Selective purification of glycosylated IgG from plasma using MiMode PuraBead® CBX1
Selective purification of glycosylated antibodies from plasma-derived feedstocks is critical for therapeutic and diagnostic applications. This study demonstrates the use of boronate chromatography to achieve targeted enrichment of glycoproteins through reversible cis-diol binding under alkaline conditions. Mild elution with sorbitol ensures high recovery and preserves antibody functionality. MiMode PuraBead® CBX1 provides a robust and scalable platform for efficient glycoprotein purification in plasma processing workflows.
The expanding application of glycoproteins, such as immunoglobulin G (IgG), in biopharmaceutical development has driven demand for chromatography methods capable of highly selective purification. Aminophenylboronate chromatography leverages reversible covalent interactions with cis-diol groups present on sugars, enabling both enrichment of glycosylated proteins and removal of diolcontaining impurities under mild conditions.
MiMode PuraBead® CBX1 is a rigid, highly porous agarosebased resin, functionalized with an aminophenylboronic acid ligand. This configuration allows specific capture of glycoproteins such as IgG, and offers excellent chemical stability, base resistance, and reusability. In this study, MiMode PuraBead® CBX1 was evaluated for the purification of glycosylated polyclonal IgG, demonstrating effective capture and elution while preserving protein integrity.
Optimization of IgG binding and elution
Purification of glycosylated IgG was performed using a 5.7 mL column packed with MiMode PuraBead® CBX1. Binding was conducted in alkaline buffer conditions known to promote boronate-diol complexation, while elution exploited the addition of D-sorbitol, a competitive diol, to disrupt these interactions gently.
Following equilibration, the IgG feed was loaded at a controlled flow rate. Post-load wash with equilibration buffer removed unbound and weakly associated proteins before elution. Elution fractions were collected and analyzed by SDSPAGE, confirming efficient recovery of IgG.
Chromatographic conditions
Parameters
Conditions
Column 5.7 mL MiMode PuraBead® CBX1-packed column
Equilibration buffer
Load
Elution buffer
50 mM glycine/NaOH, 0.1 M NaCl, pH 9.0
70 mL polyclonal IgG at 1 mg/ mL in equilibration buffer
50 mM glycine/NaOH, 0.1 M NaCl, 200 mM D-sorbitol, pH 9.0
Clean-in-place 1 M NaOH
The chromatogram (Figure 1) shows clear demarcation of load, wash, and elution phases. The major elution peak corresponded to the displacement of IgG from the resin upon sorbitol addition, confirming the boronate-diol mechanism of interaction.
High binding capacity observed at pH 9.0 enabled efficient and consistent capture of the target antibodies. The process delivered selective enrichment of glycosylated IgG with minimal co-purified contaminants. Mild elution using sorbitol preserved antibody integrity throughout the workflow. SDSPAGE analysis confirmed that the eluted fractions (E1 and E2) contained predominantly IgG chains, with very low levels of residual non-target proteins, demonstrating the effectiveness of the purification strategy.
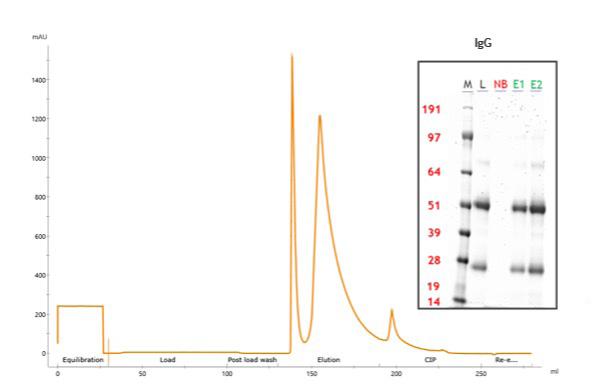
Figure 1: Chromatogram illustrating the capture and elution profile of glycosylated polyclonal IgG on MiMode PuraBead® CBX1. The inset shows SDS-PAGE analysis of load (L), nonbound fraction (NB), and elution fractions (E1, E2), confirming selective enrichment of IgG heavy and light chains with minimal co-purified proteins.
Mechanism of binding
Aminophenylboronate ligands bind glycoproteins via reversible covalent interactions with cis-diol groups of glycan moieties. Under alkaline pH (above pKa ~8.5), the boronic acid group ionizes to form a tetrahedral boronate anion, which interacts strongly with diols to form cyclic esters. This reversible complexation ensures efficient capture and allows for gentle elution by competitive displacement with sorbitol or other diols.
This binding mode enables MiMode PuraBead® CBX1 to target a wide range of glycosylated biomolecules, including antibodies, enzymes, and glycoproteins.
Application potential
The demonstrated workflow highlights the suitability of MiMode PuraBead® CBX1 for a wide range of applications, including the capture and purification of glycosylated antibodies such as monoclonal and polyclonal IgG, as well as the removal of glycosylated impurities from non-glycosylated protein preparations. It also enables the selective enrichment of glycoproteins for both analytical and preparative workflows and supports the quantification of glycation, for example, in HbA1c assays. Additionally, the resin can be used for the purification of enzymes that contain glycan structures or bind diol-containing cofactors, and for the separation of nucleic acids that possess cis-diol functionalities.
Conclusion
This application note demonstrates the successful capture and enrichment of glycosylated polyclonal IgG using MiMode PuraBead® CBX1 resin. The optimized workflow employed high-pH binding and sorbitol-mediated elution to achieve selective purification under mild conditions. This approach preserves antibody structure while delivering high purity and yield, confirming MiMode PuraBead® CBX1 as a versatile tool for glycoprotein purification and enrichment.
The performance showcased here highlights the resin’s potential for broader applications, including glycoprotein analytics, enzyme purification, and glycated protein quantification workflows.
For further details on MiMode PuraBead® CBX1 and its implementation in your purification processes, please contact our technical support team or browse our resources.
Astrea Bioseparations is a world class provider of chromatography adsorbent and resin services. With over 30 years of chromatography manufacturing expertise, we deliver a unique and trusted service in close partnership with our clients. For more information, please don’t hesitate to reach out at sales@astrea-bio.com or visit astreabioseparations.com.
