Arizona Journal of Pharmacy


OFFICIAL PUBLICATION OF ARIZONA PHARMACY ASSOCIATION | FALL 2022
PharmD, BCGP, FASCP, FAzPA AzPA President 2022–2023
Dawn Gerber
June 2023 Southwestern States Residency Conference Phoenix, AZ



2 FALL 2022
UPCOMING EVENTS
November 12, 2022 | Virtual AzPA Anticoagulation Certificate Program March 4-5, 2023 Spring Clinical Conference Phoenix, AZ
BOARD OF DIRECTORS 2022–2023
OFFICERS
President Dawn Gerber
President Elect Kimberly Langley
Past President Darren Clonts
Treasurer Jacob Schwarz Secretary Nancy Costlow Director/CEO Kelly Fine
DIRECTORS AT LARGE
Community Pharmacy
Phillip Ieng Health System Pharmacy Christopher Edwards Technician Melinda Browning Directors at Large Reasol Chino Ryan Gries Brandy DeChellis Misty Brannon Nina Vadiei
LIAISONS
University of Arizona Student Chapter Jose Espinoza Dean’s Designated Representative Nancy Alvarez
Midwestern University Student Chapter Lyndy Abdelsayed Dean’s Designated Representative Michael Dietrich
Creighton University Student Chapter Sharon Ruditser Dean’s Designated Representative Jane Stein
LEGAL COUNSEL
Roger Morris
AZPA STAFF

Chief Executive Officer Kelly Fine Education & Professional Development Dawn Gerber Events & Strategic Partnerships Cindy Esquer
Membership & Volunteer Services Hanna Wooldridge Digital Marketing & Engagement Irma Settle
Administrative Services Melina Esquer
Editor Kelly Fine

Creative Coordinator Irma Settle The interactive digital version of the Arizona Journal of Pharmacy is available for members only online at www.tinyurl.com/azjournal
(480) 838-3385 admin@azpharmacy.org
EDITOR’S NOTE: Any personal opinions expressed in this magazine are not necessarily those held by the Arizona Pharmacy Association. “Arizona Journal of Pharmacy” (ISSN 1949-0941) is published quarterly by the Arizona Pharmacy Association at: 1845 E. Southern Avenue, Tempe, AZ 85282-5831.
3 azpharmacy.org
CONTENTS
President’s Message 4 AzPA News Welcome New Members 5 University & Alumni News 24 AzPA State Advocacy 38 Editorial 2022 - 2023 Legacy Interns 6 Az-ASHP State Affiliate News 7 Preceptor Corner 8 Member Spotlight 11 Financial Forum 20 Rx and the Law 22 Arizona and COVID-19: Fall 2022 36 Continuing Education Impact of Specialty Pharmacy on Adherence to Maintenance Medications for Chronic Diseases Among Solid Organ Transplant Recipients 12 COVER STORY Dawn Gerber, PharmD, BCGP, FASCP, FAzPA AzPA President • 2022–2023
president’s message
Dawn Gerber, PharmD, BCGP, FASCP, FAzPA

Associate Professor of Pharmacy Practice at Midwestern University College of Pharmacy-Glendale, Arizona, earned her Doctor of Pharmacy degree from Drake University, Des Moines, Iowa. She completed a pharmacy practice residency at the Creighton University Medical Center-Omaha, Nebraska. Dr. Gerber is a Board Certified Geriatric Pharmacist (BCGP) and recognized as Fellow of the American Society of Consultant Pharmacists (ASCP) and Fellow of the Arizona Pharmacy Association (AzPA). She collaborates with the Banner Geriatric Medical Fellowship Multidisciplinary Rounds. She teaches geriatric pharmacotherapy topics and the required Complementary and Alternative Medicine course. She has held leadership positions with AzPA, ASCP, and American Society of Health-System Pharmacists (ASHP). She is a Pharmacy Residency Accreditation Practitioner Surveyor with ASHP.
Dear AzPA (Arizona Pharmacy Association)
Members,
I am looking forward to serving our members and leading the Board of Directors as we guide the Arizona Pharmacy Association to an exciting year of new beginnings. We look forward to seeing everyone back in-person at events during 2022-2023!
I started my career in pharmacy when I was 16 ½ years old. I started as a “Saturday girl” at my small-town hometown of 4,000 people at the local pharmacy. Tom Tremmel, the small independent pharmacy owner, introduced me to the world of pharmacy. He explained that a little white pill named Lasix removes potassium out of a patient's body and they must have a big orange vitamin tablet to replace it. I realized this is what I wanted to do with the rest of my life! I wanted to be a pharmacist! I was excited to start my education and career in pharmacy at Drake University in Des Moines, Iowa. At Drake University, we were taught that it was our responsibility to support our profession via state or national pharmacy associations with our time, money, and efforts. I am excited to fulfill my duty that was instilled in me 20 years ago by serving as your president.
I hope to pass on the values and knowledge that were ingrained in me as a student by mentoring the new generation of young pharmacists. To help guide pharmacy students as they prepare to enter the pharmacy profession, I created the AzPA Legacy Intern Program. An AzPA Legacy Intern is an AzPA student member who has served as a mentee in the AzPA Mentor Connection Program and hopes to stay in Arizona after graduation. The AzPA Board of Directors and committee chairs will mentor these students to prepare them for leadership roles in the pharmacy profession in Arizona and beyond.
To support the needs of pharmacy professionals, AzPA provides several certificate programs and events. I am excited to assist AzPA with the development of new certificate programs specializing in substance use disorders and cardiovascular risk reduction. This past June at the 2022 Annual Convention, our attendees expressed immense gratitude that the event was held in person, seeing everyone, and having the opportunity to network with friends and colleagues again. Next year, we will be hosting the 2023 Spring Clinical meeting as well as the 2023 Annual Convention in-person. We look forward to these sensational educational offerings and seeing everyone at these events!
Lastly, my vision as your board president for this coming year is to connect pharmacy professionals to come together to advance our profession and reinforce that pharmacy is a vital part of the healthcare team. I ask that all pharmacy professionals join together as one voice for one profession. Together, we will make a difference for our profession and our patients!
Sincerely, Dawn Gerber, PharmD, BCGP, FASCP, FAzPA AzPA President 2022-2023
4 FALL 2022
Welcome New Members AzPA news
2nd Year Practitioner
Amy Heinrich
Jamie Frazier
Pharmacist
Alexis Spence
Dana McKay
Jacqueline Baginski
Jacqueline Cavanagh Kaya Borg
Kellie Joy Goodlet
Madison McConnell Masoud Aliani Melissa Molitor
Stephanie Bellows Teresa Aleman Vidhi Gupta Vivian Du
Premium Pharmacist
Arti D Patel Casey Orton Christine Tran Justin R Dorotheo
Resident
Alexis Relampagos
Ayesha Shamim Jubair Hussain Meagan Pawlak Mona Modi
Olivia Benyamin Sivani Gadiraju
Student Pharmacist
Adrian Alvarez
Adrian Gonzalez
Alexander Rayis
Alexandra Holmes
Allen Dao
Allyson Prichard
Amanda Covaleski
Andrew Aguilera
Anh Thu Vuong
Annie Nguyen
Arielle Douglas Arleen Mundian Ayesha Shamim Bowen Ma
Bryton Lee Caitlyn Santos CatLinh Nguyen Celine Evbuomwan
Chloe Beeson Chrisha Gallibu Christina Yamada Claudia Kopec Crystal Viste Darius Vladeanu David Shumaker Dorothee Kom Petnkeu Eirene Phan Eli Ostovar Elisabetta Petrucci Emily DeVos Emma Banks Ghazaleh Asrari Hajer Abuzir Hannah Camano Humza Ullah Hunter Reed Isiah Ingram Ivan Gong Ivy Mac Jacob Butler
Jason Agundez
Jocelyn Contreras
Joseph Zeppa Kalyn King Katayoon Rohani Katie Oswald Kelly Nguyen Kelly Zeisel Kevin Mills
Kulbir Grewal
Kyle Norman Lacey Hunter Lauren Hudson LeAundra Murray Lee Stamper Lindsey Garcia Logan Abbott Marcus Toma Margarita Kanaeva Mariphil Paculba Gregory Matthew Butler MeShell Green Michael Khatchadourian Moneka Tawdrouse Nancy Gonzalez Vazquez Nicole Boothe Oksana Cabrera Parth Patel Sarita James-Atwater Shams Rehman Shaynia Monk Sinh Nguyen Stephanie Soklim Tanner Ramos Taylor Hardwick Tionna Garner Trang Nguyen Tyler Tran Upasna Patel Yenyi Ho Yun-Ching Chen Zahraa Jassar
Tech in Training
Steven Preston
Technician Billy Cooper Katherine Reed Miranda Murphy
5 azpharmacy.org
2022-2023
LEGACY INTERNS
ALEX ROMANO
ANNA MERAUTA
Univ Mid U
Class of 2023 University of Arizona (Phoenix)
Class of 2023 Midwestern University (Glendale)
GEORGIA MATZ JACLYN JUAREZ
Class of 2023 University of Arizona (Phoenix)
Class of 2023 University of Arizona (Phoenix)
M SA
Midw (Glendale) L acy (Di way)
JORDAN RIFFER
JO U
Class of 2023 Midwestern University (Glendale)
W H A T I S A L E G A C Y I N T E R N ?
MARISSA TREVINO
Class of 2023 Midwestern University (Glendale)
SAMANTHA ZIMMERMAN
Class of 2023 University of Arizona (Phoenix)
ZAINA ALOMRAN



Class of 2023 Midwestern University (Glendale) Mid LECOM School of Pharmacy (Distance Education Pathway)
Class of 2023 LECOM-School of Pharmacy (Distance Education Pathway)

An AzPA Legacy Intern is an AzPA student member who has participated as a mentee in the AzPA Mentor Connection Program and who hopes to stay in Arizona after graduation.
W H A T I S A L E G A C Y I N T E R N ?
An AzPA Legacy Intern is an AzPA student member who has participated as a mentee in the AzPA Mentor Connection Program and who hopes to stay in Arizona after graduation



















An AzPA Legacy Intern is an AzPA student member who has participated as a mentee in the AzPA Mentor Connection Program and who hopes to stay in Arizona after graduation.


The Legacy Intern is invited to shadow the activities of an AzPA leader, such as a Committee Chair, or a member of the AzPA Board of Directors In addition, Legacy Interns are invited to attend Board and Committee Meetings throughout the year and participate in exclusive leadership development programming and recognition during the AzPA Annual Convention. After college of pharmacy graduation, the Legacy Intern is invited to continue to be an active AzPA member and pursue engagement and leadership opportunities within the organization.
We will be accepting applications for the 2023-2024 Legacy Intern cycle in early 2023.
The Legacy Intern is invited to shadow the activities of an AzPA leader, such as a Committee Chair, or a member of the AzPA Board of Directors. In addition, Legacy Interns are invited to attend Board and Committee Meetings throughout the year and participate in exclusive leadership development programming and recognition during the AzPA Annual Convention After college of pharmacy graduation, the Legacy Intern is invited to continue to be an active AzPA member and pursue engagement and leadership opportunities within the organization.
The Legacy Intern is invited to shadow the activities of an AzPA leader, such as a Committee Chair, or a member of the AzPA Board of Directors. In addition, Legacy Interns are invited to attend Board and Committee Meetings throughout the year and participate in exclusive leadership development programming and recognition during the AzPA Annual Convention. After college of pharmacy graduation, the Legacy Intern is invited to continue to be an active AzPA member and pursue engagement and leadership opportunities within the organization.
We will be accepting applications for the 2023-2024 Legacy Intern cycle in early 2023.
We will be accepting applications for the 2023-2024 Legacy Intern cycle in early 2023.
6 FALL 2022
2022-2023 LEGACY INTERNS
W H A T A R E T H E B E N E F I T S O F B E I N G A L E G A C Y I N T E R N ?
2022-2023 LEGACY INTERNS
W H A T A R E T H E B E N E F I T S O F B E I N G A L E G A C Y I N T E R N ?
W H A T I S A L E G A C Y I N T E R N ?
W H A T A R E T H E B E N E F I T S O F B E I N G A L E G A C Y I N T E R N ?
Az-ASHP State Affiliate News

I am honored to serve as the Director of Health Systems Pharmacy for the Arizona Pharmacy Association. This group is Arizona’s state affiliate chapter for ASHP and exists to serve and represent all pharmacy professionals practicing in Arizona hospitals and health-systems.
We are currently a small group, but there are exciting things in our future!
There are a number of ways to get involved. Interested members can:
1) Join our monthly calls
We meet the third Friday of every month from 12:30pm-1:30pm to discuss issues related to health systems practice in Arizona and nationally.
This is a great way to become familiar with the group, network with leaders in pharmacy in Arizona, and find out about other opportunities to get more involved.
2) Serve in a leadership position in AzSHP
Are you a pharmacist, technician, or student? Are you interested in legislative affairs? Education? Membership? Convention Planning? If so, please let us know if you are interested in serving in a leadership position within our group!
Christopher J. Edwards, PharmD, BCPS, FASHP AzPA Board of Directors

Appointments are one year and typically require attending 1-2 meetings per month
3) Serve as a Delegate to ASHP’s House of Delegates
Are you a pharmacist who is interested in helping to shape ASHP policy? Consider running for election as a delegate to ASHP’s House of Delegates!

The House of Delegates amends and votes on ASHP policy and serves to steer the organization.
Arizona has 3 delegates and several alternative delegates.
We are looking forward to the Spring Clinical meeting, where we will be hosting a number of non-traditional, interactive sessions focused on providing high level education for advanced practitioners within the state. If you have an idea for a topic or a session, please keep an eye out for our call for education proposals, coming soon.
There are so many great ways to get involved with this awesome organization and we look forward to working with you soon!
All the best,
Christopher J. Edwards, PharmD, BCPS, FASHP AzPA Board of Directors-Health System
www.azpharmacy.org/education/azpa-podcast/

7 azpharmacy.org
editorial
AzPA Health System Special Interest Group (AzSHP)
AzPA presents an innovative podcast intended for pharmacists and pharmacy technicians to obtain continuing education credit. The podcast also provides current health information and hot topics in the evolving world of pharmacy.
editorial preceptor corner
"Students are often instructed that networking is important but are rarely taught exactly how to do this. As a preceptor, share with your learners how you have developed your own professional network and if possible, bring students along to professional organization meetings, state association meetings, and state board of pharmacy meetings."

Preceptor Pearl: Your Students Want More Professional Development
Suzanne Larson, Director of Experiential Education, Midwestern University College of Pharmacy-Glendale
Janet Cooley, Director of Experiential Education, University of Arizona College of Pharmacy
Brooke L. Griffin, Professor and Vice Chair of Pharmacy Practice, Midwestern University College of PharmacyDowners Grove Campus (CPDG)
Disclosure
The author(s) declare no real or potential conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honorarium.
Funding
This research was not funded.
8 FALL 2022
Once upon a time, there was a final year pharmacy student rotating three hours away from her college of pharmacy. She and her fellow rotation student were just a few months preceding graduation and were preparing for a school-sponsored job fair, where employers would be seeking new graduate pharmacists. Attending this fair required missing an entire day of the rotation. These two students hesitantly approached their preceptor, explained their situation, and carefully requested the day off from patient care duties to attend this fair. What was the preceptor’s response? He told the students that everything they were learning about patient care would be for naught if they didn’t have a job in which to practice these skills as a pharmacist. To this preceptor, meeting with different employers, interviewing, and eventually securing a job were just as important to the development of an Advanced Pharmacy Practice Experience (APPE) student as learning the nuances of patient care. This is a true story from author Suzanne Larson's education, and the authors chose this story to set the stage for an important, and sometimes overlooked, discussion. The authors frequently remind preceptors and students that experiential education is the time for student pharmacists to learn the fundamentals of pharmacy practice that cannot be taught in a lecture hall or with multiplechoice tests. These important lessons include how to communicate with other health care professionals, how to deal with stressful situations, how to deal with challenging patient interactions, and how to manage a successful pharmacy. It is also vital for student pharmacists to develop their own professional identities.1 One concept that may help mentors and preceptors guide their learners can be discussed under the broad term of professional development (PD).
What is professional development? The Accreditation Council for Pharmacy Education (ACPE) defines continuous professional development (CPD) as “…a self-directed, ongoing, systemic and outcomes-focused approach to lifelong learning that is applied into practice.”2 For student learners, it can be summarized as the attributes and outcomes described in ACPE Standards 2016 Standard 4, which includes the development of characteristics such as self-awareness, leadership, innovation, entrepreneurship, and professionalism.3 Schools and colleges of pharmacy must provide curricula to meet the ACPE standards in order to achieve and maintain accreditation, however, there is flexibility in “how” this content is delivered and measured.
Intuitively, pharmacists understand that a wellrounded pharmacist is not only one who is knowledgeable and competent, but also one who is caring, articulate, engaged, and an effective communicator. These traits of the affective domain are usually modeled implicitly and part of a “hidden curriculum.” These characteristics can also be nurtured and developed through a purposeful focus on professional development. Some students may lack formal mentorship, so their preceptors often serve as informal mentors. Students are usually nervous about navigating the “real world” after graduation and APPE rotations offer the perfect container to reinforce professional development concepts from their didactic curriculum. The remainder of this article will discuss several components of professional development and provide suggestions for how preceptors can incorporate elements of PD into their rotation. Additional suggestions can be found in Table 1. A simple way for a preceptor to incorporate PD into their rotation is by offering a Curriculum Vitae (CV) or resume review. In addition to reviewing and offering guidance or tips on the learner’s CV or resume, the preceptor can ask the learner for constructive feedback on their own CV or resume. Together, the learner and preceptor can discuss and share examples of how they report their activities and what they think is important to emphasize. The process of giving feedback, receiving feedback, editing, and revising can offer rich learning and development for a student pharmacist.
Offering a mock interview is another way to incorporate professional development into experiential education. Interviewing skills may have been taught on campus but practicing this with a preceptor offers real-world value. Whether this is a complete mock interview or just asking one or two practice interview questions per week, helping learners think through and answer interview questions provide valuable, impactful lessons. Practice and preparation can ease the anxiety that typically accompanies a job or residency interview.
Another important element of professional development relates to personal finances. For some preceptors, this may not be a topic they feel confident in providing information. For other preceptors, this may be a topic that they have many life lessons to share. There may be books or websites that preceptors and learners can explore together. Topics such as navigating student loans, budgeting, career transitions, and buying
9 azpharmacy.org continued
on next page
a house could bring perspective to a learner that is preparing for the transition from pharmacy student to practicing pharmacist.
In connection with personal finance, a preceptor may consider providing learners with information about pharmacist liability insurance. Is this provided by your practice site? Is this something that is reimbursed? How much does this cost? Where do you obtain your liability insurance? How did you decide how much coverage to obtain?
When completing the pharmacist licensure requirements of continuing education or CPD materials, consider including your learners. As learners transition from the didactic or classroom curriculum to experiential learning, they are developing skills of self-directed learning. However, these skills may not yet be fully developed, and learners may not have the self-awareness needed to see and address the gaps in their knowledge. Having learners work through the CPD cycle with a mentor can serve as an extremely valuable learning opportunity. This gives the learner the opportunity to set goals, find relevant learning materials, work through the materials, and re-evaluate if their learning addressed their knowledge gaps.4
APPE rotations also provide an excellent opportunity for students to develop their professional networks. Students are often instructed that networking is important but are rarely taught exactly how to do this. As a preceptor, share with your learners how you have developed your own professional network and if possible, bring students along to professional organization meetings, state association meetings, and state board of pharmacy meetings. While there, introduce them to other pharmacists. Help your learners to see the importance of advocacy for and within the profession. Together, you may want to compose a letter to your elected officials
on legislative issues that impact pharmacy. There may also be opportunities for your learner to develop or improve their own professional online profile on sites such as LinkedIn.
The NAPLEX and MPJE exams may be the most high-stakes and important exams of a future pharmacist’s life. A preceptor is in an ideal position to help a student prepare for these exams by incorporating practice questions, topic discussions, calculations practice, or review sessions with the learner. What NAPLEX/MPJE preparation activities make sense for your practice site and rotation?
The final year of pharmacy school is the perfect opportunity to mentor students as they embark on a significant transition from student pharmacist to practicing pharmacist. Their APPE preceptors are in a perfect position to provide authentic learning activities that help students develop their patient care skills, and these duties can also be augmented with practical professional development activities.
REFERENCES
1. Professional Identity Formation [Internet]. American Association of Colleges of Pharmacy; 2022 [cited 2022 Aug 24]. Available from: https://www.aacp.org/article/ professional-identity-formation
2. Continuing Professional Development [Internet]. Accreditation Council for Pharmacy Education [cited 2022 Aug 24]. Available from: https://www.acpe-accredit.org/ continuing-professional-development/
3. Accreditation Standards and Key Elements for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree (“Standards 2016”) [Internet]. Accreditation Council for Pharmacy Education; 2015 [cited 2022 Aug 24]. Available from https://www.acpe-accredit. org/pdf/Standards2016FINAL.pdf
4. Continuing Professional Development…a self-directed, ongoing, systematic and outcomes-focused approach to lifelong learning that is applied to practice. Accreditation Council for Pharmacy Education [cited 2022 Aug 24]. Available from: https://www.acpe-accredit.org/pdf/ CPDConceptsACPEWebsiteFeb2015.pdf
10 FALL 2022
Table 1. Professional Development Ideas/Activities for Preceptors Area of Focus Ideas Self-Awareness Journal Club or reflection paper on giving and receiving feedback Facilitate a 360-degree feedback experience for your student Leadership Attend a local or national pharmacy meeting such as Legislative Day, State Association Meeting, or a State Board of Pharmacy Meeting Innovation/ Entrepreneurship Exposure to pharmacists who have created or implemented new programs or clinical services Professionalism Journal Club or reflection paper on academic dishonesty Journal Club or reflection paper on Professional Identity Formation
editorial member spotlight

Misty Brannon
PharmD
College of Pharmacy: Midwestern University College of Pharmacy, Graduating Class of 2020
AzPA Membership Type: New Practitioner-2nd Year
Safeway
- Staff Pharmacist
AzPA Board of Directors
LinkedIn
@Misty Brannon
How long have you been an AzPA member?
1-5 years
What do you enjoy most about being an AzPA member?
What I enjoy most about being an AzPA member is the networking. I have met so many amazing individuals – many of whom have become direct colleagues and friends.
How were you first introduced to the world of pharmacy?
When I was a freshman in high school, I had to interview two people from the Math or Science field. My mother worked at Smitty’s at the time, so I utilized the two Pharmacists to complete my project. Both Pharmacists had opposing views about their career which intrigued me to learn more about the field.

Explain what a typical work day looks like for you.
A typical work day includes: filling prescriptions, counseling, screening and administering vaccinations, MTMs, placing and checking in controlled substance orders, general customer service, directing traffic, and keeping the technicians happy.
In your opinion, what is the most rewarding part of practicing pharmacy?
The most rewarding part of practicing pharmacy is that smile on a patient’s face when they walk away empowered with knowledge about their medication and/or disease state.
How do you give back to the profession?
I give back to the profession by being an AzPA member, a committee member, and now a Director at Large. I have advocated for the profession on the Senate lawn, I am a preceptor, and a mentor.
What do you enjoy doing in your free time?
In my free time, I enjoy: baking, working in my vegetable garden, and hanging out with my husband, 2 dogs, and 20 chickens.
What's something about you (a fun fact) that not many people know?
I used to sing competitively in high school, but I have paralyzing stage fright.
11 azpharmacy.org
Impact of Specialty Pharmacy on Adherence to Maintenance Medications for Chronic Diseases Among Solid Organ Transplant Recipients

Kellie J. Goodlet, PharmD, BCPS, BCIDP-Associate Professor in the Department of Pharmacy Practice, Midwestern University College of Pharmacy - Glendale, AZ
Brittany Cooper, PharmD-PGY1 Community Pharmacy Resident at Walgreens Specialty Pharmacy - Phoenix, AZ
Nikki Christofferson, PharmD-Specialty Pharmacy Site Manager at Walgreens Specialty Pharmacy - Phoenix, AZ Acknowledgement None
12 FALL 2022
continuing education
Funding This research was not funded.
Conflict of Interest The authors declare that there are no conflicts of interest.
Continuing Education Information: Target Audience: Pharmacists Activity Type: Knowledge Learning Objectives: 1. Describe study rationale and design as it relates to the contemporary original research article 2. Summarize the study methodology and measured outcomes 3. Interpret study results to form conclusions about primary and secondary outcomes Refer to page 19 for more continuing education details.
Abstract
Purpose: Adherence to maintenance medications for hypertension, diabetes, and dyslipidemia supports long-term allograft survival and overall health among solid organ transplant recipients. Specialty pharmacists are experienced in the comprehensive medication management of high-risk populations and may help support medication adherence. The primary objective of this study was to determine if filling both immunosuppressants and maintenance medications at one specialty location resulted in a high proportion of solid organ transplant recipients achieving good medication adherence.
Methods: Included transplant recipients filled immunosuppressants and a renin angiotensin system antagonist, non-insulin oral anti-diabetic, or statin at a single specialty pharmacy from 1/1/202012/31/2020. Good adherence was defined as a Proportion of Days Covered (PDC) score ≥ 80% (PDC-80).
Results: A total of 100 patients met inclusion criteria, with the most common transplant types being lung (42%), kidney (36%), and heart (14%). Overall, 83% of patients met the PDC-80 threshold, including 85% with an eligible hypertension prescription, 79% with an eligible diabetic prescription, and 87% with an eligible dyslipidemia prescription. Patients who were adherent were more likely to be older (median age 65 years versus 58 years, p=0.042) and were more likely to have received a lung transplant (47% versus 18%, p=0.031) compared to the non-adherent group. There were no significant differences between the groups with respect to evaluable disease states, prescribed medications or drug classes, or years post-transplantation.
Conclusion: Filling all medications at a single specialty pharmacy may have a positive impact on maintenance medication adherence among solid organ transplant recipients.
Introduction
Patients with solid organ transplants have complex medication regimens comprised of numerous different medications to keep their allograft functional and support overall health. Commonly utilized immunosuppressant medications in transplant, although necessary to prevent allograft rejection, may negatively affect blood pressure, glucose, and cholesterol, resulting in high rates of posttransplant hypertension, diabetes, and dyslipidemia in this population. For example, in one study, 19% of recipients had developed dyslipidemia, 39% developed diabetes mellitus, and 40% developed hypertension 1 year post liver transplant. The incidence of all of these metabolic disorders was associated with immunosuppressive therapy, particularly use of calcineurin inhibitors.1 In addition to the known risk for deleterious cardiovascular outcomes associated with these disorders, these comorbidities contribute to shortened graft and patient survival outcomes in the transplant population.2-5
Among solid organ transplant recipients with metabolic comorbidities such as hypertension, patients with controlled disease have been shown to have improved survival and fewer cardiovascular events compared to patients with uncontrolled disease.6 However, nonadherence is a known barrier to achieving chronic disease control among solid organ transplant recipients.7 An estimated 20-55% of solid organ recipients are nonadherent to their posttransplant regimens, though adherence rates specific to maintenance medications for chronic diseases are less known.8-10 Given the complexity of transplant medication regimens, the United Network for Organ Sharing requires that all solid organ transplant programs include at least one pharmacist within the multidisciplinary transplant team. Transplant pharmacists have been shown to positively influence adherence rates through medication education and working in collaboration with their patients to develop individualized adherence tools and strategies.11,12 However, transplant programs may lack structured processes to assess and monitor adherence in the outpatient setting, with less than 30% of centers having a prospective medication nonadherence screening protocol per a large survey of transplant healthcare professionals.12 Additionally, patients not appearing for their scheduled clinic appointments may be at greatest risk for nonadherence.13 This suggests that to achieve optimal chronic disease control, additional strategies to promote adherence within the community are needed.
13 azpharmacy.org continued on next page
Filling both immunosuppressant and chronic disease prescriptions at a single specialty pharmacy with expertise in transplant medication management may represent a “low hanging fruit” intervention to achieve good adherence rates to medications used for hypertension, diabetes, and dyslipidemia that are assessed by the Centers for Medicare and Medicaid Services (CMS) to judge Medicare Part D plans and determine their star (quality) rating.14 Specialty pharmacies offer comprehensive support in distribution and medication therapy management for specialty drugs and may improve clinical outcomes among patients with complex and chronic conditions such as organ transplantation. Among transplant recipients, filling medications with a specialty pharmacy has been associated with lower transplant-related medical costs and reduced overall healthcare costs, with improved immunosuppressant adherence.15 However, the effect of the specialty pharmacy on adherence rates to transplant recipients’ maintenance medications remains underexplored within the literature.
The primary objective of this study was to calculate the Proportion of Days Covered (PDC) scores of solid organ recipients’ maintenance medications at a local specialty pharmacy to determine if filling immunosuppressant medications and one or more qualifying maintenance medication at one specialty location results in a high proportion of patients meeting a PDC threshold of 80%, the established CMS standard for good medication adherence.14 The secondary objective was to investigate patient factors associated with failing to meet this threshold in order to identify potential barriers to adherence and support future targeted initiatives by specialty pharmacies to raise adherence rates in this population.
Methods
This retrospective cohort study was approved by the institutional review board of Midwestern University. The study population was all patients with a solid organ transplant in the clinical care program at a local specialty site. This site is not affiliated with a particular transplant center and serves patients across Arizona and primarily within the Greater Phoenix region, which is the largest metropolitan area in the Southwestern United States. The five pharmacists that are on staff at this specialty pharmacy are experienced in transplant pharmacotherapy and in obtaining and accessing specialty transplant medications. Most medications are mailed directly to patients to ease medication
access; however, medications may be also picked up on-site per patient preference. All patients receive comprehensive pharmacist counselling for new or changed prescriptions. To further promote medication adherence, the pharmacists also work to align refill dates of all medications filled at the specialty pharmacy and perform structured, monthly calls prior to the patient being scheduled to run out of medication in order to identify potential adherence issues and refill and counsel on medications as needed.
A list of transplant recipients who filled their immunosuppression medications at the specialty pharmacy from 1/1/2020 through 12/31/2020 was generated. Inclusion criteria consisted of filling immunosuppressants and at least one qualifying medication at the study location. Qualifying medications consisted of a statin medication, oral antidiabetic medication (i.e. metformin, sulfonylurea, thiazolidinedione, dipeptidyl peptidase-4 inhibitor, sodium-glucose cotransporter-2 inhibitor), or a renin-angiotensin-aldosterone system antagonist medication (i.e. angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker) at the study site. Other pharmacotherapies for the management of dyslipidemia, diabetes mellitus, or hypertension were excluded as only the aforementioned drug classes are evaluated by CMS. 14
The primary outcome was the proportion of patients achieving a PDC score of ≥ 80% (PDC-80) for all eligible maintenance medications during the study time period, which was the definition used for medication adherence. Patients not achieving this threshold for all evaluable chronic disease states were considered nonadherent. The PDC score is an objective measure of medication adherence obtained through the extraction of patients’ prescription filling patterns and calculated based on the number of days covered by a medication per day in the observation time period. The PDC score, unlike other measures of adherence, takes into account patients that are on multiple medications for the same indication by calculating the days the patient would have all medications, in which the patient is considered covered in therapy. The Pharmacy Quality Alliance (PQA) has endorsed PDC as its recommended measure of medication adherence, and CMS has incorporated PDC threshold assessments into its plan ratings. The PDC threshold is the level above which the medication has a reasonable likelihood of achieving its optimal clinical benefit. The PQA supports a threshold of 80% for most chronic medication therapies, which also represents the standard set by CMS.14,16
14 FALL 2022
continued on next page
Descriptive statistics (means/standard deviation for normally distributed continuous data, medians/IQR for skewed continuous data, and counts/percents for nominal data) were used to report patient baseline characteristics. As a secondary exploratory analysis, inferential statistics (chi-square test for nominal data, Mann-Whitney U test for skewed continuous data, or Student’s t-test for normally distributed continuous data) were used to assess for differences (e.g. age, sex, specific medications) among those patients meeting and not meeting the PDC-80 threshold (i.e. adherent and nonadherent groups).
Results
Patient characteristics
Among 285 solid organ transplant recipients on the specialty pharmacy active transplant list for 2020, 100 patients (35.1%) met inclusion criteria (Table 1). The median age was 64 years, 65% of patients were male, and the most common transplant types were lung (42%), kidney (36%), and heart (14%). The median time posttransplant was 3 years (IQR 2-4), with 13% of patients less than 1 year from their transplant date. Over 90% of patients received tacrolimus with mycophenolate mofetil or mycophenolic acid for prevention of allograft rejection. Among all patients, 86% had a qualifying prescription for dyslipidemia, 26% had a qualifying prescription for hypertension, and 14% had one or more qualifying prescriptions for diabetes mellitus. Assessment of adherence
Overall, 83% of all study participants met the PDC-80 threshold for all evaluable disease states (adherent group). In considering each individual disease state, the proportions of patients meeting the PDC-80 threshold were similar, with rates highest for dyslipidemia (75/86, 87.2%), followed by hypertension (22/26, 84.6%) and diabetes (11/14, 78.6%). By organ type, lung transplant recipients had the highest adherence rates (39/42, 92.9%), following by liver recipients (5/6, 83.3%), kidney recipients (28/36, 77.8%), and heart recipients (10/14, 71.4%). In comparing the adherent and nonadherent patient groups, the solid organ recipients who were adherent were more likely to be older (median age 65 years versus 58 years, p=0.042) and were more likely to have received a lung transplant (47% versus 17.6%, p=0.031). The percentage of patients within 1-year posttransplant trended higher in the nonadherent group but was not significant. Also, no significant differences appeared between the groups for evaluable disease states, prescribed medications, medication therapy classes, or sex.
Discussion
Per previous reports, medication nonadherence among solid organ transplant recipients is common, representing an ongoing challenge for healthcare practitioners.8-10,12 The causes of nonadherence are multifactorial, and may include barriers related to health system access and insurance, dissatisfaction with medication effects, and sociodemographic and psychosocial factors such as self-efficacy and emotional well-being.17 Among solid organ transplant recipients receiving one or more maintenance medications evaluated by CMS, 83% of patients in the present study had a PDC score of 80 or higher, suggesting that having all medications filled at a single specialty pharmacy may have a positive impact on adherence.
Although not specific to organ transplant recipients, these results are consistent with a previous study of over 9,000 older adult patients enrolled in Medicare Part D plans, which found that filling prescriptions at multiple pharmacies concurrently was associated with increased odds of nonadherence across all chronic medication classes (range 1.10 to 1.31, p<0.001), including after statistical adjustment for multiple social determinants of health.18 In addition, the calculated nonadherence rates of 27-43% (also using the PDC-80 threshold) were consistent with prior studies19-21 but numerically higher than was observed in the present study. One study of 53 kidney transplant recipients in Belgium reported an adherence rate to antihypertensive therapy of 79%, which was similar to the present study.22 However, it should be noted that the included patients all consented to urine drug dosage quantification and were far out from transplantation (mean of 9.5 years), potentially reflecting a patient population that was more likely to be adherent. Additionally, it is difficult to directly compare adherence rates of U.S. patients with those of patients living in countries with socialized medicine due to differences in healthcare practices.
In comparing patient characteristics, solid organ transplant recipients who were adherent to their maintenance medications were more likely to have received a lung transplant and were also more likely to be older. Although older adults are often regarded as a high-risk group, studies investigating the association between age and maintenance medication adherence have produced conflicting results, with some studies also reporting higher adherence rates among older adults,21,23 while others report reduced adherence.24-26 Although contemporary data among transplant recipients are limited, a single center study investigating medication adherence following liver transplantation
15 azpharmacy.org continued on next page
found that older age (≥ 65 years) was associated with higher adherence rates to immunosuppressive therapy (65% versus 42%, p=0.02) among patients ≥ 1 year post transplant.27 Although not statistically significant, there was a trend toward more recent transplantation for the nonadherent group in the present study, which, in addition to reflecting a new dramatic change in these patients’ pharmacotherapy, includes patients who received their transplant during the early COVID-19 pandemic at a time when stay-at-home orders were active across Arizona and access to in person healthcare was limited. Additionally, maintenance medications may be used for prophylactic indications among transplant recipients, such as using statins to prevent cardiac allograft vasculopathy in patients who have received a heart transplant, which could also decrease patients’ motivation to be adherent to therapy. Specialty pharmacists may help attenuate the potential increased risk for nonadherence among younger solid organ transplant recipients by helping to ensure that these patients understand the purpose of their maintenance medications and the link between control of chronic disease and allograft function and rejection.
Limitations
Limitations of the present study should be noted and serve as potential directions for future research investigating maintenance medication adherence among solid organ transplant recipients, for which there exists a paucity of data. First, this study did not include a control group of patients who did not fill at the specialty pharmacy, precluding a direct comparison of adherence rates. However, the 83% adherence rate observed in the present study was consistently in line with or higher than adherence rates reported in the published literature investigating adherence either to maintenance medications or among transplant recipients. Second, there are several different measures which may be used to assess medication adherence, including therapeutic drug monitoring, patient self-report, pill counts, and different calculations based on prescription refill rates.28 The PDC score was used in the present study due to its use in the determination of Medicare Part D star quality ratings by CMS, which is of high importance to community pharmacies. Pharmacy benefit managers (PBMs) also look at adherence rates when determining direct and indirect remuneration fees, a payment mechanism to pharmacies for the fulfillment of various quality measures or, alternatively, a fee assessed to pharmacies for noncompliance with quality measures.29 Additional benefits of the PDC
score include that it is an objective and non-invasive way to measure adherence, and it is generally considered more conservative (i.e. more likely to underestimate adherence) than other calculations such as the medication possession ratio, which provides further support to the high adherence rates observed in the present study. However, a notable limitation of the PDC score is that it only provides evidence of the drug being dispensed, but does not guarantee that the drug was actually taken by the patient as prescribed. It is possible that alternate measures of adherence would have resulted in higher or lower rates. Third, some measures that may be associated with nonadherence such as race, socioeconomic status, and insurance coverage were not available for analyses. Our results nevertheless add to the current adherence literature by identifying some groups that may be at higher risk for nonadherence in this patient population such as younger age and non-lung transplant type. Lastly, the year of data analyzed occurred at the start of a global viral pandemic. Shutdowns, stay-at-home orders, and fears of infection could have posed a barrier for patients to pick up their medications, leading to increased nonadherence during this time period. Adherence rates were high despite this limitation, with monthly pharmacy reminder calls and the availability of mailed prescriptions potentially mitigating pandemic-related medication disruptions.
Conclusion
Specialty pharmacists are experienced in the provision of comprehensive medication management for both specialty and non-specialty medications, often have increased access and integration within care teams, collaborate with PBMs and payers to support medication access, and advocate for patient-centered care and strong pharmacist-patient relationships within community and ambulatory care settings. When applying a national standard metric for assessing medication adherence, solid organ transplant recipients filling prescriptions at a single specialty pharmacy had high rates of adherence to maintenance medications commonly utilized for the management of hypertension, diabetes mellitus, and dyslipidemia. This study illustrated the potential benefits of utilizing specialty pharmacy services to support the comprehensive care of solid organ transplant recipients and complement the efforts of the multidisciplinary transplant team to promote outpatient medication adherence.
16 FALL 2022 continued on next page
Table 1
– Patient demographics and medications for solid organ transplantation recipients filling maintenance medication prescriptions at a single specialty pharmacy
Variable
All patients, n=100
Adherent groupa, n=83
Nonadherent group, n=17 p-valueb
Male sex, n (%) 65 (65) 56 (67.5) 9 (52.9) 0.253
Age, years, median (IQR) 64 (53-71) 65 (55-71) 58 (41-67) 0.042
• ≥ 75 years, n (%) 11 (11) 11 (13.3) 0 0.203
Years post-transplant, median (IQR) 3 (2-4) 3 (2-4) 3 (1-4) 0.201
• ≤ 1 13 (13) 9 (10.8) 4 (23.5) 0.227
• 2-3 25 (25) 21 (25.3) 4 (23.5) 1.00
• 4-5 19 (19) 17 (20.5) 2 (11.8) 0.516
• ≥ 6 9 (9) 8 (9.6) 1 (5.9) 1.00
• Unknown 34 (34) 28 (33.7) 6 (35.3) 0.902
Transplant type, n (%)
• Lung 42 (42) 39 (47.0) 3 (17.6) 0.031
• Kidney 36 (36) 28 (33.7) 8 (47.1) 0.297
• Heart 14 (14) 10 (12.0) 4 (23.5) 0.250
• Liver 6 (6) 5 (6.0) 1 (5.9) 1.00
• Heart/lung 1 (1) 1 (1.2) 0 1.00
• Kidney/pancreas 1 (1) 0 1 (5.9) 0.170
Immunosuppression, n (%)
• Tacrolimus 95 (95) 80 (96.4) 15 (88.2) 0.199
• Cyclosporine 2 (2) 1 (1.2) 1 (5.9) 0.313
• MMF 76 (76) 63 (75.9) 13 (76.5) 1.00
• MPA 13 (13) 11 (13.3) 2 (11.8) 1.00
• Sirolimus 4 (4) 4 (4.8) 0 1.00
• Everolimus 2 (2) 1 (1.2) 1 (5.9) 0.313
Qualifying DM med, n (%) 14 (14) 11 (13.3) 3 (17.6) 0.702
• Metformin 5 (5) 4 (4.8) 1 (5.9) 1.00
• DPP-4 5 (5) 4 (4.8) 1 (5.9) 1.00
• SGLT-2 3 (3) 2 (2.4) 1 (5.9) 0.432
• Sulfonylurea 3 (3) 2 (2.4) 1 (5.9) 0.432
• Glitazone 1 (1) 1 (1.2) 0 1.00
Qualifying HTN med, n (%) 26 (26) 21 (25.3) 5 (29.4) 0.725
• ACE-I 17 (17) 12 (14.5) 5 (29.4) 0.135
• ARB 9 (9) 9 (10.8) 0 0.351 Statin, n (%) 86 (86) 72 (86.7) 14 (82.4) 0.634
• Pravastatin 51 (51) 43 (51.8) 8 (47.1) 0.721
• Rosuvastatin 15 (15) 13 (15.7) 2 (11.8) 1.00
• Atorvastatin 15 (15) 11 (11.3) 4 (23.5) 0.279
• Lovastatin 3 (3) 3 (3.6) 0 1.00
• Simvastatin 2 (2) 2 (2.4) 0 0.432
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; DM, diabetes mellitus; DPP-4, dipeptidyl peptidase-4 inhibitor; HTN, hypertension; IQR, interquartile range; MMF, mycophenolate mofetil; MPA, mycophenolic acid; SGLT-2, sodium-glucose cotransporter-2 inhibitor a Defined as Proportion of Days Covered score ≥ 80% for all evaluable disease states b Comparison of adherent and nonadherent patient groups continued on next page
17 azpharmacy.org
REFERENCES
1. Hashim MS, Alsabaawy M, Afify S, et al. Incidence and risk factors for diabetes, hypertension and hyperlipidemia after liver transplantation. J Gastroenterol Hepatol Res. 2020;9:3077-3081.
2. Harding JL, Pavkov M, Wang Z, et al. Long-term mortality among kidney transplant recipients with and without diabetes: a nationwide cohort study in the USA. BMJ Open Diabetes Res Care. 2021;9:e001962.
3. Opelz G, Döhler B. Improved long-term outcomes after renal transplantation associated with blood pressure control. Am J Transplant. 2005;5:2725-2731.
4. Park S, Kang SJ, Lee JW, et al. Association between early post-transplant hypertension or related antihypertensive use and prognosis of kidney transplant recipients: a nationwide observational study. J Nephrol. 2021;34:14571465.
5. Sharif A, Cohney S. Post-transplantation diabetes—state of the art. Lancet Diabetes Endocrinol. 2016;4:337-349.
6. VanWagner LB, Holl JL, Montag S, et al. Blood pressure control according to clinical practice guidelines is associated with decreased mortality and cardiovascular events among liver transplant recipients. Am J Transplant. 2020;20:797-807.
7. Midtvedt K, Onsøien MO, Åsberg A. Posttransplant hypertension matters! Transplantation. 2021;105:e150.
8. Bunzel B, Laederach-Hofmann K. Solid organ transplantation: are there predictors for posttransplant noncompliance? A literature overview. Transplantation. 2000;70:711-716.
9. De Geest S, Borgermans L, Gemoets H, et al. Incidence, determinants, and consequences of subclinical noncompliance with immunosuppressive therapy in renal transplant recipients. Transplantation. 1995;59:340-347.
10. Nevins TE, Nickerson PW, Dew MA. Understanding medication nonadherence after kidney transplant. J Am Soc Nephrol. 2017;28:2290-2301.
11. Doyle IC, Maldonado AQ, Heldenbrand S, et al. Nonadherence to therapy after adult solid organ transplantation: a focus on risks and mitigation strategies. Am J Health Syst Pharm. 2016;73:909-920.
12. Patel SJ, Hofmeyer BA, Moore CA, et al. Medication nonadherence monitoring and management in adult kidney transplantation: a survey of practices and perceptions at US-based transplant programs. J Am Coll Clin Pharm. 2021;4:1100-1108.
13. Mohamed M, Soliman K, Pullalarevu R, et al. Nonadherence to appointments is a strong predictor of medication non-adherence and outcomes in kidney transplant recipients. Am J Med Sci. 2021;362:381-386.
14. PQA measure use in CMS’ Part D quality programs. Pharmacy Quality Alliance. https://www.pqaalliance.org/ medicare-part-d. Accessed November 10, 2021.
15. Tschida S, Aslam S, Khan TT, et al. Managing specialty medication services through a specialty pharmacy program: the case of oral renal transplant immunosuppressant medications. J Manag Care Pharm. 2013;19:26-41.
16. Adherence: PQA adherence measures. Pharmacy Quality Alliance. https://www.pqaalliance.org/adherencemeasures. Published March 25, 2021. Accessed February 2, 2022.
17. Doyle IC, Maldonado AQ, Heldenbrand S, et al. Nonadherence to therapy after adult solid organ transplantation: a focus on risks and mitigation strategies. Am J Health Syst Pharm. 2016;73(12):909-920. doi:10.2146/ajhp150650
18. Marcum ZA, Driessen J, Thorpe CT, et al. Effect of multiple pharmacy use on medication adherence and drug-drug interactions in older adults with Medicare Part D. J Am Geriatr Soc. 2014;62(2):244-252. doi:10.1111/jgs.12645
19. Persaud N, Bedard M, Boozary A, et al. Adherence at 2 years with distribution of essential medicines at no charge: the CLEAN Meds randomized clinical trial. PLoS Med. 2021;18(5):e1003590. doi:10.1371/journal. pmed.1003590
20. Pucci M, Martin U. Detecting non-adherence by urine analysis in patients with uncontrolled hypertension: rates, reasons and reactions. J Hum Hypertens. 2017;31(4):253257. doi:10.1038/jhh.2016.69
21. Steve Tsang CC, Browning J, Todor L, et al. Factors associated with medication nonadherence among Medicare low-income subsidy beneficiaries with diabetes, hypertension, and/or heart failure. J Manag Care Spec Pharm. 2021;27(8):971-981. doi:10.18553/ jmcp.2021.27.8.971
22. Georges CMG, Devresse A, Ritscher S, et al. Adherence to antihypertensive drug treatment in kidney transplant recipients. Blood Press. 2021;30(6):411-415. doi:10.1080 /08037051.2021.2004087
23. Spadea T, Onorati R, Baratta F, et al. Monitoring adherence to pharmacological therapy and follow-up examinations among patients with type 2 diabetes in community pharmacies. Results from an experience in Italy. PLoS One. 2021;16(9):e0256478. doi:10.1371/journal. pone.0256478
24. Bonikowska I, Szwamel K, Uchmanowicz I. Analysis of the impact of disease acceptance, demographic, and clinical variables on adherence to treatment recommendations in elderly type 2 diabetes mellitus patients. Int J Environ Res Public Health. 2021;18(16):8658. doi:10.3390/ ijerph18168658
25. Pinhati R, Ferreira R, Carminatti M, et al. Adherence to antihypertensive medication after referral to secondary healthcare: a prospective cohort study. Int J Clin Pract. 2021;75(3):e13801. doi:10.1111/ijcp.13801
26. Shruthi R, Jyothi R, Pundarikaksha HP, et al. A study of medication compliance in geriatric patients with chronic illnesses at a tertiary care hospital. J Clin Diagn Res. 2016;10(12):FC40-FC43. doi:10.7860/ JCDR/2016/21908.9088
27. Leven EA, Annunziato R, Helcer J, et al. Medication adherence and rejection rates in older vs younger adult liver transplant recipients. Clin Transplant. 2017;31(6):10.1111/ctr.12981. doi:10.1111/ctr.12981
28. Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92(2):117-122. doi:10.15386/ mpr-1201
29. Frequently asked questions about pharmacy DIR fees. NCPA website. ncpa.co/pdf/faq-direct-indirectremuneration-fees.pdf. Accessed February 16, 2022.
18 FALL 2022
continued on next page
AzPA Members may retrieve FREE CE for this article up to one year after the program release date.

The Arizona Pharmacy Association is accredited by the Accreditation Council for Pharmacy Education as providers of continuing education.
Accredited Date: 10/12/2022
Expiration Date: 10/12/2025
Thisprogramprovides0.5contacthoursofcontinuingeducationcredit.
UniversalActivityNumber(UAN): 0100-0000-22-152-H99-P
Applyforcredithere:https://www.lecturepanda.com/a/AJPFall2022
CE Questions
1. This study took place in which of the following practice settings?
a. Emergency department
b. Medical intensive care unit
c. Outpatient transplant clinic d. Specialty pharmacy
2. Which of the following justifications was provided by the study authors for examining maintenance medication adherence in the solid organ transplant population?
a. Filling medications with a specialty pharmacy has previously been associated with higher transplant-related medical costs
b. Metabolic comorbidities may contribute to impaired graft function and negatively impact patient survival
c. Pharmacists have not traditionally been integrated within the multidisciplinary teams of solid organ transplant programs
d. While 95% of solid organ transplant recipients are adherent to immunosuppressant therapy, adherence rates to maintenance medications have not been formally assessed
3. Which of the following measures was used in the assessment of patient adherence?
a.Analysis of serum drug levels
b. Immunosuppressant therapy adherence scale
c. Medication possession ratio d. Proportion of days covered
4. Which of the following anti-hypertensive medications was NOT included in the evaluation of patient adherence?
a.Amlodipine
b. Enalapril c. Lisinopril d. Losartan
5. Which two patient characteristics were associated with increased adherence to maintenance medications?
a.Older age and commercial insurance b. Older age and lung transplant recipient c. Younger age and female sex d. Younger age and kidney transplant recipient
6. Which of the following conclusions best represents the findings of Cooper et al with respect to maintenance medication adherence among solid organ transplant recipients who filled their prescriptions at a single specialty pharmacy?
a.A high proportion of solid organ transplant recipients were adherent to their maintenance medications, suggesting that having all medications filled at a single specialty pharmacy may have a positive impact on adherence
b. Solid organ transplant recipients receiving medications for dyslipidemia are at greatest risk for nonadherence and should be prioritized in future targeted efforts to improve adherence rates
c. Solid organ transplant recipients who filled their medications at a specialty pharmacy had a statistically significant increase in medication adherence compared to those who did not fill their medications at a specialty pharmacy
d. The COVID-19 pandemic may have restricted healthcare access or decreased patient motivation to adhere to therapy, resulting in a low observed rate of medication adherence
19 azpharmacy.org
editorial financial forum
Do Our Biases Affect Our Financial Choices?
Even the most seasoned investors are prone to their influence. Pat Reding and Bo Schnurr
This series, Financial Forum, is presented by PRISM Wealth Advisors, LLC and the Arizona Pharmacy Association through Pharmacy Marketing Group, Inc., a company dedicated to providing quality products and services to the pharmacy community.

Investors are routinely warned about allowing their emotions to influence their decisions. However, they are less routinely cautioned about their preconceptions and biases that may color their financial choices. In a battle between the facts & biases, our biases may win. If we acknowledge this tendency, we may be able to avoid some unexamined choices when it comes to personal finance. It may actually "pay" to recognize blind spots and biases with investing. Here are some common examples of bias creeping into our financial lives.
Letting emotions run the show. An investor thinks, "I got a great return from that decision," instead of thinking, "that was a good decision because ______."1
How many investment decisions do we make that have a predictable outcome? Hardly any. In retrospect, it is all too easy to prize the gain from a decision over the wisdom of the decision, and to, therefore, believe that the findings with the best outcomes were the best decisions (not necessarily true). Putting some distance between your impulse to make a change and the action you
20 FALL 2022
continued on next page
want to take to help get some distance from your emotions.1
Valuing facts we "know" & "see" more than "abstract" facts. Information that seems abstract may seem less valid or valuable than information that relates to personal experience. This is true when we consider different types of investments, the state of the markets, and the economy's health.2
Valuing the latest information most. In the investment world, the latest news is often more valuable than old news. But when the latest news is consistently good (or consistently bad), memories of previous market climate(s) may become too distant. If we are not careful, our minds may subconsciously dismiss the eventual emergence of the next bear (or bull) market.2
Being overconfident. The more experienced we are at investing, the more confidence we have about our investment choices. When the market is going up, and a clear majority of our investment choices work out well, this reinforces our confidence, sometimes to a point where we may start to feel we can do little wrong, thanks to the state of the market, our investing acumen, or both. This can be dangerous.3
The herd mentality. You know how this goes: if everyone is doing something, they must be doing it for sound and logical reasons. The herd mentality is what leads many investors to buy high (and sell low). It can also promote panic selling. The advent
of social media hasn't helped with this idea. Above all, it encourages market timing, and when investors try to time the market, they frequently realize subpar returns.4
Sometimes, asking ourselves what our certainty is based on and reflecting about ourselves can be a helpful and informative step. Examining our preconceptions may help us as we invest.
CITATIONS
1. CNBC.com, September 28, 2020
2. Forbes.com, March 26, 2020
3. Forbes.com, March 19, 2020
4. CNBC.com, June 26, 2020
Pat Reding and Bo Schnurr may be reached at 800-288-6669 or pbh@berthelrep.com.

Registered Representative of and securities and investment advisory services offered through Berthel Fisher & Company Financial Services, Inc. Member FINRA/SIPC. PRISM Wealth Advisors LLC is independent of Berthel Fisher & Company Financial Services Inc.
This material was prepared by MarketingPro, Inc., and does not necessarily represent the views of the presenting party, nor their affiliates. This information has been derived from sources believed to be accurate. Please note - investing involves risk, and past performance is no guarantee of future results. The publisher is not engaged in rendering legal, accounting or other professional services. If assistance is needed, the reader is advised to engage the services of a competent professional. This information should not be construed as investment, tax or legal advice and may not be relied on for the purpose of avoiding any Federal tax penalty. This is neither a solicitation nor recommendation to purchase or sell any investment or insurance product or service, and should not be relied upon as such. All indices are unmanaged and are not illustrative of any particular investment.
21 azpharmacy.org
rx and the law
Do Employed Pharmacists Need an Individual Pharmacist Professional Liability Policy?
 Don R. McGuire Jr. R.Ph., J.D.
Don R. McGuire Jr. R.Ph., J.D.
This series, Pharmacy and the Law, is presented by Pharmacists Mutual Insurance Company and the Arizona Pharmacy Association through Pharmacy Marketing Group, Inc., a company dedicated to providing quality products and services to the pharmacy community.
Most employed pharmacists believe their employer’s insurance policy protects them in the event of a professional liability claim. This is usually correct. The fact that it is not always correct is reason enough for pharmacists to consider buying their own individual professional liability policy. There are three factors, which when considered together, show the need for a pharmacist to obtain their own coverage.
1. Control – The employed pharmacist has no control over the coverage purchased by their employer. During my years as an employed pharmacist, I never saw my employer’s policy. I worked on their word that I was covered. I did not know what the coverage limits were, what services the policy covered or even if employed pharmacists were an insured under the policy.
22 FALL 2022
continued on next page
editorial
Most employed pharmacists believe their employer’s insurance policy protects them in the event of a professional liability claim. This is usually correct. The fact that it is not always correct is reason enough for pharmacists to consider buying their own individual professional liability policy.
If limits are too low or if the policy does not cover certain services, such as immunizations or MTM, the employed pharmacist is potentially left exposed. If this lack of control was not enough, the employee does not know if/when the policy lapses or if the employer fails to pay the premium. The worst time to find out these things is when you are facing a claim. While the typical individual professional liability policy is secondary or excess, it can drop down to provide primary coverage for the pharmacist when the employer’s policy is missing or inapplicable.
2. Coverage – The typical employer’s policy only provides the pharmacist with professional liability coverage for acts within the scope of their employment. In other words, the pharmacist is only covered while they are at work. For a pharmacist who volunteers at a senior center or a church, provides advice to friends and neighbors, or occasionally moonlights, their primary employer’s policy does not cover them in these situations. An individual policy, on the other hand, covers the pharmacist 24 hours a day regardless of when or where they provide pharmacy services. It is also possible that the employer's policy may choose to not cover the employed pharmacist if the pharmacist violates the employer's policies or procedures. The additional protection provided by the individual's policy allows the pharmacist to give back without worrying about their personal exposure.
3. Target – There is one additional concern often expressed by risk managers and employers. That is that the existence of
an individual professional liability policy makes the employed pharmacist a target for the plaintiff’sattorney. Our experience has shown this not to be true. The trend is that plaintiffs’ attorneys are naming the individual pharmacists as defendants many more times today than they were 20 years ago. A good plaintiff’s attorney will bring all potentially liable persons into the suit. Most often, this happens even before the existence of the individual pharmacist's policy is known. We have even had cases where the individual policy was not discussed until two or three years into the litigation process. While I believe this target idea is a myth, even if it is true, it is outweighed by the other considerations above.
The ease of application and low cost of individual professional liability coverage make this choice even easier for the employed pharmacist. It provides an extra layer of protection over and above that carried by their employer. If there is a problem with the employer’s coverage for the employed pharmacist, the pharmacist’s individual coverage can provide the missing, and much needed, protection. This is especially important when it comes to the cost of defending lawsuits. Even winning a lawsuit can be expensive. Every pharmacist should take steps to protect their own career and reputation.
© Don R. McGuire Jr., R.Ph., J.D., is General Counsel, Senior Vice President, Risk Management & Compliance at Pharmacists Mutual Insurance Company.
This article discusses general principles of law and risk management. It is not intended as legal advice. Pharmacists should consult their own attorneys and insurance companies for specific advice. Pharmacists should be familiar with policies and procedures of their employers and insurance companies, and act accordingly.
23 azpharmacy.org
University & Alumni News
Rick G. Schnellmann, PhD Dean, University of Arizona College of Pharmacy

UArizona R. Ken Coit College of Pharmacy Ranked No. 5 by American Association of Colleges of Pharmacy

More than $20 million in funding from the National Institutes of Health bolsters R. Ken Coit College of Pharmacy’s rise to No. 5. TUCSON, Arizona — More than $20 million in National Institutes of Health (NIH) grants and contracts in fiscal year 2021 propelled the University of Arizona R. Ken Coit College of Pharmacy to No. 5 in American Association of Colleges of Pharmacy (AACP) rankings.
A transformational $50 million gift from alumnus R. Ken Coit in November 2021 bolstered the college’s recent rise through the creation of six endowed chairs and four professorships. The infusion of funding has helped invigorate research endeavors of faculty throughout the college. “It’s indicative of an energized, collaborative and growing research enterprise,” said Nathan Cherrington, PhD, ATS, Associate Dean for Research in the Coit College of Pharmacy. “The hard work of our faculty is bringing to light cutting-edge approaches to health care challenges and positioning us as a leader among our peers.”
The ranking is based on information compiled from the NIH Research Portfolio Online Reporting Tool. It includes all funded research grants awarded to principal investigators in U.S. colleges and schools of pharmacy. A recent example of this success is Haining Zhu, PhD, Professor of Pharmacology and Toxicology and holder of the R. Ken and Donna Coit Endowed Chair in Aging and Neurodegenerative Diseases. His NIH-funded work focuses on understanding the molecular mechanisms for neurodegenerative diseases, including amyotrophic lateral sclerosis and frontotemporal dementia, and other diseases such as cancer.
His goal is to understand how stress response pathways contribute to diseases at a molecular level. For example, nerve cells are under stress during aging and the failure to respond properly can lead to their dysfunction and death, which ultimately results in neurodegenerative diseases. His research is to learn how to fine tune stress response pathways in nerve cells to maintain their healthy status.
24 FALL 2022 editorial news
continued on next page
University & Alumni News

Dr. Zhu said the NIH funds serve as a critical source of financial support and as a means for recruiting and retaining top talent for his lab.
The impact of NIH funding is far-reaching as students have the unique opportunity to interact with Dr. Zhu and other worldrenowned researchers.
It’s the case with Rukayat Aromokeye, a second-year graduate student in the department of pharmacology and toxicology and researcher in Dr. Zhu’s lab. She received a Young Investigator Award at the 2022 International Research Conference on Neurodegenerative Diseases in Omaha.
“It is a privilege to be a part of a wonderful institution with the best mentors I could ever wish for like Dr. Haining Zhu, Dr. Gregory Thatcher, Dr. Hongmin Li, and Martha Ackerman-Berrier,” Aromokeye said.
continued from page 24
Following graduation, Aromokeye said she hopes to contribute to more research in drug discovery and pharmacology.
“Our rise is demonstrative of our commitment to advancing the pharmaceutical sciences and ensuring current and future students gain hands-on experience from the best in our field,” said Dean Rick G. Schnellmann, PhD
The AACP represents pharmacy education in the United States and partners with the 142 schools of pharmacy across the nation to ensure quality education and training.

25 azpharmacy.org
Creighton University School of Pharmacy
 Amy Wilson, PharmD Dean, Creighton University School of Pharmacy
Amy Wilson, PharmD Dean, Creighton University School of Pharmacy
Friedman Wilson named dean of School of Pharmacy and Health Professions
Amy Friedman Wilson, PharmD, has been named the new dean of the Creighton University School of Pharmacy and Health Professions, effective immediately, the University announced on July 14, 2022. Wilson has been serving as interim dean since January 1, 2022.
"I am so pleased to announce Dr. Wilson's appointment as dean," said University Provost Mardell Wilson, EdD. The appointment followed a national search. "Amy is a wonderful leader with a deep connection to Creighton, as both a graduate of our School of Pharmacy and Health Professions and a faculty member and administrator in the school for the last 22 years. I am excited to work with her to move the school forward, particularly as we continue to expand in Phoenix."
Creighton University is welcoming its first Doctor of Physical Therapy class to its Phoenix campus this fall, joining programs in pharmacy and occupational therapy that began in Phoenix in the fall of 2021. "Dr. Wilson has been instrumental in the development and operations of the Phoenix Health Sciences Campus, participating in the leadership and planning of the site
since 2018," said Provost Wilson. "As the School of Pharmacy and Health Professions' administrative representative for Phoenix, she has had operational responsibility for our three programs located at the new state-of-the-art facility."

Wilson has served as interim dean of Creighton's School of Pharmacy and Health Professions since January, while also serving as senior associate dean for operations and associate dean for the School of Pharmacy and Health Professions Phoenix Campus.
Wilson earned her Doctor of Pharmacy from Creighton in 1995. She completed a clinical pharmacy residency at the University of Iowa Hospitals and Clinics and worked at CHI Health St. Elizabeth Regional Medical Center in Lincoln, Nebraska, and Mutual of Omaha Healthcare

26 FALL 2022
continued on next page
University & Alumni News
Management before returning to Creighton as a faculty member in 2000. She served for 10 years as director of Creighton's Center for Drug Information and Evidence-Based Practice, beginning in 2005, providing leadership and guidance on health care policy and formulary development for a number of institutions, as well as oversight of the medication information consultation service.
Wilson completed the American Association of Colleges of Pharmacy Academic Leadership Fellows Program in 2010 and, in 2015, was appointed assistant dean, and later associate dean, for Academic Affairs for Creighton's School of Pharmacy and Health Professions. She has served as interim dean twice, including a term from 2018 to 2019.
continued from page 26
Her professional affiliations include serving on the board of the Nebraska Pharmacists Association and as a site reviewer for the Accreditation Council for Pharmacy Education. She is also a member of the Rho Chi Pharmacy Academic Honor Society, Phi Lambda Sigma Pharmacy Leadership Society, American Association of Colleges of Pharmacy, Association of Schools of Allied Health Professions, American Pharmacists Association and American Society of Health Systems Pharmacists. Wilson was awarded the Mary Lucretia and Sarah Emily Creighton Award in 2014 for her support of the development and accomplishments of women at the University.

27 azpharmacy.org
Midwestern University College of Pharmacy
 Mitchell R. Emerson, PhD Dean, Midwestern University College of Pharmacy
Mitchell R. Emerson, PhD Dean, Midwestern University College of Pharmacy

• Medicinal Chemistry – Dr. Diana Morcos
• Pharmaceutics – Dr. Kristopher Carson
• Pharmacology – Dr. Jessica Leibold
• Pharmacy Administration – Dr. Tristian Diep
• Professional Skills Development –Dr. Danielle Savage
Greetings from the College of Pharmacy (CPG) at Midwestern University. We have a lot to celebrate as we wrap up a busy Summer and look forward to Fall!
The Class of 2022 celebrated an Awards Ceremony on May 13th to recognize outstanding achievement of the class. Additionally, they celebrated the Teachers of the Year, Mentor of the Year, and Preceptors of the Year. The class culminated their time at CPG with a wonderful graduation ceremony and reception on June 2nd.
Congratulations to the class of 2022 award recipients.
• Robert C. Johnson Leadership –Dr. Sinmileoluwa Okegbile

• US Public Health Service Award –Dr. Russell McCauley
• Viatris Excellence in Pharmacy Award –Dr. Olivia Benyamin
• Merck Manual Award for Academic Excellence – Dr. Georgeanne Tolmachoff, Dr. Christina Tamou, Dr. Andrea Callas, Dr Matthew McLeod
• Wolters Kluwer Award for Excellence in Clinical Communication – Dr. Sydney Lambert
• CPG Excellence Awards
• Pharmacy – Dr. Katelynd Routledge
• Patient Communication – Dr. Daisy Ma
• Therapeutics Award – Dr. Brian Clarke
• Service – Dr. Josiah Harrison
• Research Collaboration – Dr. Salfee Bhathal & Dr. Eldo Joseph
• Evidenced-Based Healthcare –Dr. Omar Iniguez Morales
Dr. Titilola Afolabi was recognized as the 2022 Mentor of the Year. Additionally, we recognized Dr. Kellie Goodlet as the Teacher of the Year for the Class of 2022 during their PS-2/3 year and Dr. Mary Gurney for the Class of 2023 during their PS-1 year.
We wanted to repeat the recognition of our fantastic preceptors as they too were highlighted at the Awards Ceremony. Congratulations to the Preceptor of the Year, Dr. Kevin Carrasco, the Rookie Preceptor of the Year, Dr. Sean McHale, and the Faculty Preceptor of the Year, Dr. Kelsey Buckley.
The CPG Alumni Council has been busy as well. The Council welcomed two new members: Dr. Kaveh Oloumi, CPG ’21 and Dr. Cassandra Anderson, CPG ’14. Welcome to our newest members!
We’re looking forward to bringing all our pharmacy alumni together for the ASHP 2022 Conference in Las Vegas this December. Look for more details in the coming month and plan to join us if your schedule allows.
The Fall quarter is beginning as we send the Class of 2023 off to APPEs and welcome back the Class of 2024 from IPPEs just in time to mentor the Class of 2025 as they begin their second quarter at CPG. The Fall will be busy, and we look forward to the cooler days ahead.

28 FALL 2022
Be Audit Ready! Clinical Notes
and Annotations: What Documentation is Essential for Audit?
By Trenton Thiede, PharmD, MBA, President at PAAS National®, expert third party audit assistance and FWA/HIPAA compliance.
Many pharmacies receive hundreds of prescriptions every day, and inevitably, some of those prescriptions will contain errors, omissions, or just be confusing enough to require clarification with the prescriber’s office. When these situations occur, make sure you have good documentation of the conversation with the prescriber’s office to successfully pass an audit that may occur years later.
Documentation that should be included in any clinical note are as follows:
• Date (and preferably time)
• Name and title of who you spoke with (some PBMs do not accept “per MD” or “per nurse”)
• The details of the clarification/conversation
• Your name or initials
This documentation should either be noted directly on the prescription or within your dispensing software. For clinical notes on controlled substance prescriptions received electronically, the clinical note must be made and retained electronically per 21 CFR 1311.200(f)1. Any notations made must be visible to the auditor at the time of audit.
Clarifications to a prescription should be made prior to dispensing. PAAS National® has seen clinical notes invalidated when the pharmacy prints an image of a prescription for audit that shows the printed date, then proceeds to handwrite a clinical note on the prescription with a date prior to the printed date of that prescription. The auditor assumes the pharmacy “made up” the clinical note after the audit notice was issued and will expect the pharmacy to prove the clinical note was valid on appeal.
PAAS Tips:
• Remember to always document the When, Who You Spoke With, What Was Discussed, and Who Documented elements of a clinical note
• A custom ink stamp containing these elements may be made to help ensure pharmacy staff documentation is complete
• Documentation should be included on the original prescription or electronically
• Documentation should be visible to the auditor at the time of audit
• Any notations made on previous prescriptions that are still valid should be carried forward to the new prescription
• Submission clarification codes and Drug Utilization Reviews must be clearly documented with details to support the override given
• Professional service code “M0” requires consultation with the prescriber and documentation to support the conversation
• If the patient directions are clarified, ensure the patient label is updated prior to dispensing to reflect the new directions
• Verbal clarifications do not change the origin code of the prescription
• PAAS Audit Assistance2 members can view the following articles on the Member Portal for additional information:

• March 2021 Newsline article, Are You Documenting DUR and Submission Clarification Codes?3
• October 2021 Newsline article, Prescriber Statements Requested to Validate Incomplete Clinical Notes4
REFERENCES
1. https://www.ecfr.gov/current/title-21/chapter-II/part1311/subpart-C/section-1311.200
2. https://paasnational.com/audit-assistance/ 3. https://portal.paasnational.com/Paas/Newsletter/Go/825 4. https://portal.paasnational.com/Paas/Newsletter/Go/918
PAAS National® is committed to serving community pharmacies and helping keep hard-earned money where it belongs. Contact us today at (608) 873-1342 or info@paasnational.com to see why membership might be right for you.
©2022 PAAS National® LLC All Rights Reserved
29 azpharmacy.org
editorial
NCPDP Updates DAW Code Definitions to Encompass Interchangeable Biosimilars
By Trenton Thiede, PharmD, MBA, President at PAAS National®, expert third party audit assistance and FWA/HIPAA compliance.
In the August 2022 version of the National Council for Prescription Drug Programs (NCPDP) Telecommunication Version D and Above Questions, Answers and Editorial Updates1, there is new guidance in section 3.1.3 regarding interchangeable biosimilar products and Dispense As Written (DAW)/Product Selection Code (408-D8) field in anticipation of the DAW code definition updates which should go into effect October 15, 2023. With more interchangeable biosimilar products set to hit the market later this year, it is important to understand how to appropriately use these DAW codes to prevent future audit issues.
Prescribed Drug
Section 3.1.3 of the NCPDP document starts by outlining the FDA definitions of a biosimilar product, interchangeable product, and reference product. It goes on to state that when a prescriber indicates DAW 1 on a prescription, substitution of the product as written is not appropriate and when the prescriber does not indicate DAW 1 on a script, DAW 0 or DAW 2-9 could be appropriate. The chart below was included in the NCPDP document to aid pharmacies in determining appropriate DAW codes to bill.
Substitution Allowed Substitution Not Allowed Dispensed/Billed Drug DAW Code (408-D8)
Reference Product Allowed
Interchangeable Biosimilar 0
Reference Product Not Allowed Reference Product 1
Reference Product Allowed Reference Product 2-9
Interchangeable Biosimilar Allowed Interchangeable Biosimilar 0
Interchangeable Biosimilar Not Allowed
Interchangeable Biosimilar Allowed
While this chart is useful, you may be asking yourself – What does this all mean?! Let us go through several examples.
Example #1 – The pharmacy receives a script for Lantus Solostar® (DAW 1) from the prescriber. Since the script was flagged “Dispense as written” by the prescriber, the pharmacy must dispense the prescribed reference product, Lantus Solostar® and should utilize DAW 1 in field 408-D8 on the claim.
Example #2 – The pharmacy received a script for Lantus Solostar® (DAW 0) from the prescriber.
Interchangeable Biosimilar 1
Reference Product 2-9
Since Lantus Solostar® has an authorized interchangeable biologic (Semglee®), and both Lantus Solostar® and Semglee® have interchangeable unbranded biologics there are some additional considerations that must be made before determining the proper item to bill: (1) State regulations regarding the substitution of interchangeable biologic agents. Many states require the least expensive biologic agent to be dispensed. To find a link to your applicable state regulation, you may reference the Cardinal Health website.2 This may mean looking at your inventory to see what you have in stock and
30 FALL 2022
editorial
continued on next page
trying to bill the least expensive product first, then following the PBM claim messaging if the claim rejects.
a. If the interchangeable biosimilar is dispensed instead of the reference product, DAW 0 is appropriate.
b. If the reference product is dispensed, due to a formulary preference, DAW 9 would be appropriate.
(2) Patient Preference. Though the prescriber allows for substitution, the patient may prefer to stay with the reference product prescribed, in this case that would be Lantus Solostar®. DAW 2 would be appropriate for this claim.
Example #3 - The pharmacy received a script for Semglee®, the interchangeable biosimilar for Lantus Solostar®, (DAW 1) from the prescriber.
Since the script was flagged “Dispense as Written” by the prescriber, the pharmacy must bill Semglee® as prescribed and a DAW 1 would be appropriate on this claim.
Example #4 - The pharmacy received a script for Semglee®, the interchangeable biosimilar for Lantus Solostar®, (DAW 0) from the prescriber.
If the claim is billed for Semglee®, DAW 0 would be appropriate. If instead the patient wishes to be on (or stay on) Lantus Solostar®, then DAW 2 should be used on the claim for Lantus Solostar®
If Semglee® is not available from your supplier and Lantus Solostar® is available, DAW 8 could be utilized when billing the claim for Lantus Solostar® to signify that the interchangeable biosimilar was unavailable from your wholesaler—for this scenario, be sure to keep record to prove Semglee® was unavailable in the event the claim is audited. This could be a copy of an invoice showing Semglee® was out of stock, or a screen shot of your wholesaler’s website showing it was unavailable.
Example #5 - The pharmacy received a script for insulin glargine 100 units/mL (DAW 0) from the prescriber.
Insulin glargine encompasses several different insulin products, include reference products Lantus Solostar® and its unbranded equivalent, Semglee® and its unbranded equivalent, Basaglar®, and RezvoglarTM. Pharmacy staff should consider clinical context, patient history, formulary preference, and cost of the medication when determining the correct product to bill and
may need to contact the prescriber’s office for additional clarification. If Lantus Solostar® (the reference product) is billed due to formulary preference, a DAW 9 would be appropriate and if Lantus Solostar® was billed due to the patient requesting brand, DAW 2 would be appropriate. If an interchangeable biosimilar was billed, DAW 0 would be appropriate.
PAAS Tips:
• PAAS Audit Assistance members3 can view the PAAS’ on-demand webinar: Understanding Interchangeability with Prescription Biologics4 (recorded 8/17/22)
• Remember to have visible documentation for all claims billed with a DAW code other than zero to support the use of the medication dispensed and DAW code utilized on the claim
• Refer to the “interchangeable” column on the Insulin Medication Chart5 and the Biologic Injectable Medication Chart5 located on the PAAS Portal for Audit Assistance members, to find FDA-approved interchangeable biologic agents
• Utilize the FDA Purple Book6 – a searchable, online database of biologic products (remember to look for matching card colors which signify interchangeability)
REFERENCES
1. https://www.ncpdp.org/NCPDP/media/pdf/VersionDQuestions.pdf
2. https://www.cardinalhealth.com/en/product-solutions/ pharmaceutical-products/biosimilars/state-regulationsfor-biosimilar.html
3. https://paasnational.com/audit-assistance/
4. https://portal.paasnational.com/paas/resource/ video?id=740511064&desc=Understanding%20 Interchangeability%20with%20Prescription%20Biologics
5. https://portal.paasnational.com/Paas/Resource/Charts
6. https://www.fda.gov/drugs/types-applications/ therapeutic-biologics-applications-bla
PAAS National® is committed to serving community pharmacies and helping keep hard-earned money where it belongs. Contact us today at (608) 873-1342 or info@paasnational.com to see why membership might be right for you.
©2022 PAAS National® LLC All Rights Reserved

31 azpharmacy.org

32 FALL 2022 trhc.com Clinical Pharmacists Impacting Patient Outcomes with MedWise® Science and MedWise Risk Score™ Backed by Science, Proven by Research: editorial Backed by Science, Proven by Research: Clinical Pharmacists Impacting Patient Outcomes with MedWise® Science and MedWise Risk Score™
Healthcare costs are rising in the U.S. According to the Centers for Medicare & Medicaid Services (CMS), National Health Expenditure increased 4.6% in 2019, reaching $3.8 trillion, with Medicare spending climbing 6.7% to $799.4 billion.1 Meanwhile, U.S. Census Bureau estimates show a growing 65-and-older age group, which increased 34.2% between 2010 and 2019.2 Among this group, the Lown Institute reports that 42% take at least five prescription medications.3
Rising healthcare costs and a growing elderly population could have significant implications when it comes to adverse drug events (ADEs). ADEs occur when an individual is harmed by medication, even when that medication is used appropriately. They are responsible for around 1.3 million emergency department visits a year, and roughly 350,000 individuals require hospitalization for additional treatment.4
Tabula Rasa HealthCare’s (TRHC) MedWise Risk Score™ is a novel approach to help clinical pharmacists target patients at risk for ADEs. Used together with TRHC’s clinical decision support technology, MedWise® Science, pharmacists can identify those patients with high risk scores and target them for risk-mitigating interventions. TRHC has found these pharmacistled interventions to result in better patient outcomes, including: reduced falls, hospital admissions, emergency department visits, medical expenditures, and deaths. Pharmacists and other healthcare providers working with TRHC clinical pharmacists can use these technologies to effectively pinpoint risk, identify medicationrelated problems, and make recommendations that advance patient outcomes and ultimately save lives. The impact of clinical pharmacist-driven interventions, under the scope of medication safety reviews, using these technologies is further illustrated in a new peer-reviewed research series in The American Journal of Managed Care.
Understanding MedWise and the MedWise Risk Score
TRHC’s MedWise Risk Score helps pharmacists determine which patients are at risk for ADEs5 by analyzing medication-related risk factors through proprietary algorithms.6 Unlike traditional medication therapy management offerings, which evaluate a number of chronic diseases, number of medications, and Medicare Part D costs, the MedWise Risk Score evaluates pharmacokinetic
and pharmacodynamic characteristics of active medication ingredients.7 This evaluation incorporates over-the-counter medications, herbals, and supplements to illuminate simultaneous, multi-drug interactions.5 Based on the data, patients are assigned a risk score ranging from 0 to 50, where 0–9 indicates minimal risk, 10–14 indicates low risk, 15–19 indicates intermediate risk, 20–29 indicates high risk, and 30 and above indicates severe risk.6
In the CMS Innovation Center’s Enhanced Medication Therapy Management (EMTM) model, TRHC clinical pharmacists used the MedWise Risk Score with MedWise Science, a clinical decision support technology, to optimize patient outcomes.7 The MedWise Matrix creates a visualization of a patient’s complete medication regimen and enables pharmacists to identify and address medicationrelated problems that can lead to ADEs.
Identifying Patients with Higher Risk for Negative Outcomes
Research has proven that MedWise Risk Scores can help pharmacists successfully identify patients whose medications—when used together— put them at greater risk for negative outcomes including ADEs, falls, higher medical expenditure, emergency department visits, hospitalizations, and death. A study by Michaud et al. included over 200,000 participants who were prescribed medication in 2018.6 A MedWise Risk Score was calculated for the year 2018 and, according to the data, there was a greater percentage of intermediate, high, and severe max scores among participants who had at least one ADE. Specifically, 18.6% of participants with one or more ADE had an intermediate score, 26.5% had a high score, and 9.9% had a severe score.
The results also showed a strong link between the MedWise Risk Score and falls. Compared to participants with a minimal max score, participants with a severe max score were most likely to experience a fall, followed by participants with a high max score, then an intermediate max score. Data trended similarly for medical expenditures Fifty percent of participants with a minimal max score had a mean yearly medical expenditure of $1,297. This cost escalated to $2,964 for participants with a low score; $4,532 for participants with an intermediate score; $6,514 for participants with a high score; and $10,352 for participants with a severe score (see Figure 1).
33 azpharmacy.org
Executive Summary
Annual medical spend
MedWise Risk Score
Figure 1: Average Annual Medical Expenditures by Risk Level
In addition, the data revealed associations between the MedWise Risk Score and emergency department visits, hospitalizations, and deaths. For example, the data showed a greater number of participants with high and severe max scores among those who died compared to those who remained living. These results confirm the MedWise Risk Score’s ability to identify which patients have the greatest risk for negative outcomes.
Pinning Down Medication-related Problems
By identifying patients with the greatest risk, a MedWise Risk Score helps to prioritize individuals for MedWise Safety Reviews. These reviews enable pharmacists to leverage the clinical decision support tools to detect medication-related problems and make recommendations to prevent dangerous drug interactions.8
Pharmacists used MedWise Risk Scores and MedWise Science to effectively lower patient risk for multi-drug interactions in a study by Bankes et al.7 During the study, analyzing MedWise Risk Scores helped pharmacists prioritize 22,696 highrisk participants for their first-ever review. Guided by MedWise Science, pharmacists detected 36,455 clinically actionable medication-related problems, roughly 85% of which were adverse drug reaction,
drug interaction, and unnecessary medication use. MedWise Science also informed pharmacist recommendations to address the medicationrelated problems, with start alternative therapy, discontinue medication, change time of medication administration, and change dose topping the list. According to the study’s results, pharmacist recommendations could reduce the average MedWise Risk Score by 4.7 points.
Improving Patient Outcomes
Insight from MedWise Safety Reviews can optimize prescribing practices and impact patient outcomes. This impact was proven by Stein et al., who studied the impacts of MedWise Safety Reviews on medical expenditures, hospitalizations, emergency department visits, and mortality.9 The research included two groups totaling 11,436 participants: 4,384 individuals who received their first-ever review in 2018 and at least one review in 2019, and 7,052 individuals who did not receive a review. According to the data, participants who received MedWise Safety Reviews benefitted from less total medical expenditure as well as fewer hospitalizations and emergency department visits, comparatively.
While mean total medical costs (Medicare Part A and Part B) increased for both groups from 2018 to 2019, participants with MedWise Safety
34 FALL 2022
Minimal $0 $2,000 $4,000 $6,000 $8,000 Low Intermediate High Severe $10,000 $12,000 $1,297 $2,964 $4,532 $6,514 $10,352
Figure 2: Patients who completed a MedWise Safety Review
improved outcomes from 2018 to 2019
Putting Results in Perspective
With over 6 billion prescriptions dispensed in 2020, the safe use of medication is critical.10 Advanced tools like MedWise Science and the MedWise Risk Score are important to shed unparalleled light on medication regimens and prescribing practices. Research has proven the MedWise Risk Score effective in identifying patients whose medication regimens put them at greater risk for negative outcomes. For high-risk patients, pharmacists can pinpoint
Reviews saved in comparison. In fact, the findings revealed an 8.5% increase for those who received MedWise Safety Reviews versus a 16% increase for those who did not, resulting in 7.5% cost savings for those who completed reviews. Similarly, emergency department visits increased in both groups, but this increase was less among participants with MedWise Safety Reviews. According to the data, the change from 2018 to 2019 was 2.3% for those who received MedWise Safety Reviews and 12.6% for those who did not, marking a 10.3% decrease for the intervention group (see Figure 2).
Meanwhile, the hospitalization rate decreased among participants who had MedWise Safety Reviews, but increased among participants who did not. Hospitalizations climbed 6.6% for those who did not complete reviews but dropped 3.4% for those who did, resulting in an overall 10% reduction for those who completed reviews (see Figure 2).
reduce risk through pharmacist-led consultations, which leverage MedWise Science.
medication-related problems and recommend measures that reduce risk through pharmacist-led consultations, which leverage MedWise Science.
As research demonstrates, this strategy ultimately leads to improving the safety of medication use and better patient outcomes. Given overarching trends surrounding healthcare costs, the elderly, and ADEs, wider use of advanced clinical decision support technology, such as MedWise Science, can help clinicians significantly improve the healthcare landscape.
As research demonstrates, this strategy ultimately leads to improving the safety of medication use and better patient outcomes. Given overarching trends surrounding healthcare costs, the elderly, and ADEs, wider use of advanced clinical decision support technology, such as MedWise Science, can help clinicians significantly improve the healthcare landscape.
REFERENCES
1. NHE fact Sheet. CMS.gov. https://www.cms.gov/ResearchStatistics-Data-and-Systems/Statistics-Trends-andReports/NationalHealthExpendData/NHE-Fact-Sheet Published December 16, 2020. Accessed August 19, 2021.
CONTACT
info@trhc.com 1-866-648-2767
2. 65 and older population grows rapidly as baby boomers age. United States® Census Bureau. https://www. census.gov/newsroom/press-releases/2020/65older-population-grows.html. Published June 25, 2020. Accessed August 19, 2021.
trhc.com
References
The data also demonstrated impact in terms of mortality. While 6.8% of participants who had their first-ever review in 2018 died by the end of 2019, 8.9% of participants who did not have a review died by the end of 2019. This marks a 2.1% difference between the groups (see Figure 2).
1 NHE fact Sheet. CMS.gov. https://www.cms.gov/ResearchStatistics-Data-and-Systems/Statistics-Trends-and-Reports/ NationalHealthExpendData/NHE-Fact-Sheet. Published December 16, 2020. Accessed August 19, 2021.
2 65 and older population grows rapidly as baby boomers age. United States® Census Bureau. https://www.census.gov/newsroom/pressreleases/2020/65-older-population-grows.html. Published June 25, 2020. Accessed August 19, 2021.
Putting Results in Perspective
3 Medication overload and older Americans. Lown Institute. https:// lowninstitute.org/projects/medication-overload-how-the-drive-toprescribe-is-harming-older-americans. Accessed August 19, 2021.
3. Medication overload and older Americans. Lown Institute. https://lowninstitute.org/projects/medication-overloadhow-the-drive-to-prescribe-is-harming-older-americans Accessed August 19, 2021.
4. Adverse drug events in adults. Centers for Disease Control and Prevention. https://www.cdc.gov/medicationsafety/ adult_adversedrugevents.html. Published October 11, 2017. Accessed August 19, 2021.
6 Michaud V, Smith M, Bikmetov R, et al. Association of a MedWise Risk Score with health care outcomes. Am J Manag Care. 2021;27(suppl 16):S280-S291
5. MedWise Risk Score™. Tabula Rasa HealthCare. https:// www.tabularasahealthcare.com/insights/medwisescience-patient-outcomes/. Accessed August 19, 2021.
7 Bankes D, Pizzolato K, Finnel S, et al. Medication-related problems identified by pharmacists in an Enhanced Medication Therapy Management model. Am J Manag Care. 2021;27(suppl 16):S292-S2999.
6. Michaud V, Smith M, Bikmetov R, et al. Association of a MedWise Risk Score with health care outcomes. Am J Manag Care. 2021;27(suppl 16):S280-S291
8 Discover the next frontier of medication safety. Tabula Rasa HealthCare. https://www.tabularasahealthcare.com/medwise-msr. Accessed August 20, 2021.
4 Adverse drug events in adults. Centers for Disease Control and Prevention. https://www.cdc.gov/medicationsafety/adult_ adversedrugevents.html. Published October 11, 2017. Accessed August 19, 2021.
5 MedWise Risk Score™. Tabula Rasa HealthCare. https://www. tabularasahealthcare.com/our-technology/medication-risk-score. Accessed August 19, 2021.
With over 6 billion prescriptions dispensed in 2020, the safe use of medication is critical.10 Advanced tools like MedWise Science and the MedWise Risk Score are important to shed unparalleled light on medication regimens and prescribing practices. Research has proven the MedWise Risk Score effective in identifying patients whose medication regimens put them at greater risk for negative outcomes. For high-risk patients, pharmacists can pinpoint medicationrelated problems and recommend measures that
9 Stein A, Finnel S, Bankes D, et al. Health outcomes from an innovative Enhanced Medication Therapy Management model. Am J Manag Care. 2021;27(suppl 16):S300-S308.
7. Bankes D, Pizzolato K, Finnel S, et al. Medication-related problems identified by pharmacists in an Enhanced Medication Therapy Management model. Am J Manag Care. 2021;27(suppl 16):S292-S2999.
10 The use of medicines in the U.S. IQVIA. https://www.iqvia.com/ insights/the-iqvia-institute/reports/the-use-of-medicines-in-the-us. Published May 27, 2021. Accessed August 19, 2021.
8. Discover the next frontier of medication safety. Tabula Rasa HealthCare. https://www.tabularasahealthcare. com/medwise-msr. Accessed August 20, 2021.
9. Stein A, Finnel S, Bankes D, et al. Health outcomes from an innovative Enhanced Medication Therapy Management model. Am J Manag Care. 2021;27(suppl 16):S300-S308.
10. The use of medicines in the U.S. IQVIA. https://www.iqvia. com/insights/the-iqvia-institute/reports/the-use-ofmedicines-in-the-us. Published May 27, 2021. Accessed August 19, 2021.
35 azpharmacy.org
saw
7.5% healthcare cost savings
10% reduction in hospital admissions
10.3% decrease in ED visits
2.1% lower mortality rate
Figure
2:
Patients who completed a MedWise Safety Review saw improved outcomes from 2018 to 2019
Arizona and COVID-19: Fall 2022
Dr. Howard J. Eng, Associate Professor in Public Health (retired)
Arizona’s COVID-19 summer case surge is over. It is expected the case numbers in early fall will be low, while the numbers in late fall will be higher. On September 22, the CDC reports Arizona and Pima County are at low-risk transmission levels (see Figures 1 and 2). Pima County has had lowrisk transmission levels since August 11.
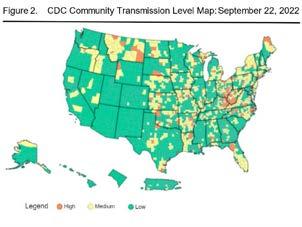
The Arizona and Pima County weekly case numbers are declining as shown on Figures 3 and 4. The United States 7-day positivity rate is 10.81% (CDC – September 21). On September 11, the state and county positivity rates were 10% and 11%, respectively (AzDHS).
On September 21, Johns Hopkins reports 613,459,898 total cases and 6,531,491 deaths associated with COVID-19 in the world. There has been an increase of 3,109,817 cases during the past week (September 15 to 21). The U.S. has 95,874,591 cases and 1,055,195 deaths. In the previous week, there has been an increase of 384,096 cases.
Tables 1 and 2 show Arizona and Pima County four-week COVID case, hospitalization, and death numbers. The state and county cases have been fluctuating. The numbers of deaths remain low.

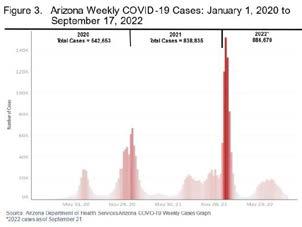
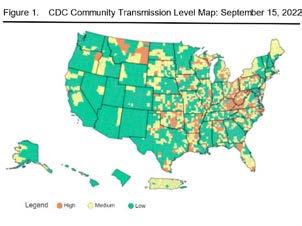
36 FALL 2022
continued on next page editorial
Table 1. Arizona Total and Weekly Numbers of COVID-19 Cases, Hospitalizations, and Deaths
Date
08-31-2022
2,254,374 8,661 116,462 555 31,114 67 09-07-2022 2,258,040 3,666 116,954 492 31,162 48 09-14-2022 2,264,159 6,119 117,341 387 31,244 82 09-21-2022 2,268,158 3,999 117,625 284 31,326 82
Source: Arizona Department of Health Services COVID-19 Dashboard. Arizona 2021 population est. is 7,285,370, July 1, 2021 – Arizona OEO.
Table 2. Pima County Total and Weekly Numbers of COVID-19 Cases, Hospitalizations, and Deaths
Date
Total Cases Weekly Case Total Hospital Weekly Hospital Total Deaths Weekly Deaths
08-31-2022 288,089 1,588 12,965 64 3,967 10 09-07-2022 288,749 660 13,039 74 3,972 5 09-14-2022 289,898 1,149 13,087 48 3,983 11 09-21-2022 290,693 795 13,129 42 3,996 13
Source: Arizona Department of Health Services COVID-19 Dashboard. Pima County 2021 population est. is 1,058,318, July 1, 2021 – Arizona OEO.
On September 21, the CDC reports 224,980,931 (67.8% of population) who completed their primary series (previously – fully vaccinated). 92.3% of the senior (65>) population (50,576,510) who completed their primary series, 35,864,236 (70.9%) received their first booster, and 15,335,531 (42.8%) received their second booster. There are 4,536,654 (62.3%) in Arizona and 717,778 (67.8%) in Pima County who are fully vaccinated (AzDHS – September 21).
New vaccine options are available. Those who are unvaccinated and reluctant to get the messenger RNA vaccines (Pfizer/BioNTech Comirnaty and Moderna Spikevax), Novavax (Nuvaxivud) offers another vaccine option that uses inactive virus to stimulate antibody production. On August 31, the Food and Drug Administration approved both Pfizer and Moderna new bivalent COVID-19 vaccines that are effective against both the original virus and Omicron BA.4 and BA.5 variants. If your vaccine protection is waning you needed to get the new vaccine to extend your immunity protection. Those high risk in getting a severe COVID case need to get their flu shot.
Many still have anxiety/depression/stress associated with the virus. The causes for the mental anguish are the uncertainty of the virus, constant emergent of new variants, vaccine
limitations, the lack control of the situation, and no end to the virus. We know most cases of the virus are mild or moderate. Few cases are severe (require hospitalization and/or result in death). Vaccines reduce the risks of getting a severe case, and therapeutics are used to treat these cases.
There are several things you can do to reduce your chances of getting a severe case of the virus. Staying healthy will keep your immunity level high and will reduce the risk of getting a severe case. You can reduce your exposure to the COVID-19 by maintaining social distancing and avoid touching your nose, mouth, and eyes with uncleaned hands. Potentially contaminated surfaces or objects need to be cleaned and disinfected. During case surges, you need to wear a N95 or multi-layer face mask in high COVID-19 risk situations (e.g., indoor low ventilated places with large crowds of people), make sure there is good ventilation and air circulation at home, change your home air filter(s) often, and stay home if you have the virus.
Even with the periodic case surges, the numbers of severe COVID-19 cases and deaths in Arizona and Pima County remain low because of the high level of population immunity, the availability of drugs in treating the virus, and most Omicron variant cases are mild or moderate. Be safe, and stay healthy, calm, and positive.
37 azpharmacy.org
Total Cases Weekly Case Total Hospital Weekly Hospital Total Deaths Weekly Deaths
AzPA state advocacy
AzPA Legislative Affairs Update

With your support, the Arizona Pharmacy Association was able to achieve success in many of our legislative priorities last session. As we prepare for the Fifty-Sixth Legislature, our ask is for YOU to get involved. There is strength in numbers, the more people involved, the louder and stronger our voice is at the capitol.
We still have work to do! As we witnessed last year with SB1016 we need to stay vigilant against those who seek to attack our ability to practice pharmacy. We need to forge ahead and continue to work on ensuring we can practice to the top of our degree and training while being fairly compensated for the products we dispense, and the patient care services we offer.
Legislative Affairs Committee
Submit a volunteer application to be part of the Legislative Affairs Committee. The focus of this committee is to propose, develop, evaluate, monitor, review, and interpret proposed and actual legislation, rules and regulations affecting the pharmacy profession in the state of Arizona, with special emphasis and activity during the Arizona State Legislative Session. The application is located in your AzPA member account, under the portal page titled: Volunteer Committee Application. This committee meets the first Wednesday of the month at 12:05PM.
Pharmacy Day at the Capitol (PD@C)
Join the AzPA at the Capitol to meet with state legislators to discuss current issues relating to pharmacy and health care. Your expertise is needed to highlight the advanced patient care services pharmacists provide throughout our community. PD@C provides a wonderful opportunity to network with other pharmacists, students, and technicians in an effort to help raise awareness about our profession. Learn more about PD@C at https://azpharmacy.org/events/ pdac/
Ways You Can Join the Efforts
Donate to PharmPAC
AzPA uses its political action funds carefully and wisely to provide support to legislators and candidates who are in favor of the pharmacist's role in delivering patient care. PharmPAC plays an important role in enhancing the advocacy efforts within the profession of pharmacy in Arizona. Our ability to interact with key legislators is vital and allows us to keep our issues visible and top of mind at the state capitol. The advancement and protection of the profession of pharmacy depends on all of us. Donate to PharmPAC by clicking here
Update Your Legislative District in Your Membership Profile
Help us with target campaigns by updating your Legislative District (LD) in your AzPA profile. Due to legislative redistricting, your district number (1-30) has changed. Unsure of your legislative district? Click here to find out! You can login to your member portal by clicking here.
Register to Vote
Every vote counts! If you are not a registered voter, register today to help support our profession. You can register online at https://servicearizona.com/ VoterRegistration.
38 FALL 2022
continued on next page
Decide which volunteer opportunity works best for you this upcoming legislative session and become involved.

























39 azpharmacy.org
Dianne McCallister AzPA Lobbyist
Kelly Fine AzPA CEO
Ken Bykowski AzPA Legislative Affairs Committee Co-Chair
Your Team COMING SOON 2023
Mark Boesen AzPA Legislative Affairs Committee Co-Chair
Arizona State Board of Pharmacy Update editorial advocacy

Board Members
Joseph Leyba, RPh, President
Lorri Walmsley RPh, Vice President
Cedar Lahann, PharmD, RPh, Member
Ted Tong, PharmD, RPh, Member
Kevin Dang, PharmD, RPh, Member
Kristen Snair, CPhT, Member
Tenille Davis, PharmD, NEW Member*
Frank Thorwald, NEW Member* (Public)
Randy Schoch, NEW Member* (Public)
The Board has Moved!
New Location: 1110 W Washington St Suite 260 Phoenix, AZ 85007
License Renewal Reminder!
If your license expires on October 31, 2022, it is time to renew your license. The last day to renew your license/permit without a renewal penalty is October 31, 2022
Continuing Education Requirements for 2022 Renewal:
PHARMACISTS: 30 total continuing education (“CE”) hours are required for renewal:
Description of CE Activity
Opioid-related, substance use disorderrelated, or addiction- related continuing education activities.
Immunizers: CE related to administering immunizations, vaccines, and emergency medications.
Pharmacists who prescribe and dispense tobacco cessation drug therapies: CE related to tobacco cessation.
Number of CE Hours
Three (3) of the thirty (30) CE hours required for renewal.
Two (2) of the thirty (30) CE hours required for renewal.
Applicable Statute/Rule
A.R.S. 32-3248.02
A.A.C. R4-23-204(A)(2)(a)
Two (2) of the thirty (30) CE hours required for renewal. A.R.S. 32-1979.03(B)
PHARMACY TECHNICIANS: 20 total CE hours are required for renewal:
Description of CE Activity
Opioid-related, substance use disorderrelated, or addiction- related continuing education activities
*Remote Dispensing Pharmacy Technicians: CE in remote dispensing site pharmacy practices.
Verification Technician: CE in patient safety.
Number of CE Hours
Applicable Statute/Rule
Three (3) of the twenty (20) CE hours required for renewal. A.R.S. 32-3248.02
Two (2) hours - these hours are in addition to the twenty (20) total CE hours required for renewal. A.R.S. 32-1923.01(C)(1)
Four (4) of the twenty (20) CE hours required for renewal. A.A.C. R4-23-1104.01(D)(4)
*Note: Remote Dispensing Pharmacy is also known as “Telepharmacy”. Refer to A.R.S. 32-1961.01. This does not apply to pharmacy technicians who work from home.
40 FALL 2022
Remote Dispensing Site Pharmacy aka Telepharmacy
For a pharmacy that is operated by a pharmacy technician and supervised remotely by a pharmacist, please note that all prescriptions for CS must be electronically prescribed. Paper prescriptions for CS cannot be filled at a remote dispensing site pharmacy. DEA is evaluating remote dispensing site pharmacies. Currently, DEA does not have a definition of telepharmacy; therefore, remote dispensing sites are being treated like online pharmacies. In its advanced notice of proposed rulemaking, DEA stated: “As telepharmacies utilize the internet to dispense controlled substances, they may constitute Online Pharmacies under the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 (Ryan Haight Act) and must therefore either: (1) Obtain a modified registration under 21 CFR 1301.19; or (2) meet one of the exceptions to an Online Pharmacy under 21 CFR 1300.04(h).”
Upcoming Board Meetings:
Date
December 7-8, 2022
November 29, 2022
REFERENCES
1. https://pharmacy.az.gov/
Meeting Type
Board Meeting
Working Remotely
Pursuant to Arizona Law Working remotely has been exercised prior to the coronavirus disease 2019 pandemic. During the health emergency, working remotely became a very reliable option to help take care of patients. This is a friendly reminder. Your employer, the permit holder, has authorized you to work from a designated location. It is the responsibility of a permit holder to ensure that the work location of the license holders is secure and void of distractions, Health Insurance Portability and Accountability Act breaches, etc. It is also the permit holder’s responsibility to monitor and ensure that employees are working from only their authorized locations. Working remotely has become a convenience for many to balance work and life. But it is considered unprofessional conduct to work from any area other than the assigned area stated in the policies and procedures of the permit holder. License holders should reach out to their employer to obtain the “work from home policy,” especially if questions exist.
Submission Deadline

November 4, 2022
Complaint Review
2. https://nabp.pharmacy/bop_members/arizona/
41 azpharmacy.org
ACCEPTING APPLICATIONS October 1 - December 1, 2022
Recipients selected will each be awarded $3,000. Up to $60,000 in scholarships may be awarded for this academic year.
Recipients selected will each be awarded $3,000. Up to $60,000 in scholarships may be awarded for this academic year.
To be eligible to apply for the Pharmacists Mutual Community Pharmacy Scholarship, students must meet the following criteria:
To be eligible to apply for the Pharmacists Mutual Community Pharmacy Scholarship, students must meet the following criteria:
• Current students must be a P3 or P4 pharmacy student in the 2023-2024 academic year
To be eligible to apply for the Pharmacists Mutual Community Pharmacy Scholarship, students must meet the following criteria:
• Eligible students must plan to practice in one of the following settings:
• Current students must be a P3 or P4 pharmacy student in the 2023-2024 academic year
• Current students must be a P3 or P4 pharmacy student in the 2023-2024 academic year




- an independent or small chain community pharmacy, or - an underserved geographic or cultural community, preferably in an independent or small chain community pharmacy
• Eligible students must plan to practice in one of the following settings: - an independent or small chain community pharmacy, or - an underserved geographic or cultural community, preferably in an independent or small chain community pharmacy
• Eligible students must plan to practice in one of the following settings: - an independent or small chain community pharmacy, or - an underserved geographic or cultural community, preferably in an independent or small chain community pharmacy
Pharmacists Mutual Insurance Company Algona, Iowa phmic.com
Pharmacists
Pharmacists
Iowa phmic.com
FOR ELIGIBILITY REQUIREMENTS AND TO APPLY: phmic.com/scholarship
Mutual Insurance Company Algona, Iowa phmic.com
FOR ELIGIBILITY REQUIREMENTS AND TO APPLY: phmic.com/scholarship
Mutual Insurance Company Algona,
FOR ELIGIBILITY REQUIREMENTS AND TO APPLY: phmic.com/scholarship
Apply Now!






































 Don R. McGuire Jr. R.Ph., J.D.
Don R. McGuire Jr. R.Ph., J.D.




 Amy Wilson, PharmD Dean, Creighton University School of Pharmacy
Amy Wilson, PharmD Dean, Creighton University School of Pharmacy



 Mitchell R. Emerson, PhD Dean, Midwestern University College of Pharmacy
Mitchell R. Emerson, PhD Dean, Midwestern University College of Pharmacy
































