INDUSTRIA BIOTEC
Biotech solutions are ready –time to change the paradigm
Frédéric Pâques, CEO of Standing Ovation SA, on why vegan cheese made with microbial casein can help save the climate.

Focus Pharma Services Record pandemic-powered profits for CDMOs & CROs predicted
Clusters

Life Sciences and Industry Magazine Winter Edition 2022 | Volume 21 | 20 € ISSN 2364-2351 | A 60711 |
in Europe Development and scaling-up at Toulouse White Biotech Interview
Crisis? Biotech Financing
Crisis? What
Better Prep!
The Nexera Prep Series Preparative Purification LC improves productivity tremendously via efficient pre parative work using application-specific LC or LC-MS solutions. It provides better prep processes for drug discovery and purification of functional components in pharmaceutical, chemical and food industries.


Ease-of-operation in a flexible set-up supported by flexible choice of detectors and efficient process automation
Simplifies the fractionation programming using the LabSolutions software’s fractionation simulation function
High sample capacity with a reduced footprint through a space-saving design with support of custom racks
www.shimadzu.eu /better-prep Preparative LC System
Fast recovery of highly purified target compounds by automation of fractionation, concentration, purification and recovery
Keep it simple, stay flexible
Why we need the switch from oil to biotech now
Our species – modern humans, or homo sapiens sapiens – has ever striven for progress. And progress was greatly enabled by fire, a fundamental chemical phenomenon that allows the fast release of large amounts of energy. In the modern age, industry began tapping into coal, oil and more recently shale gas as ‘feedstocks’ (known in biology as ‘substrates’), using heat from their fire, and high pres sure, to crack substances to yield yet other fuels and base chemi cals. Those industries use ‘prefermented’ compounds as an energy source. But processing conditions are harsh, while side-products are often unknown and, in many cases at least, toxic. The exploitation of fossil resources allowed economic growth during an era when by-products and the long-term effects on our species and the en vironment were secondary worries. Those days are over.
DR ALEXANDER KRAJETE is managing director of Krajete GmbH, a company that pioneers high-performance gas fermentation and gas purification. He holds a PhD in chemistry with postdoctoral experience from UC Berkeley. Alexander started his career in the petrochemical industry with Borealis Norway in 2004, where he was project manager for polyolefin upscaling and senior scientist for hydrocarbons. Inspired by archaea, he founded Krajete GmbH in 2012.

So why does biotechnology provide long-term solutions to the legacy of past economic growth? Why has it been so long ignored? Why has it taken so long for large corporations to dis cover the charms of enzymes and microbes, and has it real ly? Or do they just want to keep running their old profitable plants under the guise of green promises or because they lack the know-how?
Unlike chemistry that requires other catalysts, biotech employs enzymes and microbes to accomplish the transformation from component A to B. The biggest difference between the two is intimately related to energy and selectivity. Biotech processes are ‘mild’. They occur within the boundaries of life. And due to the nature of the factors that drive them, enzymatic processes are also selective, influencing for instance the amount of de sired product produced. Biotech processes additionally arise from evolved systems, and both products and ‘waste’ are com patible with Nature. Conventional industrial chemical processes, in comparison, are harsh, often involving high temperatures and high pressures, yielding byproducts and waste that is not compatible with Nature. In the balance that must be struck between a) energy demand, b) selectivity and c) overall fit with Nature, only biotechnology of fers ways to exploit resources in truly long-term and sustainable ways.
But because our societies have been powered by fossil fuels for centuries, it’s difficult for decisionmakers to understand and execute new frameworks in the modern world. It is therefore of utmost importance to look holistically at the big perspective, and ques tion economic growth as the sole key measure for ‘progress’. We need a perspective that equally values a long-term fit with the environment. The impact of a biotech-based economy would quickly become clear (in the form of a restored global ecosystem) if measures are taken now to move towards a fast ‘Global Fossil Exit’. Humanity needs to rediscover the circularity common to ancient cultures and center it again as a fun damental pillar of modern cultures. We can no longer afford to simply ignore our cen tral ally and partner – Nature – in favour of short-sighted economic gains.
3 INTRO Picture: © Krajete GmbH European Biotechnology | Winter Edition | Vol. 21 | 2022
L
COVER STORY
INSIGHT
EUROPE
AMR: Big Pharma’s TEE initiative
Banks and policymakers driving climate change
Agri-coalition for genome editing
Crisis? What Crisis?
M&A – hints of a looming crisis?
2022 looks set to be the worst year for biotech Mergers & Acquisitions since 2018, and some experts have speculated it might herald the start of a new crisis for the sector. External financing is indispensable for drug developers. In the absence of external capital, achieving growth turns into an even greater struggle, and innovation slows. We take a deeper look into the causes behind the ‘new normal’ in partner ships and licensing, and break down why a predicted M&A wave is not cresting as quickly as expected.
Analyst commentary
European Biotech Stocks
Innate immunity and its power in cancer therapy
Interview: Frédéric Pâques, CEO, Standing Ovation SA
Clinical trials
Focus Pharma Services: CDMO/CRO business set to grow
Cell factories: the heart of rAAV manufacturing
Blow-Fill-Seal technology and vaccine delivery
Interview: Christian Nafe, CFO, leon-nanodrugs GmbH
Lessons learned in the face of new health challenges
AGC Biologics doubles mammalian capacity
Wholistic approach to strategic consulting
REGIONAL NEWS
65 Special Industrial Biotech Cluster: Toulouse White Biotechnology
Northern Europe: Sweden, Denmark, Norway, Iceland and Finland
Western Europe: France, Belgium, The Netherlands and the UK
Central Europe: Germany, Switzerland and Austria
Southern Europe: Italy, Spain, Croatia and Portugal
Eastern Europe: Czech Republic SCIENCE & TECHNOLOGY
At the interface of AI, healthcare and data science
Biofilms for production
AI-enabling predictive diagnostics; Bio-based electronic devices
Ultra-low freezers: Stability of cancer biomarkers
PICK & MIX
Biopeople
News from Biotech Austria, the ECBF, YEBN and the SBA
Company index/ New product
Encore
IMPRINT European Biotechnology (ISSN 2364-2351) is published quarterly by: BIOCOM AG, Jacobsenweg 61, D-13509 Berlin, Germany, Tel.: +49-30-264921-0, Fax: +49-30-264921-11, Email: service@ european-biotechnology.com, Internet: www.european-biotechnology.com; Publisher: Andreas Mietzsch; Editorial Team: Thomas Gabrielczyk (Editor in Chief), Derrick Williams (Co-editor), Uta Mommert, Gwendolyn Dorow, Margarita Milidakis, Maren Kühr; Advertising: Oliver Schnell, +49-30-264921-45, Christian Böhm, +49-30-264921-49, Andreas Macht, +49-30-264921-54; Distribution: Lukas Bannert, +49-30-264921-72; Graphic Design: Michaela Reblin; Production: Martina Willnow; Printed at: Königsdruck, Berlin; European Biotechnology Life Sciences & Industry Magazine is only regularly available through subscription with a BIOCOM CARD. Annual subscription BIOCOM CARD Europe: €80 for private individuals (students €40) incl. VAT, €120 plus VAT for corporates. Prices includes postage & packaging. Ordered subscriptions can be cancelled within two weeks directly at BIOCOM AG. The subscription is initially valid for one calendar year and is automatically renewed every year after. The subscription can be cancelled at any time and is valid until the end of that calendar month. Failures of delivery, which BIOCOM AG is not responsible for, do not entitle the subscriber to delivery or reimbursement of pre-paid fees. Seat of court is Berlin, Germany. As regards contents: individually named articles are published within the sole responsibility of their respective authors. All material published is protected by copyright. No article or part thereof may be reproduced in any way or processed, copied, and proliferated by electronic means without the prior written consent of the publisher. Cover Photo: Garrykillian - Freepik.com; ® BIOCOM is a registered trademark of BIOCOM AG, Berlin, Germany.
4 CONTENTS European Biotechnology | Winter Edition | Vol. 21 | 2022
8
10
11
20
21
24
26
28
6
EU rules on biogas production ECONOMY
30
34
36
38
40
42
44
12
70
72
74
76
78
79
80
81
82
Picture: © LGarrykillian Freepik.com
46
83
88
89 Events 90
INDUSTRIAL BIOTECH
(Bio)economy 2.0
For years, policies promoting industrial production modelled on naturally occurring cycles have been little more than a thin green veneer used by oil and chemistry multinationals. At the new forum INDUSTRIA BIOTEC, companies presented both new ideas and longignored solutions that – in cooperation with politics – could help avert the looming gas and climate catastrophes.
 FOCUS: PHARMA SERVICES
FOCUS: PHARMA SERVICES
EDITORIAL
Empty promises?
Determined to grow
Over 40,000 on-site visitors to the CPHI in Frankfurt have shown that the COVID-19 pandemic has had a positive impact on the (bio)-pharmaceutical sector. A new CPHI annual survey predicts how business will develop in the future, with prospects for CDMOs and CROs looking rosy.


When politicians talk about climate change and the associated systemic shift from nature-exploiting to natureconserving processes, they often sound reasonable and responsible. This was also the case at COP27 in Sharm-el-Sheikh in Egypt. But the ‘er ror’ in the massively underestimated national CO2 emission budgets of the G20 countries – revealed by ex-US Vice President Al Gore’s Climate TRACE coalition – was not taken as an opportunity to decide immediately for binding production budgets. Nor was a decision made to shut down CO2 emitters, which can now be identified by satellite, to make up for lost time in decarbonising the atmosphere (see p. 8). Instead, the somewhat pointless promise was made that from now on, we would take an annual look at the depressing CO2 figures.
Biofairs Compass

In view of how little time is left to de flect the consequences of climate change and lower the world’s still ex cessive energy consumption, we could use another Al Gore to help push nextgen biotechnological processes for en vironmental and climate protection (see p. 58). Significant investments are needed to scale-up nature-compatible production processes and realise the biologisation of industries. At the pre miere of the INDUSTRIA BIOTEC con ference, experts explored rational, lob by-free ways out of the climate and energy crisis – that didn’t rely on emp ty promises.
 Thomas Gabrielczyk Editor-in-Chief
Thomas Gabrielczyk Editor-in-Chief
5 CONTENTS European Biotechnology | Winter Edition | Vol. 21 | 2022
30
58 SPECIAL
Pictures: Billionphotos Freepik.com (left below; © Informa (middle); BIOCOM AG (right) and upper left) 48 PharmaPack Europe, Paris, France 50 AMR Conference, Basel, Switzerland 52 BIO-Europe Spring 2023, Basel, Switzerland 54 Swiss Biotech Day 2023, Basel, Switzerland 56 Connect in Pharma 2023, Geneva, Switzerland
Statistics favours Big Pharma’s TEE pull initiative
AMR Boston-based consultancy firm Charles River Associates has published studies before that clouded the scientific picture in the interest of clients, including those interpreting climate-change to reflect more favourably on the oil industry. The issue of antimicrobial resistance is now in its crosshairs.
Commissioned by the umbrella organisa tion of the European pharmaceutical in dustry EFPIA, the global consulting firm Charles River Associates in Boston has as sessed the benefits of a payment model for antibiotics called Transferable Exclusiv ity Extension. TEE is a pull initiative aimed at motivating research and marketing of new antimicrobial drugs for which there is currently no business model that promises an attractive return on investment.
New global estimates
New model calculations on the benefit of TEE carried out by Charles River As sociates are based on the assumption of 400,000 deaths per year from direct and indirect effects of antimicrobial resistance (AMR) to drugs, which in Europe and the US is still limited to nosocomial infec tions in hospitals. The new estimate is based on the modelling of data published through 2019 by a group headed by the US government’s AMR consultant Mohs
en Naghavi who founded the Institute for Health Metrics and Evaluation (IHME) in L ancet in January and October 2022 (doi: 10.1016/S0140-6736(21)02724-0).
The since then widely adopted data that were e.g. cited at the Global Health Summit in October in Berlin nevertheless seem strangely impractical. The Europe an Centre for Disease Control (ECDC) in Stockholm and the World Health Organ isation (WHO) in Geneva reported com pletely different figures in their AMR Re port 2022: For the year 2020, they only arrive at around 33,000 deaths due to di rect effects of the eight most important drug-resistant microorganisms in the EU, Norway and Iceland. These figures are not based on literature research but are based on data from both EU AMR sur veillance networks, CESAR and EARSNet, and were submitted individually by the member states for each pair of active substance/microbial pathogen. Precisely, because AMR deaths have been declin ing in Europe since 2016, according to the
AMR 2022 report, the reverse increase of a good twelve-fold quoted and postulated by Charles River Associates – mainly due to indirect effects not explained in detail but illustrated in advanced illustrations –seems bizarre at first glance.
TEE: a viable option?
In an online statement published at the end of September, EFPIA – supported by the cost-benefit analysis of Charles River Associates – announces that “the TEE sys tem will revitalise antibiotic research and save Europe money”.
TEE provides that the developer of an eligible novel antibiotic receives a vouch er or coupon. With this, he can either ex tend the exclusivity of his own drug for a certain period of time and prevent com petition from cheaper generics, or he can sell the voucher to another company. Ac cording to EFPIA, the profits are to be used to finance antibiotics research. How this is supposed to work is an open ques tion in view of the recent withdrawal of the big pharmaceutical companies from antibiotics research since 2016. After the exit of Johnson&Johnson, only the com panies MSD, GlaxoSmithKline, Otsuka and Roche remain as antibiotics develop ers alongside the biotech SMEs. Over the next ten years, TEE is expected to provide two new antibiotics per year, CRA calcu lates. The study is apparently intended to dispel doubts that TEE could become an expensive affair because the regulation will cancel out savings made by health systems through the use of generics.
6 European Biotechnology | Winter Edition | Vol. 21 | 2022 INSIGHT EUROPE Picture: Charles River Associates
The table shows a modelling of potential cost savings in €m in six countries reflecting the EU27 market through a new antibiotic incentivised by the TEE over 10 years. Value for France Germany Italy Spain Greece Poland › Clinical benefit 54-75 23-33 87-120 18-25 8-11 18-25 › Productivity 69 30 111 23 10 23 › Transmission 335-466 414-575 296-411 236-327 53.74 189.262 › Diversity 57-79 70-97 50-69 40-55 9-13 32-44 › Enablement 277-644 342-648 245-489 195-390 44-88 156-312 › Insurance 43-165 54-204 38-146 30-116 7-26 24-93
L t.gabrielczyk@biocom.eu
Your research specimens can be priceless and make a difference in the world. That's why secure links at every stage of the cold chain are crucial. B Medical Systems’ products have 40 years of proven reliability in the world's most challenging environments.




Learn how we can help you maintain your specimens safe at every step of the cold chain: www.bmedicalsystems.com


B Medical Systems is a global manufacturer of medical-grade refrigera�on and transport solu�ons with over 40 years of experience.
B Medical Systems is a global manufacturer of medical-grade refrigera�on and transport solu�ons with over 40 years of experience.
B Medical Systems S.à r.l 17, op der Hei, L-9809, Hosingen, Luxembourg
B Medical Systems S.à r.l 17, op der Hei, L-9809, Hosingen, Luxembourg
Tel: +352 92 07 31 1
Tel: +352 92 07 31 1
Email: info@bmedicalsystems.com
Email: info@bmedicalsystems.com
www.bmedicalsystems.com
www.bmedicalsystems.com
LIVES THROUGH RELIABLE AND INNOVATIVE TECHNOLOGY
SAVING
LIVES THROUGH RELIABLE AND INNOVATIVE TECHNOLOGY Laboratory Refrigerators | | Ultra-Low Freezers Pharmacy Refrigerators Laboratory Freezers Transport Boxes
Because SAVING
Banks and policymakers driving climate change
COP27 Last year, the G7 heads declared to end funding for the oil industry by the end of this year. At the COP27 in November, the WHO and United Nations renewed promises to re-direct the hundreds of billions of dollars invested annually in fossil fuels to fund renewable energy initiatives.
make it impossible for the main emitters from the gas and oil industry to cheat and counter the often politically embellished estimates of national CO2 emissions with a reliable database.
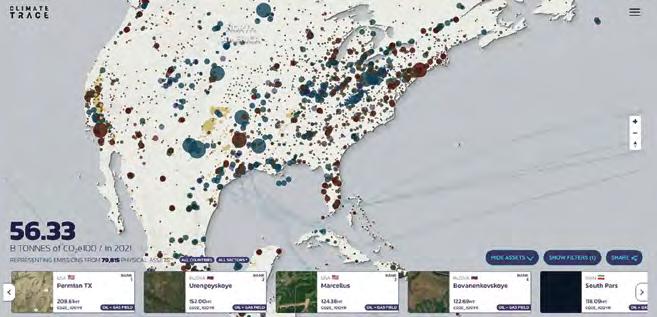
G20 countries, including China, are responsible for about 80% of global CO2 emissions. With help of Climate Trace, which relies on AI-analysis of satellite data, now the major emitters can be reliably traced.
While climate researchers worldwide appealed to the decision-makers at the COP27 climate summit in Sharm el-Sheikh for a decisive change in the energy indus try, activists demonstrated in front of the UN conference centre against the billions in subsidies with which politicians con tinue to promote the oil cartels that have long been recognised as destroying nature. “These investments are causing incredi ble damage, destroying biodiversity and devastating livelihoods across the world,” stressed Susan Huang from the NGO Oil Change International. In fact, they want ed to remind policymakers of their prom ise to end funding for fossil resources at the end of this year that was watered down by the International Energy Agency, which claimed the slow transition to renewable energies aggraved climate change. Inside the air-conditioned centre, UN Secre tary-General António Guterres criticised the ongoing investments in fossil fuels. He
pointed to an independent inventory of greenhouse gas emissions created by the ClimateTrace Coalition supported by envi ronmentalist and former US Vice-President Al Gore that could “be a wake-up call to governments and the financial sector, es pecially those that continue to invest in and underwrite fossil fuel pollution.”
World is at a turning point
António Guterres was speaking at the launch of a new independent inventory of greenhouse gas emissions created by the ClimateTrace Coalition and spearhead ed by former US Vice-President Al Gore. The tool that uses satellite data and artifi cial intelligence to make the facility-level emissions of over 70,000 sites around the world visible, will allow leaders to know the location and scope of emissions be ing released. Even today, the validated data that come from over 30,000 sensors
According to Gore, who spoke at COP27 Finance Day, the climate ca tastrophe is currently costing the glob al economy two and a half trillion dol lars annually. He said the world is at a turning point because solutions exist to reverse this dire trend (see p. 58). Ac cording to Anjali Viswamohanan, poli cy director at the Asia Investor Group, it’s not technology that is the solution but fi nance as fossil industry giants are work ing heavily to greenwash their asset and their CI. “We must have zero tolerance for net-zero greenwashing”, suggested Guterres. According to the first report of a UN high-level expert group on the top ic, net-zero pledges must be in line with the UN Intergovernmental Panel on Cli mate Change (IPCC) scenarios limiting warming to 1.5 degrees. Industry experts, however, described the IPCC figures as flawed and said behind closed doors that we would be lucky to stay below 3°C.
EU Commission President Ursula von der Leyen, on the other hand, practised verbal acrobatics and described the EU as “fully on track”, especially after the Coun cil and Parliament agreed to the Com mission’s proposal to include sectors in the CO2 emission targets that have so far not been included on the outside: trans port, buildings, waste and agriculture. The emission reduction target for these sectors by 2030 will now be raised to 40% from 2005 emissions.
8 European Biotechnology | Winter Edition | Vol. 21 | 2022 INSIGHT EUROPE
Picture:Climate Trace
L t.gabrielczyk@biocom.eu
• Ainnocence’s SentinusAITM - Powerful self-evolving artificial intelligence engine - Effectively ranks up to 1010 antibody sequences - 1-2 weeks computational HTP screening • Combined with Sino Biological ‘s high-throughput recombinant antibody development platform, top candidates can be expressed recombinantly at a lower cost and shorter lead time to generate affinity data with a high hit rate of 15% www.sinobiological.com
About Sino Biological
About SentinusAITM Sino Biological specializes in recombinant protein production and antibody development. The company routinely conducts high-throughput projects of up to 1,000 per batch. Sino Biological's manufacturing facility can also handle large-scale production at gram level.
Sino Biological US Inc. (U.S.A)
Tel: +1-215-583-7898 Email: cro_us@sinobiologicalus.com
Sino Biological, Inc. (Global)
Sino Biological Europe GmbH (Europe) Tel: +49(0)6196 9678656 Email: cro-service@sinobiological.com Hit Rate Service Highlights: Screen up to 1010 sequence space Direct access to sequence No animal use Cost effective $ Can be combined with “humanization” module Higher hit rate will be achieved on subsequent computation by incorporating wet-lab data SentinusAITM is a powerful and self-evolving protein design engine created by Ainnocence. Its database contains up to 100 million human antibody sequences as well as an extensive collection of human/animal viral antigen information, resulting in high confidence prediction of binding affinity.
Tel: +86-400-890-9989 Email: cro-service@sinobiological.com Sino Biological and Ainnocence have joined forces to establish an AI-enabled platform for antibody affinity maturation. Lead Time 4 weeks 103 increase High Affinity 15%
NEWS EU funding
In mid-October, the new MIBIREM project kicked off in Vi enna. Under the coordination of the Austrian Institute of Technology, 11 re search groups from six EU coun tries will develop a toolbox for mi crobiome-based soil remediation on contaminated sites. Conventional re mediation technologies are often too expensive and technically demand ing. Microbiomes have a high poten tial to improve such processes. They can degrade organic contaminants. Until 2027, the EU Commission will grant €6.67m to develop a sustaina ble solution to clean up the 324,000 severely contaminated sites, such as mines, landfills or petrol stations, across Europe.
Switch plastic production
After a phase of steady growth, fear is sweeping Europe’s plastics-producing industry. At the end of October, Marco ten Bruggen cate, President of Plastics Europe and VP for Packaging and Specialty Plastics at petrochemical giant Dow, warned at the K 2022 industry trade fair in Düsseldorf, Germany, that the remaining suppliers were leaving Eu rope – investments were threatening to flow overseas. He called for a vig orous approach to the “Green Deal” proclaimed by politicians. A combi nation of chemistry and biotechnol ogy seems to offer great opportuni ties. The German start-up EEDEN GmbH has developed a process to extract and reuse cellulose from tex tile waste, while the French Carbios SA (Clermont-Ferrand) can microbi ally break down PET fibres from syn thetic textiles into their monomers and re-polymerise them.
EU agri-coalition pushes for relaxing rules for genome editing
EUROPE After 17 countries worldwide, including the UK, relaxed rules on grow ing genome-edited crops, a majority of European agriculture ministers are pres suring to exempt genome-edited crops and their products from the strict EU GMO legislation.
“We only need to modify our old leg islative framework regulating modern breeding techniques,” stressed Czech agriculture minister Zdenek Nekula. Among the countries supporting the re laxation of EU GMO laws, currently un der consultation at the EU level, at an informal meeting of ministers in late Sep tember are reportedly the Netherlands, Estonia, Sweden, Lithuania, Netherlands, Malta, Ireland, Italy, Hungary, Romania and Belgium.
The main arguments for the relaxation are the current lack of fertilizers, soaring energy prices and climate change. Ris ing energy prices and the need for more irrigation alone have increased costs for farmers by 35%. “Climate change is coming faster than we are developing new crops,” Euractiv quotes Dutch farm er Hendrik Jan ten Cate. “We need new techniques. There is a big danger to food production in Europe.”
Alternative model
While in the UK the cultivation of high er-yielding crops optimised with the gene scissors seems to be a done deal, in Switzerland the relaxation targeted for 2024 is still being disputed. At the end of October, the Federal Ethics Com mittee on Non-Human Biotechnology (Ekah), which is staffed by internation al experts, announced that a restructur ing of agricultural production could yield much more than high-intensity farming with the genome-edited plants that are to be used primarily as animal feed. The experts recommend stopping the culti vation of concentrated feed, reducing
livestock and meat production and re stricting it to grassland only, as in Ireland, and using the freed-up land to grow food crops. “The state should assume political leadership responsibility in view of the urgency of the climate targets,” says the extra-parliamentary advisory body.
The Swiss also want to limit the import of the soy concentrate needed for inten sive livestock farming.
India, Russia, China, the Phillippines, Nigeria, Kenya, Canada the USA and South American soybean exporters Bra zil, Argentina, Chile, Ecuador, Colombia, Paraguay, Honduras and Guatemala are largely united in deregulating biological mutation breeding:
› If no foreign DNA is present in a ge nome-edited plant and if it could have arisen under natural conditions through random mutation, it is to be evaluated like a classically bred plant. No labelling is envisaged.
› However, if DNA segments have been inserted into the genome using ge nome editing techniques, such plants are generally considered GMOs and fall under genetic engineering laws.
Genetic engineering sceptics describe the consultation on the intended new EU regulation as fake. It is eagerly await ed how the European Commission will shape the new regulations so as not to jeopardise its goal of increasing organ ic cultivation by 30% by 2030 by placing conventional and genome-edited prod ucts on an equal footing. According to scientists at the Swiss Research Institute of Organic Agriculture (FIBL), there is currently no obligation for farmers to de clare what they grow GM crops. A labelfree cultivation of genome-edited plants in the vicinity of organic fields would make it impossible for organic farmers to provide the necessary evidence for or ganic certification.
10 European Biotechnology | Winter Edition | Vol. 21 | 2022 INSIGHT EUROPE
L
Improved biogas production
HORIZON EUROPE Biogas plays an important role in achiev ing EU energy security. Under its EU action plan, the EU Com mission aims to double biogas production through microbial fermentation of agricultural residues to 35 billion cubic me tres by 2030 to end the EU’s dependence on the Russian fos sil supply. However, the potential of methane as a renewable fuel (CNG) remains untapped. To turn biogas into a cost-ef fective alternative to fossil fuels, researchers within the Micro 4Biogas project will be developing tailored anaerobic microbi al communities recycling organic waste. “We aim to increase the yield of biogas, its quality, the speed of production and the robustness of the whole process,” says Manuel Porcar from the University of Valencia (Spain) that coordinates the Micro4 Biogas project.
Scientists from the project have already collected microbi al samples from multiple biogas plants and they are analys ing them to create microbial communities that are optimised for biogas production.
The idea is to transport the biogas in the existing gas in frastructure replacing natural gas in all scenarios and pre venting energy supply disruptions. “While both photovoltaic and wind energy have the disadvantage of depending on the weather to generate electricity, biogas can be stored and con sumed whenever it is needed,” explains Carlos Roldán, elec trical engineer from the Polytechnic University of Valencia, where biogas from the pilot plant will be used for electrici ty production. The possibility of storing biogas helps stabilise the electricity grid, facilitating the widespread use of intermit tent renewable sources.
Animal biogas
L
SigmaPlot® 15
Designed Specifically to Meet the Needs of Scientists, Professional Researchers and Engineers
SigmaPlot® is an easy-to-use scientific graphing & statistical data analysis software package for researchers, scientists and engineers who need to create precise, publication-quality graphs that best communicates their research results for presentations, technical publications, and the web. Along with advanced curve fitting, a vector-based programming language, macro capability and over 50 frequently used statistical tests, SigmaPlot also provides more than 100 different 2D & 3D graph types from which one can choose a full range of graphing options such as technical axis scales, multiple axes, multiple intersecting 3-D graphs and much more. SigmaPlot now has SigmaStat included with it which is a perfect tool to visualize and understand basic and advanced statistics.
SigmaPlot offers:
•Complete Advisory Statistical Analysis
•Award-Winning Technical Graphing Capability
•Powerful and Easy-to-use Data Analysis
•Ability to Customize every Element of Graph
•Precise Publication-Quality Graphs

•Ability to Publish your Work Anywhere
Latest updates:
•Includes a New Heat Map Macro
•Removes all dependencies on old redistributable by removing Lead Tools and uses Windows Graphics Device Interface + (GDI+) for graph export
•Uses the latest Sentinel License Manager which is compatible with the latest Microsoft Server 2022
•Uses a hosted licensing service for smooth license activation and validation
•Has a new and refreshed ribbon manager that enhances the already commendable user experience in SigmaPlot
LIVESTOCK FARMING Around €2m is being invested through Horizon Europe to unlock the biogas potential in livestock farming. In November, the ALFA project kicked off in Thessaloniki, Greece. ALFA, which is coordinated by QPlan International, promotes 50 large-scale livestock farms in Belgium, Denmark, Greece, Italy, Slovakia and Spain to set up an infrastructure for the production of biogas from un treated animal waste. Ten partners will collaborate to pro mote non-vegan methane production facilities: Agenzia per la Promozione della Ricerca Europea, AzzeroCO2, Centre for Research and Technology Hellas, European Biogas Associa tion, European Dairy Farmers, Food & Bio Cluster Denmark, Pedal Consulting, SUSTAINABLE INNOVATIONS and White Research.
North America & South America
Inpixon USA
2479 E. Bayshore Rd, Suite 195. Palo Alto, CA 94303, United States saves.sales@inpixon.com
Germany, Austria, & Eastern Europe
Inpixon GmBH Königsallee 92a, 40212 Düsseldorf, Germany saveskontakt@inpixon.com
UK & Western Europe
Inpixon UK Ltd

268 Bath Road, Slough, SLI 4DX, UK saves.sales@inpixon.com
©2022 by SYSTAT Software, Inc. SYSTAT, SigmaPlot, SigmaStat, SigmaScan, TableCurve2D, TableCurve3D and PeakFit are trademakes of SYSTAT Software, Inc. All other product or brand names are trademarks or registered trademarks of their respective holders.
11 European Biotechnology | Winter Edition | Vol. 21 | 2022 INSIGHT EUROPE
ANCOVA Global Curve Fit
Principal Component Analysis Regression
 Full house at BIO-Europe 2022 in Leipzig
Full house at BIO-Europe 2022 in Leipzig
Crisis? What Crisis?
FINANCING External financing has proven indispensable for small biotech companies in the drug arena, with the majority of firms viewing it as essential for accelerated devel opment. Where external capital is lacking, growth becomes more difficult, and innovation slows. The ‘new normal’ involves partnerships and licensing, especially in Europe, while the M&A wave is not building as fast as once predicted.
Few companies expect to be worse off in 2022 than they were before the pandemic. Fast, generous political aid and a strong recovery in demand in the first half of this year should help prevent it. Overall, 84% of companies expect sales to at least return to pre-pandemic levels in 2022. That’s the survey result across all sectors that emerged from the Euro pean Investment Bank’s (EIB) investment survey at the beginning of November. On the flip side, opinions about the fu ture sounded less positive: the Ukraine war and its consequences look set to “test the resilience of companies consid erably”. Investment conditions have also deteriorated significantly, hamstrung by the energy crisis, uncertainty and slow ing global growth. Expectations regarding the economic situation slipped again into the red (from +27% to -53%). There was also a turnaround in the assessment of the business outlook (down from +34% to +3%), as well as in the outlook for the political and regulatory climate (-40%) and access to external finance (-8%).
View of biotech/pharma
With this dim view of the future from across industries, pharmaceuticals/bio technology probably shouldn’t have high hopes, right? But depending on the inter locutor, the entrepreneurial perspective or the level of development of the com pany or its products, you hear very dif ferent sounds from the community. “On paper, the record cash reserves of the big
pharma ceutical companies, the steep, rapidly approaching patent cliff and the low public biotech valuations should lead to a buying frenzy,” wrote Melanie Sen ior in a recent commentary in nature Bio techno Logy (Nature Biotechnology Vol ume 40, 2022). And since stock markets within biotech indices are still falling, bio tech companies short of cash were hope ful the pharma industry would buy up the external innovation on offer a little more indiscriminately, Senior added.
But that’s not happening. Many pre dicted a new surge based on a wave of top valuations and stock market prices piling up during the pandemic, foresee ing a buying wave in mergers & acqui sitions (M&A). It has not failed to ma terialise completely, but hasn’t turned into the hoped-for spring tide – at least so far. Pfizer’s US$11.6bn acquisition of
Biohaven Pharmaceuticals in May was a high point in 2022. But a possible mega deal (rumoured to be worth around US$40bn) between Merck and Seagen remains stuck, whether for substantive reasons, because of differences over the valuation, or that they’re simply shying away from striking such a major deal in times like these – maybe a combination of all of the above. Outwardly, both par ties continue to claim that there are just some details left to be cleared up. How ever, without it, at the current pace, 2022 is set to be the worst year for M&A since 2018.
By the end of August, the total value of acquisitions in 2022 was just half that of 2021, which in turn was low com pared to 2020 and 2019 (Fig. 1). Accord ing to BioCentury BCIQ, the total val ue of deals was US$41.5bn by August
Fig. 1: M&A over a five year period. The average size of deals has fallen. *Through August. (Source: BioCentury BCIQ in Nat Biotechnol 40, 1546–1550, 2022)
13 European Biotechnology | Winter Edition | Vol. 21 | 2022 COVER STORY Picture: left © Ludwig Schedl for Informa Connect/BIO-Europe
3.0 2.5 2.0 1.5 1.0 0.5 0 1.2 1.8 2.7 1.7 1.1 0.9 0 20 40 60 80 100 120 140 160 2022* 2021 2020 2019 2018 2017
Total
Average deal
Average deal
108 119 132 140 146 96
Total no. deals
number of deals
size ($billions)
size
2022, compared to nearly US$97bn in 2021 and over US$140bn in 2020. And not just megadeals like the Merck/Sea gen proposition are missing. The over all number of deals is down 30%, and is significantly lower in 2022 than in pre vious years.
Patent cliff or just a bump?
Three drugs alone – Humira (adali mumab) from AbbVie, Keytruda (pem brolizumab) from Merck and Opdivo (nivolumab) from Bristol Myers Squibb (BMS) – account for annual sales of over US$45bn. Although these patents are slated to expire in the next few years, there’s been no panic buying on the part of pharmaceutical firms yet.
In financial circles it’s being said that there appears to be no sense of urgen cy on the part of Big Pharma. And when external products or entire companies are bought up, marketed or near-market products are purchased that immediately closes sales gaps. “Anything that comes earlier is not worth the risk or the addi tional R&D costs. The patent problem of Big Pharma companies will not be solved by buying a phase II company,” Gil BarNahum, managing director at Jefferies’ Global Healthcare Investment Banking Group in London, told nature Biotech no Logy. Although according to SVB Se curities, Big Pharma companies have an estimated US$350bn in cash (and up to half a trillion to spend), most appear re
luctant to adopt early-stage programmes with funding needs that might erode their bottom line in an era of double-digit in flation, fragile supply chains and an am bivalent FDA in price discussions.
Acquisitions not excluded
Exceptions of course confirm the rule, and some deals continue to go through. But in addition to product purchases that have already hit the market, a new paradigm appears to be in place. The latest algorithm seems to classify prod ucts in phase II as attractive if they are also accompanied by a groundbreaking treatment option (see table below). Most of the US$26bn Pfizer spent on acquisi tions over the past 12 months went to high-revenue companies, but the sum (which is still below COVID-19 vac cine revenues expected for 2022) also included phase I/II cancer company Trillium Therapeutics (US$2.2bn) and ReViral (US$525m), which has a phase II vaccine candidate against respirato ry syncytial virus (RSV). Bristol Myers Squibb is also less cautious than the pack. It paid US$4.1bn in June for Turn ing Point Therapeutics, which is expect ed to file for approval for the lung can cer drug repotrectinib later this year – with market approval possible in 2023. Repotrectinib adds a strategically fitting precision oncology asset to BMS’s existing portfolio: tyrosine kinase inhib itor target ROS1 and NTRK mutations,
complementing BMS’s non-small cell lung cancer therapies, including Opdi vo (nivolumab), Yervoy (ipilimumab) and Opdualag (nivolumab and relatlimab). GlaxoSmithKline’s (GSK) US$2.1bn ac quisition of Affinivax brought to the company a pneumococcal vaccine can didate that is still in Phase II. Howev er, it’s viewed as groundbreaking, and includes more pneumococcal sero types than approved vaccines. The Brit ish pharmaceutical company also spent US$1.9bn on Sierra Oncology in its bid to rebuild an oncology franchise aban doned nearly a decade ago.
All of these large-volume transactions can therefore be classified under the heading ‘long-term strategy’, with hun dreds of mid-sized biotech companies with early development pipelines re maining largely ignored. Some industry observers put restraint at the forefront of their analyses, while others see these in dividual transactions as the beginning of a larger shopping spree that could bene fit any biotech company that already has products on the market or ones that are about to be approved.
Biotech well positioned
Some biotech fund managers are cau tiously optimistic about the situation. “Many biotech companies are current ly very well positioned and highly attrac tively (low) valued. In addition, many im portant innovations are expected, with
Source: BioCentury BCIQ in Nat Biotechnol 40, 1546–1550, 2022, modified
14 European Biotechnology | Winter Edition | Vol. 21 | 2022 COVER STORY
M&A deals 2022 worth over US$1 billion Buyer-Seller Deal value ($billion) Therapy area (latest stage) › Pfizer-Biohaven 11.6 Migraine (marketed), obsessive–compulsive disorder, epilepsy, rare diseases › Pfizer-Global Blood Therapeutics 5.4 Sickle cell disease complications (marketed), blood disorders › Bristol Myers Squibb-Turning Point 4.1 Cancer (phase III) › Amgen-ChemoCentryx 3.7 Rare autoimmunity (approved) › GlaxoSmithKline-Affinivax 2.7 Viral infection, cancer (phase II) › GSK-Sierra Oncology 1.9 Myelofibrosis (submitted) › Novo Nordisk-Forma Therapeutics 1.1 Sickle cell disease, rare blood disorders (phase II/III)







Built for BiotechSM Strategic product development Full-service clinical research & development Therapeutic & special population expertise Holistic technology ecosystem Get started at premier-research.com/builtforbiotech
growing market potential of the individ ual indications,” says fund manager Lukas Leu (Bellevue Asset). He also brings the patent cliff argument. According to FDA estimates, blockbuster drugs with a total turnover of more than US$250bn will lose their patent protection by 2030. Leu esti mates that the sharp price reductions that will ensue now offer attractive entry op portunities.
In his view, areas like gene thera py are currently moving into the next generation of products and technolo gies. Other more established technol ogies, such as RNA platforms, have rapidly expanded from their initial fo cus on rare diseases to larger indica tions, and could generate significantly

higher revenues than originally antic ipated. An example of this is Bioma rin’s European launch of Roctavian, the first gene therapy for the treatment of severe haemophilia A. After achieving excellent clinical data, Alnylam is also about to receive approval for patisiran, a drug for ATTR amyloidosis with car diomyopathy. Although a niche prod uct, it could generate billions in reve nues in the future. The fund manager of Bellevue Asset Management emphasis es that solid regulatory conditions, cou pled with increasing study applications submitted to the FDA, point to further approvals in the field of drug devel opment: “Innovation is in full swing, new breakthroughs are being driven
and new products are being brought to market quickly.”
Good vibrations at BIO-Europe
At Europe’s largest annual event for bio tech partnerships this year in Leipzig, the unbroken good mood on both sides of the deal table between biotech innova tors and partners from pharma was pal pable. “This was our first personal BIOEurope in three years, and the result exceeded our expectations,” says Anna Chrisman, Managing Director Pharma and Life Sciences at Informa Connect. Around 5,000 participants came to the traditional German trade fair city to take a closer look at the projects presented, or
VC still bold in financing innovation
INTERVIEW VC funds are flush with cash. Some have set new records for closings, but inflation, war and ECB interest rates are spooking others. EuropE an BiotEchnology asked Sander Slootweg, Managing Partner and co-founder of Forbion Capital Partners, about how he’s coping with the complex and difficult times.
EuroBiotech _The BIO-Europe in Leipzig just sent a lot of positive signals. Why is the healthcare market seemingly unaf fected by all the ongoing crises?
Slootweg _There are several reasons why the life sciences venture capital field seems immune to the current challenges facing the business community. It is im portant to understand that during times of crises, a reduction in pharma sales does not occur as it would with fast moving consumer goods. Most of Big Pharma sees their innovation coming from the biotech industry, with more than 80% of newly approved drugs stemming from our indus try. Pharma also has to replenish their pipelines across several areas of interest, having set aside US$500bn collectively for acquisitions – most of which was generat ed from proceeds from COVID-19 vac cines and antivirals. This implies that they are cash rich with low leverage, and there fore not affected by rising interest rates. Another factor that plays into their resil
ience is the fact that pharma can reprice drugs annually to compensate for infla tion, showing they have pricing power.
EuroBiotech _ We have seen and are still seeing record VC fund closings. A lot of
money is being put into the pockets of venture firms. Are your investors, institu tional or otherwise, reluctant to give you new money for new funds?
Slootweg _Based on the reputation that Forbion has carefully curated in the last two decades, we have not been affected. Earlier this year we announced the first close of our Growth Opportunities Fund II at €470m. We expect a final close at €600m despite the setbacks experienced in the markets. We have big plans for 2023, and our investment relations team is confident of success.
EuroBiotech _If you look at the Forbion funds, where does the money come from, and how is this changing (if at all) by region? Do you get more money from European investors...or from the US...or from Asia...than you did in the past?
Slootweg _Forbion has always maintained strict criteria for sourcing investors. We pride ourselves in being a European firm
16 European Biotechnology | Winter Edition | Vol. 21 | 2022 COVER STORY
Picture: © Forbion Capital Partners
Sander Slootweg, Managing Partner, Forbion
to talk investment strategies with poten tial partners in the over 30,000 discus sions agreed via the partnering platform. “Our task has always been to present various biotech clusters and shed light on some of the hidden gems in Europe,” explains Chrisman about this year’s ven ue. Leipzig’s Lord Mayor Clemens Schül ke added: “For the first time, Leipzig was able to present its BioCity Campus at the event, and present new construction pro jects and laboratory space together with private investors.” Biosaxony Managing Director André Hofmann, who heads the local biotech network, was effusive. “The return of BIO-Europe to Saxony af ter 17 years has clearly shown how far we have all come,” he said. The fact that and our investor base reflects that. Hav ing said that, we are seeing keen interest from North America, and that investor base is rapidly growing. As word gets out about our success rate and the companies we have built, we are seeing institutional investors (pension funds, endowments, insurance companies) coming on board. This is a definitive reflection of the prag matic approach we have to investing, and the confidence in the capability and ex pertise of our investment professionals.
EuroBiotech _If you look at the European landscape in VC, it seems to be dominat ed by funds from France, Benelux, the UK, and some US funds with European sites. Am I missing something? Why is Germany still lagging behind?
Slootweg _Germans are known to be great engineers and can execute a clearly laid out plan to perfection. However, the road to success in biotech is never a straight line. Often one has to change course several times along the way in or der to overcome obstacles. Dealing with this type of inherent uncertainty is not a cultural strength of Germans.
EuroBiotech_At the moment the IPO-exit strategy doesn’t seem to offer a good op portunity. So does one just have to wait
the most recent Nobel laureate in med icine, Svante Pääbo, is based in Leip zig was also continuously underlined throughout the trade fair.
No drama in financing
Arno Fuchs, CEO and Owner of FCF Fox Corporate Finance GmbH, has been working in the industry for a long time analysing developments in the US and Europe. “Financing volumes have not fallen very much in the US, but the Americans are holding back somewhat in Europe, so we see a decline of 30% in overall funding volumes here,” Fuchs re ported in an interview with european Bio technoLogy Europe’s dependence on US
financing volumes is evident. However, the CEO does not view the decline as a drama, but as a normal reaction to strong growth in recent years. “The cycles are intact, there are exit opportunities aside from the stock market, there is money that is being invested. Good companies always find the money they need, even now,” Fuchs believes, though “financing rounds may take longer or be somewhat smaller.” Overall, however, the biotech industry is decoupled from other indus tries and the broader market, which suf fer more from fears of inflation and rising interest rates. With a proper and profes sionally executed strategy to approach ing the right investors in a well prepared and targeted manner, Fuchs thinks firms
this phase out? Or does it strongly influ ence strategy in your portfolio?
Slootweg _Having been in the business for over two decades, we have ‘survived’ financial downturns and also experi enced extremely profitable times. It is for this reason that Forbion developed its current strategy, which allows us to exit through M&A or IPO when the markets are buoyant, and to be conservative and invest in companies at attractive terms when the markets are not. The venturecapital business is all about timing and strategy, and of course the public mar kets are an enormous factor when con sidering the next moves for our portfolio companies. A great example I like to refer to is VectivBio, a public company that we sought to invest in two years ago. At the time, our team believed that the company was valued relatively highly on the public markets, and we decided not to invest. In 2022 the situation was differ ent, and we saw VectivBio’s valuation decrease, enabling us to make a value oriented investment. They are now part of our portfolio.
EuroBiotech _We see some smaller funds specialising in early drug development, and strongly interacting with academic institutions or focusing on very special
disease indications. Are these smaller funds possibly more affected by sur rounding critical framework conditions?
Slootweg _I would imagine that this is in deed the case. Funding at the seed stage is far less costly, and this is why you prob ably see the phenomenon of smaller funds in this arena. It is no coincidence that generally speaking, the size of the fi nancing rounds are typically larger as they progress, since there is less risk as you get further into the financing because the companies are better developed both from an IP and operational standpoint.
EuroBiotech _If you are purely optimistic for the future, why is that? Can you per haps explain with describing your key strategy what makes you so positive?
Slootweg _The 21st century is truly the century of biotech. So much more is known about what causes disease and how to best design solutions, and so many diseases are currently poorly or not treated that we believe current market conditions not to be representative of the opportunity we are chasing. We concen trate on three strategies: company crea tion, company building and company expansion. These are three distinct cate gories that drive our success, and ulti mately our positive disposition.
17 European Biotechnology | Winter Edition | Vol. 21 | 2022 COVER STORY Pictures: xxx
L
A senior finance panel discussion at BIO-Europe claimed a ‘silver lining’ in challenging times. One member was Sander Slootweg (Forbion, second from left, interview p. 16).

can also chalk up successes with the se lected family offices that are increasingly interested in the industry.
He sees the large available fund vol umes as further proof that the cycle of money in the innovation process of new active ingredients is intact. Even if these larger sums are being spent somewhat more slowly or in smaller amounts, he says institutional funding capacity re mains intact for existing portfolio com panies, also to buffer delays in clinical trials or difficulties on the logistics, lab capacity or human resources side. The mood among VC investors in many dis cussion rounds at BIO-Europe confirms this assessment. There was no trace of panic or crisis.
A less sanguine view
In her commentary in n ature B iotech no L ogy, Melanie Senior put forward some counter-arguments as to why one should move cautiously when optimism is bubbling. For example, she sees some setbacks in gene therapy candidates or in the use of cell therapeutics as one of the reasons for the reluctance to acquire gene and cell therapy companies, which have fallen sharply. By September 2022, there were only eight such mergers, less than half as many as the year be fore. 2022 deals include Vertex Pharma ceuticals’ acquisition of stem cell com
pany ViaCyte with its type 1 diabetes programme, as well as Galapagos’ ac quisition of CellPoint, whose lead CART cell therapy is in phase I/II trials. In early October, AstraZeneca indulged in a bargain, snapping up struggling gene therapy company LogicBio Therapeu tics for only US$68m, or just over US$2 per share – a fraction of what it would have had to pay for the adeno-associat ed virus -based gene therapy company during the past three years.
Senior also considers the discussion about drug prices in the US to be by no means over. Rather, the so-called Infla tion Reduction Act, which went into ef fect in August 2022, requires the govern ment-funded Medicare program (which covers people over the age of 65) to ne gotiate prices for the ten best-selling out patient drugs in 2026, and 20 best-selling outpatient drugs three years later. Other cost-control measures include rebates on all Medicare drugs whose prices are ris ing faster than inflation, caps on negotiat ed maximum prices, and – for manufac turers who refuse to negotiate – punitive taxes on the previous year’s sales.
Industry lobby group PhRMA com plains that the law will stifle innovation. However, not all drugs are affected by the negotiations. Exceptions are drugs for which there are already generics or bio similars that have been on the mar ket for less than seven years (for small
molecules) or eleven years (for biolog ics) or that will cost Medicare less than US$200m in 2021. The bill will therefore not significantly shorten the market ex clusivity periods (5-7 years for small mol ecules and 12 years for biologics). Ex pensive injectables and other products administered in hospitals will not be ne gotiated until 2028. Orphan drugs that are only approved for rare diseases are also excluded, along with drugs that ac count for over 80% of a company’s sales – a common scenario for biotech compa nies focused on rare diseases.
In Germany, too, an austerity pro gramme in the healthcare sector has the pharmaceutical associations up in arms amidst fears that it could that it could slow innovation. However, a withdraw al of the corresponding law is not up for debate.
Overall, optimism prevails
In general then, a slow trend towards in creasing acquisition activity – coupled with the strong innovation pipeline – are being seen as positive signs that the sec tor should be protected from the head winds of rising interest rates. Even if phar ma is backing off biotech temporarily in terms of acquisitions, the ‘biodollars’ are still flowing when it comes to payment after all negotiated milestones. Manuel Bauer summarised the current market situation for european BiotechnoLogy: “As part of the data collection for EY’s Ger man Biotechnology Report, we are ob serving a continuation of the noticeable downward trend in international M&A transaction activity with a significant ly reduced transaction volume. Simulta neously, there is a trend towards allianc es, so that for the German biotech sector we will see a record year for strategic al liances in 2022 with more than €8bn.”
Bauer has been with EY´s Life Sciences & Healthcare sector as Biotech Leader Ger many, an active observer on the scene for over twenty years, he’s seen plenty of ups and downs. Most of the discussion partners for this article stated, a financial crisis looks different.
18 European Biotechnology | Winter Edition | Vol. 21 | 2022 COVER STORY Pictures: © Ludwig Schedl for Informa Connect/BIO-Europe
L g.kaeaeb@biocom.eu
LISAvienna’s 20th anniversary celebration was a blast
AUSTRIA Vienna is one of Europe’s most dynamic innovation hubs in the life sciences. End of September, the life science community met to celebrate LISAvienna. For 20 years, LISAvienna has been acting as the cluster’s central point of contact. In close collaboration with Austria’s support and funding ecosystem, the platform accompanies and supports bioentrepreneurs through the ups and downs of their journey to transfer innovative business ideas into viable products and services.
LISAvienna provides advice free of charge at the interface of Austria Wirtschaftsser vice – Austria’s promotional bank boost ing innovation and growth – and the Vienna Business Agency.
Vienna’s growing biotechnology, phar maceutical, medical device and digital health community comprises more than 600 organisations, employing a work force of 41,000 people. The Austrian Federal Ministry of Labour and Econo my and the City of Vienna are fully com mitted to advancing the sector and regu larly launch thematic funding programs.
In addition, 14% research tax premi um, well-trained hands-on profession als, an outstanding quality of life and its strategically favourable location in the heart of Europe, all make Austria high ly attractive to the life science business community. In collaboration with the Austrian Business Agency, LISAvienna also provides support for international companies, that want to set up in Aus tria, expand, grow or do research here.
Up to €1m pre-seed and seed financing is available for implementing deep-tech start-ups, and early-stage drug devel opment is eligible for funding from the € 70m KHAN-I via wings4innovation.
Twenty years ago, LISAvienna was in itiated to facilitate joining forces at fed eral and state levels with the life sci ences sector. Its outstanding success proves that the founders Sonja Ham merschmid and Edeltraud Stiftinger were right on the mark! During the cele

bration at the gorgeous Vienna City Hall, they briefly looked back at the struggles in those early days and congratulated their successors on their achievements. Today, LISA – Life Science Austria –is well-known throughout Europe and LISAvienna plays a key role in that. The Austrian booth at BIO-Europe in Leipzig once again pulled in crowds. LISAvien na Managing Directors Johannes Sarx and Philipp Hainzl commented: “Bio medical research, drug and IVD devel opment and manufacturing plus all the related services still are the strongest pillars of Austria’s and Vienna’s life sci ences landscape. In addition to health tech, green and food tech are also gain ing importance, with shared scientif ic infrastructure and cutting-edge re search findings being key drivers. To put
a spotlight on the diversity of topics and actors characterising the community, we invited eight representatives to share insights on stage at our festive anni versary ceremony.” Six stakeholders in LISAvienna were also engaged digital ly in advance to present their organisa tions, tasks and personalities, and cur rent challenges. These videos and a clip based on hundreds of event photos are available on LISAvienna’s YouTube chan nel. There, you can also find a photo mo saic clip containing lots of great memo ries drawn from LISAvienna’s archive.
Contact us: Johannes Sarx und Philipp Hainzl office@LISAvienna.at www.LISAvienna.at
19 European Biotechnology | Winter Edition | Vol. 21 | 2022 ADVERTORIAL
/
Picture: © LISAvienna
Ludwig Schedl
LISAvienna’s former and current Managing Directors: Johannes Sarx, Peter Halwachs, Eva Czernohorszky, Edeltraud Stiftinger, Sonja Hammerschmid, Michaela Fritz, Philipp Hainzl
Have biotech stocks bottomed out?
CHRIS MAGGOS,
 BIOCONFIDANT
BIOCONFIDANT
SÀRL Biotechnology was dramatically rewarded in the financial markets through much of 2020 and the first half of 2021, widely credited for providing the COVID-19 vaccines. This followed four already exceptional years in the financial markets, with the Nasdaq Biotech Index (NBI) hitting its all-time high of 5449.32 on 30 August 2021.
The prolonged positive trend brutally reversed in Septem ber 2021 – the persisting COVID-19 pandemic, glob al inflation and geopoliti cal turmoil delivered repeat ed seismic shocks to financial markets, and public equity in vestors, eschewing risk, dra matically reduced their exposure to bio tech. The financing window slammed shut for biotech IPOs and venture capital in vestments dramatically slowed.
The storm in the markets over the past 14 months has been harrowing but re cently, the thunderclouds seem to have a silver lining. Despite the fact that the same fundamental issues – COVID-19, inflation, volatile geopolitics – remain, it would appear that sentiment towards bi otech has improved, as financial markets look to the future. The larger healthcare stocks maintained a more “normal” trad ing pattern, as a haven for investors dur
ing difficult market condi tions, and recently smaller biotech stocks also started to recover somewhat.
Pharmaceutical and larger biotech stocks (i.e. the larger profitable com panies with marketed products) are outperform ing the broader markets in 2022. For ex ample, Dow Jones Index is down 7.7% compared to only 1.3% for the DRG, the NYSE ARCA Pharmaceutical Index, through 15 November. The NBI is only down 9.6% compared to 28% for the Nasdaq composite during the same pe riod. This is a major change from early 2022 and late 2021, when biotech was often among the worst-performing sec tors.
The change occurred over the sum mer; NBI recovered from a low of 3508.58 on 6 June 2022 to 4248.70 at the close on 15 November, an impressive
News from the floor
Abivax SA (Euronext Paris: FR00123 33284 – ABVX), French biotechnolo gy company developing novel thera pies that modulate the immune system to treat chronic inflammatory diseases, viral infections, and cancer, announced the first patient being enrolled in the US into its global Phase III clinical program (“ABTECT program with product candi
date obefazimod for the treatment of mod erate to severe ulcerative colitis (UC).
BioNTech SE (Nasdaq: BNTX) and part ner Pfizer announced that the companies have initiated a Phase I study of a nextgeneration COVID-19 vaccine candidate that aims to enhance SARS-CoV-2 T cell responses and potentially broaden protec
740 point (21%) improvement. It is still 22% below the all-time high of 30 Aug 2021, so there is little doubt that inves tors have retrenched and begun to look for opportunities over the summer.
Nevertheless, the IPO window re mains firmly shut with the fewest num ber of deals getting done year to date in more than five years. This is likely to remain the case while the question re mains: Is this a durable recovery leading to an opening of the financing window, or the eye of the storm and just a prelude to greater losses ahead in light of worsen ing global crises?
More exciting acquisitions by larger companies could drive industry stock performance. Positive data, like the pos itive Clarity AD trial of lecanemab (Bio Arctic/Eisai/Biogen), have driven inves tors into many related neurodegeneration companies. While no company is bullet proof, the industry as a whole is stronger than ever. L
tion against COVID-19. This candidate, BNT162b4, is composed of a T cell an tigen mRNA encoding for SARS-CoV-2 non-spike proteins that are highly con served across a broad range of SARSCoV-2 variants and will be evaluated in combination with the companies’ Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine.
20 European Biotechnology | Winter Edition | Vol. 21 | 2022 STOCK MARKETS Picture: © BioConfidant Sàrl
L
COMPANY QUOTE M-CAP 52 WEEKS INDICATOR
2cureX AB 0.73 12,300k
4SC AG 1.75 17,700k
AB Science SA 8.13 376,100k
Abcam plc 15.80 3,620,000k
Abionyx Pharma SA 1.84 51,400k
Abivax SA 8.07 178,000k
Ablivia AB 0.02 26,200k
AC Immune SA 2.82 234,800k
Acticor Biotech SA 6.42 67,900k
Active Biotech AB 0.08 25,600k
Adaptimmune Therapeutics plc 2.32 386,600k
ADC Therapeutics SA 3.89 292,700k
Addex Therapeutics Ltd 0.13 5,100k
ADL Bionatur Solutions SA 0.25 13,500k
Adocia SAS 3.67 30,500k
Advicenne SA 3.75 37,400k
Aelis Farma SAS 8.67 109,100k
Affimed NV 2.08 287,600k
Akari Therapeutics plc 0.58 34,400k
ALK-Abelló A/S 13.36 2,710,000k
Alkermes plc 22.70 3,730,000k
Allarity Therapeutics A/S 0.40 4,100k
Allergy Therapeutics plc 0.17 117,100k
Alligator Bioscience AB 0.15 32,900k
Altamira Therapeutics Ltd 5.01 5,100k
Alvotech SAS 5.76 1,430,000k
Alzinova AB 0.16 6,500k
Amniotics AB 0.31 4,900k
Annexin Pharmaceuticals AB 0.09 14,300k
Aprea Therapeutics AB 0.37 19,600k
Aqua Bio Technology ASA 0.65 13,500k
Arctic Zymes Technologies ASA 6.19 320,400k
Arecor Therapeutics plc 2.72 76,600k
Argenx BV 353.80 19,770,000k
Arix Bioscience plc 1.22 157,600k
Arocell AB 0.05 12,000k
Arterra Bioscience SpA 2.13 14,300k
Asarina Pharma AB 0.08 1,800k
Ascelia Pharma AB 2.29 77,100k
Ascendis Pharma A/S 111.65 6,427,200k
Asit Biotech SA 0.12 2,500k
Autolus Therapeutics plc 2.20 206,000k
Avacta Group plc 1.34 357,500k
Avadel Pharmaceuticals plc 7.57 467,800k
Axichem AB 1.26 20,800k
Basilea Pharmaceutica AG 48.27 574,800k
Bavarian Nordic A/S 33.0 2,333,300k
Bergenbio ASA 0.08 6,000k
Bicycle Therapeutics plc 26.02 772,500k
Bioarctic AB 26.50 1,970,000k
Biocartis NV 1.02 99,000k
Bioextrax AB 0.20 1,900k
Biofrontera AG 1.62 100,800k
Biogaia AB 7.68 756,300k
Bioinvent International AB 3.24 215,000k
Biomed-Lublin SA 1.51 97,700k
Biomérieux SA 97.24 11,340,000k
BioNTech SE 161.0 38,870,000k
Biophytis SA 0.05 9,800k
low high
COMPANY
QUOTE M-CAP 52 WEEKS INDICATOR low high
Bioporto Diagnostics A/S 0.31 100,900k
Biosergen AB 1.59 41,600k
Biotage Sweden AB 16.53 1,100,000k
Bioventix plc 41.20 216,700k
Biovica International AB 0.81 17,900k
Bivictrix Therapeutics plc 0.15 9,900k
Blirt SA 0.59 9,100k
Bone Therapeutics SA 0.16 4,200k
Brain AG 5.62 118,000k
C4X Discovery Holdings plc 0.23 59,000k
Calliditas Therapeutics AB 7.64 449,800k
Camurus AB 22.50 1,250,000k
European Biotech Stocks
The unique and most complete list of share price developments of biotech companies listed in Europe – exclusively in European Biotechnology Magazine.
Cantargia AB 0.32 52,500k
Carbios SAS 32.28 360,900k
Cellectis SA 2.18 97,800k
Cellink AB 7.40 433,400k
Celon Pharma SA 2.95 161,500k
Celyad Oncology SA 1.01 23,300k
Centessa Pharmaceuticals plc 3.30 312,300k
Centogene NV 0.69 15,700k
Circassia Pharmaceuticals plc 0.39 166,100k
Cline Scientific AB 0.10 1,600k
Co.don AG 0.03 1,100k
Coegin Pharma AB 0.02 14,700k
CombiGene AB 0.79 15,300k
Cosmo Pharmaceuticals NV 60.10 998,100k
CRISPR Therapeutics AG 57.95 4,553,400k
CSL Ltd 192.22 91,900,000k
Curevac NV 8.10 1,460,000k
Cytotools AG 1.44 7,200k
Cyxone AB 0.04 3,600k
DBV Technologies SA 2.75 257,800k
Deinove SA 0.01 500k
Destiny Pharma plc 0.38 27,600k
Diagonal Bio AB 0.13 1,200k
Diamyd Medical AB 1.21 95,200k
Diasorin SpA 131.45 7,070,000k
e-Therapeutics plc 0.20 118,800k
Elanix Technologies AG 0.04 400k
Elicera Therapeutics AB 0.34 6,800k
Ellen AB 0.30 1,600k
Enzymatica AB 0.31 51,600k
Epigenomics AG 0.34 5,800k
21 European Biotechnology | Winter Edition | Vol. 21 | 2022 FINANCIAL MARKETS
COMPANY QUOTE M-CAP 52 WEEKS INDICATOR low high
Erytech Pharma SA 0.70 22,600k
Esperite NV 0.0 481,400k
Eurobio Scientific SA 17.0 195,300k
Eurocine Vaccines AB 0.06 1,000k
Eurofins Scientific SE 66.0 12,560,000k
Evaxion Biotech A/S 2.24 51,600k
Evgen Pharma plc 0.04 10,900k
Evolva SA 0.08 90,600k
Evotec SE 16.43 2,870,000k
Expres2ion Biotech Holding AB 1.03 38,600k
F-star Therapeutics Inc. 4.50 98,900k
Faron Pharmaceuticals Oy 4.16 244,000k
Fermentalg SA 1.62 65,600k
Fluicell AB 0.20 5,000k
Formycon AG 79.30 1,180,000k
Forward Pharma A/S 2.94 20,900k
Freeline Therapeutics Holding plc 0.58 39,900k
Fusion Antibodies plc 0.52 13,600k
Gabather AB 0.46 6,200k
Galapagos NV 39.35 2,610,000k
Genedrive plc 0.13 9,800k
Geneuro SA 1.68 42,000k
Genfit SA 3.67 185,800k
Genflow Biosciences plc 0.02 6,300k
GENinCode plc 0.13 12,500k
Genkyotex SA 3.22 45,100k
Genmab A/S 420.56 27,457,000k
Genomic Vision SA 0.06 4,300k
Genovis AB 3.75 245,500k
Genoway SA 4.08 36,000k
Gensight Biologics SA 3.56 166,700k
Gentian Diagnostics AS 3.63 56,000k
Genus plc 36.40 2,330,000k
Global Bioenergies SA 4.09 60,800k
Glycorex Transplantation AB 0.24 17,300k
Guard Therapeutics International AB 0.01 2,400k
Hansa Biopharma AB 4.70 209,900k
HBM Healthcare Investments AG 165.0 1,150,000k
Heidelberg Pharma AG 5.70 265,500k
Hemogenyx Pharmaceuticals plc 0.01 12,700k
Herantis Pharma Oyj 3.12 52,700k
Hofseth Biocare ASA 0.26 104,000k
Hookipa Biotech AG 1.09 56,400k
Hybrigenics SA 0.09 18,300k
Idorsia Ltd 14.45 2,591,800k
Immatics NV 9.91 751,500k
Immunic AG 1.40 54,800k
Immunicum AB 0.20 40,500k
Immunovia AB 2.95 68,100k
Immupharma plc 0.05 3,300k
Index Pharm. Holding AB 0.09 47,000k
Infant Bacterial Therapeutics AB 5.77 63,000k
InflaRx NV 2.51 108,000k
Innate Pharma SA 2.14 170,200k
Integragen SA 1.19 7,700k
Intervacc AB 2.66 134,100k
Inventiva SA 4.29 173,800k
IO Biotech Inc. 2.88 80,900k
IRLAB Therapeutics AB 3.12 163,400k
COMPANY QUOTE M-CAP 52 WEEKS INDICATOR
Isofol Medical AB 0.04 6,700k
ISR Holding AB 0.29 20,100k
Kancera AB 0.20 11,200k
Karo Pharma AB 5.39 1,470,000k
Kuros Biosciences AG 1.67 61,500k
Lipigon Pharmaceuticals AB 0.09 1,800k
Lipum AB 1.39 10,600k
Lysogene SA 0.54 10,800k
Lytix Biopharma AS 0.74 30,400k
MaaT Pharma SA 8.68 85,800k
Mabion Ltd 4.54 73,400k
Mainz Biomed BV 9.12 132,200k
Marinomed Biotech AG 71.60 105,400k
MDxHealth SA 0.76 123,800k
Medesis Pharma SA 2.60 11,100k Medigene AG 2.19 55,000k Medincell SA 5.90 146,400k Medivir AB 0.77 43,700k Merus BV 15.60 722,300k
Metabolic Explorer SA 1.67 72,300k
Midatech Pharma plc 0.04 4,400k
Mithra Pharmaceuticals SA 6.05 327,000k
Modus Therapeutics Holding AB 0.22 3,600k
Molecular Partners AG 6.53 230,200k
Morphosys AG 15.36 524,400k
Nabriva Therapeutics plc 2.16 6,600k Nanobiotix SA 3.83 132,100k Neovacs SA 0.01 100k
Newron Pharmaceuticals SpA 2.25 40,200k
Nextcell Pharma AB 0.65 22,400k
NFL Biosciences SA 2.09 10,500k Nicox SA 1.73 73,100k
NLS Pharmaceutics AG 0.67 10,200k
Nordic Nanovector ASA 0.10 11,900k Novacyt SA 0.72 50,600k
Novozymes Biopharma DK A/S 53.84 11,986,600k Noxxon Pharma NV 3.40 4,900k Nucana plc 0.91 50,700k
Nykode Therapeutics ASA 2.25 733,900k ObsEva SA 9.38 779,100k
Okyo Pharma Ltd 0.02 29,000k
Oncimmune Holdings plc 0.47 32,200k
Oncoarendi Therapeutics SA 3.17 44,400k
Oncodesign Biotechnology SA 14.34 99,300k
Oncopeptides AB 1.41 127,700k
OncoZenge AB 0.40 4,700k
Onxeo SA 0.20 22,500k
Open Orphan plc 0.12 88,600k
Optibiotix Health plc 0.21 18,300k Orphazyme A/S 0.15 5,500k
Oryzon Genomics SA 2.12 114,100k
OSE Immuno SA 6.68 123,800k
Ovoca Bio plc 0.05 4,100k
Oxford Biodynamics plc 0.25 35,800k
Oxford Biomedica plc 3.82 367,700k
Oxurion NV 0.11 11,200k
Paion AG 0.92 65,600k
Pangaea Oncology SA 1.53 46,700k
PCI Biotech Holding ASA 0.17 5,800k
low high
22 European Biotechnology | Winter Edition | Vol. 21 | 2022 FINANCIAL
MARKETS
COMPANY QUOTE M-CAP 52 WEEKS INDICATOR
Pharma Mar SA 65.22 1,190,000k
Pharming Group NV 1.14 757,000k
Pharnext SA 0.0 3,900k
Pherecydes Pharma SA 2.18 15,500k
Photocure ASA 9.09 248,200k
Physiomics plc 0.02 2,400k
Pieris Pharmaceuticals Inc. 0.92 68,700k
Plant Advanced Technologies SA 15.75 17,200k
PolyPeptide Laboratories (Sweden) AB 35.06 1,163,300k
Polyphor AG 0.46 21,300k
Poolbeg Pharma plc 0.11 55,000k
Poxel SA 1.47 43,100k
Predilife SA 5.90 21,500k
Probi AB 18.94 218,500k
Promore Pharma AB 0.07 4,600k
ProQR Therapeutics BV 1.19 84,900k
Prostatype Genomics AB 0.16 3,700k
Proteome Sciences plc 0.03 9,000k
Prothena Corporation plc 56.10 2,720,000k
Pure Biologics SA 5.51 12,200k
Q-Linea AB 1.64 47,900k
Qiagen NV 46.26 10,710,000k
Quantum Genomics SAS 0.15 5,400k
QuiaPEG Pharmaceuticals Holding AB 0.01 100k
Quotient Ltd 1.06 3,800k
Redx Pharma plc 0.62 209,300k
Relief Therapeutics Holding AG 0.03 130,400k
Reneuron Group plc 0.27 15,400k
Ryvu Therapeutics SA 8.19 152,900k
Saniona AB 0.29 20,200k
Santhera Pharmaceuticals AG 0.60 31,600k
Sareum Holdings plc 0.96 67,400k
Scancell Holdings plc 0.19 155,700k
Scandion Oncology A/S 0.12 4,800k
Selectimmune Pharma AB 0.32 5,400k
Selvita SA 16.62 305,100k
Sensorion SA 0.46 36,400k
SenzaGen AB 1.16 28,600k
Shield Therapeutics plc 0.14 34,800k
Silence Therapeutics plc 6.32 536,000k
Simris Alg AB 0.26 41,400k
Skinbiotherapeutics plc 0.26 41,000k
Softox Solutions AS 2.70 27,900k
low high
COMPANY QUOTE M-CAP 52 WEEKS INDICATOR
Sophia Genetics SA 1.89 116,000k
Sprint Bioscience AB 0.07 2,900k
Stayble Therapeutics AB 0.37 4,800k
Summit Therapeutics plc 0.96 79,000k
Swedish Orphan Biovitrum AB 19.12 6,030,000k
SynAct Pharma AB 4.87 137,500k
Synairgen Research Ltd 0.18 42,800k
Targovax ASA 0.11 21,200k
Theradiag SA 2.27 29,800k
Theranexus SA 1.82 7,800k
Tissue Regenix Group plc 0.01 50,100k
Tiziana Life Sciences plc 0.62 63,400k
Transgene SA 1.87 189,100k
Trinity Biotech plc 1.34 50,300k
Ultimovacs ASA 9.64 316,800k
uniQure NV 21.56 1,010,000k
Vaccibody AS 2.53 733,900k
Valbiotis SAS 3.60 44,900k
Valirx plc 0.21 18,600k
Valneva SE 6.92 940,700k
VectivBio AG 7.68 342,100k
Verici Dx plc 0.16 26,400k
Verona Pharma plc 12.02 911,900k
Vicore Pharma Holding AB 2.40 172,400k
Virax Biolabs Group Ltd 1.41 14,900k
Virogates A/S 7.37 26,400k
Vita 34 AG 8.76 138,700k
Vivoryon Therapeutics AG 10.0 205,800k
WntResearch AB 0.04 5,500k
X4 Pharmaceuticals Inc. 1.79 124,600k
Xbrane Biopharma AB 6.23 168,600k
Xintela AB 0.05 14,600k
Xlife Sciences AG 31.30 163,800k
Xspray Pharma AB 4.99 113,300k
Yourgene Health plc 0.03 20,300k
Zealand Pharmaceuticals A/S 24.38 1,260,000k
low high
23 European Biotechnology | Winter Edition | Vol. 21 | 2022 FINANCIAL MARKETS
All quotes are listed in Euro. All data is provided without guarantee. The effective date is 22nd November 2022. These Europe-based biotech companies are traded on European stock markets.
How do I find a new job or a new employee? Now, there is an easy solution: eurobiotechjobs.net, the Europe-wide job market for biotechnology and the life sciences. Presented by the European Biotechnology Network.
Innate immunity and its power in cancer therapy
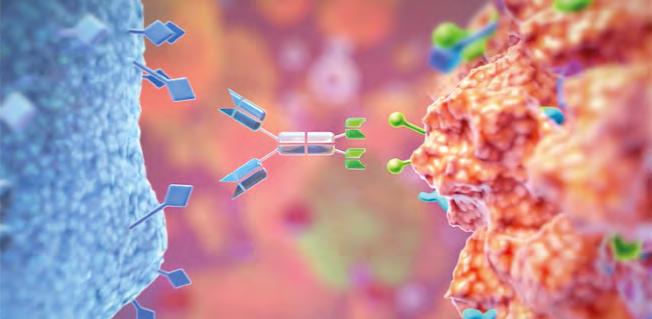
KILLER CELLS One of the most distinguishing hallmarks of tumours is their ability to avoid immune recognition. New immunotherapies have focused on breaking down those barriers, leading to treatment breakthroughs for blood cancers. While these therapies have shown great promise for some indications, we still lack effective therapies for many others. A novel route to address this is to the restore innate immunity’s ability to fight cancer.
› Arndt Schottelius – Chief Scientific Officer at Affimed NV, Heidelberg, Germany
In our body, innate and adaptive immune systems work in concert to achieve effec tive immune responses. Hence, involv ing the innate immune system in modern combination approaches could achieve a more integrated and sustained response against tumours. In recent years, employ ing the innate immune system has gath ered momentum and is now seen as a key growth area for novel cancer thera pies based also on encouraging clinical and preclinical results seen by Affimed and our peers. There are different ways to directly engage the innate immune system – using NK cells, bispecific anti bodies, or targeted molecules.
Unleashing ICE® against cancer
One of the most potent receptors for activating innate immune cells such as natural killer cells and macrophages is CD16A. Targeting this receptor mimics
the body’s natural defence against po tential threats, resulting in a cascade of defence mechanisms. Our bispecific in nate cell engagers (ICE®) are designed to directly bind CD16A and a distinct tu mour-associated antigen such as CD30 or EGFR, which is present on a specific cancer type. This enables our molecules to bring innate immune cells and cancer cells in close proximity empowering acti vated NK cells to effectively destroy ma lignant cells.
By using a proprietary variant of CD16, our ICE® demonstrate two major benefits –first, the selectivity for CD16A prevents re direction of ICE® to CD16B-carrying neu trophils, which are more abundant in our bodies, and second, the selected epitope minimizes competition with circulating IgG, reducing off-target binding. Addition ally, with their high affinity to NK cells and macrophages, Affimed can target cancers with lower antigen expression.
Several companies focus on drug devel opment in the innate immunity space with candidates in preclinical or early clinical development. Affimed is one of the most experienced clinical develop ment companies in the field. Our clini cal data have been in the spotlight at sev eral major oncology conferences with the next update planned for ASH 2022 from our lead candidate AFM13, which is currently being investigated in an ongo ing Phase I/II combination trial, precom plexed with cord blood-derived NK cells. So far, 24 patients have been treated at the recommended Phase II dose (RP2D) since the previous update in spring 2022. Patients continue to show an overall re sponse rate of 100% at the RP2D and a complete response rate of 70%. At a me dian follow-up of eight months, an overall survival rate of 83% was observed across all dose levels. Affimed is now looking to conduct a potential registration-direct ed Multicenter study with AFM13 in com bination with NK cells together with our new partner, Artiva Biotherapeutics. Af fimed is also conducting multiple clinical trials with AFM24, an EGFR-binding ICE® directed against solid tumours.
The next two years will be a tremen dously exciting time for the innate im munity field to mature and propel its first therapeutics towards registration al studies and approval. All ongoing ef forts highlight the importance of this field and its potential to improve cancer treat ments for many patients.
24 European Biotechnology | Winter Edition | Vol. 21 | 2022 ECONOMY Picture: German Research Cancer Center
L









CATAPULT Watch the 2022 Virtual Semifinal pitches to meet the best European selection of Start-ups looking for investments Pitch
one-on-one meetings
start-ups
BioTech Semifinalists MedTech Semifinalists Digital Health Semifinalists REGISTRATION TO THE PLATFORM https://eithealth.eu/catapult/ 13-19 February 2023 20-26 February 2023 27 February - 5 March 2023 23 January 2023
days include
with
and networking opportunities with investors, industry leaders, and EIT Health partners.
Gain access to the next generation of European innovators. Discover elite European start-ups in biotech, medtech and digital health that are shaping the future of healthcare. Our start-ups are pre-selected, best-in-class, and trained through the Catapult programme. Industry investors, leaders, and stakeholders work closely together challenging 40+ ambitious start-ups to optimize their business plan and strengthen their pitch deck. Join us to meet the best health-care start-ups in Europe
Making a better cheese
STANDING OVATION The French company Standing Ovation uses precision fermentation to create the protein that gives cheese its unique properties. With his pitch, co-founder and CEO Frédéric Pâques took the prize at the start-up pitch of the INDUSTRIA BIOTECH
EuroBiotech _Let me get straight to the point: Why vegan cheese?
Pâques_Well, first of all, because we real ly like cheese!
Cheese is special in two respects. First, it is a frontier in terms of technical solu tions. Before starting the company, we had tasted the alternatives we could find, and we were really not convinced. The plant-based products don’t come close to milk-based cheese, and most impor tantly, do not taste like it. But they also do not have the same nutritional value, notably in terms of proteins. Many of them are terribly expensive. So there is a need for other alternatives.
Second, cheese is also a frontier for many people when it comes to changing their diet. My son, for example, wants to be vegan but keeps going back to being vegetarian. Unlike with meat, he is still waiting for something that can really re place it. This is a very frequent situation: for many people, cheese is the reason why they will not go without animal-de rived products. They can reduce their meat consumption, sometimes even drop it. But they want their cheese!
EuroBiotech _So Standing Ovation has developed a fermentation-based alter native?
Pâques_The caseins, yes. When you want to produce animal-free cheese, caseins are the core issue. They represent 80% of milk’s proteins, and nearly all of cheese proteins, but it is not only about quantity, they also provide the functionalities, the curdling first of all, but also stretching and melting in cheese. However, caseins are difficult to produce. In comparison, whey proteins, the other milk proteins, are very well-behaved. They are very soluble, a dream for biochemists, and can be pro
FRÉDÉRIC PÂQUES CEO, Standing Ovation, Paris
duced easily. But caseins are different, so this is where we had our work cut out for us. We use precision fermentation – mi crobes that are fed with plant-based sugars – to produce the caseins.
EuroBiotech _How did Standing Ovation start out?

Pâques_Both founders, Romain Chayot and me, are scientists. We had been work ing together for seven years before, in pre cision fermentation. There was the idea, the skills, the team, and a project that made sense for us. So we started the jour ney.
EuroBiotech_What made you found?
Pâques_I believe the major reason was that it made sense. Younger people are really aware of the environmental issues, but people of my age have really seen the progressive switch from a world where you had the impression of infinite resources to another one with much
more limited perspectives. We are all aware that we need solutions to avoid dystopic scenarios where food and water supplies are severely limited.
EuroBiotech_Animal-free cheese is more sustainable?
Pâques _Cows and other animals are converting plants into meat and milk for us, at a high environmental cost: more than 14% of anthropogenic GHG, with high water consumption, and using 80% of agricultural land. Precision fer mentation consists in converting plantbased sugar into a desired compound by microbial fermentation, in a confined environment, the fermenter, with a very high yield, which means low needs for feedstock, water and soil.
For cheese making, many ingredients with low environmental footprint can be found in nature – as plant-based or min eral ingredients. But not caseins, and if you want to make real cheese with or ganoleptic and nutritional qualities, you need caseins. The solution is to make cheese with caseins made by precision fermentation.
EuroBiotech _Your approach is very suc cessful with investors – in September, you raised €12m, and in November, you announced your partnership with French cheese giant Bel Group. Tell us more!
Pâques_Standing Ovation was founded in 2020. The company was financed ini tially by the founders and love money, but we could increase our funding in 2021 thanks to Astanor Ventures. We could also get support from Bpifrance at each step. During the two first years, it was mostly about proof of concept and the first steps of process development
26 European Biotechnology | Winter Edition | Vol. 21 | 2022 INTERVIEW Picture: © Standing Ovation
and scale-up, and we could progress with relatively little money. But we have now entered the next stage, with scaleup and regulatory process, and needed more significant financing.
We launched our Series A financing round in the first half of 2022. Our capac ity to produce casein samples, and to pre sent cheese mimics made with these ca seins was key. Actually, we were looking for €10m, but the round was oversub scribed by €2m. Astanor Ventures rapidly took the lead and was joined by four oth er funds, Peakbridge, Seventure Partners, Big Idea Ventures and GoodStartup, all well-established investors, which support goes far beyond just money. They can help us with their experience and connec tions. In addition, Bel was convinced by our technology, and decided to join the round. With them, we found a partner placing innovation at the heart of its cul ture, and with real know-how in working with start-ups. We also have a partnership with them, to incorporate our caseins in Bel’s alternative cheese offerings.
EuroBiotech _What does the market look like for vegan cheese?

Pâques_The market of alternative proteins is blooming, with a very high growth, and it is expected to reach around US$300bn by 2035 (Boston Consulting Group). By then, which is tomorrow, we want to be able to produce dozens of thousands of tons of vegan caseins. Regarding dairy applications, non-animal cheese alterna tives already represent US$2.4bn, but still essentially with low-value plant-based products today. To meet the strong and sustained growth projections we can sometimes hear about, I believe the cur rent alternatives have to improve: both in taste – a real glass ceiling – and in nutri tional quality, notably protein content. Dairy alternatives need to be protein-rich, as their conventional counterparts, to ad dress the global shortage of proteins for human food. And to address both as pects, caseins will be key.
EuroBiotech_What differentiates Standing Ovation from other companies that use precision fermentation to make cheese?
Pâques_Many companies develop whey proteins, which are technically low hang ing fruits. Casein, on the other hand, is the holy grail. Our proposition is to provide casein, and at a cost compatible with food applications. Today, we are one of the very few companies capable of bring ing to the table a pot of non-animal ca seins, made with a proprietary process, and a ‘plateau de fromage’ made with these caseins. This is already a lot.
EuroBiotech _You aren’t worried about competition – either from other startups or major food industry players?
Pâques_The food industry looks more and more like the pharma industry, where partnerships between young inno vative biotech and large companies have become a standard. Whatever their strength and the quality of their R&D, large companies cannot deal with all the innovations. Meanwhile, we have our unique technology for casein produc tion. Mass production of this protein will require more financing, but having a pro prietary technology delivering what peo ple want is a good key to unlocking this door. Yet, collaboration with large food companies is certainly another key for both speed and impact. Large players can bring not only their know-how in cheese (or other dairy product) develop ment and manufacturing, but also their capacities in marketing and distribution, a costly item on the path to market, and a difficult one for a small entity. And we
have a very good partner: Our collabora tion with Bel will considerably speed up delivery to the consumer’s plate, and the number of people we can reach.
EuroBiotech _What are the next steps for your company?
Pâques_The next critical steps are the scale-up, which requires the access to large fermentation capacities, and the reg ulatory process. The path to regulatory approval is very different across countries. It takes a long time in Europe when com pared to the US or Singapore, and there is a big lag between what Europe can do and what other countries can do, which is something we regret. We are on a B2B model, positioning ourselves first as ingre dient providers, so we can approach cli ents all over the world. However, due to the difference in timelines for the regula tory path, the market will be open in the US before Europe. Beyond scale-up and regulatory affairs, we also keep doing R&D. For example, we are also working on yoghurt, ice cream and so on. Longterm, we also envision the possibility to sell our own cheese one day. But in the short term, we think about impact and speed first. Partnering with major players in the food industry is the path to bringing a product to the market rapidly, with a large diffusion. This is our top priority, which has become concrete with the partnership with Bel that we announced recently. L u.mommert@biocom.eu
27 European Biotechnology | Winter Edition | Vol. 21 | 2022 INTERVIEW
Picture: © Standing Ovation
Update of ongoing clinical trials NEWS
Safe tumour vaccine
HPV16 vaccination specialist ISA Pharmaceuticals BV and its US partner Regeneron Inc have pub lished results from a Phase I clini cal trial conducted at Leiden Uni versity Medical Center (LUMC) with their preventive HPV-16 E6 vaccine Amplivant. Amplivant is a synthet ic peptide/Toll-like receptor 2 li gand conjugate and was shown to be safe and effective in induc ing T-cell-based immune respons es in patients with HPV16 positive (pre-)malignant lesions (10.1136/jitc2022-005016) when administered intra-dermally. Amplivant-adjuvant ed oncoprotein fragments strongly enhance tumour antigen presenta tion by dendritic cells, T cell prim ing, and induce effective antitumor responses without serious adverse events as shown in the trial. Induc tion of HPV16-specific T cell im munity in pre-treated patients with HPV16-positive (pre-)malignancies showed a dose-dependent increase in systemic T cell immunity in 25 patients who received three doses intradermally, three times a week, and with a 3-week interval in four dose groups (1, 5, 20 or 50 µg per conjugated peptide).
Good lymphoma data
Affimed NV reported a 100% response rate of 24 CD30-positive lymphoma patients that received its NK-cell linker AFM13 in a US-based Phase II study. Over 71% showed a complete response. At all dose lev els tested, no severe ADEs occurred ,and there was an event-free surviv al rate of 57% and an overall surviv al rate of 83% observed at a median follow-up of eight months.
COVID-19
In mid-October, Pfizer Inc. and Mainzbased vaccine developer Bio NTech SE announced that 90 subjects in a Phase II/III study (NCT05472038) showed a sig nificant increase in neutralising antibody titres against BA.4/BA.5 following a 30 µg booster vaccination with the compa nies’ bivalent vaccine adapted to Omi cron BA.4/BA.5. The blood sera con tained similarly elevated antibody titres seven days after administration in 40 subjects in the age groups 18 to 55 years and over 55 years. Qualitatively, as ex pected, revaccination with the bivalent vaccine showed a higher, but not quan tified, increase in antibody titres com pared to a fourth dose of the monova lent Comirnaty vaccine. From the early data, the companies concluded that the bivalent vaccine provided better protec tion against the omicron BA.4/BA.5 var iants than the original vaccine. In early November, the companies followed up with data for 32 patients in the 55+ age group who received either the monova lent Comirnaty jab or the bivalent and already EU-approved BA.4/BA.5 boost er vaccine. According to the companies, the antibody titre against the Omicron variant was four times higher in those re vaccinated with the booster than with the first-generation vaccine. Data pre viously published in the n ew e ng L and JournaL of M edicine by a research group at the University of Columbia on a sim ilarly low number of subjects contradict these data. The scientists found no dif ference in antibody titer, while a group from Ohio State University reported a doubling of antibody titres going omi cron in a preprint publication. Due to the conditional marketing authorisation, Pfizer’s/BioNTech’s Phase II/III trial is on going and will provide data on a larger portion of patients that allow for better statistical evidence than small studies. At the beginning of November, BioNTech raised its forecast for the year despite a sharp decline in the number of peo ple vaccinated. At the same time, Pfizer
announced that it would quadruple the price of the booster vaccination to be tween $110 and $130 when the pandem ic emergency expires in 2023.
A nasal formulation of AstraZeneca ’s COVID-19 vaccine Vaxzevria, on the other hand, showed little efficacy in a phase I trial with 41 subjects: the mu cosal and systemic immune responses were not sufficient to protect against in fection with SARS-CoV-2.
MULTIPLE MYELOMA
GlaxoSmithKline plc ’s BCMA-targeted refractory multiple myeloma antibodydrug conjugate (ADC) Blenrep (belan tamab mafodotin) did not meet the end points in a non-inferiority pivotal Phase III trial. The drug did not improve pro gression-free survival compared to Bris tol Myers Squibb ’s Pomalyst (pomalid omide) plus low-dose dexamethasone. Belantamab mafodotin had been predict ed to become a blockbuster with estmat ed peak sales of £3bn annually. In 2020, the FDA granted accelerated approval to the drug candidate provided the compa ny proved belantamab mafodotin at least inferior to the current standard of care in terms of PFS. According to headline re sults of the ongoing Phase III trials, pa tients lived longer without disease pro gression, going 11.2 months vs. 7 months at median. belantamab mafodotin also wasn’t significantly better concerning an objective response rate of 41% com pared with 36% for the control group.
Swedish Oncopeptides AB has report ed new Phase III data in October on its formerly approved multiple myeloma medicine melflufen (formerly Pepaxto/ melphalan flufenamide) in patients with relapsed refractory disease. After a high er rate of deaths compared to the stand ard of care had been reported, the FDA halted all clinical trials related to the tri al but allowed to carry out clinical stud ies provided patients accepted the higher risk of death. In October, Oncopepides
28 REGULATORY AFFAIRS European Biotechnology | Winter Edition | Vol. 21 | 2022
AB reported data from the halted LIGHT HOUSE study that assessed the efficacy and safety of the combination of mel flufen plus daratumumab subcutaneous ly plus dexamethasone in comparison with daratumumab sc. with supportive dexamethasone in 54 patients who were refractory to at least an immunomodu latory agent and a proteasome inhibitor or have received at least three prior lines of therapy. According to the data, pro gression-free survival (PFS) was superior for the melflufen treatment arm vs. the control arm. “LIGHTHOUSE is the sec ond Phase III study (the first was called OCEAN) to confirm the clinical bene fit of melflufen in multiple myeloma pa tients with a treatment history with no stem-cell transplant or a successful prior stem-cell transplant in line with the re cent full European approval,” comment ed Jakob Lindberg, CEO, Oncopeptides. “In addition, the LIGHTHOUSE data sup port the EMA conclusion that there is no indication of absolute overall surviv al harm from treatment with melflufen.” The safety profile was in line with what was observed in the Phase I/II ANCHOR study, with predominantly clinically manageable cytopenias.
LIVER CIRRHOSIS
Copenhagen-based Galecto Inc has an nounced top-line data from its Phase

Ib/Ia trial with GB1211 in 54 patients with decompensated liver cirrhosis (NCT05009680). Data from blood testing suggest statistically significant improve ments in certain parameters (ALT/AST/ GGT) and encouraging reductions for ALP associated with liver diseases, a second ary endpoint of the study. Treatment with the oral galectin-3 modulator GB1211 ap peared not to be too toxic as only one out of evaluable 15 patients to assess safety and pharmacokinetics of GB1211 showed treatment-emergent but adverse events, which did not appear related to the drug, and demonstrated consistent signs of activ ity across biochemical liver function mark ers and markers of target engagement, ap optosis, and fibrosis, including reductions in galectin-3 and CK-18. According to the company, “these findings are unique even when compared to data from previ ous studies in patients with less severe liv er disease and treated over longer periods of time, and suggest that GB1211 provid ed liver cell protection and improved liver status, further supporting clinical develop ment in severe liver disease”.
RENAL CANCER
Heidelberg Pharma AG (Germany) an nounced in November that its licens ing partner Telix Pharmaceuticals Ltd (Melbourne, Australia, has published positive data from the pivotal ZIRCON
Phase III study with the imaging candi date TLX250-CDx. The trial met both its primary and secondary endpoints. TLX250-CDx (89Zr-DFO-girentuximab) is a zirconium-89-radiolabelled antibody and has been tested by Telix in the glob al Phase III-ZIRCON renal cancer PET imaging study since August 2019. From 284 evaluable renal cell cancer patients who were treated with TLX250-CDx, and comparison of histological tumour samples from surgical resection of clear cell renal cell cancer (ccRCC) with ref erence histological the company deter mined a sensitivity of 86% and a speci ficity of 87%. This exceeds the required thresholds to demonstrate the ability of TLX250-CDx to reliably detect the clear cell phenotype in renal cancer and pro vide a non-invasive method for the di agnosis and spread of ccRCC. The study also met the key secondary endpoint of 85% sensitivity and 89% specificity in detecting ccRCC in tumours <4 cm , which is a major clinical challenge in the diagnosis of ccRCC. Thus, TLX250-CDx offers an option for non-invasive diag nosis of clear cell renal cancer. Telix in tends to submit an MAA to the FDA and other global regulatory authorities for ap proval as a PET/CT for the characterisa tion of indeterminate renal masses previ ously identified by CT or MRI as ccRCC or non-ccRCC. L
29 REGULATORY AFFAIRS European Biotechnology | Winter Edition | Vol. 21 | 2022
Pharma industry on expansion course
CPHI At the CPHI, the world's largest pharmaceutical event, which attracted 40,405 visitors this year, it became evident that the COVID-19 pandemic has had an extremely positive impact on the (bio)pharmaceutical sector. A new CPHI annual survey shows how business will develop in the future. Particularly, the prospects for CDMOs and CROs are excellent.
Decision-makers in the biopharmaceuti cal industry expect the CRO and CDMO sectors to grow significantly over the next two years. According to the annual CPHI survey (shorturl.at/himv7), revenues of the CRO sector will increase by 7.2 %, while those of the CDMO market will grow by 8.5 % by 2024. Venture capital (VC) and private equity (PE) liquidity reserves in particular will support this expansion. The overhang of private equity in the US is $749bn, and that of venture capital is another $300m. Inflation and energy pric es, especially for parts of Europe’s gener ic and active pharmaceutical ingredient manufacturers, are the biggest challeng es, triggered by the COVID-19 pandemic and recent geopolitical issues.
Nevertheless, the CPHI Pharma In dex recorded an increase of more than 8%, the largest annual increase in the six-year history of the survey. In addi tion, record levels were recorded in the areas of growth, innovation and produc tion quality in the pharmaceutical indus try. Geographically, India saw the most significant change in score with a 15% improvement in 2021, bringing the Asian country on par with the leading Western countries. In terms of active ingredient quality, India and China made the most progress, China with 25% in 2022.
The report's authors found that near ly 36% of biopharma companies will increase their spending on outsourced R&D or biomanufacturing in the next 12 months. All 12 major pharma economies improved their growth potential scores. The US consolidated its leading position with the highest score (8.5%) in the sur
Pharma Awards ceremony at CPHI 2022

vey's history, followed by Germany at 8.1% and the UK (7.8%). 1 billion in cap ital suggests an upswing for the German contract manufacturing industry.
Changes in outsourcing
The CDMO market continued the trend to offer time-saving one-shop-stop so lutions. The demand for pharma ser vices increased sharply in the wake of COVID-19. According to the survey, the global CRO market is predicted to grow from $58bn in 2021 to $76bn by 2025 due to increased R&D spending and out sourcing. Similarly, the CDMO market is forecasted to expand from $177.2bn to $246bn over the same period of time. For biotechs, deeper partnerships in out sourcing strategies are expected than for Big Pharma companies.
A key finding of the report is that a sig nificant shift is underway in global out
sourcing strategies, with innovators now mapping a product’s full life-cycle de velopment as early as pre-clinical. A key finding is that innovators across the in dustry are planning ‘pharma-ready’ syn thetic routes much earlier in the devel opment process, with ‘phase appropriate development’ increasingly seen as out dated – and particularly inappropriate for accelerated pathways. The report’s pre dictions suggest innovators will need to choose either a single end-to-end pro vider or take a multi-provider model. CDMOs will need to rethink their ap proach both to development as well as to how they market their services to in novators and biotechs.
The type of outsourcing strategy pre sented by CDMOs helps customers iden tify their most suitable potential partners. As a result, most CDMOs will focus on marketing to specific types of customers rather than the entire market. This shift re
30 European Biotechnology | Winter Edition | Vol. 21 | 2022 FOCUS PHARMA SERVICES Picture: © Informa


www.biovian.com YourOne-Stop-ShopCDMO forBiopharmaceuticals ONE-STOP-SHOPservices ViralVectorproduction–AAV,Adenovirusetc. PlasmidDNAproduction Microbialproduction CellBankandVirusSeedStock AsepticFillandFinish ProcessDevelopment QualityAssuranceandQualityControl Biovian’sconceptofaOne-Stop-Shopistoprovideclients withGMPCDMOservicesfromgenetofinishedvialand fromthelaboratorybenchtotheclinic.
flects the different risk tolerance and col laborative capabilities of biotechs, small pharma or large pharma organisations. “Over the next few years, we anticipate many more biotechs will use CPHI to map out their potential partners long be fore IND,” commented Orhan Caglayan, Brand Director at CPHI Frankfurt.
According to the report, innovators are now looking for an outsourcing ap proach that gets them rapidly through IND to reach Phase IIb and beyond as fast as possible. The impact of this trend isn’t only on development timelines, but also on investors: pharma process readi ness will have a fundamental impact on when and how investors can exit.
At Phase II sale, a biotech with a clear plan and ready-to-scale manufacturing strategy will deliver a better validation than a promising target in need of finetuning (see report 12). The CPHI experts predict that early-stage investors will in creasingly use CMC consultants and pro cess specialist CRO/CDMOs to help en sure a smooth scale up process.
The CPHI experts warned innovators against adopting an exclusive phase-ap propriate approach, saying they risked building in severe delays to later stages of the development process.

Saving time to market
The COVID -19 pandemic taught the in dustry to speed up development. This in turn compressed timelines. The demand
for vaccines will stay high. Additional ly, the report predicts an increased de mand for self-administered injectables. A new approach for partnering strategies includes a process development partner for chemistry and improvements. Such partners offer acumen in chemistry de velopment and documentation. Inter esting for investors might be start-ups, which have a development plan from basic until commercialisation. Investors can estimate, if products can fail and they are able to map out the route to success.
Pandemic impact
The pandemic pushed drug and vaccine development along with (RNA and vec tor-based) delivery platforms. No sur prise, that a record number of new drugs entered development, especially in 2020. A more detailed look forecasts a growth for medium-sized CDMOs driven by the huge number of new compounds enter ing development.
The best growth outlook is seen for smaller CROs and CDMOs. Smaller CROs provide specialised expertise, speed, and high-touch relationships, which accom pany well with the innovative nature of emerging companies. Smaller pharma services providers tend to match up well with bigger players in the early phases of development, whereas the larger integrat ed CROs/CDMOs dominate in mid- and later stages. Due to the pandemic, the
biggest challenge is to obtain excipients timely and deliver them to the drug prod uct manufacturer. The study authors ex pect that these supply chain disruptions will remain in the future.
Specialisation in therapies
According to Alliance for Regenerative Medicine, the demand on access to pro duction capacity has raised due to the rapid expansion of cell and gene thera pies. In 2019, 1,052 clinical trials have been run on ATMPs, a 67% increase in this field driven by gene and cell therapy progress. In a ranking of biotechnological compounds, mRNA is the leading thera peutic class for early-stage investments, followed by cell and gene therapies. Ex perts forecast that biologics will account for 55% of all drug sales in 2028, where as small molecules yet dominate approv als and sales at the moment. Monoclonal antibodies as a key class of biologicals will drive this development with 46% of total sales. The USA, Germany and India reported the highest scores in growth for biologics manufacturing.
Outlook for development
What else was important? The “hybrid Asian strategy” evolved in recent years especially by CDMOs with headquarters in India and Asia allowing the manufac turing of API components in Asia but full product finishing in Europe. Big Pharma use this strategy to save resources and in creasing hit success.
The trend to make use of environmen tally questionable single-use systems (disposable bioprocessing, particular ly in early phase upstream processing) rose to 62,9% in 2021. However, the re port diagnoses a big shift towards green er manufacturing that will affect supply chain partners, CDMOs, ingredient and excipient manufacturers. “Environmental credentials are now integral to all supply chain decisions. 95% of industry execu tives suggest it is either important or ex tremely important (52%) to have visibility on supply chain partners.”
32 European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © Informa FOCUS PHARMA SERVICES
Impressions at CPHI Frankfurt 2022
L g.dorow@biocom.eu


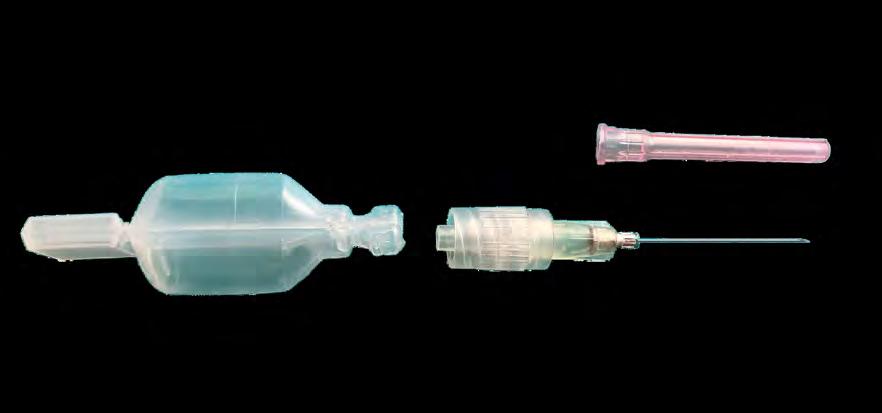


A n i n n o v a t i v e a n d c o s t e f f e c t i v e i n j e c t i o n d e v i c e BFS vial after opening Low density Polyethylene (LDPE) Connector Needle This prototype device might be subject to changes Proprietary information of Unither Pharmaceuticals +33 (0) 1 44 63 51 70 contact@unither pharma.com www.unither pharma.com I N T R O D U C I N G . . . G L O B A L C D M O W o r l d w i d e l e a d e r i n B l o w F i l l S e a l ( B F S ) L o o k i n g f o r a n i n n o v a t i v e d e v i c e t o i n j e c t y o u r v a c c i n e o r b i o l o g i c p r o d u c t ? O u r d i s r u p t i v e t e c h n o l o g y i s e a s y a n d s a f e t o u s e . W e o f f e r h i g h f i l l i n g c a p a c i t i e s a t a c o m p e t i t i v e c o s t ! Open system, free of royalties Adaptable to any type of needle Different routes of administration for injection Dedicated BSL2* building in our Amiens facility Very large capacity: up to 1 billion doses per year Competitive pricing No risk of shortage in components and capacities Opportunity for new markets (LMIC*) *Low middle income countries *Biosafety level Needle cap with safety lock option With
years of experience and constant innovation, French CDMO Unither Pharmaceuticals
worldwide leader in sterile unit doses contract manufacturing. At our sites in France, the USA, Brazil and China, we provide comprehensive innovation & development services to fulfil every
your contract pharmaceutical manufacturing needs.
more than 30
has established itself as the
aspect of
LEARN MORE
Cells: The heart of Biovian’s rAAV manufacturing
VIRAL VECTORS
Commercial-scale manufacturing demands a high yield of potent rAAV products. Understanding the metabolism during cell growth, especially at high densities, molecular effects of transfection or infection in production is vital. In addition, the choice of producer cell type determines the PTMs of the AAV capsid and potency. Successful trials in tweaking cell factories’ genes to boost rAAV particle yields set the direction for further development.
› Artur Padzik, PhD, AAV Technology Manager, Biovian
of metabolic processes. Therefore, iden tifying the critical cellular processes af fected by culture conditions and viral presence will aid in resolving the limitations in production.
Metabolomics and manufacture

At Biovian, we can manufacture rAAV vectors by introducing AAV components by transfection, infection, or genetic en gineering to harness producer cells as nano factories. Different rAAV produc tion systems are characterised by (i) cell line, (ii) culture substrate, and (iii) precur sor materials. Mammalian HEK293, HeLa, and insect Sf9 cells have successfully pro duced rAAV vectors. However, they dif fer in what product qualities they generate and how flexible they are across the entire IND development. Early stages of development focus on therapeutic ef fect, efficacy, or specificity. Later clini cal phases force a change of scale with a predictable outcome, simple produc
tion procedure, and stable behaviour of cells. High requirements of up to 1015-1016 vg per patient and the current most popu lar HEK293 platform limit of 1014 vg/L af ter purification urges the field to seek pro cess improvements. Understanding the delicate dynamics of reciprocal cell–virus interplay is a prerequisite for process op timization. Search spans from design-ofexperiments’ rational-based wet lab work to mathematical modelling. Fundamental differences exist in how the metabolism shift occurs when we use transfection or infection as a mode of AAV manufactur ing. In the former, metabolic reduction is observed, leading to protein production. In the latter, infection activates a plethora
The essential goal in Biovian’s AAV manu facturing process is maintaining metabolic homeostasis. Excessive changes in the cel lular metabolic state impede viral vector production. Process upscaling increases the overall nutrient deployment, oxygen consumption, and the accumulation of metabolites. Proteomics profiling of AAVproducing cells shows activation of gene clusters responsible for cell growth, proliferation, differentiation, defence response mechanisms, and glycerophospholipid, ri boflavin metabolism involved in energy production. Moreover, cell model-related differences in PTMs, ITR sequences, and DNA methylation patterns affect vector potency and safety in vivo.
Innovative technologies, such as cell line engineering, have been deployed to leverage cell performance. Trials im proving tolerance over metabolic waste products, such as lactate and ammonia, blocking endocytosis, modulating apoptosis-triggering signalling and cell cycle pathways enhance the production effi ciency. Alternatively, boosting intracellu lar nutrient and precursor deployment for rAAV particle production might offer an other solution for high-yield needs.
34 European Biotechnology | Winter Edition | Vol. 21 | 2022 FOCUS PHARMA SERVICES Picture: © Biovian
L


Blow-Fill-Seal technology and vaccine delivery
UNIT-DOSE
The SARS-CoV2 pandemic caused 6.3 million deaths and contaminated 545 million people across the world. In spite of the rapid development and approval of vaccines, the shortages of consumables like glass vials limited the distribution of prophylactic vaccines. Blow-Fill-Seal (BFS) is an alternative solution with advantages that could play an important role in vaccine distribution worldwide.
› Alexandre Fontayne, PhD, CSO Biologics – Unither Pharmaceuticals
In this context of sanitary crisis, Unither Pharmaceuticals, a BFS lead er with almost 30 years of experience, launched Euroject ® in Sept. 2021 com bining a BFS unit-dose vial with a needle hub for vaccine injection. This new de vice goes with a dedicated BSL2 work shop, based in Amiens (FR), which will be able by 2024 to fill 1 billion doses an nually. Meanwhile, all internal and cus tomer development tests are performed on an industrial full-size BottlePack (BP) 460 machine in Coutances (FR).

BFS history
BFS technology is originally derived from a 1963 patent application. At that time, the concept allowed the packaging of food, cosmetics, and medical device products, all in non-sterile condition.
The first BP machine was launched in 1964. In the early 1970s, the pharma ceutical industry used the equipment to pack large volumes of pharmaceutical solutions. (Oschmann & Schubert, 1999).
At the end of 1980s, the BFS technolo gy was well-established in the packag ing industry, particularly for pharmaceu tical and healthcare products. Along the 1980s and 1990s, BFS was developed and is now adopted for the unit-dose for mat of sterile solutions. Throughout early 2000s, BFS has been used for parenteral products instead of glass bottles because it is light and unbreakable. In the final EU GMP Annex 1 on the manufacture of
medicinal products, published in August of this year, BFS is one of the essential manufacturing processes for sterile prod ucts. Since multiple vials are formed by a mold and filled in a row, and because the machine can have multiple molds at once, the cadency can reach 33,000 vials per hour. Extrapolated over the year, this can lead to the production of 200 to 300 million vials per machine!
BFS is adapted to vaccines
In the BFS process, the plastic polymer granules are melted at high temperatures, around 160 °C, then extruded and blown into a vial shape. During the shape forma tion, a needle fills the dose with the drug product before sealing and demolding the
vial. The entire process is fully automat ed and performed in aseptic environment.
At first glance, the process might seem incompatible with temperature-sensitive molecules like biologics and vaccines. Indeed, the temperature is high enough to damage a biologic’s function when ex posed for too long. In fact, the full BFS process cycle is fast, only a few seconds, limiting drastically the exposure to dele terious temperature.
In the past, BFS vials have been used as primary container of vaccines, even at commercial stage. In early 2010s, Med Immune conducted a Phase III clini cal study to evaluate the immunogenic ity of an intra-nasal influenza vaccine (MEDI8662) supplied in BFS compared to FluMist ® /B, the reference supplied in
36 European Biotechnology | Winter Edition | Vol. 21 | 2022 FOCUS PHARMA SERVICES Picture: Unither Pharmaceuticals
Euroject ® device for injectable vaccine delivery is composed of Blow-Fill-Seal (BFS) unit dose vial and a connectable needle hub via luer lock connection.
a Becton Dickinson Accuspray™ device. The results showed that the BFS live-at tenuated vaccine is immunogenically non-inferior to the reference and the ad verse effect were comparable.
More recently, the vaccine against hu man rotavirus, Rotarix ® from GSK, still a live-attenuated virus, used a BFS vial for the packaging of the vaccine bulk to im prove access to this oral vaccine in lowand middle-income countries, like in My anmar where multidose BFS containers are used. No matter the container, in cluding glass vials, the stability of this vaccine over 12 months is comparable (Manjari, et al., 2016).
These examples prove that the BFS process did not affect the efficacy and the storage of the vaccine even if consid ered temperature sensitive.
Regardless, the performance of the pri mary container and its compatibility with the product must be demonstrated over the vaccine's shelf life, and this stabili ty must be evaluated through a produc tion-scale filling process. In addition, the cost to produce the vaccine in each con sidered container must be taken into con sideration. This exercise has been done by PATH for oral (Rotarix) and parenteral vaccine (IPV) (Jeff, et al., 2018). It shows that BFS is the best option for oral vac cine and just below the 10-dose glass vial for parenteral route. However, this study does not take into consideration the po tential issues of cross-contamination that can be dramatic for the population, con
sidering that in many cases it is a prophy lactic vaccination for healthy people.
Considering the advantages of BFS compared to the other solutions, the Euroject ® device has been designed to respond to the Fill&Finish of biologics and vaccines for an accurate and a costeffective delivery.
We optimized the set-up of the bot tlepack (BP) machine and its peripheric to maintain the biologic in an appropriate environment. This setup has been tested for the filling of a surrogate vaccine (Ar turo, et al., 2021). To do so, we prepared a bulk of 3L containing 60 mg of SARSCoV-2 RBD antigen. The industrial BP machine was run to dispense 0.5 ml of product per dose. The full run took less than 7 min. and produced 6,000 vials! To evaluate the filling process impact on the molecule, samples were taken along the run and were analysed by different tech niques for biochemical and functional properties. The samples were compared to a reference, which was the same prod uct before the run.
The experimental size of the RBD pro tein, evaluated by SDS-PAGE or SECHPLC, was shown to be preserved with a mass of 30 kDa and a retention time of 27.5min. The protein mass at the end of the run was 27014.6 Da and was com parable to the reference when analysed by LC-MS after reduction and PNGaseF treatment. Similarly, no degradation or aggregation were detected by SDS-PAGE or HP-SEC analysis. Biochemical charac
BFS process (A) operating in fully automated manner in a bottlepack machine (B) to produce vial filled aseptically of pharmaceutical product (C)
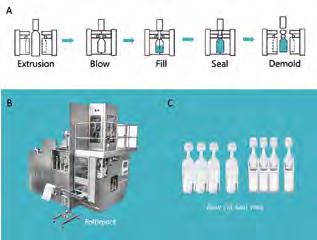
terisation was completed by nano-DSF analysis giving a Tma value between 51.8 to 52.4 °C for the samples compared to 51.9 °C for the reference, showing that the process did not impact the folding of the molecule. This was confirmed by ELISA, where the binding of RBD to E2 was conserved.
Altogether, the literature completed to our data with the SARS-CoV-2-RBD an tigen shows that the BFS process, even if it involves high temperature, is clearly compatible with biologics and vaccines. One question could remain on the new class of vaccines based on mRNA. In ab sence of data, we believe that mRNA is compatible with BFS and the temper ature is not an issue. Indeed, previous studies have shown that the mRNA vac cine against rabies can resist a tempera ture of 70°C for three months (Lothar, et al., 2017). This is largely higher than what can face a molecule in BFS process opti mized for biologics at Unither.
Impact of the BFS process on SARS-CoV-2 purity and integrity as determined by A) SDSPAGE analysis in 15% gel and by B) LC-MS after reduction and treatment by PNGaseF.
BFS is largely adopted in the pharma ceutical industry as primary container for storage and delivery of liquids. At Unith er, we are deeply convinced that BFS unit-dose and Euroject ® technology are part of the solution to store and deliver biologics and vaccines. Our R&D offer and feasibility testing combined with our new capacities will contribute to the in ternational effort to deliver drug products to the global population, by limiting the impact of primary container shortage, to better protect the world population in case of a pandemic.
37 European Biotechnology | Winter Edition | Vol. 21 | 2022 Pictures: Unither Pharmaceuticals FOCUS PHARMA SERVICES
L
Unleashing the power of LNP encapsulation
LNP ENCAPSULATION
The COVID -19 pandemic has changed perception of the value of rapid vaccine production, followed by multi-billion investments across the globe. While nanomedicines are receiving attention for enhancing stability and targeted activity of APIs, their broad availability currently suffers from the same production bottleneck as mRNA vaccines. Leon-nanodrugs, a pharma technology company, is developing a disruptive approach to unlock high-speed vaccine production.
EuroBiotech _What sets leon-nanodrugs’ (LEON) manufacturing technology for vaccines apart from what is currently on the market? Nafe_Commercial-scale production of mRNA has been pursued for only a short time and under quite a lot of pressure from the COVID -19 pandemic. Successful production of mRNA vac cines requires the mRNA to be formu lated into Lipid Nanoparticles (LNP). Naked mRNA degrades rapidly, causes serious side effects and cannot cross the cell membrane without delivery systems. LNPs are one such safe plat form: Spherical entities comprise a pro tective lipid layer around the mRNA and allow mRNA to safely reach its tar get site and enable cell transfection.
While there are machines on the mar ket that facilitate this process, and which have been used in the production of LNPs for COVID-19 mRNA vaccines, they suffer from several limitations: overly complex, cumbersome, investment inten sive, inefficient and costly. Besides this, they require months of time to validate scale-up.
The special architecture of LEON’s proprietary encapsulation technology allows a seamless scale-up from lab to large-scale GMP production and protects mRNA integrity enabling manufacturing at educt concentrations much higher than possible with technologies currently available. The applicability of LEON’s technology goes beyond mRNA encapsu lation, as it can be used for nano-formula tion of a wide range of APIs.
LEON since 2018, bringing in a wealth of experience from previous board-level positions including CEO and CFO, which he held at global companies for more than 25 years. With a focus on life sciences, he has successfully led the delivery of several M&As, financing rounds, license contracts and supply agreements.

cturing of LNP-encapsulated APIs, such as mRNA vaccines. It enables about six to eight million vaccine doses to be processed per day. It is suitable for GMP manufacture and allows real-time release of batches by PAT. NANOus was designed to be used in a high-volume and commercial production setting.
NANOme will be the first fully GMPcompliant device, using single-use disposable kits to overcome the existing capacity bottleneck in personalised medicine and will also accelerate production of, e.g., CAR-T cell therapies.
As personalised treatments often need to be prepared for specific patients, a small device like NANOme can help reduce logistics, cost and time burden. Each NANOme encapsulation device will enable approximately 35,000 patientrelated batches per year. The application is not limited to personalised medicine and can also make small biotechs more competitive by enabling in-house nano-formulation of their APIs for clinical trials.
EuroBiotech _Where do you currently stand in terms of development?
EuroBiotech _How do you implement the technology to draw out the poten tial you have just described?
Nafe_We have developed two disrup tive closed and aseptic working devi ces to realize the potential of our NANOnow product platform:
NANOus is our fully automated aseptic device for medium- to large-scale manufa-
Nafe_LEON is in the last stage of product development. Currently, we are assembling our first NANOus device for commercial application, together with the leading German device manu facturer Harro Höfliger. We expect to be market-ready by Q4/2023.
For our NANOme device, we are aiming at 2023 for production of our pro totype and 2024 for market entry. L
38 European Biotechnology | Winter Edition | Vol. 21 | 2022 INTERVIEW Picture: © Patrick Art
CHRISTIAN NAFE serves as CFO at
end-to-end manufacturing
services
SCTbio is a European global contract development and manufacturing organization for Advanced Therapy Medicinal Products (ATMPs). Founded as a spin-off from SOTIO to directly address the growing demand for cell and gene therapy manufacturing, SCTbio holds over a decade of experi ence developing best-in-class clinical manufacturing services, advancing solutions and technology transfers.

12 years
employees 80+ facility area including 420 m2 of clean rooms 2,000 m2
SCTbio
offers Needle-to-Needle Services: • GMP Manufacturing and QC • Analytical & Process Development • Logistics and Apheresis Collection • Procurement and storage • Quality and QP release • Regulator y suppo t www.sctbio.com pa tnering@sctbio.com
and development
to accelerate your cell gene therapy programs in cell and gene therapy field
Lessons learned in the face of new health challenges
CDMO SARS-COV-2 exposed our vulnerability and strategic weakness at European level, in terms of biotechnological manufacturing capacity.
› Beatriz Díaz Lorenzo, Corporate Communications & Marketing Manager, CZ Vaccines SAU, Spain
In two decades of the 21st century, we have gone through two pandemics, both caused by a coronavirus, the one caused by SARS in 2003, which spread through out the world aboard commercial flights, reaching 29 countries and which, fortu nately, had limited effects if we compare it with the devastating consequences of the current one. Since then, scientists have warned of the need to prepare for possible new viruses.

Vaccines are complex biological prod ucts that are obtained from living organ isms through long manufacturing and control processes. More than 70% of the manufacturing time of a vaccine is invested in quality controls. COVID-19 has plausibly revealed the high cost of relocation in some areas. We have seen the difficulties of manufacturing and production, especially in Europe, of es sential biotechnological products. The shortage of companies capable of pro ducing vaccines has been a reality in this crisis. The manufacture of vaccines re quires high investments, in technology,
Biotech manufacturing plant at CZ Vaccines, Spain
infrastructure and highly qualified staff. It can only be supported if the facility manufactures on a large scale.
Global biopharma CDMO
CZ Vaccines has a long history in the bi otech industry. It is one of the few com panies in Europe, and the first in Spain, authorised to produce vaccines with ca pacity – barely a dozen are. It is quali fied to work with extensive scientific ex perience, which enables them to work with different types of vaccines: genet ic engineering live attenuated vaccines, conventional vaccines, subunit vaccines (recombinant protein) or last generation DNA vaccines. CZ Vaccines produces viral vaccines, microbial vaccines and cell therapies in their biopharmaceutical facilities (GMP) prepared for this pur

pose, where a wide range of biological products are produced for third parties, from the development to commercial products, since it has a large manu facturing infrastructure and production sites, both in Spain and Portugal.
CZ Vaccines has been involved in col laborating actively on the urgent devel opment of solutions against the coro navirus disease COVID-19 through its research and manufacturing capabilities in several projects in the last years.
It must be considered of strategic im portance for Europe to have companies, qualified and authorised to manufac ture vaccines and biologicals as an axis of autonomy and strength in the face of potential future health crises. Taking advantage of the synergies and lessons learned in this pandemic is key to ad dress future problems.
40 European Biotechnology | Winter Edition | Vol. 21 | 2022 FOCUS PHARMA SERVICES Picture: CZ Vaccines
L




58% Drugs 31% Biologics 40% Rare Diseases Think Bold. Think Scientific. Think Veristat. Strategic Consulting and Trial Design to Trial Execution through Regulatory Approval and Post-Marketing Veristat harnesses knowledge, expertise, and scale to provide superior solutions and progressive approaches to mitigate risks and achieve meaningful outcomes for biopharmaceutical and medical technology companies and their patients around the globe. PRE-CLINICAL PHASE I-III REGISTRATION POST-MARKET Bold thinking and scientific-minded insights to plan your development program – from IND/CTA to NDA/MAA and beyond STRATEGIC CONSULTING Regulatory expertise that delivers the roadmap, gap analysis, agency interactions and registration support to achieve success REGUL ATORY AFFAIRS Versatile Service Model Across Entire Development Spectrum From a single service to full-service, Veristat experts support you your way.
Veristat – Bold
Results With our focus on novel therapies and more than 28 years’ experience in clinical trial planning and execution, we deliver bold approaches that make the impossible possible. To ensure your success, select Veristat as your partner. Meet Veristat at MPP 2022 ©2022 Veristat, LLC. All rights reserved. Veristat is a trademark of Veristat, LLC. All other trademarks are the property of their respective owners. ADV -VT-149 v.02 Aug-22 In 2021, Veristat supported marketing applications for 12% of all FDA Novel Drug Approvals.
Meet
Thinking That Delivers
AGC Biologics doubles mammalian capacity
CDMO With a new expansion coming online in Copenhagen, AGC Biologics is offering more capacity in Europe for mammalian biologics projects using innovative single-use bioreactor technology. This is the latest in a string of new investments from the global CDMO to expand capacity and upgrade technology at its sites in Europe, Japan and the United States.
› Ben Clauberg, Senior Director Business Development, AGC Biologics
Contract Development and Manufactur ing Organisations (CDMOs) are playing an increasingly critical role in the large molecule drug market.
The biopharmaceutical CDMO mar ket continues to grow in double digits annually. With scientific ability and in genuity, the latest technology, and copi ous manufacturing capacity, these part ners are now critical to the ecosystem. A notable example of this is AGC Biologics, one of the fastest-growing CDMOs in the life sciences space. In the last few years, the company has expanded at sites in Ja pan, USA, Germany, and Italy.
The CDMO is also finishing one of the biologics industry’s most extensive ex pansion projects, a new building at its site in Copenhagen, Denmark that is of fering developers greater access to largescale mammalian cell-culture develop ment and manufacturing services.
AGC Biologics Copenhagen site
The Denmark-based site is one of the most active biologics facilities in the AGC Biologics network.
AGC Biologics runs multiple mamma lian and microbial cGMP manufacturing lines at various scales and offers techno logical flexibility to meet drug product specifications.
The site’s core teams of scientists have been working together to develop and manufacture biologics for more than 20 years. The facility is truly one of the

top CDMO sites in Medicon Valley and is ready to take on more projects.
Mammalian capacity expansion
AGC Biologics invested in an expansion and a new dedicated building to extend the Copenhagen site’s capabilities and more than double its single-use bioreac tor mammalian cell-culture capacity.
AGC Biologics is actively having dis cussions to fill this space now, with oper ations beginning in mid-2023.
This expansion is a clear demonstra tion of AGC Biologics’ commitment and dedication to supplying innovative tech nological manufacturing solutions for de velopers and large pharmaceutical com panies. The new building will include
four levels holding about 8,000 m 2 dis tributed across manufacturing, quality control laboratories and technical and warehouse areas. The production will be focused on genetically modified organ isms, biosafety level 1, and manufactured using single-use technology in dedicated cleanrooms.
Global supply chain access
In addition to expanded capacity, one of the other benefits of working with a global CDMO is access to a greater net work of supplies. The challenge of find ing affordable supplies has grown larger because of the COVID-19 pandemic and now again with potential financial mar ket inflation. The longer it takes to secure
42 European Biotechnology | Winter Edition | Vol. 21 | 2022 FOCUS PHARMA SERVICES Picture: © christian buck
supplies for pharmaceutical develop ment, the longer it takes to get products to patients. For developers and pharma companies of all sizes, this can be the difference between being first to market or falling behind your competitors.
A CDMO like AGC Biologics has ex tensive material sourcing abilities, effec tive bargaining power, and worldwide inventory and supplies. This network of shared resources gives AGC Biolog ics more buying power and the ability to lower the total cost of goods to produce a drug product. The global supply func tion can also stand up to supply market changes – even during a pandemic or global economic strain.
With this deep supply chain and the knowledge and experience of the scien tific team at each site, AGC Biologics can help resolve any issues during the devel opment and manufacturing process and effectively guide the product through important clinical and commercial dead lines.
Sites in Europe, Japan, and USA
If you need something done outside of Copenhagen or the European continent, AGC Biologics’ seven sites have a range of specialisations, scales, and capabili ties.
In addition to traditional biologics (mammalian and microbial), the CDMO specialises in recombinant DNA, plasmid DNA (pDNA), messenger RNA (mRNA), viral vectors, and genetically engineered cells.
› Heidelberg, Germany: The Heidel berg facility serves as a hub for mi crobial systems and services for AGC Biologics’ global customers. The site has scientists with several decades of protein expression experience, which enabled AGC Biologics to set up a centre for custom pDNA and mRNA service lines at this site.

The facility’s segregated line design al lows for capacity and technological flexibility while ensuring compliance with current global guidelines needed for cGMP compliance.
› Milan, Italy: AGC Biologics Milan spe cialises in cell therapy and viral vector development and manufacturing. The site works with any cell type and len tiviral, retroviral, and adeno-associat ed viral vectors.
The facility was the first cell and gene therapy site approved in Europe for GMP manufacturing of clinical and commercial supplies. The core scien tific team has 25 years of experience in the field and unique knowledge you will not find anywhere else.
› Chiba, Japan: This multipurpose, high ly efficient facility is one of the few sites in Japan with both microbial and mammalian capabilities, backed by a global CDMO network. The facility ’s core structure and gravity-based de sign enable guaranteed flexibility and high-quality work. The Chiba teams have experience in cGMP microbi al manufacturing for many projects, ranging from E. coli to yeasts, while the site's mammalian offering guaran tees ability and technological flexibili ty, thanks to the extensive adoption of single-use systems.
› Boulder, Colorado, USA: The AGC Biologics Boulder Facility offers a sig nificantly larger production scale for mammalian-based projects. The site houses two massive stainless steel cell bioreactors, and its size, auto mation, and cost-effective capabil
ities make it well-suited for highvolume commercial production and high titer antibody processes.
In addition to cGMP commercial ser vices, the site has a stellar record in cell line development and experience with multiple mammalian cell types and expression systems.
› Longmont, Colorado, USA: Longmont is AGC Biologics’ North American Hub for cell and gene therapy services. The site's capabilities include viral vec tor development, characterisation, and manufacturing in adherent and suspen sion environments, along with cell cul ture and modification development and manufacturing using the latest industry technologies and methodologies.
› Seattle, Washington, USA: The cam pus is the headquarters and has been producing biologics products for 30 years. Here, AGC Biologics operates multiple manufacturing lines and a va riety of scales for mammalian and mi crobial biologics. With a uni-flow de sign, the campus offers capacity and technological flexibility, including extensive adoption of single-use sys tems, while ensuring compliance with current ICH guidelines required for cGMP compliance. This location is the company's center of excellence for formulation and employs the latest fed-batch and perfusion manufactur ing processes.
43 European Biotechnology | Winter Edition | Vol. 21 | 2022
FOCUS PHARMA SERVICES
Picture: © AGC Biologics
L
Wholistic approach to strategic consulting
CLINICAL TRIALS Strategic planning for the development of a novel therapy is critically important to reduce financial burdens, save time, and find the best, shortest path forwards. No two products will have the same pathway or same criterion for optimisation, but early, forward-looking planning can result in greater efficiency and a better understanding of key decisions and timepoints along the course of novel therapy development.
› Robin Bliss – VP, Strategic Consulting
Strategic consulting is the contribution of cross-disciplinary questions, ideas, and experience that increases critical thinking and forward planning towards the study and development of a novel product.
Early clinical development
Strategic input is critical in constructing the clinical development plan (CDP), a document that details the proposed path way from an initial clinical study through eventual marketing application with key decision options outlined to the extent possible. This document typically in cludes proposed indications, planned study design(s) based on the competitive landscape and other similar products and indications, proposed sample sizes, op tions for study adaptation, and the nar rowing or expanding of the study popu lation, as appropriate. The CDP is a living document that can be updated with in creasing knowledge gained during the de velopment phase with contributions made by regulatory strategists, clinical opera tions, and strategic statisticians. With in sights from experienced strategic team members, the CDP can detail the longterm plan for product development, pro viding visibility and opportunity to iden tify ‘go-no-go’ decision timepoints and efficiencies during development.
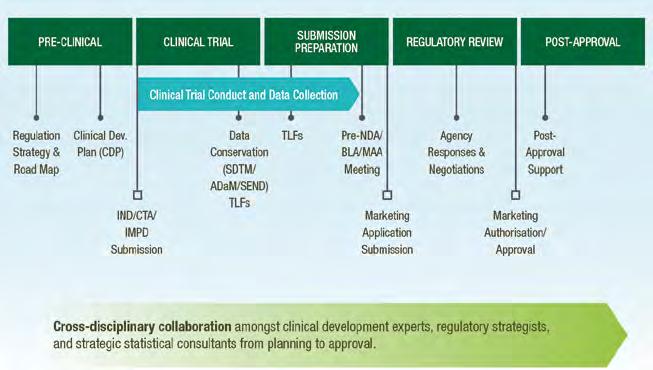
Go-no-go decisions are predefined timepoints and criteria for a decision to either continue product development,
Roadmap of strategic consulting
modify the pathway, or stop develop ment as currently planned. The timing and criteria should be prespecified with consideration to all possible pathways along a decision tree to ensure that products with promise are continued and risks for continuing the develop ment programme, including cost, time line, and likelihood of success, are well understood. The decisions are impacted by multiple disciplines, and the related risks should be considered by all par ties, requiring the merging of identified clinically meaningful results, appropri ate endpoint selection with appropriate statistical analysis methods and consid eration for Type I error control, key re quirements or objectives from regu latory agencies, and consideration of
the timeline and competing landscape with other products. Go-no-go decision planning is critical early in development to ensure that appropriate efforts are di rected to products most likely to lead to marketing authorisation.
Meetings with regulatory agencies can be planned to occur at opportune times when the clinical development team can receive valuable input to help direct the pathway towards a more efficient route for eventual marketing application and approval. As a partnering document to the CDP, a target product profile (TPP) is an organisational, planning, and commu nication tool to present the minimally ac ceptable vs ideal results for the planned indication, patient population, treatment duration, modes of administration, and
44 European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © Veristat
FOCUS PHARMA SERVICES
standards for clinical efficacy. In the ear ly stages, the TPP can be used as a con cise, early presentation of the ultimate goals of the product development to in form conversations with regulatory agen cies to ensure agreement surrounding the development plan.
Regulatory interactions
Regulatory interactions and feedback should be considered carefully in the context of the greater clinical develop ment programme. Changes to study de sign, endpoints, eligible patient popu lations, or study procedures, can have an impact on the generalised conclu sions that can be made from the clinical programme. In general, Veristat recom mends early conversation on key plan ning elements with regulatory agencies. This can be expedited with key elements identified through the careful develop ment and updating of a CDP.
Early protocol development
An early opportunity for strategic input is during development of the first-in-human or first-in-indication protocol. Strategists can advise on:
› Selection of objectives and endpoints for establishing safety and initial dem onstration of efficacy
› Identification of surrogate endpoints and/or biomarkers for prognostic or predictive effect
› Alternative study designs, including adaptive or Bayesian methods that might reduce the time on study and exposure of subjects to the novel treatment at sub-therapeutic levels
› Appropriate sample size selection with context to available information on potential safety and toxicity of the product and relevant conclusions for proof of efficacy
› Optimal pathways for progressing the clinical development pathway to sup port later regulatory requirements
Early in product development, the key to success is to think ahead – an opti mal solution needs not only to be a
good decision today, but also to ad vance the product effectively and effi ciently through the CDP without wast ing time or patients.
Rare diseases
For rare diseases, particularly where there are no, or few, alternative treat ments, strategic input is critical to iden tify an appropriate study design. Strate gists can help overcome:
› Small sample sizes
› Lack of medical experts/sites
› Limited info/consensus on standard of care
› Access to patients
› Difficulty identifying clinical out comes to measure
› Lack of control or comparator data
› No disease-specific endpoint validation
With only a limited number of patients available worldwide, an efficient ap proach must consider the total number of patients required for each study, as well as the use of appropriate controls. The gold standard clinical trial design is a double-blind, randomised, controlled tri al; however, this is not typically feasible with low patient populations worldwide. When possible, the use of non-concur rent or synthetic control arms can be beneficial. This, of course, requires ear ly and strategic interaction with regula tory agencies.
In some settings, these control arms may not be available, and single-arm noncomparative studies can be used as the key evidence for marketing application and approval.
Decisions regarding study design in this context are closely entwined with the appropriate treatment of patients and the regulatory strategy.
The cross-disciplinary considerations of statistics, clinical operations, clinical development, and regulatory strategy must be considered to create an optimal and feasible approach to product devel opment.

Conclusion
Many factors contribute to an optimal strategy, including the type of product, indication(s) of application, disease prev alence, and the competitive landscape. This is why strategic planning from an experienced, cross-disciplinary team of experts is critically important for the efficient and effective development of novel therapies.
Early, forward-looking planning, the development of tools, such as the clini cal development plan and target product profile, frequent interactions with regu latory agencies, and consideration of nu anced details that may be most effective ly handled through non-standard clinical trial design, can result in a more efficient pathway to market. L
45 European Biotechnology | Winter Edition | Vol. 21 | 2022 FOCUS PHARMA SERVICES Picture: Veristat
New created position
VALNEVA The specialty vaccine com pany Valneva, based in Saint-Herblain, France, has appointed a new Chief Com mercial Officer and Management Board member: Dipal Pa tel took over the newly created role in November. She brings more than 23 years of expe rience in the phar maceutical sector covering commer cial strategy, execu tion, market access and life cycle management. Over her ca reer, she has held roles of increasing re sponsibilities across multiple countries. Since 2019, Patel has been Global Com mercial Head of GSK’s shingles vaccine, leading a global cross-functional team establishing it as a global brand with sig nificant worldwide expansion. L

EIF Chief Executive
EIB Marjut Falkstedt has been appointed as Chief Executive of the European Invest ment Fund (EIF) by the EIF’s Board of Di rectors. She will start her new position at the beginning of January 2023. The Finn ish national, currently serving as Secretary General of the EIB, began her career at Dresdner Bank be fore joining the Di rectorate-General for Economic and Financial Affairs at the European Com mission in 1996. She held various roles in the field

of SME and infrastructure financing until 2013 and was also advisor to the Europe an Commission’s representatives on the Board of Directors of the European Invest ment Fund (EIF), part of the EIB Group, between 2001 and 2007. From 2013 to 2015, Falkstedt (previously known as Marjut Santoni) was Deputy Chief Execu tive of the EIF, before being appointed to the position of Deputy Secretary Gener al of the EIB in September 2015. She was promoted to Secretary General at the EU Bank in 2018.


Experts for Directors board
L
off from the Free University of Brussels, founded in February 2021, with focus on developing pharmaceuticals to restore blood-brain barrier function. L
Double new expertise
ANJARIUM BIOSCIENCES Swiss An jarium Biosciences AG strengthened its Board of Directors with the appoint ments of Gaurav Shah, MD, and Douglas Fambrough, PhD as independent Nonexecutive Directors. Shah is also named as Chairperson of the Board. This expan sion brings significant additional gene therapy and corporate development ex pertise to Anjarium, a biotech company, focused on creating and delivering a new class of non-viral gene therapies. L
Expanding leader team
NEUVASQ NeuVasQ Biotechnologies, based in Gosselies, Belgium, has ap pointed Emmanuel Lacroix, PhD, MBA, as Chief Executive Officer and com pany director. Lacroix’s skills as an ac complished biotech executive comple ment the expertise of NeuVasQ Founder and Chief Scientific Officer Benoit Van hollebeke. NeuVasQ also announces that Yves Ribeill, CEO, Ribogenics, and Richard Porter, Chief Business Officer, uniQure, have joined its Board as Inde pendent Directors. NeuVasQ is a spin-
REWIND THERAPEUTICS Belgian Re wind Therapeutics has strengthened its management: In September, Dr Anja Harmeier took over the position of CEO. In November, Dr Irene Knuesel joined the team as Chief Scientific Of ficer. Harmeier came from Pureos Bio ventures, where she was responsible for identifying, evaluating and manag ing investments in innovative life sci ence companies in the US and Europe, as well as raising capital. Previous ly, Harmeier held several positions at Roche. Since 2016, she was an invest ment director at the Roche Ven ture Fund. Harmei er holds a PhD in Neuroscience/Bio chemistry. The new Rewind-CSO Irene Knuesel was Head of Neuroimmunology at Roche. She brings many years of expe rience in CNS research, drug discovery and development. Prior to her career at Roche, she held numerous academ ic positions in neu robiology at the University of Zu rich, the California Institute of Tech nology, Pasadena, CA (USA) and the Swiss Federal Institute of Technology (Zurich, Switzerland). Irene Knuesel re ceived a PhD in Molecular Neuroscience and a doctorate from the Medical Facul ty of the University of Zurich. L
46 European Biotechnology | Winter Edition | Vol. 21 | 2022 BIOPEOPLE Pictures: Valneva/EIB/Rewind Therapeutics
Dipal Patel
Marjut Falkstedt
Anja Harmeier
Irene Knuesel
II Guide to Life Sciences Events

Euro B ioFa irs Compa ss
1st half-year 2023 SPECIAL
© BillionphotosFreepik.com
Pharmapack 2023
PACKAGING Pharmapack is Europe’s largest event dedicated to the pharmaceutical packaging and drug delivery industries. With an extensive conference agenda, it provides invaluable opportunities for networking and showcasing innovations.
Pharmapack 2023, taking place in hy brid format with in-person event on Feb 1st -2nd 2023, at the Paris Expo, Porte de Versailles Paris, France, is bringing together nearly 5500 pharma execu tives from 75 different countries and more than 350 exhibitors at the heart of the pharma packaging and drug deliv ery industry. The online platform (18th Jan -17th Feb, 2022) will be available to all registered participants.
Beyond the exhibition floor, 15 ses sions under Three Tracks- Device & Packaging Innovation, Large Volume
Come to Paris
GREETING The great thing about Pharmapack is that it remains the ab solute heart of the drug packaging and delivery industry, and the event retains a community- and in novation-driven at mosphere. The 2023

Drug Delivery & Combination Prod uct and Sustainability- delivered by industry experts will take place over the 2-day event to discuss the very lat est market trends in the drug delivery space. Running alongside will be work shops: Sustainable Materials, Autoin jectors, Labelling & Track & Trace or Contract Packaging and the returning ‘innovation gallery’ which will show case exhibitor innovations providing a window on the latest trends and cut ting-edge developments. The ‘learn ing labs’ will provide an opportunity to
hear from different exhibitors on their research and products.
The show floor will also feature Start-up hub, a platform for young start-up companies to exhibit their in novations, providing valuable brand visibility. All these sessions will be streamed for online audiences. In-per son attendees can also participate in guided expert-led walk-throughs on the ‘innovation tours’.
The much-awaited Pharmapack Awards will return this year celebrat ing the best products and innovations from the pharma packaging world un der two categories of ‘exhibitor inno vation’ and ‘health products’- with an awards ceremony on Feb 1st 2023. L

› QUICK FACTS
Pharmapack Europe will be online 18 th Jan - 17th Feb, and in person 1st – 2nd Feb, 2023 at the Paris Expo, Porte de Versailles, France.
In 2022, some 5000 attendees from 250 companies and 75 countries went to on the Paris Expo for what was one of the most convivial exhi bitions in memory.

edition will also feature a larger sus tainability agenda and the full return to onsite content – so already there is a real excitement amongst the indus try to return and a desire to accelerate their partnering.
Laura Murina Brand Manager, Pharmapack
› The latest content agenda for the 2023 event is available here – https://informamarkets. turtl.co/story/pharmapack2023-content-agenda/page/1.
› For more information and online registration, please visit https:// www.pharmapackeurope.com
48 EURO BIOFAIRS COMPASS European Biotechnology | Winter Edition | Vol. 21 | 2022
Pictures: Pharmapack/Informa
18 th Jan 17 th Feb´23 -online In person 1st – 2nd Feb´23 at Paris Expo, Porte de Versailles, France
2023

Pharma’s dedicated packaging & drug delivery event #PharmapackEU www.pharmapackeurope.com 1 - 2 February 2023 Paris Expo, Porte de Versailles Hall 7.2 | Paris, France 18 January - 17 February 2023 Online event and networking Innovation Networking Education Get your free* ticket at pharmapackeurope.com *Free early-bird registration applies to the standard attendee pass. Available until 17 January 2023
7th AMR Conference
16-17 March 2023 Congress Centre Basel Switzerland


The silent pandemic
AMR CONFERENCE 2023 The AMR Conference is the well established international platform for SMEs, start-ups, big pharma, academia, investors and public institutions to discuss the latest updates in innovation and related policymaking needed to bring new antimicrobial treatments and diagnostics to the market.
Being the proven successful forum to connect relevant AMR stakeholders from industry, SMEs, finance, hospi tals, science, and policy, the seventh AMR Conference will provide two days packed with 20 sessions on the most urgent topics the AMR community is
›
QUICK FACTS
› Programme – 80+ high-level speakers in 20 sessions; pre-conference day & workshops
› Partnering – 1-to-1 partnering among the 400+ participants
› Innovation – Start-up competition, poster session and exhibition
Further information: www.amr-conference.com
currently discussing: How do policy makers take over their role in estab lishing most needed pull incentives? Which new initiatives have been start ed to push forward innovations in an timicrobial therapies and diagnostics? Which approaches take investigators to improve the efficiency of preclinical models as well as clinical trial designs?
Perspective of SMEs
The goal of the conference is to catchup with the latest trends in the de velopment of AMR products. The up coming conference will provide an important platform for all stakehold ers – in particular for small and me dium enterprises and start-up compa nies – to exchange views on innovation opportunities, development paths and alternative business models. The 2023 conference edition, hosted by the Euro
pean SME organisation BEAM Alliance in cooperation with bamconn GmbH, will again be the leading meeting of the AMR community in Europe. Together with other major stakeholders such as the Novo Repair Impact Fund and oth ers, the participants will explore fur ther strategies to improve conditions for companies developing novel anti microbials or AMR diagnostics. A spe cial focus of the event is targeted at the perspective of innovative SMEs, their R&D pipeline, requirements for regu lation and policy frameworks.

A 360°-view on AMR innovation
In addition, the conference will be a platform to discuss innovative strat egies to improve preclinical R&D as well as clinical trial designs. Among the partners of the event will be the EU-funded IMI AMR accelerator which is organizing its annual meeting as a pre-conference event on 15 March. Be yond the overview of antifungal and an tibacterial innovation landscape, the conference will also propose a special focus on how India is tackling the AMR issue. Among the further highlights will be a start-up competition, hosted by the pharma-backed incubator INCATE. Founders are requested to apply un til January 15, 2023. The top ten candi dates will qualify for a pitch during the conference.
50 EURO BIOFAIRS COMPASS European Biotechnology | Winter Edition | Vol. 21 | 2022 Pictures: © BIOCOM AG; stock.adobe.com
L
Novel Antimicrobials & AMR Diagnostics
Novel Antimicrobials & AMR Diagnostics 7th AMR Conference















Conference office: bamconn GmbH • Erholungsweg 51 • 13509 Berlin Boris Mannhardt • info@bamconn.eu • Tel. +49 (0)176 101 439 86 amr-conference.com #AMRconference Mar. 16-17 2023 Congress Center Basel | Switzerland
The #AMRconference is the one-stop-shop for all AMR stakeholders from industry, SMEs, start-ups, big pharma, academia, clinics, regulatory bodies, investors and public authorities. Join us in Basel to meet and exchange! More than 400 delegates from 25+ countries A variety of topics from R&D to financing, regulation & incentives 80+ high-level speakers in 20 sessions Poster session, exhibition and prescheduled 1-to-1 partnering meetings
workshops and satellite meetings SMEs, academics and governmental agencies benefit from discounted fees Supporting Partners: Media Partner: ©ScieProstock.adobe.com
Pre-conference
20–22 March 2023
Basel Switzerland
BIO-Europe® 2023
BIO-EUROPE SPRING It’s time once again to network and facilitate partnerships face-to-face at the premier springtime event for the life science industry.


The seventeenth annual BIO-Europe Spring premier springtime partnering conference is back in-person and will be taking place March 20–22, 2023 in Ba sel, Switzerland.
After being delivered digitally over the last two years, the event is expected to bring together over 2,800 leading exec utives from around the world to engage in more than 15,000 one-to-one meet ings. The event will cater to the needs of the entire value chain with world-class workshops and panels, innovative com pany presentations, active exhibition, and a variety of networking opportuni ties making this event an unrivalled fo rum for companies across the biotech value chain to meet and do business.

EBD Group is committed to pro viding a top-tier forum for the global
biopharma community to come togeth er. With that goal in mind, this year’s event will feature three days of digital partnering in the week following the inperson event.
partneringONE® will continue to allow delegates to experience the entire event from anywhere, including one-to-one meetings, on-demand content, and con ference activities. Scheduled meetings will take place face-to-face during the conference days as well as digitally dur ing the digital partnering days with links to a video conferencing solution.

We are thrilled to welcome you back to this “must-attend” springtime part nering event—making it a special re union for some and new beginnings for others.

Come to Basel
GREETING We are all thrilled to reu nite at the next BIOEurope Spring, to meet in-person and of course to make new connections. Ex panding on the success of EBD Group’s flagship conference, BIO-Europe® this last October, we anticipate this confer ence to exceed 2019 as well.
The last couple of years have fun damentally changed business devel opment and networking. While we are happy to have established new dig ital meeting solutions and virtual conferencing, the EBD team is thrilled to launch BIO-Europe Spring as a fusion of innovative digital solutions and a vibrant onsite event of unprece dented scope. We are looking forward to welcoming both attendees in Basel and online next spring.”
Shanks Portfolio Director –European Market, EBD Group
52 European Biotechnology | Winter Edition | Vol. 21 | 2022 EURO BIOFAIRS COMPASS
EBD Group
Pictures:
L
Registration https://informaconnect.com/ bioeurope-spring/Bookingoptions/bioeuropespring.com › QUICK FACTS
Chris
RECONNECTING AND FACILITATING GLOBAL PARTNERSHIPS

The seventeenth annual BIO-Europe Spring® is back in-person and will be taking place March 20–22, 2023 in Basel, Switzerland. This premier springtime partnering conference will bring together 2,800+ leading executives from around the world to engage in 15,000+ partnering meetings, world-class workshops and panels, innovative company presentations, active exhibition and as always, a variety of networking opportunities.




Register now and take advantage of early bird rates: bioeuropespring.com

MARCH
2023 // BASEL,
Produced by:Supported by:
20–22,
SWITZERLAND
Swiss Biotech Day
SWISS BIOTECH DAY One of the most important biotech conferences in Europe, the Swiss Biotech Day, will once again attract over 1,000 senior life science experts from all over the world to Basel.

The Swiss Biotech Day in Basel is a fixed date in the biotechnology commu nity's calendar. In 2022, the conference demonstrated the impressive growth of the Swiss biotech industry and pre sented an exciting program with ideal networking opportunities across bor ders to over 1,200 participants from 36 nations.
in R&D, manufacturing, data manage ment, artificial intelligence and innova tive financing over one and a half days. Partnering allows to take advantage of opportunities and synergies arising from the close cooperation between biotech and pharma or to find interest ing partners for future projects, and to jointly drive the development of inno vative products and platform technol ogies. In addition, international biotech delegations use the "Global Village" to strengthen their ties with Switzer land as a biotech location and promote cross-border investments, public-pri vate partnerships, research and devel opment collaborations and exchange of talent.
An inspiring program with themati cally focused panel discussions, ex hibition, company presentations and partnering are the cornerstones of the lively networking event, accompanied by the trends and facts of the Swiss biotech industry and the celebration of the Swiss Biotech Success Stories Awards. L
Switzerland's most important biotech nology conference is equally attractive to biotech companies, their suppliers, investors and pharma corporations. In 2023, the Swiss Biotech Day will again offer high-level experts from the life science industry the space to network, discuss and discover trends


› Meet 1,000+ participants › 70+ exhibitors and global village with international delegations from all over the world › Swiss Biotech Success Stories Awards

Innovative biotech start-ups and medium-sized biotech companies › Thematically focused panel discussions
one-to-one partnering meetings
54 European Biotechnology | Winter Edition | Vol. 21 | 2022 EURO BIOFAIRS COMPASS Pictures: © Swiss Biotech Association
REGISTRATION
Phone:
register@swissbiotechday.ch
April 24-25, 2023 Congress Centre Basel, Switzerland
www.swissbiotechday.ch
+41-52-569-82 27
Highlights
› QUICK FACTS
›
›
SWISS BIOTECH DAY
...ONE BIOTECH CLUSTER
As one of the leading biotechnology conferences, the Swiss Biotech Day has developed into a truly global networking day, attracting many international delegations.

What you can expect:
› Meet 1,000+ senior experts from the life science industry
› 70+ exhibitors and global village with international delegations from all over the world
› Swiss Biotech Success Stories Awards
› Innovative biotech start-ups and medium-sized biotech companies
“WHITE BIOTECHNOLOGY” WORKING GROUP
› Thematically focused panel discussions
› Swiss Biotech Report 2023
Leading chemical companies are exploring the opportunities that have been opened up by modern biotechnology, especially in the field of “white” or indusbiotechnology. And they are also applying these technologies, wherever it makes sense. The SBA takes initiatives seriously and has formed a working specifically dedicated to white biotechnology.
› Pre-scheduled one-to-one partnering meetings

› General Assembly of the Swiss Biotech Association
sector is internationally visible. The project-specific participating companies (most of them young and internationally less savvy) find a comprehensive partner which is helping to put them in the public window. The participating Life Science Regions are important internal carriers of the dynamics in the Biotech sector, thus enhancing the common understanding of the industry. This and more knowledge is brought into Europa Bio, the European Biotech Association, where the SBA is an active member.
SWISS BIOTECH...
...is an alliance of four leading Biotech regions of Switzerland (Bio Alps, BioPolo Ticino, Basel Area and Greater Zurich Area). They have combined efforts to streamline interests of the national biotech sector. The SWX Swiss Exchange holds a leading position in terms of lifescience listings and offers companies from that industry – be they located in Switzerland or abroad – access to an internationally recognised financial marketplace. The initiative was co-founded by the SBA which also manages the executive office of Swiss Biotech.
Media partners:
Swiss Industrial Biocatalysis Consortium is an important partner in this effort. The group includes leading multinational companies that support white biotechnology as a pillar of economic growth. The planned activities are in agreement with OECD strategies.
partnership with the Swiss Biotechnet (see pages the SBA develops training programmes and usesupport tools for the industry. It is of importance the industry specifies its training needs so that academic side can create tailor-made education. strategy ensures that the industry gets the right workforce with the right education. The SBA profits
Domenico Alexakis is Executive Director
Organized by:
For further information please visit
April 24-25, 2023 Congress Center Basel
Save the date!
Swiss Biotech Association
©
@
22
Sign in on our website at www.swissbiotechday.ch and stay updated about any news.
Connect in Pharma


FUTURE PHARMA After a successful launch in 2022, Connect in Pharma returns to Geneva to bring together the community of professionals shaping the future of drug delivery, manufacturing, outsourcing and packaging.
Connect in Pharma aims to drive inno vation, business and new partnerships in four key areas: innovative packaging, drug delivery systems, sub-contract ing, and manufacturing. Taking place in Geneva – the centre of a major phar maceutical and biopharma cluster –Connect in Pharma is the perfect place to meet key influencers from leading pharmaceutical groups, biopharma, in dustry clusters and suppliers.
After attending the 2022 event, Ele na Obrecht, Head of Maintenance at
Come to Geneva
GREETING Europe is home to a range of companies making extraordinary ad vances across drug delivery, manufac turing and packaging, and we’re delight ed to be able to bring that community to gether through Con nect in Pharma.
Everyone who at tends Connect in Pharma can expect
Johnson & Johnson said: “I have been inspired by the newest trends in the market, including pharma and medi cal device innovations.”
With expert speakers, workshops, a gala dinner, receptions and loung es, Connect in Pharma offers unri valled opportunities to network, ne gotiate and foster connections with inspiring thought-leaders, world-wide key industry professionals and the most exciting innovators. Exhibitors and visitors can expect to connect with
to find something that inspires them, a new business lead or a solution to a technical challenge, and a host of new ideas to take back.
Lastyear’sfirst-everConnectinPhar ma event was a huge success. We can’t waittowelcomeourexhibitors,partners and visitors to an even more dynamic event in June 2023. We hope to see you in Geneva!
Renan Joel
Divisional Managing Director for Packaging at Easyfairs
14 & 15 June 2023
Palexpo, Geneva, Switzerland
www.connectinpharma.com
a curated collection of industry lead ing brands seeking to source packaging and production solutions in the phar maceutical industry.
In 2023, visitors will find even more content to support the industry’s navigation around the future of phar ma, with three stages tackling and advising on the trends and trials fac ing the pharma and biopharma com munity.
The theme of the event is creating and navigating the future of pharma.
The conference programme will ad dress key factors that will revolution ise the industry: sustainability, con nected health, AI and digitalisation, and human-centric approaches. Also, at the event, there will be speakers from Google, NASA, Forbes, GSK and Bayer who will speak about their ex periences and how the current use of technology will advance the pharma community. L

› QUICK FACTS
James Montero-MacColl
james.montero@easyfairs.com
+44 (0)20 3196 4428
https://www.connectinpharma.com/
REGISTRATION
https://www.connectinpharma.com/ visit/register-your-interest/ AGENDA
https://www.connectinpharma.com/ visit/programme/
56 European Biotechnology | Winter Edition | Vol. 21 | 2022 EURO BIOFAIRS COMPASS Pictures: © Connect in Pharma




The new event at the heart of European pharma dedicated to innovative packaging, drug delivery systems, CDMO/CMO and filling & assembling processes 14 & 15 June 2023 | Palexpo, Geneva Register your interest
This year’s INDUSTRIA BIOTEC took place in a former brewery: the KINDL Centre for Contemporary Art in Berlin. The spirit and topics are featured in a short film, which is available online. Just scan the barcode and watch. More information about this event – organised by BIOCOM AG – can be found here: ➜ https://industria-biotec.com


White Biotech: New kids on the block
INDUSTRIA BIOTEC For years, policies promoting industrial production modelled on naturallyoccurring cycles that don’t exploit natural resources but re-produce from waste have been little more than a green veneer used by oil and chemistry multinationals. At the new forum INDUSTRIA BIOTEC , companies presented both fresh and long-ignored solutions for averting the looming gas and climate catastrophes in cooperation with politics, while experts took a close, hard look at the lobbies involved.
Conceived as a showcase for modern industrial biotech nology solutions for the ener gy and climate crisis, the pre miere of INDUSTRIA BIOTECH in Berlin turned out to be unexpectedly political While the EU High-Level Bioeconomy Conference 2022 running in parallel in Brussels favoured agricultural and forest ry bio-based solutions as an alternative to petrochemicals, the biotech entrepreneurs and engineers in Berlin presented innova tive microbial and cell biological solutions that have received less political attention so far. These promise an additional mas sive reduction of CO2 emissions, which are currently rising at an annual rate of 0.65% (from 11/2021 to 11/2022). In Berlin, bio tech innovators made clear how surprising ly much they can contribute to solving the energy and climate problems. They em phasised the importance of immediate po litical support to replace nature-destroying petrochemical production with sustainable production as quickly as possible.
Industrial biotech – a driver
“We already have the technologies that will make it possible to bring atmospheric CO2 levels down to 1990 levels”, said An eri Pradhan, COO of sustainable energyfocused, $1.5bn non-commercial biotech
accelerator New Energy Nexus (Thailand). “But we finally need the support of the public sector and politics for this. Firstly, to be able to scale-up fermentation plants. Secondly, to encourage consumers to con sume more sustainably.” This astonishing ly optimistic outlook on solving the the cli mate and energy crisis was not unique at the first INDUSTRIA BIOTEC at the begin ning of October. At the event, biotech ex perts of various stripes from all over Eu rope were in unanimous agreement that the petrochemical era must be brought to
an end as soon as possible, and the bil lions in subsidies that support it should in stead quickly be pivoted to create publicly funded upscaling centres for energy- and space-saving microbial conversion and re cycling processes (see report on COP27, p. 8). In general, experts at the conference agreed that the greatest potential for CO2 savings lies in a new energy mix that in cludes biotech alternatives to oil, gas and coal burning for energy generation and industrial production, which account for 80% of global CO2 emissions. They also
Atmospheric carbon dioxide concentration has risen from 278ppm in the pre-industrial era to currently 417.95 ppm (November, 2022) as measured at the Mauna Loa observatory in Hawaii. Since 1990, when CO2 emissions hit 353ppm, levels classified as a climate threat have only grown. Growth is currently around 0.65% yearly.

59 Pictures: © BIOCOM AG (left); Climate Trace INDUSTRIA BIOTEC European Biotechnology | Winter Edition | Vol. 21 | 2022
Vegan cheese in the limelight
AWARD French food-tech company Standing Ovation SA won the first start-up pitch contest at INDUSTRIA BIOTEC (left: CEO Frédéric Pâques).

The two-year-old Paris-based biotech that produces animal-free casein in fermenters recently took a key step on its way to becoming a major player in the current €61.1bn vegan food market, which is expected to grow with a CGAR of almost 13% annually. In November it announced an equity invest ment and exclusive collaboration with a leading French cheesemaker (see p. 26). The company‘s business goal is to get in on the ground floor of GMfree sustainable animal-free milk proteins. Livestock farming currently causes 15% of all greenhouse gas emissions, while protein brewing could occupy just 1% of its land-use, leading to a reduction of the biotech prod uct’s CO2 footprint of more than 95% at the demonstration scale.
FIVE OTHER COMPANIES PARTICIPATED IN THE START-UP PITCH: Brussels-based Paleo BV develops GMO-free heme proteins through pre cision fermentation that are bioidentical with six animal proteins (chicken, beef, pork, lamb, tuna and mammoth) and give vegan protein products typi cal properties of animal-made products (taste, colour, texture). The compa ny expects to launch its first product next year.Akribion Genomics, which was spun off from German BRAIN Biotech AG in September, will commer cialise a patented non-Cas9 nuclease genome editing platform for the op timisation of microbial production strains, agricultural and pharma biotech applications.Nine-month-old Barcelona-based Esencia Foods produces fish and seafood products through solid state fermentation of fungal myce lium. The products are similar in taste and texture to real fish whose natu ral stocks will be exhausted by 2048. Berlin-based Ucaneo Biotech GmbH is developing the first cell-free Direct Air Capture technology leveraging a bi ocatalytic membrane, and aims to capture 1% of global CO2 emissions from the air by 2035. The final contestant, Mannheim-based Badische Peptide & Proteine GmbH (BPP) specialises in the solvent-free production and analy sis of cyclic peptides and proteins using green biotechnology. L

presented biotech alternatives to industri al animal-based meat production. Produc ing feed for the meat industry occupies at least 80% of all land used in agriculture.
Years of political inaction
“We are almost running out of time. As long as politicians continue to pour billions of euros in subsidies into petrochemical energy production and chemical produc tion as well as factory farming, biotech so lutions – which are compatible with natu ral cycles and save energy, CO2 and space – will not fly,” says Alexander Krajete, managing director of gas fermentation and adsorption specialist Krajete GmbH (Pas ching, Austria). His firm microbially pro duces natural gas from CO2 and hydrogen on an industrial scale (see p. 3). As long as petrochemical rather than biotech pro cesses benefit from subsidies worldwide, he and others think, biotechnological fer mentation products will be unable to com pete in price with the petrochemical prod ucts consumed by the throwaway society, and will at best only be used by privileged sections of the population.
Many second-generation innovations in focus at biotech SMEs target completely new emerging markets worth billions. One such innovation is for instace the microbial production of the milk protein casein – a crucial ingredient in authentic-tasting and sustainable vegan cheese. The fermen tation process mapped out by the win ner of the Industria Biotec start-up pitch Standing Ovation SA, which was select ed on the eve of the event, was validated in November through a production part nership and exclusive collaboration with the French Bel Group. The cheese man ufacturer achieved an annual turnover of €3.8bn in 2021, and now wants to open up new markets with products that taste and are processed like real cheese, but that aren’t tied to factory farming or un sustainable raw materials such as cashew nuts (see box left and interview, p. 26).
Five thematic streams
In five panel discussions on the second day of the conference, company repre
60 European Biotechnology | Winter Edition | Vol. 21 | 2022 INDUSTRIA BIOTEC Picture: © BIOCOM AG
sentatives, investors and bioengineers dis cussed and presented the current state of biotech solutions in sessions looking at the fields of nutrition, chemicals, waste as a resource, energy and market opportunities involving capital. Here the industry focus was on products, markets, expectations, concerns and wishes for advancing the field in Europe as quickly as in the US and Asia, where governments recognised the strategic importance of the fields early and are already creating support programmes and appropriate framework conditions for product marketing.

Food for the future
Opinions are divided when it comes to vegan meat, fish and milk substitutes. At times it feels like meat-lovers and vegans are almost at war. Some dedicated carni vores say the taste and texture of plantbased microbial or cell-culture-based meat, sausage and cheese products are abysmal. Factory-farmed meat remains cheap, and is viewed widely as a ‘natu ral’ product – even if the methods used to produce it are anything but. That said, sur veys show that more than one in five con sumers are interested in alternative protein products, and according to Google Ads, searches for vegan food grew by 47% last year.
Consumer acceptance, scaling up man ufacturing processes to produce more cheaply, and the advantages of biotech processes over livestock farming were the focus of the food panel sponsored by For mo Food GmbH, which produces ani mal-free milk protein by precision fermen tation. “We consume 2.5 million tons of meat per day,” explained Daan Luining, co-founder and CTO of Holland-based Meatable BV. “The meat supplied by farm ers is natural, but it is not good for the cli mate.” However, he said, one could also ask how natural and how good for the cli mate it is to use 80% of the world’s ad ministered antibiotics to fatten cattle with a 75% reduction in life expectancy in meat factories. Then there’s the need for the protein-rich feed grown on 80% of all farmland, which takes up 127,000 times more space to produce the same amount
of protein as producing muscle cells in a fermenter would. According to Luining, it is a widespread misconception that con ventional meat production should be abol ished. That is not in sight, even in the long term. Instead, the aim is to make the sys tem as a whole more sustainable, and bio technological solutions could play a major role in that process. “Not everything that appears natural is sustainable,” said Jo hannes Kopton from the evidence-based sustainability-focused Eco-Progressive Network. More important, he said, is what objectively brings society back into greater harmony with nature.
“The biggest lever to reduce energy consumption and agriculture’s [15%] share of global CO2 emissions is the big reduc tion in land-use change when consumers switch to plant-based protein alternatives,” said Hermes Sanctorum, CEO of Bel gian Paleo NV. “Contributing to this with biotech solutions is Paleo’s main goal,” he underlined. If freed-up agricultural land is established as new carbon sinks through reforestation, or is sown with crops that consume more CO2 than maize or soy [which have a lower photosynthesis rate than most C3 plants] and if biodiversity is increased again, a major recovery ef fect for nature and the climate can be ex pected, Sanctorum believes. According to recent publications, dairy and meat pro duction contribute almost 60% of the greenhouse gas emissions attributed to agriculture. Unlike Meatable, Paleo is not targeting the B2C end-consumer, but the
B2B market. However, its solution could still dramatically increase consumer ac ceptance of vegan protein alternatives. The company makes species-specific heme proteins secreted by the production organisms into the fermentation medium. Heme makes up only 0.2% of animal pro tein, but plays a key role in determining the typical taste and colour of beef, pork, chicken, lamb and tuna. Biotechnologi cally produced heme proteins could thus make plant-based, microbiologically or cell-based meat alternatives attractive to larger groups of buyers, thereby helping to reduce the negative impact of animal farming.
Transparency is crucial
“It takes cooperation between sever al companies and a number of bioreac tors [for scaling production] that we don’t have today to find [sustainable] solutions for agriculture,” said Roman Plewka, CEO of Formo Bio. But the negative example of Monsanto also shows how important transparency and communication are to achieving the shift toward a positive con sumer perception of biotechnologically produced foods, Luining reminded par ticipants. “When you do things and don’t talk about them, people get suspicious,” he said. The point is to use technology to benefit people, not against them: “We have reached a level of technology where we can replicate things of nature.” Sancto rum agreed that “it’s a matter of integrating
61 European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © BIOCOM AG Picture: © BIOCOM AG INDUSTRIA BIOTEC
a wide variety of technologies to change the overall system.” Plewka added that to enable this collaboration and get out of the secrecy corner, patents are needed. With out their protection, no one would make innovations publicly available and initiate collaborations. Plewka also saw anoth er challenge in the fear many consumers have of “food from the lab.” In fact, how ever, Formo’s vegan cheese comes from a fermenter identical to those used to brew beer – which needs to be communicat ed. There was also agreement that scaling up production was essential. According to Pradhan, public-sector investment is “ab solutely necessary for this. The bottleneck is not the number of innovative technolo gies, but the scaling to market maturity.”
A high-energy discussion
Bioenergy has even greater decarbonisa tion potential than agriculture. But there, too, panelists lamented a lack of support when it comes to scaling up processes. “The key by 2050 will be to decarbon ise, that is, to take the energy demand out of the system,” explained David Hamp ton, CEO of Switzerland-based Pheida
AG. “We can save the planet, and all bio energy sources will have a role to play in that,” said the Briton, who was convinced that a lot of the much-needed energy sav ings could be achieved by replacing fossilbased high-pressure, energy-consuming chemical production with mild enzymat ic processes.
Alexander Krajete from Austrian com pany Krajete GmbH explained why bio technological solutions still play a shad owy role in the production of energy carriers such as hydrogen, methane or biofuels such as ethanol or biodiesel: “As long as large, established energy compa nies continue to call the shots and are al lowed to continue operating their plants with a green coat – which have been fi nanced with decades of public money –the mild, nature-compatible processes of biotechnology will not be established. We have seen with nuclear energy that it is possible. Politicians must understand that we don’t have much time left to leave the oil era behind (...) to establish a new era with a sustainable energy mix and cycles copied from nature.” Measured in terms of their substitution potential it is certain ly worth taking a look at biotechnologi
cal processes in the energy sector. That’s because 80% of global CO2 emissions churned out by the G-20 countries are at tributable to the combustion of fossil re sources. Power generation, transport and industrial production remain nearly entire ly dependent on coal, gas and oil.
However, during the INDUSTRIA BIO TEC Energy Panel it became clear that wind, solar and hydropower, as well as the fermentation of residues into biomethane propagated by the European Commission and New Nexus Energy, will not be suf ficient on their own to satisfy the world’s steadily growing hunger for energy. Addi tional waste streams presented at the con ference, such as CO2-containing industrial waste gases or sugar-containing waste water, offer an interesting complement to biomass gasification in this respect.
To increase the efficiency of fermen tation of agri- or wood waste by anaero bic fermentation to biomethane, the 40% of CO2 produced as a by-product can be converted bacterially to almost 100% methane – provided hydrogen is externally added. The resulting biomethane can be be stored, and converted into heat, elec tricity, and mechanical energy (CNG)
Turning point for the biologisation of production
ALEXANDER KRAJETE CEO of Krajete GmbH, says the shift to biotech must happen now.

EuroBiotech _Why is the switch from fossil to biotech manufacture taking so long?
Krajete_Oil companies try to preserve their existing assets and use them for green technologies. For a complete transition to green technologies, they are lacking personnel since classic chemical engineers have no education al background in life sciences. Biologi sation of industrial production starts with mindset and the right compe tence. Economists and chemical engi
neers as drivers in big petrochemical cor porations need tools to change from a traditional chemical to a modern biological world. It is a process that requires re sources, dedication, pa tience and above all com mitment and genuity.
EuroBiotech _Where is the legislator in all of this?
Krajete_As a chemist I know that chemical products have polluted the whole world. The basic problem is that we have launched thousands of chemicals whose long-term impact on humans and the environment was unknown. REACH is
trying to shed some light on this topic. We need a global law to exit from fos sil fuels by rigorously clos ing oil wells. Only then will we compete under similar conditions with a surface carbon economy where only technology matters – politics and lob bying less so. As for now, petrochemical companies use prefermented resourc es while biotech compa nies start from scratch with CO2, meaning they’re heavily dis advantaged and unable to compete simply for energy reasons – no matter how good the technology is. L
62 European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © Krajete GmbH INDUSTRIA BIOTEC
with the latter the commercially most at tractive. All the experts on the energy pan el agreed that the cost-effective produc tion of green hydrogen – whether in the medium term through electrolysis in sunand wind-rich regions, phototrophic mi croorganisms or fermentation of waste streams – is the key to a new energy mix.
Acquiring green hydrogen
The problem is that more than 90% of the hydrogen gas available today is produced by steam reforming of fossil fuels and nat ural gas, and therefore does not offer a green alternative. According to Krajete, only about 5% of hydrogen is currently produced by electrolysis fed by regener ative energy.
“The sustainability of the methane pro duced therefore depends on how the hy drogen required for this purpose is made,” explained Juliana Rolf, process engineer at Münster University of Applied Sciences. “Hydrogen can be produced without elec tricity by dark fermentation, for example,” she added. Her team is currently scaling up a two-stage process to the 700-litre scale, and is looking for industrial partners to advance the process to industrial scale. In the first stage, H2, CO2 and – above all –acetic acid are produced without electric ity from sugar-containing wastewater from the food and textile industries through a mixed culture. In the second stage, it’s fur ther converted to CH4. Germany’s waste water alone contains 1.5 million met ric tonnes of organic carbon compounds each year.
According to Krajete, in the medium term the green hydrogen needed for bio methane could be best produced direct ly in wind- and sun-rich regions, where it could be converted very efficiently into CH 4 that is not explosive and therefore easier to transport, using high-pressure fermentation with archaea. He’s devel oped a corresponding industrial process.
However, panelists also reported pro gress at the biomass forefront. Volker Bau er, CEO of LXP Group GmbH, whose company has developed a process to ex tract cellulose and lignin from wooden residues to produce biofuels and chemi
cals, said the problem was to scale pro cesses up to cost-efficiency. “Companies will only invest €100,000 – €1bn, if they are sure to see a return on investment,” he underlined. Also on the panel was Thomas Brück, the Werner-SiemensChair of Synbio at the Technical University Munich. His team uses photosynthetic algae and yeasts to produce sustainable aviation fuel at a cost-competitive price of €1.20/l independently of agricultural land or large amounts of electricity. Brück stressed that “we need two things: scaleup facilities for scaling a process rapidly from demonstration to industrial scale as well as an appropriate regulatory frame work for timely market launch. The fact that we are miles away from this in Europe is the reason why innovation takes place primarily in the US.” Brück, Krajete and Bauer all called on policymakers to sup port innovations from industrial biotech and to stop old-boys clubs from the en ergy industry.

Green chemistry – what’s next?
In the Chemistry Panel, Tanja Meyer, Eu ropean coordinator at the Bio Base Europa Pilot Plant (BBEPP) in Ghent, Belgium, provided a model for scaling up indus trial biotech processes in Europe. “In our Multipurpose Bioprocess Hub, which was launched in 2008,” Meyer says, “we scale a wide variety of processes – from the 10-litre laboratory scale to the 75,000-litre scale – from chemical building blocks to proteins, surfactants and nutraceuticals.”
Demand has also changed at the German biorefinery hotspot Leuna, according to Christine Rasche, an expert on lignin from the Fraunhofer Centre for Chemical-Bio logical Processes: “More and more startups are asking for specifically designed production plants for scale-up,” she point ed out. One focus is on plastics, biobitumen, resin and aromatics production.
On the way to climate neutrality, the chemical industry is increasingly relying on “renewable carbon” from biomass, CO2 or recycling, explained Lars Krause from the nova-Institut (Hürth,Germany). This concept is being promoted at nova with the “Renewable Carbon Initiative”. 45 industry players have already joined it.
In the industry, chemical giant BASF is researching microbial surfactants in a publicly funded biosurfactants alliance, explained Kati Schmidt, Global Head of the firm’s Surfactants division. So far, the showcase project has only produced limit ed quantities, but industrial customers are showing great interest.
Waste as a resource
Microplastics in the environment, garbage gyres in the oceans, mountains of batteries – all signs that cradle-to-cradle and circu lar production are currently receiving lit tle more than political lip service. But at titudes are changing, and Martin Langer – the Chief Business Developer at BRAIN Biotech AG and moderator of the Waste Panel – spoke in the spirit of the new cir cular economy when he announced it
63 European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © BIOCOM AG INDUSTRIA BIOTEC
wouldn’t talk about waste, but about bio materials and resources that open up val ue creation. In the face of increasing geo political tension over access to rare metals and other resources needed for digital and eMobility, the panel looked at future ap proaches that promise to mitigate natural exploitation and damage caused by land filled or incinerated battery waste.
Because lithium, an alkaline earth metal widely used in batteries, is “the new gold” according to Gina Kuippers from BRAIN, its microbial recovery from the previous ly incinerated battery slag – along with cobalt and nickel – is a biotechnological gold-mine project for the 21st century. The current global annual battery production of 50,000 tonnes, according to Langer, is expected to rise more than tenfold “in the near future”. In a screening, Kuipers and colleagues identified more than 100 microorganisms that enable the selective extraction of lithium from the previously ground-up battery contents. The bacteria do not require any nutrient medium oth er than renewable feed residues or CO2 The commercialisation of battery recy cling will presumably be possible on a large scale by 2030 in the US. By then Volkswagen AG alone plans to sell 29 mil lion electric vehicles – a big reason why BRAIN has been working with Blue Whale Material LLC for eight years. Its CEO Rob ert Kang explained the advantages of mi crobial battery recycling over the exploita tion of natural deposits: “When we recycle batteries, it takes less exploitation of nat ural deposits.” Lithium extraction from South American sites or Australian ore mines by 314 lithium megafactories is ex pected to increase eightfold by 2030, ac cording to Kang. But natural deposits are limited and restricted to a handful of coun tries. “In terms of collecting used batteries, the US is a developing country,” Kang ex plained. “So we will set up collection cen tres and recycling points locally.” Scaling is expected to cut recycling costs in half. Kang reported that the US government has now recognised the strategic importance of lithium recovery, and has recently been “investing massive amounts of money” to create corresponding capacities. Could that provide a model for Europe?
Other recycling projects concern the mi crobial recovery of gold through bio leaching, according to Andreas Blatter, Head of R&D at the Swiss PX Group. Used printed circuit boards (WPCBs), are one source that can be exploited to further improve the recovery of the cov eted raw material. According to Langer, 950-000 tonnes of electronic waste con taining 150 ppm of rare metals are pro duced in Germany alone every year. The recovery of nitrogen, phosphorus, organ ic carbon and rare metals from wastewa ter also represents an economically in teresting material stream, explained Dirk Bogaczyk of the Emschergenossenschaft/ Lippeverband.
Thomas Haas from Evonik Industries AG is investigating the potential of artifi cial photosynthesis, which has been op timised compared to the natural model in the publicly funded Retikus project. In the project, the natural CO2 fixation ef ficiency of 1% is to be improved many times over in order to use CO2 to produce synthesis gas with the help of a bacterial mixed culture. Syngas can be further pro cessed into fine chemicals or biofuel, or used to generate energy. A pilot plant in Marl is already working on the prospect. However, Haas did not hold out the pros pect of scaling up at the conference.
Achieving capital injection
How industrial biotechnology looks through investors’ glasses was illuminated by the INDUSTRIA BIOTEC Capital Ses sion. The main message from Maximil ian Bade, founder of synthetic biology-fo cused Nucleus Capital AG, was: “Given the [gas and energy] crisis, I see the poten tial for industrial biotechnology companies based on their market promise to be high er than for ICT companies at the moment. There is no better time for a company that needs another two to three years to get to market than now, while market-ready products are having a hard time. Climate protection is a highly interesting move ment, and not just traditional life sciences investors are moving into this field.” How ever, he added that it’s important to wait until Series B/C financing to assess impact,
because 80-90% of portfolio companies fail. “Ten years after the first wave of in dustrial biotechnology and its frustrating experiences with biofuels, consumer in terest has fundamentally changed. Sus tainable products are in demand, but they still have to come closer to convention al products in terms of price,” explained Georg Lenzen, Marketing Director of b.value AG. According to Bade, the “dras tically changed openness of the young generation towards vegan and climateprotecting products” and political an nouncements about change are attracting investors – especially in the food sector.
Frederike Grosse-Holz, Scientific Direc tor of Blue Horizon Corp. AG, sees food ingredients as highly interesting, because they only make up 1-5% of products and therefore only have to be produced with manageable investments on a scale of up to 5,000 litres. The experts also saw sus tainable crop protection as highly interest ing in view of the loss of biodiversity.
Moderator Jowita Sewerska from the European Circular Bioeconomy Fund also glimpsed a great opportunity in the short er development time of only three to four years to market.
According to Bade, “countries that can not keep up with the speed of approval of novel foods and framework conditions are at a loss”. It took Finnish precision fer mentation specialist Solar Foods only six weeks to get its protein powder Solein, which is produced from CO2, approved as a novel food in Singapore. In the EU, such approvals take at least three years.
Investment into future markets
Given the EU’s political refusal to put sig nificant funding into scaling up biotech processes under Horizon 2020, awakening consumer interest in brands with demo plants can help, Lenzen believes, calling it “a way to establish products with im pact”. In terms of real transformation, ‘sig nificant’ in this context means around the scale of global annual spending on arma ments – which according to the Climate Policy Initiative currently runs at about US$2,000bn.
64 European Biotechnology | Winter Edition | Vol. 21 | 2022 INDUSTRIA BIOTEC
L t.gabrielczyk@biocom.eu
Industrial Biotech in focus: Toulouse White Biotech
TWB In terms of the bioeconomy, France has long since overtaken its neighbour Germany, which was the first country in Europe to come up with a national bioeconomy strategy. At least that is what the inventor of the EU bioeconomy told European Biotech magazine. A showcase cluster is Toulouse White Biotech, which helps start-ups to make processes ready for the market.
In the next issues of european B iotech no Logy magazine, we will be looking at Europe’s biotech clusters and their com panies in a loose sequence. The spec trum ranges from classical biopharma hubs to industrial biotech scaling cen tres and the last remaining EU agribiotech clusters. This time we turn our attention to Toulouse White Biotech –TWB for short – France’s flagship cluster for industrial or “white” biotechnology solutions, which are preparing to replace the fossil economy, provided that the de cision-makers get around to doing what they are supposed to: deciding wheth er they want to exploit even the last re sources or prefer to rely on nature-com patible cycles.
As emphasised at INDUSTRIA BIO TEC in Berlin in early October, it is not only the innovations themselves that play a major role, but above all the marketdriven scaling of biotech processes (see p. 58) – be it in the recycling of plastic residues, rare metals, etc., or tasty alter natives to meat and dairy products from factory farming through precision fer mentation (see interview p. 26), or in re ducing the toxicity and energy consump tion of chemicals such as dyes (see p. 66) or organic acids.
TWB Start-up Day
An annual showcase of achievements, which has enjoyed international popu larity since its start five years after TWB’s launch in 2012, is the TWB Start-up Day, with start-up pitches awarding services worth €50,000 to the winner.
This year, the growing pressure of cli mate change, readily available and novel decarbonisation technologies in various sectors attracted the attention of over 200 investors, CEOs and scientists at the meeting. Israeli company Ambrosia Bio and French start-up Dionymer won the “Fast track it!” pitch competition and the “Go for it!” competition among ten startup companies.
In the “Fast track it!” competition, Am brosia Bio beat four international start-ups in the development phase – Denmark‘s

Cellugy, Swiss Cultivated Biosciences, Aus tria‘s myBIOS, and Italy‘s REWOW. Am brosia Bio CEO Ziv Zwighaft pointed out the negative impact of industrial sugar on human health and emphasised that “sugar is the new tobacco”. The company‘s en zyme technology platform makes it pos sible to develop acid-stable enzymes that efficiently convert high glycaemic index sugars such as fructose in juices into rare, low-calorie sugars such as allulose, taga tose or allose, without compromising taste – a solution desperately sought by juice producers whose sales have plummeted by almost 40% in the last 15 years. Am brosia Bio received €50,000 for services related to TWB’s technology platforms and the communication facilities offered by Bi oeconomy For Change & Agri Sud-Ouest Innovation.
In the “Go for it!” competition for younger start-up companies, the French company Dionymer was successful with a process that converts grape waste into biodegradable bioplastics such as Poly hydroxyalkanoates (PHA). “Au revoir petrol” is the company‘s motto. It wants to contribute to carbon recycling. Dionymer‘s polyesters can replace petro leum-based compounds in a variety of applications.
“Consumers want to be sustainable,” stressed Rasmus von Gottberg, Genom atica’s chief growth officer, who currently sees a second wave of growth coming for industrial biotech solutions, at the event. “In our sector, we cannot drive our busi ness alone, we need partners to finance our technologies.” TWB leads the path. L
65 European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © TWB CLUSTER REPORT
t.gabrielczyk@biocom.eu
Ziv Zwighaft, CEO Ambrosia Bio, and Olivier Rolland (right), Executive Director, TWB
TWB, an original model supporting the bioeconomy
(TWB)
Rolland,
With industrial biotechnologies as its core expertise and collaboration as its strategy for sustainable materials, TWB is celebrating its 10 th year anniversa ry this year. Since its creation in 2012, TWB has been perfecting an original, unconventional model in the field of in dustrial biotechnology. Its secret weap on is collaboration through a flourishing public/private ecosystem that brings to gether all the stakeholders involved in the bioeconomy value chain. Leverag ing this ecosystem, it has been success fully supporting the development of in novative solutions, among them: plastics degradation and regeneration, biocon trol solutions or adhesive biomaterials.
Evolving in the dynamic French biotech field, which is strongly promoted by the government, TWB has managed to gath er all the ingredients needed to become one of the major biotech actors globally.


Speedy public-private partnership
TWB covers a wide range of fields, from chemistry, materials and energy to agri/ agro sectors, nutrition, and well-being. To accelerate R&D projects, TWB creat ed in 2012 a public/private consortium, which accounts today for 51 members including 35 industry players (start-ups, SMEs, large corporations), 4 investment funds, 3 research and higher education
national institutes, and local authorities. This consortium was designed to pro mote innovation and stimulate its incor poration into industry-ready solutions between entrepreneurs, laboratories and industrial companies, according to an easy-to-use contractual framework. Since 2012, 285 R&D projects have been con ducted by TWB and its partners and re sulted in significant advances, both in the form of incremental industrial applica tions and disruptive technologies. For in stance, TWB has been collaborating with Carbios SA on the end-of-life of plastics: Carbios’ process will be soon integrated into an industrial plant to process 50,000 tons of PET waste annually, or with Resicare (Michelin Affiliate) and Leaf to develop a new adhesive resin free of substances of very high concern. Thanks to its highly automated, cutting-edge fa cilities, along a continuum of knowledge from gene to product, TWB has been able to support the development of al ternative, innovative and sustainable bio logical solutions.
Catalysing future innovators
It is in TWB’s DNA to foster innova tion. In ten years, TWB invested €11m in more than 40 high-risk /high-reward projects, which fed ten product devel opment pipelines and also triggered the creation of two start-ups. Nine startups have been hosted on its premises, among them: Pili, producing sustainable dyes and pigments to reduce the envi ronmental footprint of the colour indus
European Biotechnology | Winter Edition | Vol. 21 | 2022 CLUSTER REPORT 66
Pictures: TWB / Jérémie Lortic
INDUSTRIAL BIOTECH Toulouse White Biotech
is a good example to how scaling and collaboration through production partnerships can help the industrial biotech sector to satisfy the urgent need to speed up and scale sustainable processes for industrial production.
› Olivier
Managing Director at TWB and France 2030 Ambassador
try; and Micropep, developing innovative biological crop protection solutions based on micropeptides. TWB has contributed to the growth of numerous start-ups that have raised a total of more than €250m. This year, TWB directly employs 90 peo ple, with an additional 45 staff members working for the hosted start-ups.

To further support the start-up ecosys tem, TWB designed its own internation al event in 2018, which now takes place every year: TWB START-UP DAY, dedi cated to entrepreneurs who want to ex pand their business activity, investors looking for new projects, and interna tional stakeholders in the field of indus trial biotechnology. Two pitch contests are open to entrepreneurs. Among the winners: UK based start-up Better Na ture (tempeh fermentation), Spanish start-up Zymvol (high-throughput virtu al screening, in-silico design of enzymes) and 2022 winners Israeli start-up Ambro sia Bio (production of low-calorie sugars) and French start-up Dionymer (transfor mation of organic waste into biodegrad able polymers).
A thriving French ecosystem…
As a circular bioeconomy player, France is well-endowed with biomass resourc es, significant forest and a powerful ag riculture sector, being a major produc er of cereals and sugar beet. In 2014,
France was the single largest (18% of to tal EU production) producer of agricul tural products in the EU.
Through a cooperative system and with several large agro-industrial players, such as Limagrain or Vivescia, France provides a solid basis for biomass pro duction, while internationally-renowned companies such as Lesaffre or Roquette support biomass-based manufactur ing. The country also benefits from the leadership of B2C large players such as L’Oréal, which is targeting that, by 2030, 95% of its ingredients will be derived from renewable plant sources, abundant minerals, or circular processes. To sup
port such ambitious goals, France en joys a well-organised R&D ecosystem, including national research institutes such as INRAE, CNRS and CEA, techni cal universities (INSA), incubation struc tures (Genopole), investors (Sofinnova Partners, Elaia, Truffle Capital), as well as a whole host of innovative start-ups get ting industrial (Metabolic Explorer, Glob al Bioenergies, Afyren, etc.).
… Supported by the government
The role of biomass has been public ly recognised as a key factor in the post-pandemic relaunch of the French economy. It will play a central role in helping France to green its economy and achieve ambitious targets regarding re ductions in greenhouse gas emissions and the improvement of overall sus tainability. Between 2010 and 2020, the government invested more than €500m alongside industrial companies. Since the pandemic, France has stepped up its ef forts and promoted a policy geared to wards the bioeconomy, as illustrated by the €54bn France 2030 plan that aims at decarbonising the economy and support ing emerging innovation players.
With a thriving ecosystem and a supportive government, France has all the ingredients to become one of the globally leading actors, not only in the industrial biotechnology sector. L

68 European Biotechnology | Winter Edition | Vol. 21 | 2022 CLUSTER REPORT
Pictures: © TWB / Jérémie Lortic
WE MOBILIZE CAPITAL FOR TRANSFORMATION





Innovative technology is the key to shifting from a fossil-based to a bio-based circular economy, crucial for achieving the European climate targets.




As a venture capital fund, our primary goal is to invest in scale-up companies with high potential for excellence on a pan-European or global scaledelivering both impact and financial returns at the highest level.
Our entire experience in venture capital and our investment scope spanning the circular economy and bioeconomy is focused on this purpose.
www.ecbf.vc
Transform our future with us!
Supported by:
Investors:
Brain transport
LYSOSOMAL STORAGE At the beginning of November, BioArctic AB has initiated a project with their Brain Transporter technology on enzyme re placement therapy to fight Gaucher’s disease. Gaucher’s disease is current ly an untreatable hereditary autoso mal recessive neuropathy in which the reduced function of the enzyme glu cocerebrosidase leads to a build-up of glycolipids in certain organs. Conse quently, these organs enlarge and can be restricted in their function and –more rarely – the disease comes with neurological implications like seizures or cognitive impairment. “Using our ex pertise in neurological disorders, joint with our Brain Transporter technology platform, we hope to be able to change that and help these patients,” said CEO Gunilla Osswald. While enzyme replace ment therapy is today’s care standard, it does not treat neuropathic conditions since the enzymes are unable to pass the blood-brain barrier and thus never reach the brain. By combining the prin ciple of enzyme replacement with their Brain transporter, BioArctic plans to by pass the blood-brain barrier and create a unique treatment possibility for patients with neuropathic Gaucher disease.
However it is a single-in-class ap proach, and there is competition: USbased AVROBIO Inc. has created a sin gle-shot gene therapy, code-named AVR-RD-02, that is currently in Phase I/II clinical development to halt or even pre vent Gaucher disease type 1. L
Novo expands pipeline
INVESTMENT Novo Nordisk A/S has inked a $700m licensing agreement with Ventus Therapeutics in late Septem ber to advance the development of pe ripherally restricted NLRP3 inhibitors for a broad range of diseases. According to the agreement, Novo Nordisk will make an initial payment of $70m to Ventus. Ven tus will be eligible for up to $633m in ad ditional clinical, regulatory, and commer cial milestone payments, as well as for tiered royalties, if a therapeutic devel oped through the collaboration will make it to the market. Novo Nordisk’s experi ence in cardiometabolic disorders will be combined with Ventus’ NLRP3 inhibitor candidate, VENT-01, which is expected to enter the clinical trials next year, as well
as other backup compounds in for exam ple nonalcoholic steatohepatitis.
The pattern recognition receptor NLRP3 is linked to conditions in which inflammasome-mediated processes are a major cause of pathology such as fi brotic, dermatological, rheumatological and neurological diseases. An overshoot ing immune response can be suppressed by targeting NLRP3. Nonalcoholic stea tohepatitis and chronic kidney disease are among the targeted conditions since evidence suggests that blocking NLRP3 calms the inflammation that causes liver fibrosis. The drug industry is desperately waiting for such a development and ac cording to Novo Nordisk, Ventus is offer ing a promising prospect.
Award for stem cell therapy
NLS DAYS 2022 MyoPax has won this years Nordic Life Science Days’ NLS Invest award. At the end of September, 40 Nordic start-ups had the opportuni ty to pitch their unique solutions to an audience of investors and conference delegates in Malmö as part of the con ference’s investment track NLSInvest. MyoPax Denmark ApS presented its platform for regenerative stem cell thera
pies that target the treatment of multiple muscle diseases and restoration of mus cle function, and was awarded the first prize, which included SEK50k. Their pat ented procedure allows them to extract primary muscle stem cells, repair the ge netic defects in them, multiply the im proved cells and regenerate the tissue needed for replacement implantation in the patient.

70 NORTHERN EUROPE European Biotechnology | Winter Edition | Vol. 21 | 2022
Picture: © Camille Sonally/NLSDays.
Verena Schöwel, CEO and co-founder of MyoPax, receives the NLSInvest Award at NLSDays 2022. From the left: Chelsea Ranger, Verena Schöwel, Frida Lawenius, Stephan Gauldie, and Ulrica Sehlstedt.
L
Plant-based food initiative
to solve some of the complex issues as sociated with developing plant-based foods. The focus will be to create a li brary of basic knowledge of the proper ties of crops and how they could poten tially substitute food products that are straining global recourses, like meat. It is an open collaboration platform, meaning that the research will be made publicly available, intending to create a fast-grow ing pool of market-ready plant-based food options.
COPD study
FOOD Plant2Food, a new collabora tion platform, has been launched in Den mark aiming at creating opportunities for the vegan market to expand and the CO2 emissions to be reduced. The platform is hosted by the Aarhus University, the University of Copenhagen, the Technical University of Denmark, and Wageningen University and Research in the Nether lands. The list of partners is completed with the Food & Bio Cluster of Denmark, a national network of organizations aim ing at a more sustainable and climateneutral green transition.
Plant2Food serves as a hub for re searchers and companies to collaborate

The Novo Nordisk Foundation an nounced its support of the newly launched Open Innovation in Science platform at the beginning of November. They are awarding this collaborative re search effort up to €27m over the next five years. “We need to rapidly develop foods that can feed a growing world pop ulation without over-utilising the planet’s resources.” said Claus Felby, Senior Vice President of Biotech at the Novo Nordisk Foundation. Plant2Food creates a unique opportunity to do it. That way the foun dation has ensured a spot in the evergrowing million-market of plant-based foods and shown its support for more sustainable nutrition.
Nordic Nanovector: buy or die
MERGER Nordic Nanovector ASA in early November swallowed APIM Thera peutics in the hope of avoiding bank ruptcy. After a financially strained few months, blood cancer specialist Nordic Nanovector might have found the floatie it needed. The setback from July, where the PARADIGME study of Betalutin, an anti-CD37 antibody radionuclide con jugate was terminated after phase II tri als due to recruitment problems, left the company with a slew of early-stage anti-CD37 candidates. A merger with APIM, which is also focused on oncol ogy research, appeared to be a way out of the crisis. Kostas Alevizopoulos, CEO of APIM, is going to take over as CEO of

the combined entity, which will soon be renamed and hopes to retain the public listing on Oslo Børs. Approximately 76% of the merged company will be owned by APIM shareholders.
Inflammation specialist EpiEn do Pharmaceuticals ehf received reg ulatory approval for its Phase IIa tri al of Barriolide™ (EP395) in patients with Chronic Obstructive Pulmo nary Disease (COPD). The study is conducted at the University of Man chester for the Icelandic company. Its lead molecule EP395 is an oral mac rolide with reduced antibiotic resist ance risk. With COPD being the third leading cause of death, EpiEndo takes a new approach to drug development to mitigate the symptoms of chronic respiratory diseases since Barriolide™ seems to restore the epithelial barri er insufficiency that is believed to be a key driver of COPD-induced in flammation. Today, COPD manage ment relies mainly on inhaled drugs, including long-acting adrenoceptor agonists (LABA), long-acting mus carinic receptor antagonists (LAMA), and corticosteroids (ICS).
A piece of food history
Finnish industrial biotech special ist Solar Foods Ltd has received its first novel food authorisation in Singa pore for Solein, a microbially generat ed protein powder made from CO2, O2, a few nutrients and green hydro gen and electricity. According to the company, the vegan amino acid mix can replace dairy and meat protein. Solein seems to bring the unprece dented advantage of being detached from traditional agriculture, and is now produced in fermenters taking about 0.1% of land and 1% of the wa ter needed for the production of the same amount of beef. Production is expected to start in 2024. The com pany claims it will revolutionise food production.
71 NORTHERN EUROPE European Biotechnology | Winter Edition | Vol. 21 | 2022 Pictures: © karriezhu pixabay.com; © Picography/pixabay.com
L
NEWS
No pesticides
FEASABILITY STUDY Belgian protein-based plant protection compa ny Biotalys NV and Danish Novozymes A/S announced in November, that Evo ca™, Biotalys’ first proprietary biofungi cide, offers potentially significant cost of goods and scaling advantages. “Thanks to its leading protein fermentation expertise, Novozymes has increased the efficien cy of production for Evoca,” said Patrice Sellès, the CEO of Biotalys. “We will now work on a deeper partnership with Novo zymes and explore strategic supply and commercialisation agreements for the fu ture generation of Evoca while continuing our ongoing internal development activi ties.” Evoca is Biotalys’ first protein-based biofungicide that might replace chemical pesticides. Evoca helps sustainably control economically important fungal diseases such as Botrytis and powdery mildew in fruits and vegetables. Evoca is expected to obtain approval from the US Environmen tal Protection Agency (EPA) in early 2023. Since entering into a partnership in June 2022, Novozymes has explored routes for the upscaling and production of the bioac tive protein using other production strains to those currently used by Biotalys. Sub ject to confirmation of commercial scaleup, field trial performance and regulatory procedures, these results may help reduce time to a commercially attractive version of Evoca. The companies are now enter ing into the next phase of their partnership and will explore strategic supply and com mercialization agreements for the future generation of Evoca. L
Innovation price winner

DRUG DEVELOPMENT At the beginning of November, human cell model developer Mimetas BV won the Dutch Innovation Award 2022. The 2013 spin-off from University Leiden was se lected by an expert company based on the Amsterdam Center for Business In novation’s monitor and a public vote. The company’s miniaturised organs-ona-chip technology OrganoPlate ® is be ing used to reduce animal experimen tation in pharmaceutical drug discovery and development whenever cell-based assays can replace human-relevant dis ease models. Based on its disease assays, Mimetas has grown into a multination al company with operations in Europe, Asia, and the USA that collaborates with the top 50 global pharmaceutical com panies.
L
Bones and inflammation
M-RNA Belgian Bone Therapeu tics SA has merged with French inflam matory/autoimmune company Medsenic SA in a €40m deal and changed its name into BioSenic. The merger will expand Bone Therapeutics’ allogeneic cell ther apy-based product portfolio in orthope dics by drugs to treat systemic autoim mune diseases, such as cGvHD (chronic Graft versus Host Disease), lupus erythe matosus, and sclerosis.
Under the deal, Bone Therapeutics will bring in its bone marrow-sourced mes enchymal stromal cells (MSCs), which can be stored at the point of use in hospitals. French Medsenics’ arsenic TriOxide (ATO) platform is best de scribed by a double basic effect on im mune cells: firstly, an increase in oxida tive stress in activated B, T or other cells of the innate/adaptative immune system to the point they will enter apoptosis, secondly, a boost on several pro-inflam matory cytokines involved in inflamma tory or autoimmune cell pathways. Un der the deal, Medsenic‘s shareholders
have contributed 51% of the total out standing share capital of Medsenic, val ued at €40,800,207, at a subscription price per share of €0.45, which values Bone Therapeutics at €10m. In exchange for the in-kind contribution of 51% of Medsenic‘ shares, 90,668,594 shares were issued by BioSenic to Medsenic shareholders. BioSenic will maintain its status as a Belgian-listed company.
A Phase III study of cGvHD is current ly anticipated to start in H1/2023 follow ing the Phase II clinical study with arse nic trioxide in the first-line treatment of chronic GvHD-positive results. A Phase IIa clinical trial for Lupus had previous ly confirmed safety for patients and effi ciency in the course of the autoimmune disease: A Phase IIb clinical trial for se vere Lupus is in planning stage. Also, preclinical work gives good grounds for a Phase II clinical trial on systemic scle rosis. A Phase IIb trial of ALLOB, a study in patients with high-risk tibial fractures, is still ongoing and set to report impor tant interim results in the H1/2023.
72 WESTERN EUROPE European Biotechnology | Winter Edition | Vol. 21 | 2022
L
Picture: Mimetas
Mimetas’ management team
Advanced stratification
NEUROLOGY Precision neuro science company Stalicla SA has licensed SFX-01 from British Evgen Pharma plc in neurodevelopmental disorders and schiz ophrenia. The Geneva-based precision neuroscience company, which is advanc ing the first precision medicine platform (DEPI) for patients with neurodevelop mental disorders, announced the first ap plication of the licenced compound will be in a biologically-characterised sub group of patients with Autism Spectrum Disorder (ASD). Under the agreement, Stalicla AS is going to pay $0.5m upfront and milestones of up to $160.5m in re lation to the first indication. The Swiss company will grant low to medium dou
Strategic deal
UBIQUITINYLATION Cambridgebased PhoreMost Ltd. has entered a multitarget collaboration with PROTACs leader Arvinas Inc. that investigates two target ed protein degraders (ARV-110 and - 471) in Phase II studies. Under the agreement, PhoreMost will deploy its Siteseeker ® platform to find candidates homing in on multiple therapeutic targets in oncology and neurodegeneration. The British com pany will receive research funding and will be eligible for pre-clinical, clinical, and commercial milestones. PhoreMost’s Protein Interference technology probes the entire proteome in a cell environment
ble-digit percentage royalties on sales of SFX-01, if approved. Since its foundation in 2017, the company has raised $31m to advance DEPI and its pipeline. Aggre gating molecular data and human genet ic information, DEPI has already reached validation through the clinical identifica tion of biologically defined subgroups of patients with ASD. SFX-01 is a patented composition of sulforaphane and alphacyclodextrin. Thanks to DEPI, Stalicla identified SFX-01 as the best treatment candidate for its ASD-Phenotype 2 sub group of patients. SFX-01 in-licensing fol lows the completion, in early 2022, of Phase Ib for STP1, Stalicla’s lead candi date tailored to ASD-Phenotype 1. L
NEWS
RNA investment
for novel druggable targets linked to any chosen disease, using the vast shape di versity of proprietary miniprotein libraries. The companies did not announce any fi nancial details of their deal.
A major advantage of targeted pro tein degraders is that they can mark tar get proteins with ubiquitin for destruction through the cell’s protein-shredding pro teasome. However, some tumours have been reported to become resistant to ubiquitin E3 ligases that are linked to pro tein-binding moieties of a targeted pro tein degrader (see european BiotechnoLogy magazine, Autumn 2022). L

Belgian CDMO Univercells SA has raised €44m through the first close of its Series D financing round led by new investors includ ing 3d investors; Global Health In vestment Corporation, and In-Q-Tel. In addition, existing public institu tional funds such as SFPI-FPIM (So ciété Fédérale de Participation et d‘Investissement), SRIW (Société Régionale d‘Investissement de Wall onie) and the Amerigo Fund, as well as Adjuvant Capital, AdBio Partners, and TheClubDeal participated. The proceeds will mainly support the de velopment of Quantoom Bioscienc es‘ RNA platform, the commercial expansion of Exothera‘s Jumet-based infrastructure, and the global growth of the Univercells Group.
Boost for epigenetics
Mablink Bioscience SA has bagged €31m in a Series A round led by Sofinnova Partners and Mérieux Equi ty Partners to bring its ADC MBK-103 into clinical trials. Mablink’s masked linker provides a longer ADC half-life.
Uncovering ncDNA
Cambridge-based Enhanc3D Genomics Ltd has raised £10m in a Series A financing led by BGF and Parkwalk Advisors. The company said it will use the proceeds to ac celerate the development of its Gen Link3DTM platform that allows de coding the 3D structure to uncover the non-coding genome and identi fy novel biomarkers for patient strat ification and new drug targets for cancer, ageing and autoimmune con ditions.
PhoreMost’s platform has been deployed to identify completely new E3 ligases.
73 WESTERN EUROPE European Biotechnology | Winter Edition | Vol. 21 | 2022
Picture: © Phoremost Ltd
Roche’s failure may reevaluate the Alzheimer space Spatial biology
FINANCING German Resolve Biosciences secures US$71m in funding and screws a total amount raised for this young company up to more than $100m.

The funding follows the launch of its technology for ultra-high-resolution im aging of subcellular, single-molecule and spatial biology. Called “spatial omics technology”, these analytical methods combine diverse molecular insights into a whole picture on a section of a cell, tis sue or whole (smaller) organism.
“Spatial biology is widely regarded as the most significant new field in the life sciences,” said Laura Furmanski, PhD, Partner and Head of Patient Square In sights at Patient Square Capital (Califor nia). “Resolve opens the door to a wave of scientific discoveries that will redefine our understanding of disease and lead to new therapies.”
In recent months, the workflow has been installed in a growing number of leading European and North American laboratories, including the VIB (Vlaams Instituut voor Biotechnologie) in Bel gium, the Novo Nordisk Foundation Center at the University of Copenhagen, the German Cancer Research Center (DKFZ), the Stanford Medicine Depart ment of Genetics and the European Spa tial Biology Center in Belgium.
Patient Square Capital co-led the Se ries B financing. It also includes par ticipation from EDBI, Alafi Capital and NRW.BANK as well as other existing in vestors. “Spatial omics” was described in detail in the summer issue of european BiotechnoLogy
NEURODEGENERATION The be ta-amyloid theory in Alzheimer’s dis ease took another hit when Roche an nounced that gantenerumab failed to meet the primary endpoint in two Phase III studies. While the stock of cooperation partner Morphosys (Planegg, Germany) dropped by 30%, Roche only had to en dure a small setback. In contrast, several of Roche’s competitors, including Biogen, Cassava Sciences and Eli Lilly saw their stocks rise as a result.
Gantenerumab, a fully human monoclonal IgG1 antibody that targets and binds beta-amyloid aggregates such as plaques, fibrils and oligomers, slowed clinical decline by 8% compared to pla cebo in the GRADUATE I study and 6% in GRADUATE II.
In the opposite corner and benefiting from Roche’s woes is lecanemab, being developed by Biogen and Eisai – and orig inating from Swedish BioArctic –, which,
in the Phase III CLARITY AD trial, re duced clinical decline by 27% compared to placebo after 18 months, based on the Clinical Dementia Rating-Sum of Boxes (CDR-SB) assessment.
For many experts, this is further proof that not all drugs that attack the same tar get are identical, the brain leaving space for nuances in how drugs work which need an active immune system to clear amyloid proteins and reduce inflamma tion. Biogen is now in a unique posi tion, having for all intents and purposes, passed the threshold of demonstrating ef ficacy on a large phase three study.
Eli Lilly’s donanemab has yet to yield Phase III data, expected in mid-2023. De pending on the results, donanemab could be the only competitor to Biogen, whose aducanumab (Aduhelm) struggled just months ago because of Amyloid-related imaging abnormalities (ARIA) as primary safety concern.
Golden tickets by Novo
CENTRAL EUROPE European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © BioLabs Heidelberg
news
M agazine.
74
ACCELERATION Johannes Backs & Eva van Rooij (Revier Therapeutics, middle), as well as Heiko Weyd, Peter Willinger and Björn Cochlovius (Medraxa Therapeutics, sitting right) were winning the Golden Tickets awarded by Novo Nordisk to have one year of free participation in BioLabs/BioLabs Heidelberg, Ann-Kristin Mueller (mid, BioLabs Heidelberg) celebrating both new tennants. L
Founded to reprogram
REJUVENATION Apollo Health Ventures and Paul Scherrer In stitute (Zurich, Switzerland) established Focal Biosciences, a company aiming to use the expertise of scientific found er Prof. G.V. Shivashankar to break new ground in age-related diseases.
Founder Prof. G.V. Shivashankar`s goal is to redefine the treatment of age-relat ed diseases by “reprogramming” deregu lated cellular states that drive the harmful processes underlying many serious dis eases of old age, thereby restoring them to their original healthy state. Focal Bi osciences is present at three locations:
Villigen (CH), Boston (USA) and Berlin (D). Shivashankar researched mechanogenomic mechanisms underlying cell ageing. This can be understood as the three-dimensionality of cell architecture, which is clearly different in young and older cells.
Likewise, the researchers were able to show how ageing cells can be partial ly reprogrammed and rejuvenated us ing non-genetic methods for robust ther apeutic interventions. The method uses knowledge of how to change the me chanical limitation of cells in the sur rounding tissue.
Roche enters the arena
Hookipa Pharma announc es a strategic collaboration and li censing agreement with Roche to develop a novel immunotherapy for KRAS-mutated cancers based on its proprietary arenavirus plat form. The Vienna-based compa ny stands to receive (performancebased) milestone payments of up to $930 million. This is Hookipa’s first licensing agreement in the oncolo gy space, Jörn Aldag, Chief Execu tive Officer of Hookipa comments: “This collaboration validates the po tential of our arenavirus platform.”
Female entrepreneur
Valneva: it isn’t over yet
INFECTION There’s life in the old dog yet, and that also applies to the company with the Covid denatured vac cine (“Tot-Impfstoff”), which no one wanted anymore: Valneva SE.
The French-Austrian company Valneva SE (Nasdaq: VALN; Euronext Paris: VLA) had even to increase its offering price for the issuance of 21 million ordinary shares (including 375,000 American Depositary Shares “ADSs”) from the previously an nounced amount of $40m to a whopping $99m as a result of strong demand. The reason for the unexpectedly large influx is Deep Track Capital, a new sharehold
er of the company, which has agreed to purchase a total of approximately 50% of the global offering.
Most recently, Valneva has tended to pull back on the COVID-19 vaccine, but in other indications, development has been better recently, not least due to the cooperation with Pfizer on Lyme disease, where 50% of the net proceeds from the global offering shall be put, about 40% to fund the development and commer cialisation of its Chikungunya virus vac cine candidate (VLA1553). The financial situation is excellent with cash and cash equivalents of €336.2m.
In the competition for the Aus trian Founder’s Prize PHOENIX, the winners of the various categories were chosen in November, among them two representatives of the Aus trian biotechnology scene: Vienna Textile Lab and Myllia Biotechnolo gy. The winner of the 2022 “Female Entrepreneur” category is Dr Karin Fleck, co-founder of Vienna Textile Lab. The company produces biodyes from naturally occurring bacte ria to provide the most sustainable, healthy and environmentally friendly alternative to conventional synthet ic dyes. There are already some co operations with textile companies. Myllia Biotech is working on CRIS PR/Cas as a plattform technology for single-cell modifications.
Liquid biopsy
Hedera Dx (Lausanne, Switzer land) receives €14m in seed capital to accelerate the global roll-out of its liquid biopsy sample analytics.
CENTRAL EUROPE European Biotechnology | Winter Edition | Vol. 21 | 2022
Picture: © PSI, Laboratory of Nanoscale Biology (LNB) Zurich
NEWS
75
HMF3a cell changes actin structure after treatment with TNFalpha
EIC funding
AMR The European Innovation Council (EIC) will fund the start-up Immu nethep under the umbrella of its EIC Ac celerator programme. Immunethep, which was founded in 2013 as a spin-off of the University of Porto, will receive a €2.5m grant initially, and it is eligible for addition al equity investments up to €17.5m. The company will use the funds to advance its Paragon Novel Vaccine (PNV) to firstin-human clinical trials. PNV could pro vide protection against bacterial infections caused by 5 different bacteria: Staphylo coccus aureus, Streptococcus pneumoniae, Klebsiella pneumoniae, E. coli, and Strep tococcus agalactiae. It works by neutralis ing an extracellular form of bacterial GAP DH, without blocking the human GAPDH. This will enable the host immune system to fight the infection and eliminate the bac teria. If successful, PNV will become the world’s first vaccine preventing multiple bacterial infections for all its invasive sero types, including multi-resistant strains. Immunethep is one of the 75 innovative startups selected in this EIC Accelerator cut-off among more than 1000 applica tions from all over Europe.

Undruggable no longer
FINANCING Verona-based Sibylla Biotech closed a €23m Series A financ ing round in October. The spin-off from the Italian National Institute for Nucle ar Physics, the University of Trento and the University of Perugia plans to use the funds to advance its Pharmacological Protein Inactivation by Folding Interme diate Targeting platform. The company‘s small molecule degraders target a pro tein that transiently appears during the process of protein folding and was un druggable using other pharmacological approaches. The proceeds of the Series
GMP go ahead
PRODUCTION ReiThera, a Romebased biotech specialised in developing products based on gene delivery technol ogies for advanced therapies, has opened the new €15m production area at its Cas tel Romano Technopole facility. The ex pansion will serve the large-scale produc tion of viral vectors for vaccines and gene therapy. Designed to meet the growing global demand for viral vector produc tion, the new facility is able to accommo date GMP productions based on Adeno virus (Ad), Adeno-associated virus (AAV) and Lentivirus starting from 50 litres up to a maximum of 3,000 litres.
A will allow the company to build out its pipeline of therapeutics by exploiting the protein folding simulation platform, as well as to further expand its technol ogy to a range of therapeutic areas. Sib ylla currently has several preclinical can didates under evaluation.
The financing was led by V-Bio Ven tures with participation from Seroba Life Sciences, 3B Future Health Fund, Claris Ventures, CDP Venture Capital with Evo lution Fund, VI Partners, Indaco Venture Partners, as well as the company’s seed investor, Vertis SGR.
First-in-class
FUNDING The European Investment Bank has provided Palma-based Sanifit with €20m in financing to support the de velopment of new treatments for vascu lar calcification. The disorder, an accumu lation of mineral in the vascular system, is associated with cardiovascular disease mortality, and there are currently no treat ments. Sanifit has developed SNF472, a selective inhibitor of hydroxyapatite crys tallisation, the final common pathway that leads to vascular calcification. The EIB is advancing funds for this project through a venture debt operation with Investment Plan for Europe support.
Fast track for Minoryx
LICENSE AGREEMENT Barcelonabased late-stage biotech Minoryx Ther apeutics has teamed up with speciality pharma company Neuraxpharm for the commercialisation of its lead candidate leriglitazone. Neuraxpharm will pay Mi noryx up to €258m in upfront and mile stone payments for the exclusive Euro pean rights to the novel, brain penetrant and selective PPAR gamma agonist. The two companies will also collaborate in the further development of leriglitazone,
which is under development for the treat ment of X-linked adrenoleukodystrophy (X-ALD) and other orphan CNS diseas es. It has been granted orphan drug sta tus from the EMA and the FDA as well as fast track and rare pediatric disease desig nation from the FDA for the treatment of X-ALD. Just two months earlier, Minoryx had announced that the EMA had validat ed the filing of its Marketing Authorisa tion Application for the treatment of adult male patients with X-ALD.
76 European Biotechnology | Winter Edition | Vol. 21 | 2022 SOUTHERN EUROPE Picture: © Immunethep (top)
The Immunethep team
Asabys launches €200m fund
FUNDING Barcelona-based venture capital firm Asabys is launching a new €200m biotech and healthtech fund. With it, Asabys aims to cement its presence as a leading venture capital firm in the health innovation space in Southern Europe.
The new fund, named Sabadell Asabys Health Innovation Investments II (SAHII 2), will close around the end of this year, with commitments from institutional and pri vate investors. SAHII 2 will invest in dig
ital health, medtech and biotech compa nies, developing disruptive solutions with solid scientific evidence to improve patient outcomes.
Asabys was founded by Josep Ll. San feliu and Clara Campàs in 2018 with the support of Banc Sabadell as anchor inves tor and Alantra as strategic partner. Banc Sabadell will also be the main investor in the newest fund. So far, Asabys has invest ed in 13 companies (1 exited) in the bio pharma, medtech and digital health sec tors. “Asabys was born with the mission to invest health innovation across the board and the reality is that the space has been converging technologically, creating count less opportunities to solve the challenges that medicine has nowadays by integrat ing biology or pharmacy with nanotech nologies, digitalisation or artificial intel ligence,” commented Sanfeliu. Campàs added: “With this second fund, we will continue investing in the creation of new companies, as well as consolidate existing ones, with the goal to transform disruptive science into real solutions for patients.”

Keep-out areas in the genome
RESEARCH Cell toxicity and genome instability: depending on the target ed spot of the human genome, CRISPR gene editing can have unwanted effects, IRB Barcelona researchers have found. The scientists in the Genome Data Sci ence lab detected 3,300 targeted spots of the human genome that show strong toxic effects. The report, published in n ature c o MM unications (DOI: 10.1038/ s41467-022-32285-1), finds that around 15% of the human genome contain at least one toxic editing point. This unwanted effect is mediated by the tumour suppressor protein p53 and is determined by the DNA sequence near the editing point and various epi genetic factors in the surrounding re gion. The gene editing procedure could favour cells with defective p53 func
tioning, which are prone to accumu lating further mutations, thus increas ing the risk of developing malignancies. “Our work addresses an important issue with TP53-associated toxicity of Cas9, which was a matter of some controversy recently, and it also provides guidelines on how to sidestep the problem,” group leader Fran Supek explains. “Avoiding editing in these “risky” spots would not only make CRISPR editing more effi cient but, more importantly, safer.”
Seed financing
Nanoligent SL, a Spanish biotech company specialised in the devel opment of cancer treatments based on unique protein conjugates, has completed a seed financing round of €2.8m. The final closing of the round consists of €1m investment by i&i Bi otech Fund I, an early-stage Life Sci ence fund with teams in Luxemburg and Prague which is backed by the European Investment Fund. i&i Bio joined previous Nanoligent investors, Italian Angels for Growth, the largest network of business angels in Italy, and AVANTECA Partners, a Swiss pri vately held asset management firm.
Orphan drug plant
Pfizer and Sobi inaugurated the construction of a new high-tech pro duction plant in Savski Marof, totaL croatia news reports. The two com panies are investing €100m into the plant and are planning to export in novative biological drugs for rare diseases starting in 2026. The plant, which is scheduled to be construct ed by 2024, will spread over 4,000 square metres on 11 hectares of land.
Boosting efficacy
The ”la Caixa” Foundation is funding an IRB Barcelona project seeking to boost the efficacy of im munotherapies for the treatment of cancer through machine learning. La Caixa is providing €1m under the umbrella of the 2022 CaixaResearch call for Research in Health, which has awarded a total of €23.1m to 33 promising biomedical and health pro jects undertaken in research centres and universities in Spain and Portugal.
77 European Biotechnology | Winter Edition | Vol. 21 | 2022 SOUTHERN EUROPE Pictures: © Asabys (top), Nathan Devery com/Adobe Stock (bottom)
NEWS
Asabys founders Josep Ll. Sanfeliu and Clara Campàs
BioEa(s)tsUP
BIOECONOMY At the Projec2Project event organised by EuBioNet, running and accom plished projects in bioeconomy (BI OEASTsUP, BE-Rural, BioCircular Cities and LIFT) were matched with thematically related projects that were launched in summer/autumn 2022 (ShapingBio, CEE2ACT, BIOMODEL 4REGIONS, ROBIN, BIOLOC, Blue BioCluster, BlueRev and RefreSCAR and BIOGOVnet).
George Sakellaris (BIOEAST TWG Bi oeconomy Education Leader, BIOEAST HUB CZ) moderated the thematic cor ner “Bioeconomy Communication and Education – Future Skills for the Bioec onomy” joined by about 15 participants and had a topic 6 old (Transition2Bio, BIObec, Althings.bioPRO, Biovoices, Bi oskills, EBU Label) and 4 new (GenB, BioBeo, BioGov.net, Engage4BIO) pro jects. Several issues were raised and dis cussed among the participants:
- Specific skills are important for BioIn dustry and particularly in the frames of vocational training; the vocational train ing programs should be tailored-made and on a case-by-case basis
- Educational spots for training and in creasing awareness as they are designed in the BIObec project are an excellent example and similar approaches should be adopted in other cases too.
The initiative to organize the “Pro jec2Projects” event was received very positively. It was suggested to organize this event on a regular basis. L
New addition to IL-17 receptor
IMMUNOLOGY A new player in the field of the body’s immune response has been identified by scientists at the Insti tute of Molecular Genetics of the Czech Academy of Sciences. They have suc ceeded in describing another component in the “receiver” of inflammatory signals - the CMTM4 protein. The discovery was made together with the First Faculty of Medicine of Charles University in the BI OCEV centre, and led by Peter Dráber and Ondrej Štepánek.
During an immune response, all cells must work in concert to suppress in fection and minimise collateral dam age caused to their own tissues. To ac complish this, white blood cells begin to communicate with other cells in the body. Interleukin 17 (IL-17), a protein re leased by immune cells, acts as one of the key signals that put cells on alert. A cell that receives such a signal triggers a specific type of inflammatory response that leads to the suppression of certain yeast and bacterial infections of the skin and mucous membranes. On the other hand, if this immune cell coordination fails for any reason, the autoimmune sys tem can be triggered to act on its own tissue. IL-17 plays a crucial role in some autoimmune diseases such as psoria sis and psoriatic arthritis. A cell can per
ceive the pro-inflammatory signals sent from the IL-17 protein only if it has a cor responding “receiver” or “receptor for IL17” on its surface. Using a modern ap proach, the collaborating laboratories of Ondrej Štepánek have succeeded in dis covering a previously unknown compo nent of this receptor.
“It is a protein with an almost com ic book-like name, CKLF-like MARVEL transmembrane domain-containing pro tein 4, abbreviated as CMTM4. Subse quent research has shown that CMTM4 is essential for the composition of the re ceptor for IL-17 and for the susceptibili ty of cells to this signal,” explains Peter Dráber. “Cells lacking CMTM4 lost the ability to respond to IL-17. Similarly, mice lacking CMTM4 were largely protect ed against autoimmune psoriasis,” adds Ondrej Štepánek. Given the critical role IL-17 plays in some autoimmune diseas es, there is a hypothetical possibility that CMTM4 could serve as a target for future therapies for these diseases, which has yet to be confirmed.
Publication:
Knizkova D, (...), Stepanek O, Draber P (2022). CMTM4 is a subunit of the IL-17 receptor and mediates autoimmune pa thology. Nature Immunology. https://doi. org/10.1038/s41590-022-01325-9. L
Histological analysis of the ears of laboratory mice suffering from autoimmune psoriasis. This disease is characterised by marked thickening of the skin (left). In contrast, mice lacking CMTM4 are significantly protected against this disease (right).

78 EASTERN EUROPE European Biotechnology | Winter Edition | Vol. 21 | 2022
Pictures: © Draber Lab Tiskova Zprava Institute of Molecular Genetics of the Czech Academy of Sciences
At the interface of AI, healthcare, and data science
THE AI HEALTH INNOVATION CLUSTER is bringing together leading scientists at the interface of artificial intelligence (AI), healthcare, and data science. The goal is to combine and further develop the outstanding research networks and infrastructures in modern health data sciences that have emerged in the Rhine-Neckar region in Germany in recent years.
The German Cancer Research Center (DKFZ), the European Molecular Biology Laboratory (EMBL), Heidelberg Universi ty, Heidelberg University Hospital, Uni versity Hospital Mannheim, the Central Institute of Mental Health, and the Max Planck Institute for Medical Research join forces in the AI Health Innovation Clus ter. The collaboration among these sev en institutions is intended to generate sustainable synergies in the academic, medical, and health economic sectors and together become a stronger voice for the use of AI in health research. Through a competitive fellowship pro gram, the Cluster funds interdisciplinary projects that link data infrastructures, AI methods research, ethical-legal research issues, and clinical application fields across disciplines. The top criterion for the projects is scientific excellence.
Towards data-driven medicine
Two of the 67 projects to be funded evolve around early detection of sep sis, which is one of the leading causes of death and critical illness worldwide. In one project, hyperspectral imaging will be used for microcirculatory mon itoring of patients in the intensive care unit based on a study that found a char acteristic pattern of skin microcircula tory alterations in sepsis patients. The data collected will serve to evaluate the feasibility of early automated sepsis di agnosis with a novel machine learning algorithm. The second project, which in volves two studies, is based on two ad vanced machine learning technologies
Stucture of the AI Health Innovation Cluster in Heidelberg, Germany


– deep learning and multi-task learning. One study aims to develop a novel AI approach that will allow for forward pre dictions of individual patient trajectories and advanced identification of future tip ping points in disease dynamics towards sepsis. The second study focuses on the development of a new multi-task learn ing approach to identify the risk of sepsis for polytrauma patients during hospital admission, as septic complications often occur in this patient group.
A long-term AI strategy
The funded projects, which synergistical ly link several partners and/or regional initiatives, are strategically accompanied by a cross-institutional and interdiscipli nary think tank whose goal is the further development and continuous expansion of an AI strategy in health research for
the Rhine-Neckar region while promot ing international visibility. The tasks of the think tank include developing new objectives with particularly high chanc es of success for AI applications, integrat ing existing initiatives, transferring AI into clinical practice, and organizing the par ticipation of industry and start-ups. To ensure a successful long-term concept, fellowship program and think tank are complemented by central competence teams for important core tasks that sup port the funded projects and activities and the overall strategy development. L
Contact Dr Daniela Beyer, Scientific Program Manager, daniela.beyer@dkfz-heidel berg.de
Jennifer Mogk, Communications Manag er, jennifer.mogk@dkfz-heidelberg.de
79 European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: German Cancer Research Center SCIENCE & TECHNOLOGY
Biofilms for production
BIOMANUFACTURING Researchers have presented a novel method to promote biofilm formation. They believe their method can help to increase the efficiency of biocatalysis in biotechnological production processes and thus allow cost-effective recycling of waste, as well as carbon dioxide conversion into chemical building blocks and natural gas, or amino acids.
Spanish and British researchers have pat ented a method that boosts biofilm forma tion of bacterial production strains. They hope this will open the avenue to improv ing the efficiency of biocatalysis in bio tech production. In their paper published in Materials Horizons, Paco FernandezTrillo from the University of Coruña and Dr Tim Overton from the University of Birmingham (UK) describe that the ad dition of a set of synthetic polymers car rying mildly cationic, aromatic, heter oaromatic or aliphatic moieties induced biofilm formation even in production strains that normally slowly form the pro tective shell. The researchers say that their method can significantly improve yields in the growing field of enzymatic synthe sis of pharmaceuticals, fine chemicals, or food ingredients on an industrial scale.
E. coli as a model organism
The most commonly used non-patho genic bacterial production strains are not necessarily good at forming biofilms, a sort of growth-promoting ecosystems that form a protective micro-environ ment around communities of microbes and increase their resilience and so boost productivity. Normally bio-engineers use genetic engineering to boost biofilm for mation and thus productivity. However, the new method can bypass this costly and time-consuming process.
In a screening for biofilm formation boosters, Trillo et al compared the E. coli strain MC4100, which is poor at form ing biofilms, with E. coli PHL644, a good isogenic biofilm former to reveal the chemistries that are best suited to stim ulating biofilm formation. Hydrophobic
The researchers monitored the biomass and biocatalytic activity of MC4100 and E. coli strain PHL644 (a good biofilm builder) incubated in the presence of the polymers. They found MC4100 matched and even outperformed PHL644.

polymers outperformed mildly cationic polymers, with aromatic and heteroaro matic derivatives performing much better than the equivalent aliphatic polymers.
When the research team incubated both strains in the presence of these pol ymers, they found that MC4100 outper formed PHL644. “We explored a broad chemical space and identified the best performing chemistries and polymers, resulting in a small library of synthetic polymers that increase biofilm formation when used as simple additives to micro bial culture”, according to Trillo.
Wide range of applications
According to the researcher, "to the best of our knowledge, currently there are no methods that provide this simplicity and versatility when promoting biofilms for
beneficial bacteria. These synthetic pol ymers may bypass the need to introduce the traits for biofilm formation through genome editing, which is costly, timeconsuming, non-reversible and requires a skilled person in microbiology to im plement it. We believe this approach has an impact beyond biofilms for biocataly sis. A similar strategy could be employed to identify candidate polymers for oth er microorganisms such as probiotics or yeasts, and develop new applications in food science, agriculture, bioremediation or health.”
The University of Birmingham Enter prise, the tech-transfer arm of the aca demic institution, has filed a patent ap plication for the method and polymer additives, and is seeking commercial partners for licensing.
t.gabrielczyk@biocom.eu
©
Picture:
NBIC, University of Birmingham, UK
L
80 European Biotechnology | Winter Edition | Vol. 21 | 2022 SCIENCE & TECHNOLOGY
Predictive diagnostics
METABOLOMICS Researchers at Uni versity College London and Center for Cardiovascular Diseases at Berlin-based Charité have presented impressive re sults of a big data analysis that looked at 168 metabolomic disease markers in the blood of almost 120,000 participants of the UK Biobank (nature Medicine, doi: 10-1038/s41591-022-01980-3) and linked them to their medical histories to predict disease risk scores.
The group headed by cardiologists Ulf Landmesser, John Deanfield and bioinformatician Roland Eils were able to show that the biomarker patterns in blood samples tracked over the past ten years in combination with age and sex of the participants allowed significant ly better disease prediction about eight common diseases as the current diagno sis standard. Early diagnose before dis ease onset is the holy grail of preventive medicine. The AI analysis of the meta bolic blood markers recorded by NMR
from almost 118,000 volunteers includ ed in the UK Biobank sample and data collection revealed statistically signifi cant risk scores for eight out of 24 com mon diseases that were analysed. The researchers confirmed their predictions using data from four other large popula tion studies.
“This is exactly the direction we also want to take: Motivating people to have regular check-ups after a certain age so that they can take preventive measures in good time if the worst comes to the worst,” Landmesser told European B io technoLogy magazine.

AI predicts risk of disease onset
To do so, the research consortium linked metabolite profiles to the medical health history of the volunteers, that is digitally stored in the UK until the 1990ies. Ac cording to the cardiologist, “a combi nation of several different metabolites could provide predictive information on an individual’s risk of developing a num ber of different diseases.
LANDMESSER
With a cost of less than €20 per analy sis, examining the metabolome is cheap er than genome sequencing which “will be the next step to improve our portfolio of predictors of heart failure, acute coro nary syndrome, diabetes etc.” for which his team found predictive evidence. Car diovascular diseases, the No. 1 cause of death worldwide, has a strong genetic component (over 50%).
It’s crucial to evaluate the results cautiously. However, the goal is to avoid disease manifestation through early detection. We believe that 80% to 90% of cardiovascular diseases are preventable with an optimal prevention strategy.
In fact, the the human blood metabo lome is a direct reflection of the physi ological state, according to Landmesser, whereas the expression of the genome (3d genome) together with markers for chronic stress, LDL cholesterol etc re flects the individual susceptibility to dis ease. For the future, Landmesser sees the potential for a huge reduction of mortality in high risk patients for car diovascular diseases, stroke or athero sclerosis. “ Our data are the basis for in dividual prevention programmes,“ he says.
European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © Universitätsmedizin Charite
? What potentiel bears AI-based models that allow early diagno ses of cardiovascular diseases? !
PROF. DR ULF
Director Centre for Cardiovascular Research, Charité, Berlin, Germany
L High Quality Grade Plasmid and Minicircle DNA PlasmidFactory.com PlasmidFactory GmbH & Co. KG Meisenstraße 96 | 33607 Bielefeld | Germany info@plasmidfactory.com The better way to DNA! Starting material for GMP production of mRNA, viral vectors & CAR-T cells Now in LARGE scale! grafeting gmbh stock.adobe.com
Ultra-low freezers: Stability of cancer biomarkers
COLD CHAIN Maintaining the integrity of tumour biomarkers during processing and storage is of great importance for the accurate diagnosis and prognosis of many diseases, including cancers. To safely store samples for research or clinical uses, freezing procedures are used to arrest the degradation of biomarkers. However, the stability of these molecules depends upon long-term storage conditions.
› Julian Precht, B Medical Systems
In oncology, biomarker testing identi fies genes, proteins, and other substanc es that can reveal information about a cancer type since each one has a distinct pattern of biomarkers. The data can then be used to diagnose or classify the can cer type, estimate the prognosis, or se lect an appropriate treatment.
Specimens stored at ultra-low temper atures are generally preferred for these tests because most biomarkers, such as nucleic acids and protein molecules, are prone to degradation due to fixation pro cedures. On the other hand, biospeci mens also contain degradative molecules like proteases, lipases, and nucleases, whose activities are greatly influenced by temperature.
ULTs for modern cancer research
Ultra-low freezers (also called ULTs) are essential for the storage of samples in cancer research and are especially im portant in maintaining the stability and quality of biomarkers. Therefore, the performance and the reliability of med ical devices should be carefully ana lysed, and the flexibility in offering vary ing set points should be considered as it would help medical professionals choose the right storage temperature depending on the type of biomarkers being frozen and the length of the storage required. Moreover, the ULTs must maintain a uni form temperature inside their cabinets to best maintain the samples, while su perior door opening recovery and hold
Ultra-Low Freezers can be used effectively to reliably store certain types of biomarkers.

over times will help ensure their safety even during adverse events. Certifica tions such as the EU MDR ensure com pliance with regulations that govern the production of quality medical devices. Finally, energy-efficient operations and green refrigerants will ensure that labo ratories will be able to achieve consider able sustainability levels.
Reliable ultra-low freezers
B Medical Systems offers a wide range of ULTs among its products, whose major feature is the flexibility in the operating temperature range as they can reliably reach temperatures between -86°C and -20°C. Moreover, the advanced cool ing system allowing a constant and even temperature distribution, coupled with insulated inner doors, improved gasket seals and strong insulation, ensures a re
liable storage environment for optimal sample safety. These products provide a rapid pull down, strong door opening re covery and holdover times, which help guarantee a stable internal temperature during door openings and even adverse events. B Medical Systems’ ULTs also use natural green refrigerants to reduce their environmental impact while increasing their overall cooling efficiency. These models are class II medical devices (EU MDR and US FDA), conform to EU FGas and US SNAP regulations, and the Energy Star certification is also available on some of them.
These models are trusted worldwide for their safety, quality, reliability, and energy efficiency. B Medical Systems’ ULTs are used all over the world and are currently supporting hospitals, blood banks, univer sities, and research institutes in their clini cal and biomedical research efforts. L
82 European Biotechnology | Winter Edition | Vol. 21 | 2022 SCIENCE & TECHNOLOGY Picture: © B Medical Systems
Start-up heaven




BIOCAT Catalonia’s healthcare start-ups raised €238m in 2021, which marks a new record for the Spanish region. Ac cording to the 2021 BioRegion of Cata lonia Report, which was published by Biocat and supported by CataloniaBio & HealthTech and ACCIÓ, venture cap ital was the main driver of this growth with more than €187 million, or 79% of all capital, three times as much as in 2019. Most venture capital (83%) came from international investors. Catalonia can call its own 91 research centres and more than 1,300 companies in health care innovation. In 2020, there were 54 start-ups newly founded, most of them in the biotech and digital health fields. Most start-up companies were spun off from universities and research institutes like BSC, lCFO, IDIBAPS, IJC, IGTP, IMIM, IRB, Hospital SJD, UPF and UB. Invest ment into start-up companies was €184m in 2021, up 50% from 2020.







Grim picture
SFEE Public health is under threat, while healthy entrepreneurship is still being ham pered: The annual report “The Pharmaceu tical Market in Greece: Facts and Figures 2021” paints a grim picture of Greece’s health ecosystem. The report was present ed by the Economic and Industrial Re search Foundation (IOBE) in cooperation with the Hellenic Association of Pharma ceutical Companies (SfEE). In the wake of the Covid-19 pandemic, the pharmaceuti cal sector is bearing the brunt of the rising pharmaceutical expenditure, the authors point out. They call for the State to review the funding of the public health system. “Better health leads to prosperity. Health ier people enjoy a longer and more pro ductive professional life, they contribute to the economy, and they absorb less health care expenditures”, commented Olympios Papadimitriou, President of SfEE. The asso ciation proposes far-reaching reforms. www.sfee.gr
83 European Biotechnology | Winter Edition | Vol. 21 | 2022 ASSOCIATIONS
Biotechnology is published in co-operation with the following organisations: Europe: european-biotechnology.net European Biotechnology Network Europe: medicinesforeurope.com Ireland: ibec.ie/ibia Denmark: danskbiotek.dk The Netherlands: hollandbio.nl Germany: biodeutschland.org Portugal: www.p-bio.org UK: biopartner.co.uk France: france-biotech.org Italy: assobiotec.it Sweden: swedenbio.com Hungary: hungarianbiotech.org Europe: cebr.net Spain: asebio.com Norway: biotekforum.no Finland: finbio.net Belgium: bio.be EuropE an BiotEchnology covers the biotechnology sector of the current 27 EU member states, Norway, Switzerland, and UK. If you would like to subscribe, please refer to european-biotechnology.com Council E p B R io Europe: ecbf.vc Switzerland: swissbiotech.org Austria: lifescienceaustria.at Austria: biotechaustria.org Europe: yebn.eu
European
Young European Biotech Network
WORKING GROUP
Leading chemical companies are exploring the opportunities that have been opened up by modern biotechnology, especially in the field of “white” or industrial biotechnology. And they are also applying these technologies, wherever it makes sense. The SBA takes such initiatives seriously and has formed a working group specifically dedicated to white biotechnology. The Swiss Industrial Biocatalysis Consortium is an important partner in this effort. The group includes leading multinational companies that support white biotechnology as a pillar of economic growth. The planned activities are in agreement with OECD strategies.
participating companies (most of them young and internationally less savvy) find a comprehensive partner which is helping to put them in the public window. The participating Life Science Regions are important internal carriers of the dynamics in the Biotech sector, thus enhancing the common understanding of the industry. This and more knowledge is brought into Europa Bio, the European Biotech Association, where the SBA is an active member.
A global village within the Swiss Biotech Day
In partnership with the Swiss Biotechnet (see pages 14/15) the SBA develops training programmes and useful support tools for the industry. It is of importance that the industry specifies its training needs so that the academic side can create tailor-made education. This strategy ensures that the industry gets the right workforce with the right education. The SBA profits from the marketing alliance “Swiss Biotech” (see box) in a multiplied form. Thanks to Swiss Biotech, the
NETWORKING The Global Village at the Swiss Biotech Day was successfully launched this year to support the event’s role as a truly global biotech networking platform. After this year’s success, the Global Village will remain integrated in the 2023 Swiss Biotech Day.

SWISS BIOTECH...
...is an alliance of four leading Biotech regions of Switzerland (Bio Alps, BioPolo Ticino, Basel Area and Greater Zurich Area). They have combined efforts to streamline interests of the national biotech sector. The SWX Swiss Exchange holds a leading position in terms of lifescience listings and offers companies from that industry – be they located in Switzerland or abroad – access to an internationally recognised financial marketplace. The initiative was co-founded by the SBA which also manages the executive office of Swiss Biotech.
SAVE THE DATE
For further information please visit www.swissbiotechassociation.ch www.swissbiotech.org
› 24-25 April 2023, Basel Swiss Biotech Day & Swiss Biotech Success Stories Awards
After its successful launch this year, the Global Village at the Swiss Biotech Day 2023 enables countries and regions again to strengthen their ties with the Swiss biotech hub.
It is now further developed to offer inter national biotech delegations the opportu nity to strengthen their ties with the Swiss biotech hub, facilitate cross-border in vestments, establish public-private part nerships, R&D collaborations, and talent exchange. It is directed at international delegations who want to strengthen their links to Switzerland and comprises coun try or regional partnering booths, a central roundtable, and an international recep tion. Delegations promote their offerings and connect their participants with con ference visitors. At the central roundta ble, each delegation is invited to discuss with Swiss delegates how to jointly grow the life science business and foster collab oration as well as matchmaking between the guest country and the Swiss biotech hub. As the Swiss Biotech Day attracts more than 1,000 participants from over 30 countries, the launch of the Global Vil lage platform further supports Switzer
land’s position as a country able to inte grate many cultures and people.
International delegations benefit in many ways
Organised by the Swiss Biotech Associ ation in collaboration with Switzerland Global Enterprise and BIOCOM AG, inter national delegations can actively partici pate in the Swiss Biotech Day program and increase their visibility. Focused roundtable discussions help to jointly grow the life sci ence business, establish collaborations, fa cilitate matchmaking and exchange talents. The international reception offers an excel lent opportunity to reach out to other glob al partners.
The Global Village is open to national and regional biotech clusters who are in terested in engaging with Swiss counter parts in order to identify and nurture mutually beneficial collaborations and
partnerships. Each delegation designates a lead coordinating the delegation’s pres ence and follow-on activities. This novel and effective platform connects inter national decision makers in the global bio tech community with Swiss counterparts. Ideally delegations comprise diverse stake holders: Investors, venture builders, entre preneurs, leading hospitals, clinical inves tigators, contract research organisations, universities and private research centres, government bodies with vested interest (e.g. regulatory agencies), incubators and accelerators, as well as talent developers. The international reception enables dele gations to selectively invite their guests and to connect with participants of other nations and regions. The participation in the Global Village also provides inter national delegates with full access to the event’s program and one-to-one partner ing. Furthermore, interested international delegations are offered the opportunity to meet and visit Swiss organisations active in biotech before or after the conference. This could include visits at leading uni versities, investors and enterprises in bio tech, pharma, contract development and manufacturing organisations, leading in novation centers like the Center for Trans lational Medicine and Biomedical Entre preneurship in Berne, the Biopôle Campus in Lausanne, or other Swiss biotech hot spots across Switzerland.
Interested in taking part in the Global Village?
Contact Christian Böhm & Oliver Schnell Phone: +41 52 569 82 27 marketing@biocom.de

84 European Biotechnology | Winter Edition | Vol. 21 | 2022 ASSOCIATIONS Picture: Swiss Biotech Association
@
Domenico Alexakis is Executive Director of the Swiss Biotech Association.
Synthetic DNA for the global markets
RIBBON BIOLABS Since its foundation in 2018, Ribbon Biolabs has been developing a radically new technology for the high-throughput synthesis of long DNA molecules. With the setup of their first automated produc tion line for commercial scaling at their new headquarter location in Vienna, Ribbon Biolabs is now preparing for market entry in 2023.
Ribbon Biolabs was founded to rede fine the potential of synthetic DNA and aims at being the world leading partner for the development of next-generation DNA based solutions. Synthetic biology is amongst the fastest-growing fields of research with great potential in industri al and health applications but is heavily constrained by the availability of synthet ic DNA parts of multi-Kbp lengths, the fundamental components for biological engineering. Whereas small DNA mol ecules like oligonucleotides or sequenc es of up to some hundred base pairs are readily available based on the standard phosphoramidite chemistry, existing tech niques to assemble longer and/or com plex DNA are extremely difficult and time consuming. This unmet need is exempli fied by the massively increasing demand for expression vectors and large viral vec tor payloads required in biopharmaceuti cal R&D and production of complex bio logics such as monoclonal antibodies or gene therapy genetic reagents, as well as for metabolic engineering and other ap plications in research and industry.
Ribbon Biolabs' InfiniSynth™ platform
computational and combinatorial opti mization, with enzymatic assembly re actions concerted by state-of-the art automation workflows with adaptive control systems. This allows the expo nential assembly of long DNA molecules up to several thousands of bps, with the highest accuracy, a turnover time of only days and, moreover, in high-throughput. To date, the platform has been success fully applied to provide customers with FullGenes™ solutions, including com plex, GC-rich and repetitive DNA se quences as well as long molecules of more than 20 Kbp in length. Given the high value and importance of long DNA availability to the markets, Ribbon Bio labs’ technology will not only acceler ate customers’ existing applications but also unlock numerous entirely novel ap plications.
New headquarter and automated synthesis platform
UPCOMING EVENTS
› 27 April 2023
2. BIOTECH CIRCLE AUSTRIA, Vienna www.biotechaustria.org
Ribbon Biolabs’ CTO Dr. Marc J. Brehme highlights: “We took on a milestone en deavor with the design and implementa tion of the first automated line of our In finisynth™ platform – and just now we have achieved this key milestone towards our great vision – synthetic DNA of un limited length”.

Starting operation, the automated syn thesis line will allow commercial scaling with an initial focus on customers in bio tech and biopharma. In parallel to enter ing the European markets, Ribbon Bio labs is already preparing for international expansion by establishing a US based branch on the East Coast.
Ribbon Biolabs is a proud member of BIOTECH AUSTRIA. The support and exchange within this growing network is very helpful, especially for an early stage company.

With its novel InfiniSynth™ platform technology, Ribbon Biolabs introduc es an entirely novel approach to lift the technological limitations of phosphora midite chemistry and standard assem bly techniques. InfiniSynth™ employs a unique and powerful combination of Dr Marc J. Brehme, CTO
Since its foundation, Ribbon Biolabs has grown to an international and high ly skilled team of 30 interdisciplinary ex perts. Harnessing the funds of their re cent 18 M € Series A investment round, the company is now setting up their first fully automated DNA synthesis platform for commercial production. At Ribbon Biolabs’ new headquarter in Vienna’s 11th district the highly customized stateof-the-art robotic production platform has just passed its Site Acceptance Test in November 2022.
85 European Biotechnology | Winter Edition | Vol. 21 | 2022 ASSOCIATIONS Picture: Ribbon Biolabs.
Ribbon Biolabs
The Young European Biotech Network e.V.
YEBN The YEBN is an association of students and young researchers and professionals, working to build a bridge between academia and industry, as well as foster European collaborations aiming at creating a sustainable impact on our society.

We are working on expanding our Institu tional Members, and establishing connec tions with Hungary and the Baltics, as well as developing common projects with fel low associations. These include the btS (Biotechnological Student Initiative e.V.), in Germany, or Health Valley Netherlands, the biggest Life Sciences & Health inno vation Companies Cluster in the Nether lands. We are also interested in establish ing deeper collaborations with companies, providing a positive connection with young professionals and students.
In YEBN, we work on creating tools to help young professionals and last year's students to develop themselves at a pro fessional and personal level. We want to empower and give voice to young bi oleaders concerned with having a posi tive impact on society.
Our initials spell our DNA, the charac teristics of our association:
› Young: YEBN is powered by young developing bioleaders from students and young professionals in academia and industry.
› European: we have a European target. We work hand in hand with regional and national associations, aiming for the benefit of the whole society and having an impact at an international level.
› Biotech: we focus on Biotechnology and the Life Sciences sector.
› Network: A network full of inspira tion, information, connection, best practices exchange, training, and in novation.
YEBN is currently collaborating with many partners throughout Europe. Our main stakeholders are our Institutional Mem bers or fellow associations that believe in a common objective for our associations. Our current institutional members are pre sented here:

› NGB - New Generation of Biotechnol ogists (France)
› AYB - Association of Young Biotech nologists (Poland)
› Biotecnologi Italiani - National Associ ation of Italian Biotechnologists (Italy)
› NBV - The Dutch Biotechnology Asso ciation (Netherlands)
The YEBN has a series of value pro posals, whether we target young students and professionals, partners or alumni of ours, or fellow associations. We take ac tions to create impact in four axes of the Sustainable Development Goals (or SDGs), in order to “achieve a better and more sustainable future for all”. These are “access to advanced education”, “ac cess to information & communication”, “health & wellness” and inclusiveness. YEBN acts as a catalyst and provides a platform for access and exchange of in formation and communication. We pro vide a common place for people to meet and exchange ideas in events organ ized throughout Europe, with multiple events organized in the last few years. The YEBN, together with our Institu tional Members and fellow associations, work on developing events and partner ships that create impact in these Strategic Axes, keeping in mind that at the centre of all actions we have the objective of strengthening YEBN, our institutions, and partners to foster innovation and to keep working on our common goals.
If you are an individual, an association, or a company aligned with our goals, get in touch with us. We will be thrilled to dis cuss any possible collaboration and com mon endeavour. Drop us an e-mail at con tact@yebn.eu. Get inspired, get involved!
86 European Biotechnology | Winter Edition | Vol. 21 | 2022 ASSOCIATIONS Picture: YEBN
Life After PhD programme, organized in Barcelona’s Research Park together with the Barcelona Molecular Biology Institute, IBMB (PCB, February 2020). In this event, we gathered speakers in various scientific fields to share their own experiences on their professional pathways after completing the PhD.
It’s on: the race for sustainable packaging
THE PLASTIC PROBLEM A decade ago, the news about the Great Pacific Garbage Patch went viral. Since then, a multitude of startups proliferated on a mission to create ecological alternatives to the ubiquitous fossilbased plastics. At ECBF, we are closely monitoring the emerging materials for packaging applications, by far the main use case of plastics.
There is a reason for the immense suc cess of plastics in packaging. It is sim ply a material with phenomenal charac teristics: cheap, food-grade, lightweight, flexible, easy to produce, and an ex cellent barrier against oxygen, grease and water. However, traditional plas tic does not come without flaws: it lasts an eternity in the environment and is derived from oil. Finding a solution to these problems has become a priority as the consumers are becoming more environmentally aware and policymak ers are introducing stringent regulato ry frameworks for single-use plastics. Fortunately, many brave entrepreneurs are currently embracing this challenge. Their goal is to develop cost-competi tive alternatives with similar proper ties compared to fossil-based plastics while at the same time having a low er environmental footprint. Some start ups are aiming to improve the perfor mance of existing materials, like paper, via eco-friendly additives or composites (e.g., Papkot and Qwarzo) or via nov el fiber processing technologies (e.g., PulPac and The Loop Factory). Oth ers are experimenting with complete ly new bio-based materials that focus on either biodegradability (e.g., Sulapac and Traceless) or recyclability (e.g., UBQ Materials and Woodly). The reali ty is that for each packaging application there is a different solution and there is not a universal formula that will fix all our plastic problems. Soon, we expect to witness an ecosystem of green com
panies covering the many needs of the packaging market.
The challenges of sustainability

From an investor’s perspective the road to sustainable packaging bears opportu nities and obstacles. First, some of the biobased resources used as feedstock are new and currently unexploited. This means establishing new supply chains and ensuring they are ready for scaling up. There is also a tradeoff between bi odegradability and usability. Packaging that can compost at home is hardly suit able to protect its content for long un der certain conditions. When it comes to a non-biodegradable material recy clability in existing recycling streams is key. For companies and investors alike, emerging and fast-changing regulations
related to packaging and waste manage ment is a substantial risk. What can be labelled as biodegradable today might be considered generic waste tomorrow and vice versa. Finally, even when the abovementioned green characteristics are pre sent, it is not obvious that a product has a lower carbon footprint. That is why ECBF benchmarks packaging alternatives against each other by so-called “life cy cle assessment” starting from feedstock to the end of product life.
The role of ECBF
ECBF, the biggest European fund focused on the bio-based and circular bioecon omy, is striving to participate in the sus tainable packaging revolution. We be lieve that the ecological alternatives to fossil-based packaging are a key com ponent of the green transition hence our commitment to scout and support the most promising startups in the field as an active investor.

87 European Biotechnology | Winter Edition | Vol. 21 | 2022 ASSOCIATIONS Picture: © bennymartystock.adobe.com
Guillaume Gras, Hakan Karan and Tommaso De Santis, European Circular Bioeconomy Fund
The Great Pacific Garbage Patch
AbbVie Inc. (USA) 14
Affinity Maturation Service

ANTIBODY Sino Biological and Ainno cence have joined forces to establish an AI-enabled platform for antibody affini ty maturation. Powered by a self-evolving artificial intelligence engine, Ainnocence’s SentinusAI™ is revolutionizing affinity mat uration processes. SentinusAI™ can effec tively rank up to 10^10 antibody sequenc es based on their predicted affinity towards one or more antigens within a few days. Combined with the high-throughput re combinant antibody development program at Sino Biological, top candidates can be expressed recombinantly at a lower cost and shorter lead time to generate affinity data with a high hit rate of 15%.
SentinusAI™ is a powerful and selfevolving protein design engine created by Ainnocence. Its database contains up to 100 million human antibody sequences as well as an extensive collection of hu man/animal viral antigen information, re sulting in high-confidence prediction of binding affinity. Antibody affinity matu ration is a popular module of SentinusAI. Its close-loop nature guarantees that the model creates better antibodies by learn ing from each cycle of the experimental results. It has since delivered affinity-ma tured antibody sequences with an average hit rate of 15% and successfully increased affinity by three orders of magnitude.
CONTACT

Sino Biological Europe GmbH order_eu@sinobiologicaleu.com www.sinobiological.com/ Get a Quote!
Abivax AB (F) 20
Affimed (DE) 24, 28
Affinivax (USA) 14
Afyren (F) 68
AGC Biologics (USA) 42, 46
Akribion Genomics (DE) 60
Alnylam Pharmaceuticals Inc. (USA) 16
Ambrosia Bio (IL) 65, 68
Anjarium Biosciences AG (CH) 46
APIM Therapeutics AS (NO) 71
Apollo Health Ventures (USA) 75
Arvinas Inc. (USA) 73
Asabys Partners (ES) 77
AstraZeneca (GB/SE) 18, 28
Avrobio Inc. (USA) 70
B Medical Systems S.a r.l. (LU) 7, 82
Badische Peptide & Proteine GmbH (DE) 60
BASF (DE) 63
BEAM Alliance / bamconn GmbH (DE) 50, 51
Bel Group (F) 60
Bellevue Asset Management AG (CH) 16
Better Nature (GB) 68
BioArctic Neuroscience AB (SE) 70 BIOCOM AG (DE) 58, 84
Biohaven Pharmaceuticals (USA) 13
BioMarin Europe Ltd. (UK) 16
BioNTech SE (DE) 20, 28
BioRN Cluster Management GmbH (DE) 25, 79 Biotalys NV (BE) 72
BIOVIAN Ltd (FI) 31, 34
Blue Horizon Corp. AG (CH) 64
Blue Whale Material LLC (USA) 64
Bone Therapeutics (BE) 72
Brain Biotech AG (DE) 60
Bristol-Myers Squibb (USA) 14, 28
Calecto Inc. (DK) 29
Carbios SAS (F) 10, 66
CellPoint BV (NL) 18
Cellugy (DK) 65
Charles River (USA) 6
Cultivated Biosciences (CH) 65
CZ Vaccines (Zendal Group) (ES) 35, 40
Dionymer (F) 68
Easyfairs Group, Connect in Pharma (BE) 56, 57
EBD Group (USA) 54, 55
ECBF Management GmbH (DE) 69, 87
Eeden GmbH (DE) 10
Elaia (F) 68
Emscher Genossenschaft/Lippeverband (DE) 64
Enhanc3D Genomics Ltd. (GB) 73
EpiEndo Pharmaceuticals (IS) 71
Eppendorf Austria GmbH CP4
Esencia Foods (ES) 60
European Biotechnology Network (B) 23
Evgen Pharma (UK) 73
Evonik Industries AG (DE) 64
FCF Corporate Finance GmbH (DE) 17
FGK Clinical Research GmbH (DE) 29
Forbion Capital Partners (NL) 16
Formo Bio GmbH (DE) 61
Galapagos NV (BE) 18
GlaxoSmithKline (UK) 6, 14, 28
Global Bioenergies SA (FR) 68
Heidelberg Pharma AG (DE) 29
Hookipa Biotech AG (AT) 75
Immunethep SA (PT) 76
Informa Markets CPHI/Pharmapack (UK) 48, 49, CP3
Inpixon GmbH (DE) 11
ISA Pharmaceuticals B.V. (NL) 28
Johnson & Johnson (USA) 6
Krajete GmbH (AT) 3, 60, 62
L'Oréal (FR) 68
LABORATOIRE Unither (FR) 33, 36
leon-nanodrugs GmbH (DE) 38 Lesaffre (FR) 68
Limagrain Genetics SA (FR) 68
LISAvienna (AT) 19
LogicBio Therapeutics (USA) 18 LXP Group GmbH (DE) 63 Mablink Bioscience SA (FR) 73 Meatable BV (NL) 61 Medraxa Therapeutics (DE) 74 Medsenic SA (FR) 72 Merck & Co. (USA) 13 Metabolic Explorer (FR) 68 Micropep (FR) 68 Mimetas BV (NL) 72 Minoryx Therapeutics S.L. (ES) 76 MorphoSys AG (DE) 74 MSD Sharp & Dohme (USA) 6 MyBIOS (AT) 65
Myllia Biotechnology (AT) 75 MyoPax ApS (DK) 70
Neuraxpharm Arzneimittel GmbH (DE) 76 NeuVasQ Biotechnologies (BE) 46
Nordic Nanovector AS (NO) 71 nova-Institut GmbH (DE) 63 Novo Nordisk A/S (DK) 70, 71, 74 Novozymes A/S (DK) 72 Nucleus Capital AG (DE) 64 Oncopeptides AB (SE) 28 Otsuka (JP) 6 Paleo BV (BE) 60
Patient Square Capital (USA) 74 Pfizer (USA) 13, 28, 77 Pheida AG (CH) 62 PhoreMost Ltd. (GB) 73 Pili (FR) 66
PlasmidFactory GmbH & Co. KG (DE) 81
Premier Research (USA) 15 Processium (FR) 67
Regeneron Pharmaceuticals Inc. (USA) 28 ReiThera Srl (IT) 76
Resolve Biosciences (DE) 74 Revier Therapeutics (DE) 74 Rewind Therapeutics (B) 46 REWOW (IT) 65
Ribbon Biolabs (AT) 85 Roche AG (CH) 6, 74, 75
Roquette Group (FR) 68 Sanifit Therapeutics (ES) 76 sctbio (CZ) 39
Seagen (USA) 13
Shimadzu Europa GmbH (DE) CP2
Sibylla Biotech (IT) 76
Sierra Oncology (USA) 14
Sino Biological Europe GmbH (DE) 9, 88
Sobi AB (SE) 77
Sofinnova (FR) 68
Solar Foods (FI) 64, 71
Stalicla SA (CH) 73
Standing Ovation (F) 26, 27, 60
Strategic Consulting 44
Swiss Biotech Association, SBD (CH) 52, 53, 84
Telix Pharmaceuticals Ltd. (AUS) 29
Toulouse White Biotech – TWB (FR) 65, 66
Trillium Therapeutics (USA) 14
Truffle Capital (FR) 68
Turning Point Therapeutics (USA) 14
Ucaneo Biotech GmbH (DE) 60
Univercells SA (BE) 73
Valneva SE (FR/AT) 46, 75
Ventus Therapeutics (USA) 70
VERISTAT International Ltd (UK) 41, 44
Vertex Pharmaceuticals Inc. (USA) 18
ViaCyte (USA) 18
Vienna Textile Lab (AT) 75
Vivescia (FR) 68
Volkswagen AG (DE) 64
Zymvol (ES) 68
88 European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © Sino PRODUCT/COMPANY INDEX
Biotech Showcase
9.–11.1.2023 SAN FRANCISCO The investor conference for innovators will give the opportunity to connect in-person in San Francisco or virtu al (on January 18-19, 2023). Partici pating companies are ranging from early, mid to later stage. From Start up, over Biotech and large Pharma. https://informaconnect.com/ biotech-showcase
14.-15.2.23
CLIB International Conference CIC2023, Düsseldorf (DE)
Info: Cluster Industrielle Biotechnologie e.V. www.clib-cluster.de
6.-8.3.23
UNESCO – EU H2020 LimnoPlast Conference, Paris (F) Info: University of Bayreuth, https://www.limnoplast-itn.eu

8.-9.3.23
International Conference on Cellulose Fibres 2023, Cologne (DE)/+++ online +++ Info: nova Institut, www.cellulose-fibres.eu
14.-16.3.23
Bioprocessing Summit Europe, Barcelona (ES)/+++ online +++ Info: Cambridge Healthtech Institute www.bioprocessingeurope.com/
15.-16.3.23
Future Health, London (UK) Info: Dawn Barclay-Ross, Future Health Expo Ltd. www.futurehealth.global
1.12.22
Virtual Roadshow: AI in Health Care an Medical Technologies, +++ online +++
Info: Baden-Württemberg International/ BIOPRO Baden-Württemberg GmbH https://ai-hamt.vidivent.de/landing
7.-8.12.22
BioFIT 2022, +++ online +++
Info: Margaux Satola, Eurosanté www.biofit-event.com
17.-19.1.23
GlycoBioTec 2023 - 3rd International GlycoBioTec Symposium 2023, Berlin (DE)
Info: Max Planck Institute for Dynamics of Complex Technical Systems, Magdeburg https://bit.ly/3tOFnLx
18.-21.1.23
15 th Global Forum for Agriculture – GFFA, Berlin (DE)
Info: BIOCOM AG https://gffa-berlin.de/en
20.-29.1.23
International Green Week, Berlin (DE)
Info: Messe Berlin GmbH www.gruenewoche.de/en
23.-24.1.23
20. International Conference on Renewable Mobility –Fuels of The Future , Berlin (DE)
Info: Bundesverband Bioenergie e.V. www.fuels-of-the-future.com/en
6.-7.2.23
asiaPLX 1 – Marketplace for Pharma Business Opportunities, Singapore
Info: Dr. Norbert Rau, RauCon https://www.raucon.com/asia-plx/asiaplx-1
16.-17.3.23
7 th AMR Conference on Novel Antimicrobials and AMR Diagnostics, Basel (CH) Info: Dr. Boris Mannhardt, bamconn GmbH, Berlin, www.amr-conference.eu
20.-22.3.23
BIO-Europe Spring 2023, Basel (CH) Info: Olivia Guiliana, EBD Group https://informaconnect.com/bioeurope-spring/

Forum Labo
28.–30.3.2023 PARIS Forum LABO brings together the profession als of the laboratory: companies, startups, researchers, students, learned societies but also buyers and laboratory technicians from the public sector or from the phar maceutical, chemical, agrifood, cos metics industries and biotechnologies. www.forumlabo.com/
24.-25.4.23
BioVaria 2023, Munich (DE)
Info: Esther Lange, Ascenion GmbH www.biovaria.org/

3.-4.5.23
OTC – Outsourcing in Clinical Trials Europe 2023, Barcelona (ES)
Info: Katie Giorgadze, Arena International Event Group www.arena-international.com/octeurope/
Innovation for Health
6. APRIL 2023 ROTTERDAM The con ference for key players in Health & Life Sciences, will cover ‘Digital Trans formation in Healthcare’. It provides a opportunity to meet leading innova tors, to catch up on the latest trends, to present cutting-edge innovations and to engage leaders and decision makers. www.hyphenprojects.nl/i4h
3.-4.5.23
7 th Euro BioMAT 2023 – European Symposium on Biomaterials and Related Areas, Weimar (DE), +++ online +++
Info: German Materials Society https://dgm.de/biomat
15.-16.5.23
BioEquity Europe 2023, Dublin (IRL)
Info: EBD Group/Biocentury https://conferences.biocentury.com
24.-25.5.23
Chemspec Europe , Basel (CH)
Info: Mac Brooks Exhibitions www.chemspeceurope.com
5.-8.6.23
BIO International Convention, Boston (USA)
Info: BIO – Biotechnology Innovation Organization www.bio.org/events/bio-international-convention
14.-15.6.23
11th International Bioeconomy Conference, Halle/Saale (DE)
Info: BioEconomy Cluster/Science Campus Halle/S. www.bioeconomy-conference.eu
89 EVENTS
Pictures: Walkerssk/pixabay/1496©FOUCHA-MUYARD Flickr/Hyphen Projekts
European Biotechnology | Winter Edition | Vol. 21 | 2022
Winners & losers
ANDERA PARTNERS closed a record-break ing new BioDiscovery 6 Fund with €456m, 30% plus the generation before. To date, the BioDiscovery 6 Fund has made invest ments in six companies already: Evom mune, Amolyt, TargED, Tubulis, Miner alys and ImCheck.
PSORIASIS A high place bo rate has shocked in vestors in Immunic, sending its stock down dramatically after skyrocketing only weeks before. The company blamed the response rate in the placebo arm for a disappointing interim look at blinded data from its Phase 1b psoriasis trial.
Cell tools market
I’m in biotechnology because
EU funding
DR REYK HORLAND, CEO, TissUse GmbH Berlin, Germany

“… I was part of the development of a disruptive technology to challenge the old paradigms in drugs. Now it’s time to build on that excellent scientific foundation in order to realise its tre mendous socioeconomic potential . ”
SYNERGIES MISSING The EU allocates tens of billions of euros to support research and innovation in Europe. Between 2014 and 2020, 12% of the EU budget went to research and innovation. The European Court of Auditors (ECA) examined wheth er the Commission and managing author ities in EU countries have taken adequate measures to create the necessary synergies between Horizon 2020 and the ESI Funds, examining a sample of five Member States – Portugal, Poland, Slovenia, Croatia and Romania. Horizon 2020 (€76m allocated) and ESI Funds (€41m) and their succes sors, the Cohesion Funds, were not well coordinated, the auditors said. There was insufficient data collection on projects in ESI and even today the data between Ho rizon2020 and ESI are not compatible.
The global market for cell and gene therapy tools and reagents is esti mated to grow from $8.3bn in 2022 to $13.7bn in 2027, with a com pound annual growth rate (CAGR) of 10.6% for the period of 20222027. That’s the result of a survey published by BCC Research, which claims to have “50 years’ worth of experience in market research” to provide the reader with the latest trends, opportunities, challenges, and innovations, being located just outside of Boston, Massachusetts.
#WomenLeadersInBiotech
Congratulations to the winner of the #IndustriaBiotec Start-up pitch: Frédéric Pâques from Standing Ovation!https:// standing-ovation.com. @BiocomDe

UPDATE—Just learned that Eli Lilly ex ecutives are raging and furious at losing $20bn in market cap from this stunt with @TwitterBlue. It’s too bad they don’t see their own damn insulin price gouging as the actual problem. Karma @LillyPad, karma.
@DrEricDing
The Top 25 Women Leaders in Biotechnology of 2022 published| The Healthcare Technology Report. https://
Next issue 2023
thehealthcaretechnologyreport.com/thetop-25-women-leaders-in-biotechnologyof-2022/
SPRING EDITION Pharma outsourcing and bioprocessing will be in the focus of our next issue, together with a feature on the blockbuster market of personalised cardiology. Players in these fields are invited to present their offering in our special in european BiotechnoLogy. Want to participate? Please contact Andreas Macht (+49-30-264921-54), Oliver Schnell (-45), or drop us an email: marketing@biocom. de. Publishing date is 16th of March 2023; deadline for ads is 3rd of March 2023.
ENCORE European Biotechnology | Winter Edition | Vol. 21 | 2022 Picture: © TissUse GmbH
Please follow us @EuroBiotechNews
@Joseph79120626
cell tools market 0 2 4 6 8 10 12 14 Sales in $bn CAGR 2020-2031 10,6% 8.3 2022 9.1 2023 10 2024 11.1 2025 12.3 2026 13.7 2027 10 12 14 8 6 4 2 0 90
24-26 October 2023 | Barcelona, Spain
Exclusive Entry into the Spanish Pharma Market
Pharma’s largest event is making an exciting return to Barcelona on 24-26 October 2023. Join us at CPHI Barcelona to position your company at the heart of pharma and to connect with international pharma professionals. Grow your business and expand your network in one of Europe’s leading countries in pharma.

cphi.com/europe
45,000+ Attendees 160+ Countries 2000+ Exhibitors










® and the Eppendorf Brand Design are registered
of Eppendorf SE, Germany. All rights reserved, including graphics and images. Copyright © 2022 by Eppendorf SE. https://www.eppendorf.com/bioprocess-basics/ eBook on Bioprocessing Basics Bioreactors are more than just vessels! Do you want to learn more about the key characteristics of a bioreactor and the di erences between batch, fed-batch, and continuous fermentation modes? Then download our eBook in which we provide information on the basics of bioprocessing. Get your free copy now! https://eppendorf.group/bioprocessing-basics
Eppendorf
trademarks







 FOCUS: PHARMA SERVICES
FOCUS: PHARMA SERVICES



 Thomas Gabrielczyk Editor-in-Chief
Thomas Gabrielczyk Editor-in-Chief









 Full house at BIO-Europe 2022 in Leipzig
Full house at BIO-Europe 2022 in Leipzig












 BIOCONFIDANT
BIOCONFIDANT


























































































































































