etal. CellDeathandDisease (2018)9:183
Regenerativepotentialoftonsil mesenchymalstemcellsonsurgical cutaneousdefect
Sung-ChanShin1,YoojinSeo2,HeeYoungPark1,Da-WoonJung1,Tae-HoonShin2,HaejinSon2,YoungKeumKim3, Jin-ChoonLee4,Eui-SukSung4,JeonYeobJang5,Hyung-SikKim2 andByung-JooLee1
Abstract
Astissueengineeringandregenerativemedicinehaveevolvedrecently,stemcelltherapyhasbeeninvestigatedinthe fieldofimpairedwoundhealing.Severalstudieshavereportedthatmesenchymalstemcellsderivedfromvarious tissuesincludingbonemarrowandadiposetissuecanexerttheregenerativeefficacyinthewoundhealing.Previously, wehavedemonstratedtheisolationandcharacterizationoftonsil-derivedmesenchymalstemcells(TMSCs)with excellentproliferativeproperty.Inthepresentstudy,weaimedtoevaluatetheregenerativeefficacyofTMSCsinthe woundhealingprocess.Twodistinctcutaneoussurgicaldefectsweregeneratedinthedorsumofmice.Eachwound wastreatedwithTMSCsorphosphate-bufferedsaline(PBS),respectively.Aftersacrifice,theskinandsubcutaneous tissuesaroundthesurgicaldefectwereharvestedandassessedforinflammation,re-epithelialization,dermal regeneration,andgranulationtissueformation.TheadministrationofTMSCsintowoundbedssignificantlypromoted therepairofsurgicaldefectsinmice.Especially,TMSCsefficientlycontributedtotheattenuationofexcessive inflammationinthesurgicallesion,aswellastheaugmentationofepidermalanddermalregeneration.Toelucidate theunderlyingmechanisms,TMSCswereanalyzedfortheirpotencyinimmunomodulatoryabilityonimmunecells, stimulatoryeffectontheproliferationofkeratinocytes,and fibroblasts,aswellastheregulationof fibroblast differentiation.TMSCsinhibitedthenon-specificorT-cell-specificproliferationofperipheralbloodmononuclearcells, aswellastheM1polarizationofmacrophage-likecells.Moreover,TMSCsaugmentedtheproliferationofskinconstituting fibroblastsandkeratinocyteswhiletheysuppressedthedifferentiationof fibroblastsintomyofibroblasts. Takentogether,our findingsdemonstratetheregenerativepotentialofTMSCsinwoundhealingprocessthroughthe regulationoninflammation,proliferation,andremodelingofvariousskincells,implyingthatTMSCscanbea promisingalternativeforwoundrepair.
Correspondence:H-S.Kim(hskimcell@pusan.ac.kr)orB-J.Lee (voiceleebj@gmail.com)
1DepartmentofOtorhinolaryngology-HeadandNeckSurgery,Biomedical ResearchInstitute,PusanNationalUniversitySchoolofMedicine,Pusan NationalUniversityHospital,Busan,RepublicofKorea 2BiomedicalResearchInstitute,PusanNationalUniversitySchoolofMedicine, PusanNationalUniversityHospital,Busan,RepublicofKorea Fulllistofauthorinformationisavailableattheendofthearticle S-CShinandYSeocontributedequallytothiswork. H-SKimandB-JLeecontributedequallytothiswork. EditedbyYShi.
©TheAuthor(s)2018
Introduction
Woundhealingprocessisinitiatedinresponsetovariousdeleteriousstimulitorestorethetissuehomeostasis aswellastolimitfurtherdamage1,2.Itisahighlyorganized,well-controlledprocedurewhichconsistsof somewhatoverlappingbutspecificstages:inflammation, proliferation,andmaturation/remodeling3.Severaltypes ofcellsincludingtissueresident-orrecruitedimmune cells,keratinocytes,tissuestemcells,and fibroblastsare
OpenAccess ThisarticleislicensedunderaCreativeCommonsAttribution4.0InternationalLicense,whichpermitsuse,sharing,adaptation,distributionand reproduction inanymediumorformat,aslongasyougiveappropriatecredittotheoriginalauthor(s)andthesource,providealinktotheCreativeCommonslicense, andindicateif changesweremade.Theimagesorotherthirdpartymaterialinthisarticleareincludedinthearticle’sCreativeCommonslicense,unlessindicated otherwiseinacreditlinetothematerial.If materialisnotincludedinthearticle’sCreativeCommonslicenseandyourintendeduseisnotpermittedbystatutoryregulationorexceedsthepermitteduse,youwillneedtoobtain permissiondirectlyfromthecopyrightholder.Toviewacopyofthislicense,visit http://creativecommons.org/licenses/by/4.0/

involvedinwoundrepairprocess1,4.Adequateenvironmentalcuesoriginatedfromvariousextracellularmatrix andsolublesignalingmoleculesarealsoessentialfora successfulwoundrepair5,6.Importantly,failureinany singlephaseofthenormalwoundresponsecanleadto chronicwound,resultingindelayedrecoveryand,even worse,thepermanentlossoftissue.Intheeraofacceleratedpopulationagingwiththegrowingriskofchronic orprogressivemedicalconditionssuchasdiabetes,the chronicwoundhasbecomeasignificanteconomicburden tothesociety7,8.Althoughsomesurgicaltechniques includingskingraftand flapshavebeentriedtotreatthe delayed-ornon-healingwoundincombinationwith generalsymptomaticmanagement(e.g.anti-inflammatory agentandpainreducer)9,10,thetherapeuticoutcomeis oftenlimitedwithlowfunctionalrecoveryscore.Given thatdisruptedcellularhomeostasis,includingpersistent inflammation,excessive fibrosis,anddecreasedangiogenesisunderlieschronicwoundformation,morecomprehensiveapproachesarerequiredforcompletewound repair.
Asavarietyofstudieshavebeenperformedinthe field oftissueengineeringandregenerativemedicinerecently, stemcelltherapyhasbeenwidelyappliedtointractable diseasestoovercomethelimitationofconventional treatmentoptions11.Inparticular,mesenchymalstem cells(MSCs),whichpossesslessethical-andsafetyissues thanembryonicstemcellsorinducedpluripotentstem cells,cannotonlymaintaintheirself-renewalcapacitybut alsodifferentiateintomultiplecelltypes12,13.Furthermore,throughtheinteractionwithothertypesofcellsand microenvironment,MSCscanactivelymigrateinto inflammatoryordamagedsitestosecreteanumberof paracrinefactors,includinggrowthfactorsandcytokines associatedwithregeneration,immunomodulation,and angiogenesis14–16.Becauseofthesebeneficialcharacteristics,therapeuticpotentialofMSCshasbeeninvestigated andprovenforchronicwoundrepair.Severalgroupshave reportedthatbonemarrow-derivedMSCs(BMSCs)and adiposetissue-derivedMSCscanstimulateneoangiogenesisandreduceexcessiveinflammationto improvecutaneouswoundhealing17–20.These findings supporttheadvantagesofMSCtherapyagainstwound healingimpairment;however,thetherapeuticefficacyand relevantundelyingmechanismsofMSCsfromdifferent sourceshavenotbeenfullyuncoveredandtheharvesting ofMSCsfrombonemarrowandadiposetissues,themain sourcesofadultMSCs,issomewhatinvasive.
Tonsillectomy,asurgicalproceduretoremovethe tonsilincaseofchronictonsillitisortonsillarhypertrophy,isoneofthemostcommonsurgeryinthe fieldof otolaryngology.Interestingly,severalgroupsincludingour owngrouphavesuccessfullyisolatedpalatinetonsilderivedMSCs(TMSCs)andfurtherrevealedthatTMSCs
exhibitsimilarcharacteristicswithMSCsfromothertissuesintheirmorphology,thepatternofsurfacemarker expression,aswellasthepotentialofproliferationand differentiation21,22.Furthermore,growingevidencehas suggestedthetherapeuticbenefitsofTMSCapplicationin variousdiseasessuchasliver fibrosis,allergicrhinitis,and peripheralnerveinjury23–25.Inourownpreviousstudies, wesuccessfullyisolatedandcharacterizedTMSCsand furtherelucidatedtheiruniquefunctionsrepresentedby superiorproliferationpotential,indicatingthatTMSCs mightbeapromisingsourceforthelarge-scalemanufacturingoftherapeuticsfromstemcellsortheirderivatives21,26.Toinvestigatethepossiblebeneficialefficacyof TMSCsinwoundrepairandtofurtherelucidatetheir underlyingmechanisms,wecreatedexcisionalwound splintingmodelanddeliveredTMSCsintowoundbeds.
Results
TMSCsacceleratewoundhealinginvivothroughthe suppressionofinflammationandtheinductionof regeneration
We firstinvestigatedwhetherthetransplantationof TMSCscoulddemonstrateanybeneficialeffectsagainst excisionalwoundsplintingmodel.Basedonourprevious resultsdemonstratingtheoptimaldosageanddelivery routeofMSCsfromvarioussourcesagainstmurine modelsofseveraldisordersincludingskininflammatory disease,weinfusedonemillioncellslocallyontowound bedstoexpectoptimumefficacy 27–30.Therefore,one millionTMSCswereplacedonthewoundbedsafter woundgenerationandthereductionrateofwoundarea wasmeasuredeveryotherdayuntilday12.PBSwas placedontothewoundbedsintheoppositesideof TMSC-treatedwoundasavehiclecontrolgroup.Wound areasdiminishedtime-dependentlyinbothPBS-or TMSC-treatedgroupsandTMSCspromotedthewound closurethroughoutthemonitoringperiod(Fig.1a,b). Particularly,TMSCssignificantlyacceleratedthereductionofwoundareaonday6,comparedtoPBS-treated group(Fig.1a,b).
Histopathologicalevaluationbasedonhematoxylinand eosin(H&E)stainingdemonstratedconsistentefficacyof TMSCtreatment.Ondays6and8,TMSCssignificantly downregulatedtheinfiltrationofinflammatorycells, includingneutrophils,lymphocytes,histocytes,and plasmacells,comparedtoPBS-treatedgroup(Fig.2a,b andSupplementaryFigureS1A-B).Moreover,TMSCs significantlyattenuatedtheinfiltrationofinflammatory cellsproducingtumornecrosisfactor(TNF)-α ondays6 and8,whiletheyslightlyenhancedtheproductionof interleukin(IL)-10,aprominentanti-inflammatorycytokine(SupplementaryFigureS2AandB).Epidermal thicknessandcollagendepositionwerenotsignificantly differentbetweentwogroups(Fig.2a,b).Interestingly,

Fig.1EfficacyofTMSCsinpunchbiopsywoundmodel.a Representativetime-courseimagesofwoundclosureinamurineexcisionalskin woundmodelafterTMSCsorPBSdelivery. b Thereducedwoundsize(%)relativetoday0lesion(0%;completewoundclosureisconsideredas 100%)wasdeterminedeveryotherdayfor12days.** P <0.01.Resultsareshownasmean±SD
epidermalregenerationwassignificantlyimprovedby TMSCtreatmentatdays6and8(Fig.2a,c).Moreover, TMSCtransplantationnotonlyinduceddermalregenerationsignificantlyatday8,butalsoaugmentedthe formationofgranulationtissueatdays4and6(Fig.2a,c).
Whentheef fi cacyofTMSCtreatmentwascompared tothatofadiposetissue-derivedMSCs(AMSCs),MSCs frombothsourcesexertedsimilarpotencyoftherapeuticef fi cacyinwoundrepair(SupplementaryFigureS3AandB).Wenextveri fi edtheef fi cacyof conditionedmediaharvestedfromTMSCs(TMSCCM) toassessthefunctionofsolublefactorswithoutthe transplantationofcells.However,thetopicalapplication ofTMSCCMdidnotexhibitanysupportiveortherapeuticfunctioninwoundhealingprocess(SupplementaryFigureS3CandD).
Giventhatangiogenesisiscriticalintissueregenerationaswellaswoundrepair31,wenextinvestigated whetherTMSCtreatmentcouldaffecttheneovascularizationduringwoundhealingprocess.Skinsections containingwoundbedswerestainedwithanti-CD31 antibodiesandCD31 + vasculatureswerequanti fi edby measuringstainedarea.ComparedwithPBS-treated
wounds,neovascularizationwassigni fi cantlyincreased inTMSC-treatedwoundsatdays4and8(Fig.3a,b).
Takentogether,theseresultsindicatethatTMSC treatmentcanenhancewoundrepairingrossandhistopathologicalevaluationviaimmunomodulationand regeneration,presumablymediatedbyparacrinefunction oftransplantedcellsinthewoundbeds.
TMSCsdirectmacrophagestowardanti-inflammatorytype andsuppresstheproliferationofinflammatorycells
The firststageofwoundhealingistheinflammatory phase.Thisphasebeginsimmediatelyafterwoundgenerationtopreventtheinfectionaswellasthelossofblood and fluid.After2–3days,monocytesmigratetowound areaanddifferentiateintomacrophages,keyregulatory cellsforlatereventinwoundrepair32,33.Therefore,we thenassessedthefunctionalalterationinmacrophages afterco-culturewithTMSCs.Mousebonemarrowderivedmacrophages(BMDMs)werepolarizedinthe presenceofTMSCs.Activationofmacrophage-likecells intoM1typewithlipopolysaccharide(LPS)andinterferon (IFN)-γ ledtothesecretionoftumornecrosisfactor (TNF)-α (Fig. 4a).TMSCadditioninhibitedTNF-α
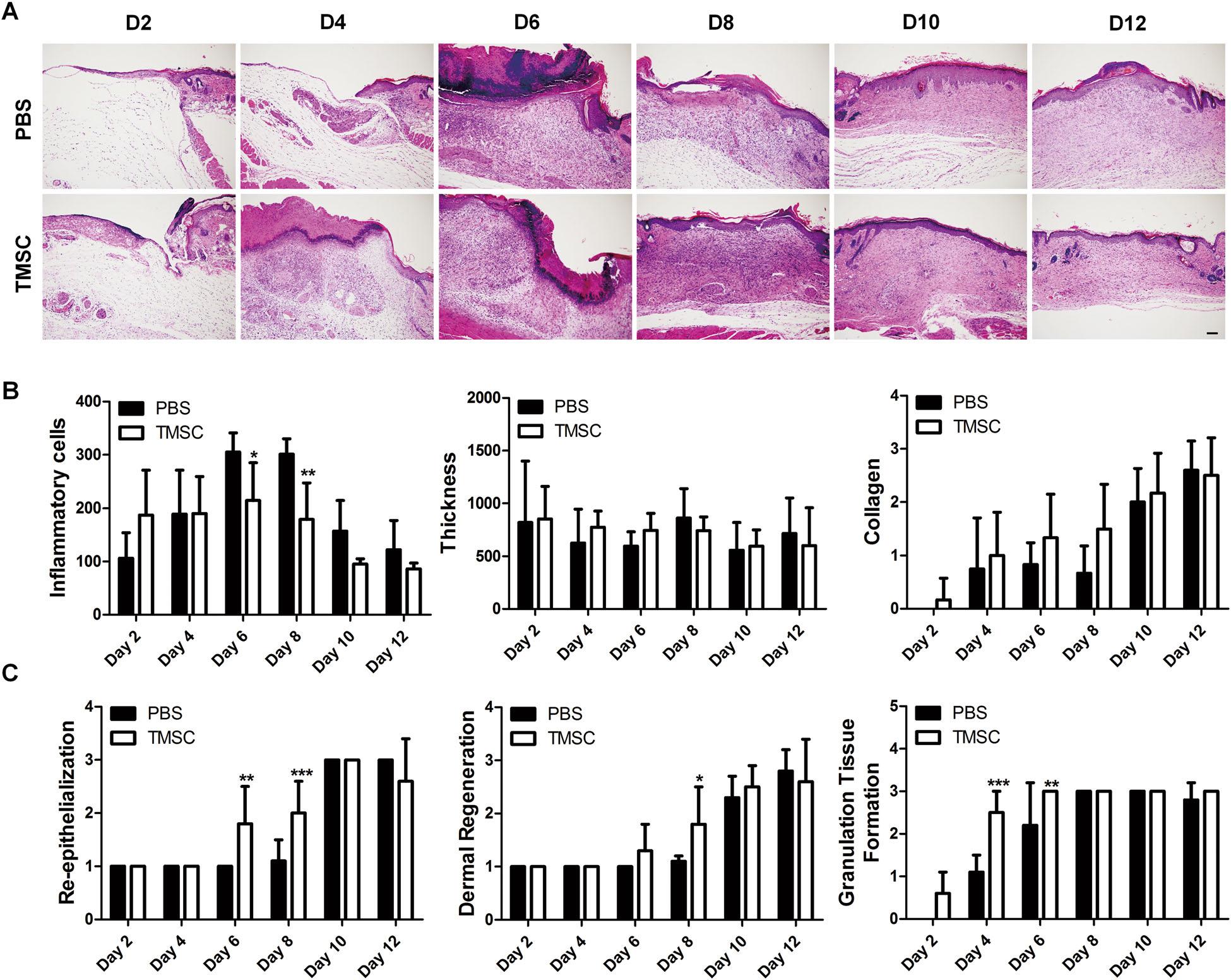
Fig.2HistopathologicalanalysisofwoundlesionafterTMSCtransplantation.a–c SkinwoundlesionswerecollectedandprocessedforH&E stainingtoperformthehistologicalassessment. a RepresentativeH&E-stainedsectionsareshown. b,c WoundhealingefficacyofTMSCscompared toPBSwasevaluatedbasedonwound-associatedindicatorsincludinginflammatorycellrecruitment,epidermalthickness/regeneration,collagen deposition,re-epithelialization,andgranulationtissueformation.TMSCsreducedlocalinflammationaswellastissuegranulationandenhanced epidermalregenerationduringthemiddlestageofwoundhealing(days4–8).*P <0.05,**P <0.01,***P <0.001.Scalebar=200 μm.Resultsare shownasmean±SD
secretionfromM1typemacrophage-likecellsinadosedependentmanner(Fig. 4a).Moreinterestingly,coculturewithTMSCsinducedthematurationofBMDMs withoutanyactivationintoIL-10secretingM2type (Fig.4b).ThisregulatoryfunctionofTMSCswereconsistentlyobservedinTMSCsfromtwodifferentdonors. ToprovethisregulatoryfunctionofTMSCsonmacrophagepolarizationinhumanmacrophages,macrophagelikecellswerematuredfromTHP-1,followedbystabilizationandactivationinthepresenceofTMSCs(Fig.4c).
Asobservedinco-cultureexperimentsusingmouse BMDMs,TMSCsdemonstratedthesimilardosedependentsuppressiveabilityontheproductionof TNF-α byM1typemacrophage-likecells(Fig. 4d). However,TMSCsdidnotsignificantlyaugmentthe
secretionofIL-10bymacrophage-likecellswithoutany stimulationforpolarization(datanotshown).
BecauseinvivoTMSCtransplantationattenuatedthe infiltrationofinflammatorycells,wenextexploredthe effectofTMSCsontheproliferationofinflammatorycells usingperipheralbloodmononuclearcells(PBMCs).All TMSCsisolatedfromdifferentdonorssignificantlysuppressedtheproliferationofPBMCsorTlymphocytes, stimulatedwithPokeweedmitogen(PWM)orantibodies forCD3andCD28,respectively(Fig.4c,d).
Takentogether,our findingsdemonstratethatTMSCs cancreateinflammatorymilieubeneficialforwound repairbythesuppressionofmacrophagepolarizationinto inflammatorytypesaswellastheproliferationofvarious inflammatorycells.

Fig.3AngiogenicfunctionofTMSCsinthewoundhealingprocess.a, b Toevaluatethepro-angiogenicimpactofTMSCsonwoundhealing, formalin-fixedskinsectionswerelabeledwithendothelialspecificmarker,CD31. a Thedistinctivesignofneovascularizationwasobservedduringthe healingprocessinbothgroups.RepresentativeCD31+ cellsareindicatedbyarrowheads. b RelativecomparisonofCD31+ areabetweenTMSCsand PBS-treatedwoundshowedthatangiogenesisoccurredmoreactivelyintheTMSCsgroupthaninthePBSgroup.***P <0.001.Scalebar=100 μm. Resultsareshownasmean±SD
TMSCssupportdermalregenerationthroughthe augmentationof fibroblastproliferationandmigration Thesecondstageofwoundrepairistheproliferation phasewhichbeginsonthethirddayafterinjuryandlasts forabout2weeks.Thisphaseischaracterizedbycellular proliferationandmigration,depositionofextracellular matrix,granulationtissueformationalongwithangiogenesis33.Inourpreviousresultsobtainedfrominvivo experiments,TMSCtransplantationontowoundbeds criticallycontributedtodermalregenerationatday8, whichisincludedintheproliferationphase.Therefore,we soughttoinvestigatewhetherTMSCscanaffectthe proliferationandmigrationof fibroblasts,whichconstitutedermis.TMSCswereco-culturedwithhuman dermal fibroblastsusingtranswellsystemtoprevent directcell-to-cellcontactwithintherangeof1:100to1:1, basedontheTMSC:fibroblastratio.Theproliferationof fibroblastswassignificantlyincreasedwhentheywerecoculturedTMSCsfromtwodifferentdonors,regardlessof theTMSC:fibroblastratio(Fig.5a,b).Todeterminethe chemotacticfunctionofTMSCstorecruit fibroblasts
towardwoundarea,migrationassaywasperformedusing transwell.Fibroblastsplatedinupperchambermigrated towardTMSCsinlowerchamber(Fig.5c,d).
OurdataproposethatTMSCsmightcontributeto dermalregenerationviathestimulationof fibroblast proliferationandtherecruitmentof fibroblastsintoclose proximitywithwoundregion.
TMSCssuppressdifferentiationof fibroblastsinto myofibroblast
Inthe finalstageofwoundrepair,theremodelingphase, dermal fibroblastsnewlygeneratedermaltissueto remodelwoundmatrix.Afterexposuretotransforming growthfactor(TGF)-β1inthewoundarea, fibroblasts differentiateintomyofibroblasts.Thesecellsproduce excessiveamountofextracellularmatrix,leadingtoscar tissueformationandpoorregeneration34.Therefore, regulatoryfunctionofTMSCsonmyofibroblastdifferentiationwasanalyzed.Differentiationof fibroblastsinto myofibroblastswasinducedbyTGF-β1treatmentandwas detectedby α-smoothmuscleactin(SMA)expressionin

Fig.4ImmunosuppressiveeffectsofTMSCsonactivationofmacrophage-likecellsandproliferationofinflammatorycells.a-b Mouse BMDMswerecu-culturedwithTMSCsandtheproductionofcytokinesrepresentingmacrophagepolarizationwasdeterminedbyELISA. a The secretionofTNF-α wasmeasuredfortheanalysisofM1polarization. b IL-10productionwasmeasuredfortheanalysisofM2polarization. c Outlineof macrophage-likecelldifferentiationfromTHP-1andco-cultureprotocolwithTMSCs. d THP-1-derivedmacrophage-likecellswereactivatedbyLPS/ IFN-γ andco-culturedwithTMSCsisolatedfromtwodifferentdonorsfor2daysatdifferentratios.ThesecretionofTNF-α wasmeasuredbyELISA. e TheproliferationofPBMCsstimulatedwithPWMorantibodiesforCD3/28wasevaluatedbyBrdUincorporationassay.*P <0.05,**P <0.01,***P < 0.001.Resultsareonerepresentativeexperimentofthreeorthecumulativeofthreeindependentexperiments.Resultsareshownasmean±SD
proteinlevelusingimmunofluorescencestaining.While TGF-β1treatmentincreasedthenumberof α-SMAexpressingmyofibroblasts,TMSCco-culturesignificantly abrogatedthegenerationofmyofibroblasts(Fig. 6a).
ExpressionofcollagenorTGF-β subtypesinmRNAlevel wasfurtherdeterminedbyqPCR.Consistentlywith immunofluorescencestaining, α-SMAexpressionin mRNAlevelwassignificantlydecreasedbyTMSCcoculture(Fig.6b).Moreover,relativeexpressionsoftype1 collagenandtype3collagenweredownregulatedby TMSCaddition(Fig.6b).TheTGF-β superfamilyhas beenreportedtobecriticallyinvolvedinscarformation andTGF-β3isknowntobemoreprofibroticthanTGF-β1 and-β235.AmongTGF-β subtypes,relativeexpressionsof TGF-β1and-β2werediminishedbyTMSCco-culture whereasTGF-β3expressionwaselevated(Fig. 6c). TMSCsisolatedfromtwodifferentdonorsexhibited similarpotencyintheregulationofgeneorprotein expressioninmyofibroblasts(Fig.6a–c).
TMSCsincreasekeratinocyteproliferationtopromote epidermalregeneration
Keratinocytesarekeyplayersinepidermalregeneration. HaCaTcell,animmortalizedcelllineofhumankeratinocyte,wasco-culturedwithTMSCsusingtranswellto allowparacrine-mediatedcell-to-cellinteractionwithout directadhesionandHaCaTcellproliferationwasassessed.TMSCssignificantlyincreasedtheproliferationof HaCaTcells,regardlessoftheTMSC:HaCaTcellratio (Fig.7a,b).Theseresultsdemonstratethestimulatory functionofTMSCsonkeratinocyteproliferation,indicatingthatTMSCsmightacceleratere-epithelializationin woundrepair.
Discussion
Inthepresentstudy,wedemonstrateforthe firsttime thathumanTMSCtransplantationcanacceleratewound repairthroughimmunomodulationandregenerationof
Our findingsimplythatTMSCscaninhibitmyofibroblastdifferentiationandregulategeneexpressionpattern, presumablyinawaybeneficialforwoundrepairwith completeregeneration.

Fig.5Regulationofdermal fibroblastproliferationandmigrationbyTMSCs.a-b Afterindirectco-cultureusingtranswell,theproliferationof humandermal fibroblastswasanalyzedby a observingphasecontrastmorphologyand b determiningMTTactivity. c-d TMSCswereplatedinto lowerchambersofthetranswellsystemandthen fibroblastswereseededintoupperchambers,subsequentlyincubatedfor6,12,and24h. Migrationof fibroblastsweredeterminedbyDAPIstaining. c Representative fluorescentimagesofmigrated fibroblastswereshownand d thetotal numberofmigratedcellswerequantified.***P <0.001.Allresultsarepresentedasmean±SDfromatleastthreeindependentexperiments
dermisandepidermisinthemurineexcisionalsplint model.Severalpreviousstudieshavereportedwound healingefficacyofMSCsandMSC-derivedconditioned mediaorexosome,isolatedfromvarioushumansources, includingbonemarrow36,adiposetissue37,andumbilical cord38–40.ShinandPeterson36showedthathumanBMSC graftsenhancedwoundhealinginmurinemodelof excisionalsplintwound.AnotherstudyfromHeoetal.37 provedthatconditionedmediafromactivatedhuman AMSCspromotedcutaneouswoundhealingthrough paracrinemechanisms37.Othergroupshavereportedthat humanumbilicalcord-derivedMSCscouldenhance woundrepairwhencellitself38,conditionedmedia39or exosome40weredeliveredontowoundbeds.Our findings areconsistentwiththesepreviousstudies.Weproved herethatTMSCtransplantationontowoundbedssignificantlyacceleratedthereductionofwoundsize.
AlthoughTMSCscouldnotdemonstratesuperiorefficacy whencomparedtoAMSCsinmurinewoundmodel, TMSCspossessadvantagesinthattheyarehighlyproliferativeandmoreresistanttosenescence,asdemonstratedinourpreviousstudy21,26,41.Theclinical applicationofMSCsrequireslarge-scaleproductionof cellsforallogeneictherapybecauseofthelimitationin obtainingsufficientquantityofautologousstemcells. Therefore,TMSCcouldbeapromisingcandidatefor bothautologousandallogeneiccelltherapy.
MSCshavebeenreportedtoregulateallthreephasesof woundrepair:theinflammation,proliferation,and remodelingphases.Duringthetransitionfromthe inflammationphasetotheproliferationphase,wound stateshouldnotregresstochronicinflammation.Inthis transitionperiod,macrophagesresidentinwoundregion playacrucialroletoshiftinflammationtoward

Fig.6InhibitionofmyofibroblastdifferentiationbyTMSCs.a–d Humandermal fibroblastswereseededonlowerchambersoftranswellplates andstimulatedwithTGF-β1todifferentiateintomyofibroblasts,followedbyincubationfor2dayswithorwithoutTMSCsfromtwodifferentdonors inupperchambers. a Theexpressionof α-SMA,aspecificmarkerformyofibroblast,wasdeterminedbyimmunocytochemistry.Representative αSMA+ cellsareindicatedbyarrowheads. b mRNAexpressionofcollagenorTGF-β subtypeswasanalyzedbyqPCR.*P <0.05,**P<0.01,***P <0.001. Resultsareshownasmean±SD

Fig.7EnhancementofkeratinocyteproliferationbyTMSCs.a-b Humankeratinocytes(HaCaTcells)wereplatedintolowerchambersofthe transwellsystem,andincubatedfor3and5daysinthepresenceorabsenceofTMSCsinthelowerchamber. a Representativephage-contrast imagesofkeratinocytesatdays3and5. b MTTassaywasperformedfordeterminingproliferationofkeratinocytesatthreedifferentratios.Dataare onerepresentativeexperimentofthreeorthecumulativeofthreeindependentexperiments.*** P <0.001.Resultsareshownasmean±SD
regeneration.Anumberofstudieshavedemonstrated thatMSCscoulddirectlyorindirectlyattenuateinflammatoryresponses.Inourpreviousstudy,wefoundthat thatMSCsfromanothersource,theumbilicalcordblood (CBSC),exhibitedmacrophage-modulatingabilityto polarizemacrophagestowardM2type,theantiinflammatoryandregenerativetypeofactivatedmacrophages41.Inthepresentstudy,weprovedthatTMSCs alsopossessedsimilarregulatoryfunctiononmacrophage polarization.TMSCssignificantlydownregulatedthe TNF-α productionfromequivalentnumberofM1macrophages.Inflammatorycellsotherthanmacrophages, includingTlymphocytes,Blymphocyte,andmastcells, areinvolvedintheprogressofskininflammationand woundrepair.Inthisstudy,TMSCsimpairedtheproliferationofPBMCsactivatedwithnon-specificmitogen, aswellastheproliferationofTlymphocytesactivated withantibodiesforCD3andCD28onT-cellsurface. Moreover,inourpreviousstudies,weshowedthatCBSCs couldnotonlyinducethegenerationofregulatory Tcells29,butalsosuppresstheactivationofmastcells28 orBcells27,bothinvitroandinvivousingrelevant immunecellsorimmune-relateddiseasemodels.One mightenvisionthatTMSCscouldpossesssimilar immunomodulatoryfunctionsviamultiplemechanisms, whichmakesTMSCsasapromisingcandidateforwound therapeutics.
Recently,severalexperimentalevidenceshaveproven thatMSCscouldsupporttheproliferationandremodelingphasestopromotewoundhealingviatheirregenerativepotential.Althoughsomestudiessuggestthat MSCscandirectlyparticipateinstructureregeneration duringwoundrepairbytrans-differentiationintokeratinocyte,endothelialcells,and fibroblasts42–44,themajority ofresearchersputgreateremphasisonparacrinefactormediatedregenerationofMSCsthandirectdifferentiation.Forinstance,studiesfromthesegroupsproposethat fibroblasts,keratinocytes,andendothelialcellsareaffectedbyMSC-mediatedparacrinesignaling,resultinginthe functionalalterationoftheirproliferation,survival,and migration45.Inthepresentstudy,TMSCsaugmentedthe epidermalanddermalregenerationinvivo,aswellasthe migrationof fibroblastsandtheproliferationofboth fibroblastsandkeratinocytesinvitrothroughthe paracrine-mediatedinteraction.Moreover,paracrinefactorsfromMSCsarereportedtoprovideanti-scarring effectinwoundrepairbybalancingtheexpressionof TGF-β1,-β2and-β346,47.Consistentwiththesereports, wedemonstratedherethatTMSCscouldpreventthe differentiationof fibroblastsintomyofibroblastsandregulatethebalanceinexpressionofTGF-β superfamily duringdifferentiation,elevatingtheexpressionofantifibroticTGF-β3.Duringtheproliferationphaseofwound repair,angiogenesisisanessentialprocesstoprovide
sufficientnutrientsfornewtissueformingcells,including fibroblasts48.OnemightdoubtthatinhibitionofTGF-β1 expressionin fibroblastsbyTMSCscanleadtothe decreaseincellularproliferation,becausecrucialroleof TGF- β1in fibroblastproliferationhasbeenreportedby severalgroupsincludingtherecentonebyXiaoetal.49 However,studiesfromothergroupsdemonstratedthat MSCscaninducetheproliferationof fibroblaststhrough thesecretionofvariousgrowthfactorsincludingbasic fibroblastgrowthfactor(bFGF)50,51.Inthepresentstudy, althoughTMSCsdownregulatedthemRNAlevelofTGFβ1in fibroblast,TMSCsaugmentedtheproliferationof fibroblasts,presumablythroughthesecretionofavariety ofgrowthfactors.Lackofadequatemicrovascularization canresultinnon-healingchronicwound.MSCshave beenreportedtosecreteparacrinefactorstopromotethe generationofmicrovascularnetworkandvascularstability52,53.Inthisstudy,TMSCsexhibitedsignificant angiogenicpotentialinproliferationphaseofwound repair.SincelotsofparacrinefactorsfromMSCssuchas matrixmetalloproteinases(MMPs),tissueinhibitorsof MMP,epidermalgrowthfactors,bFGFs,vascularendothelialgrowthfactors,andanti-inflammatorysoluble factorsarereportedtopromotewoundhealing,future studieselucidatingtheuniqueparacrinefunctionof TMSCscomparedtoMSCsfromothersourcescould uncovernovelmechanismsofTMSC-mediatedwound repairandfurthersuggesttheadditionaladvantagesof TMSCsastherapeutics.Inaddition,furtherstudiesare requiredtoexplorethedetailedconditionsforMSC treatmentincludingdosage,numberandintervalof multiplecelladministration,aswellastheoptimizationof procedureforcellmaintenanceandharvesting.Lastly, pre-clinicalandclinicalreferencesreportingtheadverse effectsofMSCtreatmentshouldbeaccumulatedto strengthentheproofforitssafety.
Thepresentstudyhasitslimitationsinconstruct validityofanimalmodelforwoundrepair.Nudemice cannotexactlymimictheprocessofwoundrepairin human.Inparticular,thismodelmightexhibitdifferent mechanismsintheinflammatoryphase,becausenude micelackcertainpopulationofimmunecells,includingT lymphocytes.However,Dandekaretal.54reportedthat thenumberandfunctionofmacrophageswereconserved andevenlimitedpopulationofextrathymicallymatured Tcellswereobservedinathymicnudemice.Giventhat macrophagesarekeyimmunecellsintheregulationofthe inflammatoryphaseinwoundrepair,themodelusedin thisstudycanpartiallyrecapitulatetherepairprocessin humanwound.Anotherlimitationalsoresidesinthe animalmodel,inthatthemodelcannotfullydescribeall threephasesofwoundrepairindependently.Therefore,it isdifficulttoelucidatetheexactmechanismsofTMSC efficacyineachphase.
Inconclusion,ourpresentstudycomprehensively revealedtheefficacyofhumanTMSCsinwoundrepair,as wellastheunderlyingfunctionsofTMSCsforeachphase ofwoundrepair.TMSCsefficientlycontributedto immunoregulationandregenerationthroughtheinteractionwithavarietyofskinconstitutingcellsand immunecells,indicatingthatTMSCsmightbeapromisingtherapeuticalternativeforwoundrepair.
Materialsandmethods
IsolationandcultureofTMSCs
TMSCswereisolatedandmaintainedasdescribedpreviously41.Briefly,TMSCswereisolatedfromexcised palatinetonsiltissueobtainedaftertonsillectomy.Tonsil tissuewasextensivelywashedwithphosphate-buffered saline(PBS),followedbythedigestionwith0.075%collagenasetypeI(Sigma,St.Louis,MO)for30minat37°C. Thepelletwasobtainedbycentrifugationat1200 g for10 minandwas filteredthrougha100 μmnylonmesh.Suspendedcellswereincubatedovernightin α-minimum essentialmediacontaining10%fetalbovineserum(FBS)at 37°Cwith5%CO2.Non-adherentcellswereremovedby extensivewashingwithPBSandadherentcellswere maintainedandsub-cultured.Allproceduresusinghuman tonsiltissueortissue-derivedcellswereconductedin accordancewithguidelinesapprovedbythePusan NationalUniversityHospitalInstitutionalReviewBoard.
WoundhealingmodelandTMSCadministration
BALB/cnudemice(female,8weeksold)wereobtained fromSamtako(Osan,RepublicofKorea)andgrouphoused underspecificpathogenic-freeconditionsintheanimal facilityofthePusanNationalUniversityHospital.All experimentswereapprovedbyandfollowedtheregulationsoftheInstituteofLaboratoryAnimalResources(No. PNU-2008-0001),PusanNationalUniversityHospital.The excisionalwoundmodelwasgeneratedandTMSCswere subcutaneouslytransplanted.Briefly,aftertheaseptical preparationofsurgicalsiteincludinghairremoval,two6mmfullthicknessskinwoundswerecreatedoneachside ofthedorsalpartusingabiopsypunch.Onemillion TMSCssuspendedin20 μLPBSwereplacedontowound bedsimmediately,whereaswoundsontheoppositeside receivedPBSasvehiclecontrolgroups.Allwoundswere coveredwithTegadermFilm(3M,Minneapolis,MN).
Grossevaluation
Digitalphotographsofwoundsweretakenatdays0,2, 4,6,8,10,and12.Woundareawasmeasuredbytracing themarginofwounds.Thepercentageofwoundsize reductionwascalculatedasthefollowing:%woundsize reduction = (areai areat)/areai × 100,whereareaidesignatestheinitialwoundareaandareatdesignatesthearea aftertimeinterval.
Histopathologicalevaluation
Atdays2,4,6,8,10,and12,miceweresacrificedand woundbedswereexcised, fixedin4%paraformaldehyde followedbyconsecutivetissueprocessingstepsand embeddinginparaffin.Sectionsof4 μmthicknesswere preparedandstainedwithH&Eoranti-CD31antibody (Abcam,Cambridge,UK).Infiltrationofinflammatory cellswasmeasuredbycountingthenumberofneutrophils,lymphocytes,histocytes,andplasmacellsintissuesectionsstainedwithH&E.Epidermalthicknessand collagendepositionweremeasured.Histologicalparametersincludingre-epithelialization(1:minimalepidermalregeneration <50%,2:moderateepidermal regeneration ≥50%,3:completeepidermalregeneration = 100%),dermalregeneration,andgranulationtissue formationwerecalculated.Angiogenesiswasassessedby measuringtheareaofCD31+ vasculaturesusingImageJ software.Histologicalevaluationwasconductedusing randomlyselected fieldsfromskintissuesections.
Co-cultureofTMSCswithactivatedmouseBMDMsand humanmacrophage-likecells
BMDMswereisolatedfromthefemurandtibiaof C57BL/6miceby flushingwithPBS.Harvestedcellswere resuspendedinRPMI-1640(GibcoBRL,GrandIsland, NY)containing1%L-glutamine,10%FBS,and10ng/mL macrophagecolony-stimulatingfactorandmaintainedfor 7daysat37°Cand5%CO2withmediumchangeevery 3–4days.Onday7,BMDMswithoutanytreatmentwere co-culturedwithTMSCswithintherangeof1:100to1:1 basedontheTMSC/M1ratio.ForM1activationof BMDMs,100ng/mLLPSand50ng/mLIFNγ weretreatedalongwiththeadditionofTMSCs.Co-culturewas maintainedfor2daysandculturemediawasharvested anddeterminedformouseTNF-α orIL-10production usingcommercialenzyme-linkedimmunosorbentassay (ELISA)kits(R&DSystems,Minneapolis,MN).Togeneratehumanmacrophage-likecells,THP-1cells(2.5 × 105/mL)weretreatedwith200nMphorbol12-myristate 13-acetate(PMA,Sigma-Aldrich)for48h,followedbythe stabilizationofPMA-treatedcellsforadditional2daysin freshmediawithoutPMA.Toinducetheactivationof macrophage-likecellsintoM1typemacrophage-likecells, LPS(1 μL/mL)andIFN-γ (20ng/mL)weretreatedwith TMSCadditionforco-culture.Cellswereincubatedfor 2daysandculturemediawasharvestedanddetermined forhumanTNF-α andIL-10productionusingELISAkits (R&DSystems).
ProliferationassayofPBMCsandTlymphocytes TMSCsweretreatedwithmitomycinC(25mg/mL)at 37°Cfor1htoinhibitcellproliferation.Thecellswere platedin96-wellplatesat1 × 104/well.Sixhourslater, humanPBMCs(1 × 105/wellin100 μLmedia;Zen-bio,
TrianglePark,NC)wereaddedwiththetreatmentof PWM(Sigma-Aldrich)orantibodiesforCD3andCD28 (e-bioscience,SanDiego,CA),fortheactivationof mononuclearcells(MNCs)orTlymphocytes,respectively.After3daysofaco-culture,MNCorTlymphocyte proliferationwasdeterminedbyacellproliferation ELISA,bromodeoxyuridinekit(Roche,Indianapolis,IN).
Proliferationassayof fibroblastsandkeratinocytes
Human fibroblastsorkeratinocytes(HaCaTcells)were seededontothelowerchamberofa12-welltranswell plate(0.4 μmporesize)at5 × 104/wellinDulbecco's modifiedEagle'smediummedia(Invitrogen,Carlsbad, CA)containing2%FBS.After6hofstabilization,TMSCs atdifferentnumber(5 × 104,5 × 103,and5 × 102/well) wereaddedontotheupperchamber.Atday3andday5, theproliferationof fibroblastsorkeratinocyteswasmeasuredbymethylthiazolylbluetetrazoliumbromide (MTT;Sigma-Aldrich).
Cellmigrationassay
FibroblastmigrationtowardTMSCswasdetermined usingtranswellwith5 μmporesize.TMSCs(1 × 104/ well)wereplatedontothelowerchamber,followedby incubationfor6h.Human fibroblastswereseededonto theupperchamberat1 × 104/well.After6,12,and24hof incubation,cellsintheupperchamberwere fixedwith 10%formaldehydefor10min,followedbywashingwith PBS.Usingcottonswabs,cellswhichhadnotmigrated throughtheporewereremoved.Migratedcellswere stainedwithDAPI.AfterwashingwithPBS,stainedcells werecountedusinga fluorescentmicroscope(Leica Microsystems,Wetzlar,Germany).
Inductionanddeterminationofmyofibroblast differentiation
Fibroblasts(5 × 104/well)wereplatedonlowerchambers of12-welltranswellplate(0.4 μmporesize).After6hof incubation,5ng/mLofrecombinanthumanTFG-β1was treatedfor48hatthepresenceorabsenceofTMSCs(5 × 104/well)inupperchambers.The fibroblastsweresubsequentlysubjectedtoimmonofluorescentstainingfor αSMA(Abcam)orRNAisolationusingTRIzolReagent (Invitrogen).Thenumberofcellsexpressing α-SMAwas determinedfrom10 fieldsofeachimage.Quantitativerealtime-PCR(qRT-PCR)wasperformedbymixingcDNA synthesizedfromisolatedRNAwithprimersandSYBR GreenPCRMaterMix(AppliedBiosystems,FosterCity, CA)usinganReal-time-PCRsystem(ABI7500,Applied Biosystems).Theprimersequencesusedwereasfollows; αSMA,GCTACTCCTTCGTGACCACAG(forward)and GCCGTCGCCATCTCGTTCT(reverse);CollagenI, CCTCAAGAGAAGGCTCACGATGGTG(forward)and AGGTCTCACCAGTCTCCATGTTGCA(reverse);
CollagenIII,GCTCTGCTTCATCCCACTATTA(forward) andTGCGAGTCCTCCTACTGCTAC(reverse);TGF-β1, AGTTGTGCGGCAGTGGTTGA(forward)andGCCATGAATGGTGGCCAGGT(reverse);TGF-β2,TAGACATGCCGCCCTTCTTCC(forward)and AGCACCTGGGACTGTCTGGA(reverse);TGF-β3, AGCACCTGGGACTGTCTGGA(forward)andCAATGTAGAGGGGGCGCACA(reverse).
Statisticalanalysis
Themeanvaluesofthedifferentgroupswereexpressed asthemean ± SD.Allstatisticalcomparisonsweremade usingoneortwo-wayANOVAfollowedbytheBonferroni posthoctestformulti-groupcomparisonsusingthe GraphPadPrismversion5.01(GraphPadSoftware,San Diego,CA).Statisticalsignificancedesignatedasasterisks isindicatedinthe figurelegends.
Acknowledgements
ThisresearchwassupportedbytheBasicScienceResearchProgram(No.NRF2016R1C1B2016140and2014R1A2A1A11052999)andBio&Medical TechnologyDevelopmentProgram(2016M3A9E8942065)throughaNational ResearchFoundationofKorea(NRF)grantfundedbytheMinistryofEducation, Science,andTechnologyandpartiallysupportedbyclinicalresearchgrant fromPusanNationalUniversityHospitalin2016.
Authordetails
1DepartmentofOtorhinolaryngology-HeadandNeckSurgery,Biomedical ResearchInstitute,PusanNationalUniversitySchoolofMedicine,Pusan NationalUniversityHospital,Busan,RepublicofKorea. 2BiomedicalResearch Institute,PusanNationalUniversitySchoolofMedicine,PusanNational UniversityHospital,Busan,RepublicofKorea. 3DepartmentofPathology, BiomedicalResearchInstitute,PusanNationalUniversitySchoolofMedicine, PusanNationalUniversityHospital,Busan,RepublicofKorea. 4Departmentof Otorhinolaryngology-HeadandNeckSurgery,BiomedicalResearchInstitute, PusanNationalUniversitySchoolofMedicine,YangsanPusanNational UniversityHospital,Yangsan,RepublicofKorea. 5Departmentof Otorhinolaryngology-HeadandNeckSurgery,AjouUniversitySchoolof Medicine,Suwon,RepublicofKorea
Conflictofinterest
Theauthorsdeclarethattheyhavenoconflictofinterest.
SupplementaryInformation accompaniesthispaperat(https://doi.org/ 10.1038/s41419-017-0248-4).
Received:23May2017Revised:3December2017Accepted:18December 2017
References
1.Eming,S.A.,Martin,P.&Tomic-Canic,M.Woundrepairandregeneration: mechanisms,signaling,andtranslation. Sci.Transl.Med. 6,265sr6(2014).
2.Gurtner,G.C.,Werner,S.,Barrandon,Y.&Longaker,M.T.Woundrepairand regeneration. Nature 453,314–321(2008).
3.Pastar,I.etal.Epithelializationinwoundhealing:acomprehensivereview. Adv. WoundCare(NewRochelle) 3,445–464(2014).
4.Shaw,T.J.&Martin,P.Woundrepairataglance. J.CellSci. 122(Pt18), 3209–3213(2009).
5.Guo,S.&Dipietro,L.A.Factorsaffectingwoundhealing. J.Dent.Res. 89, 219–229(2010).
6.Schultz,G.S.&Wysocki,A.Interactionsbetweenextracellularmatrixand growthfactorsinwoundhealing. WoundRepairRegen. 17,153–162(2009).
7.Gould,L.etal.Chronicwoundrepairandhealinginolderadults:current statusandfutureresearch. WoundRepairRegen. 23,1–13(2015).
8.Jarbrink,K.etal.Prevalenceandincidenceofchronicwoundsand relatedcomplications:aprotocolforasystematicreview. Syst.Rev. 5,152 (2016).
9.Frykberg,R.G.&Banks,J.Challengesinthetreatmentofchronicwounds. Adv. WoundCare(NewRochelle). 4,560–582(2015).
10.Werdin,F.,Tennenhaus,M.,Schaller,H.E.&Rennekampff,H.O.Evidencebasedmanagementstrategiesfortreatmentofchronicwounds. Eplasty 9,e19 (2009).
11.Trounson,A.&McDonald,C.Stemcelltherapiesinclinicaltrials:progressand challenges. CellStemCell 17,11–22(2015).
12.Hwang,N.S.,Zhang,C.,Hwang,Y.S.&Varghese,S.Mesenchymalstemcell differentiationandrolesinregenerativemedicine. WileyInterdiscip.Rev.Syst. Biol.Med. 1,97–106(2009).
13.Liu,Z.J.,Zhuge,Y.&Velazquez,O.C.Traffickinganddifferentiationof mesenchymalstemcells. J.CellBiochem. 106,984–991(2009).
14.Chamberlain,G.,Fox,J.,Ashton,B.&Middleton,J.Concisereview:mesenchymalstemcells:theirphenotype,differentiationcapacity,immunological features,andpotentialforhoming. StemCells 25,2739–2749(2007).
15.Gao,F.etal.Mesenchymalstemcellsandimmunomodulation:currentstatus andfutureprospects. CellDeathDis. 7,e2062(2016).
16.Kean,T.J.,Lin,P.,Caplan,A.I.&Dennis,J.E.MSCs:deliveryroutesand engraftment,cell-targetingstrategies,andimmunemodulation. StemCellsInt. 2013,732–742(2013).
17.Fathke,C.etal.Contributionofbonemarrow-derivedcellstoskin:collagen depositionandwoundrepair. StemCells 22,812–822(2004).
18.Jackson,W.M.,Nesti,L.J.&Tuan,R.S.Mesenchymalstemcelltherapyfor attenuationofscarformationduringwoundhealing. StemCellRes.Ther. 3,20 (2012).
19.Kim,W.S.etal.Woundhealingeffectof adipose-derivedstemcells:acritical roleofsecretoryfactorsonhumandermal fibroblasts. J.Dermatol.Sci. 48, 15–24(2007).
20.Wu,Y.,Chen,L.,Scott,P.G.&Tredget,E.E.Mesenchymalstemcellsenhance woundhealingthroughdifferentiationandangiogenesis. StemCells 25, 2648–2659(2007).
21.Lee,B.J.etal.Isolationandlocalizationofmesenchymalstemcellsinhuman palatinetonsilbyW5C5(SUSD2). CellPhysiol.Biochem. 38,83–93(2016).
22.Ryu,K.H.etal.Tonsil-derivedmesenchymalstromalcells:evaluationofbiologic,immunologicandgeneticfactorsforsuccessfulbanking. Cytotherapy 14, 1193–1202(2012).
23.Jung,N.etal.Tonsil-derivedmesenchymalstemcellsdifferentiateintoa Schwanncellphenotypeandpromoteperipheralnerveregeneration. Int.J. Mol.Sci. 17,e1867(2016).
24.Park,M.etal.Tonsil-derivedmesenchymalstemcellsameliorateCCl4-induced liver fibrosisinmiceviaautophagyactivation. Sci.Rep. 5,8616(2015).
25.Samivel,R.,Kim,E.H.,Chung,Y.J.&Mo,J.H.Immunomodulatoryeffectof tonsil-derivedmesenchymalstemcellsinamousemodelofallergicrhinitis. Am.J.Rhinol.Allergy 29,262–267(2015).
26.Choi,J.S.etal.Effectsofdonorage,long-termpassageculture,andcryopreservationontonsil-derivedmesenchymalstemcells. CellPhysiol.Biochem. 36,85–99(2015).
27.Shin,T.H.etal.Humanadiposetissue-derivedmesenchymalstemcells alleviateatopicdermatitisviaregulationofBlymphocytematuration. Oncotarget 8,512–522(2017).
28.Kim,H.S.etal.Humanumbilicalcord bloodmesenchymalstemcell-derived PGE2andTGF-beta1alleviateatopicdermatitisbyreducingmastcell degranulation. StemCells 33,1254–1266(2015).
29.Kim,H.S.etalHumanumbilicalcordbloodmesenchymalstemcellsreduce colitisinmicebyactivatingNOD2signalingtoCOX2. Gastroenterology 145, 1392–1403e1-8(2013).
30.Shin,T.H.etal.Humanumbilicalcordblood-stemcellsdirectmacrophage polarizationandblockinflammasomeactivationtoalleviaterheumatoid arthritis. CellDeathDis. 7,e2524(2016).
31.Li,J.,Zhang,Y.P.&Kirsner,R.S.Angiogenesisinwoundrepair:angiogenic growthfactorsandtheextracellularmatrix. Microsc.ResTech. 60,107–114 (2003).
32.Diegelmann,R.F.&Evans,M.C.Woundhealing:anoverviewofacute, fibrotic anddelayedhealing. FrontBiosci. 9,283–289(2004).
33.Velnar,T.,Bailey,T.&Smrkolj,V.Thewoundhealingprocess:anoverviewof thecellularandmolecularmechanisms. J.IntMedRes. 37,1528–1542(2009).
34.Bucala,R.,Spiegel,L.A.,Chesney,J.,Hogan,M.&Cerami,A.Circulating fibrocytesdefineanewleukocytesubpopulationthatmediatestissuerepair. Mol.Med. 1,71–81(1994).
35.Kishi,K.,Okabe,K.,Shimizu,R.&Kubota,Y.Fetalskinpossessestheabilityto regeneratecompletely:completeregenerationofskin. KeioJ.Med. 61, 101–108(2012).
36.Shin,L.&Peterson,D.A.Humanmesenchymalstemcellgraftsenhance normalandimpairedwoundhealingbyrecruitingexistingendogenoustissue stem/progenitorcells. StemCellsTransl.Med. 2,33–42(2013).
37.Heo,S.C.etal.Tumornecrosisfactor-alpha-activatedhumanadiposetissuederivedmesenchymalstemcellsacceleratecutaneouswoundhealing throughparacrinemechanisms. J.InvestDermatol. 131,1559–1567(2011).
38.Arno,A.I.etal.HumanWharton’sjellymesenchymalstemcellspromoteskin woundhealingthrough paracrinesignaling. StemCellRes.Ther. 5,28(2014).
39.Li,M.etal.Mesenchymalstemcell-conditionedmediumaccelerateswound healingwithfewerscars. Int.WoundJ. 14,64–73(2017).
40.Zhang,B.etal.HucMSC-exosomemediated-Wnt4signalingisrequiredfor cutaneouswoundhealing. StemCells 33,2158–2168(2015).
41.Park,G.C.etal.Roleof fibroblastgrowthfactor-5ontheproliferationof humantonsil-derivedmesenchymalstemcells. StemCellsDev. 25,1149–1160 (2016).
42.Yamaguchi,Y.etal.Bonemarrowcellsdifferentiateintowoundmyofibroblastsandacceleratethehealing ofwoundswithexposedboneswhen combinedwithanocclusivedressing. Br.J.Dermatol. 152,616–622(2005).
43.Lozito,T.P.,Kuo,C.K.,Taboas,J.M.&Tuan,R.S.Humanmesenchymalstem cellsexpressvascularcellphenotypesuponinteractionwithendothelialcell matrix. J.CellBiochem. 107,714–722(2009).
44.Fu,X.,Fang,L.,Li,X.,Cheng,B.&Sheng,Z.Enhancedwound-healingquality withbonemarrowmesenchymalstemcellsautograftingafterskininjury. WoundRepairRegen. 14,325–335(2006).
45.Hocking,A.M.&Gibran,N.S.Mesenchymalstemcells:paracrinesignalingand differentiationduringcutaneouswoundrepair. Exp.CellRes. 316,2213–2219 (2010).
46.Shah,M.,Foreman,D.M.&Ferguson,M.W.NeutralisationofTGF-beta1and TGF-beta2orexogenousadditionofTGF-beta3tocutaneousratwounds reducesscarring. J.CellSci. 108(Pt3),985–1002(1995).
47.Colwell,A.S.etal.Increasedangiogenesisandexpressionofvascularendothelialgrowthfactorduringscarlessrepair. Plast.Reconstr.Surg. 115,204–212 (2005).
48.Brown,L.F.etal.Expressionofvascularpermeabilityfactor(vascularendothelialgrowthfactor)byepidermalkeratinocytesduringwoundhealing. J.Exp. Med. 176,1375–1379(1992).
49.Xiao,L.etal.TGF-beta1induced fibroblastproliferationismediatedbythe FGF-2/ERKpathway. FrontBiosci.(LandmarkEd.) 17,2667–2674(2012).
50.Lee,E.Y.etal.Hypoxia-enhancedwound-healingfunctionofadipose-derived stemcells:increaseinstemcellproliferationandup-regulationofVEGFand bFGF. WoundRepairRegen. 17,540–547(2009).
51.Smith,A.N.etal.Mesenchymalstemcellsinducedermal fibroblastresponses toinjury. Exp.CellRes. 316,48–54(2010).
52.Lozito,T.P.,Taboas,J.M.,Kuo,C.K.&Tuan,R.S.Mesenchymalstemcell modificationofendothelialmatrixregulatestheirvasculardifferentiation. J.Cell Biochem. 107,706–713(2009).
53.Gruber,R.etal.Bonemarrowstromal cellscanprovidealocalenvironment thatfavorsmigrationandformationoftubularstructuresofendothelialcells. TissueEng. 11,896–903(2005).
54.Dandekar,A.A.&Perlman,S.Virus-induceddemyelinationinnudemiceis mediatedbygammadeltaTcells. Am.J.Pathol. 161,1255–1263(2002).
