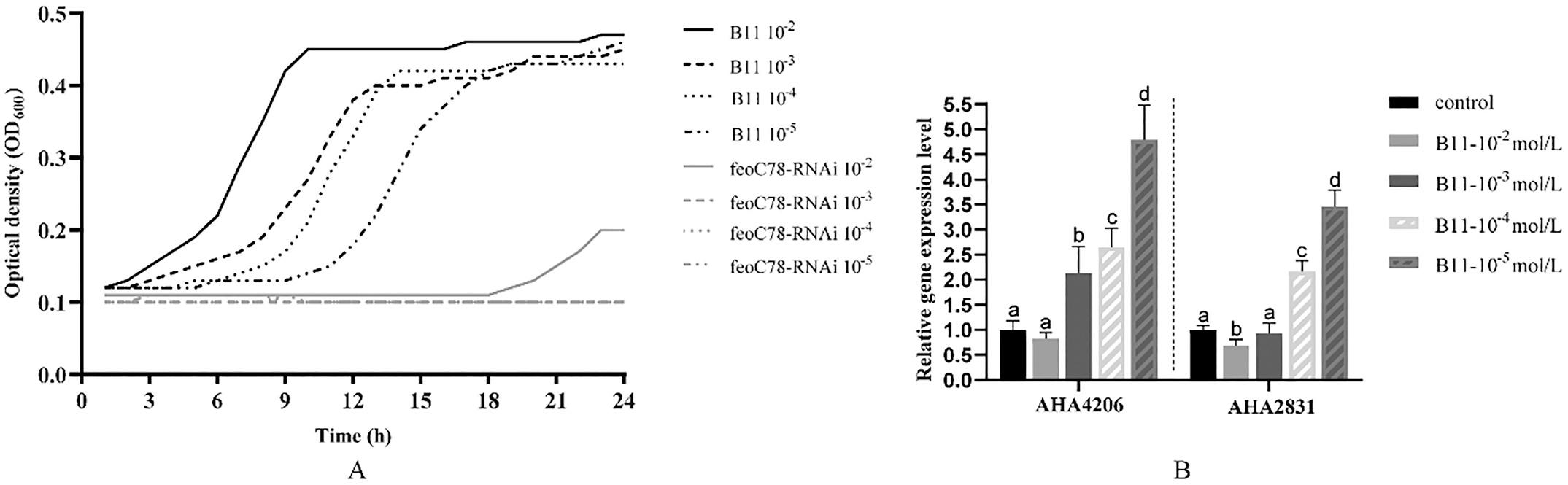
The data showed that Mg2+ has a significant effect on the growth of bacteria (Fig. 8A). Under different concentration of magnesium ion, the OD600 of the maximum growth of the wild-type strain was all lower than 0.5, and the silent strain hardly grew. Therefore, the genes expression could not evaluate in feoC-RNAi strain. And the expression of the genes mgtE and yifB tended to be up-regulated with the decrease of Mg2+
Z. Zhang
1. feoC-RNAi construction and intracellular survival in fish macrophages. (A) The efficiency of feoC silencing. (B) The growth curves of wild-type strain and feoCRNAi strain. (C) The intracellular survival of the wild-type strain and silencing strain. The data are presented as the means ± SD; Values denoted by different letters are significantly different. (P < 0.05).
Volcano plot and statistics of differentially expressed genes. (A) Volcano plot (B) The number of up-regulated genes and down-regulated genes.
Fig. 3. Histogram presentation of clusters of GO classification of 1286 DEGs.
Fig.
Fig. 2.
Z.
Table 4
The genes with significant difference in expression and closely related to the intracellular survival of A. hydrophila.
Function Number in the transcriptome Encoded protein (Gene)
Ion metabolism
AHA_2496 (Fe–S)-binding protein
AHA_3833 Bacterioferritin (bfr)
AHA_2558 iron sulfur cluster binding protein
AHA_2559 Fe-S oxidoreductase
AHA_2831 magnesium transporter (mgtE)
AHA_4206 Mg chelatase-like protein (yifB)
Chemotaxis
Type IV pilus biogenesis and binding
AHA_4123 methyl-accepting chemotaxis protein (mcp)
AHA_0937 chemotaxis sensory transducer family protein
AHA_2529 putative chemotaxis protein
AHA_1036 chemotaxis protein (chA-1)
AHA_3869 type IV pilus assembly ATPase (pilB)
AHA_0691 type IV pilus biogenesis protein (pilY)
AHA_0686 type IV pilus modification protein (pilV)
AHA_3193 type IV pilus biogenesis protein (pilN)
concentration in wild-type strain (Fig. 8B). These results suggested that the FeoC-like LuxR-type regulator could regulate the magnesium metabolism of A. hydrophila, then further involved in bacterial growth and intracellular survival.
3.4. Effects of the FeoC-like LuxR-type regulator expression on chemotaxis of A. hydrophila
From the results of transcriptome analysis and qRT-PCR verification, it can be considered that after the expression of the FeoC-like LuxR-type regulator was inhibited, a series of chemotaxis related genes were significantly down-regulated (Fig. 9A) in feoC-RNAi. Bacteria chemotaxis assay also confirmed that the chemotactic ability of feoC-RNAi was reduced by more than 60% (Fig. 9B). However, feoC-RNAi still had flagella (Fig. 10C) and motility of feoC-RNAi was not reduced compared with the wild-type strain (Data not shown). These results suggested that the FeoC-like LuxR-type regulator can regulate the chemotaxis of A. hydrophila, and then affect bacterial intracellular survival.
3.5. Effects of the FeoC-like LuxR-type regulator expression on pilus biogenesis and adhesion of A. hydrophila
The results of transcriptome analysis and qPCR verification also suggested that after the expression of the FeoC-like LuxR-type regulator was inhibited, some genes related to type IV-A pilus biogenesis were
Fig. 4. The KEGG pathway enrichment of 1286 DEGs.
Fig. 5. Verification of differentially expressed genes related to Fe and Mg metabolism. (A) The expression of the genes related to Fe metabolism; (B) The expression of the genes related to Mg metabolism.
Z.
Fig. 6. Effects of Fe2+ on growth and the expression of genes related to iron metabolism of A. hydrophila.
Fig. 7. Effects of Fe3+ on the expression of genes related to iron metabolism and growth of A. hydrophila.
Fig. 8. Effects of Mg2+ on the expression of genes related to magnesium metabolism and growth of A. hydrophila.
significantly down-regulated (Fig. 10A). Electronic microscope observations showed that pili were distributed around the cells of the wildtype strain (Fig. 10B), while no intact pilus was observed around the cells of the foeC-RNAi (Fig. 10C). Adhesion assays revealed that the adhesive ability of feoC-RNAi was reduced by about 50% (Fig. 10D, E, F). The results indicated that the FeoC-like LuxR-type regulator can regulate type IV-A pilus biogenesis and adhesion of A. hydrophila, and then affected bacterial intracellular survival.
4. Discussion
The key role of LuxR transcriptional regulator in bacteria has been wildly recognized, and it has been confirmed that LuxR can regulate the expression of a variety of genes, including those encoding Quorum Sensing (QS), virulence factors, antibiotics biosynthesis, motility, biofilm formation, and so on (Chen and Xie, 2011). The results in this study also displayed that after the expression of this FeoC-like LuxR-type regulator was inhibited, the intracellular survival of A. hydrophila was
Z.
decreased by about 67.5%, which meant luxR05735 played important role on A. hydrophila survival in fish macrophages. The role of LuxR on intracellular survival of other bacteria has also been revealed. Study on the LuxR-type regulator VjbR in Brucella suggested that this regulator directly modulated the expression of a subset of genes that not only encompass the well-studied virulence factors known to control intracellular trafficking and escape lysosome-mediated degradation, but also involve electron transport chain components (Kleinman et al., 2017). In this study, transcriptome analysis suggested that this FeoC-like LuxRtype regulator may regulate the expression of at least 1286 genes. According to the GO and KEGG analysis of these 1286 DEGs, it is speculated that this regulator may affect A. hydrophila intracellular survival by
regulating bacterial metal ion metabolism, chemotaxis, type IV pilus biogenesis and binding.
Further studies showed that this FeoC-like LuxR-type regulator could regulate the expression of some genes related to iron metabolism, but not affect the growth of A. hydrophila under the limited Fe2+ or Fe3+ concentration. At the same time, restricted expression of this FeoC-like LuxR-type regulator greatly affect the expression of mgtE and yifB and growth of bacteria under the limited Mg2+ concentration. So it is speculated this FeoC-like LuxR-type regulator play a vital role in regulating the uptake of magnesium and the homeostasis of magnesium and iron in A. hydrophila to maintain growth and intracellular survival. The role of LuxR-type regulator in the various physiological and biochemical functions of bacteria has been extensively studied and elucidated. However, the role of LuxR family regulators on regulating magnesium and iron metabolism of bacteria is rarely mentioned. As a FeoC-like regulator, the function of this regulator in bacteria remain enigmatic (Brown et al., 2021; Smith et al., 2019). FeoC has been known to be one of the three proteins that comprised FeoABC system, which is the most widely distributed and dedicated Fe2+ uptake system across the prokaryotic domain (Brown et al., 2021) and has been shown to connected most widely to the virulence of pathogenic prokaryotes (Hantke, 2003; Lau et al., 2016). Gao et al. once provided indirect evidence that FeoC is a negative regulator of the feoABC operon in Yersinia pestis (Gao et al., 2008). But Fetherston et al. found a ΔfeoC mutant did not affect transcription from the feo promoter under aerobic or micro aerobic conditions (Fetherston et al., 2012). Duo to feoC conserved helis-turn-helix (HTH) motif, it has been predicted to play a role as a transcriptional regulator (Brown et al., 2021; Kammler et al., 1993; Lau et al., 2013). However, there are no reports indicating that FeoC binds to DNA (Brown et al., 2021). Therefore, the function of FeoC remains to be elucidated.

Fig. 9. Effects of the FeoC-like LuxR-type regulator expression on chemotaxis of A. hydrophila. (A) The results of qRT-PCR; (B) The chemotaxis index of B11 and feoC-RNAi strains.
Fig. 10. Effects of the FeoC-like LuxR-type regulator expression on pilus biogenesis and adhesion of A. hydrophila.
Z. Zhang et al.
Both iron and magnesium are essential element for bacteria survival and growth and also critical for the pathogenesis (Blanc-Potard and Groisman, 2021; Leon-Sicairos et al., 2015). Magnesium is the most abundant divalent metal in living cells and is required for numerous cellular activity (Moomaw and Maguire, 2008; Shin et al., 2014) and Fe is needed in amounts 1000 to 10,000 times smaller than those of Mg in the cell (Hantke, 1997). Moreover, study once suggested that Mg2+ and ferrous iron shared a permease and ferrous iron can be taken up by the magnesium transport system independently of Feo system, especially when the medium contained a low concentration of magnesium (Hantke, 1997). Based on these point of view, it could be explained the results in this study. This regulator with both LuxR and FeoC characteristics may not direct involve in ferrous iron uptake of A. hydrophila, and this bacteria can obtain enough ferrous iron by other pathway, including magnesium transporter system. So under the limited iron concentration, foeC-RNAi could maintain the same growth trend as the wild-type strain. While under the limited magnesium condition, the growth of both wild-type strain and foeC-RNAi was inhibited, especially feoC-RNAi hardly grew.
The results in this study also showed that restricted expression of this FeoC-like LuxR-type regulator greatly affected the chemotaxis ability, pilus biogenesis and adhesion of A. hydrophila, then further reduced the ability of bacteria to survive in macrophages. The phagocytosis of pathogenic bacteria by host phagocytes was once believed to be a passive process. Recent research has found that some pathogens can actively choose host phagocytes as refuge and have evolved a number of very effective strategies that permit intracellular survival within phagocytes (Kaufmann, 2011; Roop II and Caswell, 2013). For example, inhibition of phagosome-lysosome fusion, inhibition of phagosome acidification, lysis of the phagosome and escape to enter the cytoplasm, and so on (Qin et al., 2014). Weinstein et al. once reported flagellated salmonella survived longer in macrophages than do nonflagellated salmonellae (Weinstein et al., 1984). And some the mechanisms elucidated by the authors may also be applicable to the results of this study. For example: (i) Bacteria with pili may not trigger the full cascade of early microbicidal events of macrophages. (ii) After invading macrophages, bacteria with pili or without pili may be sequestered in different compartments of cells. (iii) Bacteria with pilus may consequently be shielded from the microbicidal actions of the macrophages better than are the bacteria without pili. (iv) The presence of pili may act as a cloak to partially protect bacteria from the microbicidal enzymes, pH, and other defenses of the macrophages. And during these processes, chemotactic movement of A. hydrophila may help bacteria to find the sites suitable for survival or escape the sites for microbicidal actions.
CRediT authorship contribution statement
Zhe Zhang: Investigation and Editing. Leilei Mao: Investigation and Writing. Yingxue Qin: Investigation, Writing-Reviewing and Editing. Lingmin Zhao: Investigation. Lixing Huang: Investigation. Xiaojin Xu: Investigation. Qingpi Yan: Writing- Reviewing and Editing.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Fujian Province (Grant No. 2020J01663), the National Natural Science Foundation of China (Grant No. 31502194), and Industry-University-Research cooperation project of Xiamen (Grant No.3502Z20203049).
References
Ahmer, B.M.M., 2004. Cell-to-cell signalling in Escherichia coli and Salmonella enterica Mol. Microbiol. 52 (4), 933–945. https://doi.org/10.1111/j.1365-2958.2004.04054. x.
Awan, F., Dong, Y., Wang, N., Liu, J., Ma, K., Liu, Y., 2018. The fight for invincibility: environmental stress response mechanisms and Aeromonas hydrophila Microb. Pathog. 116, 135–145. https://doi.org/10.1016/j.micpath.2018.01.023
Blanc-Potard, A.-B., Groisman, E.A., 2021. How pathogens feel and overcome magnesium limitation when in host tissues. Trends Microbiol. 29 (2), 98–106. https://doi.org/10.1016/j.tim.2020.07.003
Boesen, H.T., Larsen, M.H., Larsen, J.L., Ellis, A.E., 2001. In vitro interactions between rainbow trout (Oncorhynchus mykiss) macrophages and vibrio anguillarum serogroup O2a. Fish & Shellfish Immunol. 11 (5), 415–431. https://doi.org/10.1006/ fsim.2000.0328
Brown, J.B., Lee, M.A., Smith, A.T., 2021. Ins and outs: recent advancements in membrane protein-mediated prokaryotic ferrous iron transport. Biochemistry 60 (44), 3277–3291. https://doi.org/10.1021/acs.biochem.1c00586
Chen, J., Xie, J., 2011. Role and regulation of bacterial LuxR-like regulators. J. Cell. Biochem. 112 (10), 2694–2702. https://doi.org/10.1002/jcb.23219
Daskalov, H., 2006. The importance of Aeromonas hydrophila in food safety. Food Control 17 (6), 474–483. https://doi.org/10.1016/j.foodcont.2005.02.009
Elwitigala, J.P., Higgs, D.S., Namnyak, S., White, J.W., Yaneza, A., 2005. Septic arthritis due to Aeromonas hydrophila: case report and review of the literature. Int. J. Clin. Pract. 59, 121–124. https://doi.org/10.1111/j.1368-504X.2005.00338.x
Fang, H., Yu, D., Hong, Y., Zhou, X., Li, C., Sun, B., 2013. The LuxR family regulator Rv0195 modulates Mycobacterium tuberculosis dormancy and virulence. Tuberculosis 93 (4), 425–431. https://doi.org/10.1016/j.tube.2013.04.005.
Fetherston, J.D., Mier Jr., I., Truszczynska, H., Perry, R.D., 2012. The Yfe and Feo transporters are involved in microaerobic growth and virulence of Yersinia pestis in bubonic plague. Infect. Immun. 80 (11), 3880–3891. https://doi.org/10.1128/ iai.00086-12
Gan, Z., Tang, X., Wang, Z., Li, J., Wang, Z., Du, H., 2019. Regulation of macrophage iron homeostasis is associated with the localization of bacteria. Metallomics 11 (2), 454–461. https://doi.org/10.1039/c8mt00301g
Gao, H., Zhou, D., Li, Y., Guo, Z., Han, Y., Song, Y., Yang, R., 2008. The iron-responsive fur regulon in Yersinia pestis J. Bacteriol. 190 (8), 3063–3075. https://doi.org/ 10.1128/jb.01910-07
Hantke, K., 1997. Ferrous iron uptake by a magnesium transport system is toxic for Escherichia coli and salmonella typhimurium J. Bacteriol. 179 (19), 6201–6204. https://doi.org/10.1128/jb.179.19.6201-6204.1997
Hantke, K., 2003. Is the bacterial ferrous iron transporter FeoB a living fossil? Trends Microbiol. 11 (5), 192–195. https://doi.org/10.1016/s0966-842x(03)00100-8
Huang, L., Qin, Y., Yan, Q., Lin, G., Huang, L., Huang, B., Huang, W., 2015. MinD plays an important role in Aeromonas hydrophila adherence to Anguilla japonica mucus. Gene 565 (2), 275–281. https://doi.org/10.1016/j.gene.2015.04.031
Hung, K.-W., Tsai, J.-Y., Juan, T.-H., Hsu, Y.-L., Hsiao, C.-D., Huang, T.-H., 2012. Crystal structure of the Klebsiella pneumoniae NFeoB/FeoC complex and roles of FeoC in regulation of Fe2+ transport by the bacterial Feo system. J. Bacteriol. 194 (23), 6518–6526. https://doi.org/10.1128/jb.01228-12
Kammler, M., Schon, C., Hantke, K., 1993. Characterization of the ferrous iron uptake system of escherichia-coli. J. Bacteriol. 175 (19), 6212–6219. https://doi.org/ 10.1128/jb.175.19.6212-6219.1993.
Kaufmann, S.H.E., 2011. Intracellular pathogens: living in an extreme environment. Immunol. Rev. 240, 5–10. https://doi.org/10.1111/j.1600-065X.2010.01001.x
Kleinman, C.L., Sycz, G., Bonomi, H.R., Rodriguez, R.M., Zorreguieta, A., Sieira, R., 2017. ChIP-seq analysis of the LuxR-type regulator VjbR reveals novel insights into the Brucella virulence gene expression network. Nucleic Acids Res. 45 (10), 5757–5769. https://doi.org/10.1093/nar/gkx165
Kohler, S., Porte, F., Jubier-Maurin, V., Ouahrani-Bettache, S., Teyssier, J., Liautard, J.P., 2002. The intramacrophagic environment of Brucella suis and bacterial response. Vet. Microbiol. 90 (1–4), 299–309. https://doi.org/10.1016/s0378-1135(02)002158
Kusdarwati, R., Rozi, Dinda, Nurjanah, D.N., Iop, I., 2017. Antimicrobial resistance prevalence of Aeromonas hydrophila isolates from motile Aeromonas septicemia disease. In: 7th ASEAN-FEN International Fisheries Symposium, Brawijaya Univ, Fac Fisheries & Marine Sci, Batu, INDONESIA. https://doi.org/10.1088/1755-1315/ 137/1/012076
Lau, C.K.Y., Ishida, H., Liu, Z., Vogel, H.J., 2013. Solution structure of Escherichia coli FeoA and its potential role in bacterial ferrous iron transport. J. Bacteriol. 195 (1), 46–55. https://doi.org/10.1128/jb.01121-12
Lau, C.K.Y., Krewulak, K.D., Vogel, H.J., 2016. Bacterial ferrous iron transport: the Feo system. FEMS Microbiol. Rev. 40 (2), 273–298. https://doi.org/10.1093/femsre/ fuv049
Leon-Sicairos, N., Angulo-Zamudio, U.A., de la Garza, M., Velazquez-Roman, J., FloresVillasenor, H.M., Canizalez-Roman, A., 2015. Strategies of Vibrio parahaemolyticus to acquire nutritional iron during host colonization. Front. Microbiol. 6 https://doi. org/10.3389/fmicb.2015.00702
Lin, G., Chen, W., Su, Y., Qin, Y., Huang, L., Yan, Q., 2017. Ribose operon repressor (RbsR) contributes to the adhesion of Aeromonas hydrophila to Anguilla japonica mucus. Microbiologyopen 6 (4). https://doi.org/10.1002/mbo3.451
Mao, L., 2019. The role of LuxR Family Proteins in the Pathogenicity of Aeromonas hydrophila and the Mechanism in the Regulation of Bacterial Intracellular Survival. MS Thesis. Jimei University, Xiamen, China. https://doi.org/10.19663/j.issn20959869.20180531001
Z.
Mao, L., Qin, Y., Kang, J., Wu, B., Huang, L., Wang, S., Yan, Q., 2020. Role of LuxR-type regulators in fish pathogenic Aeromonas hydrophila J. Fish Dis. 43 (2), 215–225. https://doi.org/10.1111/jfd.13114
McCarthy, U.M., Bron, J.E., Brown, L., Pourahmad, F., Bricknell, I.R., Thompson, K.D., Ellis, A.E., 2008. Survival and replication of Piscirickettsia salmonis in rainbow trout head kidney macrophages. Fish & Shellfish Immunol. 25 (5), 477–484. https://doi. org/10.1016/j.fsi.2008.07.005
Moomaw, A.S., Maguire, M.E., 2008. The unique nature of Mg2+ channels. Physiology 23 (5), 275–285. https://doi.org/10.1152/physiol.00019.2008
Nunez, G., Sakamoto, K., Soares, M.P., 2018. Innate nutritional immunity. J. Immunol. 201 (1), 11–18. https://doi.org/10.4049/jimmunol.1800325
Pfaffl, M.W., Horgan, G.W., Dempfle, L., 2002. Relative expression software tool (REST (c)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30 (9) https://doi.org/10.1093/nar/30.9.e36. Qin, Y., Lin, G., Chen, W., Huang, B., Huang, W., Yan, Q., 2014. Flagellar motility contributes to the invasion and survival of Aeromonas hydrophila in Anguilla japonica macrophages. Fish & Shellfish Immunol. 39 (2), 273–279. https://doi.org/10.1016/ j.fsi.2014.05.016
Roop II, R.M., Caswell, C.C., 2013. Bacterial persistence: finding the “sweet spot” Cell Host Microbe 14 (2), 119–120. https://doi.org/10.1016/j.chom.2013.07.016
Shin, J.-H., Wakeman, C.A., Goodson, J.R., Rodionov, D.A., Freedman, B.G., Senger, R.S., Winkler, W.C., 2014. Transport of magnesium by a bacterial nramp-related gene. PLoS Genet. 10 (6) https://doi.org/10.1371/journal.pgen.1004429
Smith, A.T., Linkous, R.O., Max, N.J., Sestok, A.E., Szalai, V.A., Chacon, K.N., 2019. The FeoC 4Fe-4S cluster is redox-active and rapidly oxygen-sensitive. Biochemistry 58 (49), 4935–4949. https://doi.org/10.1021/acs.biochem.9b00745
Tokunaga, Y., Liu, D., Nakano, J., Zhang, X., Nii, K., Go, T., Yokomise, H., 2015. Potent effect of adenoviral vector expressing short hairpin RNA targeting ribonucleotide reductase large subunit M1 on cell viability and chemotherapeutic sensitivity to gemcitabine in non-small cell lung cancer cells. Eur. J. Cancer 51 (16), 2480–2489. https://doi.org/10.1016/j.ejca.2015.05.013
Wang, S., Yan, Q., Zhang, M., Huang, L., Mao, L., Zhang, M., Qin, Y., 2019. The role and mechanism of icmF in Aeromonas hydrophila survival in fish macrophages. J. Fish Dis. 42 (6), 895–904. https://doi.org/10.1111/jfd.12991
Weinstein, D.L., Carsiotis, M., Lissner, C.R., Obrien, A.D., 1984. Flagella help salmonellatyphimurium survive within murine macrophages. Infect. Immun. 46 (3), 819–825. https://doi.org/10.1128/iai.46.3.819-825.1984.
Zeng, L.-R., Xie, J.-P., 2011. Molecular basis underlying LuxR family transcription factors and function diversity and implications for novel antibiotic drug targets. J. Cell. Biochem. 112 (11), 3079–3084. https://doi.org/10.1002/jcb.23262
Zhang, M., Yan, Q., Mao, L., Wang, S., Huang, L., Xu, X., Qin, Y., 2018. KatG plays an important role in Aeromonas hydrophila survival in fish macrophages and escape for further infection. Gene 672, 156–164. https://doi.org/10.1016/j.gene.2018.06.029
Zhang, M., Qin, Y., Huang, L., Yan, Q., Mao, L., Xu, X., Chen, L., 2019. The role of sodA and sodB in Aeromonas hydrophila resisting oxidative damage to survive in fish macrophages and escape for further infection. Fish & Shellfish Immunol. 88, 489–495. https://doi.org/10.1016/j.fsi.2019.03.021
Other documents randomly have different content
drinking the tempting fluid on the sly, too, for his bristling mustache was suspiciously creamy.
“What’s the matter with you?” he reiterated.
“Father, Katie has suddenly disappeared, very strangely and Mr. Jeffries is much alarmed.”
“Disappeared?”
“Yes; nothing has been seen of her since last night. She is not at home.”
“Hoh! she’s at some of the neighbors’.”
“No, she is not. I have been here ever since daybreak, and no one has left the house.”
“Ha!” and Dunbar started.
“What’s the matter?” asked Jeffries. The other came forward with a grave, solemn face, and laid his hand on his shoulder, quietly.
“See here, Rob, I’ll not beat about the bush, but will out with it. Last night, about midnight, I was awake, and as I lay quiet, I heard what I thought was an Indian yell, away down the creek. I got up and looked out the window. The moon was shining very bright, and all was still as the grave. As I stood looking, I saw something small and white glance for an instant close to your house, then a bright red light shine down by Hans’ cabin. Thinks I, something’s brewing, and I watched, but I saw nothing more. But I heard somebody away down, it seemed like, in Dead-Man’s Forest, say these words in a far-away voice:
“‘Take care! beware!’”
Jeffries started. “That voice!” he exclaimed, uneasily.
“What, did you hear it, too?”
“Go on!” and Jeffries gestured impatiently.
Dunbar stared, but went on. “It was the strangest voice I ever heard, and I can’t give any reason for it, but a cold chill ran over me, and I felt for my gun. It was a voice from the grave.”
He stopped short, and Hettie turned pale. Jeffries gave a gesture of irritation.
“Go on!” he said.
“In a few moments, say ten minutes, I saw, or imagined I saw, a dark object moving rapidly down the hill. Part of it was black, part white. I only saw it for about five seconds, when it vanished, and all was quiet again.
“I waited for some time, then, seeing no more, went back to bed, wondering. Just as I was falling asleep, I felt a draft of air pass over me, and looked up. Though seeing nothing, I was sure that a presence was near me—a thing felt, but not seen.”
He stopped, and drew Hettie protectingly to him, and grasped Jeffries’ hand.
“Now, my daughter, I don’t want to alarm you, but though I could not hear it, something seemed, ay, said:
“Trouble in Shadow Swamp—take care!”
Jeffries looked uneasy and seriously alarmed, while Hettie grew very white. Dunbar watched them both steadily, then said:
“Now, what I think is this.”
Jeffries stopped him.
“Hold! I’ve suthin’ ter say, too. It’s all about thet rascal, Danforth— thet galish feller.”
Then he related the events of the evening before; the meeting of the lovers; the quarrel between Danforth and Walter; the latter’s defeat, and the former’s disappearance; and concluded in a low, earnest tone:
“I was a-huntin’ for the villain, and was down by Hans’ cabin, whar he stops, when suthin’ said, ’pears like away off in the night:
“‘Yer air a-treadin’ on dangerous ground! tek keer, tek keer!’
“Wal, thet voice seemed so far away like, I kedn’t tell whar it was; but as I war thinkin’, it kim ag’in, clost ter my ears, loud an’ peart, right
from the bunch of willows jist above the cabin. Thinkin’ it must be Danforth hisself, I beat ’em all through, spendin’ an hour at it; but it was no go. Then, hafe scared, I kim home. Dunbar, thar’s suthin’ wrong.”
“I am afraid there is, my friend, very apprehensive. I have always given Dead-Man’s Forest a wide berth since the red-skins have been about, but I think the best thing we can do is to search in it at once for Katie—for it’s my opinion you’ll find her there.”
“That’s so, sartain. She ain’t ter hum, an’ whar she is no one knows. Great God, whar’s my pooty little gal, my little pet?” And Jeffries buried his face in his hands.
“Courage, my friend!” said his friend, kindly. “Courage, perhaps we are mistaken—perhaps something strange though not of evil might have turned up. Hettie, run to Sol Jacobs and give the alarm. Tell them to spread the men around while I go down to Hans Winkler’s cabin to see him. Gather the whole settlement and send a swift lad for Cato the Creeper—we’ll find her soon.”
Hettie sped away toward the distant cabins, making her white, bewitching ankles fly over the ground; she loved Katie dearly, and, with a woman’s lightning wit, suspected the true state of the case.
Once she had been strolling about on the border of the wood, and had overseen Danforth in close confab with a trio of villainous, desperate-looking men, all armed to the teeth. Then, again, she had seen him exchange significant glances with Cato, whom she cordially suspected of evil.
To use an uncouth but forcible phrase, Hettie was “nobody’s fool.” She linked several suspicious events, and by a little shrewd guessing picked Danforth to pieces.
Though naturally penetrating and keen, she was under the influence of the great sense-sharpener—Cupid, and was thoroughly in love with gay, handsome Captain Downing. She loved him with an ardent, whole-souled love, and could have gainsayed him in nothing. Fortunate for her it was that the unscrupulous robber did not know of her passion for him—very fortunate; for he would have caused her
bitter misery She well knew his impulsive temperament, and avoided him, knowing that to see him were only to give her love another impetus.
Stop and consider what this backwoods girl was doing, and see what a heroine she was. Cognizant of Downing’s ardent love for Katie, conscious he did not love her, knowing Katie was her successful rival, she was deliberately doing all she could to protect and save her —she who had unwillingly outstripped her in the love of the beautiful bandit—to organize a party for the apprehension and punishment of her idolized hero, though it would almost be her death-blow to see him disgraced and punished.
You see she was a very extraordinary girl—this young backwoods maid.
She soon arrived at the cabin of Sol Jacobs, and hurriedly entering told them of the story. Old Sol heard her through, heard her suspicions, conjectures and fears, then turned sharply to his son, a stalwart young fellow of twenty who would have died for Hettie, being devotedly attached to her.
“Arouse the settlement, Eben!” he said, “and make your pins fly too. Tell every man that little Katie has disappeared suddenly! that’ll bring ’em together short meter.”
Eben sprung away while Hettie lingered with the women, who, cackling all at once, plied her with questions. Old Sol took down his gun and rubbed the dust off the barrel.
The news flew like wildfire about the little settlement. Men frowned and quietly took their rifles from their pegs. Young men swore an oath or so, then clenched their teeth, and baring their arms, watched their brawny muscles as they swelled with the arm’s rise and fall. Then they clutched their guns, and uniting together, clamored to start in pursuit.
The elders, though quite as resolved and more worthy and reliable than their juniors, were men of experience, and never moved rashly, always looking before leaping. They assembled the youngers, and all uniting started for Jeffries’ cabin.
They had gone but a short distance when they discovered three forms approaching by Winkler’s cabin. They halted and waited for them on receiving a signal to that effect.
They were Cato, Eben Jacobs, and Walter Ridgely, the latter walking unsteadily. His head was bound up in Eben’s scarlet handkerchief and his face was livid and white. His eyes were bruised and purple and his nose was defaced. He was too angry and chagrined to control his anger, but allowed it free scope. The result was that he was in a dangerous state of mind.
They gathered round him, plying him with questions, which he answered moodily.
He had been walking, he said, through the spur of forest when he felt a rustle behind him, and turning had seen a man with uplifted bludgeon directly behind him. He tried to avoid the impending blow but too late; the cudgel descended squarely upon his head, and he knew no more until morning, an hour or so since, when he was stumbled upon by Eben, on his way to Cato’s cabin.
When asked if he recognized his would-be assassin he replied in the negative. But he was sure that it was not Danforth. He was a much larger man, being almost a giant.
Murmurs of indignation and menaces rose from the settlers, old and young. They had long suspected the depths of the grim forest were the haunts of evil men, and they were now sure of the fact. They were rapidly believing that quiet Danforth too was not what he should be, but was connected in some way with Katie’s disappearance, all being aware of last night’s events.
Walter was frantic when told of her sudden and strange absence, and sick with fear and doubt, raved to be gone in hot search. In this he was seconded by Jeffries, who was scarcely less alarmed and distracted. Accordingly, hasty arrangements were made; officers and scouts were chosen; Cato, the Creeper, stood ready to fix upon any trail, trace or mark; and the hearts of the whole band beat as one.
Every man was armed to the teeth, and what was better, was buoyed by the sense of being in the right—a weapon far more potent
than the steadiest rifle, the deadliest pistol, or keenest knife ever made.
Place two men of equal strength and agility upon an open field to combat, one being in the right and the other in the wrong. It will surprise you to see how soon the former will defeat his antagonist. This is solid truth.
Sol Jacobs was chosen chief, as being an old Kentucky Indianfighter. The next in command was Jeffries. The scouts and flankers were the keenest, sharpest young men in the settlement, under the supervision of Cato, the Creeper
Before long they were wending their way down the hill toward the forest, Cato grinning with delight, the only agreeable person in the party. The women stood by the little block-house watching them depart; and though many feminine hearts were sad, none were so heavy and torn as the virgin one of sweet Hettie Dunbar, watching with red, swollen eyes, the departure of cunning, earnest men, to bring to harm her lover.
In a few moments they were out of sight, and the women went back to their cabins sorrowfully. But Hettie mounted the narrow ladder in the block-house and sat drearily alone, sadly waiting, trembling lest at any moment she should see her heart’s idol brought back wounded or dying, and in disgrace and shame.
CHAPTER V.
A FIENDISH DEED.
Downing and Cato hurried away through the forest, toward Shadow Swamp, Katie meanwhile lying unconscious in her abductor’s arms. But, when they arrived at the pool, and stopped and signaled for the canoe, the cessation of the jolting motion aroused her and she opened her eyes.
At first her senses were scattered, and she did not remember the startling occurrence which had just taken place. But by degrees her wandering thoughts collected, and looking at the dark, grim trees, the still, pale light of the moon, the sable form beside her, and at her own villainous captor, she realized all and her heart sunk. The incidents, one by one, with startling distinctness rushed over her; the sudden awakening and fright; the villain’s rude and immodest grasp of her; the gradual fading away into oblivion; all, with the terrible, sickening dread of her fate to come was too much for her, and she swooned again.
When she again opened her eyes she looked upon four log walls and a roof of “brush.” She was in a cabin.
The walls were hung with skins, weapons, utensils and clothing, and last, in one corner, was a looking-glass—the pet of the dandy captain. The cabin was small, very small; but it was clean. Raising herself on her elbow, she looked around. In two corners were two piles of buffalo-skins undressed, and blankets—evidently used as beds. A round, short piece of a log stood on end in the center of the room, evidently a stool. This, with her own couch, completed the scanty furniture of the cabin.
She was lying on a bed which had been prepared for her, and she was delicately covered by skins. Her own clothing lay near.
In a few moments the door opened, and Captain Downing entered. He found her dressed and sitting vacantly on the stool without power to fly and escape. He had evidently taken some pains with his toilet, as his green coat was carefully brushed, his hair was arranged, and his boots were cleansed of all soil which generally adhered to them. He bowed gracefully, in a manner which would have reflected credit upon many a “carpet knight.”
“Ah!” he said, softly, “I am very glad to see you are able to be up and about. Please accept my sincerest wishes for your health.”
She did not raise her head, but sat as if in a trance. He went on:
“May I call you Miss Katie? Please do not be offended if I do. It seems so much more pleasant than cold, formal Miss Jeffries. Besides, my ardent regard for you causes me to use a more familiar title.”
But she did not notice him. After watching for any effect his remarks might produce, he lounged gracefully upon his pile of robes, and took a meerschaum from his pocket.
“A relic of former days,” he said, in a musing tone. “May I so far trespass upon your good-humor as to smoke? A vice to which gentlemen are much addicted. The dear ladies, however, in their sweet graciousness, not only grant their permission generally, but protest they ‘like the perfume of a good cigar.’ Here’s to the ladies— one in particular, the bonniest of them all. Having no claret to quaff their health in, I am forced to be satisfied with a meerschaum and very villainous tobacco. Miss Katie, your own health.”
He puffed out a wreath of smoke with exquisite effrontery, and smiled as a low moan escaped her lips.
“You are looking lovely to-day, Miss Katie—very enchanting. If you only knew how my heart bleeds for you in your present embarrassing situation, you would at least reward me with one of your sweet smiles. Let us hope, however, that the present place may soon become pleasant, even dear to you. I will do all in my power to make it so, I assure you.”
His last remark had the effect of partially arousing her from her apathy. She looked at him mournfully, with a glance in which were mingled grief, outraged modesty, terror and contempt. He laughed.
“You are very beautiful—very lovely. When you gazed at me so earnestly just now, my heart beat faster than its usual wont, and I imagined I could detect a sly twinkle of love, too. Was my surmise correct, Katie?”
She rocked to and fro, groaning in sheer despair and terror. His eyes snapped.
“I’m like the boy who drew the nightingale in the lottery,” he muttered. “I’ve got her, and now she won’t sing. Well, we will try the efficacy of force.”
He arose deliberately and stood before her, and their eyes met. Hers were terror-stricken, like a wounded fawn’s; his glittered like a snake’s. Nevertheless, he spoke musically and low.
“If the fair Katie is aware of the value of obedience, she will temper her stubbornness slightly.”
Her eyes wandering vacantly about, fell upon a polished pistol hanging to a peg close by; she noted it. He waited a moment, then laid his hand quietly on her shoulder.
With a wild, piercing cry she shook it off, and darting away, clutched the pistol.
Never opening her lips, but piercing him with her eye, she stood drawn to her full hight, her cheeks pale, her hands quivering, and her whole being aroused.
“Stand back, you monster!” she commenced, in a ringing, grating voice. “Don’t dare to lay your vile hands on me! Keep off, I say!”
She was thoroughly aroused, and her eyes darted angry fire. Irresistibly lovely she looked, and Downing, in spite of his chagrin at her opposition, loved her ten times more than ever. He gazed at her with his heart beating violently, he was so affected by her resolute bearing. Then his lip curled and he advanced on her.
She quickly cocked the pistol and presented it. He halted, but moved slowly around her, trying to find an opportunity for rushing in and disarming her. But, impelled by her terrified modesty, she was wary and kept him at bay. After some time spent in gliding about, he saw it was no use and changed his manner.
Dropping his arms and extending his hands, he put on, with splendid cunning, a mask of virtue. Throwing a wistful, pleading look into his comely brown eyes, he murmured, in a low voice:
“Lady, do your will and take my life! See, I am unarmed and unguarded; shoot! Oh, dear lady, to die by your hands were far sweeter than to live and see you scorn me so, my love!”
His sudden change surprised her, but she was too affrighted to lose her advantage. He saw she was in earnest, and he went on:
“I do not, I could not wish to bring myself to such a degraded level as to wish to do you harm. If you knew how passionately I love you, with what high regard I esteem your purity and courage, you would at least refuse to threaten me so. Your harsh manner cuts me to the heart. Believe me, dear lady, I do not mean you ill—if you think so, you have only to shoot and rid yourself of such a detested object as I am to you.”
He groaned as he said this, and sinking on his couch, buried his face in his hands. She watched him warily, though half melted by his protestations.
“I brought you here,” he said, with his face muffled, “to love and cherish you—to tenderly care for you. If, after a time you did not like it here, I was going to take you back. But oh! it wounds me to have you scorn me so.”
“I know too well your foul hypocrisy to be deluded by it. You have brought me here for evil, and you can not deny it. But this I tell you— that if you lay your hand on me but once, it will be your last moment upon earth. Take it in earnest, you demon, for I am terribly so.”
He groaned, then spoke, pleadingly:
“Oh, my love! please—”
“Keep your distance in language as well as in manner, for I will brook no rude familiarity from you!”
“Miss Jeffries, won’t you try and care for me? Even if you can not regard me as I would choose, you can at least endeavor to respect me.”
This last was a false move. With this last effrontery her ire and grief found a full vent.
“Dare you sit there and ask me to respect you?” she rung out, in noble wrath. “Dare you, in the name of all that is pure and holy, to ask me to look even pityingly upon you? Oh, sir, if in your mother was a spark of womanly virtue, if your father was a man of worth and honesty, if you ever had a pure sister, think of them and then of yourself at this moment!—think of them and release me from this wicked place. Take me back to my dear home; do not, oh, sir, do not bring down the wrath of Heaven upon you! Think of my poor father— of his anguish at my absence; think of the one who is to be my husband; please, sir, please pity and commiserate me. Oh, if you could imagine my grief and horror at being here, away from my friends, if you could respect or pity my sorrow, you would at once release me. Oh, sir, for the love and in the memory of your mother and sister, please do so, and let me go, and I will never tell of what I have been through here.”
He looked up in his natural expression and said, quietly:
“I will at once release you and take you safely home if you will grant me a single favor. It will not incommode you.”
“Name it!” she said, hastily, with her face lighted by a ray of hope.
“I will. It is to marry me.”
“Marry you!”
She looked at him steadily for a moment, then sunk on the stool with a shudder, wildly weeping.
“What is your answer?” he asked, with a quiet smile.
She did not answer, but sobbed and wept as if her heart was breaking.
“What is your answer?” and he smiled.
“Never!” she sobbed; “never!”
“Very well—very well.”
He arose and walked toward the door and looked out.
“By the sun I should judge the time to be ten o’clock. Now, Miss Jeffries, you will stay here twelve times twelve hours without food or water unless you accede to my desire. I do not wish to humiliate you in any manner, and will say there is a preacher about forty miles east. If you desire to unite your fortunes with mine, say the word and before night we will be at his house. Otherwise think of the terrors and anguish of slow starvation. I will give you an hour to decide. Reflect carefully, Miss Jeffries!”
He walked quietly out, leaving her a prey to the most harrowing thoughts. She had been tenderly reared and had never known the slightest grief, and this blow, dire as it was, humbled her and caused great anguish. She well knew his quiet ferocity and unrelenting disposition; she had just now seen his character in different phases; and knowing he would accomplish his purpose if it was possible, she trembled at the thought of the future.
In addition to these keen pangs was one nearly as piercing—she had no idea in what place she was. In the settlement the robber had lived in Hans Winkler’s cabin; she had often been there and knew this was not it. She was probably in some remote and obscure place, far from any path, alone with this dangerous and passionate man. She did not dream that a dozen yards from the cabin, seven or eight men, abandoned and profligate, ready to sanction and further any act of Downing’s, isolated from any thing pure or honest, were laughing and coarsely joking—even about her.
It was fortunate she did not, else she might have been unable to bear the thought, and would have swooned with fear.
She was in a critical and harrowing position, without means of escape, as she had heard him place a heavy log against the door as he went out. The door opened outward purposely in order to confine any prisoner within. Escape by the door was impossible.
As she thought upon her situation, fear lent her strength, and she began to examine the walls of the cabin. For a half-hour she beat them and pushed at the heavy logs feebly; she ran about sobbing, beating them with her delicate hands until they bled; she mounted the stool and searched the strong roof; she vainly endeavored to force the door; she called on her father and lover frantically; then, when escape was only too vain, she began to pray, half-crazed.
At the expiration of the hour Downing entered and closed the door behind him.
Then he folded his arms and quietly gazed at her as she sat on the low, rude stool, in a semi-stupor.
“Well?” he said.
She made no reply, neither did she raise her eyes; but sat motionless.
“Well,” he continued, smiling slightly, “have you made up your mind?”
He expected here that she would show some spirit, at least a little resistance; but she neither did one nor the other.
“Have you resolved which alternative you will take?”
She answered in a faint voice, “I have.”
“Well, will you be my wife and gain a protecting husband?”
“No!”
“Are you in earnest, Miss Jeffries? Think well before you speak. You know the alternative; do you choose it?”
“I do; any thing were better than being the wife of a man I loathe and detest.”
“You will find yourself mistaken before many days, mark well what I say. I am not to be deterred from my resolve.”
“I am resolved.”
“Once again I enjoin, nay entreat you to reflect. You are, metaphorically speaking, at the forks of a road. One leads, if not to perfect happiness, to at least, an easy, indolent life, well garnished with luxuries; the other to—a horrible, unknown death.” “Fiend!”
“I am, Miss Jeffries, I acknowledge it. Yet I can be most tender and agreeable when I choose. Fiend! that is a harsh word, yet I take a strange sort of pride in it. You do not know my early life. Well, I will relate it. Meanwhile you can, in listening, form some opinion of death by starvation. I love you fondly, tenderly, Miss Kate, as only one of my disposition can; and it is for this reason that I treat you so cruelly. It is one of the contradictions of my nature. But I will go on with my history.”
He lighted his quaint, costly pipe, and begging her pardon as politely as any native of France, began in his rich, round voice, occasionally making a gesture with the ease of an experienced orator.
“I am a native, of nowhere, and my parents were nobody. That is, my parents either died or deserted me when very young, as I was found, a frail infant in the middle of one of New York’s busiest thoroughfares, in early morning, by a young roystering blade, rolling home in the morning. He took me to a foundling asylum, and left me to live or die—as my nurses by their care or neglect, might will.
“I lived—after suffering all the ills and evils of babydom, and grew strong and healthy. When I arrived at the unripe and vicious age of ten, an old gentleman, a retired merchant, attracted by the comeliness of my face and form, adopted me, giving me his own name—Robert Davis.
“I was a quick-witted, jovial little chap, and if I do say it myself, was very fair and handsome. Being petted and caressed by all the women both old and young, of the neighborhood, I easily grew into the belief that I was something superhuman—in fact a genius, one day to be the President of the country. It is true, that notwithstanding my good-nature and affability, I was at times seized with fits of quiet,
inordinate cruelty, which made me a demon, and at these moments everybody avoided me.
“As years went on these attacks became more frequent and violent. Before, when under the influence of them, I restrained myself, and was content with murdering all the small animals within my reach. But now, I became more bloodthirsty and ferocious—attempting, though vainly, the lives of all my companions.
“Then they avoided me, and feared the very ground I trod. This incensed me and I grew more violent. At last, on my twentieth birthday, a fit, stronger and more uncontrollable than any before, seized me. Without provocation of any kind I fell upon a comrade and attempted his life. I failed, though he was made a cripple for life, and I was buried in an insane asylum, a monomaniac. I was not insane but only a monomaniac, yet that was sufficient to cause my incarceration.
“In five years I was pronounced cured, and was freed. I went back to my old haunts, penitent and resolving to do all in my power to alleviate any suffering I had caused. But I was too late; the friends I sought were gone. My adopted father was dead, the one whom I had made useless for life had gone, no one knew whither; and weary of lingering near the scene of so much unhappiness I went South.
“If you recollect, or if you ever knew, a most horrible robbery and murder occurred in Charleston, a few years since. The perpetrator was never discovered, though long and vigilant search was made for him, and large rewards were offered for his apprehension. I see by your face you recollect the event—it was the talk and alarm of the whole country. I will not tell you what reason the murderer had for his outrage; I need not dwell upon the subject, but will only say that he escaped scot-free, plunged into the western wilds and organized a band of robbers. Miss Jeffries, the man who stole into a banker’s house for purposes of robbery (and to gratify a grudge) and who, being discovered, took the lives of him and his servant, then made off with a rich plunder, stands before you.”
She started up wildly, then after gazing at him in terror, clasped her hands and sunk to the ground, unnerved. He smiled.