







Pierre Simonet founded Vygon in France in 1962. He was one of the first to introduce the concept of disposable medical equipment. Shortly thereafter, the company established subsidiaries in Europe, the United States, India, South America, and more. Today it is present worldwide. Vygon also relies on many production units, starting with its first plant in France and continuing on to its latest, brand-new plant that opened in Portugal in 2015.
Belgium
Czech Republic
Denmark
Finland
France
Germany
Ireland
Italy
Netherlands
Norway
Poland
Portugal
Spain
Sweden
Switzerland
Turkey
United Kingdom



Vygon, committed to its customers worldwide
Vygon is a company that designs, manufactures and markets a wide range of medical devices for 4000 customers worldwide. Its success is due to its teams, who are available and committed. They are at the service of caregivers to offer them products and solutions that improve the safety and performance of care. The company that invented the single-use medical device, a true revolution in hospital care practices, has built its offer on the complementary skills that make up its business: industrial and technological know-how, clinical knowledge and understanding of hospital structures, and its own resources in research and development. Today, Vygon is an international company but it also remains a family business. The stability and commitment of its shareholders allow for a sustainable development that brings projects to fruition and its value system is a guide.
This dedicated range of products aims to provide safe and adapted care to the smallest patients for the following treatments:
VENTILATION
Ventilation is needed when the baby has trouble breathing on its own. Invasive or non-invasive ventilation are used mainly for treatment of Respiratory Distress Syndrome (RDS).
The back of the throat, upper airways and endotracheal tubes of the patient may be obstructed by secretions such as mucus. Specific catheters, extractors and aspirators are designed by Vygon to solve each specific issue.
THORACIC DRAINAGE
Thoracic drainage is performed to evacuate liquid or gaseous effusions from pleural spaces. This operation requires adapted devices, to maximize the chances of success on the most vulnerable neonatal patients.
Vygon’s connectors are shaped to ease the link between every of our medical devices.
(2)
RDS is a breathing disorder that affects newborns, mainly premature babies.
RDS is caused by pulmonary immaturity and surfactant deficiency, resulting in respiratory in-sufficiency from soon after birth. (3)
Managing RDS remains a key component of neonatal intensive care. (3)
BREATHING SUPPORT WITH INVASIVE OR NON INVASIVE VENTILATION
• Continuous positive airway pressure (CPAP) can be used to cure RDS. It is considered as a less invasive ventilation.
• In more serious cases of RDS, a mechanical invasive ventilation may be needed to ensure life support to the premature patient.
CPAP or (s)NIPPV [synchronized Nasal Intermittent Positive Pressure Ventilation] should be started from birth in all babies at risk of RDS, such as those <30weeks of gestation who do not need intubation for stabilisation(3)
• Surfactant therapy improves survival and reduces pneumothorax and therefore plays an essential role in management of RDS
•LISA (Less Invasive Surfactant Administration) is a technique of delivering surfactant, while maintaining a noninvasive ventilation.
European data from 2014 to 2016 show that around 50% of all babies born between 22 and 32 weeks receive surfactant (3)
LISA is the preferred method of surfactant administration for spontaneously breathing babies on CPAP (3)
If a preterm baby <30 weeks of gestation requires intubation for stabilisation, they should be given surfactant (3)
• If surfactant is needed in case of an invasive ventilation, the use of ET tubes with lateral lumen may be required.
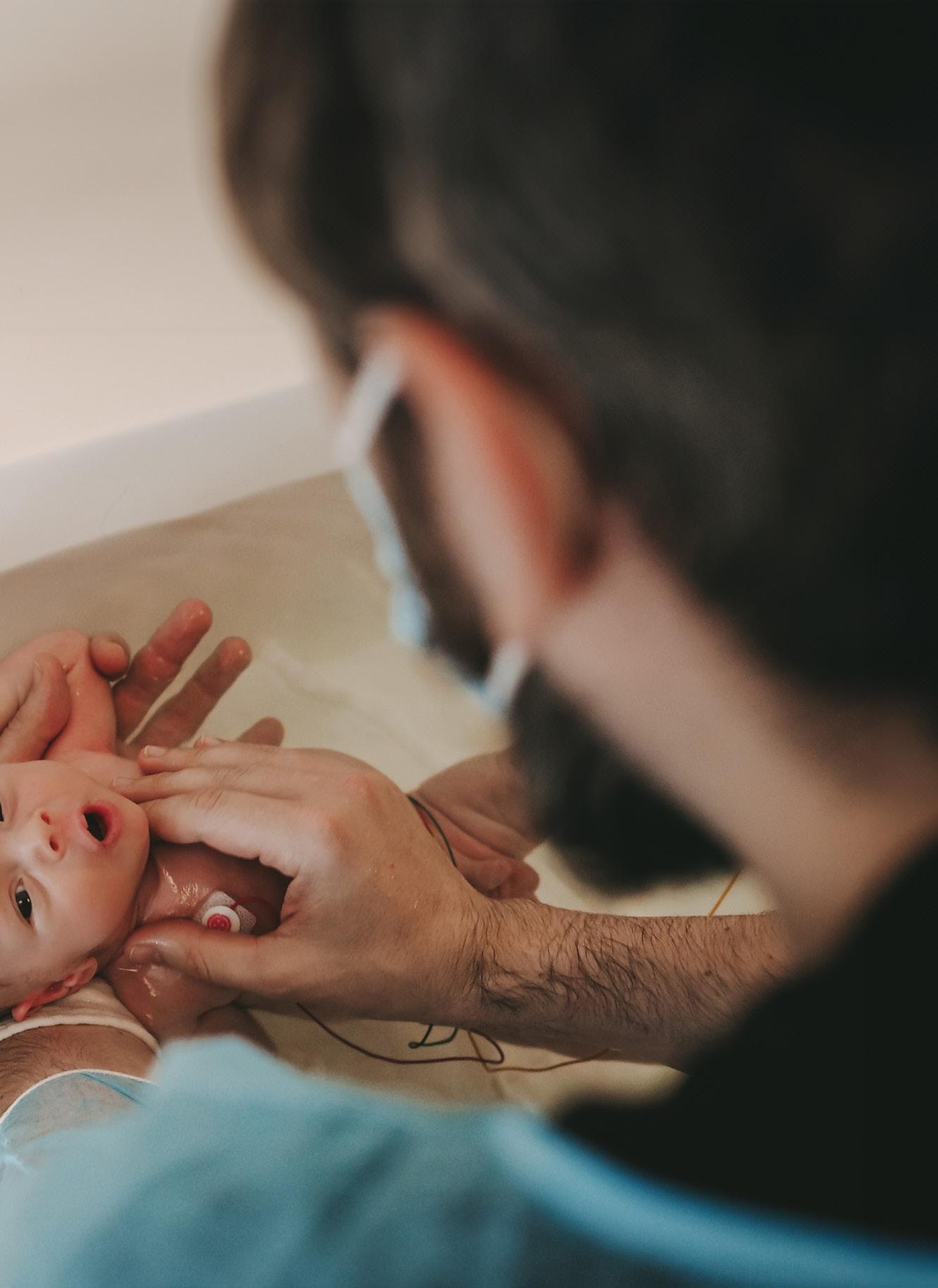
At birth the most vulnerable newborns need a trustful respiratory support to fight for their lives. Vygon offers a complete range of endotracheal tubes (ET tubes) for mechanical ventilation of the smallest patients.
The following ET tubes are designed to keep the airway open in order to provide air, oxygen, medicine or anesthesia.
Markings depending on the length and size of ET tubes
*Only for standard ET Tubes
Half-centimetre
15 mm adaptor
The ET tubes are intended to be used for oral or nasal intubation of the newborn. They are designed for ease of introduction in routine anaesthesia or emergency intubation. The soft green tubes (codes 520.xx) aim to minimise tracheal damage during long-term intubation.

15mm
Adaptor
Radiopaque
Made of PVC (DEHP <0.1%)
24mm black distal end mark, used as a mark of glottal depth
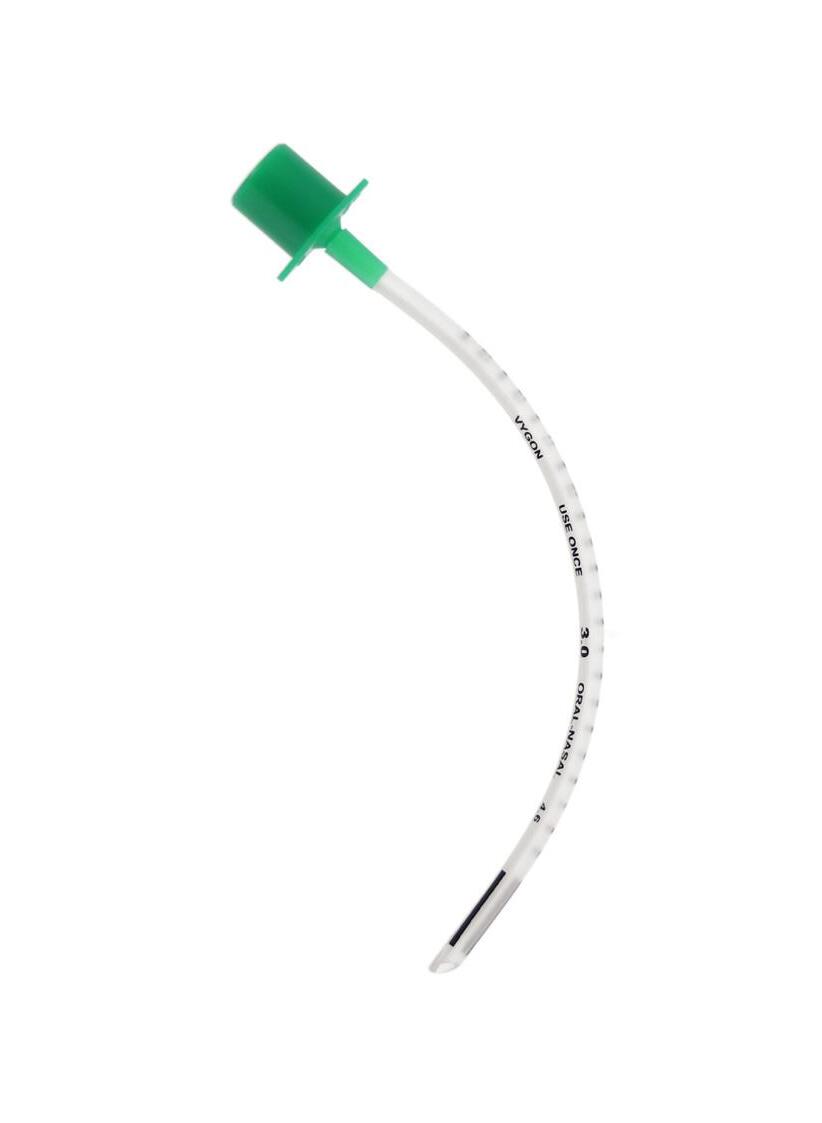
The secondary lumen is dedicated to surfactant administration or other medication, or for monitoring the airway pressure.

Code: 5520.30
15mm
Adaptor
Secondary lumen with female Luer-lock hub
Made of PVC (DEHP <0.1%)
Radiopaque
24 black distal end mark used as a mark of glottal depth
Tube distal end bevelled at 38°

Intubation introducers are used in situation where planned or unplanned intubation turns out to be difficult, and with patients presenting apnea.
The bO2ugie Boussignac is an orotracheal flexible and angled intubation guide used to position endotracheal tubes during difficult intubation.
It features two lumens:
- The first lumen (transparent) is closed at the proximal end and open at the distal end.
- The second lumen (pink) is open at the proximal end and closed at the distal end. An opening of the colored lumen, before the proximal end, allows passage of the oxygen.
This double lumen can be used to inject oxygen during guide placement. In the event of spasm, decompression openings prevent any risk of barotrauma by allowing the oxygen to escape via the side holes.

Supplied with taper connector to connect the device to oxygen source if needed
Compatible with 2,5 to 3,5 mm internal diameters endotracheal tube
Double lumen in case of oxygenation

Numerical marking every centimetre from 1 to 20
Curved with a 40° angle To follow the airway anatomy and ease the passage between vocal cords
Side holes for decompression allowing oxygen to escape and preventing any risk of barotrauma
Numerical marking every centimetre from 1 to 20
Evidence-based lung-protective management includes initiation of noninvasive respiratory support from birth […] and avoidance of intubation and mechanaical ventilation where possible (3)
‘‘
The single and double nasal tubes are intended to be used for neonatal non-invasive nasal continuous positive airway pressure (CPAP) ventilation via the respiratory system.
Non-invasive CPAP ventilation is a method of artificially delivering air or oxygen enriched air to a patient (neonate) to mimic breathing and restore or maintain a constant level of oxygen in the blood.
International recommendations are more and more in favour of a non-invasive respiratory support for neonates. Vygon offers different devices dedicated to non-invasive ventilation. 15mm

Code 2595.xx
Single nasal tube
Main indications of use:
Open and rounded distal tips

Code 2596.xx
Double nasal tube
• In case of moderate respiratory distress syndromes in neonates, mild hyaline membrane disease, temporary respiratory difficulties
• If recurrent apneas occur in premature babies
• When reduction of incubation time (replacement of the endotracheal tube)
The disposable face masks are intended to be used for
The product features are as follows:
• PVC Inflatable cushion
• 15 mm male connection
• Non-sterile

Co-invented with Dr Kribs from University Hospital of Cologne (Uniklinik Köln)
surfcath is a catheter for exogenous surfactant administration with Less Invasive Surfactant Administration (LISA) method, intended to be used for the treatment of newborns under non-invasive ventilation suffering from respiratory distress syndrome (RDS) caused by lung surfactant deficiency.
(3)
Preterm infants should be managed without mechanical ventilation where possible
LISA is the preferred mode of surfactant administration for spontaneously breathing babies on CPAP
Non invasive ventilation with early rescue surfactant by LISA technique is considered optimal management for babies with RDS
Surfactant therapy improves survival and […] plays an essential role in management of RDS
• 2 cm black distal tip: visual mark to address the inserted length
• 2 cm soft distal tip: Double softness to minimize tracheal lesion risks
• Blunt: to minimize tracheal lesion risk
• Angled: to follow the anatomy of the trachea and ease the passage between vocal cords

Transparent: to allow visualisation of surfactant

Made of PEBA: semi-rigid and bendable with no need of Magill forceps
Numerical marking every centimetre
Proximal female Luer-lock hub
Airway suctioning is a way of removing excess mucus from the back of the throat and upper airway by insertion of a catheter via the nose or mouth. In order to clean the secretions, an application of suction will be apply. (4)
Today, several issues are identified as causing too much mucus to collect in the airway. Clearing these may help the child to breathe better.
The airway suctioning is practiced when:
• There are signs of excessive secretions in the upper airway which cannot be cleared by coughing alone or,
• Secretions are causing respiratory distress and making breathing difficult(1)
Colour code for suction catheters with large connector references 70533, 70545 and 70565 according to ISO 8836.
The following catheters are used for naso-pharyngeal aspiration
Naso-pharyngeal suction catheter

with
The following catheters are used for tracheal and bronchial aspiration
Standard suction catheter

Code 533.06

Made of soft PVC
Open and rounded distal tip
Proximal end with a small funnel no colour-coded connector
Code 70565.05

Proximal end with a large funnel colour-coded connector


The mark at 16 cm from the distal tip for sizes 4 Fr to 8 Fr shows when the suction catheter has reached the bevelled tip of endotracheal tube codes 516, 520, 5516 and 5520.20/25/30/35.
Proximal connector with lateral eye for fingertip control (with integral closing cap)
Open distal tip with 2 lateral eyes

Made of PVC
Numerical marking every cm from 5 to 20
Proximal end: With large funnel connector and colour code

Made of PVC
Open and rounded distal tip with 1 lateral eye
The mucus extractors are intended to be used for aspiration and collection of mucus congesting the upper airways of the newborn or for suctioning tracheal / bronchial secretions (of new-borns, children, and adults) which will be analysed in laboratory.
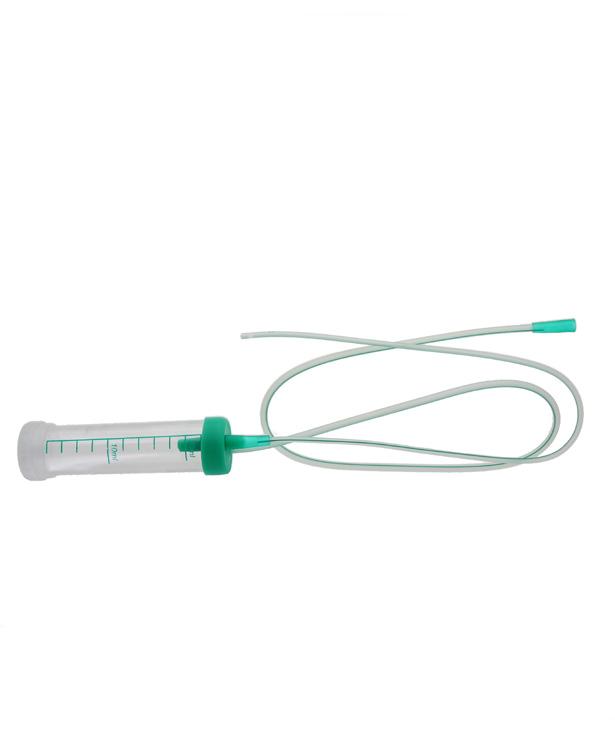
Small funnel connector

Small funnel connector
• 1 container (capacity 25ml), with graduations from 2 to 22 ml.
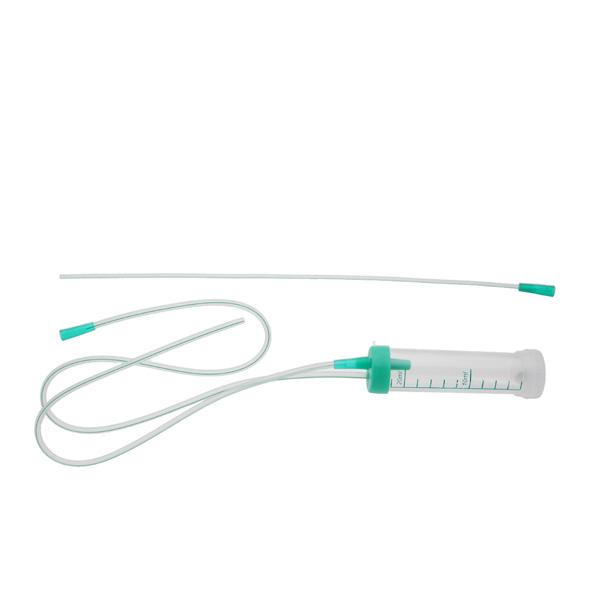
Large funnel connector
• 1 PVC extension tube with small or large funnel to connect the mucus extractor to the suction source
• 1 separate lid for safe sealing and transport of the aspirate
• 1 label
About the suction tubes :
• 534.08 has only 1 main tracheal suction tube (8 Fr)
• 534.10 and 534.101 each have 2 suction catheters:
- 1 main suction catheter connected to the container (10 Fr)
- 1 additional catheter (6 Fr), to replace the 10 Fr one if suction is carried out on a low-weight baby
The main difference between these two references is the size of their funnel connector.
Tracheal suction tube, made of PVC with open tip and no lateral eyes

Screw cap located at the bottom of the graduated container for safe sealing and transport of the aspirate
Transparent container with graduations from 0 to 30 mL, capacity of 35 mL
Container with graduations from 2 to 22 mL, capacity of 25 mL

Separate lid for safe sealing and transport of aspirate
PVC extension tube 23 cm long with connector for finger-tip suction control, to connect the device to the suction source (mechanical/wall suction)
This device is specially designed for meconium aspiration in case the baby has a meconium inhalation syndrome at birth.
This syndrome occurs during stress. The foetus inhales amniotic liquic which contains meconium, and the substance settles in his lungs. At birth, meconium can obstruct the airways and cause parts of the lungs to collapse. (5)
The meconium aspiration is done thanks to the connector with finger tip control of suction.
Green adaptor

Connector
1. Perfom a Laryngoscopy to visualize the vocal cords of the patient before the insertion of the device.
2. Place the meconium aspirator until it reaches the trachea.
3. Withdraw the laryngoscope and start the aspiration, by connecting the white connector to the hospital aspiration system.
4. Once the aspiration is done, cut the tube below the white connector with sterile scissors.
5. Then, connect the green adaptor.
6. Start the respiratory assistance of the patient by connecting the ventilation system to the green adaptor.
Thoracic trocars and drains are intended to be used for thoracic / pleural drainage in case of pneumothorax, haemothorax, chylothorax, pyothorax, pleural effusion...

Distal tip with a lateral eye
Made of transparent PVC
Numerical marking every cm

This complete tray is intended to be used for thoracic drainage. It contains all the instrumentation and accessories used during the insertion of a drain and allows to carry out this procedure under optimal aseptic conditions.

They include:
• 1 XRO trocar drain 8Fr code 625.08
• 1 dual suction valve with fitting connection
• 2 galipots (1 transparent, 1 white) for detergent and disinfectant agents
• 10 swabs (10 x 10 cm)
• 4 ball swabs
• 1 fenestrated absorbent drape (45 x 60 cm)
• 1 green scalpel
• 1 surgical gloves
• 2 blue prep forceps
• 2 luer slip syringes (10 ml)
• 1 pouch with connector for collecting the drained liquid (2 L)
• 1 extension line (6cm) for drainage pack
• 1 18G needle for drug aspiration (38 mm)
• 1 25G needle for skinwheal (15 mm)
• 1 21G needle for local anaesthesia (38 mm)
• 1 20G needle for exploratory puncture (38 mm)
• 2 suture needles (1 straight and 1 curved)
• 1m coil of non woven adhesive bandage, 7 cm wide
It allows either the double valve (code 668.00) or single valve (code 669.10) to be connected to the drain for pleural drainage (code 625) in neonates
These devices allow thoracic drainage without risk, even when transport of the patient is necessary. The non-return valve eliminates the need to clamp the drain. The valve opens when the patient breathes out, allowing the fluid or air to escape. When the patient inhales, the valve closes, preventing air from entering in the pleural space.
The valves can be connected to the drain indirectly, using connector code 800.01 (cf below p.27) and an extension tube for neonates and infants.
Dual valve for thoracic drainage
Natural rubber sleeve in “duck’s bill” shape acting as a non-return valve

Single valve for thoracic drainage, Heimlich type Flexible and transparent PUR

This two-chamber device (code 668.00) differs from the single valve (code 669.10) in that it is possible to apply suction by manual compression of the compartment marked with an arrow.
It allows either the double valve (code 668.00) or single valve (code 669.10) to be connected to the drain for pleural drainage (code 625) in neonates.

881.xx
Straight double taper connector and adaptor
881.00
Universal straight double taper connector

882.xx «T» connector

884.xx «Y» connector

CPAP: Continuous Positive Airway Pressure
ET: Endotracheal tube
LISA: Less Invasive Surfactant Administration
NIPPV: Nasal Intermittent Positive Pressure Ventilation
RDS: Respiratory Distress Syndrome
XRO: X-ray Radio-Opaque
Material
ABS: Acrylonitrile butadiene styrene
DEHP: Di(2-ethylhexyl) phtalate
EVA: Ethylene-Vinyl acetate
PA: Polyamide
PE: Polyethylene
PEBA: Polyether bloc amide
PP: Polypropylene
PUR: Polyurethane
PVC: Polyvynil chloride
Si: Silicone
(1) NIH National Heart, Lung and Blood Institute – March 24,2022 -https://www.nhlbi.nih.gov/health/respiratory-distresssyndrome
(2) John Hopkins Medicine - https://www.hopkinsmedicine.org/health/conditions-and-diseases/respiratory-distresssyndrome#:~:text=Respiratory%20distress%20syndrome%20(RDS)%20is,he%20or%20she%20is%20born
(3) Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, Te Pas A, Plavka R, Roehr CC, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GHA, Halliday HL. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology. 2023;120(1):3-23. doi: 10.1159/000528914. Epub 2023 Feb 15. PMID: 36863329; PMCID: PMC10064400.
(4) Paediatric suction (Yankeur/soft catheter – OP of NP) – Patient information-NHS Worcestershire Acute Hospitals NHS Trust.Approval Date : 01/09/2019 - https://www.worcsacute.nhs.uk/documents/documents/patient-informationleaflets-a-z/paediatric-suction-yankeur-soft-catheter-op-or-np/
(5) MSD Manual - https://www.msdmanuals.com/fr/accueil/probl%C3%A8mes-de-sant%C3%A9-infantiles/ probl%C3%A8mes-pulmonaires-et-respiratoires-chez-le-nouveau-n%C3%A9/syndrome-d-inhalation-m%C3%A9coniale