
March 2024

Dr Janale Charlotte Beckford DPM
Foot and Podiatric Surgeon
Tampa, Florida, United States of America



March 2024

Dr Janale Charlotte Beckford DPM
Foot and Podiatric Surgeon
Tampa, Florida, United States of America

Chronic wounds pose significant treatment challenges and financial burdens. Bioactive Glass Wound Matrices (BGWM) have shown promising wound healing properties in preclinical and early clinical studies. In this case series, we evaluate the potential cost savings of using BGWM as a treatment for hard-to-heal DFUs and VLUs compared to prior failed treatment modalities. The patients with long-standing wounds (3 DFUs, 1 VLU) were treated with BGWM after at least one year of other unsuccessful treatments at a tertiary wound care center. Treatment costs before and during BBBGM therapy were compared. Across the four wounds, the average treatment cost decreased by 60% with the use of BGWM compared to prior advanced modalities. All wounds achieved closure with 4-13 BGWM applications over 7-20 weeks.
Chronic wounds affect 6.5 million people in the United States, with estimated treatment costs exceeding $25 billion annually.1 Diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs) account for a significant portion of this immense clinical and economic burden. About 25% of people with diabetes will develop a foot ulcer in their lifetime, 2 and up to 2% of the adult population suffers from VLUs. 3 These wounds are notoriously challenging to heal, often persisting for months or years despite standard treatments. The associated morbidity, mortality, and treatment costs dramatically impact patient quality of life and rapidly increase healthcare costs. Bioactive Glass Wound Matrix (BGWM) has emerged as a promising therapeutic option for complex wounds. 3-16 The innovative nature of these materials with potential angiogenesis stimulating properties, enhanced cellular proliferation and differentiation, and potential antibacterial properties that promote healing make this a viable option for advanced wound care. BGWM has shown encouraging results in the treatment of DFUs and VLUs. 3,4,10 In a prospective, randomized controlled trial, BGWM demonstrated a significantly greater mean wound size reduction compared to standard care in Wagner grade 1 or 2 DFUs. 3 The unique composition and structure of BGWM enables the sustained delivery of ions that stimulate healing, while also providing a resorbable scaffold for tissue ingrowth and to maintain a moist wound environment. 11 Given the substantial costs associated with hard-to-heal wounds, there is a pressing need for effective therapies that can reduce the treatment duration and expense. The purpose of this case series is to evaluate the potential cost
savings associated with the use of a BGWM in the treatment of chronic DFUs and VLUs that were previously unresponsive to conventional modalities. By comparing the estimated costs before and during therapy, we aim to provide preliminary insights into the economic benefits that this novel matrix may offer in managing these challenging wounds.
Three patients with a total of four chronic wounds were treated. The wound types included diabetic foot ulcers (DFUs) (n=3) and a venous leg ulcer (VLU) (n=1). All patients had received standard wound care for at least one year in a tertiary wound care center prior to presentation, with multiple failed treatment modalities. Upon presentation to the wound clinic, the patients were started on a regimen including BGWM. Informed consent was obtained from all participants. Costs prior to BGWM were estimated and compared to costs during BGWM therapy. Estimates were based on top tier pricing for a large integrated delivery network. Costs prior to BGWM comprised of dressings and debridement costs, and not skin graft or cellular and tissue-based product costs, even if those used prior to BGWM. Dressing types included in the estimate were silver alginate and gelling fiber, absorbent dressings, and collagen. Costs during BGWM therapy comprised the matrix and absorbent dressing, plus collagen dressings used between final BGWM application and wound closure. Wound debridement was performed as needed prior to BGWM use, but not after BGWM was initiated; therefore, debridement was not included in BGWM cost estimates. Estimates did not consider hospitalization, antibiotics, or pain
“Bioactive Glass Wound Matrix (BGWM) has emerged as a promising therapeutic option for complex wounds. 3-16 The innovative nature of these materials with potential angiogenesis stimulating properties, enhanced cellular proliferation and differentiation, and potential antibacterial properties that promote healing make this a viable option for advanced wound.”
medication costs, or reimbursement rates.
Results
Patient 1
Clinical Presentation
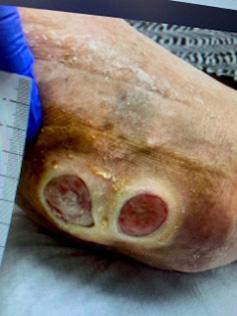
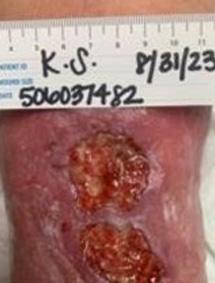
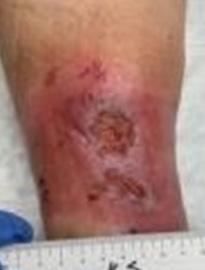
This was a 60-year-old female with two DFUs on the plantar aspect of her left foot present for 8 years without successful closure using numerous advanced therapies shown in Figure 1.
Costs
The costs calcualted prior to BGWM therapy was $89,568. The costs during BGWM therapy were $18,113, which was an 80% decrease from the cost prior to BGWM.
Time to Healing
The first DFU closed after 9 BGWM applications over 15 weeks; the second closed after 13 BGWM applications over 20 weeks.
Healing Progression
At baseline, the first DFU measured 5.4 cm2 and the second measured 2.7 cm2. After 15 weeks and 9 BGWM applications, the first DFU achieved complete closure. The second DFU closed after 20 weeks with 13 BGWM applications. The estimated pre-BGWM treatment costs were $89,568 over 8 years. The total costs during BGWM therapy were $18,113, representing an 80% cost reduction.
Results
Patient 2
Clinical Presentation
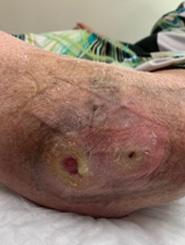
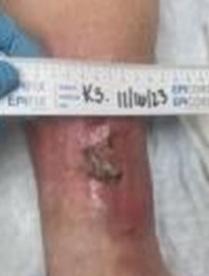
This patient was a 68-year-old male with a 1.5-year-old DFU on his right heel which was refractory to other treatment modalities shown in Figure 2.
Costs
The costs prior to BGWM were $19,080. The costs during BGWM therapy were $9,506, a 50% decrease. After 7 weeks of BGWM therapy with 7 applications, the ulcer achieved complete re-
epithelialization. The estimated pre-BGWM costs were $19,080, while the BGWM treatment costs totaled $9,506, a 50% decrease.
Time to Healing
The DFU closed after 7 BGWM applications over 7 weeks.
Healing Progression
The wound measured 4.1 cm2 upon presentation and had failed to close despite multiple treatment modalities.
Results
Patient 3
Clinical Presentation
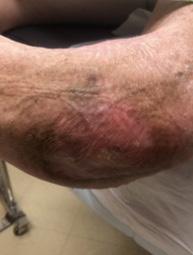
This patient was a 39-year-old female with a 1-year history of a recalcitrant VLU on her left medial malleolus which had been refractory to other advanced modalities.
Costs
The costs prior to BGWM was $10,816. The costs during BGWM therapy was $5,432, a 50% decrease.
Time to Healing
The VLU closed after 4 BGWM applications over 11 weeks.
Healing Progression
The wound size at baseline was 6.8 cm2 Following 11 weeks of treatment with 4 BGWM applications, the VLU closed completely.
“Given the substantial costs associated with hard-to-heal wounds, there is a pressing need for effective therapies that can reduce the treatment duration and expense. The purpose of this case series is to evaluate the potential cost savings associated with the use of a BGWM in the treatment of chronic DFUs and VLUs that were previously unresponsive to conventional modalities.”
“By facilitating healing of hard-to-heal wounds, BGWM reduced costs compared to prior treatment modalities. Patients reported reduced pain and improved quality of life during BGWM therapy. This case series demonstrates the potential for substantial cost savings when using a borate-based bioactive glass matrix in the treatment of hard-to-heal DFUs and VLUs.”
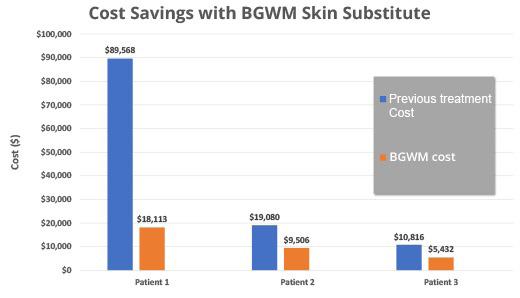
In aggregate, the average cost savings across the four wounds treated with BGWM was 60% compared to prior failed treatment modalities. The mean time to closure was 13.3 +/- 5.4 weeks, with an average of 8.3 +/3.9 BGWM applications required. Patients reported decreased pain and enhanced quality of life during the course of BGWM therapy. No adverse events or complications were noted.
“The exclusion of cellular tissue products and skin grafts from the cost analysis may underestimate the true expense of prior failed treatments. Despite these limitations, the substantial cost reductions and positive clinical outcomes observed across multiple wound types suggest that the BGWM merits further investigation as a cost-effective treatment for refractory DFUs and VLUs.”
By facilitating healing of hard-to-heal wounds, BGWM reduced costs compared to prior treatment modalities as shown in Table 1. Patients reported reduced pain and improved quality of life during BGWM therapy. This case series demonstrates the potential for substantial cost savings when using a borate-based bioactive glass matrix in the treatment of hardto-heal DFUs and VLUs. By promoting wound closure in these complex, chronic wounds, the BGWM resulted in a mean cost reduction of 60% compared to prior unsuccessful treatment modalities. The ability of this novel matrix to stimulate healing in wounds that had persisted for several months to years despite standard care is particularly noteworthy. The cost benefits observed in this study are likely attributable to the unique properties and mechanisms of action of the BGWM. The sustained release of bioactive ions from the borate-based glass fibers has been shown to stimulate angiogenesis, enhance fibroblast proliferation, and promote re-epithelialization. Additionally, the antibacterial effects of the released ions may help control bioburden and reduce the risk of infection-related complications. 11 By providing a comprehensive wound healing solution, the BGWM may decrease the need for multiple adjunctive therapies and prolonged treatment courses. Furthermore, the elimination of debridement procedures during BGWM therapy contributes to cost savings. The matrix itself may facilitate autolytic debridement through its moisturebalancing properties, obviating the need for frequent sharp or enzymatic debridement. 11 This reduction in debridement frequency not only decreases procedural costs but also minimizes patient discomfort and the risk of debridement-related complications. The positive impact of the BGWM on patient quality of life should not be overlooked. Chronic wounds are associated with significant pain, mobility limitations, and psychosocial distress. 12 By accelerating wound closure and reducing
the need for frequent dressing changes and clinic visits, the BGWM may alleviate these burdens and improve patient well-being. The reported decrease in pain and enhanced quality of life during BGWM treatment in this case series underscores the potential holistic benefits of this therapy. In this case series, the use of a borate-based bioactive glass matrix resulted in a 60% average cost reduction compared to prior failed therapies in the treatment of hard-to-heal DFUs and VLUs. The BGWM facilitated wound closure, reduced the need for debridement, and improved patient-reported outcomes. These findings highlight the potential economic and clinical benefits of this novel matrix for managing challenging chronic wounds. Future controlled studies with larger patient populations are necessary to validate these preliminary results and to further define the role of BGWM in cost-effective wound care algorithms.
Conclusion
The limitations of this study include its retrospective nature, small sample size, and lack of a randomized control group. The cost estimates were based on maximum allowable reimbursement rates within a specific healthcare system and may not be generalizable to all settings. Additionally, the exclusion of cellular tissue products and skin grafts from the cost analysis may underestimate the true expense of prior failed treatments. Despite these limitations, the substantial cost reductions and positive clinical outcomes observed across multiple wound types suggest that the BGWM merits further investigation as a cost-effective treatment for refractory DFUs and VLUs..
“Additionally, the antibacterial effects of the released ions may help control bioburden and reduce the risk of infection-related complications. 11 By providing a comprehensive wound healing solution, BGWM may decrease the need for multiple adjunctive therapies and prolonged treatment courses..”
1. Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught M, and Cartwright D. An Economic evaluation of the impact, cost, and medicare policy implications of chronic non-healing wounds. Value in Health, 21(1)
2.Armstrong DG, Boulton AJM,Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376(24):2367-2375.
3. Armstrong DG, Orgill DP, Galiano RD, et al. A multi‐centre, single‐blinded randomised controlled clinical trial evaluating the effect of resorbable glass fibre matrix in the treatment of diabetic foot ulcers. Int Wound J. 2022;19(4):791-801.
4. Jung S, Day T, Boone T, Buziak B, Omar A. Anti-biofilm activity of two novel, borate based, bioactive glass wound dressings. Biomed Glas. 2019;5(1):67-75.
5.Rahaman MN, Day DE, Sonny Bal B, et al. Bioactive glass in tissue engineering. Acta Biomater. 2011;7(6):2355-2373.
6. Sen CK. Human wounds and its burden: An updated compendium of estimates. Adv Wound Care (New Rochelle). 2019;8(2):39-48.
Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann Epidemiol. 2019;29:8-15.
7. Hench LL. The story of Bioglass®. J Mater Sci Mater Med. 2006;17(11):967
8. Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering. Acta Biomater. 2011;7(6):2355-2373.
9. Jung SB. Borate based bioactive glass scaffolds for hard and soft tissue engineering. In: Advances in Bioceramics and Porous Ceramics VI. John Wiley & Sons, Inc.; 2013:1-19.
10. Ottomeyer M, Mohammadkah A, Day D, Westenberg DJ. Broad-spectrum antibacterial characteristics of four novel borate-based bioactive glasses. Adv Microbiol. 2016;06(10):776-787.
11. Wray P. ‘Cotton candy’ that heals? Borate glass nanofibers look promising. Am Ceram Soc Bull. 2011;90(4):25-29.
12. Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019;27(1):114-125.*Mirragen® Advanced Wound Matrix, ETS Wound Care, Rolla, Missouri
13.Castillo-Garcia E, Thuy Nguyen P. Complex Refractory Wounds: How to Overcome Treatment Recalcitrance and Restore the Healing Trajectory Using Innovative Bioactive Glass. Wound Masterclass. Volume 3. March 2024.
14.Johnson ML, Armstrong DG, Ortega W. How Can Novel Bioactive Glass Wound Matrix Optimise Hard-to-Heal Venous Leg Ulcers in Geriatric Patients with Multiple Comorbidities? Wound Masterclass. Volume 3. March 2024.
15. Laurentin LA, Buck DW, Rivera D. Can Bioactive Glass Matrix be Used to Facilitate Pain Reduction and Healing for Patients with Pyoderma Gangerosum Ulcers? Wound Masterclass. Volume 3. March 2024.
16. Jung S, Schultz G, Mafiz AI, Bevels E, Jaskula K, Brownell K, Lantz E, Strickland A. Antimicrobial effects of a borate-based bioactive glass wound matrix on wound-relevant pathogens. J Wound Care. 2023 Dec 7;32(12):763. https://doi.org/10.12968/jowc.2023.32.12.763