WHAT'S IN THE PROPOSED LCD AND DRAFT POLICY ARTICLE FOR KNEE ORTHOSES? 20
AOPA NAMES EXECUTIVE DIRECTOR 16
AUTOMATING KEY STEPS OF THE CUSTOM-MADE ORTHOSIS DESIGN PROCESS 46


WHAT'S IN THE PROPOSED LCD AND DRAFT POLICY ARTICLE FOR KNEE ORTHOSES? 20
AOPA NAMES EXECUTIVE DIRECTOR 16
AUTOMATING KEY STEPS OF THE CUSTOM-MADE ORTHOSIS DESIGN PROCESS 46

3D printing and digital workflows are transforming O&P—and the practitioners who use them P.24

THE PREMIER MEETING FOR ORTHOTIC, PROSTHETIC, AND PEDORTHIC









September 3–6, 2025, for an ideal combination of top-notch education and entertainment at the 108th AOPA National Assembly in Orlando, FL, at the Orange County Convention Center (OCCC).










EXHIBITS EDUCATION NETWORKING



























CX™, the #1 small hand. The TASKA CX, available in small and medium, has a legacy of proven performance and trust. While others chase trends, the CX consistently delivers what matters most - unmatched durability, reliability, and aesthetic excellence. It’s the benchmark for quality, engineered to endure and chosen by users and prosthetists who demand more. In a crowded market, the CX doesn’t just stand out - it sets the standard. To discover more, visit fillauer.com or taskaprosthetics.com
3D printing is reshaping everything from manufacturing workflows to patient-care delivery. Practitioners who once relied on traditional hand skills are now embracing additive manufacturing, AI-assisted design, and new materials. From remote brace repairs to prosthetics that could intelligently fail to protect users in accidents, the technology is reshaping not just how devices are made, but practitioners' relationship with technology of all kinds.
By JOSEPHINE ROSSI

After leading the organization as interim executive director for six months, Teri Kuffel, JD has been appointed by the AOPA Board of Directors as full-time executive director.
Access tips for navigating recent changes to the Medicare program.
to earn CE


by taking the online quiz.
Find out how Louis-Philippe Broze, co-founder and chief executive officer of Spentys, is elevating the design process for custom-made orthoses.
Meet Alex Miller, CPO, MSPO, and learn about his collaboration with an active patient to design a custom cycling foot for

Learn what the Assembly sponsoring organizations will showcase at their booths in Orlando.
See the full list of companies exhibiting at AOPA's 2025 National Assembly.


Dear AOPA Members,

As I reflect on the past seven months serving as your interim and now executive director, I’m filled with gratitude, momentum, and a deep sense of responsibility. It is my honor and privilege to work alongside AOPA’s Board of Directors, our dedicated staff, valued members, and O&P partners to advance our shared mission: improving access to high-quality care and elevating the O&P profession.
Leadership transitions present a unique opportunity—a natural moment to pause, assess, and explore the organization through a fresh lens. That’s exactly what we’ve been doing. Together, the Board, staff, and I have been asking important questions, listening with intention, and identifying new ways to amplify AOPA’s impact.
As the first person in some time to lead AOPA with direct industry experience, I bring with me an understanding of the challenges and opportunities you face every day. That perspective has helped shape our priorities, ensuring they’re firmly grounded in the needs of our members, the profession, and, most importantly, the individuals we serve.
One theme has remained front and center: Collaboration is essential. Over the past seven months, we’ve doubled down on the power of working together—because real progress happens when our community moves as one.
Three powerful examples:
• The Medicare O&P Patient-Centered Care Act: Together with the O&P Alliance, our peer organizations, and our members across the country, we’re aligning our advocacy efforts to speak with a unified voice in Washington and beyond. The reintroduction of these bills in the House and Senate is a true testament to collaboration and grassroots advocacy at its finest. We look forward to working together to move this legislation across the finish line this session. (Read more on page 10.)
• Proposed LCD Revision to Expand Coverage of Knee Braces Used to Treat OA: After nearly two years of persistent engagement from AOPA staff and Coding and Reimbursement Committee following our formal LCD reconsideration request, the DME
MACs have released a draft revision to the Knee Orthoses Local Coverage Determination (LCD) and its corresponding Policy Article. This proposed update represents a major step forward in correcting one of the most significant omissions in Medicare coverage, orthotic treatment for osteoarthritis (OA) of the knee. AOPA will continue to use its expertise to participate in the public hearing and submit formal comments to ensure this becomes policy.
• So Every BODY Can Move (SEBCM): Together with our peer organizations, we’re working on this transformative, state-by-state initiative to expand access to activity-specific orthoses and prostheses and increase access to O&P care. With legislation already enacted in 12 states and momentum growing nationwide, we’re showing up and showing how united advocacy can drive real, life-changing results.
These efforts go beyond policy—they reflect our commitment to advancing the O&P profession, supporting your work, and improving lives across the limb loss and limb difference community. With the year halfway through, I want to share a few highlights of how we’re translating our strategic vision into meaningful results:
Communicating the power of O&P: We're continually working to amplify the voice of the profession with communications that show how O&P care transforms lives. Communicating the power of O&P is integrated into all strategic priorities, focus areas, and goals.
Increasing access to care: As mentioned prior, the AOPA advocacy team is working daily to ensure all patients have access to clinically appropriate, high-quality O&P services and that our members can be compensated fairly for that care.
Helping members navigate a changing healthcare landscape: From regulatory updates to the expansion of AOPAversity’s free and online CE courses, we’re equipping you with tools to thrive amid shifts in the healthcare environment, audits, and clinical demands.
Identifying and influencing trends and learnings that may impact O&P: We’re investing in research through the O&P Foundation for Research and Education and developing education specific to issues our members are facing that will help them in their businesses. AOPA has committed to donating $1 million over five years, demonstrating our unwavering commitment to help advance innovation, education, and research in the O&P profession.
Enhancing AOPA value, engagement, and community: AOPA is making it easier than ever to engage with benefits through the new AOPA Hub. We are improving our committee structure and increasing opportunities through new offerings at the 2025 National Assembly. We are visiting more members in offices and labs, engaging with the O&P schools so that students can acclimate to our profession; and those who aspire to do so can become rising stars.
Driving collaboration by creating strategic relationships: Our work continues beyond the O&P community. We’re working to build bridges—with allied health organizations, policymakers, regulators, and payors—to strengthen O&P’s collective impact.

Bolstering infrastructure, innovation, and staff engagement: Behind the scenes, we’re improving our systems, launching a new website, fostering staff development, and ensuring AOPA remains a nimble, responsive organization.
Each of these endeavors ties back to AOPA’s vision, “A world where orthotic and prosthetic care transforms lives,” and our core goal: supporting you, your business, and your patients. I’m proud of the progress we’ve made, excited about the momentum we’re building, and looking forward to what the future holds.
I’m especially looking forward to connecting with many of you in person at the 2025 National Assembly in Orlando. It will be a powerful opportunity to learn, collaborate, and celebrate the future of O&P—one that has never looked brighter.
As always, I welcome your feedback, questions, and ideas. Please don’t hesitate to reach out to me directly at any time for any reason at tkuffel@AOPAnet.org
Thank you for being part of the AOPA community.
Very Truly

Teri Kuffel, JD AOPA Executive Director









See through design
instructions and

SEBCM
As Paralympic athletes train to compete for gold, AOPA and state advocates are going after the goal of implementing legislation mandating insurance coverage for recreational prosthetic and orthotic care in 28 states by the 2028 Los Angeles Paralympic Games.
So Every BODY Can Move (SEBCM) is a policy and advocacy initiative with the mission to create life-changing access to orthotic and prosthetic care necessary for physical activity for individuals with disabilities. As a founding partner and steering committee member of the movement, AOPA has worked since 2022 to encourage states to adopt laws that would require state-regulated insurance plans to cover prosthetic and orthotic devices necessary for activities of daily living, like bathing, or exercise that would improve an individual’s overall health and maximize limb function. Activities such as running, biking, swimming, strength training, skiing, climbing, or surfing should be deemed medically necessary and covered by insurance as they yield both physical and mental health gains, according to SEBCM ’s model legislation.
This summer, New Jersey became the 12th state to enact its own variation of SEBCM legislation, joining Washington, Oregon, Colorado, New Mexico, Minnesota, Illinois, Arkansas, Georgia, Maine, New Hampshire, and Maryland, all of which have laws on the books. Legislation has been introduced in nine other states and advocates have plans to pursue legislation in 16 more.
“In every state, there are hundreds or thousands of bills that get introduced in any session, with a very small percentage actually getting passed, so, advocacy is generally a long game, and we expect it could take several attempts to get one of our bills passed in any state,” says Maggie Baumer, JD, a state lead and state coach for SEBCM. “However, we have also seen rapid success with this initiative, with 12 states passing the bill in just three years—pretty incredible by any standard!”
Baumer leads enterprise patient advocacy at Hanger in addition to serving as a state coach for SEBCM, guiding the process of passing, implementing, and expanding legislation to broader age groups and patient populations.
State coaches like Baumer help state teams avoid pitfalls like filing bills late in the legislative session, limiting the time they have to gain traction and support. They also help develop financial analyses that show legislators and insurance plans how SEBCM laws can reduce costs.
As activism continues to bring more states on board, a new phase of advocacy has ramped up to assist stakeholders in states with new laws in expediting their implementation. “There is often a lag

in translating requirements of new legislation into the practical,” says Morgan Fabber, MPH, AOPA’s manager of state and federal advocacy and an advisor for SEBCM. “So, the goal is to help them understand how to communicate effectively with legislators and policy makers to help it go into effect faster.”
AOPA has created an arsenal of tools to equip state leads and core teams to usher in change smoothly and efficiently:
An Implementation Guide on the SEBCM website provides practical tips for state leads to help them effectively communicate about the new law with the O&P clinics, patients, and the extended O&P community. Suggestions for creating community engagement opportunities and identifying key patients for testing insurance claims processes, and ideas for collaborating with the state insurance commission, are featured in the guide, along with template communications to make outreach simple.
Monthly implementation meetings are held to assist all states that have passed SEBCM legislation in moving it forward. Implementation sessions provide opportunities to share successes, challenges, and ideas across various states.
New orientation sessions will launch soon to brief states immediately after their bills pass. Orientation sessions will highlight the experiences of states further along in the process, where the focus has shifted to making sure patients have full access to adaptive O&P care.
Two new Frequently Asked Questions (FAQs) resources have been published, one with patient-facing information and one with clinician and billing staff-facing information. The FAQs resources answer a variety of questions received from stakeholders and offer guidance for navigating the reimbursement process and understanding the nuances of SEBCM legislation.
AOPA Director of Health Policy and Advocacy Joe McTernan, a national lead for SEBCM, says the resources merge the experience of state leads and coaches to provide actionable guidance to ease the implementation process.
Once a bill has passed, one of the main implementation hurdles is identifying the insurance plans to which the law applies, typically fully insured plans regulated at the state level, public employee plans, and, in some cases, Medicaid, Baumer says.
“The other barrier to putting the law into practice is simply adoption,” she says. “It takes time for clinicians and administrators to get used to the idea of this coverage and implement the steps to carry it forward.”

With AOPA’s implementation resources, coalitions can plan ahead to create educational materials and programs for practitioners, patients, and the limb loss and limb difference communities, greatly expediting the new law’s effective usage.
Jennifer Davis is a contributing freelance writer for O&P Almanac










On July 17, Sens. Mark Warner and Steve Daines and Reps. Glenn “GT” Thompson, Gus Bilirakis, Debbie Dingell, and Mike Thompson introduced the Medicare Orthotics and Prosthetics Patient-Centered Care Act in the Senate (S 2329) and House of Representatives (HR 4475), respectively. This bipartisan legislation represents a significant step forward in improving access, quality, and oversight in O&P care for Medicare beneficiaries.
“AOPA applauds the introduction of this critical legislation and sincerely thanks Sens. Warner and Daines and Reps. Thompson, Bilirakis, Dingell, and Thompson for their leadership,” said Teri Kuffel, JD, executive director of AOPA. “We look forward to working with Congress to advance this patient-centered bill and ensure individuals living with limb loss, limb difference, and musculoskeletal conditions receive the safe, high-quality care they deserve.”
The Medicare O&P Patient-Centered Care Act includes three essential provisions designed to improve clinical outcomes and reduce unnecessary Medicare spending:
• Prohibits drop shipping of custom orthoses and prostheses directly to beneficiaries, ensuring patients receive appropriate in-person care from certified professionals
• Exempts O&P providers from the competitive bidding program, simplifying care delivery by allowing patients to receive comprehensive services from a single provider
The postmastectomy prosthesis market, estimated at $500 million in 2025, is projected to exhibit a compound annual growth rate of 5% over the next eight years, reaching approximately $750 million by 2033.
SOURCE:

• Allows timely access to replacement custom orthoses when a patient’s clinical condition changes—removing outdated barriers that delay medically necessary care.
An independent analysis by Braid-Forbes Health Research estimates that this legislation will result in $60 million in net savings over the first 10 years of enactment, including $73 million in fraud and abuse prevention with an estimated cost of only $13 million to increase care efficiencies.
“This bill isn’t just about improving care—it’s about protecting patients and the integrity of the Medicare program,” said AOPA President Rick Riley. “Medicare beneficiaries deserve access to personalized, clinically appropriate orthotic and prosthetic services—without risk of fraud, delay, or disruption. This legislation brings us closer to that goal, and we urge Congress to act swiftly.”
In a study of individuals with unilateral lower-extremity amputation, University of Colorado researchers found that participants with bone-anchored prostheses experienced mobility advantages compared to the socket prosthesis users.
The researchers leveraged the new Colorado Limb Donning Timed Up-and-Go (COLD-TUG) test—a measurement tool that combines the time required for donning a prosthesis with the time to complete the Timed-Up-and-Go test—in studying a group of individuals with lower-limb amputation. The COLDTUG test measures time required for a series of activities: donning a prosthesis, getting up from a

chair, walking 3 meters, turning around, walking back to the chair, and sitting down again.
Participants who had undergone osseointegration had a significantly shorter mean COLD-TUG time compared to the socket group. The researchers determined that the COLD-TUG test accurately measures prosthesis donning burden in the context of functional mobility. The study was published in May in the Journal of Bone & Joint Surgery

At Hersco we pride ourselves on being at the leading edge of technology. We have mastered the art of seamlessly accepting scans, designing foot orthotics and 3d printing. We deliver industry leading quality in an eco-friendly manner.
Hersco: simplifying orthotics, one scan at a time.
Participants with above-knee amputation who participated in a clinical study at the Massachusetts Institute of Technology (MIT) found that a newly designed “tissue-integrated” prosthetic knee system offered greater stability and control, according to a new study.
The new bionic knee is directly integrated with the user’s muscle and bone tissue, using an agonist-antagonist myoneural interface surgical approach, wherein muscle pairs are reconnected during surgery so that they can dynamically communicate with each other within the residual limb, combined with osseointegration via the e-OPRA system.
The researchers measured the participants’ ability to perform several tasks, including bending the knee to a specified angle, climbing stairs, and stepping over obstacles. The new system offers the user improved control over the movement of the prosthesis, according to the research team, which included Hugh Herr, PhD, Rickard Branemark, MD, MSc, PhD, and others. Study participants reported feeling that the limb felt more like a part of their own body, compared to people who had more traditional above-knee amputations.
“A prosthesis that’s tissue-integrated—anchored to the bone and directly controlled by the nervous system—is not merely a lifeless, separate device, but rather a system that is carefully integrated into


human physiology, offering a greater level of prosthetic embodiment,” said Herr, co-director of the K. Lisa Yang Center for Bionics at MIT. “It’s not simply a tool that the human employs, but rather an integral part of self.” The study was published in July in Science
Researchers are examining how the study of axolotls can aid in the development of human limb regeneration. Axolotls are a species of salamander that are expert at regeneration. After losing a limb, adult axolotls can grow the limb back fresh and new—and they can also regrow tissue in the heart, lungs, and brain.
A team of Boston researchers studied axolotls that had been genetically engineered to glow in the dark to understand the molecular underpinnings of their ability to regenerate limbs. James Monaghan, MA, from Northeastern University’s Department of Biology, led the investigation, tracing the ability of the axolotl to regenerate to a molecule known as retinoic acid, which is responsible for telling the animal’s cells which body part to grow back.
Monaghan and his team discovered that axolotls have a gradient of retinoic acid signaling. In the arm, for example, this means axolotls have more retinoic acid in their shoulders and less retinoic acid in their hands. The retinoic acid acts as a cue to the regenerative cells, called fibroblasts, telling them what and how much to grow back, according to the researchers.
Understanding the signal for regeneration is a step toward applying these lessons to humans, Monaghan said. Further study “could help with scar-free wound healing but also something even more ambitious, like growing back an entire finger,” Monaghan said. “It’s not out of the realm [of possibility] to think that something larger could grow back like a hand.” The study was published in June in Nature
A majority (63%) of healthcare consumers trust the health information shared on social media by influencers, and 82% are likely to follow health advice given by healthcare influencers.



In a single-center study, one hospital partnered with an independent prosthesis care program to determine whether such a partnership would result in better patient outcomes for individuals who undergo transfemoral and transtibial amputation.
Over a four-year period, the researchers found that 59% of patients who participated in the prosthesis care program received prostheses within 12 months, compared with only 25% among those not in the program. Forty-seven percent of prosthesis care program participants achieved ambulation within a year, compared with 13% of nonparticipants.
“Participating in a prosthesis care program significantly improved rates of prosthesis receipt and ambulation after major amputation in this at-risk population,” reported the researchers. The study was published in June in Journal of Vascular Surgery
BY THE NUMBERS
The So Every BODY Can Move (SEBCM) program, launched in pursuit of creating equitable and life-changing access to O&P care and devices for physical activity, made Forbes’ inaugural “Accessibility 100” list. The list highlights the biggest innovators and impact makers in the field of accessibility for people with disabilities.
SEBCM, which is supported by AOPA, the Amputee Coalition, the American Academy of Orthotists and Prosthetists, and the National Association for the Advancement of Orthotics and Prosthetics, was recognized within the Law & Government category, which honors those working to protect and expand the civil rights of people with disabilities.
“This recognition is a tremendous honor for our collective movement,” said Nicole Ver Kuilen, who leads the program at the Amputee Coalition. “It belongs to all of our dedicated advocates and legislative champions—individuals who show up every day to demand change, collaborate across divides to shape policy, and prove that physical activity is a fundamental right, not a privilege.”
The Limb Loss and Preservation Registry (LLPR) is the first national registry designed to improve care for individuals with limb loss and limb difference. The LLPR collects data on limb preservation, amputation, and nerve surgical hospital procedures from hospitals, providers, and patients.

>466,000 Unique Patients

Tina Culbreth, CHC, CCEP, CHPC, CDME, has been appointed director of compliance by the Board of Certification/Accreditation (BOC). In this leadership role, Culbreth will provide strategic oversight of business activities such as accreditation eligibility reviews, corrective action processes, and surveyor communications. She also will collaborate with internal teams and stakeholders to enhance process efficiency and ensure alignment with CMS requirements and BOC’s rigorous standards. Culbreth brings more than a decade of experience in compliance leadership, most recently serving in compliance roles at Illumina and Ortho Virginia. “Tina brings a combination of strategic insight, operational excellence, and industry-specific compliance knowledge,” said Judi Knott, MA, MBA, CAE, president chief executive officer of BOC. “Her leadership and experience will further our commitment to excellence in accreditation and certification.”

Brian Hafner, PhD, and his team at the University of Washington (UW) Center on Outcomes Research in Rehabilitation, were awarded the Bryan and Joyce Blatchford Team Prize for Innovation at the International Society for Prosthetics and Orthotics World Congress in June. The prize recognizes achievements related to O&P hardware or scientifically based new techniques resulting in improved devices.
Elizabeth Halsne, PhD, MSE, CPO, LPO, has joined the faculty of the UW Prosthetics-Orthotics Division as assistant professor. This is a joint appointment between the Puget Sound VA Center for Limb Loss and Mobility and the UW Department of Rehabilitation Medicine. She also serves as program manager for the Orthotics and Prosthetics Foundation for Education and Research’s Early Career Research Grant Program.

Teri Kuffel, JD, executive director of AOPA, was honored with a Leadership Award from the National Association for the Advancement of Orthotics & Prosthetics (NAAOP) in recognition of her outstanding leadership in the O&P profession and at AOPA. NAAOP Executive Director George Breece presented the award to Kuffel in July.

Ashlie White has joined AOPA as senior director of external affairs, effective Aug. 18. White most recently served as chief strategy and programs officer at the Amputee Coalition. Previously, she served as AOPA's director of health policy and strategic alliances.
In her new role, White will direct federal and state policy initiatives, facilitate research collaborations, and lead AOPA's strategic external alliances.
Embla Medical, parent company of Össur, Fior & Gentz, and College Park, was named to Forbes’ “Accessibility 100” list in the Sports & Recreation category. “We are honored to have received this recognition as a purpose-driven company that is passionately committed to improving the quality of life for people experiencing chronic mobility challenges,” said Sveinn Sölvason, Embla Medical’s president and chief executive officer.
Hanger Clinic has been awarded a national group purchasing agreement for O&P care with Premier Inc. Under this agreement, members of Premier—a technology-driven healthcare improvement company—can access Hanger Clinic’s comprehensive services to support care delivery for patients requiring orthotic and prosthetic interventions, including orthopedic trauma, spinal trauma, stroke rehabilitation, and amputee care.
Hanger Inc. has entered into an agreement to acquire Point Designs, a Colorado-based firm offering upper-limb prostheses, including partial hand prostheses, with expertise in additive manufacturing and mechanical design. “Point Designs is the perfect partner to bring on board as part of our focus on creating the most comprehensive offering of products, services, and care for patients with upper limb loss or difference,” shared Pete Stoy, Hanger’s chief executive officer (CEO). “We are excited about the experience and knowledge the Point Designs team can bring to our ecosystem of upper-limb prosthetic innovation and patient care.”
“We are very excited about the opportunity to join the Hanger family and to expand our ability to positively impact the lives of those with upper-limb loss or limb difference,” said Levin Sliker, PhD, CEO and co-founder of Point Designs. The transaction is expected to close in Q3 of 2025.
Kuffel will lead the organization’s efforts to advance public policy, education, and member engagement
Teri Kuffel, JD, has been appointed by the AOPA Board of Directors to serve as the organization’s executive director.
Kuffel, who served as interim executive director from January through July of this year, received unanimous support from the Board. She has proven her ability to provide strong and passionate leadership that is built on her nearly three decades of experience in the O&P profession.
During her eight years of service on the AOPA Board, including her role as president from 2022 to 2023, she consistently and passionately demonstrated her commitment to AOPA’s vision,

mission, and strategic priorities, always striving to bring more value to its members. She is recognized as an effective legislative advocate and collaborator who has nurtured relationships with the leaderships of O&P associations and businesses, as well as state and national legislators.
“These past six months, Teri has continuously demonstrated her innate ability to engage with sister organizations and AOPA members. Her leadership during this transition has been steady, strategic, and forward-looking,” said Rick Riley, president of AOPA. “Teri is a business leader who has real-world experience in the daily operations of an O&P clinic. I am confident our decision to choose Teri to guide AOPA through its next chapter of growth and impact will be widely supported by O&P providers and suppliers, as well as our sister organizations, including those that comprise the O&P Alliance.”
Kuffel earned her juris doctorate from Mitchell Hamline School of Law in St. Paul, Minnesota. She co-founded and has served as vice president of Arise Orthotics & Prosthetics, a longtime AOPA member in Minnesota. She also has served on the boards of the Minnesota Society of Orthotists, Prosthetists, and Pedorthists as well as Wiggle Your Toes, an amputee nonprofit. Kuffel has led grassroots advocacy initiatives that helped achieve legislative wins benefiting patients and O&P providers in her home state.
For the past 10 years, Kuffel also has dedicated time to educating the next generation of clinicians as a business and legal ethics instructor for the Master of Science in O&P Program at Concordia University in St. Paul.
As executive director, Kuffel will lead AOPA’s efforts in advancing public policy, education, and member engagement—ensuring the association remains the leading voice for the O&P profession across the United States.
“It is my honor and privilege to serve this profession that has given me so much,” said Kuffel. “I am grateful for the trust of the Board and excited to continue working alongside our members, partners, and staff to strengthen the future of O&P.”




The only manufacturer offering a oneband guarantee, providing confidence and reliability.



Our team includes 4 experienced clinical orthotists and prosthetists who have fitted thousands of Starbands.

Access top cranial educational resources and gain CEUs to enhance your clinical expertise.



Orthomerica’s SmartSoc® and Starscanner® deliver fast, reliable, and comprehensive Star Reports®, featuring the StarScale™, multi-plane 3D data visualization, and secure patient data storage.
Navigate insurance and compliance with our support.
Over 600,000+ infants treated with Orthomerica’s cranial solutions worldwide.


Tailored for pediatricians and healthcare professionals to help grow your business.
Orthomerica offers the most FDA-cleared CROs for all types of patient-specific cranial deformities.





For those of us who live and breathe orthotics and prosthetics, the transformative power of our work is undeniable. We see it every day—in the confident stride of a veteran with a new prosthesis, the steady balance of a child with cerebral palsy in a custom orthosis, or the return to independence for a stroke survivor learning to walk again.
These are not small victories. They are life-changing moments made possible through evidence-based practice and innovative care. And yet, despite its profound impact, the orthotic, prosthetic, and pedorthic (OP&P) profession remains drastically underfunded and under-researched compared to other healthcare disciplines.
The Orthotics and Prosthetics Foundation for Education and Research is here to change that.
Founded by the OP&P community, for the OP&P community, the O&P Foundation is the only independent, 501c3 dedicated solely to advancing our field through funding programs in research, education, and professional practice. As we near the close of a historic $1 million matching opportunity from the American Board for Certification in Orthotics, Prosthetics, and Pedorthics (ABC)— which ends Nov. 30, 2025—now is the moment for the OP&P community to come together and invest in our shared vision to improve the quality of orthotic and prosthetic care.
The O&P Foundation’s mission is straightforward: to advance the art and science of orthotic and prosthetic rehabilitation. It does this by enhancing clinical practice through professional training awards, promoting education and training access via academic scholarships, strengthening the knowledge base through research grants, and inspiring excellence through recognition of scholarly achievements.
From its inception, the O&P Foundation has focused on empowering OP&P professionals at every stage—from students just entering the field, to seasoned clinicians managing unbelievable patient loads. “We exist to seed sustainability and progress in the profession,” says Fanny Schultea, MS, MSEd, CPO, LP, FAAOP(D), executive director of the O&P Foundation. “Every scholarship, every grant, every professional training award is an investment in the long-term quality and accessibility of O&P care for our patients.”
The O&P Foundation’s strategic plan for the next three years is built around five guiding priorities:
Engaging Broader and Deeper Networks: Mapping and connecting with every segment of the OP&P field—from academic programs to clinical practices and industry partners
Achieving Financial Sustainability: Maintaining strong program-to-operations ratios and driving efficient fundraising to maximize donor impact
Strategic Partnerships: Launching cross-sector collaborations that increase program reach, attract new funding sources, and elevate the profession as a whole
Demonstrating Impact Through Metrics and Storytelling: Collecting and sharing the outcomes of funded programs to show the real-world impacts of the investments in research, education, and professional practice
Building a Strong Volunteer Pipeline: Ensuring a committed, skilled, and diverse pool of volunteers and future leaders to carry forward the mission of the O&P Foundation.
Every initiative launched under this plan is designed with the future of patient care in mind—and all of the professionals who deliver it. 1 2 3 4 5
Too often, OP&P research funding lags behind other medical fields, limiting the scope and scale of studies needed to demonstrate clinical efficacy and value. The O&P Foundation is directly addressing this gap through its targeted research priorities. These include studies that improve clinical outcomes, enhance patient experience, and reduce healthcare costs—aligned with valuebased care principles and key domains of quality such as safety, equity, effectiveness, and patient-centeredness.
Importantly, the O&P Foundation’s funding structure encourages early-career researchers, clinicians, and innovators to enter the research ecosystem, often launching careers that grow into larger, federally funded projects. O&P Foundation Immediate Past Chair Shane Wurdeman, PhD, CPO, FAAOP(D), says: “The fellowship award was a springboard for my career. It helped me establish a research lab and eventually secure over $30 million in federal grants.”
With populations of the patients we serve projected to grow rapidly, the demand for OP&P care and providers is increasing parallel to the rates of diabetes, vascular disease, and aging. The O&P Foundation is working proactively to support access to clinical education and training by ensuring that funding is not a barrier to a well-trained and motivated workforce ready to meet these growing demands. Through its scholarship programs, professional training, and continuing education funding opportunities, the O&P Foundation has already helped close to 60 future clinicians complete their training and grow into their clinical and professional roles. One of those
clinicians, Janet Trujillo, CPO, says, “Without the O&P Foundation’s scholarship, I wouldn’t have completed my master’s program or participated in global service trips that deepened my commitment to equitable care.”
Similarly, Denise Hull, CPO, says, “The funding allowed me to attend conferences, explore career pathways, and accelerate my professional growth.”
The opportunity to shape the future of the profession has never been greater. Thanks to ABC’s $1 million matching challenge, every dollar donated to the O&P Foundation through Nov. 30, 2025, will be matched—doubling its impact.
Whether you’re an individual clinician, a practice owner, a manufacturer, or a researcher, you have a role to play. Your contribution will fund research that drives better outcomes, education that supports tomorrow’s workforce, and programs that advance quality and equity in patient care.
The Orthotics and Prosthetics Foundation for Education and Research isn’t just another nonprofit. It is the backbone of our collective future—built by and for the OP&P community. It exists so that every patient receives the highest quality care, and every professional is empowered to provide it.
As we count down to the close of the ABC match challenge, there’s never been a more urgent time to give and support. Together, we can create a more sustainable future, one of growth, for the O&P profession and the care we provide. Let’s move it forward—together.
Consider taking one or more of the following actions to support the O&P Foundation.

Donate: Make a one-time or recurring gift. Every dollar donated will be matched through Nov. 30, 2025.
Sponsor: Fund an existing or new scholarship, training award, or research grant aligned with shared values.
Partner: Join our industry consortium to build the evidence base and support infrastructure needed for sustainable, high-quality OP&P care.
Advocate: Share our mission and impact stories with your networks to elevate awareness and support us across the OP&P field and beyond.

Volunteer: Come share your energy, time, and talent—join the O&P Foundation as a volunteer and help drive our mission as a committee chair, committee member, reviewer, ambassador, or board member.

Connect With Us: Stay connected with O&P Foundation news and updates by signing up for our mailing list, or reach out directly to Fanny Schultea at executivedirector @oandpfoundation. org
BY JOE MCTERNAN


Change is inevitable. Whether we like it or not, whether it is comfortable or challenging, everything changes. When I look back at my 30 years in the O&P profession, the amount of change I have seen is almost incomprehensible. From changes to functional level classifications, to the advent of programs like competitive bidding and prior authorization, to regular updates to O&P coverage policies, changes have impacted the overall landscape of O&P care. Often, the difference between operational success and failure is a direct result of a company’s ability to understand change and react to it accordingly.
The year 2025 has seen a significant number of Medicare program changes that will have a direct impact on O&P care and the patients you serve. This month’s Reimbursement Page examines some of the more significant recent and proposed changes and discusses how they may impact your O&P business. Understanding these changes will help you adjust to them in a way that positively impacts your business.
On April 28, 2025, CMS announced that Cotiviti Government Services had been awarded the contract to serve as the
Region 5 Recovery Audit Contractor (RAC), responsible for all RAC audits for claims for durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) nationwide. Cotiviti replaces Performant Recovery, which will remain an administrative subcontractor to Cotiviti but will no longer be the main point of contact for RAC audits. While an official transition date from Performant to Cotiviti has not yet been released, Cotiviti is expected to begin performing active DMEPOS RAC audits soon. Cotiviti currently serves as the RAC contractor for several other Medicare product categories so it is already familiar with RAC operations. In addition, the DMEPOS
RAC contractor prior to Performant Recovery was Connolly Healthcare, a company that was later acquired by Cotiviti. This should help facilitate a fairly seamless RAC transition from Performant to Cotiviti.
Cotiviti has not yet activated its RAC resources for Region 5 DMEPOS services, but these resources will eventually be added to its website at cotiviti.com/markets/ cms-rac
AOPA will closely monitor O&P RAC activity as Cotiviti begins its work and will work with the entity to ensure that O&P providers are treated fairly and that any issues are addressed quickly and efficiently.
CMS on June 30 released its 2026 Home Health Prospective Payment System Proposed Rule, which annually includes proposed changes that CMS intends to
implement in the DMEPOS space. This year’s proposed rule contains several proposals that will significantly impact O&P operations, including changes to DMEPOS competitive bidding, facility accreditation requirements, provider enrollment processes, and Medicare prior authorization.
The proposed rule would increase the frequency of Medicare DMEPOS facility accreditation renewal form the current three-year cycle to an annual requirement. The stated purpose of this proposal is to improve efforts to combat Medicare fraud and abuse in the DMEPOS space. The proposed rule would require CMS-approved accreditation organizations (AOs) to re-accredit Medicare DMEPOS suppliers annually. While AOPA and its O&P Alliance partners fully support efforts to eliminate fraud and abuse in the Medicare program,

an annual re-accreditation requirement will result in exponentially higher administrative costs to both AOs and the providers they accredit and also will result in significantly more time that will have to be dedicated to performing accreditation review and preparing for the re-accreditation process. This may prove challenging to both AOs and accredited facilities alike.
The proposed rule also includes requirements for more frequent unannounced provider enrollment audits performed by CMS and its contractors. While this is also intended to reduce Medicare fraud and abuse, the current provider enrollment audit process is already facing significant process challenges. Simply increasing the number and frequency of enrollment audits will likely highlight and increase these challenges rather than improve them.

MT. EMEYS 9705
SIZE: 7, 7.5 - 11.5, 12-17
WIDTH: D, 4E, 6E, 9E
BREATHABILITY




Another section of the proposed rule addresses the expected return of DMEPOS competitive bidding. The original round of Medicare competitive bidding that initially impacted O&P involved 23 off-theshelf (OTS) orthosis codes and ended in December 2023. The proposed rule indicates that CMS intends to announce a new round of competitive bidding in the near future. While it is unlikely that CMS will include codes from the last competitive bidding round in future rounds, as it is unlikely that additional savings will be achieved, the remaining 22 OTS Healthcare Common Procedure Coding System (HCPCS) codes that were eligible for competitive bidding but not selected for previous inclusion are likely to be included.
AOPA has long advocated for expansion of the competitive bidding exemption currently in place for physicians and therapists to include certified and licensed orthotists and prosthetists. This would allow for O&P practitioners to provide OTS orthoses to their patient population without requiring a DMEPOS competitive bidding process. This is in the interest of holistic patient care and is one of the three main provisions of the recently reintroduced Medicare O&P Patient-Centered
Care Act in both the House of Representatives and Senate (see page 10). AOPA will continue to advocate for this legislation while also communicating why DMEPOS competitive bidding should not be expanded to additional O&P HCPCS codes.
The proposed rule also includes a pathway for providers that achieve high success rates on claims subject to Medicare prior authorization to be exempt from further prior authorization for a predetermined time. While this seems to be a positive proposal, Medicare prior authorization has been an extremely successful program, and if it continues to operate in a fair and efficient manner, providers may want to consider continuing to file for prior authorization even if an exemption is available.
AOPA will work with its O&P Alliance partners to develop and communicate relevant comments to the proposed rule in advance of the Aug. 29 deadline.
An example of a positive change for the O&P profession and its patients in 2025 involves the recently published proposed Local Coverage of Determination (LCD)
and affiliated draft Policy Article for Knee Orthoses. This was published by the durable medical equipment Medicare administrative contractors (DME MACs) July 24, and would expand Medicare coverage for certain custom-fitted and custom-fabricated knee orthoses used to treat osteoarthritis without requiring documentation of a recent injury to or surgery on the knee or objective documentation of ligamentous joint laxity.
This is a policy challenge that has existed for many years. In August 2023, AOPA and its Coding & Reimbursement Committee submitted a formal LCD reconsideration request to the DME MACs asking that they revise their policy to include coverage for osteoarthritis braces to manage pain and improve ambulation.
The proposed LCD released July 24 expands this coverage and incorporates much of the policy language suggested by AOPA; it appears to be very beneficial to Medicare beneficiaries seeking orthotic intervention to manage osteoarthritis.
CMS will hold a public hearing on the proposed LCD Aug. 27. AOPA will attend the hearing and provide relevant comments. The proposed LCD and draft Policy Article may be accessed on the CMS website.
Because this entire column was built around discussing change, I can’t think of a better way to end it than with a word of congratulations and welcome to Teri Kuffel, JD, AOPA’s new full-time executive director.
Kuffel has been a long-time member of the AOPA Board of Directors and served as president in 2022-2023, and she brings her lifetime of experience to the executive leadership role at AOPA. This is a new challenge, and we look forward to her tenure and leadership as she takes over the role of executive director. Welcome, Teri!

Joe McTernan is director of health policy and advocacy at AOPA. Reach him at jmcternan@AOPAnet.org


Coapt’s new HCPCS Level II code means more access to modern pattern recognition myo control for upper limb prosthesis users L6700
Prosthetic devices are only as good as how they are controlled. Coapt’s convenient and highly compatible system applies the remarkable power of myoelectric pattern recognition – adding personalized, modern control – to arm and hand prostheses. Scan and connect with us to find out more. www.coaptengineering.com
BY JOSEPHINE ROSSI
3D printing and digital workflows are transforming O&P—and the practitioners who use them
It’s Tuesday in a college dorm room where a student texts a photo of her broken orthosis to her orthotist six hours away. Within hours, a 3D printer is creating her replacement brace based on a digital file; it arrives by Friday without significant disruption to her schedule. This scenario, now routine for some practitioners, illustrates how additive manufacturing is transforming a hands-on field.
For the uninitiated, the 3D printing (also called additive manufacturing) workflow involves scanning a patient’s limb to create a digital model, customizing the design using specialized software, and then printing the device layer-by-layer before final finishing and delivery to the patient. What was once associated with oversized, clunky, expensive machines making toys and knickknacks has now become an efficient, commercially viable manufacturing technique used across many industries, including the fabrication of prostheses, orthoses, and their components.
Through conversations with practitioners, educators, and manufacturers, a picture emerges of a profession facing both unprecedented opportunities and fundamental challenges.
Brent Wright, CP, BOCO, vice president of EastPoint Prosthetics and Orthotics, who has been working in O&P since he was 16, says he initially dismissed digital methods. The turning point came in 2014 after an
f 3D printing has evolved from creating novelty items to efficient manufacturing for prostheses and orthotics, with some practitioners converting nearly all work to digital methods.
f Implementation varies by application and economics— complex, high-value devices like adjustable sockets benefit most, while simpler applications face reimbursement barriers.
intensive clinic in Guatemala where a large patient load left his hands “just shot” from physical demands. A colleague encouraged him to try digital work.
“That moved the needle for me to start looking as this is an alternative for clinicians to the high labor intensity work,” he says. “If you're not even looking at this technology, you are doing your patients a disservice.”
Today, nearly all of Wright’s prosthetic work involves 3D printing, though the economic realities vary significantly by application. Digital works well for complex, high-value devices requiring extensive technical work, such as adjustable sockets, upperextremity sockets, or ankle-foot orthoses (AFOs) that use struts. Simpler applications face barriers: “For what you get reimbursed, it's not worth doing,” explains Wright, who also is co-founder of Advanced 3D, which offers additive manufacturing, digitization, and reverse engineering services.
This selective implementation pattern appears across different practice settings. At Gillette Children’s Specialty Healthcare, Corey Baum CO, LO, and John Sytniak, CPO, describe a hybrid approach developed over more than a decade. Their facility uses in-house 3D printing for specialty wheelchair seating components while outsourcing printing of other applications. “One hundred percent of our cranial remolding helmets, 20% of our FOs [foot orthoses], SMOs [supramalleolar orthoses], and AFOs are 3D printed.”
f Digital workflows enable quantified, evidence-based practice by providing concrete data points that help clinicians track patient changes and reduce material waste, fitting times, and physical storage needs.
f Future materials will combine flexibility and rigidity in specific zones, with advances in multimaterial printing and "tunable" properties, while AI serves as a design tool rather than replacement for clinical judgment.

f Related emerging technologies include embedded monitoring sensors in devices, virtual reality modifications, and open-source manufacturing platforms, though practitioners emphasize patient safety must remain paramount.

The productivity gains extend beyond simple metrics. “I’m able to get my work done during normal business hours,” says Wright. He contrasts this with previous traditional workflows requiring early morning lab work, full patient schedules, and evening nursing home visits extending to 10 p.m.
“Digital workflow and fabrication have cut down on material waste with less plaster and plastic waste compared to traditional fabrication,” say Baum and Sytniak, while offering “the ability to do a wider range of modifications, create modifications macros, leading to greater consistency in modifications.”
Yet implementation isn’t necessarily easy.
One challenge is the learning curve, according to Chad Duncan, CPO, LPO, PhD, CRC, professor and program director of O&P, Salus at Drexel University. Students with backgrounds in “industrial arts, biomedical engineering” or who “tinker” with programs and printers adapt more easily, while those from backgrounds such as psychology or kinesiology, and who haven't been exposed to it, face steeper challenges navigating different software systems. Proficiency isn’t achieved until residency, he says.
“If you're not even looking at this technology, you are doing your patients a disservice.”
—BRENT WRIGHT, CPO, BOCO
As Wright notes, the reimbursement landscape adds another layer of complexity. For orthoses, the challenge is less complex since they don't require endoskeletal/exoskeletal classification, and CMS guidance from February 2024 confirmed that 3D-printed orthoses can use existing custom-fabricated codes if they comply with DMEPOS Quality Standards. However, prostheses will likely require new codes supported by data and research, and both applications face challenges with quality standards that were written 20 years ago before digital models and additive manufacturing existed.
Extensive validation also is required for commercial products, according to Herb Barrack, CPO, co-founder and chief clinical officer of Limber Prosthetics. His company’s development of “the world's first, monolithic, single-printed transtibial adjustable prosthesis” required four years to achieve FDA registration, quality management system implementation, and ISO 10328 standards compliance. “Regulatory is very costly but is a large piece to the puzzle when it comes to safely integrating [additive manufacturing] into your practice,” Barrack notes. “Products must be designed with a particular geometry and load tested in order to keep patient safe and functional while using their prosthesis.”
Beyond manufacturing methods changes, clinical thinking and educational approaches also are progressing. Duncan describes a shift from traditional procedural learning to analytical thinking;
educators want students to understand there is more than one way to address a patient’s needs. “I think that’s the [purpose of] education—[for students] to become more or better problem solvers and process-oriented versus, traditionally, we knew these steps, and we followed these steps.”
“Digital workflow and fabrication have cut down on material waste with less plaster and plastic waste compared to traditional fabrication,” while offering “the ability to do a wider range of modifications, create modifications macros, leading to greater consistency in modifications.”
Perhaps more significantly, digital workflows enable quantified evidence-based practice. Scanning, for example, provides concrete data points helping students better understand pathologies such as diabetes. “If somebody’s having dialysis, the limb changes, and they can actually see that via numbers,” Duncan says. “It could be a very small change, but those small changes do make a difference," he says.
Baum and Sytniak say that digital workflows have cut back on the time they need for fittings and the amount of physical space needed with plaster models. Similarly, Wright explains that when a patient’s traditional carbon-fiber socket breaks, the replacement process requires physical measurements, casting, and fabrication. In contrast, when a digitally designed socket needs to be replaced, “a patient does not have to come in, as long as everything was working before. And we’re able to take those digital files, make adjustments, and reprint and get them in.”
Despite historically limited research focusing specifically on O&P, materials science is advancing. “Specific material properties for O&P are materials that can be flexible in areas where we need movement in the orthosis and rigid in areas that we need support and stability,” say Baum and Sytniak. They also note advancing capabilities: “Materials for 3D printing and printers are advancing daily with capability to print with multiple materials in the same device.”
“We need materials that combine high energy return for optimal patient mobility with the ability to be thermomolded for precise customization—while also being environmentally responsible,” explains Barrack. “Advances in ultralight, high-strength polymers specifically engineered for compatibility with 3D printing will be
For 30 years, The Bremer Group Company has established a reputation for supporting practitioners and patients with quality back braces, and exceptional customer service. The VertAlign® Spinal Support System embraces these 7 Cs.
For more information visit www.bremergroup.com.


game-changing.” These materials must “remain dimensionally stable yet responsive to controlled heating, enabling practitioners to fine-tune fit and function without compromising durability or performance.”
The concept of “tuning” materials through design offers particular promise for future applications. Wright envisions materials that can be precisely calibrated through digital design—adjusted in thickness, flexibility, and structural elements to meet specific patient requirements. He and Duncan both highlight the need for population-specific material development and testing protocols.
Tuning materials through design may eventually help the profession implement controlled failure into devices. Wright advocates engineering devices that “fail safely” rather than catastrophically: He recalls one patient's car accident experience where the absence of his prosthetic leg likely saved his life—doctors said, “You weren’t wearing your prosthesis, were you? Because if you were, you wouldn't be with us right now. It would have split you in half.”
“We have always [said] we don’t want [devices] to fail, we don't want it to break, and I don’t think that’s necessarily the right way to start to look at it,” Wright explains, drawing parallels to automotive crumple zones and modern helmet design. “There’s a chance I won't ever see that in my lifetime, and I’m okay with that, as long as we start the discussion.”
The integration of artificial intelligence (AI) into design and manufacturing represents both potential and limitations. In the case of a socket, the challenge of “fitting a living, breathing, changing body” begins with variables unknown to any algorithm and extends through daily variations in human anatomy and activity. “There’s so much that gets involved with the creation of a socket, and it starts with the surgeon,” Wright explains. “Did they actually do a good job with the surgery? And we don’t know. And so this is where AI starts to fail”—and why human intelligence will always be needed.
AI could also “make us lazy,” warns Duncan. He views AI pragmatically as “just a tool” that is “not always correct.” As AI becomes more prevalent in clinical practice, he expresses concern about technological dependence and knowledge retention, citing research showing that students using AI for writing had significantly lower retention levels compared to those using traditional writing processes.
On the other hand, Wright raises an intriguing possibility about AI’s creative potential in exploratory design. “What if there was some AI hallucination, and it created a shape and it actually works? Because if you go from clinician to clinician to clinician, you have really happy patients with [very] different styles of sockets.”
Beyond materials and AI, emerging technologies promise to expand the boundaries of what O&P devices can accomplish. Baum and Sytniak describe current capabilities for embedded monitoring within 3D-printed devices: “Channels can be printed into devices to track gait, temperature, [and] pressure or monitor wear time for outcome studies.” This integration of sensing technology directly
into devices enables real-time data collection that could revolutionize both clinical care and research.
Duncan envisions even more immersive technologies, including holograms and “virtual reality (VR) modifications” where practitioners use VR headsets to modify devices in three-dimensional space.
Wright identifies broader industry trends toward technological openness that could accelerate innovation. He points to automotive manufacturers’ movement toward “open materials” platforms allowing custom material specifications rather than closed systems. “There is a trend toward intellectual property, individual ... machines that are ‘open source’ that will run these things, and away from this black box idea.” This democratization of manufacturing capability could enable widespread experimentation and rapid innovation cycles.
“Advances in ultralight, high-strength polymers specifically engineered for compatibility with 3D printing will be game-changing.”
HERB BARRACK, CPO
He also cites SpaceX’s rocket engine development as a model for simplifying complex assemblies through 3D printing: “You go from needing to source literally thousands of components to sourcing maybe tens of components … the output is still what you want, but your whole vertical now is more simplified.”
Practical implementation of 3D printing and related emerging technologies requires balancing innovation with safety and proven effectiveness. Technological capability doesn’t automatically translate to clinical superiority in all applications. “The most critical thing is to never forget that patient safety is most important,” Barrack stresses. “Not everyone can 3D print a device that is safe and functional for patients.”
Indeed, Gillette Children’s will continue with selective adoption “where it makes sense to the benefit of the patient’s outcomes,” but Wright sees particular urgency for independent practices facing competitive pressure: “If you’re an independent practice, it’s game on, and move as fast as you can because the big guys are coming, and they will catch up to you.”
Duncan also has heard the call for rapid adoption, particularly at industry conferences. He believes that while traditional and digital methods will co-exist in the future, clinical practices should be at least learning about integrating 3D printing and AI. “There are still no dumb questions in this arena. There are people way ahead, but at the same time, you can catch up.”
Josephine Rossi is editor of O&P Almanac. She couldn’t resist letting AI contribute to this article, too! Reach her a jrossi@contentcommunicators.com






Expand your network, learn the latest in O&P research and clinical care, and view cutting-edge products and services at AOPA's 2025 National Assembly

Assembly Sponsors………32
Meet the exhibitors that have signed on as title sponsors for AOPA's 2025 National Assembly
Exhibitor Directory………36
Access a complete, alphabetical listing of the exhibitors and their booth numbers

Get ready to catch some rays and shine a light on the latest advances in O&P care when AOPA's 2025 National Assembly convenes in the Sunshine State. Join AOPA Sept. 3-6 in warm and welcoming Orlando, Florida, to learn about the newest innovations in orthotics and prosthetics from the most brilliant researchers and skilled practitioners in the field.
Throughout the event, pop in to the “Ask Me Anything” sessions across from the AOPA Hub #1611 to learn directly from content experts addressing everything from new product development to insurance negotiations in a dynamic, 30-minute “office hours” experience. And enjoy more hands-on programming than ever before with our on-site lab sessions featuring technical and pedorthic demonstrations.
Cap off an exciting first day of sun-drenched programming with the Rolling out the Boardwalk: Welcome to Orlando reception from 5:30 to 7:30 p.m. in West Hall E. Enjoy boardwalk bites, carnival games, and entertainment while you browse the largest O&P exhibit hall in the U.S. and make valuable connections with O&P professionals from around the world.
Savor wine and cheese while perusing the latest research at the Poster Showcase from 5:30 to 6:30 p.m. Sept. 4, or register now to reserve your spot to network for a cause at one of two special receptions that evening. Mingle over a drink and hors d’oeuvres at the research mixer benefiting the O&P Foundation or the So Every BODY Can Move reception, and a portion of your ticket price will benefit those organizations.
Attend the Thranhardt Award Lecture Series at 8 a.m. Sept. 5 to hear the two winners present their best-in-show research. Get inspired and make connections that afternoon at the 10th annual Women in O&P Luncheon from 12 to 1:30 p.m. (Register now for this sell-out event!)
Wrap up the final day of programming with our popular Digital Showcase, which affords unique opportunities to learn about 3D design software tools from exhibitors who will present their prosthetic, orthotic, or pedorthic patient case files and fabricated molds, devices, or 3D prints.
Keep this guide handy as you navigate three sunlit days of research, education, and networking. Remember to follow AOPA’s social channels and share your National Assembly experience with #AOPA2025. See you soon in Orlando!

Wednesday, Sept. 3 | 5:30 - 7:30 p.m.


Rolling Out the Boardwalk: Welcome to Orlando Reception
Thursday, Sept. 4 | 5:30 - 6:30 p.m.
Research Mixer Supporting Poster O&P Foundation Showcase
Thursday, Sept. 4 | 6:30 - 9 p.m.
So Every BODY Can Move Activation Celebration
Wednesday, Sept. 3 5:30 - 7:30 p.m. (Welcome Reception)
Thursday, Sept. 4
9 a.m. - 6:30 p.m.
Friday, Sept. 5
9 a.m. - 4 p.m.
Thursday, Sept. 4 and Friday, Sept. 5 Noon – 2 p.m.
New Product Showcase
Explore the newest O&P innovations in the exhibit hall, then vote for your favorite to help crown this year’s Best in Show!
in the Exhibit Hall*
*For those with lunch tickets/special badges. NEW THIS YEAR
CAN’T MAKE IT TO ORLANDO
That’s OK—if you miss a session you wanted to attend, or if you cannot travel to Orlando for AOPA's 2025 National Assembly, AOPA will bring the education to you—virtually— starting Sept. 25 and ending Nov. 25. Learn on your own


Meet the sponsors of this year’s AOPA National Assembly, and learn about their latest products and services
Booth: 1203
Alps South
Booth: 1003
Area of Specialty: Prosthetic liners & sleeves
St. Petersburg, Florida easyliner.com
ALPS South is excited to connect with clinicians, innovators, and partners at this year’s AOPA National Assembly. Visit our booth to explore the UltraSeal Liner with OptiGel technology, our black reinforced Flex Sleeve, and other high-performance solutions tailored to meet diverse clinical needs. We’ll also highlight our versatile liner portfolio , featuring multiple fabric, gel, and suspension combinations. We look forward to meaningful conversations and opportunities for collaboration that will help us continue improving comfort, mobility, and outcomes for amputees everywhere. Stop by our booth to see what’s new and what’s next!
Area of Specialty: Lower & upper limb prosthetics, bracing & supports, neuro-orthotics Irvine, California ossur.com/en-us
Össur focuses its efforts and expertise on helping people to be confident, safe and mobile, regardless of injuries or conditions that could compromise their quality of life. Visit our booth, 1203, at this year’s conference. Our team looks forward to connecting with all of the clinician-customer attendees, business owners, and the extended health care provider network associated with prosthetic and orthotic patients.
Össur Americas is pleased to share the combined solutions and product offerings from our upper and lower limb Prosthetic, Bracing & Solutions and recently acquired Fior & Gentz (Neuro. Orthotic) business areas. We will be showcasing the newly launched, fully waterproof multiprocessor knee solution - Navii® in our booth along with other key product categories.
A one-of-a-kind seal-in solution.

It’s the only seal-in liner available in gel.
OptiGel has a smooth, soft texture that ensures gentle skin contact and a tailored fit around the residual limb.
*Also available in silicone
It’s made with less constrictive fabric.
Outer knitted fabric combines strength, durability, and differentiated stretch for comfortable elasticity.
It’s available in both locking and cushion.
The locking feature ensures users are provided with a suspension that is secure, firm, and comfortable.
It’s available in 3 ring heights.

American Board for Certification in Prosthetics, Orthotics, and Pedorthics Booth: 1132
Area of Specialty: Credentialing Board Alexandria, Virginia abcop.org
The ABC team looks forward to connecting with current and prospective credential holders and business owners at this year’s National Assembly. We are also thrilled to be helping with a career awareness event for local high school students interested in healthcare careers. Stop by our booth for the latest information about our programs, where we will be highlighting the WhatIsPOP career awareness initiative designed to educate and inspire the next generation of prosthetists, orthotists, and pedorthists. Visit us to learn about the benefits of care extenders, specifically through our assistant certifications, and ask all your questions about ABC credentialing.
BionicM Inc.
Booth: 1632
Area of Specialty: Powered prosthetic knee Ashburn, Virginia & Tokyo, Japan bionicm.com/en
BionicM looks forward meeting with CPOs in booth 1632 at the National Assembly. Come visit with us to discuss the benefits of our powered MPK Bio Leg.
Booth: 1228
Area of Specialty: DMEPOS facility accreditation and credential certification or DMEPOS professionals Owings Mills, Maryland bocusa.org
We look forward to welcoming you at Booth 1228, where you will meet senior Board of Certification/Accreditation (BOC) leaders to learn more about our O&P facility accreditation as well as orthotic and mastectomy fitter certifications. BOC has provided accreditation and certification credentials to professionals and facilities across the country for more than 40 years. We invite BOC-credentialed practitioners to stop by to pick up their certification and accreditation badge ribbons as well as our commemorative anniversary pin. All attendees can also add to their CEU count by attending educational sessions and visiting our booth to help complete your Exhibits Passbook. We will see you soon!
Cailor Fleming now World Insurance
Booth: 1125
Area of Specialty: Professional liability/malpractice and all business insurance Youngstown, Ohio cailorfleming.com

Spinal Technology LLC by Eqwal
Booth: 1501
Area of Specialty: Design and custom fabrication of spinal orthoses West Yarmouth, Massachusetts spinaltechnology.com
Spinal Technology is proud to once again sponsor AOPA’s National Assembly and be exhibiting many custom spinal and scoliosis orthosis samples in Booth 1501, including the Providence Nocturnal Scoliosis® Orthosis. New for 2025, we now fabricate lower-limb prostheses and offer technical guidance to prosthetists.

Spinal Technology is most excited for the Best-in-Show Contest this year and hopes everyone will have a chance to come see our new stWear™ Compliance Monitoring System.
Booth: 1707
Area of Specialty: O&P news and information Alexandria, Virginia aopanet.org/publications/opalmanac-magazine/
AOPA and O&P Almanac staff look forward to connecting with Assembly attendees and learning what’s top of mind for O&P professionals. The O&P Almanac booth will feature current and past issues of AOPA’s O&P Almanac magazine. Stop by to pick up a copy and learn about earning continuing education credits by reading.


Balance & Symmetry not just bringing the gait back, but bringing the balance back
Safe and Controlled Power brings comfortability and security
Intuitive and Natural Movement brings confidence and higher quality of life




Sunny Xiaojun Sun Founder & CEO


On the following pages is a look at the companies exhibiting at the AOPA's 2025 National Assembly. You’ll find company names, booth numbers, and website information for each exhibitor. Use this guide to get to know this year’s exhibitors and plan your visit to the exhibit hall. Visit aopaassembly.org for full exhibitor details.
n = New Exhibitor for 2025 Bold = Member of AOPA Exhibitors as of Aug. 1, 2025
n A Custom Fit iNMotion LLC Booth: 719 acustomfitinmotion.com
Aether Biomedical Booth: 819 aetherbiomedical.com
Allard USA Booth: 1337 allardusa.com
AllClaim by Ottobock Booth: 1607 shop.ottobock.us
Alps South LLC Booth: 1003 easyliner.com
Alt-Bionics Inc. Booth: 807 altbionics.com
Alternative Prosthetic Services Inc. Booth: 1036 alternativeprosthetics.com
American Academy of Orthotists and Prosthetists Booth: 1338 oandp.org
GOLD SPONSOR
American Board for Certification in Orthotics, Prosthetics, & Pedorthics Booth: 1132 abcop.org
American Central Fabrication Booth: 720 certifiedlimbandbrace.com
American Orthotic & Prosthetic Association (AOPA) Booth: 1611 aopanet.org
American Prosthetic Components Booth: 1430 americanprostheticcomponents.com
n AmerX Health Care Booth: 718 amerxhc.com
n AMPRIA Booth: 1342 ampria.net
Amputee Coalition Booth: 928 amputee-coalition.org
n Anatomic Studios Booth: 714 anatomic-studios.com
Anclote Manufacturing Co. Booth: 809 anclote.net
Anodyne Booth: 1033 anodyneshoes.com
Apis Footwear Company Booth: 906 emeys.com
ARTech Laboratory, Inc. Booth: 701 artechlab-prosthetics.com
Aspen Medical Products Booth: 812 aspenmp.com
n Aurushi Inc. Booth: 1241 aurushi.com
BASINGLEAD Booth: 937 basingtech.com
Becker Orthopedic Appliance Company Booth: 1122 beckerorthopedic.com
BILLY Footwear Booth: 1513 billyfootwear.com
Bionic Power Inc. Booth: 843 bionic-power.com

Bowman Medical International Booth: 1344 bowmanmedicalinternational.com
BionicM Inc. Booth: 1632 bionicm.com/en
Bionic Prosthetics and Orthotics Group Booth: 712 bionicpo.com
BionIT Labs Inc. Booth: 941 bionitlabs.com/us
BioSculptor Corporation Booth: 1614 biosculptor.com
Blatchford Booth: 1010 blatchfordus.com
GOLD SPONSOR
Board of Certification/Accreditation (BOC) Booth: 1228 bocusa.org
BrainRobotics Booth: 804 brainrobotics.com
Breastcare Education Kiosk Booth: 1301 trulife.com
Bulldog Tools, Inc. Booth: 911 BulldogTools.com

GOLD SPONSOR
Cailor Fleming Insurance now World Insurance Booth: 1125 cailorfleming.com/specialty/orthotics-prosthetics-insurance
Cascade Orthopedic Supply, LP Booth: 1319 cascade-usa.com
CBS Medical Billing & Consulting LLC Booth: 1329 cbsmedicalbilling.com
n Certified Orthopedics Inc. Booth: 825 certifiedortho.com

Coapt LLC Booth: 910 coaptengineering.com
College Park Industries Booth: 1103 college-park.com
Comb O&P Booth: 1230 combscan.com
Comfort Products, Inc. Booth: 915 comfortoandp.com
Coretech Orthopedics Booth: 704 coretechortho.com
Cornerstone/Proclaim Billing Services Booth: 1042 Proclaimbillingservice.com
Coyote Booth: 1237 coyote.us
CPO Labs/insoles.ai Booth: 943 insoles.ai
n Crary Shoes Booth: 1340 craryshoes.com
Curbell Plastics Inc. Booth: 1533 curbellplastics.com/materials/industries/ orthotics-and-prosthetics
Cypress Adaptive LLC Booth: 1531 cypressadaptive.com
Danmar Products, Inc. Booth: 1620 danmarproducts.com
DAW Industries Inc. Booth: 1225 daw-usa.com
Dr. Comfort Booth: 805 drcomfort.com
n Drd Kids Footwear Booth: 710 drdfootwear.com
Drew Shoe Corp. Booth: 1619 drewshoe.com
Elevate Movement Booth: 1043 elevatemovement.com
EQWAL Booth: 1501 eqwalgroup.com
Ethnocare Booth: 811 ethnocare.ca
FabCo Prosthetic Designs Booth: 1530 fabcoprostheticdesigns.com
Fillauer Companies Booth: 1109 fillauer.com
n Finger Prosthetics by Paralid Booth: 829 fingerprosthetic.com
FIOR & GENTZ GmbH Booth: 1203
FLO-TECH O&P Systems Inc. Booth: 1706 1800FLO-TECH.com
n Fred’s Legs Booth: 820 fredslegs.com
Friddle’s Orthopedic Appliances Inc. Booth: 1343 friddles.com
Grace Prosthetic Fabrication Inc. Booth: 1014 gpfinc.com
Hanger Clinic Booth: 1029 hangerclinic.com
HiTek Fabrication Booth: 1525 hitekfab.com
HP 3D Printing Booth: 1231 hp.com/us-en/printers/3d-printers.html
IBT Booth: 700 i-biomed.com
Icarus Medical Booth: 1144 icarusmedical.com
Integrum Inc. Booth: 931 integrum.se
Invent Medical USA Booth: 1425 inventmedical.com
Three Decades of Successful Development of Off-The-Shelf to Custom-Fitted to Custom Fabricated and Guidelines to Reduce AFO Insurance Claim Denials


Dynamic Contracture Management in Pediatric Gait: Increasing ROM with LLPS and Multi Motion for Optimal SWASH & AFO Outcomes





n Kanapo Prosthetics and Orthotics Ltd. Booth: 926 kanapo.ca
Kinetic Research Booth: 925 kineticresearch.com
KISS Technologies LLC Booth: 1428 kiss-suspension.com
LaunchPad O&P Booth: 1623 launchpad-op.com
n LEO G. STEIN Booth: 1621 leogstein.com
Levitate Sport Booth: 723 letslevitate.com
Life-Like Laboratory Booth: 1227 lifelikelab.com
Limb Loss and Preservation Registry Booth: 1142 llpr.org
n Limber Prosthetics Inc. Booth: 841 limberprosthetics.com
Lindhe Xtend Inc. Booth: 1325 lindhextend.com
n macu4 AG Booth: 806 macu4.com
Martin Bionics Booth: 800 martinbionics.com
Materialise Booth: 1630 materialisemotion.com
medi USA Booth: 1031 mediusa.com
n Metacarpal Booth: 845 metacarpalprosthetics.com

Mile High Orthotics Lab Booth: 1044 mholabs.com
Mosaic Mfg. 3D Printing Booth: 1243 mosaicmfg.com
MOZN Solutions LLC Booth: 803 moznsolutions.com
Myomo Booth: 922 myomo.com
National Commission on Orthotic and Prosthetic Education Booth: 1130 ncope.org
Neuro Rehab Recovery Booth: 1612 neurorehabrecovery.com
n Neuros Medical Booth: 1040 neurosmedical.com
New Step Orthotic Lab Inc. Booth: 1024 newsteporthotics.com
North Sea Plastics Ltd. Booth: 1045 northseaplastics.com
NuTech Synergies LLC Booth: 1629 nutechsyn.com
Nymbl Systems Booth: 1037 nymblsystems.com
O and P Booth: 1336 oandp.com.mx
SILVER SPONSOR
O&P Almanac Booth: 1707 aopanet.org
n O&P Cloud CAD Booth: 939 oandpcloudcad.com
O&P Insight Booth: 1027 oandpinsight.com
OP Solutions Booth: 1026 opsolutions.us
Open Bionics Inc. Booth: 823 OpenBionics.com
OPIE Software Booth: 1401 opiesoftware.com
The Icon microprocessor knee offers a user-friendly experience for patients and clinicians. Featuring responsive sensors, streamlined setup, and the intuitive Stride Studio app, this dynamic knee was designed to enhance user confidence. Clinicians can easily access all adjustments, custom modes, and more in Stride Studio. Equipped with a longlasting battery and IP68 rating, the Icon is the versatile solution for low to high activity users.






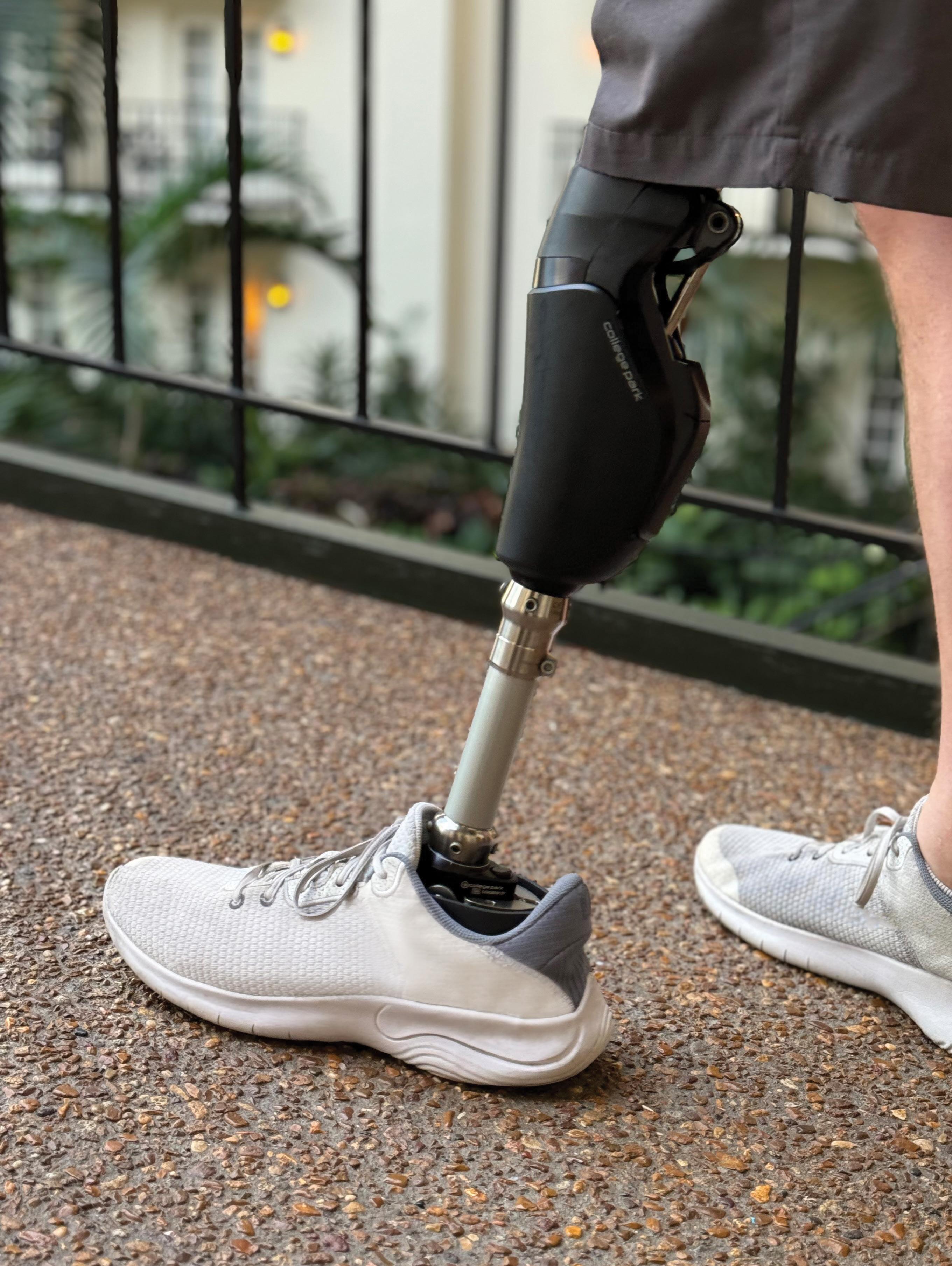


The new Odyssey® iQ is a hydraulically dampened microprocessor foot that is lightweight and incredibly low-profile. With an IP67 rating, long-lasting battery, and fast responses, it accommodates a wide range of users and activities. It also features simple setup and a unique method of spring-assisted dorsiflexion. Patients and clinicians can access valuable functions in College Park’s Stride Studio app.




Orfit Industries America Booth: 1326 orfit.com
OrthoFeet Inc. Booth: 1131 orthofeet.shoes
Orthomerica Products Inc. Booth: 1511 orthomerica.com
n OrthoPediatrics Specialty Bracing Booth: 1141 opsb.com
Orthotic & Prosthetic Group of America (OPGA) Booth: 1119 vgm.com/communities/opga
Orthotic and Prosthetic Equipment Corp. Booth: 831 optable.com
Orthotic Holdings OHI Booth: 822 ohi.net
OssKin Ortho Inc. Booth: 801 www.osskin.com
n PowerStep Booth: 1625 powerstep.com
n Propet Footwear Booth: 710 propetfootwear.com
Pro-Tech Orthopedics Booth: 1515 protech-intl.com
PROTEOR Booth: 902 & 903 us.proteor.com
n Proxy Health Partners Booth: 711 proxyhealthpartners.com
Prudential Billing & Consulting Booth: 1532 prudentialbilling.org
PSYONIC Inc. Booth: 1028 psyonic.io
Össur Americas Inc. Booth: 1203 ossur.com/en-us
Ottobock Booth: 1601 ottobock.com
Paceline, Inc. Booth: 1519 paceline.com
Pacific Medical Booth: 1333 topshelfortho.com
Pedorthic Footcare Association Booth: 927 pedorthics.org
PEL LLC Booth: 919 pelsupply.com
Phoenix Molded Shoes, Inserts, and Braces Booth: 715 phoenixmolded.com
Point Designs Booth: 1030 pointdesigns.com
Qwadra by Eqwal Booth: 1501 qwadra.com
Radii Devices Ltd. Booth: 1242 radiidevices.com
Renia GmbH Booth: 1129 renia.com
n Revopoint Global Inc. Booth: 929 revopoint3d.com
RND Softech Booth: 818 rndsoftech.net
n Roadrunnerfoot Engineering srl Booth: 821 roadrunnerfoot.com
Roboticom Booth: 1627 roboticom.us
n RockyTech Ltd. Booth: 945 rockytechs.com
Sensor Medica Corp. Booth: 1409 sensormedica.us
Join Fabtech Systems at AOPA 2025 for Exclusive Training and Education Sessions.
Join Fabtech Systems at AOPA 2025 for Exclusive Training and Education Sessions.
Join Fabtech Systems at AOPA 2025 for Exclusive Training and Education Sessions.
Learn about our products and techniques from the people that invented them.
Learn about our products and techniques from the people that invented them.
Learn about our products and techniques from the people that invented them.





Personalized Performance



Personalized Performance






Creating Bespoke Adaptive Sports Equipment
Personalized Performance
Creating Bespoke Adaptive Sports Equipment
Creating Bespoke Adaptive Sports Equipment
Thursday September 4, 2025 | 4:00 pm – 5:30 PM
Thursday September 4, 2025 | 4:00 pm – 5:30 PM
Thursday September 4, 2025 | 4:00 pm – 5:30 PM
The Role of Iterative Design and 3D Printing in O&P
The Role of Iterative Design and 3D Printing in O&P
Session: 3D Printing in O&P: From Materials to Modern Workflows
Session: 3D Printing in O&P: From Materials to Modern Workflows
Friday September 5, 2025 | 4:00 PM – 4:25 PM
The Role of Iterative Design and 3D Printing in O&P Session: 3D Printing in O&P: From Materials to Modern Workflows
Friday September 5, 2025 | 4:00 PM – 4:25 PM
Friday September 5, 2025 | 4:00 PM – 4:25 PM












The Science of Offloading


The Science of Offloading
The Science of Offloading
Justifying and Implementing Volume Management in Advanced Lower Limb Orthotics
Justifying and Implementing Volume Management in Advanced Lower Limb Orthotics
Justifying and Implementing Volume Management in Advanced Lower Limb Orthotics
Saturday September 6, 2025 | 9:15 AM – 10:45 AM


Saturday September 6, 2025 | 9:15 AM – 10:45 AM
Saturday September 6, 2025 | 9:15 AM – 10:45 AM



Transforming Practice: The Science and Application of Advanced AFO Design Principles
Transforming Practice: The Science and Application of Advanced AFO Design Principles
Transforming Practice: The Science and Application of Advanced AFO Design Principles
Discover why AFO stiffness plays a critical role in patient outcomes—and how to harness it for more effective orthotic care.
Discover why AFO stiffness plays a critical role in patient outcomes—and how to harness it for more effective orthotic care.
Discover why AFO stiffness plays a critical role in patient outcomes—and how to harness it for more effective orthotic care.
Saturday September 6, 2025 | 1:00 PM – 5:00 PM








Saturday September 6, 2025 | 1:00 PM – 5:00 PM
Saturday September 6, 2025 | 1:00 PM – 5:00 PM
Ask Us About Our Products!
Ask Us About Our Products!
Ask Us About Our Products!


Our team will be available to provide insights into our cutting-edge Reaktiv™ brace, PDE™ technology, Pluseries™ adhesives, and Restech™ epoxy resin. This is a great opportunity to see how our solutions can improve lives and make more possible.
Our team will be available to provide insights into our cutting-edge Reaktiv™ brace, PDE™ technology, Pluseries™ adhesives, and Restech™ epoxy resin. This is a great opportunity to see how our solutions can improve lives and make more possible.
Our team will be available to provide insights into our cutting-edge Reaktiv™ brace, PDE™ technology, Pluseries™ adhesives, and Restech™ epoxy resin. This is a great opportunity to see how our solutions can improve lives and make more possible.

SHINING 3D Technology Inc. Booth: 1431 shining3d.com
n Simple Medical Products Inc. Booth: 1244 simplemp.com
n Slingshot Bionics Booth: 1400 SlingshotBionics.com
Solvedge Inc. Booth: 1145 solvedge.com
n Specularis Medical Booth: 930 specularismedical.com

GOLD SPONSOR
Spinal Technology LLC by Eqwal Booth: 1501 spinaltech.com
SPRINGER AKTIV AG Booth: 1618 springer.one
SPS Booth: 1211 spsco.com

Thrive Orthopedics Booth: 833 thriveorthopedics.com
ST&G USA Corp. Booth: 1419 stngco.com
n Staffingly Inc. Booth: 722 staffingly.com
Steeper USA by Eqwal Booth: 1501 steeperusa.com
Structure Booth: 1442 structure.io
Surestep Booth: 1311 surestep.net
Tamarack Habilitation Technologies Booth: 1023 tamarackhti.com
The International Institute of Orthotics and Prosthetics Booth: 1017 iiop.edu
The Orthotics and Prosthetics Foundation for Education and Research Booth: 1128 oandpfoundation.org/home
Thermo-Ply Inc. Booth: 808 thermoplygel.com
Thuasne USA/Townsend Design Booth: 1402 thuasneusa.com
Tillges Technologies Booth: 918 teamtillges.com/technologies
TriOx Innovations Booth: 1245 cleanwithross.com
n Turtlebrace Booth: 1038 turtlebrace.com
n Vessl Prosthetics Inc. Booth: 1240 vesslpro.com
n WCBL Fabrication Booth: 932 wcblfab.com
WillowWood Global Booth: 1215 willowwood.com
Xiamen Kon Technology Co. Ltd. Booth: 827 aideastep.com





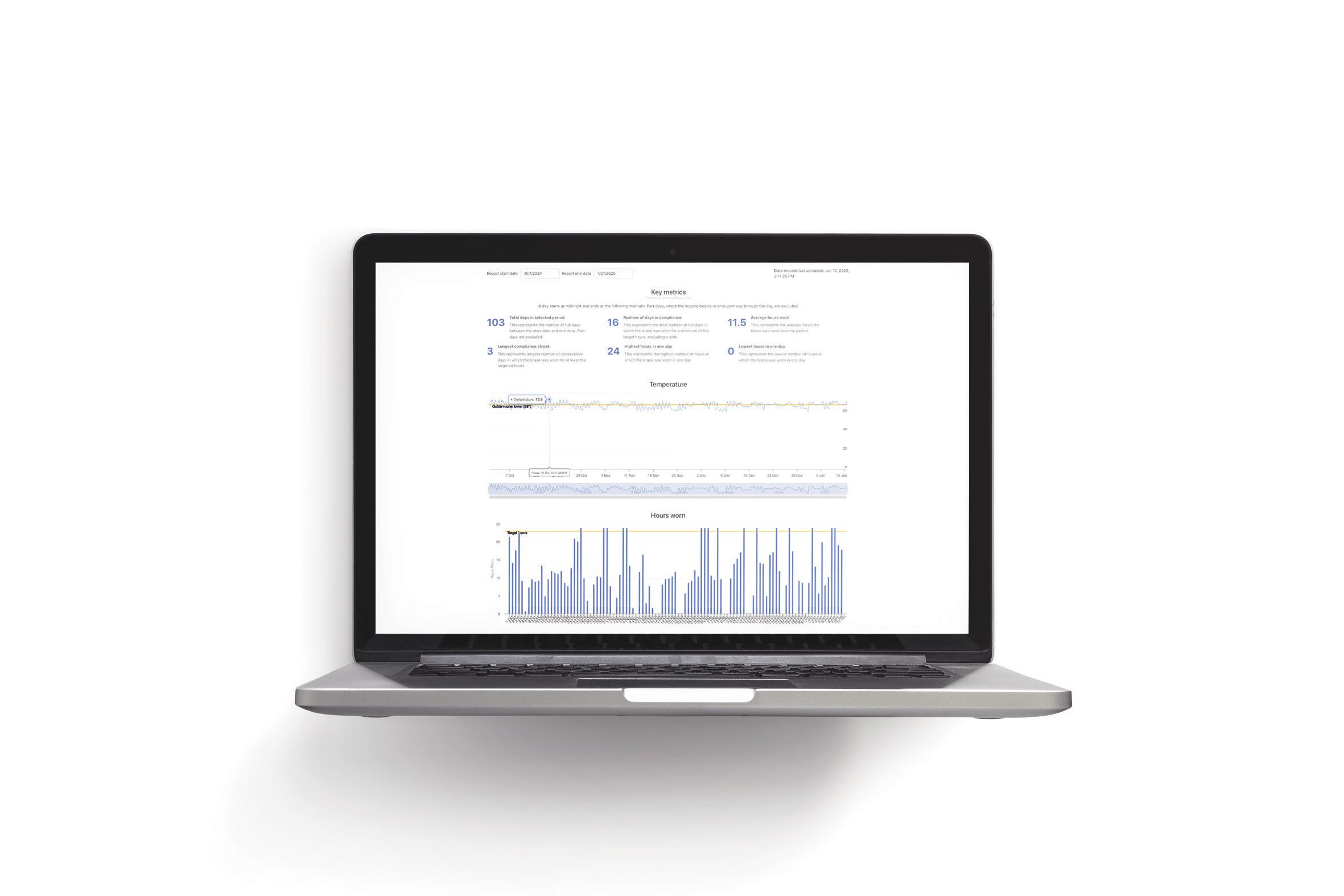




The Fresh Faces column introduces readers to prominent O&P professionals who are making an impact with their contributions to the orthotics and prosthetics profession. This month, we speak with Louis-Philippe Broze, co-founder and CEO of Spentys.
Louis-Philippe Broze is co-founder and CEO of Spentys, a company that offers software to automate key steps of the custom-made orthosis design process. Broze and co-founder
Florian de Boeck were childhood friends and budding entrepreneurs who launched the company in Forest, Belgium, seven years ago—before they had even finished earning their undergraduate degrees.

O&P Almanac: How did you get involved in O&P?
Louis-Philippe Broze: When we started Spentys, my co-founder Florian and I didn’t set out to revolutionize the O&P field. In fact, we didn’t even know certified prosthetists and orthotists existed. Coming from engineering backgrounds, what we did know was 3D technology, especially 3D printing, and we were passionate about exploring how we could use it to make a real impact in a specific industry.
O&P Almanac: How did Spentys get started?
Broze: Initially, we envisioned Spentys as a solution for trauma care. We thought 3D-printed orthopedic casts could replace traditional plaster casting in emergency departments. But we quickly discovered that the emergency care environment wasn’t ideal. 3D printing was too slow, and workflows too rigid to adopt such a disruptive change.
However, those early conversations with hospitals and surgeons opened our eyes to a broader world: O&P. It was an entire industry still heavily reliant on manual techniques, even for highly custom and complex solutions. That’s when it clicked— this is where we realized we could make a meaningful difference, and where our passion for O&P was born.
At its core, Spentys is a digital partner for the O&P profession. We provide an end-to-end software platform that enables clinicians to integrate 3D scanning, modeling, and printing into their daily workflow. Our goal is to be the bridge between clinical expertise and the power of digital manufacturing. Whether CPOs are creating a mold for carving or designing a fully 3D-printed ankle-foot orthosis (AFO), our tools make it possible to do so with more speed, customization, and consistency, without losing control over the clinical decision-making process.

A technician uses Spentys software to design a foot mold.

And that part is key: We believe automation should serve the clinician, not replace them. That’s why our software is built to automate repetitive, nonmedical steps, while still giving full control over the parts that require medical expertise.
O&P Almanac: How did you break into the U.S. market?
Broze: Expanding into the U.S. market was a major milestone for us. We knew we needed someone who understood both the clinical world and the cultural nuances of the American O&P landscape. That’s when we found Bryan Craft, a young, passionate O&P professional who shared our excitement for innovation.

Prosthetic restorations include:
Traditional prostheses have been fabricated primarily to restore function with little emphasis on the aesthetic appearance. ARTech's natural looking restorations are virtually undetectable.

Custom fitted for the ultimate patient comfort.
AMPUTEE RESTORATION TECHNOLOGIES
Custom fitted for the ultimate patient comfort.
• Custom fitted for the ultimate patient comfort.
• Custom sculpted, in great detail, to the mirror image of the sound extremity.
Custom sculpted, in great detail, to the mirror image of the sound extremity.
• Custom painted to match the sound extremity including veins, freckles, age spots, hair, scars, etc.
Custom sculpted, in great detail, to the mirror image of the sound extremity.
A.E. and B.E. Passive and Myo Coverings, Total and Partial Hands, Fingers, A.K. and B.K. Coverings, Total and Partial Feet, Toes, Ears and Noses.
Office: (888) 775-5501 Fax: (972) 775-2499
Traditional prostheses have been fabricated primarily to restore function with little emphasis on the aesthetic appearance. ARTech's natural looking restorations are virtually undetectable.
www.artechlab-prosthetics.com
ARTech silicone prostheses can improve gait and posture, ease lower back pain, relieve pressure on bone spurs and other sensitive areas, protect the tissue from further injury, improve the operation of myoelectric hands, etc.
ARTech silicone prostheses can improve gait and posture, ease lower back pain, relieve pressure on bone spurs and other sensitive areas, protect the tissue from further injury, improve the operation of myoelectric hands, etc.


We reached out to him cold via LinkedIn, and it turned out to be one of the best decisions we’ve made. Bryan helped us identify the right industry events, connect with organizations like AOPA to partner with, and navigate the U.S. market authentically. We’re still a young company, and a young team, but we’re committed to learning, listening, and building solutions that matter.
O&P Almanac: What are your current professional responsibilities?
Broze: As CEO, my role spans everything from strategy to sales to product vision. I work hand-in-hand with Florian, my co-founder, who leads our product and technology development. What I love most about my job is its diversity, no two days are alike. But what motivates me the most is seeing the innovations we’ve developed actually used in clinics, improving workflows, and ultimately enhancing patient outcomes. That’s when all the long hours and tough decisions feel truly worth it. What also drives me is the opportunity to go into schools and talk to students about digital technologies in the O&P sector. Being able to teach, share insights, and inspire the next generation of clinicians and innovators is something I find deeply rewarding. Supporting their growth and helping them see the possibilities of what’s ahead is just as important to me as building great tools, because they are the future of this profession.
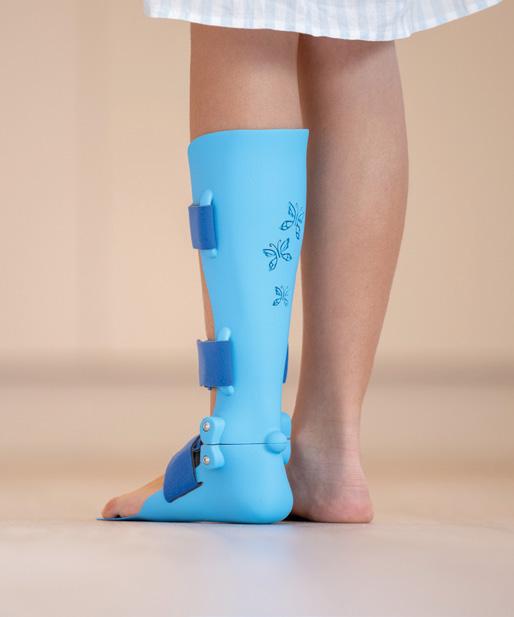
An active pediatric patient is fit with an AFO, developed through 3D modeling, which supports the child throughout daily movements.
O&P Almanac: Why do you believe that new technologies are critical to a bright future for O&P?
Broze: I firmly believe that new technologies are essential for the future of O&P. The industry faces significant challenges: rising labor and material costs, stagnant reimbursement rates, and growing patient demand, particularly with the increase in chronic conditions. At the same time, there’s

a shortage of skilled labor, which makes it harder for clinics to scale.
I believe digital tools can help bridge these gaps, making clinics more efficient, training faster, and ultimately allowing professionals to spend more time with patients instead of on production or admin tasks.
O&P Almanac: Which new technologies are having the greatest impact?
Broze: The emergence of sensor-enabled devices is game-changing. They allow clinicians to collect real-time data, like pressure maps inside a socket that can guide adjustments more precisely than ever before. This kind of objectivity improves decision making and leads to better-fitting, more comfortable devices for patients. It’s an added layer of insight that complements, rather than replaces, clinical intuition.
Looking ahead, I think technologies like artificial intelligence (AI), augmented reality (AR), and predictive modeling will further empower the profession. Imagine a scoliosis brace that not only visualizes correction in 3D but predicts long-term impact based on patient data. Or AR tools that help new practitioners learn complex fitting techniques interactively. AI may not be fully ready to deliver these capabilities today, but the groundwork is being laid, and the potential is massive.
Ultimately, my vision is an O&P industry that embraces change, not for the sake of novelty, but to solve real, pressing challenges. I often say that the biggest threat to our field isn’t technological, it’s our own reluctance to leave our comfort zones. Innovation can be uncomfortable, but it’s also where the greatest opportunities lie. By being open to new tools and ideas, we can ensure CPOs continue to thrive, and that patients receive the best care possible.
O&P Almanac: What else would you like to share with O&P Almanac readers?
Broze: I invite you to reach out, let’s start a conversation. Because if there’s one thing I’ve learned, it’s that the future of O&P isn’t built alone. It’s built together.



Colorado prosthetist collaborates with active patient to create custom cycling foot to conquer mountain trails safely

The Transformations column features the success story of an O&P clinician who has worked with an inspiring or challenging patient. This issue, we speak with Alex Miller, CPO, MSPO, who developed a specialized cycling foot prosthesis for a lifelong amputee and advocate who inspires others.
Sometimes innovation is a team sport. When Alex Miller, CPO, MSPO, set out to create a cycling foot for Angelina Martinson, the patient’s input and ideas from Instagram led to a custom device that would transform her mountain biking experience— and potentially inspire countless others.
For Miller, who works at Evergreen Prosthetics & Orthotics, the journey to becoming a clinician began in his father's lab in Belpre, Ohio. He started working there at 16, initially cleaning up but gradually learning the craft. “My father, Mark Miller, who's a prosthetist/orthotist, has been making legs my whole life, so I decided to follow in his footsteps,” Miller recalls.
While earning his undergraduate degree in biological sciences with a biomedical focus from the University of Cincinnati, Miller worked at Rocco Prosthetics & Orthotics, gaining invaluable experience with a wide variety of patients. “I was just kind of a lab rat, making whatever anyone needed," he says. "We're making foot orthotics, we’re making custom spinal braces, obviously custom prosthetics, and I would dip my hand in all those cookie jars.” He also assisted practitioners with castings, fittings, and evaluations.
After graduation, Miller worked as a lab manager for his father. Then he moved to Tampa to complete his Master of Science in Orthotics and Prosthetics from the International Institute of


Orthotics and Prosthetics and a combined residency at Superior Prosthetics & Orthotics. In 2022, he moved to Colorado, where he now works at Evergreen P&O. He shares the active lifestyle of his Colorado patients and enjoys “messing around” with devices to optimize performance for a specific activity.
Martinson’s path to Miller’s clinic was shaped by years of searching for the right prosthetic partnership. Born in Russia with clubfoot and tibial hemimelia in her right leg, she was adopted by an American family and underwent amputation at age 4-and-a-half at Shriners Children’s Hospital in St. Louis.
At Shriners, collaborative care was the norm. “They really encouraged me to be a part of my device design. I would go back to the lab with them and help build my own prosthetic,” Martinson recalls. This approach instilled a lifelong expectation that prosthetic care should be creative and possibility focused.
However, transitioning to adult care was more difficult. After moving across five states in five years, Martinson struggled to find clinics that matched Shriners’ can-do spirit. When she settled in Colorado three years ago, many clinics effectively
dismissed her goals either for insurance reasons or because her “very short” below-knee amputation with no tibia was challenging to fit.
Evergreen Prosthetics & Orthotics proved different. Miller and his colleagues welcomed Martinson’s challenges and viewed fitting her unique anatomy as a problem to solve rather than a limitation to accept.
Martinson had been cycling for years, despite significant challenges with pedal connection. “I’ve always been a bike rider, and I just trust that my foot is on the pedal—and half the time it's slipped off, and that’s very dangerous,” she explains. When she decided to tackle mountain biking two years ago, the safety stakes increased. Traditional clip-in pedals were “really scary to get out of quick enough when you don't have that kind of flexion in your ankle,” she says. Flat pedals weren’t much better—her everyday prosthetic foot, while excellent for its energy return properties, felt too soft for secure pedal contact during aggressive cornering and riding.
A potential solution emerged when a company sent Martinson, who has a degree in biomedical engineering and

experience working in an O&P lab, magnetic pedals designed to work with magnets embedded in shoes: “I was wondering if there was any way we could eliminate the shoe portion of it and just have the magnet in the foot itself,” she recalls.
Social media played a crucial role in inspiring the initial design. Miller had been following another amputee, Meg Fisher, on Instagram, who had created her own cycling-specific prosthetic foot. That, combined with Miller’s fabrication skills, started the iterative design process. With salvaged components from old SACH feet, Miller created the initial prototype using basic materials: “Instead of having a cushion heel, I took a piece of wood and screwed the male pyramid adapter onto it,” placing the magnet on the midfoot and attaching the pyramid adapter to a pylon, he says. “It was as simple as you could get.”

The first version, modeled after Fisher's shorter foot design, “didn't have enough push on the toe,” Miller recalls. The next versions were just as straightforward: “Same thing, took another piece of wood, shaved it down to the shape I wanted, and then put the magnet out further on the toe so she has a little more leverage," he says. Once they achieved the optimal length, magnet placement, and neutral alignment, Miller reinforced the prototype for longterm durability. “Locked, tightened, and torqued all the screws and wrapped the
whole thing in carbon fiber and laminated it,” he explains. Per Martinson’s request, Miller finished the final in “bright pink with sparkles.”
The entire development process took four or five appointments over two to three months, with a brief interruption for international travel. Miller’s approach emphasized starting with functional prototypes and refining through real-world testing on the bike in the office and at home.
The custom cycling foot has transformed Martinson’s mountain biking experience
both physically and psychologically. “I feel like I can feel the pedal actually, which had never been the case for me,” she says. “Having that sensation that I am connected to the pedal and feeling it when I’m pressing my foot into it, gives me a lot more confidence on my rides, makes me feel a lot more secure.” Her cycling skills also are improving.
Martinson’s own advocacy work through Adaptive Amputees, which she started in high school, continues this cycle of inspiration. Growing up, she rarely saw active amputee role models, she recalls. Her platform encourages other amputees to pursue their goals and shows prostheses as tools for daily life. Miller encourages other individuals with limb loss to “look her up, see what she's doing” in the hope that others will be motivated to ask their prosthetists for solutions to facilitate their favorite activities.
“I want to be a resource for these amputees and tell them, ‘Get up off the couch. Don’t make excuses,’” says Martinson. “You can be on the soccer team; you can play basketball. I’m not good at any of the things that I do, but I really enjoy them.”
The success of Miller’s and Martinson’s collaboration stems from aligned values and shared goals. Their partnership exemplifies how the right combination of technical skill, creative thinking, and patient input can transform possibility into meaningful patient care. Miller reminds clinicians who work with active patients that sometimes simple solutions can work—and to not be discouraged by financial realities of activity devices: “We’ve got to keep the lights on, pay everyone’s paychecks, but don’t let that limit your patients.”
The custom bright pink, sparkly foot that now connects Martinson securely to her pedals is a testament to the power of refusing to accept limitations and the importance of finding clinical partners who share that philosophy.
FACILITY:
EastPoint Prosthetics & Orthotics



“I think it’s important to be engaged with groups that have a focus on O&P to increase our awareness of issues in the field. We get that educational arm from AOPA, which allows us to be more informed and have a backing body as well.”
—Chris Baughman, CP, BOCO
OWNER: Paul Sugg, CPO, CPed, FAAOP
LOCATIONS:
Kinston, Raleigh, and Wilmington, North Carolina
IHISTORY: 17 years
t only took a few years of operating a brick-andmortar O&P clinic for Paul Sugg, CPO, CPed, FAAOP, to realize the benefits of taking his show on the road.
Sugg opened EastPoint Prosthetics & Orthotics in 2008 in Kinston, North Carolina, providing customized and prefabricated devices to help patients with mobility limitations. He partnered with Brent Wright, CP, BOCO, in 2011, expanding on Wright’s presence in Raleigh and incorporating his interests in emerging fabrication technologies and mobile services.

The facility’s onsite printing capabilities have equipped the practice to increase its volume of orthotic work while improving efficiency and controlling costs and turnaround times. EastPoint now manufactures about 50% of its prosthetic sockets via 3D printing. According to Baughman, these emerging technologies allow for greater customization of devices and provide new avenues for creative solutions to meet patients’ needs, helping them achieve better mobility and stability.
Today, with 26 employees and a fleet of 14 Ford Transit vans fully equipped with all the specialty tooling required for a multitude of O&P tasks, the practice extends its reach far beyond its Kinston and Raleigh hubs. “We basically cover the whole of eastern North Carolina, from Virginia down to South Carolina, because we’re mobile and can travel,” says Clinical Director Chris Baughman, CP, BOCO. “About 75% of what we do is on the road and 25% in the office.”
The facility provides a full spectrum of prosthetic and orthotic care, treating patients of all ages and providing services in skilled nursing facilities, rehab centers, hospitals, and patients’ own homes.
Whether it’s replacing straps, grinding sockets, or modifying devices, “probably 70% to 80% of our lab work can be done on the road,” Baughman says. “It just allows us to be a little bit more reactive to the patient when we’re there—instead of having to bring something back to the lab or the office.”
EastPoint’s mobile units are just one way they work to provide quick, nimble service. They’ve also expanded their manufacturing capabilities with new 3D printing options, under the guidance of Wright, an early adopter and innovator in the realm of additive manufacturing. EastPoint’s Kinston office houses high-quality 3D printers, including multijet fusion, selective laser sintering, and other additive manufacturing technologies that are known for producing strong, durable parts and allowing for fabrication of flexible materials.
The team recently devised a custom solution for one patient—a tow-truck driver—who struggled to find the right devices following bilateral transtibial amputation that left him with short residual limbs requiring a suction suspension system for his prostheses. Because the patient is constantly getting in and out of his vehicle, on his knees changing tires, or crawling under vehicles to perform maintenance, he tore through two or three suspension sleeves every few weeks until the EastPoint design team experimented with ways to 3D-print a new flexible inner socket that was lightweight, cool, and durable—and didn’t pinch his suspension sleeve. He now does his job with less wear-and-tear from the device and is getting two to five months from each suspension sleeve.
“It’s pretty cool to see things like that,” Baughman says. “We're always able to push those limits, run it up the flagpole with the design team—and nine out of 10 times, we can think of what we can do outside traditional fabrication to come up with a solution.”
EastPoint’s success in mobile treatment and 3D printing facilitates the company’s priority to bring O&P care to individuals in underserved areas. Both Sugg and Wright established nonprofits, and EastPoint staffers regularly travel to Africa and Guatemala to provide free prostheses and orthoses to patients through the work of LifeNabled and EP Legacy Inc. Desiring to serve their community at home as well, EastPoint hosts local high school students in its Kinston office each year to spotlight O&P career opportunities and provide a glimpse of what patient care looks like through hands-on demonstrations.
Jennifer Davis is a contributing freelance writer for O&P Almanac. Deborah Conn contributed to this article.
BY DEBORAH CONN
OWNER: Ted McDonald



FabCo specializes in high-performance lower- and upper-extremity prostheses, usually designed for highly active individuals.
Am I
“In this field, AOPA is the leader in knowing where the industry is going. We always try to stay up on developing technology and techniques, and AOPA helps us do that.”
—Aaron
Hyndman
FLOCATION:
Nashville, Tennessee
HISTORY: 14 years
abCo Prosthetic Designs began as a central fabrication facility for several O&P clinics under the umbrella of Amputee Associates, a network of providers working with surgeons. In 2020, the facility changed its name to FabCo Prosthetic Designs and became a separate entity offering central fabrication services to prosthetists throughout the country.
The company specializes in high-performance lower- and upper-extremity prostheses. Its products are intended for highly active individuals who are looking for sport-specific devices, such as prostheses designed for running, basketball, cross training, snowboarding, and other activities. Early on, FabCo partnered with Fillauer as a testing site for developing posterior mounting feet, which are used for these athletic purposes.
“Our goal is to help as many people as we possibly can and to find each one the best prosthesis possible,” says Aaron Hyndman, general manager. “The company started with three people, including the owner [Ted McDonald, chief executive officer of Amputee Associates], and we intend to continue to grow and push for better outcomes and excellent communication between prosthetists and technicians.”
Today, FabCo’s team includes 11 technicians and two administrative staffers. The business does not have a sales department, but instead gains new customers through word of mouth and by attending AOPA Assemblies and industry trade shows.
According to Hyndman, about 70% of FabCo’s jobs are fulfilled through computer-aided design and manufacturing, with many customers submitting digital scans through the company’s online portal that are sent on to automated carvers. FabCo can accommodate more traditional methods as well; about a third of the company’s customers mail in positive models. “We prefer CAD/CAM, because it cuts down on turnaround time,” Hyndman says, “and we expect that we’ll do even more digital formatting as the industry moves in that direction.”
One of FabCo’s partners is Click Medical, which offers adjustable below-knee prosthetic sockets. “We are about to roll out an order form specific to Click
devices on our website,” says Hyndman. “We can showcase all the different socket designs and make the ordering process as easy as possible.”
As the industry evolves technologically, FabCo is poised to take advantage of new developments. “We have three biomechanical engineers who work here, who follow where the industry is going and where we want to position ourselves,” says Hyndman. “There will always be a place for traditional prosthetic fabrication, but less so in this new technological era.” FabCo has 3D printers on site. “We are doing a lot more on the engineering side of it at this point, formatting and prepping the print,” he notes. “The printers are very expensive; that’s one thing holding back the technology. Another problem currently with 3D printing is overall durability. The materials are catching up quickly, but heavy activity patients require additional rigidity to accommodate their lifestyle. When it’s suitable, we’ll jump in.”
As part of its community involvement, FabCo works with Amputee BladeRunners (ABR), a Nashville-based nonprofit that provides free running and sportsspecific prostheses to individuals with limb loss, both adult and children. “We are the sole providers of their fabrication,” says Hyndman. “ABR patients are allowed to come to our facility and assist in the fabrication of their own prostheses, eliminating the wonder of how it’s made or why it’s designed like this.”
Hyndman sees FabCo as unique among central fabrication facilities in the quality of its communication with prosthetists. “We are one of the only places you can actually talk to the technician who is fabricating the device,” he says. “And that technician … is extremely skilled, with years of experience. We try to keep things as straightforward and painless as possible, to really understand what the prosthetist is trying to achieve with the patient. We try to be very open and leave no unanswered questions. As a result, both patients and prosthetists are satisfied.”
Deborah Conn is a contributing writer to O&P Almanac. Reach her at deborahconn@verizon.net


Designed to provide fast and reliable access to the latest HCPCS codes for orthoses, shoes, prostheses, and modifiers, this is a must-have, essential resource for streamlining your billing and coding process. The popular Quick Coder comes laminated so it is durable, long-lasting, and easy to store.
The 2025 Quick Coder includes the new codes that are active as of April 1. Price is $45 for AOPA members and $95 for nonmembers.
Secure yours today: aopahub.aopanet.org/merchandise.




























Sept. 4, 6:30 - 9 p.m.
Join us for an unforgettable night at AOPA's 2025 National Assembly in Orlando to support SEBCM. The evening will be filled with motivation, celebration, and connection. We'll reflect on a year of wins, hear from powerful speakers, honor the incredible community driving SEBCM, and spark fresh energy for what's next. Proceeds from ticket sales directly fuel SEBCM 's mission, supporting grassroots advocates and legislative action.
Buy tickets or donate at charity.pledgeit.org/2025ActivationCelebration

The Orthotic/Prosthetic Assistant, under the supervision of an ABC Certified Prosthetist/Orthotist, may perform procedures and related tasks in the management of patient care. The Assistant may also fabricate, repair and maintain orthoses/prostheses.
ABC Orthotist/Prosthetist
Assistant Certification
Communicate effectively with patients and care team


Handle documentation, fitting and adjustments for increased practice efficiency.
Assist with performing outcome measures to streamline workflows and reduce practitioner workloads. Ensure accurate and complete documentation crucial for audits and maintaining compliance. Help improve efficiency to allow practice to see more patients and reduce wait times.
If you’re looking to reduce workloads and increase efficiency while improving patient care and growing your business, it may be time to hire a care extender like an ABC Certified Orthotic/Prosthetic Assistant.
SCAN TO LEARN MORE:




The officers and directors of the American Orthotic & Prosthetic Association (AOPA) are pleased to present these applicants for membership. Each company will become an official member of AOPA if, within 30 days of publication, no objections are made regarding the company’s ability to meet the qualifications and requirements of membership.
A Custom Fit iNMotion LLC
757 Harbor Island Clearwater, FL 33767 863-473-1673
acustomfitinmotion.com
Mitchell Pantelides Supplier Startup
Anclote Manufacturing Co. 12580 Enterprise Blvd., Ste. A Largo, FL 33773
727-400-4807
anclote.net
Kevin McLoone Supplier Startup
Certified Orthopedics Inc.
2415 E. Mulberry Street
Fort Collins, CO, 80524
970-482-7119
certifiedortho.com
Casey Bradshaw
Supplier Startup
Charles King Consulting 52 Marion Street Cumberland, MD 21502
301-697-3052
Charles King
Supplier Consulting
Coretech Orthopedics by Vive Health
8955 Fontana Del Sol Way Naples, FL 34109-4428
239-220-5772
coretechortho.com
Juanita Gonzalez
Supplier Startup
Golden Isles Prosthetics & Orthotics
2414 Parkwood Drive Brunswick, GA 31520 912-265-2810
goldenislespando.com
Avery Stickland, CPO Patient-Care Facility
Levitate Technology
Marielumdvej 28, St. TV Herlev, 84, 2730
Denmark +45 4524658992 letslevitate.com
Lasse Werner Madsen Supplier Startup
MH3 Prosthetics
55 E. 124th Street New York, NY 10023
917-261-2830
mh3prosthetics.com
Mac Hanger, CP Patient-Care Facility
Simple Medical Products Inc. 2000 Windy Terrace, Ste. 6C Cedar Park, TX 78613 512-956-9044
simplemp.com
Erica Meyers Supplier Startup
Slingshot Bionics
220 SE 4th Street, Ste. 400 Oklahoma City, OK 73129 405-850-2069
slingshotbionics.com
Jay Martin CP, LP, FAAOP Supplier Startup
Xiamen Kon Technology Co. Ltd.
361006 Haitian Road, Huli District, 35 Fuijan, China +86 18106091282
aideastep.com
Ying Chen Supplier Startup

Enhance your skills at Allard’s Technical Workshops and Product Preview Theatres during AOPA 2025! All presentations are ABC/BOC CEU-approved.
Find out more in our Tradeshow Calendar!



Embrace the comfort of custom-fit with Mt Emey®’s Custom Shoe (Medical) Program. Our precise 3D scanning captures your unique foot contours, promising a perfect fit without the wait or waste. From stylish athletics to roomy comfort designs, our handcrafted shoes adapt to your needs. Satisfaction guaranteed before payment. Plus, qualified wholesale accounts receive a free 3D scanner. Step into the Mt Emey difference— where every shoe is made for you.









Navigate the bumpy road of life with comfort. The Terrain foot was created to achieve anatomical ankle motion and high energy return in a low-profile design. College Park uses its tri-axial technology expertise in a unique way. Versatile and service-free, the Terrain foot features a carbon fiber base and permanent bumpers inside the ankle housing for a progressive, comfortable response during low to high impact activities.
For more information, call 1-888-937-2747 or visit emeys.com
Learn more: www.college-park.com/terrain

Coyote’s Dynamic Strut was designed with varying thicknesses to flex and move, helping the patient navigate real world conditions. The strut excels at creating a comfortable natural gait for daily use, walking, working, hiking, biking, and golfing. It works great with a thermoformed orthosis and provides the needed energy response.
• Comfortable natural gait
• Works great with thermoformed AFOs
• Provides energy response
• New smaller size available.
For more information, contact Coyote at 208-429-0026 or visit www.coyote.us

The Motion Arm is a sleek, low-profile hybrid elbow with a highcapacity 3000-mAhr internal battery to power the hand longer throughout the day. Designed to accommodate higher loads, the arm supports up to 50 ft·lb in the locked position. Paired with the TASKA hand, this ultimate combination has an IP67 waterproof rating and unbeatable performance. Visit fillauer.com to find out more.

Download the Hersco app on your iPhone and connect to the lab anytime. The Hersco app and order portal makes scanning and ordering seamless. Scan patients for foot orthotics, Richie braces, and ankle gauntlets, and submit at your convenience. Our digital workflow allows you to easily track jobs, re-order devices, and manage your account. Download the app and get your username and password.
Our 3D printers produce accurate and precise orthotics, reducing landfill waste by over 90%!
Email us today at fastorthotics@hersco.com or call 800- 301-8275 to get a free product sample. Visit hersco.com





Spinal Technology is the global leader in custom spinal orthoses design and fabrication, specializing in adolescent idiopathic scoliosis. We are pleased to introduce the NEW stWear™ Compliance Monitoring System which features a sleek new design and an extended battery life. The smartphone app and web-based clinician interface improves the ability to access patient compliance data in between office visits for both practitioners and parents. The monitor button can be ordered with or without an orthosis purchase from us.
Learn more and download the stWear™ app by visiting Spinal.Tech/stWear or call 508-957-8282.




Allard USA 39 866-678-6548 allardusa.com
A large number of O&P Almanac readers view the digital issue— If you’re missing out, visit issuu.com/americanoandp to view your trusted source of everything O&P.



Scan the QR to start advertising in the O&P Almanac or visit bit.ly/AlmanacMediaKit25
ALPS South LLC 33 800-574-5426 easyliner.com
American Board for Certification 57 703-836-7114 abcop.org
Apis Footwear Co. 21 888-937-2747 apisfootwear.com
ARTech Laboratory Inc. 47 888-775-5501 artechlab-prosthetics.com
BionicM USA LLC 35 trial@bionicm.com https://bionicm.com/
Bremer Group Co. 27 800-428-2304 bremergroup.com
Board of Certification 55 877-776-2200 boc.usa.org
Coapt 23 844-262-7800 coaptengineering.com
College Park Industries 41 800-728-7950 college-park.com
Coyote Prosthetics & Orthotics 29 800-819-5980 coyote.us
Fabtech Systems 43 800-322-8324 fabtechsystems.com
Ferrier Coupler Inc. 56 810-688-4292 ferrier.coupler.com
Fillauer Companies 1 423-624-0946 fillauer.com/products/motion-foot-six/
Flo-Tech O&P Systems Inc. 9 800-356-8324 1800flo-tech.com
Hersco 11 800-301-8275 hersco.com
Naked Prosthetics 3 888-977-6693 npdevices.com
OrthoFeet Inc. C4 800-524-2485 orthofeet.shoes
Orthomerica 17 800-446-6770 orthomerica.com
Performance Laboratories 13 973-523-8610 performlab.com
Spinal Technology Inc. 45 508-957-8281 spinal.tech/almanac
TurboMed Orthotics 7 888-778-8726 turbomedorthotics.com

Monthly Events
ABC: Application Deadlines, Exams Dates, O&P Conferences, and More! Check out ABC’s Calendar of Events at abcop.org/calendar for the latest dates and event details, so you can plan ahead and be in the know. Questions? Contact us at info@abcop.org or visit abcop.org/contact-us
The Pedorthic Footcare Association: The Active Foot—From 1st Step to Last! 10-session online education program series. Approved CEs by ABC and BOC, monthly classes are 2 hours each. For more information and to register, visit pedorthics.org.
September 3–6
AOPA National Assembly. Orange County Convention Center, Orlando. For information, visit aopanet.org
October 17–18
PrimeFare Central—NEW VENUE!
Waterford Renaissance Hotel, Oklahoma City. Contact Cathie Pruitt at 901-359-3936, primecarepruitt@ gmail.com, or visit primecareop.com

For Job Seekers:
Job searching is easy with the pane-view job search page. Set up job alerts, upload your resume or create an anonymous career profile that leads employers to you.
For Employers:
Reach 4,500+ O&P professionals through the Job Flash™ email. Ensure high visibility for your open positions through this highly engaging email.
January 9–11
2026 Leadership Conference. Huntington Beach, CA. For more information, visit aopanet.org
September 9–12
AOPA National Assembly. Las Vegas, NV. For more information, visit aopanet.org Finding your next job or hire just got easier with the AOPA Career Center.
September 22–25
AOPA National Assembly. Long Beach, CA. For more information, visit aopanet.org
August 29–September 1
AOPA National Assembly. Washington, DC. For more information, visit aopanet.org




Colorado’s Medicaid Provider Rate Review Advisory Committee is hosting public quarterly meetings this year. These meetings will consider Medicaid rates for prostheses, orthoses, and disposable supplies, among other categories, and interested parties can attend and sign up to provide comments for these meetings. Several AOPA members provided comments at the first- and second-quarter public meetings.
For more information, visit colorado.gov/ rate-review-public-meetings
In late June, the Illinois Department of Healthcare and Family Services (HFS) confirmed the public notice for the 7% rate increase for 240 orthotic, prosthetic, and pedorthic custom L codes for Illinois Medicaid. This rate increase was passed in 2023 and was effective as law Jan. 1, 2025. The rate increase is available as of June 1, 2025, and the updated rates can be accessed on the Illinois HFS website.
Claims with old rates that were submitted after Jan. 1, 2025, are eligible for a retroactive updated reimbursement for the 240 listed custom L codes. As of late June, this process requires the entire initial repayment of the original claim submitted after Jan. 1, 2025, which can be difficult administratively. HFS may consider other options that have a clear process.
Minnesota Medicaid revised its Orthotics and Prosthetics Policy June 12. The updated policy includes more comprehensive criteria for Healthcare Common Procedure Coding
System codes L2005—custom-fabricated knee-ankle-foot orthoses (KAFOs) with an automatic lock and swing-phase release, and L2006—microprocessor-controlled KAFOs. To see the complete criteria, visit Minnesota Medicaid’s website.
The policy also adds new modifiers for showering and bathing orthotic devices (modifier U1) and recreational orthotic devices (modifier U2). These modifiers were already in use for prosthetic devices.
As mentioned in the May/June 2025 O&P Almanac , Assembly Bill 2520/1616, a bill that would require Medicaid managed care organizations to reimburse items that qualify as durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) at no less than 100% of the state’s Medicaid fee schedule, passed in both the Assembly and the Senate. AOPA has submitted a memorandum in support of the bill. It is expected to be delivered to the desk of Gov. Kathy Hochul for consideration in the fall.
AOPA has submitted a memorandum in support of Pennsylvania SB 537, a bill that would increase reimbursement rates and codes on the Medicaid fee schedule to match those on the Medicare fee schedule for DMEPOS items.
Gov. Greg Abbott signed House Bill 426 into law June 20. This law requires Medicaid and the Children’s Health Insurance Program to fully cover
cranial remolding orthoses for infants with plagiocephaly according to agreed-upon clinical standards.
SB 1330 also was signed into law June 20. This law will regulate billing practices for medical equipment provided to Medicare enrollees, including orthotic and prosthetic devices and supplies. It caps charges by nonparticipating suppliers at 115% of the Medicare-approved amount except when certain conditions are met, including written consent from the Medicare enrollee, and introduces penalties for violations. Similar legislation has passed in Nebraska. It does not appear that there would be a significant effect on businesses as long as an advanced beneficiary notice and proper documentation are obtained up front.
Most recently, in New Jersey, Senate Bill 1439 was signed into law July 8. New Jersey is the 12th state to pass SEBCM legislation.
So Every BODY Can Move’s 28x28 Mobility Challenge, which encouraged participants to engage in at least 28 minutes of physical activity per day from July 1-28, was an amazing success. Over 1,300 participants joined the challenge and over $160,000 was raised for So Every BODY Can Move’s continuing work.