Introductiontoblood-gastests andblood-gasphysiology
Introductionandhistoryofbloodgases
Theterm“bloodgas”referstotheparameterspH, pCO2,and pO2 measuredin blood.Notethatthelittle“p”inpHstandsfornegativelog,whiletheitalicized p in pCO2 and pO2 standsforthepartialpressureofeachofthesegases.Inaddition topH, pCO2,and pO2,modern“bloodgasanalyzers”mayalsomeasurethe hemoglobinfractions,electrolytes,andmetabolitessuchassodium,potassium, ionizedcalcium,chloride,bicarbonate,glucose,andlactate.Someanalyzers alsoincludemeasurementsofcreatinine,urea,andionizedmagnesium.
Thehistoryofbloodgasesandoximetryhasperhapstheoldest,bestdocumented,and,tosomeofus,themostinterestinghistoryofdevelopmentsin laboratorytests.Thehistoryincludesmanynotablefigures,includingJoseph Priestley,whobecamefascinatedobservingthelargevolumeofgasproducedin makingbeerandthenwentontoisolate10differentgases,includingoxygenin thelate1700s.Aroundthattime,theeccentricandexceedinglywealthy(byan unexpectedinheritance)HenryCavendish,oncedescribedas “therichestofall learnedmen,andprobablyalsothemostlearnedofalltherich, ” discovered hydrogen,characterizedcarbondioxide,andwasthefirstpersontoaccurately analyzeatmosphericair.Theearlyhistoryofbloodgasesevenincludes BenjaminFranklin,acolleagueofmanyscientistsincludingPriestleyanda foundingfatheroftheUnitedStatesofAmerica.ToparaphraseAlanGrogono: “Inadditiontopublishingnewspapers,draftingconstitutions,servingaspostmastergeneral,flyingkitesinthunderstorms,discoveringtheGulfStream,and maintainingfriendshipswithFrenchladies,BenjaminFranklinfoundtimeto makeanunfortunateguessaboutcalling“vitreous”charges“positive.” This decisionlaterledtoassigninga“negative”chargetoelectronsanda“positive” chargetohydrogenions (1).
Severaldistinguishedscientistshavecontributedtothedefinitionofan acid.Inthelate1800s,Arrheniusdefinedacidsashydrogensalts.Intheearly 1900s,LawrenceHendersonandKarlHasselbalchsequentiallycharacterized thebufferingrelationshipbetweenanacidandbasetherebycreatingthe eponymousHenderson Hasselbalchequation,butwhoneveractuallyknew
https://doi.org/10.1016/B978-0-323-89971-0.00002-1
1
2 BloodGasesandCriticalCareTesting
eachother.BrønstedandLowrysimultaneously,butseparately,definedacids assubstancesthatcoulddonateahydrogenion,andGilbertLewislater describedanacidasanycompoundthatcouldacceptapairofelectronsto formacovalentbond.
DonaldVanSlykeembracedtheideathatacid basestatuswaspartly determinedbyelectrolytes,anideathatwasexpandedbyPeterStewartinto theverycomplexStrong-Ion-Differenceexplanationofacid basebalance (2). Importantly,VanSlykeiscreditedwithexpandingchemicalanalysesintothe hospitalandisconsideredafounderof“clinicalchemistry.”Oneofhismost notablediscoverieswasthegasometricmethod,whichmeasuredreleasedO2 gasandconsequentiallytheoxygensaturationintheblood.Beforeandduring WorldWarII,KurtKramer,J.R.Squires,andGlenMillikanmadesignificant advancementsinoximetry,whichledtoitsintegratedusewithoxygendeliverysystemsenablingsaferhigh-altitudemilitaryflights.ThesedevelopmentseventuallyledTakuoAoyagitothediscoveryofpulseoximetryin the1970s,whichallowsfortheseparationofthearteriesabsorptionofhemoglobinfromtheabsorptionofthetissueusingthepulsatilenatureofthe arterialabsorptionsignal.
Theprototypeelectrodeformeasuringthepartialpressureofoxygen(pO2) wasdevelopedin1954byLelandClark,usingpolyethylenefilmandother materialsthatcostlessthanadollar.Alsoin1954,RichardStowecovereda pHelectrodewitharubberfingercoveringtodevelopaprototypeoftoday’s partialpressureofcarbondioxide(pCO2)electrodes.Thesestoriesandmany othersthatledtothedevelopmentoftheblood-gasanalyzerweredocumented inabookbyAstrupandSeveringhaus (3).
Themethodologyformeasuringclinicalbloodgaseshasevolved dramaticallyfrommostlylargelaboratory-dedicatedanalyzerstohybridanalyzersadaptabletobothlaboratoryandnear-patientsettings.Therehasalso beenahugegrowthintheuseofportablehand-heldanalyzersthataresuited forsmallerlaboratoriesandnear-patientuseinhospitals,clinics,orremote locations.Bloodgastestingiswidelyusedasatoolfordiagnosingdisorders andevaluatingtheefficacyoftherapeuticinterventions.
Explanationsofbloodgas,acid base,andcooximetry terms
pH.pHisanindexoftheacidityoralkalinityoftheblood.NormalarterialpH is7.35 7.45.ApH <7.35indicatesanacidstate,andapH >7.45indicatesan alkalinestate.Acidemiareferstotheconditionofthebloodbeingtooacidic, andacidosisreferstothemetabolicorrespiratoryprocesswithinthepatient thatcausesacidemia.Theadjectivefortheprocessisacidotic.Similarterms areusedforthealkalinestate:alkalemia,alkalosis,andalkalotic.Becauseall enzymesandphysiologicalprocessesmaybeaffectedbypH,pHisnormally regulatedwithinaverytightphysiologicrange,especiallywithinanindividual,butalsoforreferenceintervals(see Table1.1).
TABLE1.1 Referenceintervalsforvenousandarterialblood. TestAgecategory
4 BloodGasesandCriticalCareTesting
pCO2. pCO2 isameasureofthetensionorpressureofcarbondioxide dissolvedintheblood.The pCO2 ofbloodrepresentsthebalancebetween cellularproductionanddiffusionofCO2 intothebloodandventilatory removalofCO2 fromblood.Anormal,steady pCO2 indicatesthatthelungs areremovingCO2 frombloodataboutthesamerateasCO2 producedinthe tissuesisdiffusingintotheblood.Achangein pCO2 indicatesanalterationin thisbalance.CO2 isanacidicgasthatislargelycontrolledbyourrateand depthofbreathingorventilation,whichchangestomatchtherateofmetabolic productionofCO2. pCO2 isclassifiedastherespiratoryorventilatory componentofacid basebalance.
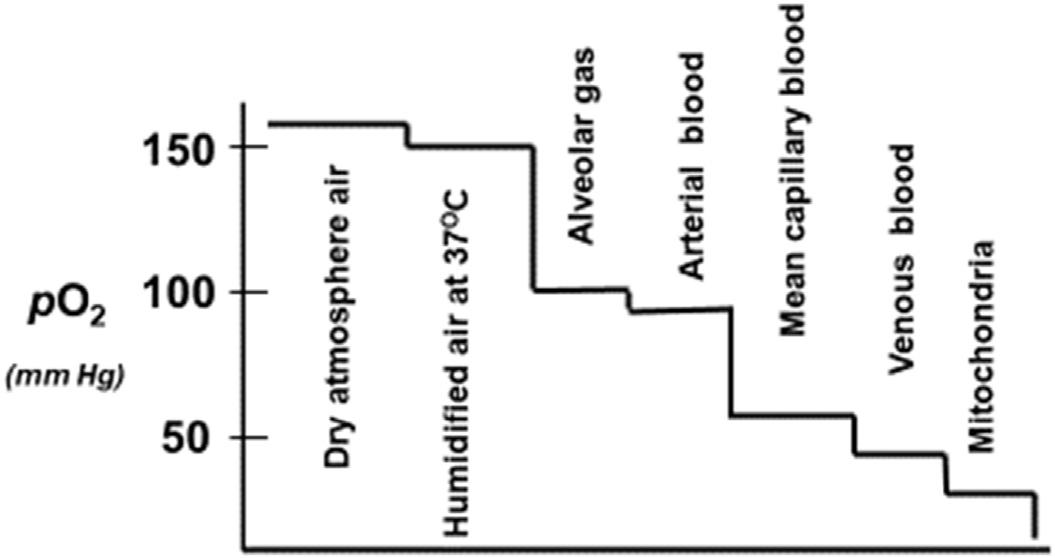
pO2. pO2 isameasureofthetensionorpressureofoxygendissolvedinthe blood.The pO2 ofarterialbloodisprimarilyrelatedtotheabilityofoxygento enterthelungsanddiffuseacrossthealveoliintotheblood.Asshownin Fig.1.1,thereisacontinualgradientof pO2 fromatmosphericair(150mmHg) tothealveoli(w110mmHg),toarterialblood(w100mmHg),capillaries (w60mmHg)andvenousblood(w40mmHg),andfinallytothemitochondriaincellswiththelowest pO2 of w8 12mmHg.Thesegradientsdrivethe movement,binding,andreleaseofoxygenamongthesesystems.
Commoncausesofadecreasedarterial pO2 arelistedbelow,withfurther detailspresentedinChapter4:
l Hypoventilation:AlveolarventilationislowrelativetoO2 uptakeandCO2 production,whichleadstodecreasedalveolar pO2 andincreasedalveolar pCO2.Example:severeobstructivelungdisease.
l Lowoxygenenvironment:Partialpressureofoxygenininspiredairisless than160mmHg.Thisismostcommonlyseenathighaltitudes.
l Ventilation/perfusion(V/Q)mismatch:Areaswithinthelungarereceiving inspiredair,buttheperfusiontothatportionofthelungislimited. Example:Pulmonaryembolism,whereaclotlodgesinapulmonaryartery tolimitbloodflowtoanotherwisefunctioninglungunit.
FIGURE1.1 Gradientsof pO2 fromatmosphericairtobloodtomitochondria.
Introductiontoblood-gastestsandblood-gasphysiology Chapter|1 5
l Shunt:Aportionofthebloodtravelsfromthevenoussystemtothearterial systemwithoutcontactwithafunctioningalveolarunit.Example:Lung diseasewherebloodflowsthroughportionsofthelungthatareunventilatedduetocompleteairwayobstruction,atelectasis,orfillingwithfluidor cells.
l Diffusionimpairment:Oxygenisunabletoefficientlytransferacrossthe blood-gasbarrierofthealveoli.Examples:Thickeningoftheblood-gas barrierduetofibrosis,edema,orinflammatorycellinfiltration.
Bicarbonate.Althoughthebicarbonateion(HCO3 )cannowbemeasured directly,someblood-gasanalyzerscalculate[HCO3 ]withtheHenderson HasselbalchequationfrommeasurementsofthepHand pCO2.Bicarbonateis anindicatorofthebufferingcapacityofbloodandisclassifiedasthemetaboliccomponentofacid basebalance.
BaseExcess.Baseexcess(BE)isacalculatedtermthatdescribesthe amountofbicarbonaterelativeto pCO2.StandardBEreflectsonlythemetaboliccomponentofacid basedisturbances.Itisbasedonthetitratablefluid volumethroughoutthebody(bothextravascularandvascular(blood))andalso includesthecontributionofHbforacid basedisturbances.
TheBEconcepthasalonghistorywithsomespiritedcontroversythatis reviewedbyJohanKofstad,aprofessionalcolleagueandmostgraciousfriend whoImetinOsloonmyfirsttriptoEuropein1983 (5).Baseexcesswas conceivedbyAstrupinthe1950sandrefinedwithequationsandnomogramsby Siggaard-Andersenin1960.In1977,attemptingtoresolvecontroversiesabout BEbetweentheAmericansandDenmark,Severinghausproposedamodified nomogram.However,thedifferentbeliefsrelatedtowhetherBEshouldbe calculatedinbloodorextracellularfluidremainunreconciledtothisday.
Equationsforcalculatingextracellularbaseexcess(BEECF)frompHand eitherHCO3 (mmol/L)or pCO2 (mmHg)appeartobedifferentbutareeerily similar.HerearetwoequationsusedforcalculatingBE (4,6):
The“normal”referenceintervalforBEis 3to þ3mmol/L.Comparison ofthecalculatedBEtothereferencerangeforBEmayhelpdeterminewhether anacid/basedisturbanceisarespiratory,metabolic,ormixedmetabolic/respiratoryproblem.Abaseexcessvalueexceeding þ3indicatesmetabolic alkalosissuchthatthepatientrequiresincreasedamountsofacidtoreturnthe bloodpHtoneutralif pCO2 isnormal.Abaseexcessbelow 3indicates metabolicacidosisandexcessacidneedstoberemovedfromthebloodto returnthepHbacktoneutralif pCO2 isnormal.Mypersonalopinionisthat theBEcalculationaddslittletothesimpleinterpretationofthedifferenceof themeasuredbicarbonate 24,especiallyforpHfrom7.3to7.5,asnoted belowandin Table1.2.
TABLE1.2 Comparisonsofbaseexcesstobicarbonatedifference.
WhenthevalueoftheBEisanegativenumber,itisfrequentlyreferredto asabasedeficit(BD).TheBDisoftenusedtoguideresuscitationinpatients sufferingfromshockwherehypoperfusionleadstoinadequatedeliveryof oxygentothetissueresultinginmetabolicacidosis (7).Asthepatientis successfullyresuscitatedandoxygendeliveryrestored,theBDwillbeginto normalize.Inpatientswhohaveundergoneacutephysicaltrauma,theBDhas asignificantprognosticvalue (8)
Table1.2 showsthattherelationshipbetweenBEandthesimpledifference of(measuredbicarbonate 24mmol/L)isusually2mmol/Lorless,especially forpHfrom7.3to7.5asnotedearlier.Weleaveittoeachreadertodetermine theclinicalimportanceofcalculatingBEversustheuseofthebicarbonate concentration.
Aniongap
Theaniongap(AG)isacalculatedtermforthedifferencebetweenthe commonlymeasuredcations(Naþ andsometimesKþ)andthecommonly
measuredanions(Cl andHCO3 ).Therefore,itrepresentstheunmeasured anionssuchasnegativelychargedproteins(particularlyalbumin)andlactate, phosphates,sulfates,urates,andketonesproducedbythebody.Exogenous toxinsanddrugs,includingmethanol,salicylate,andethyleneglycol(andits metabolites),alsocontributetotheaniongapwhenpresent.TheAGis calculatedasfollows:
AG ¼½Naþ ½Cl HCO3 ðReferenceRange : 8 16mmol = LÞ (1.3)
IfKþ isincludedinthecalculation,theformulaisasfollows:
AG ¼½Naþ þ½Kþ ½Cl HCO3 ðReferenceRange : 12 20mmol = LÞ (1.4)
CorrectingtheAGforabnormalalbuminconcentrationsisimportantasit isthegreatestcontributortotheAGinhealth.Generally,foreachg/dL decreaseinalbumin,theAGisreducedbyabout2.5mmol/L(or0.25mmol/L foreachg/Ldecreaseinalbumin)orissimilarlyincreasedforariseinserum albumin (9,10).TheAGisusefulindiagnosingametabolicacidosisand differentiatingamongthecauses,asshownin Table1.3
Whileoftenveryuseful,AGiscalculatedfromthreeorfourmeasurements andissubjecttoavariationofupto 4mmol/L.Consequentially,itmay detectelevatedlactateinonlyabouthalfofthecases (9).Eveninpatientswith anAGintherangeof20 29mmol/L,onlyabouttwo-thirdswillhavea metabolicacidosis,whileallpatientswithanAGhigherthanthiswillhavea metabolicacidosis (11,12)
Deltagapanddeltaratio
The“deltagap”isthedifferencebetweentheincreaseinAGandthedecrease inHCO3,whilethe“deltaratio”istheratiooftheincreaseinAGdividedby TABLE1.3
thedecreaseinHCO3.TheycanhelpdetermineifthehighaniongapmetabolicacidosisissolelyexplainedbythedecreaseinHCO3,orifapossible mixedacid basedisorderispresent.Thesecalculationsandtheirapplication willbediscussedin Chapter2
Strongiondifference
Thestrongiondifference(SID)isaconceptdevelopedbyPeterAStewartin 1981aimedatexplainingpHchangesandatassessingclinicalacid base disturbances (2,13,14).ItisbasedondifferencesbetweenthestronglydissociatedcationsNaþ,Kþ,Caþþ,Mgþþ,andstronglydissociatedanionsCl and lactate.Stewartusedelectroneutralityandthedegreeofdissociation(strongly orweakly)ofelectrolytestoexplainacid basephysiology.Essentially,the sumofpositivechargesmustbeequaltothesumofnegativecharges.To maintainelectroneutrality,theSIDconceptsaysthattheconcentrationsofHþ , OH ,HCO3 ,andavarietyofotherweakacidsandbases(andthereforethe pH)dependonthedifferencebetweenstronglydissociatedcations(likeNaþ andKþ)andanions(suchasCl andlactate).AhigherSIDfavorsanalkaline environmentandalowerSIDfavorsanacidicenvironment.
TheSIDconceptiscomplexmathematically,noteasilygraspedchemically,andissubjecttoanalyticalvariabilitybecausemultiplemeasurements areusedincalculatingtheSID.Evenmoreconfusingisthatmultipleequations andanalytesseemtobeusedincalculatingtheSID.Somerelativelyclear equationsforSIDare (14,15):
TherearealsoprogramsthatcalculatetheSID,withonerequiringupto11 resultsinthecalculation,includingpH, pCO2,Naþ,Kþ,Cl ,HCO3 ,lactate, phosphate,andalbumin (16).Anotherreportdescribesastrongioncalculator thatminimallyrequires[Naþ],[Kþ],[Cl ],pH,and pCO2 (17).Examplesof SIDindifferentsolutionsareshownin Table1.4.
BecauseStewartconsideredalbuminthemostimportantweakacid (2), Story,etal. (13) proposedsomerelativelysimple(butstillhardtograsp) equationstopredicttheunmeasuredioneffect(UIE)fromthestandardbase excess(SBE),theNa Cleffect,andthealbumineffect:
Thisapproachmightexplainhowadecreasedalbuminissometimes associatedwithmetabolicalkalosis.
Asanexample,theSIDcanhelpdefinehowchangesinCl concentration causesignificantpHchanges.IfCl movesacrossamembrane,itmusteither takeacationwithitorexchangeforanotheraniontomaintain
Introductiontoblood-gastestsandblood-gasphysiology Chapter|1 9
TABLE1.4 Examplesofstrongiondifferences.
Hypotheticalsolutionwith SID ¼ 0
Hypotheticalsolutionwith SID ¼ 10
Na(mmol/L)100100
K(mmol/L)00
Hþ (nmol/L) w010
Cl(mmol/L)100110
Lactate(mmol/L)00
SID(mmol/L)0 10
pHNeutralVeryacidic
electroneutrality.IfCl takesanotherstronglydissociatedionwithit,suchas Na,therewillbenonetchangeintheSIDandnochangeinpH.However,if Cl exchangesforaweakerHCO3 ion,theSIDofthesolutionreceivingCl willdecreaseandthesolutionwillbecomemoreacidic,andthesolution receivingtheHCO3 willbecomemorealkaline (13,14)
WhiletheSIDtheorymaycorrectlyexplainsomeactualionmovements andpHchanges,itisrarelyusedclinicallytounderstandthediagnosisand managementofacid basedisorders,asnotedelsewhere (18).
Hemoglobinandderivatives
Hbanditsderivativesaremeasuredbycooximetry.Theseareoxyhemoglobin (O2Hb),deoxyhemoglobin(HHb),carboxyhemoglobin(COHb),andmethemoglobin(metHb).Briefly,O2Hbhasfouroxygenmoleculesboundtoeachof thehemegroupsinthehemoglobinmolecule.HHbistheoxygen-unloaded formofhemoglobin.COHbhascarbonmonoxide(CO)boundverytightly toanO2-bindingsiteonahemegroup,whichincreasestheaffinityoftheother threehemegroupsforO2 molecules,andpreventsoxygenunloadinguntilthe COisreleased.MetHbisinactiveHb(unabletobindO2)becauseitsferrous ion(Fe2þ)hasbeenoxidizedtoaferricion(Fe3þ).Hemoglobinfunctionis furtherdiscussedlaterinthischapter.
sO2 and%O2Hb
sO2 isthepercentoxygensaturation,whichisthepercentageoffunctionalHb thatisboundwithoxygen.sO2 iscalculatedastheconcentrationofO2Hb dividedbytotalfunctionalHb.sO2 mayvaryfrom0%to100% (2).
The%O2Hb(formerlycalledthefractionalHbsaturation)isthepercentage oftotalHbthatissaturatedwithoxygen.The%O2Hbmaybeusedfor determiningtheoxygencontentofbloodand,thereforeoxygendelivery(DO2) andoxygenconsumption(VO2) (19)
ThedifferencebetweensO2 and%O2Hbisusuallyoflittleclinicalrelevance,butitcanbequitesignificantinthesettingofpathologicallyhighlevels ofCOHbormetHb.Therefore,itisimportanttounderstandthedifference betweenthesetwotermsforoxygenboundtohemoglobin(sO2 and%O2Hb) (20)
COHbandmetHb
Carboxyhemoglobin(COHb)andmethemoglobin(metHb)togethernormally makeuponly1% 2%ofthetotalHbinblood.NeithercanperformO2 carryingandreleasingfunctions.COHbisHbwithcarbonmonoxide(CO) boundverytightlytoasitethatnormallybindsO2.Exposuretocarbon monoxidewillincreasethe%COHbfromanormalbaselinelevelofabout1% to5% 10%insmokersand50%ormoreinthosewithexposuretotoxicor lethallevels,ascanoccurinthesettingofcombustioninapoorlyventilated area.MethemoglobinisHbthathasitsFe2þ ionoxidizedtoFe3þ,which makesmetHbunabletoeffectivelybindO2.
DO2 andVO2
BothDO2 (oxygendelivery)andVO2 (oxygenconsumption)areparameters forassessingcardiacfunction,metabolicdemands,andmitochondrialfunction incriticallyillpatients.Therearenowseveralwaysofdeterminingthese parameterswithlaboratorymeasurements.DO2 inmL/minrequiresmeasurementofthefollowingvaluesinarterialblood:Hbing/L,%O2Hbfrom 0to1.0, pO2 inmmHg(ofminorimportanceinthiscalculation),andcardiac output(CO)inL/min.Thecalculationisessentiallydetermininghowmuch oxygenisloadedonhemoglobinordissolvedinthebloodandpumpedtothe bodybythehearteveryminute.
whereO2a ¼ arterialoxygencontent.
VO2 isoxygenconsumptionbytheorgansandtissuesofthebody.Itisthe differenceinoxygencontentbetweenarterial(O2a)andvenous(O2v)blood timescardiacoutput(CO):
DO2 ¼ CO ð1 34 Hb %O2 Hb þ 0 03 pO2 Þ¼ CO ðO2 aÞ (1.10)
VO2 ¼ CO ðO2 a O2 vÞ (1.11)
Introductiontoblood-gastestsandblood-gasphysiology Chapter|1 11
Physiologyofacidandbaseproduction
Introduction.Mostenzymaticandmetabolicprocessesessentialtolifeare criticallydependentonpH.Thus,thehydrogenionconcentration(andpH)are tightlyregulatedinthehumanbody.Whileonlyextracellular(blood)pHis measuredforclinicalpurposes,mostpH-dependentreactionsoccurwithinthe cell,wherethepHisslightlymoreacidic (21).Metabolicacidosismay developifeitherHþ accumulatesorHCO3 islost.Whereas,metabolic alkalosismaydevelopfromeitherlossofHþ orincreaseofHCO3 .RespiratoryacidosisresultsfromincreasedCO2 intheblood,whilerespiratory alkalosisresultsfromdecreasedCO2 inblood,withbothprocesseslargely relatedtoeffectivealveolarventilationrate.
“Metabolicacid”.Thevastmajority(about20 moles perday)ofour metabolicacidisactuallyCO2 producedinthemitochondriaasoneofthe ultimatebyproductsofglucoseoxidation,withlesseramountsproducedby metabolismoffatsandaminoacids.MostCO2 combineswithH2Otoproduce carbonicacid,whichisreadilyconvertedbacktoCO2 forremovalbyalveolar ventilation.Thus,eventhoughCO2 isproducedbyordinarymetabolism,itis consideredthe“respiratory”componentofouracid-basebalancebecause alveolarventilationdirectlyaffectstheblood pCO2.
“Lactate”acidosis: Contrarytocommonbelief,lacticacidisvirtually NEVERproduced.Infact,theonlyreactioninthehumanthatproduceslactate iswhenpyruvateisconvertedtolactate,areactionthatactually consumes acid bytheparticipationofNADHandHþ beingconvertedtoNADþ (22).So wheredoestheacidosiscomefromduringanoxygendeficit?Itisproduced manybiochemicalstepslaterwhenATPisconvertedtoADP,phosphate,and hydrogenions.Normally,oxidativephosphorylationreconvertstheseproducts backtoATPwithlittlenetchangeofHions.However,withoutoxygen,the ADPcannotundergooxidativephosphorylationandHions(acid)accumulates.LactateisindependentlyproducedfromabuildupofNADHthatfavors theconversionofpyruvatetolactatebytheenzymelactatedehydrogenase. Thus,lactateisamarkerforinsufficientoxygenavailabilitytothetissues, cells,andmitochondria,orinabilityofthemitochondriatoextractoxygenin thesettingofcarbonmonoxidepoisoning,certaincytokines,reactiveoxygen species,orothercellfactorspresentinsepsis (23)
Ketoacidosis.Ketoacidosisoccursinthesettingofunregulatedketone productioncausingasevereaccumulationofketoacidsintheblood.Themost commonclinicalpresentationofketoacidosisoccurswithdiabeticketoacidosis, whichdevelopswhenapersonhastoolittleinsulinsuchthatglucosecannotenter cellstogenerateenergy.Theliverbeginstobreakdownfatforenergy,which producestoxicketoacidsformedbythedeaminationofaminoacidsandthe breakdownoffattyacids.Thetwocommonacidicketonesproducedinthis processareacetoacetateand b-hydroxybutyrate.Ketoacidosiscanalsooccur withprolongedstarvationorseverealcoholismwherethebodyhaddepleted carbohydratestoresandisforcedtobreakdownfatforsustenance.
12 BloodGasesandCriticalCareTesting
Nonvolatileacids.Theseacidsaregeneratedasphosphatesandsulfates predominantlyfromprotein,phospholipid,andnucleicacidmetabolism.Acids fromthesesourcesaccountfor <1%ofacidproducedperday (21).
Productionofbase.Generally,most“new”basiccompoundscomefrom dietarysources,withlittledirectmetabolicproductionofbicarbonateorother alkalinesubstances.BicarbonateisgeneratedwhenCO2 combineswithH2O anddissociatesintoHþ andHCO3 ,however,thereisnonetgainofanalkaline substanceunlesstheHþ isexcreted.ThekidneycanretainorexcreteHCO3 dependingonthephysiologicneed.
Ammoniametabolisminthekidneyiscomplex,butbasicallyammonia (approx98%asNH4þ and2%asNH3 atpH7.4)andHCO3 areproducedin equimolaramountsbyseveralenzymaticreactionsinvolvingglutamine(note: theterm“ammonia”referstothecombinationofbothNH4þ andNH3 in metabolism).Inresponsetoacidosis,ammoniaandbicarbonatearegenerated. WhenNH4þ isexcreted,bicarbonateremains,whichisamajormechanismof bicarbonategenerationinthekidney (24).Theproximaltubuleisthemainsite ofammoniaformation.Inchronickidneydisease,thekidneysareunableto produceandexcreteenoughammonia,whichleadstotheaccumulationofacid andthedevelopmentofmetabolicacidosis.BicarbonatecanincreaseifCl is lostbyeithervomitingorbyrenallossfromdiuretics.Generally,anylossof acidwillincreasetheproportionofHCO3 fromCO2.Bicarbonategivenin excesstotreatanacidosisisaniatrogenicsourceofbase.
Buffersystems
Bicarbonate carbondioxide(carbonicacid).Bicarbonateisthebaseinthe highestconcentration(w24mmol/L)inthebloodplasmaandisofcentral importanceinacidbufferingintheblood.CO2 isavolatileacidicgasthatis solubleinwater(solubilityfactor0.03mmol/mmHg)andisthemajoracid producedasaproductofenergymetabolism.CO2 producedbymetabolism readilydiffusesfromcellsintotheblood,whereitcombineswithH2Oto producecarbonicacid,whichimmediatelydissociatestoHCO3 andhydrogen ions.
pH,HCO3 ,and pCO2 (inmmHg)arerelatedbytheequation:
pH ¼ pK þ log HCO3 = ð0:03 pCO2 Þ (1.12)
NotethatthepKisdefinedasthepHatwhichtheHCO3 andH2CO3 (or 0.03 pCO2)areinequalconcentrations.Thatis,theirratiois1,andthelog of1isequalto0.
Atthenormalconcentrationratiointhebloodof20:1(“ideal”wouldbe 1:1),andwithapKof6.1(“ideal”wouldbe7.4),theHCO3 -H2CO3 buffer systemwouldseemtobeill-suitedtobufferingpHintheblood.However,this excessofbase(HCO3 ),alongwiththevolatilityofCO2,givestremendous abilitytopreventexcessaccumulationofacid.Thelungseffectivelymakethis
Introductiontoblood-gastestsandblood-gasphysiology Chapter|1 13
anopensystem,withtheabilitytoexhaleCO2 providingalmostunlimited bufferingcapacity.Asthepreviousequationindicates,thepHofbloodis largelydeterminedbytheratioofHCO3 toH2CO3.Thus,HCO3 :H2CO3 ina concentrationratioof15:0.75hasthesamepHasaratioof20:1.0,butwould havelessbufferingcapacity.
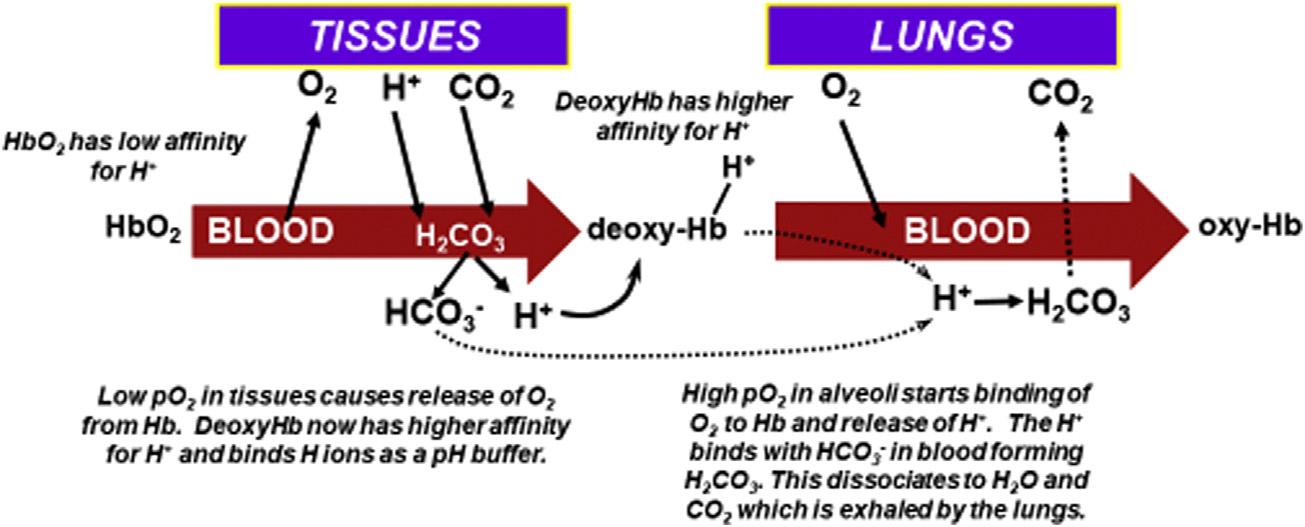
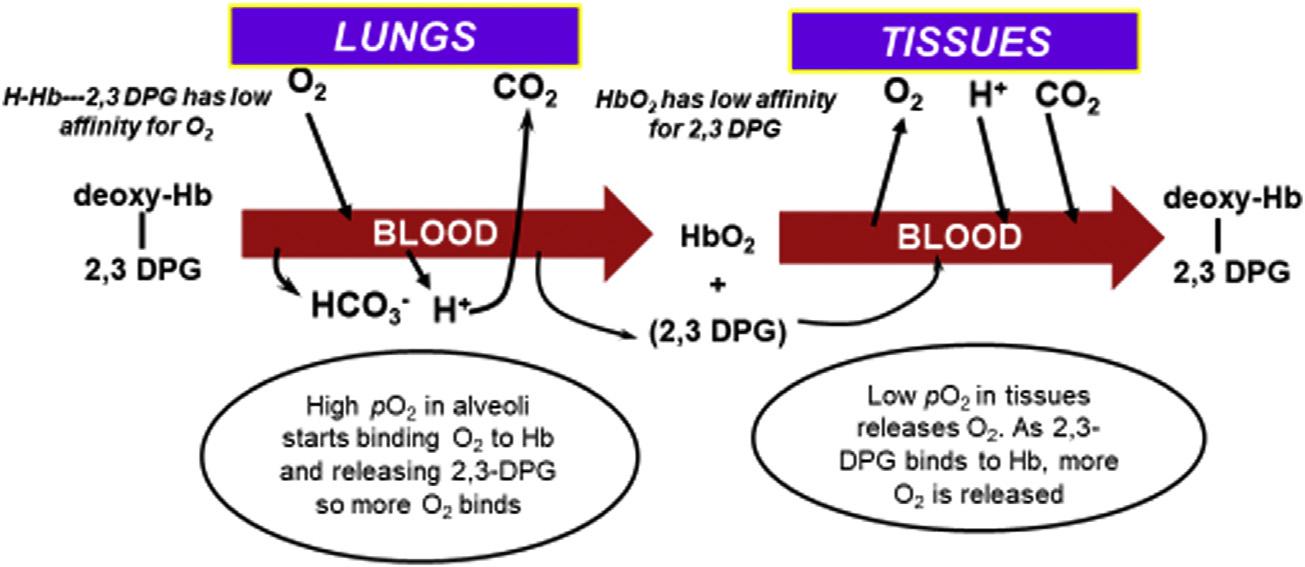
Hemoglobin.Hbactsasabufferbytransportingacidfromthetissuesto thelungs.AremarkablefeatureofHbisthatitincreasesitsaffinityfor hydrogenions(Hþ)asitreleasesoxygen.Thatis,deoxy-Hb(alsocalled reducedHb)hasagreateraffinityforHþ thandoesO2Hb.Asoxy-Hbenters tissuecapillaries,theenvironmentoflower pO2 andhigherHþ andCO2 increasesHþ bindingtoHb,thuspromotingmorereleaseofO2 tothetissues.As thedeoxyHbentersalveolarcirculationandO2 bindstoHb,itreleasesHþ , thatcombineswithHCO3 toultimatelyformCO2 thatdiffusesintothealveoli andisexhaled.2,3-Diphosphoglycerate(2,3-DPG)alsoaffectstheHbbinding ofO2 asitbindstoHbinthetissues(decreasingaffinityofHbforO2)and releasesfromHbinthealveoli(increasingaffinityofHbforO2).Theserelationshipsareshownin Figs.1.2and1.3
Phosphate.TheHPO4 2 H2PO4 bufferpairisofminorimportanceasa bufferinplasma,withaconcentrationof w1mmol/L(3.1mg/dL).Itisof greaterimportance,andinhigherconcentration,asanintracellularbuffer.
Albuminandotherproteins.Largelyduetotheimidazolegroupsonthe aminoacidhistidine,withapKof w7.4,albuminandotherproteinsalsoactas pHbuffers.Albuministhemajor“unmeasured”anioninblood,anditis normallythebiggestcontributortotheaniongap.Patientswithcriticalillness and/ormalnutritioncommonlyhavehypoalbuminemia,whichcanlowerthe AGandpotentiallyimpacttheinterpretationofthisparameter (11,25)
FIGURE1.2 Interrelationshipsofthebicarbonateandhemoglobin(Hb)systemsinbufferingHþ andinthebindingandreleaseofoxygeninlungsandtissues.Thehigh pO2 inthelungsandlow pO2 inthecapillaries,respectively,drivethebindingandreleaseofO2.Hionsparticipateby bindingtoHbinthetissuecapillariestodecreasetheaffinityofHbforO2 ordissociatingfromHb inthelungstoincreasetheaffinityofHbforO2.
FIGURE1.3 Interrelationshipsofhemoglobin(Hb),2,3-diphosphoglycerate(2,3-DPG),andHþ inbindingandreleaseofoxygeninlungsandtissues.2,3-DPGremainsinredcellsandcooperates withHbtoincreaseordecreaseHbaffinityforO2.Asdeoxy-Hbboundwith2,3-DPGenters alveolarcirculationinthelungs,O2 bindstoHb,whichreleases2,3-DPGandHþ.Thesefurther increasetheaffinityofHbforO2 somoreO2 isbound.AsO2-Hbenterstissues,theenvironmentof higherHþ andCO2 promotesthereleaseofO2 tothetissues,whichnowfavorsHbbindingof2,3DPGandHþ topromotemorereleaseofO2 fromHb.
Acid baseregulation
Normalacid basebalance,oxygenmetabolism,andtheirassociateddisorders caninvolveacomplexinterplayofseveralorgansystems,especiallypulmonary,renal,hepatic,andgastrointestinal.Thebrainmaybeoverlookedbutit playsanextremelyimportantroleinacid baseregulation (21)
Respiratory(pulmonary)system.BecausearterialCO2 isinfluenced greatlybyalveolarventilation, pCO2 isconsideredtherespiratorycomponent ofthebicarbonate-CO2 buffersystem.BecauseCO2 istheendproductof manyaerobicmetabolicprocesses,bufferingandremovalofCO2 are continuallyrequiredforpHregulation.Providedthereisasufficientgradient of pCO2 betweentissuesandblood,CO2 willreadilydiffuseintotheblood.As mentionedpreviously,CO2 combinesenzymaticallywithH2Otoformthe unstableH2CO3,whichquicklydissociatesintoHCO3 andHþ ions.DeoxyHbplaysakeyroleherebyreadilyacceptingtheHþ andtransportingitto thelungsfromthetissues.AsHþ isexchangedforoxygeninthelungs,Hþ quicklycombineswithHCO3 toproduceH2CO3,whichdissociatestoH2O anddissolvedCO2,whichdiffusesintothealveolarairforventilatoryremoval (see Fig.1.2).
Thearterial pCO2 representsabalancebetweentissueproductionand diffusionofCO2 intothebloodandpulmonaryremovalofCO2.Anelevated pCO2 usuallyindicatesinadequateventilation(hypoventilation)andarespiratoryacidosis.Conversely,adecreased pCO2 usuallyindicatesexcessive ventilation(hyperventilation)andarespiratoryalkalosis.Thereareseveral causesofrespiratoryabnormalities (26,27).
Introductiontoblood-gastestsandblood-gasphysiology Chapter|1 15
Respiratoryacidosis(ventilatoryfailure)canbecausedbythefollowing:
l Obstructivelungdisease:chronicbronchitis,emphysema,asthma
l Impairedcentralrespiratorydrive:headtrauma,sedatives,opioids, anesthesia
l Weakordisordereddiaphragmmuscles
l Hypoventilationbymechanicalventilator
Respiratoryalkalosis(hyperventilation)canbecausedbythefollowing:
l Hypoxemia(whichstimulateshyperventilation)
l Anxiety
l Pain
l Hyperventilationbymechanicalventilator
l Metabolicacidosis
l Septicemia
Metabolic(renal)system.WhentheHþ concentrationdeviatesfrom normal,thekidneysrespondbyreabsorbingorsecretinghydrogen,HCO3 , NH4þ,andotherionstoregulatethepHoftheblood.BecauseHCO3 isprimarilyregulatedbythekidney,itisconsideredthemetaboliccomponentofthe HCO3 -CO2 buffersystem.Inkidneyfailure,acidemiabecomesmorelikely becausethekidneysarelessabletogenerateammoniaandbicarbonatesothat lessNH4þ isexcretedwhichleaveslessbicarbonatetobufferthehydrogenion.
Metabolic(liver)system.Theureacycleintheliveressentiallyconverts bicarbonateandammoniatourea,whichisaneutralcompound.Because acidosisdecreasesthehepaticproductionofurea,moreammoniaisconverted toglutamine,whichsparesbicarbonateandattenuatestheacidosis (21)
Brainsystem.Thebrainmaybeanoverlookedbutveryimportantregion foracid baseregulation (21).RegulationofbrainpHdiffersfromperipheral pHregulationbyinvolvingcellularmechanismsthatallowthebrainto compensatebacktonearnormalineachoftheprimaryacid basedisturbances:metabolicacidosisandalkalosis,andrespiratoryacidosisandalkalosis.CorrectionofsystemicpHwhenbrainpHisdisturbed,suchasinstroke, maydisrupttheimportantbraincompensation (21)
Aldosteroneinacid basebalance.Aldosteroneisaverypowerful regulatorofacid basebalanceandlinksthiswithitsprincipalfunctionin regulatingsaltandpotassiumhomeostasis (28). Compensation.Compensationisahomeostaticresponsetoanacid base disorderinwhichthebodyattemptstorestorepHtowardnormalbyadjusting theHCO3 :(0.03 pCO2)backtoanormalratioof20:1.Compensationinvolveseitherarelativelyrapidventilatoryresponse(changein pCO2)toa metabolicabnormalityorarelativelyslowmetabolicresponse(changein HCO3 )toaventilatoryabnormality.Althoughachangeinalveolarventilation canalterarterialpHinminutes,thekidneysrequirehourstodaystosignificantlyaffectpHbyalteringtheexcretionofHCO3
AscompensationreturnspHtowardnormal,thepH-drivencompensation processslows,thenstopsbeforethepHisfullynormalized,whichhassurvival benefits.Mechanismsforthisarecomplex,butinmetabolicacidosis,theacid pHandlowbicarbonatestimulatethemedullarycentertoincreaseventilation. Becausehyperventilationincreasesenergyconsumption,incomplete compensationreturnspHsufficientlynearnormalwhilesavingenergyto maintainothervitalprocesses (21).
Inmetabolicalkalosis,hypoventilationoccurstodecreasethepH.However,fullcompensationislimitedbecausecoexistinghypokalemia,volume depletion,andhyperaldosteronismdevelopandmaintainaslightmetabolic alkalosis.Thebenefitsofthisincompletecompensationarethatwater,salt, andpotassiumbalancearemaintained.
Whiletherespiratorycompensationbyhyperventilationisfairlypredictableinmetabolicacidosis,therespiratoryresponsebyhypoventilationisless predictableinmetabolicalkalosis.Whilehypoventilationalmostinvariably occursinmetabolicalkalosis,otherfactorssuchaspainandhypoxemiacan stimulateventilationandovercomethehypoventilatoryeffectofmetabolic alkalosis (29).
Theexpectedcompensationforeachacid baseabnormalitywillbediscussedlaterinthesections“ClinicalDisordersofAcid-BaseBalance”and “Mixedacid-basedisorders.”
Hemoglobinfunction
Hbisaproteinof64,500Dathatconsistsoffourhememoleculesattachedto fourglobinmolecules.Hbiscertainlyahall-of-famemolecule,havingthe essentialabilitiestobind,transport,andreleaseoxygentothetissuesandto transportHþ andcarbondioxidefromthetissuestothelungs.Eachofthefour hemegroupscontainsanFeþþ ionandcanbindonemoleculeofoxygen. Structuralchangesoccurwithoxygenbindingthatresultsincolorchangesto themolecule.HHb(deoxyhemoglobin)givesthebloodadeeppurplishhue, whileO2Hbgivesthebloodascarletredappearance (30).2,3-DPGisa moleculecontainedwithinerythrocytesthatcooperateswithHbtobindor releaseO2.AsHbbindsO2 inthealveoli,thisfavorsthereleaseof2,3-DPG, whichfurtherincreasestheaffinityofHbforO2 binding(Fig.1.3).This eventuallyleadstothesaturationofHbwithoxygenandasigmoidalrelationshipbetween pO2 andsO2,asshownintheHb-oxygendissociationcurve in Fig.1.4.Hemoglobinwillbediscussedindetailin Chapter4.
Referenceintervalsforbloodgases
Higginsprovideshisusualexcellentdiscussionoftheimportanceandderivationofreferenceintervals,specificallyforbloodgases(31,32).Becausethe clinicalvalueoflaboratorytestresultsdependsonthequalityofreference
Introductiontoblood-gastestsandblood-gasphysiology Chapter|1 17
FIGURE1.4 Oxyhemoglobindissociationcurveforwholeblood.ThesolidmiddlecurverepresentsthepercentageofHbthatissaturatedwithoxygenas pO2 isincreased.Thedashedcurve representsa leftshift ofthecurvewhentheaffinityofHbforO2 isincreasedbycoolertemperature, higherpH,orreleaseof2,3-DPG.Thedottedcurverepresentsa rightshift ofthecurvewhenthe affinityofHbforO2 isdecreasedbywarmertemperature,lowerpH,orbindingof2,3-DPG.The P50istheoxygenpressure(pO2)thatsaturatesHbby50%andisanindexoftheoverallaffinityof HbforO2.A leftshift ofthecurvedecreasestheP50anda rightshift increasestheP50.
intervals,theyshouldbereviewedandupdatedregularly.Healsonotesthe lackofgoodstudiesofreferenceintervalsforarterialbloodgases,understandablyduetotheriskandpainassociatedwithcollectingarterialblood. HigginscitesastudyfromDenmarkdonein2011inwhicharterialbloodwas collectedfrom182healthyadults(96females;86males)ages20 76years (33).Ofcriticalimportancetobloodgasresults,allsampleswereanalyzed within10minofcollection.Referenceintervalsfromthisstudyareincludedin Table1.1 alongwithreferenceintervalsfromseveralsources.
Thereferenceintervalsfor pO2 requireadditionalexplanation.First, because pO2 hassomevariabilitywithage,enoughdataisprovidedtoindicate the pO2 differencesforvariousagegroups.Afterage60years,theaverage pO2 declines,althoughthereisdebateabouttherateatwhichthisoccurs.Traditionally,the pO2 isbelievedtodeclineatabout1mmHgperyearafterage60, butthishasbeenchallenged(34,35).Whilealowerreferencelimitfor pO2 of 70mmHgisreasonableat70yearsofage,thatmayalsobethelowerlimitfor personsolderthan70yearswhoareinreasonablehealth.Second,someclaim thatforpersonsbreathingroomair,thearterial pO2 shouldnotgoabove 100mmHg.Thisisbasedonthepresumptionthatalveolar pCO2 isapproximately50mmHgforavenous pCO2 of40mmHg.Becausealveolarairisa mixtureofincomingatmosphericair(pO2 w150mmHg)andwiththeCO2
contributiontoalveolarairof wpCO2 50mmHg,thealveolar pO2 shouldbe nohigherthan w100mmHg.However,inyoungerpersons,thestudiesclearly indicatethearterial pO2 canbeupto w110mmHg(31,33). Intheneonate,BrouilletteandWaxmanshowedthatbloodgaseschange rapidlyduringthefirst60minafterbirth,thenstabilizeby60 120minafter birthatlevelsthatresembleadultvalues(36).
Self-assessmentandmastery
1. Whoiscreditedwithdiscoveringtheconceptofpulseoximetry?
a. LelandClarke
b. PeterStewart
c. TakuoAoyagi
d. JohnToffaletti
e. VanSlyke
2. Whichparameterismostcloselyrelatedtobaseexcess?
a. Aniongap
b. Na
c. Cl
d. HCO3
3. Whichanionscontributetotheaniongap?
a. Albumin
b. Acetoacetate
c. Phosphate
d. Lactate
e. Alltheabove
4. Whichmoleculebindsmosttightlytohemoglobin?
a. Oxygen
b. Hydrogenion
c. CO
d. CO2
5. WhatisVO2?
a. Vanadiumdioxide
b. Breathingrateperminute
c. Oxygenconsumption
d. Oxygendelivery
e. Percentofoxygenininspiredair
6. Whichstatementisfalse?
a. Normalmetabolismproducesmorealkalinethanacidiccompounds
b. LossofHCO3 createsanacidosis
c. LossofHioncreatesandalkalosis
d. ExtracellularpHishigherthantheintracellularpH
7. Whichmetabolicproductcreatesthemostacidiccondition?
a. CO2
b. NADþ
c. ATP
d. Lactate
8. Mostammoniaisproducedas
a. NH4þ
b. Aminoacids
c. NH3
d. Creatinine
9. Whatarefunctionsofhemoglobin?Chooseallthatapply.
a. BufferspH
b. CarriesO2
c. ReleasesO2
d. Uses2,3-DPGinO2 bindingandrelease
10. Whichorganscontributetoacid baseregulation?
a. Lungs
b. Kidneys
c. Liver
d. Brain
e. Alltheabove
11. Compensationinacid baseregulationrespondstowhichofthefollowing parametersinblood?
a. Changesinlactate
b. ChangesinpH
c. Changesinsodium
d. Changesincreatinine
12. ArterialpCO2 isabalancebetween:
a. CO2 productionandbindingbyHb
b. TissueproductionandpulmonaryremovalofCO2 fromblood
c. HCO3retentionandexcretionbythekidneys
d. Hypoventilationandhyperventilation
e. LactateproductionandATPdepletion
AnswerKey:
1. c 2. d
3. e
4. c
5. c 6. a 7. a Introductiontoblood-gastestsandblood-gasphysiology Chapter|1 19
8. a
9. a,b,c,d
10. e
11. b
12. b
References
1.Grogono,A.W. Acid-BaseTutorial:Acid-BaseHistory http:/www.acid-base.com/history (accessedDecember2020).
2.Story,D.A.Bench-to-BedsideReview:ABriefHistoryofClinicalAcid-Base. Crit.Care 2004, 8, 253 258.
3.Astrup,A.;Severinghaus,J.W. TheHistoryofBloodGases,AcidsandBases; Munksgaard: Copenhagen,1986.
4.Grogono,A.W. Acid-BaseTutorial:Terminology:BaseExcess. http:/www.acid-base.com. (accessedDecember2020).
5.Kofstad,J. AllAboutBaseExcess toBEorNottoBE, July2003. www.acutecaretesting. org.
6. BaseExcess&CalculatedBicarbonate http://www-users.med.cornell.edu/wspon/picu/calc/ basecalc.htm.(accessedOctober2020).
7.Connelly,C.R.;Schreiber,M.A.Endpointsinresuscitation. Curr.Opin.Crit.Care 2015, 21(6), 512 519.
8.Ibrahim,I.;Chor,W.P.;Chue,K.M.;etal.Isarterialbasedeficitstillausefulprognostic markerintrauma?Asystematicreview. Am.J.Emerg.Med. 2016, 34(3), 626 635.
9.Kraut,J.A.;Nagami,G.T.TheSerumAnionGapintheEvaluationofAcid-BaseDisorders: WhatAreItsLimitationsandCanItsEffectivenessBeImproved? Clin.J.Am.Soc.Nephrol. 2013, 8, 2018 2024.
10.Feldman,M.;Soni,N.;Dickson,B.InfluenceofHypoalbuminemiaorHyperalbuminemiaon theSerumAnionGap. J.Lab.Clin.Med. 2005, 146, 317 320.
11.Brandis,K. Acid-BasePhysiology:TheAnionGap.From http://anaesthesiaMCQ.com/ AcidBaseBook/ab3_2.(accessedDecember2020).
12.Iberti,T.J.;Leibowitz,A.B.;Papadakos,P.J.;Fischer,E.P.LowSensitivityoftheAnion GapasaScreentoDetectHyperlactatemiainCriticallyIllPatients. Crit.CareMed. 1990, 18, 275 277.
13.Story,D.A.;Morimatsu,H.;Bellomo,R.StrongIons,WeakAcidsandBaseExcess:A SimplifiedFencl-StewartApproachtoClinicalAcid-BaseDisorders. Br.J.Anaesth. 2004, 92, 54 60.
14.Grogono,A.W. Acid-BaseTutorial:Stewart’sStrongIonDifference. http:/www.acid-base. com/strong-ion-difference.(accessedDecember2020).
15.Moviat,M.;Terpstra,A.M.;Ruitenbeek,W.;Kluijtmans,L.A.J.;Pickkers,P.;vander Hoeven,J.G.ContributionofVariousMetabolitestothe“Unmeasured”AnionsinCritically IllPatientswithMetabolicAcidosis. Crit.CareMed. 2008, 36 (3),752 758.
16. ICUCalculator;BloodGas(Stewart) https://intensivecarenetwork.com/Calculators/Files/ Gazo.html
17.Lloyd,P.StrongIonCalculator APracticalBedsideApplicationofModernQuantitative Acid-BasePhysiology. Crit.CareResusc. 2004, 6, 285 294.
Introductiontoblood-gastestsandblood-gasphysiology Chapter|1 21
18.Masevicius,F.D.;Dubin,A.HasStewartApproachImprovedOurAbilitytoDiagnoseAcidBaseDisordersinCriticallyIllPatients? WorldJ.Crit.CareMed. 2015, 4 (1),62 70.
19.Scott,M.G.;LeGrys,V.A.;Hood,J.L.ElectrolytesandBloodGases.In TietzTextbookof ClinicalChemistryandMolecularDiagnostics; Burtis,C.A.,Ashwood,E.R.,Bruns,D.E., Eds.,5thed.;ElsevierSaunders:StLouis,2012;pp807 835.
20.Toffaletti,J.;Zijlstra,W.G.MisconceptionsinReportingOxygenSaturation. Anesth.Analg. 2007, 105, S5 S9.
21.Seifter,J.L.;Chang,H.-Y.DisordersofAcid-BaseBalance:ANewPerspective. KidneyDis. (Basel) 2017, 2 (4),170 186.
22.Robergs,R.A.;Ghiasvand,F.;Parker,D.BiochemistryofExercise-InducedMetabolic Acidosis. Am.J.Physiol.Regul.Integr.Comp.Physiol. 2004, 287, R502 R516.
23.Gotts,J.E.;Matthay,M.A.Sepsis:PathologyandClinicalManagement. Br.Med.J. 2016, 353, i1585.
24.Mohiuddin,S.S.;Khattar,D. Biochemistry,Ammonia; NCBIBookshelf,July2020.
25.Kraut,J.A.;Madias,N.E.SerumAnionGap:ItsUsesandLimitationsinClinicalMedicine. Clin.J.Am.Soc.Nephrol. 2007, 2, 162 174.
26.Patel,S.;Sharma,S. RespiratoryAcidosis; NCBIBookshelf,June2020. www.ncbi.nlm.nih. gob/books/NBK482430/
27.Brandis,K. RespiratoryAlkalosis Causes http://www.anaesthesiaMCQ.com.(accessed November2020).
28.Wagner,C.A.EffectofMineralocorticoidsonAcid-BaseBalance. Nephron.Physiol. 2014, 128, 26 34.
29.Brandis,K. Acid-Basephysiology:MetabolicAlkalosis-Compensation.From http://www. anaesthesiaMCQ.com/AcidBaseBook/ab7_5.(accessedJanuary2020).
30. Why/HowisBloodRed?(ColoursofHemoglobin). https://chemistry.stackexchange.com/ questions/49940/why-how-is-blood-red-colours-of-hemoglobin.
31.Higgins,C. AdultReferenceIntervalsforBloodGases, January2012. www.acutecaretesting. org.
32.Higgins,C. CentralVenousBloodGasAnalysis, July2011. www.acutecaretesting.org
33.Klaestrup,E.;Trydal,T.;Pedersen,J.;Larsen,J.;Lundbye-Christensen,S.;Kristensen,S. ReferenceIntervalsandAgeandGenderDependencyforArterialBloodGasesandElectrolytesinAdults. Clin.Chem.Lab.Med. 2011, 49, 1495 1500.
34.Hardie,J.A.;Vollmer,W.M.;Buist,S.;Ellingsen,I.;Morkve,O.ReferenceValuesfor ArterialBloodGasesintheElderly. Chest 2004, 125, 2053 2060.
35.Sorenson,H.M.ArterialOxygenationintheElderly. EliteLearn. 2006, 19 (2),17. https:// www.elitecme.com/resource-center/respiratory-care-sleep-medicine/arterial-oxygenation-inthe-elderly.
36.Brouillette,R.T.;Waxman,D.H.EvaluationoftheNewborn’sBloodGasStatus. Clin. Chem. 1997, 43 (1),215 221.