Pink Salmon and their Profound
Impact on Ocean Ecology

ALSO IN THIS ISSUE:
WILD STEELHEAD, SALMON AND THE ESA • SEA RUN CUTTHROAT TROUT • WHAT 207 HATCHERY STUDIES HAVE TO SAY • STEELHEAD PROPAGATION AND EPIGENETICS • DOING RIGHT BY OLYMPIC PENINSULA WILD STEELHEAD


THE OSPREY
Issue
107 Winter 2024
The International Journal of Salmon and Steelhead Conservation
No.
3
4 5
6
Pink Salmon and their Profound Impact on Ocean Ecology
By Gregory T. Ruggerone and Alan M.
13
By Richard
By John McMillan
16
By Guy W. Fleischer
By


THE OSPREY
Chair
Pete Soverel
Editor
Jim Yuskavitch
Editorial Committee
Pete Soverel • Kathleen Bergeron
Greg Knox • Brian Braidwood
Rich Simms • Ryan Smith
Guy Fleischer
Scientific Advisors
Rick Williams • Jack Stanford
Jim Lichatowich • Bill McMillan
Bill Bakke • Michael Price
Design & Layout
Jim Yuskavitch
The Osprey is published by: Wild Salmon Rivers
16430 72nd Avenue, West Edmonds, WA 98026
Letters To The Editor
The Osprey welcomes letters to the editor and article proposals.
The Osprey
P.O. Box 13121
Portland, OR 97213
jyusk@bendcable.com
(541) 549-8914
General business and change of address:
https://www.ospreysteelhead.org/contact
The Osprey is a joint publication of not-for-profit organizations concerned with the conservation and sustainable management of wild Pacific salmon and steelhead and their habitat throughout their native and introduced ranges. This unique partnership includes The Conservation Angler, Fly Fishers International, Steelhead Society of British Columbia, SkeenaWild Conservation Trust, Wild Salmon Center, and Wild Steelhead Coalition. Financial support is provided by partner organizations, individuals, clubs and corporations. The Osprey is publishes three issues per year: Winter, Spring/Summer and Fall. All materials are copyrighted and require permission prior to reprinting or other use.





The Osprey © 2024
ISSN 2334-4075
2 The Osprey Contents Cover Photo by NASA; Cover Inset Image
www.taps-photography.pixels.com; Page
photo
McMillan
& News Steelhead Artificial Propagation and Epigenetics
by Preston and Teresa Cole,
3 inset
by John
Columns
A Synopsis of Hatchery Salmonid Effects
Salmonids
on Wild
Springer
14 Field Report: Olympic Peninsula Steelhead — Doing the Right Thing By Wild Fish
David Moskowitz
Sea Run Cutthroat Trout and the Environmental Factors Affecting Their Abundance
20
Stoll
Features
20 From
Perch — Editor’s Message Hits and Misses — Chair’s Corner Letters to the Editor Fish Watch: Wild Fish News, Issues and Initiatives
the
FROM THE PERCH — EDITOR’S MESSAGE
A Little Bit Wonky and a Little Bit Rock and Roll
by Jim Yuskavitch
One important way that The Osprey stands out from other conservation publications is how it balances active conservation advocacy for wild steelhead and salmon, and their habitat, along with a dedication to presenting our readers with the latest and best science that gives us the information needed to more effectively press the powers-that-be for changes that will help our wild fish survive into the future. On some occasions, it’s time to stand up and tell truth to power . At other times, presenting the cold, hard, irrefutable facts to fish managers and government agencies is the most powerful approach.
In this issue of The Osprey, we have gone to the “wonky” side. We all know by now, probably by heart, the negative impacts hatchery fish have on wild fish — genetic changes, less fitness, etc. — along with the seemingly endless production of hatchery studies as the hatchery-industrial complex continues to perk along, business as usual for the most part. What’s going on with all that?

So we called upon some Pacific salmon and steelhead scientists — experts in their fields — to look into a few of those things for us based on their own scientific research and those of their colleagues. Does dumping large numbers of hatchery fish into the enviroment impact wild fish? How about entire ecosystems, as the huge amount of hatchery pink salmon do throughout the North Pacific Ocean, affecting not just salmon but an astounding number of other ocean creatures from orcas to seabirds and more.
And what about all those studies on how hatcheries do or do not adversely affect salmonids? Another article in this issue looks at those many studies to see what they really have to say, both individually and collectively.
And genetic changes? How about a sneaky, little-studied, under-the-DNA radar gremlin that might have significant impacts on fish behavior that could work its way into wild populations. That story is here, too.
The reading is a little bit more challenging in this issue of The Osprey, but we think it will be well worth your time and effort, and will give you some real insights into hatcherywild fish relationships that often stays in the background.

How The Osprey Helps Wild Fish
The Osprey has been bringing the latest science, policy, opinion and news stories to its readers supporting wild Pacific salmon and steelhead conservation and management for 37 years. But we are much more than a publication that you subscribe to because of your own interest in wild fish conservation. The funds we receive from our subscribers allows us to send The Osprey to wild fish conservation decision-makers and influencers including scientists, fisheries managers, politicians and wild fish advocates.
Sending The Osprey to decision makers is key to our wild fish conservation advocacy. Your support makes that possible.
So when you subscribe/donate to The Osprey, you not only receive a subscription yourself, but you also help us put The Osprey into the hands of the people we need bring to our side to save our wild fish.
Please go to the subscription/donation form on page 23 or on-line at www.ospreysteelhead.org/donation and donate whatever you are able. Thank you.
Jim Yuskavitch Editor, The Osprey
Winter 2024 • Issue No. 107 3
A wild coho salmon captured in a fish trap at the Klatskanine Hatchery, Oregon about to released upstream to spawn. Photo by Jim Yuskavitch
HITS & MISSES — CHAIR’S CORNER
Wild Steelhead, Salmon and the ESA
By Pete Soverel
This issue’s Hits and Misses focuses on the Endangered Species Act (ESA), which was signed into law on December 28, 1973, and celebrated its 50th anniversary last year. The ESA is the last line of defense for conserving and restoring endangered and threatened animals, plants, and insects across the United States of America.
Hits
1. The ESA has been critical to recovery of many species, including bald eagles, gray whales, peregrine falcons, and it has saved others such as the California condor and Whooping crane from inevitable extinction. A study in 2019 (Greenwald et al. 2019, Extinction and the U.S. Endangered Species Act) reported that the ESA has prevented the extinction of 291 species since 1973 and recovered another 39 species, including 23 in the past decade (20102019).
2. The ESA has been critical to preventing the extinction of wild salmon and steelhead. Without the ESA, many of our wild salmon and steelhead populations would likely be at much greater threat of extinction or extinct.
3. Oregon Coast coho salmon and Hood Canal summer chum salmon have shown improvements in abundance since they were listed under the ESA. The ESA has also provided funding for critical research, improved monitoring, and recovery and restoration of critical habitat, such as improved flows, passage, and survival of salmon and steelhead in the mainstem Columbia and Snake Rivers.
4. Dam removal in the Elwha River reopened habitat for ESA-listed Chinook salmon, steelhead, and bull trout, all of which are showing signs of rebuilding –though as I have mentioned before, the Chinook salmon have not improved as rapidly as summer steelhead or bull trout.
Misses
1. Although the ESA has the potential to rapidly improve the status of animals, that has not been the case for Columbia River salmon and steelhead because of the actions — or inactions — of federal agencies and the lack of political will power to take necessary actions to recover these imperiled fish (e.g., remove
The Endangered Species Act has prevented the extinction of 291 species — including wild steelhead and salmon — since it was signed into law in December 1973.
the four lower Snake River dams). As I noted in the Hits section, the ESA has undoubtedly helped many stocks of wild salmon and steelhead avoid slipping further towards extinction, but a recent study by Jaeger and Scheuerell (2023, Return(s) on investment: Restoration spending in the Columbia River Basin and increased abundance of salmon and steelhead) found that despite spending 9-billion dollars on restoration efforts, the number of wild salmon and steelhead passing Bonneville Dam has
not greatly improved and their abundance is nowhere close to the 2025 goal of 5-million adults set by the Northwest Power and Conservation Council.
2. One reason the ESA has likely not achieved better results in the Columbia River basin is because the agencies flood the basin with over 140 million hatchery fish annually, which prevents the recovery of these species and undermines ESA-based recovery efforts. For example, over 90% of Chinook salmon and steelhead smolts in the Columbia River estuary are hatchery origin. Hatcheries have a wide range of adverse impacts on wild salmon and steelhead, and they also prop up unsustainable mixed-stock fisheries, which raises the question: Can we learn from our mistakes and apply our successes — from salmon to birds — to improve odds of recovery for ESA-listed salmon and steelhead in the Columbia River basin?
3. Despite decades of decline for Snake River salmon and steelhead, the four lower Snake River dams remain in place, and, as a result, most independent scientists believe these species will never recover. The good news is there is growing commitment to dam breaching. The bad news is that time is run-
Continued on next page

4 The Osprey
A wild fall Chinook salmon spawning in an Oregon coastal stream. Photo by Jim Yuskavitch
ning out for wild Snake River salmon and steelhead, and the federal government is pumping more money into hatchery operations, which prevents ESA recovery actions (e.g., habitat restoration) from buying more time for these species to hold on before the window to remove these dams and save these species closes.
4. Bipartisan Congressional efforts in the 1960s and 1970s created America’s bedrock environmental laws that have turned the tide for some of the nation’s critical resources such as clean air, clean water, and biodiversity. Yet despite 50 years of implementation and some incredible successes, the ESA is under increasing attack in Congress. There are dozens of attempts to dismantle key provisions of the ESA being advanced by regulated industries across the country — the very same industries that have brought many species to the brink of extinction and successfully extirpated others.
The ESA’s track record of wins and losses is not unlike the sporting records of our most celebrated athletes — for example Ted Williams — known as one of the best hitters in baseball — and who served his country as a fighter pilot during two world wars — did not get a hit every at-bat. It is certainly not the ESA’s fault for not hitting 100% of the time — especially when the agencies and Congress are forcing the ESA to box with one hand behind its back with inadequate recovery funding, continually pouring hatchery fish on top of wild fish trying to access restored habitat and on and on. Allowing the ESA to box with both hands might just make it the Muhammed Ali of saving salmon and steelhead. With more attention to what we know works, the ESA can have more hits than misses — and we need those hits so future generations can enjoy wild salmon and steelhead as we have during our lives.
Pete Soverel is Chair of The Osprey Management and Editorial Committee and founder and President of The Conservation Angler, one of The Osprey’s supporting partner organizations. Learn more about their work at: www.theconservationangler.org
Letters to the Editor
Skeena Enchancement Article Hits Nail on the Head
The piece by Michael Price and Kaitlin Yehle (“The Follies of Salmon Enhancement”, The Osprey, Fall 2023) hit the nail on the head in the last section entitled, “Enhancement Today.”
The only thing I would add is that the SEP Babine projects, which were and are successful in their own right, do not represent all the other wild Babine sockeye stocks, and of course, none of the wild sockeye stocks of the entire Skeena watershed. So when everybody enjoys the abundance from SEP success such as last year’s record, they think all is well with sockeye, which is not the case. Only those enhanced stocks of the Fulton and Pinkut benefit in a huge way. All other wild sockeye stocks have the same fate as all un-enhanced wild stocks of the Skeena… decades and decades of commercial fishing pressure from the non-selective methods.
Lastly, the comments made by groups such as SkeenaWild that we need habitat enhancement…well maybe in terms of calling off the logging and mining dogs, but the Skeena has vast amounts of still healthy and available spawning gravels. What those gravels need is spawners. We need to concentrate on getting spawners to the existing habitat, not enhance the empty habitat!
The same problems existed many years ago. The problem is that we haven’t done anything about those same old problems, and that’s on us all now. Lots of wasted time, energy and meetings that did nothing in the end…a total failure that everybody has to own. Can we still change things? Yes. Will we…I don’t see it yet.
Pierce Clegg
British Columbia, Canada
Attention Wild Fish Researchers and Advocates
Previous issues of The Osprey, going back to 2008, are now available on our new website, providing access to years of in-depth science, policy and legal articles pertaining to wild Pacific salmon and steelhead, their management, research and conservation written exclusively for us by experts in their fields.
Whether you are doing a literature search for a research project or preparing a wild fish conservation initiative and looking for supporting data, The Osprey is an invaluable data base of wild fish information — past and present.
Access back issues of The Osprey at:
https://www.ospreysteelhead.org/archives
Older issues available by request.

Editor’s Note: Pierce Clegg is the former owner of the famous Babine Norlakes Steelhead Camp in British Columbia and a long-time advocate for wild fish. The
Continued from previous page
Osprey
excellence
fisheries conservation
communications Winter 2024 • Issue No. 107 5
recipient of the Haig-Brown Conservation Award for
in
journalism and
Pink Salmon and their Profound Impact on Ocean Ecology
By Gregory T. Ruggerone and Alan M. Springer
In our article “Pink Salmon, the Overlord of Pacific Ocean” (The Osprey, September 2019, https://www.ospreysteelhead.org /archives), we wrote that more Pacific salmon returned to rivers and hatcheries from the North Pacific Ocean during 2005 to 2015 than at any time since 1925. Since then, Pacific salmon abundance has continued to increase, reaching nearly 1 billion fish in 2021. Think about this number. And recall that fewer than 3 million Pacific salmon typically return to the once mighty Columbia River each year, including those harvested in ocean fisheries.
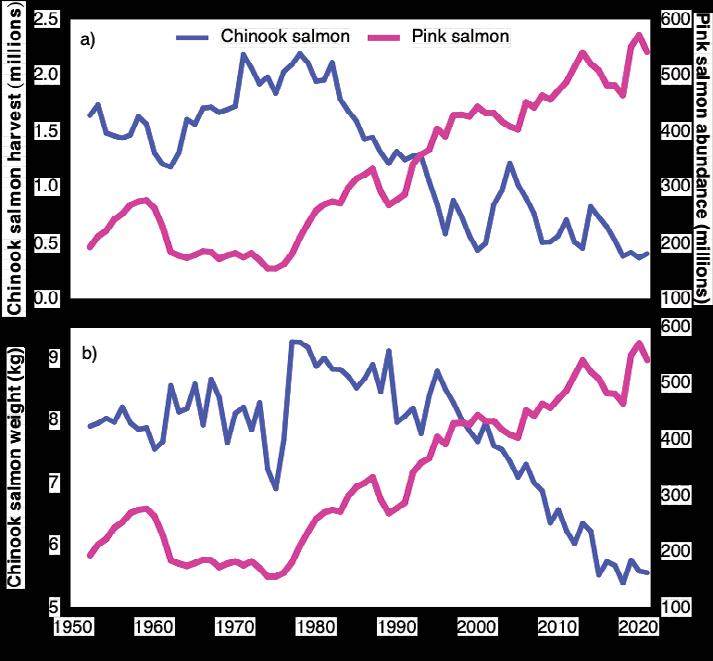
This spectacular surge is linked primarily to pink salmon (Oncorhynchus gorbuscha), the obvious winner in the unprecedented climate change sweepstakes. Pink salmon now represent up to 80% of all Pacific salmon (Fig. 1) and their abundance is highly correlated with National Oceanic and Atmospheric Administration’s (NOAA) ocean heat index (https://www.climate.gov/news-
features/understanding-climate/climate-change-ocean-heat-content). Pink salmon can thank humans for their current success: we are largely responsible for heating of the North Pacific, according to Mike Litzow and colleagues, and we release up to 1.5 billion hatchery pink salmon each year. Furthermore, pink salmon are invading and rapidly colonizing habitats throughout the North Atlantic Ocean, thanks to Russian hatchery transplants and to ocean heating.
You might ask, “Are there consequences of the rapid rise and record abundances of pink salmon for other species in the ocean? Could they impact marine ecosystems, including other species of salmon, seabirds, and marine mammals? Can the ocean support wild salmon in addition to massive numbers of hatchery salmon and wild pink salmon?” These are difficult questions to answer. Some Russian and Alaskan colleagues have said answering questions like these is like “catching a moon beam in a bottle.” However, pink

salmon life history characteristics offer a unique scientific approach for addressing these important issues.
Biennial Pink Salmon Cycle
Pink salmon abundance varies tremendously every other year throughout much of their range in both North America and Asia. Abundance is high in odd-numbered calendar years (25X or more) and low in even-numbered calendar years, owing to their fixed two-year life cycle. That biennial pattern provides a natural experiment or tool to test for interactions, such as competition for prey, between pink salmon, other salmon, and other marine species. If competition is important, then we expect to see biennial patterns in key metrics such as diet, growth, survival, and abundance of other species. It is important to note that physical oceanographic factors do not show similar biennial patterns.
In September 2023, we published, “From diatoms to killer whales: impacts of pink salmon on North Pacific ecosystems.” That synthesis of existing publications and new data revealed consistent and robust evidence for the effects of pink salmon on individual species and on marine ecosystems throughout the North Pacific and Bering Sea. We described an open ocean trophic cascade caused by pink salmon, and effects of pink salmon on forage fishes (herring, Atka mackerel, Pacific Ocean perch, and sand lance), squid, all five species of Pacific salmon and steelhead, 11 species of seabirds, humpback whales, and critically-endangered southern resident killer whales (see Table 1 in the manuscript for a summary of impacts by region). Here, we briefly summarize findings about pink salmon’s effect on ecosystem function via trophic cascades; on Chinook salmon, coho salmon, and steelhead; and on seabirds and killer whales. Evidence for pink salmon interactions with other species is available from the on-
Continued on next page
6 The
Osprey
Pink salmon returning to Prince William Sound, Alaska hatcheries have contributed to record-setting abundances in recent years and to impacts on other marine species. Photo by Preston and Teresa Cole, www.taps-photography.pixels.com
line manuscript and the supplemental documents, which are available for free as a feature article.
Pink Salmon Cause a Trophic Cascade in the North Pacific Ocean
A trophic cascade occurs when a predator population increases or decreases, leading to large reciprocal changes in its prey population, ripple effects down through the food chain, and changes in ecosystem structure. In the North Pacific and Bering Sea, pink salmon are key predators of zooplankton, which consume phytoplankton.
Using data since 1954, Japanese scientists provided the initial evidence indicating that pink salmon could cause trophic cascades. Then in 2018, Sonia Batten and colleagues analyzed 15 years of data collected by plankton recorders towed behind commercial ships in the Bering Sea and North Pacific. They found three lines of evidence supporting this hypothesis. First, in odd years when pink salmon were more abundant, zooplankton abundance was low and phytoplankton abundance was high; in even years, when pink salmon were less abundant, zooplankton abundance was high and phytoplankton abundance was low. Second, zooplankton abundance was negatively correlated with the abundance of pink salmon known to occupy this region (mostly the eastern Kamchatka stock), whereas phytoplankton abundance was positively correlated with pink salmon abundance. Third, in 2013 the runs of pink salmon returning to eastern Kamchatka declined dramatically, zooplankton were exceptionally abundant, and phytoplankton abundance was low; i.e., just the opposite of expectations for odd years when pink salmon abundance is typically high. As trophic cascades involve the primary building blocks of marine food webs, they provide the initial evidence for how pink salmon may directly and/or indirectly affect other marine species and ecosystems.
Pink Salmon Affect Chinook Salmon
Chinook salmon is a species in crisis. Chinook salmon abundance during the past several decades has declined throughout their entire range, including wild populations in pristine regions of
Alaska and Russia. This decline is associated with reduced size at age and younger age at maturation, leading to overall smaller fish and lower fecundity.
Salmon biologists and managers often believe Chinook and pink salmon do not compete for prey in the open ocean because pink salmon are considered to be planktivores and because chemical markers, which reflect trophic level, fail to detect the switch in pink salmon diet to squid and fishes during their last 2 or 3 months at sea. For example, a 10-year salmon diet study by Nancy Davis, formerly at the University of Washington, found significant diet overlap between Chinook and pink salmon, with both species consuming 60% or more high-energy squid and small fishes (Fig. 2). In odd years, when subadult pink salmon were approximately 40 times more abundant than in even years, total prey weight consumed per Chinook salmon declined 56% versus only 23% per pink salmon. In odd years, Chinook salmon consumed 72% less squid and 44% less fish, but 44% more zooplankton than in even years.


Many factors have led to declining abundance and size of Chinook salmon over the years, and evidence indicates competition with pink salmon is important. Maturing pink salmon are approximately 300 times more abundant than Chinook salmon and they reduce the amount of high energy prey required by Chinook salmon. For example, Chinook salmon body size and harvests in Alaska and British Columbia over the
past 70 years were opposite the increasing abundance of pink salmon in the North Pacific (Fig. 3). Likewise, from 1977 to 2022, increasing pink salmon abundance explained 70% of the decline in average body size and 43% of the decline in commercial harvests of Chinook salmon in Russia.
Additional evidence supports our hypothesis that pink salmon affect Chinook salmon. For example, the average lengths of all 11 populations of age-1.4 Chinook salmon and 9 of 10 age-1.3 populations for which data were available
Continued on next page
Winter 2024 • Issue No. 107 7
previous
Continued from
page
Figure 1. Pink salmon abundance in North America and Asia has reached record-high levels in response to the heating of the North Pacific Ocean, due to climate change, and hatchery production.
Fig. 2. Average weight of stomach contents of pink and Chinook salmon sampled in the central Bering Sea during oddand even years, 1991-2000 (UI = unidentified). Pink salmon were 40 times more abundant in odd years.
that most of these Chinook salmon originated from western and central Alaska, regions where abundance has plummeted.
In the Salish Sea, sub-yearling hatchery Chinook salmon smolts released from 13 hatcheries experienced a 59% decline in marine survival, on average, when released during even years with numerous juvenile pink salmon compared with those released during odd years with few juvenile pink salmon, 1984−1997 (Fig. 5a). A new analysis of scale growth of Skagit River, Washington Chinook salmon, 1961-2000, revealed significantly lower early marine growth during even years when numerous pink salmon were present (Fig. 5b). Additionally, analyses of hatchery Chinook salmon from 1983 to 2012 found lower density-dependent survival and fewer adult returns of hatchery Chinook salmon when released into the Salish Sea during even years when juvenile pink salmon were abundant.
declined with increasing pink salmon abundance. The Chinook salmon populations ranged from the Yukon River in the Bering Sea, to the Copper River in the northeastern Gulf of Alaska, and to the Unuk River in Southeast Alaska. The average age of 4 of 5 Chinook salmon populations declined with increasing abundance of pink salmon. In the Yukon, Kuskokwim, and Nushagak
rivers, Chinook salmon scale growth during the third and fourth years at sea, the percentage of returning age 6 (age-1.4) and older Chinook salmon, and their productivity tended to decline with increasing pink salmon abundance.
An analysis of Chinook salmon catches in the Japanese high seas salmon fishery in the western/central Bering Sea and North Pacific revealed a biennial pattern in the catch during 1955−1981, a 27 year period. The mean catch in odd years (254,000 Chinook salmon) was 39% lower than the catch in even years (417,000 Chinook salmon), and Chinook salmon catch was negatively correlated with pink salmon catch (Fig. 4). The biennial catch was driven by the predominance of Chinook salmon in their third year at sea in the size-selective gillnet. Scale analysis indicated
Many Chinook salmon from the Pacific Northwest migrate north to feed in the North Pacific and Bering Sea. For example, genetic analyses by Larson and colleagues indicated 11−38% of Chinook salmon sampled in the southeastern Bering Sea during 2005−2010 originated from the Pacific Northwest, raising concern by those authors that climate warming may be shifting salmon from the Pacific Northwest into a crowded Bering Sea where temperatures are cooler. In support of this hypothesis, Buckner and colleagues analyzed the growth of 48 stocks of Chinook salmon returning to hatcheries and spawning grounds in the Columbia River Basin, Oregon coast, and Washington coast (brood years 1976−2013), and found that growth of far-north migrating Chinook salmon was negatively associated with pink salmon abundance. The effect of pink salmon on Chinook salmon growth was stronger than that of oceanographic variables, which are often thought to be driving salmon production.
Our manuscript provides evidence indicating that reduced consumption of high-energy prey, growth, body size, survival, and abundance of Chinook salmon are linked to high abundances of pink salmon. In a warming ocean, bioenergetic studies by Dave Beauchamp and Nancy Davis indicated large salmon, such as Chinook salmon, require more high energy prey or greater consumption to meet metabolic
Continued on next page
8 The Osprey Continued from previous page
Figure 3. Trends in Chinook salmon harvests in Alaska and British Columbia (upper graph) and average size of Chinook salmon in Alaska (lower graph) from 1952 to 2021 are opposite average North Pacific pink salmon abundance during the four years prior to harvest, i.e., the period of species overlap.
195519601965197019751980 -300 -200 -100 0 100 200 300 400 500 600 Year OddyearEvenyear Interannual change in Chinook salmon catch (1,000s)
Figure 4. From 1955 to 1981, the Japanese high seas salmon fishery caught an average of 417,000 Chinook salmon per year in even years when few pink salmon were present, but only 254,000 in odd years when pink salmon were abundant, a 39% decline.
Continued from previous page
demands and efficiently grow compared with smaller pink salmon. Thus, ocean heating from human-induced climate change has exacerbated competition with pink salmon for high energy squid and fish prey through 1) increased abundances of pink salmon, and 2) greater bioenergetic requirements of Chinook salmon.
Pink Salmon Affect Coho Salmon
Several studies indicate pink salmon affect the growth and survival of coho salmon. We updated the analysis by Leon Shaul and Hal Geiger and found body size of coho salmon returning to Southeast Alaska exhibits a biennial pattern and is reduced in relation to increasing pink salmon abundance, 19702021 (Fig. 6). The strong biennial pattern in coho salmon body size was related to predation on squid (Berryteuthis anonychus), which exhibits biennial abundance. Pink salmon also consume squid and affect squid abundance, according to Elaina Jorgensen. In the Kuskokwim River in western Alaska, commercial coho salmon harvests in odd years averaged 33% less compared with even years (avg. 225,000 versus 336,000 fish), 1965−2007. Dick Beamish reported that juvenile coho in the Salish Sea had more empty stomachs and the abundance and survival of hatchery coho salmon declined in even years when numerous pink salmon were present, findings consistent with those of juvenile Chinook salmon in this region.
Pink Salmon Affect Steelhead
Steelhead travel farther at sea than other salmonids, and tag data indicate their distribution overlaps with abundant Russian pink salmon. Research by Megan Atcheson, Kate Myers, and others found that the percentage of steelhead with empty stomachs and their consumption of squid in the central North Pacific declined with greater abundances of pink salmon, which also consume squid. With the assistance of Canadian steelhead scientists, we found that summer-run steelhead returning to the Thompson and Chilcotin rivers in the interior Fraser River (classified as Endangered by the Committee on the Status of Endangered Wildlife in Canada) and winter-run steelhead returning to the Keogh River on northeast

Figure 5. (a) Mean smolt to adult survival of juvenile Chinook salmon released from 13 hatcheries in the Salish Sea (Washington and British Columbia) during even years (numerous juvenile pink salmon) and odd years (nearly zero juvenile pink salmon), 1984−1997, and (b) average early marine scale growth in the Salish Sea of adult Skagit River Chinook salmon during even- and odd years, 1961−2000.
Vancouver Island experienced reduced survival with increasing abundance of pink salmon during the past 35 to 40 years (Fig. 7a,b). Likewise, B-run steelhead returning to the Columbia River exhibited a strong pattern of biennial abundance, especially after year 2000 when the biennial pattern of pink salmon abundance became more extreme (Fig. 7c).
Pink Salmon Affect Seabirds
Research by Alan Springer, Gus van Vliet, and others has revealed a biennial signal in the breeding biology of 11 species of seabirds nesting in the Aleutian and Pribilof islands in the Bering Sea, the Gulf of Alaska, and Prince William Sound, and for one southern hemisphere migrant species. For example, the nesting period of several resident species is late in odd years when pink salmon abundance is high and early in even Winter 2024 • Issue No. 107

Southeast Alaska, 1970-2021, declined up to 35% with increasing abundance of North American pink salmon, while accounting for effects of the Pacific Decadal Oscillation. Coho weights in 2020 and 2021 were outliers. See manuscript.
Continued on next page
9
Figure 6. Average weight of adult coho salmon in
Pink Salmon Affect Southern Resident Killer Whales
years when pink salmon abundance is low—early nesting phenology is commonly associated with good physiological condition (Fig 8). Productivity of black-legged and red-legged kittiwakes is depressed by up to 62% in odd years compared to even years, and 30% to 40% of seabird productivity can be explained by pink salmon abundance. Migrant short-tailed shearwaters that nest in Australia spend their winter season primarily in the Bering Sea, where they have been found to be in poorer physio-
logical condition and die in greater numbers in odd years than in even years. Moreover, they commonly experience high mortality as they arrive on their nesting colonies at the end of a 7,500 km non-stop migration, and nest in fewer numbers, in odd years compared to even years. Both of those observations reveal an uncommon example of an ecological transhemispheric teleconnection. All of these seabirds compete with pink salmon for common prey.
Southern resident killer whales (SRKW) never eat pink salmon, yet this Endangered Species, which ranges from central California to mid-Vancouver Island and into the Salish Sea, shows a highly unusual biennial pattern in both successful births and mortality. From 1998−2020, mortality of newborn and older orcas was 3.1 times higher (65 versus 21 deaths) and successful births 42% lower (19 versus 33 calves) in even years than in odd years as the population decreased from 92 to only 74 individuals (Fig. 9). This biennial pattern was not apparent during the earlier period (1976-1997) when Chinook salmon, critical prey of this population, were more abundant and pink salmon were less abundant.
Recent body condition measurements of SRKW taken by drones support the pink salmon hypothesis. Joshua Stewart and colleagues found that the body condition of calves, juveniles, subadults, adult males, adult females, and senior females of the L pod was consistently less robust in September of odd years after many pink salmon were present than in even years.
We hypothesized in 2019 that pink salmon escapement to Salish Sea rivers, which increased 135% during the period of orca decline, interfered with the foraging efficiency of orcas as they attempted to capture Chinook salmon. Both Chinook and pink salmon concentrate along the west side of San Juan Island and into Boundary Pass from late July through early September, but pink salmon are only abundant in odd years. Effects of reduced foraging efficiency may be expressed approximately one year later (in even years) as suggested by earlier research. We know of no other species or environmental variable that might cause such a strong biennial pattern in SRKW.
Understanding the mechanism of this biennial pattern is crucial to the recovery of SRKW. For example, if births and mortality during even years had been similar to those during odd years, the population would have substantially increased (rather than decreased) during the past 20 years (Fig. 9).
Ocean Carrying Capacity
The synthesis of pink salmon interactions with other marine species pro-
Continued on next page
10 The
Continued from previous page
Osprey
Figure 7. Influence of North Pacific pink salmon abundance on (a) the productivity of Thompson River summer-run steelhead (Fraser River watershed, BC), and (b) smolt to adult survival of Keogh River winter-run steelhead (NE Vancouver Island). (c) Interannual change in abundances of Columbia River B-run summer steelhead (black line) and North Pacific pink salmon (pink line).
Continued from previous page
vided robust and consistent evidence that the carrying capacity of this ocean food web is limited. Many species compete for common prey, and pink salmon is a highly successful competitor. The effects of pink salmon competition span multiple decades, including the period before the 1977 ocean climate regime shift when salmon abundance was much lower. Widespread effects have been observed in the North Pacific, Bering Sea, Gulf of Alaska, Prince William Sound, and the Salish Sea. We conclude that the belief by some agencies and managers that unlimited numbers of hatchery salmon can be released into the ocean without consequences for wild salmon is misguided. Large releases of hatchery salmon have consequences for both local and distant wild salmon populations because salmon travel thousands of miles at sea.
The Ocean is Filled with Hatchery Salmon
Annual releases of Pacific salmon from hatcheries increased 6-fold from 1970 to 1990 (0.9 billion versus 5.1 billion juveniles), producing approximately 25% of all adult salmon, or 40% of the total mature and immature salmon biomass at sea. Returns of adult hatchery pink salmon averaged 82 million fish during 2005 to 2015, of which 70% was produced in Alaska (20102015). Hatchery pink salmon production was much greater than that of wild chum salmon and approximately equal
to that of wild sockeye salmon.
Alaska is a major producer of hatchery salmon, even though habitat is largely intact. Hatchery salmon releases in Alaska have increased steadily since the 1990s and have recently reached 1.9 billion fish per year (Fig. 10). Approximately 5.7 times more hatchery salmon were released by Alaska during 2018-2022 than by the combined states of Washington, Oregon, Idaho, and California where habitat is degraded.
Furthermore, the ratio of hatchery to wild salmon harvests in the Gulf of Alaska region of Alaska is very high when excluding wild pink salmon that
have benefited from climate warming. During the past five years, 5.1 hatchery salmon have been harvested for every wild salmon in the region extending from Chignik in the west to Southeast Alaska. The percentage of hatchery-origin salmon in the harvests was high for each species: chum (80%), pink (43%), coho (29%), sockeye (24%), and Chinook salmon (23%). Hatchery Chinook salmon is greatly underestimated because many non-Alaskan hatchery Chinook salmon are harvested in Southeast Alaska. These percentages exclude Chignik where no hatchery production occurs.
The reliance of Alaska on hatchery


sources: ADFG and NPAFC websites and reports.
Continued on next page
Winter 2024 • Issue No. 107 11
Figure 8. Hatching phenology of tufted puffins at Buldir Island (Aleutian Islands) during odd (black bars) and even years (open bars). Positive values are late years, negative values are early years.
Figure 9. Endangered southern resident killer whales (SRKW) have shown a strong biennial pattern in deaths and births during 1998 to 2020 consistent with the hypothesis that abundant pink salmon interfere with foraging on Chinook salmon in odd years, leading to higher death rates and fewer successful births in the following even year. Body condition of the L pod is lower in odd years after encountering numerous pink salmon in the Salish Sea.
!"# !"$ !# $ # "$ "# %$ "&'' "&&$ "&&% "&&( "&&) "&&' %$$$ %$$% %$$( %$$) %$$' %$"$ %$"% %$"( %$") %$"' %$%$ *+,-./0+,1/+234+56 +B
Figure 10. Numbers of hatchery salmon (all species, red bar) and wild salmon (blue bar) harvested annually in Alaska (excluding Bering Sea region), and the number of Alaskan hatchery salmon released into this region of the North Pacific each year, 1977 to 2022. Wild salmon harvests exclude wild pink salmon, which have been productive over time. Data
salmon production may be surprising to many people, especially since State policy requires managers to use the precautionary principle to protect wild salmon from potential adverse effects of hatchery salmon. A recent synthesis by John McMillan and colleagues on the effects of hatchery salmonids on wild salmonids reported that 83% of more than 200 peer reviewed publications concluded adverse to minimally adverse effects from hatchery salmonids. This finding is not surprising for those of us that have tracked these studies over the past few decades, but the reliance on hatchery salmon production in relatively pristine regions is surprising given what has been learned about genetic and ecological effects of hatchery salmon on wild salmon in recent decades.
Greg Ruggerone, Ph.D, is an aquatic scientist with interests in salmon ecology, population dynamics, and interactions between hatchery and wild salmon. Over the past 20 years he has used the biennial pattern of pink salmon to quantify their interactions with other species.
Alan Springer, Ph.D, is a marine ecologist with particular interests in food web structure, causes of variability, and relationships to seabird and marine mammal populations. He is Professor Emeritus at the College of Fisheries and Ocean Sciences, University of Alaska Fairbanks.
Suggested Reading
Batten, S.D., G.T. Ruggerone, and I. Ortiz. 2018. Pink Salmon induce a trophic cascade in plankton populations around the Aleutian Islands. Fisheries Oceanography 27:548-559.
Litzow, M.A., M.J. Malick, T. Kristiansen, B.M. Connors and G.T. Ruggerone. 2023. Climate attribution time series track the evolution of human influence on North Pacific sea surface temperature. Environment Research Letters 19: 014014 DOI 10.1088/1748-9326/ad0c88
McMillan, J.R., B. Morrison, N. Chambers, G.T. Ruggerone, L. Bernatchez, J. Stanford, and H. Neville. 2023).A global synthesis of peer-reviewed research on the effects of hatchery salmonids on wild salmonids. Fisheries Management and Ecology 30: 446-463. https://onlinelibrary.wiley.com/doi/10.1111/fme.12643
Ruggerone, G.T. and J.R. Irvine. 2018. Numbers and biomass of natural- and hatchery-origin pink, chum, and sockeye salmon in the North Pacific Ocean, 1925-2015. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science 10:152-168.
Ruggerone, G.T., A.M. Springer, L.D. Shaul, and G.B. van Vliet. 2019. Unprecedented biennial pattern of birth and mortality in an endangered apex predator, southern resident killer whales in the eastern North Pacific Ocean. Marine Ecology Progress Series 608:291-296.
Ruggerone, G.T., J.R. Irvine, and B. Connors. 2021. Did recent marine heatwaves and record high pink salmon abundance lead to a tipping point that caused record declines in North Pacific salmon abundance and harvest in 2020? North Pacific Anadromous Fisheries Commission Technical Report No. 17:78-82. Available: https://www.int-res.com/abstracts/meps/v719/
Ruggerone, G.T, A. Springer, G.B. van Vliet, B. Connors, J.R. Irvine, L.D. Shaul, M.R. Sloat, and W.I. Atlas. 2023. From diatoms to killer whales: impacts of pink salmon on North Pacific ecosystems. Marine Ecology Progress Series 719:1-40. https://www.int-res.com/abstracts/meps/v719/p1-40/
Shaul, L.D., and H.J. Geiger. 2016. Effects of climate and competition for off shore prey on growth, survival, and reproductive potential of coho salmon in Southeast Alaska. North Pacific Anadromous Fish Commission Bulletin 6:329–347.
Springer, A.M. and G.B. van Vliet. 2014. Climate change, pink salmon, and the nexus between bottom-up and top-down forcing in the subarctic Pacific Ocean and Bering Sea. Proceedings of the National Academy of Sciences 111 (18) E1880-E1888.

12 The Osprey
previous page
Continued from
Sea Run Cutthroat Trout and the Environmental Factors Affecting Their Abundance
By Richard K. Stoll
Several years ago, I wrote a book on sea run cutthroat trout. After some substantial literature research, there appeared not to be a lot known about these fish. The exceptions were investigations in limited geographic areas in Puget Sound, Oregon, California, and southern British Columbia. What I can say is that populations have substantially decreased from historical numbers. These declines have been coincident with coastal development.
Sea run cutthroat trout, Onchorhynchus clarkii clarkii, are one of 14 Westslope coastal cutthroat trout subspecies. They occur from the rivulets of Prince William Sound, the location of the 1989 Exxon Valdez 10.5million-gallon oil spill, to the coastal lagoons and streams of northern California that have been substantially modified by anthropogenic activities. Since sea run cutthroat have very little commercial value there has, consequently, been very little scientific research on them.
To say that sea run cutthroat are anadromous like salmon and steelhead may be somewhat of a misnomer. They are amphidromous and do not make lengthy ocean journeys, but will migrate to local saltwater environs to feed on abundant near-shore biota before returning to their natal streams to spawn.
Landlocked populations of sea run cutthroat trout occur in local beaver ponds, wetlands, and other natural watershed impoundments. It is my opinion that in some areas these may be important in maintaining population numbers. I have little doubt that post-emergent juveniles inadvertently wash downstream during high water events. This appears to be the case in the highly degraded stream that I live next to. Unlike a few clean areas in contributing wetlands, spawning substates in my stream are substantially imbricated — an alignment of course pebbles on the streambed.
Most sea run cutthroat spawn between December to February in small coastal streams or tributaries to larger streams. This is notwithstanding their occurrence in estuaries and the lower
reaches of coastal rivers at certain times of the year. Juveniles migrate out of their natal streams into adjacent salt waters when they are approximately 6 to 8 inches in length, and sometimes smaller. Sea run cutthroat rarely get much more than 16 inches in length, although a few get as large as 21-inches.
When in saltwater, most cutthroats exhibit a great deal of site fidelity. They feed in very limited near-shore areas and do not migrate far, if at all. As a result, individual fish are often caught and released by anglers who fish on
Unfortunately, many of the small streams where sea run cutthroat spawn have been heavily degraded by logging, mining, agriculture and urban development.
certain beaches. This gives the impression that local population sizes are much larger than they actually are.
Unfortunately, many of the small streams where the sea run cutthroat spawn have been heavily degraded by logging, mining, agriculture, and urban development. Further, In Puget Sound in Washington State, waterfront residential and commercial development has caused beach erosion, interruption of longshore transport of sediments, and coincident hardening of natural substrates. This has led to a decrease in diversity of the marine life sea run cutthroat feed on.
Sea run cutthroat run timing and habitat may vary substantially. While there may be some limited drift between watersheds, this is not likely common considering the distances between local populations. They most often feed along the same shallow waters along the same beaches during and between years.
While some fish have been documented to migrate distances sometimes in excess of 50 miles, this appears to be rare.
Incidental hybridization between sea run cutthroat and steelhead is also known to occur. This likely happens when male cutthroat incidentally spawn with steelhead females to produce so called “cuttbows” that have characteristics of both species. Otherwise, spawning habitat is quite different. They do not actively interbreed.
In very recent years, sea run cutthroat trout have been heavily promoted by fishing shops and guide services, particularly in the fly angling community. Sea run cutthroat are gullible and voracious fish. They often attempt to prey on anything that makes a commotion in the water. This makes them easy targets for enthusiastic anglers. Fortunately, Washington State regulations for trout in marine waters mandate barbless hook and catch-andrelease. However, more important from a conservation perspective, most Puget Sound tidelands are privately owned unlike other West Coast states and British Columbia, and provide substantial cutthroat refugia
Richard Stoll is author of the book “Sea Run Cutthroat Trout. A Saltwater Angler’s Guide to Their Biology, Prey, Angling Strategies, and Conservation” published in 2017 by West Sound Angler.
References
l Coastal Cutthroat Coalition. https://coastalcutthroatcoalition.com/ l Johnson, L. 2004. Fly-Fishing Coastal Cutthroat Trout: Flies, Techniques, Conservation. Frank Amato Publications.
l Stoll, R. 2017. Sea Run Cutthroat Trout. A Saltwater Angler’s Guide to Their Biology, Prey, Angling Strategies, and Conservation. West Sound Angler.
l Trotter, P. 2008. Cutthroat, Native Trout of the West. Second Edition. University of California Press.
Winter 2024 • Issue No. 107 13
A Synopsis of Hatchery Salmonid Effects on Wild Salmonids
By John McMillan
Hatcheries have been widely used to propagate and release salmonids to subsidize fisheries, mitigate habitat loss, and attempt to rebuild depleted populations of wild salmonids. Their widespread use has raised numerous concerns, however, because hatchery salmonids generally display lower fitness, lesser diversity, and reduced spatial distribution compared to wild populations. The debate over hatcheries is further complicated by the immensity of the literature, which spans decades, continents, life histories, and species. These complexities, and others, can make it difficult to draw conclusions about the larger body of research.
To that end, several co-authors and I conducted a global literature review of peer-reviewed research (from 1970 –May 2021) to synthesize the existing body of science on how hatchery salmonids affect wild salmonids. We also created a database of all the publications to provide access to studies we reviewed. The database is available online and can be accessed for free (see Supplemental Information at the bottom of the publication’s website).
The review process revealed over 11,000 total potential hits. After several months of analysis, we identified 206 publications that met our criteria, though our synthesis technically represented a total of 207 studies because one study included two species with different results.
We delineated the 207 publications into a variety of categories (see publication for detailed description of Methods) ranging from year and country of publication to the type of hatchery (e.g., production, supplementation, recovery) the pathway of effect (e.g., genetic, ecological, disease, fishing), and whether the study was conducted on the ocean or freshwater phase of the life cycle. Each publication was classified as having an effect the author’s found to be beneficial, adverse, or minimally adverse on the recipient wild population, or as no effect or indeterminate (both

We reviewed 207 papers published between 1970 and 2021 on hatchery salmonids’ affect on wild salmonids. Seventy percent found adverse impacts.
beneficial and adverse effects were found).
Here is what we found in a nutshell.
First, publications per year generally increased from 1970 onward and peaked in 2015. The studies spanned 12 countries, with the most publications coming from the United States, and covered 15 species – with brown trout being the most studied species, followed by steelhead and Chinook salmon.
Second, hatchery effects on wild salmonids varied, but most publications (n = 144, 70 %) reported adverse effects on wild salmonids and another 26 articles (13 %) reported minimally adverse effects. Thus, 83% of the publications found some degree of adverse effects from hatcheries.
In contrast, only seven publications (3 %) reported beneficial effects of hatchery salmonids on wild salmonids, while 17 studies (8 %) reported no hatchery effects on wild salmonids, and 13 (6 %) were classified as indeterminate.
Third, most publications focused on production hatchery programs (n = 143), which are designed to produce fish for fisheries and almost exclusively use hatchery fish for broodstock. We found 28 studies on supplementation programs that often include some portion of wild fish for broodstock and produce fish for fishing and supplementing wild spawners, 17 studies on recovery programs that typically use all wild fish for broodstock to try and recover highly depleted stocks that are close to extinction, and 19 studies fo-
Continued on next page
14 The Osprey
Juvenile wild steelhead, about 3.5 inches long. A number of studies reviewed examined recovery programs using wild fish for broodstock to restore highly depeted stocks that are close to extinction. Photo by John McMillan.
cused on a combination of production and supplementation hatcheries.
Among hatchery types, publications reporting adverse effects accounted for 74 % and 64 % of studies on production and supplementation programs, respectively, though another 17 % of the publications on production hatcheries also reported minimally adverse effects. No studies on supplementation programs reported minimally adverse effects, and only 7 % of supplementation programs reported a beneficial effect.
Publications on recovery programs was more balanced, with 30 % reporting adverse to minimally adverse effects, while 29 % found beneficial hatchery effects – though four of the five studies that reported beneficial effects were duplicative studies on the same programs. Regardless, the beneficial results were most common in situations where smaller-scale hatchery programs used all wild fish to provide a boost to highly depleted populations at great risk.
Fifth, most publications (n = 126) tested or evaluated how hatchery salmonids affected wild salmonids via genetic pathways and most of those reported adverse or minimally adverse genetic impacts to wild populations. A total of 60 studies evaluated ecological hatchery effects, with 44 reporting adverse effects, and 12 of 17 studies that looked at both genetic and ecological processes also found adverse impacts. In comparison, disease and fishery effects were far less studied.
Last, we found a large and growing body of science that indicates large re-
leases of hatchery salmonids (primarily pink salmon) are negatively correlated with growth, survival, and production of wild salmonoids in the open ocean – including some stocks of Chinook salmon and steelhead in the lower-48 of the USA.
In conclusion, our results – in addition to two similar reviews by Miller et al. 1990 and Araki and Schmid (2010) –suggest hatcheries commonly exert a range of negative genetic and ecological impacts on wild salmonids, and only rarely provide benefits. If salmon are to adapt and remain resilient through climate change, they will need all the diversity and productivity they can muster, which is why our review is topical for those entrusted with recovering wild salmonids.

John McMillan is Science Director for The Conservation Angler, one of The Osprey’s supporting organizations. Learn more about their work at www.theconservationangler.org



Continued from previous page Winter 2024 • Issue No. 107 15
A female coho salmon swims against the current at a raceway water inlet, Nehalem Hatchery, Oregon. Photo by Jim Yuskavitch
Removing eggs from a female Chinook salmon, Klaskanine Hatchery, Oregon.
Photo by Jim Yuskavitch
Hatchery workers process salmon during the spawning run, Big Creek Hatchery, Oregon. Photo by Jim Yuskavitch
“A global synthesis of peer-reviewed research on the effects of hatchery salmonids on wild salmonids,” https://doi.org/10.1111/fme.12643
Read the Paper
Steelhead Artificial Propagation and Epigenetics
By Guy W. Fleischer
In the recent paper by McMillian et al. (See McMillan’s article beginning on page 14) the authors offered an exhaustive review of the body of peer-reviewed literature to address the ongoing debate over global hatchery practices where salmonid recovery efforts are underway. They found that a demonstrable majority of the publications reported adverse impacts on natural-origin salmonids, with adverse genetic effects the most common, followed by ecological and genetic influences affecting productivity and abundance. It now can be safely expected that hatchery rearing practices routinely have adverse impacts on extant wild salmonids in freshwater and marine environments.
In conclusion, they called for future research on less studied effects to improve knowledge and management of the full extent of hatchery impacts. In this statement, they specifically identified epigenetics as one of these research areas.
Epigenetics 101
In lay terms, epigenetics refers to chemical modifications of deoxyribonucleic acid (DNA) that influence gene activity and expression without changes in DNA sequence. Epigenetic effects are not genetic mutations. They are alterations in how an organism’s genes are internally adjusted in response to its environment. Epigenetics, well established in the field of human health, is a proven molecular mechanism describing the process for modulating genetic expression and offers deeper insight of phenotypical changes observed in organisms in response to their environment.
As quick background, in the 1940s, developmental biologist Conrad Waddington of the University of Edinburgh reported that fruit flies exposed to outside changes in temperature during embryonic development resulted in varying wing structures (Waddington 1940). Waddington found that external stimuli, temperature in this case, would change wing structure and then that be-
came heritable for many generations. This phenomenon revealed that classic Mendelian genetics could not explain this change in phenotype. [Editor’s Note: Phenotype refers to an individual’s observable traits, such as height, eye color and blood type. Phenotype is determined by both genomic makeup (genotype) and environmental factors.] Wadington coined the term ‘epigenetics’ [the Greek prefix epi- implies features that are "on top of" or "in addition to" the traditional genetic basis for inheritance] because it was around the
Epigenetic effects are not genetic mutations. They are alterations in how an organism’s genes are internally adjusted in response to its environment.
DNA, not involving the genomic sequence. These observations were the basis of the field now known as epigenetics. Skinner (2014), Hanson and Skinner (2016), and Nilsson et al (2018) offer the interested reader more detail on this topic.
In a nutshell, epigenetic modulation involves primarily the process of DNA methylation. [Editor’s Note: Methylation is a chemical modification of DNA and other molecules that may be retained as cells divide to make more cells. When found in DNA, methylation can alter gene expression.] This was the first epigenetic phenomena identified. Basically, when hypermethylated a gene is turned off. If it's less methylated, the gene can turn on. Geneticists now know there's about five different epigenetic markers and they all interact with each other to regulate the genome, but the one intertwining element is DNA methylation (Figure 1). When these epigenetic marks bind to
DNA in or near genes, they alter the amount of RNA or protein made from the genes — similar, perhaps, to how modern traffic signal systems manage traffic along fixed roadways in response to changing traffic patterns. A good example of epigenetics at play is in the different cell types we all have. Even though virtually all cells in an organism contain the same genetic information, not all genes are expressed simultaneously by all cell types. It is epigenetic mechanisms that derive the variety of cells and tissues in multicellular organisms. Epigenetic mechanisms are also what allow organisms to adjust and adapt by means of gene expression to their environment.
Epigenetic modulation of gene expression, a well understood mechanism, has been broadened from human health applications to ecology and the field of conservation biology, including profound implications for our understanding of evolution (e.g., Banta and Richards 2018, Skinner and Nilsson 2021, Stajic et al 2022). Rey et al. (2019) argues, for example, that epigenetic variation, and more particularly the process of DNA methylation, represents a molecular component of biodiversity that directly links the genome to the environment. They conclude that with an epigenetic conservation perspective, this insight can give environmental managers the possibility to better define Ecologically Significant Units (ESUs). In addition to stock delineation, epigenetics has possible applications to understanding natural selection and adaptation. Chapelle and Silvestre (2022) conclude that epigenetic inheritance in wild animal populations might reshape the way ecologists generate predictive models of fitness and the capacity of organisms to adapt to changing environments. These and other studies offer one clear insight: Epigenetics, independent of the genome, allows organisms to alter gene expression and develop enzymes and proteins to response to changing environmental cues, which is, if you think about it, smart from an evolutionary
16 The Osprey
Continued on next page
Continued from previous page
and fitness point of view. And when epigenetic changes happen, they are mitotically stable, meaning they are heritable.
Epigenetics and Hatchery Practices
It is no secret that hatchery-reared salmonids differ from natural-origin fish both in phenotype and in having reduced reproductive success in the wild (e.g., Araki et al. 2007, Fraser 2008, Frankhan 2008, Brown 2016, Christie et al 2014, Christie et al 2016, Ford et al 2016, Berejikian et al 2012, Melnychuk et al 2013, Brown et al 2013, Christie et al 2016, Marchetti and Nevitt 2003). Pathologies reported include decreased fitness of hatchery-reared fish and offspring in the wild, and a myriad of altered traits that include changes in age at spawning, morphology, growth rate, brain morphology, anti-predator behavior, decreased egg size, and migration (including straying) — all linked to intensive culturing practices where hatchery-altered fish are released back into the wild.
The reduced survival of hatchery origin steelhead compared to their wild counterparts has been demonstrated in many instances (Figure 2).
To explore the underlying molecular impacts of hatcheries, several studies specifically on steelhead epigenetics have been recently published. Gavery et al. (2018) reported differences in DNA methylation between adult hatchery- and natural-origin steelhead from the same population, documenting epigenetic differences that could otherwise not be distinguished by DNA sequence variation with significant differences in epigenetic programming in both somatic and germ cells. [Editor’s Note: Germ line cells are sex cells (eggs and sperm) that sexually reproducing organisms use to pass on their genomes from one generation to the next (parents to offspring). Egg and sperm cells are called germ cells, in contrast to the other cells of the body, which are called somatic cells.] These results raised the possibility that such DNA methylation changes occurred during the first year in the hatchery and that they persist into adulthood in the form of an ‘epigenetic memory’ of the hatchery environment. The idea that epigenetic mechanisms may serve as the link between early-environmental exposures
and adult phenotypes (i.e., ‘developmental programming’) is implicated. This work was among the first to demonstrate the potential for transgenerational inheritance of epigenetic information in hatchery steelhead by reporting differences in DNA methylation in the male steelhead germline.
The observation that epigenetics could distinguish hatchery- from natural-origin steelhead from the same population formed the basis of a follow-

epigenetic
modulation of genetic information that is encoded by stretches of DNA inside the chromosomes of each cell. But another layer of information is encoded in epigenetic marks, which include chemicals such as methyl (CH3) that attach to the DNA and to the histone groups that the DNA encircles. When these epigenetic marks bind to DNA in or near genes, they alter the amount of RNA or protein made from the genes. Source: Skinner (2014)

up study by Gavery et al (2019) where they compared DNA methylation patterns between fish reared in conditions of traditional hatchery tanks and a simulated stream-rearing environment. For the simulated natural environment, fertilized eggs were placed in gravel to simulate a redd constructed by a naturally spawning steelhead. Embryos completed development within the interstitial spaces in the gravel, emerged naturally, and began exogenous feeding on natural aquatic insects and other in-
vertebrates, until they were sampled eight months later. They found substantial differences in DNA methylation patterns between hatchery- versus stream-reared fish after a year of common rearing.
More recently, Nilsson et al (2021) investigated the impacts on a molecular level of hatchery-origin steelhead as compared to wild-origin fish. Again, the researchers found negligible genetic differences between the two fish popu-
Continued on next page
Winter 2024 • Issue No. 107 17
Figure 1. Schematic representation of DNA methylation, histone and other chemical modifiers involved in
gene expression
Figure 2. Comparison of smolt survival rate of natural versus hatchery origin steelhead. Source: Coastal Steelhead Proviso Implementation Plan, Washington Department of Fish and Wildlife
lations, however they identified a distinct set of epigenetic features, called DNA methylation regions (DMRs), in both the sperm and red blood cells of the hatchery-raised steelhead that weren’t present in their wild-raised counterparts, demonstrating clearly the epigenetic alterations in gene expression. The full and precise impact of each of these DMRs could not be determined at this time even with a more genome-wide analysis, but they recognized that exposure of somatic cells leading to epigenetic changes (e.g. DNA methylation) act as red flags and likely impact the exposed individuals’ health and phenotypes, which would correspond to hatchery populations having reduced fertility, abnormal health, and survival when compared to the wild populations. The authors concluded that hatchery operations, particularly with feeding practices that promote faster growth, should consider “not allowing interbreeding with wild fish populations. If not, impacts on wild-raised steelhead trout and the environment could be dramatic and alter the future trajectory of the overall population.”
Conclusions and Additional Remarks
The reduction of fitness from hatchery practices can now be directly addressed with the advance of molecular science, specifically in observable markers due to epigenetic programming. In the case of steelhead, hatchery-origin fish exhibit well-documented epigenetic changes in both somatic and germline cells that are linked to the hatchery environment and can be inferred as driving the decrease in fitness of the fish. This fact should not come as a surprise as the environmental landscape and feeding ecology of a typical fish hatchery is deprived of all the natural features that work to select for fitness that a wild fish would encounter. The observed reduction in fitness is not news to hatchery managers. The irony is that agencies running hatcheries have always depended on natural-origin populations to bolster dwindling hatchery stocks.
Moreover, the research on steelhead epigenetics also shows that these impacts aren’t limited to the fish directly reared in a hatchery. Through epigenetic inheritance, these altered phenotypes developed in the hatchery can be
passed to the next generation of offspring when such fish reproduce in the wild, even in the absence of the original source of the alteration. This characteristic is particularly concerning for considerations of endangered or threatened populations from both within- and outside-basin fish due to not just heritability but also the well-established lack of fidelity (straying) exhibited by hatchery-origin steelhead.
 Jim Yuskavitch
Jim Yuskavitch
Hatchery managers and fishery management agencies must now face the fact that the previous genetics studies and published information are not relevant nor accurate without the integration of epigenetics. This is a powerful science, and every fishery management agency needs to come to grips with the fact that the environment in hatcheries have dramatic documented impacts on fish physiology with implications for generations to come. Nilsson et al (2021) point out that the epigenetic alterations observed could be used as biomarkers to further identify hatchery impacts on the fish. They also suggest that such markers also correlate with phenotypic alterations that likely result from migration impacts of dams, behavioral impacts from predators, water temperature and quality, and even ocean conditions. This molecular tool
also has possible use for better delineation of population diversity.
Wild steelhead display complex life histories that have been forged over eons by evolution to adapt and adjust to past natural changing conditions. Each river system, including its tributaries, supports fish with heritable characteristics and strategies uniquely adapted to the hydrology and habitats from which they were spawned, whereas hatchery practices work against this perfectly evolved diversity in favor of a blunt and conforming means strictly to support the short-sighted demands for a fishery. In a recent in-depth review regarding the role of hatcheries in supplementing wild populations of fish, Claussen and Philipp (2023) concluded that “we need to stop treating wild fish as livestock and stop pretending artificial propagation is a means to address conservation issues for wild populations.” Major changes in hatchery practices, which can be documented by epigenetic markers, are clearly needed to address their impacts on physiology and health of steelhead and reduce the creation of irregular, ill-fit phenotypes. In the meantime, the practice of diverting natural-origin steelhead to become ‘broodstock’ is simply wrong-headed: Let these proven survivors continue to propagate their derived fitness from nature.
Despite their time-tested adaptability over the eons, the one condition wild steelhead populations have not had to face until recently is the impact of the heritable epigenetic distortion leading to reduced fitness spread from rampant artificial propagation programs. This reduced fitness is particularly concerning given the challenges increasingly facing extant wild steelhead stemming from our changing climate (Wade et al. 2013, Wilson et al. 2021). Given all the evidence to date, hatchery programs need to be reined in, and quickly, where wild steelhead runs still exist.
Guy Fleischer is a retired research fishery scientist, Science Advisor to Wild Steelhead Coalition and a member of The Osprey Editorial Committee. The Wild Steelhead Coalition is one of The Osprey’s partner organizations. Learn more about thir work at:
https://www.wildsteelheadcoalition.org
Taking a scale sample from a wild steelhead, Trask Fish Hatchery, Oregon. DNA samples can be obtained from scales without harming the fish. Photo by
18 The Osprey
Continued from previous page
References
McMillan, J.R., Morrison, B., Chambers, N., Ruggerone, G., Bernatchez, L., Stanford, J. et al. 2023. A global synthesis of peer-reviewed research on the effects of hatchery salmonids on wild salmonids. Fisheries Management and Ecology, 30, 446–463. Available from: https://doi.org/10.1111/fme.12643
Waddington CH. Organisers and Genes. Cambridge: Cambridge University Press, 1940.
Skinner MK. 2014. A new kind of inheritance. Sci Am 311: 44–51.
Hanson, M.A., Skinner, M.K. 2016. Developmental origins of epigenetic transgenerational inheritance. Environ Epigenetics
https://doi.org/10.1093/eep/dvw002
Nilsson, E.E., Sadler-Riggleman, I., Skinner, M.K. 2018. Environmentally induced epigenetic transgenerational inheritance of disease. Environ Epigenetics.
https://doi.org/10.1093/eep/dvy016
Banta, J.A., Richards, C.L. Quantitative epigenetics and evolution. 2018. Heredity 121, 210–224. https://doi.org/10.1038/s41437-018-0114x
Michael K Skinner, Eric E Nilsson. 2021. Role of Environmentally Induced Epigenetic Transgenerational Inheritance in Evolutionary Biology: Unified Evolution Theory, Environmental Epigenetics, Volume 7, Issue 1, dvab012, https://doi.org/10.1093/eep/dvab012
Rey, O, Eizaguirre, C, Angers, B, et al. 2020. Linking epigenetics and biological conservation: Towards a conservation epigenetics perspective. Funct Ecol. 34: 414–427. https://doi.org/10.1111/13652435.13429
Chapelle, V.; Silvestre, F. 2022. Population Epigenetics: The Extent of DNA Methylation Variation in Wild Animal Populations. Epigenomes 6, 31. https://doi.org/10.3390/epigenomes6040 031
Dragan Stajic, Claudia Bank, Isabel Gordo. 2022. Adaptive Potential of Epigenetic Switching During Adaptation to Fluctuating Environments, Genome Biology and Evolution, Volume 14, Issue
5, May 2022, evac065, https://doi.org/10.1093/gbe/evac065
Araki H, Cooper B, Blouin MS. 2007. Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science. 318:100–3.
Fraser DJ. 2008. How well can captive breeding programs conserve biodiversity? A review of salmonids. Evol Appl. 1:535–86.
Frankham R. 2008. Genetic adaptation to captivity in species conservation programs. Mol Ecol. 17:325–33.
Brown AD, Sisneros JA, Jurasin T, Coffin AB. 2016. Effects of hatchery rearing on the structure and function of salmonid mechanosensory systems. Adv Exp Med Biol. 875:117–24.
Christie MR, Ford MJ, Blouin MS. 2014. On the reproductive success of earlygeneration hatchery fish in the wild. Evol Appl. 7: 883–96.
Ford, M.J.; Murdoch, A.R.; Hughes, M.S.; Seamons, T.R.; LaHood, E.S. 2016. Broodstock History Strongly Influences Natural Spawning Success in Hatchery Steelhead (Oncorhynchus mykiss). PLoS ONE 11, e0164801.
Berejikian, B.A.; Larsen, D.A.; Swanson, P.; Moore, M.E.; Tatara, C.P.; Gale, W.L.; Pasley, C.R.; Beckman, B.R. 2012. Development of natural growth regimes for hatchery-reared steelhead to reduce residualism, fitness loss, and negative ecological interactions. Environ. Biol. Fishes 94, 29–44.
Melnychuk Michael C., Korman Josh, Hausch Stephen, Welch David W., McCubbing Don J.F., and Walters Carl J. 2013. Marine survival difference between wild and hatchery-reared steelhead trout determined during early downstream migration. Canadian Journal of Fisheries and Aquatic Sciences. 71(6): 831-846.
https://doi.org/10.1139/cjfas-2013-0165
Christie, M., Marine, M., Fox, S. et al. 2016. A single generation of domestication heritably alters the expression of hundreds of genes. Nat Commun 7, 10676 (2016).
https://doi.org/10.1038/ncomms10676
Brown AD, Sisneros JA, Jurasin T, Nguyen C, Coffin AB. 2013. Differences in Lateral Line Morphology between
Hatchery- and Wild-Origin Steelhead. PLOS ONE 8(3): e59162. https://doi.org/10.1371/journal.pone.005 9162
Marchetti, Michael & Nevitt, Gabrielle. 2003. Effects of Hatchery Rearing on Brain Structures of Rainbow Trout, Oncorhynchus mykiss. Environmental Biology of Fishes. 66. 9-14.
Gavery, Mackenzie R., Krista M. Nichols, Giles W. Goetz, Mollie A. Middleton, and Penny Swanson. 2018. Characterization of genetic and epigenetic variation in sperm and red blood cells from adult hatchery and natural-origin steelhead, Oncorhynchus mykiss. G3: Genes, Genomes, Genetics 8, no. 11 (2018): 3723-3736.
Gavery, Mackenzie R., Krista M. Nichols, Barry A. Berejikian, Christopher P. Tatara, Giles W. Goetz, Jon T. Dickey, Donald M. Van Doornik, and Penny Swanson. 2019. Temporal Dynamics of DNA Methylation Patterns in Response to Rearing Juvenile Steelhead (Oncorhynchus mykiss) in a Hatchery versus Simulated Stream Environment. Genes 10, no. 5: 356. https://doi.org/10.3390/genes10050356
Eric Nilsson, Ingrid Sadler-Riggleman, Daniel Beck, Michael K Skinner. 2021. Differential DNA methylation in somatic and sperm cells of hatchery vs wild (natural-origin) steelhead trout populations, Environmental Epigenetics, Volume 7, Issue 1, 2021, dvab002, https://doi.org/10.1093/eep/dvab002
Claussen, J. E. & Philipp, D. P. (2023). Assessing the role of supplementation stocking: A perspective. Fisheries Management and Ecology, 30, 583–591. https://doi.org/10.1111/fme.12573
Wade, A.A., Beechie, T.J., Fleishman, E., Mantua, N.J., Wu, H., Kimball, J.S., Stoms, D.M. and Stanford, J.A. 2013. Steelhead vulnerability to climate change in the Pacific Northwest. J Appl Ecol, 50: 1093-1104. https://doi.org/10.1111/1365-2664.12137
Wilson, S.M., Buehrens, T.M., Fisher, J.L., Wilson, K.L., Moore, J.W. 2021. Phenological mismatch, carryover effects, and marine survival in a wild steelhead trout Oncorhynchus mykiss population. Progress in Oceanography, Volume 193,102533, ISSN 0079-6611, https://doi.org/10.1016/j.pocean.2021.102 533
Winter 2024 • Issue No. 107 19
Field Report: Olympic Peninsula Steelhead Doing the Right Thing By Wild Fish
By David Moskowitz
The Washington Department of Fish and Wildlife (WDFW) missed an opportunity to take action to begin rebuilding Olympic Peninsula steelhead when it refused to reconsider the 2023-24 steelhead regulations in late January.
The 2023-2024 pre-season forecast showed that Olympic Peninsula (OP) steelhead are unlikely to meet spawning escapement goals on four of seven coastal steelhead watersheds. Yet WDFW proposed liberal fishing seasons in three watersheds — one of which is predicted to be under-escaped by 120 wild steelhead. Finally, the agency pushed until the last minute to allow angling that purportedly only “targets” hatchery steelhead in a watershed that is predicted to be under escaped by over 1,100 wild fish. All of us owe thanks to the Tribal Nations for standing up to protect wild steelhead in the Chehalis watershed.
Most disappointing is WDFW’s ongoing failure to address the depletion of early returning wild steelhead by sticking with a December 1 “opener” and continuing hatchery steelhead fisheries that exacerbate angler impacts on the weakest portion of the winter return: early wild winter steelhead.
The Conservation Angler filed a petition challenging WDFW’s coastal steelhead fishing regulations the day after they were adopted on November 30, 2023. Even though anglers have now been fishing for two months, there are more than two months of fishing pressure remaining on wild steelhead. Yet, WDFW failed to take any substantive action to reduce risk to these fish when they recommended that the Fish and Wildlife Commission deny the petition on January 26, 2024.
Listening to WDFW respond to questions from the Fish and Wildlife Commission was also disappointing. WDFW’s answers confirmed that it knows there is much uncertainty surrounding escapement, in part, due to inadequate monitoring efforts. Despite these admissions, WDFW refuses to change its management actions. Fur-
ther, despite the uncertainty, WDFW claims to be on the right path and is “hoping” for better returns in coming years. In other words, WDFW is making decisions with blindfolds on and praying for a good return, which can hardly be considered management at all.
Additionally, WDFW did not address how highly informed and mobile anglers might increase encounters on the open rivers or result in crowding, which also reduces the quality of the experience.
Most disappointing is WDFW’s failure to address the depletion of early-returning wild steelhead, the weakest portion of the winter return.
The results of WDFW’s denial of TCA’s petition non-action in late January continues a decades-long pattern of mismanagement. The following points highlight our concerns about the course WDFW is on:
1. Early returning wild winter steelhead, which formerly comprised the largest portion of the overall return, are extremely depleted.
2. Sport and tribal harvest contribute to this depletion, though direct sport harvest finally ended in 2014.
3. WDFW and the Tribes began hatchery programs that use early-returning hatchery fish, and those fish have largely replaced wild steelhead that once predominated from November through January.
4. WDFW allows fishing “targeting” the returning hatchery fish (to harvest
hatchery steelhead and hoping to keep the percent of hatchery steelhead spawning with wild steelhead at levels to prevent harm to wild steelhead). There is no such activity as “targeting hatchery fish” as sport anglers cannot control whether a wild or hatchery fish is caught in a river where both wild and hatchery fish are present.
5. Early returning wild steelhead likely experience the highest encounter rates because they are in the system/fishery for longer periods, and yet WDFW still claims that anglers are not disproportionately fishing any part of the run, which is undeniably false when also considering the back end of the run is closed to fishing.
6. WDFW asserts that its regulatory regime protects wild steelhead diversity, and WDFW staff have even stated that anglers "will not encounter many wild steelhead in the early portion of the season." Well WHY is that the case? Because the fishery structure they have developed has ALREADY depleted the early return over time — NOT because there have never been many wild fish returning. WDFW’s statement is also inaccurate for other reasons:
a. The Dec. 1 opening (on the weakest part of the wild run) and the creation of the early hatchery component both contributed to the depletion of the early-return wild run (and its failure to rebuild).
b. WDFW closes fishing for the late-returning wild fish to “protect Kelts” from fishing pressure when we know that there are Kelts exiting rivers in January through June and even into July.
c. Further, WDFW allows sport and tribal angling targeting hatchery spring Chinook, which adversely impacts Kelts.
d. Lastly, diversity is but one parameter
20
Osprey
The
Continued on next page
of a species’ overall health; management must also address spatial distribution and productivity, and abundance. Unfortunately, WDFW’s regulatory regime does not protect these other critical factors.
e. WDFW does not monitor summer run steelhead on the OP even though other agency evidence shows that they are threatened with extinction, which is yet another serious risk factor for the overall health of the entire OP steelhead population.
7. WDFW’s 2023-24 regulations impose undue risk on wild steelhead:
a. WDFW’s monitoring and evaluation plan is insufficient to measure encounter rates because the creel surveys are not robust enough to keep track of basin-wide effort and encounters.
b. WDFW’s boat angling impact study proposes to learn about wild steelhead encounters by increasing effort and encounters and without a more robust study design harmful impacts continue.
c. Before allowing a specific set of angling regulations, WDFW should estimate and model the angler effort and impact to produce a preseason forecast of how many steelhead will be encountered under the proposed rule package.
d. WDFW does not account for angler effort shifts given the closures elsewhere in WA.
e. There is insufficient enforcement to reduce poaching.
f. Kelts are at risk throughout the season.
g. No effort is made to account for sublethal effects from angling on productivity.
h. WDFW is relying on the abundance of the late-running portion of the wild return to sustain the overall steelhead population when climate change will continue to diminish the productivity of this portion of the run as the glaciers melt away and streamflow continues to diminish in spring and summer.
8. Season-by-season fishing season development is a recipe for failing to ad-

dress both long-term and short-term trends. The status of wild steelhead in the Chehalis watershed is a case study for failure. WDFW estimated that the watershed would be under-escaped by more than 1,400 wild fish this year, yet WDFW vigorously pursued sport seasons to “target large numbers of hatchery steelhead.” WDFW needs to develop long-term plans for sustaining wild steelhead and rebuilding early returning life histories and increasing repeat spawner survival.
There are multiple and substantial changes that the Fish and Wildlife Commission could direct WDFW to address in the immediate future to change the trajectory for wild steelhead, including:
l Annual regulations must be based on performance standard measures and not on fishery opportunities.
l Develop a work product resulting in regional specific management plans for all 4 Viable Population parameters (diversity, spatial distribution, productivity, and abundance).
l Gather support for funding from the Washington Legislature to accomplish the work not funded by the steelhead proviso.
l Require electronic catch record cards to keep track of angler effort and encounters in-season.
l Require guide logbook entries daily through an electronic portal.
l Develop a prioritized list of fish passage barriers on coastal steelhead and OP watersheds and begin fixing them.
l Finally, Washington may need to treat wild steelhead like it treated Roosevelt Elk in the early 1900s, when hunting nearly drove them to extinction on the OP. To prevent extinction, hunting was banned for nearly 30 years. The Olympic National Park was secured in that interim, and the elk rebuilt their population in the absence of hunting.
We don’t necessarily need to close all fishing for 30 years to recover wild steelhead on the Olympic Peninsula, but we do need to realize that at some point, our generation will need to make a sacrifice for the generation(s) in the womb. Otherwise, the state fish will continue its downward decline and eventually, there will be nothing left for future citizens.
You can listen to the discussion at the Fish & Wildlife Commission at:
https://tvw.org/video/washington-fishand-wildlife-commission2024011066/?eventID=2024011066
David Moskowitz is Executive Director of The Conservation Angler, one of The Osprey’s supporting partner organizations. Learn more about their work at: www.theconservationangler.org
Winter 2024 • Issue No. 107 21 Continued from previous page
With its excellent habitat, the Olympic Peninsula is one of the Pacific Northwest’s last great places for wild steelhead. Photo courtesy National Park Service
FISH WATCH — WILD FISH NEWS, ISSUES AND INITIATIVES
BC Mining Companies Threaten
Future Wild Salmon Habitat and First Nations Land Rights
Recent research has found that mining companies are staking claims in British Columbia in areas that are experiencing glacier retreat, and are potential future salmon habitat, setting up another area of conflict between mining and the conservation of wild fish and their habitats. The study was led by Dr. Jonathan Moore and Dr. Kara Pitman of Simon Fraser University, British Columbia. Also involved were researchers from Gitanyow First Nation Hereditary Chiefs, Taku River Tlingit First Nation and University of Montana Flathead Lake Biological Station.
The study found that 25 of the 114 sub-watersheds in the transboundary region that contained future salmon habitat had more than 50 percent of that future habitat near a mining claim. More than half of British Columbia’s future salmon habitat has high or medium potential for mineral extraction. That is likely to fuel addition pressure to establish mines in these future habitat areas.
Mining regulations in British Columbia allow mining companies to stake claims with little government oversight, often violating First Nations right to be consulted on land use matters. First Nations are currently working to establish Indigenous Protected Areas that incorporate the effects of climate change into their environmental stewardship strategies.
The transboundary region is the area along the AlaskaBritish Columbia border. For additional background on mining in the transboundary region see “Mining Development Threatens Transboundary Region’s Wild Salmon, by Chris Zimmer, The Osprey, May 2014. (https://www.ospreysteelhead.org/archives.)
To learn more about how melting glaciers influence salmon habitat see: “As Glaciers Melt, Impacts on Salmon Complex,” by Ramona DeNies, The Osprey, September 2020
For more information on current research go to: https://www.jonwmoore.org/about-4 The complete paper is available at: https://www.science.org/stoken/authortokens/ST-1570/full
Pacific Gas and Electric Agrees to Remove Two Eel River Dams
On November 17, Pacific Gas and Electric Company (PG&E) released the initial draft of its plan to remove two dams on the Eel River in California. The plan calls for the complete and expeditious removal of most of the Potter Valley Project facilities. PG&E must provide the plan to federal regulators as part of the license surrender process triggered by the utility’s decision to divest from the financially unviable Project, which has not generated power since 2021. PG&E must submit a final Draft License Surrender Application (LSA) and Decommissioning Plan to federal regulators in May 2024, and a Final LSA in January 2025.
The Potter Valley Project includes two Eel River dams — the Scott and Cape Horn dams — a diversion tunnel that moves water out of the Eel River watershed and into the East

Branch of the Russian River, and a powerhouse.
Located on the Eel River 20 miles northeast of Ukiah, Scott and Cape Horn Dam are over 100 years old. Equipment failures in 2021 caused Project owner PG&E to permanently suspend hydropower operations. Water storage levels in Lake Pillsbury, the reservoir created by Scott Dam, have been reduced by more than 25% due to increased seismic safety concerns with the dam. Scott Dam completely blocks fish passage to high-quality cold-water habitat in the Eel River headwaters. The smaller Cape Horn diversion dam has a faulty fish ladder that needs to be revised to meet current environmental standards.
The Eel River once supported runs of up to a million salmon and steelhead each year, but those numbers have plummeted to a fraction of historical numbers. Scientists recognize that a healthy and free-flowing Eel River has the potential to play a key role in the rebound of these fisheries throughout the California North Coast region.
Removing the Eel River dams would make the Eel California’s longest free-flowing river and would reconnect salmon and steelhead with almost 300 miles of cold-water habitat.
PG&E’s plan also includes — as an alternative for evaluation — a revised framework proposal from a regional group to negotiate terms for a new diversion facility that could support ongoing limited water diversions into the Russian River watershed after removing the dams, provided such diversions are consistent with the full recovery of the Eel River ecosystem to self-sustaining, harvestable populations. Proponents of the proposal include the California Department of Fish and Wildlife, California Trout, Humboldt County, Mendocino County Inland Water and Power Commission, Round Valley Indian Tribes, Sonoma County Water Agency, and Trout Unlimited.
The framework proposal submitted to PG&E lays out critical elements of a deal, including: formation of a Regional Entity with the financial capacity to operate a new project; selection of an engineering solution for the new diversion works; identification of river flows and a diversion schedule that allows water diversions and full restoration of the Eel River; funding for Eel River restoration; and a permitting pathway for the new facility, among other things. Proponents
Continued on next page
22 The
Osprey
Scott Dam is one of the two dams on the Eel River slated for removal. Photo by Kyle Schwartz/California Trout
hope to resolve these matters in time for inclusion as a preferred alternative in PG&E’s May 2024 Draft and January 2025 Final License Surrender Application.
Proponents have set a target date for dam deconstruction to begin in 2028, pending review and approval from the Federal Regulatory Energy Commission, which oversees the decommissioning of hydroelectric projects. Provided a final deal can be negotiated, all proponents, including CalTrout and Trout Unlimited, would commit to seeking permits for the new diversion so that it can be built immediately following dam removal to avoid a disruption of water supplies.
The final draft License Surrender and Decommissioning Plan will be submitted to FERC by June 3, 2024. The public can then comment on that final draft in late June/early July of this year.
For additional information:
a“The Peril and Promise of California’s Eel River” by Samantha Kannry and Scott Greacen, The Osprey September 2013 (https://www.ospreysteelhead.org/archives)
a“Eel River Dams: Reconnecting California’s Lost Coast” by Charlie Schneider, The Osprey Fall 2023 (https://www.ospreysteelhead.org/archives)
aPG&E’s Initial Draft Surrender Application and Decommissioning plan can be downloaded from: https://www.pottervalleysurrenderproceeding.com/
Click the documents tab and enter password: PV_Surrender






Winter 2024 • Issue No. 107 23 To support our conservation mission and receive The Osprey three times per year, January, May and September, please fill out this coupon with your check made out to The Osprey - The Conservation Angler and mail to: The Osprey P.O. Box 13121 Portland, OR 97213 SUPPORT THE OSPREY Send My Copies By E-Mail q Send My Copies by Standard Mail q PHONE E-Mail CITY/STATE/ZIP ADDRESS Yes, I will help protect wild salmon and steelhead ❏ $15 Basic Donation/Subscription ❏ $25 Dedicated Angler Level ❏ $50 For Future Generations of Anglers ❏ $100 So There Will Always Be Wild Fish ❏ $ Other Or donate at: www.ospreysteelhead.org/donation NAME Scan the QR code with your phone to support/subscribe to The Osprey This is a gift subscription q DONATE Continued from previous page VISIT
Wild Salmon Rivers
P.O. Box 13121
Portland, OR 97213
2023 HONOR LIST
The Osprey thanks the dedicated people and organizations who gave their financial support in 2023. Our readers are our primary source of funding and without your support we could not continue publishing.Your donations are much appreciated, and used wisely.
$1,000 and Up
The Conservation Angler
Fly Fishers International
Wild Steelhead Coalition
Wild Salmon Center
SkeenaWild Conseration Trust
Steelhead Society of British
Columbia
The North Umpqua Foundation
Keith Beverly
Stanford Young
John Sager
Rick Rupp
Daniel Higman
$500 to $999
Dale Greenley
Charles Keller
David Thomas
Mary Wahl
The Steamboaters
Pat Micek
Peter Soverel
David Brown
Barbara Stewart
Washington Fly Fishing Club Foundation
Anonymous
$200 to $499
John de Yonge
Jim Maus
Barbara Phillips
Russ Giuntini
Lew Mason
Don Simonson
John MacDiarmid
Richard Mace
Scott Hagen
Evergreen Fly Fishing Club
$100 to $199
Robert Stewart
Gregg Rogers
Rick Williams
Robert Plotnikoff
Bill Lombard
Ed Wickersham
Lawrence Hill
Yancy Lind
Larry Lowe
Jim McRoberts
Richard Raisler
David Thyer
J Lee Lashway
John Swan
John Bauman
Thomas Ramsey
Vincent Nitido
James Birrell
Thomas Weseloh
Christopher Striebich
Adam Tavender
John Rogers
Bert Bowler
Christine Soverel
Charles Bucaria
Harry Griffith
Rebecca Riley
Lillooet Tribal Council
Marty Sherman
Dale Davey
James Adams
Steve Park
Mark Dullea
Frederick Buckner
David Stangland
Peter Tronquet
Joseph Ferguson
Timothy Goforth
Chris Striebich
Scott Wallace
Doug Schaad
Jay Beckstead
Arthur Carlson
Bill Bakke
Jon Lund
Joseph Jauquet
Leonard Volland
Bill Tattam
Stephen Asher
Arthur Carlson
Dale Davey
Clint Brummitt
$50 to $99
Donald Starkin
Puget Sound Fly Fishers
Andrea Warner
Brad Staples
Mark Meyocks
Jerry Reeves
James Walton
Ed Pearson
Thomas Hogye
Steve Rajeff
Thomas Haensly
Peter Gustafson
Alexander Uber
Vance Luff
Frank Collin
Yale Sacks
James Holder
C Mark Rockwell
Todd English
Donald Johnstone
Craig Smelter
Robert Zasoski
Steve Haskell
Bill Morrish
Bruce Allen
Larry Brown
Christopher Jones
Thomas Campbell
Joseph Car
Edward Robertson
William Pearcy
Herb Burton
Trinity Fly Shop
William Knight
Dr. Eric Pettine
Bill Kuest
$15 to $49
Chris Denchfield
Danny Beatty
Doug Webb
Reggie Hammond
Charles Hammerstad
William Boyer
Ron Dyslin
Joseph Brown
Jeralyn Mandell
Coleman Byrnes
Jake Campbell
Pat Ford
Darren Voctorine
Trinity Fly Guide Service
Hans Solie
Fred Carter
Richard Burge
Robert Cook
McKenzie Fly Fishers
Brad Wistrom
Ed Heidel
James Lukens
Forest Wood
Richard Bauer
Ralph Wetherell
Craig Handly
John Titland
Robert Pauli
Rick Kustich
Thomas Krughoff
Puget Sound Fly Fishers
Arlene Christy
John Kearney
David Beatty
Sam Wright
Robert Bailey
Robert Van Kirk
James Quinn
Richard Stoll
Ed Heidel
Fred Carter
Sam Wright
Justinas Savickas
Mike Brinkley
Robert E Burdick
John Warrick
Lewis and Clark College
Alan Smith
Patrick Trotter
Josey Paul
Harry MacDonald
David Scherrer
Timothy McGilvrey
Michael Dornfeld
Dennis Lockett
John Townsell
Joseph Youren
Kevin Brannon
Michael Clifton
THE OSPREY































 Jim Yuskavitch
Jim Yuskavitch





