


































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































SURGICAL GLOVES
SURGICAL GLOVES
MULTI-SPECIALTY
MULTI-SPECIALTY
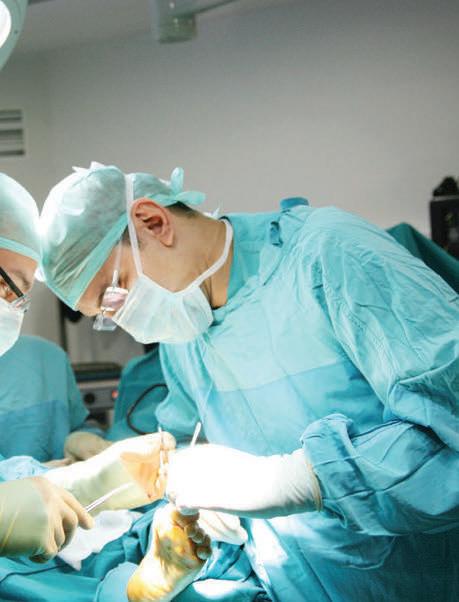
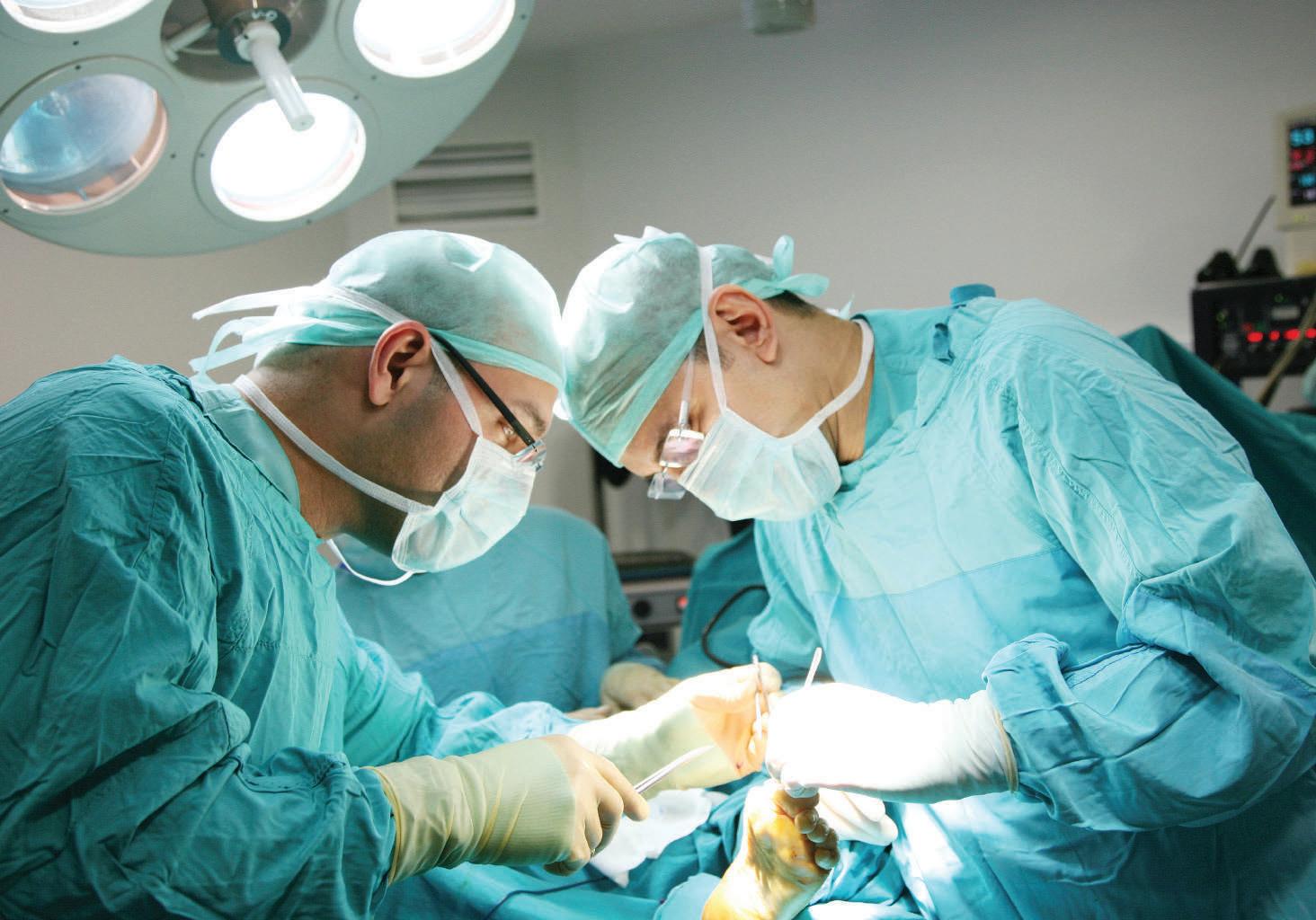
We strive to be the world’s leading surgical gloves manufacturer with excellent quality products and services that enrich and protect human lives.
SURGICAL GLOVES
SURGICAL GLOVES
MICROSURGERY & UNDERGLOVE
MICROSURGERY & UNDERGLOVE
We strive to be the world’s leading surgical gloves manufacturer with excellent quality products and services that enrich and protect human lives.
REACTIVE SURGICAL GLOVES
REACTIVE SURGICAL GLOVES
TRAUMA & HIGH RISK
TRAUMA & HIGH RISK
SAFETY
SAFETY
Use of the glove will prevent sensitisation and/or allergies to both doctor and patient.
Use of the glove will prevent sensitisation and/or allergies to both doctor and patient.
PROTECTION
PROTECTION
Patient/doctor cross contamination control
Patient/doctor cross contamination control
An impermeable barrier
An impermeable barrier
COMFORT
COMFORT
surgical outcomes. Perform well under all conditions.
surgical outcomes. Perform well under all conditions.
RISK MITIGATION
RISK MITIGATION
Reduce risks when a glove is cut or breached.
Reduce risks when a glove is cut or breached.
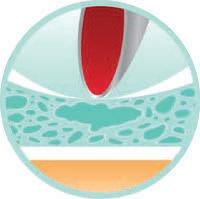
Fig.1.
Fig.1.
Cross Sectional micrograph of the Aegis® glove demonstrating outer, inner and middle layer with embedded micro-size reservoirs.
Cross Sectional micrograph of the Aegis® glove demonstrating outer, inner and middle layer with embedded micro-size reservoirs.
SAFETY
SAFETY
Zero accelerators
Zero accelerators
Zero natural latex protein
Zero natural latex protein
AQL 0.10 (highest accepted quality level) within the industry
AQL 0.10 (highest accepted quality level) within the industry

Outer layer
Outer layer
Middle layer
Middle layer
antimicrobial liquid in drop-like compartments
antimicrobial liquid in drop-like compartments
INFECTION BARRIER
INFECTION BARRIER
Inner layer
Inner layer
inherent microchannel
Skin (hand)
inherent microchannel
Skin (hand)

Grip consistency
Grip consistency

RISK MITIGATION
RISK MITIGATION
Actively responds to prevent infection
Actively responds to prevent infection


Challenge Organism:
Challenge Organism:
Human Immunodeficiency Virus Type 1
Human Immunodeficiency Virus Type 1
(HIV-1) Strain IIIB, clade B Zeptometrix
(HIV-1) Strain IIIB, clade B Zeptometrix
Fig.1.
Fig.1.
Swelling of different types of glove materials in artificial perspiration.
Swelling of different types of glove materials in artificial perspiration.
The higher Swelling % indicates higher glove hydration.
The higher Swelling % indicates higher glove hydration.
Hydration is the absorption of fluid into the interstitial areas of a glove once it is wetted and how it will adversely affect the clinical performance of the gloves.
Hydration is the absorption of fluid into the interstitial areas of a glove once it is wetted and how it will adversely affect the clinical performance of the gloves.

Accelerators and chemicals are added to cross-link polymers together to form a continuous film.
Accelerators and chemicals are added to cross-link polymers together to form a continuous film.
Standard glove manufacturing process for latex gloves, polyisoprene gloves and other synthetic glove materials today.
Standard glove manufacturing process for latex gloves, polyisoprene gloves and other synthetic glove materials today.
Irregular cross-linked polymers produce microchannels, pinhole size ranges from 5 - 12 micron traversing across the glove film.
Irregular cross-linked polymers produce microchannels, pinhole size ranges from 5 - 12 micron traversing across the glove film.
Higher rate of micro pinholes or micro perforation, allowing crossing of fluids and microorganism across glove film unnoticed.
Higher rate of micro pinholes or micro perforation, allowing crossing of fluids and microorganism across glove film unnoticed.

No accelerators and no binding chemicals are added in the manufacturing process. Glove film is cast as solid film from true solution without any cross-linking agent required.
No accelerators and no binding chemicals are added in the manufacturing process. Glove film is cast as solid film from true solution without any cross-linking agent required.
Developed using Molecular Layer Technology. Each pair represents a block of co-polymer built in multiple molecular layers.
Developed using Molecular Layer Technology. Each pair represents a block of co-polymer built in multiple molecular layers.
Uniform layers are deposited to form a strong polymer block in each glove. Virtually no pinholes.
Uniform layers are deposited to form a strong polymer block in each glove. Virtually no pinholes.
Strong wall of molecular layers, maximum protection barrier, prohibits any potential fluids or microorganism exchange across the glove film.
Strong wall of molecular layers, maximum protection barrier, prohibits any potential fluids or microorganism exchange across the glove film.
of healthcare professionals who use latex and other gloves may get irritant contact dermatitis on their hands due to chemical-related hypersensitivity
of healthcare professionals who use latex and other gloves may get irritant
contact dermatitis on their hands due to chemical-related hypersensitivity
Finessis Aegis® is a revolutionary barrier protection system. It is a reactive surgical glove with a Response Triggered Disinfecting System. Made with Flexylon™, the Aegis® glove is designed with an encapsulated layer of disinfecting liquid between the elastomer external layers.
Finessis Aegis® is a revolutionary barrier protection system. It is a reactive surgical glove with a Response Triggered Disinfecting System. Made with Flexylon™, the Aegis® glove is designed with an encapsulated layer of disinfecting liquid between the elastomer external layers.
The Disinfecting System releases the disinfecting liquid only when a glove is punctured, with the right amount to disinfect and at the location where the glove is perforated. The disinfectant is not in contact with wearer’s skin in normal glove wear, mitigating over-sensitisation of chemical compounds on the skin.
The Disinfecting System releases the disinfecting liquid only when a glove is punctured, with the right amount to disinfect and at the location where the glove is perforated. The disinfectant is not in contact with wearer’s skin in normal glove wear, mitigating over-sensitisation of chemical compounds on the skin.
Each pair of Finessis® surgical gloves is delicately engineered. Carefully deposited at each molecular layer at a time, each pair is a strong block of high performance elastomer, FlexylonTM, without the use of any binding chemicals.
FINESSIS AEGIS® surgical glove features :
FINESSIS AEGIS® surgical glove features :
Each pair of Finessis® surgical gloves is delicately engineered. Carefully deposited at each molecular layer at a time, each pair is a strong block of high performance elastomer, FlexylonTM, without the use of any binding chemicals.
Latex-Free, Powder-Free synthetic surgical glove
Latex-Free, Powder-Free synthetic surgical glove
Encapsulated with disinfecting liquid in the middle layer, which releases in the event of glove puncture
Encapsulated with disinfecting liquid in the middle layer, which releases in the event of glove puncture
Industry’s highest Accepted Quality Level (AQL) 0.10
Industry’s highest Accepted Quality Level (AQL) 0.10
Length : Min 270mm
Length : Min 270mm
Thickness (mm) : Finger (0.29 ± 0.03)
Thickness (mm) : Finger (0.29 ± 0.03) : Palm (min 0.24)
: Palm (min 0.24)
: Cuff (min 0.15)
: Cuff (min 0.15)
Latex-free, accelerator-free and with zero added chemicals, Finessis® surgical gloves eliminate all risks of irritant contact dermatitis, Type I (latex-related allergy) and Type IV (chemical-related allergy) skin hypersensitivity.
ZERO accelerators
Latex-free, accelerator-free and with zero added chemicals, Finessis® surgical gloves eliminate all risks of irritant contact dermatitis, Type I (latex-related allergy) and Type IV (chemical-related allergy) skin hypersensitivity.
ZERO accelerators
Made from FLEXYLON™ High Performance Elastomer built by Molecular Layer Technology
Made from FLEXYLON™ High Performance Elastomer built by Molecular Layer Technology
Ultra low stress design
Ultra low stress design
Low hydration
Low hydration
Colour : Light Green / White
Colour : Light Green / White
Sterilisation : Ebeam Irradiation
Sterilisation : Ebeam Irradiation
Inspection Level : AQL 0.10
Inspection Level : AQL 0.10
Product Code : STPAF
Product Code : STPAF




M g p




FINESSIS CORIUM® surgical glove features :
ZERO accelerators
Latex-Free, Powder-Free synthetic surgical glove
Industry’s highest Accepted Quality Level (AQL) 0.10
surgical glove features : tic surgical glove ality Level (AQL) 0.10



gh Perfor El tomer
Made from FLEXYLON™ High Performance Elastomer built using Molecular Layer Technology

Ultra low stress design
Low hydration




Finessis CORIUM® exerts half the stress compared to natural rubber latex and other synthetic latex gloves


Designed to d touc chieve the righ gy with zero acc with Finessis ure.
Smooth textured surface, comfortable with enhanced grip sensitivity





Length : Min 270mm


Colour : Green
Finessis CORIUM® is a glove which provides supreme tactility for double gloving and microsurgeries. Designed to deliver enhanced touch sensitivity, Corium® is perfect as an underglove to achieve the right comfort without loss of grip sensitivity. Built using Molecular Layer Technology with zero accelerators, Finessis CORIUM® poses no risk of Type I and Type IV allergies. Pair it with Finessis ZERO® for a perfect fit and impermeable barrier in every surgical procedure.
Thickness (mm) : Finger (0.19 ± 0.03)
: Palm (min 0.14) : Cuff (min 0.13)


Inspection Level : AQL 0.10
Product Code : STPCF


ce, comfor nc d c Ebeam Irradiation


Sterilisation : Ebeam Irradiation


Deep injury from a percutaneous blood exposure incident is one of the incremental risks for infection
Deep injury from a percutaneous blood exposure incident is one of the incremental risks for infection
Finessis ZERO® is the next generation of gloves, featured in the CLEAN surgical glove segment that uses ZERO accelerators and no binding chemicals in the manufacturing process. Built using Molecular Layer Technology, Finessis ZERO® is the cleanest surgical glove for your hands and poses the lowest risk for Type I and Type IV allergies.
Finessis ZERO® is the next generation of gloves, featured in the CLEAN surgical glove segment that uses ZERO accelerators and no binding chemicals in the manufacturing process. Built using Molecular Layer Technology, Finessis ZERO® is the cleanest surgical glove for your hands and poses the lowest risk for Type I and Type IV allergies.
FINESSIS ZERO® surgical glove features :
FINESSIS ZERO® surgical glove features :
Finessis AEGIS® surgical gloves are designed with a unique middle layer of disinfecting liquid. In the event of a percutaneous blood exposure incident or a needlestick injury resulting in the glove being breached the designed mechanism of the glove will release the disinfecting liquid and disinfect the needle or sharp object.
Finessis AEGIS® surgical gloves are designed with a unique middle layer of disinfecting liquid. In the event of a percutaneous blood exposure incident or a needlestick injury resulting in the glove being breached the designed mechanism of the glove will release the disinfecting liquid and disinfect the needle or sharp object.
ZERO accelerators
ZERO accelerators
Latex-Free, Powder-Free synthetic surgical glove
Latex-Free, Powder-Free synthetic surgical glove
Industry’s highest Accepted Quality Level (AQL) 0.10
Industry’s highest Accepted Quality Level (AQL) 0.10
Made from FLEXYLON™ High Performance Elastomer built by
Length : Min 280mm
Length : Min 280mm
Thickness (mm) : Finger (0.22 ± 0.02)
: Palm (min 0.18)
Made from FLEXYLON™ High Performance Elastomer built by
A surgical glove that protects WHEN NEEDED, WHERE IT IS NEEDED WITH THE RIGHT AMOUNT NEEDED.
A surgical glove that protects WHEN NEEDED, WHERE IT IS NEEDED WITH THE RIGHT AMOUNT NEEDED.
Molecular Layer Technology
Molecular Layer Technology
Ultra low stress design
Ultra low stress design
Low hydration
Low hydration
Feel fit design
Feel fit design
Thickness (mm) : Finger (0.22 ± 0.02) : Palm (min 0.18) : Cuff (min 0.17)
: Cuff (min 0.17)
Colour : White
Colour : White
Sterilisation : Ebeam Irradiation
Sterilisation : Ebeam Irradiation
Inspection Level : AQL 0.10
Inspection Level : AQL 0.10
Product Code : STPZF
Product Code : STPZF
Constriction of the soft tissue in the palm or the wrist can lead to pain in the thumb or carpal tunnel area. A properly fitted glove should fit around the wrist and be easy to don and be taken off
Constriction of the soft tissue in the palm or the wrist can lead to pain in the thumb or carpal tunnel area. A properly fitted glove should fit around the wrist and be easy to don and be taken off
Constriction of the soft tissue in the palm or the wrist can lead to pain in the thumb or carpal tunnel area. A properly fitted glove should fit around the wrist and be easy to don and be taken off
Constriction of the soft tissue in the palm or the wrist can lead to pain in the thumb or carpal tunnel area. A properly fitted glove should fit around the wrist and be easy to don and be taken off
Finessis® surgical gloves are designed with ultra-low stress. Each pair is anatomically formed and comfort is enhanced by the high viscoelasticity of FlexyonTM . This means less exertion and fatigue on hands and fingers when flexing.
Finessis® surgical gloves are designed with ultra-low stress. Each pair is anatomically formed and comfort is enhanced by the high viscoelasticity of FlexyonTM . This means less exertion and fatigue on hands and fingers when flexing.
Finessis® surgical gloves are designed with ultra-low stress. Each pair is anatomically formed and comfort is enhanced by the high viscoelasticity of FlexyonTM . This means less exertion and fatigue on hands and fingers when flexing.
Finessis® surgical gloves are designed with ultra-low stress. Each pair is anatomically formed and comfort is enhanced by the high viscoelasticity of FlexyonTM . This means less exertion and fatigue on hands and fingers when flexing.
































Finessis CORIUM® is a glove which provides supreme tactility for double gloving and microsurgeries. Designed to deliver enhanced touch sensitivity, Corium® is perfect as an underglove to achieve the right comfort without loss of grip sensitivity. Built using Molecular Layer Technology with zero accelerators, Finessis CORIUM® poses no risk of Type I and Type IV allergies. Pair it with Finessis ZERO® for a perfect fit and impermeable barrier in every surgical procedure.
Finessis CORIUM® is a glove which provides supreme tactility for double gloving and microsurgeries. Designed to deliver enhanced touch sensitivity, Corium® is perfect as an underglove to achieve the right comfort without loss of grip sensitivity. Built using Molecular Layer Technology with zero accelerators, Finessis CORIUM® poses no risk of Type I and Type IV allergies. Pair it with Finessis ZERO® for a perfect fit and impermeable barrier in every surgical procedure.
FINESSIS CORIUM® surgical glove features :
FINESSIS CORIUM® surgical glove features :
ZERO accelerators
ZERO accelerators
Latex-Free, Powder-Free synthetic surgical glove
Latex-Free, Powder-Free synthetic surgical glove
Industry’s highest Accepted Quality Level (AQL) 0.10
Industry’s highest Accepted Quality Level (AQL) 0.10
Made from FLEXYLON™ High Performance Elastomer built using Molecular Layer Technology
Made from FLEXYLON™ High Performance Elastomer built using Molecular Layer Technology
Smooth textured surface, comfortable with enhanced grip sensitivity
Smooth textured surface, comfortable with enhanced grip sensitivity
Finessis CORIUM® exerts half the stress compared to natural rubber latex and other synthetic latex gloves
Finessis CORIUM® exerts half the stress compared to natural rubber latex and other synthetic latex gloves
Ultra low stress design
Ultra low stress design
Low hydration
Low hydration
Length : Min 280mm
Length : Min 280mm
Thickness (mm) : Finger (0.19 ± 0.03)
Thickness (mm) : Finger (0.19 ± 0.03)
: Palm (min 0.14)
: Palm (min 0.14)
: Cuff (min 0.13)
: Cuff (min 0.13)
Colour : Green
Colour : Green
Sterilisation : Ebeam Irradiation
Sterilisation : Ebeam Irradiation
Inspection Level : AQL 0.10
Inspection Level : AQL 0.10
Product Code : STPCF
Product Code : STPCF
Deep injury from a percutaneous blood exposure incident is one of the incremental risks for infection
Deep injury from a percutaneous blood exposure incident is one of the incremental risks for infection
Finessis ZERO® is the next generation of gloves, featured in the CLEAN surgical glove segment that uses ZERO accelerators and no binding chemicals in the manufacturing process. Built using
Finessis ZERO® is the next generation of gloves, featured in the CLEAN surgical glove segment that uses ZERO accelerators and no binding chemicals in the manufacturing process. Built using
Molecular Layer Technology, Finessis ZERO® is the cleanest surgical glove for your hands and poses the lowest risk for Type I and Type IV allergies.
Molecular Layer Technology, Finessis ZERO® is the cleanest surgical glove for your hands and poses the lowest risk for Type I and Type IV allergies.
Finessis AEGIS® surgical gloves are designed with a unique middle layer of disinfecting liquid. In the event of a percutaneous blood exposure incident or a needlestick injury resulting in the glove being breached the designed mechanism of the glove will release the disinfecting liquid and disinfect the needle or sharp object.
FINESSIS ZERO® surgical glove features :
FINESSIS ZERO® surgical glove features :
Finessis AEGIS® surgical gloves are designed with a unique middle layer of disinfecting liquid. In the event of a percutaneous blood exposure incident or a needlestick injury resulting in the glove being breached the designed mechanism of the glove will release the disinfecting liquid and disinfect the needle or sharp object.
ZERO accelerators
ZERO accelerators
Latex-Free, Powder-Free synthetic surgical glove
Latex-Free, Powder-Free synthetic surgical glove
Industry’s highest Accepted Quality Level (AQL) 0.10
Industry’s highest Accepted Quality Level (AQL) 0.10
Length : Min 270mm
Length : Min 270mm
Thickness (mm) : Finger (0.22 ± 0.02)
Thickness (mm) : Finger (0.22 ± 0.02) : Palm (min 0.18)
: Palm (min 0.18)
A surgical glove that protects WHEN NEEDED, WHERE IT IS NEEDED WITH THE RIGHT AMOUNT NEEDED.
A surgical glove that protects WHEN NEEDED, WHERE IT IS NEEDED WITH THE RIGHT AMOUNT NEEDED.
Made from FLEXYLON™ High Performance Elastomer built by
Made from FLEXYLON™ High Performance Elastomer built by
Molecular Layer Technology
Molecular Layer Technology
Ultra low stress design
Ultra low stress design
Low hydration
Low hydration
Feel fit design
Feel fit design
: Cuff (min 0.17)
: Cuff (min 0.17)
Colour : White
Colour : White
Sterilisation : Ebeam Irradiation
Sterilisation : Ebeam Irradiation
Inspection Level : AQL 0.10
Inspection Level : AQL 0.10
Product Code : STPZF
Product Code : STPZF
































of healthcare professionals who use latex and other gloves may get irritant contact dermatitis on their hands due to chemical-related hypersensitivity
of healthcare professionals who use latex and other gloves may get irritant contact dermatitis on their hands due to chemical-related hypersensitivity
Finessis Aegis® is a revolutionary barrier protection system. It is a reactive surgical glove with a Response Triggered Disinfecting System. Made with Flexylon™, the Aegis® glove is designed with an encapsulated layer of disinfecting liquid between the elastomer external layers.
Finessis Aegis® is a revolutionary barrier protection system. It is a reactive surgical glove with a Response Triggered Disinfecting System. Made with Flexylon™, the Aegis® glove is designed with an encapsulated layer of disinfecting liquid between the elastomer external layers.
The Disinfecting System releases the disinfecting liquid only when a glove is punctured, with the right amount to disinfect and at the location where the glove is perforated. The disinfectant is not in contact with wearer’s skin in normal glove wear, mitigating over-sensitisation of chemical compounds on the skin.
The Disinfecting System releases the disinfecting liquid only when a glove is punctured, with the right amount to disinfect and at the location where the glove is perforated. The disinfectant is not in contact with wearer’s skin in normal glove wear, mitigating over-sensitisation of chemical compounds on the skin.
FINESSIS AEGIS® surgical glove features :
FINESSIS AEGIS® surgical glove features :
Each pair of Finessis® surgical gloves is delicately engineered. Carefully deposited at each molecular layer at a time, each pair is a strong block of high performance elastomer, FlexylonTM, without the use of any binding chemicals.
Each pair of Finessis® surgical gloves is delicately engineered. Carefully deposited at each molecular layer at a time, each pair is a strong block of high performance elastomer, FlexylonTM, without the use of any binding chemicals.
Latex-Free, Powder-Free synthetic surgical glove
Latex-Free, Powder-Free synthetic surgical glove
Encapsulated with disinfecting liquid in the middle layer, which releases in the event of glove puncture
Encapsulated with disinfecting liquid in the middle layer, which releases in the event of glove puncture
Industry’s highest Accepted Quality Level (AQL) 0.10
Industry’s highest Accepted Quality Level (AQL) 0.10
ZERO accelerators
Length : Min 280mm
Length : Min 280mm
Thickness (mm) : Finger (0.29 ± 0.03)
Thickness (mm) : Finger (0.29 ± 0.03)
: Palm (min 0.24)
: Palm (min 0.24)
: Cuff (min 0.15)
: Cuff (min 0.15)
Latex-free, accelerator-free and with zero added chemicals, Finessis® surgical gloves eliminate all risks of irritant contact dermatitis, Type I (latex-related allergy) and Type IV (chemical-related allergy) skin hypersensitivity.
ZERO accelerators
Latex-free, accelerator-free and with zero added chemicals, Finessis® surgical gloves eliminate all risks of irritant contact dermatitis, Type I (latex-related allergy) and Type IV (chemical-related allergy) skin hypersensitivity.
Made from FLEXYLON™ High Performance Elastomer built by Molecular Layer Technology
Made from FLEXYLON™ High Performance Elastomer built by Molecular Layer Technology
Ultra low stress design
Ultra low stress design
Low hydration
Low hydration
Colour : Light Green / White
Colour : Light Green / White
Sterilisation : Ebeam Irradiation
Sterilisation : Ebeam Irradiation
Inspection Level : AQL 0.10
Inspection Level : AQL 0.10
Product Code : STPAF
Product Code : STPAF
Fig.1.
Fig.1.
Cross Sectional micrograph of the Aegis® glove demonstrating outer, inner and middle layer with embedded micro-size reservoirs.
Cross Sectional micrograph of the Aegis® glove demonstrating outer, inner and middle layer with embedded micro-size reservoirs.
SAFETY
SAFETY
Zero accelerators
Zero accelerators
Zero natural latex protein
Zero natural latex protein
AQL 0.10 (highest accepted quality level) within the industry
AQL 0.10 (highest accepted quality level) within the industry
COMFORT
COMFORT


Grip consistency

Outer layer
Outer layer

Middle layer
Middle layer
antimicrobial liquid in drop-like compartments

antimicrobial liquid in drop-like compartments
INFECTION BARRIER
INFECTION BARRIER
Inner layer
Inner layer
inherent microchannel
inherent microchannel
Skin (hand)
Skin (hand)



RISK MITIGATION
RISK MITIGATION
Actively responds to prevent infection
Actively responds to prevent infection


Challenge Organism:
Challenge Organism:
Human Immunodeficiency Virus Type 1
Human Immunodeficiency Virus Type 1
(HIV-1) Strain IIIB, clade B Zeptometrix
(HIV-1) Strain IIIB, clade B Zeptometrix
Fig.1.
Fig.1.
Swelling of different types of glove materials in artificial perspiration.
Swelling of different types of glove materials in artificial perspiration.
The higher Swelling % indicates higher glove hydration.
The higher Swelling % indicates higher glove hydration.
Hydration is the absorption of fluid into the interstitial areas of a glove once it is wetted and how it will adversely affect the clinical performance of the gloves.
Hydration is the absorption of fluid into the interstitial areas of a glove once it is wetted and how it will adversely affect the clinical performance of the gloves.
SURGICAL GLOVES
SURGICAL GLOVES
MULTI-SPECIALTY
MULTI-SPECIALTY
SURGICAL GLOVES
SURGICAL GLOVES
MICROSURGERY & UNDERGLOVE
MICROSURGERY & UNDERGLOVE
REACTIVE SURGICAL GLOVES
REACTIVE SURGICAL GLOVES
TRAUMA & HIGH RISK
TRAUMA & HIGH RISK
SAFETY
SAFETY
Use of the glove will prevent sensitisation and/or allergies to both doctor and patient.
Use of the glove will prevent sensitisation and/or allergies to both doctor and patient.
PROTECTION
PROTECTION
Patient/doctor cross contamination control
Patient/doctor cross contamination control
An impermeable barrier
An impermeable barrier
COMFORT surgical outcomes. Perform well under all conditions.
COMFORT surgical outcomes. Perform well under all conditions.
Reduce risks when a glove is cut or breached. Don the Best for
RISK MITIGATION
RISK MITIGATION
Reduce risks when a glove is cut or breached.
