
March 2024


March 2024
This prospective study evaluates the efficacy of a novel borate-based bioactive glass wound matrix (BGWM) in the treatment of chronic, non-healing pyoderma gangrenosum (PG) ulcers. The primary objective is to assess the impact of BGWM on wound healing trajectories, pain management, and overall clinical outcomes in patients with refractory PG ulcers who have failed numerous prior treatments. By enrolling patients with longstanding, treatment-resistant PG lesions, the study aims to determine whether BGWM can overcome the challenges associated with this debilitating condition. A key secondary aim is to explore the potential mechanisms through which BGWM may facilitate wound healing in PG, including modulation of inflammation, promotion of angiogenesis, and stimulation of tissue regeneration. This investigation holds promise for identifying an effective therapeutic approach for a notoriously challenging disease process, with implications for improving quality of life for patients suffering from chronic, non-healing PG ulcers.



Pyoderma gangrenosum (PG) is a rare inflammatory skin disease, commonly associated with severe pain with significant morbidity for patients’ lives. PG is characterized by this specific, severe pain, tenderness, and the formation of necrolytic ulcers, significantly impacting patients’ quality of life. This condition poses a significant challenge in management, necessitating a delicate balance between addressing the underlying inflammation and pain while providing optimal wound care. Conventional therapies often target a broad spectrum of immunologic mediators and inflammatory cells implicated in the pathogenesis of PG, including cytokines, neutrophils, and lymphocytes.1 Despite advancements in our understanding of PG, many cases unfortunately still remain refractory to standard treatments, prompting the exploration of novel therapeutic approaches. Treatment is challenging and involves management of inflammation and pain, balanced with optimising wound care. Therapies typically target a broad spectrum of immunologic mediators and inflammatory cells shown to be involved in PG, including cytokines, neutrophils, and lymphocytes.1 A novel borate-based bioactive glass wound matrix (BGWM) (Mirragen® Engineered Tissue Solutions, MO) has been shown to promote healing in complex chronic wounds by improving angiogenesis, increasing metabolic activity and cell proliferation, and reducing bioburden.2-7 These bioactive glasses are biocompatible watersoluble materials, formulated to quickly degrade in a wound. 8-9 Recent studies have highlighted the potential of a borate-based bioactive glass wound matrix (BGWM) in promoting healing in complex chronic wounds by modulating various processes crucial for wound repair, including angiogenesis, cellular metabolic activity, cell proliferation, and bioburden reduction. These biocompatible, water-soluble bioactive glasses are formulated to rapidly integrate within the wound environment, releasing therapeutic ions that can potentially modulate the wound healing process.2-7
Signs
Symptoms of Pyoderma Gangrenosum: 1
• Painful, necrolytic skin ulcers
• Rapidly enlarging pustules or nodules
• Lesions can develop at sites of trauma/injury
• Fever, malaise, and other systemic symptoms
• Ulcers most commonly on the legs
• Severe pain and tenderness out of proportion to ulcer size
• Surrounding erythema and edema
Pyoderma gangrenosum (PG) is characterized by the rapid onset of painful, necrolytic ulcers. These ulcers possess a distinct clinical presentation, with a characteristic pathophysiological features of an undermined border and a violaceous hue. However, the underlying pathophysiology of PG remains enigmatic, further compounding the challenges associated with its management and wound reconstruction. PG is often a diagnosis of exclusion and is often reached at the end of the clinical differential diagnosis list.
While the exact etiology of PG remains elusive, it is believed to be a manifestation of dysregulated immune function, driven by a complex interplay of genetic, environmental, and immunological factors. PG is frequently associated with underlying systemic diseases, such as inflammatory bowel diseases (IBD), rheumatoid arthritis, and hematological malignancies, further complicating the clinical picture and treatment approaches. 10
PG is characterized by a self-perpetuating cycle of inflammation and tissue destruction, fueled by the infiltration of neutrophils and other inflammatory cells into the affected areas. This relentless inflammatory response leads to the progressive breakdown of skin and subcutaneous tissues, resulting in the formation of deep, undermined ulcers that are notoriously resistant to healing.
Wound reconstruction in PG presents a formidable challenge for clinicians and patients alike. The aggressive nature of the disease, coupled with the potential for pathergy (the phenomenon where trauma or injury can induce the formation of new lesions), often renders conventional wound management strategies ineffective.
The mainstay of PG treatment involves a multidisciplinary approach, combining systemic immunomodulatory therapies, such as corticosteroids, cyclosporine, and biologic agents, with meticulous wound care. However, this delicate balance between suppressing the underlying inflammation and promoting wound healing is often precarious, as excessive immunosuppression can impede the body’s natural healing processes. 11
To combat the challenges of wound reconstruction in PG, clinicians have explored various adjunctive wound care strategies, including negative pressure wound therapy (NPWT), hyperbaric oxygen therapy, and the use of advanced wound dressings and skin substitutes. While these approaches may facilitate wound healing in some cases, their efficacy remains variable, and their long-term success is often hampered by the recalcitrant nature of PG. 11
Given the immune-mediated nature of this condition, bioactive glass matrices should be considered as a viable treatment option.
Key Diagnostic Features: 1
• Rapidly enlarging, painful ulcer with violaceous border
• Pathergy phenomenon
• Cribriform or “wrinkled paper” peripheral pattern
• Exclusion of other causes like infection, malignancy, vascular disease
• Serosanguinous or purulent drainage
• Deeper ulcers may involve muscle, fascia, or even bone
• Associated with underlying systemic diseases
Recent years have witnessed a surge in research exploring novel therapeutic approaches for PG, driven by the urgent need for more effective and targeted interventions. These include the investigation of targeted biologic agents, stem cell therapies, and the use of innovative biomaterials engineered to modulate the wound healing environment.12
One such promising avenue is the exploration of bioactive glass-based wound matrices, which have demonstrated the ability to promote angiogenesis, reduce bioburden, and stimulate tissue regeneration in chronic wounds. These materials may hold the potential to address the multifaceted challenges of wound reconstruction in PG by modulating the inflammatory response, facilitating tissue repair, and providing a conducive environment for healing. 2-7

“The bioactive glass skin substitute matrix represents a remarkable advancement in the field of tissue regeneration, drawing upon decades of research and clinical application. Originally developed in the late 1960s by pioneering scientists, this bioactive glass material was engineered to facilitate bone-to-bone bonding and support healing processes, a revolutionary concept at the time.”
The primary aim of this pioneering study was to evaluate the efficacy of BGWM in the management of chronic, treatment-resistant PG ulcers.
Specifically, we sought to assess the impact of BGWM on:
• Wound healing trajectories
• Monitoring wound size reduction
• Monitoring wound depth
• Monitoring the emergence of granulation tissue
Additionally, we aimed to evaluate the potential of BGWM in alleviating the severe, debilitating pain associated with PG ulcers, which can profoundly impact patients’ quality of life. Furthermore, this study sought to explore the potential mechanisms through which BGWM may exert its therapeutic effects in the context of PG. We hypothesized that the unique properties of BGWM could modulate the dysregulated inflammatory response characteristic of PG, thereby facilitating wound healing. Moreover, we aimed to observe the potential of BGWM to promote angiogenesis and stimulate tissue regeneration, processes critical for the successful resolution of chronic, non-healing wounds.
The bioactive glass skin substitute matrix represents a remarkable advancement in the field of tissue regeneration, drawing upon decades of research and clinical application. Originally developed in the late 1960s by pioneering scientists, this unique material was engineered to facilitate bone-to-bone bonding and support healing processes, a revolutionary concept at the time.
Over the past five decades, its orthopedic applications have been rigorously tested and refined, establishing an exceptional safety
profile with no reported adverse events. Building upon this solid foundation, further investigative efforts have expanded the frontiers of bioactive glass technology, unlocking its potential for soft tissue repair and regeneration across a wide array of clinical domains. Notably, the MIRRAGEN® formulation has emerged as a promising solution for acute and chronic wound management, harnessing the material’s inherent properties to modulate the complex wound healing environment. Moreover, ongoing research continues to explore the use of bioactive glasses in nerve regeneration, dental materials, antimicrobial surfaces, bone grafting, and hemostatic devices, underscoring the vast therapeutic potential of this versatile class of biomaterials.6

“As we delve deeper into the intricacies of tissue repair and regenerative medicine, the bioactive glass skin substitute matrix stands as a testament to the synergy between scientific innovation and clinical translation, offering new avenues for enhancing patient outcomes and revolutionizing wound care practices.” 7
Methods
Study Design
This was a prospective, pilot study designed to evaluate the efficacy and safety of a novel borate-based bioactive glass wound matrix (BGWM) in the management of hard-to-heal pyoderma gangrenosum (PG) ulcers. Stringent inclusion criteria were applied to ensure participants met the diagnostic criteria for PG as outlined by the International Pyoderma Gangrenosum Network. 13
Additionally, all patients had failed to achieve satisfactory outcomes despite exhausting multiple conventional treatment modalities, including systemic immunosuppressive agents, biologic therapies, and advanced wound care strategies over an extended duration.

This study enrolled three patients presenting with a total of eight chronic PG ulcers that had proven refractory to numerous prior treatments over an extended duration. Patients were carefully screened to ensure they met the diagnostic criteria for PG 13 and had a failed course of conventional treatment modalities without achieving satisfactory outcomes. Three patients with eight different PG ulcers were treated.
Prior to BGWM application, the wounds were meticulously cleansed using a sterile wound cleansing solution to remove any debris, exudate, or necrotic tissue that could impede healing. The BGWM was then carefully shaped and contoured to fit the precise dimensions of each individual ulcer, ensuring adherence and contact with the entire wound surface. To secure the BGWM in place and manage exudate, a non-adherent dressing in combination with an alginate or a super-absorbent foam dressing with gauze was applied as a secondary dressing. The BGWM was reapplied on a weekly basis, with ongoing monitoring and assessment of wound progression, pain levels, and any potential adverse events.
“The application of BGWM yielded promising results in this cohort of patients with longstanding, treatment-refractory PG ulcers. Notably, all previously non-healing wounds in this series were returned to a healing trajectory following the introduction of BGWM therapy. The matrix was easy to apply and was used until complete wound closure was achieved for all ulcers.”
The application of BGWM yielded promising results in this cohort of patients with longstanding, treatment-refractory PG ulcers. Notably, all previously non-healing wounds in this series were returned to a healing trajectory following the introduction of BGWM therapy. The matrix was easy to apply and was used until complete wound closure was achieved for all ulcers. Patients tolerated the BGWM well, with no reported adverse events or complications. Remarkably, all patients reported significant reductions in pain levels associated with their PG ulcers, a testament to the potential analgesic properties of BGWM. Furthermore, no systemic antibiotic therapy was required during the course of treatment, suggesting a potential role for BGWM in managing wound bioburden and preventing secondary infections.
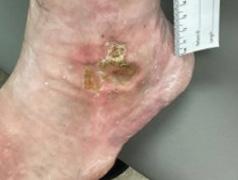
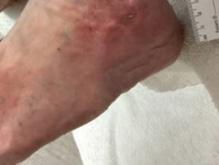
The progression of severe wounds after BGWM application was documented through this mini case series of images (Figures 2-4). One patient’s lower leg and foot wounds showed a marked improvement over a 4-week period, initially presenting as a painful, refractory PG that did not respond to treatment (Figure 2a). The wound showed signs of product integration (Figure 2b-c) before decreasing in size at 3 weeks (Figure 2c) and fully healing at 4 weeks (Figure 2d). Another patient with a partial necrolytic skin ulcer refractory to treatment (Figure 3a) demonstrated progressive healing at 1 week (3b) and 16 weeks (3c) postapplication. Additional patient cases showcased the effectiveness of BGWM in treating rapidly enlarging ulcers (Figure 4a-b), painful ulcers with deeper central defects (Figure 4c-d), interdigital PG extending from the dorsal wound space to the dorsum of the foot (Figure 4e-f), and lateral malleolar PG causing localized scarring, pain, and induration before and after treatment with BGWM is shown (Figure 4g-h).
The results of the application of the boratebased bioactive glass wound matrix (BGWM) in this prospective pilot study demonstrated promising results for the management of chronic, treatment-resistant pyoderma gangrenosum (PG) ulcers. The study enrolled three patients with a total of eight PG ulcers that had failed to respond to multiple conventional therapies over an extended period. Furthermore, the absence of systemic antibiotic therapy during the course of treatment highlights the potential role of BGWM in managing wound bioburden and preventing secondary infections.
The encouraging outcomes observed in this study suggest that BGWM may offer a promising therapeutic approach for managing chronic, treatment-refractory PG ulcers.
Figure 2a: Patient 1
This patient had refractory Pyoderma Gangrenosum which had not responded to treatment
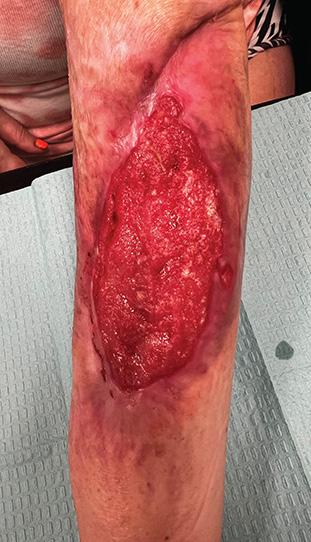
Figure 3a: Patient 2 Patient has a painful, necrolytic, skin ulcer refractory to treatment.
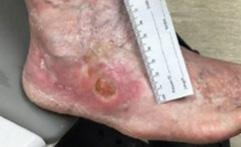
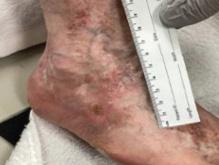
Figure 2b:
Post-application day 1 Wound shows signs of product integration
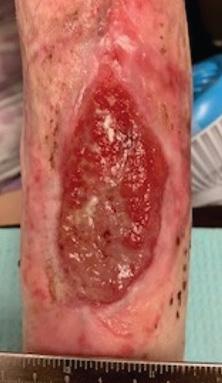
Figure 2c:
Post-application 3 weeks The defect on close monitoring has decreased in size
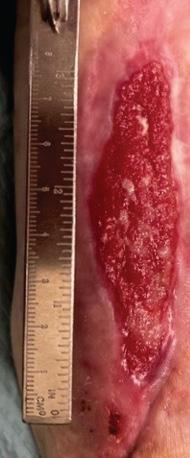
Figure 3b,c: Patient reviewed at standard post-application weekly intervals with appearances at 8 weeks (3b) and 16 weeks (3c) placeholder
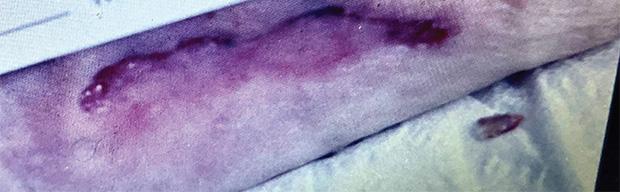
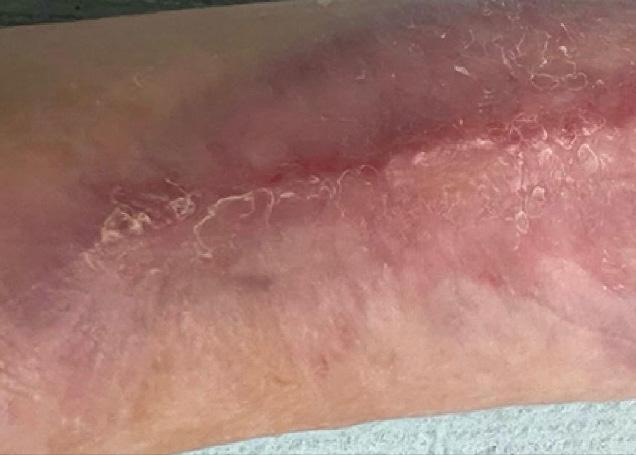

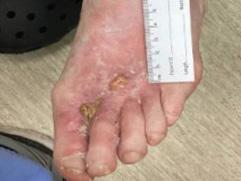


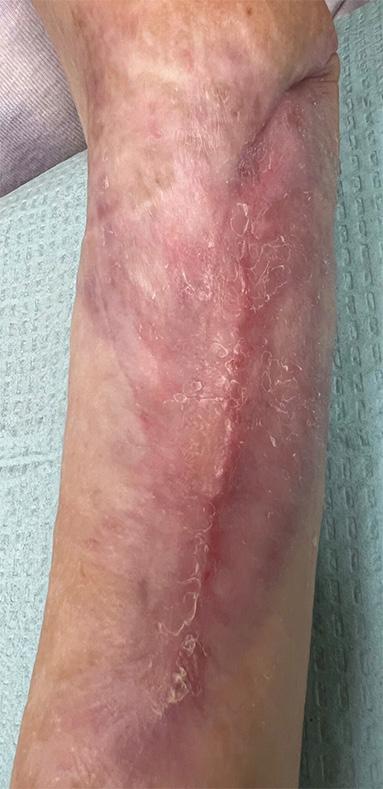
Figure 2d:
Post-application 4 weeks The wound has fully healed
“The application of BGWM yielded resulted in the successful healing of all previously non-healing wounds, with patients experiencing significant pain reduction and no adverse events. The matrix’s ease of application and its ability to promote wound clsoure without the need for systemic antibiotics highlight its potential as an adjunctive therapy in the complex management of PG.”
Pyoderma gangrenosum remains a formidable challenge in the realm of wound reconstruction and healing, necessitating a multidisciplinary approach that combines targeted immunomodulation with innovative wound care strategies. This prospective pilot study demonstrates the potential of a novel boratebased bioactive glass wound matrix (BGWM) in the management of chronic, treatment-resistant PG ulcers.
The application of BGWM resulted in the successful healing of all previously non-healing wounds, with patients experiencing significant pain reduction and no adverse events. The matrix’s ease of application and its ability to promote wound closure without the need for systemic antibiotics highlight its potential as an adjunctive therapy in the complex management of PG.
While these findings are encouraging, further research is needed to fully elucidate the mechanisms underlying the therapeutic effects of BGWM in PG. Future studies should aim to investigate the matrix’s impact on the dysregulated inflammatory response, angiogenesis, and tissue regeneration processes characteristic of PG. Additionally, larger, randomized controlled trials are warranted to validate these initial findings and assess the long-term efficacy and safety of BGWM in the management of PG ulcers.
As we continue to explore novel biomaterials and emerging technologies, the quest for more effective therapeutic interventions for PG remains a priority. The promising results of this study underscore the potential of BGWM as a valuable tool in the armamentarium of wound care specialists, offering new hope for patients suffering from this debilitating condition.
The relentless nature of the disease, coupled with the complex interplay of inflammatory processes and tissue destruction, necessitates a multidisciplinary approach that combines targeted immunomodulation with innovative wound care strategies. While progress has been made, the quest for more effective therapeutic interventions continues, fueled by the promise of novel biomaterials and emerging technologies that may unlock new avenues for managing this debilitating condition. We report our outcomes with use of BGWM in chronic PG ulcers that had failed numerous prior treatments over a prolonged duration.
1. Alavi A, French LE, Davis MD, Brassard A, Kirsner RS. Pyoderma gangrenosum: An update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017 Jun;18(3):355372. doi: 10.1007/s40257-017-0251-7. PMID: 28224502.
2. Castillo-Garcia E, Thuy Nguyen P. Complex Refractory Wounds: How to Overcome Treatment Recalcitrance and Restore the Healing Trajectory Using Innovative Bioactive Glass. Wound Masterclass. Volume 3. March 2024.
3. Johnson ML, Armstrong DG, Ortega W. How Can Novel Bioactive Glass Wound Matrix Optimise Hard-to-Heal Venous Leg Ulcers in Geriatric Patients with Multiple Comorbidities? Wound Masterclass. Volume 3. March 2024.
4. Laurentin LA, Buck DW, Rivera D. Can Bioactive Glass Matrix be Used to Facilitate Pain Reduction and Healing for Patients with Pyoderma Gangerosum Ulcers? Wound Masterclass. Volume 3. March 2024.
5. Armstrong DG, Orgill DP, Galiano RD, et al. A multi-centre, single-blinded randomised controlled clinical trial evaluating the effect of resorbable glass fibre matrix in the treatment of diabetic foot ulcers. IntWound J. 2021;1-11. doi:10.1111/iwj.13675
6. Jung S, Schultz G, Mafiz AI, Bevels E, Jaskula K, Brownell K, Lantz E, Strickland A. Antimicrobial effects of a borate-based bioactive glass wound matrix on wound-relevant pathogens. J Wound Care. 2023 Dec 7;32(12):763. https://doi.org/10.12968/jowc.2023.32.12.763
old 1. Milne J, Searle R, Styche T. The characteristics and impact of hard-to-heal wounds: results of a standardized survey. J Wound Care 2020;29:282-288.
7. Jung S, Day T, Boone T, et al. Anti-biofilm activity of two novel, borate based, bioactive glass wound dressings. Biomed. Glasses 2019; 5:67–75.
8. Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering. Acta Biomater. 2011; 7(6): 2355–2373.
9. Mehrabi T, Mesgar AS, Mohammadi Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater Sci Eng. 2020;6(10):53995430.
10. Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015 Oct;73(4):691-8. doi: 10.1016/j.jaad.2015.06.021. Epub 2015 Jul 10. PMID: 26253362. 11. Patel F, Fitzmaurice S, Duong C, He Y, Fergus J, Raychaudhuri SP, Garcia MS, Maverakis E. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015 Jul;95(5):525-31. doi: 10.2340/00015555-2008. PMID: 25387526.
12. Cañedo-Dorantes L, Cañedo-Ayala M, Coto-Preciado S, Beltrán-Rodríguez U, SeguraPeña R, García-Peña A, Hernández-Morales M, Serrano-Trasviña E, Ancer-Arellano J, RamosGaribay A. The Role of Stem Cells and Regenerative Medicine in the Treatment of Pyoderma Gangrenosum. Int J Mol Sci. 2021 May 24;22(11):5545. doi: 10.3390/ijms22115545. PMID: 34070460; PMCID: PMC8197129.