The


Surgical
The
33RD CONFERENCE OF THE EUROPEAN WOUND MANAGEMENT ASSOCIATION
WOUND CARE – FROM ART TO SCIENCE DALL’ARTE ALLA SCIENZA: L’EVOLUZIONE DELLA CURA DELLE FERITE






























The


Surgical
The
33RD CONFERENCE OF THE EUROPEAN WOUND MANAGEMENT ASSOCIATION
WOUND CARE – FROM ART TO SCIENCE DALL’ARTE ALLA SCIENZA: L’EVOLUZIONE DELLA CURA DELLE FERITE


Highlights of this issue include exploring biosurgery, optimization of oxygen therapy in your clinical practice and overcoming challenges; mastering the art of compression, and the scope of a collagen-elastin dermal replacement scaffold. We also explore the types of wounds in clinical practice amenable to fish skin technology.
Steeped in the depths of the Norwegian fjords, historian, teacher, and political scientist Christian Lous Lange came up with this famous quote. As one of the world’s foremost exponents of the theory and practice of internationalism, he was a sceptic on the role that technology would play in our future lives.
None so true as in the wound care world. This September issue is packed with articles about the latest in innovation from the wound world. However, also featured in the issue are two of the ‘oldest’ wound treatment modalities: biosurgery and compression bandage treatments. This beautiful combination of old and new enables the skilful wound care practitioner to delve deep into their armamentarium for a potential solution to the challenging wound.
Wound Masterclass as an innovative, sustainable model of publishing endeavours to bring the combination of tried and tested modalities and techniques, with an open mind, to the emerging technologies.
Digital health encompasses categories such as wearable devices, telehealth, telemedicine, mobile health, health information technology, and customized and personalized medicine.
Digital health has the ability to improve the diagnosis and treatment processes as well as supporting the clinical decisions we have to make for the benefits of patient care; we have two excellent articles exploring the future of digitizing wound care.
An alligator eye forms our cover for this issue, and the animal world continues to lead in innovation. Alligator derived hyaluronic acid quells the inflammatory phase of wound healing
through a dual interaction with the host and the microbiome, and bioactive collagen from discarded frog skin has been derived as a ‘patch’ for advancing the healing process. We wait with baited breath to see further developments with these new modalities in the future.
Don’t miss our exciting range of articles in this issue including: Biotensigrity, Jump Starting the Challenging Wound, and Navigating the PostCOVID Landscape. We also have an in-depth look at electroceutical dressings and their role in clinical care, electrical stimulation and the clinical scope of Omega3 fish skin grafts.
Our Masterclass Guides reach the breadth of variety in wound care modalities, including topical wound oxygen therapy, decellularised fish skin technology, collagen-elastin skin dermal replacement scaffold, extracorporeal shockwave therapy, and algorithm of management of c-section wounds, virtual reality in wound education and management.
We are delighted to announce our free MasterSeries of live, concise and interactive sessions in November mastering new concepts in oxygen therapy, advances in nutritional therapy and surgical site infection prevention. Don’t miss the opportunity to join in the discussion with global experts covering the most important topics!
Lastly we have just been announced as the global media partner to the Association for the Advancement of Wound Care. We look forward to this collaboration to improve education, training and wound care.
Enjoy this issue!

Understanding the mechanisms of how structures develop, how they work, how they adapt when exposed to other new or changing environments, and how they interact with one another, is essential as it can help us manage impairment and disease. Nature follows assembly rules with intention and efficiency. A specific architecture called tensegrity is how human beings are constructed from common structural elements. Tensegrity provides a mechanism to harmonically and mechanically couple interconnected structures of different size scales and locations throughout living tissue and cells; within the context of this living system, the term is biotensegrity. This article explores this concept and provides a concise overview of the impact to our clinical practice.
Carl Sagan famously stated, ‘We are made of star stuff.’ Essentially, the materials that form our physical bodies were forged in distant, long-extinguished stars. A beautiful sentiment describing the amazing complexity of the human body. Yet, in all complex machines and organisms, there lies a simplicity in the seeming chaos of form and function. Nature follows assembly rules with intention and efficiency. Patterns emerge in all structures ranging from crystals to viruses to flowers to humans and everything in between. These patterns form systems which guide biological design, organization and function at the micro and macro level. How this all works remains a mystery, however Donald Ingber has put forth a unifying theory; “An astoundingly wide variety of natural systems, including carbon atoms, water molecules, proteins, viruses, cells, tissues and even humans and other living creatures, are constructed using a common form of architecture known as tensegrity. The term refers to a system that stabilizes itself mechanically because of the way in which tensional and compressive forces are distributed and balanced within the structure.”1 Ingber describes how the principles of tensegrity are relevant to every aspect of the human body. “At the macroscopic level, the 206 bones that constitute our skeleton are pulled up against the force of gravity and stabilized in a vertical form by the pull of tensile muscles, tendons, and ligaments… In other words, in the complex tensegrity structure inside every one of us, bones are the compression struts, and muscles, tendons and ligaments are the tension-bearing members. At the other end of the scale, proteins and other key molecules in the body also stabilize themselves through the
principles of tensegrity.”1 Tensegrity provides a mechanism to harmonically and mechanically couple interconnected structures of different size scales and locations throughout living tissues and cells.2-4 When framed within the context of living systems, it is termed biotensegrity.
The question remains as to how this all works? How does this choreography maintain and sustain itself? It has been postulated that mechanical stresses play a role in tissue form and growth. Newer evidence suggests it is an interplay between the physical forces of gravity, compression, pressure, tension, and shear that influence growth and remodeling of all tissues at the cellular level.2 Interestingly, these same forces are often used medically as interventions, yet they may also contribute to cellular and tissue disruption, as seen in integumentary dysfunction. Examples include negative pressure wound therapy (NPWT) as an intervention (applying the principles of macro- and micro-strain to the tissues) and sustained pressure/ tissue deformation leading to pressure injury development. Everything is a carefully orchestrated balance to preserve biological functioning with ease. When balance is disrupted, the result is dis-ease.
As all living things are made of star stuff, all life on Earth evolved from the sea. Thirty million years ago, organisms evolved in water without a large influence of gravity. In fact, underwater, gravity is compensated by buoyancy defined as ‘the upward force exerted by a fluid that opposes the weight of an immersed object.’5 Other forces such as lift and drag become more significant in an aquatic environment as these
flow forces vary in direction and magnitude and can even reach higher values than gravity. Further, the lack of surface tension and viscous forces under immersed conditions are also important in the aqueous environment. Aquatic species counterbalance the force of gravity through the pressure exerted around them and through specialized internal organs (form and function). The swim bladder, for example, is a specialized aquatic organ evolutionarily homologous to the lungs.5 It is an internal gas filled organ with flexible walls that contract or expand according to the ambient pressure to obtain neutral buoyancy and ascend and descend to a large range of depths.5 This demonstrates life adapting to its environment and the physical forces acting upon the environment. Just consider the shape and design of aquatic organisms; they are drastically different that organisms on land as they have adapted to their environment and the forces experienced in that ecosystem (whale compared to elephant).
Gravity is a constant force on Earth. Land animals and organisms (as they emerged from the sea) began to develop adaptive mechanisms to orient themselves to the gravity vector, as the mechanical load on land organisms is ~1000 times larger than in water.5 Life on Earth developed in the presence of, and with the influence of gravity. This is rooted in the evolution of terrestrial life. Even at the cellular level, specialized structures have evolved such as statoliths in plants (cells that sense gravity) and otoliths in hair cells of the inner ear, all to respond directly to the force of gravity.
So, what happens when gravity is removed? The reduction and/ or absence of gravity can have profound effects on the physiological, biochemical, histological, and psychosocial components of the human body, or any organism for that matter. This effects every system of the body macroscopically to microscopically, resulting in transient and
permanent dysregulation and adaptation. This has been evidenced from the time of the Apollo launches beginning in the 1960s, to current research being conducted on the International Space Station (ISS) as well as through private space ventures such as SpaceX, Blue Origin and Virgin Galactic. The environment of space, essentially a vacuum devoid of physical forces, subjects those that venture out of the protective fold of the Earth, to challenges that effect the essence of biotensegrity.
Understanding the mechanisms of how structures develop, how they work, how they adapt when exposed to other new or changes in environments, and how they interact with one another is essential, as it can help us manage impairments and disease. This translational knowledge is exemplified from the lessons learned in space regarding human physiology and dysregulation, all of which are directly translatable to improving human health on terra firma. Incredibly, most bodily systems work in tandem and unison to maintain health and homeostasis, even when challenged. This natural rhythm, however, can be disrupted whether endogenously, exogenously or through iatrogenic impacts. Philosophies of treatment to maintain and restore this rhythm are embodied in therapies such as myofascial release (MFR), craniosacral therapy (CST), yoga and shiatsu, to name a few. MFR is manual pressure and stretching directed to loosen restricted movement, improving pain, mobility, and posture. CST is a gentle hands-on technique that uses touch to examine membranes and movement of the fluids in and around the central nervous system (CNS). Relieving tension in the CNS can help to eliminate pain and boost immunity. In essence, these treatments are designed to rebalance biotensegrity. These interventions and philosophies employ external forces to realign the internal forces of tensegrity (solid and liquid fascia), addressing the body as a whole, integrated system.
“Understanding the mechanisms of how structures develop, how they work, how they adapt when exposed to other new or changes in environments, and how they interact with one another is essential, as it can help us manage impairments and disease.”Biotensegrity: Its Application to Tissue Function and Dysfunction
“The movement attributed to the skin is called elasticity, yet that term belies its true intricacies. It is a rhythm connecting all components of the human body, from the smallest cell to the largest organ.”
Another example is highlighted in integumentary disruption. The skin provides the protective mantel that allows biotensegrity to work harmoniously. As all systems are connected and reliant upon one another, skin disruption can lead to numerous impairments and dysregulation throughout the host.
A video titled ‘Strolling Under the Skin’,11 provides an incredible look at biotensegrity in action viewed from the perspective of tissues, particularly the skin and its related structures.This was created by the French surgeon, Jean-Claude Guimberteau. It is geometry in motion revealing the powerful yet delicate dance of living fractals. The creators of this video, reveal this biological marvel as the multimicrovacuolar collagenic dynamic absorbing system (MCDAS). It shows the true inter-connectedness of the systems and in particular, the VAIL. The VAIL is a concept created by the author of this editorial describing how the venous, arterial, integumentary, and lymphatic systems work in unison. These are not siloed structures or systems, but inter-dependent one on another to maintain nominal function. Dysfunction in one system, can and often results in dysfunction in the other systems. For example, the concept of lymphatic dermopathy describes how disorders of the lymph system, whether systemic (macro-lymphedema) or localized (micro-lymphedema), produce cutaneous regions susceptible to infection, inflammation, and carcinogenesis.6-7 Lymphatic impairments result in skin barrier failure and this partially explains why patients with chronic edematous conditions have a propensity for infections (cellulitis in patients with lymphedema) and hypersensitivity reactions (stasis dermatitis in chronic venous disease).
The structure and function of the skin is remarkable. According to Dr. Guimberteau, the skin is more than just an organ, it is ‘a set of organs which areanatomically, physiologically, culturally, and psychically complex.’ In ‘Strolling Under the Skin’, it describes how touch is the
most fundamental of all the senses. “You can survive without smelling, seeing, or tasting, but not without touching. The skin permanently relays information; it never shuts down, blocks up or sleeps. It has an odor, a texture; it perspires, secretes, eliminates. It exchanges signals with the outside world… thanks to the skin, the body’s surface is as much a machine for communication as a protective barrier. It can change color, texture and shape, and retains the vestige of aggression such as sunburn, scars, and disease… through the hairs on its surface, you can see pseudo-geometric structures separated by strength lines allowing movement in all dimensions of space. These triangular pyramidal structures move in relation to each other…the skin undergoes this form of translation, traction and stretching; yet, with the exception of open wounds, it returns to its initial position.” Full thickness wounds and other injuries that resolve through scar tissue formation, never regain normal tensile strength, function, cosmesis, texture or pliability disrupting the skin’s biotensegrity. Fundamental and irreparable changes occur in the dermis and deep tissue structures disrupting the skin’s biological rhythm and connection to the other systems.
The movement attributed to the skin is called elasticity, yet that term belies its true intricacies. It is a rhythm connecting all components of the human body, from the smallest cell to the largest organ. Dr. Guimberteau describes that “under the dermis and hypodermis there is a highly mobile tissue encompassing everything else and penetrating what is known as the undermined plane. When this tissue is tracked upward… curious movements occur due to the bursting of vacuoles at atmospheric pressure demonstrating the existence of hydraulic systems under different levels of pressure.”
Similarly, fascia is another organ or system with a unique 3-dimensional metabolic and mechanical matrix. It consists of soft, collagen derived loose and dense connective tissue that permeates the body. “It incorporates
Biotensegrity: Its Application to Tissue Function and Dysfunctionelements such as adipose tissue, adventitia and neurovascular sheaths, aponeuroses, deep and superficial fasciae, epineurium, joint capsules, ligaments, membranes, meninges, myofascial expansions, periostea, retinacular, septa, tendons, visceral fasciae, and all the intramuscular and intermuscular connective tissue including endo-/peri-/epimysium… interpenetrates and surrounds all organs, muscles, bones and nerve fibers, endowing the body with a functional structure, and providing an environment that enables all body systems to operate in an integrated manner.”8 The solid fascia structures described above divide, support and connect the different parts of the body into a connected system; liquid fascia,8 comprised of blood and lymph, transports messages and nourishes the solid fascia. This liquid fascia also determines the health of the tissues. “As water shapes rocks, bodily fluids modify shapes and functions of the bodily structures. Bodily fluids are silent witnesses of the mechanotransductive information, allowing adaptation and life, transporting biochemical and hormonal signals.”8 A poignant example of this is demonstrated through the endothelial glycocalyx layer (EGL). Mechanotransduction is the mechanism that allows the EGL, a gel-like matrix with hairlike projections extending into the lumen of all vessels, to act like a molecular sieve by regulating fluid and macromolecule movement (liquid fascia) out of the vascular space. Blood flow shear forces (mechanotransduction) act on the vascular endothelial cells to produce and release nitric oxide. This in turn, dilates the vessel.9-10 With trauma, disease, and other conditions, this normal process of the microcirculation, can lead to modifications in the shape and function of the areas involved by inducing edema. This, when coupled with other comorbidities or issues, can result in further dysregulation in biotensegrity; problems with mobility and function, lymphatic dermopathy, and infection to name a few.
Armed with this knowledge, we can begin to look differently at preventative strategies to maintain system wide health. All things are connected; it is up to us to put the puzzle pieces together and to be open to the remarkable synergies that can be found in unlikely places to help us explain our building blocks. This in turn, will help preserve biotensegrity to allow for optimal functioning in all environments. Although form follows function, form is influenced by its environment.
1. Ingber D. The Architecture of Life. Sci Am. 1998 Jan;278(1):48-57. doi:10.1038/ scientificamerican0198-48.
2. Ingber D. Tensegrity: The architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575-599.
3. Ingber D, Jamieson J. Cells as tensegrity structures: architectural regulation of histodifferentiation by physical forces transduced over basement membrane. In Gene Expression During Normal and Malignant Differentiation, ed. LC Andersson, CG Gahmberg, P Ekblom, pp. 13=32. Orlando, FL: Academic.
4. Pienta K, Coffey D. Cellular harmonic information transfer through a tissue tensegritymatrix system. Med. Hypoth. 1991;34:88-95.
5. Narjana T, Sanchez-Esteban J. Mechanotransduction as an adaptation to gravity. Front Pediatr. 2016;4:140. doi:10.3389/fed.2016.00140.
6. Carlson J. Lymphedema and subclinical lymphostasis (microlymphedema) facilitate cutaneous infection, inflammatory dermatoses, and neoplasia: A locus minoris resistentiae. Clinics in Dermatology. 2014;32(5):599-615.
7. Ruocco V, Ruocco E, Brunetti G, Sanguiliano S, Wolf R. Opportunistic localization of skin lesions on vulnerable areas. Clinics in Dermatology. 2011;29(5):483-488.
8. Bordoni B, Marelli F, Morabito B, Castanga R. A New Concept of Biotensegrity Incorporating Liquid Tissues: Blood and Lymph. J Evidence-Based Integ Med. 2018;23:110.
9. Biddle C. Like a slippery fish, a little slime is a good thing: the glycocalyx revealed. AANA Journal. 2013;81(6):473-80.
10. Weinbaum S, Tarbell J, Damiano E. The structure and function of the endothelial glycocalyx layer. Annu Rev biomed Eng. 2007;9:121-167.
11. Dr. Jean-Claude Guimberteau. Strolling under the Skin. 2014. [Internet]. Available from: https://www.youtube.com/watch?v=eW0lvOVKDxE&t=484s
“Armed with this knowledge, we can begin to look differently at preventative strategies to maintain system wide health.”
Biotensegrity: Its Application to Tissue Function and Dysfunction

Evaluating the factors which may stall or impair healing is an essential skill to hone for effective clinical practice. This teamed with clinical wound assessment, as well as objective measurement, enables the tracking of progress of wound care patients. Establishing reversible and irreversible factors in a timely fashion enables a pathway to facilitating effective wound healing.
Wounds can become chronic and difficult to heal, and profoundly affect the clinical condition and quality of life of patients for months, years or decades. They are considered a vicious cycle within healthcare systems. A number of local and systemic factors contribute to this issue worldwide.
The elderly are an example of a target population for healing deficits. The world population grows and ages with an increased life expectancy but this is proportionally accompanied by the high prevalence of chronic diseases.
The body changes throughout life, a series of personal factors, clinical and socioenvironmental conditions; and, mainly, the knowledge that medical and nursing professionals need to have about the healing process, can influence the increase in the risks for the appearance of wounds, and also directly interfere with healing outcomes.
The awareness of health professionals must be directly related to the initial care with the maintenance of skin integrity, with the prevention of wounds and with the early treatment of the already existing wound, whether surgical or non-surgical. If we do not know how to detect patients at risk or the factors involved with the scarring deficit, we will easily miss the chance of correctly treating an acute wound to prevent this wound from becoming chronic.
Observing the patient and the healing behavior
plays an important role, since healing is influenced by several systemic factors such as advanced age, malnutrition, diabetes, chronic diseases, vascular problems, anemia, vasculitis, and drugs that interfere with immunosuppression.¹The local factors involved with healing are ischemia, edema and infection.
Essentially, the healing process involves three highly complex, interdependent and overlapping stages: the hemostasis and inflammatory phase, the proliferation phase (fibroblasts and collagen synthesis), and the maturation/ remodeling phase.1,2 The healing process depends on the correct interaction between these phases to reach the goal, the healed wound.
So the observation of the measurements (area) of the wound and the documentation of the evolution of the wound are important tools for the evolutionary follow-up. Among the criteria to be observed are the percentage reduction of the area weekly (requiring reliable metric monitoring), the appearance and maintenance of granulation tissue, control of exudates, reduction of deadspaces and perilesional edema, and stimulation of epithelialization. For all this to happen, you need an ideal nutritional condition.
It is important initially to define what a difficult to heal wound is and why this type of wound is a public health problem worldwide.
“It is believed that 6% of the world population (approximately 480 million people) have chronic wounds that are difficult to heal and that 16.4% of antibiotic prescriptions are attributed to wound care.”12,13
A wound is defined as a rupture of tissue in the normal anatomical structure with consecutive loss of function. Healing is an organized and interactive cascade of cellular events with an innate immune response to tissue injury. This already gives us an idea of the complexity of the biological reactions that are involved in this process.2
When Does a Wound Become ‘Chronic’ or ‘Hard-to-Heal’?
‘Chronic wounds’ are defined as those that have not followed the normal healing path for 4 weeks. They are trapped in the inflammatory phase of the healing process.1-4 This may have happened due to a neglected or not properly treated local infectious process, as the wounds have the ideal local conditions for the invasion of the bacterial microbiota, mainly. The incidence of infection in chronic wounds is greater than 50%, and the presence of bacterial biofilm has been identified as being between 60% - 90% of cases of biopsies in chronic wounds.1,2,5,6 The chronicity and scarring deficit of wounds are increasingly being correlated with the presence of bacterial biofilms, especially in the elderly, people with diabetes and immobilized patients. These groups are at greater risk of skin invasion by pathogenic microorganisms that violate the skin barrier.5,6
As they are trapped in the inflammatory phase of the healing process, chronic wounds are characterized by a high concentration of inflammatory cytokines; a high level of proteases, a high level of reactive oxygen species; low mitotic activity, non-responsive cells to growth factors, and poor cell migration.1,7
A chronic wound that does not heal is typically correlated with a person with comorbidities, such as diabetes, vascular insufficiency, high blood pressure, and chronic kidney disease.4
Then, after the initial tissue damage, several factors can contribute to the delay of the healing process, either due to the severity itself at the injury site, or to the poor health status of the patient.¹
‘Complex wounds’ are those accompanied by aggressive infections, with extensive skin loss, compromised tissue viability (by ischemia or necrosis), and in patients with systemic diseases that impair healing (such as diabetes or vasculitis).8-10
Population aging means a greater number of elderly people with chronic, complex and debilitating wounds, increasing the demand for professionals and tissue viability services.4,10
A ‘difficult to heal wound’ is any wound that has not reduced in area by between 40% - 50% after 4 weeks of treatment, requiring changes in therapeutic strategies. Therefore, a wound that is difficult to heal is one that failed to respond to the evidence-based standard of care.2,11,12 A wound that has not reduced in (surface) area by >40% - 50% within 4 weeks should be considered difficult to heal and referred to a wound specialist or complex wound clinic.
The diagnosis and treatment of difficult to heal wounds cannot neglect the correct treatment of the biofilm.1,2 The prevention of infections and the correct antibiofilm treatment in wounds should be an important focus of attention by health professionals and managers.
Chronic, complex and difficult to heal wounds are considered a public health problem and generate high costs for healthcare services.
It is believed that 6% of the world population (approximately 480 million people) have chronic wounds that are difficult to heal, and that 16.4% of antibiotic prescriptions are
attributed to wound care.12,13
They involve specialized care and dressings, prolonged hospitalizations, complex treatments, use of adjuvant therapies, high demand on the time of healthcare professionals, sequence of treatments in dehospitalization, and are associated with high rates of recurrence, judicializations, and chronic morbidity.1,14,15
Chronic wounds have an impact on the quality of life of patients and their families.1 The quality of life for people with wounds that do not heal is similar to that of patients with chronic obstructive pulmonary disease and cardiovascular disease, associated with pain, immobility, infection, amputations and death.1,11,12
There are a number of conditions and risk factors associated with difficult to heal wounds: age, malnutrition, diabetes, anemia, hypoxia, smoking, peripheral vascular disease (arterial and venous), neuropathy, chronic inflammation, systemic medication use, and radiation. But it is worth remembering that external factors such as immobilization, patient adherence to treatment, patient’s economic situation, and demographic and behavioral factors can also significantly affect the difficulty of healing a wound.11
If we consider vascular ulcers of the lower limbs, diabetic foot ulcers, pressure injuries and surgical dehiscence as chronic wounds and all of which are also likely to be complex and difficult to heal, we have to ask: are we really addressing the underlying causes of hard to heal wound modifiable risk factors? Evaluate the person with a wound with a holistic and much broader view of the problem of local wound damage.
There is an aging population, rising health care costs, a sharp rise in the incidence of diabetes, and an increase in obesity.4
So what do the elderly, diabetics and immobilized people have in common when we approach the topic of wounds that are difficult to heal? These 3 groups also have increased risks for malnutrition, infection and delayed healing.5,6
Initially, I draw attention to the need to assess the nutritional status of patients and ensure adequate energy and protein intake, as recommended by current guidelines,16 not forgetting the importance of correct hydration. Meeting the cellular energy demands that are necessary for healing becomes fundamental, because healing involves a series of reactions, many types of cells, enzymes, growth factors, and other substances.
It is necessary to maintain the ideal pattern to create the healing energy necessary for the healing process, which requires an adequate supply of nutrients and oxygen to cells and tissues, both to fight infection in the wound and for the reconstruction of injured tissue.17
One of the main systemic factors related to scar deficit is malnutrition, when under diagnosed, neglected and not properly treated.18 Unfortunately, less than 50% of patients identified as malnourished receive nutritional intervention19 and the elderly have a 30% greater chance of being malnourished in the hospital environment.
This happens due to the lack of attention of health professionals to malnutrition. It is also due to the lack of programs for the assessment and detection of nutritional status, with appropriate screening, and early and timely nutritional intervention. The lack of attention to these conditions collaborates so that patients with wounds and malnourishment, both in the
“Meeting the cellular energy demands that are necessary for healing becomes fundamental, because healing involves a series of reactions, many types of cells, enzymes, growth factors and other substances.”
“It is necessary to have a holistic view and an interdisciplinary approach to the patient with wounds. This involves not only the local management of the wound and the correct choice of dressing, but also the diagnosis and treatment of systemic factors that interfere with healing, such as nutritional status and hyperglycemia.”
hospital environment, in outpatient clinics and in their homes, have unfavorable outcomes.
It is essential to have professionals with knowledge related to nutrition and the wound healing process, combining clinical control with the correct indication and use of nutritional supplements, dressings and supporting technologies.
Another obstacle to the wound healing process is the control of sustained hyperglycemia present in diabetes. Hyperglycemia reduces collagen synthesis by fibroblasts, reduces immunity and the inflammatory response.20 Interest in the effects of hyperglycemia is growing and the goal of diabetes mellitus management is to normalize blood glucose levels, since controlling hyperglycemia is associated with a reduction in the development and progression of complications such as scar deficit.
Strict glycemic control is therefore essential, so that healing is not compromised and the potential for wound infection is reduced.
The loss of skeletal muscle mass associated with age and immobility is well known too. Estimates of the severe muscle loss that sarcopenia represents range from 5% - 13% for adults aged between 60 and 70 years, and from 11% - 50% for those aged 80 years and over.21 This contributes a lot to Immobility Syndrome, which is a set of signs and symptoms resulting from the suppression of joint movements and, therefore, the inability to change posture. Immobility may be associated with chronic diseases or debilitating neurological sequelae. Its aggravating factors are malnutrition, dehydration, hypoalbuminemia and anemia.
In order to flick the switch and jump start the challenging wounds it is essential to maintain a holistic view and an interdisciplinary approach to the patient with wounds. This involves not only the local management of the wound and the correct choice of dressing, but also the diagnosis and treatment of systemic factors that interfere with healing, such as nutritional status and hyperglycemia.
Bearing in mind the vital premise that local factors that negatively interfere and induce tissue hypoxia, such as ischemia, inflammation and infection, must be quickly detected, avoided and properly treated.
Patients with wounds need a systematic assistance that includes the clinical evaluation of the patient and the lesion.
Identifying that wounds with 30% - 50% area reduction in the first 2 - 4 weeks have high healing potential, it follows that we need to correctly measure the areas of the wounds and follow them up.
If the patient is not adequately nourished, blood glucose is not controlled, and the dressing is not correct, the healing goals will not be achieved.
Without this vision, we will not be able to break the vicious cycle that perpetuates the coexistence of a person with a wound that is difficult to heal.
1. Mehl AA, Schneider Jr B, Schneider FK, Carvalho BHK. Measurement of wound area for early analysis of the scar predictive factor. Rev. Latino-Am. Enfermagem. 2020;28:e3299.
2. Mehl AA, Damião AO, Viana SD, Andretta CP. Hard-to-heal wounds: a randomised trial of an oral proline-containing supplement to aid repair. J Wound Care. 2021 Jan 2;30(1):26-31. doi: 10.12968/jowc.2021.30.1.26.
3. Sen CK, Roy S, Gordillo G. Chapter 14: Wound Healing (Neligan Plastic Surgery, Vol. 1). London: Elsevier, 2017.
4. Sen CK. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv Wound Care (New Rochelle). 2021 May;10(5):281-292. doi: 10.1089/wound.2021.0026.
5. Ganesh K, Sinha M, Mathew-Steiner SS, Das A, Roy S, Sen CK. Chronic Wound Biofilm Model. Adv Wound Care (New Rochelle);4(7):382–388, Jul 1, 2015.
6. Skrlin J. Impact of biofilm on healing and a method for identifying it in the wound. Acta Med Croatica.;70(1):29-32,Mar,2016.
7. Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4:411-20.
8. Ferreira MC, Tuma P Jr, Carvalho VF, Kamamoto F. Complex wounds. Clinics (Sao Paulo). 2006 Dec;61(6):571-8. doi: 10.1590/s1807-59322006000600014.
9. Coltro PS, Ferreira MC, Batista BP, Nakamoto HA, Milcheski DA, Tuma Júnior P. Role of plasticsurgery on the treatment complex wounds. Rev Col Bras Cir. 2011 Nov-Dec;38(6):381-6. English, Portuguese. doi: 10.1590/s0100-69912011000600003.
10. Nussbaum SR, Carter MJ, Fife CE, et al. An economicevaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018;21:27–32.
11. Atkin L, Bućko Z, Montero EC, Cutting K, Moffatt C, Probst A, Romanelli M, Schultz GS, Tettelbach W. Implementing TIMERS: the race against hard-to-heal wounds. J Wound
Care Consensus Document. 2019; 28(3 Suppl 3):S1–S49. https://doi.org/10.12968/jowc.2019.28. Sup3a.S1
12. Murphy C, Atkin L, Swanson T, Tachi M, Tan YK, Vega de Ceniga M, Weir D, Wolcott R. International consensus document. Defying hard-to-heal wounds with an early antibiofilmintervention strategy: wound hygiene. J Wound Care 2020; 29(Suppl 3b):S1–28.

13. Oliveira AC, Rocha DM, Bezerra SM, Andrade EM, Santos AM, Nogueira LT. Qualidade de vida de pessoas com feridas crônicas. Acta Paul Enferm. 2019;32(2):194-201.doi. org/10.1590/1982-0194201900027
14. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that wounds impose on the national health service in the UK. BMJ Open. 2015;5,12.
15. Drew P, Posnett J, Rusling L. The cost of wound care for a local population in England. Wound Care Audit Team. Int Wound J. 2007 Jun;4(2):149-55.

16. de Oliveira KDL, Haack A, Fortes RC. Terapia nutricional na lesão por pressão: revisão sistemática. Rev. Bras. Geriatr. Gerontol. 2017; 20(4): 567-575. http://dx.doi.org/10.1590/198122562017020.160195
17. Janis JE, Harrison B. Wound Healing: Part I. Basic Science. Plast Reconstr Surg. 2016; 138:9-17S.
18. Citty SW, Cowan LJ, Wingfield Z, Stechmiller J. Optimizing Nutrition Care for Pressure Injuries in Hospitalized Patients. Advances in Wound Care, volume 8, number 7, 2019. DOI: 10.1089/wound.2018.0925)
19. Khalatbari-Soltani S, Marques-Vidal P. Impact of nutritional risk screening in hospitalized patients on management, outcome and costs: A retrospective study. Clin Nutr. 2016 Dec;35(6):1340-1346. doi: 10.1016/j.clnu.2016.02.012.
20. Arya AK, Tripathi K, Das P. Promising roleof ANGPTL4 gene in diabetic wound healing, Int. J. Low Extrem. Wounds. (2014) Mar;13(1):58-63.
21. Malmstrom TK, Miller DK, Herning MM, Morley JE. Low appendicularskeletalmusclemass (ASM) with limited mobility and poor health outcomes in middle-aged African Americans. J Cachexia Sarcopenia Muscle (2013) 4:179–186.
Join AAWC for OUTSTANDING EDUCATION, as well as NETWORKING and KNOWLEDGE-SHARING
Earn 15.25 CE credits for attending!
For Wound Masterclass readers! Buy one registration ($450) and get 50% off a second ($225) using the discount code BOGO50
The person paying full price receives a free one-year membership ($119-139 value).
Check out this list of session titles!
• PAD National Action Plan: Summit Consensus Recommendations
• Technology Update: Micro and Macro Assessment of Perfusion
• Topical Oxygen: Where Does It Fit in Your Practice?
• Advances in Addressing the Wound and Skin Microbiome
• Pathology or Culture: What Is Best for Your Patient
• Technology of Early Detection
• Transitioning Technology Into Your Practice - Navigating the Process
• Disparities in Wound Care: Social Determinants of Health, Epigenetics and the Biochemical Response to Stress
Seeing the Real Wound Status with Technology with Prof. Dr. Sebastian Probst President, European Wound Management Association (EWMA)
• Development and Psychometric Evaluation of an i-Pad Based Decision Support Tool to Prevent Community Acquired Pressure Injury for the SCI Clinic
• Vitamin D as a Marker for Diabetic Food Disease
• Walk in My Shoes and Socks - Integrating New Modalities in Care
• Wound Healing with Your Tablet: Breaking Barriers Across the Care Continuum
• Wound Bed Preparation to Optimize Topical Therapy
• Selecting Advanced Wound Bed Technologies (CTP)
• Support Surface Testing: How to Use What We Get
• Journeying Into the Future: Biomarkers and Smart Biotechnology for Chronic Wound Management
This smaller, more intimate conference means :
• More interactive sessions
• Networking time for continued learning from presenters
• Greater access to the industry’s key leaders

Integrating Technology Into Wound Care: A Toolkit for the Clinician
* Separate pre-registration
Salt Lake City is a modern city set at the foot of spectacular mountains!
• Arts and culture
• Food and drink
• Events and festivals
• Shopping
• Outdoor adventure
 Scientist, Net Health
GA, United States
Scientist, Net Health
GA, United States
Encompassing a wound care database, the largest of its kind, this review culminates data from 439 facilities treating 3.6 million wounds and representing 12 million visits. The demand for advanced wound care has almost doubled in the space of a few years; the trends during and after the pandemic will be explored further in this article.
The COVID-19 pandemic turned the world upside down. What started as rumors of a potentially highly transmissible virus in China all but crippled the global economy. Borders were closed, the stock market plummeted, and the future could not have been more uncertain. The wound care industry was not left out of this carnage.
While the mainstream media focused on the effects of the virus on familiar activities like hotel stays and air travel, there was less emphasis on esoteric industries like wound care where data might not be readily available.
Despite the absence of wound care in regular conversation, chronic wounds occur in 1 out of every 100,000 U.S. citizens, and the number of people seeking help is poised to increase. For those with wound issues, care is generally centered around closure or preventing serious complications to minimize hospitalization and surgery.
To better understand the impact of COVID-19 on the current wound care landscape, I analyzed a subset of the Net Health Wound Care database, one of the largest of its kind. The analysis included 439 facilities, which handled 3.6 million wounds representing 12 million visits dating back to 2012. In the next few paragraphs, I’ll share my observations and offer conjectures as to relevant causal or correlated factors.
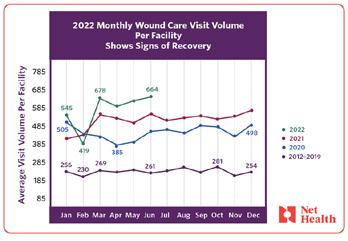
Evidently, the demand for advanced wound care is growing as average wound care volume per facility at the beginning of the year has more than doubled when comparing 2022 to
the 2012 - 2019 period (Figure 1a). It is unclear how much of this growth may be due to a shift towards more higher volume facilities being represented in the database. Anecdotally, there are fewer new U.S. hospital-based wound centers opening, so the number of new patients is likely outpacing the current wound center infrastructure. If this observation holds true, it will be important to regularly examine the data and find patterns that can lead to a better understanding of where wound care is needed, and how resources could be efficiently deployed, so that those suffering can get the help they need.
With regards to the COVID-19 effect, we see that from January 2020 to April 2020, visit volume declined by a whopping 25%. This huge drop in visit volume in the first months of 2020 reflects the onset of pandemic awareness when there was a disruption of healthcare services due to lockdown mandates. In addition, many patients were taking a wait-and-see approach for fear of entering a healthcare facility and contracting the virus.
Looking at the trend, October historically is the highest volume month, with the month ranking in the top quartile for 7 of the last 10 years. This is likely because October is one of the longest months with 31 days, and with summer being over, people are probably home and close to their primary wound clinician. February, with 28 days and cooler winter weather, generally sees the least volume of the year.
As one can imagine, perverse weather conditions and other personal priorities affect patients’ ability to make appointments. This is
supported by data, where for the 10-year period between 2012 to 2021, volumes in May, August and October tended to be higher than November, January and February, likely correlated to weather and holidays taking place during those months.
The COVID-19 pandemic created outliers to the historical data. However, once vaccinations became more widespread and people’s fears about seeing healthcare clinicians waned a bit, volume started inching towards preCOVID-19 levels. In December of 2021, visits peaked for the year, potentially due to pentup demand, vaccine availability and many COVID-19 restrictions being lifted. This was a clear deviation from the 2012 - 2019 trend where December, despite having 31 days, ranks relatively low among other months in terms of volume.
I also looked at the daily wound care visit volume per facility per month to account for working days, that is, excluding public holidays and weekends (Figure 1b). So far in 2022, wound care visits have shown signs of an uptick as fall approaches. Although the first few months of the year have been volatile, a peak between August and October followed by a relative decline in December could signal a return to trend. December declines will be in line with the thesis that patients skip on visits during holiday months. It could also be that they have exceeded their deductibles and health insurance allocations so wound care visits decrease.

Studies have suggested that the frequency with which patients visit wound care clinics influences the rate of chronic wound healing. Understanding the dynamics around patient visits in a facility could help with decisions like the best time to start recruitment for clinical trials, or to schedule system upgrades for minimal disruption. With an aging population and increasing prevalence of diabetes, the
demand for wound care is growing and an acute awareness of operational metrics like visit volume will help facilities better navigate the changing landscape.
For those who haven’t been collecting data or utilizing it properly, now is the time to do so. It helps to know your numbers. By keeping track of fluctuations and investigating underlying causes, you can identify the strengths and weaknesses of your practice. As facilities better understand the numbers behind the operations, they can be better prepared to adopt data-based clinical practices.
“With an aging population and increasing prevalence of diabetes, the demand for wound care is growing and an acute awareness of operational metrics like visit volume will help facilities better navigate the changing landscape.”
This Masterclass Guide is a concise overview exploring the modality of extracorporeal shockwave therapy and how to incorporate this into your clinical practice.
High-energy, nonthermal acoustic pressure waves generated via an electric discharge inside a fluid, which is known as the electrohydraulic method, is used.
Specially modulated shockwaves are delivered directly beyond the wound bed and periulcer area. Energy penetrates deep into and beyond the wound bed to the micro-vascular level where the problem originates, to stimulate perfusion and promote arteriogenesis and angiogenesis and form a new capilary network in the pathology of healing and wound closure.
The dermaPACE® system noninvasively delivers focused extracorporeal shockwaves that stimulate biological responses at the cellular level via mechanical stressing of tissue.
PACE® technology elicits the following responses:
Is dermaPACE®?1
■ Extracorporeal Shockwave Technology (ESWT) utilizes high energy focused acoustic shockwaves created through an electrohydraulic method to treat chronic wounds
■ Energy stimulates cells, which starts cellular intercommunication resulting in expression of multiple healing factors
■ The dermaPACE® System is the first shockwave technology cleared by the FDA in the USA for treatment of Diabetic Foot Ulcers (DFU) only
■ The dermaPACE® System is used for the repair and regeneration of skin, musculoskeletal tissue, and vascular structures
■ One treatment session typically lasts 5-7 minutes and fits in with the normal wound care routine in the physician’s office
■ It is non-invasive and well tolerated
■ Reach Beyond the Wound Bed to the micro-vascular level to establish a new capillarity network resulting in a wound healing process
■ Works with Standard of Care or alone
1 Choose an appropriate patient
2 Prepare the wound bed according to Best Practice Guidelines
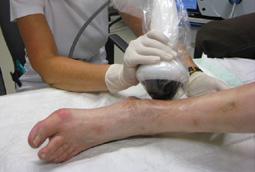
3 Use the intuitive screen to program the treatment settings
4 Apply the gel to the applicator head and the wound bed
5 The gel will allow high energy PACE® waves to penetrate the tissue as a coupling medium
6 Move the probe slowly and continuously across the surface of ulcer and periwound
7 Perform wound assessment before each treatment session, documenting the observations
8 Possible side effects can include reddening of wound, tingling and numbness
9 Remove the gel and apply standard dressing
10 Confirm treatment frequency regime with patient (Typically 1 treatment per week)

■ Clinically proven to manage and heal wounds at greater rates and frequency than standard of care alone. The dermaPACE® system’s treatments can be used in combination with other treatment modalities, without causing any negative interference
■ dermaPACE® demonstrated superiority in wound healing and wound area reduction, with an excellent safety profile. When compared to the control, superior results were achieved4:
■ A greater number of DFUs treated with dermaPACE® healed (37.79%), as compared to DFUs treated with only standard of care (26.2%). These results were statistically significant beginning at 20 weeks to 24 weeks
■ DFUs treated with dermaPACE® reached closure at a faster rate than DFUs only treated with standard of care
■ Beginning at 4 weeks, the dermaPACE® group had a higher percentage of subjects with a 50% wound reduction compared to the control (p=0.058). This advantage for dermaPACE® continued throughout the remainder of the trial
■ Banasiewicz and Cioanta note the ease of administration of the device, remarking how the noninvasive therapy was well tolerated by patients, and that a higher dosage has better results2
■ “We have been involved with dermaPACE® since 2012 after being part of the pivotal trial that demonstrated its utility in speeding wounds to closure. Since that time we have used the device extensively, with tremendously positive results not only in diabetic wound healing and closure but also incision line annealing, peri-wound edema reduction, pyoderma gangrenosum and adjunct treatment of osteomyelitis. We believe that these uses represent just the tip of the iceberg for dermaPACE®.”
Dr. Perry V Mayer, The Mayer Institute (TMI), Hamilton Ontario, Canada
■ “Results using SANUWAVE’s dermaPACE System have been very positive in treating diabetic foot ulcers. We put dermaPACE to use on complex DFUs that have not responded to the best practice applied to wounds longer than six months with a marked improvement. I highly recommend that you add this device to all advanced wound centers.”
■ “With the dermaPACE system, all of our diabetic foot ulcer patients that have completed six to 10 weeks of treatment have 100% healed. All patients had ABIs above 0.5 or had no peripheral ischemia with lower limb threatening disease.”
■ “In a patient with diabetes, we normally have decreased blood flow to the foot. This decreased blood flow starves the tissues and causes a skin breakdown and ulcerations. By applying this dermaPACE technology, we stimulate blood flow to these starved areas. When my patients were randomized in the study, we saw a remarkable rate of closure.”
Despite the development of advanced wound care products, there is still a need to find the most effective treatment for reducing the time required to close a wound. The Noninvasive Extracorporeal Shockwave Therapy system, dermaPACE®, shows well evidenced improvements in wound area reduction, financial viability when compared to other costly alternatives, and ease of use with minimal training required.Dr Brian Harper, Clinical Director, York Medical
Dr Peter P Balingit, LA County Dept. of Health Services





Millions of Americans are afflicted with painful, open, draining ulcers on their lower extremities. Venous leg ulcers (VLUs), cause significant clinical and economic burden to the health care system and society.1,2 It is not uncommon for clinicians to see patients who have suffered for years with VLUs. VLUs are the result of chronic venous insufficiency, a malady caused by an abnormality of the veins in the lower extremity.
The lower extremity venous system is composed of both a superficial and deep system connected by an elaborate series of perforating veins.2,3 Under normal conditions, valves within these veins direct blood from the superficial into the deep system, which in turn carries the blood back towards the heart. The flow in the deep system is directly impacted by the pumping action of the musculature in the legs during physical activity. A host of illnesses and disease states can directly affect the anatomic function of the venous system. Deep vein thrombosis, for example, damages the valves in the veins of the deep and superficial systems. Pregnancy increases the risk of chronic venous insufficiency and VLUs: functional and structural changes occur in the venous system secondary to elevated hormone levels and damage caused by elevated pressure on the inferior vena cava by the enlarging fetus.4 In addition, there is a hereditary predisposition toward valvular dysfunction eventually leading to the development of VLU. Obesity and sedentary lifestyle can also contribute to the
development of venous insufficiency.
The common etiology of venous insufficiency is the reversal of blood flow from the deep to the superficial venous system.2,3 This reversal of flow leads to pooling of the blood and fluid in the legs. The patient may experience swelling or edema in the lower extremities as a first sign of disease. Over time, hallmark trophic changes in the tissues appear: hyper-pigmentation, venous stasis dermatitis; hemosiderin deposits, loss of hair; thickened nails, atrophy blanch, and lipodermatosclerosis. As a result of these changes, skin breakdown can occur, resulting in ulceration. Finally, VLUs typically occur in the medial gaiter region of the lower leg. This corresponds to the position of the perforating veins connecting the superficial and deep systems.
Several competing theories aim to describe the progression from chronic venous hypertension to skin breakdown and ulceration. A popular hypothesis is that hypertension in the venous system leads to the development of a pericapillary fibrin cuff, that forms a barrier to oxygen diffusion; the skin and subcutaneous tissue become hypoxic and subsequently ulcerate.5,6 The final common pathway for all the theories is tissue ischemia: another theory suggests that white cells plug the capillaries causing tissue hypoxia.6 A more recent theory of ulcer pathogenesis suggests that increased inflammation, brought on by a cycle of chronic ischemia-reperfusion, leads to skin breakdown.7 Neutrophils, activated by repeated ischemiareperfusion, release oxygen-derived free radicals
(ROS). The ROS stimulate the formation of capillary cuffs that impair oxygenation and trap more neutrophils, creating a vicious cycle of inflammation. The repeated activation of this cascade eventually overwhelms the body’s compensatory capacity and the balance tips in the favor of tissue destruction.8 In many cases the immediate cause of the ulceration is a traumatic event; however, healing is disrupted by one or a combination of factors described above.8 Correction of the underlying venous hypertension is the crux of treatment for VLUs, but compression alone fails to allow for optimization of the wound healing environment. Even with advanced wound care, many VLUs fall short of achieving complete wound resolution.
The author/ investigator hypothesized that the addition of a wireless electroceutical dressing (WED) to standard of care could further support wound healing in chronic VLU patients. The WED harnesses V.Dox Technology (Vomaris Wound Care, Inc., Tempe, AZ), mimics the electric potential found in the skin. When skin is wounded the physiologic electric field is disrupted. Clinical evidence has shown that application of low levels of exogenous electricity can support the body’s natural electrical gradient, contribute to cell migration, and encourage wound healing.9 The contact layer of the WED is composed of elemental silver and elemental zinc, in a dot-matrix pattern on a polyester substrate. In the presence of a conductive medium such as wound exudate, sterile saline, water or a wound hydrogel, silver and zinc ions are activated and the dot-matrix pattern creates a microcell battery. Low-level electric fields are generated at the surface of the dressing. Electricity is generated via a redox reaction. This mechanism of action is dissimilar to the ‘release of ions’ seen in traditional silver dressings. The voltage
between the dots is measured to be 0.2 - 1.0 V when in contact with wound fluid. These levels are non-hazardous and support the natural skin current.10 Biofilm is believed to be one of the most common causes of wound chronicity. It has been estimated that up to 90% of chronic wounds have biofilm bacteria.11 The WED has also been shown to effectively disrupt biofilm bacteria.12 VLUs are heavily exudative and are plagued by adherent biofilm formation at the wound base. Therefore, it would stand to reason that reducing biofilm bacteria with the use of the WED dressing would support more rapid wound healing. The purpose of this study was to determine the effects of the WED on wound healing and modulating biofilm, as detected by qPCR wound cultures, to support wound healing in chronic venous leg ulcers.
This was a prospective, randomized (1:1), open label, single center pilot study to investigate healing with WED dressing in venous leg ulcers (VLU) within a 12 week time frame. The study was planned to enroll 20 patients (10 in each group). A treatment group (WED and Multi-Level Compression Therapy (MLCT), was compared to a standard of care (SOC) group (silver alginate and MLCT). The primary endpoint was wound surface area percent reduction. A secondary endpoint was to determine disruption of biofilm causing bacteria within VLUs due to the different wound treatments.
All participants included in this pilot study were ≥18 years of age, and had a non-healing venous leg ulcer present for ≥4 weeks that had failed ≥1 wound care treatments. Following the IRB approved protocol, written informed consent from the subjects was obtained by the PI prior to performance of wound assessments or data collection. Only one wound per patient was selected by the Principal Investigator (PI) as the study (target) wound. If multiple wounds
“Correction of the underlying venous hypertension is the crux of treatment for VLUs, but compression alone fails to allow for optimization of the wound healing environment. Even with advanced wound care, many VLUs fall short of achieving complete wound resolution.”
Mean
Age
Range:
were present, the largest that met eligibility criteria was selected. A review of inclusion and exclusion criteria was then performed; if deemed eligible, participants were enrolled in the Screening Phase of the study.
The study was divided into a Screening Phase and a Treatment Phase that spanned 12 weeks. Participants whose wound closed during the study or completed the Treatment Phase, but their wound did not heal, were considered as having completed the study. Once subjects successfully completed the informed consent process, they entered into the Screening Phase of the study. Standard wound measurements and photographs were obtained using the eKare device (eKare Inc., Fairfax, VA). During the Screening Phase, all wounds were treated with inert alginate as the primary dressing and multilayer compression bandage therapy (MLCT), for the entire 14 day run-in period. The Screening Phase consisted of 2 wound assessments spaced 7 days (+3 days) apart to determine patient eligibility. At each screening visit the wound was measured and photographed via the eKare device. After completion of the Screening Phase, if the target ulcer had not decreased in size by ≥20% and the subject was able to tolerate MLCT, they then advanced to randomization.
The subjects were randomized via envelop system. Both groups were seen weekly during the 12 week Treatment Phase for dressing changes, wound assessments, wound photos and measurements. qPCR wound cultures (MicroGenDX, Lubbock, TX) were collected from both cohorts at weeks 1, 4 and 8, to determine bacterial cell abundance, and taxonomic composition.
Descriptive statistics were expressed as mean and standard deviation (SD). Mean, standard deviation, or percentage change/ reduction were calculated for primary outcomes.
As a consequence of the global COVID-19 pandemic, in-person research was temporarily suspended at the author/ investigator’s institution, the grant funding for this project was not renewed and enrollment in this study was prematurely closed. A total of 6 patients were enrolled in the study prior to the work stoppage. Two in the standard of care arm (silver alginate and MLCT) and 4 in the treatment arm (WED and MLCT), completed the study. Patients’ demographics and baseline characteristics are
in
1.
being diabetics (3 out of 4).
All patients receiving the WED compression tolerated the treatment well. There were no reported adverse events. Subjects experienced no pain, irritation, or maceration associated with the use of the WED product. No adverse events were reported in the SOC group either.
One wound from each group completely closed at week 9 (SOC group), and week 10 (Treatment group). Average wound surface area in each group was reduced more than 60% by week 8, and 98% by week 12. Figure 1 illustrates wound surface measurement at baseline, week 4, 8 and 12 for all patients in both groups. Average wound surface area and wound reduction in both groups are listed in Table 2. It is worth noting that the baseline wound surface area (8.08 ± 1.81 cm2) in the SOC group is less than half the size of that in the Treatment group (17.45 ± 4.78 cm2). At week 12, all remaining wounds in both arms reached a significant reduction (97%) with average surface area of less than 0.5 cm2.
With the exception of patients #10 and #12 (Treatment group), all patients demonstrated significant improvement in their wound reduction by week 4 as noted in Figure 1. patient #12’s wound reduced significantly by week 8 while patient #10 reached a significant reduction at week 12.
Patient #10 was a 67 year-old African American male with a history of gastric bypass (2018), PVD, NIIDM, hypertension, hyperlipidemia, peripheral neuropathy, edema, varicose veins,
xerosis, and baseline wound size of 14.8 cm2 This patient also showed wound culture of a dominant campylobacter ureolyticus at week 4, which decreased slightly later with E. colipresence at week 8 (it was below detection level at week 4). At week 12, wound surface area was 1.3 cm2.
Patient #12 was a 65 year-old Caucasian male with a history of NIDDM, hyperlipidemia, COPD, edema, PVD, neuropathy, Hep C and a baseline wound size of 22.04 cm2. This patient showed wound culture of a dominant staph aureus at week 4, which decreased significantly later at week 8. At week 12, wound surface area was 0.2 cm2.
Patient #9 was a 57 year-old African American female with a history of NIDDM, hypertension, PVD and a baseline wound size of 20.88 cm2 in the right lower leg with a duration of 3 months. This wound failed prior therapies including
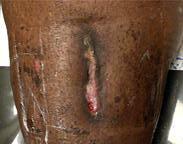
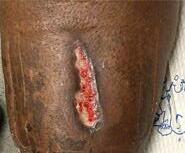
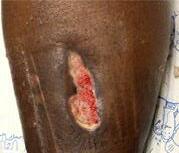
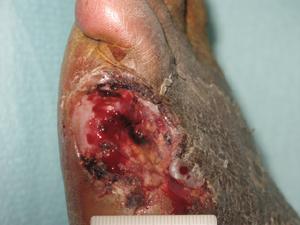
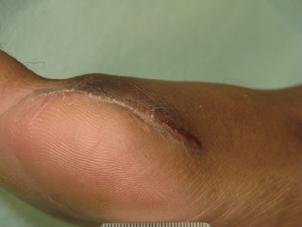
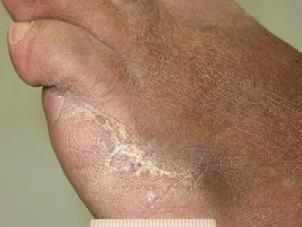
Prisma, Alginate and compression bandages. At week 10 the wound completely closed. Figure 2 shows photos of the wound at baseline, week 4, week 8, and healing at week 10.
Patient #3 was a 76 year-old Caucasian male with a history of Vit D deficiency, CHF, hypertension, PVD, A-fib, Psoriasis and a baseline wound size of 12.09 cm2. At week 12 the wound surface area was 0.09 cm2.
Patient #7 was a 61-year-old African American male with a history of Gout, Chronic venous insufficiency, edema, anemia, CAD, hypertension, hyperlipidemia, and a baseline wound size of 9.36 cm2. At week 9 the wound completely closed.
Patient #6 was a 56 year-old Hispanic female with a history of PVD, edema and a baseline wound size of 6.8 cm2. At week 12, wound surface area was 0.35 cm2
Wound swab samples were collected from all patients at week 1, 4 and 8 and were analyzed by an independent lab to assess bacterial alfa diversity (species richness) and estimated bacterial cells abundance in each sample for each patient. Estimated bacterial cell abundance for each patient at each visit are presented in Figure 3. Bacterial species noted at each sample for each patient are presented in Figure 4.
There was no significant correlation between estimated bacterial cells with each collection visit (Kruskal-Wallis test; x2x2 = 13.10, p = 0.52). There was also no significant correlation between estimated bacterial cells and treatment groups (Kruskal-Wallis test; x2x2 = 13.81, p = 0.46).
Multiple bacterial species observed in all samples in both groups show how the diversity of wound microbiota differed between each treatment group and at each visit (Figure 4). Although the data only represents the 6 patients analyzed and no statistically significant differences were observed in this study, wound surface area and microbial species diversity decreased concurrently in both groups over the 8 week study period. This observed trend,
Figure 3: Plot depicts the log q value of each treatment group over the completed study period. Coloring has been applied to indicate each treatment group and lines connect dots associated to the same patient and wound. The horizontal dashed line represents the cutoff for the qPCR analysis. Values below the 4.5 threshold did not pass the limit of detection (Ct = 30) and can be treated as zeros.
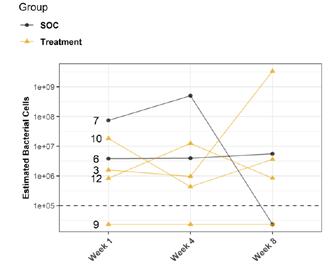
which could be due to the small number of samples, contradicts previous research on wound healing which showed that decreasing wound diversity has a significant negative linear relationship with healing rate, suggesting further research is needed.13
The main objective of this pilot study was to evaluate healing with the WED dressing and MLCT in venous leg ulcers (VLU), to gain knowledge of its performance for our clinical practice and possible further research. As noted above, due to COVID-19, the project was stopped after enrolling 6 patients. One wound from each group healed at week 9 and 10, and the remaining four wounds were reduced significantly (97%) from baseline at week 12.
Several authors reported and discussed VLUs healing rates and average monthly wounds reduction to predict wounds closure. In a retrospective review of 1323 patients enrolled in 6 prospective clinical studies, Rajhathy et al.14 identified 777 patients who were treated with compression and advanced wound dressings for VLUs, with a baseline wound surface area average of 9.8 cm2.
The authors reported wound closure in 328 (42.2%) patients by 3 months or 65.8% by 6 months, with an average monthly wound size reduction of 30%. Others suggested wound size
reduction by 20 - 40% in the first 4 weeks is predictive of complete closure by 6 months in participants treated with high compression.15,16
We find this data very helpful for clinicians to anticipate healing and recognize progress in wound size reduction at 4 weeks. In our patients, we noticed wound size reduction in the treatment group was 30% at week 4. By the end of study at 12 weeks, 97% of wound size reduction was achieved.
Polymerase chain reaction (PCR) is a chemical reaction harnessed to detect and identify trace bits of DNA, whether from a virus or bacteria to study the organism or diagnose an infection.17 Real-time PCR has been shown to be capable of detecting both live and dead bacterial DNA regardless of the inactivation procedure.18 The results of one study showed that direct qPCR resulted in an overestimation of up to 10 times of the amount of cells in the samples compared to viable counts, due to detection of DNA from dead cells.19 Therefore, it is possible that while bacteria were still detected on sampling visits, this was not representative of only live bacterial levels. In addition, variance in technique, while collecting the culture swab throughout the
study, could have resulted in higher bacterial loads. For example, greater pressure applied while sampling or more twists of the swab could result in a larger bacterial concentration.20

The COVID-19 outbreak posed an unprecedented challenge to many aspects of academic life. Cancellations and postponement of scientific events, including national and international conferences, symposiums, workshops, in-person training programs and classes, as well as scientific research. In many fields, if research was not directly related to the pandemic it was abandoned or displaced, as was the case with this project.
Pilot studies remain important for the development of new ideas and the expansion of future agenda items, for the progress of science and scientific communities. VLU healing rates are often prolonged with only 40 - 60% on average healed within 12 - 24 weeks, and once healed, 75% develop a recurrence within 3 weeks.13,19 At least 60% of VLUs become chronic.21 Hence, it is the author’s contention that although there were not enough patients participating in this study, the results are encouraging and there is opportunity
to expand on the basic concepts presented in this report. For example, piggybacking off the information gained from this pilot study, the author/ investigator initiated a 5 patient case series in her out-patient clinical practice. All patients had baseline positive fluorescence wound images, indicating pathologic levels of surface bacteria were present (≥104 CF). These patients were then treated with the WED and a secondary moisture managing dressing. Dressings were changed every other day by the patient or caregiver. wound images were then obtained one week later to determine if there was evidence of changes in bacterial contamination based on fluorescence images. All 5 wounds in this small case series showed a marked decrease in bacterial fluorescence, with just one week of WED therapy, as noted by lack of fluorescence on the images. It was concluded that the WED did decrease harmful bacteria levels initially present in the wounds of varying etiologies to less than 104 CFU. Large randomized clinical trials are needed to validate the findings of this small case series.
This was designed as an investigational pilot study. Although randomization is considered a strength, due to unforeseen circumstances brought on by the global pandemic, the study was limited due to the small sample size. Therefore, it is difficult to make widespread conclusions based on these study results alone.
The present study demonstrated that use of the WED was well tolerated in this patient population and may support wound healing in chronic venous leg ulcers. More research is needed. Real-time detection of bacteria through fluorescence imaging technology may better illustrate how the WED can help to manage bioburden and support an ideal environment for wound healing. Future studies should look
to incorporate larger sample sizes and greater durations of observation to affirm this limited pilot investigation.
This study was supported in part through a grant from the Podiatry Foundation and with the donation of the WED from Vomaris Wound Care, Inc. The authors would also like to recognize the MicrogenDX team (Nichola Sanford, PhD; Craig Tipton, and Jacob Ancira) for their assistance with the bacterial data analysis, and Stacey Coe, MS, CCRP for her help with data collection for this study.
1. Ballard JL, Bergan JJ, editors. Chronic venous insufficiency. Diagnosis and treatment. London: Springer-Verlag, 2000.
2. Negus D. Leg ulcers: A practical approach to management. 2nd ed. Oxford: Butterworth Heinemann, 1995.
3. Olivencia JF. Pathophysiology of venous ulcers: surgical implications, review and update.
Dermatol Surg 199: 25:880-885
4. Ginekol Pol. Risk factors for the development of venous insufficiency of the lower limbs during pregnancy—part 1. Review Article 2012 Dec; 83(12):939-42.
5. Browse NL. Venous ulceration. BMJ 1983; 286:1920-1922.
6. Coleridge Smith PD, Thomas P, Scurr JH, Dormandy JA. Causes of venous ulceration: a new hypothesis. BMJ 1988;296:1726-1727.
7. Coleridge Smith PD. Causes of venous ulceration-a new hypothesis. Br Med J (Clin Res Ed) 1988; 296(6638):1726-7.
8. Falanga V. The “trap” hypothesis of the venous ulceration. Lancet 1993; 341:1006-1008.
9. Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1-26. doi: 10.1016/s0070-2153(03)58001-2. PMID: 14711011.
10. Banerjee J, Ghatak PD, Roy S, et al.; Improvement of Human Keratinocyte Migration by a Redox Active Bioelectric Dressing. PLoS ONE. 2014 Mar 3;9(3):e89239.
11. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. Journal of wound care. 2017;26(1):20-25.
12. Barki KG, Das A, Dixith S, Ghatak PD, Mathew-Steiner S, Schwab E, Khanna S, Wozniak DJ, Roy S, Sen CK. Electric Field Based Dressing Disrupts Mixed-Species Bacterial Biofilm Infection and Restores Functional Wound Healing. Ann Surg. 2019 Apr;269(4):756-766.
13. Tipton CD, Wolcott RD, Sanford NE, Miller C, Pathak G, Silzer TK, et al. (2020) Patient genetics is linked to chronic wound microbiome composition and healing. PLoSPathog 16(6):e1008511.
14. Rajhathy EM, Murray HD, Roberge VA, Woo KY. Healing Rates of Venous Leg Ulcers Managed With Compression Therapy. J Wound Ostomy Continence Nurs. 2020 Sep/ Oct;47(5):477-483
15. Sibbald G, Woo K, Ayello EA. Increased bacterial burden and in¬fection: the story of NERDS and STONES. Adv Skin Wound Care. 2006;19;447-461.
16. Benigni J, Lazareth I, Parpex P, et al. Efficacy, safety and acceptability of a new two-layer bandage system for venous leg ulcers. J Wound Care. 2007;16(9):385-390.
17. Maurin M. Real-time PCR as a diagnostic tool for bacterial diseases. Expert Rev Mol Diagn. 2012 Sep;12(7):731-54. doi: 10.1586/erm.12.53. PMID: 23153240.
18. Wolffs P, Norling B, Rådström P. Risk assessment of false-positive quantitative real-time PCR results in food, due to detection of DNA originating from dead cells. J Microbiol Methods. 2005 Mar;60(3):315-23. doi: 10.1016/j.mimet.2004.10.003
19. Griffith C. Surface sampling and the detection of contamination. Handbook of Hygiene Control in the Food Industry. Elsevier; 2016:673-696
20. Abbade LP, Lastória S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol. 2005;44(6):449–56.
21. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle). 2015;4(9):560–82.
Venous
“WED did decrease harmful bacteria levels initially present in the wounds of varying etiologies to less than 104 CFU.”
Virtual reality simulation is a powerful tool that harnesses technology to improve the efficacy and precision of learning medical procedures. This Masterclass Guide will look at the role of this advanced technology in wound management and education.
■ As technology transforms all sectors in healthcare, there is a need for innovation in learning and professional development
■ Use of technology such as virtual reality in medical training allows higher levels of interactivity, and the ability to rehearse clinical procedures and refine relevant skills1

■ Education in wound care is vital to ensure that healthcare professionals (HCPs) can accurately identify, diagnose and manage wounds, ensuring that they deliver safe and effective care for patients
■ Minimizes the impact on resources such as time high cost presence in clinic that can be involved in the medical practical education requirements
■ Additionally, it is a challenge to be able to deliver meaningful training that applies to real world scenarios without putting patients at risk
■ Training using virtual reality (VR) and augmented reality (AR) has been well established in other medical fields, such as surgery, for some years. On this subject, Nassar et al. concluded that technical skills training should commence outside the operating room on a simulator, with VR found to be successfully used to provide safe training, and to validate and fine-tune acquired skills to achieve proficiency and independent practice2
■ The major strengths of VR compared to other simulation modalities, were found to be that it facilitates deliberate practice with built-in auto feedback helping to address limited staff resources2
■ HARTMANN Virtual Reality Wound Care Simulation Training was launched at the World Union of Wound Healing Societies Congress 2022, pioneering a new approach to wound care education
care
Reality Simulation
■ Facilitates realistic training without the need for involvement of real patients or putting patients at risk
■ Allows clinicians to make bold choices in a risk-free environment and learning from errors without causing patient harm
■ Provides a meaningful and memorable training experience
■ Provides an objective and granular picture of what HCPs are doing correctly and identifies areas for improvement

■ In wound care, VR training can provide a safe and effective means of HCP education, in a way that can be applied to real-world practice without causing risk of harm to patients
■ Virtual reality technology can offer a unique clinical environment and patient specific 360 degree view
■ Use of VR technology can also provide a more flexible form of training for busy HCPs4
■ HARTMANN’s Virtual Reality Training Program in Wound Management introduces a new approach to wound care education, using VR simulation to give healthcare professionals a deeper, interactive, and intuitive learning experience
■ Additionally, VR-based tools can incorporate a ‘checklist-style’ assessment measures to objectively evaluate proficiency, taking the process outside the realm of human subjectivity; thus, VR training tools offer a highly granular picture of what HCPs are doing correctly and identify areas for improvement5
■ It allows exposure of new procedures to students, nurses, doctors and other healthcare professionals
■ By immersing the user in a three dimensional virtual world it facilitates limitless opportunities for education, training and management
■ VR is the computer-generated simulation of a threedimensional image or environment where the user can interact in a seemingly real or physical way by using special electronic equipment, such as a helmet with a screen inside or gloves fitted with sensors
■ The capability of today’s VR headsets means that truly immersive virtual experiences are available for the first time in history4. These technologies are now being adapted for medical education and are enabling further interactivity, immersion, and safety in medical training1
■ Advancements in VR technology now provide the training necessary for skill acquisition and transfer to real-word practice, such as in surgical procedures2
■ Recent clinical data demonstrated VR training programmes as a more effective modality of learning styles in various medical fields6
■ In a related study, 20 participants were randomised into VR and standard learning groups. All 20 participants completed the first phase and 17 completed the second phase of the study
■ The authors concluded that VR training was more effective than standard learning in this surgical field


■ HARTMANN Virtual Reality Wound Care Training offers a unique learning experience based on Virtual Reality (VR) Simulation, providing lifelike clinical experience in Wound Management. This specific algorithm of learning offers the first of its kind 3D immersion and simulation with life like clinical experience in wound management
■ The user has the opportunity to see a patient and their wound, and to select the correct treatment for the individual patient. They will then be able to find out if they made the correct treatment choices, by tracking the wound and its progress over time, conducting dressing changes and choosing continued treatment choices at each stage, and influencing the final outcome
■ Crucially, the VR training gives the HCP the chance to explore clinical options safely and even learn through trial and error, without compromising patient safety. This provides an opportunity to build clinician confidence and, additionally, the novel experience of using the VR technology makes learning memorable. Using VR technology in wound management helps to improve the perceptive level of training, increasing its relevance to application in real-world practice
■ The VR training modules complement the Wound Management education programmes HARTMANN has already offered to HCPs via seminars, conferences and workshops. The VR modules allow remote, collaborative learning, enhancing either autonomous, peer-to-peer or group learning experiences of HCPs in wound care. The format of the training encourages interactions with peers and open seminars, conferences and workshops
■ The technology is progressive and easy to use, consisting of a headset and handheld controllers. The VR training scenarios allow HCPs to evaluate patient history, to examine and diagnose the wound type, and to select the dressings considered as the appropriate therapeutic solution for the given healing phase This way the focus of the VR training is on evaluation, clinical reasoning and decision making, designed to mirror practice in the real world. Clinical reasoning and decision making skills are improved, and the user’s hand movements and possible errors during dressing applications are corrected. The training enables users to go on to identify more easily and correctly the specific wound types more accurately and confidently, and to select the right treatment options for those wounds
■ User wears headset and handheld controllers
■ The VR training allows the user to assess the patient and their wound
■ User selects appropriate treatment for the individual patient and applies the dressing to the patient using the tutorial provided
■ User can see the wound progress, conduct dressing changes and make continued dressing choices
■ Correct or incorrect selection of dressing will be visualised as wound progress or healing regression; the user can repeat and correct their selection, as necessary
■ User receives a memorable and meaningful training experience that can be applied to real-life practice, increasing their confidence and enabling them to make accurate diagnoses and deliver safe and effective care
“Impressive method, the VR simulation scenarios push you to think like in real-life clinical conditions.” (Surgeon, Chile)
“With present legal requirements, the access to patients with a certain type of wound/s is difficult and this VR technology facilitates all steps of clinical protocol.” (Wound Nurse, EU)
“We can simulate as many clinical scenarios as we need, very useful and memorable experience.” (Head of Nursing, UAE)
“An excellent learning method for general practitioners (doctors and nurses) as sometimes it is difficult to train them, and with VR you are excited to do it again and again and you never forget because you are really doing.” (Surgeon, Poland)
“I have been using VR for surgery learning for 12 years and this is the first time seeing it applied for wound care. It is an excellent idea as the nurses need such training, you can organise the study in the library of the hospital and still they can experience real clinical cases - this is the future!” (Surgeon, Oman)



■ VR is now being used as a powerful tool in medical education, allowing higher levels of interactivity, and the ability to rehearse clinical procedures and refine relevant skills1
■ The HARTMANN Virtual Reality Training Program offers the first 3D immersion and simulation with lifelike clinical experience in wound management, serving as a powerful educational tool and complementing the existing traditional, or advanced digital learning pathways

■ The VR technology allows training in a risk free environment, and the opportunity for ‘checklist-style’ assessment can offer a granular picture of what HCPs are doing correctly and identify areas for improvement5
■ This pioneering new approach to HCP education will increase clinicians’ skills and confidence and ultimately improve experiences and outcomes for patients
■ HCPs will be equipped to assess, identify, diagnose and manage wounds based on the patient’s history and described symptoms, selecting treatment options and tracking outcomes

Three-dimensional VR training modules provide healthcare professionals with an interactive and realistic clinical learning experience that allows collaborative learning without the involvement of the patient. hartmann.info


The increasing burden of wound care is well established in evidence. It is essential for the clinician to be provided with the adequate tools to safely and effectively provide a high standard of clinical care to wound care patients. The deployment of the electronic patient record (EPR) has attempted to standardize documentation in care. However, it remains difficult to extract the suitable data required to provide evidence based medicine. An overview of the future of different digitizing wounds is explored in detail.
The increasing burden of wound care is globally recognised. Many publications cite the rising economic costs and the rapidly increasing incidence of chronic wounds, and the impact that this has upon people and healthcare systems.1,2,3 Despite the increases in demand, the workforce required to deliver wound care is not expanding at the same rate; in fact, it appears to be contracting, driven by a global shortage of these professionals. Ousey et al. predicted a decline in the nursing workforce by 2016 due to reduced education commissions, attrition, rising retirements and net emigration of trained nurses.4 By 2020, 10% of community nursing posts in the UK were vacant.5 Given the mismatch between capacity and demand, it is vital to ensure that front line clinicians are provided with the correct tools to help manage these increasingly complex caseloads in as an efficient a manner as possible. Digital Wound Management Tools have the potential to achieve this.
I qualified as a Podiatrist in 2005, and still recall with a shudder being presented with a large volume of paper records for patients that I had to visit that day. The pressures on the service were such that I often did not know who I was going to visit in advance, giving little time for any forward planning, let alone working out the logistics of completing the round.
Obtaining simple information from nonstandardised assessments on crumpled sheets of paper was challenging and time-consuming.
I often found it hard to identify the key assessments that had been undertaken, and the resulting actions from them. Equally, deciphering the previous care plans to know what had been tried before, what worked, and equally what did not work was at times impossible. Fundamental questions such as how long the patient had the wound, what it looked like last week, last month and in some cases years before, took a long time to answer.
The deployment of Electronic Patient Records (EPR) had the potential to improve this by standardising the recording of wound assessments. In my view, many of the EPRs on the market use a primary care model of recording, where often singular interventions are recorded in free text boxes and templates that lack any form of validation.
This results in variation between clinicians in how and where things are recorded. If information is not in a consistent place, clinicians will have to hunt for it, potentially increasing the amount of time it will take them to get the information that they need. The secondary effect is that key data cannot be extracted for analysis of variables, such as healing rates.
Wound dimensions are frequently used to determine the progression of a wound. Frequently, for lack of a better available method, paper rulers are used to obtain these measures, even though this can result in the over-estimation of the wound area by 40%.6
To highlight this, the United Kingdom and
Uganda have similar land areas. The UK is approximately 1000km long and 500km wide on its longest axis.7 Uganda measures approximately 790km by 480km8. When the area of each country is estimated (length x width), the UK appears to be over 30% larger than Uganda; in reality, the UK is only 0.55% larger than Uganda.9
Given the variability of wound margins, and the high levels of intra-observer variability with wound measurement, it is easy to see how subtle changes in wound area could be missed, especially when the wound is attended to by multiple professionals.
I am not advocating that we teach clinical staff advanced mathematics to estimate wound areas, but provide them with digital tools to automate this process.
The need for digitizing wounds has not been lost on clinical staff. In the absence of a system, some resorted to using smartphones and digital cameras to take images of wounds, resulting in a variety of information governance headaches!
Even if the images obtained are downloaded and stored appropriately, clinical staff reviewing them must open, manipulate, and order images to judge progress, which is likely less efficient than working with paper records.
Although a paper ruler will often be included in the image, clinicians (likely hindered by varying lighting and wound orientation), still must note or memorise the dimensions from one image to the next to track healing.
An effective system needs to be capable of obtaining more than just a photograph of the wound; it needs to automate the process of assessment and measurement of key wound variables a consistent, reliable, and valid
manner.
What is crucial is that anything automated can be overridden if deemed to be inaccurate so as not to remove the clinical judgement of staff.
The UK National Wound Care Strategy Programme aims to eliminate unnecessary variation, and this is one of the ways that this can be achieved.10
When implementing a digital wound management solution, the buy in from clinical staff is imperative. This type of solution cannot be simply taken out of the box and deployed. The culture of a team and organisation must change if it is to be used to its maximal effect.
My organisation adopted a digital wound management system in April 2020. Some early adopters of the solution became champions for the system, and through push or pull, they have enabled their teams to start to use the solution and see its benefits. Without this buy in, I would not be able to write this editorial!
Digital solution must allow for the download of data from every assessment that a clinician undertakes. The reliability of this will be facilitated by a standardized, linear assessment flow that makes it obvious where data should be recorded.
Dashboard-style information at wound level will provide the clinician with a series of aligned photographs, progress charts and assessment notes in one place, making the process of working out the status of the wound more efficient, giving some time back to put into either direct patient care, or perhaps allowing the clinician to take the break that they often do not have time for.
“What is crucial is that anything automated can be overridden if deemed to be inaccurate so as not to remove the clinical judgement of staff.”
Digitizing the Future of Wounds: What Are the Challenges?
At a more strategic level, simple questions, such as wound time, recurrences rates, etc., can be obtained with ease, accuracy, and reliability, in ways that are either impossible or complicated, currently.
In my experience, clinicians do not like collecting data for the sake of collecting it, especially if the collection of this data adds additional time pressures onto their already pressured day. There does, however, need to be a balance of recording of data pertinent to the assessment, and data that will support the wider analysis of data from the entire caseload. The ethos we have adopted is that this data needs to be turned into information that can drive decision making from front line to strategic levels.
Clinical oversight of caseloads is more important now that it has ever been. The changing staff profiles in services has left potential gaps in skills and knowledge at more senior clinical levels, leaving a smaller pool of clinicians overseeing increasingly larger and more complex caseloads of people with wounds.
The time of these staff is more of a commodity, and needs to be used in an effective manner, given the size and varying geographic areas that organisations serve. Skill mixing within workforces means that care is often provided by assistant/ associate level staff, who follow care plans developed by registered staff.
If a member of staff spots that a wound’s characteristics have changed, they will require the support of a more senior clinician to review the care plan. The availability of senior staff might mean that this review could take days (if not longer) to happen. The patient however needs that input now to potentially prevent further deterioration.
Digital Wound Management facilitates this. As soon as an image of the wound is captured, the senior clinician can view the image and may be able to provide advice and guidance there and then; this may make an additional visit from them unnecessary. In some cases, the advice may be to continue the care plan. It is incredibly frustrating for the senior clinician to have spent an hour or more travelling and not actually need to do anything - it is not a good use of their time.
The roll out of our digital solution is still underway, but early evidence suggests that the solution is supporting a different skill mix, where we can provide one fewer ‘senior’ visit to every three visits from a junior member of staff, releasing significant amounts of time that can be used more effectively. The objective is not to remove senior posts, but to ensure that their time is used effectively.
As discussed, changes to wound area can be hard to spot over periods of time. The data that our clinicians record at each assessment is downloaded and used to identify people who have been seen in the previous week, have wounds that are increasing at an average of 10% by area per week, have a pain score of 7 (quality of life is as important as healing rate) or more, or have developed a new infection.
This is currently a crude model, but identifies about 15 - 20% of people seen in the last week that need to have a review. This helps our senior clinicians identify those in most need of their time. From a patient perspective, we have anecdotal evidence of this supporting expedited referrals into Vascular Services and getting people onto the right pathway where the original diagnosis of wound aetiology may not have been correct. In other cases, this will support a visit from a senior clinician to provide further assessment, and hopefully stabilise and get the patient onto a healing trajectory.
“Early evidence suggests that the solution is supporting a different skill mix, where we can provide one fewer ‘senior’ visit to every three visits from a junior member of staff... the objective is not to remove senior posts, but to ensure that their time is used effectively.”Digitizing the Future of Wounds: What Are the Challenges?
I have always felt that many research studies are limited by small sample sizes and can only follow people over relatively short periods of time. It is difficult and expensive to put together large studies, find suitable populations, and then get clinicians to collect the data.
Clinical staff have an eye for what could work and what does not. What they often lack are the tools and the time to prove it one way or another. We now have the potential to use the data from our digital wound management solutions to support research, audit, and quality assurance reviews. The standardised assessment flows will ensure that the data is available and issues relating to inter/ intra-user variability in measurement will be eliminated.
In the longer term, machine learning techniques could be applied to these large data sets. This will not only tell us how much of a variable is present, but it may also tell us which are the variables that we need to have the greatest affect upon to change the outcome for the patient, giving us insights that may be difficult to spot in small samples using standard statistical analysis.
1. Sen CK. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv Wound Care (New Rochelle). 2019 Feb 1;8(2):39-48. doi: 10.1089/wound.2019.0946. Epub 2019 Feb 13. PMID: 30809421; PMCID: PMC6389759.
2. Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020 Dec 22;10(12):e045253. doi: 10.1136/bmjopen-2020-045253. PMID: 33371051; PMCID: PMC7757484.
3. Martinengo L, Olsson M, Bajpai R, Soljak M, Upton Z, Schmidtchen A, Car J, Järbrink K. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 2019 Jan;29:8-15. doi: 10.1016/j. annepidem.2018.10.005. Epub 2018 Nov 12. PMID: 30497932.
4. Ousey, Karen, Stephenson, John, Barrett, Simon, King, Brenda, Morton, Nicky, Fenwick, Kim and Carr, Caryn (2013) Wound care in five English NHS Trusts: Results of a survey. Wounds UK, 9 (4). pp. 20-28. ISSN 1746-6814
5. NHS Benchmarking Network-Deep
The delivery of wound care is challenging across the globe, and despite differences in the way that services are commissioned and funded, the availability of appropriately qualified staff to manage these increasingly complex caseloads is diminishing. Digital Wound Management will help overcome this by providing tools that can pro-actively manage caseloads based on real time, reliable data and reduce variations in practice. This will help to ensure that a person with a wound gets the right care, at the right time, from the right professional.
Organisations need to move from reactive ways of managing caseloads to proactive methods, that identify the people with the variables that are predictive of a poor outcome and ensure that they receive the skills and expertise of highly experienced staff to optimise their care plans and put them onto the right trajectory.
A significant amount of time and effort is required to deliver a digital wound management system, but the potential benefits of this effort are enormous, and will only benefit people with wounds and those who provide care for them.
“The potential benefits of this effort are enormous, and will only benefit people with wounds and those who provide care for them.”
What
the
Real-life wound imaging with 2D and 3D measurement technology

Real-time access to case load data and analytics
Realised efficiencies in workforce and documentation
Minuteful for Wound is a CE marked medical device which integrates with your electronic record – a smartphone is required. To request a demo scan the QR code. healthy.io/eu/services/wound | wound-uk@healthy.io

Wound care providers know the magnitude of the silent epidemic of chronic wounds. Globally, over 40 million people live with chronic wounds.1 In some countries, wound care accounts for 35 - 65% of post-acute care.2 Government spending in the United States through Medicare is estimated at $96 billion annually.3 Beyond the size of the problem, health care providers face many challenges in providing proactive, optimized wound care resulting in delayed wound healing, poorer outcomes and lower quality of life for patients with wounds.
Similar to many other industries, wound care has the opportunity to address these challenges with technology designed specifically for the needs of clinicians and patients. Solutions that provide easyto-use tools at the point of care across the healthcare continuum help empower clinicians of all skill levels to effectively manage their patients’ wounds.
The introduction of smartphones into clinical care enables easy correction of a core challenge of wound care, accurate measurement. Disposable paper rulers became an industry standard, and length and width are widely accepted as critical measures of wound healing. However, Rogers4 highlighted that wounds are not rectilinear, and using length and width for area calculation can lead to a 41% overestimation of wound sizes. Beyond the two-dimensional surface area, wounds also have depth. Smartphones now have high-quality cameras and increased computing capacity. AI-powered applications can conduct threedimensional analysis of the wound bed and identification of the deepest point.5 Submillimeter accuracy can be achieved considering the entire visible wound bed and reducing the variability between clinicians. The ability to map a wound again increases the potential to precisely assess the wound volume and closure over time. Wound closing by secondary intention requires tissue granulation at the base of the wound to occur, followed by eventual reepithelialization.6 Thus, the surface area may lag behind depth or wound volume measurements. Wound care clinicians equipped with modern digital wound assessment tools can counteract these measurement challenges, which can compound when clinicians compare wound measurements over






 Mr David Mannion
Dr José L. RamírezGarcíaLuna
Mr Robert D. J. Fraser, RN
Mr David Mannion
Dr José L. RamírezGarcíaLuna
Mr Robert D. J. Fraser, RN
time and their potential to lead to inappropriate treatment changes or failure to identify chronic wounds.
Figure 1: Kong et al. (2021) implemented an AI-powered digital wound management solution (Swift Medical Inc., Toronto) with remote patient monitoring to precisely measure wounds taken by clinicians and patients. Their results highlight how the use of a specialized wound imaging platform enabled the acquisition of images by the patient comparable to those taken by the healthcare provider while allowing him to be remotely monitored during the COVID-19 pandemic.10
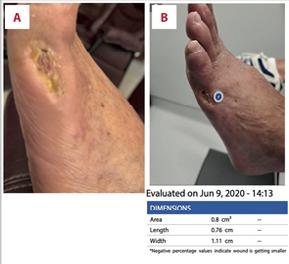
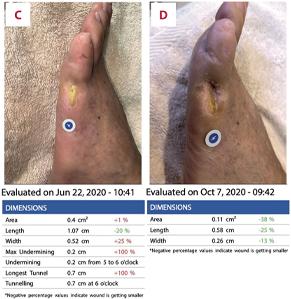
Accurate assessment over time can be further complicated by variability among clinicians. Technology can make wound assessments more consistent by reducing confounding factors such as skills and subjective judgement. Digital area and temperature measurement are two specific examples of wound documentation subject to variations. Wound size is essential to overall wound healing, and temperature indicates potential infection or delayed wound healing.7 Temperature is frequently measured subjectively in practice using palpation, and some clinicians are slowly adopting thermometry. Thermometry uses a laser to measure the temperature of a single point objectively. In contrast, thermography uses a thermal camera to measure an image that can collect temperature measurements for thousands of points.8 Wang and colleagues evaluated variation in measurement of size and temperature between clinicians across 42 wounds using traditional methods of a paper ruler and smartphone-based digital planimetry (area measurement) and thermography.9 The smartphone-based planimetry had a higher intraclass correlation coefficient and, thus, reproducibility than paper-based measurement. The smartphone-based thermography was equal to the clinically accepted reference thermometer. A notable difference was that non-expert clinicians also had higher interrater reliability. Therefore, this technology may address challenges caused by continuity of care and low wound care education in entry-level health professionals.
Patient self-monitoring is another theme across healthcare, and wound care is no exception. As shown in Figure 1, a smartphone app that securely enabled patients to upload wound photos to their medical records during the pandemic was deployed in Canada.10 The artificial intelligence (AI)-powered wound care app was simplified for patient use and would automatically enhance image quality and complete measurements for easy review
“This technology may address challenges caused by continuity of care and low wound care education in entry-level health professionals.”
by the clinicians. Patients responded positively, noting it saved time and money for them and their caregivers. Using the app reduced the need for travel to the clinic, reduced missed time from work and avoided the necessity to seek informal caregiver support for children. The clinicians could identify early warning symptoms of infection and recall patients as needed. Following this theme, a scoping review found that telehealth for wound care was not inferior to in-person care,11 indicating that clinicians will continue to adopt these methods beyond the pandemic. Making it easier for any clinician or patient to better monitor the wound allows clinicians to rethink their procedures and how frequently to schedule return visits.
Specialized wound management systems can even improve the efficiency of seasoned clinicians in high-volume wound clinics. Well-designed digital solutions can improve the measurement, capturing a wound photo and documentation, meaning even highperforming wound centers have opportunities to optimize care. A study comparing paperbased ruler measurement to smartphonebased measurement found the digital method was 57% faster than manual methods.12 Furthermore, a recent study using an AIpowered smartphone app in a high-volume wound clinic demonstrated significant time savings compared to manual measurements and digital photography.13 The wound app was 77% faster than traditional paper ruler wound measurement, and in completing all steps of documentation, the AI-powered smartphone app saved 54% of the time. This efficiency applied to the wound clinic’s regular volumes and business schedule could save 21.8 - 51.7 days of a clinician’s time annually by adopting the app. This time presents opportunities for additional patient care in a clinicians’ day.
Adopting digital wound management solutions creates further opportunities for research and innovation in wound care by creating big data
sets. Information captured by digital wound assessment and saved to the patient’s record can be used to advance our understanding of wounds and is now beginning to be accepted by regulators (e.g., United States Food and Drug Administration) for coverage of treatments.14 Leveraging this real-world data requires appropriate methods, identifying useful sources, inclusion/ exclusion criteria, and relevant cohorts, data can be transformed into usable information for calculating end-point outcomes15. For example, a recent study used real-world data insights from over 1.5 million wounds and identified over 188,675 skin tears assessments to assess their prevalence in North America.16 This study was over ten times larger than any previously identified study, demonstrating the power of digitizing wound documentation and creating representative analyses. Furthermore, rich data such as wound photographs can be used to develop better assessment tools.
Artificial intelligence and machine learning (ML) offer new possibilities for advancing wound assessment. Changes in the different tissue types within the wound hint toward a healing wound but can be limited in utility by the knowledge, skill and experience of clinicians performing this assessment. AI is ideally suited to automatically identify tissue types and perform the task of quantifying the amount, which humans perform poorly. Even when clinicians know how to identify tissue types (e.g., granulation, eschar), humans cannot accurately identify the percentage of tissue type beyond a general estimate. AI can use an image to predict wound tissue composition pixel by pixel and provide highly accurate measurements. As an example of this, Ramachandram and colleagues17 demonstrated an AI tissue-typing (called AutoTissue) that achieved a high agreement compared to a panel of expert clinicians’ tissue ratings (Figure 2). Furthermore, this AI is designed to be deployed on a mobile app that runs on entry-
“Artificial intelligence and machine learning (ML) offer new possibilities for advancing wound assessment... AI can use an image to predict wound tissue composition pixel by pixel and provide highly accurate measurements.” Digitizing Wound Assessment: Unlocking the Potential of the Wound Care Teamlevel smartphone models, therefore allowing it to be used seamlessly in clinical settings. This AI learned from thousands of wound images with labels for tissue types. The images had a wide variety of lighting conditions with diverse patient groups, reflecting the real world’s normal practices and ‘messy’ data. This type of model training results in a more robust prediction of tissue types than previous tools, which typically perform colour analyses that is trained on small data sets or in highly curated data sets. Wound tissues are not simply clear-cut red, black, or yellow. Instead, they are a mixture of cells often overlapping and presenting unique and complex patterns that AI can help analyze. Therefore, taking out the variability seen by clinicians’ assessments can improve documentation quality and, more importantly, increase the accuracy of wound assessment and prognostic scores that ultimately guide treatment.
More accurate wound healing prognostics are possible by leveraging big data available and objective wound assessments, such as AutoTissue. Gupta et al.18 used data from over 2.1 million wound evaluations to develop a model that identifies wounds at risk of delayed healing. Compared to commonly used wound scores such as the Pressure Ulcer Scale for Healing (PUSH) and Bates-Jensen Wound Assessment Tool (BWAT), their objective AI model performed better. Using advanced vision technology supports the concept of extracting objective information from wound imaging to help identify wounds that expert wound clinicians should review. Thus, AI-powered wound triage could identify hard-to-heal wounds, allowing wound specialists to be notified and assess wounds earlier if they change healing trajectory.
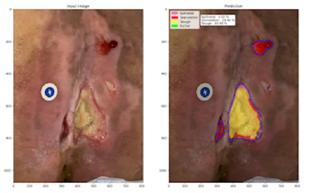
Embedding this AI in an app to be used across the continuum of care has significant benefits. Digitizing wound assessment and providing integrated AI tools empowers clinicians to work in new ways to optimize the entire health care team.
Another possibility for how wound care clinicians and teams organize themselves and achieve collective outcomes is the creation of collaborative digital wound records. One example is an inter-professional wound care team in Canada comprising ten home health provider organizations, eight hospitals and wound care centres. A regional health authority implemented a digital wound management solution to address chronic wounds in their population.19 The first phase of this project followed 248 patients that had chronic wounds. By continually monitoring the wounds for improvements using a smartphone app and escalating if no improvements were achieved in 14 days, the program attained a 3-month reduction in length of stay on home care, reduced emergency room visits by 7%, lowered readmission to home care by 50%, and savings of $1,000 (CAD) per patient per month. The savings resulted from decreased weekly visits and lower utilization of advanced wound care product spending. The wound care team reported that the digital wound management solution was intuitive, helped evaluate wound progress better, and supported better and faster decisions. Thus, it was concluded that a shared wound management tool could optimize clinician roles within and across organizations. Clinicians can quickly seek input when wounds are not progressing, and referral times can be decreased if rich standardized assessment documentation is captured in a shareable format.
Harnessing the power of digital wound assessment can translate to many positive outcomes. Home health can reduce hospitalization and less visit utilization per episode. In-patient settings can enable remote digital consultation models to provide prompt support from wound experts across their facilities. Clinicians can gain organizational support and funding by making the case through clinical and financial outcomes. Patients can be engaged in self-monitoring and self-care in the privacy of their own homes.
Digitizing Wound Assessment: Unlocking the Potential of theWound management solutions influence care beyond the initial assessment and achieve their highest potential using systems thinking. Clinicians need to understand the benefits of new digital tools in wound care to identify opportunities to supplement clinical skills and improve organizational outcomes.
The advancement of technology will continue to bring changes to wound care by challenging
and improving the methods of our current practice and developing new assessment capabilities. Over the past decades, different devices have come on the market, enriching the potential assessment of wounds. For example, bacterial fluoresces enables the ability to see microorganisms invisible to the human eye.20
Under violet light (405 nm) certain bacteria will fluoresce red or blue green
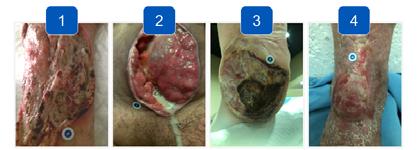
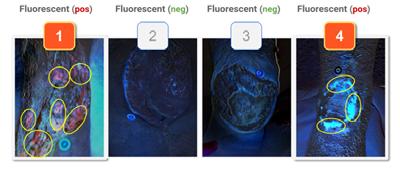
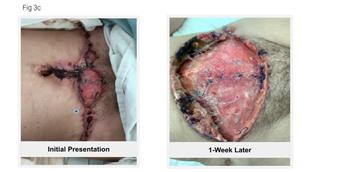
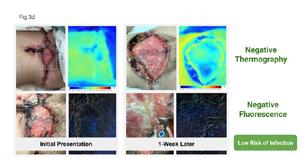
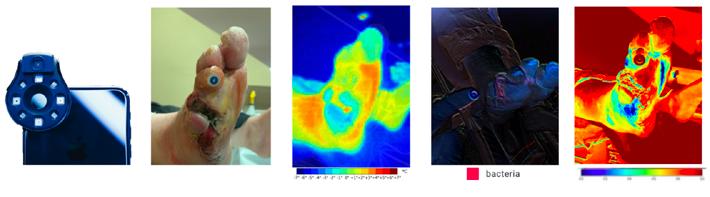
(See Figure 3). Thermography can reduce the subjective assessment of infection by human touch and give insights into areas of inflammation.21,22 Tissue oxygenation measurements of the skin show clinicians if the wound bed has sufficient perfusion.23,24 Moisture assessments can be an early indicator of pressure injury.25
Acquiring these technologies and seamlessly integrating them into practice seems out of reach due to the cost, multiple devices required that cost tens of thousands of dollars, and lack of interoperability. As technology develops, these medical imaging modalities are becoming integrated into wound management solutions in a form factor that can fit in your pocket.20 As illustrated in Figure 4, the ability to compare wound imaging modalities in seamless workflows will help advance assessment by clinicians. These changes bring the possibility of broad adoption of these advanced technology solutions.
1. Las Heras, K., Igartua, M., Santos-Vizcaino, E., & Hernandez, R. M. (2020). Chronic wounds: Current status, available strategies and emerging therapeutic solutions. Journal of Controlled Release, 328, 532–550. https://doi.org/10.1016/j.jconrel.2020.09.039
2. Welsh, L. (2018). Wound care evidence, knowledge and education amongst nurses: A semi-systematic literature review. International Wound Journal, 15(1), 53–61. https://doi. org/10.1111/iwj.12822
3. Vaughan, G., Prizeman, G., Eustace-Cook, J., & Byrne, G. (2021). Use of mHealth apps by nurses in the management of chronic wounds: A scoping review protocol. JBI Evidence Synthesis, 19(10), 2783–2789. https://doi.org/10.11124/JBIES-20-00401
4. Rogers LC, Bevilacqua NJ, Armstrong DG, Andros G. Digital planimetry results in more accurate wound measurements: a comparison to standard ruler measurements. J Diabetes Sci Technol. 2010 Jul 1;4(4):799-802. doi: 10.1177/193229681000400405. PMID: 20663440; PMCID: PMC2909508.
5. Lakdawalla, A., Phan, C., & Allport, J. (2020). Clinical Validation of Digital Wound Management as an Efficient Tool for Assessing and Monitoring True Wound Depth. 1. https://f. hubspotusercontent40.net/hubfs/2024649/+%20Conference%20Assets/SAWC%20-%20 AutoDepth.pdf
6. Beitz, J. M. (2022). Wound Healing. In L. L. McNichol, C. R. Ratliff, & S. S. Yates (Eds.), Wound, Ostomy, and Continence Nurses Society Core Curriculum: Wound Management (2nd ed., pp. 38–54). Wolters Kluwer.
7. Sibbald, R. G., Mufti, A., Armstrong, D. G., & Smart, H. (2021). Nontouch Infrared Skin Thermometry: An Underutilized Tool. Advances in Skin & Wound Care, 34(11), 614–615. https://doi.org/10.1097/01.ASW.0000795248.80980.89
8. Ramirez-GarciaLuna, J. L., Bartlett, R., Arriaga-Caballero, J. E., Fraser, R. D. J., & Saiko, G. (2022). Infrared Thermography in Wound Care, Surgery, and Sports Medicine: A Review. Frontiers in Physiology, 13, 838528. https://doi.org/10.3389/fphys.2022.838528
9. Wang SC, Anderson JAE, Evans R et al (2017) Point-of-care wound visioning technology: Reproducibility and accuracy of a wound measurement app. PLoS One 12(8): e0183139. https://doi.org/10.1371/journal.pone.0183139
10. Kong, L. Y., Ramirez-GarciaLuna, J. L., Fraser, R. D. J., & Wang, S. C. (2021). A 57-YearOld Man with Type 1 Diabetes Mellitus and a Chronic Foot Ulcer Successfully Managed with a Remote Patient-Facing Wound Care Smartphone Application. American Journal of Case Reports, 22. https://doi.org/10.12659/AJCR.933879
11. Kostovich, C. T., Etingen, B., Wirth, M., Patrianakos, J., Kartje, R., Baharestani, M., & Weaver, F. M. (2022). Outcomes of Telehealth for Wound Care: A Scoping Review. Advances in Skin & Wound Care, 35(7), 394–403. https://doi.org/10.1097/01.ASW.0000821916.26355.fa
12. Au, Y., Beland, B., Anderson, J. A. E., Sasseville, D., & Wang, S. C. (2019). Time-Saving Comparison of Wound Measurement Between the Ruler Method and the Swift Skin and Wound App. Journal of Cutaneous Medicine and Surgery, 23(2), 226–228. https://doi. org/10.1177/1203475418800942
13. Mohammed, H. T., Bartlett, R. L., Babb, D., Fraser, R. D. J., & Mannion, D. (2022). A time motion study of manual versus artificial intelligence methods for wound assessment. PLOS ONE, 17(7), e0271742. https://doi.org/10.1371/journal.pone.0271742
14. Fife, C. E., & Eckert, K. A. (2017). Harnessing electronic healthcare data for wound care research: Standards for reporting observational registry data obtained directly from electronic health records: Standards for reporting observational data from electronic health records. Wound Repair and Regeneration, 25(2), 192–209. https://doi.org/10/f99qmb
15. Madu, T. (2021). Conducting meaningful real-world data analysis in wound care—A guide. Journal of Wound Care, 30(2), 93–94. https://doi.org/10/gn7ksq
16. Fraser, R. D. J., Gupta, R., & Mohammed, H. T. (2022). Analysis of real-world data from North American skilled nursing facilities’ skin and wound records for skin tear prevalence, healing and treatment. Journal of Wound Management, 13.
17. Ramachandram, D., Ramirez-GarciaLuna, J. L., Fraser, R. D. J., Martínez-Jiménez, M. A., Arriaga-Caballero, J. E., & Allport, J. (2022). Fully Automated Wound Tissue Segmentation Using Deep Learning on Mobile Devices: Cohort Study. JMIR MHealth and UHealth, 10(4), e36977. https://doi.org/10.2196/36977
Digitization and technological advancement will continue to transform the standard of care for wound assessment. Technology is a tool to empower clinicians to perform assessments that maximize the scope of practice and potential of their wound care team. Healthcare organizations face increasing pressures. Technology needs to be integrated into their medical records and designed with empathy for the needs of clinicians and patients. Adopting digital tools into clinical practice will provide deeper insights into wound healing and help health care providers address the global burden of chronic wounds. The world has moved into the digital age. It is time for wound care teams to move there as well.
18. Gupta, R., Goldstone, L., Eisen, S., Ramachandram, D., Cassata, A., Fraser, R. D. J., Ramirez-GarciaLuna, J. L., Bartlett, R., & Allport, J. (2022). Towards an AI-based Objective Prognostic Model for Quantifying Wound Healing [Preprint]. https://doi.org/10.36227/techrxiv.21067261.v1
19. Barker, M., Perron, M., Parrott, W., & Fraser, R. D. J. (2021). Interprofessional Virtual Wound Care Impact in an Integrated Health Care System during COVID-19. https://doi.org/10/gn9nbr
20. Ramírez-GarcíaLuna, J. L., Martínez-Jiménez, M. A., Bartlett, R., Wang, S. C., Fraser, R. D. J., Saiko, G., & Gergory C Berry. (2022). Is My Wound Infected? https://doi.org/10.13140/RG.2.2.17235.66088
21. Chanmugam, A., Langemo, D., Thomason, K., Haan, J., Altenburger, E. A., Tippett, A., Henderson, L., & Zortman, T. A. (2017). Relative Temperature Maximum in Wound Infection and Inflammation as Compared with a Control Subject Using Long-Wave Infrared Thermography. Advances in Skin & Wound Care, 30(9), 406–414. https://doi.org/10.1097/01.ASW.0000522161.13573.62
22. Lin, Y.-H., Chen, Y.-C., Cheng, K.-S., Yu, P.-J., Wang, J.-L., & Ko, N.-Y. (2021). Higher Periwound Temperature Associated with Wound Healing of Pressure Ulcers Detected by Infrared Thermography. Journal of Clinical Medicine, 10(13), 2883. https://doi.org/10.3390/jcm10132883
23. Lee, L.-L., & Chen, S.-L. (2022). Assessment of Hyperspectral Imaging in Pressure Injury Healing. Advances in Skin & Wound Care, 35(8), 429–434. https://doi.org/10.1097/01. ASW.0000831888.39420.a6
24. Saiko, G., Lombardi, P., Au, Y., Queen, D., Armstrong, D., & Harding, K. (2020). Hyperspectral imaging in wound care: A systematic review. International Wound Journal, 17(6), 1840–1856. https://doi.org/10.1111/ iwj.13474
Serena, T. E., Harrell, K., Serena, L., & Yaakov, R. A. (2019). Real-time bacterial fluorescence imaging accurately identifies wounds with moderateto-heavy bacterial burden. Journal of Wound Care, 28(6), 346–357. https://doi. org/10.12968/jowc.2019.28.6.346
25. Moore, Z., McEvoy, N. L., Avsar, P., Byrne, S., Vitoriano Budri, A. M., Nugent, L., O’Connor, T., Curley, G., & Patton, D. (2022). Measuring subepidermal moisture to detect early pressure ulcer development: A systematic review. Journal of Wound Care, 31(8), 634–647. https://doi. org/10.12968/jowc.2022.31.8.634
26. Serena, T. E., Harrell, K., Serena, L., & Yaakov, R. A. (2019). Real-time bacterial fluorescence imaging accurately identifies wounds with moderateto-heavy bacterial burden. Journal of Wound Care, 28(6), 346–357. https://doi. org/10.12968/jowc.2019.28.6.346
27. Ramírez-GarcíaLuna, J. L., Fraser, R. D. J., Bartlett, R., Saiko, G., Wang, S. C., & Berry, G. K. (2022). Infrared Thermal Imaging: A New Paradigm For Wound Care. https://doi.org/10.13140/RG.2.2.19985.30560

The Health Protection Agency suggests that 1 in 10 women undergoing Caesarean section (C-section) may develop a wound infection. It is considered higher than other similar types of surgery. The risk of wound infection is increased by smoking, diabetes, poor nutrition, and being overweight. This makes emergency C-sections more challenging as the patient will not have sufficient time to reverse or minimise these risk factors.
This article provides a concise overview of Surgical Site Infection (SSI) symptoms and risks, and a pathway to prevent SSI after C-Section, to optimize healing of the surgical wound.
Wound infection is defined as the colonization of the wound by proliferating bacteria to a degree which ellicits from the host a local, spreading and/ or systemic response. The increasing number of microorganisms in the wound causes the development of a range of virulence factors which overcome the defences of the host, which leads to local tissue damage and compromises normal wound healing.1,2,3
Surgical Site Infection (SSI) is a common complication following C-section, and the procedure has higher rates of this than other surgeries, affecting 1.8 - 9.2% of patients undergoing this procedure.7 The consequences are potentially devastating for the individual and costly for hospitals and healthcare systems.
The Center for Disease Control (CDC) defines SSI as an infection at or near the site of a surgical procedure performed within the last 30 days, or within 90 days if the procedure involved the removal of an implant. The reduction of a patient’s risk factors is the main focus of strategies intended to prevent SSI. In addition to attempts to identify and reduce these individual patient risk factors, there is potential scope for the use of topical antimicrobials to prevent wound infection in high risk wounds.4 This potential should be carefully considered against the risks and established principles of antimicrobial stewardship.
• Increased erythema in the wound
• Increased tenderness in the wound
• Increased heat in the wound
• Increased swelling of the wound
• Increased induration of the wound
Seek Urgent Medical Attention - Consider Sepsis in the following settings:
• Increasing pain, erythema or heat
• Spreading induration, swelling or cellulitis
• Purulent drainage from the incision, or deep wound abscess or fasciitis
• Separation of the edges of incision exposing the deeper tissues
• Unexpected post-operative fever accompanied by increasing wound pain and/ or wound dehiscence
• Pathological blood test findings (elevated CRP, WBC counts, erythrocyte sedimentation rates, pro-calcitonin)
Seek Urgent Medical Attention - Consider Sepsis in the following settings:
• Involve any part of the anatomy other than the incision during surgery; CRP— C-reactive protein; WBC—white blood count
• Purulent drainage from a drain placed through the skin into the organ or body space
• Organ or body space abscess diagnosed by radiological or histopathological examination
• Evidence of infection involving the organ or body space seen on direct examination during surgery
• Post-operative fever
• Positive result of blood cultures, deep tissue biopsies, surgical sampling or pathological blood test findings (see deep SSI column)
Low to Moderate Risk of SSI
High Risk of SSI
•
•
•
Leave open - No further dressing required
No Wound Dehiscence
Apply a Leukomed® Sorbact® dressing using aseptic, non-touch technique, monitor for further signs and symptoms of infection and treat accordingly
a wound swab
• Dermal layer only involved; no visible subcutaneous fat
Apply a Cutimed® Sorbact® ribbon dressing with a Mepilex Border using aseptic, non-touch technique, monitor for further signs and symptoms of infection and treat accordingly. If on next wound review the wound has not improved or has deteriorated send a referral to Plastic Surgery and the TVN team
Assess level of dehiscence as per below
• Subcutaneous layer exposed, fascia not visible
Make urgent referral to Plastic Surgery and Tissue Viability, upload a wound photo. Apply a Cutimed® Sorbact® ribbon dressing and a Mepilex Border using aseptic, non-touch technique, monitor for further signs and symptoms of infection and treat accordingly
Leukomed® Sorbact® is a sterile single-use dressing developed for use in closed surgical wounds. It offers a dual effect of binding bacteria at the wound bed and protecting the wound from external contamination.
A transparent, showerproof, polyurethane film adhesive outer layer protects and keeps the wound moist, and covers an absorbent pad with a green contact layer coated with dialkylcarbamoyl chloride (DACC), (a fatty acid derivative), which irreversibly binds the bacteria (Figure 1).

The dressing utilizes a unique technology by irreversibly binding the bacteria and is proven to prevent surgical site infections (SSI).

Overall savings were realised in terms of lower SSI and reduced readmission rates, and consequently a better patient experience was achieved in a trust that incorporated Leukomed® Sorbact® in place of the standard dressings in a maternity unit.6
Leukomed® Sorbact® can reduce the rate of surgical wound infections, antibiotic usage and associated hospital admissions.
Stanirowski et al. conducted a randomized, single-blinded controlled study which assessed the efficacy of Leukomed® Sorbact® in preventing SSI in women after a C-section. The participants were randomly assigned to two groups, one given standard surgical dressings and the other group given Leukomed® Sorbact® dressings. The authors note a decreased rate of SSI in the group given Leukomed® Sorbact® dressings.7
A meta-analysis of efficacy of DACCimpregnated dressings found reductions of SSI risk (RR 0.33 [95% CI 0.14–0.77; p = 0.01]), compared to other types of advanced dressings (silver-impregnated dressings, copperinpregnated dressings and chlorhexidine gluconate dressings).8
Reduced costs in the group treated with Leukomed® Sorbact®, due to reduced need for further treatment resulting from SSI.7 The NICE (The National Institute for Health and Care Excellence) guidance for Leukomed® Sorbact® further supports the cost effectiveness, efficacy and safety in use of Leukomed® Sorbact® dressings, to prevent SSI.5
SSI
post-C-section
Evidence supports the case for adopting PICO negative pressure wound dressings for closed surgical incisions in the NHS. They are associated with fewer surgical site infections and seromas compared with standard wound dressings in a specified group. PICO negative pressure wound dressings should be considered as an option for closed surgical incisions in people who are at high risk of developing surgical site infections.
PICO 7 (Figure 2) provides negative pressure wound therapy (NPWT) which draws out excess fluid from a wound and protects the injured area. The wound dressing protects the wound from bacteria to ultimately help promote healing. It consists of a battery-operated NPWT pump connected to an absorbent gentle adhesive dressing. The dressing is applied to the wound and extra strips are placed over the outside edge to help hold the dressing in place.

The dressing may be left in place for up to seven days depending on the amount of fluid drawn from the wound. This will depend on the size, type, drainage amount and position of the wound.
An AIRLOCK◊ Technology layer distributes pressure evenly and consistently for up to 7 days, with optimal fluid management to help reduce the potential for maceration.9
A systematic literature review and meta-analysis conducted by Saunders, et al. report reductions in surgical site complications, risks and hospital stay length, including:
• Reduction of 63% In SSI risk with PICO sNPWT compared with standard care
• Reduction of 30% in dehiscence risk with PICO sNPWT compared with standard care
• Reduction of 77% in seroma risk with PICO sNPWT compared with standard care
• 1.75 day reduction in length of hospital stay seen with PICO sNPWT compared with conventional dressings10
NICE Guidance demonstrates that PICO◊ sNPWT provides better outcomes than standard care for preventing surgical site complications in high-risk patients with closed surgical incisions, with similar overall cost.
PICO◊ sNPWT reduced the incidence of SSIs by 50% in women with ≥30 BMI following C-sections compared with standard dressing (p=0.007).11
In an RCT of 876 women undergoing C-section with pre-pregnancy BMI ≥≥ 30, PICO◊ sNPWT significantly reduced the relative risk of SSIs by 50% compared with standard dressings (p=0.007).11
The evidence reporting on the prophylactic use of PICO demonstrates clinical and cost benefits in patient populations with a prepregnancy BMI of ≥30.
It was also estimated to be cost saving compared with standard dressings in women with prepregnancy BMI ≥35kg/m2, being associated with low hospital readmission rate (0.8%).12
A review of the rates of C-Section SSIs in your unit is essential to ascertain the reason for any increase in accepted rates. Assessment of wound cultures and the review of microbiology may reveal that wounds were not being infected by the same microorganisms. The presence of the types of microorganisms may elucidate the reason for the different sources of infection. Giving the wound the best chance to heal before potential exposure to microorganisms is vital.
Often times, dressings applied to the wound in theatre are only left in place for 24 hours before being removed. This leaves the wound at an increased risk is developing an infection, having only been closed 24 hours prior. Successful healing needs to have taken place before a dressing is removed.
In conclusion, consideration of incorporating Leukomed® Sorbact®, and the PICO system, into the SSI C-Section management pathway has seen many benefits; reduction of SSIs, reduction in readmittance due to an SSI; potential reduction in antibiotic usage, protecting the wound from potential organisms whilst it is at its most vulnerable, and potential cost savings due to a reduction in SSIs.
The next step would be to complete an audit on our C-Section SSI figures when the pathway has been in place for six months; this will give a deeper analysis of the aforementioned benefits.
1. Wound Infection In Clinical Practice: Principles of best practice. International Wound Infection Institute. Wounds International. 2022.
2. Siddiqui AR and Bernstein JM. Clin Dermatol, 2010; 28(5): 519-26.
3. Eberlein T. Critical colonisation and local infection - Current therapy by use of polihexanide. https://lohmann-rauscher.co.uk/downloads/clinical-evidence/SXP010-T-EberleinCriticalcolonisation-and-local-infect.pdf, 2009.
4. Dumville JC et al. Cochrane Database Syst Rev, 2016; 12(12): CD003091.
5. NICE. 2021. Leukomed Sorbact for preventing surgical site infection. NICE National Institute for Health and Care Excellence. MTG55
6. Magro, M. Towards Value-Based Healthcare: Reducing Surgical Site Infections PostCaesarean Section. 2022.
7. Stanirowski PJ, Bizoń M, Cendrowski K, Sawicki W. Randomized Controlled Trial Evaluating Dialkylcarbamoyl Chloride Impregnated Dressings for the Prevention of Surgical Site Infections in Adult Women Undergoing Cesarean Section. Surg Infect (Larchmt). 2016 Aug;17(4):427-35. doi: 10.1089/sur.2015.223. Epub 2016 Feb 18. PMID: 26891115; PMCID: PMC4960475.
8. Wijetunge S, Hill R, Katie Morris R, Hodgetts Morton V. Advanced dressings for the prevention of surgical site infection in women post-caesarean section: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021 Dec;267:226-233. doi: 10.1016/j. ejogrb.2021.11.014. Epub 2021 Nov 11. PMID: 34826671.
9. Smith & Nephew. PICO™ 7. Patient home care information. PDF. Available from: https:// www.smith-nephew.com/global/anz/awm/pico/sn14083%20-%20pico7%20patient%20info%20 booklet.secure.pdf. 2022.
10. Saunders C, Buzza K, Nherera L. 2019. A single use negative pressure system reduces surgical site complications compared with conventional dressings in closed surgical incisions: a systematic literature review with meta-analysis. Poster presented at the European Wound Management Association annual meeting, June 5-7, 2019, Gothenburg, Sweden.
11. Hyldig N, Vinter CA, Kruse M, Mogensen O, Bille C, Sorensen JA, Lamont RF, Wu C, Heidemann LN, Ibsen MH, Laursen JB. Prophylactic incisional negative pressure wound therapy reduces the risk of surgical site infection after caesarean section in obese women: a pragmatic randomised clinical trial. BJOG: An International Journal of Obstetrics & Gynaecology. 2019 Apr;126(5):628-35.
12. Norman G, Goh EL, Dumville JC, Shi C, Liu Z, Chiverton L, Stankiewicz M, Reid A. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2020 Jun 15;6(6):CD009261. doi: 10.1002/14651858.CD009261.pub6. Update in: Cochrane Database Syst Rev. 2022 Apr 26;4:CD009261. PMID: 32542647; PMCID: PMC7389520.
SSIs are the third most commonly reported type of healthcare-acquired infection and the most costly¹. They place a significant impact on patient welfare² as well as presenting a heavy financial burden for the NHS³.
NICE medical technologies guidance recommends the use of Leukomed® Sorbact® for prevention of surgical site infection (SSI) in wounds with low to


after caesarean section and vascular surgery.
https://www.nice.org.uk/guidance/mtg55
In chronic wounds, diminished endogenous bioelectric signals can be supplemented by electrical stimulation (ES) devices. The in vitro and clinical evidence for the beneficial effects of ES on wound healing are now quite substantial,2 but ES has yet to become a mainstream wound therapy. One reason is the predominant use of large, expensive, periodic, clinic-based ES treatments which are inconvenient for patients and poor business models for providers. Chronic wounds are often painful and ES therapy has frequently been shown to be able to reduce pain in patients with non-healing painful wounds.3

The aim of this evaluation was to assess the frequency of a positive response for pain and healing, in stalled non-healing chronic wounds to a portable automatic, continuously active, disposable low-voltage pulsed microcurrent ES device.*

UK)
Ion pumps separate + and - ions. Intact skin acts as a battery.

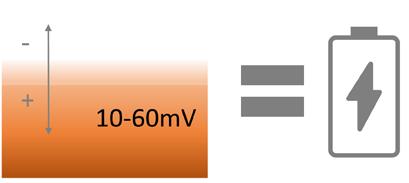 Endogenous bioelectricity is a fundamental mechanism of normal wound healing. Measurements show that microamp (μA) bioelectric currents are naturally released upon injury to the skin. Bioelectric fields operate through known pathways of cell signaling to switch on functions such as cell migration, growth factor and growth factor receptor expression and generally co-ordinate wound healing.1
Ms Gabriele Danner
Wund Pflege Management GmbH, Bad Pirawarth, Austria
Dr Robin Martin Robin Martin PhD Scientific Consulting Foggathorpe, United Kingdom
Mr Peter Kurz, RN, WDM Managing Director WPM Wound Care Management Lecturer, University of Krems Member of the board of WundD.A.CH. Bad Pirawarth, Austria
Endogenous bioelectricity is a fundamental mechanism of normal wound healing. Measurements show that microamp (μA) bioelectric currents are naturally released upon injury to the skin. Bioelectric fields operate through known pathways of cell signaling to switch on functions such as cell migration, growth factor and growth factor receptor expression and generally co-ordinate wound healing.1
Ms Gabriele Danner
Wund Pflege Management GmbH, Bad Pirawarth, Austria
Dr Robin Martin Robin Martin PhD Scientific Consulting Foggathorpe, United Kingdom
Mr Peter Kurz, RN, WDM Managing Director WPM Wound Care Management Lecturer, University of Krems Member of the board of WundD.A.CH. Bad Pirawarth, Austria
Wounded skin has a small (μA) electrical current which activates many healing functions. This dissipates as a wound becomes chronic.
1: Clinical response scores
No response 0
Minor 1 Limited 2 Modest 3 Good 4 Excellent 5
Table 2: Breakdown of wound indications
Indications
Application of external electrical stimulation can replace natural microcurrents to stimulate healing and reduce pain.
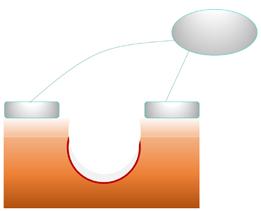
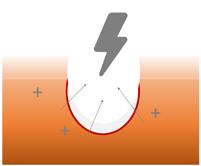
Number of Wounds
Post-surgical 5 Diabetic ulcer 5 Pressure ulcer 3 Venous ulcer 3 Arterial ulcer 1 Post trauma 3 20
This was an observational evaluation of a CE marked single-use electrical stimulation device* (see Figure 2) in a population of patients with static chronic non-healing wounds.
Following a period of 3 weeks to establish the wound was static, ES therapy was applied to all wounds for 12 days. Eight wounds subsequently received a further 12 days of ES therapy and some wounds received up to 4 x 12 days ES therapy in total.
Changes in clinical parameters such as area, depth, nature of granulation tissue, condition of peri-wound skin and pain were recorded. An overall informal clinical response, reflecting changes in these parameters, was scored on a 0 - 5 scale (where 5 is excellent and 0 indicates no clinical response), see Table 1.
Table 3: Effects of electrical stimulation on significantly painful wounds
Wounds with pain Significant pain reduced within 48 hours 10/ 20 have significant pain 9/ 10 (90%)
Mean significant pain score = 5%
Mean significant wounds pain score at 1 week = 2.0
The ES device is single-use, and delivers a preset therapy which is:
• Continuously active
Subsensory
• Pulsed μcurrent
• Low voltage
The device monitors current flow through the electrodes and automatically adjusts the voltage to ensure the same current is delivered across all patients, even if the electrodes are different distances from the wound edge. ES therapy is applied for 12 days during which time a 30min ES stimulation protocol is automatically delivered every 2 hours (odd days) and 4 hours (even days). The device can be used at the same time as any dressing or bandaging.

A total of 20 wounds were recruited in early 2021: 19 patients, 8 female, 11 male; with a mean age of 69.6 years. Wounds had been present a mean 35.7 months and static during this evaluation for a mean of 3 weeks, despite a range of interventions including wound bed preparation. The wound indications are shown in Table 2. Patients reported their assessment of pain on a 0 - 10 visual analogue scale (VAS), where 0 is no pain and 10 is worst imaginable pain. Many of the wounds (10/ 20) caused significant pain (>4).
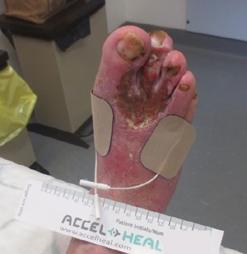
Reduction in pain, peri-wound oedema, exudate, inflammation, wound depth and area and increases in granulation tissue growth and re-epithelialisation fed into the overall rating of each patient on a 0 - 5 clinical response score.
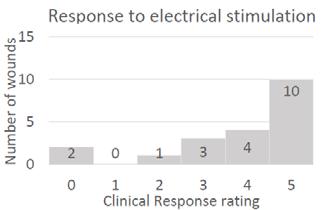
Figure 3 shows that 14/ 20 (70%) of wounds showed a significantly positive clinical response (scoring either 5 or 4), with reductions in pain, peri-wound oedema, exudate, inflammation, and increases in granulation and re-epithelialisation. Changes were typical observed within the first 14 days.
Table 3 shows that 9/ 10 (90%) of those patients with significant pain, there was reduction in pain scores within the first 48 hours.
“Reduction in pain, peri-wound oedema, exudate, inflammation, wound depth and area and increases in granulation tissue growth and re-epithelialisation fed into the overall rating of each patient on a 0 - 5 clinical response score.”This 64-year-old man with type 2 diabetes underwent hallux valgus surgery in 2020. Postoperatively, there was an infection with pronounced dehiscence. Bone and tendon parts were exposed. The diabetes mellitus is insulin dependent. HbA1c is in the normal range. Good wound healing occurred from October 2020 to February 2021, using professional wound management products. Then the wound healing stagnated for months until the patient came a second time to our practice.
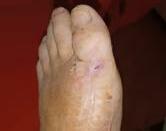
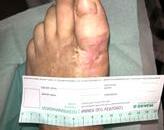
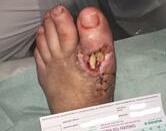
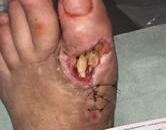
There was still a small wound with sharply defined wound edges. The bone was still exposed. With continued moist wound treatment, electrical stimulation (Accel-Heal) was started with a total of 2 treatments. After only 9 days, the bone was almost completely overgrown. 2 weeks later I started the second treatment. After 2 months, the bone was overgrown and only a small opening remained.
This case illustration is an excellent example of the possibilities offered by this easy-to-use therapy. See Figure 4.
The purpose of this investigation was to explore in greater breadth, the clinical capabilities of a small continuously active pulsed microcurrent ES* device.4 In a population of 20 static nonhealing wounds, including post-surgical and DFUs, 70% of wounds displayed significant positive responses such as reductions in periwound oedema, reductions in inflammation, wound depth and area, or increases in granulation, that had not been seen during the preceding weeks of care.
In these wounds the clinical signs suggest ES was responsible for changing the physiology of the wound and that reparative processes were in motion. In addition, 90% of patients with significant pain recorded a reduction in pain by the end of the first 48 hours.
With the ability to allow patients to receive electrical stimulation therapy at home, in comination with any type of dressing. this device will be able to greatly expand the access of patients to this ES therapy. Further studies with greater numbers of wounds should be performed to further reveal its full potential.
1. Houghton, Pamela E. 2017. “Electrical Stimulation Therapy
D. McCaig. 2014. “Harnessing the
Wound Care3(2):127–38.
Milne, Jeanette, Amelia Swift, Jennifer Smith, and Robin Martin. 2021. “Electrical
Wound Healing.” Journal of Wound
Ovens, Liz. 2017. “Electrical Stimulation Therapy and Electroceutical Treatment
of Venous Leg Ulcers.” British Journal of Community Nursing 22 Suppl3(Sup3):S28–36.
5. Zhao, Min. 2009. “Electrical Fields in Wound Healing—An Overriding Signal That Directs
Biology20(6):674–82.
“In these wounds the clinical signs suggest ES was responsible for changing the physiology of the wound and that reparative processes were in motion. In addition, 90% of patients with significant pain recorded a reduction in pain by the end of the first 48 hours.”There have been significant advances in chronic wound management treatments over the last 30 years, however, hard-to-heal wounds are an increasing problem.
Chronic wounds can also be extremely painful –between 50% and 60% of patients with a chronic wound experience persistent pain.[1,2] One major issue is that pain can make gold standard treatments, such as compression, unbearable.[3]
Electrical stimulation has been used globally in specialist clinics for more than a decade to relieve pain and accelerate healing in chronic wounds. With nine meta analyses and over 35 randomised controlled trials published, electrical stimulation is one of the most evidence-based technologies in wound management.
Electrical stimulation therapy is now available in a simple to use, single use, wearable device, enabling its more widespread use across different care settings. Accel-Heal Solo electrical stimulation wound therapy is a one-off, interventional, 12-day treatment which is applied alongside existing care to relieve pain [4] and accelerate healing [4,5]. Accel-Heal Solo improves the quality of life for the patient [5] and helps facilitate the use of compression therapy where wound pain has been an issue.[6]
To find out more about Accel-Heal Solo, please contact: customerservices@accelheal.com






■ Other tissue transplant products that are based on tissues of human and porcine origin are not ideal substitutes because of the heavy processing needed to eliminate the risk of disease transmission. This harsh, anti-viral treatment removes most of the material’s natural components, making it too dissimilar to human skin.
Kerecis® Omega3 is not subject to this treatment, leaving a more naturally intact product
■ Kerecis® has demonstrated its proficiency in creating lipidcontaining tissue matrices from fish skin, and it has been shown that the material is safe, non-toxic and structurally sound
■ A recent publication of an RCT by Lullove et. al (2022) shows 106% more healed wounds at 12 weeks compared to Collagen-Algenate dressing, a statistically significant finding
■ Compared to mammalian-based skin substitutes, Kerecis® Omega3 offers improved economics and clinical performance, as well as reduced disease transfer risk
■ Mammalian tissue carries the risk of disease transmission to humans. This is not a risk with Kerecis® Omega3
■ Kerecis® Omega3 fish skin contains lipids and proteins that, in a concentrated form, help the body regenerate damaged tissue
■ Kerecis® is focused on developing medical device applications with a predicated high return on investment and a low to medium cost of development. The company focuses exclusively on tissue regeneration and maintenance, utilizing its core Omega3 fish skin technology.
“These results will help guide clinicians to make decisions that positively impact both their patients’ outcomes and treatment costs.”
“The standard of care for treating DFUs is constantly improving... We compared the fish skin to an advanced collagen-alginate dressing to represent current alternatives. We wanted to compare it to more than just a simple wet-to-dry gauze or other, older methods.”
"The Kerecis™ Omega3 Wound matrix is a decellularized skin matrix derived from fish skin and represents an innovative concept to achieve wound healing... in this study (it) represented an effective treatment option in 25 complicated wounds."
Kerecis® Omega3 has had numerous trials supporting its efficacy in healing rates. A study regarding the use of Kerecis® Omega3 on diabetic foot ulcers (DFU) showed significantly higher healing rates compared to DFUs treated by fibrocol, a collagen-alginate dressing.
This is a retrospective review that explores the effect of hemoglobin A1C (HbA1c) levels on diabetic foot ulcer (DFU) healing in type II diabetes mellitus (DMII) patients. There is currently conflicting evidence as to whether HbA1c levels influence wound healing rate and there are no studies on the association of HbA1c levels with acellular fish skin (AFS) treatment for DFU.


This study reviewed 20 cases of chronic DFUs (defined as DFUs that did not show improvement after 30 days of standard of care (SOC) treatment). Patients were treated with AFS once a week for 4 weeks, and the wound size was recorded at the beginning and end of this process. Patients were broken into two groups, each consisting of 10 patients. One group were patients with HbA1c levels between 6 - 8, while the other group consisted of patients with HbA1c levels between 8 - 10.

The group with HbA1c 6 - 8 showed an average of -41.30% decrease in wound size. The HbA1c 8-10 group had an average of -30.41% decrease. After performing an independent t-test, t-value: -0.56, p-value: 0.299, p>0.05. HbA1c levels do not have a statistically significant effect on wound healing time.
This study confirms that fish skin grafts are an effective form of wound care treatment. It further proves that despite uncontrolled HbA1c, fish skin treatment remains effective, as HbA1c is not a factor in healing rate.
According to the Centers for Disease Control and Prevention (CDC) more than 37 million Americans have diabetes, and 90 - 95% of them have type II diabetes mellitus (DMII). One of the key areas of morbidity associated with diabetes mellitus is the diabetic foot. 15 - 25% of patients with diabetes will develop a diabetic foot ulcer (DFU) within their lifetime, accounting for a total of 9.1 to 26.1 million DFU cases worldwide.1 DFU has become one of the leading causes of limb loss as it is associated with more than 80% of diabetes-related lower extremity amputations. Additionally, the combination of high blood sugar, poor blood circulation and immune system deficiencies that diabetic patients experience increase the risk for wounds and subsequently poor wound healing.2
Hemoglobin A1C (HbA1C) measures the blood glucose levels in diabetic patients and is a standard test to monitor glycemia. Hb1AC reflects glycemia over 2 - 3 months. When hemoglobin is
Shenmei Wu University of Nevada, Las Vegas Las Vegas NV, United States Dr Ryan Huang Teaching Faculty, Valley Hospital Medical Center Residency Program Adjunct Professor, Touro University Nevada Las Vegas NV, United States Alexis Chan Northwestern University Evanston IL, United Statesglycated, there is the formation of advanced glycation end products (AGEs).3 AGEs have a high affinity for RAGE (receptor for advanced glycation end products), which have been shown to induce proinflammatory pathways.4 However, there has been conflicting evidence whether HbA1c levels affect wound healing rate in diabetic wounds. While some studies claim that the increase in HbA1c decreases healing time,5 others said the association remained unclear.6
We define a chronic DFU as one that did not show improvement after 30 days of standard of care (SOC) treatment. Once the ulcers are not healed, they may become infected and lead to amputation. Current SOC for DFU includes five principles:
1. Pressure relief
2. Debridement
3. Dressing to facilitate moist wound environment
4. Infection management
5. Treatment of arterial insufficiency7,8,9
In addition to those principles, the Society for Vascular Surgery has recommended adequate glycemic control of Hb1AC less than 7% to reduce the incidence of DFU and infections.10
The use of skin substitutes has been considered as advanced treatment for chronic, nonhealing DFU. Skin substitutes are used to cover the open chronic ulcers, create an optimal environment for the patient’s own cells to migrate into, proliferate, revascularize and promote wound healing. Among many commercially available skin substitutes originated from highly processed human and animal tissues, AFS is a recent, innovative addition. AFS is made from North Atlantic cod fish that are wildly caught in the pristine waters of Iceland. Since there is no disease transmission risk from cod fish to humans, the graft is subjected to a gentle, minimally processed manufacturing procedure
to remove cellular components while still retaining the structural integrity and bioactive compounds of the fish skin. Particularly, AFS contains a rich amount of Omega3 fatty acids that are uniquely high in docosahexaenoic acid (DHA) and eicosatetraenoic acid (EPA).11 AFS has been proven to be more effective at healing DFUs compared to the standard of treatment;12,13,14 the authors concluded that AFS was able to drive the DFU from a chronic phase into an acute wound due to its porous, homologous structure to human skin and rich omega-3 fatty acids source.
The application of advanced treatments for chronic DFU such as skin substitutes is often delayed until patients get their HbA1C level under control, as required by insurance companies to receive coverage. This process can be lengthy as blood glucose takes time to improve. It involves diet, exercise, weight loss, medication and stress management. For patients with open DFU, the delay in treatment until adequate HbA1C levels are reached can worsen the wound and expose it to a higher risk of infection. There is no evidence as to whether HbA1C interferes with a skin substitute treatment for DFU, especially with AFS. Our objective is to identify the impact of HbA1C levels in chronic DFU treated with AFS in the lower extremities, in patients with DMII.
Patient charts from Professional Wound Specialists (PWS) reviewed data from 20152021. The place of care varies from facilities, to office, to home health care. This study reviewed de-identified data of patients with DMII and chronic DFU treated by practitioners from PWS, that were treated with AFS. Exclusion criteria included patients whose wounds healed prior to four applications, expired patients, and non-compliant patients. With a total population
“With a total population of 20 patients, we created two groups of patients. One with relatively controlled HbA1c levels. between 6-8. The other group consisted of patients with HbA1c levels between 8-10. Each group consisted of 10 patients.”“The
was
For
with 6
with
and
of 20 patients, we created two groups of patients; one with relatively controlled HbA1c levels between 6 - 8, the other with HbA1c levels between 8 - 10. Each group consisted of 10 patients.
Patients’ HbA1c levels were measured within the first 3 months of their first treatment. If the levels was not obtained from the primary care physician, then they were obtained by PWS prior to the study. The patient’s chronic DFU was treated once a week using the PWS treatment standard (see next section). Wound sizes were measured before the first treatment and after 4 treatments, over a 4-week time period. Demographic information recorded includes gender and age.
The DFU wound bed was first prepared with a wound cleanser. Afterwards, a curette was used to stimulate the wound bed, as well as removing any bioburden. AFS was then rehydrated with normal saline and placed on the wound bed with the scale side facing away from the wound bed. A mesh was then placed and secured with steristrips. Finally, an outer dressing was placed over the wound. Treatment was performed on a weekly basis.
The average age was 77 years old in the HbA1C 6 - 8 patient group with 6 females and 4 males. For the HbA1C >8 patient group, the average age was 70 years old with 3 females and 7 males. See Table 1.
The average starting size for HbA1C 6 - 8 was 15.33 cm2 and was reduced to 8.46 cm2, which is a 41.3% reduction in wound size. The HbA1C average was 6.86. For the HbA1C >8 patient group, the average week 1 size was 12.17 cm2 and was reduced to 7.6 cm2, with a 30.4% reduction rate.
Table 2: Wound size change.
Patient
HbA1C
HbA1c >8
Average
The average HbA1C level in this group was 8.92. Overall, the combined average wound size was 16.81 cm2 and was reduced to 10.96 cm2. This was a 36% reduction in size with an average HgA1C of 7.9. See Table 2.
Week
HbA1c % size change
6.2 -7
6.3 -21
6.5 -85
6.6 -77
6.8 -21
7.1 -11
24.57 7.2 -39
8.68 7.3 -45
7.56 7.4 -53
2.25 7.4 -54
8.46 6.86 -41.30%
SD 10.6 7.07 0.48 26.00%
10.14 8.2 -32
2.1 8.4 -53
2.86 8.5 -90
1 8.8 -89
9.3 8.8 -57
29.07 8.9 66
0.9 9.1 -60
3.6 9.1 -10
9 9.6 -40
8.06 9.9 61
Average
7.6 8.92 -30.40
8.39 0.53 55.01
7.9 -36.00
1.98 44%
“The average wound size reduction rate was at 41.3% for the lower HbA1c level group and 30.4% for the higher HbA1c level group... The study findings support that AFS is an effective treatment for chronic DFU regardless of HbA1c level.”
Our study reviewed 20 patients with DFU who received treatment with AFS. According to our analysis, there is no correlation between HbA1c levels from two groups with their wound size reduction rate. The baseline HbA1c levels were not associated with ulcer healing. The average wound size reduction rate was at 41.3% for the lower HbA1c level group and 30.4% for the higher HbA1c level group.
This result is consistent with previous literature indicating that baseline HbA1c is not associated with lower extremity wound healing in patients with diabetes. Fesseha et al. proposed a model in which HbA1c was divided into <6.5%, 6.5-8% and >8%, and found no relationship between initial blood glucose level and healing rate.15 Margolis et al. pooled over 586 patients with DMII who had neuropathic DFUs, to predict the risk factors that affect the healing rate, and found HbA1c levels measured at the start of the study were not associated with the probability of wound healing.16 Xiang et al. also found similar results where there were no differences in wound healing rate among groups with different baselines HbA1c levels.17
Advanced biologic therapeutics for DFUs is often delayed while HbA1c levels are corrected because it is believed that the wound will not respond while the HbA1c is elevated. This delay in treatment can lead to deterioration in the wound for several weeks and leave the open wound at risk of infection. The study findings support that AFS is an effective treatment for chronic DFU, regardless of HbA1c level. The use of skin substitutes, especially those that are proven to respond to the ulcers, should be recommended as the primary treatment for DFU.
org/10.1038/s41598-021-92630-0
4. Zong H, Ward M, Madden A, Yong PH, Limb GA, Curtis TM, Stitt AW. Hyperglycaemiainduced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia. 2010 Dec;53(12):2656-66. doi: 10.1007/s00125-010-1900-z. Epub 2010 Sep 12. PMID: 20835858.
5. Christman, Andrea L et al. “Hemoglobin A1c predicts healing rate in diabetic wounds.” The Journal of investigative dermatology vol. 131,10 (2011): 2121-7. doi:10.1038/jid.2011.176
6. Fesseha, Betiel K et al. “Association of Hemoglobin A1c and Wound Healing in Diabetic Foot Ulcers.” Diabetes care vol. 41,7 (2018): 1478-1485. doi:10.2337/dc17-1683
7. Lavery LA, Davis KE, Berriman SJ, Braun L, Nichols A, Kim PJ, Margolis D, Peters EJ, Attinger C. WHS guidelines update: Diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2016 Jan-Feb;24(1):112-26. doi: 10.1111/wrr.12391. PMID: 26663430.
8. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012 Jun;54(12):e132-73. doi: 10.1093/cid/cis346. PMID: 22619242.
9. Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ; International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016 Jan;32 Suppl 1:2-6. doi: 10.1002/dmrr.2694. PMID: 26409930.
of HbA1c
10. Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, Driver VR, Frykberg R, Carman TL, Marston W, Mills JL Sr, Murad MH. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016 Feb;63(2 Suppl):3S-21S. doi: 10.1016/j.jvs.2015.10.003. PMID: 26804367.
11. Kotronoulas A, Jónasdóttir HS, Sigurðardóttir RS, Halldórsson S, Haraldsson GG, Rolfsson
Ó. Wound healing grafts: Omega-3 fatty acid lipid content differentiates the lipid profiles of acellular Atlantic cod skin from traditional dermal substitutes. J Tissue Eng Regen Med. 2020 Mar;14(3):441-451. doi: 10.1002/term.3005. Epub 2019 Dec 30. PMID: 31826323.
12. Lullove EJ, Liden B, Winters C, McEneaney P, Raphael A, Lantis II JC. A multicenter, blinded, randomized controlled clinical trial evaluating the effect of Omega-3–rich fish skin in the treatment of chronic, nonresponsive diabetic foot ulcers. Wounds. 2021;33(7):169-177.
13. Michael S, Winters C, Khan M. Acellular Fish Skin Graft Use for Diabetic Lower Extremity Wound Healing: A Retrospective Study of 58 Ulcerations and a Literature Review. Wounds. 2019 Oct;31(10):262-268. Epub 2019 Aug 21. PMID: 31730505.


14. Yang CK, Polanco TO, Lantis JC 2nd. A Prospective, Postmarket, Compassionate Clinical Evaluation of a Novel Acellular Fish-skin Graft Which Contains Omega-3 Fatty Acids for the Closure of Hard-to-heal Lower Extremity Chronic Ulcers. Wounds. 2016 Apr;28(4):112-8. PMID: 27071138.
15. Fesseha BK, Abularrage CJ, Hines KF, Sherman R, Frost P, Langan S, Canner J, Likes KC, Hosseini SM, Jack G, Hicks CW, Yalamanchi S, Mathioudakis N. Association of Hemoglobin A1c and Wound Healing in Diabetic Foot Ulcers. Diabetes Care. 2018 Jul;41(7):1478-1485. doi: 10.2337/dc17-1683. Epub 2018 Apr 16. PMID: 29661917; PMCID: PMC6014539.
16. Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Risk factors for delayed healing of neuropathic diabetic foot ulcers: a pooled analysis. Arch Dermatol. 2000 Dec;136(12):1531-5.
doi: 10.1001/archderm.136.12.1531. PMID: 11115166.
17. Xiang J, Wang S, He Y, Xu L, Zhang S, Tang Z. Reasonable Glycemic Control Would Help Wound Healing During the Treatment of Diabetic Foot Ulcers. Diabetes Ther. 2019 Feb;10(1):95-105. doi: 10.1007/s13300-018-0536-8. Epub 2018 Nov 21. PMID: 30465160; PMCID: PMC6349287.
18. Katie Glover, Alexandros Ch. Stratakos, AnikoVaradi, Dimitrios A. Lamprou, 3D scaffolds in the treatment of diabetic foot ulcers: New trends vs conventional approaches, International Journal of Pharmaceutics, Volume 599, 2021, 120423, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2021.120423.
19. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005 Jan 12;293(2):217-28. doi: 10.1001/jama.293.2.217. PMID: 15644549.
20. Younis, Bilal Bin et al. “Frequency of foot ulcers in people with type 2 diabetes, presenting to specialist diabetes clinic at a Tertiary Care Hospital, Lahore, Pakistan.” BMC endocrine disorders vol. 18,1 53. 6 Aug. 2018, doi:10.1186/s12902-018-0282-y
For more information visit Arjo.com/ Provizio
Validated efficacy1 for early assessment of Pressure Injury (PI) risk and damage in peri-operative patients.
Provizio SEM Scanner delivers objective and anatomically-specific assessment of PI risk, empowering you to deploy a targeted prevention strategy that helps minimise PI incidence and reduce overall cost and time to care. This is especially important for peri-operative patients who, due to periods of immobility, can be particularly vulnerable to the effects of pressure and shear.
Findings from the latest publication1 assessing the use of the SEM Scanner on a sample of 231 surgical patients:
• Using the SEM Scanner, 51% of patients presented high SEM readings indicating early development of PI (n=116)
• Using the Braden Scale, only 16% of patients were identified as At Risk (n=37)
”Because SEM measurement is a diagnostic tool that can detect early risk and damage, those undergoing surgery should have the areas of their bodies that have been subject to pressure/shear scanned immediately postoperatively and every day until discharge, or until SEM deltas normalise.” 1
The use of sub-epidermal moisture (SEM) assessments is recognised in the EPUAP - NPIAP - PPPIA International Clinical Practice Guidelines since 2019. 2
* Median
References: 1. Oliveira M, Lúcia A et al. Sub-epidermal moisture versus traditional and visual skin assessments to assess pressure ulcer risk in surgery patients. Journal of wound care vol. 31 Issue 3 (2022), P. 254-264. doi:10.12968/jowc.2022.31.3.254.
2. EPUAP/NPIAP/PPPIA (2019). Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. Section 5, p.78-79.
information visit www.arjo.com/Provizio

Certain conditions can cause the nutritional system to be suppressed, thereby compromising the wound healing process for those subgroups of patients. This article provides a review of two of these types of patients, the application of the Omega3 fish skin graft and subsequent wound closure.
Skin, the largest organ of the body is also one of the most important. Comprising of many layers of skin cells, in distinct layers, in a constant renewal cycle. Within the layers of the skin cells in the epidermis and dermis are a network of blood vessels providing nourishment to the skin cells and the skin’s finely tuned nervous system with temperature, pressure and pain sensors, and oil and sweat secreting glands. Healing of the skin occurs from the deepest layer up, and from the edges inward. Initial stages of healing occur when basic connective tissue cells of the skin start the cycle for production of collagen, which nests in the wound area, thus building the framework to support the rebuilding of the skin. Once this collagen construct framework is in place, the expanding leash of blood vessels migrates to it. Enhancement of the blood supply enables the skin and nerve cells to follow. If the vital structures residing in the skin survive the trauma then hair pigment and the sweat glands may regenerate. Otherwise, the new ‘healed’ skin is deficient in these features. Human skin is a vital part of the body’s immune system, forming a physical barrier between external and internal environments. Adequate nutrition is required for this active immune organ to perform its physical barrier function.
The growing incidence of chronic nonhealing wounds in aging and indigenous populations may result from a culture of poor nutrition, in what can be referred to as ‘nutritional skin failure syndrome’. Most wounds are partial thickness, i.e. involve only partial layers of skin. If the skin has a full thickness defect it may need to be addressed with an additional reconstructive option.
Additional factors can affect the healing of large wounds such as infection, which can slow the
healing process. Wound healing in a normal state involves a delicate balance between nutritional intake and the body’s metabolism. A deficiency of nutrients (or an excess) can cause a disruption to this balance. A nutritional deficiency is most often encountered in patients from a lower socio-economic background, or sometimes the deficiency has a medical cause involving malabsorption, or for example, patients recovering from bariatic surgery. Psychiatric conditions such as eating disorders are a cause of insufficient nutrition, as are drug and alcohol addiction. A state of insufficient nutrition can be identifiable in symptoms of disorders of the skin.1
Challenging wounds, such as diabetic foot ulcers and venous leg ulcers, have a significant economic impact on healthcare and are associated with elevated patient morbidity. The added nutritional deficit adds an additional layer of challenge for the surgeon.
Dermal and surgical application of minimally intact fish skin graft allows for the deposition of collagen and Omega3 fatty acids to the wound, aiding in reducing inflammatory response as well as providing nourishment to the skin, allowing for improvement in the environment that led to the chronic wound in the first place.
Dr Naz Wahab
Day
A 71 year old man with a long history of peripheral arterial disease presented with a long-standing hard-to-heal wound on the dorsal aspect of his right foot. Initial treatment
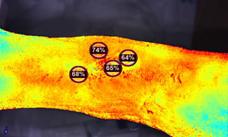
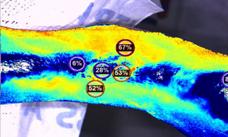
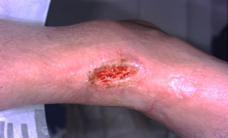
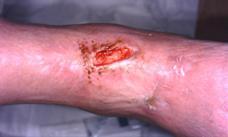
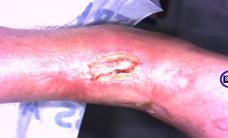
commenced with basic dressings, and the progression through the reconstructive ladder included amniotic products and a course of hyperbaric oxygen therapy, which was unfortunately poorly tolerated by the patient. The wound was eventually debrided surgically, prior to application of Omega3 fish skin grafts.
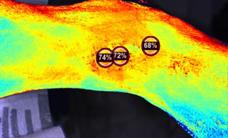
Initial
A 92 year old woman had a hard-to-heal wound following multiple attempted endovascular revascularization procedures. She presented to our clinic with a right great toe wound and an Achilles’ tendon wound. She had an aggressive surgical debridement undertaken and initial treatment with amniotic products and other skin substitutes, prior to the application of Omega3 fish skin grafts which were applied weekly.

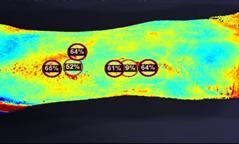

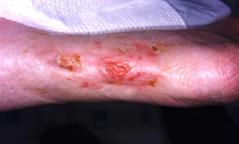
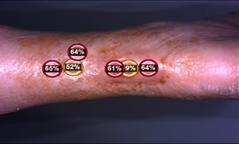
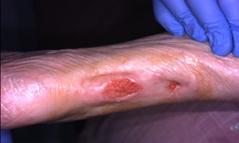



The oxygenation of the wound had improved by day seven post Omega3 fish skin graft application and continued to improve at day twenty one, in both cases. Wounds showed improved blood flow and delivery of oxygenrich nutrients to the skin, which aided in wound healing processes.

The population most often experiencing chronic wounds is the same population that socioeconomically can least tolerate them. Treating the nutritional depletion of these wounds may be useful both topically and systemically, and further study of nutrition as both treatment and prophylaxis should be performed.
To conclude by summarising the benefits of Omega3 fish skin technology: it confers the advantage of no cultural or religious barriers to clinician/ patient acceptance. It is easier to use, with larger, thicker sheets which negates the need for multi-layer grafts. The product is nonallergenic and is bio-compatible, with no known risk of disease transfer, and improved infection control; and in an advantage particularly relevant in patient groups with nutritional skin failure syndromes, the rate of absorption into the surrounding tissue is adjustable.



Compartment syndrome, acute or chronic, occurs with increased pressure within a confined space in the body, affecting areas of the body known as fascial compartments. Formed by a special tissue that forms a membrane layer around muscles, fascial compartments are highly sensitive to pressure changes. Increased pressure significantly reduces blood flow to the area, impairing oxygen delivery, ultimately causing nerve and muscle damage. Compartment syndrome can occur in the hand, forearm, upper arm and buttocks, commonly occuring in the anterior fascial compartment below the knee. Fractures, burns, bites and crush injuries can cause acute compartment syndrome where there is significant damage to the blood vessels and tissues. Repetitive tissue injury and overuse can also lead to chronic compartment syndrome. Symptomatically, severe pain (even at rest), tightness, reduced mobility, decreased function and difficulty weight bearing are are all pathognomonic of this condition.
Fasciotomy is the definitive treatment for acute compartment syndrome to decompress the involved compartments, decreasing the risk of amputation of the affected limbs. Although this is a life-saving procedure, patients are often faced with complications including long hospital stays, wound infection, and osteomyelitis due to the challenges associated with healing full-thickness wounds. With no clear consensus regarding the best methodology for closure of fasciotomy wounds, the technique applied each time is largely the surgeon’s preference.

In these case reports, the feasibility of an intact fish skin graft for definitive fasciotomy wound closure in a patient with multiple wounds is assessed, and a second case involves multiple nonhealing venous ulcers with extensive soft tissue loss and exposed tendons.
 Lower limb reconstruction remains a pivotal but challenging aspect of a wound care clinician’s workload. This article offers an overview of surgical applications of intact and fragmented fish skin grafts and Negative Pressure Wound Therapy (NPWT) for lower extremity wounds.
Dr Deepali Darji
Northern Illinois Foot & Ankle Specialists Cleveland OH, United States
Lower limb reconstruction remains a pivotal but challenging aspect of a wound care clinician’s workload. This article offers an overview of surgical applications of intact and fragmented fish skin grafts and Negative Pressure Wound Therapy (NPWT) for lower extremity wounds.
Dr Deepali Darji
Northern Illinois Foot & Ankle Specialists Cleveland OH, United States
A male patient presents with compartment syndrome of the right leg and secondary rhabdomyolysis a week after surgery. The patient received multiple fasciotomies of the leg, resulting in five wounds. The wound beds were debrided three days after the procedure
and meshed fish skin grafts were sutured in place, followed by a non-adherent dressing, before continuous NPWT (negative pressure wound therapy) at 125 mmHg setting with bridging between wound sites. The patient received four applications of Omega3 fish skin grafts to all sites, including a single application of fragmented intact fish skin graft into the larger area wounds.


1a: S/p fasciotomy. Wound bed preparation/ debridement prior to first application of Omega3 fish skin grafts.
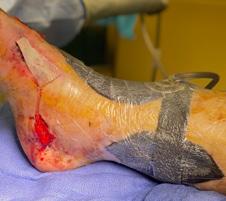
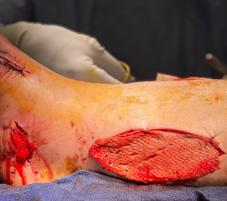
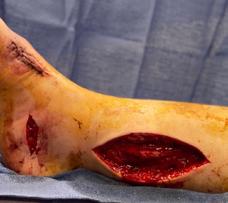
1b: 3:1 ratio meshing of fish skin Complete wound closure of all sites achieved graft.
1c: Meshed fish skin graft sutured over wound base.
1d: Non-adherent dressing placed over the graft prior to application of NPWT with bridging between wounds.
1e: Application of fragmented fish skin graft as wound size decreased and discontinued the use of NPWT.
1f: Complete wound closure of all sites achieved.
Surgical Applications of Intact and Fragmented Fish Skin Grafts and NPWT for Lower Extremity Wounds:The second case involved a male patient with chronic venous insufficiency and three large ulcers encompassing the right ankle. The patient was referred to the surgeon for
aggressive wound debridement and advanced wound care due to deterioration and active infection. After resolution of infection, the wounds were debrided prior to placement of grafts and NPWT.
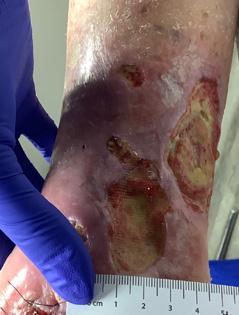
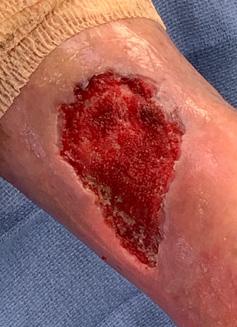
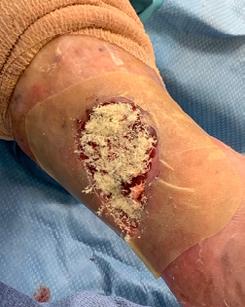
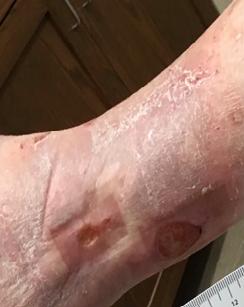
Most wound sites were epithelialized within two months. The average wound surface area reduction across the five wounds one month after the application of fish skin was over 7%. The wounds showed an earlier buildup of granulation tissue. The results had good cosmetic and functional outcomes for the patients.
Previously published clinical trials showed that full-thickness acute wounds treated with fish skin grafts healed significantly faster than wounds treated with porcine tissue.1-3
1. Lullove EJ, Liden B, Winters C, McEneaney P, Raphael A, Lantis Ii JC. A Multicenter, Blinded, Randomized Controlled Clinical Trial Evaluating the Effect of Omega-3-Rich Fish Skin in the Treatment of Chronic, Nonresponsive Diabetic Foot Ulcers. Wounds. 2021 Jul;33(7):169-177. doi: 10.25270/wnds/2021.169177. Epub 2021 Apr 14. PMID: 33872197.
3. Kirsner RS, Margolis DJ, Baldursson BT, Petursdottir K, Davidsson OB, Weir D, Lantis JC 2nd. Fish skin grafts compared to human amnion/chorion membrane allografts: A doubleblind,
Animal studies have shown that Omega-3 fatty acids in the fish skin graft have an inhibitory effect on bacterial growth, improve epithelial cell migration, angiogenesis and accelerated blood perfusion in the wound bed.4,5 By incorporating aggressive wound debridement/ preparation and application of NPWT, as well as applying the standard tenets of wound management (reduction of bioburden/ infection and adequate offloading), these wounds can heal considerably faster compared to conventional wound care.1
prospective, randomized clinical trial of acute wound healing. Wound Repair Regen. 2020 Jan;28(1):75-80. doi: 10.1111/wrr.12761. Epub 2019 Oct 25. PMID: 31509319; PMCID: PMC6972637.
4. Kotronoulas A, Jónasdóttir HS, Sigurðardóttir RS, Halldórsson S, Haraldsson GG, Rolfsson Ó. Wound healing grafts: Omega-3 fatty acid lipid content differentiates the lipid profiles of acellular Atlantic cod skin from traditional dermal substitutes. J Tissue Eng Regen Med. 2020 Mar;14(3):441-451. doi: 10.1002/term.3005. Epub 2019 Dec 30. PMID: 31826323.
5. Stone R 2nd, Saathoff EC, Larson DA, Wall JT, Wienandt NA, Magnusson S, Kjartansson H, Natesan S, Christy RJ. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int J Mol Sci. 2021 Feb 4;22(4):1590. doi: 10.3390/ijms22041590.
PMID: 33557424; PMCID: PMC7915828.
“Previously published clinical trials showed that full-thickness acute wounds treated with fish skin healed significantly faster than wounds treated with porcine tissue.”1-3 IL,
IL,
Hidradenitis suppurativa (HS) is a chronic inflammatory disease that manifests with abscesses and sinuses formations. Treatment of advanced and chronic HS includes surgical resection and skin reconstruction that is often challenging because the remaining tissue is at a poor healing stage, and lack of vascularization.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition affecting apocrine gland bearing skin in the axilllae, groin and under the breasts. It is characterised by persistent or recurrent nodules and abscesses that culminate in a purulent discharge, sinuses and scarring. Often presenting between the ages of 20 and 40, it is much more common in females and also affects individuals with a family history of this condition, smokers, patients with other inflammatory conditions such as inflammatory bowel disease, obese individuals with insulin resistance; and as a result of some medication side effects, such as lithium and biologics. It is thought to be an auto-inflammatory syndrome and can affect multiple areas anatomically including the inner thighs, as well as anogenital involvement, most commonly the scrotum perineum vulva and the perianal folds. The typical presentation is open headed comedones, with some tender papules, and nodules; in addition pustules pseudocysts and abscesses may also be present. If left untreated, the condition can progress onto hypertrophic scarring as well as sinuses. There are a number of subtypes of HS, which include scarring folliculitis, a frictional furuncle, and syndromic HS. The severity of HS is assessed using the Hurley grading system, which is an assessment tool comprising of three clinical stages.
• Stage one is a solitary or a multiple isolated abscess formation without sinus tract stage
• Stage two involves recurrent abscessing with single or multiple wide spaced lesions
• Stage three involves diffuse involvement of a anatomical area with multiple connected sinus tract and abscesses
HS can progress onto several serious complications which include superseding infection, lymphoedema, anaemia of chronic disease, and skin cancer (Squamous cell carcinoma, particularly in male anogenital HS).
A common occurrence in HS is the delay to diagnosis, which can be due to a number of factors: typically a triad of symptoms needs to be present for this diagnosis to be made; these include the presence and recurrence of lesions, specific anatomical distribution, and the characteristics of these lesions. It is important to bear in mind the differential diagnosis, which may be inflammatory bowel disease, and other types of cystic lesions.
The quality of tissues in chronic and longstanding HS is poor, which is an added reconstructive challenge for the wound care clinician.
Fish skin grafts are a unique biologic scaffold resembling the dermal extracellular matrix, promoting cellular ingrowth and neovascularization. FSG also contains Omega3, which is anti-inflammatory. We hypothesize that FSG can be used as part of the surgical intervention for HS, both as wound bed preparation and closure support, that results in faster and better healing.
A case series was conducted with 9 HS patients who received treatment with FSG at SAS Surgical LTD, performed by Dr. Michael Romberg. HS defects varied from the axilla, groin, labia and abdominal wall.
Patients received excision of the damaged tissues, followed by application of FSG to the wounds until they were ready for secondary closure or split thickness skin graft (STSG).

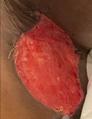

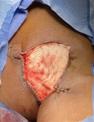
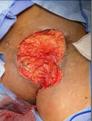
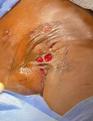
The management of HS is a multi-staged procedure, with or without STSG, and often faces a high risk of recurrence rates due to infection and patient comorbidities. Omega3 fish skin supports the preparation of the wound bed for early and successful grafting, or secondary closure. FSG is an effective alternative to conventional surgical interventions of HS that helps shorten hospital stays. The use of FSG is safe, involves no complications and results in rapid healing.
Complete healing was achieved in all cases without complications. FSG resulted in faster granulated tissues that led to successful closure or effective STSG after 1 - 3 months. Patients also noticed scar reduction, better cosmetic outcomes, and less pain.
 VA, United States
VA, United States
Oxygen therapy is a well evidenced modality of care in clinical wound practice. It can provide many benefits, such as a reduction in hospitalizations and amputations for diabetic foot ulceration patients. This article provides a practical top ten tips for the methods of optimization of oxygen therapy in your practice; taking into account factors for delivery of this modality such as activity, wound impacting factors; comfort, economic impact; geographic factors, capable participation level; compliance/ adherence, number of wounds and severity, and active infections, all of which will be discussed in this article to improve patient care delivery.
This article is the second of two I have written on oxygen therapy for Wound Masterclass; the first, ‘Optimizing Oxygen Therapy in Your Clinical Wound Practice’, published in the inaugural MayJune issue, provided a concise background and overview to this modality. This second article is intended to complement the first with a practical ‘Top Ten Tips’ of use to the clinican, in introducing this modality to their practice and how to approach the various factors involved.
How active is my patient? If they need to be active because they are still working and supporting a family you may want to consider a portable continuous diffusion device. Are they retired and have a more sedentary lifestyle, where at home delivery devices that are not as mobile may be used? Is the mobility from some delivery systems beneficial for the patient, or will the ambulation and freedom from a more portable device enable your patient to partake in more activities than you recommend? The boot systems and chambers are great for providing guaranteed non ambulatory stationary time when activity restriction is desired. The pressurized system requires the patient to sit near the condenser unit, ensuring that during device use they cannot do much, thus allowing protected offloading time. If they do need to be mobile, the continuous delivery systems offer that freedom. If you want to limit mobility, the boot/ sleeve systems require close association to the base unit and do not allow free movement while on therapy.
Some of the delivery systems differ in the ability to move edema during use, adding the benefit of fluid control. The cyclic pressurized TOT delivery system (not the continuous flow TOT systems) has the added benefit of sequential non-contact compression of the limb, which helps to reduce peripheral edema in addition to the oxygen, and promotes arterial blood flow. Wound bed dryness is another consideration. Topical oxygen requires delivery to the wound surface and requires a moist wound environment; HBOT delivers the oxygen systemically and does not have this requirement. Dry wound beds will have an issue with the diffusion of oxygen, and thick gel wound care products will inhibit diffusion of the oxygen molecules. Pressurized cyclical TOT has the added benefit of adding humidification for oxygen diffusion. Keep in mind, moist wound environments have better outcomes compared to dry wound therapy.
For some, group settings have a positive impact on patients, while it makes others withdraw from social interactions because of the wounds. HBOT requires frequent interactions in a facility and may utilize multi-place units or single full body chambers, which have the additional claustrophobic issue. If the patient is not comfortable, they will not use the therapy and thus the treatment is ineffective. It has been well documented that oxygen therapy reduces wound pain; this benefit is achieved from both HBOT and TOT. Physical discomfort may come from devices that require
seals (TOT boots/ sleeves) to achieve pressure, or from prolonged stationary positions (HBOT and high/ low pressurized TOT systems) for 60120 minutes at a time. The TOT systems have less noticeable negative side effects during use compared to HBOT, which can cause temporary myopia, glycemic control issues, middle ear barotrauma, pulmonary oxygen toxicity, and CNS oxygen toxicity.
Can your patient afford to drive or take transportation daily to your office or treatment facility, if HBOT is the recommendation? Does their insurance plan cover the therapy or is it an out-of-pocket expense? Can they maintain their job occupation under the therapy, or do they need to take time away in order to utilize the therapy?
Where is the patient located? What if your patient is in a rural area and can only make infrequent appointments? Don’t forget about the homebound patients who cannot leave, and telehealth patients who prefer to stay virtual. HBOT requires daily transportation to a specific location. TOT devices can be used at home and do not require transportation, and can also be used in skilled facilities and inpatient settings.
How involved is your patient in their care? Are they capable of performing their own dressing changes and therapy or do they rely on others to help, and are they capable of helping with the therapy prescribed? Patients that cannot perform their own care but have no problem getting transportation may do better with HBOT. Pressurized TOT systems are daily applications; patients or caregivers that can participate in donning and doffing of boot/
chamber delivery systems daily will find benefit from this therapy. Continuous TOT device systems may stay in place in the dressing for 1 - 2 weeks and do not need to be changed as often, but do require batteries that need to be charged/ changed often, which can also be problematic. Cognition deficiencies pose wound care problems in general with dressing changes, without the added daily application or battery changes.
No matter which therapy you think would be best, it all comes down to your patient’s willingness to participate. If you know your patient misses recommended appointments but oxygen therapy is indicated, then a system which requires the least amount of patient involvement would likely work better. Continuous diffusion systems do not need to be changed/ used daily and do not require daily transportation to another location. Home health agencies can preform the dressing changes and they are user friendly, for the more independent individual.
Let’s face it, some patients unfortunately have more than one wound or wounds that are very large. Continuous TOT devices are designed for single wounds or smaller wounds grouped together. They rely on tubing under occlusive dressing to deliver the oxygen. Larger wounds and patients with multiple wounds need a larger surface area of coverage, which would best be found from the pressurized devices and HBOT.
All oxygen delivery systems can be used in conjunction with treated infections but are not a substitute for antibiotic therapy. HBOT has the added benefit of being synergistic with some antibiotics due to its systemic delivery
“No matter which therapy you think would be best, it all comes down to your patient’s willingness to participate.”
where the TOT do not, and HBOT also has a specific indication for osteomyelitis. HBOT has the strongest evidence of support with severe infections to include necrotizing and clostridial. Cyclic pressurized TOT systems, along with HBOT, have been shown to increase local wound partial oxygen tension. Increasing the wound oxygen tension has a direct effect on leukyocyte activity in clearing the infection. Activated neutrophils produce NADPH oxidase to generate reactive oxygen species, which also consumes the available oxygen.
Between the cellular activity needed to fight an infection, the now increased metabolic demand of oxygen to heal damage tissue, and bacterial or fungi consumption thereof, the wound tends to be depleted of available oxygen creating a hypoxic like environment.
Chronic wounds with suspected biofilms will have a chronic increase of neutrophils, thereby high oxygen consumption that continues to impede wound healing.
HBOT can be used with any dressing choice. Topical delivery systems cannot be used in conjunction with gels, as they hinder the oxygen from getting into the wound bed (they can be wiped out before device use for the noncontinuous delivery devices). Continuous non pressurized devices require occlusive dressings to keep the oxygen locally and if not kept in check can lead to maceration of the periwound. Pressurized systems can go through breathable dressings like unna boots, compression systems, and even TCC.
Evidence Based Therapy: Demonstrated in both Randomized Controlled Trial and Real World Evidence to offer superior healing for Diabetic Foot Ulcers (DFUs).
A Unique Delivery System: Targets multiple aspects of wound healing with OXYGEN, CYCLICAL COMPRESSION and HUMIDIFICATION
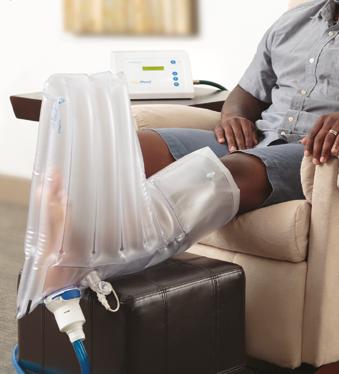

A Game Changer for Patients and Clinicians: Drives compliance with a self-administered, at-home therapy and overcomes traditional healthcare barriers. TWO2 can be used with gas permeable dressings, CCD, UNNA Boot and TCC.
Visit www.AOTInc.net for research articles, patient and physician testimonials – and more.



Oxygen is a vital component of basic and advanced wound healing. It plays a major part in intricate cellular processes involved with healing all types of wounds. Understanding the pathophysiology of a hypoxic situation at the tissue level is vital in establishing the types of wounds that will benefit from oxygen therapy. The options for comprehensive therapy are discussed in depth; indications such as venous leg ulcers and chronic venous insufficiency, as well as diabetic foot ulceration are also reviewed in this article. A summary of recent topical oxygen therapy evidence is presented, as well as the myths surrounding oxygen therapy. This guide is aimed at providing support for clinicians wishing to incorporate oxygen into their practice.
Advanced, or ‘smart’, technology continues to be utilized in all facets of healthcare, up to and including for delivery of oxygen to the body. And while the use of smart technology is said to make life increasingly more convenient from both a personal and professional standpoint, it does not relieve wound care providers of having to understand the many nuances associated with caring for patients living with hard-toheal wounds. From the mechanism of action and the administration of oxygen therapy, to the available research that offers proven pros and cons to oxygen’s use, providers must be comprehensively well versed with this modality regardless of how much today’s technology might be able to ‘think’ for us humans. What’s more, topical oxygen presents a myriad of challenges despite how sophisticated today’s technology is. This continues to place emphasis on the basic facts and best practices surrounding this important life-sustaining element.
Oxygen is crucial in all phases of the wound healing cascade of events. Cellular and biological processes depend on oxygen, especially during the repair process. These include cell proliferation, angiogenesis, collagen deposition, resistance to infection, and protein synthesis needed to restore tissue integrity and function.1 Tissue oxygenation can trigger healing responses as well. Oxygen keeps cells nourished, oxidizes food during cellular respiration, is involved in the production of cell energy, and is an overall essential element
to wound healing.2 Hypoxia, a lower level of oxygen than normal, is caused when there’s an impaired delivery of oxygen or an impaired cellular oxygen uptake.2 In the human body, normal blood oxygen level is categorized in the 94 - 98% range, while levels below 90% are considered dangerous and require intervention.3
Limiting oxygen to the cells could have negative consequences, such as impaired healing and respiratory issues. Consider chronic obstructive pulmonary disease (COPD), which compromises lung function due to damage to the airways that renders breathing difficult.
As COPD and breathing difficulties advance, a lack of oxygen to vital organs could lead to hypoxia. Symptoms include shortness of breath, frequent respiratory infection, fatigue, lower-extremity swelling, and reduced muscle strength.
Another condition that restricts the flow of oxygen and can impact wound healing is obstructive sleep apnea (OSA), a serious disorder that causes breathing to stop repeatedly during sleep when the body should be receiving extra oxygen. If left untreated, OSA can lead to hypertension, development of diabetes, heart conditions, and strokes, among other maladies. The importance of recognizing diminished oxygen levels has led clinicians to utilizing the delivery of oxygen as a treatment. COPD patients, depending on severity, can be prescribed supplemental oxygen, while patients with OSA can be treated with positive airway pressure devices that prevent breathing
interruptions.
Another form of oxygen as a treatment is hyperbaric oxygen therapy (HBOT), the roots of which actually date back to the 1600s. In the late 1930s, the military began using hyperbaric chambers to treat deep-sea divers who experienced decompression sickness. In 1967, the Undersea Medical Society, now known as the Undersea and Hyperbaric Medical Society (UHMS), was founded and later reviewed available evidence supporting the use of HBOT for a number of conditions. A listing of approved indications for HBOT by the U.S. Food & Drug Administration includes4:
Approved Indications for HBOT:
• Air and gas bubbles in blood vessels
• Anemia (severe anemia when blood transfusions cannot be used)
• Burns (severe and large burns treated at a specialized burn center)
• Carbon monoxide poisoning
• Crush injury
• Decompression sickness
• Gas gangrene
• Hearing loss (complete hearing loss that occurs suddenly and without any known cause)
• Infection of the skin and bone (severe)
• Radiation injury
• Skin graft flap at risk of tissue death
• Vision loss (when sudden and painless in one eye due to blockage of blood flow)
• Wounds (non-healing, diabetic foot ulcers)
It is reported that there are more than 8 million chronic wounds that are more likely to be stuck in the inflammatory phase.5 The longer that it takes to manage these wounds effectively will increase likelihood of complications - time is
An arsenal of modalities is available that can impact cellular dysfunction in chronic wounds. These modalities could include electrical stimulation, high frequency ultrasound, low frequency contact and non-contact ultrasound, low level laser, shockwave therapy, pulse lavage, negative pressure wound therapy, diathermy, deep oscillation devices, and others that can be conducted in conjunction with sharp debridement, compression, and complete decongestive therapy (CDT). The goal with these modalities mainly includes the ability to ‘jump start’ chronic wounds into the progressive healing cascade and removing nonviable tissue to alter the wound environment.
Altering the chronic wound environment can move the wound along the healing path. Chronic wounds have an alkalic pH level of 7.15 - 8.9, while wounds that make progress have a much lower pH level.6 If we are successful in decreasing the pH level by one numeric point, this increases the oxygenation level at the wound dramatically.6 Necrotic tissue is a medium for bacteria to proliferate and produce inflammatory mediators that inhibit healing. Without adequate local tissue oxygenation, the respiratory burst is impaired and the result is increased susceptibility to infection.7
Clinicians should also aim to assess arterial perfusion in lower-extremity wounds to establish adequate delivery of oxygen-rich blood to tissue. The delivery of oxygen is dependent on adequate blood flow at the capillary level. Without adequate perfusion, tissue death is eminent as the environment could become ischemic. Acute hypoxia is needed for cellular signaling, but chronic hypoxia will maintain the wound in an inflammatory phase. Treatment through revascularization can reestablish adequate levels of oxygen delivery to tissue.
“The delivery of oxygen is dependent on adequate blood flow at the capillary level. Without adequate perfusion, tissue death is eminent as the environment could become ischemic.”
Another important and often missed component in wound care is recognizing the negative effects of edema. Edema impairs the healing process because it delays the delivery of oxygen and nutrients to tissue. The endothelial glycocalyx plays an important role in fluid management, but increases endothelial cell permeability and leukocyte infiltration when injured. There’s also an increase in inflammatory cytokines, matrix metalloproteinases, reactive oxygen and nitrogen species, iron deposition, and tissue metabolites.8 When interstitial space increases due to endothelial glycocalyx damage or with an edematous condition, the distance from the capillaries to the cells requiring oxygen increases, therefore delaying oxygen transport. This increase in diffusion distance robs the wound of oxygen, which ultimately impairs the healing cascade of events. Patients with chronic venous leg ulcers (VLUs) and edematous lower extremities suffer from frequent infections, skin changes, and poor outcomes.
Studies have validated the fact that patients with chronic edema have a difficult time healing. Rafetto et al. describe that the incidence of VLUs is 70 - 80% of all clinical ulcers, with recurrence rates of 50 - 70% at 6 months.8 VLUs pose a significant problem to our population, but new findings indicate that the numbers will continue to increase. The standard of care for VLUs consists of compression. With the recent changes in the Starling principle, it is understood that the lymphatic system is responsible for removing fluid and resolving edema. If a patient has a healthy lymphatic system, edema management will not pose a problem. However, if a patient’s lymphatic system fatigues, fails, or is damaged, any longstanding VLU edema will lead to a condition known as phlebolymphedema. A condition that indicates venous damage, phlebolymphedema when present with ulcers will lead to difficult healing due to the presence of stagnant
protein-rich fluid and due to the longstanding fluid volume within the lower extremities. Essentially, the lymphatic system is working overtime and eventually fails. Margolis et al. discuss a prognostic model for VLUs, indicating that a wound that is < 10 cm2 and less than 12 months old at the first visit has a 29% chance of not healing by 24th week of care, while a wound > 10cm2 and > 12 months old has a 78% chance of not healing.9 This could be our answer as to why VLU patients with prolonged unresolved edema could have a poor chance of healing. If patients with VLUs have a lymphatic system that fails due to prolonged edema, compression will not suffice as the standard of care. The treatment at this point, along with compression, should be aimed at moving lymph to another quadrant with a normal lymphatic system. A 2022 article indicates that all edema is related to lymphatic dysfunction, whether transient or permanent, thereby creating a lymphedema continuum.10 Untreated lymphatic dysfunction will also lead to an increase in diffusion distance, thus decreasing the amount of oxygen delivered to the skin and/ or wound.
In their 2020 study, Dean et al. established that lower-extremity lymphedema is predominantly caused by chronic venous insufficiency (CVI) and not the commonly encountered cancer.11 This also underscores that when the cause of edema is not addressed properly, patients will encounter frequent infections, chronic wounds, and other complications while not allowing oxygen to diffuse adequately. Tests such as transcutaneous oximetry (PtcO2) can assess the peri-wound oxygen tension of wounds. Fife et al. determined oxygen tension < 40mm Hg to be defined as tissue hypoxia.12
Patients with edema, inflammation, and/ or infection could have initial Ptc02 readings < 30 mm Hg, due to the increased diffusion distance. When adding supplemental oxygen under
“If patients with VLUs have a lymphatic system that fails due to prolonged edema, compression will not suffice as the standard of care.”
normobaric conditions, if the PtcO2 is > 100mm Hg, there could be an edematous condition at fault, as opposed to an arterial condition, decreasing the delivery of O2. Noting the important role of oxygen throughout the healing process, focusing on adequate arterial perfusion and decreasing the diffusion distance of edematous legs can be of value to bringing wounds out of the inflammatory phase and into the proliferative phase. Additionally, wound bed oxygenation can increase by revascularization, debridement of necrotic tissue, address infection, and address edema.
A study published in 2003 found that the percent change in wound area at 4 weeks in those who healed was 82%, whereas the percent change in wound area was 25% in those who failed to heal.13 The percent change in foot ulcer area after 4 weeks of observation is a robust predictor of healing at 12 weeks. This study concluded that this can serve as a clinical decision point in care for diabetic foot ulcers to identify patients who may not respond to standard care requiring additional treatments. If limited or no progress is made at 4 weeks, it is recommended to reassess the wound for arterial perfusion, infection, and other factors that delay healing and potentially consider the use of advanced modalities.
If ischemia impairs the delivery of oxygen, a vascular referral is of importance, whereas edematous lower extremities require appropriate compression to decrease the larger diffusion distance to improve oxygenation.
Topical oxygen therapy (TOT) is another valuable option that has been available for some time, but early research was lacking. As such, TOT was often labeled as ‘topical hyperbaric oxygen therapy’, which is incorrect based on its function.
More recently, there has been a great deal of available research on the effectiveness of TOT and its ability to help heal chronic wounds. TOT is not likely to replace HBOT, but should continue to produce optimal evidence-based results. What follows is a list of top challenges that exist related to the use of TOT.
1. The use of TOT at Home Doesn’t Alter Treatment at an Outpatient Wound Clinic
During the past 3 years, I’ve had the opportunity to use long wave infrared thermography and Near Infrared Spectroscopy on chronic hard to heal wounds. Medical thermography has over 800 published articles and this allows me to not only look for physiological anomalies in the nonvisible light spectrum but also to assess tissue oxygenation. These objective findings assist providers with their clinical assessments, while requesting further objective tests, validates treatment interventions and utilized in preventative measures not only for pressure injury but also for patients with diabetes. As we consider chronic wounds to have a decrease in oxygenation in the wound bed, I am also noting a decrease in thermal energy when imaging chronic wounds, classifying them in a hypoperfusion state. Thermographically, it is evident that technology can assist us with determining which wounds may not progress due to a lack of oxygen, whether the lack of oxygen is due to arterial disease of a problem with increased diffusion distance, as in an edematous extremity. In addition, dehiscence of incisions may occur when there’s increased diffusion distance, therefore minimizing the amount of oxygen needed to maintain surgical incision health.
Patients can utilize TOT at home and continue wound care treatments at an outpatient clinic and/ or with home health, without altering the plan of care. Providers can continue treatment,
“If ischemia impairs the delivery of oxygen, a vascular referral is of importance, whereas edematous lower extremities require appropriate compression to decrease the larger diffusion distance to improve oxygenation.”Oxygen Therapy and Wound Management:
such as debridement and other modalities, just as if they were performing HBOT treatment. The only exception would be negative pressure wound therapy and/ or occlusive dressings, as oxygenation might not reach the wound bed due to the occlusive barrier. To be mindful of successful interventions, chronic wounds could require additional modalities, as long as the basic tenets of wound care have been implemented and not overlooked.
There is growing evidence of the benefits of TOT. A sampling of studies includes:
• Evidence of TOT promoting collagen formation and VEGF expression promoting angiogenesis26
• Use of a special probe to measure the superficial pO2 at the center of the wound (2mm depth) after exposure to topical oxygen27
• A comparison of a variety of chronic wounds being treated with HBOT versus TOT for 14 weeks and assessing healing outcomes19
• A study that examined VLUs being treated with TWO2 versus compression28
• A report that TOT can generate sustained increased in wound pO2 supporting angiogenesis and increased VEGF29
• A DFU study noting that at 12 weeks with TWO2 with a mean baseline wound area of 4.1 cm2, there was a healing rate of 82.4%, with a median of 56 days to heal; versus the control group with a mean baseline wound area of 1.4 cm2, there was a healing rate of 45.5%, with 93 median days to heal in the standard of care group30
• A study of VLUs with > 2 years onset, with no improvement over the previous year, demonstrating improved healing rates and median time to heal when using TWO231
• A multinational, multicenter, randomized, doubleblinded, sham-controlled trial supporting cyclical pressurized TOT for the treatment of DFUs32
As indicated by a recent consensus statement on guidelines for the use of TOT in the treatment of hard-to-heal wounds25, there’s a growing body of evidence suggesting that topical oxygen is valuable in healing DFUs.
As with any product, following manufacturer’s indications to ensure proper use is imperative. Indications for TOT includes the use for chronic, hard-to-heal wounds such as DFUs, VLUs, pressure ulcers (stage 3 - 4), and postsurgical and burn wounds. TCOT and/ or CDO list indications to treat such as skin ulcerations resulting from diabetes, venous stasis, postsurgical infections, gangrenous lesions, pressure ulcers, infected residual limbs, skin grafts, burns, and frostbite. Research lists wound types that are indicated and/ or have demonstrated successful outcomes for TOT,21 including diabetic ulcers, vascular ulcers, post-surgical infections, pressure injuries, amputations and infected stumps, skin grafts, ischemic tissues, burns, and frostbite. The utilization of various products and modalities as intended could alter the healing trajectory of chronically stalled hard-to-heal wounds.
Contraindications for topical oxygen include:
• Inadequate perfusion to support wound healing
• Acute thrombophlebitis
• Ulcers due to Raynaud’s disease
• Presence of necrotic tissue if debridement is not attempted. Necrotic wounds with eschar or slough
• Wounds with fistulas or deep sinus tracts with unknown depth
• Wound dressings that are occlusive, including the use of petrolatum products
“As indicated by a recent consensus statement on guidelines for the use of TOT in the treatment of hard-to-heal wounds25, there’s a growing body of evidence suggesting that topical oxygen is valuable in healing DFUs. ”
Also consider including untreated osteomyelitis, and malignant wounds.
Although there are benefits for using oxygen in the wound management field, the hazards and side effects of oxygen must be considered. Oxygen should be administered cautiously, as with any medication.
Generally, oxygen supports combustion, so smoking should not be permitted near the source of oxygen and it should be kept away from any heat source. Ensure that any electrical equipment involved is in safe working condition. Unlike precautions and possible complications with HBOT, TOT presents no risk for oxygen toxicity, ear barotrauma, pneumothorax, temporary change in vision, or a decrease in glucose levels. Devices that are worn continuously are recommended to be disconnected during a shower or bath. A recent systematic review and meta-analysis study on the efficacy and safety of TOT for DFUs concluded that TOT is effective and safe for chronic DFUs.23 Another 2022 study describes TOT to be considered safe with no known risks to the patient above moist wound therapy alone with no reported serious adverse events or reactions in the literature.21,24
Oxygen levels affect the quality of new blood vessel growth, collagen formation, and the signaling of growth factors. Chronic wounds are found to have low oxygen levels. Adequate circulation should be determined with objective testing in lower-extremity wounds to ensure there’s oxygenation and nutrients reaching tissue. The importance of oxygen is also recognized in the numerous biological processes occurring throughout the healing cascade, such as cell proliferation,
angiogenesis, protein synthesis, and resistance to infection, which are required for restoration of tissue function and integrity.21,22
It is known that chronic wound beds have higher-than-normal pH levels. Dissemond et al. acknowledge that pH values influence wound healing and that insight into this allows for more individualized therapy.14 Percival et al. mention how pH has been shown to affect matrix metalloproteinase activity (MMP), tissue inhibitors of MMP, fibroblast activity, keratinocyte proliferation, microbial proliferation, and immunological responses in a wound.15
The pH of chronic wounds has been described to be between 7.15 and 8.9 (7 being neutral, above that as alkaline and below as acidic). For perspective, a pH of 4 is 10 times as acidic as a pH of 5, representing a 10-fold in H+ concentrations.16 The same study describes how pH level decreased as the wound progresses.17 Since there’s a higher number of MMP and necrotic tissue in the wound bed, there’s also an increased metabolic load resulting in tissue hypoxia.18
The pH also influences oxygen release to the tissue. By lowering the pH, there’s an increase of oxygen diffusion. Tissue oxygen tension (pO2) > 40mmHg increases the likelihood of healing. Gordillo demonstrated that tissues must have a pO2 of at least 40 mmHg to promote the production of VEGF, collagen, and restore angiogenesis.19 Fries et al. utilized a special probe at a depth of 2mm in the center of a wound, to determine if TOT raises pO2 levels. Another study utilized an oxygen aerosol delivery system, which could continue to answer questions on oxygenation penetration.20
“The importance of oxygen is also recognized in the numerous biological processes occurring throughout the healing cascade, such as cell proliferation, angiogenesis, protein synthesis, and resistance to infection, which are required for restoration of tissue function and integrity.”21,22
There are various types of topical oxygen therapy (TOT) delivery systems, including intermittent TOT, continuous TOT (TCOT), and continuous diffusion of oxygen (CDO), to deliver oxygen to the tissue. There are differences, however, in how oxygen is supplied to the wound bed and the equipment. TOT uses a high-flow oxygen concentrator connected to a disposable boot or bag, supplying oxygen to the area for 90 minutes, five days per week. The pressure on one device that’s available cannot be controlled, while the other device can control the pressure from 0 - 50 mmHg to help oxygen diffusion to the wound bed. The concentrator is connected to a power source and it’s capable of being used at multiple settings, including the patient’s home. The patient will be immobile during therapy. While oxygen could desiccate a wound bed, a humidifier is utilized to maintain the moisture level inside this boot or bag. Oxygen transfer requires the wound to be moist. One product utilizes noncontact cyclical compression to aid in edema management and the use of humidification, to maintain a moist wound barrier to aid in oxygen diffusion while preventing desiccation.
TCOT uses oxygen generators to continuously provide oxygen from the air to the wound 24 hours per day, 7 days per week. Tubing can be placed within a wound dressing. This continuous system requires a minimum relative humidity level and the level of humidity must be met for normal operation. CDO is a lightweight, handheld device using an oxygen generator providing continuous delivery of oxygen to the wound bed. It delivers oxygen via a cannula that is placed under a moist wound dressing indicated for 24 hours, 7 days per week therapy time. There are two types of CDO systems available: CDO with oxygen distribution system. These systems will produce oxygen even at lower levels of humidity.
Topical oxygen is not and should not be classified in the same category as HBOT, nor ‘soft’ or ‘mild’ chambers. Although HBOT and TOT interventions deliver oxygen to the wound bed, HBOT mechanism of action is to deposit an increase of oxygen to the wound bed, HBOT mechanism of action is to deposit an increase of oxygen to the wound through the plasma as the patient breathes 100% oxygen. On the contrary, TOT is diffusing oxygen across the wound bed and can be utilized in various settings, including the patient’s home, making it convenient when there are barriers such as lack of transportation, rising gasoline prices, the pandemic, lack of resources, and no access to advanced modalities.
Topical oxygen pressures are slightly higher than normobaric pressure, equivalent up to 1.1 atmosphere absolute (ATA). HBOT pressures will be higher than normobaric pressures consisting of >1.4 ATA with treatments typically ranging from 2-3.0 ATA. There’s data indicating the effectiveness of TOT, especially for treating DFUs. But if the goal is to increase the tissue pO2 to a hyperoxygenated level systemically, HBOT will be the choice.
1. Castilla DM, Liu Z-J, Velazquez OC. Oxygen: implications for wound healing. Adv Wound Care (New Rochelle). 2012;1(6):225-30.
2. MacIntyre NR. Tissue hypoxia: implications for the respiratory clinician. Respir Care. 2014;59(10):1590-6.
3. Aviles Jr. F, Whitten-Byles D. Oxygen & wound healing: going beyond hyperbaric therapy. TWC. 2018;12(11):14-21.
4. Hyperbaric oxygen therapy: get the facts. FDA. 2021. Accessed online: www.fda.gov/ consumers/consumer-updates/hyperbaric-oxygen-therapy-get-facts
5. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21:27-32.
6. Gethin, Georgina. (2007). The significance of surface pH in chronic wounds. Wounds UK. 3.
7. Cole W. Wound care update: the role of topical oxygen therapy in the treatment of wounds. Lower Extremity Review. 2020; 12(5):35-8.
8. RaffettoJD, Ligi D, ManiscalcoR, KhalilRA,Mannello, F. Why venous leg ulcers have difficulty healing: overview on pathophysiology, clinical consequences, and treatment. J Clin Med. 2020;10(1):29.
9. Margolis DJ, Allen-Taylor L, HoffstadO, BerlinJA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound RepairRegen. 2004;12(2):163-8.
10. HettrickH, Aviles Jr. F. All edema is lymphedema: progressing lymphedema and wound management to an integrated model of care. Wound ManagPrev. 2022;68(1):8-15.
11. DeanSM, ValentiE, HockK, LefflerJ, CompstonA, AbrahamWT. The clinical characteristics of lower extremity lymphedema in 440 patients. J VascSurgVenous LymphatDisord. 2020;8(5):851-9.
12. FifeCE, SmartDR, SheffieldPJ, HopfHW, HawkinsG, Clarke D. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence.Undersea HyperbMed. 2009;36(1),43-53.
13. Sheehan P, Jones P, Giurini JM, Caselli A, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Plastic and Reconstructive Surgery. 2006 Jun;117(7 Suppl):239S-244S. DOI: 10.1097/01.prs.0000222891.74489.33. PMID: 16799391.
14. Dissemond, J., Witthoff, M., Brauns, T. C., Haberer, D., &Goos, M. (2003). pH-Wert
“If the goal is to increase the tissue pO2 to a hyperoxygenated level systemically, HBOT will be the choice.”
des Milieus chronischerWunden. UntersuchungenimRahmeneinermodernenWundtherapie [pH values in chronic wounds. Evaluation during modern wound therapy]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandteGebiete, 54(10), 959–965.

15. Percival, S. L., McCarty, S., Hunt, J. A., & Woods, E. J. (2014). The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society, 22(2), 174–186.
16. Tsukada K, Tokunaga K, Iwama T, Mishima Y (1992) The pH changes of pressure ulcers related to the healing process of wounds. Wounds 4(1): 16–20.
17. Solomon E, Schmidt R, Adragna P (1990) Human Anatomy and Physiology. 2nd International edn. Saunders, USA.
18. Hunt TK, Beckert S (2005) Therapeutical and practical aspects of oxygen in wound healing. In: Lee B (ed) The Wound Management Manual. McGraw-Hill Medical, New York.
19. Gordillo GM, Roy S, Khanna S, et al. Topical oxygen therapy induces vascular endothelial growth factor expression and improves closure of clinically presented chronic wounds. Clin Exp PharmacolPhysiol2008;35:957–964.
20. Petri M, Stoffels I, Jose J, et al. Photoacoustic imaging of real-time oxygen changes in chronic leg ulcers after topical application of a haemoglobin spray: a pilot study. J Wound Care. 2016; 25(2):87–91.
21. Oropallo A, Andersen CA. Topical Oxygen. [Updated 2021 Sep 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.
22. Oxygen: Implications for Wound Healing Diego M. Castilla,1 Zhao-Jun Liu,1,2 and Omaida C. Velazquez1,2, ADVANCES IN WOUND CARE, VOLUME 1, NUMBER 6 j 225-230 Copyright ª 2012 by Mary Ann Liebert, Inc. DOI: 10.1089/wound.2011.031.
23. Sun, X. K., Li, R., Yang, X. L., & Yuan, L. (2022). Efficacy and safety of topical oxygen therapy for diabetic foot ulcers: An updated systematic review and meta-analysis. International wound journal, 10.1111/iwj.13830. Advance online publication.
24. Kalliainen, L. K., Gordillo, G. M., Schlanger, R., & Sen, C. K. (2003). Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiology : the official journal of the International Society for Pathophysiology, 9(2), 81–87.
25. Serena, T. E., Andersen, C., Cole, W., Garoufalis, M., Frykberg, R., &Simman, R. (2022). Guidelines for the use of topical oxygen therapy in the treatment of hard-to-heal wounds based on a Delphi consensus. Journal of wound care, 31(Sup3), S20–S24.
26. Scott, G., & Reeves, R. (2005). 051 Topical Oxygen Alters Angiogenesis‐Related Growth Factor Expression in Chronic Diabetic Foot Ulcers. Wound Repair and Regeneration, 13(2), A4-A27.
27. Fries RB, Wallace WA, Roy S, et al. Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat Res. 2005; 579(1-2):172-81. Epub 2005 Aug 18. PMID: 16105672.
28. Tawfick W, Sultan S. Does topical wound oxygen (TWO2) offer an improved outcome over conventional compression dressings (CCD) in the management of refractory venous ulcers (RVU)? A parallel observational comparative study. Eur J VascEndovasc Surg 2009;38:125–132.

29. Gordillo GM, Sen CK. Evidence-based recommendations for the use of topical oxygen therapy in the treatment of lower extremity wounds. Int J Low Extrem Wounds 2009;8:105–111.
30. Blackman E, Moore C, Hyatt J, Railton R, Frye C. Topical wound oxygen therapy in the treatment of severe diabetic foot ulcers: a prospective controlled study. Ostomy Wound Manage 2010; 56:24–31.
31. Tawfick WA, Sultan S. Technical and clinical outcome of topical wound oxygen in comparison to conventional compression dressings in the management of refractory nonhealing venous ulcers. Vasc Endovascular Surg 2013;47:30–37.
32. Frykberg, R. G., Franks, P. J., Edmonds, M., Brantley, J. N., Téot, L., Wild, T., Garoufalis, M. G., Lee, A. M., Thompson, J. A., Reach, G., Dove, C. R., Lachgar, K., Grotemeyer, D., Renton, S. C., & TWO2 Study Group (2020). A Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers: The TWO2 Study. Diabetes care, 43(3), 616–624.
■ Most difficult to heal wounds have complicated etiologies resulting in an oxygen deficit in the wounded tissue. Furthermore, superior wound healing can only occur at oxygen levels greater than those delivered by a healthy blood supply (Figure 2).
■ The TWO2 therapy extremity system (Figure 3) is comprised of three major components: A single use Extremity Chamber (Figure 3b), Controller (Figure 3a) and oxygen source.
An oxygen flow rate of 10 LPM is used to cycle the therapy pressure between 10 and 50 mb directly at the wound site, within a sealed environment, providing an oxygen partial pressure diffusion gradient of close to 800 mmHg. The therapy offers the option of humidification with the goal of maintaining an ideal moisture balance for healing.
This allows for the maximum penetration of oxygen into the wounded tissue. Simple on-screen step-by-step instructions provide easy direction for patients and caregivers to apply the therapy at home. 12
■ The TWO2 therapy Multi-Patch system is comprised of two major components: A single use Multi-Patch chamber (Figure 3c) and an oxygen source.
An oxygen flow rate of ≈ 4 LPM applied into the chamber delivers 30 mb of non-contact pressure directly to the wound site, within a sealed and humidified environment.
The resultant oxygen partial pressure diffusion gradient of about 800 mmHg provides for maximum penetration of oxygen into the wounded tissue, penetrating into tunneling wounds, thereby stimulating healing at the deepest point of injury. 1,8
Higher pressure delivery
upregulating
in
Non-contact, cyclical-compression helps to reduce edema, enhance peripheral vascular circulation and stimulate neovascularization.
Assures
TWO2 provides the highest penetration of oxygen into the wounded tissue. The immediate availability of oxygen at a partial pressure of approximately 800 mmHg provides the greatest diffusion gradient into the tissue and supercharges cellular activity.
TWO2 propels stalled, non-healing wounds into a robust healing pathway by addressing hypoxia. Increased oxygen levels upregulate enzyme production responsible for the immune response to fight infection, increase angiogenesis, stimulate better organized collagen deposition, and epithelialization. Collectively, this allows for a faster, more organized wound closure resulting in durable sustained healing. 1
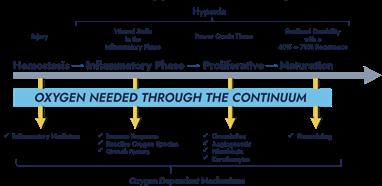
The non-contact cyclical compression provided is between 10 mb and 50 mb pressure that is equivalent to a level 3 conventional compression dressing (CCD). This helps to reduce both edema and the hydrostatic pressure in the lower leg that is a frequent component of lower extremity peripheral vascular disease. The cyclical nature of the pressure delivery eliminates concerns when treating ischemic wounds.
A medical nebulizer
particulate
to the chamber to
■ A study conducted in DFU patients found that TWO2 was associated with “...significant reductions in hospitalizations and amputations in the real-world setting” over 12 months. 2
The authors conclude: “...6.6% and 12.1% of TWO2 patients had hospitalizations and amputations, respectively, compared with 54.1% and 41.4% of NO TWO2 patients... representing 88% and 71% reductions. In matched cohorts 82% fewer TWO2 patients were hospitalized... and 73% fewer TWO2 patients had amputations... Logistic regression among matched cohorts demonstrated nearly ninefold and fivefold higher risk of hospitalization and amputation, respectively, for NO TWO2 versus TWO2.” 2
■ A robust double-blind placebo controlled RCT in DFU concluded that ”…at both 12 weeks and 12 months, adjunctive cyclical pressurized TWO2 therapy was superior in healing chronic DFUs compared with optimal SOC alone.”
■ “TWO2 is prudent, effective, and valuable in managing VLUs without the risks of full body hyperbaric chambers. The TWO2 slashes the time needed for VLU healing and is successful in pain alleviation, MRSA elimination, and management.” 3
“TWO2 radically degrades recurrence rates, thus providing an improved quality of life.” 3
■ In VLUs, TWO2 is associated with significant reduction in pain. 3
■ Patients administer TWO2 therapy at home while enjoying activities such as reading, watching television, or the like. 5,8
■ At-home therapy improves compliance and access to care helping to address health inequalities.
■ In the healthcare system, patients with chronic diseases account for the vast majority of healthcare spending. By more durably healing those ulcers and lessening the reoccurrence, TWO2 has a direct impact on health economics and cost savings. 2,9,10,11,14
■ As an at-home therapy, TWO2 can help reduce costly hospital stays. 2,9,10,11
■ TWO2 provides an easy to use and more cost-effective means of delivering oxygen to the wound. 2,9,10,11
■ Multiple studies state that it would be cost-effective compared to hyperbaric oxygen therapy. 2,9,10,11
■ In the United States, TWO2 is reimbursed throughout the Veterans Administration and other government sectors under an awarded Worldwide Federal Supply Schedule Contract. Additionally, TWO2 is also reimbursed by New York State Medicaid. 5
Multimodality cyclical-pressure Topical Wound Oxygen (TWO2) therapy has been proven in multiple clinical studies to deliver faster, better quality, and more sustainable wound healing. TWO2 is an especially effective adjunctive treatment modality for chronic non-healing and acute complex wounds. 4 Recent high-quality double-blinded, randomized controlled trial (RCT) and real-world evidence (RWE) studies have demonstrated that TWO2 therapy results in more durable healing and provides significant clinical, quality of life and cost saving benefits. 2,4,9,10,11
Evidence Based Therapy: Demonstrated in both Randomized Controlled Trial and Real World Evidence to offer superior healing for Diabetic Foot Ulcers (DFUs).
A Unique Delivery System: Targets multiple aspects of wound healing with OXYGEN, CYCLICAL COMPRESSION and HUMIDIFICATION


A Game Changer for Patients and Clinicians: Drives compliance with a self-administered, at-home therapy and overcomes traditional healthcare barriers. TWO2 can be used with gas permeable dressings, CCD, UNNA Boot and TCC.
Visit www.AOTInc.net for research articles, patient and physician testimonials – and more.



Complex, full thickness soft tissue injuries pose a great challenge to every clinician. In patients where primary closure is not possible, other options need to be considered. Traditionally, small defects were covered using a split thickness skin graft (STSG) and larger defects were resurfaced using full thickness skin grafting (FTSG). STSG and FTSG alone can compromise the final functional and aesthetic outcome. Dermal templates improve the quality of the reconstruction. This article is an overview of the application and efficacy of a dermal substitute: MatriDerm® (Medskin Solutions Dr. Suwelack AG, Germany).
Skin is a barrier from the external environment; this is its primary function. In clinical scenarios where there is a full thickness skin defect present, traditionally a full thickness skin graft (FTSG) or a flap was used for healing. However, these procedures sometimes come with complications, compromised outcomes, and the generation of donor site scars. Using a split thickness skin graft (STSG) alone however can contribute to scar contraction, contour defects, and functional deficits.1 Several dermal substitutes have been developed over the last decades, and more recently innovation in this area has led to the development of products that allow clinicians to consider long-term prognosis and aesthetic outcomes, as well as the priority of healing the wound.2,3
Dermal substitutes are biomatrices that carry out the functions of the cutaneous dermal layer of skin by providing scaffolding and thereby contributing to the control of pain and scarring. This dermal architecture also facilitates tissue growth and wound healing.4 These substitutes have an integral role in reconstructing full thickness defects in the acute and chronic setting, and also reduce scarring.
With concerns raised over the possibility of dermal substitutes potentially compromising skin grafts by acting as a barrier between the graft and the wound, many dermal substitutes have been developed with the intention of application as part of a two-step methodology;
the product is first applied to the wound in an initial procedure, and the skin graft is applied later in a second procedure.3 An ideal dermal substitute however gives the surgeon the possibility to apply the skin graft directly, without the need to wait for weeks, or in case a two-step procedure is needed already after a few days, due to the fast integration and vascularization of the dermal template.
The role of the dermal substitute is essentially to replace the injured layers of skin, which can extend from superficial epidermis down to the deep dermal layer, sometimes a full thickness skin loss, and sometimes with exposed tendon or bone. Full thickness injury is defined as involving all the layers of the skin and is deficient in keratinocytes, stem cells and fibroblasts;3 therefore the role of an ‘ideal’ dermal substitute is one that can replace the deficient layers, accelerate vascularization and cell growth and minimise post-operative complications, and should have the following properties:
• Bioabsorbable
Accelerate wound healing and wound closure
• Promote vascularization and cell growth Prevent infection
• Lead to regeneration of new skin
• Limit scar formation
Lead to functional as well as aesthetic outcome
There is evidence for the use of MatriDerm® in a broad range of indications, as well as treating
exposed structures such as tendons and bones.
Dermal templates have evolved as a blueprint for dermal repair by enhancing elasticity and pliability of the reconstructed skin. The versatility that MatriDerm® provides to the clinician is the key to its continued success in a wide scope of clinical conditions; carrying with it the added advantage of a single stage procedure where traditionally two stage procedures were needed, teamed with a tactile flexibility in the product usage, MatriDerm® leads to a reduction of economic factors such as hospitalization days and complications.
The multi-faceted challenges involved with healing these types of wounds and achieving the most satisfactory results in individual cases has led to a wide range of dermal substitutes that are now available, potentially at the clinician’s disposal depending on economic and geographic factors, encompassing synthetic, biosynthetic or biological materials providing temporary or permanent coverage of wounds.
MatriDerm® is a dermal substitute suitable for one-step as well as two-step repair of fullthickness skin defects in combination with STSG. It is associated with an accelerated wound healing that allows the skin graft to be added directly (one-step) or after a few more days (two-step), in contrast to traditional dermal substitutes where it takes weeks until the epidermal graft can be placed. The product was developed with the challenges of the two-step graft procedure in mind; naturally, a patient will appreciate as few procedures as possible, and fewer days waiting for the skin graft.
MatriDerm® is a dermal substitute of bovine origin and provides an elastic and stable neodermis generated by a three-dimensional matrix consisting of collagen type I, III, and V, additionally supplemented by elastin
hydrolysate which contributes to an improved elasticity and aesthetic outcome of the new skin.5 MatriDerm® promotes the regeneration of dermis as a scaffold and consequently decreases scar tissue formation. It also provides optimal dermal wound bed preparation as regenerated skin, with extensive formation of rete ridges and capillary loops. Min J et al. writes that the elasticity of the skin reconstructed with MatriDerm® was not found to be significantly different from that of surrounding normal skin. 6 This supports the conclusion that grafted skin in combination with MatriDerm® has an elasticity similar to normal skin.
MatriDerm® is applied to the wound bed and then covered by the physician’s preferred secondary dressing or graft. If more than one sheet is used, the sheets should overlap by around 2 - 3 mm. It is advised to utilize the dry MatriDerm® application. Essentially, the clinician must ensure that MatriDerm® is evenly attached to the wound bed and that air bubbles are gently removed, before rehydrating it with saline or Ringer’s solution that is not warmer than room temperature. Negative Pressure Therapy (NPWT) can be used as a bolster for compression and splintage to minimize movement between the wound bed and MatriDerm®. This is accomplished by encouraging blood vessel sprouting (vascularization) and cell proliferation, which improves the adhesion of the collagen elastin matrix.
MatriDerm® is useful for applications such as, burns (reconstructive and acute), trauma and acute wounds, chronic wounds, cancer excision, adhesion barrier, donor sites, mucosal defects and exposed structures.
Another practical application of MatriDerm® is in the reconstruction of post-traumatic wounds. Clinicians from Italy treated post traumatic wounds with MatriDerm® combined with skin grafting, compared with skin grafting alone. Findings showed 95% of wounds treated with MatriDerm® and skin graft showed reepithelialisation at two weeks. Furthermore, the assessments showed a reduction in wound contraction, improvement of elasticity, and quality of scar.7
Tissue loss in the finger presents a reconstructive challenge to surgeons and often requires techniques involving flaps and/ or grafting. The aesthetic outcomes are frequently less satisfactory and there are also relatively low rates of functional satisfaction. Healing times are also comparatively long.
To address this, Fulchignoni et al. conducted a study to evaluate the efficacy of MatriDerm® in finger reconstruction. The authors report that MatriDerm® is a valid solution, and that in most cases of fingertip injuries the wound was in an advanced state of healing three weeks after application of MatriDerm®, and that in these cases grafting could be avoided.3,29
Dermal substitutes are an integral part of burn care management. Acellular dermal matrices like MatriDerm® are a cost-effective alternative in the treatment of full-thickness burns.
During the acute stage of burns treatment, the usage of a dermal substitute may improve functional and cosmetic results, thereby improving the quality of life in the long term. The collagen elastin dermal substitute allows for immediate application of a dermal substitute
under a split thickness skin graft.3,8
The added advantage that MatriDerm® confers is that it can be used as a single stage procedure under a skin graft, reducing scar contracture in both early and late reconstructions.
MatriDerm® can also be applied in the facial aesthetic subunits for delayed paediatric burns reconstruction. In burn reconstruction, the use of MatriDerm® beneath split skin grafts improves both cosmetic results and functional skin movement. The strong early evidence for this method in reconstructing paediatric patients with full-thickness facial burns shows excellent cosmetic results in terms of texture and colour, as well as normal or nearly normal ocular and oral function at the 12-month followup.3,9
Deep wounds with exposed tendons are also amenable to reconstruction in a one step process with STSG and MatriDerm®. Wetzig manages to reconstruct complex wounds with tendon exposure in patients with significant co-morbidities; these types of defects with exposed tendons would normally necessitate flap reconstruction.3,28 Cervelli et al. applied MatriDerm® and STSG in a one step reconstruction of an infected diabetic foot ulcer after debridement. Utilized in conjunction with antibiotics, a significant reduction in ulcer size was achieved.7 Furthermore, MatriDerm® has documented usage in large complex post necrotising fasciitis patients with joint and tendon exposure.3,18
The suitability of MatriDerm® for use in donor site coverage is well evidenced. The donor site from a radial forearm free flap procedure involves full thickness forearm skin defects, which require a dermal substitute and skin
“Dermal substitutes are an integral part of burn care management. Acellular dermal matrices like MatriDerm® are a cost-effective alternative in the treatment of fullthickness burns.”graft. Watfa et al. conducted a study on the use of MatriDerm® in transgender patients after a radial forearm free flap reconstruction of phallus, using the contralateral arm as control. The authors conclude that application of MatriDerm® with a split-thickness skin graft significantly decreases post-operative complications, preserving sensory function and decreasing morbidity of the donor site.3,30
Similarly, Cristofari reports on a study on the efficacy of MatriDerm® on radial forearm flap donor site coverage in terms of functionality, skin quality and patient satisfaction on aesthetic outcome, finding MatriDerm® a reliable solution associated with improved DASH and VSS scores and higher mean patient and surgeon satisfaction, compared to the other techniques used.3,31
1. Bloemen MC, van Leeuwen MC, van Vucht NE, van Zuijen PP, Middelkoop E. Dermal substitution in acute burns and reconstructive surgery. A 12-year follow-up. Plast Reconstr Surg 2010; 125(5): 1450-59
2. Halim AS, Khoo TL, Yussof SJM. Biologic and synthetic skin substitutes: An overview. Indian J Plast Surg 2010; 43(Suppl): S23–S28.
3. Enoch, S., & Kamolz, L. (2010). Indications for the use of MatriDerm® in the treatment of complex wounds.
4. Lee K. H. Tissue engineered human living skin substitutes: Development and clinical application. Yonsei Medical journal. 2000; 41(6): 774. 9
5. Lempert et Al. Long-term experience with a collagen-elastin scaffold in combination with split-thickness skin grafts for the treatment of full-thickness soft tissue defects: improvements in outcome—a retrospective cohort study and case report . Langenbecks Arch Surg. 2022; 407(1): 327–335. Published online 2021 Sep 4. doi: 10.1007/s00423-021-02224-7
6. Min J et Al. The Use of Matriderm and Autologous Skin Graft in the Treatment of Full Thickness Skin Defects Arch Plast Surg. 2014 Jul; 41(4): 330–336.
7. Cervelli V et al. The use of MatriDerm and skin grafting in post-traumatic wounds. Int Wound J 2011 Aug
8. Zajicek et al. Dermal Replacement with Matriderm- First experience at the Prague Burn Centre. Acta Chir Plast.2020 Winter
9. Jackson Sh., Roman S., Matriderm and Split Skin Grafting for Full-Thickness Pediatric Facial Burns, Journal of burn care and research, 2019
10. de Vries HJ, Middelkoop E, Mekkes JR, Dutrieux RP, Wilde- vuur CH, Westerhof H. Dermal regeneration in native non- cross-linked collagen sponges with different extracellular matrix molecules. Wound Repair Regen. 1994;2:37–47.
11. de Vries HJ, Mekkes JR, Middelkoop E, Hinrichs WL, Wilde- vuur CR, Westerhof W. Dermal substitutes for full-thickness wounds in a one-stage grafting model. Wound Repair Regen. 1993;1:244–252.
12. Haslik W, Kamolz LP, Nathschlager G, Andel H, Meissl G, Frey M. First experiences with the collagen-elastin matrix Matriderm as a dermal substitute in severe burn injuries of the hand. Burns 2007;33:364–368.
13. BranskiLK,HerndonDN,PereiraC,etal.Longitudinalassess- ment of Integra in primary burn management: A randomized pediatric clinical trial. Crit Care Med. 2007;35:2615–2623.
14. Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995;21:243–248.
15. Eaglstein WH,AlvarezOM,AulettaM,etal.Acuteexcisional wounds treated with a tissueengineered skin (Apligraf). Der- matol Surg. 1999;25:195–201.
16. Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns: A multi-center randomized clinical trial. Ann Surg. 1988;208:313–320.
17. Haslik W, Kamolz LP, Manna F, Hladik M, Rath T, Frey M. Management of full-thickness
MatriDerm® is a highly versatile and reliable dermal substitute applicable to a wide range of clinical conditions, and is very well evidenced by numerous studies in terms of efficacy, healing time, and quality of functional and aesthetic outcome. The added benefit of the option of a single stage procedure, combined with tactile flexibility in the product usage, earns MatriDerm® a reputation as a world leading product in the field of wound care.
skin defects in the hand and wrist region: First long-term experiences with the dermal matrix Matriderm. J Plast Reconstr Aesthet Surg. 2010;63:360–364.
18. Ryssel H, Gazyakan E, Germann G, Ohlbauer M. The use of MatriDerm in early excision and simultaneous autologous skin grafting in burns: A pilot study. Burns 2008;34:93–97.
19. Hansbrough JF, Dore C, Hansbrough WB. Clinical trials of a living dermal tissue replacement placed beneath meshed, split-thickness skin grafts on excised burn wounds. J Burn Care Rehabil. 1992;13:519–529.
20. Sheridan RL, Hegarty M, Tompkins RG, Burke JF. Artificial skin in massive burns: Results to ten years. Eur J Plast Surg. 1994;17:91–93.
21. van Zuijlen PP, van Trier AJ, Vloemans JF, Groenevelt F, Kreis RW, Middelkoop E. Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a onestage grafting model. Plast Reconstr Surg. 2000;106:615–623.
22. Santi G., Greca C., Bruno A., the use of dermal regeneration template (Matriderm 1mm) for reconstruction of a large full-thickness scalp and calvaria exposure, Journal of burn care and research, 2016.
23. Jackson Sh., Roman S., Matriderm and Split Skin Grafting for Full-Thickness Pediatric Facial Burns, Journal of burn care and research, 2019.
24. Dunne J., Wilks D., previously discounted flap now reconsidered: Matriderm and split thickness skin grafting for tendon cover following dorsalis pedis Fascio-cutaneous flap in lower limb trauma, Journal of plastic surgery, 2014.
25. Angelis B., Gentile P., Agovino A., Chronic ulcers: MATRIDERM(®) system in smoker, cardiopathic, and diabetic patients, journal of tissue engineering, 2013.
26. Kang S., Park J., Shon Hyun, Skin graft using MatriDerm® for plantar defects after excision of skin cancer, Dovepress, 2019
27. Wood BC Caputy G. Skin grafts. 2011; available at http://emedicine.medscape.com/. Accessedarticle/1295109-overview. Accessed 06/10/2022.
28. Wetzig T, Gebhardt c, Simon JC. New Indications for artificial Collagen-Elastin Matrices? Covering Exposed Tendons. Dermatology 2009; 219(3): 272–3
Fulchignoni C, Rocchi L, Cauteruccio M, Merendi G. Matriderm dermal substitute in the treatment of post traumatic hand’s fingertip tissue loss.
“The added advantage that MatriDerm® confers is that it can be used as a single stage procedure under a skin graft reducing scar contracture in both early and late reconstructions.”


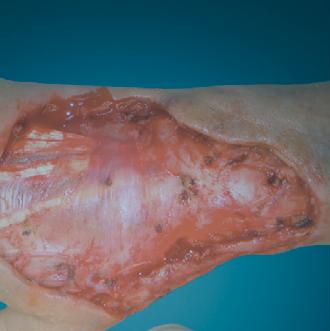



 Native collagen fibers ElastinNo chemical cross-linking
Native collagen fibers ElastinNo chemical cross-linking
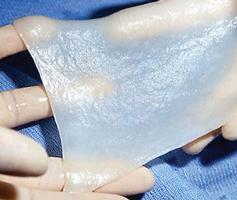

■ In an animal AV-Loop model, a number of new formed blood vessels were analyzed in wounds treated with either with MatriDerm® Dermal Matrix or a competitor product. MatriDerm® Dermal Matrix accelerates revascularization7
■ MatriDerm® Dermal Matrix limits myofibroblast formation compared to competitor products5 Myofibroblast formation is associated with wound contraction8 . Limited wound contraction is observed with MatriDerm®9,10,11
■ MatriDerm® ensures active healing. In diabetic foot ulcers the combination of MatriDerm® with STSG leads to a shorter healing period compared to STSG alone12
■ A long term follow-up study of an intra-individual comparison of patients treated with STSG alone, and with MatriDerm® Dermal Matrix and STSG, to analyze different skin parameters after 12 years of treatment, demonstrated a long lasting effect on scar quality even after 12 years by the use of MatriDerm® Dermal Matrix, compared to STSG alone13
“The native dermis-like collagen fibrillar networks exhibit premium properties for cell ingrowth, survival, and proliferation... by balancing the expression of myofibroblasts, they lead to decreased wound contraction and aberrant scar formation5.”
“FTSG with MatriDerm® improves the DASH score and the aesthetic outcomes resulting to be a reliable solution in treating full thickness forearm skin defects after RFF harvesting15.”
■ In an RCT the total surgical treatment costs of Full-thickness wounds treated with STSG alone or with MatriDerm® Dermal Matrix and STSG were analyzed. The costs do not differ significantly14
MatriDerm® is able to preserve closeness to human dermis, accelerate cell invasion, cell elongation and proliferation and limit myofibroblast formation and contraction5. This scientific performance is as a result of the Advanced CryoSafe® Method which gently preserves the native structure with no chemical crosslinking6 .DOI: https://doi.org/10.3390/
U. Collagen-Elastin and Collagen-Glycosaminoglycan Scaffolds Promote Distinct Patterns of Matrix Maturation and Axial Vascularization in Arterio venous Loop-Based Soft Tissue Flaps. Ann Plast Surg. 2017 Jul;79(1):92-100. DOI: 10.1097/SAP.0000000000001096. PMID: 28542070.
8. Kattan WM et al., J Coll Physicians Surg Pak 2017;27:38-43.
9. de Vries HJ, Middelkoop E, Mekkes JR, Dutrieux RP, Wildevuur CH, Westerhof H. Dermal regeneration in native non-cross-linked collagen sponges with different extracellular matrix molecules. Wound Repair Regen. 1994 Jan;2(1):37-47. DOI: 10.1046/j.1524-475X.1994.20107.x. PMID: 17168910.
10. De Vries HJ, Zeegelaar JE, Middelkoop E, Gijsbers G, Van Marle J, Wildevuur CH, Westerhof W. Reduced wound contraction and scar formation in punch biopsy wounds. Native collagen dermal substitutes. A clinical study. Br J Dermatol. 1995 May;132(5):690-7. DOI: 10.1111/j.1365-2133.1995.tb00712.x. PMID: 7772472.
11. Hur GY, Seo DK, Lee JW. Contracture of skin graft in human burns: effect of artificial dermis. Burns. 2014 Dec;40(8):1497-503. DOI: 10.1016/j.burns.2014.08.007. Epub 2014 Sep 28. PMID: 25270084.
12. Jeon H, Kim J, Yeo H, Jeong H, Son D, Han K. Treatment of diabetic foot ulcer using matriderm in comparison with a skin graft. Arch Plast Surg. 2013 Jul;40(4):403-8. DOI: 10.5999/aps.2013.40.4.403. Epub 2013 Jul 17. PMID: 23898439; PMCID: PMC3724003.
13. Bloemen MCT, van Leeuwen MCE, van Vucht NE, van Zuijlen PPM, Middelkoop E. Dermal substitution in acute burns and reconstructive surgery: a 12-year follow-up. PlastReconstr Surg. 2010 May;125(5):1450-1459. DOI: 10.1097/ PRS.0b013e3181d62b08. PMID: 20440164.
14. Hop MJ, Bloemen MC, van Baar ME, Nieuwenhuis MK,




Integrated pressure adjustment

This article will discuss the burden of caring for patients with venous leg ulcers. We know just how expensive and costly the impact for treating venous leg ulcers is, not just for the patients but also for the payers. It’s at least 1 bn dollars annually to treat these patients. For those of us that treat the patients, we know that they miss a ton of work every year. My patients are employed in areas such as retail, and the physical nature of this type of work can exacerbate the problems with their venous disease. These patients miss on average 14 days of work per year. The cost itself for the patients can be about $18,000 a year; many of my patients simply cannot afford this.
Looking at the actual incidence rate for Medicare itself, it affects about 2.2% of the population, and around 0.5% have private insurance. Reflecting on my own practice, many of my patients who have venous leg ulcers are not even on Medicare, and are on the Medicaids. I am seeing more and more patients who are in their 30s, 40s and 50s, so the actual incidence is much higher than 2.2%, and is going to be rising in the Medicare population as the burden increases as patients age into the Medicare age range.
Let us consider the objective truth. Looking at the source of truth for all of our evidence, we go to the gold standard. This is using the Cochrane database, which has all the meta-analyses for the evidence. We also look to resources like those provided by the Society for Vascular Surgery.


We know that compression increases ulcer healing rates when compared to no compression. We also know that all of the data tells us that multi-component systems are more effective than single-component systems. Similarly, multi-component systems containing an elastic bandage appear to more effective than those containing a mainly inelastic consistence. So, two-component bandage systems appear to perform as well as the four layer systems, as long as the two-bandage system components have
 The financial burden of venous ulceration is significant to healthcare systems around the world. In this article, an overview of compression is provided, identifying areas of challenge to the wound care clinician. Patient compliance, as well as anatomical consideration and other challenges are considered. The mechanism of continuous compression is detailed, with consideration for comfort and consistency. A detailed look at the mechanism of establishing compression is included; finally a conversion of data into real practice is presented.
Dr Lucian G. Vlad Clinical Associate Professor. Wound Care and Hyperbaric Medicine Center Winston-Salem NC, United States
Mr Evan Call Lab Director, EC Service, Inc. Centerville UT, United States
Ms Kara Couch
President-Elect, Association for the Advancement of Wound Care Associate Research Professor of Surgery, School of Medicine and Health Studies, George Washington University. Director, Wound Care Services, The George Washington University Hospital Arlington VA, United States
The financial burden of venous ulceration is significant to healthcare systems around the world. In this article, an overview of compression is provided, identifying areas of challenge to the wound care clinician. Patient compliance, as well as anatomical consideration and other challenges are considered. The mechanism of continuous compression is detailed, with consideration for comfort and consistency. A detailed look at the mechanism of establishing compression is included; finally a conversion of data into real practice is presented.
Dr Lucian G. Vlad Clinical Associate Professor. Wound Care and Hyperbaric Medicine Center Winston-Salem NC, United States
Mr Evan Call Lab Director, EC Service, Inc. Centerville UT, United States
Ms Kara Couch
President-Elect, Association for the Advancement of Wound Care Associate Research Professor of Surgery, School of Medicine and Health Studies, George Washington University. Director, Wound Care Services, The George Washington University Hospital Arlington VA, United States
“With these factors, we as clinicians have many challenges to overcome. We need to apply both long-stretch and short-stretch, we need to consider the comfort of the patient, and we need to ensure that the therapy is working whether the patient is at rest or active. In short, we need to tailor therapy to the patient’s lifestyle so that they can continue as normal.”
at least an elastic bandage. Patients who do receive the four-layer bandage heal faster than those who have the short-stretch bandage in and of itself. The inelastic bandage and the short-stretch bandage are more commonly known as the Unna’s boot, the more traditional, or as I call it the ‘old-school’ methodology of applying compression.
So, what are the present challenges that the application of compression can present? The stretch is the key issue here. The Unna’s boot has been widely used in, for instance, vascular surgery because this methodology has been passed down for decades.
An Unna’s boot is a paste bandage that does not have much give or stretch, hence the term ‘short-stretch’. It is applied wet and hardens into cast or a stiff bandage, and essentially requires the patient’s calf muscle to compress against the stiff bandage, giving the ‘squeeze’ of the veins in the cast, forcing the venous return back up to the heart. This implies that the patient would have to be an active patient; in a patient who is non-ambulatory and more sedentary, this is simply not a good option. These patients need more of an elastic component which is going to provide more of a continuous squeeze on the leg, if the calf muscle pump simply is not working. We must match the stretch to the patient’s lifestyle and activity level.
The next issue is compliance. We should consider some of the most common aspects that patients dislike about wearing bandage systems on the lower leg.
They are hot. I work in Washington DC; it is known as ‘the swamp’, not just for political reasons but because it’s around 110 degrees here in August. It can be very humid and uncomfortable. In conditions such as these, the last thing a patient will want is to do is wear a
multi-component bandage system that has lots of layers in padding. Due to the discomfort, the patient is likely to scratch the area and push or shift the bandage down, possibly leading to the point where the patient decides to take it off, compromising their treatment.
The other challenge here are unique patient factors. Not all legs are shaped the same, and not always shaped the same on the same person. We may have one person who has a primary lymphedema, or post-thrombotic syndrome, on one limb but not the other; or the limbs may simply be different sizes. It can be very challenging when wrapping both legs to achieve a consistent application on both sizes.
With these factors, we as clinicians have many challenges to overcome. We need to apply both long-stretch and short-stretch, we need to consider the comfort of the patient, and we need to ensure that the therapy is working whether the patient is at rest or active. In short, we need to tailor therapy to the patient’s lifestyle so that they can continue as normal.
One of the most important questions I ask my patients is “What do you want out of this experience? What is your goal?” Not all patients will respond that they want to be healed, but they may want to, for instance, go to church. Their goal may be to be able to attend a family function. The patient’s preference should not be optional, we do need to understand what is important to them.
If the bandage system is uncomfortable, itchy or painful the patient is simply not going to wear it. We could see this not necessarily as non-compliance, but rather that we have not fully considered the patient. We also need to address the unique patient factors, making sure that we are wrapping the leg shape correctly, we are considering ankle sizes, that we are not having slippage, and that the nurses are able to apply the bandage systems accurately and
Mastering the Art of Compressionconsistently no matter what the ankle size, or unique leg shape.
How Can We Overcome This Burden and Address the Need for Better?
What does ‘better’ look like? Firstly, we need to keep things simple. We need a system that features all elasticities, in that it has shortstretch and long-stretch; it works both at rest and during activity; and the same kit needs to work for all different ankle sizes.
Again, we need to consider patient compliance. The system should be as comfortable as possible, breathable, and tolerable night and day.
Also, we must eliminate the variables to ensure that everything is applied consistently, the first time, every time.
This leads us to consider the three principles of good compression:
• We need to apply continuous pressure 24/7
We need to ensure consistent pressure application
We need to ensure that compression is comfortable and patients can wear it
What is it that gives us this continuous pressure through the course of use? The short-stretch inner bandage confers rigidity and contributes to comfort; it also absorbs exudates, while contributing to the pressure overall. The outer, long-stretch bandage maintains pressure at rest, it holds the system in place and creates a ‘scaffolding effect’, that holds the whole structure in place on the leg. This continuous
compression effect works up to seven days, and it maintains that therapeutic pressure in all positions, whether lying, sitting, standing, walking or working.
After seven days we see this pressure maintaining the compression where we want it. We had a minimal reduction in that maximum working pressure as time goes on. That means that we achieve a smaller reduction in working pressure over time maintaining that therapeutic compression through the whole wrap cycle. Why do we see this continuous compression? With the URGO K2 and not with a two-layer system we have a unique kind of ‘work’ that occurs – the energy that is put into the tissue when you apply a compression wrap.
The padded short-stretch layer provides 80% of the compression, but because it is padded it also provides excellent force redistribution. This provides the work of compression without the edge effect – the reactive hyperemia demonstrating shear that is imparted to the tissue. We want to distribute that force without inducing shear and this is why the padded inner layer becomes so valuable.
The work of compression done by a padded layer dramatically reduces point loads. High loads at the malleolus or at the calcaneus that could potentially injure the tissue if misapplied, are dramatically reduced and we have a much better wrap and force distribution because of this padded layer.
These combine to provide compression without pressure peaks, or the edge effect that creates pain and shear. This improved comfort is all together in place while we have this rigidity of the combination of the short-stretch inner with this outer layer.
The outer layer provides 20% of the compression, it ‘traps’ the first layer and holds it in place in a scaffold relationship. The structure
“What does ‘better’ look like? Firstly, we need to keep things simple. We need a system that features all elasticities, in that it has short-stretch and long-stretch; it works both at rest and during activity; and the same kit needs to work for all different ankle sizes.”
“With this system, patients had a reduction of reported pain of 28%, together with reductions of pain intensity, and sensations of heat and itchiness. 95% of patients considered K2 to be more comfortable compared with their previous system, and 92% felt it was more comfortable at night. “
holds the wrap up on the leg, so that it is not able to slip down. The friction which interacts between the two layers tie it together and create a compression layer and a scaffold layer that protects the tissue and keeps the wrap in position. It reduces the tendency to slide, or bundle up.
The friction between the two layers creates an effect which achieves what a four-layer system is designed for, with a two-layer system; binding and performance is very similar to that of a fourlayer system.
Studies on the pressure consistency of K2 have shown that between 85% and 87% of nurses achieve the therapeutic pressure from the first application, with no prior experience. This is significantly more impressive than other kinds of wraps currently on the market, such as the four-layer bandage systems or Unna’s boot.
With this system, patients had a reduction of reported pain of 28%, together with reductions of pain intensity, and sensations of heat and itchiness. 95% of patients considered K2 to be more comfortable compared with their previous system, and 92% felt it was more comfortable at night.
A study conducted into patient compliance with K2, which compared patient compliance in winter and summer, found that 96% of patients returned for follow up visits in winter, and 92% of patients did so in summer. In my own practice, I have also found this to be true. Once the patients are wearing it, they wear it consistently; they are not taking it down, and it is not sliding down. This provides continuous pressure, 24/7, at all different levels of activity. It is possible that 87% of all nurses are achieving therapeutic compression at their first application and then
consistently matching that throughout. The wrap is comfortable, and can be worn regardless of the climate.
Let us look at two studies we conducted to measure the performance of the K2 dual layer compression system, one human subject study and one bench study.
The important starting point for this discussion is understanding the construction of the compression wraps/ bandages studied. The K2 dual-layer compression system consists of two active compression layers; a soft-padded shortstretch inner layer, KTech, that is absorbent and provides a comfortable interface with the body, conferring 80% of the compression pressure; and And a long-stretch outer layer, KPress, which is intended to apply 20% of the compression and maintain the system in place.
Additionally, one of the benefits of the K2 compression system is that both layers include reference points to guide the operator during the application process. The reference points are ovals that convert into circles as the bandage is stretched; this indicates how much the bandage should be stretched and overlapped to provide the desired pressure.
The compression mechanism consists of the interaction of the inner wrap/ layer with the outer layer to provide structure. This occurs particularly well when the inner layer is tied with the outer layer, which maintains pressure at rest and holds the combined system in place. I like to think of this structure as functioning as a scaffold, holding up a temporary structure underneath it, creating rigidity in something like fabric that wouldn’t normally be rigid . This combination makes the assembly even better by the fact that the two layers are elastically contracting into each other.
The warp and the weft of the fabric create an interface that reaches into each other’s surfaces like your fingers would when you lay your hands together and your fingers fit between each other. Together, these structures provide continuous pressure and strong structural performance. The strength of this relationship between the two layers is that it provides longterm performance compression. The K2 system is designed to reach 40 millimeters of mercury as the target pressure and stay in place without sliding down or bunching up over seven days. During the seven days, it continues to provide therapeutic compression while lying down, while sitting, and then an appropriate maximal working pressure when a person is up and ambulating.
We set out a bench test study to understand why this continuous compression occurs with the K2 and compared it with a traditional twolayer compression system. Note that in the graph of the load-deflection curves (Figure 1), you see the force at a given deflection/ stretch. The shapes of these curves predict the product’s performance over various levels of stretch. These curves show the amount of energy as the product is stretched. This gives us the understanding that the K2’s performance, at the manufacturer’s instructed stretch indicated by the circle, provides high energy delivery. This shows highly consistent performance of the compression system from application to 7-day use. Looking at the second graph, the outer or second layer applies less compression because there is more deflection with a lower load. This lower force required to deflect (stretch) the wrap applies less of the compression to the tissue, ties the two layers together, and provides a high-level wrap performance. This also creates a unique energy delivery process. The elastic, padded, and absorbent KTech stretch layer provides 80% of the compression. This means that 80% of the
Figure 1: We looked at the engineering of UrgoK2 compared to a traditional 2LB to understand why UrgoK2 provides continuous pressure.
• 2LB comprised of a foam comfort layer (Layer 1) and a shortstretch compression bandage (Layer 2)
• At recommended stretch, UrgoK2 obtained more load and work than 2LB higher work is better for maintaining sustained compression, especially after limb volume is reduced
• This shows why traditional 2-layer compression is NOT Dual Compression
“Together, these structures provide continuous pressure and strong structural performance. The strength of this relationship between the two layers is that it provides long-term performance compression. “work or energy going into that tissue to reduce the edema comes from this soft, absorbent, cushioning structure. This allows for reduced point loads, evidenced by the observation of reactive hyperemia lines pressed into the tissue when care providers remove a compression wrap. These lines make it obvious that there is an edge of the wrap pressing into the tissue. This creates a potential area of concern for the tissue and the patient’s comfort. The highly cushioned yet compressing inner KTech wrap avoids that point load at the edge of the wrap, and provides compression free of potential injury. This highly compressing inner layer combinedwith the lower compression but retaining outer layer, together combines to provide greater energy application to the tissue. However, with this greater compression, we don’t see the same edge impact; there are fewer pressure peaks and less shear-based pain. Remember, the shear of something twisting on the tissue is what generates pain in compression, so delivery of compression without that twisting shear provides effective compression while maintaining greater comfort for the patient.The high stretch outer line layer provides 20% of the compression and holds the inner layer in a scaffold relationship that keeps it in place, reducing the tendency to slide down. This stability and the relationship between inner and outer stretch layers allow it to respond to limb volume reduction without the tendency to bunch up or move. These two layers, applied together, one cushioning and compressing on the inside and the other compressing and stabilizing on the outside, further reduce the effect of the ridges created by each layer interacting with the skin. This reduces the wrap edge pressure effect responsible for redness, skin irritation, and pain.
The overlap between the two layers creates a structural binding that strengthens the scaffold effect holding the K2 in place, allowing for four-layer type performance with a two-layer compression wrap.
Looking at the theoretical pressures that compression wraps apply, using Laplace’s law to convert tensile stretch into compression pressure, it becomes evident that at the ranges of therapeutic compression, the amount of energy or work that can be applied to the edematous tissue is much greater when both layers provide compressive energy. Notice that in the total load of the K2 in Newtons of the KTech outer layer and the KPress inner layer, there is a significant difference in total compressive work. When comparing the inner wrap of K2 and the traditional two-layer wrap, in the first layer, there is an 11 to 0 compression difference for the K2, when looking at the Newtons at the stretch recommended by the manufacturers. Applied by the second layer, there is a 2.5 to 10 compression ratio favoring the traditional 2-layer wrap. Most importantly, if we consider the sum of the inner and outer for the K2 vs the sum of the inner and outer for the traditional 2-layer wrap, the K2 is providing 13.5 Newtons of compression while the traditional two-layer wrap is only applying 10 Newtons.
This amounts to 35% more work being applied to the tissue by the K2 over the traditional two-layer wrap. This construction allows higher-energy to be applied to the tissue all while maintaining a lower pain level, fewer high-pressure points, and more comfortable compression.
The understanding that comes from the bench study of the total energy applied to the tissue by the K2 raises the question,“How do we then apply this product consistently to gain the best possible compression for the patient?” We conducted a human subject study where three nurses were trained to apply the K2 and the traditional two-layer wrap. Each nurse wrapped six people, five times with both of the two compression wraps, K2 and the traditional twolayer system.
“This construction allows higher-energy to be applied to the tissue all while maintaining a lower pain level, fewer high-pressure points, and more comfortable compression.”
The target compression was 40 millimeters of mercury. We measured the sub-bandage pressure achieved with each system using a PicoPress pressure measurement device. Pressures were measured with the volunteer in the supine (sitting with legs extended), and standing positions. The pressures were used to measure consistency and proximity to the target pressure. The difference between the nurse’s performance in reaching that target pressure with the K2 and the traditional twolayer compression wraps was reported.
In Figure 2, you see that when we plot the pressures achieved by each of the nurses with each device in the resting (supine) position, compression for both wraps is centered around 40 millimeters of mercury, with two very interesting observations; Nurses B and C both achieved an average pressure closer to the target pressure using the K2 over the traditional two-layer wrap; second, despite the fact that Nurse A was further away from the target with K2 than the traditional 2-layer wrap, the pressures observed were high. The observed pressure in Nurse A’s wraps was dramatically different, and in some cases
approached the risk of tissue injury; because of this videos and photographs of the process were reviewed.
These reviewed observations showed that Nurse A applied the wrap overlapping more than was required when using the K2; because the wrap application technique is essential to achieving therapeutic compression, training was repeated, and Nurse A repeated the wrapping process. It should be noted that Nurse A is a highly skilled nurse who applies 15 - 20 wraps per day and is very experienced in using the traditional two-layer compression system without a pressure guide.
Now looking at the data (Figure 3) following the second training, the graphs on the left (Nurse B and Nurse C) are the same as the data from the previous trial. The graph on the right demonstrates that when Nurse A properly applied the pressure guide, the average pressure was almost perfectly centered on the target pressure of 40 millimeters of mercury. Again, Nurse B and Nurse C moved towards the target pressure by using the K2 versus the traditional two-layer compression system.
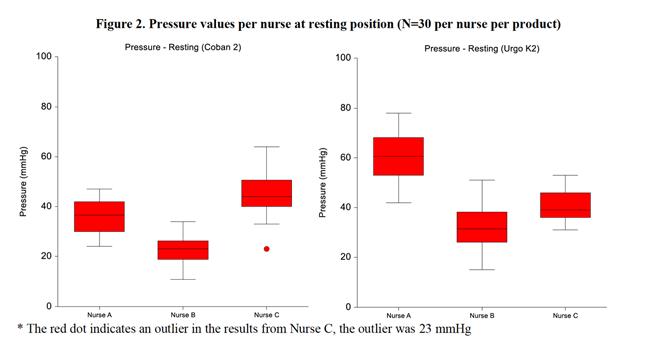
This application study using nurses and human volunteers shows that the training was most critical for the nurse that was skilled with the use of the traditional two-layer system. This difference is likely because of the difference in dynamic compression created by the K2, where both layers provide compression. With this training and using the Pressure Guide in compliance with the instructions, the K2 provides excellent consistency in reaching the target pressure. Overall, the K2 pressure guide allowed the nurses to obtain the target pressure consistently.

What does this mean for your patients? The K2 provides continuous therapeutic compression throughout the day or night, supine, ambulating, or sitting. The higher level of energy delivered by K2 and the scaffolding effect, allow it to continuously apply the target compression over a greater range of limb circumference and volume, and it is able to respond to the volume changes more effectively. This provides for an accurate and consistent therapeutic compression through the course of the use of the wrap for up to 7 days by the patient.
From a clinical perspective it is extremely important to have a treatment method that is continuous and effective. Continuous therapeutic compression means that the patient
will receive treatment day and night, regardless of ambulation status or limb size, and will move towards positive goals and especially the desired wound healing outcome.
The engineering of DCS padded short stretch layer with the overlying long stretch outer layer creates a mechanical averaging that reduces peak pressures and sheer forces at the edge of each layer, thus improving patient comfort.
Besides continuity and comfort, the consistency of therapeutic applications on every patient, regardless of limb circumference, has a synergistic affect towards positive outcomes. The visual aid printed on the wrap has a major contribution to the consistency of the application and allows verification of the correct application in the desired therapeutic range. A study conducted by Hanna shows 85% of nurses were able to apply a DCS wrap correctly on the first application.8 By comparison, the same target therapeutic range was achieved to 69%, using a standard of care four layer wrap, and to only 25% using a classic standard of care short stretch wrap. In a similar study Pilati reports that 87% of nurses hit the therapeutic range in the first application.9 DCS was far superior and easier to apply and was more comfortable compared to standard of care. To summarize, from a clinical practice perspective, this treatment approach removes the guesswork in applying accurate, therapeutic and consistent compression by
reducing the risk for undercompression, which can prolong healing time, and overcompression, which can cause harm.
Dr Hugo Partsch is one of the world’s best known experts in compression therapy. Dr Partsch recognized that compression therapy is under used, and realized provider education can and should be improved. Writing in an editorial a few years ago, he explains; “The main problem concerning compression therapy is the lack of adequately trained staff. Some new compression devices which do not require special bandaging skills may replace conventional compression materials in the future.“ Indeed, it seems we may have arrived at that future, as the technology of this treatment achieves these desired goals.
In my practice, one of the issues I encountered a few years ago when using different compression wraps and short stretch wraps, was patients requesting specific staff members to apply their compression wrap because of the inter-staff variability. These requests posed difficulty to clinical flow and created hard feelings. However, since the adoption of DCS in our clinic this problem has been eliminated, attesting to the consistency it brought.
Of recent increased clinical interest has been the application of compression wraps as an additional treatment for diabetic lower extremity chronic wounds. Diabetes continues to show an increasing trend along with obesity.1
It has been estimated that approximately 1/4 of patients with venous leg also have diabetes as a comorbidity, and almost 50% of diabetic foot ulcers have lymphedema diagnosed as a complication.2,3
There is an increasing awareness that all
diabetic wounds on lower extremities, including feet, include an element of lymphedema. Hypoglycemia leads to micro-angiopathy, which in turn leads to degradation of the glycocalyx vascular barrier, increasing capillary filtration and distance to fluid buildup in the intracellular tissues, thus impairing the lymphatic pumps and allowing fluid buildup in the intracellular space, which compromises cellular function and delays healing of chronic wounds.4
A concern regarding application of compression on lower extremity for patients with diabetes is arterial impairment and compromised perfusion. Autonomic neuropathy creates microcircuitry changes of the skin including itching, venous prominence, callus formation, loss of nails and sweating anomalies, and can make it difficult to recognize skin perfusion changes due to arterial disease. Furthermore, sensory neuropathy could contribute to under reporting of pain or other ischemic symptoms.5
However, this concern can be addressed with a thorough arterial perfusion assessment. Arterial brachial index is an initial screening tool for arterial perfusion. This test involves having the patient lie flat for about 10 minutes so that their ankle is at the same height with the arm, to eliminate hydrostatic pressure differences. Subsequently, we obtain brachial pressure and ankle pressure and, for a normal patient, that should be an arterial breaker index ABI equal to 1.
A great number of guidelines have been published for interpretation of ABI and recommendations for compression application. A summary of these guidelines from many countries has been published by the European Wound Management Association in 2017.7 See Table 1.
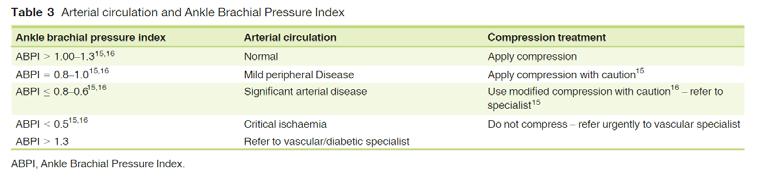
Arterial calcifications can cause limitations for obtaining adequate ABI’s calcified arteries. Higher pressure readings can allow overestimation of perfusion, or can even show normal perfusion, and patients that have significant arterial disease.
Diabetic patients are much more likely to have arterial classifications. In order to overcome this concern, additional arterial assessments such as obtaining segmental pressures, pulse volume recordings, and digital pressures can be extremely useful. These types of assessments are becoming standard of care in vascular labs equipped with better and more specialized equipment. However, with adequate training this assessment can be obtained using simple blood pressure cuff and handheld Doppler.
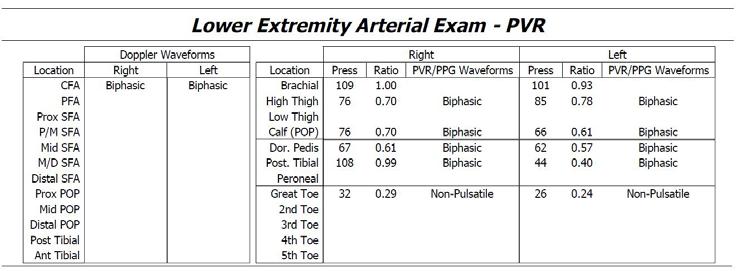
See Table 2. In this example, the patient presented with a diabetic toe ulcer and his ABI was 0.99, essentially normal. However, he failed to progress in spite of adequate treatment. Subsequent additional arterial evaluation showed low segmental pressures in his thigh, with biphasic waveforms and toe level pressures compromised and non-pulsatile. This prompted Vascular Surgery consultation and a revasculation procedure and limb salvage.
A large prospective study was published in May 2021 of 702 VLU patients using DCS and looking at wound healing rate, wound healing progression, assessment of edema and ankle mobility and local tolerability and acceptance.6 A post-publication analysis
of this study compared efficacy and safety of DCS, specifically non diabetic (n=492) versus diabetic (n=185) patients. Overall demographics were comparable between the two groups except for significantly higher percentage of comorbidities including hypertension, heart disease and diagnosed peripheral arterial disease. However no significant difference was seen in outcomes including one healing and edema reduction between the two groups. Local tolerance and acceptability were not significantly different.
We can conclude that compression is safe to use for patients with diabetic lower extremity alterations when they present with edema, venous stasis and lymphedema as comorbidities.
national-diabetes-statistics-report.pdf
2. Apelqvist J, Larsson J, Agardh CD. The importance of peripheral pulses, peripheral oedema and local pain for the outcome of diabetic foot ulcers. Diabet Med. 1990;7(7):590–594.; 2) 3. Ho TK, Leigh RD, Tsui J. Diabetic foot disease and oedema. The British Journal of Diabetes & Vascular Disease. 2013;13(1):45-50. doi:10.1177/1474651412472213;
4. Kanapathy M, Portou M, Tsui J, Richards T. Diabetic foot ulcers in conjunction with lower limb lymphedema: pathophysiology and treatment procedures. Chronic Wound Care Management and Research. 2015;2:129-136
5. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P; Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010 Oct;33(10):2285-93. doi: 10.2337/dc10-1303. Erratum in: Diabetes Care. 2010 Dec;33(12):2725. PMID: 20876709; PMCID: PMC2945176.
6. Stücker M, Münter KC, Erfurt-Berge C, Lützkendorf S, Eder S, Möller U, Dissemond
J. Multicomponent compression system use in patients with chronic venous insufficiency: a real-life prospective study. J Wound Care. 2021 May 2;30(5):400-412. doi: 10.12968/ jowc.2021.30.5.400. https:// pubmed.ncbi.nlm.nih.gov/33979221/
7. Andriessen A, Apelqvist J, Mosti G, Partsch H, Gonska C, Abel M. Compression therapy for venous leg ulcers: risk factors for adverse events and complications, contraindications - a review of present guidelines. J Eur Acad Dermatol Venereol. 2017 Sep;31(9):1562-1568. doi: 10.1111/jdv.14390. Epub 2017 Jul 31. PMID: 28602045.
8. Hanna R, Bohbot S, Connolly N. A comparison of interface pressures of three compression bandage systems. Br J Nurs. 2008;17(20):S16-24.
9. Pilati, L. Do the findings in winter of a dual compression system compression bandage’s wear compliance with patients replicate if the study is repeated in the summer months?. SAWC Fall 2021.

The cost associated with pressure ulceration are escalating, both in personal cost to quality of life and the financial burden of costs that need to be met by each healthcare system. The cost associated with the management of injury due to pressure ulcers are considered to be very significant in many countries. Education and training remain an area of focus to reduce the incidence of this condition. This article explores the clinical practice guidelines for pressure ulcer/ injury prevention.
Evidence-based practice is a fundamental approach to healthcare that helps patients to receive high quality, efficient, safe, and individualized care. Evidence-based medicine (or healthcare) can be defined as “…the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients”.1 Clinical Practice Guidelines (CPGs) are particularly useful tools to translate this evidence into practice and to support these individual patient focused decisions.
One of the first CPGs on the prevention of pressure ulcers/ injuries was published by the Agency for Health Care Policy and Research in the United States 30 years ago.2 It contained 25 recommendations including pressure ulcer/ injury risk assessment, regular repositioning, use of special support surfaces, and educational programs. Since then, numerous CPGs have been published and are regularly updated. For more than 10 years the European Pressure Ulcer Advisory Panel (EPUAP), the National Pressure Injury Advisory Panel (NPIAP), and the Pan Pacific Pressure Injury Alliance (PPPIA), have worked successfully together to develop, update, disseminate and implement international CPGs for the prevention and treatment of pressure ulcers/ injuries. These three organisations published their third and latest international edition in 2019.3
The expansion of CPGs indicates an increased interest in and the use of evidence-based pressure ulcer/ injury management on a national and international level. The international CPG alone was cited more than 2000 times in international literature, indicating wide
dissemination.4 However, successful dissemination is different from implementation in practice, and evidence indicates that pressure ulcer/ injury prevention and treatment must be improved. Although it is widely accepted that not all pressure ulcers/ injuries are preventable, a substantial proportion are. Clinicians are in charge, but much of the responsibility lies within structures and processes of organizations and their management. This also includes managing information overload and balancing conflicting recommendations, and the requirements of numerous policies at the same time.5 Developing national and international CPGs in parallel may also result in unnecessary duplication of efforts, which is problematic in times of limited resources and funding. Low quality pressure ulcer/ injury guidelines6 are unlikely to improve care.
Finally, in the field of pressure ulcer/ injury prevention and treatment are many areas of missing evidence. There is an urgent need for well-designed trials in the field.7 This is a particular challenge for guideline developers because guideline recommendations must be based on evidence, while practitioners expect guidance irrespective of the availability of evidence.8 Interestingly, basic pressure ulcer/ injury preventive approaches such as systematic skin inspection, using support surfaces, or raising heels, have not changed significantly over decades2, and are cornerstones of state-ofthe-art pressure management today.
Despite these well-known challenges, there are a number of opportunities. Strong and
established relations between the EPUAP, NPIAP and PPPIA are an excellent basis for further efficient international collaboration. Worldwide, there is an increasing acceptance of the standards for trustworthy guidelines and up-to-date guideline development methods, such as the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. There is also an increasing number of low-risk of bias confirmatory trials and high quality systematic reviews and metaanalysis, including the latest series of Cochrane support surface reviews.9 This strengthens a growing body of evidence in pressure ulcer/ injury prevention and treatment to be employed in high quality CPGs in the future.
1. Sackett DL, et al. Evidence based medicine: what it is and what it isn’t. BMJ 1996; 312(7023):71–2.https://doi.org/10.1136/bmj.312.7023.71
2. U.S. Department of Health and Human Services, Agency for Health Care Policy. Pressure Ulcers in Adults: Prediction and Prevention – Clinical Practice Guideline Number 3. Rockville, Maryland, United States of America, 1992.
3. Kottner J et al. A closer look at the 2019 International Guideline on the prevention and treatment of pressure ulcers/injuries. J Tissue Viability. 2020;29(4):225-226.https://doi. org/10.1016/j.jtv.2020.09.003
4. Kottner J, El Genedy-Kalyoncu M. The uptake of the international pressure ulcer/injury prevention and treatment guidelines in the scientific literature: A systematic analysis of two major citation databases. J Tissue Viability. 2022 Aug 7 (Online ahead of print).https://doi. org/10.1016/j.jtv.2022.07.011
5. Carthey J et al. Breaking the rules: understanding non-compliance with policies and guidelines. BMJ 2011;343:d5283.https://doi.org/10.1136/bmj.d5283
6. Gillespie BM et al. The quality and clinical applicability of recommendations in pressure injury guidelines: A systematic review of clinical practice guidelines. Int J Nurs Stud. 2021;115:103857.https://doi.org/10.1016/j.ijnurstu.2020.103857
7. Walker RM et al. Prevention and treatment of pressure injuries: A meta-synthesis of Cochrane Reviews. J Tissue Viability. 2020;29(4):227-243.

8. Haesler E, Kottner J, Cuddigan J; 2014 International Guideline Development Group. The 2014 International Pressure Ulcer Guideline: methods and development. J AdvNurs. 2017;73(6):1515-1530.https://doi.org/10.1111/jan.13241
9. Shi C et al. Beds, overlays and mattresses for preventing and treating pressure ulcers: an overview of Cochrane Reviews and network meta-analysis. Cochrane Database Syst Rev. 2021;8(8):CD013761.

“Worldwide, there is an increasing acceptance of the standards for trustworthy guidelines and up-to-date guideline development methods.”

Challenging non-healing wounds can prove a challenge to the wound care provider. As this article explores, consideration of combination therapies, which sequence different wound treatment modalities, can help flick the switch in the healing process.
Chronic non-healing wounds lead to enormous financial burden on the health care system, resulting in costs of more than $25 billion annually in the United States alone.1 These wounds are stuck in the inflammatory phase with increased levels of harmful metalloproteases.
All patients may not qualify or may decline flap reconstruction. Stage IV pressure ulcers are best covered with fascio or myocutaneous flaps. Addressing the etiology and associated risks of these wounds prior to surgery is key, since their complication and recurrence rates are very high.2 Other factors will of course influence the outcome such as compliance, nutrition status, offloading, ruling out osteomyelitis, clean wounds with no soft tissue infection, being on certain medications, and others. In patients who may not be a good candidate for flap closure but have met all the above-mentioned conditions, other therapeutic modalities and their combinations may lead to faster healing.
Cell and Tissue-Based Products (CTPs) have increased in popularity in the last two decades with their proven favorable effects on chronic hard to heal wounds. They include extra cellular matrix (ECM) replacement (including bioengineered matrices, Allo and Xeno Grafts), cell signaling products and dehydrated, cryopreserved or fresh amniotic and umbilical cord products.3
Extracellular matrix (ECM) derived from small intestinal submucosa (SIS) is widely used in clinical applications as a scaffold for tissue repair.
The SIS-ECM is made from porcine small intestinal submucosa. This product has been previously reported to produce a dressing that inactivates the tissue metalloproteases, thereby preventing destruction of the provisional ECM. Its composition stimulates cell migration, reduces inflammation, and provides moisture to the wound bed; SIS-ECM also grants structural support and enhances cellular proliferation and attachments. The SIS-ECM has been employed for a variety of wounds including:
• Chronic vascular
• Venous ulcers
Diabetic ulcers
• Partial-thickness burns
• Donor sites and skin graft preparation
Partial and full-thickness, surgical, and traumatic wounds4
In a recently published manuscript, we demonstrated with a prospective randomized controlled trial that combining ECM replacement with negative pressure wound therapy (NPWT) will hasten the healing rate in this patient population.5 In this study 16 patients were randomized to receive either the SIS-ECM plus NPWT (8 study) or NPWT alone (8 control) for stage 4 pressure ulcers treatment. Wounds were photographed and measured weekly. The experimental group had their ECM dressings changed every other week and their NPWT changed twice weekly. After the 12-week study period, the average control
Figure 1:
ECM
NPWT
“The SIS-ECM is made from porcine small intestinal submucosa. This product has been previously reported to produce a dressing that inactivates the tissue metalloproteases, thereby preventing destruction of the provisional ECM.”
patient healing rate was 45.79% as compared with the 89.98% healing rate in the study group (P < .01). The difference in healing rate between control and study patients was optimal by 12 weeks.5
The study was limited by the small number of participating patients, although it demonstrates that wound care providers, when faced with challenging non healing wounds, should consider combined therapies to improve the healing rate.
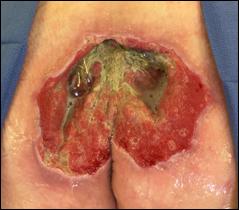
1a: Large stage IV sacral and bilateral gluteal pressure ulcer.
4
1. Frade MAC, Das PK. Chronic ulcers: updating epidemiology, physiopathology, and therapies. Ulcers. 2013. doi:10.1155/2013/964826.
2. Ravinder B. et al. Flap Reconstruction for Pressure Ulcers: An Outcomes Analysis. PlastReconstr Surg Glob Open. 2017 Jan 18;5(1):e1187. doi: 10.1097/GOX.0000000000001187.
3. Rubin JP et al. Regulatory Advocacy Update: ASPS Comments in Response to the U.S Food and Drug Administration Draft Guidance Documents on Human Cell and Tissue Products. Plast and Reconstruct Surg: May 2017, vol 139-issue 5-page 1259-1261.
4. Abouissa A, Mari W, Simman R. Clinical usage of an extracellular, collagen-rich matrix: a case series. Wounds. 2015;27(11):313–318.
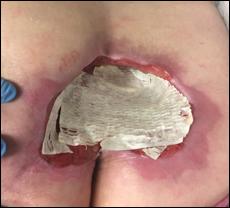
5. Mari W, Simman R, et al. Use of a Natural Porcine Extracellular Matrix with Negative Pressure Wound Therapy Hastens the Healing Rate in Stage 4 Pressure Ulcers. Wounds 2019;31(5):117–122. Epub 2019 March 15.
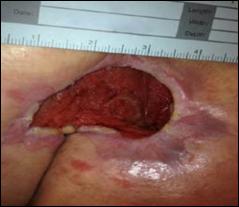
1b: SIS ECM being applied to wound bed before the application of NPWT.
1c: Ulcer surface reduction at 3 months, at the end of the study.
1a 1b
1c
Published
Stadler, F.
DOI: https://doi.org/10.11647/
It is important for healthcare practitioners to understand when to use maggot therapy. This article explains the general factors that determine the choice of wound treatment and how they apply to
therapy: firstly the wound characteristics, secondly the patient characteristics, thirdly the environment, fourthly the available resources, and fifthly the specific characteristics of each available treatment modality. Beyond the regular healthcare setting, maggot therapy can make a significant contribution to the treatment of people with wounds in compromised healthcare settings such as in times of disaster and armed conflict, in underserved populations, or in palliative care.
There is a great need for improved wound care in both resourced and compromised healthcare settings. Although not a panacea, maggot therapy can meet many of these pressing wound care needs. It is well-established that medicinal maggots have three major actions on wounds: 1) they dissolve and dislodge dead (necrotic) tissue and debris (debridement), 2) they kill microorganisms (“disinfection”), and 3) they stimulate healthy tissue to grow faster.1-3 Therefore, maggot therapy can usually assist in treating wounds that require any of these actions. Notwithstanding this broad spectrum of potential indications, it is important for healthcare policy makers, regulators, and health administrators to understand the contribution maggot therapy can make to wound care in order to support mainstreaming of this therapy.4
It is also important for healthcare practitioners to understand when to use maggot therapy.
While treatment selection can seem quite complicated at times, the following general factors should always be considered when choosing a wound treatment:
• The wound characteristics
Dr Frank Stadler Director, MedMagLabs Sydney, Australia

• The patient characteristics
The environment
• The available resources
• The specific characteristics of each available treatment modality.
Examples of how these factors might affect treatment decisions can be found in Table 1.
Using these characteristics as a guiding framework, this article explains when maggot therapy is indicated.
It should also be noted that the specific types of wounds and situations that are appropriately treated with a particular method or product are usually based on scientific studies, anecdotal reports, personal experience, and regulatory approvals. Since maggot therapy is not officially regulated in most countries, this discussion of indications for maggot therapy is based primarily on its mechanisms of action and on published experience.
Most wounds on an otherwise healthy person will heal quite well despite our choice of therapy. But when dead tissue covers or fills that wound, then the surrounding healthy tissue cannot fill the void. What’s more, microorganisms that live and feed on dead tissue, or under its cover, can multiply and spread beyond these borders, especially if they can also live in the surrounding live tissue. Even if they cannot themselves spread beyond dead tissue, their secretions (called “toxins”) may be harmful to the surrounding live tissue, and may cause that tissue, too, to become inflamed and/or die, thereby extending the wound. Although scientific proof is sparse, there is wide agreement that a wound with necrotic tissue and debris will not easily heal until the necrotic tissue and debris is removed.5,6
Table 1: Factors to consider when selecting wound treatments.
Maggots must compete fiercely with other scavengers for limited resources, and many of those competing scavengers would consume the maggots, too, if the maggots were found still on the rotting tissue. The maggots used therapeutically are those that are well-adapted to quickly remove (and ingest) as much of the necrotic tissue and debris as possible, and then leave the area. Maggot therapy debrides the necrotic wound by at least two methods: enzymatic debridement and physical
• Infection might require specific antimicrobial treatment; aggressive infection might require immediate resection or at least close daily or hourly inspection
• Dead tissue and debris often require removal (debridement) before healing can fully transpire
• If the wound cannot be visualised completely, accommodations will need to be made to ensure that even non-visualised surfaces are cleaned and disinfected
• If vital structures (major vessels, nerves and organs) are involved, accommodations will need to be made to ensure that they are not damaged in the attempt to halt the infection or remove dead tissues
• Factors that affect wound healing (i.e., malnutrition, anaemia, diabetes, peripheral vascular disease)
• Factors that affect a patient’s ability to tolerate surgery or other treatments (i.e., underlying heart, kidney, liver disease)
• Factors that affect a patient’s ability to participate in the therapy, to the degree that will be necessary (i.e., ability to consent; physical and mental limitations on the ability to do dressing changes; social support at home; financial limitations; cultural/ religious factors)
• Availability of shelter, electricity, refrigeration, clean or sterile water, transportation
• Weather/temperature extremes can impact the access to or shelf-life of certain products (i.e., medicinal maggots cannot survive extreme cold or hot temperatures unless stored and transported in temperature-resistant containers)
• Physically/structurally stable environment may be required by certain treatments; this is threatened by ongoing earthquake aftershocks, wildfires, flooding, or civil unrest and conflict
• Financial resources available to the individual
• Availability of healthcare insurance
• Level of healthcare resource availability in the country or region
• Physical ability to access those resources (i.e., transportation and accommodation to access care only available in regional centres; availability of treatment in the home)
• Availability of adequately trained wound care providers
• Policies at all levels which affect the availability, provision and financing of various treatment modalities
• Safety • Cost and likelihood of reimbursement
• Acceptability to the patient and care provider
• Indications and contraindications
• Regulatory status (i.e., whether it is locally considered to be an approved, unapproved, or investigational modality)
debridement.7
The maggot’s digestive juices, rich with proteolytic enzymes, are excreted into the wound bed and quickly dissolve dead tissue. Meanwhile, as the maggot crawls about the wound bed, its microscopic spines and its modified mandibles (‘mouth hooks’) physically dislodge some of the debris, helping the digestive juices gain access into the crevices.
Given that medicinal blowflies, if living in the wild, would inhabit corpses, faeces, and other rotting organic matter, it seems logical that the larvae would have a method for killing infectious organisms, or else the microbes might kill the maggots. The mechanisms by which the maggots accomplish microbial killing are covered by Nigam and Wilson.2 For the purpose of this discussion, let it suffice to acknowledge that medicinal maggots kill microbes through a variety of mechanisms including ingestion,8,9 the secretion of antimicrobial compounds,10-15 dissolving biofilm,12,16-21 and altering the local environment in ways that make it less hospitable to microbial pathogens.22,23 The end result is that microbial populations are reduced or eliminated,24-26 and clinical infections subside.27,28 The antimicrobial effect may even endure well beyond the life of the maggots.29
Wounds can vary greatly in wetness. Some wounds are extremely dry; the necrotic tissue covering the wound being like leather (“eschar”). Other wounds may drain serous (watery), serosanguinous (bloody), or purulent (pus-filled) liquid profusely. The same wound may switch back and forth over the course of a week. Many dressings are suitable only for moderately dry wounds, or only for very wet wounds. Some dressings will not adhere to a wet wound; others are designed to absorb excess fluid tenaciously, making them superfluous or even dangerous for use with a minimally moist wound. Medicinal maggots have no such limitations, though the dressings used to confine them to the wound may themselves need to be selected or modified to meet the moisture conditions of the wound and surrounding tissue.
Maggot therapy has been observed to enhance
wound healing, even in apparently clean but stagnant wounds, at least as far back as William Baer’s time.31
Subsequent clinicians have described, in controlled studies, the rapid proliferation of granulation tissue and hastened closing of the wound margins in previously stagnant, nonhealing wounds.32-25 The mechanisms known to be involved are described by Nigam and Wilson.3 More and more therapists are using maggot therapy to promote healing and closure of chronic wounds, even though the wounds may not appear grossly necrotic nor infected. Some of the therapists describe this indication as maintenance debridement, others say their intent is growth-stimulation. The desired endpoint is the same: not just a clean (debrided) wound but a clean wound that is healing.
Pressure alone, from lying in the same position for a prolonged time, or in combination with shear force, can starve tissue of oxygenated blood which leads to tissue death. Shear may exacerbate the injury and cause mechanical damage to the compromised tissue.36
Factors other than pressure can also prevent arterial blood from reaching tissue. Primary arterial disease, for example (i.e., vasculitis, arteritis obliterans, or thromboses) can occlude arteries and arterioles, leading to local necrosis of the skin and soft tissue. Many anecdotal reports support the use of maggot therapy for debriding these wounds.40-43 However, without adequate blood flow, healing cannot occur even in the maggot-debrided wound.40,41
Therefore, the arterial flow needs to be assessed prior to maggot therapy. Even if arterial flow
“More and more therapists are using maggot therapy to promote healing and closure of chronic wounds, even though the wounds may not appear grossly necrotic nor infected.” Wound Aetiologies, Patient Characteristics, and Healthcare Settings Amenable to Maggot Therapycannot be restored, maggot therapy can be administered to palliate ulcer symptoms such as exudate and odour and to prevent infection, thereby maintaining quality of life.
Venous disease, too, can lead to skin and softtissue wounds. Venous insufficiency results in legs, for example, with increased intravascular pressure. The result is that fluid (serum) leaks out of the blood vessels and into the soft tissue, thereby causing swelling, pain, itching (trauma and infection from scratching). Oxygen cannot easily perfuse the fluid-filled (oedematous) leg, and the normal immune mechanisms (i.e., migration of white blood cells) are similarly impaired. In the end, skin breaks down more easily, the body does not fight the infections well, and healing is impaired (Figure 1a). Venous ulcers are best prevented by compression and elevation of the affected limb. However, when a person presents with a venous ulcer, then regular debridement is necessary along with compression and elevation of the limb. Multiple controlled and non-controlled maggot therapy studies have described faster debridement (with or without faster healing) of venous ulcers.38,44-46
Diabetes results in multi-system disease. Neuropathy can lead to increased trauma, impaired immunity interferes with the body’s ability to fight infection, and blood vessel disease greatly inhibits healing (Figures 1c, 1d). DFUs require timely and expert assessment to identify the aetiology and prompt initiation of tailored management.47
When aetiology is not ascertained, management will fail to address the underlying cause, which may lead to amputation.48 Up to 85% of amputations can be prevented.49,50 Maggot therapy is often used to debride these wounds and control emerging infections. Several controlled studies32,34 and case series51 have demonstrated significant benefits.
Medicinal maggots are quite useful in debriding
traumatic and infected or necrotic post-surgical wounds (Figure 1e, 1f). In an otherwise healthy individual, these wounds usually heal quite well after initial debridement and infection management.
Unlike many other necrotic wounds, burns need to be debrided acutely (Figure 1g). When the burn area is extensive or when vital structures could be damaged by surgical debridement, maggot debridement has proven to be a good alternative.10,52-58 Burn wounds are often painful, and maggot therapy is not likely to be less painful than any other manipulation of the damaged tissue. Therefore, anaesthetics will be just as necessary during maggot debridement as during any other form of debridement, and maybe more so.
Undermining Wounds, Difficult to Reach or Visualise
Because medicinal maggots, by nature, crawl into nooks and crannies, they are very appropriate for treating wounds whose boundaries may not be easily visualised. Like miners, the larvae will crawl into the depths of undermined tissue and sinus tracts, debriding within these areas until they need to return to the surface for air, food (assuming that the area no longer contains necrotic tissue or nutritious liquid), or to moult. Treating undermining wounds with maggot therapy can avoid having to open a deep cavity for accurate visualisation and debridement.
Similar to undermined wounds and sinus tracts, medicinal maggots have been used effectively to debride body cavities with infected necrotic tissue, such as necrotising peritonitis or chronic empyema.56 If debridement is not needed in the crevices or out-of-sight areas of these cavities, it may be possible and preferable to apply the maggots within sealed mesh bags so that they stay contained and can be more easily removed.30
Bone infections (‘osteomyelitis’) were a
common indication for maggot therapy during the 1930s, when many orthopaedic surgeons used maggot therapy to perform the ‘fine debridement’ after the surgeon removed the grossly infected or dead bone.31,59,60 We now have procedures and antibiotics that make postoperative infections much less common. Maggot therapy is rarely used for osteomyelitis at all anymore; osteomyelitis is generally considered a surgical disease, treated by removing (resecting) the dead bone and by long-term antibiotics to prevent spread or recurrence of the infection. However, when surgical resection and/or antibiotics are inadequate or are not an option, maggot therapy is sometimes used to debride the bone.61-63 Similarly, infected joints and joint prostheses are generally treated by removing the infected hardware and draining the infection, along with antibiotic coverage. But when the removal of hardware is risky or impossible, maggot therapy has been used alongside the antibiotics to eliminate the infection.64,65
Medicinal maggots dissolve necrotic tissue, but not viable tissue. In that regard, they are not useful for killing live cancer cells. However, when a tumour mass outstrips its blood supply and starts to rot, maggot therapy can be a rapid and effective method for removing the necrotic mass (Figure 1h). Many cancer patients were able to return to a reasonably normal life in public only after maggot therapy removed their foul-smelling, draining, painfully infected tumours.66-68
Because blowflies kill microbes through mechanisms other than those used by typical antibiotics,2 they are effective in killing pathogens that have developed resistance to those antibiotics, such as methicillin-resistant Staphylococcus aureus (MRSA);66-68 and multiresistant Acinetobacter.69
Maggot secretions kill or inhibit representative fungi in the laboratory,70,71 though very little
Figure 1: Chronic wounds amenable to maggot therapy: a) pressure ulcer, b) venous stasis ulcer, c&d) diabetic foot ulcers, e) burn, f) surgical wound dehiscence, g) orthopaedic wound, h) malignancy.
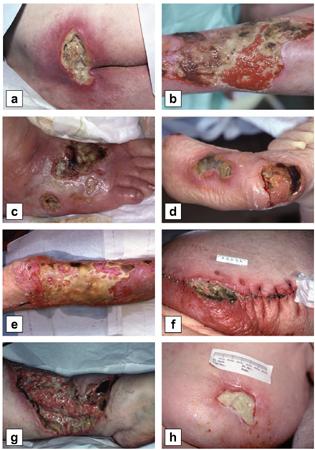
Photos: © Steve Thomas, www.medetec.co.uk.
clinical experience with fungi has been published.72
Several microbes, and especially mixed aerobic and anaerobic infections, can be highly destructive to soft tissue, causing necrosis that rapidly extends down to the deep layers of muscle and bone. Fasciitis is a surgical emergency. Even with immediate resection of the dead tissue, systemic broad-spectrum antibiotics and intensive care, the mortality of these infections can still exceed 40%.73
Frequently, repeated surgical debridement is required, ultimately leading to destruction or resection of vital structures, and the need for major anatomical reconstruction, if the patient survives at all. Maggot therapy has been used successfully to debride these wounds without unnecessarily harming the remaining viable tissue.74-77 In a small but prospective study, patients with Fournier’s gangrene that were
debrided only by maggots if they required more than one initial surgical resection, not only survived their wounds without the need for any additional surgical debridement but also avoided the need for reconstructive surgery.78
Tropical Ulcers (Mycobacteriosis and Tropical Phagedenic Ulcers)
The term ‘tropical ulcers’ collectively refers to painful wounds in typically malnourished individuals living in tropical climates. The wounds often follow trauma, and they may be colonised, if not caused, by Mycobacterium ulcerans (Buruli ulcer, mycobacteriosis) or Fusobacterium and Borrelia (Phagedenic ulcers, Naga sores).79
These are chronic wounds often with very poor outcomes, though antibiotics and improved nutrition can be curative. Buruli ulcers (Mycobacterium ulcerans wounds) often deteriorate following what appears to be appropriate antimicrobial therapy.80 Medicinal maggots were used extensively in the 1930s as an adjunct to surgical resection, in the treatment of tuberculous osteomyelitis. It is reasonable to assume that maggot therapy should adequately debride tropical ulcer wounds as well, but the efficacy and safety of maggot debridement for these wounds remain undefined.
The wounds commonly seen in patients living with leprosy (caused by Mycobacterium leprae) are generally caused by trauma; the leprosy bacterium is not necessarily within the wound.81 These wounds should also respond to maggot therapy, if needed, just like any other traumatic wound.
Medicinal maggots have demonstrated antileishmania activity in vitro and in animal models,82-85 though no human studies have been reported. The endemic trepanematoses (i.e., yaws, pinta, and bejel) can also cause problematic soft-tissue wounds, but penicillin and several other readily available antibiotics are generally curative. No experience treating these lesions with maggot therapy has been published to date.
During a disaster or military conflict, the number of victims often overwhelms the capacity of first responders. This is the perfect opportunity to recruit fly larvae to assist in wound care.86 Often there are insufficient facilities or hospital beds, and unreliable electricity and water supplies. Again, maggot therapy is well suited to these trying circumstances because the larvae require none of those amenities. However, the supply chains for getting medicinal maggots to compromised healthcare settings are hitherto non-existent. Because maggots are highly perishable and cannot be stored for more than a day or two, attention needs to be paid to their production close to the point of care in disasters and conflict, or to reliable logistics solutions that ensure timely and safe delivery of medicinal maggots.87,88
People living in sparsely populated or medically underserved regions have all the same problems as described above, but often in different proportions. Wounds as a result of infectious and parasitic diseases, burns, and traumatic injury are common, as are increasingly wounds related to cancer, diabetes and cardiovascular disease. Again, maggot therapy is a great resource when there is a severe shortage of medical staff and other healthcare resources, provided that reliable supply chains exist to make maggot therapy accessible to these communities.
Death is a part of life whether in a rural part of the world or in a big city. Most people become ill or reach an older age before dying. With aging and chronic illness, organs (including skin) begin to fail. Skin failure is a common pre-terminal occurrence.89 As already noted, maggot therapy is a simple and effective means of treating necrotic, infected, or chronic wounds in the ill or frail person.90 Maggot therapy can quickly provide surgical-quality wound care, at home or in long-term/residential care, at a fraction of the cost of a surgeon, and be costeffective when compared to existing advanced
dressings that deliver only limited wound bed improvement.
Many treatment modalities are not available to persons with wounds depending on their particular age, gender, or both. For example, certain antibiotics and other pharmaceuticals should not be used in children or in childbearing women, or even in women of a particular (‘child-bearing’) age. Often this is the result of real or theoretical complications that have been observed in humans or in animal models. Other times it is simply because the studies supporting safety and efficacy were performed in populations that excluded these high-risk individuals. Maggot therapy does not need to be withheld from anyone on the basis of gender or age.31,91
Whether due to age or underlying medical illnesses, some people are unable to endure the physical or mental stress of aggressive medical interventions such as surgery, anaesthesia, or even frequent phlebotomy (needles). Even if their cardiopulmonary status is stable, an underlying problem such as hypertension, obesity, emphysema or sub-optimally managed diabetes may make surgery and/or anaesthesia excessively risky.92 These conditions are not contraindications to maggot therapy, because maggot therapy does not require general anaesthesia, and does not put any additional physical stress on the body.
Non-healing wounds are often seen in people when they are dying. When managing wounds that cannot heal, including in people who are dying, the focus of holistic management is to
optimise quality of life and to maintain optimal dignity. Maggot therapy can be of particular benefit if wounds are infected, foul-smelling, draining, painful, in need of much care and attention.
Maggot therapy controls the infection, debrides the necrotic tissue, and thereby diminishes or eliminates the drainage, odour and pain of a necrotic wound or terminal ulcer. Maggot therapy can provide comfort in these ways, without adding additional stress to a patient in their last days of life.77
Maggot therapy can be used safely and effectively even in people receiving other drugs and treatments. Medicinal maggots and their debridement efficacy appear to be unaffected by antibiotics,93,94 and most other drugs in pharmacologic doses, and various medical and life support interventions.56,95-97 In fact, maggots have proven to be safe and effective in patients receiving anticoagulants or intensive care that made surgical debridement unsafe.56,98 Unpublished anecdotal reports suggest that medicinal maggots may not survive on animals receiving systemic or topical insecticides, such as ivermectin or flea and tick treatments. No work has been published concerning the impact of cancer chemotherapy on medicinal maggots.
One of the most favourable aspects of maggot therapy is its flexibility regarding the care setting. Patients can be treated as in-patients, as outpatients, in the community, in field hospital situations, and even in the most austere healthcare settings that lack any medical resources.31,86,99 This is because the application of, and treatment with, medicinal maggot dressings does not require energy or sophisticated equipment. Maggots are also well-adapted to unhygienic environments
“Many treatment modalities are not available to persons with wounds depending on their particular age, gender, or both... Maggot therapy does not need to be withheld from anyone on the basis of gender or age.”31,91
and therefore will perform their therapeutic action equally well in the modern hospital environment, the battlefield, and the disaster zone.
The lack of stable shelter is not an impediment to using maggot therapy.86,99 Maggot therapy will still be effective, even if exposed to the elements, as long as the maggots receive enough air and the wounds are well-drained to prevent suffocation and drowning. Extreme climatic conditions should not be a deterrent either. In general, if the weather is tolerable to the patient, it will be tolerated by medicinal maggots.100,101 Wound temperatures encountered by medicinal maggots will depend on the body region, general condition of the patient and the environmental temperature, but will in most instances not be lower than 30 or exceed 40 , which is a temperature range that promotes high activity and rapid growth in medicinal maggots.100,102 and lies well below the lethal temperature threshold of around 47°C.103 Environmental temperature is only a concern during transport of young medicinal maggots that are deprived of food until they reach the point of care and patient. To slow down activity and maintain optimal health, medicinal maggots should be kept at 6 to 25 and be applied to the wound within 48 hours of dispatch87. In other words, most limitations imposed on healthcare provision by the environment, facilities, or equipment will have little to no impact on the work of the maggots.
Social support systems are occasionally called into play to help with medical issues. In some parts of the world, patients are discharged to home while still recovering. Friends and family may need to assist in the care of their loved ones at home or in the hospital. Maggot therapy is simple enough that dressings can be applied, maintained, and/ or removed by the patient or the patient’s caregivers.104
Without financial resources, the purchase of specialised medical supplies can seem like an insurmountable problem.
Fortunately, medicinal maggots are not very costly to produce (except when regulatory agencies are involved). Many labs provide free or subsidised larvae to patients with limited finances. Some therapists procure larvae themselves when a commercial product is unavailable.99,105 Even when financial resources are sparse, maggot therapy remains a viable and cost-effective solution for wound care.
Moreover, the use and application of medicinal maggots requires no electricity, no batteries, no running or sterile water, and no refrigeration.86,99 Maggot dressings can be made from materials readily available at any facility that is modestly prepared to manage wounds (e.g., gauze and tape). While specialised or maggot-specific dressing supplies can simplify the application of maggot therapy, such amenities are not required.
While maggot therapy is not a panacea and will not resolve the underlying causes, particularly of chronic wounds, it is appropriate for the treatment of a wide variety of infected, necrotic, non-healing skin and soft-tissue wounds. Maggot therapy may also be used for acute wounds in need of urgent debridement, and non-necrotic wounds that simply will not close. In all of these situations, maggot therapy has proven to be very effective and relatively safe, at a low or reasonable cost. Maggot therapy can make a significant contribution to the treatment of people with wounds in disasters and armed conflict, in underserved populations, and in palliative care. With respect to patient characteristics, maggot therapy is also highly accommodating as it can be used to treat people of all ages, genders and levels of frailty.
Indeed, it should be a treatment of choice for debridement and wound maintenance where people are unfit to endure general anaesthesia or are at their end of life. Moreover, medicinal maggots can be deployed in both high- and lowresource care settings irrespective of healthcare infrastructure and hygiene levels as they do not require any special facilities, resources, or highlyskilled personnel. Table 2 provides a summary of the ideal wound dressing characteristics, and how maggot therapy compares favourably to that ideal.
Table 2: The degree to which maggot therapy compares with the ideal wound dressing.
The Ideal Wound Dressing ***** How Maggot Therapy Compares
Can be applied with minimal training
Safe for healthy wound tissue
Can be applied by
professionals
Can be applied with minimal training
Low
Low
impact
Safe for healthy surrounding tissue
Low
Simple to
1. Nigam, Y. and M.R. Wilson, Maggot Debridement, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 143–152, https://doi.org/10.11647/ OBP.0300.08.
2. Nigam, Y. and M.R. Wilson, The Antimicrobial Activity of Medicinal Maggots, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 153–174, https://doi.org/10.11647/OBP.0300.09.
3. Nigam, Y. and M.R. Wilson, Maggot-assisted Wound Healing, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 175–194, https://doi. org/10.11647/OBP.0300.10.
4. Bullen, B. and P. Chadwick, Clinical Integration of Maggot Therapy, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 97–118, https://doi. org/10.11647/OBP.0300.06.
5. Falabella, A.F., Debridement and Wound Bed Preparation. Dermatologic Therapy, 2006. 19(6): pp. 317–325, https://doi.org/10.1111/j.1529-8019.2006.00090.x.
6. Weir, D., P. Scarborough, and J. Niezgoda, Wound Debridement, in Chronic Wound Care: The Essentials, D.L. Krasner and L. van Rijswijk (eds). 2018, HMP Communications: Malvern, PA, pp. 63–78.
7. Sherman, R.A., Mechanisms of Maggot-Induced Wound Healing: What Do We Know, and Where Do We Go from Here? Evidence-based Complementary and Alternative Medicine, 2014. 2014: pp. 1–13, https://doi. org/10.1155/2014/592419.
8. Greenberg, B., Model for Destruction of Bacteria in the Midgut of Blow Fly Maggots. Journal of Medical Entomology, 1968. 5(1): pp. 31–38, https:// doi.org/10.1093/jmedent/5.1.31.
9. Mumcuoglu, K.Y., et al., Destruction of Bacteria in the Digestive Tract of the Maggot of Lucilia sericata (Diptera: Calliphoridae). Journal of Medical Entomology, 2001. 38(2): pp. 161–166, https://doi.org/10.1603/0022-2585-38.2.161.
10. Bian, H., et al., Beneficial Effects of Extracts from Lucilia sericata Maggots on Burn Wounds in Rats. Molecular Medicine Reports, 2017. 16(5): pp. 7213– 7220, https://doi.org/10.3892/ mmr.2017.7566.
11. Erdmann, G.R. and S.K. Khalil, Isolation and Identification of Two Antibacterial Agents Produced by a Strain of Proteus Mirabilis Isolated from Larvae of the Screwworm (Cochliomyia hominivorax) (Diptera: Calliphoridae). Journal of Medical Entomology, 1986. 23(2): pp. 208–211, https://doi.org/10.1093/jmedent/23.2.208.
12. Gordya, N., et al., Natural Antimicrobial Peptide Complexes in the Fighting of Antibiotic Resistant Biofilms: Calliphora Vicina Medicinal Maggots. PloS ONE, 2017. 12(3): e0173559, https://doi.org/10.1371/journal.pone.0173559.
13. Pöppel, A.-K., et al., Antimicrobial Peptides Expressed in Medicinal Maggots of the Blow
Fly Lucilia sericata Show Combinatorial Activity against Bacteria. Antimicrobial Agents and Chemotherapy, 2015. 59(5): pp. 2508–2514, https://doi.org/10.1128/AAC.05180-14.
14. Teh, C.H., et al., Determination of Antibacterial Activity and Minimum Inhibitory Concentration of Larval Extract of Fly via Resazurin-based Turbidometric Assay. BMC Microbiology, 2017. 17(1): pp. 36–36, https://doi.org/10.1186/s12866-017-0936-3.
15. Wright, E.A. and E.R. Pavillard, An Antibiotic from Maggots. Nature, 1957. 180(4592): pp. 916–917, https://doi.org/10.1038/180916b0.
16. Bohova, J., et al., Selective Antibiofilm Effects of Lucilia sericata Larvae Secretions/ Excretions against Wound Pathogens. Evidence-Based Complementary and Alternative Medicine, 2014. 2014: pp. 857360–857360, https://doi.org/10.1155/2014/857360.
17. Brown, A., et al., Blow Fly Lucilia sericata Nuclease Digests DNA Associated with Wound Slough/Eschar and with Pseudomonas aeruginosa Biofilm. Medical and Veterinary Entomology, 2012. 26(4): pp. 432–439, https://doi.org/10.1111/j.1365-2915.2012.01029.x.
18. Cazander, G., et al., Maggot Excretions Inhibit Biofilm Formation on Biomaterials. Clinical Orthopaedics and Related Research, 2010. 468(10): pp. 2789–2796, https://doi.org/10.1007/ s11999-010-1309-5.
19. Cazander, G., et al., The Influence of Maggot Excretions on PAO1 Biofilm Formation on Different Biomaterials. Clinical Orthopaedics and Related Research, 2009. 467(2): pp. 536–545,
https://doi.org/10.1007/s11999-008-0555-2.
20. Harris, L.G., et al., Disruption of Staphylococcus Epidermidis Biofilms by Medicinal Maggot Lucilia sericata Excretions/Secretions. International Journal of Artificial Organs, 2009. 32(9): pp. 555–564, https://doi.org/10.1177/039139880903200904.
21. van der Plas, M.J.A., et al., Maggot Excretions/Secretions Are Differentially Effective against Biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. Journal of Antimicrobial Chemotherapy, 2008. 61(1): pp. 117–122, https://doi.org/10.1093/jac/dkm407.
22. Davydov, L., Maggot Therapy in Wound Management in Modern Era and a Review of Published Literature. 2011, SAGE Publications: Los Angeles, CA, pp. 89–93.
23. Nigam, Y., et al., Maggot Therapy: The Science and Implication for CAM Part II — Maggots Combat Infection. Evidence-based Complementary and Alternative Medicine, 2006. 3(3): pp. 303–308, https://doi.org/10.1093/ecam/nel022.
24. Contreras-Ruiz, J., et al., [Comparative Study of the Efficacy of Larva Therapy for Debridement and Control of Bacterial Burden Compared to Surgical Debridement and Topical Application of an Antimicrobial]. Gaceta médica de México, 2016. 152(Suppl 2): pp. 78–87, http://www.anmm.org.mx/GMM/2016/s2/GMM_152_2016_S2_78-87.pdf.
25. Malekian, A., et al., Efficacy of Maggot Therapy on Staphylococcus aureus and Pseudomonas aeruginosa in Diabetic Foot Ulcers: A Randomized Controlled Trial. Journal of Wound, Ostomy and Continence Nursing, 2019. 46(1): pp. 25–29, https://doi.org/10.1097/ WON.0000000000000496.
26. Tantawi, T.I., et al., Clinical and Microbiological Efficacy of MDT in the Treatment of Diabetic Foot Ulcers. Journal of Wound Care, 2007. 16(9): pp. 379–383, https://doi. org/10.12968/jowc.2007.16.9.27868.
27. Armstrong, D.G., et al., Maggot Therapy in “Lower-extremity Hospice” Wound Care: Fewer Amputations and More Antibiotic-free Days. Journal of the American Podiatric Medical Association, 2005. 95(3): pp. 254–257, https://doi.org/10.7547/0950254.
28. Steenvoorde, P. and J. Oskam, Use of Larval Therapy to Combat Infection after Breastconserving Surgery. Journal of Wound Care, 2005. 14(5): pp. 212–213, https://doi.org/10.12968/ jowc.2005.14.5.26778.
29. Sherman, R.A. and K.J. Shimoda, Presurgical Maggot Debridement of Soft Tissue Wounds
Is Associated with Decreased Rates of Postoperative Infection. Clinical Infectious Diseases, 2004. 39(7): pp. 1067–1070, https://doi. org/10.1086/423806.
30. Sherman, R., Medicinal Maggot Application and Maggot Therapy Dressing Technology, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 79–96, https://doi. org/10.11647/OBP.0300.05.
31. Baer, W.S., The Treatment of Chronic Osteomyelitis with the Maggot (Larva of the Blow Fly). The Journal of Bone and Joint Surgery. American Volume, 1931. 13: pp. 438–475, https:// doi.org/10.1007/s11999-010-1416-3.
32. Markevich, Y.O., et al., Maggot Therapy for Diabetic Neuropathic Foot Wounds. Diabetologia, 2000. 43: p. Suppl 1: A15.
33. Sherman, R.A., Maggot versus Conservative Debridement Therapy for the Treatment of Pressure Ulcers. Wound Repair and Regeneration, 2002. 10(4): pp. 208–214, https://doi. org/10.1046/j.1524-475X.2002.10403.x.
34. Sherman, R.A., Maggot Therapy for Treating Diabetic Foot Ulcers Unresponsive to Conventional Therapy. Diabetes Care, 2003. 26(2): pp. 446–451, https:// doi.org/10.2337/ diacare.26.2.446.
35. Sherman, R.A., F. Wyle, and M. Vulpe, Maggot Therapy for Treating Pressure Ulcers in Spinal Cord Injury Patients. The Journal of Spinal Cord Medicine, 1995. 18(2): pp. 71–74, https://doi.org/10.1080/10790268.1995.11719382.
36. European Pressure Ulcer Advisory Panel. National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. 2019. www.internationalguideline. com/static/pdfs/Quick_ Reference_Guide-10Mar2019.pdf.
37. Gilead, L., K.Y. Mumcuoglu, and A. Ingber, The Use of Maggot Debridement Therapy in the Treatment of Chronic Wounds in Hospitalised and Ambulatory Patients. Journal of Wound Care, 2012. 21(2): pp. 78–85, https://doi. org/10.12968/jowc.2012.21.2.78.
38. Mumcuoglu, K.Y., et al., Maggot Therapy for the Treatment of Intractable Wounds:
Maggot Therapy for Intractable Wounds Pharmacology and Therapeutics. International Journal of Dermatology, 1999. 38(8): pp. 623–627, https:// doi.org/10.1046/j.1365-4362.1999.00770.x.
39. Polat, E., et al., Treatment of Pressure Ulcers with Larvae of Lucilia sericata. Türkiye Fiziksel Tıp ve Rehabilitasyon Dergisi, 2017. 63(4): pp. 307–312, https://doi.org/10.5606/tftrd.2017.851.
40. Fleischmann, W., et al., Biosurgery — Maggots, Are They Really the Better Surgeons? Chirurg, 1999. 70(11): pp. 1340–1346, https://doi.org/10.1007/ s001040050790.
41. Igari, K., et al., Maggot Debridement Therapy for Peripheral Arterial Disease. Annals of Vascular Diseases, 2013. 6(2): pp. 145–149, https://doi.org/10.3400/avd.oa.13-00036.
42. Nishijima, A., et al., Effective Wound Bed Preparation Using Maggot Debridement Therapy for Patients with Critical Limb Ischaemia. Journal of Wound Care, 2017. 26(8): pp. 483–489, https://doi.org/10.12968/jowc.2017.26.8.483.
43. Rafter, L., Larval Therapy Applied to a Large Arterial Ulcer: An Effective Outcome. British Journal of Nursing, 2013. 22(Sup4): pp. S24-S30, https://doi.org/10.12968/bjon.2013.22.Sup4.S24.
44. Dumville, J.C., et al., Larval Therapy for Leg Ulcers (VenUS II): Randomised Controlled Trial. BMJ, 2009. 338(7702): pp. 1047–1050, https://doi.org/10.1136/bmj.b773.
45. Mudge, E., et al., A Randomized Controlled Trial of Larval Therapy for the Debridement of Leg Ulcers: Results of a Multicenter, Randomized, Controlled, Open, Observer Blind, Parallel Group Study. Wound Repair and Regeneration, 2014. 22(1): pp. 43–51, https://doi.org/10.1111/wrr.12127.
46. Wayman, J., et al., The Cost Effectiveness of Larval Therapy in Venous Ulcers. Journal of Tissue Viability, 2000. 10(3): pp. 91–94, https://doi.org/10.1016/S0965-206X(00)80036-4.
47. Schaper N, et al. IWGDF Guidelines on the Prevention and Management of Diabetic Foot Disease. 2019. https:// iwgdfguidelines.org/wp-content/uploads/2019/05/IWGDF-Guidelines-2019.pdf.
48. Hingorani, A.M.D., et al., The Management of Diabetic Foot: A Clinical Practice Guideline by the Society for Vascular Surgery in Collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. Journal of Vascular Surgery, 2016. 63(2): pp. 3S-21S, https://doi.org/10.1016/j.jvs.2015.10.003.
49. International Diabetes Federation. IDF Diabetes Atlas, 7th Edition. 2015. https://www.idf.org/component/attachments/ attachments.html?id=1093&task=download.
50. Krishnan, S., et al., Reduction in Diabetic Amputations over 11 Years in a Defined U.K. Population: Benefits of Multidisciplinary Team Work and Continuous Prospective Audit. Diabetes Care, 2008. 31(1): pp. 99–101, https://doi. org/10.2337/dc07-1178.
51. Marineau, M.L., et al., Maggot Debridement Therapy in the Treatment of Complex Diabetic Wounds. Hawaii Medical Journal, 2011. 70(6): pp. 121–124, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3233395/.
52. Jun-cheng, W., et al., Maggot Therapy for Repairing Serious Infective Wound in a Severely Burned Patient. Chinese Journal of Traumatology, 2012. 15(2): pp. 124–125, https://doi.org/10.3760/cma.j.issn.1008-1275.2012.02.012.
53. Feng, X., et al., Evaluation of the Burn Healing Properties of Oil Extraction from Housefly Larva in Mice. Journal of Ethnopharmacology, 2010. 130(3): pp. 586–592, https://doi.org/10.1016/j.jep.2010.05.044.
54. Li, Q.M.D., et al., Maggots of Musca Domestica in Treatment of Acute Intractable Wound. Surgery, 2009. 145(1): pp. 122–123, https://doi.org/10.1016/j. surg.2008.08.016.
55. Namias, N., et al., Biodebridement: A Case Report of Maggot Therapy for Limb Salvage after Fourth-degree Burns. Journal of Burn Care & Rehabilitation, 2000. 21(3): pp. 254–257, https://academic.oup.com/jbcr/article-abstract/ 21/3/254/4758239?redirectedFrom=fulltext.
56. Sherman, R.A., C.E. Shapiro, and R.M. Yang, Maggot Therapy for Problematic Wounds: Uncommon and Off-label Applications. Advances in Skin & Wound Care, 2007. 20(11): pp. 602–610, https://doi.org/10.1097/01. ASW.0000284943.70825.a8.
57. Summers, J.B. and J. Kaminski, Maggot Debridement Therapy (MDT) for Burn Wounds. Burns: Journal of the International Society for Burn Injuries, 2003. 29(5): pp. 501–502, https://doi.org/10.1016/s0305-4179(03)00059-7.
58. Vistnes, L.M., R. Lee, and G.A. Ksander, Proteolytic Activity of Blowfly Larvae Secretions in Experimental Burns. Surgery, 1981. 90(5): pp. 835–841.
59. McKeever, D.C., Maggots in Treatment of Osteomyelitis: A Simple Inexpensive Method. The Journal of Bone and Joint Surgery, 1933. 15: pp. 85–93, http:// dx.doi.org/10.1007/s11999-008-0240-5.
60. Stewart, M.A., The Therapeutic Behavior of Lucilia Sericata Meig. Larvae in Osteomyelitis Wounds. Science (American Association for the Advancement of Science), 1934. 79(2055): pp. 459–460
61. Galeano, M., et al., Maggot Therapy for Treatment of Osteomyelitis and Deep Wounds: An Old Remedy for an Actual Problem [8]. Plastic and Reconstructive Surgery (1963), 2001. 108(7): pp. 2178–2179.
62. Horn, K.L., A.H. Cobb, and G.A. Gates, Maggot Therapy for Subacute Mastoiditis. Archives of otolaryngology (1960), 1976. 102(6): pp. 377–379, https://doi.org/10.1001/archotol.1976.00780110089013.
63. Mumcuoglu, K.Y., et al., [Maggot Therapy for Gangrene and Osteomyelitis]. Harefuah, 1997. 132(5): pp. 323–325, 382.
64. Townley, W.A., A. Jain, and C. Healy, Maggot Debridement Therapy to Avoid Prosthesis Removal in an Infected Total Knee Arthroplasty. Journal of Wound Care, 2006. 15(2): pp. 78–79, https://doi.org/10.12968/jowc.2006.15.2.26890.
65. Wollina, U., M. Kinscher, and H. Fengler, Maggot Therapy in the Treatment of Wounds of Exposed Knee Prostheses. International Journal of Dermatology, 2005. 44(10): pp. 884–886, https://doi.org/10.1111/j.1365-4632.2005.02366c.x.
66. Beasley, W.D. and G. Hirst, Making a Meal of MRSA—The Role of Biosurgery in Hospital-acquired Infection. 2004, Elsevier Ltd: London, pp. 6–9.
67. Bowling, F.L., E.V. Salgami, and A.J.M. Boulton, Larval Therapy: A Novel Treatment in Eliminating MethicillinResistant Staphylococcus aureus from Diabetic Foot Ulcers. Diabetes Care, 2007. 30(2): pp. 370–371, https://doi.org/10.2337/ dc06-2348.
68. Dissemond, J., et al., Treatment of Methicillin-resistant Staphylococcus aureus (MRSA) as Part of Biosurgical Management of a Chronic Leg Ulcer. Hautarzt, 2002. 53(9): pp. 608–612, https://doi.org/10.1007/s00105-002-0336-x.
69. Čeřovský, V. and R. Bém, Lucifensins, the Insect Defensins of Biomedical Importance: The Story behind Maggot Therapy. Pharmaceuticals, 2014. 7(3): pp. 251–264, https://doi.org/10.3390/ph7030251.
70. Evans, R., E. Dudley, and Y. Nigam, Detection and Partial Characterization of Antifungal Bioactivity from the Secretions of the Medicinal Maggot, Lucilia sericata. Wound Repair and Regeneration, 2015. 23(3): pp. 361–368, https://doi.org/10.1111/ wrr.12287.
71. Margolin, L. and P. Gialanella, Assessment of the Antimicrobial Properties of Maggots. International Wound Journal, 2010. 7(3): pp. 202–204, https://doi.org/10.1111/j.1742-481X.2010.00234.x.
72. Bohac, M., et al., Maggot Therapy in Treatment of a Complex Hand Injury Complicated by Mycotic Infection. Bratislava Medical Journal, 2015. 116(11): pp. 671–673, https://doi.org/10.4149/bll_2015_128.
73. Sorensen, M.D. and J.N. Krieger, Fournier’s Gangrene: Epidemiology and Outcomes in the General US Population. Urologia Internationalis, 2016. 97(3): pp. 249–259, https://doi.org/10.1159/000445695.
74. Angel, K., et al., Madentherapie bei Fournierscher Gangrän — erste Erfahrungen mit einer neuen Therapie. Aktuelle Urologie, 2000. 31(07): pp. 440–443.
75. Dunn, C., U. Raghavan, and A.G. Pfleiderer, The Use of Maggots in Head and Neck Necrotizing Fasciitis. The Journal of Laryngology & Otology, 2002. 116(1): pp. 70–72, https://doi.org/10.1258/0022215021910212.
76. Preuss, S.F., M.J. Stenzel, and A. Esriti, The Successful Use of Maggots in Necrotizing Fasciitis of the Neck: A Case Report. Head & Neck, 2004. 26(8): pp. 747–750, https://doi.org/10.1002/hed.20092.
77. Steenvoorde, P., et al., Maggot Debridement Therapy in Necrotizing Fasciitis Reduces the Number of Surgical Debridements. Wounds, 2007. 19(3): pp. 73–78.
78. Fonseca‐Muñoz, A., et al., Clinical Study of Maggot therapy for Fournier’s Gangrene. International Wound Journal, 2020. 17(6): pp. 1642–1649, https://doi.org/10.1111/iwj.13444.
79. Berger, S., Tropical Skin Ulcers: Global Status. 2017, Los Angeles: Gideon Informatics, Inc.
80. Frimpong, M., et al., Paradoxical Reactions in Buruli Ulcer after Initiation of Antibiotic Therapy: Relationship to Bacterial Load. PLOS Neglected Tropical Diseases, 2019. 13(8): e0007689-e0007689, https://doi.org/10.1371/journal. pntd.0007689.
81. Eichelmann, K., et al., Leprosy. An update: Definition, Pathogenesis, Classification, Diagnosis, and Treatment. Actas Dermo-Sifiliograficas, 2013. 104(7): pp. 554–563, https://doi.org/10.1016/j.ad.2012.03.003.
82. Arrivillaga, J., J. Rodríguez, and M. Oviedo, Preliminary Evaluation of Maggot (Diptera: Calliphoridae) Therapy as a Potential Treatment for Leishmaniasis Ulcers. Biomédica, 2008. 28(2): pp. 305–310, https://doi.org/10.7705/ biomedica. v28i2.102.
83. Cruz-Saavedra, L., et al., The Effect of Lucilia sericata- and Sarconesiopsis magellanica-derived Larval Therapy on Leishmania panamensis. Acta Tropica, 2016. 164: pp. 280–289, https://doi.org/10.1016/j.actatropica.2016.09.020.
84. Polat, E., et al., Detection of Anti-leishmanial Effect of the Lucilia sericata Larval Secretions in vitro and in vivo on Leishmania tropica: First work. Experimental Parasitology, 2012. 132(2): pp. 129–134, https://doi.org/10.1016/j. exppara.2012.06.004.
85. Sanei-Dehkordi, A., et al., Anti Leishmania Activity of Lucilia sericata and Calliphora vicina Maggots in Laboratory Models. Experimental Parasitology, 2016. 170: pp. 59–65, https://doi.org/10.1016/j.exppara.2016.08.007.
86. Stadler, F., R.Z. Shaban, and P. Tatham, Maggot Debridement Therapy in Disaster Medicine. Prehospital and Disaster Medicine, 2016. 31(1): pp. 79–84, https://doi.org/10.1017/S1049023X15005427.
87. Čičková, H., M. Kozánek, and P. Takáč, Growth and Survival of Blowfly Lucilia sericata Larvae under Simulated Wound Conditions: Implications for Maggot Debridement Therapy. Medical and Veterinary Entomology, 2015. 29(4): pp. 416–424, https://doi.org/10.1111/mve.12135.
88. Stadler, F. and P. Tatham, Drone-assisted Medicinal Maggot Distribution in Compromised Healthcare Settings, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 383–402, https://doi.org/10.11647/OBP.0300.18.
89. Ayello, E.A., et al., Reexamining the Literature on Terminal Ulcers, SCALE, Skin Failure, and Unavoidable Pressure Injuries. Advances in Skin & Wound Care, 2019. 32(3): pp. 109–121, https://doi.org/10.1097/01. ASW.0000553112.55505.5f.
90. Steenvoorde, P., et al., Maggot Debridement Therapy in the Palliative Setting. American Journal of Hospice & Palliative Medicine, 2007. 24(4): pp. 308– 310, https://doi.org/10.1177/1049909107302300.
91. Brüggmann, D., H.R. Tinneberg, and M.T. Zygmunt, [Maggot Therapy in Gynecology]. Zentralblatt für Gynäkologie 2006. 128(5): pp. 261–265, https://doi.org/10.1055/s-2006-942121.
92. Wolters, U., et al., ASA Classification and Perioperative Variables as Predictors of Postoperative Outcome. British Journal of Anaesthesia, 1996. 77(2): Pp. 217–222, https://doi.org/10.1093/bja/77.2.217.
93. Peck, G.W. and B.C. Kirkup, Biocompatibility of Antimicrobials to Maggot Debridement Therapy: Medical Maggots Lucilia sericata (Diptera: Calliphoridae) Exhibit Tolerance to Clinical Maximum Doses of Antimicrobials. Journal of Medical Entomology, 2012. 49(5): pp. 1137–1143, https://doi.org/10.1603/ME12066.
94. Sherman, R.A., F.A. Wyle, and L. Thrupp, Effects of Seven Antibiotics on the Growth and Development of Phaenicia sericata (Diptera: Calliphoridae) Larvae. Journal of Medical Entomology, 1995. 32(5): pp. 646–649, https://doi.org/10.1093/ jmedent/32.5.646.
95. Felder, J.M., et al., Increasing the Options for Management of Large and Complex Chronic Wounds with a Scalable, Closed-system Dressing for Maggot Therapy. Journal of Burn Care & Research, 2012. 33(3): pp. e169-e175, https://doi. org/10.1097/BCR.0b013e318233570d.
96. Sherman, R.A., B. Khavari, and D. Werner, Effect of Hyperbaric Oxygen on the Growth and Development of Medicinal Maggots. Undersea and Hyperbaric Medicine, 2013. 40(5): pp. 377–380.
97. Teich, S. and R.A.M. Myers, Maggot Therapy for Severe Skin Infections. Southern Medical Journal, 1986. 79(9): pp. 1153–1155.
98. Rojo, S. and S. Geraghty, Hemophilia and Maggots: From Hospital Admission to Healed Wound. Ostomy Wound Management, 2004. 50(4): pp. 30, 32, 34.
99. Sherman, R.A. and M.R. Hetzler, Maggot Therapy for Wound Care in Austere Environments. Journal of Special Operations Medicine, 2017. 17(2): pp. 154–162.
100. Grassberger, M. and C. Reiter, Effect of Temperature on Lucilia sericata (Diptera: Calliphoridae) Development with Special Reference to the Isomegalen- and Isomorphen-diagram. Forensic Science International, 2001. 120(1): pp. 32–36, https://doi.org/10.1016/S0379-0738(01)00413-3.
101. Sherman, R.A., et al., Effects of Food Storage and Handling on Blow Fly (Lucilia sericata) Eggs and Larvae. Journal of Food Science, 2006. 71(3): pp. M117-M120, https://doi.org/10.1111/j.1365-2621.2006.tb15634.x.
102. Gallagher, M.B., S. Sandhu, and R. Kimsey, Variation in Developmental Time for Geographically Distinct Populations of the Common Green Bottle Fly, Lucilia sericata (Meigen). Journal of Forensic Sciences, 2010. 55(2): pp. 438–442, https:// doi.org/10.1111/j.1556-4029.2009.01285.x.
103. Richards, C.S. and M.H. Villet, Data Quality in Thermal Summation Development Models for Forensically Important Blowflies. Medical and Veterinary Entomology, 2009. 23(3): pp. 269–276, https://doi.org/10.1111/j.1365-2915.2009.00819.x.
104. Mirabzadeh, A., et al., Maggot Therapy for Wound Care in Iran: A Case Series of the First 28 Patients. Journal of Wound Care, 2017. 26(3): pp. 137–143, https://doi.org/10.12968/jowc.2017.26.3.137.
105. Lin, G., et al., Hard Times Call for Creative Solutions: Medical Improvisations at the Israel Defense Forces Field Hospital in Haiti. American Journal of Disaster Medicine, 2010. 5(3): pp. 188–192.

The cost of health care for ulceration and amputation in diabetes is estimated at between £962 million and £1 billion: which is approximately 0.8% to 0.9% of the National Health Service (NHS) budget for England. More than 90% of expenditure was related to ulceration, and 60% was for care in community, outpatient and primary settings. For inpatients it is suggested that ulceration was associated with a length of stay 8.04 days longer than that for diabetes admissions without ulceration. For clinicians it is vital to be able to distinguish between a diabetic foot ulcer and Charcot Neuroarthropathy.1
With escalating NHS spend on diabetic foot care, it is a clinical condition that needs more focused attention. Ulceration patients are at risk to develop deformity and ulceration due to neuropathy and loss of protective mechanism. Almost 50% of patients with a new diabetic foot ulcer had at least one admission within 6 months of their assessment, and those with severe ulcers are likely to have procedures involving foot care, revascularisation and amputation.2
Diabetic foot osteomyelitis (DFO) and Charcot neuroarthropathy (CN) are two common presenting conditions. It is important to bear in mind that presentation of both can be very similar and have some overlapping features, however it is very important to differentiate between the two because of the entirely different pathology and treatment.
DFO often has as its inital presenting sign as soft tissue infection, spreading to the underlying osseus structures. CN on the other hand is a non-infectious destructive process affecting the bones, joints, and soft tissues of the foot and ankle, characterized by inflammation in the earliest phase, and if it remains untreated, leading to deformity and ulceration.
The diagnosis is even more challenging in the early red, hot swollen foot (stage 0 Eichenholtz3) in which no radiographic changes or when there is late presentation with osseous changes. In its early stage it can easily be confused with cellulitis and later with osteomyelitis. The diagnostic tools required to differentiate the
two are:
Diagnostic Tools:
• Imaging
• Biopsy
History and clinical examination both are equally important to make the diagnosis. If the presentation is with an ‘angry’ looking foot with a background of recent infection, fever, systemica illness rather than a localised hot, red, swollen foot then the likelihood of infection is higher. Sometimes in a Charcot Neuroarthropathy there is history of minor trauma which triggers the condition. On clinical examination of a Charcot neuropathy, the foot will be warmer than the contralateral side at by least >2 degrees, however the swelling and oedema in Charcot will subside after 5 - 10 mins of foot elevation which is less likely with infection.
Charcot is most commonly seen in the midfoot and is associated with neuropathy and loss in sensation. Circulation is also generally maintained. Infection is commonly associated with ulceration.
Biochemical markers are a key investigative tool in the clinicians ‘toolbox’ to assist in elucidating the difference between these two conditions.
In Charcot neuroarthropathy there will be mild increase in inflammatory markers (WBC, CRP)

compared to osteomyelitis where they will increase markedly. Glycaemic control (HbA1c) can be poor in both conditions. Blood markers seldom allow confirmatory diagnosis.4
Imaging is a modality that when used appropriately in conjunction with clinical history, examination and other tests can allow the clinician to make a more accurate diagnosis.
Different imaging modalities have different roles and help in aiding the diagnosis. Base line imaging consists of weight bearing AP, lateral and oblique view plain radiographs of the foot.
Radiographs in early phases can be entirely normal in both conditions however in later stages destructive fragmentation seen can easily be confused with infective process.
MRI scans are relatively easily available, and this modality gives reliable information to help differentiate between Charcot and Osteomyelitis which will be missed by most plain radiographs.5 However, if oedema is the only finding then it may be difficult to differentiate between the two. Multiple joint involvement can be the clue for Charcot whereas osteomyelitis will cause localised signal changes. The presence of abscesses or sinus tracts are also more likely to be suggestive of infection.
The other imaging modality to diagnose Charcot is SPECT-CT in which there will be an increase uptake in all three phases in both conditions, however in cellulitis uptake will be predominantly in early blood flow. CT part of SPECT-CT also can help in the diagnosis subtle fractures which can mimic Charcot but may requires urgent treatment.6
WBC scans (white cell labelled scans) reliably help to distinguish Osteomyelitis from
Charcot. However, they are not available in every hospital and access to these scans can be relatively difficult.
Microbiology swabs of ulceration can be deceptive due to contaminant skin flora, Deep bone/ tissue sample through the separate entry wound gives valuable information by identifying the microbes and help in treatment with targeted antimicrobials.
Bone biopsy is another invasive procedure which can be done in the outpatient clinic to differentiate infection from non-infective process. Osteomyelitis will show inflammatory cell aggregates however in Charcot, Osteoclast cells are predominant. These procedures require the setup and skill set to conduct these tests.
Diagnosis is vital to treat and protect the diabetic foot and prevent the avoidable amputations. These similar presenting conditions but different treatment can be very challenging and it important to think and get the right diagnosis before you treat. Rarely both conditions can coexist…. So stop , think and then treat!
“Utilising
and
in making the important
distinction
these
Global
surgical and reconstructive wounds. Refer to the
References: 1. Greenwood JE, et al. Eplasty. 2016. 2. Damkat-Thomas L, et al.
2019. 3. Greenwood JE, et al. Burns Open. 2018. 4. Wagstaff MJD, et al. Burns Open. 2019. ® PolyNovo
PolyNovo Biomaterials Pty Ltd.
When

comes