






The University of Rochester Clinical and Translational Science Institute (UR CTSI) helps research teams produce results better, faster and cheaper, with the ultimate goal of improving the health of communities and populations. We link researchers with the connections, resources and education they need for success. We are moving beyond the physical and virtual confines of institutions to conduct research without walls
The UR CTSI develops, demonstrates and disseminates methods and approaches to advance translational research by providing education and training, supporting transdisciplinary teams, improving quality and efficiency, and engaging community and national stakeholders.
The UR CTSI will be a replicable model environment for research across the translational spectrum, from molecules to populations, that is responsive to community priorities, conducted by transdisciplinary, patient- and community-engaged teams, and that improves population health.
The UR CTSI shares the University of Rochester Medical Center’s ICARE values: Integrity, Inclusion, Compassion, Accountability, Respect and Excellence. We believe that exceptional patient care starts with exceptional research.
The UR CTSI promotes research that is reproducible, community-engaged and compliant with rules and regulations.
The UR CTSI strives to create an inclusive and welcoming environment for all.
The UR CTSI manifests its compassion through our goal of improving population health and reducing health disparities in our communities.
The UR CTSI meets the commitments it makes to funders and the community. We are accountable to the University, funding agencies, and local and global communities, for whom we provide research and education programs. We are committed to developing metrics of accountability consistent with these values.
The UR CTSI actively solicits community input to guide its work and acts upon that input. We respect the differing values of the communities we serve: students, researchers, patients and community members. We are committed to the value of team science, which includes fostering a culture of transdisciplinary respect and active engagement with scientists of every background and perspective.
The UR CTSI strives to be a national model for excellence in translational science and research education programs. We are dedicated to training and supporting researchers to enable them to achieve the highest quality of work in their fields. We are committed to excellence in translational research at a national level, balancing efficiency, integrity and timeliness.
Over the past year, we finally began to emerge from the moment-to-moment crises of the COVID pandemic. With infection and death rates falling nationally, and a toolbox full of COVID treatments, highly effective vaccines and widely available testing, we could breathe a little easier and begin to assess our pandemic-related triumphs and failings.
The ability to quickly implement large national clinical trials was one of the nation’s greatest triumphs during the pandemic. These trials, which were no easy feat to run, allowed us to test the safety and efficacy of treatments and vaccines faster than ever before. That speed undoubtedly saved many lives – and we need to carry that momentum forward.
Each year, the UR CTSI’s Office of Clinical Research evaluates several hundred multi-site clinical trial opportunities for the Medical Center and provides vital infrastructure to help research teams manage the trials we undertake. In the past year, the office has seen incredible growth under the leadership of Nicole Mason, M.S., who was hired as the director of the office in August of 2021, and Karen Wilson, M.D., who serves as faculty strategic advisor.
After adding several new staff to her team, Mason has deftly driven the implementation of two new service lines to help clinical researchers determine the feasibility of their studies and manage research finances. Performing more cutting-edge clinical research is an institutional priority for URMC, and the Office of Clinical Research provides the infrastructure and support to make that possible.
The pandemic also laid bare the enduring health inequities in the U.S., with Black/African Americans dying at much higher rates than white Americans. This prompted an acute awareness of the need for health equity research across the nation.
In June of 2020, URMC acknowledged that need in its Equity and Antiracism Action Plan. The fifth pillar of the plan called for creating an office to foster health equity research across the Medical Center – a task which was entrusted to the UR CTSI. Earlier this year, after a nearly year-long search, we named a founding director for the new Office of Health Equity Research. Edith M. Williams, Ph.D., a Rochester native with nearly two decades of health equity research experience, joined the UR CTSI in September and immediately got to work meeting with key stakeholders across the university and local community to understand their health equity needs.
The Office of Clinical Research and Office of Health Equity Research are both shining examples of the UR CTSI’s unique role as trusted stewards of institution-wide resources. Because of our reach across the Medical Center and our ability to bring people together, URMC leaders have repeatedly called upon us to stand up programs that support high-level Medical Center priorities and help them reach sustainability. We are mindful of that role of stewardship and aim to continue to serve the research community and support institutional priorities far into the future.
 Martin Zand, M.D., Ph.D., and Nancy Bennett, M.D., M.S., Co-Directors of the UR CTSI
Martin Zand, M.D., Ph.D., and Nancy Bennett, M.D., M.S., Co-Directors of the UR CTSI
The UR CTSI is committed to offering education and fostering research that ensures all people have the opportunity to achieve their full health potential. Our newly established Office of Health Equity Research aims to go beyond documenting health inequities to develop and effectively disseminate interventions that ensure health equity.

Edith M. Williams, Ph.D., was named the founding director of the UR CTSI’s new Office of Health Equity Research in June of 2022, following a comprehensive search. A Rochester native with nearly two decades of health equity research experience, Williams was a perfect choice to lead this crucial component of the University of Rochester Medical Center’s Equity and Anti-Racism Action Plan.
“The Office of Health Equity Research will provide foundational science to inform URMC’s commitment to health equity,” said Medical Center CEO Mark Taubman, M.D. “Edith Williams brings a rich combination of research expertise, institutional experience and local knowledge that will enable her to structure this new office for success, and ultimately to elevate the role of science in achieving health equity, locally and nationally.”
Edith Williams, Ph.D., (right) working with biostatistician Sanjukta Bandyopadhyay, M.S., (left). Photo credit: John Schlia.The Office of Health Equity Research will provide foundational science to inform URMC’s commitment to health equity.
-Mark Taubman, M.D.
“Dr. Williams has the perfect constellation of research, leadership and mentorship experiences to lead this important effort at URMC,” said UR CTSI Co-Director Nancy M. Bennett, M.D., who chaired the search committee. “She has a long history of helping other researchers and community partners advance their own health equity efforts that I’m excited to see her continue here.”
Williams began her journey with health equity research while earning a doctorate in Epidemiology and Community Health at the State University of New York at Buffalo. Having grown up in the comforts of suburban Henrietta, she was shocked by the inequities she noticed in Buffalo. Her master’s thesis highlighted the lack of access to fresh, healthy foods in communities with mostly Black residents and helped obtain a grant to set up a community garden and to distribute food from the garden to local residents and food pantries.
For her doctoral research, Williams shifted her focus to lupus, a chronic autoimmune disease that disproportionately impacts Black women. Her community-based participatory research in this area included lengthy interviews with patients that moved her to devote her research career to understanding this disease and improving patients’ lives.

And she went the extra mile for those patients – literally. As a faculty member at the Medical University of South Carolina, Williams drove all over South Carolina to collect specimens from lupus patients to reduce the burden of participating in her study.
She is also accustomed to working with many partners and stakeholders across sectors and disciplines. Working with partners at historically Black colleges and universities, she developed a pipeline program to mentor students at those institutions and to provide them a path into the Medical University of South Carolina’s Public Health program.
“As URMC strives to become a leader in health equity research, Dr. Williams’ deep expertise in this area and demonstrated ability to bring researchers and community members together and to advance their goals is exactly what we need,” said Senior Associate Dean for Equity and Inclusion Adrienne Morgan, Ph.D., who co-chaired the search committee.
A key piece of the fifth pillar of the URMC Equity and Anti-Racism Action Plan, the goal of the Office of Health Equity Research is to increase capacity to conduct health equity research across the Medical Center. The office,
with Williams at the helm, will bring people together across research and clinical enterprises and the broader community to develop a learning health system – integrating community-engaged research to inform and improve equitable care for all.
“As a Rochester native, I am overjoyed to be returning home to lead an effort focused on synergizing innovative, transdisciplinary, and impactful research to improve the health of the most vulnerable residents of Rochester and beyond,” Williams said. “It’s time to move beyond simply documenting health disparities toward ensuring health equity through intervention, implementation, dissemination, and translational science.”
Dr. Williams has the perfect constellation of research, leadership and mentorship experiences to lead this important effort at URMC.
-Nancy M. Bennett, M.D.In her new post, Williams serves as a central resource for all URMC departments, maintaining strong connections to help them develop their own health equity research projects and to encourage that all research be viewed through a health equity lens. When she took up her new post in September of 2022, she immediately began a listening tour to inventory ongoing health equity research across the Medical Center and to understand researchers’ and community members’ needs.
Scientists know shockingly little about the general health and well-being of Deaf or DeafBlind people – a group that has been historically overlooked, excluded and underserved by healthcare and health research. A new survey tool developed by Deaf researchers at the UR CTSI aims to turn that around by helping scientists create surveys that are accessible in American Sign Language (ASL) or other primary languages used by Deaf and DeafBlind people.
Using the module, survey makers can also associate multiple videos for each survey item, enabling each item to be presented in different sign language modalities. For example, a survey taker could choose a video that shows the survey question in ASL, or in Signed English, allowing them to select the modality that best matches their communication preferences. These features not only improve accessibility and user experience, they also enhance the accuracy and reliability of the response data.
“We were thrilled to work with the NCDHR team on this initiative to fill an important need, empowering both researchers and research participants,” said REDCap creator Paul Harris, Ph.D., professor of Biomedical Informatics, Biomedical Engineering and Biostatistics at Vanderbilt University Medical Center. “The Rochester team has decades of experience working with Deaf and DeafBlind communities, so we were grateful for the opportunity to collaborate.”
The tool, called the Rochester Accessibility Survey, was developed by researchers in the UR CTSI’s National Center for Deaf Health Research (NCDHR), which has been conducting surveys in ASL since 2004 – with great effort and clunky, expensive systems. Wanting to make the process easier, NCDHR researchers partnered with the Vanderbilt Institute for Clinical and Translational Research, home of the REDCap online survey application.
With Deaf and DeafBlind communities in mind, the two institutions created an external module for REDCap that makes it easy to embed sign language videos in each survey question and each answer. This allows participants to take the survey in their primary language without having to hold a question and its possible answers in memory before making a selection.
That was a primary concern for Jenna Stewardson, who led the development of the new module. When they and their colleagues at the NCDHR used REDCap to produce a very large and complicated ASL survey in 2018, they recognized several ways the process could be improved for both survey makers and takers.

“Participants had a difficult time reviewing the videos to make sure they were choosing the right answer, especially when a question had a large list of options,” said Stewardson. “We wanted to break down the survey further and embed a video for each question and answer option so the participant can click on a single item and a video will appear that presents that specific item instead of the whole set of question and answer options.”
Stewardson worked closely with Harris’ REDCap Data Core team at Vanderbilt to make that happen. The NCDHR and Vanderbilt teams worked together to build and test the new module, adding features like the ability to adjust text size and color as well as background color to improve readability, and using a responsive design to ensure videos display optimally no matter what device (phone, desktop, tablet) a survey participant prefers to use.
The Rochester team has decades of experience working with Deaf and DeafBlind communities, so we were grateful for the opportunity to collaborate.”
-Paul Harris, Ph.D.REDCap provides user-friendly tools for survey makers, and the Rochester Accessibility Survey external module extends that ease-of-use to features that enhance the ability to produce and conduct surveys in ASL to an extent that has never been achieved before. While it has often been prohibitively expensive and complicated to create surveys in sign language, now it can be done with a small budget and without any special expertise in web or application development.
Stewardson hopes the Rochester Accessibility Survey will help others make surveys that are accessible for use with Deaf populations and explore the potential broader applications to reach other historically marginalized communities.
From dedicated clinical research space and staff to industry-leading clinical trial management tools, the UR CTSI is here to help researchers conduct clinical trials and other health research studies. We connect researchers with clinical trial opportunities and staff to support their studies, and help them attract and keep study participants, and navigate regulatory hurdles.
The UR CTSI’s Office of Clinical Research has seen a lot of change in the past year. With new leadership and a growing staff, the office is adding new services to help researchers manage various aspects of their clinical trials. From educational opportunities to study feasibility and financial services, the Office of Clinical Research is helping clinical researchers across the Medical Center take their studies to the next level.
In August of 2021, Nicole Mason, M.S., joined the Office of Clinical Research as its new director, bringing with her over 15 years of research experience and an extensive professional background in clinical care, research and regulations. While conducting a listening tour to ensure the office was fully supporting the clinical research community’s needs, she got right to work filling vacancies on the office’s staff and launching two service lines to help clinical researchers determine the feasibility of their studies and manage research finances.
There are a lot of things the Office of Clinical Research – and the University –already does really well. I want to use that as a spring board for growth in a way that is responsive to the community’s needs.
-Nicole Mason, M.S.“There are a lot of things the Office of Clinical Research –and the University – already does really well,” said Mason. “I want to use that as a spring board for growth in a way that is responsive to the community’s needs.”
The Study Feasibility service line helps clinical research teams determine whether their proposed studies can succeed with existing resources (finances, staff, investigators, space, equipment and patient population) and on the proposed timeline. Research teams can also get help developing meaningful measures of success for their studies and finding patients that meet their study criteria. Through its various industry connections, the Study Feasibility service line also connects researchers with multisite clinical trial opportunities, offering 339 such opportunities in the 2021-2022 fiscal year.


The Research Finance service line helps teams manage their clinical study finances from start to finish – freeing them up to focus their efforts elsewhere. More than half of the departments at the Medical Center that run clinical trials have taken advantage of the office’s financial services – and the return on investment is substantial. In the 2021-2022 fiscal year, the service line negotiated an average of $37,500 extra into each research study budget, ensuring each study had the resources it needed to succeed. Cumulatively, that amounts to an extra $375,000 over the year to test new treatments and advance our understanding of health.
Laurie Passalacqua, M.S., the director of Finance and Administration for Pediatric Adolescent Medicine, Pediatric Endocrinology and Pediatric Infectious Diseases, says the benefits of using the Office of Clinical Research’s financial services have been huge. “We are actually recouping funds for what we budgeted and negotiated.
By using OCR, we know we’re getting our startup costs paid for. We know we’re getting reimbursed for our storage costs. We know that we’re billing out for extraneous procedures that may have been done in the past,” she said.
The Office of Clinical Research has also stepped up its education and training game – supporting unlimited institutional attendance of the Advarra Onsemble conference for the first time in the fall of 2021. The conference, which is held biannually in the spring and fall, covers a wide range of topics related to conducting clinical trials, including leveraging technologies to improve the conduct of clinical research. In the fall of 2021, the office supported conference attendance of 38 University of Rochester staff, faculty or trainees. The following spring, that number nearly doubled to 60 attendees from the University.
The office is also improving its training and services related to the OnCore clinical trial management system to better meet users’ needs. The training team is focusing on creating more remote demonstration and hands-on training opportunities after 78 percent of respondents to a recent survey said they prefer these training methods.
The UR CTSI’s support for clinical research goes well beyond the Office of Clinical Research. The fast-growing URMC Research Registry and our robust regulatory support are just two examples.
URMC Research Registry Participants
Nearly 14,000 community members have signed up to be informed about clinical trials through our research registry since it launched a decade ago. The registry provides community members with opportunities to participate in research and potentially receive experimental treatments, while easing the task of recruiting trial participants for clinical researchers.
Our Office of Regulatory Support helps researchers navigate government requirements and regulatory processes to get new treatments to the public. Each year, we foster dozens of submissions to the FDA to make investigational drugs and devices available to patients through full FDA approval or expanded access.
Blood from patients who have recovered from COVID –called COVID convalescent plasma – is a safe and effective early treatment for others with the disease, according to the results of a national clinical trial published in the New England Journal of Medicine in May of 2022.
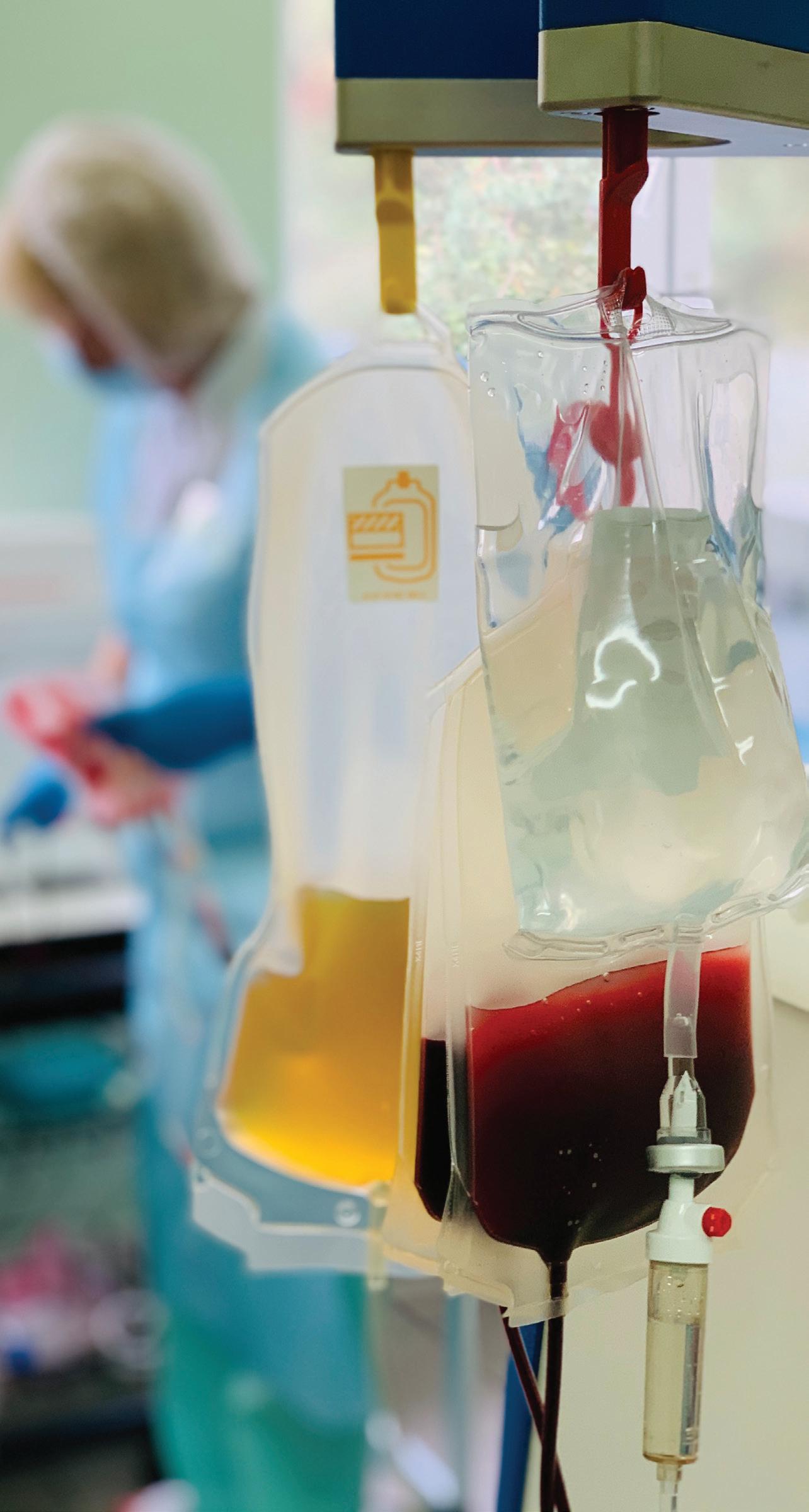
The multi-site clinical trial, which the UR CTSI took part in, showed that administering antibody-rich COVID convalescent plasma to COVID patients within nine days of their first symptoms reduced their risk of hospitalization by 54 percent. It also confirmed the safety of the treatment – with fewer side effects reported among the group who received COVID convalescent plasma compared with those who received control plasma.
Study authors believe convalescent plasma is a valuable addition to the existing arsenal of outpatient COVID treatments, especially where existing drugs and monoclonal antibodies are scarce – and in light of reports that some monoclonal antibodies are less effective against new COVID variants.
“While monoclonal antibodies contain a single purified antibody against SARS-CoV-2, convalescent plasma contains many different antibodies,” said UR CTSI Co-Director Martin Zand, M.D., Ph.D., who was an author on the study and led the local study site. “So convalescent plasma is likely to maintain effectiveness against new strains of the virus.”
Zand, who is also the senior associate dean for clinical research at the University of Rochester Medical Center, conducted the local arm of the study through the UR CTSI’s Clinical Research Center, making use of its specialized space and skilled research staff.
“The UR CTSI’s research infrastructure – particularly the Clinical Research Center – is a large part of why URMC was selected as a site for this study,” said Zand. “That enabled us to offer a treatment to our community that they might not have been able to access otherwise.”
The trial was led by Johns Hopkins Medicine and the Johns Hopkins Bloomberg School of Public Health and involved 23 study sites.
The UR CTSI provides research teams with various opportunities to partner with other clinical and translational researchers –both near and far – as well as physicians, research staff and the community. We help you make the connections you need to move your research forward.

In 2017, Jeffrey Bazarian, M.D., M.P.H., a concussion researcher at the University of Rochester Medical Center, wanted to pull together a national network of concussion clinics to foster clinical trials. He turned to the UR CTSI for help and ended up connecting with a group of researchers from across the U.S. who were working toward the same goal. In October of 2021, that group was awarded $10 million from the National Institute of Neurological Diseases and Stroke to study prolonged concussion symptoms in children and teens.
Nearly 1.9 million kids under 18 suffer from concussions in the U.S. each year and about one-third of those kids end up with concussion symptoms that last longer than normal – three months or more. The grant-funded clinical trial, which is led by the University of California, Los Angeles, is testing ways to predict which kids will develop persistent symptoms after concussion, so researchers can study how to help them recover faster.
“Prolonged concussion recovery can have an enormous impact on the lives of teens and pre-teens, often setting the stage for academic difficulties, persisting mood disorders and chronic pain,” said Bazarian, who leads the Rochester study site. “Early evaluation and treatment for kids at high risk for prolonged recovery is our best hope for preventing an acute injury from becoming chronic.”
The study, which focuses on children between the ages of 11 and 18, aims to identify biomarkers (i.e. changes in blood pressure, heart rate and pupil reactivity) associated with persistent concussion symptoms. Ultimately, the team hopes to develop an algorithm to help healthcare providers diagnose and treat concussed kids and to enable future development of therapies that could help kids recover from concussion faster.
Bazarian credits the UR CTSI for connecting him with the Trial Innovation Network (TIN), part of NIH’s Clinical and
Translational Science Awards (CTSA) Program that fosters multi-site clinical trials. Clinical trial experts at the TIN worked with Bazarian to refine his original clinical trial proposal and connected him with a new group of collaborators that made the $10 million grant and clinical trial possible.
“If I hadn’t applied to the TIN, I wouldn’t have found this group to become a part of the Four Corners Youth Consortium,” Bazarian said. “This grant will open doors for some of the country’s most brilliant concussion researchers to work together to advance concussion research and patient care.”
The Four Corners Youth Consortium is a group of research, clinical, and education entities working to address the “four corners” of a child’s life: family, school, sports/recreation and health. Together, they are building an evidence base to promote active, healthy participation in youth sports and recreation, in part by studying youth concussion.
Led by the University of California, Los Angeles, the consortium now includes URMC, University of Washington, Wake Forest School of Medicine, Children’s National Medical Center, and the University of Texas Southwestern Medical Center, which are all members of the CTSA Program.
“Working with the TIN ended up being a lot of work, but it was very helpful,” said Bazarian. “It was worth the effort to land where I have.”
The UR CTSI provides seed funding to researchers at all career stages to support highly innovative clinical and translational research. Our Pilot and Career Development grants, ranging from $10,000 - $200,000, help researchers gather preliminary information and lay a foundation for future extramurally funded research projects and programs. This early-stage research funding is critical to bridging the gap between scientific discovery and improving human health.
At the beginning of the COVID pandemic, we were hopeful that pre-existing immunity to the common cold could protect us from COVID, but a study in the Journal of Infectious Diseases in March of 2022 suggested that sometimes the opposite can happen. The UR CTSI-supported study shows that prior infection and immunity to one of the common cold coronaviruses may have put people at risk of more severe COVID illness and death.
The study examined immunity to various coronaviruses in blood samples provided by the UR CTSI’s COVID-19 Biobank, a repository of blood samples from hundreds of patients with and without COVID infections. Study authors compared 155 samples taken from COVID patients in the early months of the pandemic to 188 blood samples collected in the pre-COVID era (prior to December of 2019). Among the COVID patients who provided samples, 112 were hospitalized and donated sequential samples over the course of their hospitalization. These hospitalized patients experienced a large, rapid increase in antibodies that targeted SARS-CoV-2 and several other coronaviruses. While big boosts in antibodies –protective proteins generated by the immune system –is usually a good thing, in this case it wasn’t.
The study showed that these antibodies were targeting parts of the spike protein (which sits on the surface of coronaviruses and helps them infect cells) that were similar to common cold coronaviruses the immune system remembered from previous infections. Unfortunately, targeting those areas meant the antibodies could not neutralize the new SARS-CoV-2 virus. When levels of these antibodies rose faster than levels of SARS-CoV-2 neutralizing antibodies, patients had worse disease and a higher chance of death.
“In people who were sicker – those who were in the ICU or died in the hospital – the immune system was responding robustly in a way that was less protective,” said UR CTSI Co-Director Martin Zand, M.D., Ph.D., who was the lead author of the study. “It took those patients longer for the immune system to make protective antibodies… unfortunately, too late for some.”
This study added to a growing pool of evidence that a phenomenon called immune imprinting is at play in COVID immune responses. Zand likened this phenomenon to

‘immune distraction’: immunity to one threat (seasonal coronaviruses) hijacks the immune response to a new, but similar threat (SARS-CoV-2). Immune imprinting has been linked to poor immune responses to other viruses, like flu, and can have implications for vaccine strategies.
By some predictions, COVID is likely to be with us for a long time – with new, milder strains emerging and circulating on an annual or seasonal basis. If those predictions hold true, the study suggests that we may need to regularly develop new vaccines targeting new strains of SARS-CoV-2. COVID boosters developed by Pfizer/BioNTech and Moderna that target the omicron variant were approved by the Food and Drug Administration in September of 2022, affirming the study authors’ prediction.
“We should expect that development of new vaccines is a good thing,” said Zand. “It doesn’t mean the original science was wrong. It means nature has changed. If we want an immune system that pays attention to the right stuff, we need to teach it new tricks with different vaccines.”
Tumors and cancer cells are not the only villains in cancer. They are surrounded by a host of cells and structures, called the cancer microenvironment, that help the cancer grow. With funding from the UR CTSI, a team of researchers at the Wilmot Cancer Institute have developed a novel therapy that can uncouple cancer from this support system in mice and cellular models.
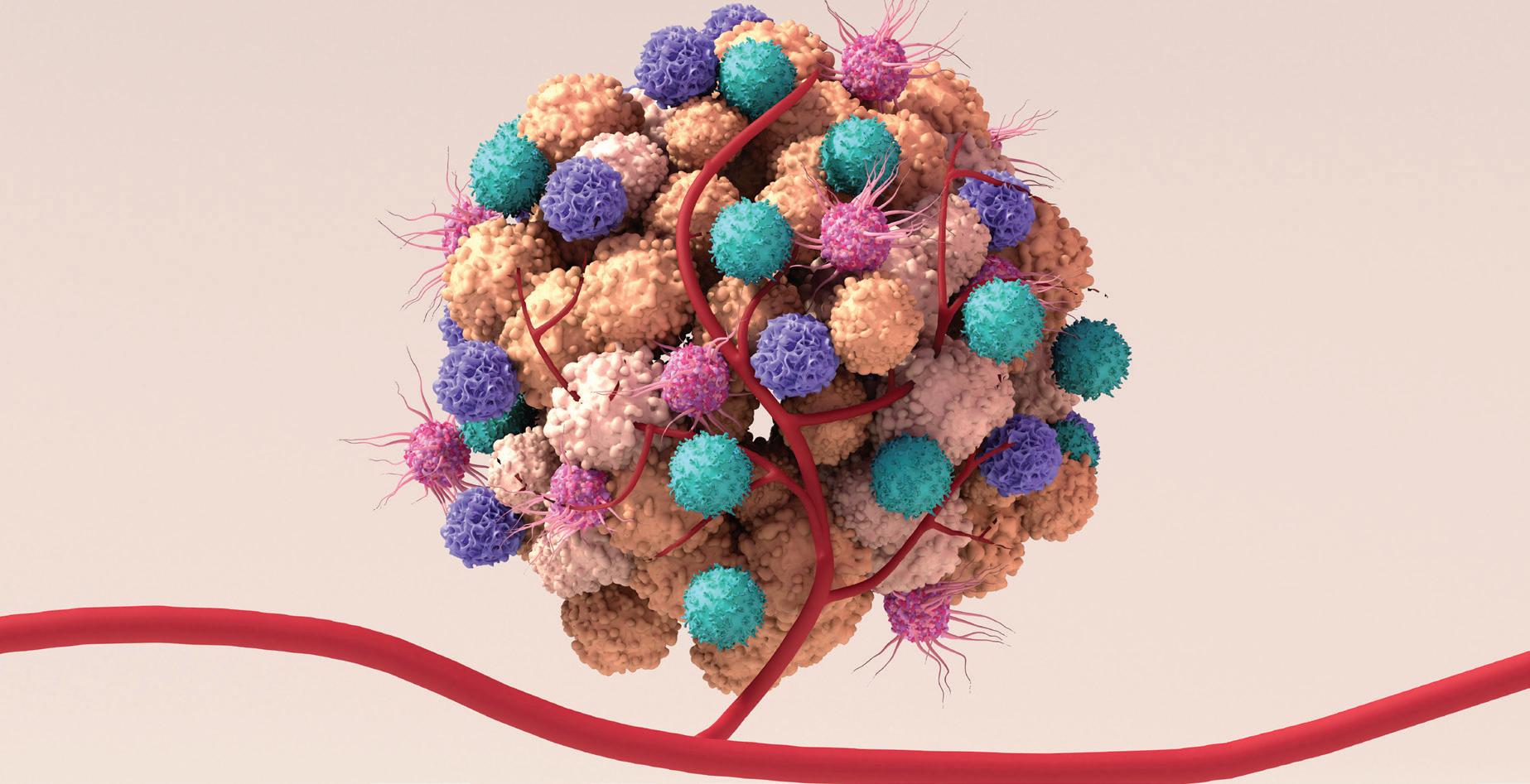
The team, led by Michael W. Becker, M.D., dean’s professor of Hematology/Oncology at Wilmot, identified a pair of immune signaling proteins, called IRAK1 and IRAK4, that enhance the collaboration between cancer cells and their microenvironment and seem to play a role in promoting cancer growth and resisting therapies. Rakesh K. Singh, Ph.D., M.Phil., research associate professor of Obstetrics and Gynecology at the University of Rochester Medical Center, created a new proprietary IRAK1/4 inhibitor, called UR241-2, which improved upon existing commercially-available inhibitors.

When their UR CTSI-funded Incubator project kicked off in July of 2021, the team already knew UR241-2 was highly effective at inhibiting IRAK1/4 in solution. With Incubator funding, they moved that investigation into cells and animals.
They found that UR241-2 disrupted the collaboration between cancer cells and their microenvironment in cellular models of pancreatic cancer and two blood cancers (myelodysplastic syndrome and acute myelogenous leukemia) – all of which are fairly common and highly incurable. A wealth of good science also suggests IRAK1/4 plays an important role in these cancers by helping cancer cells multiply.
The team then focused on studying UR241-2 toxicity: whether it targets the appropriate cells and proteins, whether it can sufficiently inhibit IRAK1/4, and if it has off-target effects in cellular and mouse models of cancer or bone marrow biopsies from patients. They also intend to investigate how well UR241-2 impairs tumor growth in these models, hoping to eventually move this research into early phase clinical trials with the ultimate goal of advancing patient care.
The team, which includes, Laura M. Calvi, M.D., the SKAWA Foundation Professor in Endocrinology and Metabolism, and Aram F. Hezel, M.D., the John and Ethel Heselden Professor and Chief of Hematology/Oncology, have already leveraged their incubator findings to apply for further funding. They also have an ongoing patent application for UR241-2, and plan to submit further grant proposals within the next year to keep this research moving forward.



In November of 2021, the UR CTSI launched a new, more secure and convenient way for researchers across the University of Rochester to access and analyze data that contain protected health information (PHI). The Secure Environment for Research Data Analytics (SERDA) provides far greater data security than previous systems and is free and easy for researchers across the University of Rochester to use.
SERDA was developed as part of a larger collaborative project between the UR CTSI and University of Rochester Medical Center (URMC ) Information Systems Division. Over the past few years, they have been building a data ecosystem at URMC that improves both the security and accessibility of patient data for research. With funding from URMC, this project is an example of the University’s investment in research infrastructure.
Keeping patient data secure is a top priority for UR CTSI leaders, but it is also essential for researchers to be able to safely and securely access data that can provide new insights and foster the development of new therapies or interventions that benefit patients.

SERDA does not allow any data containing protected health information to be downloaded or shared outside the system, but it makes it easy for researchers to collaborate and share data securely within the system alongside the software needed to analyze it. Researchers can also download de-identified data and analysis results and take advantage of new features, like the ability to dynamically refresh data.
By providing these things at no cost – and just when researchers need them – the UR CTSI is making it easier for researchers at all career stages to access clinical data and advance research.

The UR CTSI is dedicated to training the next generation of clinical and translational researchers. With educational programs tailored to undergraduate, graduate and postdoctoral trainees and faculty at all career stages, we have something for everyone. Our offerings range from certificate programs to a fully accredited doctorate program.
After serving a year as associate program director of the UR CTSI’s Translational Biomedical Science PhD Program, Juilee Thakar, Ph.D., stepped into the role of program director in July of 2022. Thakar relieved interim program director Edwin van Wijngaarden, Ph.D., who stayed on as the associate program director.
“Dr. Thakar is perfectly suited to lead this important program,” said van Wijngaarden, who is also Strategic Director for the UR CTSI’s Research and Education Branch as well as professor and associate chair of Public Health Sciences, and a professor in the Departments of Dentistry, Environmental Medicine, Pediatrics and in the Center for Community Health and Prevention. “I look forward to working with her to foster the careers of our truly exceptional trainees.”
Thakar, an associate professor of Microbiology and Immunology, Biostatistics and Computational Biology, and Biomedical Genetics, has a passion for mentoring the next generation of translational scientists. “I truly enjoy working with students, empowering them to create their own career paths, and seeing them grow,” she said.
For nearly a decade, Thakar has been working in translational and multidisciplinary research, collaborating with clinicians and basic scientists on projects big and small. These experiences, she feels, prepared her to teach the next generation about the nuances of translational research and to identify growth opportunities for early-career scientists working in these fields.
“Providing clinical and public health-related instruction to basic scientists is a unique balance that the Translational Biomedical Science PhD Program tries to achieve,” said Thakar. “I’m excited to help our students prepare for a wide range of careers and opportunities.”
Together, Thakar and van Wijngaarden recently restructured the TBS program curriculum, adding two new concentrations: clinical research methods and bioinformatics. Thakar is excited to implement the new curriculum and to bring new peer-education opportunities to the program.

Each year, the UR CTSI offers KL2 Career Development Awards to early-stage clinical and translational scientists at the University of Rochester. These awards provide two years of mentored research support to help awardees advance their careers and obtain independent funding. In July of 2022, awardees kicked off projects to test a new treatment regimen for breast cancer patients, improve clinical decision support tools, test an adapted mental health treatment for Deaf individuals, and map the brain to improve neurosurgery.
The U.S. Deaf community experiences higher rates of several mental health issues, but are much less likely to receive behavioral health treatment. During her postdoctoral training, Aileen Aldalur, Ph.D., adapted a mental health therapy for use with Deaf individuals.

The therapy, called Cognitive Behavioral Therapy for Treatment Seeking, typically involves a telephone interview with people who screen positive for mental health issues, like depression or anxiety, and encourages them to seek behavioral treatment. Aldalur’s adapted therapy uses technologies that are more accessible for Deaf people – video phone or Zoom – and factors in the unique linguistic and cultural concerns of the Deaf population, such as fear and mistrust of the health care system and lack of accessible information. With funding from the UR CTSI, Aldalur will test the feasibility and efficacy of her adapted therapy with 30 Deaf adults from across the country.

Brain tumors can be tricky to remove when they originate in white matter areas of the brain that contain nerve fibers, which carry signals throughout the brain. Surgeons can either leave a portion of a tumor, sparing nearby white matter fibers, or fully remove a tumor and risk damaging nearby white matter and causing post-operative issues.
For his Career Development Award project, Frank E. Garcea, Ph.D., will use high definition fiber tracking in 40 patients who have brain tumors originating in their white matter to identify white matter fiber pathways that control fine movements of the mouth and hands associated with speech and tool use. Mapping the location of white matter fiber pathways in relation to a brain tumor will help neurosurgeons avoid these critical structures when removing a tumor. Long-term, the study aims to understand how the protection of white matter fiber tracts can help preserve patients’ language and fine motor skills after surgery.

Machine learning and artificial intelligence have increasingly been used in health care. While they produce a lot of data, they haven’t produced meaningful outcomes for patients –possibly because machine learning or AI algorithms often aren’t validated using broad data sets from multiple centers. Clinical decision support systems, which analyze electronic health record data and provide prompts and reminders to physicians, are also often poorly designed, leading to physician alert fatigue.
With funding from the UR CTSI, Adam Dziorny, M.D., Ph.D., is testing a new way to optimize, validate and implement an existing algorithm to develop a clinical decision support tool that is designed with health care providers in mind. His practical test case will center around acute kidney injury, a dangerous condition that is common among critically ill children and is typically cared for reactively.
Dziorny will validate and refine an algorithm that predicts early acute kidney injury using two large multi-center datasets. The validated algorithm will then be used to identify children at high risk of developing acute kidney injury at the UR Medicine Golisano Children’s Hospital. Guided by user-centered design principles, an alert will be added to the patients’ electronic health record to help inform physicians of their risk and improve proactive care.
Up to 30 percent of patients with metastatic breast cancer will develop brain tumors during the course of their disease. Patients with HER2-positive breast cancer (a type of breast cancer marked by an overabundance of a protein called HER2 that contributes to tumor growth) are typically treated with a combination of chemotherapy and HER2-targeted therapies.
Therapies that target HER2 can get into the brain and prolong survival of patients whose HER2-positive breast cancer has spread to the brain.
Unfortunately, therapies typically used for patients who have HER2-negative breast cancer are not effective against brain metastases. But preliminary evidence suggests that HER2-targeted therapies may be able to help patients who have HER2-negative tumors with underlying abnormal HER2 signaling.
Ajay Dhakal, M.B.B.S., will test whether a combination of HER2-targeted therapy and chemotherapy can shrink brain metastases in a small set of patients who have HER2-negative breast cancer and hyperactive HER2 signaling.

The University of Rochester Clinical and Translational Science Institute (UR CTSI) provides funding, education, resources and services to help research teams collaborate and produce results faster. Research faculty, staff, trainees and students will all find helpful programs and services at the UR CTSI, which is centrally located in the Saunders Research Building at the University of Rochester Medical Center.
We strive to move beyond the physical and virtual confines of institutions to conduct “research without walls.” Through our many programs, we are advancing science and medicine and improving the health of communities and populations.
Not sure what you need, or how to find it? Contact the Research Help Desk at researchhelp@urmc.rochester.edu for access to research-related services and expertise provided by the UR CTSI and many other organizations across the University.
Find out what’s happening! The UR CTSI Weekly Update starts your week off right with research-related news, events and funding opportunities. The UR CTSI Stories blog provides useful information on programs, services and more.
Interact! Follow the UR CTSI on Facebook, LinkedIn, Twitter and YouTube

There is more! Check the UR CTSI website, ctsi.urmc.edu, for all the details.
Clinical and Translational Science Institute
Saunders Research Building, Suite 1.200 265 Crittenden Blvd, | Rochester, NY 14642-0708 (585) 275-0653 | ctsi.urmc.edu
