https://ebookmass.com/product/aggregation-induced-emissionaie-a-practical-guide-jianwei-xu/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Continuous Emission Monitoring 3rd Edition James A. Jahnke
https://ebookmass.com/product/continuous-emission-monitoring-3rdedition-james-a-jahnke-2/
ebookmass.com
Continuous Emission Monitoring 3rd Edition James A. Jahnke
https://ebookmass.com/product/continuous-emission-monitoring-3rdedition-james-a-jahnke/
ebookmass.com
Chronic Total Occlusions-A Guide to Recanalization, 3e (Nov 29, 2023)_(1119517273)_(Wiley-Blackwell) Ron Waksman
https://ebookmass.com/product/chronic-total-occlusions-a-guide-torecanalization-3e-nov-29-2023_1119517273_wiley-blackwell-ron-waksman/ ebookmass.com
Fanny Hill John Cleland
https://ebookmass.com/product/fanny-hill-john-cleland-3/
ebookmass.com
A Dragon's Wolf (The Hidden Realm Book 1) Heather Renee & Mystics & Mayhem
https://ebookmass.com/product/a-dragons-wolf-the-hidden-realmbook-1-heather-renee-mystics-mayhem/
ebookmass.com
The Decency Code: The Leader's Path to Building Integrity and Trust Steve Harrison
https://ebookmass.com/product/the-decency-code-the-leaders-path-tobuilding-integrity-and-trust-steve-harrison/
ebookmass.com
Core-Shell Nanostructures for Drug Delivery and Theranostics : Challenges, Strategies and Prospects for Novel Carrier Systems Focarete
https://ebookmass.com/product/core-shell-nanostructures-for-drugdelivery-and-theranostics-challenges-strategies-and-prospects-fornovel-carrier-systems-focarete/ ebookmass.com
Wong’s Nursing Care of Infants and Children – E-Book 11th Edition – Ebook PDF Version
https://ebookmass.com/product/wongs-nursing-care-of-infants-andchildren-e-book-11th-edition-ebook-pdf-version/
ebookmass.com
The Phenomenology of Spirit: Translation with Introduction and Commentary G. W. Hegel
https://ebookmass.com/product/the-phenomenology-of-spirit-translationwith-introduction-and-commentary-g-w-hegel/
ebookmass.com
The Skilled Helper: A Problem-Management and OpportunityDevelopment Approach to Helping (11th Edition) Gerard Egan
https://ebookmass.com/product/the-skilled-helper-a-problem-managementand-opportunity-development-approach-to-helping-11th-edition-gerardegan/
ebookmass.com
AGGREGATION-INDUCEDEMISSION(AIE) AGGREGATIONINDUCEDEMISSION (AIE) APracticalGuide Editedby JIANWEI XU
InstituteofMaterialsResearchandEngineering,AgencyforScience, TechnologyandResearch(A*STAR),Singapore
MING HUI CHUA InstituteofMaterialsResearchandEngineering,AgencyforScience, TechnologyandResearch(A*STAR),Singapore
BEN ZHONG TANG
SchoolofScienceandEngineering,ShenzhenKeyLaboratoryofFunctionalAggregateMaterials, TheChineseUniversityofHongKong,Shenzhen,Guangdong,China
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethods theyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhavea professionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliability foranyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,or fromanyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-12-824335-0
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionsEditor: KaylaDosSantos
EditorialProjectManager: IsabellaC.Silva
ProductionProjectManager: AnithaSivaraj
CoverDesigner: MarkRogers
TypesetbySTRAIVE,India
Contributors MassimoCametti DepartmentofChemistry, MaterialsandChemicalEngineering“Giulio Natta”,PolitecnicodiMilano,Milano,Italy
SijieChen MingWaiLauCentreforReparative Medicine,KarolinskaInstitutet,HongKong, China
XiaojieChen PCFMLab,GDHPPCLab, GuangdongEngineeringTechnologyResearch CenterforHigh-performanceOrganicand PolymerPhotoelectricFunctionalFilms,State KeyLaboratoryofOEMT,SchoolofChemistry, SunYat-senUniversity,Guangzhou,China
ZhenguoChi PCFMLab,GDHPPCLab, GuangdongEngineeringTechnologyResearch CenterforHigh-performanceOrganicand PolymerPhotoelectricFunctionalFilms,State KeyLaboratoryofOEMT,SchoolofChemistry; SchoolofMaterialsScienceandEngineering, SunYat-senUniversity,Guangzhou,China
MingHuiChua InstituteofMaterialsResearch andEngineering,A*STAR(AgencyforScience, TechnologyandResearch),Singapore, Singapore
YoshikiChujo DepartmentofPolymer Chemistry,GraduateSchoolofEngineering, KyotoUniversity,Kyoto,Japan
DanDing StateKeyLaboratoryofMedicinal ChemicalBiology,KeyLaboratoryofBioactive Materials,MinistryofEducation,andCollege ofLifeSciences,NankaiUniversity,Tianjin, China
YongQiangDong BeijingKeyLaboratoryof EnergyConversionandStorageMaterials, CollegeofChemistry,BeijingNormal University,Beijing,China
ZoranDz ˇ olic RuđerBos ˇ kovicInstitute,Zagreb, Croatia
ZhiyuanGao StateKeyLaboratoryofMedicinal ChemicalBiology,KeyLaboratoryofBioactive Materials,MinistryofEducation,andCollege ofLifeSciences,NankaiUniversity,Tianjin, China
XiangyuGe PCFMLab,GDHPPCLab, GuangdongEngineeringTechnologyResearch CenterforHigh-performanceOrganicand PolymerPhotoelectricFunctionalFilms,State KeyLaboratoryofOEMT,SchoolofChemistry, SunYat-senUniversity,Guangzhou,China
MasayukiGon DepartmentofPolymer Chemistry,GraduateSchoolofEngineering, KyotoUniversity,Kyoto,Japan
PengboHan StateKeyLaboratoryof LuminescentMaterialsandDevices, GuangdongProvincialKeyLaboratoryof LuminescencefromMolecularAggregates,AIE Institute,CenterforAggregation-Induced Emission,SouthChinaUniversityof Technology(SCUT),Guangzhou,China
KyoheiHisano DepartmentofApplied Chemistry,RitsumeikanUniversity,Kusatsu, Japan
YuningHong DepartmentofChemistryand Physics,LaTrobeInstituteforMolecular Science,LaTrobeUniversity,Melbourne,VIC, Australia
RongrongHu StateKeyLaboratoryof LuminescentMaterialsandDevices, GuangdongProvincialKeyLaboratoryof LuminescencefromMolecularAggregates,AIE Institute,CenterforAggregation-Induced Emission,SouthChinaUniversityof Technology(SCUT),Guangzhou,China
YangHu StateKeyLaboratoryofLuminescent MaterialsandDevices,GuangdongProvincial
KeyLaboratoryofLuminescencefrom MolecularAggregates,AIEInstitute,Centerfor Aggregation-InducedEmission,SouthChina UniversityofTechnology(SCUT),Guangzhou, China
ShunichiroIto DepartmentofPolymer Chemistry,GraduateSchoolofEngineering, KyotoUniversity,Kyoto,Japan
ChuenKam MingWaiLauCentrefor ReparativeMedicine,KarolinskaInstitutet, HongKong,China
BingShiLi KeyLaboratoryofNewLithium-Ion BatteryandMesoporousMaterial,Collegeof ChemistryandEnvironmentalEngineering, ShenzhenUniversity,Shenzhen,China
HongkunLi LaboratoryofAdvanced OptoelectronicMaterials,CollegeofChemistry, ChemicalEngineeringandMaterialsScience, SoochowUniversity,Suzhou,China
MengLi CenterforAIEResearch,Shenzhen University,Shenzhen;ShenzhenInstituteof AggregateScienceandTechnology,Schoolof ScienceandEngineering,TheChinese UniversityofHongKong, Shenzhen,Guangdong,China
XiangyuLi InstituteofFineChemicals,East ChinaUniversityofScienceandTechnology, Shanghai,China
Guey-ShengLiou InstituteofPolymerScience andEngineering,NationalTaiwanUniversity, Taipei,Taiwan
YantingLyu InstituteofFineChemicals,East ChinaUniversityofScienceandTechnology, Shanghai,China
JuMei KeyLaboratoryforAdvancedMaterials, FeringaNobelPrizeScientistJointResearch Center,FrontiersScienceCenterfor MateriobiologyandDynamicChemistry,Joint InternationalResearchLaboratoryforPrecision ChemistryandMolecularEngineering, InstituteofFineChemicals,Schoolof Chemistry&MolecularEngineering,East ChinaUniversityofScience&Technology, Shanghai,P.R.China
QiOu MOEKeyLaboratoryofOrganic OptoElectronicsandMolecularEngineering,
DepartmentofChemistry,Tsinghua University,Beijing,China
SupattraPanthai DepartmentofApplied Chemistry,RitsumeikanUniversity,Kusatsu, Japan
SurajKumarPathak CollegeofMaterials ScienceandEngineering,ShenzhenUniversity, Shenzhen,China
QianPeng SchoolofChemicalSciences, UniversityofChineseAcademyofSciences, Beijing,China
AndreaPucci DepartmentofChemistryand IndustrialChemistryoftheUniversityofPisa, Pisa,Italy
AnjunQin StateKeyLaboratoryof LuminescentMaterialsandDevices, GuangdongProvincialKeyLaboratoryof LuminescencefromMolecularAggregates,AIE Institute,CenterforAggregation-Induced Emission,SouthChinaUniversityof Technology(SCUT),Guangzhou,China
SoheilaSabouri DepartmentofChemistryand Physics,LaTrobeInstituteforMolecular Science,LaTrobeUniversity,Melbourne,VIC, Australia
MasakiShimizu FacultyofMolecular ChemistryandEngineering,KyotoInstituteof Technology,Kyoto,Japan
ZhigangShuai MOEKeyLaboratoryofOrganic OptoElectronicsandMolecularEngineering, DepartmentofChemistry,Tsinghua University,Beijing,China
YueSi BeijingKeyLaboratoryofEnergy ConversionandStorageMaterials,Collegeof Chemistry,BeijingNormalUniversity,Beijing, China
KazuoTanaka DepartmentofPolymer Chemistry,GraduateSchoolofEngineering, KyotoUniversity,Kyoto,Japan
BenZhongTang SchoolofScienceand Engineering,ShenzhenKeyLaboratoryof FunctionalAggregateMaterials,TheChinese UniversityofHongKong, Shenzhen,Guangdong,China
HeTian KeyLaboratoryforAdvanced Materials,FeringaNobelPrizeScientistJoint
ResearchCenter,FrontiersScienceCenterfor MateriobiologyandDynamicChemistry,Joint InternationalResearchLaboratoryforPrecision ChemistryandMolecularEngineering, InstituteofFineChemicals,Schoolof Chemistry&MolecularEngineering,East ChinaUniversityofScience&Technology, Shanghai,P.R.China
OsamuTsutsumi DepartmentofApplied Chemistry,RitsumeikanUniversity,Kusatsu, Japan
DongWang CenterforAIEResearch,Shenzhen University,Shenzhen,China
FeiWang MingWaiLauCentreforReparative Medicine,KarolinskaInstitutet,HongKong, China
JiaWang StateKeyLaboratoryofLuminescent MaterialsandDevices,GuangdongProvincial KeyLaboratoryofLuminescencefrom MolecularAggregates,AIEInstitute,Centerfor Aggregation-InducedEmission,SouthChina UniversityofTechnology(SCUT),Guangzhou, China
QiWang InstituteofFineChemicals,EastChina UniversityofScienceandTechnology, Shanghai,China
AlexY.H.Wong MingWaiLauCentrefor ReparativeMedicine,KarolinskaInstitutet, HongKong,China
JianweiXu InstituteofMaterialsResearchand Engineering,A*STAR(AgencyforScience, TechnologyandResearch),Singapore, Singapore
ChuluoYang CollegeofMaterialsScienceand Engineering,ShenzhenUniversity,Shenzhen, China
ZhanYang PCFMLab,GDHPPCLab, GuangdongEngineeringTechnologyResearch CenterforHigh-performanceOrganicand PolymerPhotoelectricFunctionalFilms,State KeyLaboratoryofOEMT,SchoolofChemistry, SunYat-senUniversity,Guangzhou,China
ZhiyongYang PCFMLab,GDHPPCLab, GuangdongEngineeringTechnologyResearch
CenterforHigh-performanceOrganicand PolymerPhotoelectricFunctionalFilms,State KeyLaboratoryofOEMT,SchoolofChemistry, SunYat-senUniversity,Guangzhou,China
BichengYao DepartmentofChemistryand Physics,LaTrobeInstituteforMolecular Science,LaTrobeUniversity,Melbourne,VIC, Australia
Hung-JuYen InstituteofChemistry,Academia Sinica,Taipei,Taiwan
LihuiZhang StateKeyLaboratoryof LuminescentMaterialsandDevices, GuangdongProvincialKeyLaboratoryof LuminescencefromMolecularAggregates,AIE Institute,CenterforAggregation-Induced Emission,SouthChinaUniversityof Technology(SCUT),Guangzhou,China
YiZhang PCFMLab,GDHPPCLab, GuangdongEngineeringTechnologyResearch CenterforHigh-performanceOrganicand PolymerPhotoelectricFunctionalFilms,State KeyLaboratoryofOEMT,SchoolofChemistry, SunYat-senUniversity,Guangzhou,China
YouhengZhang InstituteofFineChemicals, EastChinaUniversityofScienceand Technology,Shanghai,China
YucongZhang BeijingKeyLaboratoryof EnergyConversionandStorageMaterials, CollegeofChemistry,BeijingNormal University,Beijing,China
JuanZhao SchoolofMaterialsScienceand Engineering,SunYat-senUniversity, Guangzhou,China
HuiZhou InstituteofMaterialsResearchand Engineering,A*STAR(AgencyforScience, TechnologyandResearch),Singapore, Singapore
QiangZhu InstituteofMaterialsResearchand Engineering,A*STAR(AgencyforScience, TechnologyandResearch),Singapore, Singapore
Wei-HongZhu InstituteofFineChemicals,East ChinaUniversityofScienceandTechnology, Shanghai,China
Preface Thediscoveryofaggregation-induced emission(AIE)in2001hasservedasagame changerinthedevelopmentandapplication ofluminogenicfunctionalmaterials.Fundamentalunderstandingofphotophysicalprocessesandpropertiesinorganicluminogens hasbeenreshaped,creatingvastopportunitiesforawiderangeofapplicationsofAIE luminogens(AIEgens).AIEhaseffectively overcomethelimitationsofaggregationcausedquenching(ACQ)commonlyfound intraditionalluminogens.Thisnotonlycontributestoimprovementinmaterialperformanceforexistingapplicationsbutalso leadstotheemergenceofnewapplications. Therefore,researchinterestsandeffortsin theareaofAIEhavesoaredoverthepast twodecades,withanexponentialgrowth ofscientificpublicationsandcitations,servingasatestamenttotheusefulnessof AIEgens.Thisbook,therefore,aimstoprovideaholisticcoverageofbothfundamental principlesandapplicationsofAIEgens,coveringthekeyscientificprogressesintheAIE topicatanintroductory-to-intermediate level,suitableforawiderangeofscientific andacademicaudiences,bothwithinand outsidetheAIEresearchfraternity.
Thisbookbeginswiththeintroduction offundamentalconcepts,principles,and mechanismsofAIEin Chapter1.Todate,a largenumberofnovelAIEgenshavebeen designedandsynthesized,andthereforethe firstpartofthebookseekstodisplaythestructuraldiversitiesofAIEgens. Chapter2 highlightsnovelAIEgenswithboroncomplexes while Chapter3 summarizesnumerousAIEactivepolymersdevelopedthusfarforvarious
applications.Thereafter,chiralAIEgenswith circularlypolarizedluminescenceandhelical self-assemblypropertiesaredescribedin Chapter4,followedbyAIEsupramolecular gelsystemsin Chapter5
Thesecondpartofthebookseekstoshowcasetheapplicationdiversityandusefulnessof AIEgens.AIEgenscanbebroadlycategorized intofourkeydomainsintermsoftheirmainapplications:(i)stimuli-responsiveAIEsystems, (ii)optoelectronics,(iii)biomedicalsensing, and(iv)chemicalsensing.Thesubsequent chaptersarethereforearrangedinaccordance withtheirapplicationsintherespective domains.Forstimuli-responsiveAIEsystems, Chapter6 discussesmechanochromicAIEgens, whereas Chapter7 reviewsAIEgenswithphotochromicandthermochromicproperties.
AIEgensareexcellentcandidatesforoptoelectronicapplications,notablyorganic light-emittingdiodes(OLEDs)andliquid crystal(LC)opticaldisplays,duetotheir amplificationofluminescenceintensitiesin thesolidstates. Chapters8and9 introduce pureorganicAIEgensthatexhibit(aggregationandcrystallization-induced)phosphorescenceandthermallyactivateddelayed fluorescence(TADF)properties,respectively,bothofwhichexhibitimportant solid-stateluminescencepropertiesparticularlyforthedevelopmentofOLEDs.This leadsusto Chapter10,inwhichtheapplicationofAIEgensinOLEDsissummarized. TheuseofAIEgensinothernotableoptoelectronicapplicationssuchasLCopticaldisplays,electrofluorochromicdevices,and photovoltaicsisdiscussedseparatelyin Chapters11–13.
Next,thebookconcentratesonAIEgens forbiomedicalapplications,includingbiosensingandbioimaging,diagnostics,therapy,anddrugdelivery.AIEgensmaybe directlyusedasmolecularfluorescent probes(mainlyforbiosensingandimaging) orbefurtherpreparedasfluorescent nanoparticles(FNPs)andbioconjugatesto enhanceluminescenceintensity,sensitivity, andbiocompatibility.TheuseofAIE-active molecularprobes,FNPs,andbioconjugates foraspectrumofdifferentbiomedicalapplicationsiscollectivelydiscussedin Chapters 14–16,respectively.
Inadditiontobiosensing,chemicalsensingencompassesenvironmentalmonitoring ofharmfulsubstancestosafeguardpublic healthandthedetectionoftracechemicals forproductqualityassurance. Chapter17 thereforesummarizesAIE-basedfluorescent chemosensorsforexplosivedetectionwhile thedevelopmentofanAIE-basedvapor
sensortodetectanalytesinthevaporstate isdiscussedin Chapter18.Thereafter, Chapter19 highlightstheuseofAIEgensin sensingfoodstuffhazardsaswellasafluorescentthermometer.Finally, Chapter20 summarizestheluminescencemechanism ofAIEgensutilizingcomputationalsimulationandmodelingmethods,whichprovide anotherwaytohavein-depthandintrinsic understandingofthenatureofAIE.
Throughthisbook,itisenvisagedthat readerswillgainknowledgeandunderstandingaboutnotonlythefundamental principlesofAIEbutmoreimportantly howAIEluminogenscanbeincorporated invariousmolecularandpolymericmaterialstospecificallycatertotargetedneeds andapplications.
Finally,theeditorswishtoexpresstheir immenseappreciationtoallauthorsfor theirdedicatedeffortsincontributing high-qualityworktothisbook.
FundamentalprinciplesofAIE PengboHana,JiaWanga,AnjunQina,andBenZhongTangb
aStateKeyLaboratoryofLuminescentMaterialsandDevices,GuangdongProvincialKey LaboratoryofLuminescencefromMolecularAggregates,AIEInstitute,CenterforAggregationInducedEmission,SouthChinaUniversityofTechnology(SCUT),Guangzhou,China bSchoolof ScienceandEngineering,ShenzhenKeyLaboratoryofFunctionalAggregateMaterials, TheChineseUniversityofHongKong,Shenzhen,Guangdong,China
1Introduction Luminescentmaterialswithaggregation-inducedemission(AIE)featureshaveattracted tremendousattentionfortheirpotentialpracticalapplications.TheconceptofAIEwascoined in2001byTangetal.whentheyobservedauniquephenomenoninasilolederivative,which isnonemissiveindilutesolutionbutemitsbrightlywhenformingaggregates [1].
DecipheringtheunderneathmechanismsofAIEiscrucialfortheenrichmentoffundamentalphotophysicalknowledge,constructionofnewluminogens,andexplorationofpractical applications [2–4].Inprinciple,amatterabsorbinglightenergywillbepromotedtothe excitedstate,whichwillfallbacktolowerenergystatesthroughphotophysicalorphotochemicalprocesses [5].Thesephotophysicalprocessesmainlyincluderadiativetransition andnonradiativetransitionpathways,whereasthephotochemicalpathwaymainlyincludes achemicalreaction.Inthesolutionstate,theexcited-statedecayofAIEluminogens(AIEgens) ismainlythroughnonradiativephotophysicalorphotochemicalprocesses.Meanwhile,in aggregatestates,thenonradiativedecaypathwaysareblockedandtheradiativeonesare opened.ThecombinationeffectsreadilyresultintheuniqueAIEfeature.
NumerouseffortshavebeendevotedtodecipheringtheAIEworkingprincipleanda numberofpossiblemechanismshavebeenproposed,suchasJ-aggregation,conformational planarization,E/Zisomerization,twistedintramolecularchargetransfer(TICT),andexcitedstateintramolecularprotontransfer(ESIPT),butmostofthemwereonlyapplicabletolimited AIEsystems.
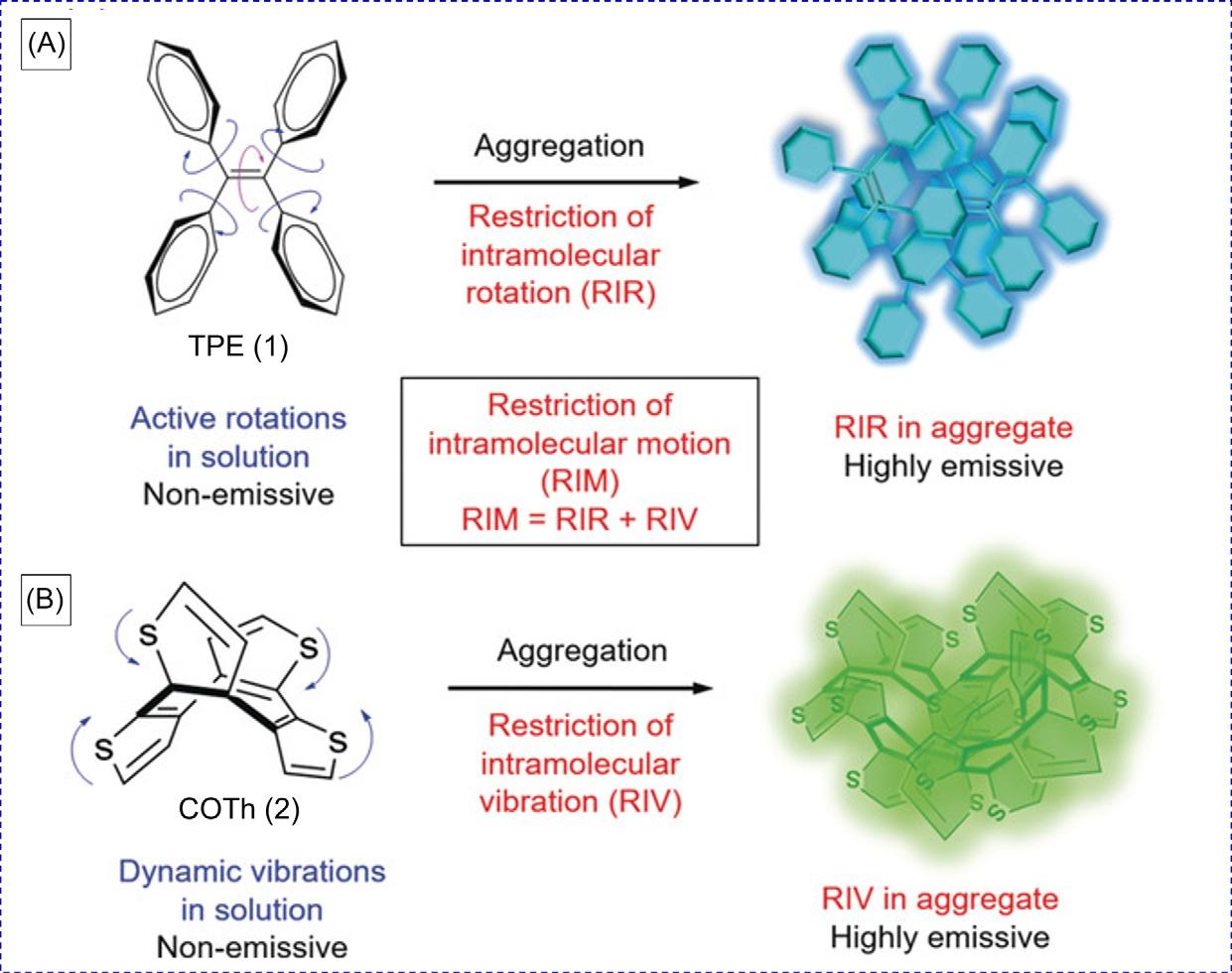
FIG.1 (A)Tetraphenylethene(TPE)isnonemissivewhenmolecularlydissolvedbutbecomesemissivewhen aggregatedduetotherestrictionofintramolecularrotations(RIR).(B)Cyclooctatetrathiophene(COTh)shows AIEactivityduetotherestrictionofintramolecularvibration(RIV)intheaggregatestate. Reproducedwithpermission fromZ.Zhao,H.Zhang,J.W.Y.Lam,B.Z.Tang,Aggregation-inducedemission:newvistasattheaggregatelevel,Angew. Chem.Int.Edit.59(2020)2.Copyright2020Wiley-VCHVerlagGmbH&Co.KGaA.
Withgreatandpersistentefforts,therestrictionofintramolecularrotation(RIR)mechanismhasbeenproposed.However,asthefamilyofAIEactivemoleculesgrows,someAIE systemswithnorotatableunitscannotfullybeexplainedbytheRIRmode.Therefore,the restrictionofintramolecularvibrations(RIV)wasraisedtoexplaintheseAIEcases.Ithas becomeclearthatRIRandRIVhavebeenrationalizedasthemaincausefortheAIEeffect. Therefore,theyareintegratedintoamorecomprehensiveAIEmechanism,i.e.,restriction ofintramolecularmotion(RIM)(Fig.1) [6]
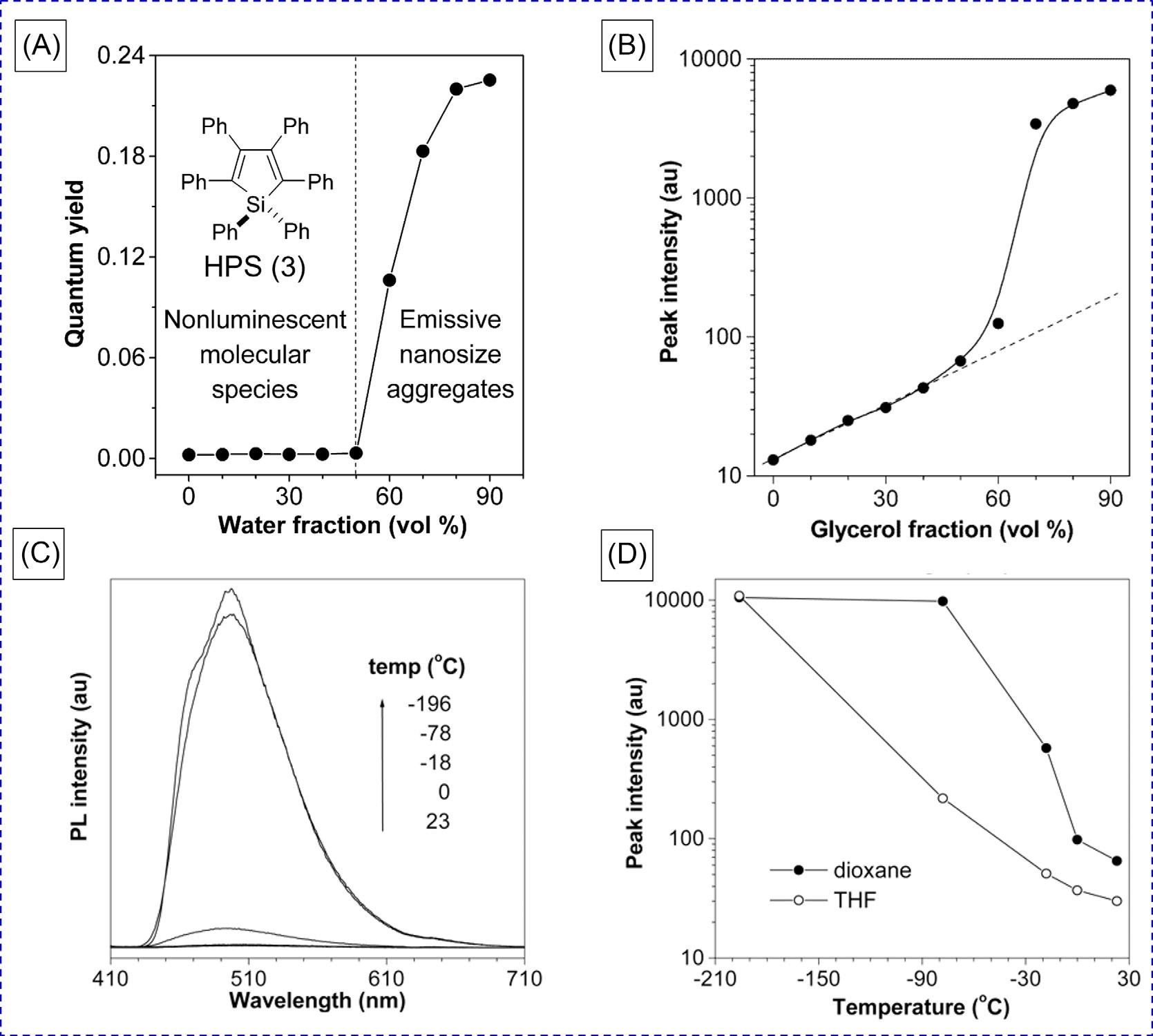
2Restrictionofintramolecularrotations TheRIRmechanismwasproposedbasedonacarefulandsystematicstudyofanarchetype ofAIEgenofhexaphenylsilole(HPS, 3) [7].HPSissolubleinorganicsolvents,suchas dichloromethane,acetone,THF,andmethanol,butinsolubleinwater.Therefore,theaggregationofHPSmoleculescanbeinducedbyaddingwaterinHPSacetonesolution,andthe photoluminescence(PL)quantumyield([Fcy]F)wasexploredinacetone/watermixtures withdifferentwaterfractions(fw).Asshownin Fig.2A,HPSisnonemissiveindiluteacetone
FIG.2 Plotsof(A)PLquantumyieldofHPSvswaterfractioninacetone/watermixturesand(B)itsPLpeakintensityvsglycerolfractioninglycerol/methanolmixtures.(C)PLspectraofHPSin1,4-dioxaneatdifferenttemperatures.(D)EffectoftemperatureonthepeakintensityofthePLofHPSindioxaneandTHF.Concentration ¼ 10 μM. ReproducedwithpermissionfromJ.Chen,C.C.W.Law,J.W.Y.Lam,Y.Dong,S.M.F.Lo,I.D.Williams,etal.,Synthesis,light emission,nanoaggregation,andrestrictedintramolecularrotationof1,1-substituted2,3,4,5-tetraphenylsiloles,Chem.Mater. 15(7)(2003)1535–1546.Copyright2003AmericanChemicalSociety.
solutionwithalow [Fcy]F ( 0.1%),whichremainsalmostunchangeduntilthe fw reaches 50vol%butstartstoincreaseswiftlyafterward.Whenthe fw increasesto90%,the [Fcy]F value isboostedto22%,whichis 200timeshigherthanthatoftheacetonesolution.Thehigher [Fcy]F valueofHPSintheaggregatestatethanthatindilutesolutionsdemonstratesits AIEeffect,whichisattributedtotheRIRmechanism.Insolution,theperipheralphenylrings ofHPScandynamicallyrotatearoundthecentralsilolering,whichmayeffectivelyconsume
theenergyoftheexcitedstate,makingtheHPSnonemissive,whileintheaggregatestatethe intramolecularrotationsarerestrictedbecauseofthephysicalconstraint.Therefore,the nonradiativechannelofdeexcitationisblockedandtheradiativedecayisactivated, makingtheHPSemitstrongly.
2.1Externalphysicalcontrolexperiments 2.1.1Viscosityeffect
TofurtherverifytherationalityoftheRIRmechanism,severalcontrolexperimentswere designedandconducted [7–9].StrongemissionisenvisionedforHPSinthemoreviscous mediabecausethehighviscositywouldretardtheintramolecularrotations.Theviscosity ofglycerol(934cPat25°C)is1720timeshigherthanthatofmethanol(0.544cPat25°C), andtheviscosityofmediawillbeenhancedbyincreasingtheglycerolpercentagein methanol.Therefore,thePLmeasurementsofHPSwereperformedinsuchmixtures.As shownin Fig.2B,thePLintensityincreasedlinearlyastheglycerolfraction(fG)increased intherangeof0–50vol%at25°C.Theemissionenhancementinthisregionshouldbepredominantlyascribedtotheviscosityeffect.When fG isfurtherincreased,thepeakintensity increasedsharplyduetotheformationofnanoaggregates.
2.1.2Temperatureeffect Sincedecreasingthesolutiontemperaturecanalsohampertheintramolecularrotations, thetemperatureeffectsontheHPSemissionwerethusstudied.Whenthedioxanesolution ofHPSwascooled,itsPLintensityincreasedaccordingly(Fig.2C).Thisisbecausethedioxanesolutionchangedtoaglassystatewhenthetemperaturecooledbelowitsmeltingpoint (11.8°C).Therefore,theintramolecularrotationofthephenylringsofHPSwouldberestricted bytherigidenvironments.Inaddition,theemissionofHPSdecreaseddrasticallywhenthe solutionwasheatedabovethemeltingpointofdioxane(Fig.2D).
TofurtherverifytheRIRprocessrestrictedatlowtemperatures,dynamicNMRexperimentswerealsocarriedout.Theveryfastconformationalexchangescausedbythestrong intramolecularrotationsgavesharpNMRsignalsatroomtemperature.However,the NMRpeakswerebroadenedatalowertemperaturebecausetheslowrotationsledtothe slowerexchanges.Therefore,bothincreasingthesolutionviscosityanddecreasingthesolutiontemperaturewouldhampertheintramolecularrotationsofphenylringsofHPS.Asa result,theradiativetransitionsofAIEgenswouldbeopenedandtheemissionintensity wouldbeboosted.
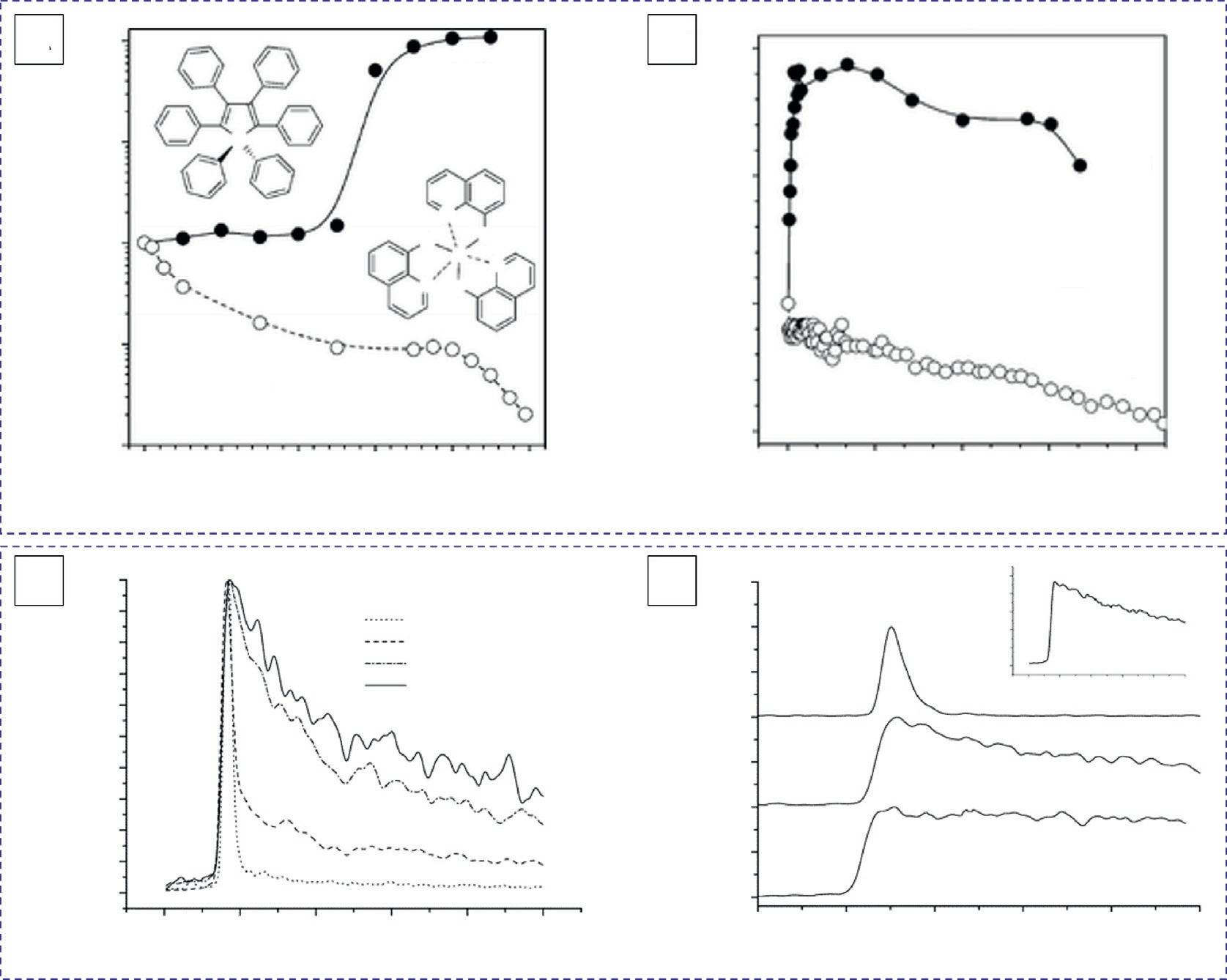
2.1.3Pressureeffect Inadditiontoviscosityandtemperature,pressurewillalsoinfluencetheemissionofHPS [8,10].Atraditionalluminophore,tris(8-hydroxyquinolinato)-aluminum(III)(AlQ3)wasused asacontrast.AlQ3 ishighlyemissiveindilutesolutionbutlessluminescentwhenaggregated (Fig.3A).Whenapplyingdifferentpressures,theemissionofHPSbecomesmorecomplicated (Fig.3B).ThePLintensityofHPSincreasedswiftlybyincreasingitspressure.However,furtherincreaseofthepressureledtothedecreaseinPLintensity.Theoretically,theexternal pressurizationdecreasestheintermoleculardistanceofHPS,thusimposingantagonistic
FIG.3 (A)ChangesinPLintensitiesofHPSandAlQ3 solutionswithwaterfractionsofaqueousmixtures.Solution concentration:10mM.(B)EffectsofpressureonthePLintensitiesofHPSandAlQ3 films.(C)Time-resolvedfluorescenceofHPSinsolutionwithdifferentfractionsofwaterandDMF.Theidenticalconcentrationforthemixturesis 1.3 10 5 mol/L.(D)Time-resolvedfluorescenceofDMFsolutionofHPS(2wt%)atdifferenttemperatures.Inset:PL decayat30Kata5nstimescale,whichshowstheslowdecaycomponentofPLatthelowtemperature. (B)Reproduced withpermissionfromX.Fan,J.Sun,F.Wang,Z.Chu,P.Wang,Y.Dong,etal.,Photoluminescenceandelectroluminescenceof hexaphenylsiloleareenhancedbypressurizationinthesolidstate,Chem.Commun.26(2008)2989.Copyright2008RoyalSocietyofChemistry.(D)ReproducedwithpermissionfromY.Ren,J.W.Y.Lam,Y.Dong,B.Z.Tang,K.S.Wong,Enhancedemissionefficiencyandexcitedstatelifetimeduetorestrictedintramolecularmotioninsiloleaggregates,J.Phys.Chem.B109(2005) 1135.Copyright2005AmericanChemicalSociety.
effects.Ontheonehand,theappliedlowpressureincreasestheintermolecularinteractionbut haslittleeffectontheintermoleculardistance.Asaresult,thefreedomofthemolecular rotationsisinhibitedandtheemissionisenhanced.Ontheotherhand,thedistancesbetween thegroupswithintheHPSmoleculewouldbeshortened,andtheformationofexcimers,etc. wouldbepromotedathighpressures,thusweakeningtheemission.Thequenchingeffect wasfoundinAlQ3 anditsPLintensitywasweakenedmonotonouslywithinthepressure rangeof1–650atm,indicatingthatthepressurizationenhancedtheunfavorablemutual interferencebetweenmolecules.
TheaggregationofamoleculecannotonlyenhanceitsemissionbutalsoinfluenceitsPL lifetime [9].Therefore,thetime-resolvedfluorescenceofHPSwasfurthermeasuredto exploretheAIEmechanism.Asshownin Fig.3C,therelaxationoftheexcitedstateof HPSisasingle-exponentialdecayindilutesolutionanditsPLlifetimeisonly40ps.The lowemissionefficiencyandshortPLlifetimeindicateastrongnonradiativerecombination process.Increasing fw causestworelaxationpathwaysofdecayduetotheformationof nanoaggregates,whichresultsinthedecayofmoremoleculesradiativelybyaslowerchannel.Inthemixturesolutioncontaining90%water,theexcitedstatemainlydecaysthroughthe slowpathwayandthePLlifetimeoftheslowcomponentrisesto 7ns.Therapidrotationof thephenylringsgreatlyconsumestheenergyoftheexcitedstateindilutesolutions,resulting inaps-scalelifetime.However,therotationsofphenylringsarelargelyrestrictedwhen aggregatesareformed,andtheradiativedecaychannelsareactivatedwithannslifetime. Inaddition,decreasingthetemperatureandincreasingthemediumviscositycanalsoenhancethePLlifetimeofHPS(Fig.3D).Theseresultssuggestthat(a)therotationofphenyl ringsconsumestheexcitedstateenergyandincreasesthenonradiativedecayrates,resulting inanonemissionstateofHPSindilutesolution,and(b)therestrictionofrotationalmotions activatestheradiativedecayprocess,thusintensifyingtheiremissioninanaggregatestate.
2.2Chemicalmodification
AllthecontrolexperimentsdescribedabovehavegreatlyproventheRIRmechanism. Moreover,theseresultsalsoconfirmthattheluminescenceofcompoundscanbecontrolled byphysicalorengineeringencapsulation.Theseobservationsimplythattheemissionof compoundsmightalsobeinfluencedbycontrollingtheirintramolecularrotationprocesses atthemolecularlevel [11–15].
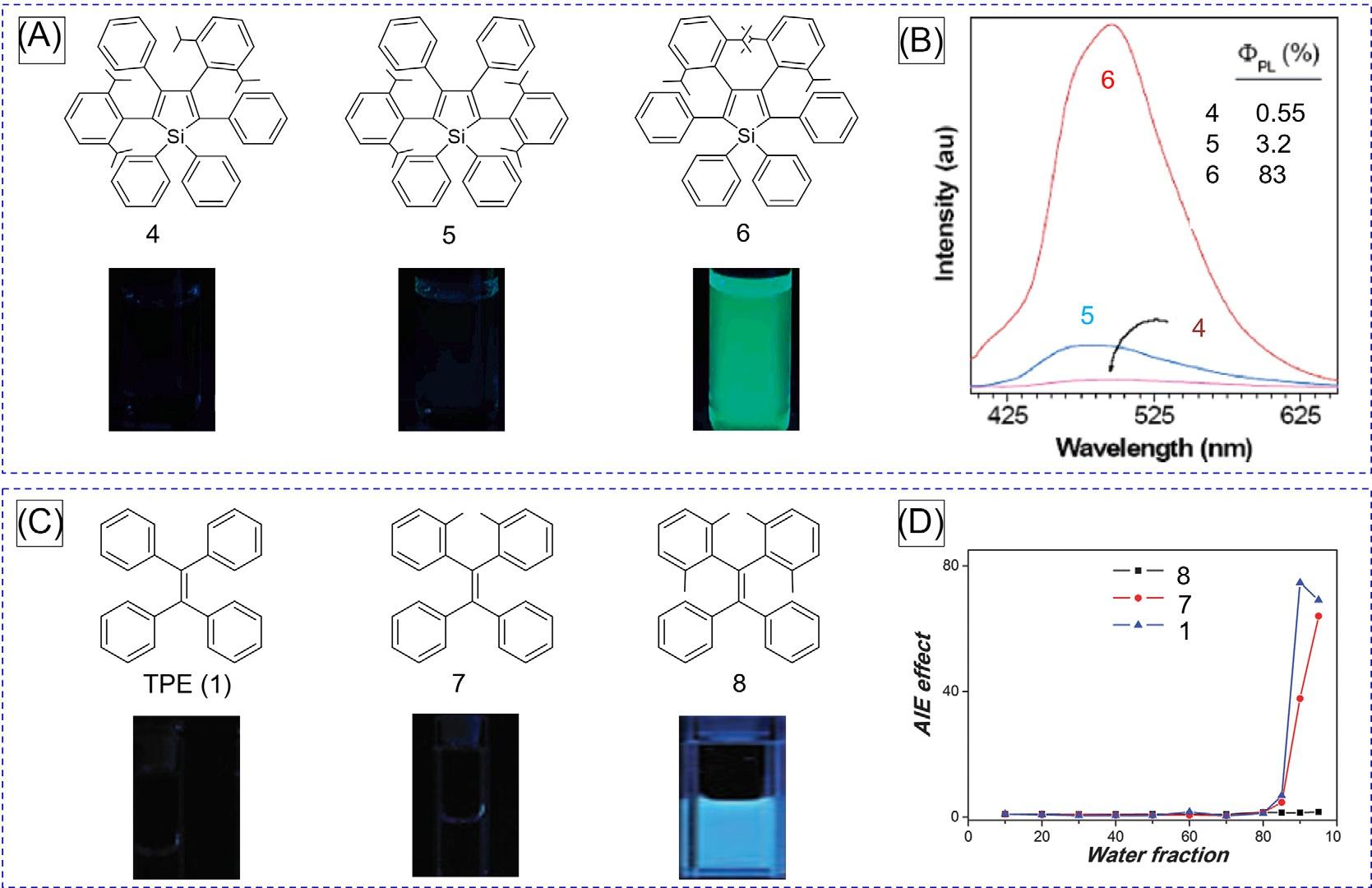
2.2.1Stericeffect Theisopropyl(iPr)groupswereattachedtothephenylringsofHPStostudyhowthesteric effectwouldaffectitsAIEbehavior [11].AseriesofHPSderivativeshavebeendesignedand synthesized(Fig.4A).UnlikeHPS,regioisomers 4–6 areluminescentindilutesolutions, althoughtheiremissionintensityvariesdramatically.Inacetone,theregioisomers 4–6 exhibit ablue-greenemissionwithincreased [Fcy]F valuesintheorderof 6 > 5 > 4 (Fig.4B).Similar resultswerealsoobtainedinothersolventssuchasTHF,whichfurtherconfirmedtheconclusionoftheorderofobservedemissionintensityinacetone.The ΦF valuesof 4–6 arehigher thanthatofHPSbecausethehigherrotationbarriersinhibittheintramolecularrotation processofthephenylrings.Itwaswellunderstoodthatamorerigidchromophoreemitsa strongeremission.Therefore,thestructuralrigidificationplaysacrucialroleinmakingthe regioisomersmoreemissivethanHPSindilutesolutions.
TofurtherverifythattheRIRprocessofAIEgenscanbeactivatedatthemolecularlevelvia facilechemicalmodification,analogousworkshavealsobeendoneinTPEsystems [12,13].The multiplemethylgroupswereintroducedattheorthopositionsofTPEtocheckhowtheintramolecularstericeffectswouldinfluenceitsphotophysicalproperties [12].TPE,atypical AIEgen,showsweakemissioninTHFsolutionduetotheactiveintramolecularrotationsof peripheralphenylrings.Whenthestericallyhinderedmethylgroupwasintroduced,the
FIG.4 (A)Chemicalstructuresandfluorescentphotographsand(B)PLspectraofsolutionsofsiloles 4–6 inacetone (10 μM).(C)Chemicalstructuresandfluorescentphotographs.(D)Plotsof I/I0 ofTPE, 7 and 8 versuswaterfractions inTHF/watermixtures(10 μM),where I0 and I arethePLintensitiesinTHFsolutionandaTHF/watermixture,respectively.Inset:fluorescencephotographsofTPE, 7 and 8 inTHFsolutions. (AandB)Reproducedwithpermissionfrom Z.Li,Y.Dong,B.Mi,Y.Tang,M.Haussler,H.Tong,etal.,Structuralcontrolofthephotoluminescenceofsiloleregioisomers andtheirutilityassensitiveregiodiscriminatingchemosensorsandefficientelectroluminescentmaterials,J.Phys.Chem.B109 (2005)10061.Copyright2005AmericanChemicalSociety.(C)ReproducedwithpermissionfromG.Zhang,Z.Chen,M.P. Aldred,Z.Hu,T.Chen,Z.Huang,etal.,Directvalidationoftherestrictionofintramolecularrotationhypothesisviathesynthesisofnovelortho-methylsubstitutedtetraphenylethenesandtheirapplicationincellimaging,Chem.Commun.50(2014) 12058.Copyright2014RoyalSocietyofChemistry.
luminescenceoftheTPEderivativeswasfurtherenhanced.The ΦF ofcompound 8 inTHF solutionishigherthanthatofTPEandcompound 7 (Fig.3C).Thecompound 8 showedabright cyanluminescenceinTHFsolution,whileweakfluorescencewasobservedbythenakedeye forbothTPEand 7.SimilartoTPE,compound 7 exhibitsobviousAIEcharacteristics.Incontrast,asthesterichindranceisfurtherenhancedbyincreasingthenumberofmethylgroups, thestericallycrowdedcompound 8 losesitsAIEfeature.Theintroductionoftetra(orthomethyl)groupsinTPEgreatlysuppressedtherotationalmotionofintramolecularphenyl rings,andthusinhibitedthenonradiativedecay,furtherverifyingtheRIRmechanism.
2.2.2Electronicconjugationeffect Besidesthestericeffect,theelectronicinteractioncanmakeacontributiontotheRIR processofAIEgens [14,15].TostudyhowelectronicconjugationaffectstheAIEbehavior
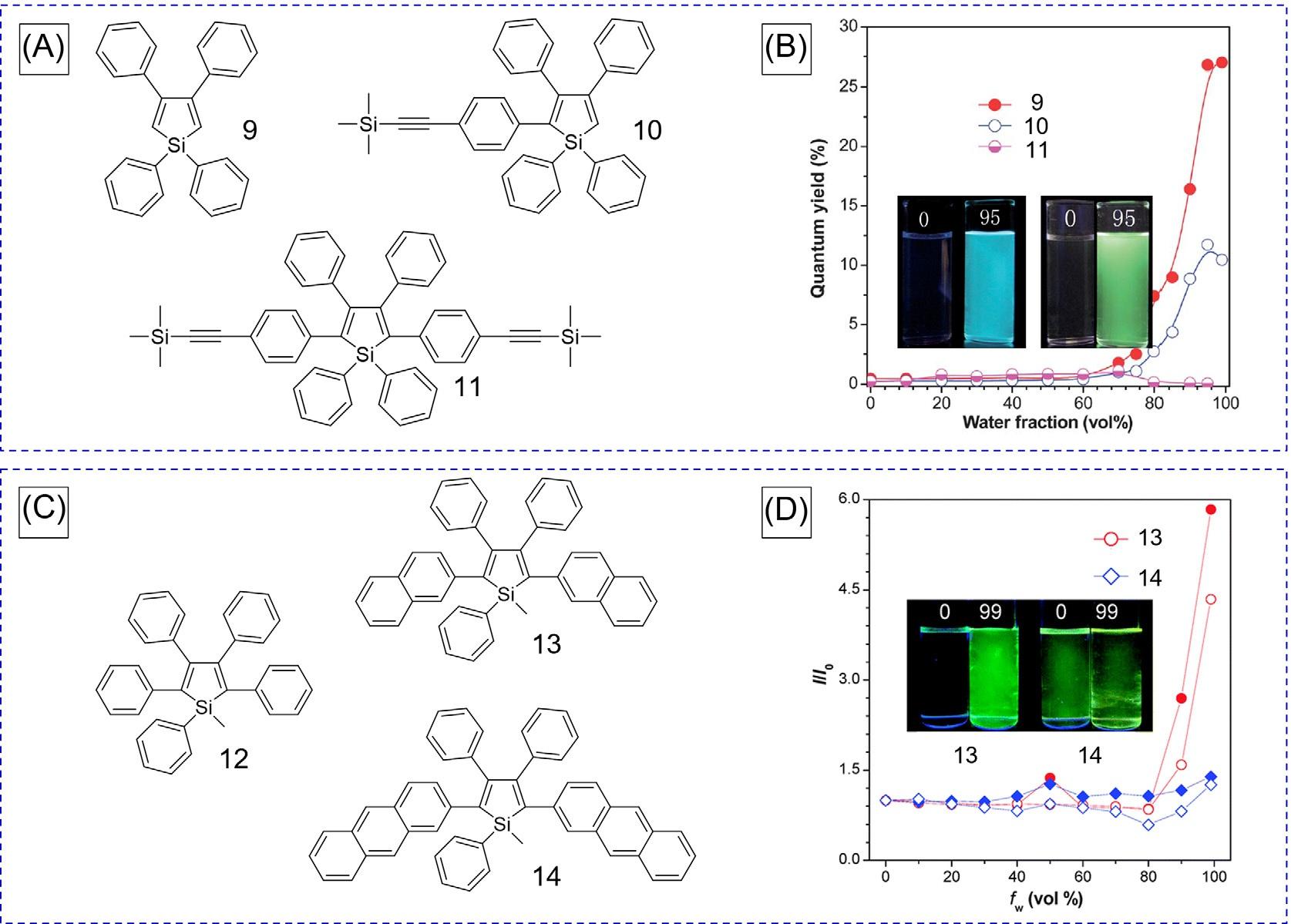
FIG.5 (A)Chemicalstructuresand(B)plotsof I/I0 of 9, 10, and 11 versuswaterfractionsinTHF/watermixtures (10 μM),where I0 and I arethePLintensitiesinTHFsolutionsandTHF/watermixtures,respectively.Inset:fluorescencephotographsof 10 and 11 inTHFsolutions.(C)Chemicalstructuresofsilolederivatives 12, 13,and 14 and (D)plotsoftheirfluorescenceintensityversuswaterfractionsinTHF/watermixtures.Inset:Fluorescentphotographs of 13 and 14 inTHF/watermixtures(fw ¼ 0,99vol%). (AandB)ReproducedwithpermissionfromE.Zhao,J.W.Y.Lam,Y. Hong,J.Liu,Q.Peng,J.Hao,etal.,Howdosubstituentsaffectsiloleemission?J.Mater.Chem.C1(2013):5661.Copyright2013 RoyalSocietyofChemistry.(CandD)ReproducedwithpermissionfromB.Chen,H.Nie,P.Lu,J.Zhou,A.Qin,H.Qiu,etal., Conjugationversusrotation:goodconjugationweakenstheaggregation-inducedemissioneffectofsiloles,Chem.Commun. 50(2014)4500.Copyright2014RoyalSocietyofChemistry.
of1,1,3,4-tetraphenylsilole(TPS, 9),compounds 10 and 11 withthetrimethylsilylethynylphenyl(TMSEP)grouponits2,5positionsweredesignedandprepared(Fig.5A) [14] WeakemissionwasobservedinbothsolutionandaggregatestatesduetothepoorconjugationofTPS.Incontrast,compounds 10 and 11 showeddistinctlydifferentemissionbehaviors. Theyarenonemissiveindilutesolutionsbutexhibitabrightblue-greenemissionataggregate states,clearlydemonstratingtheAIEproperty(Fig.5B).The ΦF of 10 insolidstatewas measuredtobe54.80%,whilegreatlyintensifiedluminescencewasfoundintwo substituent(s)atthe2,5positionsofTPS(11)witha ΦF of90.88%initssolidstate.Thesteric effectinfluencestheemissionbehaviorofTPSfortheformer(10),whiletheelectroniceffect affectstheemissionefficiencyandwavelengthofTPSforthelatter(11).
Inaddition,similarworkhasalsobeenperformedtoexaminethecontributionofelectronic effect [15].Polycyclicaromaticgroupswereattachedatthe2,5-positionsofsilole 12 and naphthyl-andanthracyl-substitutedsilolederivatives 13 and 14,respectively(Fig.5C). Compound 13 exhibitedweakemissioninthesolutionstatewitha ΦF of2.4%,whilestrong luminescencewasobservedfor 14 inthesolutionstatewitha ΦF of11.0%.Theseresultssuggestthattheemissionof 12 wasenhancedinthesolutionstatewithanincreasedconjugation effect.Luminescenceof 13 (ΦF ¼ 37.0%)inthesolidthinfilmstatewasgreatlyintensified, whilethatof 14 (ΦF ¼ 14.0%)wasbarelyenhancedrelativetoitssolutionstate.Compound 14 sufferedfromquenchinginsolidstatedueto π–π stacking,thusweakeningitsAIEeffect (Fig.5D).Theseresultsalsoindicatethatthereisalsoacompetitiverelationshipbetweenthe electronicconjugationeffectandtheintramolecularrotationprocesses.
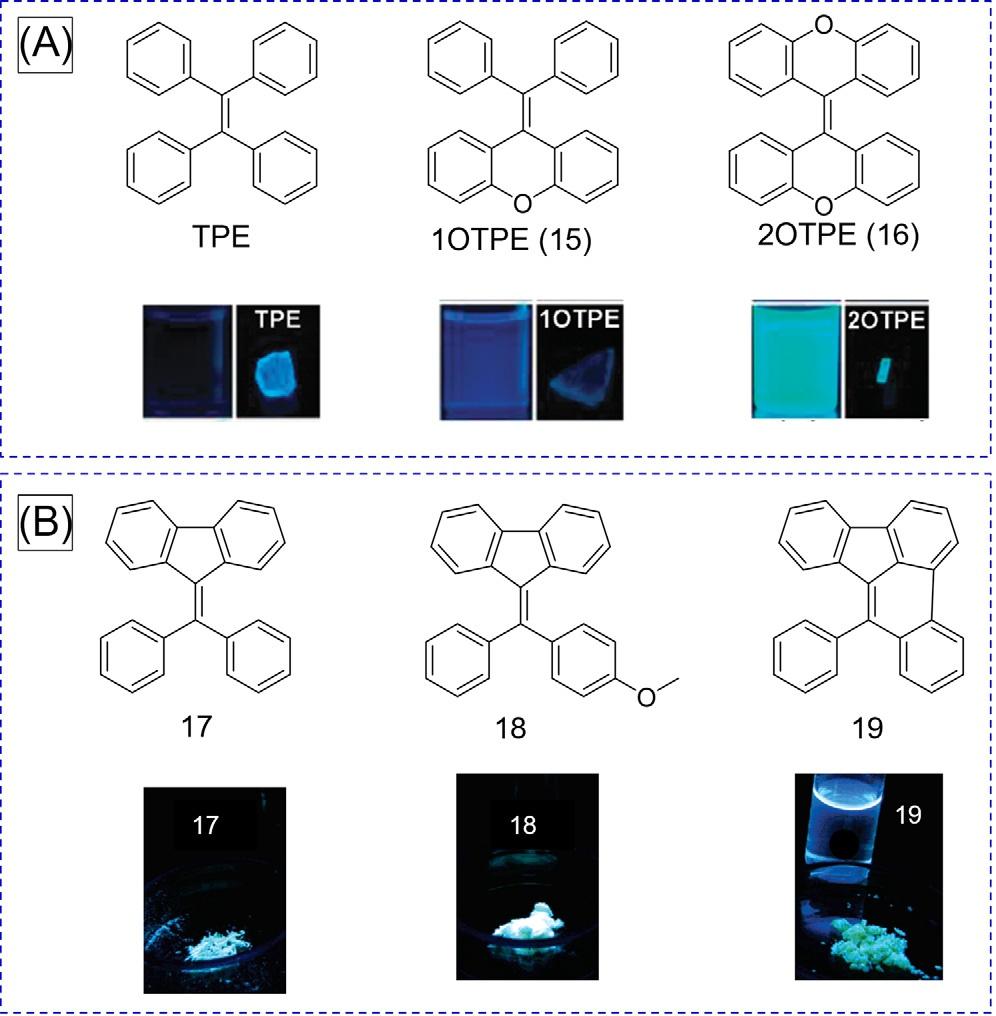
2.2.3Effectoflockingthephenylrings LockingthephenylringsofAIEgensandkeepingitstwistedconformationareeffective waystoobtainhighemissionefficiencythroughtheactivationoftheRIRprocess [16–19].Relatedstudieswereperformedwithcompounds1OTPE(15)and2OTPE(16)wherethephenyl ringsofTPEarelockedwiththe“O”atombridge(Fig.5A) [16] TPEisnonemissiveindilute solutionwithanegligible ΦF,whilethe ΦF of 15 and 16 insolutionswereincreasedto4.6%and 30.1%,respectively.ThisimpliesthatlockingthephenylringwithObridgescanrestrictthe intramolecularrotationandfurtherblockthenonradiativedecayprocess,thusmakingthe TPEderivativesemissiveinsolutionstates.Theemissionefficiencyofluminogensinsolution graduallyincreasedwiththestepwiselockingofphenylringsofTPE,thusverifyingtheRIR mechanismagain.
Asshownin Fig.6B,AIEgen 17 canalsobechangedtoanACQfluorophore(ACQphore) byblockingitsphenylring [18].Diphenyldibenzofulvene(17)isAIEactive,givingavery weakemissionindilutesolutionbutbrightbluelightinsolidstate.Thederivativeof 17, i.e., 18,inwhichamethoxylgroupwasattachedinaphenylringisalsoAIEactive.Unlike 17 and 18,itslockedformof 19 isanACQphore.Brightemissionwasobservedfor 19 in solution(ΦF ¼ 38.0%)butweakemissioninsolidstate(ΦF ¼ 5.5%).Inthesolutionstate, theintramolecularrotationof 19 isaninvalidwaytoconsumetheexcitedstateenergy.Meanwhile,thestrong π–π interactionbetweentheflatbenzo[e]acephenanthrylenestatorswas observedin 19,whicheffectivelyquencheditsemissioninthesolidstate.
2.3Supramolecularinteraction Intramolecularrotationscanalsoberestrictedthroughsupramolecularinteractions [20–22].Forexample,aseriesofTPEderivatives 20–22 wasusedfordetectingproteinand DNA(Fig.7A) [21].Theirincreased ΦF valuesintheiraggregatestatesdemonstratedtheir AIEfeature(Fig.7B).Compounds 20–22 arenonemissiveindilutesolutionsbutshowbright fluorescenceuponadditionoftheDNAandBSA(Fig.7C).Similarworkhadbeendoneforthe coordinationinteractionofanotherTPEderivative 23 andcations.Nearlynoemissionwas observedforcompound 23 indilutesolution,whilethefluorescencewasturnedonafter theadditionofHg2+ cations(Fig.7D).Moreover,theemissionofcompound 23 canalsobeenhancedbythesubsequentadditionofHSO4 intothemixtureof 23 andHg2+ (Fig.7E) [22].
FIG.6 (A)StructuresofTPEanditsderivativeswith“O”bridges(15 and 16),and fluorescentphotographsoftheirsolutions andcrystals.(B)StructuresofTPEderivatives(17, 18,and 19),andfluorescentphotographsoftheirsolutionsandcrystals. (A)ReproducedwithpermissionfromJ.Shi,N. Chang,C.Li,J.Mei,C.Deng,X.Luo,etal., Lockingthephenylringsoftetraphenylethenestep bystep:understandingthemechanismof aggregation-inducedemission,Chem.Commun. 48(2012)10675.Copyright2012RoyalSociety ofChemistry.(B)Reproducedwithpermission fromH.Tong,Y.Dong,Y.Hong,M.H € aussler, J.W.Y.Lam,H.H.Y.Sung,etal.,Aggregationinducedemission:effectsofmolecularstructure, solid-stateconformation,andmorphological packingarrangementonlight-emittingbehaviors ofdiphenyldibenzofulvenederivatives,J.Phys. Chem.C111(2017)2287.Copyright2007 AmericanChemicalSociety.
HSO4–Hq2+ Aggregation of 23 with Hg2+ Aggregation of 23 with Hg2+-HSO4–
FIG.7 (A)Chemicalstructuresoftetraphenylethenederivatives 20–22.(B)Dependenceoffluorescencequantum yieldsofsolutionsof 20 and 21 onthesolventcompositionofacetonitrile(AN)-watermixtures.(C)Plotsoffluorescenceintensitiesofbuffersolutionsof 22 at463nmversusconcentrationsofctDNAandBSA.(D)Schematicillustrationsofcoordination-inducedrestrictionofintramolecularrotationsbasedonluminogen 23.(E)Fluorescencespectra of1(10 μMinDMF)withHg2+ andafteradditionof1.0–16.0equiv.HSO4 1.Inset:fromlefttoright:photosofsolutions of1,1+4.0equiv.Hg2+,1+8.0equiv.HSO4 1,1+4.0equiv.Hg2+ +8.0equiv.HSO4 1 underUVlightillumination. (B andC)ReproducedwithpermissionfromH.Tong,Y.Hong,Y.Dong,M.Haußler,J.W.Y.Lam,Z.Li,etal.,Fluorescent“lightup”bioprobesbasedontetraphenylethylenederivativeswithaggregation-inducedemissioncharacteristics,Chem.Commun. 35(2006)3705.Copyright2006RoyalSocietyofChemistry.(DandE)ReproducedwithpermissionfromG.Huang,G. Zhang,D.Zhang,Turn-onofthefluorescenceoftetra(4-pyridylphenyl)ethylenebythesynergisticinteractionsof mercury(II)cationandhydrogensulfateanion,Chem.Commun.48(2012)7504.Copyright2012RoyalSocietyofChemistry.
3Restrictionofintramolecularvibrations
Inthesesystems,beforetheadditionofcations,theexcitedstateenergycanbeconsumed throughtheintramolecularrotations,makingitnonemissiveinthesolutionstate.However, theadditionofcationsformscoordinationcomplexes,generatingahigherrotationalbarrier fortherotatorygroupsintheligands,thusleadingtothedistinctlightemission.
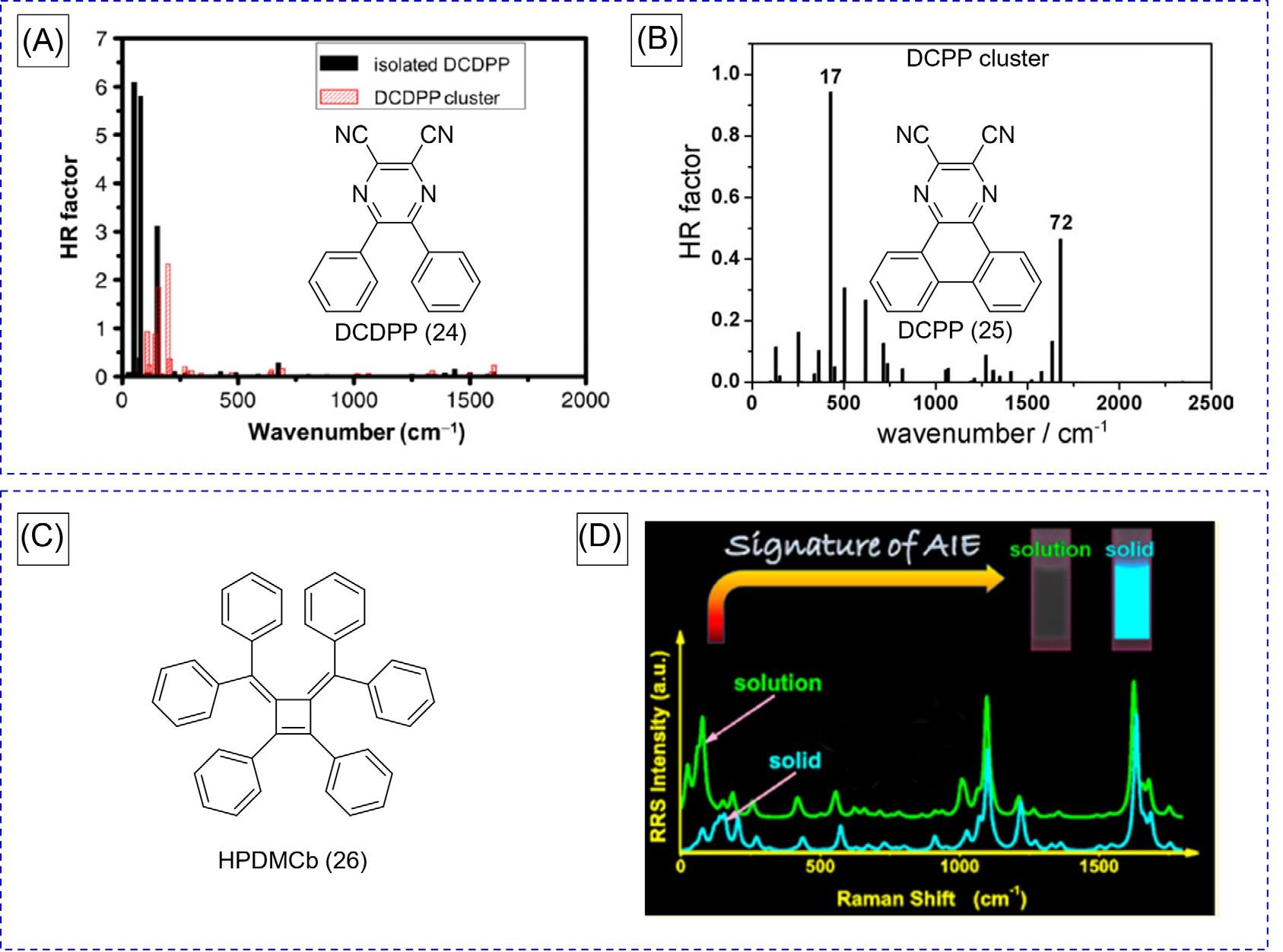
2.4Theoreticalstudies Besidesexperimentalapproaches,theoreticalstudiesoftheAIEmechanismwere performed [23–33].ItisdocumentedthatDCDPP(24)isanAIEgen,whileitslocked-form DCPP(25)isanACQphore.Shuaietal.modeledtheAIEgenof 24 anditslocked-form 25 byusingquantummechanicsandmolecularmechanics(QM/MM)approaches [26,27,29]. Thisprovidescrucialinsightsintotheirphotophysicalbehaviorsindifferentstates. Huang-Rhys(HR)factorsatdifferentnormalmodesareshownin Fig.8AandB.Threemodes withlargeHRfactorsofisolated 24 werefoundinthelowfrequencyregion,andthusthese normalmodesarethemainchanneltoconsumeitsexcitedstateenergy(Fig.8A).However, smallerHRfactorsof 24 clusterswereobservedinthehigherfrequencyregion,signifyingthat excited-stateenergyisreducedsubstantiallybythelow-frequencyvibrations,suchasthe twistingofphenylrings.Incontrast, 25 inisolatedandclusterstatesdidnotshowlowfrequencynormalmodes,butmuchsmallerHRfactorswerefoundinamuchhigherfrequencyregion(Fig.8B).TheseresultsprovideaclearsupporttoRIR.
Furthermore,resonanceRamanspectroscopy(RRS)wasalsousedtostudythemicroscopic mechanismofAIEincombinationwiththecomputationalstudies [34].TakingHPDMCb(26) asanexampleandnon-AIEactiveDCPPforcomparison,theintensitiesoflow-frequency peaksof 26 clusterinRRSobviouslydecreasedcomparedwiththehigh-frequencypeaks. However,theRRSofDCPPremainedunaffectedafteraggregation.Theseresultscanbe attributedtotheRIRofAIEgen,suchasthelow-frequencyphenylringtwisting.Therefore, theRRSwasalsoadirectapproachtoexplaintheabovetheoreticalstudiesontheRIR mechanism.
Alltheresultsabove,fromexperimentalmeasurementstotheoreticalcalculations,from externalphysicalandengineeringcontrolstointernalstructuralandchemicalmodification, providestrongevidencetoconfirmthatRIRistheworkingmechanismofAIEgenswith rotatablearomaticrings.
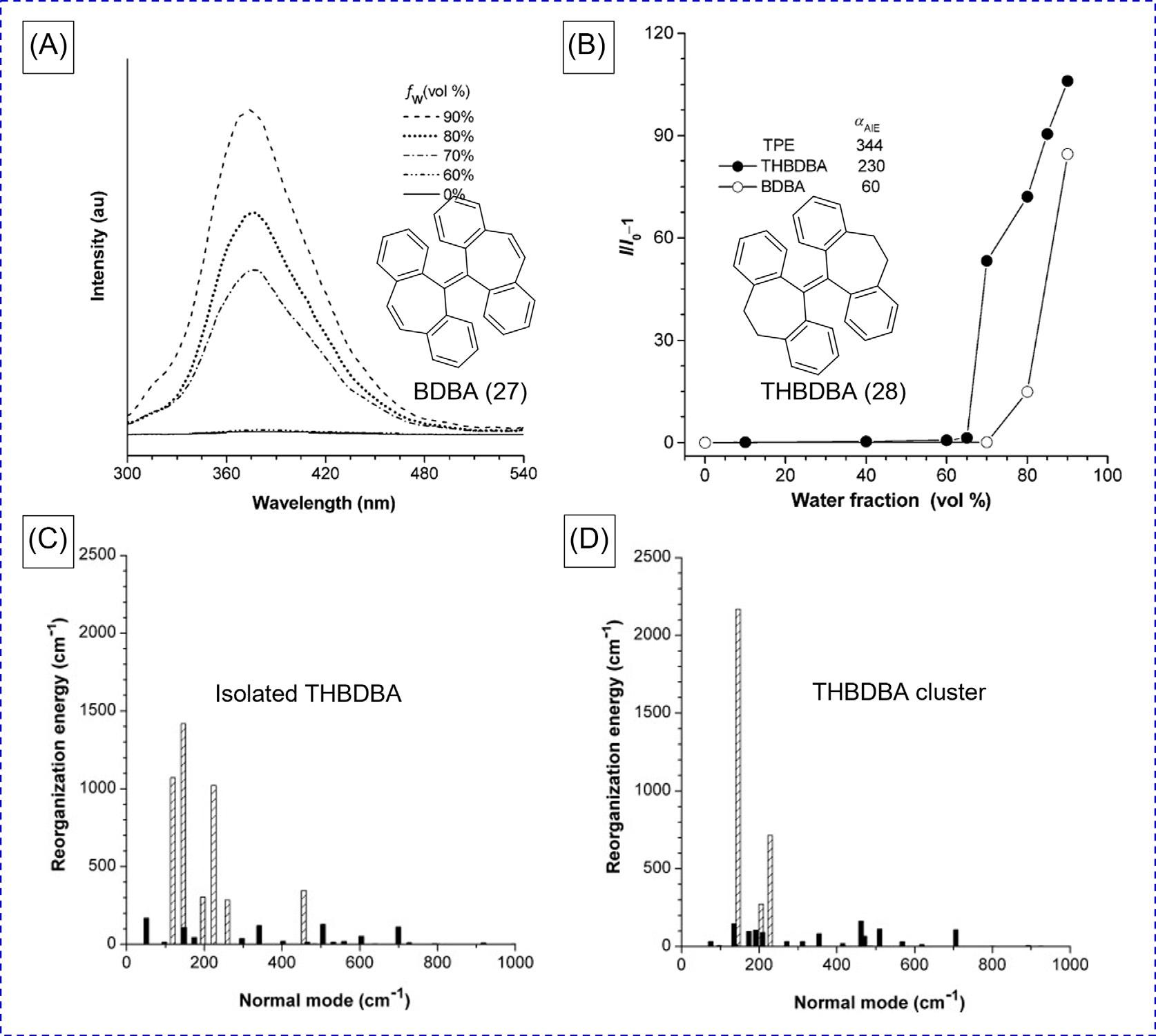
3Restrictionofintramolecularvibrations UndertheguidanceofRIR,manyAIEgenshavebeendesigned,prepared,andappliedin diverseareas.However,partofthemoleculeswiththeAIEfeaturecannotbefullyexplained bytheRIRmechanism,suchasBDBA(27)andTHBDBA(28)displayedin Fig.9[35,36].They possessnorotatableunitsbecausethetwopairsofphenylringsofBDBAandTHBDBAare lockedthroughvinyllinkagesandethylenetethers,respectively.Thesemoleculesshouldbe emissiveevenindilutesolutionsbasedonthemechanismofRIRbecausetheexcited-state energyisnolongerconsumedbynonradiativepathways.However,theyshowAIEproperty. Nearlynoflorescencewasobservedinsolutionbutbrightemissionwasrecordedin
FIG.8 CalculatedHuang-Rhys(HR)factorsversusnormalmodewavenumbersfor(A)isolatedDCDPP(24)and clusterand(B)DCPP(25)cluster.(C)ChemicalStructureofHPDMCb(26).(D)ResonanceRamanspectroscopy(RRS) intensityinbothsolutionandsolidphasesforHPDMCb. (AandB)ReproducedwithpermissionfromQ.Wu,C.Deng,Q. Peng,Y.Niu,Z.Shuai,Quantumchemicalinsightsintotheaggregationinducedemissionphenomena:aQM/MMstudyfor pyrazinederivatives,J.Comput.Chem.33(2012)1862.Copyright2012Wiley.Copyright2013ElsevierB.V.(D)Reproduced withpermissionfromT.Zhang,H.Ma,Y.Niu,W.Li,D.Wang,Q.Peng,etal.,Spectroscopicsignatureoftheaggregationinducedemissionphenomenacausedbyrestrictednonradiativedecay:atheoreticalproposal,J.Phys.Chem.C119(2015)5040. Copyright2015AmericanChemicalSociety.
aggregatestates(Fig.9AandB).Similartotherotationofphenylrings,thevibrationalmotionsshouldalsoconsumeexcitonenergy.Inotherwords,restrictionofintramolecularvibrations(RIV)isthecauseoftheAIEeffectofBDBAandTHBDBA.Computationalanalyseswere alsoemployedtoexplaintheAIEphenomenon.Asdepictedin Fig.9C,QM/MMmodeling resultsshowthattherearemainlysixnormalmodesforTHBDBAinasinglemoleculeconsumingtheenergyoftheexcitedstate.Amongthese,everyreorganizationenergyexceeded 200cm 1,resultinginatotalenergyof5679cm 1.Incontrast,THBDBAclustersonlyhave threesignificantnormalmodefrequenciesinthevicinityofthelow-frequencyrange ( 4016cm 1 ; Fig.9D).Intheclusters,thecombinationofadecreaseinthetotalreorganization energyandvibrationalchannelswaslikelythecauseoftheobservedAIEeffectofTHBDBA.
FIG.9 (A)PLspectraofBDBA(27)inTHF/watermixtureswithdifferent fw and(B)changeinthePLintensityof BDBAandTHBDBAwith fw (20 μM).Plotsofreorganizationenergyversusnormalmodewavenumbersforexcited statesof(C)molecularand(D)clusteredspeciesof 28 ReproducedwithpermissionfromN.L.C.Leung,N.Xie,W.Yuan,Y. Liu,Q.Wu,Q.Peng,etal.,Restrictionofintramolecularmotions:thegeneralmechanismbehindaggregation-inducedemission, Chem.Eur.J.20(2014)15349.Copyright2014Wiley-VCHVerlagGmbH&Co.KGaA.
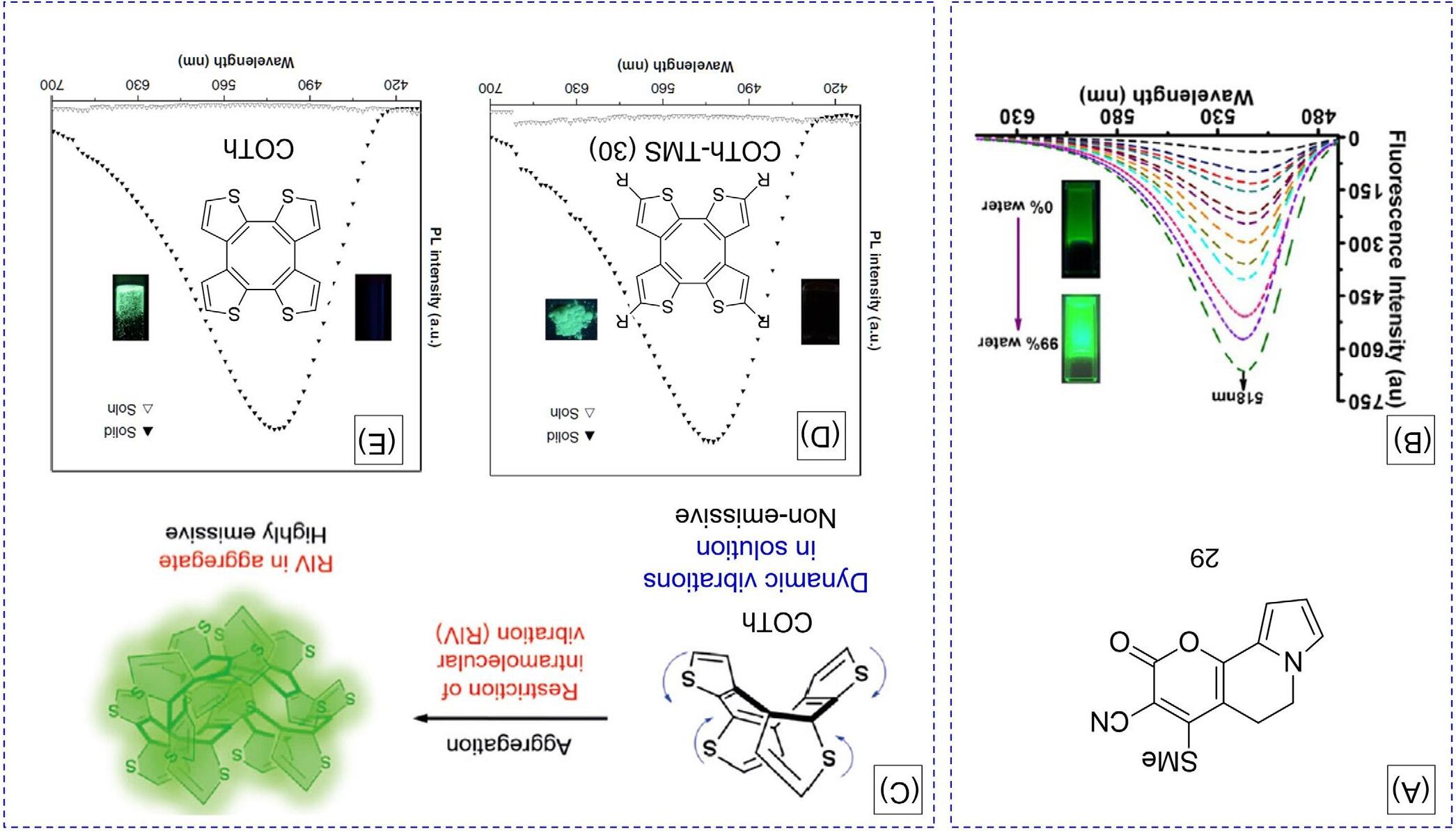
TheRIVmechanismhasbeenfurtherconfirmedinotherAIEgensystems [37–42].Goel etal.reporteda5,6-dihydro-2H-pyrano[3,2-g]indolizine(DPI)derivative 29 whichexhibits auniquesolution/soliddualemissionbehaviorwithastrongeremissioninthesolidstate (Fig.10A) [41].TochecktheAIEfeatureof 29,thePLwasrecordedinTHF/watermixtures withvarying fw.Asshownin Fig.10B,thePLintensityof 29 wasremarkablyenhancedupto 20-foldhigherthanthatofitssolutionwhenthe fw reached99%.Consideringthestructural characteristicof 29,theRIVofC2-flexuremightberesponsibleforitsAIEfeature.Fromthe perspectiveofthecrystalstructure,twostrongnoncovalentC
FIG.10 (A)Chemicalstructureof 29.(B)PLspectraof 29 inTHF/watermixtures.Inset:Fluorescentphotographsof 29 inTHF/watermixtures(fw ¼ 0, 99vol%).(C)IllustrationofcyclooctatetrathiopheneCOThthatshowstheAIEactivityduetotherestrictionofintramolecularvibration(RIV)intheaggregatestate.(D)PLspectraofCOTh-TMSinTHFsolution (emptytriangles) andsolidstates (fulltriangles).Concentration:10 μM,excitationwavelength: 365nm.Inset:thefluorescencepicturesof 30 inTHFsolution(left, dark)andsolidstates(right, greenfluorescence)takenunderanexcitationwavelengthof 365nmbyaportableUVlamp.(E)PLspectraofCOThinTHFsolution (emptytriangles) andsolidstates (fulltriangles).Concentration:10 μM,excitation wavelength:350nm.Inset:thefluorescencepicturesofCOThinTHFsolution(left, dark)andsolidstates(right, greenfluorescence)takenunderanexcitation wavelengthof365nmbyaportableUVlamp. (B)ReproducedwithpermissionfromA.Raghuvanshi,A.K.Jha,A.Sharma,S.Umar,S.Mishra,R.Kant,etal., Anonarchetypal5,6-dihydro-2H-pyrano[3,2-g]indolizine-basedsolution-soliddualemissiveAIEgenwithmulticolortunability,Chem.Eur.J.23(2017)4527.Copyright2017Wiley-VCHVerlagGmbH&Co.KGaA.(E)ReproducedwithpermissionfromZ.Zhao,X.Zheng,L.Du,Y.Xiong,W.He,X.Gao,etal.,Non-aromatic annulene-basedaggregation-inducedemissionsystemviaaromaticityreversalprocess,Nat.Commun.10(2019)1.Copyright2019NaturePublishing Group.
interactionswerefoundbetweentheadjacentmolecules.Theseinteractionseffectively restrictedthemolecularvibrationalmotionofC2-flexure,thusleadingtostrongemission insolidstate.
Cyclooctotetraene(COT)derivativessuchasCOThandCOTh-TMS(30)werealsofoundto beAIE-active(Fig.10C) [42].Weakemissionwitha ΦF of0.7%andbrightgreenluminescence witha ΦF of10%wererecordedforCOTh-TMSindilutesolutionandsolidstate,respectively (Fig.10D).Initially,theTMSgroupswereproposedtopossiblyactasrotorstoconsumethe excitonenergy.However,afurtherstudyindicatedthatCOThwithoutTMSgroupsalsoexhibitanAIEfeature,inwhichthe ΦF insolutionwasonly0.4%butenhancedto11%initssolid state(Fig.10E).TheseresultsindicatethattheAIEactivityoftheCOThsystemiscaused throughthemolecularvibrationratherthantherotationofTMSgroups.TogainadeeperinsightintotheAIEactivityofCOThderivatives,theircrystalstructureswereanalyzed.No obviousintermolecular π–π interactionwasfoundduetotheirnoncoplanarandsaddle-type conformations.Furthermore,multipleintermolecularC H ⋯ π andS ⋯ π interactionswere observedinthecrystals,whichcanrigidifythemolecularconformationresultinginsolidstateemission.Inthesolutionstate,thevibrationalmolecularmotionwouldoccurforthe COThderivativesduetothelackofaromaticitystabilization,makingthemnonemissive. However,thearomaticityoftheCOThderivativesisstabilizedbecauseoftheRIVprocess insolidstateandtheactivationoftheradiativechannel,makingthemoleculesemitbrightly. Thereversalfromthegroundstatetotheexcitedstateofaromaticityservesasadrivingforce toinducetheexcited-stateintramolecularvibrationandleadstotheAIEphenomenon.
Theexamplesabovethereforeindicatethatmolecularvibrationalmotions,including in-plane/out-of-planebending,flapping,stretching,scissoring,wagging,twisting,rocking, etc.,canalsoconsumetheexcitonenergy.SimilartoRIR,restrictionofintramolecularvibrationcanalsoturnonthefluorescenceofthemoleculeintheaggregatestates.Theproposalof theRIVmechanismcannotonlyoffernewperspectivesforphotophysicalfundamentalsbut alsoopennewwaysforthedesignanddevelopmentofnewAIEgensystems,whichwill furtherbroadenthescopeofAIEresearch.
4Restrictionofintramolecularmotions Accordingtotheabovediscussion,itbecomesclearthatRIRandRIVhavebeenrationalizedasthemaincausefortheAIEeffect.Thus,RIRandRIVcouldbeunifiedasrestrictionof intramolecularmotion(RIM).
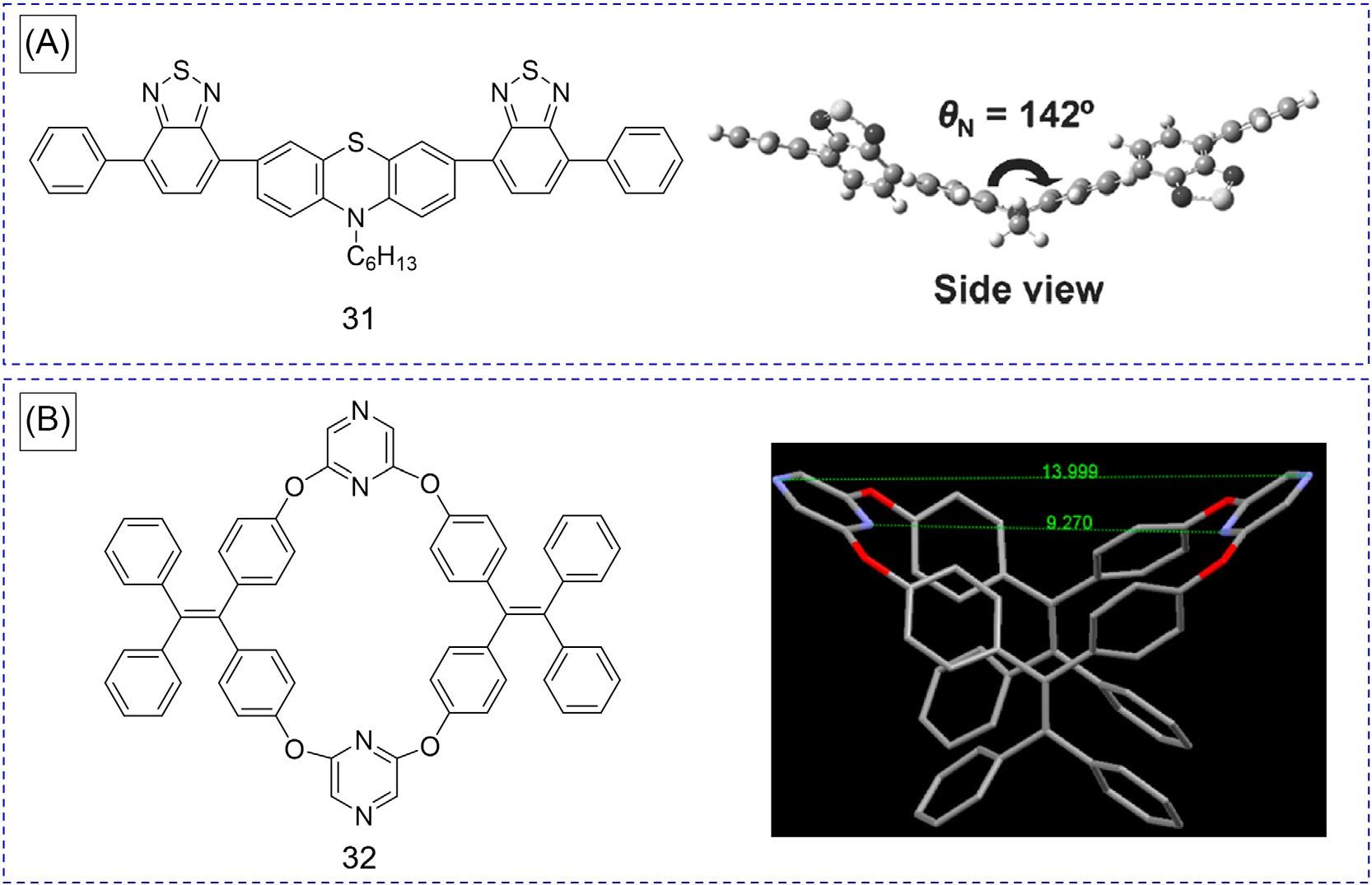
Interestingly,insomeAIEsystems,bothRIRandRIVareinvolved,andtheworkingmechanismcanonlybeascribedtoRIM [43–46].TworepresentativeexamplesofsuchAIEgensare shownin Fig.11.Maetal.reportedabutterfly-shapedphenothiazinederivative 31 (Fig.11A), whichisnotemissiveinsolution,butbrightredfluorescenceintheTHF/watermixtureswith fw 70vol%wasobserved,indicatingitsAIEeffect [43].Thetheoreticaloptimizedgroundstategeometryshowedthatthephenothiazineunitexhibitsanonplanarbutterfly-like structurewithalargeC S N Cdihedralangleof142degrees.Moreover,thereisalarge twist-linkeddistortedangleof145degreesbetweenthephenothiazineandbenzothiadiazole groups.Inthesolutionstate,itsexcited-stateenergyismainlyconsumedthroughthe
FIG.11 (A)Chemicalstructureandtheoreticallyoptimizedgeometryof 31.Tosimplifythecalculation,thehexyl groupwasreplacedwithamethylsubstituent.KGaA.(B)Chemicalandcrystalstructureof 32.Hydrogenatomswere omittedforclarity. (A)ReproducedwithpermissionfromL.Yao,S.Zhang,R.Wang,W.Li,F.Shen,B.Yang,etal.,Highly efficientnear-infraredorganiclight-emittingdiodebasedonabutterfly-shapeddonor-acceptorchromophorewithstrongsolidstatefluorescenceandalargeproportionofradiativeexcitons,Angew.Chem.Int.Ed.53(2014)2119.Copyright2014WileyVCHVerlagGmbH&Co.(B)ReproducedwithpermissionfromC.Zhang,Z.Wang,S.Song,X.Meng,Y.Zheng,X.Yang, etal.,Tetraphenylethylene-basedexpandedoxacalixarene:synthesis,structure,anditssupramoleculargridassembliesdirected byguestsinthesolidstate,J.Org.Chem.79(2014)2729.Copyright2014AmericanChemicalSociety.
vibrationalmotionsofthephenothiazinecoreandtherotationalmotionsofthe benzothiadiazoleandphenylrings.Theseintramolecularmotionswere,however,confined intheaggregatestate.Asaresult,theRIMprocessisthecauseoftheAIEeffectof 31. Inadditiontothetypicalluminogensincludingrotatableperipheryphenylringsand vibratablecores,manymacrocyclesalsoexhibittheAIEfeaturecontrolledbytheRIMprocess [47–50].Forexample,aTPE-basedoxacalixarene(32)exhibitsthetypicalAIEeffect.Itisalmostnonemissiveindilutesolution,whileemitsbrightemissionafteraggregation(Fig.11B) [47].TherotationsofphenylringsanddiphenylmethyleneunitsinTPEunitsconsumethe excitonenergy.Inaddition,theasymmetricallylinkedpyrazineunitsinthecrystalstructure of 32 adoptslantedconformations,whichallowflap-likevibratorymotionsoccurringtofurtherdissipatetheexcited-stateenergy.Asaresult,therotationandvibrationconsumethe excitonenergy,whichinturnleadstoemissionquenchingindilutesolution.However,upon theformationofaggregates,theintramolecularmotionisrestricted,andhenceitsluminescenceisturnedon.