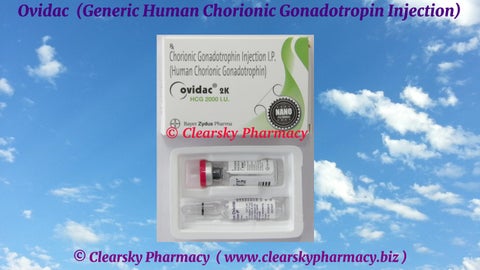
© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection)
Ovidac (Generic Human Chorionic Gonadotropin Injection) Ovidac Injection (Generic Human Chorionic Gonadotropin Injection) is used in females to help conception occur. It is usually given in combination with other medicines such as menotropins and urofollitropin. Many women being treated with these medicines usually have already tried clomiphene alone (e.g., Serophene) and have not been able to conceive yet. Human Chorionic Gonadotropin is also used in in vitro fertilization (IVF) programs. Chorionic gonadotropin is a medicine whose medical effects are almost the same as those of Luteinizing Hormone (LH), which is produced by the pituitary gland.
Clearsky Pharmacy
www.clearskypharmacy.biz

©
(
)
Ovidac

Chorionic
In males, LH and Human Chorionic Gonadotropin (Ovidac injection) stimulate the testes to produce male hormones such as Testosterone. Testosterone causes the enlargement of the penis and testes and the growth of pubic and underarm hair. It also increases the production of sperm. Although Human Chorionic Gonadotropin has been prescribed to help some patients lose weight, it should never be used this way. When used improperly, Human Chorionic Gonadotropin (Ovidac) can cause serious problems.
© Clearsky Pharmacy ( www.clearskypharmacy.biz )
(Generic Human
Gonadotropin Injection)


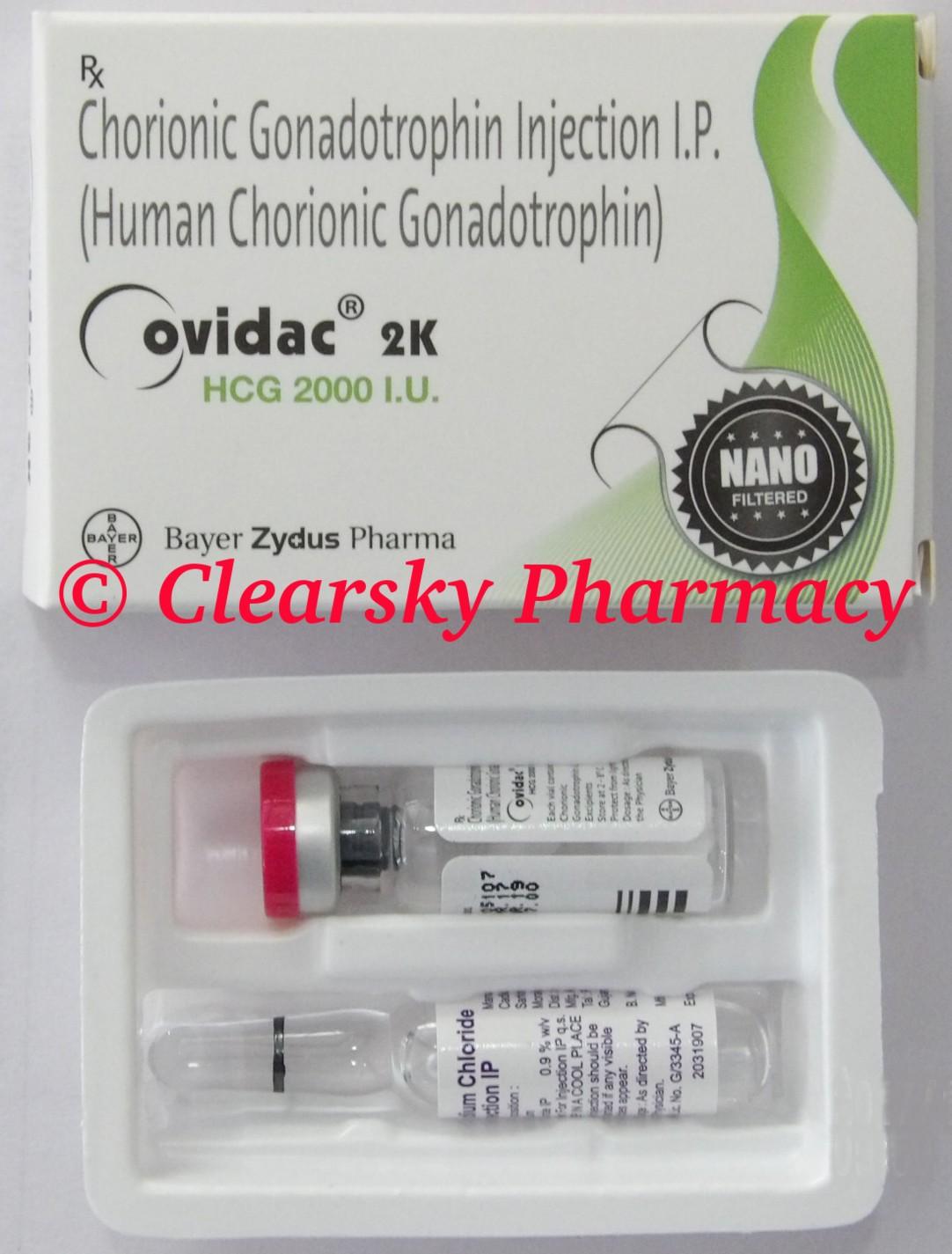
© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection) Active Ingredient The active ingredient present in Ovidac injection is Human Chorionic Gonadotropin. Each vial of Ovidac injection contains 2000 iu, 5000 iu or 10000 iu of purified Human Chorionic Gonadotropin.

© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection) Ovidac Injections are manufactured by Bayer Zydus Pharma, India in the strengths of 2000 iu, 5000 iu or 10000 iu of purified Human Chorionic Gonadotropin. (Website: https://www.bayerzyduspharma.com/en/ ) URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html

© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection) Human Chorionic Gonadotropin Injections – Brand Names Human Chorionic Gonadotropin Injections are available under various drug trade names that include Pregynl, Ovidac, Ovidrel, Novarel, Vitagon, Chorex, Corion and Choragon.
Ovidac (Generic Human Chorionic Gonadotropin Injection)

Uses of Ovidac Injections
Prepubertal cryptorchidism not due to anatomical obstruction. In general, Human Chorionic Gonadotropin is thought to induce testicular descent in situations when descent would have occurred at puberty. HCG thus may help predict whether or not orchiopexy will be needed in the future. Although, in some cases, descent following HCG administration is permanent, in most cases, the response is temporary. Therapy is usually instituted between the ages four and nine. Selected cases of hypogonadotropic hypogonadism (hypogonadism secondary to a pituitary deficiency) in males. Ovidac Injection (Generic Human Chorionic Gonadotropin Injection) is also used for induction of ovulation and pregnancy in the anovulatory, infertile woman in whom the cause of anovulation is secondary and not due to primary ovarian failure, and who has been appropriately pretreated with human menotropins.
URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html
© Clearsky Pharmacy ( www.clearskypharmacy.biz )
Ovidac (Generic Human Chorionic Gonadotropin Injection)
Ovidac Injection Dosage
The dosage of Ovidac Injection (Generic Human Chorionic Gonadotropin Injection) is given below: In the Female: Sterility due to the absence of follicle-ripening or ovulation. 5,000 IU to 10,000 IU hCG to induce ovulation, following treatment with an FSH (Follicle Stimulating Hormone) or HMG (Human Menopausal Gonadotrophins) preparation. In combination with FSH (Follicle Stimulating Hormone) or HMG (Human Menopausal Gonadotrophin), promotion of controlled superovulation in medically assisted reproduction programmes. 5,000 IU to 10,000 IU hCG 30 to 40 hours after the last FSH or HMG injection. Ovidac injection should not be administered if the following criteria have not been met: at least 3 follicles greater than 17 mm in diameter are present with 17ß estradiol levels of at least 3500 pmol/L (920 picogram/ml). Oocyte collection is carried out 32 hours to 36 hours after the hCG injection has been administered.As luteal phase support, two to three injections of 1,000 to 3,000 IU hCG each may be given within nine days of ovulation or embryo transfer, for example on day 3, 6 and 9 after ovulation induction or embryo transfer.
URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html
Clearsky Pharmacy ( www.clearskypharmacy.biz

©
)
Ovidac
Delayed
IU hCG

weekly
least
Sterility in selected cases of
Usually, 3,000 IU hCG per week in combination with an FSH or HMG preparation. This treatment must be continued for at least three months before any improvement in spermatogenesis can be expected. During this treatment
replacement therapy must be suspended. Once achieved, the improvement can sometimes be maintained by hCG alone.
© Clearsky Pharmacy ( www.clearskypharmacy.biz )
(Generic Human Chorionic Gonadotropin Injection) Ovidac Injection Dosage The dosage of Ovidac Injection (Generic Human Chorionic Gonadotropin Injection) is given below: In The Male: Hypogonadotrophic Hypogonadism 5,000 IU to 10,000 IU hCG 2-3 times weekly.
puberty associated with insufficient gonadotrophic pituitary function. 1,500
twice
for at
6 months.
deficient spermatogenesis.
testosterone
URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html

© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection) Contraindications Ovidac Injections are contraindicated in persons with a hypersensitivity (allergy) to Human Chorionic Gonadotropin or any of the other ingredients of this medicine. The other contraindications include Presence of uncontrolled nongonadal endocrinopathies (e.g. thyroid, adrenal or pituitary disorders), Breast, uterine, ovarian tumours, Vaginal bleeding of unknown cause, Known or suspected androgen-dependent tumours such as testicular tumours, carcinoma of the prostate or mammary carcinoma in males, Malformations of the sexual organs incompatible with pregnancy and Fibroid tumours of the uterus incompatible with pregnancy. URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html

© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection) Storage Instructions Ovidac Injection (Generic Human Chorionic Gonadotropin Injection) have to be stored at controlled room temperature i.e. from 20°C to 25°C (68°F to 77°F) with excursions permitted between 15° to 30°C (59° to 86°F). Keep this as well as all other medicines away from children and pets. URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html
Ovidac (Generic Human Chorionic Gonadotropin Injection)
Warnings
Ovidac injection should be used in conjunction with human menopausal gonadotropins only by physicians experienced with infertility problems who are familiar with the criteria for patient selection, contraindications, warnings, precautions, and adverse reactions. The principal serious adverse reactions are: (1) Ovarian hyperstimulation, a syndrome of sudden ovarian enlargement, ascites with or without pain and/or pleural effusion, (2) Rupture of ovarian cysts with resultant hemoperitoneum, (3) Multiple births and (4) Arterial thromboembolism. Anaphylaxis and other hypersensitivity reactions have been reported with urinary-derived hCG products. Since androgens may cause fluid retention, Human Chorionic Gonadotropin should be used with caution in patients with cardiac or renal disease, epilepsy, migraine, or asthma.

© Clearsky Pharmacy ( www.clearskypharmacy.biz )

© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection) Side Effects Ovidac (Human Chorionic Gonadotropin Injection) Side effects include Headache, irritability, restlessness, depression, fatigue, edema, precocious puberty, gynecomastia, pain at the site of injection. Hypersensitivity reactions, both localized and systemic in nature, have also been reported. URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html

© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection) Overdose The toxicity of Human Chorionic Gonadotrophic hormone is very low. However, a very high dose of Ovidac may lead to hyperstimulation of the ovaries. URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html
Chorionic
pregnancy.
has occurred.

© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection) Ovidac Injections During Pregnancy Human Chorionic Gonadotropin is classified by the US FDA as Pregnancy Category X. Defects of forelimbs and central nervous system and alterations in sex ratio have been reported in mice receiving combined gonadotropin and chorionic gonadotropin therapy in dosages to induce superovulation. Alterations in sex ratio has been reported in animal studies on combined gonadotropin and chorionic gonadotropin treatment in dosages to induce superovulation. There are no controlled data in human pregnancy. Human
Gonadotropin (HCG) is considered contraindicated during
This medicine must not be administered after conception
URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html


© Clearsky Pharmacy ( www.clearskypharmacy.biz ) Ovidac (Generic Human Chorionic Gonadotropin Injection) For more details on Ovidac Injections click here Ovidac Injection (Generic Human Chorionic Gonadotropin Injection) by Bayer Zydus Pharma, India. URL: https://www.clearskypharmacy.biz/ovidac-human-chorionic-gonadotropin-injection.html