












The Charing Cross (CX) International Symposium returns to ExCeL London this spring to tackle critical challenges in vascular disease management. Across three days, from 23–25 April, the programme reaches all corners of the vascular world—from endovascular repair of the aortic arch to new guidelines on trauma—offering faculty and delegates the opportunity to discuss and debate the best vascular care for patients worldwide.
Steered by co-chairs Dittmar Böckler (University Hospital Heidelberg, Heidelberg, Germany), Andrew Holden (Auckland City Hospital, Auckland, New Zealand) and Erin Murphy (Carolinas HealthCare System’s Sanger Heart and Vascular Institute, Charlotte, USA) alongside an expert executive board, this year’s CX agenda sees over 50 podium firsts, over 20 edited cases, and involvement from over 250 speakers.
“I have been visiting Charing Cross for more than 20 years now, since I was
a young trainee in vascular surgery, and I haven’t missed one single year,” Böckler tells Vascular News ahead of this year’s meeting, alluding to CX’s educational value for vascular specialists at all levels. Now in its 47th year, CX continues its tradition of showcasing the highest-quality education, innovation and evidence.
One of the highlights of this year’s Aortic programme is the Roger M Greenhalgh memorial lecture, honouring the legacy of the CX founder following his passing in 2023. Set to be delivered

“I think we should carry on the legacy of Professor Roger Greenhalgh and his life’s work on best medical education for optimal care of our patients with vascular disease,” says Böckler.
Continuing the theme of legacies is an Aortic session dedicated to the founding fathers of endovascular aneurysm repair (EVAR) and the impact of their contributions to modern vascular surgery.
Holden details that the Peripheral Arterial track this year covers several challenging topics.
“We’re going to have some late-breaking data on femoropopliteal interventions and drugeluting technologies,” he says, adding that the latest advances in aortoiliac occlusive disease treatment, vessel preparation, and ‘no-option’ chronic limb-threatening ischaemia (CLTI) management are also addressed.
Furthermore, this pillar of the programme sees the debate on surgical bypass versus endovascular-first
approaches for CLTI continue, with new data from BASIL-2, BASIL-3 and BEST-CLI set to be the basis of further discussion.
CX 2025 also features the launch of The Hurting Leg campaign with activities throughout the meeting looking at the issue of amputation reduction, including a CLTI session in the main programme, an amputation workshop, a diabetic limb salvage workshop and a CLI-C@CX session featuring a live case.
Elsewhere, new data from the ACCESS 2 randomised controlled trial and FLEX FIRST AV registry, as well as challenging cases, define the Vascular Access and Renal Interventions programme, while the Venous and Lymphatic programme sees new data from the STRIKE-PE study and the VenaSeal Spectrum study evaluating cyanoacrylate closure among other podium firsts.
Hot-topic debates are the highlight of this year’s Carotid and Acute Stroke programme, including a head-to-head between three experts on whether the gold standard for carotid revascularisation is carotid endarterectomy (CEA), transcarotid artery revascularisation (TCAR) or carotid artery stenting (CAS).
This year’s Vascular Trauma programme features a dedicated discussion on new guidelines from the European Society for Vascular Surgery (ESVS), ahead of the annual Innovation Showcase to close out the conference. A hot topic on the programme is artificial intelligence (AI) and the aorta, with the winner of the annual Dragon’s Den-style competition set to be announced at the end of the session. Innovation will also be showcased in the main programme via a robotics demonstration.
Complementing the main programme, there will be several hands-on workshops taking place across all three days of the meeting on topics including venous, vascular access and—new for 2025— vascular trauma.
For more information and to register for CX 2025, visit CX symposium.com.

Greetings everybody and welcome to the Charing Cross (CX) 2025 special edition of Vascular News. I hope that you enjoyed the winter months and like me are happy to see the green shoots of spring arrive.
In this special CX edition of Vascular News, there are interviews with several members of the CX executive board on highlights to be expected in the Peripheral Arterial, Aortic, Venous, Vascular Trauma, Vascular Access and Renal Interventions, and Carotid and Acute Stroke programmes of the symposium. These include several anticipated podium first presentations, debates and workshops.
There is also extensive coverage of key news items from across the vascular world. In the aortic realm, there is a piece on a report from the US Aortic Research Consortium (ARC) that indicated a steep decrease in the use of prophylactic spinal drains at the same time as a decline in spinal cord ischaemia after complex aortic endografting between 2011 and 2024. There is also coverage of a recent roundtable discussion between Ian Loftus, Ross Milner, Colin Bicknell, Tilo Kölbel and Dittmar Böckler that focuses on the latest developments in aortic disease.
An interview with Meghan Dermody discussing key evidence from the ROADSTER 3 trial on the efficacy of transcarotid artery revascularisation (TCAR) is a headline item in the carotid section, which also includes coverage of a presentation from the recent Southern Association for Vascular Surgery (SAVS) annual meeting (22–25
I hope that you will join me in welcoming the three new editorial board members for Vascular News Europe: Ian Loftus, Stephen Black, and Nicholas Inston.”


January, St. Thomas, the US Virgin Islands).
Regarding peripheral arterial disease, coverage of a recent roundtable discussion on below-the-knee (BTK) trials including ELITE-BTK, limus trials and vessel preparation technologies that involved Eric Secemsky, Thomas Zeller, Marianne Brodmann, Brian DeRubertis and Naseer Ahmad is included. There is also coverage of the 2025 Leipzig Interventional Course (LINC; 28–30 January, Leipzig, Germany), and a focus on Abbott’s AmpuNATION campaign heralded as a “a wake-up call for all stakeholders” by senior executive Samih Al Mawass.
Regarding new developments in the venous arena, Erin Murphy calls for “second-generation steps” in venous stenting—as per a presentation she delivered at the 2025 American Venous Forum (AVF; 16–19 February, Atlanta, USA). This section also includes coverage of the European Vascular Course (EVC; 9–11 March, Maastricht, The Netherlands) and new research published in the European Journal of Vascular and Endovascular Surgery
Vascular access is also featured, with Nicholas Inston, Bart Dolmatch and Kate Steiner discussing the future of endovascular arteriovenous fistulas (endoAVF).
There are invited commentaries from Michele Piazza, Ellen Dillavou and Robert Shahverdyan, as well as a feature article highlighting the profile interviews from the last four editions of Vascular News, with Bijan Modarai, Kevin Mani, Ramon Varcoe and Vincent Rowe.
In addition, I hope that you will join me in welcoming the three new members of the editorial board for Vascular News Europe: Ian Loftus, Stephen Black, and Nicholas Inston. Ian, Steve and Nick bring new and wide-ranging expertise to the team and will be sharing editorial letter-writing duties with me from now on.
Finally, like you I was saddened to hear of the recent passing of Professor Janet Powell. Janet Powell was one of the giants of vascular research and she will be missed by all vascular specialists who knew her. An obituary is presented on page 3.
I hope to see as many of you as possible at CX 2025.



ROB MORGAN is professor of interventional radiology at St George’s University Hospitals NHS Foundation Trust (London, UK) and the president of the British Society of Interventional Radiology.
Editorial board: Ian Loftus, Rob Morgan, Stephen Black and Nicholas Inston | Publisher: Stephen Greenhalgh
Content director: Urmila Kerslake
Editor: Jocelyn Hudson Jocelyn@bibamedical.com | Contributing editor: Byran Kay | Editorial contribution: Jamie Bell, Will Date and Éva Malpass | Design: Terry Hawes, Josh Lyon and David Reekie
Advertising: Rav Pankhania Rav@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News and Media, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2025. All rights reserved.
n CHALLENGING
AORTIC ANATOMY:
Michele Piazza (Padua, Italy) advises how to manage the challenging anatomical scenario of a narrow aorta during fenestrated and branched endovascular aneurysm repair (F/BEVAR), highlighting accurate, patientspecific planning and use of the correct bridging stent technology as the keys to good results.

For more on this story go to page 21.

n AMPUTATION
PREVENTION CAMPAIGN:
A new initiative from Abbott hopes to increase awareness of peripheral arterial disease (PAD) in the UK with the aim of preventing unnecessary amputations. Following the launch of the campaign, Samih Al Mawass, divisional vice president of Abbott’s vascular business for Europe, the Middle East and Africa, speaks to Vascular News to discuss the origins, execution and future of the project.
For more on this story go to page 22.
n VENOUS DISEASE RESEARCH:

Addressing the audience at the 28th European Vascular Course (EVC 2025; 9–11 March, Maastricht, The Netherlands), Ulka Sachdev-Ost (Pittsburgh, USA) set out a method to ensure scarce funding is directed to the most pressing venous disease research needs. “As everybody here in this room knows,” the presenter began, “funding for research from a federal level—at least in the USA, and I’m sure it’s similar in other countries—is actually pretty poor given the impact of venous disease for our patients and for the global population.”
For more on this story go to page 26.
If you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
Janet Powell, the clinical trial investigator who played a pivotal role in several landmark abdominal aortic aneurysm (AAA) studies, died in early March at the age of 79. At the time of her death, Powell was professor of vascular biology and medicine at Imperial College London (London, UK).
Powell was born in Oxford on 1 August 1945. After obtaining a BSc in chemistry from the University of Oxford (1968) and a PhD in biophysics in London (1972), Powell moved to the USA to study medicine at the University of Miami School of Medicine, graduating in 1981. She then returned to the UK to complete clinical training in pathology, specialising in cardiovascular risk.
Initially interested in the pathophysiology of elastic tissues, Powell told Vascular News in a 2015 profile interview that her research interests turned to the aorta specifically through her work with Roger Greenhalgh, with whom she collaborated over many decades.
As part of the Imperial College Vascular Surgery Research Group, Powell was involved in numerous clinical trials in AAA management, including the UK Small Aneurysm Trial, EVAR-1, EVAR-2 and IMPROVE.
When asked in her 2015 Vascular News profile interview which piece of research she was most proud of, Powell highlighted a central focus of her work: the patient. “Anything that improves outcomes for patients,” she responded, “including all the randomised trials of abdominal aortic aneurysm management, as well as an early piece of pathology research which showed the importance of inflammation in the developing aneurysm… a theme still being investigated by vascular biologists and evaluated in randomised trials.”
Powell was the chief investigator of the IMPROVE randomised controlled trial, which compared open repair with an endovascular strategy for ruptured AAA. Initial trial results were published in the British Medical Journal (BMJ) in 2014, with one-year results presented at the 2015 Charing Cross (CX) Symposium and published in the European Heart Journal the same year.
“We think that the results of this trial support the increasing use of endovascular repair and that it should always be available, so there’s
Powell is survived by her son Duncan (46) and daughter Tamsin (48), as well as five grandchildren. Colleagues have shared with Vascular News their memories of working and collaborating with Powell, as well as considered her legacy as a renowned clinical trial investigator.
Colin Bicknell, professor of vascular surgery at Imperial College London
“Janet delivered numerous trials over the years and has unquestionably changed the way that every vascular surgeon in the world practises daily. Not many can claim that accolade.
“She has been a mentor to me and

equity of access to care for patients,” she told Vascular News in an interview on the one-year results.
Powell was actively involved in vascular disease research until her passing. Alongside Imperial College London colleagues Colin Bicknell and Anna Pouncey, she was cochief investigator of the ongoing WARRIORS trial—her last research project—which aims to examine early endovascular aneurysm repair (EVAR) in women.
“The disadvantage of women with AAA can no longer be ignored, and we hope that you will support us, in what will hopefully be a major step towards readdressing the imbalance in AAA outcomes for women and men,” she urged Vascular News readers in an interview on the trial in 2022.
Powell has been recognised by several societies for her contributions to the vascular field. In 2012, she received a Lifetime Achievement Award from the Vascular Society of Great Britain and Ireland (VSGBI), while the European Society for Vascular Surgery (ESVS) hosts a named lecture at its annual meeting to honour Powell’s advocacy of evidence-based medicine.
UK), immediate past president of the ESVS and Vascular News editorial board member
“Professor Janet Powell was unsurpassed in her achievements, to promote evidence-based vascular practice. Her work has changed the way we work, through numerous ‘landmark’ clinical trials, but also a rigorous interpretation of scientific and clinical research, contributions to a number of ESVS clinical guidelines, and other international collaborations. She was chair of the ESVS board of directors for many years, and her contributions to the Society and its journal, were immense. It is right that these will be honoured formally at the forthcoming annual meeting in Istanbul in September.”
Pinar Ulug, clinical trial manager at Imperial College London
“Janet was committed to creating consensus and she never shied away from speaking out about difficult or unpopular issues. Her intellectual rigour, dedication to scientific excellence, and ability to foresee and resolve complex challenges in the trials she led set her apart as a brilliant leader and hugely inspirational mentor.
“She leaves behind a lasting legacy, both professionally and personally, and her influence on surgical practice is immeasurable. Janet’s passing is a tremendous loss to the vascular surgery community, but her remarkable career and extraordinary contributions will continue to inspire for years to come. She will be deeply missed by all of us who had the privilege of working with her.”
countless others, a role that she took so very seriously, building a cohort of researchers for the future. I and everyone will miss her every day, but I hope we can make her proud in delivering vascular trials in the future to practise evidence-based medicine in the way she taught us.”
Anna Pouncey, NIHR clinical lecturer in vascular surgery at St George’s, University of London (London, UK)
“When Janet moved, she moved quietly. Like pebbles dropped into a pond, the ripples of her actions were often only noticed much later. Now, as we look back, we realise that she helped shape vast swathes of our discipline. She was kind and loyal to those she cared for, rescuing and defending many a vascular surgeon in times of need. By doing so, she built not only a wealth of scientific evidence but also a living legacy within the vascular community.”
Ian Loftus, consultant and professor in vascular and endovascular surgery at St George’s University Hospitals NHS Foundation Trust (London,
Frank Veith, chair of the VEITHsymposium and professor of surgery at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University (Cleveland, USA) and New York University Medical Center (New York, USA)
“Janet was a superstar scientist and researcher and a good friend. She was a superb critical thinker with an uncanny knack to be correct on most issues. She was a major star contributor to our annual VEITHsymposium for decades. Janet was a genuinely good person and a great doctor/scientist who will be sorely missed on both sides of the Atlantic.”
Alun Davies, professor of vascular surgery at Imperial College London
“Janet was a delightful colleague with an international reputation, with whom I had worked for 30 years. She was adored by all her colleagues and students. She also had a unique long-term research collaboration with Roger Greenhalgh. Janet will be sadly missed.”
Peripheral Arterial Challenges
Peripheral Arterial & CLTI Challenges
“These crucial results will help to shape next steps in research, including ongoing randomised controlled trials comparing bioresorbable scaffolds to angioplasty, and eventually, to drugeluting stents.” These are the words of Michel Bosiers (University Hospital Bern, Bern, Switzerland), sharing his initial predictions ahead of several new data releases during the Peripheral Arterial programme at the 2025 Charing Cross (CX) Symposium (23–25 April, London, UK). Set to address critical challenges in the treatment of intermittent claudication and chronic limbthreatening ischaemia (CLTI), the programme will host over 30 podium firsts, including breaking data from the BASIL-2 and BASIL-3 trials.
Bosiers, who will present on day one of the peripheral programme, will deliver three-year results from the MOTIV bioresorbable scaffold (Reva Medical) trial. The trial aims to evaluate safety and efficacy of the device for the treatment of patients with rest pain or minor tissue loss due to the presence of lesions of a maximum length of 100mm at the level of the below-the-knee (BTK) arteries.
“Research on bioresorbable scaffolds is crucial because these devices offer unique advantages over traditional treatments, addressing some of the most significant challenges associated with managing peripheral arterial disease in chronic limb-threatening ischaemia patients,” says Bosiers, speaking to Vascular News ahead of this year’s symposium.
To address the increasing incidence of peripheral arterial disease (PAD) worldwide due to aging populations and rising diabetes diagnoses, Bosiers hopes that “these results will provide crucial insights into the long-term efficacy and safety of bioresorbable scaffolds in treating CLTI patients, significantly contributing to the investigation of advanced treatment options”.
Following Bosiers, Andrew Holden (Auckland City Hospital; University of Auckland, Auckland, New Zealand)— CX co-chair and member of the peripheral arterial executive board— will begin his series of podium first presentations during the first day of the symposium.
Set to deliver a first data release on drug-eluting resorbable scaffolds in the femoropopliteal segment, Holden comments that there has been “renewed interest” in resorbable scaffolds in lower limb arterial intervention following the LIFE-BTK trial results which facilitated the approval of the ESPIRT BTK (Abbott) scaffold. “Most companies that are investigating peripheral arterial indications for drug-eluting resorbable
vascular care,” he adds.
Later in the peripheral programme, Holden will also deliver a podium-first talk on new intravascular lithotripsy (IVL) technologies and trial data for calcified tibial artery disease. He tells Vascular News that, in several vascular territories, IVL has achieved “excellent acute luminal gain” in calcified arteries with a “low incidence of dissection of provision stenting”. Yet, Holden notes that there has been limited evidence supporting IVL use in tibial arteries, calling for a successful technology which can address calcification in these vessels and achieve better results than when treating with angioplasty.
“These data will help support discussion on the optimum management algorithm for CLTI patients with calcified tibial artery disease,” says Holden, who adds that delegates can expect interesting debate on whether the two technologies presented are “complimentary or competitive”.
Elsewhere in the day one peripheral

scaffolds are focusing on tibial artery applications. However, the advantages of drug-eluting resorbable scaffolds— which include providing an acute scaffold to optimise angioplasty results and resorbing without a permanent implant—are even more relevant in the femoropopliteal segment,” Holden describes.
In his talk, Holden will present an interim analysis from the first-in-human Efemoral-1 trial, including six-month follow-up of the Efemoral (Efemoral Medical) device, which was designed to manage the anatomic and restenotic challenges of the femoral artery. “These clinical outcomes will ultimately determine if this device translates from one of promise, to a standard tool for femoropopliteal arterial intervention. I believe these data have the potential to stimulate further interest and excitement around this important advancement in
techniques, comparing plain balloon angioplasty, IVL and atherectomy prior to the application of a drug-coated balloon (DCB).
Zayed comments: “Vessel preparation is an evolving concept which is gaining popularity amongst interventionalists, with the aim to enhance the outcomes of definitive treatment of arterial lesions. It is therefore important that we appraise the available research in order to highlight areas in need of further research to help guide our routine daily practice.”
Zayed states that their first-release data to be presented at CX 2025 will highlight that the current evidence is still heterogeneous, particularly in regard to patient cohort e.g. CLTI or intermittent claudication, lesion characteristics and complexity, as well as duration of follow-up.
During a ‘Challenges in aorto-iliac occlusive disease’ session, Maria Antonella Ruffino (IIMSI, Ente Ospedaliero Cantonale, Lugano, Switzerland) will present on new large balloon IVL in the treatment of aortic lesions. Importantly, she will question whether IVL is an effective therapy when used alone or in combination with stenting as a means of vessel preparation.
In conversation with Vascular News ahead of her presentation, she details that there is growing evidence in support of polytetrafluoroethylene (PTFE)-covered stents for the treatment of infrarenal occlusive disease of the abdominal aorta as an alternative to surgical reconstruction.
“Despite the type of stent we want to implant, suboptimal vessel expansion still represents a limit in the treatment of isolated aortic lesions, where neither high pressure balloon angioplasty nor atherectomy can significantly impact on wall calcifications,” says Antonella Ruffino.
These data will help support discussion on the optimum management algorithm for CLTI patients with calcified tibial artery disease.”
Andrew Holden
itinerary, Hany Zayed (Guy’s and St Thomas’ NHS Foundation Trust, London, UK) will present during a session dedicated to vessel preparation
Today, with the advent of ‘non-stent’ technology such as IVL that can be applied to large vessels and facilitate vessel expansion, Antonella Ruffino underlines the importance of confirming its safety and efficacy in aortic lesions. She hopes their research will highlight IVL technology as an “additional weapon” to treat very calcified isolated aortic lesions, and demonstrate that the combination of IVL and stenting could “help to overcome the risk of incomplete stent expansion with consequent restenosis”.
The CX 2025 peripheral programme will also showcase several challenging debates on technologies and techniques including vessel preparation devices versus high-quality plain balloon angioplasty, and whether endovascular techniques should only be offered to patients with common femoral artery disease who cannot undergo endarterectomy. Additionally, attendees will see the launch of the CX Hurting Leg campaign—an initiative to reduce amputations through increasing awareness in deprived communities and to promote systemic healthcare reform.







Critical challenges in open and endovascular treatment of aortic disease will be brought into focus when world leaders in cardiac, aortic, and vascular therapies return to London this spring.
CX, the world’s largest vascular meeting, has a three-year cycle of raising vascular and endovascular controversies to challenge the available evidence and reach a consensus after discussion with an expert audience.
“The highlight this year is challenges, and we have challenges in clinical practice in aortic care every day,” CX co-chair Dittmar Böckler (University Hospital Heidelberg, Heidelberg, Germany) tells Vascular News, looking ahead to the highlights on the aortic programme in 2025. “We need evidence, we need teaching, we need technical skills and tips and tricks, [and] all this will be provided by a really outstanding programme with key opinion leaders in the field of aortic disease and aortic care.”
The CX 2025 programme has sessions touching upon all vascular domains. The aortic pillar of the programme offers attendees insights from 123 presentations, augmented by 14 edited cases, with new data set to be brought to light in 16 podium first presentations, alongside debates on five hotly contested issues. There will also be opportunities for attendees to translate theory into practice through a series of hands-on workshops running throughout the three days.
Aortic techniques and technologies will be the focus on day one, with Böckler highlighting a case involving the treatment of a thoracoabdominal aneurysm using a four-inner-branched device as one to look out for. “We need off-the-shelf devices to treat these emergent and urgent patients, as customised devices are not available. How to handle those devices, how to implant them, [and] what the evidence is so far is the focus,” he says of the case. Turning to the highlights among the many podium first presentations featuring at CX 2025—with more than 50 across the full programme—Böckler mentions the SUNDAY trial, a randomised trial looking at
treatment options for uncomplicated type B aortic dissection (TBAD), as being of particular importance. “We have a lack of evidence there, and we are going to have the first insight into early outcomes of this trial,” he comments. Other podium first presentations include data on the impact of blood pressure on abdominal aortic aneurysm (AAA) growth rates, risk stratification after endovascular aneurysm repair (EVAR), a multicentre study on physician-modified endografts for very large and urgent complex AAA, and more.
The use of artificial intelligence (AI) in patient evaluation is among the “disruptive” technologies featuring alongside topics that impact daily clinical practice such as sarcopenia, the risk of abdominal
Wednesday 23 April, Theatre 2
■ Edited case: Off-the-shelf four inner branch stentgraft for thoracoabdominal and juxtarenal aortic pathologies—Dittmar Böckler (Heidelberg, Germany)
■ Edited case: Open repair of post-dissection TAAA—Drosos Kotelis (Aachen, Germany)
■ Edited case: A novel triple branch device for complete endovascular repair of the aortic arch—Alexander Zimmermann (Zurich, Switzerland)
Thursday 24 April, Theatre 1
■ Podium first: The impact of blood pressure on AAA growth rates: A prospective longitudinal cohort study—Colin Bicknell (London, UK)
cancer after EVAR, the use of proteomics to predict sac shrinkage after EVAR and quality-of-life assessment following vascular care.
“Something we have really neglected for years, in my mind, is quality-of-life assessment after vascular care, specifically in TBAD,” explains Böckler. “Many patients get conservative treatment, but we didn’t pay attention to their psychological status, how they behave, how they feel, so quality of life is something very new. And that’s in the programme of the aortic sessions this year.”
Among other highlights, Gustavo Oderich (Baylor College of Medicine, Houston, USA) will deliver the inaugural Roger M Greenhalgh memorial lecture, speaking on the challenges of 21st century aortic education, innovation and evidence. Citing this as one of the event’s must-attend presentations, Böckler says that the talk will continue CX founding chair Roger Greenhalgh’s legacy of advocating for the best medical education for the optimal care of patients with vascular disease.
“The unique and special thing about Charing Cross is the style of discussion,” Böckler adds, paying tribute to another of Greenhalgh’s CX legacies. “We have a one-to-one relationship between presentations and discussions. I love the discussion culture at Charing Cross. This makes it unique and special. We have a special style, a special atmosphere. Everybody can go to the microphone, ask questions, and you will get excellent answers from the leading physicians of the world.”
Attendees can join world-leading experts in the management and treatment of aortic disease from the cardiovascular, vascular and endovascular worlds who are participating in 2025 including CX aortic executive board members Oderich, Tilo Kölbel (University Heart Center Hamburg, Hamburg, Germany), Joseph Bavaria (University of Pennsylvania, Philadelphia, USA), Alexander Zimmermann (University Hospital Zürich, Zürich, Switzerland), Aung Oo (St Bartholomew’s Hospital, London, UK).
The highlight this year is challenges, and we have challenges in clinical practice in aortic care every day.”



■ Podium first: Recognising and treating abdominal compartment syndrome is more important than ever in the era of EVAR for rAAA— Martin Björck (Uppsala, Sweden)
■ Podium first: Risk stratification and treatment selection in patients with asymptomatic AAAs— Lorenz Meuli (Zurich, Switzerland)
Friday 25 April, Theatre 1
■ Inaugural Roger M Greenhalgh memorial lecture: “Meeting the 21st century challenges in aortic education, innovation and evidence”— Gustavo Oderich (Houston, USA)
■ The SUNDAY trial on uncomplicated type B dissection therapy—Jacob Budtz-Lilly (Aarhus, Denmark)












First indicated & dedicated platform for bridging stents in FEVAR
Precise & standardised positioning due to a dedicated fenestration marker
2-in-1 design that combines inflation & flaring in one step
No longer a ‘one-trick shop’: Venous becomes core pillar of CX amidst field’s evolution
Charting the development of the CX Venous and Lymphatic programme from a sidebar event to becoming an “integral part” of the meeting’s agenda, executive board member Stephen Black (Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, London, UK) highlights the venous education, innovation and evidence that delegates can expect at CX 2025.
BLACK HAS BEEN INVOLVED IN THE CX venous programme for over a decade, in which time he has witnessed a marked growth of the venous field. This expansion, he says—characterised by a move away from the narrow definition of varicose vein surgery towards “something that offers value to a broad range of patients”—has seen venous become a central pillar of CX. Black also cites the involvement of “world leaders” Erin Murphy (CX cochair; Carolinas HealthCare System’s Sanger Heart and Vascular Institute, Charlotte, USA), Manj Gohel (Addenbrooke’s Hospital, Cambridge, UK; Imperial College London, London, UK) and Kush Desai (Northwestern University, Chicago, USA) as his co-executive board members in “driving a really high standard of programme” to reflect the field’s evolution.
An educational cornerstone of the CX Venous and Lymphatic programme and one that has witnessed marked growth is the venous workshop, which
provides practical experience for both deep and superficial venous interventions.
Describing this as a “pioneering achievement” of Ian Franklin (London Vascular Clinic, London, UK), Black explains how the workshop offers a “unique opportunity” to delegates in representing a bridge between didactic talks and real-world practice. He encourages CX 2025 attendees to “come and learn something, come and practice it, and go back and do better for patients”.
On innovation, Black first steps back from the CX programme to consider wider trends that represent the continued growth of the venous field. January’s edition of Vascular News featured promising oneyear data on Envveno Medical’s VenoValve—an innovation Black expresses excitement for.
“A venous valve has been the holy grail of vascular surgery for as long as I can remember,” he says. “For the first time, we’ve got an almost FDA [US Food and Drug Administration]-approved device for treating
An in-depth review of new European Society for Vascular Surgery (ESVS) guidelines will headline this year’s CX Vascular Trauma session—set to take place on Friday 25 April in Theatre 3.
THE ESVS CLINICAL PRACTICE guidelines on the management of vascular trauma were published in February of this year as an Editor’s Choice paper in the European Journal of Vascular and Endovascular Surgery (EJVES)
A writing committee consisting of first author Carl Magnus Wahlgren (Karolinska University Hospital, Stockholm, Sweden) and colleagues from across Europe outlined a total of 105 recommendations.
The guidelines cover several topics, namely technical skill sets, bleeding control and restoration of perfusion, graft materials, and imaging; management of vascular trauma in the neck, thoracic aorta and thoracic outlet, abdomen, and upper and lower extremities; postoperative considerations after vascular trauma; and paediatric vascular trauma.

Unresolved vascular trauma issues and patients’ perspectives are also discussed.
Wahlgren and team state that the guidelines “provide the most comprehensive, up-to-date, evidencebased advice to clinicians on the management of vascular trauma,” whilst acknowledging some limitations affecting their generalisability.
“There is a general paucity of high-quality data and literature on vascular trauma management,” they write, noting that this applies to aspects relating to sex and ethnicity, but also conditions of low- and mediumincome countries.
“These limitations must be kept in mind when managing vascular trauma in different settings and environments.”
Wahlgren will be one of three speakers to discuss the new guidelines
patients with deep venous reflux, which has been the Achilles’ heel of venous treatment for generations.”
Homing in on some of the innovations set to grace the CX 2025 podium, Black notes those related to the concept of stent maintenance, sharing his opinion on their importance: “In venous, we do things for patients [that have] got to last 50 years. It’s not like arterial disease where the natural morbidity of patients creates an attrition rate that means your horizon for treatment is much shorter. So, maintaining whatever we do for the life of the patient is really important and we’re starting to see a lot more coming that will allow us to do that in a much better way.”
Evidence is also central to the CX venous programme, with Black pointing out that the CX 2025 programme includes a plethora of new data, in particular related to venous thromboembolism (VTE) treatments.
Black recalls that a considerable development he has seen over the past 10 years has been in the management of VTE. He shares that, in the early days, “nobody believed in it, nobody wanted to treat it”.
At CX 2025, new data are set to move VTE treatment forward. “There will be a couple of podium firsts this year, and in particular STRIKE-PE will be presented for the first time,” Black tells Vascular News. He adds that some trial results from the DEXTERITY-AFP study will be presented for the first time, which is looking at acute deep vein thrombosis treatment with adjuncts of steroid delivery to the vessel wall.
Black also points to new randomised controlled trial data on cyanoacrylate glue, set to be presented by Gohel, as another highlight of the CX programme.
This year’s CX Venous and Lymphatic session takes place on Thursday 24 April in Theatre 2.
at CX 2025, alongside Karim Brohi (The Royal London Hospital, London, UK) and CX executive board member Ross Davenport (Barts Health NHS Trust, London, UK), who are both also on the guideline writing committee.
Writing on X, formerly Twitter, Brohi was enthusiastic about the impact of the new document. He remarked that the publication represents “a massive piece of work” and one that will “bring vascular trauma care kicking and screaming into the 21st century”.
The CX session will close with a panel discussion led by CX executive board and guideline writing committee members Christopher Aylwin (Imperial College Healthcare NHS Trust, London, UK) and Davenport, as well as Todd Rasmussen (Mayo Clinic, Rochester, USA).
Davenport tells Vascular News that the new guidelines document is the first set of European guidelines for vascular clinicians to guide care for injured patients with vascular trauma.
Programme highlights:
● Debate: TEVAR debate: Early vs. delayed intervention for trauma
● Deep dive: In-depth review of the latest ESVS vascular trauma guidelines and clinical applications
“As a writing committee, our intention was to produce a novel, practical and usable set of recommendations based on the breadth of evidence available,” he says.
“In this new vascular guideline,” Davenport continues, “readers will find a number of recommendations for system organisation and training, but for the first time we have proposed a new grading system for vascular injuries, evidence summaries on method and type of revascularisation, and a very clear recommendation on revascularisation times for ischaemic limbs (less than one hour from admission).”
On the timing of the guideline publication, Davenport remarks: “With major trauma, war and conflict continuing to rise globally, we hope this document will serve not only as an important reference to inform care but be the driver for research and innovation in the field of vascular trauma.”
Members of the wider vascular community have been responding to the guidelines’ publication, with editorials from specialists in Belfast, UK, Johannesburg, South Africa, and Seattle, USA, appearing alongside the document in the EJVES
The CX 2025 Vascular Trauma session will provide further, expert insight into these first-of-their-kind guidelines.



EU: The VasQ device is intended for use as subcutaneous arteriovenous conduit support for vascular access.
US: VasQ is intended for use as an external support for upper extremity arteriovenous fistulas created for vascular access by means of vascular surgery.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events available at https://laminatemedical.com/eIFU

Vascular Access Challenges Renal Interventions Challenges
A dedicated all-day session at CX 2025 (Wednesday 23 April, Theatre 3) will address critical challenges in vascular access and renal interventions, featuring multiple podium first datasets that provide valuable insights and evidence to guide practice and the management of complex cases.
“THIS YEAR THE THEME IS CHALLENGES, and what other area is more challenging than renal disease and vascular access?” executive board member Nicholas Inston (Queen Elizabeth Hospital
Carotid & Acute Stroke Challenges
Birmingham, Birmingham, UK) posits, speaking to Vascular News ahead of CX 2025. “Renal patients are such a challenging group that most vascular surgeons and interventionalists will at some stage interact with,” he continues, going on to note that CX aims to involve everyone who sees renal patients, not just those with large practices in this area.
On what delegates can expect from this year’s meeting, Inston details: “We’ve got podium firsts, but also we’ve got the important area of case discussions, which I think is one of the highlights of the programme, where people can get together and actually discuss what issues they have with their patients.”
Inston explains that part of the programme will focus on the “big topic” of the timing of when a fistula should be created. “That’s a huge issue,” he says, “because we create a lot of fistulas that never get used and we’re actually doing patients a disservice by creating fistulas too early, but we also have a lot of fistulas that don’t mature and [those] people start on central venous catheters, which we know are detrimental in so many ways—quality of life and quantity of life.”
Executive board member Kate Steiner (East and North Herts NHS Trust, Stevenage, UK) adds that a Great Debate on the motion ‘drug-coated balloons (DCBs) should not be the standard of care for vascular access stenosis’ is set to be another programme highlight at CX 2025. “We see some variation in practice in the UK and also across the globe,” Steiner notes, adding that she anticipates this will be a “controversial” and “lively” debate that should
With challenges as its primary focus, CX 2025 will examine a number of pressing topics within carotid and acute stroke treatment via debates, first-time data presentations, and more.
TAKING PLACE IN THEATRE
3 at ExCeL London on Thursday 24 April, the Carotid and Acute Stroke Challenges programme will kick off with Ross Naylor (Leicester Vascular Institute, Leicester, UK) and Sean Lyden (Cleveland Clinic, Cleveland, USA) providing audiences with an overview of the latest carotid treatment guidelines from the European Society for Vascular Surgery (ESVS) and Society for Vascular Surgery (SVS), respectively, outlining the ‘common ground’ shared by the two documents as well as areas within which they pose contrasting perspectives.
The centrepiece of the programme will see three physicians go headto-head via a ‘Great Debate’ on the current gold standard in carotid revascularisation. CX executive board member Barbara Rantner (Ludwig Maximilians University Munich, Munich, Germany) will be the proponent of carotid endarterectomy (CEA) during this debate; CX executive
board member Christopher Metzger (OhioHealth Riverside Methodist Hospital, Columbus, USA) will present in favour of carotid artery stenting (CAS); and Michael Stoner (University of Rochester Medical Center, Rochester, USA) will put forward the argument for transcarotid artery revascularisation (TCAR).
“TCAR has achieved clinical superiority associated with its unique neuroprotection and minimal-access nature compared to other carotid anatomic treatment modalities,” Stoner told Vascular News. “Prospective trials have demonstrated safety and efficacy in both standard and high-risk patients, and these data are backed up by a large-scale real-world registry—the Vascular Quality Initiative. The safety, rapid learning curve, minimal-access technique, lower CNI [cranial nerve injury] rate, and scalability for local anaesthesia, support the motion that— after seven decades of surgical carotid revascularisation—TCAR is now the
“answer some important questions for everyone around DCB use.”
“I think it’s really good to have these controversial debates that are on peoples’ minds and to ask the questions, because it makes it so much more relevant,” Steiner comments, considering the link between CX and real-world practice. “I think we’re quite brave at Charing Cross now, addressing some of the real problems that we see on a day-to-day basis.”
Finally, Inston highlights the workshops that will be taking place on Thursday 24 April, describing these as an unmatched opportunity to engage with experts. He notes there will be several workshops this year, focused on topics ranging from endovascular and surgical arteriovenous fistula (AVF) creation to thrombectomy and ultrasound.
I think it’s really good to have these controversial debates that are on people’s minds and to ask the questions, because it makes it so much more relevant.”
Kate Steiner
gold standard.”
All three of these treatment modalities will be in focus later on in the session as well, with presentations from Enrico Ascher (New York University, New York, USA), William Gray (Main Line Health, Wynnewood, USA), and Sonya Noor (University of Buffalo, Buffalo, USA).
Another debate will see Alan Lumsden (Houston Methodist DeBakey Heart and Vascular Center, Houston, USA) and CX executive board member Adnan Siddiqui (State University of New York at Buffalo, Buffalo, USA) debate the motion, ‘Severely calcified ICA [internal carotid artery] lesions are at higher risk and are best treated by CEA’. Lumsden will deliver arguments in favour, while Siddiqui will postulate against this statement.
“Almost 75 years after the first CEA, it’s durability and efficacy has stood the test of time,” Lumsden said, speaking to Vascular News. “Can we improve on the unimprovable? Low complication rate, small incisions, speedy recovery—an operation that can be applied for almost
Programme highlight:
The centrepiece of the Carotid and Acute Stroke Challenges programme at CX 2025 will see three physicians go headto-head via a ‘Great Debate’ on the current gold standard in carotid revascularisation
every lesion and in the overwhelming majority of patients. In this debate, we will discuss calcified carotid stenosis and reaffirm the supremacy of this internationally available procedure.”
“I believe this is a historically accurate statement which is no longer true,” Siddiqui stated in response.
“Contemporary development of intravascular lithotripsy [IVL] has made endovascular management of these lesions routine. It is, in our experience, best performed with common carotid artery balloon-guided flow reversal in addition to a distal embolic filter using the 4x40 Shockwave peripheral OTW [over-the- wire] balloon. IVL allows ideal preparation of heavy calcific stenosis, which can then be treated with standard higher-pressure balloon angioplasty and stenting. We routinely perform intravascular ultrasound and, to date, have not seen any new embolic debris protruding inside the stent.”
This year’s Carotid and Acute Stroke Challenges programme will close with data from multiple significant studies related to carotid stenting; William Gray will take to the podium to give the first European presentation of two-year outcomes from PERFORMANCE II, as will Stoner to provide an update from the ROADSTER 3 study evaluating TCAR’s safety and efficacy, while Piotr Musialek (John Paul II Hospital, Krakow, Poland) is set to summarise one-year results from the CGUARDIANS pivotal trial that are— in his view—“as good as we have seen”.




■ Gore
420
With more than 55 million medical devices implanted over the course of more than 45 years, Gore builds on its legacy of improving patient outcomes through research, education and quality initiatives. Gore is joined in service with clinicians and through this collaboration we are improving lives. goremedical.com/eu
■ Medtronic
400
We lead global healthcare technology, boldly attacking the most challenging problems. Our mission—to alleviate pain, restore health, and extend life—unites a global team of 90,000+ people, and our technologies transform the lives of two people every second, every hour, every day. Expect more from us. Medtronic. Engineering the extraordinary. medtronic.com
■ Cook Medical
310
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today, we are combining medical devices, biologic materials, and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: our patients, our employees, and our communities. cookmedical.eu
■ Terumo Aortic
410
At Terumo Aortic, we partner with our customers to revolutionise aortic care. We deliver innovation, versatility and precision with the broadest range of solutions that can be personalised for every patient. We are further complementing our implantable device portfolio through the development of digital technologies. terumoaortic.com
■ Terumo Interventional Systems
410
In peripheral interventions, Terumo Interventional Systems provides a full portfolio extending from access through interventions to closure. Our product range covers endovascular solutions for carotid artery stenting,
below-the-knee and femoropopliteal procedures, regardless of the access approach, femoral or radial. terumois.com
■ Lombard Endovastec
330
Lombard Medical joined the MicroPort Endovastec family in August 2024, rebranding as Lombard Endovastec, a proud subsidiary of MicroPort Endovastec. While our commitment to minimally invasive treatment of aortic disease remains unchanged, we now offer an even broader portfolio. Our innovative solutions include thoracic endovascular aortic repair (TEVAR), fenestrated endovascular aneurysm repair (FEVAR), and EVAR. lombardmedical.com
■ Penumbra, Inc 120
Penumbra, Inc, the world’s leading thrombectomy company, is focused on developing the most innovative technologies for challenging medical conditions such as ischaemic stroke, venous thromboembolism including pulmonary embolism, and acute limb ischaemia. Our broad portfolio, which includes computer-assisted vacuum thrombectomy (CAVT), centres on removing blood clots from head to toe with speed, safety and simplicity. penumbrainc.com
■ Shockwave Medical 320
Shockwave Medical is a company focused on developing and commercialising products intended to transform the way calcified cardiovascular disease is treated. We aim to establish a new standard of care for medical device treatment of atherosclerotic cardiovascular disease through our differentiated and proprietary local delivery of sonic pressure waves for the treatment of calcified plaque, which we refer to as intravascular lithotripsy. shockwavemedical.com
■ Boston Scientific
128
Boston Scientific transforms lives through innovative medical technologies that improve the health of patients worldwide. For 45 years, we have advanced science for life by
providing a broad range of performant and minimally invasive solutions to help physicians treating indications such as peripheral arterial disease, pulmonary embolism, and deep vein thrombosis. bostonscientific.eu
■ Cordis 124
Cordis is a worldwide leader in the development and manufacture of interventional vascular technology, with a reputation for clinical acumen, training and services. Our focus is on cardiology and endovascular platforms, with high-quality products such as diagnostic and interventional catheters, balloons, self-expanding stents, guidewires, vascular closure devices and drug-eluting balloons. cordis.com
■ Inari Medical 132
The company purpose-built its products for specific characteristics of the venous system and the treatment of the two distinct manifestations of venous thromboembolism: deep vein thrombosis and pulmonary embolism. In 2025, Stryker acquired Inari Medical, with the mission to treat and transform the lives of patients suffering from venous diseases. stryker.com
■ Siemens Healthineers
130
At Siemens Healthineers, we pioneer breakthroughs in healthcare. For everyone. Everywhere. Sustainably. We enable healthcare professionals to innovate personalised care, achieve operational excellence, and transform the system of care. Our portfolio, spanning in vitro and in vivo diagnostics to image-guided therapy and cancer care, is crucial for clinical decision-making and treatment pathways. siemens-healthineers.com
■ Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 114,000 colleagues serve people in more than 160 countries. abbott.com
■ AOTI, Inc
148
AOTI’s unique multimodality TWO2 therapy is randomised controlled trial (RCT) and real-world evidence (RWE) proven to heal chronic wounds more durably, thereby significantly reducing ulcer recurrence, hospitalisations, and amputations, while lowering the total cost of care. As a patient-applied,
at-home therapy, TWO2 improves patient access to care and improves quality of life. Our Nexa multi-patient, disposable negative pressure wound therapy (NPWT) system offers unparalleled simplicity, performance, and affordability. aotinc.net
Artivion is a leader in the manufacturing, processing, and distribution of medical devices and implantable tissues used in cardiac and vascular surgical procedures focused on aortic repair. We offer a suite of aortic-centric solutions such as cryopreserved cardiac and vascular allografts, surgical sealants, prosthetic heart valves, and aortic stents and stent grafts. Artivion has over 1,250 employees worldwide, with sales representation in over 100 countries. artivion.com
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. BD and its 75,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians’ care delivery process, enable laboratory scientists to accurately detect disease and advance researchers’ capabilities to develop the next generation of diagnostics and therapeutics.
bd.com
■ Bentley 112
Bentley’s passion is the development, manufacturing and distribution of innovative implants for minimally invasive treatments of vascular diseases. Since market launch in 2012 we rapidly expanded worldwide. Thanks to our international network of exclusive distribution partners, we are represented in more than 80 countries—in some we are already market leader.
bentley.global
■ BIBA News and Media E6
BIBA News and Media is the global, multimedia, multichannel broadcast and publishing division of BIBA Medical, producing five print and three digital-only titles for vascular, venous, cardiovascular, neurointerventional, interventional radiology, renal and cardiac rhythm management specialists. New for 2025 is our broadcast offering, based in our bespoke London studio. bibamedical.com
■ Bonesupport UK Ltd 104
Bonesupport AB is a Scandinavian orthobiologics company that develops
and markets Cerament, an innovative range of radiopaque injectable osteoconductive and drug-eluting bioceramic products that have a proven ability to heal defects by remodelling to host bone in six to 12 months. bonesupport.com
■ Brainlab 444
At Brainlab, we digitise medical workflows from diagnosis to therapy, and our innovative ecosystem forms the basis for modern healthcare technology. brainlab.com
■ CX Insights E6
Insights carries out market research, globally, in the medical device industry (particularly in the vascular and cardiovascular fields), with an extensive panel of healthcare professionals built from our proprietary work, news publications and educational conferences as well as a presence at all main symposia. bibamedical.com
■ Cydar Medical 440
Cydar Medical provides an endto-end integrated solution to transform minimally invasive imageguided procedures. Using artificial intelligence (AI) to advance surgical visualisation, Cydar Medical supports remote collaboration with the referral community, pre-procedure planning, interoperative guidance and postoperative Automatic Volume Assessment (AVA). Cydar Medical is headquartered in Cambridge, UK. cydarmedical.com
■ Endologix 342
At Endologix, we believe the pursuit of disruptive innovation requires unwavering persistence. Guided by data, we pioneer interventions that address persistent unmet needs in patients with vascular disease. We empower physicians underserved by current solutions so we can confidently redefine what’s possible, together. This is our promise. Embrace the evidence. endologix.com
■ GE HealthCare
110
As a standalone company, GE HealthCare is a leader in precision care, infusing innovation with patient-focused technologies to enable better care. We’re dedicated to providing integrated solutions that make hospitals more efficient, clinicians more effective, therapies more precise, and patients healthier. Together our imaging, ultrasound, patient care solutions, and
pharmaceutical diagnostics businesses help improve patient care from prevention and screening, to diagnosis, treatment, therapy, and monitoring. gehealthcare.com
■ Haemonetics 344
Our solutions, including blood management technologies and interventional technologies are designed to help solve the everyday challenges that hospitals face. These procedure-enabling technologies are backed by an experienced team to ensure seamless implementation, while helping to increase efficiency and cost-effectiveness across the care continuum. haemonetics.com
■ Huntleigh 100
A proud member of the Arjo family, Huntleigh is committed to supporting healthcare professionals in improving outcomes and enhancing patient wellbeing since 1979. With innovation and customer satisfaction as our guiding principles, we strive for clinical excellence and improved performance within vascular assessment and treatment as well as foetal and patient monitoring. huntleigh-diagnostics.com
■ InspireMD 200
InspireMD is the developer of the CGuard carotid embolic prevention stent system (EPS) for the prevention of stroke. InspireMD seeks to utilise its proprietary MicroNet technology to make its products the industry standard for carotid stenting by providing outstanding acute results and durable, stroke-free long-term outcomes. inspiremd.com
■ Laminate Medical Technologies W4
Laminate Medical Technologies is committed to advancing arteriovenous fistula (AVF) function for haemodialysis patients globally. Laminate’s flagship product, VasQ, is an extravascular support device designed to improve surgical fistula outcomes by providing permanent reinforcement to the arteryvein connection, promoting stability against wall tension, and decreasing turbulent flow. laminatemedical.com
■ LeMaitre 146
LeMaitre is a leading global provider of devices for the treatment of cardiovascular and peripheral vascular disease and for arteriovenous access. We develop, manufacture and market
a broad range of unique disposable as well as biologic and biosynthetic implantable devices to address the needs of vascular and cardiac surgeons and interventionalists. lemaitre.com
■ LifeTech Scientific 136
LifeTech Scientific is committed to the research and development, manufacture, and sale of minimally invasive interventional medical devices for cardio-cerebrovascular and peripheral vascular diseases, with over 25 years’ experience and network penetration in nearly 120 countries and regions around the world. lifetechmed.com
■ LSO Medical 142
Driver of innovation in the treatment of venous pathology, LSO Medical is a French company specialised in the design of vascular lasers for over 20 years. Serving patients is a privilege, for which we demand the highest standards of quality and ethics. These requirements are applied to all of the company’s activities: design, manufacture and sale of our products. LSO Medical improves the quality of life of patients, by constantly optimising existing treatments. lsomedical.com
■ Merit Medical 348
Merit Medical offers an integrated portfolio of products designed to support your vascular surgery procedures, dialysis, peripheral vascular interventions, interventional radiology, and cardiology. We bring you innovative solutions for vascular challenges. Join us during our satellite symposium and CX hands-on workshops to learn more. merit.com
■ Nectero Medical
Nectero Medical is a clinical-stage biotech company pioneering novel therapies with a potential to treat aneurysmal disease and improve patients’ lives. The company is led by a highly accomplished, multidisciplinary management team with input from an experienced board of directors and renowned physician leaders in vascular surgery and interventions. necteromedical.com
■ optimed Medizinische Instrumente 144
As a German manufacturer for self-expanding nitinol stents with a patient-centric mindset, we stand for high quality and innovation in stents. Our deep expert knowledge in vascular surgery and great partnerships with surgeons worldwide help us to create unique products such as life-saving ductus stents for infants or special venous stents, like the Sinus-Obliquus. Our latest addition to the wide portfolio
is the Tentos 4F, a chronic outward force (COF)-adjusted arterial stent for infrapopliteal and superficial femoral artery (SFA) applications. optimed.com
■ Philips E4
At Philips, our purpose is to improve people’s health and wellbeing through meaningful innovation. We offer one of the broadest portfolios of interventional solutions in the industry, helping clinicians treat patients more effectively and efficiently. Only Philips offers the powerful combination of advanced imaging and specialised treatment options designed to accurately assess inside the vessel, successfully select the right treatment algorithm, and optimise outcomes for your patients. philips.com
■ PrediSurge 208
PrediSurge develops predictive software solutions for cardiovascular interventions that allow physicians and MedTech companies to improve procedural planning and medical device designs. Based on preoperative imaging, our technology enables the creation of patient-specific digital twins: numerical models simulating arteries and valves’ biomechanical behaviour. This solution allows physicians to optimise intervention strategy, by choosing a graft adapted to each patient and anticipating potential operative complications. predisurge.com
■ Scanlan International 108
The world’s finest surgical instruments designed and manufactured by the Scanlan family since 1921. Experience the Scanlan difference at Exhibit #108. scanlaninternational.com
■ Sentante E3
Sentante is empowering physicians with its tele-operated endovascular robot system to enable accuracy and precision, stability and safety, and X-ray radiation elimination for medical personnel—to transform the lives of millions of patients and providers. Sentante is setting the stage for a new era in robotic, accessible, digital, and connected healthcare. sentante.com
■ Shape Memory Medical 103
Shape Memory Medical is reshaping clinical success through the science of smart polymer. Smart polymer upgrades device performance and redefines embolisation possibilities. Our conformable smart polymer delivers unparalleled volume, returns imaging clarity, and promotes healing as the material absorbs. We continue to drive a cross-specialty portfolio to meet procedural demands. shapemem.com


■ Teleflex E5
We believe that the future of healthcare is in the people we empower. Teleflex is a global provider of medical technologies designed to improve the health and quality of people’s lives. We apply purpose-driven innovation—a relentless pursuit of identifying unmet clinical needs—to empower healthcare professionals, who constantly strive to create a healthier tomorrow. teleflex.com
■ ThinkSono W6
ThinkSono guidance enables any non-ultrasound-trained healthcare professional to scan for blood clots (deep vein thrombosis; DVT). It reduces waiting times, negative DVT cases and can be used at the point of care. thinksono.com
■ Vascular Technology, Inc 448
Vascular Technology, Inc (VTI) has been manufacturing high-quality surgical devices for over 30 years. Our TQI Doppler system is designed specifically for use in vascular surgery. By
optimising the Doppler signal for both intraoperative and transcutaneous assessment, our single-use probes allow for reliable evaluation of the vasculature from start to finish with one device. vti-online.com
■ VentureMed
350
VentureMed develops devices for endovascular therapies in patients with arteriovenous (AV) fistulas, graft stenosis, and peripheral disease. The Flex Vessel Prep system is used in kinetic endovascular micro-incision creation (KEMIC) procedures and targets optimising vessel preparation, reducing trauma, extending patency, and contributing to improved patient outcomes. Based in Minneapolis, USA. venturemedgroup.com
■ Versono Medical W5
Versono Medical, a Galway-based MedTech company, is advancing a breakthrough platform technology for endovascular treatment of complex chronic lesions in critical limb ischaemia (CLI). Now in pivotal trials in the USA,
it is designed to overcome severe occlusions, enabling revascularisation and improving outcomes for patients at high risk of amputation. versono.life
■ Vexev W13
Vexev is an Australian MedTech startup company that has developed robotic tomographic ultrasound, a new imaging modality that is able to produce CTscan like images using ultrasound. The company is currently working on a vascular access application of this novel technology and will soon also expand into the peripheral arterial disease (PAD) space.
vexev.com
■ Veryan Medical
300
Veryan has developed innovative technology to improve the performance of vascular stents by adopting the principle of biomimicry; developing structures that imitate those occurring naturally. Veryan’s vascular biomimetic stent technology involves adapting a straight stent design to a threedimensional helical shape, which more closely mimics the natural geometry of the human vascular system. veryanmed.com
■ Wisepress Medical Bookshop W1
Wisepress.com, Europe’s leading conference bookseller, attend around 200 conferences every year. We have an extensive range of books and journals relevant to the themes of this conference available at our booth. We also have a comprehensive range of scientific, technical and medical (STM) titles available on our online bookshop. Follow us on X @WisepressBooks. wisepress.com
■ Ziehm Imaging
140
Ziehm Imaging is specialised in the development and manufacture of mobile C-arms. Since 1972, we have produced technologies that enhance imaging and streamline clinical workflows. Our devices’ exceptional image quality and flexibility in the operating room serve as an important basis for treatment success. ziehm.com







The last year of Vascular News profiles has seen key leaders in the field share important lessons and advice based on notable careers. This overview of their individual in-depth interviews is an annual look back at their insights and experiences.

INTERESTED IN MEDICINE FROM A YOUNG age, Bijan Modarai (London, UK) is now professor of vascular surgery at King’s College London and a consultant vascular and endovascular surgeon at Guy’s and St Thomas’ NHS Foundation Trust, co-managing one of the largest UK practices in complex endovascular aortic repair.
Speaking to Vascular News last summer, Modarai recalled that his interest in vascular surgery dates back to his first year of medical school. “I liked the idea of operating on blood vessels and the fact that vascular surgery was a technical and holistic discipline,” he said. “I found it an exciting discipline as a significant proportion of the work was done as an emergency and therefore success or failure was often instantly apparent. I did, because of this, worry in the early days that the demands of work may impact other aspects of life but in the end decided to do what I was passionate about and have never looked back.” Modarai also spoke about his career mentors and the best advice they shared with him. He noted: “I have had the fortune of several mentors who have taught me, led by example, and advocated for me. Kevin Burnand, my predecessor at St Thomas’ Hospital, taught me how to have exacting standards and care passionately for patients. He, together with Alberto Smith, a prolific non-clinical translational scientist, supervised my PhD. Rachel Bell,

“WE NEED TO continue to build the scientific evidence base for what we do,” Kevin Mani (Uppsala, Sweden) told Vascular News in a profile interview last September, underlining what he believes to be a key priority for vascular surgery in the years ahead. Research has been central to Mani’s career so far, forming a crucial part of his surgical residency and pathway into vascular surgery and culminating in his current role as professor and chief of vascular surgery at Uppsala University Hospital. Mani is also chair of the Swedish Vascular Registry (Swedvasc) and, from this vantage point, shared his opinion on some of the benefits of registry data in vascular surgery. He said: “Vascular surgery is a field that deals with various low-incidence diseases—not necessarily the rarest diseases in the world but rare enough to be difficult to study in randomised controlled trials. Swedvasc and international registries in Vascunet have contributed significantly to the vascular surgery evidence base, with data now available on the management of various scenarios including infected
who I met as a senior house officer when she was my registrar, taught me how to be a brave surgeon and, crucially, had my back in my first year as a consultant. Julian Scott suggested I apply to the British Heart Foundation intermediate fellowship scheme in 2010 and was always a sensible source of advice. Finally, Tom Carrell taught me complex endovascular aortic surgery and handed over the reins to the service at St Thomas’ when he left to found his startup company, Cydar.”
On vascular surgery more generally, Modarai highlighted some important developments that have shaped the field over the course of his career thus far.
The fact
that we are now on the cusp of incorporating the aortic valve into our repairs is highly compelling.”
“The application of complex endovascular techniques to every aortic territory has been a big advance and the fact that we are now on the cusp of incorporating the aortic valve into our repairs is highly compelling,” he told Vascular News
Continuing, Modarai added: “We are beginning to see the application of artificial intelligence (AI) to inform case selection, planning and postoperative surveillance. Current algorithms are crude, but in time I believe this approach will revolutionise how we treat the patient. We have also become better at searching for the evidence that supports our interventions. Hand in hand with this is a greater ethos of collaboration, where colleagues are open to joining forces nationally and internationally to accrue meaningful data.”
Modarai also highlighted his achievements as president of the British Society of Endovascular Therapy (BSET), outlined some of his and his research team’s current work on radiation and DNA damage, and pointed to the inaugural Interdisciplinary Aortic Dissection Symposium, which took place in September 2024. Modarai also shared some advice for medical students, advising them “don’t listen to the naysayers” and to choose their mentors well.
grafts and ruptured internal iliac aneurysms.
There is a strong interest internationally for registries with the availability of new technologies for the management of big data.”
“We have been able to establish evidence for new technologies (e.g. endovascular aneurysm repair [EVAR] for infected aneurysms), as well as the threshold for repair for some of the pathologies involved, in studies that would be extremely difficult to conduct in any other way because we cannot find large enough cohorts of patients. The registry serves both to evaluate practice and identify best practices, as well as offer the possibility of spreading that best practice to new units and establishing evidence for new techniques that are being introduced.
“There is a strong interest internationally for registries with the availability of new technologies for the management of big data. There is also an increasing number of good quality national registries established across Europe and indeed around the world. I believe we’ve only scratched the surface when it comes to large-scale registry data for both the evaluation of practice as well as for the monitoring of new device outcomes. Clearly there is a lot of future potential.”
Mani also shared in the interview one of his most memorable cases to date, recalling the successful treatment of a young patient with complex aortic disease—an experience that for him exemplified the value of continued technological innovation and teamwork in vascular surgery.
FOLLOWING HIS PRESENTATION IN 2023 OF 12-month results from the LIFE-BTK randomised controlled trial, Ramon Varcoe (Sydney, Australia) spoke to Vascular News late last year about this research as part of his wider career in vascular surgery so far. The vascular surgeon at Prince of Wales Hospital and full professor at the University of New South Wales also considered how the VERVE Symposium—which he founded and directs— has evolved since its first iteration 12 years ago, and reflected on how past experience in professional athletics has influenced his current work.
Speaking on the LIFE-BTK trial, Varcoe commented: “This is one of the landmark randomised controlled trials in our field for several reasons. The endovascular treatment of infrapopliteal peripheral arterial disease has struggled with durability, and recent trials aimed at demonstrating that new technologies could provide better patency than simple angioplasty have largely failed. However, LIFEBTK demonstrated a 30% risk difference in the primary endpoint, favouring the Esprit drug-eluting resorbable scaffold [Abbott] over angioplasty. This success led to US Food and Drug Administration (FDA) approval and subsequent commercial availability in the USA and parts of Asia, with more regions expected to follow. This development has the potential to revolutionise treatment options for patients with chronic limbthreatening ischaemia.”
On the evolution of the VERVE symposium, Varcoe shared that the meeting has “matured significantly” over the past 12 years. “What began as a small gathering of around 100 people at Coogee Beach has now evolved into the largest vascular and endovascular congress in the region. We draw faculty and delegates from across the globe, showcasing over 300 presentations and featuring more than 15 live cases. The event also has a vibrant social aspect, with a popular harbour ferry tour and a gala dinner with live music that everyone is welcome to enjoy.”
Varcoe had a previous career in professional athletics

HAVING INITIALLY BEEN GEARED TOWARDS a career in engineering, Vincent Rowe (Los Angeles, USA) was ultimately drawn to medicine’s dual offering of intellectual stimulation and interpersonal engagement. He overcame a fear of the sight of blood to pursue what has so far been a “deeply rewarding” career in vascular surgery, culminating in his current role as professor of surgery and division chief of vascular and endovascular surgery at the University of California, Los Angeles (UCLA).
Speaking to Vascular News at the beginning of this year, Rowe reflected on his career to date and shared his thoughts on the state of surgical education, multidisciplinary care, and vascular surgery’s identity.
Rowe has received numerous teaching awards over the course of his career so far. Sharing some reflections on the state of vascular education and training at present, he remarked: “I believe surgical education has never been stronger. For much of my career, teaching was primarily

My experience as an athlete has greatly influenced my career, making me a better surgeon.”
I hope the indispensable role of vascular surgeons in achieving better patient outcomes is fully recognised and appreciated in the future.”
and told Vascular News this had a marked impact on his career as a vascular surgeon. “Professional sports impart valuable lessons, such as the importance of hard work and dedication, the pursuit of excellence, and the drive to compete at your best every day,” he said. “They teach you how to turn failure into immediate learning opportunities for future growth, which is essential for continual improvement. Additionally, sports highlight the significance of camaraderie and relationships. My experience as an athlete has greatly influenced my career, making me a better surgeon.”
Outside of medicine, Varcoe noted that sports are still a significant hobby, sharing: “Water activities are a big part of my life; I love scuba diving, sailing, boating, and deep-sea game fishing.”
confined to intraoperative instruction or didactic lectures. Today, however, surgical educators have a wealth of tools at their disposal, allowing trainees to engage with simulators, cadavers, and review relevant content or questions via mobile educational platforms. For surgical training, I believe our integrated training pathway has been revolutionary in enhancing the depth of surgical training. Surgical education is in an excellent place.”
Rowe also spoke on his experience working in a multidisciplinary limb salvage team on a day-today basis. “At my previous institution,” he shared, “we developed a strong multidisciplinary team for limb salvage, with daily collaboration among most members of the team. At my current institution, the foundational elements for a highly effective limb salvage programme were already established before my arrival. We are now focused on refining and optimising our integration to enhance the impact and efficiency of the team in delivering the best possible patient outcomes.” Rowe added that preserving limbs has for a long time been one of his main research interests.
On some of the biggest challenges currently facing vascular surgery, Rowe highlighted the issue of identity and branding, which, he said, “has not yet reached a level that reflects our critical role in patient care”.
Rowe elaborated: “This lack of recognition has, at times, led to vascular surgeons being undervalued, both within the surgical community and by the broader healthcare system. Many complex operations simply wouldn’t happen without the assurance of vascular surgery expertise, whether for direct involvement or for providing essential support. I hope the indispensable role of vascular surgeons in achieving better patient outcomes is fully recognised and appreciated in the future.”
Randomised trials and registry data were two of several topics to be addressed during a recent Vascular News Live broadcast focused on the aorta.
PONDERING SOME OF THE MOST PRESSING questions currently facing the aortic specialty were Vascular News editorial board members Ian Loftus (St George’s University of London, London, UK) and Ross Milner (University of Chicago, Chicago, USA) together with Dittmar Böckler (University Hospital Heidelberg, Heidelberg, Germany), Colin Bicknell (Imperial College London, London, UK) and Tilo Kölbel (University Heart Center Hamburg, Hamburg, Germany).
The broadcast took place in the weeks following the passing of Janet Powell (see obituary on page 3), who dedicated decades of her life to evidence-based aortic care.
On the research legacy she leaves behind, Bicknell commented that Powell “produced some of the bestknown trials in aortic surgery that have shaped the way that we all practice now”.
In addition, Kölbel remarked on Powell’s ability to “honestly study a subject” without bias, focusing not on desired outcomes but instead on what was best for the patient.
Evidence was a key theme of the ensuing
discussion, with the panel first addressing three ongoing randomised trials looking at the benefits of early intervention for uncomplicated aortic dissection: EARNEST, IMPROVE-AD and SUNDAY.
Speaking on the USA-based IMPROVE-AD trial, Milner highlighted some enrolment challenges thus far—citing in particular the difficulties of enrolling patients in the appropriate period of time—before noting efforts that are underway to “streamline” the enrolment process moving forward.
Offering a similarly hopeful nod to the future, Böckler emphasised that the “beauty” of the currently running trials as compared to some predecessors is their “very broad” inclusion criteria, ensuring “many patients can be enrolled”.
Böckler did, however, echo Milner when he questioned the possibility of pooling data from the three trials given their use of different enrolment time intervals.
Bicknell recognised that the three trials “all do have their differences” including enrolment time intervals but shared that the teams have “spent a long time” ensuring their outcome measures and follow-up are
[Janet Powell] produced some of the best-known trials in aortic surgery that have shaped the way we all practice now.”
Colin Bicknell
US ARC uncovers ‘dramatic’ decrease in use of prophylactic
Despite a “dramatic” decrease in the use of prophylactic cerebrospinal fluid drains (CSFDs) for the prevention of spinal cord ischaemia (SCI) in patients undergoing fenestrated and branched endovascular aneurysm repair (F/BEVAR), the rate of SCI has tracked a similarly dramatic decrease, according to the senior author of a new analysis of US Aortic Research Consortium (ARC) data from 2011–2024.
THE RETROSPECTIVE analysis of the ARC registry—which encompasses 10 major medical centres with individual investigational device exemption (IDE) studies for complex aortic repairs—included 2,585 patients undergoing elective F/BEVAR. Eras of repair were divided into early (2011–2013), mid (2014–2021) and late (2022–2024) based on the publication of influential papers which changed ARC practices. Patient cohorts were separated by prophylactic (n=949), therapeutic (n=27) and no CSFD (n=1,609) use. A composite variable consisting of any SCI, major CSFD complication or intracerebral haemorrhage was designated as the primary outcome. The ARC data showed that 196 patients (7.6%) experienced the
primary composite outcome and 160 (6.2%) experienced SCI.
Presenting the data, vascular surgery resident Angela Sickels (University of Alabama at Birmingham (UAB; Birmingham, USA), told the 2025 annual meeting of the Southern Association for Vascular Surgery (SAVS; 22–25

January, St. Thomas, the US Virgin Islands):
“The yearly incidence of the primary composite outcome and any SCI gradually declined over time, from a maximum of 25% [for
aligned in the interest of pooling data along those lines in the future.
The discussion then turned to the benefits of large-scale registry data to help answer some of the questions that cannot be suitably addressed by randomised trials.
Loftus highlighted that registry data allow specialists to “refine” their practices and “get much better levels of granular patient data”. He referenced the work of the US Aortic Research Consortium (ARC) on complex aortic intervention in particular, praising its “robust” data collection.
Milner was similarly complimentary of the US ARC, especially regarding its data on spinal cord drainage. “That group of investigators has really been able to show pretty clearly the decrease in the paraplegia rate and also how they’ve changed their prophylactic use of spinal cord drainage,” he said, “which I do think is impacting a lot of our decisions for many of us and I think that’s been really one of the most outstanding parts of this work.”
Loftus pointed out that, while European specialists have had access to bespoke complex aortic devices for years, a US ARC equivalent has not yet materialised on the other side of the Atlantic. “I think that’s a shame,” he commented, “but maybe we still have opportunities to do that, to get really robust data.”
On this point, Kölbel shared with the panel that a transatlantic collaboration between US ARC and European investigators is currently underway, which hopes to provide further strong evidence on complex aortic interventions.
To view the full discussion—which also spanned the thresholds and indications for intervention, the decreasing prevalence of aneurysmal disease, and stroke prevalence with thoracic endovascular aortic repair (TEVAR)—visit Vascularnews.com/videos.
both primary composite and SCI] in 2011 to 2.8% and 2.3%, respectively, in 2023.”
Meanwhile, the use of prophylactic CSFD declined from “being essentially universally done” in 2011 down to just 10.9% in 2023 “without any substantial increase in therapeutic CSFD use, which reached a maximum of just 3.5%,” Sickels said.
In high-risk patients (n=1,026), 12.9% (n=132) and 10.6% (n=109) experienced the primary composite outcome or any SCI event, respectively, the data revealed. Rates of the primary composite outcome declined from 38.5% in 2013 to 3% in 2023. Prophylactic CSFD use in high-risk patients—while nearly universal (92.9–100%) until 2016—
The yearly incidence of the primary composite outcome and any spinal cord ischaemia gradually declined over time.”
Angela Sickels
has also since been on a continuous decline, reaching a minimum of 22.6% in 2023, the research shows. “This subset of patients also saw no increase in therapeutic drain use, reaching a maximum of 5.9%,” Sickels added.
Senior author Adam Beck (UAB; Birmingham, USA) described the data as likely practice-changing for many specialists who carry out complex repairs of thoracoabdominal aortic aneurysms (TAAAs) ahead of their presentation at SAVS 2025.
Speaking to Vascular News about the significance of the findings, Beck noted how as experience of endovascular long-segment coverage of TAAAs over the last decade has expanded, discussion and education around SCI prevention protocols, too, have broadened at most large medical centres.
“With implementation of defined protocols, the rate of SCI has dropped significantly over the last five or 10 years,” he said.
The ARC data, Beck said, show a “rapid drop, especially over the last eight years or so, in the rate of SCI. In addition, interestingly people have been using less and less prophylactic spinal drains, and we think that that’s probably because the rate of SCI has dropped with the use of SCI prevention protocols and is almost equivalent to the risk of the placement of spinal drains.”
The dilemma of a narrow aorta in F/BEVAR: A matter of planning and technologies

Michele Piazza
Michele Piazza (Padua, Italy) advises how to manage the challenging anatomical scenario of a narrow aorta during fenestrated and branched endovascular aneurysm repair (F/BEVAR), highlighting accurate, patient-specific planning and use of the correct bridging stent technology as the keys to good results.
Endovascular treatment of complex abdominal aortic aneurysms (AAAs) and thoracoabdominal aortic aneurysm (TAAAs) with fenestrated and branched endografts, or F/BEVAR, today represents a valid alternative to open repair, thanks to its association with lower morbidity and mortality. Generally, these aneurysms are characterised by extensive involvement of the aorta, with visceral segment management being the real challenge. Some anatomical features such as a narrow or angulated aorta increase the complexities of repair, not only in terms of technical feasibility, but also in terms of target vessel complications and material resistance to fatigue.
The main issue with a narrow paravisceral aorta relates to the lack of space, which may lead to inadequate expansion of the main aortic endograft, as well as conflicts between or compression of the bridging stents. Also, aortic angulation is one of the major causes of target vessel instability, primarily in relation to kinking, and its association with a narrow paravisceral aorta may worsen outcomes exponentially.
To date, the only parameter that has defined a narrow paravisceral aorta is a transverse maximum diameter of ≤25mm, while a risky aortic angle is >45 degrees. However, other concomitant anatomical aspects may play a role in defining outcomes, including wall quality, narrowing extension, and the type of endograft and bridging stents used. We recently published our results demonstrating that in FEVAR, a narrow paravisceral aorta of <20mm in association with a >30 degree aortic angle or no vertical distance between the renal arteries was a predictor of target vessel instability and particularly that which causes occlusion. This is probably because a narrow paravisceral aorta can often create conflicts between the multiple flared segments of two (renal) or three (two renal and the superior mesenteric artery
above the coeliac trunk, compared to the longer coverage that would be required to allocate outer branches, thus exposing the patient to a lower risk of spinal cord ischaemia.
Other potential planning options, as well as new technological characteristics, are emerging in the market. For example, the ‘semibranch’, which is an intermediate solution between a fenestration and a branch, has possible applications in cases involving narrow and angulated aortas.
[SMA]) bridging stents protruding at the same level within the main aortic fenestrated endograft. This geometrical structure exposes the procedure to the risk of lower technical success and worsens short-term outcomes, caused by inadequate flaring of one or more bridging stents, or stent compression or collapse during other concomitant endovascular manoeuvres, such as the deployment of a distal bifurcated device. Today, several custom-made device conformations are available for elective complex AAA cases, and the possibility of switching a plan from a four-fenestrated graft to a combination of fenestration and inner branches may help to overcome this complex anatomic dilemma. A case example is depicted in Figure 1. In the case of a complex AAA with a narrow pararenal aorta and an SMA and renal arteries originating at the same level, the use of two fenestrations for the coeliac trunk and SMA and two inner branches for the renal arteries allow for the spatial differentiation of the inlet of the three vessels. The SMA will have a single flared bridging stent, protruding perpendicularly from the anterior wall of the main graft as usual, while the two renal arteries will have their inlet 2cm above, at a 90-degree clockwise position, thus avoiding cumbersome protrusion conflicts.
As previously reported by other expert centres, a potential issue in using inner branches for the renal arteries may be the higher risk of instability. For this reason, it is mandatory to use patient-specific, custom-made devices with dedicated planning, with the ostium of the renal arteries positioned in the lowest part of the diamond, to favour a smooth bridging stent transition. Also, it is of paramount importance to optimise the choice of bridging stents, using either self-expanding, balloonexpandable, or a combination of both, depending on the specific anatomical course.
In this conformation, the use of inner branches for the renal arteries also allows for a short covering length
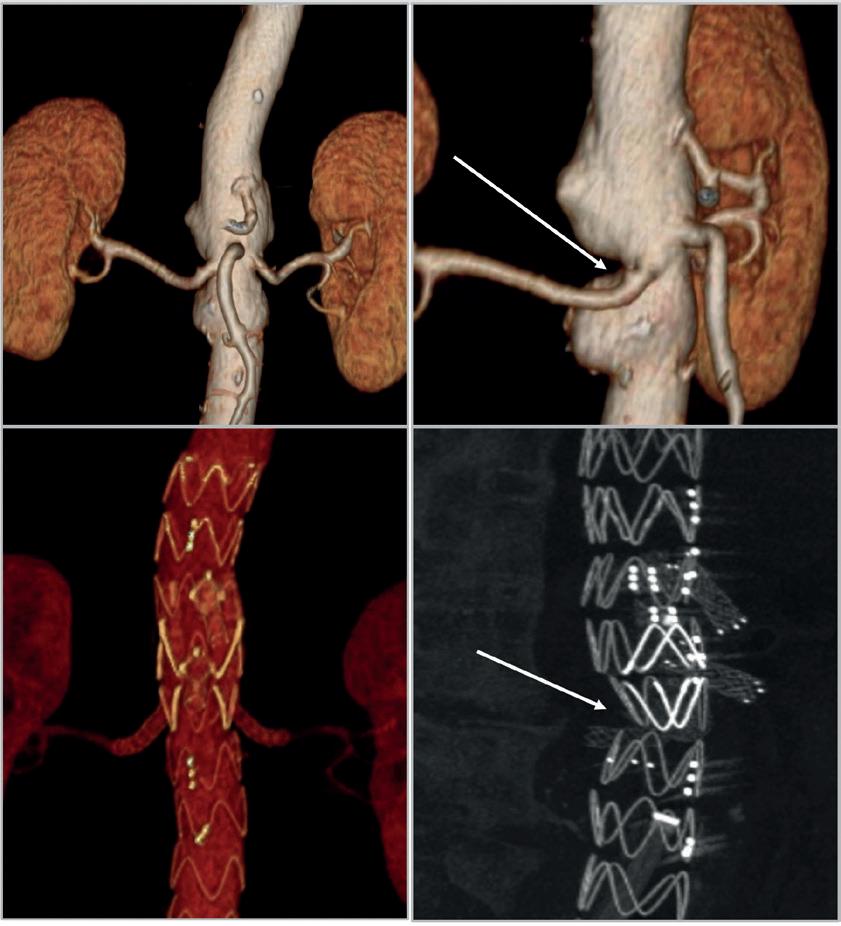

Today, FEVAR and BEVAR are both feasible in a narrow paravisceral aorta and overall provide satisfactory results.”
In extent type II and III TAAAs, which generally carry a large aneurysmal sac, planning may include several possible custom-made device conformations using fenestrations, outer branches or inner branches. To be clear, outer branches have been demonstrated to work in both large and narrow aortas with good outcomes. In particular, the use of off-the-shelf inner or outer branches is feasible with any aortic diameter, even if a theoretical landmark is historically set for outer branches at 25mm.
In our study, a narrow paravisceral aorta by itself was not a problem for outer branches; the only condition that increased the risk for occlusion was the presence of a long-segment longitudinal
extension of the narrow aorta or a narrow aorta with circumferential wall calcifications. This is clearly because there is a fixed narrow space with no adaptability for complete outerbranch expansion or multiple-branch course. When planning, do not focus on the narrowness; instead, consider the quality of the wall in the narrow region. Frequently, the narrow inner lumen represents a virtual space, with the surrounding area occupied by soft thrombus material. In terms of
switch from a four-fenestrated graft to a combination of fenestration and inner branches
Michele Piazza is a professor in vascular surgery at the University of Padua (Padua, Italy). He reports that he is a consultant for Cook, Artivion and WL Gore, with all fees paid to the Department of Cardiac, Thoracic, Vascular Science and Public Health at the University of Padua. Point
the combined use of both self-expanding and balloon-expandable stents
technology, preloaded branches may have a role; these may help in a narrow area to make catheters progress with more support.
Using a main graft with nitinol stents in the narrow area may increase conformability and be able to surround the space where branches lie rather that compressing them. What about the right bridging stent? Plan which one to use, rather than working with what you have in stock that day. Self-expanding stents guarantee adequate distal adaptability and transition to target vessel, as well as smooth conformation in cases involving tortuosity or angulation. On the other hand, balloonexpandable stents may allow for the maintenance of an adequate diameter in a severely angulated narrow aortic region, thanks to their strong radial force, and sometimes the combination of both may be useful for success (Figure 2). So, please anticipate how your main graft, branches, and fenestration will conform to the narrow anatomy and plan accordingly.
Today, FEVAR and BEVAR are both feasible in a narrow paravisceral aorta and overall provide satisfactory results. In this complex anatomic scenario, accurate patient-specific planning with both custom-made devices and off-theshelf conformations—as well as use of the correct bridging stent technology— are mandatory to ensure a higher rate of success and long-term durability.
A new initiative from Abbott hopes to increase awareness of peripheral arterial disease (PAD) in the UK, with the aim of preventing unnecessary amputations. Samih Al Mawass, divisional vice president of Abbott’s vascular business for Europe, the Middle East and Africa, speaks to Vascular News to discuss the origins, execution and future of the project.
TENS OF THOUSANDS OF amputations are carried out in Europe each year, Al Mawass shares, with the number increasing annually. He adds that diabetes is a significant cause of amputations, even though many foot amputations due to diabetes are thought to be preventable, which “comes at a huge financial, but more importantly emotional, cost”.
To highlight the scale and urgency of the issue, Abbott devised AmpuNATION—a campaign centred around a series of six portraits, shot by iconic photographer Rankin, of people living with amputation.
The portraits accompany an Abbottfunded report on the economic impact of limb salvage, authored by Athanasios Saratzis (University of Leicester, Leicester, UK) and colleagues, that was recently published in the British Journal of Surgery
On the origins of the campaign, Al Mawass notes that the idea was to do something different. “Abbott is not new to the field,” he says, “and we have been trying the usual ways of building awareness through the traditional channels, but unfortunately we have only seen baby-step improvements.”
It was also important for Abbott to reach a wider target audience than it had done with previous campaigns,
Al Mawass continues. “In the past,” he recalls, “we were trying as much as possible to build the awareness of vascular surgeons, and we saw that this was not enough, because if it was, we would not see the number of amputations increasing.”
AmpuNATION represents “a wake-up call for all stakeholders,”
Al Mawass says, from patients and physicians to healthcare systems, industry partners and societies. This multi-stakeholder approach, he explains, is necessitated by the complex, threefold nature of the problem at the campaign’s core. The issue of late referral and assessment calls for patient awareness, while a lack of standardised management for chronic limb-threatening ischaemia (CLTI) patients requires awareness among the physician community, and a lack of resources warrants the involvement of decision makers and the wider healthcare system.
Collaboration between stakeholders is also crucial, Al Mawass points out, commenting: “It is really all stakeholders that need to put their heads together”. Remarking specifically

Wyles, whose portrait features in the AmpuNATION series, attends the campaign launch at the UK National
on the importance of cooperation between industry partners, he says: “Abbott can make this buzz that you are seeing now, we can build this to a certain extent, but if we don’t collaborate with all industry players that are concerned and that can help in the treatment [of PAD patients], we will not achieve the result that we aim for, which is to ensure that these patients, the moment they have symptoms, they go into the right channel to be diagnosed and given the right treatment.”
And it is the patients and their stories that are at their centre of this project, Al Mawass stresses. “What we tried to do through the campaign was to have people share their own stories,” he says. For example, one of the portraits in the campaign is of a surgeon who, Al Mawass shares, “was trying to delay facing reality as much as possible when he first got symptoms” and “unfortunately, paid the price”. “There is no better way for a patient to speak to another patient than to tell them to go and have your diagnosis early because if not, you’re going to have what I’m having. Your life is going to change. You’re going to be in a very difficult situation that will impact not only your life

A US$5 million gift from Lorraine and Bill Dodero is set to establish the eponymously named Lorraine and Bill Dodero Limb Preservation Center at University Hospitals (UH) Harrington Heart & Vascular Institute in Cleveland, USA. Led by interventional cardiologist Mehdi Shishehbor, who is a pioneer in the field of limb preservation, the Dodero Center aims to revolutionise care for patients at risk of amputation due to peripheral arterial disease (PAD) and diabetes.
“AMPUTATION STATISTICS ARE BLEAK, and amputation should be a last resort,” said Shishehbor, president of UH Harrington Heart & Vascular Institute, and Angela and James Hambrick chair in innovation at UH. “This is why we are passionate about pursuing alternatives to amputation for our patients. When we save a limb, we save a life. We are deeply grateful to the Doderos for their heartfelt recognition and unwavering support of this worthy mission.”
The Lorraine and Bill Dodero Limb Preservation Center will aim to lessen the burden of amputation by creating a national destination for limb preservation care; discovering new and uninvestigated treatments for chronic limbthreatening ischaemia (CLTI) and diabetesassociated arterial diseases; educating more physicians at every level on innovations in vascular health and revascularisation; and ensuring patients facing amputation at UH and beyond know they
have options.
but the life of everybody around you.”
Al Mawass also touches on the fact that PAD does not affect society equally. “Certainly, parts of society are more impacted than others,” he remarks, citing differences in PAD prevalence and outcomes along lines of sex, race and ethnicity, and socioeconomic status. Indeed, statistics from a 2022 Life (Basel) paper by Horváth and colleagues, highlighted on Abbott’s AmpuNATION webpage, show that women who undergo an amputation experience a faster decline in quality of life than men and black patients face a 37% higher risk of amputation than white patients.
However, Al Mawass is also keen to point out that “the problem exists all over” and that society as a whole is “still behind from an awareness perspective”.
Reactions to the initiative have so far been positive, Al Mawass says in closing, outlining plans to take the campaign to other European markets following its UK launch. While acknowledging that these are still early days, Al Mawass is hopeful that the campaign will contribute to meaningful change. “I believe that we are definitely on the right track to build the awareness that we want to achieve.”
Shishehbor has contributed to multiple societal guidelines, published more than 200 manuscripts on this topic, and has trained hundreds of physicians worldwide. He has also served as co-principal investigator for the PROMISE II US pivotal clinical trial, which found the novel LimFlow therapy enabled most patients to avoid amputation.
“UH is committed to improving the health of all people by advancing science and human health,” said Shishehbor. “Statistically, amputees are disproportionately of lower socioeconomic status and African Americans are up to four times more likely to undergo an amputation than white Americans. All people should have the opportunity to receive the necessary medical resources and support for a healthier life, regardless of their status in the community or their race.”
As part of the new centre, UH will also coordinate an annual international conference for limb preservation in Cleveland, sharing advances and best practices with experts across the globe.
Leaders in the vascular field confronted a series of hot-button issues on peripheral arterial disease (PAD) and chronic limb-threatening ischaemia (CLTI) during a recent roundtable discussion. Vascular News editorial board member Robert Morgan (St George’s University Hospitals NHS Foundation Trust, London, UK) moderated the panel comprising fellow editorial board member Eric Secemsky (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, USA) and Marianne Brodmann (Medical University of Graz, Graz, Austria), Naseer Ahmad (Manchester University Hospitals NHS Foundation Trust, Manchester, UK), Thomas Zeller (University Heart Centre Freiburg-Bad Krozingen, Bad Krozingen, Germany) and Brian DeRubertis (NewYork-Presbyterian and Weill Cornell Medicine, New York, USA).
THE DELUGE OF DRUG-RESORBABLE scaffold trials underway, the relative importance of various antiproliferative agents such as paclitaxel and sirolimus, options for vessel preparation—and whether these make a difference to outcomes—as well as identifying the most important factors that can turn the dial down on amputation rates came under the scanner in this intense, controversial style of discussion that is “the trademark” of the Charing Cross Symposium.
Are disappearing scaffolds able to go the distance?
While DeRubertis set the scene for the enthusiasm surrounding the drug-resorbable scaffolds in the wake of the two-year LIFE-BTK clinical trial that demonstrated the sustained benefits seen with the Esprit BTK everolimus-eluting resorbable scaffold system (Abbott Vascular) in patients with the most severe form of below-the-knee (BTK) PAD, Zeller counselled vigilance on their effectiveness in long lesions.
DeRubertis highlighted the number of failed trials below the knee, and “general sense of malaise” that had accompanied endovascular treatment in this domain noting that “everyone felt we really needed a win for the space. And that’s where the LIFE-BTK trial came through” making room to usher in a number of other iterative devices coming down the pipeline. Are these going to be great solutions for BTK disease, enquired Morgan, with Zeller retorting that he was not yet “fully convinced” the future of BTK treatment will be to implant scaffolds
Zeller said: “[...] Even if it’s exciting to see the outcome of this particular trial, LIFE- BTK, it has to be shown that it’s effective also in longer lesions.”
The panel agreed that this trial is a first step in terms of this technology and to watch what the right long-term play for patients is.
Devoting dialogue to drugs and coatings
Brodmann opened the next segment by noting that limus-based coating “made a step forward” during the paclitaxel safety controversy, but how they fit into clinical practice remains to be determined since harms associated with paclitaxel have since been laid to rest. She did not see a defined need to “emulate the success” of paclitaxel-coated devices in the above-the-knee (ATK) segment, as this segment is dominated by paclitaxel DCBs. However, in the BTK space “where we do not have a really big RCT [randomised controlled trial] for paclitaxel-coated devices, […] I think there might be space, to evaluate this kind of technology.”
The issue, she put forth, is that with limus-coated devices there is a challenge in producing an efficient
balloon design to bring sufficient drug into the vessel wall. The panel agreed that the paclitaxel safety controversy propelled the limus conversation forward, and that there was potentially a little bit of a fishing expedition in terms of trying to find the right agent to match the disease state. Referencing the SELUTION4BTK RCT (Cordis) and other upcoming trials, they were optimistic about some of the properties of sirolimus such as a lowering of distal particulate embolisation issues.
The verdict was a call for more high-quality data in the domain and that “without a crystal ball” the right drug for BTK devices is currently unknown.
Vessel preparation and crackdown on calcium
The mechanical background for failure, with its high prevalence in BTK disease, and the lens of costeffectiveness became the cornerstones for discussion on the potential and relevance of vessel preparation strategies.
Morgan to conclude that the panel and Charing Cross “certainly want to focus on evidence, not just innovation for the sake of innovation.”
Strategies to lower “unacceptably high” rates of the ‘one-stop chop’ Morgan then turned to Ahmad to “tell us how we can save more legs from amputation” both in and outside of hospitals.
In the former scenario, Ahmad spotlighted the importance of “multidisciplinary teams where the service offers everything that is on offer”.
“Often people will just provide services that they can actually provide as opposed to having a team that can find all the services and then choosing the right technology [approach] for the patient themselves. So, I think having a highly functioning team that is able to offer those [the full spectrum of endovascular and surgical] services is really important,” he said. “But I think where the real difference is going to be made is outside of hospital. And that is about getting patients early in their disease. […] Often if these patients have ulcers, the prognosis is worse than with many cancers.”
Tracing the success of the story in Greater Manchester where the rates of amputation have dropped over the course of six years due to very specific interventions, he also called out the variation in different parts of the country admitting that “the story is very different” with amputation rates going up elsewhere.


Zeller posited that “severe calcification” was an obstacle to adequate biological efficacy, which invited a number of different approaches under development or already introduced into the market for vessel preparation including specialty balloons, atherectomy and intravascular lithotripsy.
Again, the panel cracked down on the early, singlearm and underpowered evidence underpinning the case for vessel preparation. New developments such as laser lithotripsy (THOR IDE trial, Philips), the Serranator cutting balloon (Cagent Vascular) and Spur self-expanding scaffold system (Reflow Medical) were mentioned in the mix alongside more established technologies.
When asked by Morgan on what he would do to take the argument forward for vessel preparation “if he had endless money and the world at his disposal”, Zeller remarked that “First of all, I don’t believe that vessel preparation as a standalone therapy will solve our problem of the durability of our peripheral interventions. It has to be in conjunction with something [definitive treatment] whether with drugcoated balloon [DCB] following the ‘leaving nothing behind’ concept or improving the environment for stent implantation for better stent expansion, [and] with this also improving stent patency. And we need definitely these trials showing that vessel preparation does improve the outcome,” he said leading
At a strategic level, Ahmad pinpointed the need for focus on not just diabetic amputations, despite these being tremendously important, but to also take non-diabetic amputations seriously as the disease appears to behave differently in Black, white and Asian populations.
“We know that half of all major amputations are in people who do not have diabetes, and 30% of minor amputations are in people who do not have diabetes. The path to an amputation begins with an ulcer. And whether you are diabetic or not diabetic, the treatment is the same,” he said.
“You need multidisciplinary teams. You need fast access to expert assessment. You need offloading, antibiotics, you know, good infection control, good vascular supply. If you are diabetic, you get access to these services really quite quickly. It is set up for people with diabetes. But if you do not have diabetes, you do not have access to these services. I think that’s one big difference that can be made, out in the community,” Ahmad reiterated.
Upcoming data from the BASIL-2 and BEST-CLI RCTs
Secemsky closed off the discussion with a finger pointing to the data to be presented at the Charing Cross Symposium (23– 25 April, London, UK) from the two landmark RCTs on angiographic scoring, cost-effectiveness data and a deep dive into a subset analysis, by stating: “We have had a really rich conversation about what is the right primary strategy for our patients. And I think that this conversation is ongoing. There is no one, perfect solution for every patient […] there is no one right strategy for every patient and lesion type. Evidently, this requires a close eye and a multidisciplinary approach. Hopefully that is where the whole field will move as these data evolve.”
Thirty-day results from the ROADSTER 3 study have demonstrated that transcarotid artery revascularisation (TCAR) is a safe and effective approach in patients who are deemed to be at ‘standard risk’ of experiencing adverse events related to carotid endarterectomy (CEA). These findings were presented at VIVA 2024 (3–6 November, Las Vegas, USA) by Meghan Dermody (Penn Medicine Lancaster General Health, Lancaster, USA), who—speaking recently to Vascular News—highlighted the importance of being able to “quote stroke rates that go beyond registry data” for this patient population.
“Since we are unable to conduct an adequately powered randomised controlled trial to study TCAR against other modalities to treat severe carotid stenosis, and especially without a TCAR arm in the forthcoming CREST-2 trial, it is imperative that we study TCAR in a standardrisk population—ideally in a prospective fashion,” Dermody said. “ROADSTER 3 is an FDA [US Food and Drug Administration]-required, post-approval study, but it allowed us the opportunity to acquire data from across the USA with multiple generations of surgeons performing the procedure.”
type II or type III aortic arch, and 17.2% of lesions had severe calcification.
atheroma; any thrombus burden within the plaque; arterial wall inflammation; and a slew of other anatomic features, should truly be what we base our shared decision-making on in the future. This requires thorough scrutiny of preoperative imaging, specifically an adequately thin-sliced and wellwindowed CT [computed tomography] angiogram.”
Dermody and colleagues also note that symptomatic status did not appear to have a statistically significant bearing on the 30-day incidence of stroke/death/ MI, which occurred at a rate of 1% in asymptomatic patients (n=3) versus 0% in symptomatic patients (n=0) in ROADSTER 3’s ITT population, and 0.7% (n=2) versus 0% (n=0) in its per-protocol analysis.

Touted by Silk Road Medical (Boston Scientific) as the firstever prospective, multicentre trial evaluating the safety and effectiveness of TCAR using the company’s Enroute transcarotid stent system (TSS) in conjunction with its Enroute transcarotid neuroprotection system (NPS) for the treatment of carotid stenosis in standard surgical risk patients, ROADSTER 3 enrolled a total of 344 patients across 53 US sites for intention-to-treat (ITT) analyses. The single-arm study’s primary endpoint is a composite of major adverse events (stroke, death or myocardial infarction [MI]) through 30 days post-procedure, plus ipsilateral stroke from day 31 to day 365 post-procedure, while incidence of cranial nerve injury (CNI) within 30 days post-procedure is described by the authors as a key secondary endpoint.


The rate of stroke/death/MI at 30 days in the study’s ITT population was 0.9%—a figure that decreased to 0.6% within per-protocol analyses involving 320 patients. The researchers found that 30-day stroke rates specifically were the sole contributor to these numbers, with no deaths or MIs being reported through the 30-day follow-up. They further relay that the incidence of CNI within 30 days was 0.6% in both the ITT and per-protocol analyses, and that all cases in both of these investigations were resolved within six months.

According to Silk Road, this constitutes—to date—the lowest reported rate of adverse event outcomes in a population of standard surgical risk patients treated via carotid revascularisation. The company also claims that, in line with the previous ROADSTER and ROADSTER 2 registry studies, ROADSTER 3 demonstrates consistently low adverse event rates across all risk levels.
“ROADSTER 3 was not powered to study differences amongst patients based on presenting symptoms, so clinical relevance is difficult to estimate,” Dermody commented. “However, based o n multiple previous publications and my own personal experience in practice, the vast majority of patients who present with symptomatic carotid disease are not on best medical therapy at the time of presentation. Adequate management of their risk factors perioperatively is more likely to result in good outcomes, regardless of preoperative
There aren’t enough data regarding surveillance of carotid stents, how or when to treat recurrent carotid stenosis within a stent, or long-term outcomes from TCAR yet.”
In ROADSTER 3’s ITT population, 75.3% of patients were younger than 75 years of age, 42.7% were female, and 16.3% had symptomatic carotid stenosis, with a quarter of these symptomatic patients experiencing a neurologic event within the two weeks preceding their TCAR procedure. Dermody and colleagues report that the mean lesion length was 23.3mm, while 47.4% of patients presented with a
“Given TCAR is less invasive than CEA and has similar stroke rates with less nerve injury risk, if a patient is able to take dual antiplatelet and statin therapy, and their anatomy is amenable, there is no reason why TCAR shouldn’t be your first-choice modality,” Dermody told Vascular News, outlining how the minimally invasive procedure may fit within existing carotid revascularisation paradigms. “But, given the anatomic and physiologic requirements needed for a successful TCAR, naturally, not all patients will be good candidates for this approach.
“Personally, I believe the treatment of carotid stenosis needs to be more focused on the lesion morphology rather than the degree of stenosis alone. The amount of calcification within or around the lesion; the amount of irregularity or ulceration to the
FFRCT diagnosis of coronary ischaemia with ischaemia-targeted coronary revascularisation more than halves five-year risk of cardiac death and MI following CEA
PREOPERATIVE DIAGNOSIS of lesion-specific ischaemia using coronary computed tomography (CT)-derived fractional flow reserve (FFRCT) and ischaemia-targeted coronary revascularisation after carotid endarterectomy (CEA) can reduce the five-year risk of cardiac death and
myocardial infarction (MI) by more than 50% and improve long-termsurvival, new data show.
The findings represent the latest results from Dainis Krievins and colleagues from Pauls Stradins Clinical University Hospital in Riga, Latvia, on the use of FFRCT in patients
symptomatic status.”
Discussing next steps, Dermody highlighted the five-year follow-up of these patients—as per a ROADSTER 3 sub-study. In addition, she stated that the future may offer opportunities to study Plavix (clopidogrel) resistance, which is considered a major contributor to post-TCAR stroke, as well as the option to utilise transcarotid flow reversal technology for intracranial neurointerventions.
“There aren’t enough data regarding surveillance of carotid stents, how or when to treat recurrent carotid stenosis within a stent, or long-term outcomes from TCAR yet,” Dermody added. “I look forward to seeing how best medical management compares to CEA and transfemoral carotid stenting in CREST-2.”
undergoing vascular procedures. The data were presented at the 2025 Southern Association for Vascular Surgery (SAVS) annual meeting (22–25 January, St. Thomas, the US Virgin Islands).
The single-centre observational study included 200 patients. Half received preoperative cardiac evaluation with FFRCT to identify asymptomatic ischaemia-producing coronary stenoses with ischaemia-targeted coronary revascularisation following CEA, compared to matched controls with standard preoperative cardiac evaluation and no elective coronary revascularisation. In the FFRCT cohort, lesion-specific ischaemia was defined as
FFRCT ≤0.80 distal to >30% stenosis, and severe ischaemia as FFRCT ≤0.75. Endpoints included all-cause death, cardiac death, MI, stroke and major adverse cardiovascular events (MACE) at five-year follow-up.
Krievins revealed that, at five years, there was a two-fold reduction in all-cause death in the FFRCT group compared to controls—24% vs. 11%. Annual mortality in the control group was 4.8% compared to 2.3% among the FFRCT cohort. Further, there was a four-fold reduction in risk of cardiac death, seven-fold reduction in the risk of MI, and three-fold reduction in MACE, he added. There was no difference in stroke rate.
A systematic review and meta-analysis of over 1,500 venous stenting procedures—said to be the first study on this topic to date—has highlighted an 18% drop in primary patency rates after one-year follow-up with declining rates beyond 12 months. As a result, researchers highlight the need for surveillance and consideration of reintervention to optimise long-term outcomes.
THE STUDY, PUBLISHED AS AN editor’s choice paper in the European Journal of Vascular and Endovascular Surgery (EJVES), aimed to appraise recent evidence assessing patency outcomes at various timepoints in patients with superior vena cava, subclavian, and brachiocephalic vein stenosis who had undergone stenting.
As a starting point, Shreya Chawla (Imperial College London, London, UK), Qingwei Zhang (St George’s University Hospital, London, UK), Adam Gwozdz (Imperial College London, London, UK) and colleagues, under the senior authorship of Stephen Black (St Thomas’ Hospital, London, UK), searched PubMed, Scopus, and Cochrane Library databases for studies up to December 2022. The researchers then measured outcomes including technical success rate, and primary,
primary-assisted, and secondary patency rates at various timepoints.
Chawla, Zhang, Gwozdz et al share that they included a total of 39 studies reporting outcomes in 1,539 patients in their meta-analysis.
The authors report that primary patency up to one year after a venous stenting procedure was 81.5%, declining to 63.2% at 12–24 months. Furthermore, they reveal that primaryassisted patency and secondary patency rates at 24 months and beyond were 72.7% and 76.6%, respectively.
The team also conducted subgroup analyses, in which they found no significant difference for pooled secondary patency rates when comparing the malignant and benign subgroups. However, they note that GRADE analysis determined the certainty of evidence for all outcomes
With the aims of predicting and comparing venous stent outcomes, aiding in communication with patients, and enhancing therapeutic decisionmaking, researchers have proposed an anatomical classification system for patients with chronic venous obstruction (CVO) of the iliofemoral tract undergoing interventional procedures.
IN A PAPER PUBLISHED IN THE EJVES, Houman Jalaie, Mohammad E Barbati (European Venous Center, Clinic of Vascular and Endovascular Surgery, RWTH Aachen University Hospital, Aachen, Germany) and colleagues outline the results of a retrospective, multicentre study on the newly proposed classification system.
The study analysed data from 13 vascular centres and included 1,033 patients with CVO who were treated between 2015 and 2019. They note that patients were classified into five category types: 1) non-thrombotic iliac vein lesion; 2) CVO of the iliac segment; 3) CVO of the iliofemoral segment above the common femoral vein confluence; 4) CVO of the iliofemoral segment extending into the femoral vein or deep femoral vein; and 5) CVO of the iliofemoral segment involving both the deep femoral vein and the femoral vein.
to be “very low”.
“Stenting is an effective intervention for benign and malignant stenosis of the superior vena cava, subclavian, and brachiocephalic veins,” Chawla, Zhang, Gwozdz and colleagues conclude. They reiterate that primary patency rates were “good” up to one year but declined after this timepoint.

“Importantly,” the authors continue, “the results suggest that reintervention before in-stent thrombosis significantly increases patency rates.”
Chawla, Zhang, Gwozdz et al note that there is a lack of high-quality evidence related to venous stenting outcomes, which necessitates further research. In particular, they stress that the merits of surveillance and reintervention programmes should be explored in the interest of bettering
Jalaie and colleagues detail that the mean age of the patients included in the study was 44 years, with just under 60% being women. They specify that a median of two stents was used for unilateral cases, while a median of five was used for bilateral cases.
Writing in EJVES, the researchers report that primary patency rates for types one to five were 94.9%, 90.3%, 80.8%, 60.6%, and 39.4%, respectively, at 12-month follow-up. “These rates were strongly correlated with the extent of CVO and showed significant differences between each type,” the authors comment.
In addition, Jalaie and colleagues reveal that univariable analysis identified predictors of primary patency loss as the type of CVO, history of deep vein thrombosis, and the total number of stents.

outcomes. “This review suggests that systematised strategies to monitor and follow up patients may help optimise long-term patency,” they write.
Chawla, Zhang, Gwozdz and colleagues acknowledge certain limitations of their systematic review and meta-analysis. They note, for example, that the 39 included studies comprised 36 case series and three cohort studies, which they recognise might have affected data quality, and that “significant data” were not reported in the included studies, such as a lack of data on the criteria for reintervention.

Highlighting some limitations to their study, Jalaie and colleagues recognise that its retrospective nature, the absence of a core lab for patency determination, and a lack of standardised outcome analysis such as post-thrombotic syndrome scoring, clinical venous severity scores, and quality-of-life scores, all impact the strength of their conclusions. Speaking to Vascular News following publication of the study, Barbati comments: “In essence, this classification system not only enhances the clinical management of patients with CVO but also contributes to the broader understanding and treatment of venous diseases, ultimately leading to improved patient care and health outcomes.”

In multivariable analysis, they continue, the significant independent predictors of primary patency loss were the type of CVO and the total number of stents.
“The decrease in primary patency over time based on the extent of pathology is indicative of the prognostic value of the proposed classification of the iliofemoral CVO in this analysis,” the authors write in their discussion.
Based on their findings, the authors conclude: “The proposed anatomical classification of iliofemoral CVO will help to predict intervention outcomes and facilitate comparison of stent outcomes in future studies.” However, they stress that “further evaluation and validation in prospective studies are needed to confirm the utility of this classification”.
The proposed anatomical classification of iliofemoral CVO will help to predict intervention outcomes and facilitate comparison of stent outcomes in future studies.”
Addressing the audience at the 28th European Vascular Course (EVC 2025; 9–11 March, Maastricht, The Netherlands), Ulka Sachdev-Ost (University of Pittsburgh, Pittsburgh, USA) set out a method to ensure scarce funding is directed to the most pressing venous disease research needs.
“AS EVERYBODY HERE IN THIS ROOM knows,” Sachdev-Ost began, “funding for research from a federal level—at least in the USA and I’m sure it’s similar in other countries—is actually pretty poor given the impact of venous disease for our patients and for the global population.”
Providing a more specific example, Sachdev-Ost homed in on chronic venous disease, which, the presenter shared, “affects quality of life for millions and millions of patients globally”. She also noted that the disease tends to be progressive and is associated with disability, depression, and immobility. “These are things that may not be life- or limb-threatening but certainly can affect how people are living their daily lives,” the presenter highlighted.
Sachdev-Ost also underlined a considerable financial burden associated with managing chronic venous disease, which is estimated to cost the US healthcare system US$15 billion per year in some analyses.
Despite these considerable patient and monetary costs, Sachdev-Ost pointed out that there is very limited research focused on chronic venous disease. Citing a PubMed search, the presenter noted that the term ‘chronic venous disease’ yields around 24,000 hits compared to 211,000 for ‘coronary artery disease’ and over three million for ‘cardiovascular disease’.
Against this backdrop, the presenter laid out her argument that venous specialists must “focus on identifying high-yield topics that would benefit from dedicated research dollars”. To do this, she proposed, venous research topics must be prioritised.
Sachdev-Ost highlighted the importance of multidisciplinary, multi-stakeholder projects to define research priorities, referring to the UK-based James Lind Alliance (JLA) as an example of such an initiative. “A big part of [the JLA] is to include
patients,” she said. “You must include stakeholders if you’re going to come up with the appropriate research priorities.”
The presenter also detailed a recent multidisciplinary, research priority-setting effort she was involved in with the American Venous Forum (AVF).
Sachdev-Ost shared that she was chair of the AVF research committee in 2024–2025 and was tasked with materialising an idea from the former chair to come up with research priorities in the management of C2 disease, which she presented at
disease and provide a foundation to design future research efforts,” she said.
The presenter explained that the forum comprised a 10-person multidisciplinary team from specialties including vascular surgery, interventional radiology, vascular medicine, and phlebology.
“We had a really, really good discussion,” SachdevOst recalled, with topics—determined based on multiple discussions with the group—focusing on varicose vein management.
“We ranked before and after the talk and found that really the aetiology of varicose veins and equity were some of our big components.”

Concluding, Sachdev-Ost shared that the next step would be to include patients in the discussion to ensure that all stakeholders are involved in setting research priorities related to venous disease.
A question from Stephen Black (Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, London, UK) during discussion time highlighted further the importance of SachdevOst’s point regarding patient involvement in setting research priorities.
Funding for research from a federal level is actually pretty poor given the impact of venous disease for our patients and for the global population.”
the recent AVF annual meeting (16–19 February, Atlanta, USA).
The aim was to approach research priorities in a “transparent” way, Sachdev-Ost told the EVC audience.
“We hypothesised that an open forum of brief presentations, open panel discussions and ranking surveys would identify scientific priorities in C2
The need for a revolutionary second generation of venous stents are among the gaps and unmet needs currently at play in deep venous surgery, the 2025 American Venous Forum (AVF; 16–19 February, Atlanta, USA) heard.
ERIN MURPHY (ATRIUM Health’s Sanger Heart and Vascular Institute, Charlotte, USA) was talking through the most common complications she deals with in practice as she identified a series of gaps in indications for venous stenting during a combined Society for Vascular Medicine-European Vascular Forum-AVF session at the AVF’s VENOUS 2025 annual meeting. Patients report with swelling and
lack of improvement, she told the meeting, and “most of the time, if we look at why, it’s because we’re treating physiologic but not pathologic compression.” Though less common at this juncture, Murphy said, stent migration represents the most dangerous of the complications venous specialists confront. Illustrating her point with a case example where a stent has migrated into the inferior vena cava (IVC), she
“In the broader context of prioritising venous disease against all the other disease states that we fight with,” Black stated, “the challenge we face is that the impact of death and limb loss for aortic and arterial disease seems to always outweigh the significant quality-of-life destruction that venous patients have, and it becomes hard to challenge that.”
Against this backdrop, he asked: “How do you go about doing that from a point of view of increasing the potential long-term impact of venous disease versus the other priorities?”
Sachdev-Ost responded: “I think that’s an area in which pulling in maybe more of the patient-centred perspective can be very helpful.
“For many years, peripheral arterial disease and the impact on the muscle was not really given a lot of attention but now a lot more interest I think is there in how much peripheral arterial disease can decrease mobility and create a whole other host of downstream problems that have enormous impacts on spending dollars etc. for the healthcare system. So, I think similar types of things can be done with venous disease and pulling in different stakeholders can really help define those metrics.”
again highlighted the spectre of compression, or lack thereof, where venogram and intravascular ultrasound (IVUS) imaging suggested compression, whereas on postmigration computed tomography (CT), there appears to be no compression. “So we have an education gap in a lot of cases,” Murphy said. This raised the potential for technology that might “help us differentiate that this is truly pathologic versus anatomic compression,” she said. There have been advances with the coming of dedicated venous stents, and ebbs and flows with the likes of the Duo venous

stent system (Philips) removed from the market, Murphy noted. But IVC stenting would seem to remain “a big gap,” with the Viafort stent (Gore) for the treatment of symptomatic IVC obstruction with or without combined iliofemoral obstruction, still under pivotal trial, “our first real big move in this direction,” she added. What about “secondgeneration steps” in venous stenting? Murphy pondered. “We haven’t seen any second moves, so is there something with drug elution like they do in the arteries that we could take advantage of; is there a gamechanger in the field? What I’ve seen is that there’s really no push for competition right now.”
Tipping the scales with less weight: Why RCTs can be overly burdensome for de novo dialysis access devices

Ellen Dillavou (Raleigh, USA) asks whether the high bar for evidence being sought to bring new de novo devices to market is stifling innovation in the field of vascular access.
In the last decade, we have seen remarkable innovation in the dialysis access space—percutaneous fistulas, external supports, drug-eluting technology, immediate access grafts and novel catheters for treating thrombosis and stenosis. However, getting these tools into the hands of practitioners is a challenging and unpredictable process.
At the last VEITH meeting (19–23 November, New York, USA) I had the privilege of debating these challenges with Robert Lee, formerly of the US Food and Drug Association (FDA) and head of the committee which was tasked with approving these novel technologies. As a clinician focused on access, the following are some of my thoughts on this topic.
While randomised controlled trials (RCTs) are the gold standard for clinical evaluation, their necessity for de novo devices, particularly in dialysis access, warrants reconsideration. While rigorous evaluation is essential, the “least burdensome” principle, enshrined in the FDA Modernization Act of 1997 (FDAMA),1 mandates that the FDA utilises the least burdensome means of demonstrating that the probable benefits outweigh the probable risks, while protecting patient safety, and the level of evidence required to tip the scales of approval is commensurate with the level of potential risk a device may pose.
2 In the context of novel dialysis access devices requiring de novo classification, the risk level is often low to moderate as device-related mortality and/or unresolvable morbidity are rarely probable. In dialysis access, RCTs can often represent an overly burdensome approach, potentially hindering innovation and delaying patient access to new technologies.
The dialysis access space presents unique challenges for RCT design. First, patients requiring dialysis often have complex comorbidities, limited treatment options, and a high risk of complications which can complicate both enrolment and interpretation of results.
The recent Vascular Therapies RCT on the juxta-anastomotic delivery of sirolimus to support
maturation—ACCESS—is a prime example of complexity in the patient population leading to a negative result. The treatment group had an overrepresentation of complex patients that skewed the endpoint in favour of the control. Even though a subgroup analysis demonstrated a potential clinical effect in the most challenging patient population, the failure of the overall endpoint resulted in the FDA requiring another RCT before a de novo approval could be granted. Luckily, Vascular Therapies was able to pull together the funding to run another study—ACCESS 2—and hopefully we will be able to offer our patients access to this therapy in a few years. However, the lack of any safety risk reported in the study combined with the probable benefit in complex patients begs the question why de novo status was not granted for at least a limited indication for use.
The ethical consideration of randomisation also cannot be ignored. In many cases, there may be a strong clinical rationale to believe a new device offers advantages over existing options. Randomising patients to a potentially inferior treatment, even temporarily, raises ethical concerns. Additionally, providing a treatment to a patient that may not align with best practices can be equally concerning. The recent WAVE trial of the Wrapsody endoprosthesis (Merit Medical) highlights this issue. Multiple RCTs have demonstrated that primary use of stent-grafts is superior to primary angioplasty of a stenosed access. However, current clinical guidelines dictate we give angioplasty a chance first because nothing is left behind that could compromise venous real estate. In the Wrapsody study design, as guided by the FDA, primary stenting was performed on patients who may have responded well to angioplasty. Conversely, angioplasty was performed on patients with multiple failed angioplasties who were indicated for a stent-graft. Neither addresses the problem that Wrapsody was designed to solve, which is the edge stenosis and in-growth failure mechanisms of legacy stent-grafts, and this randomisation
strategy potentially harmed patients in the quest for patient safety.
Finally, the FDA should give credence to physician judgement. Unlike pharma, we can directly observe how the device is performing and assess benefit on a case-by-case basis. If given sufficient data to understand the risks, we can determine what is appropriate for our patients. The endovascular arteriovenous fistula (endoAVF) cases are excellent examples of single-arm studies that allowed for the dialysis access community to make sound clinical judgements. Generally, we decided that endoAVF has value in specific circumstances for a subset of patients. However, this was only realised because the FDA allowed access to t he device in a real-world setting at an early timepoint.
The recently approved and reimbursed VasQ device (Laminate Medical) is undergoing that same process. The single-arm study demonstrated promise and comparators of fistulas created by the same surgeons in the study suggested superiority of VasQ access creations. While surgeons now have a chance to assess the value of the device in the real world, a post-market RCT is being conducted which will add to the level of evidence.
Unfortunately, the FDA defers to lone medical reviewers that may have their own biases. To be clear, this is not a criticism of these reviewers, but the process that requires them to make complex decisions on an island. No one celebrates the reviewer when a good device is approved, but everyone will let them know when an approved device leads to excessive patient harm.
Whilst having the highest level of data is enticing, the cost of RCTs and risk of design issues may stifle investment into innovation for dialysis access.”
This dynamic leads to a conservative approach that seeks comfort in an RCT rather than clinical judgement. For this reason, the reaction to the lack of endoAVF adoption may have swung the pendulum to default to RCTs for several new devices on the horizon, regardless of the safety profile.
Whilst having the highest level of data is enticing, the cost of RCTs and risk of design issues may stifle investment into innovation for dialysis access. We have seen a recent surge of investment since the successful FDA approval, Centers for Medicare & Medicaid Services (CMS) reimbursement and acquisition of the endoAVF devices. Devices currently in clinical studies like Venostent
(VenoStent), Velocity (Venova Medical), and EchoMark (Sonavex) have great potential to improve the lives of dialysis patients. However, if the barrier to enter the market is increased, we may see the renaissance end. Trials require substantial resources, including funding for patient recruitment, data collection, analysis, and regulatory submissions. For smaller companies, which are often the source of innovation in the medical device space, this can be prohibitive. This can create a barrier to entry, preventing potentially beneficial devices from reaching the market. It’s like trying to tip the scales when one side is already weighed down by immense costs.
So, what are the alternatives? The least burdensome principle calls for exploring other scientifically valid methods. In the dialysis access space, this could include:
● Well-designed single-arm studies: These studies can be valuable for evaluating the safety and effectiveness of a new device, particularly when historical controls or registries are available for comparison.
● Real-world data: Real-world data can complement data from clinical trials and provide a more comprehensive understanding of the device’s safety and effectiveness.
● Patient registries: These organised collections of data on patients with specific conditions can be used to track the long-term outcomes of devices and identify safety signals. These alternative approaches, when rigorously implemented and analysed, can provide substantial evidence of safety and effectiveness while reducing the burden on manufacturers, aligning with the intent of the least burdensome principle.
While RCTs remain a valuable tool for evaluating medical devices, they should not be considered the default or only method for demonstrating safety and effectiveness for FDA approval, especially in the challenging landscape of dialysis access.
For de novo devices in this space, requiring RCTs can often be overly burdensome, hindering innovation and delaying patient access to potentially life-saving technologies. By embracing alternative approaches, when scientifically justified, the FDA can better adhere to the least burdensome principle, tipping the scales towards a more balanced and patient-centred approach to medical device regulation.
References: 1. FDA-2017-D-6702. The Least Burdensome Provisions: Concept and Principles Guidance for Industry and FDA Staff. 2. De Novo Classification Process (Section 513(f)(2) of the FD&C Act).
Ellen Dillavou is the division chief of Vascular Surgery at WakeMed Hospitals (Raleigh, USA). She reports that she is a speaker for WL Gore and 3M/KCI, a consultant/speaker for Merit Medical and a scientific advisor for Venostent and WL Gore.
Point of View

Robert Shahverdyan (Hamburg, Germany) writes on the technologies shaping the future of percutaneous arteriovenous fistula (pAVF) creation for patients requiring haemodialysis.
The creation of a vascular access is a critical step for patients requiring haemodialysis, and traditionally, surgical AVFs have been the gold standard. However, the introduction of pAVFs in 2017 marked a significant advancement in this field. The concept of pAVFs (or endoAVFs) was revolutionary because it allowed for the creation of vascular access using endovascular techniques rather than open surgery. These minimally invasive procedures promised to create an AVF without the need for surgical incisions, reducing trauma and improving patient outcomes. Two devices received US Food and Drug Administration (FDA) and CE-mark approval and became commercially available: WavelinQ (BD) and Ellipsys (Medtronic).
Upon introduction, pAVFs generated significant excitement in the medical community. The promise of avoiding surgical trauma while achieving durable results led to increased usage, especially among early adopters and specialised centres. But despite the early enthusiasm, pAVFs failed to achieve widespread adoption worldwide. Additionally, over the past two to three
years, the initial momentum surrounding pAVFs has slowed, with a noticeable decrease in usage. Many centres that initially adopted the technology either reduced their procedural volume or abandoned it altogether. As a result, only a few highly experienced specialists remain who continue to use either WavelinQ, or Ellipsys, or both. Why? One of the primary challenges with early pAVF technology stems from the location where these fistulas are created. Unlike traditional surgical AVFs, current generation pAVFs are created in or adjacent to the deep veins, leading a significant portion of the fistula flow to return through the deep venous system instead of being directed efficiently to the superficial veins. This results in fractional flow loss due to multiple venous branches. As a result, in many dialysis centres cannulation of pAVFs can be difficult. Although this has (partially successfully) led to increased reliance on and adoption of ultrasound guidance for cannulations, there is a need for longer learning curves for dialysis staff to adapt to pAVFs and many are reluctant to put in the extended effort.
Merit Medical Systems has announced that the six-month results from the randomised arm of the Wrapsody arteriovenous access efficacy—WAVE— trial are scheduled for publication in the April issue of Kidney International, ahead of the presentation of 12-month results from the trial at the Society of Interventional Radiology’s 50th annual scientific meeting (29 March–2 April, Nashville, USA).
THE WAVE TRIAL IS A MULTICENTRE,
Another challenge is the development of juxta-anastomotic venous stenosis. This can compromise fistula maturation and delay usability for dialysis, as well as increase the risk of access failure, requiring frequent interventions to maintain patency and ensure the fistula is functional for dialysis. Hence, many patients require additional (typically interventional) procedures before successful cannulation.
The combination of device costs, procedural expenses, and the need for frequent reinterventions has made pAVFs more expensive compared to traditional surgical AVFs. While there is no large direct cost-effectiveness comparison, the overall financial burden remains a significant barrier to widespread adoption. Therefore, to increase pAVF adoption, pAVF devices should be cheaper to reduce the costs of the initial procedure, yet improve pAVF outcomes by reducing number of interventions, and hence additionally reduce follow-up expenses. More importantly, a high rate of unassisted maturation would improve usability while minimising hospitalisation costs. Finally, if the procedure is both fast and successful, it would enhance overall efficiency in patient care.
To address the above challenges, several pAVF devices are being developed, of which two—ePATH (Pathfinder Medical, UK) and Velocity (Venova Medical, USA)—are known through several conferences. The ePATH is an electronic guidance system, which employs an ultra-low-profile catheter system to enable precise placement and positioning of guidewires and catheters using a re-entry and endovascular bypass (presumably a stentgraft) technique, with an AVF creation at the wrist under fluoroscopic imaging guidance. Although this device can add new anatomical locations for pAVF creation, many uncertainties remain, specifically regarding the technical success and patency of the small and sharp (c-shaped) angled implant,
international, investigational device exemption (IDE) trial designed to evaluate the Wrapsody cell-impermeable endoprosthesis (CIE)’s safety and efficacy over two years. In the randomised arm of the trial, 245 patients on haemodialysis who experienced stenosis in the venous outflow of their AVF were treated with the Wrapsody CIE (n=122) or standard percutaneous transluminal angioplasty (PTA, n=123).
Initial results at six months demonstrated that the target lesion primary patency was significantly higher for the Wrapsody CIE vs. PTA (89.8% vs. 62.8%, p<0.0001). Similarly, the access circuit primary patency was significantly higher for the Wrapsody CIE vs. PTA (72.6% vs. 57.9%, p=0.015). No significant difference in the safety outcome was observed between treatments.
At 12 months, the Wrapsody CIE remained significantly higher than PTA for both target lesion primary patency (70.1% vs. 41.6%, p<0.0001) and access circuit primary patency (58.1% vs. 34.4%, p=0.0003).
“The Wrapsody CIE’s positive outcomes at one
covering the distal radial artery. Limited information is available on this system except that there have been successful animal and cadaveric studies.
Velocity aims to ‘streamline’ the AVF creation process, enabling rapid functionality with reduced need for reinterventions, thereby addressing patient and systemic barriers associated with current methods. It is designed for optimal flow dynamics, featuring a single outflow using a covered tapered fenestrated implant through the perforating vein into the proximal radial artery without the need for flow diversion or embolisation, which promotes fast maturation. Its advanced approach should minimise anastomotic stenosis by avoiding inflammation, thermal injury, and turbulent flow, eliminating the need for angioplasty. Additionally, it should allow procedures to be performed even faster than the current pAVF procedures and under ultrasound guidance, making it a practical and efficient option for both patients and clinicians. Recent clinical experience, such as from the VENOS-1 FIH [first-in-human] trial, has demonstrated the potential to improve unassisted maturation and primary patency rates and decrease complications. The US feasibility study (VENOS-2) was initiated in January 2025 and will hopefully confirm the excellent outcomes of the FIH trial. Whether the mid- to long-term outcomes and device costs of nextgeneration pAVFs become favourable remains to be evaluated in future years.
Robert Shahverdyan is co-founder and head of vascular access medicine at the Petridis Vascular Institute (Gefäßinstitut) Hamburg in Hamburg, Germany. He reports that he is a consultant for Medtronic, BD, Laminate Medical, Xeltis and VentureMed, has been in receipt of honoraria from Cardionovum and BrosMed Medical, and is on the scientific advisory board of Venova Medical.
year address an important knowledge gap regarding the potential durability of the device,” said Dheeraj K Rajan (University of Toronto, Toronto, Canada), WAVE trial investigator. “It is encouraging to know there is a new device available to help us prolong functional vascular access in our patients.”
The Wrapsody cell-impermeable endoprosthesis’ positive outcomes at one year address an important knowledge gap regarding the potential durability of the device.”
While Medtronic will continue to offer customers and patients support as they transition to other solutions, discontinuation of this established product prompts questions on the technique’s future.
IT WAS RECENTLY REVEALED THAT Medtronic has taken the decision to discontinue offering the Ellipsys vascular access system for arteriovenous fistula (AVF) creation in all geographies. Speaking with Vascular News in the week following this news, Nicholas Inston (Queen Elizabeth Hospital Birmingham, Birmingham, UK), Bart Dolmatch (El Camino Hospital, Palo Alto, USA) and Kate Steiner (East and North Herts NHS Trust, Stevenage, UK) ponder what’s next for endovascular arteriovenous fistulas (endoAVF), discussing possible reasons behind Medtronic’s decision and pointing to the development of secondgeneration devices. The discussion opened a Vascular News Live broadcast focusing on interesting current talking points in the vascular access field.
“It came as a great shock,” says Inston, sharing his initial reaction to the breaking news on a device he “thought was getting very established as part of practice”.
Steiner, who details that around 30 patients at her institution have undergone an Ellipsys procedure over a two-year period, expresses a similar response.
“We were quite shocked by the news and disappointed from a patient’s perspective,” she says. “It’s become part of our algorithm now.”
Beyond these first thoughts, Steiner wonders what is next. “Is it going to be the next generation of devices?” she posits.
Speculating on why Medtronic might have taken the decision it did, Steiner highlights barriers to adoption, focusing first on the difficulties associated with setting up and running a successful endoAVF programme.

a business case for [the device] is very difficult”. This has not been an issue in all geographies, however, with Inston pointing out several success stories outside of the UK, especially in insurancebased European healthcare systems. “Rob Shahverdyan in Germany’s had a big programme, Alex Mallios in France has had a big programme,” he notes.
Offering a perspective from the USA, Dolmatch considers the wider picture, highlighting “relatively low” adoption rates for percutaneous fistula creation that have prevented emerging technologies from taking hold.


“I think it’s the kind of product that, when you adopt [it], it’s not just the procedure, it’s not just the product, it’s the whole thing around it—it’s having a team able to deliver a successful endoAVF programme and to do that involves a lot of education and a degree of training around cannulation in order to be able to do that,” she explains. “I just wonder if that’s something that we can do fairly easily at some institutions, but at others there may be barriers towards adopting a programme and having it as part of your algorithm.”
PERCUTANEOUS FISTULA MARKET IS THE ELLIPSYS
He shares his opinion that for Medtronic, Ellipsys simply was not a “profitable endeavour”. In addition to its introduction during the COVID-19 pandemic, Dolmatch reiterates Steiner’s point about the difficulties of creating successful programmes that “push these [devices] into the hands of interventional radiologists and interventional nephrologists”. He continues: “These are issues that slow the adoption, and I think financially if you’re looking at it from a Medtronic standpoint, you can’t keep on running a programme that may not be making enough money to support it year after year.”
Dolmatch also points out that Medtronic is a dominant player in the small emerging market that is endoAVF creation. “I would say the Ellipsys has the lion’s share of [the market] globally,” he says. “If you really look into some of the market analyses something close to 85–90% of the percutaneous fistula market is the Ellipsys.”
On this point, Steiner adds that her institution only had approval for the Medtronic device. “Our usage was 100% Medtronic,” she says.
Against this backdrop, the Medtronic news prompts the question: is this the end of endoAVF?
Inston wonders what move BD—the other player in the endoAVF game with its WavelinQ device—will make. “It will be really interesting to see what happens with WavelinQ because this could be the end of endoAVF, but equally it could be a concentrated interest in a single device.”
works very well to create a fistula; the downside is it just takes a lot of surveillance and secondary procedures to really get most of these fistulas to be ready for cannulation for haemodialysis, so I think that with advances perhaps in a second-generation device that might reduce the number of procedures it would be much more palatable option to start a percutaneous fistula programme.”
Vascular News contacted David Moeller, president of Peripheral Vascular Health at Medtronic, in light of the Ellipsys withdrawal news. He provided the following statement: “Medtronic has made the decision to discontinue offering the Ellipsys vascular access system for [AVF] creation in all geographies. In a commitment to our customers, we will ensure they have up to 12 months of supply and up to six months of field, clinical, and cannulation support and will work closely with stakeholders to transition to alternative fistula creation solutions over the coming months.
We know the technology works very well to create a fistula; the downside is it just takes a lot of surveillance and secondary procedures to really get most of these fistulas to be ready for cannulation for haemodialysis.”
Bart Dolmatch
“This decision will not limit any patient’s ability to receive an endovascular or surgical fistula. As the current standard of care, surgical fistula creation remains an option for any patient who would be a candidate for the Ellipsys system and an alternative endovascular fistula creation technology is commercially available in the US and other regions.
“Medtronic remains committed to the arteriovenous fistula market, focusing on effective fistula maintenance with our IN.PACT AV drug-coated balloon and the Fortrex HP PTA [percutaneous transluminal angioplasty] balloon catheter. While this was a difficult decision, this strategic shift will enable us to prioritise our resources towards fostering innovation and developing new therapies where we can have the most significant impact. This decision is aligned to Medtronic’s active portfolio management work, an important lever for delivering on the company’s long-term strategic and financial objectives.”
Steiner also considers a financial obstacle, citing a cost of £4,000 per catheter in the UK’s taxpayerfunded National Health Service (NHS).
On this, Inston remarks that one of the “big problems” Ellipsys users have had in the UK is that the technology has not been deemed a cost-effective treatment option by the National Institutes for Health and Care Excellence (NICE) and therefore “setting up
Dolmatch looks to the strength of the data behind currently available technologies as a strong foundation for second-generation devices that could see the endoAVF story continue. He notes that Shahverdyan has shown a “very high” rate of creating percutaneous fistulas with both Ellipsys and WavelinQ. “The issue is just the maturation,” he comments, “the point of getting them to be functional for haemodialysis.”
Dolmatch continues: “We know the technology
Vascular News also contacted Tim Hug, vice president and general manager at BD Peripheral Intervention, who shared: “At BD, we understand that hemodialysis is a lifeline for ESKD [end-stage kidney disease] patients and are highly committed to providing innovative solutions that help create, restore, and maintain dialysis access. [...] As part of our ESKD commitment, BD has created a robust WavelinQ endoAVF system infrastructure and programme that includes training, case support, and other offerings. We welcome new opportunities and partners who align with our purpose of advancing the world of ESKD health and are committed to improving the quality of life for ESKD patients.”
technologies should be ‘de facto standard of care’ for PAD, LINC audience hears
New data from a large, real-world study support the use of drug-eluting devices to reduce amputations, readmissions, and healthcare costs in the treatment of peripheral arterial disease (PAD), Marianne Brodmann (Medical University of Graz, Graz, Austria) informed attendees at the Leipzig Interventional Course (LINC 2025; 28–30 January, Leipzig, Germany).
BRODMANN WAS, FOR THE first time, sharing the results of TRUEPTX. This study sought to evaluate the use of paclitaxel drug-eluting versus non-drug-eluting devices for the treatment of PAD in a large patient population, focusing specifically on above-ankle amputation, hospital readmission, and mortality rates at 12 months.
Several studies have demonstrated the benefits of drug-eluting therapy for femoropopliteal lesions, Brodmann noted, including sustained vessel patency, clinical improvement, and fewer reinterventions. The question of
whether these benefits transfer to realworld practice, however, remains.
Brodmann and colleagues identified 10,000 patients on the Truveta platform who had undergone either drug-eluting therapy (5,000 patients) or non-drug-eluting therapy (5,000 patients) for PAD and analysed their outcomes.
Regarding patient characteristics, Brodmann shared that 40% were female and 82% were white. The presenter also pointed out that race and ethnicity distributions varied between the treatment groups. “Drug-eluting devices were used more frequently
for white and Hispanic patients, and less frequently for Black patients,” the presenter noted.
On comorbidities, Brodmann detailed that approximately 32% of patients across the two groups had chronic limb-threatening ischaemia (CLTI), and that other comorbidities were far more prevalent in those patients treated with drug-eluting devices.
Brodmann reported that, before matching, 12-month amputation and readmission “occurred significantly less frequently for patients receiving drugeluting treatment”. The presenter added that there was no significant difference between the two groups regarding mortality. “So, once again, a severe hint that drug-eluting technology, paclitaxelcoated technology, is not relevant for mortality,” she remarked.
After propensity-score matching, there were around 4,000 patients remaining in the drug-eluting device group and around 3,700 in the nondrug-eluting device group, Brodmann relayed. The proportion of patients with CLTI stayed broadly the same, at close to 30%.
Patients in this matched cohort treated with non-drug-eluting treatment were more likely to have above-ankle amputation, any readmission, and
Pending the results of several trials and “iterative changes” to device design, bioabsorbable scaffolds are set to change the treatment paradigm for lower extremity peripheral arterial disease (PAD) within the next decade. So concluded Eric Secemsky (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, USA) during a presentation at LINC 2025.
SECEMSKY WAS SPEAKING ON THE future of bioabsorbable scaffolds, providing an overview of the key features of this “ideal” device type for the treatment of below-the-knee (BTK) disease. The first characteristic is drug elution, he noted, which inhibits restenosis and provides drugmodulated healing for sustained patency; second, a scaffold design provides transient support in the vessel and addresses recoil and dissection; and finally, resorbability ensures nothing is left behind.
“The era of bioabsorbable scaffolds has really begun with the Esprit drug-eluting resorbable scaffold (DRS),” Secemsky commented, noting that this Abbott innovation “is what created the market in the USA”. Esprit DRS is the first device of its kind to receive approval from the US Food and Drug Administration (FDA), the presenter shared, highlighting positive data from the prospective, multicentre, randomised LIFE-BTK trial that now has results available out to two years.
Secemsky stressed, however, that the technology is still in its infancy. The Esprit BTK device is “only the first step on a long pathway to solving BTK interventions for both US and non-US patients,” he told the LINC audience.
Here the presenter listed a number of other devices with bioabsorbable properties that are being evaluated in the USA, focusing in particular on the Motiv (Reva Medical) and Magnitude (R3 Vascular) scaffolds.
The Motiv device, Secemsky detailed, is CE
mark approved, has been granted FDA breakthrough designation, and is currently being assessed in the recently completed MOTIV BTK randomised controlled trial. Led by Ehrin Armstrong (Adventist Heart and Vascular Institute, St Helena, USA) and Andrej Schmidt (University of Leipzig, Leipzig, Germany), the trial enrolled its target cohort of 292 patients across 35 centres in the USA and Europe and is awaiting completion of follow-up.
“We saw results of their early postmarket trial in the EU looking at 60 limbs, 58 patients, and [the
I think we’ll continue to have these conversations over the years, see more data, and I think we’ll see many of these scaffolds make it to clinical practice.”
device] demonstrated really impressive patency outcomes through three years,” Secemsky shared. Continuing, the presenter stressed the significance of this result: “It’s hard for a BTK device in vessels that are less than 3.5mm to maintain 88% patency
readmission associated with a repeat procedure, Brodmann communicated.
The presenter summarised that, in a contemporary, real-world patient cohort with a high prevalence of CLTI, above-ankle amputation and readmission within 12 months occurred more frequently with non-drug-eluting therapy versus paclitaxel drug-eluting devices in propensity-score-matched patients.
“This large study of real-world evidence parallels the results of formal randomised controlled trials,” Brodmann said in her concluding remarks. “The TRUE-PTX results support the use of drug-eluting devices to reduce amputations and readmissions, and the results have implications for reducing healthcare costs.”
“It’s not only about patient satisfaction, about patient quality of life; it’s also about expense,” Brodmann continued. “Major amputation is expensive and avoiding amputation has the potential for substantial cost savings.”
Based on these conclusions, Brodmann shared her take-home message from the presentation: “Drugeluting technologies should be the de facto standard of care for PAD.”

Eric Secemsky presents at LINC 2025
through 36 months.”
Moving on to Magnitude, Secemsky disclosed that he is the co-principal investigator for the US pivotal trial of this device. “[Magnitude] is really as resistant to compression and supportive as a metallic stent, but has unique flexibility to optimally perform in challenging anatomical locations,” Secemsky noted, also pointing out the device’s ability to “maintain its structure in a compromised situation, including in the infrapopliteal space”.
The results of early feasibility study RESOLV I, Secemsky shared, were “very supportive of moving forward to an IDE [investigational device exemption] trial”. In total, he noted that 36 lesions in 35 limbs were treated in this trial with >90% freedom from occlusion or restenosis, which included “really impressive real-world patient and lesion characteristics”.
“Bioabsorbables have the promise to change clinical practice for infrainguinal arterial disease,” Secemsky posited, drawing his presentation to a close. “As iterative changes allow for longer scaffold lengths, larger diameters and consistent tensile strength, the treatment paradigm for lower extremity disease may look much different in the next five to 10 years.
“I think we’ll continue to have these conversations over the years, see more data, and I think we’ll see many of these scaffolds make it to clinical practice.”

DeepQure advances clinical trials of its HyperQure extravascular renal denervation system
DeepQure has announced progress of clinical trials of its extravascular renal denervation device, HyperQure.
The company is actively conducting clinical trials in both South Korea and the USA, which it says demonstrate “promising” results. In South Korea, seven patients have undergone the procedure, showing meaningful reductions in blood pressure without any adverse events during- or postoperation. The company aims to complete enrolment for its Korean clinical trial within the first quarter of 2025.
In the USA, DeepQure successfully completed its first clinical case on 17 January 2025, at the University of California, Irvine (UC Irvine). The procedure, performed by Pengbo Jiang, yielded significant blood pressure reductions one month after the operation with no reported complications. The next patient is scheduled for treatment in March, with additional institutional review board (IRB) approvals secured from institutions including Mayo Clinic and Stanford University.
DeepQure’s US clinical trial spans six university hospitals, with IRB approvals finalised. The company plans to enrol 15 patients in the first half of 2025, with the ultimate goal of initiating a pivotal study by year-end to establish the safety and efficacy of HyperQure.
Beyond hypertension, DeepQure is expanding its technology to address atrial fibrillation (AF), one of the most common cardiac arrhythmias. The company has submitted an application to South Korea’s Ministry of Food and Drug Safety (MFDS) to initiate clinical trials for AF treatment.
Currently, pulmonary vein isolation (PVI) is the standard treatment for AF, but its major drawback is a high recurrence rate. DeepQure’s renal denervation technology is expected to enhance the effectiveness of PVI procedures by reducing recurrence rates, offering a breakthrough solution in a field where drug therapy has shown limited success, a press release says.
Shape Memory Medical announces first European enrolment in AAA-SHAPE pivotal trial
Shape Memory Medical has announced the first European enrolment in the AAA-SHAPE pivotal trial, the
company’s prospective, multicentre, randomised, open-label trial to determine the safety and effectiveness of the Impede-FX RapidFill device to improve abdominal aortic aneurysm (AAA) sac behavior when used with elective endovascular aneurysm repair (EVAR). The patient was treated by Jan Heyligers and Patrick Vriens at Elisabeth-TweeSteden Hospital in Tilburg, The Netherlands.
“We congratulate Dr Heyligers, Professor Vriens, and the clinical study team at Elisabeth TweeSteden Hospital for being the first centre in Europe to enrol in the AAA-SHAPE pivotal trial,” said Marc Schermerhorn (Beth Israel Deaconess Medical Center, Boston, USA), AAA-SHAPE national principal investigator. “This milestone reflects the unwavering dedication of the physician investigators to advance embolisation solutions and enhance patient care worldwide.”
A press release details that AAASHAPE (Abdominal aortic aneurysm sac healing and prevention of expansion) will enrol 180 patients

with infrarenal AAA across up to 50 sites in the USA, Europe, and New Zealand. Key endpoints will compare sac diameter and volume change, endoleak rates, and secondary interventions.
“We are proud to be the first site in Europe to treat a patient in this groundbreaking trial,” said Heyligers. “Research shows that 60% of aneurysms either fail to regress or expand within a year after EVAR, often leading to rehospitalisations, additional interventions, and higher mortality rates. The results of this study will be essential in determining whether the Impede-FX RapidFill, with its unique properties, can significantly enhance post-EVAR AAA outcomes and elevate the standard of patient care,” continued Heyligers.
Impede-FX RapidFill, the investigational device, incorporates Shape Memory Medical’s novel shape memory polymer, a proprietary, porous, radiolucent, embolic scaffold that is crimped for catheter delivery and selfexpands upon contact with blood. In AAA-SHAPE, Impede-FX RapidFill is intended to fill the aneurysm blood lumen around a commercially available EVAR stent graft to promote aneurysm thrombosis and sac shrinkage.
The AAA-SHAPE pivotal trial builds
upon the AAA-SHAPE early feasibility study, which enrolled 35 patients in New Zealand and The Netherlands to assess the use of Impede-FX RapidFill for AAA sac embolisation during EVAR.
DEScover trial of drug-eluting vascular stent graft in AVG and AVF patients reaches enrolment milestone
Solaris Endovascular has announced an enrolment milestone in its DEScover clinical trial, assessing a sirolimuseluting covered stent graft—Solaris DE—for the treatment of patients facing challenges with dialysis access dysfunction and peripheral arterial disease (PAD).
In a press release, Solaris Endovascular announced that trial enrolment is ahead of schedule and has successfully enrolled 42 patients out of the planned 120 patients to date.
The trial’s objective is to evaluate the safety and efficacy of the Solaris drug-eluting endovascular stent graft in patients with arteriovenous fistulae (AVF) and arteriovenous grafts (AVG).
A total of 80 participants with AVF are randomised in a 1:1 ratio to receive either the Solaris DE device or standard balloon angioplasty, with a single-arm design for AVG patients (n=40) treated with Solaris DE.
The trial’s safety endpoints include the proportion of patients free from serious adverse events at 30 days, and efficacy will be determined by target lesion primary patency at six months.
With healthcare costs for end-stage kidney disease surpassing US$59 billion in 2020, effective dialysis access management is more critical than ever. Vascular access failures, particularly restenosis, remain unresolved. Solaris DE seeks to address this unmet clinical need by combining a mechanical barrier of the next-gen impermeable electrospinning PTFE with a biological barrier to block cell proliferation, the company’s press release adds.
Leonardo Harduin (Liv Care Centro Clínico, Niterói, Rio de Janeiro, Brazil), DEScover principal investigator, said: “The Solaris DE device, with its electrospinning PTFE coverage and sirolimus-eluting platform, is designed to effectively prevent restenosis and extend the functional duration of dialysis access for patients.”
Ziv J Haskal (University of Virginia School of Medicine, Charlottesville, USA), added: “Addressing edge stenosis is the next frontier in dialysis access care. This trial has the potential to significantly advance our treatment strategies.”
InspireMD and NAMSA to partner on CGUARDIANS II pivotal clinical trial InspireMD and North American Science Associates (NAMSA) recently announced that, pursuant to a previously announced strategic outsourcing partnership, the companies
are working together to conduct the CGUARDIANS II pivotal study of InspireMD’s CGuard Prime 80cm carotid stent system for use in transcarotid artery revascularisation (TCAR) procedures.
On 9 December 2024, InspireMD announced that the first patient had been enrolled into the CGUARDIANS II clinical study.
CGUARDIANS II is a prospective, multicentre, single-arm pivotal study that aims to enrol a minimum of 50 evaluable patients. Its objective is to evaluate acute device success and technical success of the CGuard Prime when used in conjunction with an FDA-cleared TCAR neuroprotection system in patients at high risk for adverse events from carotid endarterectomy.
AngioDynamics initiates AMBITION BTK RCT and registry to advance treatment for CLTI AngioDynamics has announced the initiation of AMBITION BTK, a randomised study of the Auryon atherectomy system in the treatment of below-the-knee (BTK) chronic limb-threatening ischaemia (CLTI).
AMBITION BTK is a multicentre, randomised controlled trial (RCT) designed to evaluate the clinical outcomes of the Auryon atherectomy system in combination with standard balloon angioplasty compared to standard balloon angioplasty alone for the treatment of infrapopliteal lesions in patients with CLTI.
The trial will enrol up to 200 patients across up to 30 hospital-based sites. Additionally, up to 1,500 patients treated with the Auryon atherectomy system at the same sites who do not meet the eligibility criteria of the RCT will be enrolled in a companion registry.
A press release notes that the Auryon laser can be used to treat all infrainguinal lesion types, including above-the-knee (ATK), BTK and instent restenosis (ISR) and to date, it has been used to treat more than 100,000 patients in the USA and worldwide.
“With the global rise in diabetes, we are seeing a growing number of patients with severe tibial disease,” said AMBITION BTK coprincipal investigator Anahita Dua (Massachusetts General Hospital and Harvard Medical School, Boston, USA). “In the USA, treatment options for below-the-knee lesions remain limited, often relying primarily on plain balloon angioplasty. These patients frequently present with tibial disease that can extend throughout the entire vessel. An innovative tool like the Auryon laser, which can restore laminar flow, could be a gamechanger in their care.”
AMBITION BTK builds upon the positive outcomes of a prior multicentre, prospective trial that evaluated the safety and effectiveness of the Auryon system in treating BTK lesions in patients with limb ischaemia.

Inari Medical, now part of Stryker, launches Artix thrombectomy system
Inari Medical, now part of Stryker, recently announced the launch of its Artix thrombectomy system. Purposebuilt for the distinct needs of the peripheral arterial system, Artix is a combined aspiration plus mechanical thrombectomy solution that delivers procedural control and versatility, and is designed to set a new standard for arterial thrombectomy.
Stryker notes that the Artix system builds on the success of Inari’s venous thrombectomy devices and is its inaugural entry into the arterial space. “Inari offers a comprehensive toolkit approach to arterial thrombectomy with an innovative, over-the-wire system that provides physicians with the flexibility to aspirate and/or mechanically extract a clot,” a press release reads.
Jonathan Bowman (Norwalk Hospital, Norwalk, USA) performed the first commercial case with Artix on 19 October 2024. “The Artix thrombectomy system marks a significant advancement in peripheral arterial thromboembolism treatment,” said Bowman. “With Artix, I finally have a solution that effectively addresses a wide range of clots while enabling me to maintain vessel access and retain control throughout the entire case. I expect Artix will take the place of open surgical repair in many of my arterial cases.”
Key distinctive features of the Artix system include:
● Dual mechanical thrombectomy and aspiration toolkit designed to remove acute to chronic clots in a single session via a streamlined procedure
● Aspiration-capable, 8Fr lowprofile, trackable, kink-resistant sheath in both 65cm and 90cm lengths expands treatment options by enabling more complex and distal interventions
● Innovative over-the-wire mechanical element designed to effectively collect and retrieve acute-to-chronic clot
● Covered nitinol mesh funnel provides temporary flow restriction during the procedure to minimise the risk of arterial clot migration
Philips issues Class I recall for Tack endovascular system Philips has recently announced
that it will no longer sell its Tack endovascular system in the USA following a Class I recall issued by the US Food and Drug Administration (FDA).
A Class I recall is the FDA’s most serious classification, which denotes that the continued use of the affected product could result in severe injury or death.
The FDA’s decision follows reports that the Tack endovascular system— designed to treat arterial dissections— has posed challenges for users, sometimes necessitating additional interventions to retrieve or remove the implant. The self-expanding device is composed of nitinol, a nickel-titanium alloy.
The device is used following angioplasty, a procedure that widens narrowed or blocked arteries. While rare, tears in blood vessels can be a serious complication, and the Tack implant is designed to repair these by securing damaged tissue to the artery wall.
The FDA warned that continued use of the device could lead to significant health risks. Short-term complications include partial or complete blockage of blood flow, perforations in the artery’s inner lining, or full-thickness arterial tears. Long-term consequences may involve pain, tissue loss, re-narrowing of the vessel, and, in more severe cases, bypass surgery, amputation, or death.
approach to our peripheral practices, enabling us to make cases more efficient and optimise outcomes for our patients living with PAD, especially those with more complex CLTI.”
The “forward” IVL platform is designed to modify calcium and cross calcified occlusive disease or extremely narrowed lesions where a wire will cross but devices might not. Shockwave Javelin has a working length of 150cm and features a single distal emitter that creates up to 120 shockwave pulses. Each shockwave pulse creates a spherical energy field that extends beyond the tip of the catheter. This novel design delivers lithotripsy closer to calcium than the balloon-based platform. Despite the challenging nature of the calcified lesions studied, the clinical outcomes from the FORWARD PAD investigational device exemption (IDE) trial demonstrated that Shockwave Javelin has a similar safety and effectiveness profile to balloon-based Shockwave IVL catheters, a company press release states.

Philips has reported 20 injuries linked to the Tack system but has not received any reports of fatalities.
The Tack system was initially approved by the FDA in April 2020. It was developed by Intact Vascular, a cardiovascular company acquired by Philips for US$275 million later that year.
Shockwave Medical announces US launch of intravascular lithotripsy catheter
Shockwave Medical has announced the US launch of its Shockwave Javelin peripheral intravascular lithotripsy (IVL) catheter, a platform designed to modify calcium and cross narrowed vessels in patients with peripheral artery disease (PAD).
“Physicians have faced significant challenges in tackling complex calcific lesions in narrowed peripheral vessels, and there is a growing need for more effective crossing and treatment tools,” said JD Corl, medical director of the PAD/chronic limb-threatening ischaemia (CLTI) programme at The Lindner Center for Research and Education at The Christ Hospital in Cincinnati, USA. “With proven safety and effectiveness similar to existing IVL devices, Shockwave’s new IVL platform will bring a transformative
otherwise difficult-to-reach vascular anatomy.
It expands the established benefits of Robotic Magnetic Navigation into multiple new clinical applications including minimally invasive procedures used to treat stroke, cancer, and cardiovascular disease.
“Robotic Magnetic Navigation offers significant promise to address clinical challenges we face in the neurointerventional field by enabling safe and rapid navigation through tortuous vasculature,” said Timo Krings (Beth Israel Lahey Health, Boston, USA).
“I’m excited by the opportunity to help pioneer this technology, evaluate and demonstrate its clinical value, and explore entirely new applications that may become possible. Our field is uniquely poised to benefit from robotics and we look forward to advancing the technology and clinical science over the coming years.”

“As the pioneer of IVL technology, our goal is to continue to deliver innovations that address the unmet needs of the physicians that we serve,” said Nick West, chief medical officer, Shockwave Medical. “By listening to and leveraging their valuable insights, we developed our transformational forward IVL platform with the unique capability to both modify calcium and cross extremely narrowed vessels. We are proud to be leading the charge in offering endovascular interventionalists more flexibility to address critical treatment needs and potentially reduce the risks associated with CLTI for their patients.”
Stereotaxis seeks to broaden use of its robotic magnetic navigation system into multiple endovascular fields Stereotaxis has announced a US Food and Drug Administration (FDA) regulatory submission for the first robotically navigated catheter designed to expand usage of robotic magnetic navigation into the broader endovascular field.
Emagin 5F is the first in a family of robotically navigated endovascular devices being developed by Stereotaxis. The Emagin brand— short for Endovascular Magnetic Intervention—will encompass a portfolio of robotic catheters and wires.
Emagin 5F is a catheter guide with a 5Fr diameter used to navigate tortuous venous and arterial vasculature. Robotic navigation of the catheter directly from the distal tip, using precise magnetic fields, is designed to enable efficient and safe navigation to
“The evolution of robotics in interventional cardiology is inevitable and will be of significant benefit to patients, physicians, and healthcare systems,” said Kalpa De Silva (Guy’s and St Thomas’ NHS Foundation Trust, London, UK). “I see significant promise in the use of the roboticallysteered Emagin catheter to enhance the safety, precision, and efficiency of various challenging procedures including renal denervation and complex percutaneous coronary interventions. I look forward to helping pioneer this technology.”
Stereotaxis submitted a 510(k) application for Emagin 5F with the FDA and expects to submit the catheter for European CE mark clearance in March 2025. Emagin 5F was designed and is manufactured by Stereotaxis’ fully-owned subsidiary Access Point Technologies in Minnesota (USA).
Stereotaxis expects to launch Emagin 5F following anticipated approvals in the second half of this year.
Humacyte announces commercial launch of Symvess for extremity vascular trauma
Humacyte has announced the commercial launch of Symvess (acellular tissue engineered vesseltyod) for use in adults as a vascular conduit for extremity arterial injury when urgent revascularisation is needed to avoid imminent limb loss, and when autologous vein graft is not feasible.
The US Food and Drug Administration (FDA) granted a full approval for Symvess on 19 December 2024. A press release reports that the FDA has now completed its required review of commercial batch information and has authorised Humacyte to commence commercial shipments.
“This is a great moment for Humacyte but, more importantly, for patients in urgent need of arterial repair options to help save their limbs or lives,” said Laura Niklason, founder and chief executive officer of Humacyte.







FEBRUARY 9–12, 2026


Loews Miami Beach Hotel Miami Beach, FL

Scan here to stay updated on registration information.




Life Seal Vascular appoints Matt Thompson as CEO
Life Seal Vascular has announced the appointment of Matt Thompson as chief executive officer (CEO).
A press release states that Thompson’s career as a vascular surgeon and medical device executive positions Life Seal Vascular to accelerate the development and commercialisation of its vascular technologies.
“We are thrilled to welcome Thompson as our CEO,” said Robert Mitchell, executive chairman of Life Seal Vascular. “His unique combination of clinical expertise and executive leadership in the vascular medical device industry makes him the ideal leader to drive our next phase of growth. His prior service on our board has provided him with deep insight into our company’s mission and we are excited for his leadership as we advance our technologies into clinical use.”
serving as chief medical officer at Endologix from 2016–2021 and chief executive officer from 2021–2025. He currently continues to contribute to Endologix as chief medical officer.

“I am honoured to join Life Seal Vascular at this pivotal stage,” said Thompson. “The company is developing breakthrough endovascular solutions that address critical unmet clinical needs, particularly in the prevention of type II endoleaks following endovascular aneurysm repair. I look forward to working with the team to bring these innovations to patients and clinicians worldwide.”
Xeltis announces the appointment of nephrology clinicians to its medical advisory board
Xeltis has announced the appointment of clinical nephrology experts An De Vriese and Haimanot (Monnie) Wasse to its medical advisory board.
Rush University Medical Center in Chicago, USA. A press release states that both add considerable clinical nephrology expertise to the company’s medical advisory board and will work alongside current members to offer strategic and clinical guidance as Xeltis advances its aXess vascular access conduit towards commercialisation.
Eliane Schutte, chief executive officer of Xeltis said: “We are pleased that both De Vriese and Wasse have chosen to join our medical advisory board, bolstering our already stellar team of advisors. The appointments represent significant validation of the unique and promising nature of our technology from leading experts in the field. Their experience across nephrology and vascular access will be invaluable as we continue to progress aXess through its pivotal trials in the EU and US, with primary readouts for our EU pivotal trial expected in mid2025.”
technology. AXess has an immensely promising medical profile, and I am proud to now be in a position to offer my expertise in interventional nephrology and vascular access to further the clinical development of this innovative technology.”
Currently, Xeltis is advancing pivotal trials in both the EU and the US for aXess; enrolment was recently completed in the EU while ten patients have now been implanted in the USA.
Reflow Medical expands global reach with new European subsidiary
Reflow Medical has announced the opening of its European subsidiary in Landsberg am Lech, Germany. This strategic expansion strengthens the company’s international presence and enhances its ability to serve markets outside the USA, a press release notes.
From 2002 to 2016, Thompson served as a leading vascular surgeon at St. George’s Hospital in London, UK, where he gained international recognition for his expertise in complex aortic repair, the company states. Transitioning to the medical device industry, he held key leadership roles,
3–4 April
CLI-C GLOBAL Venice, Italy cli-courses.com
3–5 April
Vascular Access Society (VAS) congress Padua, Italy vas2025.org
De Vriese is currently head of the division of nephrology and infectious disease in AZ Sint-Jan Brugge, in Brugge, Belgium and guest professor at Ghent University in Ghent, Belgium, while Wasse serves as the interim chair of the department of internal medicine, and the chief of nephrology and director of interventional nephrology at the
23–25 April
Charing Cross (CX) Symposium London, UK cxsymposium.com
8–10 May Venous Symposium New York, USA venous-symposium.com
22–23 May
“It is an honour to join Xeltis’ MAB at such a pivotal time in the company’s development,” commented De Vriese. “I am truly excited by Xeltis’ innovative technology which, through its avoidance of the frequent reinterventions and infections associated with current treatment options, has the potential to transform patient outcomes for those with endstage renal disease.”
On her recent appointment, Wasse stated: “I am familiar with the important work of Xeltis, having kept a keen eye on the continual clinical progress of its
Pacific Northwest Endovascular Conference (PNEC) Seattle, USA pnec-seattle.org
4–7 June
Vascular Annual Meeting (VAM) New Orleans, USA vascular.org/vam-2025





26–27 June
Reflow Medical Europe GmbH is led by senior vice president and general manager Knut Sauerteig. Under his leadership, the European subsidiary will focus on establishing direct sales channels and forging strategic distribution partnerships across multiple regions.
“Expanding into markets outside the USA marks a significant milestone for our company,” said Isa Rizk, cofounder and chief executive officer of Reflow Medical. “This presence allows us to better address the needs of international markets by providing direct sales solutions and forming key distribution collaborations.”
British Society of Endovascular Therapy (BSET) annual meeting Wotton-under-Edge, UK bset.co.uk/meetings/bset-annualmeeting-2025
26–28 June
European Venous Forum (EVF) annual meeting Krakow, Poland https://europeanvenousforum.org
11 September
Interdisciplinary Aortic Dissection Symposium (IADS) London, UK aorticdissectionsymposium.com
13–17 September
Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress Barcelona, Spain cirsecongress.cirse.org
GORE® VIABAHN® VBX
Balloon Expandable Endoprosthesis (VBX Stent Graft)
Same trusted performance of the VBX Stent Graft, now with greater versatility1,2 :
▪ Longest balloon expandable stent graft (up to 79 mm)
▪ Broadest range of diameter adjustability in a single device*
▪ 1 Fr profile reduction on most catheter sizes
See what’s new
* Maximum post-dilated diameter up to 16 mm with 8L or 11 mm devices.
1. Gore Medical Products Instructions for Use (IFU). W. L. Gore & Associates, Inc. Accessed September 10, 2024. https://eifu.goremedical.com/
2. EUROPEAN Device Guide - Balloon-Expandable Covered Stents. Endovascular Today European Edition. Accessed June 18, 2024. Balloon-Expandable Covered Stents - Endovascular Today (evtoday.com)
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available.
Products listed may not be available in all markets. GORE, Together, improving life, VBX, VIABAHN and designs are trademarks of W. L. Gore & Associates. © 2024 W. L. Gore & Associates GmbH 24PL2075-EN01 JUNE 2024
W. L. Gore & Associates, Inc. goremedical.com Asia Pacific +65 6733 2882


