
March 2024


March 2024
This case series evaluates the efficacy of a novel borate-based bioactive glass wound matrix (BGWM) for treating complex, refractory chronic wounds that failed to heal with multiple previous advanced therapies. Chronic non-healing wounds pose a significant health burden, and new treatment approaches are needed. BGWM is a biocompatible, water-soluble matrix designed to promote wound healing by enhancing angiogenesis, cellular proliferation, and reducing infection risk. Patients with longstanding surgical and radiation wounds of mean 13.2 month duration were treated with serial BGWM applications per a standardized protocol. Despite previously proving recalcitrant to other costly advanced wound care modalities, all three wounds achieved complete closure after a mean of 8.1 weeks of BGWM use. The mean initial wound volume of 1.9 cm3 across this cohort highlights the severity of the wounds treated. This small case series provides promising preliminary evidence that the BGWM represents an effective new therapeutic option capable of promoting healing in complex, chronic wounds refractory to other treatments.

Infectious Diseases and Wound Care Physician,
Wesley Chapel FL,

Introduction
The profound clinical and socioeconomic ramifications of chronic, non-healing wounds continue to escalate at an alarming rate, representing one of the most formidable challenges facing modern healthcare systems globally. It is estimated that chronic wound prevalence now afflicts over 6 million individuals in the United States alone, culminating in a heavy annual economic burden exceeding $50 billion in associated treatment costs.1,2 This fiscal strain is projected to intensify further as our population ages, with a concurrent rise in age-related comorbidities like diabetes mellitus, obesity, and cardiovascular disease that critically impair physiologic wound healing pathways.3 Implementing advanced, cost-effective wound care strategies that synergistically target the multifactorial molecular and cellular impediments underlying each phase of chronic wound pathogenesis is therefore imperative to promote timely and durable healing while reducing downstream healthcare utilization and expenditures.
An innovative bioactive glass fiber matrix* (BGWM) novel borate-based bioactive glass wound matrix (BGWM) , (Mirragen® Engineered Tissue Solutions, MO) composed of water-soluble borate compounds has recently emerged as a promising potential solution, demonstrating notable capabilities to facilitate healing in complex chronic wounds based on initial clinical experiences.4 This novel biomaterial represents a distinctly engineered class of biocompatible, biodegradable wound dressings designed to temporarily integrate
biomimetically support native wound repair processes over a finite resorption period.5 Extensive preclinical investigations have elucidated multiple physiologic mechanisms by which borate-based bioactive glasses may restore and augment chronic wound healing cascades, including stimulation of angiogenesis, enhancement of cellular metabolic activity, proliferation, and migration of key cell mediators like fibroblasts and keratinocytes, and intrinsic broad-spectrum antimicrobial properties that reduce bacterial burden.6,7,8 Collectively, these attributes position BGWM as a potentially powerful advanced wound care modality capable of overcoming the inherent molecular barriers that traditionally impede healing of chronic wounds. Herein, we present our preliminary clinical experience evaluating the safety, tolerability, and therapeutic efficacy of BGWM in treating a series of complex, recalcitrant chronic wounds that had proven refractory to a multitude of other advanced wound care strategies and costly interventions over an extended period.
Chronic wounds are detrimental to patient quality of life and a significant strain on the healthcare economy.1 To reduce healthcare utilization, use of advanced wound care products that address essential components of the healing phases of chronic wounds is critical. A recently developed novel boratebased bioactive glass wound matrix (BGWM) has been shown to promote healing in complex chronic wounds.2 Borate-based bioactive glasses are biocompatible water-soluble materials that have been formulated to degrade in a wound over a period of days or weeks. These bioactive glasses have shown promise in wound
“Steroids have wide-ranging metabolic effects that hinder key phases of healing— inflammation, proliferation, and remodelling. Determining appropriate candidacy for steroidal therapy requires assessing individual disease state, risk factors, and benefitrisk profile.”
healing by improving angiogenesis, increasing metabolic activity and cell proliferation, and reducing incidence of infection.3-5 We report our experience with application of BGWM in recalcitrant, non-healing wounds previously treated with multiple advanced wound care modalities.
Radiation exposure can significantly impair and delay the normal healing process of tissues and organs.6 When ionizing radiation interacts with cells, it can directly damage DNA structure as well as produce free radicals that attack cell membranes and intracellular proteins and enzymes. This cellular damage triggers programmed cell death (apoptosis) and impairs cellular reproduction.7 Additionally, radiation prompts inflammatory responses that can persist chronically. These effects inhibit the major phases of healing—inflammation, proliferation, and remodelling.
During the inflammatory phase, radiation diminishes the immune cells crucial for debris removal and fighting infection.8 Key growth factors and cytokines for new tissue formation are reduced while inflammatory factors like TGF-beta are elevated. In the proliferative phase, cell reproduction is constrained, slowing replacement of dead cells with viable ones.9 Angiogenesis is also hampered, decreasing oxygen and nutrient supply. Cells may persist in a prolonged state of metabolic stress. In remodelling, radiation causes decreased production and disorganization of the extracellular matrix while increasing stimulators of fibrosis like fibrocytes.9 The result is scar tissue formation rather than healthy replacement tissue.
The healing impairments from radiation are dose-dependent—higher exposures progressively inhibit healing capacity. The timing of radiation delivery can also influence effects, with continual exposures being more damaging than fractionated. Different
tissues display varying radiation sensitivity, related to their baseline regeneration ability and proportion of radiosensitive stem cells.10 However, the mechanisms of radiation-delayed healing are consistent across tissues. Effective strategies to mitigate radiation-impaired healing include limiting radiation dose and avoiding repeat exposures where feasible. Growth factors, stem cell therapies, oxygen radical scavengers, and pharmacologic anti-inflammatories hold promise to help kick-start the recovery process. Still the impact of radiation on healing should not be underestimated. Radiation protection principles and testing of regenerative biologics are key frontiers requiring further research and development. In healthcare, corticosteroids such as fluticasone, budesonide, hydrocortisone, and prednisone are commonly used for their anti-inflammatory and immunosuppressive properties. However, medicinal steroid use can significantly impair and delay the normal wound healing process.11 Steroids have wideranging metabolic effects that hinder key phases of healing—inflammation, proliferation, and remodelling. Determining appropriate candidacy for steroidal therapy requires assessing individual disease state, risk factors, and benefit-risk profile.
Steroids are a foundational treatment for acute phases of inflammatory bowel diseases like Crohn’s and ulcerative colitis. Oral steroids like prednisone offer rapid reprieve from flareup symptoms like abdominal pain, frequent loose stools, bleeding, and malnutrition. They induce remission in moderate or severely active IBD before transitioning patients to maintenance treatments. Caution is warranted in IBD patients with higher complication risks including osteoporosis, diabetes and glaucoma.12
Inhaled corticosteroids like fluticasone and
“Even minor wounds can demonstrate notable failure to progress through the overlapping healing stages. Strategies to restore healing progression include steroid tapering, switching to less potent steroids, and wound environment modification through moisture balance and debridement.”
budesonide are central to managing COPD symptoms and reducing exacerbation frequency. Oral steroids are deployed intermittently for COPD exacerbations to relieve acute respiratory distress without accentuating comorbidity risks associated with long-term oral steroid use. Contraindications include uncontrolled infections and avoidance in patients with significant untreated tuberculosis risk factors.13
Autoimmune disorders like rheumatoid arthritis, systemic lupus erythematosus, granulomatosis with polyangiitis and polymyalgia rheumatica are all responsive to steroids. Intramuscular or intraarticular steroid injections can provide localized efficacy. However, ubiquitous comorbidities and immune status in this population necessitates prudent consideration before sustained high dose systemic steroid administration. First line treatments are often steroid-sparing alternatives like DMARDS or biologics when feasible to minimize exposure.
In the inflammatory phase, steroids blunt the acute immune response needed to clear debris and prevent infection. They reduce key innate immune cells like macrophages and neutrophils and inhibit new vessel permeability. Steroids also lower growth factor and cytokine levels critical to signaling tissue regeneration while raising immunosuppressive factors like IL-10 and lipocortin-1.
In the proliferative phase, cellular reproduction slows under steroid influence, constraining replacement of damaged tissue. Steroids make cells less responsive to growth stimuli and more susceptible to apoptosis. Reduced angiogenesis also limits blood and nutrient supply to heal the wound area. Remodeling is impacted through decreased collagen production and impaired crosslinking, leading to weaker scar tissue formation. Matrix metalloproteinases break down scaffolding proteins faster than they can
be rebuilt.
The dose and duration of steroid exposure correlates with the severity of healing impairment—higher doses and longer usage most significantly delay healing capacity. The potency of the particular steroid also matters, as does administration route. Inhalational and topical steroids exert more localized effects on wounds than systemic treatment.
Wounds with greater baseline severity may be most vulnerable to further steroid-induced delay. However, the mechanisms depressing healing are consistent across wound types. Chronic ulcers and lacerations heal slowly anyway and are pushed to further stagnation. Even minor wounds can demonstrate notable failure to progress through the overlapping healing stages. Strategies to restore healing progression include steroid tapering, switching to less potent steroids, and wound environment modification through moisture balance and debridement. Growth factors may also aid cellular proliferation as may supplemental nutrition. Still, the multilevel impacts of steroids on recovery pathways should not be underestimated. Further research into therapeutics to counteract steroid-mediated healing retardation remains vital.
In summary, steroids remain a potent weapon against inflammatory states across varieties of conditions and patient profiles. Determining appropriateness requires carefully weighing their immunosuppressive effects against their anti-inflammatory benefits for each patient’s situation. The therapeutic index demands judicious dispensing to maximize relief while minimizing adverse events.
Methods
This prospective case series was conducted at our outpatient wound care center between January 2022 and August 2022. All patients
provided informed consent, and the study was approved by the institutional review board. Patients were eligible if they had a chronic wound that failed to achieve >40% wound area reduction after >4 weeks of appropriate treatment with advanced wound care products. Exclusion criteria included uncontrolled diabetes (HbA1c >9%), untreated malignancy, end-stage renal disease, active immunosuppression, and wounds of vascular or arterial etiology.
Three patients with a total of 3 chronic wounds meeting inclusion criteria were treated with serial applications of BGWM over the study period. All patients underwent initial wound preparation involving sharp debridement of necrotic tissue and cleansing with sterile saline solution. BGWM* was comprised of bioactive borate glass fibers suspended in an aqueous gel matrix (95% type 45S5 bioactive glass).
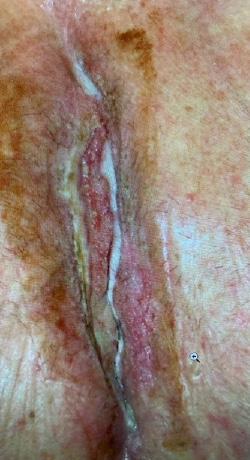
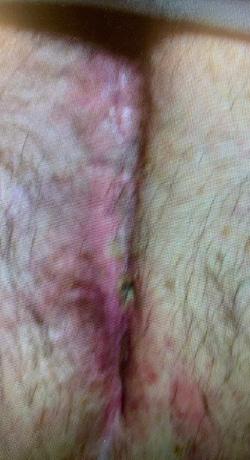
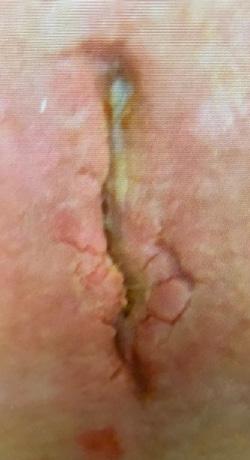
The mean age of the patients was 72 years (range 56-84 years). Two of the wounds were chronic, recalcitrant post-surgical wounds and one a chronic radiation wound. All three wounds in this series had failed healing despite multiple previous advanced wound care
To apply BGWM, the fibers were first spread evenly across the wound bed to fully contact and cover the entire wound area with a 3-4 mm overlap extending onto the peri-wound skin. An occlusive secondary dressing was applied over the BGWM to immobilize the matrix and absorb wound exudate. Dressings were changed once weekly during follow-up visits when the wound and surrounding skin were reassessed and the BGWM reapplied as needed until complete wound closure. Systemic antibiotics were prescribed based on clinical criteria for evidence of wound infection.
Wound surface area and volume measurements were obtained using standardized wound imaging and analysis software at each dressing change. Pain levels were self-reported by patients on a 10-point scale. Healing was defined as full re-epithelialization of the wound surface.
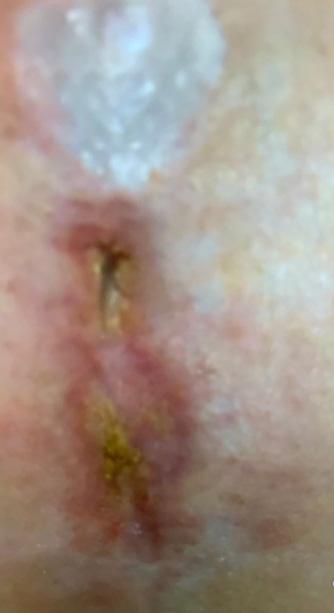


modalities including negative pressure wound therapy, bioengineered skin substitutes, and hyperbaric oxygen over a mean duration of 13.2 months (range 8-22 months). The mean initial wound size surface area and volume at the start of BGWM application were 12.7 cm2 (range 5.8-25.6 cm2) and 1.9 cm3 (range 0.8-4.1 cm3), respectively. Baseline characteristics are summarized in Table 1.
“Restoring adequate perfusion and oxygenation is critical for preventing oxidative stress, facilitating inflammatory cell influx, and supporting metabolic demands of wound healing pathways. Additionally, borate bioactive glass compositions have demonstrated potent anti-microbial and antibiofilm properties against a broad spectrum of bacterial pathogens commonly colonizing chronic wounds.”
All three wounds achieved complete closure after a mean duration of 8.1 weeks (range 5-11 weeks) with serial BGWM applications as the primary wound treatment. Figures 1-3 illustrate the sequence of healing in each case. [Insert figures showing wound healing progression]
Mean patient-reported pain scores decreased from 6.7/10 at baseline to 1.3/10 after two weeks of BGWM application. BGWM was well tolerated by all patients without any adverse events. Two wounds required a short course of oral antibiotics early in treatment for clinical signs of superficial infection prior to achieving closure.
Discussion
The results of this case series demonstrate the promising potential of the borate-based bioactive glass wound matrix (BGWM) as an effective new therapeutic modality for complex, chronic wound pathologies that have proven recalcitrant to other advanced treatment approaches. Despite the inherent challenges to wound healing posed by the advanced age and significant comorbidities afflicting the patients in this cohort, complete wound closure was ultimately achieved in all three cases after a mean of just 8.1 weeks of treatment with serial BGWM applications. This noteworthy outcome is rendered even more remarkable given that these non-healing wounds had persisted for a mean duration of 13.2 months and remained stubbornly refractory to multiple previous advanced wound care therapies and surgical interventions at considerable healthcare expense.
While the small sample size and lack of a control arm preclude definitive conclusions, these preliminary findings align with the
hypothesized wound healing capabilities ascribed to borate-based bioactive glass biomaterials based on robust preclinical data. The proposed mechanisms by which BGWM may have contributed to overcoming the chronic, non-healing state in these wounds involve strategic targeting of several key impediments underlying stalled wound repair processes. Firstly, the gradual biodegradation and ionic dissolution products released from the borate matrix have been shown to promote physiologic angiogenesis and neovascularization within the hypoxic, underperfused wound bed microenvironment.9 Restoring adequate perfusion and oxygenation is critical for preventing oxidative stress, facilitating inflammatory cell influx, and supporting metabolic demands of wound healing pathways. Additionally, borate bioactive glass compositions have demonstrated potent anti-microbial and antibiofilm properties against a broad spectrum of bacterial pathogens commonly colonizing chronic wounds. By reducing bacterial burden and virulence factors that perpetuate inflammatory cascades toxic to wound healing cells, BGWM may help resolve a pivotal barrier to constructive tissue repair.
Perhaps most crucially, borate bioactive glasses have exhibited a remarkable capacity to stimulate proliferation and activation of fibroblasts, keratinocytes, and other key cellular mediators that govern reconstitution of the extracellular matrix and epithelial resurfacing integral to wound closure. Mechanistic studies have revealed ionic dissolution products release therapeutic concentrations of metabolic enzymes cofactors like Si, Cu, and Zn that upregulate expression of growth factors like VEGF, bFGF, and TGF-β.12 This initiates a phenotypic shift in cells from a destructive, senescence associated state to a proliferative, anabolic phenotype primed for matrix remodeling and granulation tissue deposition. Taken together, the multifaceted ability of BGWM to simultaneously ameliorate
the ischemic, bacterial, and cellular/molecular barriers that have perpetuated the non-healing trajectory may have facilitated the successful wound closure observed in this challenging cohort. Looking ahead, it is imperative that results from this promising initial case series catalyze larger, rigorously-designed randomized controlled trials to definitively establish the clinical efficacy of BGWM compared to current standards of care and other advanced wound care products. Systematic cost-effectiveness and quality of life analyses will also be essential to evaluate the full socioeconomic impact and patient-centered outcomes associated with implementing this novel therapy into chronic wound management protocols. Only through judicious evidence-based validation can the full clinical potential of borate-based bioactive glass wound matrices be realized.
Considerable opportunities remain for continued innovation to further optimize composition, structural properties, and therapeutic delivery of bioactive glass wound matrices. For instance, rational design of hybrid biomaterial composites can impart complementary wound healing capabilities like soft tissue filler or guided tissue regenerative functions. Controlled-release drug delivery systems could be engineered into the resorbable glass matrix for localized administration of
1. Sen CK. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv Wound Care (New Rochelle). 2021;10(5):281-292.
2. Armstrong DG, Orgill DP, Galiano RD, et al. A multi-centre, single-blinded randomised controlled clinical trial evaluating the effect of resorbable glass fibre matrix in the treatment of diabetic foot ulcers. Int Wound J. 2022 May;19(4):791-801.
3. Jung S, Day T, Boone T, et al. Anti-biofilm activity of two novel, borate based, bioactive glass wound dressings. Biomed. Glasses 2019; 5:67–75.
4. Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering. Acta Biomater. 2011; 7(6): 2355–2373.
5. Mehrabi T, Mesgar AS, Mohammadi Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater Sci Eng. 2020;6(10):53995430.
6. Haubner, Frank, et al. “Wound Healing after Radiation Therapy: Review of the Literature.” Radiation Oncology, vol. 7, no. 1, 24 Sept. 2012, https://doi.org/10.1186/1748-717x-7-162.
7. Orlita-Kozar, Angelika, et al. “Biological Adaptations of Three-Cells to Radiation Therapy.” Frontiers in Oncology, vol. 11, 24 Nov. 2021, https://doi.org/10.3389/fonc.2021.718636.
8. Wunderlich, Roland, et al. “Ionizing Radiation Reduces the Capacity of Activated Macrophages to Induce T-Cell Proliferation, but Does Not Trigger Dendritic Cell-Mediated Non-Targeted Effects.” International Journal of Radiation Biology, vol. 95, no. 1, 21 Aug. 2018, pp. 33-43, https://doi.org/10.1080/09553002.2018.1490037.
9. Dormand, Emma-Louise, et al. “Radiotherapy and Wound Healing.” International Wound Journal, vol. 2, no. 2, June 2005, pp. 112–127, https://doi.org/10.1111/j.1742-4801.2005.00079.x
10. Ottomeyer, M., Mohammadkah, A., Day, D., & Westenberg, D. J. (2016). Broad-spectrum antibacterial characteristics of four novel borate-based bioactive glasses, Advances in
organic compounds like antimicrobials or biomolecules like growth factors and stem cells. As our understanding of chronic wound pathophysiology and the mechanisms by which bioactive glasses interact with wound environments grows, these next-generation wound dressings can be fine-tuned to present a personalized, diseased tailored therapy based on the distinctive wound characteristics and patient risk profile.
In conclusion, this preliminary case series provides compelling initial insights into the promising capacity of a borate-based bioactive glass wound matrix (BGWM) to overcome barriers to healing in complex, chronic wound pathologies that have remained refractory to other advanced treatment modalities. While inherent limitations preclude definitive conclusions, the ability to achieve full wound closure relatively rapidly in this cohort despite formidable challenges posed by patient age, comorbidities, and recalcitrance to previous therapies suggests BGWM may represent a powerful new tool in the wound care armamentarium. Larger, rigorously designed clinical trials are urgently warranted to comprehensively delineate the full therapeutic potential, patient outcomes, health economic impacts, and appropriate clinical utilization of this innovative biomaterial wound matrix therapy.
11. Wray, P. (2011). “Cotton candy” that heals? Borate glass nanofibers look promising. American Ceramic Society Bulletin, 90(4), 25-29.
12. Hu, S., Chang, J., Liu, M., & Ning, C. (2009). Study on antibacterial effect of 45S5 Bioglass®. Journal of Materials Science: Materials in Medicine, 20(1), 281-286. https://doi. org/10.1007/s10856-008-3564-5
13. Romano, C. L., Logoluso, N., Meani, E., Romanò, D., De Vecchi, E., Vassena, C., & Drago, L. (2014). A comparative study of the use of bioactive glass S53P4 and antibioticloaded calcium-based bone substitutes in the treatment of chronic osteomyelitis: a retrospective comparative study. The Bone & Joint Journal, 96-B(6), 845-850. https://doi. org/10.1302/0301-620X.96B6.33360
14. Lin, Y., Brown, R. F., Jung, S. B., & Day, D. E. (2014). Angiogenic effects of borate glass microfibers in a rodent model. Journal of Biomedical Materials Research Part A, 102(12), 44914499. https://doi.org/10.1002/jbm.a.35120
15. Zhao, S., Li, L., Wang, H., Zhang, Y., Cheng, X., Zhou, N., Rahaman, M. N., Liu, Z., Huang, W., & Zhang, C. (2015). Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials, 53, 379-391. https://doi.org/10.1016/j.biomaterials.2015.02.112
16. Jia, T. B., Fu, T., & Liu, J. (2019). The effects of copper-doped borosilicate bioactive glass on the proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Journal of Biomaterials Applications, 33(7), 1005-1018. https://doi. org/10.1177/0885328218815770