






The continuing need for strong data and the importance of providing optimal treatment for each individual patient were two salient messages to materialise from a series of aortic debates at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK).
Uncomplicated TBAD: “We have zero data” Kevin Mani (Uppsala University, Uppsala, Sweden) opened a debate on whether ‘Best medical treatment remains the standard for uncomplicated acute type B aortic dissection [TBAD]’ arguing for the motion, which he noted had the backing of society guidelines.
The presenter then turned his focus to what his opponents— Christoph Nienaber (Royal Brompton & Harefield Hospitals,
London, UK) and Firas Mussa (McGovern Medical School, Houston, USA)—would be arguing for: thoracic endovascular aortic repair (TEVAR).
“TEVAR doesn’t come risk free,” the presenter stressed, detailing its association in the acute setting with a 5% stroke risk. He also highlighted a lack of supporting data for its use in uncomplicated TBAD.
Mani went further, suggesting that Nienaber and Mussa must also agree there is no data on TEVAR for uncomplicated TBAD, evidenced by the fact they are both starting randomised trials— EARNEST and IMPROVE AD, respectively—to assess if there is a role for TEVAR. He also mentioned the SUNDAY study, which is looking to answer the same question.
Nienaber then put forward the first of two arguments for the opposition, which was that aortic dissection specialists “have a responsibility to offer more” than best medical treatment for their patients. He remarked that, while best medical therapy might be the foundation of dissection management, it should not be the final treatment to improve long-term benefit or survival.
Hence Verhagen (Erasmus Medical Center, Rotterdam, The Netherlands) put forward the second defence for the motion. He argued that the only sensible high-risk feature in uncomplicated acute TBAD to warrant TEVAR is a maximum diameter of >40mm, explaining that if a patient starts with an aneurysm, it will likely behave like an aneurysm and grow over time. He specifically made it clear to the audience that all other supposed high-risk features lack scientific basis if one reads the original papers where they were stated. Even stronger, he added: “It’s painful to see that guideline committees seem to copy each other’s statements and references without actually reading the papers. There is no scientific basis to do TEVAR for uncomplicated acute TBAD.”
Mussa closed the debate with a final argument for the opposition. However, his presentation did not oppose the

Studies highlight need for tailored treatment options for women with peripheral arterial disease
NEW CLINICAL RESULTS HAVE highlighted the need for inclusive approaches and comprehensive examinations of treatment options for peripheral arterial disease (PAD), including endovascular therapy and revascularisation. The data were presented at the Society for Cardiovascular Angiography & Interventions (SCAI) 2024 Scientific Sessions (2–4 May, Long Beach, USA).
“PAD is a prevalent and debilitating disease with serious consequences, especially for advanced cases that may have progressed due to lack of treatment, which is something that many clinicians are seeing in their patients today. Evidence-based data on treatment outcomes for all are critically important for individualised care,” said SCAI president George D Dangas (Icahn School of Medicine at Mount Sinai, New York, USA) in a press release. “SCAI and its PAD Pulse Alliance partners have worked to close these gaps through the Get a Pulse on PAD campaign, which kicked off this year with resources for physicians and patients.”
At SCAI, one presentation showed late-breaking data that support the effectiveness and safety of endovascular therapy with stent implantation as an alternative to bypass surgery in both women and
We must do better in enrolling women in PAD trials”Serdar Farhan
Greetings dear readers, and welcome to the June 2024 edition of Vascular News.
It was good to see many of you at the 2024 Charing Cross (CX) International Symposium (23–25 April) at the London ExCeL congress centre, and I hope that those of you who made it to London enjoyed it as much as I did. I thought that the new venue worked well. Moreover, it gave delegates the opportunity to visit a part of London that they may never have visited, or not explored to any extent if they had. I was struck by how the London docklands area where the ExCeL is located is unrecognisable compared with its appearance before the millennium.
I would like to convey my congratulations to the programme co-chairs, Erin Murphy (Charlotte, USA), Dittmar Böckler (Heidelberg, Germany) and Andrew Holden (Auckland, New Zealand), for putting together an excellent programme with support from the CX Executive Board members. I can confirm that next year’s CX Symposium will also take place at the London ExCeL in April 2025.
In this issue of Vascular News, several highlights from the 2024 CX programme are discussed.
The first major session of the symposium in the peripheral arterial programme discussed the recent developments on the paclitaxel device issue. Presentations were given by representatives from the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA) explaining their recent update announcements on the safety of paclitaxel-coated devices. In addition, Eric Secemsky (Boston, USA) presented data that indicated that the restriction of paclitaxel device usage by the potential mortality signal caused patient harm. There were also talks on limus-eluting devices in peripheral arterial disease, which fuelled anticipation of comparative data between limus- and paclitaxel-coated devices which should appear later this year.
(7–8 October, Vienna, Austria)
2025 (23–25 April, London, UK)
Another main podium presentation at the symposium that is also discussed in this
issue is that of the BASIL-3 trial by Andrew Bradbury (Birmingham, UK) in which there was no demonstrable benefit for drug-eluting device use in chronic limbthreatening ischaemia (CLTI) patients. In addition, there was an interesting session on bioresorbable scaffolds with a deep dive into the LIFE-BTK trial. In this issue, Abbott Vascular announces that the ESPRIT BTK scaffold investigated in the LIFE-BTK trial has been given US FDA approval for use in CLTI. It is hoped that a licence for use in Europe will not be too far behind.
There is a major feature on treatment of aortic arch lesions by endovascular therapy with contributions from Dittmar Böckler, Eric Verhoeven (Nuremberg, Germany), Hence Verhagen (Rotterdam, The Netherlands) and Ross Milner (Chicago, USA). Other aortic articles include an opinion piece from Michel Reijnen and Rianne van Rijswijk (Arnhem, The Netherlands) on creating a clinically applicable prediction model for aneurysm shrinkage after endovascular aneurysm repair, and a summary of new data on aortic dissection in England from Arun Pherwani (Stoke-on-Trent, UK) and colleagues.
Also in the issue is an interview with Linda Harris (Buffalo, USA) on the newly formed International Society for Women Vascular Surgeons and a promotion of this year’s Women’s Vascular Summit. There is also an interview with Tara Mastracci (London, UK) which focuses on the new European Society for Vascular Surgery Equality, Diversity and Inclusion task force.
This issue’s profile specialist is Bijan Modarai of Guy’s and St Thomas’ Hospital and King’s College London (London, UK).
I hope that you all enjoy reading this issue as much as the editorial team has enjoyed putting it together. Happy reading.
I would like to convey my congratulations to the [CX 2024] programme co-chairs, Erin Murphy, Dittmar Böckler and Andrew Holden, for putting together an excellent programme with support from the CX Executive Board members”

ROBERT MORGAN is professor of interventional radiology at St George’s University Hospitals NHS Foundation Trust (London, UK) and the president of the British Society of Interventional Radiology.
n AORTIC ARCH TREATMENT:

“Centralisation and interdisciplinary teams—they are the keys,” Dittmar Böckler (Heidelberg, Germany) concluded during a roundtable discussion at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK) on the future of aortic arch treatment. Böckler had been speaking with Eric Verhoeven (Nuremberg, Germany) and Hence Verhagen (Rotterdam, The Netherlands) at the CX Live Studio to hear their thoughts on what the future of aortic arch treatment, and specifically the future of endovascular therapy in the arch, looks like.
For more on this story go to page 6.

n BASIL-3:
At the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK), Andrew Bradbury (Birmingham, UK) and the BASIL-3 team of triallists presented—for the first time—the headline clinical and cost-effectiveness data from this UK National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA)-funded randomised controlled trial.
For more on this story go to page 12.
n ARTIFICIAL INTELLIGENCE:

New research on Chat generative pre-trained transformer (GPT) technology and its Vascular Education and Self-Assessment Program (VESAP) success rate provides insight into the future of artificial intelligence (AI) in vascular surgery training and practice, investigators Michael Amendola (Richmond, USA; pictured) and Quang Le (Charlottesville, USA) told Vascular News
For more on this story go to page 17.
Editors-in-chief: Robert Morgan (European Edition) and Ross Milner (North American Edition) | Publisher: Stephen Greenhalgh
Content director: Urmila Kerslake | Global commercial director: Sean Langer
Editor: Jocelyn Hudson Jocelyn@bibamedical.com | Editorial contribution: George Barker, Jamie Bell, Will Date, Bryan Kay, Éva Malpass, Brian McHugh | Design: Terry Hawes, Josh Lyon and David Reekie
Advertising: Rav Pankhania Rav@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2024. All rights reserved. Scan the QR code to subscribe
If you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
Common ground:
Continued from page 1
motion so much as highlight a lack of high-quality data in the space.
“We have zero data,” he said succinctly. “We do not have 100 patients with uncomplicated TBAD prospectively followed for five years.”
Based on this, Mussa concluded that “in 2024, we do not have a standard of care that is based on high-quality data, and that justifies the three currently funded randomised controlled trials”.
Audience polling before the debate showed strong support for the motion, with 79% voting in agreement. Following the four presentations, opinions had shifted somewhat, reducing to 65% in favour.
Prophylactic spinal drainage: Necessary or not?
A debate on prophylactic spinal drainage saw Martin Austermann (St Franziskus Hospital, Münster, Germany) and Nikolaos Tsilimparis (LudwigMaximillians-University of Munich, Munich, Germany) arguing for and against, respectively, the motion ‘Prophylactic spinal drainage cannot be justified for thoracoabdominal endovascular repair’.
Austermann stressed that spinal cord ischaemia (SCI) usually occurs hours after fenestrated or
branched endovascular aneurysm repair (F/BEVAR) and therefore there is time for it to be treated. He added that rescue treatment—including therapeutic cerebrospinal fluid (CSF) drainage—works.
Tsilimparis, on the other hand, argued for selective prophylactic use in high-risk patients. He emphasised that there is no need for “extensive” use of CSF drainage, but that its use in high-risk patients is “absolutely justified and necessary,” citing case examples in support of this.
Branched repair: “We don’t have the perfect stent yet”
Later in the same session, Gustavo Oderich (University of Texas Health, Houston, USA) and Eric Verhoeven (Paracelsus Medical University, Nuremberg, Germany) debated the motion ‘Self-expandable covered stents beat balloon-expandable stent grafts in branched endovascular aortic repair’.
Taking to the podium to argue for the motion, Oderich started by saying that both types of stent graft have performed quite well in recent years.
While sharing that balloon-expandable stent grafts have several positive aspects, he stressed that they are also associated with more endoleaks.
Verhoeven took a similar approach to the debate, underlining its friendly nature due to the multitude of choices now available in this area and the fact that both self-expandable and balloon-expandable stents perform well as a bridging stent.
He did stress, however, that there is still room for improvement. “We don’t have the perfect stent yet,” he said, going on to state that endovascular techniques are “evolving all the time”.
AAA repair: Long-term outcomes “must be our focus”
One of the final aortic debates at CX 2024 saw experts
ESVS Equality, Diversity and Inclusion task force: A step in the right direction

Point of View
Diversity and Inclusion (EDI) task force.
THE NEWS OF THE ESVS’ decision to create an EDI task force, and its recent election of members of this task force from the population of vascular surgeons across Europe, should inspire hope in young vascular surgeons. Traditionally, the ESVS has been a society that has prioritised the more mature surgical viewpoints across Europe, however in recent years, some effort has been made to increase relevance to surgeons in training and those in their early careers. One example, the large expansion of the ESVS educational portfolio has been
welcomed by many. I see the EDI task force as the next step in promoting diversity and broadening the relevance of the society.
Having representation of younger surgeons at the society level is essential to ensuring the populations of vascular surgeons leading discussion across Europe remain innovative and forward thinking. The path towards achieving diversity can be controversial and challenging and will be ineffective if we also don’t work towards embedding transparent inclusion in the process. By broadening the voices on the podium
exchange views on the motion ‘Volume-outcome relationship of AAA repair calls for a combined recommendation of EVAR and OAR [open aortic repair] hospital volumes’.
Ian Loftus (St George’s University Hospital NHS Foundation Trust, London, UK)—starting proceedings in favour of the motion—noted specific differences between the European Society for Vascular Surgery (ESVS) and Society for Vascular Surgery (SVS) guidelines on the topic.
Loftus’ argument was that it is impossible to design aortic services based on open surgical repair alone, and that endovascular and open surgical repair should be offered by the same teams, in the same hospitals.
He stressed the importance of appropriate individualised case planning, multidisciplinary team working, and the ability to rescue out of hours.
In terms of case volume, Loftus argued that there is “no magic number,” but that more is better, and that long-term outcomes “must be our focus”.
David Stone (Dartmouth-Hitchcock Medical Center, Lebanon, USA) presented the first argument against the motion, highlighting a lack of good-quality data. He concluded that there is a need for better real-world data specifically to inform the volume-outcome relationship.
On this point, the presenter pointed out that 75% of US centres would fail to meet either guideline. “Where patients would get their care if we wanted to follow these guidelines is a real-world issue,” he remarked.
Putting forward another argument for the motion, Mani stressed that “practice makes perfect” and that, from a patient’s perspective, “you want to have a centre that has expertise to do both repairs”.
Closing the debate, Maarit Venermo (Helsinki University Hospital, Helsinki, Finland) also put an emphasis on a point made earlier by Loftus, that it is imperative to give the best treatment for an individual patient.
and in leadership positions, there may be an opportunity to increase the pool of mentors, and possibly inspire young surgeons into a career path they may not have formerly considered. I am looking forward to seeing the different ways the ESVS will grow and prosper as more young surgeons get involved, and the creative and innovative ways that traditional roles can evolve to include more diverse viewpoints.
In the hope of modelling the nurturing role that inclusive leadership can have, the EDI task force has nominated a young surgeon, Vaiva Dabravolskaite, as their lead, who will be supported by the all the members of the task force, and will hopefully continue to contribute to the society as she progresses through her career.
This broadening of scope need not be limited to the Society’s political agenda, as it has relevance to our clinical practices as well. As technology evolves, and economies struggle to deliver the latest technological advances in healthcare, disparity in our patient populations will also become more apparent. Being aware of the innovative ways our colleagues across Europe are meeting challenges will inform our
local practice, and possibly broaden our research and clinical perspectives to make treatments more effective. As we struggle with the burden of social deprivation in the cardiovascular population in the UK, where I practise, we are beginning to better understand that non-clinical factors, and the broader determinants of health, have an impact on the effectiveness of the treatment we provide.
We need a deeper understanding of cultural and social context for the patients we treat to provide them with the care they need. Engaging a wider range of voices in the leadership and committees of our professional society may be the way to start building this knowledge.
The great Canadian thinker Marshall McLuhan said it best: “We become what we behold. We shape our tools, and thereafter our tools shape us.”
Tara Mastracci is the lead for endovascular aortic surgery at Barts Health NHS Trust in London, UK.
The views expressed in this article are those of Tara Mastracci and do not necessarily reflect those of the ESVS.
We need a deeper understanding of cultural and social context for the patients we treat to provide them with the care they need”
Studies
highlight need for tailored treatment options for women with peripheral arterial disease
Continued from page 1
men with PAD. A literature search identified six randomised controlled trials comparing endovascular therapy with stent implantation (bare-metal, drug-eluting, or covered stent) versus bypass surgery with vein or prosthetic material in patients with symptomatic PAD involving the femoropopliteal segment. The primary endpoint was major adverse limb events (MALE), a composite of all-cause death, major amputation, or reintervention of the target limb. Other endpoints included amputation-free survival (AFS), the individual components of MALE, and primary patency. Early complications were defined as a composite of any bleeding, infection, or all-cause death within 30 days of the procedure.
Of 639 patients investigated, 185 (29%) were female. Baseline and procedural characteristics
were comparable between patients randomised to endovascular therapy versus bypass surgery. At two years, there was no significant difference in the incidence of MALE between endovascular therapy and bypass surgery in women (40.6% vs. 42.1%, p=0.764; hazard ratio [HR] 0.93) and men (39.7% vs. 34.4%, p=0.963; HR 0.98). Similarly, there were no differences in amputation-free survival (AFS), individual components of MALE and primary patency between endovascular therapy and bypass surgery regardless of sex. Endovascular therapy compared to bypass surgery was associated with a significantly lower rate of early complications at 30 days (8.7% vs. 25.96%, p=0.002 in women and 5.9% vs. 21.5%, p<0.001 in men) and significantly shorter hospital stay in both women and men (3.7±5.7 vs. 7.2±4.3 days, p<0.001 and 2.8±3.2 vs. 7.4±5.1, p<0.001).
“While the findings of the study are of value considering the scarce data on PAD treatment in women, they are also a strong reminder that we must do better in enrolling women in PAD trials. Women remain underrepresented in PAD trials and concerted efforts are warranted to achieve adequate representation of women to improve our understanding of the disease and its management in both women and men,” said Serdar Farhan (Icahn School of
Medicine at Mount Sinai, New York, USA), lead author of the study. “Early diagnosis and guideline-directed medical therapy are key to improving outcomes of any treatment strategy for PAD.”
Another study presented at SCAI showed that women and Asian Americans are less likely to undergo endovascular revascularisation.
In this analysis, Bayesian machine learning-augmented propensity score translational (BAM-PS) statistics with multivariable regression was conducted for the largest US all-payer inpatient dataset, the National Inpatient Sample (NIS), from 2016 to 2020.
Of 148,755,036 adult hospitalisations, there were 17,173,000 (11.54%) with PAD, of whom 680,025 (3.96%) underwent inpatient endovascular revascularisation. Endovascular revascularisation prevalence increased steadily (0.46% to 0.49%, p<0.001). In BAM-PS
multivariable regression adjusting for several clinical and demographic variables, female sex (odds ratio [OR] 0.54) and Asian versus Caucasian race (OR 0.66) significantly decreased the odds of endovascular revascularisation. Medicare versus commercial insurance (OR 1.17) significantly increased the odds of endovascular revascularisation (p<0.001). There were no significant differences in endovascular revascularisation mortality and cost when analysed by sex, race, and income (p>0.05 for all).
“Although not surprising, it is frustrating to see women and Asian Americans are less likely to undergo procedures that may prevent amputations or even death,” said Awad Javaid (University of Nevada, Las Vegas, USA), lead author of the study. “The results reinforce the need to change current practice by using a more inclusive and multidisciplinary approach to [PAD] interventions.”
Although not surprising, it is frustrating to see women and Asian Americans are less likely to undergo procedures that may prevent amputations or even death”
Awad Javaid
“The incidence of acute aortic dissection in England is rising,” Arun Pherwani (University Hospitals of North Midlands NHS Trust, Stoke-on-Trent, UK) told the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK). Pherwani delivered this statement among other key findings as part of a podium-first presentation on outcomes of acute aortic dissection in the UK.
PHERWANI, WHO INCLUDED ON A LIST OF disclosures his role as clinical lead for the National Vascular Registry (NVR) and the joint clinical lead for the National Consultant Information Programme (NCIP) Vascular Surgery, first underlined “one of the biggest problems with aortic dissections”. This, he added, is the fact that there is a single code on the International Classification of Diseases 10 that codes all aortic dissections, irrespective of which section of the aorta is dissected, or where the entry tear is. “That’s why it’s very hard to find data—real data, national data—on aortic dissections,” Pherwani said.
As part of the National Consultant Information Programme (NCIP), Pherwani and colleagues decided to “hit on a novel strategy” to collect data, whereby they picked up every single admission in England as an emergency with a diagnostic code of aortic dissection. “So, we focused on the condition rather than the procedures that were done,” he outlined.
The researchers used Hospital Episode Statistics (HES) data from November 2017 to October 2022 to capture the outcomes of all aortic dissections in the UK during this time period. The project, he remarked, represents a unique collaboration of the vascular NCIP, cardiac NCIP, NVR and National Institute for Cardiovascular Outcomes Research (NICOR).
Over the five-year study period, Pherwani and colleagues identified 6,994 patients with aortic dissection admitted to English hospitals. Those with previous repair (n=76) were excluded, leaving 6,918 patients to be analysed. “Of these,” he detailed, “the ones who underwent a type A operation were just under 40%, who underwent a procedure for type B were 8.8%, and the majority were patients who we’ve loosely clubbed [together] as medically treated. This will include patients who were palliated, and even palliated patients who presented with type A aortic dissections.”
Pherwani compared these data to some from the NVR, noting that there are currently data available for a six-year period available—2016–2021— covering 540 procedures for type B aortic dissection (TBAD). The majority of these (97%) were thoracic endovascular aortic repairs (TEVAR). Pherwani shared that overall mortality among the patients treated was 8.9%, 11.1% were treated as emergencies, and that there were no data on medically treated TBAD.
The presenter then focused on HES and its role as NCIP’s key data source for procedure dashboards. Specifically, he addressed the question of why the NCIP uses HES for admitted patient care. Pherwani
listed various reasons, including its ready availability and the fact that it offers complete coverage of activity. Furthermore, he continued, HES is linkable for longitudinal analyses, has a standardised format, and is good for hard outcomes, including hospital mortality, length of stay, readmission, and reoperation.
“Data quality is NCIP’s biggest priority,” Pherwani commented.
“In conclusion,” Pherwani said, “this is the first unbiased report of all-comers with all types of aortic dissection using national data from England.” He informed the audience that these are not bespoke registry data on TEVAR for either uncomplicated or complicated TBAD alone, and that the incidence of dissection is rising.
“This methodology is reproducible in other nations,” the presenter added.
Closing his presentation, Pherwani stated: “Whilst I’ve shown you some strengths and limitations of data from administrative sources, what I’ve also been able to show you is how to identify the study population for IMPROVE-AD, for SUNDAY, and for the EARNEST trials.”
In the discussion following Pherwani’s presentation, session anchor Ian Loftus (St George’s University Hospital NHS Foundation Trust, London, UK), asked whether there is inequality in access to surgical intervention in the UK.
“What we want to do and what we’re in the process of doing is producing data regionally for the [different] sections in England, which will tell us about equality of access to services,” Pherwani said in response.
Also in the discussion, Pherwani commented on the rising incidence of aortic dissection. “One of the reasons I think the incidence of aortic dissection is rising in the UK is because of the aortic dissection awareness campaigns.”








L-R: Dittmar Böckler, Eric Verhoeven and Hence Verhagen
“Centralisation and interdisciplinary teams—they are the keys,” Dittmar Böckler (University Hospital Heidelberg, Heidelberg, Germany) concluded during a roundtable discussion at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK) on the future of aortic arch treatment.
BÖCKLER HAD BEEN SPEAKING WITH ERIC Verhoeven (General Hospital Nuremberg, Paracelsus Medical University, Nuremberg, Germany) and Hence Verhagen (Erasmus Medical Center, Rotterdam, The Netherlands) at the CX Live Studio to hear their thoughts on what the future of aortic arch treatment, and specifically the future of endovascular therapy in the arch, looks like.
“We know that, for decades, open surgery was the standard,” Böckler began, “but now we have so many other options to treat our patients.” Against this backdrop, the CX co-chair was keen to hear the experts’ opinions on the new therapeutic options that are available.
Verhoeven set the scene by acknowledging the challenges of treating the arch via endovascular means. “I think we are boarding the most difficult part [of the aorta] for endovascular treatment, but we are at the beginning, which means the future is probably bright,” he said with cautious optimism. However, while noting that progress has been made over the last couple of years, Verhoeven did acknowledge that there is “room for improvement” regarding devices.
Verhagen echoed this thought, adding that endovascular treatment of the arch is still “experimental”. He expressed a belief that, in the notso-distant future, endovascular “will take over” but that the field is “not there yet,” citing some significant issues that need to be resolved.
The conversation then moved to the evidence base for endovascular therapy, with Verhoeven and Verhagen sharing some insights from their daily practice.
Verhoeven opined that technically, endovascular treatment of the arch is “not that difficult,” thanks in large part to branched devices being “relatively forgiving” and the technique being well known among specialists. However, he also pointed out that there
remains a “substantial” risk of stroke.
Adding to that, Verhagen stated that patient selection is key. He cited promising results from the last two years that he believes are likely to do with good technique and improved patient selection. “We’re just starting to learn about which patient is suitable for this technique at the moment, and which is not,” he said.
With various endovascular solutions available, Böckler then asked whether there is one best way to treat arch disease, or whether there is a need for multiple options on the market—customised and off the shelf, branched and fenestrated.
Verhoeven emphasised the importance of having off-the-shelf devices available for arch treatment, commenting that there is “no doubt” more will be on offer soon. “[This] is a plea to the companies
This should be done only in centres where cardiac surgery is present and also cooperating with vascular surgery very well—I think that’s imperative”
Eric Verhoeven
to provide us with grafts that are readily available because many of the pathologies deserve early treatment,” he said.
Verhagen agreed, adding that an off-the-shelf device is “easier for everyone” than a custom-made
device. However, he did express uncertainty about whether it would be possible to create a device that fits at least 80% of patients.
Verhagen then highlighted something that is currently lacking with regard to endovascular devices for arch treatment. “We haven’t figured out what the materials of the branches should be,” he pointed out. “We all know that the strokes don’t stop at 30 days, they tend to keep on coming, and that may have something to do with either a suboptimal anticoagulation schedule [or] it may have something to do with the fabric of the branches—we haven’t figured it out yet.”
Having outlined the importance of learning more about patient selection and graft design, the conversation then turned to what an aortic arch treatment service should look like. “Do we need to centralise [it]? Do we need high-volume centres? Who should do these kinds of difficult and challenging procedures for our patients?” Böckler asked.
Verhoeven underscored the importance of a multidisciplinary approach. “It speaks for itself that we cannot do this without involving the cardiac surgeons,” he said, “which automatically means that this should be done only in centres where cardiac surgery is present and also cooperating with vascular surgery very well—I think that’s imperative.”
In addition, Verhoeven commented that “centralisation of these procedures is a must” in order to have a “neutral and objective evaluation of the patients to see whether they will get an open repair or any type of endovascular repair”.
Verhagen did express some caution, based on his own experience. He remarked that centralisation in The Netherlands is proving to be a “painful process” and that he is unsure whether it will succeed in the long term.
Nevertheless, he did agree with Verhoeven overall that “close cooperation with your cardiothoracic surgeons, maybe the radiologists, the cardiologists, the interventional cardiologists” is key and that a “fairly big team looking after a fairly small [number] of patients” necessitates centralisation as a key element of any successful aortic arch treatment programme.
IN MEMORIAM
Lucien Castellani, who had been professor of vascular surgery at the University of Tours (Tours, France), has passed away.
Born in Relizane in Algeria, where he began his studies, Castellani obtained his “agrégation” and then professorship in vascular surgery at the University of Tours. Among several achievements over the course of his career, Castellani was appointed as a visiting professor at the Sapienza University of Rome (Rome, Italy) and served as editor-in-chief of the Journal of Cardiovascular Surgery published by Minerva Medica for many years. He was also made a Knight of the Legion of Honor of France. In 1994, Castellani organised the first International Endovascular Workshop in Ajaccio, Corsica, which, every June for more than 15 years, received eminent surgeons from across the world. These included Jean Pierre Becquemin (Champignysur-Marne, France), Frank Veith (New York, USA), the late Ted Diethrich (Phoenix, USA) and the late Roger Greenhalgh (London, UK) to name a few.
Jacques Busquet (Paris, France) has paid tribute to Castellani in a letter. “The national and international vascular community knows what it owes you and will never forget you. We will never forget your enterprising spirit, your kindness and your good humour,” he writes.






Michel Reijnen and Rianne van Rijswijk (both Rijnstate Hospital, Arnhem, The Netherlands) write about a new consortium focused on sac regression after endovascular aneurysm repair (EVAR).
Aneurysm shrinkage after EVAR has been identified as an important predictor for treatment success.
Patients with a shrinking aneurysm have a lower likelihood of complications and reinterventions and a better survival rate. However, which factors are decisive in this process and how they influence each other is largely unknown.
The RADAR consortium was founded to specifically address this issue in the ART in EVAR study. The project is a collaboration between Rijnstate, Amsterdam UMC, University Medical Centre Groningen, Medisch Spectrum
Twente and University of Twente in The Netherlands and the Karolinska Institutet in Sweden. Besides the standard clinical and anatomical data, the computed tomography angiography (CTA) imaging will be meticulously analysed, investigating factors like intraluminal thrombus volume, distribution and constitution, and wall stress in relation to abdominal aortic aneurysm (AAA) remodelling. In collaboration with the Universitat Pompeu Fabra in Barcelona, Spain, a multi-modal artificial intelligence (AI) model will be developed with the aim
THIS ADVERTORIAL IS SPONSORED BY GORE
of predicting prior to surgery in which patients the aneurysm sac will shrink or not. Or, in other words, the model will identify patients in whom regular EVAR is sufficient AAA treatment to induce sac shrinkage, and, more importantly, identify the patients in whom regular EVAR is insufficient to ensure good outcomes. The overarching aim is to help clinicians to stratify patients who may benefit from adjunctive treatment modalities like pre-emptive embolisation of side branches, active sac management, or reinforcement of the proximal seal using endoanchors. These techniques have shown to promote AAA shrinkage but are often considered to be either too complex and/or too costly to be performed in all EVAR patients. A reliable prediction model is thus indispensable. The model could also aid stratification of the follow-up surveillance after EVAR based on the patient’s individual risk, by decreasing the frequency of followup visits in the 40–50% of the patients who will experience sac shrinkage after EVAR. Overall, the multi-modal AI prediction model aims to decrease the number of reinterventions after EVAR and associated healthcare costs, and potentially improve patient survival. The ART in EVAR study is considered to be the start of a long-term collaboration in the form of the RADAR consortium. The extensive database that is set up for this study holds the answer to a myriad of other questions, which
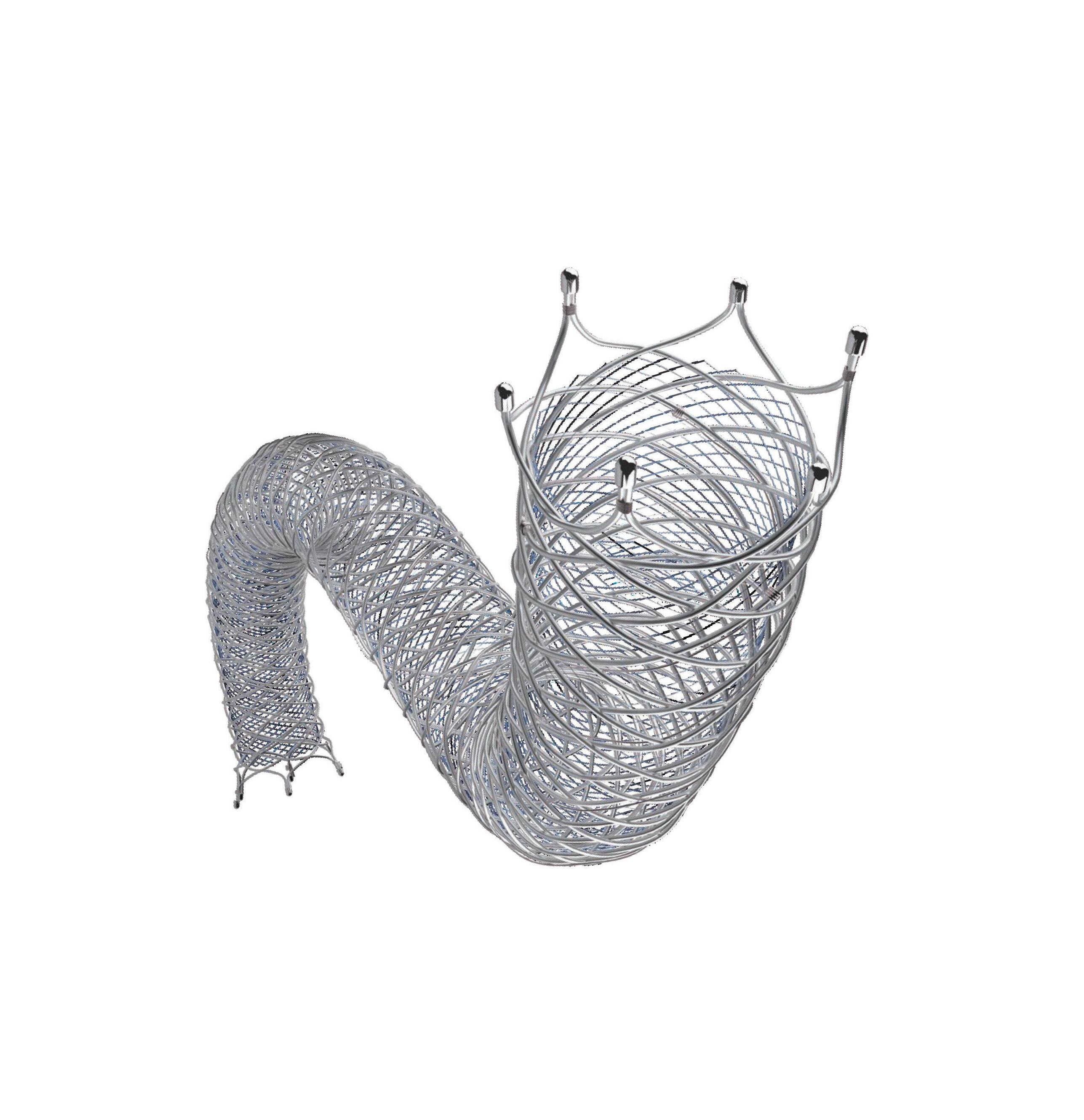
A satellite session at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK) focused on a new treatment option for left subclavian artery (LSA) preservation.
FIRST, IAN LOFTUS (ST GEORGE’S University Hospitals NHS Foundation Trust, London, UK) outlined LSA preservation guidelines and the importance of having an endovascular solution in this area. He pointed out that evidence from the literature demonstrates a “significant advantage” in preserving LSA patency, either by bypass or endovascular means. In particular, he said, the risk of stroke is reduced by around 50%. “This effect is accentuated in aneurysmal disease and is attributable to a large decrease in the risk of posterior territory strokes,” Loftus went on to remark, adding that there is also an advantage in reducing spinal cord ischaemia.
This benefit is reflected in international guidelines from both the European Society for Vascular Surgery (ESVS) and the Society for Vascular Surgery (SVS), Loftus highlighted. As current president of the ESVS, Loftus shared his insights regarding the guidelines from this society in particular. “The ESVS guidelines state that revascularisation should be considered—a class B recommendation with a level of evidence, 2a.”
Finally, Loftus underscored the potential risks associated with a traditional surgical approach for LSA preservation. “Much of our evidence relates to carotid subclavian bypass,” he noted. “These
operations are invasive and come with risk of complications in the literature of greater than 30%, including nerve damage and bleeding.” The GORE TAG Thoracic Branch Endoprosthesis (TBE), however, offers a welcome alternative. “Less invasive endovascular techniques, such as the TBE, with potentially lower rates of procedural complications, may shift the risk benefit ratio even further in favour of left subclavian preservation,” Loftus posited.
Session moderator Gustavo Oderich (University of Texas Health Science Center at Houston, Houston, USA) delivered another presentation, specifically on the clinical evidence behind the GORE TAG TBE. Oderich noted for Vascular News that two clinical trials have been conducted on the device, the first being a feasibility study that ran from 2014–2016 and involved five clinical centres in the USA. It enrolled 31 patients. “There was no mortality, there was one side-branch occlusion several years later (which was asymptomatic), stroke rate was very low, and
we aim to unravel. Our next step entails identification of the role of proteomics and peripheral blood mononuclear cells in the remodeling of the aneurysm sac after EVAR. We will investigate the biological response of sac regression versus sac growth, unravelling the reasons behind the longer survival of patients showing sac regression.
Our future aim is to open up and broaden the collaboration with other interested parties and stakeholders, like hospitals, universities, patient groups and industry, both from a device and imaging perspective. Together we aspire to improve clinical care for aneurysm patients and advance our patients’ lives.
Michel Reijnen is a consultant vascular surgeon at Rijnstate Hospital in Arnhem, The Netherlands.
Rianne van Rijswijk is a technical physician and PhD student at Rijnstate.
Patients with a shrinking aneurysm have a lower likelihood of complications and reinterventions and a better survival rate”
technical success was very high,” Oderich reported. The results of this study led to the initiation of a pivotal trial. This started in 2016 and enrolled 289 patients in just over one year, which, Oderich noted, “shows how much the device was utilised”. Technical success was “remarkably high,” he said, adding that patency was 99.2% throughout the trial.
According to Oderich, this new single-side branch device from Gore has “absolutely” changed the treatment paradigm in the USA when treating zone 2 thoracic endovascular aortic repair (TEVAR) patients. “Our numbers of carotid subclavian bypasses have dramatically decreased,” he said, “and [TBE] has become the first line of treatment for us.”
Finally, Oderich conveyed his positive outlook on the overall benefits of the GORE TAG TBE. Since US Food and Drug Administration (FDA) approval of the device in August of 2022, he underscored a “spike” in usage across the USA, with some “outstanding” results from this real-world usage.

Postoperative computed tomography (CT) scan of the first TBE patient in Europe.
Dittmar Böckler (University Hospital Heidelberg, Heidelberg, Germany), who recently performed the first implantation of the TBE in Europe, also spoke during the session, sharing his prediction that the GORE TAG TBE will “fill a gap” in aortic treatment. “It will play a big role in our armamentarium and be complementary to the customised solutions. Also, I think it will reduce the need for physician-modified solutions,” the CX co-chair remarked, concluding that the device will ultimately increase the number of aortic patients who are able to be treated.



















“We need to continue to be deliberate”: Dedicated society
The inaugural president spoke to Vascular News about the newly formed International Society for Women Vascular Surgeons ahead of this year’s Women’s Vascular Summit (3–4 May, Chicago, USA), outlining its genesis and goals, as well as some of the highlights of this year’s gathering.
LINDA HARRIS (UNIVERSITY AT Buffalo, Buffalo, USA) notes that she created the Women’s Vascular Summit back in 2019 and, more recently, the International Society for Women Vascular Surgeons, for two reasons: to address vascular health and disease in women, and to promote leadership among women vascular surgeons. Reflecting on her own experience, Harris is clear: “I’ve had some wonderful opportunities, but there have also been a lot of glass ceilings.”
She says that many of the women who came before her—as well as many of her own generation—have experienced these same glass ceilings, and there was a general feeling that “it was time for a change”.
Leadership will be a key focus of the Society and of this year’s Summit, Harris points out. “I realised early on that I never really did things like self-promote,” she explains, remarking that part of the reason why has to do with how women are brought up and conditioned. Harris continues: “There’s a lot of data showing that you can have a woman who’s more capable and more experienced and really deserves a position more than a man, and he will self-nominate and she’ll say, ‘I don’t think I’m ready.’”
Harris also considers the role of the Society and the Summit as part of a bigger picture, underscoring the importance of diversity in vascular surgery.
“Unfortunately, we are doing worse for our patients today,” were the sobering thoughts of Eric Secemsky (Beth Israel Deaconess Medical Center, Boston, USA) during a late-breaking presentation at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK) concerning the paclitaxel mortality controversy.
EARLIER PRESENTATIONS GIVEN BY Thomas Zeller (Universitäts Herzzentrum Freiburg, Bad Krozingen, Germany) and Peter Schneider (University of California San Francisco, San Francisco, USA)—concerning paclitaxel-coated balloon safety in femoropopliteal occlusive disease and paclitaxel mortality across randomised controlled trials—established the “lack” of paclitaxel-coated device mortality risk in the most recent available data. Secemsky, who presented next, gave a brief timeline of the rise and fall of paclitaxel-coated devices showing their sharp decline in use following the Katsanos et al meta-analysis which reported an increased mortality risk, and the subsequent regulatory restrictions. Previously, paclitaxel devices—driven by drug-coated balloons (DCB)—were the preferred treatment for femoropopliteal intervention for peripheral arterial disease (PAD) in the USA. Using both Medicare data and the IQVIA: Medical Device Supply Audit database, Secemsky and colleagues looked at paclitaxel device use in US hospitals between Q3 2013 and Q3 2023. He reported that, following the pivotal Katsanos et al
“It’s about having different opinions, and that’s not just male/female,” she says, also citing the importance of engaging with leadership roles in national and regional organisations.
“The new society is partnering with the Society for Vascular Surgery, the Society for Clinical Vascular Surgery, American Venous Forum and regional societies, as well as the Frank Veith society. All have been supportive of the mutual mission,” she says.
“We need to pay particular attention to diversity committees and women’s sections, utilising them to help facilitate intentional appropriate changes within these organisations, allowing for all undderrepresented groups to gain traction: women, underrepresented minorities, community practice.”
Palma Shaw (Upstate Medical University, Syracuse, USA), the vice president of the new Society, says that “the leadership looks forward to productive collaboration with the SVS, which has been very supportive.”
Meanwhile, Harris outlines some of the highlights of this year’s Summit, explaining that the agenda highlights a mixture of

meta-analysis, DCB and drug-eluting stent (DES) use did not recover to pre-meta-analysis levels until Q3 2023—a period of four and a half years—showing the close “parallel” trajectory between device use and emerging data, Secemsky said. The speaker also highlighted the “shrunken” downward trend in the overall femoropopliteal procedural counts in the USA following the controversy, despite PAD-related amputations remaining “stable” in the same period.
Then, Secemsky and colleagues followed 275,009 Medicare beneficiaries who underwent femoropopliteal intervention, comparing the risk of major amputation and death in the period prior to and following the paclitaxel controversy.
Risk of amputation or death was reported in 40.4% and 43.2% of patients in the pre- and post-paclitaxel period, respectively. Secemsky also reported a 43.9% risk of major amputation and death in the post-COVID-19 period, establishing that risks following femoropopliteal intervention were “not driven by COVID-19 alone”, he said.
talks, some geared towards vascular disease in women and others dealing with leadership and other non-clinical topics. The programme also zeros in on updates on a number of trials that are currently underway and how they apply to women.
Harris points out that, often at national and regional meetings, the conclusion is reached that women have different outcomes. At this year’s Summit, the aim was to go much further than this. “What are we doing about it?” Harris asks, underlining one of the key questions that need to be addressed. “Most of the time we end up saying we don’t have enough data, and that’s not good enough anymore.”
Finally, Harris hopes the Society and the Summit partner with international societies and offer virtual attendance to those not able to attend the US meeting. She also highlights the importance of the Society’s impact extending beyond her premiership. “When I started this, I didn’t want to have it be my society forever. I wanted it to be something that I could transition to others,” Harris adds, noting that a metric of success would be whether the Society is sustained after she steps aside.

Administration (FDA) who ensured regulatory concerns were addressed,” Secemsky finalised. “These lessons can help guide the vascular community through the next controversy and to further develop evidence to shape clinical practice.”

“Global restrictions on paclitaxel device use relegated thousands of patients to conventional device treatment,” said Secemsky. “The use of a less durable treatment increases the possibility of more frequent repeat interventions, the associated risks of reintervention and the economic burden of performing these procedures.”
Although the meta-analysis and regulatory body restrictions on paclitaxel devices caused harm, Secemsky—flipping the coin—stated that it “also did good” by reiterating the need for better clinical trial practices and complete study participant follow up.
“The paclitaxel controversy not only instigated interest in non-paclitaxel therapies such as sirolimusbased treatments, but it also demonstrated that the vascular community could quickly band together to address a dispute, and particularly displayed the collaborative nature of the US Food and Drug
The discussion was then opened to the FDA and UK Medicines and Healthcare products Regulatory Agency (MHRA). Representing the FDA, Ariel AshShakoor (Washington, USA) began by tracing the course of the regulatory body’s communication to present day. Ash-Shakoor stated that, based on the “totality of available evidence” the FDA has determined that the data do not support an excess mortality risk for paclitaxel-coated devices. However, current FDA guidance when using paclitaxel-coated devices includes, but is not limited to, routine monitoring and optimal medical therapy.
Ash-Shakoor noted that lessons can be learned from the paclitaxel mortality controversy, including the importance of long-term follow up, prespecified plans concerning missing data, and proactive patient monitoring to ensure “complete” reporting.
Providing the perspective of the MHRA, Alexander McLaren (London, UK) confirmed that no increased risk in mortality with paclitaxel-coated devices has been observed in their review of available randomised controlled trial data.
“Looking back with a critical eye to our own management of the long-running complex topic, I do feel the MHRA response was swift and decisive with the establishment and advice that we received by the expert advisory group,” McLaren stated. In his view, as “quality, robust data were slow to emerge”, delays in decision-making were inevitable. However, McLaren said that the MHRA intends on improving pre-market clinical investigation and ensuring studies are “sufficiently powered and supported” to collect real-world follow-up data.
Ozan Yazar is a vascular and endovascular surgeon with international training acquired in various countries including Belgium, The Netherlands, and Germany. He is currently working at Zuyderland Medical Center in Heerlen, The Netherlands, where he performs a comprehensive range of endovascular and vascular surgeries in aortic and peripheral arteries. He has obtained a doctorate in fenestrated and branched endovascular aneurysm repair (F/BEVAR) for complex aortoiliac aneurysms and organised numerous workshops covering topics such as vascular closure devices (VCDs), BEVAR, and iliac branched devices (IBDs). Here, Yazar speaks to Vascular News about his clinical experience using the Manta VCD (Teleflex) in the treatment of abdominal aortic aneurysms (AAAs) and thoracoabdominal aortic aneurysms (TAAAs), as well as the available data on this device.
Could you please describe your experience dealing with AAAs and TAAAs?
I have had the privilege of working in multiple tertiary centres, which has greatly enriched my expertise in vascular and endovascular surgery. During my training, I gained experience in dealing with complex (T)AAAs. In our centre, we offer a comprehensive range of treatment options for complex aortoiliac aneurysms, including open aneurysm surgery, EVAR, IBD, and F/BEVAR. On average, we have 35 ruptured AAA cases per year. If suitable, a percutaneous EVAR under local anaesthesia will be performed and we use the Manta closure device.
You were an active investigator in the study ‘Clinical outcomes of Manta closure device in percutaneous endovascular aortic aneurysm repair’ recently published in the Journal of Vascular Surgery. Could you please describe the objective, the method, and the results of this study?
most identified issue being failure to achieve complete closure of the puncture site. Overall, our findings suggested that the Manta closure device is a safe and feasible option with a high rate of technical success for vascular closure of large-bore arteriotomies in patients undergoing percutaneous EVAR.
Could you please describe when and how Manta should be selected for EVAR and thoracic endovascular aortic repair (TEVAR) procedures?
Patient selection is critical for ensuring favourable outcomes with the Manta closure device in (T)EVAR procedures. Ideal candidates have a normal body mass index (BMI), CFA diameter ≥6mm, minimal calcifications, and normal CFA bifurcation heights. One of the major benefits of the Manta device is that you don’t need to use a ‘preclose technique’. Especially in ruptured AAA cases, this prevents unnecessary time loss.

The objective of our study was to evaluate the use of the Manta closure device in percutaneous EVAR. We recognised a gap in the literature regarding comprehensive data on the application of the Manta device in EVAR patients. Most of the existing data came from the cardiology field, primarily from experiences in transcatheter aortic valve implantation (TAVI) patients. Given the distinct perspective on vascular issues, we deemed it crucial to investigate this topic within our EVAR population. Therefore, together with Maxim Peeters and our vascular team Lee Bouwman, Pieters Salemans, and ChunYu Wong, we collected data from patients who underwent elective percutaneous EVAR with the Manta device between October 2018 and December 2022. Preoperative computed tomography angiography (CTA) scans were evaluated using an adapted version of the Peripheral Artery Calcium Scoring System (PACSS) to assess arterial calcifications.
Our primary outcome measure was procedural technical success, defined as the placement of the Manta closure device resulting in vascular closure with a patent common femoral artery (CFA), without the need for immediate open or endovascular surgery. In total, we included 152 patients who underwent percutaneous EVAR with 291 CFA closures using the Manta device. Our results demonstrated a high technical success rate of 96.6%. Major vascular complications occurred in 4.5% of cases, with the
We advocate that (T)EVAR procedures are well-suited for the Manta device, particularly due to their relatively shorter duration. This contrasts with more complex aortic surgeries, where downsizing of the sheath may be preferred in longer procedures. In our practice, we prioritise a percutaneous-first approach for EVAR. We

consistently utilise ultrasound guidance for puncturing the CFA. We base our decision on the preoperative CTA and/or ultrasound. If the CFA shows a diameter of <6mm or severe calcification, we opt for a femoral cut-down approach. However, it’s important to note that patient selection isn’t always straightforward, and the final decision may be influenced by the preference or experience of the surgeon.
How does the learning curve define and impact the clinical outcomes in EVAR?
While we don’t have clear data on the learning curve for the Manta closure device, we do recognise its importance. In our experience, the Manta closure device is easy to learn and shares similarities in other closure mechanisms. Teleflex offers support and certification programmes for Manta, which we highly recommend physicians take advantage of. Certification ensures that surgeons receive proper training and guidance, ultimately contributing to safer and more successful procedures.
By actively engaging in certification programmes and continuous learning, surgeons can enhance their skills, reduce procedural complications, and ultimately improve patient outcomes in (T)EVAR procedures. We organise annual VCD workshops because we recognise the importance of this final step in closing the artery hole for a successful operation and clinical outcome.
We organise annual VCD workshops because we recognise the importance of this final step in closing the artery hole for a successful operation and clinical outcome”
INDICATIONS
The 14F MANTA is indicated for closure of femoral arterial access sites following the use of 10-14F devices or sheaths (maximum OD/profile of 18F), and the 18F MANTA device is indicated for closure of femoral arterial access sites following the use of 15-18F devices or sheaths (maximum OD/profile of 25F).
The MANTA Device is contraindicated in the following:
• Severe calcification of the access vessel.
• Severe peripheral artery disease.
• Puncture in the origin of the profundal femoral artery, above the inguinal ligament, or above the most inferior border of the epigastric artery (IEA).
• Sheath insertion in vessel other than the femoral artery.
• Marked tortuosity of the femoral or iliac artery.
• Marked obesity or cachexia (BMI >40 or <20).
• Blood pressure >180mmHg.
• Patients who cannot be anti-coagulated for the procedure.
Disclaimers:
• This advertorial is intended for healthcare professionals only, please consult your doctor/healthcare professional regarding the suitability of the device
• This information is provided for clinical education purposes and is not intended to be a substitute for sound clinical judgment or decision making, or professional experience relative to diagnostic and treatment options of a specific patient’s medical condition. Refer to package insert provided with the product for complete warnings, indications, contraindications, precautions, potential complications, and instructions for use.
• Results from case studies are not predictive of results in other cases. Results in other cases may vary. Please see the instructions for use for complete product information.
BASIL-3 does not provide evidence to support drug-eluting technology use for

In addition, BASIL-3 found that DCB with or without bare metal stenting is unlikely to be costeffective at the UK National Health Service (NHS) National Institute for Health and Care Excellence (NICE) willing-to-pay threshold. DES, on the other hand, is potentially cost-effective at this threshold.
Andrew Bradbury (University of Birmingham, Birmingham, UK) and the BASIL-3 team of triallists shared this and other key findings at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK), addressing the question of which endovascular strategy is best in the femoropopliteal segment. The investigators presented—for the very first time—the results of this long-awaited, only completed, fully publicly funded RCT in this clinical space.
“This was a pragmatic, ‘real-world’ UK trial whose outcomes are likely to be a realistic representation of what can be reasonably achieved across the UK NHS,” Bradbury shared during the session.
Following an introduction to the trial from Bradbury, Lewis Meecham (Cardiff and Vale University Health Board, Cardiff, UK) shared the evidence that was available for the use of paclitaxel DCB and DES in CLTI before BASIL-3; Matthew Popplewell (University of Birmingham, Birmingham, UK) put the trial into context, speaking specifically about the BASIL Prospective Cohort Study (PCS); and Gareth Bate (University Hospitals Birmingham, Birmingham, UK) and Jack Hall (University of Birmingham, Birmingham, UK) shared details of BASIL-3 clinical and statistical methodologies, respectively.
Against this backdrop, Catherine Moakes (University of Birmingham, Birmingham, UK) reported the headline clinical results of the BASIL-3 trial.
Between 29 January 2016 and 26 August 2021, the trial enrolled 481 patients, with 160 randomised to plain balloon angioplasty with or without bare metal stenting, 161 to DCB angioplasty with or without bare metal stenting, and 160 to DES.
In the intention-to-treat analysis, Moakes reported that 106/160 (66%) patients in the plain balloon angioplasty group reached the primary endpoint of major (above-the-ankle) amputation of the index limb or death from any cause, whichever occurred first (no
In the UK National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA)-funded BASIL-3 randomised controlled trial (RCT), neither drug-coated balloon (DCB) angioplasty with or without bare metal stenting nor drug-eluting stenting (DES), when used in the femoropopliteal segment in patients with chronic limb-threatening ischaemia (CLTI), conferred the a priori hypothesised clinical benefit over femoropopliteal plain balloon angioplasty with or without bare metal stenting.
amputation-free survival [AFS]), compared to 97/161 (60%) in the DCB angioplasty arm and 93/160 (58%) in the DES arm.
In a per-protocol analysis of only adherent participants, Moakes reported that 91/140 (65%) patients in the plain balloon angioplasty group met the primary endpoint, compared to 74/122 (61%) in the DCB angioplasty group and 71/118 (60%) in the DES group.
In a cost-utility analysis, Jesse Kigozi (University of Birmingham, Birmingham, UK) reported that DCB angioplasty with or without bare metal stenting when compared to plain balloon angioplasty with or without bare metal stenting was less costly by -£250.71 and less effective by -0.007 quality-adjusted life years (QALYs). Kigozi then reported that in a cost-utility analysis, the differences observed in the costs and outcomes between the DES and plain balloon angioplasty with or without bare metal stenting-first strategies were minimal.
This was a pragmatic, ‘realworld’ UK trial whose outcomes are likely to be a realistic representation of what can be reasonably achieved across the UK NHS”
However, Kigozi added that DES was the dominant strategy because, when compared to plain balloon angioplasty with or without bare metal stenting, DES was less costly by -£724 and resulted in additional 0.048 QALYs.
Kigozi summarised that there were minimal incremental differences in costs and outcomes in terms of QALYs out to two years and amputationfree life years out to seven years when the DCB angioplasty with or without bare metal stenting or the DES-first revascularisation strategies were compared to the plain balloon angioplasty with or without bare
metal stenting-first strategy in the cost-utility and cost-effectiveness analyses.
Kigozi said that, while there is uncertainty overall, the results show DCB angioplasty with or without bare metal stenting is unlikely to be cost-effective when compared to plain balloon angioplasty with or without bare metal stenting, while DES is potentially cost-effective when compared to plain balloon angioplasty with or without bare metal stenting. He added that these findings were generally consistent over different scenarios and analyses and across different patient subgroups.
Summarising, Bradbury emphasised that the trial had sufficient power (exceeding 90%) to detect the 40% effect size set a priori, with more than the 291 required primary outcomes observed. Bradbury added that follow-up was longer and better than anticipated, with only seven patients withdrawing prior to the primary endpoint. Cause of death was available for all deceased patients, he continued, adding also that most (35) UK vascular units had randomised patients.
Bradbury also outlined some potential limitations of the trial, highlighting among these the effects of the “Katsanos pause”— referring to the fallout from the controversial 2018 meta-analysis on paclitaxel devices—and the effects of COVID-19.
Discussion following the presentations included examination of the choice of primary endpoint.
Michael Conte (University of California San Francisco, San Francisco, USA), for example, remarked that there have now been three BASIL trials, but not one has shown a difference in limb outcomes.
“While I recognise that AFS is a critical endpoint, I think the way in which you’ve powered it doesn’t take into account that death is a noise and the outcome in the limb is what you’re actually looking to differentiate.”
CX co-chair Andrew Holden (Auckland City Hospital, Auckland, New Zealand), who comoderated the session, asked Bradbury what should be taken away from these trial results in 2024. “We would like to have had a more clear-cut result,” he admitted. “Maybe over the next few days, weeks, months, as we digest it, as the publications come out and as we present more data, people will begin to think about what it means for their practice, in their country, in their healthcare system.”

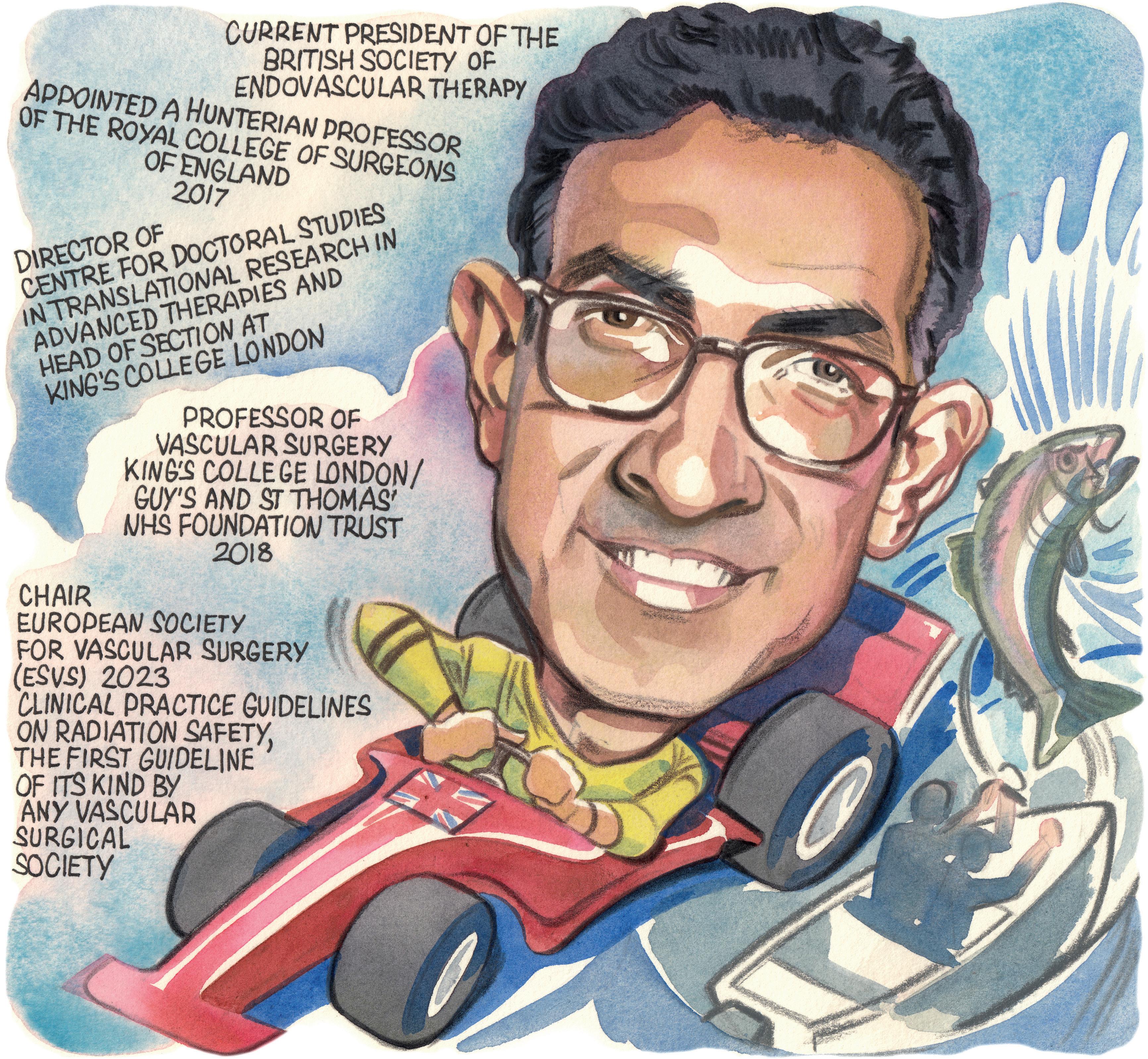
Interested in medicine from a young age, Bijan Modarai (London, UK) is now professor of vascular surgery at King’s College London and a consultant vascular and endovascular surgeon at Guy’s and St Thomas’ NHS Foundation Trust, where he co-manages one of the largest UK practices in complex endovascular aortic repair. In this interview, Modarai charts the course of his career in vascular surgery so far, highlighting his achievements as current president of the British Society of Endovascular Therapy (BSET), outlining some of his and his research team’s current work on radiation and DNA damage, and pointing to the inaugural Interdisciplinary Aortic Dissection Symposium later this year. Modarai also shares some advice for medical students, advising them “don’t listen to the naysayers” and to choose their mentors well.
Why did you decide to pursue a career in medicine and why, in particular, did you choose to specialise in vascular surgery?
I was interested in medicine from a very young age. My father, an emeritus professor of chemistry, had always wanted to be a medic and thought this would be a great career so his influence may have contributed to this.
I became interested in vascular surgery in the first year of medical school. I liked the idea of operating on blood vessels and the fact that vascular surgery was a technical and holistic discipline. I found it an exciting discipline as a significant proportion of the work was done as an emergency and therefore success or failure was often instantly apparent. I did, because of this, worry in the early days that the demands of work may impact other aspects of life but in the end decided to do what I was passionate about and have never looked back.
Who were your career mentors and what was the best advice that they gave you?
I have had the fortune of several mentors who have taught me, led by example, and advocated for me. Kevin Burnand, my predecessor at St Thomas’ Hospital, taught me how to have exacting standards and care passionately for patients. He together with Alberto Smith, a prolific non-clinical translational scientist, supervised my PhD. Rachel Bell, who I met as a senior house officer when she was my registrar, taught me how to be a brave surgeon and, crucially, had my back in my first year as a consultant. Julian Scott suggested I apply to the British Heart Foundation intermediate fellowship scheme in 2010 and was always a sensible source of advice. Finally, Tom Carrell taught me complex endovascular aortic surgery and handed over the reins to the service at St Thomas’ when he left to found his startup company, Cydar.
What have been some of the most important developments in vascular surgery over the course of your career so far?
The application of complex endovascular techniques to every aortic territory has been a big advance and the fact that we are now on the cusp of incorporating the aortic valve into our repairs is highly compelling. We are beginning to see the application of artificial intelligence (AI) to inform case selection, planning and postoperative surveillance. Current algorithms are crude but in time I believe this approach will revolutionise how we treat the patient.
We have also become better at searching for the evidence that supports our interventions. Hand in hand with this is a greater ethos of collaboration, where colleagues are open to joining forces nationally and internationally to accrue meaningful data.
What are the biggest challenges currently facing vascular surgery?
I believe our greatest challenge remains appropriate selection of patients for the plethora of procedures and devices that we can offer them. We are entering an era of personalised medicine that will be facilitated by intelligent data analysis. We are likely to find better ways of assimilating multi-modal data, including anatomical, physiological, and genomic, to allow better treatment selection and prediction of expected outcomes. Along these lines is the fact that most of the patients we treat as vascular surgeons are afflicted with systemic atherosclerosis and prolonging their longevity is a key remaining challenge. After aortic aneurysm repair, for example, our patients are succumbing to non-aortic diseases, including cardiovascular conditions that could be pre-emptively managed to prolong their life.
How do you hope the European Society for Vascular Surgery (ESVS) 2023 clinical practice guidelines will influence radiation safety in vascular surgery?
The guidelines—published in February 2023 in the European Journal of Vascular and Endovascular Surgery—were the first of their kind commissioned by any vascular surgical society and it was a privilege to cochair this endeavour with Professor Stéphan Haulon. First and foremost, the fact the ESVS commissioned these guidelines and the work they have done to promote them since being published has raised awareness in the field about the dangers of radiation exposure and what we, as operators, can do to protect ourselves and our patients. Our committee consisted of an expert interdisciplinary group and their synthesis of evidence in this space, in one document, provides an invaluable reference for our colleagues and highlights to institutions what safety standards they should be adhering to.
Could you outline some of your current research in the area of radiation safety?
As far as my group’s research is concerned, we have been fortunate enough to partner with the UK Health Security Agency and work
CURRENT APPOINTMENTS (SELECTED)
2023–present: President, British Society of Endovascular Therapy (BSET)
2018–present: Professor of vascular surgery, King’s College London/ Guy’s and St Thomas’ NHS Foundation Trust (London, UK)
2012–present: Senior lecturer and consultant vascular and endovascular surgeon, Guy’s and St Thomas’ NHS Foundation Trust (London, UK)
EDUCATION
2010: Completed the Fellowship of the Royal College of Surgeons of England
2006: Completed a PhD in Biochemistry from the University of London (London, UK)
1998: Graduated from the United Medical and Dental Schools of Guy’s and St Thomas’ Hospitals (London, UK)
AWARDS (SELECTED)
2017: British Heart Foundation Senior Clinical Research Fellowship
2017: Hunterian Professorship, Royal College of Surgeons of England
2013: President’s Early Career Award, Circulation Foundation
2011: British Heart Foundation Intermediate Clinical Research Fellowship
with the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Chemical and Radiation Threats and Hazards at Imperial College London. We have important findings, which will be disseminated this year, pertaining to cytogenetic analysis in patients who have undergone endovascular aortic repair. Early indications are that patients show evidence of chronic DNA damage consequent to radiation exposure. Allied to that is a large-scale cancer incidence study we are engaged with that interrogates Hospital Episode Statistics data and the UK Cancer Registry to determine malignancy rates in patients after aortic repair. We hope that the latter project will determine whether the chronic DNA damage we have found has clinical consequences in terms of an increased incidence of malignancy.
What have been some of the highlights of your 2023–2024 presidency of the British Society of Endovascular Therapy (BSET)? I have thoroughly enjoyed working with a dynamic council who have a “can do” attitude and together we have pushed through a number of initiatives with two recent examples being closer working with interventional radiology (IR) colleagues and the British Society of Interventional Radiology (BSIR) and a new research pump priming grant. BSET held an over-subscribed endovascular training course in March this year and it was a pleasure to teach a highly engaged and well-informed group of trainees.
The annual meeting, taking place 27–28 June (the National Vascular Training Day will be held on 26 June for vascular and IR trainees), has attracted the largest number of abstracts to date for any BSET meeting and will undoubtedly be a highlight with a number of high-profile international and national speakers attending. Finally, it has been a real pleasure to work with Jeanette Oliver who has expertly spearheaded the administration and organisational aspects of BSET for a number of years.
You are programme chair for the new Interdisciplinary Aortic Dissection Symposium. Could you outline the reasons behind setting up this event and what delegates can expect from the 2024 meeting?
There are very few meetings that are fully focused on aortic dissection, and we therefore thought it would be useful to put together this one-day symposium dedicated to the condition. According to the latest data, the incidence of aortic dissection is rising, and so it is a priority for vascular and cardiothoracic surgeons, interventional radiologists, and medical colleagues to optimise its management. I envisage the symposium as an interdisciplinary forum in which to discuss the best practices for treating aortic dissection, including contemporary surgical and medical treatments. We will discuss upcoming trials, encourage debate and highlight real-life difficult-to-manage cases.This year the
symposium is going to be held on 6 September at Bush House, a King’s College London campus, and we are fortunate enough to have several internationally recognised experts in aortic dissection speaking at the event. For more information, you can visit www. aorticdissectionsymposium.com.
Could you outline one of your most memorable cases?
It is a privilege for a person to agree to be put to sleep and leave themselves in your hands with full trust. That focuses the mind for each and every case, but I suppose one remembers best the cases where you have to think outside the box and use a collaborative approach to achieve success. I remember a difficult emergent endovascular aortic arch repair that the team and I embarked on with trepidation and after some deliberation in a patient who was not a candidate for open repair. This was a difficult operation, including a cardiac arrest during the procedure that was expertly managed by cardiology and anaesthetic
colleagues. The repair was successful, and it was hugely satisfying to give her more time with her family.
I also vividly remember being coached through my first significant operation, an appendicectomy, on Christmas Eve 1998 in my first year after graduating from medical school.
What advice would you give to someone looking to start a career in medicine?
Don’t listen to the naysayers. Despite the pressures, medicine remains a very fulfilling career—a fulfilment that goes beyond what can be measured in monetary terms. Choose your mentors well. There will be lots of voices whispering in your ear as you navigate a way forward and these mentors will help you choose and focus on the worthwhile voices and advocate for you. Finally, remember that medicine is a multi-faceted career—it can afford you the chance of international exchanges, interacting with highly expert and interesting colleagues. You will acquire skills that can be applied to many other
“I believe the greatest challenge remains appropriate selection of patients for the plethora of procedures and devices that we can offer them”
arenas in life. On a related note, I believe that as a community we should be doing more to promote surgery in medical schools, highlighting how it can lead to a rewarding career. This is necessary to ensure we have a strong talent pipeline and continue to attract those with potential to be the role models and key opinion leaders of the future.
What are your hobbies and interests outside of medicine?
I am an avid Formula One fan, and I have followed the sport for many years. As a youngster I spent a lot of time go-karting and I was a big fan of the Brazilian driver Ayrton Senna, whose career was cut short in a fatal crash at the San Marino Grand Prix in 1994. I believe he remains the greatest Grand Prix driver of all time. Last year I took my son to the British Grand Prix for the first time and we both had a wonderful time. I am also keen on fishing. I used to fly fish but more recently I have spent many enjoyable hours fishing by boat off the English coastline.
The recent, first-time European presentation of the PERFORMANCE II trial’s findings— delivered by Ralf Langhoff (Sankt Gertrauden-Krankenhaus, Berlin, Germany) at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK)—led delegates to discuss the role of stenting in carotid artery disease treatment, and how this has evolved in light of newer technologies.
Piotr Musialek (Jagiellonian University, Kraków, Poland) later noted that “the landscape has changed” recently, owing to the fact modern carotid stents are also ‘cerebral protectors’, while Christopher Metzger (OhioHealth, Columbus, USA) asserted that carotid artery stenting (CAS) is now competitive with carotid endarterectomy (CEA) when used appropriately.
PERFORMANCE II in Europe
“The implications from the results of the PERFORMANCE II trial are that procedural embolic protection is the most important aspect in minimising stroke during CAS, and the Neuroguard three-inone carotid stent system [Contego Medical] with 40-micron protection will be a transformational technology for the treatment of carotid artery disease.”
Those were the words of Langhoff, speaking to Vascular News on the eve of CX 2024 to outline the significance of the study and its findings. Langhoff, who is the European principal investigator for PERFORMANCE II, took to the CX podium during the Acute Stroke and Carotid Controversies programme on Wednesday 24 April to discuss these data in greater detail—a European first, following their initial presentation at last year’s Vascular Interventional Advances (VIVA) meeting (30 October–2 November 2023, Las Vegas, USA).
The prospective, multicentre PERFORMANCE II trial was set up to evaluate the safety and effectiveness of the Neuroguard integrated embolic protection (IEP) system, enrolling carotid disease patients with stenosis ≥50% if symptomatic or stenosis ≥70% if asymptomatic. According to Contego, the company behind this technology, the Neuroguard IEP system incorporates a novel, closed-cell nitinol stent, a balloon, and a filter with 40-micron pores, into a 6Fr device intended to capture micro-emboli and provide protection against stroke risks.
Langhoff’s podium-first presentation at CX saw him report low rates of minor (1.3%) and major (0%) stroke at 30 days across a total of 305 patients at 40 different clinical sites in PERFORMANCE II’s intention-to-treat (ITT) analysis, as well as a myocardial infarction (MI) rate of 0.7% and a death rate of 0.3%. He also relayed that no major strokes, contralateral strokes or neurological deaths ultimately occurred at 30 days.
Moving onto the trial’s primary endpoint (any stroke, death or MI at 30 days, and ipsilateral stroke from day 31 to 12 months), which was assessed across 282 patients at one year, Langhoff detailed a rate of just 2.8% in ITT analysis and 2.5% in the perprotocol analysis.
The presenter went on to share rates of 3.7% for instent restenosis, 0% for neurological death, and 1.1% for target-lesion revascularisation, as per one-year ITT analyses. No major strokes nor instances of stent thrombosis occurred at one year either, according to Langhoff, and only one minor stroke was observed from day 31 to 12 months.
Putting these findings into context, Langhoff then
pointed out that the 30-day ‘all stroke’ rate of 1.3% seen in PERFORMANCE II compares favourably to those observed with CEA in trials like ACST-2 (2.4%) and CREST 1 (2.3%), and is marginally better than in ACT 1 (1.4%) as well. This is especially notable, in Langhoff’s view, as all three of these studies included patient populations generally considered to be ‘standard risk’, while PERFORMANCE II exclusively enrolled patients deemed ‘high risk’ for CEA. The speaker further noted that similar trends were present regarding the primary endpoint outcome of PERFORMANCE II (2.8%), in comparison with the same measures for CEA in CREST 1 (6.6%) and ACT 1 (3.3%), at one year.
Langhoff’s concluding message was that—across a “challenging, highrisk” cohort of carotid artery stenosis patients—the PERFORMANCE II trial has demonstrated the durability, safety and effectiveness of the Neuroguard IEP system at both 30 days and one year. The presenter also averred that “these are the lowest one-year event rates reported for an adequately powered multicentre trial of any type of carotid revascularisation, regardless of patient risk”.
The latest technologies
analyses—one comparing first- and second-generation carotid stents, and another comparing secondgeneration stents to contemporary CEA data—that have provided indications of the short- and long-term clinical benefits these more novel devices may enable.
“There is always a role for surgery,” he added, “but the landscape has changed. Today, patients need to be fully informed and involved in the decision of how they are revascularised, and the decision in a given centre should be determined by a multispecialty neurovascular team. Today’s carotid stents are cerebral protectors and, with respect to clinical decisionmaking, historical data have mostly historical value— we are treating patients today, not 20 years ago.”
A closing message from Musialek was that, in wider medicine, there is an “evolution” towards less invasive treatments, and this point was corroborated by Metzger during the session’s final presentation.
“Am I going to tell you that stenting is equivalent [to CEA] for every patient with obstructive carotid artery disease? Absolutely not,” he began. “In fact, it should only be performed if the patient has favourable anatomy and appropriate history for CAS, and the operator themselves is experienced, uses good technique, and has considered all options and considers stenting to be best. That said, CAS has matured to where it is an overall equivalent strategy, and this has developed under very close scrutiny for a long period of time.”

Two subsequent talks attempted to take a closer look at the technological advances seen in stenting over the past few years.
Firstly, Musialek took to the podium, initially outlining the relevance of the timing of CAS-related stroke, as roughly 50% of strokes occurred postprocedurally in CREST-1, with a similar trend being observed in the 2007 CAPTURE trial and other large studies. The problem, according to Musialek, related to the limitations of the conventional, single-layer carotid stents being used in those early trials, and their inferior ability to fully eliminate plaque as compared to surgery.
“So, it’s not surprising that the key opinion leaders in vascular surgery said that—with the technology available at that time—CAS cannot be considered equivalent to CEA,” he noted. “But, if the technologies improve, we will come back and reevaluate.”
This provided a fitting introduction to discussions of several newer, second-generation ‘mesh’ carotid stents, such as the Hybrid Vascular Graft (Gore), the Casper/Roadsaver stent (Terumo), and the CGuard embolic prevention system (EPS; InspireMD). Musialek stated that, despite some similarities, these devices are mechanically different, and some of the said differences may translate into varying clinical outcomes. However, he went on to highlight that recent studies have generally found significant reductions in incidence of embolic material in filters, filter load, and CAS-related cerebral injury, with these newer-generation stents.
Musialek also drew attention to two meta-
Metzger went on to posit that treatment strategies including CEA, CAS, and also best medical therapy, should be viewed as “complementary, rather than competitive”, and emphasised the benefits of an individualised approach for each and every carotid stenosis patient.
Next, he touched on the fairly recent introduction of the aforementioned Neuroguard and CGuard stents, both of which have been specifically designed to reduce embolic events.
In addition to once again highlighting “excellent” data from PERFORMANCE II, Metzger recapped the findings of the prospective, multicentre C-GUARDIANS trial evaluating the investigational CGuard device in 316 patients enrolled at a total of 25 US and European sites. He stated that—across a patient population that is, “by definition”, high-risk for endarterectomy, 25% of whom were symptomatic— the study produced a 0.95% rate of stroke/death/MI at 30 days in its ITT analysis, and an event lower rate of 0.63% in per-protocol analyses. As with PERFORMANCE II, these findings were presented for the first time at VIVA 2023 and, according to Metzger, compare “very favourably” with historical endarterectomy outcomes.
“CAS is an excellent strategy, which is equivalent to the other options in well-selected patients treated by experienced operators—and new technology may lead to even better results,” Metzger concluded.
Attention is now likely to turn to upcoming results from trials assessing these ‘third-generation’ stent technologies. One-year, primary-endpoint data from C-GUARDIANS—pertaining to the incidence of major adverse events including any stroke/death/MI through 30 days post-index procedure, or ipsilateral stroke from day 31 to day 365 post-procedure—are expected later this year, and may instigate US Food and Drug Administration (FDA) approval of the CGuard device.
In addition, while PERFORMANCE II saw Neuroguard placed via transfemoral or transradial access, the first patients have already been enrolled in the investigational device exemption (IDE) PERFORMANCE III study evaluating the same stent when implanted via direct transcarotid access.
New research on Chat generative pre-trained transformer (GPT) technology and its Vascular Education and Self-Assessment Program (VESAP) success rate provides insight into the future of artificial intelligence (AI) in vascular surgery training and practice, investigators Michael Amendola (Richmond, USA) and Quang Le (Charlottesville, USA) told Vascular News
LE, A MEDICAL STUDENT AT the University of Virginia School of Medicine and first author of the research, explained that the project began with a petition to the Society for Vascular Surgery (SVS) SelfAssessment Committee, which granted access to the fourth edition of VESAP (VESAP4) in April 2023.
Subsequently, VESAP4 materials— namely 385 non-imaging questions, separated into 10 domains of vascular surgery knowledge—were submitted to the GPT-3.5-Turbo (GPT 3.5) large language model. Two independent reviewers examined AI-generated responses for accuracy and content and compared them to provided key answers. Application programming interface (API) requests were triplicated to evaluate consistency.
The research, recently presented as a moderated poster presentation at the Southern Association of Vascular Surgery (SAVS) annual meeting (24–27 January, Scottsdale, USA), showed that GPT 3.5 provided the correct answer to 49.4% of questions, and that 77.8% of correct responses were similar across all three queries.
Le reported that GPT 3.5 performed best in questions on radiation safety, achieving a 54.4% correct rate, while it performed worst in questions on dialysis access, answering only 39% of questions correctly.
Of the incorrectly answered questions, Le noted that the most common cause of inaccuracy was retrieval of false information or failure to retrieve important facts.
The team conducted further research, due to be presented as a poster at the 2024 Society for Vascular Surgery (SVS) Vascular Annual Meeting (VAM; 19–22 June, Chicago, USA), which found that while GPT 3.5 had an accuracy rate of about 48%, the corresponding figure for a later iteration of the model, GPT 4, was about 63%. However, the researchers also found that consistency was limited, with GPT 3.5 only consistent 55% of the time across three query attempts. GPT 4 was consistent in 90% of answers.
“Unfortunately, we found that industry updates have had conflicting effects,” Le added. He shared that,
while accuracy rates remained stable in the time between the releases of these two models—June 2023 and November 2023—consistency increased to 65% in GPT 3.5 but dropped to 79% in GPT 4 in this period.
challenges due to the legislative consideration as well as integration into existing health systems and health record systems,” said Le, with Amendola adding that privacy and Health Insurance Portability and Accountability Act (HIPPA) concerns are “limiting the infiltration of a lot of these models at large healthcare systems”.
Perhaps the biggest drawback at present, though, according to Le, is the tendency for models such as the one used in the aforementioned research to “hallucinate”. He explained: “Our large language models sometimes make up information in a way that might be harmful when used in a clinical setting.”
opportunity to individualise care, Le noted. He added that AI in general could improve the efficiency of vascular practice and education, citing the rapid drafting of medical documentation and the distillation of historical medical data as examples. Large language models, Le continued, could build realistic clinical situation simulations for training purposes.
“We’re using this technology now in a lot of other parts of our lives, and eventually it will become part and parcel of what we see at the bedside and within our practice,” Amendola posited.
Alongside this potential, though, Amendola was keen to stress the need for safety measures. “I think there’s a lot of promise and opportunity, but there needs to be a lot of policy, and we’re going to have to put some guardrails on what exactly some of these models are able to look at.”

Amendola, professor of surgery at Virigina Commonwealth University School of Medicine and chief of the Division of Vascular Surgery at Central Virginia VA Health Care System, as well as senior author of the research, highlighted a key takeaway from the
I think there’s a lot of promise and opportunity, but there needs to be a lot of policy”
Michael Amendola
project: “The interesting finding in this is that [ChatGPT] was not as good as we thought it ought to be.”
This comment laid the foundations for a wider discussion on the future of AI—an umbrella term covering a variety of different technologies, from large language models like ChatGPT to machine learning algorithms—in vascular surgery.
Both Amendola and Le highlighted various challenges that need to be addressed at this early stage of development before wider implementation into training and practice can be successful.
“A lot of this technology is experimental right now,” Le pointed out, noting that its application is currently highly varied by institution and by country.
Both Le and Amendola underline regulatory issues as one limitation.
“Integration of these tools—AI and more specifically large language models—continues to pose significant
Amendola also highlighted the impact of early-iteration AI on vascular training, stressing that educational institutions are grappling with how to effectively handle the use of AI among students. “Can you generate your own AI-based position paper or personal statement for an application?” he asked, highlighting a key question at the centre of this conundrum.
Data are also an issue. Le pointed out that large language models are influenced by training data, which “may harbour hidden bias”. In addition, he noted that predictive

I think as these technologies develop, the barrier to entry and the learning curve will continue to decrease” Quang Le
machine learning modelling needs large amounts of clean data, which often are not available.
“Unfortunately,” he stressed, “data capture during clinical care tends to be of low quality, for various reasons, with lots of missing or poorly documented information, which reduces the strength of such predictive modelling.”
Overall, however, both researchers expressed cautious optimism about the potential of AI technology in the vascular surgery landscape of the future.
“While our study has been about understanding the limitation of these new large language models, it’s only a stepping stone towards the innovative application of these tools,” Le said, putting his and Amendola’s research into context. Machine learning offers a significant
Commenting finally on adoption of AI at the physician level, Le emphasised the generally user-friendly nature of most current popular large language models, and that learning curves will become shorter as time goes on. “Overall, I think as these technologies develop, the barrier to entry and the learning curve will continue to decrease,” he commented.
“It will be an aid,” Amendola said as a closing remark, citing as one of his key messages the fact it is not a question of if but when with the adoption of AI, urging colleagues to embrace this new set of technologies. “One of the best quotes I’ve heard about AI is that AI will not replace doctors or surgeons, but the surgeon or the doctor who doesn’t use AI will be replaced.”
While GPT 3.5 had an accuracy rate of about 48%
the corresponding figure for a later iteration of the model, GPT 4, was about 63%.
This year’s Charing Cross (CX) International Symposium (23–25 April, London, UK) featured a debate on whether there is a role for resuscitative endovascular balloon occlusion of the aorta (REBOA) after the UK-REBOA trial. With Nigel Tai (Royal London Hospital, London, UK) arguing for the use of REBOA and Tim Stansfield (Leeds Teaching Hospitals NHS Trust, Leeds, UK) putting forward a case for moving away from it, the debate had the audience split nearly down the middle; poll results showed 58% of attendees voting against the use of REBOA, with 42% voting for it, which moderator of the session Ross Davenport (Barts Health NHS Trust, London, UK) highlighted was not decisively in favour of either option.


registry propensity-matched studies use a more comprehensive matching protocol and stricter calibration criteria there is a signal of survival advantage” he states. “Similarly, where REBOA is deployed in high-volume single centre settings such as Baltimore, the advantages can be evidenced.”
However, balloon inflation comes at a cost of ischaemia reperfusion. Further, it shouldn’t be difficult to spot those that may benefit from REBOA, but in sick trauma patients, trauma mimics continue to surprise even experienced clinicians.”
PRESENTING HIS CASE IN favour of REBOA first, Tai’s argument was based primarily on the ongoing requirement to address poor outcomes in sub-diaphragmatic traumatic haemorrhage, the evidence that REBOA can lead to rapid improvements in physiology and the benefit and lessons from high volume trauma centres from the USA. Whilst praising the need for and pragmatic design of the UKREBOA study, “one that I was proud to participate in”, there were “several features of the study that, to my mind mean that we must caution against overzealous application of the study message to all settings.” He argued that, due to extended pre-hospital and pre-surgery times, difficulty in technical application of REBOA and an insufficient per-centre case load, the results of the UK-REBOA trial may instead reflect the reality of attempting to introduce a novel technology in a system that is not optimised for the care of major haemorrhage.
Tai added that he thinks where REBOA is targeted in a more proscriptive manner the picture is different. There is a “message to be gleaned” from two analyses, both of which are propensity-matched cohort studies conducted on the same Japanese trauma registry. “Where observational
Conversely, Stansfield’s stance was that the most relevant element to this question should be framed as “would you advocate placing a REBOA resuscitation pack in your local trauma centre”. He argued that, should a major trauma centre have REBOA bags placed in them “someone will use it,” but they are not likely to “hit the sweet spot of REBOA usage, whatever that might be”. He instead suggested that they might be more likely to cause harm.
Stansfield also disputed Tai’s use of evidence to support the use of REBOA. “As we have heard, animal studies have demonstrated REBOA provides preferential pressure values to the coronary and carotid territories.
“We know which DCB to use”—but many other questions remain in vascular access
A first-time presentation at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK) of five-year data from the IN.PACT AV Access study evaluating the IN.PACT AV (Medtronic) drug-coated balloon (DCB) led principal investigator and CX co-chair Andrew Holden (Auckland City Hospital, Auckland, New Zealand) to conclude that “we know which DCB to use” now. However, discussions during the CX Vascular Access Masterclass did raise several other questions that are yet to be answered conclusively.
WITH IN.PACT AV ACCESS HAVING demonstrated superior outcomes at three years with the IN.PACT AV DCB versus plain balloon angioplasty for dialysis access—as per a presentation from Holden at last year’s CX Symposium—the speaker initially noted that it is currently the only randomised pivotal study to have produced “consistent and sustained clinical benefit” with an arteriovenous fistula (AVF) treatment device at such a long-term follow-up.
According to Holden, 98 patients—57 in the DCB group and 41 in the standard percutaneous transluminal angioplasty (PTA) group—had follow-up data available at five years. He reported an all-cause mortality rate (post vital status update) of 41% and 46.5%, respectively, across these two groups, describing these figures as “comparable”. Holden highlighted the fact that both of these figures are significantly lower than a 60.4% five-year mortality rate among US haemodialysis patients derived from the United States Renal Data System (USRDS). “Durable, long-term data suggest the use of IN.PACT AV DCB as a standard of care for AVF maintenance in patients with ESKD [end-stage kidney disease],” Holden concluded, also remarking that “we
now know which DCB to use”, and reporting that the device is enabling prolonged time to reintervention in dialysis access patients.
These findings may—in Holden’s view, at least— have drawn a line under debates over the optimal DCB in dialysis intervention, but further discussions throughout the Vascular Access Masterclass revealed several other areas yet to be fully addressed. The first of two controversies tackled within the session alluded to the fact that, despite recommendations in the 2019 update to the Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guideline for Vascular Access, ‘we don’t know what the right access, at the right time, for the right patient, is’.

One question that was posed to both presenters that did elicit a small amount of agreement, albeit with some caveats, was whether there was a role for the use of REBOA outside of hospitals and major trauma centres. As the first to respond, Tai stated: “My instinct is yes, […] but with a lot of preconditions. For instance, we run the Defence Medical Services training courses for REBOA, and we currently only teach one indication, which is essentially that if you have a patient who is occupying the single operating table in your farforward surgical tent, and you receive another casualty who is bleeding, then you can use REBOA to bridge that patient to surgery.”
Stansfield’s response was also along a similar vein; he stated that, although he is not a pre-hospital doctor, he feels that the people that use REBOA pre-hospital are “exceptionally well trained at diagnosing haemorrhage”. However, he did urge caution, saying: “I think we’ve just got to be careful. If we say there is a role for REBOA, it has to have some very, very strong caveats to it in terms of patient selection and training.”
Presentations here saw Maarten Snoeijs (Maastricht University, Maastricht, The Netherlands) assert that “access registries are not adequate, currently”; Karen Stevenson (University of Glasgow, Glasgow, UK) highlight the need for ‘minimally disruptive vascular access’ owing to the immense burden experienced by dialysis patients; and David Kingsmore (University of Glasgow, Glasgow, UK) advocate arteriovenous grafts (AVGs) over AVFs, drawing dissent from Robert Shahverdyan (Asklepios Klinik Barmbek, Hamburg, Germany). A more futuristic outlook was conveyed via a presentation from Koen van der Bogt (Leiden University Medical Centre, Leiden, The Netherlands), who updated delegates on progress towards developing a ‘dynamic AVF’ that is able to normalise flow rates between dialysis sessions, thus reducing patients’ cardiac burden. While this project remains in its infancy, CX Executive Board member Nicholas Inston (Queen Elizabeth Hospital Birmingham, Birmingham, UK) described the concept as “really innovative and futuristic”.
Following this, speakers debated the notion: ‘endoAVFs are a failed experiment’. Tobias Steinke (Schön Klinik, Dusseldorf, Germany) and Shannon Thomas (Prince of Wales Hospital, Sydney, Australia) delivered arguments favouring this statement, while Shahverdyan and Monnie Wasse (Rush University Medical Center, Chicago, USA) were in opposition.
The pro-endoAVF arguments accrued marginally more support, with an audience poll revealing that 54% of those in attendance disagreed with the suggestion that endovascular fistulas are a ‘failed experiment’. CX Executive Board member Kate Steiner (East and North Herts NHS Trust, Stevenage, UK) weighed in on these discussions, noting that “it would be impossible to take [endoAVFs] away now”—especially given the number of patients she sees who would not be able to receive a fistula if only surgical options were available.
Our Launch Pad columnist Sarah Sillito (Newcastle, UK), who ran the Rouleaux Club Introduction to Vascular Surgery Course at the recent Charing Cross (CX) International Symposium (23–25 April, London, UK), writes about the importance of there being “mutual respect and trust between teacher and learner” when it comes to surgical education.
It was great to be down at the CX Symposium again this year. It was another hugely successful conference, and I cannot get my head around the organisation that must be involved in running such an event!
This year at CX, I ran the Rouleaux Club Introduction to Vascular Surgery Course. It’s become an annual staple at the event and places
on the course were sold out again. I always enjoy meeting the delegates and getting to know how they have become interested in vascular surgery and seeing how keen they are to learn, even sometimes showing off some of the amazing surgical skills they have learnt so far. Teaching and education are something that I enjoy hugely. Having the opportunity to see someone grasp a new concept or skill, or simply just sharing the knowledge and experience you have gained is so rewarding. As doctors, teaching is an inherent part of our jobs whether this be teaching medical students in the classroom or trainees within theatre. It’s how we have all got to where we are today. It’s often hard to find the time within our busy work schedules to spend a dedicated amount of time with students or trainees, which is one of the reasons I enjoy running the Introduction to Vascular Surgery Courses as part of the Rouleaux Club.
In my current role as a core surgical trainee, I’m required to rotate through different surgical specialties to help me gain a broad base of competencies before specialising in vascular surgery. Working in a specialty that you don’t have much experience in can be tough and the learning curve is often steep. I try to approach my placements with an open mind and get stuck in, even though I know that specialty may not to be my long-term career. I make it known to those around me that I am enthusiastic to learn, and this usually results in being given


the opportunity to try new things, but not always. Learning in the workplace often relies heavily on a culture of reciprocity which unfortunately isn’t always met or encouraged for whatever reason. Having been both a learner and an educator, I know how frustrating this can be and how detrimental it is to the teacher-learner relationship. I certainly do not claim to be perfect when I wear either of these hats, but I have become more mindful of how I behave. Fostering a culture of reciprocity by having mutual respect and trust between teacher and learner is a key to success. Both parties must be present and in agreement to participating otherwise the learning will be diminished. Being mindful and encouraging of this is one way to nurture the culture of reciprocity thus creating meaningful educational experiences for the teacher and learner.
Having the opportunity to see someone grasp a new concept or skill, or simply just sharing the knowledge and experience you have gained is so rewarding”
Patients undergoing cyanoacrylate glue closure for superficial venous disease reported higher periprocedural satisfaction than those who were treated via surgical stripping, data from a pair of randomised controlled trials (RCTs) assessing the VenaSeal system (Medtronic) reveal.
THE MODALITY ALSO SHOWED GREATER improvement in disease-specific quality-of-life compared to surgery or endothermal ablation. However, at 30 days participants in both the glue closure and stripping arms were similarly satisfied post-procedurally, the primary investigators told the Charing Cross (CX) International Symposium 2024 (23–25 April, London, UK) in the first-time release of results from the Spectrum Program trials. Similarly, principal investigators Kathleen Gibson (Lake Washington Vascular, Bellevue, USA) and Manj Gohel (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK) reported that in the RCT comparing the VenaSeal system to endothermal ablation, similar patient satisfaction was demonstrated between the two procedures. The results emerged during the Superficial Venous Controversies session at CX 2024.
The peri- and postprocedural primary endpoints were measured using the novel Venous Treatment Satisfaction Questionnaire (VenousTSQ) tool, Gohel said. Data from the RCTs also showed that VenaSeal performed similarly to both surgical stripping and endothermal ablation in terms of elimination of truncal reflux, the third primary endpoint. Results from the secondary endpoint measure of modified Aberdeen Varicose Vein Questionnaire (AVVQ) at 30 days showed trends in favour of the VenaSeal system compared to both surgical stripping and endothermal ablation.
Meanwhile, six-month effectiveness data showed that VenaSeal was non-inferior to surgical stripping,

with similar anatomic closure rates (97.9% vs. 92.9%; p=0.00041). The equivalent measure was not tested between VenaSeal and endothermal ablation in the second RCT, but the trial investigators reported similar effectiveness in each arm.
The VenaSeal versus surgical stripping study included 88 patients, with 46 treated with the former and 42 the latter. In the second trial, VenaSeal (82) and
New data from the APEX-AX trial demonstrated that catheterdirected mechanical thrombectomy is safe and effective in patients with acute intermediate-risk pulmonary embolism (PE) with significant improvement in the right ventricle (RV) function and minimal major adverse events. The safety and efficacy results from the prospective trial were presented as late-breaking science at the Society for Cardiovascular Angiography & Interventions (SCAI) 2024 Scientific Sessions (2–4 May, Long Beach, USA).
APEX-AV IS A PROSPECTIVE, single-arm, multicentre investigational device exemption trial in which patients with acute intermediate-risk PE were treated with the AlphaVac F1885 aspiration system (AngioDynamics). The primary efficacy endpoint was a change in the right ventricle-to-left ventricle (RV/LV) ratio from baseline to 48 hours post-procedure on core lab-adjudicated computed tomography angiography. The primary safety endpoint was a composite of 48 hours post-procedure major adverse events: device-related death, major bleeding, and devicerelated serious adverse events.
mean percentage reduction in the overall clot burden from the baseline at 48 hours post-procedure. Five patients had major adverse events within the 48-hour visit and no death was reported. Lastly, procedural times were short with a mean procedural time of 37.2±17.7 minutes.
“Pulmonary emboli can be extremely dangerous and require effective and prompt action,” said William Brent Keeling (Emory School of Medicine, Atlanta, USA), lead author of the study.

Among the 122 patients treated at 25 US sites, the average reduction in the RV/LV ratio was 0.45 with a 35.5%
“Catheter-directed mechanical thrombectomy using a new aspiration system can achieve excellent thrombus removal with a wonderful safety profile, thus enabling more tools in our armamentarium for the treatment of acute PE.”
endothermal ablation (81) combined for a total of 163 participants. All patients were Clinical, Etiological, Anatomical and Pathophysiological (CEAP) classification 2–5.
“This is an innovative study design, incorporating all of the usual elements we have seen in other trials, including quality of life measures and closure rates, but also includes new outcomes measures that are very patient focused on how they feel about their procedure and their outcomes,” Gibson said. “We are interested to see going forward whether we see any differences when we follow them out further.
“Participants were satisfied with their treatment. The VenaSeal patients—and all of the patients in general—had good quality-of-life improvements at 30 days. The VenaSeal patients had similar elimination of truncal reflux and closure rates compared to what are the worldwide gold standard. There was a low incidence of adverse events, and very similar to what we see in published literature. We did not find any new adverse events that had not been previously reported.” Despite VenaSeal not meeting all of the patient-specific primary endpoints, “it is notable that there were tends towards advantages with VenaSeal and it performed comparably with our standards of care,” Gibson added.
The Spectrum Programme also contains a third single-arm study set to measure VenaSeal’s impact on venous leg ulcers (VLUs) in patients with C6 disease. It will measure time to ulcer healing through 12 months. “This is a series of three studies, all with traditional outcome measures—a very robust safety assessment—but then with additional focus on patient satisfaction,” said Gohel. “The patients were similar, although there were some subtle differences.”
Late-breaking data from the ENGULF trial showed that a novel dual-action thrombectomy device was effective and safe in treating acute pulmonary embolism (PE). The safety and effectiveness results were presented as late-breaking science at the SCAI 2024 Scientific Sessions.
THE ENGULF TRIAL IS A prospective, single-arm, first-inhuman, safety and feasibility study evaluating a novel embolectomy catheter system for the treatment of acute PE with a steerable and expandable funnel and an internal agitator, the Hēlo PE thrombectomy system (Endovascular Engineering).

Patients underwent a pre- and 48-hour post-procedural computed tomography (CT) scan. The primary efficacy outcome was the percent difference in the pre-topost procedural right ventricleto-left ventricle (RV/LV) ratios.
The primary and secondary safety outcomes were all-cause mortality, major life-threatening bleeding, device-related serious adverse events, pulmonary or cardiac injury, and clinical decompensation at 48 hours and 30 days post-procedure.
All 25 patients from eight centres underwent successful embolectomy.
The mean RV/LV ratio was 1.53±0.27 at baseline and 1.15±0.18 at 48 hours post-procedure (23.2%±12.81% change). Of note, there were no major adverse events at 48 hours and no deaths at 30 days.
“For the field of interventional PE therapies to fully reach its promise, continued innovation is needed to optimise our procedural workflows across the wide array of patients affected by this disease,” said Jay Giri (Hospital of the University of Pennsylvania, Philadelphia, USA), senior author and national principal investigator of the study.
“The ENGULF trial is an important step in this process, demonstrating that a novel, purpose-built PE thrombectomy catheter can achieve excellent results even among its earliest users.”










Interim analysis provides early window into real-world outcomes for Endologix’s Alto
In a podium-first presentation on the final day of the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK), Sean Lyden (Cleveland Clinic, Cleveland, USA) disclosed an interim analysis from the JAGUAR trial and, in doing so, gave delegates an early window into potential real-world outcomes with the Alto stent graft (Endologix).
Lyden initially noted that the device in question has been designed to treat a wide range of abdominal aortic aneurysm (AAA) cases via endovascular aneurysm repair (EVAR), even in some of the most difficult anatomical features in these patients. He also described JAGUAR as a postmarket, prospective, multicentre randomised controlled trial (RCT), intending to enrol a total of 450 patients (300=Alto, 150=comparator) across 60 US and European sites, with planned follow-up out to five years.
As of February 2024, the study had enrolled 272 (180=Alto, 92=comparator) of its target of 450 patients, with 28 sites having already been activated. According to Lyden, enrolment is expected to be complete by Q2 2025, while investigators estimate five-year data being available in 2030.
Regarding relevant events through 30 days in JAGUAR—as per an interim analysis of the study’s findings to date—Lyden relayed a slightly higher rate of complications in the comparator group versus the Alto group. However, the Alto group patients have experienced a slightly greater rate of Type Ib and II endoleaks at 30 days, he added, also noting no conversions, occlusions or ruptures in either group.
Moving on to relevant one-year events, Lyden said there has been a slightly higher rate of total secondary interventions with Alto, while there was a marginally increased presence of Type II endoleaks in the comparator compared to the Alto group. Another observation he reported was the fact that the Type Ib endoleaks in the Alto group at 30 days were no longer present at one year.
Prior to concluding, Lyden informed the CX audience that there was very little increase in sac diameter sizes at six months, and one year, across both groups, and that there were no reports of neck dilatation noted for any patients
in the trial at the time of this interim analysis.
In summary, the presenter stated that anticipated core-lab analyses at one year will provide information on aneurysm-related complications and robust neck dilatation data on Alto, when comparing Alto with other commercially available devices. He also noted that JAGUAR will provide insights on quality, long-term, level-one evidence, and its results “will shape optimal treatment strategies” and shed light on enhancing patient outcomes.
New data show Penumbra’s Indigo aspiration system used in a single session without overnight thrombolytics is safe and effective for patients with lower extremity ALI
During the Society of Interventional Radiology (SIR) 2024 annual meeting (23–28 March, Salt Lake City, USA), newly presented data from a subgroup analysis of the STRIDE study showed that Penumbra’s Indigo aspiration system used in a single session without the need for overnight tissue plasminogen activator (tPA) is safe and effective for patients with lower extremity acute limb ischaemia (ALI).
“Although the use of tPA following aspiration thrombectomy with [the] Indigo system was not limited during the STRIDE study, the majority of patients did not receive it and still experienced excellent outcomes,” said STRIDE investigator Jayer Chung (Baylor College of Medicine, Houston, USA).
“These findings demonstrate that the use of aspiration mechanical thrombectomy, without overnight tPA, yields high procedural success, low complication rates, and high target limb salvage rates in [lower extremity] ALI.”
A press release details that, although widely used, there are many complications associated with tPA and other clot-busting drugs. In the case of the overnight lytic group, tPA was also

associated with an increased need for intensive care unit (ICU) monitoring as shown by the higher median length of ICU stay among those patients.
“The high risk of associated major bleeding is a notable limitation of thrombolytics as an intervention
across many disease states, and [lower extremity] ALI is no exception,” said Thomas Maldonado (New York University of Langone Health, New York, USA), national principal investigator of the STRIDE study. “The data show that aspiration mechanical thrombectomy is a safe and effective minimally invasive procedure, which may offer an option to eliminate thrombolytic use for some patients.”
STRIDE is an international, prospective, single-arm, multicentre, observational study of patients with lower extremity ALI and using the Indigo aspiration system as a frontline intervention. The latest findings include:
• No significant differences were detected between patients who received overnight tPA and those who did not for target limb salvage rate at 30 days, patency at 30 days and 30-day mortality.
• ICU resource utilisation was significantly lower in single-session Indigo patients.
Penumbra notes that ALI is associated with a high risk of amputation and death. Studies have shown that ALI patients treated with catheter-directed thrombolysis often risk further vascular complications, such as major bleeding.
First patient enrolled in IDE study of Aquedeon Medical’s Duett vascular graft system
Aquedeon Medical has announced the initiation of an investigational device exemption (IDE) clinical trial to study the Duett vascular graft system.
A press release reveals that, this February, the first enrolled patient underwent open surgical aortic arch reconstruction at the University of Pennsylvania Presbyterian Medical Center (Philadelphia, USA). During the surgery, the Duett vascular graft system was successfully deployed to connect the native left common carotid artery to the surgical graft.
Aortic arch surgical reconstruction involves removal of the diseased aortic segment and replacement with a synthetic (e.g. polyester) surgical aortic vascular graft. Typically, the three vessels, which provide blood flow to the brain and the left arm, are resected from the diseased aortic arch and reattached to the surgical aortic graft with a series of hand sewn, circumferential surgical anastomoses by suturing. A sutured surgical anastomosis can take upwards of 15 minutes per vessel to complete, while Aquedeon Medical claims that its Duett vascular graft system may enable surgeons to connect vessels in substantially less time.
“This procedure is done under hypothermic circulatory arrest (HCA), in which the body temperature of the patient is brought down from 37°C to 28°C with the heart stopped and the body supported with a cardiopulmonary bypass machine. During this period of HCA, the brain and the lower body are subjected to temporary periods of
decrease blood flow, and therefore, the longer the patient is subjected to HCA, the greater the risk for neurological complications” said Wilson Szeto, chief of Cardiovascular Surgery at Penn Presbyterian Medical Center and professor of Surgery at the University of Pennsylvania, who is serving as the principal investigator for the clinical trial. He added: “I’m very excited about this technology. Suturing vessels by hand adds time to the procedure, as these vessels are often deep within the thoracic cavity and difficult to access. The Duett enables us to significantly reduce the surgical time associated with creating an anastomosis.”
“The patented Duett vascular graft system has been developed in collaboration with leading cardiothoracic surgeons and the device is aimed at helping to address the complexities and intricacies of thoracic aortic surgeries. Reducing anastomosis time is one major step in addressing this long and complex procedure,” said Tom Palermo, chief operations officer and general manager of Aquedeon Medical. “This is a significant milestone for our team, and we look forward to continued patient enrollment and use of the device at our other clinical sites.”
BD announces first patient enrolled in PAD vascular covered stent trial
BD has announced the enrolment of the first patient in the AGILITY investigational device exemption (IDE) study, which will assess the safety and effectiveness of the BD vascular covered stent for the treatment of peripheral arterial disease (PAD).
The investigational vascular covered stent is a self-expanding, low-profile, polytetrafluoroethylene encapsulated nitinol implant. It is deployed from a delivery system that provides controlled stent release.
“When we’re addressing advanced PAD, a self-expanding covered stent can play an important role,” said Sean Lyden (Cleveland Clinic, Cleveland, USA) national principal investigator of the study. “We need a stent that can track to the lesion, opposes the vessel wall and ultimately provides long-term durability. We’re excited to see how this technology performs.”
According to BD, the global, prospective, multicentre, single-arm, non-randomised AGILITY clinical study will include 315 patients at up to 40 clinical study sites across the USA, Europe, Australia and New Zealand. Follow-up for all treated patients will be performed at various points after treatment—starting at one month and ending at 36 months.
REGISTER BY JULY 11 TO SECURE EARLY TUITION RATES viva-foundation.org/register
14th Annual Venous Endovascular INterventional Strategies
NOVEMBER 2 - 3, 2024 •
• 2 days of venous education featuring sessions on medical management, cases & complications, DVT, and more
• The 5th Annual Blue Sky Breakfast providing a first look at the future of venous care
• Late-Breaking Clinical Trials session featuring first-on-podium data
• PE Summit open to all VIVA and The VEINS attendees offering a foundational understanding of the current state of PE, high-value RCT results, and strategies to improve patient outcomes
22nd Annual V ascular I nter V entional A dvances
NOVEMBER 3 - 6, 2024 • WYNN LAS VEGAS
• 5 Pre-Courses: CLTI, Aortic, Dialysis, PharmacoRx, and PE Summit
• The Global Theater featuring international live cases, groundbreaking clinical trials, great debates, and disruptive tech sessions
• Dynamic case discussions in Strategies on the Frontline
• Roundtable forums on AI/robotics, carotid intervention, and renal denervation
• Case presentations by the next generation of vascular leaders in the Face-Off Competition
• Simulator Showcase and Hands-on Cadaver Lab

Learn more, view the agenda, and register here
Abbott’s Esprit BTK scaffold system given US FDA approval for CLTI treatment

Abbott has announced that the US Food and Drug Administration (FDA) has approved the Esprit BTK everolimuseluting resorbable scaffold system (Esprit BTK system), a breakthrough innovation for people with chronic limb-threatening ischemia (CLTI) below-the-knee (BTK).
The Esprit BTK System is designed to keep arteries open and deliver a drug (everolimus) to support vessel healing prior to completely dissolving.
Until today, there were no stents or drug-coated balloons approved for use below the knee in the USA. The standard of care has been balloon angioplasty, which relies on a small balloon delivered via a catheter to the blockage to compress it against the arterial wall, opening the vessel and restoring blood flow. However, blockages treated only with balloon angioplasty have poor short- and longterm results, and in many instances the vessels become blocked again, requiring additional treatment.
The Esprit BTK system is a firstof-its-kind dissolvable stent and is comprised of material similar to dissolving sutures. The device is implanted during a catheter-based minimally invasive procedure via a small incision in the leg. Once the blockage is open, the Esprit BTK scaffold helps heal the vessel and provides support for approximately three years until the vessel is strong enough to remain open on its own.
“The FDA approval of Abbott’s Esprit BTK System marks a significant milestone in our fight against peripheral artery disease below the knee and should usher in a new era of improved outcomes for people worldwide,” said Sahil A Parikh, (Columbia University Irving Medical Center, New York, USA) and one of the principal investigators of the LIFEBTK trial. “By introducing a treatment option that is superior to balloon angioplasty, Abbott is changing the landscape of CLTI therapy.”
The LIFE-BTK trial, which evaluated Abbott’s Esprit BTK system, was presented in October 2023 as a late-breaking clinical trial at the 35th Transcatheter Cardiovascular Therapeutics (TCT) conference (23–26 October, San Francisco, USA) and simultaneously published in the New England Journal of Medicine. The results of the trial demonstrated that the Esprit BTK System reduces
disease progression and helps improve medical outcomes compared to balloon angioplasty, the current standard of care.
“At Abbott, we’ve recognised the significant burden of disease and limited treatment options available for people living with the most severe form of peripheral arterial disease (PAD). That’s why we’re revolutionising treatments with resorbable scaffold technology below the knee,” said Julie Tyler, senior vice president of Abbott’s vascular business. “Our resorbable program is focused on meeting unmet needs in the peripheral anatomy to help people live better and fuller lives.”
Provisio Medical announces US FDA clearance of the Provisio SLT IVUS system
Provisio Medical has announced US Food and Drug Administration (FDA) 510(k) clearance of the Provisio sonic lumen tomography (SLT) intravascular ultrasound (IVUS) system.
According to a press release, SLT technology addresses a critical unmet need for vascular specialists by providing automatic, real-time, accurate, numeric measurements of the flow lumen of blood vessels without the complexities of image interpretation.
Provisio Medical states that its catheter is the world’s first integrated intravascular imaging and support crossing catheter and enables vessel lumen measurement and visualisation simultaneously with guidewire support and delivery of radiopaque contrast agents.
It has been demonstrated repeatedly that the current standard-of-care of using angiographic information by itself is insufficient to accurately assess vessel size in the approximately 20 million people in the USA with peripheral vascular disease, the company notes. It goes on to say that the use of intravascular imaging has been shown to improve accuracy of vessel sizing and thereby improve clinical outcomes.
By incorporating its technology into a front-line support crossing catheter, Provisio claims to simplify the acquisition and presentation of vessel sizing data without changing the physician’s workflow, while potentially reducing the time for data acquisition, use of contrast media, and exposure to radiation.
“Clinical outcomes in peripheral vascular disease have consistently been shown to benefit from accurate intravascular measurements, yet adoption has been limited by the additional procedure time and training required to interpret images” noted S Eric Ryan, chief executive officer.
“Thanks to the ease-of-use of SLT IVUS, which can be incorporated more efficiently in the peripheral vascular workflow, we believe there is the
possibility of increased adoption and therefore improved outcomes for many more patients with potentially devastating peripheral vascular disease.”
The Provisio SLT IVUS system consists of the SLT IVUS P1 system and the SLT IVUS support crossing catheter. The SLT IVUS support crossing catheter is an over-the-wire IVUS catheter with an ultrasound transducer array at the distal end that also functions as a support crossing catheter. The information from the ultrasound signal is used similarly to sonar technology to measure vessel dimensions in real-time and visualise the peripheral vessel’s flow lumen.
Penumbra announces FDA clearance of Lightning Flash 2 for the treatment of PE Penumbra has announced the US Food and Drug Administration (FDA) clearance and launch of Lightning Flash 2, the next generation computer assisted vacuum thrombectomy (CAVT) system to remove venous thrombus and treat pulmonary embolism (PE), a press release states.
Lightning Flash 2 features advanced Lightning Flash algorithms, designed for increased speed and sensitivity to thrombus and blood flow. These new features combined with Penumbra’s novel catheter technology allow physicians to better navigate the body’s complex anatomy and deliver high power for clot removal with possible minimal blood loss.

“Based on what we’ve seen in the initial launch, Lightning Flash 2 has significantly improved procedure time by shortening the aspiration time. It has also shown reductions in blood removed during aspiration. These advantages can improve patient safety, provide better outcomes for the patients, and streamline efficiency for physicians treating the patients,” said James F Benenati, chief medical officer at Penumbra. “As adoption of thrombectomy becomes more widespread, Lightning Flash 2 will provide physicians with the confidence that CAVT is a valuable frontline option.”
With streamlined audio-visual feedback, Lightning Flash 2 enables physicians to have a better understanding of what is occurring at the tip of the catheter during a procedure, the press release details. This enhanced feedback loop results in a more intuitive thrombus removal experience for the physician.
“Lightning Flash 2 now combines an
optimally sized catheter with the latest algorithm technology designed to more efficiently remove blood clots while maintaining a high level of safety,” said Adam Elsesser, president and chief executive officer of Penumbra. “Our ongoing innovation around CAVT underscores our commitment to advancing patient care so that more patients suffering from these complicated conditions can benefit from this advanced therapy.”
Lightning Flash 2 is part of Penumbra’s Indigo system with Lightning portfolio. The company’s Lightning products are the only computer assisted mechanical thrombectomy systems currently available in the USA and early clinical data has demonstrated improvement in patient clinical outcomes and quality of life.
Silk Road adds Enroute NPS Plus device to TCAR portfolio Silk Road Medical has announced the launch of its Enroute transcarotid neuroprotection system Plus (NPS Plus), which the company describes as a key component of its transcarotid artery revascularisation (TCAR) portfolio.
As per a Silk Road press release, this next-generation device builds upon the prior Enroute transcarotid neuroprotection system to deliver smoother arterial sheath insertion, greater flow precision, and a simplified prep experience for surgical teams— all while maintaining “unparalleled” neuroprotection during the TCAR procedure.
“With the launch of the NPS Plus, we’re thrilled to empower our TCARtrained physicians with a solution that addresses their insights and feedback to further strengthen and streamline the TCAR procedure,” said Chas McKhann, chief executive officer of Silk Road. “Our focus on new product innovation reflects our ongoing commitment to leadership in the treatment of carotid disease.”
The release goes on to state that TCAR combines direct carotid artery access with robust blood flow reversal, providing neuroprotection akin to open surgical techniques in a less invasive, more patient-friendly approach to treating carotid artery disease. During TCAR, the surgical team inserts a tube-like sheath into the carotid artery and connects it to the Enroute neuroprotection system, which temporarily reverses blood flow. The system filters potentially dangerous debris from the blood to reduce intraprocedural stroke risk while the team uses a TCAR-specific balloon and stent to open the artery, and contain the carotid artery disease.


“With over 500 TCAR procedures performed by our team, we view TCAR as a first-line treatment option for carotid disease and we’re excited about what these improvements mean for patient care,” stated Brian Peterson (St Luke’s Heart and Vascular Institute, St Louis, USA).




Nectero Medical announces completion of US$96 million Series D financing Nectero Medical recently announced the closing of its US$96 million Series D financing round.
Financing was led by Norwest Venture Partners, with large investments from Boston Scientific Corporation, BioStar Capital, Cadence Healthcare Ventures, Aphelion Capital and other firms.
A press release reports that proceeds will be utilised to accelerate the execution of the phase 2/3 stAAAble trial and to support the submission of a New Drug Application (NDA) with the US Food and Drug Administration (FDA) for the Nectero endovascular aneurysm stabilisation treatment (Nectero EAST) system.
Upon completion of the Series D financing, Zack Scott, of Norwest Venture Partners, and Alan Davis, of BioStar Capital, have joined the Nectero Medical board of directors.
“We are pleased to have raised the funds required to complete the necessary work to bring our potentially transformative technology to market,” said Jack Springer, president and chief executive officer, Nectero Medical. “In addition, we are fortunate to add two highly knowledgeable and extremely
23–24 May Pacific Northwest Endovascular Conference (PNEC) Seattle, USA pnec-seattle.org
28–31 May
Leipzig Interventional Course (LINC) Leipzig, Germany leipzig-interventional-course.com
insightful members to our already strong and accomplished board of directors.”
The Nectero EAST system is an investigational, single-use, endovascular system that delivers pentagalloyl glucose (PGG) locally into the aneurysmal wall, where it binds to elastin and collagen to strengthen the aortic vessel wall and to potentially reduce further degradation. The procedure takes less than an hour to complete and leaves no permanent implant behind. In January 2024, Nectero Medical initiated the stAAAble trial to investigate the safety and efficacy of the Nectero EAST system in patients with infrarenal abdominal aortic aneurysms (AAAs), maximum diameter 3.5–5cm.
“We are honoured and excited to be the lead investor in the Series D financing to fund the efforts necessary to bring the Nectero EAST system to market,” said Zack Scott, general partner, Norwest Venture Partners.
“The Nectero EAST system has the potential to have a profound impact on the hundreds of thousands of patients living with aneurysmal disease and would represent the most significant advancement in the treatment of AAA in over 25 years.”
“[The] FDA has granted
19–22 June Vascular Annual Meeting (VAM) Chicago, USA vascular.org/2024-vascularannual-meeting
27–28 June British Society of Endovascular Therapy (BSET) annual meeting South Gloucestershire, UK bset.co.uk

Breakthrough Therapy designation for the Nectero EAST system, acknowledging that the preliminary clinical evidence indicates that the combination product may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” said Alan Davis, managing director, BioStar Capital. “This potentially transformational treatment is a perfect addition to the BioStar’s investment portfolio centered around our deep-seated commitment to fund cutting-edge innovations that improve patients’ lives.”

Johnson & Johnson to acquire Shockwave Medical Johnson & Johnson is to acquire Shockwave Medical, it has been announced. Under the terms of the transaction, Johnson & Johnson will acquire all outstanding shares of Shockwave for US$335.00 per share in cash, corresponding to an enterprise value of approximately US$13.1 billion including cash acquired. The transaction was approved by both companies’ boards of directors.
The acquisition of Shockwave further extends Johnson & Johnson MedTech’s position in cardiovascular intervention and accelerates its shift into higher-growth markets, Johnson
6 September Interdisciplinary Aortic Dissection Symposium London, UK aorticdissectionsymposium.com
14–18 September Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress Lisbon, Portugal cirsecongress.cirse.org/about/ theannualcongress



& Johnson said in a press release. The acquisition will expand Johnson & Johnson’s medtech cardiovascular portfolio into coronary artery disease (CAD) and peripheral arterial disease (PAD). The transaction follows Johnson & Johnson MedTech’s acquisitions of Abiomed, a leader in heart recovery, and more recently Laminar, an innovator in left atrial appendage elimination for patients with non-valvular atrial fibrillation (AF).
Shockwave is a provider of intravascular lithotripsy (IVL) technology for the treatment of calcified CAD and PAD. IVL is a minimally invasive, catheter-based treatment for calcified arterial lesions, which can reduce blood flow and cause pain or heart attack.
In addition to its IVL platform, Shockwave also recently acquired Neovasc, developer of the the Reducer system, a novel product focused on symptom relief of refractory angina. The Reducer system is currently undergoing clinical studies in the USA and is CE marked in the European Union and the United Kingdom.
Joaquin Duato, chairman and chief executive Officer of Johnson & Johnson, said: “The acquisition of Shockwave and its leading IVL technology provides a unique opportunity to accelerate our impact in cardiovascular intervention and drive greater value for patients, shareholders and health systems.”
24–27 September European Society for Vascular Surgery (ESVS) annual meeting Kraków, Poland esvs.org/events/annual-meeting
7–8 October CX Aortic Live Vienna, Austria cxaortic.com
3–6 November Vascular Interventional Advances (VIVA) Las Vegas, USA viva-foundation.org/vivaprogramming
19–23 November VEITHsymposium New York, USA veithsymposium.org/index.php



چ 3 piece system provides maximum planning flexibility
چ Low profile expands patient applicability (19F OD)
Cannulate from above for procedural ease and efficiency
Ability to axially and radially reposition graft while partially expanded
technical success 1 90% target vessel patency 1 98%
(146/149)
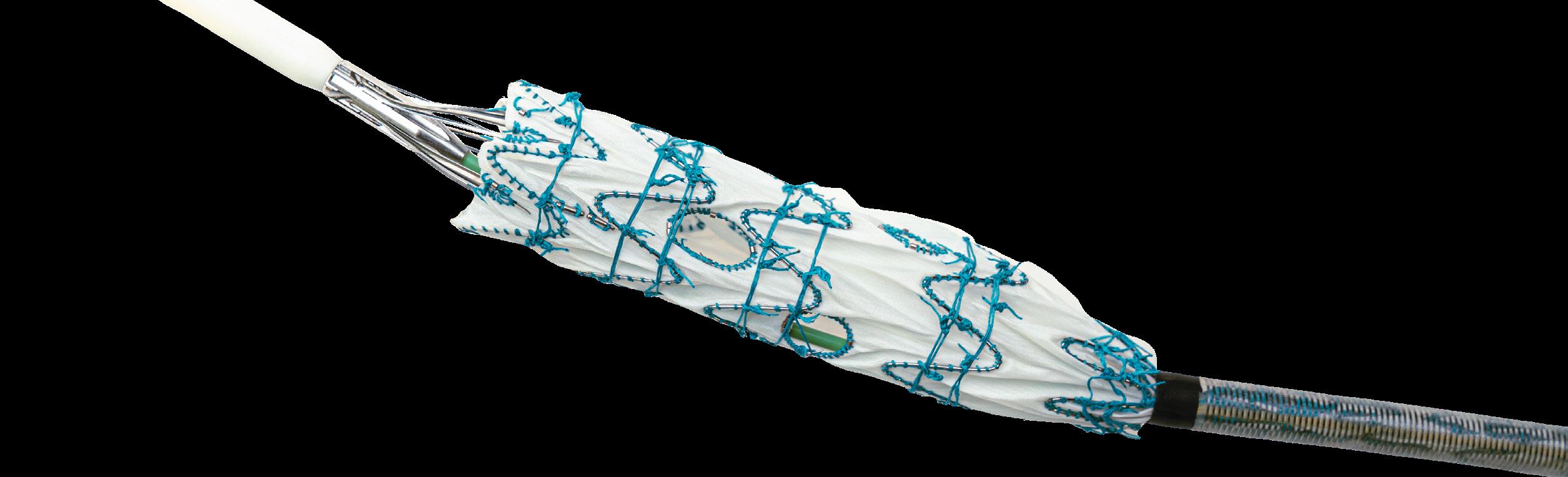
Freedom to position up to 5 fenestrations accommodates a variety of anatomies
Radiopaque markers for improved visualisation and alignment accuracy
Fenestration position and main body design can be widely customised according to specific preoperative anatomy. 2