



State-of-the-art mammalian cell culture manufacturing facilities in Europe and Asia for clinical and commercial material, ranging from 150 to 13,000 liters.
Customized solutions and efficient handling for your microbial-based products, with upstream and downstream capabilities ranging from 150 to 40,000 liters.
High-quality plasmid DNA and RNA manufacturing for various types of RNA drugs (including siRNA), mRNA vaccines and innovative gene and cell therapies, up to a scale of 3,000 liters.
Rely on a leading Cell & Gene therapy manufacturer with an excellent team and high-tech facilities.
Producing viral vectors for vaccines or cell and gene therapy in a state-of-the-art facility.

A fully integrated set of services and solutions for aseptic fill and finish of medicinal products. Available packaging systems and volumes include:
• Vials (liquid, lyophilized)
• Pre-filled syringes
• Low-level siliconized syringes
• Droptainers
• Ampules
• Tubes
Manufacturing master cell and working cell banks to enable high-quality clinical and commercial production.
We follow a comprehensive Novartis quality assurance system, with all our facilities operating in compliance with cGMP regulations.
To find out how we can help, get in touch at biotech.cooperations@novartis.com www.novartis.com/globalbiotechcooperations
Biotechnology is helping us tackle challenges such as emerging diseases, the impact of ageing in healthcare, population growth, climate change, and the green transition of the economy. Precision medicines, advanced therapies, mRNA and gene editing technologies are transforming the way diseases can be diagnosed and treated, providing life-saving solutions. Biotechnology is also at the forefront of building a sustainable economy, developing better crops, providing new protein sources, and producing bio-based materials.
ION AROCENA is the CEO of AseBio, the Spanish Bioindustry Association. He holds a Bachelor’s degree in Biology from the Complutense University of Madrid, graduating with honours (2003), and a Master’s degree in Business Administration from EOI (2010). He worked in a life sciences venture capital firm for almost a decade before joining AseBio in 2016.

It’s time to place biotechnology at the core of a new, more sustainable European growth model. Since July 1st, Spain holds the Presidency of the Council of the EU with a programme focused on four key pillars: re-industrialisation of the EU and ensuring its strategic autonomy, advancing in the green transition, promoting greater social and economic justice, and strengthening European unity. Biotechnology can play a fundamental role in achieving these objectives. The COVID -19 pandemic highlighted both the vulnerabilities and strengths of the EU life sciences ecosystem. It may be too late or costly to revert some of the latter, but with the lessons learnt in mind, we could still strengthen our capabilities in key technologies for the medicines of tomorrow, such as biologics, cell and gene therapies. While the EU remains a scientific powerhouse in these areas, we struggle to develop technology, launch ventures and grow them into global players. Europe needs to boost investment in R&D and enabling infrastructure, generate and grow more innovative companies, and increase biomanufacturing capacity in this space. On the other hand, biotechnology is crucial for the green transition. New Genomic Techniques (NGTs) are very precise and efficient, allowing us to modify the genome of plants and microorganisms specifically. NGTs are key tools for developing plant varieties that can be climate resilient, pest resistant, require fewer fertilisers and pesticides, or ensure higher yields, contributing to the goals of the green deal and meeting the increasing demand. Their application in microorganisms can also lead to new strains to produce raw materials for other industries, reduce the demand for natural or fossil resources and contribute to the green transition.
Europe is clearly at a turning point that will determine our competitiveness and resilience for the next generation. Legislative proposals affecting the biotech ecosystem are currently under discussion. To mention two, the EC launched a proposal for a new regulation on plants obtained by NGTs paving the road to enable the use of NGTs in Europe. Secondly, the proposal of the Commission for the revision of the EU Pharmaceutical Legislation includes provisions aimed at improving the EU’s regulatory framework and promoting ‘novel technologies, although some may undermine the predictability and stability of EU’s incentives regime and may hamper the industry’s ability to innovate. It is a critical moment for Europe to establish regulatory frameworks which are fit for purpose and enable innovations to happen, allowing their potential to be fulfilled. Only by doing so will Europe be able to lead in global innovation and transition towards a more sustainable economy, thereby strengthening its strategic autonomy.
Cell and Gene Therapies (CGTs) have emerged as one of the fastest growing sectors in the life sciences industry, even though supply chains in the field are more complex than in traditional pharma. That’s because CGTs for rare diseases mostly can’t be produced centrally, and still require a lot of handson work, including directly in clinics. For firms to t ake advantage of Europe’s excellent science base, networking stakeholders and safe reimbursement are proving to be crucial factors. The EU has some catching up to do.
6 NGT deregulation: The end of breeding standstill is in sight
10 EU Commission: Gun-jumping fine for Illumina; Pharmaceutical legislation; EU Commission to cut red tape; Pandemic update
17 Interview: VC m odel fi ts Europe Daniel Parera, M.D. and Dr. Peter Neubeck, Kurma Partners
20 Analyst commentary
21 European Biotech Stocks
24 Artificial intelligence –to ol or trouble?
28 Update on clinical trials
30 Precision therapeutics: optimising early drug discovery
34 Infrastructure makes the difference
57 Spain – an e merging European biotech champion
58 An overview of a sector that has to be proven strategic
60 Paving the way to internationalisation
62 BIOSPAIN 2023: Biotech as a critical industry
64 Northern Europe: Sweden, Denmark, Norway and Finland
66 We stern Europe: France, Belgium, The Netherlands and the UK
68 Central Europe: Germany, Switzerland and Austria
70 Southern Europe: It aly, Spain, Croatia, Slovenia and Portugal
72 Ea stern Europe: Poland, Hungary, Czech Republic & biotech powerhouse Lithuania
75 Topical hair growth inducer
76 Bot for drug delivery
77 Biodiversity: Gene drive for nature protection
PICK & MIX
42 Biopeople
81 News from Biotech Austria,SBA
85 ICPO Forum
86 PharmaLab Congress
88 Company index, New products
89 Events
90 Encore
IMPRINT European Biotechnology (ISSN 2364-2351) is published quarterly by: BIOCOM Interrelations GmbH, Jacobsenweg 61, D-13509 Berlin, Germany, Tel.: +49-30-264921-0, Fax: +49-30-264921-11, Email: service@european-biotechnology.com, Internet: www.european-biotechnology.com; Publisher: Andreas Mietzsch; Editorial Team: Thomas Gabrielczyk (Editor in Chief), Derrick Williams (Co-editor), Dr. Georg Kääb, Uta Mommert, Gwendolyn Dorow, Margarita Milidakis, Maren Kühr; Advertising: Oliver Schnell, +49-30-264921-45, Christian Böhm, +49-30-264921-49, Wolfgang Gutowski, +49-30-264921-54; Distribution: Lukas Bannert, +49-30-264921-72; Graphic Design: Michaela Reblin; Production: Martina Willnow; Printed at: Königsdruck, Berlin; European Biotechnology Life Sciences & Industry Magazine is only regularly available through subscription with a BIOCOM CARD. Annual subscription BIOCOM CARD Europe: €80 for private individuals (students €40) incl. VAT, €120 plus VAT for corporates. Prices includes postage & packaging. Ordered subscriptions can be cancelled within two weeks directly at BIOCOM AG. The subscription is initially valid for one calendar year and is automatically renewed every year after. The subscription can be cancelled at any time and is valid until the end of that calendar month. Failures of delivery, which BIOCOM AG is not responsible for, do not entitle the subscriber to delivery or reimbursement of pre-paid fees. Seat of court is Berlin, Germany. As regards contents: individually named articles are published within the sole responsibility of their respective authors. All material published is protected by copyright. No article or part thereof may be reproduced in any way or processed, copied, and proliferated by electronic means without the prior written consent of the publisher. Cover Photo: Ruslan Batiuk - stock.adobe.com; ® BIOCOM is a registered trademark of BIOCOM AG, Berlin, Germany.
Biotech start-ups that want to completely decouple protein production from agriculture due to environmental and animal protection benefits have been blocked by the EU’s stubbornly slow Novel Food Regulation. This summer, the Dutch Government paved the way to giving consumers early and rapid access to cellular agriculture products through unique tasting events.
Except in Spain and Portugal, the commercialisation of biotech crops stalled in Europe for about 15 years. In July, the EU Commission tabled a draft regulation, backed by agriculture ministers at an informal meeting to incentivise researchers and farmers to make use of new genomic techniques in plant breeding.
Survival of the fittest – that’s how Charles Darwin formulated the idea that the species best adapted to prevailing environmental conditions will win out. In the fastest-growing field in the pharma biotech sector – cell and gene therapy – China now looks set to overtake the US, the biotech world market leader – at least as far as clinical trials are concerned. And Europe? The bloc continues to suffer from a lack of communication/networking and seemingly ineradicable bureaucratic hurdles when it comes to bringing ideas from bench to bedside –with exceptions such as the UK or Spain (see Country Focus, p. 57). Biotech entrepreneurs want to turn that around with historic opportunities for reform of the EU’s pharmaceutical legislation (see. p. 36).
Their biotech colleagues in the boom market of cellular agriculture and precision fermentation – in short, vegan protein substitutes – feel the same. This summer, the Netherlands opted out of the EU system, and will now allow preapproval tastings of novel sustainable foods (see. p. 12).


The EU Commission has already moved on the issue of biotech breeding, and was able to get a majority in the EU Council of Agri-Ministers behind its proposal to create the most liberal set of regulations in the world for approving crops produced with new techniques (see p. 6). Now we have to see how adaptable Europe is in other fields.
Thomas Gabrielczyk Editor-in-Chief


NEW GENOMIC TECHNIQUES With an aggressive legislative proposal to exempt crops modified biotechnologically within the species’ own gene pool from the strict controls of EU genetic engineering rules, the EU Commission wants to bring Europe back to the top of the world in plant breeding. If its draft regulation goes through unchanged, the EU would have one of the most liberal regulations in the world. Most EU agricultural ministers support the draft.
A renaissance of European plant research, once a leader in precision breeding, is promised by the EU Commission’s current attempt to facilitate the use of “new genomic techniques” (NGT). Ex-Commission Vice President Frans Timmermans said in Brussels “farmers will get access to more resilient crops through the new breeding techniques, for which fewer pesticides need to be used and which are better adapted to climate change.” Of 484 development projects, however, just 38 are aimed at improving crop resilience to climate-related abiotic stresses, according to data released by the German Academy of Sciences Leopoldina in mid-August.

At the heart of the Commission’s proposal are plant varieties bred using new
genomic techniques – an internationally unfamiliar term under which the EU Commission subsumes all cis-genetic and genome-editing techniques by which individual nucleotides or DNA sequences in the plant genome can be mutated, deleted, reversed, or reinserted from closely related species using homologous end-joining. While governments worldwide, including the USA or UK regulations, only allow a single targeted change to the plant genome to be deregulated, the EU Commission wants to greenlight 20 of the above changes in any combination for each plant under newly defined, so-called equivalence criteria. With these, the Commission demarcates Class 1 NGT plants from Class 2 NGTs. NGT1 breeds – after formal official confirmation of their status within 30 days – will be treated in the same way as conventional breeds and will only be labelled as NGTs in a seed database but
won‘t be traceable like GMOs are. The deregulation is expected to save seed breeders up to €11,000 per approved variety and a lot of administrative burden, according to the Commission.
Although NGT-2 plants contain only species-specific DNA or that of closely related species, they are considered nonequivalent because they could not even theoretically be produced by conventional breeding methods. They therefore undergo a simplified approval and risk classification procedure compared with transgenic plants containing genetic material foreign to the species.
In contrast to plants containing foreign genes, the EU Commission wants to promote the cultivation and release of these NGT2 varieties:
› if the plants promise improved yields with less fertilizer and pesticide use,
› are better able to withstand abiotic and biotic stress,
› can be better stored and transported, or
› require less water and nutrients for the same yield.
Yet, they are supposed to be subject to labelling and traceability requirements – in short, to contribute to the climate goals of the Green Deal. That this is not in the cards is claimed by a study from Switzerland published at the end of August. “Swiss agriculture should not rely too much on novel genetic engineering” writes the Federal Ethics Committee on Non-Human Biotechnology (Ekah) in its 36-page study as a clear majority of the commission [...] considers the potential
Keep it simple, stay flexible

The Nexera Prep Series Preparative Purification LC improves productivity tremendously via efficient preparative work using application-specific LC or LC-MS solutions. It provides better prep processes for drug discovery and purification of functional components in pharmaceutical, chemical and food industries.
Ease-of-operation in a flexible set-up supported by flexible choice of detectors and efficient process automation
Simplifies the fractionation programming using the LabSolutions software’s fractionation simulation function
High sample capacity with a reduced footprint through a space-saving design with support of custom racks
Fast recovery of highly purified target compounds by automation of fractionation, concentration, purification and recovery

of genetic engineering methods in arable farming to be too insignificant to rely on these methods in view of Switzerland and especially in view of the urgency of the climate goals as a whole”. Instead of relying on NGT breeding, the experts see more potential to mitigate GHG emissions from agriculture in reducing the number of animals in livestock farming, establish grassland farming and expand plant-based nutrition. The report does not cover whether the Paris goals could be achieved by means of biotechnologically and microbially produced protein substitutes. Manufacturers of vegan protein, on the other hand, emphasize that their fermenter-supported processes reduce CO2 emissions by more than 85% and water consumption by at least 60% compared to conventional agriculture and livestock farming, and therefore want to completely decouple protein production from animals and land use
Science and seed industry officials rejoiced at the solution put forward by the EU Commission in the first week of July. “It’s a big step; I’m quite happy,” said Agnès Ricroch, secretary of the life Sciences section of the Academy of Agriculture of France researching at AgroParisTech. “It has been difficult to get money to carry out research using genetically modified organisms and permission for field trials”, she said. “I think this is going to change,” Euroseeds Secretary General Garlich von Essen stressed that “Europe today is the leading developer and exporter of seed worldwide. It is good to see that the foundation
of this success is enshrined in the Commission’s proposal.” Plant breeders, however, criticised that the EC’s draft regulation excludes the question of patents on breeds, from the regulation and delayed a decision on it not earlier than in 2026. According to the European Patent Office, today there are more than 3,000 patents on GMOs, most of them in the hands of multinational seed companies such as BASF, Bayer/ Monsanto or ChinaChem/Syngenta. European Landowners’ Organization (ELO) welcomed the proposals for an EU regulation on plants produced by New Genomic Techniques. However, ELO “strongly advocates for maintaining the integrity of organic production through clear labelling and traceability.” There is a need for more details on both authorisation and notification procedures and their key elements to ensure a swift implementation of the regulation, ELO officials stressed.
Opponents of genetic engineering and consumer protection groups showed the familiar defensive reflexes against GMOs. GMO opponents such as Green MEP Martin Häusling warned that if this draft becomes law, consumers will have “no way of knowing whether they are eating GM food”. According to said Jan Plagge, president of IFOAM Organics Europe, “this proposal is a massive accelerator for a lucrative business.”

In this context, the promise that new bio tech breeds could slow down cli -
mate change shows a value-based split in the anti-GM front, which has successfully kept agribiotech products off the EU market and fields for more than 15 years. Seemingly “green” organisations such as RePlanet that describes itself as “a grassroots citizen organisation driven by science-based solutions to climate change, biodiversity collapse and the need to eliminate poverty” stressed that “there is simply no need to regulate NBTs more strictly than traditional methods.”
In view of the fact that in its draft regulation the Commission can impose releases on EU member states if an NGT2 plant has proven to be harmless in a release in another EU state, there have been efforts by GMO-averse member states to form a blocking minority in the Council of Europe. In addition, the draft regulation prohibits organic farmers from using NGT1 plants, but at the same time offers no practicable coexistence rules for organic farmers. However, at an informal Council meeting, a majority of agricultural ministers backed the draft (see p.57).
Just as in Switzerland, Austria is committed to GMO-free cultivation and wants to block the EU Commission’s deregulation proposal, as it does not contain any effective measures for the coexistence of NGT breeding and organic farming. For the EU, the goal of achieving a 30% organic farming share until 2030 in the absence of appropriate coexistence rules would no longer be achievable and would make the EU largely dependent on imports. As a means to achieve coexistence of organic farming and NGT crops, the Swiss Research Institute of Organic Agriculture proposes a cultivation cadastre that shows before sowing on which field NGT seeds are to be used. This would enable farmers to reach agreements without the need for mandatory labelling of NGT products.
Sino Biological provides rapid and quality guaranteed monoclonal antibody humanization services using complementaritydetermining region (CDR) grafting technology and computer-aided molecular modeling, based on its deep expertise and over 15 years of experience in antibody development.
Short
3-4
• Comparable to Parental Antibodies or Higher •
Affinity Validated by ELISA and SPR/BLI
• Integrated Analysis Platforms
Mouse Antibody CDR and FR Identification
Affinity Validation


Antibody Modeling Identify Key Residues
Human V Gene
Antibody Modeling Search
Antibody Expression
Can be combined with AI-powered affinity maturation service
CDR Grafting
Back Mutations
Align
Learn more about antibody humanization services
As part of the Pharma legislation reform, the European Commission is preparing simplifications in the approval of medicines in Europe. Short approval times for patented medicines is crucial for the profits of biopharma companies which select where to go depending on the quality of regulatory expertise, openness to industry collaboration, clarity of communication, and a positive regulatory track record. The Commission wants to cut red tape and shorten the time needed for benefit-risk assessments by authorities co-funded by manufacturers. Following stakeholder consultation in September, the Commission will present a legislative proposal to this end. The current rules in Regulation EC 1234/2008 on the scope of data and technical information to be provided by manufacturers will be adapted. The initiative also proposes to extend the risk-based approach for categorising changes to certain biologic medicines, and in particular to update the rules for biologics and active substance changes.
In September, the European Commission has authorised an updated COVID-19 vaccine from BioNTech SE and the company’s global commercialisation partner Pfizer Inc. It targets the still dominant Omicron XBB.1.5 variant. However, a potentially new variant called Pirola (BA.2.86), which differs in 35 mutations from XBB.1.5. is currently spreading globally. Though, the US CDC mark its high potential for immune escape, the authority claims to already know that the XB.1.15 vaccine protects against them.
COMPETITION LAW The European Commission has fined US next generation sequencing (NGS) world market leader Illumina Inc. with €432m for intentionally breaching EU merger regulations when taking over cancer diagnosis pioneer Grail Inc for US$8bn despite the bloc’s authorities ongoing probing of the beleaguered deal. Grail says, its Galleri cancer test can detect a signal shared by more than 50 types of cancer with 99.5% specificity and 88% sensitivity.
The fine corresponds to 10% of Illumina’s annual sales and is thus the highest amount possible under EU antitrust law. Grail will receive a symbolic fine of €1,000. Such a high fine for Illumina is necessary because the company “delib -
erately and intentionally” breached the standstill obligation set out in EU merger control law by closing early, the EU Commission said.
Illumina had completed the €8bn acquisition of Grail in August 2021 and put aside the fine to be paid in 2024 after announcing it in September 2020, without notifying EU competition regulators, who had announced in July 2021 that the review would take longer than expected. In September 2022, the Commission blocked the transaction over concerns that it would have significant anticompetitive effects, stifling innovation and reducing choice in the emerging market for blood-based early cancer detection tests. L

BIODIVERSITY In a memorable vote, the European Parliament confirmed a core element of the EU’s biodiversity strategy by a razor-thin majority. The MEPs rejected an application of German EPP leader Manfred Weber to reject the Commission’s proposal (s. photo). For the first time globally, the renaturation law recognises services provided by nature, such as buffering anthropogenic CO2 emissions through carbon sinks, pollinating plants by insects, etc., which are accounted for as free in previous economic models. The bill will allow for 30% of all former peatlands currently exploited for agriculture (excluding cattle farms) to be restored and partially shifted to other use by the end of the decade, a figure rising to 70% by 2050. L

The novel food sector has gotten a boost in The Netherlands. Tasting has grown much easier, helping start-ups get customer feedback early on – a seemingly simple but important step.

PRECISION FERMENTATION As plant-based alternatives to meat take off, novel foods are no longer looking so novel – even when it comes to those that come only indirectly from animals. Many start-ups are pursuing the lab-based development of milk, meat or fish. What’s missing is the customer experience. The Netherlands is now seizing a position at the centre of the novel food movement in Europe by allowing early tasting events.
Food is no longer just food. It may come from traditional agriculture, which now includes products from land that’s extensively or intensively farmed with the digital help of drones to optimise pesticide use. But the term ‘food’ also covers products farmed according to dynamic-organic regulations that explicitly exclude chemistry, or ones that follow traditional methods, like planting and harvesting during certain phases of the moon. Not even to mention genetically modified crops. But much of the ‘food’ we buy from the supermarket may soon no longer require farmland at all. It can be blended by cultivation devices into a product that outwardly resembles the original fruit, vegetable, meat, fish or milk.
Sounds disgusting, inhuman or unnatural? Well, it’s already happening. It would just be a further step down a road that many foods in our industrialised world have taken. We often mix different ingredients and some natural raw materials to create products that taste like what is pictured on the packet. Just look at the ingredients list on the ice cream you buy in your favourite shop. Do you recognise them, or know what they are for?
The new movement of novel foods originating in the lab is actually in most cases aimed at giving consumers more insight, and talking openly about the processes of cultivation or fermentation. But the landscape is also hard to parse. On the one hand, there’s a lot of investor interest in cultured meat, precision fermentation and cow-less milk or cheese. On
the other, the sector is cooling down because the gulf between product and consumer is still pretty deep and wide. At least that’s true in Europe, where regulation of novel food approvals coordinated by European Food Safety Authority (EFSA) is very slow, to say the least. Consumer experiences of cultivated meat were first celebrated in London (2013), and have since also happened in Singapore. The cost of that complex first gen -
eration burger made of diced cellular matrices was a whopping €330,000.
The field has come a long way since those early days. Professor Mark Post from the University of Maastricht in the Netherlands cultured that first burger from muscle stem cells in fetal calf serum, so the method didn’t meet slaughter-free expectations from the start. But Post and his team at Mosa Meat have since learned to use other ingredients for cultivation, and now guarantee results where no animal was harmed.
So far, only California-based Good Meat (formerly known as Eat Just) has managed to have a cultured meat product approved for public sale. Regulators in Singapore – until recently the only country in the world to allow the sale of such products – greenlighted their cultured chicken in December 2020. “Cultured meat is real meat, but you don’t have to slaughter an animal,” said Josh Tetrick, CEO of Good Meat, in a BBC interview.
?Your comment about the new legislation in The Netherlands?
!We thank all 127 members of the Tweede Kamer who voted in favour of finding a way to make food from laboratories possible. Mosa Meat will use these controlled tastings to gather feedback, but also to educate stakeholders on the role that cellular agriculture can play in Europe to meet food sovereignty and sustainability goals.
Now his home country is following suit. At the end of last June, the US Department of Agriculture gave a first-ever approval for cell-cultured meat produced by two companies: Good Meat and Upside Foods. Both grow small amounts of chicken cells into slaughter-free slabs. It was the final regulatory thumbs-up the California-based companies needed to sell and serve their products in the United States. The approval came less than a year after the US Food and Drug Administration (FDA) declared the companies’ products safe to eat, and is a major mile -

stone for the burgeoning cultured meat industry.
But that doesn’t mean cultivated steaks will be hitting supermarket shelves tomorrow. For now, both companies have just been given the go-ahead to sell chickenonly products to a select handful of restaurants. Marketing cell-cultured beef, pork or seafood will require further approvals.
The Netherlands is now building on the early successes of Mark Post and his team, and has taken up the baton from the US approval. In July, the announcement came that it will soon be possible to taste cultivated meat and seafood in the Netherlands. The Dutch government – in collaboration with producers Meatable and Mosa Meat, alongside industry representative Hollandbio – has successfully developed a ‘code of practice’ to allow tastings in controlled environments. The normal novel food approval process does not allow for pre-tasting. This proviso is what drives start-ups to countries and locations, like Singapore, where there is more openness to food innovation.
The historic decision makes the Netherlands the first country in the EU to allow pre-approval tastings of foods derived directly from animal cells prior to novel food approval in the bloc. It follows the government’s ‘National Growth Fund’, which has committed €60m to build a robust cellular agriculture ecosystem to make the Netherlands a global hub for the emerging technology.
The organisation set up to implement the National Growth Fund plan, Cellular Agriculture Netherlands, will be responsible for implementing the code of practice. It includes hiring a panel of experts to evaluate requests from companies to conduct tastings of cultured meat and seafood.

The code of practice was created following an intervention by the Dutch
House of Representatives in 2022. A motion sponsored by MPs Tjeerd de Groot (D66) and Peter Valstar (VVD) called on the government to enter into consultations with Dutch cellular farming producers to allow for pre-approval tastings under controlled and safe conditions. Companies welcomed the move.
Krijn de Nood, CEO of Meatable, commented on the new framework: “This is great news for the Netherlands. We know that cultured meat can make a significant contribution to reducing climate change. By making it possible to taste cultured meat, the Netherlands continues to lead the way in Europe and beyond. For Meatable, this means that we can give consumers the opportunity to taste and experience our products, and use their feedback to make our products even better. Our goal is to make tasty cultured meat, indistinguishable from conventional meat, available to everyone, without harming people, animals or our planet.”
Maarten Bosch, CEO of Mosa Meat, added: “We thank all 127 members of the Tweede Kamer who voted in favour of finding a way to make this possible, and Minister Kuipers, Minister Adema and their teams for their professionalism and cooperation in making this happen. Mosa Meat will use these controlled tastings to gather invaluable feedback on our products and to educate key stakeholders on the role that cellular ag-
riculture can play in helping Europe to achieve its food sovereignty and sustainability goals.”
The motion was supported by 14 of the 17 voting political parties, including the VVD, BBB, CDA, D66, Christen Unie, PvdA, GroenLinks and others. But it has been a long road to the new legislation, as Timen van Haaster from the Dutch biotech industry association Hollandbio told EuropE an BiotEchnology. “It took years, starting with MPs Tjeerd de Groot (D66) and Peter Valstar (VVD) raising the issue in the Dutch parliament,” said van Haaster. In the end, almost two years ago, they tabled a motion calling on the Dutch government to make room for tasting cultured meat in a safe and responsible way before applying for a Novel Food procedure.
Meatable and Mosa Meat are members of Hollandbio, but the cellular farming sector remains small. Because of the long history and the new global dynamics in the field, these few companies and the industry organisation felt they had to start putting cultured meat on the Dutch political agenda. That created more urgency to implement policies that would stimulate development in the field in the Netherlands. Without it, start-ups might have started looking abroad for friendlier policies. After the motion was passed by a
fairly wide margin in the Dutch parliament, the country’s Ministries of Agriculture and Health – in cooperation with Meatable, Mosa Meat, Hollandbio and a regulatory expert – started working on a protocol to enable tastings. This process resulted in a ‘code of practice’ to which companies will adhere. It includes a procedure for assessing the application by independent experts in a committee that will be part of the Cellular Agriculture Netherlands Foundation.
The tasting of cultured meat and fish will be considered a pilot project that will run for a year, and will be evaluated throughout. The pilot project could then be extended for another year. The decision could then also be taken to make it common practice, or to abolish it if there are unresolvable issues.

A committee of several independent experts within the Cellular Agriculture Netherlands Foundation is expected to be set up in September. Once this committee is up and running, applications can be submitted, and the committee will have one month to respond. This means that the Dutch government’s original expectation that a first tasting could take place before the end of 2023 is still on track. For the year of the pilot tastings, around 10-12 tast-
ings are planned, with up to 30 people attending. “The tasting is not a commercial launch; it’s really about tasting and getting customer feedback early on. We think it will be quite an event, the first tastings of their kind in Europe,” says van Haaster. He believes the impact of the events will go beyond the people in the room where the product is offered. “It’s no longer Singapore or the US. Now we have such an event in Europe. It will stimulate the industry,” he adds.
Allowing tastings and gathering data and insights could prove to be a first useful step for companies in the sector, to gain final EFSA Novel Foods approval.
Hollandbio has received a lot of enthusiastic feedback from companies in the cultured meat and fish industry, including – and perhaps especially – from outside the Netherlands. Many start-ups want to know about the process framework, and how it might also work for them. For example, whether they would have to have an R&D site in the Netherlands before being allowed to apply for a tasting proposal (yes). So far, only the cellular meat and seafood sector is included in the code of practice for tastings, but the demand from other sectors is inevitable. “Start-ups, scale-ups and es-
–innovative solutions
– highest quality standards


– expert technical support for optimizing reliability of your immunoassays











tablished companies from other areas of biotechnology have also asked how this
tion, testing and approval of these novel foods in the EU and Germany, and has

not only to a thriving biotech sector, but more importantly to healthier and more sustainable food production.
A pan-European organisation in the novel food industry, Food Fermentation Europe (FFE) is using the new regulation as an opportunity to step up calls for improved framework conditions. The new industry alliance represents companies like Better Dairy, Formo, Imagindairy, Onego Bio, Standing Ovation, Those Vegan Cowboys, and others. FFE spokesman Christian Poppe told EuropE an BiotEchnology that the organisation welcomes the Netherland initiative with open arms. “This is also a demand that we make of German politics,” he said.
“Food Fermentation Europe (FFE) enthusiastically supports the Dutch initiative, which allows innovators in the cultivated meat sector to conduct pre-market tastings of their novel products. This not only provides consumers with an invaluable opportunity to engage with these products but also proves crucial as these companies approach the brink of com -


VENTURE CAPITAL Dusk or dawn for European biotech companies? Is the US funding downturn affecting the European community, and how? EUROPEAN BIOTECHNOLOGY NEWS spoke to the Munich based team of Kurma Partners, Daniel Parera, M.D. and Dr Peter Neubeck.

EuroBiotech _Dear Mr. Parera, Mr. Neubeck how do you assess the funding situation in Europe?
Parera _There is enough funding available and most companies are still well capitalised. But of course, there is still a Corona backlog. A good part of the companies have not been able to reach the planned milestones, to generate all the data they had planned to have on hand by now.
EuroBiotech _There is still a Corona gap?
Neubeck _There is a difference between US and European companies. In particular in the US, there is a resulting cautiousness of investors to not invest at pre-Corona levels. As a result, an increasing number of companies are coming under pressure and need refinancing. But you can’t generalise. European biotechs are being set-up with a slightly different principle - less capital intense, more capital efficient - because in Europe there is typically less overall capital available for biotech.
EuroBiotech _So everything is fine, please stop complaining?
Parera _European companies have to continue to sharpen their focus, they have to deliver a good clinical programme that ticks all the boxes for Big Pharma. In the US, it is typically the goal to build big companies, that have fully staffed in-house functions. Now, with the above described delays, you see organisational adjustments, smaller refinancings, or indication adjustments. This adjustment now takes time and requires additional capital.
EuroBiotech _All of Europe is Kurma’s core territory - or just certain parts of it?
Neubeck_Kurma is a European fund with French origins. We have been expanding across Europe, including Scandinavia, Southern Europe and, increasingly, East-
ern Europe. We are less active in the US, perhaps more with the growth fund. Especially in early-stage syndication, we tend to be the driver and bring in the partner funds.
Parera (adds)_It is a bit different across our funds. Peter does early-stage projects with Kurma Biofund, and with my fund, Kurma Growth Opportunities Fund we do growth-stage financing. Growth is more often in global consortia, and we are also more active in Asia. Kurma funds are “comparably smaller but mighty”. The other big funds have a lot more money. We are making up for it by bringing our deep scientific and operational expertise to the company, which is very much in demand these days.
EuroBiotech_How does Kurma approach early-stage projects?
Neubeck_Kurma has done company creation since its inception and we have continually refined the way we do this. Since Kurma Biofund II we have been creating companies straight out of academia leveraging our internal competencies and finding suitable industrial partners depending on the technology requirements of the specific project. In addition we conceived and created Argobio, an incubator or startup studio, which we set up in early 2021 together with Bpifrance, the French national investment bank, Angelini Pharma, a private international pharmaceutical company, Evotec SE, and Institut Pasteur. The main rationale for creating Argobio was to scale our proven model of company creation with a dedicated team of experienced entrepreneurs and project managers. Since its inception, Argobio has

now more than 10 projects in incubation and the first two projects will be ready for Series A financing still this year. These two projects will be the first deals we will fund with our upcoming Kurma Biofund IV.
EuroBiotech_How do you plan your early projects, is an exit foreseeable at all?
Neubeck _Exits require planning, execution, good timing and some luck. In our current example with Emergence Therapeutics, it was not easy to put together the initial company creation syndicate and the initial development had the usual roller coaster moments. Once we had good data with our lead project, things moved fast and we were able to put together a nice Series A which was one of the largest ever for a German biotech company with close to €90m. If you then add a very hyped ADC market and many Pharmas on the lookout for good projects in the space, you can end up with an attractive early exit as we delivered with Emergence.
Parera (adds)_It is rather rare that very early-stage projects exit soon. That may be a dream, but it is not the rule. A growth opportunities fund in the same house is one helpful way of being able to participate sufficiently over the life-time of a company and several financing rounds.
EuroBiotech _So something like the Emergence exit is for the most part luck?
Neubeck_We are not speculating on a big preclinical deal. The fact that it turned out this way was a very welcome exception in our eyes. But we had laid the groundwork in the way we had built the company and had made very good progress towards the clinic, so it wasn’t “just” luck.
EuroBiotech _ Is Pharma paying more money, for early-stage projects now?
Parera_The pharma landscape in general hasn’t changed that much. The big players still need external innovation for their pipelines. What has changed is the greater diversity of modalities. There are less and better validated modalities and there are pharma companies taking more or less risk and choosing to be active in completely new disease areas and indications or rather in comparably less risky ones. We see that more validated targets and modalities are in higher demand at the moment. It just takes a long time and a couple of approach generations for something completely new being sufficiently validated and generate prevailing data.
EuroBiotech_But if Kurma is rather “smaller but mighty”, then you cannot cover the whole diversity and have to exclude certain areas, I guess?
Parera _We are already diverse in our portfolio, in terms of indications and modalities. We just don’t invest as large tickets in one area as the larger funds. But diversity does not depend on size. Neubeck (adds)_If you just look at the Argobio pipeline, you can already see a great diversity. We are looking for “best in class” and can initially use up to €2.5m, which we can normally double up with non-dilutive funding opportunities and grants. With these €5m to start with, you can take a lot of risk out of these early project ideas and deliver pharma-quality data early on. This is what the bigger VCs are looking for, risk-adjusted projects ready for Series A funding and this is also what guarantees a proper Pharma-grade data package when we get to the point of exit, which remains per definition at clinical PoC.
EuroBiotech _You’ve mentioned that in the US, Flagship or Arch Ventures are building large companies from the start,
fully staffed in-house. Why is this not happening in Europe?
Neubeck _I think we are just doing things a bit differently and there are two main reasons for that – there is still less money available for early stage ideas and the science is usually even earlier-stage, e.g., just a new target without a clear idea of how to address it. Argobio is build exactly to address and exploit this difference in the respective ecosystems on both sides of the Atlantic.
EuroBiotech _How does Kurma invest?
Parera _The market for the investment is important, but you need a team that understands that market and can reach it with the project. So it is very important to pay attention to the composition of the team, as otherwise you simply lose time if you have to restructure.
Neubeck (adds)_We are investing in excellent science and innovation addressing a clear unmet medical need. If these boxes are checked, you either have to have or to create a team that can design and execute a smart development plan.
Parera (again)_You need different qualities within the team: Visionaries and people who can build a well-functioning organisation. With our investment, we are not aiming to help people catch up on their entrepreneurial training from scratch. So we are looking for both the conductor and the orchestra, and it has to be set up well for the perfect sound.
EuroBiotech _Where are you now? Are you looking for funding or projects?
Neubeck _Actually, we are just about to do our final deal with Biofund III and, in parallel, are working on a first closing of our latest fund, Kurma Biofund IV. We are aiming for €250-300m. For this fund we are lining up investment opportunities which will be a mix of good external opportunities and our own proprietary deal flow coming from Kurma and Argobio.
Parera (adds)_We are moving towards final closing of the Growth Opportunities Fund and have already invested in half a dozen projects. Those who deliver good data in difficult times will shine. L
g.kaeaeb@biocom.eu

PIERRE-YVES GAUTHIER, HEAD OF STRATEGY, ALPHAVALUE Stock markets love investment concepts, calling the robust ones ‘megatrends’. Golden ageing is one such megatrend banking on the fact that business can extract more from people living longer. Such megatrend investments help – up to the moment when rising interest rates crash the party.

A decade ago AlphaValue had a cynical go at the ‘Older & Fatter’ megatrend which speaks for itself: stocks addressing the good-living excesses of the West and their unhappy outcomes. The idea of that ‘Older & Fatter’ came when Novo Nordisk was already making a fortune from diabetescontaining drug, which later on morphed into weight-containing drugs. The point was made about such a ‘Older & Fatter’ megatrend. In the CNS space, another Danish company, H. Lundbeck, was also quickly widening its offer, to tackle schizophrenia, depression, Parkinson etc.
Today we discovered that the strategy was not worth the effort when measuring on an equal-weighted basis, the performance of the relevant universe made of 21 large European stocks. The outper-
formance is only 25% over 10 years, which is insufficient to cover the risk associated with such a specialist exposure. The sorry point is that this well-oiled investment strategy has been losing money since late 2021 when rates edged up. So much for the megatrend: its attraction is only as great as the cheap money punchbowl of the Fed or ECB.
Indeed things turned nasty from summer 2021 as highlighted by the relative performance decline on an equal-weight basis. On a weighted basis, everyone seems to be in the money, courtesy of Novo Nordisk and the craze for its weight-loss drugs. Remember that back in 2013, Novo was worth €57bn. Now expect to pay €400bn, or 7x its 2013 valuation (but LVMH manages 7x too).
Valneva SE (Nasdaq: VALN; Euronext Paris: VLA) and Pfizer Inc. (NYSE: PFE) announced positive immunogenicity and safety data for their investigational Lyme disease vaccine candidate VLA15 in children and adolescents when given as a booster. These results from the Phase 2 VLA15-221 study showed a strong anamnestic antibody response for all serotypes in children (5 to 11 years), adolescents (12 to 17 years) and adults (18 to 65 years) one month
after administration of a booster dose (month 19). But no boosting at the stock exchange was observed after these still early results (see also page XY).
Abcam plc US Danaher Corporation (NYSE: DHR) has entered into a definitive agreement to acquire all outstanding shares of Abcam plc (NASDAQ: ABCM) for $24.00 per share in cash, or a total enterprise value of approximately $5.7bn, including assumed debt and net of cash
To begin with, our 10-year review is a reminder that ultimately investment concepts matter less than the cost of money or the odd systemic accident such as COVID-19. For that ‘Older & Fatter’ strategy to offer the best relative return one would have had to exit by late 2019. COVID-19 had no positive impact – possibly because it led to an early departure of the targeted audience.
Then, the universe of listed companies associated with ‘Older & Fatter’ is simply as expensive as anything related to health. The underlying organic growth is too thin to justify ‘concept’-type valuation multiples (25x and above). Remember that valuations differ substantially whether on an average, median or equalweighted basis.
Anyway, more of a correction is needed before boarding that ‘Older & Fatter’ slow train again L
acquired. This represents a premium of approximately 50% to the average recent trading price of Abcam’s shares. Founded in 1998 and headquartered in Cambridge, UK, Abcam provides antibodies, reagents, biomarkers and assays to target biological pathways critical to the advancement of drug discovery, life science research and diagnostics to a customer base of approximately 750,000 researchers, as the company stated. L
The unique and most complete list of share price developments of biotech companies listed in Europe – exclusively in European Biotechnology Magazine.
All quotes are listed in Euro. All data is provided without guarantee. The effective date is 12 th September 2023. These Europe-based biotech companies are traded on European stock markets.






DATA SCIENCE CONSULTING Depending on who you talk to, artificial intelligence (AI) is either a saviour or an enslaver. The concerns range from loss of jobs through to robots running society. However, in the life sciences, the use of AI and machine learning (ML) have created some excitement over their potential applications.
› Dr Liesbeth Ceelen, CEO and board member of BioLizard, Belgium
While AI has been steadily evolving in the IT community for decades, for the general public it seems to have only recently made the leap from science fiction to reality. Authorities have identified the need for regulation of AI, while technology-savvy entrepreneurs have already created applications for both highly-skilled personnel and the average consumer, the most prominent example being ChatGPT.
This has led to an ongoing critical review of AI to investigate its uses in and effects on industry and society, such as cost savings, innovation and employment. New applications of AI are also steadily being developed, and data-intensive disciplines in the natural sciences, such as biology or medicine, can particularly benefit from the new possibilities, which enable users to process more data than ever before.
To demonstrate the impact of AI, Mount Sinai Hospital in the US is among a group of leading hospitals pouring hundreds of millions of dollars into AI software. They are buoyed by a growing body of scientific literature – such as a recent study finding that AI readings of mammograms detected 20% more cases of breast cancer than radiologists – along with the conviction that AI is a valuable component of the future of medicine.
As most scientists are neither programmers nor informaticians, a new consulting industry is budding. Supporting the evolution and application of AI in science, and providing the interdisciplinary expertise required to translate the story of any research undertaking into code, they become a gateway for
accessing AI, supplementing a difficultto-find expertise.
In the evolution of medicine, technology has always played a part. In the 1990s and early 2000s, AI algorithms began deciphering complex patterns in X-rays, CT scans and MRI images to spot abnormalities. Likewise, companies incorporated algorithms that scanned masses of patient data to spot trends when developing tailored treatments. AI has generally been welcomed by life science companies for its ability to work with massive amounts of data, and to generate datadriven results such as new drug targets.
Several AI-native drug discovery companies have progressed AI-based mole -
cules into clinical trials, reporting greatly accelerated timelines and reduced costs, and raising high expectations in the R&D community. This performance has been discussed in a recent Nature Reviews article, where the disclosed discovery programmes and preclinical assets of the top 20 of these companies were compared with the top 20 of Big Pharma. Astoundingly, these young AI-native companies already have a combined pipeline with close to 50% of the number of assets that the top 20 of big pharma are running.
Being involved in bringing many projects and innovations from concept to user, the BioLizard team has experienced and worked with a wide range of requests and ideas for the application of AI across the biotech and pharma space.
To give an example of how AI is implemented from concept to product, one recent project for a client was an end-toend, data-driven solution for predicting clinical outcomes in transplantation patients. The main focus was on using RNA sequencing (RNASeq) data to predict different types of organ transplant rejection in patients. The task was to expand and improve the efficiency of the approach by providing an end-to-end workflow, from input of raw RNASeq data through to the prediction of transplantation rejection.
The data analytics & AI team first set out to create predictive models for both acute and long-term organ rejection using a dataset of 60,000 genes from 150 patients. However, the complexity of


Unify and streamline:
Quality Processes
Content Management





















Validation Management

Role-Based Training
QC Lab Operations
Reduce Cycle Time
Improve Usability

Maximize Efficiency
Connect to External Partners



the data, compounded by the biological variability among different ethnicities, genders, and ages of the patients, at first made it difficult to derive meaningful biological insights.
Consequently, the team narrowed things down to a more manageable set of genes that were found to have the highest predictive value, and then worked with the client to choose a subset of clinical features that would also be included in the predictive models. This way, the resulting models would take into account not only information related to gene expression, but also other potentially predictive variables, such as the presence of patient comorbidities.
BioLizard improved and tested the models over time, and the final result presented to the client was a highly standardised and data-driven product that is now being validated for use in a clinical setting to predict patient outcomes. This case is a great example of how human input is still necessary to make the most out of AI: by selecting the best set of predictive genes and a complementary subset of clinical information, a client was being enabled to apply AI to its highest potential.
Regulation is a huge consideration in the development and use of AI. The FDA, in a recent discussion paper, acknowledged current and potential use of AI in the field, and agreed that AI/ML has the potential
to accelerate drug development and make clinical trials safer and more efficient. However, the importance of assessing if AI/ML introduces risks is also noted. For example, AI/ML algorithms could amplify errors and pre-existing biases present in underlying data sources and thereby, when the findings are extrapolated outside of the testing environment, raise issues related to generalisability and ethical considerations. These concerns have resulted in the development of standards for AI to address areas such as explainability, reliability, privacy, safety, security, and bias mitigation.
The European Commission also promotes the use of AI, while emphasising the need for data protection. The current draft framework of the EU Artificial Intelligence Act is still evolving, which is why the European AI Alliance has been created to provide a forum for open policy dialogue. Some of the concerns expressed by healthcare AI stakeholders have already been addressed, but one key issue remains. As it stands, provisions for governance of data appear to exclude most real-world data as a source of evidence. This would greatly diminish the applicability of AI in healthcare, as most data from the laboratory or clinical studies could then not be used to train AIs to develop predictive capability. At the time of publication, the regulator is still open for discussion to reach a pragmatic solution. Another real obstacle to the application of AI can be misconceptions. AI is not magic, and cannot provide reliable re -
sults if not fed with the right data and human insights, and if the output is not interpreted correctly. As with all analysis methods, it is important to consider where the data comes from. If an experiment was conducted in a way that is not applicable to the question at hand, AI won’t be able to draw reliable conclusions. On the other hand, applying the vast amounts of public data resources available can be a path forward, when using an applicable and relevant, not necessarily perfect, data set as basis. Identifying the right input data for the question and ensuring careful interpretation of the output information – both of which may be challenging as this requires both biological understanding and expert knowledge in data science – is of crucial importance, and part of the expertise BioLizard is providing.
Another misconception that can easily be overcome is related to costs. Many consider AI to be expensive, while not considering that just like in the wet lab, it is possible to start with small proof-ofconcept studies to see how and where AI will add value to a company. For this reason, BioLizard assists clients by examining their unique situations and pinpointing key areas that could be streamlined using AI/ML. While not always applicable, often opportunities for improvement arise, and BioLizard can help clients decide where AI can add the most value. There can be little doubt that AI is here to stay and, as it evolves, it has the potential to positively transform the life sciences sector. However, regulation and risk mitigation are clearly important, and the industry needs to be a part of that ongoing discussion.
As with any new technology, utilising AI and ML appropriately and wisely is critical to successful implementation. This is why it is important to know who to turn to in order to navigate this new and exciting path towards the ultimate goal – a more efficient life sciences industry that provides new and better solutions for patients. L
Fördergesellschaft IZB mbH Am Klopferspitz 19 82152 Planegg/Martinsried

Tel. + 49 (0)89.55 279 48-0




Fax + 49 (0)89.55 279 48-29
info@izb-online.de www.izb-online.de

Site: 26,000 m2, S1 laboratories










Real estate and facility management on site







Faculty Club and conference rooms for up to 100 people


Kindergarden (Bio Kids), Chemistry School Elhardt
Hotel CAMPUS AT HOME
Restaurant SEVEN AND MORE, The Bowl
On the Martinsried Campus: over 50 start-ups in the IZB, two Max Planck Institutes, ten faculties of the LMU, Clinic of the University Munich





In August, CureVac NV announced that dosing for its Phase II study with a modified COVID-19 mRNA vaccine candidates in collaboration with GlaxoSmithKline plc has been started. A first data read-out of the study that includes a mono- and a bivalent vaccine candidate using modified RNA is expected early in the first half of 2024. The monovalent candidate, CV0601, exclusively encodes the spike protein of the omicron BA.45 variant, while bivalent candidate, CV0701, additionally includes the original SARS-CoV-2 strain.
Swedish biotech company Hansa Biopharma A/S has dosed the first of ten patients with imlifidase in an investigator-initiated single arm Phase II study at Charité Berlin in anti-neutrophil cytoplasmic antibody (“ANCA”)-associated vasculitis caused by pulmonary hemorrhage. Imlifidase acts by cleaving IgGs enzymatically. Efficacy and safety of imlifidase will be assessed by evaluating ANCA antibody seroconversion and titers, adverse events, mortality, as well as amelioration of lung and renal function over a 6-month observation period.
Eli Lilly’s Alzheimer’s drug donanemab has quickly removed amyloid plaques in a pivotal Phase III trial halting disease progression for one year in half of patients. Donanemab is the second antibody, after Eisai’s/Biogen’s accelerated approved lecanemab, that can slow the progression of dementia
Finnish immuno-oncology specialist Valo Therapeutics Oy and its CRO Texcell SA (Evry, France) have agreed to evaluate immune responses in ValoTx’s Phase I study that is designed to assess the immunogenicity of the company’s lead candidate PeptiCRAd-1. ValoTx’s lead platform (Peptide-coated Conditionally Replicating Adenovirus) was developed out of the laboratory of Professor Vincenzo Cerullo at the University of Helsinki. It turns oncolytic adenoviruses into powerful activators of systemic anti-tumour cytotoxic T-cell immunity without the need to generate and manufacture multiple genetically modified viruses. PeptiCRAd-1 is the company’s lead product made up of its virus VALO-D102, which expresses the immuno-stimulatory proteins CD40L and OX40L and is coated with MAGEA3 and NY-ESO-1 peptide derivatives. In the ongoing open-label Phase I study of up to 15 patients in German centres ValoTx will assess the safety and immunological mechanism of action of PeptiCRAd-1 in combination with Merck Sharp & Dohme’s checkpoint inhibitor pembrolizumab in multiple cancer types. The collaboration with Texcell aims at providing insights on PeptiCRAd-1’s ability to modulate immune responses and the mechanism of action of VALO-D102.
Stockholm-based Calliditas Therapeutics AB has reported interim data from its Phase IIb trial in patients with squamous cell carcinoma of the head and neck who were treated with its lead NOX 1 and 4 inhibitor product candidate, setanaxib. The company assumes an encouraging trend in early clinical progression-free survival (PFS) results and sees hints to the presumed anti-fibrotic mode of action of setanaxib. On the basis of 12 evaluable biomarker profiles from patients with recurrent or metastatic SCCHN, transcriptomic analysis supported an anti-fibrotic mode of ac-
tion. The Idiopathic Pulmonary Fibrosis Signaling Pathway and the Hepatic Fibrosis/Hepatic Stellate Cell Activation Pathway were affected. Furthermore, pathologist found preliminary evidence of an increase in immunological activity of treated patients, with favourable changes in Foxp3 and PDL-1 CPS. Additionally, seven out of the 16 evaluable patients were progression-free with either stable disease or partial response. The study is expected to read out final data in 2024.
Immuno-oncology specialist Scancell Holdings plc has opened its ModiFY trial for expansion in combination with checkpoint inhibitors (CPI) and in the neoadjuvant setting. Following the successful completion of Cohort 4, where three patients received at least two doses of Modi-1 combined with CPI, the safety review committee approved on 31 July expansion into two cohorts of patients with renal or head and neck cancer who receive CPI as standard of care. Twenty-one patients will be recruited into each cohort. Patients with triple negative breast cancer will not be included in this part of the study as these patients receive checkpoints in combination with chemotherapy which may induce citrullination in normal cells and induce toxicity. Additionally, recruitment into the neoadjuvant arm of the Modi-1 trial in combination with CPI was also approved. This study will recruit 30 patients who will be randomised at diagnosis to receive either two doses of Modi-1 three weeks apart or two doses of Modi-1 plus one dose of CPI. Tumour biopsies will be taken prior to immunisation and from the tumour resection six weeks following the initial vaccination. The two tumour samples will allow the extent of T cell infiltration and activation pre- and post-Modi-1 vaccination to be assessed with and without a checkpoint inhibitor.
Berlin-based TME Pharma NV has reported in July that one out of six glio -
blastoma patients enrolled in the company’s GLORIA Phase I/II expansion arm showed a complete response after having dosed with the CXCL12 inhibitor NOX-A12 in combination with standard of care radiotherapy and anti-VEGF, bevacizumab. In addition to the patient, whose tumour was no longer detectable by MRI and previously had shown best response of 89.9% tumour shrinkage, two patients were reported to show near-complete reductions (>99%) in tumour size. NOX-A12 (olaptesed pegol) is an intravenously administered, PEGylated L-stereoisomer RNA aptamer that targets CXCL12
PARKINSON'S DISEASE Bayer AG’s gene and cell therapy subsidiary BlueRock Therapeutics has met all safety endpoints in an open-label Phase I study with its Parkinsion’s cell therapy candidate bemdanepro-cel (BRT-DA01). However, there were two severe adverse events that were unrelated to bemdaneprocel, one seizure attributed to the surgical procedure and one COVID-19 case. Both resolved without sequelae. Twelve patients received surgical transplantation of bemdaneprocel cells to the post-commissural putamen bi-laterally, and administration of a one-year immunosuppression regimen. Cohort A (5 subjects) received a dose of 0.9 million cells per putamen. Cohort B (7 subjects) received 2.7 million cells per putamen. Safety and tolerability were assessed at one year, along with evidence of cell survival and motor effects. The feasibility of transplantation was also assessed. All assessments will continue over 24 months. According to BlueRock Therapeutics, no major safety issues were observed in all dosed patients through 12 months. Furthermore, at one-year, exploratory clinical endpoints improved overall, with participants in the high dose cohort showing greater improvement. Data from all 12 patients demonstrated feasibility of transplantation, cell survival, and engraftment. The company announced to begin enrolling participants in H1/2024. Bemdaneprocel (BRT-DA01) is an investigational cell therapy designed to replace
the dopamine-producing neurons that are lost in Parkinson’s disease. These do paminergic neuron precursors are differ entiated from human embryonic stem cells. In a surgical procedure, these neu ron precursors are implanted into the brain of a person with Parkinson’s dis ease. When transplanted, they are hoped to reform neural networks that have been severely affected by Parkinson’s and restore motor and non-motor function to patients.
In mid-August, Lytix Biopharma A/S ’ (Oslo) licensing partner Verrica Pharmaceuticals Inc has reported lesion clearance data from Part 1 of an ongoing Phase II study of VP-315 (LTX 315) for the treatment of basal cell carcinoma (BCC). Consistent clinical and histological clearance of treated BCC lesions was observed by Day 49 post-treatment with the 8 mg dose of VP-315, in four out of six patients (67%) showing complete tumour clearance, and two subjects showing a partial response in tumour burden reduction. Optimisation of the 8 mg dosing regimen is under investigation in Part 2 of the study.
At the end of July, Sanofi SA and BioNTech SE announced the termination of SAR441000 (BNT131), a Phase I program that is part of a multimillion-dollar research collaboration on RNA anticancer agents. SAR441000 is a mixture of mRNAs encoding the four proteins IL12sc, IL-15sushi, IFN-alpha2b and GMCSF. While preclinical data had encouraged Sanofi and BioNTech to clinically investigate SAR441000 as a monotherapy and in combination with Regeneron’s licensed PD-1 blocker, Libtayo, an unpublished interim analysis of the results has now brought the program to an end. Positive interim results with 17 patients with advanced-stage melanoma, cutaneous squamous cell carcinoma or head and neck squamous cell carcinoma presented three years ago had motivated the companies to expand the trial to 77 patients. L
Partner with a leading global CDMO for aseptic fill & finish
For 40+ years, pharma and biotech companies around the world have relied on Vetter to put their parenteral medications on a path to success. We’re proud to help advance your innovative therapy with flexible, robust, scalable processes and services that set our partnership apart:

• Specialized support for your unique clinical development program
• High-quality aseptic filling at both clinical and global commercial scale
• Strategic packaging and device assembly solutions that span your product’s life cycle
• Comprehensive technical, analytical, and regulatory expertise at every step
TARGETED ONCOLOGY THERAPIES Precision medicine tailors therapeutics to individual patients’ diseases, and is seen as the future of clinical practice. With significant time and financial investments being made in developing therapeutics, the ultimate success of patient-data driven precision therapies hinges on three main factors: the quality of the tissue samples, the associated molecular data, and the analysis used in their development.
A foundational aspect of precision oncology is the data-driven understanding of cancer, which requires high-quality biospecimen. Use of suboptimal specimen can therefore lead to loss of vital information critical for biomarker and therapeutic target identification. Obtaining freshly frozen and consistently collected and processed biospecimen with high sample integrity is crucial for generating clinically relevant patient-based data. Accurate therapeutic target identification and defining patient subgroups are critical in precision medicine. To ensure success, the tissue type and patient subgroup should be optimised for each therapeutic target during preclinical research.
Analysing the data is as essential as sample quality. The emerging field of multi-omics offers promise by analytically combining distinct datasets that together provide broader insights into the disease. However, large and complex datasets can present challenges, which machine learning and cloud-based computing tools can help address, facilitating efficient target selection.
In-vitro target validation is necessary before progressing through discovery pipelines. Patient-derived cellular models, like organoids, enhance preclinical validation efforts, optimising chances of success.

Preclinical research presents unique challenges in that it is expensive, laborious, and marked by a high failure rate in identifying therapeutic targets. To address and overcome these challenges, Indivumed Therapeutics developed nRavel ®, a discovery and development platform. Based on the standardised tissue and clinical data collection in partner clinics and the extensive multi-omic data processing, nRavel® sifts through patterns and identifies, characterises, and validates targets using AI-integrated data analytics and patient information, alongside patient based cellular model experimental results.
It utilises the same tissue and corresponding data throughout the entire drug
development process to gain insights, drive therapeutic target and biomarker discovery and validation, and lead development and clinical trial performance to facilitate the discovery and development of targeted therapies.
Starting and ending with the patient in mind, Indivumed Therapeutics is the only company possessing such a vast amount of high-quality, deep molecular data. The company remains at the forefront of therapeutic development, with actionable targets currently being validated using patient-derived organoids across ten cancer types and the ultimate goal of ensuring the global delivery of effective precision therapies to patients in need.
INCUBATOR For 25 years now, BioM has been the network organisation of the biotechnology sector in Munich and Bavaria. To shape the future of biotechnology, BioM identifies relevant and sustainable local, regional and global trends and creates a unique ecosystem for innovation and growth.
Biotechnology has undergone a remarkable transformation in recent years, becoming a dynamic and multifaceted field in which the convergence of areas such as biology, medicine, computer science, and data science plays a central role.

The new tasks of biotechnology lie in increased, interdisciplinary and interactive collaboration. Moreover, the seamless integration of data analysis, cutting-edge digital technologies, such as artificial intelligence and machine learning, is pivotal to staying at the forefront of innovation and ensuring the successful application of biotechnological advancements.
BioM is addressing this trend within its new concept AI4Biotech, by which BioM aims to leverage artificial intelligence and advanced (digital) technologies including data science to revolutionise the field and various aspects of nanoand biotechnology in particular for drug development and delivery.
The innovative field of Advanced Therapy Medicinal Products (ATMPs) and the scaling and manufacturing of cell and gene therapeutics will also have a major impact in therapy and cure of diseases. By developing and fostering scientific networks, Bio M is contributing to unlock the possibilities of these promising approaches.
To achieve the greatest benefit for society, it is important to carry these
trends into all areas and phases of biotechnological developments – and to bring together all players and disciplines. This is what Bio M is doing. We bring together all the stakeholders in biotechnology: scientists, founders, entrepreneurs, investors, and politics.
In addition to our tailored support for founders, we support selected Bavarian pre-seed and early-stage startup teams from the life sciences and healthtech sectors with the Munich Accelerator Life Sciences & Medicine (MAxL), our unique co-creation startup incubator. MAxL offers exclusive high-end infrastructure on 900m2 , access to a vibrant start-up community and BioM 's extensive network. We also offer partnerships with pharma, big biotech, deeptech and other industry
players like strategic partners, CROs and investors.
In an increasingly interconnected world, BioM operates from a global perspective. We empower and support Bavarian biotech companies of all stages to broaden their horizons and make a difference through cross-border collaborations on a global and interdisciplinary level. We will continue to drive the future of biotechnology, not only in Bavaria, but throughout Europe and the world. Together, we will continue to unlock the potential of biotechnology.
Contact us:
Prof. Ralf Huss
BioM Biotech Cluster Development GmbH
Am Klopferspitz 19a
D-82152 Martinsried
info@bio-m.org
LOCATION Austria has gained an outstanding reputation as a research location in the field of life sciences – both as a vibrant hub for science and as a cluster for research-oriented and manufacturing companies. The ABA links international companies with this ecosystem.
The figures speak for themselves: Austria is home to almost 1,000 life sciences companies with a workforce of more than 60,000 employees, alongside 55 research institutions with close to 25,000 employees, and about 77,000 students focusing just on life sciences. It is no exaggeration to say that Austria – a country with just nine million inhabitants – has emerged as one of Europe’s most important research locations for pharmaceuticals and biotechnology. In 2020, the country’s pharmaceutical and biotech sector generated €25bn in revenue, showing 10% annual increases year after year.
Research and development are driven by 35 big pharma players and numerous innovative biotech firms. These include big names like Boehringer Ingelheim, Takeda and Novartis as well as many smaller, innovative companies, service providers, suppliers and sales organisations. The excellent infrastructure in Austria (including laboratory space), the large number of highly qualified employees and the availability of data and donors support the boom as a location for medical research.
Another yet even more important aspect is that foreign and national companies as well as research institutions highly value the unbureaucratic funding along with the intensive linkage between science and industrial research in the country.
Austria
As a life sciences hotspot, Austria has succeeded in creating a rather unique symbiosis fusing science, education, business relocation and cross-cutting business promotion. In terms of its R&D to GDP ratio, Austria is ranked third among all EU member states at 3.26%. The country improved to sixth place in the European Innovation Scorecard and thus leads the group of “strong innovators” within the EU. The good underlying conditions are attracting an increasing number of firms – from multinationals to startups.
› Vienna serves as the centre for cancer research of Boehringer Ingelheim. Boehringer Ingelheim already put a production plant for active pharma -

ceutical ingredients into operation and will open a new cancer research building in 2024.
› The Swiss company Novartis located its largest single facility for research, development and production in Tyrol. The site has made a name for itself in the media because it is home to Europe’s only complete production chain for oral antibiotics.
› The Mainz-based company BioNTech SE, which gained global fame during the coronavirus crisis, acquired PhagoMed, the Viennese developer of precision antibiotics, thus expanding its portfolio of promising anti-infectives.
› 4,500 employees in Vienna, Linz and Orth an der Donau conduct research on gene therapies, recombinant proteins, plasma-based therapies and innovation technology for the Japanese compa -
ny Takeda, a global biopharmaceutical player. Furthermore, Takeda also operates twelve plasma centres in Austria and produces gene therapy products, biologics and other medical products.
› The MedTech startup Sarcura founded in 2019 focuses on developing personalised cell therapies on a large industrial scale. At the end of last year, it succeeded in raising a further €7m from existing and new investors.

› Myllia Biotechnology in Vienna makes use of the CROP-Seq workflow (CRISPR droplet sequencing) developed by Myllia‘s co-founder Christoph Bock to map the impact of thousands of genetic perturbations on the global transcriptome at single-cell resolution. This approach is widely used in identifying novel drug targets, elucidating unknown mechanisms of actions of drugs and understanding genetic variants associated with risks of disease.
Besides political and financial incentives, the interface linking science on the level of institutes to highly innovative companies is perhaps the most important argument of all in favour of Austria as a research location. Vienna and Innsbruck are home to the biggest clusters. Half of all biopharmaceutical firms operate in the capital city. The Eric Kandel Institute - Center for Precision Medicine is being set up at the Medical University of Vienna in close proximity to Vienna General Hospital, home to the world’s largest university clinics. Vienna with its Co-Location Center Austria as a startup incubator for life sciences companies is also one of the hubs in the European Institute for Innovation & Technology Health.
Other science locations also stand for strong life sciences expertise in addition to Vienna. For example, the Medical University of Graz is known as a headquarter for blood banks. Graz is home to one of the biggest clinical biobanks anywhere and the EU headquarters of BBMRI-ERIC, the European research infrastructure for biobanking.
The Medical University of Linz is positioned as an interface for science, software and health offering a good home for eHealth startups and the Medical University of Innsbruck as a research centre for oncology, neurosciences and genetics.
Health and pharmaceutical research including technological research is one of the major issues in this century. Continents and nations are engaged in a fierce competition for the best ideas, minds and business models.
In this regard, Austria has developed a particularly attractive funding policy compared to many other nations, directly paying an uncapped research premium of 14% of R&D expenditures to researching companies. This not only applies to in-house research but also contract research, staff costs and much
more. In this way about €1bn in funding has been paid out in recent years.
An “immigration allowance” further enhances Austria’s attractiveness for companies and research facilities from abroad. This foreign researchers’ 30% tax deduction limited to five years applies to all income from scientific work. The Austrian Science Fund (FWF) supports basic research, the Austrian Research Promotion Agency (FFG) offers target funding packages to researching companies for the entire development cycle and clinical studies, and as the government’s promotional bank, Austria Wirtschaftsservice GmbH (aws) provides grants and loans.
Contact us:

Austrian Business Agency (ABA), Birgit Reiter-Braunwieser
Telephone: +43 1 588580
E-Mail: b.reiter-braunwieser@aba.gv.at https://investinaustria.at/
Anyone thinking about setting up a life sciences company or research location in Austria is in the right place with ABA. Austria’s national business location consultancy Austrian Business Agency informs and advises interested parties free of charge – but also does much more. It provides comprehensive support to companies in the initial phase and offers subsequent business development assistance. ABA’s services include market and industry research, site search (also for laboratory space), networking with research institutes, information on establishing and expanding companies and consulting on potential funding, tax aspects and financing options. Finally, ABA also helps in searching for qualified skilled workers. ABA’s overall package enormously facilitates the decision in favour of a business location in Austria. L
TECH-PARKS A well connected and effective infrastructure, a high density of research and development as well as close networking are of great value, not only to founders. Within the successful healthcare cluster Berlin-Brandenburg, these include numerous technology parks in the region. The parks offer optimal space including state of the art laboratories to start-up and grown-up companies.
› Dr Kai Uwe Bindseil; Head of Division Healthcare, Industry, Infrastructure at Berlin Partner for Business and Technology GmbH
With a total of eight technology parks in the life sciences, HealthCapital BerlinBrandenburg is unique in Germany in terms of size and diversity: These include the Berlinbiotechpark Charlottenburg, the Biopark Luckenwalde, the Campus Berlin-Buch, the Innovation Park Wuhlheide, the GO:IN Innovation and Startup Center in the Potsdam Science Park in Golm, the Innovation Forum Henningsdorf and the Science and Business Location Adlershof (WISTA). These parks provide a total of about 250,000 square meters.
To meet the strong demand for space of growing companies in the metropolitan region and to increase the attractiveness for start-ups in the life sciences, several expansions of the centers and new
space offers for founders are already being implemented or planned.
The BerlinBioCube on the Campus Berlin-Buch will open this year as a new start-up center on October 11th and will create space for up to 400 new jobs. Flexible and modern laboratory office space is offered on a total of 8,000 square meters. The start-up center is already the fourth construction phase in the BiotechPark and will host start-ups in the fields of innovative therapies and diagnostics.
Also, the Potsdam Science Park in Potsdam-Golm is expanding: Upcoming laboratory complexes will provide 78,000 square meters of rental space for start-ups and companies from life sciences and biotechnology in the immediate vicinity of
the seven hectares of commercial properties at the new Technology Campus.
The FUBIC Innovation Center (Business and Innovation Center next to the Free University Campus) is currently being built in the direct environment of FU Berlin. It will be equipped with offices, co-working space and laboratories for up to 85 start-ups and young companies from the life sciences, healthcare and IT sectors.

Complementing these expansions, new, highly specialised sites are being built, such as SEE:LAB – a competence center for biomaterials on the Campus Teltow-Seehof – with over 2,000 square meters. Currently, 55% of the building, which opened in late 2022, is leased. By the end of 2023, 75-80% is expected. Together with Hereon Helmholtz Zentrum, a start-up ecosystem is being developed in the life sciences and bioeconomy sectors.
The Chemical Invention Factory at the Technical University of Berlin plans to host faculty spin-offs in the future. The mission of CIF is to boost start-up activities based on the excellence of the chemical sciences in the Berlin region. Being located at the University’s campus in the middle of Berlin, CIF is offering earlystage teams the necessary laboratory- and office-space as well as access to ecosystem-partners such as mentors, coaches, funding and public authorities. L

INNOVATIVE ECOSYSTEM Individually customised therapies for every patient – this is the vision of the future which numerous companies, research institutions, and clusters focusing on precision medicine in Saxony are working along the entire value creation chain. They are primarily active in oncology and have gained national and international acclaim and renown both in highly specialised cancer diagnostics and innovative treatment methods.
SaxoCell, the Saxon cluster for precision medicine, wants to become an international leader in innovative cell and gene therapeutics. Towards this end, the network, an alliance of leading research institutes and medical facilities from Leipzig, Dresden, and Chemnitz as well as national enterprises, is working, for example, on specific projects which are designed to optimise the CAR-T cell therapy and adapt it for use in other therapies.
In Dresden, experts from the National Center for Tumor Diseases (NCT), the National Center for Radiation Research in Oncology (OncoRay), the German Consortium for Translational Cancer Research (DKTK), and the Else Kröner Fresenius Center for Digital Health (EKFZ) are investigating new therapies for cancer and tumor treatment by pursuing different approaches and research methods. The institutes’ research activities are geared towards the application of artificial intelligence for the evaluation and interpretation of complex image data and the application of high-precision radiation therapies with a specific focus on particle and proton therapy. The Dresden University of Technology's (TU Dresden) new degree programs in Biomedical Engineering and Medical Informatics both heavily incorporate artificial in -
Fluorescence microscopy in the new Centre for Radiopharmaceutical Tumour Research of HelmholtzZentrum Dresden-Rossendorf (HZDR)
telligence in medicine as a pioneering scientific field.
New diagnostic approaches and methods for fighting cancer raise the demand for so-called radiopharmaceuticals. The new Saxon “Radiopharmaceutical Valley” seeks to close this gap with innovative solutions. It is a highly specialised niche in which such companies as Rotop Pharmaka, ABX advanced biochemical compounds, and TRIMT as well as the CUP Laboratories Radeberg are active together with the research center Helmholtz-Zentrum DresdenRossendorf (HZDR). The new clinical
area of application of “theranostics” combines molecular imaging with radioligand therapy. It is currently evolving into one of the most promising targeted therapies with surprisingly low side effects.
The Molecular Diagnostics Group (MDG), which consists of the three companies BIOTYPE (molecular precision diagnostics), qualitype (software & data) as well as Rotop Pharmaka (molecular imaging), develops and promotes innovative analysis methods and diagnostics for precision medicine. Coordinated from Dresden, MDG is developing a number of innovative cancer diagnostics in strategic partnerships at a national and international level.
When it comes to precision medicine, an innovative ecosystem has emerged in Saxony which offers a very attractive environment not only for cooperations, and new business partners, but also for startups and business setups. Saxony Trade & Invest is happy to be your contact for this.
Contact us:
Saxony Trade & Invest
Wirtschaftsförderung Sachsen GmbH
(WFS)
+49-351-2138-0
E-Mail: info@wfs.saxony.de
www.business-saxony.com
Cell-based cancer immune therapies look set to become a new cash cow for developers of cell and gene therapies.
BIOMEDICAL RESEARCH Cell and Gene Therapies (CGTs) have emerged as one of the fastestgrowing sectors in the life sciences industry. Supply chains in the field are more complex than in traditional pharma, due to the fact that CGTs for rare diseases mostly can’t be produced centrally, and still require a lot of hands-on work. For companies to take advantage of Europe’s excellent science base, networking stakeholders and safe reimbursement are proving to be crucial factors. Approaches in other regions have shown how to turn concepts into therapies.
Since Novartis received FDA approval for the very first (CAR-)T cell-based blood cancer therapy (Kymriah) in 2017, the Advanced Therapy Medicinal Products (ATMPs) that involve cell, tissue engineered and gene therapies have really come into their own – outperforming annual growth rates of the most lucrative indications three to fourfold (see Fig. 1). ATMPs, many of which have been optimised by researchers for at least 30 years, are now providing hope for cures to many diseases that in the past were difficult if not impossible to treat with conventional medications. Examples include patient-derived CAR-T cell therapies that have knocked out several blood cancers, as well as gene therapies against haemophilia A, spinal muscular atrophy or the heritable immune deficiency ADA-SCID.
However, due to both their nature and very small patient populations, CGTs face several challenges (see p. 39) compared to centrally manufactured biologics such as antibodies. These include scaleability and immune rejection of gene therapy vectors or administered cells, the many manual steps in engineering the cells suited to a patient’s genetic make-up, and the logistics of autologous cells on the hospital site – to name just a few.
CGT developers in the EU have in -
creasingly lost ground to the US and China when it comes to clinical development and funding in the field, despite a historically superior research base. But in an effort to advance the lucrative cellular immunotherapies for cancer, which make up the bulk of the clini-
cal CGT pipeline, there are now at least good role models in Europe demonstrating what changes in the recently updated EU Pharmaceutical Legislation could help EU biotechs advance.
In retrospect, when it comes to CGTs at least, the EU may regret Brexit. In the UK, remarkable things are currently happening in the rapidly evolving field – although China and the US remain far ahead of Europe in terms of numbers of preclinical and clinical CGT studies, investment and developers (see Fig. 2, p. 38). In June, for instance, UK-based Adaptimmune Therapeutics plc shook up the sector by completing its US marketing application for afami-cel, the world’s first TCR-T cell therapy to treat solid tumours.
Fig. 1: Revenues with CGT including RNA, CRISPR, gene and cell therapeutics have been growing at an annual rate of 60% since 2017 – three to six times more than in the top 10 therapy areas by value (including the lucrative US$184bn cancer market). This also benefits GCT CDMOs, whose revenues are expected to grow from currently US$4.8bn to US$15.4bn by 2030.
Solid tumours, unlike the blood cancers for which all engineered immune cell therapies (mostly T- or NK cell-based) have been approved, account for 90% of all cancers. That makes them a potential goldmine for Adaptimmune and the rest of the pack. Big news came in June with a company announcement at the ASCO, the world’s most important cancer con-
Fig. 2: China and North America are outperforming the EU in terms of CGT development, even though 120,000 papers published in the field were affiliated with a European institution in 2019 – a year that saw only 72,000 US papers and 100,000 Chinese-authored ones. But as research needs funding, this is currently changing.
gress. Adaptimmune reported that two years after a single application of the subject’s own genetically engineered TCR-T cells, 70% of terminally ill patients with synovial sarcoma were still alive. The British firm had previously taken over TCR 2 Therapeutics Inc, forming the world’s first company exclusively developing cell therapies against solid tumours. While other cell-based cancer immune therapies have to be injected intro-tumourally, TCR 2 has stabilised TCR-Ts to the point that they are able to migrate through the blood and tumour stroma into the targeted carcinoma.
“Together, we will advance our industry-leading pipeline, making cell therapy a mainstream option for people with cancer,” said Adrian Rawcliffe, Adaptimmune’s CEO, upon the acquisition. “This starts with gaining approval for the first engineered T-cell therapy for a solid tumour – afami-cel for the treatment of synovial sarcoma – and progressing our Phase II trials for patients with ovarian cancer with both ADPA2M4CD8 and gavo-cel.”
The UK did its homework concerning what biotech companies need to relocate, short approval timelines for clinical trials, clustered excellent science, investment, potential partners, CDMOs/CROs, tech providers, and experts to solve logistic or regulatory problems. And not only when it comes to
CGTs: “We have seen that drug development can be accelerated without taking shortcuts if everyone works together seamlessly towards the same goal,” said BioNTech SE’s CEO Ugur Sahin, after he announced a strategic partnership with the UK government for the development and testing of the company’s RNA-based cancer vaccines in early January (see EuropE an BiotEchnology I/2023).
Adaptimmune was also one of the first partners at the world’s third-largest CGT cluster in Stevenage, which spun out a stem cell-focused subsidiary in Edinburgh in June. Clusters like CGT Catapult seem to have a magnetic effect on biotech companies and their service providers, like CGT CDMO Rentschler ATMP
Ltd, which was spun out in 2021 from German CDMO Rentschler Biopharma SE. “We decided to establish our facility for cell and gene therapy in the UK, driven by our corporate strategy to make new and innovative modalities available to our clients,” said Dr. Christian Schetter, CSO at Rentschler Biopharma SE, in a talk with Europ E an B iot E chnology.
“The UK has the largest industry cluster for CGT outside North America, offering comprehensive support and funding for academic and biotech research. And CGT has a good standing in society. We were also impressed by the Catapult Initiative from Innovate UK, which provides infrastructure for ATMP process development and cGMP manufacturing. By locating our facility in Stevenage, we are close to established CGT biotechs, as well as to clients and talent. Moreover, the UK is an ideal hub for our global activities, also from a US perspective,” Schetter added.
According to the US-headquartered Alliance for Regenerative Medicine (ARM), however, one factor is crucial to whether and where the potential of CGTs will be realised – the ability to hire qualified staff. The alliance cites a tripling of CGT companies (to 2,700) since 2018, and points
Fig. 3: As of Q4/2022, 32 cell and gene therapies had been approved in the US, compared to 25 in Europe. According IQVIA/EMEA, there were 767 industrysponsored CGTs in development, mostly cell-based modalities against cancer. Cell therapies (at 40%) account for the lion’s share, followed by gene and RNA therapies – exclusive of prophylactic vaccines – at 32% and 28% respectively. The approximate success rate for CGTs from Phase II through approval was 14%, while those for small molecule drugs stood at 43%. According to the Alliance for Regenerative Medicine’s (ARM) August 2023 sector snapshot, however, there have been six further regulatory approvals for CGTs divided between the US and the EU in 2023 so far.
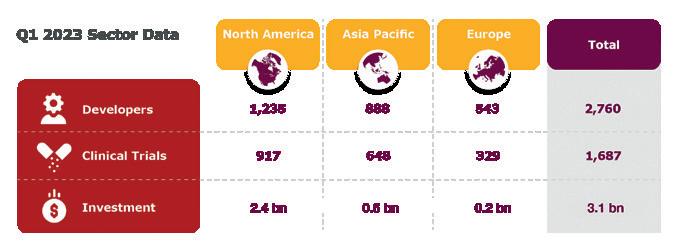
at growth in the number of clinical trials (from 1,000 then to 1,600 currently) as reasons for it. “The sector needs more talent to keep up,” the organisation concluded in its August sector update.
An earlier comparison of patent applications in 2019 showed that Europe struggles to translate strength in research into market leadership. In fact, between 2005-2018, Europe accounted for just 16% of total CGT patent registrations. The remaining 28% and 56% came from the US and China, respectively.
According to an analysis by Charles River Associates that was commissioned by the European Federation of Pharmaceutical Industries and Associations (EFPIA), Europe is falling behind the competition because it’s not prioritising life sciences hubs for investment. The study authors recommend that “the EU Commission should consider more strategic allocation of [funding] resources to foster growth of world-leading research centres” like those in the UK, Switzerland, or Denmark. 200 new life sciences companies set up shop at the latter’s Øresund cluster between 2017-2022.
The study stresses that the EU Commission’s Pharmaceutical Strategy for Europe may improve major shortcomings of
As of Q4/2022, according to Evaluate Pharma, ATMP clinical trials included 64.8% cell therapies (33% gene-modified cells, 31.8% cell therapies), and 35.2% gene therapies (18.3% of these DNAand RNA therapies, 15.5% gene therapies, and 1.4% CRISPR-based gene therapies in cancer). Other indications were cardiovascular disorders (6.2%), immunology (5.7%), dermatology (4.7), neurology (4.3%), infections (4%), musculoskeletal (3.7) and other (11.5%) diseases. The challenge with cell-based cancer immune therapies is that they are generally very costly (median €360,000/patient), so companies have to pass drug approval and reimbursement hurdles to market them. Manufacturing costs are linked
closely to production and application in a hospital setting, because personalised CAR-T/NK/TCR cell therapies rely on patient-derived immune cells that are genetically engineered to recognise individual tumour antigens, instead of being centrally manufactured from donor cells (allogeneic). High prices are often not accepted by reimbursers. The same is true for gene therapies (cost up to €1.9m per patient) against orphan and ultra-rare conditions. But to make gene therapies a viable business proposal, they can be only offered for high prices per patient. Besides current AAV-vector-based therapies (60%), mRNA or tRNA-based candidates are on the horizon that will be much cheaper to produce.
the past, but fails to recognise that competition for investment starts with attracting research and the creation of innovation clusters. Because it’s often more cost- and time-effective – and less risky –to continue to invest in a location where human resources, company culture, expertise and infrastructure have already been established, the Commission has to
channel its funding toward CGT clusters. Much as the US does for instance with CABIM in Boston, where R&D, CROs, CDMOs, investors and consultants colocate. The report released at the end of August concludes that currently “the countries with the highest EU research spend per population are not the centres of innovation.”

Conventional assays do not distinguish between dying target or effector cells
The HiBiT Target Cell Killing Bioassay is designed for precise and sensitive measurement of target cell death in mixed cell cultures

The assay works with all formats: from monoclonal and BiTE antibodies to TCR-T and CAR-T cells
Fig. 4: How interconnectivity between research, clinical development and manufacturing of Investigational Medicinal Products (IMPs) drives investment, according to Charles River Associates
According to Charles River, the CGT Catapult or the Wallonia region are good examples of how Europe can improve. As half of all CGTs in Europe gained market approval with Phase II instead Phase III trials, the consulting firm also recommends using adaptive regulatory and reimbursement frameworks that create a supportive environment for long-term, registry-based clinical trials – particularly for gene therapies that target rare and ultra-rare conditions. The current version of Europe’s revamped Pharmaceutical Strategy does not emphasise the interconnectedness of pharma manufacturing and supply chains with research and clinical testing. Developers, however, want to have the certainty of high regulatory standards in order to keep investing in Europe.
Just last April, the 330 CGT member companies in ARM sent recommendations from a developer perspective to the European Commission on how to create a forward-looking legal framework for ATMP development within the EU. Based on previous inputs from the EU regulatory authority EMA and patient advocacy groups, they recommended harmonising the so-called hospital exemption in Member States, which allows the use of ATMPs before market approval in hospi-
tal settings under the control of national medicine agencies. “The intent of the exemption is to provide individual patients […] (with) ATMPs created in hospitals, for which there is no EMA-authorised treatment available,“ stressed ARM spokesman Paolo Morgese. “However, in practice, EU member states interpret the exemption differently, and have a variety of regulatory standards under which they approve therapies, sometimes for larger patient populations than was intended by the regulation. Ultimately, this risks undermining the high regulatory standards of the EMA and creating two sets of rules – one for hospitals and one for developers.“
Experts also told Europ E an B iot E chnology that “Germany had some trials under hospital exemption enrolling up to 70 patients in orphan diseases who – depending on the inclusion criteria – are no longer available for sponsored clinical trials in the same indication.“ At the other end of the spectrum are advanced clinical trials that have been abandoned by the sponsor – e.g. for economic reasons – but for which there is nevertheless an unmet medical need. Furthermore, some ultra-rare conditions simply do not offer companies sound business footing. In these cases, academic investigators are dependent on funding from health insurance funds.






As a second measure, the ARM called on the EU Commission to provide guidance on the methodology for so-called Joint Clinical Assessment (JCA), conducted collaboratively by the EMA and national health technology assessment agencies (HTAs) to provide faster and more consistent access to innovative medicines such as cancer drugs and ATMPs for patients across the EU. The aim of JCAs is to assess the clinical benefit and cost-effectiveness of innovative ATMPs or cancer meds, and to provide recommendations on pricing and reimbursement decisions at the national level. However, according to Nikola Vesic, Merck’s Director of Global

Value Access & Pricing Operations Europe for Cardiology, Metabolism & Endocrinology, the JCAs’ “success in practice will depend on effective implementation, sufficient resourcing, and flexibility to accommodate diverse local needs and preferences. Manufacturers will need to closely monitor the progress of the JCA and work closely with local stakeholders to ensure that drugs are accessible and affordable to patients in different regions and countries.“ ARM spokesman Morgese adds that “there are clearly challenges related to many HTA processes, which were developed for traditional small molecule medicines and often require randomised control trials to establish benefit over the current standard of care,” and emphasised that “these trials are often not feasible or ethical in the rare disease populations that many CGTs treat. That’s why the implementation of the new JCA process at the EU level is so important for the future of CGTs in Europe. However, if the new JCA simply adapts the most conservative approaches in use in some Member States, the evaluations of ATMPs will be inconclusive, impeding access to ATMPs.”
trial in the meantime – that is equally beneficial to the patient.
› If a harmonised definition of what is a ‘non-routine use’ is given.
› If all HE applications are made transparent through a register or EMA reports, and investigators are obliged to perform a long-term follow-up. The paper concludes that academia plays an important role in the development of ATMPs and helps drive innovations in the field, but cannot unlock the potential of ATMPs on its own. In September 2022, the EMA launched a pilot for academia and non-profit organisations developing ATMPs to apply for EMA support in meeting regulatory requirements.
In July, the ARM, EFPIA, EuropaBio, EUCOPE (European Confederation of Pharmaceutical Entrepreneurs) and the ICST (International Society of Cell & Gene Therapy) published a joint paper with recommendations on how to revamp the EU’s Hospital Exemption (HE) Scheme for ATMPs. In particular, they urge that under the EU’s Pharma Legislation, HE should be only legitimate if there are no suitable approved therapies or ongoing clinical trials. In concrete terms:
› If the HE is limited to one year and may only be continued if a review has shown that no centrally approved CGT has been approved for treatment in the meantime – or there is a clinical
For companies trying to build a business on ATMPs, the increasing number of HEs is a significant threat, as compounds developed under hospital exemption are significantly cheaper than centrally authorised therapies. ARI-0001, a chimeric antigen receptor T cell therapy targeting CD19 that was initially developed by hematopoietic stem cell specialist Orchard Therapeutics plc (Boston/London) is a good example of a very low-cost CAR-T that received authorisation from the Spanish drug agency outside the EU’s centralised marketing authorisation pathway. It achieved decentralised uptake from hospitals in Barcelona at a price just one-third that of commercial CAR-Ts already marketed in Spain. ARI-0001 is the first CART cell therapy participating in the new EMA pilot aimed at supporting the translation of basic research developments into medicines that could make a difference in patient lives under the EMA’s PRIME scheme. The pilot is open to five academic sponsors and non-profit organisations developing ATMPs that need guidance through the regulatory process.
Thus, the evolving HE environment could also be regarded as an opportunity for the biopharma industry to establish new partnership models – ones that will be sustainable for both companies and public organisations.






L

t.gabrielczyk@biocom.eu
High Quality Grade Plasmid & Minicircle DNA












Customized High Quality Grade DNA for GMP production of viral vectors, RNA and CAR-T cells







QC including CGE service pDG/pDP plasmids for AAV production


2 plasmid system
Serotypes including AAV8 & AAV9 GFP transfer plasmids

ITRRESCUETM In Stock service
The better way to DNA! ask for
“The EU has led before –and can lead once again … but the time to act is now.“
VIROCELL BIOLOGICS London-, Dublin- and New York-based ViroCell Biologics has appointed Brian Collins as Chief Financials Officer. The last four years he has served as CFO of the Cell and Gene Therapy Catapult (CGT Catapult”), an UKbased organisation supporting companies to develop, deliver and commercialise advanced therapies. Prior to CGT Catapult, he was CFO of Coveris Packaging and he hold numerous financial and operational roles of increasing seniority at companies, includin MSX International, HB Fuller and Avery Dennison. L



Chief Operating Officer. There he was responsible for structuring and launching the CDMO’s cell therapy activities. L
CELONIC GROUP CDMO Celonic Group, with sites in Basel and Heidelberg, has appointed Dr Samanta Cimitan as Chief Executive Officer (CEO). She succeeds Dr Konstantin Matentzoglu, who is stepping down after nine years in this position. He intends to remain a member of the Celonic Board of Directors and will support the new CEO during the transition period. With more than 20 years of experience in working, teaching and studying life sciences, Cimitan is an expert in the CDMO industry. She joined Celonic in June 2022 as Chief Business Officer (CBO). Prior to that, Samantha Cimitan spent almost 13 years at Lonza, where she held several leadership positions in various commercial and strategic areas, including Vice President of Lonza Group Operations Strategy, where she developed and led transformation programmes in R&D, Operations and Commercial. L
ceeds Helena Strigård, who is acting as an advisor to Hoogstraate in this crucial phase before she takes on a new leadership role in another company. Hoogstraate holds a PhD in biopharmaceutical sciences from Leiden University in the Netherlands. She has conducted research at the University of Utah and the University of Iowa, USA. Since 1995 Hoogstrate has worked in the pharmaceutical industry in research and all phases of development, as well as in local and global leadership positions with full budgetary responsibility.L
PLECO THERAPEUTICS
Jérôme
BédierFLASH THERAPEUTICS The French company Flash Therapeutics, a specialist in the design and bioproduction of highly pure, highly concentrated DNA and RNA lentiviral solutions for the biopharmaceutical industry, has a new president. Jérôme Bédier (49), brings extensive experience in the field of bioproduction and CDMO. He held positions in business development and operational management before taking over the strategic leadership of the Novartis biopharmaceutical business and its three European Locations. In 2021, Bedier joined Cell-Easy as
NORTHX BIOLOGICS The new CEO of development and manufacturing company NorthX Biologics AB, Dr Janet Hoogstraate, took over her new position effective at the end of August. Prior to joining NorthX, Hoogstraate was CEO of Valneva Sweden. At NorthX she suc-

Nijmegen-based specialty pharmaceutical company Pleco Therapeutics BV, announces the appointment of Dr Henno Welgemoed in the newly created role of Chief Medical Officer (CMO). Welgemoed joins Pleco Therapeutics from the UK-based Clinigen Group. There he served as the Global Director of Medical Affairs for the past seven years, after he moved from South Africa to UK. At Pleco Therapeutics he will be a member of the global leadership team, taking over responsibility for the Company’s clinical development activities. The physician brings 15 years of clinical expertise and over two decades of pharmaceutical experience to Pleco. Throughout his career, he has fulfilled various roles across commercial, new business development, and medical affairs globally.


Jurassic Park stormed theaters in ‘93
4 Non Blondes, Culture Beat, Bon Jovi and Meat Loaf ruled the world of music
Adobe publishes the first version of the PDF format

Francisco Mojica first characterizes what becomes later known as the CRISPR locus
MLM Medical Labs started its operations to grow into a global full-service central and specialty lab in Mönchengladbach
MLM has been delivering high-quality lab services to support pharma and biotech for 30 years
Where were you in ‘93
Submit your favorite photo from 1993 for a chance to win one of 4 really cool prizes.
Scan for more info
www.mlm-labs.com
CUSTOM SERVICES Since 2006, the share of biomanufacturers producing everything in-house have declined from 57.6% to 34.9% in 2022. According to biopharmaceutical companies, the percentage is to decrease to 20% by 2026, according to the 19th biomanufacturer’s survey from Bioplan Associates International.
As production capacity demand for gene and cell therapies has been growing since 2021 by about 20% per year, qualified personnel is increasingly becoming a growthlimiting factor, according to current figures of Bioplan Associates International.
This is especially true for the USA, which accounts for 27.8% of global production capacities, and for Europe with a share of 30.8%. In contrast, China's 13% production capacity is facing an order boom. According to data from BioPlan 's annual industry survey, more than 45% of CDMOs want to order services there. When biomanufacturing professionals were asked to identify which major constraining factors limited their organisation's growth, 34.2% answered, "the inability to hire new, experienced technical and production staff”. "Another
finding we saw this year was an inability to retain experienced scientific staff, which increased by 13.3% (32.5% in 2022 vs. 19.2% in 2021). In addition, the concern for retention of experienced technical and production staff increased by 10% from 2021 to 2022,” says Bioplan research associate Shriya Bhatkhande.
On top of that, new advances like cellular therapy are pulling experienced employees from mainstream biologicals such as antibody production. Large US companies, thus have begun to hire experts in Europe and other parts of the world.
Bioprocessing efficiency and productivity improvements concerning titer levels increased to an average of 2.92 g/l, up from 2.65 g/lL in 2021. The average cost-per-gram monoclonal antibody was at its lowest point on record. The market has been expanding at a rate of 12% to 13% per year over the past decade, virtually doubling in size every five years.
With this growth, efficiencies and economies of scale have resulted in lower unit costs. Cost savings from process automation and other factors continue to expand.
International biomanufacturing and offshoring are growing, especially in major markets and Asia. An upcoming wave of facilities making biologics, as well as pandemic and biodefense preparedness products, is increasing regional capacity, and growth among regional CDMOs, especially in Asia. The demand for biologics in these regions is also creating readymade markets for innovative therapies.
The number of cell and gene therapy pipeline products and facilities, including commercial manufacturing, is increasing. However, more than 50% of the industry is having trouble finding qualified employees. As this continues to get worse each year, market success more and more depends on a company’s ability to hire experts. L
t.gabrielczyk@biocom.eu
Profound understanding of customer requirements based on 15+ years of experience

UNLOCK PICHIA® toolbox – broad set of expression tools and strategies
Process intensification with methanol-free Pichia allowing shorter batch times Industry proven and regulatory compliant expression platform
Tailor made glycoproteins – UNLOCK PICHIA® boosting Pichia GlycoSwitch®


TRANSATLANTIC Clinical trials have to be coordinated as a safe process where monitoring, analytics and handling of patient samples is streamlined – even cross oceans. German MLM Medical Labs is quickly establishing itself as an emerging central and specialty lab in the U.S.
EuroBiotech_Mr. Houlton, let´s start with the ecosystem of central and specialtiy labs, where MLM is entering the crowd. Scott Houlton_MLM has a track record of three decades, that´s quite a number and much has happend. In today’s landscape, the industry is made up of a broad base of very diverse, specialised suppliers, the same is true for the customer base. Typically the largest pharmaceutical companies like to work with the really large vendors. The size fits them well.
EuroBiotech _Where did MLM find its position?
Houlton_Although the large vendors do a lot of things very well, still no one is perfect. The larger companies are not good in providing nimble customised service that many of the customers want and complex studies require. These customers, the smaller to mid sized biopharmaceutical and biotech companies, don´t feel like they get the attention they need, and frankly want, from the large vendors. And that´s the niche, we are trying to go after: to provide highly customised and flexible services.
EuroBiotech_Now you are ‘copying’ the German, European success story of MLM as a blueprint for the US markets, is it really that simple?
Houlton_So what we wanted to do was to take what MLM is already doing very well and help broaden the scope and the geographic reach to help MLM grow. That was really the focus and the thesis for the investment to help MLM expand into the US in 2020, which we have done. We acquired two labs in the U.S. and have mirrored our offerings from Mönchengladbach, Germany, to Memphis, Tennessee, and are now
Scott Houlton, CEO MLM, has over 25 years of leadership experience in the pharmaceutical services industry across the drug development, clinical trial and commercial segments. He was most recently CEO of Clinical Supplies Management and has held past leadership roles at Catalent, Aptuit, Quintiles and Cardinal Health. He is a member of the CEO Advisory Board at Great Point Partners.

expanding both - our revenue and customer base.
EuroBiotech_Are you changing your offering regarding other market needs?
Houlton _In late 2020 we acquired a business offering preclinical testing and histology services as a means to provide begining-to-end support along the drug development timeline. However, our primary focus remains on the clinical trials market. We want to continue with what we are good at in the safety and biomarker testing world and focus on drug development.
EuroBiotech_Since the company is in different positions on both sides of the ocean, the CEO must fill also different roles transatlantically, I guess?
Houlton_Yes. In the US, it is really about getting people on board, training them and getting operations ramped up efficiently. When I am working with the German team, it´s how do I help them expand and scale their business even further.
EuroBiotech_What can you take to the other side of the ocean, practically?
Houlton _We were lucky that we were able to take the operating systems that Germany had developed over the last few decades and transfer it to the US. A huge benefit for us. In our world, our customers want the labs to be harmonised so that they get comparable results. We really mirror the German set-up with processes, equipment and shared IT-systems.
EuroBiotech_What is it you are adding to the US ecosystem?
Houlton _We now add the central lab which is a good complimentary offering in the US. Together, this will help us to grow the customer basis very fast. Most of our activity is on phase one through phase four trials. We do have a small part of our business focused on preclinical research.
EuroBiotech_But the sample is still the core of your service, the 'tissue is the issue'?
Houlton_The sample is the starting point. Collecting samples on a global scale is not an easy job. We have developed a process for collecting samples from over 80 countries via validated collection kits and tracking those samples at every step of their journey from collection to real-time, digital reporting of test results through our mlm online® platform. L
g.kaeaeb@biocom.eu

ANTIBODY ENGINEERING The development of therapeutic antibodies is a time and cost intensive process. Biological and physicochemical properties of the lead molecules influence almost every step in the development. Investing into antibody engineering and lead optimisation during early development pays off in later steps and does not only accelerate the programme, but also increases the chance of success.
Therapeutic antibodies have become the most important class of biopharmaceutic drugs and are indispensable for the treatment of many diseases. Fully human and humanised antibodies can be generated by different technologies, such as in vitro display technologies from naïve or immune libraries or from transgenic animals. Selection and screening of early antibody candidates can be tailored to define many if not most properties of the final drug, such as target specificity and selectivity, or important features for preclinical development such as cross-reactivity with the animal model. However, it is unlikely, that the first antibody lead possesses all favourable features that are required for a therapeutic drug. Of-

ten problems occur in later development stages such as manufacturing, process development, formulation, or clinical development.
Early introduction of antibody engineering and lead optimisation that take these stages in consideration can help to improve functional properties and to address manufacturing or formulation issues in the early development process. Therefore, performing optimisation steps at early stages of drug development can save valuable time, costs and de-risk decisions on critical paths in upcoming process steps.
Exchanging only a single amino acid in an antibody sequence can influence functional properties, stability, expression levels, aggregation, and immunogenicity, all critical properties which can decide the fate of an entire drug programme. Many properties are interdependent, for example higher stability often improves expression levels and facilitates process and formulation development, or lower aggregation propensity may allow high dose formulations, a prerequisite for the subcutaneous administration route, and reduces the likelihood of immunogenicity.
Engineering and optimisation of early lead candidates need to be done in the context of each individual molecule. Optimising lead panels instead of single lead candidates increases the chance of success instead of relying on one mole-
cule. The combination of computer-aided predictions, for example to generate focused libraries, with experimental studies accelerates antibody engineering and lead optimisation compared to pure bioinformatic approaches or random in vitro evolution. The employment of next generation sequencing offers a much deeper analysis of screening outputs and accesses larger pools of sequence variants. The access to more data also opens the door for machine learning and artificial intelligence, which will become an essential part of drug development in the future.
In conclusion, the introduction of antibody engineering and lead optimisation very early in drug development by using a combination of experimental and bioinformatic approaches does not only accelerate the entire process, it also reduces costs, avoids failures, and increases the chance of success.

CDMO Within the competitive CDMO market, Richter-Helm has developed into a highly valued partner when it comes to the manufacturing of biopharmaceuticals. Richter-Helm is well recognised by pharmaceutical companies, including Big Pharma, as a professional partner offering flexible CDMO solutions from gene to product, all from one source, including consultancy services. This makes Richter-Helm one of the key players in CDMO business.
business growth, the implementation of new technologies, and the establishment of expertise in certain areas. Richter-Helm is using this positive development to expand both its production and development capacities along with its technology pipeline.
According to various reports and surveys, the biologics market is constantly growing. Pharmaceutical companies focus on bringing their pipeline products to the market quickly and with minimum risk, and therefore increase their outsourcing activities to reliable partners, such as Richter-Helm. On the other side, CDMOs compete to win the project(s). In the end, decision-making is dependent on the most suitable fit between two partners. The trend seems to go in the di rection of one-stop-shop offerings, where projects are outsourced to a single partner able to provide services from process development to analytical development, manufacturing, QC testing and filling of finished product. The goal is to build strong and long-term partnerships on equal footing to strengthen and extend business.
With a 35-year-long track record in GMP manufacturing, Richter-Helm pro-
vides its clients with a unique knowledge base in process and analytical validation, process performance qualification (PPQ) procedures, commercial production of therapeutic proteins and peptides, antibody-like scaffolds (e.g., VHH/ Nanobodies, Fab-fragments), bacterial vaccines, and plasmid DNA (pDNA) products.
On the company's sites, new projects can either begin from scratch for full development or involve the direct transfer of existing process technologies into large-scale production sites for market supply or to support with materials for clinical studies and the development of new products.
The increasing demand for further services offers very promising new opportunities for CDMO companies like Richter-Helm with respect to the
To serve the demand for drug production and offer attractive manufacturing solutions for especially large and commercial scales, Richter-Helm is installing a new multipurpose facility, which is designed for maximum flexibility and opens opportunities for new projects. A total area of about 10,000 m2 enables Richter-Helm to take on projects at 300L and 1,500L fermentation volumes for microbial production, as well as related mid- and downstream operations, ensuring high product yields. The new site includes additional space for huge analytical laboratories, warehousing, and technical areas designed for further growth. That makes the delivery of technically advanced services at different scales, especially large clinical scales up to commercial scale, possible.
Via the application and implementation of digital technologies (e.g., digitalisation of process design and targeted process characterisation to facilitate process development) into the whole manufacturing process, the biomanufacturing efficiency at Richter-Helm´s production sites increase further. L


FR-JET TECHNOLOGY Manufacturing of COVID vaccines showed that large-scale production of lipid nanoparticles (LNPs) as a delivery vehicle for mRNA vaccines is already possible. As nanocarrier-based formulations became mainstream, however, insufficiencies in existing technologies for routine GMP manufacturing became apparent. LEON is committed to bridging these technology gaps through its unique FR-JET technology and innovative manufacturing equipment.
EuroBiotech_How accessible are LNPs for developing and manufacturing new therapeutic modalities?
Dr Setu Kasera_The two most popular techniques used for lipid-based nanocarrier formulations are jet impingement and microfluidics, using mixing processes that operate in very different flow regimes. Jet impingement, which was the primary method used for making COVID vaccines, has clearly distinguished itself due to superior output of product volume. Yet, problems with the technique also persist, which need to be solved to enable its full utilisation. For example, finding the right process parameters to make nanoparticles with desired properties and quality currently is a tedious process, mostly based on trial-and-error. There can also be batch-to-batch variability and other issues with consistent particle quality. These issues can largely be attributed to poor mixer design.
The aim of LEON is to fully enable access to these nanocarrier-based formulations. Our FR-JET technology features a near-ideal mixer geometry constructed in a modular fashion, where different segments of the mixing system can be adjusted depending on client’s needs. This design offers precise control over the process parameters and makes it possible to approach process development in a systematic manner. Importantly, the same mixer is used on the bench scale and commercial scale. This approach de-risks batch scale up and eliminates the need to invest
Dr Setu Kasera serves as chief scientific officer at leon-nanodrugs GmbH. With nearly a decade of hands-on experience in nanotechnology, she is directing research at LEON and is responsible for managing product development and data generation as well as strategic collaborations. She received her PhD in chemistry from the University of Cambridge, UK, followed by positions in research and business strategy in the biotech industry, with a focus on nanotechnology, drug development, CMC and science management.

time and resources for intermediate scale up and pilot equipment. Our devices are built especially for aseptic GMP-compliant manufacturing, featuring closed designs and using sterile single use components among others. Our NANOme® manufacturing device is a gamechanger, designed
such that batch changeover (including product changeover) takes less than a few minutes. Its operation is similar to inserting a cartridge into a printer and pressing a few buttons, bringing us much closer to making beside manufacturing a reality.
EuroBiotech_How close is your product portfolio to maturity?
Dr Setu Kasera_The development of our lab scale and GMP manufacturing equipment and their performance testing have been concluded successfully. We are now in the final stages of functional testing with industry partners. The benchtop NANOlab® device for process development and our small-scale GMP NANOme® device for on-site manufacturing are planned for market rollout in 2024. We are already prospecting customers for our larger, high-volume GMP manufacturing device NANOus®, which has been designed in partnership with leading pharma production equipment manufacturer, Harro Höfliger.
EuroBiotech_How do you enable access to your technology for potential clients?
Dr Setu Kasera_Our pre-market benchtop units are already accessible for conducting feasibility projects in our laboratory in Munich. We can also ship the equipment to the client’s site. Given the plug-and-play nature of our systems, installation and operations are not complicated and support from our expert team is available.
Richter-Helm is a Germany-based GMP manufacturer specialized in products derived from bacteria and yeasts, with a proven 30-year track record.

Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide have already benefited from our commitment to good manufacturing practice and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.
Richter-Helm consistently works to the highest standards of pharmaceutical quality.
Contact us
+49 40 55290-801
www.richter-helm.eu
BIOMANUFACTURING When businesses receive a proposal from a contract development and manufacturing organisation (CDMO), it's much like a first date: first impressions matter. The proposal process not only reveals a CDMO’s services but also hints at the potential relationship's quality. This is gauged mainly through metrics like timing, quality, and price.
ences offer insights into the CDMO's recent performance, aiding decisionmaking.
Technical Call Significance: Technical calls help prospective clients understand the CDMO’s capabilities. The presence of the right personnel and detailed note-taking during these calls is vital for project success.
Transparency is Key: A CDMO's proposal should be clear about risks and solutions. Access to the CDMO's sites and management team further emphasises transparency.
Flexibility of a CDMO: Adaptability is essential. A good CDMO will work collaboratively, even when unexpected challenges arise, showcasing their problem-solving skills.
CDMO Response Time Matters: The speed of a CDMO's response is critical in an industry where timely responses to requests for proposals (RFP) and timely revisions indicate a CDMO’s dedication to timelines.
Qualities of a Good Proposal: A standout proposal is customised, focusing on the client's unique needs, rather than a one-size-fits-all approach. It begins ambitiously, then hones details, rather than the other way around.
Evaluating CDMO Responsiveness: Initial interactions, even before the RFP, can indicate a CDMO's respon -
siveness. How quickly they handle preliminary processes and customise their proposals speaks volumes about future collaborations.
Understanding a CDMO's Qualification: An efficient proposal process reflects a CDMO’s project management skills. Speedy communication between a CDMO’s business and technical teams is a positive sign. CDMOs should also convey enthusiasm, connecting their technical staff with the project’s larger mission.
Checking CDMO References: Potential clients should verify a CDMO’s track record. Current, relevant refer -
The Final Proposal Revisions: The end revisions transform proposals from generic to specific, ensuring clients know exactly what to expect. This step prevents unforeseen costs and ensures clarity.
Prospective CDMO clients can gauge a lot from the proposal process, from responsiveness and creativity to the depth of technical understanding. Proactive CDMOs, like Northway Biotech, stay ahead by anticipating client needs, offering innovative solutions, and prioritising clear, swift communication. L

State-of-the-art mammalian cell culture manufacturing facilities in Europe and Asia for clinical and commercial material, ranging from 150 to 13,000 liters.
Customized solutions and efficient handling for your microbial-based products, with upstream and downstream capabilities ranging from 150 to 40,000 liters.
High-quality plasmid DNA and RNA manufacturing for various types of RNA drugs (including siRNA), mRNA vaccines and innovative gene and cell therapies, up to a scale of 3,000 liters.
Rely on a leading Cell & Gene therapy manufacturer with an excellent team and high-tech facilities.
Producing viral vectors for vaccines or cell and gene therapy in a state-of-the-art facility.

A fully integrated set of services and solutions for aseptic fill and finish of medicinal products. Available packaging systems and volumes include:
• Vials (liquid, lyophilized)
• Pre-filled syringes
• Low-level siliconized syringes
• Droptainers
• Ampules
• Tubes
Manufacturing master cell and working cell banks to enable high-quality clinical and commercial production.
We follow a comprehensive Novartis quality assurance system, with all our facilities operating in compliance with cGMP regulations.
To find out how we can help, get in touch at biotech.cooperations@novartis.com www.novartis.com/globalbiotechcooperations
April 22-23, 2024
As one of the leading biotechnology conferences, the Swiss Biotech Day has developed into a truly global networking day, attracting many international delegations.

What you can expect:
› Meet 1,800+ senior experts from the life science industry
› 70+ exhibitors and Global Village with international delegations from all over the world
› Swiss Biotech Success Stories Awards
“WHITE
› Innovative biotech start-ups and medium-sized biotech companies
› Thematically focused panel discussions
› Swiss Biotech Report 2024
› Pre-scheduled one-to-one partnering meetings
› General Assembly of the Swiss Biotech Association
Media partners:
Leading chemical companies are exploring the opportunities that have been opened up by modern biotechnology, especially in the field of “white” or industrial biotechnology. And they are also applying these technologies, wherever it makes sense. The SBA takes such initiatives seriously and has formed a working group specifically dedicated to white biotechnology. The Swiss Industrial Biocatalysis Consortium is an important partner in this effort. The group includes leading multinational companies that support white biotechnology as a pillar of economic growth. The planned activities are in agreement with OECD strategies.
In partnership with the Swiss Biotechnet (see pages 14/15) the SBA develops training programmes and useful support tools for the industry. It is of importance that the industry specifies its training needs so that the academic side can create tailor-made education. This strategy ensures that the industry gets the right workforce with the right education. The SBA profits from the marketing alliance “Swiss Biotech” (see box) in a multiplied form. Thanks to Swiss Biotech, the
sector is internationally visible. The project-specific participating companies (most of them young and internationally less savvy) find a comprehensive partner which is helping to put them in the public window. The participating Life Science Regions are important internal carriers of the dynamics in the Biotech sector, thus enhancing the common understanding of the industry. This and more knowledge is brought into Europa Bio, the European Biotech Association, where the SBA is an active member.
Sign in on our website at www.swissbiotechday.ch and stay updated about any news.

...is an alliance of four leading Biotech regions Switzerland (Bio Alps, BioPolo Ticino, Basel and Greater Zurich Area). They have combined forts to streamline interests of the national sector. The SWX Swiss Exchange holds a position in terms of lifescience listings and companies from that industry – be they located Switzerland or abroad – access to an internationally recognised financial marketplace. The initiative was co-founded by the SBA which also manages the executive office of Swiss Biotech.
Host:
© Swiss Biotech AssociationSPAIN In just one decade, Spain has managed to develop its excellent basic research into an emerging translational hub in the life sciences. Unlike elsewhere in Europe, the regional clusters focus not only on pharmabiotech, but also on agribiotech, cellular agriculture and precision fermentation.
Spain has become an emerging life sciences hub due to its concentration of skilled talent, research institutions, hospitals and the government’s support policies for technology transfer. According to ICEX Trade and Investment, Spain ranks 12th in the world in terms of scientific output and participates in nearly 20% of international clinical trials. Spain’s success is reflected by the growing attraction of private equity investments into its biopharma sector, which tripled from 2016 to 2021 (€180m), according to figures from the national biotech industry association AseBio. This seems little at first, compared to the UK or the US. By 2025 the government, however, wants to channel €8bn to support biopharmaceutical innovation. And: the dynamics in the regional life sciences cluster regions and private-public hubs such as BioBask, BioCat or in Madrid are far greater than in other EU states. There is a great will to innovate and an increasingly strong motivation to network internationally, despite a sometimes retarding pride in one’s own region.
“We were surprised ourselves at how many of our students are drawn to Spain,” says Prof Michael Rossbach, a professor in the Bio-Star translation cluster in Singapore, who in 2018 founded Ikxinta Pte Ltd in Bilbao, which helps academic founders in the US, Spain, Portugal, Germany, Singapore and China commercialise their scientific breakthroughs. Dr Matthias Meergans, Managing Director R&D of the German pharmaceutical association vfa, considers Spain to be a more attractive location for multicentre clinical trials than Germany, thanks to the removal of administrative obstacles. The majority of Spanish biotech SMEs, as elsewhere in Europe, are

focused on pharmaceutical biotechnology. “We believe in consolidating our country as a centre of attraction for intellectuals, talents and investments dedicated to science for the benefit of all,” says Asebio President Ion Arocena (see p. 3, 58).
However, Spain has long been known as a bastion of agri-biotechnology, and during its EU presidency, it wants to help the EU Commission’s current draft on the de-
regulation of so-called New Genomic Techniques (NGT) for plant breeding to succeed. “The majority of member states welcome the NGT proposal with interest,” stressed the Spanish Minister of Agriculture Luis Planas on the fringes of the first informal meeting of EU agriculture ministers under the Spanish EU Presidency in Cordoba (see p. 6). According to Planas, the ministers see great potential to create crops adapted to climate change in a short time and to reduce the use of fertilisers and pesticides. If the world’s most liberal genetic engineering law for precision breeding goes through, Spain would be strengthened as an EU centre for agribiotechnology. In addition to the EU NGT regulation, decarbonisation of agriculture within the framework initiative on sustainable food systems are also high on the list of priorities for the Spanish EU presidency. Spain has also already positioned itself in the billion-dollar market for vegan meat, cultivated through cellular agriculture and precision fermentation. The Iberian Peninsula already offers some of the largest production facilities for first-generation products: insect- and plant-based protein foods. Now, in June, the non-profit organisation AgriCultura Celular España was formed “to make Spain a global player in cell culturebased protein production. Companies like GrowMeat (Malaga), Cocuus (Navarra) or Cubiq Foods (Barcelona) need support.
The next chance for the biotech allround location Spain to prove that it can act faster than the EU, which with 18-48 months approval time currently forces companies to go to market in the USA or Singapore (see p.12).
t.gabrielczyk@biocom.eu
SPAIN After two years of unprecedented growth driven by the pandemic, investment attracted by Spanish biotech companies has stabilised, and they face significant challenges, positioning biotechnology as a strategic sector for Spain.
Biotechnological innovations define each society. Biotechnology is present in all aspects of one's life, and the disruptive innovations it generates have a direct impact on social and economic well-being, as well as the preservation of the planet's sustainability.
Deep tech is driving profound changes in the world through technologies such as advanced therapies, messenger RNA, the microbiome, precision nutrition, waste valorization, CRISPR, or advanced diagnostics, among others.
In recent years, biotechnology has demonstrated great potential in addressing global challenges such as the pandemic caused by COVID-19 through the development of vaccines. The health crisis has positioned biotechnology as a cornerstone of responses and actions in the face of the challenges on the horizon, as reflected in the current landscape of the biotechnology sector in Spain, which has undergone significant evolution over the past two decades.
During this transformative period, the Spanish biotechnology sector has grown from having around 50 companies to nearly 900 companies, following the 4.2% growth recorded in 2021. Over the last decade, an average of 48 ‘biotech’ companies have been added to the expanding list annually. The Spanish biotech business landscape is predominantly composed of small and micro-enterprises, with a geographical

distribution where Catalonia stands out as the leading region with 24.5% of biotechnology companies. Madrid follows with 17%, and Andalusia with 14%.
Human health is the primary area of activity for Spanish biotechs, accounting for over 50%, followed by food (39%), and, to a lesser extent, those engaged in activities related to agriculture and forestry production (15.4%).
In terms of investment, the situation in the biotechnology sector in Spain is positive. According to the figures gathered in the latest AseBio Report, Spanish biotechs attracted €142m in investment in 2022.
After two years of unprecedented growth driven by the pandemic, the in -
vestment captured by Spanish biotech companies has stabilised following two years of record-breaking investments. Although the numbers indicate a decrease compared to the previous two years, they reflect sustained growth levels that exceed those recorded in the years before the onset of the health emergency.
In 2021, the impact on the GDP of biotechnology companies amounted to €11,183m, representing 1% of the GDP. That same year, Spanish biotechs increased their revenue by nearly 8%, surpassing €13,000m (1.1% of the total GDP).
In terms of employment, Spanish biotechnology companies contribute to 118,000 jobs. This figure represents 0.65% of total national employment. In 2021, biotechnology companies invested €1,038m in R&D, marking a 16% growth
compared to the previous year. Biotech companies also stand out as the industrial sector with the highest percentage of researchers among the total workforce, at 14.62%. Studies conducted in Spain position the country as the ninth-ranking global power in biotechnological scientific production, making Spain a global leader in this field. This is demonstrated by the fact that Spanish biotechnology accounts for 2.6% of global production in the area and is cited 20% more than the worldwide average.
Talent is one of the key drivers of technological development. The AseBio 2022 Report confirms that an increasing number of Spanish students are looking to biotechnology for their future careers. Since the 2015-2016 period, the number of students enrolled in undergraduate or master's biotechnology programs has in-
creased by 25%. In the 2021-2022 academic year, the latest period with available data, there are over 8,900 students enrolled in master's and undergraduate programs. Among these 8,900 students, the number of women enrolled in these programs continues to rise, with a 3% increase compared to the previous year, reaching 62%. In this regard, it's worth noting that 60% of individuals working in biotechnology companies are women, and they make up 28.4% of the management teams in biotech companies.
The data presented in these lines demonstrate how biotechnology has established itself in Spain as a cornerstone due to the scientific potential it has made evident, as well as being a driver of economic growth and social well-being. Biotechnology is crucial, as was made obvious by the C OVID-19 pandemic, but its position in Spain as the strategic sector it is must continue to be strengthened.
TRANSLATIONAL MEDICINE Ikxinta, a life sciences advisory firm that originated in the Basque Country in 2018, collaborates with scientists, industry innovators, and investors to propel clients in scientific frontiers

As scientists, the founders Dr. Pablo Lizana Allende and Dr. Michael Rossbach, bring a science mindset to strategy consulting. Life sciences has emerged as one of the most dynamic sectors in the global economy, and Spain, with its rich history of research, has become a significant player in the field. The expansion of Spain’s biotech sector can be attributed to government funding, innovation initiatives, and a commitment to academic excellence. Institutions like the Spanish National Research Council, the Institute for Health Carlos III in Madrid, or The University of the Basque Country have made significant contri -
butions to the sector. Ikxinta connects such institutions with partners in academia, clinics, or in the bio/pharmaceutical industry to drive innovation from research into clinical practice.
Today, many scientific projects focus on genomics, cell-based therapeutics, drug discovery, bio-IT, or medical devices – they benefit from supportive ecosystems that include accelerators, and venture capital. Notable success stories are Grifols in Barcelona (a glob -
In this regard, one of the major challenges identified in the biotechnology sector is its recognition as a key sector for achieving the strategic autonomy of Spain and Europe. Spain needs to advance in developing a comprehensive government agenda that promotes deep tech industries like biotechnology, which are strategic in addressing economic, social, and environmental challenges.
To this challenge the issue of financing is added. While the figures presented are positive, it is essential to establish a fund for strategic deep tech that strengthens and maximizes the potential of the Spanish biotechnology industry. Spain must work to overcome the financial and industrial barriers that still exist and hinder the financing of biotech companies. Without a strong commitment to biotechnology at the national level, a sector that has proven to be strategic, we will continue to be vulnerable to future challenges. L
al leader in plasma-derived therapies), or Progenika Biopharma in the Basque Country (innovative diagnostics for rare or complex genetic diseases).
In the dynamic landscape of the global biotech sector, Spain is well-positioned to sustain its leadership, driving advancements that transcend national boundaries. This underscores the potential for scientific innovation in regions dedicated to R&D, benefiting both their own nations and the broader global healthcare community.
Our Ikxinta team, spanning across Portugal, Spain, France, the Netherlands, Germany, the United Kingdom, China and Singapore, comprises consultants, entrepreneurs, investors, and scientists. We actively nurture unique collaborations, introduce emerging technologies to the market, and create new ventures – with a hands-on approach.
Contact: Prof. Dr. Michael Rossbach, rossbach@ikxinta.com
LIFE SCIENCES CLUSTER Due to the global niche character of pharmaceutical biotechnology, research collaborations and market entries on an international level are essential. Agility and market knowledge are required to maintain an overview in a rapidly evolving environment and to initiate a sustainable internationalisation process.
› Stephanie Füller, PR Manager, BioRN Network e.V.The decision to expand into international markets must be based on careful consideration. This includes issues about the long-term vision for building an international business, the unmet need of patients in the international market and available resources that can be devoted - considering the challenges and priorities in the home market. This requires profound experiences and know-how. BiointeRNational, the new service unit of the BioRN Life Science Cluster RhineNeckar, supports SMEs and start-ups in risk assessment as well as market analysis and instructions to secure this project.
Sciomics´ internal development pipeline for novel diagnostics in precision medicine is on track for growth. The Heidelberg based company's project on Perioperative Acute Kidney Disease (AKI) has been successfully validated and the next steps to develop a diagnostic device are being planned. To this end, an internationalisation strategy for tackling the US market is to be developed.
"To take advantage of global opportunities, companies first need to understand a number of complex country-level factors. This is why we have carried out expert interviews with key opinion leaders in the relevant market", says Arantxa Encinas-López, startup project manager at BioRN.
BiointeRNational takes over the project management and sets, in constant
Christoph Schröder, CEO Sciomics GmbH and Arantxa Encinas-Lopéz, BioRN

exchange with the project partners, the necessary course to achieve the relevant results. "It was initially important for me to get a better feeling for the complex diagnostics market in the US and to understand how reimbursement via the health care system works there. BioRN put us in touch with the right partners and paved access to key contacts in the target market", says Christoph Schröder, CEO of Sciomics.
Based on the results of the expert interviews together with secondary research, a go-to market strategy has been developed – further steps to tackle the market are already initiated.
In addition to the door-opening function into foreign markets, BiointeRNa -
tional also supports European SMEs and start-ups on the development of a softlanding strategy within Germany. This includes the identification of key opinion leaders and distributors, introduction and support throughout the collaboration, market introduction with tailor-made workshops about the German healthcare system, reimbursement pathways and regulatory, and marketing support.
PROGEN, well-established and experienced provider of an exclusive portfolio of AAV antibodies and analytical tools based in Heidelberg, was recently introduced to a Spanish startup with an innovative and very interesting technology. The introduction by BiointeRNational formed the perfect basis for the establishment of a possible long-term supply relationship.
"BiointeRNational facilitates the flow of information about companies you would normally not necessarily hear about, e.g. due to a different research area they are focused on. Keeping track of innovations, especially when these take place in different focus areas, can sometimes be tedious and BiointeRNational makes it very easy to find the right match and establish a relationship with foreign companies," says Katja Betts, CEO at PROGEN.
Further information:
https://www.biorn.org/biointernational


EVENT The new edition of the international reference event of the Spanish biotech sector will be held in Barcelona from 26 to 28 September, in collaboration with Biocat, the Barcelona City Council and the Government of Catalonia. Its programme, made up of more than 60 sessions, will focus on the main challenges and opportunities of the biotech industry.
60 sessions, national and international experts will focus on health, the future of food and agriculture, industrial transformation, green transition, advanced therapies, antibiotic resistance, financing, the challenge of Europe's strategic autonomy, and drug discovery, among others.
BIOSPAIN celebrates its 20 th anniversary in 2023, positioned as an international benchmark event in the biotechnology sector and an exceptional witness to how biotechnology has evolved over the past two decades, ultimately becoming a deep tech that is transforming the world.
BIOSPAIN returns to Barcelona, the primary hub for Life Sciences in Southern Europe, to demonstrate how biotechnology is a critical and emerging industry fundamental for ensuring Europe's strategic autonomy, its role as a guarantor of social and economic well-being, and how it has become one of the cornerstones in preserving the sustainability of the planet.
An environment where more than 1,700 attendees from 850 entities representing over 30 countries will converge. BIOSPAIN provides the ideal ecosystem for collaboration between companies and investors, the discovery of talent, and finding solutions to the pressing challenges facing the biotechnology sector. It is an internationally recognised event that brings together major indus -
try players, innovative startups and spinoffs, and leading investors with the goal of enhancing partnering and collaboration among various stakeholders in the biotechnology industry.
The partnering platform of BIOSPAIN 2023 is designed to promote and enhance collaboration opportunities with potential business partners and will facilitate up to 4,000 one-to-one meetings.
The BIOSPAIN 2023 programme offers a comprehensive overview of the challenges and opportunities shaping the pace of the biotech industry. Through more than
BIOSPAIN ensures all the ingredients that are attractive to a company: collaboration with other companies, investment opportunities, and discussions of interest on the key global challenges facing the biotechnology sector. Speaking of BIOSPAIN is talking about the history of biotechnology. It‘s about how ‘biotechs,’ despite the difficulty and high cost of innovations that can take years to reach the market, continue to develop disruptive solutions to ensure the health and sustainability of the planet.
In its twentieth anniversary, BIOSPAIN solidifies its position as one of the international flagship events for the biotechnology sector. A comprehensive showcase of the industry's potential and its presence in all areas of society. These are the reasons why it is a key date on the agendas of all those interested in biotechnology.



ETHICS Former Karolinska Institutet surgeon Paolo Macchiarini has been sentenced to two years and six months in prison for aggravated assault. In 2011 and 2012, he had performed experimental surgeries on three patients at the renowned research institute, implanting artificial windpipes seeded with stem cells. The operation was hailed as groundbreaking at the time, however, patients suffered grueling complications and died. When the scandal broke in 2016, it severely scarred the reputation of the Karolinska Institutet.
A year ago, a district court had found the physician guilty in only one of the cases. Both the defence and the prosecution had appealed the verdict. Now, the court of appeal increased the sentence. The Stockholm court ruled that physical harm and suffering had been inflicted on the patients in all three cases. Macchiarini continues to deny any wrongdoing and stated that the operations were life-saving measures and the only alternative for the patients. However, the court ruled that it was not a life-or-death situation in the first two cases: both patients could have lived for a considerable time without the surgeries. And by the time he operated the third patient, he was well aware of the issues arising from the procedure. Macchiarini acted with criminal intent, the court ruled, following the prosecution’s take that the surgeon’s actions were little more than experiments on humans. Macchiarini’s legal team said they plan to appeal the verdict to Sweden’s high court.
MANUFACTURING Insulin manufacturer Novo Nordisk A/S is expanding its Active Pharmaceutical Ingredient (API) production facility in Hillerød to the tune of a whopping DK15.9bn (around €2.1bn).
The new facility, spanning around 65,000m 2, will give the company more production capacity to handle the future market demands and play a major role in developing their future clinical latephase products. “This important investment will ensure the continuous development of our late-phase pipeline into deliveries of important medicines for treatments to patients worldwide,” said Henrik Wulff, executive vice president of Product Supply, Quality & IT.
While Novo Nordisk has long operated on a global level, around half of its workforce – more than 21,000 staff – is still in Denmark. The expansion will add another 340 positions once completed. Construction is underway and the facility is expected to start producing API by early 2029.
OBESITY Novo Nordisk A/S is aiming to maintain its leadership in the thriving weight loss drug market with the acquisition of Canadian endocannabinoid specialist Inversago Pharma for up to US$1.075bn (around €995m). The pharma company, facing intensifying competition for its blockbuster GLP1-receptor blocker semaglutide, is getting access to Inversago’s promising CB1 receptor blocker INV-202, potentially beneficial for treating obesity, diabetes, and related metabolic disorders.
INV-202 plays a pivotal role in regulating metabolism and appetite in various peripheral tissues. The compound has exhibited weight loss potential in a Phase 1b trial and is currently undergoing Phase 2 trials for diabetic kidney disease. Novo Nordisk is also exploring INV-202’s potential applications in addressing obesity and obesity-related complications.
OPHTHALMOLOGY Danish biopharma Breye Therapeutics has joined forces with Milan-based biotech incubator Golgi Neurosciences to develop a treatment programme for retinal disorders, focusing on early-stage diabetic retinopathy (DR) and dry-AMD, both of which often lead to vision loss. The P2X7 receptor, an ATP-gated ion channel, plays a crucial role as an inflammation switch, with inflammation being a significant factor in both diabetic retinopathy and AMD.
The collaboration aims to address the demand for more effective and patientfriendly drugs in the ophthalmic field.
“We are pleased to collaborate to advance the P2X7 receptor antagonist programme for retinal disorders, where there is a large unmet medical need for more effective and less burdensome therapies,” Breye Therapeutics’ CEO, Ulrik Mouritzen, commented. Breye Therapeutics ApS focuses on novel oral therapies for retinal vascular diseases. Breye has received financial support from various institutions, including Novo Holdings, Sound BioVentures, the BioInnovation Institute, the Danish Growth Foundation (Vækstfonden), and the Danish Innovation Foundation (Innovationsfonden).
BIOGAS Nordic energy firm Gasum is expanding its biogas holdings by purchasing a 66% stake in Liquidgas Biofuel Genesis AB. The Swedish company operates a biogas upgrading facility in Helsingborg, southern Sweden. Currently, the plant produces approximately 80 gigawatt hours of upgraded biogas annually, using raw biogas from biodegradable waste collected from households and industries in the area. Gasum is also considering the possibility of onsite gas liquefaction in the future. Gasum CEO Mika Wiljanen sees this acquisition as a strategic move to increase the availability of renewable gas in the Nordic market.
DIGESTIVE HEALTH Probiotic expert Probi AB has launched a partnership with Clasado Biosciences, a UK company known for its prebiotics research. The companies plan to collaborate to develop synbiotic products. While probiotics are live health-promoting microorganisms, prebiotics are substrates selectively utilised by those microorganisms. Synbiotics combine both, leading them to enhance each
other’s effects on the gut microbiome and general health. There is a growing demand for such products – a market that the companies are now set to tackle. Initially, they will focus on advancing the understanding of prebiotic and probiotic combinations, but are also looking for future opportunities in terms of research, development, and commercialisation of a joint synbiotic technology, Lund-based Probi said.

CARBON CAPTURE Researchers in Norway are spearheading an effort to harness the carbon-absorption capabilities of seaweeds. The project, named Seaweed Carbon Solutions, seeks to convert seaweeds collected off the Trøndelag coast into biocoal, a substance that captures carbon and enriches agricultural soil. “If we are to meet our climate change mitigation targets, we have no time to lose in removing CO2 from the atmosphere,” Jorunn Skjermo, research scientist at SINTEF, emphasises the urgency of carbon capture efforts.
The project involves cultivating macroalgae seedlings on ropes, where they absorb CO2 during their growth phase. After harvest, the seaweeds are convert-
ed into biocoal through pyrolysis, i.e. heating them to about 600 degrees in an oxygen-free atmosphere. The resulting biocoal is resistant to degradation and, when combined with seaweed-based fertilisers, can enhance soil porosity, water retention, and microbial growth, benefiting agriculture. Production at the test facility is expected to be in the region of 600 tonnes of seaweed, which in turn will yield 25 tonnes of biocoal. SINTEF has calculated that a facility one square kilometre in size will produce 20,000 tonnes of seaweed annually, equivalent to the capture of 3,000 tonnes of CO2 Skjermo believes that it is realistic to upscale this approach to an industrial facility by 2030.
Karolinska University Hospital and Karolinska Institutet plan to create a joint center focused on advanced cell, gene, and tissue therapies, or “Advanced Therapy Medicinal Products” (ATMP). These therapies have the potential to treat and even cure diseases that were previously untreatable. The centre, named Karolinska ATMP Centre, will operate in Huddinge/Flemingsberg and Solna.
Lund-based biotechAlligator Bioscience has been granted Orphan Designation from the European Medicines Agency (EMA) for its primary asset, mitazalimab, for treating pancreatic cancer. Mitazalimab, a monoclonal antibody targeting CD40, is designed to enhance tumour sensitivity to chemotherapy and activate dendritic cells, B cells, and macrophages to promote immune-mediated tumour destruction. It is currently undergoing assessment in OPTIMIZE-1, a Phase 2 multicentre study.
Biovian Oy, a CDMO specialising in biopharmaceuticals, is investing more than €50m to extend its manufacturing facility in Turku. The new facility will be used in the development, manufacturing, and testing of ATMPs (Advanced Therapy Medicinal Products) such as adenoviral and AAV (adeno-associated viral) therapies. The new facility is expected to be finished by 2024 and fully operational in 2025.
SUSTAINABILITY Oil-eating bacteria form dendritic biofilms that reshape oil droplets to speed up the rate of consumption, a French-British team of microbiologists reported in August. Their discovery of how Alcanivorax borkumensis optimises oil biodegradation and consumption might boost research on environmentally-friendly ways to clean up nature from left-overs from the crude oil industry. Since deciphering its genomes in 2006, these aerobic marine bacteria have been genetically optimised to play a greater role in the bioremediation of spilled petroleum worldwide. During the consumption of alkanes, the bugs form biofilms around oil droplets.
For the first time, the team led by Jean-Francois Rupprecht from University Toulon used a microfluidic device that allows to monitor the bacteria-covered oil droplets. In cultures new to oil exposure, they found the bacteria to form a thick spherical biofilm that grows outward from the oil, and the oil droplet remained mostly spherical as it was consumed. However, cultures that had longer exposure to oil formed a thin biofilm with finger-like protrusions, socalled call dendritic biofilms. Dendritic biofilms alter the oil-water interfacial tension and buckle and reshape the oil droplets as bacterial cells proliferate. This leads to an increase in the surface area of a droplet, optimising and accelerating consumption by the ever-growing population of bacteria.
ATMPS The UK is making a €15m investment in its biopharmaceutical manufacturing industry via its national innovation agency Innovate UK. The agency announced that seventeen new projects will receive funding for cutting edge medicine manufacturing, delivered by the Innovate UK Transforming Medicine Manufacturing programme. The investments focus on nucleic acid medicines, intracellular drug delivery and digitalisation and
automation to accelerate medicines manufacturing. Among the projects awarded funds, many focus on innovative technologies to make better, faster vaccines. For example, one of the funds will go towards a project to streamline production of mRNA and regulatory RNA drugs and vaccines at reduced cost, another in nanosieves for oligonucleotide synthesis. As gene and cell therapies require intracellular delivery another focus is on vector-free delivery technologies. Furthermore, new methods for the optimisation of pharmaceutical freeze-drying processes, in the production of injectable drug products (medicines) and powder gene therapy formulation will be funded along with optimisation of self-amplifying RNA production for novel vaccines and therapeutics. Among the funded project are also some machine-learning-based tools for oligobased therapeutics characterisation and manufacturing.
AMR A Dutch-German research team together with US biotech company Novobiotics Inc (Cambridge, US) seem to have found a novel antibiotic in soil bacteria that eliminates antibiotic-resistant Staphylococcus aureus. The researchers from the Universities of Bonn (Germany) and Utrecht (The Netherlands) discovered clovibactin in the thought-tobe uncultured soil bacterium Eleftheria terrae, subspec. Carolin. Using Novobiotics’ microfluidics iChip, solid-state nuclear magnetic resonance, and atomic force microscopy, the team dissected the drugs’ mode of action. Clovibactin blocks cell wall synthesis by targeting pyrophosphate of multiple essential peptidoglycan precursors (C55PP, lipid II, and lipid IIIWTA). The novel antibiotic uses an unusual hydrophobic interface to tightly wrap around “immutable” pyrophosphate but bypasses the variable structural elements of precursors, accounting for the lack of
resistance. Selective and efficient target binding is achieved by the sequestration of precursors into supramolecular fibrils that only form on bacterial membranes that contain lipid-anchored pyrophosphate groups. According to the researchers, “this potent antibiotic holds the promise of enabling the design of improved therapeutics that kill bacterial pathogens without resistance development”.
“We are very confident that the bacteria will not develop resistance to clovibactin as quickly,” said first author Tanja Schneider from University Bonn. She added that the combination of the different mechanisms of action results in “exceptional resistance to resistance.” Next, the research team plans to use its findings to date to further increase the effectiveness of clovibactin. However, Schneider says there is still a long way to go before the new antibiotic reaches the market.
IMMUNOLOGY In 2022, the Coalition for Epidemic Preparedness Innovations (CEPI) launched its 100 Days Mission of pandemic preparedness. The goal of the private-public partnership is to produce and distribute vaccines that fight “Disease X” within just 100 days of a virus with pandemic potential emerging. For comparison: the first COVID-19 mRNA vaccine manufactured by Pfizer/BioNTech took a record 326 days to complete, but this time period was still too long, according to CEPI, which was established in 2016 in the wake of the Ebola virus outbreak in west Africa.
At the end of August CEPI and the University of Oxford have entered into a strategic partnership to decrease the development time of such globally accessible vaccines and to strengthen Oxford’s existing network of clinical trial sites around the world against pandemic outbreaks. Under the collaboration with vaccine experts from the Jenner Institute and AstraZeneca, CEPI will provide up to US$80m for the optimisation of existing prototype vaccines against CEPI priority pathogens Lassa, MERS and Nipah virus, which could be swiftly adapted if a new viral threat is identified.
Under the agreement, researchers at the Jenner Institute in Oxford will use its chimpanzee adenovirus vaccine platform ChAdOx, to improve such prototype vaccines. While different researchers developed the COVID-19 vaccine that was produced and distributed by Astra-Zeneca at self-cost price, a team led by Sandy
Douglas developed a simple method of manufacturing any vaccine based upon the ChAdOx platform, choosing techniques suitable for scale-up and transfer to larger-scale manufacturing facilities if required in an outbreak.
“CEPI’s strategic partnership with the University of Oxford will make a vital contribution to our work to drive forward the 100 Days Mission,” said Dr Richard Hatchett, CEO of CEPI.
Key to the success of the 100 Days Mission are rapid response vaccine technology platforms – some of which were clinically validated for the first time during the COVID-19 pandemic – that can be used to design vaccines in a matter of days. ChAdOx is one of only a handful of these technologies with proven capability as a platform on which safe and effective vaccines can be quickly developed and manufactured at scale and low cost.
The ChAdOx platform was the basis for AstraZeneca’s COVID-19 vaccine which saved over 6 million lives in the first year of its rollout. CEPI and the University of Oxford’s prototype vaccines will be key components of the Global Vaccine Library: a repository of knowledge, data and resources which can be pulled ‘off the shelf’ by researchers next time Disease X strikes and used to dramatically accelerate the development of life-saving vaccines. L
The European Patent Office (EPO) in Munich has granted a patent to Strasbourg-based Domain Therapeutics SA’s Phase I candidate DT-9081 designed to reverse PGE2mediated immunosuppression used by tumours to bypass the immune system. DT-9081, a selective EP4 receptor antagonist in Phase I clinical development, preclinically synergised with PD1-checkpoint blockers and shrank tumours by reversal of prostaglandin E2-mediated immunosuppression (PGE2).
Donaldson Company Inc. (Minneapolis) has acquired the viral vector production specialist Univercells Technologies SA (Nivelles) from the US-based life sciences tools provider Gamma Biosciences for €136m.

Exeliom Biosciences SA has got an €8m extension of its previous €16m Series A financings to advance clinical development of its microbial immunotherapy EXL01 to fight gastric, lung and hepatocellular cancer and C. difficile infections as well as Crohn’s disease. New investor Crescent Enterprises-Ventures (United Arab Emirates) led the Series A extension, joining existing investors including Auriga Partners and UI Investissement, and Biocodex SA. EXL01 is a oncedaily, first-in-class immunomodulatory therapy containing the major commensal bacterium Faecalibacterium prausnitzii that is developed to broaden the response to existing treatments.
FUNDING Tyrol sees itsself as a successful life sciences region with numerous established companies. “These include global market leaders as well as niche specialists and hidden champions,” explains Mario Gerber, Provincial Councillor for Economy, Tourism and Digitalisation. The majority of these companies is active in medical engineering. In order to further expand the Health Hub Tyrol, centered in Innsbruck, a funding initiative has been launched to support innovation activities with broader perspectives including digital and therapeutics. This funding initiative is endowed with €2.4m (selection of companies):
› Occyo GmbH
Occyo GmbH develops innovative solutions for imaging the surface of the eye. The aim is to develop an AI-based telemedical solution for diagnosing and monitoring diseases of the ocular surface.
› KinCon biolabs GmbH
KinCon biolabs GmbH aims to accelerate the development of personalised medicines through the use of unique molecular reporters and - in the case of Parkinson’s disease - to enable drug discovery through its patented technology.
› ViraTherapeutics GmbH
ViraTherapeutics GmbH is engaged in innovative virus-based immunotherapeutics. The aim of the project is to gain a deeper understanding of the interaction between different immune cells and cancer cells. These findings should help to make therapy with oncolytic viruses more efficient.
PATENTS A first hearing at the Düsseldorf Regional Court marked the start of the lawsuit brought by the Tübingen-based RNA company Curevac N.V. against Biontech SE of Mainz. In July last year, Curevac filed a lawsuit accusing Biontech, the succesfull developers of a COVID-19 mRNA-vaccine, of infringing patents in the use of its mRNA technology.
According to its own information, Curevac N.V. had deliberately delayed the clarification of the complicated situation surrounding the patents on the mRNA technology until “after” the pandemic in order not to hinder the development of the vaccine. However, Curevac founder Ingmar Hörr in particular had already repeatedly emphasised in the hot phase of the competition for an
effective Corona vaccine that he sees himself as the actual inventor of an mRNA active substance that can be applied by injection.
So far, Biontech has not let this stop it from developing the successful vaccine Comirnaty, and has also shown itself only little impressed by the threatened and finally filed lawsuit. Biontech said it had used an “original” mRNA technology. However, since Curevac is interested in “fair compensation” and thus a share in the enormous sales of the Mainz vaccine, Biontech has also retaliated and filed its own lawsuit against Curevac in the USA. Biontech has already fought various patent disputes concerning the mRNA technology and is not afraid of the lawsuit from Tübingen albeit preparing for the next vaccination seeason to come.
PLANT RESEARCH With an investment of €220m in research and development at the Monheim site, Bayer is underlining its commitment to sustainable agriculture. It is the company’s largest single investment in its crop protection business in Germany since the foundation stone of the Monheim campus was laid in 1979. In the presence of Bayer´s new CEO Bill Anderson (Foto: 3rd from left), Bayer’s Agricultural Division opened the construction site for the new buildings in Monheim with a traditional groundbreaking ceremony. The investment will create a new building complex with laboratories, offices and a greenhouse, providing space for around 200 employees. The five-storey main building will have a usable area of 28,000 square metres. Construction of the new buildings is expected to take around three years till the end of 2026.


Austrian Invios has received regulatory approval for a multi-center trial of novel autologous cell therapy APN401 leveraging two GMPcompliant manufacturing sites. Patient recruitment has started. Invios was awarded up to 45% funding of trial costs by Austrian Research Promotion Agency (FFG) to support Phase 1b RP2D trial.
CLIMATE HEALTH French-Austrian vaccine developer Valneva (Nasdaq: VALN; Euronext Paris: VLA) said mid of August, the US Food and Drug Administration (FDA) has pushed back the Prescription Drug User Fee Act (PDUFA) deadline for a decision on the company’s chikungunya virus vaccine candidate from the end of August to the end of November. No additional data, but more time would be needed to plan for postapproval surveillance of use.
The FDA postponed the PDUFA to allow sufficient time to coordinate and agree on the phase IV programme required for the accelerated approval process. Additional clinical data for the approval process have not been requested, Valneva stressed.
As this vaccine candidate is currently the only one that is so advanced in the approval process and has convincing phase III data, Valneva’s CMO, Juan Carlos Jaramillo, is relaxed: “We are proud that, if approved by the BLA, VLA1553 will be the first vaccine candidate to be approved under the accelerated approval process for an epidemic disease and that
the required phase IV activities will then set a future standard.” Secretly, however, the company is still speculating on an earlier approval: “We continue to work closely and in partnership with the FDA and believe it may be possible to obtain approval before the new PDUFA date,” Jaramillo said.
“Chikungunya” is a Kimakonde word of the Makonde tribe in Africa describing the disease symptoms of a virus infection, and can be translated as “disease that bends up the joints”. The causative chikungunya virus (CHIKV) belongs to the genus Alphavirus of the Togavirdae family, first described in 1952. In the long race for approval of a vaccine – going on for these 70 years –, US Merck withdrew in spring because Valneva seemed to be uncatchably in clinical development. Other institutions and companies are still in early clinical phases. The current diversity of vaccine modalities could be greatly reduced after approval if investors lose interest in the field. Valneva´s vaccine is an attenuated, modified virus variant that was proven to be safe but triggering a sufficient immune response.
Martinsried-based Bind-X GmbH has successfully closed a €10m Series B round of funding from national and international investors (HTGF, HG Ventures, K&K 1, Saxovent, Greeneering Invest). With its technology platform Bind-Tech®, the start-up, formerly known as Dust Biosolutions, aims to make entire industries more sustainable. Bind-X has developed a technology based on microbiologically induced calcite precipitation. This leads to a consolidation of dry or dusty material. Applications reach from weed management in agriculture (only deep-rooted plants get their nutrients, growth of weeds is suppressed), “ecological dust suppression” (by giving the soil of lorry ruts in mining a higher strength) or reduction of bitumen in construction.
Haya Therapeutics from Lausanne has outperformed 99 other Swiss start-ups from different sectors in the Venture Lab´s annual award. Haya is developing compounds against cardiac fibrosis, targeting the non-coding region of the genome.
GENE DELIVERY AAVantgarde Bio srl has secured €61m in Series A financing to advance its gene delivery programme towards clinical trials. This funding marks the largest Series A round in the history of Italian biotechnology. The Milanbased company, specialises in two exclusive adeno-associated viral vector platforms designed for delivering large genes. One utilises DNA recombination, called dual hybrid, and the second is based on protein trans-splicing.
EMA APPROVAL The Italfarmaco Group SpA has received validation from the European Medicines Agency (EMA) for its Marketing Authorization Application for givinostat as a potential treatment for Duchenne Muscular Dystrophy (DMD). The submission was based on the Phase III EPIDYS clinical trial’s safety and efficacy findings, which assessed givinostat, an HDAC inhibitor, in DMD patients. This submission to the EMA follows a New Drug Application submitted to the US Food and Drug Administration (FDA) earlier this year, which received priority review status. The Phase III trial involved 179 ambulant DMD patients aged six and older, and Givinostat showed positive results in improving muscle function. The treatment targets the deregulated activity of HDACs in DMD patients, mitigating muscle degeneration and disease progression.
FUNDING Slovenian biotech firm GenePlanet doo. secured €20m in Series B funding in July. The investment, with contributions from BlackPeak Capital, Darwin AG, Kolektor d.d., FMR doo, and Designed Impact Ventures GmbH, is aimed at expanding the company’s global presence and enhancing genetic testing capabilities. GenePlanet plans to use the funds to diversify its product line-up, target acquisitions in medical DNA testing, invest in big data infrastructure, and develop advanced AI systems for genome interpretation. CEO Marko Bitenc anticipates profitable operations in 50 countries over the next five years. Established in 2008, the company specialises in clinical prenatal, and lifestyle genetic testing based on whole genome technology.
FINANCING Oniria Therapeutics S.L. secured a total of €1.28m in funding. This financial injection will enable the company to continue its progress in the development of ONR-001, a drug designed to eliminate persistent tumour cells that remain following conventional treatments. These latent cells are the root cause of cancer relapses and metastasis. The company has conducted preclinical studies involving various TET2 activator drugs, demonstrating their effectiveness in animal models for colon cancer, melanoma, and acute myeloid leukaemia. The funding round was spearheaded by Mind the Gap, the Botin Foundation’s biotechnology entrepreneurship program, and included contributions from Banco de Sabadell, SA, and public funding.
ARTIFICIAL BEEF In June, JBS SA, the Brazilian meat conglomerate, announced that its subsidiary BioTech Foods has initiated the construction of a sizeable labgrown meat facility in Spain, slated for completion by mid-2024. This plant, touted as the world’s largest of its kind, is set to produce over 1,000 metric tons of labgrown beef annually, with plans to expand to 4,000 metric tons in the near future. JBS acquired a 51% stake in BioTech in a $100m deal in 2021, allocating $41m
for this facility in San Sebastián. BioTech’s CEO, Iñigo Charola, highlighted the potential of lab-grown protein to enhance food security and global protein production amid supply chain challenges. BioTech aims to scale up production to meet rising consumer demand, targeting key markets like Australia, Brazil, the European Union, Japan, Singapore, and the United States. Their unique approach involves cultivating tissue from livestock cell samples, promising innovation in the meat industry.


BLUE BIOECONOMY The latest study of the PROFIUS Project, in collaboration with the Maltese AquaBioTech Group, was published in July and reveals potential by-products from tuna farming, including collagen, enzymes, fishmeal, and fish oil. The three-year PROFIUS Project, involving universities and companies from Denmark, Malta, Norway, and Iceland, aims to improve biomass quality through preservation methods. The focus is on optimizing sidestreams from tuna processing, addressing sustainability challenges. The article highlights the potential of tuna processing sidestreams as a valuable raw
material for resource recovery. Identifying and integrating these technologies into the production process can ultimately lead to increased economic circularity and sustainability in tuna fisheries. Fish by-products offer sustainable sources of protein and lipids for fish feed. In Malta, AquaBioTech collaborates with Aquaculture Resources Ltd to develop rendering facilities for tuna by-products. This project, funded by the EU’s Horizon 2020 program and the Malta Council for Science and Technology, promotes resource recovery and sustainability in tuna farming (10.1007/s11356-02328610-w).
MEDICINE PRODUCTION Construction of the Greek pharmaceutical industry’s first modern Biotechnology research centre is now underway in Agios Stefanos, Attica. In mid-March, the Interministerial Committee for Strategic Investments (DESE) granted approval for the pharmaceutical firm DEMO ABEE to be included in the Strategic Investments program. This program focuses on the Industrial Research and Development of two biosimilar monoclonal antibodies designed for treating malignant neoplasms, with a total budget amounting to €89m. DEMO has ambitious plans to initiate the research and de-
velopment of three monoclonal antibodies by the year 2027. These Biotechnology investments constitute a pivotal component of DEMO SA’s comprehensive investment plan spanning 2021-2027, with a substantial budget totalling €356m. These ongoing projects encompass various sectors, including raw material production, active generic drug manufacturing, research and development, and biotechnology. Notably, DEMO’s strategic investments bear tremendous significance for the National Economy, as they establish the entirely new sector in Greece, of Biotechnological medicine production.
The drug discovery initiative, PalmitoMET, aims to address the enzyme linked to elevated palmitic acid metabolism. Under the leadership of Dr. Salvador Aznar Benitah at the Institute for Research in Biomedicine Barcelona, this project has secured €150,000 in funding for an 18-month duration. In July, the European Research Council unveiled funding support for 66 recipients, with the goal of harnessing the commercial and societal impact of their research endeavours.
In July, Quince Therapeutics Inc. acquired EryDel SpA, including its Phase III asset aimed at treating Ataxia-Telangiectasia, a condition currently lacking approved treatments, with an estimated peak sales potential exceeding $1bn. This acquisition will be completed through a stock-for-stock upfront exchange, along with potential future milestone cash payments.
Nuage Therapeutics SL has secured €12m in seed financing, led by Sofinnova Partners SAS and Sabadell Asabys Health Innovation Investments, SA. The company has developed a technology enabling the targeting of intrinsically disordered proteins using small drug-like molecules. The funds raised in this seed financing will be utilised to advance the lead program, focusing on castration-resistant prostate cancer, and to validate the effectiveness of its drug discovery platform.
FINANCING Turbine Ltd, a Hungarian-British biopharmaceutical company specialising in cell simulations for drug discovery and precision medicine, has added €5.5m in funding from MassMutual Ventures (MMV) to its Series A financing round. This brings the Series total to €25.5m and broadens the group of USbased investors. Turbine also announced the appointment of four independent directors to its board.
This development follows Turbine’s recent progress, including establishing lab facilities in Budapest and a partnership with Cancer Research Horizons. The funds will support Turbine’s internal programmes and expand its biology simulation platform. Supported by companies such as Bayer and MSD, Turbine has multiple in vivo validated novel assets in its pipeline.
AMR Proteon Pharmaceuticals SA has inaugurated its Center for Bacteriophage Biotechnology in Łód ź , a stateof-the-art laboratory. The Łód ź ’-based bacteriophage expert invested nearly PLN6m in the facility, which spans 1600 m 2. Here, Proteon aims to explore bacteriophages’ antibacterial properties to pioneer sustainable health solutions. The company, which has secured nearly PLN37.5m in EU grants, aims to revolutionise animal health and food safety while reducing antibiotic usage.
R&D Clinical stage biotechnology company Molecure SA has received clearance from the FDA on its Investigational New Drug Application to conduct Phase II clinical testing of its lead candidate OATD-01, a first-in-class CHIT1 inhibitor for the treatment of sarcoidosis. In Phase I studies, OATD-01 demonstrated to be safe and to have a novel therapeutic mechanism of action, simultaneously targeting inflammation and fibrosis. The Phase II clinical trial of OATD-01 is expected to be a multi-center, randomised, double-blind, placebo-controlled study assessing the drug’s safety and effectiveness in treating approximately 90 pulmonary sarcoidosis patients. The first patients in this Phase II study are scheduled to begin receiving treatment within 2023.
MANUFACTURING Mabion SA has finalised a €3.2m agreement with Global Life Sciences Solutions Poland sp. zoo for the purchase and installation of Xcellerex XDR bioreactors with conventional mixing technology at its Konstantynów Łódzki facility. This move aligns with Mabion’s 2023-2027 strategy for technological diversification. Mabion also aims to complete its plant modernisation by year-end, further boosting its capabilities. “We are actively working to acquire new business partners, and the broader panel of available bioreactor technologies together with the planned modernisation of the existing plant [...] significantly support this direction of our activities”, commented Krzysztof Kaczmarczyk, President of the Management Board of Mabion.

M&A PPF Biotech is joining forces with eureKING, a French SPAC dedicated to bioproduction, with the goal to build a new European CDMO leader in biotechnology. In August, PPF Biotech agreed to hand over the assets of its full-service CDMO SCTbio in exchange for a minority stake in eureKING. The proposed transaction values SCTbio assets at approximately €19.5m, with eureKING acquiring 67% of SCTbio shares from PPF Biotech for €13.08m in cash. The remaining 33% stake will be settled
through newly issued eureKING shares, resulting in full ownership of SCTbio by eureKING. “We intend to combine our distinctive and complementary skills and capabilities to create the foundations for a CDMO network with industry credentials”, said Lud ě k Sojka, CEO of SCTbio. “Having a new, fully integrated bioCDMO leader is the key to reinforcing the Europe’s strategic biomanufacturing capabilities.” served patient populations through efficient, simulation-guided development.”
LITHUANIA In recent years, Lithuania‘s life sciences sector has defied geopolitical and economic challenges, emerging as a global leader in biotech and medtech. Amidst political and financial turbulence, Lithuania‘s life sciences ecosystem is experiencing unprecedented growth, with innovative breakthroughs at its core.
While the average funding per start-up is decreasing globally, central, and Eastern Europe, with Lithuania leading the charge since 2017, sees an increase in enterprise value. Despite the investment in deeptech increasing across the Baltic region — with $90.3m received in 2022, in comparison to 2021’s $69.4m — it still significantly lags behind other European regions. In the past few years, with government incentives, a rich talent pool, and close ties to their multiple research institutions, Lithuania is rapidly becoming a haven for innovation and a central nexus for state-of-the-art science and technology.
The country’s strategic central location in Europe has piqued the interest of foreign companies seeking manufacturing hubs. Lithuania is actively luring expatriates through public campaigns like “Move to Vilnius,” a move that has already bolstered the city's population by over 5% in 2022, with expectations of reaching 830,000 residents by 2030. At the heart of the industry lies an audacious government target: to contribute 5% of Lithuania‘s GDP by 2030. Currently, the sector already makes a substantial 2.7% contribution to the nation‘s GDP, a feat achieved through steadfast government support and a collaborative ethos shared among academia, businesses, and government bodies. Beyond mere aspirations, the Lithuanian life sciences sector is delivering measurable progress, exemplified by an enviable annual growth rate
Lithuania's rapidly growing life sciences sector is making remarkable progress through a mix of innovations, including genetically modified yeast for psychedelic compounds, tremor-reduction tech, and advanced gene editing.
of 30%, a statistic that many European counterparts can only envy.
In just a few years, Lithuania‘s life sciences sector has undergone a seismic transformation. Its value, which stood at €500m in 2018, has surged to a staggering €8bn, becoming the nurturing ground for over 740 startups. Notably, the COVID-19 pandemic catalysed this evolution, propelling a flurry of innovation. Lithuanian life sciences witnessed a significant uptick in patent applications from 2019 to 2021, reflecting the nation's commitment to combat the pandemic head-on. To achieve this, family-friendly policies, a high quality of life, and progressive approaches to work
and gender equality are attracting global talent.
In OECD rankings, Lithuania secures the second spot among member countries with an impressive 49% representation of women in science, technology, and innovation. Initiatives such as Women in Tech, Women4Cyber, and the businesswomen association Leaders Lithuania are empowering women in STEM fields, encouraging their participation in the biotech sector, and thus bolstering the growing deeptech ecosystem.
Moreover, Lithuania‘s favourable tax and legal framework provide significant empowerment to tech companies, facilitating growth and attracting invest-

Designed Specifically
SigmaPlot® is an easy-to-use scientific graphing & statistical data analysis software package for researchers, scientists and engineers who need to create precise, publication-quality graphs that best communicates their research results for presentations, technical publications, and the web. Along with advanced curve fitting, a vector-based programming language, macro capability and over 50 frequently used statistical tests, SigmaPlot also provides more than 100 different 2D & 3D graph types from which one can choose a full range of graphing options such as technical axis scales, multiple axes, multiple intersecting 3-D graphs and much more. SigmaPlot now has SigmaStat included with it which is a perfect tool to visualize and understand basic and advanced statistics.
SigmaPlot offers:
•Complete Advisory Statistical Analysis
•Award-Winning Technical Graphing Capability
•Powerful and Easy-to-use Data Analysis
•Ability to Customize every Element of Graph
•Precise Publication-Quality Graphs
•Ability to Publish your Work Anywhere
Latest updates:
•Includes a New Heat Map Macro
•Removes all dependencies on old redistributable by removing Lead Tools and uses Windows Graphics Device Interface + (GDI+) for graph export
•Uses the latest Sentinel License Manager which is compatible with the latest Microsoft Server 2022
•Uses a hosted licensing service for smooth license activation and validation
•Has a new and refreshed ribbon manager that enhances the already commendable user experience in SigmaPlot
ments. The country‘s burgeoning momentum is anticipated to trigger substantial exits, creating a cascading effect as tech founders, flush with capital from successful ventures, reinvest in new startups.
The government's incentives for innovation encompass various measures, including a one-year corporate income tax exemption for small enterprises, tax deductions for companies investing in digital transformation, and shorter depreciation periods for assets dedicated to research and development. Policies like the startup visa program, launched in 2017, have attracted hundreds of entrepreneurs to Lithuania.
Presently, Lithuania boasts over 100 active life sciences companies, with one standout star, Caszyme LLC, gaining international recognition. Established in 2017, Caszyme has rapidly ascended as a prominent player in Lithuania‘s biotech sector, focusing on Crispr-Cas9-based molecular gene editing technology. The company envisions using its technology to combat complex diseases, revolutionise agriculture, and contribute to climate change mitigation. The public‘s response to gene editing remains mixed, with concerns about its potential risks. While discussions continue, gene therapy trial regulations remain stable, with 2023 marking the first approval for a CRISPR gene therapy.
North America & South America
Inpixon USA
2479 E. Bayshore Rd, Suite 195. Palo Alto, CA 94303, United States saves.sales@inpixon.com
Germany, Austria, & Eastern Europe
Inpixon GmBH
Königsallee 92a, 40212 Düsseldorf, Germany saveskontakt@inpixon.com
UK & Western Europe
Inpixon UK Ltd
268 Bath Road, Slough, SLI 4DX, UK saves.sales@inpixon.com
©2022 by
In the burgeoning landscape of mental health therapeutics, Vilnius-based biotech firm Psylink is making waves with its groundbreaking approach to psychedelic drug development. Amid growing optimism about the potential of psychedelics in reshaping mental healthcare, Psylink stands out as a pioneer. Their strategy involves harnessing genetically modified yeast cells to recreate biosynthetic pathways for psychedelic compounds, thereby providing a sustainable, scalable, and cost-effective means of production. Despite challenges related to sourcing and manufacturing these substances, Psylink‘s 2021 licensing to work with Schedule I substances positions it as a key player in the evolving field. As research progresses and regulations evolve, Psylink envisions the development of standardised protocols for psychedelic-assisted therapy, potentially integrating these substances into mainstream mental healthcare practices.
All the players of Lithuanias Life science scene will meet later this week at the Life Sciences Baltics organised by the Innovation Agency Lithuania. This year‘s event, scheduled for September 20 th -21st in Vilnius, will bring together 800 life sciences professionals, offering a platform for networking and collaboration within the thriving Baltic ecosystem.
Lithuania remains unwavering in its dedication to sculpting a brighter future through the transformative power of life sciences. The sector not only buoyed the economy but also raised global awareness about Lithuania‘s capabilities in cutting-edge science and technology. L
m.milidakis@biocom.eu
ANDROGENETIC ALOPECIA German university spin-out Mallia Therapeutics GmbH has raised seed money to accelerate preclinical research of a potentially curative baldness cure based on soluble CD83. Promising preclinical data show that the immunomodulator stimulates both hair growth and the formation of new hair follicles via a dual mechanism of action without evidence of otherwise common systemic side effects.
Good news for all those with genetic hormonal hair loss (androgenic alopecia and alopecia areata): according to the patent specification, an anti-inflammatory soluble peptide called soluble CD83 (sCD83) may not only help patients with arthritis, diabetic foot ulcer or poorly healing wounds, but it also makes the hair on bald heads grow back – at least a little in mice.
Preclinically, sCD83 prevented the dihydro-testoterone-induced micro-inflammation of hair follicles and subsequent hair loss in favour of boosting regulatory T cells and pro-regenerative macrophages leading to hair growth. The programme, which already was awarded €500,000 at the m4 award of the Bavarian State Ministry of Economic Affairs two years ago, led to the founding of Mallia Therapeutics GmbH (former working name MalliaBiotech) in Erlangen and triggered a seed financing in an undisclosed amount in mid-July. The company, spun
off in June and led by Institute Director Alexander Steinkasserer (Univ. Erlangen) and Manfred Gröppel is based on the work of Dmytro Royzman and published a European patent two years ago that covers the application areas of anti-inflammation and hormonally induced hair loss – the latter affects around 50% of hairless people. Unlike previous products, the sCD83 formulation stimulates the formation of new hair follicles.
Worldwide, over 50% of men as well as 50% of postmenopausal women are affected by hormone-induced androgenetic alopecia (pattern baldness) and another 147 million suffer from autoimmune-mediated alopecia areata. Current treatments do not induce new hair growth but only extend the life cycle of existing hair or work to systemically suppress the immune system with associated adverse effects including severe infections. Mallia expects to address both these shortcomings with sCD83 with a superior safety profile and a dual mechanism of action to preserve hair and induce new hair follicles: Firstly, it induces an anti-
inflammatory environment at the hair follicle via regulatory T cells (Tregs), which interact with follicular stem cells and thereby activate hair growth. Secondly, sCD83 directly binds to follicular stem cells where it induces the formation of new hair follicles.
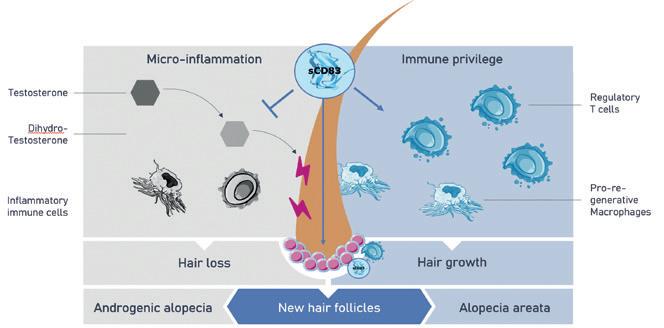
In a presentation at the EHRS Conference, the co-founder and scientific director of Mallia Therapeutics Dmytro Royzman presented data from a preclinical androgenetic hairloss model showing that sCD83 accelerated the hair growing phase (anagen phase) and induced new hair growth. In addition, using a human ex vivo system, sCD83 treatment prolonged the growing phase of human hair, and hair growth-associated pathways are induced. Further, sCD83 application led to the expansion of a stem cell population within the human hair follicles. According to Managing Director Manfred Groeppel, Mallia Therapeutics is going to use its seed funding to bridge the phase until closing a Series A round maybe this year. “Given the promising results of our ongoing preclinical studies including results using tissue samples from patients, we hope to move into the clinic and start treating patients within the next two years,” he said.
Androgenetic alopecia is the most common form of hair loss. It is due to genetic and hormonal factors. In men it often leads to receding hairline and baldness on the top of the head, in women to thinning hair on the crown of the head. Alopecia areata results in circular hair loss on the scalp, face or other parts of the body. It occurs when the immune system “mistakenly” attacks hair follicles, resulting in autoimmune-related hair loss. sCD83 counteracts both of these. L
t.gabrielczyk@biocom.eu
Picture: © Alexander Steinkasserer, Univeristy of Erlangen Mode of action of soluble CD83For biotechnologists, Europe is a collaborative research area. For the biotech industry it is a huge common market with almost 500 million potential customers. The 13th edition of the European Biotechnology Guide presents an attractive cross-section of companies and institutions from 16 nations.

ROBOTICS A new smart soft robotics system tackles a key problem faced when using implantable devices for longterm drug delivery: the build-up of fibrotic tissue, which can obstruct the delivery of essential drugs such as insulin. At the end of August, an Irish-US research team presented a solution (10.1126/scirobotics. abq4821).
During tests, the system used machine learning to monitor fibrosis in rats, and to distribute varying amounts of blue dye –as a proxy for drugs – into gel dishes, as permeability changed in the gel. While the design has yet to be examined in the context of drug delivery, it showcases a new purpose for self-adaptable robotics.

“The use of machine learning in the implant represents a notable example of the potential for artificial intelligence (AI) in medical device development and function,” biomedical engineer Tejal Desai from Brown University in Berkeley (USA) and Nanomedicine Chair Alessandro Grattoni at Houston Methodist Research Institute told Europ E an B iot E chnology
Over time, medical implant devices can trigger immune inflammation and fi-
brosis. This can cause devices to become encapsulated, when collagen-based fibrotic tissue encircles the device and obstructs drug delivery. For example, with insulin pumps, this process can reduce the amount of insulin that is released into the body, preventing patients from getting their needed dosage.
Thus, first author Rachel Beatty and study co-leaders Garry Duffy from the AMBER centre at the University of Galway and Ellen Roche from the MIT in Cambridge, USA, developed the FibroSensing Dynamic Soft Reservoir (FSDSR) as an answer to these challenges. By using machine learning and electrical impedance spectroscopic sensors, FSDSR can spot the beginning of fibrotic encapsulation and adjust the dose accordingly. The researchers tested FSDSR’s potential in vivo using rats, establishing that the design could monitor inflammation and the beginning stages of fibrosis over the course of seven days. Then, they conducted in vitro experiments with blue dye in agar gel and found that the system could sense changes in the gel’s permeability, prompting it to adapt how much blue dye it distributed. Overall, the findings support future investigations into FSDSR’s efficacy with actual drugs like insulin.
184 pages, available in all bookshops and at biospheria.shop/en
ISBN 978-3-928383-87-5
19.80 Euro

GENE DRIVE Invasive species often compete with native species for resources, posing a significant ecological challenge. Can synthetic gene drives provide a solution to this problem?
The gene drive technology holds tremendous potential in combatting diseases like malaria, dengue, and yellow fever. According to the World Health Organization (WHO), in 2021, approximately 619,000 people succumbed to malaria. Up to now, the most effective remedy has been mosquito nets treated with insecticides. However, a new vaccine, R21/ Matrix-M, offers hope. It is the successor to Mosquirix, which was approved in 2019 but demonstrated only a 35% protective effect. The new vaccine achieves up to 80% protection against malaria and is currently undergoing WHO approval. Since the malaria vector Plasmodium falciparum is challenging to combat, and mosquitoes are developing resistance to insecticides, novel control methods are urgently needed. The WHO reports that the healthcare costs for malaria control, at €3.3bn, are only half of what is required. Gene modification methods have proven
successful in agriculture for pest control. Synthetic gene drives also come into play in the realm of nature conservation. They can be employed to contain invasive species that harm ecosystems, transmit diseases to other species, and damage crop plants, such as the Mediterranean fruit fly. Gene drives accelerate the spread of a desired trait within a population by bypassing Mendelian inheritance rules.
Synthetic gene drives are a subset of genome editing applications that allow for alterations in the genome. Up to now,
this technique has been tested in a limited number of species (mosquitoes, yeast, and fruit flies) in laboratory experiments. The desired gene is not transmitted to just 50% of offspring, but to all of them, leading to rapid establishment of the desired feature within a few generations.

For over 50 years, scientists have been exploring this technique. The advent of CRISPR/Cas9 genome editing in 2012 greatly facilitated the creation of gene drives. This technique is more versatile, precise, easier, faster, and cost-effective than other methods. With gene drives, mosquitoes can be immunised against malaria or rendered sterile (see figures p.78/79). As mosquitoes develop resistance to insecticides, gene drives offer a potential alternative. CRISPR/Cas9 can generate genetically modified organisms

easily and prevent the loss of introduced traits through Mendelian rules.
Previous research has primarily focused on different mosquitoes, Drosophila melanogaster, and mice. The X-Shredder, using a homing-endonuclease-based CRISPR/ Cas9 gene drive in the Anopheles mosquito, selectively cuts only the X-chromosome during spermatogenesis. This leads to a skewed gender ratio, with over 95% of descendants being male. This technique proves effective in controlling population expansion if the X-shredder is located on the Y-chromosome.
Synthetic gene drive systems theoretically hold potential for saving endangered species. Laboratory experiments with the Anopheles mosquito, which transmits Plasmodium spec. to its host, have served as a model.
Between 1986 and 2022, invasive species caused approximately €16.5bn in damages worldwide, with Oceania and the Pacific Islands bearing the brunt (63%). Islands, like New Zealand, face a particularly high threat from invasive species due to their exceptionally diverse ecosystems.
In New Zealand, introduced rodents such as house mice and rats pose a threat to native birds, reptiles, and plants. Mice consume the eggs and chicks of 28 native bird species. To protect bird populations and vegetation on the main island of New Zealand, the government allocated €2.4m in 2016 for mouse pest control. The “Predator Free 2050” programme aims to eradicate invasive species in New Zealand by 2050. Traditional methods involving poison are expensive and can have unintended consequences for other animals and plants. Genetic approaches may offer a more effective, long-lasting, and cost-efficient solution.
Hawaii faces similar challenges, with introduced rats, pigs, avian malaria transmitted by mosquitoes, and habitat destruction all contributing to the decimation of bird populations. Domestic pigs introduced to the island also create breeding pools for mosquitoes by digging holes
in the ground. Mosquitoes, introduced just 250 years ago, have no natural predators in Hawaii. As a result, 70% of the island's bird species have gone extinct, including the Kauai ‘O’o, a type of Hawaiian honeycreeper.
Some organisms naturally possess gene drives, as seen in certain mosquito species, fruit flies, and yeasts. In 1907, Edmund B. Wilson observed parasitic or selfish genetic elements in hemipterans. These selfish genes promote their own transmission at the expense of the host organism, even if it harms the host. In 1976, Richard Dawkins described such selfish genes in his book The Selfish Gene. These selfish DNA elements are transmitted through non-Mendelian rules, with more than 50% inheritance. They have no apparent benefit for the host organism and propagate in the genome by copying themselves.
The idea of spreading a desired trait through selfish genes within a population emerged in 1960. It involved controlling populations through the distribution of chromosomal translocations and the use of selfish genes. The use of homing endonucleases for constructing selfreplicating gene drives was introduced in 2003 by Austin Burt. However, practical applications in nature are yet to be realised. Initial developments for malaria control using RNA interference, zinc-finger nucleases, transcription activator-like effector nucleases (TALEN), and meganucleases received funding from governments and foundations. The Melinda & Bill Gates Foundation, for example, aims to eradicate malaria through gene drive technology.
There are various types of selfish DNA, such as transposons, B-chromosomes (redundant chromosomes), or meiotic drives (deviations from the second Mendelian rule). The term meiotic drive was first coined by Larry Sandler and Edward Novitski in 1957.
One instance of meiotic drive was observed as an SD-system (segregation distorter) in the fruit fly Drosophila mela-
nogaster. Another example is seen in t-haplotypes (transmission ratio distortion) found in house mice, and the spore-killer system (Sk), observed in moulds of the genus Neurospora. The yellow fever mosquito ( Aedes aegypti ) possesses a naturally occurring gene drive that skews the gender ratio in favour of male offspring. This is known as the Killer-Y chromosome element, which cuts the X-chromosome during gametogenesis. Even in plants, there is non-random segregation of specific chromosomes, as seen in maize.
Researchers at the University of Zurich are harnessing the t-haplotype, a naturally occurring selfish DNA sequence in mice. It contains a combination of specific genes that are consistently transmitted together. In males, this gene is transmitted at an amplified rate, up to 95%. This occurs due to a distortion in heredity rates known as transmission ratio distortion (TRD).
Scientists led by Dr. Jan-Niklas Runge have observed that young mice carrying the gene exhibit increased migratory behaviour and seek contact with other mouse populations. The selfish gene can self-eliminate if it becomes too prevalent in a population, as a homozygous t-haplotype is lethal for the mouse. The researchers noted that the larger the population, the more pronounced the migratory behaviour of the mice became. This suggests that the t-haplotype spreads by manipulating mouse migration behaviour to ensure its own survival. Consequently, mutations that lead to infertility within the t-haplotype could rapidly disseminate within a mouse population.
Researchers at the University of Adelaide utilised the genetic traits of t-haplotypes in mice to introduce a population-specific gene drive using CRISPR/Cas9 on some New Zealand islands. They altered a gene responsible for female mouse fertility, specifically targeting genes unique to the island populations. This approach aims to
In sexually-reproducing species, most genes are present in two copies, either one of which has a 50% chance of passing to a descendant (B, left-hand side). By biasing the inheritance of particular altered genes, through double strand breaks and subsequent repair by homologous recombination (A), synthetic gene drives could spread alterations through a population (B, right-hand side).
prevent the gene drive from spreading to adjacent islands and regionalise its impact.
Establishing a gene drive within a population requires many sexually reproduced generations. Factors influencing gene drive spread include the organism's generation time, the efficiency of repair mechanisms, how the gene drive affects the organism's fitness, and the population's reproduction rate. To solidify a gene drive in a population, new individuals carrying the gene drive must be introduced to replace incorrect versions. The technical challenges are substantial, given the complexity of an organism's genetic profile and the generation time.
Resistance to gene drives can arise due to individual mutations at the target sequence. Incomplete or erroneous copying during the repair process can lead to resistance. The formation of resistances can be mitigated by selecting various target sequences.
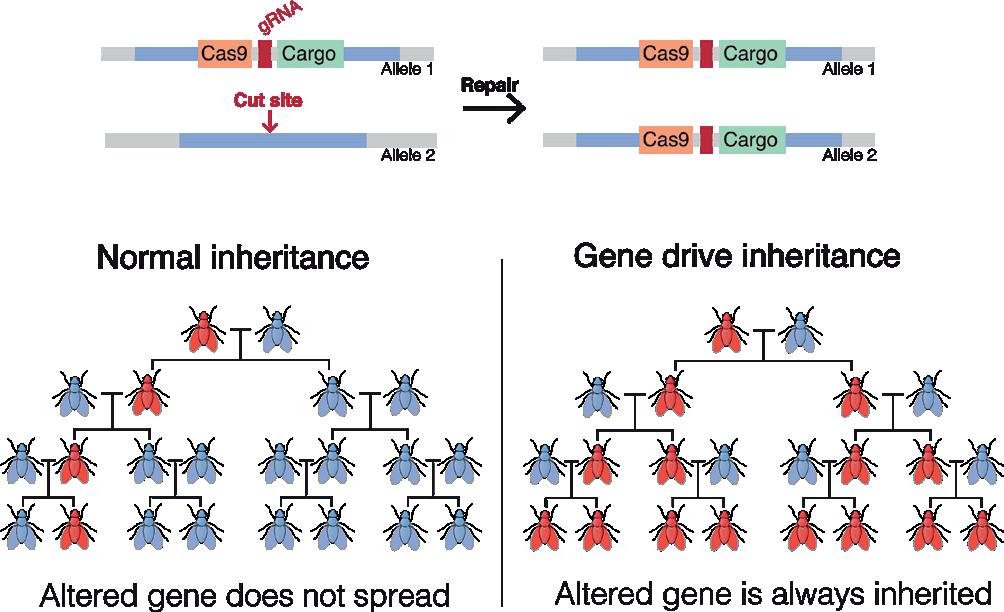
Synthetic gene drives represent a significant intervention in nature, and therefore, their positive and negative effects must be carefully assessed. Ethical and social considerations are paramount as well.
Given the profound and potentially irreversible impact of gene drive technology on society, transparency in research is crucial. The public should be well-informed about the technology and participate in discussions regarding its application. Experts like Kevin Esvelt from the Massachusetts Institute of Technology (MIT) advocate for transparency within the research community and toward the public. Empirical data and predictive models should be openly accessible in a comprehensible and transparent format. The technology's advantages and disadvantages must be rigorously evaluated and discussed within the scientific com -
munity and in the public sphere. Concerns include the potential for misuse in military and terrorist contexts. Once released into the wild, a gene drive is virtually impossible to eradicate. It selfreplicates and undergoes evolutionary processes. The effects of mutations and selection on gene drive development are unpredictable. Consequently, measures are being considered to enhance the environmental security of gene drives.
Proponents highlight several advantages of gene drive technology. It requires fewer resources and coordination efforts compared to conventional methods of species conservation. Opponents, on the other hand, fear the complete eradication of certain species and ecosystem collapse. Once introduced, gene drive organisms can spread uncontrollably, potentially giving rise to new invasive species. For instance, reducing mosquito populations through a suppression drive could impact entire ecosystems. Even laborato -

› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2023
› Guide to German Biotech Companies 2023
› conveniently accessible ePapers
› Discount for BIOCOM events for 1 person
› additional partner offers
Price: 80 Euro p.a. (incl. VAT)
Students 50% discount (subject to proof of enrolment)
› European Biotechnology Magazine Subscription
› European Biotechnology Guide 2023
› Guide to German Biotech Companies 2023
› conveniently accessible ePapers
› Discount for BIOCOM events for 2 persons
› additional partner offers
Price: 120 Euro p.a. (plus VAT)
ry animals may escape, posing a risk. In the event of resistance formation against a gene drive, the spread of genetic changes cannot be ruled out. Safety measures are necessary in case of unintended gene drive organism releases. The European Union's genetic engineering law governs gene drive systems based on molecular techniques. An environmental risk assessment, following the guidelines of the European Food Safety Authority (EFSA), is conducted for the risk assessment of genetically modified animals. The Cartagena Protocol is an international regulation addressing gene drives under the Convention on Biological Diversity. Experts are debating whether the existing evaluation criteria are sufficient for assessing the risks posed by gene drives.
Various proposals aim to limit potential damage caused by unintended gene drive releases and uncontrolled gene drive spread. Gene drives specific to a particular population (local drive systems) can spread within that defined population. For instance, a daisy chain drive links several genetic elements together, with each element activating the next. Over time, elements are sequentially lost, potentially allowing control and containment within a population. Other proposals include splitdrive systems, where only one genetic component of the drive construct is transmitted, while the other adheres to Mendelian inheritance rules. Reversal (or overwriting) drives can eliminate gene drive constructs, and immunising drives aim to make other populations resistant to gene drives by modifying the target sequence.
( Aedes aegypti ) and the Asian tiger mosquito ( Aedes albopictus), carriers of diseases like Zika, Dengue, Chikungunya, and Yellow fever, this technique has already been developed.
Sterile Insect Technology (SIT) involves releasing artificially reared sterile insects that outcompete naturally occurring males. This reduces the number of wild descendants. SIT successfully eradicated the tsetse fly, a carrier of sleeping sickness, in Zanzibar in 1997. However, SIT is not suitable for all insect species, requiring continuous releases of genetically altered males.
In 2016, the British biotech company Oxitec Ltd reported field trials with genetically modified Egyptian tiger mosquitoes (Aedes aegypti, known to transmit yellow fever, dengue, chikungunya, and Zika) and Anopheles in various regions. These mosquitoes carried a dominant lethal gene passed on by males, rendering females nonviable. The goal was to control diseases like Dengue and Malaria transmitted by this mosquito species. Field trials of Oxitec’s mosquitoes have reduced Anopheles aegypti mosquito populations 80-95% and dengue fever cases by as much as 91%.
Recent research from the University of California, San Diego, introduced a genetic method where male Anopheles mosquitoes pass on a gene that is lethal to female mosquitoes. This gene, known as “Femaleless” (FLE), is crucial for the development of female larvae. As female mosquitoes transmit the Malaria pathogen, they are the primary target. Computer simulations suggest that this approach could reduce mosquito populations by 90%. Unlike gene drives, the genetic modification introduced using this method naturally disappears from the mosquito population over time.
european-biotechnology.com/card
An alternative to synthetic gene drives for population control is the RIDL approach (Release of Insects Carrying a Dominant Lethal Allele). This technique involves releasing insects carrying a dominant lethal gene in large quantities. The offspring produced by these insects are not viable. For species like the yellow fever mosquito
Alternative methods to synthetic gene drives exist but have their own complexities, particularly the need for mass releases of individuals. Further research is required to determine the effectiveness of these methods and to weigh the advantages and disadvantages of gene drives comprehensively.
g.dorow@biocom.eu
European Biotechnology Network
Europe: european-biotechnology.net
Young
Europe: yebn.eu

Austria: lifescienceaustria.at
Austria: biotechaustria.org

Germany: gasb.de

Switzerland: swissbiotech.org

BIO DEUTSCHLAND The Netherlands are now the first EU country to allow tastings of biotechnologically cultivated meat and seafood. The German biotech industry’s association applauded its neighbour country’s decision and urged the German government to follow suite. BIO Deutschland advocated for improved framework conditions for the development, production, tasting and market approval of these novel foods in a position paper, stressing its potential benefits. Foods produced sustainably through biotechnology, the association pointed out, can aid in reducing factory farming, combatting climate change and preserving biodiversity. “Biotechnologically produced chicken meat has already been approved for consumption and sale in Asia and the USA […],” Oliver Schacht, Chairman of the Board of BIO Deutschland, said. “In Europe, however, it’s not even clear how to go about submitting a successful application for approval in Brussels. Our companies have to figure this out by trial and error. Something urgently needs to change here.“
Europe: medicinesforeurope.com
Ireland: ibec.ie/ibia
Denmark: danskbiotek.dk
The Netherlands: hollandbio.nl
Germany: biodeutschland.org
Portugal: www.p-bio.org
UK: biopartner.co.uk
France: france-biotech.org
Italy: assobiotec.it

Sweden: swedenbio.com
Hungary: hungarianbiotech.org
Europe: cebr.net
Spain: asebio.com
Norway: biotekforum.no
Finland: finbio.net
Belgium: bio.be
EUROPEAN BIOTECHNOLOGY covers the biotechnology sector of the current 27 EU member states, Norway, Switzerland, and UK. If you would like to subscribe, please refer to european-biotechnology.com
ASSOBIOTEC Biotechnology is an industry on the rise in Italy. According to the new ENEA-Assobiotec report “Biotechnology companies in Italy,” there are more than 800 companies with 13,700 employees in Italy, with a turnover exceeding 13 billion euros. According to the findings, biotech enterprises in Italy have experienced substantial expansion in recent years, with a notable increase in both revenue and job creation. This growth has positioned the sector as a key driver of economic development in the country. Government officials and industry experts have welcomed the report’s findings, recognising the pivotal role of biotech in shaping Italy’s future.

PARTNERING The Swiss Biotech Day 2023 was the biggest and most international edition so far, demonstrating the strengths and global reach of the Swiss biotech hub. Its Global Village platform for international delegations supports the event’s role as a truly global biotech networking event, fostering collaboration with mutual benefit and a lasting impact.
In 2023 the Swiss Biotech Association welcomed roughly 1,800 participants, with an impressive 39% joining from abroad, representing 44 countries. For international delegations seeking to strengthen their connections with the Swiss biotech hub, foster cross-border investments, establish public-private partnerships, collaborate on research and development initiatives, and exchange talent, the Global Village serves as an effective partnering tool. With delegation booths, a central roundtable, and an exclusive international reception, delegates get the perfect opportunity to showcase their offerings and engage with conference visitors. During the roundtable, each delegation can engage in focused
discussions with Swiss delegates, exploring ways to collectively expand the life science business, nurture collaboration, and facilitate matchmaking between their home country and the Swiss biotech hub. Given its great success, the Global Village will again be an important element of the Swiss Biotech Day 2024.
“It’s the personal touch that makes the Swiss Biotech Day so special”, said one of the plenary speakers, Briton Daniel, Chancellor of consulting firm Citeline. From Brazil to Australia and from Spain to Taiwan – all participants of the Global Village demonstrated that life sci-
› April 22-23, 2024
Basel
Swiss Biotech Day & Swiss Biotech Success Stories Awards
ence R&D connects people internationally. They all have the same goal of sharing knowledge, establishing contacts and exploring collaboration and business opportunities.
The Global Village, organized by the Swiss Biotech Association, Switzerland Global Enterprise, and BIOCOM, empowers each delegation to appoint a leader to coordinate their presence and subsequent activities. Ideally, delegations comprise diverse stakeholders: Investors, venture builders, entrepreneurs, hospitals, clinical investigators, contract research organisations, universities, private research centres, government bodies with vested interest (e.g. regulatory agencies), incubators and accelerators, as well as talent developers. At the international reception delegations selectively invite guests to connect with participants of other nations and regions. The delegates have full access to the conference’s program and the one-to-one partnering. Additionally, they can connect with and explore Swiss biotech organizations prior to or following the conference. This includes visits at leading universities, investors and enterprises in biotech, pharma, contract development and manufacturing, leading innovation centres like the Center for Translational Medicine and Biomedical Entrepreneurship in Berne, the Biopôle Campus in Lausanne, and other Swiss biotech hotspots across Switzerland.
Interested to take part in the Global Village?
Contact the Swiss Biotech Association at globalvillage@swissbiotech.org

› October 12-13, 2023








Med Uni Graz
1. Biotech Summit Austria
INDUSTRY EVENT On October 12-13, the first BIOTECH SUMMIT AUSTRIA will take place at Med Uni in Graz. The premier of this major event is being organized by BIOTECH AUSTRIA together with Human.technology Styria.


As the Austrian industry association, BIOTECH AUSTRIA has set itself the goal of representing the interests of companies and start-ups in biotech towards political as well as national and international decision-makers. A regular exchange of knowledge and experience among members is promoted throughout the year at lectures, seminars and networking events.
With the BIOTECH SUMMIT AUSTRIA, a two-day summit for the entire industry is now being launched for the first time. On these days, top-class representatives of their specific fields will discuss with participants on current topics and trends. An exhibition area will be available for the presentation of companies and start-ups as well as their projects. Students are also welcome to attend the Career & Recruiting Sessions. In addition, on the first day of the event, a dinner followed by a party will provide an informal setting for casual
networking. The Summit consists of these three pillars:
Conference
› Key notes
› Panel Discussions
› Short Talks
› Workshops
› Pitches Exhibition
› Start-Up Corner


› Company Presentation Area
Networking
› 1:1 Partnering Session
› Dinner & Party
› Recruiting & Career Sessions
On the agenda are topics such as:

› Trends and developments in biotech
› The dealmaking vision of big pharma
› Funding & financing for start-ups and established companies



› Regulatory landscape and best practices for clinical trials
Key note speakers include personalities such as Mark Kotter (Founder and CEO bit.bio), Ulrich Granzer (Granzer Regulatory Services), Regina Hodits (Wellington Partners Life Sciences) and Joachim Vogt (AbbVie). The focus of the event is both on the continuing education of individual participants and on the progress of the industry as a whole. Only with a strong community do niche topics attract greater public attention. That is why BIOTECH AUSTRIA and Human.technology Styria, two renowned partners, have joined forces to create a relevant platform. Cooperation partners like ics and the enterprise europe network support this project. Special thanks go to the sponsors, whose commitment makes the first edition of a seminal event possible: first and foremost Platinum Sponsor ZETA as well as Johanneum Research, GNN Group and the WKO with the initiative Go international.
We are looking forward to a lively exchange and networking of researchers, investors and international key players of the biotech scene in October in Styria. Leading representatives of Austrian and international biotechnology companies are expected to attend. Young talents and newcomers are also welcome: Students get access to the Job Board and Career Sessions with free admission, startups find the opportunity to present themselves and establish contacts with investors in the “Start-Up Corner”. Conference tickets are available from €100.
Detailed program, sponsoring information and registration: biotech-summit-austria.com
EVENT Discover the Future of Precision Oncology at ICPO 2023 Forum (12–13 October 2023, in Garching near Munich) by the ICPO Foundation!


US, Canada, and other locations around the globe, while more than 15 industry sponsors are kindly supporting the event. The goal is to connect participants across all horizons, including industry, academia, as well as medical and patient societies, associations, foundations, investors, and philanthropists.
Since 2019 the International Centers for Precision Oncology (ICPO) is a German foundation working to enable growing numbers of Cancer Patients worldwide to get access to highly effective diagnostics and treatments in Radiomolecular Precision Oncology.
ICPO believes that scaling patient access can best be achieved through building a global community and international network of Precision Oncology Centers based on shared passion, knowledge, standards, training, and blueprints, essentially building a common operating model for dedicated and optimised Precision Oncology Centers.
On October 12th and 13th, 2023, the ICPO Foundation will be hosting its 3rd ICPO Forum for Theranostics in Precision Oncology physical in the heart of the Science Campus in Garching Munich next to the medical isotopes production reactors that is the world largest Lutetium-177 production facility recently opened. Over 200 hundred participants are expected to attend from Europe, India, China, the Middle East, the
Stay ahead: Learn about the latest trends in expanding patient access to Theranostics in Precision Oncology.
› Global Connections: Exchange ideas with colleagues from the international ICPO Community.

› Diverse Programme: Immerse yourself in a stimulating programme, spanning from scientific innovation to tangible patient outcomes.
With its 3rd Forum edition, the ICPO stands more than ever for its mission to support the community, science, training, and expansion of patient access worldwide in Precision Oncology in an inclusive and collaborative approach! Don‘t miss this opportunity to be part of something truly remarkable in the world of Precision Oncology. Mark your calenders, and secure your spot. L
Available for 3 EUR exclusively at the biospheria.shop/en

EVENT In 2023, the PharmaLab Congress will again offer a diverse programme to professionals from analytical and microbiological quality control in industry, from contract laboratories, from authorities and service providers – making available a platform for the exchange of knowledge and experience.
› Axel H. Schroeder, Operations Director, Concept HeidelbergThe Congress will take place as an international on-site event in Neuss/Düsseldorf, Germany, and invites participants to a direct exchange with colleagues, speakers and exhibitors.
A pre-conference workshop on 20 November and five parallel conference tracks with more than 70 speakers on 21-22 November 2023 will provide delegates with important and new information on analytics, bioanalytics and microbiology. Sessions will cover the following topics:
› Laboratory optimisation, automation and digitisation

› Outsourcing in pharmaceutical laboratories
› Analytical method validation and life cycle management – ICH Q14/Q2 (R2)
› Endotoxin and pyrogen testing
› Alternative and rapid microbiological methods
› Cell and gene therapies/ ATMPs –quality and safety.
Track 1 focuses on current topics that concern the modern laboratory such as the optimisation of structures and processes, the possibilities of automation, outsourcing and the associated opportunities in continuous improvement (CIP). The lectures will also present modern approaches to saving costs while at the same time complying with GMP guidelines.
In March 2022, the European Medicines Agency (EMA) published the ICH Q2(R2) guidelines on the validation of analytical methods and ICH Q14 on the development of analytical methods for public consultation.
During the second track, experts from industry and laboratories will present on the status of these revisions, the contents of the guidelines and report about their own experiences with implementation.
Track 3 is dedicated to endotoxin and pyrogen testing – an area that has seen major changes in recent years. The track is divided into different sections dealing with the LAL test as well as with recombinant factor C (rFC) and the monocyte activation test (MAT).
Additionally, PharmaLab will focus on the important topic of alternative microbiological control and detection methods, especially related to the growing importance of cell and gene therapies – which have often short shelf lives or small batch sizes. Real-time or online systems are also gaining importance in cleanroom and media monitoring.
Accordingly, the fourth track is dedicated to alternative/rapid microbiologi-
cal methods, including next-generation sequencing (NGS).
Track 5 addresses manufacturers and developers of cells, tissues, cell- and tissue-based products or advanced therapy medicinal products (ATMPs). Sessions will deal with microbiological and analytical quality requirements, suitable methods and test systems as well as their implementation and validation. The speakers will explain the current regulatory requirements and report about their experiences during inspections and the implementation of the requirements.
One-day or multi-day tickets allow attendents to PharmaLab to visit parts of the congress or the entire congress – and thus to put together a programme according to their individual needs and interests. L
For all information, please visit www. pharmalab-congress.com/congress-2023
20 November: Pre-Conference Workshop
4th International Mycoplasma qPCR Testing User Day

21/22 November 2023: Congress & Exhibition With 5 Parallel Conference Tracks Düsseldorf, Germany
Highlights
 Method Validation and Life Cycle Management
 Laboratory Optimisation and Automation
 Modern and Alternative Microbiological Methods
 Cell and Gene Therapies/ATMPs - Quality and Safety
 Endotoxin and Pyrogen Testing
 Mycoplasma Detection
 Outsourcing in Pharmaceutical Laboratories

Premium Sponsor 2023:

CRO Antibody drugs including monoclonal antibodies (mAb), bispecific antibodies, antibody-drug conjugates (ADCs), and nanobodies have sparked a new surge in research and development.
The bioactivity of mAbs is often confirmed by rigorous testing of efficacy and potency. This forms an essential part of the antibody drug evaluation system and plays an important role in determining the mechanism of action (MOA) of drugs, providing a foundation for preparing investigational new drug (IND) submissions.
Owing to the importance of in vitro screening and target validation during the early stages of antibody discovery, Sino Biological has established in vitro activity assay platforms for streamlining antibody drug development projects. Sino Biological provides comprehensive reagents and extensive in vitro efficacy evaluation services to fulfill diverse testing needs.

Discover More Now!

Sino Biological Europe GmbH
Düsseldorfer Str. 40, 65760 Eschborn, Germany +49(0)6196 9678656 cro-service@sinobiological.com www.sinobiological.com
26.–28.09.2023 BARCELONA BIOSPAIN brings together innovative organisations working in health, sustainable agrifood and industrial transformation to find solutions to climate change. It is one of the leading events in the biotech sector in Europe and one of the largest by the number of one-to-one meetings.
https://biospain2023.org
24.-26.10.23
CPhI Worldwide 2023, Barcelona (ES)
Info: CPhI Global Office, https://www.cphi.com
24.-25.10.23
European Forum for Industrial Biotechnology & the Bioeconomy – EFIB, Rotterdam (NL) Info: EuropaBio https://efibforum.com
24.-25.10.23
Future of Biofuels 2023, Copenhagen (DK) Info: FORTES Media Group Sp. z o.o. https://fortesmedia.com
25.-26.10.23
New Food Conference, Berlin (DE) Info: ProVeg e.V. https://www.new-food-conference.com/berlin
6.-7.11.23
7 th International BioSC Symposium, Bonn (DE)
Info: BioSC-Geschäftsstelle c/o FZ Jülich www.biosc.de
8.11.23
Bioscience 2023, Stockholm (SE)
Info: Maria Eriksson, Life Science Sweden www.bioscienceevent.com
26.-28.9.23
ILMAC 2023, Basel (CH)
Info: Sandy Mauch, MCH Group https://www.ilmac.ch/de
2 8.9.23
Life Science Investor’s Day, Heidelberg (DE)
Info: BioRN et al. https://www.life-science-investors-day.vc/
29.-30.9.23
International Forum on Industrial Biotechnology and Bioeconomy – IFIB, Florence (IT)
Info: SPRING – Italian Circular for Bioeconomy Cluster/ ASSOBIOTEC https://ifibwebsite.com/
12.-13.10.23
1st BIOTECH SUMMIT AUSTRIA, Graz (AT)
Info: BIOTECH AUSTRIA/Human.technology Styria www.biotech-summit-austria.com
15.-17.10.23
World Health Summit 2023, Berlin (DE)/digital
Info: WHS Foundation GmbH www.worldhealthsummit.org
19.-20.10.23
European Conference of the Global Biotech, Pharma & Healthtech Supply Chain Community – BSMA, Brussels (B)
Info: BSMA – Bio Supply Management Alliance https://www.bsmaeurope.com/
22.-25.10.23
Medical Biodefense Conference, Munich (DE)
Info: Bundeswehr Institute of Microbiology www.biodefense.de
14.-15.11.23
Bio-Europe Digital 2023, +++ online +++ Info: EBD Group https://informaconnect.com/bioeurope/

29.-30.11.23
Nordic Life Science Days 2023, Copenhagen (DK) Info: Olivier Duchamp, SwedenBio www.nlsdays.com
06.–08.11.2023 MUNICH With over 5,000 participants from 60 countries and more than 2,220 companies, BIO-Europe is a major industry gathering of biopharma professionals in Europe. They can meet potential partners and hear the latest trends from key biotech and pharma leaders. https://informaconnect. com/bioeurope/

5.12.23
Pharma Outsourcing – Find the right partner, Stockholm (SE)
Info: Maria Eriksson, Life Science Sweden www.pharmaoutsourcing.eu/
14.12.23
New Horizons in Biologics & Bioprocessing, Stockholm (SE)
Info: Maria Eriksson, Life Science Sweden www.bioprocessing.se
12.–13.12.2023 MARSEILLE At BioFIT academia-industry collaborations get started. Together with big pharma, biotech and diagnostic companies, BioFIT operates as a platform to build partnerships for all public and private actors.
www.biofit-event.com
21.-22.2.24
CLIB International Conference CIC2024, Düsseldorf (DE)
Info: Cluster Industrielle Biotechnologie e.V. www.clib-cluster.de
6 .-7.3.24
8 th AMR Conference Novel Antimicrobials and AMR Diagnostics, Basel (CH)
Info: Boris Mannhardt, bamconn GmbH www.amr-conference.com
13.-14.3.24
Cellulose Fibres Conference 2024, Cologne (DE)/ +++ online +++
Info: nova Institut
www.cellulose-fibres.eu
25.-27.3.24
8 h Global Congress on Plant Biology and Biotechnology 2023, Singapore/+++ online +++
Info: Magnus Grou
https://plantbiologyconference.com
22 .-23.4.24
Swiss Biotech Day, Basel (CH)
Info: SBA/ bamconn GmbH
https://swissbiotechday.ch/
BIONTECH SE is poised to lead the $2bn mRNAbased oncology therapy market by 2029, with an expected market share of 44.6% and projected sales of $885m, according to GlobalData plc. They’re developing various mRNA drugs for solid tumours, including prostate, and pancreatic cancer.
BIONTECH SE and Sanofi S.A. have scrapped their mRNA-based cancer therapy, BNT131, following disappointing results in a phase I trial. This therapy was designed to use four mRNAs to express specific cytokines in the tumour microenvironment, with the goal of enhancing the immune system’s ability to target cancer cells, but fell short of expectations, leading to its discontinuation.
“...I believe in harnessing science’s potential to revolutionise healthcare, bridging the gap between research and clinical innovation.”
SUSTAINABLE MATERIALS Fibenol OÜ successfully completed its Imavere biorefinery project, marking a significant step towards sustainable materials in the chemical and materials industry. The company aimed to shift from fossil-based chemicals to biobased materials derived from sustainable sources, like woody biomass. Despite past challenges, Fibenol’s Sunburst® technology can now convert wood chips into sugars and lignin fractions. This achievement comes after seven years of strategic efforts, with the Imavere biorefinery in Estonia entering the production ramp-up phase in 2023. The lignin fraction, LignovaTM, has shown promise in applications like asphalt and plywood adhesives. C5 and C6 sugars are used for bio-ethanol, proteins, and other biodegradable products. Fibenol’s efforts are laying the foundation for more sustainable materials.
The United States had the largest area of genetically modified (GM) crops in the world in 2019, with 71.5 million hectares (MHa), followed by Brazil with just over 52.8 MHa. The top five GM crop producing countries account for around 90% of the global area under GM crops. Within a country, the percentage of GM cropped area compared to the total available arable land varies dramatically (see table). Paraguay, Uruguay, Argentina and Brazil lead the way in this respect
In recent years, sustainability in food has become a thought-provoking topic the relationship between food waste & processed foods as well as the connectedness of biotechnology with food affordability & accessibility. Eat Local & Reduce Waste. @theshantanumum
According to Swiss bioethicists, deregulation of genetic engineering rules for plants optimised through targeted mutation and

cisgenetics will make little contribution to climate protection by 2050. shorturl.at/ jCET3J @EuroBiotechNews
Please
The EU insists: At COP28 we need to talk about fossil phase out. Fossil peak needs to be reached soon, says Europe.
tinyurl.com/t4srk2f7
@SustEnvNet
WINTER EDITION Life Sciences hubs, R&D centres and clusters can present themselves in our next issue. Additionally, we will focus on precision fermentation/CellAG. Players in these fields are invited to present their offering in our special in EuropEan BiotEchnology. Want to participate? Please contact Christian Böhm (+49-30-26492149), Oliver Schnell (-45), or drop us an email: marketing@biocom.de. Publishing date is 7th December, 2023; deadline for ads is 24th November, 2023.

Utilize our flexible, light-filled, and modern laboratory and office space (8,000 m² in total)

Be part of the international life science community at Campus Berlin-Buch
Benefit from the proximity to outstanding biomedical research, biotech companies, high-tech platforms, and clinics
Share thoughts and ideas with other entrepreneurs and scientists
Meet bright minds during “lunch and learn”, at the gym, or in the barista café
Work and relax on our green campus
Contact us: Campus Berlin-Buch GmbH



Dr. Christina Quensel
rental@campusberlinbuch.de
Phone +493094892511
www.berlinbiocube.de
The “Establishment of the BerlinBioCube Start-up Center on Campus Berlin-Buch” measure was funded by the federal and state governments as part of the “Joint Task for the Improvement of Regional Economic Structure” (GRW).