Featured in this issue:
ROUNDTABLE DISCUSSION
Stroke transfer protocols
What does the road ahead look like?
page 4
ARISE I recommends multidisciplinary approach to managing brain aneurysms, chronic SDHs and AVMs

‘No-reflow’ phenomenon after successful thrombectomy
page 11

www.neuronewsinternational.com
Profile Adnan Siddiqui
page 14
Semmes-Murphey Clinic, Memphis, USA), told NeuroNews
“Although in its infancy, artificial intelligence [AI]—already having been applied in detecting brain aneurysms—and robotics, will gradually become important in our field. A continuous, educational dialogue between all stakeholders, including the US FDA [Food and Drug Administration], NIH [National Institutes of Health], MedTech industry, and the scientific community, is mandatory to advance the field further.”
Intracranial aneurysms
Aneurysms
The first of these papers—led by Stavropoula Tjoumakaris (Thomas Jefferson University, Philadelphia, USA) and colleagues—relates specifically to the management of intracranial aneurysms. It initially notes that these cases constitute a challenging neurological diagnosis associated with significant morbidity and mortality, also detailing that—despite “a plethora of microsurgical and endovascular techniques for the treatment of both ruptured and unruptured aneurysms”— there is currently no definitive consensus as to the best of these options.
Highly variable carryover effect confirmed in SCS patients
page 23
First European PERFORMANCE II release highlights carotid stenting’s durability in highrisk patients
Three papers published in the journal Stroke have highlighted multidisciplinary care and global, collaborative efforts as “paramount” in the management of intracranial aneurysms, chronic subdural haematomas (cSDHs), and brain arteriovenous malformations (AVMs). These recommendations were led by the Aneurysm/AVM/cSDH Roundtable Discussion with Industry and Stroke Experts (ARISE) group, which facilitates conversations between academia, industry, and regulators, and ultimately intends to optimise acute stroke care.
“AChronic SDH
Brain AVMs
s the neurovascular and neuroendovascular field continues to evolve rapidly with the introduction of new therapies—for example, in cSDH—into our discipline, which prove to be highly effective and safe through large, multicentre randomised controlled clinical trials, it remains critical that complex neurovascular pathologies like brain AVMs are managed in a multidisciplinary fashion at major neuroscience centres,” Ajay Wakhloo (Tufts University School of Medicine, Boston, USA), coorganiser of the ARISE series of meetings alongside Adam Arthur (University of Tennessee Health Science Center/
Experts therefore convened to discuss the latest research, approaches and devices with the aim of improving outcomes for brain aneurysm patients. Key among their suggestions is the further incorporation of AI technologies as a means for capturing sequential aneurysm growth, identifying predictors of rupture, and using risk-rupture predictions to guide treatment options. The ARISE consensus has also “strongly recommended” nationwide, systemic data collection for radiographic images of unruptured aneurysms, to facilitate the analysis and development of machine learning algorithms designed to anticipate rupture risks.
Tjoumakaris et al’s recommendations highlight optical coherence tomography (OCT) and magnetic resonance (MR) contrast-enhanced 3T vessel wall imaging as “promising technologies”, but go on to note that “more data are needed” to define their role in aneurysm management. In addition, their consensus paper voices support for centres of excellence through which multicentre, preclinical trials—in areas including genetics, cellular composition and radiogenomics— can be conducted. Regarding the role of multidisciplinary, collaborative approaches, the authors state that ruptured aneurysms are “best managed” at larger, high-volume centres that should ideally incorporate comprehensive patient management, and expertise across microsurgery, endovascular
Continued on page 7
THE RECENT, FIRST-TIME European presentation of the PERFORMANCE II trial’s findings— delivered by Ralf Langhoff (Sankt Gertrauden-Krankenhaus, Berlin, Germany) at the 2024 Charing Cross (CX) International Symposium (23–25 April, London, UK)—led delegates to discuss the role of stenting in carotid artery disease treatment, and how this has evolved in light of newer technologies.
Piotr Musialek (Jagiellonian University, Kraków, Poland) later noted that “the landscape has changed” recently, owing to the fact modern carotid stents are also ‘cerebral protectors’, while Christopher Metzger (OhioHealth, Columbus, USA) asserted that carotid artery stenting (CAS) is now competitive with carotid endarterectomy (CEA) when used appropriately.
In addition, while Musialek, Metzger and several other delegates were in firm agreement that there is still a place for surgery in carotid stenosis treatment, William Gray (Lankenau Medical Center, Wynnewood, USA)—global principal investigator (PI) for PERFORMANCE II—also suggested
Continued on page 18

NEWLYPHYSICIANSDISCUSS INTRASACCULARLAUNCHEDARTISSE DEVICETurntopage8 May 2024 | Issue 54
Stroke triage, ICH surgery, carotid stenting, and more
Welcome to another edition of NeuroNews!
When I was asked to write a short introductory letter for this issue, I knew there would be no shortage of potential talking points and timely content to highlight.
Among so many important topics, recent editions of NeuroNews have featured the latest randomised trial data on middle meningeal artery (MMA) embolisation for chronic subdural haematoma (cSDH), mechanical thrombectomy for large-core infarcts with large vessel occlusion (LVO), and selection of general versus local anaesthesia for stroke interventions. These subjects are all highly relevant to the neurointerventional field; and, no doubt, they will be the subjects of ongoing study and debate in the coming years.
Outside the catheterisation lab, the way we triage and transport acute stroke patients is another topic of great significance. The fact that the brain is exquisitely metabolically active and oxygen-sensitive is well known. As such, acute stroke is one of our most time-sensitive emergencies. The general population is ageing, and the potential stroke cohort is growing, but assessment and triage of these patients remain complicated. Our emergency medical services (EMS) are capable of great speed at times but become handicapped by long rural distances or heavy urban traffic. Some centres readily incorporate emergency stroke services and excel; other facilities struggle with even the most basic stroke services for a variety of reasons, despite widely available guidelines.
In a recent substudy of the RACECAT trial, the authors have shown that—above all else—revascularisation speed is essential, particularly for LVOs. Similarly, in some ways, SELECT2 investigators have also demonstrated that the speed to revascularisation using mechanical thrombectomy is paramount, although many additional specialised hospital services are necessary to achieve optimal patient outcomes in acute stroke. These new data are summarised and discussed on pages 4–5.
Having initially been presented in conference last year, now-published findings from the ENRICH trial in a peerreviewed journal indicate that good outcomes can be achieved with minimally invasive surgical evacuation of intracranial haemorrhage (ICH). Our takeaway is outlined on page 13.
I believe that the ENRICH results pave the way to something neurosurgeons have sought
for many years; that is, an indication to intervene on ICH. However, it also puts them on notice that haemorrhagic stroke must be treated like acute ischaemic stroke, i.e. very quickly. This will be an important test of emergency neurosurgical services. Some centres have 24-hour neurosurgical coverage that responds immediately to emergencies. However, many US centres contract or employ neurosurgeons who are satisfied by their electively scheduled work and eschew sudden emergencies, for a variety of reasons. It will be interesting to see if operative neurosurgery will embrace ICH as a timesensitive emergency.
Stories published in this issue of NeuroNews also allude to the deep divisions among experts in carotid revascularisation following the US Centers for Medicare & Medicaid Services (CMS) decision to expand its coverage of stenting procedures.
There are strong opinions on both sides of the debate, despite so many technical advances and increasing diffusion of operator knowledge. All participants in these debates clearly hold their patients’ best interests at heart. However, such divisions, or differences of opinion, occur when the scientific data are incomplete. In the meantime, some very expensive—but necessary—trials are ongoing. Enrolment remains important, especially when equipoise persists. Paradoxically, perhaps, the rush to market with new stent technologies threatens to undermine both trial enrolment and access to answers that are so badly needed.
The eventual publication of the CREST-2 trial and its findings will undoubtedly be key in creating a more complete body of data on carotid revascularisation, not only regarding the role of carotid stenting or surgical endarterectomy, but also how contemporary medical management fits into this complex picture alongside revascularisation.
These are, of course, just a snapshot of the big-ticket items in today’s neurointerventional space, and ongoing and future studies promise to shed light on additional important matters.
How far will stroke thrombectomy expand beyond the patients and situations in which it is currently recommended by the guidelines? How large is the treatable stroke population? What are the true roles of artificial intelligence (AI) and other innovative technologies in modern neurological imaging and patient care? To what extent will more novel intravenous thrombolytic drug candidates impact acute stroke treatments? How do we raise public awareness of the underlying threat of brain aneurysms and, ultimately, optimise care as the burden of haemorrhagic stroke continues to grow?
In time, we should have answers to these and other burning questions—and, when we do, NeuroNews will provide succinct and relevant updates that capture the ongoing debates and discussions.

PHILIP M MEYERS is a neurointerventionist, endovascular neurosurgeon, and diagnostic and interventional neuroradiologist, based in the USA. He is also former president of the Society of NeuroInterventional Surgery (SNIS).
NEWS IN BRIEF THE LATEST
STORIES FROM THE NEURO WORLD
n TRANSRADIAL NONINFERIOR TO FEMORAL ACCESS IN STROKE THROMBECTOMY:
In stroke patients undergoing a mechanical thrombectomy procedure, access via the radial artery may be non-inferior to access through the femoral artery in terms of final recanalisation-related outcomes. However, reduced procedural delays associated with the latter mean transfemoral access may still be favourable as a default, first-line approach, as detailed by the authors of a randomised clinical trial published recently in the journal Stroke.
For more on this story go to page 6.
n ENRICH TRIAL RESULTS PUBLISHED IN FULL:
The entirety of the results from the ENRICH trial evaluating the early surgical removal of intracranial haemorrhage (ICH) have been published in the New England Journal of Medicine, demonstrating that early minimally invasive parafascicular surgery (MIPS) is safe and superior compared to the current standard of care.

For more on this story go to page 13.
n GROWING HAEMORRHAGIC STROKE BURDEN IN USA REPRESENTS “MAJOR CHALLENGE”:
The past three decades have seen a “notable rise” in the burden of haemorrhagic stroke across the USA and this, coupled with the increasing burden of stroke more generally, represents an “evolving and substantial public health challenge” for the country to contend with. These are among the key findings from a cross-sectional analysis of the 2019 Global Burden of Disease study, published recently in JAMA Neurology by Daniela Renedo (New Haven, USA) and colleagues.
For more on this story go to page 17.

Editor-in-chief: Prof Philip M Meyers | Publisher: Stephen Greenhalgh
Editor-in-chief: Prof
Content director: Urmila Kerslake | Global commercial director: Sean Langer
Content Kerslake Global commercial Sean
Editor: Jamie Bell jamie@bibamedical.com | Editorial contribution: Jocelyn Hudson and Bryan Kay
Editor: Bell jamie@bibamedical.com Editorial contribution: and Bryan Kay
Design: Terry Hawes, Josh Lyon and David Reekie
Design: Terry Lyon Reekie
Advertising: Michael Broughton michael@bibamedical.com
Advertising: michael@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
Published News, a subsidiary BIBA Europe, Fulham Road, SW6 United Kingdom Tel: 7736 BIBA North America, 155 North Wacker Drive, States +1 708-770-7323
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2024. All rights reserved.
Printed regarding newspaper should to the at the address. © Medical 2024.
neuronews linkedin.com/company/neuronews/
neuronews linkedin.com/company/neuronews/ @NN_publishing Subscribe here
have comments this suggestions for
If you have comments on this issue or suggestions for upcoming editions write to jamie@bibamedical.com
2
May 2024 | Issue54
Subscribe
C M Y CM MY CY CMY K
INTRODUCTORY EDITORIAL

Challenges Update Vascular & Endovascular SAVE THE DATE CXSYMPOSIUM.COM 23–25 APRIL 2025 WEDNESDAY-FRIDAY EXCEL, LONDON UNITED KINGDOM INNOVATION EDUCATION EVIDENCE CONTROVERSIES CHALLENGES CONSENSUS PERIPHERAL ARTERIAL ACUTE STROKE & CAROTID AORTIC VENOUS & LYMPHATIC VASCULAR ACCESS VASCULAR TRAUMA THE HURTING LEG
Stroke transfer protocols: What does the road ahead look like?
A number of somewhat conflicting studies have been published over the past year regarding triage and transport protocols for stroke. In light of the most recent of these publications, NeuroNews speaks to Ashutosh Jadhav (Barrow Neurological Institute, Phoenix, USA) in an attempt to make sense of the existing data.
Last year, new data from the TRIAGE-STROKE and RACECAT studies created a level of uncertainty regarding the advantages of directly triaging acute stroke patients to thrombectomy-capable centres. The former suggested a trend towards functional outcome benefits in patients transferred directly to comprehensive stroke centres (CSCs), but was ultimately unable to reach statistical significance on this front, while the latter indicated that bypassing a primary stroke centre (PSC) may even be harmful in patients with a final diagnosis of intracranial haemorrhage (ICH).
Speaking to NeuroNews following publication of these datasets, leading authors for both—Anne Behrndtz (Aarhus University Hospital, Aarhus, Denmark) and Anna Ramos-Pachón (Hospital Germans Trias i Pujol, Barcelona, Spain)—were in agreement that further research is needed to improve stroke diagnosis ‘in the field’, and to ascertain which patients are most likely to benefit from bypassing protocols. These data were followed towards the end of last year by a statement from the Society of NeuroInterventional Surgery (SNIS), in which several leading US neurointerventionists asserted that the entire body of evidence to date—including findings from the aforementioned trials—points towards the fact that direct triage to a CSC is beneficial in stroke patients with emergent large vessel occlusions (LVOs). The statement urged against misinterpreting or inappropriately applying the data, noting that TRIAGESTROKE produced results “overwhelmingly in favour” of direct triage, despite its lack of power, and attributing RACECAT’s somewhat negative outcomes to geographical and healthcare system-related factors in Catalonia, Spain—where the study was conducted— that are “vastly different” to those seen across much of the USA.
Recent updates
The beginning of 2024 has already seen two further publications that contribute additional data to this ever-growing body of evidence. In February, Amrou Sarraj (Case Western Reserve University, Cleveland, USA) delivered one-year outcomes from the SELECT2 trial at the International Stroke Conference (ISC; 7–9 February, Phoenix, USA), and his presentation was accompanied by a prespecified analysis of the study’s transfer protocols being published in JAMA Neurology Across a total of 352 enrolled patients, all of whom had severe ischaemic strokes—meaning an Alberta stroke programme early computed tomography score (ASPECTS) of 3–5, or a core volume of ≥50mL on imaging, or both—59.9% were transferred from a PSC to receive thrombectomy, while 40.1% presented directly to a CSC to undergo the procedure.
Sarraj and colleagues’ analysis showed that the positive treatment effect of thrombectomy versus medical management was observed across both groups. Treatment effect estimates were maintained and continued to favour thrombectomy in patients with low ASPECTS at referring hospitals, and in those who demonstrated a loss of ≥2 ASPECTS points during transfer.

The authors also note that, while improved functional outcomes were numerically better in patients presenting directly to a CSC for thrombectomy versus those triaged via a PSC, this did not constitute a significant effect modification. Another of their findings was that thrombectomy treatment effect estimates were lower in patients with transfer times of three or more hours, as compared to those with shorter transfer times.
Sarraj and colleagues conclude their paper by stating that “these findings emphasise the need for rapid identification of patients suitable for transfer and expedited transport”.
More recently, in March, the team behind RACECAT offered another contribution to the existing body of data in this space, publishing a substudy of the trial in Stroke: Vascular and Interventional Neurology (SVIN). This secondary, post-hoc analysis sought to evaluate factors that may influence functional outcomes among stroke patients initially assessed at a local stroke centre—either a telestroke centre or a PSC—compared to a thrombectomy-capable CSC.
Leading author Marta Olivé-Gadea (University Hospital Vall d’Hebron, Barcelona, Spain) and colleagues determined that, across a modified intention-to-treat population of 903 acute ischaemic stroke patients, 90-day functional outcomes were associated with door-to-needle times and local hospital level of care. More specifically, they observed a trend favouring direct transport to a CSC for patients whose assigned local stroke centre was a telestroke centre, rather than a PSC, and those whose door-to-needle time was greater than the global median of 31 minutes. The authors also found that these benefits of direct transfer to a thrombectomy-capable CSC were “more evident” in patients with a confirmed LVO.
“Direct transport to thrombectomy-capable centres may be preferable in areas primarily covered by telestroke or local stroke centres with poorer performance, especially in patients with LVO,” OlivéGadea and colleagues conclude. “These findings can contribute to refining prehospital triage strategies and optimising stroke systems of care.”
Deciphering the data
“It is confusing, because there are multiple studies that show slightly different things,” says Jadhav, reflecting on these varying recent publications. “For LVO stroke patients, the only thing that we’ve shown helps is to open up the blood vessel quickly. There’s no doubt that, at an individual level, the faster you take out the blockage, the better. But, apart from opening up the vessel quickly, nothing else has really been proven as something we can modify to improve outcomes.”
Here, Jadhav draws comparisons between these debates and similar discussions over percutaneous coronary intervention (PCI) triage for myocardial infarction (MI) in the cardiology space. However, while a rough “inflection point” of two hours has been settled on regarding direct PCI triage, there are additional challenges preventing the translation of similar findings into the neuro world.
Firstly, according to Jadhav, ‘how’ stroke triage is actually conducted is relevant, and door-in-door-out (DIDO) times constitute an “important metric and potential bottleneck”.
“Ideally, the patient receives lytics and then goes straight to a thrombectomy-capable centre,” he adds, “but, if DIDO times are long and the system is inefficient, that can be to the detriment of the patient, because you’re spending a lot of time giving them lytics and doing advanced imaging.”
On this front, stroke triage is generally more complex and ultimately “much slower” compared to the equivalent process for MI, as it involves telemedicine consultations, a greater imaging burden, and more discussions around lytics and other medical options. In addition, the second key discrepancy Jadhav highlights between stroke and MI triage relates to a point right at the heart of debates surrounding stroke transport protocols: the uncertainty over whether a patient is suffering from an ischaemic or haemorrhagic stroke, which is estimated by emergency responders using stroke severity scales but cannot be diagnosed with certainty until they undergo in-hospital imaging.
According to Jadhav, this is one of the major factors behind RACECAT’s perceived failure to produce data supporting the direct triage of stroke patients to thrombectomy-capable hospitals. The study used Rapid arterial occlusion evaluation (RACE) scores to approximate an LVO diagnosis but, in doing so— because RACE scoring cannot discriminate between stroke patients with or without brain bleeds—saw many haemorrhagic strokes bypassing local hospitals and heading straight to CSCs.
“Most EMS [emergency medical service] triage protocols, if they assume it’s an ischaemic stroke caused by a blockage, tend to advise letting the patient’s blood pressure ride high, because we want that patient to perfuse their brain,” Jadhav notes. “But, then, you have patients with a high RACE score being driven around for an extra hour with uncontrolled blood pressure and, potentially, a brain haemorrhage. That’s why RACECAT saw that difference where harm was caused to the haemorrhage population.”
As such, the appropriate triage option for a given individual patient may be clear but, “at a population level”, the answer is far less simple. Jadhav believes that, “if you don’t have a diagnostic tool beyond just RACE or NIHSS [National Institutes of Health stroke scale], there are a lot of LVO mimics that you’re potentially going to be harming [via direct triage]”. According to Jadhav, the “ideal” comparative stroke triage study will initially exclude haemorrhagic patients and include, for example, a “very clean population” of hundreds of LVO patients who are then randomised to different strategies. However, such a study would be “hard to do” with current technologies and will likely require more sophisticated prehospital tools capable of discerning those with brain bleeds from more ‘standard’ ischaemic stroke patients.
4 May 2024 | Issue54 Feature
STROKE TRIAGE
Ashutosh Jadhav
Real-world factors
Touching on the potential future role of mobile stroke units (MSUs) in this space, Jadhav adds that—while “bringing the ED [emergency department] to the patient would be the ideal solution”—the reality is that many places cannot afford to deploy MSUs on a large enough scale for the approach to be used en masse. He feels this will be a “huge limiting factor”, stating that “while, conceptually, it may be what we want, practically, it’s unlikely to happen”.
Even if a cheaper, more practical alternative to MSUs—for example, thermal energy- or transcranial Doppler (TCD)-based methods for distinguishing between stroke types—can be introduced into ambulances, the prehospital phase remains “politically, very complicated”, particularly in the USA. Jadhav notes the “major challenge” posed by the fact the country’s Centers for Medicare & Medicaid Services (CMS) does not provide coverage for the prehospital setting as a place of care.
“So, who pays for it?” he asks. “That means the appetite to introduce expensive technologies in the prehospital setting is not going to be very high, without a financial pathway to do it. MSUs have struggled with this issue as well.”
Returning to the topic of RACECAT, Jadhav highlights another discrepancy brought about by regional variations.
“Before that study, I think a lot of people thought: ‘it’s definitely better to bypass the local ED’, because we know it’s going to take a while to get patients triaged,” he says. “One thing we learned from RACECAT is that the Catalonia region is phenomenally efficient at triaging and transporting patients. Their DIDO times were very good and, in a way, that actually hurt their study hypothesis because they did not have these long delays that we see in
other regions. Their times are definitely faster than in the USA, for example.”
This was confirmed, in a sense, by the RACECAT group’s more recent March 2024 publication in the SVIN journal. Jadhav says this paper essentially showed that direct triage to a CSC is more beneficial when it comes to less efficient systems with longer DIDO times at local centres, because “you’re spending way too much time at the primary hospital”.
“Bypassing protocols might be favourable in one town but not another, and it really depends on some of these drivers of workflow efficiency. If you’re in an area like Catalonia where processes are superefficient, bypass may not be necessary and could even be detrimental. The approach to triage is not going to be generally applicable to everybody—what works in
I think we’re trying to solve this problem the same way cardiology did, but cardiology had a different set of considerations and was able to get to a more definitive answer. The efficiency of stroke triage is so multivariable that it’s hard to translate results between regions.”
Catalonia may not be what works across the rest of Europe, or even in other parts of Spain. We know there are differences between urban and rural environments. And, within countries and between countries, there are huge differences in how triage works.
“I think we’re trying to solve this problem the same way cardiology did, but cardiology had a different set of considerations and was able to get to a more definitive answer. The efficiency of stroke triage is so multivariable that it’s hard to translate results between regions.”
Another emerging stroke workflow paradigm is the direct-to-angio approach, bypassing the emergency room at the thrombectomy-capable centre and directly admitting a confirmed or suspected acute thrombectomy candidate to the angiography suite with the hope of achieving faster recanalisation. Jadhav looks forward to the results of the upcoming DIRECT trial, which will compare the efficacy of the direct-toangio approach to traditional protocols triaging via the ED. Jadhav closes the conversation by emphasising the bearing of robust evidence on real-world stroke triage, and discussions around it.
“Distinct considerations arise when considering changes to the prehospital system, separate from those within the confines of a hospital,” he concludes. “While actions within a hospital may be more controlled, altering prehospital protocols requires a robust foundation of data, as there will be impact on additional stakeholders including paramedics and local hospitals. Many anticipated that RACECAT would unequivocally advocate for bypassing, based on their local experience, and the results were surprising to many.
“Getting the right patient to the right hospital at the right time remains a significant area of uncertainty in the rapidly evolving stroke landscape.”





5 Issue54 | May 2024 Feature *Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the neuro interventional arena Editorially independent Subscribe today Available in print and digital formats and through our social channels Visit neuronewsinternational.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription**
Early technique switches “should be contemplated” following failed thrombectomy attempts
A retrospective analysis including data from close to 3,000 stroke patients has concluded that early changes in mechanical thrombectomy strategies may be associated with higher reperfusion rates and therefore “should be contemplated” following failed attempts with first-line standalone contact-aspiration or stentretriever approaches.
THE ANALYSIS IN QUESTION—NOW published in the Journal of NeuroInterventional Surgery (JNIS) by Diogo Haussen, Pedro Martins (both Emory University School of Medicine, Atlanta, USA) and colleagues—was predicated on the fact that, in the authors’ view, although switching to a different thrombectomy technique after initially unsuccessful passes is a common occurrence, its effect remains undetermined.
Haussen, Martins and his colleagues set out to evaluate the association between early changes in thrombectomy approach and reperfusion, doing so via a multicentre, retrospective analysis of prospectively collected data. Their analysis concerned patients who underwent a thrombectomy procedure for occlusions in the intracranial internal carotid, middle cerebral (M1/
M2) or basilar artery. In total, 2,968 patients (median age, 66 years; 52% men) were included. Changes in thrombectomy technique after either one or two failed passes with a stent retriever alone, contact aspiration alone, or a combined technique incorporating the two, were compared with repeating the previous strategy. The primary outcome of the analysis was complete or near-complete reperfusion, defined as an expanded thrombolysis in cerebral infarction (eTICI) score of 2c–3, following each of the second and third thrombectomy passes.
The authors found that changing from a standalone stent-retriever technique to isolated contact aspiration on the second or third pass did not ultimately influence eTICI 2c–3 rates, although changing from a stent retriever to the combined technique after two failed passes led to an increased chance of eTICI 2c–3 (odds ratio [OR], 5.3; 95% confidence interval [CI], 1.9–14.6). Switching techniques was seen to have had an even more notable impact when changing from a contact aspirationonly approach to a combined technique, owing to a higher likelihood of immediate eTICI 2c–3 after one failed attempt (OR, 2.9; 95% CI, 1.6–5.5) and also after two failed attempts (OR, 2.7; 95% CI, 1.0–7.4). However, switching from contact aspiration to a stent retriever alone was not associated with a significant change in reperfusion outcomes.
reduced chance of eTICI 2c–3 (OR, 0.3; 95% CI, 0.1–0.9). A final observation that the authors relay in their JNIS report is that rates of functional independence were not significantly different across the analyses— irrespective of the initial thrombectomy approach and any subsequent technique switch.


Another of Haussen, Martins and colleagues’ primary outcome findings was that, following one or two failed combined-technique attempts, switching to a stent retriever-only approach was not associated with different reperfusion rates. Changing to a contact aspiration-only approach after two failed combinedtechnique attempts was, however, associated with a
Transradial achieves non-inferiority but procedural
delays may mean femoral access remains default in stroke thrombectomy
In stroke patients undergoing a mechanical thrombectomy procedure, access via the radial artery may be non-inferior to access through the femoral artery in terms of final recanalisationrelated outcomes. However, reduced procedural delays mean transfemoral access may still be favourable as a default, first-line approach, according to authors of a recent study.
WRITING IN THE JOURNAL
Stroke, Manuel Requena (University Hospital Vall d’Hebron, Barcelona, Spain) and colleagues detail that— between September 2021 and July 2023—they conducted a randomised clinical trial at their centre to directly compare transradial and transfemoral thrombectomy approaches.
“Transfemoral access is predominantly used for mechanical thrombectomy in stroke patients with a large vessel occlusion,” they explain. “Following the interventional cardiology guidelines, routine transradial access has been proposed as an alternative, although its safety and efficacy remain controversial.”
Against this backdrop, Requena and colleagues sought to evaluate the potential non-inferiority of a radial access approach, as compared to a femoral access approach, doing so via
an investigator-initiated, single-centre, evaluator-blinded trial whereby 120 patients were initially assigned to one of the two techniques. Specifically, stroke patients undergoing thrombectomy, with a patent femoral artery and a radial artery diameter ≥2.5mm, were randomised on a 1:1 basis into transradial (n=60) and transfemoral (n=60) groups. The binary outcome that served as the primary endpoint for their study was the rate of successful recanalisation—defined as an expanded thrombolysis in cerebral infarction (eTICI) score of 2b–3 and assigned by blinded evaluators. Requena and colleagues note that they established a non-inferiority margin of -13.2%, considering an acceptable reduction of 15% in the expected recanalisation rates. Ultimately, 116 patients with a confirmed intracranial occlusion on initial angiogram—58 in each group—
“Although we acknowledge that clinical outcomes are the most relevant outcome variable, our study was not designed or powered for such analysis,” said Martins, addressing this last detail in conversation with NeuroNews. “Since most patients achieved excellent reperfusion in up to three passes despite changing or repeating strategies, changing by itself is unlikely to account for a huge difference in clinical outcomes. Additionally, time to reperfusion may vary amongst techniques and play a role in the final clinical outcomes. Despite that, we believe that reperfusion remains a valuable intermediate outcome, and our study cannot rule out a potential effect on functional independence.”
“We did not design the study to compare the optimal moment for switching [technique]—the key finding is that changing before three failed passes, which was the paradigm employed in the landmark trials comparing thrombectomy strategies, may be beneficial,” Haussen added, responding to NeuroNews’ question on precisely when it might be best to consider an alternative approach during a thrombectomy case. “Considering that earlier reperfusion has been previously shown to be associated with better clinical outcomes and improved safety, it is reasonable to consider early changes in thrombectomy strategy. Whether and how angioarchitectural, clot composition or clinical characteristics lead to greater responsivity to specific techniques still needs to be established.”
were included in the trial’s intentionto-treat analysis. The aforementioned outcome of successful recanalisation was achieved in 96.6% of patients in the radial access group (n=56) and 87.9% in the femoral access group (n=51). Stemming from this, the researchers found an adjusted, one-side risk difference of -5% that indicated non-inferiority of transradial versus transfemoral access in the trial (95% confidence interval [CI], -6.61% to 13.1%).
Another key factor measured in Requena and colleagues’ analysis related to procedural delays. Their study revealed a median time from angiosuite arrival to first thrombectomy attempt of 41 minutes in the radial group (interquartile range [IQR], 33–62 minutes) and 30 minutes in the femoral group (IQR, 25–37 minutes; p<0.001).
A similar trend of increased delays with radial-access thrombectomy was seen in terms of median time from angiosuite arrival to recanalisation—59.5 minutes with radial (IQR, 44–81 minutes) versus
42 minutes with femoral (IQR, 28–74 minutes; p<0.05).
Finally, regarding complication and conversion rates, the authors relay that one severe, access-related complication occurred in each group, and there was no statistically significant difference in the rate of access conversion, at 12.1% with radial (n=7) and 8.6% with femoral access (n=5; p=0.751).
“In our study, radial access was associated with longer procedural time, without any advantage in terms of safety,” Requena told NeuroNews “These results must be taken with caution, because it is an unpowered trial to detect safety differences between groups. Radial access for selected patients may be the first-line approach, despite our results, but we have no data to defend radial access as a default for all mechanical thrombectomy patients.”
These data from what has been dubbed the ‘SFERA’ trial were originally presented at the 2024 International Stroke Conference (ISC; 7–9 February, Phoenix, USA).
Radial access for selected patients may be the first-line approach, despite our results, but we have no data to defend radial access as a default for all mechanical thrombectomy patients.”
6 May 2024 | Issue54 Thrombectomy Approaches
Pedro Martins
Diogo Haussen
ARISE I recommends multidisciplinary approach to managing brain aneurysms, chronic SDHs and AVMs
Continued from page 1
surgery, neurology and neurocritical care as well.
“The future of intracranial aneurysm diagnosis and monitoring could be enhanced by the incorporation of AI, and national radiographic and biologic registries,” they conclude. “A collaborative effort between academic centres, government regulators, and the device industry, is paramount for the adequate management of intracranial aneurysms and the advancement of the field.”
Chronic SDHs
In a second paper published recently by the ARISE group, Peter Kan (The University of Texas Medical Branch, Galveston, USA) et al provide a consensus statement on the optimal management of cSDHs—focusing primarily on new data from the EMBOLISE, MAGIC-MT and STEM trials, which all produced outcomes favouring the use of middle meningeal artery (MMA) embolisation. The authors note that multiple randomised controlled trials have now produced high-level evidence demonstrating
MMA embolisation is a “potent” adjunct to surgical and non-surgical standard care paradigms in neurologically stable cSDH patients.
“Pooled data analyses following the formal conclusion and publication of these trials will form a robust foundation upon which guidelines can be strengthened for cSDH treatment modalities, and optimal patient selection, as well as delineate future lines of investigation,” they add.
Brain AVMs
A third paper published by Edgar Samaniego (University of Iowa, Iowa City, USA) et al on behalf of the ARISE group pertains to the management of brain AVMs—complex, rare arteriovenous shunts that present with a wide range of signs and symptoms.
According to the authors, “despite prior societal position statements”, there is no consensus on how these patients should be managed.
Emphasising the importance for evidencebased approaches and an enhanced understanding of brain AVMs, ARISE discussions identified the need to develop scales to predict the risk of rupture in brain AVMs, and the use of “common data elements” to execute prospective registries and clinical studies. And—similarly to intracranial aneurysms—they also highlight the vital role of comprehensive patient

As the neurovascular and neuroendovascular field continues to evolve rapidly with the introduction of new therapies, […] it remains critical that complex neurovascular pathologies like brain AVMs are managed in a multidisciplinary fashion at major neuroscience centres.”
management at specialised centres, as well as a multifaceted care approach with expertise spanning cranial and spinal microsurgery, neurological endovascular surgery, and stereotactic radiosurgery.

Finally, the group deems collecting prospective, multicentre data and gross specimens as “essential” for improving brain AVM characterisation, genetic evaluation, and phenotyping. The paper also highlights the need for multidisciplinary frameworks and collaborative research across multiple centres, with a view to harnessing “collective expertise and centralisation of resources”, in brain AVM management.
ARISE II
“Since all stakeholders gave very
AI language model shows “encouraging” potential in locating brain lesions after stroke
Artificial intelligence (AI) may serve as a future tool for neurologists to help locate the part of the brain in which a stroke occurred, as per the findings of a new study. In the study, AI—more specifically, a large language model called generative pre-trained transformer 4 (GPT-4)—processed text from health histories and neurologic examinations to locate lesions in the brain. These results were published recently in an online issue of Neurology Clinical Practice.
“Not everyone with stroke has access to brain scans or neurologists, so we wanted to determine whether GPT-4 could accurately locate brain lesions after stroke based on a person’s health history and a neurologic exam,” said study author and American Academy of Neurology (AAN) member Jung-Hyun Lee (State University of New York [SUNY] Downstate Health Sciences University, Brooklyn, USA).
The study in question used 46 published cases of people who had stroke. Researchers gathered raw text from patients’ health histories and neurologic exams, and fed it into GPT-4. They then asked it to answer three questions relating to: whether a patient had one or more lesions; on which side of the brain lesions were located; and in which region of the brain the lesions were found. They repeated these questions for each patient three times and results from GPT-4 were then compared to brain scans for each patient.
The researchers found that GPT-4 processed the text from the health histories and neurologic exams
to locate lesions in many patients’ brains, identifying which side of the brain the lesion was on, as well as the specific brain region—with the exception of lesions in the cerebellum and spinal cord. For the majority of people, GPT-4 was able to identify on which side of the brain lesions were found with a sensitivity of 74% and a specificity of 87%. It also identified the brain region with a sensitivity of 85% and a specificity of 94%.
When looking at how often the three tests had the same result for each patient, GPT-4 was consistent for 76% of patients regarding the number of brain lesions. In addition, it was consistent for 83% of patients for the side of the brain, and for 87% of patients regarding the brain regions. When combining its responses to all three questions across all three times, GPT-4 provided accurate answers for 41% of patients.
“While not yet ready for use in the clinic, large language models such as generative pre-trained transformers have the potential not only to assist in locating lesions after stroke, but they may also reduce
positive feedback on the ARISE meeting last year, Dr Arthur and I decided to move the ARISE II meeting—initially planned for 2025—to this year,” Wakhloo stated.
The ARISE II meeting (23–24 May 2024, Washington DC, USA) will see stroke experts including representatives of medical academia, the healthcare industry and government agencies discuss intracranial atherosclerotic disease (ICAD), intracranial haemorrhage (ICH), and cerebral venous diseases (CVDs).
Conversations at ARISE II will pertain to the improved acute and elective management, treatment, and prevention, of these disease areas. The group intends to develop new approaches to overcome barriers impeding drug and device development, and also identify, clarify and communicate the implications of new research for cerebrovascular diseases, with a view to subsequently publishing more consensus recommendations in an effort to address these concerns.
healthcare disparities, because they can function across different languages,” Lee noted. “The potential for use is encouraging—especially due to the great need for improved healthcare in underserved areas across multiple countries where access to neurologic care is limited.”
The researchers do, however, report a key limitation of their research: the fact that the accuracy of GPT-4 depends on the quality of the information it is provided with, and—while researchers had access to detailed health histories and neurologic exam information for each patient in the present study—such information is not always available for everyone who has a stroke.
While not yet ready for use in the clinic, large language models such as generative pretrained transformers have the potential not only to assist in locating lesions after stroke, but they may also reduce healthcare disparities, because they can function across different languages.”
7 Issue54 | May 2024 Artificial Intelligence
COVER STORY
Adam Arthur Ajay Wakhloo
Conformability, softness, simplicity and simulation enable Artisse to ‘personalise’ treatments in intrasaccular aneurysm care
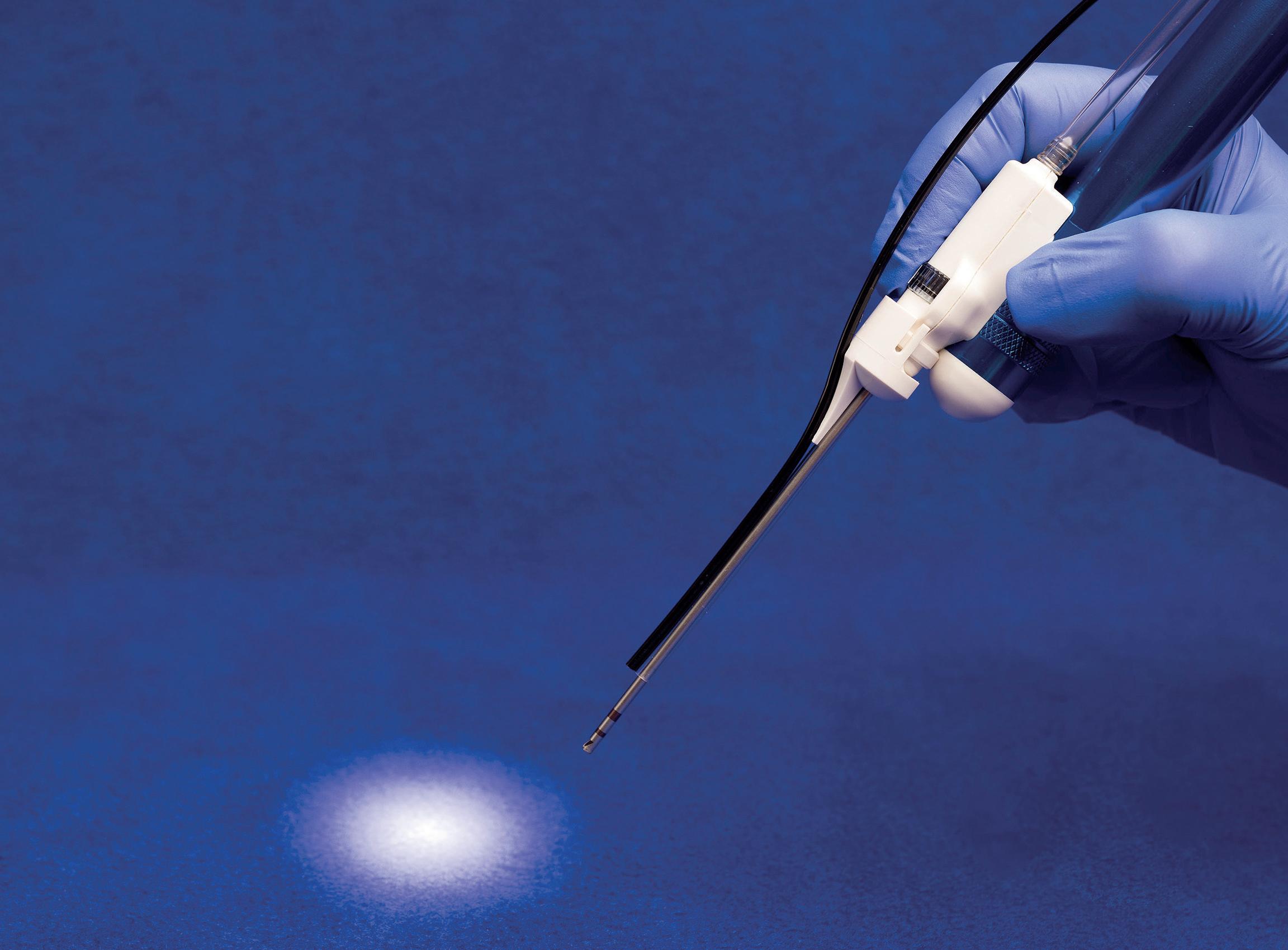
In light of the official European launch of the Artisse aneurysm embolisation system (Medtronic) at LINNC Paris 2024 (3–5 June, Paris, France), NeuroNews speaks to three of the device’s earliest users—Monika Killer-Oberpfalzer (Salzburg University Hospital, Salzburg, Austria), Markus Möhlenbruch (Heidelberg University Hospital, Heidelberg, Germany) and Vincent Costalat (University Hospital of Montpellier, Montpellier, France)— to discuss their experiences to date, and find out how Artisse differs from other intrasaccular technologies.
“When I first saw it, I thought, ‘wow, this is exactly what we need!’”, KillerOberpfalzer recalls. “It combines the things that are already on the market, in a better way compared to what was available before.”
Artisse—a reconfigured and enhanced update to the previous Luna aneurysm embolisation system— is a self-expanding, braided, ovoid implant made from a double layer of nitinol wire mesh. While this description alone may not sound drastically different to those of prior-generation intrasacculars like the Woven EndoBridge (WEB; Microvention/Terumo) or Contour (Cerus Endovascular/Stryker), KillerOberpfalzer believes Artisse features many of the benefits of these more established technologies while also carrying a few even more unique advantages of its own.
She notes that, during treatments she has performed with Artisse, the device has displayed an ability to be positioned at the neck of the aneurysm both effectively and easily—meaning it does not “compromise” the parent artery—and can also “remodel” itself in order to truly conform to the shape of the aneurysm in question.
“That is a real advantage,” Killer-Oberpfalzer continues. “And, another advantage—my favourite— is the softness of Artisse. Usually, when you push an intrasaccular device into an aneurysm, your microcatheter gets pushed back and you may lose your position in the aneurysm, but that doesn’t happen with Artisse because it’s so soft. The microcatheter almost doesn’t move at all. It is very stable and easy to handle, and that makes it much easier to position the device.
“I always say that a monkey should be able to use the device. The easier the device is to use, the easier and shorter the procedure is, and the less likely you are to have complications. If a device gives you a better chance of not having complications, I think it is the next step and the next generation of device that should be used, and that’s why I’m really happy to have it available at the moment.”
While it remains unlikely that we will see a nonhuman primate in the angio suite any time soon, even with the latest technological advancements, Killer-Oberpfalzer believes the “self-explanatory, user-friendly” nature of Artisse means it should be relatively straightforward for less experienced clinicians and other first-time users to train with the device. Many of the outstanding benefits of Artisse that Killer-Oberpfalzer alludes to here— conformability, softness and overall ease of use— among others, are also endorsed by her peers.
“The ease of use of this new device is very
high,” Möhlenbruch comments. “I don’t think it’s a big deal whether or not you are an experienced neurointerventionist. If you know how to use other intrasaccular devices then, after you have seen one or two [Artisse] cases, you can easily use this device in a safe way. You can also use any 021 microcatheter you want with Artisse. There’s no need to use a specific microcatheter so, if you are more familiar with ‘microcatheter A’, you can use it; if you prefer ‘microcatheter B’ at a different centre, then you can also use it, which I think is a nice advantage.”
“In comparison to the WEB, which was the benchmark for us, we found the device to be extremely soft and ‘non-aggressive’—especially at the dome of the aneurysm,” Costalat adds. “The WEB tends to be very stiff before becoming soft again, whereas Artisse is soft immediately after starting the delivery. So, in this aspect, we have found the device to be extremely safe to use, particularly at the first step of deployment. The other thing we found to be extremely interesting was its ability to remodel and modify its shape according to the aneurysm. And, sometimes, you will fill part of the aneurysm that you may not expect to fill, because of its softness. It is surprisingly good in this sense. The ease of use is extremely high too, and I think it offers a great simplification of complex situations.”
Early experiences
All three physicians relay similar stories in terms of their usage of the Artisse device to date. KillerOberpfalzer and her colleagues in Salzburg began using it in the final months of 2023 and have completed a total of 17 cases with it thus far, at time of writing in early May 2024. She reports having treated ruptured and unruptured aneurysms alike to date, and almost “everything that is available” in terms of aneurysm locations: most commonly in the middle cerebral artery (MCA), but also the anterior and posterior communicating arteries (AComA/PComA), basilar artery, carotid artery, and posterior inferior cerebellar artery (PICA).
“The first case was a very difficult one,” she says. “I selected the aneurysm very carefully: a nice, small basilar tip. But, the access to the aneurysm was so difficult. It was the first case, so the whole team was there, and I said, ‘If this device pushes my microcatheter out of this aneurysm, I’ll coil it’. But then it was so easy—as I said before, [the microcatheter] did not move—it was unbelievable. Since then, in whatever case we’ve done, it’s astonishing how similar it is. It just, simply, works.” Killer-Oberpfalzer goes on to state that her experience has seen “one small problem” relating to
detachment of the device and, in light of this, advises prospective users of the importance of cleaning the delivery system carefully before the procedure. In addition, she says she is yet to experience any difficulties relating to the actual positioning of the Artisse device, leading to it becoming her go-to intrasaccular tool of choice.
“You always expect that something has to go wrong because, after a few cases, there’s always something that happens—there’s no perfect device in the world,” she comments. “But, so far, either I’ve been really lucky or, I think, it’s easier to use than the other intrasacculars that are available. All of my Artisse cases have been perfect, from the first one onwards.”
Möhlenbruch recounts that he and his colleagues in Heidelburg performed one of the first Artisse cases anywhere in Europe, which took place towards the end of 2022. This and every subsequent treatment at their centre have been completed as part of the INSPIRE-A registry—a real-world, postmarket database set up to evaluate the safety and efficacy of Medtronic’s various aneurysm therapies across Europe. And, similarly to Killer-Oberpfalzer, Möhlenbruch says they have treated “almost all” bifurcation aneurysms, with locations mainly consisting of the MCA, AComA, and basilar artery tip.
“We were one of the first centres using the Artisse device, and we are still one of the centres performing the largest number of treatments,” he notes. “We have treated close to 20 patients [as of early May 2024]—I have performed about two thirds of those cases—and we are very happy with the device.”
In addition, having initially had the opportunity to “play” with the device during its development phase, via in-silico testing, Costalat and his team in Montpellier began performing clinical cases with Artisse in the second half of 2023. Their centre has since used the device in a total of 30 cases, predominantly involving wide-neck bifurcation aneurysms in the MCA and anterior cerebral artery (ACA), but also some in carotid and basilar-tip locations as well.
Costalat, like Killer-Oberpfalzer and Möhlenbruch, further states he has seen the Artisse device used mainly in unruptured cases, as well as a smaller number of ruptured ones, with a consistent safety profile being observed across both.
“We have some six-month follow-up data coming, and almost for one year as well, and the follow-up up to now is pretty encouraging, showing no aneurysm recurrence at the moment,” Costalat says. “But, of course, we need mid-term and long-term follow-up to ensure that Artisse will be stable over time.”
Killer-Oberpfalzer corroborates these views, noting that six-month follow-up data from a total of roughly 50 patients are available to date, and the device has demonstrated a good safety profile therein.
“We need to have more data than that—I believe the device could be ‘less dangerous’ [meaning it may produce fewer complications], but that’s just my impression,” she continues. “We need to prove it, and we need long-term follow-up in terms of clinical outcomes and recanalisation rates. It is very early, but what we have seen so far is very promising.”
Here, Möhlenbruch claims that the Artisse device is especially well-suited to certain patient anatomies; specifically, aneurysms found in more vertical, ‘Y-shaped’ bifurcations.
“The proximal part of Artisse is also shaped like a ‘V’, and this may have some flow-diverting impact at the level of the bifurcation—which may also lead to a lower rate of recurrence of the aneurysm over time,” he adds. “We do not know this for sure at this time, but this is hopefully what we’ll see when we look to the one- and two-year follow-up data.”
Möhlenbruch goes on to highlight multiple positive characteristics of Artisse: “The overall friction when
8 Advertorial May 2024 | Issue54 THIS ADVERTORIAL IS SPONSORED BY MEDTRONIC
you introduce the Artisse device into the microcatheter is actually very low—the force you need in order to be able to push the system to the location you want is very low. This makes the whole procedure very safe, because it gives you much more control.
“Another thing is the visibility, which is always very important for us, as we like to see what we’re doing and how the devices act in the aneurysm. And, from my point of view, the visibility of the Artisse device is highly sufficient. You see when the device starts to open, you see when the device is completely open, and this also adds more safety to the whole procedure.
“The last point where I also see benefits is with the detachment system. You have a proximal marker located on the device, and the marker on the delivery wire, and—immediately after detachment—you see that there is a greater distance between these two markers, and you know that the device is detached. Again, this contributes to even greater procedural safety. In all but one of our cases, it has been very easy to detach, and there was no movement afterwards

either. This is also important because you will ideally have the same device morphology before and after you detach it. There is no change in the morphology of the Artisse device when you retrieve the delivery wire.
“From my personal experience, this is the best detachment system when we speak about intrasaccular devices. It’s very reliable—it takes a little longer, but then it is reliable.”
Simulating for success
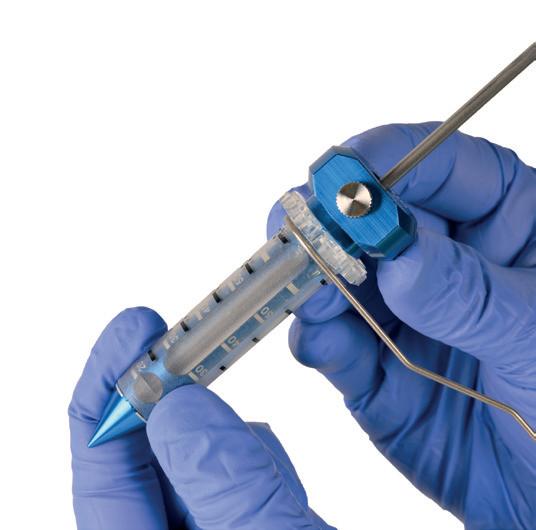
Once again, there is broad agreement between the three physicians over what the main limitation—and probably the only current limitation—of the Artisse system is. Killer-Oberpfalzer reports that only aneurysms up to a certain size, between 3mm and 6.5mm in diameter, can be treated using the device configurations that are currently available. Costalat agrees that concerns around sizing constitute the only real limitation of Artisse in its current form, as it cannot yet be used for very small aneurysms and its profile of delivery is still limited to 021 microcatheters.
In addition, when it comes to choosing the right device size for a given aneurysm, there is consensus between Killer-Oberpfalzer and Möhlenbruch on the importance of ‘oversizing’ slightly, in order to fill the aneurysm completely. If unsure between two different sizes, Möhlenbruch’s advice would be to go for the
larger of the two options, in order to ensure that the aneurysm can be completely filled. He attributes this to the softness and the ability of the device to configure, even to an irregular aneurysm morphology, “very well”.
However, both feel that these decisions are relatively simple and, in the words of the former, are made “even easier” by the artificial intelligence (AI)-powered preoperative planning tool Sim&Size (Sim&Cure), which Medtronic makes available to all Artisse users. Killer-Oberpfalzer also avers that combining the customary sizing chart provided by Medtronic with this simulation technology gives the operator “a really good selection base”.
“What we’ve found extremely valuable is the utilisation of the device with simulation assistance, as the simulation helps us to understand the final deployment of the device into the aneurysm sac as well as the level of compression of the device against the wall of the aneurysm,” Costalat contributes. “I would advise using the simulation software to size

the device, because only simulation can show you the wall apposition. We have learned that this is a very important risk factor for aneurysm recurrence—if the device is not apposed to the wall, or if you do not reach a certain level of compression. You should use simulation assistance, because you want to reduce the chance of making a mistake and you will add safety to the procedure.”
Wider implications and a positive outlook
Touching on the more general upsides of newer intrasaccular aneurysm treatments, Killer-Oberpfalzer says that ‘one-and-done’ devices like Artisse lead to shorter, smoother procedures that are often completed after one deployment—whereas coiling can take two hours or more, and may require the placement of several coils consecutively. She also relays that she has not needed to supplement any of her Artisse treatments to date with a stent. And, as is the case with other intrasacculars, Killer-Oberpfalzer highlights the avoidance of dual antiplatelet therapy (DAPT)—a requirement when treating intracranial aneurysms using a flow diverter—as another “big advantage” carried by Artisse.
“Comparing it to coiling—which is also an intrasaccular treatment—we know that coils recanalise, especially in wide-neck aneurysms,” she
adds. “Here, with Artisse, we have an intrasaccular device that acts like a flow diverter. Its stability, once [the aneurysm] is occluded, should be the same as with a flow diverter. So, less recanalisation, less medication, easier to use, shorter procedure times—there’s nothing more that we can really ask for!”
Möhlenbruch also weighs in on this point, stating:
“The goal of an intrasaccular device is to avoid using a stent or flow diverter in the parent artery, and, here, the major benefit of using an intrasaccular device is that we can reduce or avoid antiplatelet therapies, so that there is no additional risk created by the medication you have to give to the patient. The risk is low but, if you give DAPT to the patient, you do add some risk. And the beauty of intrasaccular devices like Artisse is that there’s no need to do this. Therefore, from my point of view, if we’re able to show that we can achieve stable results with Artisse, then I think it’s the future of treatment—the perfect treatment—for aneurysms that fit to the size of the device.”
“At the moment, I think it’s the best and the easiest

to use but, if you’d asked me a year ago, I would’ve said Contour is the only one,” Killer-Oberpfalzer says next. “There are always new things coming, and I’m pretty sure [Medtronic] will also develop Artisse further and that they already have ideas for how to make it even better. With further development and good ideas, it has a real future.”
“The first and most important point at the moment is that we can already prove that the device is very safe,” Möhlenbruch adds. “This is what we have seen from the INSPIRE registry’s safety data analysis—the rate of complications, periprocedural and postprocedural, is super low. So, it’s a safe device. The second question: is it effective? This is something we do not know for certain at the moment, because we are still following the patients. For this question, I would like to see one-year follow-up data from a GCP [good clinical practice] trial—like INSPIRE—and we are looking forward to these data right now.”
“Improving the safety profile of neurovascular interventions—for example, with Artisse—will push us in the direction of global screening for populations at risk of carrying aneurysms, and treating them in a systematic fashion, with very low risks,” Costalat concludes. “So, the growing generation of new intrasaccular devices may help us to reach the point where the preventive treatment of aneurysms will be far safer and can be offered at a large scale.”
9 Advertorial Issue54 | May 2024
DISCLAIMER: The data and content included in this article express only the clinical perspectives of the interviewees. They are completely independent and do not necessarily reflect the opinions of Medtronic.
Monika Killer-Oberpfalzer
Markus Möhlenbruch
Vincent Costalat
SNIS 21st Annual Meeting & Fellows Course
July 22 – 26, 2024
The Broadmoor I Colorado Springs, CO

in
with: European Society of Minimally Invasive Neurological Therapy
Programming
conjunction
REGISTER NOW

No-reflow in clinical studies: Causes, rates and implications for patient outcomes

In a guest article for NeuroNews, Adnan Mujanovic (Bern, Switzerland) discusses a relatively unknown but potentially significant factor in stroke patients experiencing poor clinical outcomes following a successful endovascular therapy (EVT) procedure—the ‘no-reflow’ phenomenon.
EVT HAS REVOLUTIONISED the treatment for patients with acute ischaemic stroke. Yet, despite these advancements, more than half of patients undergoing EVT end up with a poor clinical outcome. Researchers are looking into different causes of this ‘reperfusion failure’, and one potential reason for having a poor outcome despite successful reperfusion could be due to the no-reflow phenomenon. This phenomenon occurs when, despite successful macrovascular reperfusion and restoration of the blood flow in principle blood vessels, flow is not fully restored on the microvascular level in the affected brain tissue. The compromised microcirculation
in affected areas results in persistent ischaemia, exacerbating neuronal injury and impaired tissue recovery, which all leads to a poor outcome. Therefore, a comprehensive understanding of the underlying mechanisms of no-reflow is crucial.
Voyage into microvasculature
Several proposals have been put forward to explain the pathophysiology of noreflow. Damage to the microvasculature can lead to the capillary vasospasm and constriction of the pericapillary pericytes. Conversely, sudden restoration of the blood flow can trigger an inflammatory response, which leads
Early DAPT, statin therapy and stent patency among predictors of good post-thrombectomy outcomes
An analysis involving some 300 patients with anterior-circulation acute ischaemic strokes caused by tandem lesions has determined a number of factors that may be predictive of good clinical outcomes at three months following an endovascular mechanical thrombectomy procedure. Said factors include admission glycaemia; initiating dual antiplatelet therapy (DAPT) and statin therapy within 12 and 24 hours, respectively, post-procedure; and stent patency within the first 30 days.
WRITING IN CARDIOVASCULAR AND Interventional Radiology (CVIR), Daniel Šaňák (University Medical School and University Hospital Olomouc, Olomouc, Czech Republic) and colleagues note that these predictors of positive postthrombectomy outcomes were present in their study
to the aggregation of neutrophiles and leucocytes that can obstruct the capillary lumen. Lastly, clots can get fragmented during the intervention and distal emboli may cause downstream occlusion leading to hypoperfusion of distal regions. These different hypotheses on the cause of no-reflow have made it hard to identify molecules and biomarkers that may be targeted for prognostic or therapeutic purposes.
From angiograms to echoes
Another topic of discussion regarding the no-reflow phenomenon is how to detect it. Perfusion imaging has been widely accepted to detect changes in tissue blood flow; however, it is not yet clear which perfusion maps might be most suitable to detect subtle changes in the microvasculature. Digital subtraction angiography imaging has also been suggested as an imaging modality for detection of no-reflow due to its widespread use and convenience, although its potential to show tissuelevel changes is uncertain. Others have reported the use of transcranial Doppler and laser speckle contrast imaging for measurement of microvascular resistance and blood flow. This diverse array of imaging modalities makes it challenging to estimate true prevalence of no-reflow.
Chronological currents
The choice over the timing for measurements of no-reflow is also one of the crucial points for its accurate assessment. Prevalence of no-reflow seems to be highest when measured immediately after the intervention, followed by a decreasing tendency until reaching a plateau once 24 hours have passed since the intervention. However, a serial assessment of no-
“beside the generally known ones”.
“However,” the authors add, “it remains unclear whether early start of DAPT and statin therapy after [mechanical thrombectomy] could be affected by the preceding early neurological worsening followed with or without sICH [symptomatic intracranial haemorrhage] in some patients. Thus, a further large prospective study is needed.”
Initially averring that tandem lesions in the anterior circulation currently represent a “clinical challenge” in stroke thrombectomy treatment, Šaňák and colleagues attempted to assess potential predictors of good clinical outcomes in these patients via the multicentre, retrospective ASCENT study.
In ASCENT, a ‘good’ three-month outcome was defined as a score of 0–2 on the modified Rankin scale (mRS), while successful recanalisation was defined as a score of 2b–3 on the thrombolysis in cerebral infarction (TICI) scale. SICH was assessed using Safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST) criteria. In addition, logistic regression analysis was used to evaluate possible predictors of mRS 0–2, with adjustment for potential confounders. Ultimately, a total of 300 patients with a median National Institutes of Health stroke scale (NIHSS) score of 17—of whom 68.7% were male and who had a mean age of 67.3 years—were analysed in the study. Šaňák and colleagues note that recanalisation was achieved in 290 patients (96.7%), and 176 (58.7%) had an mRS score of 0–2.
reflow at different timepoints is still lacking. In an ideal study, a patient would systematically undergo imaging at several timepoints—starting from immediately after the intervention until the 24-hour threshold. Timepoints for estimating no-reflow in current studies seem to be guided more by institutional stroke-imaging protocols rather than by research-driven motives to discover the natural evolution of no-reflow.
Clinical compass
Despite these uncertainties, one thing on which the vast majority seem to agree is the notion that there is a clear association between no-reflow and poor outcomes. No-reflow has been associated with reduced rates of functional independence in multiple studies, and a recent meta-analysis of clinical data has also shown this association (odds ratio [OR], 0.2; 95% confidence interval [CI] 0.1–0.3).
This should further motivate strokeologists to define a comprehensive definition of what constitutes a ‘noreflow’, including clear outlines on imaging modalities, potential biomarkers and post-interventional timepoints that can be used for its assessment.
This journey into the waters of no-reflow is a dynamic one, but having a clear definition will help us effectively tackle this next frontier in acute stroke therapy.
References for this article can be viewed online.
Adnan Mujanovic works as a clinical research fellow at the University Hospital Bern Inselspital in Bern, Switzerland.
The author declared no relevant disclosures.
Besides the more well-known predictors of postthrombectomy outcomes, including age, NIHSS on admission, and sICH, the researchers identified that the following, less established factors were also present in ASCENT: lower admission glycaemia (p=0.005; odds ratio [OR], 0.884); stent patency within the first 30 days after thrombectomy (p=0.0003; OR, 0.219); DAPT started within 12 hours after thrombectomy (p<0.0001; OR, 5.006); and statin therapy started within 24 hours after thrombectomy (p<0.0001; OR, 5.558).
Prior to concluding, Šaňák and colleagues do acknowledge multiple limitations of their study, including its retrospective design and lack of central, blinded assessment of imaging findings.
They also note that thrombectomy-related strategies—including carotid artery stenting (CAS), periprocedural antiplatelet regimen and postprocedural management—were not standardised or unified across all participating centres, and the possibility that these differences may have impacted their results cannot be excluded.
In addition, they state that the fact some patients did not receive DAPT due to early neurological worsening and/or presence of ICH on computed tomography (CT) imaging performed earlier than the first 12 hours after thrombectomy may limit the interpretation of their findings. However, no association between starting DAPT early and sICH occurrence was found in the study, they add.
11 Issue54 | May 2024 Stroke Care
Point of View
Adnan
Mujanovic
“Education is the most powerful weapon you can use to change the world”
Nelson Mandela
Congress Presidents: Alessandra Biondi, France
Elisa Ciceri, Italy
Congress Vice-President: Paolo Machi, Switzerland
Congress Vice-President: Christian Taschner, Germany

4 – 6 September 2024 Palais du Pharo – Marseille, France
In collaboration with EANS, ESO and SNIS




www.esmint.eu

ENRICH trial results published in full in New England Journal of Medicine
The entirety of the results from the ENRICH trial evaluating the early surgical removal of intracranial haemorrhage (ICH) have been published in the New England Journal of Medicine (NEJM). As stated in a Nico Corporation press release, ENRICH demonstrated early minimally invasive parafascicular surgery (MIPS) intervention using the company’s BrainPath and Myriad technologies to treat ICH is safe and superior compared to the current standard of care: guideline-based medical management (MM) alone.
The ENRICH trial—a randomised, multicentre, adaptive clinical study—met its primary endpoints, indicating that MIPS improved outcomes for ICH patients owing to a statistically significant improvement in utility-weighted modified Rankin scale (UWmRS) scores at 180 days (MIPS, 0.458 vs. MM, 0.374). This difference showed a 98.1% posterior probability of superiority of MIPS compared to MM (95% confidence interval [CI], 0.005–0.163). In addition, MIPS was seen to have led to a reduced mortality rate at 30 days compared to MM (9.3% vs. 18.1%, respectively), and the approach also produced significantly decreased intensive care unit (ICU) length of stay (LOS) and hospital LOS with reductions of 2.8 days, and 3.1 days, respectively.
“The results of the ENRICH trial not only demonstrate the efficacy and safety of MIPS, but they also herald a transformative milestone for the entire stroke community, changing the ICH treatment paradigm through a standardised approach and advanced technology,” said Gustavo Pradilla (Emory University School of Medicine/ Grady Memorial Hospital, Atlanta, USA), co-lead investigator for ENRICH. “The ability to maximise the amount of clot evacuated in a safe manner is a pivotal advancement. We are steadfast in our commitment to collaborate with the medical community to educate on these interdisciplinary practices, and foster their widespread adoption across institutions and specialties. Together, we aspire to significantly improve the outcomes and lives of ICH patients, caregivers and loved ones.”
The ENRICH trial was set up to evaluate the efficacy, safety and economics of a standardised, early MIPS approach—performed within 24 hours—
across 300 patients with spontaneous haemorrhagic stroke. Of these patients, 92 had a haemorrhage in the anterior basal ganglia (ABG) location and 208 had a haemorrhage in the lobar location.
The trial enrolled patients at 37 stroke centres across the USA, with these patients being randomised to either a treatment group involving MIPS or a control group involving MM. The treatment group saw patients undergo procedures utilising two of Nico’s patented technologies: BrainPath, which the company claims is “the world’s first and only” system that achieves minimally disruptive access using a trans-sulcal and parafascicular surgical approach, and Myriad, which provides automated, non-ablative tumour removal and haemorrhage evacuation.
The primary intention-to-treat analysis of ENRICH evaluated whether average disability outcomes at 180 days, assessed via UWmRS scores, were superior in patients treated with MIPS compared to those who received MM alone. Safety endpoints were mortality at 30 days, and changes in haemorrhage volume from index to 24-hour computed tomography (CT) scan, while economic endpoints pertained to quality-adjusted life years (QALY) at 90, 120 and 180 days post haemorrhage.
Nico’s recent press release states that, overall, ENRICH showed that early MIPS intervention led to improved functional outcomes and increased safety, and demonstrated statistically significant economic improvements. As per one of the trial’s prespecified secondary endpoints, at each timepoint (7, 30, 90, 120 and 180 days), ordinal logistic regression analysis showed that the mRS-measured treatment effect was favourable in the MIPS group. Another prespecified secondary endpoint saw a
procedures were performed in 20% of MM group patients compared to only 3.3% of MIPS group patients.
Another finding from ENRICH was that in-hospital mortality was 4.7% in patients treated with surgery, which compared favourably to the 12.7% seen in the control group. All-cause mortality at final follow-up was similar between the two groups, as 30 patients in the MIPS group (20%) and 35 in the MM group (23%) had died at 180 days, giving rise to a combined mortality rate of 21.7% across the whole study. ENRICH investigators also found that the treatment group experienced 15% fewer serious adverse events versus the control group (MIPS, 95 [63.3%] vs. MM, 118 [78.7%]). Five patients treated with MIPS experienced rebleeding with clinical deterioration after surgery—a rate of 3.3%—and, “notably”, according to Nico’s recent release, there were nine patients with reported cardiac arrest in the surgical group compared to just two in the control group.
The results of the ENRICH trial not only demonstrate the efficacy and safety of MIPS, but they also herald a transformative milestone for the entire stroke community, changing the ICH treatment paradigm through a standardised approach and advanced technology.”
Gustavo Pradilla
significant reduction of 44mL in average haemorrhage volume within the MIPS group, compared to an increase of 4mL in average volume in the MM group— and the end-of-treatment volume goal of <15mL was achieved in 73% of patients in the MIPS group as well.
The company’s release also highlights multiple exploratory clinical endpoint findings from the trial, with one of these being that the mean number of ventilator days was significantly lower in the MIPS group versus the MM group, as per a reduction of 3.5 days. Furthermore, decompressive hemicraniectomy
The primary outcome results of the ENRICH trial were initially reported last year, at the 2023 American Association of Neurological Surgeons (AANS) annual scientific meeting (21–24 April, Los Angeles, USA), and demonstrated that the trial met its primary efficacy and safety endpoints at six months. Nico claims that, based on the trial design, the MIPS approach was found to be superior to MM for haemorrhages in the lobar location and statistically neutral in the ABG location—leading investigators to conclude their NEJM paper by stating that “the effect of surgery appeared to be attributable to intervention for lobar haemorrhages”.
The company is also expecting to announce further results from ENRICH, including economic outcome results that will quantify the cost per QALY gained through the MIPS treatment approach at specified timepoints. In addition, Nico has said it is anticipating a surgical outcome paper that will consist of a complete review of all 150 surgically treated patients.
“With its high rates of morbidity and mortality, and the combined cost of both acute treatment and long-term recovery, ICH is the costliest, most deadly and debilitating form of stroke—but, despite these facts, no surgical approach has produced level-one evidence to intervene until now,” said Jim Pearson, president and chief executive officer of Nico. “The success of our trial on MIPS for ICH demonstrates the pivotal role of safe and effective clot removal using our technology, coupled with early intervention. With intervention initiated within 24 hours—an average of 16 hours for trial participants—the MIPS group achieved a significant, 88% median haematoma volume reduction and improved mortality compared to the standard of care. The results from the trial are clear: the more effectively and quickly we remove blood off of the brain, the greater the patient’s chance of functional recovery and survival.”
13 Issue54 | May 2024 Intracranial Haemorrhage
NEUROSURGERY
Myriad (above), BrainPath (inset)
ADNAN SIDDIQUI
While he is a neurosurgeon by trade, Adnan Siddiqui (Buffalo, USA) has delved into an increasing number of domains throughout his career—not only through his interest in endovascular treatments and the neurosciences, but also via significant involvement in clinical trials, translational research, and even entrepreneurship. Here, the chief executive officer (CEO) and chief medical officer (CMO) of the Jacobs Institute, who is also a distinguished professor and vice-chairman of neurosurgery for the University at Buffalo (UB), speaks to NeuroNews to chart his journey and provide insights on several major topics.
What initially drew you to medicine, and the field of neurointervention specifically?
My first real encounter with neurosurgery was after a car accident in Pakistan. I was on vacation with my family in the Himalayas, and our Jeep fell off a mountain. My brother died from a spinal cord injury, and my sister was severely hurt due to closed head trauma— so, I had to deal with neurosurgeons every single day and I distinctly remember feeling that they had no idea as to what the outcome was going to be. It was just this incredibly primitive field and, because of that, I wanted to become a neurosurgeon who could give different answers to the ones I had received. That was right before I started medical school in Pakistan, at Aga Khan University. I wasn’t sure what I wanted to do in medical school—I thought maybe cardiac surgery, but all I was doing as a student was holding the heart in an ice-cold bucket, freezing my hands and not having fun! I didn’t particularly like general surgery or urology. Then, one day, I saw a neurosurgeon operating on a brain arteriovenous malformation (AVM). It was my first time seeing the living human brain and I thought it was the most magnificent, beautiful thing I’d ever seen. The brain ‘beats’ at its own pace—it makes us who and what we are. So, that was a very profound experience, and I was hooked. I said: “Okay, I’m going to become a neurosurgeon”.
Who have your key mentors been and how have they impacted your career? My first mentor was neuroscientist Shirley Joseph. I did my PhD with her at the University of Rochester. She was an anatomist by training, and I absolutely loved anatomy, but I also learned a lot about electrophysiology, molecular biology and such. Translational research has subsequently been a big part of my life, and I still run the Canon Stroke and Vascular Research Center doing entirely translational, basic science work. My neurosurgeon mentor was Charlie Hodge. He was neurosurgery chair at Upstate Medical University in Syracuse. He did complicated skull-base and vascular surgery, and he really inspired me in terms of the practices that I have on the cranial and open neurosurgical side. On the endovascular side, it’s somebody I later joined rather than trained with: Nick Hopkins. It’s not so much that I learnt particular techniques with him—it was that mindset, where you bring all the forces to bear. You bring your education, research background, cranial skills, and endovascular skills, to instigate change through innovation, through discovery, through entrepreneurship.
Having all those facets, all at the same time, is all Nick Hopkins. I would say, of all my mentors, he’s the one that I’m most indebted to in terms of who I am now.
The Jacobs Institute has become a centre of excellence for medical research in recent years—how have you and your colleagues achieved this?
The incredible imagination of Nick Hopkins led to this concept. At a time when many people were focused on neuroscience institutes, he thought we needed vascular institutes where cardiologists, interventional (neuro)radiologists, vascular surgeons, cardiac surgeons and neurosurgeons could pool their resources and experiences. That’s how the Gates Vascular Institute in Buffalo was born and, then—again, Nick’s genius—he convinced the university to build the Clinical and Translational Research Center, dedicated to neuro and vascular translational research, above the hospital. He also wanted to create a partnership-focused centre that could utilise hospital and university resources to optimise the field through entrepreneurship. That’s the genesis of the Jacobs Institute. Today, our goal is to create new medical devices that improve outcomes for patients who have neurologic or vascular diseases, and we’ve done a variety of things to help us achieve this. Early on, we created training programmes, leveraging incredible physicians like Elad Levy (neurosurgery), Vijay Iyer and David Zlotnick (cardiology), Sonya Noor (vascular surgery), and many others. We’ve developed a high level of competency training advanced practitioners—not just physicians, but also engineers, industry and regulators—on how particular disease states can be managed. Now led by Pam Marcucci, that’s been incredibly successful. We also gained an interest in advanced 3D-printed models of the brain to simulate the human anatomy, as well as good laboratory practice (GLP) animal studies, early feasibility studies (EFS) and other ways to test devices to support regulatory approval. We currently have eight first-in-human studies going on here in Buffalo. When Carlos Peña joined us from the US Food and Drug Administration (FDA), that was a godsend too, as he’s built an extremely robust regulatory services programme for companies aspiring to get their devices approved in the USA. The last part, we call ‘idea to reality’ (i2R), where someone comes to us with a concept, and we develop and prototype it for an equity share. That’s been quite a recent development but, now, we can essentially make any device for vascular or neurosurgical applications. So, you can see there’s an ecosystem that has evolved
FACT FILE
CURRENT APPOINTMENTS
CEO and CMO, Jacobs Institute (Buffalo, USA)
Distinguished professor and vice-chairman of Neurosurgery, UB Director, Canon Stroke and Vascular Research Center
Director of Neuroendovascular Critical Care and Neurosurgical Stroke Service, Kaleida Health
EDUCATION
1992: MBBS, Medicine/Surgery (Aga Khan University)
2003: PhD, Neuroscience (University of Rochester)
1999–2004: Resident, Neurosurgery (SUNY Upstate Medical University)
2005–2006: Fellow, Cerebrovascular Surgery, INR and Neurocritical Care (Thomas Jefferson University)
HONOURS (SELECTED)
2004: Arnold P Gold Foundation National Award for Humanism and Excellence in Teaching
2012: George Thorn Young Investigator Award, Jacobs School
2017: Inaugural recipient of Ahuja Endowed Clinical Professorship of Cerebrovascular Surgery
2021–2022: SUNY Chancellor’s Award for Excellence in Scholarship and Creative Activities
and, hopefully, it won’t be long before we have a successful exit for one or more of the startups in our i2R Center.
Of all the clinical studies you’ve been involved with, which do you think has had the greatest impact?
From close to 800 publications, a few really stand out. The earliest one is PREMiSe, which was my first true clinical trial. It assessed venous angioplasty—via the ‘liberation’ procedure—in multiple sclerosis (MS) patients. I learned the technique and did a double-blinded, sham-controlled, randomised trial, which demonstrated that, not only did the procedure not help, but it actually caused harm. So, that trial was negative but also incredibly impactful, because we helped prevent a lot of people with MS from undergoing unnecessary surgery. I’m also very proud of my participation in the PUFS trial that led to the approval of the Pipeline (Medtronic) device, as I think flow diversion has been absolutely transformational for aneurysm therapies. I was really frustrated with the 2013 stroke thrombectomy trials, but we were anticipating they may not be successful, and I worked with Jeff Saver and Mark Turco to design the SWIFT-PRIME trial alongside Elad Levy—he was national principal investigator (PI) and I remained on the steering committee. And, we all know how important that study was in proving that mechanical thrombectomy was the right thing to do in stroke and helping to open up the entire thrombectomy space. The most recent ones that I’m really proud of are the ENRICH trial, which has finally proven the benefits of minimally invasive clot removal in intracranial haemorrhage—and in which I was an active participant—and the EMBOLISE trial demonstrating middle meningeal artery embolisation as a potential treatment option in chronic subdural haematoma, which I developed alongside national PIs Jason Davies and Jared Knopman.
How do you see the neuroendovascular space evolving in the future?
We are at the very beginning of switching from our roles as ‘plumbers’ to people who actually interact with and manipulate the brain. Endovascularly, we’ve essentially been focusing on things that are ruptured or blocked, but I think brain-machine interface, brain neurophysiology and structural diseases are going to be the next transformational stage. Adel Malek and Carl Heilman’s idea of putting a tiny tube through the inferior petrosal sinus, into the cerebellopontine angle, to drain cerebrospinal fluid (CSF) and treat
14 Interview PROFILE May 2024 | Issue54
communicating hydrocephalus is absolutely brilliant, and there’s no question in my mind that it’s a gateway technology for others to use this transvascular route to do things in the brain. That could be to drain CSF, like Cerevasc, or to place devices inside—for example, to avoid doing a craniotomy entirely when implanting a brain-machine interface, like Synchron. So, just as cardiology was revolutionised by electrophysiology and structural heart, I think that’s coming to the neuro space now.
Which research areas could have the greatest impact on neurointerventional surgery over the next 10 years?
I’m currently focusing a lot of what I do at the Canon Stroke and Vascular Research Center on endovascular treatments for glioblastoma multiforme, and using the vascular route to deliver chemotherapy to maintain, shrink or even cure the tumour. We’ve begun a line of research testing this in rats to see which agents work and I think that’s going to be an incredible opportunity for neuro-oncology—
it’s probably a few years away, but it’s coming. I’m also really fascinated by the work of Maiken Nedergaard, who discovered the glymphatic system, and showed that CSF is absolutely critical in every aspect of brain functionality. We now have the ability to potentially manipulate these ‘glymphatics’ to improve outcomes for disorders not considered at all neurosurgical; degenerative diseases like Alzheimer’s and cognitive dementias, or normal-pressure hydrocephalus. These are all just hypotheses and, again, currently at the animal-testing stage, but I think that’s going to be a whole new area and I’m really excited about it.
What are your hobbies and interests outside of medicine?
I love playing squash with my sons and hoping they go easy on me! I enjoy travelling to new places, and I’m fortunate that I’m invited all over the world and can gain that experience. I love watching good movies—Gladiator is probably my favourite—and the only type I don’t enjoy is horror. Finally, I must admit I’m
"We are at the very beginning of switching from our roles as ‘plumbers’ to people who actually interact with and manipulate the brain.”
addicted to binge-watching a good TV series at night.
What is your favourite place to visit?
I have a strong bias towards Jackson Hole; it’s one of my favourite places on the planet. When I go, it just lowers my heartrate by 10–15 beats and, someday, I’d like to have a place there.
If you had not opted for a career in medicine, what do you think you would be doing for a living today?
When I applied, I promised myself that—if I did not match in a neurosurgical programme the very first time—I would quit medicine and science entirely, and go into foreign affairs, and some kind of international peacekeeping profession. As a neurosurgeon, I’ve done 20,000 or so cases, and hopefully helped most of those patients, but the impact diplomats and peacekeepers can have is logarithmically even greater. I’ve always been fascinated by that area and, if I have enough life in me after neurosurgery, maybe I’ll still do something like that.
15 Interview alisonlang.com Issue54 | May 2024
ESMINT issues ‘call for experts’ to streamline EU medical device certification
The European Society of Minimally Invasive Neurological Therapy (ESMINT) has issued a call for experts in neurointerventions to work with notified bodies over the certification process of medical devices within the European Union (EU).
THE SOCIETY RECENTLY established a Medical Device Regulation (MDR) Task Force, chaired by ESMINT treasurer Christian Taschner (University Hospital Freiburg, Freiburg, Germany), with the aim of supporting the CE-certification process for neurointerventional medical devices by establishing a database of experts in this field.
As noted in a recent ESMINT press release, these experts will work with EU notified bodies—organisations designated by EU member states or through specific agreements to assess the compliance of medical devices before they reach the market— providing technical input and advice in the certification process. According to
ESMINT, this addresses an “urgent need”: certification processes for medical devices in the EU are increasingly delayed due to a shortage of experts to advise notified bodies across Europe.
The society has therefore initiated a call for clinical experts who will be able to leverage their clinical and scientific expertise to participate in the

Meta-analysis deems venous sinus stenting
a safe
and effective option in selected IIH patients
A meta-analysis published recently in the Journal of the Neurological Sciences has concluded that venous sinus stenting (VSS) appears to be a safe and effective treatment option in certain idiopathic intracranial hypertension (IIH) patients—specifically, those who do not respond to medical therapy or have significant visual symptoms.
“PATIENTS SHOWED SIGNIFICANT improvement in IIH symptoms post-VSS, with 79% experiencing reduced headaches, 89% improvement in papilloedema, and 88% relief in diplopia or visual disturbances,” write corresponding author Ahmed Azzam (Albert Einstein College of Medicine, New York, USA) and colleagues. “VSS demonstrated a promising potential as a minimally invasive treatment for IIH, especially in patients who did not respond to medical therapy or had significant visual symptoms. It significantly reduced symptoms with a relatively low complication rate.”
CE-certification process of medical devices for neurointerventions. A database of experts will be compiled, and notified bodies will be able to select experts according to their particular areas of expertise in the neurointerventional field. ESMINT details in its recent release that, in collaboration with the engineers and technicians at the notified bodies, these experts will ensure a comprehensive evaluation of medical devices, ultimately guaranteeing the safety of users—both patients and doctors alike.
“This initiative highlights ESMINT’s unique role in the field and
This initiative highlights ESMINT’s unique role in the field and in Europe.”

in Europe,” said ESMINT president Zsolt Kulcsár (University Hospital of Zürich, Zürich, Switzerland). “And, Professor Taschner is singularly qualified to move this forward, given his role at the European Medicines Agency’s [EMA] Expert Panels, where he serves as member of the coordination committee as well as vice-chair for the expert panel on vascular implants.”
New BCI registry launched to support motorimpaired patients
Synchron has announced the launch of a community-centred brain-computer interface (BCI) registry to bring patients, carers and clinicians together to learn about how BCI technology is being utilised to provide benefit to people with limited mobility.
The company’s BCI technology— primarily seen in the endovascularly implanted Stentrode device—is designed to decipher the neural code of the brain and find new ways to restore motor intent to control digital devices, ultimately restoring functionality and independence in patients with limited mobility.
“There is a grass-roots movement happening with BCI,” said Synchron chief executive officer and founder Tom Oxley. “We are creating an avenue for potential users and their physicians to engage, and stay connected, while we prepare for the next stage of clinical trials.”
“By controlling digital devices through one’s own thoughts, BCI offers a transformative path towards performing daily tasks with greater ease and efficiency,” added David Lacomis (University of Pittsburgh, Pittsburgh, USA), the registry’s principal investigator. “From communication to accessing essential services online, BCI technology represents a groundbreaking frontier for individuals and their families.”
The researchers also report that, post-VSS, they observed a “significant reduction” in the transstenotic gradient pressure and the lumbar puncture cerebrospinal fluid opening pressure—both of which are indicative of the effective alleviation of intracranial pressure. In addition, their analysis found a major complication rate of 3.93% and a minor complication rate of 2.72% post-VSS, indicating that the procedure
is “relatively safe with manageable risks”. In light of the fact that the safety and effectiveness of VSS to treat IIH remains somewhat “controversial”, Azzam and colleagues conducted a systematic review and meta-analysis following Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines that involved searching multiple databases for studies evaluating the use of VSS for this condition, and pooling the subsequent data. A total of 36 studies involving 1,066 patients who underwent VSS were included in the researchers’ final analysis. In addition to the aforementioned improvements regarding headaches, papilloedema and visual disturbances, Azzam and colleagues report a 95% rate of improvement in tinnitus symptoms as well. However, they continue, some 13.7% of patients experienced either treatment failure or complications, with 8.35% of this accounting for treatment failure characterised by worsening symptoms and IIH recurrence. The authors further note an overall complication rate of 5.35%, with these complications including subdural haemorrhage, urinary tract infection, and stent thrombus formation, among others.
Azzam and colleagues conclude that, while their findings suggest VSS constitutes a safe and effective option in the treatment of IIH patients who are unresponsive to medical therapy or have significant visual symptoms, the long-term outcomes and safety relating to the procedure “require further investigation”.
16 May 2024 | Issue54 Medical Devices
Post-VSS improvements in IIH symptoms 79% Reduced headaches 89% Improvements in papilloedema 88% Relief of diplopia or visual disturbances
ESMINT
Left: Zsolt Kulcsár and Christian Taschner
Growing haemorrhagic stroke burden in USA represents “major challenge” moving forward
The past three decades have seen a “notable rise” in the burden of haemorrhagic stroke across the USA and this, coupled with the increasing burden of stroke more generally, represents an “evolving and substantial public health challenge” for the country to contend with. These are among the key findings from a cross-sectional analysis of the 2019 Global Burden of Disease study, which was published recently in JAMA Neurology
RESEARCHERS DANIELA RENEDO
(Yale School of Medicine, New Haven, USA) and colleagues aver that, while decreases in the agestandardised rates of ischaemic and haemorrhagic stroke between 1990 and 2019 “show progress”, their analysis also identified disparities across different age groups and geographic regions—highlighting the need for targeted interventions.
“Stroke is a leading cause of death and disability in
the USA,” Renedo and colleagues write, outlining the study’s significance. “Accurate and updated measures of stroke burden are needed to guide public health policies.”
The researchers’ in-depth analysis of the 2019 Global Burden of Disease study—from which results were initially released in October 2020—encompassed estimates for various types of strokes, including all-cause strokes, ischaemic strokes, intracranial haemorrhages (ICHs), and subarachnoid haemorrhages (SAHs). Its primary focus centred on both overall and age-standardised estimates of stroke incidence, prevalence, mortality and disability-adjusted life years (DALYs) per 100,000 individuals.
In 2019, the USA was found to have recorded a total of 7.09 million prevalent strokes, with more of these happening in women (57.4%) than in men (42.6%). In addition, the majority of these stroke cases were ischaemic in nature (82.7%), with prevalence also including roughly 660,000 ICHs and 850,000 SAHs.
“Although the absolute numbers of stroke cases, mortality and DALYs surged from 1990 to 2019, the age-standardised rates either declined or remained steady,” Renedo et al report. “Notably, haemorrhagic strokes manifested a substantial increase, especially in mortality, compared with ischaemic strokes.”
The authors go on to note that the incidence of ischaemic stroke rose by 13% across this near-30-year period, while incidence rates for ICH and SAH—two prevailing types of haemorrhagic stroke—increased by 39.8% and 50.9%, respectively.
“The downturn in stroke mortality plateaued in the recent decade [since 2010],” Renedo and colleagues continue. “There was a discernible heterogeneity in
Advancing neurosurgery inside and outside the operating room

Point of View
Brian Nahed (Boston, USA) outlines the latest advances in neurosurgery, and why it is important for those working in the field to keep up to date and continually hone their skills, in a guest article for NeuroNews.
Training and education are the foundation of progress in every area of science; even more so in surgical specialties, in which the craftsmanship component is implied.1 Experiencing an exponential rate of expansion within neurosurgery is what makes the specialty both challenging and fulfilling.
Neurosurgery training: successes and challenges
Neurosurgery training is typically structured around an expertapprentice approach. Trainees perform
different surgeries several times throughout a seven-year residency programme.1 Today, the field is moving towards not only qualifying residents to become neurosurgeons, but also quantifying the experience, ensuring they can perform surgery safely and effectively as future leaders. Programmes nationwide in the USA have focused on holistic neurological surgery training—which includes developing the best surgical skills while also providing leadership training, mentoring and coaching so that neurosurgeons can play a large role both
stroke burden trends, with older adults aged 50–74 years experiencing a decrease in incidence in coastal areas (decreases up to 3.9% in Vermont), in contrast to an uptick observed in younger demographics aged 15–49 years in the South and Midwest USA (with increases up to 8.4% in Minnesota).”
Delivering their concluding messages, the analysis’ authors note that declining age-standardised stroke rates over the past three decades suggest recent progress has been made when it comes to managing stroke-related outcomes.
“However,” they add, “the increasing absolute burden of stroke—coupled with a notable rise in haemorrhagic stroke—suggests an evolving and substantial public health challenge in the USA. Moreover, the significant disparities in stroke burden trends across different age groups and geographic locations underscore the necessity for region- and demography-specific interventions, and policies to effectively mitigate the multifaceted and escalating burden of stroke in the country.”
Notably, haemorrhagic strokes manifested a substantial increase, especially in mortality, compared with ischaemic strokes.”
inside and outside the operating room.
Residents receive an immense amount of support and encouragement around their research and academic interests, especially when it comes to improving surgical techniques and better understanding the disease pathology neurosurgeons are likely to see every day.
In this specialty, technology and techniques are constantly evolving. Current and future neurosurgeons are challenged to stay on top of the latest advancements while keeping a broad representation of surgical skills.
Hot topics in brain tumours
According to the National Brain Tumor Society (NBTS), an estimated 94,390 people will have received a new primary brain tumour diagnosis in 2023.2 While an estimated 71% of discovered tumours in 2023 will be considered benign, approximately 29% will be found to be malignant. Transformation around a diagnosis that impacts such a sizeable portion of our population is crucial.
Recent immunotherapy and surgical technology advances have allowed for safer and more effective outcomes in the treatment of brain tumours. Traditionally, neurosurgery had focused on treating neurological issues using invasive techniques. Today, neurosurgeons are using minimally invasive and even some non-surgical approaches to treat neurological diseases. In functional neurosurgery, surgeons have found innovative methods to modulate or add functionality as well. Vascular neurosurgery has made significant advances with the
development of minimally invasive endovascular techniques to treat very difficult vascular lesions. Patients are now often sent home the same day after a procedure, and with great outcomes. With continuing advances and innovations, conditions that were once difficult to treat surgically can be treated endovascularly with lower morbidity rates.
The field of liquid biopsy has grown significantly in the detection of cancers. Neurosurgeons have led the development of liquid biopsies using blood or cerebrospinal fluid so that they can diagnose brain tumours and potentially identify recurrence of tumours, and help guide immunotherapy to provide real-time treatment based upon a patient’s tumour and specific mutations.
With the rapid advancements currently happening in neurosurgery, it will be critical for surgeons to continue to improve their skillset and awareness around the latest news to help ensure their ability to provide patients with the safest, and most effective, treatment options.
References: 1. Bongetta D, Zoia C. Editorial: Training and education in neurosurgery: Challenges and strategies for the next ten years. Front Surg. 2022; 9: 984208. 2. Porter K R, McCarthy B J, Freels S et al. Prevalence Estimates for Primary Brain Tumors in the United States by Age, Gender, Behavior, and Histology. Neuro Oncol. 2010; 12(6): 520–7.
Brian Nahed is a neurosurgeon and director of the neurosurgery residency programme at Massachusetts General Hospital in Boston, USA.
The author declared no relevant disclosures.
17 Issue54 | May 2024 Future Prospects
Brian Nahed
First European PERFORMANCE II release highlights carotid stenting’s durability in high-risk patients
Continued from page 1
that “there aren’t really any controversies left” relating to CAS, and its ability to produce outcomes equivalent to CEA.
PERFORMANCE II in Europe
“The implications from the results of the PERFORMANCE II trial are that procedural embolic protection is the most important aspect in minimising stroke during CAS, and the Neuroguard three-inone carotid stent system [Contego Medical] with 40-micron protection will be a transformational technology for the treatment of carotid artery disease.”
Those were the words of Langhoff, speaking to NeuroNews on the eve of CX 2024 to outline the significance of the study and its findings. Langhoff, who is the European PI for PERFORMANCE II, took to the CX podium during the Acute Stroke and Carotid Controversies programme on Wednesday 24 April to discuss these data in greater detail—a European first, following their initial presentation at last year’s Vascular Interventional Advances (VIVA) meeting (30 October–2 November 2023, Las Vegas, USA).
The prospective, multicentre PERFORMANCE II trial was set up to evaluate the safety and effectiveness of the Neuroguard integrated embolic protection (IEP) system, enrolling carotid disease patients with stenosis ≥50% if symptomatic or stenosis ≥70% if asymptomatic. According to Contego, the company behind this technology, the Neuroguard IEP system incorporates a novel, closed-cell nitinol stent, a balloon, and a filter with 40-micron pores, into a 6Fr device intended to capture micro-emboli and provide protection against stroke risks.
Langhoff’s podium-first presentation at CX saw him report low rates of minor (1.3%) and major (0%) stroke at 30 days across a total of 305 patients at 40 different clinical sites in PERFORMANCE II’s intention-to-treat (ITT) analysis, as well as a myocardial infarction (MI) rate of 0.7% and a death rate of 0.3%. He also relayed that no major strokes, contralateral strokes or neurological deaths ultimately occurred at 30 days.
Moving onto the trial’s primary endpoint (any stroke, death or MI at 30 days, and ipsilateral stroke from day 31 to 12 months), which was assessed across 282 patients at one year, Langhoff detailed a rate of just 2.8% in ITT analysis and 2.5% in the per-protocol analysis. The presenter went on to share rates of 3.7% for in-stent restenosis, 0% for neurological death, and 1.1% for target-lesion revascularisation, as per oneyear ITT analyses. No major strokes nor instances of stent thrombosis occurred at one year either, according to Langhoff, and only one minor stroke was observed from day 31 to 12 months.
Putting these findings into context, Langhoff then pointed out that the 30-day ‘all stroke’ rate of 1.3% seen in PERFORMANCE II compares favourably to those observed with CEA in trials like ACST-2 (2.4%) and CREST 1 (2.3%), and is marginally better than in ACT 1 (1.4%) as well. This is especially notable, in Langhoff’s view, as all three of these studies included patient populations generally considered to be ‘standard risk’, while PERFORMANCE II exclusively enrolled patients deemed ‘high risk’ for CEA.
The speaker further noted that similar trends were present regarding the primary endpoint outcome of
PERFORMANCE II (2.8%), in comparison with the same measures for CEA in CREST 1 (6.6%) and ACT 1 (3.3%), at one year.
Langhoff’s concluding message was that—across a “challenging, high-risk” cohort of carotid artery stenosis patients—the PERFORMANCE II trial has demonstrated the durability, safety and effectiveness of the Neuroguard IEP system at both 30 days and one year.
The presenter also averred that “these are the lowest one-year event rates reported for an adequately powered multicentre trial of any type of carotid revascularisation, regardless of patient risk”.
Stenting versus surgery
Discussions during the same session at CX 2024 also gave delegates—vascular surgeons, interventional cardiologists and neurointerventionists alike—a chance to address the current state of play in carotid disease treatment.
“I was asked to speak about carotid stenting controversies, and I would submit that there aren’t really any controversies left,” said Gray, alluding to the multitude of global trials, spanning high/low-risk and symptomatic/asymptomatic patients, that indicate “there’s really no difference between endarterectomy and stenting”. He cited data from more than 6,700 asymptomatic carotid artery stenosis patients across CREST 1, ACT 1, SPACE-2 and ACST-2, and a further 3,000 symptomatic patients from the CREST 1 and ICSS trials of 2010, to support the notion that transfemoral CAS appears to be at least equivalent to CEA.
While Gray went on to highlight comparable 30-day rates of any stroke/death/MI, and of major stroke, between CAS and CEA in seminal studies like CREST 1 and ACT 1, he did acknowledge lingering concerns over minor stroke rates, which were roughly twice as high with stenting in both trials.
“Where’s the controversy? I think it lies here,” Gray added. “I should note that minor stroke in these patients is typically recovered [from] within a week or two, and certainly by one month. Nevertheless, it’s not something we aspire to have.”
One of Gray’s key points subsequent to this was that “all is not lost”, as he drew the audience’s attention to the fact that—even prior to the technological advances seen in stenting over the past few years— improvements in patient selection and procedural techniques may have already instigated better CASrelated outcomes.
Here, he referenced 2019 data from the CREST-2 registry, which reported “remarkable” stroke/ death rates of 1.4% in asymptomatic and 2.8% in symptomatic patients; “about as low as has ever been reported [with] endarterectomy or stenting”.
The latest technologies
Two subsequent talks attempted to take a closer look at the technological advances alluded to previously by Gray.
Firstly, Musialek took to the podium, initially outlining the relevance of the timing of CAS-related stroke, as roughly 50% of strokes occurred postprocedurally in CREST-1, with a similar trend being observed in the 2007 CAPTURE trial and other large studies. The problem, according to Musialek, related to the limitations of the conventional, single-layer carotid stents being used in those early trials, and their inferior ability to fully eliminate plaque as compared to surgery.
“So, it’s not surprising that the key opinion leaders in vascular surgery said that—with the technology available at that time—CAS cannot be considered equivalent to CEA,” he noted. “But, if the

technologies improve, we will come back and reevaluate.”
This provided a fitting introduction to discussions of several newer, second-generation ‘mesh’ carotid stents, such as the Hybrid Vascular Graft (Gore), the Casper/Roadsaver stent (Terumo), and the CGuard embolic prevention system (EPS; InspireMD).
Musialek stated that, despite some similarities, these devices are mechanically different, and some of said differences may translate into varying clinical outcomes.
However, he went on to highlight that recent studies have generally found significantly reductions in incidence of embolic material in filters, filter load, and CAS-related cerebral injury, with these newergeneration stents.
Musialek also drew attention to two metaanalyses—one comparing first- and second-generation carotid stents, and another comparing secondgeneration stents to contemporary CEA data—that have provided indications of the short- and long-term clinical benefits these more novel devices may enable.
“There is always a role for surgery,” he added,
18 May 2024 | Issue54 Carotid Stenting
COVER STORY continued
These are the lowest one-year event rates reported for any adequately powered multicentre trial of any type of carotid revascularisation, regardless of patient risk.”
Ralf Langhoff

“but the landscape has changed. Today, patients need to be fully informed and involved in the decision of how they are revascularised, and the decision in a given centre should be determined by a multispecialty neurovascular team. Today’s carotid stents are cerebral protectors and, with respect to clinical decisionmaking, historical data have mostly historical value— we are treating patients today, not 20 years ago.”
A closing message from Musialek was that, in wider medicine, there is an “evolution” towards less invasive treatments, and this point was corroborated by Metzger during the session’s final presentation.
“Am I going to tell you that stenting is equivalent [to CEA] for every patient with obstructive carotid artery disease? Absolutely not,” he began. “In fact, it should only be performed if the patient has favourable anatomy and appropriate history for CAS, and the operator themselves is experienced, uses good technique, and has considered all options and considers stenting to be best. That said, CAS has matured to where it is an overall equivalent strategy, and this has developed under very close scrutiny for a long period of time.”
Metzger went on to posit that treatment strategies including CEA, CAS, and also best medical therapy, should be viewed as “complimentary, rather than competitive”, and emphasised the benefits of an individualised approach for each and every carotid stenosis patient.
Next, he touched on the fairly recent introduction of the aforementioned Neuroguard and CGuard stents, both of which have been specifically designed to reduce embolic events. In addition to once again highlighting “excellent” data from PERFORMANCE II, Metzger recapped the findings of the prospective, multicentre C-GUARDIANS trial evaluating the investigational CGuard device in 316 patients enrolled at a total of 25 US and European sites. He stated that—across a patient population that is, “by definition”, high-risk for endarterectomy, and 25% of whom were symptomatic—the study produced a 0.95% rate of stroke/death/MI at 30 days in its ITT analysis, and an even lower rate of 0.63% in per-protocol analyses.
As with PERFORMANCE II, these findings were presented for the first time at VIVA 2023 and,
according to Metzger, compare “very favourably” with historical endarterectomy outcomes.
“CAS is an excellent strategy, which is equivalent to the other options in well-selected patients treated by experienced operators—and new technology may lead to even better results,” Metzger concluded.
Attention is now likely to turn to upcoming results from trials assessing these ‘third-generation’ stent technologies.
One-year, primary-endpoint data from C-GUARDIANS—pertaining to the incidence of major adverse events including any stroke/death/MI through 30 days post-index procedure, or ipsilateral stroke from day 31 to day 365 post-procedure—are expected later this year, and may instigate US Food and Drug Administration (FDA) approval of the CGuard device.
In addition, while PERFORMANCE II saw Neuroguard placed via transfemoral or transradial access, the first patients have already been enrolled in the investigational device exemption (IDE) PERFORMANCE III study evaluating the same stent when implanted via direct transcarotid access.
19 Issue54 | May 2024 Carotid Stenting
Ralf Langhoff presents PERFORMANCE II data in front of CX executive board members Barbara Rantner and Domenico Valenti
Hans-Henning Eckstein: “bridge-builder” between vascular surgery and neurology dies aged 68
Hans-Henning Eckstein, the vascular surgeon who played a leading role in the SPACE and SPACE-2 randomised controlled trials on the treatment of carotid stenosis, and founder of the Munich Vascular Conference (MAC), died on 24 February at the age of 68. Colleagues have paid tribute to an “excellent doctor, outstanding researcher and university professor” as well as a “bridge-builder between vascular surgery and neurology”.
ECKSTEIN HAD BEEN CHAIR and professor of the Department of Vascular and Endovascular Surgery at the University Hospital Rechts der Isar of the Technical University of Munich (Munich, Germany), and was widely recognised for advancing research and knowledge in the field of vascular surgery. He was awarded an honorary doctorate from the Medical Faculty of the University of Larissa (Larissa, Greece) in 2017 and, since 2019, had also been a visiting professor at the Medical School of Pittsburgh (Pittsburgh, USA) and Stanford University (Stanford, USA).
After studying medicine at Ruprecht-Karls University Heidelberg (Heidelberg, Germany), Eckstein
completed his PhD in 1986 and two years later acquired his postdoctoral teaching qualification in Heidelberg. From 1999 until 2003, Eckstein was medical director of the Clinic for Vascular Surgery at Ludwigsburg Hospital (Ludwigsburg, Germany). In 2004, he became director of the Department of Vascular Surgery at the University Hospital Rechts der Isar of the Technical University of Munich and, five years later, was appointed the first holder of the newly created chair for vascular and endovascular surgery at the university—a role he held until his retirement in 2023.
A statement on the Technical University of Munich’s website mourning the loss of Eckstein reflects
on his impact at the institution: “Prof Eckstein made the clinic known far beyond the borders of Munich, and developed it into a leading centre and attraction for patients with vascular diseases.”
Eckstein played a leading role in research including randomised controlled trials on symptomatic and asymptomatic carotid stenosis, which—a statement on the European Stroke Organisation (ESO) website reads—made him “well-known” in vascular neurology. He was co-chair of the steering committee of SPACE and SPACE-2, representing vascular surgery in both multicentre trials.
(Vascular Surgery). Eckstein was also involved in vascular education and founded the MAC, the 12th iteration of which took place in December 2023 and received international attention.

Furthermore, Eckstein was heavily involved in the creation of international guidelines in his field, including the German S3 guideline for the diagnosis, therapy and follow-up care of patients with carotid stenosis, and the ESO guideline for carotid stenosis.
Eckstein’s involvement in professional organisations included his 2009–2010 presidency of the German Society of Vascular Surgery and Vascular Medicine (DGG), as well as his editorship of the journal Gefässchirurgie
Several colleagues have paid tribute to Eckstein and highlighted his legacy in the fields of vascular surgery and neurology. “We are deeply saddened by the death of Prof Eckstein,” a statement on the Technical University of Munich’s website reads. “With Prof Eckstein, we are losing an extremely committed colleague and excellent doctor, outstanding researcher, and university professor.”
Eckstein is also remembered in a statement on the ESO website, with Werner Hacke, senior professor of neurology at Ruprecht-Karls University of Heidelberg, and Peter Ringleb, academic researcher at University Hospital Heidelberg (both Heidelberg, Germany), stating: “In Hans-Henning Eckstein, we have lost an extremely dedicated colleague and excellent physician, an outstanding researcher, university lecturer, bridgebuilder between vascular surgery and neurology, and a friend.”




20 May 2024 | Issue54 Vascular Surgery *Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the vascular arena Editorially independent Subscribe today Available in print and digital formats and through our social channels Visit vascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription**
TRIBUTE
Hans-Henning Eckstein
New data underscore physician experience levels required to derive benefit from transfemoral carotid artery stenting
Data from a new study that maps out the levels at which adverse postoperative events decrease following transfemoral carotid artery stenting (TfCAS) based on depth of physician experience with the procedure represent an “absolute minimum threshold of learning”, according to the lead author.
This analysis comes in the wake of the US Centers for Medicare & Medicaid Services (CMS) decision to expand coverage for carotid stenting procedures and, in doing so, loosen restrictions on the use of TfCAS.
Findings from an analysis of the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative (VQI)— which were presented at the 2024 Society for Clinical Vascular Surgery (SCVS) annual meeting (16–20 March, Scottsdale, USA)—showed that the number of procedures a physician had to perform in order to get an in-hospital stroke and death rate below 4% in symptomatic patients was 235. Similarly, to achieve
a stroke and death rate below 2% in asymptomatic patients, the threshold physicians had to reach to derive benefit was 13 procedures.
Rates were based on the fact the best available VQI registry data relate to in-hospital stroke and death, not the 30-day stroke and death thresholds of 6% and 3% for symptomatic and asymptomatic patients, respectively.
Marc Schermerhorn (Beth Israel Deaconess Medical Center, Boston, USA), the study’s senior author, raised concerns over how the recent CMS coverage expansion will interplay with data reporting and, ultimately, the tracking of stroke and death rates going forward.
“Now that CMS has done away with all the regulations and restrictions on who can do [TfCAS] procedures, where [they can do them] etc., people may not submit data to VQI anymore, unfortunately,” he said.
“There have been a lot of other studies that have shown transfemoral stenting outcomes are not as good as TCAR [transcarotid artery revascularisation] and CEA [carotid endarterectomy], but I think the concern now is, by opening up the doors for anybody to do carotid stenting, there could be a lot of people who have never done carotid stenting before who now decide to get into the game.
“I think that the way things were before, when you had to be an approved centre and you had to have credentialing committees that had to collect data, monitor that local data, and submit it to Medicare, that prevented a lot of people from doing the procedure. So, it concentrated in the people who have the most experience.”
However, the respective thresholds for symptomatic and asymptomatic cases represent the basement of learning, in Schermerhorn’s view.
“You have to remember that transfemoral stenting has been around since the turn of the century—and even before that,” he continued. “The VQI didn’t start collecting data on carotid stenting until 2005, and that was only in New England. It wasn’t until 2011 that

we started to collect national data. So, most people who were in the carotid stent registries in the VQI had experience before they started submitting data, and so we actually don’t think this is a full representation of the learning curve.
“We know almost certainly most people had been doing cases prior to entry of data into the VQI, so this is an absolute minimum threshold of learning curve, and the true learning curve is likely larger. I don’t know how much, but it’s higher numbers.”
Schermerhorn said that, with the possibility of “a lot more novices” performing TfCAS in the wake of CMS coverage expansion, his team’s latest study clearly demonstrates “results are highly dependent on experience”.
Even supposing a lot of TfCAS experience, he went on to state that “the results don’t seem to be as good as with either TCAR or endarterectomy”.
Despite the potential for TfCAS entries into the VQI registry to decrease, Schermerhorn expects monitoring to continue, including via administrative databases, albeit with a lower level of clinical detail available, but expressed hope enough interest would persist in submitting data to the VQI.
“That’s my other concern,” he noted. “The people who are interested in submitting data are probably the ones who are more committed to quality improvement and striving toward knowing what their outcomes are—and improving them.”
That raises another dimension of the study presented at SCVS 2024, noted Schermerhorn: the research team also looked at whether those who had bad outcomes when they first started doing procedures were less likely to continue performing TfCAS. They found that this was indeed the case.
“The people who went on to do more than 25 procedures had better outcomes than the people who quit at up to 25 and then didn’t do any more,” he said.
“We presume that there is some feedback to people and that, if they’re finding that their own results are not that good, then they don’t continue to do the procedure.
When you have a procedure with a low adverse event rate, it’s hard for people to gain personal perspective.”
If people don’t track their outcomes somewhere, then they may not know.
“That’s the problem. When you have a procedure with a low adverse outcome event rate, it’s hard for people to gain personal perspective.”
Further concerns
Reservations over the CMS’ expansion of coverage for TfCAS have also been expressed recently by SVS president Joseph Mills (Baylor College of Medicine, Houston, USA), who told Vascular News that “this decision will likely increase the number of strokes in the USA in the near future”.
“Unrestricted access to TfCAS can be predicted to result in an increase in the number of operators performing the procedure at a low annual rate, slowly making their way through the learning curve, and struggling to achieve annual procedure numbers, with less-than-acceptable results for Medicare beneficiaries,” Mills stated.
“We believe the [CMS] decision was premature, especially given that the taxpayer-funded CREST-2 trial is not yet completed and may well shed important insights on the issue of who benefits from carotid revascularisation by stenting versus CEA compared to best medical therapy.”
21 Issue54 | May 2024 Vascular Surgery
Marc Schermerhorn






*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the spinal arena Editorially independent Visit spinalnewsinternational.com and click ‘Subscriptions’ for and e-newsletter subscription* Subscribe today Available in digital format and through our social channels
Carryover effect exists and has “remarkably high” variability in spinal cord stimulation patients
An international, multicentre study has confirmed the existence of the carryover or ‘echo’ effect—an interval before patients perceive the return of pain after their spinal cord stimulation (SCS) system has been deactivated—and also indicated a “remarkably high” degree of variation between individuals regarding this phenomenon.
Kaare Meier (Aarhus University Hospital, Aarhus, Denmark) and colleagues, who recently published these findings from the EChO study in the journal Neuromodulation: Technology at the Neural Interface, state that their results suggest the magnitude of carryover may be correlated to the nature of the pain condition and, possibly, stimulation paradigms as well. The authors also highlight cyclic stimulation approaches, crossover study designs and optimisation of SCS therapies as areas where their findings may have impactful implications.
“It has long been assumed that, when deactivating SCS, there is a variable interval before the patient perceives the return of the pain—a phenomenon often termed echo or carryover effect,” Meier and colleagues initially relay. “Although the carryover effect has been problematised as a source of error in crossover studies, no experimental investigation of the effect has been published.”
With this gap in the current literature in mind, researchers conducted the open, prospective, international, multicentre EChO study in an attempt to systematically document, quantify and investigate the phenomenon of the carryover effect in chronic pain patients receiving SCS therapy.
The rationale for the study was relatively simple: eligible patients whose SCS treatment had a beneficial effect were instructed to deactivate their device in a home setting, and to reactivate it when their pain returned. The study’s primary outcome was the duration of carryover time, defined as the time interval from deactivation to reactivation. Meier and colleagues note that central clinical parameters—including age, sex, indication for SCS, SCS

treatment details and pain score—were registered and correlated with carryover time using nonparametric tests for categorical data, and linear regression analysis for continuous data.
A total of 158 patients from nine European centres, and one in Canada, were included in EChO analyses between April 2018 and March 2021. Across these patients, a median carryover time of five hours (interquartile range, 2.5–21 hours) was found. Tests comparing patient subgroups found some notable, statistically significant differences: those with complex regional pain syndrome (CRPS) displayed the shortest carryover time, and those with back pain experienced the longest, while patients with radicular pain, painful neuropathy and peripheral nerve damage pain showed carryover times between these two extremes. Meier and colleagues relay that a higher pain score at the time of SCS deactivation was correlated with longer carryover time, but that this correlation was not replicated in linear regression analyses. Patients receiving highfrequency (10kHz) stimulation also experienced statistically significantly longer carryover effects than those on tonic or burst stimulation therapies—although the authors note, on this point, that patients treated with high-frequency stimulation only constituted a small fraction of the included patients, and had a higher proportion of back pain patients as a possible confounder.
Among other key findings of EChO’s analyses was the fact that SCS lead location did not seem to be a predictor for any change in carryover time. In addition, neither the age of the patients, nor the duration of their
This study is, to our knowledge, the first to investigate the carryover effect in SCS in an experimental setting.”
painful condition or SCS treatment, was associated with carryover time. Here, the authors also note that SCS patients with previous experience of rapid pain onset after deactivation may be less inclined to participate, leading to overestimation of the carryover time. Finally, regarding safety outcomes, the authors report that no adverse events were recorded in the study.
“This study is, to our knowledge, the first to investigate the carryover effect in SCS in an experimental setting,” Meier and colleagues write in summary. “The data presented here confirm the existence of a carryover effect, quantify the duration of the carryover effect, and investigate possible correlations with clinical parameters. In our study population, there was a remarkably large interindividual variation among patients with SCS, with a significant fraction of patients having carryover duration of >24 hours—a finding with both clinical and profound scientific implications.”
Study reinforces view that ECT is safe and effective for severe mental illness
Researchers have found that electroconvulsive therapy (ECT) can reduce the severity of mental illnesses, as per a study presented at the 32nd European Congress of Psychiatry (6–9 April, Budapest, Hungary).
“Our findings from this large, naturalistic study across Scotland from over an 11-year period reinforce the widely held but nonetheless underexplored view that ECT is both a safe and effective treatment when delivered to appropriate groups of people with severe mental illness,” said Julie Langan Martin (University of Glasgow, Glasgow, UK). “Monitoring of side-effects, especially cognitive side-effects, should be undertaken carefully and rigorously in all patients receiving ECT.”
The study examined ECT use across Scotland over an 11-year period—from 2009 to 2019—via data from the Scottish ECT Audit Network (SEAN). It assessed the efficacy of, and side-effects associated with, ECT across a range of common mental illnesses, such as depression, bipolar depression, schizophrenia, and mania.
In the study, ECT was shown to be effective in reducing illness severity, as measured by the Clinical Global Impression Scale (CGI-S)—a validated, clinician-administered assessment tool that measures illness severity. Some 2,920 ECT episodes had CGI-S scores recorded for patients before and after treatment. The mean CGI-S score prior to treatment indicated a ‘marked’ illness severity of 5.03 (95% confidence interval [CI], 4.99–5.07), whilst, after treatment, the mean CGI-S score was reduced to 2.07 (95% CI, 2.03–2.11), indicating a reduction to ‘borderline’ illness severity.
Assessing the side-effects associated with ECT, the study found that anaesthetic complications and prolonged seizures were rare, occurring in <1% of treatment episodes. Cardiovascular complications were reported in 2.2%, nausea in 7.2% and muscle aches in 12%. In addition, confusion was reported in 19% and cognitive side-effects in 26.2%.
“This study on ECT presents compelling evidence of its effectiveness in reducing the severity of mental illnesses, with major side-effects found to be rare,” added Julian Beezhold (Norfolk and Suffolk NHS Foundation Trust, Norwich, UK), secretary general of the European Psychiatric Association (EPA). “It challenges common misconceptions and stigmas associated with ECT, providing valuable insights that can reshape public perceptions and stimulate informed discussions among healthcare professionals.”
23 Issue54 | May 2024
Kaare Meier
Study elucidates how patient age and electrode connectivity impact cognitive effects of DBS
A study presented at the recent American Academy of Neurology (AAN) annual meeting (13–18 April, Denver, USA) has gone some way to elucidating how two factors—patient age, and connectivity of electrodes to the subiculum-retrosplenial cortex (SBC-RSC)—may impact the cognitive effects of deep brain stimulation (DBS) among Parkinson’s and Alzheimer’s patients.
DELIVERING THESE FINDINGS ON BEHALF of his co-investigators, Calvin Howard (Harvard Medical School, Boston, USA) outlined that he and his colleagues endeavoured to gain a better understanding of how patient age and electrode connectivity influence various cognitive outcomes with DBS across both of these neurological diseases.
According to Howard and his colleagues, recent research has demonstrated that brain lesions and DBS
electrodes functionally connected to the SBC-RSC impair cognition—while other pieces of research have shown what appears to be the opposite effect in DBS for Alzheimer’s.
For the present study, researchers examined electrode locations and cognitive outcomes in a total of 33 patients who received subthalamic nucleus DBS for Parkinson’s and 46 patients who received fornix DBS for Alzheimer’s. They then related the observed DBS sites to the lesion-based, a-priori SBC-RSC region of interest, and tested whether connectivity between DBS sites and the SBC-RSC was significantly correlated with memory outcomes, as well as whether the difference between Parkinson’s and Alzheimer’s was significant. The researchers also used whole-brain mapping to assess if both diseases were driven by the same region in the SBC-RSC and, finally, investigated the influence of patient age upon outcomes.
As reported by Howard at AAN 2024, connectivity to the SBC-RSC was simultaneously correlated with cognitive decline in Parkinson’s disease patients and cognitive improvement in Alzheimer’s disease patients. He and his colleagues also found that the difference between the two diseases was statistically significant. In addition to this, a data-driven, whole-brain analysis of connections correlating with cognitive decline revealed that both diseases were driven by the same SBC-RSC region.
Among other key findings was the fact
Short-pulse DBS appears more effective than conventional DBS in tremor but not in Parkinson’s
In a systematic review and meta-analysis of the current literature, deep brain stimulation (DBS) with a short pulse width has been shown to potentially induce more tremor reduction in essential tremor patients, but does not appear to offer a notably different level of therapeutic efficacy for Parkinson’s disease, as compared to more conventional DBS therapy.
Sara Smeets (University Hospitals Leuven, Leuven, Belgium) and colleagues also report that—as per their findings, now published in the journal Brain Stimulation—short-pulse DBS enlarges the therapeutic window with a wider amplitude range in both Parkinson’s and tremor treatment, but was not found to alter therapeutic windows defined by charge per pulse (CPP).
“To maximise clinical benefit and minimise stimulation-induced sideeffects, optimising DBS parameters is paramount,” the authors state in their report.
“Recent literature suggests a potential benefit of short pulse-width DBS [≤40μs] over conventional pulse-width DBS [≥60μs] in movement disorders.”
Smeets and colleagues go on to assert that, based on clinical and neurophysiological measurements and theoretical models, some researchers have suggested that the ideal pulse width for DBS in movement disorders like Parkinson’s and tremor is shorter than the conventional 60μs.
Here, the authors also highlight the fact that longer pulse widths typically narrow the therapeutic window and increase energy consumption.
“Recently, novel devices capable of delivering pulse widths as low as 10μs have been commercialised, enabling researchers to corroborate these predictions,” they continue.
“In a small number of recently published case series and small randomised controlled trials [RCTs], short pulse-width DBS indeed appears to widen the therapeutic window— defined as therapeutic amplitude range—and lower energy consumption versus conventional DBS.
“Nevertheless, short-pulse DBS has not yet been widely adopted in clinical practice and therapeutic window defined by CPP/TEED [total electrical energy delivered] is often neglected in those studies.”
With this in mind, the researchers set out to compare the therapeutic window, therapeutic efficacy, side-effects, and energy consumption, between shortpulse and conventional DBS therapy in movement disorders.
On 12 July 2022, they performed a systematic search of Medline, Embase, Cochrane Library and Web of Science, with appropriate paired analyses being applied to their results.
Their search returned a total of 11 Parkinson’s disease studies and six essential tremor studies eligible for
that—as per subsequent regression analyses—both groups were found to have demonstrated significant interactions with patient age. High SBC-RSC connectivity was also associated with improved cognitive outcomes in older patients compared to impaired cognition in younger patients, while low SBC-RSC connectivity had the opposite effect on these age groups. Lastly, Howard relayed that further data-driven analysis identified the inflection point as occurring at 65 years of age in both Parkinson’s and Alzheimer’s.
Howard concluded by noting that the cognitive effects of DBS, in both of these diseases, are “unified” when considering connectivity to the SBC-RSC, and also patient age. He further stated that the “magnitude of impact” upon cognition primarily correlates with the degree of DBS connectivity to the SBC-RSC—but whether DBS impairs or improves cognition is dependent on patient age.

Following this presentation at AAN, Howard spoke to NeuroNews to relay that he and his colleagues are now actively working to expand upon these findings. They recently collected additional data from retrospective cohorts and have since validated their results, with their final planned step being to translate these findings into neuroanatomical targets to guide DBS electrode implantation by neurosurgeons, and electrode programming by neurologists.
systematic review—of which nine Parkinson’s disease studies consisting of 143 patients and four essential tremor studies consisting of 26 patients were included in a meta-analysis.
Smeets and colleagues’ search did also pertain to dystonia patients but, ultimately, none of these studies met the inclusion criteria. The authors note that all those included were crossover studies published between 2015 and 2022.
Among the key findings of their metaanalysis was the fact that the therapeutic window, when defined as therapeutic amplitude range, was larger with short-pulse versus conventional DBS in both Parkinson’s (standardised mean difference [SMD], –1.04; p<0.001) and tremor (SMD, –0.71; p<0.001). However, the therapeutic window between the two DBS types did not differ in terms of CPP.
Additionally, across short-pulse and conventional stimulation therapies, Smeets and colleagues found no difference in Parkinson’s patients regarding a range of therapeutic and side-effect metrics—including Movement Disorder Society unified
This is, to our knowledge, the first comprehensive literature study comparing shortpulse DBS to conventional DBS in movement disorders.”
Parkinson’s disease rating scale-part III (MDS-UPDRS-III); speech and gait; dyskinesia; non-motor symptoms, and quality of life.
They did, conversely, find that scores on the Fahn-Tolosa-Marin tremor rating scale in tremor patients were lower with short-pulse compared to conventional DBS (SMD, 0.36; p<0.001), indicating improved clinical outcomes.
A qualitative analysis undertaken as part of the study also suggested fewer stimulation-induced side-effects with short-pulse DBS.
“This is, to our knowledge, the first comprehensive literature study comparing short-pulse DBS to conventional DBS in movement disorders,” Smeets and colleagues note, before acknowledging fairly short times between pulse-width adjustment and outcome evaluation in some Parkinson’s trials, a relatively small number of essential tremor studies and patients, and a lack of studies assessing the more long-term effects of short-pulse DBS, as key limitations of their analysis.
“Despite preliminary promising results of short-pulse DBS, further research should be undertaken to prospectively evaluate [its] real-life, long-term value in larger patient cohorts,” the authors continue, also averring that “several questions remain unanswered”—including the precise underlying neurophysiological effects of pulse width—and, as such, “before any changes in clinical pathways are recommended, stronger evidence should be provided”.
They go on to identify Parkinson’s patients receiving globus pallidus internus (GPi)-DBS and dystonia patients as additional populations in which the effects of short-pulse DBS “remains to be investigated”.
24 May 2024 | Issue54
Calvin Howard
Vagus nerve stimulation demonstrates promise as non-medical treatment for narcolepsy
An initial evaluation of vagus nerve stimulation (VNS) in narcolepsy patients has linked this neuromodulation modality to a “significant improvement” in daytime sleepiness, leading researchers to aver that VNS could be a promising non-medical treatment option for the condition.
Writing in the journal Brain Stimulation, Yaroslav Winter (Johannes Gutenberg University, Mainz, Germany) and colleagues note that VNS is known to trigger arousal- and wake-promoting side-effects in epilepsy and depression, and these effects have also been demonstrated previously in animal experiments. However, up to now, no clinical study has directly assessed VNS therapy in patients with narcolepsy.
“A further search for new nonpharmacological treatment options is necessary,” the authors state, citing limitations around efficacy and adherence with drug therapies, as well as the various comorbidities associated with narcolepsy. “Neurostimulation has never been investigated in narcolepsy until now. It would improve the adherence and reduce the drug load in these patients. In order to prove its efficacy, neurostimulative approaches should consider the minimal important difference of about 2.5 on the Epworth sleepiness scale (ESS) or ≥25% reduction of excessive daytime sleepiness (EDS) compared to baseline, which are known from approvals of narcolepsy therapy by the [US] Food and Drug Administration or European Medicines Agency.”
As such, Winter and colleagues attempted to evaluate the therapeutic effect of VNS on narcolepsy symptoms like daytime sleepiness and cataplexies—sudden, involuntary attacks of muscle flaccidity brought on by strong emotions. The researchers’ open-label, prospective, comparative study enrolled narcolepsy patients who were treated with VNS because of epilepsy or depression, and compared them to a control group of patients without narcolepsy who were also receiving VNS therapy due to epilepsy or depression. A total of 36 patients (average age, 31.5 years; 55.6% female) were enrolled, with 18 being included in each group. No significant differences in baseline characteristics, including disease duration, number of medications, and comorbidities, were present between the two groups. Daytime sleepiness, as per ESS scores, and number of cataplexies per week were evaluated as key outcome measures in the study, being recorded prior to implantation of a VNS device, and at three- and six-month follow-ups.
Major findings
Winter and colleagues ultimately found that—compared to an average score of 15.9 at baseline—ESS scores in patients with narcolepsy showed a significant improvement, decreasing to 11.2 at three months (p<0.05) and 9.6 at six months (p<0.001). In addition, there was a trend towards a reduction in cataplexy symptoms in patients with narcolepsy, indicated by a weekly cataplexy rate (WCR) improvement at six months (1.8) compared to baseline (3.9) that did not reach statistical significance (p=0.09).
Conversely, in the study, there was no
therapeutic effect of neurostimulation increases over time, or by minimal fluctuations in ESS scores due to the study’s small sample size. They go on to state that the difference between ESS values at three and six months was both small, and below the minimum difference for importance of 2.5 points. As such, they believe the longitudinal effect of VNS in narcolepsy “should be investigated in multicentre, randomised controlled studies with larger study populations”, adding that such studies may also help to establish the effect of VNS on cataplexies.
A comparison of VNS with best medical treatment in narcolepsy may have been “out of the scope” of the researchers’ study, they concede, but they were able to register a 39.6% improvement in ESS scores at six-month follow-up versus baseline—a rate that is higher than the recommended clinically important difference for this measure (≥25%). Similarly, the absolute difference of 6.3 points on ESS between baseline and six months was higher than the 2.5-point minimal important difference, as was the absolute difference between patients with and without narcolepsy in their study at six months (3.6 points).
ESS scores in narcolepsy patients treated with VNS
significant ESS improvement observed in patients without narcolepsy—scores only decreased slightly, from 14.9 at baseline to 13.6 at three months, and 13.2 at six months (p=0.2).
Furthermore, Winter and colleagues note that ESS improvements in patients with narcolepsy undergoing VNS therapy were found to be independent from changes in Beck depression inventory II (BDI-II) scores, and that multiple regression analysis confirmed this phenomenon. The authors also observed no significant differences in the frequency of side-effects between patients with and without narcolepsy (p>0.05), with the majority of those in both study groups experiencing no adverse side-effects.
Discussing their findings in greater granularity, Winter and colleagues highlight the positive effects of VNS on daytime sleepiness in narcolepsy patients being more notable with increased duration of the therapy— something that could be explained by the “well-known fact” that the
teratogenic effects of medication could be avoided [via] the use of neurostimulation in pregnancy. Further investigation of neurostimulation in narcolepsy could provide new ways to treat this disease and to search for synergistic effects of medication in combination with neurostimulation, as is already being done in epilepsy.”
The authors do also highlight multiple limitations to the present study, including data collection not being blinded and the lack of a shamcontrolled group, and note that the number of patients enrolled was not sufficient to perform subgroup analyses of different narcolepsy types; for example, across patients with or without cataplexy. In addition, Winter and colleagues say that—with an open-label design not involving sham stimulation—their study only provides level-three evidence.
However, while the exact mechanisms behind this are yet to be fully elucidated, they reiterate that their findings support assumptions of an “alerting effect” with VNS and, possibly, a subsequent positive impact on EDS in narcolepsy patients.
“In conclusion,” the researchers state, “VNS could be a safe, complementary treatment option for patients with narcolepsy who did not respond to drug treatment. Our data suggest a positive effect of VNS on daytime sleepiness in patients with narcolepsy of both types. Our open-label study should motivate future randomised, sham-controlled studies on VNS in narcolepsy.”
Following the publication of this study, Winter spoke to NeuroNews to outline the wider implications of his and his colleagues’ findings, and the next steps they are planning.
“Neurostimulation is a new and promising approach to treat not only narcolepsy but also other sleep disorders,” he said.
Neurostimulation is a new and promising approach to treat not only narcolepsy but also other sleep disorders. Neurostimulation strategies in this medical field would help to reduce side-effects of pharmacological treatments and to promote neuroplasticity.”
Wider
significance
“Neurostimulation as an innovative approach to treat EDS in narcolepsy has a potential to significantly improve the management of this disease,” Winter and colleagues write.
“It would prevent polytherapy in drug-resistant cases and improve adherence. Considering the data on refractory cases and poor adherence, approximately 30% of patients could be possible candidates for neurostimulation. In addition,
“Neurostimulation strategies in this medical field would help to reduce sideeffects of pharmacological treatments and to promote neuroplasticity. Our working group is planning a sham-controlled study of VNS in narcolepsy, and also investigating other neurostimulative treatments—such as deep brain stimulation and direct focal cortical stimulation by means of EASEE system [Precisis]—for other clinical indications in the field of sleep medicine.”
25 Issue54 | May 2024
NARCOLEPSY at baseline 15.9 at 3 months 11.2 at 6 months 9.6
Industry News

Penumbra launches Midway intermediate catheters, expands European stroke portfolio
Penumbra has announced the launch of its Midway 43 and Midway 62 delivery catheters, which are designed to provide ideal tracking and a stable platform to support the delivery of various embolisation therapies in the neurovasculature.
Based on the company’s Red catheter technology, Penumbra describes its newly launched Midway intermediate catheters as a “significant advancement” in the intermediate access device market—which has seen “minimal innovation over the last decade”.
These Midway catheters are compatible with Penumbra’s Benchmark 71, BMX 81 and BMX 96 access systems, and have been engineered to enable the precise delivery of distal microcatheters, embolisation coils, intrasaccular devices, and flow diverters, providing a critical balance between trackability and support for each of these therapeutic approaches, according to a company press release.
“Penumbra’s portfolio of Midway intermediate catheters now provides us with highly engineered technology that offers an optimal balance of support and trackability in a wide range of applications,” said John Chaloupka (Mount Sinai Medical Center, Miami, USA).
This news came on the same day (7 May) that Penumbra also announced the CE-mark certification and European launch of three of its reperfusion catheters: Red 43, Red 72 with Sendit technology, and Red 78. In a second press release, the company said these launches will expand the company’s neurovascular offerings designed for acute ischaemic stroke.
“I’m thoroughly impressed with the currently available Red aspiration catheters’ precision in navigating and removing clots,” said Levansri Makalanda (Barts Health NHS Trust, London, UK). “I’m eagerly anticipating the Red 43, Red 72 Sendit and Red 78, which promise to enhance both the reach and efficiency of clot extraction.”
Penumbra claims that, optimising science-based aspiration thrombectomy (S-BAT), these devices are engineered with the latest in trackability and
aspiration to address a wide range of large vessel occlusions—including the company’s Redglide technology, and a full-length polytetrafluoroethylene (PTFE) liner.
RapidPulse publishes data indicating cyclic aspiration technology is safe and effective RapidPulse, a spinout of medical device incubator Syntheon 2.0, has announced that a study recently published in the journal Interventional Neuroradiology has provided multicentre trial results that demonstrate the promise of using precise cyclic aspiration in the treatment of acute ischaemic stroke.
The RapidPulse feasibility study saw researchers assess their ability to restore blood flow in large vessel occlusion (LVO) ischaemic stroke patients using standard (static) aspiration versus with the company’s new technology, which precisely cycles aspiration to increase the removal rate of blood clots in the brain. RapidPulse sponsored the study, which enrolled 40 patients across Turkey, Brazil, Spain, Latvia and Denmark in its RapidPulse arm, and included a control arm comprised of 40 eligible patients treated previously using commercially available aspiration catheters for comparative data.
The study compared the percentage of patients who achieved almost complete (modified thrombolysis in cerebral infarction [mTICI] 2c) or complete (mTICI 3) reperfusion at the first aspiration attempt between these groups, and ultimately found that the mTICI ≥2c rate after the first pass was 60% among patients treated with RapidPulse who satisfied all eligibility criteria versus 38.5% in the corresponding control arm. This >21-point improvement in first-pass clot removal rates led the authors to conclude that the RapidPulse system is “highly effective and safe for the removal of LVOs”.
“This is an exciting improvement for the treatment of ischaemic stroke due to large vessel occlusion,” said study author Serdar Geyik (Istanbul Aydin University, Istanbul, Turkey).
“Higher recanalisation rates on the first attempt are correlated with better outcomes for patients. This cyclic aspiration technology enabled fast and remarkable rates of excellent first-pass recanalisation rates. This may lead to reduced use of stent retrievers.”
The RapidPulse technology used in this study was comprised of an external valve box connected to a commercially available aspiration pump. This valve box was programmed to deliver a precise cyclic algorithm to the distal tip of the catheter, where it can have “maximal effect” on clot removal, according to the company. Future studies of a new version of the RapidPulse technology—which is not
currently available for sale in the USA or other markets—are expected to begin enrolling patients later in 2024.
Silk Road expands TCAR portfolio with new Enroute device launches Silk Road Medical has announced the launch of its Enroute transcarotid neuroprotection system Plus (NPS Plus), which the company describes as a key component of its transcarotid artery revascularisation (TCAR) portfolio.
As per a Silk Road press release, this next-generation device builds upon the prior Enroute transcarotid neuroprotection system to deliver smoother arterial sheath insertion, greater flow precision, and a simplified prep experience for surgical teams—all while maintaining “unparalleled” neuroprotection during the TCAR procedure. TCAR delivers periprocedural stroke rates of less than 1% and a better patient recovery experience than open surgery, the company further claims.
“Silk Road Medical’s responsiveness to our feedback has provided us with better procedure predictability and a more streamlined workflow for our entire surgical team,” said Brian Peterson (St Luke’s Heart and Vascular Institute, St Louis, USA). “With over 500 TCAR procedures performed by our team, we view TCAR as a first-line treatment option for carotid disease and we’re excited about what these improvements mean for patient care.”

Silk Road also recently announced the launch of its tapered Enroute transcarotid stent system to hospitals in the USA, expanding upon the company’s prior Enroute transcarotid stent system and offering additional configurations to better tailor the TCAR procedure to patient anatomy.
“The new tapered stent is a welcome addition to my TCAR toolkit, further differentiating the company’s core product offering,” said Joseph Lombardi (Cooper University Health Care, Camden, USA). “The Enroute stent has always provided comprehensive lesion coverage and durable procedural outcomes, but I now have more options to customise treatment to each patient’s anatomy.”
The Enroute transcarotid stent system features an optimised cell design and short delivery system purpose-built for TCAR, and is the only commercially available transcarotid stent system indicated for patients at high risk and standard risk for adverse events from carotid endarterectomy (CEA), as per a Silk Road press release.
Route 92 gains US FDA 510(k) clearance for FreeClimb 54 reperfusion system
Route 92 Medical has announced receipt of US Food and Drug Administration (FDA) 510(k) clearance for the FreeClimb 54 reperfusion system, comprised of the FreeClimb 54 aspiration catheter paired with a Tenzing 5 delivery catheter.
This new system is part of a growing portfolio of neurovascular devices from Route 92, designed to work harmoniously as a complete endovascular thrombectomy solution to address a broad range of neurovascular interventions, as per a company press release.
The FreeClimb 54 system can ‘telescope’ through the company’s complementary products, allowing physicians to rapidly, predictably reach and remove stroke-causing clots during endovascular thrombectomy procedures. Route 92’s recent press release also notes that FreeClimb 54’s robust tip and shaft design facilitates use for multiple passes, and compatibility with other technologies. Additionally, the system may be used to remove distal clots when first-line therapies fail to completely remove the clot.
“The FreeClimb 54 system is a gamechanger,” added Ronald Budzik (Riverside Methodist Hospital, Columbus, USA). “It is a simple system capable of getting a larger bore aspiration catheter to distal anatomy, especially around tighter turns.”
Viz.ai announces new collaborations in ICH and postcryptogenic stroke care
Viz.ai has announced a collaboration with Nico Corporation to improve the lives of patients with intracranial haemorrhages (ICHs), leveraging the former’s artificial intelligence (AI)-powered disease detection and intelligent care coordination products, and the latter’s modern, minimally invasive interventional technologies for neurosurgery.
Through Viz.ai’s technology, hospitals will be able to detect suspected disease earlier and receive accurate volume measurements of brain bleeds that are crucial for assessing the severity of ICH cases. In addition, Nico’s technology can provide early and safe access to the brain with its BrainPath device, and maximal evacuation of the clot with its Myriad device. The combination of both companies’ technologies will allow for greater efficiencies and enhanced patient care, as stated in a recent press release.
With the AI-powered Viz ICH Plus module, hospital and health systems can automate the process of identifying, labelling and quantifying the volume of segmentable brain structures on noncontrast computed tomography (NCCT) images. Radiologists, neurologists and neurosurgeons can incorporate Viz ICH Plus seamlessly into their workflows, detect brain bleeds, and automate the
26 May 2024 | Issue54 Market Watch
Enroute device
Midway catheters
manual process of measuring brain bleeds, Viz.ai claims.
Viz.ai also recently announced a strategic collaboration with Medtronic to improve the coordination of patient care for cryptogenic stroke—strokes with an unknown cause—between neurology and cardiology teams in the USA.
For stroke patients who are at risk of atrial fibrillation (AF) post-stroke and may need additional cardiac monitoring, stroke care teams will have the opportunity to use the Viz Connect solution—a software tool that automates the communication across disciplines, including neurology and cardiology. A second Viz.ai press release cites the Medtronic-led DiVERT Stroke clinical study—in which only 16% of stroke patients from community hospitals and 34% of patients at academic centres received a cardiology consult—as evidence of the need for stronger, standardised care pathways between neurology and cardiology to ensure that stroke patients receive guideline-directed therapy.
According to Viz.ai, Viz Connect has demonstrated impact on improving patient access to cardiac care after a cryptogenic stroke. Examples detailed by the company include an average increase of more than 50% in in-patient cardiology follow-up, and an average time of under five minutes from when
Conference calendar
3–5 June 2024
LINNC Paris Paris, France linnc.com/Course-information/ LINNC-Paris-2024
17–21 June 2024
The European Course in Minimally Invasive Neurological Therapy (ECMINT) 5.3 Oxford, UK esmint.eu/ecmint
the notification is sent from neurology to when it is reviewed by a cardiologist.
MindRhythm announces first official publication of EPISODE study data
MindRhythm has announced that results from the pilot phase of its threephase clinical study, EPISODE, have been published in Academic Emergency Medicine. While pilot EPISODE data have been presented in various academic society forums, this is the first official publication of results from the study, according to a press release from the company.
The company claims that its family of EPISODE studies, assessing MindRhythm’s Harmony technology in the prehospital detection of ischaemic stroke, is the largest evaluation of a prehospital stroke device, with eight geographies, 17 hospitals and 14 emergency medical services (EMS) groups participating in this research.
Results from the EPISODEPS-COVID pilot phase reveal that Harmony—a cranial accelerometerybased device—is a feasible tool for use in the EMS-prehospital setting, and can reliably identify large vessel occlusion (LVO) stroke based on ‘headpulse’ measurements. Use of the device algorithm incorporating subjective data from the commonly deployed Los Angeles motor scale (LAMS),
19–20 June 2024 7th International Symposium on Mechanical Thrombectomy Acute Stroke (SYMTAS) Santiago de Compostela, Spain symtas2024.compostelacongresos. com
19–22 June 2024
Vascular Annual Meeting (VAM24) Chicago, USA vascular.org/2024-vascular-annualmeeting

and Harmony’s cranial accelerometery data, resulted in “significantly higher sensitivity without reduced specificity when compared to the use of LAMS alone”.
The company was granted a Breakthrough Device designation by the US Food and Drug Administration (FDA) as a result of the data from EPISODE-PS-COVID, and combined datasets from the pilot and pivotal phases of the ultimate EPISODE study will be used for MindRhythm’s FDA submission.
Cerenovus launches Cereglide 71 aspiration catheter for acute ischaemic stroke in Europe Cerenovus, part of Johnson & Johnson MedTech, has announced the launch of its Cereglide 71 aspiration catheter in Europe—an aspiration catheter equipped with TruCourse technology and indicated for the
29 June–2 July 2024 10th Congress of the European Academy of Neurology (EAN) Helsinki, Finland ean.org/congress2024
22–26 July 2024 Society of NeuroInterventional Surgery (SNIS) 21st Annual Meeting & Fellows Course Colorado Springs, USA snisonline.org/meetings



revascularisation of patients suffering from acute ischaemic stroke. The device is set to be included in the next phase of Cerenovus’ real-world EXCELLENT registry studying stroke-causing blood clot removal via mechanical thrombectomy.
The Cereglide 71 aspiration catheter is the latest innovation in a planned Cereglide family of catheters to join the Cerenovus stroke solutions portfolio. As per a company press release, Cereglide 71 is optimised for effective, direct aspiration of blood clots and for the delivery of compatible stent retrievers, including the Embotrap III revascularisation device and Cerenovus’ Nimbus geometric clot extractor, into the neurovasculature.
“The introduction of the Cereglide 71 aspiration catheter in Europe is an important step in endovascular thrombectomy, as it allows swift, targeted clot access and removal during stroke treatment,” said Kyriakos Lobotesis (Imperial College London, London, UK). “With Cereglide 71 aspiration catheter, we can now access occlusion sites and aspirate clots more rapidly and efficiently, maintaining a smooth interaction with stent retrievers when used in co-aspiration. This leads to the prompt restoration of blood flow in the patient’s brain, which has the potential to save more lives and improve long-term clinical outcomes.”
4–6 September 2024 16th Congress of the European Society of Minimally Invasive Neurological Therapy (ESMINT) Marseille, France esmint.eu
18–22 September 2024 European Society of Neuroradiology (ESNR) Annual Meeting Paris, France esnr.org/en/scientific/annualmeetings/18-09-2024-47th-esnrannual-meeting
28 September–
2 October 2024 Congress of Neurological Surgeons (CNS) Annual Meeting Houston, USA cns.org/2024
3–4 October 2024 British Society of Neuroradiologists (BSNR) Annual Scientific Meeting Bristol, UK bsnr.org.uk/annual-meeting
27 Issue54 | May 2024 Sign up for a free print subscription* and e-newsletter subscription** www.neuronewsinternational.com NeuroNews is a trusted, independent source of news and opinion in the neurointerventional and neurosurgical world. *Available for US and EU readers only ** Available worldwide Market Watch
Harmony headset

























































DELIVERING EXCELLENCE Penumbra System™ RED Reperfusion Catheters Photographs taken by and on file at Penumbra, Inc. Devices shown at same scale. Procedural and operative techniques and considerations are illustrative examples from physician experience. Physicians’ treatment and technique decisions will vary based on their medical judgment. Individual results may vary depending on patient-specific attributes and other factors. Product availability varies by country. Prior to use, please refer to the Instructions for Use (IFU) for complete product indications, contraindications, warnings, precautions, potential adverse events, and detailed instructions for use. Please contact your local Penumbra representative for more information. Copyright ©2024 Penumbra, Inc. All rights reserved. The Penumbra P logo, RED, and Penumbra System are registered trademarks or trademarks of Penumbra, Inc. in the USA and other countries. 30084, Rev. A 05/24 OUS For the complete Penumbra IFU Risk Statements, please scan QR code or visit: peninc.info/risk Access Catheter 62 RED 43 RED






















































































































