Established July 2023










Manual for Ocelot (Leopardus pardalis) Breeding and
Reintroduction for Recovery in the United States Version 1
Executive Summary and Forward
Reintroducing an additional population of federally endangered ocelots (Leopardus pardalis) into suitable habitat within the ocelot’s historical but now unoccupied range in Texas forms a pioneering, exciting, and valuable conservation action. Reintroduction will aid in ocelots’ recovery from the Endangered Species List and will help ensure the species’ continued existence in both Texas and the United States This Manual provides the first version of procedures and protocols for breeding and wilding ocelots, releasing ocelots onto reintroduction sites within their historical range in southern Texas, and monitoring the released populations. All ocelot breeding, wilding, releasing, and monitoring activities shall be conducted consistent with any requirements defined in U.S. Fish and Wildlife Service (USFWS) and/or Texas Parks and Wildlife Department (TPWD) permits.
The breeding of ocelots will rely on source ocelots and genetic material available from zoological institutions and from wild ocelot populations in Texas, Mexico and elsewhere in Central America. The approach of using multiple sources of ocelots for breeding maximizes genetic exchange and will provide a sufficient number of ocelots to support reintroduction. Ocelot breeding will occur at a secure Ocelot Conservation Facility to be established in Kingsville, Texas. Ocelots at the Ocelot Conservation Facility will also receive appropriate veterinary care, and individuals genetically eligible for release will receive behavioral preparation (“wilding”) for release into the wild. The behavioral preparation program at the Ocelot Conservation Facility will support the development of natural ocelot behaviors, such as hunting live prey, securing denning sites, and avoiding humans. Ocelots produced at the Ocelot Conservation Facility who are ultimately deemed genetically, physically, and behaviorally suitable for release may be transferred to identified reintroduction sites for release into the wild and subsequent long-term monitoring.
The Captive Propagation Team within the ocelot reintroduction program (RecoverTexasOcelots.org), comprised of carnivore and ocelot ecologists; veterinarians; and conservation practitioners from various university, zoological, and agency backgrounds, developed the procedures and protocols in this document based on their knowledge of ocelots, professional expertise, and conservation experiences. This team incorporated existing protocols produced by institutions within the Association of Zoos and Aquariums and the International Union for Conservation of Nature. The team also studied past reintroduction projects for Iberian lynx (Lynx pardinus), Canadian Lynx (Lynx canadensis), jaguars (Panthera onca), and Persian leopards (Panthera pardus tulliana). to inform goals and best practices for ocelot breeding and reintroductions.
In Texas and the United States, the translocation and reintroduction of wild or captive-born ocelots has never occurred. This project, if implemented, will be a novel and experimental effort. Therefore, the procedures and protocols described herein will guide management of the ocelot reintroduction effort but should be considered dynamic and subject to revision based on program results, lessons learned, and changing circumstances.
2
Ocelot Reintroduction Study Captive Propagation Team: Manual Authors and Experts
Arturo Caso, Ph.D.; Predator Conservation A.C.; President; IUCN SSC Cat Specialist Group
Ashley Reeves, D V M , Ph.D.; The East Foundation; Research Veterinarian and Reproductive Scientist
Clayton Hilton, M S , D V M ; Caesar Kleberg Wildlife Research Institute at Texas A&M UniversityKingsville; Wildlife Veterinarian and Professor
Fernando Najera, M S , D V M , Ph D ; University of California Davis Karen C. Dryer Wildlife Health Center; California Carnivore Program Lead
Grant Harris, PhD; U.S. Fish and Wildlife Service Southwest Regional Office; Chief of Biological Sciences
Jan Janecka, Ph.D., Duquesne University; Associate Professor of Biology; IUCN SSC Cat Specialist Group
Janess Vartanian, M S ; U.S. Fish and Wildlife Service Southwest Regional Office; Recovery Biologist
Jason Lombardi, Ph D ; California Department of Fish and Wildlife; Large Carnivore Research Coordinator; IUCN SSC Cat Specialist Group
Ken Kaemmerer, M.S.; Pittsburgh Zoo & Aquarium; Curator of Mammals (retired)
Laura de la Garza, U.S. Fish and Wildlife Service Southwest Regional Office; Ocelot Species Lead
Lindsay Martinez, Texas A&M University-College Station; Graduate Research Assistant
Lisanne Petracca, Ph.D.; Caesar Kleberg Wildlife Research Institute at Texas A&M University-Kingsville; Assistant Professor of Carnivore Ecology; IUCN SSC Cat Specialist Group
Michael Tewes, Ph D ; Caesar Kleberg Wildlife Research Institute at Texas A&M University-Kingsville; Research Scientist and Regents Professor
Sarah Lehnen, Ph.D.; U.S. Fish and Wildlife Service Southwest Regional Office; Biometrician
Tyler Campbell, Ph D ; The East Foundation; Science Manager
William Swanson, D.V.M., Ph.D.; Lindner Center for Conservation and Research of Endangered Wildlife at the Cincinnati Zoo; Director of Animal Research
The use of trade, firm, or product names in this Manual is for descriptive purposes only and does not imply endorsement by the authors or their organizations, including the U.S. Government
3
4 Table of Contents Genetic Management and Monitoring of Ocelot Breeding and Reintroduction..................................................... 5 Ocelot Conservation Facility for Breeding and Wilding...................................................................................... 11 Natural Breeding and Assisted Reproductive Technologies (ARTs) 16 General Health Monitoring, Preventive Medicine, Pathogen Surveillance, and Quarantine 31 Transportation of Ocelots ..................................................................................................................................... 75 Pregnancy, Parturition, and Kitten Rearing in Ocelots 96 Behavioral Preparation (“Wilding”) Program .................................................................................................... 108 On-site Ocelot Release........................................................................................................................................ 129 Standard Field Monitoring Protocols.................................................................................................................. 142 Appendices 157
Genetic Management and Monitoring of Ocelot Breeding and Reintroduction
Without known access to wild ocelots that can be translocated to the reintroduction site to establish a viable new population, an ocelot breeding program is needed to produce a source stock of ocelots for reintroduction in southern Texas. The planned genetic management of the ocelot breeding and reintroduction programs is based on several program-specific guiding principles as well as: the 2013 International Union for the Conservation of Nature (IUCN) Guidelines for Reintroductions and Other Conservation Translocations [1], the 2016 Ocelot Recovery Plan [2], 2017 IUCN taxonomic revision of the ocelot [3], and logistics associated with establishing and implementing a breeding program
Goal and objectives of genetic management
The ultimate genetic goal of the breeding program is to create a source stock of ocelots for reintroduction that has the most genetically appropriate and diverse composition to support ocelot population establishment in the reintroduction site in Texas. Objectives for meeting this goal include (1) incorporating geographically relevant genetics from southern Texas or from the ocelot subspecies that is native to Texas, as this will support ocelot adaptation to the environment in southern Texas, and (2) maintaining high levels of genetic diversity and minimizing inbreeding to avoid long-term reductions in adaptive capacity and fitness, respectively.
Guiding principles for meeting genetic objectives and goal
To address objective 2, throughout the breeding and reintroduction program, genetic monitoring (initially of microsatellites and in the future, as possible, monitoring of additional genetic markers) and monitoring of phenotypes will inform genetic management of ocelot breeding to promote high genetic diversity and to minimize inbreeding or genetic defects. Meanwhile, Section 5.1.4 of the IUCN Reintroduction Guidelines [1] will be used to guide the selection of founders for a breeding program, with prioritization of adequate genetic diversity as well as individuals from areas that are from the ocelot subspecies native to Texas and geographically nearest the reintroduction site. Unfortunately, the ocelot breeding and reintroduction program cannot source ocelots solely from Texas; the small populations in Texas cannot support reintroduction on their own [4] and wild ocelot populations in Texas have undergone genetic erosion and need genetic augmentation [5].
Regarding the ocelot subspecies that is native to Texas, the Ocelot Recovery Plan [4] recommended that, until both a range-wide genetic evaluation of the ocelot is conducted and subspecies or evolutionarily significant units are further refined, the U.S. Fish and Wildlife Service (USFWS) should focus ocelot conservation in the United States according to historical taxonomic classifications of Leopardus pardalis albescens (in Texas and northeastern Mexico) and L.p. sonoriensis (in Arizona). However, in 2017, the IUCN Cat Specialist Group revised the taxonomy of the ocelot, recognizing two subspecies and acknowledging the designations as provisional [3]. These two subspecies include a northern subspecies L. p. pardalis, hereafter referred to as
5
northern ocelot, found in North and Central America and L.p. mitis, primarily found in South America. Because they are the same subspecies, northern ocelots from Central America may provide ecologically similar genetics to those found in Texas.
Establishment of an ocelot breeding program requires the acquisition of northern ocelots or their genetic material, which is dependent upon cooperation with foreign governments, national and international permitting procedures, and field logistics. Such efforts can be challenging, and they require consideration of potential impacts to wild source populations. For example, for the past decade, USFWS and partners in Mexico have considered translocating ocelots from northeastern Mexico into Texas for genetic augmentation of existing ocelot populations in Texas [2]. However, this effort has been logistically challenging, and translocation has not yet been implemented. While incorporation of northern ocelots from elsewhere within the range of L. p. pardalis should contribute relevant genetics to the breeding program, reliance solely on northern ocelots that are difficult to access may limit the number of genetic founders in the breeding program.
A newly established ocelot breeding program as well as the reintroduced population in the wild will be relatively small, which will limit offspring production as well as introduce concerns about low genetic diversity and vulnerability to inbreeding. To address the issues of small population size, program managers should maximize the genetic diversity of founders in the ocelot breeding program Maximizing in this way is also important because Population Viability Analysis (PVA) results suggest that at least 2 ocelots should be released to the reintroduction site every year for at least 10 years to establish a viable new population, equating to the need to produce 20+ releasable ocelots over 10+ years [6]. These should be considered only minimum numbers, with greater numbers increasing likelihood of success.
The breeding and reintroduction program must source individuals that offer the highest likelihood of creating a genetically viable reintroduced population. Multiple sources of genetics for a reintroduction are recommended by the IUCN in cases where the use of local populations alone is not sufficient for building a source stock for reintroduction and where a balance can be struck between local genetics and other sources. In these cases, IUCN Guidelines suggest that multiple source populations can be used in reintroduction to increase genetic diversity and decrease the risk of inbreeding. IUCN further states that multiple sourcing may be used if outbreeding depression is considered unlikely and if offspring are monitored to identify any evidence of outbreeding depression [1]
To optimize founder numbers and genetic diversity, it is suggested to incorporate zoo-based ocelots into the breeding program at the Ocelot Conservation Facility Ocelots from Association of Zoos and Aquariums (AZA) zoos are considered “generic”- they are a blend of ocelots sourced from the historical pet trade whose origins and current genetic makeup are unknown [W. Swanson, Lindner Center for Conservation and Research of Endangered Wildlife, personal communication]. Zoo-based ocelots will contribute to the acquisition of a sufficient number of individuals and genetic diversity in the breeding program.
The IUCN does not provide recommendations for genetic composition of reintroduced individuals when combing genetics from multiple sources [1]. The ocelot breeding and reintroduction program should be managed to propagate ocelots who have at least 75% northern ocelot subspecies genetics. Individuals with this genetic makeup will be eligible for release into the reintroduction site in Texas. Additionally, the ocelot breeding program must manage for increasing levels of northern genetics over time as the availability of northern ocelot genetics increases. The 75% threshold is based on: (1) IUCN guidelines to use primarily local/ecologically similar genetics and introduce decreasing amounts of unique genetics that can contribute genetic diversity but not swamp local genetics, (2) the need to balance maximizing northern genetics while
6
minimizing inbreeding that could occur in small populations, (3) increased resource (time and space) costs of higher northern ancestry proportions, and (4) USFWS precedence for outbreeding listed species at similar levels to promote recovery. This precedence includes translocations of West Texas cougars into endangered Florida panther populations to achieve 80% Florida genetics and 20% Texas genetics in Florida populations [7] and breeding programs for the Columbia Basin distinct population segment of pygmy rabbit, which managed for 75% Columbia Basin genetics and 25% other populations’ genetics until managers lowered the 75% threshold due to poor results [8]. While individuals in the reintroduced ocelot population may have up to 25% of their genetics from zoo-based ocelots, the reintroduced population will be geographically distinct from existing populations in Texas, so connectivity with and gene flow into the existing populations will likely not occur without human intervention.
In total, the approved sources of ocelots to pursue as founders for a breeding program include:
• L.p. pardalis (northern) ocelots or their genetic material from Texas or northeastern Mexico
• L.p. pardalis (northern) ocelots or their genetic material from elsewhere in Mexico or Central America
• Zoo-based ocelots or their genetic material that are considered “generic” (i.e., a blend of ocelots sourced from the historical pet trade whose origins and current genetic makeup are unknown)
Over the course of the management of the breeding program, managers must make decisions regarding which sources of ocelots to focus acquisition efforts on at any time, given availability and genetic composition of individuals. Additionally, based on factors such as individuals’ genetic ancestry, heterozygosity, phenotype, and behavioral disposition, managers must determine which individual ocelots to breed, which individuals to match together in breeding pairs, and which breeding procedures to use. Breeding of individuals in the program may include natural (unassisted) breeding or assisted reproduction methods, such as semen banking, artificial insemination, and embryo transfer.
Ocelot offspring with at least 75% northern ocelot genetic ancestry are eligible for release to the reintroduction site. The determinations of whether to maintain an ocelot in the breeding program or to release it to the wild (and if so, whether to release it to the reintroduction site or into occupied ocelot habitat) should also be based on an individual’s genetic ancestry, heterozygosity, phenotype, and behavioral disposition, as well as overall ocelot reintroduction program needs.
Operating group for decision-making
A group comprised of the USFWS ocelot species lead, a USFWS research or recovery biologist; the manager of the Saving Animals From Extinction (SAFE) Association of Zoos and Aquariums program for the ocelot; and an ocelot population geneticist, ecologist, and veterinarian will be convened to make the necessary, real-time decisions - in a timely manner - on the genetic management of the ocelot breeding, behavioral preparation, and release/reintroduction programs based on the goals/objectives and guiding principles established here. The flexibility to make real-time decisions allows managers to use adaptive management and base decisions on situational factors. These may include ocelot welfare; the availability of live animals or their genetic material given logistics, costs, and permitting; any given strategy’s potential for success; and program outcomes. Decisions by this group will be operationalized into the breeding and reintroduction efforts through collaborative partnership between the Service and partner organizations.
7
Genetic Monitoring
It is critical to monitor the genetics of small populations, particularly populations such as the ocelot breeding program population that will grow from a limited number of founders. There are several basic goals for genetic monitoring and management in species conservation, including maximizing genetic variation to enable future adaptability to changing environments, maintaining natural levels of variation, and reducing inbreeding depression (i.e., loss of fitness due to reduction of heterozygosity). Genetic monitoring of the breeding program and reintroduced ocelot populations is needed to assess achievement of these goals and should also be used to elucidate important population genetic parameters and processes including census size, effective population size (i.e., the number of breeders), changes in effective population size, dispersal, migration, inbreeding, and social structure. Additionally, having a DNA “fingerprint” on each individual ocelot in the reintroduction program can provide opportunities for non-invasive tracking of ocelots via collection of scat and hair, as well as identifying deceased individuals from their tissue remains. Finally, when sampling successive generations in captive-bred and reintroduced populations, each individual’s contribution to the entire gene pool can be estimated and tracked.
Testing Procedures
Appropriate genetic samples should be collected from each individual ocelot in the breeding program to enable a broad array of potential DNA- and RNA-based analyses, as well as future establishment of cell lines.
Analysis types
(1) Microsatellite and mitochondrial DNA (mtDNA) analysis: This basic population genetic analysis provides information on genetic diversity and relatedness. The molecular markers used will be the 25 variable microsatellites analyzed in the remnant free-ranging ocelot populations in Texas by Janecka et al. [5, 9] and Eizirik et al. [10]. This analysis will provide a low-cost way to obtain general information on heterozygosity (alleles present in individuals). The applications of mtDNA analysis include selecting the most heterozygous ocelots for breeding or reintroduction; making specific ocelot breeding pairings to maximize diversity and minimize genetic similarity; estimating pedigrees; relating mtDNA data to reproductive success or other fitness measures; and using mtDNA to assess population dynamics such as population structure, dispersal, and effective population size.
(2) Genome-wide Single Nucleotide Polymorphisms (SNPs) and Copy Number Variants (CNVs): SNPs and CNVs may be generated by a combination of whole genome sequencing (30X coverage) or targeted enrichment Illumina sequencing (e.g., RADseq, multiplexed amplicons, or hybridization libraries). This analysis would provide the same estimates as strategy (1), but with much higher resolution and more robust estimates. In addition, it would provide information on functional SNPs and CNVs that affect phenotypes, disease resistance, heritable diseases, and reproductive parameters.
(3) Gene expression: Analysis of gene expression using RNAseq enables monitoring of functional aspects of ocelot genetics. Gene expression profiles are monitored to provide information on the immune system. For example, blood samples would include white blood cell RNA that plays an important role in fighting viruses, bacteria, and parasites.
(4) Cell culture: Samples will be processed to maintain live viable cells for future cell culture, source of nucleic acids, and biobanking. This would also enable preservation of live cells that could later be immortalized into a cell line for virtually unlimited sources of DNA and RNA from that individual. If technology advances to a point where ocelot cloning becomes feasible, preserved samples may have application for cloning. Such
8
activities would occur at the direction of and in partnership with USFWS Prior to using cloning, functional studies examining the health and fitness impacts of various genotypes and phenotypes are necessary.
Necessary samples
For genetic analysis, the following samples will be collected from ocelots and prepared for shipment to a partnered laboratory facility for analysis.
(1) Microsatellite and mtDNA analysis: 500 µL of blood in 1.5 mL of Longmire’s Lysis buffer. Samples can be transported at room temperature and stored long term at -20°C. (Store prepared 5-mL tubes containing 1.5 mL of Longmire’s buffer at room temperature prior to use.)
(2) Genome-wide SNPs and CNVs: 3 mL of blood in EDTA tube frozen in liquid nitrogen and stored at -80°C.
(3) Gene expression: 1 mL of blood in 3 mL of RNAlater. Samples are maintained at room temp for 24 hours and then frozen at -80°C. (Store prepared 5 mL tubes containing 1.5 mL of RNAlater buffer at room temp prior to use)
(4) Cell culture: If the partnered lab will start tissues cultures within two days of sample collection, use tissue protocol A (Hank’s at room temperature). If tissue culture will not be done within two days, use tissue protocol B to store viable tissue (Freezing media in liquid nitrogen)
a. Tissue Protocol A. Small tissue punch biopsy in 10 mL Hank's Balanced Salt Solution (sterile) with 200 µl of Antibiotic-Antimycotic solution (store the buffer at -20°C in 15-mL conical tube, thaw before adding samples). Keep tissue at room temperature (20°C) and transport to lab so the tissue can be cultured within two days of collection.
b. Tissue Protocol B. Small tissue biopsy in Freezing media (store the buffer at -20°C in 2-mL cryotube, thaw before adding samples). Freeze immediately in liquid nitrogen. Store long-term in liquid nitrogen or -80°C freezer.
Buffers
Hank’s solution for tissue culture
• In a sterile cell culture hood, mix in a 15-mL sterile conical tube:
o 10 mL Hank's Balanced Salt Solution (sterile)
o 200 µL of Antibiotic-Antimycotic solution (sterile)
• Store the tubes ready-to-go at -20°C.
• Reagents
o Hanks' Balanced Salt Solution (HBSS) (1X), liquid, Invitrogen SKU# 24020-117, 500 mL, $15.50
▪ Contains calcium and magnesium.
• Antibiotic-Antimycotic (100X), liquid, Invitrogen (Gibco) SKU# 15240-062, 100 mL, $29.00
o Contains 10,000 units of penicillin (base), 10,000 µg of streptomycin (base), and 25 µg of amphotericin B/mL utilizing penicillin G (sodium salt), streptomycin sulfate, and amphotericin B as Fungizone® Antimycotic in 0.85% saline.
9
Longmire’s Lysis Buffer, 500mL
• 0.1M Tris-HCl, pH 8.0, 0.1M EDTA-Na2•2H2O, pH 8.0, 0.01M NaCl, 0.5% w/vol SDS
If using Powders: If using Solutions:
• If making a buffer from powders, adjust pH to 8.0 and bring to 500 mL, store at room temp. If making it from solutions, not necessary to adjust the pH, just bring buffer to 500 mL. Autoclave.
• Make 5 mL ready-to-go tubes with 1.5 mL of Longmire’s buffer
Freezing media
• Gibco Recovery Cell Culture Freezing Medium, Catalog # 12648010 ($152/50mL)
o Complete cryopreservation medium for mammalian cell culture.
References
[1] IUCN Species Survival Commission. 2013. Guidelines for Reintroduction and Other Conservation Translocations. Version 1.0. Gland, Switzerland.
[2] Translocation Team (A Subcommittee of the Ocelot Recovery Team). 2009. Plan for Translocation of Northern Ocelots (Leopardus pardalis albescens) in Texas and Tamaulipas.
[3] Kitchener AC, Breitenmoser-Würsten C, Eizirik E, Gentry A, Werdelin L, Wilting A, Yamaguchi N, Abramov AV, Christiansen P, Driscoll C, Duckworth JW, Johnson W, Luo SJ, Meijaard E, O’Donoghue P, Sanderson J, Seymour K, Bruford M, Groves C, Hoffmann M, Nowell K, Timmons Z, Tobe S. 2017. A revised taxonomy of the Felidae. The final report of the Cat Classification Task Force of the IUCN/SSC Cat Specialist Group. Cat News Special Issue 11, 80 pp.
[4] U.S. Fish and Wildlife Service. 2016. Recovery Plan for the Ocelot (Leopardus pardalis), First Revision U.S. Fish and Wildlife Service, Southwest Region, Albuquerque, New Mexico.
[5] Janečka JE, Tewes ME, Laack LL, Caso A, Grassman Jr LI, Haines AM, Shindle DB, Davis BW, Murphy WJ, Honeycutt RL. 2011. Reduced genetic diversity and isolation of remnant ocelot populations occupying a severely fragmented landscape in southern Texas. Animal Conservation 14(6): 608-616.
[6] Martinez LA, Lombardi JV, Parker ID, East F, Campbell TA, Lopez R. In Review. Evaluation of strategies for ocelot reintroduction in Texas, United States.
[7] Hedrick PW, Fredrickson R. 2010. Genetic rescue guidelines with examples from Mexican wolves and Florida panthers. Conservation Genetics 11: 615-626.
[8] Becker PA, Hays DW, Sayler RD. 2011. Columbia Basin Pygmy Rabbit (Brachylagus idahoensis) Reintroduction and Genetic Management Plan. Washington Department of Fish and Wildlife, Olympia.
[9] Janečka JE, Walker CW, Tewes ME, Caso A, Laack LL, Honeycutt RL. 2007. Phylogenetic relationships of ocelot (Leopardus pardalis albescens) populations from the Tamaulipan biotic province and implications for recovery. The Southwestern Naturalist 52(1): 89-96.
[10] Eizirik E, Bonatto SL, Johnson WE, Crawshaw Jr PG, Vié JC, Brousset DM, O'brien SJ, Salzano FM (1998). Phylogeographic patterns and evolution of the mitochondrial DNA control region in two Neotropical cats (Mammalia, Felidae). Journal of Molecular Evolution 47(5): 613-624.
10
Tris-HCl
6.06g 1M Tris-HCl,
50ml EDTA-Na2•2H2O 18.6g 0.5M EDTA 100ml NaCl 0.29g 5M NaCl 1.0ml SDS 2.5g/300ml ddH2O 10% SDS 5ml
(or Trizma base)
pH 8.0
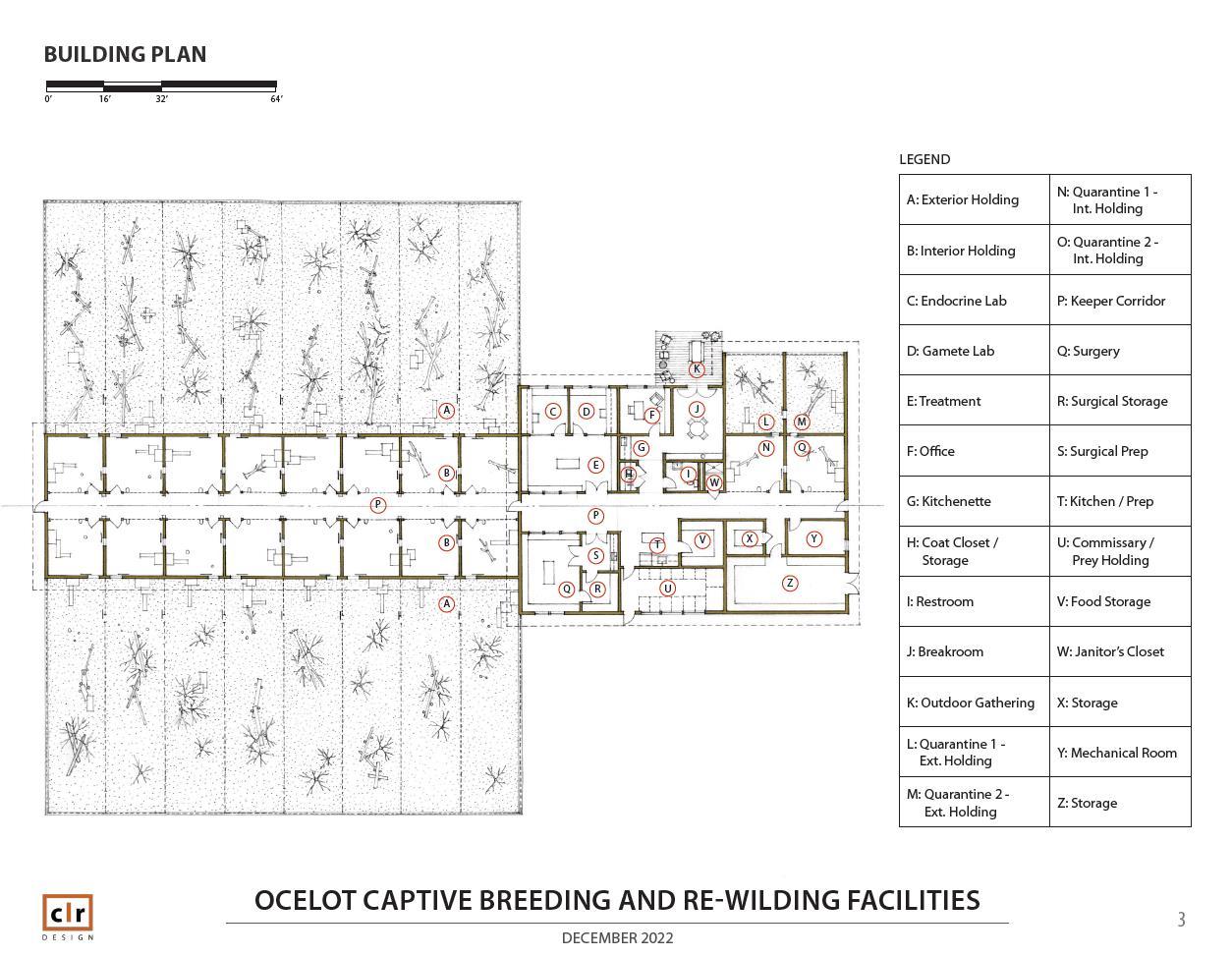
Ocelot Conservation Facility for Breeding and Wilding
To support ocelot reintroduction in Texas, an Ocelot Conservation Facility will be established in Kingsville, Texas. The Ocelot Conservation Facility will serve to breed a source stock of ocelots for reintroduction and to behaviorally prepare ocelot offspring for life in the wild. The Ocelot Conservation Facility will be housed on and around a six-acre lot at the Tio and Janell Kleberg Wildlife Research Park at Texas A&M UniversityKingsville. The breeding portion of the Ocelot Conservation Facility will be used to house breeding female and male ocelots; females and males slated for artificial insemination or gamete collection, respectively; individuals in quarantine; and new dams with offspring during the offspring’s first weeks of life. The behavioral preparation or “wilding” portion of the Ocelot Conservation Facility will contain enclosures that house dams and offspring and/or other adult felids. Ocelot health and behavior will be monitored in wilding enclosures, which will be designed to allow ocelots to acquire hunting skills and other wild behaviors in preparation for release to the wild. Appropriate spaces for veterinary care of ocelots and laboratory processing of ocelot samples will also be present at the Ocelot Conservation Facility. Facilities proposed for ocelot breeding and wilding are based on wild felid breeding programs for Iberian lynx (Lynx pardinus) in Spain and Portugal [1] and the small cat breeding facility plans developed by the Lindner Center for the Research and Conservation of Endangered Wildlife [2].
Ocelot Conservation Facility Objectives
The objectives of the Ocelot Conservation Facility that support the overall goal of propagating a source stock of ocelots available for reintroduction into Texas include: (1) house ocelots for breeding purposes, (2) support natural breeding and assisted reproductive technologies to produce offspring, (3) provide space for medical care, reproductive care, and laboratory practices for sample/gamete assessment and long-term storage, (4) enable ocelot offspring to develop and learn hunting skills and other natural behaviors, limit interactions with humans, and prepare for release into reintroduction sites in the wild, and (5) provide a secure and safe location to quarantine ocelots that have been transferred to the Ocelot Conservation Facility from other locations.
Ocelot Conservation Facility Design
Breeding, Quarantine, and Veterinary and Laboratory Spaces (Figure 1)
The Ocelot Conservation Facility (Figure 1) will contain sixteen breeding enclosures in two rows of eight enclosures opposite a middle walkway. Each enclosure will consist of an indoor space [5 m (L) X 5 m (W) X 3 m (H)] that is connected to an outdoor space [20 m (L) X 10 m (W) X 3 m (H)]. The outdoor space will include multiple fans with misting capabilities and space heaters. Both the indoor space (Figure 2) and outdoor space will contain multiple nest boxes (with cameras inside for monitoring) as options for denning. Each indoor space will have a shift door that leads into the connected outdoor space. There will be two quarantine enclosures of
11
the same spacing and structure as the breeding enclosures, but the quarantine enclosures will be located separately from all other enclosures. All breeding enclosures will be connected to each other by a continuous shift lane between the indoor parts of the enclosures. A door will separate each enclosure from another, and the shift lanes will allow ocelots to be moved from one enclosure to the next. Visual blocking material (e.g., concrete, PVC panels, etc.) will line all indoor and outdoor enclosure spaces up to the full height of the enclosure. Individual ocelots can be housed adjacently, in every other enclosure, or in a combination thereof depending on purpose for holding the ocelots, breeding plans, and individual demeanor. Meanwhile, breeding pairs of ocelots can be housed together in an enclosure, and pairs can be housed in every other enclosure to allow space between pairs. See the following sections of this manual for description of treatment of ocelots in the breeding enclosures.
The Ocelot Conservation Facility will also consist of a veterinary research building with indoor spaces for veterinary and laboratory procedures and other necessary activities. Indoor spaces will include: (1) endocrine laboratory for performing estrogen and progesterone monitoring of females; (2) procedure room for non-sterile procedures; (3) procedure room for sterile surgical procedures; (4) gamete lab for cryostorage of gametes, postthaw sperm assessments and gamete handling; (5) storage space for supplies with a -80°C freezer for media and biomaterials, liquid nitrogen (-196°C) storage equipment, -20°C freezer for media and biomaterials, and a refrigerator; (6) food storage with a large freezer for ocelot food; (7) a separate holding room for live prey; and (8) employee room with restrooms and a shower. While this building and the indoor parts of ocelot enclosures will be under the same roof, only the veterinary research building will have air conditioning.
Wilding
The Wilding portion of the Ocelot Conservation Facility (Figure 1) will have at least four chain-link enclosures [0.25 acres2 X 3 m (H)] that can be expanded, sub-divided, or connected as needed. Enclosures will be topped with 2-4 foot, 45° positive cantilevers angled toward the interior of the enclosure. The cantilevers will be constructed of a smooth material (plexiglass, tin, aluminum, bobbins, spindles etc.) or chain link wire and topped with electric wire to prevent ocelot escape from the enclosures On the outside perimeter of the enclosures, two electric wires will be placed to deter wildlife from approaching the enclosures and interacting through the fence. The electric wires will be layered vertically at different heights above the ground (e.g., 5 inches, 1 foot, 2 feet, etc.) with the possible addition of a secondary set of wires for two layers horizontally. These enclosures are expected to hold a dam and her kittens. Inside the enclosures, there will be multiple plumbed water features for ocelot use. Within or around the enclosures, there may be smaller prey pens [1] with a water source to hold live prey species. Tunnels [1] leading from the prey pens to the enclosures may be used to supply the ocelots with live prey while also minimizing the association of humans with feeding. Smooth surface barriers, or other designs options, will be placed along the bottom of the enclosure fencing to decrease the chances of prey escape from the wilding enclosures. The enclosures will contain natural vegetation found in southern Texas to encourage ocelots’ use of natural cover for denning, hunting, protection, or other behaviors. Cameras will be spaced throughout the enclosure to monitor ocelots’ behavioral development and health to assess ocelots’ suitability for release to the wild.
12


13
Figure 1: General planned draft design of Ocelot Conservation Facility, including breeding enclosures, quarantine, indoor building spaces, and wilding enclosures Figures designed by John Collins and Gregg Leicester: CLR Designs. Drawn figures are not to scale.
Ocelot Conservation Facility Emergency Responses
Backup generators will be present at the Ocelot Conservation Facility to provide electricity to electric fencing, security measures, freezers, refrigerators and any other necessary items in the case of an emergency. Along with electrical outages, fire, hurricane, tornado, envenomation, Africanized bees, and animal escape and recapture are among the emergency events that may impact the Ocelot Conservation Facility. Each emergency has a unique protocol for standard operating procedures. These include initial emergency contact names and numbers, emergency response contacts (fire station, police department), evacuation plans and locations, and the response team for each situation. In the case where an emergency does occur, program managers (including the attending veterinarian) will be contacted to determine response steps appropriate to the emergency. For emergencies that can be predicted (e.g., hurricanes that can be forecast), there will be prevention and/or preparedness planning in anticipation of the event.
In the case of an evacuation of the Ocelot Conservation Facility, it may be necessary to transport animals to a safe location. Institutions available for holding and providing care and resources during an
14
Figure 2: General planned draft design of breeding enclosures, quarantine, and veterinary and laboratory building space at the Ocelot Conservation Facility.
emergency will be identified when the Ocelot Conservation Facility is constructed. Additionally, in Texas, Zoological Disaster Response, Rescue, and Recovery (ZDR3) is a network that can provide support, upon request, to zoos, aquariums, sanctuaries, and other exotic animal operators before, during, and after significant emergency incidents, based on available network resources and operator needs. This network can assign a communications command post as the main communication point between the affected facility and the necessary resources and/or response teams to respond to an emergency. The network operates 24-7-365 and when contacted, can gather basic information to forward to a coordinator. In the case of a mass evacuation of the Ocelot Conservation Facility, the ZDR3 network can be utilized to assist in transport of animals from the facility, identification of interim holding facilities, and other resource needs.
References
[1] A. Vargas, F. Martínez, J. Bergara, L. D. Klink, J. M. Rodríguez, D. Rodríguez, A. Rivas. Manual for the management of the Iberian Lynx in captivity: Breeding Center Operation Program. 2011-2016.
[2] Linder Center for Conservation and Research of Endangered Wildlife. 2012. Cat Canyon: Small Cat Breeding Facility (Bowyer Farm) SCaRCe Design Overview.
15
Natural Breeding and Assisted Reproductive Technologies (ARTs)
While studies have shown that ‘wild-born’ carnivores have a better chance of survival once released compared to captive-born carnivores, dam-reared kittens produced (if possible) by wild-born dams may provide the best scenario for ocelot breeding and reintroduction in Texas. The Ocelot Conservation Facility will allow the use of both natural breeding and assisted reproduction techniques using available live wild-born and captive-born ocelots, and their genetic material. Using both natural breeding and assisted reproduction approaches offer the best methods for increasing the opportunity to produce ocelot pregnancies and ultimately propagating a source population of ocelots for reintroduction. Laparoscopic Oviductal-Artificial Insemination may be conducted using semen samples collected from wild ocelots with desirable genetic backgrounds. For semen collection,

16
Photo courtesy Ashley Reeves, East Foundation.
urethral catheterization provides a simple approach that is most adaptable to field use, though complications with urine contamination and low sperm recovery may occur and further investigation to mitigate urine contamination is warranted. The availability of the electroejaculation method allows recovery of additional semen samples and maximizes sperm recovery from potentially genetically valuable males. Collected semen samples can be frozen for future Artificial Insemination procedures, and although the catheter collection and Ultra Rapid Freezing (URF) of semen in combination would be useful in field settings, electroejaculation with straw sperm freezing is recommended in field settings where an individual may only be captured one time. The combination of electroejaculation and URF freezing has not been studied at this time but could be an additional approach to field semen recovery and cryopreservation. This simplification of the semen cryopreservation process may be particularly valuable for banking semen samples in other countries (e.g., Mexico) under lessthan-optimal field conditions. Finally, in-Vitro Fertilization and Laparoscopic Oviductal-Embryo Transfer may also be considered for ocelot breeding, and the techniques provide ways to conserve the female genome and conduct embryo freezing with later transfer into desirable females.
Source Populations (Wild vs Captive-born) for Reintroduction
Many reviews investigating the use of captive-born versus wild-caught carnivores for reintroductions have found evidence to support that wild-caught animals are more likely to survive after release and create a selfsustaining population [36-40]. Wild-caught carnivores in reintroduction programs survive more (53%) than captive-born carnivores (32%) [40]. The differences may be due to captive-born carnivores being more susceptible to starvation or disease plus unable to avoid predators/competitors [40]. Additionally, the survival of captive-reared carnivores may be low due to poor ability or inability to search for and kill prey, recognize suitable home/habitat sites, migrate with seasons, raise young, and avoid humans [39]. For example, captiveraised pumas (Puma concolor) released in Florida had less fear of humans and were more likely to engage in human and livestock encounters than wild-caught animals [41]; however, captive-reared Eurasian lynx (Lynx lynx) released in Germany did not show the same engagement and the release of captive-bred lynx did result in a successful reintroduction [40]. Furthermore, the reintroduction of Eurasian lynx in Germany using captivesourced animals had a high percentage of founders surviving (68%) compared to others in Poland and France [40]. Including a quarantine period to recognize and treat disease prior to release and using soft releases where animals have the opportunity to acclimate to the release site could mitigate some issues with poor survival of reintroduced captive-bred animals. Additionally, as depicted in 1998 International Union for Conservation of Nature guidelines (modified from Macdonald 2009, 420-421), to prevent or mitigate the lack of shyness to humans, captive-bred individuals should be allowed to acquire necessary training in their captive environment to enable survival in the wild and care should be taken to ensure captive-bred animals are not so confident in the presence of humans that they would pose a threat to local inhabitants or their livestock [42,43].
Although wild-sourced populations may be the best source of animals for reintroduction, acquisition of wildsourced individuals may not be feasible for every reintroduction effort. Additionally, there may not be enough wild-sourced individuals for use of wild-sourced individuals alone to produce suitable numbers for a reintroduction. Behavior preparation programs, or “wilding,” of offspring produced from wild-born or captiveborn females at the Ocelot Conservation Facility will be necessary to prepare captive-bred offspring for life in the wild.
17
Natural Breeding of Ocelots Under Human Care
Estrus and Breeding Behavior
Behavioral estrus typically lasts 2-5 days in female ocelots and is indicated by an increase in flehmen responses and sniffing of females’ anogenital region by males; plus cheek rubbing, urine marking, and vocalization from males and females [44]. When a female becomes receptive to a male, she will allow him to mount in which he will grasp her at the nape of the neck, straddle her and begin thrusting for 1-5 minutes; and intromission for 510 seconds is signaled by the “copulatory cry” by the female. At this point she will throw the male off and begin rolling on her back for 5-30 seconds.
Introductions for Breeding
During the 2-5 days of behavioral estrus, the female will be receptive to the male, allowing him to mount and copulate over 24-96 hours during this time. If male and female are to be housed separately, observation of both individuals must be made to pair them together when the female comes into estrus. If housed separately, the two must have a common wall adjacent to one another to observe behavioral responses. When possible, the male will be introduced into the female’s habitat and closely monitored. Keepers should be prepared to intervene during introductions.
Sources (Who) – Options for Natural Breeding at the Ocelot Conservation Facility
1) Wild-born ocelots bred to one another
• Wild females will be able to teach their kittens wild behaviors that will aid them in hunting and surviving.
2) Captive-born ocelots bred to one-another
3)Wild-born ocelots bred to captive-born ocelots
Advantages of Natural Breeding
● Natural breeding allows for ocelots’ normal physiological processes to determine the success of pregnancy. These processes may include ovarian follicular growth, estrus behavior, breeding-induced ovulation, post-coital sperm transport, conception, implantation, fetal growth and parturition.
● Minimal sedation events are involved.
Disadvantages of Natural Breeding
● Breeding of wild-born ocelots with captive-born ocelots may not be feasible due to individual demeanor, which may lead to behavioral and physical incompatibilities between pairs of one captive-born and one wild-born individual.
● The stress induced by bringing wild ocelots into captivity may decrease ovarian cyclicity, likelihood of conception following breeding, completion of term pregnancy, or libido of both males and females.
18
Assisted Reproduction Techniques
Laparoscopic Oviductal Artificial Insemination (LO-AI)
The standard protocol for artificial insemination in non-domestic feline species that produces the best chance of successful pregnancy requires exogenous gonadotropin treatment to synchronize follicular growth and ovulation with insemination procedures [45-46]. Because ocelots are induced ovulators (i.e., requiring mating to stimulate ovulation), exogenous hormones are necessary when natural mating does not occur. High pregnancy percentages (~70%) have been obtained in domestic cats using a LO-AI approach with low sperm numbers in females treated with gonadotropins before the procedure [47]. Porcine luteinizing hormone (pLH) has proven effective in inducing ovulation in feline species when compared to other gonadotropins and reduces the formation of unwanted secondary ovarian structures that could disrupt the endocrine environment and oviductal transport [47-50].
For felines, several variations of artificial insemination exist, including placement of sperm into the vagina, uterine horn, uterine body, and/or the oviduct [24, 51-57]. However, many of these techniques require the use of high sperm numbers [24, 51-57], limiting their application to many feline species. LO-AI presents a technique that can bypass anatomical barriers, which and reduces the need for both high sperm numbers [32-33, 51] and sperm transport to the site of fertilization in the ampulla [51, 58-59]. Furthermore, multiple LO-AI procedures can be performed with a single ejaculate [51]
Since 1995, in zoo-based ocelots in the United States, there have been three successful pregnancies using artificial insemination with frozen semen collected from zoo-based males, and 6 pregnancies using non-frozen semen from zoo-based males [W. Swanson, Lindner Center for the Conservation and Research of Endangered Wildlife, personal communication]. Since 2019, there have been eight artificial insemination procedures conducted in zoo-based female ocelots using semen collected from free-ranging male ocelots trapped in Texas. None of the eight procedures resulted in a pregnancy, likely due to a combination of low-quality sperm collected from male ocelots in Texas and further damage by cryopreservation [A. Reeves, East Foundation, unpublished data]. Continued assessment of artificial insemination techniques in ocelots using non-frozen sperm and/or sperm of higher quality could make this technique more successful in the ocelot breeding program.
Ovarian Synchronization and Ovulation Induction [32,60]
For ocelots, oral progestin (Regumate; 0.044 mg/kg BW) is fed daily for 30 consecutive days and then discontinued during a 7-day withdrawal period. On the 8th day of withdrawal, 400 IU of equine chorionic gonadotropin (eCG) is administered intramuscularly (IM). Approximately 82 hours post eCG injection, 3000 IU of porcine luteinizing hormone (pLH) is administered IM ~ 38 hours prior to the laparoscopic oviductal artificial insemination (LO-AI) procedure.
Semen Thawing Procedure
For thawing of straw-frozen semen samples, the straw is removed from the liquid nitrogen canister and held in room air for 10 seconds, then transferred to a 37°C water bath for 30 seconds for thawing. The straw is removed, wiped dry, and the sample placed into an Eppendorf tube. FOCM-Hepes medium (100 μL) is slowly added to each straw sperm sample and all straw samples are combined into one Eppendorf tube. Initial postthaw motility is assessed under light microscopy at 400X, and then an aliquot is spread across a slide and allowed to air dry for later post-thaw acrosome assessment. A small aliquot is also diluted (1:400) in water in an
19
Edperdorf tube for a concentration count under light microscopy. The semen sample is split equally into two tubes and centrifugated for 8 minutes at 300g. The supernatant is removed, each pellet is measured, and, if necessary, resuspended to 15-20 μL total for insemination. The entire volume of one pellet is placed onto a sterile petri dish and aspirated into the sterile AI needle. The AI needle is inserted through an 18-gauge catheter into the oviduct for LO-AI (below). Residual sperm is flushed from the AI needle to assess final motility.
LO-AI Procedure [22, 32, 51-52, 61]
The female’s hair is clipped on her ventral abdomen (from xiphoid to pubis), the surgical field is prepped with betadine and alcohol alternating 3 repetitions, a sterile drape placed over the surgical field, and the drape cut to expose the clipped area. The Verres needle is placed in the right caudal abdomen approximately 1 inch caudal and lateral to the umbilicus by tenting the skin and using manual force to enter the abdomen. A hand pump is attached to the Verres needle to insufflate the abdominal cavity with room air to a uniform tautness. An ~ 1 cm incision is made ~ 2-3 cm cranial to the umbilicus and the surgical table is tilted at a 20-30° angle. The skin caudal to the incision is grasped and the trocar-cannula assembly is inserted at a 60° angle to the ventral abdomen with a sharp thrusting motion.
The trocar is removed, and the laparoscope, with a camera attached, is inserted and attached by a fiberoptic cable to a light source. The laparoscope (10 mm diameter) is used to visualize the ovaries, oviduct, and/or uterus for various procedures, using the Verres needle to manipulate abdominal contents as necessary. Both ovaries are examined for follicles, corpus luteum, corpus albicans, and cysts. The uterine horns are assessed for tone, symmetry, and size. The oviducts are examined for distinctness and presence of adequate fimbrial tissue for grasping. The Verres needle is removed, and the accessory trocar is inserted in the same location into the abdomen for placement of the grasping forceps. The grasping forceps are inserted through the accessory cannula and the oviductal tissue picked up using the grasping forceps to lift the bursa laterally.
An 18-gauge (18 g, 3.2 cm length; Terumo Medical Corporation, Elkton, MD, USA) catheter is placed into the abdomen lateral and caudal to the ovary on the left side. The needle is removed from the catheter and a blunted, artificial insemination needle (22 g, 6.8 cm length), derived from the stylet within an i.v. catheter (20 g, 5.0 cm length; Sherwood Medical Co.), is attached to a 1-mL syringe and placed through the catheter. The AI needle is inserted into the ampulla via the oviductal opening and the sperm injected as the insemination needle is retracted from the oviduct. The same procedure is performed on the right ovary. Following the AI procedure, surgical closure of each skin incision requires 1–2 sutures and a small amount of tissue adhesive. Cats typically return to normal activities shortly following anesthetic recovery.
Sources (Who) – Options for LO-AI
1) Captive-born ocelot females with wild-born fresh or frozen-thawed male ocelot semen
2) Captive-born females with captive-born fresh or frozen-thawed male ocelot semen
3) Wild-born females with captive-born fresh or frozen-thawed male ocelot semen
4) Wild-born females with wild-born fresh or frozen-thawed male ocelot semen
Advantages of LO-AI
● Selecting the parents of the offspring without having to account for behavioral mismatches in breeding pairs.
● Use frozen-thawed sperm collected from wild or captive ocelots to increase genetic diversity in the breeding program.
20
● For both male and females, procedures are minimally invasive and virtually no recovery period is needed.
● The opportunity to use freshly collected sperm obtained from a nearby wild-born (or captive-born) ocelot could increase pregnancy success.
Disadvantages of LO-AI
● For AI of wild females, the procedure requires manipulation of the ovarian cycle to induce follicular growth and ovulation in time with the scheduled AI procedure without the advantage of ovarian synchronization (using Regumate treatment)
● If performing an AI procedure on wild-born ocelots, females must be maintained in a captive environment for ~1 week to induce follicular growth and ovulation before AI
● Stress from maintenance in captivity may affect ovarian response or pregnancy success in wild-born females
Laparoscopic Oviductal-Embryo Transfer (LO-ET)
Embryo transfer (ET) has shown great promise for allowing propagation of non-domestic feline species [45,6263]. Live offspring have been produced following ET in ten cat species (domestic cat, wild cat, tiger, ocelot, serval, caracal, fishing cat, black-footed cat, sand cat, cheetah) [33,67-69]. Most of the earlier ET procedures required intra-abdominal laparotomy to gain access to the reproductive structures [33]. The ability to perform these techniques laparoscopically has helped to alleviate animal welfare concerns and health concerns during intra-abdominal reproductive procedures [64]. Laparoscopic oocyte collection has provided a minimally invasive technique to collect oocytes from numerous feline species [45,52,65] and has allowed the creation of embryos for more than 20 felid species following in vitro fertilization. Additionally, techniques to access the oviduct via laparoscopic approaches have been developed to conduct LO-ET, similar to laparoscopic oviductal artificial insemination (LO-AI) [46,47, 66]. The success of this procedure, like LO-AI, depends on the use of exogenous gonadotropins to induce ovulation for the timed-ET.
Over the last few decades, the Lindner Center for the Conservation and Research of Endangered Wildlife (CREW) has investigated the use of LO-ET in domestic cats for potential application to the propagation of nondomestic feline species [33]. This approach has been successfully used for the production of multiple pregnancies and viable offspring in the ocelot and sand cat (Felis margarita) [33]. A total of 5 pregnancies and 5 term kittens have been born from 10 LO-ET attempts with frozen-thawed In-Vitro Fertilization (IVF) embryos in ocelots in the United States and Brazil [33]. Additionally, one pregnancy leading to the birth of two sand cat kittens born has resulted from 4 attempts of LO-ET with non-frozen embryos [33].
Oocyte collection [70]
Females are treated intramuscularly first with equine chorionic gonadotropin (eCG) (Sigma Chemical Co. or Sioux Biochemical Inc., Sioux Center, IA), and then 84-86 hours later, with human chorionic gonadotropin (Sigma Chemical Co. or Sioux Biochemical Inc.). At 24-47 hours after hCG administration, in vivo-matured oocytes are collected using techniques for laparoscopic oocyte recovery [20, 71]. Mature follicles (≥2 mm) are aspirated using a 22-gauge needle with aspiration pressure (~ 1.5 mm Hg) provided by a vacuum pump, and follicular contents are collected in a sterile glass tube containing FOCMH with 40 units/mL of heparin (ElkinsSinn Inc., Cherry Hill, NJ). Only oocytes with dark homogeneous cytoplasm that are surrounded by expanded cumulus cells (grade 1) are used for IVF.
21
In-Vitro Fertilization (IVF) [72-74]
For IVF, spermatozoa (~ 1 X 106 mL-1) are co-incubated with oocytes in 5% CO2 in air at 38°C under mineral oil (#4008, Sage BioPharma, Bedminster, NJ, USA) in Tyrode’s solution containing 6 mg/mL BSA, 15 mM NaHCO3, 0.36 mM pyruvate, 2.2 mM calcium lactate, 1.0 mM glutamine and 50 µg/mL gentamicin (IVF medium 8). At 5-6 hours post-insemination, oocytes are rinsed and cultured in 500 µl of Tyrode’s solution that contains supplements used for IVF along with 1% MEM non-essential amino acids (NEAA) and BSA reduced to 3 mg/mL (IVC(in-vitro culture)-1 medium) in an atmosphere of 5% CO2, 5% O2, 90% N2 at 38°C. Embryos are cultured in a three-step system: 1) culture in IVC-1 medium until day 2; (2) culture in fresh IVC-1 medium containing 1% EAA (IVC-1A) until day 5; (3) culture in IVC-2 medium until cryopreservation or transfer to a recipient.
Embryo cryopreservation and thawing for transfer [72-73, 75]
On IVC day 5, if embryos are to be cryopreserved, embryos are equilibrated in a cryoprotectant solution consisting of 1.4 M propylene glycol, 0.125 M sucrose, 10% dextran 70 and 10% FBS in HeTy (CPS) and cooled at a slow, controlled rate to 30°C before storage in liquid nitrogen. Embryos are warmed by holding the straw in air for 2 min and CPS is removed in five steps of 3 min each. Then, embryos are cultured in IVC-2 medium until transferred to a recipient within 2–24 h.
LO-ET Procedure [33]
The female is clipped from xiphoid to pubis, the surgical field is prepped with betadine and alcohol alternating 3 repetitions, a sterile drape placed over the surgical field, and the drape cut to expose the clipped area. The Verres needle is placed in the right caudal abdomen approximately 1 inch caudal and lateral to the umbilicus by tenting the skin and using manual force to enter the abdomen. A hand pump is attached to the Verres needle to insufflate the abdominal cavity with room air to a uniform tautness. An ~ 1 cm incision is made ~ 2-3 cm cranial to the umbilicus and the surgical table is titled at a 20-30° angle. The skin caudal to the incision is grasped and the trocar-cannula assemble is inserted at a 60° angle to the ventral abdomen with a sharp thrusting motion. The trocar is removed, the laparoscope is inserted and attached by a fiberoptic cable to a light source. The laparoscope (10 mm diameter) is used to visualize the ovaries, oviduct, and/or uterus for various procedures, using the Verres needle to manipulate abdominal contents as necessary. Both ovaries are examined for follicles, corpus luteum, corpus albicans, and cysts. The uterine horns are assessed for tone, symmetry, and size. The oviducts are examined for distinctness and presence of adequate fimbrial tissue for grasping. The camera is attached to the end of the scope. The Verres needle is removed, and the accessory trocar placed in the same location in the abdomen for the grasping forceps. The grasping forceps are placed through the trocar opening and the oviductal tissue picked up using the grasping forceps and rolling the bursa laterally. A polypropylene i.v. catheter (20 g, 5 cm length; Sherwood Medical Co. St. Louis, MO, USA) is placed through the ventral abdominal wall dorsal to the ovary and inserted through the oviductal ostium into the distal oviduct. Polyethylene tubing (25 cm length, PE10; Bectin Dickinson Co Sparks, MD, USA), attached to a blunted 30 g (1.25 cm) needle and 1 ml syringe, is passed through the catheter and, with continued forward pressure on the tubing, the catheter is completely withdrawn from the oviduct. Embryos (n=3-7, depending on species), contained in 5-10 μL of culture medium at the distal end of the transfer tubing, are expelled deep into the oviductal lumen with slight air pressure from the syringe. Surgical closure of each skin incision requires 1–2 sutures and a small amount of tissue adhesive, and cats typically return to normal activities shortly following anesthetic recovery.
22
Sources (Who) – Options for Embryo Transfer
1) Wild-born female oocytes with captive or wild-born male semen, and the embryo is placed into captive-born or wild-born females
2) Captive-born female oocytes with wild-caught semen and embryo placed into wild-born females
Advantages of LO-ET
● LO-ET could allow use of additional source of female oocytes to increase genetic diversity in the breeding program
● Embryos can be frozen for subsequent transfer into a different female. For example, use of wild-born ocelot female eggs could be used to produce embryos for transfer into captive females.
● Fertilization is assured since the fertilization process is conducted in vitro.
Disadvantages of LO-ET
● Involves multiple additional steps compared to other techniques and options
● Involves additional sedation events for females to collect oocytes and transfer embryos
● Involves substantial lab techniques to properly fertilize and culture embryos prior to transfer or freezing
● Embryo freezing can compromise viability relative to non-frozen embryos
Semen collection and cryopreservation
Although various techniques and technologies are utilized for semen collection in different species, there are two collection techniques of interest in ocelots: electroejaculation and urethral catheterization. Electroejaculation has been the long-accepted standard for many species and while effective, utilization requires advanced skill and equipment. Urethral catheterization is a relatively new technique (established in 2008 in domestic cats) that requires less equipment and expertise and is most adaptable to field use. Catheterization has been studied in wild ocelot populations in southern Texas since 2019, but many samples collected so far have proved nonviable due to urine contamination during sampling and low sperm recovery [A. Reeves, East Foundation, unpublished data]. Potential ways to improve semen recovery by catheter must be further investigated to mitigate urine contamination for optimal sperm recovery. Meanwhile, when collecting samples by electroejaculation, the inclusion of seminal fluids creates a potential buffer against the negative impacts of urine on seminal quality. The seminal fluids are either not present or are present in small amounts in semen samples collected by catheterization. Semen collection opportunities for wild male ocelots are limited to those occurring at the time of capture, and an individual ocelot may be captured only once in its lifetime. Given its efficiency and reliability, electroejaculation is currently the preferred method for semen collection in wild ocelots.
When cryopreserving semen samples, straw freezing is a complicated and time-consuming technique, but results suggest that this technique may produce superior post-thaw sperm traits and be more effective for insemination procedures in ocelots compared to ultra-rapid freezing (URF, A. Reeves, East Foundation, unpublished data). The combination of electroejaculation and URF has not been studied at this time but could be an additional approach to field semen recovery and cryopreservation. The simplification of the semen collection and cryopreservation process may be particularly valuable for banking semen samples in other countries (e.g., Mexico) under less-than-optimal field conditions.
23
Semen Collection Techniques
Specific pharmaceutical agents are typically used to successfully collect semen in many species. Pharmacological methods have been studied with success in species such as the horse [1-3], domestic felids [4], domestic canids [5], and white rhinoceros [6]. When conducting field-based studies on wild felids, immobilization of the animal can be achieved using many combinations of pharmaceuticals, especially alphaadrenergic agonists (α2 agonists). Alpha-2 adrenergic agonists are reported to influence erection [6,7], and the ejaculatory reflex [7] and induce action at the level of the vas deferens [8]. Medetomidine, a potent α2 agonist, is thought to induce smooth muscle contraction of the vas deferens, which then forces semen into the pelvic urethra [9]. An alternative method for semen collection that was recently developed and reported to be successful in domestic felids is urethral catheterization of male cats after they have been sedated with medetomidine [4]. This technique has allowed for the recovery of high sperm numbers in domestic cats [4], jungle cats (Felis chaus) [10], Amur leopard cats (Prionailarus bengalensis euptilurus) [11], lions (Panthera leo) [12], and other wild felids. Urethral catheterization under medetomidine sedation could provide a costeffective and simplified technique that could be valuable for imperiled felid conservation globally.
One confounding factor recently observed in semen collection in ocelots is urine contamination of cathetercollected samples [A. Reeves, East Foundation, unpublished data]. Due to the lack of seminal fluids in catheter samples and the high osmolarity, the acidity of the urine damages the sperm and decreases its viability, even if immediately diluted and centrifuged to remove urine. Samples collected by electroejaculation include more seminal fluids and thus have higher alkalinity when compared to catheter samples. The seminal fluids may provide a better buffer against urine contamination. Spermatozoa characteristics of frozen-thawed semen samples do not differ between the catheterization and electroejaculation methods [4]. Overall, electroejaculation provides another opportunity to obtain a semen sample if the initial catheter sample is compromised.
Electroejaculation has been performed successfully in domestic cats and virtually all wild felid species [13-16] maintained under human care in zoos, ranging in body size from the tiger (Panthera tigris) [16] to the blackfooted cat (Felis nigripes) [15]. During electroejaculation, a lubricated rectal probe, varying in diameter based on species size, and an electro stimulator are used to deliver 80 -140 electrical stimuli (voltage range 2-6 V) divided into 3 to 5 separate series [13-16]. During collection, cats typically extend their hind limbs due to electrical stimulation of peripheral motor nerves. Rarely, they may vocalize, but because cats are anesthetized with a dissociative anesthetic, they do not have any conscious perception of muscular stimulation or experience any associated discomfort. The electroejaculation semen collection method has been used in cats for over 40 years and has been applied to thousands of domestic and wild felids by CREW and by other investigators. Safety and health concerns for felids are almost nonexistent assuming proper techniques are used. Scientists from the CREW lab have conducted more than 200 electroejaculation procedures in ocelots within zoos without any reported adverse effects. In ocelots, electroejaculation remains the most effective approach for recovering semen. In a recent study in ocelots (n=7) assessing semen collection with urethral catheterization followed by immediate electroejaculation (during the same anesthetic event), it was determined that electroejaculation produced significantly greater seminal volume and more than doubled the total sperm numbers collected across males [16]. These findings suggest that high numbers of residual sperm may be safely collected from ocelots by conducting electroejaculation immediately after urethral catheterization.
Electroejaculation (EEJ) has been used extensively as part of captive propagation and reintroduction programs for other wildlife species, such as the black-footed ferret [17,18]. EEJ in ferrets requires four sets of electrostimulations (20-30 stimuli per set over a 2-5 V range) using a 6 mm diameter rectal probe [18]. As in ocelots, no negative impacts on the animal’s health have been reported [17,18]. The black-footed ferret is one of
24
the most intensively managed mammals in North America and, with the use of EEJ and artificial insemination, the black-footed ferret reintroduction program has created a model for applying assisted reproduction to address challenges posed by a small number of founders available to support species reintroduction programs [17]. Much like ocelots, ferret populations are challenged by population declines due to genetic restriction, and the black-footed ferret was listed as endangered in 1967 [14]. Since then, 30 generations of successful ferret kit births and more than 9,100 ferrets have been produced in the ex-situ breeding program [19], including >100 offspring produced from artificial insemination with freshly collected and frozen-thawed semen. Use of EEJ procedures in free-ranging ocelots in Texas has the potential to improve success in obtaining viable semen samples from this imperiled population and ultimately supporting breeding of ocelots for reintroduction.
Urethral Catheterization Procedure [4]
Males are immobilized and maintained at a light anesthetic plane for semen collection. The anesthetic protocol consists of an injectable combination of ketamine hydrochloride with medetomidine or dexmedetomidine followed by partial reversal with atipamezole. Approximately 25-40 minutes after anesthetic injection, the penis is extruded with manual manipulation and sterile gloves. Debris on the penis and in the preputial cavity is removed with water-soaked gauze. A 3.5- or 5- French urinary catheter (dependent on age, size, and species) is advanced approximately 15 cm into the urethra, left in place for 30 seconds and slowly removed. The sample is placed into an Eppendorf vial using a one -mL syringe and a small amount of air.
Electroejaculation Procedure [13, 20, 21]
A lubricated probe is gently inserted into the rectum with the electrodes directed ventrally. A warmed, sterile collection cup is placed over the end of the penis. The electro ejaculator is turned on (after ensuring that the voltage rheostat is turned to zero). A series of 1 to 3 electrical stimulations will occur, beginning at 2 volts and progressing to 5 or 6 volts (Series 1, ~ 30 stimulations; Series 2, ~ 30 stimulations; Series 3, ~ 20 stimulations) with 10 stimulations at each voltage and 3–5-minute rests between series. Initial electrical stimulation is applied by slowly increasing voltage from 0 to 2 volts, pausing momentarily, and then abruptly returning to 0 volts. This stimulation is repeated 10 times, then voltage is increased to 3 volts for another 10 stimulations, and then voltage occurs at 4 volts for another 10 stimulations. When the series is completed, the electro ejaculator is switched off. The cup is removed from the penis and any additional liquid adhering to the penis is collected with a sterile pipettor. The probe is removed from the rectum. The total semen volume is measured and transferred into a sterile, warm Eppendorf tube.
Semen Cryopreservation Techniques
Previous semen cryopreservation efforts in other feline species have used either sperm pelleting on indentations in dry ice [22-25] or straw freezing over liquid nitrogen vapor [23, 26-28] to preserve semen samples for later use. Despite fairly substantial acrosome damage to semen post-thaw after pelleting on dry ice, frozen-thawed spermatozoa in ocelots may be functionally competent [22], similar to findings in fishing cats (Prionailurus viverrinus) [29]. In an earlier study, one nulliparous ocelot female treated with exogenous gonadotropins and inseminated with thawed spermatozoa, previously frozen by pelleting, conceived and gave birth to a healthy kitten 78 days later [22]. However, compared to freshly collected inseminates used for artificial insemination in ocelots, the amount of frozen-thawed sperm for artificial insemination must be increased to compensate for the acrosome damage to frozen-thawed sperm [22]. In another study comparing freshly collected and frozen-thawed ocelot semen, frozen-thawed spermatozoa showed similar values for progressive motility status but had decreased percentages of normal sperm morphology and lower percentages of intact acrosomes [13]. However, higher numbers of spermatozoa were bound to fertilized domestic cat oocytes when using the pelleted dry ice
25
treatment compared to straw freezing of samples over liquid nitrogen and storage in a dry shipper [13]. Furthermore, semen samples frozen by pelleting on dry ice exhibit higher motility or viability immediately postthaw compared to samples frozen in straws over liquid nitrogen [13].
A newer sperm cryopreservation approach, ultra-rapid freezing or URF, offers simplicity and minimal equipment needs because it requires only URF-specific medium and liquid nitrogen [30,31]. The URF process involves extending the spermatozoa into a soy-lethicin-based medium with 0.2 M sucrose, equilibrating, and directly pipetting into an open container of liquid nitrogen. Then, the sperm pellets are transferred into labeled cryovials and stored until thawing. A comparative study in domestic cats involving catheter-recovered sperm samples frozen by URF and by conventional straw freezing reported no difference in post-thaw motility and acrosome status of URF-catheter samples over time when compared to straw-frozen samples [9]. Preliminary in-vitro fertilization (IVF) results indicated that URF-catheter sperm is capable of fertilizing oocytes in vitro, and fertilization success with URF sperm for all inseminated oocytes (30%) did not differ from success observed with straw-frozen samples (57%). However, based on mature oocytes (M2 cell or cleaving), fertilization success with URF sperm (35%) was slightly lower than that of sperm frozen in straws (65%) [9].
With laparoscopic oviductal artificial insemination (LO-AI), sperm function and motility over time are not as critical as with intravaginal or intrauterine AI. High pregnancy rates (70-80%) have been obtained with LO-AI using low sperm numbers (~ 1 million motile/oviduct) for insemination, including semen that was frozen using standard straw cryopreservation methods [32-34].
From 2019 to 2022, semen samples were collected by catheter collection from free-ranging ocelots in Texas to compare the URF and straw techniques for sperm freezing (A. Reeves, East Foundation, unpublished data). Preliminary findings suggested similar results between the two freezing techniques (URF and straw) for motility and forward progression of sperm over time. However, heterologous IVF results suggested that the straw technique was superior to URF for fertilization success.
In the study, additional analysis and sample collection further explored the results of semen collection and cryopreservation methods. While there was not a significant difference in pre- and post-thaw parameters and quality of semen, most catheter-collected semen samples were contaminated by urine and were not of adequate quality for cryopreservation. Although the catheter-collection/ URF cryopreservation combination provides a simple field technique for collecting and storing sperm from free-ranging ocelots using minimal equipment, it is not recommended as a first-line technique for semen collection, especially when the capture of a wild male ocelot could be a one-time occurrence. Further assessment of the catheterization combination is needed to mitigate urine contamination and ensure best sample collection practices before this technique is used in field settings While straw freezing is a more complicated and time-consuming semen cryopreservation technique, results suggest this technique may produce superior post-thaw sperm traits and be more effective for insemination procedures in ocelots (A. Reeves, East Foundation, unpublished data). Additionally, the use of electroejaculation yielded semen samples of higher quality for cryopreservation when employed in the field and therefore, has become the preferred method to be utilized in field collections.
26
Straw freezing
For straw freezing, the raw semen is diluted 1:5 with FOCM-Hepes medium. The diluted straw sample is centrifuged at 600Xg for 8 minutes and the resulting sperm pellet is resuspended in soy lethicin with 4% glycerol to a concentration of 50 X 106 motile sperm/mL and loaded into 0.25 mL straws (30-50 μL/straw). The ends of the straws are heat sealed, transferred into a sealable plastic bag, submerged in room-temperature water (100 mL) within a glass container, and cooled in a refrigerator to 4°C for a minimum of 2 hours in a refrigerator. Straws are then frozen using a modified two-step protocol [29, 35]. In this protocol, two metal racks are placed in a polystyrene foam container partially filled with liquid nitrogen (LN2). Cooled straws are placed on the top rack (7.5 cm above the LN2 surface) for one minute and then transferred to the bottom rack (2.5 cm above the LN2 surface) for one minute before plunging directly into liquid nitrogen for storage until thawing.
Ultra-rapid freezing (URF)
For URF, the raw semen is diluted 1:5 with soy-lethicin 0.2 M sucrose medium and allowed to equilibrate at room temperature for 5 minutes. The diluted URF sample is cryopreserved using a micropipette, pipetting one ~ 20 μL drop at a time directly into liquid nitrogen and allowing the pellet to sink to the bottom before the next drop is added to the LN2 container. This process is repeated for the entirety of the volume. The pellets are recovered using forceps and placed into a labeled cryovial for storage in liquid nitrogen until thawing.
Pregnancy Monitoring
Fecal samples are collected three days per week beginning two months prior to an assisted reproduction or natural breeding procedure, with sample collection continued for 85 days after assisted reproduction or natural breeding Fecal samples are placed into labeled (name, studbook number, institution name, date) plastic bags and immediately frozen (-20°C) for storage until processing. Samples are then lyophilized via a freeze dryer (Labconoco Corp., Kansas City, MO, USA) in their plastic bags, pulverized into a fine powder, and then weighed (250± 5 mg) into labeled 15 mL-polypropylene conical tubes. Each sample is then extracted by adding 2.5 mL of 90% ethanol (or a 1:10 weight:volume) overnight on a mechanical rocker (≥12 hours). Extracted samples are centrifuged (1000g, 15 min, Eppendorf, Enfield, CT, USA), supernatants are removed, and samples are stored in 2.0-mL cryovials at -20 °C until analysis.
Procedures for enzyme immunoassays (EIAs) used by Herrick et al. (2010) [76] and Bateman et al. (2009) [77] are recommended for assessing pregnancy status. Arbor Assays progesterone mini-kit (ISWE003, Arbor Assays, Ann Arbor, MI, USA) will be used to determine progestogens (this kit included both antibody and horseradish peroxidase) and been previously used in the CREW endocrine laboratory and validated for ocelots Similarly, fecal estrogen metabolites can be quantified using specific EIAs to monitor natural or induced ovarian follicular activity [76]. EIAs may be conducted in endocrine laboratories to be established at the Ocelot Conservation Facility or at partnered institutions.
References
[1] McDonnell SM. 2001. Oral imipramine and intravenous xylazine for pharmacologically-induced ex copula ejaculation in stallions. Animal Reproduction Science 68: 153-159.
[2] Calvero TMS, Papa FO, Schmith RAM, Scheeren VFC, Canuto LEF, Gobato MLM, Rodrigues LT, Freitas-Dell’aqua CP. 2019. Protocols using detomidine and oxytocin induce ex copula ejaculation in stallions. Theriogenology 140: 93-98.
[3] Card CE, Manning ST, Bowman P, Leibel T. 1997. Pregnancies from imipramine and xylazine-induced ex copula ejaculation in a disabled stallion. Canadian Veterinary Journal 38(3): 171-174.
27
[4] Zambelli D, Prait F, Cunto M, Iacono E, Merlo B. 2008/ Quality and in vitro fertilizing ability of cryopreserved cat spermatozoa obtained by urethral catheterization after medetomidine administration. Theriogenology 69(4): 485-490.
[5] Kuczmarski AH, Alves de Barros M, Souza de Lima LF, Motheo TF, Bento HJ, Iglesias GA, Sonego DA, Rodrigues da Paz RC. 2020. Urethral catheterization after pharmacological induction for semen collection in dog. Theriogenology 153: 34-38.
[6] Silinski S, Walzer C, Schwarzenberger F, Slotta-Bachmayr L, Stolla R. 2002. Pharmacological methods of enhancing penile erection for ex-copula semen collection in standing white rhinoceros (Ceratotherium simum simum). European Association of Zoo and Wildlife Veterinarians (EAZWV) 4th Scientific Meeting
[7] Hafez ES. 2013. Anatomy of male reproduction. In: Hafez B, Hafez ESE, editors. Reproduction in Farm Animals Maryland. Lippincot Williams & Wilkins; 3-11.
[8] MacDonald A, McGrath JC. 1980. The distribution of adrenoceptors and other drug receptors between the two ends of the rat vas deferens as revealed by selective agonists and antagonists. British Journal of Pharmacology 71:696-701.
[9] Swanson WF, Bateman HL, Vansandt LM. 2017 Urethral catheterization and sperm vitrification for simplified semen banking in felids. Reproduction in Domestic Animals 52(Suppl 2): 255-260.
[10] Kheirkhah MS, Mollapour sisakht M, Mohammadsadegh M, Moslemi HR. 2017. Sperm evaluation of Jungle Cat (Felis chaus) obtained by urethral catheterization (CT) after medetomidine administration Theriogenology 91: 17-20.
[11] Jeong DH, Kim JH, Na KJ. 2018. Characterization and cryopreservation of Amur leopard cats (Prionailarus bengalensis euptilurus) semen collected by urethral catheterization. Theriogenology 119: 91-5.
[12] Lueders I, Luther I, Scheepers G, van der Horst G. 2012. Improved semen collection method for wild felids: urethral catheterization yields high sperm quality in African lions (Panthera leo). Theriogenology 78(3): 696-701.
[13] Stoops MA, Bond JB, Bateman HL, Campbell MK, Levens GP, Bowsher TR, Ferrell ST, Swanson WF. 2007. Comparison of different sperm cryopreservation procedures on post-thaw quality and heterologous in vitro fertilization success in the ocelot (Leopardus pardalis). Reproduction, Fertility and Development 19: 685-694.
[14] Swanson WF, Johnson WE, Cambre RC, Citino SB, Quigley KB, Brousset DM, Morais RN, Moreira N, O’Brien SJ, Wildt DE. 2003. Reproductive Status of Endemic Felid Species in Latin American Zoos and Implications for Ex Situ Conservation. Zoo Biology 22: 421-441.
[15] Vansandt LM, Bateman HL, Miller AG, Herrick JR, Moresco A, Gonzalez R, Iwaniuk ME, Swanson WF. 2021 Cross-species efficacy of a chemically defined, soy lecithin-based cryomedium for semen banking in imperiled wild felids. Theriogenology 159: 108-115.
[16] Swanson WF, Vansandt LM, Citino S, Larsen RS, Cole GA, Moresco A. 2017. Addressing the Challenge of Do-It-Yourself (DIY) Semen Banking in Wild Felids. Abstract in 2017 49th AAZV Annual Conference Proceedings
[17] Santymire RM, Lonsdorf EV, Lynch SM, Wildt DE, Marinari PE, Kreeger JS, Howard JG. 2019. Inbreeding causes decreased seminal quality affecting pregnancy and litter size in the endangered black-footed ferret. Animal Conservation 22: 331-340. © 2018
The Zoological Society of London.
[18] Howard JG, Lynch C, Santymire RM, Marinari PE, Wildt DE. 2016. Recovery of gene diversity using long-term cryopreserved spermatozoa and artificial insemination in the endangered black-footed ferret. Animal Conservation 19: 102-111.
[19] Marinari P. 2017. North American regional black-footed ferret studbook. Front Royal: Smithsonian National Zoological Park and Conservation Biology Institute.
[20] Herrick JR, Bond JB, Magarey GM, Bateman HL, Krisher RL, Dunford SA, Swanson WF. 2007. Development of a feline optimized culture medium: effects of ions, carbohydrates, essential amino acids, vitamins and serum on the development and metabolism of IVF-derived feline embryos relative to embryos grown in vivo Biology of Reproduction 76: 858-870.
[21] Howard JG, Bush M, Wildt DE. 1986. Semen collection, analysis and cryopreservation in nondomestic mammals. In: Morrow D, editor. Current therapy in theriogenology Philadelphia, PA: WB Saunders Co. 1047-53.
[22] Swanson WF, Howard JG, Roth TL, Brown JL, Alvarado T, Burton M, Starnes D, Wildt DE. 1996a. Responsiveness of ovaries to exogenous gonadotrophins and laparoscopic artificial insemination with frozen
thawed spermatozoa in ocelots (Felis pardalis). Journal of Reproduction and Fertility 106(1): 87-94.
[23] Swanson WF, Roth TL, Blumer E, Citino SB, Kenny D, Wildt DE. 1996b. Comparative cryopreservation and functionality of spermatozoa from the normospermic jaguar (Panthera onca) and teratospermic cheetah (Acinonyx jubatus). Theriogenology 45(1): 241.
[24] Platz CC, Wildt DE, Seager SW. 1978. Pregnancy in the domestic cat after artificial insemination with previously frozen spermatozoa. Journal of Reproduction and Fertility 52(2): 279-282.
[25] Donoghue AM, Johnston LA, Seal US, Armstrong DL, Simmons LG, Gross T, Tilson RL, Wildt DE. 1992. Ability of thawed tiger (Panthera tigris) spermatozoa to fertilize conspecific eggs and bind and penetrate domestic cat eggs in vitro. Journal of Reproduction and Fertility 96: 555-564.
[26] Zambelli D, Caneppele B, Castagnetti C, Belluzzi S. 2002. Cryopreservation of Cat Semen in Straws: Comparison of Five Different Freezing Rates Reproduction in Domestic Animals 37(5): 310-313.
[27] Hay MA, Goodrowe K. 1993. Comparative cryopreservation and capacitation of spermatozoa from epididymides and vasa deferentia of the domestic cat. Journal of Reproduction and Fertility 47: 297-305.
[28] Byers AP, Hunter AG, Seal US, Binczik GA, Graham EF, Reindl NJ, Tilson RL. 1989. In-vitro induction of capacitation of fresh and frozen spermatozoa of the Siberian tiger (Panthera tigris). Journal of Reproduction and Fertility 86(2): 599-607.
28
–
[29] Thiangtum K, Swanson WF, Howard J, Tunwattana W, Thongthainan D, Wichasilpa W, Patumrattanathan P, Pinyopoommintr T. 2006. Assessment of basic seminal characteristics, sperm cryopreservation and heterologous in vitro fertilisation in the fishing cat (Prionailurus viverrinus). Reproduction, Fertility, and Development 18(3): 373-82.
[30] Rosato MP, Iaffaldano N. 2013. Cryopreservation of rabbit semen: Comparing the effects of different cryoprotectants, cryoprotectant-free vitrification, and the use of albumin plus osmoprotectants on sperm survival and fertility after standard vapor freezing and vitrification. Theriogenology 79(3): 508-516.
[31] Isachenko E, Isachenko V, Weiss JM, Kreienberg R, Katkov II, Schulz M, Lulat AG-MI, Ridopatron MJ, Sanchez R. 2008. Acrosomal status and mitochondrial activity of human spermatozoa vitrified with sucrose. Reproduction (Cambridge, England) 136(2): 167.
[32] Conforti VA, Bateman HL, Schook MM, Newsom J, Lyons LA, Grahn RA, Deddens JA, Swanson WF. 2013. Laparoscopic oviductal artificial insemination improves pregnancy success in exogenous gonadotropin-treated domestic cats as a model for endangered felids. Biology of Reproduction 89: 1-9.
[33] Swanson WF. 2012. Laparoscopic Oviductal Embryo Transfer and Artificial Insemination in Felids – Challenges, Strategies and Successes. Reproduction in Domestic Animals 47(S6): 136-140.
[34] Swanson WF, Newsom J, Lyons LA, Grahn RA, Bateman HL. 2014. Ovarian Down-Regulation with Oral Progestin for FixedTime Laparoscopic Oviductal Artificial Insemination with Freshly-Collected and Frozen-Thawed Spermatozoa in Domestic Cats. Reproduction, Fertility, and Development 26(1): 143.
[35] Crosier AE, Pukazhenthi BS, Henghali JN, Howard J, Dickman AJ, Marker L, Wildt DE. 2006. Cryopreservation of spermatozoa from wild-born Namibian cheetahs (Acinonyx jubatus) and influence of glycerol on cryosurvival. Cryobiology 52: 169-181.
[36] Griffith B, Scott JM, Carpenter JW, Reed C. 1989. Translocation as a species conservation tool: status and strategy. Science 245: 477-480.
[37] Wilson SM. 2018. Lessons Learned from Past Reintroduction and Translocation Efforts with an Emphasis on Carnivores. Action
A.4: Elaboration of plans for reinforcement of the Dinaric – SE Alpine population and for creation of new “stepping stone” population – Guidelines for Lynx Reinforcement in Preventing the extinction of the Dinaric-SE Alpine lynx population through reinforcement and long-tern conservations. p 1-43.
[38] Wolf CM, Griffith B, Reed C, Temple S. 1996. Avian and mammalian translocations: update and reanalysis of 1987 survey data. Conservation Biology 10: 1142–1154.
[39] Fischer J, Lindenmayer DB. 2000. An assessment of the published results of animal relocations. Biological Conservation 96: 111.
[40] Jule KR, Leaver LA, Lea SEG. 2008. The effects of captive experience in reintroduction survival in carnivores: a review and analysis. Biological Conservation 141: 355-363.
[41] Beldon RC, McCown JW. 1996). Florida panther reintroduction feasibility study. Final Report, Study no. 7507. Tallahassee, FL: Florida Game and Fresh Water Fish Commission.
[42] IUCN/SSC. 2013. Guidelines for Reintroductions and other Conservation Translocations. Version 1.0, Gland, Switzerland: IUCN Species Survival Commission, viii + 57pp.
[43] Macdonald DW. 2009. Lessons Learnt and Plans Laid: Seven Awkward Questions for the Future of Reintroductions. Pgs. 411448. In M.W. Hayward and M. Somers, editors. Reintroduction of Top-Order Predators. John Wiley & Sons, Incorporated. Hoboken, USA.
[44] Mellen JD. 1993. A comparative analysis of scent-marking, social and reproductive behavior in 20 species of small cats (Felis). American Zoologist 33, 151–166.
[45] Howard JG, Wildt DE. 2009. Approaches and efficacy of artificial insemination in felids and mustelids. Theriogenology 71:130148.
[46] Lambo CA, Grahn RZ, Lyons LA, Bateman HL, Newsom J, Swanson WF. 2012. Comparative fertility of freshly collected vs frozen-thawed semen with laparoscopic oviductal artificial insemination in domestic cats. Reproduction in Domestic Animals 47: 284288.
[47] Conforti VA, Bateman HL, Vick MM, Lyons LA, Grahn RA, Deddens JW, Swanson WF. 2011. Improved fertilization success using laparoscopic oviductal artificial insemination with low sperm numbers in domestic cats. Proceedings of the Society for the Study of Reproduction 40 [abstract no. 173).
[48] Swanson WF, Wolf BA, Brown JL, Martin-Jiminez T, Riviere JE, Roth TL, Wildt DE. 1997. Pharmokinetics and ovarian stimulatory effects of equine and human chorionic gonadotropins administered singly and in combinations in the domestic cat. Biology of Reproduction 57: 295-302.
[49] Graham LH, Swanson WF, Birchard GF, Brown JL. 2000. Chorionic gonadotropin administration in domestic cats causes an abnormal endocrine environment which disrupts oviductal embryo transport. Theriogenology 54: 1117-1131.
[50] Magarey GM, Bond JB, Herrick JR, Stoops MA, Swanson WF. 2005. Improved recipient synchronization protocol for embryo transfer in the domestic cat (Felis silvestris catus) using equine chorionic gonadotropin (eCG) and porcine luteinizing hormone (pLH). Proceedings of the 38th Society for the Study of Reproduction 91 [Abstract no. 48].
[51] Swanson WF. 2019. Practical application of laparoscopic oviductal artificial insemination for the propagation of domestic cats and wild felids. Reproduction, Fertility and Development 31: 27-39.
29
[52] Howard JG, Barone MA, Donoghue AM, Wildt DE. 1992.The effect of preovulatory anesthesia on ovulation in laparoscopically inseminated domestic cats. Journal of Reproduction and Fertility 96: 175-186.
[53] Tsutsui T, Tanaka A, Takagi K, Nakagawa K, Fujimoto Y, Murai M, Anzai M, Hori T. 2000. Unilateral intrauterine horn insemination of fresh semen in cats. Journal of Veterinary Medical Science 62: 1241-1245.
[54] Tsutsui T, Tanaka A, Hori T. 2001. Intratubal insemination with fresh semen in cats. Journal of Reproduction and Fertility Supplement 57: 347-351. PMID: 11787173.
[55] Toyonaga M, Sato Y, Sasaki A, Kaihara A, Tsutsui T. 2011. Artificial insemination with cryopreserved sperm from feline epididymides at 4℃. Theriogenology 76: 532-537.
[56] Zambelli D, Cunto M. 2005. Transcervical artificial insemination in the cat. Theriogenology 64: 698-705.
[57] Chatdarong K, Axner E, Manee-In S, Thuwanut P, Linde-Forsberg C. 2007). Pregnancy in the domestic cat after vaginal or transcervical insemination with fresh and froze semen. Theriogenology 68: 1326-1333.
[58] Chatdarong K, Lohachit C, Linde-Forsberg C. 2004. Distribution of spermatozoa in the female reproductive tract of the domestic cat in relation to ovulation induced by natural mating. Theriogenology 62: 1027-1041.
[59] Coy P, Garcia-Vazquez FA, Viaconti PE, Aviles M. 2012. Roles of the oviduct in mammalian fertilization. Reproduction 144: 64-660.
[60] Swanson WF, Howard JG, Roth TL, Brown JL, Alvarado T, Burton M, Starnes D, Wildt DE. 1996. Responsiveness of ovaries to exogenous gonadotropins and laparoscopic artificial insemination with frozen-thawed spermatozoa in ocelots (Felis pardalis). J. Reproduction and Fertility 106 :87-94.
[61] Lambo C, Conforti V, Goff D, Gyimesi Z, Campbell M, Lewandowski A, Limoges MJ, Bateman H, Swanson WF. 2011. Improving pregnancy success with laparoscopic artificial insemination in Brazilian ocelots (Leopardus pardalis mitis). Proceedings of the American Association of Zoo Veterinarians 76-78.
[62] Pope CE, Gomez MC, Dresser BL. 2006. In vitro production and transfer of cat embryos in the 21st century. Theriogenology 66: 59–71.
[63] Swanson WF. 2006. Application of assisted reproduction for population management in felids: the potential and reality for conservation of small cats. Theriogenology 66: 49–58.
[64] Wildt DE, Kinney GM, Seager SWJ. 1977. Laparoscopy for direct observation of internal organs in the domestic cat and dog. American Journal of Veterinary Research 38: 1429–1432.
[65] Goodrowe KL, Wall RJ, O’Brien SJ, Schmidt PM, Wildt DE. 1988. Developmental competence of domestic cat oocytes after fertilization in vitro. Biology of Reproduction 39: 355–372.
[66] Swanson WF, Bond JB, Steinetz B, McRae MA. 2001. Fetal and neonatal development of domestic cats produced from in vitro fertilization and laparoscopic oviductal embryo transfer versus natural mating. Theriogenology 55: 371.
[67] Pope CE, Gómez MC, Dresser BL. 2006a. In vitro embryo production in domestic and nondomestic cats. In Special issue of Theriogenology: Proceedings of the Fifth International Symposium of Canine and Feline Reproduction, v.66, 1518-1524
[68] Pope CE, Gómez MC, Galiguis J, Dresser BL. 2012. Applying embryo cryopreservation technologies to the production of domestic and black-footed cats. Reproduction in Domestic Animals v.47(Suppl. 6), 125-129
[69] Pope CE. 2019. Forty years of assisted reproduction research in non-domestic, wild and endangered mammals. Anais do XXIII Congresso Brasileiro de Reprodução Animal (CBRA-2019); Gramado, RS, 15 a 17 de maio de 2019.
[70] Herrick JR, Campbell M, Levens G, Moore T, Benson K, D’Agostino J, West G, Okeson DM, Coke R, Portacio SC, Leiske K, Kreider C, Polumbo PJ, Swanson WF. 2010/ In vitro fertilization and sperm cryopreservation in the black-footed cat (Felis nigripes) and sand cat (Felis margarita). Biology of Reproduction 82: 552-562.
[71] Howard JG. 1998. Assisted reproductive techniques in nondomestic carnivores. In: Fowler ME, Miller RE (eds.) Zoo and Wild Animal Medicine, IV. Philadelphia: WB Saunders; 449-457.
[72] Pope CE, Gómez MC, Cole A, Dumas C, Dresser BL. 2006b. Oocyte recovery, in vitro fertilization and embryo transfer in the serval (Leptailurus serval). Reproduction, Fertility, and Development, v.18, p.223 (Abstract).
[73] Pope CE, Gómez MC, Dresser BL. 2006c In vitro production and transfer of cat embryos in the twenty-first century. In: Special Issue of Theriogenology: Feline Reproduction, v.66, p.72-81.
[74] Pope CE. 2004. In vitro fertilization and embryo transfer in felids. In: Schatten H, editor. Methods in molecular biology germ cell protocols molecular embryo analysis live imaging transgenesis and cloning, vol. 2. Totowa, NJ: The Humana Press, Inc.; 227
[75] Gomez MC, Pope CE, Harris R, Mikota S, Dresser BL. 2003. Development of in vitro matured, in vitro fertilized domestic cat embryos following cryopreservation, culture and transfer. Theriogenology 60: 239–51.
[76] Herrick JR, Bond JB, Campbell M, Levens G, Moore T, Benson K, D’Agostino J, West G, Okeson DM, Coke R, Portacio SC, Leiske K, Kreider C, Polumbo PJ, Swanson WF. 2010. Fecal endocrine profiles and ejaculate traits in black-footed cats (Felis nigripes) and sand cats (Felis margarita). General and Comparative Endocrinology 165: 204-214.
44.
[77] Bateman HL, Bond J, Campbell M, Barrie M, Riggs G, Snyder B, Swanson W 2009. Characterization of basal seminal traits and reproductive endocrine profiles in North American river otters and Asian small-clawed otters. Zoo Biology 28: 107-126.
30
–
General Health Monitoring, Preventive Medicine, Pathogen Surveillance, and Quarantine
All general health monitoring data captured in ocelots at the Ocelot Conservation Facility and in free-ranging ocelots in the wild in Texas will be used to inform necessary veterinary care for ocelots held at the Ocelot Conservation Facility and reintroduced to the wild. Health monitoring will also be required for ocelots entering the Ocelot Conservation Facility’s quarantine facility to assure that they do not introduce health concerns to ocelots already held at the Ocelot Conservation Facility. Finally, all ocelots considered for release to the wild should have a health assessment and be tested for pathogens. Results will be used to determine if an ocelot is

31
Photo courtesy Caesar Kleberg Wildlife Research Institute
suitable for release, and to provide baseline health information should the individual be captured in the wild in the future and reevaluated.
General Health Monitoring and Preventative Medicine
Visual examinations of ocelots will be performed periodically in individual ocelots present in the Ocelot Conservation Facility. Meanwhile, physical examinations of adult ocelots in the Ocelot Conservation Facility will take place under full anesthesia periodically (Tables 1-2), and in all cases prior to an ocelot’s transfer to a reintroduction site. Additionally, ocelots captured in the wild for monitoring of wild populations will undergo similar general health monitoring. Physical exams will include blood draws for health and pathogen testing, and assessment of body condition, weight, and specific body systems described below. General health monitoring may occur during regularly occurring exams or when otherwise warranted, such as if an individual is ill.
Tables 1 and 2 provide general recommendations for health monitoring and preventative medicine procedures and their frequency of performance based on information provided by Felid Taxon Advisory Group (TAG) veterinarians and other zoo veterinarians working at Association of Zoos and Aquariums (AZA) institutions that currently care for ocelots. Table 1 is for “individuals that will not be released” and Table 2 for “individuals that will be considered for release.”
32
Table 1. For ocelots to be human-managed and not considered for release, recommendations for general health monitoring and preventative medicine.
Physical Examination
Neonate 8, 12, 16 weeks
Adult Q2-3years or when warranted
Vaccination
FVRCP Neonate 8, 12, 16 weeks
Adult 1 annual; then Q3 years
RABIES Neonate 16 weeks
Adult 1 annual; then Q3 years
FeLV Neonate 12 and 16 weeks
Adult Annually if using recombinant; Q3 if using killed
Fecal Exam
Intestinal Parasite Screening Q6 months
Hormone Testing When warranted
Ectoparasites During examination
Urinalysis
CBC/CHEM/Bank Samples
Disease Testing
Dental Prophy
Ultrasound Exam (M/F)
Heartworm Antigen Testing
Q2-3Years or when warranted
Neonate exam; Annual for 3 years; then Q2-3 years or when warranted
Situational
Q2-3 years
Annual/ when warranted
Annual
Ivermectin Monthly
Deworming
Radiographs
Q6 months/ when warranted
Q2-3 years
33
Table 2. For ocelots considered for release, recommendations for health monitoring and preventative medicine
Physical Examination
Neonate 8, 12, 16 weeks
Adult Annual until release
Vaccination
FVRCP Neonate 8, 12, 16 weeks
Adult Annual until release
RABIES Neonate 16 weeks
Adult Annual until release
FeLV Neonate 12 and 16 weeks
Adult Annual booster if warranted before release; recommend killed vaccine due to longevity of rebooster
Fecal Exam
Intestinal Parasite Screening Q6 months
Hormone Testing When warranted
Ectoparasites During examination
Urinalysis
CBC/CHEM/Bank Samples
Disease Testing
Dental Prophy
Reproductive Ultrasound Exam (M/F)
Heartworm Antigen Testing
Annual until release/ when warranted
Neonate exam/ Annual until release/ when warranted
30 days prior to release/ when warranted
Never unless warranted
Annual until release/ when warranted
Annual until release
Ivermectin Monthly until release (tentative)
Deworming
Radiographs
Preventative Medicine
Q6 months until release/ when warranted
At least once prior to release
Vaccinations of zoo-managed non-domestic felids are based on both recommendations made by the American Association of Feline Practitioners (AAFP) and on the specific risks to non-domestic species. These vaccinations are divided into core (recommended for all felids) and non-core (optional vaccinations where use
34
is dependent on risk factors, Table 3). In domestic cats, studies have shown protection for 3 years with certain vaccines: killed rabies and killed and modified live combination (MLV) vaccines (panleukopenia, calicivirus, herpesvirus) [236-237]. However, specific information relating to vaccines for non-domestic species is generally lacking, and therefore, most AZA institutions recommend a frequency of vaccination every 1-3 years for core vaccines [238]. There have been several cases of disease in non-domestic species after the administration of MLV vaccines, such as feline herpesvirus (FHV), feline calicivirus (FCV), and canine distemper virus (CDV), As such, MLV vaccines are not recommended in cubs or kittens [239]. It is recommended to always record the site of injection for each vaccine to document any reactions that may occur.
Table 3: Core and non-core vaccines for felines as recommended by the American Association of Feline Practitioners.
Vaccines Vaccine Type
Core
Rabies Killed (Imrab 3®, Merial); recombinant canarypox-vectored (PureVax Rabies®, Merial)
Feline panleukopenia, calicivirus, herpesvirus (FVRCP) Killed (Fel-O-Vax®, Elanco)
Non-Core
Canine distemper virus (CDV)
Recombinant canarypox-vectored (PureVax Ferret Distemper®, Merial); MODIFIED LIVE NOT RECOMMENDED
FeLV Killed; Recombinant (PureVax®, Merial)
General Anesthesia
General anesthesia for physical exams or other veterinary procedures will consist of medication combinations used frequently in feline species and specifically designed for ocelots (Appendix 5). Current protocols include a combination of ketamine (200 mg/mL), medetomidine (10 mg/ mL), midazolam (5 mg/ mL), and/or butorphanol (10 mg/ mL). Many combinations of these medications have been utilized successfully in ocelots at varying dosages, depending on individual physiology and veterinarian preference. Other anesthetic medications within similar drug classes may also be utilized at the veterinarian’s discretion, and anesthetic medications are not limited to the above medication list. Medication use will be identified by the attending veterinarian and may be informed by resources provided by the zoo community or other researchers. Resources may include but are not limited to, unpublished zoo records housed in zoological institutions, published anesthetic regimes, and direct conversation with other researchers or managers of captive ocelots
Physical examinations
Physical examinations of ocelots at the Ocelot Conservation Facility will be performed under general anesthesia assessing the following body systems/parameters: general appearance, respiratory system, eyes/ears/nose/throat, peripheral lymph nodes, nervous, musculoskeletal, and body condition score (Figure 1). Additional items to assess include:
Gastrointestinal: Fecal samples assessed for intestinal parasites and fecal consistency
35
Genitourinary: For males, palpation of the testes for tumors or abnormalities, collection of sperm sample for assessment of seminal parameters, assessment of penis for spine development and abnormalities (persistent frenulum, etc.). For females, vulvar and mammary examinations for tumors, assessment of discharge/discoloration and lactation status
Cardiac: Echocardiogram (ECG) for arrhythmias. Indirect blood pressure should be measured with a cuff, sized at 30-40% of the limb circumference, and placed either on the forelimb (below or above the elbow), hind limb (above the hock), or at the base of the tail. Hypotension will be defined as a systolic arterial pressure (SAP) of less than 90 mmHg, mean arterial pressure (MAP) less than 70 mmHg and/or diastolic arterial pressure (DAP) less than 50 mmHg [17-19].
Dentition: Tooth wear, discoloration, fractures, missing teeth; aging [20]. Dental radiographs should be taken at sedation events when possible. (Table 4)
Integument: Skin scraping, or hair culture as indicated by skin lesions or patterns.
36
Figure 1. Feline Body Condition Guidelines by the Felid Taxon Advisory Group.
Dentition/Aging
https://link.springer.com/article/10.1007/s10344-018-1198-6
http://www.toothvet.ca/PDFfiles/Cat_Chart.pdf
http://www.toothvet.ca/PDFfiles/Cat_Deciduous_Chart.pdf
Hematology Profile
A complete blood count (CBC) evaluates the cells that circulate within the blood, including red blood cells (RBCs), white blood cells (WBCs), and platelets [1]. The results describe the number, size, and shape of each cell type [1]. Elevated white blood cell counts indicate the presence of inflammation or infection, while a decrease in red blood cells and platelets can indicate the presence of anemia or decreased clotting abilities [2]. A blood chemistry profile evaluates internal organ function and electrolyte status and can inform about disease processes affecting the internal organs of that individual [3-4]. In combination, these two analyses can provide information regarding the health of an individual and a population.
Minimal baseline hematology (Table 5) and biochemical parameters (Table 7) exist for ocelots currently, with one set originating from free-ranging ocelots in Brazil [5] and another from zoo-managed ocelots included in the Species360 online database [6]. Baseline hematology (Table 6) and biochemical parameters (Table 8) have also been collected for free-ranging ocelot populations in Texas [A. Reeves, East Foundation, unpublished data, n=24 ocelots]. Blood samples should continue to be collected in free-ranging ocelots and captive-held ocelots in the Ocelot Conservation Facility to establish a reliable health monitoring system and to assess the health of captive-held ocelots. Blood parameters may vary between free-ranging and captive populations [7-11], between sex [8, 12-14], and age of the individual at time of collection [7-8, 13-16].
Sample Type and Collection Method
Whole blood (2-3 mL) will be collected into an Ethylenediaminetetraacetic acid (EDTA) blood tube (typically with a lavender top). For best results, this sample should either be tested the same day of collection or kept on ice/placed into the refrigerator until testing is possible. Four blood smears will be created per individual using the EDTA whole blood, including one for differential white blood cell count and platelet estimation, one for disease testing, and two for sample archive or other use. See Table 9.
Sample Testing
Complete Blood Count analysis will be either tested in laboratory space at the Ocelot Conservation Facility using the IDEXX Procyte® Hematology Analyzer (IDEXX Laboratories, Inc., Westbrook, Maine USA) using whole blood in EDTA or will be sent to a testing laboratory at the discretion of the veterinarian.
If white blood cell differential counts are performed at the Ocelot Conservation Facility, the following procedures will take place (http://www.vspn.org/Library/Misc/VSPN_M02362.html):
1) A single blood smear will be stained with Diff-Quik Stain (Jorgensen Laboratories, Inc. Loveland, CO, USA) and a differential white blood cell count will be performed under 1000X oil immersion using a biological compound light microscope. One hundred white blood cells (neutrophils, lymphocytes,
37
Parameter PDF Reference
Table 4 Dentition and aging information for ocelots.
macrophages, eosinophils, basophils) will be counted and a percentage of 100 will be reported. Additionally, abnormal cells and/or pathogen inclusion bodies will be noted.
2) Platelet counts will be performed under 1000X oil immersion using a biological compound light microscope. The smears will be assessed for platelet number by taking the average number of platelets in 5 microscopic fields and multiplying them by 15,000 and 20,000 for an estimated platelet range (http://www.vspn.org/Library/Misc/VSPN_M02362.htm ).
Table 5 Available hematology parameters of free-ranging ocelots, captive ocelots, and domestic felines [5-6, 21].
MCV=mean cell volume; MCH=mean cell hemoglobin; MCHC=mean cell hemoglobin concentration
* Student’s t-test: statistically significant differences (P<0.05) between means for wild and captive ocelots.
38
Free-Ranging Ocelots Captive Ocelots Domestic cat Parameter Units Mean (median) ±SD of the mean 95% CI of the mean Range n Mean ±SD Range n Range Red blood cells* 109/L 6.4 (6.5)±0.5 5.6-6.9 5.5-7.1 7 7.37±1.12 5.10-10.8 107 5.0-10.0 Hemoglobin g/L 114.3 (117)±11.5 103.6-124.9 95-131 7 125±17 94-171 114 980-154 Hematocrit L/L 0.35 (0.35)±0.03 0.32-0.37 0.30-0.40 8 0.38±0.05 0.27-0.53 136 0.30-0.45 MCV fl 52.3 (52.9)±6.6 46.8-57.8 42.25-60.0 8 51.2±4.3 42.9-62.8 106 39-55 MCH* pg/ce ll 18.4 (18.5)±3.0 15.9-20.9 13.4-22.4 8 17.0±1.7 12.7-21.8 105 13-17 MCHC g/L 333.1 (327)±19.9 314.6-351.5 311.8363.9 7 332±23 238-396 113 300-360 White blood cells* 109/L 17.7 (18.6)±2.6 15.5-19.9 12.1-19.8 8 10.16±3.46 4.62-23.30 134 5.5-19.5 Bands* 109/L 0.34 (0.36)±0.15 0.21-0.48 0.18-0.57 7 0.19±0.13 0.00-0.50 21 0.0-0.3 Neutrophils* 109/L 12.0 (12.4)±2.9 9.5-14.4 7.4-15.9 8 6.96±2.86 0.105-20.7 121 2.5-12.5 Lymphocytes* 109/L 4.3 (3.6)±2.4 2.3-6.3 1.5-8.7 8 2.48±1.57 0.46-7.61 126 1.5-7.0 Eosinophils 109/L 0.1 (0.0)±0.31 -0.13-0.34 0.0-0.9 7 0.42±0.48 0.00-3.63 100 0.0-0.8 Basophils* 109/L 0.0 (0.0)±0.0 0.0 0.0 8 0.16±0.13 0.00-0.37 7 0.0-0.2 Monocytes* 109/L 0.9 (0.87)±0.35 0.6-1.2 0.5-1.6 8 0.28±0.30 0.05-2.62 102 0.0-0.9 Platelet count* 109/L 396.7 (336)±147.1 273.7-519.7 280-694 8 293.0±105. 0 88.0-581.0 38 300-800
Table 6 Available hematology parameters for free-ranging ocelots in Texas) [A. Reeves, East Foundation, unpublished data, n= 24 ocelots]
Free-Ranging Texas Ocelots
RI, reference interval; LRL, lower reference limit; URL, upper reference limit; ALT; RBC= red blood cell; Conc.= concentration; MCV=mean cell volume; MCH=mean cell hemoglobin; MCHC=mean cell hemoglobin concentration; WBC= white blood cell.
*90% confidence interval around the 95% LRL and URL are shown these columns
39
Parameter Units LRL of RI* URL of RI* Range n RBC conc. 106/µL 5.4-6.5 8.5-9.7 5.9-9.2 23 Hemoglobin g/dL 9.8-11.0 13.4-15.5 10.3-13.9 20 Hematocrit % 11.2-29.7 50.0-68.1 20.2-59.3 24 MCV fl 35.0-42.6 61.5-69.8 38.9-65.7 24 MCH pg 13.7-14.6 17.2-18.2 14.1-17.7 24 MCHC g/dL 21.7-25.7 35.7-39.4 23.4-37.6 24 WBC conc. 103/µL 6.8-10.2 18.3-23.3 8.1-20.8 21 Neutrophils 103/µL 3.2-6.9 17.6-22.5 4.9-20.3 24 Lymphocyte s 103/µL 0-0.87 2.1-3.6 0.11-2.9 24 Eosinophils 103/µL 0-0 0.1-0.8 NA Basophils 103/µL 0-0 0.28-1.6 0-0.95 24 Monocytes 103/µL 0-0.05 0.34-0.47 0-0.41 24 Platelet conc. 103/µL 157.9279.4 527.3629.5 219.3-581.9 21
Sample Type and Collection Method
Whole blood (4-6 mL minimum) will be collected into a plain red top serum tube or a serum separator tube. The serum tube will be allowed to clot for a minimum of 20 minutes and centrifuged for 10 minutes at ≥ 2000g and the serum removed for serum chemistry analysis. Serum will be maintained in a refrigerator until analysis can be performed on the day of collection or stored in the refrigerator for a maximum of 7 days prior to shipping to a partner laboratory for completion. This sample should not be frozen prior to biochemical profile completion.
Sample Testing
Chemistry analysis may be conducted on-site using the IDEXX Catalyst One® Chemistry Analyzer and a Chem 17 CLIP with electrolytes (IDEXX Laboratories, Inc., Westbrook, Maine USA) or may be sent to a testing laboratory at the discretion of the veterinarian. The CHEM 17 clip contains albumin (ALB), albumin/globulin ratio (ALB/GLOB), globulins (GLOB), alkaline phosphatase (ALKP), alanine transaminase (ALT), amylase (AMYL), cholesterol (CHOL), glucose (GLU), blood urea nitrogen (BUN), creatinine (CREA), blood urea nitrogen/ creatinine ratio (BUN/CREA), lipase (LIPA), gamma-glutamyl transferase (GGT), phosphate (PHOS), total bilirubin (TBIL), calcium (Ca), and total protein (TP) (IDEXX Laboratories, Inc., Westbrook, Maine USA).
The electrolyte clip contains chloride (Cl), potassium (K), sodium (Na), and sodium/potassium ratio (Na/K) (IDEXX Laboratories, Inc., Westbrook, Maine USA).
40
Biochemical Profile
Table 7 Available biochemical parameters of free-ranging ocelots, captive ocelots, and domestic felines [5-6, 22]
ALKP= alkaline phosphatase; ALT=alanine aminotransferase; GGT=gamma-glutamyl transferase; BUN= blood urea nitrogen; TBIL= total bilirubin
* Student’s t-test: statistically significant differences (P<0.05) between means for wild and captive ocelots.
41
Free-Ranging Ocelots Captive Ocelots Domestic cat Parameter Units Mean (median) ±SD of the mean 95% CI of the mean Range n Mean ±SD Range n Range Albumin g/L 25.0 (24.5)±2.16 23.2-26.8 22.1-28.2 8 33±4 22-46 108 28-39 Globulin g/L 66.5 (67.5)±12.9 55.7-77.3 50.0-90.9 8 41±7 24-67 107 26-51 Albumin/globulin ratio - 0.4 (0.42)±0.07 0.32-0.45 0.24-0.48 8 - - -ALKP IU/L 27.4 (28.5)±6.8 21.7-33.0 13.0-35.0 8 39±44 4-243 128 0-45 ALT IU/L 65 (60.5)±28.8 40.9-89.19 33.0-117.0 8 66±41 19-269 129 25-97 Phosphate mmol/L 1.3 (1.2)±0.55 0.9-1.8 0.81-2.36 8 1.52±0.42 0.74-2.71 103 1.0-2.0 Amylase U/L - - - 550-1,458 Cholesterol mmol/L 4.5 (4.6)±0.84 3.8-5.2 3.4-6.1 8 8.21±2.28 3.39-14.54 132 1.8-4.0 Glucose mmol/L - - - 3.3-6.7 BUN mmol/L 31.7 (32.3)±4.4 28.0-35.4 24.6-37.3 8 11.42±3.2 1 5.35-22.85 131 6.8-12.1 Creatinine µmol/L 93.9 (88.4)±17.6 79.2-108.7 61.9-114.9 8 159±44 71-283 128 80-194 BUN/creatinine ratio - - - - - - - -Lipase - - - - - - - -GGT IU/L 5.13(5.0)±1.55 3..8-6.4 2-7 8 2±2 0-10 43 1.8-12 TBIL µmol/L 2.5(2.8)±0.92 1.6-3.5 1.2-3.4 6 5±3 0-15 119 0-1.7 Calcium mmol/L 2.5(2.4)±0.37 2.2-2.8 2.1-3.2 8 2.5±0.18 2.10-3.00 128 2.2-2.9 Total Protein g/L 91.5(94.4)±12.9 80.7-102.4 74.0-113.0 8 74±7 56-100 120 60-79 Chloride mmol/L 114.4(110)±7.3 107.6-121.2 107-126 7 120±6 107-137 110 115-130 Potassium mmol/L 4.8(5.0)±0.26 4.6-5.1 4.5-5.2 8 4.2±0.5 2.8-5.8 113 3.7-6.1 Sodium mmol/L 149.9(150)±2.1 148.1-151.6 147-154 8 153±5 139-166 116 146-156 Sodium/Potassium ratio - - - - - - - - -
Table 8 Available values for biochemical parameters of free-ranging ocelots in Texas [A. Reeves, East Foundation, unpublished data, n=24 ocelots]
Free-Ranging Texas Ocelots
RI, reference interval; LRL, lower reference limit; URL, upper reference limit; ALT, alanine transaminase. *90% confidence interval around the 95% LRL and URL are shown in these columns.
42
Parameter Units LRL of RI* URL of RI* Range n Albumin g/dL 2.4-2.6 3.2-3.4 2.5-3.3 24 Globulin g/dL 3.8-4.3 5.9-6.6 4.0-6.3 24 Albumin/globulin ratio - 0.34-0.43 0.70-0.78 0.39-0.74 24 ALT U/L 17.3-46.1 122.8-154.1 31.2-139.9 24 Glucose mg/dL 83.8-121.4 205.9-247.0 102.4-228.3 21 BUN mg/dL 8.7-18.4 40.3-51.7 13.4-46.8 24 Creatinine mg/dL 0.25-0.67 1.5-1.9 0.5-1.8 24 BUN/creatinine ratio - 0-8.69 43.6-63.4 0.69-53.7 24 Total Protein g/dL 6.5-7.1 8.9-9.6 6.9-9.2 24
Table 9. Recommended blood collection volume by ocelot weight (kg) and use of blood for testing based on weight. 1% of kg weight is the maximum volume of blood that may be collected from an animal, but collection of that volume rarely necessary. This upper limit will be most important for young kittens and small individuals.
Urine Diagnostics
The use of urinalysis in felids can help detect or characterize renal (kidney) disease; monitoring kidney disease once diagnosed; detecting diabetes and complications of undiagnosed or unregulated diabetes; or identifying signs of malnourishment, starvation, urinary tract disorder, or other pathologies [23-25]. Further examination of urine sediment after centrifugation can reveal crystals, renal casts, bacteria, and abnormal cells which can identify diet-related issues, urinary tract infections, kidney damage and certain urogenital and renal neoplasia (cancers) [23-24]. This information can be coupled with the complete blood count and biochemical analysis to characterize and/or add to information about organ function [24].
43
Weight in kg (lb) Max Blood Collected (mL) Actual Blood Collected (mL) Priority Testing Opportunistic Testing 0.25 (0.55) 2.5 1.25- 2.5 Genetics CBC, CHEM, Other Disease 0.5 (1.1) 5 2.5-5 Genetics, CBC, CHEM Other Disease 1 (2.2) 10 3-5 CBC, CHEM, Genetics, FeLV, FIV Other Disease 2 (4.4) 20 8-10 CBC, CHEM, Genetics, FeLV, FIV Other Disease 3 (6.6) 30 12-15 CBC, CHEM, Genetics, FeLV, FIV Other Disease 4 (8.8) 5 (11) 6 (13.2) 7 (15.4) 8 (17.6) 9 (19.8) 10 (22) 11 (24.2) 12 (26.4) 13 (28.6) 14 (30.8) 15 (33) 40 50 60 70 80 90 100 110 120 130 140 150 18-20 CBC, CHEM, Genetics, All Disease
Sample Collection Method
During anesthesia, urine of ocelots at the Ocelot Conservation Facility can be collected by cystocentesis. A 1.5inch, 22-gauge needle will be attached to a 3 to 6 mL syringe and passed through the abdominal wall into the bladder, where 3 to 6 mL of urine will be aspirated into the syringe. A second approach to collect urine is to place a pan in an area of consistent elimination and attempt to capture urine voluntarily.
Sample Testing (Table 10)
Testing of urine may be performed by sending samples to a partner lab or by performing the analysis at the Ocelot Conservation Facility. If sending a sample to another laboratory, collection in a non-additive, sterile container (white top tube) is preferred, though urine can also be collected and stored in a red top (non-additive) tube or urine sample cup. This sample should be shipped chilled on ice packs the same day it is collected or stored in the refrigerator to be shipped within 7 days for routine testing [10].
IDEXX UATM Strip Test: 1 drop of sample is placed onto each pad, left to develop for the specified duration for a particular pad, and then evaluated based on the strip test results provided on the package. The duration for each pad and evaluation results will differ for each company in which the strip tests are purchased. Follow package instructions that accompany each strip pad test.
Urine Specific Gravity (Refractometer): 1 drop is placed onto the window of the calibrated refractometer and the upper window closed to spread the drop. The refractometer is held toward a light and a blue line guides the concentration of the urine. There are multiple readings on the refractometer depending on the type purchased. Instructions will be provided as to which reading coincides with specific gravity.
Urine Sediment Examination [24]: The rest of the urine sample is centrifuged for 5-10 minutes at ~250g, the supernatant poured off, and the remaining pellet is mixed in the bottom of the container. A drop is placed onto a microscope slide (with or without urine sediment stain, dependent on personnel) and read on a compound light microscope using the 10X and 40X objectives. Number of cells, bacteria, and other abnormalities are recorded as described in resources provided in Table 10.
44
Table 10: Urinalysis Test Assessments and Supplemental Information [23-25]
Urine Test Assessment Guide Screens for:
Visual Assessment Color, turbidity
Urine Specific Gravity Concentrating ability of the kidneys
Urine Sediment Crystals, white blood cells, red blood cells, bladder epithelial cells, sperm, kidney casts, neoplastic cells
https://eclinpath.com/urinalysis/visual-features/ Infection, toxicity
https://www.bristol.ac.uk/medialibrary/sites/vetscience/documents/clinicalskills/Urinalysis%20Specific%20Gravity.pdf
https://eclinpath.com/urinalysis/cell-quick-quide/
https://eclinpath.com/urinalysis/cellular-constituents/
https://eclinpath.com/urinalysis/crystals/
https://eclinpath.com/urinalysis/crystal-quick-guide/
https://eclinpath.com/urinalysis/casts/
https://eclinpath.com/urinalysis/infectious-agents/
Kidney disease (acute or chronic)
Infection, toxicity, kidney disease (acute or chronic), neoplasia, muscular injury, nutrition, parasites
Urine Strip Test Organ function and urine metabolites
(https://www.idexx.com/files/urine-sedimentguide.pdf)
Vaginal Cytology, Abdominal Ultrasonography, and Hormone Analysis
blood, myoglobin, renal disease, toxicity, others
Vaginal cytology can be a useful tool for staging the current phase of a female’s reproductive cycle through microscopic examination of stained vaginal epithelial cells. Combining findings gained from cytology, abdominal ultrasonography, hormone assessment will provide relevant information regarding ovarian cyclicity, pregnancy status, and measurement of fetal parameters. These techniques may also provide insight into causes of reduced fertility and reproductive failures. Standardized methods to evaluate vaginal cells and to perform progesterone testing relative to the stage of the ovarian cycle are useful for detecting estrus in wild ocelots or captive-held ocelots who may be used for artificial insemination procedures.
Currently, there is no standardized stain recommended for application with felid vaginal cytology slides. Although Wright-Giemsa stain (Rapidiff Fixative®, Clinical Sciences Diagnostics CC, South Africa) has been assessed in lions [26], this has not been compared to other types of stains. In humans, Papanicolaou (Pap) stain has long been used for assessing vaginal epithelial cells and provides an approved standard stain for these types of cells [27]. This stain has multiple steps for completion and can be technically complicated (https://www.biovision.com/documentation/datasheets/K1440.pdf), whereas the Rapidiff stain is a simple, three step process (https://vetlabsupplies.co.uk/assets/Rapi-diff-Instructions-For-Use.pdf). Comparing the use of the Pap stain to the Wright-Giemsa stain in ocelots could be informative for improving vaginal cytology staining.
Meanwhile, ultrasonography of the abdomen is used for many animals to confirm pregnancy, approximate fetal age, and assess the reproductive tract. In canids and felids, measurements such as biparietal diameter, thoracic diameter, and gestation sac diameter have been used to evaluate stages of fetal development relative to specific time points during pregnancy [28-30]. Currently, there is no standard fetal measurement tool used for nondomestic felids, though it could be beneficial for future pregnancy evaluations in the field or other situations when the date of mating/conception is unknown. Measuring these parameters at different time points throughout
45
an ocelot’s gestation (i.e., 79-85 days) [31] relative to known breeding/artificial insemination date or parturition date could standardize methods for pregnancy detection and estimations of gestational ages. This information would be helpful when preparing for births or for recognizing delayed parturition or dystocia.
Various methods for monitoring endocrine activity in serum, feces, and urine have been assessed in domestic cats [32-33] and many non-domestic cat species [34-41]. Monitoring steroid hormones in fecal samples is preferred over monitoring in urine samples because steroid hormones are almost exclusively excreted in the feces [41-45] and this method provides the most non-invasive method of sample collection. Fecal hormone assessment can also be used to assess responses to ovarian synchronization protocols, ovulation, and pregnancy following artificial insemination procedures [40]. Progesterone and estrogen (and their metabolites in fecal samples) are the primary hormones of interest for assessing feline reproductive cycles. In ocelots, fecal estrogen and progesterone metabolite profiles have been described in captive ocelots throughout their reproductive cycle [40, 45] (Figure 2). In studies of wild ocelots in Texas and captive ocelots, the primary interest for hormone analysis is for tracking fecal progesterone metabolites during cycle manipulations for artificial insemination and during pregnancy. Hormone monitoring also could provide valuable information about normal and/or abnormal cyclicity in study females. In non-domestic felids, fecal progestin levels are indistinguishable between pregnant and non-pregnant luteal phases; however, non-pregnant luteal phases are characterized by a shorter duration of progestin elevation (~1/3 to 1/2 of the gestation period) than pregnant luteal phases [36, 44, 46-49]. In ocelots, the non-pregnant luteal phase lasts for ~45 days post-ovulation compared to a typical 79-day to 85-day gestation length after natural breeding.
Sample Collection Method
Vaginal cytology: When an ocelot is under anesthesia, a sterile, moistened cotton-tipped applicator will be passed through the vulva and into the vagina, rotated 360°, and withdrawn. The end of the applicator would be rolled onto two microscope slides to recover vaginal cells for staining and the swab stored in a plain tube.
Abdominal ultrasonography: When an ocelot is under anesthesia, an ultrasound probe will be placed on the abdomen using gel and alcohol to improve visualization of the reproductive tract. Dating of fetal parameters can be initiated for the ocelot by using the following guidelines (https://www.imv-imaging.es/educacion/educacionanimales-pequeno/tracto-reproductivo/canine-and-feline-foetal-ageing/) and establishing fetal measurements for this species based on known gestation dates and time points
Hormone analysis: Feces (~1 gram) will be collected after natural voiding or, if necessary and for anesthetized ocelots, via a lubricated rectal loop. Feces will stored in a cryovial or plastic specimen bag at -20°C or lower until analysis. In ocelots at the Ocelot Conservation Facility, fecal samples for hormone analysis should be collected three times weekly (every other day) by non-invasive methods of collection (from the enclosure they reside in) either (1) continually for a year to assess ovarian cyclicity (normal versus abnormal) or (2) beginning one month prior to gonadotropin treatment until at least 85 days after insemination. Serum (0.5 mL) will be stored in a cryovial at -20°C or lower until analysis.
Sample Testing
Vaginal cytology: The assessment of vaginal epithelial cells has not been standardized for ocelots. A recent study with lions (Panthera leo) [26] established vaginal cytology parameters as one component of their slide evaluation methods, and these can be useful for assessing vaginal cytology of other felid species [50]. These parameters can be viewed in Table 11. Examples of epithelial cell classifications in domestic cats have been
46
described [50]. Detailed measurements of epithelial cells and standardization of cell sizes for ocelots may be possible using methods described for lions [26].
Table 11. Vaginal Cytology Evaluation Parameters [26, 50]
Hormone analysis: Fecal and serum samples can be utilized for estrogen and/or progesterone hormone analysis. However, because serum sample collection requires anesthesia, few serum samples are expected to be available for analysis. If serum is collected, analysis of serum samples for progesterone (and/or estrogen) can be performed at a partnered endocrine laboratory or at the Ocelot Conservation Facility, possibly after validating progesterone testing using an in-house IDEXX analyzer. Fecal samples will be stored at -20°C in a cryovial or specimen bag labeled with the date, animal ID, and location or institution. Samples shipped to a partnered laboratory should be shipped (Monday to Wednesday only) on dry ice (~5-6 pounds/shipment) using next-day delivery An example ocelot endocrine profile is in Figure 2.

47
Mucus/Cellular Debris (40x and 200x) Cellular distribution (40x and 200x) Epithelial Cell Classification (200 cells on 1000x) Epithelial Cell Quantity (40x and 200x) PMN’s (1000x) Microbial Presence (1000x) 0-1 minimal quantity/no debris 0 single epithelial cells Basal 0-20% small Small- 0-2/100 epithelial cells Minimal- <10 per field 1-2.5 small quantity/debris 1 small clusters of epithelial cells Parabasal 20-50% moderate Moderate- 20-100/100 epithelial cells Small/few- 10-25/ field 2.5-4.5 moderate quantity/debris 2 moderate clusters of epithelial cells Intermediate 50-100% large Large- 100-400/100 epithelial cells Moderate- 20100/field 4.5-6 large quantity/debris 3 large clusters or piles of epithelial cells Superficialnucleated/enucleat ed Very large- >400/100 epithelial cells Large- >5/ field
Figure 2: Ocelot fecal endocrine profile [40]. Longitudinal profile of fecal estrogens (open triangle) and progestogens (closed circle) in a singleton ocelot.
Fecal parasite evaluation
Since 2019, fecal samples have been collected from free-ranging ocelots in Texas to assess parasitic prevalence. In wild ocelots in Texas, the most common intestinal parasites identified were the roundworm, Toxascaris leonina, a protozoon, Cystoisospora felis, and an unknown species of capillarid [A. Reeves, East Foundation, unpublished data]. Any released ocelots will likely be exposed to these parasites upon release to the wild. Parasites found in the wild or in ocelots held in the Ocelot Conservation Facility should be taken into consideration when employing deworming practices.
Sample Collection Method
Fecal samples will be collected from ocelots at the Ocelot Conservation Facility by obtaining it from their enclosures, and samples will be collected from wild ocelots caught in a box trap (if available). If a wild ocelot does not defecate in the trap, a sample may be obtained from the ocelot once anesthetized by passing a lubricated fecal loop (approximately 3/4 inch in diameter) approximately 2 to 4 inches into the rectum, rotating it, and removing the loop. Digital collection from the rectum of an anesthetized ocelot is also an option. Three cryovials will be stored at -20°C or lower for intestinal parasite sequencing (and fecal hormone analysis as described previously). Additionally, 1 gram of feces will be placed into formalin for future fecal flotation and intestinal parasite identification and load if fecal flotation cannot be performed on the day of collection. A fresh fecal sample also may be sent to a partnered laboratory for flotation and intestinal parasite identification.
Sample Testing
Testing will be conducted via fecal flotation by centrifugation (Table 12). Fecal direct smears will be made by mixing a small amount of fecal material with saline and placing a coverslip over the mixture. Fecal direct examination and flotation will be evaluated at 100X, 400X, and/or 500X magnification using light microscopy, and intestinal parasite ova identified based on literature (Table 12). Further speciation can be confirmed by polymerase chain reaction (PCR) on frozen feces.
Test PDF Reference
Fecal Centrifugation
https://www.jorvet.com/wp-content/uploads/2017/05/LITCentrifuge-Fecal-Protocal.pdf
https://www.midamericaagresearch.net/documents/Internal%20Parasite%20Manual%20for%20dogs.pdf
Intestinal Ova
Identification
Ectoparasites
https://veteriankey.com/reference-to-common-parasite-ova-and-forms-seen-in-veterinary-medicine/
https://www.jorvet.com/wp-content/uploads/2011/12/Fecal-Study-Gastrointestinal-Parasites-Guide.pdf
Since 2019, ectoparasites were collected from free-ranging ocelots in Texas to assess parasite prevalence. In this population, the most common ectoparasites identified were the tick species Dermacentor variabilis and an unknown species of Pulex flea [A. Reeves, East Foundation, unpublished data]. Any ocelots released to the reintroduction site will likely be exposed to these parasites upon release. Monitoring of ectoparasites and related pathologies in free-ranging ocelots and ocelots held at the Ocelot Conservation Facility is necessary.
48
Table 12: Intestinal parasite testing and identification references.
Sample Collection Method
While an ocelot is sedated, ectoparasites (ticks, fleas, lice, etc.) will be collected with forceps and placed into a cryovial containing alcohol for preservation and future identification.
Sample Testing
Ectoparasites will be identified to species level (Table 13) under a dissecting scope in collaboration with a partnered parasitology laboratory. If an individual ocelot tests positive for a vector-borne pathogen, the collected vectors (if present at the time of positive test) can be tested for the pathogen as well. Depending on the pathogen of interest, storing ectoparasites in a cryovial without alcohol at -20 ℃ is recommended [266]. It is recommended to identify best methods of storage based on the specific pathogen of interest.
Table
Test
Ectoparasite Identification
PDF Reference
https://vlm.ub.ac.id/pluginfile.php/46102/mod_resource/content/1/Veterinary%20Ectoparasites%20%20Biology%2C%20Pathology%20and%20Control%20%28VetBooks.ir%29.pdf
https://todaysveterinarypractice.com/feline-arthropods/
https://todaysveterinarypractice.com/parasitology-expertise-from-the-ncvp-parasitology-expertise-from-thencvp-feline-tick-borne-diseases/
https://www.fleatickrisk.com/sites/fleatickrisk/files/docs/Under%20the%20microscope.pdf
Pathogen Surveillance
A variety of pathogens may affect ocelots at the Ocelot Conservation Facility as well as ocelots or other freeranging carnivores in the wild. It is important to conduct routine pathogen testing using samples collected during health monitoring events to assess health risks for ocelots, prepare any necessary veterinary responses, and be aware of possible health risks to humans working on the ocelot reintroduction program Additionally, some pathogens potentially impacting ocelots may be transmissible to or by humans who contact ocelots. To support the health and safety of both people and ocelots, personnel working with ocelots should follow the most updated and available guidance from the Center for Disease Control and Association of Zoos and Aquariums to avoid transmission of the diseases between personnel and ocelots.
Pathogen surveillance in ocelots is necessary to inform veterinary treatment of ill ocelots as well as the use of vaccines or other preventative medicines. Additionally, pathogen testing must occur for all ocelots prior to transfer into the Ocelot Conservation Facility or into the wild to avoid introducing health risks to ocelot populations in the Ocelot Conservation Facility or in the wild. The following are pathogens that ocelots could be screened for. Use of specific pathogen screenings will be dependent on the risk assessments of particular pathogens on ocelots in the Ocelot Conservation Facility and the wild. In all cases of pathogen testing, if the testing will be performed within 7 days of collection, the samples can remain refrigerated (~4℃). If the testing will be performed after 7 days of collection, the samples should be frozen and stored at -40℃ until testing will be completed. When working with new partner laboratories, pathogens or testing methods, communication with the partnered laboratory on the preferred method of storage and preservation is recommended.
49
13. Ectoparasite identification references
Feline Immunodeficiency Virus (FIV)
FIV is a lentivirus that has variable prevalence in cat populations across the world [51]. FIV is primarily transmitted through bite wounds with infected saliva entering the blood, though it can be spread in the absence of fighting in immunosuppressed individuals or from dam to kitten [52]. Although felids can contract this disease at all ages, infection is likely to be the most severe in kittens due to the immaturity of their immune systems. Typically, FIV causes a decline, rebound, and repeated decline of CD4 and CD8 T-lymphocytes, leading to a decline in cell-mediated immunity, which acutely can cause fever, lymphadenopathy, and lymphopenia [52]. FIV is a prevalent disease in wild felid populations [53-56], with most infectious strains being considered species-specific [57] and tending to group geographically rather than in relation to the affected species [58]. Although cross-species transmission has been reported [58-59], it has rarely been documented in managed-care settings where risk of contact is higher [58].
Testing for FIV is important when planning movement of individuals between populations. While FIV could be endemic in one population, movement of individuals from other populations may introduce a strain of FIV that could cause detrimental effects in the recipient population. All ocelots should be tested for FIV when determining suitability for transfer into the Ocelot Conservation Facility or transfer to the wild.
Feline Leukemia Virus (FeLV)
FeLV has not been identified in free-ranging Texas ocelots studied since 2019 (n=24, A. Reeves, East Foundation, unpublished data), and a select number of ocelot samples from South Texas and dated from 1985 to 2012 (n=9) [Reeves et al., unpublished data, in prep.] also were negative for FeLV Though FeLV is not currently present in Texas ocelot populations, it is important to continue screening for FeLV in free-ranging ocelot populations in Texas and at the Ocelot Conservation Facility given the potential health impacts of FeLV.
FeLV is a gammaretrovirus [60] that can be transmitted vertically to kittens and horizontally among other adult cats [52]. Once infected, FeLV spreads from local lymphoid tissues, where it eventually infects the bone marrow [52] and can cause tumors, bone marrow suppression syndromes, and increased susceptibility to secondary infections due to suppression of the immune system [61]. FeLV infections have been categorized as abortive, regressive, progressive, or focal based on how well the virus is contained during the early stage of infection [52, 61]. Kittens are more susceptible to infections becoming progressive, leading to a greater chance of associated disease and death [52]. Approximately 2-4% of domestic cats test positive for FeLV in the United States [52, 62]. There is currently a vaccination protocol for domestic felines, based on their level of risk, established by the American Association of Feline Practitioners [52].
FeLV is rarely noted in non-domestic felines [55] and most cases have been reported in captive individuals that have been previously exposed to infected domestic cats [63-64]. The only wild species known to have endemic FeLV in their populations is the European wildcat (Felis silvestris) [65]. Additionally, FeLV has been found in other non-domestic free-ranging feline populations including Florida panthers (Puma concolor coryii) [66-67], and Iberian lynx (Lynx pardinus) [68]. While most non-domestic felids experience no adverse effects or just the immunosuppressive effects of FeLV as seen in domestic cats [69], there have been reported outbreaks leading to mortality indicating the existence of a pathogenic form of the disease [66-68] and a strain causing significant disease in Florida panthers documented cross-species infection from domestic felines in the area [66].
For ocelots in southern Texas, the high number of feral domestic cats surrounding current populations presents concern for transmission of FeLV from domestic cats into ocelot populations. If FeLV infection produces
50
decreased immune fitness in ocelots, it could impact population health or present the possibility of increased susceptibility to other pathogens. Additionally, decreasing genetic diversity documented in other species (Iberian lynx and Florida panther) show that lack of a robust immune system may increase the potential risk of introduction of disease, cross-species transmission of pathogens, and mortality.
Sample Type and Testing (FIV/FeLV)
Testing for FeLV and FIV will use the IDEXX SNAP Feline Leukemia Virus Antigen-Feline Immunodeficiency Virus Antibody Test Kit (IDEXX Laboratories, Inc., Westbrook, Maine USA). The IDEXX SNAP FIV/FeLV Combo test has a sensitivity and specificity of 96-100% for domestic cats [70]. Whole blood can produce false positive results for FIV, so it is recommended to use serum for testing. For individuals under 6 months of age, retesting should take place after 6 months of age as maternal antibodies against FIV can cause a false positive result (https://catvets.com/public/PDFs/PracticeGuidelines/RetrovirusGLS-Summary.pdf ; https://www.vet.cornell.edu/departments-centers-and-institutes/cornell-feline-health-center/healthinformation/feline-health-topics/feline-immunodeficiency-virus-fiv). Additionally, confirmatory testing is recommended for FeLV positive snap tests, as more information about the type of infection can be identified (https://www.idexx.com/files/updates-diagnosis-management-felv.pdf ). Furthermore, PCR testing for FeLV will be performed to identify potential regressive infections in ocelots in the Ocelot Conservation Facility or in the wild in Texas. PCR testing for FeLV can be performed on whole blood through many laboratories. “Realtime PCR (FeLV RealPCR™ Test) detects proviral DNA and, therefore, is useful to confirm the presence of FeLV infections that have progressed to the bone marrow” [71-72]. Three drops of serum and four drops of conjugate (anti-FELV/FIV Ag: HRPO conjugate) are mixed in a vial and poured into the sample well. When the solution reaches the activation circle, the activator is pushed firmly until flush with the device body and the test is read in 10 minutes. Positive, negative, and invalid test results are interpreted by the parameters set forth in Figure 3 Confirmation of a positive test requires additional testing methods such as a Western Blot. (https://www.idexx.com/files/fiv-diagnostic-algorithm.pdf; https://www.dvm360.com/view/a-practical-guideto-feline-retrovirus-testing ).

51
Figure 3: FeLV/FIV Snap Test IDEXX Laboratories, Inc., Westbrook, Maine USA Snap Combo Package Insert https://www.idexx.com/files/SNAP_COMBO_package_insert_032917.pdf
Toxoplasma gondii
Since 2019, Toxoplasma gondii antibodies were identified in free-ranging Texas ocelots (9/22; 40.9%) [A. Reeves, East Foundation, unpublished data] as well as in a select number of samples dating from 1985 to 2012 (4/9; 44.4 %) [A. Reeves et al., unpublished data, in prep.]. This pathogen is consistently present within freeranging ocelot populations and reintroduced ocelot populations are likely to be exposed to the parasite. While previous research suggests low concern for either negative side effects or population level impacts, T. gondii should be continually monitored for in ocelots at the Ocelot Conservation Facility and in the wild to identify possible pathology due to infection.
Toxoplasmosis is a zoonotic disease caused by infection with the protozoan parasite T. gondii. Its definitive host is all members of the family Felidae, and the primary intermediate hosts are rodent prey species, but any warmblooded animal, including humans, may serve that role [73-74]. Transmission of T. gondii can occur horizontally through ingestion of bradyzoites encysted in undercooked animal tissue, or sporulated oocysts from cat feces that have contaminated water or food supplies [75-76]. Transmission can also occur vertically through transplacental and lactogenic infection by tachyzoites [74-75].
In members of the family Felidae, such as bobcats (Lynx rufus) and ocelots, horizontally transmitted toxoplasmosis most commonly causes either mild clinical signs such as diarrhea or no clinical signs at all in individuals who are otherwise healthy [77]. If there is concurrent immunosuppression or decreased fitness, more severe clinical signs might occur [77-79]. These clinical signs could include fever, cough, dyspnea, jaundice, neurologic signs, ocular signs, and even death [77]. Vertically transmitted toxoplasmosis is usually asymptomatic in kittens but has the potential to result in more severe signs in otherwise healthy, definitive host species [77]. In bobcat kittens specifically, clinical signs have included myocarditis, hepatitis, and encephalitis, eventually leading to death [80].
In healthy intermediate hosts, specifically in humans, horizontal transmission can result in flu-like symptoms, decrease reaction times to outside stimuli, or cause no symptoms at all [74]. However, immunosuppression can make symptoms much worse. The most common clinical sign of toxoplasmosis in immunosuppressed humans is toxoplasmic encephalitis [76]. Vertical transmission in intermediate hosts, such as humans, has been reported to lead to a variety of clinical signs depending on the time during gestation that the infection occurred [81-82]. In humans, the risk of transplacental transmission increases as the length of gestation increases [81-82]. However, the consequences of congenital infection are more severe the earlier the transmission occurs in gestation, meaning the severity of disease is inversely correlated with the risk of transmission [81-82].
Symptoms in humans due to congenital transmission can vary but tend to target the eyes and brain [83-85]. Abortion is also possible with congenitally transmitted toxoplasmosis [74].
Due to the threat that toxoplasmosis poses to inbred, non-domestic feline populations, understanding the prevalence of toxoplasmosis in ocelot populations in Texas is important for the reintroduction program as well as the protection of the humans and wild or domestic animals who interact with them directly or indirectly. Currently, there is published information on T. gondii antibody prevalence in bobcat populations in Minnesota, Mississippi, and Pennsylvania, all of which indicate that T. gondii has high prevalence in these populations [8688]. One study of T. gondii prevalence in ocelot populations in Mexico found that this population had high prevalence of antibodies as well [89].
52
Sample Type and Testing
Toxoplasma gondii titers will be evaluated using the Toxoplasma gondii MAT test Kit (TgMAT Kit; University of Tennessee Research Foundation) for detection of anti-Toxoplasma IgG antibodies in serum [90-91]. The antigen used in the test kit is formalin-treated Toxoplasma tachyzoites. The presence of anti-Toxoplasma IgG antibodies in the samples will cause the suspension to form a diffuse cellular mat in the bottom of the well, whereas a negative sample will produce a smaller pellet on the well bottom. Methods will be performed and interpreted according to the recommended protocol for MAT testing provided by the University of Tennessee (https://volweb.utk.edu/~csu1/MATprotocol.pdf; https://volweb.utk.edu/~csu1/TgMATModified_Agglutination_Test.html) or another laboratory of the veterinarian’s choosing. A titer equal to or greater than 1:25 is considered a positive result.
Trypanosoma cruzi
Two non-domestic cat species, ocelots and pumas (Felis concolor) have been reported to be serologically positive for Trypanosoma cruzi [99-101]. In pumas, T. cruzi infection rates were shown to increase significantly with the number of vertebrate species present in their diet. Similarly, ocelots with a high dietary presence of a diverse assemblage of mammalian species were shown to be consistently infected as well [99], and more recently T. cruzi was identified in 3/21 (14.3%) of ocelots in a southern Texas population [102]. This study identified PCR-positive samples from muscle, heart tissue, and a blood clot from ocelot carcasses collected from roadway mortalities from 2010-2017 around Laguna Atascosa National Wildlife Refuge, with one individual showing anti-T. cruzi antibodies [102]. Although the parasite was identified, the clinical significance remains unknown. Over the last three years, T. cruzi antibodies were not identified in free-ranging Texas ocelots (A. Reeves, East Foundation, unpublished data, n=24) and a select number of samples dating from 1985 to 2012 (n=9) [A. Reeves et al., unpublished data, in prep]. Continuing to screen for Trypanosoma cruzi and potential negative side effects pre-mortem and post-mortem in ocelots could enhance the understanding of the clinical significance of T. cruzi in ocelots and dictate any necessary management of this disease.
T. cruzi, a zoonotic parasite, causes Chagas disease in humans and other mammalian species. The vector, a triatomine bug, must take a blood meal to ingest the trypomastigotes in the bloodstream of a given mammal, which was initially infected by oral ingestion of the infected bug’s feces, contaminated food products, or by consuming an infected insect or animal [92]. This parasite is endemic in the southern half of the US, including southern Texas, with well-established zoonotic cycles [93]. Although there is little known about T. cruzi in felines, a seroprevalence of ~ 30 % is reported in domestic cats in Mexico and Argentina [94-97]. In southern Texas, there are reports of 11.4% seropositivity in domestic cats [98]; however, little is known about the clinical progression of this disease in cats and further studies are needed to determine the clinical impact of this disease in feline species.
Sample Type and Testing
One mL of serum is transferred into a non-additive tube and placed on ice for shipping overnight to a partnered laboratory. If shipping is not performed on the same day, this sample should remain in the refrigerator. Antibodies to Trypanosoma cruzi are detected by indirect fluorescent antibody testing (IFA) with serial dilutions (https://tvmdl.tamu.edu/tests/trypanosoma-cruzi-ifa/).
53
Cytauxzoon felis
Cytauxzoon felis is the causative agent of the protozoal disease, cytauxzoonosis, which is transmitted among felids by ticks [103]. The schizogenous phase, or tissue phase, occurs after the tick has fed on a reservoir host (e.g., bobcats) and bites a new, uninfected host [104]. This phase develops in macrophages within the lungs, spleen, liver, and lymph nodes of an infected individual [105], and can progress to a chronic, erythrocytic (red blood cell) piroplasm phase and become established in the bloodstream of the individual [104-105]. The American dog tick, Dermacentor variabilis, and the lone star tick, Amblyomma americanum, have been confirmed as competent vectors of this disease in a laboratory setting, although the lone star tick is considered the primary vector of Cytauxzoon felis [104]. The range of infection of C. felis includes south-central, midcentral, and mid-Atlantic regions of the United States, which correlates with the range of the lone star tick [104]
Cytauxzoon felis was first reported in 1976 in a domestic cat in Missouri and since then, cases have been reported in other states, including Texas [105]. Infected domestic cats are known to suffer from severe and often fatal illness due to large amounts of parasitic replication, leading to obstructive blood flow, organ damage, and disseminated intravascular coagulation, making them a dead-end host [104, 106]. In non-domestic felids in the United States, C. felis infection has been reported in pumas [103], Florida panthers (Puma concolor coryi) [103], and bobcats [104], while outside of the United States, the parasite is reported in Iberian lynx [103] and other wild felid populations including ocelots, pumas, and jaguars (Panthera onca) [103]. Infected bobcats usually undergo a brief illness and then recover, becoming lifelong carriers and reservoir hosts, although there are reports of rare fatalities [104]. As the ocelot populations share much of their habitat in southern Texas with bobcats and may come into contact with feral domestic cats as well, investigation of the prevalence of infection in ocelots, the effect on their health, and possible sources of transmission would benefit ocelot conservation and health.
Sample Type and Testing
EDTA whole blood (minimum 0.5 mL) is used to create a blood smear for staining using the Wright-Giemsa Stain Solution (Thermo Scientific “Richard Allan Scientific”, Kalamazoo, Michigan, USA) that is examined using standard light microscopy at 400x, 500x, and 1000x magnification with oil for intracytoplasmic and intranuclear inclusions of parasitic origin. PCR testing will be performed using whole blood at a partnered laboratory
Coronavirus
Feline coronavirus (FCoV) is a common virus among domestic cat populations. Transmission is via the fecaloral route and is more common with a higher density of cats [107]. Lower prevalence has been found in stray and feral cats compared to companion animals due to lower population densities and less exposure to contaminated fecal material [107-111]. Cats are most likely to spread FCoV during the primary stage of infection (7-18 months) in which the level of viral shedding is highest [112]. Following the primary stage of infection, cats can become intermittent shedders (70-80%), consistent shedders (10-15%), or non-shedders (<5%) [112-114].
FCoV is divided into two biotypes: common asymptomatic or mild feline enteric coronavirus (FECV) and the possibly fatal feline infectious peritonitis virus (FIPV) [115-118]. FECV and FIPV are nearly identical antigenically and genetically dependent on the body system affected, but differ from other geographic isolates, leading to the assumption that FIPV arises from the FECV within an individual [115, 117-122]. With the FECV
54
biotype, there may be vomiting and diarrhea in a small proportion of adult cases and kittens [123]; however, most individuals remain asymptomatic. Cats infected with FCoV have a 5-12% chance of developing feline infectious peritonitis (FIP) which is a highly lethal systemic immune-mediated disease, characterized by the depletion of T-lymphocytes [107, 109, 124-127]. Of these, sexually intact males and young cats are at the greatest risk of developing FIP [128]. In addition, certain cat lineages [129-131], individuals with preexisting immunosuppressive conditions such as FIV or FeLV [125, 132-133], and those under stress are at greater risk [134].
There are two forms of FIP: non-effusive/dry form and an effusive/wet form [135-136]. The effusive/wet form presents abundant clear, protein-rich, straw-colored peritoneal effusions [137] and large amounts of thick exudative fluid abundant in fibrin, which causes a distended abdomen, and a perivascular inflammatory reaction [107,130]. The non-effusive/dry form has little to no effusion but is marked by perivascular granulomatous lesions with or without vasculitis in multiple organs [107]. As certain feline lineages have shown to have an increase in mutation of FECV to FIP, monitoring of ocelot populations as it pertains to the individual, lineage, and population should be considered.
In non-domestic felids, FCoV testing has been performed on multiple species, including cheetahs (Acinonyx jubatus), leopards, tigers (Panthera tigris), lions, lynx, ocelots, jaguars, and bobcats by fecal RT/nPCR and serum FCoV-specific antibodies [138]. Of the 75 individual felids tested, 24 of 72 (32%) were positive for FCoV using RT/nPCR and 29 of 63 (46%) were seropositive [138]. Of the species tested in this study, none of the ocelots tested positive by either test. However, because bobcats were among the positive individuals, FCoV should be monitored in ocelots in the wild and at the Ocelot Conservation Facility since bobcats live in proximity to ocelots in Texas, and theoretically, could share infectious agents with ocelots. Additionally, FIP has been described in multiple non-domestic feline species [139-148], and FCoV antibodies have been reported in two, free-ranging ocelots in Brazil [149-150].
Sample Type and Testing
For screening of cats that may be shedding the virus in feces, 2-5 grams of fresh feces of five consecutive fecal samples are submitted for reverse transcription polymerase chain reaction (RT-PCR) screening. For clinical cases, RT-PCR can be performed on abdominal effusion, whole blood, plasma, serum, or fresh tissues (if necropsy is being performed). The amount of fluid submitted should be 1-2 mL (abdominal effusion, whole blood, serum, or plasma) or 1-2 grams of fresh tissues. It is important to note that a positive test will diagnose infection with FCoV but does not differentiate between FIP and FCoV. The cat must be exhibiting signs of FIP to identify it. A positive FCoV test without clinical signs of FIP diagnoses a FCoV infection only.
(https://www.vet.cornell.edu/animal-health-diagnostic-center/veterinary-support/disease-information/felinecoronavirus). Additionally, there are two types of coronaviruses (I and II) and the only laboratory that tests for antibodies against both virus types is the University of Tennessee (http://www.vet.utk.edu/diagnostic/virology/index.php). If the above samples are not available, a rectal swab can be substituted for testing.
Feline Calicivirus (FCV)
FCV is a highly contagious virus causing mild to severe respiratory infections and oral ulcers in domestic cats that occurs most commonly in shelters or areas of high density [151-152]. All members of the Felidae family are susceptible to FCV infection [153]. Most domestic cats will recover from a calicivirus infection, but in rare cases this disease can be fatal [151]. Several strains circulate in domestic and wild cat species and mutate
55
readily, leading to new strains with varying severity of disease that may compromise the effectiveness of vaccines [151, 154]. Rare outbreaks of mutant strains of FCV (e.g., FCV-associated virulent systemic disease or FCV-VSD) have been reported and cause serious disease with multi-system organ failure and death [151].
FCV spreads through direct contact with saliva, nasal mucus, ocular discharge, and aerosol droplets [151]. Shedding lasts around 2-3 weeks after infection, but some individuals will become long-term carriers and continue to shed on and off for months [151]. This virus is very hardy in the environment and can be spread by objects and personnel working with infected cats [151]. Transplacental infection has not been documented, but FCV has been isolated from aborted fetuses [155]. Symptoms can range from a mild cold (sneezing, nasal congestion, fever, drooling) to oral ulceration, lethargy, lameness, inappetence and even pneumonia [151]. Symptoms of FCV-VSD can be more severe including swelling of the head and legs, crusting sores, hair loss, and liver damage causing jaundice [151]. Severity of clinical signs vary depending on age, exposure route, concurrent infections, immune status of the host, and vaccination history [156].
Although serological detection has been reported in many wild cat species [65, 150, 157-160], including ocelots [161], RT-PCR detection of FCV was unsuccessful in 21 ocelot samples from 1999-2011 in Brazil [162]. However, serological detection of FCV was reported in areas where domestic cats were not present, suggesting FCV to be endemic in ocelot populations and not acquired from domestic cats in that region [160]. Clinically, there were no reports of physical ailments as it pertains to an infection with FCV or FCV-VSD in ocelots.
Given the population density of ocelots held at the Ocelot Conservation Facility and wild ocelots surviving in limited habitat space, the monitoring of this pathogen is important for future assessment of transmission and the health impact it may have on current or future populations. Given present difficulties in detection using RTPCR, identification of new sequences for detection should be explored or considered. Additionally, concurrent serological testing should be performed to differentiate between exposure and infection.
Sample Type and Testing
A Feline Calicivirus- RT-PCR may be conducted using a conjunctival or oral swab, or 1 g fresh tissue (biopsy, lesion) (https://tvmdl.tamu.edu/tests/feline-calicivirus-rtpcr/). Detection of antibodies requires 1 mL of serum (https://tvmdl.tamu.edu/tests/feline-calicivirus-vn/). It is important to note that commercial testing cannot distinguish mild strains of FCV from FCV-VSD. Detection of antibodies can be performed on serum, but presence of antibodies only confirms exposure, and not active infection [156] and should be performed with concurrent RT-PCR testing.
Feline Herpesvirus (FHV)
FHV is the agent of feline viral rhinotracheitis and although only one serotype is described, virulence can differ between strains [163]. In domestic felids, FHV replicates in the epithelial cells of the upper respiratory tract, neurons, and conjunctiva [164]. Neuronal infection allows the virus to establish a lifelong latency in the trigeminal ganglia after a primary infection [164]. This latent infection can undergo intermittent reactivation, creating viral shedding in oronasal and conjunctival secretions, which is mainly associated with stressful events [164]. Although related to other herpesviruses known to infect other species, cross-species transfer is not known to occur [165]. The domestic cat is the main host of FHV, but it has been documented in other felids such as cheetahs, lions, and pumas [164].
FHV is shed by cats with an acute infection or a latent infection that has been reactivated [166]. Although transplacental infection has not been documented, queens with a latent infection may transmit FHV to their
56
offspring as parturition and lactation create stress within the queen, leading to viral reactivation and subsequent shedding [164]. In domestic cats, risk is higher in shelters where close quarters and high numbers of cats can make disinfection and separation of viral shedders difficult. Once the virus has entered the nasal/oral cavity or conjunctiva, it can spread throughout the rest of the respiratory tract causing a transient viremia [163, 167], and viral excretion, beginning ~ 24 hours after infection and lasting one to three weeks [164].
Acute disease typically resolves in 10 to 14 days; however, some individuals may develop chronic lesions in the upper respiratory tract and/or ocular tissues [164]. Conjunctivitis can be associated with corneal ulcers, chronic sequestrum, stromal keratitis, and damage to the nasal turbinate’s leading to chronic rhinitis [163]. Clinical signs are associated with the disease type and many types have been described: classical acute disease (cytolytic), atypical acute disease, chronic immune-mediated disease, and possible FHV-related diseases [164]. Clinical signs can range from a mild upper respiratory infection with nasal discharges, sneezing, corneal ulcers, and dermatitis to pneumonia, coughing, fading kitten syndrome, and blindness, with disease typically more severe in kittens [164].
Most kittens acquire immunity via colostrum ingestion; however, this protection will wane between 6 to 10 weeks of age [168-169]. Additionally, natural infection does not result in complete immunity, protecting against disease but not infection, and clinical signs can return with re-infection, although generally mild [170]. The FHV vaccine for domestic cats provides protection against clinical signs and reduces viral shedding within a week of administration [171] but does not provide full protection [163]. Most vaccines are combined with FCV or additional agents in both modified-live and inactivated parenteral (injectable) vaccines; subunit FHV and modified intranasal vaccines also are available in many countries [164]. Two vaccinations, an initial injection and booster, are recommended for domestic feline kittens starting around nine weeks of age with a four-week interval, followed by annual boosters [164]. Other protocols from the American Association of Feline Practitioners recommend vaccinations beginning between 6 to 10 weeks with boosters every 3-4 weeks until 16 to 20 weeks for age (i.e., 8, 12 and 16 weeks)
Among non-domestic felids, cheetahs are particularly susceptible to FHV-1 (alpha herpesvirus) [172-174] and although clinical signs can be like those described previously, some infections can cause severe clinical disease and death, especially in young animals [175-177]. In studies of other non-domestic cat species, FHV is reported to be endemic in East African free-ranging lions [160] but was not detected in ocelots in the Bolivian Chaco [161].
Sample Type and Testing
The preferred method of detection is PCR (conventional, nested, and real-time) testing of conjunctival, corneal, or oropharyngeal swabs, corneal scraping, aqueous humor, corneal sequestra, blood or biopsy specimens [178187]. This test should be interpreted given observed clinical signs and is important to note that PCR tests can detect FHV DNA in modified-live vaccines [188]. A positive PCR result may indicate low level shedding or viral latency but does not necessarily correlate clinical signs with the actual viral infection [167]. When using quantitative real-time PCR [185], the amount of virus detected can suggest active replication and involvement of the virus in the present clinical signs. Recommended laboratories for serology (serum neutralization) testing is the Veterinary Diagnostic Laboratory at Cornell University (www.diaglab.vet.cornell.edu) and for PCR, the University of Tennessee College of Veterinary Medicine (http://www.vet.utk.edu/diagnostic/virology/index.php).
57
Canine Distemper Virus (CDV)
CDV is a highly contagious paramyxovirus seen in dogs worldwide [189]. Although domestic dogs are the reservoir hosts, many species are susceptible to infection, including large felids [189]. The main route of spread is via aerosol droplet secretions shed by infected animals for several months [189]. Clinically, a canine infected with CDV will present with a fever, leukopenia, gastrointestinal and respiratory signs, pneumonia, and neurologic signs [189]. Classic neurologic signs include muscle twitching, convulsions, salivation, and chewing movements of the jaw, and may include circling, a head tilt, nystagmus, paresis to paralysis, and seizures [189]. Nasal discharge is serous with an associated mucopurulent ocular discharge, lethargy, and anorexia [189]. If an animal survives the infection, it may have hyperkeratosis of the footpads and nasal planum and enamel hypoplasia of incompletely erupted teeth [189]. Since there is no curative treatment and only supportive care can be attempted, prevention of canine distemper is necessary by widespread vaccination of domestic dogs.
CDV has been reported in both wild and captive populations of nondomestic felids [190-193] and recent identification of this virus in wild populations of Amur tigers (Panthera tigris altaica) present conservation concerns for this endangered felid [194-196]. CDV vaccination is currently effective at managing infection in domestic dogs and other species [192, 197], and is recommended for tigers in captive settings [198]. The PureVax Ferret Distemper vaccine, a monovalent canarypox-vectored canine distemper vaccine has been demonstrated to be effective and safe in several wild carnivore species [199-201]. Although parenteral vaccination may be the most effective way to manage this virus in captive animals, another vaccine delivery method may be more effective for widespread vaccination of wild animals. Similar to the oral-transmucosal rabies vaccination method used for raccoon rabies in the eastern United States, a recent study sought to examine the immune response of tigers following parenteral and oral-transmucosal vaccination. Their results showed a poor serologic response to this vaccine using both administration routes [202]. Similarly, other vaccines have shown similar poor serological responses in tigers [203]. Both papers suggest these results could be due to a dose-dependent effect when comparing tigers to domestic cats as they were given the same standard vaccine dose across highly differing body weights [202]. The best vaccination route for CDV prevention in the wild is still being explored in wild cats, with large felids apparently the main species of Felidae that are susceptible to this virus.
In ocelots, a few studies have assessed the prevalence of CDV in free-ranging populations by various methods: serology of 10 ocelots reported 7/10 samples were seropositive for CDV (70%) in the Bolivian Chaco [161], serology of two free-ranging ocelots reported 0/2 seropositive samples (0%) in Brazil [204], and 3/71 freeranging ocelots in Costa Rica tested positive by fecal RT-PCR [205]. However, there were no reports of clinical illness associated with the positive tests where the individual underwent a concurrent physical examination. Small cats are not thought to be susceptible to disease caused by CDV, but some may seroconvert once infected [206]. Although unlikely to cause illness in small to medium-sized felids, this virus has the potential to cause detrimental effects to populations. Additionally, a decline in genetic diversity and heterozygosity could present a situation in which a typically asymptomatic disease could impact an immunocompromised individual or population. Screening for this virus is recommended to assess the potential for introduction into a potentially naïve population.
Sample Type and Testing
One mL of serum in a plain red top tube should be submitted for serology using the serum neutralization assay for CDV. Cornell Diagnostic Laboratory has utilized the laboratory assay to diagnose CDV in felids and multiple other species. Additionally, Canine Distemper PCR can be performed to detect acute infection in
58
individuals with clinical disease. This test recommends using 5 mL of urine, 1 gram of tissue, or a swab of nasal or ocular discharge. The swab must be placed into a non-additive tube with a few drops of sterile saline to prevent desiccation (www.diaglab.vet.cornell.edu).
COVID-19
COVID-19 (SARS-CoV-2) is a recently identified coronavirus that is differentiated from the canine coronavirus (CCoV) or feline coronavirus (FCoV) [207]. Although humans can infect cats and dogs with the same coronavirus that causes COVID-19 in humans, there is little evidence that animals can infect humans with this same virus [207], except for transmission from mink (Neovison vison) to humans reported in the Netherlands and Denmark [208-209]. However, there is no evidence that dogs and cats can infect humans at this time. Dogs infected with this virus do not appear to show clinical signs of illness; however, some domestic cats have demonstrated mild illness with respiratory and gastrointestinal symptoms [207]. However, many other diseases can cause similar symptoms, such as fever, coughing, difficulty breathing, lethargy, sneezing, nasal discharge, ocular discharge, vomiting and/or diarrhea [210]. At this time, routine testing of companion animals is not recommended; however, testing may be recommended on a case-by-case basis [207].
Non-domestic animals and wildlife species that have been reported to be infected with the virus include several cat species [211]. Most of these cases were reported following contact with people infected with COVID-19, including owners, caretakers, or others in close proximity to the cats [211]. Additionally, cats have been shown to be able to transmit this virus among other individuals of the same species [211]. In the United States, several large cat species have become infected with SARS-CoV-2 including tigers, lions, Amur leopards (Panthera pardus orientalis), snow leopards (Panthera uncia), pumas and a fishing cat (Prionailurus viverrinus) after being exposed to people with COVID-19 [212]. At this time, SARS-CoV-2 fatalities have been confirmed in a lion and several snow leopards [212]. The Felid Taxon Advisory Group (TAG) supports vaccination of all nondomestic felids with the new Zoetis vaccine specifically formulated for vaccinating at-risk non-domestic species in zoological collections [212]; however, efficacy of this vaccine has not been established due to its recent availability.
While most cases of SARS-CoV-2 infection in cats (domestic and non-domestic) have been mild and most have recovered with supportive care, the risk of transmission and significant disease remains. The startling deaths of multiple snow leopards and increase in infections at captive facilities raises concern for ocelots in captive settings, although infection of ocelots with SARS-CoV-2 is yet to be documented. Monitoring ocelots in the Ocelot Conservation Facility for infection with this virus, as well as transmission from personnel, will be vital to ensure viruses are not shared among species or introduced into the wild, where supportive care would not be possible.
Sample Type and Testing [208]
Concurrent antibody and PCR testing are recommended to determine exposure versus active infection. PCR testing can be performed on swabs of the respiratory tract (nasal swab or oropharyngeal swab) and/or rectal swabs. Antibody testing can be performed on serum.
Panleukopenia
Feline panleukopenia virus (FPLV; also known as feline distemper or feline parvo) is a highly contagious parvovirus of cats (domestic and non-domestic) [213-214]. Although historically the leading cause of death in cats, the availability and use of effective vaccines have made the disease uncommon today [213]. Kittens are
59
most affected by the virus, as it kills cells that are rapidly growing and dividing, such as bone marrow, intestinal cells, and developing fetuses [213]. This virus is present in the environment, and virtually all felines (adult and kittens) are exposed at some point in their lives [213]. Kennels, pet shops, shelters, and unvaccinated feral cat colonies are the main reservoirs of FPLV, and it is seen more commonly in high density areas [213].
This virus is shed in the urine, stool and nasal secretions of infected cats and can transmit rapidly when another uninfected individual encounters these infected secretions [213]. Although shedding is short-lived (one to two days), the virus can survive up to a year in the environment, infecting individuals without direct contact with another infected cat [213, 215]. The virus causes damage to the intestinal lining, bone marrow and lymph nodes resulting in decreases in all red and white blood cell types and causing panleukopenia and anemia [213, 216]. Clinical signs include depression, loss of appetite, high fever, lethargy, vomiting, severe diarrhea, nasal discharge, and dehydration [213, 216]. In pregnant females, abortion can occur or kittens may have cerebellar damage causing incoordination (cerebellar ataxia) presenting as severe tremors or shaking [213, 216]. There are no current medications to successfully kill this virus, so the mainstay of treatment is supportive care while the individual’s immune system fights the virus, as with many other viral infections. Strict isolation from other cats is required due to the high transmissibility of this virus.
FPLV-like viruses have been documented in many non-domestic felid species including the cheetah [217], European wild cat [218], lion [219], African wild cat (Felis lybica), ocelot, puma, Amur tiger and southern tigrina (Leopardus tigrinus) [150], and Eurasian lynx (Lynx lynx) [243], bobcat [241], and Iberian lynx [242243] in which mortality events were linked to FPLV infection. Although strains can differ between species as this virus can mutate within the body of the host, an outbreak of FPLV in cheetah cubs involved a previously unreported strain of FPLV that was also found in a deceased lion, African black footed cat (Felis nigripes), caracal (Caracal caracal), ocelot and serval (Leptailurus serval) [214]. The likelihood of mutation and transmission increases concern over introductions into wild populations from captive felines and vice versa. Detection of this pathogen can be challenging for diagnosis due to the short-lived shedding and identical presentation to other viral diseases. Typically, a presumptive diagnosis is made from clinical signs, blood work abnormalities and lack of vaccination, prior to a laboratory diagnosis.
Sample Type and Testing
Diagnosis is typically made based on clinical signs, leukopenia on the complete blood count, and lack of vaccination. Fecal antigen and/or PCR testing is the recommended test for this pathogen, but the virus is only detectable for a short time and false negatives are common. Additionally, false positives can occur if vaccinated within 5-12 days of the test. An antibody test is available; however, it only documents exposure and may result in a false positive due to vaccination.
Canine heartworm
Dirofilaria immitis (canine heartworm) antibody testing may be performed prior to release of an ocelot as well as opportunistically during other physical exams to screen for this pathogen. Monthly preventative for canine heartworm is not possible, so infection with canine heartworm must be tested for. For captive individuals that will not be released, heartworm tests can be performed with the general blood screening conducted during physical examinations or sooner if warranted.
60
Highly Pathogenic Avian Influenza Virus
Influenza viruses, including Highly Pathogenic Avian Influenza Virus (HPAI), have been reported in many domestic and non-domestic species. In domestic and captive cats, the World Health Organization (WHO) reported HPAI H5N1 strain in domestic cats in Thailand in 2004 and in at least one case the cat was known to be in contact with deceased chickens (see https://www.who.int/.) Laboratory studies confirmed domestic cat susceptibility though experimental inoculation, through feeding of birds infected with H5N1 avian influenza viruses, or through horizontal transmission between cats [244-246]. Not all infections caused severe disease or death, and some subclinical infections were apparent [247]. In laboratory studies, challenging cats with an attenuated strain of H5N6 low pathogenic avian influenza virus (LPAI) resulted in subclinical infections and viral shedding and further protected them against HPAI H5N1 viruses [248-249], suggesting a level of crossstrain protection [250]). In the United States, an outbreak occurred in 2016 among shelter cats in New York [251-252]. This virus most closely matched the avian H7N2 virus that has circulated in New York historically, and compared to the avian lineage, the feline isolate showed improved replication in mammalian cell lines [253]. Disease in these cases were mild [254].
In non-domestic cat species, further cases in Thailand in 2004 were documented in captive tigers and leopards who were fed infected chickens [255] although some tigers became infected even without consuming chickens, suggesting cat-cat transmission [256]. Many other species have been observed with influenza within the order Carnivora, including ferrets [257-258], minks [259-261], and seals and related Pinnipeds [262-264].
In 2022 and 2023, HPAI began emerging in many wild carnivore species in the United States, including many wild cats. In the United States, there have been cases reported in Amur leopards (n=1; NY), Amur tigers (n=1; NE), bobcats (n=6; WI, WA, CA, CO), and mountain lions (n=16; NE, CO, WY, CA, MT, UT) (https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/avianinfluenza/hpai-2022/2022-hpai-mammals; https://www.cdc.gov/flu/avianflu/spotlights/2022-2023/h5n1technical-report.htm#infections-among-mammals). In December 2022, a bobcat’s remains were collected in California and cause of death was confirmed as a result of HPAI H5N1 (https://wildlife.ca.gov/News/avianinfluenza-detected-in-deceased-bobcat#gsc.tab=0). Although two mountain lions found deceased in December 2022 and January 2023 tested positive for the pathogen (https://wildlife.ca.gov/News/avian-influenza-detectedin-deceased-mountain-lions#gsc.tab=0), HPAI has not yet been ruled the sole cause of death. Although reports of infection have been reported in many cat species thus far, these cases are the most recent reports of death caused by the current outbreak. Currently, infection of wild mammals with HPAI appears to be rare with periodic detections throughout the United States. The main route of infection in mammals appears to be through infected prey items, specifically wild birds. The monitoring and testing of bird prey items is recommended where direct ingestion could be possible. Where direct ingestion is not possible, aerosolized transmission from birds has not yet been shown to be an effective mode of HPAI transmission.
Sample Type and Testing
In poultry prey items, the detection of HPAI can be monitored using the Applied Biosystems™ VetMAX™Gold AIV Detection Kit. The Thermo Fisher Scientific USDA-licensed avian influenza diagnostic kit is widely available for testing and provides a qualitative, one-step, real-time RT-PCR assay to detect AI virus (AIV) in RNA isolated from individual poultry oropharyngeal/tracheal swab samples.
In felines, it is recommended to collect antemortem nasal and oropharyngeal swabs for testing. In the event of mortality, nasal, oropharyngeal, tracheal, intestinal, rectal, and tissue swabs should be collected and placed in viral transport media and tissue samples should be stored refrigerated or frozen were fixed in 10% neutral
61
buffered formalin [265]. Samples can be submitted to the National Veterinary Services Laboratory (NVSL) in Ames, Iowa. Sample collection and storage should be in accordance with NVSL guidelines: https://www.aphis.usda.gov/animal_health/lab_info_services/downloads/WIAV0020.pdf.
Other Vector Borne Pathogens (Ehrlichia, Babesia, Rickettsia rickettsia, Hepatozoon felis, Anaplasma spp)
Various vector-borne pathogens have been identified in several mammalian species, with few causing disease [220]. Although they may not cause significant disease in certain animals, many vector-borne pathogens carry a zoonotic concern for humans, specifically A. phagocytophilum, Ehrlichia chaffeensis, Ehrlichia ewingii, and Rickettsia conorii, ricketsii, felis, and typhi [220] Anaplasma, Ehrlichia and Rickettsia genera are vector-born members of the Rickettsiales order infecting humans and many domestic and non-domestic animals worldwide [221]. Among these pathogens, little information is available on the pathogenesis of these agents in cats, with Anaplasma phagocytophilum being the most important pathogen for cats [220]. Table 14 shows current areas where these pathogens have been noted in cats around the world [220, 222]. Although many of these pathogens have not been detected in cats in the United States, the table only lists published reports, and it is likely these pathogens exist in countries that have not published the data or are not currently testing for the pathogens.
Most tick-borne illnesses will present with similar clinical signs so testing for the pathogen is the only way to discern the causative agent. Little information is available on the pathogenesis of these agents in cats; however, mild reductions in white blood cell counts, red blood cell counts, and liver values have been detected [223]. Other domestic cat cases with vector-borne infections, following experimental exposure, only showed a transient lymphopenia during 13 weeks of observation after tick infestation with a normal general appearance, appetite, body temperature and cell blood count otherwise reported [224]. Other clinical signs reported in cats can range from thrombocytopenia, joint swelling, fever, anorexia, anemia, dehydration, lethargy, epistaxis, and pain on abdominal palpation [225-231]. The main indication for diagnosis of a Rickettsial disease is a febrile cat exposed to ticks in an endemic area of the pathogen, especially outdoor or free-ranging felids that cannot be protected by ectoparasiticides. Certain antibiotics, such as doxycycline and similar compounds, are the recommended therapy for treating these infections [220]. Otherwise, supportive care is indicated.
Hepatozoonosis of domestic cats has been reported in many countries, however infection is often subclinical. Although the vector for domestic cats is unknown, it is suspected to be the same vector as documented in canines (Table 8) and the infection has been described in the same regions where canine infection is documented [222]. Transplacental transmission of H. felis has been suggested and could be an important route of transmission [232]. At this time, the pathogenesis has not been described in cats. Although the infection of myocardial and skeletal muscle is common, the infection does not lead to significant inflammatory reactions, so clinical signs are rarely noted [232-234]. No specific treatment is recommended but a case in a domestic cat has been successfully treated with imidocarb and doxycycline [222, 235]. Babesia is not currently reported in cats in the United States; however, although the vector is unknown, it is assumed to be transmitted by the same tick species as canine Babesiosis (Dermacentor reticulatus; Rhipicephalus sanguineus and Haemaphysalis leachi).
Although most of the vector-borne pathogens discussed previously do not produce clinical disease in cats or have not been documented in the United States, screening of these pathogens intermittently for their arrival provides important information regarding the spread of emerging pathogens and diseases. This testing becomes especially important when translocating individuals from a country with endemic disease to a country with no known detection of such disease, pathogen, or vector of causative agents.
62
Since 2019 Babesia spp. and Rickettsia spp. (PCR, gel electrophoresis) were not identified in free-ranging ocelots in Texas (n=22, A. Reeves, East Foundation, unpublished data) nor a select number of samples dating from 1985 to 2012 (n=9) while Hepatozoon spp. (5/22; 22.7%) was identified in ocelots in Texas and a select number of samples dating from 1985 to 2012 (2/9, 22.2%) [A. Reeves et al, unpublished data, in prep.]. Leishmania spp. (2/22; 9%) was identified in ocelot populations in Texas, however, it was not identified prior to 2021 [A. Reeves, East Foundation, unpublished data]. For some of the listed species, continuing to screen for emergence within the United States could provide information about emerging infectious pathogens. For others, the clinical significance remains unknown as these were one time captures and a snapshot in time of the individual’s health. It is important to note the pathogens that released individuals may be exposed to and screen for negative impacts to their health.
Table 14: Rickettsial and Vector-Borne Pathogens, Countries of Detection in Cats, and Known Vectors
Pathogen Genus and Species
Countries of Detection Vector
Genus Ehrlichia
E. canis Canada, USA, Brazil, Portugal
E. chaffeensis USA, Brazil
E. ewingii USA
Ehrlichia spp. Italy, USA, Kenia, France
Genus Anaplasma
A. phagocytophilum USA, Sweden, Finland, Poland, Switzerland, Germany, Italy, Spain
The brown dog tick (Rhipicephalus sanguineus), the lone star tick (Amblyomma americanum), and the blacklegged tick (Ixodes scapularis)
Ixodes ricinus
A. platys USA Brazil Rhipicephalus sanguineus (suspected)
A. platys-like Italy
A. bovis Japan
Genus Rickettsia
R. rickettsii USA
R. conorii Spain
R. massiliae Spain
Rickettsia spp. Italy
Hepatozoon spp. India, South Africa, Nigeria, USA, Brazil, Israel, Spain, France, Portugal, Italy,
Dermacentor andersoni (W) D. variabilis (MW and E)
Rhipicephalus sanguineus
Rhipicephalus sanguineus (brown dog tick) suspected; but unknown in felines
63
Turkey, Cape Verde archipelago, Cyprus, Switzerland, Austria
Sample Type and Testing [220- 222]
Both Anaplasma and Ehrlichia spp. give rise to cytoplasmic inclusion bodies: small elementary bodies (0.2-0.4 μm diameter) and larger reticulate bodies and morulae (up to 2-6 μm). These inclusion bodies are mostly found in neutrophils. Antibodies to rickettsial infections can be detected by IFA and ELISA; however, cross-reaction between other species can occur, antibody detection may not be possible early in infection, and some cats may not have yet seroconverted. Blood PCR analysis is a sensitive and specific method for confirming the diagnosis at the onset of acute clinical disease before starting therapy; however, this early infection will likely result in a negative antibody test. The use of genus-inclusive primers is suggested, followed by sequencing of any resulting PCR products to determine the infecting species. The diagnosis of hepatozoonosis in cats can be made by observation of the parasite gamonts in blood smears within the neutrophils and monocytes, meronts in muscles by histopathology and detection of parasite DNA in blood and tissue by PCR.
Quarantine
Any ocelots entering the Ocelot Conservation Facility from an AZA or similar institution or from the wild will have a pre-entry health examination, a quarantine period, and a post-quarantine examination prior to any future transfer and/or contact with other ocelots in the Ocelot Conservation Facility or in the wild. When possible, ocelots should be tested for disease prior to transfer to the Ocelot Conservation Facility. Disease testing may not be possible for transfer of ocelots originating from the wild, and supervising veterinarians at the Ocelot Conservation Facility will have discretion on whether to accept an ocelot to the Ocelot Conservation Facility based on its origin and available disease testing results. Upon an individual’s arrival to the Ocelot Conservation Facility, the minimum components of the quarantine examination (arrival exam) should include a physical examination under anesthesia, weight, blood collection (for use in CBC, blood chemistry, disease, and genetic testing), confirmation or insertion of a permanent identification applied (PIT tag), and fecal exam. Additionally, recommended components include a urinalysis, thoracic and abdominal radiographs, and ultrasound exam. After the arrival exam, quarantine is important to protect resident animals from contracting disease from outside sources. Quarantine at the Ocelot Conservation Facility must occur in an area separate from other ocelots. There must be proper sanitation plus footbaths and removable clothing for staff to use prior to working at quarantine facilities. Quarantine length will be determined based on individual circumstance and the results of health testing. The minimum length of time separated from the main population is 30 days, and quarantine may occur for up to 45 days, or longer. At the end of the quarantine period, the same required components of the prearrival exam should be repeated, with the exception of sample collection for genetic testing and placement of a PIT tag.
References
[1] American Association for Clinical Chemistry. Lab Tests Online: Complete Blood Count (CBC) 2015. Available at: https://labtestsonline.org/tests/complete-blood-count-cbc. Accessed April 1, 2021.
[2] Canadian Cancer Society: Complete blood count (CBC). 2021. Available at: https://www.cancer.ca/en/cancernformation/diagnosis-and-treatment/tests-and-procedures/complete-blood-count-cbc/?region=on. Accessed April 1, 2021.
[3] American Association for Clinical Chemistry. Lab Tests Online: Chemistry Panels. 2014. Available at: https://labtestsonline.org/tests/chemistry-panels. Accessed April 1, 2021.
64
[4] Canadian Cancer Society: Blood chemistry tests. 2021. Available at: https://www.cancer.ca/en/cancer-information/diagnosis-andtreatment/tests-and-procedures/blood-chemistry-tests/?region=on. Accessed April 1, 2021.
[5] Widmer CE, Matushima ER, de Azevedo FCC. 2016. Clinical Evaluation, Hematology, and Serum Chemistry of Ocelots (Leopardus pardalis) in the Atlantic Forest of Brazil. Journal of Wildlife Diseases. 52(4): 916-621.
[6] ISIS (International Species Information System). 2002. Ocelot (Leopardus pardalis) physiological reference ranges (both sexes, all ages). ISIS, Apple Valley, Minnesota.
[7] English AW, Lepherd EE. 1981. The haematology and serum biochemistry of wild fallow deer (Dama) in New South Wales. Journal of Wildlife Diseases 17(2): 289-95.
[8] Huber D, Kusak J, Zvorc Z, Rafaj RB. 1997. Effects of sex, age, capturing method, and season on serum chemistry values of brown bears in Croatia. Journal of Wildlife Diseases 33(4): 790-94.
[9] Broughton H, Govender D, Shikwambana P, Chappell P, Jolles AE. 2017. Bridging gaps between zoo and wildlife medicine: establishing normal reference ranges for free-ranging African lions (Panthera leo). Journal of Zoo Wildlife Medicine 48(2): 298-311.
[10] Moen R, Rasmussen JM, Burdett CL, Pelican KM. 2010. Hematology, serum chemistry, and body mass of free-ranging and captive Canada lynx in Minnesota. Journal of Wildlife Diseases 46(1): 13-22.
[11] Constable P, Hinchcliff K, Demma N, Callahan M, Dale B, Fox K, Adams L, Wack R, Kramer L. 1998. Serum biochemistry of captive and free-ranging gray wolves (Canis lupus). Journal of Zoo Wildlife Medicine 29(4): 435-440. PMID: 10065853.
[12] Couch CE, Movius MA, Jolles AE, Gorman ME, Rigas JD, Beechler BR, Serrano Ferron E. 2017. Serum biochemistry panels in African buffalo: Defining reference intervals and assessing variability across season, age and sex. PLos ONE. 12 (5).
[13] Borjesson DL, Christopher MM, Boyce WM. 2009. Biochemical and hematologic reference intervals for free-ranging desert bighorn sheep. Journal of Wildlife Diseases 36(2): 294-300.
[14] Bush M, Smith EE, Custer RS. 1981. Hematology and serum chemistry values for captive dorcas gazelles: variations with sex, age and health status. Journal of Wildlife Diseases 17(1): 135-43.
[15] Doornenbal H, Tong AK, Murray NL. 1988. Reference values of blood parameters in beef cattle of different ages and stages of lactation. Canadian Journal of Veterinary Research 52(1): 99.
[16] Marler RJ. 1975. Some hematologic and blood chemistry values in two herds of American bison in Kansas. Journal of Wildlife Diseases 11(1): 97-100.
[17] Dodham J. 2020. Anesthetic monitoring: blood pressure (direct pressure). Felis ISSN 2398-2950. Vetstream Ltd https://www.vetstream.com/treat/felis/freeform/anesthetic-monitoring-blood-pressure-(direct-pressure).
[18] Robertson SA, Gogolski SM, Pascoe P, Shafford HL, Sager J, Griffenhagen GM. 2018. AAFP Feline Anesthesia Guidelines. Journal of Feline Medicine and Surgery 20: 602-634.
[19] Barnette C. 2020. Veterinary Anesthesia Monitoring: Cheat Sheet and FAQs. Dispomed.
[20] Marti I, Ryser-Degiorgis MP. (2018) A tooth wear scoring scheme for age estimation of the Eurasian lynx (Lynx lynx) under field conditions. European Journal of Wildlife Research 64: 37.
[21] Aiello SE, Moses MA, editors. 2012a. The Merck veterinary manual online. Hematologic reference ranges. Merck, Sharp, and Dohme Corp. New York, New York.
http://www.merckvetmanual.com/mvm/appendixes/reference_guides/hematologic_reference_ranges.html. Accessed December 2021.
[22] Aiello SE, Moses MA, editors. 2012b. The Merck veterinary manual online. Serum biochemical reference ranges. Merck, Sharp, and Dohme Corp. New York, New York.
http://www.merckvetmanual.com/mvm/appendixes/reference_guides/serum_biochemical_reference_ranges.html. Accessed December 2021
[23] DeNicola DB, Cowell RL, Frye M. 2014. Urine Sediment Guide. IDEXX Laboratories, Inc. 09-80958-00. https://www.idexx.com/files/urine-sediment-guide.pdf
[24] ECLINPATH. Cornell University College of Veterinary Medicine. 2020. https://eclinpath.com/urinalysis/.
[25] Chung PH. 2020. Urinalysis and Urine Culture in Merck Manual Consumer Version.
https://www.merckmanuals.com/home/kidney-and-urinary-tract-disorders/diagnosis-of-kidney-and-urinary-tract-disorders/urinalysisand-urine-culture
[26] Callealta I, Ganswindt A, Lueders I. 2020. Reproductive cycle stage assessment using vaginal cytology evaluation in African lions (Panthera leo). Animal Reproduction Science 213: 106260
[27] Raju K. 2016. Evolution of Pap Stain. Biomedpress 3(2): 490-500.
[28] Kutzler, MA, Yeager, AE, Mohammed, HO and Meyers-Wallen, VN. 2003. Accuracy of canine parturition date prediction using fetal measurements obtained by ultrasonography. Theriogenology 60: 1309-1317.
[29] Nyland, TG and Mattoon, JS. 1995. Veterinary diagnostic ultrasound. W.B. Saunders Company, Philadelphia.
[30] O’Brien, R and Barr, F (Eds) 2009. BSAVA Manual of canine and feline abdominal imaging. British Small Animal Veterinary Association, Gloucester.
[31] Mondolfi E. 1986. Notes on the biology and status of the small wild cats in Venezuela. In: Miller SD, Everett DD (Eds.). Cats of the world: biology, conservation and management. Washington, D.C.: National Wildlife Federation. pp. 85-124.
[32] Goodrowe KL, Howard JG, Schmidt PM, Wildt DE. 1989. Reproductive biology of the domestic cat with special reference to endocrinology, sperm function and in-vitro fertilization. Journal of Reproduction and Fertility Supplement 39: 73-90. PMID: 2695644
[33] Wildt DE, Chan SYW, Seager SWJ, Chakrabory PK. 1981. Ovarian activity, circulating hormones, and sexual behavior in the cat. I. Relationships during the coitus-induced luteal phase and the estrous period without mating. Biology of Reproduction 25: 15–28.
65
[34] Schmidt AM, Nadal LA, Schmidt MJ, Beamer NB. 1979. Serum concentrations of oestradiol and progesterone during the normal oestrous cycle and early pregnancy in the lion (Panthera leo). Journal of Reproduction and Fertility 57: 267-72.
[35] Schmidt PM, Chakraborty PK, Wildt DE. 1983. Ovarian activity, circulating hormones and sexual behavior in the cat. II. Relationships during pregnancy, parturition, lactation and the postpartum estrus. Biology of Reproduction 28(3): 657–71.
[36] Schmidt AM, Hess DL, Schmidt MJ, Lewis CR. 1993. Serum concentrations of oestradiol and progesterone and frequency of sexual behavious during the normal oestrous cycle in the snow leopard (Panthera uncia). Journal of Reproduction and Fertility 98: 91-5.
[37] Bonney RC, Moore HDM, Jones D. 1981. Plasma concentrations of oestradiol-17β and progesterone, and laparoscopic observations of the ovary in the puma (Felis concolor) during oestrus, pseudopregnancy and pregnancy. Journal of Reproduction and Fertility. 63: 523-31.
[38] Seal US, Plotka ED, Smith JD, Wright FH, Reindl N, Taylor RS, Seal MF. 1985. Immunoreactive luteinizing hormone, estradiol, progesterone, testosterone, and androstenedione levels during the breeding season and anestrus in Siberian tigers. Biology of Reproduction 32: 361-8.
[39] Schramm RD, Briggs MB, Reeves JJ. 1995. Spontaneous and induced ovulation in the lion (Panthera leo). Zoo Biology 13: 3017.
[40] Brown JL. 2006. Comparative endocrinology of domestic and nondomestic felids. Theriogenology. 66:25-36.
[41] Lasley BL, Kirkpatrick JF. 1991. Monitoring ovarian function in captive and free-ranging wildlife by means of urinary and fecal steroids. JJournal of Zoo and Wildlife Medicine. 22: 23-31.
[42] Shille VM, Wing AE, Lasley BL, Banks JA. 1984. Excretion of radiolabeled estradiol in the cat (Felis catus, L): a preliminary report. Zoo Biology 3: 201-209.
[43] Shille VM, Haggerty MA, Shackleton C, Lasley BL. 1990. Metabolites of estradiol in serum, bile, intestine and feces of the domestic cat (Felis catus). Theriogenology. 34: 77994.
[44] Brown JL, Wasser SK, Wildt DE, Graham LH. 1994. Comparative aspects of steroid hormone metabolism and ovarian activity in felids, measured noninvasively in feces. Biology of Reproduction 51(4): 776–86.
[45] Moreira N, Monteiro-Filho ELA, Moraes W, Swanson WF, Graham LH, Pasquali OL, Gomes MLF, Morais RN, Wildt DE, Brown JL. 2001. Reproductive steroid hormones and ovarian activity in felids of the Leopardus genus. Zoo Biology 20: 103-16.
[46] Braun BC, Frank A, Dehnhard M, Voigt CC, Vargas A, Goritz F, Jewkenow K. 2009. Pregnancy diagnosis in urine of Iberian lynx (Lynx pardinus). Theriogenology 71:754-761.
[47] Brown JL, Graham LH, Wu J, Collins D, Swanson WF. 2002. Reproductive endocrine responses to photoperiod and exogenous gonadotropins in the Pallas’ cat (Otocolobus manul). Zoo Biology 21: 347-64.
[48] Brown JL, Wildt DE, Graham LH, Byers AP, Barrett S, Howard JG. 1995. Comparison of natural versus chorionic gonadotropininduced ovarian responses in the clouded leopard (Neofelis nebulosa) assessed by fecal steroids. Biology of Reproduction 53: 93-102.
[49] Brown JL, Wildt DE, Wielebnowski N, Goodrowe KL, Graham LH, Wells S, Howard JG. 1996. Reproductive activity in captive female cheetahs (Acinonyx jubatus) assessed by faecal steroids. Journal of Reproduction and Fertility 106: 337-46.
[50] Johnston SD, Root Kustritz MV, Olson PNS. 2001. Vaginal Cytology. In: Johnston SD, Root Kustritz MV, Olson PNS (Eds.), Canine and Feline Theriogenology, first ed. Saunders, Philadelphia. 32-40.
[51] Ammersbach M, Bienzle D. 2011. Methods for assessing feline immunodeficiency virus infection, infectivity and purification. Veterinary Immunology and Immunopathology 143(3): 202–214.
[52] Little S, Levy J, Hartmann K, Hofmann-Lehmann R, Hosie M, Olah G, Denis K. 2020. AAFP Feline Retrovirus Testing and Management Guidelines. Journal of Feline Medicine and Surgery 22(1): 5–30.
[53] Sacristán I, Acuña F, Aguilar E, García S, López MJ, Cabello J, Hildalgo-Hermoso E, Sanderson J, Terio KA, Barrs V, Beatty J, Johnson WE, Millán J, Poulin E, Napolitano C 2021. Cross-species transmission of retroviruses among domestic and wild felids in human-occupied landscapes in Chile. Evolutionary Applications 14: 1070–1082.
[54] Franklin SP, Kays RW, Moreno R, TerWee JA, Troyer JL, VandeWoude S. 2008. Ocelots on Barro Colorado Island Are Infected with Feline Immunodeficiency Virus but Not Other Common Feline and Canine Viruses.Journal of Wildlife Diseases 44(3): 760–765.
[55] Gomerčić T, Perharić M, Kusak J, Slijepčević V, Starešina V, Stevanović V, Mojcec V, Toplicanec I, Sindičić M. 2021. A retroviral survey of endangered Eurasian lynx (Lynx lynx) from Croatia. Veterinarski arhiv. 91(1): 65-71.
[56] Filoni C, Adania CH, Durigon EL, Catão-Dias JL. 2003. Serosurvey for feline leukemia virus and lentiviruses in captive small neotropic felids in São Paulo state, Brazil. Journal of Zoo and Wildlife Medicine 34(1): 65-68.
[57] Zhang Z, Gu Q, Marino D, Lee KL, Kong IK, Häussinger D, Münk C. 2018. Feline APOBEC3s, Barriers to Cross-Species Transmission of FIV? Viruses 10(4): 186.
[58] Troyer JL, VandeWoude S, Pecon-Slattery J, McIntosh C, Franklin S, Antunes A, Johnson W, O’Brien SJ. 2008. FIV crossspecies transmission: an evolutionary prospective. Veterinary Immunology and Immunopathology 123(1-2):159-166.
[59] VandeWoude S, Troyer J, Poss M. 2010. Restrictions to cross-species transmission of lentiviral infection gleaned from studies of FIV. Veterinary Immunology and Immunopathology 134, 25–32.
[60] Tandon R, Cattori V, Willi B, Lutz H, Hofmann-Lehmann R. 2008. Quantification of endogenous and exogenous feline leukemia virus sequences by real-time PCR assays. Veterinary Immunology and Immunopathology. 123(1-2): 129
[61] Hartmann K. 2012. Clinical aspects of feline retroviruses: a review. Viruses. 4(11): 2684-710.
133.
66
–
[62] Burling AN, Levy JK, Scott HM, Crandall MM, Tucker SJ, Wood EG, Foster JD. 2017. Seroprevalences of feline leukemia virus and feline immunodeficiency virus infection in cats in the United States and Canada and risk factors for seropositivity. Journal of the American Veterinary Medical Association 251: 187–194.
[63] Cunningham MW, Brown MA, Shindle DB, Terrell SP, Hayes KA, Ferree BC, McBride RT, Blankenship EL, Jansen D, Citino SB, Roelke ME, Kiltie RA, Troyer JL, O’Brien SJ. 2008. Epizootiology and management of feline leukemia virus in the Florida puma. J. Wildl. Dis. 44: 537-552.
[64] Kennedy-Stoskopf S. 1999. Emerging viral infections in large cats. In: Zoo and Wild Animal Medicine (Fowler, M. E., R. E. Miller, Eds.), W. B. Saunders, Philadelphia. 401-410
[65] Leutenegger CM, Hofmann-Lehmann R, Riols C, Liberek M, Worel G, Lups P, Fehr D, Hartmann M, Weilenmann P, Lutz H. 1999. Viral infections in free-living populations of the European wildcat. J. Wildl. Dis. 35: 678–686.
[66] Brown MA, Cunningham MW, Roca AL, Troyer JL, Johnson WE, O’Brien SJ. 2008. Genetic characterization of feline leukemia virus from Florida panthers. Emerging Infectious Diseases. 14 (2). Gale Academic OneFile. link.gale.com/apps/doc/A175285235/AONE?u=googlescholar&sid=bookmark-AONE&xid=42d74442.
[67] Chiu ES, Kraberger S, Cunningham M, Cusak L, ROelke M, VandeWoude S. 2019. Multiple Introductions of Docestic cat feline leukemia virus in endangered Florida panthers. Emerging Infectious Diseases 25 (1) 92-101.
[68] Meli ML, Cattori V, Martínez F, López G, Vargas A, Palomares F, López-Bao JV, Hofmann-Lehmann R, Lutz Hans. 2010. Feline leukemia virus infection: a threat for the survival of the critically endangered Iberian lynx (Lynx pardinus). Veterinary Immunology and Immunopathology 134(1-2):61-67.
[69] Lamberski N. 2015. Felidae. Fowler's Zoo and Wild Animal Medicine. 8: 467-476.
[70] Levy JK, Crawford PC, Tucker SJ. 2017. Performance of 4 Point-of-Care Screening Tests for Feline Leukemia Virus and Feline Immunodeficiency Virus. Journal of Veterinary Internal Medicine 31: 521-526.
[71] Tandon R, Cattori V, Gomes-Keller GH, Meli ML, Golder MC, Lutz H, Hoffmann-Lehmann R. 2005. Quantitation of feline leukemia virus viral and proviral loads by TaqMan real-time polymerase chain reaction. Journal of Virological Methods 130: 124-132.
[72] Hoffmann-Lehmann R, Huder JB, Gruber S, Boretti F, Sigrist B, Lutz H. 2001. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. Journal of General Virology 82: 1589-1596.
[73] Attias M, Teixeira D, Benchimol M, Vommaro R, Crepaldi P, De Souza W. 2020. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites & Vectors 13(1): 588–588.
[74] Robert-Gangneux F, Darde M. 2012. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clinical Microbiology Reviews 25(2): 264–296.
[75] Hill D, Dubey J. 2002. Toxoplasma gondii: transmission, diagnosis, and prevention. Clinical Microbiology and Infection 8(10): 634–640.
[76] Al-Malki ES. 2021. Toxoplasmosis: stages of the protozoan life cycle and risk assessment in humans and animals for an enhanced awareness and an improved socio-economic status. Saudi Journal of Biological Science. 28(1): 962–969.
[77] Brister J, Shell L. 2019. Vincyclopedia of Diseases: Toxoplasmosis. Vincyclopedia of Diseases https://www.vin.com/Members/Associate/Associate.plx?from=GetDzInfo&DiseaseId=814&pid=607.
[78] Charlesworth D, Willis JH. 2009. The genetics of inbreeding depression. Nature Reviews: Genetics 10(11): 783–796.
[79] Spielman D, Brook BW, Briscoe DA, Frankham R. 2004. Does Inbreeding and Loss of Genetic Diversity Decrease Disease Resistance? Conservation Genetics 5(4): 439–448.
[80] Dubey J, Quinn W, Weinandy D. 1987. Fatal neonatal toxoplasmosis in a bobcat (Lynx rufus). Journal of Wildlife Diseases 23(2): 324–327.
[81] Desmonts G, Couvreur J. 1974. Congenital toxoplasmosis. A prospective study of 378 pregnancies. The New England Journal of Medicine 90(20): 1110–1116.
[82] Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. 1999. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet (London, England). 353(9167): 1829
1833.
[83] Delair E, Latkany P, Noble AG, Rabiah P, McLeod R, Brézin A. 2011. Clinical manifestations of ocular toxoplasmosis. Ocular Immunology and Inflammation. 19(2): 91–102.
[84] Remington JS, McLeod R, Thulliez P, and Desmonts G. 2001. Toxoplasmosis. In: Remington JS and Klein J (ed), Infectious diseases of the fetus and newborn infant, 5th ed. WB Saunders, Philadelphia, PA. 205–346.
[85] Roberts F, Mets MB, Ferguson DJ, O'Grady R, O'Grady C, Thulliez P, Brézin AP, McLeod R 2001. Histopathological features of ocular toxoplasmosis in the fetus and infant. Archives of ophthalmology. (Chicago, Ill. : 1960). 119(1): 51–58. PMID: 11146726
[86] Verma SK, Minicucci L, Murphy D, Carstensen M, Humpal C, Wolf P, Calero-Bernal R, Cerqueira-Cézar CK, Kwok OC, Su C, Hill D, Dubey JP. 2016. Antibody Detection and Molecular Characterization of Toxoplasma gondii from Bobcats (Lynx rufus), Domestic Cats (Felis catus), and Wildlife from Minnesota, USA. The Journal of Eukaryotic Microbiology 63(5): 567–571.
[87] Verma SK, Sweeny AR, Lovallo MJ, Calero-Bernal R, Kwok OC, Jiang T, Su C, Grigg ME, Dubey JP. 2017. Seroprevalence, isolation and co-infection of multiple Toxoplasma gondii strains in individual bobcats (Lynx rufus) from Mississippi, USA International Journal for Parasitology 47(5): 297–303.
[88] Humphreys JG. 2006. Seroprevalence of Antibodies to Toxoplasma gondii in the Pennsylvania Bobcat (Lynx rufus rufus). Journal of Wildlife Diseases 42(1): 188–191.
67
–
[89] Rendón-Franco E, Caso-Aguilar A, Jiménez-Sánchez NG, Hernandez-Jauregui DMB, Sandoval-Sánchez AL, Zepeda-López HM. 2012. Prevalence of anti-Toxoplasma gondii antibody in free-ranging ocelots (Leopardus pardalis) from Tamaulipas, Mexico. Journal of Wildlife Diseases 48(3): 829–831.
[90] Desmonts G, Remington JS. 1980. Direct agglutination test for diagnosis of Toxoplasma infection: Method for increasing sensitivity and specificity. Journal of Clinical Microbiology 11: 562-568.
[91] Dubey JP, Desmonts G. 1987. Serological responses of equids fed Toxoplasma gondii oocysts. Equine Veterinary Journal 19:337-339.
[92] Jansen A M, Roque ALR. 2010. Domestic and wild mammalian reservoir. In: Telleria J, Tibayrenc M, editors. American Trypanosomiasis Chagas Disease-one hundred years of research. London: Elsevier. 249-276.
[93] Curtis-Robles R, Hamer SA, Lane S, Levy MZ, Hamer GL. 2018. Bionomics and spatial distribution of triatomine vectors of Trypanosoma cruzi in Texas and other southern states. USA. American Journal of Tropical Medicine and Hygiene 98: 113-121.
[94] Gurtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. 2007. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwest Argentina. Parasitology 134: 69-82.
[95] Enriquez GF, Cardinal MV, Orozco MM, Wirth S, Schijman AG, Gurtler RE. 2013. Detection of Trypanosoma cruzi infection in naturally infected dogs and cats using serological, parasitological, and molecular methods. Acta Tropica 126(2): 11-217.
[96] Enriquez GF, Bua J, Orozco MM, Wirth S, Schijman AG, Gurtler RE, Cardinal MV. 2014. High levels of Trypanosoma cruzi DNA determined by qPCR and infectiousness to Triatoma infestans support dogs and cats are major sources of parasites from domestic transmission. Infectection, Genetics and Evolution 25: 36-43.
[97] Jiminez-Coello M, Acosta-Viana KY, Guzman-Marin E, Gomez-Rios A, Ortega-Pacheco A. 2012. Epidemiological survey of Trypanosoma cruzi infection in domestic owned cats from the tropical southeast of Mexico. Zoonoses and Public Health 59: 102-109.
[98] Zecca IB, Hodo CL, Slack S, Auckland L, Rodgers S, Killets KC, Saunders AB, Hamer SA. 2020. Prevalence of Trypanosoma cruzi infection and associated histologic findings in domestic cats (Felis catus). Veterinary Parasitology 278: 109014.
[99] Rocha FL, Roque ALR, Saab de Lima J, Cheida CC, Lemos FG, Cavalcanti de Azevedo F, Arrais RC, Bilac D, Herrera HM, Mourao G, Jansen AM. 2013a. Trypanosoma cruzi infection in neotropical wild carnivores (mammalia : carnivora): at the top of the T. cruzi transmission chain. PLOS ONE 8(7): e67463.
[100] Rocha FL, Roque AL, Arrais RC, Santos JP, Lima VD, Chagas Xavier SC, Cordeir-Estrela P, D’Andrea PS, Jansen AM. 2013b. Trypanosoma cruzi TcI and TcII transmission among wild carnivores, small mammals and dogs in a conservation unit and surrounding areas, Brazil. Parasitology 140: 160-170.
[101] Herrera HM, Rocha FL, Lisboa CV, Rademaker V, Mourao GM, Jansen AM. 2011. Food web connections and the transmission cycles of Trypanosoma cruzi and Trypanosoma evansi (Kinetoplastida, Trypanosomatidae) in the Pantanal region, Brazil.
Transactions of the Royal Society of Tropical Medicine and Hygiene 105: 380-387.
[102] Zecca IB, Hodo CL, Swarts HM, DeMaar TW, Snowden KF, Prestridge HL, Light JE, Hamer SA. 2021. Trypanosoma cruzi and incidental Sarcocystis spp. in endangered ocelots (Leopardus pardalis) of south Texas, USA. Journal of Wildlife Diseases. 57(3): 667671.
[103] Andre MR, Adania CH, Machado RZ, Allegretti SM, Felippe PAN, Silva KF, Nakaghi ACH, Dagnone AS. 2009. Molecular Detection of Cytauxzoon Spp. in Asymptomatic Brazilian Wild Captive Felids. Journal of Wildlife Diseases 45(1): 234–237
[104] Lewis K. 2011. Cytauxzoon felis: An emerging feline pathogen and potential therapy. University of Missouri- Columbia, Ann Arbor.
[105] Meinkoth J, Kocan AA, Whitworth L, Murphy G, Fox JC, Woods JP. 2000. Cats Surviving Natural Infection with Cytauxzoon Felis: 18 Cases (1997–1998). Journal of Veterinary Internal Medicine 14(5): 521–525.
[106] Gallusová M, Jirsová D, Mihalca AD, Gherman CM, D'Amico G, Qablan MA, Modrý D. 2016. Cytauxzoon Infections in Wild Felids from Carpathian-Danubian-Pontic Space: Further Evidence for a Different Cytauxzoon Species in European Felids. The Journal of Parasitology 102(3): 377–380.
[107] Drechsler Y, Alcaraz A, Bossong FJ, Collisson EW, Diniz PPVP. 2011. Feline coronavirus in multicat environments. Veterinary Clinics of North America: Small Animal Practice 41(6): 1133-69.
[108] Pedersen NC. 2009. A review of feline infectious peritonitis virus infection: 1963–2008. Journal of Feline Medicine and Surgery 11(4):2 25–258
[109] Pedersen NC. 1976. Serologic studies of naturally occurring feline infectious peritonitis. American Journal of Veterinary Research 37(12): 1449
1453. PMID: 793459
[110] Cave TA, Golder MC, Simpson J. 2004. Risk factors for feline coronavirus seropositivity in cats relinquished to a UK rescue charity. Journal of Feline Medicine and Surgery 6(2): 53–58.
[111] Luria BJ, Levy JK, Lappin MR. 2004. Prevalence of infectious diseases in feral cats in Northern Florida. Journal of Feline Medicine and Surgery 6(5): 287–296.
[112] Pedersen NC, Allen CE, Lyons LA. 2008. Pathogenesis of feline enteric coronavirus infection. Journal of Feline Medicine and Surgery 10(6): 529–541.
[113] Addie DD, Jarrett O. 2001. Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Veterinary Record 148(21): 649–653.
[114] Addie DD, Paltrinieri S, Pedersen NC. 2004. Recommendations from workshops of the second international feline coronavirus/feline infectious peritonitis symposium. Journal of Feline Medicine and Surgery 6(2): 125–130.
68
–
[115] Han JI, Kang SY, Yoon KJ, Na KJ. 2014. Nucleic acid-based differential diagnostic assays for feline coronavirus. Journal of Virological Methods. 208: 21-25.
[116] Pedersen NC, Black JW, Boyle JF 1984a. Pathogenic differences between various feline coronavirus isolates. Advances in Experimental Medicine and Biology 173: 365–380.
[117] Pedersen NC, Evermann JF, McKeirnan AJ. 1984b. Pathogenicity studies of feline coronavirus isolates 79-1146 and 791683. American Journal of Veterinary Research 45(12): 2580–2585. PMID: 6084432
[118] Pedersen NC. 1987. Virologic and immunologic aspects of feline infectious peritonitis virus infection. Adv Exp Med Biol. 218:529–550.
[119] Chang HW, de Groot RJ, Egberink HF. 2010. Feline infectious peritonitis: insights into feline coronavirus pathobiogenesis and epidemiology based on genetic analysis of the viral 3c gene. J Gen Virol. 91(Pt 2): 415–420.
[120] Poland AM, Vennema H, Foley JE 1996. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. J Clin Microbiol. 34(12): 3180–3184.
[121] Vennema H, Poland A, Foley J. 1998. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virolog. 243(1): 150–157.
[122] Pedersen NC, Floyd K. 1985. Experimental studies with three new strains of feline infectious peritonitis virus FIPV-UCD2, FIPV-UCD3, and FIPV-UCD4. Compendium Continuing Education Practicing Veterinarians. 7:1001–1011.
[123] Sharif S, Arshad SS, Hair-Bejo M, Omar AR, Zeenathul NA, Hafidz MA. 2009. Prevalence of feline coronavirus in two cat populations in Malaysia. Journal of Feline Medicine and Surgery, 11 (12): 1031-1034.
[124] Addie DD, Toth S, Murray GD. 1995. Risk of feline infectious peritonitis in cats naturally infected with feline coronavirus. American Journal of Veterinary Research 56(4): 429–434. PMID: 7785816
[125] Foley JE, Poland A, Carlson J. 1997. Risk factors for feline infectious peritonitis among cats in multiple-cat environments with endemic feline enteric coronavirus. Journal of the American Veterinary Medical Association 210(9): 1313–1318. PMID: 9143536
[126] Addie DD, Jarrett O. 1992. A study of naturally occurring feline coronavirus infections in kittens. Veterinary Record 130(7): 133–137.
[127] Herrewegh AA, Mahler M, Hedrich HJ. 1997. Persistence and evolution of feline coronavirus in a closed cat-breeding colony. Virology 234(2): 349–363.
[128] Pesteanu-Somogyi LD, Radzai C, Pressler BM. 2006. Prevalence of feline infectious peritonitis in specific cat breeds. Journal of Feline Medicine and Surgery 8(1): 1–5.
[129] Foley JE, Pedersen NC. 1996. The inheritance of susceptibility to feline infectious peritonitis in purebreed catteries. Veterinary Practitioner 24(1): 14-22.
[130] Pedersen NC. 2009. A review of feline infectious peritonitis virus infection: 1963–2008. Journal of Feline Medicine and Surgery 11(4): 225–258.
[131] Rohrbach BW, Legendre AM, Baldwin CA. 2001. Epidemiology of feline infectious peritonitis among cats examined at veterinary medical teaching hospitals. Journal of the American Veterinary Medical Association 218(7): 1111–1115.
[132] Hardy WD, Jr. 1982. Immunopathology induced by the feline leukemia virus. Seminars in Immunopathology 5(1): 75–106.
[133] Cotter SM, Hardy WD, Jr, Essex M. 1975. Association of feline leukemia virus with lymphosarcoma and other disorders in the cat. Journal of the American Veterinary Medical Association 166(5): 449–454. PMID: 163223
[134] Addie D, Belak S, Boucraut-Baralon C. 2009. Feline infectious peritonitis: ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery 11(7): 594–604.
[135] Hartmann K. 2005. Feline infectious peritonitis. Veterinary Clinics of North America: Small Animal Practice 35(1): 39-79
[136] Kipar A, May H, Menger S. 2005. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Veterinary Pathology 42(3): 321–330.
[137] Andrew SE. 2000. Feline infectious peritonitis. Veterinary Clinics of North America: Small Animal Practice 30(5): 987–1000.
[138] Kennedy M, Citino S, McNabb AH, Moffatt AS, Gertz K, Kania S. 2002. Detection of Feline Coronavirus in Captive Felidae in the USA. Journal of Veterinary Diagnostic Investigation 14 (6): 520-2.
[139] Stout AE, André NM, Whittaker GR. 2021. Feline coronavirus and feline infectious peritonitis in nondomestic felid species. Journal of Zoo and Wildlife Medicine 52(1): 14-27.
[140] Juan-Sallés C, Domingo M, Herráez P, Fernandez A, Segalés J, Fernández J. 1998. Feline infectious peritonitis in servals (Felis serval). Veterinary Record.
[141] Stephenson N, Swift P, Moeller R, Worth S, Foley J. 2013. Feline Infectious Peritonitis in a Mountain Lion (Puma concolor), California, USA. Journal of Wildlife Diseases 49(2): 408-12.
[142] Kennedy M, Kania S, Stylianides E, Bertschinger H, Keet D, van Vuuren M. 2003. Detection of feline coronavirus infection in southern African nondomestic felids. Journal of Wildlife Diseasess 39(3): 529-35.
[143] Van Rensburg IB, Silkstone M. 1984. Concomitant feline infectious peritonitis and toxoplasmosis in a cheetah (Acinonyx jubatus). Journal of the South African Veterinary Association 55(4): 205-207
[144] Evermann J, Heeney J, Roelke M, McKeirnan A, O’Brien S. 1988. Biological and pathological consequences of feline infectious peritonitis virus infection in the cheetah. Archives of Virology 102(3-4): 155-171.
[145] Horhogea C, Floristean V, Lazăr M, Creţu C, Solcan C. 2017. Immunohistochemical Methods to Diagnose Atraumatic Spleen Rupture in Feline Infectious Peritonitis of Tiger (Panthera tigris). Revisita de Chimie-Bucharest-Original Edition 68(5): 1055.
69
[146] Mwase M, Shimada K, Mumba C, Yabe J, Squarre D, Madarame H. 2015. Positive immunolabelling for feline infectious peritonitis in an African lion (Panthera leo) with bilateral panuveitis. Journal of Comparative Pathology 152 (2): 265-268.
[147] McOrist S, Boid R, Jones T, Easterbee N, Hubbard A, Jarrett O. 1991. Some viral and protozool diseases in the European wildcat (Felis silvestris). Journal of Wildlife Diseases 27(4): 693-6.
[148] Watt N, Macintyre N, McOrist S. 1993. An extended outbreak of infectious peritonitis in a closed colony of European wildcats (Felis silvestris). Journal of Comparative Pathology 108(1): 73-9.
[149] Schmitt AC, Reishak D, Clavac CL, Monforte CHL, Couto FT, Almeida BPF, Santos DGG, Souza L, Alves C, Vecchi K. 2003. Infection by feline leukemia virus and feline infectious peritonitis in a wild and captive felid from the pantanal region of mato grosso. Acta Scientiae Veterinariae 31(3): 185
188.
[150] Filoni C, Catão-Dias JL, Bay G, Durigon EL, Jorge RSP, Lutz H, Hoffman-Lehmann R. 2006. First evidence of feline Herpesvirus, Calicivirus, Parvovirus, and Ehrlichia exposure in Brazilian free-ranging felids. Journal of Wildlife Diseases 42(2): 470477.
[151] Cornell University: Baker Institute for Animal Health. 2016. “Feline Calicivirus” https://www.vet.cornell.edu/departmentscenters-and-institutes/baker-institute/our-research/feline-calicivirus
[152] Radford AD, Sommerville L, Ryvar R, Cox MB, Johnson DR, Dawson S, Gaskell RM. 2001. Endemic infection of a cat colony with a feline calicivirus closely related to an isolate used in live attenuated vaccines. Vaccine 19(31): 4358-4362.
[153] Radford AD, Coyne KP, Dawson S, Porter CJ, Gaskell RM. 2007. Feline calicivirus. Veterinary Research 38(2): 319-335.
[154] Henzel A, Lovato LT, Weiblen R. 2015. Epidemiological status of felid herpesvirus type-1 and feline calicivirus infections in Brazil. Cienca. Rural: Microbiology 45(6).
[155] Ellis TM. 1981. Jaundice in a Siamese cat with in utero feline calicivirus infection. Australian Veterinary Journal 57 (8): 383385
[156] Radford AD, Addie D, Belák S, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, Hartmann K, Hosie MJ, Lloret A, Lutz H, Marsilio F, Pennisi MG, Thiry E, Truyen U, Horzinek MC. 2009. Feline calicivirus infection ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery 11(7): 556-564.
[157] Henzel A, Brum MCS, Lovato LT, Widblen R. 2013. Serological Survey of Feline Calicivirus and Felid herpesvirus in Rio Grande do Sul, Brazil. Acta Scientiae Veterinariae 41:1-6.
[158] Ostrowski S, Vuuren MV, Menain DM, Durand A. 2003. A serologic survey of wild felids from central west Saudi Arabia. Journal of Wildlife Diseases 39: 696–701.
[159] Thalwitzer S, Wachter B, Robert N, Wibbelt G, Müller T, Lonzer J, Meli ML, Bay G, Hofer H, Lutz H. 2010. Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian farmland. Clinical and Vaccine Immunology 17:232–238.
[160] Hoffman-Lehmann R, Fehr D, Grob M, Elgizoli M, Packer C, Martenson JS, O’Brien SJ, Lutz H. 1996. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infection in free-ranging lions in east Africa. Clinical and Diagnostic Lab Immunology 3(5): 554-562.
[161] Fiorello CV, Noss AJ, Deem SL, Maffei L, Dubovi EJ. 2007. Serosurvey of small carnivores in the Bolivian Chaco. Journal of Wildlife Diseases 43(3): 551-557.
[162] Furtado MM, de Ramos Filho JD, Scheffer KC, Coelho CJ, Cruz PS, Ikuta CY, de Almeida Jácoma AT, de Oliviera Porfírio GE, Silveira L, Sollmann R, Tôrres NM, Neto JSF. 2013. Serosurvey for selected viral infections in free-ranging jaguars (Panthera onca) and domestic carnivores in Brazilian cerrado, pantanal and Amazon. Journal of Wildlife Diseases 49: 510–521.
[163] Gaskell R, Dawson S, Radford A, Thiry E. 2007. Feline herpesvirus. Veterinary Research 38: 337-354.
[164] ABCD Feline Herpesvirus infection. 2017. Journal of Feline Medicine and Surgery 17: 570-582
[165] Gaskell R, Dawson S, Radford A. 2006. Feline respiratory disease. Greene CE, ed. Infectious diseases of the dog and cat. Missouri: WB Saunders, 145-154.
[166] Gaskell RM, Povey RC. 1982. Transmission of feline viral rhinotracheitis. Veterinary Record 111: 359-362.
[167] Westermeyer HD, Thomasy SM, Kado-Fong H, Maggs DJ. 2009. Assessment of viremia associated with experimental primary feline herpesvirus infection or presumed herpetic recrudescence in cats. American Journal of Veterinary Research 70: 99-104.
[168] Johnson RP, Povey RC. 1985. Vaccination against feline viral rhinotracheitis in kittens with maternally derived feline viral rhinotracheitis antibodies. Journal of the American Veterinary Medical Association 186:149-152. PMID: 2982774.
[169] Dawson S, Willoughby K, Gaskell RM, Woog G, Chalmers WCK. 2001. A field trial to assess the effect of vaccination against feline herpesvirus, feline calicivirus and feline panleukopenia virus in 6-week-old kittens. Journal of Feline Medicine and Surgergy 3: 17-22.
[170] Gaskell RM, Povey RC. 1979. The dose response of cats to experimental infection with feline viral rhinotracheitis virus Journal of Comparative Pathology 89: 179-191.
[171] Jas D, Aeberle C, Lacombe V, Guiot AL, Poulet H. 2009. Onset of immunity in kittens after vaccination with a non-adjuvanted vaccine against feline panleukopenia, feline calicivirus and feline herpesvirus. The Veterinary Journal 182: 86–93.
[172] Munson L, Marker L, Dubovi E, Spencer JA, Evermann JF, O'Brien SJ. 2004. Serosurvey of viral infections in free-ranging Namibian cheetahs (Acinonyx jubatus). Journal of Wildlife Diseases 40(1): 23–31.
70
–
[173] Thalwitzer S, Watcher B, Robert N, Wibbelt G, Müller T, Lonzer J, Meli ML, Bay G, Hofer H, Lutz H. 2010. Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian farmland. Clinical Vaccine Immunology 17(2): 232–238
[174] Van Vuuren M, Goosen T, Rogers P. 1999. Feline herpesvirus infection in a group of semi-captive cheetahs: case report. Journal of the South African Veterinary Association 70(3): 123–134.
[175] Munson L, Wack R, Duncan M, Montali RJ, Boon D, Stalis I, Crawshaw GJ, Cameron KN, Mortenson J, Citino S, Zuba J, Junge RE. 2004. Chronic eosinophilic dermatitis associated with persistent feline herpes virus infection in cheetahs (Acinonyx jubatus). Veterinary Pathology. 41(2): 170–176.
[176] Junge RE, Miller E, Boever WJ, Scherba G, Sundberg J. 1991. Persistent cutaneous ulcers associated with feline herpesvirus type 1 infection in a cheetah. Journal of the American Veterinary Medical Association 198(6): 1057–1058. PMID: 1851739
[177] Flacke GL, Schmidt-Kuntzel A, Marker L. 2015. Treatment of chronic herpesviral dermatitis in a captive cheetah (Acinonyx jubatus) in Namibia. Journal of Zoo and Wildlife Medicine 46(3): 641–646.
[178] Hara M, Fukuyama M, Suzuki Y, Kisikawa S, Ikeda T, Kiuchi A, Tabuchi K. 1996. Detection of feline herpes virus 1 DNA by the nested polymerase chain reaction. Veterinary Microbiology 48: 345-352.
[179] Nasisse MP, Weigler BJ. 1997. The diagnosis of ocular herpes virus infection. Veterinary and Comparative Ophthalmology 7: 44-51.
[180] Stiles J, McDermott M, Bigsby D, Willis M, Martin C, Roberts W, Greene C. 1997a. Use of nested polymerase chain reaction to identify feline herpesvirus in ocular tissue from clinically normal cats and cats with corneal sequestra or conjunctivitis. American Journal of Veterinary Research 58: 338-342. PMID: 9099374
[181] Stiles J, McDermott M, Willis M, Roberts W, Green C. 1997b. Comparison of nested polymerase chain reaction, virus isolation, and fluorescent antibody testing for identifying feline herpesvirus in cats with conjunctivitis. American Journal of Veterinary Research 58: 804-847. PMID: 9256959
[182] Weigler BJ, Babineau A, Sherry B, Nasisse MP. 1997. High sensitivity polymerase chain reaction assay for active and latent feline herpesvirus-1 infections in domestic cats. Veterinary Record 140: 335-338.
[183] Maggs DJ, Lappin MR, Nasisse MP. 1999. Detection of feline herpesvirus-specific antibodies and DNA in aqueous humor from cats with and without uveitis. American Journal of Veterinary Research 60: 932-936. PMID: 10451199
[184] Sykes JE, Allen JL, Studdert VP, Browning GF. 2001. Detection of feline calicivirus, feline herpesvirus 1 and Chlamydia psittaci mucosal swabs by multiplex RT-PCR/PCR. Veterinary Microbiology 81: 95-108.
[185] Vögtlin A, Fraefel C, Albini S, Leutenegger CM, Schraner E, Spiess B, Lutz H, Ackermann M. 2002. Quantification of feline herpesvirus 1 DNA in ocular fluid samples of clinically diseased cats by real-time TaqMan PCR. Journal of Clinical Microbiology 40: 519-523.
[186] Helps C, Reeves N, Egan K, Howard P, Harbour D. 2003. Detection of Chlamydophila felis and feline herpesvirus by multiplex real-time PCR analysis. Journal of Clinical Microbiology 41: 2734-2736.
[187] Marsilio F, Di Martino B, Aguzzi I, Meridiani I. 2004. Duplex polymerase chain reaction assay to screen for feline herpesvirus1 and Chlamydophila spp. in mucosal swabs from cats. Veterinary Research Communications 28: 295-298.
[188] Maggs DJ, Clarke HE. 2005. Relative sensitivity of polymerase chain reaction assays used for detection of feline herpesvirus type 1 DNA in clinical samples and commercial vaccines. American Journal of Veterinary Research 66: 1550-1555.
[189] Creevy KE. (2020) Canine Distemper Overview in Merck Manual: Veterinary Manual. https://www.merckvetmanual.com/generalized-conditions/canine-distemper/canine-distemper-overview
[190] Appel MJG, Yates RA, Foley GL, Bernstein JJ, Santinelli S, Spelman LH, Miller LD, Arp LH, Anderson M, Barr M, PearceKelling S, Summers BA. 1994. Canine distemper epizootic in lions, tigers, and leopards in North America. Journal of Veterinary Diagnostic Investigation 6(3): 277-288.
[191] Blythe LL, Schmitz JA, Roelke M, Skinner S. 1983. Chronic encephalomyelitis caused by canine distemper virus in a Bengal tiger. Journal of the American Veterinary Medical Association 183(11): 1159-1162. PMID: 6685717
[192] Deem SL, Spelman LH, Yates RA, Montali RJ. 2000. Canine distemper in terrestrial carnivores: a review. Journal of Zoo and Wildlife Medicine 31 (4): 441-451.
[193] Martinez-Gutierrez M, Ruiz-Saenz J. 2016. Diversity of susceptible hosts in canine distemper virus infection: a systematic review and data synthesis. BMC Veterinary Research 12(1): 78.
[194] Gilbert M, Miquelle DG, Goodrich JM, Reeve R, Cleaveland S, Matthews L, Joly DO. 2014. Estimating the potential impact of canine distemper virus on the Amur tiger population (Panthera tigris altaica) in Russia. PLoS One 9(10): e110811.
[195] Gilbert M, Soutyrina SV, Seryodkin IV, Sulikhan N, Uphyrkina OV, Goncharuk M, Matthews L, Cleaveland S, Miquelle DG.
2015. Canine distemper virus as a threat to wild tigers in Russia and across their range. Integrative Zoology 10(4): 329-343.
[196] Goodrich J, Lynam A, Miquelle D, Wibisono H, Kawanishi K, Pattanyibool A, Htun S, Tempa T, Karki J, Jhala Y, Karanth K. 2015. Panthera tigris. In: The IUCN red list of threatened species. Available from www.iucnredlist.org
[197] Montali RJ, Tell L, Bush M, Cambre RC, Kenny D, Sutherland-Smith M, Appel MJG. 1994. Vaccination against canine distemper in exotic carnivores: successes and failures. In: Proc Am Assoc Zoo Vet Ann Conf: Pittsburgh, PA; 340–344.
[198] Association of Zoos and Aquariums. Association of Zoos and Aquariums tiger species survival plan® 2016. tiger care manual. Silver Spring (MD): Association of Zoos and Aquariums; 2016.
[199] Bronson E, Deem SL, Sanchez C, Murray S. 2007. Serologic response to a canarypox-vectored canine distemper virus vaccine in the giant panda (Ailuropoda melanoleuca). Journal of Zoo and Wildlife Medicine 38(2): 363-366.
71
[200] Connolly M, Thomas P, Woodroffe R, Raphael BL. 2013. Comparison of oral and intramuscular recombinant canine distemper vaccination in African wild dogs (Lycaon pictus). JJournal of Zoo and Wildlife Medicine 44(4): 882-888.
[201] Vickers TW, Garcelon DK, Fritcher DL, Christianson M. 2004. Evaluation of an oral and food-based canarypox-vectored canine distemper vaccine in endangered Channel Island foxes (Urocyon littoralis). In: Proceedings of the American Association of Zoo Veterinarians and the American Association of Wildlife Veterinarians, Wildlife Disease Association Joint Conference 87-88.
[202] McEntire M, Ramsay EC, Kania S, Prestia P, Anis A, Cushing AC, Wilkes RP. 2020. Tiger (Panthera tigris) and domestic cat (Felis catus) immune responses to canarypox-vectored canine distemper vaccination. Journal of Zoo and Wildlife Medicine 50(4): 798-802.
[203] Sadler RA, Ramsay EC, McAloose D, Wilkes RP. 2016. Evaluation of two canine distemper vaccines in captive tigers (Panthera tigris). JJournal of Zoo and Wildlife Medicine. 47(2): 558-563.
[204] Nava AFD, Cullen Jr L, Sana DA, Nardi MS, Filho JDR, Lima TF, Abreu KC, Ferreira F. 2008. First evidence of canine distemper in Brazilian free-ranging felids. EcoHealth 5: 512-518.
[205] Avendano R, Barrueta F, Soto-Fournier S, Chavarria M, Monge O, Gutierrez-Espeleta GA, Chaves A. 2016. Canine distemper virus in wild felids in Costa Rica. Journal of Wildlife Diseases 52(2): 373-377.
[206] Ikeda Y, Nakamura K, Miyazawa T, Chen M, Kuo T, Lin JA, Mikami T, Kai C, Takahaski E. 2001. Seroprevalence of canine distemper virus in cats. Clinical & Diagnostic Laboratory Immunology 8: 641–644.
[207] Gollakner R, Kimmerlein A. Coronavirus Disease (COVID-19). VCA Animal Hospitals. https://vcahospitals.com/know-yourpet/coronavirus-disease-covid19
[208] Hammer AS, Lauge Quaade M, Bruun Rasmussen T, Fonager J, Rasmussen M, Mundbjer K, LohseL, Strandbygaard B, Svaerke Jorgensen C, Alfaro-Nuñes A, Rosenstierne MW, Boklund A, Halasa T, Fomsgaard A, Belsham GJ, Botner A. (2021). SARS-CoV-2 transmission between mink (Neovison vison) and humans, denmark. Emerging Infectious Diseases 27: 547–551.
[209] Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, Munger E, Molenkamp R, van der Spek A, Tolsma P, Rietveld A, Brouwer M, Bouwmeester-Vincken N, Harders F, Hakze-van der Honing R, Wegdam-Blans MCA, Bouwstra RJ, GeurtsvanKessel C, van der Eijk AA, Velkers FC, Smit LAM, Stegeman A, van der Poel WHM, Koopmans MPG. (2021).
Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371: 172–177.
[210] CDC Centers for Disease Control and Prevention. Animal Testing Guidance. 2021. https://www.cdc.gov/coronavirus/2019ncov/animals/animal-testing.html#content-3
[211] CDC Centers for Disease Control and Prevention. Animals and COVID-19. 2022. https://www.cdc.gov/coronavirus/2019ncov/daily-life-coping/animals.html
[212] Terio KA, Bronson E, McAloose D. 2021. Felid TAG guidance for working around non-domestic felid species during the SARS CoV 2 pandemic. 1-2. https://cdn.ymaws.com/www.aazv.org/resource/resmgr/docs/Guidance_for_working_around_.pdf
[213] AVMA. American Veterinary Medical Association. Feline panleukopenia. https://www.avma.org/resources-tools/petowners/petcare/feline-panleukopenia
[214] Lane EP, Brettschneider H, Caldwell P, Oosthuizen A, Dalton DL, du Plessis L. 2016. Feline panleukopenia virus in captive non-domestic felid in South Africa. Onderstepoort Journal of Veterinary Research 83(1): a1099.
[215] Nakamura K, Sakamoto M, Ikeda Y, Sato E, Kawakami K, Miyazawa T, Tohya Y, Takahashi E, Mikami T, Mochizuki M. 2001. Pathogenic potential of canine parvovirus types 2a and 2c in domestic cats. Clinical and Diagnostic Laboratory Immunology 8(3): 663-668.
[216] Squires RA. 2020. Merck Manual Veterinary Manual. Feline Panleukopenia. https://www.merckvetmanual.com/generalizedconditions/feline-panleukopenia/feline-panleukopenia
[217] van Vuuren M, Steinel A, Goosen T, Lane E, van der Lugt J, Pearson J, Truyen U. 2000. Feline panleukopenia virus revisited: Molecular characteristics and pathological lesions associated with three recent isolates. Journal of the South African Veterinary Association 71(3): 140-143.
[218] Wasieri J, Schmiedeknecht G, Forster C, Konig M, Reinacher M. 2009. Parvovirus infection in a Eurasian lynx (Lynx lynx) and in a European wildcat (Felis sylvestris silvestris). Journal of Comparative Pathology 140(2-3): 203-207.
[219] Chandran NDJ, Saravanabava K, Albert A, Venkatesan RA. 1993. Occurrence of parvoviral infection in lions. Indian Journal of Animal Science 63(7): 727-728.
[220] Pennisi MG, Radford A, Tasker S, Belák S, Addie DD, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, Hartmann K, Horzinek MC, Hosie MJ, Lloret A, Lutz H, Marsilio F, Thiry E, Truyen U, Möstl K. 2017. Anaplasma, Ehrlichia, Rickettsia Infections. Journal of Feline Medicine and Surgery 19(5): 542-548.
[221] Allison RW, Little SE. 2013. Diagnosis of rickettsial diseases in dogs and cats. Veterinary Clinical Pathology 42: 127-144.
[222] Lloret A. 2020. Hepatozoonosis in European Advisory Board on Cat Diseases (ABCD). http://www.abcdcatsvets.org/hepatozoonosis/
[223] Foley JE, Leutenegger CM, Dumler JS, Pedersen NC, Madigan JE. 2003. Evidence for modulated immune response to Anaplasma phagocytophila sensu lato in cats with FIV-induced immunosuppression. Comparative Immunology, Microbiology & Infectious Diseases 26: 103-113.
[224] Lappin MR, Chandrashekar R, Stillman B, Liu J, Mather TN. 2015. Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats after exposure to wild-caught adult Ixodes scapularis. Journal of Veterinary Diagnostic Investigation 27: 522-525.
72
[225] Bjöersdorff A, Svendenius L, Owens JH, Massung RF. 1999. Feline granulocytic ehrlichiosis – a report of a new clinical entity and characterization of the infectious agent. Journal of Small Animal Practice 40: 20-24.
[226] Lappin MR, Breitschwerdt EB, Jensen WA, Dunnigan B, Rha JY, Williams CR, Brewer M, Fall M. 2004. Molecular and serological evidence of Anaplasma phagocytophilum infection in cats in North America. Journal of the American Veterinary Medical Association 225: 893-896.
[227] Schaarschmidt-Kiener D, Graf F, von Loewenich FD, Müller W. 2009. Anaplasma phagocytophilum infection in a cat in Switzerland. Schweiz Arch Tierheilkd 151: 336-341.
[228] Heikkilä HM, Bondarenko A, Mihalkov A, Pfister K, Spillmann T. 2010. Anaplasma phagocytophilum infection in a domestic cat in Finland: case report. Acta Veterinaria Scandinavica 52: 62.
[229] Adaszek L, Górna M, Skrzypczak M, Buczek K, Balicki I, Winiarczyk S. 2013. Three clinical cases of Anaplasma phagocytophilum infection in cats in Poland. Journal of Feline Medicine and Surgery 15: 333-337.
[230] Savidge C, Ewing P, Andrews J, Aucoin D, Lappin MR, Moroff S. 2015. Anaplasma phagocytophilum infection of domestic cats: 16 cases from northeastern USA. Journal of Feline Medicine and Surgery Feb 13. pii: 1098612X15571148.
[231] Tarello W. 2005. Microscopic and clinical evidence for Anaplasma (Ehrlichia) phagocytophilum infection in Italian cats. Veterinary Record 156: 772-774.
[232] Baneth G, Sheiner A, Eyal O, Hahn S, Beaufils JP, Anug Y, et al (2013): Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood from morphology, and possibly transplacental transmission. Parasite Vectors 6: 102.
[233] Klopfer U, Nobel TA, Neumann F 1973. Hepatozoon-like parasite (schizonts) in the myocardium of the domestic cat. Veterinary Pathology 10(3): 185-190.
[234] Beaufils JP, Martin-Granel J, Jumelle P 1998 Hepatozoon spp. parasitemia and feline leukemia virus infection in two cats. Feline Pract 26: 10–13. ISSN : 1057-6614
[235] Basso W, Görner D, Globokar M, Keidel A, Pantchev N 2019. First autochthonous case of clinical Hepatozoon felis infection in a domestic cat in Central Europe. Parasitology International 72: 101945.
[236] Scott FW, Geissinger CM. (1999) Long-term immunity in cats vaccinated with an activated trivalent vaccine. American Journal of Veterinary Research 60: 652-658.
[237] Mouzin DE, Lorenzen MJ, Haworth JD, King VL. (2004) Duration of serologic response to three viral antigens in cats. Journal of the American Veterinary Medical Association 224: 61-66
[238] Bronson E, Terio K. (2018) 2018 Felid Taxon Advisory Group Preventative Medicine Recommendations.
http://www.felidtag.org/Husbandry
[239] Rivas AE, Langan JN, Colegrove KM, Terio K, Adkesson MJ. (2015) Herpesvirus and calicivirus infection in a black-footed cat (Felid nigripes) family group following vaccination. Journal of Zoo and Wildlife Medicine 46: 141-145.
[240]Schmidt-Posthaus H, Breitenmoser-Wörsten C, Posthaus H, Bacciarini L, Breitenmoser U. (2002). Causes of mortality in reintroduced Eurasian lynx in Switzerland. Journal of Wildlife Diseases 38: 84–92.
[241] Wassmer DA, Guenther D, Layne JN. (1988). Ecology of the bobcat in South-Central Florida. Bulletin of the Florida Museum of Natural History 33: 176–178.
[242] López G, López-Parra M, Garrote G, Fernández L, del Rey-Wamba T, Arenas-Rojas R, García-Tardío M, Ruiz G, Zorrilla I, Moral M, Simón MA (2014) Evaluating mortality rates and causalities in a critically endangered felid across its whole distribution range. European Journal of Wildlife Research 60: 359–366.
[243] Nájera F, Grande-Gómez R, Peña J, Vázquez A, Palacios MJ, Rueda C, Corona-Bravo AI, Zorrilla I, Revuelta L, Gil-Molino M, Jiménez J. (2021). Disease surveillance during the reintroduction of the Iberian Lynx (Lynx pardinus) in southwestern Spain. Animals 11(2): 547.
[244] Nakamura J, Iwasa T. 1942. On the fowl-pest infection in cat. Japanese Journal of Veterinary Science 4: 511–523.
[245] Kuiken T, Rimmelzwaan G, van Riel D, van Amerongen G, Baars M, Fouchier R, Osterhaus A. 2004. Avian H5N1 influenza in cats. Science 306: 241.
[246] Rimmelzwaan GF, van Riel D, Baars M, Bestebroer TM, van Amerongen G, Fouchier RAM, Osterhaus ADME, Kuiken T. 2006. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. American Journal of Pathology 168: 176–183.
[247] Leschnik M, Weikel J, Möstl K, Revilla-Fernández S, Wodak E, Bagó Z, Vanek E, Benetka V, Hess M, Thalhammer JG. 2007. Subclinical infection with avian influenza A (H5N1) virus in cats. Emerging Infectious Diseases 13: 243–247.
[248] Vahlenkamp TW, Harder TC, Giese M, Lin F, Teifke JP, Klopfleisch R, Hoffmann R, Tarpey I, Beer M, Mettenleiter TC. 2008. Protection of cats against lethal influenza H5N1 challenge infection. Journal of General Virology 89: 968–974.
[249] Vahlenkamp TW, Teifke JP, Harder TC, Beer M, Mettenleiter TC. 2010. Systemic influenza virus H5N1 infection in cats after gastrointestinal exposure. Influenza and Other Respiratory Viruses 4: 379–386.
[250] Ayyalasomayajula S, DeLaurentis DA, Moore GE, Glickman LT. 2008. A network model of H5N1 avian influenza transmission dynamics in domestic cats. Zoonoses and Public Health 55: 497–506.
[251] Lee CT, Slavinski S, Schiff C, Merlino M, Daskalakis D, Liu D, Rakeman JL, Misener M, Thompson C, Leung YL,Varma JK, Fry A, Havers F, Davis T, Newbury S, Layton M, Influence A(H7N2) Response team. 2017. Outbreak of influenza A(H7N2) among cats in an animal shelter with cat-to-human transmission New York City, 2016. Clinical Infectious Diseases 65: 1927
1929.
73
–
[252] Newbury SP, Cigel F, Killian ML, Leutenegger CM, Seguin MA, Crossley B, Brennen R, Suarez DL, Torchetti M, TooheyKurth K. 2017. First detection of avian lineage H7N2 in Felis catus. Genome Announc 5: e00457–17. doi:10.1128/genomeA.00457-17
[253] Wasik BR, Voorhees IEH, Parrish CR. 2021. Canine and Feline Influenza. Cold Spring Harbor Perspecies in Medicine. Cold Spring Harbor Laboratory Press.
[254] Hatta M, Zhong G, Gao Y, Nakajima N, Fan S, Chiba S, Deering KM, Ito M, Imai M, Kiso M, Naktsu S, Lopes TJ, Thompson AJ, McBride R, Suarez DL, Macken CA, Sugita S, Neumann G, Hasegawa H, Paulson JC, Tooher-Kurth KL, Kawaoka Y. 2018. Characterization of a feline influenza A(H7N2) virus. Emerging Infectious Diseases 24: 75–86.
[255] Keawcharoen J, Oraveerakul K, Kuiken T, Fouchier RAM, Amonsin A, Payungporn S, Noppornpanth S, Wattanodorn S, Theambooniers A, Tantilertcharoen R, Pattanarangsa R, Arya N, Ratanakorn P, Osterhaus DME, Poovorawan Y. 2004. Avian influenza H5N1 in tigers and leopards. Emerging Infectious Diseases 10: 2189–2191.
[256] Thanawongnuwech R, Amonsin A, Tantilertcharoen R, Damrongwatanapokin S, Theamboonlers A, Payungporn S, Nanthapornphiphat K, Ratanamungklanon S, Tunak E, Songserm T, Vivatthanavanich V, Lekdumrongsak T, Kesdangsakonwut S, Tunhikorn S, Poovarawan Y. 2005. Probable tiger-to-tiger transmission of avian influenza H5N1. Emerging Infectious Diseases 11: 699–701.
[257] Bouvier NM, Lowen AC. 2010. Animal models for influenza virus pathogenesis and transmission. Viruses 2: 1530
1563.
[258] Belser JA, Barclay W, Barr I, Fouchier RAM, Matsuyama R, Nishiura H, Peiris M, Russell CJ, Subbarao K, Zhu H, Yen, H-L. 2018. Ferrets as models for influenza virus transmission studies and pandemic risk assessments. Emerging Infectious Diseases 24: 965–971.
[259] Berg M, Englund L, Abusugra IA, Klingeborn B, Linné T. 1990. Close relationship between mink influenza (H10N4) and concomitantly circulating avian influenza viruses. Archives of Virology 13: 61–71.
[260] Yoon K-J, Schwartz K, Sun D, Zhang J, Hildebrandt H. 2012. Naturally occurring influenza A virus subtype H1N2 infection in a Midwest United States mink (Mustela vison) ranch. Journal of Veterinary Diagnostic Investigation 24: 388–391.
[261] Jiang W, Wang S, Zhang C, Li J, Hou G, Peng C, Chen J, Shan H. 2017. Characterization of H5N1 highly pathogenic mink influenza viruses in eastern China. Veterinary Microbiology 201: 225–230.
[262] Geraci JR, St Aubin DJ, Barker IK, Webster RG, Hinshaw VS, Bean WJ, Ruhnke HL, Prescott JH, Early G, Baker AS, Madoff S, Schooley RT. 1982. Mass mortality of harbor seals: pneumonia associated with influenza A virus. Science 215: 1129–1131.
[263] Anthony SJ, St Leger JA, Pugliares K, Ip HS, Chan JM, Carpenter ZW, Navarrete-Macias I, Sanchez-Leon M, Saliki JT, Pedersen J, et al. 2012. Emergence of fatal avian influenza in New England harbor seals. mBio 3: e00166– 00112.
[264] Bodewes R, Bestebroer TM, van der Vries E, Verhagen JH, Herfst S, Koopmans MP, Fouchier RAM, Pfankuche VM, Wohlsein P, Siebert U, et al. 2015. Avian influenza A (H10N7) virus–associated mass deaths among harbor seals. Emerging Infectious Diseases 21: 720–722.
[265] Elsmo EJ, Wünschmann A, Beckmen KB, Broughton-Neiswanger LB, Buckles EL, Ellis J, Fitzgerald SD, Gerlach R, Hawkins S, Ip HS, Lankton JS, Lemley EM, Lenoch JB, Killian ML, Lantz K, Long L, Maes R, Mainenti M, Melotti J, Moriarty ME, Nakagun S, Ruden RM, Shearn-Bochsler V, Thompson D, Torchetti MK, Van Wettere AJ, Wise AG, Lim AL. 2023. Pathology of natural infection with highly pathogenic avian influenza virus (H5N1) clade 2.3.4.4b in wild terrestrial mammals in the United States in 2022. bioRxiv.
[266] Savage HM, Burkhalter KL, Godsey Jr MS, Panella NA, Ashley DC, Nicholson WL, Lambert AJ. (2017). Bourbon virus in field-collected ticks, Missouri, USA. Emerging Infectious Diseases 23(12): 2017-2022
74
–
Transportation of Ocelots

75
Photo courtesy Ken Kaemmerer, Pittsburgh Zoo
The following guidelines for transporting zoo-based ocelots, wild-caught ocelots, and ocelots born at the Ocelot Conservation Facility have been adapted/modified from the Association of Zoos and Aquariums Felid Taxonomic Advisory Group. [In prep. Small Felids (Felidae) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums.] In addition to the information presented here, many zoo registrars are well experienced to advise or set up animal shipments and have great resources available to guide any transfers of ocelots.
Ocelot transportation must be conducted in a manner that adheres to all laws and permits, is safe, and minimizes risk to the animal(s), employees, and general public. Safe ocelot transport requires the use of appropriate conveyance and equipment that is in good working order. It is imperative to include copies of appropriate permits and authorizations in transport documentation. Contact with the management of both the sending and receiving institutions to obtain permission and initiate relevant documentation should be made at least 30 days prior to setting a transport date.
If considering shipping via a commercial airline, domestic commercial flights have procedures in place and should be contacted well in advance for requirements. For land transportation across state boundaries, compliance with the wildlife regulations for each state on the travel itinerary is necessary. Contacting the cooperating state wildlife agency’s law enforcement division is an important first step. At the federal level, transport conditions for mammals are regulated through the United States Department of Agriculture Animal and Plant Health Inspection Service (USDA-APHIS); these are noted below. The International Air Transport Association (IATA) specifies details of shipping containers, and all professional airlines abide by IATA’s manual for both domestic and international flights in addition to USDA regulations.
All transport equipment must provide adequate containment, life support, comfort (sized to allow the animal space to stand, sit up, turn around, lay flat, or curl up), temperature control, food/water, and safety for the animal(s). Care should be taken with the size, shape, and spacing of openings of containers to minimize opportunities for individuals to damage teeth and claws while inside the container. Animals should not be able to contact any edges or surfaces that can cut or abrade them. Food and water containers should be fixed off the floor to prevent soiling near the front of the container. There should be a safe way to access food and water from the outside to refill the containers. The height of the transport container must allow the ocelot to stand erect with its head extended, and the length should permit the animal to lie in a prone position. A good rule of thumb is to allow a 10 cm (4 inch) clearance around the animal when standing.
Constructed to IATA guidelines, containers will provide adequate space. However, it is important not to make transport containers too large. Injuries may occur if space permits animals to leap or be thrown about within the crate or if the vents are large enough to encourage the animals to attempt to escape.
In addition to transport container(s), state and federal permits, transaction paperwork, health certificates of veterinary inspection and related documents, scheduling and coordination of preparation, and execution of the move are important components. Travel logistics for animals and staff, whether by land, air, or water, should be organized and confirmed well in advance of shipment. Depending on the mode of transport, tools, equipment, vehicles, and backup options should be prepared and assembled. These are general considerations and are not intended to be an exhaustive list. Preparation of a checklist of tasks and timing is recommended for animal transport. Examples of checklists are included (Figures 1-2).
76
Health certificates, transaction paperwork, air-bills, and all other relevant documents should be shipped along with animals and attached to the shipping container. A document outlining details of the sending institution’s husbandry procedures, diet, and behavior notes along with husbandry and medical records is an important component of this paperwork. The Animal Data Transfer (ADT) Form, a form printed by the American Association of Zoo Keepers, has been commonly used by zoos. Although the animals sourced for the ocelot reintroduction program may or may not originate from zoos, the ADT form (Figure 3) may be a useful method to provide relevant information. For information on recommended pre-shipment medical evaluations, see the health section of this Manual.
Safe transport also requires the assignment of an adequate number of appropriately trained personnel (by institution or contractor) who are equipped and prepared to handle contingencies and/or emergencies that may occur during transport. Planning and coordination for animal transport requires good communication among all affected parties, plans for a variety of emergencies and contingencies that may arise, and timely execution of the transport. Consideration and identification of emergency veterinary services should be made in advance of travel with the agreement of the identified veterinarians and notice of the travel date. At no time should the ocelot(s) or people be subjected to unnecessary risk or danger.
Because anesthesia and sedation carry risks of negative reaction (e.g., overheating, cardiac or respiratory arrest, injury due to impairment of coordination during recovery), transporting any cat while they are under the influence of such drugs is discouraged. Oftentimes, airlines will not ship an animal unless it is fully recovered from sedation. Due to risk of injury to both animals and people, direct contact with individuals during shipment should be minimized. Unless conditions become life-threatening, standard practice is to maintain focus on reaching the destination once the animals are secured in shipping containers
Specific Transport Guidelines
Transport Within Ocelot Conservation Facility
Containers should be of suitable size and strength to house the cat comfortably while being moved within the facility. Appropriate locking and safety mechanisms must be in place. Handles or carrying devices must be in place to allow correct positioning of the carrier and to prevent handlers from placing fingers in the container. Cloth, such as burlap or a fine mesh, should be placed over the windows and door to prevent human fingers from intruding as well as to lessen outside light and stimulation from human activities.
Transport by Air and Ground
The International Air Transport Association (IATA) provides transportation regulations for shipping live animals. Container requirement 82 (see below) describes regulations for shipping small felids including ocelots While IATA regulates international shipping of live animals, domestic air carriers also follow IATA standards, so the shipper is encouraged to contact the potential domestic carrier for their requirements in shipping an ocelot by commercial air carrier.
77
For air, ground, or other transport, the USDA APHIS publication 9 CFR Ch. 1 Subchapter A – Animal Welfare provides additional transportation regulations for shipping live animals. Subpart F section 3.134–3.142 provides transport specifications (Animal Welfare Act and Animal Welfare Regulation). All specifications should be reviewed in preparation for shipping and are summarized below.
Type of Transport Container
The transport crate should be made of heavy-duty plastic, wood, metal, welded mesh, and/or wire mesh. Sky kennels (Sky Kennels Ltd can provide all your pet relocation products & service needs) or Vari kennels (Vari Kennel Pet Crate – Pet Crates Direct (#300 - Intermediate 32 in. L x 22.5 in. W x 24 in. H or #400 - Large 36 in. L x 25 in. W x 27 in. H) made of heavy-duty plastic, metal mesh windows and a front metal mesh threepoint lockable swinging door, have been commonly used for young and adult ocelots in zoos and in the field. Instead of a swing door, there are companies that produce an attachable vertical or horizontal sliding guillotine door to replace the swinging door (e.g., Adj - Kennel Door info | c2si-1 (carter2systems.com) In addition, K. Kaemmerer (Pittsburgh Zoo, personal communication) developed a design modification for Sky or Vari kennels to be temporarily attached back-to-back with each kennel modified to have rear guillotine transfer doors allowing the transfer of an animal from one kennel to another (Figures 5-6).
Appropriate Size of Transport Container
The height of the container must allow the animal to stand in a natural position with its head extended and the width must permit it to turn around and lie down comfortably. The actual measurements will vary with the individual involved.
Provision of Food and Water During Transport
Cats do not normally require additional feeding or watering during the 24 hours following the time of dispatch. If feeding is required due to an unforeseen delay, food should be provided, but care should be taken not to overfeed.
Provision of Bedding or Substrate in Transport Container
If a rigid plastic Vari kennel or pet carrier is used, absorbent materials should be provided. Wood shavings, hay or straw, shredded wood (wood wool) and shredded paper are common options. However, for international travel, consult customs authorities, both export and import, as natural organic materials may not be permitted due to a risk from agricultural pests or disease. Materials used should be familiar to the cat to help prevent ingestion.
Mechanism(s) for Separating Animals from Urine and Feces During Transport
Absorbent materials should be provided (see above for examples) unless the shipping container has a floor designed to allow urine and feces to pass through it into a removable tray below
78
Appropriate Temperature Range During Transport
Ambient temperature for cats that have not been acclimated to lower temperatures should be maintained at a temperature above 10 °C (50 °F). Auxiliary ventilation, such as exhaust fans and vents or air conditioning, should be provided when the ambient temperature is 30 °C (85 °F) or higher. Airlines may require a veterinarian to submit a letter of acclimation.
Appropriate Group Size or Need for Separation of Individuals During Transport
If more than one cat is to be shipped, each should have a separate crate/container. An exception is hand-reared young and/or dam/kitten pairings; they may be transported in the same container if they are compatible and of appropriate size. Verify the age cutoff with IATA and check the divided container.
Access to Animals During Transport
If an ocelot comes from a captive or zoo background, it can be beneficial to have a familiar keeper travel with the cat that is being transported, especially for air travel if the cat must wait inside a freight terminal for the next flight connection. Most healthy, adult animals seem to transport with minimal stress, especially if they have been acclimated to their container before shipping. Animal managers should make every effort to give the animal prior access to the container (at least one week) and to take measures (e.g., baiting with food, training) to encourage the cat to enter the container voluntarily.
Maximum Duration of Transport Allowable Before Temporary Transfer to “Normal Housing” is Required
Most healthy adult cats appear to be able to tolerate being away from “normal housing” for 24–48 hours. After that period, the cat should be moved to “normal housing.”
Appropriate Timing of Release, Size, and Type of Enclosure at Transport Destination
Upon arrival, the animal should be provided with fresh water and food, and preferably transferred into the appropriate enclosure as soon as possible. Prior to transport, a discussion and decision should be made on the type of quarantine. Should a direct transfer to the quarantine area not be possible, the animal can be kept in the transport carrier for an additional six hours with fresh food and water in an isolated, quiet location.
Ventilation and Environment
Per USDA Animal Welfare Regulations (Part 3, Subpart F - 3.137) as stated: “there are ventilation openings located on two opposite walls of the primary enclosure and the ventilation openings on each such wall shall be at least 16 percent of the total surface area of each such wall, or there are ventilation openings located on all four walls of the primary enclosure and the ventilation openings on each such wall shall be at least 8 percent of the total surface area of each such wall. Provided, however, that at least one-third of the total minimum area required for ventilation of the primary enclosure shall be located on the lower one-half of the primary enclosure and at least one-third of the total minimum area required for ventilation of the primary enclosure shall be located on the upper one-half of the primary enclosure.”
79
All efforts should be made to maintain and not exceed exposure to normal light levels. Covering doorways and ventilation areas with breathable, open-weave material such as burlap, cotton or light plastic mesh will help control both light and noise levels. Additionally, securing coverings will avoid flapping of material in wind, which could cause stress to animals, or the tearing of coverings during transport. Any coverings must still allow viewing of the animal to ensure safety.
Handles
Adequate handholds or other devices for lifting should be provided on the exterior of the primary enclosure to enable the carrier to be lifted without tilting and to ensure that the carrier handler will not be in contact with the animal.
Labeling
Primary enclosures should be marked on top and on one or more sides with the words “Live Animal” or “Wild Animal” and their studbook or other identification number in large letters. Additionally, arrows or other markings should indicate the correct upright position of the container. Labels may be available through air cargo; this should be verified with the airline before transport. Copies of supporting documents (see below) should also be attached to the container.
Primary Conveyances
The primary conveyance should be designed and constructed to protect the health and ensure the safety and comfort of live animals. The ingress of engine exhaust fumes and gasses should be prevented. An adequate supply of fresh air sufficient for normal breathing should be available. Primary enclosures should be positioned such that in an emergency the live animals can be removed as soon as possible.
Documents
When shipping animals, documents accompanying the shipment should be attached in an easily accessible manner to the outside of the primary enclosure.
Permits/Required Documents
● Interstate Health Certificate, USDA APHIS form 7020.
● When appropriate, a permit for transporting an animal is regulated by the U.S. Endangered Species Act (www.fws.gov/endangered) and CITES (www.cites.org).
● Some states require a permit, issued by their state wildlife agency, to transport endangered species; verify with state authorities before shipping.
● Other helpful documents include Species 360 Zoological Information Management Systems or other similar specimen reports with all data relevant to a studbook and institutional records, and an American Association of Zookeepers animal data transfer form to relay husbandry, behavioral, and other pertinent information.
80
Import
Verify and obtain all import permits for shipping and receiving countries, including CITES when required. It is likely that an International Health Certificate from the import country will be necessary. A declaration of importation/exportation form 3-177 is needed and unless receiving the shipment through a valid customs port of entry, in which case a designated port exception permit is needed. For imports, a broker is required. Brokers at shipping and receiving ports routinely deal with customs and simplify handling at ports. Upon arrival, quarantine imported cats for the required number of days (can be within permitted facility).
Export
When exporting, verify that pre-shipment quarantine requirements have been met with the receiving country. An international health certificate signed by the USDA state veterinarian is needed, and a declaration of importation/exportation form 3-177 unless sending through a valid customs port of export, which will require a designated port exception permit. Finally, a proforma or commercial invoice may be required Brokers are knowledgeable about all aspects of imports/exports and can facilitate the transfer of an animal through checkpoints and onto travel carriers. When using wooden crates, verify with regulations of the country of import pertaining to the use of wood and type of bedding substrate.
International Air Transport Association Container 82 requirements applicable to ocelots
Container Construction
Materials
Heavy-duty plastic, wood, metal, synthetic materials, weld mesh and wire mesh.
Principles of Design
The following principles of design must be met in addition to the General Container Requirements outlined at the beginning of this section.
Dimension
The height of the container must allow the animal to stand in a natural position with its head extended and the width must permit it to turn around and lie down comfortably. The actual measurements will vary with the species involved.
Frame
The frame must be made from solid wood or metal parts bolted or screwed together. It must be constructed so that it cannot be damaged from continual biting or scratching at the corners. If the total weight of the container plus animal exceeds 60 kg (132 lb) metal bracing must be added to the frame.
81
Sides
The sides and door must be made of metal or solid wood. The front of the container must be constructed of weld mesh. The mesh must have a diameter that will prevent the animal protruding its nose or paws to the outside. The whole front must be covered by a sliding shutter which can be raised and lowered to permit feeding and watering. It must have two observation holes of at least 10 cm (4 in) in the upper part and ventilation holes, with a minimum diameter of 2.5 cm (1 in), spread over the remainder of the surface in order to give good ventilation but at the same time leave the animal in semi-darkness.
Floor
The floor must be slatted, over a leak-proof droppings tray or, if slatted floor is not required for that species, it must be leak proof and covered by sufficient absorbent material in order to prevent any excreta escaping.
Roof
May be solid wood or metal
Doors
A sliding door must be provided, it can be made from the welded steel meshed ventilation front if required. It must have a secure means of fastening so that it cannot be opened accidentally
Ventilation
The main ventilation front must be supplemented by meshed openings along the upper part of the container walls and/or holes with a minimum diameter of 2.5 cm (1 in) spread over the top third of the sides and the whole of the back. These holes must be spaced both horizontally and vertically at intervals of approximately 10 cm (4 in) center to center. At least one-third of the total ventilation openings should be on both the lower and upper half of each ventilated wall. Palletised shipments must have the containers made entirely of weld mesh of a suitable dimension that no part of the animal can protrude in order to ensure good ventilation
The total ventilated area must be at least 20% of the total area of the surface of all four sides. More ventilation and the use of larger meshed openings is permitted but the animal must not be able to protrude its nose or paws to the outside from any opening. If the mesh is fixed to the interior of the container, all sharp edges must be protected.
Spacer Bars/Handles
Must be made to a depth of 2.5 cm (1 in), must be present on the sides of the container as shown in the illustration.
Feed and Water Containers
Food and water containers must be provided with a means of access from the outside.
82
Forklift Spacers
Must be provided if the total weight of the container plus the animal exceeds 60 kg (132 lb).
Rigid Plastic Pet Containers
The following modifications must be made:
● the grill door must be covered with securely fixed weld mesh and all ventilation openings covered with wire mesh;
● the door of the larger containers must have secure fastenings at the top and the bottom;
● a curtain, that can be raised and lowered and does not impede ventilation, must be fixed over the door to reduce light inside the container;
● there must be ventilation openings on the rear of the container, extra ventilation openings may have to be made in order that the total ventilation area is at least 20% of the four sides;
● The leak-proof floor must be covered with absorbent material;
● food and water containers must be fixed inside with access from the outside;
● the container must be correctly labelled;
● if a container has wheels, they must be removed or rendered inoperable.
83
Travel Packet Checklist
Animal: _____________________________
Destination:_____________________________________
□ Health Certificate – pink copy
□ APHIS 7020 – Return one signed copy to the Zoo performing the transfer
□ Specimen report
□ Animal Data Transfer Form
□ Animal Transportation Trip log
□ Transport Plan
Figure 1. Preparation of a checklist of tasks and timing for transfer of zoo-based or wild ocelots (H. Terrell, Registrar Pittsburgh Zoo and Aquarium; personal communication).
84
Animal transfer and transaction worksheet.
85
Type of Transaction (check) ☐INCOMING ☐OUTGOING Timeline for Transaction Other Inst. Name of Institution Contact Person Title, Address Phone USDA License# Fax AZA IN# E-Mail CBW Prt# Alternate Contact: Curator/Manager Terms of Transaction Loan/trade/sale terms/price Sex Common Name Scientific Name ZOO ID# House Name Other IDs Notes . . . . . . Documentation in Advance of Shipment Received/ sent Document date requested/sent date received Notes ☐ USFW/SSP approval ☐ Loan Agreement
86 ☐ Specimen Report ☐ Diet Sheet ☐ Medical Records ☐ Permit(s) list: ☐ ATC ☐ Crate Documentation Accompanying Shipment Received/ sent Document Date requested Date received Notes ☐ Health Certificate ☐ ADT form ☐ Shipper’s Certif. ☐ Air Way Bill AWB # ☐ USDA form ☐ Invoice invoice # ☐ Other: Shipping Arrangements method of shipment carrier/transporter flight number(s) air way bill number
departs from departure date departure time to be dropped off by arrives at arrival date arrival time to be picked up by
number
cost
Notes: Figure 2. Transfer and transaction worksheet for transfer of zoo-based or wild ocelots (H. Terrell, Registrar Pittsburgh Zoo and Aquarium, personal communication).
87
crate
other
of crates crate size(s)
weight(s)
shipment information
other
of shipment paying institution payment account #
payment information
ANIMAL DATA TRANSFER FORM
1. Curator’s copy of information on new arrival
2. Keeper’s copy of information on new arrival Date:
3. Copy for zoo files and/or veterinarian

***Please send a copy of this form to shipping institution and state condition of animal(s)***
Previous Institution (s):
Current Institution :
Contact Person: Title: Email: Phone/Fax:
Receiving Institution:
Common name:
88
Scientific name: Zoo ID# House Name Sex Hatch/Birth Date* Tattoo Band/Tag# Weight* Transponder Studbook # (Regional/International)
*Note if it is actual or estimated
DIET: Present diet and supplements, favored items, problem foods, feeding procedures.
BEHAVIORAL HISTORY & SPECIFICATIONS: Please list any unique behavioral traits, problems with aggression, safety concerns, or other behavioral problems that may affect management.
General Disposition (skittish, prefers males over females, imprinted, aggressive, etc.):
Stereotypic behavior (frequency, severity, duration, triggers)
Methods used for managing stereotypic behavior:
Does the animal have a history of aggression towards keepers and/or other animals? ☐ no ☐ yes
What are the conditions and behavioral precursors to the aggression?
What successful strategies are used for dealing with the aggression?
General comments or describe other behaviors that require further explanation:
MEDICAL HISTORY OR PHYSICAL CONDITION: Medication techniques, immobilization techniques, chronic medical problems, Vet Contact.
PREVIOUS ENCLOSURE DATA: If applicable, exhibit dimensions and description, disinfection/cleaning needs, temperature and climate control needs.
Exhibit Features: (When offered or provided, please list or check where applicable. Add comments where necessary)
Substrates: sand ☐ gunite ☐ mulch ☐ leaf litter ☐ soil ☐ other:
Exhibit Furniture: deadfall ☐ live trees ☐ rockwork ☐ perching ☐ termite mounds ☐ other:
89
Water features:
Holding Area: indoor ☐ outdoor ☐ none (see above) ☐
Substrates: ☐ sand ☐ mulch ☐ leaf litter ☐ soil ☐ other:
Holding Furniture:
How frequently rotated:
SOCIAL HISTORY (check all that apply)
Rearing type: ☐ dam, parent or family reared ☐ hand reared (☐ with conspecifics ☐ without conspecifics) ☐ puppet
☐ supplemental ☐ foster reared (☐ by same species ☐ by different species) ☐ none ☐ autonomous
☐ colony/peer
Comments:
Animal housed:
☐ individually ☐ with conspecifics (list #_____) ☐ with mixed species [List species and # of each ]
☐ other, please describe:
☐ Housed on exhibit ☐ off-exhibit ☐ access to both
Comments:
90
REPRODUCTIVE HISTORY: Relevant information, introduction techniques, behavior toward young, specific concerns.
ENRICHMENT HISTORY (Please attach any relevant schedules, approved item lists, sample calendars, etc.)
Goals for the enrichment:
Enrichment activities offered in exhibit:
Enrichment offered: ☐ daily ☐ weekly ☐ monthly ☐ scheduled ☐ other:
How frequently rotated:
Enrichment activities offered in holding (if different from those offered on exhibit):
Food Enrichment
Diet Presentation:
# of feedings per day: Varied times: ☐ When: Food scattered ☐ Hidden ☐
Novel Foods (please list or attach approved list of food items, frequency and amounts offered and presentation):
Enrichment Devices/Items
☐ PVC feeders ☐ Tires ☐ Burlap/towels ☐ Plastic containers ☐ Puzzle feeders
☐ Cardboard boxes/tubes/bags ☐ Ropes/vines/fire hose ☐ Balls/kegs/barrels ☐ Toys (Kong® , dog chews, etc.)
91
Attachments methods used (chain, rope, bungee):
Preferred enrichment for this animal (list):
Safety Concerns (ingests cloth, has become impacted, displays at cage mates with large items, etc.):
General Comments (including expanding on any of the data entered, above):
TRAINING OR BEHAVIORAL CONDITIONING:
Training goals for this animal (list general behavioral goals and indicate which goals have been achieved and/or which goals were partially shaped but not complete at time of shipment):
How long has animal participated in a behavioral conditioning program?
Frequency and Duration of Training Sessions:
☐ once daily ☐ twice daily ☐ once weekly ☐ twice weekly
☐ other, please specify:
Average length of training session (minutes):
Animal attitude/demeanor towards/during training:
Level of contact between the keeper and animal: ☐ free contact ☐ protected contact
Social arrangement during training sessions:
☐ housed individually and trained individually ☐ separated from conspecifics for training
☐ trained with conspecifics present ☐ trained with mixed species present
☐ other, please describe:
Animal conditioned to enter crate/chute/cage for transport? (circle appropriate device*) ☐ No ☐ Yes
Length: Width: Height:
*Attach pictures if necessary to describe training area or device (crates, chutes, etc.).
Reinforcers: ☐ verbal ☐ food List type and amount used:
92
☐ tactile ☐ combination of all of the above
Bridging stimulus: ☐ clicker ☐ verbal, describe:
☐ whistle ☐ other, describe:
How are undesirable behaviors addressed? ☐ Time-out ☐ ignore ☐ re-direct ☐ incompatible behavior ☐ other (describe):
Which methods have been most successful?:
BEHAVIORS TRAINED (Provide a summary. More detail can be added in subsequent section)
Behavior Verbal cue/command Visual cue Criteria for reinforcement Devices used
Please attach list of behaviors if more room is needed
General training comments:
Figure 3. American Association of Zoo Keepers Animal Data Transfer Form for use in transfer of zoo-based ocelots.
93

94
Figure 4. Example container meeting International Animal Transport Association (IATA) Container 82 requirements.


95
Figure 5. Modification of the rear of two kennel crates to allow them to be attached together for transfer to each other. Photo courtesy K. Kaemmerer, Pittsburgh Zoo.
Figure 6. Side view of two crates attached at the rear to each other allowing transfer of animal(s) from one to the other. Photo courtesy K. Kaemmerer, Pittsburgh Zoo.
Pregnancy, Parturition, and Kitten Rearing in Ocelots

96
Photo courtesy Cincinnati Zoo & Botanical Garden
Information provided in this section was directly referenced from the Association of Zoos and Aquariums (AZA) Small Felids (Felidae) Care Manual (draft, in prep.) [1] and modified for the purposes of the ocelot reintroduction program. Information here provides a guide for managing pregnant dams and kittens at the Ocelot Conservation Facility. However, every dam and kitten in the Ocelot Conservation Facility will act and respond differently, requiring fluidity to methods and the ability to adapt to each situation. This information here is meant to serve as a reference guide for handling and managing kittens, including in the case of assisted rearing, and for monitoring proper parent-rearing.
For those ocelots that will remain in the Ocelot Conservation Facility or other captive environment indefinitely, they should be provided the same care as those in AZA institutions, as recommended by the Small Felids (Felidae) Care Manual (In prep.)[1]. Ocelot kittens that are eligible for future release to the wild must be reared appropriately in a suitable environment by the biological dam or a surrogate feline to support their ability to be reintroduced into the wild in the future. Human rearing of kittens will be an emergency action based on the dam’s physical ability to rear kittens and the need of a kitten to be pulled due to failure to thrive. Hand- rearing is not without risk, as it can reduce future reproductive success [2] and may have contributed to development of umbilical herneation, perianal gland inflammation, and epilepsy in Iberian lynx kittens [3]. If a kitten must be hand-reared, the items below outline actions to decrease kittens’ habituation to humans, develop natural behaviors, and allow continued participation in the ocelot breeding program in southern Texas.
Environment for pregnancy, parturition, and kitten rearing
Temperature and humidity recommendations
Temperature extremes in climate-controlled areas should generally not exceed those of the cats’ native habitats [1]. In areas not climate-controlled, recommendations for providing shelter for warming should follow U.S. Fish and Wildlife Service (USFWS) trapping guidelines for live trapping ocelots. For example, if temperatures drop below 40ºF, live trapping for ocelots is paused so that ocelots do not get captured in a live trap and become unable to escape the elements or burrow for warmth under a tree or shrub. Kittens in the Ocelot Conservation Facility who are exposed to temperatures below 40ºF should be allowed an area to bed down in and/or to use to get out of the elements. For severe elevations in temperature (above 90ºF), shade should be provided and plenty of water, though use of misters or fans that will not be present in the wild should be avoided for kittens that may be released into the wild. This will allow kittens to adapt to the native habitat and environment prior to release. An appropriate shelter in an outside enclosure would provide the kitten with protection and shelter from the cold and heat, protection from direct sunlight, wind, and rain, and contain clean, dry substrate (e.g., hay, straw, wood shavings) if the temperature drops below 40ºF.
Nest Boxes and Design (Figure 1.)
Two to three nest boxes will be provided inside the breeding enclosures (and/or outside if temperatures and precipitation are moderate) for dams to choose a location for parturition or be able to move their kittens around. The nest boxes should not be more than 3 feet above the ground to prevent kittens from falling from high levels or the dam dropping them, resulting in injury or death. Wood shavings, shredded paper, soft grassy hay, good quality straw, towels and carpeting have been used successfully to provide comfort, traction, and warmth to
97
kittens in the den boxes. An optional heat lamp or solid heat pad can be added to cover only a portion of the box so they can move away from the heat (but may not be necessary depending on the time of year). See images below for nest box examples.

Other Enclosure Considerations
Kittens will start leaving the nest box around five weeks of age and the enclosure areas should be prepared for this exit. All enclosure areas should be assessed for any potential hazards to kittens in the weeks prior to the birth window so that any modifications can be made prior to parturition. Modifications include, but are not limited to, draining water pools, keeping water bowls with a low level of water, cover drains and small holes in walls or fencing, limit access to elevated areas and soft substrates under areas that they are likely to climb.
Pregnancy and Parturition
Ocelots demonstrate non-seasonal reproduction. Their estrous cycle lasts 18.04 ± 1.6 days with estrus (heat) lasting 1 to 6 days [AZA Regional Collection Plan]. The gestation length in an ocelot is 79-85 days. [5-7]. Prior to breeding and pregnancy confirmation, the Ocelot Conservation Facility should coordinate with zoological or other institutions to develop a plan for cross-fostering kittens into a surrogate feline litter should this become necessary when kittens are born
98
Figure 1. Example nest box for ocelots.
Physical and behavioral changes during pregnancy [1]
Weight Gain
Weight charts and body condition scores can be helpful as an indication of pregnancy. Scales can be placed within the enclosure where the dams would be fed, and the dam can be monitored daily during pregnancy. Although some felines can show changes in the abdomen (pendulous abdomen) from growing kittens in utero, those with a singleton are less likely to do so. Ocelots typically have 1-2 kittens (rarely 3-5), making this change less common, but still possible.
Appetite Change
Increased appetite prior to birth is normal, though appetite may vary depending on the individual. Monitoring body condition to maintain adequate condition without too much weight gain is important. Prior to giving birth, the dam may go off food for 1-2 days and may not resume eating for several days following birth.
Milk Production
This may start as early as 10 days prior to birth but can be difficult (or impossible) to confirm, depending on fur and behavior. Teat development may also be apparent less than 2 weeks prior to birth.
Pre-parturient Estrus
Some ocelot dams exhibit a late pregnancy “estrus, ” as progesterone decreases close to birth and ovarian follicular activity and estrogen production rebounds Signs of pre-parturient estrus can be variable, with the full complement of normal estrous behaviors or more subdued or discrete activities or even silent estrus. If a female shows estrus signs but is not yet at 82 days post-breeding (or assisted reproduction), it does not mean that she is not pregnant. However, if she is past 85 days, then likely she is not pregnant [K. Kaemmerer, Pittsburgh Zoo, personal communication].
Signs of Impending Birth
Within 24-48 hours of birth, the dam may increase grooming, especially of the vagina and teats, become agitated or aggressive, lose interest in food, begin spending time in a nest box or seclusion, stretching, hairplucking, and become restless with increased respirations and muscle contractions around the abdomen.
Birth
This process can take minutes to hours and normally occurs overnight. The dam will normally eat the placenta and should begin licking the kittens until they are dry and inspecting them separately. Kittens should be seen nursing within the first few hours after birth. The dam will often remain with her kittens for 24 hours without leaving to drink or eat and should be left alone and monitored remotely via camera during the first 24 hours Kittens should nurse, while sleep and vocalizations will be quiet and infrequent. Kittens should establish a nursing pattern of every 2-3 hours, and this pattern can be monitored remotely via camera.
99
Additional Considerations
It is important to minimize disturbances, changes to the dam’s surroundings, and changes to the dam’s routine in the weeks leading up to birth. If a pregnant dam is being housed with a male, the male should be removed 2-3 weeks prior to the first expected birth date while the dam should be left in the area that she has become familiar with. To minimize staff member interaction with the dam, a single person should take over husbandry care. Camera installations and nest box modifications should be completed by 2 weeks or earlier than the first possible birthing date. After parturition, routine cleaning can begin gradually as the dam’s behaviors indicate comfort with a singular familiar keeper in the vicinity of the young. It is important to not remove the dam’s scent during the cleaning process, so only the necessary removal of feces and leftover carcasses or food should be removed. No cleaning solvents should be used during the maternal-infant bonding time. The dam’s body condition should be monitored during lactation as more food may be required during this time. Offering food two or more times per day will provide fresh food and the opportunity for the dam to leave the kittens for short durations.
Parturition preparation, equipment, and birth plan [1]
A birth window will be established from the first possible date to the last possible date the dam could give birth.
● List of habitat and nest-area preparations should be created including dates in which they must be completed by.
● A list of potential problems and appropriate interventions should such problems arise.
● Coordination with partner institutions to determine potential cross-foster opportunities, should they become necessary
● A review of normal behaviors and indications that intervention may be needed.
● A detailed review of current husbandry practices and what alterations should take place before, during and immediately after birth.
● A detailed plan if the dam is not providing adequate care.
● List of personnel for impending birth response and communication.
● If hand-rearing is an option- a list of needed supplies, feeding schedules, and estimated timeline completed.
● A schedule of expected milestones (eyes opening, diet increases, weaning) should be created and include medical evaluations and vaccinations.
Monitoring
Nest box cameras will be placed inside of the nest boxes for continual monitoring of dams nearing parturition and for monitoring of dams and kittens after the kittens’ birth. These low-light infrared cameras should allow for complete visualization of the nest box. Bright lights that may stress the dam or kittens should be avoided Digital recording of camera images should be considered to permit later review of birthing and post-birthing activities.
100
Problems associated with parturition [7]
Pre-Partum Vaginal Hemorrhage
Bleeding in the first 2-8 weeks of gestation can indicate fetal resorption or abortion. After 8 weeks, this may be a sign of premature birth.
Dystocia
Nonproductive contractions for more than 60 minutes or a kitten being visible in the vestibule without delivery may be indicative of dystocia. Causes of dystocia include large/misshapen fetuses, narrow maternal pelvis, torsion of the uterus, or cessation of contractions prior to kitten delivery.
Post-Partum Vaginal Hemorrhage
Small amounts of hemorrhage in the first 3 weeks post-partum are not unusual; however, excessive amounts of bleeding with associated declines in red blood cell concentration or hematocrit, or thick, mucopurulent discharge can be a cause for concern.
Retained Placenta
This is difficult to observe as the dam usually consumes the placenta. Dams that develop a fever, become anorexic, or stop nursing should be assessed for a retained placenta. If nest box cameras are used, review of recorded data is helpful to determine if the placenta was consumed by the dam.
Acute Metritis
This occurs within 12 to 96 hours of parturition, secondary to retained fetal tissue or trauma during birth. Dams may become listless, anorexic, and ignore kittens.
Uterine Prolapse
This may occur during parturition or within 48 hours post-partum.
Veterinary procedures associated with parturition: Caesarean Section [8-10]
Once a dam goes into active labor (with abdominal contractions), she should produce a kitten within an hour. If she is continuing to strain and/or no kittens are produced, a veterinarian may choose to intervene. Veterinary intervention, such as caesarean section, maybe advised to save a dam’s life or that of genetically valuable offspring.
Pre-medications
Injectable opioids, alpha-2 agonists, and ketamine can be administered in low doses to allow the individual to become heavily sedated for handling. Once the kittens are born, they can be administered naloxone, naltrexone, atipamezole and/or flumazenil to reverse the effects of the opioids, alpha-2 agonists and ketamine. Once under sedation, intubation is required, and the anesthesia can be maintained by isoflurane gas delivered via endotracheal tube with oxygen.
101
Procedure
Once the patient is intubated, they will be placed in dorsal recumbency, and the hair clipped from xiphoid to pubis and lateral to the inguinal fold on both sides. Clean the surgical area with 3 rounds of alcohol and chlorhexidine or povidone iodine, rotating between the two antiseptics. Do one final prep with alcohol and the surgeon will drape the surgical site. A local midline block can be applied sterilely for anesthesia. The dose of anesthetic (specifically lidocaine) is not to exceed 10 mg/kg of body weight. Make a midline incision starting at the umbilicus and just cranial to the pubis to expose the uterus. Use blunt dissection to enter the abdominal cavity and exteriorize the uterus by lifting it out of the abdomen. Place sterile saline-soaked lap sponges under the uterus to prevent fluids from within the uterus from flowing into the abdominal cavity. Make an incision in the nonvascular portion of the uterine body and milk each fetus from the horn through the opening in the body of the uterus. Remove the amniotic sac from each fetus, place two hemostats on the umbilical cord and cut between the hemostats. One hemostat will remain with the kitten and the other attached to the dam. The kitten is handed off aseptically to an assistant and the surgeon continues this process until all kittens are removed. The uterus is sutured in a 1 to 2-layer closure with 2-0, 3-0, or 4-0 absorbable suture material (polyglactin 910, poliglecaprone 25) in a full thickness, appositional (simple continuous pattern) or inverting (Cushing pattern). Lavage of the uterus and of the abdomen (if needed) will be conducted with warm, sterile saline and a hemostasis check completed for bleeding. The uterus will be placed back into the abdomen and the incision closed. For the abdominal closure, a three-layer closure is preferred (abdominal wall, subcuticular, and intradermal) with skin edges apposed using surgical adhesive. No skin sutures will be placed to prevent irritation, kittens from nursing the sutures, or the dam from ripping out skin sutures.
Additional medications:
(1) Oxytocin can be given after the completion of the closure to help reduce the uterine size.
(2) Pain medication in the form of an injectable or topical opioid.
Kitten care
A heat source should be created in a shallow tub covered with towels. Suction bulbs will be used to clear the nasal and oral cavity of fluid. Small hand towels will be used to dry the kittens and stimulate them to breath or cry. The umbilicus will be disinfected, and the clamp removed at a minimum of 5 minutes after being handed off for hemostasis. If warranted, a single encircling suture can be placed around the umbilicus for hemostasis. Each kitten will be examined by a veterinarian and prior to the dam being recovered, the kittens will be allowed to nurse for a single meal. Then, the dam will be recovered, and the kitten(s) placed with her for further care. This will allow the kitten(s) to have their first meal and then be sleeping while the dam recovers. Take caution placing the kitten(s) with the dam too soon in the recovery as she may accidentally injure the newborns They can be placed in the den box and, following anesthetic recovery, the dam can be released back into the area with the den box.
102
Kitten Rearing
Parent Rearing [1]
Ocelots are typically successful in caring for their kittens, though young, first-time, or otherwise inexperienced dams may not have strong maternal instincts. Although not common, instances of maternal abandonment or injury, cannibalism, inadequate milk supply, or illness may occur in ocelots: hypothermia and injury of the kitten by the dam are the two most likely causes of neonatal death in captivity. In most cases, intervention should not occur to limit potential for habituation of kittens to humans and increase success of the dam’s future litters. To avoid habituation or imprinting on humans, kittens for potential release into the wild should be parent reared by the dam or reared by a surrogate feline dam, with minimal or no human intervention. Although loss of kittens could occur, inexperienced dams can learn from failed litters and future maternal care is likely to improve [18, R. Mossotti, St. Louis Zoo, pers. comm.]. Kittens for inclusion in the breeding program and life in captivity may receive additional human care or intervention if necessary.
If a dam is incapable of providing maternal care, cross-fostering kittens into a surrogate feline species litter at a partnering institution could be a potential solution. Cross-fostering can occur in the early weeks of life (typically from birth to approximately 2 weeks of age) if a surrogate litter is available and kittens are placed in the surrogate litter within 48 hours of separation from the natal dam [11-12; R. Mossotti, St Louis Zoo pers. comm.; S. Dicks, U.S. Fish and Wildlife Service, pers. comm.). During a cross-fostering event, supplemental feeding (i.e., tube feeding) may be needed prior to placement into a surrogate litter (S. Dicks pers. comm.). Kittens cross-fostered into a surrogate feline species litter and then housed with or near conspecifics for socialization and learning, may be considered for release into the wild if their behaviors (e.g., hunting and fear of humans) are equivalent to those exhibited by maternal-reared release candidates and determined to be sufficient for release. If kittens are not exposed to conspecifics during the sensitive period for socialization (2 to 7 weeks) they may not develop appropriate adult behavior such as interest in mating with their own species upon reaching sexual maturity [13-14].
Enrichment and Training Wild Behavior
See wilding procedures.
Assisted Rearing [1, 19]
Hand-rearing
The goals of hand-rearing should be defined at the start of the hand rearing process; the neonate must be identified as an individual that will become a possible captive-held ambassador animal for the species that should be habituated to humans or if it will become a non-habituated individual incorporated into the Saving Animals From Extinction (SAFE) or Species Survival Plan (SSP) breeding programs as a breeder, housed at an approved zoological institution. Assessment of the kitten’s future will help determine the frequency, duration, and type of human or ocelot socialization needed for the neonate. Because early socialization is important [2022] hand-reared kittens should be raised in groups. Hand-reared kittens may be reared with conspecifics or other small felids as long as a medical examination has been undergone and no contagious infectious diseases are
103
present. Hand-rearing of a singleton is not recommended as they do not receive proper early social development and can lack species specific behaviors appropriate to interacting with its environment and conspecifics [14-16, S. Dicks, U.S. Fish and Wildlife Service, pers. comm.]. Contact with humans is not thought to alter development of reproductive behavior as long as socialization with conspecifics occurs early in life [17]; however, in some carnivores, including felids, hand-rearing can cause behavioral abnormalities that result in decreased copulation and lower breeding success [2, 14-15, 18].
If a kitten is removed from the dam, it is important to create a rich and varied environment for any ocelot kitten so that can kittens have the opportunity to develop natural behavior and avoid habituation to humans. Providing ocelots with opportunities for interactions with other ocelots or domestic cats, stuffed animals, or other enrichment may be important for creating environmental enrichment. It is important to have management flexibility to pursue strategies that can aid behavioral development and avoid habituation. Strategies such as playing recordings of mews and sounds of ocelots recorded from other dams or housing kittens close enough to other ocelots in the facility to hear them may be used. Once kittens’ eyes open, methods to avoid visualization of humans should be in place to decrease habituation and association with food. Humans should avoid skin contact to ocelots and work in silence in the presence of neonates.
The goals of hand-rearing should be defined at the start of the hand rearing process. Options include 1) raising the individual to become a possible captive-held ambassador animal for the species with habituation to humans or 2) raising the ocelot kitten to become a non-habituated individual that is used for breeding at the Ocelot Conservation Facility or for reintroduction into the wild. Assessment of the kitten’s future will help determine the frequency, duration, and type of human or ocelot socialization needed for the neonate.
Environment
Even as newborns, hand-raised kittens need to have the ability to use their muscles to move around. They should not be left alone laying on the floor on the isolette with no other stimuli. Kittens should be provided with stuffed toys to climb over while in the isolette or with a surrogate animal for social stimulation. Even though kittens’ eyes and ears are not open, there is a skin to skin or fur to fur touch that helps promote normal neonate behavior. The use of a surrogate domestic cat has been successful with an ocelot kitten in which one of the domestic kittens was rubbed on the ocelot kitten until urinated on by the domestic kitten. When returned to the foster domestic dam, the ocelot was accepted with the domestic kitten [24]. The ocelot did need supplementation in feeding as weight loss was apparent with the domestic cat [24]. When the ocelot kitten was weaned, the domestic cats were removed and the ocelot kitten placed with another ocelot for company [24].
Equipment, and methods for hand rearing of kittens
● Isolette®/incubator (set at 85° F / humidity 45%) or its equivalent. Monitor the neonate for overheating i.e. panting or skin tenting. Normal rectal temperatures in kittens are 95° F in the first week of life, 97° F - 99° F from 2-4 weeks, and 101° F – 102.5 ° F from 6 months to adulthood.
● Polyester fleece/receiving blankets for warming. Kittens may suck on materials, but they should not be allowed to ingest them.
104
● Heating pad placed in a corner of the enclosure, not covering the entire surface. Heating pads should be set on low with a double thickness of bedding to allow the neonate to choose what is comfortable. Test the temperature of the heating pad by placing a hand on it.
● Bottles/nipples; https://www.squirrelsandmore.com
● Milk replacer: Formulas used successfully in ocelot kittens include Milk Matrix 33/40, Milk Matrix 42/25, KMR, and Esbilac®.
● Supplemental lactase enzyme (Lactaid®) decreases gastrointestinal upsets
● Electrolytes may be used in place of sterile water for the first few feedings or if diarrhea develops. Distilled water should never be used as a part of formula.
● Scale to measure weights daily in grams. Typical ocelot kittens likely will weigh 215-250 grams at birth, but weights may vary between 200 and 300 grams
● Body temperature should be monitored daily to determine when the neonates are able to maintain their own body temperature. Once they can maintain it, the isolette can be turned down a degree at a time until reaching room temperature. Neonate’s body temperature should be monitored the entire time.
● Thermometer and hygrometer are used within the room to monitor ambient temperature and humidity.
Formula Preparation
Analysis of maternal milk from domestic cats and lynx shows that milk contains 18 to 22% solids, 28 to 29% fat, 41 to 47% protein, and 21 to 28% carbohydrates [23]. Formulas used successfully in ocelot kittens include Milk Matrix 33/40, Milk Matrix 42/25, KMR, and Esbilac®. Additionally, domestic cat kittens are known to receive 95% of their maternal antibodies from the colostrum. The gut “closes” and no longer allows the transfer of immunoglobulins within 16 hours of birth [23]. If kittens are rejected by their dam or removed from the dam immediately after birth without nursing, kittens will need supplemental immunoglobulins [23]. Administration (IP or SQ) of 75
150 mL/kg of serum from conspecific adults will provide kittens with some immune protection [23]. Veterinary staff should bank and screen serum, so sufficient, disease-free serum stock is available.
Sterile or bottled water (but not distilled water) may be used to prepare formula from powder. Enough formula should be made for a 24-hour period. Warm the bottle of formula for the feeding in a warm water bath, and do not microwave the formula. Only the formula used for that feeding should be warmed, and the temperature of the formula should be 100°F or lukewarm on the wrist. After each feeding, discard any leftovers in the bottle. Bottles and nipples should be washed thoroughly before and after each use by submerging the utensils in metal bowl filled with water and adding Chlorhexidine Gluconate 2% Solution (Nolvasan) to the water at the ratio of 5ml per 480ml (2c).
Feeding
There should be 2 to 3 caregivers to assist with hand-rearing of kittens. In the first few weeks of life, when the kittens are not mobile, care staff should be spending about thirty minutes to an hour with kittens at each feeding time. This includes time for cleaning their bedding, as needed.
An ocelot kitten should weigh around 200-300 grams at birth and gain around 25-50 grams per day. Kittens should be fed every 2 to 3 hours so that the total amount fed in a 24-hour period is 15-20% of their body weight.
105
–
Neonates should be weighed every morning to determine the amount of diet they are fed that day. Since neonates have a stomach capacity of 3-5%, so it is important not to overfeed them. After the neonate begins to nurse consistently, the feedings can be divided into six daily feedings. Feedings should be given between 6 AM and 10 PM to decrease overnight disturbances. A heating pad should be placed under the kitten while they are feeding to keep them warm. When feeding kittens, they should be placed sternal, on a natural substrate, and with the head at a 45-degree angle to decrease risk of aspiration. Kittens’ front feet can push against a soft stuffed animal or towel to simulate kneading when suckling. After all feedings, the formula concentration, amount offered, amount consumed and any important information should be recorded.
The caregiver(s) should stimulate the kitten to urinate after each feeding by gently rubbing the area under their tail. Additionally, caregivers should stimulate the kittens(s) to defecate twice a day by gently stimulating under the kitten’s tail with a wet gauze. Fecal description is very important and must be recorded. The first normal stool will be dark black and thick with mucus (termed meconium). After this, a soft formed, pale yellow, toothpaste consistency stool should appear (termed the milk stool). If the frequency, color, consistency, or diameter of fecal matter changes, it should be recorded and reported to a veterinarian. If diarrhea persists past one feeding, the veterinarian should also be alerted.
If diarrhea persists, possible veterinary interventions include:
1. One feeding of electrolytes and a 50% dilution with electrolytes over the next 8-12 hours.
2. Oral electrolytes (60 mL/kg/day) for 12 to 18 hours.
3. If feedings are omitted, subcutaneous fluids will be provided at 40 mL/kg/day.
Once diarrhea subsides, the formula should be reintroduced gradually and increased daily until back to the suggested weight-based regiment.
Meat can be introduced at four weeks of age in very small amounts (pea size). At four weeks, meat substitutes (turkey flavored baby food) or ground meat in a slurry from prey items may be placed into the formula and should be offered in gradually increasing amounts until the diet can be switched to only meat without formula. Monitoring of stool consistency is important for adjusting the diet. At four weeks, converting kittens to a bowl for formula lapping is also recommended since kittens will start to chew on the nipples. Providing formula in a bowl to simulate drinking water can also begin to acclimate kittens to drinking water once they are on a solid diet and no longer receiving formula. These practices will also assist in the weaning process, which can happen as early as six weeks and no later than 11 weeks.
At seven to eight weeks, introduction of solid meat can be introduced with some formula coating it or alone, dependent on the progress of the kitten. Kittens should be off formula and eating solid food around 10-12 weeks of age.
Cleaning and Cleanliness
Caregivers must wear gloves when handling neonatal kittens. They should also wash hands prior to handling kitten(s) as well as afterward to decrease potential disease transmission to both the kitten(s) and the caregiver. Until the kitten(s) have their first vaccination, caregivers should wear scrubs or surgical gown when handling.
106
Any caregiver that feels sick should not prepare formula or interact with the neonate until they have recovered. If they must interact with a kitten, a caregiver who is sneezing, coughing, or has a runny nose must wear a facemask at all times when interacting with the kitten. Kittens’ bedding should be changed each morning and as it becomes soiled throughout the day.
References
[1] AZA Felid TAG. (In prep.). Small Felids (Felidae) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums.
[2] Hampson, MC, Schwitzer, C. 2016. Effects of hand-rearing on reproductive success in captive large cats Panthera tigris altaica, Uncia uncia, Acinonyx jubatus, and Neofelis nebulosa
[3] Martinez F, Lopez G, Pastor J, Zorrilla I, Munoz A, Garcia I, Peña L, Jimenez MA, Perez MJ, Molina I, Aguilar JM, Quevedo MA, Meli ML, Lutz H, Vargas A. 2009. Integrating health issues into the conservation of the Iberian lynx Lynx (pardinus). In Iberian lynx ex situ conservation: an interdisciplinary approach. Vargas A, ed.
[4] Moreira N, Monteiro-Filho ELA, Moraes W, Swanson WF, Graham LH, Pasquali OL, Gomes MLF, Morais RN, Wild DE, Brown JL. 2001. Reproductive steroid hormones and ovarian activity in felids of the Leopardus genus. Zoo Biology 20: 103
116.
[5] Mellen JD 1993. A comparative analysis of scent-marking, social and reproductive behaviour in 20 species of small cats (Felis). American Zoologist 33(2): 151-166.
[6] Mondolfi E. 1986. Notes on the biology and status of the small wild cats in Venezuela. In: Miller SD, Everett DD (Eds.). Cats of the world: biology, conservation, and management. Washington, D.C.: National Wildlife Federation. 85-124.
[7] Feldman, EC, Nelson RW. 2004. Feline reproduction. In R. Kersey & D. LeMelledo (Eds.), Canine and Reline Reproduction (pp. 1016
1045). St. Louis, MO: Saunders.
[8] Onclin KJ, Verstegen JP. 2008. Cesarean section in the dog. In: Reproductive medicine/Surgery. NAVC Clinician’s Brief https://files.brief.vet/migration/article/1512/08_may_article_12-1512-article.pdf
[9] Hesser A. 2019. Canine Caesarian Section-Tips and Tricks. World Small Animal Veterinary Association Congress Proceedings. Canadian Veterinary Medical Association. https://www.vin.com/apputil/content/defaultadv1.aspx?id=9382876&pid=24437&
[10] Zeltzman P. 2013. The 3 Surgical Options for -Sections. Veterinary Practice News
https://www.veterinarypracticenews.com/the3-surgical-options-for-c-sections/
[11] Kitchen AM, Knowlton FF. 2006. Cross-fostering in coyotes: evaluation of a potential conservation and research tools for canids. Biological Conservation 129(2): 221-225.
[12] Dicks S, Mossotti R, Rein T. 2021. USFWS and SSP Pup Fostering Handling Procedures for Mexican wolf (Canis lupus baileyi). U.S. Fish and Wildlife Service.
[13] Rivas A, Martinez F, Sanchez I, Aguilar JM, Quevedo MA, Bergara J, Vazquez dicksE, Cuadrado M, Vargas A. 2009 Handrearing of Iberian lynx cubs. In Iberian lynx ex situ conservation: an interdisciplinary approach. Vargas A, ed.
[14] Mellen, JD.1989. Reproductive behavior of small captive exotic cats (Felis spp.). Doctoral Dissertation. University of California, Davis.
[15] Mellen, JD. 1992. Effects of early rearing experience on subsequent adult sexual behavior using domestic cats (Felis catus) as a model for exotic small felids. Zoo Biology 11(1): 17-32.
[16] Meier JE. 1986. Neonatology and hand-rearing of carnivores. In Zoo and wild animal medicine (2nd edition). Fowler ME, ed.
[17] Advisory Group on Health Aspects of the Iberian Lynx. 2004. Iberian lynx cub breeding manual.
[18] Carlstead K. 1996. Effects of captivity on the behavior of wild mammals. In Wild mammals in captivity: principles and techniques. Kleman DG, Allen ME, Thompson KV, Lumpkin S, eds.
[19] Mellen JD, Wildt E. (Eds) 1998. Husbandry manual for small felids. Lake Buena Vista, FL: Disney's Animal Kingdom, in association with the American Zoo and Aquarium Association Felid Taxon Advisory Group.
http://www.felidtag.org/pages/Reports/Husbandry%20Manual%20for%20Small%201998/husbandry.htm
[20] Caro TM. 1995. Short term costs and correlates of play in cheetahs. Animal Behaviour 49: 333 345.
[21] Casey RA, Bradshaw JWS. 2008. The effect of an additional socialization for kittens in a rescue centre on their behaviour and suitability as a pet. Applied Animal Behavior Science 114: 196-205.
[22] Rochlitz I. 2000. Recommendations for the housing and care of domestic cats in laboratories. Laboratory Animals 34: 1-9
[23] Hedberg G. 2002. Exotic felids. In L. J. Gage (Ed.), Hand-rearing wild and domestic mammals (pp. 207–220). Ames, IA: Iowa State Press.
[24] Dunn GL. 1974. Use of a domestic cat as a foster mother for an ocelot: Felis pardalis International Zoo Yearbook
107
–
–
Behavioral Preparation (“Wilding”) Program
Individuals produced at the Ocelot Conservation Facility who are genetically suitable for reintroduction must be behaviorally prepared for life in the wild. Large, outdoor, and quasi-natural “wilding” enclosures will be established at the Ocelot Conservation Facility to allow captive-bred ocelots to develop the normal, natural behaviors - such as exploring the environment, socializing, successfully hunting and consuming prey, and fearing humans - that will be necessary for their success in the wild after release. Ocelots will be wellmonitored within these enclosures to evaluate their behavioral development, inform management of the ocelots, and determine whether they are prepared for release to the wild. Guidelines for rewilding ocelots have been developed based on wilding programs for captive-bred Iberian lynx (Lynx pardinus) [1], Persian leopards (Panthera pardus tulliana) [2], and jaguars (Panthera onca) [3-4].

108
Photo courtesy Fin and Fur Films
Wilding Enclosures
Enclosure Size and Materials
The wilding enclosures are located within the Ocelot Conservation Facility. There will be at least four 0.25-acre (~1,012 m2) enclosures that will provide a quasi-natural environment for development of wild behaviors in captive-bred ocelots. As needed, each enclosure can be connected to adjacent enclosures to provide larger spaces or subdivided to provide more individual enclosures. Enclosures should be approximately three meters in height and though not top-covered, should contain positive cantilevers to avoid ocelot escape through the top of the enclosures. Enclosures should also have a series of electric fencing to prevent ocelot escape at the ground level and to prevent other animals from entering. To prevent excessive escape of prey items from inside the enclosures, smooth surface barriers will be placed along the bottom of the enclosure fencing. Other designs options may also be considered to decrease the chances of prey escape.
To promote development of social awareness and behavior, ocelots in the wilding enclosures should be allowed to live with their dam and siblings and to make visual - but not physical - contact with other unrelated ocelots in nearby enclosures. There must be physical distance or actual physical barriers between adjacent enclosures to completely prevent any direct physical contact between unrelated ocelots. Holding unrelated ocelots in the same enclosure (except for breeding pairs or surrogate dam/kitten groups) is not recommended in order to avoid ocelot-ocelot conflict.
General Environment/Habitat of Enclosures
The wilding enclosures should be environmentally rich to support ocelots’ ability to develop wild behaviors [56] The environment should include free-ranging live native prey and a vegetation community that will mimic the community present at the reintroduction site: Tamaulipan thornscrub. Raising ocelots in a similar environment to that they will experience in the reintroduction site will support their ability to prepare for life at the reintroduction site. Additionally, the presence of a variety of natural materials (including plant structures, logs, rocks, shelves, etc.) will allow ocelots to experience a three-dimensional space and ultimately develop behavioral and psychomotor skills necessary for successful movements in the wild.
Tamaulipan thornscrub habitat is characterized by diverse woody and herbaceous thorny shrub communities with canopy cover that ranges from open to dense and can be 3 to 10 meters high. Shorter woody vegetation may form an understory below an upper story of Texas ebony (Ebenopsis ebano), mesquite (Prosopis glandulosa), or live oak (Quercus virginiana) trees. Woody and shrub understory species may include whitebrush (Aloysia gratissima), lime prickly ash (Zanthoxlyum fagara), cat-claw acacia (Acacia greggii), blackbrush (Acacia rigidula), and a variety of cacti [7-8]. Dense, medium-height native and invasive grasses often occur interspersed between woody communities and can provide suitable cover for ocelots’ exploratory and hunting movements [9-10]. Existing vegetation already present at the wilding enclosures can be maintained there during facility construction and other species may be planted and grown as necessary to make the environment more similar to the reintroduction site.
109
The density of vegetation in the wilding enclosures must be balanced with monitoring needs. Monitoring is needed to evaluate behavioral development of ocelots to determine whether they are prepared for release to the wild. While ocelots may use highly dense vegetation in the wild, especially for daytime resting sites, vegetation throughout the enclosure cannot be so dense that video monitoring of ocelots is impossible; there must be some openings in vegetation for monitoring.
Environmental Enrichment
Complex environmental enrichment, beyond vegetation and free ranging live prey, is necessary to mimic the habitat at the reintroduction site, avoid ocelot habituation to captivity, and create an overall stimulating environment for ocelot’s behavioral development. There should be standing environmental enrichment always present in the enclosures, while introducing new and variable environmental enrichment will be important in cases where ocelots show signs of abnormal or repetitive stress behaviors and thus a need for increased stimulation [11-12]. For any enrichment added to an enclosure, the desired outcome of its use should be clear, and personnel should evaluate whether the environmental enrichment will contribute to the development of a specific natural wild ocelot behavior and if the enrichment is appropriate for a given individual based on age, experience, and personality.
Standing environmental enrichment should include:
● Free-ranging live native prey species (small mammals) that ocelots can catch and consume. See sections below for further information on prey.
● Natural objects to touch such as sand, water, leaves, grass, stones, etc. and logs to scratch or use for marking of territory.
● Areas such as wildlife trails, open areas, and refuge spaces (under branches, on ledges, etc.) that provide places for hiding, eating, resting, and sheltering from weather conditions.
○ Refuge spaces should be created from natural, not human-made, materials except for a nest box or den for dams with young kittens. A human-made but natural-like nest box or den can be provided for dams with kittens, and it can be removed or remotely closed once kittens have aged to encourage the dam and kittens to seek more natural refuge spaces. The nest box can be replaced or re-opened as needed.
● Objects to climb on (to explore, see the habitat from above, develop motor skills, and experience the enclosure three-dimensionally) such as trees (fallen and upright), series of connected logs, slopes and valleys/ditches, and rocks.
○ Any climbable objects should not allow ocelots to escape from the enclosure.
● Other ocelots
○ Siblings and the dam should be held in the same enclosure to allow natural social behaviors, though the dam must be captured and removed from the enclosure to separate her from the kittens after they have matured and developed sufficiently (see more in sections below)
■ Cross fostering of kittens to other ocelot dams may be warranted due to a negligent or aggressive dam or other issues (e.g., maternal health concerns, lactational failure, etc.). Fostered kittens are typically within 2-3 weeks of age of and similar in size to the neonatal kittens of the foster dam. A dam ocelot with similarly aged kittens may not be available for
110
cross fostering. The Ocelot Conservation Facility should coordinate with partner zoological institutions during breeding to assess the availability of possible surrogate feline species for cross foster opportunities. If no cross-fostering matches are available, use of a domestic cat and kittens to aid in raising ocelot kittens and developing their behavior may be possible Supplement feeding may be necessary for ocelots raised by domestic cats, that may not produce enough milk for ocelot kittens. See Kitten Rearing section for more information. Any surrogate felines used for cross fostering must be screened for disease so they do not introduce any health risks to ocelots.
○ Otherwise, ocelots are typically not held with unrelated individuals in the same enclosure unless they are a male-female pair being housed together for breeding or surrogate dam/kitten groups
○ Ocelots in nearby enclosures are physically separated from each other, though individuals should be able to see, smell, and hear one another. Removing visual access between enclosures or reducing visual access to small viewsheds by using a shade cloth may be necessary to obscure views into neighboring enclosures if viewing of other ocelots is causing individuals to pace or exhibit other stereotypy or abnormal behavior.
Additional environmental enrichment may be added on a case-by-case basis to the wilding enclosures and may include:
● Movement of ocelots to different enclosures
○ Ocelots can be moved to different wilding enclosures to provide novel enrichment via new environmental interactions. Moving ocelots to different enclosures may also be needed to relocate ocelots who are causing stress to others or to resolve abnormal behavior in an ocelot.
■ For example, ocelots can be transferred into recently unoccupied enclosures so they can experience the scents, pawprints, etc. of the ocelot who was there before.
● Olfactory stimuli from potential prey or other ocelots
○ All scents must be free of any health risks.
○ Feces or urine of other unknown ocelots may be placed in the enclosure.
■ Scents may be captured from the wild or elsewhere in the Ocelot Conservation Facility and placed in an enclosure.
■ Some feces or urine of ocelots who previously lived in the enclosure can be left behind when new ocelots enter the enclosure.
○ Prey scat, urine, or parts (such as feathers, tails, ears, etc.)
■ Can be collected from the wild or the prey colony.
○ Plant parts collected from the reintroduction site.
○ Bobcat, coyote, or puma (potential predator/competitor) urine or feces
■ Smells from these carnivores should only be used minimally and for short periods to prevent ocelots from becoming habituated to other carnivores’ smells absent of any actual interaction with the other species. Scent objects used will be removed following brief introduction to ensure its does not linger in the environment resulting in habituation to the smell.
● Auditory stimuli (sound recordings) from potential prey, from other ocelots, and from other carnivores
111
○ As for scents, noises should only be played minimally to avoid habituation or excessive stress to an ocelot.
● Novel human objects for interaction
○ Any human object used as enrichment must support development of a specific behavior, such as exploring the environment or learning to use livestock or wildlife water tanks that will be available in the reintroduction site. The purpose/goal of the enrichment must be established prior to its use and there should be defined criteria for evaluation of the effectiveness of human objects as enrichment. Additionally, prior to the use of any human-made objects for enrichment, a defined process and schedule for presenting the objects must be established.
○ Positive enrichment items, such as hanging balls or bags with smells or meat inside can be used to stimulate ocelots as well as help them to practice motor skills. Objects such as these should be provisioned at random times, moved around the enclosure, and eventually removed completely so that any human objects remain novel, and ocelots do not become habituated to them.
○ Commercially available pet toys such as balls should not be used.
● Negative enrichment for behavioral training
○ Negative enrichment can be used to teach ocelots about dangerous objects. A dangerous item, for example, a human-made object, a domestic animal, or a predator/competitor species could be presented safely but with a negative reinforcer (e.g., loud noise or other harassment) to discourage possible interactions with that dangerous object in the future [13].
Abnormal Behavior
Abnormal behavior, including stereotypy, could be a sign of behavioral estrus in females or simply the ocelot patrolling its home range. However, it could be a sign of a behavioral issue for an ocelot. Should an abnormal behavior such as stereotypy occur in an ocelot in the wilding facility, the ocelot should first be further monitored and evaluated by personnel trained in behavioral studies. Personnel should assess the details of the ocelot's behavior, the ocelot’s emotional state, the location of the behavior, the potential cause of the behavior (i.e., keepers, noises, ocelots in neighboring enclosures, etc.), and any impacts of the behavior to other ocelots. Multiple complete observations of an abnormal behavior should be evaluated by necessary personnel to determine an individualized behavioral intervention, which may include the introduction of additional environmental enrichment presented above.
Water
Multiple water sources will be present in every wilding enclosure to assure access to clean drinking water. Small, natural-like water pools underneath vegetative cover should be constructed, if possible, to provide water to ocelots and prey animals. Water at these sources will be non-flowing and will be monitored for algal growth and mosquito larvae. Additionally, each enclosure should have one or more tanks that are plumbed for running water. These tanks will mimic existing cattle/wildlife water tanks present on ranches in southern Texas that could be used by ocelots upon release to the wild. If necessary, water tanks can be placed close to the perimeter of the enclosure to ensure the ability to refill from the outside of the enclosure.
112
Cleaning
Given that enclosures are large and should be wild-like, cleaning needs will be minimal. Cleaning of enclosures may occur as necessary but must be balanced with the need to limit human interaction with ocelots in the wilding enclosures. Removing food remains, waste, or other biological materials and cleaning of water pools and/or tanks may occur, when necessary, at the discretion of veterinarian and keepers. The best opportunity for cleaning is likely after an ocelot has been removed from the enclosure and is being held elsewhere.
Humans at the Wilding Enclosures
Human intervention in the wilding enclosures should be minimized as much as possible so that ocelots can be allowed to cope with natural elements and the various challenges of living in a natural environment without assistance. Human-ocelot contact must be minimized at the wilding enclosures. Enclosures should only be visited by authorized personnel on official business; interested persons cannot observe ocelots in the wilding enclosures in-person (although they can watch live video surveillance obtained from cameras in the wilding enclosures). Enclosure size (just over 1,000 m2) and the presence of natural cover will reduce the likelihood of a keeper encountering an ocelot when entering an enclosure for feeding, watering, maintenance, or other activities. However, if keepers do encounter an ocelot while working in or around an enclosure, they should scare the animal away and introduce a negative stimulus by yelling, throwing water, or throwing other objects near the ocelot, for example. Keepers may also periodically walk the perimeter of an enclosure to test whether any ocelot approaches and should scare away any ocelots that do approach people or fail to flee. An ocelot that shows signs of a positive or neutral response to human presence, even with negative stimuli, may ultimately be considered unsuitable for release to the wild.
Feeding
Prey Species and Sources
Provisioning a variety of live prey to ocelots in the wilding enclosures is essential for allowing ocelots to learn to catch and consume different types of prey, as they must do upon release to the wild. Ocelots being trained for release to the wild should be provided live prey as much as possible, and hunting and feeding behaviors should be monitored with video surveillance to assess hunting and feeding proficiency. Feeding ocelots with chunks of meat that are not associated with a whole live prey animal should be avoided where possible. If meat must be fed to ocelots, it is recommended to use meat from native prey animals found in Texas. Prey species native to southern Texas will be utilized for feeding in the wilding enclosures to avoid the possibility of introducing nonnative species to the environment. Prey escape from the wilding enclosures will be minimized by placing surface barriers at the bottom of enclosures.
In Texas, ocelots are known to have a generalist diet that includes birds (roadrunners and passerines), small mammals (shrew, rabbit, mouse, gopher, rat), meso mammals (white-tailed deer fawns), and reptiles (lizards) [14]. The wilding enclosures should be managed to retain as much naturally occurring live wild prey inside to way to mimic conditions in the natural environment. Additional prey animals may be placed inside the wilding enclosures and will be sourced from a prey colony at the Ocelot Conservation Facility. The colony will raise native species of mice, rats, and rabbits. Prey can also be trapped from wild sources and brought to the wilding
113
facility. All food must be appropriately checked by a veterinarian or other staff to verify safety and cleanliness before providing the prey to ocelots. To the best of their ability, keepers should record every source of prey and its estimated weight or age category (e.g., adult, juvenile, or neonate prey) that is provided to ocelots. The results of feeding (fully eaten, mostly eaten, partially eaten, or not eaten) should be recorded, if observed, after feeding.
Food Delivery
Live, free-ranging prey animals should be living in the wilding enclosures prior to the placement of any ocelots there. The method for delivery of additional live prey into the enclosure can be through a tunnel system or other remote system. In such systems, ocelots will not associate humans or human activities with the provisioning of food. In a tunnel system, prey is placed into the top of the tunnel outside of the enclosure, moved through the enclosure, and are ultimately released inside. Multiple tunnels are recommended for each enclosure to increase the complexity of food provisioning and to avoid any habituation; different tunnels should be utilized randomly and at diverse times so that ocelots do not habituate to a single location or time for prey provisioning. Additionally, to avoid ocelots becoming habituated to noise in tunnels leading to the appearance of a prey item, routine use of tunnels and noisemaking without the provisioning of prey is recommended. Prey provisioning can be scheduled to occur mostly when ocelots are active, likely after 4 PM, and at different times to avoid habituation. Video monitoring should occur at the bottom of each tunnel to capture hunting behavior in cases where the prey does not move far from the end of the tunnel.
Types of Food
Four possible types of food for ocelots - meat, dead prey, easy prey, and difficult prey - are summarized in Table 1. The four options represent an increasing level of difficulty for ocelots, who should be encouraged to hunt and eat difficult live prey. The progression from easier to more difficult food sources will be an important part of behavioral development in ocelots. It is recommended to provide as much difficult live prey to ocelots as possible so there are always ample opportunities to practice hunting behaviors. Ocelots who have problems feeding and develop poor health or body condition should be transitioned to easier food types as appropriate.
Meat
In the wilding enclosures, meat should only be provided as a necessary health intervention to support ocelots who are unable to eat whole prey. It is preferable to source meat from native live prey animals, though meat from other sources could be used as necessary. In the event meat alone is provided for substantial periods of time (greater than a few days), supplementation of minerals and vitamins may be recommended. At that time, personnel will consult with nutritionists and zoo-based feeding systems.
Dead Whole Prey
Dead whole prey can be provided as a health intervention for ocelots that prove unable to catch live prey as well as to kittens that should be beginning to eat meat and play with prey (beginning at approximately 8 weeks age) but whose dams are unable to kill prey and bring it to the kittens. When dead prey must be provided, unskinned carcasses are recommended, as this will require ocelots to learn to remove the skin. The exception is for kittens
114
needing to start eating meat but whose dams will not skin dead prey; unskinned carcasses may be provided instead.
Dead prey can be added to the enclosure through a tunnel at random times and locations, it may be placed by a keeper in hidden or difficult-to-access places as appropriate, or it could be thrown into an enclosure Providing prey in diverse locations will reduce the likelihood of ocelot habituation to any location, including to the bottom of tunnels. Placing dead prey (for sub adult or adult ocelots, not kittens) in difficult places will require ocelots to explore and search for food to find it and then exert energy to get it. Dead prey can be placed high up or hung to require some climbing or jumping to get the prey. Program managers must consider a dam’s ability to find dead prey when providing it for the benefit of kittens. Case-by-case management of the feeding of dam and young kittens, including where and when to place dead prey and whether it should be skinned, is expected to be necessary.
Easy Live Prey
Easy live prey should be provided to young ocelots that are beginning to learn to hunt or to ocelots that are having challenges with hunting and need easier hunting opportunities. Easy prey may include young, domesticated, or more habituated animals that do not fear predators or have the skills to escape from them efficiently. Additionally, small prey of a consistent variety may be easier for ocelots to catch. Easy live prey can be added to the enclosure through the tunnel system. The prey will be naive to the enclosure upon placement there, potentially making the prey animals easier to catch. If an ocelot is unable to catch easy prey that is freeranging, program managers may consider placing prey in confinement so that ocelots can easily catch the prey and develop hunting skills. Small, open-top cages that an ocelot can climb or jump into, but the prey cannot escape from, can be used, though care should be taken to minimize disruption to ocelots while placing this prey and to avoid any habituation to prey at the cage. Habituation can be avoided by changing the location of the cage or the timing of prey placement.
Difficult Live Prey
Ocelots who are at higher stages of behavioral development should be able to sustain themselves with only difficult prey, including free-ranging prey already living in the enclosure and additional prey animals added through the tunnel system or other remote systems. The most difficult prey to catch in the enclosure will be wild, live, free-ranging prey that has been established in the enclosure prior to ocelots’ placement there. This prey may already be adapted to the enclosure and may prove the most difficult for ocelots to find and kill. Such prey should always be present in the enclosures to provide enrichment for ocelots through constant opportunity to catch and eat prey. Surface barriers will be placed along the bottom of the enclosure fencing to reduce prey escape. Difficult live prey can also be added to the enclosure through the tunnel system. To make this prey difficult to catch, the prey animals should be healthy adult animals. A variety of native wild – not domesticprey species, including larger ones, should be provided. Wild-trapped prey may prove more difficult to catch than prey sourced from the prey colony.
115
Table 1. Categories of food for ocelots at the wilding facility, with meat being the easiest source of food for ocelots that do not hunt and difficult live prey the most challenging food for ocelots being prepared for life in the wild.
Food Category Meat
Types Chunks of meat, preferably from native prey animals
Dead Prey “Easy Live Prey” “Difficult Live Prey”
Unskinned, dead whole animals
Skinned (for nursing or young, but no longer nursing, kittens)
Young, small, domestic breed, confined to cage, naive to enclosure, same species always
Adult, larger, healthy, wild breed, freeranging, adapted to enclosure, variety of species, wild trapped.
Uses Only in cases of health concern where ocelots will not eat other food sources.
For ocelots learning to eat whole prey but not yet hunting and whose dams do not bring them dead prey
For ocelots beginning to learn to hunt, needing easy opportunities to successfully kill prey, and not yet ready for more difficult prey
For individuals showing success in hunting easy prey
Placement Tunnels
Placed by keeper in hidden or difficult-toaccess location
Tunnels
Placed by keeper in hidden or difficult-toaccess location
Tunnels
Placed by keeper in confinement, if necessary
Treatment of Female Breeding Ocelots at the Wilding Facilities
Tunnels
Free-ranging in enclosure before ocelots are placed there.
The ability to hold adult females in wilding enclosures before they breed or give birth will depend on the availability of open wilding enclosures. If possible, adult female ocelots, especially captive-born individuals, should be exposed to the wilding enclosures prior to giving birth to kittens. Spending time in the wilding enclosures will support practice of natural behaviors - such as hiding, exploring, and hunting - that the females can practice and then teach to their offspring (though it is possible that kittens may be able to learn to hunt even if the dam does not hunt). Females who have experienced the wilding facility and/or show better inclination to hunt and search for food may be preferred for breeding.
Adult breeding females can be held alone in wilding enclosures before they breed to become familiar with the more natural environment present there. This pre-exposure to wilding enclosures will give them experience utilizing the enclosures and it may reduce stress following relocation to the enclosures with kittens. Additionally, live breeding events with males can occur in the wilding enclosures. This may work well for consistently breeding pairs or for wild-sourced individuals who may prefer to breed in the wilding enclosures versus in the breeding enclosures.
116
Program managers and veterinarians will determine, based on individual behavior and space needs, when it is appropriate for a breeding female to be in a wilding enclosure, and if she should breed and give birth in the large wilding enclosures or the smaller breeding enclosures. It is not recommended to move ocelots to different enclosures during pregnancy due to possible stress. First time, inexperienced, or previously unsuccessful dams should give birth in the breeding enclosures, which are easier to monitor, while proven dams that have already raised one or more successful litters may give birth in wilding enclosures if program personnel are comfortable with this. While in the wilding enclosures, females should be encouraged to catch and eat live wild prey animals. Live, free-ranging prey animals (i.e., difficult prey) should be consistently available in the wilding enclosure when a female is present so that hunting and feeding skills are practiced.
An individualized feeding plan based on health and hunting abilities will be developed for each female, who will be monitored through video surveillance within enclosures. Food for females can range from the dead whole prey to difficult live prey. If an adult female is initially unable to successfully sustain herself by hunting live prey, easy prey will be provided to encourage development of hunting and feeding skills. The “difficulty” of live prey should be increased over time as hunting and feeding proficiency increases and body condition is maintained. Additionally, the location and timing of prey provisioning can also become increasingly random as the female develops hunting and eating behaviors. Fasting periods (1-3) days can be used to stimulate hunger and hunting behavior. However, if a female is unable to hunt and shows signs of declining body condition, easier prey should be provided to maintain the ocelot’s health. As described previously, both the types of prey provided to ocelots and the degree of ocelots’ consumption should be recorded so that managers and veterinarians can evaluate the efficacy of the feeding plan and make adjustments as needed.
Timeline for Management of Releasable Ocelot Offspring
The following is a suggested timeline for management of releasable ocelots in the breeding and wilding enclosures (summarized in Table 2). The timelines presented here are general and are based on what is known of ocelot biology (including anticipated age of motor skill development, hunting skill development, and dispersal from the dam, for example) by field ecologists and zoological managers. However, all individual management of ocelots should ultimately be dictated not by the exact timelines here but by the actual behavior and development of the individuals. For example, the exact timing of separating a dam from the kittens should be determined based on the offspring’s ability to hunt and its degree of independence from the dam, including time spent away from her. The determination of when exactly to remove an ocelot from the wilding facility for transfer to the reintroduction site will depend on its “readiness,” which includes its success in hunting and consuming prey, exploring habitat, and avoiding humans. When the program is implemented, managers will gain experience in identifying the development of key behaviors or other signals that indicate it is an appropriate time to transition management. Creating a behavioral development timeline for each dam and her kittens will establish baseline information used to inform subsequent management.
The dam and offspring should be kept together in the breeding and wilding enclosures so the offspring can learn from the dam and socialize together normally. Throughout their time in the enclosures, siblings should have the opportunity to play, compete for space or food, stay close to each other, move farther apart from each other, or even avoid each other. The social interactions between kittens and dam should be monitored to evaluate the growing independence of kittens as they age. Only when offspring reach approximately 10-12 months old and
117
show proper independence and hunting ability should the dams be separated from the offspring. Decisions will be made based on behavioral criteria evaluated by program managers.
Table 2. General suggested timelines for management of captive-bred ocelots from birth to release. Ocelots are born in a breeding or wilding enclosure, live in the wilding enclosure with their dam until they have developed necessary behavior, and are separated from their dam for several months to practice skills without the dam, and are finally transferred to the reintroduction site.
Offspring approximate age
Birth until 6-12 weeks (kitten)
Location Breeding enclosures or wilding enclosures, depending on circumstances
Offspring behavior
Kittens nursing and staying in den with siblings and dam
6-12 weeks up to 10-12 months (kitten)
Wilding enclosures
From 10-12 months up to 1218 months (subadult)
Wilding enclosures
From 12-18 months (subadult)
Reintroduction site (soft-release facility or hard release)
Kittens, with dam and siblings, transferred to wilding enclosures (if not already there) to develop exploratory, hunting, and social behaviors
Once behaviorally ready to be separated from the dam, offspring are separated from their dam to practice hunting on their own
Soft or hard release of ready subadult ocelots
Birth
Until 6-12 Weeks: Breeding or Wilding Enclosures with Dam
Kittens may be born in breeding enclosures or in the wilding enclosures based on veterinarian and facility manager evaluation of factors such as the dam’s behavior, veterinary needs, and the availability of enclosures. For ocelot kittens who are intended to be released to the wild, preparation for life in the wild will begin at birth. Even in the first weeks of life when kittens are blind and moving minimally, kittens should be exposed to natural sounds, smells, and surfaces so that their senses can begin developing naturally. For example, once kittens begin to stand and walk, they should be able to touch various surfaces (such as sand, pebbles, vegetation) on the floor/ground so they can gain sensory knowledge of different surfaces and develop their motor skills. Further, kittens should not be allowed to habituate to human presence, as this could cause a lack of fear of humans. Human contact (including touching, sounds, or smell) with kittens and their dam should be limited to necessary veterinary care. Around 8-10 weeks, kittens should receive a full physical exam, blood
118
workup, and vaccinations. This process is likely aversive for the kittens because human handling during the exam will be stressful. While kittens are in breeding enclosures and during any care, personnel must avoid any unnecessary touching or talking when in close proximity to kittens. Kittens that are intended to be released into the wild should not be raised to develop positive relationships with people. The number of caretakers in any area should be limited to three or fewer people as possible to limit the number of unique humans who may interact with ocelots. Ocelots may become accustomed to specific humans from their visual, auditory, and/or olfactory cues and may develop better with a smaller number of keepers. Additionally, limiting the number of humans that interact with an ocelot may improve wilding of captive reared ocelots.
Two or more nest boxes or den structures must be provided for the birth of the kittens and for shelter in their early weeks of life before they are moving. Providing multiple options for nests gives options to the dam. Nest boxes can be elevated approximately one foot off the ground where possible and around vegetative cover to allow ocelots to use the cover below the nest box. Each nest box/den structure should be well-monitored by a video camera, preferably with a wide-angle low light camera inside and with a camera outside to monitor activity directly outside the structure. If the dam is giving birth in the wilding enclosure, the nest box/den structure should be as natural as possible. Additionally, there should be a variety of substrates and climbing surfaces present near the nest box/den so that once kittens begin walking, they can experience different environments without moving far from the den. If kittens are born in the smaller indoor-outdoor breeding enclosures, there must be some natural elements added to the space, such as natural materials, sounds, smells, and different floor surfaces. The dam of newborn kittens can be given either meat, whole dead prey, easy live prey, or difficult live prey depending on the environment and its own abilities. Dams must be provided with sufficient food to avoid nutritional stress while nursing the kittens and caring for them.
Between 6-12 Weeks and 10-12 Months: Wilding Enclosures with Dam
Nursing Kittens
Kittens open their eyes around 3-4 weeks old. After 6 weeks, they may begin nursing less, gaining more interest in food, becoming more mobile, and showing greater interest in exploring their surroundings. At this point, the dam may allow the kittens more opportunity to leave the den. Once the dam is consistently allowing the kittens to leave the nest box to move around and once the kittens’ veterinary exam has been conducted, the kittens and their dam may be removed from the breeding enclosure and transferred to an available wilding enclosure (if kittens were not born there). The exact timing of a transition will be determined by program managers based on the health and behavior of the ocelot dam and kitten. When the kittens and dam are moved to a wilding enclosure, the nest box should also be moved there. It should be placed near different structures and surfaces that kittens can experience proximate to the nest box. Camera monitoring of the nest box should continue until dam and kittens no longer use the location. If there are no negative effects of the nest box, it may be left in the enclosure. The nest box may be equipped with a remote device to open and close it. This may assist in capturing dam and kittens and transferring them to appropriate locations.
When the dam and offspring are in the wilding enclosure, the dam should continue to have access to prey/food based on its hunting skill level and energetic needs. When kittens are still nursing, they should start to develop single elements of hunting behavior in their play, such as hiding and stalking. Since kittens may initially be
119
afraid of moving live prey, any delivery of live prey should occur away from the kittens. Though kittens will not yet accompany the dam on hunts, the dam should begin bringing her kittens dead prey or prey parts (tails, ears, etc.) so they can learn to play with prey parts. For dam who cannot do this, prey parts can be delivered to the kittens through remote systems, by tossing prey parts inside the enclosure, or by hand by keepers
Once kittens begin the process of being weaned from the dam (after 6-12 weeks) and can begin eating solid food, the proportion of wild meat in the kittens’ diet should be maximized. The dam should be provided additional food as necessary, including easier and smaller live prey if that helps her bring more to the kittens and encourage their curiosity about live prey. Program managers should determine on a case-by-case basis what prey should be provided for the dam, how that prey should be provided, and if food needs to be delivered more directly to the kittens to support interest in prey, meat eating, and playing with prey or prey parts. All data regarding provisioning and consumption of prey will be recorded to the degree possible. As kittens age, develop, play with prey, or prey parts, and eat more meat, they should also begin to show the first signs of the ability to perform sequences of hunting behavior while playing and moving around the enclosure.
Weaned Kittens Developing Wild Behaviors
When kittens no longer are receiving milk from the dam, they will begin learning to hunt by playing with prey brought to them by their dam and then by following their dam and participating in hunts. Once kittens begin to participate in hunts, dead prey or meat should no longer be provided, unless this is necessary to rescue declining body condition. If dead prey or meat must be fed, it should be hidden in different areas around the enclosure, delivered at random times, and/or placed in difficult-to-access locations so that the kittens must search for the prey and expend energy to get it.
When kittens are first participating in hunts with the dam, a large amount of easy live prey should be provisioned in the enclosure so that kittens have ample opportunities to learn. Initially, kittens should learn how to catch prey, though they may have challenges with properly killing and eating it. As kittens develop skills in hunting and feeding themselves, prey provisioning should transition to more difficult prey items. Management of this transition must be dictated by the kittens’ hunting success and body conditions. As kittens improve in hunting ability, they should begin hunting and successfully catching prey independently. Managers may explore temporarily separating the dam from the kittens for short periods to encourage the kittens to hunt on their own and to evaluate their ability to hunt independently. This would require capture and removal of the dam or holding of the dam in parts of the enclosure where doors can be remotely closed.
In all cases, it is important for any provisioning of prey through the tunnel system to occur at random times and locations around the enclosure so that kittens learn to search for prey and do not become habituated. Additionally, periods of 1-3 days with no provisioning of additional prey can be introduced to stimulate hunger, hunting behavior, and attempts to catch the free-ranging prey in the enclosure. Finally, since hunting failures may stimulate hunger and further hunting behaviors, failures should not automatically result in the feeding of easier prey, including dead prey or meat. Evaluation of both individual behavior and body condition is necessary for making management decisions on feeding.
120
From 10-12 Months Until Release: Wilding Enclosures Without Dam
Eventually, subadult ocelots and their siblings should be permanently separated from their dam to further their independence, test the offspring’s ability to support itself, and allow the dam to breed again. The exact timing of separation will be dictated by the subadults’ “readiness” for separation and is expected to occur sometime after 10-12 months of age. Once offspring are separated from the dam, they should not be reunited with the dam, though siblings may stay together. As such, offspring and the dam should not be separated until the offspring shows proper development of behavior and readiness for independence.
Readiness for separation should be determined by several factors. First, subadults must be observed spending an increasing amount of time away from their dam, including during both active and resting periods. This signals advancing degrees of independence. When subadults are spending time with the dam, they should be at a farther distance apart from her than they were at younger ages and they should engage in fewer positive social interactions (licking, grooming, etc.) than they did as younger kittens. Negative interactions during close contact may also increase as the dam and offspring are becoming ready to be separated. Separation should not occur if dam and offspring are still closely tied socially. Second, subadults should be spending more time exploring the enclosure without the accompaniment from the dam. They should be observed using a larger extent of available space in the enclosure than they did as younger kittens. While exploring, ocelot subadults may also begin performing marking behaviors (e.g., urine spray, scratching). Finally, subadults must be able to consistently catch, kill, and eat “difficult” live prey and maintain their body condition independent from the dam’s help or human intervention.
When a subadult is ready to be separated, the dam can be removed from the wilding enclosure and placed back into the breeding enclosures. Siblings may stay together after the dam has been removed if both show the ability to hunt and feed themselves. The siblings may continue to interact with each other and hunt together or by themselves. In cases where there is concern that one sibling hunts and the other only steals food from its sibling, siblings can be separated to test the hunting abilities of all siblings independently. Finally, in a case where one subadult is ready and other sibling is not, the ready subadult could be separated from the sibling and dam while the sibling who is unable to hunt can stay with the dam Once separated from the dam, the ocelot subadults should be fitted with a Global Positioning System (GPS) collar with an accelerometer for monitoring purposes
Upon separation from the dam, subadults should be held in the wilding enclosures for approximately two additional months, or more, without their dam to continue developing skills and to ensure that they are ready for release. Subadults in the wilding enclosures without their dam should be provisioned with only live, difficult prey for the remainder of their time in the wilding enclosures. Subadults should not be transitioned to easier prey unless their body conditions decline, and easier prey is needed to maintain health. Once offspring have been separated from the dam, they should continue to practice their natural behaviors in the wilding enclosures up until they are ready to transfer to the reintroduction site. Ocelots’ health, exploratory behavior, hunting skills, reaction to humans, and other factors as necessary will be evaluated to determine if they are ready for release to the wild.
121
Selection of Ocelots for Release
Only healthy ocelots who clearly display proper wild behaviors are suitable for transfer to the reintroduction site and release to the wild. Wild behaviors will be monitored using cameras, radio collars or other methods in the wilding enclosures. Health may be observed remotely or after capture and full assessment, under anesthesia, of the ocelot. If there are concerns about the readiness of an ocelot for release, regardless of its age, it should not be transferred out of the wilding facility. An ocelot should be kept in the wilding facility to continue its behavioral development with individualized management until it is ready for release. Animals that never reach levels of behavior that are clearly suitable for release could be maintained at the facility for breeding or could be transferred to cooperating zoological institutions.
Characteristics of releasable ocelots include:
● Have no health/physiological problems currently being treated or otherwise undesirable for reintroduction to the wild (disease, injury, body condition, defect, etc.)
○ Ocelots should be sedated for a full health check and disease screening prior to release to the wild
● Ideal body condition score of 2.5-3/5 or 4-5/9 that, along with its estimated weight, is maintained by the ocelot’s own hunting efforts.
● Proper hunting behaviors
○ Consistent successful hunting behavior, including searching, stalking, catching, killing, and eating multiple types of difficult live prey.
● Proper exploratory behaviors
○ Use of a large extent of the enclosure to explore, look for prey, etc.
○ Full command of different forms of locomotion (walking, running, climbing, stalking, pouncing, etc.)
● Fear of humans
○ Never approaches humans.
○ Flees or hides from humans if it sees them.
Monitoring
It is vital to monitor ocelots’ health and behavior in the wilding enclosure to inform management practices, including feeding, use of enrichment, and transition of ocelots to new settings, for example.
Camera monitoring
Since the enclosures will include Texas-Tamaulipan thornscrub communities, direct visual observation of ocelots in the enclosures will be difficult. Direct observation may also be disruptive to ocelots. Instead, remotely accessible (cellular signal-equipped) cameras should be placed around the enclosure to provide video monitoring of ocelot behavior in all areas of the enclosure. A grid system of cameras should be used to cover the entire enclosure. Additional cameras should be placed at high-use areas, such as watering points, the bottoms of feeding tunnels, the inside of nest boxes/dens, entrances of nest boxes/dens, and the area
122
surrounding nest boxes/dens. Prior to an ocelot’s placement in an enclosure, the vegetation there should be managed as necessary to ensure that the enclosure is not so densely vegetated that monitoring with camera traps is impossible (there must be some openings in vegetation to allow ocelots to be monitored by camera trap).
Feeding locations
The wilding facilities may also have locations where ocelots are fed above a hidden scale that is monitored by camera. Attracting an ocelot to food where it will have to step onto the scale and into live video monitoring will allow remote assessment of health. This area may also include doors that can be closed remotely to capture the individual for veterinary intervention.
Scat analysis
Ocelot scat may be collected from the wilding enclosures for analysis of diet and identification of prey species consumed. Scat may also be used for other health monitoring procedures.
Radio collar monitoring
Ocelots in the wilding enclosures can also be collared with expandable collars that automatically adjust with individual growth Ocelots must be large enough in size (typically collar weight cannot exceed 3% of individual body weight) to be monitored via collar; accordingly, kittens would not be collared. Collar monitoring of dams with kittens will provide additional activity data to supplement monitoring via camera. Though kittens will initially be too small to wear collars, collars could be placed once they become larger. Further, their collars could be used only for a short period of time and designed to drop off or expand in circumference to respond to growth. Subadults separated from their dams and appearing ready for transfer to the reintroduction site could be collared to allow more detailed monitoring of their behavior and suitability for release to the wild. Prior to transfer to the reintroduction site, all individuals should receive a new radio collar with full battery life. Collar monitoring of ocelots in the wilding enclosures can also be useful for collecting baseline activity data (resting, walking, jumping, hunting, etc.) that can inform analysis of wild-activity data obtained from ocelots who are collared and released to the wild.
Behaviors to monitor
Video monitoring (and collar monitoring where possible) should allow daily monitoring of ocelots’ behavior in the wilding enclosures. For each ocelot, a journal that contains a checklist of behaviors with additional detailed notes on the behaviors will allow for tracking of behavioral changes of individuals over time, assessment of behavioral development, and comparison between individuals. A professional animal behaviorist from a zoological or academic background, with the assistance of graduate student(s) and technicians as necessary, will be engaged to provide metrics for behavioral monitoring and to evaluate monitoring data based on these metrics. All monitoring will be used to inform management of ocelots in the facility and ultimately determine whether ocelots are suitable for release to the wild.
Behaviors to monitor in the wilding enclosures should include (though are not limited to) the following. The frequency, location, and time length of all behaviors should be recorded. A time budget for each individual
123
should be constructed regularly to record percent of time spent performing various behaviors, identity of conspecifics interacted with during the behaviors or proximity to other ocelots when behaviors were performed, and where the behavior occurred (three-dimensionally) in the enclosure.
Health
● Body condition score (see veterinary section)
● Signs of injury or illness
● Drinking water
○ Location
○ Source (tank versus natural pool)
● Vocalizations
○ Type
○ Social context
● Defecation and urination, potentially as marking.
Feeding
● Nursing
● Eating meat
● Eating dead or live prey
○ How prey was delivered to enclosure
○ Type of live prey (easy or difficult)
○ Where prey was obtained
○ How prey was obtained (who caught the prey)
● Feeding results
○ Fully, mostly, or partially ate item
○ Did not eat item
● Searching for food
● Hunting (stalk, rush, kill, consume), see below
● Stealing food from sibling or dam
● Etc.
Hunting behaviors
Socializing
● Social activities and whether each is positive, negative, or neutral
○ Social playing with other ocelot (younger age)
○ Solitary play with objects (older age)
○ Social grooming
○ Self grooming
○ Threats (hissing, growling)
○ Fighting
○ Urine marking
○ Flehmen
● Participants
○ Who initiated social action
○ Who received interaction
○ Who stopped the interaction
● Time spent together versus apart
○ By activity type
● Distance between offspring and dam during different activities
Play hunting
● Playing with prey or prey parts
● Fear of live prey
● Performance of single elements of hunting behavior, including stalk, rush, kill, or consume
● Performance of full series of hunting, from stalk to rush to kill to consume
Accompanying the dam who is hunting
● Watching the hunt
● Participating in the hunt
● Characterization of participation
● Catching prey
● Killing prey
● Playing with prey
● Etc.
Independent hunting without the dam
● With sibling or not
● Type of prey pursued
● Use of and success of elements of hunting behavior
● Searching, Waiting/watching, Stalking, Chasing, Pouncing, Catching, Killing, Eating
● Moving dead prey to a new location to eat
● Sharing prey with sibling or dam
● Etc.
124
● Play hunting with siblings/dam (stalking, jumping, etc.)
● Etc.
Resting or sleeping
• Time spent resting versus active
• Location of resting or sleeping
• In nest box/den
• Other location if not in nest box/den
• Distance between sleeping offspring and dam
Movements – measures of dexterity
● Use of different geographic locations in enclosure
● Climbing
● Jumping
● Etc.
Interactions with objects
● Scratching (potentially as marking) for claw sharpening
● Smelling objects
● Manipulating items with paws, nose, mouth
● Urine marking
Human interactions or other dangers
Interacting with man-made items
• Enrichment items
• Nest box
• Cameras
• Water tank
• Fence lines
• Etc.
References
Contact with people or dangerous objects
• Positive (approaching people), neutral, or negative (fleeing or hiding) response to people or any dangerous objects presented (actual or simulated predators, competitors, human objects, etc.)
• How ocelots were scared away from dangerous objects or people
Behavior of person scaring ocelots
• Yelling
• Throwing objects
• Etc.
[1] Vargas A, Breitenmoser C, Vreitenmoser U. eds. 2009. Iberian Lynx Ex situ Conservation: An interdisciplinary approach. Fundación Biodiversidad in collaboration with IUCN Cat Specialist Group.
[2] Rozhnov VV, Yachmennikova AA, Dronova NA, Pkhitikov AB, Magomedov M-RD, Chestin IE, Mnatsekanov RA, Blidchenko EY, Voshchanova IP, Alshinetski MV, Alibekov AB 2020. The restoration of persian leopard in the Caucasus (scientific approach). Moscow. KMK Scientific Press Ltd. 219 p.
[3] Tamboni T, Solís G, Peña J, Ríos Noya M, Di Martino S, Carro N, Paviolo A, De Angelo C, Di Bitetti M, Quiroga Ve, Donadio, E 2018. Proyecto de reintroducción del yaguareté (Panthera onca) en el Parque Iberá, Corrientes, Argentina. The Conservation Land Trust.
127
[4] Solís G, Peña J, Spørring K, Boixader J, Jiménez I. 2014 Programa de funcionamiento del centro experimental de cría de yaguaretés en la Reserve Iberá. Version 3.0. The Conservation Land Trust.
[5] McDougall PT, Réale D, Sol D, Reader SM. 2006. Wildlife conservation and animal temperament: causes and consequences of evolutionary change for captive, reintroduced, and wild populations. Animal Conservation 9: 39-48.
[6] Tetzlaff SJ, Sperry JH, DeGregori BA. 2019. Effects of antipredator training, environmental enrichment, and soft release on wildlife translocations: A review and meta-analysis. Biological Conservation 236: 324-331.
[7] French, JT, Silvy, NF, Campbell, TA Tomeček, JM. 2022. Divergent predator activity muddies the dynamic landscape of fear. Ecosphere 13: e3927.
[8] Leivers S, Campbell T, Bodenchuk M, Tomeček J. 2023. Behavior of wild pigs toward conspecific carcasses: implications for disease transmission in a hot, semiarid climate. Transboundary and Emerging Diseases, 4195199.
[9] Sergeyev M, Holbrook JD, Lombardi JV, Tewes ME, Campbell TA. 2022. Behaviorally mediated coexistence of ocelots, bobcats, and coyotes using hidden Markov models. Oikos e09480.
[10] Lombardi JV, Sergeyev M, Tewes ME, Schofield LR, Wilkins RN. 2022. Spatial capture-recapture and LiDAR-derived vegetation metrics reveal high densities of ocelots on Texas ranchlands. Frontiers of Conservation Science 3: 1003044.
[11] Santymire R. 2019. Saving the Black-Footed Ferret from Extinction: In Theory and Practice. In A. Kaufman, M. Bashaw, & T. Maple (Eds.), Scientific Foundations of Zoos and Aquariums: Their Role in Conservation and Research (pp. 440-474). Cambridge: Cambridge University Press.
[12] Swaisgood R, Shepherdson D. 2006. Environmental enrichment as a strategy for mitigating stereotypies in zoo animals: a literature review and meta-analysis. In Stereotypical Animal Behavior: Fundamentals and Applications to Welfare, 2nd edition. eds. Mason, G., & Rushen, J.
[13] Booth-Binczik, SD, Bradley RD, Thompson CW, Bender LC, Huntley JW, Harvey JA, Mays JL. 2013. Food habits of ocelots and potential for competition with bobcats in southern Texas. Southwestern Naturalist 58: 403–410.
128
On-site Ocelot Release
Methods described here guide the release of ocelots into the reintroduction site identified in southern Texas. Success of ocelot releases will be measured by the released ocelots’ ability to successfully survive, establish home ranges, and reproduce in the wild. As protocols are perfected in this effort, they may be used for future ocelot reintroductions at other sites in Texas or elsewhere. Development of these protocols was supported by study of reintroduction programs for Iberian lynx [1] and Canada lynx [2]. The release procedures documented

129
Photo courtesy Caesar Kleberg Wildlife Research Institute
here should remain dynamic and be updated as the program evaluates release practices and improves methods. Release procedures should maximize the welfare and survivability of individual ocelots given their unique backgrounds and behaviors.
Source Ocelots for Initial Reintroduction
Origin of Ocelots
The ocelot reintroduction program is anticipated to release ocelots that are sourced from the Ocelot Conservation Facility to be established in Kingsville, Texas. There, ocelots will be bred to have the appropriate genetic background and behavior for release into the wild in southern Texas.
Selection of Ocelots for Release
Individual ocelots who are chosen for release (rather than maintenance in captivity for breeding) may be transferred to the reintroduction site. There, ocelots will be released using either hard or soft releases, with the chosen method depending on the status of the reintroduced population at the reintroduction site (see sections below) and success of methods already used.
Only individuals that meet the genetic, behavioral, and physical criteria for release will be transferred to the reintroduction site and released into the wild. No ocelot should be brought to the reintroduction site if there are any reservations about its suitability for release to the wild. Once ocelots are transferred to the reintroduction site, it is assumed that they will not need to return to the Ocelot Conservation Facility.
General baseline criteria for released ocelots include:
Genetics
Must meet the genetic thresholds established for the breeding and reintroduction program (recommended at least 75% northern ocelot subspecies genetics and approaching or above 0.600 level of heterozygosity.) See the genetics recommendations/management document for more information.
Age
Generally, released ocelots should be subadults between 1 and 2 years old, as this is within the natural age for ocelots to disperse from their dam (offspring dispersal behavior was recorded between 13-35 months [3]). Ocelots from the Ocelot Conservation Facility that have reached 1-2 years of age will have had time to develop natural behaviors necessary for life in the wild. Releasing ocelots of this age will also preclude ocelots from spending extensive time in captive facilities in the care of humans, where they could become habituated to people or to life in captivity. A similar strategy, to release 1-2-year-old subadult cats from a breeding program, has proved successful for Iberian lynx (Lynx pardinus) reintroduction [4]. Release of younger carnivores has proved more successful than release of older carnivores, likely due to younger individuals’ increased behavioral plasticity and their reduced likelihood of homing to their previous location [5].
130
Behavior
Ocelots suitable for release must be documented displaying appropriate wild behaviors in the wilding enclosures. The two most critical behaviors that ocelots must display to be eligible for release include successful hunting and consuming of prey and fear of and aversion to humans.
Hunting/consuming live prey
The most important behaviors are associated with hunting. In the wilding enclosures, ocelots who are to be released must consistently practice successful hunting behaviors, including the ability to find prey and then stalk, pursue, catch, and consume live native prey. Ocelots must be able to maintain their body condition and health through their own hunting behavior without relying on provisioning of dead or disabled prey by keepers. Ocelots that only scavenge dead prey or steal prey from others without catching their own must be further assessed and tested on their individual hunting abilities and suitability for release before transfer to the reintroduction site.
Fear of/aversion to humans
Ocelots chosen for release to the wild must have a fear of or aversion to humans. They cannot be habituated to people, and they should not associate people with food. At the Ocelot Conservation Facility, any possible habituation to people will be avoided by minimizing ocelots’ contact with humans and by having humans scare ocelots in the wilding enclosures if any contact does occur. Ocelots currently showing habituation behaviors, such as not fleeing or hiding from people or even pursuing contact with people, are not suitable for release.
Health
If there is any concern about the health or well-being of an ocelot, the individual should not be transferred to the reintroduction site. Ocelots with health problems may perform poorly in the wild and should not be released until the health problem is resolved. Health standards for releasable individuals include:
• No release of ocelots with visual, auditory, cardiac, or other major physical abnormalities.
• No release of ocelots currently being treated for injury or disease.
o This includes no release of ocelots currently infected with a disease that may affect their overall fitness, thus providing additional challenges to survival and adaptation to life in the wild upon release.
o It also includes no release of an animal infected with a disease or disease strain that is not known to be present in the reintroduction site and may introduce a risk to the ecosystem there. Caution must be taken to avoid introducing a novel disease into the reintroduction site.
• No release of ocelots with a low body condition score or failing to maintain their body weight. Body condition should be 2.5-3/5 or 4-5/9 (i.e., ideal with lean or muscular appearance with obvious but not exaggerated limb delineations, see General Health Monitoring document). In the Ocelot Conservation Facility, ocelots must be able to maintain ideal body condition and their weight through their own efforts to hunt and consume live prey. Ocelots with poor body condition or who are losing weight and need human assistance with prey should not be brought to the reintroduction site
131
In summary, only ocelots that exhibit the desired genetic, behavioral, and physical characteristics will be chosen for release.
Preparation of Ocelots for Release
Prior to transport to the reintroduction site, ocelots selected for release should be sedated for a final health examination, which should include a screening for infectious disease. When sedated, ocelots must be collared (or re-collared if they had one while in the wilding enclosures) with a radio collar for monitoring at the reintroduction site after release. (Further details about monitoring can be found in the Monitoring Protocol.) If acceptable health testing results are obtained, collared ocelots may be transferred to the reintroduction site. (See transportation document for transport information).
Release Location
The initial release location for reintroduced ocelots is in habitat identified in the southern portion of the East Foundation’s San Antonio Viejo Ranch (SAVR) [6]. The East Foundation, in cooperation with other partners (including Caesar Kleberg Wildlife Research Institute, Texas Parks and Wildlife Department, and U.S. Fish and Wildlife Service) as needed, will be responsible for the release of ocelots onto this property. Releases may take place in locations on SAVR that have been identified as ocelot habitat [6]. Specific release sites (whether for hard or soft releases) should meet the following conditions:
● Occur close to known sources of refugia (vegetative cover), water, and native prey availability. This will provide released ocelots with immediate access to resources after they are released.
● Not occur at the edges of the identified habitat within the reintroduction site
● Not occur adjacent to any likely human threats to ocelots, including roadways (especially high-speed paved roads) or other human infrastructure.
● As possible, not occur within existing ocelot territories (inhabited by territorial individuals who were released to the wild earlier). While releases close to existing territories may promote site fidelity, releases within the known territory of another individual could lead to intraspecific conflict that could negatively impact the ability of the newly released ocelots to establish in the reintroduction site. Over time as ocelots establish at the reintroduction site, all available release sites may be incorporated into established territories, and it will no longer be possible to avoid releases in occupied territories.
Number of Ocelots to be Released
The goal of the reintroduction program is to conduct sufficient releases to establish a self-sustaining ocelot population at the reintroduction site. An initial population viability analysis (PVA) was created to evaluate the outcomes of 20 simulated strategies that ranged from 1- to 16-year programs [7]. Population abundance and extinction risk after 30 years was recorded for each release strategy to investigate the merits of possible release strategies. The exact number of ocelots available for release year to year cannot be anticipated and the availability will likely fluctuate annually. To be conservative, the PVA modeled a limited number of ocelots available for release. In the first year of releases, the release of either 6 (four females and two males) or 3 (two
132
females and one male) individuals was tested. In subsequent years, either 4 (two females and two males) or 2 (one female and one male) individuals were modeled as available to release in any year.
The PVA simulated ocelot reintroduction into the selected reintroduction site (SAVR) and evaluated the effects of the number of ocelots initially released in the first year as well as the design of supplemental releases. Supplemental releases strategies continuing for 5, 10, and 15 years were evaluated with variation in the number of ocelots released per year (2 or 4) and the consistency of releases (annually versus not). It was estimated that ocelots would have a 33% chance of mortality in the first year after release based on mortality rates for release of captive-bred Eurasian lynx (Lynx lynx) that ranged from 32%-70% [8] and evidence from the Canada lynx introduction program in Colorado that showed that improved design of release protocols, such as using soft releases, results in increased survival of reintroduced cats [2].
It must be noted that the initial PVA model lacks life history parameters on actual ocelot survival, reproduction, and dispersal in the reintroduction site. As such, the PVA assumed that ocelot life history parameters will be the same as those observed in existing ocelot populations in Texas [9]. The model was also not spatially explicit regarding distinct ocelot use of habitat patches; it considered all habitat identified in geospatial analysis as continuous habitat that ocelots can persist in. It also assumed that the carrying capacity of the habitat is consistent with known densities of ocelots in existing populations in Texas. In total, while the PVA provided the below recommendations for how to structure ocelot releases to create a viable population, the model must be updated with field data collected from the reintroduction program. For example, the actual survival of released ocelots in the reintroduction program in Texas must be monitored to provide an accurate measure of postrelease survival in released ocelots. Updating the model may revise the recommendations for the number of ocelots to be released and the timeline for releases. At this time, the following general guidelines for the number of ocelots to be released are provided based on results from the PVA [7]. The actual structure of releases should be revised as the program is implemented and results are gathered.
Initial Release of Founders
The PVA highlighted the importance of initially reintroducing more, rather than less, ocelots in the first year of releases. In the PVA, releasing 6 individuals in the first year (four females and two males) was preferable to releasing only 3 ocelots (two females and one male) [7].
Supplemental Releases (Population Reinforcement)
PVA model results indicated the need for population reinforcement via consistent supplemental releases of ocelots for at least 10 years beyond the initial releases. It is recommended that the program seek to release at least 2 ocelots (1 male and 1 female) but preferably 4 ocelots (2 males and 2 females) every year for at least 10 years to create a self-sustaining population [7]. Releasing more individuals on more consistent timelines for a longer time period is preferable, though actual availability of ocelots will likely vary year-to-year.
133
Timing of Releases
Daily timing
On the day an ocelot is released to the wild, a soft release enclosure or hard release carrier (see more below) should be opened when ocelots are most likely to be active, likely after 4:00 PM. It is recommended that weather conditions on the day of the release be between 40- and 90-degrees Fahrenheit with no other adverse weather, such as a severe storm. This will avoid introducing any weather-related stress to the ocelots on the day they are released.
Yearly timing of releases
Ocelots should be released into the wild during cooler months (late fall through early spring) minimizing the likelihood of ocelots experiencing extreme summer heat or wildfires immediately after release. Additionally, it may be ideal to release ocelots at times of highest prey availability, as hunting may be easiest then. Native prey (small mammals, rabbits, birds, or lizards) availability can be monitored and evaluated to identify any seasonal peaks in prey abundance and then prioritize ocelot releases at these times. Such an approach was taken in the Iberian lynx reintroduction program, which preferred to release lynx closer to spring when the abundance of European wild rabbits (Oryctolagus cuniculus, the lynx’s favored prey), was highest (F. Najera, University of California Davis Karen C. Dryer Wildlife Health Center, personal communication) and in the most successful iteration of Canada lynx reintroductions in Colorado [2].
An additional consideration in timing is the overlap between releases and any live trapping efforts at the reintroduction site necessary for monitoring. It is recommended to avoid trapping free-ranging ocelots at the same time as releasing ocelots. This addresses potential concern that newly released ocelots who are initially exploring and adapting to the environment may be highly susceptible to being trapped due to wider movement around the landscape before they have established a home range. Ocelot live trapping also occurs in the cooler seasons between the months of November and April to minimize concerns of hot temperatures. At the reintroduction site, ocelots should generally not be released until live trapping efforts have concluded for the season, or vice versa.
Summary of Hard and Soft Release Methods
Soft and hard releases are two possible methods for release of ocelots into the wild at the reintroduction site Soft release allows acclimation to the surrounding environment for a period before being released, and it usually consists of an enclosure placed at the site of release for acclimation. Hard release consists of releasing an individual directly into the environment without acclimation prior to the release. Generally, it is recommended to initiate the ocelot reintroduction with soft releases because these will allow ocelots time to acclimate to the unoccupied reintroduction site. Additionally, soft releases have proven more effective than hard releases in encouraging animals to establish at the release site [5, 10]. They provide individuals with time to recover from stress of capture and/or transport before they are fully released [11].
134
Once multiple ocelots have been established at the reintroduction site the program may transition to direct, hard releases. At that point, the presence of established individuals should tie newly released individuals to the area so long as there are still sufficient resources present [1]. The success of soft and hard releases given their exact design should be evaluated as the program is implemented to make any changes to the protocols recommended here, as for the Canada lynx reintroduction in Colorado, which went through 4 different iterations of release protocols to find the best strategy [2]. These may include longer periods of soft release, preference for soft over hard releases, or other changes.
Soft release
At the beginning of the reintroduction program when releasing the first ocelots into an area that is completely unoccupied by ocelots, soft release methods will be used. The main objective of soft release enclosures is to promote successful survival and home range establishment at the reintroduction site but outside of the soft release enclosure. Initially ocelots should be maintained for approximately two weeks (though the exact timing will depend on behavior) in soft releases enclosures encompassing at least 2,500 meters2 so they may safely acclimate to their new environment and develop fidelity to the reintroduction site while still under human observation. Monitoring of ocelot’s post-release can provide information on whether longer soft release periods may be warranted.
While in the soft release enclosures, ocelots will be provisioned with live prey and water while also being protected from competitors or predators. Ocelots in soft release enclosures also will be actively monitored via remote cameras to assure they continue to show proper health and behavior. After approximately two weeks, the enclosure will be opened to allow the ocelot to exit the enclosure and enter the wild at will. The enclosure can remain open and reliable food and water can continue to be provisioned inside the enclosure for a few days in the case that the ocelot chooses to return in the initial days following release for access to known resources. Further information on management of the soft release facilities and monitoring of the ocelots present there are in the sections below.
Hard release
Hard releases are a cheaper, faster, and easier way to release animals. In hard releases, ocelots would be transported to the reintroduction site and immediately be released from carriers at a selected location. Initially, it is recommended that hard releases should not occur until multiple male and female ocelots have successfully established territories at the reintroduction site and near the site of the soft release enclosures. The presence of established ocelots at the reintroduction site may create fidelity to the reintroduction site for newly released animals, precluding the need for continued use of the soft release strategy. The comfort of program managers in conducting hard releases, given concerns about ocelot welfare, may determine the frequency of the use of hard versus soft releases.
When planning hard releases, monitoring data (including from collars and cameras) should be used to identify the locations and sizes of existing ocelot territories in the reintroduction site. These data will be used to determine optimal locations for hard releases, which should be in areas near existing territories that have enough habitat for new, similarly sized territories. As possible, hard releases should occur in sites adjacent to -
135
but not within - the territories of other ocelots to avoid any intraspecies conflict directly after an ocelot is released. Since released animals will be subadults, they are expected to set up their own home ranges near the established ones, though they may also compete for existing territories to achieve access to mates, space, or prey. Ultimately, ocelots may be expected to partition space on their own regardless of exact release locations; males may expand their territories to encompass additional females and some animals may lose their territories to others. The reintroduction habitat is large - spanning 363 km2 of suitable low shrub cover for ocelots [6] so space will not be limited at first. Based on the known density of ocelots in a part of the Ranch Ocelot Population in Texas of 17.6 ocelots/100km2 (95% Confidence Interval 10.32-29.18) [12] the carrying capacity of the reintroduction site is estimated at 63.8 ocelots (95% Confidence Interval 37.46-105.9). However, because ocelot densities were derived from areas on the Texas coast with higher primary productivity, it is possible that densities at the reintroduction site will be different. Further, densities at the reintroduction site were estimated based only on the extent of suitable low shrub cover for ocelots and did not include any areas of suitable woody patch structure without suitable low shrub cover or any areas within 1 km of a roadway.
Soft Release Facility Design
Size and capacity
The soft release facility will be 1 hectare (10,000 meters2) and can be split into 2 or 4 separate portions. Each ocelot in the soft release enclosure should be provided with at least 0.25 hectares (2,500 meters2) of its own space, so up to four ocelots can be maintained in the soft release enclosure at once. Individual ocelots should be held in separate spaces within the soft-release enclosure to avoid inter-ocelot aggression. The only exception to this is that related female ocelots can be held in the same enclosure at the same time since these individuals will likely tolerate each other. Multiple males will not be held in the same enclosure.
Materials and structure
Soft release enclosures can be constructed using common fencing materials. Corner posts will be set with concrete while line posts and t-posts will be used around the enclosure’s perimeter to stabilize the structure. Right-angle corners of the enclosure will be rounded off with fencing materials to minimize potential ocelot interactions with hard corners. As much mixed-to-dense natural vegetation should be retained as possible inside the enclosure to provide natural habitat conditions. Shade cloth can be used to provide additional cover as needed inside the enclosure.
To keep potential competitors or predators out of the enclosure, it should be surrounded by a solar-powered electric fence wire(s) to provide double containment. Given the enclosure’s size and the abundance of vegetation greater than six feet high throughout the habitat, enclosures will not be covered at the top. To minimize potential predators from digging into the enclosure, panels or other fencing materials may be trenched up to two feet deep or fencing materials may be laid flat away from but connected to the upright fence. This apron will be attached to chain-link panels above the soil surface. Finally, to minimize escape of small prey
136
animals from the enclosure, methods such as placing smooth surface barriers along the bottom of the enclosure fencing should be used.
Location/habitat
Soft release facilities should be constructed within suitable habitat identified in the reintroduction site. Soft release enclosures should be remote but still accessible to personnel and suitable for the construction of facilities. They should not be constructed near any obvious threat to ocelots (particularly roadways) or near the boundary of the reintroduction site
The soft release enclosures must include natural vegetation that ocelots will use in the reintroduction site. The habitat should include areas for ocelots to sleep under and dig into but constructed nest boxes or other humanmade objects (other than supplement feeding stations, below) are not recommended. Vegetation in the enclosure should include dense cover for use during resting periods as well as more heterogeneous vegetation (woody and herbaceous) communities and open areas for exploring. Enclosures should be constructed to include a heterogeneous mixture of vegetation that will best represent the natural environment of the reintroduction site. Vegetation communities may include diverse woody shrub communities (<5 m tall); tall-mixed grasses; cacti; open areas; and live oak, ebony, brasil, or mesquite trees. Since ocelots’ health and behavior will have been well-monitored in the wilding facility prior to their transport to the release site and since they will be collared before transfer to the soft-release enclosure, constant monitoring by camera trap or visual observation is less crucial at the soft release facility. This requires less open space for monitoring and allows the soft release enclosure to be densely vegetated.
If reintroduced ocelots establish territories around the existing soft release enclosure, ocelots must be released in different locations using a soft or hard release method as appropriate. Ocelots should be released outside the territories of established individuals to avoid a newly released ocelot coming into conflict with another individual.
Management
Human contact
Once in the soft release enclosure, ocelots’ exposure to humans should be minimized. Fortunately, the reintroduction site occurs in remote private ranchlands where contact with any humans outside of the reintroduction personnel is unlikely. When soft release enclosures are occupied by ocelots, they should be visited only by necessary program personnel who are monitoring or maintaining the enclosure. When arriving at the enclosure in a vehicle, personnel should park far enough away so that the ocelot is not likely to hear the approach. Should program personnel encounter an ocelot when visiting the enclosure, they should make attempts to scare the ocelot by yelling, making movements, or throwing objects or water. Meanwhile, enclosures should not be visited by members of the public in order to avoid disruption to ocelots. However, the soft release facility will be monitored by camera traps so videos of ocelots living in the facility and leaving the facility for the wild may be made available for public viewing.
137
Watering
Multiple water sources will be present in the enclosure and drinking water should be continuously provisioned. Water should be provided by gravity tank-float systems that mimic the shape and structure of existing cattle and wildlife water structures that are present around the reintroduction site. Such water systems are utilized by ocelots occupying ranches in South Texas. Water structures in the enclosures can be smaller than those used for cattle, but the design should be similar to those present on ranches to help ocelots acclimate to using the water structures already present in the landscape.
Feeding
Ocelots in the soft release enclosure should have access to live, native prey species within the enclosure. The presence of native prey is recommended to help ocelots acclimate to the wild prey that will be present in the reintroduction site. The use of non-native prey, meanwhile, may be risky considering the potential for escape into the wild. Possible prey animals could include native small rodents and rabbits. While some prey animals will naturally occur in the soft release enclosure and will provide enrichment for ocelots in the enclosure, prey may be also purchased, trapped from the wild, or raised in a close-by prey colony for additional provisioning as needed. Choices of prey species type for ocelots may be informed by results at the Ocelot Conservation Facility. Finally, bird seed or grain can be placed into soft release enclosure to attract native birds as well as encourage small mammal prey animals to enter or stay in the soft release enclosure.
Live, free-ranging prey will always be present in the soft release enclosures and ocelots may hunt and consume free-ranging prey. Also, during the initial soft releases of ocelots, ocelots in the soft release enclosures may be provided food at two or more supplemental feeding stations inside the enclosures. The object of a supplemental feeding station is to ensure that there is a definite way to monitor whether ocelots are eating consistently and maintaining their body condition in the soft release enclosure [1]. There is no need to continue to test whether ocelots are capable of hunting free-ranging prey once brought to the soft release enclosure since they must have already demonstrated successful hunting behavior in the wilding enclosures at the Ocelot Conservation Facility.
A supplemental feeding station consists of a small cage that confines a prey animal or multiple prey animals inside and a nearby camera for monitoring [1]. The station can be on the ground or on an elevated platform The cage should have enough room for an ocelot to enter from the top, turn around, and catch the prey. The recommended cage size is approximately 2 meters wide, 4 meters long, and 1.5 meters high. It should have an open top so an ocelot may enter through the top from the outside by climbing up a log and jumping in, for example. The prey must be confined inside the enclosure and unable to escape through the top or other parts of the cage. Since prey at the station will be confined in the cage and thus much easier to catch than any freeranging prey animal in the enclosure, ocelots likely will take this prey.
A keeper will enter the enclosure multiple times a day at variable times to place live native prey inside one of the supplemental feeding stations. Varying the station used and the timing of placing the prey will avoid ocelots becoming habituated to a feeding pattern. Further, since the soft release enclosure will be large, the keeper placing a prey item in the supplemental feeding station is unlikely to encounter the ocelot while inside the enclosure. If the keeper does see an ocelot, they should scare the ocelot with shouting or movements. Keepers should only approach the feeding station if the ocelot is not already present there.
138
Ocelots may consume multiple (approximately 4) meals (1-2 prey items) throughout each day and night. These meals could include prey taken from the feeding station as well as other free-ranging prey caught inside the enclosure. The amount of prey placed at the feeding station should be determined based on monitoring of ocelot health and behavior, including whether it is taking the prey at supplemental feeding stations or if it is catching other prey. Fasting days could be used to stimulate hunger and hunting behaviors, but they should never be introduced if an animal appears stressed or has a decline in body condition. Additionally, depending on an ocelot’s energy needs and the amount of free-ranging prey it catches and consumes, the ocelot may need more or less prey to be placed at the supplement feeding station. A remotely accessible camera, if possible, should be placed at the supplement feeding station to continuously monitor if ocelots take the prey item and if they consume the prey. Ocelots must consistently maintain their body condition throughout maintenance in the soft release enclosure to be released.
Timeline
During their time in the soft release facilities, ocelots will be allowed to continue their natural, wild behaviors while getting comfortable with the new environment. The general guideline for the amount of time an ocelot should be held in the soft release enclosure is approximately two weeks. Two weeks provides the opportunity for the ocelot to become familiar with the environment without requiring so much time that the ocelot may become too closely tied to the soft release enclosure, which could lead the ocelot to form its home range inside the enclosure or become dependent on the resources there. The impact of the length of soft release maintenance on post-release survival should be evaluated to make any adaptations to the length of the soft release period [2].
The exact timing of release should depend on the behavior of the individual, which will be monitored throughout the time in the enclosure (see below). For example, if an ocelot shows signs of distress while in the soft release enclosure, such as excessive pacing behavior, the individual can be released earlier than two weeks. Since a stressed ocelot may attempt to escape from the enclosure and injure itself during the attempt, it should be released immediately so the ocelot can remove itself from the stressful situation. Alternatively, an ocelot may be held longer than two weeks if it needs additional time to successfully consume live prey in the enclosure and move around the enclosure.
It is recommended that a decision-making committee (including U.S. Fish and Wildlife Service ocelot species lead and a research/recovery biologist, ocelot ecologist, ocelot geneticist, ocelot veterinarian, and ocelot Association of Zoos and Aquariums Saving Animals From Extinction program manager) make decisions on exact timing of ocelot releases based on monitoring of individual behavior and any other situational factors that arise. When it is determined to release an ocelot, a door/gate at the soft release enclosure will be opened and the ocelot can disperse into the wild at will. The gate should be monitored by camera to know when the ocelot left the enclosure and if it subsequently returns to the enclosure for food, water, or refuge.
139
Post-release management of enclosure
After release, ocelots’ continued return to the soft release enclosure is not expected because released ocelots will have access to wild prey and other resources in the reintroduction site. However, if the ocelot is seen returning inside the enclosure, prey can continue to be provisioned at the supplemental feeding station for 24 to 48 hours after the ocelot has been released to ensure the ocelot’s access to food for the critical first few days after release. If the ocelot does not return to the enclosure or feeding station within 1-2 days, no additional supplementation will be provided. If ocelots (or other wild animals) display habituation to the enclosure, including continued visitation to the enclosure for greater than 3 days, the entrance may need to be closed. Supplemental feeding of ocelots at other locations can be used to promote an ocelot’s use of other habitats by following similar procedures as outlined above It is recommended to wait 1-2 weeks after the released ocelot has stopped visiting the enclosure to place another individual.
Monitoring
Since ocelots will be extensively monitored at the wilding facility and do not leave that facility until they display proper wild behaviors, it will be assumed that ocelots transferred to the soft release enclosure will be behaviorally ready for release. Visual monitoring of ocelots’ every movement in the soft release enclosure is not needed and would be difficult given the size of the enclosure and the density of native vegetation present. Rather, monitoring is needed to generally assess if (1) ocelots are displaying stressed behavior that would necessitate an early release, (2) they are maintaining their health (including good body condition and no signs of injury or illness), (3) they are using the full extent of the enclosure to search for food and water and mark territory, (4) they are consistently eating live native prey, and (5) they successfully flee or hide from humans [1].
Monitoring of these five factors will be conducted using GPS collars placed on the ocelots upon their transfer from the Ocelot Conservation Facility and with cameras. First, collars may be programmed to provide frequent fixes (for example, multiple times per hour) during the soft release period to capture detailed behavior. This fix rate can be adjusted once the animal is released, as the fix rate must be decreased to preserve battery life. Meanwhile, cameras should be placed in soft release enclosures at the supplemental feeding stations, the gate, the water sources, the edges/fence lines where they will have wide views of the enclosures, and other strategic locations. Cameras may be remotely accessible using cellular signal or other technology or can be periodically checked by personnel.
Post-release management of ocelots
In the case of ocelots with behavioral or health problems following release, it is recommended that managers first pursue field-based management interventions. Managers should only trap and remove ocelots from the reintroduction site (for transfer to another release location or back to the Ocelot Conservation Facility) as a last resort or in the case of emergency, such as extremely poor health situations (e.g., poor body condition or obvious injury) that require trapping and removal to human care. Outside these emergencies, the goal is to manage ocelots in the wild so long as the life of an individual is not at risk.
140
Potential behavioral management interventions could be necessary if released ocelots are continuously approaching highways or other human infrastructure or pursuing other risky behaviors. In these cases, individual ocelots displaying dangerous behaviors should first be monitored more closely. Then, managers may determine if interventions are needed to scare ocelots away from risky situations or attract them to new areas with supplemental feeding stations. Supplemental feeding stations can also be used to encourage use of areas of habitat or provide support to individuals with declining body condition.
Potential experimental release practices
For the first ocelots released to the unoccupied reintroduction site, the use of ocelot urine, scat, or other scents (collected from the Ocelot Conservation Facility or the wild in Texas) could be used to promote site fidelity to areas in the reintroduction site that are outside of the soft release enclosures. Similarly, releasing females prior to males could be tested to promote male fidelity to sites where females are present. The ability to test the impacts of the sex ordering of releases will depend on the availability of ocelots for release. These techniques were not necessary for Iberian lynx or Canada lynx releases but could be attempted as experimental efforts. Continued use can be evaluated based on results.
References
[1] Iberlince. (No date.) Protocolo de liberaciones de lince ibérico. http://www.iberlince.eu/images/docs/3_InformesLIFE/ProtocoloLiberaciones_M.Iberlince.pdf
[2] Devineau OT, Shenk TM, Dohert PF, White GC, Kahn RH. 2011. Assessing release protocols for Canada lynx reintroduction in Colorado. Journal of Wildlife Management 75: 623–630.
[3] Laack LL. 1991. Ecology of the ocelot (Felis pardalis) in South Texas. Texas A&I University, Master’s Thesis.
[4] Rueda C, Jiménez J, Palacios MJ, Margalida A. 2021. Exploratory and territorial behavior in a reintroduced population of Iberian lynx. Scientific Reports 11: 14148.
[5] Thomas S, van der Merwe V, Carvalho WD, Adania CH, Černe R, Gomeričić T, Krofel M, Thompson J, McBride Jr. RT, Hernandez-Blanco J, Yachmennikova A, Macdonald DW, Farhadinia MS 2023. Evaluating the performance of conservation translocations in large carnivores across the world. Biological Conservation 279: 109909.
[6] Martinez LA, Lombardi JV, Powers G, Anderson AD, Campbell TA, Lopez R. In Review. Assessing ecological and socio-political factors in site selection for ocelot reintroduction in Texas.
[7] Martinez LA, Lombardi JV, Parker ID, East, F, Campbell TA, Lopez R. In Review. Evaluating strategies for ocelot reintroduction in Texas, United States using population viability analysis.
[8] Jule KR, Leaver LS, Lea SEG. 2008 The effects of captive experience on reintroduction survival in carnivores: a review and analysis. Biological Conservation, 141, 355-363.
[9] Haines AM, Tewes ME, Laack LL, Grant WE, Young J. 2005. Evaluating recovery strategies for an ocelot (Leopardus pardalis) population in the United States. Biological Conservation 126: 512–522.
[10] Tetzlaff SJ, Sperry, JH, DeGregori BA. 2019. Effects of antipredator training, environmental enrichment, and soft release on wildlife translocations: A review and meta-analysis. Biological Conservation 236: 324-331.
[11] Montalvo VH, Hagnauer I, Cruz Díaz JC, Morera B, Lloyd K, Sáenz-Bolaños C, Fuller TK, Carrillo E. 2022. Experimental release of orphaned wild felids into a tropical rainforest in southwestern Costa Rica. Veterinary Science 9: 468.
[12] Lombardi JV, Sergeyev M, Tewes ME, Schofield LR, Wilkins RN. 2022. Spatial capture-recapture and LiDAR-derived vegetation metrics reveal high densities of ocelots on Texas ranchlands. Frontiers in Conservation Science 3: 1003044.
141
Standard Field Monitoring Protocols

142
Photo courtesy Fin and Fur films
Ocelots from the Ocelot Conservation Facility who are suitable for release will be transferred to the reintroduction site, where they will be released to the wild using soft and hard release methods as needed. This document provides the necessary protocols for monitoring the reintroduced ocelot population. Protocols for methods such as live trapping and collaring, camera trap monitoring, data processing, and individual ocelot identification are included. Monitoring data collected from these methods is critical for evaluating success of the ocelot reintroduction program and for informing adaptive management practices to support the successful establishment of a new population.
Ocelot Monitoring Objectives in Reintroduction site
Pre-Reintroduction
Phase: Baseline Ecology of Sympatric Carnivores and Prey
Prior to the reintroduction of ocelots, it is important to understand the ecology (including population abundances, space use, resource selection, and movements) of sympatric carnivores (bobcats, coyotes, and potentially pumas) as well as ocelot prey (small and medium-sized mammals, birds, reptiles, etc.) at the reintroduction site. Carnivore and prey data can be used to evaluate the necessity of management practices to support ocelot reintroduction.
The ocelot reintroduction program has conducted camera trapping and/or live trapping and collaring of animals to collect data on sympatric carnivores as well as small- and medium-sized mammals that may be potential prey for ocelots. Additionally, collaboration with the East Foundation’s existing small mammal monitoring program at the San Antonio Viejo Ranch (SAVR) property within the reintroduction site is recommended to better understand small mammal abundances.
Monitoring metrics may include but are not limited to:
1. Carnivore population abundance (density and size), survival, and reproduction
2. Carnivore space use and resource utilization/selection
3. Carnivore movements and diel activity patterns based on age and sex class
4. Carnivore energetics (accelerometers)
5. Carnivore dietary analysis (genetic analysis of fecal matter for prey choice)
6. Carnivore health and disease (disease prevalence and transmission in the populations)
7. Carnivore interspecific interactions
8. Small mammal and meso mammal abundances and distributions
Reintroduction Phase: Survival and Ecology of Released Ocelots
Ocelots released to the wild should be monitored on their survival, reproduction, and use of the reintroduction site after release by using radio collars or other geolocation methods. Ocelots should be monitored with collars for at least the first year after release and in subsequent years if collars can be replaced. Monitoring during the initial first year is necessary to gather information on the performance of released ocelots and then adapt management, as necessary, in subsequent ocelot releases. Monitoring during this phase can also be used to refine the initial habitat suitability model used to identify potential reintroduction habitat in Texas as well as the
143
population viability analysis model of ocelot reintroduction that provides recommendations for release strategies. Even after the population has established and begun reproducing, any new ocelots released into the landscape over the years should be collared and be monitored for at least one year after release (and subsequent years if possible) using collars to track their survival and collect basic ecological information.
Monitoring of sympatric carnivores and prey (described above) should also continue once ocelots are released to understand how other species are adjusting to ocelots’ presence on the landscape and how these species may be impacting ocelots. Recommended monitoring for newly released ocelots includes, but is not limited to, the metrics listed above in the Pre-Introduction Phase for monitoring baseline carnivore metrics.
Monitoring Methods
The primary methods of ocelot monitoring in the reintroduction site will be the geographic location of ocelots via collars and camera trapping of ocelots. All ocelots released to the reintroduction site will be collared to monitor their movements and population parameters. Released ocelots will also receive Passive Integrated Transponders (PIT-Tags) and will be photographed to aid in identification of individuals. As ocelots’ collars drop off and as new ocelots are born in the wild, efforts should be made to trap uncollared ocelots and place collars on them. Live trapping will also provide the opportunity for collection of other biological samples and data from ocelots. Meanwhile, camera trapping will serve as an additional method to monitor uncollared ocelots as well as other carnivores and potential prey species. When breeding occurs, den site identification and visitation can be used to monitor reproduction in the reintroduction site. Finally, necropsies must be conducted for all ocelot mortalities.
The following sections provide recommendations for ocelot monitoring, though this protocol is not intended to be regulatory or permanent. All ocelot breeding, reintroduction, monitoring activities must be conducted consistent with any requirements defined in U. S. Fish and Wildlife Service (USFWS) and/or Texas Parks and Wildlife Department (TPWD) permits. Some aspects of ocelot monitoring (i.e. specificities of live trapping and handling of ocelots) will be regulated through annual permitting.
Radio Collaring
Radio Collar Basics
Caesar Kleberg Wildlife Research Institute, the East Foundation, and the U.S. Fish and Wildlife Service use radio collars to monitor existing ocelot populations in Texas. Global Positioning System (GPS) collars that remotely upload GPS locations via satellite iridium have been used recently. (For example, Telonics TGW4177-4 GPS/Iridium collars with a CR-7B drop-off mechanism have been used). GPS location data gathered by the collars and sent to satellites may be downloaded as needed from the satellites, allowing collars to be used for real-time monitoring of ocelots’ movements upon release at the reintroduction site. If an ocelot is captured in the wild, the individual should be collared or re-collared to place a collar that has full battery life and can continue to collect data for the full life of the battery. Dropped collars should always be retrieved from the field (Appendix 2) to obtain any GPS location data that was not successfully sent to satellites. GPS locations can be
144
used to monitor ocelots’ movements inside or outside the reintroduction site, dispersal movements, or establishment of home ranges.
GPS collars should also be equipped with ultra-high-frequency (UHF) capabilities that can be customized to send a signal at different periods (hourly, daily, weekly, etc.). In-the-field UHF monitoring is an additional method to track animals and to find collars that are no longer transmitting GPS satellite signals (since UHF battery life will normally last beyond the life of the GPS capabilities of the collar). It is recommended to activate UHF signals 1-2 days a week to conserve battery life while still allowing opportunities for UHF monitoring.
Collars should be scheduled to drop-off the animal around the time that the GPS battery is predicted to run out. If the ocelot is still alive at the scheduled drop-off point, the collar will drop off and send a mortality signal, allowing monitoring personnel to find the dropped collar using previous GPS points and current UHF signals. Collars should also have the ability to be dropped-off remotely via satellite any time. This may be necessary if a collar does not send points to the satellite or has other malfunctions. In case the drop-off mechanism fails, it is advisable to also have a cotton or leather spacer on the collar to serve as a detachment failsafe.
Collars are also equipped with an accelerometer, and if the accelerometer finds that the collared ocelot is deceased, the collars will send a UHF mortality signal. The deceased ocelot and collar can be found using previous GPS points and the UHF mortality signal. In the event of an ocelot mortality, coordination with the USFWS ocelot species lead and appropriate medical personnel is required, and personnel must comply with appropriate USFWS permitting regarding reporting, necropsy procedures for determining cause of death (see more information on necropsies in later section), and any necessary notification of law enforcement.
Collar Use
We provide the following suggestions for collar use, though these are suggestions and should be adapted as needed to meet management needs or logistical dimensions of the ocelots or collars.
1. Ocelots in wilding enclosures at the Ocelot Conservation Facility (Testing and Baseline Data)
a. Collars with GPS, UHF, and accelerometer capabilities can be placed on male and female adult and subadult ocelots at the Wilding Enclosures to test the collars, and to collect data on baseline activity patterns via accelerometer data. This will allow evaluation of the functionality of collars in a wild setting and will provide data on ocelot behaviors that are relevant to ocelot field studies. To date, no captive ocelot baseline data with accelerometers exists.
2. Ocelots released at reintroduction site (monitoring survival and ecology post-release)
a. When ocelots are to be transferred to the reintroduction site for release via hard or soft release methods, they must be collared. If they were already collared at the Ocelot Conservation Facility, any necessary collar adjustments (i.e., size, fit, or programming of collar) should be made and a collar with full battery life should be placed during the last handling event before the animal is transferred.
145
b. Collars on released ocelots need to be programmed to allow at least one year of post-release monitoring. Collars should be scheduled to automatically drop-off when the GPS battery life is predicted to run out.
c. It is generally recommended to record 6 locations per day (one every 4 hours) of newly released ocelots for at least the first year after their release to monitor ocelots’ survival and general movements. However, fix rates for collars are subject to battery limitations and there is a tradeoff between maximizing the amount of time a collar can be active and the number of locations it records per day. Fix rates may need to be varied as necessary given battery constraints and management or monitoring needs.
d. As the expected end of radio collar battery life approaches, as collars fail, or as collars drop off, attempts should be made to recapture ocelots and place new collars.
3. Free-ranging ocelots in reintroduction site (monitoring ecology and reproduction of established ocelots)
a. Once ocelots are established in the reintroduction site, the programming of collars for freeranging individuals can be modified, as necessary, to better monitor fine-scale spatio-temporal movement patterns at the reintroduction site. Use of a more frequent fix schedule (more points collected per day) or a mixed fix schedule (where there are more fixes during active periods and fewer fixes during inactive periods) can allow finer-scale monitoring, though more frequent fixes will decrease the amount of time the battery will last.
b. Throughout the program, ocelots in need of a new radio collar should be live-trapped and recollared, if possible. Additionally, ocelots that are born in the wild and have not been collared should be trapped and collared if possible.
Radio Collar Design and Size
Radio collars will have programmable (fixed or remotely adjustable) GPS fix rates and all collars will be equipped with a drop-off unit. Total collar weight is impacted by collar model, battery type and size, drop-off unit type, belting width and material, and adjustment range. Collars should typically not exceed more than 3% of the individual’s known body weight. There are several trade-offs in collar use, including maximizing battery life by using a larger battery or minimizing weight of the collar. Collar design for an individual will depend on its size. Additionally, for young ocelots that may grow while wearing the collars, unique custom-fit, and expandable collars (using spacers) should be placed.
Collar Testing
When collars are not in use, the drop-off mechanism should be deactivated, and the radio collar should be turned off Ensure that the drop-off is not activated. Before deployment, all collars should be tested for proper functioning, the accuracy of locations (<10 m error), and connection to the satellite. Testing should be done at least one month before the collar is to be used. To test a collar, it should be turned on and activated. It should then be placed in different vegetation types, such as woody and herbaceous cover, and the actual location of the collar should be recorded. The collar should be left out for at least five days to ensure that the collar is functional, connecting to satellites, and sending the correct UHF frequency. Once collar testing is complete, calculate the average and 75th percentile distance from the collar to the centroid of 90 test locations. When a collar is ready to be deployed, the collar must be turned on and activated.
146
Collar Data Processing
Dropped collars must be recovered from the field because there may be more data stored on the collar than was uploaded to the satellites. Once collars have been recovered from the field, collars should be inspected to ensure that the prongs or parts required to download data are intact. If collars are not intact, they may need to be shipped back to the manufacturer to extract GPS and/or activity data from the collar. GPS collar data can be opened in Google Earth, ArcPRO, R, or similar programs for immediate viewing, processing, and analysis. A copy of all raw data for each ocelot collar should be kept for backup and future analysis (if needed). After extracting data, collars can be refurbished and deployed on a new individual.
Live Trapping
Live Trapping Basics
Live trapping of ocelots should be conducted to replace radio collars and to target deployment of radio collars on previously uncollared (i.e., wild-born) ocelots, to collect samples, or collect additional information on sympatric carnivores at the reintroduction site. The live trapping protocols listed in this document can also serve as a model for live trapping and collaring of bobcats, coyotes, or mountain lions occurring in the ocelot reintroduction site. Trapping supplies are provided in Appendix 4.
Ocelot Safety Measures During Trapping
Live trapping must be conducted in a manner that will not threaten the life or health of any individual ocelots. Further, live trapping and ocelot releases should not occur at the same time and location to ensure that released ocelots are not incidentally trapped soon after being released onto the landscape.
U.S. Fish and Wildlife Service (USFWS) ocelot live trapping permits provide safety specifics for live trapping ocelots. These include:
● A veterinarian must be available to attend an ocelot capture to provide on-site veterinary support if needed in the case of a severe injury or other emergency with a captured ocelot.
● Individual ocelots recaptured less than 45 days after undergoing chemical immobilization and handling must be released without handling, except in the case of an emergency requiring veterinary intervention or a situation requiring emergency intervention in the field (e.g., the collar shifted from the neck to around the mouth of the animal).
● The exact timing of trapping periods is dependent on temperature conditions. Live trapping for ocelots can typically be conducted from November through the end of April. There is documentation of ocelot mortality in live traps due to heat stress, and trapping during these months reduces concern of hot temperatures. Additionally, current USFWS ocelot trapping permits require ocelot traps to be closed when temperatures reach 90 degrees Fahrenheit for more than 3 consecutive days and traps cannot reopen until temperatures drop below 90 degrees Fahrenheit for more than 3 consecutive days. Finally, current permits also stipulate that traps must be closed when temperatures are expected to be at or below 40 degrees Fahrenheit to minimize the risk of hypothermia.
147
● Current permits require traps to be opened after 3 PM and checked between 7 AM and 10:30 AM, with traps closed during the day. Program managers and USFWS could explore the possibility of keeping ocelot traps open during other times during cool temperatures.
Trap Types
In areas where ocelots are known to occur or may occur based on habitat, the primary trapping method will be single-door, 108 × 55 × 40 cm steel wire Tomahawk box traps (Tomahawk Trap Company, Tomahawk, WI, USA) with attached steel mesh bait box to the back of the trap to contain a pigeon or other live bait animal. If Tomahawk traps are used, an insertable squeeze should be used to limit captured animal movements when they are sedated. Other small/tighter guillotine style traps could be explored as well.
Trap transmitter technology such as cellular cameras can be placed at ocelot traps to allow monitoring staff to respond to the capture of an animal as soon as the capture has occurred. Such technology diminishes the amount of time an individual will be held in a trap and thus minimizes the risk of any possible injuries during capture.
No leghold traps or snares should be used for ocelot trapping in the reintroduction site, but other methods for live trapping ocelots that are permitted by USFWS may be explored and used.
Bait Animals
Since 1982, ocelot research has used tomahawk live box traps that are connected to a separate wire mesh bait compartment that houses a live chicken or pigeon. The bird is provided water, food, and a roost while the trap is deployed in the field. The ocelots or any carnivore can smell, see, and hear the pigeons but cannot physically attack the pigeons once trapped. Other taxa (e.g., rabbits or rats) have not previously been used for trapping efforts and may provide an alternative to birds. Bait animals can be purchased from private sellers and ranches that raise them for food or hunting. Bait animal facilities may require approval from an Institutional Animal Care and Use Committee and an ethical practices standard operating procedure must be followed (example in Appendix 4). Roadkill may also be hung inside a trap, but use of roadkill is only advised during cooler months, would require a constant application, and requires approval from USFWS and TPWD.
Non-Animal Visual Lures
At a trap station, non-animal visual lures can be used in combination with a live animal to pull the interest of the ocelot to the trap site or make it stop near the trap to notice the bait animal. For wild cat research, hanging shiny/reflective objects above the trap site has proven effective in attracting individuals to stop in front of the trap. These may include feathers hung with fishing lines above the trap.
Olfactory Lures
Olfactory lures can be used in combination with bait animals and may prove able to catch ocelots without a bait animal, although further field testing is needed. A few drops of an olfactory lure can be placed directly in front of the trap to stop a passing individual and more drops may be placed at the back of the trap to prompt the animal to investigate. Ocelot urine is likely the preferred type of olfactory lure, though men’s cologne (e.g., Calvin Klein Obsession), commercial carnivore lures, or bobcat glands could also be explored, and methods could be tested at the Ocelot Conservation Facility. Ocelot urine collected from wild or captive individuals
148
could help with the capture of ocelots who prove difficult to capture but who may be interested in urine from another male or female not known in that area. Long-distance call lures (i.e., gusto, skunk junk) could also be applied to trees above the trap site to allow the wind to carry the smell. The use of olfactory lures must be coordinated with USFWS and the type of lure and number of days a lure may be used, for example, will be detailed in the USFWS ocelot trapping permit.
Trapping Locations
Collar and camera data can inform trap locations and target the trapping of specific wild ocelots as needed. GPS locations of traps should be recorded and made accessible to trapping staff and program managers.
It is recommended to place traps in or adjacent to patches of woody cover. Habitat types that ocelots have historically used in South Texas include:
Open, mixed, & dense Tamaulipan thornscrub communities
● Old growth live oak or palm forest
● Old-growth oak forest with sporadic shade-tolerant thornscrub species
● Oak Forest with thornscrub understories
● Mesquite woodlands with cordgrass, thornscrub or tall grasses.
● Riparian deciduous forests with dense-mixed understories
● Juniper-ashe forest
Traps should be covered with natural vegetation to conceal the trap and prevent other animals from harassing trapped ocelots (or other trapped animals). Traps should also be thoroughly shaded to protect both captured animals and bait animals. Trapping personnel should use a shovel or gloves to put sand/dirt on the floor of the trap to obscure the steel mesh and provide natural bedding for cats that enter.
Trapping personnel
An ocelot trapping team generally consists of: East Foundation and Caesar Kleberg Wildlife Research Institute scientists, Wildlife Veterinarian(s), Graduate Student(s), and Research Technician(s). All personnel conducting ocelot trapping activities should be trained in setting and deploying traps in the field. At least one ocelot trapping team member must be qualified as an “independent primary handler” who is listed in a USFWS permit as qualified to independently set traps; monitor their own trap line; sedate, handle, collar, sample, and release ocelots. Qualifications for independent primary handlers are outlined in USFWS permits and are typically based on number of experiences handling other non-listed felids and handling ocelots under supervision of trained handlers. Those who do not qualify as independent primary handlers may, once trained, set and check traps independently but required supervision by an independent primary handler to handle (i.e., sedate, radio collar, and collect biological data and samples) wild caught ocelots.
Trap Lines
A trap line is managed by at least one primary handler who is approved by USFWS. The primary handler, with the assistance of other trained individuals as necessary, processes a captured ocelot. Trap stations are defined as
149
a single trap or 2 adjacent traps that are separated by no more than 10 meters. USFWS permits will outline the number of trap stations that may be used in one trapline, where each trapline has at least one primary handler. Past permits have limited a trap line to 25 stations in locations where ocelots are known to occur and 40 stations in locations where ocelots are not known or suspected to occur.
1-2 trained individuals may set and check traps for the primary handler, but the independent primary handler must be present for ocelot handling and processing. Those checking traps should immediately notify members (cell phone, radio, GPS text messaging etc.) of the capture team of an ocelot (or other target animal) capture so that teams can mobilize. Individuals setting and checking traps may release non-target animals that are captured.
Photographs
If an ocelot is captured, photographs must be taken of the ocelot for an age and sex estimate (adult, subadult, kitten; male, female), and any potential injuries. These photos should be provided to the primary handler immediately for decision on whether to process or release the ocelot.
Procedure Prior to Team Arrival
Those checking the trap should cover it with a dark towel to calm the animal until the team arrives. Individuals who were checking the traps that day should set up the capture and handling equipment near the trap site at an appropriate distance from the captured cat to minimize stress and disturbance.
Attending Team
Upon final notification from field trapping personnel, capture and handling team leaders (primary handler, scientists, veterinarian, graduate students) should meet at a staging location and then move to the trap location. Upon arrival to the trap location, they will assess animal condition and decide to release or process the individual animal. Ocelots should be released without handling if the individual is too young, stressed, or already captured (within 45 days of previous capture event or three times within a 30-day period). Veterinarians and project supervisors are granted discretion in making the choice to process or release animals.
Handling of Captured Animal
If a determination to proceed with handling is made, animal weight will be visually estimated to generate recommended dosage for sedation. The following procedures will follow:
• Captured ocelots should be chemically immobilized using only established anesthetic drugs and dosages accepted for use on target species and under the supervision of individuals designated in the federal permit. See Appendix 5 for chemical immobilization dosage information and see Appendix 6 for regulations of the use of these controlled substances.
• Once the cat is at a stable anesthetic plane, immobilized cats should be transported to a processing site in a large carrier.
• Measures to prevent corneal drying of the eyes (e.g., ophthalmic ointment and/or covering the eyes with a cloth) should be applied.
150
• Internal body temperature, respiration rates, percent oxygen saturation, and pulse rates should be monitored regularly (recommend 5-minute intervals). The vetcorder used in field trapping efforts monitors continual pulse rate, electrocardiogram readings, internal body temperature, and percent oxygen saturation. Respiratory rates are taken manually. The body temperature should be maintained within 3 degrees of normal (average: 99-102.5).
o If the temperature exceeds 3 degrees of normal (hyperthermia), measures to reduce temperature (e.g., application of water or alcohol to foot pads, placement of ice bottle against abdomen) should be used.
• If the temperature drops more than 3 degrees below normal (hypothermia), measures to increase temperature (e.g., covering with a blanket, and placing thermal/heating pads against the abdomen) should be used.
• Place or replace appropriately sized and programmed collar.
• Check for a PIT Tag with a universal reader. Place PIT Tag if the animal has not previously been tagged. Current ocelot PIT tags are purchased from AVID Technologies.
• Morphometric data and biological samples should be collected as follows (Anesthesia/ Capture Record Sheet presented in Appendix 7):
o Body measurements
o Sex, age, reproductive status, and actual weight (kg)
o Photographs of all aspects of each individual, including left and right body and face, chest, hind end, forehead and direct face photo, back/spine, tail, abdomen, inside of all limbs (groin and axillary regions), teeth (left and right arcades and incisors), and current or previous injuries. See photos in Appendix 8 for examples. Photos should be taken of ocelots every time they are captured for individual identification and to monitor growth and changes in body size or mass over time.
o Health, including general coat condition, body condition (See Body Condition Scoring chart in General Health Monitoring), and any injuries.
o Ectoparasites are sampled from the ears, head, or on the body. Store unrefrigerated/unfrozen in 2-mL cryovial with 70% isopropyl alcohol. If testing of select pathogens does not recommend alcohol storage, ectoparasites can be stored frozen at -20℃ without alcohol (See General Health and Pathogen Testing chapter).
o Scat is collected from the trap or with 2 lubricated fecal loops and stored in a bag. Fill 3, 2-mL cryovials with 1 gram (g) of scat each and store 1 gram of feces in formalin if performing intestinal parasite ova assessment. Freeze remaining feces until desiccating for diet analysis.
o Blood samples (for health assessment, genetic monitoring, and disease profile). Total blood collection should generally not exceed 20 mL and should be divided as such:
▪ Serum sample: whole blood (minimum 2 mL, but ideally 8-9 mL) is placed into a red- or tiger-top blood tube. Keep cool and allow the sample to clot for ≥ 20 minutes. Centrifuge for 10 min @ 4,000 RPM and pipette serum only into 2-mL cryovials, 1 mL per vial
Store 1 vial in the refrigerator for CBC/chemistry if not performing that day and store all remaining vials frozen at -40℃.
▪ Whole blood sample: collect a minimum of 1 mL, ideally 3-7 mL of whole blood and place it into a purple-top EDTA blood tube. Invert the sample 8-10 times and keep cool.
151
Then, pipette entire contents into 2-mL cryovials, 1 mL per vial. Before freezing, create 3
4 blood smears on slides with microhematocrit tubes. Store 1 vial in the refrigerator for CBC/chemistry if not performing that same day and store the remaining frozen at -40℃.
o Genetic Samples: collect 2 mL of whole blood for DNA testing and put it into a tube with 6 mL of Longmire’s solution. Collect 2 mL of whole blood for RNA testing and put it into a tube with RNAlater. Store samples frozen at -40℃.
o Hair samples (including follicle) may be collected, stored in an envelope pouch, and refrigerated for additional genetic material.
o Vaginal cytology, where appropriate, should be collected with 1–2 sterile swabs. Wet swab with sterile water, lubricate plastic wand, insert the swab 2-3 inches into the vulva and rotate 360º Create 2 smears on microscope slides (with frosted end for labeling) and store unrefrigerated/unfrozen.
o Other biological samples: Nasal and oropharyngeal swabs, rectal swab, and conjunctival swabs can be taken for suspected active viral infections and/or consistently for monitoring of active infections in the population. (See General Health and Pathogen Testing chapter for more information).
o Abdominal ultrasonography will be performed, when appropriate, to assess pregnancy status and/or any other abnormalities noted on physical examination.
o Semen collection, where appropriate. See assisted reproduction technologies section above.
Recovery and Release
Captured animals should be released at the capture site. Once handling is completed, cats will be placed in a pet carrier, box trap, or other safe, secure container. Reversal drugs will be administered at that time and the cat should be allowed to recover completely from the effects of immobilization drugs before release (recovery requires the ocelot to be fully aware, alert, and able to function and move normally). A recovering cat can be released as soon as it is fully mobile if conditions arise indicating that the safety/welfare of the animal is otherwise jeopardized. For example, if the ocelot is overheated as evidenced by constant panting for more than 15 minutes, it may be released as soon as it is fully mobile. The recovery area must be isolated from human disturbance (other than visual monitoring by handlers). Recovering cats should be placed such that they are shaded and partially hidden, but so that they can still have airflow and be visually monitored from a distance as they recover. Monitoring should be done quietly and from a distance to avoid stimulating the recovering animal unless necessary. Monitoring should include a visual assessment of head/body control, thermoregulation, respiration, alertness and visual focus, muscle control, and mobility to determine when the animal is ready for release.
Capture Data
All data from capture and anesthesia datasheets should be recorded in the field and saved digitally in secure directories. Photos from captures should be added to an ocelot identification library that combines all photograph types into one centralized system to allow for identification purposes.
152
–
Camera Trapping
Camera traps provide support in monitoring collared animals, and they are necessary for monitoring uncollared ocelots and other uncollared sympatric animals. Camera trapping data will aid in population monitoring (abundance, size, survival, age-sex structure), current activity patterns, diel activity monitoring, occurrence of competitors and prey species, and ocelots’ interactions with these other species.
Camera monitoring can be used before ocelots are released onto the landscape to assess the baseline of wildlife communities before ocelots are reintroduced. Once ocelots are initially released, camera data will continue to provide wildlife data and it will provide supplemental information to complement ocelot collar data. After ocelots have established in the reintroduction site and begin breeding, camera trapping will be more crucial as released ocelots will no longer have their initial collars (though there will be attempts to capture ocelots and recollar them) and some new ocelots may be born.
Camera Grid
An ocelot camera trap monitoring program can be established at the reintroduction site. Initially, this program should cover SAVR, where ocelots will initially be released. Expansion of the camera trap grid to other nearby ranches is recommended if ocelots establish home ranges on neighboring properties and if landowners authorize camera monitoring on their properties. Ideally, a camera trap program can be developed to cover the extent of identified ocelot habitat as well as an approximately 1 km buffer around the habitat in case individuals move beyond identified habitat in the proposed reintroduction site.
The camera grid can follow standardized USFWS survey design for ocelot population monitoring: 1 x 1 km2 cells with one camera trap station (two camera traps) per cell [1-3]. Use of this system will allow compatibility and comparability to existing ocelot monitoring in the Ranch and Refuge Ocelot Populations in Texas.
It is recommended to begin camera monitoring 6 months to 1 year before any ocelot release to inform a baseline mammal community dataset and derive potential occupancy or densities of potential competitors and prey.
Recommended Camera Settings
Cameras should be programmed to take a multi-shot burst, which assists in capturing multiple angles of an animal when it passes through the detection window. A multi-shot burst of 3 photographs with a 30-second delay between captures may be used. Cameras should have a “high” sensitivity setting and a 24-hour detection window will ensure detection of all potential individuals. All cameras and memory cards should be labeled based on location, and cards should be cleared before deployment.
Camera Station Placement
Each camera station includes two camera traps to aid in the individual identification of ocelots (and other animals, if needed) by getting photos of both sides of the animal.
Cameras should be attached to woody shrub trunks or branches (or to t-posts if woody vegetation is not present, is not at the correct position, or is not large enough to hold a camera trap) at approximately 0.50 m high. The pair of cameras should be offset to avoid interference with each other. Cameras should be directed to game
153
trails or open areas with a clear field of observation. As needed, the woody and herbaceous vegetation in front of a camera can be cleared using common tools to ensure a clear view-shed (camera lens and infrared sensor) out to approximately 5 meters from the camera lens. The GPS locations of all cameras should be recorded and cameras must be armed and tested with at least one photograph burst upon deployment.
Camera grid maintenance
The frequency of camera checking is based on data needs, camera battery life, memory card storage capacity, and field logistics Given field logistics, cameras may be checked as frequently as necessary for meeting monitoring needs and downloading photos. Batteries and memory cards should be changed at each camera check. Camera sites that capture many photos should be checked more often as battery life and memory storage are depleted faster. Additionally, frequent visits to camera site at any sites in open areas where removal of tall herbaceous cover may be needed to clear the view field.
Camera Trap Image Processing
Protocols for wild cat camera trapping projects available from the Caesar Kleberg Wildlife Research Institute can be used for image pre-processing, photo sorting using artificial intelligence, and post-processing. Given privacy concerns related to ocelot reintroduction on private lands, caution should be taken when using an online repository program, such as Wildlife Insights, for processing ocelot camera data. Private property privacy concerns must be considered when distributing any ocelot photos.
Individual Ocelot Identification from Photos
All ocelots released to the reintroduced site, live captured, and photographed on camera traps should be individually identified based on photos, which should be added to a library that will be used for long-term population monitoring. Project personnel should create folders for each individually identified ocelot from all angles for each year of the cat’s life to track growth and to allow comparison of photographs over time to aid in identification. See Appendix 8 for recommended methodology for identifying individual ocelots based on coat patterns.
Den Site Identification and Monitoring
Den sites can be used to monitor reproduction in the reintroduced population. If ocelots are confirmed or suspected to be pregnant, potential dens can be distinguished by UHF locations showing a female not leaving an immediate area and by GPS location clusters of females that persist for more than 10-14 days with characteristic flower-petal foraging patterns (i.e., dam making large forays at night but returning to a centralized location). Based on previous field observations in Texas, ocelots typically select a patch of extremely dense cover, rocky areas, or even dense cordgrass for den sites. Ocelots may keep this den location for a few weeks after kittens are born, but as kittens grow older, the dam may move the kittens to additional den sites.
Camera Monitoring of Den Sites
If establishing camera monitoring around a den site, create a circular transect around the den at least 100 m from the predicted site. Set cameras to enclose this outer area to capture potential movement of the dam, kittens,
154
and additional ocelots into the area. Additional cameras may be placed around trails that extend from the den site.
Den Visitation
A site with a cluster of female ocelot location points may indicate denning and birthing behavior. Ocelot kittens may be monitored via camera traps. It is also possible to visit ocelot dens to observe kittens, if it is determined that there are minimal risks to the ocelot dam and kittens and that there are benefits of monitoring the kittens more closely. In initial efforts, it is recommended that 2-5 weeks after a predicted parturition date at the site of GPS clusters, a den site is searched for [4]. If the den and kittens are found, the location should be recorded and kittens’ sexes, weights, and lengths can be recorded, photographs can be taken for kittens’ coat patterns, and biological samples (scat) can be collected as needed for genetic analysis and health assessment. Individual identification methods, such as PIT Tags, can also be applied to kittens at this time. The use of any den visitation methods should be conservative to avoid negative impacts to ocelots, and methods should be adapted as necessary. After a den has been abandoned, it should be revisited to record more detailed characteristics of the den site.
Mortality
Mortality protocols for ocelots defined in the USFWS-issued Endangered Species Permit for ocelots should be followed in the event of an ocelot mortality (or injury). This includes notifying and coordinating with the USFWS ocelot species lead and medical personnel on proper treatment of the dead or injured ocelot, including whether it is necessary to alert the proper authorities (i.e., USFWS Officer, and Texas Parks and Wildlife Department Officer) so an investigation can be conducted, if warranted, and any further procedures can be completed prior to a necropsy.
Ocelot carcasses should be sent to an appropriate lab to conduct a necroscopy for determining cause of death and to collect tissue samples. If the necropsy must be delayed, the ocelot should be placed in a freezer to preserve the body, but since it is more difficult to manipulate and process tissues once frozen, it is recommended to perform the necropsy as soon as possible and store tissues in formalin. In order to provide the best possible samples and identification of abnormalities upon ocelot mortality, a necropsy should be performed within the first 24 hours of death. The necropsy can be conducted and tissues, and whole carcass can be collected, processed, and stored at an approved partner facility (e.g., Cesar Kleberg Wildlife Research Institute, East Foundation, USFWS, or TPWD). Tissues will be collected and stored in 10% formalin for further testing, if warranted. After the necropsy, a report should be created that includes (but is not limited to) the cause of death, abnormalities noted on necropsy, sex, age, and individual identification (if possible). The necropsy report and tissue samples can be provided to a selected lab for further evaluation of cause of death should the cause not be obvious at time of necropsy.
155
Data Storage
As stated in the programmatic Safe Harbor Agreement for ocelot reintroduction, ocelot monitoring data should be held by Caesar Kleberg Wildlife Research Institute, East Foundation, and/or TPWD to protect landowner privacy regarding ocelot presence and behaviors on their land. USFWS may access and be provided ocelot data, but data will not be held by USFWS since it would be subject to Freedom of Information Act requests that could disclose landowner information. Partners holding property-specific information about ocelots should not disclose property-specific information to the public without a landowner’s approval.
Ocelot data (raw and sorted/processed) can be securely stored by partners in both cloud-based and cold storage to provide multiple backups and prevent data loss. Cloud-based data held on secure network servers has the benefit of allowing researchers to access data remotely from anywhere, including while at field sites.
References
[1] Lombardi JV, Haines, AM, Watts III GW, Grassman LI, Janečka JE, Caso A, Carvajal S, Wardle ZM, Yamashita TJ, Stasey WC, Branney AB, Scognamillo DG, Campbell TA, Young Jr. JH, Tewes ME. 2022. Status and distribution of jaguarundi in Texas and Northeastern México: Making the case for extirpation and initiation of recovery in the United States. Ecology and Evolution 12: e8642.
[2] Lombardi J, Perotto-Baldivieso HL, Sergeyev M, Veals AM, Schofield L, Young JH, Tewes ME. 2021. Landscape structure of woody cover patches for endangered ocelots in southern Texas. Remote Sensing 13: 4001.
[3] Lombardi JV, Sergeyev M, Tewes ME, Schofield LR, Wilkins RN. 2022. Spatial capture-recapture and LiDAR-derived vegetation metrics reveal high densities of ocelots on Texas ranchlands. Frontiers of Conservation Science, 3, 1003044.
[4] Laack, LL, Tewes ME, Haines AM, Rappole JH. 2005. Reproductive life history of ocelots (Leopardus pardalis) in southern Texas. Acta Theriologica 50: 505-514.
156
Appendices
Appendix 1: Field Safety
Risks of field work in South Texas include but are not limited to heat exhaustion or heat stroke, dehydration, rattlesnakes, coral snakes, scorpions, wood ants, fire ants, brown recluse and black widow spiders, and Africanized bees. Make sure all liability, safety, and property rule forms are signed before entering any property. If staying on a property, make sure all lodging and vehicle (truck or Polaris UTV) use is coordinated with property administration.
The following are general guidelines for safety to be used in conjunction with existing property rules and safety procedures:
(1) Bring a GPS capable of sending text messages and an SOS beacon in case of emergencies.
(2) Safety Dress: Proper dress is needed when conducting fieldwork in mixed-to-dense thornscrub communities. Field pants and a long-sleeve durable brush jacket or heavy-duty button-down shirt are recommended. Snake boots or snake gaiters, leather gloves, safety glasses, and hats are recommended.
(3) Temperatures: Do not conduct collar recovery dives or similar intensive monitoring if the heat index is over 100 degrees F; you may quickly overheat in the brush. If a monitoring activity is needed in the summer, recommend attempting it only in the early morning before the heat of the day.
(4) Communication and Safety: A buddy system should be used when working in the field. Before conducting ocelot fieldwork, coordinate plans with reintroduction program managers and/or landowners so they know where you will be going and when you are expected back. Communicate upon safe return from the field. Be aware of designated HALO-Helicopter flight pad locations for emergencies.
(5) Field packs: Have plenty of water (e.g., 2 liters at all times, with an extra 1-2 gallons of water in the vehicle). Printed map and compass, knife, space blanket, and extra food.
(6) Vehicles: Make sure trucks and Polaris are filled with a full tank of gas before entering the field. Make sure researchers follow guidelines supplied by EAST, CKWRI, or others for proper truck care and maintenance. Vehicles should have tow ropes, functional spare tires, jack, shovels, and jumper cables. To avoid starting a fire, do not drive into tall grass or forbs. When returning vehicles, use an air pressure blower to clean out loose vegetation and grass from under and inside rangers and fill up the fuel tanks to full.
157
Appendix 2: Radio Collar Field Retrieval
Following the detection of a radio collar mortality signal, radio collars must be retrieved from the field to collect all data from the collar and to refurbish and redeploy it on another animal.
Collar retrieval steps:
(1) Locate collar by using last GPS locations and UHF signal (collar dive must take place at time when UHF signal has been programmed to be active)
(2) Once at a point identified by GPS and UHF, make concentric circles scanning the area to ensure complete coverage of the immediate area to find the collar. Often, collars may not be obvious in the brush. If collars are left out for extended periods, they may be dragged into woodrat middens or under dense herbaceous vegetation.
(3) Record GPS location of collar
(4) Describe the vegetation and area where the animal was found with pictures and notes that capture any unusual findings.
(5) Photograph and report the deceased ocelot, if needed
158
Appendix 3: Ocelot and Carnivore Live Trapping Supplies
Recommended trapping supplies and amounts are given below. Before each trapping season, the monitoring staff should inventory and check the functionality of all supplies in the capture kit and toolbox for the upcoming trapping period. During trapping, supplies should be inventoried every day after capture and weekly to ensure adequate supplies are available. Supplies should be restocked as necessary.
Capture Boxes
The list below encompasses what should be present in a capture box for processing an animal. There should be 2 capture boxes fully stocked and a large extra tote for refills of the capture boxes.
Capture Box Recommended Inventory: (Exact numbers of syringes and needles may not fit and having a few less than recommended is acceptable. Boxes should be filled to capacity with materials.)
EDTA Purple Top Tubes (10) 23 G Butterfly Needles (6)
Serum Red Top Tubes (10) 22 G 1 inch Needles (15)
White Plain Top Tubes (4) 20 G 1 inch Needles (15)
12 mL syringes (10)
6 mL syringes (10)
3 mL syringes (10)
1 mL syringes (8)
Size 10 blades (10)
Thermometer (1)
Eye lube (1)
Whirl packs (5)
Cryovials (10)
Gauze pad (5)
Medical tape roll (1)
Sterile swabs for biological sampling (10)
18 G 1 inch Needles (15)
Fluid Bags (2)
Fluid Lines (4)
Fecal Loops (3)
Tweezers (2)
Lube Packets (10)
Measuring tape (1)
Large tube of lube (1)
Calipers (1)
Biopsy punch 4 mm (ears) (5)
Blue-top (Falcon) tube (2)
Tourniquet (1)
Eraser (for ear biopsy)
9 V Battery (reader) (1)
Scissors (1)
Blindfold with clip (1)
Microscope slides (1 box)
PIT Tages (5)/ Reader (1)
Cotton balls (snack bag)
Zip ties (5)
Pen/Pencil/Sharpie (2 each)
Triple antibiotic ointment (1)
Stethoscope (1)
Sterile water (2)
Vaginal cytology swabs (5)
Biopsy punch 2 mm (spots) (5)
Mineral Oil (1 ounce)
Coin envelopes/zoologix cards
159
Small Tote Recommended Inventory:
Digital Scale (2)
Weigh Tarp (1)
Safety Glasses (4)
AAA Batteries
Orange Vests (4)
Face Masks (10)
Blindfold (2)
Spare batteries (2 9V; 4 AAA; 2 caliper battery)
Extra Tote Recommended Inventory: These items are intended to restock capture kits. Put items in separate quart Ziploc bags for easy access, to save space, and to prevent smashing of boxes. There are not specific amounts of every item that need to be in the extra tote, but a small bag of the smaller items (i.e., whirl packs) will suffice.
EDTA Purple Top Tubes (20) 23 G Butterfly Needles (40)
Paper Towels and S, M, L Gloves
Serum Red Top Tubes (20) 22 G 1 inch Needles (1 box) Scissors (1)
White Plain Top Tubes (20) 20 G 1 inch Needles (1 box)
12 mL syringes (40)
6 mL syringes (40)
3 mL syringes (40)
1 mL syringes (40)
Size 10 blades
Thermometer
Eye lube
Whirl packs
Cryovials
Gauze pack (1)
Biopsy punch 4 mm (5)
18 G 1 inch Needles (1 box)
Fluid Bags (10)
Fluid Lines (20)
Fecal Loops (12)
Tweezers
Lube Packets
9 V Batteries
Large tube of lube
Calipers
Handling gloves
Biopsy punch 2mm (5)
PIT Tags and Reader
Cotton balls
Zip ties
Pen/Pencil/Sharpie
Triple antibiotic ointment
Alcohol (2-3 bottles)
Peroxide (2-3 bottles)
Black trash bags
Sharps container (2)
Vaginal cytology swabs (12)
Clipboards (2)
Human forehead thermometer
160
Emergency Kit Inventory: An emergency box will be prepared by the veterinarian and brought to each capture in the event of an emergency.
Extra Inventory for Field Truck:
161
12 mL syringes (10) 24 G 3/4-inch IV Catheter (10) ET Tube (Size 8.5) 6 mL syringes (10) 22 G 3/4-inch IV Catheter (10) ET Tube (Size 9.0) 3 mL syringes (10) 20 G 3/4-inch IV Catheter (10) Pen/Pencil/Sharpie (2 each) 1 mL syringes (8) ET Tube (Size 5.0) Nasal Cannulas (5) 22 G 1 inch Needles (15) ET Tube (Size 5.5) ET Tube Stylets (3) 20 G 1 inch Needles (15) ET Tube (Size 6.0) IV Tape (4 rolls) 18 G 1 inch Needles (15) ET Tube (Size 6.5) ET Tube Gauze (small package) 22 G 1.5-inch Needles (15) ET Tube (Size 7.0) Atropine (1 bottle) 20 G 1.5-inch Needles (15) ET Tube (Size 7.5) Dopram (1 bottle) 18 G 1.5-inch Needles (15) ET Tube (Size 8.0) Epinephrine (1 bottle) Injection caps for catheter (10) Ambu Bag Oxygen
Gloves
Trap grate
sedation
Tent Tent Weights (2 tubs with walls)
Tables (4)
(field gloves) Dog Carrier (1)
for
injections
Trap Checking and Re-opening Inventory:
Towels (4-6)
Gloves (field gloves)
Face masks (2) Log List and Clipboard
Pigeon Food and Water
Pliers
Bucket and shovel Orange Flagging
Alcohol (1) Hydrogen Peroxide (1)
Collar Box Inventory:
Scissors (1) Sharpie (2)
Packing tape for applying collar magnets: 1 roll
Spare hardware (nylon-coated hex nuts, plates, brackets, extenders, magnets) and a telemetry receiver
Nut driver 5/16 inch (1)
Nut driver 5.5 mm (1)
Utility knife/box cutter (1)
Collar/drop-off programming cable
Thermal blanket
162
Appendix 4: Live Trapping Bait Animal Facilities
Potential On-site Facility
Permanent bait animal facilities on site at the reintroduction site will be logistical and cost-effective compared to housing pigeons elsewhere. Facilities should be large enough to house pigeons and roosting structures, nest boxes, and adequate feed and water sources. Management of bait animal facilities can be modeled on the aviary described below.
Duane Leech Aviary at Texas A&M University-Kingsville (TAMUK)
The following are general guidelines for pigeon care at the Duane Leech Aviary at the Caesar Kleberg Wildlife Research Institute Wildlife Center (also the location of the proposed Ocelot Conservation Facility). Pigeons are permanently held at the Aviary and may be temporarily shifted to field sites during trapping periods. For more specific details, please refer to TAMUK Animal Use Protocols. Typically, undergraduate student workers are hired to care for pigeons, but graduate students should be aware of and know how to care for animals. IACUC training is required of all personnel caring for pigeons (hired workers, undergraduate or graduate students, supervisors, etc.). Pigeon keeping duties and responsibilities:
● Aviary must be checked daily (including weekends and holidays)
● Workers should sign in and sign out, record daily duties, and initial.
● Provide and monitor food and water daily. Check the health of pigeons and notify the aviary supervisor of sick, injured, or deceased birds.
● Sweep aviary pens and halls daily, disposing of trash. Deep cleaning should be performed twice weekly.
● In the case of inclement weather (e.g., sub-zero temperatures are predicted), hang black tarps outside of the aviary to protect from cold wind chill temperatures.
Pigeon use for live trapping in the field requires prior approval from the aviary supervisor for the numbers of pigeons needed and where they will be located. Pigeons will remain under the supervision, care and responsibility of the approved researcher and care is not to be outsourced to other institutions, researchers, or other projects without approval from the aviary supervisor. Pigeons are kept in approved bait boxes at field sites and supplied with feed, water, and a roost throughout the entire time period. Pigeon mortalities in the field must be reported. Pigeons must be removed from bait boxes and returned to the aviary or other holding area if trapping ceases for extended periods (more than 4 days) due to inclement weather or other factors. Pigeons can remain in their current bait box with continual monitoring and care for short periods (2-3 days).
163
Appendix 5: Ocelot Sedation Protocols
As of spring 2023, the current approved sedation regime for ocelots uses a medetomidine-ketamine mixture that is partially reversed with atipamezole. USFWS, EAST, and CKWRI veterinarians and biologists should coordinate to develop any necessary changes to the sedation regime. Ketamine (IM) target dosage range 5 to 8 mg/kg and Medetomidine (IM) target dosage range 0.05-0.07 mg/kg. 5 mg Atipamezole (IM) per 1 mg Medetomidine given. Medications can be administered IV if instructed by the veterinarian.
OCELOT (dose in mL) 5 mg/kg Ketamine; 0.05 mg/kg Medetomidine
164
WEIGHT (lb.) WEIGHT (kg) KETAMINE 200 mg/mL MEDETOMIDINE 10 mg/mL ATIPAMEZOLE 25 mg/mL 8 3.64 0.09 0.02 0.04 10 4.55 0.11 0.025 0.05 12 5.45 0.14 0.03 0.06 14 6.36 0.16 0.03 0.06 16 7.27 0.18 0.035 0.07 18 8.18 0.20 0.04 0.08 20 9.09 0.23 0.045 0.09 22 10.00 0.25 0.05 0.10 24 10.91 0.27 0.055 0.11 26 11.82 0.30 0.06 0.12 28 12.73 0.32 0.065 0.13 30 13.64 0.34 0.07 0.14
OCELOT (dose in mL) 8 mg/kg Ketamine; 0.05 mg/kg
165
Medetomidine WEIGHT (lb.) WEIGHT (kg) KETAMINE 200 mg/mL MEDETOMIDINE 10 mg/mL ATIPAMEZOLE 25 mg/mL 8 3.64 0.15 0.02 0.04 10 4.55 0.18 0.025 0.05 12 5.45 0.22 0.03 0.06 14 6.36 0.26 0.03 0.06 16 7.27 0.29 0.035 0.07 18 8.18 0.33 0.04 0.08 20 9.09 0.36 0.045 0.09 22 10.00 0.40 0.05 0.10 24 10.91 0.44 0.055 0.11 26 11.82 0.47 0.06 0.12 28 12.73 0.51 0.065 0.13 30 13.64 0.55 0.07 0.14
Appendix 6: Regulations for Chemical Immobilization Drugs
Ketamine is a controlled substance. A list of other controlled substances can be found at https://www.dea.gov/drug-information/drug-scheduling and https://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf Controlled substances must remain behind two structures of containment at the approved location of the address listed on the DEA registration holder’s documentation (for example: a lock box inside of a locked cabinet with different keys or codes for each containment source). When traveling to field sites with controlled substances, substances must be transported in a locked safe and should not be left in a vehicle unattended. A controlled substance log must be kept that includes substance purchases, substance use per individual animal, initials of personnel using the substance, and substance disposal. Each substance (drug) should have its own separate log and each vial should have a unique bottle number.
Example of Controlled Substance Check-In
Example of Controlled Substance Use Form (KETAMINE)
166
PRODUCT NAME ASSIGNED BOTTLE NO. AMOUNT DATE RECEIVED EXP DATE SUPPLIER LOT OR SERIAL NO. INVOICE NUMBER INITIALS Ketamine TK2-5 10 mL 7 DEC 2021 OCT 2022 ZooPharm, LLC K212710 159148 ARW
DATE ANIMAL IDENTIFICATION # DRUG VIAL # SPECIES INITIAL S AMT DISPENSED AMT DISCARDED NEW BALANCE 10 mL 1 2 JAN 2022 E13M TK2-5 OCELOT ARW 0.5 mL 0.0 mL 9.5 mL
Appendix 7: Example of Ocelot Capture Data Sheets

167

168
Appendix 8: Ocelot Photo Identification Methods

Ocelots have unique asymmetrical pelage patterns on each flank (Figures A-B) and unique forehead facial patterns when viewed head-on (Figure C) that allow individual identification. If forehead marks are available in an image, the marks provide a fast and straightforward way to easily identify an individual. Meanwhile, a minimum of 3 individual marks, spots, or rosettes are recommended to identify individuals when using patterns on right or left flanks. The identifier must consider distortion, distance from the camera lens, and abrasions when comparing camera trap photos to capture photos (Figure C). Additionally, when comparing capture and camera trap images, the angle of the photo, blurriness, and how far the animal is from the camera can distort the image in a way that an ocelot that seems different is the same cat and vice versa (Figures C-D). Problem areas include the upper shoulder to the forelimb and back legs. When ocelots walk, these entire areas are visible. However, when on a table during a capture, the upper shoulder and back legs are often rolled toward the top and not stretched out, which can obscure the true markings in photos. When taking photos of a captured ocelot, pull the limbs down to see the entire coat pattern. If an ocelot cannot be identified due to poor angle, obstructed view or grainy image, it can be classified as unknown.
The process of identifying individuals is done in two steps: human-verified assessment using the information above followed by use of the machine-learning algorithm HotSpotter to confirm identifications. A two-step process with a double confirmation minimizes identification errors. It is suggested that researchers use the program Timelapse to help tag photos in this process.
169
Figure A. Comparison of spotting patterns of three different ocelots. The left panel shows unique rosettes and spotting patterns from a single female, whereas the two photos on the right are of two different unique ocelots.
Figure B. Confirmed identity of a female ocelot. Red circles indicate 3 unique rosette and spotting patterns on the shoulder, forehead, back, and lower side. The top photo is from May 2017 and the lower photo is from Nov 2021. Sunlight, coat condition, angle, and image resolution can differ in different photos of the same individual.

170

171
Figure C. Zoomed-in camera trap and capture photos of a single female ocelot. Distortions in angle, sunlight, abrasions, image resolution/blurriness, and distance from the camera can cause individuals to appear to be different cats. The camera trap photo is from November 2021 and the captured photo is from January 2022.
































