




Acknowledgment: This program was created through an educational partnership. Handbook created by: MARY K GURNEY, RPh, PhD, BCPA and ROGER MORRIS, RPh, JD Laws and Regulations were obtained through the Arizona State Board of Pharmacy Law Book and the Arizona Secretary of State website.
©2024 AzPA All Rights Reserved
Note: To ensure our learners are reading the most current version of the law we have hyperlinked the relevant laws and regulations instead of copying the current text into the e-book. As laws/regulations change the hyperlinks will take you to most current version.

Title 32 - Professions and Occupations
• Chapter 18-Pharmacy
• Chapter 31-Regulation of Health Professions
• Chapter 32-Health Professionals
• Chapter 43-Licensure, Certification and Registration of Military Members
• Chapter 48-Licensing Authority
Sec: 32-1901-32-1997
Article 1 Board of Pharmacy
• 32-1901
• Definitions
• 32-1901.01
• 32-1902
• 32-1903
• 32-1904
• 32-1905
• 32-1906
• 32-1907
• 32-1908
• 32-1909
• Definition of unethical conduct and unprofessional conduct; permittees; licensees
• Arizona state board of pharmacy; immunity
• Organization; meetings; quorum; compensation of board; executive director; compensation; powers and duties
• Powers and duties of board; immunity
• Meetings; time and place; annual report
• Membership in national associations; official attendance at professional meetings
• Arizona state board of pharmacy fund
• Scope of chapter
• Donated medicine; donors; authorized recipients; requirements; immunity; definitions
• 32-1910
• 32-1921
• 32-1921.01
• 32-1922
• 32-1923
• 32-1923.01
• 32-1924
• 32-1925
• 32-1926
• 32-1926.01
• 32-1927
• Emergencies; continued provision of services
• Exempted acts; exemption from registration fees; definition
• Disclosures on applications; licensees; registrants; applicability
• Qualifications of applicant; reciprocity; preliminary equivalency examination; honorary certificate; fee
• Interns and intern preceptors; qualifications; licensure; purpose of internship
• Pharmacy technicians; pharmacy technician trainees; qualifications; remote dispensing site pharmacies
• Licenses; fees; rules; signatures; registration; online profiles
• Renewal of license of pharmacists, interns and pharmacy technicians; fees; expiration dates; penalty for failure to renew; continuing education
• Notice of change of information required
• Change in residency status; written notice required
• 32-1927.01
• 32-1927.02
• 32-1927.03
• Pharmacists; pharmacy interns; disciplinary action
• Pharmacy technicians; pharmacy technician trainees; disciplinary action
• Permittees; disciplinary action
• Persons required to be permitted; formal hearing; disciplinary action
• 32-1928
• 32-1929
• 32-1930
• 32-1931
• 32-1932.01
• 32-1933
• 32-1934
• 32-1935
• Hearings; restraining order; judicial review
• Biennial registration of pharmacies, wholesalers, thirdparty logistics providers, manufacturers and similar places; application
• Types of permits; restrictions on permits; discontinuance of pharmacy permit
• Permit fees; issuance; expiration; renewals; online profiles
• Substance abuse treatment and rehabilitation program; private contract; funding
• Display of license or permit
• Remote hospital-site pharmacy permittee; requirements
• Approval of schools and colleges of pharmacy
• 32-1936
• 32-1937
• 32-1939
• 32-1940
• 32-1941
Article 3 Regulation
• 32-1961
• 32-1961.01
• 32-1962
• 32-1963
• 32-1963.01
• Mandatory continuing professional pharmacy education
• Exceptions to continuing education requirements
• Condition of probation; repayment of inspection costs
• Investigations; hearings; conferences; records; confidentiality
• Third-party logistics providers; permit required; designated representative; fingerprinting requirements
• 32-1964
• Limit on dispensing, compounding and sale of drugs
• Remote dispensing site pharmacies
• New drug; compliance with federal act; exception
• Liability of manager, proprietor or pharmacist in charge of a pharmacy; variances in quality of drugs or devices prohibited
• Substitution for prescription drugs or biological products; requirements; label; definitions
• Record of prescription orders; inspections; confidentiality
• 32-1965
• 32-1966
• 32-1967
• 32-1968
• 32-1969
• 32-1970
• 32-1971
• 32-1972
• 32-1973
• 32-1974
• 32-1975
• Prohibited acts
• Acts constituting adulteration of a drug or device
• Acts constituting misbranding of a drug or device; exceptions; interpretation of misleading label; definition
• Dispensing prescription-only drug; prescription orders; refills; labels; misbranding; dispensing soft contact lenses; opioid antagonists
• Filling foreign prescription orders; records; exception
• Collaborative practice agreements; requirements; rules; definitions
• Compounding pharmacies; certain medications; requirements; definitions
• Poison or hazardous substances; misbranding and labeling; prohibitions; exemption
• Pharmacies; quality assurance
• Pharmacists; administration of immunizations, vaccines and emergency medications; authorization; reporting requirements; advisory committee; definition
• Legend drug products; listing; code identification; exemption; definitions
• 32-1976
• 32-1977
• 32-1978
• 32-1979
• 32-1979.01
• 32-1979.02
• 32-1979.03
• Dispensing replacement soft contact lenses; prescription
• Sale of methamphetamine precursors by a pharmacy permittee; electronic sales tracking system; violation; classification; state preemption
• Sale of dextromethorphan; age requirement; exception; violation; civil penalty; definitions
• Pharmacists; dispensing opioid antagonists; board protocols; immunity
• Self-administered hormonal contraceptives; requirements; rules; immunity; definition
• Oral fluoride varnish; prescription and administration authority; requirements
• Tobacco cessation drug therapies; prescription authority; requirements; definition
• 32-1981
• 32-1982
• 32-1983
• 32-1985
• Definitions
• Full-service wholesale permittees; bonds; designated representatives; fingerprinting requirements
• Restrictions on transactions
• Injunctive relief
Article 4 Enforcement of Chapter; Penalties
• 32-1991
• 32-1992
• Enforcement of chapter
• Provisions of marijuana, prescription-only drugs, narcotics, dangerous drugs or controlled substances laws not invalidated by this chapter; medicated feed not included
• 32-1993
• 32-1994
• 32-1995
• 32-1996
• 32-1997
• Authorization to seize certain drugs, counterfeit drugs and equipment; disposition of seized equipment
• Authorization to embargo adulterated or misbranded drugs or devices; condemnation; destruction; costs
• Injunctions; restraining orders
• Violations; classification; civil penalty
• Misbranding; promotion of off-label use; definitions

• Chapter 18-Pharmacy
• Chapter 31-Regulation of Health Professions
• Chapter 32-Health Professionals
• Chapter 43-Licensure, Certification and Registration of Military Members
• Chapter 48-Licensing Authority
NOTE: Not all sections of this chapter are applicable to the MPJE Exam, but this chapter is relevant to practicing Pharmacy Professionals, so it is included for that reason.
Sec: 32-3101-32-3124
Article 1 General Provisions
• 32-3101
• Definitions
• 32-3102
• 32-3103
• 32-3104
• 32-3105
• 32-3106
• 32-3107
• Non applicability of chapter
• Regulation of health professions; legislation; criteria
• Health professional groups; written report; legislative informational hearings; proposed legislation
• Health professional groups; proposed regulation; factors
• Health professional groups; proposed increased scope of practice; factors; legislation
• Continuing education requirements; evidence of effectiveness
• 32-3108
Article 2 Licensure
• 32-3121
• Grievance process; public testimony
• Definitions
• 32-3122
• Rules
• 32-3123
• 32-3124
• Board delegation; executive director
• Temporary licensure; rules; fee; applicability

• Chapter 18-Pharmacy
• Chapter 31-Regulation of Health Professions
• Chapter 32-Health Professionals
• Chapter 43-Licensure, Certification and Registration of Military Members
• Chapter 48-Licensing Authority
NOTE: Not all sections of this chapter are applicable to the MPJE Exam, but this chapter is relevant to practicing Pharmacy Professionals (refer to highlighted sections), so it is included for that reason.
Sec: 32-3201-32-3249.01
Article 1 General Provisions
• 32-3201
• Definitions
• 32-3201.01
• 32-3202
• 32-3203
• 32-3204
• 32-3205
• 32-3206
• 32-3207
• 32-3208
• 32-3209
• 32-3210
• Definition of medication-assisted treatment
• License or certificate suspension
• Malpractice claim investigation
• Experimental diagnosis, therapy or treatment; implied consent; definition
• Board disciplinary action; voting requirements
• Disciplinary action; information; disclosure
• Health professionals disease hazard; testing; petition; definition
• Criminal charges; mandatory reporting requirements; civil penalty; exceptions
• Release of information; fees
• 32-3211
• 32-3212
• 32-3213
• 32-3214
• 32-3215
• 32-3216
• 32-3217
• 32-3218
• 32-3219
• 32-3220
• 32-3221
• Billing for laboratory costs; unprofessional conduct; definition
• Medical records; protocol; unprofessional conduct; corrective action; exemptions
• Umbilical cord blood; patient information; definition
• Health professionals; disclosure; unprofessional conduct; definition
• Board actions; public access to records; website
• Medical marijuana; unprofessional conduct; annual reports; identifying information
• Health care providers; charges; public availability; direct payment; notice; definitions
• Volunteer health services registration; health professionals; free medical clinic
• Health profession regulatory boards; members; training; definitions
• Licensure; renewal; notification; definitions
• Health professionals; requirements for licensure; prohibition
• 32-3222
• 32-3223
• 32-3224
• 32-3225
• 32-3226
• 32-3227
• 32-3228
• 32-3229
• 32-3229.01
• Lawful health care services; patient education; exceptions; definitions
• Health profession regulatory boards; terms of members; board meeting recordings; employment opportunities; websites
• Health profession regulatory boards; non disciplinary confidential monitoring programs
• Complaints; time limit on filing; exceptions
• Types of disciplinary action; reimbursement
• Address of record; disclosure; telephone number or email address; definition
• Unauthorized practice of a health profession; verification; posting; violation; classification; definition
• Informed consent; breast implant surgery; requirements; unprofessional conduct; work group; definition
• Unprofessional conduct; informed consent; pelvic examinations
• Health professional wellness programs; confidentiality; definition
• 32-3230
• Prohibition of irreversible gender reassignment surgery for minors; definitions
• 32-3230.01
• Health professionals; practice; employment; business entities
Article 2 Cosmetic Laser and Injection Procedures
• 32-3231
• 32-3232
• 32-3233
• 32-3234
• Definitions
• Supervision
• Lasers; IPL devices; authorized use; authorized supervision
• Laser safety fund
Article 3 Medical Licensure Compact
• 32-3241
• 32-3242
• 32-3243
• 32-3244
• 32-3245
• Medical licensure compact
• Subpoenas from member boards or courts in member states
• Participation in compact as condition of employment; prohibition
• Open meeting requirements
• Arizona medical board; Arizona board of osteopathic examiners in medicine and surgery; notice of commission actions; expenditure of certain monies prohibited
• 32-3246
• Conditional repeal; notification; withdrawal from compact; request for review
4 Controlled Substances
• 32-3248
• 32-3248.01
• 32-3248.02
• Health professionals; controlled substances; initial prescriptions; limits; exceptions; definition
• Schedule II controlled substances; dosage limit; exceptions; morphine; opioid antagonist; definitions
• Health professionals; substance use or addiction continuing education
Article 5 Health Professionals Workforce Database
• 32-3249
• 32-3249.01
• Definitions
• Designated database information; collection; transfer; confidentiality

• Chapter 18-Pharmacy
• Chapter 31-Regulation of Health Professions
• Chapter 32-Health Professionals
• Chapter 43-Licensure, Certification and Registration of Military Members
• Chapter 48-Licensing Authority
NOTE: Not all sections of this chapter are applicable to the MPJE Exam, but this chapter is relevant to practicing Pharmacy Professionals, so it is included for that reason.
Sec: 32-4301-32-4304
Article 1 General Provisions
• 32-4301
• 32-4302
• 32-4303
• 32-4304
• License, certificate or registration expiration; military active duty; one hundred eighty-day extension
• Out-of-state applicants; residents; military spouses; licensure; certification; exceptions; notice
• Military education, training and experience
• Occupational and professional licenses; websites; reporting; definition
Sec: 32-4801
Article 1 Public Meetings
• 32-4801
• Public meetings; digital recordings; posting; definition

• Chapter 20-Abortion
• Chapter 27-Controlled Substance Act
• Chapter 28-CSPMP
NOTE: Not all sections of this chapter are applicable to the MPJE Exam, but this chapter is relevant to practicing Pharmacy Professionals (refer to highlighted sections), so it is included for that reason.
Sec: 36-2151-36-2164
Article 1 General Provisions
• 36-2151
• Definitions
• 36-2152
• 36-2153
• 36-2153.01
• 36-2154
• 36-2155
• 36-2156
• 36-2157
• Parental consent; exception; hearings; time limits; violations; classification; civil relief; statute of limitations
• Informed consent; requirements; information; website; signage; violation; civil relief; statute of limitations
• Website information; agencies providing support for pregnant women; adoption information
• Right to refuse to participate in abortion; abortion medication or emergency contraception
• Performance of an abortion by individual who is not a physician; prohibition; definitions
• Informed consent; ultrasound required; violation; civil relief; statute of limitations
• Affidavit
• 36-2158
• Informed consent; fetal condition; website; unprofessional conduct; civil relief; statute of limitations; definitions
• 36-2159
• 36-2160
• Abortion; gestational age; violation; classification; unprofessional conduct; civil relief; statute of limitations
• Abortion-inducing drugs; definition
• 36-2161
• 36-2162
• 36-2162.01
• 36-2163
• 36-2164
• Abortions; reporting requirements
• Complications; reporting requirements
• Informed consent; reporting requirements
• Reports; confidentiality; annual statistical report; violations; classification; unprofessional conduct; penalties
• Construction of article

• Chapter 20-Abortion
• Chapter 27-Controlled Substance Act
• Chapter 28-CSPMP
Sec: 36-2501-36-2552
Article 1 General Provisions
• 36-2501
• Definitions
Article 2 Schedules
• 36-2511
• Nomenclature
• 36-2512
• 36-2513
• 36-2514
• 36-2515
• 36-2516
• 36-2517
• 36-2517.01
• 36-2518
• Substances in schedule I; rules
• Substances in schedule II; rules
• Substances in schedule III; rules; definition
• Substances in schedule IV; rules
• Substances in schedule V; rules
• US FDA; approved medication
▪ US FDA; approved medication; rescheduling
• Schedule exemptions; rules
• 36-2521
• Rules
• 36-2522
• 36-2523
• 36-2524
• 36-2525
Article 4 Offenses and Penalties
• 36-2531
• Registration requirements
• Records of registrants; inspection; confidentiality
• Order forms
• Prescription orders; labels; packaging; definition
• Prohibited acts; classification
Article 5 Enforcement and Administration
• 36-2541
• 36-2542
• 36-2543
• 36-2544
Article 6 Miscellaneous
• 36-2551
• Administrative inspections and warrants
• Cooperation of agencies
• Review
• Education; research; public notices
• Pending proceedings
• 36-2552 Continuation of rules

• Chapter 20-Abortion
• Chapter 27-Controlled Substance Act
• Chapter 28-CSPMP
Sec: 36-2601-36-2610
Article 1 General Provisions
• 36-2601
• Definitions
• 36-2602
• 36-2603
• 36-2604
• 36-2605
• Controlled substances prescription monitoring program; contracts; retention and maintenance of records
• Computerized central database tracking system task force; consultation on electronic prescribing; membership
• Use and release of confidential information; definitions
• Controlled substances prescription monitoring program fund
• 36-2606
• 36-2607
• 36-2608
• 36-2609
• 36-2610
• Registration; access; requirements; mandatory use; annual user satisfaction survey; report; definitions
• Disciplinary action
• Reporting requirements; waiver; exceptions
• Use of information; civil immunity
• Prohibited acts; violation; classification

Title 20 – Insurance
• Chapter 25- Pharmacy Benefits
• Chapter 31- Step Therapy
Sec: 20-3321-20-3343
Article 1 Auditing
• 20-3321
• 20-3322
• 20-3323
• 20-3324
▪ (Definitions)
• Audit procedures; interest prohibition
• Audit reports
• Applicability
Article 2 Pharmacy Benefit Managers
• 20-3331
• 20-3332
• Pharmacy benefit managers; requirements; applicability
• Prohibition against claim adjudication process fees; 20-3333
▪ Certificates of authority; issuance; revocation; renewal 20-3334
▪ Records retention; schedule
Article 3 340B Pharmacies
• 20-3341
• 20-3342
• 20-3343
• Definitions
• Applicability
• 340B drug program; 340B covered entities; pharmacies; drug coverage

Title 20 – Insurance
• Chapter 25- Pharmacy Benefits
• Chapter 31- Step Therapy
Sec: 20-3651-20-3654
Article 1 General Provisions
• 20-3651
• Definitions
• 20-3652
• 20-3653
• 20-3654
• Applicability
• Clinical review criteria
• Exceptions; process

Title 44 – Trade and Commerce
• Chapter 11- Regulations Concerning Particular Businesses
o Article 10.1-Pharmacy Benefit Managers
Sec: 44-1601-44-1799.96
Article 10.1 Pharmacy Benefit Managers
• 44-1751
• Definitions
• 44-1752
• 44-1753
• 44-1754
• Pharmacy benefits managers; prohibitions; applicability
• Prescription medications; ninety-day fill; exceptions
• Delivery of prescription drugs; disclosure; exception

Title 4 – Professions and Occupations
• Chapter 23-Board of Pharmacy
Ch. 23 4 A.A.C. 23 Board of Pharmacy

Title 9 – Health Services
• Chapter 6- Communicable Diseases (Article 13)
Ch. 06 9 A.A.C. 06 Department of Health ServicesCommunicable Diseases and Infestations
Note: Article 13 will be updated to reflect changes to the law reflected in 32-1974

• These laws have not yet made it into the text of the book because they were just signed into law.
STATUTES:
Refer to 2024 56th Legislature Bills Signed Report- These laws go into effect 9-1424)
REGULATIONS: CLICK HERE
This Chapter contains rules that were filed to be codified in the Arizona Administrative Code between the dates of January 1, 2024 through March 31, 2024
R4-23-101. General ................................................................. 4
R4-23-119. Subpoenas .......................................................... 13
R4-23-201. General ............................................................... 15
R4-23-202. Licensure by Examination ................................. 15
R4-23-203. Licensure by Reciprocity ................................... 16
R4-23-205. Fees and Charges ............................................... 17
R4-23-301. Intern Licensure .................................................18
R4-23-302. Training Site; Intern Preceptors; Training Time 19
R4-23-303. Repealed .............................................................20
R4-23-304. Repealed .............................................................20
R4-23-305. Repealed.............................................................20




Acknowledgment: This programs was created through an educational partnership.Handbook created by: MARY K GURNEY, RPh, PhD, BCPA and ROGER MORRIS, RPh, JD Laws and Regulations were obtained through the Arizona State Board of Pharmacy Law Book and the Arizona Secretary of State website.
©2024 AzPA All Rights Reserved




Gurney MK, Morris R, and Midwestern University. 2024.
You are free to copy, distribute and transmit this work for non‐commercial purposes. The licensor permits others to distribute derivative works only under the same license or one compatible with the one that governs the licensor's work, i.e., to freely share for non‐commercial purposes.
This work is licensed under the Creative Commons Attribution‐NonCommercial‐ShareAlike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by‐nc‐sa/4.0 or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA.
After attending the lecture and completing the assigned readings, the student should be able to define all of the terms presented below.
Act: a law passed by the legislature
Administer: direct application (by injection, inhalation, ingestion, etc ) of a drug to the body of a patient by a practitioner or his authorized agent or by the patient at the direction of the practitioner
Adjudication: a formal determination or judgment
Bioequivalent: A term describing products that are pharmaceutical equivalents or pharmaceutical alternatives that display comparable bioavailability; the parameters evaluated regarding bioavailability are the rate and extent of absorption.
Biosimilar: The biological product that is approved based on a showing that it is highly similar to an FDA-approved biological product, known as a reference product, and has no clinically meaningful differences in terms of safety and effectiveness from the reference product.
Biosimilar, interchangeable An interchangeable biological product is biosimilar to an FDA-approved reference product and meets additional standards for interchangeability. An interchangeable biological product may be substituted for the reference product by a pharmacist without the intervention of the healthcare provider who prescribed the reference product. (The following terminology may also be used: interchangeable biosimilar).
CI - CV: denotes a controlled substance in Class I, Class II, etc., as determined in the CSA
CARA Comprehensive Addiction and Recovery Act (2016)
CE: continuing education
CFR: Code of Federal Regulations
CGMP: current good manufacturing practices; the standard established by the FDA and applied to all drug manufacturers
Closed system: as applied to the CSA; refers to the distribution of controlled substances among registrants only
2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Common law: refers to law developed from decisions of the courts
Compounding: Preparation, mixing, assembling, packaging, or labeling of a drug/device by a pharmacist or by a practitioner in the course of professional practice. Compounding includes the preparation of drugs/devices as the result of a practitioner order or initiative, as well as in anticipation of prescriptions of medication orders based on routine prescribing patterns. Compounding does not include the preparation of commercially available products from bulk compounds, or the preparation of drugs/devices for sale to pharmacies, practitioners or any other agent for the purpose of dispensing or distribution.
Compounding Quality Act Title 1 of DQSA (Drug Quality and Security Act)
CSA: Controlled Substances Act; federal CSA is enforced by the DEA
DEA: Drug Enforcement Administration is under the jurisdiction of the Department of Justice; concerned with controlled substances only
DEA Form 222: Drug Enforcement Administration Form 222 for the ordering of CI and CII controlled substances between registrants
Direct supervision of a pharmacist: (in Arizona) pharmacist is present
Dispense: deliver medication to an ultimate user on the lawful order of a practitioner
Distribute: deliver, other than by administering (directly to a patient by the practitioner) or dispensing (on the order/prescription of the practitioner)
DQSA: Drug Quality and Security Act. The Act has 2 titles. Title 1: Compounding Quality Act Title 2: The Drug Supply Chain Security Act
Drug: articles, recognized in the official compendium, intended for the use in the diagnosis, treatment, mitigation, cure, or prevention of disease; also articles other than food intended to affect the structure or function of the body
Drug Product: is a finished dosage form, e.g., tablet, capsule, or solution, that contains a drug substance, generally, but not necessarily, in association with one or more other ingredients.
DSCSA Drug Supply Chain Security Act (Title 2 of DQSA; Drug Quality and Security Act); aka “Track and Trace”
DUE: drug use evaluation
Due process: law in its regular course of administration through the courts; fundamental fairness
DUR: drug utilization review/drug use review
2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Ex post facto: after the fact
Ex post facto law: law passed after the occurrence of an act which retroactively changes the legal consequences of the act
FDA: Federal Food and Drug Administration
FDCA: Federal Food, Drug and Cosmetic Act; enacted to protect public health; enforced by the FDA
Generic equivalent: contains an identical amount of the same active chemical ingredients in the same dosage form and which, if administered in the same amounts, will provide comparable therapeutic effects; this does not include any drug that the FDA lists as having unresolved bioequivalence concerns; see all therapeutic equivalent and pharmaceutical equivalent
GMP: good manufacturing practices; mandated by the FDCA, with standards for manufacturers promulgated in regulations by the FDA
Internship: the practical, experiential, hands-on training of a pharmacy intern under the supervision of a preceptor
Jurisdiction: (a) the power to decide a matter; (b) the geographic area over which someone has authority
Label: written, printed, or graphic matter immediately attached to the container for sale
Labeling: includes the label and any accompanying material to the container for sale (may be inside the container, like a package insert)
Legend drug: a drug that is available by prescription or medication order only
Licensee A person who is licensed to practice a profession
NDA: New drug application: filed with the FDA to get approval for marketing in interstate commerce in the US; the drug must be proven safe and effective to be approved.
NDC #: National Drug Code; a unique identifier number placed on the label of drugs, containing 9 - 11 digits arranged to specify the manufacturer, the product, the package size and dosage form; is required as part of the manufacturer prescription label with the passage of the Food Drug Administration Amendments Act 2007 (Public Law 110-85)
New drug: drug not generally recognized by qualified experts as safe and effective for use
OBRA '90: federal Omnibus Budget Reconciliation Act of 1990; mandates that pharmacists (a) provide DUR of prescription orders; (b) offer to counsel patients; and maintain a written patient history for the state to receive federal funds for Medicaid programs; first effective patient counseling rule
2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Off-label use: use of an approved drug for other than the approved indication
Official compendium: the United States Pharmacopeia and the National Formulary (USP/NF); official listing of recognized drugs in the US; and Homeopathic Pharmacopoeia of the United States; official compendia for homeopathic products in the US
Orphan drug: safe & effective drug; but the number of patients requiring the drug is so small as not to provide a reasonable return on investment to a manufacturer; thus the FDA provides incentives to companies that will market the drug
Original state of licensure: any state from which the license is obtained by examination and not by reciprocity
Orange Book: the "Approved Drug Products with Therapeutic Equivalence Evaluations", published by the federal Department of Health and Human Services (DHHS); lists all approved drugs in the US as well as therapeutic equivalence information; it has an orange cover hence its nickname
OTC: over-the-counter; medications/devices not requiring a prescription for sale
PDMA: Prescription Drug Marketing Act
PDMP/PMP: Prescription Drug Monitoring Program/Prescription Monitoring Program
Permittee: one who holds a permit, aka a business.
Pharmaceutical equivalent: drug products that contain the same active ingredient(s), same dosage form, same route of administration, and are identical in strength or concentration; they may have different shapes, coloring, flavoring, or release mechanisms
Pharmaceutical alternative: drug products that contain the same therapeutic moiety but are different salts, esters, complexes, dosage forms, or strengths
PIC: pharmacist in charge
PPPA: Poison Prevention Packaging Act; enacted to decrease accidental poisonings of children; the guidelines supersede any state legislation for packaging; administered by the US Consumer Product Safety Commission
Prescription (or prescription order): an order for medication or vaccine that is dispensed to or for an ultimate user but does not include an order for medication that is dispensed for immediate administration to the ultimate user (i.e., an order to dispense a drug to a patient in the hospital for immediate administration is a medication order; not a prescription order). See ARS 32-1901 (77) for complete definitions.
Promulgate: to announce officially; to make a law known; to put a law into action or to force
Purple Book: The “Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations” was published by CDER/FDA and has a “purple cover”, hence its name.
4 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Quid pro quo: something for something; giving one valuable thing for another; exchange
Reciprocity: transfer of licensure from one state to another; the state from which the license is transferred must be an original state of licensure
Registrant: one who is registered; often refers to registration with the DEA
Regulation: official guidelines promulgated by agencies of the executive branch (e.g., State Board of Pharmacy) which interpret and define statutes; although not laws, they hold the strength of statutes
Retroactive: applying to the time prior to the enactment
RPh: registered pharmacist
Rule: same as regulation
Statute: a law; promulgated by elected officials (the legislature)
Summary suspension: suspension of a license without/before a hearing; only for extreme circumstances where there is demonstrated immediate danger to public safety (e.g., RPh reports to work while intoxicated)
Supersede: to override; render unnecessary; to displace
The act: usually referring to the Food, Drug, and Cosmetic Act (FDCA)
Therapeutic alternate: drug product containing different therapeutic moieties but which are of the same pharmacological and/or therapeutic class that can be expected to have similar therapeutic effects when administered to patients in therapeutically equivalent doses
Therapeutic equivalent: drugs that are pharmaceutical equivalents AND which can be expected to have the same clinical effect and safety profile when administered under the conditions specified in the labeling AND which are bioequivalent; all generic equivalents are therapeutic equivalents; when used by the FDA, therapeutic equivalent does not mean different therapeutic moieties used for the same condition
Therapeutic moiety: the portion or part of a drug that produces or causes a therapeutic effect or action.
USP: the United States Pharmacopeia; one of the two official compendium
5 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
After attending the lecture and completing the assigned readings the student should be able to:
✓ Identify the reasons why society regulates medication, as well as the limitations of regulations
✓ Distinguish and describe the sources and types of laws in the United States (statutory, judicial, administrative/regulatory)
✓ Describe the federal and state legislative process (how laws get passed)
✓ Describe the structure and function of the US judicial system
✓ Identify how administrative agencies get their responsibilities (enforcement and rulemaking)
✓ Identify and describe the various aspects of judicial law (court system, civil versus criminal, parties involved, statute of limitations)
✓ Identify the various players who determine pharmacy law
✓ Define and describe public policy
✓ Identify and describe the phases of the public policy life cycle, who is involved in the public policy cycle, the types of policy instruments that may be used and their purposes,
✓ Abood R. Chapter 1: The law and the legal system. Pharmacy Practice and the Law, 9th ed. Sudbury, MA: Jones and Bartlett Publishers; 2019
✓ The Beginner's Guide to Digital Participation: Get Started with Online Citizen Participation in Your Community. Brussels, Belgium: citizenlab. Accessed 28 February 2022. Guide_beginner_digital_engagement_CitizenLab_2021.pdf. https://www.citizenlab.co/. Posted on Canvas.
✓ Mackay M and Shaxton L. Understanding and Applying Basic Public Policy Concepts. Ontario, Canada: University of Guelph. https://www.politicipublice.ro/uploads/understanding_public_policy.pdf Accessed 4 March 2022. Posted in Canvas.
“A body of rules of conduct of binding legal force and effect, prescribed, recognized, and enforced by controlling authority” Reference: West's Encyclopedia of American Law, edition 2. (2008). Retrieved March 1 202 http://legal-dictionary.thefreedictionary.com/law
6 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Figure 1: Who Determines Law? (Federal or State)
Citizens
• Individuals, society, culture, or community identify a real or perceived need for protection, order, or behavioral standards.
• Agree as a group to submit to a recognized authority in exchange for certain protections


Statutory Law Administrative Law Case Law
Legislative Branch

Executive Branch
Judicial Branch Makes Laws (Statutes; aka Acts) Carries Out Laws Evaluates Laws
Congress, House of Representatives, Senate President, Vice-President, Cabinet, Federal Agencies (Governor, Lt. Governor if one, State Agencies)
Constitutional and Statutory Law
Fed – Supreme Court, Court of Appeals (Circuit Courts), District Courts, and other Courts
Laws made by legislative bodies (aka Congress is one example)
Constitutional Law (Federal)
✓ US Constitution – the highest law of the country
o Preamble (Introduction)
o 7 Articles
o 27 Amendments
▪ Bill of Rights (1– 10)
Statutory Law (Federal)
✓ Federal Statutes
o Referred to as US Code (USC)
o Public Law
o Applies nationwide
Constitutional Law (State)
✓ AZ State Constitution – highest law in the state
o Preamble (Introduction)
o 30 Articles
Statutory Law (State)
✓ State Statutes
o Referred to as AZ Revised Statutes (ARS)
o Applies statewide and to any other bodies that affect AZ citizens
Examples Examples
✓ Food Drug and Cosmetic Act (FDCA) (21 USC 1 -2252)
✓ Controlled Substances Act (CSA) (21 USC 801-971)
✓ Arizona Pharmacy Act (ARS Title 32 –Chapter 18)
✓ Uniform Controlled Substances Act (ARS Title 36 – Chapter 27)
7 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Administrative Law (aka Rules and Regulations) Laws made by administrative (executive branch) agencies
Administrative laws (rules and regulations) are promulgated by administrative agencies (executive branch)
2: Federal Rules/Regulation Example
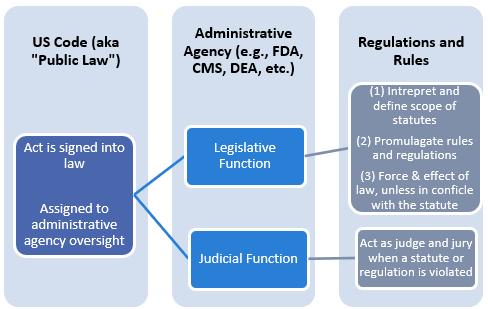
How Do Administrative Agencies Get Their Authority?
ENABLING LEGISLATION
Legislation that gives officials and/or agencies the authority (jurisdiction) to implement or enforce the law
Determined by US or State Statutes
ENABLING LEGISLATION THAT CREATED FDA AND ARIZONA STATE BOARD OF PHARMACY
7 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Federal Level State Level
Federal Rules and Regulations (Final)
✓ Final rules published in the Code of Federal Regulation (CFR)
Examples
21 CFR 1 – 1299 Food and Drug Regulations
21 CFR 1300 – 1399 Controlled Substances Regulations
42 CFR 400 – 699 Centers for Medicare & Medicaid Services
State Rules and Regulations (Final)
✓ Final rules published in AZ Administrative Code (AAC)
Examples
Arizona Board of Pharmacy – AAC R4-23-101
Arizona Medical Board R4-16-101
Arizona Board of Osteopathic Examiners in Medicine and Surgery R4-22-101
Arizona Regulatory Board of Physicians Assistants R4-17-101
City/County Level
Municipal rules (Ordinances)
Examples
Zoning rules
Local taxes
Pseudoephedrine rules
Proposed Federal rules will be found in the Federal Register
Proposed Arizona State rules will be found in the Arizona Administrative Register
Example of ASBP Final Rulemaking Notice in Arizona Administrative Register
Process to Create Administrative Law (DEA and CARA partial fills for CIIs) Process infographic with more details
Case Example:
LAW: Comprehensive Addiction and Recovery Act (CARA) passed by Congress and signed into law by the President.
• Allowed for partial fills of CII medications beyond current (at the time) DEA regulations.
Federal Agency: Drug Enforcement Administration (DEA)
Federal Register: Proposed Regulations Published on 12/04/2020
Code of Federal Regulations: Final Regulation Published on 07/21/2023 with effective date of 08/21/2023.
Code of Federal Regulations (CFR): 21 CFR 1306.13 (b) partial filling of a prescription for a schedule II controlled substance at the request of the prescribing practitioner or patient
8 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Step 1: Administrative Agency Publishes Proposed Rule/Regulation in the Federal Register
•DEA publishes proposed regulations in the Federal Register
Step 2: Public Comment Period Set in the Federal Register
•Public Comment Period open until 02/02/2021 11:59 pm EST
Step 3: Agency staff review comments
Step 3: If regulation has had major revisions, it may be republished by the agency and open for another comment period.
Step 4: Final regulation published in the Federal Register with effective date
•DEA published final notice on 07/21/2023
•Final regulation includes explanations to comment themes for the final regulation
For a regulation to be valid, it must meet the following criteria (tests):
The regulation must:
✓ be within the scope of the agency’s authority.
✓ be based on a statute that gives the agency the authority to promulgate the regulation.
✓ bear a reasonable relationship to public health, safety, and welfare.
Figure 3: Federal Agencies that Enforce Administrative Laws from a Pharmacy Perspective (not all inclusive)
9 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Dept of Health Services (ADHS)
Executive Branch (Governor)
Figure 4: State Agencies that Enforce Administrative Laws from a Pharmacy Perspective (not all inclusive)
✓ Reminder: Administrative Agencies have legislative and judicial functions





(Look at Judicial Process section for additional information of the Abood textbook).
✓ Judicial opinions = force of law (case law)
Stare Decisis (See Abood et al. under “common law” for complete explanation)
Stare Decisis = Decide to abide by decided cases - sets precedent
Purpose:
1. Establish continuity of decisions
2. Expedite judicial decision-making
Application:
✓ Only applies to lower courts within the jurisdiction that the precedent has been set
Example:
✓ US 9th Circuit Court of Appeals (9th Circuit) has jurisdiction over the following states and territories: AK, AZ, CA, HI, ID, MT, NV, OR, WA, Guam, and Northern Mariana Islands
✓ Decisions made by the 9th Circuit only apply to the states and territories in the 9th Circuit

The Judicial Process
WHO ARE THE PARTIES IN A LAWSUIT?
CRIMINAL, CIVIL, ADMINISTRATIVE LAW
Criminal Law (Think Law & Order) Civil Law (Think Judge Judy)
Administrative Law (ASBP in the matter of: Defendant –Pharmacist, Intern, Tech, Permittee, etc.)
Those involved: State vs. Accused Plaintiff vs. Defendant ASBP in the matter of: (Defendant)
Reason for case: Violation of a statute One party sues another alleging an injury Violation of statute, rule, or regulation
Objective: Punish for violation Compensate injured party for damages
Determination: “Guilty beyond a reasonable doubt.” Decisions based on 51:49%; must have “preponderance”
Potential Consequences: Accused can go to jail, fined
Defendant can be prohibited from doing certain activities, fined
Determine nature of violation and if sanctions to be imposed
Based on determination of Administrative Agency (i.e., ASBP)
Defendants may be fined, sanctioned, prohibited from certain activities, etc.
Adjudication: Various courts Various courts; arbitration Hearing officer; Defendant may appeal to agency panel; May appeal to civil court if needed
11 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
A time limit in which a suit must be file and brought before a governing body from the date when the problem first started
What is the statute of limitations in AZ? 2 years from the time known or should have known for the majority of issues.
Exception: If under the age of 18 – the clock does not start until you turn 18.
1. Pay a fee
2. File a complaint in court
3. “Serve” complaint to the defendant
4. Discovery process
5. Motions made
Approximately ½ of all civil lawsuits are settled by a judge.
Two choices: ✓ Jury ✓ Judge
12 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale

CAN YOU IDENTIFY SITUATIONS THAT MAY HAVE PROMPTED OUR SOCIETY TO AGREE TO THE EXISTENCE OF LAWS DEALING WITH DRUG PRODUCTS?
✓ Misbranded products
✓ Adulterated products
✓ No standards in place
✓ Product misrepresented
13 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
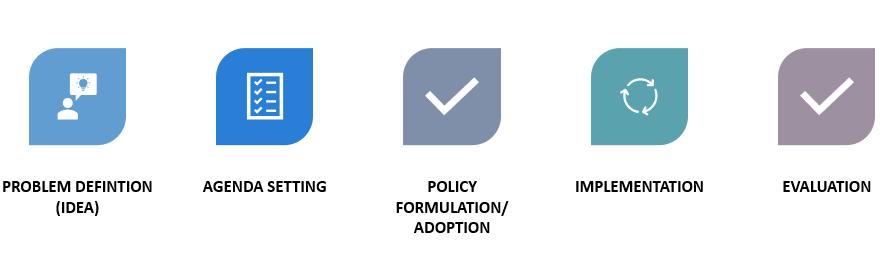
It is a decision made by the government to either act, or not act in order to resolve a problem. Examples include:
General statements about priorities
Written regulations or guidelines Court rulings
Procedures and/or standards to be achieved Ballot measures
Source: Northern California Grantmakers. Public Policy Grantmaking Toolkit. [NCG website]. Available at: http://www.ncg.org/toolkit/html/gettingstarted/index.html Accessed 1 Sept 2008
PHASE OF THE PUBLIC POLICY CYCLE WHAT IT’S ABOUT
Problem identification (idea)
Emergence of a problem that requires the attention of the public and decision-makers
Agenda Setting Who sets the agenda?
• Policy makers
• Elected officials
• Citizens Places the problem on the government’s (or organization’s) agenda in order to find a solution
Policy Formulation/Adoption
Formulation of alternatives to resolve the problem or address the idea. Includes:
• Policy analysis – the environment and internal context
o Research and analysis
• Policy determination – decide which challenges will be tackled and goals to be achieved
o Community organizing
o Advocacy
• Action planning – clearly state how will realize the goals
Implementation Creating dialogue about the plan, proposal, or decision Methods:
• Federal register/Arizona Administrative Register
• Congress – Bills
• Public health departments
• Standards of care
Evaluation Evaluate the outcomes of the policy(ies); restart the cycle Litigation may be involved
14 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Actor Role
Government Social control of behavior; coercion based on power
Cabinet Limited number of people with power;
Think Secretary of Health and Human Services, Secretary of Homeland Security, Secretary of the Treasury, etc.
Public Servants
Political Parties
Interest Groups
Civil servants working in various government agencies – provide technical knowledge and policy advice; service providers
Think: FBI, CIA, DOJ, FDA, CMS, ATF, ICE, Border Patrol, IRS, CDC, etc.
Develop relationships in exchange for political support
Seek to advance interests of members; can have major influence
Can force policy network to react
Legal System Interpret laws; acts independently
Public Elects government, forms opinions, joins interest groups and coalitions, relies on various media sources for information
Policy Instruments
Policy instruments – techniques at the government’s disposal to implement policy objectives.
Purpose of policy instruments:
✓ Achieve behavior change within individuals
✓ Realize social, political, or economic conditions
✓ Provide services to the public
Regulation(s) Government’s role to command and prohibit actions – defines norms and acceptable behavior or limits activities
Acting directly Provide a direct service to achieve outcome (instead of working through citizens or organizations to achieve goals).
Examples: education; parks and recreation, public health/CDC
16 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
CURRENT PHARMACY PUBLIC POLICY ISSUES
✓ Gain provider status for pharmacists under the Social Security Act (which already recognizes other healthcare providers, including dieticians, nurse practitioners, physician assistants, nurse midwives, and clinical social workers).
✓ Opioid epidemic – federal responses, including the CDC Update to 2022 CDC Clinical Practice Guidelines for Prescribing Opioids
✓ CARA
✓ US PREP Act
✓ US “Test to Treat” for COVID 19 and Pharmacy Involvement
WHY ARE THESE IMPORTANT TO THE PHARMACY PROFESSION AND THE GENERAL PUBLIC?
Provider Status Comprehensive Addiction Recovery Act (CARA) of 2016
May 2011 with Improving Patient and Health System Outcomes Through Advanced Pharmacy Practice: A Report to the Surgeon General 2011
2013 – Pharmacy coalition established (AACP, ACCP, AMCP, APhA, ASCP, ASHP, CPNP, FMI, IACP, NACDS, NASPA, NCPA, Rite Aid Pharmacy, SNHPA, Walgreens)
✓ APhA – dedicating $1.5 million dollars towards this initiative
2014 – Several states have passed state statues (acts) that have given provider status to pharmacists within their state (California, Arizona, Kansas, Wisconsin, etc.)
July 2016 CARA became a federal law
Title VII Sec 702 amended the Controlled Substances Act to allow pharmacists to partially fill CII (schedule II) medications if:
(1) such partial fills are not prohibited by state law;
(2) a partial fill is requested by the patient or prescribing practitioner; and
(3) the total quantity dispensed in partial fillings does not exceed the quantity prescribed
In 2017 and 2018 US Senators Warren (D-Mass), Capito (R-WVA), Grassley (R – IA), Feinstein (DCA) and Representatives Clark (D-Mass) and Stivers (R-OH) wrote joint letters to the DEA urging them to issue regulations and guidance for partial filling of CII medications as approved under CARA.
17 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
The Pharmacy and Medically Underserved Areas Enhancement Act
Introduced in the following legislative sessions:
✓ 2015 – 16 HR592/S109)
✓ 2016 - 17
✓ 2017 – 18
✓ 2020 – 21
✓ 2021 – 22 (HR 2759/S1362)
✓ 2022 – HR 7213
NACDS RxIMPACT: https://www.nacds.org/advocate/rximpactgrassroots/
February 2022 – New coalition website and name: https://pharmacycare.org/
Dec 2020 DEA Published a notice of proposed rulemaking for the partial dispensing of CII prescription medications.
Comments were open until February 2, 2021
Final rule published July 2023. Effective in August 2023.
WHAT DO YOU THINK ARE THE REASONS THAT PROVIDER STATUS LEGISLATION HAS NOT PASSED YET?
WHAT DO YOU THINK NEEDS TO HAPPEN TO GET PROVIDER STATUS FOR PHARMACISTS PASSED IN THE US CONGRESS?
TO GET MORE INFORMATION ON THE PUSH FOR PHARMACIST PROVIDER STATUS SEE:
Future of Pharmacy Coalition: https://pharmacycare.org/
Membership includes:
• Abbott
• American Pharmacists Association (APhA)
• American Society of Health-System Pharmacists (ASHP)
• AmerisourceBergen
18 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
• Cardinal Health
• CVS Health
• Good Neighbor Pharmacy
• Health Mart
• Kroger Health
• McKesson
• Medicine Shoppe International
• National Association of Chain Drug Stores (NACDS)
• Walgreens
19 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Defending public health
After attending the lecture and completing the assigned readings, the student should be able to:
✓ Identify the significant historical events that have shaped the current federal Food, Drug and Cosmetic Act (FDCA), including: (1) Pure Food and Drug Act, (2) FDCA, (3) Durham-Humphrey Amendment, (4) Kefauver-Harris Amendment, (5) federal Controlled Substances Act (CSA), (6) Poison-Prevention Packaging Act (PPPA), (7) Medical Device Amendment, (8) Prescription Drug Marketing Act (PDMA), and (9) Dietary Supplement Health and Education Act (DSHEA).
✓ Distinguish between the definitions of (1) food, (2) drug, (3) dietary supplement, (4) cosmetic, and (5) device.
✓ Identify and describe the prohibited acts, penalties and enforcement mechanisms given to the FDA.
✓ Identify and distinguish between adulteration and misbranding (definitions, examples).
✓ Identify and describe the provisions of the Durham-Humphrey Amendment and how the provisions affect drug classification and pharmacy practice (e.g., Rx vs. OTC, refills, prescription order transmittal)
✓ Identify and describe how the Kefauver-Harris Amendment amended the FDCA.
✓ Identify what the Prescription Drug Marketing Act did (including definitions, enforcement, and restrictions)
✓ Recognize and be able to distinguish the requirements and definitions established by the Poison Prevention Packaging Act (including exceptions and exemptions).
✓ Identify the various parts of OBRA ’90. Identify and describe OBRA ’90, including rebates, demonstration projects, DUR (Retrospective, Prospective reviews, and educational programs)
✓ Identify AZ statutes and regulations (chapters – e.g., Pharmacy Practice Act, etc.).
✓ Describe why the community/society would want pharmacy regulated and why pharmacists would want regulation.
✓ Identify and describe the roles and functions of the FDA. FTC, DEA, and CMS.
✓ Identify and describe the roles, responsibilities, and functions of the Arizona State Board of Pharmacy (ASBP), including purpose, board membership and terms, ASBP staff, roles, the disciplinary process, State Board inspections, and other board functions.
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Abood RR, Burns KA, and Frankhauser F. Pharmacy Practice and the Law, 10th ed. Sudbury, MA: Jones and Bartlett; 2025.
• Chapter 1: Federal vs. State Law
• Chapter 2: Historical Overview of the Federal Food, Drug and Cosmetic Act (p. e32-37); Food and Drug Administration (p. e38); Prohibited Acts, Penalties, and Enforcement (p. e51 - 53); Adulteration (p. e54-55); Misbranding (p. e56 -60; through Batch Certification)
• Chapter 3: The Durham-Humphrey Amendment (p. 111 – 116 - Through Expiration or Beyond Use Dating); Prescription Drug Marketing Act (p. e144 - 148); Inspections Under the FDCA and Related Laws to FDCA (p. e152); Related Laws to the FDCA (p. e152 – 154)
• Chapter 4: State versus Federal Authority (p. e170 – 171
• ‘Chapter 6: OBRA ’90 (p. e257 - 263); Miscellaneous Federal Laws Related to Pharmacy Practice (p. 288 – 290)
• Chapter 7: State Boards of Pharmacy (p. e323)
Arizona State Statutes
• ARS 32-1904: Power and duties of the board
• ARS 32-1927: Pharmacists; pharmacy interns; graduate interns; disciplinary action
• ARS 32-1927.01: Pharmacy technicians; pharmacy technician trainees; disciplinary action
• ARS 32-1927.02: Permittee; disciplinary action
• ARS 32-1928: Hearings; restraining order; judicial review
AKA – “preemptive doctrine” – whichever is stronger is the one that takes precedence. Laws included: statutory, administrative, and case law at either federal or state level
Federal Law Stricter than State: State Law Stricter than Federal: State Federal







©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Example:
Federal: Controlled Substances Act & DEA regulations – Hydrocodone/APAP was rescheduled to a CII (Controlled substance schedule 2) from a CIII.
✓ On the date this went regulation into effect – all Hydrocodone/APAP products were changed to CII regardless of state laws (statutory or administrative) had them listed as a CIII.
Example:
State law: Mississippi Law – Pseudoephedrine. In MS, all pseudoephedrine products are a considered a CIII (Controlled substance schedule 3).
✓ This means that in Mississippi, a patient must have a prescription from a prescriber with a DEA registration to obtain a pseudoephedrine-containing product.
Federal law: Pseudoephedrine is available overthe-counter (OTC), with some record-keeping requirements.
Interstate Commerce Claus gives Congress the power to regulate commerce between the US and:
✓ Interstate Commerce Clause. The Commerce Clause refers to Article 1, Section 8, Clause 3 of the U.S. Constitution, which gives Congress the power “to regulate commerce with foreign nations, and among the several states, and with the Indian tribes.” The Constitution enumerates certain powers for the federal government.
o US Congress and federal administrative agencies derive their authority to regulate drug distribution from this clause of the Constitution.
o Intrastate commerce is also governed by the Interstate Commerce Clause
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ Figure: Example of how the “Interstate Commerce Clause” works.

Federal and State Authority (Primary Authority/Responsibility)
Federal Level State Level

Regulates medication manufacturing, advertising, & controlled substances

Regulates the practice of pharmacy
Pure Food and Drug Act of 1906
Before the Pure Food and Drug Act it was:


Catalyst: Upton Sinclair’s “The Jungle” about the meat packing industry
Enactment of the Pure Food and Drug Act of 1906
• Addressed mostly food safety
o Failed to regulate cosmetics or devices
• Prohibited adulteration & misbranding of foods and drugs
• Drug regulation remained secondary and inadequate
Food, Drug & Cosmetic Act (FDCA) of 1938 (and amendments)
Durham-Humphrey Amendment
Kefauver-Harris Amendment Medical Device Amendments
Food Drug and Cosmetic Act (FDCA)
Catalyst: The sulfanilamide elixir tragedy of 1937

What this law does:
✓ Established the Food and Drug Administration (FDA)
✓ Primarily regulates drug development, manufacturing, and marketing –focus on quality
✓ Only safe and properly labeled drugs may be introduced into interstate commerce
✓ Defined label and labeling (see terminology); will discuss in depth in the next section
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Labels must contain adequate directions for use and warning if habit-forming
✓ Expanded the definition of misbranding and adulteration
o Applies to drugs, cosmetics, and devices
o US Supreme court ruled that FDCA applies to drugs sold by the pharmacy to the consumer
o Ruled that the outer container must tell consumers of the contents, otherwise, it is misbranded
✓ Defines what is considered a “food,” “drug,” “cosmetic,” “dietary supplement,” and “devices” (see p. 37-45 in Abood & Burns, and Terminology section of handbook/lecture notes).
Devices and cosmetics now regulated by the FDA
Note: The FDCA also regulates the practice of pharmacy, but this is primarily done at the state level.
AND MISBRANDING (SECTION 501 & 502 OF THE FDCA
The FDCA is the nucleus of almost all federal law relevant to pharmacists.
Almost every violation of the FDCA or amendments is an instance of one or both of these:
Regarding a drug’s strength, purity, and quality Regarding representations made by the manufacturer on the label or labeling
A product IS adulterated if:
⬧ It consists of any filthy, putrid, or decomposed substance
⬧ It has been prepared, packed, or held under unsanitary conditions or not in conformity with current good manufacturing (GMP) practices
⬧ If its container is composed of any poisonous substance
⬧ The drug strength, quality or purity of the drug differs from that on the label
⬧ The drug strength, quality or purity of the drug differs from compendia standards (unless variations stated on the label)
⬧ Contains an unsafe color additive
A drug may be deemed adulterated even if it is pure if:
A product is misbranded if:
⬧ Its labeling is false or misleading in any way (includes healthcare economic information)
⬧ Required information is missing from the label or labeling (not an inclusive list)
Established name of drugs (generic names) of both active and inactive ingredients
Adequate directions for use (p. e5657 for complete list)
Adequate information for use (see p. e56 - 57 for complete lists)
Name and place of business of the manufacturer, packer, or distributor
“Rx only” or “Caution: Federal law prohibits dispensing without a prescription” on the label
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
⬧ It has been prepared, packed or held in conditions where it MAY have been contaminated
⬧ It was exposed to a container that may have been contaminated
⬧ It was manufactured under conditions that do not conform to current GMP
“Rx only” missing from this label

1. A bottle of Phenergan VC Cough Syrup breaks in the shipping tote, it gets all over the inside of the tote, and appears to have seeped into the bottles of Crestor ® 10 mg Tablets.
2. As the drug order is being put away by the pharmacy technician and the pharmacy intern, they notice that the label on several bottles of Lexapro 10 mg Tablets are missing the address of the manufacturer.
Durham-Humphrey Amendment of 1951 aka the "Prescription Drug Amendment"
Background:
All drugs require "adequate directions for use," but many drugs are not safe for use without medical supervision
What this law does:
✓ Established two classes of drugs
o Prescription (Rx)
o Over-the-Counter (OTC)
✓ Prescriptions may be orally-transmitted (aka by telephone)
✓ Prescribers may authorize refills of the prescription order
o original written Rx or orally-transmitted
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ Require medical supervision
✓ Rx only drugs sold in pharmacies are exempt from the labeling requirements
✓ Prescription drugs in commercial containers are exempt from the "adequate directions for use" requirement as long as they contain the legend statement:
"Caution: Federal law prohibits dispensing without a prescription." or "Rx only"
✓ When dispensed by a pharmacist, a label containing directions from the prescriber satisfies "adequate directions for use."
Kefauver-Harris Amendment of 1962 (link to FDA discussion of K-F Amendment) aka the “Drug Efficacy Amendment”
Catalyst: Prompted by the thalidomide drug tragedy of the late ’50s in which the drug caused thousands of “flipper babies” (infants with phocomelia)
(Note: Hyperlink to original image source) What this law does:
✓ Established that drugs must be effective

Therefore – Drugs must be SAFE (FDCA) and EFFECTIVE after the passage of the Kefauver-Harris Amendment
✓ Established Current Good Manufacturing Practices (CGMP)
✓ Switched jurisdiction for Rx drug advertising from the FTC → FDA
✓ Added more extensive controls for clinical investigations by requiring informed consent of research subjects
✓ Added requirement for adverse drug event (ADRs and ADEs) reporting (Example: Medwatch, FDA Adverse Event Reporting System (FAERS), Consumer Medication Errors Reporting Program (ISMP C-MERP))
✓ DESI Drugs of 1938 - 1962 (drugs that were deemed safe, but not effective) and are to be reviewed for efficacy
Current information (https://www.fda.gov/drugs/enforcement-activities-fda/drugefficacy-study-implementation-desi) states there are approximately 11 chemical entities/combinations with open DESI hearings. Here is a sampling:
▪ Donnatal Tablets (Phenobarbital, Hyoscyamine Sulfate, Atropine Sulfate, Scopolamine Hydrobromide) used for IBS
▪ Librax (chlordiazepoxide HCl and clindinium bromide)
Actions of the National Academies of Sciences/National Research Council (NAS/NRC) review committees are published in the Federal Register
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Medical Device Amendment of 1976 (Hyperlinked to FDA website explanation)
Background:
Before this amendment, the FDA had little authority over devices
What the law does:
✓ Classification of devices according to their function
✓ Premarket approval and testing
✓ Establishment of performance standards
✓ Conformance with GMP regulations
✓ Adherence to record and reporting requirements
Classification (Based on risk)
Examples of Types of Devices (Lowest (I) to highest (III))
Class I Needles, scissors, examination gloves, stethoscopes, toothbrushes
Class II Insulin syringes, infusion pumps, thermometers, diagnostic reagents, tampons, electric heating pads
Class III Pacemakers, soft contact lenses, replacement heart valves and all “new” devices regardless of classification
FYI: Looking for more information on the medical device approval process (not going to be tested on) –check out this website and infographic: https://library.ccny.cuny.edu/c.php?g=346739&p=2337102
More on this later. Probably the most important law relating to the dispensing of prescription medications and the operation of a pharmacy.
Orphan Drug Act of 1983,
Drug Price Competition and Patent Term Restoration Act of 1984, & Prescription Drug Marketing Act of 1987
These three acts impacted pharmaceutical manufacturers to a great extent.
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Orphan Drug Act of 1983 (hyperlinked article)
✓ Provides tax and exclusive licensing incentives for the treatment of “rare diseases or conditions” (those affecting fewer than 200,000 Americans).
✓ Currently, 855 drugs approved under the ODA
✓ Selection of drugs approved under the Orphan Drug Act (to find others go to https://www.accessdata.fda.gov/scripts/opdlisting/oopd/listResult.cfm
bedaquiline (Sirturo by Jansen) – drug-resistant TB
Emflaza (deflazacort by Marathon Pharmaceutical) – Duchene muscular dystrophy
Emtricitabine and Tenofovir alafenamide (Descovey by Gilead) for HIV-1 in adults and pediatric patients
Example of what you would see on the FDA Orphan Drug Database search for deflazacort (EMFLAZA)
Deflazacort Tx of Duchenne muscular dystrophy 08/16/2013 02/09/2017 02/09/2024 Tx of Duchenne Muscular Dystrophy in patients 5 years of age or older Tx of Duchenne Muscular Dystrophy patients 5 years of age or older
Deflazacort Tx of Duchenne muscular dystrophy 08/16/2013 06/07/2019 06/07/2026 Tx of Duchenne muscular dystrophy (DMD) in patients 2 years of age & older
For the tx of Duchenne muscular dystrophy in patients 2 years of age to less than 5 years of age
Drug Price Competition and Patent Term Restoration Act of 1984
Will talk about this one in detail in lecture 3.
Prescription Drug Marketing Act of 1987 (hyperlinked to FDA information) aka “Dingle Bill” or PDMA
Background:
Reasons for the passage of this legislation
✓ Protection of public health
✓ Secondary wholesale distribution schemes
✓ Unfair competition
✓ Re-importation of prescription drugs
✓ Distribution of Rx samples
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ States must license drug wholesalers
✓ Banned the re-importation of drugs, except by the manufacturer
✓ Banned the sale, trade, purchase of prescription drug samples
✓ Set standards for distribution, storage, records of prescription drug samples
No pharmacy can distribute samples
Exception to the rule: hospital or healthcare entity pharmacy where a practitioner authorizes the sample and adequate records are kept
✓ Banned trafficking in prescription drug coupons
✓ Prohibited the resale of drugs purchased by hospitals or health care facilities
Defined as: a unit of drug intended not to be sold but used to promote sales
Before PDMA, a federal study determined that:
Too many samples were being distributed
Inadequate records were being kept
Samples were being improperly stored by sales reps and health care providers
ENFORCEMENT OF PDMA
✓ Enforced by state boards of pharmacy and the FDA

✓ Consequences for pharmacists and community pharmacies if they break this federal law
Up to 10 years imprisonment and/or $250,000 fine
FDA Guidance document on drug samples:
https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/UCM342665.pdf
ASE EXAMPLES:
1. Sally Ride is the pharmacy manager for ABC Pharmacy which is located in a small rural town in Northern Arizona. There are five physicians located within a one block radius of ABC Pharmacy. They have asked Sally if she would maintain and dispense the drug samples in the pharmacy that the physicians acquire from the drug reps. Is this legal for Sally to do? If yes, what has to occur?
2. Let’s change the location. Denzel Washington is the pharmacy manager for Hilltop Community Hospital Pharmacy. The Medical Director and the staff physicians have asked Denzel if he would maintain and dispense the drug samples in the pharmacy that the physicians acquire from the drug reps. Is this legal for Denzel to do? If yes, what has to occur?
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Supplement Health and Education Act of 1994 (hyperlink to FDA explanation)
What this law does:
✓ Provided a new definition of “dietary supplement.”
✓ A dietary supplement is a product intended for ingestion, is intended to supplement the diet, and contains any one or more of the following:
A vitamin
A mineral
An herb or other botanical
An amino acid
A dietary substance for use by humans to supplement the diet by increasing the total dietary intake
A concentrate, metabolite, constituent, extract, or combination of the previous
✓ 4 claims that dietary supplement suppliers are allowed to make under DSHEA:
✓ A benefit relating to a classic nutrient deficiency disease; must disclose prevalence of the disease in the US
✓ Describe how a product affects any part of the structure of the human body, (e.g., "Glucosamine helps promote connective tissue"), or any function of the human body (e.g., “Echinacea helps support the immune system");
✓ Characterize the mechanism of action by which a product acts to maintain body structure or function;
✓ Describers the general well-being from consumption
✓ Even if an item is called a supplement, it may be considered a drug by the FDA if there are drug claims in the item's promotion (i.e., cure, treatment and/or prevention claims are made)
✓ The product must contain the disclaimer on the label, in regards to any statements about the product.
This product has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
Unfortunately, this act may blur the line between foods and drugs; and may complicate any of the FDA's enforcement over dietary supplements.
✓ Stripped FDA of premarket approval authority
✓ Federal Trade Commission (FTC) has ramped up actions against dietary supplement manufacturers and homeopathic manufacturers over the past 10 – 15 years (see list of actions at https://www.ftc.gov/news-events/media-resources/truth-advertising/health-claims).
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Food and Drug Administration Modernization Act 1997 (hyperlinked to FDA info)
What this law does:
✓ FDAMA was passed primarily to streamline regulatory procedures at the FDA
✓ Extended the FDA’s authority over over-the-counter (OTC) drugs and new labeling requirements
✓ Created a fast-track approval process for drugs treating serious or life-threatening illnesses
✓ Created databank of information on clinical trials (see www.clinicaltrials.gov)
✓ Expanded rights of manufacturers to disseminate off-label use information
Food and Drug Administration Amendments Act of 2007 (FDAAA 2007) (hyperlinked to FDA info)
What this law does:
✓ FDAAA reauthorized and amended many of the drug and medical device provisions that were set to expire
✓ Increased funding for the FDA
✓ Enhanced the FDA’s responsibilities and authority to regulate drug safety
Can require post-market clinical studies to assess risk
Can require manufacturers to implement Risk Evaluation and Mitigation Strategies (REMS)
Poison Prevention Packaging Act of 1970 (PPPA) (hyperlinked to CPSC)
Enforcement Agency:
✓ Consumer Product Safety Commission (CPSC)
What this law does:
✓ Protect children from accidental poisonings with “household substances ”
A hazardous substance in the Federal Hazardous Substances Act

An economic poison under the Federal Insecticide, Fungicide, and Rodenticide Act
A household fuel when stored in a portable container
A food, drug or cosmetic under the FDCA
▪ Prescription Drugs – Specifically those for human ORAL use and others as deemed necessary by the CPSC.
Many household substances were impacted, and some were banned. Those not banned are required to be in special “child-resistant packaging," including many OTC medications. (See pages 144-145 for list).
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
DRUGS AND DRUG CATEGORIES COVERED BY THE PPPA
✓ Human ORAL prescription drugs
✓ Controlled drugs
✓ Drugs switched from Rx to OTC status
✓ Aspirin and acetaminophen
✓ Methyl salicylate
✓ Iron-containing drugs and dietary supplements
✓ Lidocaine, dibucaine, and minoxidil
Defined as: 80% of children less than 5 years old CANNOT open it, but 90% of adults CAN open it

PPPA AND IMPACT ON OTC DRUGS
ALL ORAL HUMAN prescription drugs, controlled substances, and OTC drugs MUST be packaged in childresistant containers (prescription bottles, and non-prescription packaging)
Note: There are exceptions to the rule (see below and next page)
Exemptions/Exceptions for OTCs and Child-resistant Packaging (PPPA)

One (1) package size of an OTC product for the elderly or handicapped may be in non-compliant packaging (aka non-child-resistant packaging)
Packaging must contain the following statement:
This Package for Households Without Young Children”
or if the label is too small, it may contain “Package Not Child-Resistant.”
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
An example of how the McNeil company might do this for its Tylenol Products:
Here are 4 different Tylenol products
✓ One size of each of these Tylenol products may have one (1) package size (any size) that is in non-child-resistant packaging
✓ What might this look like? (Package sizes are not what McNeil has actually done)

Here are 4 different Tylenol products
One size of each of these Tylenol products may have one (1) package size (any size) that is in non-childresistant packaging
What might this look like?
Tylenol 8 hour – 100 count bottle
Tylenol Rapid Release Gels –250 count bottle
Tylenol Regular Strength Liquid Gels – 100 count bottle
Tylenol Extra Strength Tablets – 250 count bottle
PPPA AND IMPACT ON PRESCRIPTIONS DISPENSED FROM A PHARMACY
✓ Child resistant containers MUST not be reused
Exception: glass or threaded plastic containers; if the container is reused it MUST be dispensed with new safety closure
Threaded vials

Non-threaded vials

©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ Individual patients may make a blanket request that all of their prescriptions be filled in non-child-resistant containers
✓ Prescribers may request that a single prescription order be in non-safe packaging (non-child-resistant), but they CANNOT make a blanket request



Exemption: Drugs dispensed and administered to institutionalized patients
Hospital, long-term care, nursing home, rehab facility, etc.
Exceptions for specific legend and controlled substances from the PPPA
Sublingual nitroglycerin tablets
Cyclically administered oral contraceptives, conjugated estrogens, and norethindrone acetate tablets in manufacturer’s memory-aid (mnemonic) dispenser packages
Preparations in aerosol containers intended for inhalation therapy
Methylprednisolone tablets containing not more than 84 mg per package (e.g.., Medrol ®Dosepak)
Potassium supplements in unit dose forms, including effervescent tablets, unit dose vials of liquid potassium, and powdered potassium in unit dose packets containing no more than 50 mEq/unit dose
(See Abood, Burns, and Frankhauser for a complete list of exceptions/exemptions)
Pharmacies often use alcohol in compounding (e.g. rubbing alcohol, ethyl alcohol, and isopropyl alcohol). Community pharmacies CANNOT legally obtain tax-free alcohol; must use federally taxed alcohol.
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
OBRA '90 (hyperlinked to US Pharmacist article)
aka Omnibus Reconciliation Act of 1990
Background:
Congress believed that improving drug therapy could reduce health care costs
Until this law took effect, no federal law had affected the counseling standards of pharmacists
What this law does:
✓ Requires pharmacists to provide Drug Utilization Review (offer to counseling and other requirements for Medicaid patients)
✓ Funded studies to measure the effectiveness of pharmacist-provided DUR
✓ Manufacturer rebates to Medicaid
** More on OBRA ’90 in the Dispensing Medications and Caring for Patients lecture **
State Statutes
Arizona Pharmacy Practice Act (ARS Title 32 – Chapter 18)
Uniform Controlled Substances Act (ARS Title 36 – Chapter 27)
Controlled Substance Prescription Monitoring Program (ARS Title 36 – Chapter 28)
Community wants regulation for:
✓ Public safety
✓ Decreased health care costs
✓ Quality healthcare
✓ Inhibiting the criminal abuse of drugs
State Regulations
Arizona Administrative Code (AAC) Title 4 –Chapter 23 (Referred to as AAC; R4-23-XXX)
Pharmacists want regulation for:
✓ Job security
✓ Organization at work
✓ Limits competition
✓ Ability to charge for pharmaceutical services
✓ Enforcement agency for the FDCA & amendments
✓ Establishes and enforces Current Good Manufacturing Practices (CGMP)
✓ Sets standards for label and labeling of OTC and prescription medications
✓ Creates OTC monographs
✓ Approves patient package inserts, MedGuides, and REMS (Risk Evaluation and Mitigation Strategies)
✓ Authority to seize misbranded or adulterated drugs (or for other reasons determined by law)
✓ Authority to inspect facilities where food, drugs, medical devices, or cosmetics are made or processed
✓ Authority to perform limited inspections of pharmacies in certain circumstances
✓ Regulates prescription drug advertising
FDA is the federal agency responsible for ensuring that foods are safe, wholesome and sanitary; human and veterinary drugs, biological products, and medical devices are safe and effective; cosmetics are safe; and electronic products that emit radiation are safe. FDA also ensures that these products are honestly, accurately and informatively represented to the public. Some of the agency's specific responsibilities include: Biologicals
product and manufacturing establishment licensing
safety of the nation's blood supply research to establish product standards and develop improved testing methods
safety
labeling
product approvals OTC and prescription drug labeling drug manufacturing standards
labeling
safety of all food products (except meat and poultry)
bottled water
37 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
premarket approval of new devices
manufacturing and performance standards
tracking reports of device malfunctioning and serious adverse reactions
livestock feeds
pet foods
veterinary drugs and devices
radiation safety performance standards for microwave ovens, television receivers, diagnostic x-ray equipment, cabinet x-ray systems (such as baggage x-rays at airports), laser products, ultrasonic therapy equipment, mercury vapor lamps, and sunlamps, cell phones
accrediting and inspecting mammography facilities
FDA Inspections (hyperlinked to FDA Inspections database and other information)
FDA inspectors DO NOT NEED a warrant to perform an inspection during business hours
They DO NOT NEED to state the reason for the inspection
The FDA rarely inspects pharmacies. Most states operate under a "Memorandum of Understanding" with the FDA
✓ Two Arizona Board of Pharmacy employees are credentialed as commissioned officers of the FDA, allowing the FDA to share secure information with them
✓ State Board Inspectors, like FDA agents, do not need a warrant to perform an inspection during business hours and they don't have to give a reason for inspection
Administrative agency for the Federal Trade Commission Act
✓ Regulates OTC drug advertising
✓ Enforces unfair business practices and anti-trust violations
Established by the Controlled Substances Act (will talk about later with Controlled Substances)
✓ Regulatory and enforcement agency
✓ Operates under the jurisdiction of the U.S. Department of Justice (DOJ) and the US Attorney General
38 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Established by the 1965 Medicare Act
✓ Responsible for rules and regulations pertaining to Medicare and Medicaid
✓ Housed in the U.S. Department of Health and Human Services
Enabling Legislation: Arizona Pharmacy Practice Act
The purpose of the Board:
• 9 members make up the ASBP
o 1 pharmacy technician
o 6 pharmacists
▪ 1 of the 6 must be a hospital pharmacist.
▪ 1 of the 6 must be a community pharmacist.
o 2 consumers (lay public)
• Terms (length of appointment) for Board Members
o 5-year terms
o May serve maximum of 2 consecutive terms; need to be off of ASBP for one (1) 5-year term and then may be re-appointed.
• Governor of the state (in this case AZ) appoints the members of the board
Requirements to be on the ASBP
✓ Practicing pharmacy technician in AZ or other jurisdiction for at least 5 years
✓ Licensed as a pharmacy technician and resident of AZ for at least 5 years
✓ Licensed for at least 10 years (AZ or other jurisdictions)
✓ Licensed and resident of AZ for at least 5 years
✓ A resident of AZ for at least 5 years
• Residency must be the 5 years immediately prior to appointment
ASBP Staff
Executive Director – Kam Gandhi
Administrative employees
Pharmacy inspectors (aka "compliance officers")
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Roles of the ASBP (ARS 32-1904 and others) responsibility of ASBP)
1. Licenses and Permits
✓ Pharmacists
✓ Technicians
✓ Interns
✓ Pharmacies
✓ Manufacturers
✓ Wholesalers
✓ Non-resident pharmacies, etc. (see article 2 of the Pharmacy Practice Act)
2. Establishing standards of practice
✓ Define pharmacy practice
✓ Collaborative practice agreements
✓ Mandatory patient counseling
✓ Patient medication records
3. Investigations and disciplinary action against license and permit holders (see discussion below)
✓ Will investigate patient complaints
✓ Discipline license and permit holders based on complaints
Roles of the ASBP with other state agencies
1. Regulation of hospital pharmacies
2. Regulation of long-term care facilities (LTCF)
3. Regulation of outcomes
4. Regulation of third-party plans
With the AZ Department of Health Services (ADHS)
With the AZ Department of Insurance
Disciplinary Action Process (ARS 32-1927, ARS 32-1928)
May Discipline:
• License Holders (Pharmacists, technicians, and interns)
• Permittees (Pharmacies, wholesalers, etc.,)
Discplinary actions are taken to:
• Prevent similar problems in the future; educate about the mistake
• Punish the mistake
40 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
1
•Complaint is filed by the patient or other interested party about a pharmacist/pharmacy/technician or board initiates investigation based on other information
•Board contacts the person filing the complaint
•Was anyone hurt?
2
3
4
5
•Board contacts the licensee/permittee alerting them to the active complaint
•Licensee/permittee must prepare a written response to the complaint
•Must be appropriate, professional, and timely
•Compliance inspector asks follow-up questions
•Prepares written summary of what he or she found from the complaint
6
•3 person subcommittee (1 consumer, 1 pharmacy technician, and 1 pharmacist)
•Reads inspector's summary and makes recommendations to the board
•Summation: no action, probation, revokation, etc.
•"Notice of hearing" is sent to the pharmacist
•Only sent if recommendation is more than a "no action" or a warning letter
7
8
9
•Board deliberates; reviews penalty decisions
•President of board is considered the "Judge"
•Board has a lawyer from the State District Attorney's Office
•Decision is given to pharmacist in question
•If the licensee/permittee feels the disciplinary action is undue, they can appeal to a higher court. In AZ this would be_________________________
✓ A license may be summarily suspended pending a hearing if:
✓ the board has reasonable grounds to believe and finds that the licensee (RPh, Intern, or Tech) has been guilty of deliberate and willful violations, or that the public health, safety, and welfare requires immediate action and
✓ Incorporates findings (evidence/information) to that effect in its order (the summary suspension)
✓ Board shall serve the licensee with written notice of the complaint and charges and inform the licensee of their right to a hearing.
✓ Hearing must occur within 10 days of ordering the summary suspension.
In Arizona, pharmacies are inspected annually
✓ Look for areas in which education is needed
✓ Look for outdated drugs
Warrantless inspections allowed under Arizona State Law
✓ Federal courts have viewed this as permissible
✓ All a board member needs to come in the pharmacy is a Board of Pharmacy Badge
Records must be readily retrievable (Prescription orders, invoices, other records as requested)
✓ Within 72 hours of a request by ASBP (ARS 32-1968; ARS 36-2525; AAC R4-23-407(A); AAC R423-408)
✓ The board of pharmacy produces a quarterly newsletter that updates the pharmacy community on the following: available on the board website – https://pharmacy.az.gov/
✓ New drug information
✓ Providers who lost their prescription writing privileges
✓ Recent law changes and updates
✓ Recent impaired pharmacists and prescribers
Impaired from drugs, alcohol, or mental condition
Practicing privileges were taken away
✓ AZ board established the PAPA program
✓ For impaired pharmacists
✓ Those who enter voluntarily are not subject to discipline from the board
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
And they are not identified on the monthly newsletter
✓ Those who are forced to enter into PAPA are subject to board discipline
Must relinquish their pharmacist license
Name is listed in the newsletter
✓ Board sets forth Practice Regulations
✓ Established generic substitution rules
✓ People have the freedom of choice to go to any drugstore they choose to, or go to "any willing provider"
✓ Adopted and upholds OBRA '90
✓ Board has very limited rules on hospital pharmacies
✓ State Board of Health has this responsibility
After attending the lecture and completing the assigned readings, the student should be able to:
✓ Define “new drug,” “reference listed drug,” “generic drug,” “authorized generic,” label, labeling
✓ Identify the official compendia (as identified in the FDCA), if they are public or private organizations, how they are recognized as the legal source of drug standards and what they establish.
✓ Identify and differentiate between the following: food vs. special dietary foods vs. medical foods.
✓ Describe the drug approval process and what occurs during each stage (pre-clinical, clinical, NDA review, and post-market).
✓ Describe the difference between an IND, NDA, SNDA, ANDA
✓ Explain which law allowed the treatment of patients with investigational drugs fast-track approval. Explain the purpose of the “fast-track” approval process What is the requirement of the manufacturer?
✓ Explain market exclusivity and its controversies.
✓ Identify and describe the three ways that a prescription drug can be switched from a prescription to an OTC.
✓ Describe and differentiate the two methods that an OTC can go to market.
✓ Describe the role of post-marketing surveillance and who is responsible
✓ Identify and describe the purpose of REMS; identify and differentiate the 4 types of REMS actions/activities.
✓ Differentiate between the label and labeling requirements for prescription and OTC drugs (including the intended user).
✓ Identify what an NDC number is, what it indicates, and which law requires it to be on the label and bar code requirements
✓ Describe the Federal Anti-Tampering Act
✓ Describe in general what Current Good Manufacturing Practices (CGMP) are and who must abide by them, and who is exempt from the rules.
✓ Describe product recalls, who may initiate them, what authority the FDA does and doesn’t have, the classifications of drug recalls and the manufacturers’ and pharmacies/pharmacists’ responsibilities, and other actions the FDA may take.
✓ Describe why pharmacies are exempt from being registered as manufacturers; describe and identify the two titles for the Drug Quality and Security Act, their purposes, what they do, who they affect, and what are the components/requirements and time frame retention if applicable.

✓ Describe the difference between advertising and labeling, requirements of drug advertising, advertising to medical professionals, the legality of off-label use and the promotion of “off-label use,” and direct-to-consumer advertising
Abood RR, Burns KA, Frankhauser F Pharmacy Practice and the Law, 10th ed. Sudbury, MA: Jones and Bartlett; 2025
Chapter 2
✓ Defining and Distinguishing Drugs from Foods, Dietary Supplements, Devices, and Cosmetics (p. e39 - 52)
✓ Product Recalls (p. e54)
✓ New Drug Approval (p. e66 - e79)
✓ Marketed Unapproved Drugs (p. e80)
✓ Drugs Intended to Treat Serious and Life-Threatening Diseases (p. e81 – e83)
✓ Biologics, Medical Devices, and Cosmetics (p. e83 - e84)
✓ Drug Advertising and Promotion (p. e90 – e100)
Chapter 3:
✓ Switch of Prescription Drugs to Over-the-Counter Drugs (p. 109 - 111);
✓ Pharmacy Compounding Versus Manufacturing & DQSA Title I (p. e129 - 131);
✓ Drug Supply Chain Security & Prescription Drug Marketing Act of 1987 (p. e132 – e139);
✓ Drug Advertising by Pharmacies (p. e145 - 148)
Drugs – FDCA Definition (21 US Code § 321(g))
“Drug” = (A) articles recognized in the official United States Pharmacopoeia, official Homeopathic Pharmacopoeia of the United States, or official National Formulary, or any supplement to any of them; and
(B) articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man or other animals; and
(C) articles (other than food) intended to affect the structure or any function of the body of man or other animals; and
(D) articles intended for use as a component of any articles specified in clause (A), (B), or (C).

Recognized by the FDA as a legal source of drug standards
1. Establish quality standards for drugs and related items
2. Establish standards for storage and packaging of drugs
1. United States Pharmacopeia and National Formulary
a. USP- monographs for drugs, drug products, dosage form, and compounded preparations
b. NF - Monographs for expicients
2. HPUSP - Monographs for homeopathic drugs and ingredients
United States Pharmacopeia and National Formulary (USP/NF)
Homeopathic Pharmcopeia of the United States (HPUS)
Private organizations have developed these compendia; they are recognized as the legal source of drug standards in the FDCA.
Monographs from the USP/NF
Diclofenac Acid (USP Monograph) Anhydrous Lactose (NF Monograph)



Diclofenac Sodium Delayed-Release Tablets, USP 75 mg

Carbamazepine Tablets, USP 200mg

Food (21 USC § 321(f))
“Food” =
(1) articles used for food or drink for man or other animals,
(2) chewing gum, and
(3) articles used for components of any such article
Manufacturers place USP information on the drug manufacturer label, such as:
• Temperature
• Protect from moisture.
• Dispense in a tight, light-resistant container
1. The purple box contains USP information.
2. Notice the “Meets USP Dissolution Test 2” information is also included
3. The USP reference with the generic (established) name indicates that it should meet the USP standards.

Special Dietary Foods (see textbook and 21 CFR § 105.3 for definitions)
Include but are not limited to:
✓ Supplying special dietary needs that exist by reason of physical, physiological, pathological, or other conditions

Medical Foods = “a food which is formulated to be consumed (orally) or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” (21
2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Includes:
• Infant formula
• Artificial sweeteners
• Caloric supplements

Nutritional Labeling and Education Act and FDAMA allowed the FDA to approve certain health claims for foods based on guidelines and petitions from the supplier that supports the claim.
(See FDA FAQ on Qualified Health Claims for Food: https://www.fda.gov/food/foodlabeling-nutrition/questions-and-answershealth-claims-food-labeling)
U.S.C. 360ee(b)(3) (Note – a prescription order is not required)
Includes:
• Used under medical supervision
• Special dietary management of a disease or condition
o Food without phenylalanine for phenylketonuria


There are two types of health food claims to receive either, the manufacturer must petition the FDA for approval and receive that approval before advertising any such claim.
Authorized claims
• Significant scientific agreement among qualified experts for the substance/disease relationship
Qualified health claims
• Example: “Diets low in sodium may reduce the risk of high blood pressure…”
• Other authorized health claims:
• Calcium, Vitamin D, and Osteoporosis
• Dietary Lipids (Fat) and Cancer
• Dietary Saturated Fat and Cholesterol and Risk of Coronary Heart Disease
• Folic Acid and Neural Tube Defects

Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Dietary supplements = a product taken by mouth that contains a "dietary ingredient" intended to supplement the diet. The "dietary ingredients" in these products may include:
• vitamins,
• minerals,
• herbs or other botanicals,
• amino acids, and
• substances such as enzymes, organ tissues, glandulars, and metabolites.
Reminder: DSHEA allows manufacturers to make four (4) types of claims for dietary supplements (see Lecture 2).
✓ Provision of educational material and other information regarding dietary supplements is allowed.
✓ The location of these materials is restricted by DSHEA
o Educational materials and/or publications must be kept physically separate from the dietary supplements (different locations)
✓ Devices defined and regulated by FDA with the passage of the Medical Device Amendment to the FDCA.
✓ Device used in conjunction with a drug – legal distinction between the two blurs.
Examples:
✓ Medicated stents for angioplasty – debate, still currently regulated as a device.
✓ Barium sulfate as a radiological contrast – originally approved as a device and now is viewed and approved as a drug.
Cosmetics = by their intended use, as "articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body...for cleansing, beautifying, promoting attractiveness, or altering the appearance" [FD&C Act, sec. 201(i)].
Among the products included in this definition are:
• skin moisturizers,
• perfumes,
• lipsticks,

• fingernail polishes,
• eye and facial makeup preparations,
• cleansing shampoos,
Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
• permanent waves,
• hair colors, and
• deodorants, as well as
• any substance intended for use as a component of a cosmetic product.
There is a fine line between cosmetics and drugs based on new technology and formulations - Think cosmeceuticals.
✓ FDA warning letter to Lancôme: http://wayback.archiveit.org/7993/20161022071552/http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012 /ucm318809.htm. Note: the FTC also took action as well: https://www.ftc.gov/news-events/pressreleases/2014/06/loreal-settles-ftc-charges-alleging-deceptive-advertising-anti
✓ FDA warning letter to Peter Thomas Roth: https://www.fda.gov/inspections-complianceenforcement-and-criminal-investigations/warning-letters/peter-thomas-roth-labs-llc-49598409182017
✓ List of companies sent warning letters across categories: https://www.fda.gov/cosmetics/warningletters-related-cosmetics/warning-letters-address-drug-claims-made-products-marketed-cosmetics
Most of us use the term "new drug" in conversation to describe a completely new active ingredient. However, FDA approval of an active ingredient in one drug product does not mean it is approved for another drug product. Almost any difference from something already on the market, no matter how slight, can require a drug product to first gain FDA approval. This includes different or novel combinations of drugs, new dosage forms, new indications, etc...
New active ingredient
New combination of drugs
A “new drug” is one that is not generally recognized by qualified experts as safe and effective for use under the given conditions.
Proportion of ingredients in combination changed

Zavzpret® (zavegepant) is a calcitonin gene-related peptide receptor antagonist indicated for acute treatment of migraines with or without aura in adults.
Airsupra® (albuterol sulfate; budesonide) for the as-needed treatment or prevention of bronchoconstriction and to reduce the risks of exacerbations in patients with asthma 18 or older
Vicodin® (hydrocodone/acetaminophen). Was HC 5 mg/APAP 500 mg → HC 5 mg/APAP 300 mg
2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
New intended use of drug Ozempic® (semaglutide) new indication for reducing the risk of major adverse cardiovascular event in adults with Type 2 diabetes and known heart disease. Wegovy® (semaglutide) new patient population, new indication for weight loss
*Note – The two brands are the same and sold by Novo Nordisk.
New dosage, method, or duration of administration or application
Pre-1938 drugs may be determined to be a “new drug”
Valtoco® (diazepam nasal spray – CIV) is a new dosage form for use in the treatment of acute seizures
Levothyroxine products. Levoxyl (King Pharmaceuticals; a subsidiary of Pfizer) Applied for NDA in 2001; Synthroid (Abbott) was the last to apply for an NDA in 2002
No new drug can be legally marketed in interstate commerce without first receiving approval by the FDA.
The approval process requires human and animal testing to demonstrate the safety and efficacy of the new drug product.

Molzon, J. The common technical document: the changing face of the new drug application. Nature Reviews Drug Discovery. 2003;2(1):71-74.

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Investigational New Drug Application (IND) – What is an IND and what is required for an IND application?
Primary Purpose of IND: Protection of human subjects in clinical trials
Requirements for the IND:
✓ Name of drug
✓ Composition
✓ Methods of manufacturing and quality control
✓ Information from preclinical investigations regarding pharmacological, pharmacokinetic, and toxicological evaluations
An IND is submitted for FDA approval to begin clinical trials in humans. Provides evidence that:
✓ The product is reasonably safe to be tested in humans.
✓ The clinical trials are adequately designed to permit an evaluation of the drug's safety and effectiveness.
✓ There are adequate protections of the human subjects, including informed consent.
FDA may terminate the IND at any time if studies show the drug is too toxic
Decision is final and NOT subject to appeal.
Clinical Trial Studies – all require participant informed consent.
PRE-MARKET CLINICAL TRIALS
Phase I – small number of healthy subjects – determines adverse effects.
Phase II – limited number patients with disease – determine efficacy and dosage range.
Phase III – larger numbers of patients with disease (hundreds to thousands); often randomized controlled trials versus placebo and/or drug already on the market.
✓ If Phase III results are favorable, drug's sponsor may submit an NDA to the FDA
POST-MARKET CLINICAL TRIALS
Phase IV – FDA may require a manufacturer to conduct additional studies after a drug's initial approval to assess additional risks.
• Most diabetes drugs are required to do phase 4 cardiovascular studies.

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Submitted for FDA approval to market a new drug. Provides evidence that:
✓ The drug is safe and effective for the listed indications based on data from human clinical trials.
Prescription Drug User Fee Act of 1992 (PDUFA)
✓ Requires a large fee upon submitting an NDA.
✓ Covers the expense of FDA reviewers.
✓ Encourages sponsors to apply only if they have a high probability of success. FDA may withdraw its approval of an NDA if future evidence uncovers unacceptably high risks.
✓ Examples: Vioxx ®, Rezulin ®, etc.
✓ The FDA assigns a priority rating to all new INDs based on chemical type and therapeutic potential.
•1-New molecular entity
•2-New active ingredient
•3-New Dosage form
•4-New combination of compounds
•5-New formulation or new manufacturer
•6-New indication for use
•7-Drug already marketed without an approved NDA
•8-OTC switch
•9-New indication submitted as distinct NDA, consolidated with original NDA
•10-New indication submitted as distinct NDA, NOT consolidated
•P (Priority) – for drugs that represent a significant advance over existing treatment
•No other effect drugs are available
•It is more effective or safe than drugs currently used
•It has important advantages in special populations
•S (Standard) – for drugs similar to available drugs (aka “Me too drugs”
•O (Orphan) – drug treats a rare disease affecting fewer than 200,000 Americans
✓ FDA has 180 days to rule on a submitted NDA.
✓ Delays past the 180-day limit are normal, but litigation would be self-defeating and is therefore uncommon.
The following sections of FDAMA (Food and Drug Administration Modernization Act) allowed for drugs to be used for treatment that were still in the investigational stages and/or fast tracked the approval process.

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
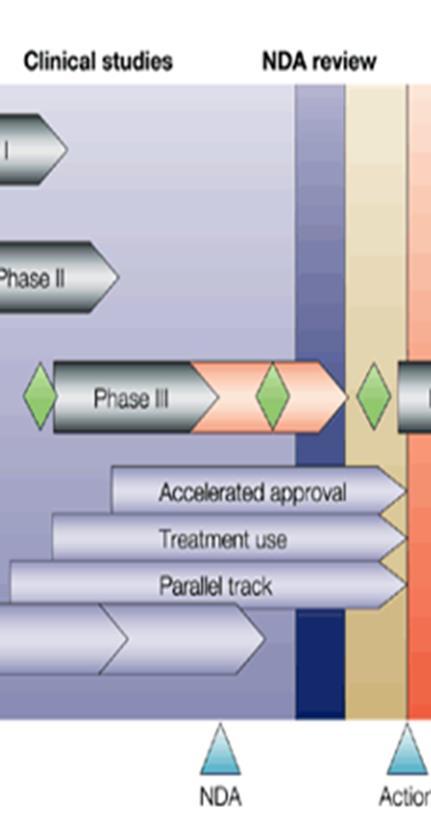
Expedited Approval of Drugs Intended to Treat Life-Threatening Illnesses (“Fast Track Approval” ~ Accelerated Approval) (§506)
▪ The “Fast Track Approval” was passed to expedite the development, evaluation, and marketing of new drugs intended to treat serious or life-threatening illnesses.
▪ Ex: REMDESIVIR by Gilead Sciences Inc
Widespread Patient Treatment with Investigational Drugs (Treatment Use IND's ~ Treatment Use) (§561)
▪ Approval to treat several patients.
Individual Patient Access to Investigational Drugs for Serious Diseases ("Parallel Track Policy") (§561)
▪ Approval to treat an individual patient.
▪ Approval is conditional upon completion of postmarketing (Phase IV) clinical studies.
Initial patents for drugs: 20 years from date patent is granted (previously was 17 years)
Occurs during the IND or phase 1 of the clinical trial phase of the NDA.
Patents = awarded by the US Patent and Trademark Office
Exclusivity = exclusive marketing rights granted by the FDA upon approval of a drug.
Exclusivity is a period of time when a brand-name drug is protected from generic drug competition. There are different exclusivities for different situations. Exclusivity is designed to promote a balance between new drug innovation and generic drug competition.
• Exclusivity and a patent may or may not run concurrently.
• See FDA Exclusivity and Generic Drugs: What Does It Mean? Infographic or see the link in the lecture section on Canvas. Also https://www.fda.gov/drugs/development-approval-processdrugs/frequently-asked-questions-patents-and-exclusivity for more detail
PTRA did the following:
▪ Created the ANDA (Abbreviated New Drug Application) which is used for generic drug approvals (more discussion below).
▪ Allows the FDA to grant innovator drugs patent-term extensions.
o Only are given if the patent has not expired.

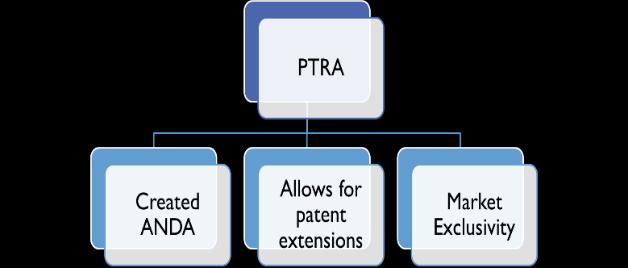
Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
▪ Provides market exclusivity.
o Market exclusivity prevents generic drug applications from being submitted and granted until after exclusivity expires.
o Exclusivity is granted at the time the NDA is approved.
TYPES OF MARKET EXCLUSIVITY
(for ANDAs
CONTROVERSIES
✓ Payments made by innovator (brand) manufacturers to generic manufacturers to prevent or postpone the ANDA from a generic manufacturer.
o “Pay-to-delay” or “reverse payments.”
✓ Product hopping is when the brand manufacturer tactically reformulates the drug and repatents the new formulation.
o Ambien ® → Ambien CR ®
✓ Abuse of the patent system, including reformulations to extend patent life.
o Humira® (AbbVie current owner)
Normally, after approval, a manufacturer cannot make any changes to the drug, its production, or its labeling.
✓ Submitted to change or update information regarding an already approved drug.
✓ Commonly used to add additional indications.
✓ Also used for the Rx-to-OTC switch

"Reference listed drug" (aka “innovator drug”) = an approved drug product to which new generic versions are compared to show that they are bioequivalent.
A drug company seeking approval to market a generic equivalent must refer to a single Reference Listed Drug (RLD) in its Abbreviated New Drug Application (ANDA). The RLD serves as the standard to which the generic must be shown to be bioequivalent. By doing this, the FDA hopes to avoid possible significant variations among generic drugs and their brand name counterpart.
"Generic drug" = A generic drug is the same as a brand name drug in:
• dosage,
• safety,
• strength,
• how it is taken,
• quality,
• performance,
• and intended use.
Before approving a generic drug product, FDA requires many rigorous tests and procedures to assure that the generic drug can be substituted for the brand name drug. The FDA bases evaluations of substitutability, or "therapeutic equivalence," of generic drugs on scientific evaluations. By law, a generic drug product must contain identical amounts of the same active ingredient(s) as the brand name product. Drug products evaluated as "therapeutically equivalent" can be expected to have equal effect and no difference when substituted for the brand name product.
Source: Drugs@FDA Glossary of Terms available at http://www.fda.gov/Drugs/informationondrugs/ucm079436.htm
EXAMPLE: OZEMPIC

EXAMPLE: AUGMENTIN


Bioequivalence – (will cover more in filling prescriptions and caring for patients lecture)
Generic equivalent may vary in rate and extent of absorption by ±20 percent from the reference listed drug (https://www.fda.gov/downloads/drugs/guidances/ucm070244.pdf)
In reality, the average difference is much less
1. ABBREVIATED NEW DRUG APPLICATION (ANDA) (I.E. A GENERIC DRUG APPLICATION)
Not required to prove safety and effectiveness through clinical studies. This is why generics are cheaper.
Must prove bioequivalence to the innovator drug (RLD).
FOUR TYPES OF ANDA APPLICATIONS MADE BY GENERIC MANUFACTURERS
1. NDA holder did not file information on the patent to the FDA.
2. Patent has expired.
3. Patent date of expiration (goal – be 1st in line with ANDA for when innovator patent expires)
4. Patent is invalid or will not be infringed by the manufacture, use, or sale of the generic applicant's drug.
✓ Applicant must notify the patent owner.
✓ If the patent owner sues, the FDA is prevented from approving the ANDA until either 30 months passed or the court rules on the issue.
✓ The first approved Paragraph IV ANDA for a given drug awards the applicant 180 days of market exclusivity.
The manufacturer of an innovator drug may produce a generic version of its drug without submitting an ANDA.
This gives the manufacturer of the innovator drug a way to compete with the 180-day market exclusivity a generic manufacturer receives upon successfully filing a Paragraph IV ANDA
✓ Ex: Advair Diskus 100/50 (GlaxoSmithKline) NDC # 00173-0695-00; authorized generic Fluticasone Propionate/Salmeterol 100/50 (Prasco) NDC 66993-584-97 (manufactured by GSK and distributed by Prasco)

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy

✓ Wixela and the generic by Prasco were allowed to be sold at the same time because the Prasco version was the authorized generic for GSK.
✓ No other generics were allowed to be sold during the 180-day exclusivity period.

Wixela (Mylan)
1st generic with 180-day exclusivity

There are 3 ways that over-the-counter drugs can get to market:
✓ Rx-to-OTC Switch
✓ OTC monograph
✓ New Drug Application (NDA)
Three ways to switch a drug from Rx to OTC:
1. The manufacturer may request the switch by submitting a supplemental application (SNDA) to its approved NDA.
2. The manufacturer or other entity may petition the FDA to add or amend an OTC monograph.
a. Ex: Citizen petition – caused Rx-to-0TC switch for Claritin, Allegra, and Zyrtec (Link)
3. The FDA may add or amend an OTC monograph through OTC drug review.

Comparison of the OTC NDA Process and the OTC Monograph Process (Adapted from: FDA program on “Bringing an Over-the-Counter (OTC) Drug to Market”. Available at: http://www.accessdata.fda.gov/scripts/cder/training/OTC/topic3/topic3/da_01_03_0190.htm )
NDA Process (similar to Rx drugs)
Pre-market approval
FDA review and approves formulation and labeling prior to marketing
Confidential filing
Drug product specific
May require a user fee
Potential for marketing exclusivity
Mandated FDA review timelines
May require clinical studies
Label comprehension
Actual use
Approved labeling is unique to the drug
Approved NDA is your “license” to market
Trade name reviewed prior to marketing
OTC Monograph Process (Recipe Book)
No pre-market approval
FDA sets forth conditions for GRASE (generally recognized as safe and effective), or in the case of developing monograph, sets forth conditions that allow for continued marketing pending a final monograph.
Oversight occurs on a post-marketing basis
Public process
Active ingredient specific and evaluated by OTC drug category
No user fees
No marketing exclusivity
Manufacturers responsible for ensuring compliant product with no FDA-mandated review (pre- or post-market).
Generally, does NOT require clinical studies
Label comprehension and actual use studies not required for ingredients already covered by final or tentative final monograph
Labeling is defined by the monograph. Once marketed, FDA can review the complete labeling at any time to determine whether truthful or misleading.
Final monograph is open to anyone
No review of trade name prior to marketing. Once marketed, FDA can review the trade name at any time.
✓ An OTC monograph is like a "recipe book," covering acceptable ingredients, doses, formulations, and labeling. Once in a monograph, any manufacturer can market the drug as an OTC product without an individual product license.
o See 21 CFR 331 – 358 for OTC monographs

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
✓ Similar to the DESI review of prescription medications
✓ Applies to post-1962 OTC products.
✓ Process
1. FDA appoints advisory panel of qualified experts to review drugs by class (e.g., analgesics, antacids)
2. FDA publishes recommendations in the Federal Register asking for public comment.
3. FDA then publishes proposed rule in the Federal Register
4. FDA then publishes a monograph in the CFR.
Post-marketing Surveillance
MANUFACTURER RESPONSIBILITIES
Manufacturers must report any serious adverse drug reactions and any new information regarding a drug's safety and efficacy.
✓ Adverse Event Reporting System (AERS) – computerized database of reports of adverse events for all drugs and biologics (https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/Adverse DrugEffects/ucm070093.htm)
PHARMACIES/PHARMACISTS RESPONSIBILITIES
MedWatch – The FDA Safety Information and Adverse Reporting System (http://www.fda.gov/Safety/MedWatch/default.htm) (PDF of form: https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf)
✓ Database that contains information on prescription and OTC drugs, medical devices, and special nutritional products
✓ Health care professionals and consumers can report serious problems they suspect.
Institute for Safe Medication Practices (ISMP) Medication Errors Reporting Program (MERP) –MedWatch Safety Partner (https://www.ismp.org/report-medication-error)
✓ ISMP is a certified patient safety organization (PSO)
✓ ISMP-MERP healthcare provider reporting form https://www.ismp.org/form/merp-form
✓ ISMP-MERP consumer reporting form https://www.ismp.org/form/cmerp-form

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Risk Evaluation and Mitigation Strategies (REMS)
✓ Used for exceptionally high-risk drugs.
✓ The FDA may require a manufacturer to implement special procedures to reduce these risks (Any to all of these may be required) https://www.fda.gov/drugs/drug-safety-and-availability/riskevaluation-and-mitigation-strategies-rems and https://www.fda.gov/drugs/risk-evaluation-andmitigation-strategies-rems/whats-rems
1. Communication Plan
▪ Educates, informs, and raises awareness of risks.
▪ Target – healthcare providers
2. Medication Guide (dispensing function – will talk about in future lecture)
▪ Provides additional information beyond what is in Consumer Medication Information (CMI)
▪ Patient focused and in lay language.
▪ Target – patients
3. Elements to Assure Safe Use (ETASU)
▪ Required medical interventions or other actions healthcare professionals need to execute prior to prescribing or dispensing the drug to the patient. Some actions may be required for the patient to continue treatment.
4. Implementation System
▪ Drug’s sponsor may be required to take reasonable steps to monitor and evaluate those in the healthcare system for implementing ETASU measures, if certain ETASU are required.
The manufacturer must submit periodic reports to the FDA regarding how well the drug's risks are being managed.
✓ See http://www.accessdata.fda.gov/scripts/cder/rems/index.cfm for REMS that are in the process of being approved or have been approved. (Using Buprenorphine as the drug search term)


Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
The display of written, printed, or graphic matter upon the immediate container and/or wrapper
Example:

The following information must be displayed on the commercial container of all prescription drug product:
Drug product name
Established name (generic or chemical)
Strength
Quantity
Recommended dosage (or reference to package insert)
Lot number
Expiration Date
For MM/YYYY format, drug expires on the last day of that month
Legend statement
"Rx only" or "Caution: Federal law prohibits dispensing without a prescription"

The label plus any 'accompanying' information such as the package insert, the patient package insert or other materials
Example:


The following sections apply to the manufacturer package insert for all prescription drug products:
Highlights
Boxed warning (only if required by the FDA)
Indications and usage
Dosage and administration
Dosage forms and strengths
Contraindications
Warnings and precautions
Adverse reactions
Drug interactions
Use in specific populations
Drug abuse and dependence (as appropriate) +
Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Type of container to be used when dispensing
Name and address of manufacturer/packager/distributor
For drugs not for oral use...
The following additional information is required:
Route of administration
Names of inactive ingredients (with certain exceptions)
For containers that are too small/unable to accommodate label requirements...
The following may be contained in other labeling on or within an outer container:
Recommended dosage
Route of administration
Inactive ingredients
Type of container to be used when dispensing
For unit dose packaging...
Label requirements are less comprehensive:
Established name (generic or chemical)
Strength
Lot number
Expiration Date
Name of manufacturer/packager/distributor
Any statements required by a compendia if an official drug, or for unofficial drugs, any pertinent statement regarding special characteristics
Overdosage
Description
Clinical pharmacology
Nonclinical toxicology
Clinical studies
References
How supplied/storage and handling
Patient counseling information
Pregnancy Warnings
Other labeling includes: (more on these in the Dispensing Medications and Caring for Patient section)
Patient package insert (PPI)
Medication Guide (MedGuide)
Consumer Medication Information (CMI)
Proposal for universal patient information
NACDS, NCPA and APhA have all asked the FDA to pursue a universal patient information guide to replace the PPI, MedGuide and Consumer Medication Information (2011) – stalled in Congress and FDA




1. The established name (generic) of each active ingredient(s), including the amount in each dosage unit.
2. The purpose of the product (general pharmacological category(ies) or the principal intended actions.
3. The uses (indications) for the product.
4. Specific warnings, including when the product should not be used under any circumstances, and when it is appropriate to consult with a doctor or pharmacist. This section also describes side effects that could occur and substances or activities to avoid.
o Route warnings "For external use only", "For rectal use only", "For vaginal use only" (if applicable)
o Specific warnings detailed in section such as (not complete list):
▪ Reye’s syndrome
▪ Allergic reaction and asthma alert warnings
▪ Liver warning
o Do not use... (contraindications)
o Ask a doctor before use if you have... (list all conditions/situations when the product should not be used)
o Ask a doctor or pharmacist before use if you are... (listing of all drug-drug and drug-food interactions)
o When using this product... (listing of possible side effects and listing of substances and activities to avoid)
o Stop use and ask a doctor if... (listing of signs of toxicity and other reactions requiring immediate discontinuation)
o "If pregnant or breast feeding" warning.
o "Keep out of the reach of children" and accidental overdose/ingestion warning.
5. Dosage instructions when, how much, and how often to take the product.
o Think quantity, frequency, duration, time of administration, preparation necessary for use (e.g., shake well, dilution, etc.)
6. Other information such as storage requirements
7. The product's inactive ingredients, important information to help consumers avoid ingredients that may cause an allergic reaction.
8. Questions and Comments? Must provide a telephone number for consumer to ask questions.
In addition, this information is required on the OTC label per FDCA.
9. The name and address of the manufacturer, packager, or distributor
10. The net quantity of the contents of the package
FDA may require a manufacturer to update a drug’s labeling with additional warnings or safety-related information when it becomes aware of a serious new drug risk.

• Black box warning on anti-depressants
• MedGuide Update for buprenorphine – update information regarding dental problems dissolved in mouth to treat opioid use disorder and pain.
✓ Serves as a universal product identifier for human drug products.
✓ Presence of an NDC number DOES NOT indicate the drug has received FDA approval.
Currently, the FDA uses a 10-digit NDC standard (21 CFR 201.2 and 207.35); HIPAA requires an 11-digit NDC standard.
12345 - 6789 - 00
Identifies: Manufacturer/Labeler
Identifies: Drug Product
Identifies: Package Size and Type
✓ Segment 1: Manufacturer/Labeler ID and is assigned by the FDA.
✓ Segment 2: Drug product segment identifies:
o Drug name
o Specific strength
o Dosage form
o Formulation
✓ Segment 3: Package size and type of drug
Typically, NDC numbers are used to:
✓ Process 3rd party prescription claims
✓ Track distribution of products
Bar Code



The NDC is required to be in linear bar code format according to FDA rules
2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy

• Response to the cyanide product tampering of Tylenol in 1982
• Requires that certain OTC drugs, food, cosmetics, and devices be manufactured wit6h tamper-resistant packaging.
• Tamper-resistant packaging = barrier to entry, which if breached or missing, will provide a visual to the consumer to not use the product and that tampering has occurred
21 CFR Part 111 (CGMP for Dietary Supplements) and 21 CFR Part 211 (CGMP for Finished Pharmaceuticals)
✓ Set of regulations that establish minimum requirements for the methods, facilities, or controls used in the manufacture, processing, packaging, or holding of a dietary supplement (Part 111) or drug product (Part 211)
✓ Manufacturers are registered with the FDA and are inspected once every 2 years for compliance with CGMP.
✓ CGMP does not apply to pharmacies.
UNLESS the FDA determines that the pharmacy is manufacturing (see manufacturing vs. compounding)
Note: FDA has mandatory recall authority for foods under FSMA (Food Safety Modernization Act) – this is different from their authority for drug products (Rx and OTC)
✓ Recalls are actions taken by a firm to remove a product from the market that is exhibiting problems or is in violation of laws (e.g., misbranding, adulteration, other false claims, etc.).
✓ Is an alternative to an FDA-initiated court action for removing or correcting products in violations of laws
✓ Recalls are almost always conducted on a firm's own initiative; the FDA may also formally request a recall in writing.
✓ The FDA does NOT have statutory authority to mandate a recall for ALL drug products.
✓ FDA DOES have statutory authority to mandate certain recalls for the following types of products (per the FDA Regulatory Procedures Manual – see

http://www.fda.gov/downloads/iceci/compliancemanuals/regulatoryProceduresManual/UCM07431 2.pdf and http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm221671.htm updated 8/2013)
o FDA Ordered Recalls (Mandatory recalls)
✓ Devices
✓ Biologicals
✓ Human tissue intended for transplantation.
✓ Infant formula
✓ Interstate milk shipments
✓ Tobacco products
✓ If the manufacturer refuses to recall a drug, the FDA may file a criminal suit that would likely result in seizure of the drug product or a court-ordered recall.
FDA classification of drug recalls – based on the product's potential to cause harm.
•Drug product may cause serious, adverse health consequences including death. Recall should include stocks in pharmacies and notification of patients to whom the drug has been dispensed.
•Drug product may cause temporary or reversible effects and the probability of serious adverse effects is remote, recall includes stock in pharmacies.
•Drug product is unlikely to cause any adverse health consequences; recall may be due to something as simple as misspelling on the label.
✓ It is the manufacture's responsibility to send written recall notices to all wholesalers and pharmacies for recalls in class I and II and some class III.
✓ It is the pharmacist's responsibility to be aware of all recalls and, if necessary, contact patients taking the recalled product
Recalls available at: http://www.fda.gov/Safety/Recalls/EnforcementReports/default.htm


2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy

If an ingredient on an OTC drug or prescription drug is determined to be potentially dangerous, FDA may request the manufacture to discontinue sales or remove the product from the market; requests for voluntary discontinuation are invariably honored.
FDA request to discontinue sales: Examples: Vioxx ®, Cylert ®, Zelnorm ®
Market withdrawal: occurs when a product has a minor violation that would not be subject to FDA legal action. The firm removes the product from the market or corrects the violation. For example, a product removed from the market due to tampering, without evidence of manufacturing or distribution problems, would be a market withdrawal.
Medical device safety alert: issued in situations where a medical device may present an unreasonable risk of substantial harm. In some case, these situations also are considered recalls.
Section 501(g) of FDCA – pharmacies are exempt from registering as manufacturers if they do not manufacture, prepare, propagate, compound, or process drugs or devices for sale other than in the regular course of their business of dispensing or selling drugs or devices at retail (i.e., based on an individual prescription order or anticipation of an individual prescription order).
✓ This exempts pharmacies from the manufacturing definitions of misbranding, adulteration and new drug provisions in the FDCA

Title I – Drug Compounding (applies to compounding for human consumption only)
Title II – Drug Supply Chain Security
Title 1: “Compounding Quality Act” (Pharmacy Compounding for Human Consumption/Use Only)
Purpose: Ensure the safety of compounded drugs
What it does:
1. Amends the Food, Drug, and Cosmetic Act with respect to the regulation of compounded drugs for human use.
2. Reinstates section § 503A that were not considered unconstitutional regarding compounded drugs and “traditional compounding” by community pharmacists and practitioner
a. Pharmacists and physicians are exempt from the following manufacturer requirements of the FDCA:
i. cGMPs (Section 501(a)(2)(B) of FDCA)
ii. Labeling and adequate directions for use (Section 502(f)(1) of FDCA)
iii. NDA and ANDA approvals for “new drugs” compounded (Section 505 of FDCA)
b. Limited compounded quantities are prepared by a licensed pharmacist or practitioner based on the receipt of a valid prescription order or anticipated receipt of a valid prescription order.
3. Creates section § 503B which applies to “outsourcing facilities”.
a. Specifically, if doing large scale compounding and/or compounding for office use, hospitals, etc. and not based on an individual patient prescription order.
b. Must be in compliance with cGMP.
c. Compounded drugs are exempt from new drug requirements, labeling requirements, and track and trace requirements if the drug is compounded by or under the direct supervision of a licensed pharmacist in a registered outsourcing facility and meets applicable requirements.
d. Compliance with the DQSA labeling requirements for each container.
e. Must report products that are compounded and any adverse events.
Who it applies to: Licensed pharmacist, practitioner, outsourcing facility.
Components: (4 of 6 are presented below)
Component Explanation
Voluntary Outsourcing Facilities
✓ Pharmacies may apply and register as a “voluntary outsourcing facility” if they want to supply compounded drugs to other facilities and prescribers.
✓ Only one geographic location may engage in the compounding of sterile products.
✓ Annual registration ($15,000)

Penalties
Enhanced Communication
✓ Biannual (June and Dec) reporting to Sec of HHS regarding drugs compounded at facility.
✓ Submit adverse event reports.
✓ Subject to risk-based inspection schedule
✓ Added definition of misbranded drugs; deems a compounded drug to be misbranded if its advertising or promotion is false or misleading in any way.
✓ Prohibits the resale of a compounded drug labeled “not for resale” or the intentional falsification of a prescription for a compounded drug
✓ Requires State boards of pharmacy to send reports to the Sec of HHS that:
o Describe any disciplinary actions taken against compounding pharmacies for any recall of a compounded drug.
o Express concerns if a compounding pharmacy may be violating the FDCA
Severability ✓ Repeals prohibitions on advertising and promotion of compounded drug by compounding pharmacies.
✓ Repeals the requirement that prescriptions filled by a compounding pharmacy be unsolicited.
Purpose: Ensure drugs are safe in the drug supply
What it does:
1. Enable verification of the legitimacy of the drug product identifier down to the package level.
2. Enhance detection and notification of illegitimate products in the drug supply chain; and
3. Facilitate more efficient recalls of drug products.
Who must comply?
✓ Manufacturers
✓ Wholesale drug distributors (wholesalers)
✓ Re-packagers
✓ Third-party logistics providers
✓ Dispensers (community pharmacies, hospital pharmacies, group of chain pharmacies under common ownership, physician dispensers, etc.)
What documentation is required?
1. Transaction History (TH) (originates with manufacturer) – is a single document that contains all changes of ownership.

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
2. Transaction Information (TI)
a. NDC
b. Product name
c. Strength
d. Dosage form
e. Container size
f. Number of containers
g. Lot number
h. Transaction date
i. Shipment date (if > 24 hours from Transaction Date)
j. Transfer from Party (business name and address)
k. Transfer to Party (business name and address)
l. Wholesaler Contact Information (for drop shipment)
3. Transaction Statement (TS) – Statement attesting that party transferring ownership:
a. Is authorized and registered.
b. Received product from authorized, registered party.
c. Received TI and TS from the prior owner.
d. Did not knowingly ship suspect or illegitimate product.
e. Has a system and process in place to comply with verification requirements?
f. Did not knowingly provide false information.
g. Did not knowingly alter the transaction history.
Length of Time to keep records: 6 years.
Diagram of the track and trace process

Source: Steiber D. Patient and Product Security: It's the New Law. Specialty Pharmacy Times. 2014; March/April. http://www.specialtypharmacytimes.com/publications/specialty-pharmacy-times/2014/April-2014


✓ Labeling is information that is intended to be used by health professionals.
✓ Advertising must meet an "adequate provision" requirement - allows advertisements on broadcast media to only present the major risks of the drug and make "adequate provisions" to obtain further information.
✓ FDA = Prescription drug marketing
✓ FTC = OTC drug marketing (consults with FDA)
First amendment protects free speech versus government regulation of speech
To permit government regulation of speech, the following factors must be met
1. The speech must not be misleading or related to an unlawful activity
2. The government interest in the regulation must be substantial
3. The regulation must directly advance the government interest asserted (e.g. public safety)
4. The restriction of speech cannot be more extensive than necessary to serve that interest
ALL ADVERTISEMENTS FOR PRESCRIPTION DRUGS MUST INCLUDE:
1. Established name of the drug
2. The formula, showing quantitatively each ingredient.
3. A "brief summary" of other information related to side effects, contraindications, and effectiveness (aka “a true statement”)
FDA has also issued more detailed regulations

A MANUFACTURER HAS NOT MET THE “TRUE STATEMENTS” REQUIREMENT IF THE ADVERTISING:
1. Is false or misleading
2. Biases effectiveness information over side effects and contraindications
3. Excludes material facts
Exceptions:
1. Reminder advertisements
2. Substances for manufacturing or compounding (cannot make any claims of safety or effectiveness)
✓ Journal advertisements
✓ Industry-supported educational programs
o If the manufacturer has any control or influence over content, the entire program is subject to FDA regulation
o The biggest downside is that off-label uses could not be discussed
o Funding from the manufacturer should be given to an independent CE provider that conducts the educational program without their influence
o PhRMA Code on Interactions with Health Professionals
✓ Industry gifting
o Manufacturers should not give out items, even of minimal value, that do not advance education (pens, note pads, mugs, stethoscopes, etc.)
Legality and rationale for “off-label use”
✓ A prescriber may lawfully prescribe a drug for uses other than the ones included in the approved labeling; he/she may also vary the dose and route of administration.
✓ Unapproved uses may be appropriate and rational in certain circumstances.
o Off-label use situations can range from drug uses that have not yet been studied to those that have been thoroughly studied, but the drug manufacturer must get FDA approval for the new indication.
✓ Before adding a new indication to the package insert, the manufacturer must get FDA approval by demonstrating the effectiveness of the new use.
1. Prescriber may ask for information from the manufacturer
2. Manufacturer may distribute unabridged pee-reviewed articles that have not been influence by the company.

o Address well-controlled clinical investigations considered by experts as scientifically sound
o Disseminated separate from promotional materials
o Accompanied by
▪ A comprehensive bibliography of publications regarding the off-label use
▪ A representative publication that reaches contrary or different conclusions (if in existence)
▪ Disclosure that the FDA has not approved the use
▪ Disclosure of any relationship between the company and the author of the article
✓ FDA Guidance document available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/uc m387652.pdf
✓ 2012 US Circuit Court of Appeals – 2nd Circuit in U.S. v Caronia decided that manufacturer offlabel use is protected free speech.
✓ 2011 US Supreme Court – Sorrell vs. IMS Health
o In 2007 State of VT had passed legislation that would have prohibited the sharing of physician prescribing information with drug companies for marketing purposes
o US Supreme Court determined that this act impinged on data mining companies and drug manufacturers’ free speech rights.
Three (3) types of DTC ads
✓ Product claim ads - name the drug and the conditions it is approved to treat - subject to FDA regulations
✓ Reminder ads - name the drug but not it's uses - not subject to FDA regulations
✓ Help-seeking ads - educational - mention the name of the company, but not the drug - not subject to FDA regulations; are subject to FTC regulations
Prescription drug advertising must:
1. Be accurate
2. Balance the risk and benefit information
3. Be consistent with prescribing information approved by the FDA
4. Only include information that is supported by strong evidence

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
See FDA website for good and bad examples of each type of advertisings: http://www.fda.gov/Drugs/ResourcesForYou/Consumers/PrescriptionDrugAdvertising/default.htm
FDA may require a preview of a DTC advertisement. After doing so, the FDA may make recommendations to the company, but can only require a change if the issue is related to serious health risks associated with the drug's use.
Kosty, T. “Track and Trace Legislation: Preview and Challenges”. American Society for Automation in Pharmacy 2014 Midyear Conference. Palm Beach, FL. June 26-28. http://www.asapnet.org/files/June2014/Presentations/ASAPJune14_Presentations07_Kosty.pdf.
Morris R. “The Year in Review: 2014 Pharmacy Law Update”. Arizona Pharmacy Association 2014 Annual Meeting. June 26-29. Litchfield Park, AZ. http://c.ymcdn.com/sites/www.azpharmacy.org/resource/resmgr/Annual_Convention/LawPresentation -R.Morris.pdf.
Tracelink. “DSQA Compliance and Data Exchange Requirements Webinar”. March 27, 2014. http://www.tracelink.com/resources/dqsa-regulations-requirements-and-readiness-for-2015compliance-on-demand-webinar
Topoleski CJ. “The Drug Quality & Security Act: What’s Next for Sterile Compounding and Outsourcing.” American Society of Health-Systems Pharmacists. Accessed March 17, 2017. Available at: http://www.ashp.org/DocLibrary/Policy/Compounding/Webinar-Handout-Drug-Quality-Security-Act.pdf

L ICENSING OF P HARMACIES , P HARMACISTS AND OTHER LICENSE AND PERMIT
After attending lecture and completing the assigned readings the student should be able to:
✓ Know the definitions and terminology in the Pharmacy Practice Act and the Administrative Code
✓ Describe the eligibility requirements and requirements for licensure for the following: Pharmacy intern, pharmacy preceptor and training site, pharmacist, pharmacy technician and pharmacy technician trainee (across all mechanisms)
✓ Identify and describe renewal information for licenses and permits; continuing education requirements for pharmacists and pharmacy technicians; special CE requirements; and any exceptions
✓ Describe the requirements for license holders and permit holders regarding the following: change in employer or home address; pharmacist-in-charge, residency status (include time frames)
✓ Identify the specific types of permits that are issued by the ASBP and requirements
✓ Identify and describe renewal information for permit holders
✓ Identify and describe the two types of physical location and 5 pharmacy permit categories and what additional information is required on the application for a hospital pharmacy
✓ Describe what type of compensation is allowed to be paid to medical practitioners by a permit holder and any consequences for any actions.
✓ Describe the responsibilities of the pharmacy permit holder and the pharmacist-in-charge.
✓ Describe the procedures for discontinuing a pharmacy
✓ Identify and describe community pharmacy specific requirements including: pharmacy area, personnel and security, pharmacy facilities, and equipment
✓ Identify and describe hospital pharmacy specific requirements including: general information, personnel, hours of operation and staffing, absence of a pharmacist, physical facility, sanitation and equipment, and security.
✓ Identify the five (5) types of limited-service pharmacies that are discussed in the ARS and AAC.
✓ Describe the requirements for the display of permits and licenses
✓ Describe unethical and unprofessional conduct discussed in 32-1901.01.

ARS = Arizona Revised Statute; AAC = Arizona Administrative Code
✓ ARS §32-1901.01. Definition of unethical and unprofessional conduct
✓ ARS §32-1921 Exempted acts; exemption from registration fees; definitions
✓ ARS 32-1921 (A) (1 –6). Exempted acts; exemption from registration fees; definition
✓ ARS §32-1922. Qualifications of applicant, reciprocity; preliminary equivalency examination; honorary certificate; fee and (See updated posted on Blackboard for this specific section)
✓ AAC R4-23-201 – R4-23-205 – Article 2. Pharmacist licensure
✓ ARS §32-1923. Interns and intern preceptors; qualifications; licensure; purpose of internship
✓ AAC R4-23-301 – R4-23-305 – Article 3. Intern training and pharmacy intern preceptors
✓ ARS §32-1923.01. Pharmacy technicians, pharmacy technician trainees; qualifications and
✓ AAC R4-23-1101 – R4-23-1106. Article 11. Pharmacy technicians
✓ ARS §32-1924 Licenses; fees; signatures
✓ ARS §32-1925. Renewal of license of pharmacists, interns and pharmacy technicians; fees; expiration dates; penalty for failure to renew; continuing education
✓ ARS §32-1926. Notice of change of employer or home address; termination of responsibility
✓ ARS §32-1926.01. Change in residency status; duty to report
✓ ARS §32-1927. Pharmacists; pharmacy interns; graduate interns; disciplinary action
✓ ARS §32-1927.01. Pharmacy technicians, pharmacy technician trainees; disciplinary action
✓ ARS §32 -1927.02. Permittees; disciplinary action
✓ ARS §32-1929. Biennial registration of pharmacies; wholesalers, manufacturers and similar places; application.
✓ ARS §32-1930. Types of permits, restrictions on permits; discontinuance of pharmacy permit
✓ ARS §32-1931. Permit fees; issuance; expiration; renewals
✓ AAC R4-23-601 – R4-23-693. Article 6. Permits and distribution of drugs and
✓ AAC R4-23-701 – AAC R4-23-703. Article 7. Non-pharmacy licensed outlets – general provisions
✓ ARS §32-1933. Display of license or permit
✓ ARS §32-1934. Pharmacies operated by hospital
✓ ARS §32-1936. Mandatory continuing professional pharmacy education
✓ ARS §32-1937. Exceptions to continuing education requirements
✓ ARS §32-1973. Pharmacies; quality assurance and
✓ AAC R4-23-620. Continuous quality assurance

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
✓ ARS $32-1981 – ARS §32-1984 – Article 3.1. Regulation of full-service wholesale permittees
✓ ARS §32-1991 – ARS §32-1996 – Article 4. Enforcement of chapter; penalties
Required to do by law, rules or regulations Nice to do; not a requirement
Terminology Definition
Pharmacist An individual currently licensed by the board to practice the profession of pharmacy in this state
Intern means a pharmacy intern
Pharmacy Intern A person who has all of the qualifications and experience prescribed in section 321923.
Pharmacy Technician A person licensed pursuant to this chapter.
Pharmacy Technician Trainee A person licensed pursuant to this chapter
Pharmacy Interns (ARS 32-1904 and ARS 32-1923 and AAC R4-23-301 – 305)
ELIGIBILITY REQUIREMENTS (AAC R4-23-301 (B))
1. Current enrollment and in good standing in a Board-approved college/school of pharmacy, OR
2. Graduation from a college or school of pharmacy not approved by the Board, AND/OR
3. Proof that the applicant is certified by the Foreign Pharmacy Graduate Examination Committee (FPGEC); OR
4. By order of the Board if the Board determines the applicant needs intern training
5. Complete a fingerprint clearance card (per ARS 32-1904 (A) (6))


IF A PHARMACY INTERN STOPS ATTENDING SCHOOL BEFORE GRADUATING….
✓ Must immediately stop practicing as a pharmacy intern
✓ Surrender their intern license to the Board no later than 30 days after the date of the last attended class.
✓ If a student re-enters a pharmacy program and wishes to continue internship training, must re-apply for a pharmacy intern license.


2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
INTERN LICENSE APPLICATION PROCESS
• Complete a non-IVP fingerprint clearance card application and obtain the card (Cost = $67.00)
• Complete and submit the online intern license application
• Pay a fee of $60.00 to the Arizona State Board of Pharmacy (ASBP)
INTERN LICENSE DURATION (ARS 32-1923; ARS 32-1924; AND AAC R4-23-301
• Valid for 5 years from the date issued
• If an intern does not complete their degree within 6 years of the date of issue, the intern is not eligible for relicensure as an intern unless approved by the ASBP.
Reporting Internship Hours (ARS 32-1923; AAC R4-23-304)
Hours within the Experiential Program (IPPE/APPE) (PharmD Curriculum)
Reported by Pharmacy School to ASBP (and other state BOPs)
School certifies and submits hours after graduation.
Includes the following information:
✓ Name, address, and phone number of the training site
✓ Dates and training hours
✓ Name of pharmacy intern preceptor
✓ Signed and dated by the OEE Director
Hours earned outside of IPPE and APPE (Paid hours ONLY) – New rules went into effect June 26, 2019 (AAC R4-23-302/304)
For AZ – Not required to be reported by the pharmacy intern
NOTE: If applying for licensure in a state that has a higher total hour requirement than AZ, your paid work preceptor will need to sign off on your paid work hours using documents from that state’s Board of Pharmacy
Examples:
Alaska = 1500 hours (only 1000 hours count from school)
Florida = 2080 hours (obtained prior to graduation)
Kansas = 1740 hours
Maine = 1740 hours
Michigan = 1600 hours

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Minnesota = 1600 hours
Mississippi = 1600 hours
Nevada = 1740 hours
Ohio = 1740 hours
South Dakota = 2000 hours (1740 hours recognized from the experiential program)
Tennessee = 1700 hours
Vermont = 1740 hours
Pharmacy Intern Preceptors & Training Sites (ARS 32-1923, AAC R4-23-302)
Training Sites
ELIGIBILITY REQUIREMENTS
✓ Holds a valid AZ pharmacy permit; or
✓ It is an alternative training site established and monitored by a Board-approved college or school of pharmacy or other nonpharmacy site where pharmacy related activities are performed.
Pharmacy Intern Preceptors
Preceptor definition: “a pharmacist who is serving as the practical instructor of an intern and who complies with ARS 32-1923.”
ELIGIBILITY REQUIREMENTS (SEE AAC R4-23-302)
1. The pharmacist shall hold a current unrestricted pharmacist license.
2. Have a minimum of one (1) year of experience as an actively practicing pharmacist
3. If found guilty of violating any federal or state law relating to the practice of pharmacy, drug or device distribution or recordkeeping, or unprofessional conduct, enter into an agreement satisfactory to the Board that places restrictions on the pharmacist’s license
NOTE: (added 4/9/2021) – there is no longer an intern/pharmacist ratio.
Responsibility of the Pharmacy Intern Preceptor (R4-23-302 (F))
✓ Assumes responsibilities as teacher and mentor
✓ Review policy and procedures with intern
✓ Responsible for the related actions of the intern during the training period

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
✓ Shall give intern opportunity for skill development and provide timely and realistic feedback regarding progress
Pharmacists (ARS 32-1922 - 1923 and AAC R4-23-201 -203)
Original Exam
By Exam
NAPLEX Exam State MPJE, f required
Score Transfer State MPJE, if required
Eligibility and Application process for Arizona
Licensure by EXAMINATION (Original Board License) (ARS 32-1922 & AAC R4-23-202)
✓ Pharmacy degree from an ACPE accredited school/college/program or qualify under the requirements of ARS 321922 (D)

Pharmacist Licensure
By Reciprocity
Licensure by RECIPROCITY
(Pharmacist already has a board license in another state) (ARS 32-1922 (B) (1-5) & AAC R4-23-203)
Use Original Board License State MPJE, if required
Universal Recogniztion
Based on Original Board License State MPJE, if required
Licensure by UNIVERSAL RECOGNITION (ARS 32-4302)
✓ Be of good moral character
✓ Licensed as a pharmacist in a state that provides reciprocity to AZ licensees
✓ Provides satisfactory evidence of having the
✓ Must reside in AZ to be eligible and provide proof
✓ Licensed in at least one other state for at least 1 year at the same practice level
© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
✓ Successfully complete 1500 hours of intern training under the direct supervision of a licensed pharmacist approved by the Board; No more than 500 hours may be obtained from an alternative training site (e.g., non-patient care; AZPA rotation, law rotation)
o Note – other states may vary in their internship hour requirements.
✓ Complete a fingerprint clearance card
✓ Pass the NAPLEX and MPJE exams (within 12 months of submitting application):
o NAPLEX
▪ Sitting for AZ exam, or
▪ Score transfer
o MPJE (AZ)
✓ Pay application and exam fees = $1205 total for one state
o AZ = $250
o NABP = $955
▪ Eligibility to Test = $85
▪ NAPLEX = $100 +$520
▪ MPJE per state = $100 + $150
required education and training
✓ Presents proof to the board of initial licensure, the license is in good standing, and passed NAPLEX
✓ Presents proof to the board that no licenses have been suspended, revoked, or restricted
✓ If applicant has not actively practiced pharmacy for over a year, the Board MAY required the applicant complete 400 hours of internship UPDATED 3/29/2024 (See ARS 321922 H)
✓ Complete a fingerprint clearance card
✓ Passes MJPE
✓ Pay application fees
o AZ = $350
o NABP =
▪ Eligibility to Test = $85
▪ MPJE (AZ) = $100
✓ Met minimum education requirements in the original state
✓ Previously passed an examination required for licensure by the other state
✓ Presents proof that license is in good standing and no complaints, allegations or investigation is pending
✓ Presents proof that license has not been revoked nor voluntarily surrendered
✓ If another jurisdiction has taken regulatory action, if the cause for the action has been NOT been resolved, the AZ state regulating entity may NOT issue a license until the matter is resolved
✓ Person does not have a disqualifying criminal history pursuant to section 411093.04
✓ Complete a criminal background check
✓ Take and pass MPJE
✓ Pay all applicable fees

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
✓ At the time you take your original licensure (NAPLEX) exam, you MAY also transfer your score to one or more states
✓ OR
✓ Within 89 days of taking the exam
✓ Pay NABP a $75 score transfer fee for each state you want to score transfer
Pass the MPJE for the other state(s)
Application (original exam or score transfer) is valid for 12 months from the date the application is received (this includes the time frame available to take exams).
Application is valid for 12 months from the date the application is received (this includes the time frame available to take exams).
Not currently clear if the 12month rule applies for the application process.
✓ If fail either exam three (3) times, the applicant shall petition the board for permission to retake either of the exams (NAPLEX or MPJE).
o Board shall evaluate petition and determine if additional educational training will be required before retaking an exam
o The maximum number of times the board will allow either exam to be taken is five (5)
✓ NAPLEX – allowed to retake after 45 days
✓ MPJE – allowed to retake after 30 days

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
(ARS 32-1923.01, ARS 32-1924, and AAC R4-23-1101 – 1106)
To work in a pharmacy as a pharmacy technician or pharmacy technician trainee in AZ you must:
1. Have a pharmacy technician or pharmacy technician trainee license issued by the ASBP
2. Read and discuss with the PIC (pharmacist in charge) of the pharmacy the Board rules concerning pharmacy technician and pharmacy technician trainees, job description, and policies and procedures.
3. Date and sign a statement that the person has complied with subsection (A) (2) (R4-23-1101)
Pharmacy Technician Trainee
Eligibility requirements:
✓ Be of good moral character
✓ Be at least 18 years of age, and
✓ High school diploma or equivalent (GED)
✓ Fingerprint clearance card submitted.
Pharmacy Technician
Eligibility requirements:
✓ Meet requirements of pharmacy technician trainee
✓ Complete a pharmacy technician training program prescribed by board rules
✓ Pass the Pharmacy Technician Certification Board (PTCB) or another Board-approved exam
✓ Fingerprint clearance card submitted.
License Duration: 36 months from the date issued
✓ If a pharmacy technician trainee doesn’t complete the technician eligibility requirement, the trainee is no longer eligible for licensure as a pharmacy technician and shall not practice as a pharmacy technician or trainee.
✓ There is no renewal of the technician trainee license
Before Working at Remote Dispensing Site:
✓ Complete a 2-hour CE program on remote dispensing site pharmacy practices
✓ Have at least 1000 hours of experience as a pharmacy technician in an outpatient pharmacy setting
Currently Working at a Remote Dispensing Pharmacy:
✓ Shall maintain an active, nationally recognized pharmacy technician certification approved by ASBP
✓ May not perform extemporaneous sterile or nonsterile compounding
✓ May prepare commercially available medications for dispensing, including the reconstitution of orally administered powder antibiotics

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Temporary Licenses (Pharmacist, Intern, Pharmacy Technician) (AAC R4-23-413)
The ASBP (and other healthcare boards) may issue a temporary license to the following when a state of emergency has been declared:
✓ Pharmacists
✓ Interns
✓ Pharmacy Technicians
Temporary License
✓ Valid for 30 days from issue or earlier if the application denied
✓ Temporary licenses MAY NOT be renewed
Requirements for Temporary License
✓ License in other state(s) is (are) in good standing (active, unrestricted)
✓ No suspensions or revocations
✓ Has not surrendered license in another state
✓ Not engaged in criminal activity or unprofessional conduct
✓ Not the subject of an unresolved complaint
✓ Paid applicable fees
✓ Submit notarized attestations that other state license meets requirements
License Renewal (Pharmacists & Technicians) (ARS 32-1925)
Biennial renewal (every 2 years)
✓ Renewal date – on or before November 1st
o Licenses ending in EVEN number renew in EVEN numbered year
o Licenses ending in ODD number renew in ODD numbered year
✓ Failure to renew results in a suspended license
✓ Must pay fees and penalties to have license re-instated
NOTE: fees are no longer prorated


© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Before renewing your license, you must complete a certain amount of approved continuing education hours
These are the general continuing education requirements
✓ 3.0 CEU (30 hours)
o 0.3 CEU (3 hours) of opioidrelated, substance abuse disorder-related, or addictionrelated CE
o ACPE approved provider
o CE renewal period is the two years between license renewals
✓ Newly licensed pharmacists are exempt from the CE requirement for their 1st renewal
✓ 2.0 CEU (20 hours)
o 0.3 CEU (3 hours) of opioidrelated, substance abuse disorder-related, or addictionrelated CE
o ACPE approved provider
o CE renewal period is for the two years between license renewals
✓ Newly licensed pharmacy technicians are exempt from the CE requirement for their 1st renewal
SPECIAL CE REQUIREMENTS (ARS 32-1974, ARS 32-1979, ARS 32-3248.02; AAC R4-23-411)
• Immunizations (AAC R4-23-411 (H) & AAC R4-23-1106)
o For Pharmacists: 0.2 CEU (2 hours) every biennium (2 years)
o For Technicians: 0.2 CEU (2 hours) every biennium (2 years)
• Tobacco Cessation Prescribing (ARS 32-1979.03) = 0.2 CEU (2 hours) every biennium (2 years)
• Naloxone Dispensing (AAC R4-23-407.1) = No CE required; Must complete 1 hour of training (Available from AzPA for free)
• Oral contraceptive dispensing (AAC R4-23-204) = 0.3 CEU (3 hours) every biennium (2 years)
EXCEPTIONS TO THE CE REQUIREMENT FOR PHARMACISTS
✓ Board may make exceptions in an emergency or hardship cases or for a good cause – based on written request
o If Board exception granted, pharmacist shall satisfactorily pass a written examination approved by the Board
NABP AND CE CREDITS
✓ All pharmacists, technicians, and technician trainees must obtain an NABP e-Profile ID
o Go to www.nabp.net

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
✓ This must be used when completing and/or attending CE programs so that CE is tracked by NABP
Licensee Responsibilities: Notice of Change of Employer or Home Address; Termination of Responsibility; or Change in Residency Status (AAC R4-23-301(L); R4-23-304; R4-23-608; ARS 32-1926)
(Home) address
Change in Pharmacist-inCharge (PIC) status Immediatelyb
Change in residency status
Shall give written notice to the ASBP If residency status ceases to be authorized, the licensee voluntarily terminates the license
aFor interns, this includes stopping or starting a paid training site and changing the base store within a corporation bChange in PIC status is starting or terminating; immediately is within 24 hours
Specific types of permits issued by ASBP (See ASBP website for all permit applications)
✓ For each permit type – the permit applicant must indicate if: Resident or Non-Resident permit
✓ Businesses must have an individual permit to engage in each of these activities
✓ Each site (specific address/location) requires a separate permit

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Permit Type
Pharmacy
Remote Pharmacy Permit/Kiosks (Resident ONLY)
Manufacturer
Wholesaler
Medical Gas/Durable Medical Equipment (DME)
Secondary Categories on the Permit Application
✓ Chain
✓ Independent
✓ Government
✓ Hospital
✓ Limited Service
✓ Remote dispensing OR
✓ Kiosk
✓ Virtual
✓ 503B Outsourcing Facility
✓ Repackager
✓ Full service (includes virtual)
✓ Non-prescription
✓ Gas distributor
✓ Gas supplier/DME
✓ Gas supplier
✓ DME
3PL – Third Party Logistics Provider
Renewal of Permits (ARS-32-1931)
✓ Permits are renewed on a biennial basis
✓ Renewal date – on or before November 1st
o If late on renewal, permit will be suspended until all fees and penalties are paid
✓ Permits are non-transferable
Pharmacy Permits
Application – Example – Pharmacy Permit


© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Physical Location
Secondary Type
Additional Requirements for Hospital Rx














Healthcare providers (MD, DO, etc.) and Pharmacy Permits




Healthcare providers are allowed to own community pharmacies. They cannot direct patients to the pharmacies in which they have ownership interest.

No “kickbacks” (compensation) to medical practitioners from the pharmacy as a result of the practitioner’s prescription orders
The Board shall deny or revoke a pharmacy permit if this activity is occurring.

Normal compensation is allowed to be paid to a medical practitioner if they are the owner of the building or space being leased for the pharmacy as long as compensation is at the prevailing rate (rent/lease).
Responsibilities of the Permit Holder (Permittee)
Continuous Quality Assurance (CQA) Programs & Pharmacies (ARS 32-1973 and AAC R4-23-620)
1. ALL pharmacies shall implement or participate in a continuous quality assurance(CQA) program
✓ Review pharmacy procedures to identify methods for addressing pharmacy medication errors
2. Records generated as a component of a pharmacy’s ongoing CQI are considered peer review documents and are not subject to subpoena or discovery in an arbitration or civil proceeding
3. A pharmacy automatically meets this requirement if it holds a current general, special, or rural general hospital license from the Department of Health Services and is any of the following:
✓ Certified by CMS to participate in Medicare or Medicaid programs
✓ Accredited by Joint Commission
✓ Accredited by the American Osteopathic Association

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Permittee or PIC shall ensure that:
✓ The pharmacy develops, implements, and utilizes a CQA program
✓ Policies and procedures are reviewed biennially, and if necessary revised
✓ Policies and procedures must be available within the pharmacy for employee reference and inspection by the Board or its staff
✓ Medication error data generated is utilized and reviewed on a regular basis (periodically, at minimum annually)
✓ Training records, policies and procedures, and other program records or documents, other than medication error data, are maintained for a minimum of 2 years.
CQA POLICIES AND PROCEDURES REQUIREMENTS SHALL ADDRESS A PLANNED PROCESS TO:
✓ Train all pharmacy personnel in relevant phases of the CQA program
✓ Identify and document medication errors
✓ Record, measure, and analyze data collected to:
o Assess the causes and any contributing factors to medication errors
o Improve the quality of patient care
o Utilize findings to develop pharmacy systems and workflow processes designed to prevent or reduce medication errors
o Communicate findings to pharmacy personnel
Notifications to ASBP and Other Responsibilities of the Pharmacy Permittee (Permit holder) (AAC R4-23-606; ARS 32-1901.01)
NOTIFICATION OF CHANGE OF PERSONNEL
Notify ASBP: (by mail, fax, or electronic mail) Permittee
Employing or terminating a pharmacist 10 days
Designating or terminating a PIC Immediately
OTHER RESPONSIBILITIES
1. Ensure that pharmacists, interns, and other pharmacy employees comply with state and federal laws and administrative rules;

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
2. May not overrule a pharmacist in matters of pharmacy ethics and interpreting laws pertaining to the practice of pharmacy or the distribution of drugs and devices
Board may suspend or revoke a pharmacy permit if the owner or management violates item #2 (is considered unethical conduct)
Scenario: State of Utopia Pharmacy sold its pharmacy business to Drugs R Us. The sale included all controlled substances, non-controlled substances, and over-the-counter (non-prescription) medications and the prescription files.
What is each pharmacy responsible for doing regarding the discontinuation of State of Utopia Pharmacy (aka Utopia Rx) as identified by the ASBP?
See form on ASBP website: https://drive.google.com/file/d/1406eBiNRNo9aoTBRddcjQyNQevRtjXl/view
1. Permittee and/or PIC (in this case –Utopia Rx) shall provide written notice to the DEA at least 14 days prior to close of business with the following information
a. Name, address, pharmacy permit number and DEA registration of pharmacy that is closing (Utopia Rx)
b. Name, address, pharmacy permit number and DEA registration (if applicable) to whom all drugs (controlled substances (CS), non-CS and OTCs) are being sold or transferred (Drugs R Us)
c. Name and address location where closing pharmacy’s records will be stored (This depends – it could be Drugs R Us or another location to be determined)
d. Name and address location where pharmacy’s prescription and patient files will be kept and person responsible for these records. (Drugs R Us
e. Proposed date of closing of business (Example – April 14, 2019)
2. Permittee ensures removal of all pharmacy signs and symbols (inside and outside of business) (Utopia Rx)
3. Permittee or PIC (in this case Utopia Rx) ensures that all state permits and certificates of registration are returned to the Board and that DEA registration certificates and unused DEA Form 222s are returned to the DEA office in Phoenix
4. PIC of closing business ensures (Utopia Rx):
a. Only a pharmacist has access to Rx only and CS drugs until they are transferred to the designated licensee, permittee or registrant being transferred to
b. All CS, Rx only, OTCs and chemicals are removed from the closing pharmacy before the date of closing
c. All CS are transferred as follows:

i. CS inventory taken by closing PIC or pharmacist
ii. Copy of inventory included with CSs being transferred
iii. Keep original inventory with discontinued pharmacy’s records (invoices, etc.)
iv. Use of DEA Form 222 to transfer CII CS
v. Transfer of CS that need destruction is the same for all CS
5. Upon receipt of outdated or damaged CS from a closing pharmacy, the receiving licensee, permittee or registrant shall contact a DEA registered reverse distributor. (Drugs R Us)
6. Records received shall be maintained for 3 years from date of receipt (If Drugs R Us receives record)
7. Prescription orders shall be maintained for 7 years from date the last original or refill prescription was dispensed. (Drugs R Us)
PHARMACY AREA Graphic from: www.scriptpro.com

1. Minimum area of community pharmacy = 300 sq ft
✓ Maximum of 3 people may work simultaneously
o any combination of RPh:Technician:Intern
o There is no RPh: Intern Ratio or RPh:Tech Ratio
✓ May add 1 person for each additional 60 sq ft
2. Compounding and dispensing counter

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
3. Working area for compounding and dispensing
4. Area for patient counseling
5. Narcotic cabinet or safe
6. Building security standards
✓ Permanent barrier or partition from floor or counter to structural ceiling or roof
✓ Entry doors that can be securely locked
✓ Only pharmacist can access the pharmacy area (keys)
7. Drug storage and security
✓ Includes temperature, ventilation and USP standards
✓ Additional storage must meet strict requirements
Community Pharmacy Personnel and Security Procedures (R4-23-610)
RESPONSIBILITIES OF THE PIC
1. Every pharmacy shall have a PIC
2. Ensure communication and compliance of Board directives to management, other pharmacists, interns and technicians.
3. Ensure that all pharmacy policies and procedures are prepared, implemented, and complied with
4. Review biennially, and if necessary, revise all pharmacy policies and procedures
5. Policies and procedures manual must be available to all pharmacy and Board members or staff
6. Responsible for the quality of drugs and devices sold or dispensed in the pharmacy
✓ This includes the OTCs in the area outside of the pharmacy (see Substantive Policy Statement from the ASBP)
✓ Except for those sold in original packages of the manufacturer
7. Provide within four (4) working days of receiving from the Board a request relating to the acquisition or disposal of prescription only and controlled substances: (ARS 32-1963)
✓ Invoices
✓ Stock transfer documents
✓ Product tracking records (think DQSA documentation)
✓ Merchandise returns
✓ Other related documentation

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
1. A pharmacist is the only person permitted to unlock the pharmacy area or any additional storage area except in an extreme emergency
2. Pharmacy permittee (or agent) or the PIC shall ensure that Rx only and controlled substances received in an area outside of the pharmacy are immediately transferred unopened to the pharmacy area.
3. Pharmacy permittee or PIC may provide a slot/small opening for written prescription orders or prescription medication containers to be refilled by be left in the prescription area when the pharmacist is not present
4. A pharmacist shall ensure that prescription medication is not left outside of the prescription area or picked up by a patient when the pharmacy is not present by either:
✓ Delivering the prescription medication to the patient, or
✓ Securing the prescription medication inside the locked pharmacy, except when using an automated storage and distribution system that complies with the requirements of R4-23614
5. All personnel must wear identification badges, including name and position whenever on duty.
Pharmacy Facilities – Key pieces of information (AAC R4-23-611)
✓ Restroom – either in the pharmacy or within 100 feet (walking distance) from the pharmacy
✓ Sink with hot and cold running water (other than that in the restroom) for preparing drug products
✓ Regular review of drugs and chemicals for expiration dates and said drugs are moved to a “quarantined” area until can be returned or destroyed.
Pharmacy Equipment Requirements (AAC R4-23-612)
1. Adequate refrigeration equipment
2. A CV controlled substance register, if CV controlled substances are sold without a prescription order
3. Graduates in assorted sizes
4. One mortar and pestle (not required if applicant states in application that there will be no compounding at this site)

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
5. Spatulas of assorted sizes including one nonmetallic
6. Prescription balance, Class A with weights or an electronic balance (not required if applicant states in application that there will be no compounding at this site)
7. One ointment tile or equivalent (not required if applicant states in application that there will be no compounding at this site)
8. Current hard copy or access to a current electronic copy of the Arizona Pharmacy Act and administration rules and Arizona Controlled Substances Act
9. Professional reference library
10. Assortment of labels, including prescription labels, transfer labels for controlled substances, and cautionary and warning labels
11. A red “C” stamp if CIII – CV controlled substance invoices are not filed separately from other invoices
12. Current antidote and drug interaction information
13. Regional poison control phone number prominently displayed in the pharmacy area
Administration Giving a dose of a medication to a patient as a result of an order by a prescriber.
Dispensing for hospital inpatient
Floor stock
Interpreting, evaluating, and implementing a medication order including preparing for delivery a drug or device to an inpatient or inpatient’s agent in suitable container appropriately labeled for subsequent administration to, or use by, an inpatient (dispensing).
Supply of essential drugs not labeled for a specific patient and maintained and controlled by the pharmacy at a patient care area for the purpose of timely administration to a patient of the hospital.
Medication order Written, electronic, or verbal order from a prescriber or their agent for administration of a drug or device.
Satellite pharmacy
Work area in a hospital setting under the direction of a pharmacist that is a remote extension of a centrally licensed hospital pharmacy and owned and dependent upon the centrally licensed hospital pharmacy for administration control, staffing, and drug procurement

Hospital Pharmacy
Hammond Henry Hospital partial floor plan http://www.hammondhenry.com/Portals/HHH/Foundation/Documents/DeptLayout.pdf
✓ Must be registered with the Board of Pharmacy
✓ Shall meet the following criteria:
1. Hospitals ≥ 50 beds pharmacy shall be under continuous supervision of a pharmacist during the time it is open for pharmacy services
a. The ASBP may establish requirements to allow a pharmacist to be in other areas of the hospital that are located outside the pharmacy
2. Hospital ˂ 50 beds with written approval and recommendations of the Board, a pharmacist shall be required on a part-time basis
3. In pharmacist’s absence from the hospital, the supervisory registered nurse (RN) may obtain from the pharmacy necessary doses of drugs that are ordered by a prescriber and are needed by a patient in an emergency, according to procedures approved by the Board for the hospital
4. All discharge medications shall be dispensed by a pharmacist and be properly labeled.
5. The PIC is responsible for policies and procedures to provide for the administrative and technical guidance in all matters pertaining to the acquisition, stocking, recordkeeping and dispensing of drugs and devices

Hospital Pharmacy Personnel: Professional and Technician
PHARMACIST-IN-CHARGE (PIC) IS EITHER:
✓ The Director of Pharmacy, or
✓ A pharmacist appointed by the Director of Pharmacy
✓ Responsible for all activities of the hospital pharmacy and for meeting requirements of the AZ Pharmacy Act and rules;
✓ Ensure policies and procedures are prepared, implemented and complied with;
✓ Review biennially and if necessary, revise policies and procedures
✓ Document the review
✓ Compile policies and procedures in a written manual (printed) or other approved method
✓ Make policies and procedures available within the pharmacy for employees and Board inspection
✓ Responsible for the quality of drugs and devices sold or dispensed in the pharmacy
✓ Provide within four (4) working days of receiving from the Board a request relating to the acquisition or disposal of prescription only and controlled substance medication: (ARS 32-1963)
o Invoices
o Stock transfer documents
o Product tracing records
o Merchandise returns
o Other related documentation
HOURS OF OPERATION AND STAFFING (AAC R4-23-651 – 653)
✓ Pharmacist shall be in the hospital during the time the pharmacy is open for pharmacy services; except for an extreme emergency as defined in R4-23-110
✓ Pharmacy services shall be provided for a minimum of 40 hours/week
o Exception: <40 hours may only be done by written request and express permission of the Board
✓ Hospital pharmacy’s not open 24 hours Pharmacist shall be “on-call” when the pharmacy is closed
✓ Director of Pharmacy may be assisted by other personnel approved by the Director of Pharmacy in order to operate the pharmacy competently, safely, and adequately to meet the needs of the hospital’s patients.

© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Director of Pharmacy and/or PIC shall:
✓ If a pharmacist will not be on duty in the hospital:
o Arrange prior to the pharmacist’s absence, for the medical staff and other authorized personnel to have access to drugs in the remote drug storage area or in the hospital pharmacy.
o A pharmacist shall be on-call during all absences
o Arrange prior to the pharmacist’s absence, for the medical staff and other authorized personnel of the hospital to have telephone access to an on-call pharmacist
✓ Ensure that the hospital pharmacy is not without a pharmacist on duty in the hospital for more than 72 consecutive hours
✓ Maintain a remote drug storage area, which includes the following:
o Develop and maintain an inventory listing of the drugs to be included
o Develop, implement, review, and revise and comply with policies and procedures that ensure proper storage, access, and accountability for drugs in this area
✓ Access to pharmacy when pharmacist absent; policies and procedures need to be created to allow one supervisory nurse on each shift to have access, including recordkeeping policies.
✓ Within 4 hours after a pharmacist returns from an absence, the pharmacist shall verify all records of drug removal that occurred during the pharmacist absence.
1. Current baseline minimum is 500 sq. ft.
a. Minimum area for a hospital pharmacy is dependent on:
i. Type of hospital
ii. Number of beds
iii. Pharmaceutical services
b. Board may require more than the minimum if they deem appropriate.
2. Must be securely locked
3. Must ensure proper sanitation, ventilation, temperature control, segregation (of products), and security

1. Professional reference library appropriate for the scope of services provided by the hospital
2. Sink, with hot and cold running water
3. Refrigerator AND freezer
4. Laminar air flow hood and other supplies required for preparation of sterile products
1. No one is permitted in the pharmacy unless a pharmacist is present except as provided in section R4-23-647 and R4-23-654.
o If only one pharmacist is on duty in the pharmacy and needs to leave the pharmacy for an emergency or patient care duties, non-pharmacist personnel may remain in the pharmacy to perform duties outlined in R4-23-653, as long as CII controlled substances are secured to prohibit access by other than a pharmacist, and that the pharmacist remains available in the hospital
2. All pharmacy areas are kept locked by key or programmable lock
3. All personnel must wear identification badges, including name and position whenever on duty.
4. Prescription blanks shall be secured, and policies and procedures developed for safe distribution and control of the prescription blanks bearing hospital identification.
1. Limited-service Correctional Pharmacy
2. Limited-service Mail-Order Pharmacy
3. Limited-service Long-Term Care Pharmacy
4. Limited-service Sterile Pharmaceutical Products Pharmacy
5. Limited-service Nuclear Pharmacy
Only need to know the different types of limitedservice pharmacies for class – will need to know specifics for MPJE

2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
✓ Permits shall be conspicuously displayed in the location to which it applies.
✓ Current renewal license or duplicate current renewal license shall be in the practice site for inspection by the board or review by the public
✓ If a licensee practices in more than one place, the board may issue one or more duplicate current renewal licenses to the licensee for a fee
Applies to: Permittees (any permit holder) Licensee holders (pharmacists, interns, technicians)
Location of conduct:
Defined as:
Applies to actions in or outside of the state Applies to actions in or outside of the state
✓ Committing a felony with conviction by a court or a plea of no contest is conclusive evidence
✓ Committing an act that is related to qualifications, functions, or duties of a permittee and that demonstrates either a lack of good moral character or an actual or potential unfitness to hold a permit in light of the public’s safety.
✓ Working under the influence of alcohol or other drugs.
✓ Addiction to the use of alcohol or other drugs to such a degree to render the permittee unfit to perform the permittee’s employment duties.
✓ Violating a federal or state law or administrative rule relating to the manufacture, sale or distribution of

✓ Addiction to the use of alcohol or other drugs to such a degree as to render the licensee unfit to practice the profession of pharmacy (or hold a technician license)
✓ Working under the influence of alcohol or drugs
✓ Violating any federal or state law, rule or regulation relating to the manufacture or distribution of drugs and devices or the practice of pharmacy
✓ Knowingly dispensing a drug without a valid prescription order as required by ARS 32-1968 (A).
✓ Failing to report in writing to the Board any evidence that a pharmacist, pharmacy intern, graduate intern, pharmacy technician, or pharmacy technician trainee is or may be professionally incompetent, is or may
© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy

drugs, devices, poisons, hazardous substances or precursor chemicals.
✓ Violating state or federal recordkeeping requirements
✓ Failing to report in writing to the Board any evidence that a pharmacist, pharmacy intern, graduate intern, pharmacy technician or pharmacy technician trainee is or may be professionally incompetent, is or may be guilty of unprofessional conduct or may be mentally or physically unable to safely practice as such.
✓ Committing an offense in another jurisdiction that if committed in this state would be grounds for discipline.
✓ Committing fraud
✓ Failing to notify the board of a change of ownership, management, or pharmacist- in-charge.
✓ Over-ruling or attempting to overrule a pharmacy in matters of pharmacy ethics or interpreting laws pertaining to the practice of pharmacy of the distribution of laws and devices.
✓ …..and more…..
be guilty of unprofessional conduct or is or may be mentally or physically unable to safely practice as such
✓ Failing to report in writing to the Board any evidence that a permittee or a permittee’s employee is or may be guilty of unethical conduct or is or may be in violation of this chapter or a rule adopted under this chapter.
✓ Committing fraud
✓ …….and more……
After attending lecture and completing the assigned readings the student should be able to:
✓ Identify and describe the difference between a medication order and a prescription order
✓ Identify who has prescriptive authority in Arizona and what, if any restrictions are on their ability to prescribe; if prescriptive authority can be delegated; who can transmit/communicate prescription and/or refill authorization, if allowed, on behalf of the prescriber
✓ Identify under what circumstances a prescription order is valid or not valid.
✓ Identify if prescribers are allowed to write for themselves or family members.
✓ Identify and describe prescription requirements; how prescriptions may be received into the pharmacy and by whom.
✓ Identify and describe the rules and regulations regarding the transfer of prescription orders
✓ Identify and describe the storage requirements for prescription orders.
✓ Identify and describe the requirements for filling prescriptions (documentation; generic substitution, including FDA Orange Book; drug utilization review, storage/counting devices and FDA Purple Book)
✓ Identify and describe the label requirements for outpatient and inpatient pharmacy labels.
✓ Identify and describe central fill pharmacies rules
✓ Define “dispense”; identify and describe who has dispensing authority and exceptions.
✓ Identify and describe consultation requirements
✓ Identify and describe the DUR (Retrospective, Prospective reviews and educational programs) components of OBRA ’90 and state law
✓ Identify and describe patient package inserts and MedGuides and their requirements; identify and describe the role of risk management programs
✓ Identify and describe the rules for returning drugs and devices
✓ Identify and describe the statute and rules and regulations for pharmacist administered immunizations
✓ Identify and describe the rules and requirements for drug therapy management
✓ Identify and describe the rules and regulations for consultant and provider pharmacists/pharmacies in long-term care facilities (LTCF).
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Abood RR, Burns KA, Frankhauser F. Pharmacy Practice and the Law, 10th ed. Sudbury, MA: Jones and Bartlett; 2025
Chapter 3
✓ “Professional Practice Considerations,” (part of Durham -Humphrey Amendment) pgs. e117-e119
✓ “Prescription Drug Labeling Information for the Patient”; pgs. e122 – e126
✓ "The Orange Book and Generic Substitution”; pgs. e140 - e144
Chapter 6
✓ "Components of Prospective Drug Use Review” (aka DUR); pgs. e261 – e264
✓ “Federal regulation of long-term care”; pgs. e295 – e299
Arizona Revised Statutes and Arizona Administrative Code
✓ ARS §32-1901. Definition of prescription order and medication order
✓ ARS §32-1963.01. Substitution for prescription drugs; requirements; label; definitions
✓ ARS §32-1968. Dispensing prescription only drug; prescription orders; refills; labels, misbranding; dispensing soft contact lenses.
✓ ARS §32-1969. Filling foreign prescription orders; records, exceptions.
✓ ARS §32-1970. Implementing, monitoring, and modifying drug therapy and use; conditions; definitions
✓ ARS §32-1974. Pharmacists; administration of immunizations, vaccines and emergency medications; certification, reporting requirement
✓ AAC R4-23-402. Pharmacist, Graduate Intern, Pharmacy Intern
✓ AAC R4-23-407. Prescription requirements
✓ AAC R4-23-408. Computer records
✓ AAC R4-23-411. Pharmacist-administered or pharmacy or graduate intern-administered immunizations
✓ AAC R4-23-614. Automated storage and distribution systems
✓ AAC R4-23-615. Mechanical storage and counting devices for a drug in solid, oral dosage form
✓ AAC R4-23-616. Mechanical counting device for a drug in solid, oral dosage form
✓ AAC R4-23-621. Shared services (aka central fill)
✓ AAC R4-23 -701 – 703 (Article 7) Non-pharmacy licensed outlets.
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale

Defining "Prescription orders" vs. "Medication orders" (ARS 32-1901)
Prescription Order Medication Order
✓ Order for medication dispensed to or for the ultimate user.
✓ Typically used in the out-patient setting

✓ Order for medication which is dispensed for immediate administration to the ultimate user
✓ Typically used in the in-patient setting (written or verbal)

95 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Prescriptive authority (AZ)
Prescriptive authority varies by state
In ARIZONA: These prescribers are exemept from licensure by the ASBP
NOTE: Know the limitations/restrictions on each of the presribers, if there are any.

No Restrictions
No prescribing restrictions based on scope of practice OR additional requirements beyond original licensure.
• Allopathic Physician (MD)
• Osteopathic Physician (DO)

Additional Educational Training (Same authority as MD/DO)

Additional Educational Training & Board Approval (Same authority as MD/DO)

Limitations based on Scope of Practice (Same authority as MD/DO within their scope of practice)

Restricted Prescriptive Authority (Prescribing restrictions based on profession).
• Homeopathic Medical Doctor (HMD)
• (Must be an MD or DO)
• Registered Nurse Practitioner (RNP; also known as APP or APNP)
• Dentist (DDS/DMD)
• Podiatrist (DPM)
• Veterinarian (DVM)
• Naturopathic Doctor (NMD)
• Doctor of Optometry (OD)
• Physician Assistant (PA)
PRESCRIPTIVE AUTHORITY and AGENCY


Prescriptive authority CANNOT be delegated by the prescriber to an employee or agent of the prescriber
UNLESS state law has specifically granted the person this authority (e.g. PA's, pharmacists, etc.)
A physician’s employee or agent CAN transmit or communicate the prescription or refill authorization on behalf of the prescriber as long as designated as “Agent.”
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Agency = fiduciary relationship that arises when one person (“registered prescriber”) gives authorization to another person (“agent” or employee) to act on the registered prescriber’s behalf and subject to the registered prescriber’s control and the agent/employee consents. (We will talk more about “Agency” in the controlled substance lectures).
(Full size PDF of this chart posted on Canvas) ASBP Prescriptive Authority Chart – Last updated 2/2019

Prohibited acts:
✓ A prescription order issued on the basis of an internet-based questionnaire or an internet-based consultation without a medical practitioner-patient relationship is not legal in Arizona.
✓ MDs in Arizona are not allowed to write controlled substance prescriptions for themselves or for family members. It is considered "unprofessional conduct." (ARS 32-1401 (27) (g)(h)) (Medicine)
27. "Unprofessional conduct" includes the following, whether occurring in this state or elsewhere:
(g) Using controlled substances except if prescribed by another physician for use during a prescribed course of treatment.
(h) Prescribing or dispensing controlled substances to members of the physician's immediate family.
Similar rules are in place for all other health care professionals (DOs, PAs, RNPs, Naturopaths, DPM, ODs, DVMs, DDS/DMD)
Prescription order requirements (aka prescriptions)
Expiration Date of Non-controlled Prescription Orders
All non-controlled prescription orders expire 1 year from the date of issue unless otherwise noted (maybe shorter – not longer)

All (non-CS and CS) prescriptions must meet the following requirements: (ARS 32-1968 and AAC R423-407) and Abood and Burns “Prescriptive Authority” – pgs. 111-114)
99 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Be issued for a legitimate medical purpose
Be written by a practitioner acting in the usual course of his/her professional practice
Examples:
❖ Would a prescription written by a veterinarian (DVM) for a human for amoxicillin 500mg be valid?
❖ Would a prescription written by a podiatrist (DPM) for Astelin (azelastine) for allergic rhinitis be valid?
❖ Would a prescription written by a dentist (DDS) for Tylenol #3 (acetaminophen 300mg/codeine 30mg) for knee pain be valid?
❖ Would a prescription written by a psychiatrist (MD) for Norco 5/325 (hydrocodone 5 mg/acetaminophen 325mg) for TMJ be valid?
VALIDITY OF NON-CONTROLLED PRESCRIPTION ORDERS ISSUED BY MEDICAL PRACTITIONERS IN FOREIGN COUNTRIES (ARS 32-1969)

Prescription orders for non-controlled substances issued by a medical practitioner in any foreign country may be dispensed by an Arizona pharmacy as long as the practitioner is licensed by the appropriate licensing board in their foreign country.
Example from France and the EU https://www.cleiss.fr/particuliers/partir/soins/ue/prescriptions_en.html
Example from Australia https://www.medicalboard.gov.au/~/link.aspx?_id=b19bed3ca4d34c86a4e692db96f77eb8&_z=z
TAMPER-RESISTANT PRESCRIPTION PAD REQUIREMENT FOR AHCCCS PRESCRIPTION ORDERS
As of November 1, 2012, all written (hand-written or EMR generated) AHCCCS prescription orders (non-CS and CS) must be on tamper-resistant prescription pads/paper per CMS.
Written and non-electronic prescriptions (EMR generated) must contain ALL three of the following characteristics for AHCCCS prescriptions:
1. Prevent unauthorized copying of a completed or blank prescription form
o E.g., thermo chromic ink (when photocopied displays “void” pattern)
2. Prevent erasure or modification of information written on the prescription by prescriber
o E.g., tamper-resistant background ink showing erasures or attempts to change written information
3. Prevent use of counterfeit prescription forms
o Example: Sequentially numbered prescription blanks
100
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale

Graphic from: http://www.rxpaper.com/pads1.jpg
2. Full name and address of the patient or owner of an animal & species of the animal
All prescriptions are required to have the following information on the FACE of the prescription order (upon receipt [receiving] in the pharmacy): (ARS 32-1968 (A) and (C) and ARS 36-2525) 1. Date of issue
3. Full name, address, and telephone of the prescriber
Drug name, strength, and dosage form 5. Quantity
Prescriber signature 8. Refills, if authorized
DEA number if for a controlled substance

1. Date issued
2. Full name and address of the patient
For an animal, it is the owner's name and species of animal
Example: Mary Gurney, feline
3. Full name, address, and telephone number of the prescriber
4. Drug name, strength, and dosage form
If the dosage form is missing and there is only one dosage form available – the pharmacist may add this without verifying with the prescriber
o e.g., Rx written as Lexapro 10 mg; RPh adds “tablets” to the prescription order.
If the dosage form is missing and there are multiple dosage forms/routes available for a drug name – the pharmacist needs to call the prescriber and verify what they want dispensed
o Examples
▪ Ciprofloxacin HCL solution – this could be OTIC or OPTHALMIC;
▪ Fluoxetine HCL 20 mg is available as a TABLET and a CAPSULE
If the salt form of the established (generic) name is missing when multiple salts are available –the pharmacist needs to call the prescriber and verify what medication they want dispensed
o Examples
▪ Metoprolol – available as tartrate and succinate salts with similar strengths
▪ Hydroxyzine – available as hydrochloride (HCL)(tablet) or pamoate (capsule)
5. Quantity
6. Directions for use
7. Prescriber's signature
Manual signature; or
Electronic/digital signature in electronically-transmitted prescriptions; or
Electronic only signature on prescription orders generated by electronic media (EMR/EHR) MUST be printed on security paper that prevents copying or alteration; or
Electronic signature with manual signature/initials are allowed for prescription orders generated by electronic media (EMR/EHR) on regular paper are allowed; or
Not required for orally-transmitted prescriptions (RPh signs for prescriber)
8. Refills if authorized
9. DEA number if a controlled substance
By default…

…this information must be written directly on the hard-copy (original if written)
With computerized records…


…a print-out of the patient and prescription information entered into the computer system and attached to the hard copy may satisfy the above requirements.
Does this prescription order have all of the required information?
If the system can scan and store the image of the original prescription order…

…this information must be directly associated with the scanned prescription.

Receiving prescriptions from prescribers/patients (ARS 32-1968, AAC R4-23-407 and R4-23-1104)
A pharmacist, intern, or tech (interns and techs have some limitations) can accept written, faxed, or eprescriptions
Method
NEW RX
Brought in by PATIENT
Faxed or emailed by PATIENT (ARS 32-1968 (A)(8))
Orally transmitted prescription from prescriber (or agent)
Who can accept Rx into the pharmacy
Pharmacist, Intern, or Technician
Pharmacist, Intern, or Technician
Pharmacist or Intern
Additional Requirements
Faxed by prescriber (Applies to all Rx, CS, and OTCs).
Note: CIIs have special rules that we will talk about in lectures 8 - 10
Electronic transmission (All RX, CS – including CII orders)
Pharmacist, Intern, or Technician
To pick up the medication, the patient MUST present the original prescription order with the prescriber’s manual signature.
Must be immediately reduced to writing.
Document who called in the Rx order, date, and time (AAC R4-23-402 (A)(2))
Faxed to the pharmacy of the patient’s choice
Contains all information required in ARS 32-1968 and ARS 36-2525
Faxed from prescriber practice location
Exception: Hospital, LTCF, in-patient hospice for a patient in these facilities
Additional information required:
Date prescription order faxed
Fax number of prescriber or facility (if hospital, LTCF, or inpatient hospice) and telephone number of the facility
Name of person transmitting the fax (if other than prescriber)
Pharmacist, Intern, or Technician
Contains all information required in ARS 32-1968 (all) and ARS 36-2525 (for CS)
Date of transmission
Prescriber name and/or authorized agent
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
AUTHORIZATION OF REFILL(S)
Orally transmitted prescription
Who can accept RX into the pharmacy
Pharmacist, Intern, or Technician
Maintains prescription order as specified in ARS 32-1964 (record of prescription order) OR R4-23-408(H)(2)
Order transmitted to pharmacy of patient’s choice
Additional requirements
Technician – only if there are NO changes to the order
Document who called in the Rx order, date, and time (AAC R4-23-402 (A)(2))
Medical practitioner’s telephone number and fax number
Faxed by prescriber
Pharmacist, Intern, or Technician
Electronic transmission
Pharmacist, Intern, or Technician
Medical practitioner’s signature or medical practitioner’s agent’s name
Date of authorization
Contains all information required in ARS 32-1968 (all) and ARS 32-2525 (for CS)
Date of transmission
Prescriber name and/or authorized agent
Maintains prescription order as specified in ARS 32-1964 (record of prescription order) OR R4-23-408(H)(2)
Order transmitted to pharmacy of patient’s choice
105 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale

Example of an Electronic Prescription (e-prescribe)
Transferring prescriptions (AAC R4-23-407 (E))
General Information
✓ Both the original and the transferred prescription order are kept for 7 years after the last dispensing date (aka date of last fill)
✓ Number of transfers limited to the number of original refills for non-controlled substances.
Example – A prescription for Lisinopril 10 mg Tablets #30 with 12 refills can be transferred 12 times (once for each refill)
✓ Controlled substances prescription orders with refills may only be transferred once.
Exception: Pharmacies sharing a common, real-time online database (i.e., Walgreens, CVS).
107 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Type of Rx Transfer
(AAC R4-23-407 (E) (4), (5), (6), and (7))
Non-Controlled Rx (within Arizona)
CS III, IV, V Rx (within Arizona)
Non-Controlled Rx (outof-state)
CS III, IV, V Rx (out-ofstate)
RPh = Pharmacist (Licensed)
Verbal Electronic (between pharmacies with the same owner/ company) using a common or shared database.
✓ 2 RPhs
✓ RPh and Intern
✓ 2 Interns
✓ 2 RPhs
✓ RPh and Intern
✓ 2 Interns
✓ 2 RPhs
✓ RPh and Intern
✓ 2 Interns
✓ 2 RPhs
✓ RPh and Intern
✓ 2 Interns
Intern = Pharmacy intern (Licensed)
Tech = Pharmacy technician (Licensed)
Tech Trainee = Technician Trainee (Licensed)
✓ RPh
✓ Interns ✓ Tech
✓ Tech Trainee
✓ 2 RPhs
✓ RPh ✓ Interns ✓ Tech ✓ Tech Trainee
✓ 2 RPhs
✓ 2 RPhs
✓ RPh and Intern
✓ 2 Interns
Not allowed by Fax (per DEA)
✓ 2 RPhs
✓ RPh and Intern
✓ 2 Interns
Not allowed by Fax (per DEA)
Transfer (Verbal, Electronic, and Faxed) – The Sending Pharmacist
The sending pharmacist must record:
The word "VOID" on the face of the transferred prescription
For a pharmacy with computerized prescription records, this can be substituted with a record of the transfer in the system that invalidates the prescription
Date of transfer
Name of receiving pharmacist (or grad intern or pharmacy intern)
Name of the receiving pharmacy
Store no. or address and phone of the receiving pharmacy
Name of sending pharmacist (or grad intern or pharmacy intern)
DEA number of receiving pharmacy if a controlled substance
108 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
NOTE: Even though your copy of the prescription is now void, you still have to maintain it for the 7 years required by AZ law!
Example of “Voided” Prescription Order after it has been transferred:
Rx originally filled at Midwestern Pharmacy and is being transferred to Utopia Avenue Clinic Pharmacy.

Back of Original (Hard copy): 09/23/2024
Transfer to Utopia Ave Clinic Pharmacy
623-572-1212
19555 N 59th Avenue, Glendale, AZ 85308
Utopia Ave Pharmacy: Roger Morris, RPh
Midwestern University Pharmacy: Mary Gurney, RPh
Verbal Transfer - The Prescription Order from the Receiving Pharmacist Perspective
The receiving pharmacist must record:
All information required on a prescription
The word "Transfer" on the face of the prescription
For e-prescriptions, the transfer record itself is sufficient
Date of transfer
Prescription number from sending pharmacy
Date of issuance
Date first dispensed
Original number of refills
Number of refills remaining and date last dispensed
Date(s) and locations of previous refill(s) for CS (21 CFR 1306.25 – Fed stricter than the state)
The name, address, DEA registration number, and prescription number from the pharmacy that originally filled the prescription, if different than the one currently transferring the
109 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
prescription to the receiving pharmacy (DEA Rule – 21 CFR 1306.25 http://www.deadiversion.usdoj.gov/21cfr/cfr/1306/1306_25.htm)
Name of sending pharmacist (or grad intern or pharmacy intern)
Name of the sending pharmacy
Store no. or address and phone of the sending pharmacy
Name of receiving pharmacist
DEA number of transferring pharmacy if a controlled substance
Example of Transfer Prescription Pad


Example of a Transferred Prescription
110 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Date of Transfer: 09/23/2024
Original Rx #: 123456
Original date of issue: 04/03/2024
Date of first fill: 04/03/2024
Original refills: PRN
Date of last fill: 08/23/2024
Midwestern Pharmacy 6235551212
67th and Beardsley, Glendale, AZ 85308
Midwestern RPh: Mary Gurney
Utopia RPh: Roger Morris

What circumstances are we talking about (See Substantive Policy Statement from the ASBP for additional information:
https://drive.google.com/file/d/12J3PqRSQf9wNNmYqu191blr1sA5DiV6d/view & https://drive.google.com/file/d/11eVT5pWmQp55cLfEj_H-8mClhYzxCPoC/view
Pharmacy A receives Rx by phone or fax from the prescriber
Pharmacy A does not have the drug OR the patient chooses to go to Pharmacy B to get the medication
How does Pharmacy B get the prescription information?
Pharmacy A (Original receiving pharmacy)
•Run prescription through the computer system so that it is assigned an Rx number (serial number)
•Void the prescription in the computer system
•Transfer prescription to Pharmacy B following the transfer rules
•Must maintain the hard-copy and computer records; indicate that the original and refill information was transferred to Pharmacy B and that no quantity of drug was dispensed
•If original Rx was a faxed prescription, fax the prescription to pharmacy B after completing the verbal transfer.

Pharmacy B (Pharmacy to which Rx is being tranferred
•Document transfer as the receiving pharmacy
•If receive the faxed prescription from Pharmacy A, place faxed Rx with transfer Rx documentation in pharmacy files.
All prescription orders must be maintained for a minimum of 7 years from the date of last fill (AZ Law)
General federal requirement is 2 years from date of last fill (DEA)
Medicare prescription retention requirement is 10 years from date of last fill (Federal)
The original of every prescription order must be serially numbered (Rx number), dated, and filed in the order in which the drugs were compounded or dispensed.
112 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
If an electronically transmitted prescription order automatically populates a pharmacy's computer system fields, the automated record constitutes the original prescription, and a hard copy or electronic image is not required.
All prescription records shall be made available to the Board within 72 hours of a Board request. (AAC R423-408(I)
Electronic imaging recordkeeping system AAC R4-23-408(H)
Some pharmacies can scan and save images of original prescriptions
The prescription image and all associated annotations must be maintained for 7 years from the date last dispensed
The original hard copy for a non-controlled substance is not required to be retained (NEW)
The original hard copy for all controlled substance prescription orders (CII, CIII, CIV, and CV) MUST be maintained for 7 years from the date of last fill (NEW)
All hard copies of prescription orders from a foreign country must be maintained for 7 years from the date of last fill.
Documenting a fill (AAC R4-23-407 (A))
NEW RX: THE FOLLOWING MUST ALWAYS BE DOCUMENTED:
All legal requirements of a prescription order (ARS 32-1968 (non-control) and ARS 36-2525 (CS)
Serial number (Rx number)
Date of dispensing
Quantity dispensed, if different than what was prescribed
Name or initials of the dispensing pharmacist or intern
Name of the drug's or device's manufacturer or distributor if the prescription order is written generically or a substitution is made
113 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale

REFILL INFORMATION:
Serial number (Rx number)
Date of dispensing (refilled)
Quantity dispensed
Back of RX:
Rx # 545454
Date filled: 04/03/2024
Qty: 180
Dispensed: metoprolol tartrate 50 mg tablets (Teva)
RPh: mkg
Name or initials of the dispensing pharmacist or intern
Name of the drug or device manufacturer or distributor if the prescription order is written generically or a substitution is made
Refill “Tag” for refill records example – print/hard copy (this is the required information)
Rx #545454
Date filled: 05/03/2024
Qty dispensed: 180
RPh: rm
Manuf: Teva Labs (required if filled generically or generic substitution made)
By default, this information must be recorded on the back of the original prescription. However...
COMPUTERIZED DOCUMENTATION OF REFILLS
Most pharmacies today maintain computerized prescription records. In this case, refill information is recorded electronically, instead of on paper. However, each dispensing pharmacist must document daily that the computer data of the refills he or she dispensed were reviewed for accuracy. This is done in one of two forms:
1. A hard-copy printout of each day's original and refill data
2. A log book or separate file of daily 'statements' (e.g. "I attest the accuracy of the original and refill data for prescriptions I dispensed on [DATE]")
114 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
And records the following information:
1. States that original and refill data for prescriptions dispensed by each pharmacist were reviewed for accuracy
2. Printed name of each dispensing pharmacist
3. Signed and initialed by each dispensing pharmacist
Daily Log Book Example:
Date New (N) /Refill R)
04/03/24 R 123457 David Letterman Cyclobenzaprine 10 mg Watson #30 MKG Dr. Smith
04/03/24 N 123458 Susie Picabo Cartia XT 180mg Andrx #30 SJC Dr. Crowe
04/03/24 N 123459 David Letterman Celexa 10mg Forest Labs #30 MKG Dr. Smith
I attest to the accuracy of the original and refill data for prescriptions dispensed on this date _04/03/2024
Mary K Gurney____________________________ _Mary K Gurney
Printed name of Pharmacist
Pharmacist signature or initials
I attest to the accuracy of the original and refill data for prescriptions dispensed on this date 04/03/2024
Stephanie J Counts _______________________ _Stephanie J Counts
Printed name of Pharmacist Pharmacist signature or initials
Drug substitution (ARS32-1963.01)
If a medical practitioner prescribes a brand name drug and does not indicate an intent to prevent substitution, a pharmacist may fill the prescription with a generic equivalent drug. "Dispense as Written"
Substitution is NOT permissible if the prescriber has somehow indicated his or her intent to prevent it. Some statements that indicate the prescriber's intent to prevent generic substitution include…
DAW Do Not Substitute If not indicated in any way, substitution is allowed
Dispense as Written Medically Necessary
115 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
The FDA Orange Book
aka Approved Drug Products with Therapeutic Equivalence Evaluations (yes you need to know both the books official name and how it is usually referred to).
Guide for healthcare professionals to determine therapeutic equivalence
Published annually with monthly supplements (electronic version at http://www.fda.gov/cder/ob/)

NOTE: There is a mobile app for the FDA Orange Book – check out Google Play or the Apple App Store.
EQUIVALENCY
Terminology
Bioequivalent
Definition
Products display comparable bioavailability (rate and extent of absorption) at the site of action under similar conditions.
Pharmaceutical Equivalent Drug products that have the same:
✓ form,
✓ route,
✓ strength, and
✓ active ingredients
Therapeutic Equivalent = (Federal terminology) (TE Code)
Generic Equivalent (AZ terminology – ARS 321963.01)
Pharmaceutically equivalent + Bioequivalent
Term applied to therapeutic equivalent drug products approved for generic substitution
116 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
THERAPEUTIC EQUIVALENCE CODE
✓ Equivalency is indicated using a two-letter "therapeutic equivalency" (TE) code.
✓ Drugs under the same heading with the same equivalence code are considered generic equivalents.
✓ A number is added to a TE code in situations where more than one reference listed drug of the same strength has been designated under the same heading.
A-rating
Bioequivalent to the reference listed drug
Categories
AA = drug in conventional dose forms, no equivalency problems
AN = solution and powders for aerosolization with no known equivalency problems
AO = injectable oil solutions with no known equivalency problems
AP = injectable aqueous solutions with no known equivalency problems
AT = topicals with no known equivalency problems
AB = drugs meeting necessary bioequivalence requirements
✓ AB ✓ AB1 ✓ AB2, etc
B-rating
NOT bioequivalent to the reference listed drug
Categories
BC = time-release drugs with bioequivalence issues
BT = topicals with bioequivalence issues
BX = insufficient data to determine therapeutic equivalence
B* = no determination of equivalence made, unresolved questions
NOTE: Bioequivalency and effectiveness are not the same thing!
Each state has its own language for defining what drugs are or are not equivalent. In Arizona, a "generic equivalent" is…
…a drug that has an identical amount of the same active chemical ingredients in the same dosage form, that meets applicable standards of strength, quality and purity according to the United States pharmacopeia or other nationally recognized compendium and that, if administered in the same amounts, will provide comparable therapeutic effects. Generic equivalent or generically equivalent does not include a drug that is listed by the federal food and drug administration as having unresolved bioequivalence concerns according to the administration's most recent publication of approved drug products with therapeutic equivalence evaluations.
117 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
This is taken directly from the Orange Book. In summary, it means A or AB-rated products may be substituted and B-rated products may not. However, it also leaves open the possibility to allow substitutions of drug products that satisfy the above requirements but are not listed in the Orange Book.
AZ Prescription Order Adaptation – Non-controlled substances ONLY (New) AAC R4-23-407(C) (2) –Effective 3/14/2020
Prescription order adaptation: for non-controlled substances, a pharmacist using professional judgment, may make the following adaptations to a prescription order if the pharmacist documents the adaptation in the patient’s record:
1. Change the prescribed quantity if the prescribed quantity is not a package size commercially available from the manufacturer
2. Change the prescribed dosage form or directions for use if the change achieves the intent of the prescribing medical practitioner
3. Complete missing information on the prescription order if there is sufficient evidence to support the change; and
4. Extend the quantity of a maintenance drug for the limited quantity necessary to achieve medication refill synchronization for that patient
EXAMPLES from the FDA “Orange Book”
The products that are AB rated will have match to the RLD or RS on the following:
✓ Same drug
✓ Same strength
✓ Same dosage form
The RLD that they had been referenced to has been discontinued by the manufacturer (Benzaclin)


Reference Listed Drugs (RLDs) are what other products are compared to for bioequivalence



Reference Standard (RS): A reference standard is the drug product selected by FDA that an applicant seeking approval of an ANDA must use in conducting an in vivo bioequivalence study required for approval.
DILTIAZEM HCL EXTENDED 120 MG EXTENDED RELEASE CAPSULES
BX rated drugs are not allowed to be substituted
The products highlighted in yellow are substitutable with each other (AB3 ratings)
The Diltiazem HCL extended-release (AB1 (purple), AB2 (green) & AB 4 (red/pink)) are not substitutable with/for any AB3 rated diltiazem.
AB1 = AB1
AB2 = AB2
AB3 = AB3
AB4 = AB4






Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations (FDA)
CDER and CBER both maintain lists for the Purple Book based on their internal jurisdiction.
(URL:
http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApp lications/TherapeuticBiologicApplications/Biosimilars/ucm411418.htm)
The “Purple Book” lists biological products, including any biosimilar and interchangeable biological products licensed by FDA under the Public Health Service Act (the PHS Act).
To be used in a similar capacity as the Orange Book.
The lists are designed to enable users to see whether a particular biological product has been determined by the Food and Drug Administration (FDA) to be biosimilar to or interchangeable with a reference biological product.
A “reference product” is the single biological product licensed by FDA under section 351(a) of the PHS Act against which a proposed biological product is evaluated in an application submitted under section 351(k).
“biosimilar” or “biosimilarity” means that the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components, and there are no clinically meaningful differences between the biological product and the reference product in terms of safety, purity and potency of the product.

120

B = Biosimilar; I = Interchangeable
(ARS 32-1963.01)
The four following conditions MUST be met for a pharmacist to substitute a biological product for the biological that was prescribed:
1. The United States Food and Drug Administration has determined the substituted product to be an interchangeable (emphasis added) biological product.
2. Substitution is not prohibited by the prescriber (same methods as medications)
3. The pharmacy informs the patient or person presenting the prescription of the substitution of the price difference.
4. Within five business days after dispensing a biological product, the dispensing pharmacist or the pharmacist's designee makes an entry of the specific product provided to the patient, including the name of the product and the manufacturer. The communication shall be conveyed by making an entry that is electronically accessible to the prescriber through an interoperable electronic medical records system, an electronic prescribing technology, a pharmacy benefit management system, or a pharmacy record. Entry into an electronic records system as described in this paragraph is presumed to provide notice to the prescriber. Otherwise, the pharmacist shall communicate the biological product dispensed to the prescriber using fax, telephone, electronic transmission or other prevailing means, except that communication is not required if one of the following applies:
a. There is no interchangeable biological product approved by the United States food and drug administration for the product prescribed.
b. A refill prescription is not changed from the product dispensed on the prior filling of the prescription.
Retrospective Review
✓ DUR board composed of physicians and RPhs reviews patient profiles to see if actual medication use conforms to a predetermined ideal (standard of practice)
Example: drug therapy duplicates, is drug therapy appropriate
Prospective Review
The 3 components of Prospective DUR:
1. Screen of prescriptions before dispensing
2. Patient counseling by pharmacist
3. Pharmacist documentation of relevant information

Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
ACTIVITIES THAT MUST BE COMPLETED DURING THE SCREENING OF PRESCRIPTIONS
A pharmacist MUST verify the feasibility of dispensing a drug based upon:
Drug allergies
Incompatibilities with a patient's current medications
o Therapeutic duplication
o Contraindications
o Drug-drug interactions (including OTCs)
Use of unusual quantities of dangerous drugs or narcotics
Frequency of refills
o Possible indication of improper dosing
Patient profile MUST contain the following information: (R4-23-402)
Name, address, telephone number, date of birth (or age), and gender
Known diseases and medical conditions
Known drug allergies or drug reactions
A comprehensive list of medications currently taken (if available)
Medical devices currently used (if available)

A pharmacist must also keep record of comments relevant to the patient's drug therapy.
Documentation should be complete enough to:
Serve as a reminder to the RPh writing the information
Provide info for other pharmacists working in the same pharmacy
Show a record of what was done, so that someone may be able to connect an action with an outcome
Show that OBRA’90 and state law requirements are being met
123 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
EXAMPLE OF A COMMUNITY PHARMACY DATA ENTRY SCREEN FOR PRESCRIPTION ORDERS AND DUE ACTIVITIES

Special regulations exist for these devices regarding when and how they can be used. For the most part, regulations require pharmacies to develop and implement policies and procedures for their use.
"Mechanical storage and counting device for a drug in solid, oral dosage form"

A mechanical device that stores and counts and may package or label drugs in solid, oral dosage forms for dispensing.
Mechanical counting device for a drug in solid, oral dosage form"

"Automatic storage and distribution system"

A mechanical device that counts drugs in solid, oral dosage forms for dispensing and includes an electronic balance when used to count drugs.
A mechanical system that performs operations or activities other than counting, compounding, or administration, relative to the storage, packaging, or distributing of drugs or devices and that collects, controls, and maintains all transaction information.
Label requirements (ARS 32-1968 (D) and R4-23-402 (A)).
Outpatient pharmacy labels
Pharmacy name and address
Rx number (Serial Number)
Date of dispensing
Prescriber name
Patient name
For an animal, the name of the owner & the species of the animal
Directions for use and any cautionary statements, if any, contained in the prescription order

125 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
In addition, for AZ:
Manually initial the finished label as indication of final accuracy check
This is waived if the pharmacy computer system complies with the computer documentation requirements
FOR MEDICATIONS WHERE GENERIC SUBSTITUTION OCCURS (ARS 32-1963.01)
Required to be on the label:
Generic equivalent for (Brand or trade name)
FOR COMPOUNDED PRODUCTS (AAC R4-23-409) DISPENSED TO A PATIENT
The above requirements, plus…
Beyond use date
Statement or symbol designating a compounded pharmaceutical product, AND
Accompanied by a list of all active ingredients
FOR A COMPOUNDED PRODUCT DESIGNATED "FOR OFFICE USE ONLY" (AAC 4-23-409)
Written list of the compounded pharmaceutical products active ingredients is provided with the product
Pharmacy name, address and telephone number
Pharmaceutical product's name with statement/symbol/designation/or abbreviation that identifies it as a compounded product
Beyond use date
Lot/control number
The statement "Not for Dispensing" AND
The statement "For Office or Hospital Administration Only"
Inpatient pharmacy labels (Including LTCF)
FOR UNIT-DOSE PACKAGES
Drug name, strength, and dosage form
Lot number and beyond-use-date
Appropriate auxiliary labels
126 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Drug name, strength, and dosage form
Lot number and beyond-use-date
Appropriate auxiliary labels
Mechanism to identify pharmacist accountable for repackaging
Patient's name and location
Name and quantity of the basic parenteral solution
Name and amount of drug added
Date of preparation
Beyond-use-date and time
Guidelines for administration
Appropriate auxiliary label or precautionary statement
Initials of pharmacist responsible for admixture preparation
Child-resistant containers (~ this is review from PPPA)
By default, all drug products must be dispensed in some form of child-resistant packaging.
There are several drug products exempt from this requirement (e.g. sublingual nitroglycerin, oral contraceptives, etc.)
Child-resistant packaging is not required in institutional settings where the medication will be dispensed by a licensed health care professional.
NOTE: You CANNOT reuse child-resistant closures (reuse compromises the effectiveness of the closure)!
An individual prescription may be dispensed in noncompliant packaging if requested by either the prescriber or the patient
All prescriptions for a patient may be dispensed in noncompliant packaging only at the patient's request.
127 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Definitions
Shared order filling ✓ Preparing, packaging, compounding, or labeling an order, or any combination of these functions.


Shared order processing ✓ Interpreting the order, performing order entry verification, drug utilization review, drug compatibility and drug allergy review, final order verification, and when necessary, therapeutic intervention, or any combination of these order processing functions.
✓ After order processing is completed, returning the processed order to the requesting pharmacy for order filling and delivery to the patient or patient's caregiver or, at the request of this pharmacy, returning the processed order to another pharmacy for order filling and delivery to the patient or patient's care-giver.
Shared services ✓ Shared order filling or shared order processing, or both.
NOTE: Shared services may be provided outside of a pharmacy (e.g. pharmacists that work from home)
Requirements for shared service providers (R4-23-621)
1. Participating pharmacies must meet one of the following prerequisites:
Same owner, OR
Written contract, AND
Shared common electronic /technology prescription database
2. Patients must be notified that their prescriptions may be filled by another pharmacy. (Does not apply to patients in facilities where medications are administered by licensed health care professionals)
3. The prescription must identify every person (Pharmacist, Intern, and Technician) that processed, filled, dispensed, and counseled on the prescription.
128 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
"Dispense" means to:
deliver to an ultimate user or research subject;
based on a lawful prescription or medication order; by or pursuant to the lawful order of a practitioner,
includes the prescribing, administering, packaging, labeling, or compounding necessary to prepare for that delivery.
Dispensing authority
By default, prescription drugs may be dispensed to an individual
only pursuant to a prescription, or
administered directly by the physician or other authorized prescriber.
NOTE: physician dispensing is not prohibited in AZ or by the federal government. Other states do restrict physician dispensing.
Dispensing in Institutional Settings
In an institutional setting, drugs are dispensed from the hospital pharmacy only upon a medication order from an authorized medical practitioner.
The medication order must first be reviewed by a pharmacist before administration.
EXCEPTIONS
✓ A facility that does not have a 24-hour pharmacy may dispense medications without prior review by a pharmacist while the pharmacy is closed.

✓ In an emergency, when the pharmacy is closed, a supervisory nurse may be allowed access to the pharmacy to obtain the necessary medications.
✓ In these cases, the pharmacy must review all medication orders within 4 hours of reopening.
PATIENT SELF-ADMINISTRATION
By default, a patient cannot self-administer medications.
129 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
This is only allowed if specifically ordered by the medical practitioner and if the patient is given proper training beforehand.
DRUGS BROUGHT IN BY THE PATIENT
If a patient brings in drugs from home, they can only be administered to the patient after being identified by the pharmacy and with a medication order by a medical practitioner.
If the drugs are not to be administered to the patient, they must be packed and sealed by the pharmacy and either stored to be given to the patient at discharge or given to the patient's agent for removal from the hospital.
Specific hospital policies may restrict this practice further.
Oral consultations
WHEN AM I REQUIRED TO GIVE AN ORAL CONSULT? (OBRA ’90 AND AZ AAC R4-23-402 (C) – (H))
1. New drug for the patient
2. Change in drug (strength, directions, etc.)
3. Requested by patient
4. Professional judgment warrants a consult
A patient or patient's caregiver may refuse consultation at any time.
A prescription medication or prescription-only device, delivered to a patient at a location where a licensed health care professional is responsible for administering the prescription medication to the patient, is exempt from consultation requirements.
WHAT INFORMATION ARE PHARMACIST REQUIRED TO CONSULT ON? (AAC R4-23-402 (C) (1 – 4)
Name and strength of medication
Direction for use
Route of administration
Indication
Any special instructions and explanation of auxiliary labels
Written information must also be provided regarding side effects, procedure for missed doses, and storage requirements
WHAT ADDITIONAL INFORMATION MAY THE PHARMACIST PROVIDE? (AAC R4-23-402 (E) (1-4)
Common severe adverse effects, interaction, or therapeutic contraindications, and the action required if they occur
Self-monitoring techniques
130 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Duration of drug therapy
Prescription refill information
WHAT IF FOR SOME REASON THE PHARMACIST CAN'T CONSULT THE PATIENT DIRECTLY? (AAC R4-23402 (D) (1 -3)
Provide written information to the patient or patient's caregiver that summarizes the information that would normally be orally communicated
Document why oral consultation was omitted
Provide the patient or patient's caregiver a method to communicate with a pharmacist at a later time
DOCUMENTING A CONSULT (AAC R4-23-402 (G) AND (H)
When an oral consult is required, regardless of whether or not it is provided, the pharmacists or intern must document this with his or her name or initials
Whether or not consultation was given
If not give, documentation of why (patient refusal, not necessary in professional judgment, etc.)
Initial and sign documentation
FDA may require a manufacturer to employ special procedures for certain drug products aimed at improving patient safety. These procedures typically involve pharmacist by requiring them to dispense additional information to the patient or verify the patient's health status prior to dispensing the medication.
Failure by a pharmacist/pharmacy to comply with REMS requirements constitutes MISBRANDING
Patient Package Inserts (PPIs)
Required for all oral contraceptive drugs, estrogens, progesterones (including medroxyprogesterone)
Informational leaflet for patients regarding medication uses, risks, and precautions
Manufacturers must provide one for each package it intends to be dispensed
Pharmacists in a community pharmacy must dispense a PPI each time the medication is filled/refilled
Pharmacists in an institutional pharmacy must dispense a PPI prior to administering the first dose and once every 30 days thereafter
Medication Guides (MedGuides)
Required for specific drugs posing a "serious and significant concern"
131 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Informational leaflet for patients including indications, contraindications, serious adverse reactions, proper use, cautions, and other general information
Manufacturers must provide a sufficient quantity of MedGuides to be dispensed
Pharmacists must dispense a MedGuide each time the medication is filled/refilled http://www.fda.gov/Drugs/DrugSafety/ucm085729.htm
Risk Management Programs
Required for a few especially hazardous drugs
Examples
RevAssist® online database for Revlimid (lenalidomide)
Risk: Lenalidomide is a potent teratogen – avoid any fetal exposure
Women must have record of negative pregnancy test prior to each dispensing
Men must be counseled about contraceptive practices prior to each dispensing
Caprelsa® (vandetanib) used in treatment of symptomatic or progressive medullary thyroid cancer
Risk: CAPRELSA can prolong the QT interval and cases of Torsades de pointes and sudden death were reported in clinical trials.
Prescribers must be certified to prescribe the drug.
Pharmacies must be enrolled to dispense the drug
A drug product dispensed to a patient may only be returned for resale if the pharmacist determines that:
✓ The drug is in its original, manufacturer's, unopened container.
✓ The drug or its container has not been subject to contamination or deterioration.
A device may only be returned for resale if the pharmacist determines that:
✓ The device is inspected and free of defects
✓ The device is rendered incapable of transmitting disease
✓ If resold or reused, the device is not claimed to be new or unused
In a LTCF, a drug may be returned and re-dispensed if the pharmacist determines that:
✓ The drug has been stored in compliance with the official compendium
✓ The drug is not obviously contaminated or deteriorated
132 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Hospitals may develop their own policies and procedures for return of unused medications to the pharmacy
Otherwise – community pharmacies ARE NOT allowed to take medications back into the pharmacy unless it is related to a misfill.
This is different from the drug take back programs facilitated by the DEA that include drop-off kiosks in pharmacies and police stations.
Pharmacist Administered Immunizations/Vaccines (ARS 32-1974; AAC R4-32-411)
Requirements
For Pharmacist or Intern to administer immunizations, vaccines, or emergency medications they must be certified by the ASBP to immunize. To be certified a pharmacist or intern MUST meet the following requirements:
Current, unrestricted license
Completion of a training program (specified in AAC R4-23-411 (D))
Current CPR Certificate
Immunization certification renewal – 2-year cycle based on date of issue
CE requirement for renewal = 2 contact hours related to immunizations (0.2 CEU)/renewal cycle (every 2 years with pharmacist license renewal)
Current CPR Certificate
Interns = to administer immunizations/vaccines MUST do so in the presence and under the immediate personal supervision of a pharmacist certified to provide immunizations/vaccines.
What are licensed and certified pharmacist and interns allowed to do regarding immunizations/vaccines/emergency medications?
ASBP Guide for Prescription Requirements – Certified Pharmacist/Intern Administered Immunizations
Booster Dose –Primary Adolescent Series (Ages 11-12)
COVID-19 (PREP Act)
Recommended by CDC for Minors
COVID-19 (PREP Act)
Recommended by CDC for Adults/ Travelers
COVID-19 (PREP Act)
Excludes any listed in (AZ) R9-6-1301
133 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Rx Required
Influenza ONLY; no others approved for the age group
Any not listed above, per CDC immunization schedule, including 1st Dose Primary Adolescent Series (Ages 11 -12)
Any not included in CDC immunization schedule
Any not included in CDC immunization schedule
Any on R9-6-1301 list
✓ Japanese Encephalitis
✓ Yellow Fever
✓ Typhoid
✓ Rabies
✓ Cholera
All: Administer epinephrine or diphenhydramine to manage an acute allergic reaction to an immunization or vaccine.
(Available: https://pharmacy.az.gov/node/5101)
Recordkeeping Requirements for Vaccines for each administration… AAC R4-23-411(F)
Patient's name, address, and date of birth
Date of administration
Injection site
Product name, dose, lot number, and expiration date of the vaccine, immunization, epinephrine, or diphenhydramine
Primary care provider's name and address
Prescriber's name and address (if different from the patient's primary care provider)
Pharmacist's or intern’s name administering vaccine/immunization or emergency medication
Record of patient consultation to determine that patient is eligible to receive the vaccine
Consultation or other professional information provided to the patient by the pharmacist
Name of the vaccine information sheet provided to the patient
In addition, for immunizations or vaccines given to an eligible minor patient:
Consent form signed by minor’s parent or guardian
Additional Requirements related to Vaccine Administration
A Vaccine Administration Record (VAR) containing the above information must be sent to the PCP (written or electronically)
o Pharmacy shall document the time and date the report is sent
Report to Arizona State Immunization Information System (ASIIS)
134 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Administration records must be maintained for 7 years from immunization administration date
Participate in any federal vaccine adverse event reporting system or successor database
Drug therapy management (ARS 32-1970 aka "Collaborative Practice Agreements"
✓ Allowed with a written drug therapy management protocol developed and approved by the provider (MD, DO or RNP);
✓ Each protocol will stipulate what the pharmacist can do – which may include the following:
Assess a patient's status,
Order and interpret lab tests, and
Modify, implement, and monitor a patient's drug therapy.
Dr. Joy Smith
Patient LP
Patient MS
Patient MP
Mr. Mark Johnson, RNP
(AAC R4-23-701-703)
Consultant pharmacists (AAC R4-23-701)
The responsibilities of a consultant pharmacist at a LTCF include:
✓ Developing policies and procedures for…
Patient SW
Pharmacist assistance in drug-related emergencies on a 24-hour basis
Controlled substance accountability
Prescription order requirements
Approved abbreviations
135 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Stop-orders
Pass and discharge prescription orders
Emergency drug supply units
Drug formularies
…and everything else listed below
✓ Storage, distribution, and procurement of drugs and biologics
✓ Evaluation and verification of prescription orders
✓ Compliance with labeling and packaging requirements
✓ Retrospective DUR as required by CMS and DHHS
✓ Maintenance of records and reports of drug use
✓ Proper disposal of discontinued and outdated drugs
✓ Arranging for emergency access to drugs
✓ Procedures for identifying and reporting to proper authorities any drug irregularities and dispensing errors
Quick List:
1. Determiningdrug recordsinareinorder
2. Controlledsubstances recordsare maintained
3. Monthly patientchart reviewstoensuredrug regimensare appropriate
4. Ensuringeach resident’sdrug therapyisfreefrom unnecessarydrugs
This type of pharmacist is not responsible for the dispensing of medication from a provider pharmacy (i.e. filling and labeling of a prescription for a patient).
Provider pharmacies (R4-23-701.01)
The responsibilities of pharmacists at a provider pharmacy to a LTCF include:
Properly filling and labeling medications pursuant to a prescription order for a patient at a LTCF
Labels comply with ARS 32-1963.01 (C) and (I), 32-1968, and 36-2525 and applicable parts of R4-23-658 (D) AND contain:
Drug name, strength, dosage form and quantity, AND
Beyond-use-date
Emergency drug units
Ensuring available at the LTCF
Properly labeled
Properly disposed of
Educating LTCF staff on proper use and storage
Evaluating emergency prescription drug orders within 72 hours of the first administration
Therapeutic Substitution – Nursing Care Facilities (ARS 36-447.02)
136 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
✓ A pharmacist must be a member of the nursing care facilities quality assessment and assurance committee or the committee created to establish therapeutic substitutions (aka formulary) that will be used during a patient’s stay in the facility
Definitions:
Terminology
Tele-health (or tele-medicine)
Tele-pharmacy
Automated-dispensing machines
Definition
“The delivery of healthcare services, where distance is a critical factor, by all healthcare professionals using information and communication technologies for the exchange of valid information for diagnosis, treatment and prevention of disease and injuries, research and evaluation, and for the continuing education of healthcare providers, all in the interests of advancing the health of individuals and their communities.” (WHO)
“the use of electronic information and telecommunications technologies to support remote clinical healthcare, patient and professional health-related education, public health and health administration” (US Department of Health and Human Services)
“the provision of pharmacist care by registered pharmacies and pharmacists located within U.S. jurisdictions through the use of telecommunications or other technologies to patients or their agents at distances that are located within U.S. jurisdictions” and provides definitions of related terms (i.e., coordinating pharmacy, remote pharmacy, remote dispensing site). (The Model State Pharmacy Act and Model Rules of the National Association of Boards of Pharmacy (Model Act))
decentralized medication distribution systems that provide computer-controlled storage, dispensing, and tracking of medications.
Range from machines like Pyxis and Omnicell to the MedAvail and ScriptPro kiosks.
Types of Tele-Pharmacy
Mail order
Patient counseling by telephone
Central processing and remote order entry
Remote supervision of technician dispensing
137 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
Medication Therapy Management (MTM) Automated dispensing systems
Collaborative drug management Medication Kiosks with 24/7 RPh Counseling
Source: Edward Rickert, RPh, JD, Quarles & Brady, and Phil Wickizer, DaVita Rx
Automated prescription-dispensing kiosks – community/out-patient setting
MedAvail Video: https://www.youtube.com/watch?v=kwa-TdDfPSE
138 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy - Glendale
After attending lecture and completing the assigned readings the student should be able to:
✓ Identify the elements of a contract.
✓ Compare the difference between a contract and a gift.
✓ Identify and define the following terms: actual damages, consequential damages, Reliance damages, liquidated damages, warranty terms, breach of contract.
✓ Describe the various ways contracts can be made and which ones are required to be in writing.
✓ Describe the three outcomes that may happen once a contract offer is made.
✓ Describe how contracts are enforced.
✓ Compare unilateral versus mutual mistakes in contracts.
✓ Identify issues related to mailing contracts.
✓ Describe an options contract.
✓ Define “contract adhesion”.
✓ Compare intentional torts versus unintentional torts.
✓ Identify and describe the five categories of “intent”.
✓ Identify, define, and describe the four elements necessary for a negligence claim to move forward.
✓ Identify and explain what is necessary for a plaintiff to win a malpractice claim.
✓ Describe “affirmative defenses” – contributory negligence, comparative fault, “Res ipsa loquitor”.

contract: An agreement between two or more parties to do (or not do) something.
✓ Contracts are legally binding and enforceable in a court of law
✓ Anything can be placed in a contract, as long as it's a legal activity.

(Source: http://smallbusiness.findlaw.com/business-forms-contracts/business-forms-contractsoverview/business-forms-contracts-overview-common.html)
• Bill of Sale
• Agreement for the Sale of Goods
• Purchase Order
• Warranty
• Limited Warranty
• Security Agreement
EmploymentRelated Contracts
• Employment Agreement
• Employee Noncompete Agreement
• Independent Contractor Agreement
• Consulting Agreement
• Distributor Agreement
• Sales Representative Agreement
• Confidentiality Agreement
• Reciprocal Nondisclosure Agreement
• Employment Separation Agreement
• Real Property Lease
• Equipment Lease
General Business Contracts
• Franchise Agreement
• Advertising Agency Agreement
• Indemnity Agreement
• Covenant Not to Sue
• Settlement Agreement
• Release
• Assignment of Contract
• Stock Purchase Agreement
• Partnership Agreement
• Joint Venture Agreement
• Agreement to Sell Business
Element
What it means
Offer If you do X, I will agree to do Y
Example
"If you pay me $5, I will give you a hotdog"
Acceptance To agree or refuse an offer (Move on to see if a consideration is there) "Sounds good!"
Consideration The bargained-for exchange (Choosing what you want out of the offer)
Once an offer is made – 3 things can happen
•Then, Consideration (the exchange happens)
Example:
"You pay the $5 and receive a hotdog"
•The offer is dead, reuires a counter offer or re-offer
•Counter-offer is a new offermade after an origianl offer is rejected
•Period of time in which the contract is NOT being decided upon
You decide to purchase a house. The buyer makes an offer to the seller. The seller can do one of the following:
1. Accept the offer; closing day is the act of consideration
2. Rejection: the seller can reject the offer and make a counter-offer.
• If this happens, the buyer can accept the offer, reject the offer, and make another counter-offer OR
3. Languish: Either party can let the offer or counter-offer NOT be decided upon
Something not enforced in court, and you can't sue to get a gift
Example: I am giving you $10 because I like you. If I decide not to give you the money, you CANNOT sue me for it.
✓ This can happen when the promised quality in the offer is not present.
✓ Must assume there are reasonable terms.
o For example, timing, the price paid is valid for a limited time only.
Terminology Definition and Example
Actual Damages
Consequential Damages:
Damages that arise directly and inevitably from breach of contract; would theoretically be incurred by every injured party regardless of circumstance.
Any damages that aren't considered actual; damages incurred as a result of the injured party's circumstances.
✓ Reasonable attorney fees, time off work to interview substitutes for hire, time off work to look for another apartment contract, etc.
Breach of Contract Purposefully broken.
✓ Some parties do this because there is an incentive to breach the contract, there may be a better deal elsewhere, more $$ elsewhere.
Damages awarded for losses suffered in reasonable reliance on a promise.
Reliance Damages (Types of damage for breach of contract)
Liquidated Damages
✓ Reliance damages are calculated by asking what it would take to restore the injured party to the economic position occupied before the party acted in reasonable reliance on the promise. Reliance damages may be awarded after a breach of contract or by way of promissory estoppel.
A defined amount of damages, set forth in a contract, to be paid by the party breaching the contract; a predetermined estimate of actual damages from a breach.
✓ If you breach this contract, then I get $ XXX.
Warranty Terms: A written warranty for a product you bought supersedes any verbal warranty stated by a salesman.
✓ Percentage points, credit issues, return issues, etc.




Verbal contracts are legal, valid, AND enforceable
✓ If seeking PUNITIVE DAMAGES, a verbal contract is not sufficient. To seek punitive damages, the contract must be in writing.


Verbal Contracts Contracts REQUIRED to be in writing to be VALID AND ENFORCEABLE
1. Real estate purchases
2. Real estate property leases that run for more than a year
3. Agreements to pay someone’s debt obligation
4. Promises to be married
o Damages can be sought if there is a breach of contract, especially when an expensive engagement ring has been purchased or the wedding has been paid for.
5. Sales of goods greater than $500
6. Contracts that cannot be performed within one year
o Ex: "I'll pay you $1,000 for life to maintain my yard" –is a contract that doesn't need to be in writing because my life could end in less than a year.
o Ex: "I'll pay you $1,000 for 2 years to maintain my yard" –is a contract that needs to be in writing because it can't be done in one year. (It’s a 2 year agreement)
Try to put parties back into a monetary/situational position where they were before the contract was broken.
This is for the best benefit of the party that didn't breach the contract
Example: I agree to sell you a Rx for $30 and realize that I don’t have the full amount in the filling process. You then have to go to another pharmacy and pay $40 for the Rx.
Technically, the damages I have to pay you is $10
Example: Jessica agrees to pay Nate $10 to cut her lawn, but Nate decides that it is too much work and he doesn’t do it (Breach of contract)
Someone else is hired to mow the lawn and costs Jessica $100; a 3rd party (courts, arbitration) can come in and make Nate pay Jessica $90.
✓ Arise when parties don't understand what they are agreeing to in a contract.
✓ "Seller beware"; seller needs to know the value of what s/he is selling
Unilateral Mistake: A mistake made by only one of the parties to the contract.
✓ A unilateral mistake occurs when only one party is mistaken as to the subject matter, or the terms contained in the contract agreement.
o In other words, a unilateral mistake occurs when only one of the parties misinterprets the subject matter or meaning of the terms contained in the contract agreement.
✓ The law does not protect the person who made the mistake.
o Contract is enforceable against the mistaken party.
✓ This mistake is enforceable in court UNLESS:
o Other party knew or should have known that a mistake was made OR
o Mistake was due to substantial mathematical error made inadvertently and without
✓ Examples of unilateral mistakes:
o Mistakes regarding the quantity of a product to be delivered (especially for large numerical values)
o Misunderstandings of certain trade terms and technical phrases
o Mistakes involving words that have several meanings, pronunciations, or spellings (such as "four" vs. "for")
o Errors regarding the quality or description of a product
Mutual Mistake: Both parties misunderstood each other and are at cross purposes.
✓ The problems arise when it comes to admitting to the misunderstanding, the degree of truthfulness often decreases.
✓ Contract can be rescinded by either party; this mistake is unenforceable in court (Video: HarvardX: Unilateral and Mutual Mistakes https://www.youtube.com/watch?v=9Mp0_iaWxAs)
✓ Contracts often used to be mailed.
✓ The contract would go into effect when it was put in the mail, even though it had not been received and agreed upon.
✓ This often led to contract issues.

Where you make a contract to keep another contract open.
✓ Ex: I will give you $1000 in 30 days, if you give me the option to buy your house.
o You are basically buying the time to have the option open.

Definition: A standardized contract offered on a “take it or leave it” basis without giving the purchaser an opportunity to bargain for terms that are more favorable.
Courts don’t like to enforce this contract modification.
✓ In AZ, a law was passed that said any product bought here should work for 4 years.
✓ If a product you buy doesn’t last that long, and the manufacturer said it could only last one year, you can go to the courts and try to get a 4-year warranty on a 1-year product.
A legal term meaning a wrongful act resulting in injury or damage
A tort is a civil wrong. A civil wrong involves a breach of a duty owed to someone else, as opposed to criminal wrongdoing which involves a breach of a duty owed to society. Torts are civil wrongs other than breaches of contract and certain equitable wrongs.
• Breach of duty to someone else
1. Intentional
2. Negligence (Unintentional)
3. Strict Liability
Levels of Intent
Level of Intent
Definition and Example
• Breach of duty to society
Actual intent: Person purposefully engages in conduct and believes or hopes that will harm/hurt.
✓ Example: Pick up a girl off her chair and throw her on the floor
Knowledge: Person knowingly allows an action to happen that will lead to a specific consequence.
© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
✓ Example: know that chair is broken and I tell someone to sit in that chair; chair breaks and person falls on the floor
Recklessness Person is aware of rules or situation and decides to engage in conduct that a “law-abiding person” would have refrained from.
✓ Example: Speeding at 100 mph in a 50 mph zone and you know the speed limit.
Negligence: Person fails to comply with a duty; “reasonable person” would have been aware
No intent: A true accident
All four elements must be proven by the plaintiff to win the claim
The plaintiff must prove… Example
1. Duty Owed (aka Duty of Care) Duty owed based on statutes, regulations, and standards of care AZ law requires a pharmacist to consult a patient receiving a new drug
2. Breach of Duty Failure to give the ordinary care owed to the patient Pharmacist forgot to consult this patient
3. Causation The misconduct actually caused the alleged damages The patient was unaware of that the medication is sedating and got into an accident because he didn't know not to
4. Damages Damages must be a legally recognized harm Medical expenses, present and future lost wages, pain and suffering, etc.
1. Duty Owed (aka Duty of Care)
✓ Duty owed based on statutes, regulations, and standards of care
o Standards of care are defined by professional organizations/associations, local organizations, what is reasonably prudent.
o Pharmacists, in comparison to other health care professionals, may be the only professionals who are legally required to practice with an "error-free" standard. This has led to high legal standards.
o “Duty of care”: The Pharmacist must use the degree of care that a “prudent and reasonable person” would use under similar circumstances.
147 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy –Glendale
o Pharmacist duty is to accurately fill, counsel
A pharmacist has a duty to monitor and intervene in a patient's care if:
1. The relationship between pharmacist and patient is of the kind that should give rise to an expanded duty.
2. Harm to the patient is reasonably foreseeable to a pharmacist.
3. Public policy concerns (such as increased health care costs and diminished patient confidence in physicians) favor recognizing such an expanded duty
a. Preventing intentional and unintentional drug abuse
b. Not jeopardizing the physician-patient relationship
c. Avoiding unnecessary health costs
HOOKS-SUPERX VS MCLAUGHLIN (PGS 346 – 349)
✓ Established duty to warn patient and/or provider if errors/concerns about the prescription order
✓ Established duty to warn patient of adverse side effects/warnings
SEE HAPPEL VS WAL-MART STORES CASE DISCUSSION (PGS 366-368)
✓ Established duty to warn patient regarding contraindications when the pharmacy and pharmacist have the patient’s drug allergies and have documented them
✓ Established duty to warn patient contraindications in certain situations
✓ Failure to give the ordinary care owed to the patient
Doctrine of "negligence per se"
If a pharmacist clearly violates a statute or regulation, he or she is considered to have automatically violated (1) duty owed and (2) breach of duty.
Example: A pharmacist incorrectly fills a prescription other than the way it was ordered by a prescriber
Example: A pharmacist fails to counsel the patient on a common side effect
✓ The misconduct actually caused the alleged damage
Proving causation is a two-step process:
1. Actual cause. The plaintiff must prove that the defendant's conduct was a substantial factor in the harm that occurred.
2. Proximate cause The plaintiff must also prove that the defendant's actions most directly caused the damages
Example: Pharmacist failed to give patient his correct anti-platelet medication and the patient died of a pulmonary embolism. (Could be viewed as either actual or proximate)
Type of Damages
Actual Damages:
Explanation and Examples
Purpose of actual damages is compensation for such things as: present and future loss of wages, medical expenses incurred, impaired vision, severe pain for a month, home health care, emotional damage, pain and suffering.
✓ In AZ, there is no limit to the amount of $ that can be awarded for damages.
✓ In CA the limit is $250,000
Pain and suffering is provided through testimony by MD, psychiatrist, counselor
Punitive damages: Purpose of punitive damages is to punish or make an example of the defendant.
✓ Awarded only if defendant showed reckless disregard to the plaintiff's rights, society deems the defendant's actions outrageous.
✓ Ex: RPh cover-up of a dispensing error.
When it comes to damages, and the awards for the damages-
✓ Plaintiffs favor judge reviewing the case due to legal focus.
✓ Defendants favor jury for review of the case due to emotional presentation.
✓ Plaintiff must prove all 4 elements of negligence to win their claim
o Duty owed
o Breach of duty
o Causation
o Damages
✓ If not proven, there will be no legal liability for the defendant
Defendant tries to show one or more "affirmative defenses"
Type of Negligence (Affirmative Defense)
Contributory negligence
Comparative fault (negligence)

“Res ipsa loquitor”

Explanation
Argues that the injured party (plaintiff) plays some part in the negligence.
Ex: a patient received the wrong medication, he knew it was wrong, but took it anyway.
a. This leads to no legal liability
When the plaintiff and defendant both carry fault. If percent assignments can be made, damages can be figured.
Ex: RPh was 60% at fault, patient was 40% at fault; RPh owes 60% of the damages.
b. Plaintiff has to be <50% at fault to bring the case to court.
When only duty owed, breach of duty & causation are proven; who caused the damages is unknown.
Statute of Limitations In AZ the statute of limitations is 2 years from the time knowing about the harm done or when they should have known.

Example: What is the statute of limitations for a 13 year old who receives the wrong medication and wants to bring a negligence suit?
“Respondeat superior”: places liability upon the employer without regard to any negligence on the part of the employer.
✓ Employers are liable for the negligent acts of their employees.
The US has a "no duty" or "no relationship" law to help your neighbor if they need it.
✓ Especially true when the action needed is out of your jurisdiction.
Example: Your neighbor lies down in your yard and is having a heart attack; you have no duty to help that person.
150 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
AZ Good Samaritan Law (ARS 36-2263. Civil liability; limited immunity; Good Samaritan)
✓ Applies to health care providers and persons administering emergency aid.
✓ If you help a person who needs emergency care at a public gathering, in good faith, you shall not be liable for any civil or other damages if harm still comes to the injured person.
✓ If the health care provider is guilty of gross negligence during emergency care, they can be held liable.
✓ If an emergency arises at work, you have the duty to respond and call an ambulance.
✓ If you provide pharmaceutical advice to a patient, if it is not correct or appropriate advice, you could be held negligent if the patient took your advice and was harmed.
Almost 97% of negligence cases never go to trial due settlements.
Types of Settlements /Judgments
Settlements
Summary Judgment
Jury Judgment:
Explanation
Decisions are made before a judge or jury determination.
✓ Does not set a precedent
Trial with a judge as the decision maker
✓ Sets a precedent because the final decision is made by a judge.
The benefit of having a jury is that 8 - 12 people are hearing and deciding upon the case. (Criminal= 12 or civil = 8)
✓ Sets precedent because decided by a jury.
Usually they are:
✓ Employees and consumers
✓ Retired
✓ Citizens
✓ Rarely are they college educated
Excused (generally – not always): mothers and students
© 2024 Mary K Gurney, Roger Morris, and Midwestern University College of Pharmacy
Glendale
1. Defamation/slander
2. Battery
3. False imprisonment
4. Infliction of emotional stress
5. Product liability claims
✓ As a pharmacist who has sold pharmacy products that have injured a patient, you could be liable for battery and negligence claims.
Central premise for Drug Product Liability
Drug Product Defect
✓ To have manufacturer be held liable – the drug product must be defective AND unreasonably dangerous
Three categories of defect:
1. Design defect
2. Manufacturing defect
3. Warning defect
Based on:
1. Negligence (see prior discussion)
2. Breach of warranty
3. Strict liability
Breach of Warranty
✓ Based on the allegation that a seller violated either an express or an implied agreement that the goods would not be harmful to the buyer.
✓ Holds that the seller of a product is responsible for an injury caused by its defective product, even if the seller was not negligent in any manner and exercised all possible care in the design, manufacture, and distribution of the product.
✓ Reasonableness of conduct is irrelevant to an action for strict liability.
✓ Less of an issue for manufactured dosage forms
✓ Becoming an issue for pharmacist compounded products
Monitor decision and update the risk management
Establish the context
Select an appropriate risk management strategy and implement the technique
Identify and analyze the risks
Evaluate and prioritize risks
1. Be correct Accurately convey written information (includes recordkeeping)
2. Be complete Documentation should contain all information that is necessary to provide continuing high levels of care to patients.
3. Be concise This means not including everything and the kitchen sink.
4. Be consistent
Breaks in patterns can be interpreted in a way that was not intended. “If it isn’t documented – it didn’t happen.”
5. Be cautious To avoid misinterpretation
Parts of CQA/CQI:
✓ Generation and use of incident reports
o Near misses
o Misfills dispensed/administered to the patient.
✓ Periodic evaluation
✓ Some elements of a CQI report form:
o Date of report
o Name of pharmacy employees involved.
o Type of incident
o Description of steps taken or to be taken to prevent the recurrence of an incident.
o Signature of employees involved.
✓ Professional malpractice insurance is a necessity in pharmacy practice today.

After attending the lecture and completing the assigned readings, the student should be able to:
✓ Identify the legislation that created Medicare and Medicaid and the administrative agencies that oversee the programs.
✓ Differentiate between who is eligible for Medicare and Medicaid, who are dual-eligible?
✓ Identify and describe the purpose, coverage, and if premiums are required for Medicare Part A, B, C, and D.
✓ Describe the two different ways of obtaining Part D coverage.
✓ Identify who is eligible for Part D Medicare Drug Coverage.
✓ Identify and describe what Medicare Part D covers, its limitations, and what patient is required to pay during the year.
✓ Identify and describe the terms: yearly deductible, copayment, and coinsurance, coverage gap, catastrophic coverage
✓ Identify and describe the late enrollment penalty for Medicare Part D, including how the penalty is calculated.
✓ Identify and describe the other pharmacy-related provisions of the Medicare Modernization Act (MMA) and Medicare Improvements for Patients and Providers Act (MIPPA).
✓ Identify what is required coverage under the Medicaid program and what is optional coverage.
✓ Identify and describe the Medicaid Drug Rebate Program and know which state is excluded.
✓ Identify examples of Medicare/Medicaid fraud and possible repercussions to pharmacies and/or pharmacists.
✓ Identify and describe the provisions under EMTALA (Emergency Medical Treatment and Labor Act), including those for women in labor.
✓ Define monopoly, price fixing, collusive price fixing, boycotting, and tying arrangements.
✓ Identify and describe the two sections of the Sherman Anti-Trust Act.
✓ Identify and describe the two rules that the courts use to determine a violation of the Sherman Anti-Trust Act; which types of actions would fall under the per se rule?
✓ What is the legality of exclusive contracts?
✓ Identify and describe the Robinson-Patman Act.
✓ Identify and describe the Nonprofit Institutions Act; who are exempt entities, what is the “own use” doctrine”; what are permissible activities under NPIA, what are not permissible activities under NPIA; know if outpatient and inpatient pharmacy stock may intermingle.

155 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Abood R and Burns K. Chapter 6 sections:
✓ Medicare – p. 256 - 264
✓ Medicaid – p. 264 - 269
✓ Medicare/Medicaid Fraud and Abuse – p. 270 - 273
✓ Federal Antitrust Laws – p. 278 - 287
LEGISLATION
Social Security Act Amendments of 1965 (Title XVIII of the Social Security Act of 1935) created Medicare
Medicare is health insurance for people:
• Age 65 or older
• Under 65 with certain disabilities
• Any age with ESRD (End-Stage Renal Disease)*
• Exposed to environmental health hazards and developed chronic illness(es)+ *ESRD is permanent kidney failure requiring dialysis or a kidney transplant.
+Health hazards include: asbestos, mesothelioma, or malignancies of the lung, colon, rectum, larynx, stomach, esophagus, pharynx, or ovary. Black lung disease (coal miners, for the most part) has a separate program administered by the US Department of Labor
The different parts of Medicare help cover specific services if the person meets certain conditions.
ADMINISTRATIVE AGENCY
The Centers for Medicare and Medicaid Services (CMS) (formerly the Health Care Financing Administration; HCFA)
✓ October 15 – December 7, 20XX
✓ New coverage begins January 1, 20XX
156 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Medicare Part A: Hospital Insurance
Medicare Part B: Medical Insurance
• No cost to eligible beneficiaries
• Helps cover inpatient care in hospitals (Includes critical access hospitals, inpatient rehabilitation facilities, and long-term care hospitals)
• Helps cover skilled nursing facility (not custodial or long-term care), hospice, and home health care services.
• Inpatient care in religious nonmedical healthcare institution(s)
• Only covers drugs given to patients while they are in the hospital
• Monthly premiums (Std. premium = $174.70/mo in 2024, based on income; premium range $174.70 - $594.00/mo)
• Helps cover medically necessary doctor services, outpatient care, durable medical equipment, mental health, & home health care
• Helps to cover some preventative services to help maintain a person's health and keep certain illnesses from worsening (e.g., x-rays, lab tests, durable medical equipment, colostomy care, diabetic supplies, etc.)
• Generally, covers 80% of the Medicare-approved amount for covered services after meeting the yearly deductible
• Covers a limited # of outpatient prescription drugs (e.g., injections received in the provider's office, drugs used with durable medical equipment (e.g. nebulizers or external infusion pumps), certain oral anticancer drugs, intravenous Immune Globulin for home use, certain drugs you receive in a hospital outpatient setting, drugs for ESRD - End Stage Renal Disease)
What is NOT covered by Medicare Parts A and B?
• Most dental care
• Eye exams
• Dentures
• Cosmetic surgery
• Massage therapy
• Routine physical exams
• Hearing aids and exams for fitting
• Long-term care
• Concierge care (direct primary care, concierge medicine, etc.)
• Covered items you get from a provider that has opted out of Medicare participation.
157 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Medicare Part C: Medicare Advantage Plans
• Monthly premium (varies by plan; Range $0 - $125.00 in Arizona) (37 Plans with a Star Rating of 4 or higher)
• Includes all benefits and services covered under Part A and Part B
• Most plans include Medicare prescription drug coverage (Part D) as part of their plans
• Run by Medicare-approved private insurance companies (HMO, PPO, PFFS, Special Needs Plan)
• May include extra benefits and services for an extra cost
Medicare Part D: Prescription Drug Coverage
• Monthly premium = $0.00 – $156.60. (Possible surcharge of ($12.40$81.00) + plan premium) (based on income and plan) (12/20 plans with a Star Rating between 3 – 3.5)
• Run by private companies approved by Medicare, either Medicare Advantage Plans (MA-PDs) or separate Medicare Prescription Drug Plans (PDPs).
• Helps cover the costs of prescription drugs
• Each plan can vary in cost and drugs covered
ELIGIBILITY: For either choice, you must live in the service area for the Medicare Part D Drug Plan.
Original Medicare Coverage
• Part D as a separate stand-alone policy
• Known as Medicare Prescription Drug Plans (PDPs)
• Requires Medicare Part A OR B
Medicare Advantage Plan (HMO or PPO) (aka Part C)
• Part D as part of Medicare Advantage Plans
• Known as Medicare Advantage Plan with Drug Coverage (MA-PDs)
• Requires Medicare Part A AND B
158 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale

Source: https://www.kff.org/medicare/fact-sheet/an-overview-of-the-medicare-part-d-prescriptiondrug-benefit/
There are now late enrollment penalties for Medicare Parts A, B, C, and D.
LATE ENROLLMENT PENALTY FOR PART B
✓ If you don’t sign up for Part B when you are first eligible, you may have to pay a late enrollment penalty for as long as you have Part B.
✓ Penalty: Monthly premiums may go up 10% for each 12-month period that you could have been covered by Part B but didn’t enroll.
Example: Mr. Smith’s initial enrollment period ended in December 2020. He waited to enroll in Part B until March 2023 (during the General Enrollment Period), and his coverage begins on July 1, 2023.
✓ Mr. Smith was without coverage for 27 months.
✓ Penalty is based on 12-month full-cycle non-coverage.
✓ Therefore, Mr. Smith’s Part B premium penalty is 20% (for 2 cycles = 24 months);
✓ He will pay this 20% premium penalty as long as he has Part B.
Let’s say Mr. Smith has standard Part B coverage, and his base premium is $174.70. What is his total monthly premium?
✓ Monthly premium with penalty for 2023: $174.70 + (174.70 * 0.20) = 174.70 + 34.94 = $209.64
159 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ The late enrollment penalty is an amount added to an enrollee’s Part D premiums.
✓ Enrollees may owe a late enrollment penalty if:
o Don’t join a Medicare drug plan when first eligible for Medicare and didn’t have other creditable prescription drug coverage
o Don’t have Medicare prescription drug coverage or other creditable prescription drug coverage for 63 days or more in a row.
✓ Penalty = (1% of national base premium) * number of full, uncovered months enrollee eligible but didn’t have drug coverage.
✓ This is added to the monthly premium (recalculated every year based on the national base premium)
OF LATE ENROLLMENT PENALTY (FROM CMS MEDICARE AND YOU BOOKLET)
2024 National Base Beneficiary Premium = $34.70
✓ Mrs. Martinez did NOT enroll in Part D coverage when she was first eligible on May 31, 2021.
✓ She doesn’t have prescription drug coverage from any other source.
✓ Joined a Medicare drug plan during the 2023 open enrollment period (November); her coverage began on January 1, 2024.
✓ Time without creditable prescription drug coverage: June 2021 – Dec 2023 = 31 months
Since Mrs. Martinez was without creditable prescription drug coverage from June 2021 – December 2023, her penalty is 31% (1% for each of the 31 months) of $34.70 (national base beneficiary premium for 2024).
0.31 (31% penalty) x $34.70 (2024 premium) = $10.75
Premium is rounded to the nearest $0.10 = Mrs. Martin’s premium is $10.80 per month
Mrs. Martinez’s total monthly premium, including the penalty, if she has a plan with the national base average premium:
$34.70 (Monthly premium) + $10.80 (penalty) = $45.50/month
OTHER PHARMACY RELATED PROVISIONS OF THE MEDICARE MODERNIZATION ACT (MMA) AND MEDICARE IMPROVEMENTS FOR PATIENTS AND PROVIDERS ACT (MIPPA)
1. Covered drugs and plan formularies established
✓ Part D drugs must be:
o Approved by the FDA for sale in the US
o Available only by prescription
o Medically necessary and for a “medically accepted indication”
160 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ Cover “all or substantially all” drugs in these 6 categories (Protected Classes or Classes of Clinical Concern)
1. Antidepressant medications
2. Antipsychotic drug medications
3. Anticonvulsant medications
4. Antineoplastic drugs (used by cancer patients)
5. Antiretroviral (used by patients with HIV or other viral infections)
6. Immunosuppressant (used by transplant patients)
✓ Cover at least 2 options for all other drug categories (includes barbiturates and benzodiazepines)
✓ Also cover (Changes due to the Inflation Reduction Act of 2022):
o Biologicals
o Insulin (Insulin cost cap = $35/month max) and insulin syringes
o Smoking cessation drugs
2. Drugs excluded from coverage
1. Non-prescription drugs (OTC)
2. Drugs for the treatment of anorexia, weight loss or weight gain
o Exception: drugs used to treat AIDS wasting and cachexia due to other diseases are not considered to be for cosmetic purposes and therefore are NOT excluded for these conditions.
3. Drugs used to promote fertility
4. Drugs used for cosmetic purposes or hair growth (Note: drugs to treat acne, psoriasis, rosacea and vitiligo are not considered cosmetic)
5. Drugs used for the relief of cough and cold symptoms
6. Drugs used for the treatment of sexual or erectile dysfunction (e.g., Viagra, Cialis, Levitra, and Caverject) except as medically necessary and approved by the FDA to treat conditions other than sexual or erectile dysfunction (i.e., Cialis for BPH)
7. Prescription vitamins and minerals (except prenatal vitamins, fluoride preparations, niacin products, and Vitamin D analogs when used for medically accepted indication)
8. Outpatient drugs that the manufacturer requires testing or monitoring
9. DESI drugs
10. Brand drugs (i.e., drugs with a FDA application type “NDA” or “BLA”) that are not under a manufacturer discount agreement
11. Drugs purchased in another country
12. Drugs that may be covered under Part A or Part B of Medicare, even if coverage is not actually available
161 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
3. Pharmacy access
✓ PDPs and MA-PDs must ensure that beneficiaries have convenient access to a network of pharmacies.
✓ “Any willing provider” requirement – though patients may pay a higher co-pay or coinsurance if use a non-preferred pharmacy.
4. Electronic prescribing (e-prescribing)
✓ Participation is required unless hardship is identified by the prescriber (SUPPORT ACT)
✓ CMS rules preempt state laws that restrict e-prescribing.
5. Pharmacy reimbursement: MIPPA requires:
✓ That plans pay electronic pharmacy claims within 14 days of submission
✓ Plans pay within 30 days for claims submitted by other means (think Universal Claim Form, CMS 1500 Form)
✓ Plans update their drug cost databases weekly to reflect accurate market prices
6. Medication Therapy Management (MTM)
✓ Requires plans to provide coverage for medication therapy management programs
✓ Pharmacists are named as one of the providers of MTM services
7. Plan and Provider Marketing Limitations
Pharmacies can:
✓ Inform patients of the plans in which they participate
✓ Distribute plan marketing materials and applications
✓ Display brochures and posters about particular plans, provided they include all plans in which they participate
✓ Distribute CMS approved plan finder information and any information from the CMS website (www.cms.gov) and the Medicare website (www.medicare.gov)
Pharmacies CANNOT:
✓ Direct, urge, or steer patients to a particular plan
✓ Compare different plan benefits unless created by CMS
✓ Collect or accept Medicare enrollment applications
✓ Accept compensation for conducting enrollment or marketing activities
162 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
8. Fraud and Abuse
Pharmacies must:
✓ Have policies and procedures in place to identify fraud, waste, and abuse
✓ Certify that the data they submit are true, accurate and complete
✓ Keep records for 10 years
✓ Check employees against an exclusion list of persons issued by the Office of the Inspector General (OIG) (https://oig.hhs.gov/exclusions/index.asp)
9. Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS)
MMA mandated that HHS establish and implement quality standards for DMEPOS suppliers (including pharmacies) URL: http://www.cms.gov/Outreach-and-Education/Medicare-LearningNetwork-MLN/MLNProducts/downloads/DMEPOS_Pharm_FactSheet_ICN905711.pdf
Allows pharmacies to bill CMS for Medicare Part B
Process:
1. Pharmacy Accreditation
Accredited by CMS-approved independent national Accreditation Organization (AO)
Cost = ~ $2599 - 4799/3 year accreditation cycle
Example: NABP DMEPOS Single Pharmacy Accreditation Fees
2. Pharmacy Accreditation Exemption
For the exemption, pharmacies that meet ALL of the following criteria may file an accreditation exemption statement to supply DMEPOS without being accredited:

163 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Social Security Act Amendments of 1965 (Title XVIX of the Social Security Act of 1935) created Medicaid
Medicaid provides for the health care costs of the:
✓ Poor,
✓ Blind,
✓ Disabled, and
✓ Those on welfare (members of families with dependent children).
AZ - Arizona Health Care Cost Containment System (AHCCCS)
CMS – Center for Medicaid and Medicare Services
Eligibility requirements
Determined by an individual’s INCOME and ASSETS
Dual-eligible: Medicare and Medicaid eligible
Medicaid coverage
Required coverage of services includes (MUST cover):
✓ Inpatient and outpatient hospital services
o Prescription drugs in these settings ~ Medicare Part B drugs
✓ Physician, midwife, and nurse practitioner services
✓ Lab and x-ray services
✓ Nursing facility and home health care for individuals age 21+
✓ Early and periodic screening, diagnosis, and treatment for children under age 21
✓ Family planning services and supplies
✓ Rural health clinic/federally qualified health centers
164 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Optional coverage includes (MAY cover):
✓ Prescription drugs (outpatient) ~ Medicare Part D
✓ Dental care
✓ Durable medical equipment
✓ Personal care services (Assistance with Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs)
OBRA ’90 created the Medicaid Drug Rebate Program.
✓ Allows for the generation of funds for state Medicaid programs
✓ If manufacturers want their drugs on the Medicaid formulary, they must agree to rebate the difference between their best price and the lowest price at which they sell the product to any customer, to each state Medicaid agency.
✓ Essentially means that Medicaid programs won’t have to pay top dollar for prescription medications.
Approximately 600 pharmaceutical companies currently participate in this program. All fifty states and the District of Columbia cover drugs under the Medicaid Drug Rebate Program. If a pharmaceutical manufacturer is going to participate in the Medicaid Rebate Program, they must also sign up to participate in these two programs:
✓ Section 340B Drug Pricing – pricing used for community health centers (low-income, federally qualified underserved populations
✓ Federal Supply Schedule – VA pricing
FALSE CLAIMS ACT
Prohibits:
1. Knowingly presenting or causing to be presented a false or fraudulent claim for payment or approval
2. Knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim
False claims to either program made by pharmacies/pharmacists/technicians/interns may result in serious disciplinary action (including expulsion from the Medicare, Medicaid programs and Federal Qualified Health Centers), imprisonment and fines.
✓ If found guilty of fraud – exclusion based on OIG list (available at: https://oig.hhs.gov/exclusions)
✓ May be on the OIG Exclusion List for up to 10 years
165 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale

Examples of fraud include:
✓ Billing for nonexistent prescriptions
✓ Billing for the brand drug when a generic was dispensed
✓ Billing for prescriptions that are filled and never picked up
✓ Splitting prescriptions in order to receive additional dispensing fees
✓ Inappropriate use of dispense-as-written codes
News from (4/12/18) regarding Banner Health (Source: Modern Healthcare: http://www.modernhealthcare.com/article/20180412/NEWS/180419967?itx[idio]=5792679&ito=79 2&itq=c6592edb-3354-49cc-91c5-cf191a458feb)
✓ Banner Health will pay $18 million to settle a federal False Claims Act case accusing the notfor-profit health system of admitting Medicare patients who could have been treated as outpatients, the U.S. Justice Department announced Thursday.
✓ Banner, which operates 28 acute-care hospitals, entered into a five-year corporate integrity agreement with HHS' Office of Inspector General that requires the system to retain an independent review organization to monitor the accuracy of its claims for services provided to patients covered under federal healthcare programs.
✓ Former Banner employee Cecilia Guardiola filed the lawsuit under the FCA's whistle-blower provisions, and will receive roughly $3.3 million, according to the Justice Department.
(References for this section: 42 USC § 1395dd[a]) and Texas Medical Association Office of the General Counsel. Emergency Medical Treatment and Active Labor Act (EMTALA) Document # 0004-LS. Austin, TX: Texas Medical Association; 2007.)
166 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Medicare website:
https://www.medicare.gov/hospitalcompare/results.html#dist=25&state=AZ&lat=0&lng=0
American College of Emergency Physicians FACT SHEET on EMTALA https://www.acep.org/life-as-aphysician/ethics legal/emtala/emtala-fact-sheet/
EMTALA was enacted in 1986 by Congress to ensure that emergency services are accessible to the public regardless of their ability to pay.
✓ Enacted in response to “patient dumping” where a hospital refuses admission to persons lacking health insurance.
1.
For any person who comes to a hospital emergency department, "the hospital must provide for an appropriate medical screening examination . . . to determine whether or not an emergency medical condition exists" (see 42 USC § 1395dd[a]).
✓ Emergency department is defined by regulation to be ANY department or facility of the hospital (including anywhere on hospital property; parking lot, sidewalk, driveway and hospital owned urgent care facilities), regardless of whether it is located on or off the main campus that meets at least one of the following elements:
o It is licensed by the state as an emergency room, OR
o It is held out to the public as a place that provides care for emergency conditions on an urgent basis without appointment; OR
o It provides at least one-third of all out-patient visits for the treatment of emergency medical conditions on an urgent basis.
✓ Appropriate medical screening examination is the process of determining whether an emergency medical condition exists. Triage does not constitute an appropriate medical screening examination.
✓ Emergency medical condition is a condition manifesting itself by acute symptoms of sufficient severity (including severe pain) such that the absence of immediate medical attention could reasonably be expected to result in:
o Placing the health of the individual (or unborn child of a pregnant woman) in serious jeopardy, or
o Serious impairment to bodily functions; or
o Serious dysfunction of any bodily organ or part
If the screening examination reveals an emergency medical condition, the hospital must "stabilize the medical condition" before transferring or discharging the patient.
167 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ Stabilize = medical treatment of the condition is provided as necessary to assure that no “material” deterioration of the condition is likely to result from or occur during the transfer or, with respect to a pregnant woman, to deliver.
✓ Until the medical condition is stabilized – transfers to other facilities are restricted by law.
✓ All laboring patients are considered unstable and are thereby deemed to have an emergency medical condition.
✓ Stabilization may be achieved in any one of the following 3 ways:
o The physician declares the labor to be false.
o Labor ceases.
o The infant and placenta are delivered.
✓ Transfer rules apply equally to women in labor. Therefore, a woman in labor who has not been stabilized (achieved delivery of infant and placenta) may be transferred only if the benefits of transfer outweigh the risks.
Designed to protect competition and keep prices low.
Monopoly = only one supplier of a service, or a single business holding greater than 40% of the market share
✓ Examples: power company (APS, SRP), water company, Microsoft, Google, Alphabet, etc.
Price fixing = an agreement (written, verbal, or inferred from conduct explicit or implicit) among competitors or a single business to affect the price or allocation of services whether prices are fixed at a minimum, maximum, or within some range; usually done in necessity services
✓ Good for business, bad for consumers
Collusive (deceitful) price fixing = when 2 or more businesses join together and price fix to benefit themselves
✓ Usually deceitful in nature; intent is to defraud
168 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
LINK: Federal Trade Commission (FTC) on Anti-trust: https://www.ftc.gov/tips-advice/competitionguidance/guide-antitrust-laws/antitrust-laws
Passed in 1890; 2 sections (Section 1 – restraint of trade; Section 2 – monopolies)
Sherman Anti-Trust Act – Section 1
Makes every contract, combination, or conspiracy in restraint of trade unlawful
A single competitor CANNOT violate Section 1
o Example: A chain pharmacy (e.g. Rite-Aid) can reject a third party plan
2 or more competitors can violate Section 1 if they have an informal or formal agreement between them
o Example: 2 or more independent pharmacies together agree to reject a third-party plan
o Example: 1996 Merck-Medco lawsuit against Rite-Aid, Giant, NeighborCare and EPIC
Sherman Anti-Trust Act – Section 2
Prohibits monopolies, attempts to monopolize, or conspiracies to monopolize
Just being a monopoly is NOT illegal
o It is illegal for a monopoly to exploit its power for the purpose of harming competition
Deciding if an activity violates the Sherman Act – Section 1 and/or 2
In deciding a violation, the courts apply the rule of reason or the per se rule
✓ Is the net effect of the action promoting competition or suppressing it?
✓ This question is answered by examining:
o The pro-competitive and anticompetitive effects of the activity
o The purpose, nature, duration, effect, and justification of the activity
o Market power of the parties involved
PER SE RULE
✓ Essentially, “if you did it, you are guilty of doing it”
✓ If an agreement is illegal under anti-trust rules it is a per se offense.
✓ Violations include price fixing, boycotting, and tying arrangements (see below for further information)
169 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Price fixing = an agreement (explicit or implicit) among competitors or a single business to affect the price or allocation of services; usually done in necessity services
✓ In pharmacy, deprives consumer of competition that has lower prices and/or better services
✓ Could make it very difficult for other competitors to start a business in this area.
Boycotting = an agreement among competitors not to deal with another party
✓ Example: 2 or more independent pharmacies (or any pharmacy ownership type) agree to reject a third-party prescription plan
Tying arrangements = conditions the purchase of one product (the “tying” item) on the purchase of another (the “tied” item)
✓ This arrangement is ONLY illegal when the seller can force a buyer to purchase the tied product. A tying arrangement is presumed to be illegal where:
o The tying and tied products are separate goods (rather than components for a single product)
o The availability of the tying item is conditioned on the purchase (or rental or license of the tied item, as the case may be), and
o The business imposing the tie is in a position to use its strength in the market for the tying to harm competition in the market for the tied product
o Example of an illegal tying arrangement (from the FTC Guide to The Antitrust Laws available at: http://ftc.gov/bc/antitrust/factsheets/antitrustlawsguide.pdf)
▪ Drug maker required patients to purchase its blood monitoring services along with its medicine to treat schizophrenia.
▪ The drug maker was the only producer of the medicine, but there were many companies capable of providing blood-monitoring services to patients using the drug.
▪ FTC and DOJ claimed that tying the drug and monitoring services together raised the price of medical treatment and prevented independent providers from monitoring patients taking the drug.
▪ Drug maker settled the charges by agreeing NOT to prevent other companies from providing blood-monitoring services
✓ Legitimate tying arrangement: Roche (the makers of Accu-check ® blood glucose meters and strips) sold the following:
o One box that tied the purchase of the meter and strips together
o Boxes of strips (without meter) in varying package sizes of 25 strips, 50 strips, 100 strips.
✓ Is this an illegal tying arrangement?
170 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
o Is it illegal for a single grocery store to require a customer to buy a box of cookies with every gallon of milk purchased?
✓ Exclusive contracts are generally legal under anti-trust laws
o A contract in which a seller agrees to sell its product only to a particular buyer (think suppliers for Apple products or the original OnStar program on GM vehicles)
LINK to FTC Explanation: https://www.ftc.gov/tips-advice/competition-guidance/guide-antitrustlaws/price-discrimination-robinson-patman
Passed in 1936
✓ Makes it unlawful for a manufacturer to charge 2 different prices to 2 purchasers who are getting the exact same product in the exact same quantity and are considered competing buyers…..if it substantially injures competition
o Volume discounts are allowed
✓ Was passed to protect small business grocery stores against chain operators who were engaging in unfair purchasing operations
✓ Therefore, hospital in-patient pharmacies are not considered competitors of retail pharmacies
This Act provides an exemption from the Robinson-Patman Act to the following types of institutions when purchases are for “own use”:
✓ Nonprofit schools
✓ Public libraries
✓ Universities Not considered competitors of retail pharmacies
✓ Churches
✓ Charitable institutions
“Own Use” Doctrine = “what may be regarded as used by the hospital in the sense that it such use is part of and promotes the hospital’s intended institutional operation in the care of persons who are patients”.
171 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
PERMISSABLE ACTIVITIES UNDER THE “OWN USE” DOCTRINE
✓ Sale of drugs to:
o Inpatients, ER patients, and outpatients (who receive treatment or consultation on premises) for use on hospital premises.
o Inpatients or ER patients on discharge and for personal use away from the premises.
o Outpatients for personal use away from the premises
o Hospital employees and their dependents, students at the hospital and their dependents, and medical staff of the hospital and their dependents, all for their own personal use.
IMPERMISSABLE ACTIVITIES UNDER THE “OWN USE” DOCTRINE (NOT ALLOWED)
✓ Sale of drugs to:
o Former patients for obtaining refills
o Medical staff for resale in their practice
o Walk-in customers who have no connection with the hospital
SEPARATION OF “OUTPATIENT PHARMACY STOCK” AND “INPATIENT PHARMACY STOCK”
Hospitals and other exempt entities may have some areas of activities that DO NOT qualify for NPIA exemption.
Let’s look at an example:
Hospital A is a non-profit that provides inpatient pharmacy services and also has an outpatient pharmacy that provides prescriptions to all hospital employees, medical staff, and anyone person who chooses to fill their prescriptions there (regardless if they have been a patient or not.)
Inpatient pharmacy = is covered by the exemptions listed in NPIA
Outpatient pharmacy = in this case IS NOT covered by the exemptions listed in NPIA and therefore may not purchase medications as an exempt NPIA entity; the outpatient pharmacy must purchase medications at general market prices
172 © 2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
After attending the lecture and completing the assigned readings, the student should be able to:
✓ Identify the state and federal laws that govern controlled substances and key information.
✓ Identify and describe the differences between the five schedules of controlled substances, the scheduling process and which departments and/or agencies have responsibility for scheduling.
✓ Identify the 10 activities requiring DEA registration; who must be registered under which activity; identify who is exempt from registration.
✓ Define dispensing and distributing.
✓ Identify what the DEA number is and the types of numbers a dispensing registrant would have vs. a mid-level practitioner; be able to calculate the check-digit in the DEA; identify who are “dispensing” registrants and who are considered mid-level practitioners.
✓ Identify the additional information that must be on the manufacturer’s label for a controlled substance.
✓ Identify the various records of receipt for controlled substances, including DEA Form 222 and invoices, how forms or invoices are filled out, what needs to be included on each, who has authority to sign, if required, how long they must be maintained, where they may be maintained, how they may be maintained.
✓ Describe the Controlled Substance Ordering System (CSOS), what it is used for, how is allowed to have a “key”, what to do if a key is lost.
✓ Identify and describe the storage requirements for controlled substances
✓ Describe the requirements for a complete controlled substances inventory including: when they must occur, what information has to be documented, which counts have to be exact and which may be estimated; under what circumstances is a partial inventory done?
✓ Identify and describe the requirements that controlled substance prescription orders must meet to be valid; the requirements for controlled substance prescription orders (paper, eprescriptions, faxed prescriptions).
✓ Identify the expiration and refill limits for CII, CIII, CIV and CV prescription orders
✓ Describe the rules for writing multiple CII prescriptions at a time for the same CII drug for the same patient.
✓ Identify the various changes from the AZ Opioid Epidemic Act and the two clean-up bills.
✓ Identify the information that may be edited on CII, CIII, CIV, and CV Rx orders
173 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ Identify the various ways controlled substance prescription orders may be received into the pharmacy (paper, phone, fax, e-prescription), if there are any restrictions or exceptions, what they are and what documentation is needed
✓ Describe what to do if the transmission of an e-prescription fails and what the pharmacy needs to do.
✓ Identify and describe the transfer requirements for controlled substance prescription orders (CII –CV) and who may transfer controlled substance prescription orders.
✓ Explain the concept of corresponding responsibility and how to evaluate the validity of a prescription order.
✓ Identify and describe the organization and storage requirements for controlled substance prescription orders (including e-prescriptions, electronic imaging recordkeeping systems).
✓ Identify how long CS prescription orders are to be maintained.
✓ Identify the CS recordkeeping requirements for hospital pharmacies.
✓ Identify who is allowed to dispense controlled substances; the information that needs to be documented by pharmacies dispensing CS, requirements if pharmacy uses computerized documentation.
✓ Identify and describe if partial filling is allowed for CII, CIII, CIV and/or CV medications, if yes, under what circumstances and what needs to be documented.
✓ Identify the label requirements for dispensed controlled substances medications (CII –CV)
✓ Identify the requirements for central fill pharmacies related to controlled substances
✓ Identify and describe the rules for mailing controlled substances in the United States.
✓ Identify and describe the requirements for sale of non-prescription CV substances.
✓ Identify and describe how distribution of controlled substances occurs by pharmacies. What are the requirements for pharmacies to be able to distribute without a distribution registration?
✓ Identify how controlled substances are disposed of or destroyed. What is the process? What form needs to be used?
✓ Identify and describe what a pharmacy report for a theft or loss of controlled substances.
✓ Identify and describe the enforcement activities of the DEA (audits, inspections)
✓ Identify and describe the rules for opioid treatment programs (OTP) and requirements for treatment outside of an OTP for prescribers and pharmacies; requirements for the prescribing of Suboxone, Subutex, and Zubsolv off-label.
✓ Identify and explain how pharmacists can dispense naloxone without a prescription.
✓ Identify and describe the requirements, quantity limits and recordkeeping requirements for ephedrine, PSE and PPA.
✓ Identify and describe the AZ Controlled Substance Prescription Monitoring Program, who has to report and frequency of reporting, who is allowed access, and mandatory look requirements.
✓ Abood RR, Burns KA, Frankhauser F. Chapter 4 “The Closed System of Controlled Substance Distribution”
174 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ Abood RR, Burns KA, Frankhauser F. Chapter 5 “Dispensing Controlled Substances”
✓ AZ Controlled Substances Act, Title 36 – Chapter 27
✓ Prescription Monitoring Program: Title 36 – Chapter 28
✓ Arizona Administrative Code: Title 4 – Chapter 23; Articles 4, 5 and 10
Federal Comprehensive Drug Abuse Prevention and Control Act of 1970 aka the "Controlled Substances Act" (CSA) (21 CFR 1300 et al )
Establishes a "closed system" for manufacturing, distribution, and dispensing of controlled substances; only persons registered with the DEA are allowed to engage in these activities




Divided into 3 titles
Title I - rehabilitation program for drug abusers
Title II - registration and distribution of controlled substances (this title governs us)
Title III - import and export regulations for controlled substances
Arizona Controlled Substances Act (Title 36 – Chapter 27; ARS 36-2501 – 36-2552)
Each state has a "uniform" CSA that is intended to mirror federal law...
Establishes a drug schedule for the state
Regulates manufacturing, distribution, and dispensing of controlled substances within the state
Establishes DEA is the administrative agency that regulates controlled substances at the federal level
Establishes the ASBP is the administrative agency that regulates controlled substances in Arizona Some significant differences do exist...
✓ For the most part, state laws concur or act in addition to federal law
✓ If and where two laws conflict, the more stringent will prevail
175 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Controlled substances are divided into 5 'schedules' (I – V) according to the potential of addiction with prolonged use.
Federal Level
Scheduling authority is given to the U.S. Attorney General (per CSA)
The U S Attorney General delegates this task to the Drug Enforcement Administration (DEA)
Prior to scheduling, the Secretary of the Department of Health and Human Services must perform a medical evaluation of the drug and makes a recommendation
The Secretary of DHHS delegates this task to the Food and Drug Administration (FDA)
State Level - Arizona
Arizona State Legislature and the Arizona State Board of Pharmacy (ASBP)
Changes to the Controlled Substances Act (AZ) are done through the legislative and rule making process.
Ways a drug may become a controlled substance:
Step 1: Scheduling (Scheduling, rescheduling, or unscheduling)
• DEA
• FDA
• Congress
• Petition
Step 2: Attorney General or DEA requests HHS/FDA perform a scientific & medical evaluation of the drug
• FDA recommends if the drug should or should NOT be scheduled.
o If the FDA does not recommend scheduling the drug, the DEA MUST comply with the FDA’s recommendation.
Step 2A: DEA begins its own investigation and collects necessary data
Step 3: DEA proposes the scheduling (scheduling, rescheduling, or unscheduling) in the Federal Register
• This allows for public comments.
Step 4: DEA publishes the final rule in the Federal Register and includes the effective date for the regulation.
176 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Exception to the process of consulting with HHS/FDA: The DEA can place a drug in Schedule 1 (CI) without consulting HHS/FDA if there is an “imminent hazard to public safety.”
Schedule
No recognized medical use in this country
Has a high potential for abuse
Lack of accepted information on safety, even under medical supervision
Investigational purposes only
Has a high abuse potential
Has a currently accepted medical use in treatment in the US
Abuse of the drug or other substance may lead to severe physical or psychological dependence
Has an abuse potential less than C-I or C-II drugs
Has a currently accepted medical use in treatment in the US
When abused may lead to moderate or low physical dependence or high psychological dependence
Has a low abuse potential relative to drugs in C-III
Has a currently accepted medical use in treatment in the US
When abused may lead to limited physical or psychological dependence relative to drugs in C-III
Have an abuse potential less than C-IV drugs
Have a currently accepted medical use in treatment in the US
When abused may lead to limited physical or psychological dependence relative to drugs in C-IV
Heroin
LSD
Peyote
Single-entity
narcotics/ stimulants
Codeine
Morphine
Oxycodone
Fentanyl
Amphetamines
Methylphenidate
Combination
Hydrocodone products
Dronabinol (Syndros)
Products containing limited quantities C-II drugs in combination with other therapeutically active ingredients
Tylenol w/ Codeine
Dronabinol (Marinol)
Anabolic steroids*
Benzodiazepines
Ambien
Lunesta
Tramadol (Ultram)
Carisoprodol (Soma)
Products containing limited quantities of narcotics/stimula nts (generally for antitussive, antidiarrheal and analgesic purposes)
Lomotil
Tylenol w/Codeine Elixir
Epidiolex ® (Epi – dio – lex) (CBD oil)
NOTE: *Anabolic steroids are C-III substances in order to discourage trafficking, but are not scientifically confirmed to be addiction prone
174
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Rule of thumb: ALL narcotics are controlled substances; not all controlled substances are narcotics
From the Greek 'narkos' meaning 'to numb.' It is an archaic medical term that can refer to any drug that dulls the senses, relieves pain, and induces profound sleep. The term mostly used in lay-language to describe any illicit substance. The DEA also utilizes this term in its regulations, but they limit the definition of 'narcotic' to include opium and cocaine and all of their derivatives.
In addition, it is important to note that while all 'narcotics,' by the DEA's definition, are controlled substances, not all controlled substances are narcotics. Some examples of nonnarcotic controlled substances include benzodiazepines, stimulants, steroids, etc.
This becomes an important distinction regarding CII prescription orders and faxed Rx orders, hospice and LTCFs.
Arizona adds cannabis to this definition
Opioid
An opioid is a substance that binds to opioid receptors. This includes all natural (e.g. morphine, codeine) and synthetic (e.g. oxycodone, fentanyl) derivatives of opium poppy. The term 'opioid' is the preferred term for use by medical professionals. For example, 'narcotic analgesics' is how we commonly refer to a certain class of medications, but it is more accurate to describe them as 'opioid analgesics.'
(TIP: Do not confuse this with the term 'opiate,' which is a more specific term referring to natural opioids such as morphine and codeine that are extracted directly from the opium poppy.)


175 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
A note on “Medical Marijuana: (Adapted from https://www.ncsl.org/research/health/statemedical-marijuana-laws.aspx)
✓ Marijuana is currently a schedule 1 at the federal level
✓ 38 states and 3 territories, and DC have adopted laws legalizing marijuana in some form. (As of 2023)
o 38 states, District of Columbia, and territories have enacted laws allowing patients with a legitimate medical need as certified by a physician the right to possess or grow marijuana
o 18 states, District of Columbia, and 2 territories allow for recreational use (nonmedical use)
✓ Pharmacies with DEA registrations and the pharmacists who work for them are not allowed to be part of the medical marijuana distribution system because it is still federally a Schedule I (CI)
✓ AR, CT, LA, MD, MN, NH, NY, PA, and UT: pharmacists have some role in dispensing in medical cannabis dispensaries or are involved in other ways.

176 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Each CS-related activity requires a separate registration
1. Manufacturing CI - CV
2. Distributing CI – CV
3. Reverse distributing controlled substances CI - CV
4. Dispensing (or Instructing) CII – CV
5. Research with CI
disPENSing
"Delivering a controlled substance to an ultimate user pursuant to a lawful order of a practitioner, including:
❖ prescribing and administering,
❖ packaging,
❖ labeling, and
❖ compounding necessary to prepare for such delivery."
6. Research with CII – V
7. Narcotic Treatment Program (including compounding) using any narcotic drug listed in CII – V
8. Chemical Analysis (CI – CV)
9. Importing CS (CI - CV)
10. Exporting CS (CI - CV)
vs. disTRIBUTing
"Delivering a controlled substance other than by administration or dispensing."
❖ Includes movement of CS between
o Manufacturer
o Wholesaler
o Pharmacy
CSA definition of MANUFACTURE =
✓ Production, preparation, propagation, compounding, or processing of a drug, either directly or indirectly.
✓ Packaging, repackaging, labeling or relabeling.
Manufacturers, under their DEA registration for Manufacturing, may do the following:
✓ Distribute CI – CV it is registered to manufacture
✓ May conduct chemical analysis and preclinical research
Issues for pharmacies and pharmacists
Pharmacists and pharmacies that compound, repackage, or re-label controlled substances need to be aware that the DEA views this differently than the FDA.
✓ FDA in general allows pharmacies to compound
177 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ DEA rules (21 CFR 1301.13 (e) (l)(iii)) allow pharmacies to compound controlled substances if the final product does not have more than 20% of a narcotic substance.
✓ DEA definition of compounding (dispensing) and manufacturing is different from the FDA if for office use or administration by prescriber to patient.
o DEA views compounding of controlled substances for “office use” or for administration by the prescriber as DISTRIBUTING and not dispensing.
Manufacturers/ Wholesalers/Distributors/ Repackagers
Generally, have CI-CV DISTRIBUTION registration
Each distributing location (physical address) requires a separate registration
Requires annual renewal
Pharmacies
Generally, have CII – CV DISPENSING registration
Each dispensing location requires a separate registration (including internet, remote dispensing, and central fill pharmacies)
Requires renewal every 3 years
Individual Practitioners
Generally, have CII – CV DISPENSING registration
Required if the practitioner prescribes, orders, or dispenses any CS
If a prescriber has multiple offices, and maintains supplies of CS, administers, or dispenses from each of those offices – each location must have a separate DEA registration (See DEA Practitioners Manual page 8)
Requires renewal every 3 years
1. An agent or employee of any registered manufacturer, distributor or dispenser, including...
Individual pharmacists, because they are agents of the registered pharmacy
Practitioners that are employees of a hospital
Note: Practitioners may be assigned the DEA # of the institution they work for, with a suffix to identify themselves when prescribing CS (numbers, letters, or combination)
For example – Marvin Smith, a 1st year resident at Sun Valley Hospital, has not yet applied for his own DEA registration.
• He is allowed to write CS prescription orders using the hospital’s DEA number with a suffix that identifies him DEA e.g., AS1234563-012
Current list of internal codes and corresponding individual practitioners is kept by the hospital/institution and is always available to other registrants and law enforcement
178 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
2. Practitioners that serve in the military (Army, Navy, Marines, Air Force) or other federal service (PHS, Bureau of Prisons, Coast Guard)
If engage in private activities involving CS (think moonlighting outside of their primary employer), must have an individual DEA registration
3. A common or contract carrier or warehouse employee
FEDEX, UPS, USPS, etc.
McKesson, Cardinal, Walgreens warehouse, etc.
4. An ultimate user who possess a CS lawfully (patient)
5. Law enforcement officials acting in the course of enforcing any law related to CS
including AZ DPS, ASBP, local law enforcement, FBI, ATF
6. Civil defense officials and disaster relief officials
May only dispense CS during times of proclaimed emergencies or disasters
An alpha-numeric code is assigned for each DEA registration AZ9754652
Registration type 1st letter of last name or company name Randomly generated Validity check
Dispensing registrants = A, B, F, or G
Midlevel practitioners = M If a business name starts with a number – this is a number instead of a letter (2nd spot – is a letter or number)
(Practitioners are not required to obtain new DEA number if change their last name)
Who are Midlevel practitioners?
Homeopathic Physicians Naturopathic Physicians Optometrists
Physician Assistants
Pharmacists (if granted prescribing authority in state of licensure)
Registered Nurse Practitioners
179 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
To check if the DEA number is valid:
1) Add the first, third, and fifth digits 9 +5 +6 = 20
2) Add the second, fourth, and six digits and multiply by 2 (7+ 4 + 5) * 2 = 32
3) Add the two sums together 20 + 32 = 52
4) The last digit of the summed total; last digit of the DEA # 2
Physicians with multiple DEA numbers and multiple offices (Prescribe AND administer OR dispense CS) (Reference: https://www.deadiversion.usdoj.gov/faq/locum_tenens.htm)
✓ A prescriber is required to have an individual DEA registration for each location where they do the following regarding CS:
o Prescribe, AND
o Administer, OR
o Dispense
✓ If a prescriber has an individual DEA number for an individual office location, the DEA number on the prescription order will need to be the one for the specific office location where the prescriber “wrote” the prescription order.
Example: Dr. Miranda Bailey, DO has several office locations and PRESCRIBES and ADMINISTERS controlled substances (CS) from the following office locations:
Dr. Miranda Bailey, DO
1815 N Central Avenue, Phoenix, AZ 85004
19680 N 59th Avenue, Glendale, AZ 85308
5460 W Thunderbird, Glendale, AZ 85306
DEA # FB2246468
DEA # FB5466823
DEA # FB8457322
Result: The pharmacy receives a CS prescription order written by Dr. Bailey from the office on 59th Avenue. The DEA number to use for this prescription order is FB5466823.
Physicians with one (1) DEA number and multiple offices (who only PRESCRIBES CS)
✓ A prescriber, if they only PRESCRIBE controlled substances from any given location, in a given state, is ONLY required to have one (1) DEA number for all of the office locations in that state.
✓ The address to be used when filling the controlled substance prescription order is to be the one that the prescriber was at when they wrote the prescription order.
181 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Example: Dr. Derek Shepard, MD has several office locations and only PRESCRIBES controlled substances (CS).
Dr. Derek Shepard
1815 N Central Avenue, Phoenix, AZ 85004
19680 N 59th Avenue, Glendale, AZ 85308
5460 W Thunderbird, Glendale, AZ 85306
DEA # GS2413492
Same DEA is used for all locations
Result: The pharmacy receives a controlled substance prescription order from Dr. Shepard written from his office on N Central Avenue. When filling the prescription order, the address that must be matched to the prescription order is the N Central address.
All commercial containers of a controlled substance drug must be labeled with identification symbols showing the schedule in which the drug is placed




General Information on Recordkeeping
Keys to compliance with CSA: Know the law
Document completely
Keep records readily retrievable
Violations can result in fines up $10,000 per incident (even if unintentional)
182 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Three types of records must be maintained (including receipt and dispersal)
Record
Record Type (Receipt or Dispersal)
1. Inventory Records Receipt
2. Records of drugs received (Invoices, DEA Form 222) Receipt
3. Records of drugs dispersed (dispensed, transferred, sold, etc.) Dispersal
Ordering C-II medications (Form is used for CI & CIIs)
DEA Form 222 – NEW Single Sheet Form (21 CFR 1305…..)
(Unless you use CSOS...keep reading)
183 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Purchaser Information is pre-printed on DEA Form 222
Step 9
Supplier Completes
Steps 1- 3

Step 4






Steps 5 - 7


Supplier completes this part







Step 8 Or Pharmacist
184 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
1. Purchaser information is pre-printed by the DEA with the information of the registrant
a. Pharmacy’s name and address
b. DEA registration number
c. Registered as: Dispensing/Dispenser
d. Controlled Substance Schedules registered: 2, 2N, 3, 3N, 4, and 5 (can choose which ones the site wants to deal with)
e. Order Form Number (will be sequentially numbered)
f. Date issued by DEA
2. Fill in the name and address of the supplier (Steps 1 – 3)
3. Complete today’s date (Step 4)
4. Write the # of packages, package size, and name and description/strength of the drug being ordered. (Steps 5 – 7)
5. Last Line Completed – write the number of the last line completed (Step 8)
6. Printed name, and signature of the purchaser
a. if not the DEA registrant, must have power of attorney at that site
7. Before sending the form to the supplier, pharmacy must make a copy of the completed DEA Form 222 and retain the copy (either paper or electronic format)
NOTE: If you make an error on the order form, it CANNOT be corrected! Write "VOID” on the form, retain it with your records, and start a new one.



WHO CAN SIGN DEA FORM 222?
The DEA registrant OR whoever has been granted power of attorney
POWER OF ATTORNEY TO SIGN AN OFFICIAL ORDER FORM (FROM 21 CFR SECTION 1305.05)
Any registrant (pharmacy) may authorize one or more individuals, whether or not they are located at the registered location, to obtain and execute DEA Forms 222 by granting a power of attorney to each such individual. The power of attorney must be signed by the same person who signed the most recent application for registration or renewal registration, as well as the individual being authorized to obtain and execute the DEA Forms 222.
The power of attorney may be revoked at any time by the person who granted and signed the power of attorney. Only if the renewal application is signed by a different person is it necessary to grant a new power of attorney when the pharmacy completes a renewal registration. The power of attorney should
185 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
be filed with executed DEA Forms 222 as a readily retrievable record. The power of attorney is not submitted to DEA.
IN A PHARMACY, WHICH OF THE FOLLOWING INDIVIDUALS WOULD BE ABLE TO HOLD POWER OF ATTORNEY FOR SIGNING THE DEA 222 FORM?
• Pharmacist
• Pharmacy Technician
• Pharmacy Technician Trainee
• Store Manager
• Assistant Store Manager
• Clerk
• Any other employee of the company
SUGGESTED FORMATS FOR GRANTING AND REVOKING A POWER OF ATTORNEY FOLLOW:

186 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Completing DEA Form 222 at Your Pharmacy (aka closing the loop)
Upon receipt of CII drug products, the following items must be documented on the photocopy of the original DEA Form 222
1. Number of containers received for each line item ordered
2. Date containers received.
If the supplier partially fills the order, the supplier has 60 days from the date of execution of DEA Form 222 to supply the balance. After 60 days of date of execution – the order form becomes invalid.

This is where you (the purchaser) document the number of containers received and the date (it is also strongly suggested that the person includes their initials)
187 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Supplier Responsibilities with DEA Form 222

Supplier Completes
Supplier completes this information (NDC, # shipped, date)
THE SUPPLIER NEEDS TO COMPLETE THE FOLLOWING ON THE ORIGINAL COPY THEY RECEIVED FROM THE PURCHASER:
1. Supplier DEA number
2. The NDC, number of packages shipped, and the date shipped for each CII product shipped to the purchaser.
3. Report purchase and sales transactions to the Automation of Reports and Consolidated Orders Systems (ARCOS)
a. If the suppler is NOT required to report purchase and sales transactions to the Automation of Reports and Consolidated Orders Systems (ARCOS) (such as a practitioner or pharmacy),
1. MUST make and submit a copy of the original DEA Form 222 to the DEA
a. Mail to the Registration Section OR
b. Email to DEA.Orderforms@usdoj.gov
4. Supplier MUST retain the original DEA Form 222 in their records
No DEA forms are required for purchase of C-III – C-V substances However, the DEA does require that you maintain a record of receipt/invoice from the supplier
ALL CS Invoices must contain the following information: (CII – CV)
Drug name
Strength
Dosage form
Quantity/Package size (# of dosage units or volume of container)
Number of commercial containers received
Date of receipt
Supplier name, address, and DEA number
In addition, if controlled substances are listed with other non-controlled substances on the invoice, they must be clearly identifiable in some manner (marked with a red asterisk or underlined in red)
Always note the date of receipt on the invoice and verify the accuracy of the order!
189 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale

✓ CSOS allows registrants to transmit orders for any controlled substances electronically (C-I to C-V)
✓ Every individual person who is the DEA registrant or who has been granted power of attorney and wants to sign electronic orders for controlled substances must first enroll with DEA to acquire his or her own personal CSOS "key" (certificate)
✓ Orders are signed electronically using your "private key" stored on a physical device that is under sole control of the registered individual
✓ Report the loss of your key to the CSOS Certification Authority within 24 hours
✓ If you're interested in learning about digital signatures (for CSOS, e-prescribing, etc.), visit: http://www.deaecom.gov/pki.pdf

The SUPPORT Act requires all DEA registrants that distribute controlled substances to report suspicious orders to the DEA. (CS sales between DEA registrants). Required to report are:
Distributor Hospital/Clinic
Mid-Level Practitioner –Ambulance Service
Manufacturer Teaching Institution Researcher
Importer
Pharmacy
Practitioner
Analytic Lab
Mid-Level Practitioner Narcotic Treatment Program
191 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
What are suspicious orders?
May include:
1. An order of a CS of unusual size
2. An order of a CS deviating substantially from a normal pattern
3. Orders of CS of unusual frequency as per 21 USC 802(57) and 21 CFR 1301.74(b)
Organizing invoices & forms (AZ and Fed the same)
C-II invoices & completed DEA Form 222s must be maintained separately from all other invoices
C-III, IV, and V invoices may be…
a. maintained separately from all other invoices OR
b. Combined with non-CS invoices, but marked with a red "C"
OPTION 1: 3 File Folders for INVOICES



CII Invoices and Executed DEA Form 222s CIII – CV Invoices




OPTION 2: 2 File Folders for INVOICES
CII Invoices and Executed DEA Form 222s
Non-Controlled Substances Invoices






CIII – CV (stamped with Red C) and Non-CS Invoices


192 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Invoices and executed DEA 222 Forms must be maintained for a minimum of 3 years (AZ)
Federal DEA requirement is only 2 years
DQSA Requirement = 6 years (all inventory records)
Medicare Requirement = 10 years
Executed DEA 222 Forms and annual CS inventory must be maintained on site
Invoices may be maintained at a central location, after notifying the DEA (see DEA Pharmacist’s Manual for complete explanation)
Records (invoices) must be readily retrievable
Comply with a DEA request within 2 business days.
Two approved methods:

In a securely locked, substantially constructed cabinet

Dispersed throughout the stock of unscheduled medications
193 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
When do I need to take inventory of my CS stock? (ARS 36-2523 & AAC R4-23Article 10 and Abood and Burns Chapter 5 – Recordkeeping)
Arizona law requires CS inventory every year on May 1st
(Federal requirement is only every 2 years after the initial inventory)
Other circumstances that require a complete CS inventory:
Opening a new pharmacy
Discontinuation of a pharmacy

When your pharmacy accepts all CS stock from another pharmacy that is closing
Change in ownership
Within 10 days of change in PIC (AZ)
Within 10 days of incidence of theft (AZ)
As directed by ASBP (AZ)
In addition, when a drug is newly scheduled, an inventory of that drug must be performed on the day it is rescheduled.
Link to the AZ CS Inventory Book: https://drive.google.com/file/d/0ByEEsGueI9GREJYbE1JcDJtOGk5ejRGQVlxX2wyV0JmMVhR/view
Drug name, strength, dosage form, and most importantly quantity
C-II drugs require EXACT counts
C-III – C-V drugs in containers of > 1000 dosage units require EXACT counts
C-III – C-V drugs in containers of ≤ 1000 dosage units may be estimated
CS inventory records must be maintained on site for a minimum of 3 years (AZ)
(Federal DEA requirement is only 2 years; DQSA = 6 years; Medicare = 10 years)
194 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
1. Prescription orders for CS
2. Non-prescription CV sales
3. Distributions from a pharmacy to another practitioner (DEA registrant)
4. Institutional medication records
5. Disposal or destruction of CS
6. Return of CS to pharmacies for disposal
7. Records of theft or loss
CS prescription requirements
All CS prescriptions must meet the following requirements: (Fed and ARS 36-2525)
C-II prescriptions must be written in ink, indelible pencil, or printed
Be issued for a legitimate medical purpose
Be written by a practitioner acting in the usual course of his/her professional practice
The practitioner must have conducted at least one in-person medical evaluation of the patient
Must be written for an ultimate user (i.e. writing a prescription "For Office Use" is prohibited)
CIIs – only one (1) drug order per prescription blank (AZ) (ARS 36-2525 A)
Must be from a prescriber licensed in the U.S. (i.e. no CS prescriptions from any foreign country or from out-of-state prescribers whose profession does NOT have prescriptive authority in AZ)
Clinical Psychologists have prescribing authority in the following states: Idaho (ID), Illinois (IL), Iowa (IA), Louisiana (LA), and New Mexico (NM)
AZ recognizes out-of-state prescribers (note not all states do)
All CS prescription orders must include the following information: (AAC R4-23-407)
Full name and address of the patient
For an animal, owner's name (AZ) and species of animal
Full name, address and telephone number of the prescriber
DEA number of the prescriber
Remember that some prescribers are exempt from DEA registration and will instead use an identifier at their institution
Date issued
195 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Drug name, dosage form, strength, and quantity
Directions for use
Prescriber's signature
Must be manually signed (pre-printed or stamped signatures are prohibited) or
Electronic/digital signature in electronically-transmitted prescriptions or
Electronic only signature on prescription orders generated by electronic media (EMR/EHR) MUST be printed security paper that prevents copying or alteration or
Electronic signature with manual signature/initials are allowed for prescription orders generated by electronic media (EMR/EHR) on regular paper are allowed or
Not required for orally-transmitted prescriptions (RPh signs for prescriber)
Refills if authorized
Additional Information Required for Oral Prescriptions (AZ)
Telephone number of prescriber
Additional Information Required for e-Prescriptions: (AZ)
Date of transmission
Name of individual transmitting the prescription, if not the prescriber
Additional Information Required for Faxed Prescriptions: (AZ)
Date of transmission
Telephone number of prescriber
Fax number
Name of individual faxing the prescription, if not the prescriber
The maximum number of refills a CS can be written for and the date after which the prescription can no longer be filled or refilled changes depending on the schedule of the drug.
* The date of issuance is considered day 0 (ARS 36-2525 (D))
196 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Federal Law: Currently, NO quantity limitations on the number of dosage units (# capsules/ tablets) or volume of ANY controlled substance that may be prescribed on an individual prescription order
One exception to this rule
Due to insurance limitations, many patients are prohibited from obtaining more than a 30-day supply at a time. C-II prescriptions cannot be refilled and can only be partially filled in a few circumstances. The DEA created regulations to alleviate this inconvenience for patients.
In addition to the requirements of any CS prescriptions:
3 identical Rx orders can be written on the same date of issue
Limited to a 90-day supply (total) (30 days per Rx order x 3 = 90 days)
The practitioner must write instructions on each prescription (other than the first) as to the earliest date on which the prescription may be dispensed
For example:
• Notice all 3 rx orders have the same date of issue;
• The total day supply across the 3 rx orders is 90 days (30 + 30 + 30 = 90);
• The 2nd and 3rd prescription orders have specific dates on which they are able to be filled.



197 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Opioid Epidemic Act and Clean-up Bills
Dispensing CII Opioid Prescriptions
Initial Prescription for a CII opioid 5-day supply limitation
Definition of “initial prescription”
Exemption to the initial prescription supply limitation
Dosing limitation
supply limitation
Initial fill is defined as not previously received by the patient within 60 days of the pharmacy filling the current prescription
✓ Active oncology diagnosis
✓ Traumatic injury, excluding surgical procedure
✓ Receiving hospice care, end-of-life care, palliative care, treatment for burns, or skilled nursing care
✓ Receiving medication-assisted treatment (MAT) for a substance abuse disorder; or
✓ Infant being weaned off opioids at the time of hospital discharge
Rx dosing cannot exceed 90 morphine milligram equivalents (MME)/day
✓ Exception: prescriber may issue RX for more than 90 MME/day if a consulting physician, board-certified in pain or opioid call service agrees with the higher dosage.
✓ Exempts prescribers who are boardcertified pain specialists from consultation requirements.
Exempt from the 90 morphine milligram equivalent (MME)/ day rule
Exceptions to the 90 MME dosing limitation:
✓ Rx is continuation of a prior Rx issued within the previous 60 days
✓ An opioid with a maximum approved daily dose in the FDA approved labeling
✓ Patient with an active oncology diagnosis or traumatic injury, not including a surgical procedure
✓ Patient who is hospitalized
198 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ Patient receiving hospice care, end-oflife care, palliative care, skilled nursing facility care, or treatment for burns
✓ Patient receiving medication-assisted treatment (MAT) for a substance abuse disorder
Naloxone or another opioid antagonist ✓ Co-prescribed to any patient receiving more than 90 MMEs/day
Opioid Epidemic Act & Clean-Up Bills – Pharmacist Responsibilities Regarding CII Opioid Rx Orders
✓ CII opioid prescriptions that exceed the 5-day supply or a new prescription that exceeds 90 MMEs/day is presumed to be exempt from statutory limitations
o What this means – pharmacists are not required to verify a prescription’s exemption status with the prescriber
Receiving CS prescriptions
Paper from the physician's office
Phone
Fax
E-prescription
Faxed C-II prescriptions

(w/ exceptions)
(w/ exceptions)

C-III, C-IV & C-V




Under normal circumstances, a faxed C-II order allows the pharmacist to begin filling. However, the pharmacist must receive the original hardcopy before releasing the CS medication from the pharmacy
199 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
EXCEPTION: A FAXED C-II PRESCRIPTION MAY SERVE AS THE ORIGINAL HARDCOPY WHEN: (ARS 32-2525 AND 21 CFR 1306.11 (FED STRICTER THAN STATE)
• CII narcotic for a compounded injectable solution (parenteral, IV, IM, SQ, or intraspinal) and will be directly administered to patient in any setting
• Any CII for a resident of LTCF (long-term care facility)
Hospice (in-patient or out-patient)
• CII narcotic issued for patient enrolled in Medicare certified hospice care program
Receiving C-II's in an emergency (Telephone)
In an emergency, a C-II prescription may be orally transmitted by a prescriber, however...
1. The quantity prescribed and dispensed must be limited to the amount necessary to adequately treat the patient during the emergency period
Typically, 72 hours-worth of medication (not a hard limit)
2. The prescriber who authorizes the emergency C-II must deliver a written prescription to the dispensing pharmacist within 7 days of the authorization
If delivered by mail, it must be postmarked within the 7-day period
3. The prescription must have “Authorization for Emergency Dispensing” written on the face and the date of authorization.

If the prescriber fails to deliver the written prescription, the dispensing pharmacist must notify the DEA and ASBP (AZ)
AN "EMERGENCY SITUATION" EXISTS ONLY WHEN THESE THREE FACTORS ARE PRESENT...
Immediate administration of the CS is necessary for proper treatment
No appropriate alternative treatment is available, including administration of a controlled substance that is not a C-II
It is not feasible for the prescriber to provide a written prescription prior to the dispensing the CS
200 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
E-prescribing
Electronic prescribing or “e-prescribing” is the computer-based electronic generation, transmission and filling of a prescription (a fax is NOT an electronic prescription)
DEA rules allowing for e-prescriptions for controlled substances (CSERx) became effective as of June 1, 2010
HOWEVER, any system transmitting or receiving CSERx must be audited and approved by a third-party (this means prescribers and pharmacies)
(Check out interactive graphic at: http://surescripts.com/products-and-services/e-prescribing-ofcontrolled-substances
PHARMACY REQUIREMENTS
✓ First recipient of the e-Rx (pharmacy or application service provider) must digitally sign and the pharmacy must archive the e-Rx.
✓ If transmission of an e-prescription fails... (you'd only know this if you receive an e-prescription, but the data is somehow corrupt)
o Inform prescriber of failed/corrupt transmission
o Prescriber may write a copy of the transmitted prescription and sign it
▪ Copy must indicate that it was originally transmitted to a specific pharmacy and that the transmission failed.
o Pharmacy must check records to ensure that the e-Rx was not received or dispensed (either at its pharmacy or another pharmacy) before it dispenses based on the paper prescription.
✓ If the electronic version was received
o If the paper/oral prescription and e-prescription are at different sites, mark the paper prescription as void
o If the paper/oral prescription and e-prescription are at the same site, you make mark either prescription as void, but remember that a paper CS prescription must comply with all DEA requirements for any paper prescription, including a manual signature
PRESCRIBER REQUIREMENTS
✓ Two-factor authentication method; prescriber must choose two of three factors → acts as a digital signature
✓ Factors
o Something you know (such as a password or PIN); or
o Something you have (a hard token separate from the computer such as a personal digital assistant, cell phone or flash drive), or
o Something you are (biometrics)
✓ An agent may enter the information into the system, the agent cannot submit for approval and authentication – only the prescriber can do the approval and authentication process.
OPIOID EPIDEMIC ACT AND E-PRESCRIBING (UPDATED 2019) (AZ)
✓ all CII opioid MUST be e-prescribed effective January 1, 2020
201 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
✓ Prescribers EXEMPT:
o Veterinarians
o Out-of-state prescribers
o Federal facilities (VA, IHS, DOD)
CMS RULE CHANGE– E-PRESCRIBING OF CS FOR MEDICARE REQUIREMENT (EFFECTIVE JAN 1, 2023)
✓ All CS prescription orders should be E-prescribed unless the practitioner is currently exempt
✓ In 2023, CMS will begin initial compliance EPCS actions
o Sending notices to non-compliant prescribers
o In 2025, increased penalties will be enforced for non-compliance with EPCS for Medicare
(Federal and ARS 36-2525)
Having consulted with the prescriber, a pharmacist may change:
Date of issue
Drug strength
Dosage form (may change from immediate release to controlled release or vice versa)
Drug quantity
Directions for use
To help clarify, the DEA specifically mentions three things a pharmacist cannot alter on C-II prescriptions:
Patient's name
Drug name = Controlled substance prescribed (except for generic substitution as permitted by state law) (Means if morphine prescribed – must dispense morphine)
Prescriber’s name = Prescriber/prescriber signature
Always document the time, date, and name of the person giving authorization for corrections, and ensure that the prescriber has documented the changes in the patient’s chart!
Oral telephone orders are allowed for CIII – CV prescription orders. Thus, if a pharmacist has questions on a CIII – CV prescription order, they may call the prescriber and either change information per consultation with the prescriber or take a new telephone prescription order from the prescriber and void the original prescription order received.
202 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Summary Chart
Prescription Order
Patient Name
Drug Name
Drug Strength
Dosage Form
Drug Quantity
Directions for use
Date of issue
Prescriber/ prescriber signature
C-III, C-IV, and C-V prescriptions may be transferred one time only!
Pharmacies sharing a real-time online database may transfer up to the maximum refills permitted by law and the prescriber’s authorization
CIIs may never be transferred
Telephone CS transfers – licensed pharmacists or interns
Electronic CS transfers – licensed pharmacists only
The sending pharmacist must record:
The word "VOID" on the face of the transferred prescription
For a pharmacy with computerized prescription records, this can be substituted with a record of the transfer in the system that invalidates the prescription
Date of transfer
Name of receiving pharmacist
Name of sending pharmacist
Pharmacy’s name, address, telephone number, and DEA number of the receiving pharmacy
NOTE: Even though your copy of the prescription is now void, you still have to maintain it for the amount of time required by law! (7 years)
203 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
The receiving pharmacist must record:
All information required on a CS prescription (see the section entitled "All CS prescriptions must include the following information:")
The word "Transfer" on the face of the prescription
For e-prescriptions, the transfer record itself is sufficient
Date of transfer
Prescription number from sending pharmacy
Date of issue
Date first dispensed
Original number of refills
Number of valid refills remaining and date(s) and location(s) of previous refill(s).
Dates and locations of previous fills for CS (21 CFR 1306.25 – Fed is stricter than state)
The name, address, DEA registration number and prescription number from the pharmacy that originally filled the prescription, if different than the one currently transferring the prescription to the receiving pharmacy (21 CFR 1306.25)
Pharmacy’s name, address, telephone number, and DEA registration of the sending pharmacy
...and of the pharmacy where the prescription was first filled, if different
Name of sending pharmacist
Name of receiving pharmacist
Corresponding Responsibility
Lawful dispensing of controlled substances not only dependent on obtaining a facially-valid prescription order!
The pharmacist has a corresponding responsibility to evaluate the validity of a prescription for a controlled substance prior to dispensing. This requires using professional judgment to discern whether or not a prescription is for a legitimate medical purpose and prescribed in the usual course of practice.
Tips for spotting illegitimate prescriptions:
Abnormally large quantities
Prescriber writes for significantly more CS prescriptions than do other providers in that field
Stimulant and depressant medications being prescribed for the same patient (Drug abusers often ask for both "uppers" and "downers")
Patient presents prescriptions written in the names of other people
204 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
A number of patients (often people who are not regular patients) appear within a short period of time, all bearing similar prescriptions from the same prescriber
Visit the DEA's "Pharmacist Guide to Prescription Fraud" online http://www.deadiversion.usdoj.gov/pubs/brochures/pharmguide.htm
In evaluating the validity of a CS prescription, the pharmacist also has a responsibility to use professional judgment to spot possible forgeries.
Tips for spotting a forged prescription:
Misspelled drug name
Grossly unusual quantity or unusual directions for use
Person is not a regular patient at your pharmacy
Variety of ink colors or handwriting
Prescription appears to have been photocopied
Date issued was a long time ago
Drug quantity or number of refills obviously changed
Prescription is presented on the weekend/holiday/late night when practitioners are generally unavailable
A DEA registration no. that fails the confirmatory test
Chronic use of opioids can build drug tolerance and a physical dependence, so dosing, strength, or refill frequency alone is not proof of fraud or abuse. Base your decision to dispense on patient safety, not liability.
Be aware that there are state and national initiatives and guidelines that have been developed that address the treatment of non-malignant chronic pain.
A sample list includes: (AZ specific guidelines can be found at https://pharmacypmp.az.gov/)
2013 Arizona Guidelines for Dispensing Controlled Substances
2013 Arizona Prescription Prescribing Guidelines
2014 Arizona Opioid Prescribing Guidelines – ADHS
AZ Medical Board Guidelines for Treatment of Chronic Pain (SPS7)
AZ Osteopathic Board of Examiners Guidelines for Treatment of Chronic Pain
CDC Common Elements in Guidelines for Prescribing Opioids for Chronic Pain (infographic) and (complete document)
205 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
If you're suspicious...
1. Ask for a photo ID
Tell them that it is store policy and you can’t fill the prescription without ID
If patient says "My ID is in the car," then leaves, it's a good bet they won’t return!
2. Attempt to contact the prescriber
Always use the phone number in the phone book or in your computer, NOT the one on the prescription!
If you say you need to clarify the order, and the patient leaves the pharmacy, there is a good chance it is a forged prescription
3. If you confirm a forgery, get a description of the person and call the local police
4. If you notice a pattern of prescription abuses, contact the DEA or the Board of Pharmacy
Remember, you have the option of not filing any questionable prescription!
The original of every prescription order must be serially numbered, dated and filed in the order in which the drugs were compounded or dispensed.
ASBP and the DEA have approved two methods for maintaining CS prescriptions:
Option 1
3-file system
One for C-II's
One for C-III – C-V's
One for non-CS prescriptions
Option 2
2-file system
One for C-II's
One for C-III – C-V's and for nonCS prescriptions †C-III – C-V's must be marked with a red "C"
† For pharmacies with computerized prescription records, the red "C" is no longer required
E-prescriptions
If a pharmacy's computer system fields are automatically populated by an electronically transmitted prescription order, the automated record constitutes the original prescription, and a hard-copy or electronic image is not required
206 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Electronic imaging recordkeeping system
Some pharmacies have the capability of scanning and saving the image of the original prescription.
The prescription image and all associated annotations must be maintained for 7 years from the date last dispensed
The original hard copy must be maintained for 7 years for CII – CVs from date last dispensed
How long do I have to retain CS prescription order records?
CS prescription orders must be maintained on-site for a minimum of 7 years from the date of last fill (AZ) (Fed = 2 years)
Prescriptions covered by Medicare = 10 years from the date of last fill
Institutional CS medication order records
CS medication records must be maintained for a minimum of 7 years from the date of the last patient visit/encounter.
Who is allowed to dispense CS prescriptions?
Pharmacists
Registered individually
Employed by a registered pharmacy
Employed by a registered institutional practitioner
Individual Practitioner
If authorized by state law ~ office dispensing; AZ allows
Opioid Epidemic Act:
✓ A prescriber who treats humans is PROHIBITED from dispensing from their office any CII opioid prescription medications
✓ Two Exemptions:
o Exemption 1: implantable devices (MD, DO, PA, RNP, Certified Nurse Midwife)
o Exemption 2: Opioid for medication-assisted treatment (MD, DO, RNP)
✓ Veterinarians are allowed to dispense CII opioids from their office practices.
207 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Upon filling (and refilling) a CS, the following must always be documented:
All legal requirements of a prescription order
Serial number (Rx number)
Date of dispensing
Quantity prescribed (and quantity dispensed if different from amount originally prescribed)
Name or initials of dispensing RPh or Intern
Name of the drug’s or device’s manufacturer if the prescription order is written generically or a substitution is made (AZ)
By default, this information must be recorded on the back of the original prescription. However...
Most pharmacies today maintain computerized prescription records. In this case, refill information is recorded electronically instead of on paper. However, each dispensing pharmacist must document daily that the computer data of the refills he or she dispensed were reviewed for accuracy. This is done in one of two forms:
1. A hard-copy printout of each day's original and refill data
2. A log book or separate file of daily 'statements' (e.g. "I attest the accuracy of the original and refill data for prescriptions I dispensed on [DATE]") and records the following information:
1. States that original and refill data for prescriptions dispensed by each pharmacist were reviewed for accuracy
2. Printed name of each dispensing pharmacist
3. Signed and initialed by each dispensing pharmacist
Occasionally, patients will only want you to fill a portion of the prescribed quantity of medication (usually due to cost or estimated length of therapy).
All CS prescriptions may be partially filled, however C-II prescriptions have some special requirements and exceptions…...
May not exceed the total number of dosage units prescribed over the life of the prescription order (applies to all CS)
208 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Mr. Smith brought in this prescription and only wants 30 tablets on the first fill.
The original prescription has 60 tablets x 6 fills (original +5) = 360 tablets
After the initial fill of 30, Mr. Smith has 5 refills of 60 + 30 tablets remaining.
Is Mr. Smith allowed to get his prescription refilled every 2 weeks until the 360 tablets have been dispensed in the 6-month time frame? If so, how many times would the pharmacist fill and dispense this prescription for Mr. Smith?


209 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
1. If the partial fill is for a terminally ill patient or a patient in an LTCF...
The prescription may be partially filled for 60 days from the date of issue
Pharmacist must write “terminally ill” or “LTCF patient”
The following additional information must be recorded on the back of the prescription
Date
Quantity dispensed
The remaining quantity authorized for that fill
Identification of the dispensing pharmacist


2. If you don’t have enough medication to fill the prescription order
The balance of the prescription becomes void after 72 hours from the partial fill
If you cannot or do not fill the balance, you must notify the prescriber that the prescription was not completed
Note: DEA interpretation of “unable to dispense full amount” discussion from Abood, Burns, and Frankhauser book: “… when a pharmacist was unable to supply the full quantity and provided a partial fill, the pharmacist was required to note the quantity supplied on the face of the prescription and provide the balance of the prescription amount within 72 hours after the partial filling. If the pharmacist was unable to supply the remaining quantity within 72 hours, the pharmacist had to notify the prescriber and no further quantity was to be supplied beyond 72 hours without a new prescription. Historically, “unable to supply” under the DEA rule meant the pharmacy did not have enough of the medication in stock to supply at the time of dispensing. Subsequently, however, the DEA decided that other situations would also be appropriate, including when the drug was in stock, but the pharmacy was waiting for verification of the legitimacy of the prescription; when the patient could not afford to pay for the entire amount; or when the patient did not want the entire amount for some other reason.” https://www.jblearning.com/blog/jbl/2019/05/20/filling-schedule-iiprescriptions-changes-in-federal-law
3. CII Partial Fill Requirements under CARA (Comprehensive Addiction and Recovery Act 2016 – 21 USC 829) – DEA rule effective 8/21/2023. See 21 CFR 1306.13
A prescription for a CII may be partially filled if:
1. it is not prohibited by State law
2. the prescription is written and filled in accordance with this title, DEA regulations, and State law
210 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
3. is requested by the patient, one acting on behalf of the patient (parent or legal guardian of a minor patient, or caregiver of an adult patient named in a medical power of attorney), or by the practitioner who wrote the prescription
Practitioner request: The practitioner must specify to quantity to be dispensed in each partial fill on the (1) face of the written prescription order, (2) verbally by telephone in consultation with the pharmacist, or on the (3) electronic prescription order
• If partial dispensing is based on consultation with the pharmacist, the pharmacist must note the following: “Authorized by Practitioner to Partial Fill”, the name of the practitioner, the date and time of the discussion, and the pharmacist’s initials
• If the prescriber has made the request, the patient or agent may not receive more than the amount of a partial fill at any one time.
Patient or agent (parent or legal guardian, or caregiver of adult patient with medical power of attorney): may be made (1) in person, (2) in writing, or by (3) phone call
• If partial dispensing is based on patient or agent request, the pharmacist must note the following: “The (patient, parent, legal guardian, or person with medical power of attorney) requested a partial fill on “date request was made” and the quantity dispensed
4. The total quantity dispensed in all partial fillings does not exceed the total quantity prescribed
5. Partial fills shall be allowed up to 30 days from the date of issue
6. Pharmacist documentation: The pharmacist must make an annotation on the face of the prescription order or in the pharmacy’s electronic records, which includes (1) the quantity dispensed, (2) the date of dispensing, and (3) the name or initials of the individual who dispensed

211 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Effective date – March 24, 2020
HHS: Administrative Simplification: Modification of the Requirements for the Use of Health Insurance Portability and Accountability Act of 1996 (HIPAA) National Council for Prescription Drug Programs (NCPDP) D.0 Standard
What it does:
Enables covered entities (pharmacies, insurers, PBMs, etc.) to distinguish if a prescription is a ‘‘partial fill,’’ where less than the full amount prescribed is dispensed, or a refill, where the full amount prescribed is dispensed, in the HIPAA retail pharmacy transactions.
• This change was made in conjunction and consultation with NCPDP because it is a field code issue in the data transmission process
All CS medications dispensed pursuant to a prescription order must be legally labeled with the following information:
Pharmacy name and address
Rx number
Patient name
For an animal, the name of the owner & the species of the animal
Prescriber name
Directions for use and any cautionary statements, if any, contained in the prescription order
Manually initial the finished label for final accuracy
Date of filling
In addition, for all C-II, C-III, and C-IV medications dispensed pursuant to a prescription order must carry the following warning:
"Caution: Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed"

Must be dispensed with a RED CAP and a warning label;
o Warning label (or similar) Caution: Opioid Risk of overdose and addiction

212 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Central fill pharmacies (https://deadiversion.usdoj.gov/GDP/(DEA-DC046R1)(EO-DEA154R1)_Pharmacist's_Manual_DEA.pdf)
Central filling of CS prescriptions IS legal in Arizona. State laws regulate this practice, but the DEA also has some requirements.
Central fill pharmacies CANNOT accept prescriptions directly from patients or practitioners
Central fill pharmacies CANNOT dispense directly to patients or practitioners
Controlled substance prescription can only be faxed or sent electronically to the central fill location from the pharmacy that will ultimately dispense to the patient or practitioner
The U.S. Postal Service does allow controlled substances to be mailed, but packages must meet the following requirement:
The inner container of any parcel containing controlled substances must be marked and sealed pursuant to any relevant provisions in the CSA and its regulations, and placed in a plain outer container or securely wrapped in plain paper.
If the controlled substances consist of prescription medicines, the inner container must also be labeled to show the name and address of the pharmacy practitioner, or other person dispensing the prescription.
The outside wrapper or container must be free of markings that would indicate the nature of the contents.
Schedule V substances may be purchased without a prescription. However, there are some special requirements for dispensing:
1. Only a pharmacist or pharmacy intern may dispense/sell non-prescription C-V products
2. Purchaser must be at least 18 years of age
If purchaser not known by the pharmacist, must furnish suitable identification, including proof of age
3. Must be for a legitimate medical purpose
4. There are limitations on the quantity that can be purchased by a single person...
No more than 240 ml (8 oz.) or 48 dosage units of an opium-containing controlled substance may be dispensed at retail to the same purchaser with any given 48-hour period
No more than 120 ml (4 oz.) or 24 dosage units of any other controlled substance may be dispensed at retail to the same purchaser within any given 48-hour period
No more than 100 dosage units of any single active ingredient ephedrine preparation may be dispensed to the same purchaser in any given 30-day period (AZ)
213 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
5. Sale must be recorded in a bound record book showing:
Name and address of purchaser
Date of sale
Name and quantity of product sold
Name or initials of dispensing pharmacist or pharmacy intern Report any unusual sales to the Board of Pharmacy!
Example:
Name
6. The CV record book must be maintained for 3 years from the last sale/purchase date
On occasion, a pharmacy may wish to distribute controlled substances from one registrant to another (e.g. a nearby pharmacy runs out of a CS medication, so you sell them some from your own stock).
Pharmacies may distribute controlled substances without having a DEA distribution registration if the following are met:
1. The registrant to whom the drug is being distributed has a DEA dispensing registration
2. The distribution (sale) is recorded with the proper information for both the distributor and recipient (Think invoice requirements for CII – CV and if CII – DEA Form 222)
Drug name, strength, dosage form, and quantity
Number of commercial containers distributed
Date distributed (seller)
Date of receipt (purchaser)
Name, address and DEA number of the pharmacy or practitioner to whom it was distributed
3. The total number of dosage units distributed does not exceed 5% of the total number of CS dispensed and distributed in 1 calendar year (21 CFR 1307.11)
214 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Example:
Pharmacy A:
Dispensed 520,000 CS dosage units in 2023
Distributed 26,000 CS dosage units in 2023
Maximum amount that may be distributed w/o a distributor DEA registration is:
(520,000 + 26,000) * 0.05 = 27,300
Pharmacy does not need to register as a distributor.
Pharmacy B
Dispensed 520,000 CS dosage units in 2023
Distributed 32,000 CS dosage units in 2023
Maximum amount that may be distributed w/o a distributor DEA registration is:
(520,000 + 32,000) * 0.05 = 27,600
Pharmacy needs to register as a distributor
Prescribers in an institutional setting write medication orders for inpatients, not prescription orders. Since medication orders are not prescription orders, they do not have to meet the same legal requirements. Institutions must still maintain acceptable records of dispersal and make them readily retrievable. All records maintained for 7 years from last filled or patient encounter.
Note: Take-home medications for a discharged patient must be dispensed pursuant to a prescription order.
Because take-home medications are not administered by hospital staff – they cannot be written for as a medication order.
The DEA recommends contacting the nearest DEA Diversion Field Office for disposal instruction.
There are 4 methods allowed by the DEA
1. Promptly destroying the substance onsite in the presence of a DEA agent or authorized person (use DEA Form 41)
2. Transfer the items to a 'reverse distributor' registered with the DEA (This is the DEA-preferred method)
Requires an invoice for C-III – C-V drugs or a DEA Form 222 for C-II drugs
Can be done at any time
3. Promptly sending returns or recalled substances to the registered manufacturer of the substance(s) or another registrant authorized by the manufacture
Requires an invoice for C-III – C-V drugs or a DEA Form 222 for C-II drugs
Can be done at any time
4. Request assistance from the Special Agent in Charge (SAC) of the DEA in the area where the registrant is located.
215
©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Step 1: Complete DEA Form 41 online and print form and send to SAC where the registrant is located
Step 2: The SAC will then instruct the registrant in one of the following manners:
By transfer to a registrant authorized to transport or destroy the substance
By delivery to a DEA agent or the nearest DEA field office
By destruction in the presence of a DEA agent or authorized person
If registrant is a hospital or clinic and is required to dispose of CS on a regular basis, the DEA may authorize the registrant to dispose of them without prior approval in each instance.
Registrant must keep records of the disposals and file periodic reports with the DEA.
Currently – pharmacies are not allowed to take back controlled substances directly into the pharmacy.
Exception: misfills – the pharmacy needs to take back the incorrect medication and document what happened
o Medication is not returned to stock
o Medication goes with other CS to be destroyed.
National Take-Back Programs: DEA collaboration with local law enforcement (www.takebackday.dea.gov)
Two-Pronged Program:
1. Two National Take-Back Days (April and October)
2. Allows local police departments and pharmacies to have take-back program lock boxes in their facilities.
Data to date: (Fall 2023)
Law enforcement participation = 4383
Total collection sites = 4675
Fall 2023 collection: 599,897 lbs. (300 Tons)
Total collected as of Fall 2023: 17.9 million lbs. (8950)
Most recent take-back day: April 27, 2024


216 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Authorized Collector (21 CFR 1317.40; https://www.deadiversion.usdoj.gov/drug_disposal/fact_sheets/disposal_regis trant.pdf)
What is an authorized collector?
= Authorized DEA registrants that choose to maintain collection receptacles for drug-take back/disposal by patients
Who can be an authorized collector?
Manufacturers Reverse Distributors Hospitals/Clinics with on-site pharmacies
Distributors Retail Pharmacies Narcotic Treatment Programs
How to become an authorized collector?
Modify current DEA registration to obtain authorization to be a collector.
Must have authority to handle CIIs
Restriction:
Not authorized to conduct take-back events. Law enforcement may conduct take-back events at any time.
What can I collect as an authorized collector?
Maintain collection receptacles inside their registered location
All pharmaceutical CS and non-controlled substances; may co-mingle in a single collection receptacle
CS collected from ultimate users SHALL NOT co-mingle with a registrant’s inventory/ stock of CS.
No illicit drugs are to be collected
In the event of a theft or significant loss*:
*Significant Loss is defined in the DEA Pharmacist’s Manual p. 17 and in Abood, etal.)
1. Notify the DEA office within one business day of discovery
2. Notify the ASBP within 10 days (AAC R4-23-Article 9)
3. Notify the AZ DPS Narcotic Division
a. Good practice to also notify the local police
4. Complete DEA Form 106 (Report of Theft or Loss of Controlled Substances) and submit within 45 days after the discovery of the theft or loss. (NEW submit time frame 2023)
217 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
a. Indicate on DEA 106 if theft/loss was investigated by police
b. Submit copies to:
i. DEA
ii. Narcotic Division of DPS
iii. ASBP
Information to be included on DEA Form 106:
a. Name and address of the firm (pharmacy),
b. DEA registration number,
c. Date of theft or loss (or when discovered if not known),
d. Name and telephone number of local police department (if notified),
e. Type of theft (e.g., night break-in, armed robbery),
f. List of identifying marks, symbols, or price codes (if any) used by the pharmacy on the labels of the containers, and
g. A listing of controlled substances missing, including the strength, dosage form, and size of the container (in milliliters if liquid form) or corresponding National Drug Code numbers.
In the event of Breakage or Spillage NOT RECOVERABLE (Does not constitute a “significant loss” of CS)
1. Document what happened in the inventory records
2. Two witnesses must sign the inventory records
3. Save broken bottles/containers and salvage the product if possible
4. Contact the local DEA Diversion Field Office for further instructions for disposal OR use a reverse distributor
5. Complete DEA Form 41 (Registrants Inventory of Drugs Surrendered)
All records must be readily retrievable within 48 hours of receiving a written request from the DEA
The DEA may enter and inspect any place where controlled substance or their records are kept or where persons are registered under the CSA
*****YES, Page Numbers jump from 217 to 224 – is an MS Word issue I haven’t been able to fix*****
224 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Upon inspection, a DEA agent/inspector is REQUIRED to:
Present owner or pharmacist with agent’s credentials
Present written notice of inspection (DEA Form 82)
State the purpose of the inspection
Obtain a written statement of informed consent to the search, signed by the owner or PIC
Unless consented to in writing by the owner or PIC, agents are PROHIBITED from inspecting:
Financial data
Sales data, other than shipment data
Pricing data
To avoid the consent requirement, a DEA agent/inspector may obtain an Administrative Inspection Warrant (AIW)
Registrants may not refuse an Administrative Inspection Warrant
Some practical considerations…
For any non-routine inspection, consider contacting your employer, who may wish to then contact an attorney.
If you deny consent (when consent is required) but the agent insists on inspecting anyway, document the conversation, obtain the agent's signature, and allow the agent to continue the inspection.
DOCUMENT all that is said and done during the inspection.
NEVER refuse inspection when presented with an AIW.
NEVER sign anything that you do not COMPLETELY understand.
OTPs are accredited and certified by the Substance Abuse and Mental Health Services Administration (SAMHSA) ~ Narcotic Treatment Programs (DEA registration category)
OTPs must be reaccredited and recertified every 3 years by SAMHSA
Physical
State of adaptation in which withdrawal occurs when the drug is stopped or quickly decreased
Tapering down an opioid analgesic to avoid withdrawal symptoms Vs.
Psychological
Compulsive use of a substance, despite harm, a loss of control, and a preoccupation with obtaining the substance
Tapering down an opioid analgesic to detoxify an addict
225 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
These are the only drugs approved by the FDA for the treatment of opioid addiction:
Methadone [C-II]
LAAM [C-II]
Buprenorphine products
Brixadi® [CIII] (Buprenorphine-Sustainedrelease injectable)
Bunavail® [C-III] (Buprenorphine-naloxone buccal film)
Buprenorphine [C-III] (sublingual) (Generic for Subutex)
Cassipa® [CIII] (buprenorphine and naloxone sublingual film )
Probuphine ® [C-III] (Implant - subdermal) (Restricted access REMS program – prescribers only)
Suboxone® [C-III] (Buprenorphine-naloxone)
Sublocade® [C-III] (Buprenorphine extendedrelease injection) (Restricted access REMS program need to be registered to dispense)
Zubsolv® [C-III] (Buprenorphine-naloxone sublingual tablet)
LUCEMYRA (lofexidine) tablets (non-controlled)
Vivitrol (naltrexone) injectable (noncontrolled)
Methadone and LAAM cannot be prescribed for "maintenance" or "detoxification" outside of an OTP and cannot be dispensed by a community pharmacy for OTP patients.
Methadone may be prescribed in the in-patient or out-patient setting for the treatment of pain.
Have official FDA indications for the “maintenance” and “detoxification” treatment outside of an OTP by a qualified prescriber
LUCEMYRA is a central alpha-2 adrenergic agonist indicated for mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults.
An opioid antagonist with highest affinity for the mu opioid receptor.
Used for opioid or alcohol dependence
226 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
BUPRENORPHINE PRODUCTS THAT ONLY HAVE AN INDICATION FOR THE TREATMENT OF PAIN – CANNOT BE USED FOR MAT (MEDICATION ASSISTED TREATMENT) FOR OUD
Belbuca [C-III] [Buccal film) All three of these products ONLY have an indication for pain.
Buprenex [C-III] (Injectable)
Butrans [C-III] (Transdermal patch)
CANNOT be used off-label for maintenance or detoxification in an OTP situation per DEA because the drug and dosage forms have not been approved for OTP.
Drug Addiction Treatment Act (DATA); Comprehensive Addiction and Recovery Act (CARA) and the Substance Use-Disorder Prevention Opioid Recovery and treatment for Patients and Communities (SUPPORT) Act (SAMHSA website updated 4/21/2022)
Permits maintenance and detox outside of an OTP
Section 1262 of the Consolidated Appropriates Act of 2023: (effective 3/22/2023)
o Removed the federal requirement for practitioners to have an “X” waiver to prescribe buprenorphine
o All practitioners with a current DEA DISPENSING registration that includes CIII authority may prescribe buprenorphine for OUD (Opioid Use Disorder) in their practice if permitted by their state
▪ Includes clinical nurse specialists (CNS), certified nurse anesthetists (CRNA), and certified nurse midwives, physician assistants. (This group was originally included with the SUPPORT Act, and its authority was to sunset on October 1, 2023.)
o Removed limitations on the number of people a practitioner may treat (panel)
Training: (Requirements effective 6/27/2023). Have at least one of the following
o 8 hours of training on opioid or other substance use disorders for practitioners renewing or newly applying for registration from the DEA to prescribe CII – CV
o Board certification in addiction medicine or addiction psychiatry from the American Board of Medical Specialties, American Board of Addiction Medicine, or the American Osteopathic Association
o Graduation within 5 years and status in good standing from medical, advanced practice nursing, or physician assistant school in the US that included successful completion of an opioid or other substance use disorder curriculum of at least 8 hours.
227 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Per ARS 32-1979, 36-2228 and 36-2266 and AAC R4-23-407.1
Pharmacists may dispense, pursuant to a standing order, and according to protocols developed by the ASBP, naloxone HCL or any other opioid antagonist that is approved by the US FDA. Recipients may be:
Any person who is at risk of experiencing an opioid-related overdose, or
A family member, or
A community member is anyone who is in a position to assist that person.
o Community member includes:
▪ emergency first responders,
▪ peace officers or other law enforcement,
▪ fire department personnel,
▪ school district employees, and/or
▪ personnel of a facility or center that provides services to individuals at risk of experiencing opioid-related overdose.
Establish written policies and procedures
Documentation Requirements for Dispensing Opioid Antagonists Based on the Standing Order (See AAC R4-23-407.1)
Include information required under R4-23-407(A)(1)(a,c,d,f, and l) and (A)(2)
o Date of issuance;
o Drug name, strength, and dosage form or device name;
o Name of the drug's or device's manufacturer or distributor if the prescription order is written generically or a substitution is made;
o Date of dispensing;
o Name or initials of the dispensing pharmacist;
o A prescription order is kept by the pharmacist or pharmacy permittee as a record of the dispensing of a drug or device for seven years from the date the drug or device is dispensed
Also include the following:
o Quantity dispensed
o Directions for use; and
o The patient’s name, address, telephone number, and birth date; OR
228 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
o Name, address, telephone number, and birth date of a family member in position to assist the individual at risk of an opioid-related overdose; OR
o Name, address, telephone number, and employer of a community member position to assist an individual at risk of an opioid-related overdose; and
o Name of the individual providing the education required under subsection (B)(2)
How to prevent an opioid-related overdose
How to recognize an opioid-related overdose
How to administer an opioid antagonist safely to an individual experiencing an opioid-related overdose
Precautions:
o Potential side effects and
o Possible adverse events associated with administration of the opioid antagonist; AND
Confidentiality, security, and privileged nature of documentation of opioid antagonists dispensed under ARS 32-1979.
Pharmacist shall complete an opioid prevention and treatment training program that includes the following information:
How to recognize the symptoms of an opioid-related overdose,
How to respond to a suspected opioid-related overdose,
How to administer all preparations of an opioid antagonist, and
The information need by an individual to whom an opioid antagonist is dispensed, and
Comply fully with policies and procedures
The trained pharmacist may administer an opioid antagonist to an individual the pharmacist believes is experiencing an opioid-related overdose, and is exempt from civil liability
Dispensing an opioid antagonist under ARS 32-1979 by invoice to a community member is not wholesale distribution as defined at ARS 32-1981.
229 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
• Narcan nasal spray was approved for OTC sale on March 29, 2023, by the FDA
o OTC dose – 4mg
o All Narcan with “Rx only” on the label must be treated as prescription only
▪ May use the state naloxone standing order to dispense Rx only Narcan and other naloxone products
• Availability of OTC Narcan = summer 2023
• Current cost of two doses of Narcan = ~$50

Special legislation regulates the OTC sale of these three substances
The Combat Methamphetamine Epidemic Act of 2005 (aka “The Patriot Act”)
Created a new substance schedule
"Schedule listed chemical products"
Requirements
Purchaser must present valid photo ID
Product must be stored behind the counter or in a locked cabinet (customer does not have direct access)
QUANTITY LIMITS (FEDERAL)
No more than 3.6 g of each purchased by the same individual on any given day (solids and liquids)
If obtained via shipping (U.S. Postal Service, FedEx, UPS, etc...)
No more than 7.5 g of each purchased by the same individual in any given 30-day period
Otherwise...
No more than 9 g of each purchased by the same individual in any given 30-day period
230 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
(FED AND AZ – ARS 32-1977)
The following warning statement must appear in the logbook or displayed by the logbook:
WARNING: Section 1001 of Title 18, United States Code, states that whoever, with respect to the logbook, knowingly and willfully falsifies, conceals, or covers up by any trick, scheme, or device a material fact, or makes any materially false, fictitious, or fraudulent statement or representation, or makes or uses any false writing or document knowing the same to contain any materially false, fictitious, or fraudulent statement or entry, shall be fined not more than $250,000 if an individual or $500,000 if an organization, imprisoned not more than five years, or both.
The following information must be recorded for each sale (Note: some cities or counties may have additional requirements):
Name and address of purchaser
Drug name and quantity purchased
Purchaser ID type and number
Date and time of sale
Signature of purchaser
• Information must be recorded in a physical (paper) logbook or electronically
• Information must be recorded by the seller (except for purchaser signature)
• Maintain logbook for at least 2 years from the date of last sale.
All 50 states and the District of Columbia have enacted electronic prescription monitoring programs for controlled substances. The generated data is used to monitor diversion, fraud, and abuse.
1. All Prescribers (except for Veterinarians) and pharmacies with current DEA registration must be registered in the CSPMP. (AZ)
2. All Pharmacists with a valid ASBP license and employed by a facility with a valid DEA registration MUST be REGISTERED with the CSPMP. (AZ)
Registration timelines apply to all:
CSPMP registration does not expire
A suspended registrant is prohibited from accessing information in the CSPMP
231 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Prescriber mandatory look: Before prescribing an opioid analgesic or a benzodiazepine CS listed in Schedule II, III, or IV:
shall obtain a patient utilization report for that patient for the preceding 12 months
at the beginning of each new course of treatment, and
at least quarterly while the prescription is part of the treatment.
Pharmacist mandatory look in AZ CSPM; before dispensing a CII drug:
shall obtain a patient utilization report for that patient for the preceding 12 months at the beginning of each new course of treatment.
Exemptions from the prescriber and pharmacist mandatory look for AZ CSPMP– This is for ALL opioid analgesics (includes Tylenol with Cod (# 2, 3, and 4)) and benzodiazepines (ARS 36-2606 (H) and (I))
Patient is in hospice or palliative care,
Patient is being treated for cancer, a cancer-related illness or condition, or dialysis,
Prescriber is administering the CS,
Patient is receiving CS during the course of inpatient or residential treatment in a hospital, nursing care facility, assisted living facility, correctional facility or mental health facility
Prescriber is prescribing a 5-day supply or less of a CS for an invasive medical or dental procedure that results in acute pain,
Prescriber is prescribing a 5-day supply or less of a CS for a patient who has suffered an acute injury or medical or dental disease process that is diagnosed in an ED (emergency department) and is for acute pain;
Prescriber or pharmacist uses an electronic medical record (EMR) that integrates data from the CSPMP, a review of the EMR with the integrated data shall be deemed compliant with the review of the CSPMP.
Pharmacies, dispensing prescribers, and health care facilities are required to report DAILY (2014) to the board information about the controlled substances they dispensed, including: (AAC R4-23-502)
Name, address, telephone number, prescription number, and DEA registration number of the dispenser
Name, address, gender, date of birth, and telephone number of the person or, if for an animal, the owner of the animal for whom the prescription is written
232 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
Name, address, telephone number, and DEA registration number of the prescribing medical practitioner (DEA and address need to match)
Quantity and National Drug Code (NDC) number of the Schedule II, III, IV, and CV controlled substance dispensed
Date the prescription was dispensed
Number of refills, if any, authorized by the medical practitioner
Date the prescription was issued
Method of payment - cash or third-party
New or refilled Rx
AZ CSPMP FAQ: https://docs.google.com/document/d/1MeqoqZ_I9WTbYWyfE8Ed-nbFJj87FfFibptPRvrRcE/edit
AZ CSPMP ACCESS: The CSPMP is not open to the public. Only the following people can access these records:
A person who is authorized to prescribe or dispense a controlled substance to assist that person to provide medical or pharmaceutical care to a patient or to evaluate a patient
o Authorized persons (prescribers and pharmacists) may delegate the ability to access the CSPMP to the following personnel:
Prescriber Delegates Pharmacist Delegates
Licensed personnel (e.g., RN, PA, Pharmacist, etc.)
Unlicensed personnel
Licensed personnel only (Intern, Technician, and Technician Trainee)
An individual who requests his or her own controlled substance prescription information
A professional licensing board
Local, state, or federal law enforcement or criminal justice agency when the information is necessary for an open investigation or complaint
The Arizona Health Care Cost Containment System Administration regarding individuals who are receiving their services
A person serving a lawful order of a court of competent jurisdiction
The Board staff for purposes of administration and enforcement
Use of CSPMP is voluntary by pharmacists for CIII, CIV, and CVs
✓ DEA considers accessing the CSPMP is part of the pharmacist’s corresponding responsibility.
233 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale
When access the AZ CSPMP database, prescribers and pharmacists have access to 47 states, DC, Puerto, St. Louis County, MO, and the Defense Health Agency PMP program databases.

234 ©2024 Mary K Gurney, Roger Morris, and Midwestern University College of PharmacyGlendale