TheProject



Editor & Contributor, David W. McMillan, Ph.D.
Managing Editor, Scott Roy
Graphic Editor, Robert Camarena
Contributors, Jeanette Bajo, Dalton Dietrich, Ph.D., Vania Almeida, Alejandra Hernandez
Photographs & Graphics, Robert Camarena, David McMillan, Ph.D., Aya Hanna and Bassel Awada, The Ali Lab.
Website: www.themiamiproject.org
Physical Address: 1095 NW 14th Terrace, Miami, FL 33136
Phone: 305-243-6001
Mailing Address: Post Office Box 016960, R-48 Miami, FL 33101


Marc Buoniconti President
Dr. Allan D. Levi
Clinical Director, Professor and Chair of the Department of Neurological Surgery
Suzanne M. Sayfie Director Major Gifts Buoniconti Fund
Diana Berning Senior Manager, Business Operations
Gigi Giobio Director, Sponsored Programs
David McMillan, Ph.D. Director, Education and Outreach
Dr. Barth A. Green Chairman, Professor of Neurological Surgery
Dr. Mark Nash Associate Research Director
Teresa De Jesus Arcay Assistant to Marc Buoniconti
Teri I. Bendell-Bou Director, Web Services, Auction, and Database
Cynthia Jones Manager, Facilities and Operations
Randy L. Medenwald Senior Director, Advocacy and Donor Relations
Alejandra Hernandez Executive Assistant to Dr. W. Dalton Dietrich
Rebecca Avshalom
Javier Burgos
Maria Chagoyen
Frank Diaz
Evelyn Gomez

On the Cover - Art meets Science: staining cells with a collection of dyes enables robust visualization of cellular structures and sensitive detection of thousands of features from each individual cell. The technique, dubbed Cell Painting, generates massive amounts of interpretable data and is being used at the Miami Project to accelerate drug discovery. Aya Hanna and Bassel Awada, The Ali Lab.
Dr. W. Dalton Dietrich Scientific Director, Professor of Neurological Surgery
Stephanie Sayfie Aagaard Executive Director
Jeanette Bajo Center Administrator
Robert Camarena Senior Graphic Designer
Jacqueline Manzano Manager, Marketing
Scott P. Roy Director, Public Relations and Communications
Taimy Trujillo Assistant to Dr. Barth A. Green
Alejandro Labrada Carole Robbins
Stephanie Lacayo Rodriguez
Paul Sanchez
Sean Sadler
Scientific Staff & Trainees
Dr. Alexander Marcillo Post-doctoral Fellows Graduate Students
Medical/Residents/Observorships Undergraduate Students Volunteers
Other Students Research Staff
By seven months, he could open his fingers readily, a milestone he says changes “literally everything” about his daily life.
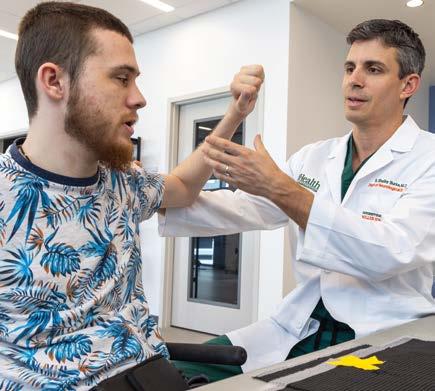
For Tommy, a 28-year-old man living with tetraplegia from a motor vehicle accident, the ability to open a medicine bottle or grip his wheelchair’s handrims represents more than medical progress—it’s a restoration of independence. “Literally everything changed,” he says, describing life after participating in a nerve transfer surgery study led by S. Shelby Burks, M.D., Assistant Professor Department of Neurological Surgery and The Miami Project to Cure Paralysis scientist. While peripheral nerve transfers have been an existing option for people living with spinal cord injury (SCI), the federally funded multi-site study, led locally by site-PI Dr. Burks, adds key innovation in the postoperative neurorehabilitation to maximize functional gains that might only be realized by the combination of surgery and rehabilitation.
Dr. Burks’ trial targets individuals with high tetraplegia in the sub-acute to early-chronic phase, a critical period when neuroplasticity remains primed for retraining. The surgery involves rerouting operational nerves from above the injury site - often the triceps or biceps - to dormant nerves that would otherwise be controlling hand muscles if not for SCI. The surgery, a procedure that has existed for some time, involves boldly cutting a working nerve from a nerve root exiting the cord above where SCI disrupts signals, and suturing that nerve into a nerve that originates below the SCI. Because nerves below the level of injury are not dead but simply inactive, the rerouted rostral nerve has a channel down which it can regenerate into a precise target. In Tommy’s case, muscles that allow his fingers to grasp again. While the surgery immediately connects the nerves, the regenerative process—unique to peripheral nerves only once they leave the spinal cord—occurs at a rate of ~1 inch per month. “It didn’t make me upset, because I was already assuming that this isn’t going to work instantly,” Tommy said when reflecting on the duration between the surgery and regaining function. A scientific target addressed by this study is optimization of the regenerative environment in this post-operative window, to maximize the functional gains resulting from the regeneration that establishes new connection.
The first nerve transfer surgeries were performed in the 1960s, but despite an effort in the early 1980s remain underutilized to our current time. This trend, which this multi-site study led by Wilson Z. Ray, M.D. at Washington University School of Medicine aims to reverse, likely occurred because while the surgical procedure restores anatomical connectivity, optimization of post-operative regenerative environment has yet to be fully explored. Thus Dr. Burks team employs intensive post-operative neurorehabilitation in collaboration with Matija Milosevic, Ph.D., Assistant Professor of Neurological Surgery and Biomedical Engineering and principal investigator of the Neuromodulation Engineering & Therapeutics (NeuroMET) Lab. The study puts to the test the hypothesis that combinatorial neurorehabilitation unlocks the potential of the surgery.
As a case, Tommy’s progress is illustrative of the incremental but transformative potential of this combined surgery and rehabilitation. At four months post-surgery, he noticed the first flicker of movement in his left hand. Tommy recalls, “I was laying in my bed trying to open my hand, and it just barely started to move. And that was my first realization that it’s working, because they didn’t move at all before. So even just that slight flicker was very like, it’s working!” By seven months, he could open his fingers readily—a milestone he says changes “literally everything” about his daily life. “Before,
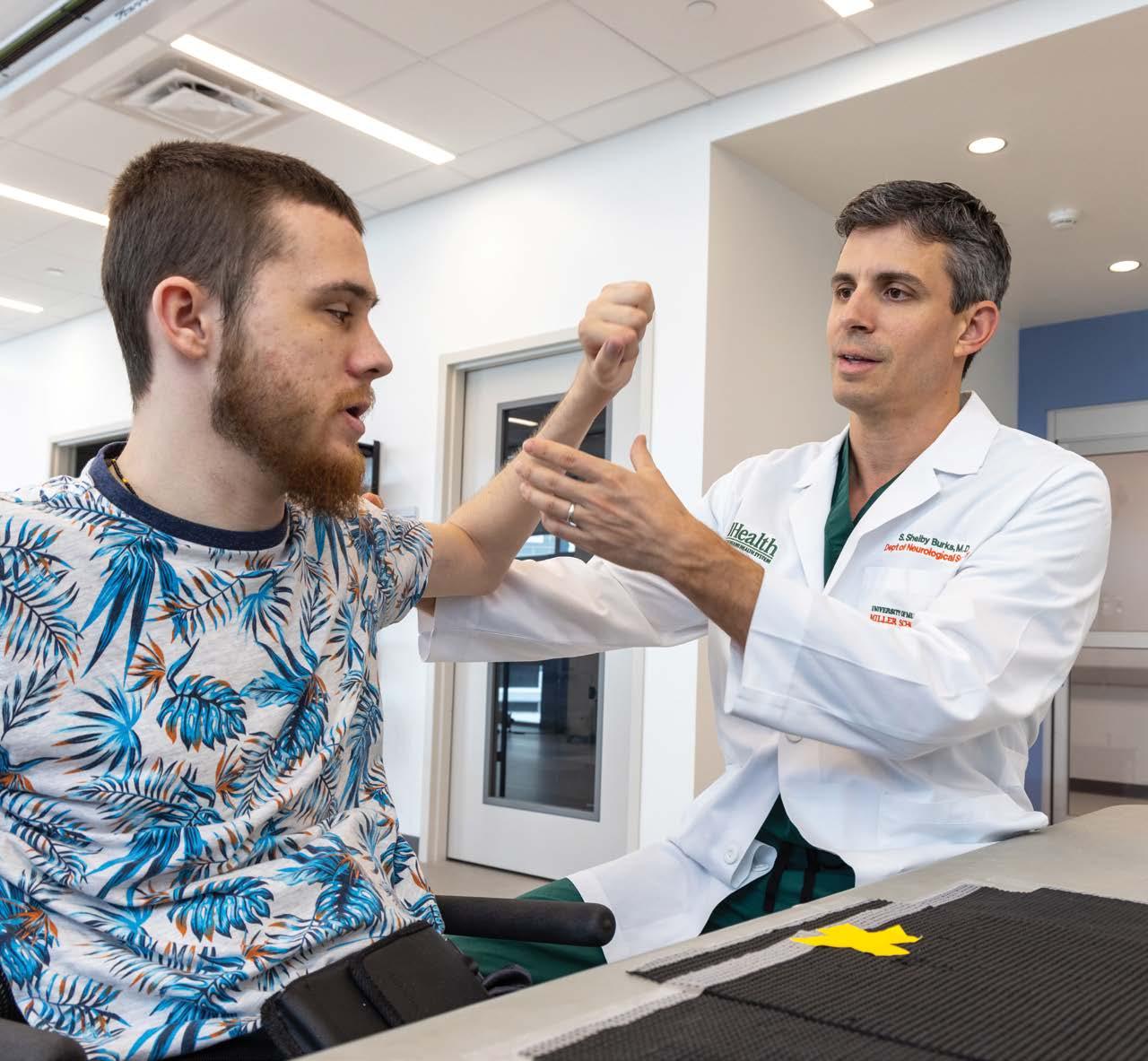
Dr. Shelby Burks examines Tommy.
I used my palms to push my chair, now I can grip the rims,” Tommy said while also listing a litany of tasks he can perform that were unavailable to him before participating in the study, everything from independently eating to selfadministering his daily medications.
With early success in restoring hand function in tetraplegia, Dr. Burks’ team is exploring lateral translation of the approach to other diagnoses such as brachial plexus injuries in collaboration with the plastic surgeons who routinely handle these cases. Concurrently, much innovation remains
to be discovered in the optimization of pre- and postoperative rehabilitative techniques employed in SCI nerve transfers. While not yet enacted, continuing the trend Drs. Burks and Milosevic might someday layer therapeutics onto the process, combining a variety of neuromodulation devices developed in Dr. Milosevic’s NeuroMET Lab with the existing surgery and neurorehabilitation. If you have an interest in this combinatorial approach, stay tuned and reach out to The Miami Project’s Office of Education and Outreach for any updates or to see if you, or someone you know, qualifies for existing studies.

“The real challenge isn’t just understanding these metabolic changes—it’s figuring out how to intervene in a way that fits the reality of living with SCI.”
Gary J. Farkas, Ph.D., MSCTI, Assistant Professor of Physical Medicine & Rehabilitation and principal investigator at The Miami Project, operates at the intersection of metabolic physiology and eating behavior in spinal cord injury (SCI) research. Dr. Farkas joins The Miami Project to address one of the most pressing yet overlooked challenges in neurorehabilitation – the metabolic storm of “neurogenic obesity,” a term he coined with his colleague and mentor, the late David R. Gater, Jr, M.D., Ph.D., MS. Dr. Farkas’s path to The Miami Project was as the lead postdoctoral fellow of Dr. Gater’s research team. Now, as a principal investigator, he carries on decoding the health implications of altered energy metabolism imposed by SCI while adding a new dimension of appetite and dietary intervention as a therapeutic target.
Dr. Farkas attacks neurogenic obesity on two fronts: energy metabolism and appetite. On energy metabolism, SCI drastically reduces the capacity for energy output due to various factors, such as withering of muscle tissue, hormonal changes like low testosterone in men, and obligate sedentary behavior characterized by reduced physical activity secondary to paralysis. Due to the specific ways SCI reduces the capacity to modulate energy expenditure, Dr. Farkas sees food intake as the key variable to achieve a negative energy balance in the energy balance equation required for good metabolic health. Previous research, including Dr. Farkas’s work, has well-documented the negative impact of SCI on energy expenditure. Yet, far less attention has been given to the intake side of the equation. Dr. Farkas’s research has identified overeating relative to reduced energy expenditure and nutrient
deficiencies. However, the mechanisms driving this disconnect between energy needs, intake, and appetite regulation remained unclear. Thus, his work focuses on understanding diet and eating behavior in SCI, using a mechanistic approach to uncover the factors contributing to these paradoxical findings. Dr. Farkas emphasized, “The real challenge isn’t just understanding these metabolic changes—it’s figuring out how to intervene in a way that fits the reality of living with SCI. We need solutions that are both scientifically sound and practical for daily life. This is why behavior is so important.”
Thanks to his prior studies, we know that people living with SCI experience altered hunger cues and rely less on physiological signals, such as fullness, instead depending more on cognitive reward control to regulate their food intake during meals. This may explain why they eat fewer

meals but have more frequent eating occasions, driven by increased snacking, compared to people without SCI. “Snacks are pre-packaged, easy to consume quickly, and energy-dense,” says Dr. Farkas. “These factors are known to contribute to overeating and obesity.” His recent work further supports this, showing that people with SCI are less likely to grocery shop independently and more reliant on others or delivery services—a reality that makes sense given the inaccessibility of grocery stores and kitchens needed for affordable, healthy meal preparation.
While these broad characterizing studies set an important justification, Dr. Farkas’s home is in the laboratory, and he has been concurrently designing and conducting experiments that bring to bear the latest technologies. “We are not just correlating physiology to behavior,” says Dr. Farkas, “we are measuring the biobehavioral responses to
food intake to understand the mechanisms contributing to obesity in chronic SCI.” In his postprandial laboratory studies conducted at The Miami Project, Dr. Farkas first showed delayed gut transit in people consuming a compulsory fixed meal along with a motility capsule, which tracks the movement of the meal through the body’s digestive system. This delayed transit time was associated with increased hunger and decreased fullness, the opposite pattern observed in people without SCI. This disconnect between gut processing and perception-based eating behavior led Dr. Farkas to recognize that the gut-brain axis, the neurological and humoral signaling pathway between the gut and brain, was a key target for interrogation in SCI. For these reasons, he views both metabolism and eating behavior as critical factors in appetite regulation, making it a key and largely unaddressed co-therapeutic target for people living with SCI.
Along with these characterizations of the fundamental metabolic pathophysiology of SCI, Dr. Farkas also studies the practical medical implications of metabolic changes post-SCI. In an ongoing study, his first funded as an Assistant Professor, Dr. Farkas and his team are examining relationships between fat depots around select organs in the chest and abdomen and blood biomarkers in the form of classic cardiovascular disease (CVD) measures and novel inflammatory analytes. Using advanced imaging techniques, fat depots around and within key metabolic organs are regionally quantified and correlated with blood biomarkers to determine which fat depots have the strongest relationship to CVD risk in SCI and the general population without SCI. This organ-specific fat mapping could help uncover the mechanisms driving the uniquely high CVD risk in people with SCI and identify potential therapeutic targets. Additionally, this work will help determine which fat depot poses the greatest risk—both in SCI and nonSCI populations—and whether this risk is driven by inflammation or other underlying factors.
Dr. Farkas has started his professorship at a blistering pace by robustly characterizing the mechanisms of altered energy metabolism in SCI. Building on his foundational understanding of the spinal cord-injured body as a lowthroughput energy system, Dr. Farkas has established a strong platform for developing and testing targeted interventions to promote a healthy lifestyle in SCI by focusing on appetite regulation and energy metabolism, integrating dietary and behavioral strategies that recognize the critical need to address metabolic imbalance in people with SCI.

Every time he goes to work, Damien Pearse, Ph.D., sees the people his research aims to help. Dr. Pearse, a professor of neurological surgery at the University of Miami Miller School of Medicine, is a faculty member at The Miami Project to Cure Paralysis. The building that houses the project includes spaces for people with spinal cord injuries to access wellness programs and participate in clinical studies.
“Being able to frequently interact with people affected with spinal cord injury and other forms of paralysis is inspiring as a researcher, because often as scientists working in the laboratory, you may feel somewhat disconnected from the people you’re trying to help,” said Dr. Pearse, the John M. and Jocelyn H.K. Watkins Distinguished Chair in Cell Therapies.

Damien Pearse, Ph.D., advising a graduate student.
Dr. Pearse is an expert in the biology of Schwann cells, the peripheral nervous system cells that play a key role in axon growth and myelination.
His lab investigates how to use Schwann cell transplants to repair the injured spinal cord. Along with fellow Miami Project researchers, Dr. Pearse has been involved in clinical trials that used Schwann cells from injured people’s own bodies to regenerate and reconnect damaged axons.
About 18,000 people per year in the United States have a new spinal cord injury, according to the National Spinal Cord Injury Statistical Center. But more than 300,000 have chronic spinal cord issues, which are at least a year from the time of initial injury. It’s difficult to promote regeneration in these cases, Dr. Pearse said, because scar tissue blocks axons from regrowing.
“This research is long and labor-intensive,” he said. “But the majority of individuals with spinal cord injury have chronic injury, so we must continue to try to find an effective treatment.”
Dr. Pearse initially studied biotechnology, but pivoted to biomedicine while pursuing his Ph.D. During a research internship in 1999, he worked on peripheral nervous system regeneration.
“Unlike our central nervous system, brain and spinal cord, which has deficiency in regenerating after it’s injured, the peripheral nervous system can regenerate,” he explained. “Schwann cells are an integral part of that repair process.”
At a conference after his internship, Dr. Pearse met Professor Mary Bartlett Bunge, Ph.D., who performed pioneering research on Schwann cells with her husband, Richard. Dr. Pearse decided this work was his calling. In 2000, he joined The Miami Project as a postdoctoral researcher.
He was thrilled to be part of a group of dedicated, knowledgeable scientists. He began studying therapies that combined Schwann cells with other approaches and continued this research when he joined the faculty in 2004.
After performing pre-clinical investigations on Schwann cell transplants, Miami Project researchers received the OK from the U.S. Food and Drug Administration in 2012 to conduct Phase 1 clinical trials. They found that transplantation using a person’s own Schwann cells was feasible and safe for several types of injuries in different parts of the spine — an important leap forward.
Now, Dr. Pearse’s lab is working on the next step. They’re gathering data to support FDA approval for Phase 1b/2 clinical trials aimed at demonstrating that Schwann cell transplants combined with biomaterials or drugs can be effective in improving function.
“To give us the best chance of success, we want to build upon our previous work, which is showing that, when Schwann cells are combined with other promising therapies, we get a much more robust, reparative effect,” he said.
“We want to make sure that the approach we select to use in a Phase 2 efficacy trial is the best, the one that we think has the highest chance of succeeding and potentially restoring the most function,” he said.
The approaches are designed to address challenges with Schwann cell transplants: their survival rate and ability to migrate from the injury site. In spring 2024, Dr. Pearse received a Department of Defense grant to use Harvard University-based Gel4Mede’s biomaterial platform to create a “protective shield” and prevent cell damage during and after a transplant, as well as overcome the scar.
Another promising approach uses a molecule called polysialic acid. Dr. Pearse collaborated with the Urs Rutishauser lab at Memorial Sloan Kettering Cancer Center to demonstrate that Schwann cells with polysialic acid on their surface can migrate from an injury site and guide axons originating from the brain into and across the site to improve function.
Dr. Pearse is now trying to identify a biological method for attaching polysialic acid to Schwann cells in a way that prevents cells from over migrating. Another approach avoids cell transplants entirely, instead using exosomes — nano-sized extracellular vesicles that transport material between cells.
“Exosomes from Schwann cells have been shown to carry beneficial cargos that promote axon protection and regeneration,” Dr. Pearse said.
In fall 2024, W. Dalton Dietrich, Ph.D., scientific director of The Miami Project and professor of neurological surgery at the Miller School, and a team including Dr. Pearse received a DOD grant to study the safety and benefits of these exosomes in spinal cord injury.
Determining the best approach to move toward clinical trials will take time. Dr. Pearse estimates he needs two to three years for the cell-based approaches and slightly less for the exosome-based approach. Dr. Pearse said he’s

honored to take his research from the lab to clinical trials, and grateful that he gets to find exciting discoveries with the team at The Miami Project.
“The main goal of my research would be to find a treatment that progresses into a medicine that can be used in people with spinal cord injury or similar conditions,” he said.
“Reaching such a milestone would be a tremendously humbling experience after the relentless pursuit of this goal.”


The Miami Project To Cure Paralysis at The University of Miami
Miller School of Medicine Selected As a U.S. Site
For Neuralink Clinical Trial
The Miami Project to Cure Paralysis and the Department of Neurological Surgery at the University of Miami (UM) Miller School of Medicine have been selected to become the second U.S.-based site for Neuralink’s PRIME Study, an investigational medical device clinical trial for Neuralink’s ground-breaking brain-computer interface.
The Neuralink PRIME (Precise Robotically Implanted Brain-Computer Interface) Study is an early feasibility study to assess the safety and functionality of Neuralink’s N1 Implant, an intracortical brain-computer interface (BCI) implant that establishes a wireless, digital link between the brain and computers. This link is designed to help restore autonomy to people with paralysis by enabling them to control external devices with their thoughts, without the need for wires or physical movement. The study will also evaluate Neuralink’s R1 Robot, a surgical robot that precisely and rapidly places the N1 Implant’s ultra-fine electrode threads within microns of targeted neurons.
“We hope this partnership is another significant step in finding meaningful solutions for the millions living with paralysis and other significant motor deficits.”
The implant procedure will be carried out by Neuralink scientists and a multidisciplinary team of neurosurgeons, neuroscientists and biomedical engineers at The Miami Project and the Miller School of Medicine.
“We are very excited about working with the Neuralink team. This announcement is a testament to our multidisciplinary approach for advancing the latest research in neural interfaces and neurorehabilitation,” said W. Dalton Dietrich, Ph.D., scientific director of The Miami Project to Cure Paralysis, co-director of the University of Miami Neural Engineering Institute and professor of neurological surgery at the Miller School.
The Department of Neurological Surgery team will include UM’s principal investigator of the trial Jonathan Jagid, M.D., professor of clinical neurological surgery, neurology, orthopedics and rehabilitation and co-investigators Allan Levi, M.D., Ph.D., professor and chair of neurological surgery, Michael Ivan, M.D., associate professor of neurological surgery and Seth Tigchelaar, M.D., Ph.D., a Miller School neurosurgery resident will oversee the surgical approaches performed at UHealth Tower, the flagship hospital of the UHealth University of Miami Health System.
“This study will use the latest technological advances in BCI approaches for improving function and quality of life in individuals living with the consequences of chronic spinal cord injury, as well as amyotrophic lateral sclerosis (ALS),” said Dr. Levi.
“The Miami Project to Cure Paralysis and the University of Miami are renowned for their pioneering research in neural interfaces for the treatment of debilitating neurological conditions such as spinal cord injury and ALS. We look forward to partnering with them as a site for our PRIME Study,” said DJ Seo, co-founder, president and COO at Neuralink.
For the PRIME Study (NCT06429735), Neuralink is specifically looking for patients who have limited or no ability to use both hands due to cervical spinal cord injury or ALS. Individuals interested in learning whether they may qualify for this trial can join Neuralink’s United States Patient Registry.
“This collaboration represents another great opportunity to combine our clinical research expertise with the forwardthinking team at Neuralink. We hope this partnership is another significant step in finding meaningful solutions for the millions living with paralysis and other significant motor deficits,” said Marc Buoniconti, president of The Miami Project.

If you would like to be considered for these, or future Miami Project trials or studies, please see our Register for a Research Program section of our website, send us a message, or call The Miami Project Education Office at 305-243-7108.
PRIME STUDY: PRECISE ROBOTICALLY IMPLANTED
BRAIN-COMPUTER INTERFACE
Purpose The purpose of this Research Study is to evaluate the initial clinical safety and device functionality of the N1 Implant and R1 Robot device designs. During the study, the R1 Robot will be used to surgically place the N1 Implant in a region of the brain that controls movement intention. Participants will be asked to use the N1 Implant and N1 User App to control a computer and provide feedback about the system.
Qualification summary: Have quadriplegia (limited function in all 4 limbs) due to spinal cord injury or amyotrophic lateral sclerosis (ALS) and are at least 1-year post-injury (without improvement); Are at least 22 years old; Have a consistent and reliable caregiver Status: Enrolling
Principal Investigator: Jonathan R. Jagid, M.D.
Contact: Letitia Fisher 305-243-3056
SAFETY AND EFFICACY OF AUTOLOGOUS HUMAN SCHWANN CELL (AHSC) AUGMENTATION OF NERVE AUTOGRAFTS AFTER SEVERE PERIPHERAL NERVE INJURY - This is open only to people with severe injury to a peripheral nerve (in leg or arm) and is NOT open to people with a spinal cord injury
Purpose: To evaluate the safety of injecting one’s own Schwann cells along with nerve autograft (eg. sural nerve – the sural nerve is a sensory nerve in the leg) after a severe injury to a major nerve has occurred. The secondary purpose of this research project is to evaluate whether transplanted Schwann cells can enhance recovery of sensory and motor function.
Qualification summary: This study is for people aged 18-65 with a severe recent nerve injury in their arm, leg, or sciatic nerve, but excludes people with certain medical conditions, pregnancy, or substance abuse. Status: Enrolling
Principal Investigators: Allan D. Levi, M.D., Ph.D.
Contact: George Jimsheleishvili, M.D. | phone: +1-305-243-4781| email: gxj150@med.miami.edu
SYSTEMIC HYPOTHERMIA IN ACUTE CERVICAL SPINAL
Purpose: This clinical trial investigates the use of intravascular hypothermia to improve neurological outcomes in acute cervical spinal cord injury patients. By potentially reducing dependence on caretakers and increasing patient productivity, this approach could benefit both patients and society.
Qualification summary: This study is for adults 18-70 with a moderate or severe spinal cord injury (AIS A-C) from a non-penetrating blow, who can receive cooling treatment within 24 hours. It excludes people with very severe injuries, pre-existing health conditions, or who are pregnant. (contact for more information)
Status: Enrolling
Principal Investigator: Allan D. Levi, M.D., Ph.D.
Contact: George Jimsheleishvili, M.D. | phone: 305-243-4781| email: gxj150@med.miami.edu
SPINAL CORD INJURY REGISTRY (NORTH AMERICAN CLINICAL TRIALS NETWORK)
Summary: The NACTN Spinal Cord Injury Registry aims to understand the natural course of recovery and complications in newly injured patients by collecting one-year follow-up data. This information will help design future clinical trials by identifying high-risk periods and expected recovery patterns.
Qualification summary: This study is for men or women 18 or older with a new traumatic spinal cord injury causing paralysis, weakness, or numbness, who haven’t received prior medical care for this injury at a specific type of hospital, and can consent to participate. (contact for more information)
Status: Enrolling
Principal Investigator: James D. Guest, M.D., Ph.D.
Contact: George Jimsheleishvili, M.D. | phone: 305-243-4781| email: gxj150@med.miami.edu
STAKEHOLDER PERCEPTIONS AND CLINICAL ASSESSMENT OF CARDIOMETABOLIC DISEASE/ SYNDROME AFTER SPINAL CORD INJURY
Purpose: This study investigates whether individuals with spinal cord injury (SCI) are aware of their risk for heart disease, diabetes, and other health problems, and how these risks change over two years post-injury. Researchers will compare participants’ self-reported awareness with actual measurements of their risk factors.
Qualification summary: Motor complete or incomplete, C5-L1, traumatic spinal injury; 18 – 70 years old; within 2 months of discharge from initial rehabilitation post-injury. (contact for more information)
Status: Enrolling
Principal Investigator: Mark S. Nash, Ph.D.
Contact: Katherine Martinez | phone: (786)-540-4104 | email: kxm940@miami.edu
MALE FERTILITY EVALUATION
Purpose: To determine the cause of low sperm motility in men with SCI.
Qualification summary: Adult men with spinal cord injury. (contact for more information)
Status: Enrolling
Principal Investigator: Emad Ibrahim, M.D.
Contact: Orrey Padilla | phone: +1-305-243-1491 | email: oxp210@ med.miami.edu
BODY COMPOSITION, POSTPRADNIAL FAT METABOLISM,
Purpose: To study relationships between body composition (e.g., percent body fat), chronic pain, and measures of inflammation in blood in individuals with chronic spinal cord injury (SCI).
Qualification summary: Age 18-65 years; SCI occurring at least 2 years prior to study entry; neurological level of injury (LOI) between C4 and L2; American Spinal Injury Association Impairment Scale (AIS) A-D. (contact for more information)
Status: Enrolling
Principal Investigators: Elizabeth Felix, Ph.D.
Contact: Lais Vidotto | phone: 305-243-3860 | email: lxv429@miami. edu
NERVE TRANSFER FOR RESTORING UPPER EXTREMITY MOTOR FUNCTION AFTER TETRAPLEGIA
Purpose: The purpose of the study is to investigate the effectiveness of nerve transfer surgery in improving hand function for spinal cord injury patients. The study will assess hand strength, range of motion, and other factors to determine if the surgery offers functional benefits.
Qualification summary: This study is for people 18-65 with chronic tetraplegia who haven’t improved in 3 months and meet specific muscle function criteria. It excludes people with active infections, ongoing recovery, certain medical conditions, and other specifics. (contact for more information)
Status: Enrolling
Principal Investigator: S. Shelby Burks, M.D.
Contact: George Jimsheleishvili, M.D. | phone: 305-243-4781| email: gxj150@med.miami.edu
HAND FUNCTION THERAPY USING BRAIN-CONTROLLED EPIDURAL SPINAL STIMULATION AFTER INCOMPLETE SCI
Purpose This study aims to improve hand and arm function by investigating the use of a non-invasive brain-computer interface (BCI) to detect motor intention-based cortical signals, which will trigger epidural spinal cord stimulation (ESCS) in individuals with SCI.
Qualification Summary AIS B-D, Injury level C4-C6, more than 6 months post-injury, and willing to obtain minimally invasive-surgical spinal cord stimulation.
Status: Enrolling
Principal Investigator: Matija Milosevic, Ph.D.
Contact: Letitia Fisher | phone: 305-243-3056 | email: lfisher@med. miami.edu
CUNEIFORM NUCLEUS DEEP BRAIN STIMULATION FOR GAIT FACILITATION FOLLOWING SCI
Purpose: This study aims to investigate the effectiveness of deep brain stimulation (DBS) of the cuneiform nucleus in improving walking ability and other functions for people with incomplete spinal cord injury. Researchers will implant electrodes in 6 participants and assess changes in gait speed, endurance, and quality of life.
Qualification summary: This study is for people with incomplete spinal cord injury (walking difficulties but some leg movement) who are at least 1 year post-injury, mentally sound, and able to commit to a 6-month training program. It excludes people with severe depression, cognitive impairment, substance abuse, certain medical conditions, or pregnancy. (contact for more information)
Status: Approved, not yet recruiting
Principal Investigator: Brian R. Noga, Ph.D. and Jonathan R. Jagid, M.D.
Contact: Letitia Fisher | phone: 305-243-3056 | email: lfisher@med. miami.edu
MECHANISMS OF NEUROPLASTICITY FOR RESTORATION OF WALKING AFTER INCOMPLETE SCI
Purpose: This short-term study aims to improve leg function by investigating the neuroplastic mechanisms and optimization of noninvasive brain-computer interface controlled transcutaneous spinal cord stimulation (BCI-TSCS) in individuals with SCI.
Qualification summary: AIS B-D, Injury level T10 and above, more than 6 months post-injury
Status: Enrolling
Principal Investigator: Matija Milosevic, Ph.D.
Contact: Patricia Graham | phone: 305-243-5119 | email: pgraham1@ med.miami.edu
SELF-REPORT ACCURACY OF THE ASIA CLASSIFICATION EXAM IN SCI
Purpose: This study aims to compare how people with spinal cord injuries perceive their own level of function compared to how doctors assess it through a standardized exam. Researchers will ask participants about their injury and then conduct a detailed physical examination to measure nerve damage.
Qualification summary: Adults with a spinal cord injury or disease from any cause. (contact for more information)
Status: Enrolling
Principal Investigator: W. Dalton Dietrich, Ph.D.
Contact: Nilanjana Datta, M.D. | phone: 305-243-7108 | email: nxd693@med.miami.edu
EQUITY AND QUALITY IN ASSISTIVE TECHNOLOGY (EQUATE)
Purpose: To study the assistive technology used by individuals with spinal cord injury (SCI) and those with diagnoses that affect the spinal cord.
Qualification summary: This study is for adults (18+) with a spinal cord injury (affecting movement or sensation) from at least a year ago, who can understand English and have internet access to participate. (contact for more information)
Status: Enrolling
Principal Investigators: Elizabeth R. Felix, Ph.D.
Contact: Matthew DeVlieger | phone: 786-496-0448 | email: mxd4401@med.miami.edu
ASSOCIATION OF SCI PHYSICAL ACTIVITY GUIDELINES AND HEALTH OUTCOMES
Purpose: To understand whether meeting the spinal cord injury physical activity guidelines is associated with positive health-related outcomes.
Qualification summary: Adults with chronic spinal cord injury. (contact for more information)
Status: Enrolling
Principal Investigators: Elizabeth R. Felix, Ph.D.
Contact: Katherine Martinez | phone: (786)-540-4104 | email: kxm940@miami.edu
BODY COMPOSITION, POSTPRADNIAL FAT METABOLISM AND PAIN IN SPINAL CORD INJURY
Purpose To study relationships between body composition (e.g., percent body fat), chronic pain, and measures of inflammation in blood in individuals with chronic spinal cord injury (SCI).
Qualification summary Age 18-65 years; SCI occurring at least 2 years prior to study entry; neurological level of injury (LOI) between C4 and L2; American Spinal Injury Association Impairment Scale (AIS) A-D. (contact for more information)
Status: Enrolling
Principal Investigator: Elizabeth Felix, Ph.D.
Contact: Lais Vidotto | phone: 305-243-3860 | email: lxv429@miami. edu
Purpose: The purpose of this study is to determine the relationship between nutritional needs, metabolic health, and body composition of individuals with and without spinal cord injury. Participants will maintain a diet log at home, interview with a registered dietician, complete a body composition analysis, and participate in fasting blood draw and medical screening.
Qualification summary: This study is for adults (18-65 years old) with complete motor loss (AIS A, B, C) in their spine (C4-L2 region) from an injury at least one year ago. (contact for more information)
Status: Enrolling
Principal Investigator: Gary Farkas, Ph.D.
Contact: Gary J. Farkas, Ph.D. | phone: 305-243-4581 | email: gjf50@ med.miami.edu
ALTERED BODY REPRESENTATION IN PEOPLE WITH SPINAL CORD INJURY AND ITS ASSOCIATION WITH PAIN SENSATION
Purpose: To define changes in body ownership underlying compromised multisensory integration in spinal cord injury (SCI) individuals by using the rubber hand illusion procedure (RHI); ii) to determine the relation between compromised multisensory integration after SCI and its impact on pain.
Qualification summary: This study is recruiting three groups of adults (18-50 years old) for a pain research visit: people with incomplete spinal cord injury and chronic pain, people with incomplete spinal cord injury but no pain, and healthy volunteers with no history of injury. (contact for more information)
Status: Enrolling
Principal Investigator: Eva Widerstrom-Noga, Ph.D.
Contact: Linda Robayo Riofrio, Ph.D. | phone: 305-243-0233 | email: linda.e.robayo@miami.edu
EFFECTS OF BODILY ILLUSION AND TDCS ON SCIRELATED NEUROPATHIC PAIN
Purpose: To determine to what extent neuropathic pain following spinal cord injury (SCI) is reduced after a non-pharmacological treatment involving bodily illusion (BI) and transcranial Direct Current Stimulation (tDCS).
Qualification summary: This study seeks men and women aged 18-70 with incomplete spinal cord injuries in the neck (cervical) region and chronic pain lasting at least 3 months (moderate or worse, rated 4 or higher on a 0-10 scale). (contact for more information)
Status: Enrolling
Principal Investigator: Eva Widerstrom-Noga, Ph.D.
Contact: Roberta Vastano, Ph.D. | phone: 305-243-0233 | email: rxv331@med.miami.edu
PHYSIOLOGICAL AND BEHAVIORAL REGULATION OF FEEDING AFTER SPINAL CORD INJURY
Purpose: The goal of this study is to determine the effects of spinal cord injury on appetite and gut metabolism following 2 meals in adults with and without SCI.
Qualification summary: Adult men and women with complete and incomplete C3-L3 chronic (at least 1-year post-injury) spinal cord injury (AIS A, B, and C). (contact for more information)
Status: Concluding recruitment
Principal Investigators: Gary J. Farkas, Ph.D.
Contact: Gary J. Farkas, Ph.D. | phone: 305-243-4581 | email: gjf50@ med.miami.edu
COMPARING CLINICALLY VALIDATED UPPER-EXTREMITY ASSESSMENTS IN SCI
Purpose: This study aims to compare four common assessments used to measure upper limb function in adults with chronic tetraplegia. Researchers will assess participants using the GRASSP, CUE-T, ARAT, and Wolf Motor tests and compare their effectiveness in evaluating hand and arm function.
Qualification summary: This study is for adults (over 18) with chronic spinal cord injury in the neck (C2-C7) for over a year, regardless of injury severity (ASIA A-D), but excludes people with severe upper arm spasticity, fractures/contractures, or who cannot consent. (contact for more information)
Status: Enrolling
Principal Investigator: David W. McMillan, Ph.D.
Contact: Nilanjana Datta, M.D. | phone: 305-243-7108 | email: nxd693@med.miami.edu
TRANSFORMATIVE POTENTIAL OF ADAPTED OCEAN ACTIVITIES
Purpose: This study aims to investigate the impact of open water activities (sailing, paddling, etc.) on health and wellbeing in disabled adults. Researchers will use surveys to assess self-reported changes in quality of life, self-efficacy, and other health domains following participation in these activities.
Qualification summary: Adults with disability who have participated at least one time in one of the adapted ocean activities being studied. (contact for more information)
Status: Enrolling
Principal Investigator: David W. McMillan, Ph.D.
Contact: David W. McMillan, Ph.D. | phone: 305-243-5569 | email: dmcmillan@med.miami.edu
ACCURACY OF SELF-REPORT PATIENT INFORMATION RELEVANT TO SPINAL CORD INJURY (ASPIRE-SCI)
Purpose: This study investigates the accuracy of self-report across a variety of health measures in people living with spinal cord injury (SCI). Researchers will compare self-reported measures with validated clinical assessments per the SCI Common Data Elements (CDEs). Furthermore, we analyze how factors like time since last measurement and injury severity influence the accuracy of self-reporting.
Qualification summary: Adults with spinal cord injury or disease from any cause. (contact for more information)
Status: Enrolling
Principal Investigator: David W. McMillan, Ph.D.
Contact: Nilanjana Datta, M.D. | phone: 305-243-7108 | email: nxd693@med.miami.edu
DEVELOPING A MULTI-CHANNEL QUANTITATIVE AUTONOMIC DIAGNOSTIC TO ASSESS THE SYMPATHETIC LEVEL OF INJURY
Purpose: The study seeks to adapt the sympathetic skin response (SSR) test to determine the sympathetic level of injury (SLOI) in individuals with and without spinal cord injury (SCI), aiming to understand the correlation between sympathetic and somatic levels of injury. This investigation addresses the need for a clinically useful method to quantify sympathetic LOI, potentially enhancing practitioners’ understanding of changes in involuntary bodily functions following SCI.
Qualification summary: Adults with and without spinal cord injury. (contact for more information)
Status: Approved, not yet recruiting
Principal Investigator: David W. McMillan, Ph.D.
Contact: David W. McMillan, Ph.D. | phone: 305-243-5569 | email: dmcmillan@med.miami.edu
URSOLIC ACID FOR MUSCLE STRENGTH AND GLYCEMIC CONTROL IN SCI
Purpose: The purpose of this pilot study is to assess the potential of Ursolic Acid (UA) in mitigating muscle wasting and insulin resistance in individuals with chronic spinal cord injury (SCI). By investigating UA’s effects on muscle mass, strength, and metabolic parameters, the study aims to provide insights into potential therapeutic strategies for improving health outcomes in the SCI population.
Qualification summary: This study is for men and women aged 1865 with chronic (over 1 year) spinal cord injury, either complete or incomplete (AIS A/B/C), in either the thoracic (T2-T8) or cervical (C4-C7) spine. It excludes pregnant or breastfeeding women. (contact for more information)
Status: Concluding recruitment
Principal Investigator: Mark S. Nash, Ph.D.
Contact: Patricia Graham | phone: 305-243-5119 | email: pgraham1@ med.miami.edu
EXPLORING EFFECT OF MINDFULNESS ON QUALITY OF LIFE IN SCI
Purpose: The purpose of the study is to explore the impact of a onemonth mindfulness intervention on physiological stress markers and quality of life in individuals with spinal cord injury (SCI). By measuring changes in heart rate, blood pressure, metabolism, and quality of life scores before and after the intervention, the study aims to provide insights into the potential benefits of mindfulness practices for SCI patients.
Qualification summary: This study is for adults (18-70) with spinal cord injury (C5-T10, any severity), who are healthy and able to consent, but excludes pregnant women and those on certain medications. (contact for more information)
Status: Approved, not yet recruiting
Principal Investigator: Mark S. Nash, Ph.D.
Contact: Patricia Graham | phone: 305-243-5119 | email: pgraham1@ med.miami.edu
BRAIN-CONTROLLED SPINAL SIMULATION THERAPY FOR RESTORATION OF WALKING AFTER INCOMPLETE SCI
Purpose: The purpose of this pilot clinical trial is to investigate the therapeutic effects of non-invasive Brain-Computer Interface (BCI)Transcutaneous Spinal Cord Stimulation (TSCS) therapy for improving neuromotor recovery in individuals with incomplete spinal cord injury (SCI). Through exploring effective therapy methods and optimizing
brain decoding algorithms, the study aims to establish the feasibility and efficacy of long-term BCI-TSCS locomotor therapy, potentially offering a novel approach to enhance walking function in this population.
Qualification summary: This study is for adults (21-70 years old) with incomplete spinal cord injury (more than 6 months old) who have some leg movement and can commit to a 6-month training program. It excludes people with other neurological problems, severe spasticity, certain medical conditions, or metal implants. (contact for more information)
Status: Enrolling
Principal Investigator: Matija Milosevic, Ph.D.
Contact: Patricia Graham | phone: 305-243-5119 | email: pgraham1@ med.miami.edu
RELATION BETWEEN RESPIRATORY PERFORMANCE AND SEATED BALANCE IN SCI
Purpose: This study aims to investigate the relationship between breathing and balance in individuals with chronic spinal cord injury (SCI), focusing on how these functions impact quality of life and daily activities. Despite the known connection between breathing and balance, this link is not well-understood, especially in chronic SCI patients.
Qualification summary: Adults with spinal cord injury. (contact for more details)
Status: Re-opening for enrollment
Principal Investigator: Mark S. Nash, Ph.D.
Contact: David W. McMillan, Ph.D. | phone: 305-243-5569 | email: dmcmillan@med.miami.edu
THE MICROBIOME AFTER SCI: GASTROINTESTINAL DYSFUNCTION MECHANISMS AND MARKERS OF GI STATUS
Purpose: The purpose of this study is to investigate the impact of spinal cord injury on the intestinal microbiome and epithelium, aiming to uncover mechanisms underlying gastrointestinal dysfunction and identify potential biomarkers. By examining alterations in gut microbiome composition and intestinal tissue function, the study seeks to inform the development of personalized therapeutic interventions for individuals with spinal cord injury.
Qualification summary: This study is for people aged 18-65 with spinal cord injury (tetraplegia or paraplegia) and constipation or bowel problems, or for healthy people of the same age range. It excludes people with certain medical conditions, implants, or recent surgery, and those who are pregnant or breastfeeding. (contact for more information)
Status: Enrolling
Principal Investigator: Sylvia Daunert, PharmD, Ph.D.
Contact: Gregory O’Connor, Ph.D. | phone: 305-243-6282 | email: goconnor@miami.edu
IMPROVEMENT OF REPRODUCTIVE FUNCTION IN MEN WITH SPINAL CORD INJURY (SCI)
Purpose: This study focuses on infertility, a common issue among men with spinal cord injuries. While most men with spinal cord injuries have a normal sperm count, they often experience reduced sperm motility, meaning their sperm have difficulty swimming. This research aims to assess whether an oral medication called “PROBENECID” can improve sperm motility in men with spinal cord injuries.
Qualification Summary: Inclusion criteria include male subjects aged 18 years or older with traumatic spinal cord injury.
Status: Enrolling
Principal Investigator: Emad Ibrahim, M.D., HCLD(ABB)
Contact: Odaro Ugbo B.Sc. | oxu32@med.miami.edu
The Office of Education and Outreach at The Miami Project to Cure Paralysis continues its quest to engage any and all who share in The Miami Project’s mission to understand, care for, and eventually cure paralysis from neurotrauma and other causes. This year, the Office maintained core programming while also expanding into new communities and realms.
The 2024 South Florida BrainBee, a neuroscience competition for teens hosted by the Office, draws young talent with a distinct curiosity for the central nervous system. This year’s winner, Archith Venkat, demonstrated an exceptional grasp of neuroscience and earned the opportunity to advance to the BrainBee’s national-level competition. The Office once again hosted its popular BrainFair exhibit at the Miami-Dade County Public Schools’ STEAM Expo. The BrainFair remains a valuable collaborative touchstone for local neuroscientists at Florida International University, Barry University, and St. Thomas University to come together via the Office and inspire younger generations to study neuro-related topics.
The Miami Project’s ongoing partnership with Shake-A-Leg Miami (SALM) was expanded upon through University of Miami’s flagship Innovation, Technology, and Design (ITD) program. David McMillan, Ph.D., co-taught, with Kirsten Schwarz, the experiential project-based course Design Challenge 5 & 6: Iterate and Prototype (ITD256). The class centered around the theme of disability and aquatic technologies, and developed projects on this topic in partnership with Royal Caribbean Cruises and Shake-A-Leg Miami. Students worked closely with these partners to address real-world design challenges using design thinking paradigm. Each partner organization defined a unique design challenge specific to their disability maritime needs. With existing accessibility rapport on their boats, Royal Caribbean put forth a project not on their cruise ships but on their private island. Once on the water, Royal had already implemented an array of accessibility
Opposite page: Medical students learn to use a Hoyer lift in a clinical skills workshop on safe


solutions that give them a great reputation in the disability community and made them a perfect co-partner alongside SALM’s aquatic disability beat. But once on their island, patrons were facing challenges participating in all the tropical activities that are at the core of what imagined in a Caribbean vacation. On SALM’s side, the organization was recently gifted 68-ft houseboat that was in the process of being made accessible to align with their mission and cater to their long-standing community of people living with disability. The design challenge included a wheelchair lift to access the top deck, and a mixed-use event space for a list of educational, social, and developmental programming will be enacted by SALM. As summarized by Dr. McMillan, “I could not be more thrilled to be educating a group of talented undergraduate students, in a unique program, by way of outreach to organizations that have a proven history of providing accessible services to the disability community.”
Riding the wave of excitement for Neuralink’s PRIME trial, the Office has been facilitating the robust recruitment efforts for this study while fielding inquiries from interested


Matija Milosevic, Ph.D. shows his Neuromodulation Engineering & Therapeutics Laboratory to participants at the 2024 Open House
parties involving the burgeoning topic of implanted braincomputer interface (iBCI). Along with the high content recruitment needs, Dr. McMillan delivered intramural and extramural presentations to interested neuroscience undergraduates, medical students, therapists and residents, and many other educational stakeholders in The Miami Project’s network. Along with conducting the PRIME trial, the broad audience wrapped up in excitement about iBCI offers an education and outreach opportunity to invite new stakeholders to join us on “team neuro.”
The Office facilitated TMP’s ninth open house, the first since the pandemic and the first including the new facilities at Christine E. Lynn Rehabilitation Center

2024 Henry G. Steinbrenner Scholars Program participants
for The Miami Project to Cure Paralysis at UHealth/ Jackson Memorial. The open house welcomed the paralysis community for a day of research updates, laboratory tours, and hands-on demonstrations. Attendees enjoyed information on innovations in neuromodulation, robotics, and drug discovery, including an update from Dr. Hassan Al-Ali on the exciting Blueprint Neurotherapeutics Network (BPM) for Small Molecule federal award that he leads for development of a drug for restoring the damaged central nervous system. After morning lectures, attendees, including some who traveled from out of state to participate, transitioned to the Lynn Rehabilitation Center to directly interact with scientists in the growing laboratories in the new state-of-the-art hospital.
Educationally, 2024 marked another successful year of the Henry G. Steinbrenner Scholar Program, and the 2025 cohort has been selected. The Miami Project welcomes Lauren Bickel, Selene Cabrera, Aashi Chhabra, Adriana Guerra, Hai Anh Le, Benjamin McCulley, Evdokia Pechlivanidou, Hunter Rosenzweig, Lauren Spencer, and Gabriel Barros to the Steinbrenner Program and the Office looks forward to stewarding the Scholars through the immersive, competitive, funded, research-driven summer internship.

For more information about these programs or how you can get involved, contact The Miami Project’s Office of Education and Outreach at 305-243-7108 or mpinfo@ miami.edu.
Each year, scientists at The Miami Project seek funding for their research by submitting proposals to the National Institutes of Health, the Department of Defense, and other funding agencies and foundations.
Their scientific peers rate the merits of the proposed experiments in a highly competitive process, and only the best projects are funded. The agencies and organizations listed here supported research at The Miami Project during 2024.
Grants & Contracts
Restricted Gifts
Endowment
IDC Allocation
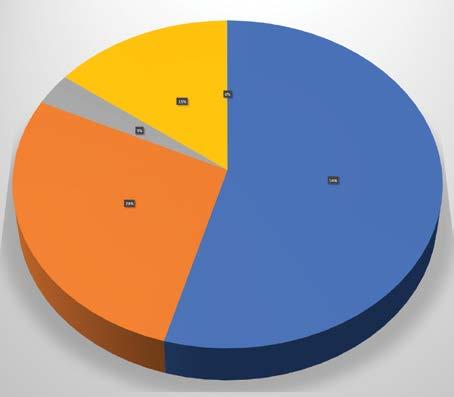
Research Programs
Fund Raising
General Administration Income Expenses
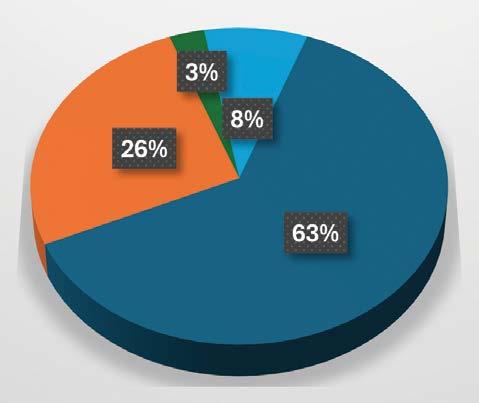
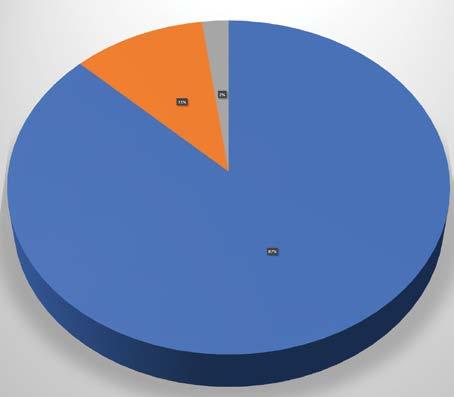
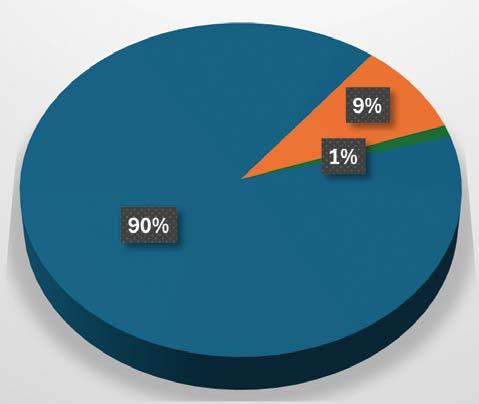
National Institute of Neurological Disorders and Stroke (NINDS)
Prasad A (PI) Effects of Iron Accumulation in Intracortical Implants and Protection by Iron Chelation
Levi A (PI) University of Miami Neurosurgery eDucation Strategy (UMINDS)
Liebl D (PI) Stabilizing the tripartite synaptic complex following TBI
Ali H (PI) Developing a kinase inhibitor drug to treat spinal cord injury
Lee J (PI) Targeting cell-type specific disease phenotypes to promote CNS repair
Lee J (PI) Targeting lipid clearance pathways to promote repair after SCI
Ganzer P (PI) Enhancing Sympathetic Outflow Control Following Spinal Cord Injury Using Targeted Plasticity Therapy
Starke R (PI) Endothelial cell dysfunction in cerebral aneurysm pathogenesis
Lemmon V (PI) Pan-Neurotrauma Data Commons
Dietrich W (PI) and de Rivero Vacari JP (Co-I) The Importance of Abnormal Inflammasome Activation as a Risk Factor between Traumatic Brain Injury and Alzheimer’s Disease
Tsoulfas P (PI) (Marquette University) Combinatorial Manipulation of Transcription Factors to Promote CNS Regeneration
Tsoulfas P (PI) (Marquette University) Global Analysis of the supraspinal connectome after spinal cord injury
National Institutes of Health (NIH)
Dietrich W (PI) Human Schwann Cell-Derived Exosome Treatment for Traumatic Brain Injury
Lee J (PI) Regeneration-permissive glia after spinal cord injury
National Institute of General Medicine Sciences (NIGMS)
Dumont C (PI) Glycosaminoglycanenabled technologies to reprogram chronic inflammatory states
National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR)
Nash M (Co Project Director) South Florida Spinal Cord Injury Model Systems
National Science Foundation (NSF)
Dumont C (PI) CAREER: Engineering the nanoparticle interface for tunable biomolecular aggregation
National Safety Council (NSC)
Hotz G (PI) Enhancing and promoting active school travel through a safe system approach
Department of Defense (DOD) and United States Army Medical Research Acquisition Activity (USAMRAA)
Levi A (PI) Systemic Hypothermia in Acute Cervical Spinal Cord Injury – A Prospective Case Controlled Study
Levi A (PI) Clinical feasibility and potential efficacy of Autologous Human Schwann Cell (ahSC) augmentation after severe Peripheral
Nerve Injury (PNI) involving volumetric muscle loss by tissue regeneration
Noga B (PI) DBS Orientation Selective Pathway Activation for Gait Ignition Following SCI
Pearse D (PI) Targeting the arginylation pathway Neuroprotection After SCI
Pearse D (PI) Functionalized Peptide Gels and Schwann Cells for Repair of the Injured Spinal Cord
Ibrahim E (PI) A Novel Therapy to Improve Reproductive Potential in Men with Spinal Cord Injury (SCI)
Widerström-Noga E (PI) Consumer perspectives on a multimodal approach to make neuropathic pain manageable after SCI

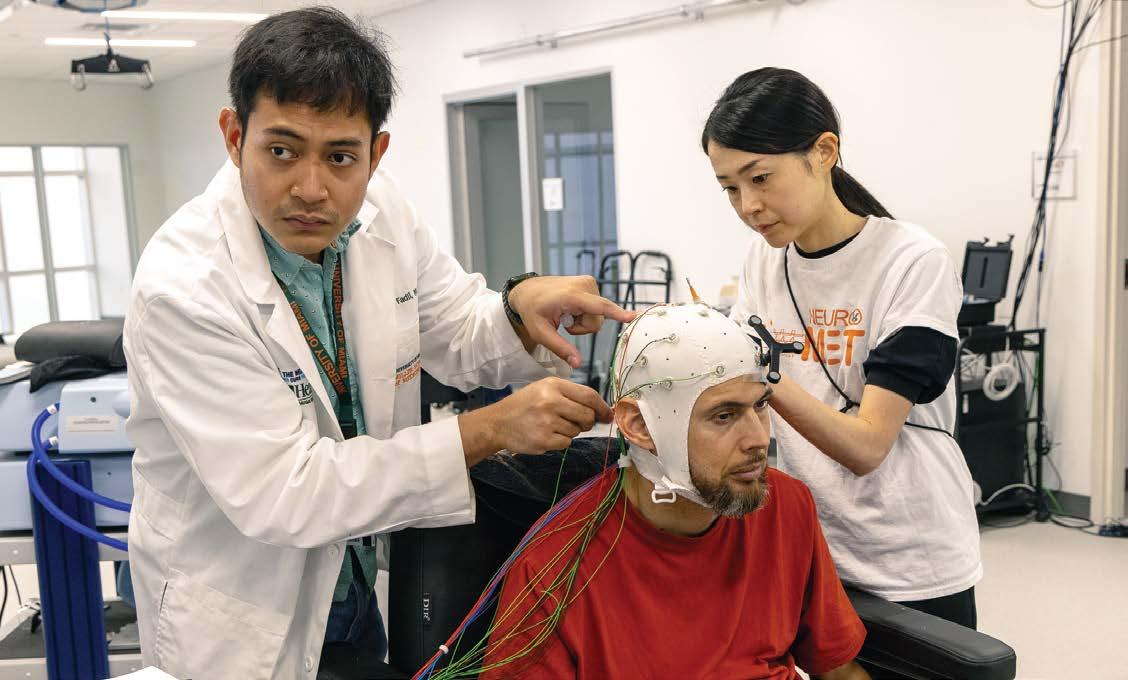
Rizaldi Ahmad Fadli and Akiko Yuasa prepare a participant for brain-computer interface controlled neuromodulation.
Widerström-Noga E (PI) Mechanisms and Utility of Multisensory Body-Representation in SCI and SCI-Related Neuropathic Pain
Sagen J (PI) Development of human iPSC-derived chromaffin cell transplants for the treatment of chronic pain
Sagen J (PI) Alleviation of chronic SCI neuropathic pain using novel engineered human iPSC-derived chromaffin cell grafts
Sagen J (PI) (SUNY) Testing RNA aptamers as analgesic candidates in rat model of SCI pain
Dietrich W (PI) US Army Battlefield Exercise and Combat Related Traumatic Brain and Spinal Cord Injury Research
Lee J (PI) Macrophage-Specific Drug Targets for Treating SCI
Burks S (Site PI) Nerve transfers to improve upper extremity function and quality of life in tetraplegic patients
Burks S (PI) Nerve Transfers in high tetraplegia to improve patient function and quality of life
Veterans Affairs Medical Center (VAMC) & U.S. Department of Veteran Affairs (VA)
Kerr N (Co-I) (Raval A) IPA Therapeutic interventions for poststroke rehabilitation
Dietrich W (Co-I) (Raval A) IPA Therapeutic interventions for poststroke rehabilitation
Bramlett H (PI) Novel Pharmacological and NonPharmacological Interventions for Bone Loss in SCI
Sagen J (PI) Evaluation 6SHG/EM1 as a treatment for spinal cord injuryinduced neuropathic pain in a pig model
Lemmon V (Co-I) (Hao S) IPA The molecular mechanisms of astrocytesneurons interaction in morphine use disorder
Florida Department of Transportation (FDOT)
Hotz G (PI) BikeSafe Middle School Clubs in Miami-Dade County
Hotz G (PI) BikeSafe Elementary School Program in Miami-Dade County
Hotz G (PI) Sustainability and Expansion of the WalkSafe and BikeSafe Programs for K-8 Grades in Miami-Dade County
Florida Department of Health (FDOH)
Bramlett H (PI) Post-stroke combination of therapeutic hypothermia (TH) and whole bodyvibration (WBV) improves cognition in nicotine-exposed rats
Starke R (PI) Cigarette smoke induces endothelial dysfunction leading to cerebral aneurysm pathogenesis
Dietrich W (PI) SOF Alzheimer’s Disease COHFA
Vontell R/de Rivero Vaccari (PI’s) Project 1 – The importance of ASC as a diagnostic biomarker for AD in MCI Patients
Loewenstein D/Curiel R (PI’s) Project 2 – Highly sensitive surrogate markers for the diagnostic and treatment of Alzheimer’s Disease
Cortes D (PI) Project 3 –Development and testing of a radioligand ASC tracers for detection of early AD
Kerr N/Bramlett H/Dietrich W (PI’s) Project 4 – The role of the gut-brain axis after TBI in Alzheimer’s Disease
Dietrich W (PI) State of FL Brain and SCI Trust Fund - Brain and Spinal Cord Injury Research
Dietrich W (PI) SOF General Revenue COPBV
Dietrich W (PI) The Administrative Core
Lee J/Ali H (PI’s) Project 1Mechanisms of lipid accumulation in glia after spinal cord Injury
Pearse, D (PI) Project 2 –Enhancing the reparative efficacy of Schwann cells after SCI
Ali, H/Brambilla, R (PI’s) Project 3 - Phenotypic assays to accelerate the discovery of CNS injury therapeutics with synergistic modes-of-action
Atkins, C (PI) Project 4Dissecting the Functional Role of ApoE in the Pathogenesis of TBIInduced Alzheimer’s Disease
De Riveo JP/Ibrahim E (PI’s) Project 5 – Effects of inflammasome activation on male infertility after SCI in rats
Milosevic M (PI) Project 6 –Optimizing spinal stimulation to improve lower limb neuromodulation
State of Florida (MMJ Outcomes)
Widerström-Noga (PI) Effects of cannaboidiol on resting state EEG and neuropathic pain severity in people with spinal cord injury
New Jersey Commission on Spinal Cord Research (Rutgers University)
Atkins C (PI) Defining PDE2A directed mitochondrial dysfunction in TBI-related Alzheimer’s disease
Lee J (PI) Effective Modulation of Inflammation for Enhanced SCI Repair using a Hybrid LNP-based Peptide Hydrogel DDS
Craig H. Neilsen Foundation (CNF)
Sagen J (PI) Human iPSC-derived chromaffin cell transplants for alleviation of chronic neuropathic SCI pain
Lemmon V (PI) Bixby J (Co-PI)
Ali H (Co-PI) Novel and potent compounds that promote axon growth
Christopher & Dana Reeve Foundation (CRF)
Guest J (Center P.I. and Co-chair) North American Clinical Trials Network for the Treatment of SCI
Florida Breast Cancer Foundation
de Rivero Vaccari JP (PI) Inflammatory Responses in Radiosensitivity and Breast Cancer Prognosis Disparity
Bryon Riesch Paralysis Foundation
Ghosh M (PI) Targeting innate immune response to promote histopathological and locomotor function after spinal cord injury repair
Milosevic M (PI) Brain-Controlled Spinal Neuromodulation System for Walking
Alzheimer’s Association
Kerr N (PI) Pyroptosis-induced gut-brain disruption after Stroke in Alzheimer’s disease
Fondazione Italiana Sclerosi Multipa (FISM)
Brambilla R (PI) Cognitive dysfunction in multiple sclerosis: role of astroglial TNFR2 signaling
NeuroTrauma Sciences
Atkins C (PI) Screening of Novel Neuroactive Steroids for Traumatic Brain Injury
Elsa U. Pardee Foundation
Shah A (PI) Targeting Epigenetic Regulators of Endogenous Retroviruses to potentiate Immunotherapy for Glioblastoma
NASA Headquarters
de Rivero Vaccari J (PI) Inflammation and brain health for astronauts
Onward Medical Inc.
Guest J (PI) Clinical Assessment of upper extremity performance in
individuals with spinal cord injury using the LIFT System to deliver non-invasive electrical stimulation (ARC Therapy)
Friends of Underline (FUL)
Whitte M (PI) The Way Kids Roll: Safety
Miccosukee Corporation
Hotz G (PI) SS4A Planning and Demonstration Grant for the Miccosukee Tribe in Florida
University of California SF (Abbott Laboratories)
Hotz G (PI) Clinical Evaluation of the i-STAT TBI Test
The faculty of The Miami Project are a talented multidisciplinary team. In the following Profiles, each faculty member describes their specific research focus and highlights of recent progress.

W. DALTON DIETRICH, PH.D.
Scientific
Director
Kinetic Concepts Distinguished Chair in Neurosurgery
Senior Associate Dean for Discovery Science
Co-Director, Institute for Neural Engineering
Professor, Neurological Surgery, Neurology, Biomedical Engineering and Cell Biology
Neuroprotection and Improved Recovery of Function following CNS Trauma
My laboratory is focused on clarifying the pathophysiology of brain and SCI with the goal of developing new therapies to protect and enhance functional recovery. My colleagues and I have studied the cellular and molecular injury mechanisms underlying various neurological disorders including stroke, TBI, SCI, and neurodegenerative diseases. We have advanced our pre-clinical therapeutic hypothermia findings into the clinic where we are conducting clinical trials in patients with SCI. Recently, we have also investigated the importance of abnormal inflammasome activation in the brain and SCI and identified a novel therapeutic target for neuroprotection. We are using novel cellular and drug treatments to promote reparative processes and functional recovery. My laboratory is funded by the NIH and DoD and I also train the next generation of scientists and clinicians in neurotrauma research

ALLAN D. LEVI, M.D., PH.D., FACS
Clinical Director, The Miami Project
Professor and Chairman Department of Neurological Surgery
Professor, Orthopedics, and Rehabilitation Medicine
Chief of Neurosurgery, Jackson Memorial Hospital
Robert Buck Distinguished Chair in Neurological Surgery
Cellular Transplantation Strategies after SCI/Systemic Hypothermia after Acute SCI
My clinical research interests currently focus on developing cellular transplantation strategies to repair injuries within both the human central and peripheral nervous system. I am currently Co-PI on our clinical trials involving the transplantation of autologous human Schwann cells (SCs), which represent first-in-man studies of autologous human SCs for patients with sub-acute and chronic SCI. We are also conducting a phase I trial evaluating SCs for peripheral nerve injuries with long segmental defects, which follows up on our previous two single patient experiences. Hypothermia continues to show promise in a variety of acute central nervous system injuries. There are various factors that need to be considered with systemic cooling of the SCI patient, including methods of cooling, window from injury to initiation, duration and depth of hypothermia, rate of re-warming, etc. While profound levels of hypothermia (<32°C) can be difficult to administer and are subject to increased complication rates, mild (modest) levels of hypothermia (32-34°C) have been shown to provide significant protection against traumatic and ischemic neuronal cell death. I am currently the PI of our institutional protocol as well as a multi-center Department of Defense funded randomized trial studying systemic hypothermia induced via an intravascular catheter and continued for 48 hours after acute cervical SCI.

BARTH A. GREEN, M.D., FACS
Professor of Neurological Surgery, Neurology, Orthopedics, and Rehabilitation
Co-Founder and Chairman, The Miami Project to Cure Paralysis
Executive Dean for Global Health and Community Service
Professor, Neurological Surgery, Neurology, Radiology, Orthopaedics and Rehabilitation Medicine
Translational Interventions
Over the recent years my research efforts have mainly involved taking the cutting edge basic neuroscience work product and data created by our Miami Project team from the bench to our UM affiliated clinics and hospitals. A good example of such translational research efforts has included the use of modest hypothermia for neuroprotection both in cases of acute spinal cord injury and for use in the operating room for patients undergoing high risk spinal cord surgery. I am also privileged to be able to collaborate with The Miami Project cellular transplantation programs and have been working on projects involving adult mesenchymal stem cells, as well as being part of the major effort transforming our successful Schwann cell laboratory model into clinical trials. Other areas of research and clinical interest include the diagnosis and treatment of tethered cord syndrome, spinal cord cysts and Chiari I malformation.
HASSAN A ALI, PH.D.
Associate Professor, Department of Neurological Surgery and Medicine
Drug Discovery for CNS Repair
As a chemical and computational biologist, my lab focuses on identifying pharmacological targets that can induce robust axon regeneration in the injured central nervous system. To accomplish this, my lab developed a unique drug discovery platform that combines phenotypic screening, target-based profiling, and sophisticated machine learning algorithms. The approach identified a promising drug candidate that is now in preclinical development. We continue to develop these methodologies to advance drug discovery in spinal cord injury, as well as in other therapeutic areas including cancer and kidney disease.

COLEEN ATKINS, PH.D.
Associate Professor, Department of Neurological Surgery

Developing Novel Therapies for Traumatic Brain Injury and Spinal Cord Injury
The research in my laboratory focuses on developing novel therapeutic interventions for traumatic brain injury (TBI) and spinal cord injury (SCI). The research goal of my laboratory is to enhance rehabilitation and recovery by manipulating synaptic plasticity at specific levels of the neuroaxis following TBI and SCI. We have found that specific synaptic plasticity signaling pathways are altered after TBI, and we are currently using pharmacotherapies to target those pathways to improve behavioral recovery after TBI.

ROBERTA BRAMBILLA, PH.D.
Associate Professor, Department of Neurological Surgery
Modulation of the Neuro-Immune Response in Neurologic Disease
The main focus of my research is to understand the role of neuroinflammation in the pathophysiology of neurodegenerative disorders (e.g., multiple sclerosis, spinal cord injury and stroke), with a specific interest in the contribution of glial cells. We study astrocytes and microglia for their involvement in the neuro-inflammatory response to injury, and oligodendrocytes and oligodendrocyte precursor cells for their role in axon myelination, metabolic support of neurons and myelin repair. Currently, our primary lines of research in the area of neuroimmunology are centered on: (1) investigating the role of tumor necrosis factor and its receptors in the processes of neuroinflammation, demyelination and remyelination, and (2) understanding how mitochondrial dysfunction in oligodendrocytes may be involved in the etiopathology of multiple sclerosis.
HELEN M. BRAMLETT, PH.D.
Professor, Departments of Neurological Surgery and Psychology, Undergraduate Neuroscience
Program Director, and Health Scientist Veterans Affairs
The Pathophysiology and Treatment of CNS Injury
The focus of my neurotrauma laboratory is to investigate both acute and long-term consequences of brain and spinal cord trauma. My current research interests are on the pathophysiology of traumatic brain and spinal cord injury with an emphasis on the pathogenesis of progressive white matter damage as well as the benefits of therapeutic hypothermia. My laboratory is also investigating mechanistic events leading to the development of posttraumatic epilepsy. Additionally, our current work is also focusing on complex traumatic brain injury models that mimic polytrauma as this type of injury has become more prevalent in combat areas.

S. SHELBY BURKS, M.D., FAANS
Assistant Professor, Department of Neurological Surgery

Spinal Cord Injury, Peripheral Nerve Regeneration, Schwann Cells, and Exosomes
Our lab emphasizes both clinical and preclinical research. We are conducting a clinical trial exploring a combination of surgery, regeneration, and rehabilitation to recover function after paralysis. Specifically, we are actively investigating nerve transfer in those with spinal cord injury to restore reaching and grasping function. Additionally, we are exploring challenges in traumatic peripheral nerve injury clinically. Peripheral nerve injury, especially injury-in-continuity, possess challenges for the nerve surgeon. By combining a host of technologies and translational research, we aim to improve outcomes for patients suffering from severe peripheral nerve injury.
JUAN PABLO DE RIVERO VACCARI, PH.D., M.S.B.A.
Associate Professor, Department of Neurological Surgery
Underlying Mechanisms of the Immune Response and Contributions to Various CNS Diseases
My research focuses on understanding early inflammatory events in central nervous system (CNS) injury and disease, as well as aging. Currently, my laboratory studies how natural-aging produces inflammation in the brain, a phenomenon known as brain inflammaging, which potentially precedes the onset of age-related neurodegenerative diseases. In addition, we are studying the mechanism by which brain injury causes systemic inflammation such as acute lung injury. Moreover, we also study the prognostic and diagnostic potential of inflammasome proteins as biomarkers of CNS injury and disease, including brain and spinal cord injury, stroke, multiple sclerosis, mild cognitive impairment and depression.



COURTNEY M. DUMONT, PH.D.
Assistant Professor, Department of Biomedical Engineering
Biomaterial Approaches for Spinal Cord Injury Repair
We are currently exploring the integration of nanoscale biomaterials with our scaffolds to improve on-demand pharmacological agent localization within the injury site as well as cytokine scavenging capabilities to reduce inflammation. Together, these strategies will help to move the field of neural tissue engineering forward, while also providing essential information about the underlying cellular biology and injury pathophysiology.
GARY J. FARKAS, PH.D., MSCTI
Assistant Professor, Department of Physical Medicine and Rehabilitation
Neurogenic Obesity, Energy Metabolism, and Appetite in Spinal Cord Injury
My lab is dedicated to understanding and addressing neurogenic obesity, diet/nutrition, appetite, eating behavior and their association with cardiovascular complications in individuals with spinal cord injury (SCI). We investigates the underlying neurobiobehavioral mechanisms contributing to obesity and food intake after SCI and develops and tests interventions to improve the management of these conditions and overall health outcomes. Our laboratory employs a multidisciplinary approach encompassing physiological, behavioral, and neurological techniques to test hypotheses. His research aims to translate findings into effective prevention and treatment strategies that enhance the quality of life for individuals with SCI while also reducing cardiovascular risk.
PATRICK D. GANZER, PH.D.
Assistant Professor, Department of Biomedical Engineering
Neural Engineering, Brain-Computer Interfaces, Bioelectronic Medicines and Machine Learning
Our overall research is focused on developing neurotechnology to treat disease and dysfunction. Our preclinical research focuses on using neuromodulation to promote neuroplasticity of damaged control systems and subsequent recovery. Here we use a variety of techniques including brain mapping, 2-photon microscopy, optogenetics, transsynaptic tracing, tissue clearing, nerve stimulation, and awake-behaving physiological recordings. Our clinical research compliments these preclinical studies and is conducted at the nationally ranked Christine E. Lynn Rehabilitation Center located on the Miller School of Medicine campus. Importantly, several of our efforts are in various stages of clinical testing to help promote recovery of lost function in patients.


MOUSUMI GHOSH, PH.D.
Research Associate Professor, Department of Neurological Surgery
Altering Host Glial Responses following CNS Injury and Disease to Promote Repair
My research is focused on altering the hostile environment of the injured or diseased CNS to one that is conducive to repair through altering inflammation. Our work focuses on delineating the intrinsic and extrinsic signals present after injury that antagonize the conversion of activated microglia and macrophages to a reparative phenotype in experimental models of CNS injury and disease. We are also interested in understanding how altering the immunophenotypical profile of macrophages and microglia can modulate spinal cord injury induced central neuropathic pain, affect host glial responses, including glial scar formation, as well as influence the ability of transplanted cells, such as Schwann cells and stem cells, to mediate neurorepair.

JAMES D. GUEST, M.D., PH.D., FACS
Clinical Professor, Department of Neurological Surgery
Augmented Recovery after SCI; Application of Therapeutic Combinations in Preclinical Studies, and Early Phase Clinical Trials
The current focus of the our lab is on the transplantation of autologous glial cells to repair spinal cord injuries. We utilize several types of animal models with an emphasis on solving translational questions related to human clinical application. We also emphasize minimally-invasive surgical lesion-making and transplantation techniques. Sophisticated outcome assessment techniques are used to evaluate transplant effects in both the acute and chronic state of injury. These include kinematic assessment of hand function and gait, electrophysiologic study of conduction across lesion sites, and sensory testing. Other areas of research include studies of human post-mortem spinal cord tissue, intraoperative human spinal cord conduction studies, and research design for human clinical trials.
GILLIAN A. HOTZ, PH.D.
Research Professor, Department of Neurological Surgery
Director, KiDZ Neuroscience Center; Director, Concussion, WalkSafeTM & BikeSafeTM Programs
Injury Prevention and Concussion Management
As a behavioral neuroscientist my clinical interests have always been investigating the neurocognitive deficits of those individuals that have sustained a traumatic and acquired brain injury. I have co-authored two neurocognitive tests, The Brief Test of Head Injury for adults and the Pediatric Test of Brain Injury for children. My research has focused on developing evidence based injury prevention programs in order to prevent brain and spinal cord injuries in children. In 2003, our team developed the WalkSafe program, which has been shown to decrease the number of elementary school age children that get hit by cars, and in 2009 we developed the BikeSafe program which educated middle school age children on bicycle safety skills. As the Director of the Concussion Program, we have spent many years developing and implementing a comprehensive countywide high school sports concussion care program, which includes neurologic evaluation, neuroimaging, neuropharmacological management, neuropsychological testing, and baseline test with ImPACT, a computerized neurocognitive screening measure. We also have developed a Concussion Injury Surveillance system. Our program is multidisciplinary and assesses and treats athletes from all levels of play. I am also the PI on many local and federal grants: Safe Routes to School initiatives, Transportation Alternative Programs, GE/NFL MRI Phase 2 study, Brainscope EEG study, one of the TRACK TBI sites, and a new project that will study the Effects of Cannabinoids on Mild TBI.

EMAD IBRAHIM, M.D., HCLD

Assistant Clinical Profesor, Departments of Urology and Neurological Surgery
Male
The research is focused on understanding and improving the impairments to male fertility which occur following spinal cord injury (SCI). Following SCI, most men, but not women, experience impaired fertility. Specifically, most men with SCI are anejaculatory, and some experience erectile dysfunction. Although their semen may be obtained by medically-assisted ejaculation procedures, in most cases, semen quality is impaired, specifically, sperm motility and viability are abnormally low, although sperm numbers tend to be normal.

JONATHAN R. JAGID, M.D., B.S., M.E.
Clinical Professor, Department of Neurological Surgery, Neurology, Orthopedics, Rehabilitation Medicine
Chief and Fellowship Director, Functional and Epilepsy Neurosurgery
Chief and Fellowship Director, Neuro-Trauma Division
Deep Brain Stimulation and Hypothermia
Research interests and experience include multi center studies in deep brain stimulation, hypothermia for both spinal cord injury and head injury, and laser ablation for refractory epilepsy. Current position as faculty at The Miami Project to Cure Paralysis and has played a significant role in translational research, as exemplified by his work in deep brain stimulation of novel targets for both intractable neuropathic pain in spinal cord injury and gait in neurodegenerative disorders. Additionally, Brain Machine Interface (BMI) studies to help restore limb function in spinal cord injury.
STANISLAVA JERGOVA, PH.D.
Research Assistant Professor, Department of Neurological Surgery
Advancing Chronic Pain Relief: Recombinant Human Cells and Gene Strategies
Our lab’s research goals involve investigating potential cell and gene therapies for chronic pain induced by spinal cord injuries (SCI) and other injuries of the central nervous system. We aim to better understand the mechanisms of chronic pain and develop new treatment options that can provide long-lasting relief for patients. As chronic pain is a multifactorial condition with several pathways involved, simultaneous targeting of different pathways with precise localization is essential. Recombinant cells and designed analgesic genes provide a great tool to achieve our goal. We explore different biomaterials as delivery vehicles to develop safe and potent approach to combat chronic pain.

VIVEK V. KANUMURI, M.D.

Assistant Professor, Departments of Otolaryngology and Biomedical Engineering
Neural Engineering and Bioelectronic Medicines
My overall research is focused on using non-invasive neuromodulation to promote neuroplasticity of damaged control systems and subsequent recovery. We specifically focus on enhancing recovery following spinal cord injury and tinnitus. We use a variety of techniques including brain mapping, 2-photon microscopy, optogenetics, transsynaptic tracing, tissue clearing, intraoperative nerve stimulation, and awake-behaving physiological recordings.
ROBERT W. KEANE, PH.D.
Professor, Departments of Physiology & Biophysics, Neurological Surgery, and Microbiology and Immunology
Regulation of Innate Immunity after CNS Trauma
Innate immunity is the first line of defense against pathogens and host-derived signals of cellular stress. My research focuses on investigating mechanisms that direct normal innate immunity and its dysregulation in central nervous system injury and disease, including (1) agonists and activation mechanisms of inflammasomes, (2) regulatory mechanisms that potentiate or limit inflammasome activation after injury, and (3) emerging data linking inflammasome proteins as biomarkers for CNS injury.


NADINE A. KERR, PH.D.
Research Assistant Professor, Department of Neurological Surgery
Mechanisms of Systemic Organ Dysfunction after Central Nervous System Injury
The focus of our research is to examine the pathomechanisms of the systemic inflammatory response and non-neurological organ dysfunction following Central Nervous System (CNS) injury, particularly traumatic brain injury (TBI) and stroke. After CNS injury and damage to the blood-brain barrier (BBB), inflammatory mediators are released into the circulation and contribute to systemic organ damage. This can then lead to further neurological damage resulting in bidirectional communication between the brain and systemic organs. The two main organ systems that we are currently investigating are the respiratory and gastrointestinal systems.
JAE K. LEE, PH.D.
Professor, Department of Neurological Surgery
Christine E. Lynn Distinguished Professor in Neuroscience
Glial and Fibrotic Scar Formation in the CNS; Axon Regeneration
After traumatic injury to the central nervous system (CNS), such as spinal cord injury or traumatic brain injury, non-CNS cells such as hematogenous immune cells and fibroblasts enter the CNS parenchyma and cause tissue damage. In response, glial cells attempt to form a protective barrier but during this process, these reactive glial cells (often called the glial scar) create an environment that is not conducive to tissue repair. The primary goal of our laboratory is to investigate how these CNS and non-CNS cells interact to form the scar in hopes that a better understanding of this complex process will help promote cellular repair and axon regeneration.

VANCE LEMMON, PH.D.
Walter G. Ross Distinguished Chair in Developmental Neuroscience

Professor, Department of Neurological Surgery, Center for Computational Science, Hussmann Institute for Human Genomics, Sylvester Cancer Center
High Content Screening and Functional Genomics of the Nervous System
Our laboratory has developed methods to test thousands of genes or chemicals in hundreds of thousands of neurons each week to obtain quantitative information about cell morphology and gene expression. This “high throughput” capability allows us to tackle questions about axon growth and regeneration using systems biology approaches, and to query the results in animal models of injury. The Lemmon-Bixby lab has several ongoing projects related to axon regeneration. One project is to test the roles of known signaling proteins called protein kinases. In this screen we have tested >1600 kinase inhibitors, dozens of which strongly promote neurite growth in vitro. Using bioinformatics, biochemistry, and machine learning we can identify key kinases and their signaling networks as well as potential lead therapeutic compounds, one of which has proven active in two different models of spinal cord injury. A second project is based on the observation that injured peripheral sensory neurons initiate a genetic program appropriate for axonal regeneration. Our laboratory has combined next-generation sequencing with cell-based phenotypic screening to identify genes, especially transcription factors, that appear to mediate this genetic program, and is testing them in vitro and in vivo. The last project is to test several hit compounds identified in a screen testing 440 million different small molecules to see if the hits promote axon regeneration.

DANIEL
J. LIEBL, PH.D.
Professor, Department of Neurological Surgery
Molecular Mechanisms that Regulate Cellular Dysfunction and Death Following CNS Injury, and Mechanisms to Promote Regeneration and Recovery
The goal of my laboratory is to identify the mechanisms that lead to CNS pathophysiology and its regenerative potential. We focus on growth and guidance molecules, which play important roles in the developing, regenerating, and injured nervous systems. Specifically, we are currently interested in areas of adult neurogenesis, neuroprotection, apoptotic cell death, synaptic plasticity, angiogenesis, regeneration, and therapeutic strategies. Overall, our approach is to develop novel strategies to minimize CNS damage and maximize regeneration/tissue repair, which can be best achieved through a comprehensive mechanistic approach.
Clinical Professor, Departments of Neurology, Neurological Surgery, and Physical Medicine & Rehabilitation
Pathophysiology and Treatment of Secondary Complications in Spinal Cord Injury
My research interests focus on common complications that are seen following spinal cord injury: pain, spasticity, syringomyelia, and tethered cord syndrome. My interests include investigating the basis for the development of the different spasticity and pain profiles in the spinal cord injured population and to study potential novel treatments for those conditions.

DAVID W. MCMILLAN, PH.D.
Director of Education and Outreach, The Miami Project

Research Assistant Professor, Department of Neurological Surgery Understanding and Treatment of Secondary Complications of SCI; Community Partnership My dissertation pertained to the role of the autonomic nervous system in the absorption, trafficking, and fates of dietary fat. I am now refocusing my efforts to align with local opportunities seen from my Outreach role—aiming at the disproportionate risks and rewards posed by the Tropical Atlantic on people living with SCI (PwSCI). Regarding risks, I study the vulnerabilities of PwSCI to climate hazards in the form of flooding, heat, and hurricanes. Regarding rewards, I study the transformative potential that adapted ocean activities—such as sailing, scuba diving, swimming, and others—carries for adults with physical disability.
MATIJA MILOSEVIC, PH.D.
Director of Neuromotor Rehabilitation, The Miami Project
Assistant Professor, Departments of Neurological Surgery and Biomedical Engineering
Neuromotor Rehabilitation
My research is focused on developing neuromodulation technologies and therapeutic approaches to restore and improve motor function of individuals living with spinal cord injuries. My research program applies a transdisciplinary approach, spanning the fields of neural engineering, neuroscience, and clinical translation to develop and test techniques and approaches for neuromotor recovery. My research team is developing approaches for therapeutic application of electrical stimulation to activate and elicit neuroplastic changes to spinal and corticospinal neural circuits.


LESLIE MORSE, D.O.
Chair and Professor of the Miller School’s Department of Physical Medicine & Rehabilitation Joins The Miami Project to Cure Paralysis as a faculty member. Dr. Morse serves as co-director for the South Florida Spinal Cord Injury Model Systems (SCIMS) Center as she did for SCIMS centers at previous stops at Spaulding-Harvard and Minnesota Regional.
JENNIFER C. MUNOZ PAREJA, M.D.
Associate Professor Pediatric Critical Care & Associate Program Director, Pediatric Critical Care, University of Miami Biomarkers in Acute Brain Injury
The overarching goal of my laboratory is to gain a comprehensive understanding of the diverse pathomechanisms contributing to adverse outcomes in pediatric acute brain injury. We strive to identify specific biomarkers that can be employed as objective measures for assessing the severity of pediatric acute brain injury, as well as predicting patient outcomes. Moreover, recognizing neuroinflammation as a central pathophysiological aspect of acute brain injury and acknowledging that a limited understanding of the intricate cellular and molecular sequelae has hindered previous translational efforts, my current focus centers around studying the role of the inflammasome in the innate immune response following injury. By accomplishing this, we aim to enhance our ability to evaluate injury severity and provide valuable prognostic and therapeutic information to this vulnerable population.

MARK S. NASH, PH.D., FACSM

Associate Scientific Director for Research, The Miami Project to Cure Paralysis
Vice-Chair for Research, Department of Physical Medicine & Rehabilitation
Professor, Departments of Neurological Surgery, Physical Medicine & Rehabilitation and Physical Therapy
Co-Director, DHHS-NIDILRR South Florida SCI Model System
Physiological Assessment of Secondary Complications following SCI: Electrical Stimulation, Cardiometabolic and Vascular Physiology, Cardioendocrine Pathology and Intervention, and Exercise and Nutritional Biochemistry
One of the enduring goals of The Miami Project has been to test and then translate strategies that optimize health of persons with SCI. A significant target for this strategy has focused on physical activity to lessen secondary risks of SCI associated with physical deconditioning. We also examine complementary themes to optimize exercise prescription after SCI, identify optimal nutritional intake, and use prescription and nonprescription agents that reduce hazards of fasting and postprandial lipid disorders, dysglycemia, and vascular inflammatory stress.
BRIAN
R. NOGA, PH.D.
Research Professor, Department of Neurological Surgery
Brain and Spinal Mechanisms Controlling Walking
Neuromodulation technologies are increasingly looked at as potential treatment options for paralysis associated with spinal cord injury (SCI). Deep brain stimulation is one such method that so far has had little or no application in persons with SCI even though most new and chronic injuries are incomplete. Recent work in our laboratory has pointed to a brain target for controlling walking. We are currently investigating the usefulness of stimulating this site to enhance walking in a translational large animal model of SCI.


DAMIEN D. PEARSE, PH.D.
Professor, Department of Neurological Surgery, Health Scientist Veterans Affairs
John M. and Jocelyn H.K. Watkins Distinguished Chair in Cell Therapies
Repairing the Injured Nervous System with Cell-Based Approaches and Modulators of Protein Modification Pathways
My laboratory focuses on several key aspects of CNS injury repair, including (1) the utility and clinical translation of exogenous and endogenously harnessed cell therapeutics (particularly when used in combinatory approaches), (2) understanding the role of, and developing therapies for, altered cyclic AMP (adenylyl cyclase, phosphodiesterases, and PKA) and MAPK signaling in neurons and glia after CNS injury, (3) the use of nanotherapeutics for multifunctional and site-directed gene/drug targeting to the injured CNS, and (4) the application of methodologies for improved imaging of axonal regeneration and cell integration within the injured CNS such as 3D ultramicroscopy and diffusion tensor imaging.
ABHISHEK PRASAD, PH.D.
Associate Professor of Biomedical Engineering
Brain and spinal cord machine interfaces for spinal cord injury
Our lab is investigating better and alternate materials for electrodes and insulation that will minimize the corrosion and delamination problem. To mitigate the foreign body response due to implanted materials, we are using therapeutic and pharmacological approaches to reduce the neuroinflammation and oxidative stress that occurs after an electrode implant.


SUHRUD M. RAJGURU, PH.D.
Professor, Departments of Biomedical Engineering and Otalaryngology
Assistant Vice Provost for Research Workforce Development, University of Miami Director, University of Miami CTSI Team Science
NeuroTherapeutics Lab
Our research is focused on characterizing, diagnosing and treating disorders and traumas that affect the inner ear functions of hearing and balance. Recent preclinical and clinical work in the NeuroTherapeutics group is focused on medical devices including cochlear and vestibular implantation, infrared neural stimulation, noise- and blast-induced hearing loss and vestibular dysfunction, and applications of mild therapeutic hypothermia for protection and preservation of neural substrate and functions.
JACQUELINE SAGEN, PH.D., M.B.A.
Professor, Department of Neurological Surgery
Cellular Implants and Gene Therapy for the Alleviation of Chronic Pain and CNS Injury
Our laboratory is exploring novel and more effective strategies in the therapeutic management of chronic debilitating pain. Our recent research is focused on (1) identification of more effective analgesic agents and combinations for alleviating pain using SCI and peripheral neuropathic pain models and (2) development of emerging therapeutic interventions, including cell transplantation and gene therapy, which have the potential to provide long-term alleviation in people with intractable pain, overcoming the need for repeated pharmacologic administration.


PANTELIS TSOULFAS, M.D.
Associate Professor, Departments of Neurological Surgery and Cell Biology & Anatomy Neurotrophins: Specificity of Action
My laboratory is interested in two areas of neurobiology that are significant for developing new strategies for spinal cord injury repair. Over the past years, we have worked to modify neurotrophins that are better suited for use in SCI. We are also interested in understanding the processes involved in maintaining and differentiating neural stem cells.
MICHAEL Y. WANG, M.D., FACS
Professor, Departments of Neurological Surgery and Rehabilitation Medicine
Chief of Neurosurgery, University of Miami Hospital
Spinal Cord Injury Outcomes
My primary research has been in the investigation of SCI Outcomes. I work with Miami Project researchers Drs. Allan Levi and Barth Green in studying the clinical effects of Hypothermia. Currently, a multi-center randomized, prospective study on the effects of hypothermia in SCI is underway. In addition, I am studying the clinical application of SCI biomarkers to predict the effects of both injuries as well as therapeutic interventions with Drs. Dalton Dietrich and Ross Bullock.

EVA WIDERSTRÖM-NOGA, D.D.S., PH.D.

Research Professor, The Miami Project to Cure Paralysis, the Departments of Neurological Surgery and Physical Medicine & Rehabilitation
Interdisciplinary Research Approaches to Improve Neuropathic Pain Management after Neurotrauma
My research program is interdisciplinary and includes the identification of clinical correlates of underlying mechanisms of neuropathic pain associated with neurological trauma as a way to facilitate the translation of basic research findings to treatments tailored to specific mechanisms. A recent line of research in my program aims to better understand cortical mechanisms of multisensory integration and how to manipulate these mechanisms by visual illusion and transcranial electrical stimulation to reduce pain. In addition, we have examined SCI stakeholders (people with SCI and neuropathic pain, their significant others and SCI healthcare providers) perspectives on barriers and facilitators to optimal management of neuropathic pain. Based on this work we have developed a preliminary pain education tool that is now further refined based on structured stakeholder feedback. My research program is highly collaborative and includes extensive interdisciplinary protocols for a multimodal evaluation of self-reported pain symptoms and its psychosocial impact, quantitative assessment of neurological function, and biomarkers including non-invasive brain imaging and electrophysiology.
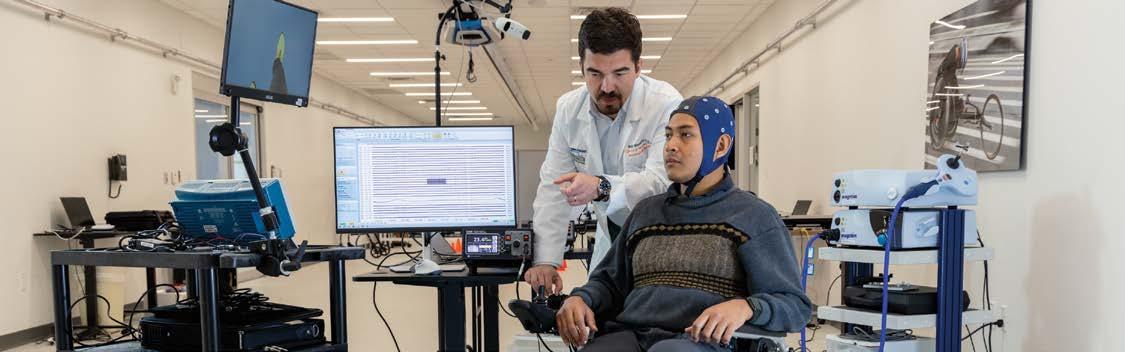

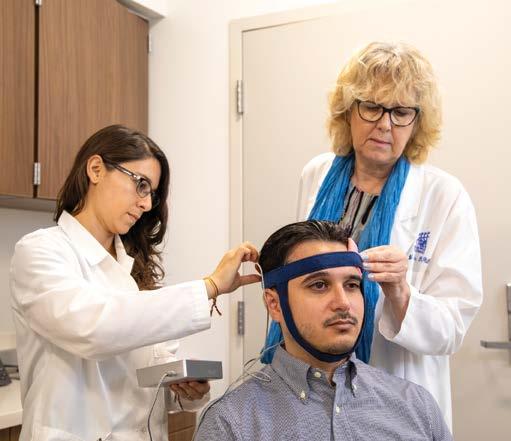




Published studies that have passed the test of peer review are the benchmark of scientific progress. Listed here are research publications by Miami Project scientists and colleagues.
Adebiyi E, Pareja JC, Alba-Sandoval M, Almodovar M. Impact of congenital heart disease on mortality and other associated outcomes in children hospitalised for acute asthma exacerbation. Cardiology in the Young. 2024;34(4):884-90.
Agarwal N, DiGiorgio A, Michalopoulos GD, Letchuman V, Chan AK, Shabani S, Lavadi RS, Lu DC, Wang MY, Haid RW, Knightly JJ. Impact of educational background on preoperative disease severity and postoperative outcomes among patients with cervical spondylotic myelopathy. Clinical spine surgery. 2024;37(3):E137-46.
Almeida T, Monaco BA, Vasconcelos F, Piedade GS, Morell A, Ogobuiro I, Lepski GA, Furlanetti LL, Cordeiro KK, Benjamin C, Jagid JR. Everything old is new again. revisiting hypophysectomy for the treatment of refractory cancer-related pain: a systematic review. Neurosurgical review. 2024;47(1):111.
Arai S, Sasaki A, Tsugaya S, Nomura T, Milosevic M. Inhibition of tibialis anterior spinal reflex circuits using frequency‐specific neuromuscular electrical stimulation. Artificial Organs. 2024;48(8):891-901.
Ascona MC, Tieu EK, GonzalezVega E, Liebl DJ, Brambilla R. A deep learning-based approach for unbiased kinematic analysis in CNS injury. Experimental neurology. 2024;381:114944.
Balleste AF, Alvarez JC, PlaceresUray F, Mastromatteo-Alberga P, Torres MD, Dallera CA, Dietrich WD, Parry TJ, Verdoorn TA, Billing Jr CB, Buller B. Improvement in edema and cognitive recovery after moderate traumatic brain injury with the neurosteroid prodrug NTS-104. Neurotherapeutics. 2024;21(6):e00456.
Balleste AF, Sangadi A, Titus DJ, Johnstone T, Hogenkamp D, Gee KW, Atkins CM. Enhancing cognitive recovery in chronic traumatic brain injury through simultaneous allosteric modulation of alpha-7 nicotinic acetylcholine and alpha-5 GABAA receptors. Experimental Neurology. 2024;379:114879.
Bigford GE, Lehmann DA, Betancourt LF, Maher JL, Mendez AJ, Nash MS. Modification of the Diabetes Prevention Program Lifestyle Intervention in Persons with Spinal Cord Injury: Efficacy for Reducing Major Cardiometabolic Risks, Increased Fitness, and Improved
Health-Related Quality of Life. Journal of spine research and surgery. 2024;6(1):10-26502.
Blight AR, Guest JD, Hamer J, Hsieh JT, Jones L, Magnuson DS, Pfleeger K. Data Safety Monitoring Boards: Overview of Structure and Role in Spinal Cord Injury Studies. Topics in spinal cord injury rehabilitation. 2024;30(3):67-75.
Bourguignon L, Lukas LP, Guest JD, Geisler FH, Noonan V, Curt A, Brüningk SC, Jutzeler CR. Studying missingness in spinal cord injury data: challenges and impact of data imputation. BMC medical research methodology. 2024;24(1):5.
Brüningk SC, Bourguignon L, Lukas LP, Maier D, Abel R, Weidner N, Rupp R, Geisler F, Kramer JL, Guest JD, Curt A. Prediction of segmental motor outcomes in traumatic spinal cord injury: Advances beyond sum scores. Experimental neurology. 2024;380:114905.
CLB Ferreira B, Hannard M, Lozano-Garcia M, Aston L, Tejeda G, Domena JB, Bernard B, Chen J, Bartoli M, Rech Tondin A, Zhou Y. Investigating the Significances of Thiol Functionalities in SARS-CoV-2 Using Carbon Dots for Viral Inhibition. ACS applied materials & interfaces. 2024;16(43):58439-51.
Cozzone IG, Ortega VL, Dumont CM. Engineered Tools to Advance Cell Transplantation in the Nervous System Towards a Clinical Reality. Current transplantation reports. 2024;11(4):222-32.
Cyr B, Cabrera Ranaldi ED, Hadad R, Dietrich WD, Keane RW, de Rivero Vaccari JP. Extracellular vesicles mediate inflammasome signaling in the brain and heart of Alzheimer’s disease mice. Frontiers in molecular neuroscience. 2024;17:1369781.
Cyr B, Curiel Cid R, Loewenstein D, Vontell RT, Dietrich WD, Keane RW, de Rivero Vaccari JP. The Inflammasome Adaptor Protein ASC in Plasma as a Biomarker of Early
Cognitive Changes. International journal of molecular sciences. 2024;25(14):7758.
Davies BM, Khan DZ, Barzangi K, Ali A, Mowforth OD, Nouri A, Harrop JS, Aarabi B, RahimiMovaghar V, Kurpad SN, Guest JD. We choose to call it ‘degenerative cervical myelopathy’: findings of AO Spine RECODE-DCM, an international and multi-stakeholder partnership to agree a standard unifying term and definition for a disease. Global spine journal. 2024;14(2):503-12.
Davies BM, Yang X, Khan DZ, Mowforth OD, Touzet AY, Nouri A, Harrop JS, Aarabi B, RahimiMovaghar V, Kurpad SN, Guest JD. A minimum data set—Core outcome set, core data elements, and core measurement set—For degenerative cervical myelopathy research (AO Spine RECODE DCM): A consensus study. PLoS medicine. 2024;21(8):e1004447.
de Monaco BA, Piedade GS, Doomi A, Jagid JR, Almeida T, Cordeiro JG. Retrograde thoracic spinal cord stimulation paddle placement for complex persistent spinal pain syndrome type 2. Pain Practice. 2024;24(3):483-8.
de Rivero Vaccari JP, Cabrera Ranaldi ED, Johnson NH, Kerr NA, Bramlett HM, Keane RW, Dietrich III WD. Inflammasome Signaling in Alzheimer’s Disease after Traumatic Brain Injury. Alzheimer’s & Dementia. 2024;20:e089769.
Desu HL, Thougaard E, Carney BN, Illiano P, Plastini MJ, Florimon Y, Mini A, Guastucci C, Kang B, Lee JK, Lambertsen KL. TNFR2 signaling in oligodendrocyte precursor cells suppresses their immune-inflammatory function and detrimental microglia activation in CNS demyelinating disease. Brain, behavior, and immunity. 2024;123:8198.
Dickey EM, Bianchi A, Amirian H, Hosein PJ, Faustman D, Brambilla R, Datta J. Transmembrane TNF-
TNFR2 signaling as a critical immunoregulatory node in pancreatic cancer. Oncoimmunology. 2024;13(1):2326694.
Echevarria‐Cruz E, McMillan DW, Reid KF, Valderrábano RJ. Spinal Cord Injury Associated Disease of the Skeleton, an Unresolved Problem with Need for Multimodal Interventions. Advanced biology. 2024:2400213.
Egawa J, Lemmon VP, Someya T. Effects of autism spectrum disorder (ASD) risk genes on phenotypes of each hierarchy. Frontiers in neurology. 2024;15:1508494.
Errante EL, Smartz T, Costello MC, Schaeffer EA, Kloehn AJ, Yunga Tigre J, Khan A, Pressman Y, Levi AD, Burks SS. Assessing Survival, Distribution, and Optimal Loading Technique of Schwann Cell–Derived Exosomes Into Second-generation Axon Guidance Channels. Military medicine. 2024;189(3):63-6.
Evaniew N, Davies B, Farahbakhsh F, Fehlings MG, Ganau M, Graves D, Guest JD, Korupolu R, Martin AR, McKenna SL, Tetreault LA. Interventions to optimize spinal cord perfusion in patients with acute traumatic spinal cord injury: an updated systematic review. Global spine journal. 2024;14(3):58S-79S.
Farber SH, Walker CT, Zhou JJ, Godzik J, Gandhi SV, de Andrada Pereira B, Koffie RM, Xu DS, Sciubba DM, Shin JH, Steinmetz MP. Reliability of a novel classification system for thoracic disc herniations. Spine. 2024;49(5):341-8.
Farkas GJ, Cunningham PM, Sneij AM, Hayes JE, Nash MS, Berg AS, Gater DR, Rolls BJ. Reasons for meal termination, eating frequency, and typical meal context differ between persons with and without a spinal cord injury. Appetite. 2024;192:107110.
Fehlings MG, Hachem LD, Tetreault LA, Skelly AC, Dettori JR, Brodt ED, Stabler-Morris S, Redick BJ, Evaniew N, Martin AR, Davies B, Guest JD, Graves D, Korupolu R, McKenna SL, Kwon BK. Timing of
decompressive surgery in patients with acute spinal cord injury: systematic review update. Global spine journal. 2024;14(3):38S-57S.
Fernandez GE, Anderson KD, Vastano R, Frank SI, Robayo LE, Cherup NP, Kochen W, WiderströmNoga E. Perspectives of people with spinal cord injury on a pain education resource. Frontiers in public health. 2024;12:1385831.
Futch BG, Seas A, Ononogbu-Uche F, Khedr S, Kreinbrook J, Shaffrey CI, Williamson T, Guest JD, Fehlings MG, Abd-El-Barr MM, Foster NA. Shifting Trends in the Epidemiology of Cervical Spine Injuries: An Analysis of 11,822 Patients from the National Electronic Injury Surveillance System over Two Decades. Journal of neurotrauma. 2024;41(17):2158-67.
Ghosh M, Lee J, Burke AN, Strong TA, Sagen J, Pearse DD. Sex Dependent Disparities in the Central Innate Immune Response after Moderate Spinal Cord Contusion in Rat. Cells. 2024;13(7):645.
Ghosh M, Pathak S, Maity D. On Z pr Z ps Z pt-additive cyclic codes exhibit asymptotically good properties. Cryptography and Communications. 2024;16:1559-1580.
Ghosh M, Pearse DD. The Yin and Yang of Microglia-Derived Extracellular Vesicles in CNS Injury and Diseases. Cells. 2024;13(22):1834.
Gober R, Dallmeier J, Davis D, Brzostowicki D, de Rivero Vaccari JP, Cyr B, Barreda A, Sun X, Gultekin SH, Garamszegi S, Scott W. Increased inflammasome protein expression identified in microglia from postmortem brains with schizophrenia. Journal of Neuropathology & Experimental Neurology. 2024;83(11):951-66.
Guest J. Tissue bridge widths and outcome after spinal cord injury. The Lancet Neurology. 2024;23(8):756-7.
Hachem LD, Zhu M, Aarabi B, Davies B, DiGiorgio A, Evaniew N, Fehlings MG, Ganau M, Graves
D, Guest J, Ha Y. A practical classification system for acute cervical spinal cord injury based on a threephased modified delphi process from the aospine spinal cord injury knowledge forum. Global spine journal. 2024;14(2):535-45.
Hejrati N, Moghaddamjou A, Pedro K, Alvi MA, Harrop JS, Guest JD, Kwon BK, Fehlings MG. Current practice of acute spinal cord injury management: a global survey of members from the AO spine. Global spine journal. 2024;14(2):546-60.
Hejrati N, Srikandarajah N, Alvi MA, Quddusi A, Tetreault LA, Guest JD, Marco RA, Kirshblum S, Martin AR, Strantzas S, Arnold PM, Guest JD. The Management of Intraoperative Spinal Cord Injury–A Scoping Review. Global spine journal. 2024;14(3):150S-65S.
Hong VM, Rade A, Syed Z, Yousuf MS, Liebl DJ, Martin S, Price TJ, Kolber BJ. Increased Mechanical Hypersensitivity Resulting from the Loss of Sigma-2 Receptor/ Transmembrane Protein 97 Expression in Both Germline and NociceptorSpecific Context. The Journal of Pain. 2024;25(4):15.
Hong VM, Rade AD, Yan SM, Bhaskara A, Yousuf MS, Chen M, Martin SF, Liebl DJ, Price TJ, Kolber BJ. Loss of sigma-2 receptor/TMEM97 is associated with neuropathic injury-induced depression-like behaviors in female mice. Eneuro. 2024;11(7).
Hotz G, Griffin JR, Ke H, Crittenden R, Chileuitt A. Comparing Public vs. Private High School Sports-Related Concussions from a Countywide Concussion Injury Surveillance System. The sport journal. 2024:1.
Ibrahim E. Penile vibratory stimulation in men with spinal cord injury: an educational video demonstration. Fertility and sterility. 2024;121(3):545-7.
Jaleh Z, Rahimi B, Shahrezaei A, Sohani M, Sagen J, Nasirinezhad F. Exploring the Therapeutic Potential of Mesenchymal Stem Cells-derived conditioned medium: An In-depth Analysis of Pain Alleviation, Spinal CCL2 Levels, and Oxidative Stress. Cell biochemistry and biophysics. 2024;82(3):2977-88.
Javeed S, Zhang JK, Greenberg JK, Botterbush K, Benedict B, Plog B, Gupta VP, Dibble CF, Khalifeh JM, Wen H, Chen Y, Burks SS, Levi AD, Ray WZ. Impact of upper limb motor recovery on functional independence after traumatic low cervical spinal cord injury. Journal of neurotrauma. 2024;41(9-10):1211-22.
Ji W, Nightingale TE, Zhao F, Fritz NE, Phillips AA, Sisto SA, Nash MS, Badr MS, Wecht JM, Mateika JH, Panza GS. The clinical relevance of autonomic dysfunction, cerebral hemodynamics, and sleep interactions in individuals living with SCI. Archives of physical medicine and rehabilitation. 2024;105(1):166-76.
Kerr NA, Higueras AF, Sanchez‐Molano J, Walters W, Vaccari JP, Keane RW, Bramlett HM, Dietrich III WD. Extracellular‐Vesicle mediated pyroptosis leads to gut‐brain axis after stroke in a mouse model of Alzheimer’s Disease. Alzheimer’s & Dementia. 2024;20:e089237.
Kerr NA, Sangaletti R, Walters W, Rajguru SM. Association of Blast‐induced Hearing Loss with Progression of Alzheimer’s Disease Pathology. Alzheimer’s & Dementia. 2024;20:e089629.
Khalafallah AM, Tigre JY, Rady N, Starke RM, Saraf-Lavi E, Levi AD. Evaluating the diagnostic accuracy of 3D contrast-enhanced magnetic resonance angiography versus digital subtraction angiography in spinal dural arteriovenous fistulas. Neurosurgical focus. 2024;56(3):E10.
Kobeissy F, Arja RD, Munoz JC, Shear DA, Gilsdorf J, Zhu J, Yadikar H, Haskins W, Tyndall JA, Wang KK. The game changer: UCH-L1 and
GFAP-based blood test as the first marketed in vitro diagnostic test for mild traumatic brain injury. Expert Review of Molecular Diagnostics. 2024;24(1-2):67-77.
LaVela SL, Berryman K, Kale I, Farkas GJ, Henderson GV, Rosales V, Eisenberg D, Reyes L. Potential barriers to the use of anti‐obesity medications in persons with spinal cord injuries and disorders. Obesity Science & Practice. 2024;10(4):e784.
LaVela SL, Farkas GJ, Berryman K, Kale IO, Sneij A, Felix ER, Reyes L. Health consequences associated with poor diet and nutrition in persons with spinal cord injuries and disorders. Disability and rehabilitation. 2024:1-2.
LaVela SL, Wu J, Nevedal AL, Frayne SM, Harris AH, Arnow KD, Davis K, Farkas GJ, Reyes L, Eisenberg D. Nutrition and eating beliefs and behaviors among individuals with spinal cord injuries and disorders: Healthy or misconceived? Rehabilitation Psychology. 2024;70(1):1-14.
Ledesma BR, Velasquez DA, Egemba C, Molina M, Ibrahim E, Costantini-Mesquita F, Deebel NA, Han S, Reis IM, Saltzman R, Ramasamy R. A phase 2 randomized, placebo-controlled crossover trial to evaluate safety and efficacy of platelet-rich plasma injections for Peyronie’s disease: clinical trial update. International journal of impotence research. 2024;9(1):11-13.
Levy AS, Jamshidi AM, Costello MC, Levi AD. Structure-sparing resection for the management of cervical chordomas: a retrospective institutional series. Neurosurgical focus. 2024;56(5):E6.
Levy AS, Jamshidi AM, Merenzon MA, Levi DJ, Levi AD. Predictors of clinical and structural outcomes after surgery for spinal nerve sheath tumor resection: a retrospective analysis. Journal of Neurosurgery: Spine. 2023;40(1):11-8.
Li L, Hashemi L, Eid J, Tao W, Campoverde L, Yu A, Farooqi AA, Al-Ali H, D’Amato G, Hornicek F, Duan Z. High-Throughput Drug Screening in Chondrosarcoma Cells Identifies Effective Antineoplastic Agents Independent of IDH Mutation. International journal of molecular sciences. 2024;25(23):13003.
Liebmann K, Castillo MA, Jergova S, Best TM, Sagen J, Kouroupis D. Modification of mesenchymal stem/ stromal cell-derived small extracellular vesicles by calcitonin gene related peptide (CGRP) antagonist: potential implications for inflammation and pain reversal. Cells. 2024;13(6):484.
Lightfuss OB, Kumar P, Newsam AD, Li L, Younes PA, Shastri T, Alaoui AY, Parets DE, Manara P, Bilbao D, Schürer S. The Cyclin-G Associated Kinase (GAK) Is a Critical Targetable Dependency for Successful Mitosis By Diffuse Large B-Cell Lymphomas Linked to Retinoblastoma Loss of Function. Blood. 2024;144:2781.
Maddy KS, Tigre JY, Lu VM, Costello MC, Errante EL, Levi AD, Burks SS. Influence of instrumentation type on outcomes after surgical management of spondylodiscitis: a systematic review and meta-analysis. European spine journal. 2024;33(8):3175-90.
Mikol D, Toews J, Mulcahy S, DePaul M, Collett N, Guest J, Perez M. NVG-291 Phase 1 Results and Phase 1b/2a Study Design in Individuals with Spinal Cord Injury. Archives of physical medicine and rehabilitation. 2024;105(4):e18.
Minor A, Klein BR, Sowah MN, Etienne K, Levi AD. Pars interarticularis fractures treated with minimally invasive surgery: a literature review. Journal of clinical medicine. 2024;13(2):581.
Mooney J, Nathani KR, Zeitouni D, Michalopoulos GD, Wang MY, Coric D, Chan AK, Lu DC, Sherrod BA, Gottfried ON, Shaffrey CI. Does diabetes affect outcome or reoperation rate after lumbar decompression or arthrodesis? A matched analysis of
the Quality Outcomes Database data set: Presented at the 2023 AANS/ CNS Joint Section on Disorders of the Spine and Peripheral Nerves. Journal of Neurosurgery: Spine. 2023;40(3):331-42.
Moritz C, Field-Fote EC, Tefertiller C, van Nes I, Trumbower R, KalsiRyan S, Purcell M, Janssen TW, Krassioukov A, Morse LR, Zhao KD, Guest JD, Marino RJ, Murray LM, Wecht JM Markus, Rieger, Pardarelli, Turner A D’Amico R, Squair JW, Courtine G. Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: a safety and efficacy trial. Nature medicine. 2024;30(5):1276-83.
Munoz Pareja JC, de Rivero Vaccari JP, Chavez MM, Kerrigan M, Pringle C, Guthrie K, Swaby K, Coto J, Kobeissy F, Avery KL, Ghosh S. Prognostic and diagnostic utility of serum biomarkers in pediatric traumatic brain injury. Journal of neurotrauma. 2024;41(12):106-22.
Mylavarapu R, Albuquerque E, Farkas G, McMillan D, Ganzer P. Comparisons of Evoked Skin Vasomotor and Sudomotor Responses to Single Pulse Median Nerve Stimulation. Physiology. 2024;39(S1):1095.
Nakashoji K, Sasaki A, Kaneko N, Nomura T, Milosevic M. Effects of finger pinch motor imagery on short-latency afferent inhibition and corticospinal excitability. NeuroReport. 2024;35(6):413-20.
Nandakumar B, Mylavarapu RV, Harris R, Albuquerque ER, Yan Z, Herter C, McMillan DW, Kanumuri VV, Ganzer PD. State dependent vagus nerve stimulation for targeted plasticity therapy: challenges and considerations. Frontiers in Control Engineering. 2024;5:1452442.
Nath S, Martínez Santamaría JC, Chu YH, Choi JS, Conforti P, Lin JD, Sankowski R, Amann L, Galanis C, Wu K, Deshpande SS. Interaction between subventricular zone microglia and neural stem cells impacts the
neurogenic response in a mouse model of cortical ischemic stroke. Nature Communications. 2024;15(1):9095.
Nekanti U, Sakthivel PS, Zahedi A, Creasman DA, Nishi RA, Dumont CM, Piltti KM, Guardamondo GL, Hernandez N, Chen X, Song H. Multichannel bridges and NSC synergize to enhance axon regeneration, myelination, synaptic reconnection, and recovery after SCI. npj Regenerative Medicine. 2024;9(1):12.
Newman J, Tong X, Tan A, Yeasky T, De Paiva VN, Presicce P, Kannan PS, Williams K, Damianos A, Tamase Newsam M, Benny MK, Brambilla R, Schmidt AF. Chorioamnionitis accelerates granule cell and oligodendrocyte maturation in the cerebellum of preterm nonhuman primates. Journal of neuroinflammation. 2024;21(1):16.
Nishimura K, Sanchez-Molano J, Kerr N, Pressman Y, Silvera R, Khan A, Gajavelli S, Bramlett HM, Dietrich WD. Beneficial effects of human Schwann cell-derived exosomes in mitigating secondary damage after penetrating ballistic-like brain injury. Journal of neurotrauma. 2024;41(2122):2395-412.
Odeh M, Sajrawi C, Majcher A, Zubedat S, Shaulov L, Radzishevsky A, Mizrahi L, Chung WK, Avital A, Hornemann T, Liebl DJ. A new type of blood–brain barrier aminoacidopathy underlies metabolic microcephaly associated with SLC1A4 mutations. Brain. 2024;147(11):387489.
Patel SH, Bramlett HM, Raval AP. Post-stroke whole body vibration therapy alters the cerebral transcriptome to promote ischemic tolerance in middle-aged female rats. Neurochemistry international. 2024;180:105843.
Peng Y, Bramlett HM, Dietrich WD, Marcillo A, Sanchez-Molano J, Furones-Alonso O, Cao JJ, Huang J, Li AA, Feng JQ, Bauman WA. Administration of low intensity
vibration and a RANKL inhibitor, alone or in combination, reduces bone loss after spinal cord injury-induced immobilization in rats. Bone Reports. 2024;23:101808.
Perez-Roman RJ, Bashti M, Silva MA, Govindarajan V, Boddu J, Sheinberg DL, Cowan M, Shah A, Levi AD. The role of emboli detection studies in acute inpatient vertebral artery dissection. Journal of Clinical Neuroscience. 2024;127:110748.
Ponzano M, Nash MS, Bilzon J, Bochkezanian V, Davis GM, Farkas GJ, de Groot S, Jin J, Larsen CM, Laskin J, Ma J, Nightingale T, Postma K, Smith BM, Strom, Berg-Emons R, Wouda M, Martine-Ginis KA. Consensus-Based Recommendations for Designing, Delivering, Evaluating, and Reporting Exercise Intervention Research Involving People Living With a Spinal Cord Injury. Archives of physical medicine and rehabilitation. 2024;000:1-16.
Pradhyumnan H, Patel SH, FuronesAlonso O, Zhao W, Bramlett HM, Raval AP. Electronic cigarette vape exposure exacerbates post-ischemic outcomes in female but not in male rats. Stroke. 2024;55(3):735-46.
Pradhyumnan H, Perez GG, Patel SH, Blaya MO, Bramlett HM, Raval AP. A Perspective on Hormonal Contraception Usage in Central Nervous System Injury. Journal of neurotrauma. 2024;41(5-6):541-51.
Raffaele S, Thougaard E, Laursen CC, Gao H, Andersen KM, Nielsen PV, Ortí-Casañ N, Blichfeldt-Eckhardt M, Koch S, Deb-Chatterji M, Magnus T, Brambilla R. Microglial TNFR2 signaling regulates the inflammatory response after CNS injury in a sexspecific fashion. Brain, behavior, and immunity. 2024;116:269-85.
Ranjbar Hameghavandi MH, Khodadoust E, Hassan Zadeh Tabatabaei MS, Farahbakhsh F, Ghodsi Z, Rostamkhani S, Ghashghaie S, Abbaszade M, Arbabi A, Hossieni SM, Sadeghi-Naini M, Guest JD, Fehlings MG, Rahimi-
Movaghar V. Challenges in traumatic spinal cord injury care in developing countries–a scoping review. Frontiers in public health. 2024;12:1377513.
Ray C, Farkas GJ, Reyes L, WK Wong A, Heinemann AW, Eisenberg D, Burns J, LaVela SL. A Literature Review of Nutrition Knowledge Measurement Items Used in Persons Living with Spinal Cord Injuries and Disorders. Topics in spinal cord injury rehabilitation. 2024;30(4):66-79.
Reisner A, Zohdy YM, Chern JJ, Blackwell LS, Lepard JR, Alawieh A, Verma MS, Kobeissy F, Mulugeta MG, Tyndall JA, Klein BJ, Munoz Pareja JC, Wang KK. The utility of biomarkers in traumatic brain injuries in children: opportunities and challenges. Journal of Neurosurgery: Pediatrics. 2024;34(2):199-203.
Rincon Sabatino S, Rivero A, Sangaletti R, Dietrich WD, Hoffer ME, King CS, Rajguru SM. Targeted therapeutic hypothermia protects against noise induced hearing loss. Frontiers in neuroscience. 2024;17:1296458.
Rincon Sabatino S, Sangaletti R, Griswold A, Dietrich WD, King CS, Rajguru SM. Transcriptional response to mild therapeutic hypothermia in noise-induced cochlear injury. Frontiers in neuroscience. 2024;17:1296475.
Rujeedawa T, Mowforth OD, Davies BM, Yang C, Nouri A, Francis JJ, Aarabi B, Kwon BK, Harrop J, Wilson JR, Martin AR, Guest JD, Fehlings MG, Dotter MR. Degenerative thoracic myelopathy: a scoping review of epidemiology, genetics, and pathogenesis. Global spine journal. 2024;14(5):1664-77.
Ryan CB, Choi JS, Kang B, Herr S, Pereira C, Moraes CT, Al-Ali H, Lee JK. PI3K signaling promotes formation of lipid-laden foamy macrophages at the spinal cord injury site. Neurobiology of disease. 2024;190:106370.
Sangadi DK, Sangadi A, Placeres-Uray F, Titus DJ, Johnstone T, Hogenkamp D, Gee KW, Atkins CM. Enhancing cognitive function in chronic TBI: The Role of alpha-7 nicotinic acetylcholine receptor modulation. Experimental neurology. 2024;372:114647.
Sangari S, Chen B, Grover F, Salsabili H, Sheth M, Gohil K, Hobbs S, Olson A, Eisner‐Janowicz I, Anschel A, Kim K, Guest JD. Spasticity predicts motor recovery for patients with subacute motor complete spinal cord injury. Annals of neurology. 2024;95(1):7186.
Scott XO, Kerr NA, Sanchez-Molano J, de Rivero Vaccari JP, Hadad R, De La Cruz A, Larsson HP, Dietrich WD, Keane RW. CatecholamineInduced Inflammasome Activation in the Heart Following Photothrombotic Stroke. Translational Stroke Research. 2024:10.1007/s12975-024-01311-3.
Sinder SB, Sharma SV, Shirvaikar IS, Pradhyumnan H, Patel SH, Diaz IC, Perez GG, Bramlett HM, Raval AP. Impact of menopauseassociated frailty on traumatic brain injury. Neurochemistry international. 2024:105741.
Snider B, Kirshblum S, Rupp R, Schuld C, Biering-Sorensen F, Burns S, Guest JD, Jones L, Krassioukov A, Rodriguez G, Schmidt Read M. International Standards for Neurological Classification of Spinal Cord Injury: Case Examples Reinforcing Concepts from the 2019 Revision. Topics in Spinal Cord Injury Rehabilitation. 2024:10.46292/sci2400049.
Solinsky R, Park K, Betancourt L, Schmidt-Read M, Owens M, Schwab JM, Dusseau II NB, Szlachcic Y, Sutherland L, Taylor JA, Nash MS. Patient perceptions and clinical assessments of cardiometabolic disease after subacute spinal cord injury. Archives of physical medicine and rehabilitation. 2024;105(5):901-5.
Starke N, Challa NV, Yuan H, Chen S, Duncan MR, Cabrera Ranaldi ED, de Rivero Vaccari JP, Schott A, Aguilar
AC, Lee YS, Khan A. Extracellular Vesicle ASC: A Novel Mediator for Lung–Brain Axis in Preterm Brain Injury. American journal of respiratory cell and molecular biology. 2024;71(4):464-80.
Tapia M, Levay K, Tsoulfas P, Park KK. Retrograde AAV-mediated gene modulation reveals chloride intracellular channel proteins as potent regulators of retinal ganglion cell death. Experimental neurology. 2024;377:114810.
Taylor RR, Keane RW, Guardiola B, López-Lage S, Moratinos L, Dietrich WD, Perez-Barcena J, de Rivero Vaccari JP. Inflammasome Proteins Are Reliable Biomarkers of the Inflammatory Response in Aneurysmal Subarachnoid Hemorrhage. Cells. 2024;13(16):1370.
Tigre JY, Begera M, Errante EL, Burks SS. Exploration of the right peroneal nerve after a gunshot wound. Surgical neurology international. 2024;15:10.
Tigre JY, Levi DJ, Lu VM, Kloehn AJ, Thorson W, Abulaban A, Burks SS, Levi AD. A novel leucine zipperlike transcriptional regulator 1 variant identified in a pair of siblings with familial schwannomatosis. Surgical neurology international. 2024;15:285.
Tigre JY, Puerto A, Khalafallah AM, Burks SS. Timing of surgical intervention in peripheral nerve injuries from gunshot wounds: Management and review of the literature. Surgical Neurology International. 2024;15:178.
Tretter BL, Dolbow DR, Ooi V, Farkas GJ, Miller JM, Deitrich JN, Gorgey AS. Neurogenic Aging After Spinal Cord Injury: Highlighting the Unique Characteristics of Aging After Spinal Cord Injury. Journal of Clinical Medicine. 2024;13(23):7197.
Vontell RT, Barreda A, Blennow K, Zetterberg H, Vaccari JP, Bramlett HM, Dietrich III WD, Keane RW, Davis DA, Rundek T, Sun X. Reduction of neurogranin
protein correlates with increases of inflammasome proteins in post mortem cases of intermediate Alzheimer’s disease. Alzheimer’s & Dementia. 2024;20:e089239.
Vontell RT, Gober R, Dallmeier J, Brzostowicki D, Barreda A, Blennow K, Zetterberg H, Kvartsberg H, Gultekin SH, de Rivero Vaccari JP, Bramlett HM. Association of region‐specific hippocampal reduction of neurogranin with inflammasome proteins in post mortem brains of Alzheimer’s disease. Alzheimer’s & dementia: translational research & clinical interventions. 2024;10(1):e12444.
Williams E, Minesinger K, Gallagher H, Stefanson JR, Bridges N, Jackson N, Stark V, Coto J, Rajguru S, Yankaskas K, Rogers R. Examining the utility of near infrared light as pre-exposure therapy to mitigate temporary noise-induced hearing loss in humans. Frontiers in neurology. 2024;15:1366239.
Witte MM, McEvoy JM, Hotz GA. TikTok is an effective platform for bicycle safety injury prevention education. E-learning and digital media. 2024:20427530241268385.
Yamagata S, Yamaguchi T, Shinya M, Milosevic M, Masani K. Comparison of sensitivity among dynamic balance measures during walking with different tasks. Royal Society Open Science. 2024;11(1):230883.
Yang E, Mummaneni PV, Chou D, Izima C, Fu KM, Bydon M, Bisson EF, Shaffrey CI, Gottfried ON, Asher AL, Coric D, Wang MY. Is Upper Extremity or Lower Extremity Function More Important for Patient Satisfaction? An Analysis of 24-Month Outcomes from the QOD Cervical Spondylotic Myelopathy Cohort. Clinical Spine Surgery. 2024;37(4):188-97.
Yepes MF, Hoffer ME, Chiossone JA, Soejima N, King CS, Rajguru SM. Noninvasive Targeted Temperature Management of the Inner Ear: Numerical Simulations
and Experimental Measurements in a Human Cadaver Model. Otology & Neurotology. 2024:10.1097/ MAO.0000000000004476.
Yu K, Portes P, Morris GS, Huang L, Felix ER, Farkas GJ, Molinares D, Tiozzo E. The role of exercise in aromatase inhibitor‐induced arthralgia. PM&R. 2024;16(12):1406-16.
Yuan H, Chen S, Duncan MR, de Rivero Vaccari JP, Keane RW, Dalton Dietrich W, Chou TH, Benny M, Schmidt AF, Young K, Park KK. Ic100, a humanized therapeutic monoclonal anti-asc antibody alleviates oxygen-induced retinopathy in mice. Angiogenesis. 2024;27(3):423-40.
Zhu H, Guest JD, Dunlop S, Xie JX, Gao S, Luo Z, Springer JE, Wu W, Young W, Poon WS, Liu S. Surgical intervention combined with weightbearing walking training promotes recovery in patients with chronic spinal cord injury: a randomized controlled study. Neural regeneration research. 2024;19(12):2773-84.


The Miami Project to Cure Paralysis was established in 1985 to develop new therapies to improve function in paralyzed individuals. We are very enthusiastic about our current accomplishments and multi-disciplinary research programs. In addition, we are most eager about the future as we continue to move new treatments forward to treat paralysis.