







November 2025
Kiaora,
Welcome to the November 2025 edition of Supplyline. The NZSSA Conference held in Rotorua in early September was wonderful, many thanks to Britta from MTANZ and the NZSSA Conference Committee. It was wonderful to see so many presenters from within our own field talk about a variety of subjects, congratulations to all of you.
Next year’s conference is going to be held in Christchurch and it will be our 50th Annual conference. So it is a huge achievement for the NZSSA. I hope to see many of you there.
Inside this edition is a variety of information including the NZSSA’s Strategic plan; various articles and adverts; and a number of updates. I hope you enjoy this edition. If there is anything that you would like to see included in Supplyline in upcoming editions, please contact either Donna Dador or myself
I wish you all a Merry Christmas and a happy New Year. Please be safe over the holiday period and all the best for 2026.
Ngã mihi
Aileen Derby
Co-Editor
NZSSA Supplyline

Kia ora koutou,
How time flies! It’s hard to believe we’ve already reached the final edition of Supplyline for 2025. This year has been nothing short of exciting and inspiring, from registration announcements and engaging education events, to the well-recognized video competition(which I have to say was a personal highlight!), and welcoming new committee member who bring fresh energy and perspectives to our community.
I want to take a moment to say thank you. Thank you for continuously making your mark in the healthcare industry, for your unwavering commitment to promoting and advocating for our patients, especially in an environment that is becoming increasingly complex and ever-evolving. It’s been incredible to witness how our profession continues to grow, gain recognition, and be celebrated for the vital role we play. The passion and dedication you bring to Sterile Services is truly making a difference. There’s so much to look forward to in the coming year, and I’m excited for what’s in store for us. Let’s keep the momentum going, support one another, and continue to raise the profile of our profession.
Ngã mihi nui, Donna Dador
As I reflect on the past year, I am incredibly proud of what our members and committees have achieved together. The NZSSA continues to strengthen its role as a leader in sterile sciences, with membership now exceeding 800. We have made significant progress in supporting education, professional development, and the registration of our members under the regulatory body. We have also established a partnership with the DAA and continued to share knowledge and expertise across Aotearoa. Our collective efforts have helped ensure excellence in reprocessing standards and patient safety nationwide.
This year’s conference was very well attended, receiving excellent feedback and enthusiasm from delegates. We were fortunate to have outstanding support from our trade partners, and our speakers delivered high-quality and engaging presentations throughout the event.
Looking ahead, next year’s conference will be held in Christchurch at Te Pae from 23–25 September 2026, with the conference dinner at the Wigram Air Force Museum. The evening will feature an aviation theme, and we look forward to another inspiring and successful event.
In closing, I sincerely thank all our members, committees, and regional representatives for their hard work and dedication throughout the year. Your continued commitment to excellence and collaboration is what keeps the NZSSA strong and respected. Together, we will continue to grow our profession, support one another, and uphold the highest standards in sterile sciences across Aotearoa.
Ngã mihi, Martin Bird President, NZSSA

Ernst Dennhöfer
Devices to be used in a sterile state must be released after reprocessing and labelled as sterile. “Devices labelled as ‘sterile’ shall be processed, manufactured, packaged and sterilized by means of appropriate, validated methods” [1]. “Where possible, heat sterilization is the method of choice.”[2] Microorganisms are eliminated exponentially by steam sterilization; it is very unlikely that a microorganism will survive the use of a validated and correspondingly safe process with moist heat at 135.5 °C. A sterility assurance level (SAL) < 10 -6 is usually achieved.
A sterilization process is characterized by the process parameters and their tolerances, i.e. by the circumstances whose changes affect the microbicidal activity. The process is controlled such that the process parameters are reached for every cycle. The measured values are recorded with adequate accuracy and precision [2].
In the healthcare setting parametric release should be the rule because “no sample or indicator testing is required for parametric release” [3]. Parametric release takes place immediately after sterilization and is valid for the documented process and the entire sterilized supplies.
EN ISO 14937 defines parametric release as follows: “Parametric release is a declaration that a product is sterile based on records demonstrating that the process variables were delivered within specified tolerances”. The following must be documented:
the plateau period which must always be longer than the holding time, the temperature during the holding time, the water activity a w during the holding time, the pressure prevailing in the chamber at the time the specified cycle parameters are reached, the amount of non-condensable gases which e.g. is monitored by a steam analyser, and
Table 1: To be used for release
Method Steam EO* LTSF**
Documented measured values Required Required Required
Biological indicators Not required Required in principle Required in principle
Chemical indicators auxiliary, not required auxiliary, not required auxiliary, not required
* Ethylene oxide
** Low-temperature steam formaldehyde
the assessment at appropriate intervals of the basic bioburden on the devices and their sterile packaging.
“Sterilization records should be available for each sterilization run”. Dedicated sensors are used for these records because “control instrumentation should normally be independent of monitoring instrumentation and recording charts”[2]. If parametric release is not possible, e.g. in the case of gas sterilization, between 10 and 30 biological indicators (BIs) are distributed for the release in each load, see Table 1.
The bioburden on the devices should be kept stable and low before sterilization [3]. Biological indicators contain many spores, and each additional indicator increases the bioburden of the load. Additional tests and additional indicators do not
* This text, written by E. Dennhöfer for “Central Service 20 years on”, has been revised and updated.
enhance the sterilization process performance. Therefore, the EU Guide to Good Manufacturing Practice states: “If biological indicators are used strict precautions should be taken to avoid transferring microbial contamination from them” [2].
The partial pressure of the air is not part of the process variables. BIs and chemical indicators (CIs) come into contact with the ambient air before and after use and must not react with the normal air. However, air can give rise to air pockets hampering steam penetration. Therefore, a steam penetration test is routinely performed.
Steam and air separate as demonstrated by experiences with the gravity-displacement steam sterilization cycles. However, for a dynamic air removal method an air amount corresponding to 35 ml NCGs in 1 kg steam [5] is not considered problematic. According to Wichmann, only a probability can be given for the presence, extent, intensity and stability of an air pocket for a particular measuring point [6]. Often, air pockets are detected only by chance. It is therefore advisable to structure the process such that air and other NCGs are systematically eliminated instead of searching for a random device defect.
In routine operations the reaction of chemical indicators is assessed immediately at the end of the cycle and the user takes account of this result for the release.
Indicators are chosen by the operator. They provide information that takes effect only if it is noted and heeded by the user; they do not eliminate risks. A used indicator is disposed of; the assessment is not verified. Indicators that have been exposed to a sterilization process are not archived. Besides, process technology solutions that are independent of the properties of the inoculated carrier and the quality of chemical indicators should be chosen for parametric release.
For BIs, EN ISO 11138-7 (formerly: EN ISO 14161) recommends an incubation time of at least 7 days [7]. If BIs are used for release, the entire load must be kept in quarantine for this period. The use of CIs is not required, but process indicators are usually used to distinguish between sterilized and unsterilized units.
Indicators should be assessed without delay because they can change after exposure if the exposure time, temperature or the moisture were not adequate. For example viable microorganisms can subsequently grow or die, and in the case of CIs the colour may darken or fade over time.
BIs are produced with spores that are difficult to sterilize and have the same D and z values and the same resistance [8]. The D value, expressed in minutes, is the exposure time after which only one in every ten BIs exhibits growth. The resistance of the biological indicator is the product of the D value and the log of the baseline microbial count N0 (FBio = log N0 × D). The z value, expressed in °C, is the change in (exposure) temperature that causes a 10-fold change in the D value of a BI.
The logarithmic representation of the survival curve in which the log of the still viable microorganisms is plotted against the exposure time is extrapolated to the required sterility assurance level (SAL) and should be a straight line. The resistance is given for the indicator, not for the spore suspension used to produce the BI. To demonstrate the sterilizability
devices are inoculated with a spore suspension and then serve themselves as inoculated carriers. With care products, the effect comes from the care product; with hot, anhydrous oil, for example, dry heat has an effect. For dry heat N0 = 106 spores, D160 ≥ 2 min and z ≤ 20 °C are calculated, see EN ISO 11138-4 [9].
To check the performance of BIs, the survival and inactivation times are tested (see Table 2). For the survival time D × (log N0 – 2) 99 % of t he BIs should exhibit growth, for the inactivation time D × (log N0 + 4) 99 % of t he BIs should not show growth.
The manufacturer describes either the graduated response, allowing assessment of the effect of one or several critical process variables, or the unambiguous change in the chemical indicator, the “endpoint”, i.e. the point at which the specified stated values (SV) have been reached or exceeded. Chemical indicators are not suitable for demonstration of sterilization effectiveness, even in any combination with a process challenge device (PCD), because “these indicators show only that the sterilisation process has occurred; they do not indicate product sterility or achievement of the required sterility assurance level.” [2] and “the endpoint of a chemical indicator
Table 2: Biological indicators for the “full cycle“ at 135.5 °C
BI with 105 spores and FBio = 12 min at 121 °C z = 6 °C (ISO 11138-3) z = 10 °C (usual)
Survival time at 135.5 °C 1.7 s 15.3 s
Inactivation time at 135.5 °C 5.0 s 46.0 s
does not prove that the item accompanied by the indicator is sterile; it demonstrates that the item has been subjected to certain conditions” [10].
Several publications have reported that even standardized CIs can respond differently. For example, the intensity of the CI reaction may depend on contact with superheated steam, coloured textiles, wet metal and other foreign substances in its vicinity, even when the CIs have the same purpose and are exposed to the same process in the same sterilizer [11].
The advantages of chemical indicators are their low costs, easy assessment and rapid availability of the results. But CIs designed to reach an endpoint cannot be adjusted; they can only be “discoloured” or “not discoloured”. An ambiguous reaction can cause uncertainty for the persons entrusted with release.
CIs are assigned to a class depending on their intended use. Process indicators belonging to class 1 as per EN ISO 11140-1 [12] are used to distinguish between sterilized and unsterilized packaging units, they respond to conditions occurring in the chamber during the specified sterilization process. Therefore, a process indicator must always be placed on the outside of the packaging where it is visible.
Class 2 CIs are PCDs. “Internal” indicators belonging to classes 3, 4, 5 and 6 are placed inside the packaging and at
times in lumens, where they are erroneously designated as “sterilization indicators”. The usefulness of internal indicators in routine operations is questionable since they are generally not visible at the time of release.
The implications of freely available moisture for steam sterilization are often underestimated. Water bound to devices through adsorption can also be freely available, for example, because a steam layer forms above the liquid, and the effectiveness of steam sterilization depends on the ratio of the absolute pressure of the steam on the device to the absolute pressure of the steam above pure water. This ratio is called the water activity a w
Murrel and Scott [13] investigated the resistance of various spores at different water activity a w They found major differences. For G. stearothermophilus spores the lowest D values were identified at 0.8–1.0 aw, the highest D values were around 20-fold higher and were found at 0.2–0.4 a w For type E C. botulinum spores the D values at 0.2–0.3 a w were much higher than at 1.0 a w For intensively dried spores the D values declined again at < 0.2 a w The D values of the dried C. botulinum spores were nevertheless higher than the resistance of likewise dried G. stearothermophilus spores. Young [14] confirmed that the D values of BIs with B. atrophaeus spores were around 2 to 3-fold higher against dry heat than the resistance of BIs with G. stearothermophilus spores.
Spicher et al. [15] identified the maximum D values for B. atrophaeus spores overheated to 28 °C or at 0.3–0.35 aw, the D values were 119-fold higher than at 1.0 a w At < 0.5 aw, i.e. at less than 10 °C overheating, the D values of G. stearothermophilus spores remained almost equally high. Therefore, G. stearothermophilus spores are not the measure of all things, as assumed in several guidelines and standards.
The least favourable site
Many standards stipulate that measuring probes and indicators be placed at the “least favourable site”. For steam sterilization a site at which a necessary condition, e.g. a temperature, is reached later than at other sites or which deviates more from a set point can be defined as “unfavourable”.
But there are always several unfavourable sites, and in the event of malfunction favourable sites may become unfavourable. There is no one site that is the least favourable site under all circumstances. For example, steam will not necessarily overheat where condensate forms a puddle. An unfavourable site may change if an air pocket moves or condensate drains off. Condensate can remain in place, drip off, collect in deepenings or crevices, be adsorbed or absorbed at different rates. Air is preferentially accumulated in places where steam condenses. However, non-condensable gases (NCGs) are almost always heavier than steam and move downwards. Air does not concentrate where there is already air, but rather at sites where there is no air to hinder condensation.
For the holding time, the water activity a w and the bioburden, the difference versus experimentally verified values can be taken into account with a safety factor of 3 or 103
Process challenge devices (PCDs) are used to investigate whether a process assures a performance defined by the PCD,
e.g. a steam penetration test is used to detect an air pocket. But EN ISO 15882 warns: “There is no universal PCD that can be used for all sterilizer types and sterilization procedures” [10].
The PCD for the Bowie & Dick test [5] consists of a standard test pack and an indicator sheet [16] based on EN ISO 111403, allowing objective evaluation of steam penetration. While this test is not proof of sterilization effectiveness, it is of major significance because EN ISO 17665-1 does not mandate any other performance test with standardized criteria [4]. Albeit, we identified the coldest site in the centre only with 17 % probability [17], and over the plateau time the air pocket moves down and towards the axis of the test pack.
For the steam penetration test in almost all cases only alternative systems to the Bowie and Dick-type test, inoculated with G. stearothermophilus spores, or a chemical indicator as detector are used anymore. But the sterilizer operating cycles differ from the standardized test cycles described in EN ISO 11140-4 [18]. Therefore, as a first step, the performance of the alternative system chosen by the operator should be compared with the performance of the Bowie & Dick test.
In principle, hollow devices can be sterilized with the universal programme. But hollow devices make different demands on the process compared with the standard test pack and the fictive dilution ratio Vf is not suitable for assessment of air removal from hollow devices. Peters et al. noted: “A vacuum-steam-vacuum system suitable for porous items is not always suitable for hollow devices; the opposite is also true” [19]. D. Kaiser [20] stated: “Air removal from hollow devices unfolds very differently in dynamic-air-removal steam sterilization cycles than e.g. within porous items or empty spaces”. That was also our experience.
According to Borchers and Mielke [22], air removal from tubes with a diameter of between 6 and 8 mm is considerably more difficult than from tubes with a 2 mm diameter. They believe this is due to overheating of the steam. Tests were done at 100 °C for 5 minutes with B. atrophaeus spores in various pipes and tubes closed at one end. D. Kaiser tested with chemical indicators and obtained the same results.
Hollow devices are devices with drill holes or gaps as well as tubes, bottles and other receptacles. Many lumens are capillary lumens/cavities, e.g. the thread in an 8 mm high M 10 nut has the shape of a double helix measuring 300 mm long. Objects with capillaries retain condensate while heat and condensate is distributed differently in capillaries than on objects from which the condensate drips off. In my opinion the hollow device systems described in EN ISO 11140-6 [21] are arbitrarily defined. While the least favourable site in the lumen is fixed, the standardized systems are not representative of either the standard test pack or specific devices.
On the first steam pulse, the mixture of steam and non-condensable gases (NCGs) located just before the opening penetrates into the lumen. On the outside, the condensate drips off as if from a solid object. On the inside, where condensate and air insulate the inside wall, there is less steam condensation and the condensate drips off only if there is a gradient. On the inside too, drops are formed in the vicinity of the opening, as seen in the Lautenschläger video “News from the test sterilizer”. If the capillary action allows it, this condensate collects at the deepest site and can trap NCGs.
Overpressure test programme (Vf = 15,000)
Standard test pack suitable
Hollow device not suitable
Programme for liquid waste(Vf = 15,000)
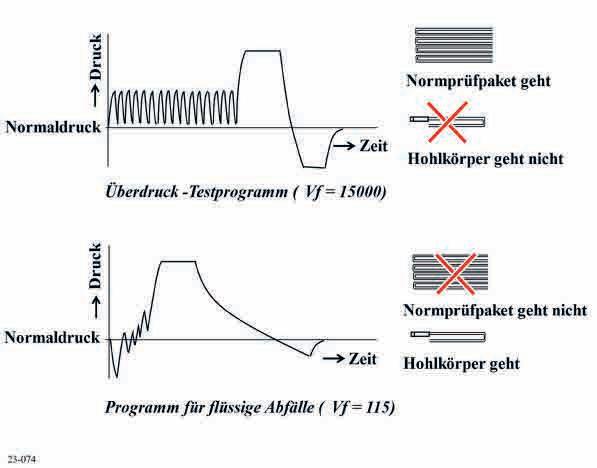
Fig. 1: The suitability of the operating cycle
Standard test pack not suitable
Hollow device suitable
The total amount of condensate depends on, among other things, the wall thickness but even if the wall thickness is the same everywhere, the condensate is distributed unevenly. No characteristic air pocket is formed within the lumen since the hollow device is heated from the outside. For thermometric tests it should be noted that NCGs that are forced by the steam down into the lumen do not have a sterilizing effect. But in the hot environment they delay humidification of the inside walls and of other objects in the lumen.
Summary
When developing devices and processes, viable spores and biological indicators can be used to detect the presence of the sterilising agent. In particular, a change in the D values can provide important information. But indicators, which can only be evaluated in a laboratory after 7 days’ incubation, are not recommended for routine operations in the healthcare setting. Chemical indicators are useful for distinguishing between sterilized and unsterilized items of packaging. They are needed for the Bowie & Dick test but are not suitable for demonstrating proof of sterilization.
References
1. Regulation (EU) 2017/745 of the European Parliament and of the Cou ncil of 5 April 2017 on medical devices
2. Commi ssion Directive (EU) 2017/1572: Guidelines for Good Manufacturing Practice for medicinal products for human use - Annex 1: Manufacture of Sterile Medicinal Products
3. EN ISO 14937:2009 – Sterilization of health care products – General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices (ISO 14937:2009)
4. EN ISO 17665:2006 – Sterilization of health care products – Moist heat – Part 1: Requirements for the development, validation and routine control of a sterilization process for medical devices (ISO 17665-1:2006)
5. EN 285:2015+A1:2021 – Steam sterilizers – Large sterilizers
6. Wich mann R, Dennhöfer E, Dennhöfer S: Steam Sterilization – The Response of the Test Pack. Biomed Instrumen Technol. 1993; 27: 412–418.
7. EN ISO 11138-7:2019 – Sterilization of health care products – Biological indicators – Part 7: Guidance for the selection, use and interpretation of results (ISO 11138-7:2019)
8. EN ISO 11138-1:2017 – Sterilization of health care products – Biological indicators – Part 1: General requirements (ISO 11138-1:2017)
9. EN ISO 11138-4:2017 – Sterilization of health care products – Biological indicators – Part 4: Biological indicators for dry heat sterilization processes (ISO 11138-4:2017)
10. EN ISO 15882:2008 – Sterilization of health care products – Chemical indicators – Guidance for selection, use and interpretation of results (ISO 15882:2008)
11. Denn höfer, E: Testbögen für den Dampf-Durchdringungstest, eine Untersuchung handelsüblicher Indikatoren; Indikatoren. Hyg Med 1989; 14: 128–134.
12. EN ISO 11140-1:2014 – Sterilization of health care products –Chemical indicators – Part 1: General requirements (ISO 111401:2014)
13. Murrell W G, Scott W J: The heat resistance of bacterial spores at various water activities. J. Gen. Microbiol. 1966; 43: 411–425.
14. Young J H: Sterilization of Various Diameter Dead-Ended Tubes. Biotechnol and Bioeng, 1993; 42: 125–132.
15. Spic her G, Peters J, Borchers U: Microbicidal efficacy of superheated steam. I. Communication: Results with Spores of Bacillus subtilis und Bacillus stearothermophilus and with Spore Earth, Zent. bl.Hyg.Umweltmed. 1998/99; 201: 541–553.
16. EN ISO 11140-3:2009 – Sterilization of health care products –Chemical indicators – Part 3: Class 2 indicator systems for use in the Bowie and Dick-type steam penetration test (ISO 111403:2007, including Cor 1:2007)
17. Meurer S, Arnold T, Dennhöfer E, Peter R: Kritische Größe des Prüfpaketes für Dampfsterilisatoren mit 80 Liter Kammervolumen. Zentr Steril 2000; 8: 278–282.
18. EN ISO 11140-4:2007 – Sterilization of health care products –Chemical indicators – Part 4: Class 2 indicators as an alternative to the Bowie and Dick-type test for detection of steam penetration (ISO 11140-4:2007)
19. Peters J, Schwebke I, Simon P G, Bönisch M: Zum Einsatz von Hohlkörper-Prüfmodellen bei VDV-Verfahren zur Dampfdesinfektion bzw. -sterilisation. Hyg Med 1997; 22 (9): 444–462.
20. Kaiser D: Experimentelle Analyse der Entlüftung von Hohlkörpern in Dampfsterilisationsprozessen. Thesis Techn. Univ. Berlin, 2013; Sächsische Landesbibliothek – Staats- und Universitätsbibliothek Dresden, XD 2104 K13, 2013 (201 p.)
21. EN ISO 11140-6:2022 – Sterilization of health care products –Chemical indicators – Part 6: Type 2 indicators and process challenge devices for use in performance testing of small steam sterilizers (ISO 11140-6:2022)
22. Borchers U, Mielke M: Einfluss von Material, Länge, Wandstärke und Durchmesser von Hohlkörpern auf das Inaktivierungsverhalten von Bioindikatoren bei der Behandlung mit feuchter Hitze, Zentr Steril 2004; 12: 314–322.





Scope Washer Rinse Water AFER


Phoebe Malley
Senior Microbiologist & Business Development Representative
Auckland & Northland
M: 027 286 6129
E: phoebe.malley@scbio.co.nz
Aaron Watson
Senior Microbiologist & Managing Director
New Zealand
M: 021 114 0079
E: awatson@scbio.co.nz

Tena koutou,
I would like to share a little about my journey in the sterilising field.
For several years, I have worked as a Sterilising Technician at the Te Kaupeka Pūniho | Otago Faculty of Dentistry. This role has given me a deep appreciation for the highest standards in both large-scale dentistry and hospital practices. I’ve also had the chance to formalise my skills by completing the New Zealand Certificate (Level 4) and Diploma (Level 5) in Sterilising Technology, which has been incredibly rewarding.
Beyond my work, my dedication to organisation and community is clear. As the social media administrator for
the New Zealand Para Darts Association, I help a diverse group of people connect and thrive. I also organise and run a pre-loved clothing market, a creative outlet that has sharpened my project management and relationshipbuilding skills.
I am passionate about precision and continuous learning in our field, and I’m eager to bring my drive and commitment to the Executive Team. I look forward to contributing to the goals of the Sterile Sciences Association.
Ngã mihi nui.
Shelley Morrison
Sterilising Technician
Te Kaupeka Puniho | Otago Faculty of Dentistry
As part of the Te Whe Programme, we had the opportunity to engage with Year 11 students to introduce them to the vital work carried out in the Central Sterilising Unit (CSU). Our session aimed to raise awareness of the behind-thescenes roles in healthcare and inspire interest in healthrelated careers.
We explained the importance of sterilisation in maintaining patient safety and preventing infection and gave students a hands-on look at the processes involved—from receiving used surgical instruments to cleaning, inspecting, packing, and sterilising them for reuse. The students were particularly interested in the technology we use, such as autoclaves and tracking systems, and how strict protocols ensure everything is safe and traceable.
It was a rewarding experience to see the students engage with curiosity and ask thoughtful questions. We hope this session helped them appreciate the critical role CSU plays in supporting surgical teams and patient care, and
perhaps sparked interest in future careers in health sciences or sterile services.

MATERIALS YOU CAN PROCESS ONLY IN A V-PRO STERILIZER VISIT OUR DEVICE MATRIX FOR A GUIDED DEVICE SEARCH
We’re pleased to confirm there is a potential educational pathway for Level 5 Diploma Sterilising technology graduates with us at Toi Ohomai. Our Post Graduate Diploma (leading to Masters) in Applied Professional Studies – with strands in Leadership and Mentoring and Adult Teaching, can be enrolled in without a degree, if the applicant can provide clear evidence of experience in that area, as well as the requisite English language requirements. Evidence of experience may include but is not limited to, relevant internal or external training, and peer and management attestations. Please email cassandra.elder@toiohomai.ac.nz with any expressions of interest, and these will be passed on to the relevant programme manager for assessment and potentially an interview.
There has been interest shown in a sterilising technology micro-credential which covers Standard AS5369 and its implication in practice, and other cultural aspects of working in Health in New Zealand. This has been raised with the Executive dean and we are currently in discussions about the financial viability of this initiative.
Strategic growth areas for 2026:
Our current course success for the Level 4 Certificate, are: 94.9% (overall), 88.9% (Mūori), 84.6% (Pasifika). While we are very pleased with these outcomes, we are always striving to make improvements to this key indicator, and to this end - we have identified two strategic growth areas, as outlined below:
Our programme coordinator Alison Stewart’s current research is on the influence of shift work on distance learning. Early findings suggest that what we call “student readiness” - students being mentally prepared for their
studies - is essential to their eventual success.
With this in mind, we are in the process of creating an information video for sterilisation managers to watch alongside their new enrolling technicians. The video will be short and accessible, and touch on key points which we have observed over several years to contribute most directly to student success. The video is currently in the last stages of scripting, and will be recorded and disseminated by December, in preparation for Semester 1, 2026 enrolments.
We have also opened a discussion with the NZSSA executive on creating a content review panel of our Level 4 and 5 Sterilising Technology programmes, to support Alison Stewart’s ongoing work in this space. The review panel would be established at the start of 2026, and would:
• include representation of leaders in sterilising technology from private hospitals, public hospitals, and dental practices
• ensure Toi Ohomai students have the most up to date information from the industry
• work towards ensuring all courses in both programmes are fully refreshed by the end of 2027
Keep an eye out for an announcement and call for expressions of interest, we would love to hear from you.
Enrolments are open for 2026 for both the Level 4 and Level 5 programmes, so we look forward to welcoming you to the Toi Ohomai student experience!
https://www.toiohomai.ac.nz/study/course/new-zealandcertificate-sterilising-technology-level-4
https://www.toiohomai.ac.nz/study/course/new-zealanddiploma-sterilising-technology-level-5
Appl icati ons clo se 30 Apri l 2026, 5pm For full details of the scholarships and application forms visit the Scholarship page: https://nzssa.co.nz/scholarship/
Written by: Richard Murray (National Medical Device Reprocessing Manager at Whiteley)
September 2025
“The Importance of Manual Cleaning” emphasises the critical role of manual cleaning in reprocessing reusable medical devices, such as endoscopes and CSSD instruments, to prevent hospital-acquired infections (HAIs) and ensure patient safety. The presentation aligns with AS 5369:2023 and GENCA guidelines. It addresses microbial contamination, point-of-use cleaning, biofilm challenges with enzymatic detergents, PPE, cleaning solutions, and innovations to enhance infection prevention and control.
Post-procedure, endoscopes harbor 8–10 billion microbes, exceeding the global population, while CSSD instruments carry 10^6–10^9 pathogens, including Staphylococcus aureus and hepatitis viruses. The complex designs of endoscopes, with channels and elevator mechanisms, are linked to 281 historical and over 130 recent infection outbreaks due to inadequate cleaning. The presentation illustrates high microbial loads (>10^4 CFU for endoscopes, >10^3 for CSSD), underscoring HAI risks.
Manual cleaning is essential for removing blood, tissue, and biofilms, preventing cross-contamination. Unlike automated methods, it enables thorough cleaning of intricate areas like endoscope channels and CSSD hinges, which machines may miss, and allows inspection for wear or damage to ensure device functionality. Point-of-use cleaning, performed immediately post-use, prevents soil drying, which forms insoluble protein barriers, increasing biofilm and infection risks. Delays exceeding one hour, particularly with external CSSDs (30–120 min transport), exacerbate these issues.
Biofilm, a microbial community in a protective matrix, adheres to surfaces and resists cleaning that shield pathogens like Pseudomonas aeruginosa. Detergents, specifically biofilm-removing formulations, target proteins, lipids, and polysaccharides in the biofilm matrix, significantly enhancing removal when combined with thorough manual cleaning. These detergents disrupt the structural integrity of biofilms, reducing infection risks. PPE, including gloves, gowns, face shields, and masks, protects staff from aerosols carrying pathogens during cleaning.
Manual cleaning achieves 2–6 log reductions for endoscopes (10^10 to 10^4–10^6) and 2–5 for CSSD
(10^9 to 10^3–10^5), enabling effective high-level disinfection for endoscopes and sterilization for CSSD. Challenges such as human error and complex designs are addressed through training, IFUs, and automation. AS 5369:2023 and GENCA emphasise rigorous protocols. The presentation advocates for immediate precleaning, biofilm-removing detergents, and adherence to standards to mitigate HAI risks, ensuring a safer healthcare environment.
Partner with Whiteley to implement our market-leading biofilm-removing detergents, expert-led training, and compliance solutions. Together, we can enhance manual cleaning processes, reduce HAI risks, and protect patients and staff. Contact Whiteley today to strengthen your infection prevention strategy.
Manual cleaning achieves 2–6 log reductions for endoscopes (10^10 to 10^4–10^6) and 2–5 for CSSD (10^9 to 10^3–10^5), enabling effective high-level disinfection for endoscopes and sterilization for CSSD (Rutala & Weber, 2019). Challenges such as human error and complex designs are addressed through training, IFUs, and automation (Ofstead et al., 2021). AS 5369:2023 and GENCA emphasise rigorous protocols. The presentation advocates for immediate precleaning, biofilm-removing detergents, and adherence to standards to mitigate HAI risks, ensuring a safer healthcare environment.
Partner with Whiteley to implement our market leading biofilm removing detergents, expert-led training, and compliance solutions. Together, we can enhance manual cleaning processes, reduce HAI risks, and protect patients and staff. Contact Whiteley today to strengthen your infection prevention strategy.
**Footnotes**
1. Kremer et al., *Scientific Reports* (2025), DOI: 10.1038/s41598025-13740-7.
2. Alfa et al., *American Journal of Infection Control* 42, no. 3 (2014): 234–240.
3. Muscarella, *Endoscopy* 28, no. 5 (1996): 409–415; Ofstead et al., *American Journal of Infection Control* 47, no. 6 (2019): 649–655.
4. Dancer et al., *Clinical Microbiology Reviews* 28, no. 4 (2015): 1108–1135.
5. Kremer et al., *Scientific Reports* (2025).
6. Ofstead et al., *American Journal of Infection Control* 47, no. 6 (2019): 649–655.
7. Vickery et al., *Journal of Hospital Infection* 56, no. 2 (2004): 113–118; Vickery et al., *Infection Control & Hospital Epidemiology* 35, no. 4 (2014): 434–436.
8. Ofstead et al., *American Journal of Infection Control* 48, no. 4 (2020): 392–398.
9. Rutala & Weber, *Infection Control & Hospital Epidemiology* 40, no. 9 (2019): 1013–1019.
10. Ofstead et al., *American Journal of Infection Control* 49, no. 2 (2021): 267–273.
11. Barakat et al., *Infection Control & Hospital Epidemiology* 45, no. 1 (2024): 45–50.






























Safety: Classified as NON Dangerous and NON Hazardous for a safer workplace.
Compliance: Meets AS/NZS 5369:2023 standards.
Visibility: Opti-Pro’s Light blue colour makes it easy to see full coverage on your instruments.

PLEASE SPEAK TO YOUR HEALTHCARE MANAGER FOR FURTHER INFORMATION
ECOLAB HEALTHCARE ANZ
4B Pukekiwiriki Place, East Tamaki, Auckland 2013, New Zealand NZ: 0800 425 529 www.healthcare-nz.ecolab.com
This is not a scientific paper. A scientific paper is to be written when the end of this saga occurs. This a cautionary tale of how reverse osmosis (RO) water can go horribly wrong. However I have included some of the scientific results in order for the reader to understand the issues encountered.
The author would like to thank Smitha Allookaran whose knowledge of microbiology and dedication to the water testing regime has been critical to getting where we need to be with our RO water.
My hospital first had an (RO) unit installed for CSSD use when new washers were installed back in 2007. Little was known about those systems at that time and we happily went along with it and allowing our hospital engineers maintain the system.
adjusted(increased or decreased) according to the test results to ensure that they stayed within the specified ranges of Table 7.2
Forward onto 2011 and CSSD moved into a new custom designed unit with four washers instead of the previous two. The original RO water system was transferred to the new unit and provided RO water to the washers and to a tap place above the sinks in the decontamination area. As Facilities and their team were maintaining this we had no reason to question to much as they told us everything was ok.
Final Rinse water TVC (total viable count) had to be undertaken monthly for the first 12 months , after which testing could be done annually as long as the results remained within the range specified in table 7.2.
Then as we know in 2014 AS/NZS4187 told us all in Table 7.2 that our water quality should meet certain requirements for cleaning and final rinse water and Table 8.1 told us how often this should occur. However the guidance on how we should be achieving these requirements left many of us confused particularly around Endotoxin testing. Our facilities team had told us they were doing the water testing which we took at their word. Upon reflection, Red flags should have been raised in our thoughts as when I requested the water results they were never forthcoming.
Fast forward to 2019 and as we all know Covid turned our world upside down.
Final Rinse Water Endotoxin this could be undertaken annually, with the frequency o f testing being able to be adjusted(increased or decreased) according to the test results to ensure that they stayed within the specified ranges .
At this time there was also an update to AS/NZS 4187 and there were changes to the water monitoring requirements in Table 8.1. Now we were required to not just be testing water quality, there were specific areas that were identified as required to be tested.
As it was the Covid era little happened with regards to wat er testing especially from our Facilities team as no one was available to undertake this.
Tests for both Supply Water Hardness and Chloride and Final Rinse Water Conductivity changed from monthly to monthly with the frequency of testing being able to be adjusted(increased or decreased) according to the test results to ensure that they stayed within the specified ranges of Table 7.2.
Final Rinse water TVC (total viable count) had to be undertaken monthly for the first 12 months , after which testing could be done annually as long as the results remained within the range specified in table 7.2.
Final Rinse Water Endotoxin this could be undertaken annually, with the frequency of testing being able to be adjusted(increased or decreased) according to the test results to ensure that they stayed within the specified ranges.
Towards the end of 2022 I approached our facilities team and talked with the plumber whose role it was to maintain the RO water system and ensure that it was clean, functioning and the filters were regularly changed. Unfortunately due to the shutdown it appeared that the RO system had been neglected and that by raising the matter with the plumber and his response even more Red f lags were raised.
As it was the Covid era little happened with regards to water testing especially from our Facilities team as no one was available to undertake this.
At this time the CSSD Quality F acilitator and I decided that we had to t ake charge of the situation and contacted SC Bio to undertake initial water testing in line with the requirements of the standards.
Towards the end of 2022 I approached our facilities team and talked with the plumber whose role it was to maintain the RO water system and ensure that it was clean, functioning and the filters were regularly changed. Unfortunately due to the shutdown it appeared that the RO system had been neglected and that by raising the matter with the plumber and his response even more Red flags were raised.
Samples of final rinse water from the washers and water from the wall mounted RO water tap were collected under aseptic conditions and sent off for testing. The results that came back were of concern. Total Viable Count for the final rinse water was above the specification of ≤100cfu/100ml. as seen in Table 1.
At this time the CSSD Quality Facilitator and I decided that we had to take charge of the situation and contacted SC Bio to undertake initial water testing in line with the requirements of the standards.
Samples of final rinse water from the washers and water from the wall mounted RO water tap were collected under aseptic conditions and sent off for testing. The results that came back were of concern. Total Viable Count for the final rinse water was above the specification of ≤100cfu/100ml. as seen in Table 1.
Table1: Initial final rinse water results
Table1: Initial final rinse water results

We raised the issue with management, infection prevention and control (IPC) and our Facilities team. More testing was going to have to happen and experts were brought in to
system, and they specifically mentione d rodents. The experts advised f acilities of remedial action that they could make to the system , and it was agreed that this would occur.
A decision was agreed upon by my Quality Facilitator (a qualified microbiologist) and the expert who had come to inves tigate the RO system that we should test the water from the RO tanks.
We raised the issue with management, infection prevention and control (IPC) and our Facilities team. More testing was going to have to happen and experts were brought in to look at the RO water system. More testing was undertaken at the CSSD end but results were still above the required threshold.
A decision was agreed upon by my Quality Facilitator (a qualified microbiologist) and the expert who had come to inves tigate the RO system that we should test the water from the RO tanks.
Samples were carefully taken from the tanks and sent off for analysis and we eagerly awaited for the results.
The experts brought into review the RO system agreed that the system was old outdated and not fit for purpose. There were areas where contaminants could potentially enter the system, and they specifically mentioned rodents. The experts advised facilities of remedial action that they could make to the system, and it was agreed that this would occur.
Samples were carefully taken from the tanks and sent off for analysis and we eagerly awaited for the results.
The results from the three RO tanks did not read well. The bioburden levels in the tanks were very high as shown in Table 2.
A decision was agreed upon by my Quality Facilitator (a qualified microbiologist) and the expert who had come to investigate the RO system that we should test the water from the RO tanks.
Samples were carefully taken from the tanks and sent off for analysis and we eagerly awaited for the results.
The results from the three RO tanks did not read well. The bioburden levels in the tanks were very high as shown in Table 2.
The results from the three RO tanks did not read well. The bioburden levels in the tanks were very high as shown in Table 2.

At this point we decided that in the interests of patient safety we would switch off the RO water and resort to town water until we could finally resolve the matter. Before we did this samples were taken of the town water and RO tap water for testing an d comparison. The tap water passed the test while the RO water did not, Table 3.
At this point we decided that in the interests of patient safety we would switch off the RO water and resort to town water until we could finally resolve the matter. Before we did this samples were taken of the town water and RO tap water for testing and comparison. The tap water passed the test while the RO water did not, Table 3.
Table 3: RO tap water vs town supply tap water
Table 3: RO tap water vs town supply tap water
At this point we decided that in the interests of patient safety we would switch off the RO water and resort to town water until we could finally resolve the matter. Before we did this samples were taken of the town water and RO tap water for testing an d comparison. The tap water passed the test while the RO water did not, Table 3.
Table 3: RO tap water vs town supply tap water

The remedial work was undertaken on the existing RO water system and before it was put into use we tested the water in the tanks. At this point we were sure the problem would be solved.
The remedial work was undertaken on the existing RO water system and before it was put into use we tested the water in the tanks. At this point we were sure the problem would be solved.
Testing continued after the remedial work was completed, yet we were still getting negative results. Something was going on and we had to find out once and for all. It was decided that we would request DNA testing on the samples to identify what was causing the issues.
By now it was Mid-2024 and we still had no answers.
The remedial work was undertaken on the existing RO water system and before it was put into use we tested the water in the tanks. At this point we were sure the problem would be solved.
Testing continued after the remedial work was completed, yet we were still getting negative results. Something was going on and we had to find out once and for all. It was decided that we would request DNA testing on the samples to identify what was causing the issues .
A sample was submitted from the RO water tap as testing here continue to give high readings. The pipes had been flushed several times with water from the RO tanks so we hoped we would find something as by now it was not showing in the tanks.
`Growing in the RO water was discovered:
By now it was Mid-2024 and we still had no answers.
Testing continued after the remedial work was completed, yet we were still getting negative results. Something was going on and we had to find out once and for all. It was decided that we would request DNA testing on the samples to identify what was causing the issues .
1. Purpureocillium lilacinum - commonly isolated from soil, decaying vegetation, insects, and nematodes and as a laboratory contaminant. It is also a causative agent of infection in human and other vertebrates.
By now it was Mid-2024 and we still had no answers.
2. Cupriavidus metallidurans - is a non-spore-forming, Gram-negative bacterium which is adapted to survive several forms of heavy metal stress.
A sample was submitted from the RO water tap as testing here continue to give high readings. The pipes had been flushed several times with water from the RO tanks so we hoped we would find something as by now it was not showing in the tanks.
A sample was submitted from the RO water tap as testing here continue to give high readings. The pipes had been flushed several times with water from the RO tanks so we hoped we would find something as by now it was not showing in the tanks.
3. Caulobacter species - is a genus of Gram-negative bacteria in the class Alphaproteobacteria. Its best-known member is Caulobacter crescentus, an organism ubiquitous in freshwater lakes and rivers; many members of the genus are specialized to oligotrophic environments
4. Staphylococcus capitis - Staphylococcus capitis is a coagulase-negative species of Staphylococcus. It is part of the normal flora of the skin of the human scalp, face, neck, scrotum, and ears.
Shelagh Thomas/CSSD/Hutt Hospital/ October 2025.
When we realised that we were dealing with a bacterial overload a solution was urgently needed. The facilities team called in the team from Sterile Solutions NZ to come in assess the situation and to flush the entire system with a solution
Shelagh Thomas/CSSD/Hutt Hospital/ October 2025.
After flushing a further sample was taken in order to determine that the solution had been successful.
The results demonstrated that three out of the four bacteria had been eliminated but that the Cupriavidus Metallidurans had flourished resulting in a result of 13,800cfu/100ml
that would kill the bacteria. After approximately 3 days the system was flushed from the RO water tanks and out via the RO wall tap. After flushing a further sample was taken in order to determine that the solution had been successful. The results demonstrated that three out of the four bacteria had been eliminated but that the Cupriavidus Metallidurans had flourished resulting in a result of 13,800cfu/100ml. A new RO water system was installed in October 2024 and again hopes were raised that the solution had been found. This joy was short lived when the water testing showed that the tanks were clear, yet at the RO wall tap the results showed high TVC readings. Table 4.
A new RO water system was installed in October 2024 and again hopes were raised that the solution had been found. This joy was short lived when the water testing showed that the tanks were clear, yet at the RO wall tap the results showed high TVC readings. Table 4.
Table 4: TVC readings from new installation
Table 4: TVC readings from new installation

We are now three quarters way through 2025 and we are still running on town water for our final rinse water in our washers. Work began on replacing the pipes in late September however, appears to have come to a halt.
I had previously had my suspicions that the problem was never the RO water tanks, rather the pipes from the water tanks to CSSD. This idea grew from the fact that some of the taps in CSSD were running very slowly and when the plumber investigate, he found that they were full of pieces of green thermos labile plastic which was coming from the pipes. These pipes as they begin to deteriorate forms grooves down their lengths which are ideal breeding grounds for bacteria. It was agreed that the pipes would need to be replaced however in the current fiscal climate of health this was not deemed a high enough risk by the decision maker, even though there was compelling evidence of bacteria that could harm patients. We are now three quarters way through 2025 and we are still running on town water for our final rinse water in our washers. Work began on replacing the pipes in late September however, appears to have come to a halt.
Water testing was undertake n early October from the RO Unit, RO Water tank and RO wall tap. Unfortunately as the system has been sitting idle it would now appear that the tank is contaminated (Table 5) as well as the pipe and this may be difficult to eliminate.
Water testing was undertaken early October from the RO Unit, RO Water tank and RO wall tap. Unfortunately as the system has been sitting idle it would now appear that the tank is contaminated (Table 5) as well as the pipe and this may be difficult to eliminate.
Table 5: October 2025 RO Water testing Results
I had previously had my suspicions that the problem was never the RO water tanks, rather the pipes from the water tanks to CSSD. This idea grew from the fact that some of the taps in CSSD were running very slowly and when the plumber investigate , he found that they were full of pieces of green thermos labile plastic which was coming from the pipes. These pipes as they begin to deteriorate forms grooves down their lengths which are ideal breeding grounds for bacteria. It was agreed that the pipes would need to be replaced however in the current fiscal climate of health this was not deemed a high enough risk by the decision maker, even though there was compelling evidence of bacteria that could har m patients.
Hutt Hospital is due to recommence ophthalmic surgeries within the next few weeks. This is of concern to me. It is critical that the changes to the pipework from the RO tanks to the washers and the RO wall tap are completed at least three to four weeks in advance of these surgeries commencing. Time is required to have the system up and running, pipes and tanks flushed and several rounds of water testing completed to our satisfaction with results reading within specified ranges. Without satisfactory completion of the works we cannot undertake the reprocessing of ophthalmic instruments as the risk to the patient from water borne contamination and potential TAS is too great to contemplate.
Lessons learnt to date:
• Understand the beast you are dealing with. RO water systems are complicated and need regular maintenance.
• Do not rely on others. Set up a schedule for taking water samples and have staff who are trained to assist with this.
• Get to know your testing laboratory so they can understand your requirements.
• Meet with your Facilities team to ensure that they have a schedule of maintenance for the RO system and they are following through on it and reporting the results to CSSD.
Hutt Hospital is due to recommence ophthalmic surgeries within the next few wee ks. This is of concern to me. It is critical that the changes to the pipework from the RO tanks to the washers and the RO wall tap are completed at least three to four weeks in advance of these surgeries commencing. Time is required to have the system up and running, pipes and tanks flushed and several rounds of water testing completed to our satisfaction with results reading within specified ranges. Without satisfactory completion of the works we cannot undertake the reprocessing of ophthalmic instruments as the risk to the patient from water borne contamination and potential TAS is too great to contemplate.
• Do not give up. When you recognise there is a problem keep pushing until you get the required outcome.
Lessons learnt to date:
• Test, test, test your RO water.
Shelagh Thomas/CSSD/Hutt Hospital/ October 2025.
Understand the beast you are dealing with. RO water systems are complicated and need regular maintenance.
Do not rely on others. Set up a schedule for taking water samples and have staff who

As many of you may be aware, particularly those who attended the NZSSA conference in September, Open Polytechnic is in the process of developing a programme of study for the New Zealand Certificate in Sterilising Technology Level 4 for delivery in 2026.
I had the pleasure of attending the conference and staffing the Open Polytechnic booth, where I met many of you face-to-face. It was a wonderful opportunity to raise awareness of the proposed programme and to gather feedback from a wide range of technicians working in the industry. Support for the programme was overwhelmingly positive, reinforcing that the approach we’ve taken so far, reflects both industry expectations and the realities of the workplace.
Designing a new programme of study takes time. Once the initial design is drafted, it goes through several rounds of consultation. Industry leaders, educators, technicians, and Maori and Pasifika stakeholders have all been asked for feedback (you may remember me handing out sheets and QR codes at the conference) to ensure the course content is relevant, realistic, and reflective of real workplace practice while meeting the strategic purpose and graduate profile outcomes of the qualification as approved by NZQA.
At Open Polytechnic, we also have an internal approval process that involves academic committees and quality teams checking every aspect of the programme - from the learning outcomes to the assessment methods and student support. It’s a detailed and collaborative process, which means that by the end, we can be confident the programme is robust, relevant, and designed to support both learners and industry.
We’re getting close to the next phase of programme development that involves writing course materials. We are therefore, seeking one or more Subject Matter Experts (SME) and a content reviewer to join our project team.
If you have experience in sterile sciences and an interest in contributing to the future of education in sterilising technology, we’d love to hear from you. Please email PDO. Team@openpolytechnic.ac.nz with the subject line Subject Matter Expert/Content Reviewer EOI NZ3219.Attach a cover letter and CV outlining your relevant experience and indicate which role you are applying for (Subject Matter Expert or Content Reviewer). For further information or enquiries about these roles, please contact us via the same email address.


Email: secretary@nzssa.co.nz
Website: https://nzssa.co.nz/
Association Membership
TOTAL MEMBERSHIP
Life Members
791 (up 90% from last year)
Lorraine Arrowsmith Marilyn Clark Aileen Derby
Lorraine Eldershaw Mary Overwheel Jill Parker
Cathy Rackley
Jean Taylor
Jackie Skudder
Bev Turner
Executive Officers (2025)
Alison Stewart
Te Whatu Ora Southern V
Southern Cross Healthcare
Te Whatu Ora Hawkes Bay
Te Whatu Ora Capital, Coast and Hutt Valley
Te Whatu Ora Counties Manukau
J
Te Whatu Ora Hauora a Toi Bay of Plenty
Te Whatu Ora Counties Manukau
Southern Cross Hospitals, Gillies Avenue
J
Te Whatu Ora -Te Pae Hauora of Ruahine o Tararua
Toi Ohomai Institute of Technology
Abhishek Sharma
Almira Blancaflor
Alyss Campbell
Amber Finlayson
Amelia Ward
Amy Bennett
Andrew France
Anna Dunlevey
Anna-Lise Ferguson
Arturo Layson
Bayron Villegas
Bonifacio Tamayao
Braille Bordon
Breanna Weastell
Briana Harvey-Bride
Brianna Kent
Brigid Malone
Chelsi Silva
Crystal Cruz
Daryl Lapa
Deidre Tait
Dylan Jones
Edgar Dela Cruz
Ellota Rodrigues
Elsie Nivo
Ericson Balba
Erwin Venzuela
Ghlory Caingcoy
Gloria Carlos
Godofredo Jr Pablo
Hannah Lammers
Janny Tautahi
Jennifer Manaois
Jerick Canada
Joanna Mendoza
Jobelle Ayban
Joffre Basallote
John Dones
Jojo Ponnola Thomas
Julian Bucay
Justin Lim
Kavita Nair
Keshav Thapa
Keyzie Torres
Kimberley Simmons
Kirsten Greenhalgh
Leah Razon
Louise Cook
Manu Joy
Marc Ytom
Maria Luciano
Matthew Baltazar
Maya Zullikar
Meg McCone
Amandeep Kang
Carla Coetzee
Francis Kunnathe
Janine Warren
Jennifer Northover
Jianwen Shi
Katy Grimshaw
Mariza Limas
Michael Consador
Midhun Jacob Kattamala
Monique Samson-Dayao
Nadine Wells
Natalia Potic
Nicky Blagrove
Pauline Coman
Piper Keast
Policarpo Jr. Viola
Rachel James
Relain Manrique
Renju Paul Mukkam
Samantha Starkey
Sanggetha Sengodan
Savnil Deo
Shayna Robinson
Smanu Koshy
Smriti Singh
Taiaroa Mahan
Tayla Chadwick
Taylor Stewart
Te Awanui Wihongi
Thanz Lachica
Ugyana Karmacharya
Victoria Lutton
Xiaorui Hu
Paul Susamma Robin
Rizalyn Gregorio
Sharron Shields
Smitha Alookaran
Kia ora koutou,
Welcome to the 29th Annual General Meeting of the New Zealand Sterile Sciences Association.
The past year has been one of significant progress and collaboration for the New Zealand Sterile Science Association (NZSSA).
C o l l a b o r a t i o n a n d S t a n d a r d s
On 11 June 2025, Shelagh Thomas met with Cathy Cummings from DAA to explore closer collaboration between NZSSA and DAA. Discussions focused on Quality Management Systems and the use of auditing and gap analysis tools. Following this, a reference group has been established to examine auditing requirements in Sterile Services. Shelagh and I will represent NZSSA on this group at its upcoming meeting.
A l l i e d H e a l t h E n g a g e m e n t
Aileen attended the Professional Leaders meeting with Jacqui Lunday, National Chief Allied Health, Scientific & Technical. At this meeting, Allied Health leaders across New Zealand were assigned portfolios aligned with Health NZ’s priorities.
Of particular importance, Hannah McCarrison, Chief Allied Health, Scientific and Technical Officer, has been given a portfolio focused on the Allied Health professions. She is keen to engage with us, learn about our work, and understand the support we require. She is also reviewing our Terms of Reference. Once available, Aileen will share information from this meeting with members.
C o m p u l s o r y R e g i s t r a t i o n
One of the most significant milestones for NZSSA has been official recognition by the Ministry of Health as the regulatory body for our members. This achievement is the result of Shelagh’s tireless work and years of negotiation and advocacy.
As the regulator, NZSSA now requires all qualified sterile technicians working in New Zealand to submit a professional portfolio for registration assessment. Portfolios are assessed on initial submission and must be resubmitted every three years thereafter
G o v e r n a n c e a n d C o n s t i t u t i o n
Aileen has played a key role in updating our Constitution and Rules to reflect our regulatory responsibilities. She also continues her outstanding work as editor of Supply Line and Donna. C
This year’s conference has been a true team effort, with John, Jenny, Charanjeet, Britta, and myself all closely involved in its organisation.
Across New Zealand, NZSSA has delivered a series of well-attended study days and STEAM meetings, providing valuable opportunities for professional development and networking.
• Christchurch STEAM Meeting – organised by Donna, Deputy President
• Palmerston North Study Day – organised by Shelagh and John
• Auckland Study Day – organised by Aileen and Charanjeet
• Waikato Study Day – organised by Jenny and Maureen
• Dunedin Study Day – organised by Kelly and Lisa
These events highlight the commitment of our members and the strength of our professional community.
C o m m u n i c a t i o n a n d P r o f i l e
We have continued to strengthen our communication channels and professional profile:
Expansion of our social media presence on LinkedIn and Facebook, helping us connect with a wider audience.
Development of a new NZSSA website, led by Antony and assisted by Alison, a major achievement that modernises our online presence.
by Charanjeet and Maureen Scott.
A c k n o w l e d g e m e n t s
The progress made over the past year reflects the dedication and teamwork of our Executive and members nationwide. We are proud of what has been achieved and confident in the continued growth and recognition of our profession.
Ngā mihi, Martin Bird
NZSSA President
The current NZSSA Executive Committee has just completed their first year since being ratified at the AGM in 2024, and what a year it has been. We have had eight executive committee meetings (mostly via Teams) since November 2024 to date. Some new executive members have been learning on the go with assistance from the more experienced executive members and great strides have been made. We had one resignation, and one new appointment to the executive after advertising and interviewing. Most of the past year’s highlights have been mentioned in the president’s report.
Membership has grown significantly this past year, and we would like to thank all our members for their ongoing support and dedication to the profession. As this year’s conference theme is GROWING A PROFESSIONAL PROFESSION, we would like to congratulate those members who have undertaken study in this past year and obtained their level 4 certificates and their level 5 diplomas. We would also like to acknowledge those who considered the level 3 course and would like to encourage you to enroll for level 4, as there are many professional benefits to doing so.
Continuing education and growth are very important and therefor regional education days have been held, a video competition has been introduced, conference is held, scholarships offered, and information made available on the Website, Supplyline and on professional and social media platforms like LinkedIn and Facebook. Please do access all the resources made available.
Thank you also to everyone who has taken the time to complete their professional portfolios and get registered this year as we strive toward becoming a self-regulated and professional profession. We hope that registration would also allow you to realise how much you have grown, learnt, how much you contribute, how valuable you are as technicians in the sterile sciences field, and how important the work is that you do as you reflect upon the quality improvement initiatives you have all been a part of in your places of work.
As the Executive committee members are all volunteering our time to run and guide the association and all work full-time, we ask for your patience in possible delays in replies and actions needed. We also ask and encourage your thoughts, feedback, ideas, suggestions and participation, so please do approach us or contact us as we move forward and grow together.
Now, go out and conquer. Carla.
The figures presented in this report are as at 31 March 2024 for the financial year 2024 –2025. The accounts were prepared by McIntyre Dick & Partners and audited by Accounting for Charities Trust.
The Association maintains a strong financial position which was boosted each year through a successful national conference. The Executive has managed the finances well enabling the Association to provide educational opportunities to its members.
The accounts operate on an accrual system in line with the Association reporting as a Tier 3 registered charity.
• T o t a l a c c u m u l a t e d f u n d s $ 2 4 8 , 9 1 2
• Assets $267,987
• Liabilities $ 20,007
Statement of Comprehensive Income (Profits and Losses)
• Revenue $ 227,518
• Expenses $ 184,927
• S
• Net cash flow from operating activities $ 50,183
• Net cash flow from other activities $ (7,429)
• Cash at end of the year (bank balance) $ 80,009
Deliverables
Education
• 3 x Supplyline journal publication
• 1 x Conference scholarship to WFHSS Santiago
• 4 x Conference scholarship to NZSSA Auckland
• 2 x NZ Diploma in Sterilising Technology
The audited performance report demonstrates an overall surplus of $42,591 This surplus was achieved through strong results from the national conference and wonderful support from industry through sponsorship The Association plans to put the money into more faceto-face education opportunities in the coming financial year.
The financial trend of the Association is holding in a good space. The Covid years are behind us, and the focus is on maintaining a solid financial position to continuously give back to members
Financial year 2021 – 2022
Financial year 2022 – 2023
Financial year 2023 – 2024
Financial year 2024 – 2025
Deficit $ 3,894
Surplus $ 9,379
Deficit $ (915)
Surplus $ 42,591
The Executive is focused on ensuring the members get value for their membership. To maintain low expenses for the operation of the Association, the executive conducts most of its business via online meetings. Two face-to-face executive meetings are held per year.
The executive would like suggestions from the membership on what they want from their Association. As a member of the Association your voice is important. Just email the Secretary or President with your thoughts.
The Association strategic plan for the financial year 2025 – 2026 is included after the financial summary.
Each year the Executive, as the management arm of the Association, allocates funds to scholarships and activities for members to promote educational opportunities and advocate for the profession.
Congratulations to the following for being awarded scholarships for 2024-2025:
• WFHSS Conference Scholarship – Luke Rabjohns
• NZSSA Conference Scholarship – Kathy King, Natalia Potic, Kirsten Greenhalgh and Christine Walker
• Education Scholarship – Chantelle Harper and Katherine Hije
There continues to be low uptake of the scholarships which cover all upfront costs for an event or education opportunity. These will continue to be promoted through the website, Facebook, LinkedIn and Supplyline and to students undertaking study.
Planning was undertaken for the 2025 calendar year to include delivery of regional meetings throughout the North and South Islands. Make sure to monitor the website and social media to avoid missing opportunities for ongoing education.
Auditor’s Report
• Qualified Opinion Report
o It is the opinion of the auditor that the performance report gives a true and fair view, in accordance with Public Benefit Entity Simple Format Reporting – Accrual (NotFor-Profit).
• Basis for Opinion
o This opinion is based on the belief that the audit evidence obtained was sufficient and appropriate. The audit was conducted in accordance with International Standards on Auditing (ISAs).
• Key Audit Matters
o Key audit matters are those of most significance in the audit of the performance report for the current period (1/4/2024 – 31/03/2025). Those matters were addressed in the context of the audit as a whole and informing the auditor's opinion and a separate opinion is not provided on these matters.
• Going Concern
o In the opinion of the Executive Committee the Association is a going concern for the foreseeable future.



Visit https://healthcare.steris.com/en-nz/nz or Email:
The strategic direction for the current financial year is presented in this document.
Providing education opportunities is a significant focus for the Association. This is also a direct way of giving back to the membership. Avenues of providing the education for the benefit of members and the wider industry are:
• hosting the annual conference and providing scholarships for conference attendance both nationally and internationally.
• Scholarships for formal education such as the NZ Diploma in Sterilising Technology
• Association journal, Supplyline, printed three times per year and delivered online
• Regional meetings as opportunities to network in person and learn from speakers on current topics
• Virtual leaders’ meetings via TEAMS supporting the leadership community to keep up with current affairs.
The executive plan of work focuses on putting processes in place to become formally self-regulated and build the Association’s reputation as the national membership body for people involved in reprocessing reusable medical devices.
This plan of work is intended to create a robust framework for the Association to;
• assess overseas qualifications against NZ qualifications to enable immigrating technicians and employers to have clarity for positive and safe employment practices,
• advocate on the behalf of members on appropriate reprocessing practices,
• build relationships with the Ministry of Health (MoH), Ministry of Business, Innovation and Employment (MBIE), and other key entities, and
• ensure members are receiving value as a member of the NZSSA
The following table presents the plan initiated in 2024 and continuing through to 2027.
Self-regulation
Shelagh (Aileen)
Develop the Association into a self-regulating body for sterile science technicians in NZ
Liaising with Ministry of Health Shelagh Aileen
Martin
Liaising with Allied Health NZ
Executive quality manual
Martin
Charanjeet
Shelley
Antony
Carla
A relationship with open communication enabling a lasting relationship
1/4ly meeting with CAPO for Health NZ
Potential for Certification of sterile sciences departments.
Advocate for Sterile Sciences as an allied health profession.
A quality manual for the Association forming a foundation for the current and future executives to work to.
Documents will be held in TEAMS
MoH recognise the Association as self-regulating.
The actions identified in this plan are required to develop a robust framework as a self-regulated body.
Working with Allied health self-regulating groups on a submission on the planned changes
Aileen & Shelagh putting in a NZSSA submission from the profession.
NZSSA requires all qualified technicians to be registered by 1 January 2026 as a start.
Connections have been built with MoH resulting in sterilising technicians being included on immigration green list.
Engagement underway for healthcare workforce development
Martin will begin to build a relationship with Allied Health NZ
Robust procedures under development.
Complaints process Aileen Jenny
A clear robust process accessible by members for making complaints
Review of Association constitution and rules Aileen Have a constitution and rules document that conforms with the Incorporated Societies Act 2022 and Charities Act 2005 (although is under review)
MBIE Immigration Green List requirements Alison, Aileen, Shelagh
Education plan Per names alongside meetings
Robust process for technician qualifications to be reviewed by the NZSSA for equivalency to NZ qualifications
Provide face to face education events for members of NZSSA to develop as professionals.
The process has been created and tested.
Will be finalised once new Website is live.
Incorporated Societies Act 2022 takes effect October 2023, Reregistration required between 5 October 2023 and 5 April 2026
Review completed and ratified by NZSSA membership. To be registered with Incorporated Societies.
Framework established for qualification review May 2023 with regular review. Applicants are being processed within 6 weeks of submission.
Micro credential for cultural and standards knowledge being investigated
2x Christchurch – Martin, Shelley, Donna
1x Dunedin – Martin, Shelley, Donna
1x Auckland – Aileen, Charanjeet, Antony
1x Wellington – Shelagh, John
1x Hamilton/Tauranga – Jenny, Maureen
1x Gisborne – Alison, Carla
1x Northland (?) – Antony, Steven Grant
3x Virtual leaders meetings – Martin, Carla
NZSSA Annual Conference Martin Charanjeet
John Donna Shelagh
NZSSA Website Antony, Alison
Representation by Executive at the WFHSS Congress
Tertiary providers Martin Jenny Aileen
Sterile Sciences
Certification - DAA Shelagh Martin
Delivery of a conference each year which prompts the industry and provides exposure to new and innovative ideas and products
Team for conference delivery reviewed each year.
Maintain a website that is easy to use and current.
A presence at the WFHSS maintains connection with the international market and means the scholarship recipient has support.
Usually the President attends. If they are unavailable, attendance is decided by executive vote.
Liaising with qualification providers to ensure qualifications meet industry need.
Establishment of certification of sterile sciences departments across all hospitals
2026 Conference – Christchurch, 23-25 September.
Upgrade of the current website is underway and plans to be delivered in 2025. A new more modern flexible platform is selected to reflect current user needs.
Nearing completion.
Each year the NZSSA will be represented at the WFHSS.
Information being sought in regard to OP delivering NZ3219 Level 4
Attend Local Advisory Committee with Toi Ohomai.
Oversight committee established and led by Martin.
Replacement Treasurer Martin Alison Find a replacement Treasurer before September 2027
Alison identifying hours required and review role description – February 13th meeting (TEAMS)
Advertising for the role will commence following confirmation of need.
Competitions Donna Quality improvement initiative Donna will create advertising for QI competition
Marketing Antony Shelley Carla Shelagh
Scholarships Antony Jenny Alison Carla
International Sterile Sciences Day (ISSD) April 10 annually – promotion of the day
NZSSA Poster
Promotion of the profession
Update scholarship documents for 2026
Receive Applications
Review applications and award scholarships
Shelagh will follow up on a contact re communications package for ISSD
February 13th meeting finalise for ISSD:
· costs for advertising on digital platforms, eg. Stuff
· radio talks
· posters / communications package
Alison update scholarship documents and load onto the website – applications close 30 April 2026.

23-25 SEPTEMBER 2026
bustling streets of Santiago to the engaging discussions contributed to an unforgettable experience.
Sterilisation Sciences (WFHSS) event expanded my professional growth. From the technology, every moment and hospitality of the Chilean provided a breathtaking backdrop conveniently located in the upmarket and learning among dances from across Chile and prevention. This celebration of both
Upon arriving in Santiago, I was immediately embraced people. The city, nestled against the stunning Andes filled with vibrant culture and rich history. The congress suburb of Vitacura, displayed excellent facilities that delegates.
Article by: Luke Rabjohns – NZSSA International Scholarship Recipient 2024 – WFHSS Santiago, Chile
Reflecting on my experience at the World Federation for Hospital Sterilisation Sciences (WFHSS) Congress 2024 in Santiago, Chile, I am reminded of how profoundly this event expanded my understanding of global sterilisation practices and enriched my professional growth. From the bustling streets of Santiago to the engaging discussions around sterilising technology, every moment contributed to an unforgettable experience.
First Impressions of Santiago
Upon arriving in Santiago, I was immediately embraced by the warmth and hospitality of the Chilean people. The city, nestled against the stunning Andes Mountains, provided a breathtaking backdrop filled with vibrant culture and rich history. The congress venue, conveniently located in the upmarket suburb of Vitacura, displayed excellent facilities that fostered interaction and learning among delegates.
The opening ceremony was particularly memorable, featuring cultural dances from across Chile and keynote speakers who are leaders in sterilisation and infection prevention. This celebration of both culture and science set a motivating tone for the days ahead.

Each particularly resonated
practices
with me was
in sterility assurance.
The breadth of topics covered at the WFHSS Congress was impressive. Each session was well-organised and featured expert panels, workshops, and poster presentations that delved deeply into essential aspects of hospital
innovative methods for validation and sterilisation processes, addressing faced in various healthcare
The opening ceremony was particularly memorable, keynote speakers who are leaders in sterilisation and culture and science set a motivating tone for the days
sterilisation practices. I was captivated as I explored the latest advancements and strategies in sterilisation sciences.
The breadth of topics covered at the WFHSS Congress session was well-organised and featured expert panels, poster presentations that delved deeply into essential sterilisation practices. I was captivated as I explored the advancements and strategies in sterilisation sciences.
One session that particularly resonated with me was a workshop on best practices in sterility assurance. Experts shared innovative methods for validation and monitoring in sterilisation processes, addressing common challenges faced in various healthcare settings. Learning about successful programmes and tools implemented in different countries inspired me to consider ways to enhance our practices back home. The discussions surrounding compliance with international standards were both enlightening and crucial for improving patient safety.

Equally impactful was a presentation focused on the integration of AI technologies in sterilisation. With rapid advancements in sterilisation equipment, hearing from practitioners who had successfully adopted these technologies was invaluable. The challenges of training staff and ensuring consistent quality were thoroughly examined, reinforcing the idea that technology must be supported by appropriate training and quality assurance protocols to be effective.
One session a workshop Experts monitoring common settings. tools to consider home. international crucial
Equally impactful was a presentation focused on the With rapid advancements in sterilisation equipment, adopted these technologies was invaluable. The challenges quality were thoroughly examined, reinforcing the idea appropriate training and quality assurance protocols
One of the most rewarding aspects of attending the congress was the opportunity to connect with a diverse network of professionals and exhibiting trades from around the globe. The collaboration of delegates at the congress saw the exchange of experiences, ideas, and sparked interesting discussions and new friendships.
music, and ventured out vibrant street restaurants offered enjoyed every and it did not of cold meats and cheese,

tried a Completo
alpaca in Peru, and indulged in Argentina’s famous Chorizo (pictured).
Beyond the congress venue, my time in Santiago was also marked by cultural exploration. The city’s rich tapestry of art, music, and culinary traditions added depth to my experience. I ventured out to explore nearby neighbourhoods, known for their vibrant street art and lively atmosphere. The local shops and restaurants offered an authentic taste of Chilean life, and I thoroughly enjoyed every moment.
Food is a significant part South American culture, and it did not
disappoint. I enjoyed traditional Chilean breakfasts of cold meats and cheese, tried a Completo Italiano (Chile’s take on the hot dog), dined on alpaca in Peru, and indulged in Argentina’s famous empanadas and perfectly grilled steak – Bife de Chorizo (pictured).
Cultural Exploration After the Congress
After the congress, I seized the opportunity to explore some of South America’s most iconic sites. My first stop ideas, and sparked interesting marked and out street offered every not meats and cheese, tried a Completo and indulged in Argentina’s famous (pictured). opportunity to explore some of South My first stop was Iguazu Falls a on the border of Argentina and Brazil.

stonework, admiring the breathtaking views and rich history were simply incredible. In Cusco, I immersed myself in the local culture, history, cuisine and connected with the friendly locals at the markets.
stonework, admiring the
history
seized the opportunity to explore some of South sites. My first stop was Iguazu Falls — a wonder on the border of Argentina and Brazil.
Buenos Aires, Argentina. The vibrant energy the historic architecture in San Telmo asado (traditional Argentine BBQ with experiences and lessons learned. I left with colleagues and friends incredible opportunity to attend the 2024
After America’s breathtaking Next, Peru, privilege Machu (pictured) historic Exploring ruins
breathtaking
were simply incredible. In Cusco,
myself in the
Concluding my journey, I explored the bustling streets of Buenos Aires, Argentina. The vibrant energy of the city, from the colourful neighbourhood of La Boca to the historic architecture in San Telmo, was captivating. I indulged in delicious Argentine cuisine, asado (traditional Argentine BBQ with chorizo sausage).
local culture, history, connected with the friendly locals at
As the congress ended, I found myself reflecting on the experiences and lessons learned. I left Santiago not only equipped with practical knowledge but with colleagues and friends from around the globe that I have stayed
Concluding

my journey, I explored the from the colourful neighbourhood was captivating. I indulged in delicious
I would like to thank the NZSSA for awarding me this incredible opportunity to attend the 2024 WFHSS
As the congress ended, I found myself not only equipped with practical connected the NZSSA for WFHSS Congress. I am forever grateful.



Innovation and Collaboration in
By Donna Dador, National Sterile Services Advisor (Southern Cross Healthcare) and NZSSA Vice President
Innovation and collaboration are at the heart of sterile services, and this year, the New Zealand Sterile Sciences Association (NZSSA) proudly celebrated both through its Quality Improvement Video Competition. Designed to spotlight creative solutions and best practices, the competition invited teams from across the country to share initiatives that enhance patient safety, streamline processes, and elevate professional standards.
Celebrating the Grand Winner: Bidwill Hospital
Bidwill Hospital took out the top honor with their project titled “No Shortcuts, Just Standards.” This entry stood out not only for its message but for its remarkable collaboration. The video featured contributions from SSD staff and their supportive theatre team, demonstrating that quality improvement is truly a team effort. Bidwill’s project focused on reinforcing adherence to

standards without compromise, ensuring that every step in the sterilization process meets the highest benchmarks. Their achievement resonated beyond the NZSSA community, earning recognition in their local newspaper for winning a national quality improvement award.
Christian Apacible receiving the award during the NZSSA conference held in Rotorua, New Zealand, presented by Antony Owens, NZSSA committee.
First Runner-Up: Grace Hospital’s Practical Innovation
Grace Hospital secured the first runner-up position with an initiative that combined simplicity and effectiveness. Their improvement involved creating a visual and listbased system in the sterile store and packing area featuring pictures of instrument sets alongside detailed lists of their contents. This approach makes it easier

her colleagues (Marvin,Daryl,Relain and Brigid) during the NZSSA Conference in Rotorua. Their presence on stage highlighted the team’s collaborative spirit and commitment to quality improvement.
The Quality Improvement Video Competition was more than a showcase of ideas, it was a celebration of the dedication sterile sciences professionals bring
to healthcare every day. These projects remind us that continuous improvement is not just a goal but a responsibility, and that innovation often begins with collaboration and creativity.
NZSSA is excited to announce that another competition will be launched next year to further promote our profession and drive ongoing improvement.

Watch this space! Your innovation could be the next to make headlines!
If you haven’t seen the videos yet, head over to our media platforms! LinkedIn, Facebook, and of course, our website nzssa.co.nz, and check them out now!
Martin Bird (President)
CSSD Manager
Dunedin Hospital
Dunedin
martin.bird@southendhb.govt.nz
Carla Coetzee (Secretary)
SSD
Health NZ Hawkes Bay
Hastings
Email: secretary@nzssa.co.nz
Aileen Derby
CSSD Manager Counties Manukau District
Auckland aileen.derby@tewhatuora.govt.nz
Charanjeet Kaur Sidhu
CSSD Quality Lead Clinical Counties Manukau District
Auckland Charanjeet.Sidhu@TeWhatuOra.govt.nz
Jenny Carston
Team Leader CSU
Tauranga Hospital Tauranga Jenny.Carston@bopdhb.govt.nz
Shelley Morrison
CSSD
University of Otago Faculty of Dentistry Dunedin 9054
shelley.vivian@otago.ac.nz
General Enquiries
Donna Belle Dador (Vice President)
National Sterile Services Adviser Southern Cross Healthcare Christchurch donna.dador@southerncrosshospitals.co.nz
Shelagh Thomas CSSD
Hutt Valley Capital, Coast and Hutt Valley shelagh.thomas@huttvalleydhb.org.nz
Antony Owens
Team Leader
Southern Cross Healthcare
Southern Cross Gillies Hospital Auckland antony.owens@schl.co.nz
John Barnacott
Manager Sterile Services Unit
Mid Central Palmerston North Hospital Palmerston North John.Barnacott@midcentraldhb.govt.nz
Alison Stewart (Treasurer)
NZSSA Treasurer
28 Brighton Street, Island Bay Wellington 6023 Email: accounts@nzssa.co.nz
secretary@nzssa.co.nz
Overseas qualification and membership inquiries accounts@nzssa..co.nz
Complaints complaints@nzssa.co.nz
Registration Assessors –Charanjeet Sidhu
Maureen Scott
Donna Dador
Shelagh Thomas
Charanjeet.Sidhu@TeWhatuOra.govt.nz Maureen.Scott@waikatodhb.health.nz donna.dador@southerncrosshospitals.co.nz shelagh.thomas@huttvalleydhb.org.nz