The Essential Role of Column Packing for Successful Chromatography



Nicola Dickson, Catherine Nguyenngo, Christine Khan, and Marc Hummersone
Achieving efficient biomolecule purification during downstream purification is critical to producing safe, high-quality biotherapeutics. Among the techniques used in this phase of manufacturing, chromatography stands out as a key operation. It plays a pivotal role in separating target molecules from impurities based on differences in size, charge, or affinity to a particular ligand, helping to ensure the quality, purity, and consistency of final products. Achieving consistent separation performance relies on many factors, including how a chromatography resin is packed into a column.
Creating an optimal packed bed requires a blend of technical proficiency and practical experience. An improperly packed column can have ripple effects throughout the purification process, leading to inefficiencies, increased costs, and compromised product integrity. This article explores key considerations for chromatography column packing, the challenges of a do-it-yourself approach, and the benefits of investing in prepacked columns for process consistency and efficiency.
The basic principle of chromatography is simple: The method exploits differences in the interactions with a packed resin bed as a process stream is passed through the column. Components either travel at different rates or are retained by the resin. That causes separation to occur and enables separated components to be collected. Effective separation occurs only if the components have ample opportunity to interact with the resin particles, making column geometry and packing quality critical factors. In many chromatographic separation processes, the overarching goal is to maximize opportunities either for target molecules to adsorb to the resin while unwanted impurities flow through the column or for impurities to be retained while target molecules flow through the column and are collected. How a chromatography column is packed determines the distribution of flow through the

column. An optimally packed column should exhibit not only a uniform flow distribution across the column diameter and throughout the column length, but also a minimal pressure drop. Those properties promote plug flow within the column, with molecular components moving through the column at the same velocity and direction. That leads to full resin use. A column that is packed too loosely may result in poor flow distribution, reducing separation efficiency and increasing buffer-volume requirements. If the middle of the packed bed is too fluid, whereas the outer edges remain rigidly packed, flow channels through the center of the column can be established, where preferential flow occurs following pathways of least resistance. Although separation still might be achieved, the separation efficiency would be reduced and/or target molecules would be within a higher elution volume of buffer. That would require additional downstream processing to achieve the desired separation or concentration. Extra procedures increase timelines, costs, and manufacturing complexity. Moreover, target molecules can denature, experience shear, or lose other properties during additional fluid contact, thus reducing final yield. Conversely, overpacking a column can compress the resin excessively, restricting flow. That can limit access to the whole resin bead, cause resin damage, and create blockages.
The packing process is complicated by the number of possible techniques to generate a packed bed within the column — e.g., gravity settling, flow packing, and axial compression — and by varying physical properties across resins. Resins can be compressible, semicompressible, or rigid, as with glass beads; they also can be spherical or irregular in shape, and they can be present as a monodisperse or wider particle-size population. Furthermore, the functional groups or ligands attached to the base beads can change how a resin behaves under different flow and pressure conditions. Even within a family of resins, each material can react differently to flow or pressures.
When packing columns, it is important to recognize that the conditions listed in resinmanufacturers’ packing guides are general recommendations and may not translate directly to all column sizes or to a customer’s specific process. For example, the manufacturer of a commonly used ion-exchange resin recommends packing with water or 0.1 M NaCl. When tested at a user facility, applying 0.1 M NaOH improved resin performance.
Standard qualification procedures ensure that the performance of each column packing can be quantified, allowing consistency and reliability to be established in any given workflow. One common method for checking packed-bed quality involves injection of a small tracer molecule — often NaCl — at a specific concentration into the column. The volume applied to the column is small in comparison to the column volume, often 1–2% column volume. Under controlled flow conditions, the NaCl solution passes through the packed bed. Elution of NaCl is monitored by detecting changes in conductivity postcolumn and reviewing results from a chromatogram. Chromatograms typically show signal intensity of a detector as a function of time or elution volume and have a peak shape.
Analysis of the peak produces quantifiable and comparable results. The theoretical number of plates and peak asymmetry are calculated by analysing the chromatogram. The peak geometry and degree of asymmetry reflect how well a bed is packed, which affects flow distribution and dilution within the column. Symmetrical peaks indicate even flow and consistent performance. A symmetrical but broad peak indicates even flow but with dilution. And an asymmetric peak indicates a poorly packed column. Borrowed from the distillation industry, height equivalent to a theoretical plate (HETP) and number of plates per unit length serve as further measures of
Precision fermentation is transforming how we produce proteins, enzymes, and other bio-based materials. By using genetically engineered microorganisms such as bacteria, yeast, or fungi, this innovative process allows for the creation of specific molecular compounds.
Unlike traditional fermentation processes used for products such as bread and alcohol, precision fermentation is designed to deliver targeted results, whether that is for plant-based proteins for alternative meats, therapeutic proteins, or critical dairy proteins, all without relying on conventional farming.
Not only is precision fermentation efficient and scalable, but it also has lower environmental impacts compared with traditional biomanufacturing processes. However, it does come with challenges, particularly the risk of endotoxins released by microorganisms during harvesting, which is a critical concern for therapeutic proteins, vaccines, and food ingredients.
Take control of endotoxin removal with EtoxiClear resin in prepacked in Astrea Bioseparations’ columns for up to 99.99% endotoxin removal — from bench scale to commercial production. EtoxiClear resin is a scalable, efficient, and robust solution for ensuring endotoxin removal from your biotherapeutics.
column efficiency. The higher the number of plates that a column has, the greater is its ability to separate target molecules — hence more effective purification. Furthermore, incorporating the resin particle size into the analysis enables calculation of the reduced plate height, allowing for comparisons across resin types.
Although it is difficult to give exact parameters, a column with a reduced plate height (h) of 1.5 to 2 and an asymmetry factor (AS) between 0.8 and 1.8 is generally acceptable when using porous chromatography media for biopharmaceutical applications. An AS of <0.8 indicates column overpacking, whereas an AS of >1.8 indicates an underpacked bed. In both cases, the column should be repacked.
For biopharmaceutical manufacturers, the consequences of improperly packed columns can be far-reaching. Poor packing can lead to a host of downstream issues, from increased buffer consumption to additional purification steps. Among the most significant drawbacks is the potential need to repack or replace a column that has failed
standard qualification tests. Column failure requires unpacking, repacking, and retesting, causing delays in production and potentially halting an entire bioprocess.
Such delays are particularly costly during largescale manufacturing, where other parts of the production process may be forced to wait for chromatography steps to be completed. Moreover, extended processing times expose sensitive biomolecules to shear forces and prolonged contact with reagents, which can diminish product yield and quality.
Compounding the difficulty of column packing is the fact that even with detailed standard operating procedures (SOPs), results can vary based on the skill and experience of the technician. Such variability underscores the importance of consistency, especially in large-scale manufacturing, during which reproducibility is key to meeting regulatory standards. Many biopharmaceutical companies choose to pack their chromatography columns in house despite the significant challenges involved, the substantial internal resources required, and the potential for significant operational delays. Self-packing not only involves the column packing step itself, but also requires validation through efficiency testing and stringent bioburden checks to protect against contamination in downstream processes. For instance, packed columns must undergo a
microbiological clearance process to ensure their sterility, a process that can take five to seven days. Any issues with bioburden could lead to additional delays and lost productivity.
Validation of self-packed columns also involves multiple departments, including quality assurance (QA) and microbiology functions. Each team’s work involves unique expertise and incurs distinctive costs. Moreover, requiring those teams to perform validation activities uses internal resources that otherwise could be allocated to value-adding activities, such as process optimization and product development.
r ecognizing The Far - r eaching implicaTion S oF poor packing Efficient column packing is a critical yet often overlooked aspect of chromatography, with farreaching implications for the overall success of downstream processing and biomanufacturing. Properly packed columns ensure optimal separation, process consistency, and product quality during downstream processing, whereas poorly packed columns can result in significant inefficiencies, increased costs, and potential production delays. Although the do-it-yourself approach to column packing might seem cost-effective, it can introduce process variability, resource constraints, and hidden operational costs that can outweigh any initial savings.
Nicola Dickson and Catherine Nguyenngo
Successful chromatographic separations in the downstream processing workflow rely on effective column packing. By ensuring uniform flow distribution, minimizing pressure drops, and maintaining consistent packing conditions, biopharmaceutical manufacturers can improve process efficiency and safeguard the integrity of their therapeutic products. As biopharmaceutical demand continues to rise, optimizing column packing processes will remain critical to the success and sustainability of operations in the competitive biomanufacturing landscape.
The variability of chromatographic resin properties and the complex interplay of many packing parameters underscore why column packing is as much an art as it is a science. Being able to optimize packing for different resins, flow rates, and purification objectives requires not only technical expertise, but also hands-on experience.
By outsourcing column packing to Astrea Bioseparations, biopharmaceutical companies benefit from timely delivery of prequalified, process-ready columns that are accompanied by all necessary documentation confirming bioburden clearance. Thus, columns can be integrated quickly into downstream processing lines, saving valuable time and resources.
Accelerated Delivery: Extensive expertise and experience in column packing, qualification, and testing sets Astrea Bioseparations apart as a trusted partner for biopharmaceutical companies. By combining technical know-how with customercentric service, the company delivers a unique blend of flexibility, reliability, and efficiency for both drug innovators and contract development and manufacturing organizations (CDMOs).
Astrea Bioseparations offers a column packing service with two different options to provide prepacked columns. Prepacked columns can be provided as disposable, single-campaign products or in multiuse format. Both options deliver plug-andplay, process-ready column solutions, enabling timely and seamless integration of columns into processes without incurring additional packing or qualification time.
With global packing facilities in Europe (Isle of Man, UK) and the United States (Canton, MA), Astrea Bioseparations provides rapid turnaround times and localized support, reducing delays caused by international shipping. Packed columns typically arrive in as little as 21 days.
For CDMOs, use of column packing services helps to maintain tight production schedules and optimize facility utilization. Astrea Bioseparations’ approach to reducing lead times ensures consistent delivery schedules, preventing unexpected delays that could disrupt processes and lead to facility downtime.
Flexible Resin Selection: Biopharmaceutical developers and CDMOs use a breadth of chromatography resins throughout their bioprocessing workflows. Recognizing that factor, Astrea Bioseparations offers comprehensive columnpacking services, not only for its proprietary resins, but also for those from other suppliers. That approach creates a seamless, one-stop solution, streamlining operations, reducing sourcing and packing complexity, and ultimately, accelerating development and manufacturing timelines.
Maximizing Resin Use: Given the high cost of chromatography resins, many companies want to ensure that they have maximized resin use. Companies might consider repacking used resins. Recovering resins from columns or repacking and reusing stored resin can be options to maximize resin use. Astrea Bioseparations offers a column packing service that can repack used resins into new columns, and resin recovery is possible from those columns.

These services can provide significant cost savings by extending the life of valuable resin materials while maintaining high- quality performance.
In the repacking of used resins, stringent stringent protocols are applied when handling used resin to prevent cross-contamination and ensure consistent results. However, not all used resins are fit for repacking. Astrea Bioseparations thoroughly evaluates each resin’s condition before proceeding to ensure that client requirements are met without compromising on process quality. In some cases, resins might be unsuitable for primary production but still have utility in process development.
Expert Technical Support: Astrea Bioseparations’ global team of field application specialists (FAS) brings a wealth of experience in chromatography and biomanufacturing. Dedicated to delivering superior customer support, they ensure seamless integration of column packing services into customer workflows. Many FAS team members have firsthand experience in bioprocessing and chromatography, offering an insider perspective that enhances communication and problem-solving.
By leveraging deep technical knowledge and a customer-focused approach, the FAS team helps to ensure that prepacked columns deliver the downstream-purification performance that customers expect while optimizing efficiency and maximizing process outcomes.
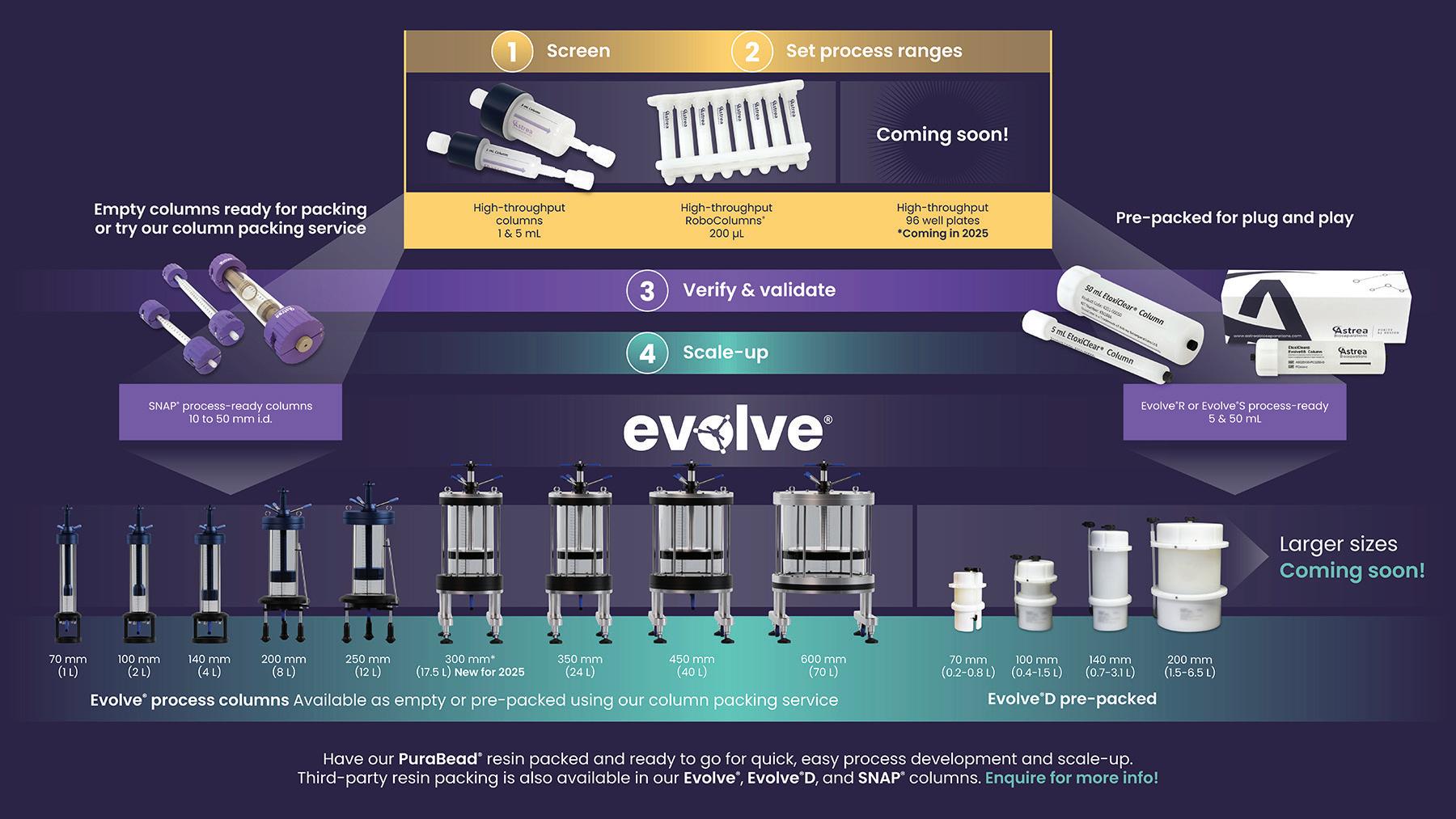
The biomanufacturing industry demands both flexibility and consistency in downstream purification solutions to accommodate different stages of biomolecule development. To address those needs, Astrea Bioseparations has developed a comprehensive portfolio of process-ready, Bioprocesssuitable chromatography columns that are designed to streamline purification processes from early stage research to full-scale production.
Astrea Bioseparations’ PuraBead resins offer a highperformance, sustainable alternative for bioseparations. Made from seaweed-derived agarose, they provide an environmentally friendly option compared with traditional resins, without compromising on efficiency or purity. PuraBead resins are designed for exceptional stability, reproducibility, and binding capacity, making them ideal for many bioprocessing applications.
Engineered for versatility, these resins support multiple purification techniques, including ion exchange, hydrophobic interaction, and affinity chromatography. Their robust structure ensures consistent performance from research to commercial scale. With sustainability at the forefront, PuraBead resins help biomanufacturers to meet their purification goals while reducing reliance on synthetic materials.
SNAP Columns — A Flexible Solution for Research and Development (R&D): SNAP columns offer flexible, scalable solutions for challenges faced in small-scale research and process development, both outside and inside cleanroom environments. Columns are available in diameters of 10, 15, 25, 35, and 50 mm and in a range of bed heights from 125 mm to 1 m. Their versatility enables process development scientists to investigate an array of process variables and resin types to optimize specific separation requirements. Furthermore, SNAP columns are available as prepacked columns to allow scientists to focus on exploring different purification strategies before moving into more controlled, regulated production environments.
SNAP Process-Ready Columns — Bridging the Gap to Clinical Production: SNAP columns are also available in a process-ready configuration with all the necessary material certification to allow their use in regulated production environments. Coupling that configuration with the packing service produces process-ready columns that have been packed in ISO 7 cleanroom environments using validated procedures and purified water.
Prepacked SNAP process-ready columns are designed to transition seamlessly from research to clinical production, and they are compliant with industry standards. Those features enable users to move received columns directly into a production suite without additional preparation. Streamlined transition from research to clinical trials is especially beneficial for clients who need reliable and consistent results while navigating the complexities of clinical-scale manufacturing.
The biomanufacturing industry demands both FLEXIBILITY and CONSISTENCY in downstream purification solutions to accommodate different stages of drug development.
Evolve Columns — Scaling Up for Commercial Production: Astrea Bioseparations’ Evolve Process and Evolve D columns offer scalable, high-quality solutions for diverse bioprocessing needs, from pilot to large-scale manufacturing.
Evolve Process columns are ready-to-use, reusable columns that provide a low-maintenance and adaptable solution for biopharmaceutical applications. Available in nine diameters, they blend the benefits of prepacked and self-packed formats, ensuring column integrity over multiple cycles. Designed for extended use, they enable efficient resin removal, maximizing cycle usage and supporting long-term studies while reducing costs, making them ideal for large-scale GMP applications.
For projects requiring a single-use option, Evolve D columns offer a ready-to-use solution for biomolecule purification and contaminant removal (see the final page of this report). Prepacked with a choice of resin and manufactured in an ISO 7 environment, they ensure purity and consistency. Each column is supplied with a certificate of analysis (CoA) and a regulatory support file (RSF), providing a reliable and cost-effective option for streamlined bioprocessing and biomanufacturing workflows.
Both column formats maintain Astrea Bioseparations’ high quality standards, offering flexibility based on production scale, regulatory requirements, and process efficiency.
In addition to extensive expertise in packing a breadth of resins, Astrea Bioseparations places a strong emphasis on column engineering and hardware development. The columns’ design and construction are optimized to meet the stringent demands of biomanufacturing, with features such as
• bioprocess-ready materials that have been selected to ensure compatibility with pharmaceutical manufacturing
• optimized inlet and outlet sizes, with configurations that are designed to integrate smoothly into existing production environments
• rigorous testing and quality checks to ensure that columns meet both performance and regulatory standards.
By combining high-quality hardware with expert packing services, Astrea Bioseparations empowers clients to achieve consistent results from development through to full-scale production. That holistic approach is a key differentiator in an industry where precision, speed, and reliability are essential for success.
In the biomanufacturing industry, consistent and efficient chromatography processes are tied fundamentally to column packing success. Whether for process development or large-scale production, the right expertise in column packing can reduce risk and enhance bioprocess efficiency significantly.
With over 30 years of industry expertise, Astrea Bioseparations stands out as a trusted partner by offering a unique combination of technical expertise, customer-focused service, and flexible solutions that are tailored to the needs of both biopharmaceutical
Evolve D chromatography columns were designed to deliver true plug-and-play solutions with efficiency and scalable performance in mind. The columns retain the flowpath geometry of the Evolve Process columns, providing consistent high-performance and flexibility across processes. The unique yet simple Evolve D design allows the columns to be packed to a customer’s specified bed height and offers a versatile option for resin recovery, thus maximizing resin use. The costeffective Evolve D product line will now include diameters of >20 cm.
developers and CDMOs. Companies can rely on Astrea Bioseparations’ regulatory expertise, robust supply chain, and short lead times. By outsourcing column packing to Astrea Bioseparations, biopharmaceutical companies can concentrate on core R&D and production activities with confidence, knowing that their chromatography needs will be expertly managed.
Nicola Dickson is subject matter expert for chromatography columns, Catherine Nguyenngo is senior project manager, and Christine Khan is a product manager for resins, all at Astrea Bioseparations. Marc Hummersone is chief scientific officer at Biotage. For inquiries, please visit; https://www. astreabioseparations.com/en/support/Contact-Us.