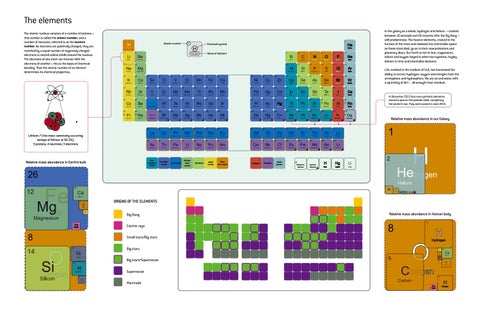
The elements The atomic nucleus consists of a number of protons – this number is called the atomic number; and a number of neutrons, referred to as the neutron number. As neutrons are positively charged, they are matched by a equal number of negatively charged electrons in nested orbital shells around the nucleus. The electrons of one atom can interact with the electrons of another – this is the basis of chemical bonding. Thus the atomic number of an element determines its chemical properties.
Atomic number
In the galaxy as a whole, hydrogen and helium – created between 10 seconds and 20 minutes after the Big Bang – still predominate. The heavier elements, created in the furnace of the stars and released into interstellar space as those stars died, go on to form new protostars and planetary discs. Our Earth is rich in iron, magnesium, silicon and oxygen forged in other star systems, hugely distant in time and interstellar distance.
Chemical symbol Name of element
Life, evolved in the medium of H2O, has harnessed the ability to extract hydrogen, oxygen and nitrogen from the atmosphere and hydrosphere. We are air and water, with a sprinkling of dirt ... all wrought from stardust.
In December 2015 four new synthetic elements earned a spot on the periodic table, completing the seventh row. They were named in June 2016.
N P N N N P
Nh
Mc
Nihonium
Moscovium
P
Ts
Og
Tennessine
Oganesson
Relative mass abundance in our Galaxy
Lithium-7 (the most commonly occurring isotope of lithium at 92.5%): 3 protons; 4 neutrons; 3 electrons
Posttransition metals
Relative mass abundance in Earth’s bulk
Transition metals
Lanthanoids
Alkaline earth metals
Metaloids
Alkali metals
Other non-metals
Halogens
Actinoids
Noble gasses
Radioactive elements
Synthetic elements
The Rest
ORIGINS OF THE ELEMENTS Big Bang The Rest
Relative mass abundance in Human body
Cosmic rays Small stars/Big stars Big stars Big stars/Supernovae Supernovae Manmade
The Rest