CELEBRATING OUR

CELEBRATING 60 YEARS OF SERVICE TO THE VETERINARY PROFESSION IN 2025 Follow Tom Hungerford’s ‘goanna track to success’…

NEXT LEVEL PROTECTION for dogs
The most complete parasite protection, all in one tasty, monthly chew







C&T
Issue 321 | December 2025
Control & Therapy Series
PUBLISHER
Centre for Veterinary Education
Veterinary Science Conference Centre
Regimental Drive
The University of Sydney NSW 2006 + 61 2 9351 7979 cve.marketing@sydney.edu.au cve.edu.au
Print Post Approval No. 10005007
CVE Director
Associate Professor Kate Patterson kate.patterson@sydney.edu.au
EDITOR
Lis Churchward elisabeth.churchward@sydney.edu.au
VETERINARY EDITOR
Dr Richard Malik
VETERINARY SUB-EDITOR
Dr Jo Krockenberger joanne.krockenberger@sydney.edu.au
DESIGNER
Samin Mirgheshmi
ADVERTISING
Lis Churchward elisabeth.churchward@sydney.edu.au
To integrate your brand with C&T in print and digital and to discuss new business opportunities, please contact: MARKETING & SALES MANAGER
Ines Borovic ines.borovic@sydney.edu.au
DISCLAIMER
All content made available in the Control & Therapy (including articles and videos) may be used by readers (You or Your) for educational purposes only.
Knowledge and best practice in this field are constantly changing. As new research and experience broadens our knowledge, changes in practice, treatment and drug therapy may become necessary or appropriate. You are advised to check the most current information provided (1) on procedures featured or (2) by the manufacturer of each product to be administered, to verify the recommended dose or formula, the method and duration of administration, and contraindications.
To the extent permitted by law You acknowledge and agree that:
I. Except for any non-excludable obligations, We give no warranty (express or implied) or guarantee that the content is current, or fit for any use whatsoever. All such information, services and materials are provided ‘as is’ and ‘as available’ without warranty of any kind.
II. All conditions, warranties, guarantees, rights, remedies, liabilities or other terms that may be implied or conferred by statute, custom or the general law that impose any liability or obligation on the University (We) in relation to the educational services We provide to You are expressly excluded; and
III. We have no liability to You or anyone else (including in negligence) for any type of loss, however incurred, in connection with Your use or reliance on the content, including (without limitation) loss of profits, loss of revenue, loss of goodwill, loss of customers, loss of or damage to reputation, loss of capital, downtime costs, loss under or in relation to any other contract, loss of data, loss of use of data or any direct, indirect, economic, special or consequential loss, harm, damage, cost or expense (including
PerSPective no. 167
Engage With Your Profession
The Control & Therapy Series was established in 1969 by Director Dr Tom Hungerford. His aim was to publish uncensored and unedited material contributed by vets writing about:
...not what he/she should have done, BUT WHAT HE/SHE DID, right or wrong, the full details, revealing the actual “blood and dung and guts” of real practice as it happened, when tired, at night, in the rain in the paddock, poor lighting, no other vet to help.
The C&T forum gives a ‘voice’ to the profession and everyone interested in animal welfare. You don’t have to be a CVE Member to contribute an article or reply to a 'What's YOUR Diagnosis?'. We welcome contributions from Vets, Techs, Nurses, allied professionals and anyone interested in animal welfare—Non CVE Members included.
Submit your C&T article
A template and information about uploading high resolution images for print can be found here cve.edu.au/submit-article
Questions?
Please contact cve.marketing@sydney.edu.au
Join In!
The C&T is not a peer-reviewed journal. Rather, it is a unique forum allowing veterinary professionals to share their cases and experiences with their colleagues. We are keen on publishing short, pithy, practical articles (a simple paragraph is fine) that our members/readers can immediately relate to and utilise. Our editors will assist with English and grammar if required.
I enjoy reading the C&T more than any other veterinary publication.
-Terry King, Veterinary Specialist Services, QLD
Thank You to All Contributors & Advertisers
The C&T Series thrives due to your generosity.
Major Winners
Prize: A CVE$500 voucher
—Moira van Dorsselaer page 3
—Christopher Simpson page 8
Winners
Prize: A CVE$300 voucher
Sandra Hodgins page 11
Madison Newton page 25
Q&A Best Answer
Prize: A CVE$300 voucher
Nurazlin Binti Che Mat ariffin page 35
Best Visuals
Prize: A CVE$300 voucher
Robert Johnson page 38

Centre for Veterinary Education Est. 1965
One of the great privileges of this role is seeing what happens when members of our community choose to step forward to share what they know. It’s not just information; it’s lived experience and practical wisdom that you can only learn from someone who has been there with their sleeves rolled up. Every contribution helps someone else take the next step with a little more clarity and confidence.
In that spirit, I want to acknowledge three remarkable colleagues: Drs Zoe Lenard (Diagnostic Imaging), Jane Yu (Feline Medicine) and Mark Newman (Surgery).
Next year they will be pursuing new opportunities and stepping back from teaching our Distance Education programs. Their impact is hard to capture in a sentence; they have taught with a generosity and depth that has helped to shape the practice and confidence of so many veterinarians in our community. They will be missed, and I thank them for their important contributions over the years with CVE.
I’m delighted to share some wonderful news. In our milestone 60th anniversary year, Dr Kim Kendall (longtime CVE member and contributor to C&T and with another article in this issue on P30) has made a generous gift that will fund a project dedicated to making C&T more accessible, searchable and properly archived. Thank you Kim – this is an incredible investment in preserving our shared knowledge and ensuring it remains useful for decades to come. This ambitious project will launch in 2026 and we are looking forward to sharing the progress with you.
This issue also features Dr Robert Johnson’s goanna article, a timely reminder of our founding Director Dr Tom Hungerford’s message: follow the goanna track to success. Robert’s piece captures exactly what that phrase has always meant; 'Veterinarians need to identify an area of interest and devote time to it, listening, learning, and building what he called a "tree of knowledge". Climbing that tree offered a unique vantage point from which other opportunities for growth and development could be identified.'
As we close out another year, I hope you can take pride in the progress made, the challenges met, and the learning shared. And as we look toward 2026, there is genuine excitement ahead.

Associate Professor Kate Patterson Director
Small animal
MAJOR Winner
The prize is a CVE$500 voucher
Herpesvirus Keratitis
Moira
van Dorsselaer BVSc
The Cat Clinic Hobart
150 New Town Rd
New Town TAS 7008
e. moira@catvethobart.com.au
t. +613 6227 8000
C&T No. 6097

Moira van Dorsselaer is the Principal Veterinarian and proud owner of The Cat Clinic Hobart, which she founded in 2013 to give cats the calm, purpose-built environment they deserve. After graduating from the University of Queensland in 1998, she worked as a small animal clinician before following her heart into a feline-only practice — the best career decision she’s ever made. Moira is unashamedly passionate about cats and the people who love them, and she enjoys the mix of complex medical cases, surgery, and everyday care that comes with this unique role. Outside the clinic, she’s happiest when spending time with her family, watching her daughter play field hockey, and loving life in Australia’s most liveable city. Cats may rule her workday, but they’ve also shaped her life.
Gizmo, an 800g kitten, was presented to our clinic by a local rescue with severe flea infestation, alopecia (suspected ringworm), scabbing, and diarrhoea. Fur samples were collected for fungal culture, and she was started on Pro-Kolin by the rescue.
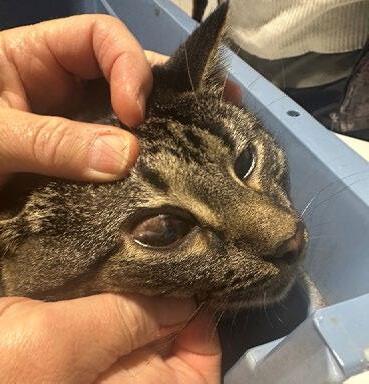
Two weeks later, she returned with minimal weight gain (200g; BCS 3/9), poor appetite, and severe upper respiratory infection consistent with feline herpesvirus. She had been started on doxycycline paste by another clinic. Clinical signs included bilateral ocular discharge with lids adhered shut, marked chemosis, fullthickness positive fluorescein staining, and pyrexia. We recommended hospitalisation for IVFT, analgesia, and serum eye drops, but the rescue opted for outpatient care with doxycycline (10 mg/kg SID PO), famciclovir (90 mg/kg TID PO), and lubricating eye drops (chloropt) TID.
One week later, Gizmo re-presented in respiratory distress with severe bilateral ocular pathology, including chemosis, bulging conjunctiva, corneal clouding, and extensive fluorescein uptake. She was tachypnoeic, tachycardic, normothermic, and still BCS 3/9. She was admitted for supportive care, analgesia, and accurate administration of ophthalmic and systemic medications.
In-hospital treatment included:
i. Doxycycline (10 mg/kg SID PO)
ii. Famciclovir (90 mg/kg TID PO)
iii. Buprenorphine (0.015 mg SC BID)
iv. Meloxicam (0.05 mg/kg SID PO)
v. Ocular therapy every 2 hours: Hyloforte, chloropt, Systane
vi. Twice-daily steam therapy to relieve nasal congestion
vii. Cidofovir ophthalmic drops 1 drop/eye TID
She showed rapid clinical improvement, with increased appetite, comfort, and activity. Cidofovir ophthalmic drops were initiated upon arrival (1 drop/eye TID), alternated with Hyloforte and chloropt at night. Due to the rescue’s capacity limitations, Gizmo stayed with me for continuous care. She gained 400g in 4 days.
After two weeks, all medications were tapered and discontinued. At one month, she received her first vaccination and remained active, with improved ocular signs. Persistent corneal scarring and possible adhesions were noted, but she retained functional vision.
Over subsequent months, Gizmo was desexed, completed her vaccination course, and was adopted alongside her companion kitten, Slipper. She now lives comfortably with minor residual corneal opacities, near normal vision and no ongoing ocular concerns.
Reflections and Lessons Learned:
– Persistence pays off: With appropriate medications and supportive care, even severely affected kittens can recover remarkably.
– Analgesia is critical: Pain management transformed Gizmo’s demeanour and initiated her clinical improvement. See video.
– Cidofovir is invaluable: Treat early and often; I feel antiviral ocular therapy was key to preserving her vision.
– Multimodal therapy matters: Combining doxycycline, famciclovir, and cidofovir was essential in managing infection and preventing enucleation.
Special thanks to Dr Richard Malik for his guidance and encouragement throughout Gizmo’s care and ensuring I did not give up.


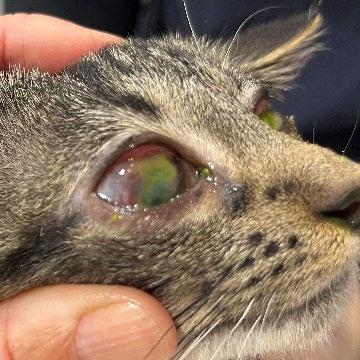

Video: Gizmo 24 hours after hospitalization and pain relief etc started—you can see despite how horrible her eyes look she is so happy cve.edu.au/gizmo

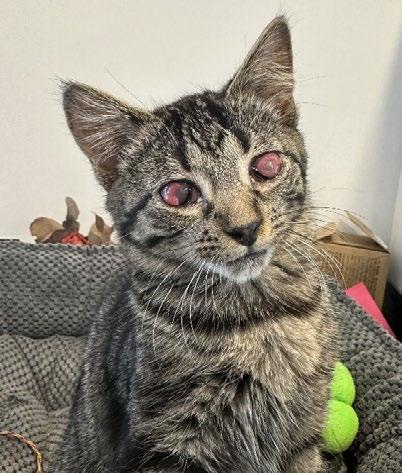

Figures 1 A,B & C. Eyes on presentation
2. Cidofovir 0.5% eye drops
3. Gizmo March 2024
4. Gizmo May 2024


EDITOR’S NOTES
1. I think starting early is critical—vets who see shelter cases should always have a bottle in the fridge— YOU CANNOT AFFORD THE 3 days wait to have it delivered.
2. BOVA make 0.5% Cidofovir. At time of writing the price is $102.
3. The dose of Famvir listed in Plumbs is 40-90mg/ kg per os every 8-12 hours; however, I never give more than 40 mg/kg BID or TID. The Davis group recommend 90 mg/kg BID to get decent levels in tears, but the levels are never all that good—even at that dose, it’s expensive and the tablets are BIG and not that easy to give.
4. The Famvir treats the whole cat including the respiratory system—and blood levels of 40 mg/ kg are not that different to 90 mg/kg because the cat's liver has a ceiling rate of conversion of famciclovir to the active drug penciclovir.
5. Cidofovir is a STRONGER antiviral, but it’s too toxic to give systemically; however, it works a treat TOPICALLY.
6. Doxycycline has benefits in relation to healing, and adding eye drops with hyaluronic acid is helpful as herpes wipes out the Goblet cells in the conjunctiva.
BOTTOM LINE
When severe ocular herpetic disease is present—it’s more cost effective to give Famvir at 40 mg/kg BID to TID and cidofovir eye drops BID, than giving massive doses of famciclovir systemically to get decent levels in tears.

Patient care is important from the moment they come
into the clinic to the time they leave. When drawing up a medication, we check the drug, double check the dose and label the syringe with the appropriate sticker to indicate who the drug is for and what is in the syringe.
So why would we not want the same safety check with our infusion lines?
Enhancing Safety and Efficiency in Veterinary Infusion Therapy: Introducing Colour-Coded Minimum Volume Tubes
Our patients rely on us to give them the best care possible. They, and their owners, trust that what we use to deliver their treatments will not cause them any harm. In the fast-paced environment of veterinary hospitals, clarity and precision in drug administration are essential—not just for outcomes, but for peace of mind. The introduction of colour-coded minimum volume IV tubing marks a meaningful step forward in infusion therapy—designed to support clinicians in delivering safe, efficient, and error-reduced care.
Veterinary teams often manage multiple high-potency drugs simultaneously. Colour-coded tubing enables clear drug assignment and rapid identification at a glance, helping reduce the risk of medication errors and enhancing workflow efficiency during critical procedures.
In addition to offering three vibrant colours (BLUE MAGENTA, and GREEN), Perfusor™ tubing retains these vital, yet sometimes overlooked characteristics:
1. Low priming volume (≤1.27 mLs) minimizes drug waste and ensures rapid therapy initiation.
2. Pressure resistance up to 2 bar supports reliable performance with syringe drivers, even in demanding clinical scenarios.
3. Luer-Lock fittings ensure secure connections across all standard syringe driver systems.
Safe Materials for Sensitive Patients
Made from polyethylene (PE), the Perfusor™ Line is free from PVC, DEHP, and latex, making it a safer choice for sensitive animal patients. Its low sorbing properties and kink-resistant design further support consistent drug delivery—because every patient deserves the best we can offer.
Optimized for Veterinary Workflow
Available in two lengths (150 cm and 200 cm), the Perfusor™ Line adapts to varied clinical setups. Whether managing anaesthesia, critical care, or post-operative recovery, veterinary professionals can rely on its excellent start-up characteristics and minimal residual volume to maintain therapeutic precision.
What Materials Are Your Current Infusion Lines Made From?
DEHP-Free Infusion Therapy: A Safer Standard for Veterinary Hospitals
In veterinary medicine, where precision and patient safety are paramount, the materials used in infusion therapy can have a profound impact—especially in high-risk procedures involving neonates, critical care, and long-duration infusions. One such material under global scrutiny is DEHP (Di(2-ethylhexyl) phthalate), a plasticiser commonly used in PVC-based medical devices.
Why DEHP-Free Matters
DEHP has been classified by the European Union as a Substance of Very High Concern, specifically toxic to reproduction. Studies have shown that DEHP can leach from PVC tubing during medical procedures, potentially affecting the testes, liver, and kidneys in animal models, and is suspected to impair fertility in humans—particularly in critically ill male neonates, unborn children of pregnant women, and nursing infants exposed to high levels of DEHP. [DEHP-Free A4]
Global health authorities have responded decisively:
– EU: Banned DEHP in toys and baby articles since 2007; mandatory labelling for medical devices since 2010. [DEHP-Free A4]
USA (FDA): Issued public health notifications and precautionary guidelines to reduce DEHP exposure. [DEHPFree A4]
– Australia: Declared products with >1% DEHP unsafe for children; DEHP in medical devices can reach up to 40%. [DEHP-Free A4]
– Canada: Requires manufacturers to disclose DEHP content exceeding 0.1%. [DEHP-Free A4]
B. Braun’s Commitment to Safety
Although not mandated in Australia, B. Braun has proactively converted its entire infusion therapy portfolio to DEHP-free materials—with no change in part numbers or pricing. This reflects a deep commitment to patient safety, environmental responsibility, and the professionals who care for animals every day. [DEHP-Free A4]
References:
1. EU directive 67/548/EEC
2. Directive 93/42/EEC and its amendment 2007/47/EG
3. FDA Public Health Notification 12/07/2002
4. The Commonwealth of Australia Consumer protection Notice No. 11 of 2011
5. NICNAS - Existing chemicals information sheet 01-2010
6. Health Canada notice 08-111801-312, May 2008
7. Safety Assessment of DEHP, released from PVC Medical Devices FDA, 2001
8. SCENIHR - “Opinion on the safety of medical devices containing DEHP-plasticised PVC or other plasticisers on neonates and other groups possibly at risk”, Feb 2008
9. Umwelt bundesamt (Germany) “Phthalates - Useful plasticisers with undesired properties”, Feb 2007
10. K. Ruzidckova, M. Cobbing, M. Rossi, T. Belazzi, “Preventing Harm from Phthalates, Avoiding PVC in Hospitals”, Health Care without Harm, June 2004
MAJOR Winner
The prize is a CVE$500 voucher
Colonic FIP in a Cat
Christopher Simpson BVSc MANZCVSc
Victoria Veterinary Clinics
Hong Kong
e. simpson_christo@icloud.com
C&T No. 6098

Dr Christopher Simpson is a graduate of The University of Melbourne, but has practiced in Melbourne, Sydney, Canberra, the US, the UK, and even the South Pacific island of Vanuatu! Since 2015, however, he has found his spiritual home in Hong Kong, where he is attracted to the thrilling pace of life, Cantonese food, language, trail-running, and of course the fascinating caseload that only tropical Asia can offer! He is a member of the Australian & New Zealand College of Veterinary Scientists, has completed a Residency in Small Animal Internal Medicine at Melbourne University, and contributed scientific papers to the Australian Veterinary Journal, the Journal of Feline Medicine and Surgery, and several veterinary textbook chapters. He is also a regular contributor to the Control & Therapy series and enjoys sharing the rich and varied experiences of practicing in Hong Kong.

Cha Cha is a 9-month-old male neutered Domestic Shorthair cat. He was initially seen by another clinic for straining to defecate and blood in the stool. He was otherwise very bright, active, eating well and in good body condition.
An ultrasound at the other clinic identified marked thickening of the descending colon.
The owners were advised that this was most likely to be neoplastic and were advised to undertake a subtotal colectomy.
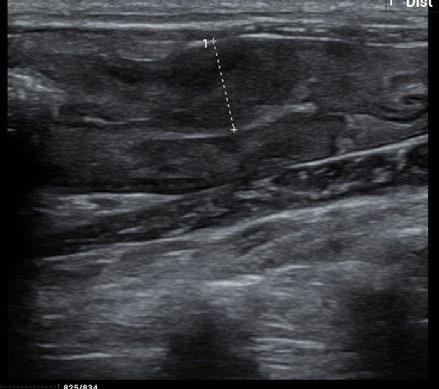
The owners came to us for a second opinion. Our ultrasound confirmed the findings of the previous clinic. There was a marked focal thickening of the descending colon:

Figure 2. Ultrasound image of the caudal abdomen showing marked thickening (10.9 mm) of the descending colon
Although neoplasia was indeed considered in the differential diagnosis, a recent publication (Müller et al JFMS 2023—Abdominal ultrasonographic findings of cats with feline infectious peritonitis—an update) had also indicated that this was amongst the increasingly varied recognised presentations for FIP. Indeed, historically it was referred to as ‘focal FIP’.
We considered the lesion to be accessible to colonoscopic biopsy, and so this was conducted, in an attempt to obtain a definitive diagnosis.
The lesion was readily accessible to endoscopic biopsy but unfortunately the results were inconclusive. Lymphoma was considered unlikely, and there was no indication of any other neoplasia.
Several years ago, we would have recommended exploratory laparotomy at this point.
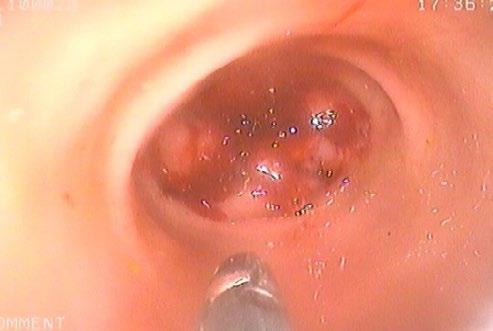
Without a definitive diagnosis, and with FIP still considered a fatal disease, excision biopsy would have been the most appropriate clinical recommendation.
However, over the last few years, the treatment of FIP has been revolutionised by highly effective antiviral medications with excellent safety profiles. After a difficult period in which these medications were only available from the black market and of dubious quality, these drugs have now become safe and readily available in most jurisdictions.
We discussed with Cha Cha’s owner the option of a therapeutic trial with the antiviral nucleoside analogue GS-441524, now known affectionately all over Hong Kong simply as ‘四四一’ (Cantonese for ‘four four one’). Keen to avoid invasive surgery at all costs, the owners readily consented.
The results were nothing less than stunning.
After weeks of relentlessly worsening dyschezia, Cha Cha’s symptoms disappeared within 2 days.
A repeat ultrasound after 10 days of treatment indicated early evidence of a reduction of the colonic lesion.
The treatment was extended.
Treatment protocols for FIP have been rapidly refined in a short space of time due to the sudden widespread availability of effective antivirals, such as GS-441524.
After an initial recommendation for 84 days of treatment, a recent study showed 42 days to be equally effective (Zuzzi-Krebitz et al Viruses 2024—Short Treatment of 42 Days with Oral GS-441524). Worthy of note is the fact that cases in this study were predominantly ‘wet’, and there is some evidence to suggest that longer courses may still be required for successful and definitive treatment of other forms (e.g. ‘dry’, focal, CNS, and/or ocular disease).
We planned to provide 42 days of treatment and serially assess the lesion by ultrasound to determine the effect of the treatment.
There were no side effects from the medications at any time.
Here are the sequential images:
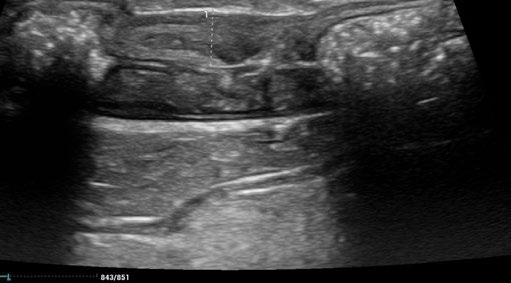

At the time of writing, 1 month after completion of a 42 day course of medication, Cha Cha is asymptomatic, with no clinical, clinical pathological, or sonographically detectable signs of disease. This response is very difficult to attribute to anything other than highly effective treatment of focal, colonic ‘dry’ FIP with GS-441524.
We have set up a schedule of serial repeat scans in the event that there is disease recurrence, and further treatment is required. We tentatively intend to re-scan at 1 month, 3 months, and then 6 months, or sooner at any time if clinical signs recur.
Despite inconclusive histopathology, we elected to use ‘441’ in this case due to accumulating evidence that this is a highly safe and effective medication, to the point that a therapeutic trial with the drug represents an absolutely
valid diagnostic test in itself, especially when other available options are expensive, invasive, or hazardous.
We submit that the landscape for managing FIP has undergone almost unrecognisable change in the last short few years, and as a profession, we should adjust our approaches accordingly…
Certainly in Hong Kong at least, the word on the street is that ‘四四一’ is close to a magical drug…
Perhaps we should allow ourselves to enjoy the remarkable power we have been given to treat this once brutal and incurable disease.
Have You Seen Any Cases Like This?
Melissa Barbuto
Tropical Queensland Cat Clinic e. info@tqcatclinic.com
C&T No.6099
I own a feline-only clinic in North QLD Australia and we run a foster kitten program. We have seen a number of orphan kittens aged between 3-5 weeks (around weaning) demonstrating acute onset (minutes to 2 hours) neurological symptoms not consistent with fading kitten syndrome.

Read here for more information and description of the cases cve.edu.au/any-cases-like-this

We would love to hear from any other vets who have seen similar cases.
Please email me at info@tqcatclinic.com and copy in cve.marketing@sydney.edu.au so that we can share your case with C&T readers.




Filgrastim in Feline Panleukopenia
A Case Series from General Practice
Sandra Hodgins
Summer Hill Village Vet (NSW)
29 Grosvenor Crescent
Summer Hill NSW 2130
e. contact@summerhillvillagevet.com
t. (02) 9797 2555
C&T No. 6100
Panleukopenia in rescue kittens remains one of the most heartbreaking presentations we see in general practice. The disease can devastate entire litters, and while we often do our best with fluids, antibiotics, antiemetics, and nursing care, survival is far from guaranteed— particularly in the underweight, under-vaccinated kittens that typically arrive via rescue.
This case series describes the use of filgrastim—a recombinant granulocyte colony-stimulating factor (G-CSF), a drug discovered in Australia by Don Metcalf at the Walter and Elisa Hall Institute—in three littermates with confirmed feline panleukopenia, treated in general practice with limited diagnostic resources but a high level of commitment from a rescue group. It’s not a magic bullet, but in this instance, it may have tipped the scales.
Case Summary
Three 14-week-old kittens arrived at Maggie’s Rescue care on 4 May 2025, with no known vaccination or parasite history. They were wormed and given an inactivated F3 vaccine on arrival by the cat co-ordinator. One kitten—’Professor Fuzzball’—presented to us within 24 hours with vomiting, diarrhoea, pale mucous membranes, and hypothermia. His body weight was 1.3 kg. A SNAP Parvo test was strongly positive, but given the recent vaccination, we also proceeded with a complete blood count (CBC) which revealed profound panleukopenia. See all the kittens CBC results here cve.edu.au/kittens-cbcs
Based on clinical presentation, the positive test, and his likely exposure during the impounding process, we diagnosed feline panleukopenia virus (FPV). Having previously used filgrastim in a dog with chemotherapyinduced neutropenia (accidental CCNU overdose), and with a recent Romanian paper [ Animals. 2024;14(24)] citing its successful use in FPV-infected cats (6 µg/kg SC for three days), we elected to treat.
Treatment Protocol
All three kittens were treated with:
– Filgrastim: 6 µg/kg SC SID
– IV fluids: 2 mL/kg/hr
– Piperacillin–tazobactam: 50 mg/kg IV BID
– Maropitant: 1 mg/kg IV SID
Vomiting in the two littermates (‘Zoomie’ and ‘Turbo’) was more severe. They also received:
– Ondansetron: 1 mg/kg IV BID
– Buprenorphine: 0.01 mg/kg IV TID
Zoomies developed worsening diarrhoea but improved rapidly on oral metronidazole (10 mg/kg BID). Turbo—just 0.9 kg—also required metronidazole after Day 6 due to persistent soft stools and perineal dermatitis.
Turbo’s recovery was complicated by persistent ataxia, possibly due to cerebellar hypoplasia or post-viral cerebellitis. A video consult with Prof. Richard Malik suggested the likely FPV-associated neuropathy was due to destruction of granule cells in the cerebellum. Turbo remains otherwise well and will be rehomed as a special needs kitten.
Outcome & Reflections
All three kittens survived and were recently desexed and are ready to be rehomed.
We were fortunate to have filgrastim on hand and a rescue willing to fund its use. As a GP vet working with rescue groups who work hard for every dollar they fundraise, I can’t always justify the use of PCR panels or referral, but clinical judgment, patient history, and a good CBC can still guide us to effective interventions.
A question I have for the cat specialists: Would earlier CBCs on the asymptomatic littermates and pre-emptive filgrastim have changed their course? We might need more data on that.
For now, this experience has given me one more tool to consider in treating a disease that too often ends in despair. The cost for treatment of each kitten with filgrastim in this case was approximately $25 and, if access can be managed, it may be a worthwhile adjunct for rescue kittens with confirmed or high-risk panleukopenia—especially when the will to try is there.

Editor’s Note
Filgrastim (a recombinant human granulocyte colonystimulating factor, (G-CSF) shows promising efficacy in treating parvoviral infections in both kittens and puppies, particularly for managing severe leukopenia/ neutropenia.
Below is a synthesis of evidence from peer-reviewed studies:
Use in Kittens with Feline Panleukopenia Virus (FPV)
Dosage and Protocol
Administered subcutaneously at 5–6 µg/kg for 3 consecutive days, with hematological evaluation on day 5.1 2
A break day (Day 4) is typically included before reassessment.1 2
Efficacy
100% survival rate observed in a study of 22 FPV-infected cats treated with Zarzio® (filgrastim).1 2
Significant improvement in WBC, neutrophils, lymphocytes, and monocytes (p < 0.01).1 2 3
Leukocytosis noted in 31.8% of cats, resolving without intervention.1 2
Side Effects
Transient reduction in RBC, hemoglobin, hematocrit, and platelets (p < 0.01).1 2
No long-term adverse effects reported.1 3
Use in Puppies with Canine Parvoviral Enteritis (CPE)
Dosage and Protocol
10 µg/kg subcutaneously for 3 days alongside standard supportive care (fluids, antibiotics).4
Efficacy
84.2% recovery rate (16/19 dogs) vs. lower rates in standard treatment alone.4
Significant increase in WBC, neutrophils, and lymphocytes by day 5 ( p < 0.001).4
Faster resolution of fever and clinical symptoms.4
Mechanism
Counters virally induced bone marrow suppression, accelerating neutrophil recovery.4
Key Considerations
– Off-Label Use: Filgrastim is not FDA-approved for veterinary use but is clinically adopted.1 ,3,4
– Timing: Early intervention is critical; administer at diagnosis of severe leukopenia. 1 3,4
– Species-Specific Responses: Cats show higher survival rates than dogs in current studies, possibly due to protocol differences or disease progression. 1,3
Filgrastim is a promising adjunct therapy for parvoviral infections, significantly improving survival and hematological recovery in both species when combined with standard supportive care. Further large-scale trials could optimize dosing and long-term safety.

References
1. Dascalu MA, Daraban Bocaneti F, Soreanu O, Tutu P, Cozma A, Morosan S, Tanase O. Filgrastim Efficiency in Cats Naturally Infected with Feline Panleukopenia Virus. Animals (Basel). 2024 Dec 11;14(24):3582. doi: 10.3390/ani14243582. PMID: 39765486; PMCID: PMC11672453.
2. Loya, K., Muhee, A., & Hussain, S. A. (2025). Effects of filgrastim on severe leucopenia associated with feline panleucopenia in cats Applied Veterinary Research , 3(2), 2024009. doi.org/10.31893/ avr.2024009
3. Ekinci, G., Tüfekçi, E., Abozaid, A.M.A., Kökkaya, S., Sayar, E., Onmaz, A.C., Çitil, M., Güneş, V., Gençay Göksu, A. and Keleş, İ., 2024. Efficacy of filgrastim in canine parvoviral enteritis accompanied by severe leukopenia. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 30(4), pp.433-443. DOI: 10.9775/kvfd.2023.31456.
4. Punia, S., Kumar, T., Agnihotri, D., and Sharma, M. (2021). A study on effect of filgrastim in severe leucopenia associated with hemorrhagic gastroenteritis in dogs. The Pharma Innovation Journal , SP-10(11), pp.868-870. Available at: thepharmajournal.com [Accessed 15 Oct. 2021].
AIM, NSAID Nephrotoxicity & Genetic Susceptibility in Cats
—A hypothesis that can be readily tested! Matthew Wun, Andrea Harvey & Richard Malik
e. m.wun@hotmail.com
e. richard.malik@sydney.edu.au
e. andrea.harvey@sydney.edu.au
C&T No.6101
What is AIM?
AIM stands for Apoptosis Inhibitor of Macrophage (also known as CD5L). It is a circulating glycoprotein produced mainly by tissue macrophages. Under normal physiological conditions, AIM binds to the IgM pentamer in serum, which stabilizes the glycoprotein and prevents its rapid renal clearance. During acute kidney injury (AKI) in most mammals, AIM dissociates from IgM, appears in the urine, and plays a critical role in renal repair.
Normal Physiology of AIM
In health, AIM contributes to homeostasis by supporting macrophage survival and regulating lipid metabolism. In the context of kidney injury, AIM is particularly important. When tubular epithelial cells undergo necrosis, intraluminal debris accumulates within the nephron. AIM facilitates the clearance of this debris by enhancing macrophage activity and promoting its excretion via the urine. This process prevents tubular obstruction, supports tubular regeneration, and helps limit the transition from AKI to chronic kidney disease (CKD).
Species Differences: Cats vs Humans
Cats are unusually susceptible to chronic kidney disease, and AIM biology might provide a possible explanation. In humans and most other mammals, AIM has relatively low affinity for IgM, allowing it to dissociate efficiently during AKI and reach the renal tubules. In cats, however, AIM binds IgM with much higher affinity, preventing its release into the urine. As a result, cats cannot clear tubular debris as efficiently, leaving them predisposed to ongoing nephron loss and progression to CKD after renal insults.
The fAIM Exon 3 Variant
Recent work from Washington State University (WSU) (Evangelista, Court, Mealey, Villarino et al., 2025) has demonstrated that many cats carry duplication(s) of exon
Renal Hypoperfusion + Tubular Epithelial Injury
Wild-type AIM (2 copies exon 3)
AIM released from IgM Debris cleared from tubules
Tubular Patency Restored Renal Function Recovers
3 in the feline AIM (fAIM) gene. This leads to production of a 4-domain AIM protein. Cats with 4 copies of exon 3 were shown to have significantly higher odds of CKD progression compared to cats with only 2 copies. This suggests that the exon 3 duplication further compromises AIM function and debris clearance.
NSAID Nephrotoxicity in Cats
Non-steroidal anti-inflammatory drugs (NSAIDs), such as meloxicam, are widely used analgesics. In cats, however, high doses (0.1-0.3mg/kg SCI) or repeated administration have been associated with AKI (The association between injectable non-steroidal anti-inflammatory drugs and acute kidney injury in dogs and cats, Veterinary Anaesthesia and Analgesia (https://www.sciencedirect. com/science/article/pii/S1467298725002223). The conventional explanation has been that prostaglandin inhibition when combined with hypotension (e.g. due to low cardiac output under anaesthesia or dehydration) results in reduced renal perfusion (failure of autoregulation). Yet not all cats are equally affected, not all cases have been associated with anesthesia or clinically apparent dehydration, and some develop catastrophic AKI while others are unaffected or get mild reversible AKI and readily recover.
Linking AIM Genetics and NSAID Susceptibility
The new understanding of fAIM variants provides a potential unifying explanation for this variability. Cats with wild-type AIM (2 copies of exon 3) may experience transient renal ischemia in association with NSAID use but can readily release AIM into the tubules to clear debris and recover renal function. In contrast, cats with exon 3 duplications (3 or 4 copies) have defective AIM activity: so, in theory, if they are exposed to NSAID-induced ischemia, they may not efficiently clear necrotic tubular debris, leading to persistent obstruction, nephron loss, and progression from AKI, leading to CKD.
Clinical and Research Implications
AIM remains bound to IgM Debris not cleared
Persistent Tubular Obstruction Progression to AKI CKD
framework for understanding why some cats develop AKI after receiving meloxicam, while most cats do not.
If you have had a cat with reversible or irreversible acute kidney injury after meloxicam or another NSAID—please write to one of the authors by e-mail so we can arrange a blood specimen or cheek swab from affected cats in order to conduct genomic testing.
We propose collecting blood samples or cheek swabs from cats with documented episodes of AKI associated in time with meloxicam administration. While our primary focus is on AKI following injectable meloxicam, cases occurring after other injectable NSAIDs (e.g. robenacoxib) and oral therapy would also be highly relevant.
Once an adequate cohort is assembled, these samples could be batch-tested at WSU for the fAIM exon 3 variant. Approximately 20% of the general cat population in the Pacific Northwest of the USA is homozygous for this variant in the WSU DNA bank.
If a substantially higher proportion is identified among a cohort of cats with NSAID-associated AKI, this would provide strong support for our hypothesis. We would then get a random population of 100 cats from Australia to see what the prevalence of the variant was in the Australian feline population.
If our hypothesis proves to be the case, it could become prudent for veterinarians to determine a cat’s AIM genotype before procedures where high doses of meloxicam are planned as part of postoperative analgesia, or for ongoing management of osteoarthritis.
Conclusion
AIM biology provides a powerful lens to understand why cats are uniquely vulnerable to CKD and NSAID nephrotoxicity. The discovery of exon 3 duplication variants might provide a cogent explanation as to why some cats are particularly prone to irreversible AKI following meloxicam exposure. These insights open the door to genetic risk stratification, more judicious use of NSAIDs, and potentially transformative new therapeutics based on recombinant AIM or AIM modulators. Such work is ongoing in Japan. NSAID Exposure (High-dose meloxicam)
This combined model—NSAID administration as the environmental trigger and AIM genetic status as the determinant of recovery—provides a compelling
The Mind of a Surgeon: Beyond the Scalpel
Bronwyn Fullagar & Chris Tan
CVE Surgery Distance Education Tutors
Follow on IG @drbronfullagar
C&T No. 6102

When most people picture a great surgeon, the image is of steady hands, perfect sutures and a complicationfree caseload. Technical skill matters, of course. But after years in theatre, we’ve learned that the scalpel is only part of the story.
What really defines a good surgeon is mindset: the way we approach uncertainty, complications, and the very human side of operating.
As neurosurgeon Henry Marsh once wrote: ‘Operating is the easy part. The hard part is the decision-making.’
The growth mindset: ‘I can’t do it… yet’
Surgery isn’t about being born with gifted hands. It’s about recognising that skill evolves with time and practice. Students often say, ‘I’ll never be a surgeon—I’m terrible with my hands.’ The truth is, everyone starts that way; driving a car feels impossible at first too, yet with practice, nearly everyone can do it.
The difference lies in how you frame the learning curve. A growth mindset turns ‘I can’t do this’ into ‘I can’t do this yet.’ That shift gives permission to make mistakes, reflect, and constantly improve.
This mindset doesn’t just shape careers—it protects mental health. Early on, many surgeons are devastated when things don’t go perfectly and a complication can feel like personal failure. With experience, we learn to care just as much about outcomes, but to ruminate less. Reflection becomes constructive: What could I have done differently? rather than, I’m not cut out for this
Are surgeons born or made?
Ask around, and you’ll hear the stereotype of the surgeon as confident, arrogant, maybe even a little ruthless. Historically, those personalities often thrived but outcomes tell a different story. The ‘cowboy’ surgeon, willing to try anything and operating completely independently, generally produces worse results than the thoughtful, reflective surgeon who works within a team.
Modern surgery is moving towards the pilot-in-a-cockpit model: confident enough to take control, but humble enough to listen to their crew. Decision-making improves when the whole team feels empowered to speak up.
That’s why surgery isn’t reserved for a narrow personality type. The real requirements are enjoying incremental improvement, being comfortable with learning in public, and holding yourself accountable to the highest standard—even when no one else is watching.
Complications, confidence, and perspective
Every surgeon remembers the sting of early complications. For some, it’s enough to avoid surgery altogether—the mental burden outweighs the enjoyment. But confidence grows when you’ve built enough skill to know that a complication doesn’t mean you’re incompetent. As we progress through our surgery careers, we become better at avoiding complications in straightforward cases, and better at managing the inevitable complications that come with taking on more advanced or unusual procedures.
Surgeons also learn to strike a balance between selfcriticism and self-compassion. We want to hold high standards—there’s always something to improve—but endless rumination isn’t helpful. The sweet spot is reflection and introspection that feeds growth without paralysing us for the next case. One helpful strategy used by professional athletes is to focus on ‘winning the next point’. When faced with an acute moment of stress or an unexpected complication during a day of surgery, being able to recover quickly and focus fully on the next step of the procedure (or the next case of the day) is a key skill to ensure we stay present in the moment. Later, it can be helpful to debrief the case with a trusted coach or mentor, when there is time to reflect on opportunities for improvement.
The art of ‘chunking’
One of the most useful mental strategies for tackling difficult or unfamiliar cases is ‘chunking.’ Rather than seeing a daunting procedure as one giant hurdle, break it down into familiar steps.
Take a splenectomy. If you can open and close an abdomen, ligate vessels, and maintain sterility, the only
new step is the specific anatomy of the spleen. Suddenly it’s not a terrifying new frontier, but a set of skills you’ve already practised with one or two new crux points.
This approach works at every level. Recently, one of us tackled a case where a six-month-old puppy had swallowed a stick that lodged in its stomach, burrowed up through the oesophagus, pierced a lung lobe, and ended up sitting beside the aorta. At first glance, it was overwhelming, but broken into chunks—gastrotomy, lung lobectomy, thoracic exploration—it became a sequence of known procedures with only a couple of truly ‘unknown’ moments to manage.
The skill of breaking a new or challenging procedure into more manageable, familiar steps helps experienced surgeons to walk into new territory without panicking.
Speed versus efficiency
New surgeons often equate speed with competence. Practices sometimes reinforce that view, measuring surgical proficiency by time taken to complete a procedure, or number of procedures completed in a given day. In reality, surgeons who have achieved mastery are efficient and effortless, not ‘fast’. Every movement is purposeful. Every instrument is the right one. They don’t flap, backtrack, or redo steps.
‘Slow is smooth, and smooth is fast.’
True mastery comes from thousands of hours of deliberate practice and there are few shortcuts. To become efficient over time, first practice the fundamentals: thorough preoperatively planning, gentle tissue handling, precise instrument selection, and avoiding wasted motion.
Feedback from an experienced surgical coach can help new surgeons to identify opportunities to improve efficiency. Speed will come naturally with experience. If speed is the primary focus too early, technical skills will plateau and surgical outcomes will be suboptimal.
Fundamentals and equipment
Great surgeons don’t neglect the basics. Instrument and tissue handling, preservation of anatomy and precise surgical planning directly affect patient outcomes.
Equipment matters. Too many vets fight their way through surgery with blunt scissors, worn-out needle drivers, or drapes the size of a tea towel. Small drapes limit movement and compromise sterility. Cheap instruments force clumsy tissue handling. The difference in cost between poor and good quality instruments is minor compared to the cost of inefficiency, tissue damage, and surgeon stress.
Create a clear visual field: position the patient on the surgical table to allow the best visualisation, clip wide, drape wide, set the table to a comfortable height, and ensure adequate lighting. This isn’t ‘demanding behaviour’—it’s about making surgery more efficient and safer for patients, and more enjoyable for the surgeon and team.
Checklists and culture
– One of the simplest but most powerful tools in surgery is the surgical safety checklist. The WHO surgical safety checklist has saved countless lives in human medicine, and veterinary adaptations are just as valuable. Cray et al (Vet Surg, 2018) found that using a checklist in a veterinary teaching hospital significantly reduced total perioperative and postoperative complications from 40.9% to 29.3%.
– An effective surgical safety checklist helps to improve patient safety by preventing medical errors that are clearly identifiable, preventable and serious in their consequences—for example, operating on the wrong limb, or unintentionally leaving a surgical gauze inside a patient.
– Checklists foster a culture of teamwork and communication and a shift towards shared accountability for ensuring patient safety. Importantly, checklists need ‘stop points’, where the team member in charge of the checklist—usually a nurse—feels empowered to pause proceedings at key steps to ensure that the list is completed. Introducing checklists isn’t always smooth. Some clinics resist them as ‘extra paperwork’ or a time-waster. The truth is, they save time by preventing errors and inefficiencies.
They also send a clear message: we’re all human, and we are all responsible for each patient’s safety.
Mentorship and culture shift
Surgeons and surgical training are undergoing a gradual cultural shift. Along with a shift towards more diversity in surgeon demographics and backgrounds, there is now an increased awareness of the importance of mental health, resilience and emotional regulation. Modern surgical training values introspection, constructive feedback and emotional as well as technical debriefs following each case. Younger surgeons and students are pushing for supportive mentorship, open conversations about complications, and kinder workplaces.
The goal is a safe space where surgeons can say, ‘This didn’t go well—what could I have done differently?’ without fear of judgment.
That cultural shift matters. Surgery is stressful enough without adding unnecessary shame or competition. If we can normalise debriefing, support, and shared reflection, we’ll not only produce better surgeons—we’ll keep them in the profession longer.
Take-home message
Being a great surgeon involves more than just advanced technical skills. A growth mindset, seeking constructive feedback, working efficiently with a team, and a commitment to lifelong learning are just as important to the development of true expertise.
The knot tying is just the beginning.
Surgery Distance Education now includes free access to A Cut Above podcast
Mastering surgery takes time, repetition, and reflection — both in and out of the theatre.
In 2026, participants in the CVE Surgery Distance Education course (cve.edu.au/surgery) will all receive free access to A Cut Above, a podcast from Dr Hubert Hiemstra and The Vet Vault team featuring A/Prof Chris Tan, Drs Bronwyn Fullagar, Mark Newman, and Justin Ward, bringing perspectives from specialists, examiners, and successful Membership candidates.
The podcast episodes have been carefully integrated to complement the learning in every Surgery DE module, helping you strengthen key concepts, reinforce understanding, and apply knowledge with confidence.
Designed for veterinarians preparing for Surgery Membership examinations, residents, and anyone eager to take their surgical skills to the next level, A Cut Above offers rich, conversational insights into the why behind surgical principles—not just the how.

Not “Naughty”, Not “Normal” Behaviour Conference + Masterclass
9 - 13 February 2026 | Early Bird rate expires 31 Dec 2025
This dynamic 5-day program covers a range of important topics that integrate behaviour as a multidisciplinary and holistic discipline.
cve.edu.au/a-cut-above

International keynote speakers Gonçalo da Graça Pereira (Portugal) and Shana Gilbert-Gregory (USA) are supported by an impressive line-up of local experts: Rachel Basa, Gabby Carter, Isabelle Resch, Jacqui Ley, Sally Nixon, Kersti Seksel, Rich Seymour & Bronwen Bollaert.

CVE Members enrol at discounted rates
Combine your members savings: Professional 20%, Part-Time Professional, Recent Graduates & Nurses 40% with the EB rate. Better in your wallet!
cve.edu.au/behaviour
Neuronal Ceroid Lipofuscinosis (NCL)
Elliot Wynne 5th year vet student Harper & Keele Veterinary School United Kingdom
e. x4p20@students.hkvets.ac.uk
C&T No. 6103
Meet the Patients
Sometimes, a case just doesn’t sit right.
When Storm and Talon, two 9-month-old littermates, both of whom were rescued as kittens, presented to practice with bizarre neurological signs including marked ataxia, intermittent seizures, tremors, ‘rearing up like meerkats’ and falling over backwards, their vet knew something was seriously amiss. Symptoms began in the siblings a mere two days apart and were progressive, prompting their owners to get them checked out.
Neurological cases presenting to practice can be challenging at the best of times for clinicians to work up; add in a set of odd presenting signs and the sibling relation, and this case proved a diagnostic challenge for all involved.

Diagnostic Workup & Differentials
History taking from the owners revealed the cats to be ‘fit and healthy’ prior to the onset of symptoms. When probed about access to potential environmental toxins this seemed unlikely. Routine haematology and biochemistry panels came back unremarkable, and the cats remained afebrile, muddying the picture further!
Given the progressive nature of the condition and lack of systemic abnormalities, an initial differential diagnoses list consisted of:
– Metabolic disorders (e.g. thiamine deficiency, hepatic encephalopathy)
– Structural abnormalities (e.g. cerebellar hypoplasia, hydrocephalus)
– Infection (e.g. toxoplasmosis, FIP, viral encephalitis)
– Toxicity (e.g. heavy metals)
– Neurodegenerative/Storage Diseases
It was at this point that the attending vet opted to trial treatment for a few of the treatable common differential diagnoses.
– Thiamine supplementation—no response
– Clindamycin for potential toxoplasmosis—no response
– Keppra (levetiracetam) for a primary seizure disorder— minimal response
Biochemistry including fasting ammonia and pre-prandial bile acids, serology (FeLV, FIV, feline coronavirus, toxoplasma), CSF analysis and MRI may be helpful to narrow differentials in similar cases.
However, with progressive neurological decline in the cats and no clear diagnosis, it was at this stage decided that euthanasia was the kindest option.
Post-Mortem Findings
Samples of both cats’ brains were submitted to Finns Pathologists with the hopes of finding a cause for the cats’ condition.
No gross changes were visible on initial assessment, and it was only at a histological level that clues started to show.
Throughout the brain, particularly the cerebellum and hippocampus, neurones were found to be filled with deeply eosinophilic-glassy material that formed circular bodies within the cytoplasm (Figure 2) Purkinje cell loss and white matter degeneration throughout the cerebellum was also noted.
It was at this stage that the pathologists began to strongly suspect some form of storage disease. Storage diseases are a group of inherited metabolic disorders, with the central idea being that affected animals are deficient in certain enzymes (often the result of a genetic mutation) leading to accumulation of metabolic products within cells of the body. The predominant two types of storage disease in animals can be split into lysosomal storage diseases and glycogen storage diseases, the associated conditions of which are detailed in Figure 3. Given the wide variety of these diseases documented in veterinary medicine, further testing was required to identify the specific type seen in Storm and Talon’s case.
Neuronal Ceroid Lipofuscinosis
Lipopigments (lipofuscin, ceroid) Dogs, cats, sheep, cattle, exotic spp.
Progressive neurological decline, seizures, blindness
GM1 Gangliosidosis GM1 Ganglioside Dogs, cats, sheep, cattle Progressive neurological decline, ataxia, dysmetria
GM2 Gangliosidosis
Fucosidosis
Mucopolysaccharidoses (I-VII)
GM2 Ganglioside Dogs, cats, pigs, exotic spp.
Fucose containing substances Dogs & cats
Mannosidosis Oligosaccharides
cats, cattle, goats
Progressive neurological decline, tremors, stiff gait, dysphagia
Progressive neurological decline, behaviour changes, ataxia, dysphagia
Stunted growth, skeletal deformities, facial dysmorphia, corneal opacity
neurological decline, ataxia, paralysis
GSD Type 1-a (Von Gierkes Disease)
GSD Type 3 (Cori’s
GSD Type 4 (Andersen’s Disease)
(Norwegian forest cats)
Depression, failure to thrive, death
Muscle tremors, gait abnormalities, death Stillbirth, flexural limb deformities, seizures GSD Type 5 (McArdle’s Disease)
a GSD and lysosomal storage disease
seizures, vision loss, ataxia, cognitive decline
Table 1. Tables highlighting the different categories and presentations of storage disorders currently documented in veterinary

Figure 2. Histopathology of neuronal tissue showing eosinophilic cytoplasmic inclusions in neurones (green arrows)
A Light in the Dark
Special stains were the next step needed to crack this case.
– Periodic Acid Schiff (PAS)—Negative for glycogen (ruled out glycogen type storage diseases)
– Luxol Fast Blue—Negative for myelin breakdown (ruled out leukodystrophies or other primary myelin disorders)
It was under fluorescence microscopy at the RVC that the light bulb moment (literally!) happened for pathologists working on the case. Green autofluorescence was detected, indicating accumulated lipofuscin within affected neurones (Figure 4). This last result led to the diagnosis of NCL, a hereditary storage disease in both cats.
What is NCL?
NCLs are autosomal recessively inherited neurological disorders that have so far been diagnosed in a wide array
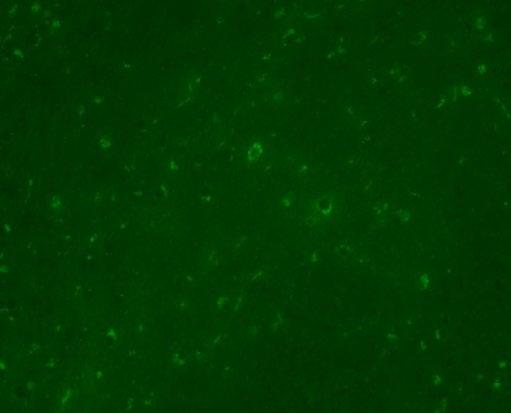
Figure 4. Autofluorescent inclusions consistent with NCL
of veterinary species including dogs, goats, a Vietnamese pot-bellied pig, a mallard duck and a cynomolgus monkey to name but a few! Little is known about the disease in cats with only a handful of cases documented in the literature. The disease causes mutations in genes involved with lysosomal function. This leads to dysfunctional lysosomes that cannot break down certain cell waste products efficiently. As a result, lipopigments start to build up inside neurones, eventually becoming toxic and leading to progressive neurological signs. The mutation leading to the disorder has been documented to vary between cats, with a defect in the CLN6 and MFSD8 genes both noted in case reports.
Clinical signs in cats can be unusual but tend to have a neurological focus. The following have been noted by clinicians in the few documented feline cases:
– disorientation
– partial and generalised seizures
– auditory +/- tactile hyperesthesia
– visual defi cits (blindness, absent menace response)
– compulsive pacing
– sporadic myoclonus
– cataplexy
– progressive weight loss
– abnormal posture
– altered behaviour
It is interesting to note that although not seen in Storm and Talon’s case, gross changes of an affected feline brain may be seen in some cases post-mortem with a study by M.D Chalkley et al (2014) noting findings in three affected cats of ‘mild to moderate, diffuse, symmetrical cerebral and cerebellar atrophy’ as well as ‘extensively flattened and narrow cortical gyri and cerebellar folia’ and ‘a dull brown discoloration of the entire cerebral
Diagnosis of feline NCL as seen in Storm and Talon’s case remains a challenge. With no known gold standard antemortem test for the condition, a definitive diagnosis is only possible postmortem. With that being said, by following a comprehensive differential diagnosis list and ruling out common things in turn, clinicians may gain confidence to suspect a presumptive diagnosis of a storage disease earlier in these cases.
A recent study at Liverpool University Small Animal Teaching Hospital exploring MRI findings of a feline NCL case has bought to light the use of MRI as a possible part of ante-mortem testing. The study did fi nd identifiable MRI changes in an affected patient and so the use of MRI may be benefi cial to support a diagnosis of NCL where there is already strong clinical suspicion. Although it should be noted that lack of MRI fi ndings would not completely rule out the condition (mutation type, early stages etc).
The future of NCL prevalence in cats is unknown, and it is only through documentation of cases and molecular investigation of the different forms and causative gene defects that pathologists will be able to build a bank of knowledge about the condition. As more mutations are identified and any trends recorded, it is pathologists’ upmost hope that targeted breeding and treatment protocols may be explored.
To any vet in practice who stumbles across this article, I would urge you to encourage owners to submit any similar case for pathological analysis. It is only by knowing about these bizarre cases that we can try and understand more about lesser-known conditions like NCL.
With special thanks to Storm and Talon’s owners, M elanie Dobromylskyj of Dobropath dobropath.com/about-1, and Pete Coleshaw from Jaffa Vets for information regarding the case

Neurological abnormalities in a South Australian Huyacaya Alpaca
Read the full article and watch the video here: cve.edu.au/alpaca and cerebellar cortices’. White C, et al (2018) also documented ‘moderate atrophy with widening of sulci’ in a feline NCL patient (Figure 5)
Figure 5. NCL affected cat brain showing macroscopic changes, White C, et al (2018)

References are available here: cve.edu.au/6103
Zoe J K Adams BVSc BHSc MaVBM MRCVS
Meadows Veterinary Centre e. meadowsvetcentre@gmail.com
Jessica Scriven DVM
Kate Werfel DVM
Zoe Hamilton e. zham6032@gmail.com
C&T No. 6104
A 3-year-old female Huacaya alpaca presented with progressive ataxia, stiffness, and proprioceptive deficits, unresponsive to initial thiamine supplementation.
This case highlights the value of clinical reasoning in neurological disease, as well as the importance of remaining vigilant to potential biosecurity risks posed by expanding feral deer populations.


Congratulations to the Distance Education Class of 2025!
For your dedication and commitment, especially when juggling study commitments with work and family, to complete this vigorous but rewarding continuing education. —CVE Tutors & Staf f
Anaesthesia & Analgesia
Tutors: Christina Dart & Eduardo Uquillas
—Fundamentals
Sandra Borg-Ryan, Australia
Callie Burnett, Australia
Kaiser Dawood, Australia
Nigel Dougherty, Australia
Christopher Godfrey, Australia
Nicole Hawken, Australia
Caroline Houston, Australia
Kate Jackson, Australia
Tuovi Joona, Australia
Derek Keeper, Australia
Craig Kelly, Australia
Phil Kowalski, Australia
Kayoko Kuroda, Australia
Alice Liaw, Singapore
Chloe McGillivray, Australia
Panida Nasongkhla, Thailand
Madeleine Nicholls, Australia
Luana Nieman, Australia
Finn Parker, Australia
Claire Phillips, Australia
Tim Portas, Australia
Siwachaya Sattapanyo, Thailand
Nuntarat Varatorn, Thailand
Thanaphan Vimooktanon, Thailand
Martine Visser, Australia
Stephanie Wong, Australia
—Compromised Patients
Jodi Bailey, South Africa
Timothy Choi, Hong Kong
Craig Kelly, Australia
Eleanor Lam, Australia
Kwei-Farn Liu, Australia
Panida Nasongkhla, Thailand
Alexandra Sharp, Australia
Philippa Simms, Australia
Olivia Thiris, Australia
Nuntarat Varatorn, Thailand
Thanaphan Vimooktanon, Thailand
—Unusual Pets
Sandra Borg-Ryan, Australia
Brett Fong, Australia
Craig Kelly, Australia
Jessica Kenworthy, Australia
Anna Muffert, Australia
Pradyumna Sathishkumaar, Australia
Jade Wastell-Stevens, Australia
Beef Production Medicine
Tutor: Paul Cusack
Katie Benson, Australia
Alan Forte, Australia
Ian Hodge, New Zealand
Chris Nel, Australia
Renee Nesser, Australia
Daniel Reiner, Australia
Josh Robinson, Australia
Rory Speirs, Australia
Behavioural Medicine
Tutors: Kersti Seksel, Jacqui Ley, Barbara Lindsay, Sally Nixon & Isabelle Resch
Emily Balch, Australia
Fauve Buckley, Australia
Ting-Yu Chen, Taiwan
Ying Cheung Andy Chiu, Hong Kong
Michelle Clinch, Australia
Lesley Hawson, Australia
Johnny He, Taiwan
Melissa Holmes, Australia
Yun Sun Hong, Hong Kong
Nathanon Hooncharoen, Thailand
Kai-Lin Huang, Taiwan
Phattanan Korjaranjit, Thailand
Fei Kuo, Taiwan
Ping Kwan, Australia
Nadia Lau, Australia
Sally Pegrum, Australia
Damien Rixon, Australia
Timothy Ruschena, Australia
Outi Tulenheimo, Finland
Brittany Ward, Australia
Cardiorespiratory Medicine
Tutor: Niek Beijerink & Mariko Yato
Alexandra Blayden, Australia
Kayniga Chodsudsaneh, Thailand
Samantha Christian, New Zealand
Pichaya Chumpholanomakhun, Thailand
Kate Johnson, Australia
Jansen Ng, Singapore
Nualsakul Noipheng, Thailand
Yanisa Pakdeenit, Thailand
Victor Poon, Hong Kong
Krorakan Thongsombat, Thailand
Jacqueline Tong, Hong Kong
Clinical Neurology
Tutors: Laurent Garosi & Simon Platt
Madeleine Baird, Australia
Manita Benyasut, Thailand
Bishal Bhattarai, Russia
Sirikanda Boonsaeng, Thailand
Yu-An Chang, Taiwan
YI AN Chen, Taiwan
Nuanrat Nateetaweesak, Thailand
Carol O’Donoghue, Australia
Preeyavart Rattanakom, Thailand
Tanakon Ruangklad, Thailand
Gentiana Seicaru, Romania
Benjamin Stewart, Australia
Clinical Pathology
Tutor: Sandra Forsyth & Karen Jackson
Carmen Chu, Australia
Heather D’Mello, Australia
Chun Ki Ho, Hong Kong
Sarah Hopf, Australia
Dermatology
Tutors: Ralf Mueller, Sonya Bettenay,
Stefan Hobi & Callum Bennie
—Advanced
Patsara Aryatawong, Thailand
Suchaya Bua-u-rai, Thailand
Tawanwad Leelaporn, Thailand
Pranpariya Lertponrat, Thailand
Phrut Phlapsawat, Thailand
Rinyarat Punpattrapong, Thailand
Maneekarn Singharat, Thailand
Eleanor Street, Australia
Namida Techamathuchartnan, Thailand
Waritha Thosaksith, Thailand
—Infectious Skin Disease
Patsara Aryatawong, Thailand
Suchaya Bua-u-rai, Thailand
Bongkoch Chonglomkrod, Thailand
Alisha Gilmore, Australia
Tawanwad Leelaporn, Thailand
Siew Nee Lim, Australia
Phrut Phlapsawat, Thailand
Rinyarat Punpattrapong, Thailand
Sasakorn Sakulpolwat, Thailand
Maneekarn Singharat, Thailand
Margaret Sung, Australia
Namida Techamathuchartnan, Thailand
—Pruritic Skin Disease
Patsara Aryatawong, Thailand
Suchaya Bua-u-rai, Thailand
Bongkoch Chonglomkrod, Thailand
Ellen James, Australia
Tawanwad Leelaporn, Thailand
Pranpariya Lertponrat, Thailand
Deborah Phillips, Australia
Phrut Phlapsawat, Thailand
Rinyarat Punpattrapong, Thailand
Rebecca Quam, United States
Nicole Rooke, Australia
Sasakorn Sakulpolwat, Thailand
Margaret Sung, Australia
Tiffany Tagros, Philippines
Qiao Yoke Tan, Australia
Namida Techamathuchartnan, Thailand
Waritha Thosaksith, Thailand
Michel Tomlinson, Australia
Diagnostic Imaging
—Abdominal
Tutor: Zoe Lenard
Jordana Capriotti, Australia
Tammy Chan, Australia
Kathryn Elliott, Australia
Rebecca Fusaro, New Zealand
Connell Healy, Australia
Chun Ki Ho, Hong Kong
Rasmeet kaur, Australia
Veronica Law, Hong Kong
Kaytlyn Playford, Australia
Isabelle Seah, Australia
Carla Werrin, Australia
—Musculoskeletal
Tutor: Xander Huizing
Emma Burpee, Australia
Jordana Capriotti, Australia
Tammy Chan, Australia
Doris Cho, Hong Kong
Rianna Dinon, Australia
Rebecca Fusaro, New Zealand
Connell Healy, Australia
Chun Ki Ho, Hong Kong
Samantha Leaver, New Zealand
Gunilla McPherson, Australia
Isabelle Seah, Australia
Johan Steyn, Australia
Maithreyi Sundaresan, Australia
Lyddy van Gyen, Australia
—Thoracic
Tutor: Belinda Hopper
Zoe Allison, Australia
Belinda Bertram, Australia
Tegan Boyd, Australia
Jordana Capriotti, Australia
Giulia Cerone, Australia
Tammy Chan, Australia
Tsz Ching Cheung, Australia
Jess Divall, Australia
Kathryn Elliott, Australia
Ameerunnisa Esmail, Singapore
Daniel Gerrard, Australia
Vanessa Grose, Australia
Chun Ki Ho, Hong Kong
Alex Hockton, Australia
Bruce Krumm, Australia
Yin Wai Leung, Australia
Rebecca Lewis, Australia
Jamie Liu, Australia
Eva Lorenz, Australia
Michelle Pacey, Australia
Carla Pendini, Australia
Cathy Pheiffer, Australia
Gabriella Robinson, Australia
Lily Simpson-Kennedy, Australia
Michael Stern, Australia
Johan Steyn, Australia
Valencia Jia Xuan Tan, New Zealand
Helen Tanzer, Australia
Amy Underwood, Australia
Katherine Wells, Australia
Emergency Medicine
Tutors: Yenny Indrawirawan & Sophia Morse
Nikita Besseling, Australia
Amy Brown, Australia
Sharlet John, Australia
Prach Maihasap, Thailand
ANIL MURARI, United States
Ngar Ting Denise Ying, Hong Kong
Feline Medicine
Tutors: Rachel Korman, Katherine
Briscoe, Michael Linton, Katie McCallum, Kerry Rolph, Ashlie Saffire, Samantha
Taylor, Jane Yu, Alison Jukes, Petra
Cerna, Sally Coggins, Ellie Mardell & Christina Maunder
Vanessa Aird, Australia
Tuva Akerjord, Norway
Michael Auld, Australia
Maria Azevedo, Portugal
Tabitha Baibos-Reyes, United States
Manuel Bajo Marijuan, Spain
Alae Bhoola, South Africa
Roberta Bocedi, UK
Jessica Brown, United States
Darren Burgess, Australia
Ester Caires, Ireland
Alexandra Carleton, Australia
Kongpop Choijinda, Thailand
Hannah Colley, United Kingdom
Fiona Coutts, United Kingdom
Steven Crowe, Ireland
Lalida Damrongchonlatee, Thailand
Joyce Doornhein, Netherlands
April Fegredo, Australia
Ines Fernandez Zapata, United Kingdom
Elise Fookes, Australia
Revathi G, Australia
Michael Garner, Germany
Kristin Gjerde, Norway
Helena Gomes, Portugal
Julie Hermansen, United States
Lindsay Hodgson, UK
Rachel Johnson, Canada
Anja Kiessler, New Zealand
Erja Knuth, Finland
Michelle Korhonen, Finland
Inga Kozlovaite, United Kingdom
Joanna Kwok, Australia
Vanessa Lambert, United Kingdom
Fiona Le Surf, Australia
Sam Leaney, UK
Waranpat Lengvilas, Thailand
Siri Linde, Sweden
Hailey MacDonald, Canada
Sarah Mitchell, Australia
M Belen Montoya Jiménez, Spain
Bastian Ness, Australia
Tiina Nikupeteri, Finland
Sofia Nóbrega, Portugal
Kathryn Orr, New Zealand
Peerakarn Pongleardrit, Thailand
Kasamyra Sainsbury, Australia
Roelie Schrijnders, Australia
Shannen Schultz, Australia
Pornkamol Siriphattarasophon, Thailand
Angie Smith, United States
Nally Tan, Singapore
Jutamas Udomkiattikul, Thailand
Charlotte Wells, Australia
Anneleen Wröbel, Belgium
Internal Medicine: A Problem Solving Approach
Tutors: Jill Maddison, Sue Bennett & Susan Carr
Alexandra Carey, Australia
Tori Carroll, Australia
Alice Chen, Australia
Alicia Cheong, Australia
Chak Hei Justin Cheung, Hong Kong
Yan Kiu Damien Cho, Hong Kong
Meagan Coolahan, Australia
Jaclyn Fung, Hong Kong
Natalia Gomez, Australia
Julien Grosmaire, Australia
Guen Hodgkiss, Australia
Lachlan Indian Manning, Australia
Melinda Khong, Australia
Ting En Khor, Singapore
Anastasia Kusumo, Australia
Yan Kei Lai, Hong Kong
Alice Lawson, Australia
Samantha Lo, Australia
Emily Sandow, Australia
Sarah Schultz, Australia
Eugenia See, Singapore
Olga Sjatkovskaja, Estonia
Yanling Tan, Singapore
Marcel Walsh, Australia
Margot Walton, Australia
Danlei Wang, Australia
Kin Yee Wong, Hong Kong
Internal Medicine: Keys to Understanding
Tutors: Darren Merrett, Steve Holloway & Jen Brown
Vanesa Bai, Australia
Emma Croser, Australia
Paige Cross, Australia
Natsuko Druery, Australia
Michele Goh, Australia
Matilda Hunter, Australia
Madhuka Nishany Kola Baddage, Australia
Santiago Neumann, Australia
Liesel Park, Australia
Jonathan Pratt, Australia
Katherine Simpson, UK
Melanee Suksamranthaweerat, Thailand
Laura Wood, Australia
Frank Yuen, Australia
Ophthalmology
Tutors: Robin Stanley & Matthew Sanders
Chanida Assawamachai, Thailand
Charlotte Cavanagh, Australia
Nadzariah Cheng-Abdullah, Malaysia
Arunluck Darunsawat, Thailand
Rachel Greenhill, Australia
Kathleen Ho, Australia
Weejarin Paphussaro Ittarat, Thailand
Ueakarn Luckkate, Thailand
Sarah Monaghan, Australia
Tanakanok Ngampongsai, Thailand
Pareeyaporn Pomjucksin, Thailand
Natchapol Preedarat, Thailand
John Rigley, Australia
Saranya Srisawat, Thailand
Sutawee Suksin, Thailand
Chayapa Udomthanakij, Thailand
Patidta Veerasai, Thailand
Kwun Yuet Wong, Hong Kong
Surgery
Tutors: Chris Tan, Mark Newman & Wendy Archipow
Celia Alman, Australia
Chris Andersen, Australia
Eloise Bartlett, Australia
Kitanjali Batliwalla, Australia
Hiu Chung Cheryl Chan, Hong Kong
Anthony Wing Chung Chow, Australia
Nicci Da silva, Australia
Charlotte Dilnutt, Australia
Amanda Dunn, Australia
Tom Harsant, New Zealand
Prabkirat Kaur, Singapore
Eleanor Lewis, New Zealand
May Lyn Lim, Malaysia
Janae Lo, Australia
Harrison Mackenzie, Australia
Chelsea Manley, Australia
Amos Nichols, Australia
Sarah Orpin, Australia
Kathleen Rebgetz, Australia
Madeleine Richard, Australia
Kristin St. John, Australia
Marena Stols, Australia
Sabrina Ying, Australia
VPOCUS: Practical Applications for GPs Distance Education
Tutors: Soren Boysen & Serge Chalhoub
Sanchi Aggarwal, Australia
Olivia Allen, Australia
Camille Armstrong, Australia
Clementine Barton, New Zealand
Elizabeth Bayne, Singapore
Cheryl (Li Wen) Cheah, Australia
Ashley Cheang, Australia
Jonathan Chee, Australia
Jessica Chen, Australia
Shirley Chow, Australia
Coty Chum, Hong Kong
Louise Clohesy, Australia
Melissa Closter, Australia
Eliza D’Arcy-Moskwa, Australia
Nina De Goede, Australia
Alisdair Eddie, Australia
Alannah Fitzgerald, Australia
Tiffany Fitzpatrick, Australia
Rebecca Fusaro, New Zealand
Kathy Gillies, Australia
Eleanor Gomersall, Australia
Tegan Hadley, Australia
Bronwen Harper, Australia
Nathan Harris, Australia
Melissa Hayde, Australia
Sarah Heineman, United States
Yan Wing Ho, Australia
Chun Ki Ho, Hong Kong
Kara Holt, Australia
Hui Ku, Australia
Crystal Mak, Australia
Kamila Osiak, Australia
John Pascall, Australia
Christine Powell, Australia
Ying Pung, Australia
Anne Quain, Australia
Natalia Ramos Reyes, Australia
Simon Roberts, Australia
Emma Shepherd, Australia
Kellie Steven, Australia
Ruby Thorn, Australia
Kanako Toda, Australia
Fumie Tokonami, Australia
Christiane Weingart, Germany
Nina Zhang, Australia
2026 Distance Education enrolments still open!
Anaesthesia & Analgesia
University of Sydney Micro-credentials
Tutors: Christina Dart & Eduardo
Uquillas
Fundamentals
Compromised Patients
Unusual Pets
Behaviour Medicine
Tutors: Kersti Seksel, Jacqui Ley, Barbara Lindsay, Sally Nixon & Isabelle Resch
Clinical Neurology
Tutors: Laurent Garosi & Simon Platt
Clinical Pathology
Tutors: Sandra Forsyth & Karen Jackson
Cardiorespiratory Medicine
Tutors: Niek Beijerink & Mariko Yata
Dermatology
Tutors: Sonya Bettenay, Stefan
Hobi, Ralf Mueller & Callum Bennie
Infectious Skin Disease
Pruritic Skin Disease
Advanced Dermatology
Diagnostic Imaging
Abdominal Imaging
Tutor: To be announced
Musculoskeletal Imaging
Tutor: Xander Huizing
Thoracic Imaging
Tutor: Belinda Hopper
Emergency Medicine
Tutors: Yenny Indrawirawan & Sophia Morse
Feline Medicine
Tutors: Katherine Briscoe, Petra Cerna, Sally Coggins, Alison Jukes, Rachel Korman, Michael Linton, Ellie Mardell, Christina Maunder, Katie McCallum, Kerry Rolph, Ashlie Saffire & Sam Taylor
Internal Medicine: Keys to Understanding
Tutors: Jen Brown, Steven Holloway & Darren Merrett
Internal Medicine: A Problem Solving Approach
Tutors: Jill Maddison, Sue Bennett & Susan Carr
Ophthalmology
Tutor: Robin Stanley & Matthew Sanders
Ruminant Nutrition
Tutor: Paul Cusack
Surgery
Tutors: Chris Tan, Wendy Archipow, Bronwyn Fullagar & James Crowley
Practical Oncology
Tutor: Katrina Cheng
VPOCUS: Practical Applications for GPs
Tutors: Soren Boysen & Serge
Chalhoub
cve.edu.au/distance-education
Winner
Entitled to a CVE$300 voucher
Unusual Fungal Infection in a Cat
Madison Newton
Tropical Vets
184 Queen Street, Ayr QLD 4807
e. Ayr@TropicalVets.com.au
t. 07 4783 2055
C&T No. 6105
‘The Big Boy’ is a 5-year-old male neutered Ragdoll cat who presented to me as a second opinion after a 6-month history of pigmented skin lesions which appeared and then grew, never resolving. They were originally thought to be cat fight wounds by another vet clinic and were treated with meloxicam and Amoxyclav.
The lesions then progressed to being multifocal over most of the body, including pinna edges, nares, trunk and abdomen, down the tail, on the paw pads …etc.
On presentation the nasal lesions had been affecting his upper respiratory tract for about a month with the owners noticing noisy breathing and sneezing. He did not open mouth breathe, was still eating well and had no nasal discharge. He was FIV / FeLV negative and had a relatively unremarkable CBC / Biochem / UA / T4.
The lesions were biopsied with a disposable skin biopsy punch and submitted for histopathological examination and mycological culture.
Diagnosis: Severe Chronic nodular pyogranulomatous dermatitis with intralesional pigmented fungi
Cutaneous infection with fungal organisms is often opportunistic and usually the result of traumatic implantation or wound contamination.
Disseminated infection is uncommon and when seen is often associated with immune dysfunction. In animals developing this condition while receiving immunosuppressive therapy, tapering and discontinuing these medications is often necessary for successful treatment of the fungal infection. In some cases, complete excision of solitary or multifocal cutaneous lesions may be curative; however, recurrence at the same or new sites is fairly common, sometimes months or even years following initial excision.
In this instance, the disseminated nature of lesions is most likely the result of a fight in which there were

1A

1B

1C
numerous bites and scratch injuries, which enabled impregnated fungal elements to ‘set up shop’ and develop into pigmented lesions. The melanised nature of the lesions was a big tip off for the presence of a dematiaceous (pigmented) fungal pathogen, with melanin elaborated by the fungus giving rise to the pigmented nature of the lesions.
PCR testing
Unfortunately PCR was subsequently unable to be run on the submitted samples.
Fungal culture
Exophiala species isolated.
Exophiala spp. is a genus of melanised fungi which can be isolated from plant debris and soil and has been known to cause mycetoma and phaeohyphomycosis in humans and in rare cases, animals.
Most infections are in immunocompromised patients and include subcutaneous cystic lesions ,endocarditis, and neurological disease.
In dogs, this fungus has been isolated in cases of peritonitis, keratitis, osteomyelitis and dermatomycosis. This result should be correlated with any cytology or histopathology on the tissue to correctly interpret the relevance of this organism.
Treatment
Big Boy commenced Posaconazole 100mg sustained release tablets at a dose of one half a tablet every second day, with a plan to do a trough level test at 3 weeks after commencing therapy. He did not have any adverse effects from the treatment or any improvement in the first week.

21/5/2025
Approx 2 months recheck The Big Boy was going very well. Most lesions were greatly improved or resolved. He still had some lesions on his tail and paws, but his face was much better. His respiratory signs had resolved, and he did not suffer any adverse effects from the medication.
Unfortunately, in the lead up to his recheck, where we were going to test posaconazole trough testing, the owners missed three medication doses due to travel / pet sitter unable to find the cat. Hence, the trough level testing was delayed.
10/6/25
The Big Boy's lesions had completely resolved, and we planned to continue his posaconazole for 2 further months post lesion resolution.
Big Boy's trough posaconazole levels were measured at 6659 µg/L (ideally should be between 1,500 and 5,000), so we reduced his dosing to Mondays, Wednesdays and Fridays, and planned to re-test in a few weeks.



Discussion
Exophiala spp. are opportunistic, melanized fungi capable of causing subcutaneous and, less commonly, systemic infections in cats. These infections are rare but clinically significant, often presenting as chronic cutaneous or subcutaneous nodules. Management has historically required an integrated approach, including surgical excision and systemic antifungal therapy.
Exophiala species are found ubiquitously in the environment, particularly in soil, decaying vegetation, and damp habitats. While infections are more frequently reported in humans, Exophiala spp are recognized as emerging pathogens in veterinary medicine, particularly in cats, where they cause a form of phaeohyphomycosis. Due to their infrequent occurrence and nonspecific clinical appearance, these infections can be underdiagnosed or misdiagnosed, often mimicking neoplasia, abscesses, or sterile granulomas.
Feline infections typically arise from traumatic inoculation through the skin, such as punctures, abrasions, or insect bites. Once established, Exophiala spp. induce a chronic granulomatous inflammatory response. Lesions often appear as firm, subcutaneous nodules, sometimes ulcerated or accompanied by draining tracts. While cutaneous and subcutaneous infections predominate, systemic dissemination may occur in immunocompromised animals or those with delayed diagnoses.
Diagnosis relies on a combination of clinical suspicion, histopathology, and mycological testing:
– Cytology and histopathology: reveal pigmented, septate hyphae or yeast-like cells within granulomatous inflammation. The pigmentation is often best seen in unstained sections or smears.
– Fungal culture: confirms the diagnosis and enables speciation; Exophiala spp. form slow-growing, darkcoloured colonies. They often grow better at room temperature or 30°C rather than 37°C.
– Molecular diagnostics (PCR/sequencing): is increasingly used for precise species identification and antifungal susceptibility profiling, unfortunately in The Big Boy's case, the sample was not suitable.
Treatment
Strategies
Surgical Management
Wide surgical excision of localised lesions is the mainstay of therapy in many instances. Incomplete resection is associated with a high risk of recurrence. Histologic margin assessment is recommended when feasible.
Antifungal Therapy
Systemic antifungal agents are often used adjunctively and are essential in disseminated or inoperable cases.
The following agents are most commonly employed:
–
–
Itraconazole (10 mg/kg PO q24h): First-line antifungal due to cost and availability. Variable efficacy reported; requires liver enzyme monitoring. Never use compounded itraconazole; use human generics of veterinary formulations, or the human formulation Lozanoc (but at only 5 mg/kg/day).
Voriconazole (2.5–5 mg/kg PO q12h): Broader spectrum and greater activity against dematiaceous fungi. Limited use due to neurotoxicity concerns. Animals on this drug need to keep out of the sun. Can be effective, but therapeutic index is narrow and therapeutic drug monitoring strongly recommend. Rarely used in cats because it’s so tricky.
– Posaconazole is much safer and generally tolerated much better in cats, dogs and people. Use the sustained release formulation at a dose rate of approximately 3-4mg/kg once a day with food. The suspension is far, far more costly.
–
Terbinafine (10–20 mg/kg PO q12- 24h): May be used in combination with azoles; good safety profile but limited standalone efficacy.
– Amphotericin B can be used in refractory cases; focal lesions are often treated effectively by intralesional therapy with amphotericin B.
Role of Posaconazole
Posaconazole is a second-generation triazole with broad-spectrum activity and superior efficacy against many dematiaceous fungi, including Exophiala spp. Its advantages include:
–
Potent in vitro activity: Lower minimum inhibitory concentrations (MICs) compared to itraconazole and fluconazole. Better tolerated, higher therapeutic index, no photosensitivity, and generally very well tolerated.
– Clinical use: Recommended for refractory, disseminated, or severe infections where other azoles have failed.
– Dosing in cats: Off-label, with reported doses ranging from 3-4 mg/kg of the sustained release tablet given every day or 5 mg/kg given every other day. Delayedrelease tablets offer more consistent absorption than oral suspensions.
– Tolerance: Generally well tolerated; monitor for hepatic enzyme elevations and gastrointestinal upset.
Prognosis
With a dedicated owner, these infections generally respond very well to either medical therapy or a combination of surgery and medicine. Long treatment duration is common, and we usually recommend therapy extend 2 months past complete resolution of the lesions to minimise risk of recurrence.
The Use of Photobiomodulation Therapy in Wound Management
Kathy Le
Sugarloaf Animal Hospital
67 Carrington Street
West Wallsend NSW 2268
e. kathy@sugarloafanimalhospital.com.au
C&T No. 6106
Initial presentation
A 4.5-year-old female neutered Australian Kelpie presented to the clinic for evaluation of periorbital swelling. This had reportedly occurred three weeks earlier but subsided with no treatment or intervention. The patient frequently spent time outside, and the family did have another dog. The dogs would occasionally fight. The patient had no prior health conditions and was up to date with broad spectrum parasite preventatives.
Physical examination
On examination, the dog was bright and alert, although had quite a nervous temperament and some aspects of examination were difficult. There was moderately firm swelling around the periorbital region and the dog was unreactive to palpation; there were no puncture wounds or superficial lesions noted. Examination of the eye revealed no abnormalities. Deep examination of the ear canal was not possible due to temperament. A FNA of the lesion was not performed at this time. The remainder of the physical exam was unremarkable.
Differential diagnoses for the swelling included an inflammatory lesion, hypersensitivity (e.g. insect bite), trauma, abscessation.
Initial treatment
The patient was treated with an antihistamine and anti-inflammatory medication but was advised that further investigation would be required if there was no improvement or worsening of the condition.
The patient re-presented 5 days later as the lesion had significantly worsened over that period. By this stage, the lesion had declared itself as an abscess which was occupying approximately 40% of the region overlying the left cranium.
Surgical treatment
The patient was admitted for surgical debridement under general anaesthesia. No foreign body was found, and a tissue biopsy was also collected at the time. Due to the large nature of the abscess and the necrotic skin present, primary closure of the wound was not possible. Histopathology (Vetnostics) results revealed marked neutrophilic to mixed cellulitis and myositis. Other diagnostics performed in-house at the time including haematology and biochemistry revealed a mild hyperglobulinemia consistent with inflammation, but were otherwise unremarkable. Topical therapeutics were applied following the procedure which included the use of silver sulfadiazine cream (Flamazine) and Stratamed®, and a light head bandage. The patient was discharged with amoxicillin clavulanate, meloxicam, and paracetamol post operatively for 5-7 days.
Additional therapies
Due to the large wound deficit remaining, the wound was treated as an open wound. To assist with healing, it was elected to use photobiomodulation therapy as a noninvasive treatment option with several benefits including pain relief, improved tissue regeneration, antimicrobial properties, and faster wound healing. The first session was performed 24 hours following the surgical procedure to avoid stimulation of any further bleeding immediately post operatively.
Photobiomodulation therapy was performed using the Vets1laser Veterinary Therapy Laser System machine. (Model VETS1-15FJ, Input ~100-240V). The settings employed were those pre-programmed for acute wound management (1.8W, total energy 171J, treatment time total 1 min 27 sec). Treatment was repeated every 24 hours for 7 sessions, with topical application of silver sulfadiazine cream, Stratamed® and light bandage following laser therapy. These treatments were then performed every 2-3 days for an additional 5 sessions until wound healing was complete.
Discussion
Photobiomodulation therapy (laser therapy), was found to reduce healing time for management of a large cutaneous lesion in this patient when used in conjunction with topical therapies. Oral medications were not extended past their initial course following surgical debridement. The use of laser therapy was minimally invasive, quick to perform, non-painful and well tolerated by the patient despite a slightly nervous temperament.


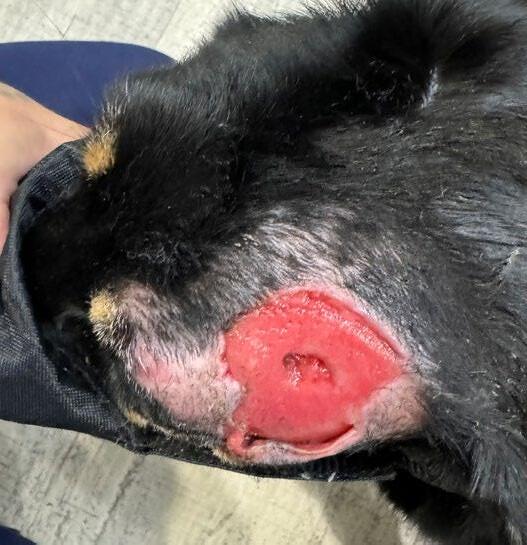
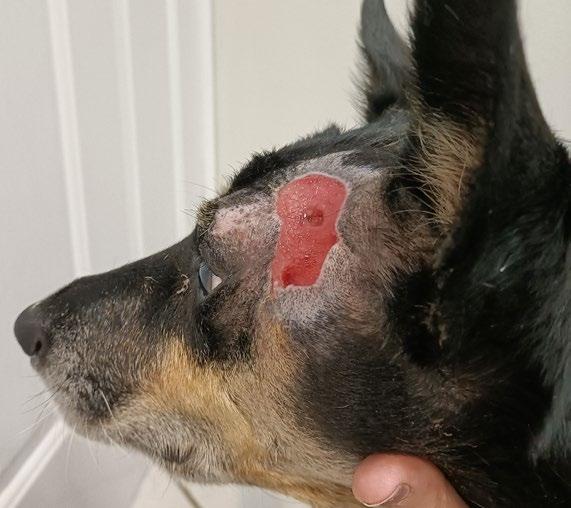
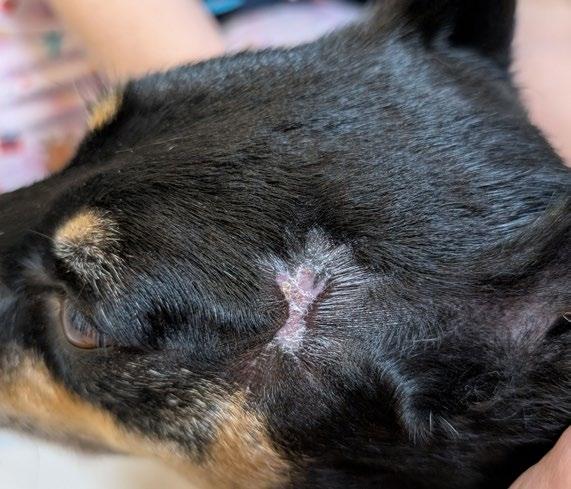
Figure 1. Surgical debridement of the abscess (Day 0)
Figure 2. Day 1 of laser therapy
Figure 3. Day 7 of laser therapy
Figure 4. Day 9 of laser therapy
Figure 5. Approximately one month after surgical debridement.
Entropion Surgery
Kim Kendall BVSc MANZCVSc in Cat Medicine
Examiner in Cat Medicine MANZCVS in Animal Behaviour
The Chatswood Cat Palace
Unit 18 / 30 - 32 Barcoo St Roseville NSW 2069
m. 0400 756 331
w. thecatpalace.com.au
C&T No. 6107
I have had a run of entropions to fix recently (in cats). This technique was demonstrated to me last century by Jeff Smith and I have used it successfully ever since. I use Allis Tissue Forceps in various other ways (see long ago C&T No. 4331, April 2001, Unauthorised Use of Allis Tissue Forceps), much like screwdrivers have several uses other than undoing screws.
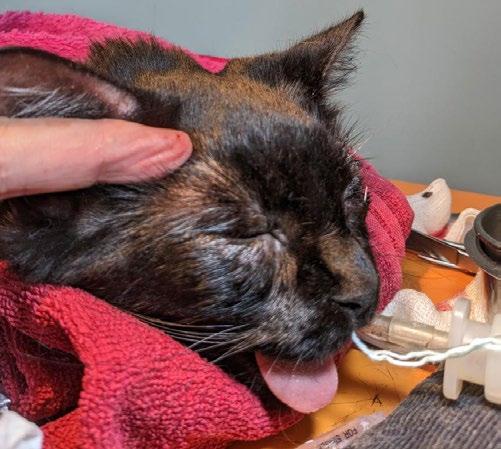


3 1 2

4
Figure 4. Then IP fluid— I am a big fan of this technique. IV fluids are gold standard, particularly if using gaseous anaesthetic, to keep blood pressure up. However, you will drop the cat’s temperature with fluids (no matter which way they get administered) and at least with IP you are not going to ‘break their heart’ with fluid overload in a cooled down cat.
Figure 2. In addition, under the towel the cat lies on is a SnuggleSafe® microwavable heat pad. Keeping them warm is the name of the game.
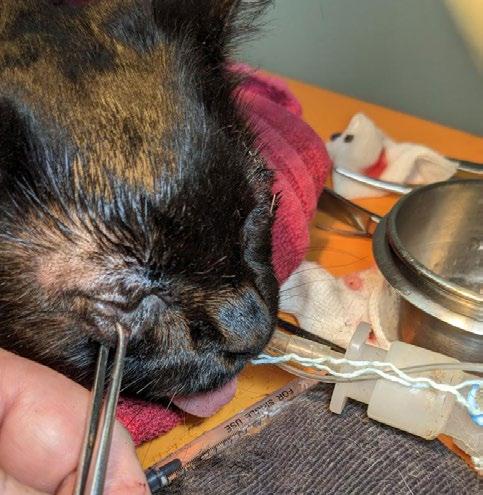
Figure 5. Next is the Allis Tissue Forceps being used to delineate the area to be removed. It also means you can ‘test’ whether the eyelids will close afterwards.
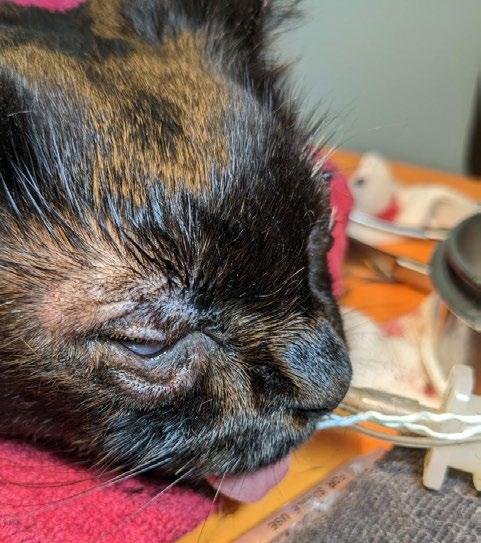
6. It is a bit hard to photograph, but you can see the ridge produced by the forceps in the next photo
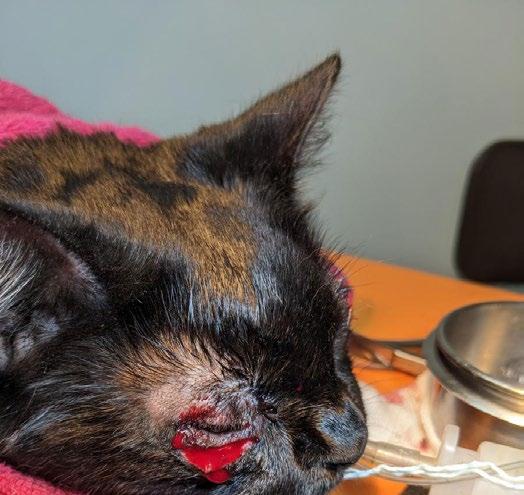

8. Suture however takes your fancy. Once finished, atipamezole reversing agent at half (or even 1/4) of the medetomidine volume.
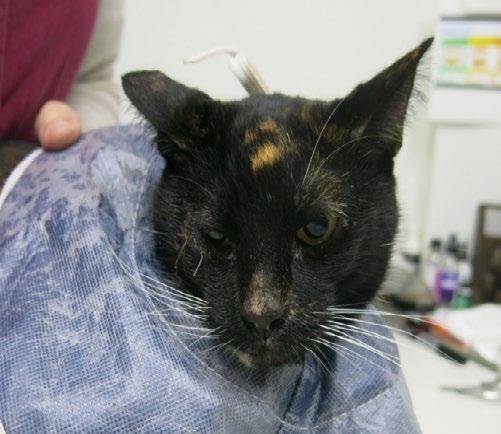
Figure 9. An E-collar and some meloxicam once eating and a few days later—final photo—take stitches out at about 4 - 5 days

Figure 10. Here is a variation on the theme for entropion–I used some EMLA cream on the skin below the eye, then pinched (with Allis Forceps per previous photo) and stapled. This cat’s cornea is badly scarred with moderate enophthalmos, and the eyelid is very thickened, and I have already removed a fair crescent. I am going to see how it goes with just the staples, but I may end up enucleating. She does open her left eye when at home.
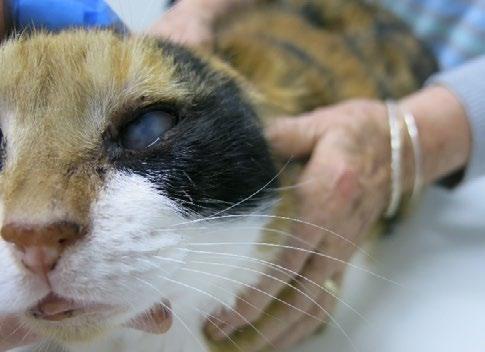

Figures 11 A & B. This is the result of the stapling of the lower lid to correct entropion. These photos were taken 4 weeks after the staples were put in. So far so good—no return of the rolled in eyelid.

Read C&T No. 4331 cve.edu.au/cnt-4331
Comment courtesy of Jeff Smith
Eye Clinic For Animals
Vets & Veterinary Surgeons
Artarmon, NSW 2064
e. info@eca-aus.com.au
I enjoyed Kim’s article and of course appreciated her kind reference to me in the intro.
4 things I would have said in my surgical description:
1. Apply the Allis Forceps closer to lid margin leaving 2-3 mm to place the single interrupted sutures
2. Make sure the skin excision parallels the curved line of the eyelid
3. The width of the excision is dictated by the degree of eyelid in-turning
4. My preferred suture material is 6/0 PDS (absorbable)
QUESTION
Seen an intriguing case recently?
Send us a high resolution image/s to pose the Q. and an article on how you treated it to appear in the following issue.
A Case of Eosinophilic Granuloma Complex in a Domestic Shorthair Cat
Answer to C&T No. 6087
How Would You Treat This Case?
Jorid Nordaker
Clinical Veterinary Registrar
Caitlin Lowe, Final year vet student
University of Sydney Veterinary Teaching Hospital
e. Jorid.nordaker@sydney.edu.au
e. clow8900@uni.sydney.edu.au
C&T No. 6108
Introduction
Eosinophilic granuloma complex (EGC) encompasses a spectrum of feline dermatoses characterised by eosinophilic inflammation. It is commonly associated with hypersensitivity disorders such as flea allergy dermatitis, food hypersensitivity, and atopic dermatitis.
Lesions may manifest as eosinophilic plaques, indolent ulcers, or eosinophilic granulomas, either individually or concurrently. Management of EGC can be difficult, as lesions often prove recurrent, chronic, and costly to treat.
This report describes a case of a 4-year-old neutered male domestic shorthair cat with plantar granulomas and oral ulceration, treated conservatively under significant financial constraints.
Case History
Mortimer, a 4-year-old neutered male domestic shorthair, was presented with a long-standing dermatological problem that had intermittently recurred
ANSWER
The best and most complete answer to any Questions posed in an issue wins a CVE$300 Voucher.
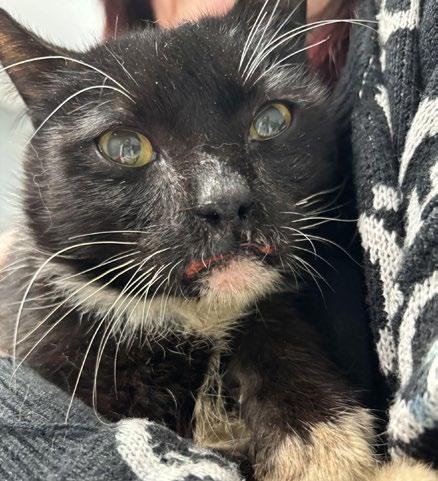
over several years. Prior consultations at a community veterinary service had resulted in medication being dispensed, although detailed records were unavailable. Mortimer resided indoors with access to a balcony and was occasionally involved in fights with cohabiting cats. He was up to date with core vaccinations but was not receiving regular parasite prophylaxis. His diet consisted primarily of commercial dry food. The owner reported a normal appetite.
On examination, Mortimer’s coat appeared unkempt, and both plantar surfaces bore raised, crusted lesions. Despite the evident discomfort, he remained calm and tolerant of handling.
Clinical Examination
Mortimer weighed 5.0 kg and was in good body condition. His coat was poorly maintained. Dermatological assessment revealed bilateral plantar lesions consisting of raised, alopecic, smooth, irregular dermal masses with marked crusting.
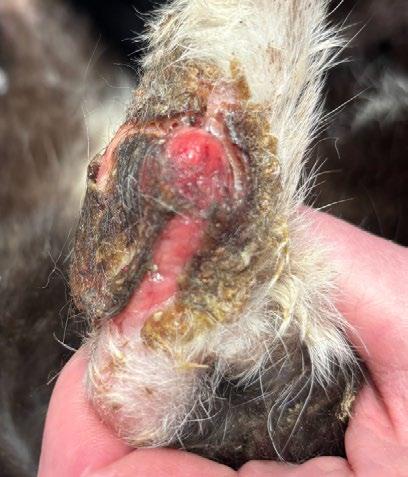
Oral examination identified indolent ulcers on the upper lip as well as multifocal ulceration of the hard palate and tongue. Bilateral submandibular lymphadenomegaly was noted, but the remainder of the physical examination was unremarkable.
Diagnostic Assessment
Cytological examination of impression smears demonstrated a mixed inflammatory population including eosinophils, neutrophils, and bacteria. Retroviral

testing was negative for both FIV and FeLV. Haematology and serum biochemistry revealed a moderate hypochromic, microcytic, non-regenerative anaemia, mild hypoalbuminaemia (A:G ratio 0.60 with high-normal globulins), mild hyperbilirubinaemia, and a moderate eosinophilia. Repeat testing after 21 days of treatment indicated partial improvement in these abnormalities.
Assessment
The clinical findings were consistent with several components of the eosinophilic granuloma complex, specifically eosinophilic granulomas affecting the plantar surfaces and indolent ulcers on the lips and likely collagenolytic granulomas on the palate. Differential diagnoses for underlying allergy included flea allergy dermatitis, insect bite hypersensitivity, cutaneous adverse food reaction, atopic dermatitis, feline herpesvirus-associated ulceration, and squamous cell carcinoma. The haematological and biochemical abnormalities were interpreted as secondary to chronic inflammation. The patient’s poor grooming was attributed to underlying discomfort, especially within the oral cavity.
Therapeutic Management
A pragmatic treatment plan was developed to balance clinical welfare with financial limitations.
Antimicrobial therapy: Cefovecin sodium (8 mg/kg SC) was administered on Days 0 and 14, providing 28 days of systemic antibiotic coverage. This long-acting cephalosporin was chosen due to compliance concerns, the owner’s inability to medicate orally, and the presence of painful oral lesions. Culture and susceptibility testing was not performed due to cost constraints, although this would have been preferable.
Corticosteroid therapy: Methylprednisolone acetate (10 mg IM) was given at presentation, with transition to oral prednisolone (1 mg/kg PO q24h) initiated on Day 21. Risks of corticosteroid-associated side effects were discussed with the owner. Longer-term alternatives such as ciclosporin (Atopica®) were considered but were not financially feasible at this stage.
Analgesia: Pregabalin (2 mg/kg PO q12h) was prescribed for chronic pain management.
Topical therapy: Silver sulfadiazine (Flamazine®) cream was applied to the plantar lesions after saline cleaning, accompanied by use of a soft Elizabethan collar.
Parasite control: Selamectin-sarolaner (Revolution Plus®) was administered, with recommendations for strict flea control in all in-contact cats.
Dietary management: Transition to a hydrolysed protein diet (Anallergenic®) was initiated, with an eight-week elimination trial planned to investigate food hypersensitivity.
Outcome and Follow-Up
By Day 21, Mortimer’s coat condition had improved, and his granulomas appeared ‘cleaner’, although significant lesions persisted. The next phase of management included repeat cytology, consideration of fine needle aspirates or biopsy, and Wood’s lamp examination. Assessment of prednisolone response with gradual tapering was planned, alongside the introduction of antihistamines. Funding support was being sought to facilitate progression to ciclosporin and clindamycin or referral to a specialist dermatologist.
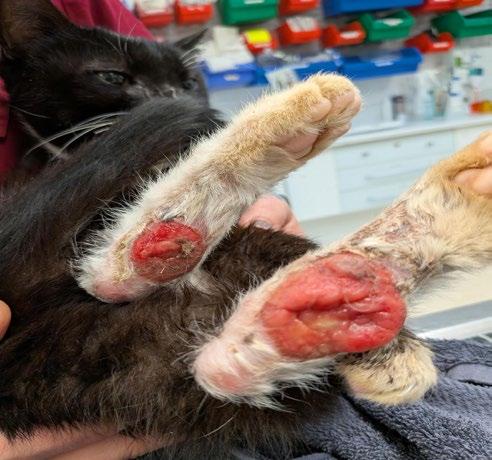
Figure 4. Mortimer’s lesions on day 21—the lesions are cleaner and his coat healthier but lesions remain significant
Discussion
This case illustrates the complexity of managing feline eosinophilic granuloma complex when financial resources are limited. Optimal investigation would have included histopathology and fungal culture to exclude neoplasia or infectious aetiologies, as well as culture and susceptibility testing to inform antimicrobial selection. In an ideal setting, topical antimicrobials and safer longterm immunomodulatory therapies could have been implemented earlier.
Systemic corticosteroids remain the cornerstone of EGC management, but alternative therapies such as ciclosporin are increasingly utilised and supported by
controlled trials. Additional emerging treatments include JAK inhibitors, allergen-specific immunotherapy, and monoclonal antibody therapies, although robust felinespecific data remain limited. Dietary trials and rigorous flea control continue to underpin effective management strategies.
This case highlights the importance of tailoring therapy to both the clinical and financial context, while maintaining a focus on patient welfare and quality of life.
Acknowledgements
The authors gratefully acknowledge the support of the City of Sydney Council’s Animals in Need program, which provided financial assistance for Mortimer’s care. We also thank colleagues at UVTHS for their contributions to clinical management and discussion of this case.
Best Answer
C&T No. 6087 (Sept 2025) How Would You Treat This Case?
Nurazlin Binti Che Mat ariffin
Vet Partners Malaysia e. nurazlinchemat@gmail.com
The patient is presented with bilateral raised, ulcerative masses on proximo-caudal metatarsals surrounding a yellowish central core. At the same time, there is a well-demarcated, severe, right upper lip ulcer consisting of erythematous tissue and a central area of brownish erosion or necrosis. This is loosely termed as rodent ulcer. Upon cytologic examination, impression smear of the ulcerated mass on both metatarsals revealed the presence of many eosinophils.
The main differential in such cases would be Eosinophilic granuloma complex (EGC). It is complex of type 1 and 4 hypersensitivity. The cat is first exposed to an allergen such as flea antigen. Langerhans cells and dendritic cells process the allergen and present it to naive T cells in lymph nodes. This activates and sensitizes memory T cells specific to that allergen. On subsequent exposure, these memory T cells recognize the allergen and release inflammatory cytokines, which recruit and activate macrophages, eosinophils, and other inflammatory cells to the skin or mucosa. Activated macrophages and eosinophils release enzymes and toxic mediators that damage local tissues, forming granulomatous inflammation characteristic of eosinophilic granuloma
complex. On presentation, feline eosinophilic granuloma complex has several manifestations including indolent ulcers, eosinophilic plaques, and eosinophilic granulomas. Treatment options may include antiinflammatory or immunosuppressive therapy. For instance, prednisolone at 1mg/kg to 2mg/kg per os daily for 1 month may be indicated.
Methylprednisolone may be another option if the patient does not tolerate oral prednisolone. Where it Prednisolone can be administered at 1mg/kg daily subcutaneously or 5-10mg/kg every 3-4 weeks subcutaneously. In cases that are resistant to the effects of prednisolone, cyclosporine at 5-10mg/kg once daily assist in selectively inhibiting the activation of T-lymphocytes. Cyclosporine blocks calcineurin, a key enzyme involved in T-cell activation. By suppressing T-cell activity, cyclosporine reduces the release of inflammatory cytokines which decreases the recruitment and activation of eosinophils and other inflammatory cells involved in the disease.
Supportive treatments may include antibiotics depending on the result of bacterial culture and antimicrobial sensitivity test. Imperative antimicrobial sensitivity testing must be performed in all cases to ensure prudent use of antibiotics and should be started if secondary bacterial infection is diagnosed. Wound care is also compulsory to reduce contamination of ulcers and speed up the healing process. Daily chlorhexidine wash on metatarsal ulcers with post-cleaning application using topical silver sulfadiazine or povidone iodine may be indicated. Daily topical cream for ulcerated lips using sodium hyaluronate 0.01% may be opted as adjunct therapy.
What other diagnostic tests would you do?
Further diagnostic measures should be attempted prior to treatment, including biopsy to confirm the presence of eosinophilic granuloma complex and to rule out squamous cell carcinoma and deep cutaneous fungal infection. On biopsy, eosinophilic granuloma complex usually presents with dermis and subcutaneous eosinophilic infiltration surrounded by degenerated collagen fibres and eosinophil granules.
Mixed inflammatory infiltrate may be present, depending on chronicity and secondary bacterial infection comprising of eosinophils, macrophages, lymphocytes, mast cells and bacteria. Bacterial and fungal cultures should be performed to rule out bacterial and fungal infection. There are reports of deep cutaneous infections that can present similarly by dermatophytes, histoplasma and cryptococcus. Depending on the sample
collected from the lesion, poor sampling may yield a false negative result on cytology. Therefore, fungal culture would be a more accurate diagnostic method.
In cats, chronic inflammation from EGC can lead to non-regenerative anaemia. Cytokines such as IL-6, TNF- α , and IL-1 are released during inflammation. These stimulate production of hepcidin, a liver peptide that decreases intestinal iron absorption and traps iron inside macrophages. Even though total body iron is adequate, it becomes unavailable to the bone marrow, so red blood cell production is reduced causing normocytic normochromic anaemia. The only dilemma in this case is hyperbilirubinemia is also present in this patient. Therefore, it is necessary for a Coombs test or saline agglutination test to be performed to rule out immune mediated haemolytic anaemia. A blood smear should be performed to screen for presence of hemoparasites that may infected red blood cells, subsequently causing red cell destruction and contributing to hyperbilirubinemia. Should haemoparasites such as mycoplasma be present in blood smear findings, this increases the likelihood that the ECG may be the result of flea bites.
Editor’s Note
These lesions are severe and located in an atypical region for EGC-associated granulomas.
The most appropriate terminology for these large eosinophilic lesions on the cat’s limb is Feline Eosinophilic Granuloma Complex (EGC). This umbrella term encompasses three patterns: eosinophilic plaques, indolent (rodent) ulcers, and eosinophilic granulomas (the latter sometimes historically called collagenolytic or linear granulomas). The term ‘collagenolytic’ reflects the characteristic histologic finding of collagen breakdown caused by eosinophil degranulation. Therefore, eosinophilic granuloma within the EGC framework is the most current and accurate diagnosis.
Pathogenesis and Triggers
EGC lesions are not infectious in origin. They represent an allergic or hypersensitivity reaction driven by eosinophilic inflammation. Common triggers include:
– Flea allergy dermatitis (very common)
– Mosquito or other insect hypersensitivity
– Food hypersensitivity
– Environmental allergens (e.g., dust mites, pollens)
– Less commonly: underlying genetic predisposition, immune dysregulation or stress
Therapeutic Approach
Management typically involves:
1. Systemic glucocorticoids, usually prednisolone, as initial immunosuppression.
2. Steroid-sparing immunomodulatory therapy such as cyclosporine (or tacrolimus) to improve long-term control and reduce relapse.
3. Adjuncts such as antihistamines or topical therapies for symptomatic relief.
4. Rigorous flea control and dietary/environmental investigation to identify allergens.
5. In refractory or severe disease, combined immunosuppressive protocols are often required.
Specific Recommendations for This Cat
Given the bulkiness and chronicity of the lesions, aggressive combination therapy is warranted. If finances permitted:
– I would start by surgically debulking the proliferative granulation tissue. This is not cosmetic surgery—the goal is simply to remove the excessive mass and achieve haemostasis.
– If systemic steroids are required (and this cat has already received substantial doses), I would use prednisolone at ~2 mg/kg once daily with a judicious taper.
– Introduce cyclosporine at 4–5 mg/kg BID, ideally using therapeutic drug monitoring to optimise trough concentrations. The liquid formulations (Atopica/ Neoral) allow the most accurate dosing.
– Because immunosuppressed cats are at increased risk of toxoplasmosis, I routinely co-administer clindamycin 7.5–10 mg/kg BID during induction.
– If improvement is incomplete, add topical tacrolimus (0.1%) to the affected areas.
– Ensure excellent flea control using a top-tier isoxazoline product.
– Feed a high-quality wet or cooked meat-based diet, avoiding dry extruded foods, which may perpetuate inflammation in some hypersensitive cats.
Creating a Cat Friendly Feline-Only Ward
Nikki Hammond
ISFMCertFN ISFMDipFN RVN
C&T No. 6109


Delivering high-quality feline care requires an in-depth understanding of feline behaviour and emotional wellbeing. Even in the most advanced clinical environments, stress and fear can profoundly influence a cat’s physiological parameters, diagnostic results, and response to treatment. Recognising and managing these factors is central to achieving optimal outcomes in feline medicine.
In this article, Nikki Hammond discusses strategies for recognising and minimising stress in hospitalised cats. Topics include recognising subtle behavioural cues, adapting handling approaches, optimising ward design, and providing appropriate environmental resources.

Read the full article in cve.edu.au/cat-friendly-ward
The International Cat Care Veterinary Society (formerly ISFM) is the veterinary membership division of pioneering cat welfare charity International Cat Care (iCatCare), bringing together a global community of veterinary professionals dedicated to improving the lives of cats worldwide. Trusted by vets and nurses, it provides professional development and CPD through access to expert knowledge resources, including the Journal of Feline Medicine and Surgery (JFMS). The iCatCare website is also a trusted resource of information and guidance for veterinary professionals, cat owners and caregivers.
iCatCare Research Roundup
Welcome to Research Roundup where we bring you summaries of the latest feline research. This month, we review two studies on analgesia for neutering, which emphasise the benefit of locoregional techniques. We also include a study looking at the challenges in diagnosing chronic enteropathy in cats and a fascinating report on CT findings in pyothorax.

Read here: cve.edu.au/rr-december-25
PerSPective no. 167
All About Goannas
Robert Johnson AM BVSc MANZCVS (Feline Medicine) CertZooMed BA FAVA
Note: We’re indebted to Robert who wrote this C&T article at our request to celebrate the CVE’s motif—the goanna—in our 60th anniversary year.
FOR VETERINARY EDU
PROUD MEMBER
Tom Hungerford’s Goanna Track
Veterinarians need to identify an area of interest and devote time to it, listening, learning, and building what he called a ‘ tree of knowledge’. Climbing that tree offered a unique vantage point from which other opportunities for growth and development could be identified. (Figure 1)
Never get between a tree and a goanna!
Robert Johnson
Introduction
Goanna/noun any of various Australian varanid (monitor) lizards as the common lace monitor, Varanus varius [from IGUANA]. The Macquarie Dictionary, Third Edition.
The lace monitor, V. varius, is a predominately arboreal species of monitor lizard found in a variety of habitats in the Australian mainland, ranging from rainforest to open woodland and scrub. V. varius is a hunter and a scavenger.¹ (Figure 2)
The Dreaming
Gugaa, the Lace Monitor, V. varius is the totem of the Wiradjuri Peoples, the largest Aboriginal Nation in New South Wales and plays a vital role in Aboriginal Culture including art and Dreaming stories.
Signifying a mutual and spiritual relationship between Aboriginal people and nature, First Nations’ Totems may be an object, plant, or animal.
An Aboriginal person will typically have four or more totems (nation/s, clan/s, family/families and personal). The Aboriginal people of Wiradjuri Country all have the Gugaa as their ‘nation’ totem. Gugaa through the Dreaming connects all people, past and present, and future of Wiradjuri lands and waters.²
Natural History of the Goanna
Known commonly in Australia as goannas, and elsewhere in the world as monitors or monitor lizards, all Australian
members of the family Varanidae belong to the same genus Varanus (Genus Varanus Merrem, 1820), a group of about 35 lizards found in Africa, Asia, the IndoPapuan Archipelago, and Australia. All are egg-laying, diurnal, raptorial (limbs adapted for seizing prey) lizards, including the largest lizard in the world, the Komodo dragon, Varanus komodoensis, and Australia’s largest lizard, the Perentie, Varanus giganteus.³
The limbs and tails of monitors are muscular and powerful, the eyes are protected by movable eyelids, while the tongue is forked like that of a snake and is flicked in and out constantly when the lizard is alert.³ (Figure 3)
Monitors are alert and agile diurnal carnivorous lizards which search for prey, relying on acute vision and extremely sensitive chemoreception. They are superb predators and are the most advanced and intelligent of all lizards.
All monitors are similar in morphology but vary considerably in size (20g to over 50kg). (Figure 4, 5)
As ectotherms they function at body temperatures like or greater than those of mammals, with cardiovascular and respiratory systems enabling extended periods of continuous activity. Their olfactory senses are acute. Mammals rely mostly on receptors in the nasal epithelium while monitors, like snakes, use their tongues to deliver compounds to the vomeronasal gland in the rostral palate.4
Depending on size, monitors feed on a broad range of prey including insects, reptiles, small mammals, possums, wallabies and nestling birds, eggs, and carrion.⁵
Most will forage on the ground but will take to a tree when disturbed, spiralling upward around a tree trunk, keeping to the opposite side of the tree from the observer or aggressor. Lace monitors generally lay 6-12 eggs, usually within the protection of a hole dug into a termite nest; the termites seal up the hole, and while the young can usually manage to burrow their way out of the nest there are reports of the female returning to the nest to free the hatchlings.³
Did You Know?
Endurance
Monitors are capable of high levels of aerobic activity during periods of continuous locomotion.⁶
There is a noticeable variance between species inhabiting different habitats. Species living in dry habitats (xeric) have higher endurance than tropical species; the reason for this difference is unknown. It may be a combination of climate-related foraging behaviours (species living in dry environments tend to forage more
widely) or it may reflect differences in behavioural motivation between the lizards from different climatic regions. For example, desert species may show greater flight response, while tropical species show a greater fight response.⁷
The heart
Where is the heart? The location of the heart usually at the level of the axilla in most lizard species is quite cranial compared with mammals, whereas the varanid heart is more caudally located. Monitors are the only lizard family to have a functionally divided cardiac ventricle. This enables them to sustain higher systemic blood pressures and higher metabolic rates than other reptiles of comparable size with a three-chambered heart. Like snakes, monitors maintain high systemic and low pulmonary circulatory blood pressures.⁸ (Figure 6)
Tongue function and morphology
Monitors are the only group of lizards which use the tongue exclusively for sensory function. The varanid tongue is extremely protrusible and smooth compared with most other lizards.⁵ (Figure 7)
Monitors are also unusual among lizards in their feeding behaviour, ingesting prey entirely by inertial feeding, as the tongue is not involved in food transport.
Inertial feeding involves moving the head over the food or prey item based on inertia alone, much like a snake. The food is held stationary in the mouth. Each time the mouth is slightly opened, the head is thrust forward over the food.⁹
Venom
Until recently, the established view was that reptile venoms were restricted to two groups of squamates (lizards and snakes): Serpentes and Heloderma ssp. (venomous lizards). Effects of the toxins from Varanus have usually been attributed to bacterial infections, but more recent systematic and toxinological analyses have discovered the presence of venom glands and venom in Varanus spp.10, 11
Four main functions of reptile venom are recognised:
– a defensive mechanism,
– an aid to digestion of prey,
– to assist in the maintenance of oral hygiene via an antimicrobial effect,
– to assist in prey capture by killing or immobilising prey.12
It has been suggested by some researchers that the primary function of venom in monitor lizards is to increase the speed and or efficiency of digestion.12
Parthenogenesis in monitors
Parthenogenesis, the production of offspring without fertilisation by a male is rare in vertebrate species; however, it has been reported in multiple species of reptiles. In wild Komodo dragons parthenogenesis could be an adaptation, as viable offspring are always male, and sexual reproduction can resume, although between related individuals, in a colony founded by a single unfertilised female. Fewer than 4,000 Komodo dragons remain in the wild, of which fewer than 1,000 are mature females.13
Parental care
Lace monitors lay their eggs in termite mounds and are known to return at hatching time, 290 days later, to dig into the mound to release the young.14
Scavengers
Lace monitors are scavengers and frequent visitors to camping sites where they search for food items such as discarded bones, meat, and garbage. They are largely carnivorous and opportunistic in their eating habits.15 Frye (1991) lists Varanus spp. as the only lizard genus with a preference for eating carrion.⁶
Veterinary Care of Goannas & Common Presentations
Monitor species are satisfying and interesting patients to treat. A comprehensive understanding of their natural history, anatomy and physiology is necessary to ensure optimal medical, surgical and welfare outcomes.
Handling
When handling monitors there are three key areas to consider: teeth, tail, and claws. Most Australian monitors can be restrained by firmly holding the neck with one hand and the hindlimbs and tail base with the other. Ensure that the animal is held away from the handler’s body. (Figure 8)
Physical examination
Large monitors, e.g. lace monitors, are best sedated to enable a thorough physical examination. Examine the skin for wounds and lacerations, check for fractures and examine the mouth carefully, paying particular attention to the tongue. Small monitors are easily handled and examined. Ensure that the head is held securely to avoid being bitten.
Sex identification
Sex identification can be conducted by various means. i. Physical examination by an experienced herpetologist or veterinarian noting certain morphological traits which vary between the sexes.




Figure 1. Lace monitor, Varanus varius Photo courtesy of Á. Lumnitzer
Figure 2. Lace monitors are keen foragers and hunters. Photo courtesy of Á. Lumnitzer
Figure 3. A lace monitor’s tongue is long and constantly flicking. Photo courtesy of A. Quain
Figure 4. Mature free-living Komodo dragon(V. komodoensis) Photo courtesy of J. Roffey
Figure 5. The pygmy monitor is the smallest member of the Varanidae family. Photo courtesy of D. Kirshner
Figure 6. Necropsy of a lace monitor showing the location of the heart.
Figure 7. A foraging Komodo dragon. Photo courtesy of J. Roffey
Figure 8. Restraint of a lace monitor ensuring a firm but gentle grip on the neck, tail and hindlimbs.
Figure 9. Hemipenes everted during the examination of a Gould’s goanna(V. gouldii) 1 4 5








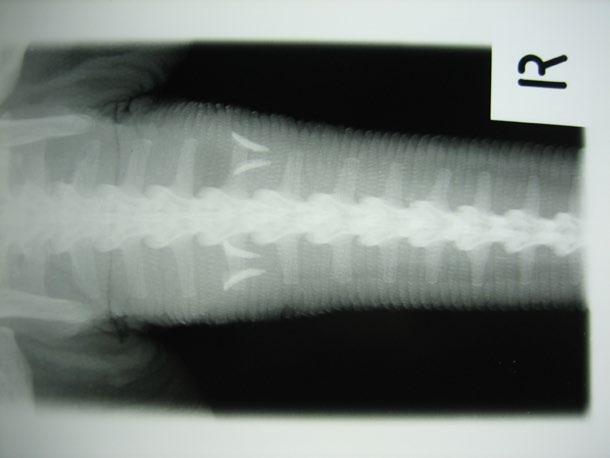

11 12 13 14 15

Figure 10. Male lace monitors have a more thickened tail base compared with females.
Photo courtesy of D. Kirshner
Figure 11. The tail base of the female lace monitor is more slender than the male.
Photo courtesy of D. Kirshner
Figure 12. The head of the male lace monitor is more robust in shape.
Photo courtesy of D. Kirshner
Figure 13. The head of the female lace monitor is more gracile in shape.
Photo courtesy of D. Kirshner
Figure 14. Hemibacula are radiographically opaque features within the tail base of mature male goannas.
Figure 15. Blood sampling of a sedated male perentie (V. giganteus)
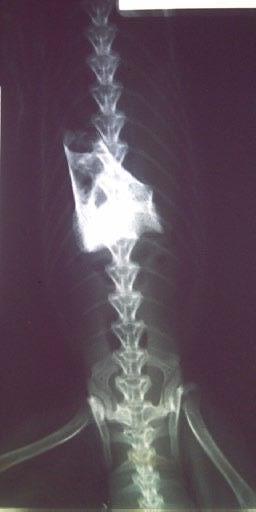

17


Figure 16. Gastric foreign body (lamb bone) in the stomach of a free-living lace monitor
Figure 17. Egg retention in a dehydrated captive monitor
Figure 18. Severe skeletal deformity in a juvenile lace monitor suffering from a metabolic bone disease due to poor diet and abysmal husbandry
Figure 19. A captive aged lace monitor with spondylosis enjoying some personalised care
a. females have a more slender tail base compared with males which contain the inverted hemipenes on each side, (Figures 9, 10 ,11)
b. females have a more gracile appearance to their head. (Figures 12, 13)
ii. Manual eversion of the hemipenis in a sedated animal
iii. Transillumination of the tail base in small monitors using a bright, cool light source to detect the presence (or absence) of the hemipenis.17
iv. Radiographic examination of the tail base of mature monitors to detect ossifications within the body of the hemipenes (hemibacula). The shape of the hemibacula will vary according to species. Absence of this feature may indicate a female or an immature male. (Figure 14)
Venipuncture
Venipuncture for intravenous injection and blood collection is easily accessed via the ventral caudal vein (Figure 15 ). Syringe and needle size will vary according to patient size.
Anaesthesia
The author’s preferred induction agent is intravenous alfaxalone (5mg/kg). A combination of medetomidine (50-100µg), ketamine (5mg/kg) and midazolam (1-1.5mg/ kg) is a reliable intramuscular option. Intubation is routine as the glottis is quite rostral compared with most other lizard species. Use only uncuffed endotracheal tubes. Secure the patency of the airway with the use of a mouth gag. Gaseous anaesthesia is maintained using isoflurane or sevoflurane. Masked induction in unsedated patients is not usually effective due to breath holding.
Cardiac monitoring
Doppler flow detection is used for audible monitoring of blood flow. The Doppler flow detector probe should be placed directly over the heart. The lubricated probe can also be turned dorsally and inserted into either the oesophagus or cloaca to detect blood flow.
Ventilation
Although in some cases monitors are known to breathe spontaneously under anaesthesia, most reptiles require assisted ventilation, either manual or mechanical. The tidal volume in reptiles is larger than that in mammals, but with a lower respiratory rate. There is a tendency to over-inflate the lungs during assisted ventilation. A low pressure should be maintained in reptiles due to their very fragile lungs and air sacs.
Pain perception in reptiles
Pain perception in lower vertebrates is likely to be analogous to that of mammals. For this reason alone, invasive, and painful procedures should always be accompanied by appropriate pain relief and anaesthesia.
Although specific doses have not been established in clinical trials, clinicians should attempt to provide lower vertebrates with appropriate analgesia during painful procedures.
Common conditions
Free-living
Traumatic injury is common in free living monitors presented for veterinary treatment. Diagnostic procedures and surgeries differ little from those used in mammalian medicine. Tongue injuries are common, and consideration should be given to the chances of rehabilitation and release of injured individuals.
Intestinal obstruction
Gorging of food by monitors is common and often essential, particularly in southern free-living populations where temperature constraints limit activity and as a result hunting time.¹ Consequently, large bone fragments or other indigestible items may be ingested causing intestinal obstruction. (Figure 16)
Captive and pet
Husbandry-related conditions (Figure 17)
Unfortunately, many conditions which are seen in animals under human care could be avoided by the provision of optimal and appropriate husbandry.
Metabolic Disease
The term Metabolic Bone Disease (MBD) is not a single disease entity but rather a term used to describe a collection of medical disorders that affect the integrity and function of bones. Metabolic bone diseases likely comprise the most common disease conditions affecting captive reptiles.
Monitors are diurnal in their habits and when kept in human care require proper UV light exposure and a balanced diet containing suitable amounts of calcium and vitamin D. Larger monitors are often housed outdoors and will have access to sunlight. In these cases, it is important to provide adequate shade and shelter. Smaller species which eat invertebrate prey should be provided with gut-loaded calcium rich food items. Common signs include weakness, musculoskeletal deformities, anorexia, poor growth or weight loss, paresis/paralysis, and neurological signs. (Figure 18)
Infectious Diseases
Infectious diseases caused by bacteria, fungi, protozoa, or viruses may infect monitors. Attention to husbandry, biosecurity and early diagnosis will help to prevent or mitigate any infection.
Obesity
Overfeeding is common in captive and pet reptiles. In the wild, monitors lead active lives foraging for food and mates. Care should be taken with dietary intake and the
provision of opportunities to climb, run, and search for food. (Figure 19)
Acknowledgement
The contribution of Dr Jeanine Leane, Wiradjuri elder, in providing Aboriginal cultural information is appreciated.
References
1. Vincent M, Wilson S. Australian Goannas. New Holland, Sydney. 1999.
2. City of Wagga Wagga. Wiradjuri and First Nations Community: Cultural Protocols https://wagga.nsw.gov.au/imagesfiles/ documents/services/WWCC_WFNC_CulturalProtocols_WEB.pdf
3. Cogger H. Reptiles and amphibians of Australia. CSIRO publishing; 2014 Mar 3.
4. S Smith KK. Morphology and function of the tongue and hyoid apparatus in Varanus (Varanidae, Lacertilia). Journal of Morphology. 1986 Mar;187(3):261-87. doi: 10.1002/jmor.1051870302. PMID: 3701871. https://doi.org/10.1002/jmor.1051870302
5. Jessop TS, Urlus JA, Lockwood T, Gillespie GR. Preying possum: assessment of the diet of lace monitors (Varanus varius) from coastal forests in southeastern Victoria. Biawak. 2010;4(2):59-63.
6. Frappell PB, Schultz TJ, Christian KA. The respiratory system in varanid lizards: determinants of O2 transfer. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2002 Oct 1;133(2):239-58. https://doi.org/10.1016/ s1095-6433(02)00147-2
7. Clemente CJ, Withers PC, Thompson GG. Metabolic rate and endurance capacity in Australian varanid lizards (Squamata: Varanidae: Varanus). Biological Journal of the Linnean Society. 2009 Jul 1;97(3):664-76.
8. Hanemaaijer J, Gregorovicova M, Nielsen JM, Moorman AF, Wang T, Planken RN, Christoffels VM, Sedmera D, Jensen B. Heart development in the lizards (Varanidae) with the greatest extent of ventricular septation. bioRxiv. 2019 Feb 28:563767. bioRxiv 563767; https://doi.org/10.1101/563767
9. Laurie J. Vitt, Janalee P. Caldwell, Chapter 10 - Foraging Ecology and Diets, Editor(s): Laurie J. Vitt, Janalee P. Caldwell, Herpetology (Third Edition), Academic Press, 2009, Pages 271-296, ISBN 9780123743466, https://doi.org/10.1016/B978-0-12-3743466.00010-9
10. Vidal N. and S.B. Hedges. 2005. The phylogeny of squamates reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein-coding genes. Comptes Rendus Biologies 328: 1000-1008. https://doi.org/10.1016/j.crvi.2005.10.001
11. Fry BG, Vidal N, Norman JA, et al. Early evolution of the venom system in lizards and snakes. Nature . 2006 Feb 2;439(7076):584-8. https://doi.org/10.1038/nature04328
12. Arbuckle KE. Ecological function of venom in Varanus, with a compilation of dietary records from the literature. Biawak. 2009;3(2):46-56.
13. Watts PC, Buley KR, Sanderson S, Boardman W, Ciofi C, Gibson R. Parthenogenesis in Komodo dragons. Nature. 2006 Dec 21;444(7122):1021-2. https://doi.org/10.1038/4441021a
14. Carter DB. Nesting and evidence of parental care by the lace monitor Varanus varius. Mertensiella . 1999;11:137-47.
15. Greer AE. The Biology and Evolution of Australian Lizards. Surrey, Beatty and Sons, Sydney; 1989.
16. Frye FL, 1991. A Practical Guide for Feeding Captive Reptiles. Krieger, Malabar; 1991.
17. Brown DA. Hemipenal transillumination as a sexing technique in Varanids. Biawak. 2009 Mar;3(1):26-9.
18. Carmel B, Johnson R. Nutritional and metabolic diseases. Reptile medicine and surgery in clinical practice. 2017 Dec 26:185-95.
A Novel Approach to Evaluate the Hoof Capsule in Thoroughbred Racehorses
Peter Kerkenezov BM
BVSc DipAppSc Cert Equine Surgery e. equivet@nor.com.au
C&T No. 6110
The welfare and performance of Thoroughbred racehorses are linked to the biomechanics of their hoof capsules, an often underestimated but crucial factor in lameness and injury.
The 2023/2024
NSW Racing Season data exposing the probability of an estimated 37 deaths potentially associated with forelimb hoof capsule imbalances draws urgent attention to this issue. Forelimb foot imbalance, occurring in both dorsopalmar and mediolateral planes, significantly challenge the structural integrity of the equine foot under the intense forces generated during racing. This imbalance not only affects the hoof itself but can propagate injury up the limb and beyond, compromising a horse’s racing career and wellbeing.
This article introduces a fresh biomechanical perspective, applying the Theorem of the Parallelogram of Forces to understand how hoof horn tubules—keratin structures aligned with resultant vertical forces—reflect and influence hoof loading patterns. Drawing on five decades of veterinary experience, the author outlines the criteria of the ‘ideal foot’ characterized by specific hoof and palmar angles, crucial for withstanding the rigorous demands of racing. The discussion suggests how deviations from these angles, particularly zero or negative palmar angles, can alter force vectors in problematic ways that can trigger a cascade of pathological conditions ranging from laminitis to degenerative joint disease.

Read the full article here cve.edu.au/Hoof-Capsule
General The Overton Window in Veterinary Medicine:
Expanding What Our Profession Thinks Is Possible
Alex Harrison Vetquity Consultancy
e. alex@vetquity.com.au
C&T No. 6111
For most of my career, I’ve seen the same thing happen when someone asks for support. Not resistance, not cruelty. Just… discomfort. Doubt. Delay. That response rarely comes from bad intent. It comes from the frame we’ve inherited. Inclusion still feels ‘too much’ in many veterinary workplaces—not because people don’t care, but because it still sits outside what the profession has historically recognised as normal.
There’s a name for that invisible frame: the Overton window. In political science, it describes the range of ideas considered socially acceptable, professionally valid, and worth acting on. Inside the window, ideas are seen as reasonable and achievable. Outside it, they’re labelled unrealistic, radical, or unthinkable. Crucially, the window is not fixed - over time, ideas can move from ‘impossible’ to ‘inevitable.’
Veterinary medicine has its own Overton window—and it has shifted many times before.
How the Window Operates in Veterinary Medicine
Consider some of the issues we now take for granted:
– Parental leave: Once won only after individual negotiation, now a standard expectation in most clinics.
– Mental health awareness: Once taboo, now central to workforce sustainability discussions.
– Flexible work: Once dismissed as incompatible with veterinary life, now increasingly offered.
A generation ago, raising these issues risked being seen as unrealistic, disruptive, or weak. Today, they are professional common sense—and embedded in conference programs, wellbeing frameworks, and clinic policies.
This did not happen by accident. It happened because people—often those directly affected—refused to let the window remain narrow. They spoke, wrote, collected data, trialled new ways of working, and challenged the idea that ‘this is just how it is.’ In doing so, they widened the frame of what the profession could imagine.
The Window & DEI in Our Profession
When it comes to diversity, equity, and inclusion (DEI), veterinary medicine is still negotiating the edges of its Overton window. Compared to other sectors, our view of inclusion remains narrow. We’ve made plenty of space for high standards, long hours, stoicism, and selfsacrifice. Yet we’ve left far less room for:
– Disability inclusion: Adjustments are still treated as exceptions to the norm, not expectations. ‘Reasonable’ is often interpreted as the bare minimum. Ableist narratives of ‘one body, one mind, one pace’ remain deeply ingrained.
– Neurodivergence: Conversations about ADHD, autism, or executive function challenges are increasing, but stigma and misunderstanding are still common.
– LGBTQIA+ inclusion: Progress is visible in some spaces, yet safety and belonging remain uneven across the profession.
– Faith, culture, gender identity, or caregiving needs: Requests for adjustments here are often treated as secondary and peripheral to professional identity.
– Mental health and burnout: Still spoken about cautiously today, and often framed as individual resilience rather than systemic reform.
– Equity in recruitment and leadership: Structural barriers continue to shape who enters, who stays, and who advances.
These issues are no longer outside the profession’s imagination, but they sit at contested edges of the window. Some leaders see them as central to workforce sustainability. Others still treat them as ‘extras’— important only after the ‘real work’ is done. And some dismiss them as ‘politics’ or ‘activism’, ignoring the tangible impacts exclusion has on real people and workplaces.
Not long ago, the idea of a profoundly deaf veterinarian using real-time captioning at conferences would have been dismissed as unrealistic. So would building a wheelchair-accessible mixed-animal hospital. Or recruiting a clinic manager from outside the profession. Yet today, each of these examples exists. What was once ‘too much’ is now normal practice somewhere.
Where Our Window is Stuck
The Overton window also explains some of the unspoken rules still shaping veterinary life:
– Debates about whether part-time vets can be ‘real contributors’.
– The expectation that new graduates must work 50+ hours a week to ‘prove themselves’.
– Recruitment processes built around in-person interviews during business hours, excluding many talented candidates before they even start.
None are immutable truths. They are simply markers of where our window currently sits.
Why This Matters for Leaders
Understanding the Overton window helps explain why change feels slow, or why ideas that seem self-evident to some are treated as unrealistic by others. It reminds us that professional culture is not fixed. What feels ambitious today may become the baseline tomorrow.
Leaders, educators, and practice owners have a choice:
– Work within the window we inherit—keeping conversations safe, familiar, and comfortable.
– Or help shift the window itself—making space for reforms that allow more people to thrive.
The second path requires courage. It often means enduring discomfort or pushback. But it is how we ensure the profession remains relevant, humane, and sustainable.
It’s also how we prepare for the workforce of the future. Generation Z is already here, and Generation Alpha is only a few years away from entering the veterinary workforce. These cohorts bring higher expectations of inclusion, flexibility, and belonging. If our window remains too narrow, they will simply choose other professions.
The Danger of a Shrinking Window
It’s important to note: the window can shift backwards too. If leaders dismiss inclusion as ‘political’ or resist change, we risk normalising exclusionary norms once again. That’s not just morally problematic—it’s strategically dangerous. In a competitive workforce market, a shrinking window means fewer people feel they belong in veterinary medicine, and more leave.
So Who Moves the Overton Window?
Not those guarding the centre. Not the people asking, ‘Is everyone comfortable with this?’
The window shifts because someone—often an outsider— names a truth the system isn’t ready to hear. Someone
builds what doesn’t exist yet. Someone keeps showing up with ideas that sound ‘too much’… until they don’t.
At first, these people are told: ‘This isn’t the right forum’, ‘Let’s not get political’, or ‘That’s just how it’s always been.’ But when they keep showing up—and others join them—the cracks become too loud to ignore.
The window moves not because the centre suddenly becomes brave, but because people on the edges refused to stay silent. They ask questions no one else is asking. They challenge language everyone else has learned to live with. They advocate not just for individuals, but for systems that work better for everyone.
How Leaders Can Push the Edges
So what does it look like to help shift the Overton window in veterinary medicine? Some starting points:
– Visible examples: When we see a vet in a wheelchair performing surgery, or a neurodivergent leader running a thriving practice, it changes what we believe is possible.
– Language shifts: Moving from ‘accommodations’ to ‘inclusive design’, or from ‘culture fit’ to ‘culture add’, signals a change in baseline expectations.
– Naming inequities: Speak openly about issues that are often silenced—whether that’s ableism in hiring or exclusion in leadership pipelines.
– Modelling alternatives: Pilot flexible rostering, run accessibility audits, or integrate trauma-informed, equity-centred leadership training.
– Using data and stories: Combine research with lived experience to show why inclusion matters for retention, morale, and patient care.
– Supporting those at the edges: Listen to colleagues whose needs or identities are marginalised. Their realities are often the first signals of where reform is needed.
– Normalising what was once radical: Treat adjustments, diverse leadership, or inclusive policy design not as exceptions but as everyday professional practice.
– Leveraging policy change: New psychosocial hazards legislation and explicit DEI requirements in accreditation standards can help reframe what’s ‘reasonable.’
– Recognising economic drivers: Workforce shortages make once-dismissed ideas—like job sharing or teletriage—suddenly viable.
– Collaborating across sectors: Other professions have shifted their windows further. Learning
from them challenges the veterinary myth of the ‘omnicompetent vet’ and opens new solutions. Each of these actions helps move ideas from ‘unthinkable’ ‘radical’ ‘acceptable’ ‘expected’.
A Wider, Braver Future
When I first read the Control & Therapy Series as a new graduate 25 years ago, I remember how much it shifted my thinking. Richard Malik’s regular contributions often challenged assumptions and reframed what was possible in practice. Looking back, I can see the Overton window at work—changing how we thought about medicine and surgery.
The same is true today. The Overton window reminds us that our profession is not bound by tradition alone. It is shaped by what we collectively decide to consider possible. When we broaden that window to include equity, accessibility, and belonging, we don’t dilute veterinary excellence—we redefine it.
The question is not only how we work within the window we’ve inherited, but also how far we are willing to push its edges. Because the future of veterinary medicine depends on the space we create today. Our choices decide who enters, who stays, and who leaves.
By expanding the window, we move from discomfort and delay toward inclusion as routine, expected, and woven into the very definition of veterinary excellence.

Engaging
You earn unstructured CPD points simply by reading the C&T. BUT, you can earn STRUCTURED CPD points by contributing a C&T or Perspective article.
The Australasian Veterinary Boards Council (AVBC) recommends that Continuing Professional Development (CPD) be undertaken by all registered veterinarians in Australia and New Zealand.
60 points required over 3 years 15 structured & 45 unstructured
Earn up to 1 unstructured point per 2 hours of reading the C&T Series.
Earn structured points for contributing a C&T or Perspective – 1 point per hour of preparation time with a cap of 4 points per paper.
So, next time you see an interesting case, please consider taking some photos or a video and write it up for the C&T. Your colleagues will benefit and you will be contributing practical information and expertise to the profession to benefit animal welfare. Best of all, you will earn structured CPD points at no cost, all without leaving your practice or home!
Submit your article to cve.marketing@sydney.edu.au

Original Perfusor™ Line
Coloured coded minimum volume extension tubing for use with Syringe drivers
Features
· Luer Lock fitting enables compatibility to all syringe drivers
· Pressure resistant up to 2 bar
· Made with PE (Polyethylene), does not container PVC, DEHP or LATEX
· Small tube diameter (1.0 x 2.0 mm) to reduce residual volume
· Available in 3 colours and two lengths
· Low sorbing properties
· Excellent start-up characteristics
· Kink resistant



