Clinicalapplicationsofstemcell-derivedexosomes
FeiTan
1,2,3,4 ✉,XuranLi1,2,ZhaoWang1,JiaojiaoLi1,2,KhawarShahzad1,2 andJialinZheng 5,6

Althoughstemcell-basedtherapyhasdemonstratedconsiderablepotentialtomanagecertaindiseasesmoresuccessfullythan conventionalsurgery,itneverthelesscomeswithinescapabledrawbacksthatmightlimititsclinicaltranslation.Comparedtostem cells,stemcell-derivedexosomespossessnumerousadvantages,suchasnon-immunogenicity,non-infusiontoxicity,easyaccess, effortlesspreservation,andfreedomfromtumorigenicpotentialandethicalissues.Exosomescaninheritsimilartherapeuticeffects fromtheirparentalcellssuchasembryonicstemcellsandadultstemcellsthroughverticaldeliveryoftheirpluripotencyor multipotency.Afterathoroughsearchandmeticulousdissectionofrelevantliteraturefromthelast fiveyears,wepresentthis comprehensive,up-to-date,specialty-specificanddisease-orientedreviewtohighlightthesurgicalapplicationandpotentialof stemcell-derivedexosomes.Exosomesderivedfromstemcells(e.g.,embryonic,inducedpluripotent,hematopoietic,mesenchymal, neural,andendothelialstemcells)arecapableoftreatingnumerousdiseasesencounteredinorthopedicsurgery,neurosurgery, plasticsurgery,generalsurgery,cardiothoracicsurgery,urology,headandnecksurgery,ophthalmology,andobstetricsand gynecology.Thediversetherapeuticeffectsofstemcells-derivedexosomesareahierarchicaltranslationthroughtissue-specific responses,andcell-specificmolecularsignalingpathways.Inthisreview,wehighlightstemcell-derivedexosomesasaviableand potentalternativetostemcell-basedtherapyinmanagingvarioussurgicalconditions.Werecommendthatfutureresearch combineswisdomsfromsurgeons,nanomedicinepractitioners,andstemcellresearchersinthisrelevantandintriguingresearch area.
SignalTransductionandTargetedTherapy (2024)9:17 ;https://doi.org/10.1038/s41392-023-01704-0
INTRODUCTION
Stemcellsareapopulationofundifferentiatedcellswithunique abilitiestoself-renewandrecreatefunctionaltissues.Theyare primarilyclassifiedbytheirdifferentiationpotential,originand lineageprogression.Accordingtotheirpotency,stemcellscanbe totipotent,pluripotent,multipotent,oligopotentandunipotent.1 Stemcellsexistbothinembryosandadultcells.Embryonicstem cells(ESCs)andinducedpluripotentstemcells(iPSCs)arebest examplesofpluripotentstemcells,2 whereasadultmultipotent stemcellsareexemplifiedbyhematopoieticstemcells(HSCs),3 mesenchymalstemcells(MSCs),4 neuralstemcells(NSCs),5 and endothelialstem/progenitorcells(EPCs)6 (Fig. 1a).Allthese subtypesofstemcellshavebeenextensivelytrialedforthe treatmentofhumandiseases.
Stemcell-basedtherapy,asamodalityofregenerative medicine,hasgeneratedtremendousattention,asitoffers newoptionsforpatientssufferingfrompreviouslyincurable diseases.Subsequently,thousa ndsofrelatedclinicaltrialshave beenregistered,coveringawidespectrumofmedical problems,suchasmusculoskeletalandneurologicaldisorders, immunediseases,hematologicaldysfunctions,anddegenerativeconditions. 7 However,sometrialshavefailedtoshowany bene fi tintheclinic.Thisislikelyduetotheinevitable limitationsofstemcelltherap y,suchasinfusiontoxicity, immunogenicity,tumorigenicpo tentialsandethicalissues. 8 Exosome,secretedbyalmostallcelltypesincludingstemcells
(Fig. 1 a),hasbeenpositedasasaferandmoreversatile alternativetostemcelltherapy. 9
Exosomesarenanoscale,spherical,andlipidbi-layeredsingle membraneextracellularvesicles,whichactasintercellular messengers.10 Exosomeshavebeenregardedasminiature versionsoftheirparentalcells,partiallybecauseexosomesfrom acertaincelltypeprovidecell-specificoruniquesetsof biomolecules.Inaddition,thestemcellshavebeenfoundto functioninaparacrinefashionthroughtheirsolublesecretome includingexosomes.11 Inotherwords,stemcell-derivedexosomes (SC-Exo)inheritsimilartherapeuticeffectsfromtheirparentalcell oforigin,e.g.,anti-inflammation,immunomodulationandtissue regeneration.12 Collectively,stemcell-derivedexosomesarea potentsurrogateforstemcelltherapywithoutexhibitingthe disadvantagestheircellularcounterpartspresent13 (Table 1).
Priortoclinicalapplications,exosomesmustbepreparedand optimizedintermsofproduction,purification,andmodification (Sections2.3and2.4).Awiderangeofmedicalreviewsanalyzing theseupstreammeasuresofexosometherapyhavebeen publishedinrecentyears.Nevertheless,someresearchavenues remainunder-investigated:inparticular,systematicinvestigation dedicatedtodownstreamclinicalapplicationsislacking,especially fromasurgicalperspective.Tissuesthathavebeendamaged, whetherbydiseaseorasurgeon’sscalpel,respondbyinflammatoryandregenerativedynamics,14 makingsurgeryaperfectarena forstemcell-derivedexosometherapy.15 Stemcell-derived
1DepartmentofORL-HNS,ShanghaiFourthPeople’sHospital,andSchoolofMedicine,TongjiUniversity,Shanghai,China; 2PlasmaMedicineandSurgicalImplantsCenter,Tongji University,Shanghai,China; 3TheRoyalCollegeofSurgeonsinIreland,Dublin,Ireland; 4TheRoyalCollegeofSurgeonsofEngland,London,UK; 5CenterforTranslational NeurodegenerationandRegenerativeTherapy,TongjiHospitalaffiliatedtoTongjiUniversitySchoolofMedicine,Shanghai,Chinaand 6ShanghaiFrontiersScienceCenterof NanocatalyticMedicine,TongjiUniversity,Shanghai,China Correspondence:FeiTan(iatrologist@163.com)
Received:23July2023Revised:15October2023Accepted:12November2023
© TheAuthor(s)2023

Fig.1 Illustrationoftheupstreammeasuresofexosometherapy(figuregeneratedusingAutodesk3dsMax2023). a productionand purificationofexosomes(MSCsandNSCsareusedasexamplesformultipotentstemcells). b contentofnaturalexosomes. c modificationof exosomes.(BMbonemarrow,DCdendriticcell,IACimmunoaffinitychromatography,iPSCinducedpluripotentstemcell,MHCmajor histocompatibilitycomplex,miRNAmicroRNA,MSCmesenchymalstemcell,MVBmultivesicularbody,NSCneuralstemcell,SECsize-exclusion chromatography,UCumbilicalcord)
Table1. Thecomparisonbetweenstemcelltherapyandstemcell-derivedexosometherapy
TreatmentmodalityAdvantagesLimitations
Stemcelltherapymultilineagedifferentiationpotentialshort-livedviabilityandlowengraftmentafter injection applicabletothetreatmentforawiderangeofdiseasesstringentstorageandtransportrequirements extensiveaccumulationoflaboratoryandclinicaldatatumorigenicpotential easytoisolateandpossibleformass-productioninfusiontoxicity well-developedregulatoryguidelinesimmunogenicity ethicalissues
Stemcell-derivedexosome therapy comparabletherapeuticeffectstostemcellsbutmuch smaller batch-to-batchinconsistency moreconcentratedfunctionalcargos,e.g.,cytokinesnostandardizedprotocolforpurificationandstorage modifiableatitssurfaceandinitscargosrelativelylowyieldforlargescalemanufacturing versatiledeliverymodalitiesnoindustry-standardqualityspecifications stableforlong-termstorageandtransportinsufficientregulatorycontrol negligibleriskoftumorigenesisandimmuneresponse lackofethicalissues
exosomesinheritsimilartherapeuticeffectsfromtheirparental celloforigin,e.g.,tissueregeneration,anti-inflammationand immunomodulation.12,16–18
Inthiswork,wewilldissectrelevantpublicationsfromthelast fiveyearsinordertopresentacomprehensive,up-to-date, specialty-specificanddisease-orientedreview(Fig. 2).Ouraimis tobridgethegapthatcurrentlyexistsbetweensurgeons, nanomedicinepractitioners,andstemcellresearchers.
GENERALBACKGROUNDOFEXOSOMESANDEXOSOME THERAPY
Biogenesis,composition,anduptakeofexosomes
Exosomesdifferfromothertypesofprimaryextracellularvesicles (e.g.,apoptoticbodiesandmicrovesicles)intermsofsize,content, andproductionmechanism.19 Themostpopularlyaccepted mechanismofexosomeformation,i.e.,anendosomalroute,isas follows(Fig. 1a).Theinitialendosomesareproducedbycell membraneinvaginationduringwhichthebioactivesubstances begintoaccumulatewithintheearlysortingendosomes.Thelate sortingendosomesthenformmultivesicularbodies(MVBs)aftera secondindentation.Finally,theMVBsfusewiththecell membrane,releasingthecarriedexosomestotheoutside.Nonendosomalrouteofexosomebiogenesis,suchasplasma membranebudding,hasalsobeenreported.20
Asthethreemajorexosomedatabases(i.e.,ExoCarta,Vesiclepedia,andEVpedia)summarize,exosomescontainnumerous molecules,includingproteins,glycoconjugates,lipids,nucleic acids,metabolites,andotherbioactivesubstances(Fig. 1b).The examplesofeachcategoryandthecorrespondingfunctionshave beenthoroughlyreviewedelsewhere.21,22 Ontheonehand, exosomescompriseacomplexproteinnetworkincludingexternal proteins(e.g.,tetraspanins,antigen-presentingcomplexes,and adhesionmolecules)andinternalproteins(e.g.,heatshock proteins,ESCRTmachinery,cytokinesandchemokines,and membranetransporters).23 Ontheotherhand,asthemost abundantinhumanexosomalnucleicacids,microRNA(mRNA) couldparticipateinhematopoiesis,exocytosis,andnerveand vascularregenerationthroughexosome-mediatedcellular communication.24
Therearevariousuptakemechanismsonceexosomesreachthe recipientcell,allofwhichcanbecategorizedintomembrane fusion,receptorinteraction,andinternalization21 (Fig. 1b).Finally, theexosomalcargosarereleasedintothecytoplasm,theprocess ofwhichdependsonthesourceoftheexosome,natureofthe
cargo,andthemetabolicstateoftherecipientcell.25 Theentire lifecyclefromexosomebiogenesistouptakeandintracellular signalingcanbetrackedusing fluorescent,luminescent,and radioactivetechniques.26 27
Sourceandclassificationofexosomes
Dependingonwhetherexosomeshavebeenartificiallymodified, theyarebroadlyclassifiedintonaturalexosomesandengineered exosomes(Section2.4).Dependingonthespeciesoforigin, exosomesaredividedintoanimal-derivedandplant-derived exosomes.Currently,exosomesaremainlyclassifiedaccordingto thetypeoftheirparentalcells.Almostalltypesofhumancellscan produceexosomes.Theseinclude,butarenotlimitedto, macrophages,dendriticcells(DCs),platelets,stemcells,andeven tumorcells28 (Fig. 1a).
Forexample,macrophage-derivedexosomescontributeto diseaseprogression(e.g.,diabetes,atherosclerosisandheart failure)29 anddiseasetreatment(e.g.,cutaneouswound,inflammatoryboweldisease,andfungalandviralinfection).30 However, theyseemtoplayparadoxicalrolesinsuppressingandpromoting tumors.31 LikeDCs,DC-derivedexosomes(Dex)couldalsointeract withimmunecells(e.g.,Tcells,Bcells,andNKcells)throughtheir surfaceproteinssuchasmajorhistocompatibilitycomplexes (MHCs).32 Somepreclinicalandclinicaltrialshavedemonstrated theeffectivenessandsafetyofDex-basedimmunotherapyfor cancers.33 Furthermore,tumor-derivedexosomes(Tex)notonly areinvolvedduringtumorproliferation,invasion,metastasis,and immunitybutalsocanbeusedasbiomarkersforcancerdiagnosis andtreatment.34 Lately,Texhasbeenusedasananti-tumordrug andanantigenpresenterforDCvaccination,servingasa promisingcell-freecancerimmunotherapy.35 Finally,theclinical applicationsofstemcell-derivedexosomeswillbediscussedin detailinthefollowingsections.
Exosomescanbefoundinallbody fluidssuchasblood,saliva, urine,plasma,tears,semen,amniotic fluid,andevenbreastmilk.36 Body fluid-derivedexosomesareahighlystablereservoirof diseasebiomarkers,assistingliquidbiopsyinvariousclinical settingssuchascancers,cardiovasculardiseases,andperinatal disorders.37 38 However,thecoexistingcontentsandavailabilityof eachtypeofbody fluidmightcreatechallengestoexosome isolation.
Production,isolationandpurificationofexosomes
Oneofthemajorobstaclespreventingexosome-basedtherapeuticsfromenteringclinicalpracticeisthelowyieldandefficiencyof

Fig.2 Illustrationofthedownstreamsurgicalapplicationsofexosometherapy(figuregeneratedusingAdobePhotoshop2023andAdobe Illustrator2023).Thetherapeuticeffectsofexosomesareahierarchicaltranslationthroughdisease-specifictissueresponses,tissue-specific cellularalterations,andcell-specificmolecularsignalingpathways
exosomes.Forexample,onlylessthan1 μgexosomalprotein couldbeharvestedfrom1mlculturemediuminalaboratory setting.39 Therearevariousmethodsofupscalingexosome production,whicharecategorizedintobiochemicalstrategies (e.g.,LPS,BMP-2,HIF-1α,andIFN-γ andTNF-α),physicalstrategies (hypoxia,thermalstress,andstarvation),mechanicalstrategies (shearstressand3Dculturing)andinstrumentalstrategies (hollow-fiberbioreactorsandstirredtankbioreactors).40
Exosomesareheterogeneousintermsofsize,content,surface markers,andsource,whichmakestheirisolationdifficult.The currentlyavailabletechniquesforexosomeisolationandpurificationarebasedontheirsize,surfacecharge,orimmunoaffinity26
(Fig. 1a).However,thereisno ‘one-fits-all’ approachasthese techniquesallhaveadvantagesanddisadvantages.
Forexample,ultracentrifugationisdeemedthegoldstandard forexosomeextraction.Althoughitrequiresminimalreagentsand expertize,thetimeconsumption,highcost,lowefficiency,and lipoproteinco-separationhavelimiteditslarge-scaleuse.41 Immunoaffinitychromatographyisaseparationtechnologybased onthespecificbindingofantibodiesandligands.Itisrapidand provideshighpurity,specificity,andyield.However,theantigen/ proteincouplingusedneedstobeexpressedonthesurfaceof exosomes.19 Size-basedisolationtechniquesmainlyreferto ultrafiltrationandsize-exclusionchromatography,bothofwhich
arequickandsuitableforlarge-scaleapplications.Butpore clogging,exosomeloss,andlowpurityaremakingthismethod difficulttopopularize.42 Althoughnosingletechniqueisperfect, combiningtheabovetechniqueswithothers(e.g.,precipitationbasedandmicrofluidics-based)mightbeasolutiontosimultaneouslymeetmultiplerequirementsforexosomeisolationand purification.
Modificationofexosomes
Exosomescanbebiochemicallymodifiedtobroaden,change,or improvetheirtherapeuticeffects.Themodificationofexosomesis classifiedintointernalstrategies(e.g.,drugloading)andexternal strategies(e.g.,surfacemodification).Ontheonehand,exosomes maybeanidealtherapeuticcarriertodeliverdrugs,nucleicacids, andvaccinesduetotheiradvantagesinstability,non-immunogenicity,andtargetingrecipientcells.43 Therearevariouscargo loadingtechniquesincludingpre-productionloadingmethods (e.g.,transfection,co-incubation,andelectroporation)andpostproductionloadingmethods(e.g.,freeze-thawcycles,incubation, sonication,extrusion,andhypotonicdialysis)dependingon whethertheyareappliedbeforeorafterexosomebiogenesis10,26,44–46 (Fig. 1c).Forexample,Tianetal.loadeddoxorubicin inDexusingelectroporationforthetreatmentofbreastcancer.47 Kimetal.loadedpaclitaxelinRAW264.7-derivedexosomesusing incubationandsonicationtoovercomemultidrugresistancein cancercells.48 Ohnoetal.loadedantitumorlet-7amiRNAin HEK293-derivedexosomesusingtransfectiontomanagebreast cancer.49
Ontheotherhand,surfacemodificationofexosomesis exemplifiedbygeneticengineeringofexosomalmembraneor parentalcells,chemicalconnectionoftargetingligands,electrostaticinteraction,andmagneticnanoparticletechnology.10 The mainpurposeofsurfacemodificationistoselectivelydeliver exosomestotargetcellsforprecisetreatment.Forexample, Alvarez-Ervitietal.modifiedDCsusinggeneticengineeringto expressLamp2bandRVGpeptides,therebytargetingthecentral nervoussystem(CNS).50 Zhuetal.insertedtumor-targeting peptides,c(RGDyK),intotheexosomesurfaceusingachemical reactiontotargetglioblastoma.51 Nakaseetal.boundexosomes withacomplexformedbypH-sensitivefusionpeptideand cationiclipidusingelectrostaticinteraction,therebyachieving enhancedcytosolicdelivery.52
Characterizationandverificationofexosomes Exosomesneedtoundergocharacterizationandverification beforetherapeuticapplications.Currentmethodsusedfor exosomecharacterizationmainlyfocusonthesize,morphology, andcargoprofileofexosomes.43 Size-orientedverification includesnanoparticletrackinganalysis(NTA),dynamiclight scattering(DLS),andtunableresistivepulsesensing(TRPS), whereasmorphology-orientedanalysisincludesscanningelectron microscopy(SEM)andtransmissionelectronmicroscopy(TEM).19 Inaddition,cargoprofilingisfurthersubdividedintoproteomic, lipidomic,andgenomicanalysesincludingwesternblotting,ELISA, flowcytometry,massspectroscopy,andPCR.36 Sinceeachofthe abovecharacterizationmethodshasadvantagesanddisadvantages,itisauniversalpracticetocombineanalysesfromthree differentaspects,e.g.,apackageofTEM,NTA,andwestern blotting,toidentifyisolatedexosomes.
Storageofexosomes
Thecurrentlyusedpreservationmethodsforlong-termstorageof exosomesmainlyincludecryopreservation,lyophilization,and spray-drying.10 Temperatureandantifreezearethetwomost importantingredientsforcryopreservation.Storageat4°Cmight weakenthebiologicalactivityandreducetheproteincargoof exosomes,whereas 80°Cisconsideredtheoptimaltemperature causingtheleastimpactonexosomemorphologyandcontent.57,58 Non-permeabledisaccharideantifreeze,especiallytrehalose,representsthebestchoiceasitpreventsexosome aggregationandcryodamage.59 Heat-sensitivematerials,e.g., exosomesandvaccines,treatedbylyophilizationoffreezedryingcanbeeasilystoredandreconstitutedbysimplyadding water.ArecentstudyshowedthatlyophilizationwithcryoprotectantcouldretaintheactivityofexosomalproteinsandRNAfor approximately4weeksevenwhenstoredatroomtemperature.60 Finally,incontrasttofreeze-drying,spray-dryingisasingle-step process,therebyreducingtheneedforexpensiveequipmentand lengthymulti-stepmilling.However,coreparametersofspraydryingsuchasexosomefeedingrate,atomizationpressure,and outlettemperature,canallaffectexosomestabilityandcargo integrity.61
ORTHOPEDICANDTRAUMASURGERYANDSC-EXOTHERAPY Fracture
Fracturesarethemostcommontraumaticlarge-organinjuries, andapproximately10%healimproperly.62 Fracturehealing involvesananabolictissue-bulkingphaseandacatabolictissueremodelingphase,whicharecontrolledbyvariousfactorssuchas stemcells,innateandadaptiveimmunefunctions,andstability.63 Biopharmacologicaltreatmentforfracturescanbegivenlocally (e.g.,bonemorphogeneticprotein,BMP)orsystemically(e.g., parathyroidhormone,PTH).Asapromisingalternative,exosome therapyforfracturehealingmostlyutilizesbonemarrow-derived MSCsasacellularsupplier(Table 2).
ThepresumedmechanismofhowMSC-derivedexosomes promotefracturehealingisasfollows.Firstly,theprogressionof bonerepairneedsavarietyofcells,e.g.,inflammatorycellsinthe inflammationstage,endothelialandmesenchymalprogenitor cellsinthe fibrovascularstage,osteoblastsandchondrocytes duringboneformation,andosteoclastsduringcallusremodeling.62 Secondly,mostofthesecellscanuptakeexosomes, especiallyosteoblastsandvascularendothelialcells,64 whichare mostrelatedtofracturehealing.Lastly,uponexosomeabsorption, thegeneexpressionoftherecipientcellsismodified,thereby activatingvarioussignalingpathways(Fig. 3a),causingvarious cellularandtissueresponses(Fig. 3b)andultimatelyleadingto improvedfracturehealing.
Earlyresearchhasemployedvariousanimalmodelsoffracture healing.Inatransversefemoralshaftfracturemodel,exosomes werefoundtonotonlypromoteosteogenesisinwild-typemice, butalsorescueretardationoffracturehealinginCD9 / mice,a strainknowntohavealowerboneunionrate.65 Inafemoral nonunionmodel,exosomesenhancedfracturehealingby promotingosteogenesisandangiogenesispossiblyviatheBMP2/Smad1/RUNX2pathway.66 Inatibialdistractionosteogenesis model,exosomessecretedbyyoungMSCspromotedosteogenic capacityofolderMSCsandenhancednewboneformationin olderrats.67 Inaddition,EPC-derivedexosomesacceleratedbone regenerationduringdistractionosteogenesisbystimulating angiogenesis.68
Forexample,microscopy-basedmethods,suchasSEMand TEM,candirectlyvisualizethesurfacetopographyandinternal structure,respectively.However,TEMisnotsuitableforquick measurementofalargenumberofsamplesduetocomplicated operationandtedioussamplepreparation. 53 NTAfacilitatesfast detectionandreal-timeexosomeobservationwhilehavinga higherresolutionthan fl owcytometry.Themaindisadvantage ofNTAisitsdif fi cultyindistinguishingexosomesfrom contaminatedproteins. 54 Asamaturetechnique,western blottingcanqualitativelyandquantitativelydetecttheexpressionofexosomalproteinbiomark ers,especiallyexosomesfrom cellculturemedia.However,itistime-consumingandnot suitableforthedetectionofexosomesfrombiological fl uids. 55 , 56
Table2. Stemcell-derivedexosomesforthetreatmentofdiseasesinorthopedicsurgeryandrelatedspecialties
TargetdiseaseExosomeInvitromodel& fi ndingsInvivomodel& fi ndingsRefs.
65
micemodel;rescued retardationoffracturehealinginCD9/mice;promotedbonehealing inwild-typemice
FractureBM-MSC-exoN/Afemoralfracturemodel,wildtypeandCD9/ -
66
BM-MSC-exoHUVECs,MC3T3-E1cells;improvedproliferation&migrationratmodeloffemoralnonunion;enhancedosteogenesisand angiogenesisviaBMP-2/Smad1/RUNX2pathway
68
67 EPC-exoHUVECs;enhancedproliferation,migrationviamiR-126distractionosteogenesisratmodel;acceleratedboneregeneration withbettermechanicalpropertiesintibiaswithhighervascular density
70
71
youngBM-MSC-exoolderBM-MSCs;enhancedproliferation&osteogenic differentiation distractionosteogenesisratmodel;acceleratedboneregeneration withbettermechanicalpropertiesintibias
BM-MSC-exoMC3T3-E1cells;promotedproliferation&differentiationmice;exosomalmiR-136-5ppromotedfracturehealingbytargeting LRP4toactivateWnt/ β -cateninpathway
72
mice;exosomalmiR-25regulatedubiquitinationanddegradationof Runx2bySMURF1topromotefracturehealing
BM-MSC-exoMC3T3-E1cells;acceleratedosteogenicdifferentiation, proliferation,andmigration
obesity-inducedfracturemousemodel;exosomallncRNAH19 improvedfracturehealingviamiR-467/HoxA10axis
BM-MSC-exoBM-MSCs;high-fatdietinhibitedexosecretion&osteogenic markers
73
CBS-heterozygousmice;exosomallncRNA-H19absorbedmiR-106and restoredboneformationandmechanicalquality
BM-MSC-exoHUVECs,BM-MSCs;promotedangiogenesis&osteogenesis viaangiopoietin-1/Tie2-NOpathway
75
DMOG-stimulatedBM-MSC- exo HUVECs;promotedproliferationandtubeformationcalvarialdefectratmodel;improvedboneregenerationand neovascularizationbyactivatingAkt/mTORpathway
76
mice;miR-29a-loadedexopromotedangiogenesisandosteogenesis byincreasingtrabecularbonemass
BM-MSC-exoHUVECs;exosomalmiR-29apromotedproliferation, migration,andtubeformationbyvasohibin-1
118
UC-MSC-exoHUVECs;hypoxiaenhancedexoproductionviaHIF-1 α ; improvedproliferation&tubeformation femoralfracturemousemodel;hypoxicexopromotedfracturehealing bytransferringmiR-126toagreaterextentthannormoxicexo
N/A
Osteoporosisadipose-MSC-exoMLO-Y4cells;reducedhypoxia/serumdeprivation-induced osteocyteapoptosisandosteocyte-mediated osteoclastogenesis
119
UC-MSC-exoBM-MSCs;inhibitedapoptosisHLU-induceddisuseosteoporosisratmodel;actedviamiR-1263/ Mob1/Hipposignalingpathway
120
UC-MSC-exoosteoblasts;promotedcellproliferationandosteogenic differentiation estrogen-de fi cientosteoporosismodelmice;improvedtibialdensity andreversedosteoporosis;miR-2110andmiR-328-3paremost importantosteogenesisregulatoryexosomalmRNAs
mice;exosomalmiR-100-5pamelioratedOAseveritybyprotecting articularcartilageandamelioratinggaitabnormalitiesviainhibitionof mTOR
79 MSC-exochondrocytes;promotedproliferationandinhibited apoptosis
OsteoarthritisIPFP-MSC-exochondrocytes;inhibitedapoptosis&autophagy,and enhancedmatrixsynthesis
mice;exosomallncRNA-KLF3-AS1protectedchondrocytesviamiR- 206/GIT1axis
N/A
80 synovial-MSC-exoprimarychondrocytes;miR-320c-enhancedchondrogenesis viaADAM19
81 chondrogenicMSC-exochondrocytes;increasedcellproliferationandmatrix synthesisviatargetingWnt5a mice;exosomalmiR-92a-3pinhibitedcartilagedegradationinOA animalmodel
82 synovial-MSC-exohumanprimarychondrocytes;enhancedproliferation& migrationviaWnt/YAPsignaling rats;miR-140-5p-oe-exopreventedOAbydecreasingjointwearand cartilagematrixloss
84
83 TGFβ -stimulatedMSC-exoC5.18cells;exosomalmiR-135bincreasedcellviabilityby regulatingspeci fi cityprotein-1 rats;promotedcartilagerepairbydecreasingOARSIscoreand increasingnumberofchondrocytes
Table2. continued TargetdiseaseExosomeInvitromodel& fi ndingsInvivomodel& fi ndingsRefs.
85
iPSC-exo,MSC-exohumanchondrocytes;stimulatedproliferation&migrationcollagenase-inducedOAmice;iPSC-MSC-exoshowedastronger therapeuticeffectonOAthansynovialmembraneMSC-exo
86
BM-MSC-exochondrocytes;decreasedin fl ammatoryfactors&glutamine metabolicproteins rats;increasedmice ’ sexercisecapacity,improvedchondrocyte functionandglutamatemetabolism,anddecreasedcartilagedamage andin fl ammation,therebyalleviatingOAprogression
87 MSC-exochondrocytes;increasedproliferation,matrixsynthesisand regenerativeimmunephenotype ratosteochondraldefectmodel; ↑ CD163 + M2and ↓ CD86 + M1 macrophages,andreducedpro-in fl ammatorycytokinesIL-1 β &TNFα
89
BM-MSC-exoosteoblasts;promotedcellproliferationandosteogenic differentiationbyreducingElf3 mice;exosomalmiR-206amelioratedin fl ammationandincreased osteocalcinandBMP2infemoraltissue;
88 gingival-MSC-exoCD4 + T-cells;inhibitedIL-17AandpromotedIL-10collagen-inducedarthritismicemodel;reducedincidenceandbone erosionofarthritisviainhibitingIL-17RA-Act1-TRAF6-NFκ Bpathway
94
SpinalcordinjuryIGF-1stimulatedNSC-exoPC12cells;inhibitedapoptosisandpromotedneural proliferation®eneration rats;reducedlesionsizeandpromotedfunctionalrecovery,causedby miR-219a-2-3p-dependentinhibitionofYY1
95
mice,subarachnoidinjection;enhancedfunctionalrecovery
miR-enclosedNSC-exoHT22cells;attenuatedneuronalapoptosisbyactivating autophagyviamiR-374-5p/STK-4axis
96
mice;enhancedrepairofneurologicalfunctionsvialncGm36569/miR- 5627-5p/FSP1axis
MSC-exoHT-22&HEK-293hypoxiccellmodel;suppressedneuronal ferroptosis
98
97 hypoxicpreconditionedBM- MSC-exo BV2microglia;hypoxiapromotedexoreleasefromMSC;exo uptakebyBV2dependedonoxygenstatus
99
BM-MSC-exomacrophages;takenupbyasubsetofM2macrophagesrats;bothMSCintravenousinfusionandfractionatedMSC-exo promotedM2macrophagepolarization,upregulatedTGFβ ,and reducedBSCBleakage
mice;promotedfunctionalbehavioralrecoverybyshiftingmicroglial M1/M2polarization;exosomalmiR-216a-5pregulatedviaTLR4/NFκ B/ PI3K/Aktpathway
EF-MSC-exoN/Arats;improvedneurologicalfunctionalrecoveryandreducedlesion volumebyinhibitingNLRP3in fl ammasome
100
EPC-exomacrophages;promotedanti-in fl ammatorymacrophagesmice;exosomalmiR-222-3ppromotedfunctionalrepairviaSOC3/ JAK2/STAT3pathway
101
mice;acceleratedmicrovascularregeneration,reducedspinalcord cavity,andimprovedfunctionalrecovery
NSC-exoSCMECs;enrichedinVEGF-Aandenhancedangiogenic activity
102
FTY720-loadedNSC-exoSCMECs;protectedbarrierfunctionofSCMECsunder hypoxicconditionsviaPTEN/Aktpathway rats;amelioratedhindlimbfunctionandreducedin fl ammatory in fi ltrationbydownregulatingBaxandaquaporin-4andupregulating claudin-5andBcl-2
104
BM-MSC-exopericyte;pre-Txwithexoreducedpericytepyroptosisand increasedpericytesurvivalrate ratmodelofT10SCI;improvedneuronsurvival,nerve fi berextension, BSCBintegrity,reducedcaspase1&IL-1 β ,andacceleratedlocomotor functionalrecovery
105
rats;miR-146a-5p-modi fi edexopromotedmorelocomotorfunctionof hindlimbsthanunmodi fi edexobytargetingneurotoxicastrocytes
miR-modi fi edUC-MSC-exoPC12cells;reducednegativeeffectsofneurotoxicastrocytes onPC12cellviabilityandneurites
107
106 placental-MSC-exoNSCs;promotedcellproliferation,andincreased phosphorylatedlevelsofMEK,ERKandCREB rats;promotedendogenousneuralstem/progenitorcellsproliferation, neurogenesis,andimprovedlocomotoractivityandbladder dysfunction
123
BM-MSC-exo-oe-NGFNSC;promoteddifferentiationofNSCsintoneurons&axonal regeneration mice;promotedrecoveryofspinalfunction&spinalcordregeneration
122 adipose-MSC-exoSchwanncells;promotedproliferation,migration, myelination,&neurotrophicfactors rats;improvedaxonregeneration&myelination,andrestored denervationmuscleatrophy
Sciaticnerveinjuryadipose-MSC-exoDRGneurons;increasedneuriteoutgrowthrats;enhancedaxonalregenerationandwalkingbehavior;discover ed neuralgrowthfactorstranscriptsinexo
Table2. continued
TargetdiseaseExosomeInvitromodel& fi ndingsInvivomodel& fi ndingsRefs.
124
rats;acceleratedfunctionalrecovery,axonregenerationand remyelination
LPS-treatedBM-MSC-exoRAW264.7cells;enhancedM2macrophagepolarizationvia TSG-6/NFκ B/NLRP3pathway
111
mousemodelofcardiotoxin-inducedmuscleinjury;promotedmuscle regeneration;exosomalmiR-494enhancedmyogenesisandmigration activity
Muscle&tendontearMSC-exoC2C12myoblasts,HUVECs;promotedmyogenesisand angiogenesis
112
Achillestendonrepairrabbitmodel;improvedmechanicalstrengthby upregulatingdecorinandbiglycan
adipose-MSC-exorabbitprimarytenocytes;enhancedproliferationand migration
114
adipose-MSC-exoN/Aratmodelofmassiverotatorcufftear;preventedatrophy,fatty in fi ltration,in fl ammation,andvascularizationofmuscles;elevated myo fi berregenerationandbiomechanicalproperties
115
adipose-MSC-exoN/Arabbitmodelofchronicrotatorcufftear;decreasedfattyin fi ltration, promotedtendon-bonehealing,andimprovedbiomechanical properties
116
BM-MSC-exoHUVECs,U937cells;promotedproliferation&angiogenic tubeformation;reducedM1polarization rats;increasedbreakingloadandstiffnessofrotatorcuffafter reconstructioninrats,reducedangiogenesisaroundrotatorcuff endpoint,andpromotedtendon-bonehealing
rats;intradiscalinjectionofexoalleviatednucleuspulposusapoptosis andIVDdegenerationbasedonhistologyandMRI
127
126 ESC-exonucleuspulposuscells;exosomalmiR-302cinhibited pyroptosis rats;ameliorateddamageinIVDdegenerationviadownregulating NLRP3in fl ammasome
128
BM-MSC-exonucleuspulposuscells;exosomalmiR-21alleviatedapoptosis viaPTEN/PI3K/Aktpathway
N/A
BM-MSC-exonucleuspulposuscells;alleviatedcompression-induced apoptosis&mitochondrialdamagebyinhibitingoxidative stress
Intervertebraldisc degeneration
Akt proteinkinaseB, BM bonemarrow, BMP bonemorphogeneticprotein, BSCB blood-spinalcordbarrier, CBS cystathionine β -synthase, CREB cAMPresponseelementbinding, DMOG dimethyloxaloylglycine, DRG dorsalrootganglion, EF epiduralfat, Elf E74-likefactor, EPC endothelialprogenitorcell, ERK extracellularsignal-regulatedkinase, ESC embryonicstemcell, exo exosome, FSP fi broblast-speci fi cprotein, GIT G-proteincoupledreceptorkinaseinteractingprotein, HIF hypoxia-induciblefactor, HLU hindlimbunloading, HUVEC humanumbilicalveinendothelialcell, IGF insulingrowthfactor, IL interleukin, IPFP infrapatellarfatpad, IVD intervertebraldisc, KLF Krüppel-likefactor, LPS lipopolysaccharide, LRP lipoproteinreceptorrelatedprotein, MEK mitogen-activatedproteinkinase, miR microRNA, mTOR mechanistic targetofrapamycin, NFκ B nuclearfactor-kappaB, NGF nervegrowthfactor, NLRP nucleotide-bindingdomain-likereceptorprotein, NP nucleuspulposus, NSC neuralstemcell, oe overexpressing, PI3K phosphoinositide3-kinase, PTEN phosphatase&tensinhomolog, Runx runt-relatedtranscriptionfactor, SCMEC spinalcordmicrovascularendothelialcell, SMURF smadubiquitinationregulatoryfactor, TGF transforminggrowthfactor, Tie tyrosinekinasereceptor, TLR Toll-likereceptor, TNF tumornecrosisfactor, TSG TNFstimulatedgene, Tx treatment, UC umbilicalcord, VEGF vascularendothelialgrowthfactor, YAP yes-associatedprotein, YY yinandyang
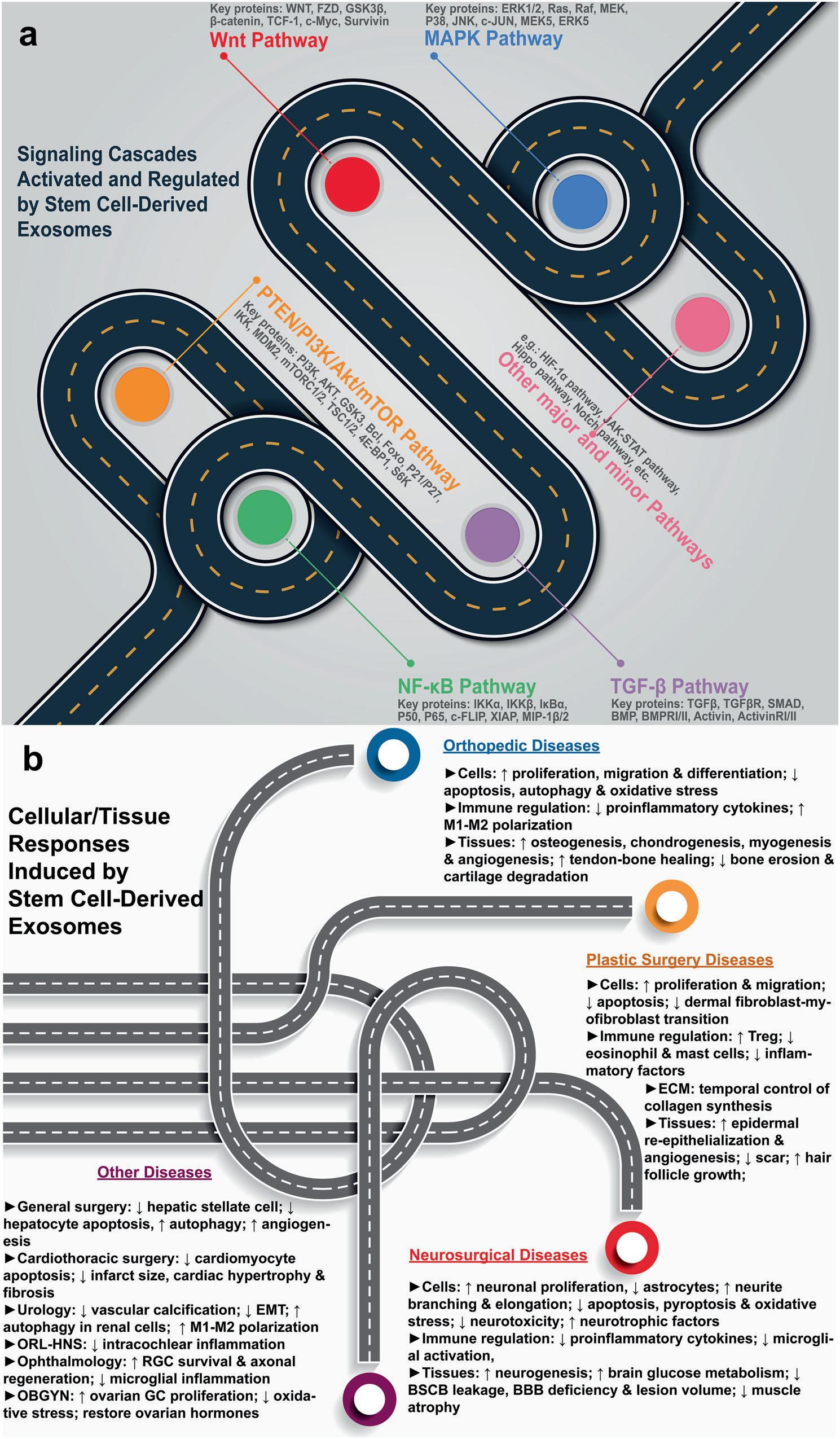
Fig.3 Mechanismsofstemcell-derivedexosometherapy(figuregeneratedusingAdobePhotoshop2023andAdobeIllustrator2023). a activationandregulationofvarioussignalingpathways. b disease-specificcellularandtissueresponses
SignalTransductionandTargetedTherapy(2024)9:17
Asamajorcargoofexosomes(Section2.1),RNAcanalter recipientcellgeneexpressionandphenotypicfunction,with microRNA(miRNA)andlongnon-codingRNA(lncRNA)beingthe mostwidelystudied.69 Fromtheperspectiveofanexosomal miRNA,onegroupdiscoveredthatmiR-136-5pfrombonemarrow MSC-derivedexosomespromotedosteoblastproliferationand differentiationinvitro,therebypromotingfracturehealing invivo.70 Thiswasachievedbyinhibitingthedownstreamtarget geneofmiR-163-5p,low-densitylipoproteinreceptor-related protein4(LRP4),throughtheWnt/β-cateninpathway.Theother groupfoundthatMSC-derivedexosomalmiR-25couldregulate theubiquitinationanddegradationofRunt-relatedtranscription factor2(Runx2)bySmadubiquitinationregulatoryfactor1 (SMURF1)topromotefracturehealinginmice.71 FromalncRNA perspective,especiallythebone-specificlncRNAH19,aChinese grouprevealedthatalthoughahigh-fatdietreducedosteogenic differentiationandweakenedfracturehealing,thiscouldbe reversedbyMSC-derivedexosomallncRNAH19viamiR-467/ HoxA10axisinanobesity-inducedfracturemodel.72 Inaddition, anAmericangroupdemonstratedthatexosomallncRNAH19not onlyimprovedosteogenesisbutalsoangiogenesisthroughthe angiopoietin1/Tie2-NOsignalingpathwayinanimmunocompromisednudemousemodel.73
InsteadofusingnaturallyderivedexosomesfromMSCs,some researchershaveconductedpre-isolationmodificationofexosomestoachievebetterresults.Liangetal.preconditionedMSCs withlowdosesofdimethyloxaloylglycine(DMOG),asmall angiogenicmolecule,topreparetheexosomesforanenhanced angiogenesisandboneregenerationinacritical-sizedcalvarial defectmodelbytargetingtheproteinkinaseB/mechanistictarget ofrapamycin(Akt/mTOR)pathway.74 Alternatively,Luetal.loaded MSC-derivedexosomeswithmiR-29a,whichshowedarobust abilityinpromotingangiogenesisandosteogenesisbytargeting vasohibin1.75 Furthermore,umbilicalcordMSC-derivedexosomes demonstratedcomparableresultstotheirbonemarrowcounterpartsduringfracturehealing.76 Inaddition,exosomesderived fromMSCsunderhypoxiaexhibitedbettereffectsonbone fracturehealingthanthoseundernormoxia.Mechanistically, hypoxiapreconditioningenhancedtheproductionofexosomal miR-126throughtheactivationofhypoxia-induciblefactor1(HIF1α).Variousstudieshaveshownthathypoxiapreconditioning representsaneffectiveandpromisingoptimizationofthe therapeuticeffectsofMSC-derivedexosomesforbonefracture healing.
Osteoarthritis
Osteoarthritis(OA)isthemostcommonjointdiseaseandmost frequentreasonforactivitylimitationinadults,affecting approximately240millionpatientsglobally.77 Thepathologyof OAhasevolvedfrombeingviewedascartilage-onlytoamultitissuediseasethataffectsallcomponentsofthewholejoint, includingbone,synovium,muscle,ligament,andperiarticular fat.78 Clinicaltrialshavesuccessfullyrevealedsystemiccompoundsthatarreststructuralprogression(e.g.,cathepsinKand Wntinhibitors)orreduceOApain(e.g.,nervegrowthfactor inhibitors).AsapotentialtreatmentoptionforOA,mostMSCderivedexosometherapyusedchondrocytesasatargetininvitro models.TheseMSCscouldoriginatefromvarioustissues,suchas bonemarrow,synovium,gingiva,andinfrapatellarfatpads(IPFPs). Somestudiesfocusingonchondrogenesisdemonstrateda particularinterestintheroleofmiRNA.Wuetal.foundthatIPFP MSC-derivedexosomesprotectarticularcartilagefromdamage andameliorategaitabnormalityinOAmicebymiR100-5pregulatedinhibitionofmTOR-autophagypathway.79 Sinceitis easytoretrievehumanIPFPfromOApatientsbyarthroscopic operationwithinaclinic,thistypeofexosometherapymight simplifyandacceleratetheprocessfrombenchtobedside.Liu etal.discoveredthatMSC-derivedexosomescouldpromote
proliferationandinhibitapoptosisofchondrocytesvialncRNAKLF3-AS1/miR-206/GIT1axisinOA.80 Thecellularworkconducted byKongetal.showedthatsynovialMSC-derivedexosomalmiR320ccouldenhancechondrogenesisbytargetingADAM19.81 In addition,Maoetal.suggestedthatexosomalmiR-92a-3pfrom chondrogenicMSCscouldenhancechondrogenesisandsuppress cartilagedegradationviatargetingWnt5a.82 Incontrasttothese studiesusingoriginalexosomes,fewgroupsmodifiedexosomes priortotheirsystemicadministration.Taoetal.modified exosomesbytransfectingsynovialMSCswithmiR-140-5pand foundthatexosomalmiR-140-5p-overexpressioncouldenhance cartilagetissueregenerationandpreventOAofthekneeinarat model.83 Meanwhile,Wangetal.usedTGF-β1tostimulateMSCs, andtheresultantexosomalmiR-135bincreasedchondrocyte proliferationbyregulatingspecificityprotein-1.84 Inacomparative study,Zhuetal.demonstratedthatexosomesfromiPSC-derived MSCscouldprovideastrongertherapeuticeffectonOAthan synovialmembraneMSC-derivedexosomes.85
Otherstudieshavefocusedonnotonlychondrogenesisbut alsoanti-inflammationandimmunemodulationduringOA treatment.Forexample,MSC-derivedexosomesinhibitedinflammatoryfactors,glutaminemetabolicactivity-relatedproteins, glutamine,andGSH/GSSGratioinvitro,whileimprovingmice’s chondrocytefunction,tissueinflammation,andexercisecapacity invivo,therebyalleviatingOAprogression.86 Usingaholistic approach,recentstudieshaveshiftedtheattentionawayfrom cartilagetowardsothertissues(e.g.,bone)inadiarthrodialjoint. Firstly,bonemarrowMSC-derivedexosomalmiR-206promoted proliferationandosteogenicdifferentiationofosteoblastsinOA byreducingE74-likefactor3(Elf3),andamelioratedinflammation andincreasedexpressionofosteocalcinandBMP2inmouse femoraltissues.87 Secondly,MSCexosome-treatedosteochondral defectsdemonstratedaregenerativeimmunephenotype,characterizedbyahigherinfiltrationofCD163+ M2macrophagesover CD86+ M1macrophages,withaconcomitantreductioninproinflammatorysynovialcytokinesIL-1β andTNF-α 88 Lastly,gingival MSC-derivedexosomesprovedtobeimmunosuppressivein preventingcollagen-inducedarthritis.89 Comparedwithparental cells,theseexosomeshadthesameorstrongereffectsin inhibitingIL-17AandpromotingIL-10,reducingincidencesand boneerosionbyarthritis,viainhibitingtheIL-17RA-Act1-TRAF6NF-κBsignalingpathway.
Currently,thereisnosingle ‘onesize fitsall’ drugthatmaybe suitableforallOApatients.Disease-modifyingOAdrugs (DMOADs)mightbecomethenext-generationOAtreatment.90 It isveryvaluableandrelevantthatMSC-derivedexosometherapy forOAcoincideswithDMOADs:botharecapableoftargeting inflammatorycytokines,matrix-degradingenzymes,andtheWnt pathway.Thus,emergingapproachesforDMOADdevelopment, suchasmiRNA-basedmodalityandtargetingcellularsenescence, mightalsobeusedtorefineMSC-basedexosometherapyforOA.
Spinalcordinjury
Traumaticspinalcordinjury(SCI)isadevastatingglobalhealth issuethatposesasignificantfunctionalandeconomicburden bothonthepatientandsociety.91 ThepathophysiologyofSCI includesprimaryinjuriescausedbymechanicaltraumaand secondaryinjurycascadecharacterizedbyapoptosis,edema, ischemia,inflammatorycellinfiltration,andexcitotoxicity.92 Despitesurgicalintervention,clinicalstudiesinvolvingpharmacotherapycanbebroadlyclassifiedaseitherneuroprotectiveor neuroregenerative.93 Targetingeacheventoftheabovemechanisticchain,bothMSC-andNSC-derivedexosometherapycould exertabeneficialinfluenceonspinalcordprotectionand regeneration.
Somegroupshavetargetedneuronalcelldeath.Maetal. revealedthatinsulin-likegrowthfactor1(IGF-1)-stimulatedNSCderivedexosomescouldinhibitneuronalapoptosiswhile
promotingfunctionalrecoveryafterSCIthroughamiR-219a-2-3p/ YY1pathway.94 Alternatively,Zhangetal.discoveredthat subarachnoidinjectionofNSC-derivedexosomescouldsuppress neuronalcellapoptosisbyactivatingautophagyviamiR-374-5p/ STK-4axisforenhancedfunctionalrecoveryinSCI.95 Shaoetal. exploredotherformsofcelldeath(e.g.,ferroptosis)usingMSCderivedexosomes,andfoundthatexosomallncGm36569could inhibitneuronalcellferroptosisviamiR-5627-5p/FSP1axis, therebydecreasingneuronaldysfunction.96
Somegroupshavetargetedanti-inflammationandimmunomodulation.Nakazakietal.discoveredthatfractionatedintravenousinfusionofMSC-derivedexosomescouldtargetM2 macrophagesandupregulateTGF-β,therebystabilizingmicrovesselsandimprovingfunctionalrecovery.97 Similarly,Liuetal. demonstratedthatinadditiontohypoxiaincreasingexosome productionfrombonemarrowMSCs,preconditionedexosomal miR-216a-5pcouldalsorepairtraumaticSCIbyshiftingmicroglial M1/M2polarizationviatheTLR4/NF-κB/PI3K/Aktpathway.98 Huangetal.valuablyprovedthatepiduralfatMSC-derived exosomescouldattenuateNLRP3inflammasomeandimprove functionalrecoveryinSCI.99 ComparedtoMSC-derivedexosomes, exosomesderivedfromEPCscouldprovidecomparableantiinflammatoryeffect.Yuanetal.showedthattheexosomalmiR222-3pfromEPCscouldpromoteanti-inflammatorymacrophages viatheSOC3/JAK2/STAT3pathwayandimprovemousefunctional repairafterSCI.100
Somegroupshavetargetedangiogenesisandblood-spinalcord barrier(BSCB)integrity.Forexample,Zhongetal.usedunmodified NSC-derivedexosomes,foundthattheywerehighlyenrichedin VEGF-A,andcouldthereforeenhancetheangiogenicactivityof spinalcordmicrovascularendothelialcells(SCMECs).101 Incomparison,Chenandco-workersmodifiedNSC-derivedexosomeswith FTY720,animmunemodulatorandmicrovascularregulator,to protectthebarrierfunctionofSCMECsviathePTEN/Aktpathway, therebyamelioratinghindlimbfunction.102 Itiswell-knownthat theconnectionbetweenthemicrovascularendotheliumofthe spinalcordandthepericyteiscrucialinmaintainingthestructural integrityofBSCB.103 Thus,Zhou’steamattemptedtoverifytherole ofexosometherapyinpericytehomeostasis.104 Theyprovedthat bonemarrowMSC-derivedexosomescouldreducepericyte pyroptosisandincreasepericytesurvivalrateinvitro,while improvingBSCBintegrityandlocomotorrecoveryinvivo. Finally,somegroupshavetargetedotheraspectsduring neuroprotectionandneuroregeneration,suchasneurotoxic astrocytesandendogenousNSCsustainability.Laietal.proved thathumanumbilicalcordMSC-derivedexosomescouldfacilitate recoveryofspinalcordfunctionbytargetingneurotoxic astrocytes.105 Inaddition,miR-146a-5p-modifiedexosomes exertedamorepowerfuleffectthanunmodifiedexosomes.Li etal.discoveredthatexosomesderivedfromnervegrowthfactor (NGF)-overexpressingbonemarrowMSCscouldenhanceneuronal differentiationofNSCsandaxonalregeneration.106 Zhouetal. demonstratedthatplacentalMSC-derivedexosomescould promotetheactivationofproliferatingendogenousNSCs,thereby improvingbothlocomotoractivityandbladderdysfunction,107 whichisafrequentsequelaethatcouldfurtherworsenthequality oflifeofSCIpatients.108
Muscleandtendontear
Muscleandtendontearscanresultfromeitheracutetrauma(e.g., fractures,Section3.1)orchronicoveruse(e.g.,sportsinjury).109 Healingofmusclestrainandtendontearfollowsthetypical woundhealingcourse,involvingtheinflammatory,proliferative, andremodelingphases.Multiplenon-surgicalstrategieshave beentrialedtoimprovehealing,includingcell-basedandgrowth factor-basedtherapies.110 Thefollowingproof-of-conceptstudies indicatethatMSC-derivedexosomescouldbecomethenextgenerationmusculoskeletaltreatment.
Ontheonehand,somegroupshavefocusedonindividual componentsofthemuscle-tendon-boneunit.Nakamuraetal. claimedthatMSC-derivedexosomescouldimproveinvitro myogenesisinC2C12myoblastsandangiogenesisinHUVECs, whileacceleratinginvivoskeletalmuscleregenerationina cardiotoxin-inducedmuscleinjurymodel.111 Thesebenefitswere atleastinpartmediatedbymiRNAssuchasmiR-494.Chenetal. discoveredthatexosomesfromadiposeMSCscouldenhancethe proliferationandthemigrationofprimarytenocytes,whilealso improvingmechanicalstrengthofrepairedtendonsbyupregulatingdecorinandbiglycaninarabbitAchillestendonrupture model.112
Ontheotherhand,somegroupshaveregardedthemuscletendon-boneunitasasinglefunctionalsystemandusedrotator cufftearasthediseasemodel,whichisthemostcommon shoulderconditionforwhichpatientsseektreatment.113 One groupofresearcherspublishedtwoconsecutivestudiesusing adiposeMSC-derivedexosomes.Inaratmodelofmassiverotator cufftear,exosometherapycouldpreventtheatrophy,inflammation,andvascularizationofmuscles.114 Inarabbitmodelof chronicrotatorcufftear,exosometherapycouldpreventfatty infiltrationandimprovebiomechanicalproperties.115 Another groupreportedthatbonemarrowMSC-derivedexosomescould increasethebreakingloadandstiffnessoftherotatorcuffafter reconstruction,induceangiogenesisaroundtherotatorcuff endpoint,andpromotegrowthofthetendon-boneinterface.116
Otherorthopedicdiseases
Osteoporosisisametabolicbonediseasecharacterizedbylow bonedensityandweakeningofbonearchitecture,whichincrease theriskoffractures.Itresultsfromosteoclasticboneresorption undercompensatedbyosteoblasticboneformation.117 Inacellular study,adiposeMSC-derivedexosomescouldantagonizehypoxia/ serumdeprivation-inducedosteocyteapoptosisandosteocytemediatedosteoclastogenesis.118 Furtheranimalstudiesrevealed thatumbilicalcordMSC-derivedexosomescouldinhibitbone marrowMSCapoptosisandpreventdisuseosteoporosisviamiR1263/Mob1/Hippopathway,119 andimprovetibialdensityand reverseestrogen-deficientosteoporosisviamiR-2110andmiR328-3p.120
ComparedtoSCI,damagetoperipheralnerve(e.g.,sciaticnerve injury)isconsiderablymorecommon.Thesubsequentnerve regenerationiscontrolledbytheinterplaybetweenneuronsand Schwanncells,andfurthercomplicatedbyinflammatorycell infiltration.121 ItwasshownthatadiposeMSC-derivedexosomes couldtargetneuronsbyincreasingneuriteoutgrowthinvitroand axonalregenerationandwalkingbehaviorinvivo.122 Adipose MSC-derivedexosomescouldtargetSchwanncellsbypromoting theproliferation,migrationandsecretionofneurotrophicfactors invitroandrestoredenervationmuscleatrophyinvivo.123 LPSpreconditionedMSC-derivedexosomescouldtargetinflammatory cellsbyenhancingM2macrophagepolarizationinvitroand accelerateperipheralnerveregenerationinvivo.124
Intervertebraldisc(IVD)degenerationisamajorcauseoflower backpainwhichistheleadinginjuryintotalglobalyearslived withdisability.Itsmolecularprocessesincludeextracellularmatrix (ECM)degeneration,inflammation,oxidativestress,apoptosis, senescenceandreducedautophagy.125 Theemergingavenuesof exosometherapyattempttosolvesomeoftheseissues.Cheng etal.demonstratedthatintradiscalinjectionofbonemarrowMSCderivedexosomescouldinhibitnucleuspulposuscell(NPC) apoptosisandalleviateIVDdegenerationviaexosomalmiR-21.126 Ontheotherhand,Chenetal.discoveredthathumanESC-derived exosomescouldinhibitNLRP3inflammasometoalleviate pyroptosisinnucleuspulposuscellsbydeliveringmiR-302c.127 Inadditiontocelldeathandmitochondrialdamage,oxidative stressinNPCswasalsofoundtobeinhibitedbyMSC-derived exosomes.128 SinceIVDdegenerationandOAshareacommon
moleculardiseasespectrum125 thepositiveresultsofOAtreatmentusingMSC-derivedexosomes(Section3.2)couldbeusedas areferenceforIVDdegenerationresearch.
Osteonecrosis,aka.,avascularnecrosis,ofthefemoralhead (ONFH)isadisablingconditionaffectingayoungerpopulation, whichoftenresultsintotalhiparthroplasty.129 Glucocorticoid (GC)-inducedosteonecrosisisoneofthemostcommoncausesof ONFH,whosepathogenesisismanifestedintwoaspects: compromisedbloodsupplytothefemoralheadanddampened osteogenicactivity.Liuetal.showedthatexosomesfromiPSCderivedMSCscouldpreventGC-inducedONFHbypromoting angiogenesisandosteogenesisviathePI3K/Aktpathway.130 Zuo etal.demonstratedthatmiR-26a-overexpressingexosomes derivedfromHSCscouldprovidesimilartherapeuticeffects.131
NEUROSURGERYANDSC-EXOTHERAPY
Ischemicstroke
Strokesarethesecondhighestcauseofdeathandthethird leadingcauseofdisabilityglobally,withischemicstrokebeingthe mostcommonsubtype.132 Thekeyeventsduringtheischemic cascadeincludeneuronaldysfunction,excitotoxicity,neurochemicalinjury,andneuroinflammation.133 Intermsoftreatment,anew generationofclinicaltrialsisnowunderway,whichuses cytoprotectivedrugs,suchasimmunomodulators,IL-6receptor antagonists,Rhokinaseinhibitors,andfreeradicalscavengers.134 Targetingeacheventoftheabovepathophysiology,nearlyall subtypesofSC-exodemonstratedpotenttherapeuticeffectson strokerecovery(Table 3).
Somegroupshavetargetedneuroprotectionandneurogenesis. Firstly,SC-exotherapycouldinhibitneuronalcelldeath.Luoetal. foundthatNSC-derivedexosomescouldinhibitapoptosiswhile promotingtheproliferationofSH-SY5Ycellsbothundernormal andoxygen-glucosedeprivation(OGD)conditions.135 Thiswas alsotestedinamiddlecerebralarteryocclusion(MCAO)modelas areducedinfarctionareaandneuronalapoptosisviaexosomal miR-150-3p.OtherinvitroandinvivostudiesshowedsimilarantiapoptoticeffectsusingEPC-derivedexosomes.136,137 Zhangetal. discoveredthattheexosomalanti-apoptoticeffectcouldbe improvedbypreconditioningtheparentalNSCswithinterferon gamma(IFN-γ).138 Secondly,SC-exotherapycouldprotectcellsof theCNS.Kangetal.revealedthatexosomesderivedfrombone marrowMSCscouldrescueOGD-inducedinjuryinneuralcellsby suppressingNLRP3inflammasome-mediatedpyroptosis.139 Exosomessourcedfromhypoxiccultureshadamorepronounced neuroprotectiveeffectthantheircounterpartsfromnormal cultures.Similarly,Lietal.discoveredthatexosomesderived fromhumaniPSC-derivedneuralprogenitorcellsexhibiteda neuroprotectiveeffectonOGDneuronsandneuriteoutgrowth.140 Thisprotectionofneuronalfunctionunderischemicconditions wasregulatedthroughthePTEN/Aktpathway.Inaddition,Sun etal.provedthatNSC-derivedexosomescouldalsoprotect astrocytes,whichbecomesupportingreactiveastrocytes(RAs) afterstrokes.141 Thirdly,SC-exotherapycouldimprovepost-stroke neurogenesis.Weietal.suggestedthatZeb2/Axin2frombone marrowMSC-derivedexosomescouldimprovepost-stroke neurogenesis,neuralplasticity,andspatialmemoryandnerve function,likelyviatheSOX10,Wnt/β-catenin,andendothelin-3/ EDNRBpathways.142 Wangetal.illustratedthatmiR-126-modified EPC-derivedexosomescouldalleviateacutebraininjuryand promotefunctionalrecoveryafterstrokebyenhancing neurogenesis.143
Somegroupshavetargetedtheinhibitionoftheneuroinflammation.Firstly,unmodifiedSC-exotherapyexhibitedanantiinflammatoryeffectthroughexosomalmiRNAs.Dongetal. showedthatbonemarrowMSC-derivedexosomescouldinduce BV2microgliadeactivationandM2polarizationinvitro,while reducinginfarctsizeandimprovingneuronalfunctioninvivovia
transferringmiR-23a-3p.144 Similarly,Zhangetal.unveiledthat umbilicalcordMSC-derivedexosomalmiR-146a-5pcouldattenuatemicroglia-mediatedneuroinflammationafterOGDinvitro, whileimprovingbehavioraldeficitsandmicrogliaactivation invivoviatheIRAK1/TRAF6signalingpathway.145 Secondly,the anti-inflammatoryeffectofSC-exotherapycouldbeenhancedby modifyingtheexosomes.Yoonandco-workersestablishedtumor susceptibilitygene(TSG)101-overexpressinghumanNSCs,thereby increasingexosomesecretion.146 Theengineeredexosomesnot onlyattenuatedLDHreleaseandproinflammatoryfactorsinvitro, butalsoreducedinfarctionvolume,inhibitedDNA-damage pathway,andupregulatedneurotrophicfactorsinvivo.Furthermore,Tian’steambrokenewgroundbyingeniouslyattaching RGDpeptideontoanNSC-derivedexosomemembrane,which targetedthelesionregionoftheischemicbrainafterintravenous administration,therebysuppressingtheinflammatoryresponse aftercerebralischemiabyinhibitingtheMAPKpathway.147 Interestingly,Gaoetal.usedinducedNSCs(iNSCs)reprogrammed frommouse fibroblastsforstroketreatment.Theyshowedthat iNSC-derivedexosomes,bearingsimilartherapeuticeffectswith NSC-derivedones,couldnotonlypromoteneurogenesisbutalso inhibitneuroinflammation.148
Finally,somegroupshavetargetedotheraspectsduringstroke recovery,suchasneurochemicalinjuryandoxidativestress.Zhu etal.loadedbrain-derivedneurotrophicfactor(BDNF)into exosomesderivedfromNSCstoconstructengineeredexosomes.149 InamodelofH2O2-inducedoxidativestress,exosome therapysignificantlyenhancedNSCsurvival.InaratMCAOmodel, exosometherapynotonlyinhibitedmicroglialactivation,butalso boostedthedifferentiationofendogenousNSCsintoneurons. Collectively,BDNF-basedmodificationofNSC-derivedexosomes hasimprovedeffectsinthetreatmentofischemicstroke.Onthe otherhand,miR-210-modifiedEPC-derivedexosomescould protectneuronsfromhypoxiaandreoxygenation(H/R)-induced apoptosis,oxidativestress,anddecreasedviability,thereby supportingthetreatmentofischemicstroke.150,151 Theexosomal miR-17-5pfromACE2-enrichedEPC-derivedexosomescould amelioratecerebralischemicinjuryinagedmice.152 Inan intriguingstudyconductedbyXuandco-workers,combination ofNSC-exoandEPC-exowithmiR-210andmiR-123overexpressionexertedbettertherapeuticeffectsonischemicstrokeby protectingH/RinjuredneuronsthroughtheBDNF-TrkBandNox2/ ROSpathways.153
Incontrasttoischemicstroke,hemorrhagicstrokeposesa deadlierthreatandworsedisabilityinmostsurvivors.154 miR-137 overexpressionwasfoundtoboosttheneuroprotectiveeffectsof EPC-derivedexosomesagainstapoptosis,ferroptosis,andmitochondrialdysfunctioninoxyhemoglobin-treatedSH-SY5Ycells,an invitrohemorrhagicstrokemodel,partiallythroughtheCOX2/ PGE2pathway.155
Traumaticbraininjury
Approximately70millionpatientssufferfromtraumaticbrain injury(TBI)globallyeachyear,whichposesseriousphysical, psychosocialandeconomicthreats.156 TBIcanbecategorizedas primaryinjuries(e.g.,axonaldeath,neuroinflammation,neurochemicalchange,andmetabolicdysfunction)andsecondary injuries(e.g.,ischemicandhypoxicdamage,cerebraledema, raisedintracranialpressure,hydrocephalus,andinfection).157 Each patientwithaTBIhasauniquesetofcircumstancesdependingon variablessuchasthelocationandseverityoftheinjury,making medicalandsurgicaltreatmentquitechallenging.158 Therefore, systemictherapyusingSC-exomaybecomea ‘one-size-fits-all’ optionformanagingTBI.
Aseriesofanimalstudiespublishedinitiallyfocusedonthe functionalrecoveryandmacroscopicaspectsofMSC-derived exosometherapy.InaratTBImodel,exosome-treatedanimals showedsignificantimprovementinspatiallearningand
Table3. Stemcell-derivedexosomesforthetreatmentofdiseasesinneurosurgeryandrelatedspecialties
TargetdiseaseExosomeInvitromodel& fi ndingsInvivomodel& fi ndingsRefs.
ratMCAOmodel;reducedinfarctionareaandneuronapoptosis, exosomalmiR-150-3penhancedneuroprotectiveeffectsbytargeting CASP2
IschemicstrokeNSC-exoSH-SY5Ycells;inhibitedapoptosisandpromoted proliferationbothinnormalandOGDconditions
EPC-exoN/AratMCAOmodel;reducedinfarctsize,neurologicaldefectscore,and percentageofapoptoticcells,butincreasedCD31andVEGF
ratMCAOmodel;promotedbehavioralandstructuraloutcomes; in fl ammatoryfactorIFNγ preconditionedexoweremorepotent
IFNγ inducedNSC-exoNSCs;increasedcellproliferation&survival,andreduced cellapoptosis
BM-MSC-exoOGDN2acells,ratprimarycorticalneurons; neuroprotectiveagainstNLRP3in fl ammasome-mediated pyroptosis
iPSC-exoratprimarycorticalneurons;improvedneuronalsurvival andneuriteoutgrowthviaPTEN/Aktpathway
mice;reducedinfarctvolume
NSC-exo,iCM-exoprimarymousecorticalastrocytes,neuronalcells; protectedafterOGDischemia;NSC-exo>iCM-exo
142
OGDratneuron;increasedneuritebranching&elongationratMCAOmodel;improvedpost-strokeneurogenesis,neuralplasticity, andspatialmemoryandnervefunction,likelyviaSOX10,Wnt/ β -catenin,andendothelin-3/EDNRBpathways
Zeb2/Axin2enrichedBM- MSC-exo
143
miR-126-EPC-exoN/AdiabeticmouseMCAOmodel;improvedacutebraininjuryand functionalrecoveryafterstrokebypromotingneurogenesis
144
BM-MSC-exoBV2microglia;inducedmicrogliadeactivationandM2 polarization ratMCAOmodel;reducedinfarctsizeandimprovedneuronalfunction viatransferringmiR-23a-3p
145
UC-MSC-exoBV2microglia;attenuatedmicroglia-mediated in fl ammationafterOGD mice;reducedinfarctvolume,behavioralde fi cits,andameliorated microgliaactivation;exosomalmiR-146a-5preduced neuroin fl ammationviaIRAK1/TRAF6pathway
TSG101-oe-NSC-exoN2Acells;attenuatedLDHreleaseandproin fl ammatory factors, ratMCAOmodel;reducedinfarctionvolume&in fl ammatorycytokines, inhibitedDNA-damagepathway,andupregulatedneurotrophicfactors 146
RGDNSC-exoReN&BV2cells;showedintrinsicanti-in fl ammatoryactivitymice;targetedischemicbrainregionsandsuppressedpostischemia in fl ammatoryresponse;exosomalmiRsinhibitedMAPKpathway 147
149
ratMCAOmodel;inhibitedtheactivationofmicroglia,promotedthe differentiationofendogenousNSCsintoneurons,andimproved behavioralfunction
BDNF-NSC-exoH 2 O 2 -inducedoxidativestressinNSCs;reducedapoptosis andincreasedneurogenicdifferentiation
150
153
N/A
miR-210-EPC-exoH/RinjuredSH-SY5Ycells;protectedfromapoptosis& oxidativestress
mouseMCAOmodel;exosomalmiR-17-5pinhibitedapoptosis, oxidativestress&braindysfunctionviaPTEN/PI3K/Aktpathway
ACE2-enrichedEPC-exoH/Rinjuredmousebrainmicrovascularendothelialcells; inhibitedsenescence
159
ratMCAOmodel;reducedinfarctvolume&neurologicalde fi citsscore viaNox2/ROS&BDNF/TrkBpathways
152 NSC-exo + EPC-exoH/RinjuredSH-SY5Ycells;protectedfromapoptosis& oxidativestress
TraumaticbraininjuryMSC-exoN/Arats;improvedspatiallearning&sensorimotorfunctionand neurovascularplasticity
160
BM-MSC-exoN/Arats;improvedspatiallearning,and3D>2Dcultureconditions; enhancedsensorimotorrecovery;increasedendothelialcells& neurons,andreducedneuroin fl ammation
161
MSC-exoN/Aprimarymotorcortexmonkeymodel;animalsreturnedtopre- operativegrasppatterns&latencytoretrievefoodrewardinthe fi rst 3 –5weeksofrecovery
Table3. continued
TargetdiseaseExosomeInvitromodel& fi ndingsInvivomodel& fi ndingsRefs.
162
MSC-exoN/AcombinedTBI&HSswinemodel;attenuatedseverityofneurologic injuryandallowedforfasterneurologicrecovery
163
adiposeMSC-exoprimaryratmicroglia&neuron;suppressedmicroglia activationbyinhibitingNFκ B&MAPK rats;promotedfunctionalrecovery,suppressedneuroin fl ammation, reducedneuronalapoptosis,andincreasedneurogenesis;exomainly takenupbymicroglia/macrophages
164
BM-MSC-exoBV2microglia;promotedM1toM2phenotypeand upregulatedanti-in fl ammatorycytokines mice;reducedcorticaltissueapoptosisandinhibited neuroin fl ammation,possiblybyexosomalmiR-181bviaIL-10/STAT3 pathway
166
165 MSC-exoN/Arats;improvedangiogenesis&neurogenesis,andsensorimotor& cognitivefunction,reducedneuroin fl ammation&hippocampal neuronalcellloss;100µg&1daywereoptimal
179
NSC-exoNSCs;exosuperiortoparentalcellsrats;improvedneurobehavioralperformance,inhibitedastrocyte neuroin fl ammation,enhanceddoublecortinneurogenesis,while maintainingSOX2&Nestinstemness
Alzheimer ’ sdiseaseBM-MSC-exoN/Aearly-stageADmice;reducedA β plaqueburden&dystrophicneurites; carriedneprilysin
180
BM-MSC-exoprimaryneuron;reducedA β -inducediNOSexpressionmice;rescuedsynapticimpairmentandimprovedcognitivebehavior
182
181 heatshock-inducedNSC- exo HC2S2cells;exhibitedgreaterneuroprotectionagainst oxidativestressandA β -inducedneurotoxicity N/A
183
184
185
186
NSC-exoN/AADtransgenicmice;enhancedmitochondrialfunction,sirtuin1 activation,synapticactivity,decreasedin fl ammatoryresponse,and rescuedcognitivede fi cits
ADtransgenicmice;improvedbrainglucosemetabolismandcognitive function;upregulatedsynapse-relatedgenes&downregulatedHDAC4 expression
MSC-exoSH-SY5YwithFADmutations;reducedA β expressionand restoredneuronalmemory
N/A
AF-MSC-exoBV2microglia,SH-SY5Ycells;mitigatedneuroin fl ammatory microglialinjuryandrecoveredneurotoxicityfromA β
UC-MSC-exoBV2microglia;reducedin fl ammatoryreaction&induced alternativemicroglialactivation mice;alleviatedneuroin fl ammationandreducedA β depositionby modulatingmicroglialactivation;increasedspatiallearning&memory function
188
MSC-exoN/Amice;stimulatedneurogenesisinsubventricularzoneandalleviated A β -inducedcognitiveimpairment
189
193
5xFADmousemodel;BBBbreakdownoccurredat4monthsofage, whichcouldbemimickedwithaninvitroBBBmodel
NSC-exo5xFADprimarycerebralendothelialcells;reversedAD- causedBBBde fi ciency
RVG-taggedMSC-exoN/AtransgenicAPP/PS1mice;improvedCNS-targeteddelivery;reducedA β deposition&astrocytes,andimprovedcognitivefunction;RVG-exo werebetter
Parkinson ’ sdiseaseBM-MSC-exoSH-SY5Y&SK-N-SHcells;exosomalTSG-6attenuated MPP + -inducedneurotoxicityviaSTAT3/miR-7/NEDD4axis N/A
194
6-hydroxydopamine-inducedPDmice;protecteddopaminergiccell viabilityviaexosomalmiR-182-5p,miR-183-5p,&miR-9
197
EAEratmodel;reducedin fl ammationanddemyelinationofCNSby regulatingpolarizationofmicrogliafromM1toM2;decreased neurobehavioralscoresandpreventedweightloss
198
NSC-exoSH-SY5Y&BV2cells;anti-oxidativestress,anti- in fl ammatory&anti-apoptoticeffects
MultiplesclerosisBM-MSC-exoHAPImicroglia;downregulatedTNFα &iNOSand upregulatedIL-10,TGFβ andarginase-1
BM-MSC-exoN/A2micemodels:EAE&CPZ;improvedneurologicaloutcome,increased OPCdifferentiation&remyelination,decreasedneuroin fl ammationvia TLR2pathway
Table3. continued TargetdiseaseExosomeInvitromodel& fi ndingsInvivomodel& fi ndingsRefs.
199
VasculardementiaNSC-exoN/Arats;exo-derivedMIATimprovedlearningability&memoryviamiR- 34b-5p/calbindin-1axis
200
201
202
203
204
205
N/A
NSC-exorescuedcellularviabilityinHIV-damagedneurons,and inhibitedapoptosisandin fl ammatoryfactorsecretion
HIV-associated neurocognitivedisorders
NSC-exoN/Amice;exosomalmiR-124improvedexercise&fearbehavior,reversed cognitiveimpairment,andreducedneuroin fl ammation
Radiation-inducedcognitive dysfunction
NSC-exoN/Amice;protectedhostneurons,enhancedneurotrophicfactors& synapticsignaling,andreducedneuroin fl ammation
EpilepsyBM-MSC-exoN/Amice;reducedhippocampalin fl ammation,andpreventedabnormal neurogenesis&memorydysfunction
L5spinalnerveligationratmodel;increasedpawwithdrawalthreshold andlatency,reducedapoptosisandin fl ammationinspinaldorsalhorn
MechanicalallodyniaBM-MSC-exomicroglia;downregulatedNOTCH2whichistargetedby exosomalmiR-150-5p
Spinabi fi daapertaNSC-exoBM-MSCs;promotedneuronaldifferentiationofMSCsratembryomodel;exosomalNetrin1promotedneuronal differentiationofMSCs&NSCsbyupregulatingHand2/Phox2b
DepressionBM-MSc-exoN/Arats;suppressedapoptosis&boostedproliferationinhippocampal tissuesbyupregulatingexosomalmiR-26a 206
207
StressUC-MSc-exoN/Amiceacutebraindisordermodel;increasedadiponectin,improved cognitivefunctionandhippocampalneurogenesisthatwas suppressedbystreptozotocininjection
208 NSC-exoNSCs;HFDdownregulatedCREB/BDNF/TrkBsignalingmice;intranasaladministrationrestoredCREBtranscriptionalactivity, rescuedbothBDNF&HFD-dependentmemoryde fi cits
BrainageingNSC-exoNSCs;rescuedIRS-1/FoxOactivationandcounteracted reducedproliferationandsenescence mice;intranasaladministrationcounteractedHFD-dependent impairmentofadulthippocampalneurogenesisbyrestoringbalance betweenproliferatingandsenescentNSCs
210
209 hypothalamicNSC-exoN/ANSC-alation-inducedmousemodel;exosomalmiRNAsreduced hypothalamicin fl ammation,andsloweddownageing,independentof foodintake
211
MSC-exoprimaryratbrainendothelialcells;rescuedOGD-induced injury&inhibitedTLR4/NLRP3/caspase-1/NFκ Bpathway N/A
Deephypothermic circulatoryarrest
A β amyloidbeta, AD Alzheimer ’ sdisease, AF amniotic fl uid, BBB bloodbrainbarrier, BDNF brain-derivedneurotrophicfactor, BM bonemarrow, CREB cAMPresponseelementbinding, CNS centralnervoussystem, EAE experimentalautoimmuneencephalomyelitis, EDNRB endothelinreceptortypeB, exo exosome, FAD familialAlzheimer ’ sdisease, FoxO ForkheadboxO, H/R hypoxiaandreoxygenation, HAPI highlyaggressive proliferatingimmortalized, HDAC histonedeacetylase, HFD highfatdiet, HS hemorrhagicshock, iCM inducedpluripotentstemcell-derivedcardiomyocyte, IFN interferon, IL interleukin, iNOS induciblenitricoxide synthase, iPSC inducedpluripotentstemcell, IRAK interleukin1receptorassociatedkinase, IRS insulinreceptorsubstrate, LDH lactatedehydrogenase, MAPK mitogen-activatedproteinkinase, MCAO middle cerebralarteryocclusion, MIAT myocardialinfarctionassociatedtranscript, miR microRNA, MPP + 1-methyl-4-phenylpyridinium, MSC mesenchymalstemcell, NEDD4 neuronallyexpresseddevelopmentallydownregulated4, NFκ B nuclearfactor-kappaB, NLRP NOD-,LRR-andpyrindomain-containingprotein, NSC neuralstemcell, oe overexpressing, OGD oxygen-&glucose-deprived, SOX Sry-Boxtranscriptionfactor, STAT signaltransducerandactivatoroftranscription, TGF transforminggrowthfactor, TLR Toll-likereceptor, TNF tumornecrosisfactor, TRAF TNFreceptorassociatedfactor, TrkB tropomyosinreceptorkinaseB, TSG TNF stimulatedgene, UC umbilicalcord, Zeb zinc fi ngerE-boxbindinghomeobox
sensorimotorfunction.159 Inaddition,exosometreatmentsignificantlyincreasedthenumberofnewbornendothelialcellsin thelesionboundaryzone,andnewbornimmatureandmature neuronsinthedentategyrus.InanotherratTBImodelwithsimilar findings,exosomesderivedfromMSCsculturedina3Dsystem providedbetteroutcomesthanthoseinaconventional2D condition.160 InamonkeymodelofTBItotheprimarymotor cortex,exosome-treatedanimalsreturnedtopre-operativegrasp patternsandlatencytoretrieveafoodrewardinthe first 3–5weeksofrecovery.161 Inanevenmorecomplicatedand clinicallyrealisticlargeanimalmodel,inwhichbothTBIand hemorrhagicshockwereinvestigated,exosometherapyattenuatedtheseverityofneurologicinjuryandenabledfaster neurologicrecovery.162
Incomparison,studiescompletedinrecentyearsshednew lightonthemolecularmechanismsunderlyingSC-exotherapyfor TBI.Chenetal.reportedthatadiposeMSC-derivedexosomes couldpromotefunctionalrecovery,suppressneuroinflammation, reduceneuronalapoptosis,andincreaseneurogenesis.Thiswas achievedthroughtheuptakeofexosomesspecificallybymicroglia andsuppressionoftheiractivationbyinhibitingtheNF-κB& MAPKpathways.163 Wenetal.showedthatbonemarrowMSCderivedexosomescouldreducecellapoptosisincorticaltissueof mousemodelsofTBI,inhibitneuroinflammation,andpromotethe transformationofmicrogliatotheanti-inflammatoryphenotype. ThiswasrealizedbytheactionofmiR-181bontheIL-10/STAT3 pathway.164 Abedietal.provedthatNSC-derivedexosomescould improveneurobehavioralperformance,inhibitastrocyteneuroinflammation,enhanceneurogenesis,whilemaintainingNSCstemness.165 Avaluableadditional findingwasthatexosomesseemed tobesuperiortotheparentNSCsintermsofsensorimotor functionalrecovery.Finally,adose-responseandtherapeutic windowdemonstratedthatMSC-derivedexosomescouldimprove angiogenesisandneurogenesis,andsensorimotorandcognitive function,whilereducingneuroinflammationandhippocampal neuronalcellloss.166 Although100µgand1daymightbethe optimaldoseandtherapeuticwindowrespectively,exosomes exhibitedawiderangeofeffectivedosesfortreatmentofTBI withinatherapeuticwindowofatleast7dayspost-injury.
TBIandSCIaretwoofthemostsevereCNStraumas,whichare increasinglyrecognizedasglobalhealthpriorities.Theemerging evidencepresentedinSections3.3and4.2aremutuallybeneficial forthesetwocloselyrelatedresearchsubspecialties.Henceforth, futureresearchonSC-exotherapyforTBIandSCIcouldbeeither mechanism-based(e.g.,theroleofbrain-gutaxis167 transcriptional factors168 inflammasome169 andthecomplementsystem170)or modification-based(loadingexosomeswithdrugs,e.g.,immunomodulators171 antioxidants172 circularRNAs173 andmicroRNAs174).
Alzheimer’sdisease
UnlikeTBIandSCI,whicharetraumaticinnature,Alzheimer ’s disease(AD)andParkinson’sdisease(PD)arethemostcommon neurodegenerativediseases(NDD).ThehallmarksofNDDinclude, butarenotlimitedto,pathologicalproteinaggregation,synaptic andneuronalnetworkdysfunction,aberrantproteostasis,cytoskeletalabnormalities,alteredenergyhomeostasis,DNAandRNA defects,in flammation,andneuronalcelldeath.175 ADisthemost commonformofdementiagloballyandaccountsfor25million cases.176 Currently,onlytwoclassesofdrugsareapprovedfor symptomaticADtreatment,includingcholinesteraseinhibitors andNMDAantagonists.Althoughseveraltherapeuticsare activelyundergoingclinicaltrials,noneofthemarenearcurative forAD.177 Thechallengesofbrain-drugdelivery,e.g.,thebloodbrainbarrier(BBB)andpharmacokineticdrawbacks,areverylikely tobesolvedbynanosizedexosomes,whichareadditionally packagedwithpotentbiomolecules.MostSC-exotherapy involvesamyloid- β (Aβ ),whichispositionedatthecenterofAD pathophysiology.178
Theinitialworkfocusedontheclearanceofaggregationofthe pathologicalprotein,Aβ peptide.Theintracerebralinjectionof MSC-derivedexosomesbyEliaandco-workersreducedAβ plaque burdenanddystrophicneuritesinboththecortexand hippocampusintheearlystagesofapreclinicalmodelofAD.179 Inaddition,usingimmunoblotting,theauthorsconfirmedthe presenceofNeprilysin,aneutralendopeptidasecapableofAβ degradation,intheexosome’slysatesanditsmRNA.
Someteamshavefocusedonrelievingsynapticdysfunction andoxidativestress.Wangetal.foundthatMSC-derived exosomescouldrescuesynapticimpairmentandimprove cognitivebehaviorinAPP/PS1mice,whilealleviatingexogenous Aβ-inducedinduciblenitricoxidesynthase(iNOS)expression.180 InsteadofusingMSC-derivedexosomes,Lietal.administered NSC-derivedexosomesandenhancedmitochondrialfunction, sirtuin1activation,synapticactivity,andrescuedcognitive deficits.181 Usingalternativemethods,Huberetal.noticedthat heatshock-inducedexosomesderivedfromNSCsexhibited greaterneuroprotectionagainstoxidativestressaswellas Aβ-inducedneurotoxicity.182
Someteamshavecenteredtheirresearcharoundenergy homeostasis.Chenetal.foundthatMSC-derivedexosomescould improvebrainglucosemetabolismandcognitivefunctioninAD transgenicmiceusing 18F-FDGPET/CTimagingandNORtesting, respectively.183
Someteamshavefocusedonmicroglialneuroinflammation.In Zavatti’scellularstudy,itwasfoundthatamniotic fluidMSCderivedexosomescouldmitigateneuroinflammatorymicroglial phenotypeandrecoverneurotoxicityfromAβ usingLPSstimulatedBV2microgliaandSH-SY5Yneuroblastomacellsas models,respectively.184 Dingetal.showedthatumbilicalcord MSC-derivedexosomescouldalleviateneuroinflammationand reduceAβ depositionbymodulatingmicroglialactivation,thereby increasingspatiallearningandmemoryfunctioninADmice.185
Someteamshavefocusedonneuronalcelldeathand neurogenesishopingtocounteractADprogression.RezaZaldivarandco-workersgaveMSC-derivedexosomestoADmice andtheSC-exotherapystimulatedneurogenesisinthesubventricularzoneandalleviatedAβ-inducedcognitiveimpairment.186 TheseeffectsarecomparabletothoseshownintheMSCs.
SometeamshavefocusedontheBBB,thedysfunctionofwhich leadstoincreasedpermeability,microbleeds,impairedglucose transport,anddegenerationofpericytesandendothelialcells.187 Liuetal.indicatedthatBBBbreakdownin5xFAD(familial Alzheimer’sdisease)miceoccurredat4monthsofage,andmore importantly,treatmentwithNSCs-derivedexosomesreversedADcausedBBBdeficiency.188
Finally,somegroupshavefocusedonimprovingthetechnicality ofSC-exotherapyforAD.Whenexosomesareinjectedintravenously,theycouldbetrackedinotherorgansinsteadofthetargeted regionsinthebrain.Cuietal.conjugatedMSC-derivedexosomes withCNS-specificrabiesviralglycoprotein(RVG)totargetthemto thebrainoftransgenicADmice.189 Themodifiedexosomesnotonly exhibitedincreaseddeliverytothecortexandhippocampus,but alsosignificantlyimprovedlearningandmemorycapabilitieswith reducedAβ deposition.Ontheotherhand,Gaoetal.obtainediNSCs throughsomaticcellreprogramming,whichopenedanewwindow forsourcingtherapeuticexosomes.TheydemonstratedthatiNSCderivedexosomes,bearingcomparabletherapeuticeffectswith NSC-derivedones,couldmitigatevariousADphenotypes,e.g., cognitivefunction,Aβ deposition,neuroinflammation,andneuroregeneration,inapreclinicalmousemodel.190
Parkinson’sdisease
Parkinson’sdiseaseisthesecondmostcommonneurodegenerativediseaseamongtheelderly,affectingmorethan6million patientsworldwide.191 PDiscausedbythenecrosisofdopaminergicneuronsinthesubstantianigraandthepresenceofprotein
inclusionsnamedLewybodies.Themolecularpathophysiology includes α-synucleinproteostasis,mitochondrialdysfunction, oxidativestress,calciumimbalance,andneuroinflammation.192
InastudyusingbonemarrowMSC-derivedexosomes,Huang etal.discoveredthatexosome-derivedTNF-stimulatedgene-6 (TSG-6)couldattenuate1-methyl-4-phenylpyridiniumion(MPP + , metaboliteofaneurotoxinMPTP)-inducedneurotoxicity.Inthis invitroPDmodelusingSH-SY5YandSK-N-SHcells,theexosomal anti-PDprogressioneffectwasfoundtobemediatedthroughthe STAT3/miR-7/NEDD4axis.193
InastudyusingNSC-derivedexosomes,Leeetal.revealedthat SC-exotherapycouldhelptopreventtheneuropathologyand progressionofPD.194 WorkinginvitroonSH-SY5YandBV2cells, NSC-derivedexosomescouldreducetheintracellularreactive oxygenspecies(ROS)andassociatedapoptoticpathways.Working invivoon6-hydroxydopamine-inducedPDmice,NSC-derived exosomescoulddownregulatepro-inflammatoryfactorsand significantlyreducedopaminergicneuronalloss.Thepresenceof NSC-specificmicroRNAs,suchasmiR-182-5p,miR-183-5p,miR-9 andlet-7,wasconfirmedandfoundtobeinvolvedincell differentiation,neurotrophicfunction,andimmunemodulation.
Multiplesclerosis
Multiplesclerosis(MS)isthemostcommonnon-traumatic, neurodegenerative,anddisablingCNSdiseaseaffectingyoung adults.ThepathologicalhallmarkofMSistheformationof demyelinatinglesionsinthebrainandspinalcord,withan inflammatoryandautoimmuneinvolvement.195 Currentlylicensed disease-modifyingtherapiesincludeinterferon-based,immunomodulatory,immunosuppressive,andimmunereconstitution drugs.196 Afewpreliminarystudieshavehighlightedthepotential ofMSC-derivedexosomesforMStreatment.
Inananimalexperimentusingexperimentalautoimmune encephalomyelitis(EAE)rats,Lietal.showedthatSC-exotherapy significantlydecreasedneuralbehavioralscores,reducedthe infiltrationofinflammatorycellsintotheCNS,anddecreased demyelination.197 Inaddition,exosometreatmentupregulated M2-relatedcytokineswhiledownregulatingM1-relatedonesby regulatingthepolarizationofmicroglia.
Inananimalstudyusingtwomousemodelsofdemyelination (theEAEmodelandthecuprizonedietmodel),Zhangetal.found thatSC-exotherapycouldpromoteremyelinationbyactingboth directlyonoligodendrocyte(OL)progenitorsandindirectlyon microglia.198 MSC-derivedexosomescouldimproveneurological outcomes,increasethenumbersofnewlygeneratedandmature OLs,decreaseAβ precursorproteindensity,decreaseneuroinflammationbyshiftingfromM1toM2phenotype,andinhibitthe TLR2/IRAK1/NF-κBpathway.
Otherneurosurgicalandrelateddiseases
ThesurgicalpotentialofMSC-andNSC-derivedexosometherapyin fourmajortypesofneurosurgicalorneurologicaldiseaseshasbeen thoroughlydiscussedabove.Inadditiontovasculardisruption-, trauma-,neurodegeneration-,and autoimmune-relateddisorders, otherdiseaseshavebeenprovensuitabletargetsforSC-exotherapy recently(Table 3).Theseinclude,butarenotlimitedto:1,dementia, suchasvasculardementia.199 HIV-associatedneurocognitivedisorders200 andradiation-inducedcognitivedysfunction201,202;2, functionaldisorders,suchasepilepsy203 andmechanicalallodynia204;3,congenitalabnormalities,suchasspinabifidaaperta205;4, neuropsychologicalconditions,suchasdepression206 andstress207; 5,brainaging208–210;6,iatrogenicbrainproblems,suchasdeep hypothermiccirculatoryarrest.211
PLASTICSURGERYANDSC-EXOTHERAPY
Intheinflammatorystage,exosomescouldinhibitthe proliferationofperipheralbloodmononuclearcellsandpromote thetransformationofregulatoryTcellsinvitro,andreducethe numberoflymphocyticinfiltrationsintheskin.214 Inaddition, exosomescouldreduceIgE,eosinophilandmastcellcount,and downregulateinflammatorycytokines.215 Intheangiogenicstage, educatedexosomes(e.g.,atorvastatinanddeferoxamine)could promoteangiogenesisindiabeticwoundsviatheAkt/eNOSand PTEN/PI3K/Aktpathways.216–218 EPC-derivedexosomescould acceleratecutaneouswoundhealingbypromotingangiogenesis219 throughtheErk1/2pathway220 andp53pathway.221 Inthe proliferativestage,stemcell-derivedexosomescouldpromotethe proliferationandmigrationof fibroblastsandkeratinocytes.Some wereachievedthroughthePI3K/Akt222 Akt/HIF-1α223 ERK1/2224 andWnt/β-catenin225 pathways,whileothersthroughinhibitionof LATS2226 PPARγ 227 andAIFnucleustranslocation228 Inthe final remodelingstageofwoundhealing,granulationtissueisreplaced bypermanentscar,duringwhichabnormalwoundhealingmight occur(e.g.,keloidsandhypertrophicscars).MSC-derivedexosomescouldsuppress fibroblast-myofibroblasttransitionviathe TGF-β/Smad2pathway229,230 andincreasecollagensynthesisin earlystageandreduceinlatestage231 therebyreducingscar formation.
Furthermore,ESC-derivedexosomeswerefoundtoexertsimilar therapeuticeffectforwoundhealingtoMSC-derivedones.Chen etal.usedhumanESC-derivedexosomestohelphealingof pressureulcer.232 Theynoticedthatexosomescouldameliorate endothelialsenescencebyactivatingNrf2andrecoveragingrelatedangiogenicdysfunction,therebyacceleratingwound healing.232 Inaddition,Baeetal.revealedthattheexosomal mmu-miR-291a-3pfromESCscouldinhibitcellularsenescencein humandermal fibroblaststhroughtheTGF-β receptor2pathway, therebyacceleratingtheexcisionalskinwoundhealingprocess.233
Inadditiontowoundhealing,otherplasticsurgery-related diseaseshavebeenproventobesuitabletargetsforSC-exo therapy(Table 4).Theseinclude,butarenotlimitedto:1,skin grafting,suchasskin flaps234;2,tissueloss,suchascraniofacial defect235;3,autoimmuneskindiseases,suchasscleroderma236;4, skininfections,suchasleishmaniasis237;5,hairtransplantation, suchasforalopecia238;6,skinaging239
GENERALSURGERYANDSC-EXOTHERAPY
Asamajorsubspecialtyofgeneralsurgery,hepatobiliarysurgery hasattractedtremendousattentiontoSC-exotherapy.Firstly, acuteliverinjury(ALI)/acuteliverfailure(ALF)isararebut challengingsyndromemanifestedbyhepaticdysfunction,coagulopathy,encephalopathy,andmultiorganfailure.About60%of caseswithALFrequireandundergoorthotopiclivertransplantationorresultindeath.240 Inonestudy,Lin’steamfocusedonthe celldeathaspectofALI,andfoundthatMSC-derivedexosomes couldprotectagainstferroptosisviastabilizationofSLC7A11in carbontetrachloride-inducedALI.241 Alternatively,Shao’steam focusedonthepre-isolationmodificationoftheexosomes,and revealedthatexosomesderivedfromumbilicalcordMSCscould ameliorateIL-6-inducedALIthroughexosomalmiR-455-3p.242 Secondly,incontrasttoALI,liver fibrosisoccurswhentheliver sustainsachronicinjury,whichmayprogressintocirrhosis,liver failure,hepatocellularcarcinoma,andevendeath.243 Maetal. discoveredthatMSC-originatedexosomalcircDIDO1couldsuppresshepaticstellatecellactivationbymiR-141-3p/PTEN/Akt
Woundhealingoccursinallpartsofthehumanbody,with cutaneouswoundsbeingthemostcommon.Thehighestwoundrelatedexpenseswereattributedtosurgicalwoundsfollowedby diabeticulcers.212 Theoverallbutoverlappingphasesofwound healingincludehemostasis,inflammation,angiogenesis,proliferationandremodeling,eachofwhichisgovernedbydistinctcell typesandmodulatedbyvarioussignalingpathways.213 Morethan halfofrelevantworkusingSC-exotherapytoboostcutaneous woundhealingisMSC-based(Table 4).
Table4. Stemcell-derivedexosomesforthetreatmentofdiseasesinplasticsurgeryandrelatedspecialties
TargetdiseaseExosomeInvitromodel& fi ndingsInvivomodel& fi ndingsRefs.
214
eczemamousemodel;acceleratedwoundclosurewithmorenewepidermis& dermisandlessscar;reducedintegralscoreofskininjuryandno.of lymphocytein fi ltrationinskin
WoundhealingUC-MSC-exoPBMCs;inhibitedcellproliferation,promotedTreg transformation&formationofendothelialtube
215
adipose-MSC-exoN/AHDM-inducedmousemodel;reducedIgE,eosinophil&mastcellcount,and downregulatedin fl ammatorycytokines
218
221
educatedBM-MSC-exoHUVECs;promotedangiogenesisviaAkt/eNOSpathwaymice;promotedcutaneouswoundhealing
streptozotocin-induceddiabeticwoundratmodel;exosomalmiR-221-3p facilitatedwoundrepairbyenhancingangiogenesisviaAkt/eNOSpathway
216 atorvastatin-treatedBM-MSC- exo HUVECs;promotedproliferation,migration,tube formation,andVEGFlevel
streptozotocin-induceddiabeticwoundratmodel;acceleratedcutaneous woundhealingbypromotingangiogenesis
deferoxamine-stimulatedBM- MSC-exo HUVECs;activatedthePI3K/AktpathwayviamiR-126- mediatedPTENdownregulation
streptozotocin-induceddiabeticwoundratmodel;acceleratedcutaneous woundhealingbypromotingangiogenesisviaErk1/2pathway
EPC-exoHMECs;enhancedproliferation,migration&tubule formation
EPC-exoN/Astreptozotocin-induceddiabeticwoundmousemodel;exosomalmiR-221-3p acceleratedcutaneouswoundhealingviap53pathway
adipose-MSC-exoHDFs;improvedproliferation&migrationmice;promotedwoundhealingviaPI3K/Aktsignalingpathway
225
mice;promotedwoundhealing,whichwaseliminatedbyinhibitionofp Akt andHIF
N/A
adipose-MSC-exoHaCaTcells;promotedproliferation&migrationby activatingAkt/HIF-1 α pathway
iPSC-exo,MSC-exoHDFs,HaCaTcells;acceleratedproliferationviaERK1/2 pathway
N/A
MALAT1-adipose-MSC-exoHaCaTcells&HDFs;promotedwoundhealingbymiR- 124viaWnt/ β -cateninpathway
MSC-exoBJcells;promoted fi broblastsmigrationrats;exosomalmiR-135apromotedcutaneouswoundhealingbyinhibiting LATS2expression
228
diabeticmice;acceleratedwoundhealingviadownregulatingPPAR γ
EPC-exoHaCaTcells;promotedproliferation&migration,and inhibitedapoptosis
mice;attenuatedfull-thicknessskinwoundingbyenhancingepidermalre- epithelializationanddermalangiogenesisviasuppressingAIFnucleus translocation
UC-MSC-exoH 2 O 2 -treatedHaCaTcells;increasedproliferation& migration,andsuppressedapoptosis
UC-MSC-exoN/Amice;suppressedmyo fi broblastdifferentiationbyinhibitingTGFβ /Smad2 pathwayduringwoundhealing;miR-21,-23a,-125b,-145responsiblefor preventingscarformation
N/A
UC-MSC-exoHDFs;suppresseddermal fi broblasts-myo fi broblasts transitionviaTGFβ /Smad2/3pathway
mice;exorecruitedtowoundarea,andacceleratedcutaneouswoundhealing; increasedcollagenI&IIIinearlystageandinhibitedcollageninlatestageto reducescarformation 231
adipose-MSC-exoprimaryHDFs;stimulatedproliferation,migration,and collagensynthesis
232
D-galactose-inducedagingmice;exosomalmiR-200aacceleratedwound closureandenhancedangiogenesisviaNrf2activation
ESC-exoHUVECs;amelioratedsenescence,proliferation,and migration
233
ESC-exoHDFs;inhibitedcellularsenescenceviaTGFβ receptor2 pathway mice;exosomalmmu-miR-291a-3pacceleratedexcisionalskinwoundhealing process
234
I/Rinjuryratmodel;increased fl apsurvival&capillarydensity,anddecreased in fl ammatoryreaction&apoptosis;H 2 O 2 -conditionedexowerebetter
fl apadipose-MSC-exoHUVECs;increasedcellproliferation,migrationwithmore cord-likestructures
Skin
235
CraniofacialdefectSCAP-exoHUVECs;improvedangiogeniccapacityandcell migration mice;promotedcraniofacialsofttissueregenerationbyenhancingCdc42- mediatedvascularization
SclerodermaUC-MSC-exoN/A
broblastactivationandcollagendepositionindermal
mice;attenuatedmyo
β /Smadsignalingpathway
fi brosisbydownregulatingtheTGF-
237
L.majorpromastigotes&amastigotes;inhibitedfor4 –10folds;combinations superiortoexoalone
fi cialwoundmodel;healed72% woundin24h
LeishmaniasisUC-MSC-exo + Aloe-EmodinL929&J744cells,arti
depilation-inducedmicehairregenerationmodel;promotedhairfollicle growthbyactivatingWnt/ β -cateninpathway
AlopeciaNSC-exodermalpapillacells;exosomalmiR-100promotedcell proliferation
238 AIF apoptosis-inducingfactor, Akt proteinkinaseB, BM bonemarrow, eNOS endothelialnitricoxidesynthase, EPC endothelialprogenitorcell, ESC embryonicstemcell, exo exosome, HDF humandermal fi broblasts, HDM housedustmite, HIF hypoxia-induciblefactor, HMEC humanmicrovascularendothelialcell, HUVEC humanumbilicalveinendothelialcell, I/R ischemia-reperfusion, LATS largetumorsuppressor, MALAT metastasisassociatedlungadenocarcinomatranscript, miR microRNA, MSC mesenchymalstemcell, NSC neuralstemcell, PBMC peripheralbloodmononuclearcell, PI3K phosphoinositide3-kinase, PTEN phosphatase&tensinhomolog, SCAP stemcellsfromapicalpapilla, TGF transforminggrowthfactor, Treg regulatoryTcell, UC umbilicalcord, VEGF vascularendothelialgrowthfactor
pathwayinhumanliver fibrosis.244 Inaddition,Wangetal.found thatexosomesderivedfrom3DhumanESCspheroidscould attenuatehepaticstellatecellactivationandinhibitliver fibrosis throughinactivationoftheSmadpathwaybyexosomalmiR-67663p.245 Forthosewithend-stageliver fibrosisneedingaliver transplant,liverischemiareperfusioninjury(IRI)isaserious complicationforgraftdysfunctionandorganrejection.246 Yang etal.demonstratedthatbonemarrowMSC-derivedexosomes couldrelievehepaticIRI,reducehepatocyteapoptosis,and decreaseliverenzymelevelsbyenhancingautophagy.247 Du etal.showedthatexosomesfromiPSC-derivedMSCscould protectliveragainsthepaticIRIviaactivatingsphingosinekinase andthesphingosine-1-phosphatepathway.248 Thirdly,nonalcoholicfattyliverdisease(NAFLD)isknowntoadverselyaffect strokerecovery.Usingatype2diabetesmellitusmousemodel, Venkatetal.demonstratedthatHSC-derivedexosomescould simultaneouslyreduceliverdysfunctionandimproveneurological andcognitivefunction.249 Lastly,acutepancreatitisisan unpredictableandpotentiallylethaldisease,theprognosisof whichmainlydependsonwhetheritdevelopsintomultipleorgan dysfunctionsyndrome.250 Chenetal.revealedthatexosomesfrom iPSC-derivedMSCscouldimprovemyocardialinjurycausedby severeacutepancreatitisthroughtheAkt/Nrf2/HO-1pathway.251 Peripheralarterydiseaseaffects200millionpatientsworldwide and,initsmostseverestage,cancausecriticallimbischemia, subjectingpatientstoincreasedriskofcardiovascularevents, amputationanddeath.252 Asacell-freetherapy,placentaMSCderivedexosomeinfusioncouldenhanceangiogenesisina murineauricleischemicinjurymodelusinglaserDopplerblood flowanalysis.253 Mechanistically,MSC-derivedexosomesnotonly promotetube-likestructureformationinvitro,butalsomobilize endothelialcellsintosubcutaneousMatrigelpluginvivo,mainly throughexosomalpro-angiogenicmicroRNAs,suchasmiR-30b.254 Inaddition,HSC-derivedexosomescouldrepairischemichindlimb inmicebyimprovinglimbperfusion,capillarydensity,motor functionandtheiramputation.255 Thiswasmostlikelycausedby internalizationofexosomalmiR-126-3pbyendothelialcells relativetosmoothmusclecellsand fibroblasts.HumaniPSCderivedexosomesdemonstratedsimilarneoangiogeniceffect throughtheexosomalmiR-199b-5p.256 Ontheotherhand, endovascularre-canalizationisincreasinglybeingusedto reestablishblood flowtoischemicareasandrestoretissueloss organgreneforpatientswithperipheralarterydisease.257 Three independentteamsallprovedthatEPC-derivedexosomescould promotevascularrepairandacceleratereendothelializationinrat modelsofballoon-inducedvascularinjurybyenhancingendothelialcellfunction.258–260 Inaddition,Kongetal.demonstrated similarprotectiveeffectofEPC-derivedexosomesagainstballoon injurybyinhibitingneo-intimalhyperplasia.261 Thiswasachieved throughpromotionofreendothelializationandsuppressionof restenosisratherthanthroughthedirectinhibitionofproliferation andmigrationofsmoothmusclecells.
McCullohandhisco-workerspublishedanintriguingstudyon theSC-exotherapyfornecrotizingenterocolitis(NEC)whichhas anoverallmortalityofover30%forprematureinfantsrequiring surgery.262 Theauthorscomparedthetherapeuticeffectof exosomesderivedfromfourdifferenttypesofstemcells,i.e., amniotic fluidMSCs,bonemarrowMSCs,amniotic fluidNSCsand neonatalentericNSCs.263 Wheninjectedataconcentrationofat least4×108,alltypesofSC-exowereshowntoreducethe incidenceandseverityofexperimentalNECaseffectivelyastheir parentalstemcells.
Sepsisisadeadlyandpotentiallypreventablecomplicationin generalsurgery,inwhichmicrovasculardysfunctionleadsto multi-organfailureandmortality.264 Usingamurinesepsismodel bycecalligationandpuncture(CLP),Zhouandco-workers demonstratedthatEPC-derivedexosomescouldimprovesepsis outcome.265 Thiswasmanifestedbyreducedlungandrenal Table4.
vascularleakage,improvedorganfunction,andincreasedsurvival throughtheexosomalmiR-126-5pandmiR-126-3p.Similarly,Liu etal.exhibitedprotectiveeffectofEPC-derivedexosomeson sepsis-inducedorgandamageandimmunesuppressionbythe exosomalmiR-382-3pthroughtheIκBα/NF-κBpathway.266
CARDIOTHORACICSURGERYANDSC-EXOTHERAPY
Theworld’sleadingmortalityisischemicheartdisease(IHD)which isprimarilycausedbyobstructivecoronaryatherosclerosis.The ruptureofanatheroscleroticplaqueisthemostcommontrigger ofacutearterialthrombosiscausingmyocardialinfarction(MI). Prolongedoxygendeprivationtothemyocardiumcanleadto cardiomyocytedeath.Althoughtimelyreperfusionisessential, myocardialIRImightoccur,thusmitigatingthebeneficialeffects ofreperfusion.Despitemoderncoronaryreperfusion,themortality andmorbidityassociatedwiththedevelopmentofheartfailureas aconsequenceofacuteMIremainsubstantial,highlightingthe importanceofnext-generationcardioprotectivetherapies,suchas SC-exo.267
TheworkconductedbyXingetal.focusedonatherosclerosis. TheadiposeMSC-derivedexosomalmiR-342-5pwasshownto protectendothelialcellsagainstatherosclerosisbytargeting PPP1R12BinaH2O2-challengedHUVECmodel.268 Thework conductedbyPengetal.andGaoetal.focusedonMI.The exosomalmiR-25-3pfromMSCscouldalleviateMIbytargeting pro-apoptoticproteinsandEZH2inanOGDcardiomyocytes modelandleftanteriordescendingarteryligationanimal model.269 Similarly,humaniPSC-derivedexosomescouldimprove recoveryfromMIwithoutincreasingthefrequencyofarrhythmogeniccomplicationsinaswinemodel.270 Theworkconductedby Wenetal.andSantosoetal.focusedonthedeathof cardiomyocytes.MSC-derivedexosomescouldamelioratecardiomyocyteapoptosisinhypoxicconditionsthroughmiR-144by targetingthePTEN/Aktpathway271 whereasiPSC-derivedexosomescouldregulateautophagyinhypoxiccardiomyocytes.272 BothKatsuretal.andChenetal.focusedonmyocardialIRI.The formerteamfoundthatexosomesderivedfromnoncardiomyocyte-relatedcells,i.e.,CTX0E03NSCs,couldreduce infarctsizewhiledelayingcardiomyocytemitochondrialpermeabilitytransitionporeopeningthroughtheJAK/STATpathway.273 ThelatterteamdiscoveredthatMSC-derivedexosomalmiR-1433pcouldsuppressmyocardialIRIbyregulatingautophagyviathe CHK2-Beclin2pathway.274 Finally,Chenandco-workersproved thatbonemarrowMSC-derivedexosomescouldattenuatecardiac hypertrophyand fibrosisinpressureoverload-inducedremodeling,therebyprovidingapromisingpotentialtreatmentforheart failure.275
IncomparisontoMSC-derivedexosomes,exosomesderived fromESCsexhibitedcomparabletherapeuticeffectforcardiac conditions.Intermsofprotectionofcardiomyocytes,Khanetal. discoveredthattheESC-derivedexosomalmiR-294couldimprove cardiomyocytesurvival,promoteneovascularizationandinhibit fibrosisafterMI,therebyaugmentingpost-MIcardiacfunction.276 Inaddition,TavakoliDargagnietal.demonstratedthatESCderivedexosomescouldalleviatedoxorubicin-inducedcardiotoxicitybyinhibitingTLR4-NLRP3-mediatedpyroptoticcelldeathin cardiomyocytes.277 Similarly,Singla’steamshowedthatESCderivedexosomescouldimprovecardiacremodelingbyenhancinganti-inflammatoryM2macrophagesandreducing inflammation-inducedpyroptosis.278 Intermsofmanagementof heartfailure,Pangetal.exhibitedthatESC-derivedexosomes couldattenuateheartfailure,improvecardiacfunctionand promotemyocardialangiogenesisthroughtheFGF2signalingin atransverseaorticconstriction-inducedheartfailuremodel.279 Usingacoronaryarteryocclusion-inducedheartfailuremodel, Kervadecetal.showedthatexosomessecretedbyESC-derived cardiovascularprogenitorscouldrecovercardiacfunctionssuchas
reducedleftventricularend-systolicandend-diastolicvolumes.280 Finally,thesameresearchgrouplaterdemonstratedthat exosomesderivedfrommorereadilyavailablecellsources,e.g., iPSCs,werecapableofcardioprotectiveeffectssimilartothose offeredbyESC-derivedones.281
OtherimportantsubtypesofSC-Exo,suchasiPSC-exo,HSC-exo andEPC-exo,havealsoexhibitedcardio-protectiveeffects.For example,exosomessecretedbyiPSCscouldexertcytoprotective effectsonmaintainingintracellularCa2+ homeostasisand promotingcardiomyocytesurvival,therebyimprovingrecovery fromMI.282 HSC-exocouldreducethecardiacinjury-related indicesandthedegreeofcardiac fibrosiswhileelevatingthe ejectionfractioninananimalmodelofheartfailure.283 Inaddition, systemicinfusionofHSC-derivedexosomescouldimprove ischemiccardiomyopathyinaratmodelofacuteMI,with additionalbenefitsintreatingthesideeffectssuchaskidney damage.284 ModificationofHSCsusingsonichedgehog(Shh),an angiogenicfactor,couldpreservecardiacfunctionafteracuteMI bydeliveryofexosomalShhtoischemicmyocardium.285 Keetal. provedthatEPC-derivedexosomescouldenhancetheproliferationandangiogenesisofcardiac fibroblastsbyactivating mesenchymal-endothelialtransitionanddecreasingtheexpressionofHMGB1286 andlaterrevealedthattheexosomalmiR-2185pandmiR-363-3pfromEPC-derivedexosomescouldameliorate MIbytargetingthep53/JMYpathway.287 Inaninterestingstudy byYueetal.,IL-10deficiency-inducedsystemicinflammationwas foundtocompromisethereparativepropertiesofEPC-derived exosomesonmyocardialrepairbyupregulatingintegrin-linked kinase(ILK)enrichmentinexosomes,andILK-mediatedactivation ofNF-κBpathwayinrecipientcells.288
Intermsoftreatmentofthoracicdisorders,Liuetal.provedthat humanESCs-derivedexosomescouldalleviateinflammation, preventexcessivecollagendepositionandpreservealveolar architectureinthelungsofmicewithbleomycin-induced pulmonary fibrosis.289 Thiswasachievedbytheexosomalmi-175ptargetingthrombospondin-2.Similarly,Zhouetal.demonstratedthattheexosomalmiR-302a-3pfromiPSC-derived exosomescouldsuppressM2macrophagesviaTET1,thereby mitigatingpulmonary fibrosis.290 Liuetal.showedthatEPCderivedexosomescouldinhibitpulmonaryarterysmoothmuscle cellsproliferationandtheirresistancetoapoptosisbyregulating theMitofusin-2andRas-Raf-ERK1/2pathways,therebyactingasa potentialtherapeuticcandidateforthetreatmentofpulmonary arterialhypertension.291 Twoindependentteamsbothrevealed thathumanEPC-derivedexosomescouldimproveoutcomesof theLPS-inducedacutelunginjurypartiallythroughthedeliveryof miR-126intotheinjuredalveolus.292,293 Zhangetal.foundthat EPC-derivedexosomescouldimprovethebioactivityofpulmonarymicrovascularendothelialcellsandprotectthemfrom hyperoxicinjuryinthedevelopinglungvasculature,thereby contributingtothetreatmentofbronchopulmonarydysplasia.294 Moreover,Montay-Grueletal.demonstratedthathumanESCderivedexosomescouldimprovetheadverselatenormaltissue complicationsassociatedwithexposureofthelungstoionizing radiation,suchasthoseencounteredduringpostoperative treatmentoflungcancer.295
UROLOGYANDSC-EXOTHERAPY
Chronickidneydisease(CKD)isasyndromecharacterizedby persistentchangesinkidneystructure,function,orboth,affecting 10 14%oftheglobalpopulation.296 Themostcommon pathologicalfeatureand finalmanifestationofCKDissomeform ofrenal fibrosis.Kidney fibrosisoccurswhenwoundhealingis deregulated,whichleadstoexcessiveaccumulationofECM proteins,suchascollagenand fibronectin.Intheirstudy,Liuetal. discoveredthatbonemarrowMSC-derivedexosomescould alleviatevascularcalcification,adetrimentalindicatorofmorbidity
andmortalityinCKD.297 ThiswasachievedthroughexosomalmiR381-3pbytargetingNFAT5,whichwasfurtherverifiedinsevere arterialcalcificationindialysispatients.Inalternativestudies, severalgroupsdemonstratedthecapacityofbonemarrowMSCderivedexosomesintreatingrenal fibrosis,eachwithadistinct mechanisticinterpretation.Inacellularstudy,Yinetal.foundthat exosomescouldpreventTGF-β1-inducedepithelial-mesenchymal transitionofrenaltubularepithelialcellsbytransportingNedd4L, whichactivatesautophagy.Ina5/6subtotalnephrotomyrat model,Liuetal.revealedthatexosomescouldimproverenal functionandreduce fibroticsizebyregulatingtheSmurf2/Smad7 axis.298 Inaunilateralureteralocclusion-inducedinterstitial fibrosis mousemodel,Luetal.demonstratedthatexosomescould improverenal fibrosisbyreducingthepolarizationofM1andM2 macrophagesbyactivatingEP2receptors.299
Acutekidneyinjury(AKI)andCKDarecloselyconnected,with eachariskfactorfordevelopingtheother.RenalIRIisaleading causeofAKIandacutekidneyfailure.300 Limetal.provedthat exosomesfromiPSC-derivedMSCscouldcorrectserumcreatinine level,tubularnecrosis,apoptosis,inflammatorycytokineproduction,andoxidativestressinAKImicebyactivatingtheERK1/ 2signalingpathway.301 InasimilarworkbyZhangetal.,the exosomalmiR-21-5pfromEPCswerefoundtoalleviatesepsisinducedAKIbyinhibitingRUNX1expressioninCLPrats.302
OTORHINOLARYNGOLOGYANDHEAD&NECKSURGERYAND SC-EXOTHERAPY
Hearinglossisthemostcommonsensoryde fi citworldwide, affectingnearly20%oftheglobalpopulation. 303 Thecausesof sensorineuralhearingloss(SNHL)canbeverydiverse,suchas presbycusis,ototoxicmedicati on-induced,noise-induced,and idiopathicsuddenSNHL.Tsai ’ steamdemonstratedthatumbilicalcordMSC-derivedexosomescouldrescuethelossofouter haircellsandrepaircochleardamageincisplatin-induced hearingloss. 304 TheunderlyingmechanismforthecochleaprotectiveeffectsismediatedbythemiRNAs(e.g.,miR-125a-5p andmiR-125b-5p)andremodelingfactors(e.g., fi bronectinand galectin-3).
CochlearIRIisoneofthemainreasonsforidiopathicsudden andnoise-inducedSNHL,whichcanleadtoirreversibledamageof sensoryhaircellsandbipolarcochlearspiralganglion.305 Its pathophysiologyincludesoxidativestress,excesscelldeathand dysregulatedinflammation.Haoetal.discoveredthatexosomes derivedfrommiR-21-overexpressingNSCscouldpreventhearing lossfromIRIbyinhibitingtheinflammatoryprocessinthemouse cochlea.306 Thiswasevidencedbyareducedauditorybrainstem responsethreshold,upregulatedIL-10anddownregulatedTNF-α andIL-1β.
Hypothyroidismisaverycommondiseasewhichcouldresult fromthyroidectomyorradioactiveablationtotreathyperthyroidismorthyroidcancer.307 Stemcelltherapyandstemcell-derived exosometherapyhaveemergedasapromisingmanagementfor hypothyroidismthroughthyroidregeneration.Usinganinvitro culturesystemofthyroidlobes,Degosserieetal.suggestedthat EPC-derivedexosomescouldfacilitatethyrocyteorganizationinto thyroidfolliclesandlumenexpansion(i.e.,folliculogenesis),which waspromotedbylaminin-α1.308
Temporomandibularjoint(TMJ)disordersarethesecondmost commonmusculoskeletalconditionaffecting31%ofadultsand 11%ofchildren.309 Likeothersynovialjointsinthebody,TMJis alsopronetoOA,sharingcommonpathophysiologicalprocesses (Section3.2).Zhangandco-workersprovedthatMSC-derived exosomescouldalleviateTMJOAinanimmunocompetentrabbit modelbyattenuatinginflammationandrestoringmatrixhomeostasis.310 Theexosome-mediatedjointrepairwasattributedto adenosineactivationofAkt,ERKandAMPKsignaling,aswellas enhanceds-GAGsynthesis.
OPHTHALMOLOGYANDSC-EXOTHERAPY
Acquiredopticneuropathyisamajorcauseofblindnessinadults, andhasvariousetiologies,suchasvascular,inflammatory, traumatic,toxic,compressive,andnutritionaletiologies.Retinal ganglioncell(RGC)lossisthehallmarkofopticneuropathies,via multiplecelldeathpathways.311 MeadandTomarevfoundthat bonemarrowMSC-derivedexosomescouldpromotesurvivalof RGCs,andregenerationoftheiraxons,inaratopticnervecrush model.312 Theexosomalneuroprotectiveandneuritogeniceffects wereaccomplishedthroughexosomalmiRNAs,demonstratinga cell-freepotentialfortraumaticanddegenerativeoculardiseases. Retinaldegenerativediseases(e.g.,age-relatedmaculardegenerationandretinitispigmentosa)aretheleadingcauseofbilateral irreversiblevisionlossworldwide.313 Itischaracterizedby progressivedegenerationofphotoreceptors,RGCsorretinal pigmentepithelium(RPE)cells.Bian’steamdiscoveredthat exosomesderivedfromNSCscouldpreservephotoreceptors, visualfunctionandpreventthinningoftheouternuclearlayerin anRCSretinaldegenerationratmodel.314 Thiswasachievedby markedinactivationofmicroglialinflammationviaexosomal targetingofTNF-α,IL-1β andCOX-2.Intwoconsecutivestudies, Gao’steamshowedthatESC-derivedexosomescouldalleviate retinaldegenerationbyenhancingtheproliferationandretrodifferentiationofretinalMüllercellsasreplacementretinal neuronalprecursors.Ononehand,thiswasaccomplishedby regulatingtheexpressionofOct4inMüllercellsthroughexosomal HSP90.315 Ontheotherhand,activationoftheWntsignaling pathwaybydeliveringBDNFproteintoMüllercellsalsoplayedan importantrole.316 Furthermore,usingaratmodelofinherited retinaldegeneration,Parketal.demonstratedthatbothsubretinal andintravitrealinjectionofhumanHSC-derivedexosomescould providefunctionalrescueofadegeneratingretina.317
Failedhealingofcornealdefectoftenleadstocornealblindness whichhasbeenreportedassecondonlytocataractintheleading causesofblindness.318 Themostsevereandrecalcitrantcases wouldneedcornealtransplantation.Wangetal.compared exosomesderivedfromiPSCsandMSCsastherapeuticproviders forthetreatmentofcornealepithelialdefects.319 Itwasfoundthat bothtypesofexosomescouldpromoteproliferation,cellcycle progressionandmigrationwhileinhibitingapoptosisinvitro,and acceleratecornealepitheliumdefecthealinginvivo.More importantly,theiPSC-derivedexosomeshadastrongertherapeuticeffectthantheMSC-derivedexosomes.
Cornealtransplantationisoneofthemostsuccessfulformsof solidorgantransplantation.However,graftrejectioncanoccurin upto90%ofhigh-riskrecipients.320 Bothinnateandadaptive immunityarethepredominantreasonforgraftfailure.Immunosuppressivedrugshaveshownonlypartialeffectiveness.Jiaetal. showedthatMSC-derivedexosomescouldcrossbiological barriersandprolonggraftsurvivaltimeinaratmodelofcorneal allograftrejection.321 Thiswaslikelycausedbyinhibitionofthe infiltrationofCD4+ andCD25+ TcellsandthereductionofIFN-γ andCXCL11viatheTh1signalingpathway.
OBSTETRICSANDGYNECOLOGYANDSC-EXOTHERAPY
Primaryovarianinsufficiency(POI),orprematureovarianfailure (POF),isdefinedaslossofovarianfunctionbeforetheageof40. Non-geneticcausesofPOIincludeautoimmunedisorders, metabolicconditions,infections,andiatrogenicprocedures(e.g., chemotherapy,radiotherapy,andsurgery).WomenwithPOIsuffer fromvariouscomplications,suchasosteoporosis,infertility, cardiovasculardisordersanddepression.322 Althoughpromptly initiatinghormonereplacementtherapyiscriticaltocontrolthese symptomsandcomplications,itfailstorestoreovarianfunction. Currently-testedexperimentaltherapiesincludemitochondrial activation,invitroactivation,stemcelltherapy,andexosome therapy.323
TheworkconductedbyLietal.showedthatumbilicalcord MSC-derivedexosomescouldimproveovarianfunctionina cyclophosphamide(CTX)-inducedPOImousemodel.324 TheSCexotherapynotonlyrestoredovarianfunction-relatedhormone levelsandthenumberofovarianfollicles,butalsoimprovedthe reproductiveabilityofPOImice.Inaddition,theexosomes promotedtheproliferationofovariangranulosacells(GCs)by regulatingtheHippopathway,andtheeffectwasneutralizedbya YAPinhibitor.Similarresultswereobtainedusingexosomesfrom iPSC-derivedMSCs.325 TheworkperformedbyDingetal. illustratedthatumbilicalcordMSC-derivedexosomescould restoreovarianphenotypeandfunctioninaPOImousemodel, promoteproliferationofCTX-damagedhumanGCsandoocytes, andalleviateROSaccumulationbydeliveringexosomalmiR-17-5p andtargetingitsdownstreammRNASIRT7.326 Itwasfurther elucidatedthatmiR-17-5pdown-regulatedPARP1, γH2AX,and XRCC6expressionbyinhibitingSIRT7.Lastly,theworkcompleted byYangetal.revealedthatbonemarrowMSC-derivedexosomes couldrecovertheestruscycle,increasethenumberofbasaland sinusfollicles,increaseestradiolE2andanti-Mullerianhormone levels,andreducefolliclestimulatinghormoneandluteinizing hormonelevelsinachemotherapy-inducedPOFratmodel.327 Mechanistically,thiswasachievedbyexosomalmiR-114-5pthat targetsPTEN.
FROMPRECLINICALSTUDIESTOCLINICALTRIALSOF EXOSOMETHERAPY
Manypreclinicalstudies,asdiscussedinSections3to11,have confirmedtheadvantagesofMSC-derivedandNSC-derived exosomestotreatmanydiseasesspanningthesubspecialtiesof surgicalpractice.Withoutrestrictingthescopetostemcellderivedexosomesonly,manyclinicaltrialshavedemonstratedthe roleofexosomestobetwofold:biologicalmarkersand therapeuticagents.AsearchonClinicalTrials.govusing ‘exosome therapy’ , ‘exosometreatment’,and ‘exosome’ askeywords generated188records.However,only60(32%)ofthesedirectly relatetointerventionalstudiesusingexosomesastherapeutic agents(Table 5).Therest,especiallyoncology-relatedtrials,mostly usedexosomesasbiomarkers,suchaskeyplayersduringdisease pathogenesis(e.g.,NCT04288141,NCT04154332),diagnostic markersandguidancebeforetreatment(e.g.,NCT04629079, NCT03791073,NCT05451342,NCT03432806),monitoringindices andpredictivetoolsfortreatmentefficiency(e.g.,NCT05427227, NCT04499794,NCT04852653,NCT05370105,NCT03800121, NCT05370105,NCT05328089),andprognosticindicatorsafter treatment(e.g.,NCT06026735,NCT05705583,NCT05411445, NCT04167722,NCT05575622).Theclinicalapplicationsofexosomesasbiomarkershavebeenextensivelyexploredinother reviews328–332 andarethereforebeyonddiscussioninthisreview. Intermsoftheclinicalcharacteristicsofthe60clinicaltrialson exosometherapy(Table 5),thereareseveralhighlightsworth mentioning.
Firstly,thespectrumofdiseasescoveredisverybroad,asboth surgicalandmedicalconditionsareincluded.Theseincludemany surgicaldisordersdiscussedinSections3to11,suchasorthopedic diseases(osteoarthritisofthekneeinNCT05060107,boneloss inNCT04998058,andintervertebraldiscdegenerationin NCT04849429),neurosurgicaldiseases(ischemicstrokein NCT03384433andAlzheimer’sdiseaseinNCT04388982),plastic surgicaldiseases(cutaneouswoundhealinginNCT02565264and NCT05475418),generalsurgicaldiseases(livercirrhosisin NCT05871463),cardiothoracicdiseases(myocardialinfarctionin NCT05669144),andophthalmologydiseases(retinitispigmentosa inNCT05413148).Inotherwords,somepreclinicalstudieshave notyetdevelopedintoclinicaltrials.Theseinclude,butarenot limitedto,exosometherapyforfracture333 spinalcordinjury334 traumaticbraininjury335 acuteliverinjury336 andhearing
loss304,306 whichmightserveasfuturedirectionsforexosome therapy-relatedclinicaltrials.
Secondly,anincreasingnumberofclinicaltrialsfocusedontwo medicalconditions,i.e.,COVID-19(16trials,27%)andcancer(5 trials,8%).MSC-derivedexosomescanmanageviralinfectionand lungdamageinCOVID-19throughbothreparativeactionsand regenerativeeffects.337 Theformermanifestsasblockageofviral entryandreplication,andsuppressionofthecytokinestorm, whereasthelatteraspreventionofinflammation,and fluidclearanceandrestorationoflungpermeability.Inaddition, exosomescanbeengineeredintoadrug(e.g.,CD24,apotent immuneregulator)deliveryplatform338 andevenavaccine339 to combatCOVID-19.IncontrasttotheMSC-derivedexosomesfor COVID-19management,exosomesusedforcancertreatment mainlyrelyonnon-stemcellsandcargoengineering.Thisis partiallybecauseMSC-derivedexosomesdemonstratecontroversialeffectsontumorigenesisandmetastasis.340,341 Althoughthe exactinteractionbetweenMSC-derivedexosomesandtumorcells remainsopentodebate,scientistshaveovercomeseveral obstaclesbymodifyingexosomalcargostodeliverantioncogenic nucleicacidsandanticancermedications,andexosomalmembranesforspecifictumortargeting.342–346
Finally,amongthe40clinicaltrialsusingstemcell-derived exosomesfordiseasetreatment,38(95%)usedMSCand2(5%) usediPSCasthecellularsourceforexosomes.However,thisdiffers significantlyfromthepreclinicalstudiesdiscussedinSections3to 11.ThelackofuseofNSC-derivedexosomesinclinicaltrialsmight bepartiallybecauseofthesupplyconstraintsoftheirparental cells.347 Currently,NSCscanbeobtainedfromthreesources348 (Fig. 1a):1,isolationfromprimaryCNStissues(e.g.,adultandfetal brain);2,differentiationfrompluripotentstemcells(e.g.,iPSCsand ESCs);3,reprogrammingofsomaticcells(e.g., fibroblastsand bloodcells)toiNSCs.349 Recentstudieshavedeveloped fibroblastderivediNSCs,openinganewwindowforobtainingexosomes fromNSC-likecells.TheseiNSC-exocouldnotonlypromotecell survivalandproliferationnolessthanNSC-exoinvitro350,351 but alsoenhancerecoveryafterischemicstroke148 andmitigateADlikephenotypesinpreclinicalmodels.190 Therefore,iNSCsmight beanexcellentcellularsourcetoproduceclinical-gradeexosomes inclinicaltrials.
Insummary,theprogressionfrompreclinicalstudiestoclinical trialsofexosometherapyhasbeenexpeditious.However,issues likeinsufficientclinicalindicationsforexosometreatmentand limitedsourcesforparentalstemcellsremaintobeaddressed.In addition,oncethelimitations(Table 1)inupscalingofmanufacturing,compliancewithgoodmanufacturingpractice,and regulatoryframeworkareovercome329 stemcell-derivedexosome therapywillsoonbeincorporatedintoclinicalpracticeandserve atthepatient’sbedside.
CONCLUSIONANDFUTUREPERSPECTIVES
Exosomeshavebeenpursuedrecentlyasacell-freealternativeto stemcell-basedtherapy.ESC-,iPSC-,HSC-,MSC-,NSC-andEPCderivedexosomesareofparticularinterest,partiallyduetothe pluripotencyormultipotencyoftheirparentalcells.Aftergoing throughproductionandpurificationwithorwithoutmodification, stemcell-derivedexosomeshavedemonstratedtremendous potentialintreatingnumerousdiseasesencounteredduring surgicalpractice.Theseareexemplifiedbydisordersinorthopedic surgery(e.g.,fracture,osteoarthritis,andspinalcordinjury); neurosurgery(e.g.,ischemicstroke,traumaticbraininjury,and Alzheimer’sdisease);plasticsurgery(e.g.,woundhealing);general surgery(e.g.,acuteliverinjury);cardiothoracicsurgery(e.g., myocardialinfarction);urology(e.g.,chronickidneydisease);head andnecksurgery(e.g.,sensorineuralhearingloss);ophthalmology (e.g.,acquiredopticneuropathies),andgynecology(e.g.,primary ovarianinsufficiency).Mechanistically,thediversetherapeutic
Table5. Clinicaltrialsofexosometherapy
CategoryofconditionsSpecificdiseaseNCTnumberSourceofexosomeGenderAgePhasesEnrollmentCountry
Behavior&mental disorders depression&anxietyNCT04202770MSCall>18N/A300USA
Blood&lymphconditionscoagulopathyNCT02594345redbloodcellall18–80N/A18Germany
Digestivesystemdiseasesperianal fistulaNCT05499156MSCall18
701/280Iran
NCT05402748MSCall18–701/280Iran livercirrhosisNCT05871463MSCall18
75215Iran
IBSNCT04879810MSCall>18N/A4USA
DiseasesatorbeforebirthELBWbirthNCT05490173MSCall1
EyediseasesdryeyediseaseNCT05738629MSCall18
NCT04213248MSCall18
3daysN/A10Russia
701/212China
701/227China macularholesNCT03437759MSCall<80early144China retinitispigmentosaNCT05413148MSCall18
Gland&hormone-related diseases T1DMNCT02138331MSCall18
Heart&blooddiseasesMINCT05669144MSC,mitochondriaall35
702/3135Turkey
602/320Egypt
801/220Iran aorticdissectionNCT04356300MSCall20
Mouth&toothdiseasesperiodontitisNCT04270006MSCall18
80N/A60China
50early110Egypt oralmucositisNCT01668849plant,grapeall20
MusculoskeletaldiseasesmeniscalinjuryNCT05261360MSCall30
OA,kneeNCT05060107MSCall30
85160USA
50230Turkey
70110Chile degenerativediscdiseaseNCT04849429PRPall18
60130India bonelossNCT04998058MSCall>351/220Brazil
NeoplasmsmetastaticpancreascancerNCT03608631MSC + KRASG12D siRNA all>18128USA
coloncancerNCT01294072plant,curcuminall>20135USA
NSCLCNCT01159288tumorantigenloadedDC all18–70241France
bladdercancerNCT05559177chimericexosomal tumorvaccines all18–85early19China
HCCNCT05375604CDK-004all>1819USA
NervoussystemdiseasesfocalepilepsyNCT05886205iPSCall18–70early134China craniofacialneuralgiaNCT04202783N/Aall>18N/A100USA ischemicstrokeNCT03384433MSCall40–801/25Iran
ADNCT04388982MSCall>501/29China
Nutritional&metabolic diseases familial hypercholesterolemia
NCT05043181LDLRmRNAdeliveryall18–45130China
RespiratorytractdiseasesCOVID-19NCT04276987MSCall18–75124China
NCT05787288MSCall18–75early1240China
NCT05808400MSCall18–80early180China
NCT05216562MSCall18–752/360Indonesia
NCT04602442MSCall18
NCT04491240MSCall18
NCT04493242MSCall18
65290Russia
651/230Russia
852102USA
NCT04798716MSCall>181/255USA
NCT05387278MSCall18–75120USA
NCT04389385COVID-19specificT cell all18–75160Turkey
NCT04747574EXO-CD24all18–85135Israel
NCT04969172EXO-CD24all18
NCT04902183CovenD24all18
802155Israel
80290Greece
NCT04384445Zofinall>181/220USA
NCT04657406Zofinall>18N/AN/AUSA
NCT05228899Zofinall>181/230USA
Table5. continued
CategoryofconditionsSpecificdiseaseNCTnumberSourceofexosomeGenderAgePhasesEnrollmentCountry
ARDSNCT04602104MSCall18–701/2169China NCT05354141MSCall18–653970USA NCT05947747EXO-CD24all>18290Israel COPDNCT05643729Zofinall40–801/220USA pulmonaryinfection,drugresistant NCT04544215MPCall18–751/260China
Skin&connectivetissue diseases
atopicdermatitisNCT05969717iPSCall18–70early120China psoriasisNCT05523011MSCall>21110Singapore dystrophicepidermolysis bullosa NCT04173650MSCall>61/210USA
skinagingNCT05813379MSCfemale35–651/220Iran chroniculcerNCT04134676MSC-CMall18–80138Indonesia cutaneouswoundNCT02565264plasmaallallearly15Japan
NCT05475418MSCall18–60N/A5China alopeciaNCT05658094MSCall25–65N/A20Iran
Urinarytract&sexual organsconditions PCOSNCT03493984plant,ginger&aloefemale18–40N/AN/AUSA
DataobtainedfromClinicalTrials.govusing ‘exosometherapy ’ , ‘exosometreatment ’,and ‘exosome’ askeywords,asof2023-09-08.Thecategorizationof diseasesfollowsthesystembyClinicalTrials.gov AD Alzheimer ’sdisease, ARDS acuterespiratorydistresssyndrome, CM conditionedmedium, COPD chronicobstructivepulmonarydisease, DC dendriticcell, DM diabetesmellitus, ELBW extremelylowbirthweight, HCC hepatocellularcarcinoma, IBS irritablebowelsyndrome, iPSC inducedpluripotentstemcell, LDLR low-densitylipoproteinreceptor, MI myocardialinfarction, MPC mesenchymalprogenitorcell, MSC mesenchymalstemcell, NSCLC non-smallcelllungcancer, OA osteoarthritis, PCOS polycysticovarysyndrome, PRP platelet-richplasma, siRNA smallinterferingRNA
effectsofstemcell-derivedexosomesareachievedthrough disease-specificcellularandtissueresponses(e.g.,tissueregeneration,anti-inflammation,anti-celldeath,immunomodulation, andanti-oxidativestress)andtissue-specificmolecularsignaling pathways(e.g.,Wnt/β-catenin,PTEN/PI3K/Akt/HIF-1α,MAPK,and JAK/STATpathways).Collectively,stemcell-derivedexosome therapyhasbeenproventobeapotentandversatilesurrogate tostemcelltherapyinthesurgicalarena.
Futureemphasisofclinicalapplicationsofstemcell-derived exosomesshouldbeplacedonvariousnodesofthistherapeutic pipeline.Firstly,targetingthepretherapeuticlarge-scaleproduction ofexosomes,ahigh-throughputcellularsourceaswellasa reproducibleandscalableproductionandisolationprotocolare required.Comparedtothestaticsystemgrowingmonolayercells, dynamicsystemintheformofbioreactor,e.g.,hollow-fiber bioreactorandstirredtankbioreactor,canincreasetheefficiency byproducingcopiouscellsandexosomesinashortperiodoftime. However,thephenotypeofparentalcellsandderivedexosomes mightchangeduetophysicalandshearstressencounteredina reactor.Thus,theworkingparametersofthebioreactormustbe optimizedtofacilitatelarge-scaleproductionofstemcell-derived exosomes.Secondly,targetingthetherapeuticmodalityofexosomes,deliverymethodsotherthansystemicadministrationneedto beexplored.Whendeliveredthroughthevenoussystem,exosomes arerapidlyclearedfrombloodcirculationandaccumulateinthe liver,spleenandlungs,whichcanbeovercomebylocaldelivery. Variousbiomaterialshavebeenrecentlyusedtoprotect,assistand augmentlocallydeliveredexosomestomaximizetheirtherapeutic effects.Thesebiomaterialscouldbedesignedaccordingtotheir sources(e.g.,natural,synthetic,andhybridpolymers),format(e.g., scaffold,patch,spray,andmicroneedle),andresponsiveness(e.g., temperature,pH,andprotein),therebyallowingdisease-specific customization.Lastly,targetingthetherapeuticindicationsof exosometherapy,morediseasesthantheonesdiscussedinthis reviewshouldbeincludedintofuturepreclinicalstudiesandclinical trials.Forexample,airwayinflammatoryconditions(e.g.,allergic rhinitisandasthma)couldbesuitablecandidatesforexosome
therapy,consideringtheimmunomodulatoryeffectofMSC-derived exosomes.Disordersthatarebesttreatedusingsurgicalimplants (e.g.,cochlearimplant,intraocularlenses,andcontraceptive intrauterinedevices)couldbemanagedintheformofimplantbasedlocalreleaseofexosomes.Inadditiontoprimarydiseases, secondaryconditionsincludingsurgicaloperation-andgeneral anesthesia-relatedcomplications(e.g.,cognitiveimpairment,wound paresthesia,andmalignanthyperthermia)mightbecometherapeutictargetsofexosometherapy.Collectively,effortstoupscale exosomeproductioninconjunctionwithmultimodalexosome deliverywillacceleratetheclinicalapplicationsofstemcell-derived exosomesinarapidlyexpandingdiseasespectrum.
ACKNOWLEDGEMENTS
ThisworkissponsoredbytheFundamentalResearchFundsfortheCentral Universities.ItwasalsosupportedbytheNationalNaturalScienceFoundationof China(No.82271192).
AUTHORCONTRIBUTIONS
F.T.:conceptualization,formalanalysis,investigation,resources,writing,visualization, fundingacquisition;X.L.:software,methodology;Z.W.:datacuration;J.L.:validation; K.S.:resources;J.Z.:projectadministration,supervision.Allauthorshaveread, discussedandapprovedthe finalversionofthisarticle.
ADDITIONALINFORMATION
Competinginterests: Theauthorsdeclarenocompetinginterests.
REFERENCES
1.Kimbrel,E.A.&Lanza,R.Next-generationstemcells-usheringinaneweraof cell-basedtherapies. Nat.Rev.Drug.Discov. 19,463–479(2020).
2.Puri,M.C.&Nagy,A.Concisereview:embryonicstemcellsversusinduced pluripotentstemcells:thegameison. StemCells. 30,10–14(2012).
3.Ng,A.P.&Alexander,W.S.Haematopoieticstemcells:past,presentandfuture. CellDeathDiscov. 3,17002(2017).
4.Naji,A.etal.Biologicalfunctionsofmesenchymalstemcellsandclinical implications. CellMol.LifeSci. 76,3323–3348(2019).
5.Tang,Y.,Yu,P.&Cheng,L.Currentprogressinthederivationandtherapeutic applicationofneuralstemcells. CellDeathDis. 8,e3108(2017).
6.Chambers,S.E.J.etal.Currentconceptsonendothelialstemcellsdefinition, location,andmarkers. StemCellsTransl.Med. 10,S54–S61(2021).
7.Hoang,D.M.etal.Stemcell-basedtherapyforhumandiseases. SignalTransduct.TargetTher. 7,272(2022).
8.Zakrzewski,W.,Dobrzynski,M.,Szymonowicz,M.&Rybak,Z.Stemcells:past, present,andfuture. StemCellRes.Ther. 10,68(2019).
9.Zhang,K.&Cheng,K.Stemcell-derivedexosomeversusstemcelltherapy. Nat. Rev.Bioeng. 1,608–609(2023).
10.Zhang,Y.etal.Exosome:AReviewOfItsClassification,IsolationTechniques, Storage,DiagnosticAndTargetedTherapyApplications. Int.J.Nanomed. 15, 6917–6934(2020).
11.Vizoso,F.J.etal.Mesenchymalstemcellsecretome:towardcell-freetherapeutic strategiesinregenerativemedicine. Int.J.Mol.Sci. 18,1852–1875(2017).
12.Ren,K.Exosomesinperspective:apotentialsurrogateforstemcelltherapy. Odontology. 107,271–284(2019).
13.Hastuti,S.etal.hUMSCvs.hUMSC-Exosome:whichoneisbetterforepilepsy? Pharmaceuticals 15,1247–1261(2022).
14.Carr,N.J.Thepathologyofhealingandrepair. Surgery 40,13–19(2022).
15.Peshkova,M.etal.Targetinginflammationandregeneration:scaffolds,extracellularvesicles,andnanotechnologiesascell-freedual-targettherapeutic strategies. Int.J.Mol.Sci. 23,13796–13813(2022).
16.Wang,H.,Huber,C.C.&Li,X.P.Mesenchymalandneuralstemcell-derived exosomesintreatingalzheimerasdisease. Bioengineering 10,253–266(2023).
17.Li,X.etal.Neuralstem/progenitorcell-derivedextracellularvesicles:anovel therapyforneurologicaldiseasesandbeyond. MedComm 4,e214(2023).
18.Norouzi-Barough,L.,Shirian,S.,Gorji,A.&Sadeghi,M.Therapeuticpotentialof mesenchymalstemcell-derivedexosomesasacell-freetherapyapproachfor thetreatmentofskin,bone,andcartilagedefects. ConnectTissueRes. 63,83–96 (2022).
19.Doyle,L.M.&Wang,M.Z.Overviewofextracellularvesicles,theirorigin, composition,purpose,andmethodsforexosomeisolationandanalysis. Cells 8, 727–750(2019).
20.Pegtel,D.M.&Gould,S.J.Exosomes. Annu.Rev.Biochem. 88,487–514(2019).
21.Gurung,S.,Perocheau,D.,Touramanidou,L.&Baruteau,J.Theexosomejourney: frombiogenesistouptakeandintracellularsignalling. CellCommun.Signal. 19, 47(2021).
22.Zhang,Y.,Liu,Y.,Liu,H.&Tang,W.H.Exosomes:biogenesis,biologicfunction andclinicalpotential. CellBiosci. 9,19(2019).
23.GholamiFarashah,M.S.etal.Bonemarrowmesenchymalstemcell’sexosomes askeynanoparticlesinosteogenesisandboneregeneration:specificcapacity basedoncelltype. Mol.Biol.Rep. 49,12203–12218(2022).
24.Qing,L.,Chen,H.,Tang,J.&Jia,X.ExosomesandtheirMicroRNAcargo:newplayers inperipheralnerveregeneration. Neurorehabilit.NeuralRepair. 32,765–776(2018).
25.Kwok,Z.H.,Wang,C.&Jin,Y.Extracellularvesicletransportationanduptakeby recipientcells:acriticalprocesstoregulatehumandiseases. Processes 9, 273–294(2021).
26.Kimiz-Gebologlu,I.&Oncel,S.S.Exosomes:large-scaleproduction,isolation, drugloadingefficiency,andbiodistributionanduptake. J.ControlRelease 347, 533–543(2022).
27.Hamzah,R.N.,Alghazali,K.M.,Biris,A.S.&Griffin,R.J.Exosometraceabilityand cellsourcedependenceoncompositionandcell-cellcrosstalk. Int.J.Mol.Sci. 22,5346–5362(2021).
28.Li,M.etal.Exosomesfromdifferentcells:characteristics,modifications,and therapeuticapplications. Eur.J.Med.Chem. 207,112784(2020).
29.Shan,X.etal.Thebiogenesis,biologicalfunctions,andapplicationsof macrophage-derivedexosomes. Front.Mol.Biosci. 8,715461(2021).
30.Wang,Y.etal.Macrophage-derivedextracellularvesicles:diversemediatorsof pathologyandtherapeuticsinmultiplediseases. CellDeathDis. 11,924(2020).
31.Liu,J.,Wu,F.&Zhou,H.Macrophage-derivedexosomesincancers:biogenesis, functionsandtherapeuticapplications. Immunol.Lett. 227,102–108(2020).
32.Elashiry,M.,Elsayed,R.&Cutler,C.W.Exogenousandendogenousdendritic cell-derivedexosomes:lessonslearnedforimmunotherapyanddisease pathogenesis. Cells 11,115–136(2021).
33.Pitt,J.M.etal.Dendriticcell-derivedexosomesforcancertherapy. JClinInvestig. 126,1224–1232(2016).
34.Kok,V.C.&Yu,C.C.Cancer-derivedexosomes:theirroleincancerbiologyand biomarkerdevelopment. Int.J.Nanomed. 15,8019–8036(2020).
35.Naseri,M.etal.Tumor-derivedexosomes:thenextgenerationofpromisingcellfreevaccinesincancerimmunotherapy. Oncoimmunology 9,1779991(2020).
36.Kluszczynska,K.etal.Methodsforthedeterminationofthepurityofexosomes. Curr.Pharm.Des. 25,4464–4485(2019).
37.Zhou,B.etal.Applicationofexosomesasliquidbiopsyinclinicaldiagnosis. SignalTransductTargetTher. 5,144(2020).
38.Boukouris,S.&Mathivanan,S.Exosomesinbodily fluidsareahighlystable resourceofdiseasebiomarkers. ProteomicsClin.Appl. 9,358–367(2015).
39.Gurunathan,S.etal.Reviewoftheisolation,characterization,biologicalfunction,andmultifarioustherapeuticapproachesofexosomes. Cells 8,307–342 (2019).
40.Bei,H.P.etal.Bone-a-Petite:engineeringexosomestowardsBone,osteochondral,andcartilagerepair. Small. 17,e2101741(2021).
41.Yang,X.X.,Sun,C.,Wang,L.&Guo,X.L.Newinsightintoisolation,identification techniquesandmedicalapplicationsofexosomes. J.ControlRelease 308, 119–129(2019).
42.Kalluri,R.&LeBleu,V.S.Thebiology,function,andbiomedicalapplicationsof exosomes. Science 367,640–654(2020).
43.Li,X.etal.Challengesandopportunitiesinexosomeresearch-Perspectivesfrom biology,engineering,andcancertherapy. APLBioeng. 3,011503(2019).
44.Khayambashi,P.etal.Hydrogelencapsulationofmesenchymalstemcellsand theirderivedexosomesfortissueengineering. Int.J.Mol.Sci. 22,684–698(2021).
45.Hussen,B.M.etal.Strategiestoovercomethemainchallengesoftheuseof exosomesasdrugcarrierforcancertherapy. CancerCellInt. 22,323(2022).
46.Shao,J.,Zaro,J.&Shen,Y.Advancesinexosome-baseddrugdeliveryandtumor targeting:fromtissuedistributiontointracellularfate. Int.J.Nanomed. 15, 9355–9371(2020).
47.Tian,Y.etal.Adoxorubicindeliveryplatformusingengineerednaturalmembranevesicleexosomesfortargetedtumortherapy. Biomaterials 35,2383–2390 (2014).
48.Kim,M.S.etal.Developmentofexosome-encapsulatedpaclitaxeltoovercome MDRincancercells. Nanomedicine 12,655–664(2016).
49.Ohno,S.etal.SystemicallyinjectedexosomestargetedtoEGFRdeliverantitumormicroRNAtobreastcancercells. Mol.Ther. 21,185–191(2013).
50.Alvarez-Erviti,L.etal.DeliveryofsiRNAtothemousebrainbysystemicinjection oftargetedexosomes. Nat.Biotechnol. 29,341–345(2011).
51.Zhu,Q.etal.Embryonicstemcells-derivedexosomesendowedwithtargeting propertiesaschemotherapeuticsdeliveryvehiclesforglioblastomatherapy. Adv.Sci. 6,1801899(2019).
52.Nakase,I.&Futaki,S.CombinedtreatmentwithapH-sensitivefusogenicpeptideandcationiclipidsachievesenhancedcytosolicdeliveryofexosomes. Sci. Rep. 5,10112(2015).
53.Wu,Y.,Deng,W.&Klinke,D.J.2ndExosomes:improvedmethodstocharacterizetheirmorphology,RNAcontent,andsurfaceproteinbiomarkers. Analyst. 140,6631–6642(2015).
54.Maas,S.L.etal.Possibilitiesandlimitationsofcurrenttechnologiesforquantificationofbiologicalextracellularvesiclesandsyntheticmimics. J.Control Release. 200,87–96(2015).
55.Thery,C.etal.Minimalinformationforstudiesofextracellularvesicles2018 (MISEV2018):apositionstatementoftheInternationalSocietyforExtracellular VesiclesandupdateoftheMISEV2014guidelines. J.ExtracellVesicles. 7,1535750 (2018).
56.Witwer,K.W.etal.UpdatingMISEV:evolvingtheminimalrequirementsfor studiesofextracellularvesicles. J.ExtracellVesicles. 10,e12182(2021).
57.Maroto,R.etal.Effectsofstoragetemperatureonairwayexosomeintegrityfor diagnosticandfunctionalanalyses. J.ExtracellVesicles. 6,1359478(2017).
58.Yamashita,T.,Takahashi,Y.&Takakura,Y.Possibilityofexosome-basedtherapeuticsandchallengesinproductionofexosomeseligiblefortherapeutic application. Biol.Pharm.Bull. 41,835–842(2018).
59.Bosch,S.etal.Trehalosepreventsaggregationofexosomesandcryodamage. Sci.Rep. 6,36162(2016).
60.Charoenviriyakul,C.,Takahashi,Y.,Nishikawa,M.&Takakura,Y.Preservationof exosomesatroomtemperatureusinglyophilization. Int.J.Pharm. 553,1–7(2018).
61.Kusuma,G.D.etal.Toprotectandtopreserve:novelpreservationstrategiesfor extracellularvesicles. Front.Pharmacol. 9,1199(2018).
62.Bahney,C.S.etal.Cellularbiologyoffracturehealing. J.Orthop.Res. 37,35–50 (2019).
63.Einhorn,T.A.&Gerstenfeld,L.C.Fracturehealing:mechanismsandinterventions. Nat.Rev.Rheumatol. 11,45–54(2015).
64.Yang,Z.etal.Exosomes:afriendorfoeforosteoporoticfracture? Front. Endocrinol. 12,679914(2021).
65.Furuta,T.etal.Mesenchymalstemcell-derivedexosomespromotefracture healinginamousemodel. StemCellsTransl.Med. 5,1620–1630(2016).
66.Zhang,L.etal.Exosomesfrombonemarrowmesenchymalstemcellsenhance fracturehealingthroughthepromotionofosteogenesisandangiogenesisina ratmodelofnonunion. StemCellRes.Ther. 11,38(2020).
67.Jia,Y.etal.Exosomessecretedbyyoungmesenchymalstemcellspromotenew boneformationduringdistractionosteogenesisinolderrats. Calcif.TissueInt. 106,509–517(2020). Clinicalapplicationsofstemcell-derivedexosomes
68.Jia,Y.etal.Exosomessecretedbyendothelialprogenitorcellsacceleratebone regenerationduringdistractionosteogenesisbystimulatingangiogenesis. Stem CellRes.Ther. 10,12(2019).
69.O’Brien,K.etal.RNAdeliverybyextracellularvesiclesinmammaliancellsandits applications. Nat.Rev.Mol.CellBiol. 21,585–606(2020).
70.Yu,H.,Zhang,J.,Liu,X.&Li,Y.microRNA-136-5pfrombonemarrow mesenchymalstemcell-derivedexosomesfacilitatesfracturehealingbytargetingLRP4toactivatetheWnt/beta-cateninpathway. BoneJointRes. 10, 744–758(2021).
71.Jiang,Y.,Zhang,J.,Li,Z.&Jia,G.Bonemarrowmesenchymalstemcell-derived exosomalmiR-25regulatestheubiquitinationanddegradationofRunx2by SMURF1topromotefracturehealinginmice. Front.Med. 7,577578(2020).
72.Wang,Y.etal.ObesityregulatesmiR-467/HoxA10axisonosteogenicdifferentiationandfracturehealingbyBMSC-derivedexosomeLncRNAH19. J.Cell Mol.Med. 25,1712–1724(2021).
73.Behera,J.,Kumar,A.,Voor,M.J.&Tyagi,N.ExosomallncRNA-H19promotes osteogenesisandangiogenesisthroughmediatingAngpt1/Tie2-NOsignalingin CBS-heterozygousmice. Theranostics. 11,7715–7734(2021).
74.Liang,B.etal.Dimethyloxaloylglycine-stimulatedhumanbonemarrow mesenchymalstemcell-derivedexosomesenhanceboneregenerationthrough angiogenesisbytargetingtheAKT/mTORpathway. StemCellRes.Ther. 10,335 (2019).
75.Lu,G.D.,Cheng,P.,Liu,T.&Wang,Z.BMSC-derivedexosomalmiR-29apromotesangiogenesisandosteogenesis. Front.CellDev.Biol. 8,608521(2020).
76.Liu,W.etal.Hypoxicmesenchymalstemcell-derivedexosomespromotebone fracturehealingbythetransferofmiR-126. ActaBiomater. 103,196–212(2020).
77.Katz,J.N.,Arant,K.R.&Loeser,R.F.Diagnosisandtreatmentofhipandknee osteoarthritis:areview. JAMA. 325,568–578(2021).
78.Martel-Pelletier,J.etal.Osteoarthritis. Nat.Rev.Dis.Primers. 2,16072(2016).
79.Wu,J.etal.miR-100-5p-abundantexosomesderivedfrominfrapatellarfatpad MSCsprotectarticularcartilageandameliorategaitabnormalitiesviainhibition ofmTORinosteoarthritis. Biomaterials 206,87–100(2019).
80.Liu,Y.etal.MSC-derivedexosomespromoteproliferationandinhibitapoptosis ofchondrocytesvialncRNA-KLF3-AS1/miR-206/GIT1axisinosteoarthritis. Cell Cycle. 17,2411–2422(2018).
81.Kong,R.etal.Synovialmesenchymalstemcell-derivedexosomalmiR-320c enhanceschondrogenesisbytargetingADAM19. FutureMed.Chem. 14,81–96 (2022).
82.Mao,G.etal.ExosomesderivedfrommiR-92a-3p-overexpressinghuman mesenchymalstemcellsenhancechondrogenesisandsuppresscartilage degradationviatargetingWNT5A. StemCellRes.Ther. 9,247(2018).
83.Tao,S.C.etal.ExosomesderivedfrommiR-140-5p-overexpressinghuman synovialmesenchymalstemcellsenhancecartilagetissueregenerationand preventosteoarthritisofthekneeinaratmodel. Theranostics 7,180–195(2017).
84.Wang,R.,Xu,B.&Xu,H.TGF-β1promotedchondrocyteproliferationbyregulatingSp1throughMSC-exosomesderivedmiR-135b. CellCycle 17,2756–2765 (2018).
85.Zhu,Y.etal.Comparisonofexosomessecretedbyinducedpluripotentstem cell-derivedmesenchymalstemcellsandsynovialmembrane-derived mesenchymalstemcellsforthetreatmentofosteoarthritis. StemCellRes. Ther. 8,64(2017).
86.Jiang,K.,Jiang,T.,Chen,Y.&Mao,X.Mesenchymalstemcell-derivedexosomes modulatechondrocyteglutaminemetabolismtoalleviateosteoarthritisprogression. Mediat.Inflamm. 2021,2979124(2021).
87.Huang,Y.etal.Bonemarrowmesenchymalstemcell-derivedexosomalmiR-206 promotesosteoblastproliferationanddifferentiationinosteoarthritisbyreducingElf3. J.CellMol.Med. 25,7734–7745(2021).
88.Zhang,S.etal.MSCexosomesmediatecartilagerepairbyenhancingproliferation,attenuatingapoptosisandmodulatingimmunereactivity. Biomaterials. 156,16–27(2018).
89.Tian,X.etal.Gingivalmesenchymalstemcell-derivedexosomesareimmunosuppressiveinpreventingcollagen-inducedarthritis. J.CellMol.Med. 26, 693–708(2022).
90.Cho,Y.etal.Disease-modifyingtherapeuticstrategiesinosteoarthritis:current statusandfuturedirections. Exp.Mol.Med. 53,1689–1696(2021).
91.Ding,W.etal.Spinalcordinjury:theglobalincidence,prevalence,anddisability fromtheglobalburdenofdiseaseStudy2019. Spine 47,1532–1540(2022).
92.Ahuja,C.S.etal.Traumaticspinalcordinjury. Nat.Rev.Dis.Primers. 3,17018 (2017).
93.Ahuja,C.S.&Fehlings,M.Concisereview:bridgingthegap:novelneuroregenerativeandneuroprotectivestrategiesinspinalcordinjury. StemCells Transl.Med. 5,914–924(2016).
94.Ma,K.etal.Insulin-likegrowthfactor-1enhancesneuroprotectiveeffectsof neuralstemcellexosomesafterspinalcordinjuryviaanmiR-219a-2-3p/YY1 mechanism. Aging 11,12278–12294(2019).
95.Zhang,L.&Han,P.Neuralstemcell-derivedexosomessuppressneuronalcell apoptosisbyactivatingautophagyviamiR-374-5p/STK-4axisinspinalcord injury. J.Musculoskelet.NeuronalInteract. 22,411–421(2022).
96.Shao,C.etal.Mesenchymalstemcellderivedexosomessuppressneuronalcell ferroptosisVialncGm36569/miR-5627-5p/FSP1axisinacutespinalcordinjury. StemCellRev.Rep. 18,1127–1142(2022).
97.Nakazaki,M.etal.Smallextracellularvesiclesreleasedbyinfusedmesenchymal stromalcellstargetM2macrophagesandpromoteTGF-betaupregulation, microvascularstabilizationandfunctionalrecoveryinarodentmodelofsevere spinalcordinjury. J.ExtracellVesicles. 10,e12137(2021).
98.Liu,W.etal.Exosome-shuttledmiR-216a-5pfromhypoxicpreconditioned mesenchymalstemcellsrepairtraumaticspinalcordinjurybyshiftingmicroglialM1/M2polarization. J.Neuroinflamm. 17,47(2020).
99.Huang,J.H.etal.Extracellularvesiclesderivedfromepiduralfat-mesenchymal stemcellsattenuateNLRP3inflammasomeactivationandimprovefunctional recoveryafterspinalcordinjury. Neurochem.Res. 45,760–771(2020).
100.Yuan,F.etal.Endothelialprogenitorcell-derivedexosomespromoteantiinflammatorymacrophagesviaSOCS3/JAK2/STAT3axisandimprovetheoutcomeofspinalcordinjury. J.Neuroinflamm. 20,156(2023).
101.Zhong,D.etal.Neuralstemcell-derivedexosomesfacilitatespinalcordfunctionalrecoveryafterinjurybypromotingangiogenesis. Exp.Biol.Med. 245, 54–65(2020).
102.Chen,J.etal.ExosomesderivedfromnervestemcellsloadedwithFTY720 promotetherecoveryafterspinalcordinjuryinratsbyPTEN/AKTsignal pathway. J.Immunol.Res. 2021,8100298(2021).
103.Zhu,S.etal.Versatilesubtypesofpericytesandtheirrolesinspinalcordinjury repair,bonedevelopmentandrepair. BoneRes. 10,30(2022).
104.Zhou,Y.etal.Exosomesderivedfrombonemarrowmesenchymalstemcells protecttheinjuredspinalcordbyinhibitingpericytepyroptosis. NeuralRegen. Res. 17,194–202,(2022).
105.Lai,X.etal.miR-146a-5p-modifiedhUCMSC-derivedexosomesfacilitatespinal cordfunctionrecoverybytargetingneurotoxicastrocytes. StemCellRes.Ther. 13,487(2022).
106.Li,S.etal.ExosomesderivedfromNGF-overexpressingbonemarrow mesenchymalstemcellsheetpromotespinalcordinjuryrepairinamouse model. Neurochem.Int. 157,105339(2022).
107.Zhou,W.etal.Exosomesderivedfromhumanplacentalmesenchymalstem cellsenhancedtherecoveryofspinalcordinjurybyactivatingendogenous neurogenesis. StemCellRes.Ther. 12,174(2021).
108.Perez,N.E.etal.Neurogenicbladderphysiology,pathogenesis,andmanagementafterspinalcordinjury. J.Pers.Med. 12,968–982(2022).
109.VilaPouca,M.C.P.,Parente,M.P.L.,Jorge,R.M.N.&Ashton-Miller,J.A.Injuries inmuscle-tendon-boneunits:asystematicreviewconsideringtheroleofpassivetissuefatigue. Orthop.J.SportsMed. 9,23259671211020731(2021).
110.Thomopoulos,S.,Parks,W.C.,Rifkin,D.B.&Derwin,K.A.Mechanismsoftendon injuryandrepair. J.Orthop.Res. 33,832–839(2015).
111.Nakamura,Y.etal.Mesenchymal-stem-cell-derivedexosomesaccelerateskeletalmuscleregeneration. FEBSLett. 589,1257–1265(2015).
112.Chen,S.H.etal.Extracellularvesiclesofadipose-derivedstemcellspromote thehealingoftraumatizedachillestendons. Int.J.Mol.Sci. 22 ,12373 –12388 (2021).
113.Brindisino,F.etal.Rotatorcuffrepairvs.nonoperativetreatment:asystematic reviewwithmeta-analysis. J.ShoulderElbowSurg. 30,2648–2659(2021).
114.Wang,C.etal.Exosomesisolatedfromadipose-derivedstemcells:anewcellfreeapproachtopreventthemuscledegenerationassociatedwithtornrotator cuffs. Am.J.SportsMed. 47,3247–3255(2019).
115.Wang,C.etal.Adiposestemcell-derivedexosomesdecreasefattyinfiltration andenhancerotatorcuffhealinginarabbitmodelofchronictears. Am.J.Sports Med. 48,1456–1464(2020).
116.Huang,Y.etal.Bonemarrowmesenchymalstemcell-derivedexosomespromoterotatorcufftendon-bonehealingbypromotingangiogenesisandregulatingM1macrophagesinrats. StemCellRes.Ther. 11,496(2020).
117.Compston,J.E.,McClung,M.R.&Leslie,W.D.Osteoporosis. Lancet 393, 364–376(2019).
118.Ren,L.etal.Adiposemesenchymalstemcell-derivedexosomesameliorate hypoxia/serumdeprivation-inducedosteocyteapoptosisandosteocytemediatedosteoclastogenesisinvitro. Biochem.Biophys.Res.Commun. 508, 138–144(2019).
119.Yang,B.C.etal.Humanumbilicalcordmesenchymalstemcell-derivedexosomesactviathemiR-1263/Mob1/Hipposignalingpathwaytopreventapoptosisindisuseosteoporosis. Biochem.Biophys.Res.Commun. 524,883–889 (2020).
120.Yahao,G.&Xinjia,W.Theroleandmechanismofexosomesfromumbilicalcord mesenchymalstemcellsininducingosteogenesisandpreventingosteoporosis. CellTransplant. 30,9636897211057465(2021).
121.Gordon,T.Peripheralnerveregenerationandmusclereinnervation. Int.J.Mol. Sci. 21,8652–8675(2020).
122.Bucan,V.etal.Effectofexosomesfromratadipose-derivedmesenchymalstem cellsonneuriteoutgrowthandsciaticnerveregenerationaftercrushinjury. Mol. Neurobiol. 56,1812–1824(2019).
123.Chen,J.etal.Exosomesfromhumanadipose-derivedstemcellspromotesciatic nerveregenerationviaoptimizingSchwanncellfunction. J.CellPhysiol. 234, 23097–23110(2019).
124.Li,C.etal.ExosomesfromLPS-preconditionedbonemarrowMSCsacceleratedperipheralnerveregenerationviaM2macrophagepolarization:involvementofTSG-6/NF-kappaB/NLRP3signalingpathway. Exp.Neurol. 356 , 114139(2022).
125.Fine,N.etal.Intervertebraldiscdegenerationandosteoarthritis:acommon moleculardiseasespectrum. Nat.Rev.Rheumatol. 19,136–152(2023).
126.Cheng,X.etal.MesenchymalstemcellsdeliverexogenousmiR-21viaexosomes toinhibitnucleuspulposuscellapoptosisandreduceintervertebraldisc degeneration. J.CellMol.Med. 22,261–276(2018).
127.Yu,Y.etal.Humanembryonicstem-cell-derivedexosomesrepressNLRP3 inflammasometoalleviatepyroptosisinnucleuspulposuscellsbytransmitting miR-302c. Int.J.Mol.Sci. 24,7664–7678(2023).
128.Hu,Y.etal.Exosomesderivedfrombonemesenchymalstemcellsalleviate compression-inducednucleuspulposuscellapoptosisbyinhibitingoxidative stress. Oxid.Med.CellLongev. 2021,2310025(2021).
129.Petek,D.,Hannouche,D.&Suva,D.Osteonecrosisofthefemoralhead: pathophysiologyandcurrentconceptsoftreatment. EFORTOpenRev. 4,85–97 (2019).
130.Liu,X.etal.Exosomessecretedfromhuman-inducedpluripotentstemcellderivedmesenchymalstemcellspreventosteonecrosisofthefemoralheadby promotingangiogenesis. Int.J.Biol.Sci. 13,232–244(2017).
131.Zuo,R.etal.ExosomesderivedfromhumanCD34(+)stemcellstransfected withmiR-26apreventglucocorticoid-inducedosteonecrosisofthefemoralhead bypromotingangiogenesisandosteogenesis. StemCellRes.Ther. 10,321 (2019).
132.Campbell,B.C.V.&Khatri,P.Stroke. Lancet. 396,129–142(2020).
133.Campbell,B.C.V.etal.Ischaemicstroke. Nat.Rev.Dis.Primers. 5,70(2019).
134.Fisher,M.&Savitz,S.I.Pharmacologicalbraincytoprotectioninacuteischaemic stroke renewedhopeinthereperfusionera. Nat.Rev.Neurol. 18,193–202 (2022).
135.Luo,H.etal.miR-150-3penhancesneuroprotectiveeffectsofneuralstemcell exosomesafterhypoxic-ischemicbraininjurybytargetingCASP2. Neurosci.Lett. 779,136635(2022).
136.Pan,J.,Wu,T.,Chen,B.&Wu,H.Exosomesderivedfromendothelialprogenitor cellsameliorateglyoxylatedeprivation(OGD)-inducedneuronalapoptosisby deliveringmiR-221-3p. Histol.Histopathol. 38,423–430(2023).
137.Huang,R.,Cheng,T.&Lai,X.Mechanismofischemicbraininjuryrepairby endothelialprogenitorcell-derivedexosomes. Mol.Med.Rep. 26,269–278 (2022).
138.Zhang,G.etal.Exosomesderivedfromhumanneuralstemcellsstimulatedby interferongammaimprovetherapeuticabilityinischemicstrokemodel. J.Adv. Res. 24,435–445(2020).
139.Kang,X.etal.Exosomesderivedfromhypoxicbonemarrowmesenchymalstem cellsrescueOGD-inducedinjuryinneuralcellsbysuppressingNLRP3 inflammasome-mediatedpyroptosis. Exp.CellRes. 405,112635(2021).
140.Li,W.Y.etal.Exosomesderivedfromhumaninducedpluripotentstemcellderivedneuralprogenitorcellsprotectneuronalfunctionunderischemicconditions. NeuralRegen.Res. 16,2064–2070,(2021).
141.Sun,X.etal.Stemcell-derivedexosomesprotectastrocyteculturesfrominvitro ischemiaanddecreaseinjuryaspost-strokeintravenoustherapy. Front.Cell Neurosci. 13,394(2019).
142.Wei,R.etal.Zeb2/Axin2-EnrichedBMSC-Derivedexosomespromotepoststrokefunctionalrecoverybyenhancingneurogenesisandneuralplasticity. J. Mol.Neurosci. 72,69–81(2022).
143.Wang,J.etal.ExosomesfrommiRNA-126-modifiedendothelialprogenitorcells alleviatebraininjuryandpromotefunctionalrecoveryafterstroke. CNSNeurosci. Ther. 26,1255–1265(2020).
144.Dong,C.etal.Mesenchymalstemcell-derivedexosomesimprovedcerebral infarctionviatransferringmiR-23a-3ptoactivatemicroglia. Neuromol.Med. 24, 290–298(2022).
145.Zhang,Z.etal.Humanumbilicalcordmesenchymalstemcell-derivedexosomal miR-146a-5preducesmicroglial-mediatedneuroinflammationviasuppression oftheIRAK1/TRAF6signalingpathwayafterischemicstroke. Aging 13, 3060–3079(2021).
146.Yoon,E.J.etal.TheneuroprotectiveeffectsofexosomesderivedfromTSG101overexpressinghumanneuralstemcellsinastrokemodel. Int.J.Mol.Sci. 23, 9532–9546(2022).
147.Tian,T.etal.Targeteddeliveryofneuralprogenitorcell-derivedextracellular vesiclesforanti-inflammationaftercerebralischemia. Theranostics 11, 6507–6521(2021).
148.Gao,G.etal.Inducedneuralstem/progenitorcell-derivedextracellularvesicles promoterecoverypost-stroke. Clin.Transl.Med. 12,e936(2022).
149.Zhu,Z.H.etal.Neuralstemcell-derivedexosomeasanano-sizedcarrierfor BDNFdeliverytoaratmodelofischemicstroke. NeuralRegen.Res. 18,404–409, (2023).
150.Yerrapragada,S.M.etal.TheprotectiveeffectsofmiR-210modifiedendothelial progenitorcellsreleasedexosomesinhypoxia/reoxygenationinjuredneurons. Exp.Neurol. 358,114211(2022).
151.Ma,X.etal.LoadingMiR-210inendothelialprogenitorcellsderivedexosomes booststheirbeneficialeffectsonhypoxia/reoxygeneation-injuredhuman endothelialcellsviaprotectingmitochondrialfunction. CellPhysiol.Biochem. 46, 664–675(2018).
152.Pan,Q.etal.MiR-17-5pmediatestheeffectsofACE2-Enrichedendothelial progenitorcell-derivedexosomesonamelioratingcerebralischemicinjuryin agedmice. Mol.Neurobiol. 60,3534–3552(2023).
153.Xu,X.etal.CombinationofEPC-EXsandNPC-EXswithmiR-126andmiR-210 overexpressionproducesbettertherapeuticeffectsonischemicstrokebyprotectingneuronsthroughtheNox2/ROSandBDNF/TrkBpathways. Exp.Neurol. 359,114235(2023).
154.Morotti,A.&Goldstein,J.N.Diagnosisandmanagementofacuteintracerebral hemorrhage. Emerg.Med.Clin.N.Am. 34,883–899(2016).
155.Li,Y.etal.miR-137booststheneuroprotectiveeffectofendothelialprogenitor cell-derivedexosomesinoxyhemoglobin-treatedSH-SY5Ycellspartiallyvia COX2/PGE2pathway. StemCellRes.Ther. 11,330(2020).
156.Wiles,M.D.Managementoftraumaticbraininjury:anarrativereviewofcurrent evidence. Anaesthesia. 77,102–112(2022).
157.McKee,A.C.&Daneshvar,D.H.Theneuropathologyoftraumaticbraininjury. Handb.Clin.Neurol. 127,45–66(2015).
158.Galgano,M.etal.Traumaticbraininjury:currenttreatmentstrategiesandfuture endeavors. CellTransplant. 26,1118–1130(2017).
159.Zhang,Y.etal.Effectofexosomesderivedfrommultipluripotentmesenchymal stromalcellsonfunctionalrecoveryandneurovascularplasticityinratsafter traumaticbraininjury. J.Neurosurg. 122,856–867(2015).
160.Zhang,Y.etal.Systemicadministrationofcell-freeexosomesgeneratedby humanbonemarrowderivedmesenchymalstemcellsculturedunder2Dand 3Dconditionsimprovesfunctionalrecoveryinratsaftertraumaticbraininjury. Neurochem.Int. 111,69–81(2017).
161.Moore,T.L.etal.Mesenchymalderivedexosomesenhancerecoveryofmotor functioninamonkeymodelofcorticalinjury. Restor.Neurol.Neurosci. 37, 347–362(2019).
162.Williams,A.M.etal.Mesenchymalstemcell-derivedexosomesprovideneuroprotectionandimprovelong-termneurologicoutcomesinaswinemodelof traumaticbraininjuryandhemorrhagicshock. J.Neurotrauma. 36,54–60(2019).
163.Chen,Y.etal.MSC-derivedexosomespromoterecoveryfromtraumaticbrain injuryviamicroglia/macrophagesinrat. Aging 12,18274–18296(2020).
164.Wen,L.etal.Exosomesderivedfrombonemarrowmesenchymalstemcells inhibitneuroinflammationaftertraumaticbraininjury. NeuralRegen.Res. 17, 2717–2724,(2022).
165.Abedi,M.,Hajinejad,M.,Atabi,F.&Sahab-Negah,S.Exosomederivedfrom humanneuralstemcellsimprovesmotoractivityandneurogenesisinatraumaticbraininjurymodel. Biomed.Res.Int. 2022,6409346(2022).
166.Zhang,Y.etal.Mesenchymalstemcell-derivedexosomesimprovefunctional recoveryinratsaftertraumaticbraininjury:adose-responseandtherapeutic windowstudy. Neurorehabil.NeuralRepair. 34,616–626(2020).
167.Wallace,D.J.etal.Spinalcordinjuryandthehumanmicrobiome:beyondthe brain-gutaxis. Neurosurg.Focus. 46,E11(2019).
168.Kumar,S.etal.Transcriptionalfactorsandproteinbiomarkersastargettherapeuticsintraumaticspinalcordandbraininjury. Curr.Neuropharmacol. 18, 1092–1105(2020).
169.Mortezaee,K.,Khanlarkhani,N.,Beyer,C.&Zendedel,A.Inflammasome:itsrole intraumaticbrainandspinalcordinjury. J.CellPhysiol. 233,5160–5169(2018).
170.Roselli,F.,Karasu,E.,Volpe,C.&Huber-Lang,M.Medusa’shead:thecomplementsystemintraumaticbrainandspinalcordinjury. J.Neurotrauma. 35, 226–240(2018).
171.Putatunda,R.,Bethea,J.R.&Hu,W.H.Potentialimmunotherapiesfortraumatic brainandspinalcordinjury. Chin.J.Traumatol. 21,125–136(2018).
172.Bains,M.&Hall,E.D.Antioxidanttherapiesintraumaticbrainandspinalcord injury. Biochim.Biophys.Acta. 1822,675–684(2012).
173.Yuan,J.etal.Roleofcircularribonucleicacidsinthetreatmentoftraumatic brainandspinalcordinjury. Mol.Neurobiol. 57,4296–4304(2020).
174.Sun,P.etal.MicroRNA-basedtherapeuticsincentralnervoussysteminjuries. J. Cereb.BloodFlowMetab. 38,1125–1148(2018).
175.Wilson,D.M.3rdetal.Hallmarksofneurodegenerativediseases. Cell. 186, 693–714(2023).
176.Erkkinen,M.G.,Kim,M.O.&Geschwind,M.D.Clinicalneurologyandepidemiologyofthemajorneurodegenerativediseases. ColdSpringHarbPerspect. Biol. 10,3118–3163(2018).
177.Breijyeh,Z.&Karaman,R.Comprehensivereviewonalzheimerasdisease:causes andtreatment. Molecules. 25,5789–5816(2020).
178.Hampel,H.etal.Theamyloid-betapathwayinAlzheimer’sdisease. Mol.Psychiatry. 26,5481–5503(2021).
179.Elia,C.A.etal.Intracerebralinjectionofextracellularvesiclesfrommesenchymal stemcellsexertsreducedabetaplaqueburdeninearlystagesofapreclinical modelofAlzheimer’sdisease. Cells. 8,1059–1078(2019).
180.Wang,S.S.,Jia,J.&Wang,Z.Mesenchymalstemcell-derivedextracellular vesiclessuppressesinosexpressionandamelioratesneuralimpairmentinAlzheimer’sdiseasemice. J.AlzheimersDis. 61,1005–1013(2018).
181.Li,B.etal.Impactofneuralstemcell-derivedextracellularvesiclesonmitochondrialdysfunction,sirtuin1level,andsynapticdeficitsinAlzheimer’sdisease. J.Neurochem. 154,502–518(2020).
182.Huber,C.C.etal.Heatshock-inducedextracellularvesiclesderivedfromneural stemcellsconfermarkedneuroprotectionagainstoxidativestressandamyloidbeta-causedneurotoxicity. Mol.Neurobiol. 59,7404–7412(2022).
183.Chen,Y.A.etal.Mesenchymalstemcell-derivedexosomesameliorateAlzheimer’sdiseasepathologyandimprovecognitivedeficits. Biomedicines. 9, 594–612(2021).
184.Zavatti,M.etal.Exosomesderivedfromhumanamniotic fluidmesenchymal stemcellspreservemicrogliaandneuroncellsfromAbeta. Int.J.Mol.Sci. 23, 4967–4980(2022).
185.Ding,M.etal.Exosomesisolatedfromhumanumbilicalcordmesenchymal stemcellsalleviateneuroinflammationandreduceamyloid-betadepositionby modulatingmicroglialactivationinAlzheimer’sdisease. Neurochem.Res. 43, 2165–2177(2018).
186.Reza-Zaldivar,E.E.etal.Mesenchymalstemcell-derivedexosomespromote neurogenesisandcognitivefunctionrecoveryinamousemodelofAlzheimer’s disease. NeuralRegen.Res. 14,1626–1634,(2019).
187.Sweeney,M.D.,Sagare,A.P.&Zlokovic,B.V.Blood-brainbarrierbreakdownin Alzheimerdiseaseandotherneurodegenerativedisorders. Nat.Rev.Neurol. 14, 133–150(2018).
188.Liu,Y.,Huber,C.C.&Wang,H.Disruptedblood-brainbarrierin5xFADmouse modelofAlzheimer’sdiseasecanbemimickedandrepairedinvitrowithneural stemcell-derivedexosomes. Biochem.Biophys.Res.Commun. 525,192–196 (2020).
189.Cui,G.H.etal.RVG-modifiedexosomesderivedfrommesenchymalstemcells rescuememorydeficitsbyregulatinginflammatoryresponsesinamouse modelofAlzheimer’sdisease. Immun.Ageing. 16,10(2019).
190.Gao,G.etal.Neuralstemcell-derivedextracellularvesiclesmitigateAlzheimer’s disease-likephenotypesinapreclinicalmousemodel. SignalTransduct.Target Ther. 8,228(2023).
191.Marino,B.L.B.etal.Parkinson’sdisease:areviewfrompathophysiologyto treatment. MiniRev,Med,Chem. 20,754–767(2020).
192.Poewe,W.etal.Parkinsondisease. Nat.Rev.Dis.Primers. 3,17013(2017).
193.Huang,D.,Zhang,M.&Tan,Z.Bonemarrowstemcell-exo-derivedTSG-6 Attenuates1-Methyl-4-Phenylpyridinium +-InducedNeurotoxicityviathe STAT3/miR-7/NEDD4/LRRK2Axis. J.Neuropathol.Exp.Neurol. 81,621–634(2022).
194.Lee,E.J.etal.Humanneuralstemcell-derivedextracellularvesiclesprotect againstParkinson’sdiseasepathologies. J.Nanobiotechnol. 20,198(2022).
195.Filippi,M.etal.Multiplesclerosis. Nat.Rev.Dis.Primers. 4,43(2018).
196.Dobson,R.&Giovannoni,G.Multiplesclerosis-areview. Eur.J.Neurol. 26,27–40 (2019).
197.Li,Z.etal.ExosomesderivedfrommesenchymalstemcellsattenuateinflammationanddemyelinationofthecentralnervoussysteminEAEratsbyregulatingthepolarizationofmicroglia. Int.Immunopharmacol. 67,268–280(2019).
198.Zhang,J.etal.Exosomesderivedfrombonemarrowmesenchymalstromalcells promoteremyelinationandreduceneuroinflammationinthedemyelinating centralnervoussystem. Exp.Neurol. 347,113895(2022).
199.Qi,D.etal.HNSCexosome-derivedMIATimprovescognitivedisordersinrats withvasculardementiaviathemiR-34b-5p/CALB1axis. Am.J.Transl.Res. 13, 10075–10093(2021).
200.Branscome,H.etal.Retroviralinfectionofhumanneurospheresanduseofstem CellEVstorepaircellulardamage. Sci.Rep. 12,2019(2022).
201.Leavitt,R.J.,Acharya,M.M.,Baulch,J.E.&Limoli,C.L.ExtracellularvesiclederivedmiR-124resolvesradiation-inducedbraininjury. CancerRes. 80, 4266–4277(2020).
202.Smith,S.M.etal.Functionalequivalenceofstemcellandstemcell-derived extracellularvesicletransplantationtorepairtheirradiatedbrain. StemCells Transl.Med. 9,93–105(2020).
203.Long,Q.etal.IntranasalMSC-derivedA1-exosomeseaseinflammation,and preventabnormalneurogenesisandmemorydysfunctionafterstatusepilepticus. Proc.Natl.Acad.Sci.USA. 114,E3536–E3545(2017).
204.Li,S.etal.Bonemarrowmesenchymalstemcell-derivedexosomesshuttling miR-150-5palleviatesmechanicalallodyniainratsbytargetingNOTCH2in microglia. Mol.Med. 28,133(2022).
205.Ma,L.etal.Neuralstemcell-derivedexosomalnetrin1contributestoneuron differentiationofmesenchymalstemcellsintherapyofspinalbifidaaperta. StemCellsTransl.Med. 11,539–551(2022).
206.Guo,H.etal.Bonemarrowmesenchymalstemcells-derivedexosomesimprove injuryofhippocampalneuronsinratswithdepressionbyupregulating microRNA-26aexpression. Int.Immunopharmacol. 82,106285(2020).
207.Niu,Y.,Wang,X.,Li,M.&Niu,B.Exosomesfromhumanumbilicalcord Mesenchymalstemcellsattenuatesstress-inducedhippocampaldysfunctions. Metab.BrainDis. 35,1329–1340(2020).
208.Natale,F.etal.Neuralstemcell-derivedextracellularvesiclescounteractinsulin resistance-inducedsenescenceofneurogenicniche. StemCells 40,318–331 (2022).
209.Spinelli,M.etal.Neuralstemcell-derivedexosomesrevertHFD-dependent memoryimpairmentviaCREB-BDNFSignalling. Int.J.Mol.Sci. 21,8994–9008 (2020).
210.Zhang,Y.etal.Hypothalamicstemcellscontrolageingspeedpartlythrough exosomalmiRNAs. Nature 548,52–57(2017).
211.Kong,L.Y.etal.Mesenchymalstemcell-derivedexosomesrescueoxygenglucosedeprivation-inducedinjuryinendothelialcells. Curr.Neurovasc.Res. 17, 155–163(2020).
212.Almadani,Y.H.,Vorstenbosch,J.,Davison,P.G.&Murphy,A.M.Woundhealing: acomprehensivereview. Semin.PlastSurg. 35,141–144(2021).
213.Rodrigues,M.,Kosaric,N.,Bonham,C.A.,Gurtner,G.C.&WoundHealing:a cellularperspective. Physiol.Rev. 99,665–706(2019).
214.Wang,M.,Zhao,Y.&Zhang,Q.Humanmesenchymalstemcell-derivedexosomesacceleratewoundhealingofmiceeczema. J.Dermatolog.Treat. 33, 1401–1405(2022).
215.Cho,B.S.,Kim,J.O.,Ha,D.H.&Yi,Y.W.Exosomesderivedfromhumanadipose tissue-derivedmesenchymalstemcellsalleviateatopicdermatitis. StemCellRes. Ther. 9,187(2018).
216.Qiu,X.etal.Exosomesreleasedfromeducatedmesenchymalstemcells acceleratecutaneouswoundhealingviapromotingangiogenesis. CellProlif. 53, e12830(2020).
217.Yu,M.etal.Exosomesderivedfromatorvastatin-pretreatedMSCaccelerate diabeticwoundrepairbyenhancingangiogenesisviaAKT/eNOSpathway. Stem CellRes.Ther. 11,350(2020).
218.Ding,J.etal.Exosomesderivedfromhumanbonemarrowmesenchymalstem cellsstimulatedbydeferoxamineacceleratecutaneouswoundhealingby promotingangiogenesis. Biomed.Res.Int. 2019,9742765(2019).
219.Li,X.,Jiang,C.&Zhao,J.Humanendothelialprogenitorcells-derivedexosomes acceleratecutaneouswoundhealingindiabeticratsbypromotingendothelial function. J.DiabetesComplications. 30,986–992(2016).
220.Zhang,J.etal.Exosomesderivedfromhumanendothelialprogenitorcells acceleratecutaneouswoundhealingbypromotingangiogenesisthroughErk1/ 2signaling. Int.J.Biol.Sci. 12,1472–1487(2016).
221.Xu,J.etal.miRNA-221-3pinendothelialprogenitorcell-derivedexosomes acceleratesskinwoundhealingindiabeticmice. DiabetesMetab.Syndr.Obes. 13,1259–1270(2020).
222.Zhang,W.etal.Cell-freetherapybasedonadiposetissuestemcell-derived exosomespromoteswoundhealingviathePI3K/Aktsignalingpathway. Exp.Cell Res. 370,333–342(2018).
223.Zhang,Y.etal.Adiposemesenchymalstemcellexosomespromotewound healingthroughacceleratedkeratinocytemigrationandproliferationbyactivatingtheAKT/HIF-1alphaaxis. J.Mol.Histol. 51,375–383(2020).
224.Kim,S.,Lee,S.K.,Kim,H.&Kim,T.M.Exosomessecretedfrominducedpluripotentstemcell-derivedmesenchymalstemcellsaccelerateskincellproliferation. Int.J.Mol.Sci. 19,3119–3134(2018).
225.He,L.etal.ADSC-ExoscontainingMALAT1promoteswoundhealingbytargetingmiR-124throughactivatingWnt/beta-cateninpathway. Biosci.Rep. 40, 549–561(2020).
226.Gao,S.etal.ExosomalmiR-135aderivedfromhumanamnionmesenchymal stemcellspromotescutaneouswoundhealinginratsand fibroblastmigration bydirectlyinhibitingLATS2expression. StemCellRes.Ther. 11,56(2020).
227.Li,P.etal.EndothelialprogenitorcellderivedexosomesmediatedmiR-182-5p deliveryacceleratediabeticwoundhealingviadown-regulatingPPARG. Int.J. Med.Sci. 20,468–481(2023).
228.Zhao,G.etal.MSC-derivedexosomesattenuatecelldeaththroughsuppressing AIFnucleustranslocationandenhancecutaneouswoundhealing. StemCellRes. Ther. 11,174(2020).
229.Fang,S.etal.Umbilicalcord-derivedmesenchymalstemcell-derivedexosomal MicroRNAssuppressmyofibroblastdifferentiationbyinhibitingthetransforminggrowthFactor-β/SMAD2pathwayduringwoundhealing. StemCellsTransl. Med. 5,1425–1439(2016).
230.Hu,J.,Chen,Y.,Huang,Y.&Su,Y.Humanumbilicalcordmesenchymalstemcellderivedexosomessuppressdermal fibroblasts-myofibroblatstransitionvia inhibitingtheTGF-beta1/Smad2/3signalingpathway. Exp.Mol.Pathol. 115, 104468(2020).
231.Hu,L.etal.Exosomesderivedfromhumanadiposemensenchymalstemcells acceleratescutaneouswoundhealingviaoptimizingthecharacteristicsof fibroblasts. Sci.Rep. 6,32993(2016).
232.Chen,B.etal.Humanembryonicstemcell-derivedexosomespromotepressure ulcerhealinginagedmicebyrejuvenatingsenescentendothelialcells. StemCell Res.Ther. 10,142(2019).
233.Bae,Y.U.etal.Embryonicstemcell-derivedmmu-miR-291a-3pinhibitscellular senescenceinhumandermal fibroblaststhroughtheTGF-betaReceptor2 pathway. J.Gerontol.A 74,1359–1367(2019).
234.Bai,Y.etal.Adiposemesenchymalstemcell-derivedexosomesstimulatedby hydrogenperoxideenhancedskin flaprecoveryinischemia-reperfusioninjury. Biochem.Biophys.Res.Commun. 500,310–317(2018).
235.Liu,Y.etal.Exosomesderivedfromstemcellsfromapicalpapillapromote craniofacialsofttissueregenerationbyenhancingCdc42-mediatedvascularization. StemCellRes.Ther. 12,76(2021).
236.Li,M.etal.Mesenchymalstemcell-derivedexosomesamelioratedermal fibrosis inaMurinemodelofBleomycin-InducedScleroderma. StemCellsDev. 30, 981–990(2021).
237.Koken,G.Y.,Abamor,E.S.,Allahverdiyev,A.&Karaoz,E.WhartonJellyderived mesenchymalstemCell’sexosomesdemonstratesignificantantileishmanialand woundhealingeffectsincombinationwithAloe-Emodin:aninvitrostudy. J. Pharm.Sci. 111,3232–3242(2022).
238.Cao,L.etal.Neuralprogenitorcell-derivednanovesiclespromotehairfollicle growthviamiR-100. J.Nanobiotechnol. 19,20(2021).
239.Oh,M.etal.Exosomesderivedfromhumaninducedpluripotentstemcells amelioratetheagingofskin fibroblasts. Int.J.Mol.Sci. 19,1715–1732(2018).
240.Stravitz,R.T.&Kramer,D.J.Managementofacuteliverfailure. Nat.Rev.Gastroenterol.Hepatol. 6,542–553(2009).
241.Lin,F.etal.MesenchymalstemcellsprotectagainstferroptosisviaexosomemediatedstabilizationofSLC7A11inacuteliverinjury. CellDeathDis. 13,271 (2022).
242.Shao,M.etal.Exosomesderivedfromhumanumbilicalcordmesenchymal stemcellsameliorateIL-6-inducedacuteliverinjurythroughmiR-455-3p. Stem CellRes.Ther. 11,37(2020).
243.Kisseleva,T.&Brenner,D.Molecularandcellularmechanismsofliver fibrosis anditsregression. Nat.Rev.Gastroenterol.Hepatol. 18,151–166(2021).
244.Ma,L.etal.Mesenchymalstemcell-originatedexosomalcircDIDO1suppresses hepaticstellatecellactivationbymiR-141-3p/PTEN/AKTpathwayinhumanliver fibrosis. DrugDeliv. 29,440–453(2022).
245.Wang,N.etal.3DhESCexosomesenrichedwithmiR-6766-3pamelioratesliver fibrosisbyattenuatingactivatedstellatecellsthroughtargetingtheTGFbetaRIISMADSpathway. J.Nanobiotechnol. 19,437(2021).
246.Hirao,H.,Nakamura,K.&Kupiec-Weglinski,J.W.Liverischaemia-reperfusion injury:anewunderstandingoftheroleofinnateimmunity. Nat.Rev.Gastroenterol.Hepatol. 19,239–256(2022).
247.Yang,B.etal.Bonemarrowmesenchymalstemcell-derivedhepatocyte-likecell exosomesreducehepaticischemia/reperfusioninjurybyenhancingautophagy. StemCellsDev. 29,372–379(2020).
248.Du,Y.etal.ExosomesfromHuman-InducedPluripotentStemCell-Derived MesenchymalStromalCells(hiPSC-MSCs)protectliveragainsthepaticischemia/ reperfusioninjuryviaactivatingsphingosinekinaseandSphingosine-1Phosphatesignalingpathway. Cell.Physiol.Biochem. 43,611–625(2017).
249.Venkat,P.etal.TherapeuticeffectsofCD133 + Exosomesonliverfunctionafter strokeintype2diabeticmice. Front.Neurosci. 17,1061485(2023).
250.Boxhoorn,L.etal.Acutepancreatitis. Lancet 396,726–734(2020).
251.Chen,M.etal.Exosomesfromhumaninducedpluripotentstemcellsderived mesenchymalstemcellsimprovedmyocardialinjurycausedbysevereacute pancreatitisthroughactivatingAkt/Nrf2/HO-1axis. CellCycle. 21,1578–1589(2022).
252.Uccioli,L.etal.Criticallimbischemia:currentchallengesandfutureprospects. Vasc.HealthRiskManag. 14,63–74(2018).
253.Komaki,M.etal.Exosomesofhumanplacenta-derivedmesenchymalstemcells stimulateangiogenesis. StemCellRes.Ther. 8,219(2017).
254.Gong,M.etal.MesenchymalstemcellsreleaseexosomesthattransfermiRNAs toendothelialcellsandpromoteangiogenesis. Oncotarget 8,45200–45212 (2017).
255.Mathiyalagan,P.etal.AngiogenicMechanismsofHumanCD34(+)StemCell ExosomesintheRepairofIschemicHindlimb. Circ.Res. 120,1466–1476(2017).
Clinicalapplicationsofstemcell-derivedexosomes Tanetal.
256.Ye,M.etal.Exosomesderivedfromhumaninducedpluripotentstemcellsendotheliacellspromotespostnatalangiogenesisinmicebearingischemic limbs. Int.J.Biol.Sci. 15,158–168(2019).
257.Lazar,A.&Morrissey,N.Recentadvancesinendovasculartreatmentofperipheralarterialdisease. F1000Res. 9,122–126(2020).
258.Li,X.etal.Exosomesderivedfromendothelialprogenitorcellsattenuatevascularrepairandacceleratereendothelializationbyenhancingendothelial function. Cytotherapy. 18,253–262(2016).
259.Hu,H.etal.Endothelialprogenitorcell-derivedexosomesfacilitatevascular endothelialcellrepairthroughshuttlingmiR-21-5ptomodulate Thrombospondin-1expression. Clin.Sci. 133,1629–1644(2019).
260.Hu,H.,Jiang,C.,Li,R.&Zhao,J.Comparisonofendothelialcell-andendothelial progenitorcell-derivedexosomesinpromotingvascularendothelialcellrepair. Int.J.Clin.Exp.Pathol. 12,2793–2800(2019).
261.Kong,J.etal.Exosomesofendothelialprogenitorcellsinhibitneointimaformationaftercarotidarteryinjury. J.Surg.Res. 232,398–407(2018).
262.Thyoka,M.etal.Advancednecrotizingenterocolitispart1:mortality. Eur.J. Pediatr.Surg. 22,8–12(2012).
263.McCulloh,C.J.etal.Treatmentofexperimentalnecrotizingenterocolitiswith stemcell-derivedexosomes. J.Pediatr.Surg. 53,1215–1220(2018).
264.Moore,L.J.etal.Sepsisingeneralsurgery:adeadlycomplication. Am.J.Surg. 198,868–874(2009).
265.Zhou,Y.etal.Exosomesfromendothelialprogenitorcellsimprovetheoutcome ofamurinemodelofsepsis. Mol.Ther. 26,1375–1384(2018).
266.Liu,Y.etal.Protectiveeffectofendothelialprogenitorcell-derivedexosomal microRNA-382-3ponsepsis-inducedorgandamageandimmunesuppressionin mice. Am.J.Transl.Res. 14,6856–6873(2022).
267.Heusch,G.Myocardialischaemia–reperfusioninjuryandcardioprotectionin perspective. Nat.Rev.Cardiol. 17,773–789(2020).
268.Xing,X.etal.Adipose-derivedmesenchymalstemcells-derivedexosomemediatedmicroRNA-342-5pprotectsendothelialcellsagainstatherosclerosis. Aging 12,3880–3898(2020).
269.Peng,Y.etal.ExosomalmiR-25-3pfrommesenchymalstemcellsalleviates myocardialinfarctionbytargetingpro-apoptoticproteinsandEZH2. CellDeath Dis. 11,317(2020).
270.Gao,L.etal.ExosomessecretedbyhiPSC-derivedcardiaccellsimproverecovery frommyocardialinfarctioninswine. Sci.Transl.Med. 12,317–331(2020).
271.Wen,Z.etal.Mesenchymalstemcell-derivedexosomesamelioratecardiomyocyteapoptosisinhypoxicconditionsthroughmicroRNA144bytargeting thePTEN/AKTpathway. StemCellRes.Ther. 11,36(2020).
272.Santoso,M.R.etal.Exosomesfrominducedpluripotentstemcell-derived cardiomyocytespromoteautophagyformyocardialrepair. J.Am.HeartAssoc. 9, e014345(2020).
273.Katsur,M.etal.Exosomesfromneuronalstemcellsmayprotecttheheartfrom ischaemia/reperfusioninjuryviaJAK1/2andgp130. J.Cell.Mol.Med. 25, 4455–4465(2021).
274.Chen,G.etal.Mesenchymalstemcell-derivedexosomalmiR-143-3psuppresses myocardialischemia-reperfusioninjurybyregulatingautophagy. LifeSci. 280, 119742(2021).
275.Chen,F.etal.Bonemarrowmesenchymalstemcell-derivedexosomes attenuatecardiachypertrophyand fibrosisinpressureoverloadinduced remodeling. InVitroCell.Dev.Biol.Anim. 56,567–576(2020).
276.Khan,M.etal.Embryonicstemcell-derivedexosomespromoteendogenous repairmechanismsandenhancecardiacfunctionfollowingmyocardialinfarction. Circ.Res. 117,52–64(2015).
277.TavakoliDargani,Z.&Singla,D.K.Embryonicstemcell-derivedexosomes inhibitdoxorubicin-inducedTLR4-NLRP3-mediatedcelldeath-pyroptosis. Am.J. Physiol.HeartCirc.Physiol. 317,H460–H471(2019).
278.Singla,D.K.,Johnson,T.A.&TavakoliDargani,Z.Exosometreatmentenhances anti-inflammatoryM2macrophagesandreducesinflammation-inducedpyroptosisindoxorubicin-inducedcardiomyopathy. Cells. 8,1224–1244(2019).
279.Pang,Y.etal.Embryonicstemcell-derivedexosomesattenuatetransverseaortic constrictioninducedheartfailurebyincreasingangiogenesis. Front.Cardiovasc. Med. 8,638771(2021).
280.Kervadec,A.etal.Cardiovascularprogenitor-derivedextracellularvesicles recapitulatethebeneficialeffectsoftheirparentcellsinthetreatmentof chronicheartfailure. J.HeartLungTransplant. 35,795–807(2016).
281.ElHarane,N.etal.Acellulartherapeuticapproachforheartfailure:invitro productionofextracellularvesiclesfromhumancardiovascularprogenitors. Eur. HeartJ. 39,1835–1847(2018).
282.Li,H.etal.Exosomessecretedbyendothelialcellsderivedfromhumaninduced pluripotentstemcellsimproverecoveryfrommyocardialinfarctioninmice. StemCell.Res.Ther. 14,278(2023).
283.Li,H.etal.IsolationofswinebonemarrowLin-/CD45-/CD133 + cellsandcardioprotectiveeffectsofitsexosomes. StemCell.Rev.Rep. 19,213–229(2023).
284.Angulski,A.B.B.etal.SystemicinfusionofexpandedCD133(+)cellsand expandedCD133(+)cell-derivedEVsforthetreatmentofischemiccardiomyopathyinaratmodelofAMI. StemCellsInt. 2019,4802578(2019).
285.Mackie,A.R.etal.Sonichedgehog-modi fi edhumanCD34 + cellspreserve cardiacfunctionafteracutemyocardialinfarction. Circ.Res. 111 ,312– 321 (2012).
286.Ke,X.etal.Humanendothelialprogenitorcell-derivedexosomesincrease proliferationandangiogenesisincardiac fibroblastsbypromotingthe mesenchymal-endothelialtransitionandreducinghighmobilityGroupBox1 proteinB1expression. DNACellBiol. 36,1018–1028(2017).
287.Ke,X.etal.ExosomalmiR-218-5p/miR-363-3pfromendothelialprogenitorcells amelioratemyocardialinfarctionbytargetingthep53/JMYsignalingpathway. Oxid.Med.Cell.Longev. 2021,5529430(2021).
288.Yue,Y.etal.Interleukin-10deficiencyaltersendothelialprogenitorcell-derived exosomereparativeeffectonmyocardialrepairviaintegrin-linkedkinase enrichment. Circ.Res. 126,315–329(2020).
289.Liu,Q.etal.ExosomalmiR-17-5pfromhumanembryonicstemcellsprevents pulmonary fibrosisbytargetingthrombospondin-2. StemCell.Res.Ther. 14,234 (2023).
290.Zhou,Y.etal.Exosomesderivedfrominducedpluripotentstemcellssuppresses M2-typemacrophagesduringpulmonary fibrosisviamiR-302a-3p/TET1axis. Int. Immunopharmacol. 99,108075(2021).
291.Liu,P.etal.Endothelialprogenitorcell-derivedexosomesinhibitpulmonary arterysmoothmusclecellinvitroproliferationandresistancetoapoptosisby modulatingtheMitofusin-2andRas-Raf-ERK1/2signalingpathway. Eur.J. Pharmacol. 949,175725(2023).
292.Zhou,Y.etal.Exosomesfromendothelialprogenitorcellsimproveoutcomesof thelipopolysaccharide-inducedacutelunginjury. Crit.Care. 23,44(2019).
293.Wu,X.etal.Exosomesderivedfromendothelialprogenitorcellsameliorate acutelunginjurybytransferringmiR-126. Exp.CellRes. 370,13–23(2018).
294.Zhang,X.etal.Exosomessecretedbyendothelialprogenitorcellsimprovethe bioactivityofpulmonarymicrovascularendothelialcellsexposedtohyperoxia invitro. Ann.Transl.Med. 7,254(2019).
295.Montay-Gruel,P.etal.Extracellularvesiclesforthetreatmentofradiationinducednormaltissuetoxicityinthelung. Front.Oncol. 10,602763(2020).
296.Huang,R.,Fu,P.&Ma,L.Kidney fibrosis:frommechanismstotherapeutic medicines. Signal.Transduct.TargetTher. 8,129(2023).
297.Liu,Y.etal.Bonemarrowmesenchymalstemcell-derivedexosomalmicroRNA381-3palleviatesvascularcalcificationinchronickidneydiseasebytargeting NFAT5. CellDeathDis. 13,278(2022).
298.Liu,Y.etal.Bonemarrowmesenchymalstemcell-derivedexosomesimprove renal fibrosisviaregulatingSmurf2/Smad7. Front.Biosci. 27,17(2022).
299.Lu,Y.etal.Bonemarrowmesenchymalstemcell-derivedexosomesimprove renal fibrosisbyreducingthepolarisationofM1andM2macrophagesthrough theactivationofEP2receptors. IETNanobiotechnol. 16,14–24(2022).
300.Kellum,J.A.etal.Acutekidneyinjury. Nat.Rev.Dis.Primers. 7,52(2021).
301.Lim,S.W.etal.Alleviationofrenalischemia/reperfusioninjurybyexosomes frominducedpluripotentstemcell-derivedmesenchymalstemcells. KoreanJ. Intern.Med. 37,411–424(2022).
302.Zhang,Y.etal.Endothelialprogenitorcells-derivedexosomalmicroRNA-21-5p alleviatessepsis-inducedacutekidneyinjurybyinhibitingRUNX1expression. CellDeathDis. 12,335(2021).
303.Sheffield,A.M.&Smith,R.J.H.Theepidemiologyofdeafness. ColdSpringHarb Perspect.Med. 9,3258–3273(2019).
304.Tsai,S.C.etal.Umbilicalcordmesenchymalstromalcell-derivedexosomes rescuethelossofouterhaircellsandrepaircochleardamageincisplatininjectedmice. Int.J.Mol.Sci. 22,6664–6687(2021).
305.Tabuchi,K.etal.Ischemia-reperfusioninjuryofthecochlea:pharmacological strategiesforcochlearprotectionandimplicationsofglutamateandreactive oxygenspecies. CurrNeuropharmacol. 8,128–134(2010).
306.Hao,F.etal.ExosomesderivedfrommicroRNA-21overexpressingneuralprogenitorcellspreventhearinglossfromischemia-reperfusioninjuryinmicevia inhibitingtheinflammatoryprocessintheCochlea. ACSChem.Neurosci. 13, 2464–2472(2022).
307.Chotigavanich,C.etal.HypothyroidismafterHemithyroidectomy:theincidence andriskfactors. J.Med.Assoc.Thai. 99,77–83(2016).
308.Degosserie,J.etal.Extracellularvesiclesfromendothelialprogenitorcells promotethyroidfollicleformation. J.Extracell.Vesicles. 7,1487250(2018).
309.Valesan,L.F.etal.Prevalenceoftemporomandibularjointdisorders:asystematicreviewandmeta-analysis. Clin.Oral.Investig. 25,441–453(2021).
310.Zhang,S.etal.MSCexosomesalleviatetemporomandibularjointosteoarthritis byattenuatinginflammationandrestoringmatrixhomeostasis. Biomaterials 200,35–47(2019).
311.Yang,Y.&Sun,X.Retinalganglioncelldeathinglaucoma:advancesand caveats. Curr.EyeRes. 48,1–10(2023).
312.Mead,B.&Tomarev,S.Bonemarrow-derivedmesenchymalstemcells-derived exosomespromotesurvivalofretinalganglioncellsthroughmiRNA-dependent mechanisms. StemCells.Transl.Med. 6,1273–1285(2017).
313.VanGelder,R.N.etal.Regenerativeandrestorativemedicineforeyedisease. Nat.Med. 28,1149–1156(2022).
314.Bian,B.etal.Exosomesderivedfromneuralprogenitorcellspreservephotoreceptorsduringretinaldegenerationbyinactivatingmicroglia. J.Extracell. Vesicles. 9,1748931(2020).
315.Ke,Y.etal.Humanembryonicstemcell-derivedextracellularvesiclesalleviate retinaldegenerationbyupregulatingOct4topromoteretinalMullercellretrodifferentiationviaHSP90. StemCell.Res.Ther. 12,21(2021).
316.Gao,Y.etal.Embryonicstemcells-derivedexosomesenhanceretrodifferentiationofretinalMullercellsbydeliveringBDNFproteintoactivateWnt pathway. Immunobiology 227,152211(2022).
317.Park,U.C.etal.SubretinalversusintravitrealadministrationofhumanCD34+ bonemarrow-derivedstemcellsinaratmodelofinheritedretinaldegeneration. Ann.Transl.Med. 9,1275(2021).
318.Ong,E.S.&Jeng,B.H.Currentandfuturetherapiesforpersistentcorneal epithelialdefectsandneurotrophickeratopathy. Curr.Opin.Ophthalmol. 32, 262–267(2021).
319.Wang,S.etal.Comparisonofexosomesderivedfrominducedpluripotentstem cellsandmesenchymalstemcellsastherapeuticnanoparticlesfortreatmentof cornealepithelialdefects. Aging 12,19546–19562(2020).
320.Zhu,J.etal.Roleofimmunecelldiversityandheterogeneityincornealgraft survival:asystematicreviewandmeta-analysis. J.Clin.Med. 10,4667–4686(2021).
321.Jia,Z.etal.Mesenchymalstemcellderivedexosomes-basedimmunological signatureinaratmodelofcornealallograftrejectiontherapy.Front.Biosci. 86, (2022).
322.Huang,Q.Y.etal.Therapeuticoptionsforprematureovarianinsufficiency:an updatedreview. Reprod.Biol.Endocrinol. 20,28(2022).
323.Na,J.&Kim,G.J.Recenttrendsinstemcelltherapyforprematureovarian insufficiencyanditstherapeuticpotential:areview. J.OvarianRes. 13,74(2020).
324.Li,Z.etal.Humanumbilicalcordmesenchymalstemcell-derivedexosomes improveovarianfunctionandproliferationofprematureovarianinsufficiency byregulatingthehipposignalingpathway. Front.Endocrinol. 12,711902(2021).
325.Zhang,L.etal.Humanpluripotentstemcell-mesenchymalstemcell-derived exosomespromoteovariangranulosacellproliferationandattenuatecell apoptosisinducedbycyclophosphamideinaPOI-likeMouseModel. Molecules. 28,2112–2129(2023).
326.Ding,C.etal.ExosomalmiRNA-17-5pderivedfromhumanumbilicalcord mesenchymalstemcellsimprovesovarianfunctioninprematureovarian insufficiencybyregulatingSIRT7. StemCells. 38,1137–1148(2020).
327.Yang,M.etal.Bonemarrowmesenchymalstemcell-derivedexosomalmiR-1445pimprovesratovarianfunctionafterchemotherapy-inducedovarianfailureby targetingPTEN. Lab.Investig. 100,342–352(2020).
328.Chen,Y.S.,Lin,E.Y.,Chiou,T.W.&Harn,H.J.Exosomesinclinicaltrialandtheir productionincompliancewithgoodmanufacturingpractice. CiJiYiXueZaZhi. 32,113–120(2020).
329.Perocheau,D.etal.Clinicalapplicationsforexosomes:Arewethereyet? Br.J. Pharmacol. 178,2375–2392(2021).
330.Rezaie,J.,Feghhi,M.&Etemadi,T.Areviewonexosomesapplicationinclinical trials:perspective,questions,andchallenges. CellCommun.Signal. 20,145 (2022).
331.Wang,X.etal.Recentprogressinexosomeresearch:isolation,characterization andclinicalapplications. CancerGeneTher. 30,1051–1065(2023).
332.Santos,P.&Almeida,F.Exosome-basedvaccines:history,currentstate,and clinicaltrials. Front.Immunol. 12,711565(2021).
333.Hao,Z.C.Stemcell-derivedexosomes:apromisingstrategyforfracturehealing. CellProlif. 50,359–368(2017).
334.Yang,Z.L.etal.TheroleofexosomesandexosomalnoncodingRNAsfrom differentcellsourcesinspinalcordinjury. Front.Cell.Neurosci. 16,882306 (2022).
335.Hade,M.D.,Suire,C.N.&Suo,Z.Mesenchymalstemcell-derivedexosomes: applicationsinregenerativemedicine. Cells. 10,1959–2006(2021).
336.Lou,G.,Chen,Z.,Zheng,M.&Liu,Y.Mesenchymalstemcell-derivedexosomes asanewtherapeuticstrategyforliverdiseases. Exp.Mol.Med. 49,e346(2017).
337.Krishnan,A.,Muthusamy,S.,Fernandez,F.B.&Kasoju,N.Mesenchymalstem cell-derivedextracellularvesiclesinthemanagementofCOVID19-associated lunginjury:areviewonpublications,clinicaltrialsandpatentlandscape. Tissue Eng.Regen.Med. 19,659–673(2022).
338.Tsioulos,G.etal.InsightsintoCD24andexosomephysiologyandpotentialrole inviewofrecentadvancesinCOVID-19therapeutics:anarrativereview. Life. 12, 1472–1487(2022).
339.Yoo,K.H.Possibilityofexosome basedcoronavirusdisease2019vaccine (Review). Mol.Med.Rep. 25,3625–3633(2022).
340.Yassine,S.&Alaaeddine,N.Mesenchymalstemcellexosomesandcancer: controversiesandprospects. Adv.Biol. 6,e2101050(2022).
341.Vakhshiteh,F.,Atyabi,F.&Ostad,S.N.Mesenchymalstemcellexosomes:atwoedgedswordincancertherapy. Int.J.Nanomed. 14,2847–2859(2019).
342.Lin,Z.etal.Mesenchymalstemcell-derivedexosomesincancertherapy resistance:recentadvancesandtherapeuticpotential. Mol.Cancer. 21,179 (2022).
343.Xu,Z.,Zeng,S.,Gong,Z.&Yan,Y.Exosome-basedimmunotherapy:apromising approachforcancertreatment. Mol.Cancer. 19,160(2020).
344.Nam,G.H.etal.Emergingprospectsofexosomesforcancertreatment:from conventionaltherapytoimmunotherapy. Adv.Mater. 32,e2002440(2020).
345.Kim,H.Recentadvancesinexosome-baseddrugdeliveryforcancertherapy. Cancers. 13,4435–4457(2021).
346.Dai,J.etal.Exosomes:keyplayersincancerandpotentialtherapeuticstrategy. Signal.Transduct.TargetTher. 5,145(2020).
347.Nie,L.etal.Directionalinductionofneuralstemcells,anewtherapyforneurodegenerativediseasesandischemicstroke. CellDeathDiscov. 9,215(2023).
348.Fernandez-Munoz,B.,Garcia-Delgado,A.B.,Arribas-Arribas,B.&Sanchez-Pernaute,R.Humanneuralstemcellsforcell-basedmedicinalproducts. Cells 10, 2377–2402(2021).
349.Shahbazi,E.,Mirakhori,F.,Ezzatizadeh,V.&Baharvand,H.Reprogrammingof somaticcellstoinducedneuralstemcells. Methods 133,21–28(2018).
350.Ma,Y.etal.Inducedneuralprogenitorcellsabundantlysecreteextracellular vesiclesandpromotetheproliferationofneuralprogenitorsviaextracellular signal-regulatedkinasepathways. Neurobiol.Dis. 124,322–334(2019).
351.Ma,Y.etal.Inducedneuralprogenitorcell-derivedextracellularvesiclespromoteneuralprogenitorcellsurvivalviaextracellularsignal-regulatedkinase pathway. CNSNeurosci.Ther. 27,1605–1609(2021).
OpenAccess ThisarticleislicensedunderaCreativeCommons Attribution4.0InternationalLicense,whichpermitsuse,sharing, adaptation,distributionandreproductioninanymediumorformat,aslongasyougive appropriatecredittotheoriginalauthor(s)andthesource,providealinktotheCreative Commonslicense,andindicateifchangesweremade.Theimagesorotherthirdparty materialinthisarticleareincludedinthearticle’sCreativeCommonslicense,unless indicatedotherwiseinacreditlinetothematerial.Ifmaterialisnotincludedinthe article’sCreativeCommonslicenseandyourintendeduseisnotpermittedbystatutory regulationorexceedsthepermitteduse,youwillneedtoobtainpermissiondirectly fromthecopyrightholder.Toviewacopyofthislicense,visit http:// creativecommons.org/licenses/by/4.0/
©TheAuthor(s)2023
