Genetics

reversed Scientists develop tool to speed up worldwide COVID-19 research



reversed Scientists develop tool to speed up worldwide COVID-19 research

Texas Biomedical Research Institute was built on the dream of our visionary founder, Tom Slick, Jr. to develop a “great center for human progress through scientific research.”
While not a scientist by training, Tom was a scientist at heart. We at the Institute understand his deep desire to make a difference, which is what propels us forward in our quest to find answers to complex diseases.
To find those answers, we often need to develop the right tools or model systems. In this edition of TxBiomed, we are delighted to introduce you to our newest Assistant Professor Tori Baxter, a CATALYST who is passionate about this topic.
Our COVER STORY takes you inside one of our labs that developed genetics-based tools now helping scientists around the world learn more about the virus that causes COVID-19. The tools are also helping screen a large suite of antivirals, antibody cocktails and vaccine candidates.
Along with dedication, hard work and careful execution, research like this requires specialized facilities and oversight. We are pleased to feature Dr. Anthony Wang, Texas Biomed’s Director of Environmental Health and Safety, in this edition’s PROFILE. The work he and his staff perform is integral to our mission and values, and underscores how safety is at the forefront of everything we do.
This past year has been extraordinary for science

education and our next generation of researchers. We’re excited to share stories that FOCUS on competitive grants won by our early career researchers, and a national education grant enabling us to provide science training for teachers and thus benefit thousands of students in our COMMUNITY.
We are proud to serve our region and make an IMPACT far beyond San Antonio. Read about how our veterinary faculty and staff trained colleagues from Ethiopia on techniques for monitoring infectious disease in wild baboons. This partnership, in collaboration with The Carter Center, is expected to expand with more joint research projects.
Research is truly a team effort, and that includes our community of supporters. As a growing nonprofit, we rely on your enthusiasm and financial support to fulfill our mission to protect you, your family and the global community from the threat of infectious disease. We hope that as you read about the lifesaving research underway at Texas Biomed, and the fascinating people behind the stories, you will be inspired to make a contribution.
Your gift will make a difference, shaping healthier futures for all — because Health Starts with Science.
Joanne Turner, PhD Executive Vice President, Research
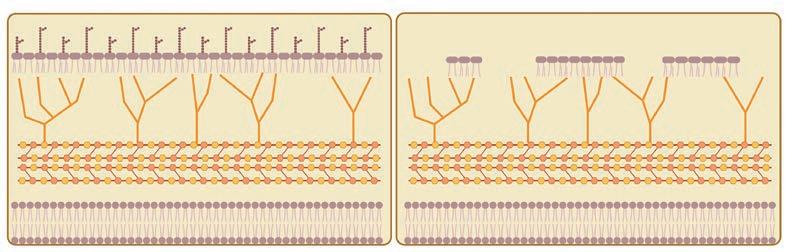
A modified tuberculosis (TB) vaccine developed at Texas Biomed could help treat a form of bladder cancer, called non-muscle invasive bladder cancer. The Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccine, developed for TB in the 1920s, has been used to treat this type of cancer since the late 1970s. It is highly effective, but up to 84% of patients cannot tolerate the strong side effects. Texas Biomed Professor Jordi B. Torrelles, PhD, and his team have removed some lipids from the outer layer of bacteria cells in the vaccine. Studies in mouse models and human cells showed the modified vaccine produces a well-regulated immune response but minimizes severe inflammation and tissue damage. The research, conducted in close collaboration with UT Health San Antonio, was published in Cancer Immunology, Immunotherapy. The group is excited to proceed to human clinical trials.
A graphical illustration shows the original BCG vaccine (left) compared with the modified vaccine developed at Texas Biomed (right).


Tuberculosis is battling with COVID-19 for the dubious distinction as the world’s leading killer by a single infectious agent. More effective TB vaccines are desperately needed.
Texas Biomed Adjunct Associate Professor Gillian Beamer, VMD, PhD, DACVP, received a phased National Institutes of Health grant for up to $3.5 million to develop and test new TB vaccines. Partners at The Access to Advanced Health Institute (AAHI), a nonprofit research institute in Seattle, Washington, will develop various vaccine candidates, which will be tested at Texas Biomed. The research will rely on a unique group of lab mice that better represent genetic diversity of the human population. This diversity will help identify vaccines that protect those most vulnerable to TB.

Finding a cure for HIV has been extremely difficult, in part because the virus hides from antiretroviral treatments in one of the hardest-toreach places: the brain. Texas Biomed Associate Professor Binhua “Julie” Ling, MD, PhD, and her collaborators are working on a treatment that can pass through the protective blood-brain barrier, and cut out the virus from infected brain cells. They will use the latest gene-editing technology, CRISPR, combined with old-school virology techniques. The National Institutes of Health provided a five-year, $3.9 million grant to advance the research. If lab and preclinical animal studies show promising results, the treatment could progress to human clinical trials.

Texas Biomed will help map the developing brain with unprecedented detail for the National Institutes of Health’s BRAIN Initiative Cell Atlas Network (BICAN). The NIH awarded a total of $500 million to 11 teams that will build a 3D brain atlas at singlecell resolution over the next five years. The final map will reveal precisely how billions of cells are situated in different structures in the brain and how they work together. Texas Biomed Associate Professor Marcel Daadi, PhD, is part of a team led by the University of California, San Francisco. The group will map the developing brain — identifying cell types, activities and locations as they differentiate during development and change throughout childhood and into adolescence. By learning more about healthy development, they can then better understand how diseases like autism, schizophrenia and Parkinson’s emerge.

With late nights, long days and some friendly bacteria, they did it. Texas Biomed researchers developed a powerful genetics-based tool now powering COVID-19 research worldwide.
What do we need to study this novel coronavirus?
That was the question Professor Luis Martinez-Sobrido, PhD, posed to Staff Scientist Chengjin Ye, PhD, in February 2020. Dr. Martinez-Sobrido, an expert in virology, vaccines and antiviral research, had just relocated his lab and team of researchers from the University of Rochester to Texas Biomed.
“We’ll probably need reverse genetics,” Dr. Ye replied.
It was still in the very early days, before the COVID-19 pandemic was officially declared. More and more countries were reporting their first cases as SARS-CoV-2 spread around the globe. The clock was ticking to learn about the virus’s strengths and weaknesses, and develop treatments and vaccines to prevent people from dying.
Fast forward to current day and the particular reverse genetics system developed by Dr. MartinezSobrido, Dr. Ye and their colleagues has drawn worldwide interest from the scientific community. Texas Biomed has shared the tool with more than 100 vetted labs across the globe, empowering more scientists as far away as Australia and Japan to advance their own COVID-19 research.
This is the fastest we’ve ever had to work. We weren’t done developing reverse genetics for one variant before the next one emerged.
— Dr. Luis Martinez-SobridoReverse genetics is a powerful tool. It allows researchers to systematically study the virus, to learn how it works and find ways to stop it. Specifically, it enables them to make changes to the virus’s genetic code. For example, researchers can use the system to knock out a gene to see if a virus becomes less harmful, or they can add a fluorescent marker so it is super easy to see if treatments or vaccines are working in cells and in animal models.
“Classical genetics starts with an outcome, such as hair color, and then looks for the gene responsible,”
Dr. Ye says. “Reverse genetics starts with the genes and then sees what happens.”
Reverse genetics is so powerful, it must be very carefully done in regulated biosafety laboratories. It involves a multi-step process that starts with a noninfectious blueprint of the virus, and ultimately results in a version of the virus making copies of itself in cells.
Because of Texas Biomed’s expertise in generating and using these critical reverse genetics tools, numerous national and international groups are now
collaborators. For example, Dr. Martinez-Sobrido is an investigator in three of the nine Antiviral Drug Discovery (AViDD) Centers for Pathogens of Pandemic Concern. The National Institutes of Health (NIH) is funding these large centers to accelerate development of treatments for COVID-19 and other viruses that could cause future pandemics.
“We have to prepare for next time,” Dr. MartinezSobrido says. “By establishing the tools, methods and networks now, we will have the necessary systems and collaborations in place to respond faster to future

Not a moment to waste Chengjin Ye, PhD, pours bacteria food into a large flask, before adding a few bacteria cells, which will begin making copies of themselves. This replication process takes 16 hours. During the height of the pandemic, Dr. Ye would return to the lab as soon as 16 hours was up so he could proceed to the next step, even if it was the middle of the night.

outbreaks and a suite of antiviral medications at the ready.”
The viruses generated with the reverse genetics system in Dr. Martinez-Sobrido’s lab are playing a key role in screening those medications. For example, glowing reporter viruses help researchers quickly see if an antiviral drug is neutralizing the virus, or not. Essentially, no glowing means no virus and the treatment worked.
Speed has been everything in this pandemic.
“This is the fastest we’ve ever had to work,” Dr. Martinez-Sobrido says. “We weren’t done developing reverse genetics for one variant before the next one emerged. We had to prioritize and focus only on the dominant variants like Delta and Omicron.”
Many of the molecular tools common in biology labs are for working with DNA. That is a challenge for virologists, because most viruses are made out of RNA. If researchers want to edit the virus’s genetic code, they have to first convert RNA into DNA, edit the DNA, and then transcribe it back into RNA.
Another challenge is the size of the SARS-CoV-2 genome. It is the biggest among RNA viruses; three times bigger than influenza and packaged in a single molecule. In order to work with it, researchers must cut up the genome, make several copies of the fragments, and then carefully reassemble the pieces.
Dr. Ye worked with an external company to do part of this process — obtaining the DNA sequences that correspond with the viral RNA genome. He designed the protocol for how the DNA sequences should be divided into five fragments and how to put them back together. The fragments have unique markers on either end so they can be reassembled in the correct order.
“The markers are essential,” Dr. Martinez-Sobrido says. “This is the only way that we can reassemble the virus.”
Small vials containing different DNA fragments that when recombined form a complete SARS-CoV-2 genome. The DNA is noninfectious so is safe to handle in the BSL-2.
A small number of DNA fragments are shipped to Texas Biomed — since it is DNA, they are not infectious and can be handled safely in the biosafety level-2 (BSL-2) lab. To make lots of copies, a process called amplification, Dr. Ye turns to a virologist’s best friend: bacteria.

Bacteria are replicating machines, cloning themselves every 30 to 50 minutes. If you want to make a lot of DNA copies, bacteria are among your best bets.
“We trick the bacteria into making copies for us by using a bacterial artificial chromosome, or BAC for short,” Dr. Ye says. Think of BAC like a vehicle used to transport genetic information. It is a circular
Biosafety levels are ranked 1 to 4, with 1 being the lowest and 4 being the highest. The levels define the required safety equipment and protocols based on how pathogens spread and what treatments are available. Texas Biomed hosts numerous BSL-2 and BSL-3 labs, and the first independently run BSL-4 in the nation.
molecule that looks and behaves like a bacterial chromosome and is easy for bacteria cells to replicate.

Dr. Ye inserts the first DNA fragment into the BAC, then puts the BAC into harmless bacteria cells in a petri dish. Once the bacteria clone themselves a few times, he carefully scoops out the tan blobs and runs genetic tests to confirm the bacteria contain the BAC with the fragment. Then, he must scale up from a few BACs to a test tube full, in order to have enough material to work with. He places a few bacteria cells into a large flask filled with a golden liquid — yummy bacteria food — and leaves it on a shaking platform for 16 hours.
During the height of the pandemic, Dr. Ye would come back to the lab when the 16 hours were done to proceed to the next step, even if it was in the dead of night, the middle of the weekend or a holiday.
“There was not a minute to waste,” he says. “The whole team was working around the clock. We were trying to catch up with the virus.”
While moving the process along as quickly as possible, it still takes two to three weeks to complete. Dr. Ye must repeat all the steps for each of the five fragments — carefully checking his work along the way to ensure that, one by one, each fragment is added correctly to the BAC, until finally, he has a complete DNA sequence in the BAC.
Up until now, all the work has been on DNA, which is noninfectious. Now, it is time to go into the more restrictive BSL-3, where a small amount of BAC is placed in petri dishes filled with one million mammalian cells. Once the BAC successfully enters the cells, the cells’ normal machinery begins to translate the DNA into RNA… voilá, now that’s the virus.
Each clear spot in the dish was a COVID-19 virus particle. Only after being washed in sterilizing liquid that kills all virus particles, do the plates come out of the BSL-3.
It was a Sunday morning in June 2020 when Kevin Chiem — a graduate student who had moved with the group from New York to Texas and will soon complete his doctoral degree — called Dr. Ye to tell him the cells in the petri dishes showed signs of infection.


Genetic tests confirmed the carefully prepared protocol had worked and they had “rescued” a recombined version of SARS-CoV-2. This recombinant SARS-CoV-2 was virtually identical to the original strain and behaved the same. The team published the work a few months later, noting it was the first time this had been done for SARS-CoV-2 using a BAC. They used the same approach to generate glowing reporter viruses, which they published in 2021.
Their system presents advantages to others; primarily, they use cells to transcribe DNA into RNA, rather than having to do this manually. Once cells make the first viral RNA copy, the RNA is translated into viral proteins and then it is off to the races. Not only are the cells helping the virus replicate, the viral particles are also replicating away.
“All we need is a little bit of BAC, and after this, we don’t have to do anything, they will help themselves,” Dr. Ye says.
All that virus — which is securely stored in the BSL-3 — can then be used to conduct experiments to understand how the virus works, and to evaluate vaccines and medicines.
For example, the generated viruses have since helped test the effectiveness of antiviral drugs, vaccine candidates and monoclonal antibodies, including a cocktail developed by Texas Biomed and the University of Alabama at Birmingham that Aridis Pharmaceuticals is working to advance to clinical trials. They have also helped develop animal models needed to study COVID-19.
The viruses have facilitated hundreds of studies at Texas Biomed and beyond. The BAC, which by itself is not infectious, has been shared with more than 100 groups with access to BSL-3 facilities. Dr. Ye has a -80ºC freezer full of BAC ready to ship as new requests come in.
Kevin Chiem moved with Dr. Martinez-Sobrido’s lab from New York to Texas to complete his graduate studies. He will soon earn his doctoral degree from UT Health San Antonio’s Graduate School of Biomedical Sciences (GSBS). In recognition of his teamwork and accomplishments, notably authoring and contributing to 28 scientific papers during his graduate program, he was named the GSBS 2022 Senior Student of the Year.
The reverse genetics system developed at Texas Biomed has been shared with more than 100 vetted labs in 18 U.S. states and 19 countries and territories.
“We have received requests every single week since we published the work in the scientific literature,” Dr. Martinez-Sobrido says.
With the main protocol established, researchers can now make specific changes to the DNA and test what happens, for example knocking out one or two genes. They pop out the original fragment from the BAC and pop in the edited version, like changing the ink cartridge in a printer.
That is how Dr. Martinez-Sobrido and his team were able to discover knocking out two genes resulted in an attenuated, or weakened, virus that does not cause illness nor death in two different animal models. They recently received approval from the NIH Office of Science Policy to work with this nonharmful strain in the BSL-2 — believed to be the first such approval in the U.S. — which will enable them to screen drug candidates even faster and safer.
“We can now try to answer important questions about the biology of the virus, which was not allowed before because of the risk involved,” Dr. Martinez-
Sobrido says. “This attenuated virus enables us to do those types of studies safely.”
They are also exploring if it can work as a live attenuated vaccine. Live attenuated vaccines could provide longer-lasting protection and are used for many other viruses, including chickenpox, smallpox and polio.
“Historically, it took decades to discover attenuated strains that could work for vaccines,” Dr. Ye says. “With reverse genetics, we are able to do this in a matter of months.”
Key to all of this was the BSL-3 laboratory. Without it, the studies would not have been able to proceed safely. For Dr. Martinez-Sobrido, Dr. Ye and the team, moving to Texas Biomed to have access to a BSL-3 made all the difference.
“If we had not come to Texas Biomed, we would have been an audience to the pandemic,” Dr. Ye says. “While of course we wish there had not been a pandemic, we are glad we could make some important contributions to help get us to where we are today and to prepare for the next one.”
Our vision for a new central research building has a fresh name and look. The planned Global Center for Bioscience will represent the epicenter of our campus and provide a home for multi-disciplinary team science that bridges research, community and culture.

The SEPA program aims to give teachers tools and resources to inspire students about the wide variety of careers in STEM.

It’s a common refrain: Texas schools aren’t keeping pace with preparing young people for in-demand STEM careers. Educators want to equip students with knowledge and skills to gather data and evaluate evidence, but it’s difficult making connections in the classroom that apply to real-world scenarios.

“I think one of the most dreaded questions a teacher can get is, ‘When am I ever going to use this information?’” says Rosemary Riggs, PhD, Texas Biomed’s Education Outreach Program Manager, who also was a high school science teacher for 10 years. “A lot of students can’t understand why balancing an equation or learning about molecular structure might be important because they don’t think it relates to everyday situations.”
Texas Biomed aims to change that, with the help of a $1.25 million science education grant designed to impact more than 9,500 students in local, middle and high schools over the next five years. The project, with funding from the National Institutes of Health (NIH), supplements teaching practices and promotes scientific literacy, thereby strengthening the STEM careers pipeline.
“Winning this federal grant was a competitive process and it complements the Institute’s overall Discovery
& Learning Initiative,” says Joanne Turner, PhD, Texas Biomed’s Executive Vice President for Research. “We are very proud to be partnering with local school districts serving mostly economically disadvantaged students — places that tend to be under-resourced when it comes to the kind of teaching and hands-on learning it can take to get kids interested in science for the longterm.”
The Texas Biomed Discovery & Learning Initiative encourages scientific skills and knowledge through education and mentoring programs for area K-12 students, as well as undergraduate internships and graduate education and training.
The goal of the NIH grant, known as the Science Education Partnership Award (SEPA), is to facilitate relationships among biomedical researchers, teachers and schools in order to provide opportunities for students from underserved communities to consider careers in basic or clinical research.
The Texas Biomed SEPA program is built around paid, professional development for teachers that occurs year-round. This intensive teacher training takes place in the summers with biomedical
education specialists and scientists at the Institute. Each year, 10 teachers from different school districts and four teacher program leaders will take part.

“Our ultimate goal is to have a positive impact on teaching practices,” says Dr. Riggs, who directs the grant program. “This will empower them to integrate current scientific information and issues into the existing curriculum, which will have an exponential impact on students over the lifetime of each teacher’s career. It’s really about allowing teachers to take ownership over what they are teaching and make connections to the real world so students can see all the exciting career paths that are available.”
Over the past summer, in preparation for the project rollout, the first cohort of teacher leaders completed the intensive training at Texas Biomed. They studied Texas Biomed research on infectious disease, and toured the labs where studies and experiments are conducted. They also learned first-hand from scientists about making observations, gathering data and analyzing results. Throughout the school year, the teachers will maintain relationships with scientists, who will, at times, help co-teach their classes, mentor
students, make classroom presentations and provide STEM career advice at the middle and high schools.
“It’s pretty amazing that I will be using journal articles about things like tuberculosis from Texas Biomed in my class this year and then later the kids will be able to learn from the very scientists who did those studies,” says Kelsey Harrison, an AP environmental and chemistry teacher at Southside High School. “It makes it real — and that’s more than half the battle in getting kids excited about learning.”
All activities will enrich, not supplant, existing curriculum, Dr. Riggs says. They also align with education standards, including the Texas Essential Knowledge and Skills (TEKS).
“We’re not asking teachers to redo their curriculum, we’re just showing ways to enhance what they’re doing in the classroom,” she says. “From there, we will measure and see whether there is an increase in student awareness and interest in STEM in general. It’s a very deliberate approach that I believe will have major ripple effects in our community for decades to come.”
It’s a very deliberate approach that I believe will have major ripple effects in our community for decades to come.
— Dr. Rosemary Riggs



What she did enjoy? Research. Especially, research in biosafety level-3 (BSL-3) labs on pathogens that cause severe disease in humans.
Assistant Professor Tori Baxter, DVM, PhD, has found her sweet spot. Growing up in Dallas, Dr. Baxter spent a lot of time at her parents’ small animal veterinary clinic. She loved animals, but also didn’t want to run her own practice. She thought maybe she’d become a doctor instead, but through a summer internship discovered she didn’t enjoy working with human patients directly.
“I love everything about it,” Dr. Baxter says. “Other people might think it is weird or scary, but I feel way safer in the BSL-3 than in the real world.”
She is in good company, then, at Texas Biomed, home to multiple BSL-3 labs and the first independent BSL-4 in the nation.
“Texas Biomed is the mecca of infectious disease research, especially high containment infectious disease research,” says Dr. Baxter, who joined the faculty in November.

Texas Biomed’s newest professor combines passion for animals, high containment labs and infectious disease research.
Dr. Baxter brings with her a wealth of experience in caring for lab animals, developing and optimizing animal models, and infectious disease research. After studying genetics and veterinary medicine at Texas A&M University, she became board certified in lab animal medicine and earned a PhD in viral immunology at Johns Hopkins University.
“A lot of emerging infectious diseases come from animals,” Dr. Baxter says. “It is fascinating to me how these pathogens adapt to their new host, one-upping us and our immune systems.”
She is also fascinated by how immune reactions are both protective and harmful. Sometimes it is not the virus causing the damage to a person, but the person’s own immune response. She is particularly drawn to studying this phenomenon in the brain.
“The brain adds an extra layer of challenge because it is largely inaccessible, especially in human patients,” Dr. Baxter says. “Cue the need for animal models.”
For the past six years at the University of North Carolina at Chapel Hill, she has been developing a mouse model for chikungunya virus. A mosquitoborne pathogen that has spread to many parts of the world, chikungunya can cause debilitating joint pain. But in some cases, it affects the brain and causes neurological problems. She wants to understand what is different about those cases and find potential treatments, so she is developing the necessary animal model.
“I am passionate about using my veterinary and lab animal experience to determine what is the best animal model for the research question a scientist is trying to answer,” Dr. Baxter says. “This way we are being respectful and minimize the number of animals needed.”
She is also improving animal models. For decades, lab mice have been bred free of pathogens, to ensure
prior exposure to bacteria, viruses or parasites do not affect how they respond to a vaccine or treatment. But researchers have since realized these mice have very immature immune systems, which is also not ideal for studying how a body will respond to a medicine. Dr. Baxter and others are developing protocols to mature a lab mouse’s immune system in a controlled way, so it better mimics a human immune system. She’ll bring these mice to Texas Biomed, which can support many researchers studying vaccines and therapies.
She can’t wait to get started.
“I really like that I’ll be working with folks focused on collaboration, with everybody bringing their individual expertise towards a common goal,” Dr. Baxter says.
Along with joining the Texas Biomed team, she is also excited to return to her home state of Texas with her family and three dogs.
“My whole family is here, my husband’s family is here,” she says. “After 12 years on the East Coast, we are so glad to be coming back to Texas.”

Other people might think it is weird or scary, but I feel way safer in the BSL-3 than in the real world.
— Dr. Tori Baxter
Four Texas Biomed staff scientists have received funding from the National Institutes of Health (NIH) to take on exploratory research projects with potential for significant impact. Each R21 grant provides about $400,000 for researchers to test hypotheses and gather more data over two years.
“These are very important grants that help advance the careers of our up-and-coming investigators,” says Joanne Turner, PhD, Texas Biomed’s Executive Vice President for Research. “Often, work completed with these grants provides the initial results necessary to then successfully compete for larger, five-year grants. Having four of our staff scientists receive R21s this year is an excellent indicator of Texas Biomed’s commitment to and success with training the next generation.”
These grants also display the wide range of research taking place at Texas Biomed and how basic science is critical for tackling some of the world’s greatest health challenges.
A lung tissue sample highlighting healthy cells in red and cells in distress in green.

Staff Scientist Riti Sharan, PhD, is investigating how HIV reactivates latent tuberculosis (TB) infection, worsening the outcome for patients battling two diseases. People with HIV are 18 times more likely to develop TB infection than those without HIV, and TB is the leading cause of death for people living with HIV. In 2020, an estimated 214,000 people with HIV died of TB
Dr. Sharan is seeking to identify the exact process that leads to TB reactivation in individuals with HIV, which could then suggest new treatment targets. Specifically, her R21 will enable her to conduct singlecell sequencing, which will provide a detailed map

of what different cell types are doing at key points during the reactivation process.
“Single-cell sequencing is extremely expensive, but provides so much data,” Dr. Sharan says. “I think this is going to answer a lot of questions.”

Dr. Sharan will use cells collected from nonhuman primates during previous studies. The samples have been preserved, capturing a moment in time before, during and after infection and treatment with antiretroviral drugs. The single-cell RNA sequencing will be completed at Texas Biomed, which will generate a wealth of data about what genes are active, or turned on, versus turned off, in each individual cell. This reveals what cells are doing in a great level of detail.
“I am excited to dive deeper into my findings and hopefully identify the specific pathways and mechanisms that lead to HIV reactivating TB and how antiretroviral therapy affects that,” she says.
Staff Scientists Winka Le Clec’h, PhD, and Frédéric Chevalier, PhD, are co-principal investigators on an R21 to study the microbiomes of snails, in search of new insight to combat parasites that infect more than 200 million people worldwide and is estimated to kill more than 200,000 every year. The parasitic worms, called schistosomes, must spend part of their lives in certain freshwater snails before infecting humans. Understanding how the snail microbiome influences the snail and the parasite could reveal new ways to block parasitic infection before it starts.

The researchers will compare microbiomes of different snail populations in their lab, and how these microbiomes could affect snail growth, survival and reproductive rates, as well as the schistosome infection.
“Perhaps we will identify a particular microbiome that makes snails more resistant to the schistosomes, and this could provide a nice strategy to control the parasites in the field that is more ecologically friendly than chemicals,” Dr. Chevalier says.
A key part of the project will be to try to generate snails without microbiomes, formally called germ-free snails, by raising them in a sterile environment, and see how they compare to snails with microbiomes. Other researchers tried this in the late 1950s — long before studying microbiomes was all the rage. Now, with much more sophisticated genetic sequencing techniques
Understanding how the snail microbiome influences the snail and the parasite could reveal new ways to block parasitic infection before it starts.
Checking snails for parasite infection.
available, the Texas Biomed team will be able to gather more detailed data and insights.
“Germ-free snails could provide a powerful model to decipher the particular interactions between the host and specific bacteria, so we are excited to follow up on this old experiment, apply the latest technologies, and see what new answers we can uncover,” Dr. Le Clec’h says.
Even though HIV is a descendant of an ancient virus found in monkeys, HIV itself does not replicate in monkeys. That means researchers studying HIV in animal models must rely on the monkey version of the virus, called simian immunodeficiency virus (SIV), and modified versions that combine some HIV genes into SIV. However, this makes it difficult to determine how well cures, treatments and vaccines might work, especially if they are designed to target specific genetic sequences of HIV, before testing them in human clinical trials. Staff Scientist Rajesh Thippeshappa, PhD, is seeking to solve this problem.

“It is very straightforward, but very challenging,” Dr. Thippeshappa says.
Instead of inserting HIV genes into SIV, he’s doing the opposite: adding just one gene from SIV to HIV, in order to help it replicate in pigtail macaques. While it works, virus replication levels are still too low to mimic human disease. His R21 will enable him to try to overcome this obstacle and achieve higher replication rates.
To do this, he will rely on the natural evolution of viruses, which mutate and adapt over time and as they encounter new hosts. By passing the virus through three pairs of monkeys — as few animals as possible — he anticipates replication will increase enough to finally have a more realistic model, or example system, of the human disease.
“It won’t be perfect,” Dr. Thippeshappa says. “The goal is to develop a reasonably good model that can help us test therapies, vaccines and cures.”

The model will also help researchers study what makes viruses jump from one species to another, and what mutations are required to begin replicating in a new host at levels that cause disease. As humans increasingly expand into wildlife habitat, the likelihood of encountering novel viruses also increases, which means understanding virus adaptation dynamics and having reliable research models will be even more critical.
The model will also help researchers study what makes viruses jump from one species to another.
Below: An electron microscopy image shows HIV viral particles, the small black dots, along the surface of a cell.Fitsum Lemma, Joe Jimenez, Aurora Shingledecker, Carrie McCabe, RoseAn Thienpont, Marques Robinson, Laura Condel, Dr. Desalegn Getahun, Kassahun Demissie.

Texas Biomed has joined a unique public health collaboration with The Carter Center aimed at eradicating the painful parasitic disease caused by Guinea worm in Ethiopia.
The decades-long effort to wipe out Guinea worm disease in humans has been nearing success, thanks in large part to the efforts of community health workers and the global humanitarian center founded by former President Jimmy Carter. But in Ethiopia, infections discovered in wild baboons have created the need for increased disease surveillance to better track and understand transmission. The worms that infect animals are the same species that infect humans, so eradication requires stopping infections in both.
Because of its 1,000 baboons — the largest such research colony in the world — Texas Biomed was selected to host representatives from the Ethiopian Public Health Institute and The Carter Center Ethiopia this past spring to train in evidence-based research and disease control. The team included wildlife veterinarians Drs. Fitsum Lemma, Desalegn Getahun and Endalkachew Birhanu, and Kassahun Demissie, a lab technician and national eradication program coordinator.
The team trained for a month at Southwest National Primate Research Center (SNPRC) based at Texas Biomed, learning surveillance and monitoring techniques.
The skills will be of great use in the field — typically a heavily forested area of Ethiopia — where baboons and other animals are regularly checked for evidence of disease, says Fernando Torres-Velez, DVM, PhD, The Carter Center’s associate director for research in the Guinea Worm Eradication Program.
“Texas Biomed’s extensive experience and subject expertise has been a great asset as we continue deploying a surveillance system in Ethiopia’s Gambella region,” he says. “The trainings mean that Ethiopia will benefit from more robust technical capacity and further development of surveillance and research programs.”
Texas Biomed and SNPRC faculty and staff were proud to share their knowledge. All those involved, including The Carter Center, are exploring ways for the international exchange to continue through future travel and potential research collaborations.
“Texas Biomed is the only institute in the United States that could provide this baboon-focused, handson training,” says Professor Olga Gonzalez, DVM, a veterinary pathologist at SNPRC. “We hope to have a direct impact in efforts to eradicate this horrible disease and are excited about follow-up projects in the works.”
Laura Condel, a veterinary research supervisor at SNPRC, describes the relationships built as invaluable.
“As much as they expressed that they learned from us, we learned just as much from them about the differences in the field and about ideas we could share to improve the work and care for animals,” she says.
Guinea worm disease is a debilitating condition that most often affects those living in poor communities. There is no drug to treat it and no vaccine for prevention.
People contract the parasite by drinking water containing microscopic fleas carrying Guinea worm larvae. They experience burning sensations and ulcers, and about a year later, a long worm erupts through the skin on the leg or foot. To alleviate the pain, people often seek relief in local water sources, which completes the parasite’s lifecycle.
Dr. Birhanu is optimistic that strategies learned at SNPRC will help overcome one of the last remaining impediments to eradicating Guinea worm disease in Ethiopia: baboons as reservoirs of infection.

“The trainings helped for the real situations in approaching wild baboons in a systematic way to understand their behavior and ecology,” he says.
Guinea worm disease is an important example of the complex interplay between animal and human health, and how research in animals is required to benefit both. This work can reveal how animals harbor pathogens and how to interrupt transmission between species. Animal models are also critical to understand infection processes involving many organ systems and to identify ways to prevent, treat and cure disease.
It didn’t matter the age, each family here faced a profound health challenge. Scientific research gave them a future. Learn how health starts with science at txbiomed.org



You can support lifesaving research and shape healthier futures for all. Give today.

There are many things you should know about Hsiang-Ming (Anthony) Wang, PhD, Texas Biomed’s Director of Environmental Health and Safety (EHS). He and his wife are proud parents of two children. He is a figure skater, photographer and first-time cat dad. He enjoys building computers and 3D-printing his own contraptions. And, he is a total people person.
In a way, that is why Dr. Wang ended up in biosafety management.
Dr. Wang studied medical technology at the National Taiwan University. He was drawn to scientific research and understanding the signaling pathways triggered by UV light and cholesterol. But after earning a PhD in molecular and cellular biology from the University of Vermont and conducting postdoctoral research at The Ohio State University, he came to a critical realization.
“I really am not a big fan of sitting in front of the bench,” he says. “I began thinking to myself, do I want to work with samples or do I want to work with people?”
Dr. Wang enjoyed helping scientists, but wasn’t sure how to make that a career. At the time, Texas Biomed’s President/CEO Larry Schlesinger, MD, directed Ohio State’s Center for Microbial Interface Biology, which oversaw the university’s biosafety level-3 (BSL-3) laboratory program. He suggested Dr. Wang manage the lab.
“I had no biosafety background at the time,” Dr. Wang says. “He took a chance on me.”
Dr. Wang has been in biosafety ever since.
He learned the ropes from several mentors, including others who have also since joined Texas Biomed: Joanne Turner, PhD, Executive Vice President for Research, and Mark Behr, Biocontainment Facilities Manager. After managing the BSL-3 and other research operations at Ohio State, Dr. Wang spent five years at the University of Chicago as a biosafety officer and assistant director of research safety. These roles expanded his experience with a wider variety of research labs, from cancer to chemistry.
“I even helped one researcher safely ship compressed volcanic gas samples from Japan,” he says.
Along the way, Dr. Wang has become an expert in the regulations and best practices for safely conducting research. He also earned an MBA, so he often combines his scientific thinking with business analysis to consider cost, benefit and risk all together. He’s excelled in the industry, being recently elected a councilor of ABSA International: The Association of Biosafety and Biosecurity.
Dr. Wang was recruited to Texas Biomed in January 2020 as the COVID-19 pandemic began. He quickly became a key member of the Pandemic Response Team, working with leaders across the Institute to keep both employees and research operations safe.
“You have to make some decisions without all the data. But we took the data that we did have, and made the best decisions we could,” he says. “In stepping back to review our response, I didn’t find anything I would have done differently because every decision was made with careful analysis.”
A safer work environment: Dr. Wang regularly tests the well water on campus to ensure it is safe to drink.

Safety is one of Texas Biomed’s five core values. You don’t see that everywhere. Here it is a part of our DNA.
— Dr. Anthony Wang
COVID-19 is just one of many safety issues Dr. Wang now helps manage. He is responsible for knowing the thick manuals outlining the rules for studying pathogens, from Ebola to Zika, in secure laboratories. The research and facilities are highly regulated, and he works with regulators and teams on campus to implement solutions that are both in compliance and practical.
Dr. Wang and his EHS team work to protect all on campus by ensuring they have the tools, skills and support to do their jobs safely. They also protect the surrounding community and environment, making sure campus activities do not pollute the air, soil or water.
Biosafety levels are ranked from 1 to 4, with 4 being the highest. The levels represent requirements for the facility, personal protective equipment and research protocols, based on how pathogens spread and what treatments are available. Texas Biomed hosts several BSL-2 and BSL-3 labs, and one BSL-4.
To achieve a safer work environment, Dr. Wang especially focuses on building relationships and trust.
“We want our people to know it is OK to say when they are having an off day,” Dr. Wang says. “When you have something on your mind, or are feeling extra tired, that’s when accidents are more likely to happen, and that’s what we want to avoid. We are here to help.”
One of the main reasons Dr. Wang wanted to join Texas Biomed is the emphasis on safety.
“Safety is one of Texas Biomed’s five core values,” Dr. Wang says. “You don’t see that everywhere. Here it is a part of our DNA.”
He is also impressed with the unique combination of infrastructure, expertise and vision at Texas Biomed. “You aren’t going to find the full pipeline to do infectious disease research in very many places,
especially on high-consequence pathogens,” he says. “Some of them have the potential to cause the next pandemic. You need to prepare for that. You don’t want to wait until something happens before you start this research.”
Dr. Wang is delighted to have come full circle, rejoining the colleagues who set him on the biosafety path, and supporting high-impact science in such a collaborative environment.
“We have so much talent here,” he says. “It’s very much like a family, both my team and Texas Biomed as a whole.”

Fireside
Texas Biomedical Research Institute and The Argyle have a historically unique connection. The Argyle was established as a private dinner club in 1956 by Betty and Lew Moorman, the sister and brother-in-law of Texas Biomed’s founder Tom Slick, Jr. Membership directly supports the mission of Texas Biomed, and Argyle members have long enjoyed a front row seat to lifesaving stories that have emerged as a result of the Institute’s research.
These Fireside Chats relaunched in September after a pandemic hiatus. Texas Biomed President/ CEO Larry Schlesinger, MD, shared his heroic journey combatting oral cancer. Dr. Schlesinger is in remission, but his cancer is most likely the direct
Texas Biomed President/CEO Larry Schlesinger, MD, shared his journey combatting oral cancer at a Fireside Chat this fall.




result of a human papillomavirus (HPV) infection acquired in his youth. The links between infectious diseases and cancer, heart disease, diabetes and more continue to grow. The research at Texas Biomed is shaping our understanding of these connections.
Texas Biomed Associate Professor Binhua “Julie” Ling, MD, PhD, also presented in October about her work on a cure for HIV using the latest gene-editing technology, and a software tool she developed to spot relationships between a host and its microbiome.
Fireside Chats at The Argyle are an intimate way to connect the Texas Biomed team with those who care deeply about the Institute’s mission. Texas Biomed looks forward to the 2023 program.

Chats at The Argyle are an intimate way to connect the Texas Biomed team with those who care deeply about the Institute’s mission.
The Texas Biomedical Forum has been an integral part of the Institute community since its founding in 1970. Well before affinity groups were a buzz term, the Forum established itself as an important affinity organization of strong women with the shared interest of promoting the science of Texas Biomed and raising monies to support big scientific ideas.
In 2021, the Forum supported seven pilot grants for research on COVID-19, Ebola, inflammation, malaria, schistosomiasis and tuberculosis. Many of these diseases have plagued the world for years, and each pilot grant gives researchers a few more answers needed to develop solutions.
Scientists use Forum pilot funds to take those answers and turn them into larger research projects. In its more than 50-year history, the Forum has awarded more than $3.7 million to Texas Biomed, which has resulted in more than $75 million in grant funds from government agencies and other nonprofits.
In the Forum’s 2021-2022 fiscal year, the organization raised nearly half a million dollars for pilot grants and to support the education program at Texas Biomed. Members of the Forum recently presented the check to Texas Biomed President/CEO Larry Schlesinger, MD, and Vice President for Development Akudo Anyanwu, MD. From left to right: Avril Byrne, Lauren Biegler, Dr. Schlesinger, Amelita Mauzé, Rebecca Nathan, Dr. Anyanwu, Bonnie Muecke and Callie Price.


