



























1. List the properties that you observe of theiceimaginary cube. magina g
Chemical properties
Are these properties determined without changing the identity of the substance? Yes
Physical properties
Does the property depend on the amount of substance? How does the substance react to the presence of the following:
• Air
• Acid
• Base
• Water
• Other chemicals Intensive physical property
• Color
• Melting point
• Boiling point
• Density
• Shape Extensive physical property
• Mass • Volume
• Length












2. Find an object near you. Describe three physical yDescri properties of physica the object.





3. Metals are malleable and ductile. Think of an object that utilizes metals’ malleability.











Filters reflect UV radiation
4. How do propertieschemical differ from properties?physical m physica






























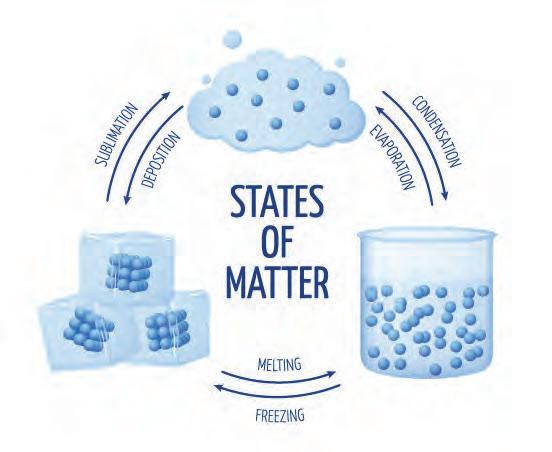
5. The ice temperaturecube’s rose, and it melted in your hand. Describe how melted the state of matter (liquid) is a physical property, not a chemical property.











Physical Properties
• Solid
• Silver color
• Metallic luster


Physical Properties
• Gas
• Pale green color
• Pungent odor
1. Why are the physical properties y of NaCl different propertie ycal prop from the propertiesphysical of Na m the physica y and Cl2?

Physical Properties
• Crystalline solid
• White color
• Salty taste
















2. How are physicaldifferentproperties prope from properties?chemical
Think about It



















































3. Why do some chemical reactions y produce changes that can be seen?

















HX (Acid) + MOH (Base) H2O + MX (Salt)
1 mole of hydrochloric acid reacts with 1 mole of sodium hydroxide to produce 1 mole of water and 1 mole of sodium chloride.
HCl + NaOH H2O + NaCl










4. How can you tell that a chemical an you te reaction has occurred if no new products are seen?
Stephanie Kwolek, Inventor of Kevlar





















Why are the properties of products different from the properties of reactants?



Physical Properties
• Solid
• Silver color
• Metallic luster

Physical Properties
• Gas
• Pale green color
• Pungent odor
5. Describe one change that can be seen as evidence of nge n be a chemical reaction and one change that cannot be seen. ne change th

Physical Properties
• Crystalline solid
• White color
• Salty taste





















1. How would you organize all the ow elements in the ganize universe, even the elements that have not been discovered yet?











Time Line of the Periodic Table




















2. Why were the ideas of early y w scientists important in forming the modern Periodic Table? ng the mode g
The same number of valence electrons indicates similar chemical properties. The number at the top (1–8) equals the number of valence electrons in the outer energy level. Number of Valence Electrons




3. State how many valence electrons tate te h each of elementsthese has: potassium, sulfur, chlorine, and argon.
Electrons and Reactivity
Electrons and Conductivity


Valence Electrons and the Periodic Table







4. How are electrons related to the chemical properties and patterns of elements on the Periodic Table?

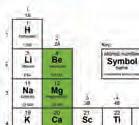
































































Principles of Organic Chemistry y Principles of Chemistry
How can we predict the properties of an unknown element?

5. How would you predict the properties of an ypredict unknown element?
























1. How do you think the atomic model w do thin changed over time?

toic heory n altons ostulates o atoic theory heled to deine the structure and nature o the ato
lha articles
hin gold oil
to nucleus
Most alha articles ass straight through ut a e are delected through ide angles hen reelled y the nucleus
hin gold oil
article ath
article source
e articles are delected through large angles e articles are delected through large angles
Circular screen that luoresces hen articles collide ieing icroscoe that is oved in a ciruclar arc
Most articles ass straight through

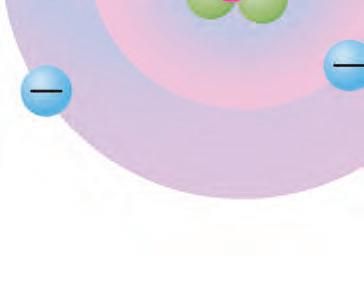


2. Do you believe that some of the models o th elieve t have limitations? If so, what limitations do you notice?




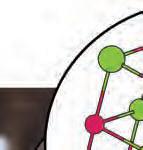
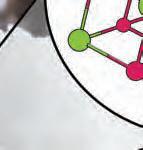
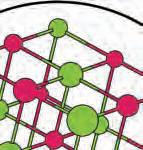
3. What are the betweendifferences the three subatomic particles?



4. What do you think causes an atom to gain or lose electrons? m
How did the atomic model change over time?








5. improvementsWhatcould be made to the current atomic model to provide a understandingbetter of the atom? standing g








































1. Why do people see color? y do pe p
Wavelength (in meters)
Size of a wavelength
Common name of wave



Sources
Frequency (waves per second)
Energy of one photon (electron volts)









2. Why wavelengthare are and frequency inverselyvelengthrelated? d que
Property
Reflection
Refraction
Polarization
Diffraction
Interference
Photoelectric effect

or particles?
3. Why are energy and frequency hy are ener y directly related? d dfrequency
Wave Model Particle Model
Compton effect



Ground state sortion o hoton cited state

4. What happens when there is a mixture of elements that are experiencing a transition from excitedexperiencing state to ground state?


















Why do people see color?

5. How can someone be color-blind?



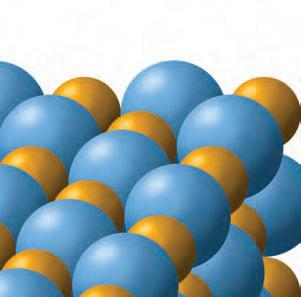
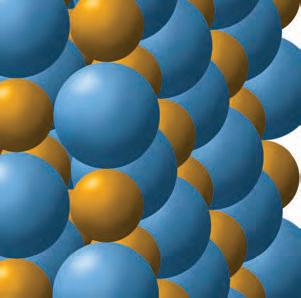


















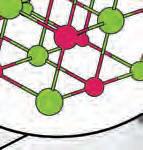
1. Why do objects stick together?object How does this attractionktogether occur?













2. What role do electrons play in bonding? trons pla s
Electron released-
Energy needed = Ionization energy
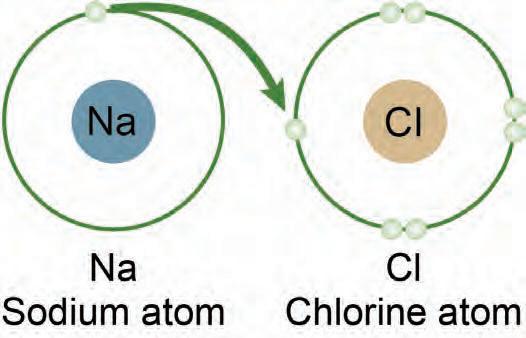

3. How does ionization energy impact chemical bonds? ergyypact p+ = 11 n0 = 12




4. What is the difference between ionization energy and electronegativity? zation z How do these two conceptselectronegativity differ when it comes to chemical bonding?




























In copper (Cu), freely moving valence electrons (red) form an electron sea around the positively charged copper ions (blue).
5. What are three types of chemical bonds? How do the ypes of che intramolecular forces impact these three different types of bonds? What ferent erent determines the type of bond in any substance? ype nd in



6. Describe what would happen in a water in terms of intermolecular forces.





































Have you felt the push or pull of two magnets?

































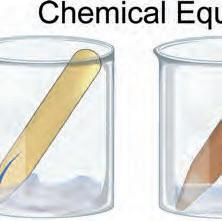
1. How would you write an equation for the production of a s’more?
A chemical equation is written by placing the reactants on the left side, the products on the right side, and a reaction arrow in the middle.




2. You conduct an experiment in which you drop a 2 g piece xperiment p of magnesium in 10 g a ydrop of hydrochloric acid, magnesium in 10 g and you notice that hydrochloric acid, ydrochloric acid the solution bubbles d notice th afterward. You weigh your solution after the fterward. d. You weig bubbling has stopped and record a mass of ubbling ling 11.75 g. Where is the remaining 0.25 g?th here is th




3. How do you observe the law of y conservation of mass in everyday life?
Synthesis
combine synthesize
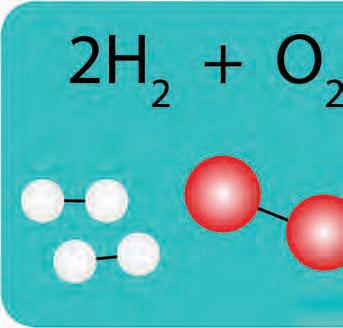


decomposes breaks down
ol o coer caronate decooses to ol coer oide and ol caron dioide gas +

y +

ol o ethane coust ith ol o oygen roducing ol o caron dioide gas and ol o ater vaor
oleeplaceet eactio
lthough there are ive general tyes o cheical reactions to tyes o doulerelaceent reactions are given ehasis hese are reciitation reactions and acidase reactions


4. What type of reaction(s) would you type expect to see in the human body?














Acid-Base Reactions
Double-Replacement Reaction: Acid-Base Reaction H Cl + Na OH Acid Base Water Salt (H+ donor) (OH- donor)






















5. Where did the name reactionprecipitation come from? n

idation and reduction alays occur together
n this cheical reaction the coound that gains an oygen e has een oidied and the coound that loses an oygen CuS has een reduced
n this cheical reaction ethane C is oidied as it urns oring oides o caron and hydrogen
n this cheical reaction agnesiu Mg is the reducing agent as it loses electrons to sulur s sulur S accets the electrons it is the oidiing agent





6. Why are double- replacement reactions redox reactions?








Connect It
How would you write an equation for making a s’more?












7. How does the structure of a chemical equation indicate the behavior of that reaction?






































1. What are some times in your daily life when es es proportions or ratios can be useful?
Molar Mass




Molar mass is the mass (in grams) of 1 mol of a substance.
The atomic mass based on the Periodic Table of Elements is equal in number to the molar mass, but the units
Molar mass is expressed as g /mol; therefore, cobalt can be said to have a molar mass of 58.93g /mol.
Use the Periodic Table Atomic Mass for Molar Mass Number

When 1 mole of CH4 reacts with 2 moles of O2, 1 mole of CO2 and 2 moles of H2O are produced.


be helpful for a Why would it ywou chemist to know the correct proportion of reactants to products in a reaction?

Start ith the given
se olar ass to go ro gras to oles
se ole ratio to go ro oles to oles
se olar ass to go ro oles to gras

The molar volume for all gases at STP is 22.4 liters per mole of gas, or 22.4 L/mol. Each gas at STP will have the same molar volume.


Why do all gases have the same volume at STP?

3. Why does 1 mole of hydrogen gasmo hy take up the same amount of space as 1 mole of gas?oxygen o
What is a limiting reactant?





Determining the Limiting Reactant in a Chemical Equation


4. Why is it important to be able is to calculate yield, or the amount of product formed by a chemical reaction? duct formed ed by




Plant tissues
Assimilation by plant cells
Weathering of rock
Animal tissues and feces
Decomposition by fungi and bacteria
Urinel
Phosphates in solution
Loss in drainage
Phosphates in soil
Incorporation into sedimentary rock; geologic uplift moves this rock into terrestrial environments.
5. How does a balancedequationchemical help chemistsquantitiescalculate of reactants needed or products produced in a chemical reaction?





































1. Would it be easier desert or a tundra?































2. Which of of the kinetic theory explain the compressibility of ry th th gas? Explain. pressibility sibil






Charles’ Law
















3. Using the terms pressure and volume s , e describe what happens when you squeeze a balloon. ppens


he total ressure o a iture o gases is the su o the artial ressures o the gases contained ithin that iture

4. Dalton’s law is used when determining whether an environment is suitable for human life. How would this law be used on Mars?
Which is easier, flying a balloon in a desert or a tundra?
5. Explain which gas law relates to hot air ballooning. glaw t

































1. Where else have you seen water create surface tension?


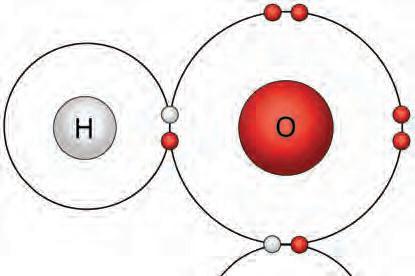

Cohesion

Hydrogen bonding causes water molecules to stick together as a tetrahedral pyramid. This cohesion of water molecules also results in macroscopic structures, such as waterdrops or a water bulge over a glass rim.

2. How is relatedelectronegativity to polarity? ctronegativity tronega
like dissolves like
Water is a good solvent due to its polarity.



3. Can a solution have more than one solute?



4. Rock candy is made with a solution.supersaturatedcandysugar
Describe how persaturated sugar prsaturated candymakers create a supersaturated dymakers creat solution.










































What water property allows bugs to walk on a pond?

5. What might have to happen for a bug gt ha to break the water’s surface tension?



























1. What are some exampleseveryday of acids me everyday er and bases you are familiar with? d a













2. Describe the pros and cons of the different methods used to test pH level.

3. Is this solution an acid or a base? How do you know?

4. Why are ofconcentrationsthe hydrogen and hydroxidehydrogenequaland yrogen an in a sample of ydroxide equal qa pure water?
tro aci colete dissociation o ions
ea aci artial dissociation o ions
5. What is the betweendifference the strength and the concentration of ngth an acid? opari















Why are some acids safer than others?














1. What would happen to the temperature of a glass of ice water if it were left on the kitchen counter for four hours?









2. Think about items at your home that are examples ms s ho of thermodynamic t example are ex systems. What is asystem?ermodynam What are ystems. s. What theystem?surroundings? yem? How might heat and e surroundings? oundin mattermighttransfer heat an ght between them?






























































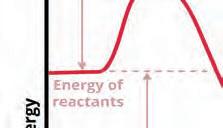
reaction in hich energy is asored y atter
Surroundings System (reactants)
increases
Thermochemistry is the study of energy changes that occur in chemical systems.
eaction athay
reaction in hich energy is released y atter
eactants nergy Products
eaction athay





3. A block of ice is placed on the sidewalk in summer and melts. Classify this enthalpy change dmelts. as endothermic or chang exothermic. Explain your answer using heatanswertransfer.usin






































4. Consider events in your daily life thatyourdemonstrate one of these laws. Describe one in terms of energy transfers and how ms f ene law you chose.











































5. Does this enthalpy value makethalpysense?value Would the process of (turningevaporation liquid water into gaseous water) absorb or r gaseou ga release heat?
























































































ccording to the second la o therodynaics nature avors an increase in disorder
How can something stay warm or cool for a long time?

6. What could you do to keep your cocoa warm based on ep your coc yr your knowledge thermodynamics?of o edge

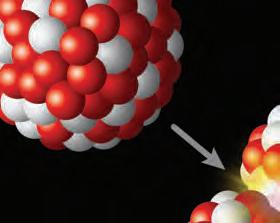








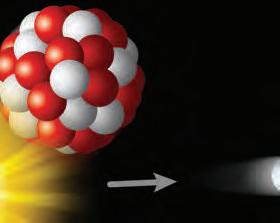
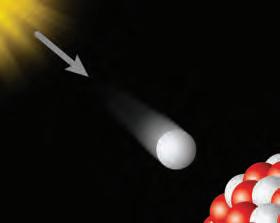





1. How do you think an element would be ow think thin affected if there were changes to its nucleus?ges

Mass number
Atomic number 4 2
Atomic symbol








2. How would losing two protons and two neutrons affect the atomic number and mass number of an atom?


nstale nucleus
Ne nucleus
nstale nucleus
lha article
nstale nucleus
Gaa radiation Ne nucleus Ne nucleus
eta article
lpha he ato decays into a ne ato and eits an alha article to rotons and to neutrons the nucleus o a heliu ato
eta he ato decays into a ne ato y changing a neutron into a roton and an electron he astoving highenergy electron is called a eta article
aa ter or decays surlus energy is soeties eitted his is called gaa radiation and has a very high reuency ith a short avelength he ato is not changed
4 2 He e alpha ( ).
Proton Neutron
Beta particle (electron)
Heavy, unstable element (e.g., uranium)
Mass number: 238 = 4 + 234
Atomic number: 92 = 2 + 90
Spontaneous decay
Alpha particle (He nucleus)
Gamma ray
Heavy, more stable element (e.g., thorium)
Protons
Neutrons
Mass number: 14 = 0 + 14
Atomic number: 6 = -1 + 7
The high-energy electron is a beta particle.
gamma (
Atomic number: 92 = 2 + 90 238 92 U 4 2 He + 234 90 Th + 2 h 0 0
Mass number: 238 = 4 + 234






















3. Why do the threeWhydifferent y types of radioactive decay have different ypes effects on an yhave differ y atom’s nucleus?




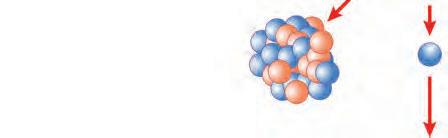


Mass number: 1 + 235 = 141 + 92 + 3
Atomic number: 92 = 56 + 36 + 0
0 n + 235 92 U Ba + 92 36 Kr + 31 0 n
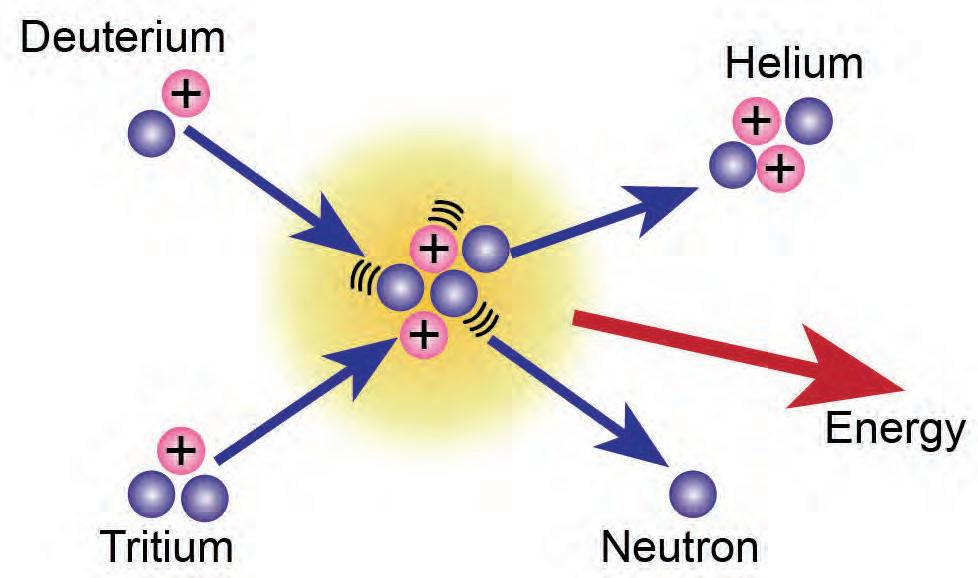
4. Why do neutrons emitted during hy neutr itted tte a chain reaction, while the neutron emitted during nuclear fusion ttedddduring does not?






5. Which of the two nuclear processes, you think is easier to control? Explain. ythink eas




Why do changes to the nucleus affect an atom’s identity? adioactive ecay in raniu Series

6. Why do radioactive decay, dioactive dioactive de change the identity of the element? nge identit ge











The speed of the reaction is called the rate of reaction.
Rusting

Baking

Exploding

The speed of different chemical reactions varies hugely. Some reactions are very

1. Why do you think some happenchanges quickly e changes while other ppen quick changes take a longgestime?take


he oard ecae a irdhouse ut is still ade o ood


he aric ecae a dress ut is still ade o cotton


he cues ecae a liuid ut are still ade o ater











2. Why is a physical changeconsidered yal chan an a change if the substance hange isn’t changed?


















3. Why is it important that a chemical change produce chemicalobservable chang emical or measurable evidence?



















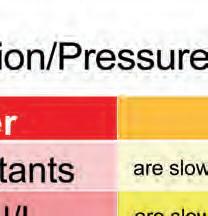
his is hy e ote heat p reactats i orer to ecorae a reactio are sloer than are sloer than otter eeratures
Cold reactantsot reactants
Cold environents ot environents



























4. Why is being able to control Why is g the rate of change useful?











































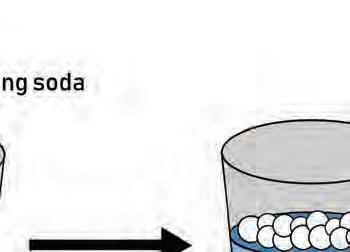
o to osere eeale a oreeale esorces
Why do some changes happen quickly while other changes take more time?

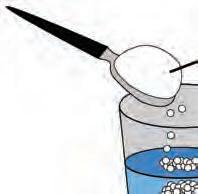




5. How can you slow the rate ow can yo at which baking soda reacts ich baki with vinegar?

































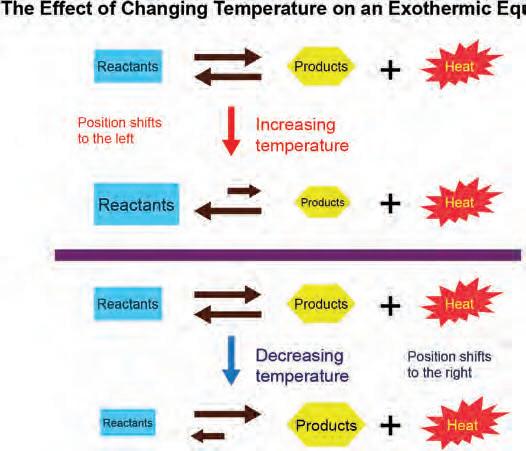
1. Why is hyimportant?equilibrium is eq











Rate of reaction
H2 + I2 2HI (Forward)
Equilibrium
2HI H2 + I2 (Reverse)
Time





2. What characterizes a reaction as being in ngequilibrium?dynamic dy in dy

ncreasing Concentration esults in Ne uili riu Point
Concentration
Products eactants
ie hours
dded reactants cause an instantaneous increase in reactant concentration ollo ed y a read ust ent as ore reactants react to roduce roducts according to e Chatelier s rinci le ne e uili riu osition is esta lished in hich the concentration o reactants added and roducts roduced is higher than e ore








3. How does the human body use water to equilibrium?maintain













Why is equilibrium important?


















4. What is another example of notherequilibrium? examp other
acid anion
acid: a compound that produces hydrogen ions in solution, donates hydrogen ions, or is an electron-pair acceptor
acid-base reaction: a type of double-replacement reaction; occurs when equal amounts of an acid are added to a base so that the acid and the base neutralize each other, forming water and salt; the general equation is HX (acid) + MOH (base) → H2O (l) + MX (salt)
acidic solution: a solution in which the hydrogen concentration is greater than the hydroxide concentration
actual yield: a measured quantity of the actual amount of product produced during a chemical reaction
alkali metal: any of the univalent elements belonging to Group 1A of the Periodic Table
alkaline earth metal: any of the bivalent metals belonging to Group 2A of the Periodic Table
agitation: the stirring or mixing of a solution so as to increase the particle movement of the solute particles in solution
alpha decay: radioactive decay in which an atomic nucleus emits an alpha particle, reducing the mass number by 4 and the atomic number by 2
alpha particle: a positively charged particle identical to the helium atom nucleus, with two protons and two neutrons, that is emitted from radioactive decay of a nucleus
amplitude: the height of a wave from the origin to the crest or trough
anion: any atom or group of atoms with a negative charge
atomic mass chemical bond
atomic mass: the weighted average mass of an element based on the abundances of the naturally occurring isotopes of that element
atomic number: the number of protons found in the nucleus of an element, represented by the letter Z
base: a compound that produces hydroxide ions in solution, accepts hydrogen ions, or donates an electron pair
basic solution: a solution in which the hydroxide concentration is greater than the hydrogen concentration; has a pH higher than 7
beta decay: the type of radioactive decay in which the neutron-to-proton ratio is too great in the nucleus and causes instability, resulting in a neutron being transformed into a proton and an electron
beta particle: a negatively charged electron emitted from the radioactive decay of a nucleus
boiling point: the temperature at which the vapor pressure of the liquid is equal to the pressure above the surface of the liquid, causing the liquid to become a vapor
Boyle’s law: a scientific law that states that the pressure of a gas varies inversely with volume at constant temperature; this is expressed as the equation P1V1 = P2V2
catalyst: a substance that speeds up or promotes a chemical reaction without being chemically changed by the reaction
cation: any atom or group of atoms with a positive charge
Charles’ law: the temperature of a gas varies directly with volume at constant pressure; expressed as the equation V1/T1 = V2/T2
chemical bond: an attraction between atoms that holds atoms together
chemical change: a change that occurs when a new substance is created with different properties; observable as a color change, the production of gas or a precipitate, or the release of heat or light
chemical equation: a representation of a chemical reaction using numbers and symbols of the elements to represent atoms and molecules
coefficient: a number representing the number of molecules in an equation; placed in front of a chemical symbol or formula to balance the equation according to conservation of mass
color change: a change in the substance’s appearance; usually an indication that a reaction has occurred
chemical property: characteristic that can only be observed or measured through a chemical reaction
chemical reaction: the process by which one or more substances change to produce one or more different substances
combustion reaction: a reaction that is an oxidation process in which a compound containing carbon, hydrogen, and sometimes oxygen reacts with oxygen gas to produce carbon dioxide gas and water; the general equations are C x H y O z + O2 → CO2 + H2O or C x H y + O2 → CO2 + H2O
closed system: a system that does not exchange matter but does exchange energ y with its surrounding environment
concentration: a measurement of the amount of solute that is dissolved in a given quantity of solvent
conduction: the transfer of energy from one medium to another through direct contact
conductivity: the property of a substance that allows an electric current or heat to move through the substance
convection: the transfer of thermal energ y through currents in fluids
covalent bond: a form of chemical bond that is characterized by the sharing of pairs of electrons between atoms
directly proportional: a relationship bet w ee n va ri ables in w h i ch o n e va ri able i n c reases as the othe r i s in c reased a n d v ice v e r sa
Dalton’s law of partial pressure: the law stating that the total pressure of a mixture of gases is the sum of the partial pressures of the gases contained within that mixture
ductility: a property that describes the ability of the material to be pulled into thin wire without breaking conduction
decomposition reaction: a reaction in which a single compound on the reactant side breaks down into two or more products during a chemical change; the general equation is AX → A + X
dissolution: the process in which molecules or compounds split into smaller pieces in solution
dissolve: to become incorporated into a liquid to form a solution
double-replacement reaction: a che mi cal reaction where two compounds exchan ge chemical bonds to form two products wit h b on d in g properties simi l ar to t h ose o f the reactants; the general equation is AX + BY → AY + BX
dynamic equilibrium endothermic
dynamic equilibrium: a state that exists once a reversible reaction ceases to change its ratio of reactants to products but substances move between the chemicals at an equal rate, meaning there is no net change
elastic collision: an encounter between objects in which the total kinetic energy of the objects before and after the collision is equal
electrolyte: a substance that forms ions when dissolved in a solvent
electron: a negatively charged subatomic particle of the electron cloud; involved in the formation of chemical bonds
electromagnetic radiation: energ y in the form of oscillating electric and magnetic fields that is released in electromagnetic processes; characterized based on the frequency and wavelength of the oscillations as radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays
electromagnetic spectrum: a continuum of all electromagnetic waves arranged according to frequency and wavelength
electron cloud: all of the area inside an atom surrounding the nucleus where electrons are found
electronegativity: the tendency of an atom or group of atoms to attract electrons
element: one of the primar y substances that make up matter and cannot be chemically broken down or converted into other substances; defined by the atomic number
endothermic: requiring or involving a net input of heat
enthalpy gamma ray
enthalpy: the total heat content of a system; equal to the internal energ y plus the product of the pressure and volume
entropy: a measure of disorder within a system
equilibrium: a condition in which all competing influences are balanced
excited electron: an electron that momentarily occupies an energ y state higher than its ground state
exothermic: requiring or involving a net output of heat
extensive property: property of a substance that is dependent on the amount of the substance present
first law of thermodynamics: the law stating that the total change in the internal energ y of a system is the sum of the work done on the system and the heat energ y added to it : ΔE = q + w
forward reaction: looking at a reversible reaction, these reactants are on the left side of the yield sign, while the products are on the right side freezing point: the temperature at which a liquid turns into a solid when cooled
frequency: the number of wave cycles that pass a given point per unit of time
gamma decay: the type of radioactive decay in which the nucleus of an atom is at too high an energ y, resulting in a reduction of energ y state and the emission of a highenerg y photon
gamma ray: ionizing electromagnetic
radiation of very high frequency and energ y emitted from the radioactive decay of a nucleus
gas
gas: a state of matter where the shape and volume are variable and dependent on the shape of the closed container, and where the particles are completely independent of each other
ground state: an electron at the lowest possible energ y for an atom
groups: the columns on a Periodic Table that arrange the elements by the number of electrons that are in the outside shell
halogen: any of the electronegative, nonmetallic elements in Group 7A of the Periodic Table
hydrogen bond: an attraction between a hydrogen atom that is covalently bonded to a highly electronegative atom (e.g., oxygen or nitrogen) and another highly electronegative atom to which the hydrogen is not covalently bonded
intramolecular forces
ideal gas: a theoretical gas that perfectly follows the ideal gas model
ideal gas law: an equation describing the behavior of a gas in terms of temperature, pressure, volume, and number of moles; expressed as PV = nRT
intensive property: property of a substance that is not dependent on the amount of the substance present
intermolecular forces: f o r ces o f attraction or repulsion that act between nei ghb orin g partic l e s
intramolecular forces: forces of attraction or repulsion that act within a molecule
inversely proportional
inversely proportional: a relationship between variables in which one variable decreases as the other increases and vice versa
ions: atoms (or groups of atoms) that have an electric charge due to having different numbers of protons and electrons
ionic bond: a form of chemical bond that is characterized by the electrostatic attraction that binds oppositely charged ions together
ionization energy: the energ y required to remove one electron from a neutral atom of an element in the gaseous state
isolated system: a system that does not exchange matter or energ y with its surrounding environment
kinetic energy: the energ y of motion
Le Chatelier’s principle
kinetic molecular theory: a theory stating that a gas is made of particles that collide with each other and with the walls of the container that holds them law of conservation of energy: a law stating that energ y cannot be created or destroyed—it can only change forms
law of conservation of mass: a law stating that mass is conserved and is neither created nor destroyed in a nonnuclear change; the total mass of the reactants equals the total mass of the products
Le Chatelier’s principle: a principle stating that if a constraint (such as a change in pressure, temperature, or concentration of a reactant) is applied to a system in equilibrium, the equilibrium will shift to counteract the effect of the constraint
light emission molecule
light emission: giving off light
limiting reactant: any reactant that is the first to be completely consumed in a chemical reaction, therefore limiting the amount of product that can be produced
liquid: a state of matter where the shape is variable and dependent on the bottom of the container, where the volume is constant, and where the particles move independently within the liquid
lone pairs of electrons: pairs of valence electrons that are not bonded to other atoms
metallic bond: the force of attraction holding metal atoms together; the attraction occurs between positively charged ions and free-floating, nonlocalized valence electrons
metals: elements that are typically solid, shiny, malleable, and good conductors of heat and electricity
molar mass: a general expression used to refer to the mass (in grams) of a mole of any substance, expressed as grams per mole, or g /mol
mass number: the total number of protons and neutrons in the nucleus of an atom
melting point: the temperature at which the phase change between solid and liquid takes place
molecule: a g roup o f two or more atoms bonded to gether by chemical forces, representin g t h e sma ll est possi bl e unit o f a chemical compound that can partici pate in a reactio n
neutralization reaction oxidation
neutralization reaction: a reaction in which equal amounts of an acid are added to a base so that the acid and the base neutralize each other, forming water and salt, the general equation being HX (acid) + MOH (base) → H2O (l) + MX (salt)
neutron: a subatomic particle of the nucleus of an atom that is without charge and contributes to the mass of an atom
nuclear fusion: a reaction in which two light nuclei combine to produce a nucleus of heavier mass, resulting in the release of a large amount of energy
nuclear reaction: an interaction between two atomic nuclei or between an atomic nucleus and a subatomic particle that results in a change in the properties of the interacting particles
noble gas: any of the elements found in Group 8A of the Periodic Table; each has the s and p sublevels of its outermost energ y level filled
nonelectrolyte: a substance that does not form ions when dissolved in a solvent
nucleus: the tiny, ver y dense, positively charged region in the center of an atom made up of protons and neutrons
open system: a system that exchanges matter and energ y with its surrounding environment
nuclear equation: an equation that shows how a nucleus gains or loses subatomic particles
nuclear fission: the splitting of a nucleus into smaller fragments that results in the release of neutrons and a large amount of energy
oxidation: a reaction that causes a molecule or atom to lose electrons
oxidation-reduction reaction physical change
oxidation-reduction reaction: a chemical reaction that involves the transfer of electrons between atoms or molecules, changing the oxidation state of the reactants; the atom or molecule that loses electrons is oxidized, and the atom or molecule that gains electrons is reduced
percent yield: the ratio of the actual yield to the theoretical yield for a chemical reaction, expressed as a percentage; shown as actual yield / theoretical yield x 100
Periodic Table of Elements: a table in which all the known elements are arranged by properties and are represented by one or two letters, referred to as chemical symbols
periodicity: the quality or state of being periodic, or occurring at regular intervals
periods: the rows in a Periodic Table that classify the elements by the number of atomic shells
pH scale: a logarithmic scale that measures the hydrogen ion concentration in an acid or base
phase change: the change from one state of matter to another
photoelectric effect: the observation that when certain wavelengths of light strike a piece of metal, electrons are emitted, potentially creating an electric current
photon: an elementar y particle that is the smallest possible amount of light and all other forms of electromagnetic radiation that can interact with anything
physical change: any alteration to a substance that does not change its chemical identity; examples include changing phase, dissolving into a solution, or breaking up into smaller pieces
physical properties radioactive decay
physical properties: properties that describe matter, including color, feel, smell, boiling point, melting point, and density
plasma: a physical state of matter that exists at extremely high temperatures in which all molecules are dissociated and most atoms are ionized
polar: describes a molecule in which one or more atoms is slightly negative and one or more is slightly positive
polymer: a large molecule formed by the bonding of smaller molecular units
potential energy: the energ y that an object has because of its position relative within a force field or because of the relative positions of its components
precipitate: an insoluble solid formed from a chemical reaction
precipitation reaction: a reaction that results in the precipitation of a solid from a solution; the general equation is AX (aq) + BY (aq) → AY (aq) + BX (s)
pressure: force per unit area
product(s): the ending substance(s), written on the right side of the chemical reaction arrow, that are created during a chemical change
proton: a positively charged subatomic particle of the nucleus of an atom that contributes to the mass of the atom
radiation: the emission or transmission of energ y as particles or waves
radioactive decay: the spontaneous emission of radiation by an unstable nucleus
reactant(s): the starting substance(s), written on the left side of the chemical reaction arrow, which will be consumed during a chemical change reaction rate: the measure of the change in concentration of the reactants or the change in concentration of the products per unit of time
redox reactions: all chemical reactions in which atoms have their oxidation state changed
reverse reaction: looking at a reversible reaction, these reactants are on the right side of the yield sign, while the products are on the left side.
second law of thermodynamics: the law stating that, when left to itself, a system’s entropy always increases, never decreases; also known as the law of entropy
single-replacement reaction: a redox reaction where one element or ion in a compound is replaced by another element or ion; the general equation is
A + BX → AX + B
saturated: containing the maximum amount of solute for a given amount of solvent at a constant temperature and pressure
solid: a state of matter where the shape is constant and rigid, where the volume is constant, and where the particles vibrate in a fixed position
solute: a substance that dissolves in another substance (solvent) to form a homogeneous mixture
solution: a homogeneous mixture where the solute particles are separated at the molecular or atomic level
solvent temperature change
solvent: a substance in which another substance (solute) is dissolved to form a homogeneous mixture standard temperature and pressure (STP): values of 0°C, or 273 kelvin (K), for temperature and 1 atmosphere (1 atm), or 760 torr, for pressure
stoichiometry: t h e n u m e ri cal relationships found in balanced chemical equations t h at re l ate t h e re l ative quantities o f the substances takin g part in a che mi cal react i on substances: elements or compounds that can only be separated or combined to make substances with new properties by means of a chemical reaction
surface tension: the elastic tendency of a liquid that makes it acquire the least surface area possible
synthesis reaction: also known as a combination reaction; a reaction in which two or more reactants combine during a chemical change to create one product; the general equation is A + X → AX
supersaturated: a solution that contains more solute than it can theoretically hold at a given temperature
system: a group of interacting, interrelated, or interdependent elements forming a complex whole temperature: a measure of the average amount of kinetic energ y of the molecules of a substance temperature change: an increase or decrease of heat energ y in a substance, which may be evidence of a new substance formed during chemical change
theoretical yield zeroth law of thermodynamics
theoretical yield: a quantity that is calculated rather than measured; shows the theoretical amount of product that could be produced in an ideal chemical reaction in which there is a complete conversion of reactants to products
thermodynamics: the study of the changes in the thermal properties of a system
third law of thermodynamics: the law stating that as the temperature of a system approaches absolute zero (-273.15°C, or 0 K), the entropy of the system will also approach zero
titration: the process of determining the concentration of a solution by adding a known concentration and volume of another solution to produce an observable reaction
transition elements: the Group B elements (Groups 3–12) found on the Periodic Table; also known as transition metals
unsaturated: a solution that contains less than the maximum amount of solute that it can hold at a given temperature and pressure
valence electrons: the electrons in the outermost energ y level of an atom that influence how an element will react with other substances
volume: a measure of the space that matter occupies
wavelength: the distance between two adjacent crests or troughs of a wave
work: the change in energy of an object that is caused by the application of a force on the object over a specific distance or displacement
zeroth law of thermodynamics: a thermodynamic law that explains thermal equilibrium, or a system that does not gain or lose thermal energ y

